User login
POISE-3 backs wider use of tranexamic acid in noncardiac surgery
The antifibrinolytic tranexamic acid (TXA) reduced serious bleeding without a significant effect on major vascular outcomes in patients undergoing noncardiac surgery at risk for these complications in the POISE-3 trial.
TXA cut the primary efficacy outcome of life-threatening, major, and critical organ bleeding at 30 days by 24% compared with placebo (9.1% vs. 11.7%; hazard ratio [HR], 0.76; P < .0001).
The primary safety outcome of myocardial injury after noncardiac surgery (MINS), nonhemorrhagic stroke, peripheral arterial thrombosis, and symptomatic proximal venous thromboembolism (VTE) at 30 days occurred in 14.2% vs.. 13.9% of patients, respectively (HR, 1.023). This failed, however, to meet the study›s threshold to prove TXA noninferior to placebo (one-sided P = .044).
There was no increased risk for death or stroke with TXA, according to results published April 2 in the New England Journal of Medicine.
Principal investigator P.J. Devereaux, MD, PhD, Population Health Research Institute and McMaster University, Hamilton, Ontario, Canada, pointed out that there is only a 4.4% probability that the composite vascular outcome hazard ratio was above the noninferiority margin and that just 10 events separated the two groups (649 vs.. 639).
“Healthcare providers and patients will have to weigh a clear beneficial reduction in the composite bleeding outcome, which is an absolute difference of 2.7%, a result that was highly statistically significant, versus a low probability of a small increase in risk of the composite vascular endpoint, with an absolute difference of 0.3%,” a nonsignificant result, Dr. Devereaux said during the formal presentation of the results at the hybrid annual scientific sessions of the American College of Cardiology.
The findings, he said, should also be put in the context that 300 million adults have a major surgery each year worldwide and most don’t receive TXA. At the same time, there’s an annual global shortage of 30 million blood product units, and surgical bleeding accounts for up to 40% of all transfusions.
“POISE-3 identifies that use of TXA could avoid upwards of 8 million bleeding events resulting in transfusion on an annual basis, indicating potential for large public health and clinical benefit if TXA become standard practice in noncardiac surgery,” Dr. Devereaux said during the late-breaking trial session.
TXA is indicated for heavy menstrual bleeding and hemophilia and has been used in cardiac surgery, but it is increasingly being used in noncardiac surgeries. As previously reported, POISE showed that the beta-blocker metoprolol lowered the risk for myocardial infarction (MI) but increased the risk for severe stroke and overall death, whereas in POISE-2, perioperative low-dose aspirin lowered the risk for MI but was linked to more major bleeding.
The cumulative data have not shown an increased risk for thrombotic events in other settings, Dr. Devereaux told this news organization.
“I’m a cardiologist, and I think that we’ve been guilty at times of always only focusing on the thrombotic side of the equation and ignoring that bleeding is a very important aspect of the circulatory system,” he said. “And I think this shows for the first time clear unequivocal evidence that there’s a cheap, very encouraging, safe way to prevent this.”
“An important point is that if you can give tranexamic acid and prevent bleeding in your cardiac patients having noncardiac surgery, then you can prevent the delay of reinitiating their anticoagulants and their antiplatelets after surgery and getting them back on the medications that are important for them to prevent their cardiovascular event,” Dr. Devereaux added.
Discussant Michael J. Mack, MD, commented that TXA, widely used in cardiac surgery, is an old, inexpensive drug that “should be more widely used in noncardiac surgery.” Dr. Mack, from Baylor Scott & White Health, Dallas, added that he would limit it to major noncardiac surgery.
International trial
PeriOperative ISchemic Evaluation-3 (POISE-3) investigators at 114 hospitals in 22 countries (including countries in North and South America, Europe, and Africa; Russia; India; and Australia) randomly assigned 9,535 patients, aged 45 years or older, with or at risk for cardiovascular and bleeding complications to receive a TXA 1-g intravenous bolus or placebo at the start and end of inpatient noncardiac surgery.
Patients taking at least one long-term antihypertensive medication were also randomly assigned to a perioperative hypotension- or hypertension-avoidance strategy, which differ in the use of antihypertensives on the morning of surgery and the first 2 days after surgery, and in the target mean arterial pressure during surgery. Results from these cohorts will be presented in a separate session on April 4.
The study had planned to enroll 10,000 patients but was stopped early by the steering committee because of financial constraints resulting from slow enrollment during the pandemic. The decision was made without knowledge of the trial results but with knowledge that aggregate composite bleeding and vascular outcomes were higher than originally estimated, Dr. Devereaux noted.
Among all participants, the mean age was 70 years, 56% were male, almost a third had coronary artery disease, 15% had peripheral artery disease, and 8% had a prior stroke. About 80% were undergoing major surgery. Adherence to the study medications was 96.3% in both groups.
Secondary bleeding outcomes were lower in the TXA and placebo groups, including bleeding independently associated with mortality after surgery (8.7% vs. 11.3%), life-threatening bleeding (1.6% vs. 1.7%), major bleeding (7.6% vs. 10.4%), and critical organ bleeding (0.3% vs. 0.4%).
Importantly, the TXA group had significantly lower rates of International Society on Thrombosis and Haemostasis major bleeding (6.6% vs. 8.7%; P = .0001) and the need for transfusion of 1 or more units of packed red blood cells (9.4% vs. 12.0%; P <.0001), Dr. Devereaux noted.
In terms of secondary vascular outcomes, there were no significant differences between the TXA and placebo groups in rates of MINS (12.8% vs. 12.6%), MINS not fulfilling definition of MI (both 11.5%), MI (1.4% vs. 1.1%), and the net risk-benefit outcome (a composite of vascular death and nonfatal life-threatening, major, or critical organ bleeding, MINS, stroke, peripheral arterial thrombosis, and symptomatic proximal VTE; 20.7% vs. 21.9%).
The two groups had similar rates of all-cause (1.1% vs. 1.2%) and vascular (0.5% vs. 0.6%) mortality.
There also were no significant differences in other tertiary outcomes, such as acute kidney injury (14.1% vs. 13.7%), rehospitalization for vascular reasons (1.8% vs. 1.6%), or seizures (0.2% vs. <0.1%). The latter has been a concern, with the risk reported to increase with higher doses.
Subgroup analyses
Preplanned subgroup analyses showed a benefit for TXA over placebo for the primary efficacy outcome in orthopedic and nonorthopedic surgery and in patients with hemoglobin level below 120 g/L or 120 g/L or higher, with an estimated glomerular filtration rate less than 45 mL/min/1.73 m 2 or 45 mL/min/1.73 m 2 or higher, or with an N-terminal pro– B-type natriuretic peptide level below 200 ng/L or 200 ng/L or higher.
For the primary safety outcome, the benefit favored placebo but the interaction was not statistically significant for any of the four subgroups.
A post hoc subgroup analysis also showed similar results across the major categories of surgery, including general, vascular, urologic, and gynecologic, Dr. Devereaux told this news organization.
Although TXA is commonly used in orthopedic procedures, Dr. Devereaux noted, in other types of surgeries, “it’s not used at all.” But because TXA “is so cheap, and we can apply it to a broad population, even at an economic level it looks like it’s a winner to give to almost all patients having noncardiac surgery.”
The team also recently published a risk prediction tool that can help estimate a patient’s baseline risk for bleeding.
“So just using a model, which will bring together the patient’s type of surgery and their risk factors, you can look to see, okay, this is enough risk of bleeding, I’m just going to give tranexamic acid,” he said. “We will also be doing economic analyses because blood is also not cheap.”
The study was funded by the Canadian Institutes of Health Research, National Health and Medical Research Council (Australia), and the Research Grant Council (Hong Kong). Dr. Devereaux reports research/research grants from Abbott Diagnostics, Philips Healthcare, Roche Diagnostics, and Siemens. Dr. Mack reports receiving research grants from Abbott Vascular, Edwards Lifesciences, and Medtronic.
A version of this article first appeared on Medscape.com.
The antifibrinolytic tranexamic acid (TXA) reduced serious bleeding without a significant effect on major vascular outcomes in patients undergoing noncardiac surgery at risk for these complications in the POISE-3 trial.
TXA cut the primary efficacy outcome of life-threatening, major, and critical organ bleeding at 30 days by 24% compared with placebo (9.1% vs. 11.7%; hazard ratio [HR], 0.76; P < .0001).
The primary safety outcome of myocardial injury after noncardiac surgery (MINS), nonhemorrhagic stroke, peripheral arterial thrombosis, and symptomatic proximal venous thromboembolism (VTE) at 30 days occurred in 14.2% vs.. 13.9% of patients, respectively (HR, 1.023). This failed, however, to meet the study›s threshold to prove TXA noninferior to placebo (one-sided P = .044).
There was no increased risk for death or stroke with TXA, according to results published April 2 in the New England Journal of Medicine.
Principal investigator P.J. Devereaux, MD, PhD, Population Health Research Institute and McMaster University, Hamilton, Ontario, Canada, pointed out that there is only a 4.4% probability that the composite vascular outcome hazard ratio was above the noninferiority margin and that just 10 events separated the two groups (649 vs.. 639).
“Healthcare providers and patients will have to weigh a clear beneficial reduction in the composite bleeding outcome, which is an absolute difference of 2.7%, a result that was highly statistically significant, versus a low probability of a small increase in risk of the composite vascular endpoint, with an absolute difference of 0.3%,” a nonsignificant result, Dr. Devereaux said during the formal presentation of the results at the hybrid annual scientific sessions of the American College of Cardiology.
The findings, he said, should also be put in the context that 300 million adults have a major surgery each year worldwide and most don’t receive TXA. At the same time, there’s an annual global shortage of 30 million blood product units, and surgical bleeding accounts for up to 40% of all transfusions.
“POISE-3 identifies that use of TXA could avoid upwards of 8 million bleeding events resulting in transfusion on an annual basis, indicating potential for large public health and clinical benefit if TXA become standard practice in noncardiac surgery,” Dr. Devereaux said during the late-breaking trial session.
TXA is indicated for heavy menstrual bleeding and hemophilia and has been used in cardiac surgery, but it is increasingly being used in noncardiac surgeries. As previously reported, POISE showed that the beta-blocker metoprolol lowered the risk for myocardial infarction (MI) but increased the risk for severe stroke and overall death, whereas in POISE-2, perioperative low-dose aspirin lowered the risk for MI but was linked to more major bleeding.
The cumulative data have not shown an increased risk for thrombotic events in other settings, Dr. Devereaux told this news organization.
“I’m a cardiologist, and I think that we’ve been guilty at times of always only focusing on the thrombotic side of the equation and ignoring that bleeding is a very important aspect of the circulatory system,” he said. “And I think this shows for the first time clear unequivocal evidence that there’s a cheap, very encouraging, safe way to prevent this.”
“An important point is that if you can give tranexamic acid and prevent bleeding in your cardiac patients having noncardiac surgery, then you can prevent the delay of reinitiating their anticoagulants and their antiplatelets after surgery and getting them back on the medications that are important for them to prevent their cardiovascular event,” Dr. Devereaux added.
Discussant Michael J. Mack, MD, commented that TXA, widely used in cardiac surgery, is an old, inexpensive drug that “should be more widely used in noncardiac surgery.” Dr. Mack, from Baylor Scott & White Health, Dallas, added that he would limit it to major noncardiac surgery.
International trial
PeriOperative ISchemic Evaluation-3 (POISE-3) investigators at 114 hospitals in 22 countries (including countries in North and South America, Europe, and Africa; Russia; India; and Australia) randomly assigned 9,535 patients, aged 45 years or older, with or at risk for cardiovascular and bleeding complications to receive a TXA 1-g intravenous bolus or placebo at the start and end of inpatient noncardiac surgery.
Patients taking at least one long-term antihypertensive medication were also randomly assigned to a perioperative hypotension- or hypertension-avoidance strategy, which differ in the use of antihypertensives on the morning of surgery and the first 2 days after surgery, and in the target mean arterial pressure during surgery. Results from these cohorts will be presented in a separate session on April 4.
The study had planned to enroll 10,000 patients but was stopped early by the steering committee because of financial constraints resulting from slow enrollment during the pandemic. The decision was made without knowledge of the trial results but with knowledge that aggregate composite bleeding and vascular outcomes were higher than originally estimated, Dr. Devereaux noted.
Among all participants, the mean age was 70 years, 56% were male, almost a third had coronary artery disease, 15% had peripheral artery disease, and 8% had a prior stroke. About 80% were undergoing major surgery. Adherence to the study medications was 96.3% in both groups.
Secondary bleeding outcomes were lower in the TXA and placebo groups, including bleeding independently associated with mortality after surgery (8.7% vs. 11.3%), life-threatening bleeding (1.6% vs. 1.7%), major bleeding (7.6% vs. 10.4%), and critical organ bleeding (0.3% vs. 0.4%).
Importantly, the TXA group had significantly lower rates of International Society on Thrombosis and Haemostasis major bleeding (6.6% vs. 8.7%; P = .0001) and the need for transfusion of 1 or more units of packed red blood cells (9.4% vs. 12.0%; P <.0001), Dr. Devereaux noted.
In terms of secondary vascular outcomes, there were no significant differences between the TXA and placebo groups in rates of MINS (12.8% vs. 12.6%), MINS not fulfilling definition of MI (both 11.5%), MI (1.4% vs. 1.1%), and the net risk-benefit outcome (a composite of vascular death and nonfatal life-threatening, major, or critical organ bleeding, MINS, stroke, peripheral arterial thrombosis, and symptomatic proximal VTE; 20.7% vs. 21.9%).
The two groups had similar rates of all-cause (1.1% vs. 1.2%) and vascular (0.5% vs. 0.6%) mortality.
There also were no significant differences in other tertiary outcomes, such as acute kidney injury (14.1% vs. 13.7%), rehospitalization for vascular reasons (1.8% vs. 1.6%), or seizures (0.2% vs. <0.1%). The latter has been a concern, with the risk reported to increase with higher doses.
Subgroup analyses
Preplanned subgroup analyses showed a benefit for TXA over placebo for the primary efficacy outcome in orthopedic and nonorthopedic surgery and in patients with hemoglobin level below 120 g/L or 120 g/L or higher, with an estimated glomerular filtration rate less than 45 mL/min/1.73 m 2 or 45 mL/min/1.73 m 2 or higher, or with an N-terminal pro– B-type natriuretic peptide level below 200 ng/L or 200 ng/L or higher.
For the primary safety outcome, the benefit favored placebo but the interaction was not statistically significant for any of the four subgroups.
A post hoc subgroup analysis also showed similar results across the major categories of surgery, including general, vascular, urologic, and gynecologic, Dr. Devereaux told this news organization.
Although TXA is commonly used in orthopedic procedures, Dr. Devereaux noted, in other types of surgeries, “it’s not used at all.” But because TXA “is so cheap, and we can apply it to a broad population, even at an economic level it looks like it’s a winner to give to almost all patients having noncardiac surgery.”
The team also recently published a risk prediction tool that can help estimate a patient’s baseline risk for bleeding.
“So just using a model, which will bring together the patient’s type of surgery and their risk factors, you can look to see, okay, this is enough risk of bleeding, I’m just going to give tranexamic acid,” he said. “We will also be doing economic analyses because blood is also not cheap.”
The study was funded by the Canadian Institutes of Health Research, National Health and Medical Research Council (Australia), and the Research Grant Council (Hong Kong). Dr. Devereaux reports research/research grants from Abbott Diagnostics, Philips Healthcare, Roche Diagnostics, and Siemens. Dr. Mack reports receiving research grants from Abbott Vascular, Edwards Lifesciences, and Medtronic.
A version of this article first appeared on Medscape.com.
The antifibrinolytic tranexamic acid (TXA) reduced serious bleeding without a significant effect on major vascular outcomes in patients undergoing noncardiac surgery at risk for these complications in the POISE-3 trial.
TXA cut the primary efficacy outcome of life-threatening, major, and critical organ bleeding at 30 days by 24% compared with placebo (9.1% vs. 11.7%; hazard ratio [HR], 0.76; P < .0001).
The primary safety outcome of myocardial injury after noncardiac surgery (MINS), nonhemorrhagic stroke, peripheral arterial thrombosis, and symptomatic proximal venous thromboembolism (VTE) at 30 days occurred in 14.2% vs.. 13.9% of patients, respectively (HR, 1.023). This failed, however, to meet the study›s threshold to prove TXA noninferior to placebo (one-sided P = .044).
There was no increased risk for death or stroke with TXA, according to results published April 2 in the New England Journal of Medicine.
Principal investigator P.J. Devereaux, MD, PhD, Population Health Research Institute and McMaster University, Hamilton, Ontario, Canada, pointed out that there is only a 4.4% probability that the composite vascular outcome hazard ratio was above the noninferiority margin and that just 10 events separated the two groups (649 vs.. 639).
“Healthcare providers and patients will have to weigh a clear beneficial reduction in the composite bleeding outcome, which is an absolute difference of 2.7%, a result that was highly statistically significant, versus a low probability of a small increase in risk of the composite vascular endpoint, with an absolute difference of 0.3%,” a nonsignificant result, Dr. Devereaux said during the formal presentation of the results at the hybrid annual scientific sessions of the American College of Cardiology.
The findings, he said, should also be put in the context that 300 million adults have a major surgery each year worldwide and most don’t receive TXA. At the same time, there’s an annual global shortage of 30 million blood product units, and surgical bleeding accounts for up to 40% of all transfusions.
“POISE-3 identifies that use of TXA could avoid upwards of 8 million bleeding events resulting in transfusion on an annual basis, indicating potential for large public health and clinical benefit if TXA become standard practice in noncardiac surgery,” Dr. Devereaux said during the late-breaking trial session.
TXA is indicated for heavy menstrual bleeding and hemophilia and has been used in cardiac surgery, but it is increasingly being used in noncardiac surgeries. As previously reported, POISE showed that the beta-blocker metoprolol lowered the risk for myocardial infarction (MI) but increased the risk for severe stroke and overall death, whereas in POISE-2, perioperative low-dose aspirin lowered the risk for MI but was linked to more major bleeding.
The cumulative data have not shown an increased risk for thrombotic events in other settings, Dr. Devereaux told this news organization.
“I’m a cardiologist, and I think that we’ve been guilty at times of always only focusing on the thrombotic side of the equation and ignoring that bleeding is a very important aspect of the circulatory system,” he said. “And I think this shows for the first time clear unequivocal evidence that there’s a cheap, very encouraging, safe way to prevent this.”
“An important point is that if you can give tranexamic acid and prevent bleeding in your cardiac patients having noncardiac surgery, then you can prevent the delay of reinitiating their anticoagulants and their antiplatelets after surgery and getting them back on the medications that are important for them to prevent their cardiovascular event,” Dr. Devereaux added.
Discussant Michael J. Mack, MD, commented that TXA, widely used in cardiac surgery, is an old, inexpensive drug that “should be more widely used in noncardiac surgery.” Dr. Mack, from Baylor Scott & White Health, Dallas, added that he would limit it to major noncardiac surgery.
International trial
PeriOperative ISchemic Evaluation-3 (POISE-3) investigators at 114 hospitals in 22 countries (including countries in North and South America, Europe, and Africa; Russia; India; and Australia) randomly assigned 9,535 patients, aged 45 years or older, with or at risk for cardiovascular and bleeding complications to receive a TXA 1-g intravenous bolus or placebo at the start and end of inpatient noncardiac surgery.
Patients taking at least one long-term antihypertensive medication were also randomly assigned to a perioperative hypotension- or hypertension-avoidance strategy, which differ in the use of antihypertensives on the morning of surgery and the first 2 days after surgery, and in the target mean arterial pressure during surgery. Results from these cohorts will be presented in a separate session on April 4.
The study had planned to enroll 10,000 patients but was stopped early by the steering committee because of financial constraints resulting from slow enrollment during the pandemic. The decision was made without knowledge of the trial results but with knowledge that aggregate composite bleeding and vascular outcomes were higher than originally estimated, Dr. Devereaux noted.
Among all participants, the mean age was 70 years, 56% were male, almost a third had coronary artery disease, 15% had peripheral artery disease, and 8% had a prior stroke. About 80% were undergoing major surgery. Adherence to the study medications was 96.3% in both groups.
Secondary bleeding outcomes were lower in the TXA and placebo groups, including bleeding independently associated with mortality after surgery (8.7% vs. 11.3%), life-threatening bleeding (1.6% vs. 1.7%), major bleeding (7.6% vs. 10.4%), and critical organ bleeding (0.3% vs. 0.4%).
Importantly, the TXA group had significantly lower rates of International Society on Thrombosis and Haemostasis major bleeding (6.6% vs. 8.7%; P = .0001) and the need for transfusion of 1 or more units of packed red blood cells (9.4% vs. 12.0%; P <.0001), Dr. Devereaux noted.
In terms of secondary vascular outcomes, there were no significant differences between the TXA and placebo groups in rates of MINS (12.8% vs. 12.6%), MINS not fulfilling definition of MI (both 11.5%), MI (1.4% vs. 1.1%), and the net risk-benefit outcome (a composite of vascular death and nonfatal life-threatening, major, or critical organ bleeding, MINS, stroke, peripheral arterial thrombosis, and symptomatic proximal VTE; 20.7% vs. 21.9%).
The two groups had similar rates of all-cause (1.1% vs. 1.2%) and vascular (0.5% vs. 0.6%) mortality.
There also were no significant differences in other tertiary outcomes, such as acute kidney injury (14.1% vs. 13.7%), rehospitalization for vascular reasons (1.8% vs. 1.6%), or seizures (0.2% vs. <0.1%). The latter has been a concern, with the risk reported to increase with higher doses.
Subgroup analyses
Preplanned subgroup analyses showed a benefit for TXA over placebo for the primary efficacy outcome in orthopedic and nonorthopedic surgery and in patients with hemoglobin level below 120 g/L or 120 g/L or higher, with an estimated glomerular filtration rate less than 45 mL/min/1.73 m 2 or 45 mL/min/1.73 m 2 or higher, or with an N-terminal pro– B-type natriuretic peptide level below 200 ng/L or 200 ng/L or higher.
For the primary safety outcome, the benefit favored placebo but the interaction was not statistically significant for any of the four subgroups.
A post hoc subgroup analysis also showed similar results across the major categories of surgery, including general, vascular, urologic, and gynecologic, Dr. Devereaux told this news organization.
Although TXA is commonly used in orthopedic procedures, Dr. Devereaux noted, in other types of surgeries, “it’s not used at all.” But because TXA “is so cheap, and we can apply it to a broad population, even at an economic level it looks like it’s a winner to give to almost all patients having noncardiac surgery.”
The team also recently published a risk prediction tool that can help estimate a patient’s baseline risk for bleeding.
“So just using a model, which will bring together the patient’s type of surgery and their risk factors, you can look to see, okay, this is enough risk of bleeding, I’m just going to give tranexamic acid,” he said. “We will also be doing economic analyses because blood is also not cheap.”
The study was funded by the Canadian Institutes of Health Research, National Health and Medical Research Council (Australia), and the Research Grant Council (Hong Kong). Dr. Devereaux reports research/research grants from Abbott Diagnostics, Philips Healthcare, Roche Diagnostics, and Siemens. Dr. Mack reports receiving research grants from Abbott Vascular, Edwards Lifesciences, and Medtronic.
A version of this article first appeared on Medscape.com.
FROM ACC 2022
Surgery groups push back on VARC-3 valve trial definitions
Five international cardiac surgery associations have banded together to address “substantive concerns” regarding the recently updated Valve Academic Research Consortium 3 (VARC-3) clinical endpoint definitions for aortic valve research.
The VARC-3 update was a multidisciplinary effort that included more than a dozen new or modified definitions for use in transcatheter and surgical aortic valve replacement (TAVR/SAVR) clinical trials, but drew criticism last year from surgeons that some of its definitions favor TAVR over surgery and that its writing committee had deep ties to industry and lacked diversity.
The new surgical associations’ position statement calls out five specific VARC-3 definitions – rehospitalization, valve thrombosis, bleeding, myocardial infarction (MI), and left bundle-branch block (LBBB).
The statement was jointly issued by the Society of Thoracic Surgeons (STS), the American Association for Thoracic Surgery, the European Association for Cardio-Thoracic Surgery, the Asian Society for Cardiovascular and Thoracic Surgery, and the Latin American Association of Cardiac and Endovascular Surgery.
It was copublished in Annals of Thoracic Surgery, the Journal of Thoracic and Cardiovascular Surgery, the European Journal of Cardio-Thoracic Surgery, and the Asian Cardiovascular and Thoracic Annals.
“We hope that this message can be seen, even if it’s somewhat difficult to hear sometimes, as positive constructive criticism compared to some of the dialogue that we’ve had on social media,” lead author Patrick O. Myers, MD, Lausanne (Switzerland) University Hospital, said in an interview. “It’s not criticizing people or the process but just trying to make these definitions better to ensure the good design of clinical trials.”
The president of each surgical association recommended representatives to help write the position statement, and once completed over Zoom meetings, it received formal endorsement from each association prior to publication, he said.
Reached for comment, VARC-3 lead author Philippe Généreux, MD, Gagnon Cardiovascular Institute, Morristown (N.J.) Medical Center, said, “I was pleasantly surprised that their comments were actually pretty minor and that most of these comments are really more a reflection, not of the validity of the definitions, but rather their applications.”
He noted that all the potential issues with the definitions were already discussed during the making of VARC-3 and resolved by consensus of more than 50 experts including the STS president at the time, Food and Drug Administration officials, and experts from the community.
“To be quite honest, I’m not sure they have consensus,” Dr. Généreux said. He added that the writing committee welcomes input from anyone, but “we’re not going to change the definitions to please eight individuals if we strongly believe by consensus of experts in the field that this is not the right thing to do.”
Rehospitalizations and valve thrombosis
The surgical associations praise VARC-3 for providing a standardized definition of bioprosthetic valve failure, but say they will not endorse the inclusion of rehospitalization as a component of the primary efficacy composite endpoint along with all-cause mortality, stroke, and quality of life.
They note that rehospitalizations outnumber mortality events, especially in short follow-up trials, and that the superiority of TAVR at 1 year in the PARTNER 3 trial of low-risk patients was driven primarily by more rehospitalizations in the surgical arm, but that this superiority was waning at 2 years of follow-up.
“The first thing we are calling for is that it shouldn’t be part of the primary composite outcome measure,” Dr. Myers said. But if it really has to be included, a 30-day blanking period for rehospitalization “would acknowledge that there’s a greater risk of rehospitalization during the acute phase of recovering from surgery.”
Dr. Généreux said that VARC-3 provides granular details for defining the different types of hospitalizations, but that a 30-day blanking period makes no sense. “If you close your eyes to anything within 30 days because you don’t like it, you’re missing the opportunity to improve your procedure, to improve your treatment, and to characterize precisely what happened with your patient.”
The new document lauds VARC-3’s focus on patient-centered and clinically relevant endpoints but questions the definition of valve thrombosis as a “clinically significant” thrombus. It points out that the incidence of valve thrombosis was significantly higher with TAVR versus SAVR in PARTNER 3 using the older VARC-2 definition, which did not require evidence of clinical sequelae (2.6% vs. 0.7%; P = .02). Under the new definition, however, half of the thrombi would be relabeled as “nothing there,” Dr. Myers said.
“As we’re doing this in younger and younger patients who will survive longer, there is a question of thrombus having an effect on the valve and leading to earlier structural valve deterioration,” he added. “All this is conjecture. We don’t have the data. So mainly what we’re advocating is that all thrombi should be reported.”
MIs, bleeding, and LBBB
The policy statement also criticizes VARC-3’s decision to define periprocedural (type 5) MI using a biomarker-only definition without need of clinical confirmation. Such definitions have been shown to have a very poor prognostic significance in surgical series compared with the Universal Definitions of Myocardial Infarction, Dr. Myers said.
“What’s interesting is that for thrombus and bleeding, they require clinical correlation, but on the perioperative MI they now use a definition that does not require clinical significance, meaning no ECG changes, no regional wall motion abnormalities or things like that,” he observed.
The decision also seems to disregard the EXCEL trial controversy that illustrated how outcomes and a trial’s message can change depending on which definition of periprocedural MI is used.
With regard to bleeding, the surgical associations agree with the VARC-3 recommendation to use different thresholds when bleeding is integrated into a composite endpoint (type 2 or greater for TAVR and types 3 or greater for SAVR) but suggest this important point should be featured in the chapter on bleeding rather than the section on composite endpoints.
The surgical associations say VARC-3 also got it right adding the need for a new permanent pacemaker to the early composite safety endpoint, but that it was a “missed opportunity” not to include new left bundle-branch block in the safety composite, despite recognizing that this may become an important endpoint to consider in the future.
Dr. Myers said that left bundle-branch block could have implications for survival as TAVR moves into lower-risk, younger patients, as some data with 1-year follow-up suggest it has a prognostic impact, even in the higher-risk older patients with more competing risks.
Finally, the surgical associations point out that only two of the 23 VARC-3 authors were practicing cardiac surgeons and say that a more diverse writing group “may help mitigate issues related to the duality of interests.”
Dr. Généreux said that the final author list is not a reflection of the rigorous work done by 11 cardiac surgeons including the two surgeon authors. The VARC-3 writing committee also had a good representation of women, unlike the surgical position statement, which was penned by eight men.
Dr. Myers reported no relevant financial relationships. Coauthors disclosed ties with EACTS, Edwards Lifesciences, Medtronic, Abbott Vascular, Boston Scientific, CryoLife, Shockwave, and JenaValve. Dr. Généreux disclosed ties with Abbott Vascular, Abiomed, Boston Scientific, Cardinal Health, Cardiovascular Systems, Edwards Lifesciences, Medtronic, Opsens, Siemens, SoundBite Medical Solutions, Sig.Num, Saranas, Teleflex, Tryton Medical, Pi-Cardia, and Puzzle Medical.
A version of this article first appeared on Medscape.com.
Five international cardiac surgery associations have banded together to address “substantive concerns” regarding the recently updated Valve Academic Research Consortium 3 (VARC-3) clinical endpoint definitions for aortic valve research.
The VARC-3 update was a multidisciplinary effort that included more than a dozen new or modified definitions for use in transcatheter and surgical aortic valve replacement (TAVR/SAVR) clinical trials, but drew criticism last year from surgeons that some of its definitions favor TAVR over surgery and that its writing committee had deep ties to industry and lacked diversity.
The new surgical associations’ position statement calls out five specific VARC-3 definitions – rehospitalization, valve thrombosis, bleeding, myocardial infarction (MI), and left bundle-branch block (LBBB).
The statement was jointly issued by the Society of Thoracic Surgeons (STS), the American Association for Thoracic Surgery, the European Association for Cardio-Thoracic Surgery, the Asian Society for Cardiovascular and Thoracic Surgery, and the Latin American Association of Cardiac and Endovascular Surgery.
It was copublished in Annals of Thoracic Surgery, the Journal of Thoracic and Cardiovascular Surgery, the European Journal of Cardio-Thoracic Surgery, and the Asian Cardiovascular and Thoracic Annals.
“We hope that this message can be seen, even if it’s somewhat difficult to hear sometimes, as positive constructive criticism compared to some of the dialogue that we’ve had on social media,” lead author Patrick O. Myers, MD, Lausanne (Switzerland) University Hospital, said in an interview. “It’s not criticizing people or the process but just trying to make these definitions better to ensure the good design of clinical trials.”
The president of each surgical association recommended representatives to help write the position statement, and once completed over Zoom meetings, it received formal endorsement from each association prior to publication, he said.
Reached for comment, VARC-3 lead author Philippe Généreux, MD, Gagnon Cardiovascular Institute, Morristown (N.J.) Medical Center, said, “I was pleasantly surprised that their comments were actually pretty minor and that most of these comments are really more a reflection, not of the validity of the definitions, but rather their applications.”
He noted that all the potential issues with the definitions were already discussed during the making of VARC-3 and resolved by consensus of more than 50 experts including the STS president at the time, Food and Drug Administration officials, and experts from the community.
“To be quite honest, I’m not sure they have consensus,” Dr. Généreux said. He added that the writing committee welcomes input from anyone, but “we’re not going to change the definitions to please eight individuals if we strongly believe by consensus of experts in the field that this is not the right thing to do.”
Rehospitalizations and valve thrombosis
The surgical associations praise VARC-3 for providing a standardized definition of bioprosthetic valve failure, but say they will not endorse the inclusion of rehospitalization as a component of the primary efficacy composite endpoint along with all-cause mortality, stroke, and quality of life.
They note that rehospitalizations outnumber mortality events, especially in short follow-up trials, and that the superiority of TAVR at 1 year in the PARTNER 3 trial of low-risk patients was driven primarily by more rehospitalizations in the surgical arm, but that this superiority was waning at 2 years of follow-up.
“The first thing we are calling for is that it shouldn’t be part of the primary composite outcome measure,” Dr. Myers said. But if it really has to be included, a 30-day blanking period for rehospitalization “would acknowledge that there’s a greater risk of rehospitalization during the acute phase of recovering from surgery.”
Dr. Généreux said that VARC-3 provides granular details for defining the different types of hospitalizations, but that a 30-day blanking period makes no sense. “If you close your eyes to anything within 30 days because you don’t like it, you’re missing the opportunity to improve your procedure, to improve your treatment, and to characterize precisely what happened with your patient.”
The new document lauds VARC-3’s focus on patient-centered and clinically relevant endpoints but questions the definition of valve thrombosis as a “clinically significant” thrombus. It points out that the incidence of valve thrombosis was significantly higher with TAVR versus SAVR in PARTNER 3 using the older VARC-2 definition, which did not require evidence of clinical sequelae (2.6% vs. 0.7%; P = .02). Under the new definition, however, half of the thrombi would be relabeled as “nothing there,” Dr. Myers said.
“As we’re doing this in younger and younger patients who will survive longer, there is a question of thrombus having an effect on the valve and leading to earlier structural valve deterioration,” he added. “All this is conjecture. We don’t have the data. So mainly what we’re advocating is that all thrombi should be reported.”
MIs, bleeding, and LBBB
The policy statement also criticizes VARC-3’s decision to define periprocedural (type 5) MI using a biomarker-only definition without need of clinical confirmation. Such definitions have been shown to have a very poor prognostic significance in surgical series compared with the Universal Definitions of Myocardial Infarction, Dr. Myers said.
“What’s interesting is that for thrombus and bleeding, they require clinical correlation, but on the perioperative MI they now use a definition that does not require clinical significance, meaning no ECG changes, no regional wall motion abnormalities or things like that,” he observed.
The decision also seems to disregard the EXCEL trial controversy that illustrated how outcomes and a trial’s message can change depending on which definition of periprocedural MI is used.
With regard to bleeding, the surgical associations agree with the VARC-3 recommendation to use different thresholds when bleeding is integrated into a composite endpoint (type 2 or greater for TAVR and types 3 or greater for SAVR) but suggest this important point should be featured in the chapter on bleeding rather than the section on composite endpoints.
The surgical associations say VARC-3 also got it right adding the need for a new permanent pacemaker to the early composite safety endpoint, but that it was a “missed opportunity” not to include new left bundle-branch block in the safety composite, despite recognizing that this may become an important endpoint to consider in the future.
Dr. Myers said that left bundle-branch block could have implications for survival as TAVR moves into lower-risk, younger patients, as some data with 1-year follow-up suggest it has a prognostic impact, even in the higher-risk older patients with more competing risks.
Finally, the surgical associations point out that only two of the 23 VARC-3 authors were practicing cardiac surgeons and say that a more diverse writing group “may help mitigate issues related to the duality of interests.”
Dr. Généreux said that the final author list is not a reflection of the rigorous work done by 11 cardiac surgeons including the two surgeon authors. The VARC-3 writing committee also had a good representation of women, unlike the surgical position statement, which was penned by eight men.
Dr. Myers reported no relevant financial relationships. Coauthors disclosed ties with EACTS, Edwards Lifesciences, Medtronic, Abbott Vascular, Boston Scientific, CryoLife, Shockwave, and JenaValve. Dr. Généreux disclosed ties with Abbott Vascular, Abiomed, Boston Scientific, Cardinal Health, Cardiovascular Systems, Edwards Lifesciences, Medtronic, Opsens, Siemens, SoundBite Medical Solutions, Sig.Num, Saranas, Teleflex, Tryton Medical, Pi-Cardia, and Puzzle Medical.
A version of this article first appeared on Medscape.com.
Five international cardiac surgery associations have banded together to address “substantive concerns” regarding the recently updated Valve Academic Research Consortium 3 (VARC-3) clinical endpoint definitions for aortic valve research.
The VARC-3 update was a multidisciplinary effort that included more than a dozen new or modified definitions for use in transcatheter and surgical aortic valve replacement (TAVR/SAVR) clinical trials, but drew criticism last year from surgeons that some of its definitions favor TAVR over surgery and that its writing committee had deep ties to industry and lacked diversity.
The new surgical associations’ position statement calls out five specific VARC-3 definitions – rehospitalization, valve thrombosis, bleeding, myocardial infarction (MI), and left bundle-branch block (LBBB).
The statement was jointly issued by the Society of Thoracic Surgeons (STS), the American Association for Thoracic Surgery, the European Association for Cardio-Thoracic Surgery, the Asian Society for Cardiovascular and Thoracic Surgery, and the Latin American Association of Cardiac and Endovascular Surgery.
It was copublished in Annals of Thoracic Surgery, the Journal of Thoracic and Cardiovascular Surgery, the European Journal of Cardio-Thoracic Surgery, and the Asian Cardiovascular and Thoracic Annals.
“We hope that this message can be seen, even if it’s somewhat difficult to hear sometimes, as positive constructive criticism compared to some of the dialogue that we’ve had on social media,” lead author Patrick O. Myers, MD, Lausanne (Switzerland) University Hospital, said in an interview. “It’s not criticizing people or the process but just trying to make these definitions better to ensure the good design of clinical trials.”
The president of each surgical association recommended representatives to help write the position statement, and once completed over Zoom meetings, it received formal endorsement from each association prior to publication, he said.
Reached for comment, VARC-3 lead author Philippe Généreux, MD, Gagnon Cardiovascular Institute, Morristown (N.J.) Medical Center, said, “I was pleasantly surprised that their comments were actually pretty minor and that most of these comments are really more a reflection, not of the validity of the definitions, but rather their applications.”
He noted that all the potential issues with the definitions were already discussed during the making of VARC-3 and resolved by consensus of more than 50 experts including the STS president at the time, Food and Drug Administration officials, and experts from the community.
“To be quite honest, I’m not sure they have consensus,” Dr. Généreux said. He added that the writing committee welcomes input from anyone, but “we’re not going to change the definitions to please eight individuals if we strongly believe by consensus of experts in the field that this is not the right thing to do.”
Rehospitalizations and valve thrombosis
The surgical associations praise VARC-3 for providing a standardized definition of bioprosthetic valve failure, but say they will not endorse the inclusion of rehospitalization as a component of the primary efficacy composite endpoint along with all-cause mortality, stroke, and quality of life.
They note that rehospitalizations outnumber mortality events, especially in short follow-up trials, and that the superiority of TAVR at 1 year in the PARTNER 3 trial of low-risk patients was driven primarily by more rehospitalizations in the surgical arm, but that this superiority was waning at 2 years of follow-up.
“The first thing we are calling for is that it shouldn’t be part of the primary composite outcome measure,” Dr. Myers said. But if it really has to be included, a 30-day blanking period for rehospitalization “would acknowledge that there’s a greater risk of rehospitalization during the acute phase of recovering from surgery.”
Dr. Généreux said that VARC-3 provides granular details for defining the different types of hospitalizations, but that a 30-day blanking period makes no sense. “If you close your eyes to anything within 30 days because you don’t like it, you’re missing the opportunity to improve your procedure, to improve your treatment, and to characterize precisely what happened with your patient.”
The new document lauds VARC-3’s focus on patient-centered and clinically relevant endpoints but questions the definition of valve thrombosis as a “clinically significant” thrombus. It points out that the incidence of valve thrombosis was significantly higher with TAVR versus SAVR in PARTNER 3 using the older VARC-2 definition, which did not require evidence of clinical sequelae (2.6% vs. 0.7%; P = .02). Under the new definition, however, half of the thrombi would be relabeled as “nothing there,” Dr. Myers said.
“As we’re doing this in younger and younger patients who will survive longer, there is a question of thrombus having an effect on the valve and leading to earlier structural valve deterioration,” he added. “All this is conjecture. We don’t have the data. So mainly what we’re advocating is that all thrombi should be reported.”
MIs, bleeding, and LBBB
The policy statement also criticizes VARC-3’s decision to define periprocedural (type 5) MI using a biomarker-only definition without need of clinical confirmation. Such definitions have been shown to have a very poor prognostic significance in surgical series compared with the Universal Definitions of Myocardial Infarction, Dr. Myers said.
“What’s interesting is that for thrombus and bleeding, they require clinical correlation, but on the perioperative MI they now use a definition that does not require clinical significance, meaning no ECG changes, no regional wall motion abnormalities or things like that,” he observed.
The decision also seems to disregard the EXCEL trial controversy that illustrated how outcomes and a trial’s message can change depending on which definition of periprocedural MI is used.
With regard to bleeding, the surgical associations agree with the VARC-3 recommendation to use different thresholds when bleeding is integrated into a composite endpoint (type 2 or greater for TAVR and types 3 or greater for SAVR) but suggest this important point should be featured in the chapter on bleeding rather than the section on composite endpoints.
The surgical associations say VARC-3 also got it right adding the need for a new permanent pacemaker to the early composite safety endpoint, but that it was a “missed opportunity” not to include new left bundle-branch block in the safety composite, despite recognizing that this may become an important endpoint to consider in the future.
Dr. Myers said that left bundle-branch block could have implications for survival as TAVR moves into lower-risk, younger patients, as some data with 1-year follow-up suggest it has a prognostic impact, even in the higher-risk older patients with more competing risks.
Finally, the surgical associations point out that only two of the 23 VARC-3 authors were practicing cardiac surgeons and say that a more diverse writing group “may help mitigate issues related to the duality of interests.”
Dr. Généreux said that the final author list is not a reflection of the rigorous work done by 11 cardiac surgeons including the two surgeon authors. The VARC-3 writing committee also had a good representation of women, unlike the surgical position statement, which was penned by eight men.
Dr. Myers reported no relevant financial relationships. Coauthors disclosed ties with EACTS, Edwards Lifesciences, Medtronic, Abbott Vascular, Boston Scientific, CryoLife, Shockwave, and JenaValve. Dr. Généreux disclosed ties with Abbott Vascular, Abiomed, Boston Scientific, Cardinal Health, Cardiovascular Systems, Edwards Lifesciences, Medtronic, Opsens, Siemens, SoundBite Medical Solutions, Sig.Num, Saranas, Teleflex, Tryton Medical, Pi-Cardia, and Puzzle Medical.
A version of this article first appeared on Medscape.com.
Congress opens investigation into FDA’s handling of a problematic heart device
A congressional oversight subcommittee is investigating the Food and Drug Administration’s regulation of a high-risk heart pump, citing safety issues detailed by ProPublica.
The HeartWare Ventricular Assist Device, created to treat patients with severe heart failure, stopped meeting key federal standards as early as 2014. But the FDA took no decisive action even as those problems persisted, and thousands of Americans continued to be implanted with the pump.
By the end of 2020, the FDA had received more than 3,000 reports of deaths related to the HeartWare device, according to a ProPublica data analysis. A father of four died as his children tried to resuscitate him when his device suddenly stopped. A teenager died after vomiting blood in the middle of the night, while his mother struggled to restart a faulty pump.
“I am concerned by FDA’s slow action, over multiple administrations, to protect patients from this product despite early warning signs,” Rep. Raja Krishnamoorthi, D-Ill., said in a scathing letter sent March 22 to the agency’s commissioner, Robert Califf, MD.
Mr. Krishnamoorthi, the chairman of the U.S. House Committee on Oversight and Reform’s Subcommittee on Economic and Consumer Policy, requested information on how the FDA made regulatory decisions related to the HeartWare device and why it didn’t take further action.
The FDA did not provide comment to ProPublica on the subcommittee’s investigation and said it would respond directly to Mr. Krishnamoorthi. It also reiterated its response to ProPublica’s findings and said the agency had been closely overseeing the HeartWare device since 2012, with patient safety as its “highest priority.”
Medtronic, the company that acquired HeartWare in 2016, took the device off the market in June 2021. The company said that new data showed a competing heart pump had better outcomes. In response to the ProPublica investigation 2 months later, the company said it took the FDA’s inspections seriously and had worked closely with the agency to address issues with the device.
Medtronic declined to comment on the subcommittee’s investigation.
Mr. Krishnamoorthi asked in the letter if any steps were being taken to address how patients, doctors and other federal agencies are notified of problems that the FDA finds with medical devices.
Many patients told ProPublica they were never informed of issues with the HeartWare pump before or after their implants. Some people who still have the device said they weren’t told when it was taken off the market. Medtronic said in December it had confirmed 90% of U.S. patients had received notification of the HeartWare discontinuation, but that it was still working to reach the other 10%.
About 2,000 patients still had HeartWare pumps as of last year. The FDA and Medtronic recommended against removing those devices barring medical necessity because the surgery to do so carries a high risk.
In his letter, Mr. Krishnamoorthi gave the FDA a deadline of April 5 to respond.
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive their biggest stories as soon as they’re published.
A congressional oversight subcommittee is investigating the Food and Drug Administration’s regulation of a high-risk heart pump, citing safety issues detailed by ProPublica.
The HeartWare Ventricular Assist Device, created to treat patients with severe heart failure, stopped meeting key federal standards as early as 2014. But the FDA took no decisive action even as those problems persisted, and thousands of Americans continued to be implanted with the pump.
By the end of 2020, the FDA had received more than 3,000 reports of deaths related to the HeartWare device, according to a ProPublica data analysis. A father of four died as his children tried to resuscitate him when his device suddenly stopped. A teenager died after vomiting blood in the middle of the night, while his mother struggled to restart a faulty pump.
“I am concerned by FDA’s slow action, over multiple administrations, to protect patients from this product despite early warning signs,” Rep. Raja Krishnamoorthi, D-Ill., said in a scathing letter sent March 22 to the agency’s commissioner, Robert Califf, MD.
Mr. Krishnamoorthi, the chairman of the U.S. House Committee on Oversight and Reform’s Subcommittee on Economic and Consumer Policy, requested information on how the FDA made regulatory decisions related to the HeartWare device and why it didn’t take further action.
The FDA did not provide comment to ProPublica on the subcommittee’s investigation and said it would respond directly to Mr. Krishnamoorthi. It also reiterated its response to ProPublica’s findings and said the agency had been closely overseeing the HeartWare device since 2012, with patient safety as its “highest priority.”
Medtronic, the company that acquired HeartWare in 2016, took the device off the market in June 2021. The company said that new data showed a competing heart pump had better outcomes. In response to the ProPublica investigation 2 months later, the company said it took the FDA’s inspections seriously and had worked closely with the agency to address issues with the device.
Medtronic declined to comment on the subcommittee’s investigation.
Mr. Krishnamoorthi asked in the letter if any steps were being taken to address how patients, doctors and other federal agencies are notified of problems that the FDA finds with medical devices.
Many patients told ProPublica they were never informed of issues with the HeartWare pump before or after their implants. Some people who still have the device said they weren’t told when it was taken off the market. Medtronic said in December it had confirmed 90% of U.S. patients had received notification of the HeartWare discontinuation, but that it was still working to reach the other 10%.
About 2,000 patients still had HeartWare pumps as of last year. The FDA and Medtronic recommended against removing those devices barring medical necessity because the surgery to do so carries a high risk.
In his letter, Mr. Krishnamoorthi gave the FDA a deadline of April 5 to respond.
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive their biggest stories as soon as they’re published.
A congressional oversight subcommittee is investigating the Food and Drug Administration’s regulation of a high-risk heart pump, citing safety issues detailed by ProPublica.
The HeartWare Ventricular Assist Device, created to treat patients with severe heart failure, stopped meeting key federal standards as early as 2014. But the FDA took no decisive action even as those problems persisted, and thousands of Americans continued to be implanted with the pump.
By the end of 2020, the FDA had received more than 3,000 reports of deaths related to the HeartWare device, according to a ProPublica data analysis. A father of four died as his children tried to resuscitate him when his device suddenly stopped. A teenager died after vomiting blood in the middle of the night, while his mother struggled to restart a faulty pump.
“I am concerned by FDA’s slow action, over multiple administrations, to protect patients from this product despite early warning signs,” Rep. Raja Krishnamoorthi, D-Ill., said in a scathing letter sent March 22 to the agency’s commissioner, Robert Califf, MD.
Mr. Krishnamoorthi, the chairman of the U.S. House Committee on Oversight and Reform’s Subcommittee on Economic and Consumer Policy, requested information on how the FDA made regulatory decisions related to the HeartWare device and why it didn’t take further action.
The FDA did not provide comment to ProPublica on the subcommittee’s investigation and said it would respond directly to Mr. Krishnamoorthi. It also reiterated its response to ProPublica’s findings and said the agency had been closely overseeing the HeartWare device since 2012, with patient safety as its “highest priority.”
Medtronic, the company that acquired HeartWare in 2016, took the device off the market in June 2021. The company said that new data showed a competing heart pump had better outcomes. In response to the ProPublica investigation 2 months later, the company said it took the FDA’s inspections seriously and had worked closely with the agency to address issues with the device.
Medtronic declined to comment on the subcommittee’s investigation.
Mr. Krishnamoorthi asked in the letter if any steps were being taken to address how patients, doctors and other federal agencies are notified of problems that the FDA finds with medical devices.
Many patients told ProPublica they were never informed of issues with the HeartWare pump before or after their implants. Some people who still have the device said they weren’t told when it was taken off the market. Medtronic said in December it had confirmed 90% of U.S. patients had received notification of the HeartWare discontinuation, but that it was still working to reach the other 10%.
About 2,000 patients still had HeartWare pumps as of last year. The FDA and Medtronic recommended against removing those devices barring medical necessity because the surgery to do so carries a high risk.
In his letter, Mr. Krishnamoorthi gave the FDA a deadline of April 5 to respond.
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive their biggest stories as soon as they’re published.
Death of pig heart transplant patient is more a beginning than an end
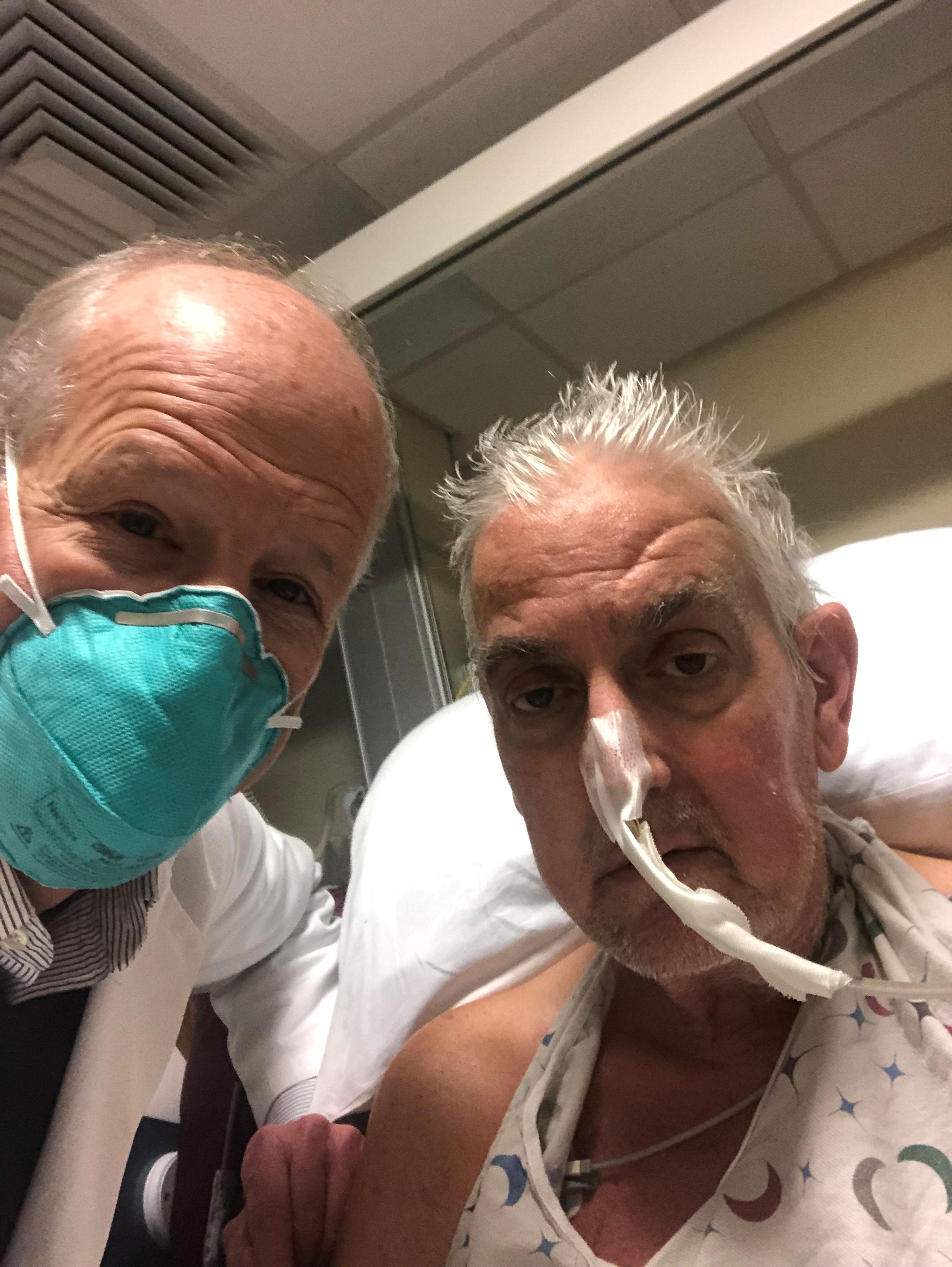
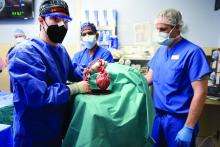
The genetically altered pig’s heart “worked like a rock star, beautifully functioning,” the surgeon who performed the pioneering Jan. 7 xenotransplant procedure said in a press statement on the death of the patient, David Bennett Sr.
“He wasn’t able to overcome what turned out to be devastating – the debilitation from his previous period of heart failure, which was extreme,” said Bartley P. Griffith, MD, clinical director of the cardiac xenotransplantation program at the University of Maryland, Baltimore.
Representatives of the institution aren’t offering many details on the cause of Mr. Bennett’s death on March 8, 60 days after his operation, but said they will elaborate when their findings are formally published. But their comments seem to downplay the unique nature of the implanted heart itself as a culprit and instead implicate the patient’s diminished overall clinical condition and what grew into an ongoing battle with infections.
The 57-year-old Bennett, bedridden with end-stage heart failure, judged a poor candidate for a ventricular assist device, and on extracorporeal membrane oxygenation (ECMO), reportedly was offered the extraordinary surgery after being turned down for a conventional transplant at several major centers.
“Until day 45 or 50, he was doing very well,” Muhammad M. Mohiuddin, MD, the xenotransplantation program’s scientific director, observed in the statement. But infections soon took advantage of his hobbled immune system.
Given his “preexisting condition and how frail his body was,” Dr. Mohiuddin said, “we were having difficulty maintaining a balance between his immunosuppression and controlling his infection.” Mr. Bennett went into multiple organ failure and “I think that resulted in his passing away.”
Beyond wildest dreams
The surgeons confidently framed Mr. Bennett’s experience as a milestone for heart xenotransplantation. “The demonstration that it was possible, beyond the wildest dreams of most people in the field, even, at this point – that we were able to take a genetically engineered organ and watch it function flawlessly for 9 weeks – is pretty positive in terms of the potential of this therapy,” Dr. Griffith said.
But enough questions linger that others were more circumspect, even as they praised the accomplishment. “There’s no question that this is a historic event,” Mandeep R. Mehra, MD, of Harvard Medical School, and director of the Center for Advanced Heart Disease at Brigham and Women’s Hospital, both in Boston, said in an interview.
Still, “I don’t think we should just conclude that it was the patient’s frailty or death from infection,” Dr. Mehra said. With so few details available, “I would be very careful in prematurely concluding that the problem did not reside with the heart but with the patient. We cannot be sure.”
For example, he noted, “6 to 8 weeks is right around the time when some cardiac complications, like accelerated forms of vasculopathy, could become evident.” Immune-mediated cardiac allograft vasculopathy is a common cause of heart transplant failure.
Or, “it could as easily have been the fact that immunosuppression was modified at 6 to 7 weeks in response to potential infection, which could have led to a cardiac compromise,” Dr. Mehra said. “We just don’t know.”
“It’s really important that this be reported in a scientifically accurate way, because we will all learn from this,” Lori J. West, MD, DPhil, said in an interview.
Little seems to be known for sure about the actual cause of death, “but the fact there was not hyperacute rejection is itself a big step forward. And we know, at least from the limited information we have, that it did not occur,” observed Dr. West, who directs the Alberta Transplant Institute, Edmonton, and the Canadian Donation and Transplantation Research Program. She is a professor of pediatrics with adjunct positions in the departments of surgery and microbiology/immunology.
Dr. West also sees Mr. Bennett’s struggle with infections and adjustments to his unique immunosuppressive regimen, at least as characterized by his care team, as in line with the experience of many heart transplant recipients facing the same threat.
“We already walk this tightrope with every transplant patient,” she said. Typically, they’re put on a somewhat standardized immunosuppressant regimen, “and then we modify it a bit, either increasing or decreasing it, depending on the posttransplant course.” The regimen can become especially intense in response to new signs of rejection, “and you know that that’s going to have an impact on susceptibility to all kinds of infections.”
Full circle
The porcine heart was protected along two fronts against assault from Mr. Bennett’s immune system and other inhospitable aspects of his physiology, either of which could also have been obstacles to success: Genetic modification (Revivicor) of the pig that provided the heart, and a singularly aggressive antirejection drug regimen for the patient.
The knockout of three genes targeting specific porcine cell-surface carbohydrates that provoke a strong human antibody response reportedly averted a hyperacute rejection response that would have caused the graft to fail almost immediately.
Other genetic manipulations, some using CRISPR technology, silenced genes encoded for porcine endogenous retroviruses. Others were aimed at controlling myocardial growth and stemming graft microangiopathy.
Mr. Bennett himself was treated with powerful immunosuppressants, including an investigational anti-CD40 monoclonal antibody (KPL-404, Kiniksa Pharmaceuticals) that, according to UMSOM, inhibits a well-recognized pathway critical to B-cell proliferation, T-cell activation, and antibody production.
“I suspect the patient may not have had rejection, but unfortunately, that intense immunosuppression really set him up – even if he had been half that age – for a very difficult time,” David A. Baran, MD, a cardiologist from Sentara Advanced Heart Failure Center, Norfolk, Va., who studies transplant immunology, said in an interview.
“This is in some ways like the original heart transplant in 1967, when the ability to do the surgery evolved before understanding of the immunosuppression needed. Four or 5 years later, heart transplantation almost died out, before the development of better immunosuppressants like cyclosporine and later tacrolimus,” Dr. Baran said.
“The current age, when we use less immunosuppression than ever, is based on 30 years of progressive success,” he noted. This landmark xenotransplantation “basically turns back the clock to a time when the intensity of immunosuppression by definition had to be extremely high, because we really didn’t know what to expect.”
Emerging role of xeno-organs
Xenotransplantation has been touted as potential strategy for expanding the pool of organs available for transplantation. Mr. Bennett’s “breakthrough surgery” takes the world “one step closer to solving the organ shortage crisis,” his surgeon, Dr. Griffith, announced soon after the procedure. “There are simply not enough donor human hearts available to meet the long list of potential recipients.”
But it’s not the only proposed approach. Measures could be taken, for example, to make more efficient use of the human organs that become available, partly by opening the field to additional less-than-ideal hearts and loosening regulatory mandates for projected graft survival.
“Every year, more than two-thirds of donor organs in the United States are discarded. So it’s not actually that we don’t have enough organs, it’s that we don’t have enough organs that people are willing to take,” Dr. Baran said. Still, it’s important to pursue all promising avenues, and “the genetic manipulation pathway is remarkable.”
But “honestly, organs such as kidneys probably make the most sense” for early study of xenotransplantation from pigs, he said. “The waiting list for kidneys is also very long, but if the kidney graft were to fail, the patient wouldn’t die. It would allow us to work out the immunosuppression without putting patients’ lives at risk.”
Often overlooked in assessments of organ demand, Dr. West said, is that “a lot of patients who could benefit from a transplant will never even be listed for a transplant.” It’s not clear why; perhaps they have multiple comorbidities, live too far from a transplant center, “or they’re too big or too small. Even if there were unlimited organs, you could never meet the needs of people who could benefit from transplantation.”
So even if more available donor organs were used, she said, there would still be a gap that xenotransplantation could help fill. “I’m very much in favor of research that allows us to continue to try to find a pathway to xenotransplantation. I think it’s critically important.”
Unquestionably, “we now need to have a dialogue to entertain how a technology like this, using modern medicine with gene editing, is really going to be utilized,” Dr. Mehra said. The Bennett case “does open up the field, but it also raises caution.” There should be broad participation to move the field forward, “coordinated through either societies or nationally allocated advisory committees that oversee the movement of this technology, to the next step.”
Ideally, that next step “would be to do a safety clinical trial in the right patient,” he said. “And the right patient, by definition, would be one who does not have a life-prolonging option, either mechanical circulatory support or allograft transplantation. That would be the goal.”
Dr. Mehra has reported receiving payments to his institution from Abbott for consulting; consulting fees from Janssen, Mesoblast, Broadview Ventures, Natera, Paragonix, Moderna, and the Baim Institute for Clinical Research; and serving on a scientific advisory board NuPulseCV, Leviticus, and FineHeart. Dr. Baran disclosed consulting for Getinge and LivaNova; speaking for Pfizer; and serving on trial steering committees for CareDx and Procyrion, all unrelated to xenotransplantation. Dr. West has declared no relevant conflicts.
A version of this article first appeared on Medscape.com.
The genetically altered pig’s heart “worked like a rock star, beautifully functioning,” the surgeon who performed the pioneering Jan. 7 xenotransplant procedure said in a press statement on the death of the patient, David Bennett Sr.
“He wasn’t able to overcome what turned out to be devastating – the debilitation from his previous period of heart failure, which was extreme,” said Bartley P. Griffith, MD, clinical director of the cardiac xenotransplantation program at the University of Maryland, Baltimore.
Representatives of the institution aren’t offering many details on the cause of Mr. Bennett’s death on March 8, 60 days after his operation, but said they will elaborate when their findings are formally published. But their comments seem to downplay the unique nature of the implanted heart itself as a culprit and instead implicate the patient’s diminished overall clinical condition and what grew into an ongoing battle with infections.
The 57-year-old Bennett, bedridden with end-stage heart failure, judged a poor candidate for a ventricular assist device, and on extracorporeal membrane oxygenation (ECMO), reportedly was offered the extraordinary surgery after being turned down for a conventional transplant at several major centers.
“Until day 45 or 50, he was doing very well,” Muhammad M. Mohiuddin, MD, the xenotransplantation program’s scientific director, observed in the statement. But infections soon took advantage of his hobbled immune system.
Given his “preexisting condition and how frail his body was,” Dr. Mohiuddin said, “we were having difficulty maintaining a balance between his immunosuppression and controlling his infection.” Mr. Bennett went into multiple organ failure and “I think that resulted in his passing away.”
Beyond wildest dreams
The surgeons confidently framed Mr. Bennett’s experience as a milestone for heart xenotransplantation. “The demonstration that it was possible, beyond the wildest dreams of most people in the field, even, at this point – that we were able to take a genetically engineered organ and watch it function flawlessly for 9 weeks – is pretty positive in terms of the potential of this therapy,” Dr. Griffith said.
But enough questions linger that others were more circumspect, even as they praised the accomplishment. “There’s no question that this is a historic event,” Mandeep R. Mehra, MD, of Harvard Medical School, and director of the Center for Advanced Heart Disease at Brigham and Women’s Hospital, both in Boston, said in an interview.
Still, “I don’t think we should just conclude that it was the patient’s frailty or death from infection,” Dr. Mehra said. With so few details available, “I would be very careful in prematurely concluding that the problem did not reside with the heart but with the patient. We cannot be sure.”
For example, he noted, “6 to 8 weeks is right around the time when some cardiac complications, like accelerated forms of vasculopathy, could become evident.” Immune-mediated cardiac allograft vasculopathy is a common cause of heart transplant failure.
Or, “it could as easily have been the fact that immunosuppression was modified at 6 to 7 weeks in response to potential infection, which could have led to a cardiac compromise,” Dr. Mehra said. “We just don’t know.”
“It’s really important that this be reported in a scientifically accurate way, because we will all learn from this,” Lori J. West, MD, DPhil, said in an interview.
Little seems to be known for sure about the actual cause of death, “but the fact there was not hyperacute rejection is itself a big step forward. And we know, at least from the limited information we have, that it did not occur,” observed Dr. West, who directs the Alberta Transplant Institute, Edmonton, and the Canadian Donation and Transplantation Research Program. She is a professor of pediatrics with adjunct positions in the departments of surgery and microbiology/immunology.
Dr. West also sees Mr. Bennett’s struggle with infections and adjustments to his unique immunosuppressive regimen, at least as characterized by his care team, as in line with the experience of many heart transplant recipients facing the same threat.
“We already walk this tightrope with every transplant patient,” she said. Typically, they’re put on a somewhat standardized immunosuppressant regimen, “and then we modify it a bit, either increasing or decreasing it, depending on the posttransplant course.” The regimen can become especially intense in response to new signs of rejection, “and you know that that’s going to have an impact on susceptibility to all kinds of infections.”
Full circle
The porcine heart was protected along two fronts against assault from Mr. Bennett’s immune system and other inhospitable aspects of his physiology, either of which could also have been obstacles to success: Genetic modification (Revivicor) of the pig that provided the heart, and a singularly aggressive antirejection drug regimen for the patient.
The knockout of three genes targeting specific porcine cell-surface carbohydrates that provoke a strong human antibody response reportedly averted a hyperacute rejection response that would have caused the graft to fail almost immediately.
Other genetic manipulations, some using CRISPR technology, silenced genes encoded for porcine endogenous retroviruses. Others were aimed at controlling myocardial growth and stemming graft microangiopathy.
Mr. Bennett himself was treated with powerful immunosuppressants, including an investigational anti-CD40 monoclonal antibody (KPL-404, Kiniksa Pharmaceuticals) that, according to UMSOM, inhibits a well-recognized pathway critical to B-cell proliferation, T-cell activation, and antibody production.
“I suspect the patient may not have had rejection, but unfortunately, that intense immunosuppression really set him up – even if he had been half that age – for a very difficult time,” David A. Baran, MD, a cardiologist from Sentara Advanced Heart Failure Center, Norfolk, Va., who studies transplant immunology, said in an interview.
“This is in some ways like the original heart transplant in 1967, when the ability to do the surgery evolved before understanding of the immunosuppression needed. Four or 5 years later, heart transplantation almost died out, before the development of better immunosuppressants like cyclosporine and later tacrolimus,” Dr. Baran said.
“The current age, when we use less immunosuppression than ever, is based on 30 years of progressive success,” he noted. This landmark xenotransplantation “basically turns back the clock to a time when the intensity of immunosuppression by definition had to be extremely high, because we really didn’t know what to expect.”
Emerging role of xeno-organs
Xenotransplantation has been touted as potential strategy for expanding the pool of organs available for transplantation. Mr. Bennett’s “breakthrough surgery” takes the world “one step closer to solving the organ shortage crisis,” his surgeon, Dr. Griffith, announced soon after the procedure. “There are simply not enough donor human hearts available to meet the long list of potential recipients.”
But it’s not the only proposed approach. Measures could be taken, for example, to make more efficient use of the human organs that become available, partly by opening the field to additional less-than-ideal hearts and loosening regulatory mandates for projected graft survival.
“Every year, more than two-thirds of donor organs in the United States are discarded. So it’s not actually that we don’t have enough organs, it’s that we don’t have enough organs that people are willing to take,” Dr. Baran said. Still, it’s important to pursue all promising avenues, and “the genetic manipulation pathway is remarkable.”
But “honestly, organs such as kidneys probably make the most sense” for early study of xenotransplantation from pigs, he said. “The waiting list for kidneys is also very long, but if the kidney graft were to fail, the patient wouldn’t die. It would allow us to work out the immunosuppression without putting patients’ lives at risk.”
Often overlooked in assessments of organ demand, Dr. West said, is that “a lot of patients who could benefit from a transplant will never even be listed for a transplant.” It’s not clear why; perhaps they have multiple comorbidities, live too far from a transplant center, “or they’re too big or too small. Even if there were unlimited organs, you could never meet the needs of people who could benefit from transplantation.”
So even if more available donor organs were used, she said, there would still be a gap that xenotransplantation could help fill. “I’m very much in favor of research that allows us to continue to try to find a pathway to xenotransplantation. I think it’s critically important.”
Unquestionably, “we now need to have a dialogue to entertain how a technology like this, using modern medicine with gene editing, is really going to be utilized,” Dr. Mehra said. The Bennett case “does open up the field, but it also raises caution.” There should be broad participation to move the field forward, “coordinated through either societies or nationally allocated advisory committees that oversee the movement of this technology, to the next step.”
Ideally, that next step “would be to do a safety clinical trial in the right patient,” he said. “And the right patient, by definition, would be one who does not have a life-prolonging option, either mechanical circulatory support or allograft transplantation. That would be the goal.”
Dr. Mehra has reported receiving payments to his institution from Abbott for consulting; consulting fees from Janssen, Mesoblast, Broadview Ventures, Natera, Paragonix, Moderna, and the Baim Institute for Clinical Research; and serving on a scientific advisory board NuPulseCV, Leviticus, and FineHeart. Dr. Baran disclosed consulting for Getinge and LivaNova; speaking for Pfizer; and serving on trial steering committees for CareDx and Procyrion, all unrelated to xenotransplantation. Dr. West has declared no relevant conflicts.
A version of this article first appeared on Medscape.com.
The genetically altered pig’s heart “worked like a rock star, beautifully functioning,” the surgeon who performed the pioneering Jan. 7 xenotransplant procedure said in a press statement on the death of the patient, David Bennett Sr.
“He wasn’t able to overcome what turned out to be devastating – the debilitation from his previous period of heart failure, which was extreme,” said Bartley P. Griffith, MD, clinical director of the cardiac xenotransplantation program at the University of Maryland, Baltimore.
Representatives of the institution aren’t offering many details on the cause of Mr. Bennett’s death on March 8, 60 days after his operation, but said they will elaborate when their findings are formally published. But their comments seem to downplay the unique nature of the implanted heart itself as a culprit and instead implicate the patient’s diminished overall clinical condition and what grew into an ongoing battle with infections.
The 57-year-old Bennett, bedridden with end-stage heart failure, judged a poor candidate for a ventricular assist device, and on extracorporeal membrane oxygenation (ECMO), reportedly was offered the extraordinary surgery after being turned down for a conventional transplant at several major centers.
“Until day 45 or 50, he was doing very well,” Muhammad M. Mohiuddin, MD, the xenotransplantation program’s scientific director, observed in the statement. But infections soon took advantage of his hobbled immune system.
Given his “preexisting condition and how frail his body was,” Dr. Mohiuddin said, “we were having difficulty maintaining a balance between his immunosuppression and controlling his infection.” Mr. Bennett went into multiple organ failure and “I think that resulted in his passing away.”
Beyond wildest dreams
The surgeons confidently framed Mr. Bennett’s experience as a milestone for heart xenotransplantation. “The demonstration that it was possible, beyond the wildest dreams of most people in the field, even, at this point – that we were able to take a genetically engineered organ and watch it function flawlessly for 9 weeks – is pretty positive in terms of the potential of this therapy,” Dr. Griffith said.
But enough questions linger that others were more circumspect, even as they praised the accomplishment. “There’s no question that this is a historic event,” Mandeep R. Mehra, MD, of Harvard Medical School, and director of the Center for Advanced Heart Disease at Brigham and Women’s Hospital, both in Boston, said in an interview.
Still, “I don’t think we should just conclude that it was the patient’s frailty or death from infection,” Dr. Mehra said. With so few details available, “I would be very careful in prematurely concluding that the problem did not reside with the heart but with the patient. We cannot be sure.”
For example, he noted, “6 to 8 weeks is right around the time when some cardiac complications, like accelerated forms of vasculopathy, could become evident.” Immune-mediated cardiac allograft vasculopathy is a common cause of heart transplant failure.
Or, “it could as easily have been the fact that immunosuppression was modified at 6 to 7 weeks in response to potential infection, which could have led to a cardiac compromise,” Dr. Mehra said. “We just don’t know.”
“It’s really important that this be reported in a scientifically accurate way, because we will all learn from this,” Lori J. West, MD, DPhil, said in an interview.
Little seems to be known for sure about the actual cause of death, “but the fact there was not hyperacute rejection is itself a big step forward. And we know, at least from the limited information we have, that it did not occur,” observed Dr. West, who directs the Alberta Transplant Institute, Edmonton, and the Canadian Donation and Transplantation Research Program. She is a professor of pediatrics with adjunct positions in the departments of surgery and microbiology/immunology.
Dr. West also sees Mr. Bennett’s struggle with infections and adjustments to his unique immunosuppressive regimen, at least as characterized by his care team, as in line with the experience of many heart transplant recipients facing the same threat.
“We already walk this tightrope with every transplant patient,” she said. Typically, they’re put on a somewhat standardized immunosuppressant regimen, “and then we modify it a bit, either increasing or decreasing it, depending on the posttransplant course.” The regimen can become especially intense in response to new signs of rejection, “and you know that that’s going to have an impact on susceptibility to all kinds of infections.”
Full circle
The porcine heart was protected along two fronts against assault from Mr. Bennett’s immune system and other inhospitable aspects of his physiology, either of which could also have been obstacles to success: Genetic modification (Revivicor) of the pig that provided the heart, and a singularly aggressive antirejection drug regimen for the patient.
The knockout of three genes targeting specific porcine cell-surface carbohydrates that provoke a strong human antibody response reportedly averted a hyperacute rejection response that would have caused the graft to fail almost immediately.
Other genetic manipulations, some using CRISPR technology, silenced genes encoded for porcine endogenous retroviruses. Others were aimed at controlling myocardial growth and stemming graft microangiopathy.
Mr. Bennett himself was treated with powerful immunosuppressants, including an investigational anti-CD40 monoclonal antibody (KPL-404, Kiniksa Pharmaceuticals) that, according to UMSOM, inhibits a well-recognized pathway critical to B-cell proliferation, T-cell activation, and antibody production.
“I suspect the patient may not have had rejection, but unfortunately, that intense immunosuppression really set him up – even if he had been half that age – for a very difficult time,” David A. Baran, MD, a cardiologist from Sentara Advanced Heart Failure Center, Norfolk, Va., who studies transplant immunology, said in an interview.
“This is in some ways like the original heart transplant in 1967, when the ability to do the surgery evolved before understanding of the immunosuppression needed. Four or 5 years later, heart transplantation almost died out, before the development of better immunosuppressants like cyclosporine and later tacrolimus,” Dr. Baran said.
“The current age, when we use less immunosuppression than ever, is based on 30 years of progressive success,” he noted. This landmark xenotransplantation “basically turns back the clock to a time when the intensity of immunosuppression by definition had to be extremely high, because we really didn’t know what to expect.”
Emerging role of xeno-organs
Xenotransplantation has been touted as potential strategy for expanding the pool of organs available for transplantation. Mr. Bennett’s “breakthrough surgery” takes the world “one step closer to solving the organ shortage crisis,” his surgeon, Dr. Griffith, announced soon after the procedure. “There are simply not enough donor human hearts available to meet the long list of potential recipients.”
But it’s not the only proposed approach. Measures could be taken, for example, to make more efficient use of the human organs that become available, partly by opening the field to additional less-than-ideal hearts and loosening regulatory mandates for projected graft survival.
“Every year, more than two-thirds of donor organs in the United States are discarded. So it’s not actually that we don’t have enough organs, it’s that we don’t have enough organs that people are willing to take,” Dr. Baran said. Still, it’s important to pursue all promising avenues, and “the genetic manipulation pathway is remarkable.”
But “honestly, organs such as kidneys probably make the most sense” for early study of xenotransplantation from pigs, he said. “The waiting list for kidneys is also very long, but if the kidney graft were to fail, the patient wouldn’t die. It would allow us to work out the immunosuppression without putting patients’ lives at risk.”
Often overlooked in assessments of organ demand, Dr. West said, is that “a lot of patients who could benefit from a transplant will never even be listed for a transplant.” It’s not clear why; perhaps they have multiple comorbidities, live too far from a transplant center, “or they’re too big or too small. Even if there were unlimited organs, you could never meet the needs of people who could benefit from transplantation.”
So even if more available donor organs were used, she said, there would still be a gap that xenotransplantation could help fill. “I’m very much in favor of research that allows us to continue to try to find a pathway to xenotransplantation. I think it’s critically important.”
Unquestionably, “we now need to have a dialogue to entertain how a technology like this, using modern medicine with gene editing, is really going to be utilized,” Dr. Mehra said. The Bennett case “does open up the field, but it also raises caution.” There should be broad participation to move the field forward, “coordinated through either societies or nationally allocated advisory committees that oversee the movement of this technology, to the next step.”
Ideally, that next step “would be to do a safety clinical trial in the right patient,” he said. “And the right patient, by definition, would be one who does not have a life-prolonging option, either mechanical circulatory support or allograft transplantation. That would be the goal.”
Dr. Mehra has reported receiving payments to his institution from Abbott for consulting; consulting fees from Janssen, Mesoblast, Broadview Ventures, Natera, Paragonix, Moderna, and the Baim Institute for Clinical Research; and serving on a scientific advisory board NuPulseCV, Leviticus, and FineHeart. Dr. Baran disclosed consulting for Getinge and LivaNova; speaking for Pfizer; and serving on trial steering committees for CareDx and Procyrion, all unrelated to xenotransplantation. Dr. West has declared no relevant conflicts.
A version of this article first appeared on Medscape.com.
Man who received first modified pig heart transplant dies
He passed away March 8, according to a statement from the University of Maryland Medical Center (UMMC), Baltimore, where the transplant was performed.
Mr. Bennett received the transplant on January 7 and lived for 2 months following the surgery.
Although not providing the exact cause of his death, UMMC said Mr. Bennett’s condition began deteriorating several days before his death.
When it became clear that he would not recover, he was given compassionate palliative care and was able to communicate with his family during his final hours.
“We are devastated by the loss of Mr. Bennett. He proved to be a brave and noble patient who fought all the way to the end. We extend our sincerest condolences to his family,” Bartley P. Griffith, MD, who performed the transplant, said in the statement.
“We are grateful to Mr. Bennett for his unique and historic role in helping to contribute to a vast array of knowledge to the field of xenotransplantation,” added Muhammad M. Mohiuddin, MD, director of the cardiac xenotransplantation program at University of Maryland School of Medicine.
Before receiving the genetically modified pig heart, Mr. Bennett had required mechanical circulatory support to stay alive but was rejected for standard heart transplantation at UMMC and other centers. He was ineligible for an implanted ventricular assist device due to ventricular arrhythmias.
Following surgery, the transplanted pig heart performed well for several weeks without any signs of rejection. The patient was able to spend time with his family and participate in physical therapy to help regain strength.
“This organ transplant demonstrated for the first time that a genetically modified animal heart can function like a human heart without immediate rejection by the body,” UMMC said in a statement issued 3 days after the surgery.
Thanks to Mr. Bennett, “we have gained invaluable insights learning that the genetically modified pig heart can function well within the human body while the immune system is adequately suppressed,” said Dr. Mohiuddin. “We remain optimistic and plan on continuing our work in future clinical trials.”
The patient’s son, David Bennett Jr, said the family is “profoundly grateful for the life-extending opportunity” provided to his father by the “stellar team” at the University of Maryland School of Medicine and the University of Maryland Medical Center.
“We were able to spend some precious weeks together while he recovered from the transplant surgery, weeks we would not have had without this miraculous effort,” he said.
“We also hope that what was learned from his surgery will benefit future patients and hopefully, one day, end the organ shortage that costs so many lives each year,” he added.
A version of this article first appeared on Medscape.com.
He passed away March 8, according to a statement from the University of Maryland Medical Center (UMMC), Baltimore, where the transplant was performed.
Mr. Bennett received the transplant on January 7 and lived for 2 months following the surgery.
Although not providing the exact cause of his death, UMMC said Mr. Bennett’s condition began deteriorating several days before his death.
When it became clear that he would not recover, he was given compassionate palliative care and was able to communicate with his family during his final hours.
“We are devastated by the loss of Mr. Bennett. He proved to be a brave and noble patient who fought all the way to the end. We extend our sincerest condolences to his family,” Bartley P. Griffith, MD, who performed the transplant, said in the statement.
“We are grateful to Mr. Bennett for his unique and historic role in helping to contribute to a vast array of knowledge to the field of xenotransplantation,” added Muhammad M. Mohiuddin, MD, director of the cardiac xenotransplantation program at University of Maryland School of Medicine.
Before receiving the genetically modified pig heart, Mr. Bennett had required mechanical circulatory support to stay alive but was rejected for standard heart transplantation at UMMC and other centers. He was ineligible for an implanted ventricular assist device due to ventricular arrhythmias.
Following surgery, the transplanted pig heart performed well for several weeks without any signs of rejection. The patient was able to spend time with his family and participate in physical therapy to help regain strength.
“This organ transplant demonstrated for the first time that a genetically modified animal heart can function like a human heart without immediate rejection by the body,” UMMC said in a statement issued 3 days after the surgery.
Thanks to Mr. Bennett, “we have gained invaluable insights learning that the genetically modified pig heart can function well within the human body while the immune system is adequately suppressed,” said Dr. Mohiuddin. “We remain optimistic and plan on continuing our work in future clinical trials.”
The patient’s son, David Bennett Jr, said the family is “profoundly grateful for the life-extending opportunity” provided to his father by the “stellar team” at the University of Maryland School of Medicine and the University of Maryland Medical Center.
“We were able to spend some precious weeks together while he recovered from the transplant surgery, weeks we would not have had without this miraculous effort,” he said.
“We also hope that what was learned from his surgery will benefit future patients and hopefully, one day, end the organ shortage that costs so many lives each year,” he added.
A version of this article first appeared on Medscape.com.
He passed away March 8, according to a statement from the University of Maryland Medical Center (UMMC), Baltimore, where the transplant was performed.
Mr. Bennett received the transplant on January 7 and lived for 2 months following the surgery.
Although not providing the exact cause of his death, UMMC said Mr. Bennett’s condition began deteriorating several days before his death.
When it became clear that he would not recover, he was given compassionate palliative care and was able to communicate with his family during his final hours.
“We are devastated by the loss of Mr. Bennett. He proved to be a brave and noble patient who fought all the way to the end. We extend our sincerest condolences to his family,” Bartley P. Griffith, MD, who performed the transplant, said in the statement.
“We are grateful to Mr. Bennett for his unique and historic role in helping to contribute to a vast array of knowledge to the field of xenotransplantation,” added Muhammad M. Mohiuddin, MD, director of the cardiac xenotransplantation program at University of Maryland School of Medicine.
Before receiving the genetically modified pig heart, Mr. Bennett had required mechanical circulatory support to stay alive but was rejected for standard heart transplantation at UMMC and other centers. He was ineligible for an implanted ventricular assist device due to ventricular arrhythmias.
Following surgery, the transplanted pig heart performed well for several weeks without any signs of rejection. The patient was able to spend time with his family and participate in physical therapy to help regain strength.
“This organ transplant demonstrated for the first time that a genetically modified animal heart can function like a human heart without immediate rejection by the body,” UMMC said in a statement issued 3 days after the surgery.
Thanks to Mr. Bennett, “we have gained invaluable insights learning that the genetically modified pig heart can function well within the human body while the immune system is adequately suppressed,” said Dr. Mohiuddin. “We remain optimistic and plan on continuing our work in future clinical trials.”
The patient’s son, David Bennett Jr, said the family is “profoundly grateful for the life-extending opportunity” provided to his father by the “stellar team” at the University of Maryland School of Medicine and the University of Maryland Medical Center.
“We were able to spend some precious weeks together while he recovered from the transplant surgery, weeks we would not have had without this miraculous effort,” he said.
“We also hope that what was learned from his surgery will benefit future patients and hopefully, one day, end the organ shortage that costs so many lives each year,” he added.
A version of this article first appeared on Medscape.com.
Fewer than half with severe aortic stenosis get new valves
The chance that patients with severe aortic stenosis (AS) will receive aortic valve replacement (AVR) is worse than the flip of a coin, even a decade after the gamechanging transcatheter option became available, a new study suggests.
Of the study’s 6,150 patients with an indication or potential indication for AVR, 48% received the procedure at Massachusetts General Hospital and its partner institution Brigham and Women’s Hospital, both in Boston – both of which have active, high-volume transcatheter and surgical AVR (TAVR/SAVR) programs.
“Essentially, this is a best-case scenario. So, unfortunately, I think on the national level we are likely to see rates that are far worse than what we observed here,” senior author Sammy Elmariah, MD, PhD, Massachusetts General Hospital, told this news organization.
The volume of AVR increased more than 10-fold over the 18-year study period (2000 to 2017), driven by the exponential growth of TAVR, he noted. However, the graying of America led to an even greater increase in the number of patients with severe AS and an indication for AVR.
The study, led by Shawn X. Li, MD, MBA, of Mass General, was published in the March 8 issue of the Journal of the American College of Cardiology.
Previous research has provided equally compelling data on the undertreatment of AS, including a 2021 study using natural language processing (NLP) that found AVR use was just 35.6% within 1 year of diagnosis and varied wildly among managing cardiologists.
The present study used NLP tools to identify symptoms consistent with severe AS in the medical record coupled with echocardiographic data from 10,795 patients with severe AS (valve area <1 cm2). Patients were divided into four AS subtypes and then classified as having a class 1 indication (high-gradient AS with symptoms or reduced ejection fraction [EF]) or a potential class 2a indication (low-gradient AS with symptoms) for AVR.
Among patients with high-gradient AS and class 1 indication for AVR, 1 in 3 did not receive AVR over the study period, including 30% with a normal EF and 47% with a low EF.
In those with low-gradient AS, 67% with a normal EF and 62% with a low EF did not receive AVR. The low-gradient groups were significantly less likely to receive AVR both in the entire study period and in the more contemporary period from 2014 to 2017, despite the valvular heart disease guideline 2014 update indicating AVR was “reasonable” in patients with low-gradient AS – a 2a recommendation upgraded to class 1 in the most recent 2020 update.
Better survival
In patients with a class 1 or potential class 2a indication, AVR was associated with a significantly lower risk of mortality in all four AS subgroups:
- High gradient/normal EF: 3% vs. 15%; adjusted hazard ratio, 0.42
- High-gradient/low EF: 16% vs. 72%; aHR, 0.28
- Low-gradient/normal EF: 5% vs. 14%; aHR, 0.73
- Low-gradient/low EF: 11% vs. 34%; aHR, 0.48; P < .001 for all
“I think what we need to do is change the paradigm, such that patients with a valve area that is less than or equal to 1 [cm2] is severe aortic stenosis until proven otherwise, and that essentially establishes a premise by which we default to treat these patients unless we can prove that it is in fact moderate,” Dr. Elmariah said.
Unfortunately, the opposite is currently true today, he said, and the default is not to treat and put patients through surgery or an invasive TAVR procedure unless physicians can definitively prove that it is severe AS. But they’re not always correct and don’t always have the ability to truly differentiate moderate from severe disease.
“The question, therefore, is ‘What do we do with those patients?’” Dr. Elmariah asked. “I think if a patient has symptoms, then we are obligated to intervene, given the stark difference in mortality that one sees when these patients go undertreated.”
Sounding the alarm
Robert Bonow, MD, a professor of cardiology at Northwestern University in Chicago and a writing committee member for the 2014 guideline update, said the study is a “big wake-up call” and “the take-home message is that we are missing some patients who have treatable aortic stenosis.”
The sheer magnitude of the problem, however, can be difficult to fully ascertain from administrative data like this, he said. Notably, patients who did not receive AVR were significantly older, with 37% aged 81-90 years and 12% over age 90, and had a lower hematocrit and lower estimated glomerular filtration rate. But it’s not clear how many had cancer, end-stage renal disease, or severe lung disease, which could have factored into the decision to undergo AVR.
“What’s also an issue is that over 50% of patients had low gradient disease, which is very problematic and takes careful assessment in an individual patient,” said Dr. Bonow, who is also editor-in-chief of JAMA Cardiology. “That’s all being generated by a low valve area of less than 1 cm2 from echo reports, so that’s not necessarily a careful prospective echo assessment ... so some of the patients with low-gradient disease may not have true severe aortic stenosis.”
Dr. Elmariah agreed that echocardiogram reports are not always clear cut and pointed out that referral to a valve specialist was highly predictive of whether or not a patient underwent AVR, supporting the class 1 guideline recommendation.
He also noted that Mass General is launching the DETECT-AS trial to determine whether electronic physician notifications highlighting clinical practice guideline recommendations will improve AVR utilization over standard care in 940 patients with severe AS on echocardiogram, defined by a valve area less than 1 cm2.
Reached for comment, Catherine Otto, MD, director of the Heart Valve Clinic at the University of Washington, Seattle, and a fellow member of the 2014 guideline writing committee, said “this adds to the data [that] we’re undertreating severe aortic stenosis, and it continues to be surprising given the availability of transcatheter options.”
The biggest challenge is trying to find out why it persists, which is difficult to determine from these data, she said. Whether that’s because the diagnosis is being missed or whether there are barriers to access because cardiologists aren’t understanding the indications or patients aren’t understanding what’s being offered, isn’t clear.
“The other [issue], of course, is are there inappropriate inequities in care? Is it fewer women, age-related, ethnic/racial-related; is it financial? Do people have coverage to get the treatment they need in our country?” Dr. Otto said. “All of those issues are areas that need to be addressed, and I think that is a concern we all have.”
An accompanying editorial points out that the “key lever” in combating undertreatment of AS is getting patients seen by a multidisciplinary heart team and details other possible solutions, such as adding process metrics regarding evaluation and treatment of AS to hospital performance.
“We track quality when AVR is performed (desirable), but how a hospital system performs in getting individuals treated who would benefit from AVR remains a complete blind spot,” write Brian Lindman, MD, MSc, and Angela Lowenstern, MD, MHS, both of Vanderbilt University Medical Center, Nashville, Tenn.
“Is it appropriate to consider the hospital ‘high performing’ when data from Li et al. show a 2-year absolute mortality difference from 9% to 56% based on treatment versus nontreatment with AVR for various AS patient subgroups?” they add.
Dr. Lindman and Dr. Lowenstern observe that having a 50% utilization rate for an effective therapy for a deadly cancer or stenting of ST-segment elevation myocardial infarction (STEMI) would generate negative headlines and a collective commitment to swift action by multiple stakeholders to address what would be “incontrovertibly unacceptable.”
“In one of America’s leading health care systems, there was evidence of an overwhelming reduction in the risk of death with AVR in all AS subgroups examined, but <50% of patients with AS with an indication or potential indication for AVR were treated with an AVR. Let that set in; hear and internalize the alarm. The status quo is unacceptable. What will you do? What will we do?” they conclude.
The study was funded by Edwards Lifesciences. Dr. Elmariah has received research grants from the American Heart Association, National Institutes of Health, Edwards Lifesciences, Svelte Medical, Abbott Vascular, and Medtronic, and has received consulting fees from Edwards Lifesciences. Dr. Bonow and Dr. Otto have disclosed no relevant financial relationships. Dr. Lindman has received investigator-initiated research grants from Edwards. Dr. Lowenstern has received consulting fees from Edwards.
A version of this article first appeared on Medscape.com.
The chance that patients with severe aortic stenosis (AS) will receive aortic valve replacement (AVR) is worse than the flip of a coin, even a decade after the gamechanging transcatheter option became available, a new study suggests.
Of the study’s 6,150 patients with an indication or potential indication for AVR, 48% received the procedure at Massachusetts General Hospital and its partner institution Brigham and Women’s Hospital, both in Boston – both of which have active, high-volume transcatheter and surgical AVR (TAVR/SAVR) programs.
“Essentially, this is a best-case scenario. So, unfortunately, I think on the national level we are likely to see rates that are far worse than what we observed here,” senior author Sammy Elmariah, MD, PhD, Massachusetts General Hospital, told this news organization.
The volume of AVR increased more than 10-fold over the 18-year study period (2000 to 2017), driven by the exponential growth of TAVR, he noted. However, the graying of America led to an even greater increase in the number of patients with severe AS and an indication for AVR.
The study, led by Shawn X. Li, MD, MBA, of Mass General, was published in the March 8 issue of the Journal of the American College of Cardiology.
Previous research has provided equally compelling data on the undertreatment of AS, including a 2021 study using natural language processing (NLP) that found AVR use was just 35.6% within 1 year of diagnosis and varied wildly among managing cardiologists.
The present study used NLP tools to identify symptoms consistent with severe AS in the medical record coupled with echocardiographic data from 10,795 patients with severe AS (valve area <1 cm2). Patients were divided into four AS subtypes and then classified as having a class 1 indication (high-gradient AS with symptoms or reduced ejection fraction [EF]) or a potential class 2a indication (low-gradient AS with symptoms) for AVR.
Among patients with high-gradient AS and class 1 indication for AVR, 1 in 3 did not receive AVR over the study period, including 30% with a normal EF and 47% with a low EF.
In those with low-gradient AS, 67% with a normal EF and 62% with a low EF did not receive AVR. The low-gradient groups were significantly less likely to receive AVR both in the entire study period and in the more contemporary period from 2014 to 2017, despite the valvular heart disease guideline 2014 update indicating AVR was “reasonable” in patients with low-gradient AS – a 2a recommendation upgraded to class 1 in the most recent 2020 update.
Better survival
In patients with a class 1 or potential class 2a indication, AVR was associated with a significantly lower risk of mortality in all four AS subgroups:
- High gradient/normal EF: 3% vs. 15%; adjusted hazard ratio, 0.42
- High-gradient/low EF: 16% vs. 72%; aHR, 0.28
- Low-gradient/normal EF: 5% vs. 14%; aHR, 0.73
- Low-gradient/low EF: 11% vs. 34%; aHR, 0.48; P < .001 for all
“I think what we need to do is change the paradigm, such that patients with a valve area that is less than or equal to 1 [cm2] is severe aortic stenosis until proven otherwise, and that essentially establishes a premise by which we default to treat these patients unless we can prove that it is in fact moderate,” Dr. Elmariah said.
Unfortunately, the opposite is currently true today, he said, and the default is not to treat and put patients through surgery or an invasive TAVR procedure unless physicians can definitively prove that it is severe AS. But they’re not always correct and don’t always have the ability to truly differentiate moderate from severe disease.
“The question, therefore, is ‘What do we do with those patients?’” Dr. Elmariah asked. “I think if a patient has symptoms, then we are obligated to intervene, given the stark difference in mortality that one sees when these patients go undertreated.”
Sounding the alarm
Robert Bonow, MD, a professor of cardiology at Northwestern University in Chicago and a writing committee member for the 2014 guideline update, said the study is a “big wake-up call” and “the take-home message is that we are missing some patients who have treatable aortic stenosis.”
The sheer magnitude of the problem, however, can be difficult to fully ascertain from administrative data like this, he said. Notably, patients who did not receive AVR were significantly older, with 37% aged 81-90 years and 12% over age 90, and had a lower hematocrit and lower estimated glomerular filtration rate. But it’s not clear how many had cancer, end-stage renal disease, or severe lung disease, which could have factored into the decision to undergo AVR.
“What’s also an issue is that over 50% of patients had low gradient disease, which is very problematic and takes careful assessment in an individual patient,” said Dr. Bonow, who is also editor-in-chief of JAMA Cardiology. “That’s all being generated by a low valve area of less than 1 cm2 from echo reports, so that’s not necessarily a careful prospective echo assessment ... so some of the patients with low-gradient disease may not have true severe aortic stenosis.”
Dr. Elmariah agreed that echocardiogram reports are not always clear cut and pointed out that referral to a valve specialist was highly predictive of whether or not a patient underwent AVR, supporting the class 1 guideline recommendation.
He also noted that Mass General is launching the DETECT-AS trial to determine whether electronic physician notifications highlighting clinical practice guideline recommendations will improve AVR utilization over standard care in 940 patients with severe AS on echocardiogram, defined by a valve area less than 1 cm2.
Reached for comment, Catherine Otto, MD, director of the Heart Valve Clinic at the University of Washington, Seattle, and a fellow member of the 2014 guideline writing committee, said “this adds to the data [that] we’re undertreating severe aortic stenosis, and it continues to be surprising given the availability of transcatheter options.”
The biggest challenge is trying to find out why it persists, which is difficult to determine from these data, she said. Whether that’s because the diagnosis is being missed or whether there are barriers to access because cardiologists aren’t understanding the indications or patients aren’t understanding what’s being offered, isn’t clear.
“The other [issue], of course, is are there inappropriate inequities in care? Is it fewer women, age-related, ethnic/racial-related; is it financial? Do people have coverage to get the treatment they need in our country?” Dr. Otto said. “All of those issues are areas that need to be addressed, and I think that is a concern we all have.”
An accompanying editorial points out that the “key lever” in combating undertreatment of AS is getting patients seen by a multidisciplinary heart team and details other possible solutions, such as adding process metrics regarding evaluation and treatment of AS to hospital performance.
“We track quality when AVR is performed (desirable), but how a hospital system performs in getting individuals treated who would benefit from AVR remains a complete blind spot,” write Brian Lindman, MD, MSc, and Angela Lowenstern, MD, MHS, both of Vanderbilt University Medical Center, Nashville, Tenn.
“Is it appropriate to consider the hospital ‘high performing’ when data from Li et al. show a 2-year absolute mortality difference from 9% to 56% based on treatment versus nontreatment with AVR for various AS patient subgroups?” they add.
Dr. Lindman and Dr. Lowenstern observe that having a 50% utilization rate for an effective therapy for a deadly cancer or stenting of ST-segment elevation myocardial infarction (STEMI) would generate negative headlines and a collective commitment to swift action by multiple stakeholders to address what would be “incontrovertibly unacceptable.”
“In one of America’s leading health care systems, there was evidence of an overwhelming reduction in the risk of death with AVR in all AS subgroups examined, but <50% of patients with AS with an indication or potential indication for AVR were treated with an AVR. Let that set in; hear and internalize the alarm. The status quo is unacceptable. What will you do? What will we do?” they conclude.
The study was funded by Edwards Lifesciences. Dr. Elmariah has received research grants from the American Heart Association, National Institutes of Health, Edwards Lifesciences, Svelte Medical, Abbott Vascular, and Medtronic, and has received consulting fees from Edwards Lifesciences. Dr. Bonow and Dr. Otto have disclosed no relevant financial relationships. Dr. Lindman has received investigator-initiated research grants from Edwards. Dr. Lowenstern has received consulting fees from Edwards.
A version of this article first appeared on Medscape.com.
The chance that patients with severe aortic stenosis (AS) will receive aortic valve replacement (AVR) is worse than the flip of a coin, even a decade after the gamechanging transcatheter option became available, a new study suggests.
Of the study’s 6,150 patients with an indication or potential indication for AVR, 48% received the procedure at Massachusetts General Hospital and its partner institution Brigham and Women’s Hospital, both in Boston – both of which have active, high-volume transcatheter and surgical AVR (TAVR/SAVR) programs.
“Essentially, this is a best-case scenario. So, unfortunately, I think on the national level we are likely to see rates that are far worse than what we observed here,” senior author Sammy Elmariah, MD, PhD, Massachusetts General Hospital, told this news organization.
The volume of AVR increased more than 10-fold over the 18-year study period (2000 to 2017), driven by the exponential growth of TAVR, he noted. However, the graying of America led to an even greater increase in the number of patients with severe AS and an indication for AVR.
The study, led by Shawn X. Li, MD, MBA, of Mass General, was published in the March 8 issue of the Journal of the American College of Cardiology.
Previous research has provided equally compelling data on the undertreatment of AS, including a 2021 study using natural language processing (NLP) that found AVR use was just 35.6% within 1 year of diagnosis and varied wildly among managing cardiologists.
The present study used NLP tools to identify symptoms consistent with severe AS in the medical record coupled with echocardiographic data from 10,795 patients with severe AS (valve area <1 cm2). Patients were divided into four AS subtypes and then classified as having a class 1 indication (high-gradient AS with symptoms or reduced ejection fraction [EF]) or a potential class 2a indication (low-gradient AS with symptoms) for AVR.
Among patients with high-gradient AS and class 1 indication for AVR, 1 in 3 did not receive AVR over the study period, including 30% with a normal EF and 47% with a low EF.
In those with low-gradient AS, 67% with a normal EF and 62% with a low EF did not receive AVR. The low-gradient groups were significantly less likely to receive AVR both in the entire study period and in the more contemporary period from 2014 to 2017, despite the valvular heart disease guideline 2014 update indicating AVR was “reasonable” in patients with low-gradient AS – a 2a recommendation upgraded to class 1 in the most recent 2020 update.
Better survival
In patients with a class 1 or potential class 2a indication, AVR was associated with a significantly lower risk of mortality in all four AS subgroups:
- High gradient/normal EF: 3% vs. 15%; adjusted hazard ratio, 0.42
- High-gradient/low EF: 16% vs. 72%; aHR, 0.28
- Low-gradient/normal EF: 5% vs. 14%; aHR, 0.73
- Low-gradient/low EF: 11% vs. 34%; aHR, 0.48; P < .001 for all
“I think what we need to do is change the paradigm, such that patients with a valve area that is less than or equal to 1 [cm2] is severe aortic stenosis until proven otherwise, and that essentially establishes a premise by which we default to treat these patients unless we can prove that it is in fact moderate,” Dr. Elmariah said.
Unfortunately, the opposite is currently true today, he said, and the default is not to treat and put patients through surgery or an invasive TAVR procedure unless physicians can definitively prove that it is severe AS. But they’re not always correct and don’t always have the ability to truly differentiate moderate from severe disease.
“The question, therefore, is ‘What do we do with those patients?’” Dr. Elmariah asked. “I think if a patient has symptoms, then we are obligated to intervene, given the stark difference in mortality that one sees when these patients go undertreated.”
Sounding the alarm
Robert Bonow, MD, a professor of cardiology at Northwestern University in Chicago and a writing committee member for the 2014 guideline update, said the study is a “big wake-up call” and “the take-home message is that we are missing some patients who have treatable aortic stenosis.”
The sheer magnitude of the problem, however, can be difficult to fully ascertain from administrative data like this, he said. Notably, patients who did not receive AVR were significantly older, with 37% aged 81-90 years and 12% over age 90, and had a lower hematocrit and lower estimated glomerular filtration rate. But it’s not clear how many had cancer, end-stage renal disease, or severe lung disease, which could have factored into the decision to undergo AVR.
“What’s also an issue is that over 50% of patients had low gradient disease, which is very problematic and takes careful assessment in an individual patient,” said Dr. Bonow, who is also editor-in-chief of JAMA Cardiology. “That’s all being generated by a low valve area of less than 1 cm2 from echo reports, so that’s not necessarily a careful prospective echo assessment ... so some of the patients with low-gradient disease may not have true severe aortic stenosis.”
Dr. Elmariah agreed that echocardiogram reports are not always clear cut and pointed out that referral to a valve specialist was highly predictive of whether or not a patient underwent AVR, supporting the class 1 guideline recommendation.
He also noted that Mass General is launching the DETECT-AS trial to determine whether electronic physician notifications highlighting clinical practice guideline recommendations will improve AVR utilization over standard care in 940 patients with severe AS on echocardiogram, defined by a valve area less than 1 cm2.
Reached for comment, Catherine Otto, MD, director of the Heart Valve Clinic at the University of Washington, Seattle, and a fellow member of the 2014 guideline writing committee, said “this adds to the data [that] we’re undertreating severe aortic stenosis, and it continues to be surprising given the availability of transcatheter options.”
The biggest challenge is trying to find out why it persists, which is difficult to determine from these data, she said. Whether that’s because the diagnosis is being missed or whether there are barriers to access because cardiologists aren’t understanding the indications or patients aren’t understanding what’s being offered, isn’t clear.
“The other [issue], of course, is are there inappropriate inequities in care? Is it fewer women, age-related, ethnic/racial-related; is it financial? Do people have coverage to get the treatment they need in our country?” Dr. Otto said. “All of those issues are areas that need to be addressed, and I think that is a concern we all have.”
An accompanying editorial points out that the “key lever” in combating undertreatment of AS is getting patients seen by a multidisciplinary heart team and details other possible solutions, such as adding process metrics regarding evaluation and treatment of AS to hospital performance.
“We track quality when AVR is performed (desirable), but how a hospital system performs in getting individuals treated who would benefit from AVR remains a complete blind spot,” write Brian Lindman, MD, MSc, and Angela Lowenstern, MD, MHS, both of Vanderbilt University Medical Center, Nashville, Tenn.
“Is it appropriate to consider the hospital ‘high performing’ when data from Li et al. show a 2-year absolute mortality difference from 9% to 56% based on treatment versus nontreatment with AVR for various AS patient subgroups?” they add.
Dr. Lindman and Dr. Lowenstern observe that having a 50% utilization rate for an effective therapy for a deadly cancer or stenting of ST-segment elevation myocardial infarction (STEMI) would generate negative headlines and a collective commitment to swift action by multiple stakeholders to address what would be “incontrovertibly unacceptable.”
“In one of America’s leading health care systems, there was evidence of an overwhelming reduction in the risk of death with AVR in all AS subgroups examined, but <50% of patients with AS with an indication or potential indication for AVR were treated with an AVR. Let that set in; hear and internalize the alarm. The status quo is unacceptable. What will you do? What will we do?” they conclude.
The study was funded by Edwards Lifesciences. Dr. Elmariah has received research grants from the American Heart Association, National Institutes of Health, Edwards Lifesciences, Svelte Medical, Abbott Vascular, and Medtronic, and has received consulting fees from Edwards Lifesciences. Dr. Bonow and Dr. Otto have disclosed no relevant financial relationships. Dr. Lindman has received investigator-initiated research grants from Edwards. Dr. Lowenstern has received consulting fees from Edwards.
A version of this article first appeared on Medscape.com.
Organ transplantation: Unvaccinated need not apply
I agree with most advice given by the affable TV character Ted Lasso. “Every choice is a chance,” he said. Pandemic-era physicians must now consider whether a politically motivated choice to decline COVID-19 vaccination should negatively affect the chance to receive an organ donation.
And in confronting these choices, we have a chance to educate the public on the complexities of the organ allocation process.
A well-informed patient’s personal choice should be honored, even if clinicians disagree, if it does not affect the well-being of others. For example, I once had a patient in acute leukemic crisis who declined blood products because she was a Jehovah’s Witness. She died. Her choice affected her longevity only.
Compare that decision with awarding an organ to an individual who has declined readily available protection of that organ. Weigh that choice against the fact that said protection is against an infectious disease that has killed over 5.5 million worldwide.
Some institutions stand strong, others hedge their bets
Admirably, Loyola University Health System understands that difference. They published a firm stand on transplant candidacy and COVID-19 vaccination status in the Journal of Heart and Lung Transplant. Daniel Dilling, MD, medical director of the lung transplantation program , and Mark Kuczewski, PhD, a professor of medical ethics at Loyola University Chicago, Maywood, Ill., wrote that: “We believe that requiring vaccination against COVID-19 should not be controversial when we focus strictly on established frameworks and practices surrounding eligibility for wait-listing to receive a solid organ transplant.”
The Cleveland Clinic apparently agrees. In October 2021, they denied a liver transplant to Michelle Vitullo of Ohio, whose daughter had been deemed “a perfect match.” Her daughter, also unvaccinated, stated: “Being denied for a nonmedical reason for someone’s beliefs that are different to yours, I mean that’s not how that should be.”
But vaccination status is a medical reason, given well-established data regarding increased mortality among the immunosuppressed. Ms. Vitullo then said: “We are trying to get to UPMC [University of Pittsburgh Medical Center] as they don’t require a vaccination.”
The public information page on transplant candidacy from UPMC reads (my italics): It is recommended that all transplant candidates, transplant recipients, and their household members receive COVID-19 vaccination when the vaccine is available to them. It is preferred that transplant candidates are vaccinated more than 2 weeks before transplantation.
I reached out to UPMC for clarification and was told by email that “we do not have a policy regarding COVID-19 vaccination requirement for current transplant candidates.” Houston Methodist shares the same agnostic stance.
Compare these opinions with Brigham and Women’s Hospital, where the requirements are resolute: “Like most other transplant programs across the country, the COVID-19 vaccine is one of several vaccines and lifestyle behaviors that are required for patients awaiting solid organ transplant.”
They add that “transplant candidates must also receive the seasonal influenza and hepatitis B vaccines, follow other healthy behaviors, and demonstrate they can commit to taking the required medications following transplant.”
In January 2022, Brigham and Women’s Hospital declared 31-year-old D.J. Ferguson ineligible for a heart transplant because he declined to be vaccinated against COVID-19. According to the New York Post and ABC News, his physicians resorted to left ventricular assist device support. His mother, Tracy Ferguson, is quoted as saying: “He’s not an antivaxxer. He has all of his vaccines.” I’ll just leave that right there.
Unfortunately, Michelle Vitullo’s obituary was published in December 2021. Regardless of whether she received her liver transplant, the outcome is tragic, and whatever you think of this family’s battle playing out in the glare of the national spotlight, their loss is no less devastating.
The directed-donation aspect of this case poses an interesting question. A news anchor asked the mother and daughter: “If you both accept the risks, why doesn’t the hospital just let you try?” The answers are obvious to us clinicians. Performing a transplantation in an unvaccinated patient could lead to their early death if they became infected because of their immunocompromised state, would open the door for transplantation of any patient who is unvaccinated for anything, including influenza and hepatitis B, which could result in the preventable waste of organs, and puts other vulnerable hospitalized patients at risk during the initial transplant stay and follow-up.
That’s not to mention the potential legal suit. Never has a consent form dissuaded any party from lodging an accusation of wrongful death or medical malpractice. In the face of strong data on higher mortality in unvaccinated, immunocompromised patients, a good lawyer could charge that the institution and transplant surgeons should have known better, regardless of the donor and recipient’s willingness to accept the risks.
The Vitullo and Ferguson cases are among many similar dilemmas surrounding transplant candidacy across the United States.
University of Virginia Health in Charlottesville denied 42-year-old Shamgar Connors a kidney transplant because he is unvaccinated, despite a previous COVID-19 infection. In October 2021, Leilani Lutali of Colorado was denied a kidney by UCHealth because she declined vaccination.
As Ted Lasso says: “There’s a bunch of crazy stuff on Twitter.”
Predictably, social media is full of public outcry. “Some cold-hearted people on here” tweeted one. “What if it was one of your loved ones who needed a transplant?” Another tweeted the Hippocratic oath with the comment that “They all swore under this noble ‘oat’, but I guess it’s been forgotten.” (This was followed with a photo of a box of Quaker Oats in a failed attempt at humor.) These discussions among the Twitterati highlight the depths of misunderstanding on organ transplantation.
To be fair, unless you have been personally involved in the decision-making process for transplant candidacy, there is little opportunity to be educated. I explain to my anxious patients and their families that a donor organ is like a fumbled football. There may be well over 100 patients at all levels of transplant status in many geographic locations diving for that same organ.
The transplant team is tasked with finding the best match, determining who is the sickest, assessing time for transport of that organ, and, above all, who will be the best steward of that organ.
Take heart transplantation, for instance. Approximately 3,500 patients in the United States are awaiting one each year. Instead of facing an almost certain death within 5 years, a transplant recipient has a chance at a median survival of 12-13 years. The cost of a heart transplant is approximately $1.38 million, according to Milliman, a consulting firm. This is “an incredibly resource intensive procedure,” including expenditures for transportation, antirejection medication, office visits, physician fees, ICU stays, rejection surveillance, and acute rejection therapies.
Transplant denial is nothing new
People get turned down for organ transplants all the time. My patient with end-stage dilated cardiomyopathy was denied a heart transplant when it was discovered that he had scores of outstanding parking tickets. This was seen as a surrogate for an inability to afford his antirejection medication.
Another patient swore that her positive cotinine levels were caused by endless hours at the bingo hall where second-hand smoke swirled. She was also denied. Many potential candidates who are in acute decline hold precariously to newfound sobriety. They are denied. A patient’s boyfriend told the transplant team that he couldn’t be relied upon to drive her to her appointments. She was denied.
Many people who engage in antisocial behaviors have no idea that these actions may result in the denial of an organ transplant should their future selves need one. These are hard lines, but everyone should agree that the odds of survival are heavily in favor of the consistently adherent.
We should take this opportunity to educate the public on how complicated obtaining an organ transplant can be. More than 6,000 people die each year waiting for an organ because of the supply-and-demand disparities in the transplantation arena. I’m willing to bet that many of the loudest protesters in favor of unvaccinated transplant recipients have not signed the organ donor box on the back of their driver’s license. This conversation is an opportunity to change that and remind people that organ donation may be their only opportunity to save a fellow human’s life.
Again, to quote Ted Lasso: “If you care about someone and you got a little love in your heart, there ain’t nothing you can’t get through together.” That philosophy should apply to the tasks of selecting the best organ donors as well as the best organ recipients.
And every organ should go to the one who will honor their donor and their donor’s family by taking the best care of that ultimate gift of life, including being vaccinated against COVID-19.
Dr. Walton-Shirley is a native Kentuckian who retired from full-time invasive cardiology. She enjoys locums work in Montana and is a champion of physician rights and patient safety. She disclosed no relevant conflicts of interest. A version of this article first appeared on Medscape.com.
I agree with most advice given by the affable TV character Ted Lasso. “Every choice is a chance,” he said. Pandemic-era physicians must now consider whether a politically motivated choice to decline COVID-19 vaccination should negatively affect the chance to receive an organ donation.
And in confronting these choices, we have a chance to educate the public on the complexities of the organ allocation process.
A well-informed patient’s personal choice should be honored, even if clinicians disagree, if it does not affect the well-being of others. For example, I once had a patient in acute leukemic crisis who declined blood products because she was a Jehovah’s Witness. She died. Her choice affected her longevity only.
Compare that decision with awarding an organ to an individual who has declined readily available protection of that organ. Weigh that choice against the fact that said protection is against an infectious disease that has killed over 5.5 million worldwide.
Some institutions stand strong, others hedge their bets
Admirably, Loyola University Health System understands that difference. They published a firm stand on transplant candidacy and COVID-19 vaccination status in the Journal of Heart and Lung Transplant. Daniel Dilling, MD, medical director of the lung transplantation program , and Mark Kuczewski, PhD, a professor of medical ethics at Loyola University Chicago, Maywood, Ill., wrote that: “We believe that requiring vaccination against COVID-19 should not be controversial when we focus strictly on established frameworks and practices surrounding eligibility for wait-listing to receive a solid organ transplant.”
The Cleveland Clinic apparently agrees. In October 2021, they denied a liver transplant to Michelle Vitullo of Ohio, whose daughter had been deemed “a perfect match.” Her daughter, also unvaccinated, stated: “Being denied for a nonmedical reason for someone’s beliefs that are different to yours, I mean that’s not how that should be.”
But vaccination status is a medical reason, given well-established data regarding increased mortality among the immunosuppressed. Ms. Vitullo then said: “We are trying to get to UPMC [University of Pittsburgh Medical Center] as they don’t require a vaccination.”
The public information page on transplant candidacy from UPMC reads (my italics): It is recommended that all transplant candidates, transplant recipients, and their household members receive COVID-19 vaccination when the vaccine is available to them. It is preferred that transplant candidates are vaccinated more than 2 weeks before transplantation.
I reached out to UPMC for clarification and was told by email that “we do not have a policy regarding COVID-19 vaccination requirement for current transplant candidates.” Houston Methodist shares the same agnostic stance.
Compare these opinions with Brigham and Women’s Hospital, where the requirements are resolute: “Like most other transplant programs across the country, the COVID-19 vaccine is one of several vaccines and lifestyle behaviors that are required for patients awaiting solid organ transplant.”
They add that “transplant candidates must also receive the seasonal influenza and hepatitis B vaccines, follow other healthy behaviors, and demonstrate they can commit to taking the required medications following transplant.”
In January 2022, Brigham and Women’s Hospital declared 31-year-old D.J. Ferguson ineligible for a heart transplant because he declined to be vaccinated against COVID-19. According to the New York Post and ABC News, his physicians resorted to left ventricular assist device support. His mother, Tracy Ferguson, is quoted as saying: “He’s not an antivaxxer. He has all of his vaccines.” I’ll just leave that right there.
Unfortunately, Michelle Vitullo’s obituary was published in December 2021. Regardless of whether she received her liver transplant, the outcome is tragic, and whatever you think of this family’s battle playing out in the glare of the national spotlight, their loss is no less devastating.
The directed-donation aspect of this case poses an interesting question. A news anchor asked the mother and daughter: “If you both accept the risks, why doesn’t the hospital just let you try?” The answers are obvious to us clinicians. Performing a transplantation in an unvaccinated patient could lead to their early death if they became infected because of their immunocompromised state, would open the door for transplantation of any patient who is unvaccinated for anything, including influenza and hepatitis B, which could result in the preventable waste of organs, and puts other vulnerable hospitalized patients at risk during the initial transplant stay and follow-up.
That’s not to mention the potential legal suit. Never has a consent form dissuaded any party from lodging an accusation of wrongful death or medical malpractice. In the face of strong data on higher mortality in unvaccinated, immunocompromised patients, a good lawyer could charge that the institution and transplant surgeons should have known better, regardless of the donor and recipient’s willingness to accept the risks.
The Vitullo and Ferguson cases are among many similar dilemmas surrounding transplant candidacy across the United States.
University of Virginia Health in Charlottesville denied 42-year-old Shamgar Connors a kidney transplant because he is unvaccinated, despite a previous COVID-19 infection. In October 2021, Leilani Lutali of Colorado was denied a kidney by UCHealth because she declined vaccination.
As Ted Lasso says: “There’s a bunch of crazy stuff on Twitter.”
Predictably, social media is full of public outcry. “Some cold-hearted people on here” tweeted one. “What if it was one of your loved ones who needed a transplant?” Another tweeted the Hippocratic oath with the comment that “They all swore under this noble ‘oat’, but I guess it’s been forgotten.” (This was followed with a photo of a box of Quaker Oats in a failed attempt at humor.) These discussions among the Twitterati highlight the depths of misunderstanding on organ transplantation.
To be fair, unless you have been personally involved in the decision-making process for transplant candidacy, there is little opportunity to be educated. I explain to my anxious patients and their families that a donor organ is like a fumbled football. There may be well over 100 patients at all levels of transplant status in many geographic locations diving for that same organ.
The transplant team is tasked with finding the best match, determining who is the sickest, assessing time for transport of that organ, and, above all, who will be the best steward of that organ.
Take heart transplantation, for instance. Approximately 3,500 patients in the United States are awaiting one each year. Instead of facing an almost certain death within 5 years, a transplant recipient has a chance at a median survival of 12-13 years. The cost of a heart transplant is approximately $1.38 million, according to Milliman, a consulting firm. This is “an incredibly resource intensive procedure,” including expenditures for transportation, antirejection medication, office visits, physician fees, ICU stays, rejection surveillance, and acute rejection therapies.
Transplant denial is nothing new
People get turned down for organ transplants all the time. My patient with end-stage dilated cardiomyopathy was denied a heart transplant when it was discovered that he had scores of outstanding parking tickets. This was seen as a surrogate for an inability to afford his antirejection medication.
Another patient swore that her positive cotinine levels were caused by endless hours at the bingo hall where second-hand smoke swirled. She was also denied. Many potential candidates who are in acute decline hold precariously to newfound sobriety. They are denied. A patient’s boyfriend told the transplant team that he couldn’t be relied upon to drive her to her appointments. She was denied.
Many people who engage in antisocial behaviors have no idea that these actions may result in the denial of an organ transplant should their future selves need one. These are hard lines, but everyone should agree that the odds of survival are heavily in favor of the consistently adherent.
We should take this opportunity to educate the public on how complicated obtaining an organ transplant can be. More than 6,000 people die each year waiting for an organ because of the supply-and-demand disparities in the transplantation arena. I’m willing to bet that many of the loudest protesters in favor of unvaccinated transplant recipients have not signed the organ donor box on the back of their driver’s license. This conversation is an opportunity to change that and remind people that organ donation may be their only opportunity to save a fellow human’s life.
Again, to quote Ted Lasso: “If you care about someone and you got a little love in your heart, there ain’t nothing you can’t get through together.” That philosophy should apply to the tasks of selecting the best organ donors as well as the best organ recipients.
And every organ should go to the one who will honor their donor and their donor’s family by taking the best care of that ultimate gift of life, including being vaccinated against COVID-19.
Dr. Walton-Shirley is a native Kentuckian who retired from full-time invasive cardiology. She enjoys locums work in Montana and is a champion of physician rights and patient safety. She disclosed no relevant conflicts of interest. A version of this article first appeared on Medscape.com.
I agree with most advice given by the affable TV character Ted Lasso. “Every choice is a chance,” he said. Pandemic-era physicians must now consider whether a politically motivated choice to decline COVID-19 vaccination should negatively affect the chance to receive an organ donation.
And in confronting these choices, we have a chance to educate the public on the complexities of the organ allocation process.
A well-informed patient’s personal choice should be honored, even if clinicians disagree, if it does not affect the well-being of others. For example, I once had a patient in acute leukemic crisis who declined blood products because she was a Jehovah’s Witness. She died. Her choice affected her longevity only.
Compare that decision with awarding an organ to an individual who has declined readily available protection of that organ. Weigh that choice against the fact that said protection is against an infectious disease that has killed over 5.5 million worldwide.
Some institutions stand strong, others hedge their bets
Admirably, Loyola University Health System understands that difference. They published a firm stand on transplant candidacy and COVID-19 vaccination status in the Journal of Heart and Lung Transplant. Daniel Dilling, MD, medical director of the lung transplantation program , and Mark Kuczewski, PhD, a professor of medical ethics at Loyola University Chicago, Maywood, Ill., wrote that: “We believe that requiring vaccination against COVID-19 should not be controversial when we focus strictly on established frameworks and practices surrounding eligibility for wait-listing to receive a solid organ transplant.”
The Cleveland Clinic apparently agrees. In October 2021, they denied a liver transplant to Michelle Vitullo of Ohio, whose daughter had been deemed “a perfect match.” Her daughter, also unvaccinated, stated: “Being denied for a nonmedical reason for someone’s beliefs that are different to yours, I mean that’s not how that should be.”
But vaccination status is a medical reason, given well-established data regarding increased mortality among the immunosuppressed. Ms. Vitullo then said: “We are trying to get to UPMC [University of Pittsburgh Medical Center] as they don’t require a vaccination.”
The public information page on transplant candidacy from UPMC reads (my italics): It is recommended that all transplant candidates, transplant recipients, and their household members receive COVID-19 vaccination when the vaccine is available to them. It is preferred that transplant candidates are vaccinated more than 2 weeks before transplantation.
I reached out to UPMC for clarification and was told by email that “we do not have a policy regarding COVID-19 vaccination requirement for current transplant candidates.” Houston Methodist shares the same agnostic stance.
Compare these opinions with Brigham and Women’s Hospital, where the requirements are resolute: “Like most other transplant programs across the country, the COVID-19 vaccine is one of several vaccines and lifestyle behaviors that are required for patients awaiting solid organ transplant.”
They add that “transplant candidates must also receive the seasonal influenza and hepatitis B vaccines, follow other healthy behaviors, and demonstrate they can commit to taking the required medications following transplant.”
In January 2022, Brigham and Women’s Hospital declared 31-year-old D.J. Ferguson ineligible for a heart transplant because he declined to be vaccinated against COVID-19. According to the New York Post and ABC News, his physicians resorted to left ventricular assist device support. His mother, Tracy Ferguson, is quoted as saying: “He’s not an antivaxxer. He has all of his vaccines.” I’ll just leave that right there.
Unfortunately, Michelle Vitullo’s obituary was published in December 2021. Regardless of whether she received her liver transplant, the outcome is tragic, and whatever you think of this family’s battle playing out in the glare of the national spotlight, their loss is no less devastating.
The directed-donation aspect of this case poses an interesting question. A news anchor asked the mother and daughter: “If you both accept the risks, why doesn’t the hospital just let you try?” The answers are obvious to us clinicians. Performing a transplantation in an unvaccinated patient could lead to their early death if they became infected because of their immunocompromised state, would open the door for transplantation of any patient who is unvaccinated for anything, including influenza and hepatitis B, which could result in the preventable waste of organs, and puts other vulnerable hospitalized patients at risk during the initial transplant stay and follow-up.
That’s not to mention the potential legal suit. Never has a consent form dissuaded any party from lodging an accusation of wrongful death or medical malpractice. In the face of strong data on higher mortality in unvaccinated, immunocompromised patients, a good lawyer could charge that the institution and transplant surgeons should have known better, regardless of the donor and recipient’s willingness to accept the risks.
The Vitullo and Ferguson cases are among many similar dilemmas surrounding transplant candidacy across the United States.
University of Virginia Health in Charlottesville denied 42-year-old Shamgar Connors a kidney transplant because he is unvaccinated, despite a previous COVID-19 infection. In October 2021, Leilani Lutali of Colorado was denied a kidney by UCHealth because she declined vaccination.
As Ted Lasso says: “There’s a bunch of crazy stuff on Twitter.”
Predictably, social media is full of public outcry. “Some cold-hearted people on here” tweeted one. “What if it was one of your loved ones who needed a transplant?” Another tweeted the Hippocratic oath with the comment that “They all swore under this noble ‘oat’, but I guess it’s been forgotten.” (This was followed with a photo of a box of Quaker Oats in a failed attempt at humor.) These discussions among the Twitterati highlight the depths of misunderstanding on organ transplantation.
To be fair, unless you have been personally involved in the decision-making process for transplant candidacy, there is little opportunity to be educated. I explain to my anxious patients and their families that a donor organ is like a fumbled football. There may be well over 100 patients at all levels of transplant status in many geographic locations diving for that same organ.
The transplant team is tasked with finding the best match, determining who is the sickest, assessing time for transport of that organ, and, above all, who will be the best steward of that organ.
Take heart transplantation, for instance. Approximately 3,500 patients in the United States are awaiting one each year. Instead of facing an almost certain death within 5 years, a transplant recipient has a chance at a median survival of 12-13 years. The cost of a heart transplant is approximately $1.38 million, according to Milliman, a consulting firm. This is “an incredibly resource intensive procedure,” including expenditures for transportation, antirejection medication, office visits, physician fees, ICU stays, rejection surveillance, and acute rejection therapies.
Transplant denial is nothing new
People get turned down for organ transplants all the time. My patient with end-stage dilated cardiomyopathy was denied a heart transplant when it was discovered that he had scores of outstanding parking tickets. This was seen as a surrogate for an inability to afford his antirejection medication.
Another patient swore that her positive cotinine levels were caused by endless hours at the bingo hall where second-hand smoke swirled. She was also denied. Many potential candidates who are in acute decline hold precariously to newfound sobriety. They are denied. A patient’s boyfriend told the transplant team that he couldn’t be relied upon to drive her to her appointments. She was denied.
Many people who engage in antisocial behaviors have no idea that these actions may result in the denial of an organ transplant should their future selves need one. These are hard lines, but everyone should agree that the odds of survival are heavily in favor of the consistently adherent.
We should take this opportunity to educate the public on how complicated obtaining an organ transplant can be. More than 6,000 people die each year waiting for an organ because of the supply-and-demand disparities in the transplantation arena. I’m willing to bet that many of the loudest protesters in favor of unvaccinated transplant recipients have not signed the organ donor box on the back of their driver’s license. This conversation is an opportunity to change that and remind people that organ donation may be their only opportunity to save a fellow human’s life.
Again, to quote Ted Lasso: “If you care about someone and you got a little love in your heart, there ain’t nothing you can’t get through together.” That philosophy should apply to the tasks of selecting the best organ donors as well as the best organ recipients.
And every organ should go to the one who will honor their donor and their donor’s family by taking the best care of that ultimate gift of life, including being vaccinated against COVID-19.
Dr. Walton-Shirley is a native Kentuckian who retired from full-time invasive cardiology. She enjoys locums work in Montana and is a champion of physician rights and patient safety. She disclosed no relevant conflicts of interest. A version of this article first appeared on Medscape.com.
VARC-3 TAVR technical failure definition ‘highly clinically relevant’
A new study offers early validation of the recently released Valve Academic Research Consortium 3 (VARC-3) definition of technical success after transcatheter aortic valve replacement (TAVR) and highlights its role in patient prognosis.
Results show that one in 10 patients (11.6%) undergoing TAVR with contemporary devices and techniques experiences technical failure, according to VARC-3.
At 30 days, patients with technical failure had significantly higher rates of the composite of cardiovascular (CV) death or stroke (11.5% vs. 3.5%), CV death (6.0% vs. 1.0%), and stroke (7.2% vs. 2.9%), compared with those with technical success.
Technical failure after TAVR was also independently associated with a twofold higher risk for CV death or stroke at 1 year (20.0% vs. 10.3%; hazard ratio, 2.01; 95% CI, 1.37-2.95).
Other independent predictors were history of peripheral artery disease (HR, 1.97), New York Heart Association III or IV disease (HR, 1.86), baseline moderate or greater mitral regurgitation (HR, 1.48), atrial fibrillation (HR, 1.40), and Society of Thoracic Surgeons predicted mortality risk (HR, 1.04).
“We were expecting that we were getting better over time with device iterations, with more experience, so we weren’t surprised by the result. But I think what is somewhat surprising is how much of an impact it has on the outcome,” senior study author Thomas Pilgrim, MD, Inselspital, University of Bern, Switzerland, told this news organization.
The VARC-3 document, introduced last year to some controversy, features a heavier focus on patient outcomes, as well as composite safety and efficacy endpoints. The definition of technical success after TAVR includes freedom from death; successful access, delivery of the device, and retrieval of the delivery system; correct positioning of a prosthetic heart valve into the proper anatomical location; and freedom from surgery or intervention related to the device or to an access-related or cardiac structural complication.
The composite endpoint is meant to replace the VARC-2 definition of “device success,” which also included freedom from death and correct valve positioning but required echocardiographic evaluation. With VARC-3, there is an “immediate measure” of success without having to wait for echocardiography, observed Dr. Pilgrim.
As reported in the Journal of the American College of Cardiology Cardiovascular Interventions, TAVR was a technical success in 1,435 of 1,624 (88.4%) patients. Technical failure occurred in 189 patients related to either vascular complications (8.6%) or procedural death or cardiac complications (3.0%).
The VARC-2 endpoint of device success was observed in 66.1% of patients. The high rate of device failure was largely attributed to a 28% incidence of prosthesis-patient mismatch.
“If you use the VARC-2 device success [definition], you include this patient–prosthesis mismatch, the [valve] gradients, [and] regurgitation and then device success is always lower,” Dr. Pilgrim said.
Asked whether the VARC-3 definition may be missing case failures, he replied: “At this stage, we don’t know how important these echocardiographic parameters are for hard clinical endpoints. Maybe the VARC-2 endpoint was too sensitive or the VARC-3 endpoint is not sensitive enough. This is something we just don’t know at this stage.”
Marco Barbanti, MD, an interventional cardiologist at Rodolico Polyclinic University Hospital-San Marco, Catania, Italy, and author of an accompanying editorial, said VARC-3 represents a more accurate indicator of immediate success of the procedure.
“It’s a more pertinent definition according to what really has an impact on prognosis, and, according to the results of this paper, actually, the calibration of this new definition is quite good,” Dr. Barbanti said in an interview.
Patients with VARC-3 technical failure were older, had a higher body mass index, and had more advanced heart failure symptoms than those with technical success. There were no significant differences between the two groups in echocardiographic or CT data, anesthetic strategy, valve type or size, or use of pre- or post-dilation.
All patients underwent TAVR with current balloon-expandable (Sapien 3/Sapien Ultra, Edwards Lifesciences) or self-expanding (Evolut R/PRO [Medtronic], Portico [Abbott], Symetis ACURATE/ACURATE neo [Boston Scientific]) devices between March 2012 and December 2019. A transfemoral approach was used in 92.5% of patients.
In a landmark analysis with the landmark set at 30 days, the effect of technical failure on adverse outcome was limited to the first 30 days (composite endpoint 0-30 days: HR, 3.42; P < .001; 30-360 days: HR, 1.36; P = .266; P for interaction = .002).
At 1 year, the composite of CV death and stroke endpoint occurred in 24.1% of patients with cardiac technical failure, in 18.8% of patients with vascular technical failure, and in 10.3% of patients with technical success.
In multivariate analyses, cardiac and vascular technical failures were independently associated with a 2.6-fold and 1.9-fold increased risk, respectively, for the composite of cardiovascular death and stroke at 1 year.
Female sex, larger device landing zone calcium volume, and earlier procedures (March 2012 to July 2016) were associated with a higher risk for cardiac technical failure, whereas, consistent with previous studies, higher body mass index and use of the Prostar/Manta versus the ProGlide closure device predicted vascular technical failure.
The findings “underscore that technical success is highly clinically relevant and may serve as one of the pivotal endpoints to evaluate the improvement of TAVR or for head-to-head comparisons of new devices in future clinical trials,” the authors conclude.
The findings reflect the experience of a single high-volume center with highly experienced operators in the prospective BERN TAVR registry, however, and may not be generalizable to other heart centers, they note. Although the registry has standardized follow-up, independent analysis of echocardiographic and CT, and independent event adjudication, vascular anatomy was not systematically assessed, and the potential exists for confounding from unmeasured variables.
Dr. Pilgrim reports research grants to the institution from Edwards Lifesciences, Boston Scientific, and Biotronik, personal fees from Biotronik and Boston Scientific, and other from HighLife SAS. Dr. Barbanti is a consultant for Edwards Lifesciences and Boston Scientific.
A version of this article first appeared on Medscape.com.
A new study offers early validation of the recently released Valve Academic Research Consortium 3 (VARC-3) definition of technical success after transcatheter aortic valve replacement (TAVR) and highlights its role in patient prognosis.
Results show that one in 10 patients (11.6%) undergoing TAVR with contemporary devices and techniques experiences technical failure, according to VARC-3.
At 30 days, patients with technical failure had significantly higher rates of the composite of cardiovascular (CV) death or stroke (11.5% vs. 3.5%), CV death (6.0% vs. 1.0%), and stroke (7.2% vs. 2.9%), compared with those with technical success.
Technical failure after TAVR was also independently associated with a twofold higher risk for CV death or stroke at 1 year (20.0% vs. 10.3%; hazard ratio, 2.01; 95% CI, 1.37-2.95).
Other independent predictors were history of peripheral artery disease (HR, 1.97), New York Heart Association III or IV disease (HR, 1.86), baseline moderate or greater mitral regurgitation (HR, 1.48), atrial fibrillation (HR, 1.40), and Society of Thoracic Surgeons predicted mortality risk (HR, 1.04).
“We were expecting that we were getting better over time with device iterations, with more experience, so we weren’t surprised by the result. But I think what is somewhat surprising is how much of an impact it has on the outcome,” senior study author Thomas Pilgrim, MD, Inselspital, University of Bern, Switzerland, told this news organization.
The VARC-3 document, introduced last year to some controversy, features a heavier focus on patient outcomes, as well as composite safety and efficacy endpoints. The definition of technical success after TAVR includes freedom from death; successful access, delivery of the device, and retrieval of the delivery system; correct positioning of a prosthetic heart valve into the proper anatomical location; and freedom from surgery or intervention related to the device or to an access-related or cardiac structural complication.
The composite endpoint is meant to replace the VARC-2 definition of “device success,” which also included freedom from death and correct valve positioning but required echocardiographic evaluation. With VARC-3, there is an “immediate measure” of success without having to wait for echocardiography, observed Dr. Pilgrim.
As reported in the Journal of the American College of Cardiology Cardiovascular Interventions, TAVR was a technical success in 1,435 of 1,624 (88.4%) patients. Technical failure occurred in 189 patients related to either vascular complications (8.6%) or procedural death or cardiac complications (3.0%).
The VARC-2 endpoint of device success was observed in 66.1% of patients. The high rate of device failure was largely attributed to a 28% incidence of prosthesis-patient mismatch.
“If you use the VARC-2 device success [definition], you include this patient–prosthesis mismatch, the [valve] gradients, [and] regurgitation and then device success is always lower,” Dr. Pilgrim said.
Asked whether the VARC-3 definition may be missing case failures, he replied: “At this stage, we don’t know how important these echocardiographic parameters are for hard clinical endpoints. Maybe the VARC-2 endpoint was too sensitive or the VARC-3 endpoint is not sensitive enough. This is something we just don’t know at this stage.”
Marco Barbanti, MD, an interventional cardiologist at Rodolico Polyclinic University Hospital-San Marco, Catania, Italy, and author of an accompanying editorial, said VARC-3 represents a more accurate indicator of immediate success of the procedure.
“It’s a more pertinent definition according to what really has an impact on prognosis, and, according to the results of this paper, actually, the calibration of this new definition is quite good,” Dr. Barbanti said in an interview.
Patients with VARC-3 technical failure were older, had a higher body mass index, and had more advanced heart failure symptoms than those with technical success. There were no significant differences between the two groups in echocardiographic or CT data, anesthetic strategy, valve type or size, or use of pre- or post-dilation.
All patients underwent TAVR with current balloon-expandable (Sapien 3/Sapien Ultra, Edwards Lifesciences) or self-expanding (Evolut R/PRO [Medtronic], Portico [Abbott], Symetis ACURATE/ACURATE neo [Boston Scientific]) devices between March 2012 and December 2019. A transfemoral approach was used in 92.5% of patients.
In a landmark analysis with the landmark set at 30 days, the effect of technical failure on adverse outcome was limited to the first 30 days (composite endpoint 0-30 days: HR, 3.42; P < .001; 30-360 days: HR, 1.36; P = .266; P for interaction = .002).
At 1 year, the composite of CV death and stroke endpoint occurred in 24.1% of patients with cardiac technical failure, in 18.8% of patients with vascular technical failure, and in 10.3% of patients with technical success.
In multivariate analyses, cardiac and vascular technical failures were independently associated with a 2.6-fold and 1.9-fold increased risk, respectively, for the composite of cardiovascular death and stroke at 1 year.
Female sex, larger device landing zone calcium volume, and earlier procedures (March 2012 to July 2016) were associated with a higher risk for cardiac technical failure, whereas, consistent with previous studies, higher body mass index and use of the Prostar/Manta versus the ProGlide closure device predicted vascular technical failure.
The findings “underscore that technical success is highly clinically relevant and may serve as one of the pivotal endpoints to evaluate the improvement of TAVR or for head-to-head comparisons of new devices in future clinical trials,” the authors conclude.
The findings reflect the experience of a single high-volume center with highly experienced operators in the prospective BERN TAVR registry, however, and may not be generalizable to other heart centers, they note. Although the registry has standardized follow-up, independent analysis of echocardiographic and CT, and independent event adjudication, vascular anatomy was not systematically assessed, and the potential exists for confounding from unmeasured variables.
Dr. Pilgrim reports research grants to the institution from Edwards Lifesciences, Boston Scientific, and Biotronik, personal fees from Biotronik and Boston Scientific, and other from HighLife SAS. Dr. Barbanti is a consultant for Edwards Lifesciences and Boston Scientific.
A version of this article first appeared on Medscape.com.
A new study offers early validation of the recently released Valve Academic Research Consortium 3 (VARC-3) definition of technical success after transcatheter aortic valve replacement (TAVR) and highlights its role in patient prognosis.
Results show that one in 10 patients (11.6%) undergoing TAVR with contemporary devices and techniques experiences technical failure, according to VARC-3.
At 30 days, patients with technical failure had significantly higher rates of the composite of cardiovascular (CV) death or stroke (11.5% vs. 3.5%), CV death (6.0% vs. 1.0%), and stroke (7.2% vs. 2.9%), compared with those with technical success.
Technical failure after TAVR was also independently associated with a twofold higher risk for CV death or stroke at 1 year (20.0% vs. 10.3%; hazard ratio, 2.01; 95% CI, 1.37-2.95).
Other independent predictors were history of peripheral artery disease (HR, 1.97), New York Heart Association III or IV disease (HR, 1.86), baseline moderate or greater mitral regurgitation (HR, 1.48), atrial fibrillation (HR, 1.40), and Society of Thoracic Surgeons predicted mortality risk (HR, 1.04).
“We were expecting that we were getting better over time with device iterations, with more experience, so we weren’t surprised by the result. But I think what is somewhat surprising is how much of an impact it has on the outcome,” senior study author Thomas Pilgrim, MD, Inselspital, University of Bern, Switzerland, told this news organization.
The VARC-3 document, introduced last year to some controversy, features a heavier focus on patient outcomes, as well as composite safety and efficacy endpoints. The definition of technical success after TAVR includes freedom from death; successful access, delivery of the device, and retrieval of the delivery system; correct positioning of a prosthetic heart valve into the proper anatomical location; and freedom from surgery or intervention related to the device or to an access-related or cardiac structural complication.
The composite endpoint is meant to replace the VARC-2 definition of “device success,” which also included freedom from death and correct valve positioning but required echocardiographic evaluation. With VARC-3, there is an “immediate measure” of success without having to wait for echocardiography, observed Dr. Pilgrim.
As reported in the Journal of the American College of Cardiology Cardiovascular Interventions, TAVR was a technical success in 1,435 of 1,624 (88.4%) patients. Technical failure occurred in 189 patients related to either vascular complications (8.6%) or procedural death or cardiac complications (3.0%).
The VARC-2 endpoint of device success was observed in 66.1% of patients. The high rate of device failure was largely attributed to a 28% incidence of prosthesis-patient mismatch.
“If you use the VARC-2 device success [definition], you include this patient–prosthesis mismatch, the [valve] gradients, [and] regurgitation and then device success is always lower,” Dr. Pilgrim said.
Asked whether the VARC-3 definition may be missing case failures, he replied: “At this stage, we don’t know how important these echocardiographic parameters are for hard clinical endpoints. Maybe the VARC-2 endpoint was too sensitive or the VARC-3 endpoint is not sensitive enough. This is something we just don’t know at this stage.”
Marco Barbanti, MD, an interventional cardiologist at Rodolico Polyclinic University Hospital-San Marco, Catania, Italy, and author of an accompanying editorial, said VARC-3 represents a more accurate indicator of immediate success of the procedure.
“It’s a more pertinent definition according to what really has an impact on prognosis, and, according to the results of this paper, actually, the calibration of this new definition is quite good,” Dr. Barbanti said in an interview.
Patients with VARC-3 technical failure were older, had a higher body mass index, and had more advanced heart failure symptoms than those with technical success. There were no significant differences between the two groups in echocardiographic or CT data, anesthetic strategy, valve type or size, or use of pre- or post-dilation.
All patients underwent TAVR with current balloon-expandable (Sapien 3/Sapien Ultra, Edwards Lifesciences) or self-expanding (Evolut R/PRO [Medtronic], Portico [Abbott], Symetis ACURATE/ACURATE neo [Boston Scientific]) devices between March 2012 and December 2019. A transfemoral approach was used in 92.5% of patients.
In a landmark analysis with the landmark set at 30 days, the effect of technical failure on adverse outcome was limited to the first 30 days (composite endpoint 0-30 days: HR, 3.42; P < .001; 30-360 days: HR, 1.36; P = .266; P for interaction = .002).
At 1 year, the composite of CV death and stroke endpoint occurred in 24.1% of patients with cardiac technical failure, in 18.8% of patients with vascular technical failure, and in 10.3% of patients with technical success.
In multivariate analyses, cardiac and vascular technical failures were independently associated with a 2.6-fold and 1.9-fold increased risk, respectively, for the composite of cardiovascular death and stroke at 1 year.
Female sex, larger device landing zone calcium volume, and earlier procedures (March 2012 to July 2016) were associated with a higher risk for cardiac technical failure, whereas, consistent with previous studies, higher body mass index and use of the Prostar/Manta versus the ProGlide closure device predicted vascular technical failure.
The findings “underscore that technical success is highly clinically relevant and may serve as one of the pivotal endpoints to evaluate the improvement of TAVR or for head-to-head comparisons of new devices in future clinical trials,” the authors conclude.
The findings reflect the experience of a single high-volume center with highly experienced operators in the prospective BERN TAVR registry, however, and may not be generalizable to other heart centers, they note. Although the registry has standardized follow-up, independent analysis of echocardiographic and CT, and independent event adjudication, vascular anatomy was not systematically assessed, and the potential exists for confounding from unmeasured variables.
Dr. Pilgrim reports research grants to the institution from Edwards Lifesciences, Boston Scientific, and Biotronik, personal fees from Biotronik and Boston Scientific, and other from HighLife SAS. Dr. Barbanti is a consultant for Edwards Lifesciences and Boston Scientific.
A version of this article first appeared on Medscape.com.
FROM JACC: CARDIOVASCULAR INTERVENTIONS
CVS Caremark formulary change freezes out apixaban
Patients looking to refill a prescription for apixaban (Eliquis) through CVS Caremark may be in for a surprise following its decision to exclude the direct oral anticoagulant (DOAC) from its formulary starting Jan. 1.
The move leaves just one DOAC, rivaroxaban (Xarelto), on CVS’ commercial formulary and is being assailed as the latest example of “nonmedical switching” used by health insurers to control costs.
In a letter to CVS Caremark backed by 14 provider and patient organizations, the nonprofit Partnership to Advance Cardiovascular Health (PACH) calls on the pharmacy chain to reverse its “dangerously disruptive” decision to force stable patients at high risk of cardiovascular events to switch anticoagulation, without an apparent option to be grandfathered into the new plan.
PACH president Dharmesh Patel, MD, Stern Cardiovascular Center, Memphis, called the formulary change “reckless and irresponsible, especially because the decision is not based in science and evidence, but on budgets. Patients and their health care providers, not insurance companies, need to be trusted to determine what medication is best,” he said in a statement.
Craig Beavers, PharmD, vice president of Baptist Health Paducah, Kentucky, said that, as chair of the American College of Cardiology’s Cardiovascular Team Section, he and other organizations have met with CVS Caremark medical leadership to advocate for patients and to understand the company’s perspective.
“The underlying driver is cost,” he told this news organization.
Current guidelines recommend DOACs in general for a variety of indications, including to reduce the risk of stroke and embolism in nonvalvular atrial fibrillation and to prevent deep vein thrombosis, but there are select instances where a particular DOAC might be more appropriate, he observed.
“Apixaban may be better for a patient with a history of GI bleeding because there’s less GI bleeding, but the guidelines don’t necessarily spell those things out,” Dr. Beavers said. “That’s where the clinician should advocate for their patient and, unfortunately, they are making their decision strictly based off the guidelines.”
Requests to speak with medical officers at CVS Caremark went unanswered, but its executive director of communications, Christina Peaslee, told this news organization that the formulary decision “maintains clinically appropriate, cost-effective prescription coverage” for its clients and members.
“Both the American Heart Association/American College of Cardiology/Heart Rhythm Society and 2021 CHEST guidelines recommend DOACs over warfarin for treatment of various cardiology conditions such as atrial fibrillation, but neither list a specific agent as preferred – showing that consensus clinical guidelines do not favor one over the other,” she said in an email. “Further, Xarelto has more FDA-approved indications than Eliquis (e.g., Xarelto is approved for a reduction in risk of major CV events in patients with CAD or PAD) in addition to all the same FDA indications as Eliquis.”
Ms. Peaslee pointed out that all formulary changes are evaluated by an external medical expert specializing in the disease state, followed by review and approval by an independent national Pharmacy & Therapeutics Committee.
The decision to exclude apixaban is also limited to a “subset of commercial drug lists,” she said, although specifics on which plans and the number of affected patients were not forthcoming.
The choice of DOAC is a timely question in cardiology, with recent studies suggesting an advantage for apixaban over rivaroxaban in reducing the risk of recurrent venous thromboembolism, as well as reducing the risk of major ischemic or hemorrhagic events in atrial fibrillation.
Ms. Peaslee said CVS Caremark closely monitors medical literature for relevant clinical trial data and that most clients allow reasonable formulary exceptions when justified. “This formulary exceptions process has been successfully used for changes of this type and allows patients to get a medication that is safe and effective, as determined by their prescriber.”
The company will also continue to provide “robust, personalized outreach to the small number of members who will need to switch to an alternative medication,” she added.
Dr. Beavers said negotiations with CVS are still in the early stages, but, in the meantime, the ACC is providing health care providers with tools, such as drug copay cards and electronic prior authorizations, to help ensure patients don’t have gaps in coverage.
In a Jan. 14 news release addressing the formulary change, ACC notes that a patient’s pharmacy can also request a one-time override when trying to fill a nonpreferred DOAC in January to buy time if switching medications with their clinician or requesting a formulary exception.
During discussions with CVS Caremark, it says the ACC and the American Society of Hematology “underscored the negative impacts of this decision on patients currently taking one of the nonpreferred DOACs and on those who have previously tried rivaroxaban and changed medications.”
The groups also highlighted difficulties with other prior authorization programs in terms of the need for dedicated staff and time away from direct patient care.
“The ACC and ASH will continue discussions with CVS Caremark regarding the burden on clinicians and the effect of the formulary decision on patient access,” the release says.
In its letter to CVS, PACH argues that the apixaban exclusion will disproportionately affect historically disadvantaged patients, leaving those who can least afford the change with limited options. Notably, no generic is available for either apixaban or rivaroxaban.
The group also highlights a 2019 national poll, in which nearly 40% of patients who had their medication switched were so frustrated that they stopped their medication altogether.
PACH has an online petition against nonmedical switching, which at press time had garnered 2,126 signatures.
One signee, Jan Griffin, who survived bilateral pulmonary embolisms, writes that she has been on Eliquis [apixaban] successfully since her hospital discharge. “Now, as of midnight, Caremark apparently knows better than my hematologist as to what blood thinner is better for me and will no longer cover my Eliquis prescription. This is criminal, immoral, and unethical. #StopTheSwitch.”
A version of this article first appeared on Medscape.com.
Patients looking to refill a prescription for apixaban (Eliquis) through CVS Caremark may be in for a surprise following its decision to exclude the direct oral anticoagulant (DOAC) from its formulary starting Jan. 1.
The move leaves just one DOAC, rivaroxaban (Xarelto), on CVS’ commercial formulary and is being assailed as the latest example of “nonmedical switching” used by health insurers to control costs.
In a letter to CVS Caremark backed by 14 provider and patient organizations, the nonprofit Partnership to Advance Cardiovascular Health (PACH) calls on the pharmacy chain to reverse its “dangerously disruptive” decision to force stable patients at high risk of cardiovascular events to switch anticoagulation, without an apparent option to be grandfathered into the new plan.
PACH president Dharmesh Patel, MD, Stern Cardiovascular Center, Memphis, called the formulary change “reckless and irresponsible, especially because the decision is not based in science and evidence, but on budgets. Patients and their health care providers, not insurance companies, need to be trusted to determine what medication is best,” he said in a statement.
Craig Beavers, PharmD, vice president of Baptist Health Paducah, Kentucky, said that, as chair of the American College of Cardiology’s Cardiovascular Team Section, he and other organizations have met with CVS Caremark medical leadership to advocate for patients and to understand the company’s perspective.
“The underlying driver is cost,” he told this news organization.
Current guidelines recommend DOACs in general for a variety of indications, including to reduce the risk of stroke and embolism in nonvalvular atrial fibrillation and to prevent deep vein thrombosis, but there are select instances where a particular DOAC might be more appropriate, he observed.
“Apixaban may be better for a patient with a history of GI bleeding because there’s less GI bleeding, but the guidelines don’t necessarily spell those things out,” Dr. Beavers said. “That’s where the clinician should advocate for their patient and, unfortunately, they are making their decision strictly based off the guidelines.”
Requests to speak with medical officers at CVS Caremark went unanswered, but its executive director of communications, Christina Peaslee, told this news organization that the formulary decision “maintains clinically appropriate, cost-effective prescription coverage” for its clients and members.
“Both the American Heart Association/American College of Cardiology/Heart Rhythm Society and 2021 CHEST guidelines recommend DOACs over warfarin for treatment of various cardiology conditions such as atrial fibrillation, but neither list a specific agent as preferred – showing that consensus clinical guidelines do not favor one over the other,” she said in an email. “Further, Xarelto has more FDA-approved indications than Eliquis (e.g., Xarelto is approved for a reduction in risk of major CV events in patients with CAD or PAD) in addition to all the same FDA indications as Eliquis.”
Ms. Peaslee pointed out that all formulary changes are evaluated by an external medical expert specializing in the disease state, followed by review and approval by an independent national Pharmacy & Therapeutics Committee.
The decision to exclude apixaban is also limited to a “subset of commercial drug lists,” she said, although specifics on which plans and the number of affected patients were not forthcoming.
The choice of DOAC is a timely question in cardiology, with recent studies suggesting an advantage for apixaban over rivaroxaban in reducing the risk of recurrent venous thromboembolism, as well as reducing the risk of major ischemic or hemorrhagic events in atrial fibrillation.
Ms. Peaslee said CVS Caremark closely monitors medical literature for relevant clinical trial data and that most clients allow reasonable formulary exceptions when justified. “This formulary exceptions process has been successfully used for changes of this type and allows patients to get a medication that is safe and effective, as determined by their prescriber.”
The company will also continue to provide “robust, personalized outreach to the small number of members who will need to switch to an alternative medication,” she added.
Dr. Beavers said negotiations with CVS are still in the early stages, but, in the meantime, the ACC is providing health care providers with tools, such as drug copay cards and electronic prior authorizations, to help ensure patients don’t have gaps in coverage.
In a Jan. 14 news release addressing the formulary change, ACC notes that a patient’s pharmacy can also request a one-time override when trying to fill a nonpreferred DOAC in January to buy time if switching medications with their clinician or requesting a formulary exception.
During discussions with CVS Caremark, it says the ACC and the American Society of Hematology “underscored the negative impacts of this decision on patients currently taking one of the nonpreferred DOACs and on those who have previously tried rivaroxaban and changed medications.”
The groups also highlighted difficulties with other prior authorization programs in terms of the need for dedicated staff and time away from direct patient care.
“The ACC and ASH will continue discussions with CVS Caremark regarding the burden on clinicians and the effect of the formulary decision on patient access,” the release says.
In its letter to CVS, PACH argues that the apixaban exclusion will disproportionately affect historically disadvantaged patients, leaving those who can least afford the change with limited options. Notably, no generic is available for either apixaban or rivaroxaban.
The group also highlights a 2019 national poll, in which nearly 40% of patients who had their medication switched were so frustrated that they stopped their medication altogether.
PACH has an online petition against nonmedical switching, which at press time had garnered 2,126 signatures.
One signee, Jan Griffin, who survived bilateral pulmonary embolisms, writes that she has been on Eliquis [apixaban] successfully since her hospital discharge. “Now, as of midnight, Caremark apparently knows better than my hematologist as to what blood thinner is better for me and will no longer cover my Eliquis prescription. This is criminal, immoral, and unethical. #StopTheSwitch.”
A version of this article first appeared on Medscape.com.
Patients looking to refill a prescription for apixaban (Eliquis) through CVS Caremark may be in for a surprise following its decision to exclude the direct oral anticoagulant (DOAC) from its formulary starting Jan. 1.
The move leaves just one DOAC, rivaroxaban (Xarelto), on CVS’ commercial formulary and is being assailed as the latest example of “nonmedical switching” used by health insurers to control costs.
In a letter to CVS Caremark backed by 14 provider and patient organizations, the nonprofit Partnership to Advance Cardiovascular Health (PACH) calls on the pharmacy chain to reverse its “dangerously disruptive” decision to force stable patients at high risk of cardiovascular events to switch anticoagulation, without an apparent option to be grandfathered into the new plan.
PACH president Dharmesh Patel, MD, Stern Cardiovascular Center, Memphis, called the formulary change “reckless and irresponsible, especially because the decision is not based in science and evidence, but on budgets. Patients and their health care providers, not insurance companies, need to be trusted to determine what medication is best,” he said in a statement.
Craig Beavers, PharmD, vice president of Baptist Health Paducah, Kentucky, said that, as chair of the American College of Cardiology’s Cardiovascular Team Section, he and other organizations have met with CVS Caremark medical leadership to advocate for patients and to understand the company’s perspective.
“The underlying driver is cost,” he told this news organization.
Current guidelines recommend DOACs in general for a variety of indications, including to reduce the risk of stroke and embolism in nonvalvular atrial fibrillation and to prevent deep vein thrombosis, but there are select instances where a particular DOAC might be more appropriate, he observed.
“Apixaban may be better for a patient with a history of GI bleeding because there’s less GI bleeding, but the guidelines don’t necessarily spell those things out,” Dr. Beavers said. “That’s where the clinician should advocate for their patient and, unfortunately, they are making their decision strictly based off the guidelines.”
Requests to speak with medical officers at CVS Caremark went unanswered, but its executive director of communications, Christina Peaslee, told this news organization that the formulary decision “maintains clinically appropriate, cost-effective prescription coverage” for its clients and members.
“Both the American Heart Association/American College of Cardiology/Heart Rhythm Society and 2021 CHEST guidelines recommend DOACs over warfarin for treatment of various cardiology conditions such as atrial fibrillation, but neither list a specific agent as preferred – showing that consensus clinical guidelines do not favor one over the other,” she said in an email. “Further, Xarelto has more FDA-approved indications than Eliquis (e.g., Xarelto is approved for a reduction in risk of major CV events in patients with CAD or PAD) in addition to all the same FDA indications as Eliquis.”
Ms. Peaslee pointed out that all formulary changes are evaluated by an external medical expert specializing in the disease state, followed by review and approval by an independent national Pharmacy & Therapeutics Committee.
The decision to exclude apixaban is also limited to a “subset of commercial drug lists,” she said, although specifics on which plans and the number of affected patients were not forthcoming.
The choice of DOAC is a timely question in cardiology, with recent studies suggesting an advantage for apixaban over rivaroxaban in reducing the risk of recurrent venous thromboembolism, as well as reducing the risk of major ischemic or hemorrhagic events in atrial fibrillation.
Ms. Peaslee said CVS Caremark closely monitors medical literature for relevant clinical trial data and that most clients allow reasonable formulary exceptions when justified. “This formulary exceptions process has been successfully used for changes of this type and allows patients to get a medication that is safe and effective, as determined by their prescriber.”
The company will also continue to provide “robust, personalized outreach to the small number of members who will need to switch to an alternative medication,” she added.
Dr. Beavers said negotiations with CVS are still in the early stages, but, in the meantime, the ACC is providing health care providers with tools, such as drug copay cards and electronic prior authorizations, to help ensure patients don’t have gaps in coverage.
In a Jan. 14 news release addressing the formulary change, ACC notes that a patient’s pharmacy can also request a one-time override when trying to fill a nonpreferred DOAC in January to buy time if switching medications with their clinician or requesting a formulary exception.
During discussions with CVS Caremark, it says the ACC and the American Society of Hematology “underscored the negative impacts of this decision on patients currently taking one of the nonpreferred DOACs and on those who have previously tried rivaroxaban and changed medications.”
The groups also highlighted difficulties with other prior authorization programs in terms of the need for dedicated staff and time away from direct patient care.
“The ACC and ASH will continue discussions with CVS Caremark regarding the burden on clinicians and the effect of the formulary decision on patient access,” the release says.
In its letter to CVS, PACH argues that the apixaban exclusion will disproportionately affect historically disadvantaged patients, leaving those who can least afford the change with limited options. Notably, no generic is available for either apixaban or rivaroxaban.
The group also highlights a 2019 national poll, in which nearly 40% of patients who had their medication switched were so frustrated that they stopped their medication altogether.
PACH has an online petition against nonmedical switching, which at press time had garnered 2,126 signatures.
One signee, Jan Griffin, who survived bilateral pulmonary embolisms, writes that she has been on Eliquis [apixaban] successfully since her hospital discharge. “Now, as of midnight, Caremark apparently knows better than my hematologist as to what blood thinner is better for me and will no longer cover my Eliquis prescription. This is criminal, immoral, and unethical. #StopTheSwitch.”
A version of this article first appeared on Medscape.com.
What does a pig-to-human heart transplant mean for medicine?
Scientific achievements usually raise big new questions, and the remarkable surgery that took place on Jan. 7, when Maryland resident David Bennett was transplanted with a genetically modified heart from a pig, has been no different.
The 57-year-old with end-stage heart failure had been repeatedly turned down for a standard transplant and was judged a poor candidate for a ventricular assist device. Now his new heart is beating soundly and apparently accepted by his immune system as Mr. Bennett, his physicians at the University of Maryland where the procedure took place, and indeed the world set out on a journey with far more unknowns than knowns.
“I think even just a couple of years ago, people felt that xenotransplantation for the heart and other organs was still a long way off. And it seems like it’s started to move very quickly,” Larry A. Allen, MD, University of Colorado, Aurora, said in an interview.
Demand for donor hearts far outstrips supply, and despite advances in the development of ventricular assist pumps and artificial hearts, “there are still significant limitations to them in terms of clotting, stroke, and infection. We’ve seen the use of those devices plateau,” Dr. Allen said. “So, the concept of a nonhuman source of organs is exciting and very much in need, if people can get it to work.”
“I really credit the surgeons at the University of Maryland for courageous clinical work and a brilliant scientific innovation,” Clyde W. Yancy, MD, MSc, Northwestern University, Chicago, said in an interview. “But it’s always in the implementation that we have to hold our breath.” Heart xenotransplantation is an old idea that “has never before been successful,” he said. And standard heart transplantation has set a high bar, with a 1-year survival of about 90% and low 1-year risk for rejection. Whether the new procedure can meet that standard is unknown, as is its potential for complications, such as chronic rejection or cancers due to long-term immunosuppression. Those are “major questions requiring more time and careful follow-up.”
‘Still a nascent technology’
“This is an exciting and courageous step forward in heart transplantation, and kudos to the team at the University of Maryland,” said Mandeep R. Mehra, MD, Brigham and Woman’s Hospital, Boston. But “there are many challenges here.”
The procedure’s 10 gene modifications were reportedly aimed at preventing hyperacute rejection of the heart and its excessive growth after transplantation, and making the organ less immunogenic, Dr. Mehra said in an interview. But even if those goals are met, could the same changes potentially impede the heart’s adaptation to human physiology, such as during ambulation or stress?
That kind of adaptation may become important. For example, Dr. Mehra observed, normally a pig heart “provides flow in a four-footed configuration, and pig temperature is inherently higher than humans by several degrees, so it will be functioning in a relatively hypothermic environment.”
Transplantation remains the gold standard for patients with advanced heart failure despite modern medical and device therapy, Dr. Allen agreed. But “if we can raise pig hearts that provide the organ, and it can be implanted with a surgery that’s been done for 50 years, and rejection can be managed with gene editing and tailored immunosuppression, then it’s not hard to think about this very rapidly replacing a lot of what we do in the advanced heart failure and transplantation world.”
Certainly, it would be a major advance if the gene editing technique successfully improves the heart’s immunologic compatibility, Dr. Yancy noted. But do we have enough genomic knowledge to select gene deletions and insertions in the safest way for a successful outcome? “We have to appreciate that this is still a nascent technology, and we should be careful that there might be consequences that we haven’t anticipated.”
For example, he said, the xenotransplantation and gene-modifying techniques should be explored in a range of patients, including older and younger people, women and men, and people of different ethnicities and races.
“There may be some differences based on ancestry, based on gender, based on aging, that will influence the way in which these engineered donor hearts are experienced clinically,” Dr. Yancy said.
The xenotransplantation technique’s potential impact on health equity should also be considered, as it “almost assuredly will be a very expensive technology that will be utilized in a very select population,” he noted. “We need to have a really wide lens to think about all of the potential ramifications.”
‘This field needs to evolve’
Dr. Mehra also flagged the procedure’s potential cost should it become mainstream. Perhaps that would promote dialogue on how to primarily use it “after legitimately exhausting all available options, such as total artificial heart support.”
It might also teach the field to take greater advantage of the many donated hearts discarded as suboptimal. “The general usage rate for offered organs is around a third,” despite opportunities to expand use of those that are “less than perfect,” Dr. Mehra said. “I think that the field will grow with the community focusing on reduced discards of current available heart organs, and not necessarily grow because of the availability of ‘xeno-organs.’ ”
“This field needs to evolve because we’re actively transplanting patients today. But in my mind, the real future is to have such a sufficient understanding of the biology of left ventricular dysfunction that transplantation is a rare event,” Dr. Yancy proposed.
“I’m not certain that heart transplantation per se is the endgame. I think the avoidance of transplantation is the real endgame,” he said. “This may be controversial, but my vision of the future is not one where we have a supply of animals that we can use for transplantation. My vision of the future is that heart transplantation becomes obsolete.”
A version of this article first appeared on Medscape.com.
Scientific achievements usually raise big new questions, and the remarkable surgery that took place on Jan. 7, when Maryland resident David Bennett was transplanted with a genetically modified heart from a pig, has been no different.
The 57-year-old with end-stage heart failure had been repeatedly turned down for a standard transplant and was judged a poor candidate for a ventricular assist device. Now his new heart is beating soundly and apparently accepted by his immune system as Mr. Bennett, his physicians at the University of Maryland where the procedure took place, and indeed the world set out on a journey with far more unknowns than knowns.
“I think even just a couple of years ago, people felt that xenotransplantation for the heart and other organs was still a long way off. And it seems like it’s started to move very quickly,” Larry A. Allen, MD, University of Colorado, Aurora, said in an interview.
Demand for donor hearts far outstrips supply, and despite advances in the development of ventricular assist pumps and artificial hearts, “there are still significant limitations to them in terms of clotting, stroke, and infection. We’ve seen the use of those devices plateau,” Dr. Allen said. “So, the concept of a nonhuman source of organs is exciting and very much in need, if people can get it to work.”
“I really credit the surgeons at the University of Maryland for courageous clinical work and a brilliant scientific innovation,” Clyde W. Yancy, MD, MSc, Northwestern University, Chicago, said in an interview. “But it’s always in the implementation that we have to hold our breath.” Heart xenotransplantation is an old idea that “has never before been successful,” he said. And standard heart transplantation has set a high bar, with a 1-year survival of about 90% and low 1-year risk for rejection. Whether the new procedure can meet that standard is unknown, as is its potential for complications, such as chronic rejection or cancers due to long-term immunosuppression. Those are “major questions requiring more time and careful follow-up.”
‘Still a nascent technology’
“This is an exciting and courageous step forward in heart transplantation, and kudos to the team at the University of Maryland,” said Mandeep R. Mehra, MD, Brigham and Woman’s Hospital, Boston. But “there are many challenges here.”
The procedure’s 10 gene modifications were reportedly aimed at preventing hyperacute rejection of the heart and its excessive growth after transplantation, and making the organ less immunogenic, Dr. Mehra said in an interview. But even if those goals are met, could the same changes potentially impede the heart’s adaptation to human physiology, such as during ambulation or stress?
That kind of adaptation may become important. For example, Dr. Mehra observed, normally a pig heart “provides flow in a four-footed configuration, and pig temperature is inherently higher than humans by several degrees, so it will be functioning in a relatively hypothermic environment.”
Transplantation remains the gold standard for patients with advanced heart failure despite modern medical and device therapy, Dr. Allen agreed. But “if we can raise pig hearts that provide the organ, and it can be implanted with a surgery that’s been done for 50 years, and rejection can be managed with gene editing and tailored immunosuppression, then it’s not hard to think about this very rapidly replacing a lot of what we do in the advanced heart failure and transplantation world.”
Certainly, it would be a major advance if the gene editing technique successfully improves the heart’s immunologic compatibility, Dr. Yancy noted. But do we have enough genomic knowledge to select gene deletions and insertions in the safest way for a successful outcome? “We have to appreciate that this is still a nascent technology, and we should be careful that there might be consequences that we haven’t anticipated.”
For example, he said, the xenotransplantation and gene-modifying techniques should be explored in a range of patients, including older and younger people, women and men, and people of different ethnicities and races.
“There may be some differences based on ancestry, based on gender, based on aging, that will influence the way in which these engineered donor hearts are experienced clinically,” Dr. Yancy said.
The xenotransplantation technique’s potential impact on health equity should also be considered, as it “almost assuredly will be a very expensive technology that will be utilized in a very select population,” he noted. “We need to have a really wide lens to think about all of the potential ramifications.”
‘This field needs to evolve’
Dr. Mehra also flagged the procedure’s potential cost should it become mainstream. Perhaps that would promote dialogue on how to primarily use it “after legitimately exhausting all available options, such as total artificial heart support.”
It might also teach the field to take greater advantage of the many donated hearts discarded as suboptimal. “The general usage rate for offered organs is around a third,” despite opportunities to expand use of those that are “less than perfect,” Dr. Mehra said. “I think that the field will grow with the community focusing on reduced discards of current available heart organs, and not necessarily grow because of the availability of ‘xeno-organs.’ ”
“This field needs to evolve because we’re actively transplanting patients today. But in my mind, the real future is to have such a sufficient understanding of the biology of left ventricular dysfunction that transplantation is a rare event,” Dr. Yancy proposed.
“I’m not certain that heart transplantation per se is the endgame. I think the avoidance of transplantation is the real endgame,” he said. “This may be controversial, but my vision of the future is not one where we have a supply of animals that we can use for transplantation. My vision of the future is that heart transplantation becomes obsolete.”
A version of this article first appeared on Medscape.com.
Scientific achievements usually raise big new questions, and the remarkable surgery that took place on Jan. 7, when Maryland resident David Bennett was transplanted with a genetically modified heart from a pig, has been no different.
The 57-year-old with end-stage heart failure had been repeatedly turned down for a standard transplant and was judged a poor candidate for a ventricular assist device. Now his new heart is beating soundly and apparently accepted by his immune system as Mr. Bennett, his physicians at the University of Maryland where the procedure took place, and indeed the world set out on a journey with far more unknowns than knowns.
“I think even just a couple of years ago, people felt that xenotransplantation for the heart and other organs was still a long way off. And it seems like it’s started to move very quickly,” Larry A. Allen, MD, University of Colorado, Aurora, said in an interview.
Demand for donor hearts far outstrips supply, and despite advances in the development of ventricular assist pumps and artificial hearts, “there are still significant limitations to them in terms of clotting, stroke, and infection. We’ve seen the use of those devices plateau,” Dr. Allen said. “So, the concept of a nonhuman source of organs is exciting and very much in need, if people can get it to work.”
“I really credit the surgeons at the University of Maryland for courageous clinical work and a brilliant scientific innovation,” Clyde W. Yancy, MD, MSc, Northwestern University, Chicago, said in an interview. “But it’s always in the implementation that we have to hold our breath.” Heart xenotransplantation is an old idea that “has never before been successful,” he said. And standard heart transplantation has set a high bar, with a 1-year survival of about 90% and low 1-year risk for rejection. Whether the new procedure can meet that standard is unknown, as is its potential for complications, such as chronic rejection or cancers due to long-term immunosuppression. Those are “major questions requiring more time and careful follow-up.”
‘Still a nascent technology’
“This is an exciting and courageous step forward in heart transplantation, and kudos to the team at the University of Maryland,” said Mandeep R. Mehra, MD, Brigham and Woman’s Hospital, Boston. But “there are many challenges here.”
The procedure’s 10 gene modifications were reportedly aimed at preventing hyperacute rejection of the heart and its excessive growth after transplantation, and making the organ less immunogenic, Dr. Mehra said in an interview. But even if those goals are met, could the same changes potentially impede the heart’s adaptation to human physiology, such as during ambulation or stress?
That kind of adaptation may become important. For example, Dr. Mehra observed, normally a pig heart “provides flow in a four-footed configuration, and pig temperature is inherently higher than humans by several degrees, so it will be functioning in a relatively hypothermic environment.”
Transplantation remains the gold standard for patients with advanced heart failure despite modern medical and device therapy, Dr. Allen agreed. But “if we can raise pig hearts that provide the organ, and it can be implanted with a surgery that’s been done for 50 years, and rejection can be managed with gene editing and tailored immunosuppression, then it’s not hard to think about this very rapidly replacing a lot of what we do in the advanced heart failure and transplantation world.”
Certainly, it would be a major advance if the gene editing technique successfully improves the heart’s immunologic compatibility, Dr. Yancy noted. But do we have enough genomic knowledge to select gene deletions and insertions in the safest way for a successful outcome? “We have to appreciate that this is still a nascent technology, and we should be careful that there might be consequences that we haven’t anticipated.”
For example, he said, the xenotransplantation and gene-modifying techniques should be explored in a range of patients, including older and younger people, women and men, and people of different ethnicities and races.
“There may be some differences based on ancestry, based on gender, based on aging, that will influence the way in which these engineered donor hearts are experienced clinically,” Dr. Yancy said.
The xenotransplantation technique’s potential impact on health equity should also be considered, as it “almost assuredly will be a very expensive technology that will be utilized in a very select population,” he noted. “We need to have a really wide lens to think about all of the potential ramifications.”
‘This field needs to evolve’
Dr. Mehra also flagged the procedure’s potential cost should it become mainstream. Perhaps that would promote dialogue on how to primarily use it “after legitimately exhausting all available options, such as total artificial heart support.”
It might also teach the field to take greater advantage of the many donated hearts discarded as suboptimal. “The general usage rate for offered organs is around a third,” despite opportunities to expand use of those that are “less than perfect,” Dr. Mehra said. “I think that the field will grow with the community focusing on reduced discards of current available heart organs, and not necessarily grow because of the availability of ‘xeno-organs.’ ”
“This field needs to evolve because we’re actively transplanting patients today. But in my mind, the real future is to have such a sufficient understanding of the biology of left ventricular dysfunction that transplantation is a rare event,” Dr. Yancy proposed.
“I’m not certain that heart transplantation per se is the endgame. I think the avoidance of transplantation is the real endgame,” he said. “This may be controversial, but my vision of the future is not one where we have a supply of animals that we can use for transplantation. My vision of the future is that heart transplantation becomes obsolete.”
A version of this article first appeared on Medscape.com.