User login
Derms in survey say climate change is impacting their patients
in which the majority of participants said their patients are already being impacted.
Almost 80% of the 148 participants who responded to an electronic survey reported this belief.
The survey was designed and distributed to the membership of various dermatological organizations by Misha Rosenbach, MD, and coauthors. The results were published in the British Journal of Dermatology.
Asked also about specific types of climate-driven phenomena with a current – or future – impact on their patients, 80.1% reported that they believed that increased exposure to ultraviolet radiation (UVR) is impactful, or will be. Changes in temporal or geographic patterns of vector-borne illnesses were affirmed by 78.7%, and an increase in social displacement caused by extreme weather or other events was affirmed by 67.1% as having an impact on their patients currently or in the future.
Other phenomena affirmed by respondents as already having an impact or impacting patients in the future were an increased incidence of heat exposure or heat-related illness (58.2%); an increase in rates of inflammatory skin disease flares (43.2%); increased incidence of waterborne infections (42.5%); and increased rates of allergic contact dermatitis (29.5%).
The survey was sent to the membership of the American Society of Dermatologic Surgery, the Society for Pediatric Dermatology, the Society for Investigative Dermatology, and the American Academy of Dermatology’s Climate Change Expert Resource Group (ERG), among other organizations.
The study design and membership overlap made it impossible to calculate a response rate, the authors said, but they estimated it to be about 10%.
Almost all respondents were from the United States, and most (86.3%) practiced in an academic setting. The findings are similar to those of an online survey of members of the International Society of Dermatology (ISD), published in 2020, which found that 89% of 158 respondents believed climate change will impact the incidence of skin diseases in their area.
“Physicians, including dermatologists, are starting to understand the impact of the climate crisis on both their patients and themselves ... both through lived experiences and [issues raised] more in the scientific literature and in meetings,” Dr. Rosenbach, associate professor of dermatology at the University of Pennsylvania, Philadelphia, said in an interview.
A majority of participants in the U.S. survey agreed they have a responsibility to bring awareness of the health effects of climate change to patients (77.2%) and to policymakers (88.6%). (In the ISD survey, 88% said they believed that dermatologists should play an advocacy role in climate change-related issues).
Only a minority of respondents in the U.S. survey said that they would feel comfortable discussing climate change with their patients (37.2%). Almost one-third of the respondents said they would like to be better informed about climate change before doing so. And 81.8% said they would like to read more about the dermatological effects of climate change in scientific journals.
“There continues to be unfilled interest in education and advocacy regarding climate change, suggesting a ‘practice gap’ even among dermatologists,” Dr. Rosenbach and his colleagues wrote, noting opportunities for professional organizations and journals to provide more resources and “actionable items” regarding climate change.
Some dermatologists have been taking action, in the meantime, to reduce the carbon footprint of their practices and institutions. Reductions in facility energy consumption, and reductions in medical waste/optimization of recycling, were each reported by more than one-third of survey respondents.
And almost half indicated that their practice or institution had increased capacity for telemedicine or telecommuting in response to climate change. Only 8% said their practice or institution had divested from fossil fuel stocks and/or bonds.
“There are a lot of sustainability-in-medicine solutions that are actually cost-neutral or cost-saving for practices,” said Dr. Rosenbach, who is a founder and co-chair of the AAD’s ERG on Climate Change and Environmental Issues.
Research in dermatology is starting to quantify the environmental impact of some of these changes. In a research letter also published in the British Journal of Dermatology, researchers from Cardiff University and the department of dermatology at University Hospital of Wales, described how they determined that reusable surgical packs used for skin surgery are more sustainable than single-use packs because of their reduced cost and reduced greenhouse gas emissions.
Such single-site reports are “early feeders” into what will become a stream of larger studies quantifying the impact of measures taken in dermatology, Dr. Rosenbach said.
Across medicine, there is evidence that health care professionals are now seeing climate change as a threat to their patients. In a multinational survey published last year in The Lancet Planetary Health, 77% of 3,977 participants said that climate change will cause a moderate or great deal of harm for their patients.
Climate change will be discussed at the AAD’s annual meeting in late March in a session devoted to the topic, and as part of a broader session on controversies in dermatology.
Dr. Rosenbach and two of the five authors of the dermatology research letter are members of the AAD’s ERG on climate change, but in the publication they noted that they were not writing on behalf of the AAD. None of the authors reported any disclosures, and there was no funding source for the survey.
in which the majority of participants said their patients are already being impacted.
Almost 80% of the 148 participants who responded to an electronic survey reported this belief.
The survey was designed and distributed to the membership of various dermatological organizations by Misha Rosenbach, MD, and coauthors. The results were published in the British Journal of Dermatology.
Asked also about specific types of climate-driven phenomena with a current – or future – impact on their patients, 80.1% reported that they believed that increased exposure to ultraviolet radiation (UVR) is impactful, or will be. Changes in temporal or geographic patterns of vector-borne illnesses were affirmed by 78.7%, and an increase in social displacement caused by extreme weather or other events was affirmed by 67.1% as having an impact on their patients currently or in the future.
Other phenomena affirmed by respondents as already having an impact or impacting patients in the future were an increased incidence of heat exposure or heat-related illness (58.2%); an increase in rates of inflammatory skin disease flares (43.2%); increased incidence of waterborne infections (42.5%); and increased rates of allergic contact dermatitis (29.5%).
The survey was sent to the membership of the American Society of Dermatologic Surgery, the Society for Pediatric Dermatology, the Society for Investigative Dermatology, and the American Academy of Dermatology’s Climate Change Expert Resource Group (ERG), among other organizations.
The study design and membership overlap made it impossible to calculate a response rate, the authors said, but they estimated it to be about 10%.
Almost all respondents were from the United States, and most (86.3%) practiced in an academic setting. The findings are similar to those of an online survey of members of the International Society of Dermatology (ISD), published in 2020, which found that 89% of 158 respondents believed climate change will impact the incidence of skin diseases in their area.
“Physicians, including dermatologists, are starting to understand the impact of the climate crisis on both their patients and themselves ... both through lived experiences and [issues raised] more in the scientific literature and in meetings,” Dr. Rosenbach, associate professor of dermatology at the University of Pennsylvania, Philadelphia, said in an interview.
A majority of participants in the U.S. survey agreed they have a responsibility to bring awareness of the health effects of climate change to patients (77.2%) and to policymakers (88.6%). (In the ISD survey, 88% said they believed that dermatologists should play an advocacy role in climate change-related issues).
Only a minority of respondents in the U.S. survey said that they would feel comfortable discussing climate change with their patients (37.2%). Almost one-third of the respondents said they would like to be better informed about climate change before doing so. And 81.8% said they would like to read more about the dermatological effects of climate change in scientific journals.
“There continues to be unfilled interest in education and advocacy regarding climate change, suggesting a ‘practice gap’ even among dermatologists,” Dr. Rosenbach and his colleagues wrote, noting opportunities for professional organizations and journals to provide more resources and “actionable items” regarding climate change.
Some dermatologists have been taking action, in the meantime, to reduce the carbon footprint of their practices and institutions. Reductions in facility energy consumption, and reductions in medical waste/optimization of recycling, were each reported by more than one-third of survey respondents.
And almost half indicated that their practice or institution had increased capacity for telemedicine or telecommuting in response to climate change. Only 8% said their practice or institution had divested from fossil fuel stocks and/or bonds.
“There are a lot of sustainability-in-medicine solutions that are actually cost-neutral or cost-saving for practices,” said Dr. Rosenbach, who is a founder and co-chair of the AAD’s ERG on Climate Change and Environmental Issues.
Research in dermatology is starting to quantify the environmental impact of some of these changes. In a research letter also published in the British Journal of Dermatology, researchers from Cardiff University and the department of dermatology at University Hospital of Wales, described how they determined that reusable surgical packs used for skin surgery are more sustainable than single-use packs because of their reduced cost and reduced greenhouse gas emissions.
Such single-site reports are “early feeders” into what will become a stream of larger studies quantifying the impact of measures taken in dermatology, Dr. Rosenbach said.
Across medicine, there is evidence that health care professionals are now seeing climate change as a threat to their patients. In a multinational survey published last year in The Lancet Planetary Health, 77% of 3,977 participants said that climate change will cause a moderate or great deal of harm for their patients.
Climate change will be discussed at the AAD’s annual meeting in late March in a session devoted to the topic, and as part of a broader session on controversies in dermatology.
Dr. Rosenbach and two of the five authors of the dermatology research letter are members of the AAD’s ERG on climate change, but in the publication they noted that they were not writing on behalf of the AAD. None of the authors reported any disclosures, and there was no funding source for the survey.
in which the majority of participants said their patients are already being impacted.
Almost 80% of the 148 participants who responded to an electronic survey reported this belief.
The survey was designed and distributed to the membership of various dermatological organizations by Misha Rosenbach, MD, and coauthors. The results were published in the British Journal of Dermatology.
Asked also about specific types of climate-driven phenomena with a current – or future – impact on their patients, 80.1% reported that they believed that increased exposure to ultraviolet radiation (UVR) is impactful, or will be. Changes in temporal or geographic patterns of vector-borne illnesses were affirmed by 78.7%, and an increase in social displacement caused by extreme weather or other events was affirmed by 67.1% as having an impact on their patients currently or in the future.
Other phenomena affirmed by respondents as already having an impact or impacting patients in the future were an increased incidence of heat exposure or heat-related illness (58.2%); an increase in rates of inflammatory skin disease flares (43.2%); increased incidence of waterborne infections (42.5%); and increased rates of allergic contact dermatitis (29.5%).
The survey was sent to the membership of the American Society of Dermatologic Surgery, the Society for Pediatric Dermatology, the Society for Investigative Dermatology, and the American Academy of Dermatology’s Climate Change Expert Resource Group (ERG), among other organizations.
The study design and membership overlap made it impossible to calculate a response rate, the authors said, but they estimated it to be about 10%.
Almost all respondents were from the United States, and most (86.3%) practiced in an academic setting. The findings are similar to those of an online survey of members of the International Society of Dermatology (ISD), published in 2020, which found that 89% of 158 respondents believed climate change will impact the incidence of skin diseases in their area.
“Physicians, including dermatologists, are starting to understand the impact of the climate crisis on both their patients and themselves ... both through lived experiences and [issues raised] more in the scientific literature and in meetings,” Dr. Rosenbach, associate professor of dermatology at the University of Pennsylvania, Philadelphia, said in an interview.
A majority of participants in the U.S. survey agreed they have a responsibility to bring awareness of the health effects of climate change to patients (77.2%) and to policymakers (88.6%). (In the ISD survey, 88% said they believed that dermatologists should play an advocacy role in climate change-related issues).
Only a minority of respondents in the U.S. survey said that they would feel comfortable discussing climate change with their patients (37.2%). Almost one-third of the respondents said they would like to be better informed about climate change before doing so. And 81.8% said they would like to read more about the dermatological effects of climate change in scientific journals.
“There continues to be unfilled interest in education and advocacy regarding climate change, suggesting a ‘practice gap’ even among dermatologists,” Dr. Rosenbach and his colleagues wrote, noting opportunities for professional organizations and journals to provide more resources and “actionable items” regarding climate change.
Some dermatologists have been taking action, in the meantime, to reduce the carbon footprint of their practices and institutions. Reductions in facility energy consumption, and reductions in medical waste/optimization of recycling, were each reported by more than one-third of survey respondents.
And almost half indicated that their practice or institution had increased capacity for telemedicine or telecommuting in response to climate change. Only 8% said their practice or institution had divested from fossil fuel stocks and/or bonds.
“There are a lot of sustainability-in-medicine solutions that are actually cost-neutral or cost-saving for practices,” said Dr. Rosenbach, who is a founder and co-chair of the AAD’s ERG on Climate Change and Environmental Issues.
Research in dermatology is starting to quantify the environmental impact of some of these changes. In a research letter also published in the British Journal of Dermatology, researchers from Cardiff University and the department of dermatology at University Hospital of Wales, described how they determined that reusable surgical packs used for skin surgery are more sustainable than single-use packs because of their reduced cost and reduced greenhouse gas emissions.
Such single-site reports are “early feeders” into what will become a stream of larger studies quantifying the impact of measures taken in dermatology, Dr. Rosenbach said.
Across medicine, there is evidence that health care professionals are now seeing climate change as a threat to their patients. In a multinational survey published last year in The Lancet Planetary Health, 77% of 3,977 participants said that climate change will cause a moderate or great deal of harm for their patients.
Climate change will be discussed at the AAD’s annual meeting in late March in a session devoted to the topic, and as part of a broader session on controversies in dermatology.
Dr. Rosenbach and two of the five authors of the dermatology research letter are members of the AAD’s ERG on climate change, but in the publication they noted that they were not writing on behalf of the AAD. None of the authors reported any disclosures, and there was no funding source for the survey.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Phototoxic Contact Dermatitis From Over-the-counter 8-Methoxypsoralen
To the Editor:
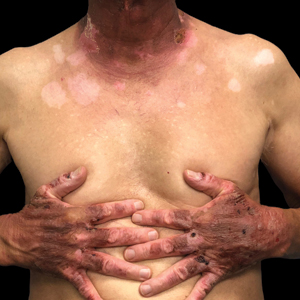
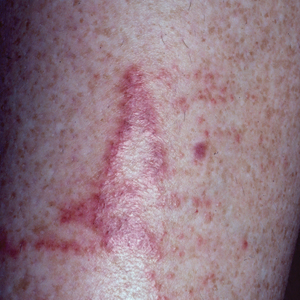
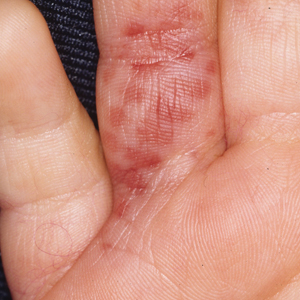
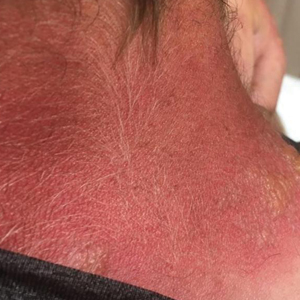
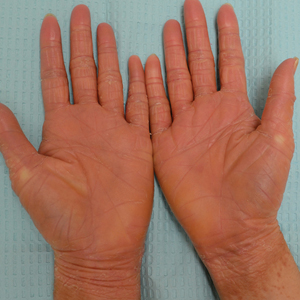
A 71-year-old Hispanic man with a history of vitiligo presented with an acute-onset blistering rash on the face, arms, and hands. Physical examination demonstrated photodistributed erythematous plaques with overlying vesicles and erosions with hemorrhagic crust on the face, neck, dorsal aspects of the hands, and wrists (Figure). Further history revealed that the patient applied a new cream that was recommended to treat vitiligo the night before the rash onset; he obtained the cream from a Central American market without a prescription. He had gone running in the park without any form of sun protection and then developed the rash within several hours. He denied taking any other medications or supplements. The involvement of sun-protected areas (ie, upper eyelids, nasolabial folds, submental area) was explained when the patient further elaborated that he had performed supine exercises during his outdoor recreation. He brought his new cream into the clinic, which was found to contain prescription-strength methoxsalen (8-methoxypsoralen), confirming the diagnosis of acute phototoxic contact dermatitis. The acute reaction had subsided, and the patient already had discontinued the causative agent. He was counseled on further avoidance of the cream and sun-protective measures.
The photosensitizing properties of certain compounds have been harnessed for therapeutic purposes. For example, psoralen plus UVA therapy has been used for psoriasis and vitiligo and photodynamic therapy for actinic keratoses and superficial nonmelanoma skin cancers.1 However, these agents can induce severe phototoxicity if UV light exposure is not carefully monitored, as seen in our patient. This case is a classic example of phototoxic contact dermatitis and highlights the importance of obtaining a detailed patient history to allow for proper diagnosis and identification of the causative agent. Importantly, because prescription-strength topical medications are readily available over-the-counter, particularly in stores specializing in international goods, patients should be questioned about the use of all topical and systemic medications, both prescription and nonprescription.2
- Richard EG. The science and (lost) art of psoralen plus UVA phototherapy. Dermatol Clin. 2020;38:11-23. doi:10.1016/j.det.2019.08.002
- Kimyon RS, Schlarbaum JP, Liou YL, et al. Prescription-strengthtopical corticosteroids available over the counter: cross-sectional study of 80 stores in 13 United States cities. J Am Acad Dermatol. 2020;82:524-525. doi:10.1016/j.jaad.2019.10.035
To the Editor:
A 71-year-old Hispanic man with a history of vitiligo presented with an acute-onset blistering rash on the face, arms, and hands. Physical examination demonstrated photodistributed erythematous plaques with overlying vesicles and erosions with hemorrhagic crust on the face, neck, dorsal aspects of the hands, and wrists (Figure). Further history revealed that the patient applied a new cream that was recommended to treat vitiligo the night before the rash onset; he obtained the cream from a Central American market without a prescription. He had gone running in the park without any form of sun protection and then developed the rash within several hours. He denied taking any other medications or supplements. The involvement of sun-protected areas (ie, upper eyelids, nasolabial folds, submental area) was explained when the patient further elaborated that he had performed supine exercises during his outdoor recreation. He brought his new cream into the clinic, which was found to contain prescription-strength methoxsalen (8-methoxypsoralen), confirming the diagnosis of acute phototoxic contact dermatitis. The acute reaction had subsided, and the patient already had discontinued the causative agent. He was counseled on further avoidance of the cream and sun-protective measures.

The photosensitizing properties of certain compounds have been harnessed for therapeutic purposes. For example, psoralen plus UVA therapy has been used for psoriasis and vitiligo and photodynamic therapy for actinic keratoses and superficial nonmelanoma skin cancers.1 However, these agents can induce severe phototoxicity if UV light exposure is not carefully monitored, as seen in our patient. This case is a classic example of phototoxic contact dermatitis and highlights the importance of obtaining a detailed patient history to allow for proper diagnosis and identification of the causative agent. Importantly, because prescription-strength topical medications are readily available over-the-counter, particularly in stores specializing in international goods, patients should be questioned about the use of all topical and systemic medications, both prescription and nonprescription.2
To the Editor:
A 71-year-old Hispanic man with a history of vitiligo presented with an acute-onset blistering rash on the face, arms, and hands. Physical examination demonstrated photodistributed erythematous plaques with overlying vesicles and erosions with hemorrhagic crust on the face, neck, dorsal aspects of the hands, and wrists (Figure). Further history revealed that the patient applied a new cream that was recommended to treat vitiligo the night before the rash onset; he obtained the cream from a Central American market without a prescription. He had gone running in the park without any form of sun protection and then developed the rash within several hours. He denied taking any other medications or supplements. The involvement of sun-protected areas (ie, upper eyelids, nasolabial folds, submental area) was explained when the patient further elaborated that he had performed supine exercises during his outdoor recreation. He brought his new cream into the clinic, which was found to contain prescription-strength methoxsalen (8-methoxypsoralen), confirming the diagnosis of acute phototoxic contact dermatitis. The acute reaction had subsided, and the patient already had discontinued the causative agent. He was counseled on further avoidance of the cream and sun-protective measures.

The photosensitizing properties of certain compounds have been harnessed for therapeutic purposes. For example, psoralen plus UVA therapy has been used for psoriasis and vitiligo and photodynamic therapy for actinic keratoses and superficial nonmelanoma skin cancers.1 However, these agents can induce severe phototoxicity if UV light exposure is not carefully monitored, as seen in our patient. This case is a classic example of phototoxic contact dermatitis and highlights the importance of obtaining a detailed patient history to allow for proper diagnosis and identification of the causative agent. Importantly, because prescription-strength topical medications are readily available over-the-counter, particularly in stores specializing in international goods, patients should be questioned about the use of all topical and systemic medications, both prescription and nonprescription.2
- Richard EG. The science and (lost) art of psoralen plus UVA phototherapy. Dermatol Clin. 2020;38:11-23. doi:10.1016/j.det.2019.08.002
- Kimyon RS, Schlarbaum JP, Liou YL, et al. Prescription-strengthtopical corticosteroids available over the counter: cross-sectional study of 80 stores in 13 United States cities. J Am Acad Dermatol. 2020;82:524-525. doi:10.1016/j.jaad.2019.10.035
- Richard EG. The science and (lost) art of psoralen plus UVA phototherapy. Dermatol Clin. 2020;38:11-23. doi:10.1016/j.det.2019.08.002
- Kimyon RS, Schlarbaum JP, Liou YL, et al. Prescription-strengthtopical corticosteroids available over the counter: cross-sectional study of 80 stores in 13 United States cities. J Am Acad Dermatol. 2020;82:524-525. doi:10.1016/j.jaad.2019.10.035
Practice Points
- Phototoxic contact dermatitis is an irritant reaction resembling an exaggerated sunburn that occurs with the use of a photosensitizing agent and UV light exposure.
- A range of topical and systemic medications, plants, and natural products can elicit phototoxic reactions.
- With the wide availability of prescription-strength over-the-counter medications, a detailed history often is necessary to identify the causative agents of phototoxic contact dermatitis and ensure future avoidance.
Contact Allergy to Topical Medicaments, Part 2: Steroids, Immunomodulators, and Anesthetics, Oh My!
In the first part of this 2-part series (Cutis. 2021;108:271-275), we discussed topical medicament allergic contact dermatitis (ACD) from acne and rosacea medications, antimicrobials, antihistamines, and topical pain preparations. In part 2 of this series, we focus on topical corticosteroids, immunomodulators, and anesthetics.
Corticosteroids
Given their anti-inflammatory and immune-modulating effects, topical corticosteroids are utilized for the treatment of contact dermatitis and yet also are frequent culprits of ACD. The North American Contact Dermatitis Group (NACDG) demonstrated a 4% frequency of positive patch tests to at least one corticosteroid from 2007 to 2014; the relevant allergens were tixocortol pivalate (TP)(2.3%), budesonide (0.9%), hydrocortisone-17-butyrate (0.4%), clobetasol-17-propionate (0.3%), and desoximetasone (0.2%).1 Corticosteroid contact allergy can be difficult to recognize and may present as a flare of the underlying condition being treated. Clinically, these rashes may demonstrate an edge effect, characterized by pronounced dermatitis adjacent to and surrounding the treatment area due to concentrated anti-inflammatory effects in the center.
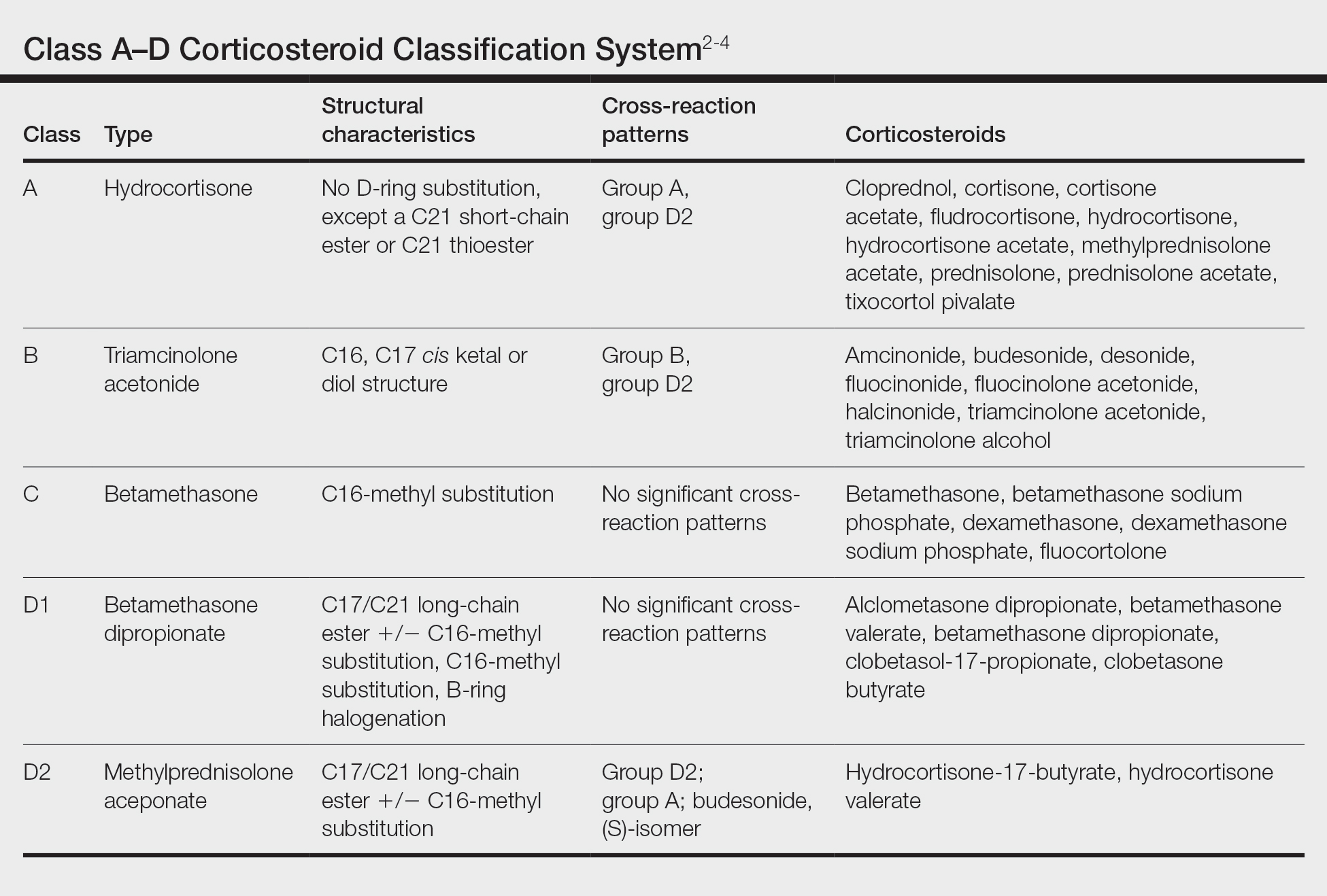
Traditionally, corticosteroids are divided into 4 basic structural groups—classes A, B, C, and D—based on the Coopman et al2 classification (Table). The class D corticosteroids were further subdivided into classes D1, defined by C16-methyl substitution and halogenation of the B ring, and D2, which lacks the aforementioned substitutions.4 However, more recently Baeck et al5 simplified this classification into 3 main groups of steroids based on molecular modeling in combination with patch test results. Group 1 combines the nonmethylated and (mostly) nonhalogenated class A and D2 molecules plus budesonide; group 2 accounts for some halogenated class B molecules with the C16, C17 cis ketal or diol structure; and group 3 includes halogenated and C16-methylated molecules from classes C and D1.4 For the purposes of this review, discussion of classes A through D refers to the Coopman et al2 classification, and groups 1 through 3 refers to Baeck et al.5
Tixocortol pivalate is used as a surrogate marker for hydrocortisone allergy and other class A corticosteroids and is part of the group 1 steroid classification. Interestingly, patients with TP-positive patch tests may not exhibit signs or symptoms of ACD from the use of hydrocortisone products. Repeat open application testing (ROAT) or provocative use testing may elicit a positive response in these patients, especially with the use of hydrocortisone cream (vs ointment), likely due to greater transepidermal penetration.6 There is little consensus on the optimal concentration of TP for patch testing. Although TP 1% often is recommended, studies have shown mixed findings of notable differences between high (1% petrolatum) and low (0.1% petrolatum) concentrations of TP.7,8
Budesonide also is part of group 1 and is a marker for contact allergy to class B corticosteroids, such as triamcinolone and fluocinonide. Cross-reactions between budesonide and other corticosteroids traditionally classified as group B may be explained by structural similarities, whereas cross-reactions with certain class D corticosteroids, such as hydrocortisone-17-butyrate, may be better explained by the diastereomer composition of budesonide.9,10 In a European study, budesonide 0.01% and TP 0.1% included in the European Baseline Series detected 85% (23/27) of cases of corticosteroid allergies.11 Use of inhaled budesonide can provoke recall dermatitis and therefore should be avoided in allergic patients.12
Testing for ACD to topical steroids is complex, as the potent anti-inflammatory properties of these medications can complicate results. Selecting the appropriate test, vehicle, and concentration can help avoid false negatives. Although intradermal testing previously was thought to be superior to patch testing in detecting topical corticosteroid contact allergy, newer data have demonstrated strong concordance between the two methods.13,14 The risk for skin atrophy, particularly with the use of suspensions, limits the use of intradermal testing.14 An ethanol vehicle is recommended for patch testing, except when testing with TP or budesonide when petrolatum provides greater corticosteroid stability.14-16 An irritant pattern or a rim effect on patch testing often is considered positive when testing corticosteroids, as the effect of the steroid itself can diminish a positive reaction. As a result, 0.1% dilutions sometimes are favored over 1% test concentrations.14,15,17 Late readings (>7 days) may be necessary to detect positive reactions in both adults and children.18,19
The authors (M.R., A.R.A.) find these varied classifications of steroids daunting (and somewhat confusing!). In general, when ACD to topical steroids is suspected, in addition to standard patch testing with a corticosteroid series, ROAT of the suspected steroid may be necessary, as the rules of steroid classification may not be reproducible in the real world. For patients with only corticosteroid allergy, calcineurin inhibitors are a safe alternative.
Immunomodulators
Calcipotriol is a vitamin D analogue commonly used to treat psoriasis. Although it is a well-known irritant, ACD to topical calcipotriol rarely has been reported.20-23 Topical calcipotriol does not seem to cross-react with other vitamin D analogues, including tacalcitol and calcitriol.21,24 Based on the literature and the nonirritant reactive thresholds described by Fullerton et al,25 recommended patch test concentrations of calcipotriol in isopropanol are 2 to 10 µg/mL. Given its immunomodulating effects, calcipotriol may suppress contact hypersensitization from other allergens, similar to the effects seen with UV radiation.26
Calcineurin inhibitors act on the nuclear factor of activated T cells signaling pathway, resulting in downstream suppression of proinflammatory cytokines. Contact allergy to these topical medications is rare and mainly has involved pimecrolimus.27-30 In one case, a patient with a previously documented topical tacrolimus contact allergy demonstrated cross-reactivity with pimecrolimus on a double-blinded, right-vs-left ROAT, as well as by patch testing with pimecrolimus cream 1%, which was only weakly positive (+).27 Patch test concentrations of 2.5% or higher may be required to elicit positive reactions to tacrolimus, as shown in one case where this was attributed to high molecular weight and poor extrafacial skin absorption of tacrolimus.30 In an unusual case, a patient reacted positively to patch testing and ROAT using pimecrolimus cream 1% but not pimecrolimus 1% to 5% in petrolatum or alcohol nor the individual excipients, illustrating the importance of testing with both active and inactive ingredients.29
Anesthetics
Local anesthetics can be separated into 2 main groups—amides and esters—based on their chemical structures. From 2001 to 2004, the NACDG patch tested 10,061 patients and found 344 (3.4%) with a positive reaction to at least one topical anesthetic.31 We will discuss some of the allergic cutaneous reactions associated with topical benzocaine (an ester) and lidocaine and prilocaine (amides).
According to the NACDG, the estimated prevalence of topical benzocaine allergy from 2001 to 2018 was roughly 3%.32 Allergic contact dermatitis has been reported in patients who used topical benzocaine to treat localized pain disorders, including herpes zoster and dental pain.33,34 Benzocaine may be used in the anogenital region in the form of antihemorrhoidal creams and in condoms and is a considerably more common allergen in those with anogenital dermatitis compared to those without.35-38 Although cross-reactions within the same anesthetic group are common, clinicians also should be aware of the potential for concomitant sensitivity between unrelated local anesthetics.39-41
From 2001 to 2018, the prevalence of ACD to topical lidocaine was estimated to be 7.9%, according to the NACDG.32 A topical anesthetic containing both lidocaine and prilocaine often is used preprocedurally and can be a source of ACD. Interestingly, several cases of ACD to combination lidocaine/prilocaine cream demonstrated positive patch tests to prilocaine but not lidocaine, despite their structural similarities.42-44 One case report described simultaneous positive reactions to both prilocaine 5% and lidocaine 1%.45
There are a few key points to consider when working up contact allergy to local anesthetics. Patients who develop positive patch test reactions to a local anesthetic should undergo further testing to better understand alternatives and future use. As previously mentioned, ACD to one anesthetic does not necessarily preclude the use of other related anesthetics. Intradermal testing may help differentiate immediate and delayed-type allergic reactions to local anesthetics and should therefore follow positive patch tests.46 Importantly, a delayed reading (ie, after day 6 or 7) also should be performed as part of intradermal testing. Patients with positive patch tests but negative intradermal test results may be able to tolerate systemic anesthetic use.47
Patch Testing for Potential Medicament ACD
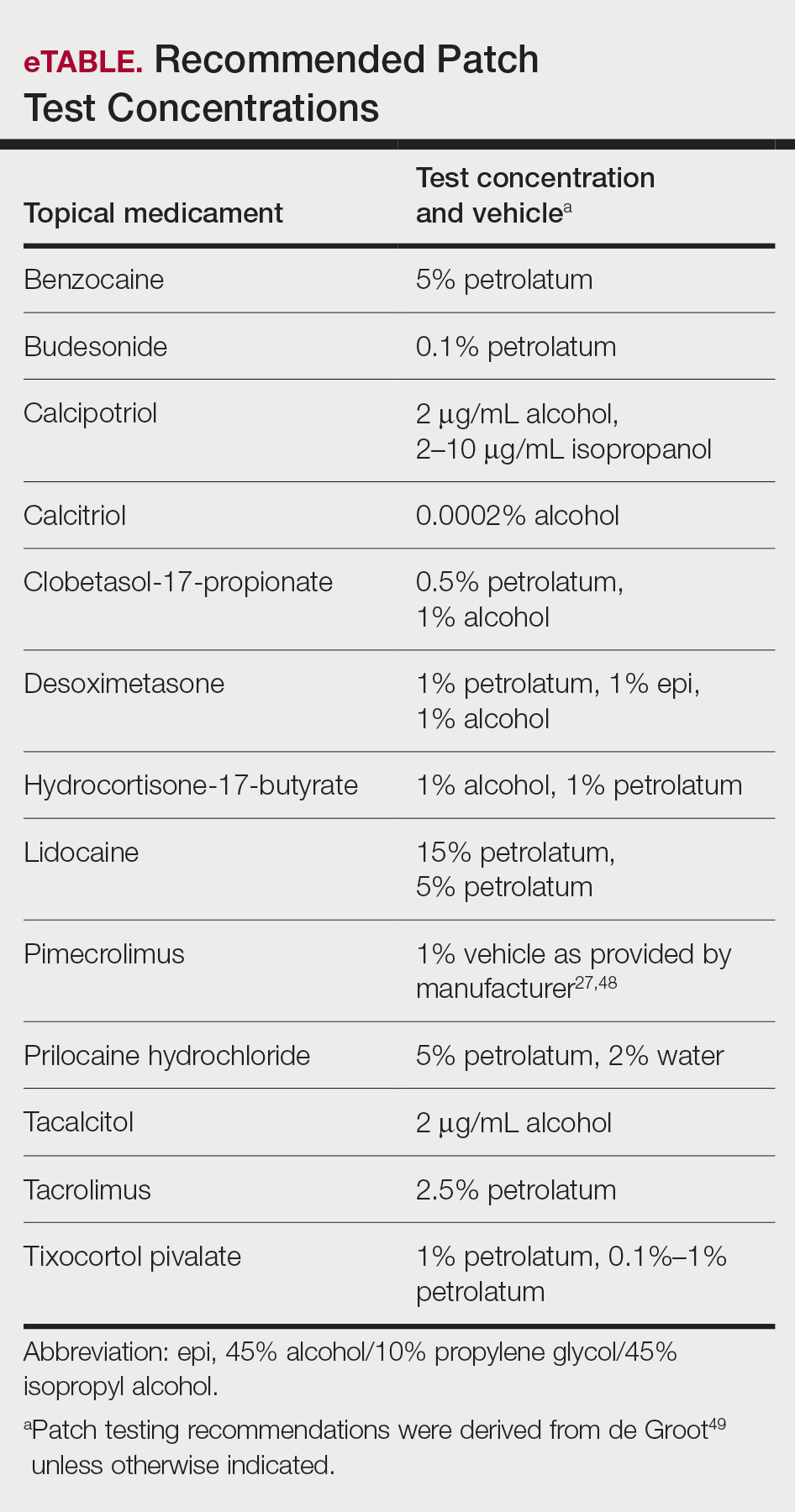
In this article, we touched on several topical medications that have nuanced patch testing specifications given their immunomodulating effects. A simplified outline of recommended patch test concentrations is provided in the eTable, and we encourage you to revisit these useful resources as needed. In many cases, referral to a specialized patch test clinic may be necessary. Although they are not reviewed in this article, always consider inactive ingredients such as preservatives, softening agents, and emulsifiers in the setting of medicament dermatitis, as they also may be culprits of ACD.
Final Interpretation
In this 2-part series, we covered ACD to several common topical drugs with a focus on active ingredients as the source of allergy, and yet this is just the tip of the iceberg. Topical medicaments are prevalent in the field of dermatology, and associated cases of ACD have been reported proportionately. Consider ACD when topical medication efficacy plateaus, triggers new-onset dermatitis, or seems to exacerbate an underlying dermatitis.
- Pratt MD, Mufti A, Lipson J, et al. Patch test reactions to corticosteroids: retrospective analysis from the North American Contact Dermatitis Group 2007-2014. Dermatitis. 2017;28:58-63. doi:10.1097/DER.0000000000000251
- Coopman S, Degreef H, Dooms-Goossens A. Identification of cross-reaction patterns in allergic contact dermatitis from topical corticosteroids. Br J Dermatol. 1989;121:27-34. doi:10.1111/j.1365-2133.1989.tb01396.x
- Jacob SE, Steele T. Corticosteroid classes: a quick reference guide including patch test substances and cross-reactivity. J Am Acad Dermatol. 2006;54:723-727. doi:10.1016/j.jaad.2005.12.028
- Matura M, Goossens A. Contact allergy to corticosteroids. Allergy. 2000;55:698-704. doi:10.1034/j.1398-9995.2000.00121.x
- Baeck M, Chemelle JA, Goossens A, et al. Corticosteroid cross-reactivity: clinical and molecular modelling tools. Allergy. 2011;66:1367-1374. doi:10.1111/j.1398-9995.2011.02666.x
- Shaw DW, Maibach HI. Clinical relevance of tixocortol pivalate-positive patch tests and questionable bioequivalence of different hydrocortisone preparations. Contact Dermatitis. 2013;68:369-375. doi:10.1111/cod.12066
- Kalavala M, Statham BN, Green CM, et al. Tixocortol pivalate: what is the right concentration? Contact Dermatitis. 2007;57:44-46. doi:10.1111/j.1600-0536.2007.01136.x
- Chowdhury MM, Statham BN, Sansom JE, et al. Patch testing for corticosteroid allergy with low and high concentrations of tixocortol pivalate and budesonide. Contact Dermatitis. 2002;46:311-312. doi:10.1034/j.1600-0536.2002.460519.x
- Isaksson M, Bruze M, Lepoittevin JP, et al. Patch testing with serial dilutions of budesonide, its R and S diastereomers, and potentially cross-reacting substances. Am J Contact Dermat. 2001;12:170-176.
- Ferguson AD, Emerson RM, English JS. Cross-reactivity patterns to budesonide. Contact Dermatitis. 2002;47:337-340. doi:10.1034/j.1600-0536.2002.470604.x
- Kot M, Bogaczewicz J, Kre˛cisz B, et al. Contact allergy in the population of patients with chronic inflammatory dermatoses and contact hypersensitivity to corticosteroids. Postepy Dermatol Alergol. 2017;34:253-259. doi:10.5114/ada.2017.67848
- Isaksson M, Bruze M. Allergic contact dermatitis in response to budesonide reactivated by inhalation of the allergen. J Am Acad Dermatol. 2002;46:880-885. doi:10.1067/mjd.2002.120464
- Mimesh S, Pratt M. Allergic contact dermatitis from corticosteroids: reproducibility of patch testing and correlation with intradermal testing. Dermatitis. 2006;17:137-142. doi:10.2310/6620.2006.05048
- Soria A, Baeck M, Goossens A, et al. Patch, prick or intradermal tests to detect delayed hypersensitivity to corticosteroids?. Contact Dermatitis. 2011;64:313-324. doi:10.1111/j.1600-0536.2011.01888.x
- Wilkinson SM, Beck MH. Corticosteroid contact hypersensitivity: what vehicle and concentration? Contact Dermatitis. 1996;34:305-308. doi:10.1111/j.1600-0536.1996.tb02212.x
- Isaksson M, Beck MH, Wilkinson SM. Comparative testing with budesonide in petrolatum and ethanol in a standard series. Contact Dermatitis. 2002;47:123-124. doi:10.1034/j.1600-0536.2002.470210_16.x
- Baeck M, Goossens A. Immediate and delayed allergic hypersensitivity to corticosteroids: practical guidelines. Contact Dermatitis. 2012;66:38-45. doi:10.1111/j.1600-0536.2011.01967.x
- Isaksson M. Corticosteroid contact allergy—the importance of late readings and testing with corticosteroids used by the patients. Contact Dermatitis. 2007;56:56-57. doi:10.1111/j.1600-0536.2007.00959.x
- Tam I, Yu J. Delayed patch test reaction to budesonide in an 8-year-old. Pediatr Dermatol. 2020;37:690-691. doi:10.1111/pde.14168
- Garcia-Bravo B, Camacho F. Two cases of contact dermatitis caused by calcipotriol cream. Am J Contact Dermat. 1996;7:118-119.
- Zollner TM, Ochsendorf FR, Hensel O, et al. Delayed-type reactivity to calcipotriol without cross-sensitization to tacalcitol. Contact Dermatitis. 1997;37:251. doi:10.1111/j.1600-0536.1997.tb02457.x
- Frosch PJ, Rustemeyer T. Contact allergy to calcipotriol does exist. report of an unequivocal case and review of the literature. Contact Dermatitis. 1999;40:66-71. doi:10.1111/j.1600-0536.1999.tb05993.x
- Gilissen L, Huygens S, Goossens A. Allergic contact dermatitis caused by calcipotriol. Contact Dermatitis. 2018;78:139-142. doi:10.1111/cod.12910
- Foti C, Carnimeo L, Bonamonte D, et al. Tolerance to calcitriol and tacalcitol in three patients with allergic contact dermatitis to calcipotriol. J Drugs Dermatol. 2005;4:756-759.
- Fullerton A, Benfeldt E, Petersen JR, et al. The calcipotriol dose-irritation relationship: 48-hour occlusive testing in healthy volunteers using Finn Chambers. Br J Dermatol. 1998;138:259-265. doi:10.1046/j.1365-2133.1998.02071.x
- Hanneman KK, Scull HM, Cooper KD, et al. Effect of topical vitamin D analogue on in vivo contact sensitization. Arch Dermatol. 2006;142:1332-1334. doi:10.1001/archderm.142.10.1332
- Shaw DW, Maibach HI, Eichenfield LF. Allergic contact dermatitis from pimecrolimus in a patient with tacrolimus allergy. J Am Acad Dermatol. 2007;56:342-345. doi:10.1016/j.jaad.2006.09.033
- Saitta P, Brancaccio R. Allergic contact dermatitis to pimecrolimus. Contact Dermatitis. 2007;56:43-44. doi:10.1111/j.1600-0536.2007.00822.x
- Neczyporenko F, Blondeel A. Allergic contact dermatitis to Elidel cream itself? Contact Dermatitis. 2010;63:171-172. doi:10.1111/j.1600-0536.2010.01764.x
- Shaw DW, Eichenfield LF, Shainhouse T, et al. Allergic contact dermatitis from tacrolimus. J Am Acad Dermatol. 2004;50:962-965. doi:10.1016/j.jaad.2003.09.013
- Warshaw EM, Schram SE, Belsito DV, et al. Patch-test reactions to topical anesthetics: retrospective analysis of cross-sectional data, 2001 to 2004. Dermatitis. 2008;19:81-85.
- Warshaw EM, Shaver RL, DeKoven JG, et al. Patch test reactions associated with topical medications: a retrospective analysis of the North American Contact Dermatitis Group data (2001-2018)[published online September 1, 2021]. Dermatitis. doi:10.1097/DER.0000000000000777
- Roos TC, Merk HF. Allergic contact dermatitis from benzocaine ointment during treatment of herpes zoster. Contact Dermatitis. 2001;44:104. doi:10.1034/j.1600-0536.2001.4402097.x
- González-Rodríguez AJ, Gutiérrez-Paredes EM, Revert Fernández Á, et al. Allergic contact dermatitis to benzocaine: the importance of concomitant positive patch test results. Actas Dermosifiliogr. 2013;104:156-158. doi:10.1016/j.ad.2011.07.023
- Muratore L, Calogiuri G, Foti C, et al. Contact allergy to benzocaine in a condom. Contact Dermatitis. 2008;59:173-174. doi:10.1111/j.1600-0536.2008.01359.x
- Sharma A, Agarwal S, Garg G, et al. Desire for lasting long in bed led to contact allergic dermatitis and subsequent superficial penile gangrene: a dreadful complication of benzocaine-containing extended-pleasure condom [published online September 27, 2018]. BMJ Case Rep. 2018;2018:bcr2018227351. doi:10.1136/bcr-2018-227351
- Bauer A, Geier J, Elsner P. Allergic contact dermatitis in patients with anogenital complaints. J Reprod Med. 2000;45:649-654.
- Warshaw EM, Kimyon RS, Silverberg JI, et al. Evaluation of patch test findings in patients with anogenital dermatitis. JAMA Dermatol. 2020;156:85-91. doi:10.1001/jamadermatol.2019.3844
- Weightman W, Turner T. Allergic contact dermatitis from lignocaine: report of 29 cases and review of the literature. Contact Dermatitis. 1998;39:265-266. doi:10.1111/j.1600-0536.1998.tb05928.x
- Jovanovic´ M, Karadaglic´ D, Brkic´ S. Contact urticaria and allergic contact dermatitis to lidocaine in a patient sensitive to benzocaine and propolis. Contact Dermatitis. 2006;54:124-126. doi:10.1111/j.0105-1873.2006.0560f.x
- Carazo JL, Morera BS, Colom LP, et al. Allergic contact dermatitis from ethyl chloride and benzocaine. Dermatitis. 2009;20:E13-E15.
- le Coz CJ, Cribier BJ, Heid E. Patch testing in suspected allergic contact dermatitis due to EMLA cream in haemodialyzed patients. Contact Dermatitis. 1996;35:316-317. doi:10.1111/j.1600-0536.1996.tb02407.x
- Ismail F, Goldsmith PC. EMLA cream-induced allergic contact dermatitis in a child with thalassaemia major. Contact Dermatitis. 2005;52:111. doi:10.1111/j.0105-1873.2005.00498e.x
- Pérez-Pérez LC, Fernández-Redondo V, Ginarte-Val M, et al. Allergic contact dermatitis from EMLA cream in a hemodialyzed patient. Dermatitis. 2006;17:85-87.
- Timmermans MW, Bruynzeel DP, Rustemeyer T. Allergic contact dermatitis from EMLA cream: concomitant sensitization to both local anesthetics lidocaine and prilocaine. J Dtsch Dermatol Ges. 2009;7:237-238. doi:10.1111/j.1610-0387.2008.06932.x
- Fuzier R, Lapeyre-Mestre M, Mertes PM, et al. Immediate- and delayed-type allergic reactions to amide local anesthetics: clinical features and skin testing. Pharmacoepidemiol Drug Saf. 2009;18:595-601. doi:10.1002/pds.1758
- Ruzicka T, Gerstmeier M, Przybilla B, et al. Allergy to local anesthetics: comparison of patch test with prick and intradermal test results. J Am Acad Dermatol. 1987;16:1202-1208. doi:10.1016/s0190-9622(87)70158-3
- Fowler JF Jr, Fowler L, Douglas JL, et al. Skin reactions to pimecrolimus cream 1% in patients allergic to propylene glycol: a double-blind randomized study. Dermatitis. 2007;18:134-139. doi:10.2310/6620.2007.06028
- de Groot A. Patch Testing. 3rd ed. acdegroot publishing; 2008.
In the first part of this 2-part series (Cutis. 2021;108:271-275), we discussed topical medicament allergic contact dermatitis (ACD) from acne and rosacea medications, antimicrobials, antihistamines, and topical pain preparations. In part 2 of this series, we focus on topical corticosteroids, immunomodulators, and anesthetics.
Corticosteroids
Given their anti-inflammatory and immune-modulating effects, topical corticosteroids are utilized for the treatment of contact dermatitis and yet also are frequent culprits of ACD. The North American Contact Dermatitis Group (NACDG) demonstrated a 4% frequency of positive patch tests to at least one corticosteroid from 2007 to 2014; the relevant allergens were tixocortol pivalate (TP)(2.3%), budesonide (0.9%), hydrocortisone-17-butyrate (0.4%), clobetasol-17-propionate (0.3%), and desoximetasone (0.2%).1 Corticosteroid contact allergy can be difficult to recognize and may present as a flare of the underlying condition being treated. Clinically, these rashes may demonstrate an edge effect, characterized by pronounced dermatitis adjacent to and surrounding the treatment area due to concentrated anti-inflammatory effects in the center.
Traditionally, corticosteroids are divided into 4 basic structural groups—classes A, B, C, and D—based on the Coopman et al2 classification (Table). The class D corticosteroids were further subdivided into classes D1, defined by C16-methyl substitution and halogenation of the B ring, and D2, which lacks the aforementioned substitutions.4 However, more recently Baeck et al5 simplified this classification into 3 main groups of steroids based on molecular modeling in combination with patch test results. Group 1 combines the nonmethylated and (mostly) nonhalogenated class A and D2 molecules plus budesonide; group 2 accounts for some halogenated class B molecules with the C16, C17 cis ketal or diol structure; and group 3 includes halogenated and C16-methylated molecules from classes C and D1.4 For the purposes of this review, discussion of classes A through D refers to the Coopman et al2 classification, and groups 1 through 3 refers to Baeck et al.5

Tixocortol pivalate is used as a surrogate marker for hydrocortisone allergy and other class A corticosteroids and is part of the group 1 steroid classification. Interestingly, patients with TP-positive patch tests may not exhibit signs or symptoms of ACD from the use of hydrocortisone products. Repeat open application testing (ROAT) or provocative use testing may elicit a positive response in these patients, especially with the use of hydrocortisone cream (vs ointment), likely due to greater transepidermal penetration.6 There is little consensus on the optimal concentration of TP for patch testing. Although TP 1% often is recommended, studies have shown mixed findings of notable differences between high (1% petrolatum) and low (0.1% petrolatum) concentrations of TP.7,8
Budesonide also is part of group 1 and is a marker for contact allergy to class B corticosteroids, such as triamcinolone and fluocinonide. Cross-reactions between budesonide and other corticosteroids traditionally classified as group B may be explained by structural similarities, whereas cross-reactions with certain class D corticosteroids, such as hydrocortisone-17-butyrate, may be better explained by the diastereomer composition of budesonide.9,10 In a European study, budesonide 0.01% and TP 0.1% included in the European Baseline Series detected 85% (23/27) of cases of corticosteroid allergies.11 Use of inhaled budesonide can provoke recall dermatitis and therefore should be avoided in allergic patients.12
Testing for ACD to topical steroids is complex, as the potent anti-inflammatory properties of these medications can complicate results. Selecting the appropriate test, vehicle, and concentration can help avoid false negatives. Although intradermal testing previously was thought to be superior to patch testing in detecting topical corticosteroid contact allergy, newer data have demonstrated strong concordance between the two methods.13,14 The risk for skin atrophy, particularly with the use of suspensions, limits the use of intradermal testing.14 An ethanol vehicle is recommended for patch testing, except when testing with TP or budesonide when petrolatum provides greater corticosteroid stability.14-16 An irritant pattern or a rim effect on patch testing often is considered positive when testing corticosteroids, as the effect of the steroid itself can diminish a positive reaction. As a result, 0.1% dilutions sometimes are favored over 1% test concentrations.14,15,17 Late readings (>7 days) may be necessary to detect positive reactions in both adults and children.18,19
The authors (M.R., A.R.A.) find these varied classifications of steroids daunting (and somewhat confusing!). In general, when ACD to topical steroids is suspected, in addition to standard patch testing with a corticosteroid series, ROAT of the suspected steroid may be necessary, as the rules of steroid classification may not be reproducible in the real world. For patients with only corticosteroid allergy, calcineurin inhibitors are a safe alternative.
Immunomodulators
Calcipotriol is a vitamin D analogue commonly used to treat psoriasis. Although it is a well-known irritant, ACD to topical calcipotriol rarely has been reported.20-23 Topical calcipotriol does not seem to cross-react with other vitamin D analogues, including tacalcitol and calcitriol.21,24 Based on the literature and the nonirritant reactive thresholds described by Fullerton et al,25 recommended patch test concentrations of calcipotriol in isopropanol are 2 to 10 µg/mL. Given its immunomodulating effects, calcipotriol may suppress contact hypersensitization from other allergens, similar to the effects seen with UV radiation.26
Calcineurin inhibitors act on the nuclear factor of activated T cells signaling pathway, resulting in downstream suppression of proinflammatory cytokines. Contact allergy to these topical medications is rare and mainly has involved pimecrolimus.27-30 In one case, a patient with a previously documented topical tacrolimus contact allergy demonstrated cross-reactivity with pimecrolimus on a double-blinded, right-vs-left ROAT, as well as by patch testing with pimecrolimus cream 1%, which was only weakly positive (+).27 Patch test concentrations of 2.5% or higher may be required to elicit positive reactions to tacrolimus, as shown in one case where this was attributed to high molecular weight and poor extrafacial skin absorption of tacrolimus.30 In an unusual case, a patient reacted positively to patch testing and ROAT using pimecrolimus cream 1% but not pimecrolimus 1% to 5% in petrolatum or alcohol nor the individual excipients, illustrating the importance of testing with both active and inactive ingredients.29
Anesthetics
Local anesthetics can be separated into 2 main groups—amides and esters—based on their chemical structures. From 2001 to 2004, the NACDG patch tested 10,061 patients and found 344 (3.4%) with a positive reaction to at least one topical anesthetic.31 We will discuss some of the allergic cutaneous reactions associated with topical benzocaine (an ester) and lidocaine and prilocaine (amides).
According to the NACDG, the estimated prevalence of topical benzocaine allergy from 2001 to 2018 was roughly 3%.32 Allergic contact dermatitis has been reported in patients who used topical benzocaine to treat localized pain disorders, including herpes zoster and dental pain.33,34 Benzocaine may be used in the anogenital region in the form of antihemorrhoidal creams and in condoms and is a considerably more common allergen in those with anogenital dermatitis compared to those without.35-38 Although cross-reactions within the same anesthetic group are common, clinicians also should be aware of the potential for concomitant sensitivity between unrelated local anesthetics.39-41
From 2001 to 2018, the prevalence of ACD to topical lidocaine was estimated to be 7.9%, according to the NACDG.32 A topical anesthetic containing both lidocaine and prilocaine often is used preprocedurally and can be a source of ACD. Interestingly, several cases of ACD to combination lidocaine/prilocaine cream demonstrated positive patch tests to prilocaine but not lidocaine, despite their structural similarities.42-44 One case report described simultaneous positive reactions to both prilocaine 5% and lidocaine 1%.45
There are a few key points to consider when working up contact allergy to local anesthetics. Patients who develop positive patch test reactions to a local anesthetic should undergo further testing to better understand alternatives and future use. As previously mentioned, ACD to one anesthetic does not necessarily preclude the use of other related anesthetics. Intradermal testing may help differentiate immediate and delayed-type allergic reactions to local anesthetics and should therefore follow positive patch tests.46 Importantly, a delayed reading (ie, after day 6 or 7) also should be performed as part of intradermal testing. Patients with positive patch tests but negative intradermal test results may be able to tolerate systemic anesthetic use.47
Patch Testing for Potential Medicament ACD
In this article, we touched on several topical medications that have nuanced patch testing specifications given their immunomodulating effects. A simplified outline of recommended patch test concentrations is provided in the eTable, and we encourage you to revisit these useful resources as needed. In many cases, referral to a specialized patch test clinic may be necessary. Although they are not reviewed in this article, always consider inactive ingredients such as preservatives, softening agents, and emulsifiers in the setting of medicament dermatitis, as they also may be culprits of ACD.

Final Interpretation
In this 2-part series, we covered ACD to several common topical drugs with a focus on active ingredients as the source of allergy, and yet this is just the tip of the iceberg. Topical medicaments are prevalent in the field of dermatology, and associated cases of ACD have been reported proportionately. Consider ACD when topical medication efficacy plateaus, triggers new-onset dermatitis, or seems to exacerbate an underlying dermatitis.
In the first part of this 2-part series (Cutis. 2021;108:271-275), we discussed topical medicament allergic contact dermatitis (ACD) from acne and rosacea medications, antimicrobials, antihistamines, and topical pain preparations. In part 2 of this series, we focus on topical corticosteroids, immunomodulators, and anesthetics.
Corticosteroids
Given their anti-inflammatory and immune-modulating effects, topical corticosteroids are utilized for the treatment of contact dermatitis and yet also are frequent culprits of ACD. The North American Contact Dermatitis Group (NACDG) demonstrated a 4% frequency of positive patch tests to at least one corticosteroid from 2007 to 2014; the relevant allergens were tixocortol pivalate (TP)(2.3%), budesonide (0.9%), hydrocortisone-17-butyrate (0.4%), clobetasol-17-propionate (0.3%), and desoximetasone (0.2%).1 Corticosteroid contact allergy can be difficult to recognize and may present as a flare of the underlying condition being treated. Clinically, these rashes may demonstrate an edge effect, characterized by pronounced dermatitis adjacent to and surrounding the treatment area due to concentrated anti-inflammatory effects in the center.
Traditionally, corticosteroids are divided into 4 basic structural groups—classes A, B, C, and D—based on the Coopman et al2 classification (Table). The class D corticosteroids were further subdivided into classes D1, defined by C16-methyl substitution and halogenation of the B ring, and D2, which lacks the aforementioned substitutions.4 However, more recently Baeck et al5 simplified this classification into 3 main groups of steroids based on molecular modeling in combination with patch test results. Group 1 combines the nonmethylated and (mostly) nonhalogenated class A and D2 molecules plus budesonide; group 2 accounts for some halogenated class B molecules with the C16, C17 cis ketal or diol structure; and group 3 includes halogenated and C16-methylated molecules from classes C and D1.4 For the purposes of this review, discussion of classes A through D refers to the Coopman et al2 classification, and groups 1 through 3 refers to Baeck et al.5

Tixocortol pivalate is used as a surrogate marker for hydrocortisone allergy and other class A corticosteroids and is part of the group 1 steroid classification. Interestingly, patients with TP-positive patch tests may not exhibit signs or symptoms of ACD from the use of hydrocortisone products. Repeat open application testing (ROAT) or provocative use testing may elicit a positive response in these patients, especially with the use of hydrocortisone cream (vs ointment), likely due to greater transepidermal penetration.6 There is little consensus on the optimal concentration of TP for patch testing. Although TP 1% often is recommended, studies have shown mixed findings of notable differences between high (1% petrolatum) and low (0.1% petrolatum) concentrations of TP.7,8
Budesonide also is part of group 1 and is a marker for contact allergy to class B corticosteroids, such as triamcinolone and fluocinonide. Cross-reactions between budesonide and other corticosteroids traditionally classified as group B may be explained by structural similarities, whereas cross-reactions with certain class D corticosteroids, such as hydrocortisone-17-butyrate, may be better explained by the diastereomer composition of budesonide.9,10 In a European study, budesonide 0.01% and TP 0.1% included in the European Baseline Series detected 85% (23/27) of cases of corticosteroid allergies.11 Use of inhaled budesonide can provoke recall dermatitis and therefore should be avoided in allergic patients.12
Testing for ACD to topical steroids is complex, as the potent anti-inflammatory properties of these medications can complicate results. Selecting the appropriate test, vehicle, and concentration can help avoid false negatives. Although intradermal testing previously was thought to be superior to patch testing in detecting topical corticosteroid contact allergy, newer data have demonstrated strong concordance between the two methods.13,14 The risk for skin atrophy, particularly with the use of suspensions, limits the use of intradermal testing.14 An ethanol vehicle is recommended for patch testing, except when testing with TP or budesonide when petrolatum provides greater corticosteroid stability.14-16 An irritant pattern or a rim effect on patch testing often is considered positive when testing corticosteroids, as the effect of the steroid itself can diminish a positive reaction. As a result, 0.1% dilutions sometimes are favored over 1% test concentrations.14,15,17 Late readings (>7 days) may be necessary to detect positive reactions in both adults and children.18,19
The authors (M.R., A.R.A.) find these varied classifications of steroids daunting (and somewhat confusing!). In general, when ACD to topical steroids is suspected, in addition to standard patch testing with a corticosteroid series, ROAT of the suspected steroid may be necessary, as the rules of steroid classification may not be reproducible in the real world. For patients with only corticosteroid allergy, calcineurin inhibitors are a safe alternative.
Immunomodulators
Calcipotriol is a vitamin D analogue commonly used to treat psoriasis. Although it is a well-known irritant, ACD to topical calcipotriol rarely has been reported.20-23 Topical calcipotriol does not seem to cross-react with other vitamin D analogues, including tacalcitol and calcitriol.21,24 Based on the literature and the nonirritant reactive thresholds described by Fullerton et al,25 recommended patch test concentrations of calcipotriol in isopropanol are 2 to 10 µg/mL. Given its immunomodulating effects, calcipotriol may suppress contact hypersensitization from other allergens, similar to the effects seen with UV radiation.26
Calcineurin inhibitors act on the nuclear factor of activated T cells signaling pathway, resulting in downstream suppression of proinflammatory cytokines. Contact allergy to these topical medications is rare and mainly has involved pimecrolimus.27-30 In one case, a patient with a previously documented topical tacrolimus contact allergy demonstrated cross-reactivity with pimecrolimus on a double-blinded, right-vs-left ROAT, as well as by patch testing with pimecrolimus cream 1%, which was only weakly positive (+).27 Patch test concentrations of 2.5% or higher may be required to elicit positive reactions to tacrolimus, as shown in one case where this was attributed to high molecular weight and poor extrafacial skin absorption of tacrolimus.30 In an unusual case, a patient reacted positively to patch testing and ROAT using pimecrolimus cream 1% but not pimecrolimus 1% to 5% in petrolatum or alcohol nor the individual excipients, illustrating the importance of testing with both active and inactive ingredients.29
Anesthetics
Local anesthetics can be separated into 2 main groups—amides and esters—based on their chemical structures. From 2001 to 2004, the NACDG patch tested 10,061 patients and found 344 (3.4%) with a positive reaction to at least one topical anesthetic.31 We will discuss some of the allergic cutaneous reactions associated with topical benzocaine (an ester) and lidocaine and prilocaine (amides).
According to the NACDG, the estimated prevalence of topical benzocaine allergy from 2001 to 2018 was roughly 3%.32 Allergic contact dermatitis has been reported in patients who used topical benzocaine to treat localized pain disorders, including herpes zoster and dental pain.33,34 Benzocaine may be used in the anogenital region in the form of antihemorrhoidal creams and in condoms and is a considerably more common allergen in those with anogenital dermatitis compared to those without.35-38 Although cross-reactions within the same anesthetic group are common, clinicians also should be aware of the potential for concomitant sensitivity between unrelated local anesthetics.39-41
From 2001 to 2018, the prevalence of ACD to topical lidocaine was estimated to be 7.9%, according to the NACDG.32 A topical anesthetic containing both lidocaine and prilocaine often is used preprocedurally and can be a source of ACD. Interestingly, several cases of ACD to combination lidocaine/prilocaine cream demonstrated positive patch tests to prilocaine but not lidocaine, despite their structural similarities.42-44 One case report described simultaneous positive reactions to both prilocaine 5% and lidocaine 1%.45
There are a few key points to consider when working up contact allergy to local anesthetics. Patients who develop positive patch test reactions to a local anesthetic should undergo further testing to better understand alternatives and future use. As previously mentioned, ACD to one anesthetic does not necessarily preclude the use of other related anesthetics. Intradermal testing may help differentiate immediate and delayed-type allergic reactions to local anesthetics and should therefore follow positive patch tests.46 Importantly, a delayed reading (ie, after day 6 or 7) also should be performed as part of intradermal testing. Patients with positive patch tests but negative intradermal test results may be able to tolerate systemic anesthetic use.47
Patch Testing for Potential Medicament ACD
In this article, we touched on several topical medications that have nuanced patch testing specifications given their immunomodulating effects. A simplified outline of recommended patch test concentrations is provided in the eTable, and we encourage you to revisit these useful resources as needed. In many cases, referral to a specialized patch test clinic may be necessary. Although they are not reviewed in this article, always consider inactive ingredients such as preservatives, softening agents, and emulsifiers in the setting of medicament dermatitis, as they also may be culprits of ACD.

Final Interpretation
In this 2-part series, we covered ACD to several common topical drugs with a focus on active ingredients as the source of allergy, and yet this is just the tip of the iceberg. Topical medicaments are prevalent in the field of dermatology, and associated cases of ACD have been reported proportionately. Consider ACD when topical medication efficacy plateaus, triggers new-onset dermatitis, or seems to exacerbate an underlying dermatitis.
- Pratt MD, Mufti A, Lipson J, et al. Patch test reactions to corticosteroids: retrospective analysis from the North American Contact Dermatitis Group 2007-2014. Dermatitis. 2017;28:58-63. doi:10.1097/DER.0000000000000251
- Coopman S, Degreef H, Dooms-Goossens A. Identification of cross-reaction patterns in allergic contact dermatitis from topical corticosteroids. Br J Dermatol. 1989;121:27-34. doi:10.1111/j.1365-2133.1989.tb01396.x
- Jacob SE, Steele T. Corticosteroid classes: a quick reference guide including patch test substances and cross-reactivity. J Am Acad Dermatol. 2006;54:723-727. doi:10.1016/j.jaad.2005.12.028
- Matura M, Goossens A. Contact allergy to corticosteroids. Allergy. 2000;55:698-704. doi:10.1034/j.1398-9995.2000.00121.x
- Baeck M, Chemelle JA, Goossens A, et al. Corticosteroid cross-reactivity: clinical and molecular modelling tools. Allergy. 2011;66:1367-1374. doi:10.1111/j.1398-9995.2011.02666.x
- Shaw DW, Maibach HI. Clinical relevance of tixocortol pivalate-positive patch tests and questionable bioequivalence of different hydrocortisone preparations. Contact Dermatitis. 2013;68:369-375. doi:10.1111/cod.12066
- Kalavala M, Statham BN, Green CM, et al. Tixocortol pivalate: what is the right concentration? Contact Dermatitis. 2007;57:44-46. doi:10.1111/j.1600-0536.2007.01136.x
- Chowdhury MM, Statham BN, Sansom JE, et al. Patch testing for corticosteroid allergy with low and high concentrations of tixocortol pivalate and budesonide. Contact Dermatitis. 2002;46:311-312. doi:10.1034/j.1600-0536.2002.460519.x
- Isaksson M, Bruze M, Lepoittevin JP, et al. Patch testing with serial dilutions of budesonide, its R and S diastereomers, and potentially cross-reacting substances. Am J Contact Dermat. 2001;12:170-176.
- Ferguson AD, Emerson RM, English JS. Cross-reactivity patterns to budesonide. Contact Dermatitis. 2002;47:337-340. doi:10.1034/j.1600-0536.2002.470604.x
- Kot M, Bogaczewicz J, Kre˛cisz B, et al. Contact allergy in the population of patients with chronic inflammatory dermatoses and contact hypersensitivity to corticosteroids. Postepy Dermatol Alergol. 2017;34:253-259. doi:10.5114/ada.2017.67848
- Isaksson M, Bruze M. Allergic contact dermatitis in response to budesonide reactivated by inhalation of the allergen. J Am Acad Dermatol. 2002;46:880-885. doi:10.1067/mjd.2002.120464
- Mimesh S, Pratt M. Allergic contact dermatitis from corticosteroids: reproducibility of patch testing and correlation with intradermal testing. Dermatitis. 2006;17:137-142. doi:10.2310/6620.2006.05048
- Soria A, Baeck M, Goossens A, et al. Patch, prick or intradermal tests to detect delayed hypersensitivity to corticosteroids?. Contact Dermatitis. 2011;64:313-324. doi:10.1111/j.1600-0536.2011.01888.x
- Wilkinson SM, Beck MH. Corticosteroid contact hypersensitivity: what vehicle and concentration? Contact Dermatitis. 1996;34:305-308. doi:10.1111/j.1600-0536.1996.tb02212.x
- Isaksson M, Beck MH, Wilkinson SM. Comparative testing with budesonide in petrolatum and ethanol in a standard series. Contact Dermatitis. 2002;47:123-124. doi:10.1034/j.1600-0536.2002.470210_16.x
- Baeck M, Goossens A. Immediate and delayed allergic hypersensitivity to corticosteroids: practical guidelines. Contact Dermatitis. 2012;66:38-45. doi:10.1111/j.1600-0536.2011.01967.x
- Isaksson M. Corticosteroid contact allergy—the importance of late readings and testing with corticosteroids used by the patients. Contact Dermatitis. 2007;56:56-57. doi:10.1111/j.1600-0536.2007.00959.x
- Tam I, Yu J. Delayed patch test reaction to budesonide in an 8-year-old. Pediatr Dermatol. 2020;37:690-691. doi:10.1111/pde.14168
- Garcia-Bravo B, Camacho F. Two cases of contact dermatitis caused by calcipotriol cream. Am J Contact Dermat. 1996;7:118-119.
- Zollner TM, Ochsendorf FR, Hensel O, et al. Delayed-type reactivity to calcipotriol without cross-sensitization to tacalcitol. Contact Dermatitis. 1997;37:251. doi:10.1111/j.1600-0536.1997.tb02457.x
- Frosch PJ, Rustemeyer T. Contact allergy to calcipotriol does exist. report of an unequivocal case and review of the literature. Contact Dermatitis. 1999;40:66-71. doi:10.1111/j.1600-0536.1999.tb05993.x
- Gilissen L, Huygens S, Goossens A. Allergic contact dermatitis caused by calcipotriol. Contact Dermatitis. 2018;78:139-142. doi:10.1111/cod.12910
- Foti C, Carnimeo L, Bonamonte D, et al. Tolerance to calcitriol and tacalcitol in three patients with allergic contact dermatitis to calcipotriol. J Drugs Dermatol. 2005;4:756-759.
- Fullerton A, Benfeldt E, Petersen JR, et al. The calcipotriol dose-irritation relationship: 48-hour occlusive testing in healthy volunteers using Finn Chambers. Br J Dermatol. 1998;138:259-265. doi:10.1046/j.1365-2133.1998.02071.x
- Hanneman KK, Scull HM, Cooper KD, et al. Effect of topical vitamin D analogue on in vivo contact sensitization. Arch Dermatol. 2006;142:1332-1334. doi:10.1001/archderm.142.10.1332
- Shaw DW, Maibach HI, Eichenfield LF. Allergic contact dermatitis from pimecrolimus in a patient with tacrolimus allergy. J Am Acad Dermatol. 2007;56:342-345. doi:10.1016/j.jaad.2006.09.033
- Saitta P, Brancaccio R. Allergic contact dermatitis to pimecrolimus. Contact Dermatitis. 2007;56:43-44. doi:10.1111/j.1600-0536.2007.00822.x
- Neczyporenko F, Blondeel A. Allergic contact dermatitis to Elidel cream itself? Contact Dermatitis. 2010;63:171-172. doi:10.1111/j.1600-0536.2010.01764.x
- Shaw DW, Eichenfield LF, Shainhouse T, et al. Allergic contact dermatitis from tacrolimus. J Am Acad Dermatol. 2004;50:962-965. doi:10.1016/j.jaad.2003.09.013
- Warshaw EM, Schram SE, Belsito DV, et al. Patch-test reactions to topical anesthetics: retrospective analysis of cross-sectional data, 2001 to 2004. Dermatitis. 2008;19:81-85.
- Warshaw EM, Shaver RL, DeKoven JG, et al. Patch test reactions associated with topical medications: a retrospective analysis of the North American Contact Dermatitis Group data (2001-2018)[published online September 1, 2021]. Dermatitis. doi:10.1097/DER.0000000000000777
- Roos TC, Merk HF. Allergic contact dermatitis from benzocaine ointment during treatment of herpes zoster. Contact Dermatitis. 2001;44:104. doi:10.1034/j.1600-0536.2001.4402097.x
- González-Rodríguez AJ, Gutiérrez-Paredes EM, Revert Fernández Á, et al. Allergic contact dermatitis to benzocaine: the importance of concomitant positive patch test results. Actas Dermosifiliogr. 2013;104:156-158. doi:10.1016/j.ad.2011.07.023
- Muratore L, Calogiuri G, Foti C, et al. Contact allergy to benzocaine in a condom. Contact Dermatitis. 2008;59:173-174. doi:10.1111/j.1600-0536.2008.01359.x
- Sharma A, Agarwal S, Garg G, et al. Desire for lasting long in bed led to contact allergic dermatitis and subsequent superficial penile gangrene: a dreadful complication of benzocaine-containing extended-pleasure condom [published online September 27, 2018]. BMJ Case Rep. 2018;2018:bcr2018227351. doi:10.1136/bcr-2018-227351
- Bauer A, Geier J, Elsner P. Allergic contact dermatitis in patients with anogenital complaints. J Reprod Med. 2000;45:649-654.
- Warshaw EM, Kimyon RS, Silverberg JI, et al. Evaluation of patch test findings in patients with anogenital dermatitis. JAMA Dermatol. 2020;156:85-91. doi:10.1001/jamadermatol.2019.3844
- Weightman W, Turner T. Allergic contact dermatitis from lignocaine: report of 29 cases and review of the literature. Contact Dermatitis. 1998;39:265-266. doi:10.1111/j.1600-0536.1998.tb05928.x
- Jovanovic´ M, Karadaglic´ D, Brkic´ S. Contact urticaria and allergic contact dermatitis to lidocaine in a patient sensitive to benzocaine and propolis. Contact Dermatitis. 2006;54:124-126. doi:10.1111/j.0105-1873.2006.0560f.x
- Carazo JL, Morera BS, Colom LP, et al. Allergic contact dermatitis from ethyl chloride and benzocaine. Dermatitis. 2009;20:E13-E15.
- le Coz CJ, Cribier BJ, Heid E. Patch testing in suspected allergic contact dermatitis due to EMLA cream in haemodialyzed patients. Contact Dermatitis. 1996;35:316-317. doi:10.1111/j.1600-0536.1996.tb02407.x
- Ismail F, Goldsmith PC. EMLA cream-induced allergic contact dermatitis in a child with thalassaemia major. Contact Dermatitis. 2005;52:111. doi:10.1111/j.0105-1873.2005.00498e.x
- Pérez-Pérez LC, Fernández-Redondo V, Ginarte-Val M, et al. Allergic contact dermatitis from EMLA cream in a hemodialyzed patient. Dermatitis. 2006;17:85-87.
- Timmermans MW, Bruynzeel DP, Rustemeyer T. Allergic contact dermatitis from EMLA cream: concomitant sensitization to both local anesthetics lidocaine and prilocaine. J Dtsch Dermatol Ges. 2009;7:237-238. doi:10.1111/j.1610-0387.2008.06932.x
- Fuzier R, Lapeyre-Mestre M, Mertes PM, et al. Immediate- and delayed-type allergic reactions to amide local anesthetics: clinical features and skin testing. Pharmacoepidemiol Drug Saf. 2009;18:595-601. doi:10.1002/pds.1758
- Ruzicka T, Gerstmeier M, Przybilla B, et al. Allergy to local anesthetics: comparison of patch test with prick and intradermal test results. J Am Acad Dermatol. 1987;16:1202-1208. doi:10.1016/s0190-9622(87)70158-3
- Fowler JF Jr, Fowler L, Douglas JL, et al. Skin reactions to pimecrolimus cream 1% in patients allergic to propylene glycol: a double-blind randomized study. Dermatitis. 2007;18:134-139. doi:10.2310/6620.2007.06028
- de Groot A. Patch Testing. 3rd ed. acdegroot publishing; 2008.
- Pratt MD, Mufti A, Lipson J, et al. Patch test reactions to corticosteroids: retrospective analysis from the North American Contact Dermatitis Group 2007-2014. Dermatitis. 2017;28:58-63. doi:10.1097/DER.0000000000000251
- Coopman S, Degreef H, Dooms-Goossens A. Identification of cross-reaction patterns in allergic contact dermatitis from topical corticosteroids. Br J Dermatol. 1989;121:27-34. doi:10.1111/j.1365-2133.1989.tb01396.x
- Jacob SE, Steele T. Corticosteroid classes: a quick reference guide including patch test substances and cross-reactivity. J Am Acad Dermatol. 2006;54:723-727. doi:10.1016/j.jaad.2005.12.028
- Matura M, Goossens A. Contact allergy to corticosteroids. Allergy. 2000;55:698-704. doi:10.1034/j.1398-9995.2000.00121.x
- Baeck M, Chemelle JA, Goossens A, et al. Corticosteroid cross-reactivity: clinical and molecular modelling tools. Allergy. 2011;66:1367-1374. doi:10.1111/j.1398-9995.2011.02666.x
- Shaw DW, Maibach HI. Clinical relevance of tixocortol pivalate-positive patch tests and questionable bioequivalence of different hydrocortisone preparations. Contact Dermatitis. 2013;68:369-375. doi:10.1111/cod.12066
- Kalavala M, Statham BN, Green CM, et al. Tixocortol pivalate: what is the right concentration? Contact Dermatitis. 2007;57:44-46. doi:10.1111/j.1600-0536.2007.01136.x
- Chowdhury MM, Statham BN, Sansom JE, et al. Patch testing for corticosteroid allergy with low and high concentrations of tixocortol pivalate and budesonide. Contact Dermatitis. 2002;46:311-312. doi:10.1034/j.1600-0536.2002.460519.x
- Isaksson M, Bruze M, Lepoittevin JP, et al. Patch testing with serial dilutions of budesonide, its R and S diastereomers, and potentially cross-reacting substances. Am J Contact Dermat. 2001;12:170-176.
- Ferguson AD, Emerson RM, English JS. Cross-reactivity patterns to budesonide. Contact Dermatitis. 2002;47:337-340. doi:10.1034/j.1600-0536.2002.470604.x
- Kot M, Bogaczewicz J, Kre˛cisz B, et al. Contact allergy in the population of patients with chronic inflammatory dermatoses and contact hypersensitivity to corticosteroids. Postepy Dermatol Alergol. 2017;34:253-259. doi:10.5114/ada.2017.67848
- Isaksson M, Bruze M. Allergic contact dermatitis in response to budesonide reactivated by inhalation of the allergen. J Am Acad Dermatol. 2002;46:880-885. doi:10.1067/mjd.2002.120464
- Mimesh S, Pratt M. Allergic contact dermatitis from corticosteroids: reproducibility of patch testing and correlation with intradermal testing. Dermatitis. 2006;17:137-142. doi:10.2310/6620.2006.05048
- Soria A, Baeck M, Goossens A, et al. Patch, prick or intradermal tests to detect delayed hypersensitivity to corticosteroids?. Contact Dermatitis. 2011;64:313-324. doi:10.1111/j.1600-0536.2011.01888.x
- Wilkinson SM, Beck MH. Corticosteroid contact hypersensitivity: what vehicle and concentration? Contact Dermatitis. 1996;34:305-308. doi:10.1111/j.1600-0536.1996.tb02212.x
- Isaksson M, Beck MH, Wilkinson SM. Comparative testing with budesonide in petrolatum and ethanol in a standard series. Contact Dermatitis. 2002;47:123-124. doi:10.1034/j.1600-0536.2002.470210_16.x
- Baeck M, Goossens A. Immediate and delayed allergic hypersensitivity to corticosteroids: practical guidelines. Contact Dermatitis. 2012;66:38-45. doi:10.1111/j.1600-0536.2011.01967.x
- Isaksson M. Corticosteroid contact allergy—the importance of late readings and testing with corticosteroids used by the patients. Contact Dermatitis. 2007;56:56-57. doi:10.1111/j.1600-0536.2007.00959.x
- Tam I, Yu J. Delayed patch test reaction to budesonide in an 8-year-old. Pediatr Dermatol. 2020;37:690-691. doi:10.1111/pde.14168
- Garcia-Bravo B, Camacho F. Two cases of contact dermatitis caused by calcipotriol cream. Am J Contact Dermat. 1996;7:118-119.
- Zollner TM, Ochsendorf FR, Hensel O, et al. Delayed-type reactivity to calcipotriol without cross-sensitization to tacalcitol. Contact Dermatitis. 1997;37:251. doi:10.1111/j.1600-0536.1997.tb02457.x
- Frosch PJ, Rustemeyer T. Contact allergy to calcipotriol does exist. report of an unequivocal case and review of the literature. Contact Dermatitis. 1999;40:66-71. doi:10.1111/j.1600-0536.1999.tb05993.x
- Gilissen L, Huygens S, Goossens A. Allergic contact dermatitis caused by calcipotriol. Contact Dermatitis. 2018;78:139-142. doi:10.1111/cod.12910
- Foti C, Carnimeo L, Bonamonte D, et al. Tolerance to calcitriol and tacalcitol in three patients with allergic contact dermatitis to calcipotriol. J Drugs Dermatol. 2005;4:756-759.
- Fullerton A, Benfeldt E, Petersen JR, et al. The calcipotriol dose-irritation relationship: 48-hour occlusive testing in healthy volunteers using Finn Chambers. Br J Dermatol. 1998;138:259-265. doi:10.1046/j.1365-2133.1998.02071.x
- Hanneman KK, Scull HM, Cooper KD, et al. Effect of topical vitamin D analogue on in vivo contact sensitization. Arch Dermatol. 2006;142:1332-1334. doi:10.1001/archderm.142.10.1332
- Shaw DW, Maibach HI, Eichenfield LF. Allergic contact dermatitis from pimecrolimus in a patient with tacrolimus allergy. J Am Acad Dermatol. 2007;56:342-345. doi:10.1016/j.jaad.2006.09.033
- Saitta P, Brancaccio R. Allergic contact dermatitis to pimecrolimus. Contact Dermatitis. 2007;56:43-44. doi:10.1111/j.1600-0536.2007.00822.x
- Neczyporenko F, Blondeel A. Allergic contact dermatitis to Elidel cream itself? Contact Dermatitis. 2010;63:171-172. doi:10.1111/j.1600-0536.2010.01764.x
- Shaw DW, Eichenfield LF, Shainhouse T, et al. Allergic contact dermatitis from tacrolimus. J Am Acad Dermatol. 2004;50:962-965. doi:10.1016/j.jaad.2003.09.013
- Warshaw EM, Schram SE, Belsito DV, et al. Patch-test reactions to topical anesthetics: retrospective analysis of cross-sectional data, 2001 to 2004. Dermatitis. 2008;19:81-85.
- Warshaw EM, Shaver RL, DeKoven JG, et al. Patch test reactions associated with topical medications: a retrospective analysis of the North American Contact Dermatitis Group data (2001-2018)[published online September 1, 2021]. Dermatitis. doi:10.1097/DER.0000000000000777
- Roos TC, Merk HF. Allergic contact dermatitis from benzocaine ointment during treatment of herpes zoster. Contact Dermatitis. 2001;44:104. doi:10.1034/j.1600-0536.2001.4402097.x
- González-Rodríguez AJ, Gutiérrez-Paredes EM, Revert Fernández Á, et al. Allergic contact dermatitis to benzocaine: the importance of concomitant positive patch test results. Actas Dermosifiliogr. 2013;104:156-158. doi:10.1016/j.ad.2011.07.023
- Muratore L, Calogiuri G, Foti C, et al. Contact allergy to benzocaine in a condom. Contact Dermatitis. 2008;59:173-174. doi:10.1111/j.1600-0536.2008.01359.x
- Sharma A, Agarwal S, Garg G, et al. Desire for lasting long in bed led to contact allergic dermatitis and subsequent superficial penile gangrene: a dreadful complication of benzocaine-containing extended-pleasure condom [published online September 27, 2018]. BMJ Case Rep. 2018;2018:bcr2018227351. doi:10.1136/bcr-2018-227351
- Bauer A, Geier J, Elsner P. Allergic contact dermatitis in patients with anogenital complaints. J Reprod Med. 2000;45:649-654.
- Warshaw EM, Kimyon RS, Silverberg JI, et al. Evaluation of patch test findings in patients with anogenital dermatitis. JAMA Dermatol. 2020;156:85-91. doi:10.1001/jamadermatol.2019.3844
- Weightman W, Turner T. Allergic contact dermatitis from lignocaine: report of 29 cases and review of the literature. Contact Dermatitis. 1998;39:265-266. doi:10.1111/j.1600-0536.1998.tb05928.x
- Jovanovic´ M, Karadaglic´ D, Brkic´ S. Contact urticaria and allergic contact dermatitis to lidocaine in a patient sensitive to benzocaine and propolis. Contact Dermatitis. 2006;54:124-126. doi:10.1111/j.0105-1873.2006.0560f.x
- Carazo JL, Morera BS, Colom LP, et al. Allergic contact dermatitis from ethyl chloride and benzocaine. Dermatitis. 2009;20:E13-E15.
- le Coz CJ, Cribier BJ, Heid E. Patch testing in suspected allergic contact dermatitis due to EMLA cream in haemodialyzed patients. Contact Dermatitis. 1996;35:316-317. doi:10.1111/j.1600-0536.1996.tb02407.x
- Ismail F, Goldsmith PC. EMLA cream-induced allergic contact dermatitis in a child with thalassaemia major. Contact Dermatitis. 2005;52:111. doi:10.1111/j.0105-1873.2005.00498e.x
- Pérez-Pérez LC, Fernández-Redondo V, Ginarte-Val M, et al. Allergic contact dermatitis from EMLA cream in a hemodialyzed patient. Dermatitis. 2006;17:85-87.
- Timmermans MW, Bruynzeel DP, Rustemeyer T. Allergic contact dermatitis from EMLA cream: concomitant sensitization to both local anesthetics lidocaine and prilocaine. J Dtsch Dermatol Ges. 2009;7:237-238. doi:10.1111/j.1610-0387.2008.06932.x
- Fuzier R, Lapeyre-Mestre M, Mertes PM, et al. Immediate- and delayed-type allergic reactions to amide local anesthetics: clinical features and skin testing. Pharmacoepidemiol Drug Saf. 2009;18:595-601. doi:10.1002/pds.1758
- Ruzicka T, Gerstmeier M, Przybilla B, et al. Allergy to local anesthetics: comparison of patch test with prick and intradermal test results. J Am Acad Dermatol. 1987;16:1202-1208. doi:10.1016/s0190-9622(87)70158-3
- Fowler JF Jr, Fowler L, Douglas JL, et al. Skin reactions to pimecrolimus cream 1% in patients allergic to propylene glycol: a double-blind randomized study. Dermatitis. 2007;18:134-139. doi:10.2310/6620.2007.06028
- de Groot A. Patch Testing. 3rd ed. acdegroot publishing; 2008.
Practice Points
- Allergic contact dermatitis (ACD) should be suspected in patients with persistent or worsening dermatitis after use of topical medications.
- Cross-reactions commonly occur between structurally similar compounds and occasionally between molecules from different drug classes.
- Some cases of topical medicament ACD remain elusive after patch testing, particularly drugs with potent immunomodulating effects.
Aquatic Antagonists: Jellyfish Stings
Jellyfish stings are one of the most common marine injuries, with an estimated 150 million stings occurring annually worldwide.1 Most jellyfish stings result in painful localized skin reactions that are self-limited and can be treated with conservative measures including hot water immersion and topical anesthetics. Life-threatening systemic reactions (eg, anaphylaxis, Irukandji syndrome) can occur with some species.2-4 Mainstream media reports do not reflect the true incidence and variability of jellyfish-related injuries that are commonly encountered in the clinic.3
Characteristics of Jellyfish
There are roughly 10,000 known species of jellyfish, with approximately 100 of them posing danger to humans.5 Jellyfish belong to the phylum Cnidaria, which is comprised of 5 classes of both free-floating and sessile animals: Staurozoa (stauromedusae), Hydrozoa (hydroids, fire corals, and Portuguese man-of-war), Scyphozoa (true jellyfish), Anthozoa (corals and sea anemones), and Cubozoa (box jellyfish and Irukandji jellyfish).1,2,6 Jellyfish typically have several tentacles suspended from a free-floating gelatinous body or bell; these tentacles are covered with thousands of cells unique to Cnidaria called nematocytes or cnidocytes containing specialized stinging organelles known as nematocysts. When triggered by physical (eg, human or foreign-body contact) or chemical stimuli, each nematocyst ejects a hollow filament or barb externally, releasing venom into the victim.7,8

The scyphozoan, hydrozoan, and cubozoan life cycles generally consist of a bottom-dwelling, sessile polyp form that produces multiple free-swimming ephyrae through an asexual reproductive process called strobilation. These ephyrae grow into the fully mature medusae, recognizable as jellyfish (Figure 1).5 Additionally, jellyfish populations experience cycles of temporal and spatial population abundance and crashes known as jellyfish blooms. In 2017, Kaffenberger et al9 reviewed the shifting landscape of skin diseases in North America attributable to major changes in climate and weather patterns, including the rise in jellyfish blooms and envenomation outbreaks worldwide (eg, Physalia physalis [Portuguese man-of-war][Figure 2] along the southeastern US coastline, Porpita pacifica off Japanese beaches). Some research suggests jellyfish surges relate to climate change and human interactions with jellyfish habitats by way of eutrophication and fishing (removing predators of jellyfish).9,10

Clinical Presentation
Jellyfish injuries can vary greatly in clinical symptoms, but they do follow some basic patterns. The severity of pain and symptoms is related to the jellyfish species, the number of stinging cells (nematocysts) that are triggered, and the potency of the venom that is absorbed by the victim.11-13 Most stings are minor, and patients experience immediate localized pain with serpiginous raised erythematous or urticarial lesions following the distribution of tentacle contact; these lesions have been described as tentaclelike and resembling a string of beads (Figure 3).12 Pain usually lasts a couple hours, while the skin lesions can last hours to days and can even recur years later. This pattern fits that of the well-known hydrozoans P physalis and Physalia utriculus (bluebottle), which are endemic to the Atlantic and Indo-Pacific Oceans, respectively. The scyphozoan jellyfish causing similar presentations include Pelagia noctiluca (Mauve stinger), Aurelia aurita (Moon jellyfish), and Cyanea species. The cubozoan Chironex fleckeri (Australian box jellyfish or sea wasp) also causes tentaclelike stings but is widely considered the most dangerous jellyfish, as its venom is known to cause cardiac or respiratory arrest.4,11 More than 100 fatalities have been reported following severe envenomations from C fleckeri in Australian and Indo-Pacific waters.6
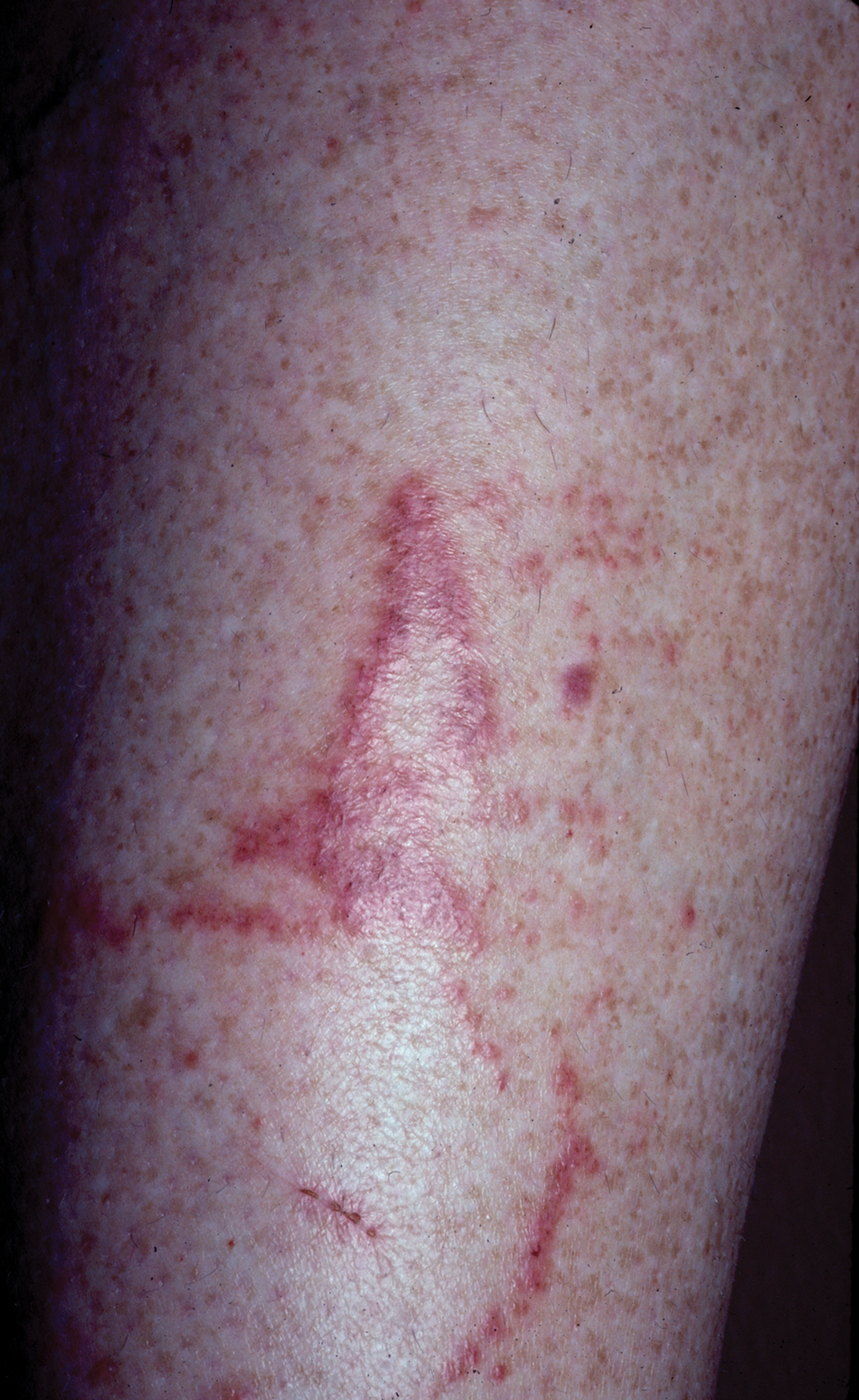
Stings from another box jellyfish species, Carukia barnesi, cause a unique presentation known as Irukandji syndrome. Carukia barnesi is a small box jellyfish with a bell measuring roughly 2 cm in diameter. It has nematocysts on both its bell and tentacles. It inhabits deeper waters and typically stings divers but also can wash ashore and injure beach tourists. Although Irukandji syndrome usually is associated with C barnesi, which is endemic to Northern Australian beaches, other jellyfish species including P physalis rarely have been linked to this potentially fatal syndrome.6,11 Unlike the immediate cutaneous and systemic findings described in C fleckeri encounters, symptoms of Irukandji-like stings can be delayed by up to 30 minutes. Patients may present with severe generalized pain (lower back, chest, headache), signs of excess catecholamine release (tachycardia, hypertension, anxiety, diaphoresis, agitation), or cardiopulmonary decompensation (arrhythmia, cardiac arrest, pulmonary edema).6,11,14.15 Anaphylactic reactions also have been reported in those sensitized by prior stings.16
Management
Prevention of drowning is key in all marine injuries. Rescuers should remove the individual from the water, establish the ABCs—airway, breathing, and circulation—and seek acute medical attention. If immediate resuscitation is not required, douse the wound as soon as possible with a solution that halts further nematocyst discharge, which may contain alcohol, vinegar, or bicarbonate, depending on the prevalent species. General guidance is available to providers through evidence-based, point-of-care databases including UpToDate and DynaMed, as well as through the American Heart Association (AHA) or a country’s equivalent council on emergency care if residing outside the United States. Pressure immobilization bandages as a means of decreasing venom redistribution is no longer recommended by the AHA because animal studies have shown increased nematocyst discharge after pressure application.17 As such, touching or applying pressure to the affected area is not recommended until after a proper rinse solution has been applied. Tentacles may be removed mechanically with gloved hands or sand and seawater with minimal compression or agitation.
When acetic acid is appropriate, such as for cubozoan stings, commercially available vinegar (5% acetic acid in the United States) is preferred.16,17 Tap water can cause discharge of nematocysts, and seawater is preferred when no other solution is available.18 Most marine venoms are heat labile. Immersion in hot water can produce pain relief, but ice can be just as efficacious and is preferred by some patients. Prior reports of patients stung by Physalia species demonstrated greater pain relief with hot water immersion compared to ice pack application.18,19
In the setting of anaphylaxis, patients should receive epinephrine and be transported to a hospital with appropriate hemodynamic monitoring and supportive care. If the species of jellyfish has been identified, species-specific antivenin also may be available in certain regions (eg, C fleckeri antivenin manufactured in Australia), but it is unclear if it improves outcomes when compared with supportive care alone.6,16
Conclusion
Following jellyfish stings, most skin lesions will spontaneously resolve. Patients likely will present days to weeks following the inciting event with mild cutaneous symptoms that are amenable to topical corticosteroids. Recurrent dermatitis following a jellyfish sting is uncommon and is thought to be due to an immunologic mechanism consistent with type IV hypersensitivity reactions. Patients may require multiple courses of treatment before complete resolution.20
Patient education regarding marine envenomation and mechanical barriers such as wetsuits or stinger suits can reduce the risk for injury from jellyfish stings. Sting-inhibiting lotions also are commercially available, though more research is needed.21 Many beaches that are known to harbor the dangerous box jellyfish provide stinger nets to direct travelers to safer waters. Complete avoidance during jellyfish season is recommended in highly endemic areas.1
- Cegolon L, Heymann WC, Lange JH, et al. Jellyfish stings and their management: a review. Mar Drugs. 2013;11:523-550.
- Hornbeak KB, Auerbach PS. Marine envenomation. Emerg Med Clin North Am. 2017;35:321-337.
- Ward NT, Darracq MA, Tomaszewski C, et al. Evidence-based treatment of jellyfish stings in North America and Hawaii. Ann Emerg Med. 2012;60:399-414.
- Burnett JW, Calton GJ, Burnett HW. Jellyfish envenomation syndromes. J Am Acad Dermatol. 1986;14:100-106.
- Brotz L, Cheung WWL, Kleisner K, et al. Increasing jellyfish populations: trends in large marine ecosystems. Hydrobiologia. 2012;690:3-20.
- Ottuso PT. Aquatic antagonists: Cubozoan jellyfish (Chironex fleckeri and Carukia barnesi). Cutis. 2010;85:133-136.
- Lakkis NA, Maalouf GJ, Mahmassani DM. Jellyfish stings: a practical approach. Wilderness Environ Med. 2015;26:422-429.
- Li L, McGee RG, Isbister G, et al. Interventions for the symptoms and signs resulting from jellyfish stings. Cochrane Database Syst Rev. 2013;12:CD009688.
- Kaffenberger BH, Shetlar D, Norton SA, et al. The effect of climate change on skin disease in North America. J Am Acad Dermatol. 2017;76:140-147.
- Purcell JE, Uye S, Lo W. Anthropogenic causes of jellyfish blooms and their direct consequences for humans: a review. Marine Ecology Progress Series. 2007;350:153-174.
- Berling I, Isbister G. Marine envenomations. Aust Fam Physician. 2015;44:28-32.
- Tibballs J, Yanagihara AA, Turner HC, et al. Immunological and toxinological responses to jellyfish stings. Inflamm Allergy Drug Targets. 2011;10:438-446.
- Tibballs J. Australian venomous jellyfish, envenomation syndromes, toxins and therapy. Toxicon. 2006;48:830-859.
- Stein MR, Marracini JV, Rothschild NE, et al. Fatal Portuguese man-o’-war (Physalia physalis) envenomation. Ann Emerg Med. 1989;18:312-315.
- Burnett JW, Gable WD. A fatal jellyfish envenomation by the Portuguese man-o’war. Toxicon. 1989;27:823-824.
- Warrell DA. Venomous bites, stings, and poisoning: an update. Infect Dis Clin North Am. 2019;33:17-38.
- Neumar RW, Shuster M, Callaway CW, et al. Part 1: executive summary: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132(18 suppl 2):S315-S367.
- Wilcox CL, Headlam JL, Doyle TK, et al. Assessing the efficacy of first-aid measures in Physalia sp. envenomation, using solution- and blood agarose-based models. Toxins (Basel). 2017;9:149.
- Wilcox CL, Yanagihara AA. Heated debates: hot-water immersion or ice packs as first aid for cnidarian envenomations? Toxins (Basel). 2016;8:97.
- Loredana Asztalos M, Rubin AI, Elenitsas R, et al. Recurrent dermatitis and dermal hypersensitivity following a jellyfish sting: a case report and review of literature. Pediatr Dermatol. 2014;31:217-219.
- Boulware DR. A randomized, controlled field trial for the prevention of jellyfish stings with a topical sting inhibitor. J Travel Med. 2006;13:166-171.
Jellyfish stings are one of the most common marine injuries, with an estimated 150 million stings occurring annually worldwide.1 Most jellyfish stings result in painful localized skin reactions that are self-limited and can be treated with conservative measures including hot water immersion and topical anesthetics. Life-threatening systemic reactions (eg, anaphylaxis, Irukandji syndrome) can occur with some species.2-4 Mainstream media reports do not reflect the true incidence and variability of jellyfish-related injuries that are commonly encountered in the clinic.3
Characteristics of Jellyfish
There are roughly 10,000 known species of jellyfish, with approximately 100 of them posing danger to humans.5 Jellyfish belong to the phylum Cnidaria, which is comprised of 5 classes of both free-floating and sessile animals: Staurozoa (stauromedusae), Hydrozoa (hydroids, fire corals, and Portuguese man-of-war), Scyphozoa (true jellyfish), Anthozoa (corals and sea anemones), and Cubozoa (box jellyfish and Irukandji jellyfish).1,2,6 Jellyfish typically have several tentacles suspended from a free-floating gelatinous body or bell; these tentacles are covered with thousands of cells unique to Cnidaria called nematocytes or cnidocytes containing specialized stinging organelles known as nematocysts. When triggered by physical (eg, human or foreign-body contact) or chemical stimuli, each nematocyst ejects a hollow filament or barb externally, releasing venom into the victim.7,8

The scyphozoan, hydrozoan, and cubozoan life cycles generally consist of a bottom-dwelling, sessile polyp form that produces multiple free-swimming ephyrae through an asexual reproductive process called strobilation. These ephyrae grow into the fully mature medusae, recognizable as jellyfish (Figure 1).5 Additionally, jellyfish populations experience cycles of temporal and spatial population abundance and crashes known as jellyfish blooms. In 2017, Kaffenberger et al9 reviewed the shifting landscape of skin diseases in North America attributable to major changes in climate and weather patterns, including the rise in jellyfish blooms and envenomation outbreaks worldwide (eg, Physalia physalis [Portuguese man-of-war][Figure 2] along the southeastern US coastline, Porpita pacifica off Japanese beaches). Some research suggests jellyfish surges relate to climate change and human interactions with jellyfish habitats by way of eutrophication and fishing (removing predators of jellyfish).9,10

Clinical Presentation
Jellyfish injuries can vary greatly in clinical symptoms, but they do follow some basic patterns. The severity of pain and symptoms is related to the jellyfish species, the number of stinging cells (nematocysts) that are triggered, and the potency of the venom that is absorbed by the victim.11-13 Most stings are minor, and patients experience immediate localized pain with serpiginous raised erythematous or urticarial lesions following the distribution of tentacle contact; these lesions have been described as tentaclelike and resembling a string of beads (Figure 3).12 Pain usually lasts a couple hours, while the skin lesions can last hours to days and can even recur years later. This pattern fits that of the well-known hydrozoans P physalis and Physalia utriculus (bluebottle), which are endemic to the Atlantic and Indo-Pacific Oceans, respectively. The scyphozoan jellyfish causing similar presentations include Pelagia noctiluca (Mauve stinger), Aurelia aurita (Moon jellyfish), and Cyanea species. The cubozoan Chironex fleckeri (Australian box jellyfish or sea wasp) also causes tentaclelike stings but is widely considered the most dangerous jellyfish, as its venom is known to cause cardiac or respiratory arrest.4,11 More than 100 fatalities have been reported following severe envenomations from C fleckeri in Australian and Indo-Pacific waters.6

Stings from another box jellyfish species, Carukia barnesi, cause a unique presentation known as Irukandji syndrome. Carukia barnesi is a small box jellyfish with a bell measuring roughly 2 cm in diameter. It has nematocysts on both its bell and tentacles. It inhabits deeper waters and typically stings divers but also can wash ashore and injure beach tourists. Although Irukandji syndrome usually is associated with C barnesi, which is endemic to Northern Australian beaches, other jellyfish species including P physalis rarely have been linked to this potentially fatal syndrome.6,11 Unlike the immediate cutaneous and systemic findings described in C fleckeri encounters, symptoms of Irukandji-like stings can be delayed by up to 30 minutes. Patients may present with severe generalized pain (lower back, chest, headache), signs of excess catecholamine release (tachycardia, hypertension, anxiety, diaphoresis, agitation), or cardiopulmonary decompensation (arrhythmia, cardiac arrest, pulmonary edema).6,11,14.15 Anaphylactic reactions also have been reported in those sensitized by prior stings.16
Management
Prevention of drowning is key in all marine injuries. Rescuers should remove the individual from the water, establish the ABCs—airway, breathing, and circulation—and seek acute medical attention. If immediate resuscitation is not required, douse the wound as soon as possible with a solution that halts further nematocyst discharge, which may contain alcohol, vinegar, or bicarbonate, depending on the prevalent species. General guidance is available to providers through evidence-based, point-of-care databases including UpToDate and DynaMed, as well as through the American Heart Association (AHA) or a country’s equivalent council on emergency care if residing outside the United States. Pressure immobilization bandages as a means of decreasing venom redistribution is no longer recommended by the AHA because animal studies have shown increased nematocyst discharge after pressure application.17 As such, touching or applying pressure to the affected area is not recommended until after a proper rinse solution has been applied. Tentacles may be removed mechanically with gloved hands or sand and seawater with minimal compression or agitation.
When acetic acid is appropriate, such as for cubozoan stings, commercially available vinegar (5% acetic acid in the United States) is preferred.16,17 Tap water can cause discharge of nematocysts, and seawater is preferred when no other solution is available.18 Most marine venoms are heat labile. Immersion in hot water can produce pain relief, but ice can be just as efficacious and is preferred by some patients. Prior reports of patients stung by Physalia species demonstrated greater pain relief with hot water immersion compared to ice pack application.18,19
In the setting of anaphylaxis, patients should receive epinephrine and be transported to a hospital with appropriate hemodynamic monitoring and supportive care. If the species of jellyfish has been identified, species-specific antivenin also may be available in certain regions (eg, C fleckeri antivenin manufactured in Australia), but it is unclear if it improves outcomes when compared with supportive care alone.6,16
Conclusion
Following jellyfish stings, most skin lesions will spontaneously resolve. Patients likely will present days to weeks following the inciting event with mild cutaneous symptoms that are amenable to topical corticosteroids. Recurrent dermatitis following a jellyfish sting is uncommon and is thought to be due to an immunologic mechanism consistent with type IV hypersensitivity reactions. Patients may require multiple courses of treatment before complete resolution.20
Patient education regarding marine envenomation and mechanical barriers such as wetsuits or stinger suits can reduce the risk for injury from jellyfish stings. Sting-inhibiting lotions also are commercially available, though more research is needed.21 Many beaches that are known to harbor the dangerous box jellyfish provide stinger nets to direct travelers to safer waters. Complete avoidance during jellyfish season is recommended in highly endemic areas.1
Jellyfish stings are one of the most common marine injuries, with an estimated 150 million stings occurring annually worldwide.1 Most jellyfish stings result in painful localized skin reactions that are self-limited and can be treated with conservative measures including hot water immersion and topical anesthetics. Life-threatening systemic reactions (eg, anaphylaxis, Irukandji syndrome) can occur with some species.2-4 Mainstream media reports do not reflect the true incidence and variability of jellyfish-related injuries that are commonly encountered in the clinic.3
Characteristics of Jellyfish
There are roughly 10,000 known species of jellyfish, with approximately 100 of them posing danger to humans.5 Jellyfish belong to the phylum Cnidaria, which is comprised of 5 classes of both free-floating and sessile animals: Staurozoa (stauromedusae), Hydrozoa (hydroids, fire corals, and Portuguese man-of-war), Scyphozoa (true jellyfish), Anthozoa (corals and sea anemones), and Cubozoa (box jellyfish and Irukandji jellyfish).1,2,6 Jellyfish typically have several tentacles suspended from a free-floating gelatinous body or bell; these tentacles are covered with thousands of cells unique to Cnidaria called nematocytes or cnidocytes containing specialized stinging organelles known as nematocysts. When triggered by physical (eg, human or foreign-body contact) or chemical stimuli, each nematocyst ejects a hollow filament or barb externally, releasing venom into the victim.7,8

The scyphozoan, hydrozoan, and cubozoan life cycles generally consist of a bottom-dwelling, sessile polyp form that produces multiple free-swimming ephyrae through an asexual reproductive process called strobilation. These ephyrae grow into the fully mature medusae, recognizable as jellyfish (Figure 1).5 Additionally, jellyfish populations experience cycles of temporal and spatial population abundance and crashes known as jellyfish blooms. In 2017, Kaffenberger et al9 reviewed the shifting landscape of skin diseases in North America attributable to major changes in climate and weather patterns, including the rise in jellyfish blooms and envenomation outbreaks worldwide (eg, Physalia physalis [Portuguese man-of-war][Figure 2] along the southeastern US coastline, Porpita pacifica off Japanese beaches). Some research suggests jellyfish surges relate to climate change and human interactions with jellyfish habitats by way of eutrophication and fishing (removing predators of jellyfish).9,10

Clinical Presentation
Jellyfish injuries can vary greatly in clinical symptoms, but they do follow some basic patterns. The severity of pain and symptoms is related to the jellyfish species, the number of stinging cells (nematocysts) that are triggered, and the potency of the venom that is absorbed by the victim.11-13 Most stings are minor, and patients experience immediate localized pain with serpiginous raised erythematous or urticarial lesions following the distribution of tentacle contact; these lesions have been described as tentaclelike and resembling a string of beads (Figure 3).12 Pain usually lasts a couple hours, while the skin lesions can last hours to days and can even recur years later. This pattern fits that of the well-known hydrozoans P physalis and Physalia utriculus (bluebottle), which are endemic to the Atlantic and Indo-Pacific Oceans, respectively. The scyphozoan jellyfish causing similar presentations include Pelagia noctiluca (Mauve stinger), Aurelia aurita (Moon jellyfish), and Cyanea species. The cubozoan Chironex fleckeri (Australian box jellyfish or sea wasp) also causes tentaclelike stings but is widely considered the most dangerous jellyfish, as its venom is known to cause cardiac or respiratory arrest.4,11 More than 100 fatalities have been reported following severe envenomations from C fleckeri in Australian and Indo-Pacific waters.6

Stings from another box jellyfish species, Carukia barnesi, cause a unique presentation known as Irukandji syndrome. Carukia barnesi is a small box jellyfish with a bell measuring roughly 2 cm in diameter. It has nematocysts on both its bell and tentacles. It inhabits deeper waters and typically stings divers but also can wash ashore and injure beach tourists. Although Irukandji syndrome usually is associated with C barnesi, which is endemic to Northern Australian beaches, other jellyfish species including P physalis rarely have been linked to this potentially fatal syndrome.6,11 Unlike the immediate cutaneous and systemic findings described in C fleckeri encounters, symptoms of Irukandji-like stings can be delayed by up to 30 minutes. Patients may present with severe generalized pain (lower back, chest, headache), signs of excess catecholamine release (tachycardia, hypertension, anxiety, diaphoresis, agitation), or cardiopulmonary decompensation (arrhythmia, cardiac arrest, pulmonary edema).6,11,14.15 Anaphylactic reactions also have been reported in those sensitized by prior stings.16
Management
Prevention of drowning is key in all marine injuries. Rescuers should remove the individual from the water, establish the ABCs—airway, breathing, and circulation—and seek acute medical attention. If immediate resuscitation is not required, douse the wound as soon as possible with a solution that halts further nematocyst discharge, which may contain alcohol, vinegar, or bicarbonate, depending on the prevalent species. General guidance is available to providers through evidence-based, point-of-care databases including UpToDate and DynaMed, as well as through the American Heart Association (AHA) or a country’s equivalent council on emergency care if residing outside the United States. Pressure immobilization bandages as a means of decreasing venom redistribution is no longer recommended by the AHA because animal studies have shown increased nematocyst discharge after pressure application.17 As such, touching or applying pressure to the affected area is not recommended until after a proper rinse solution has been applied. Tentacles may be removed mechanically with gloved hands or sand and seawater with minimal compression or agitation.
When acetic acid is appropriate, such as for cubozoan stings, commercially available vinegar (5% acetic acid in the United States) is preferred.16,17 Tap water can cause discharge of nematocysts, and seawater is preferred when no other solution is available.18 Most marine venoms are heat labile. Immersion in hot water can produce pain relief, but ice can be just as efficacious and is preferred by some patients. Prior reports of patients stung by Physalia species demonstrated greater pain relief with hot water immersion compared to ice pack application.18,19
In the setting of anaphylaxis, patients should receive epinephrine and be transported to a hospital with appropriate hemodynamic monitoring and supportive care. If the species of jellyfish has been identified, species-specific antivenin also may be available in certain regions (eg, C fleckeri antivenin manufactured in Australia), but it is unclear if it improves outcomes when compared with supportive care alone.6,16
Conclusion
Following jellyfish stings, most skin lesions will spontaneously resolve. Patients likely will present days to weeks following the inciting event with mild cutaneous symptoms that are amenable to topical corticosteroids. Recurrent dermatitis following a jellyfish sting is uncommon and is thought to be due to an immunologic mechanism consistent with type IV hypersensitivity reactions. Patients may require multiple courses of treatment before complete resolution.20
Patient education regarding marine envenomation and mechanical barriers such as wetsuits or stinger suits can reduce the risk for injury from jellyfish stings. Sting-inhibiting lotions also are commercially available, though more research is needed.21 Many beaches that are known to harbor the dangerous box jellyfish provide stinger nets to direct travelers to safer waters. Complete avoidance during jellyfish season is recommended in highly endemic areas.1
- Cegolon L, Heymann WC, Lange JH, et al. Jellyfish stings and their management: a review. Mar Drugs. 2013;11:523-550.
- Hornbeak KB, Auerbach PS. Marine envenomation. Emerg Med Clin North Am. 2017;35:321-337.
- Ward NT, Darracq MA, Tomaszewski C, et al. Evidence-based treatment of jellyfish stings in North America and Hawaii. Ann Emerg Med. 2012;60:399-414.
- Burnett JW, Calton GJ, Burnett HW. Jellyfish envenomation syndromes. J Am Acad Dermatol. 1986;14:100-106.
- Brotz L, Cheung WWL, Kleisner K, et al. Increasing jellyfish populations: trends in large marine ecosystems. Hydrobiologia. 2012;690:3-20.
- Ottuso PT. Aquatic antagonists: Cubozoan jellyfish (Chironex fleckeri and Carukia barnesi). Cutis. 2010;85:133-136.
- Lakkis NA, Maalouf GJ, Mahmassani DM. Jellyfish stings: a practical approach. Wilderness Environ Med. 2015;26:422-429.
- Li L, McGee RG, Isbister G, et al. Interventions for the symptoms and signs resulting from jellyfish stings. Cochrane Database Syst Rev. 2013;12:CD009688.
- Kaffenberger BH, Shetlar D, Norton SA, et al. The effect of climate change on skin disease in North America. J Am Acad Dermatol. 2017;76:140-147.
- Purcell JE, Uye S, Lo W. Anthropogenic causes of jellyfish blooms and their direct consequences for humans: a review. Marine Ecology Progress Series. 2007;350:153-174.
- Berling I, Isbister G. Marine envenomations. Aust Fam Physician. 2015;44:28-32.
- Tibballs J, Yanagihara AA, Turner HC, et al. Immunological and toxinological responses to jellyfish stings. Inflamm Allergy Drug Targets. 2011;10:438-446.
- Tibballs J. Australian venomous jellyfish, envenomation syndromes, toxins and therapy. Toxicon. 2006;48:830-859.
- Stein MR, Marracini JV, Rothschild NE, et al. Fatal Portuguese man-o’-war (Physalia physalis) envenomation. Ann Emerg Med. 1989;18:312-315.
- Burnett JW, Gable WD. A fatal jellyfish envenomation by the Portuguese man-o’war. Toxicon. 1989;27:823-824.
- Warrell DA. Venomous bites, stings, and poisoning: an update. Infect Dis Clin North Am. 2019;33:17-38.
- Neumar RW, Shuster M, Callaway CW, et al. Part 1: executive summary: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132(18 suppl 2):S315-S367.
- Wilcox CL, Headlam JL, Doyle TK, et al. Assessing the efficacy of first-aid measures in Physalia sp. envenomation, using solution- and blood agarose-based models. Toxins (Basel). 2017;9:149.
- Wilcox CL, Yanagihara AA. Heated debates: hot-water immersion or ice packs as first aid for cnidarian envenomations? Toxins (Basel). 2016;8:97.
- Loredana Asztalos M, Rubin AI, Elenitsas R, et al. Recurrent dermatitis and dermal hypersensitivity following a jellyfish sting: a case report and review of literature. Pediatr Dermatol. 2014;31:217-219.
- Boulware DR. A randomized, controlled field trial for the prevention of jellyfish stings with a topical sting inhibitor. J Travel Med. 2006;13:166-171.
- Cegolon L, Heymann WC, Lange JH, et al. Jellyfish stings and their management: a review. Mar Drugs. 2013;11:523-550.
- Hornbeak KB, Auerbach PS. Marine envenomation. Emerg Med Clin North Am. 2017;35:321-337.
- Ward NT, Darracq MA, Tomaszewski C, et al. Evidence-based treatment of jellyfish stings in North America and Hawaii. Ann Emerg Med. 2012;60:399-414.
- Burnett JW, Calton GJ, Burnett HW. Jellyfish envenomation syndromes. J Am Acad Dermatol. 1986;14:100-106.
- Brotz L, Cheung WWL, Kleisner K, et al. Increasing jellyfish populations: trends in large marine ecosystems. Hydrobiologia. 2012;690:3-20.
- Ottuso PT. Aquatic antagonists: Cubozoan jellyfish (Chironex fleckeri and Carukia barnesi). Cutis. 2010;85:133-136.
- Lakkis NA, Maalouf GJ, Mahmassani DM. Jellyfish stings: a practical approach. Wilderness Environ Med. 2015;26:422-429.
- Li L, McGee RG, Isbister G, et al. Interventions for the symptoms and signs resulting from jellyfish stings. Cochrane Database Syst Rev. 2013;12:CD009688.
- Kaffenberger BH, Shetlar D, Norton SA, et al. The effect of climate change on skin disease in North America. J Am Acad Dermatol. 2017;76:140-147.
- Purcell JE, Uye S, Lo W. Anthropogenic causes of jellyfish blooms and their direct consequences for humans: a review. Marine Ecology Progress Series. 2007;350:153-174.
- Berling I, Isbister G. Marine envenomations. Aust Fam Physician. 2015;44:28-32.
- Tibballs J, Yanagihara AA, Turner HC, et al. Immunological and toxinological responses to jellyfish stings. Inflamm Allergy Drug Targets. 2011;10:438-446.
- Tibballs J. Australian venomous jellyfish, envenomation syndromes, toxins and therapy. Toxicon. 2006;48:830-859.
- Stein MR, Marracini JV, Rothschild NE, et al. Fatal Portuguese man-o’-war (Physalia physalis) envenomation. Ann Emerg Med. 1989;18:312-315.
- Burnett JW, Gable WD. A fatal jellyfish envenomation by the Portuguese man-o’war. Toxicon. 1989;27:823-824.
- Warrell DA. Venomous bites, stings, and poisoning: an update. Infect Dis Clin North Am. 2019;33:17-38.
- Neumar RW, Shuster M, Callaway CW, et al. Part 1: executive summary: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132(18 suppl 2):S315-S367.
- Wilcox CL, Headlam JL, Doyle TK, et al. Assessing the efficacy of first-aid measures in Physalia sp. envenomation, using solution- and blood agarose-based models. Toxins (Basel). 2017;9:149.
- Wilcox CL, Yanagihara AA. Heated debates: hot-water immersion or ice packs as first aid for cnidarian envenomations? Toxins (Basel). 2016;8:97.
- Loredana Asztalos M, Rubin AI, Elenitsas R, et al. Recurrent dermatitis and dermal hypersensitivity following a jellyfish sting: a case report and review of literature. Pediatr Dermatol. 2014;31:217-219.
- Boulware DR. A randomized, controlled field trial for the prevention of jellyfish stings with a topical sting inhibitor. J Travel Med. 2006;13:166-171.
Practice Points
- Jellyfish stings occur an estimated 150 million times annually worldwide, with numbers expected to rise due to climate change.
- Most stings result in painful self-limited cutaneous symptoms that resolve spontaneously. Box jellyfish (Cubozoa) stings carry a greater risk for causing severe systemic reactions.
- Treatment of skin reactions includes removal of tentacles and hot water immersion. Vinegar dousing for at least 30 seconds is recommended for box jellyfish stings. Supportive care and monitoring for cardiovascular collapse are key. The role of antivenin is uncertain.
What’s Eating You? Caterpillars
Causes of Lepidopterism
Caterpillars are wormlike organisms that serve as the larval stage of moths and butterflies, which belong to the order Lepidoptera. There are almost 165,000 discovered species, with 13,000 found in the United States.1,2 Roughly 150 species are known to have the potential to cause an adverse reaction in humans, with 50 of these in the United States.1Lepidopterism describes systemic and cutaneous reactions to moths, butterflies, and caterpillars; erucism describes strictly cutaneous reactions.1
Although the rate of lepidopterism is thought to be underreported because it often is self-limited and of a mild nature, a review found caterpillars to be the cause of roughly 2.2% of reported bites and stings annually.2 Cases increase in number with seasonal increases in caterpillars, which vary by region and species. For example, the Megalopyge opercularis (southern flannel moth) caterpillar was noted to have 2 peaks in a Texas-based study: 12% of reported stings occurred in July; 59% from October through November.3 In general, the likelihood of exposure increases during warmer months, and exposure is more common in people who work outdoors in a rural area or in a suburban area where there are many caterpillar-infested trees.4
Most cases of lepidopterism are caused by caterpillars, not by adult butterflies and moths, because the former have many tubular, or porous, hairlike structures called setae that are embedded in the integument. Setae were once thought to be connected to poison-secreting glandular cells, but current belief is that venomous caterpillars lack specialized gland cells and instead produce venom through secretory epithelial cells located above the integument.1 Venom accumulates in the hemolymph and is stored in the setae or other types of bristles, such as scoli (wartlike bumps that bear setae) or spines.5 With a large amount of chitin, bristles have a tendency to fracture and release venom upon contact.1 It is thought that some species of caterpillars formulate venom by ingesting toxins or toxin precursors from plants; for example, the tiger moth (family Arctiidae) is known to produce venom containing biogenic amines, pyrrolizidine, alkaloids, and cardiac glycosides obtained through food sources.5
Even if a caterpillar does not produce venom, its setae might embed into skin or mucous membranes and cause an adverse irritant reaction.1 Setae also might dislodge and be transported in the air to embed in objects—some remaining stable in the environment for longer than a year.2 In contrast to setae, spines are permanently fixed into the integument; for that reason, only direct contact with the caterpillar can result in an adverse reaction. Although it is mostly caterpillars that contain setae and spines, certain species of moths also might contain these structures or might acquire them as they emerge from the cocoon, which often contains incorporated setae.2
Reactions in Humans
Lepidopterism encompasses 3 principal reactions in humans: sting reaction, hypersensitivity reaction, and lonomism (a hemorrhagic diathesis produced by Lonomia caterpillars). The type and severity of the reaction depends on (1) the species of caterpillar or moth and (2) the individual patient.2 There are approximately 12 families of caterpillars, mainly of the moth variety, that can cause an adverse reaction in humans.1 Tables 1 and 2 list examples of species that cause each type of reaction.6
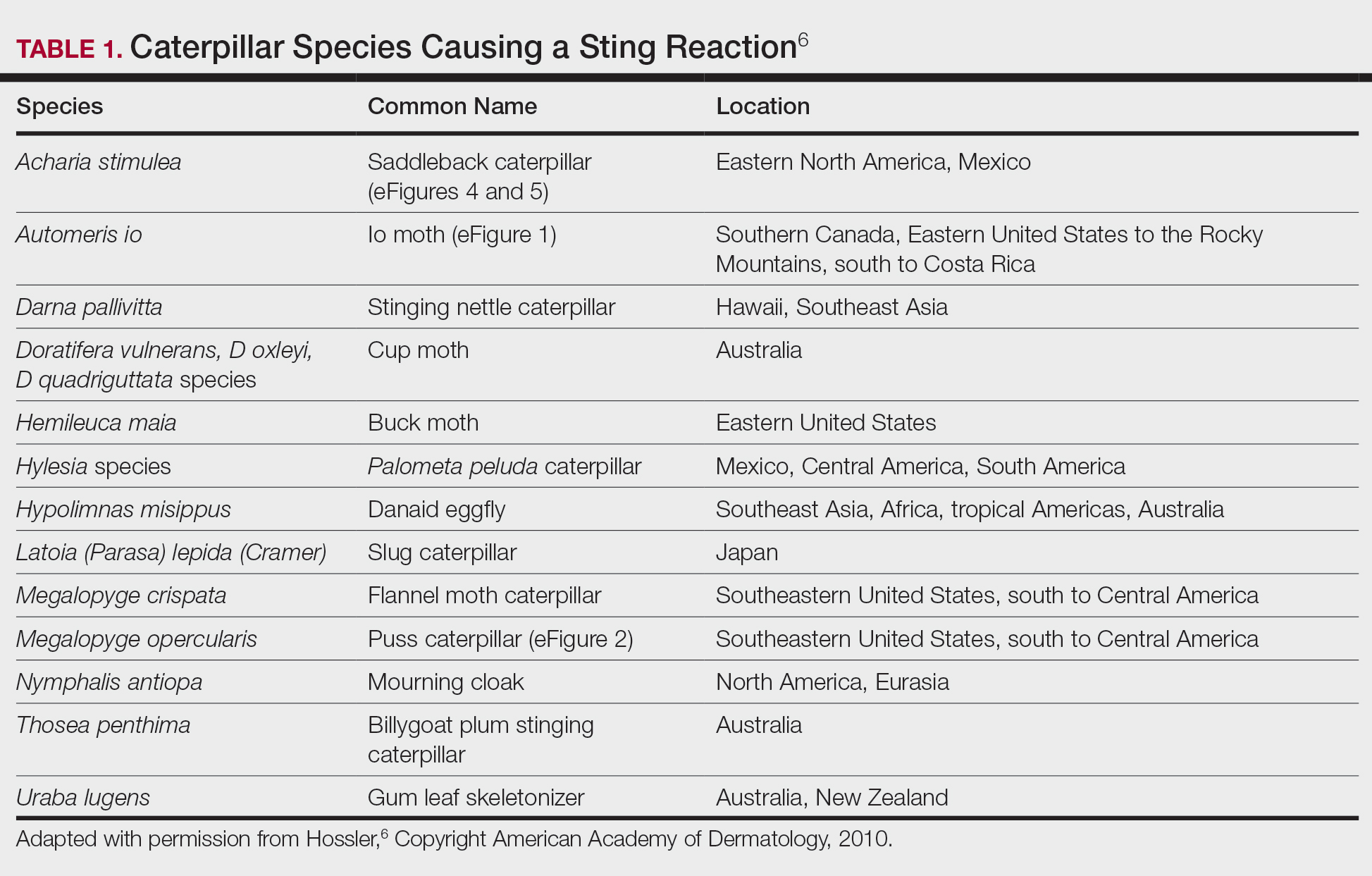
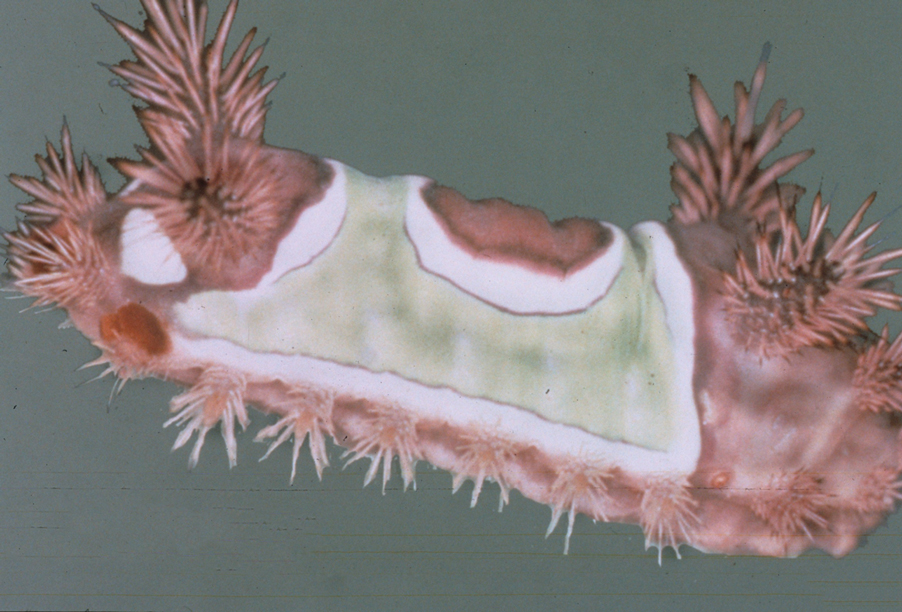
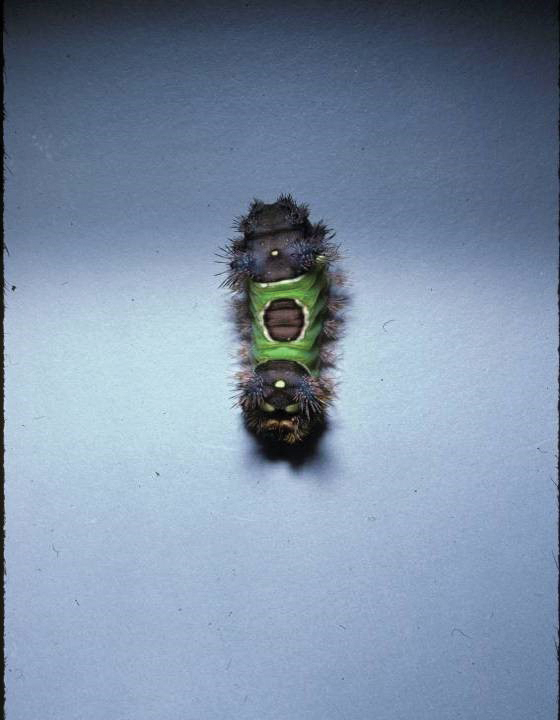
Chemicals and toxins contained in the poison of setae and spines vary by species of caterpillar. Numerous kinds have been isolated from different venoms,1,2 including several peptides, histamine, histamine-releasing substances, acetylcholine, phospholipase A, hyaluronidase, formic acid, proteins with trypsinlike activity, serine proteases such as kallikrein, and other enzymes with vasodegenerative and fibrinolytic properties
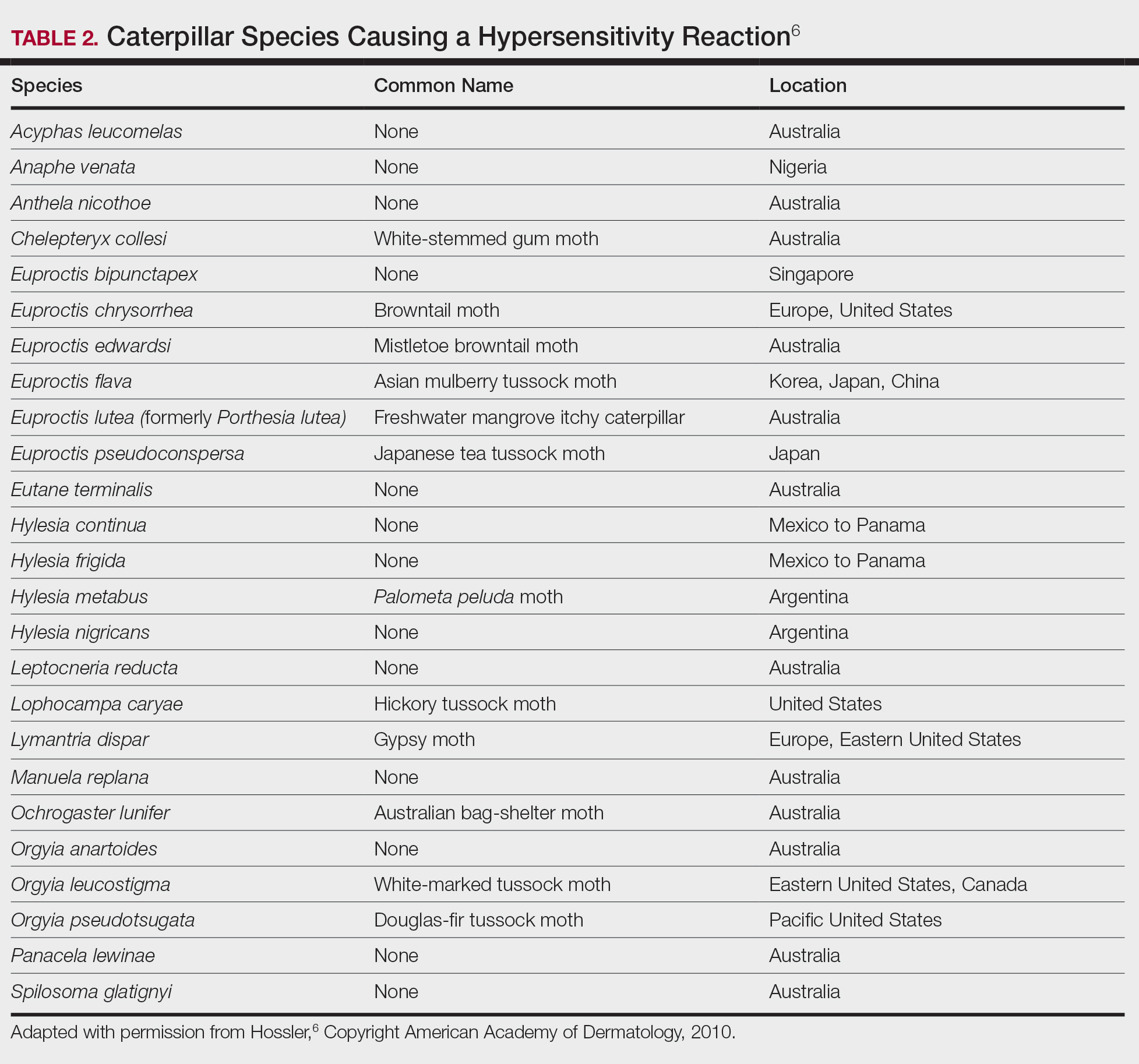
Stings: An Immediate Adverse Reaction—Depending on the venom, a sting might result in mild to severe burning pain, accompanied by welts, vesicles, and red papules or plaques.2 Figure 1 demonstrates a particularly mild sting from a caterpillar of the family Automeris, examples of which are seen in Figures 2 and 3 and eFigure 1. Components of the venom determine the mechanism of the sting and the pain that accompanies it. For example, a recent study demonstrated that the venom of the Latoia consocia caterpillar induces pain through the ion-channel receptor known as transient receptor potential vanilloid 1, which integrates and sends painful stimuli from the peripheral nervous system to the central nervous system.7 It is thought that a variety of ion channels are targets of the venom of caterpillars.



One of the most characteristic sting patterns is that of the caterpillar of family Megalopygidae (flannel moth)(eFigures 2 and 3). The stings of these caterpillars create a unique tram-track pattern of hemorrhagic macules or papules (Figure 4).4 A study found that 90% of reported M opercularis envenomations consist primarily of cutaneous symptoms, with 84% of those symptoms being irritation or pain; 45% a puncture or wound; 29% erythema; and 15% edema.3 Systemic findings can include headache, fever, adenopathy, nausea, vomiting, abdominal pain, and chest pain.4 Symptoms normally are self-limited, though they can last minutes or hours.


Hypersensitivity Reaction—Studies demonstrate that the symptoms of this reaction are a mixture of type I hypersensitivity, type IV hypersensitivity, and a foreign-body response.2 The specific hypersensitivity reaction depends on the venom and the exposed individual—most commonly including a combination of pruritic papules, urticarial wheals, flares, and dermatitis.2 A reaction that is a result of direct contact with the caterpillar or moth will appear on exposed areas; however, because setae embed in linens and clothing, they might cause a reaction anywhere on the body. Although usually self-limited, a hypersensitivity reaction might develop within minutes and can last for days or weeks.
Stings and hypersensitivity reactions to caterpillars and moths tend to lead to a nonspecific histologic presentation characterized by epidermal edema and a superficial perivascular lymphocytic infiltrate, often with eosinophils.6 After approximately 1 week, a foreign-body response to setae can lead to tuberculoid granulomas accompanied by neutrophils in the dermis and occasionally in subcutaneous tissues (Figures 5 and 6).8 If setae have not yet been removed, they also might be visible in skin scrapings.
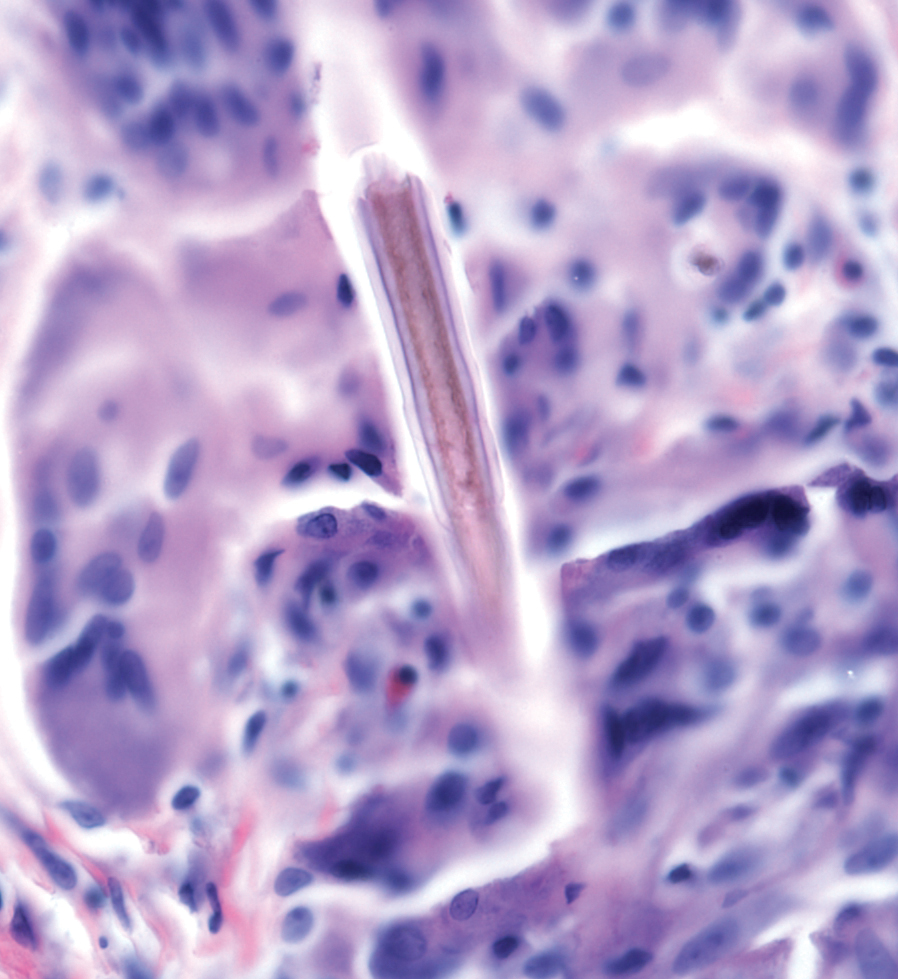
Additional complications can accompany the hypersensitivity reaction to setae or spines. Type I hypersensitivity reactions can lead to severe reactions on second contact due to previously sensitized IgE antibodies. Although the first reaction appears mild, second contact might result in angioedema, wheezing, dyspnea, or anaphylaxis, or a combination of these findings.9 In addition, some patients who come in contact with Dendrolimus caterpillars might develop a condition known as dendrolimiasis, characterized by dermatitis in addition to arthritis or chondritis.6 The arthritis is normally monoarticular and can result in complete destruction of the joint. Pararamose, a condition with a similar presentation, is caused by the Brazilian moth Premolis semirufa.6
Contact of setae or spines with mucous membranes or inhalation of setae also might result in edema, dysphagia, dyspnea, drooling, rhinitis, or conjunctivitis, or a combination of these findings.6 In addition, setae can embed in the eye and cause an inflammatory reaction—ophthalmia nodosa—most commonly caused by caterpillars of the pine processionary moth (Thaumetopoea pityocampa) and characterized by immediate chemosis, which can progress to liquefactive necrosis and hypopyon, later developing into a granulomatous foreign-body response.2,10 The process is thought to be the result of a combination of the thaumetopoein toxin in the setae and an IgE-mediated response to other proteins.10
Due to their harpoon shape and forward-only motion, setae might migrate deeper, potentially even to the optic nerve.11 Because migration might take years and the barbed shape of setae does not always allow removal, some patients require lifetime monitoring with slit-lamp examination.Chronic problems, such as cataracts and persistent posterior uveitis, have been reported.10,11
Lonomism—One of the most serious (though rarest) reactions to caterpillars is lonomism, a condition caused by the caterpillars of Lonomia achelous and Lonomia obliqua moths. These caterpillars have a unique combination of toxins filling their branched spines, which ultimately leads to the same outcome: a hemorrhagic diathesis.
The toxin of L achelous comprises several proteases that degrade fibrin, fibrinogen, and factor XIII while activating prothrombin. In contrast, L obliqua poison causes a hemorrhagic diathesis by promoting a consumptive coagulopathy through enzymes that activate factor X and prothrombin.
With initial contact with either of these Lonomia caterpillars, the patient experiences severe pain accompanied by systemic symptoms, including headache, nausea, and vomiting. Shortly afterward, symptoms of a hemorrhagic diathesis manifest, including bleeding gums, hematuria, bleeding from prior wounds, and epistaxis.5 Serious complications of the hemorrhagic diathesis, such as hemorrhage of major organs, leads to death in 4% of patients.5 A reported case of a patient whose Lonomia caterpillar sting went unrecognized until a week after the accident ended with progression to stage V chronic renal disease.12
Recent research has focused on the specific mechanism of injury caused by Lonomia species. A study found that the venom of L obliqua causes cytoskeleton rearrangement and migration in vascular smooth muscle cells (VSMCs) by inducing formation of reactive oxygen species through activation of nicotinamide adenine dinucleotide phosphate oxidase.13 Thus, the venom directly contributes to the proinflammatory phenotype of endothelial cells seen following envenomation. The same study also demonstrated that elevated reactive oxygen species trigger extracellular signal-regulated kinase pathway activation in VSMCs, leading to cell proliferation, re-stenosis, and ischemia.13 This finding was confirmed by another study,14 which demonstrated an increase in Rac1, a signaling protein involved in the extracellular signal-regulated kinase pathway, in VSMCs upon exposure to L obliqua venom. These studies propose potential new targets for treatment to prevent vascular damage.
Reactions to Adult Organisms—Although it is more common for the caterpillar form of these organisms to cause an adverse reaction, the adult moth also might be capable of causing a similar reaction by retaining setae from the cocoon or by their own spines. The most notable example of this is female moths of the genus Hylesia, which possess spines attached to glands on the abdomen. The poison in these spines—a mixture of proteases and chitinase—causes a dermatitis known as Caripito itch—the name derived from a river port in Venezuela where this moth caused a memorable epidemic of moth-induced dermatitis.7,15 Caripito itch is known for intense pruritus that most commonly lasts days or weeks, possibly longer than 1 year.
Diagnostic Difficulties
The challenge of diagnosing a caterpillar- or moth-induced reaction in humans arises from (1) the lack of clinical history (the caterpillar might not be seen at all by the patient or the examiner) and (2) the similarity of these reactions to those with more common triggers.
When setae remain embedded in the skin or mucous membranes, skin scrapings allow accelerated diagnosis. On a skin scraping prepared with 20% potassium hydroxide, setae appear as tapered and barbed hairlike structures, which allows them to be distinguished from other similar-appearing but differently shaped structures, such as glass fibers.
When setae do not remain embedded in the skin or when the cause of the reaction is due to spines, the physician is left with a nonspecific histologic picture and a large differential diagnosis to be narrowed down based on the history and occasionally the pattern of the skin lesion.
A challenge in sting diagnosis is differentiating a caterpillar or moth sting from that of another organism. In certain cases, such as those of the family Megalopygidae, specific patterns of stings might assist in making the diagnosis. Hypersensitivity reactions are associated with a wider differential diagnosis, including irritant or allergic dermatitis from other causes, scabies, eczema, lichen planus, lichen simplex chronicus, seborrheic dermatitis, and tinea corporis, to name a few.6 Skin scrapings can be examined for other features, such as burrows in the case of scabies, to further narrow the differential.
Stings and hypersensitivity reactions lacking a proper history and associated with more severe systemic symptoms have caused misdiagnosis or led to a workup for the wrong condition; for example, the picture of abdominal pain, nausea, vomiting, tachycardia, leukocytosis, hypokalemia, and metabolic acidosis can simulate appendicitis.16 Upon discovery of a puss caterpillar sting in a patient, her symptoms resolved after treatment with ondansetron, morphine, and intravenous fluids.16
In lonomism, the diagnosis must be established by laboratory measurement of the fibrinogen level, clotting factors, prothrombin time, and activated partial thromboplastin time.4 The differential diagnosis associated with lonomism includes disseminated intravascular coagulation (DIC), snakebite, and a hereditary bleeding disorder.4 The combination of laboratory tests and an extensive medical history allows a diagnosis. Absence of a personal or family history of bleeding excludes a diagnosis of hereditary bleeding disorder, whereas the absence of known causes of DIC or thrombocytopenia allows DIC to be excluded from the differential.
Treatment Options and Prevention
Treatment—The first step is to remove any embedded setae from the skin or mucous membranes. The stepwise recommendation is to remove any constricted clothing, detach setae with adhesive tape, wash with soap and water, and dry without touching the skin.1 Any remaining setae can be removed with additional tape or forceps; setae tend to be fragile and are difficult to remove in their entirety.
Other than removal of the setae, skin reactions are treated symptomatically. Ice packs and isopropyl alcohol have been utilized to cool burning or stinging areas. Pain, pruritus, and inflammation have been alleviated with antihistamines and topical corticosteroids.1 When pain is severe, oral codeine or local injection of anesthetic can be used. For severe and persistent skin lesions, a course of an oral glucocorticoid can be administered. Intramuscular triamcinolone acetonide has been shown to treat pain, dermatitis, and subcutaneous nodules otherwise refractory to treatment.8
Antivenin specific for L obliqua exists to treat lonomism and is therefore effective only when lonomism is caused by that species. Lonomism caused by L achelous is treated with cryoprecipitate, purified fibrinogen, and antifibrinolytic drugs, such as aprotinin.6 Whole blood and fresh-frozen plasma have been noted to make hemorrhage worse when utilized to treat lonomism. Because the mechanism of action of the venom of Lonomia species is based, in part, on inducing a proinflammatory profile in endothelial cells, studies have demonstrated that inhibition of kallikrein might prevent vascular injury and thus prevent serious adverse effects, such as renal failure.17
Prevention—People should wear proper protective clothing when outdoors in potentially infested areas. Measures should be taken to ensure that linens and clothing are not left outside in areas where setae might be carried on the wind. Infestation control is necessary if the population of caterpillars reaches a high enough level.
Conclusion
Several species of caterpillars and moths cause adverse reactions in humans: stings, hypersensitivity reactions, and lonomism. Although most reactions are self-limited, some might have more serious effects, including organ failure and death. Mechanisms of injury vary by species of caterpillar, moth, and butterfly; current research is focused on further defining venom components and signaling pathways to isolate potential targets to aid in the diagnosis and treatment of lepidopterism.
- Goldman BS, Bragg BN. Caterpillar and moth bites. Stat Pearls [Internet]. StatPearls Publishing. Updated August 3, 2021. Accessed November 4, 2021. https://www.ncbi.nlm.nih.gov/books/NBK539851/
- Hossler EW. Caterpillars and moths: part I. Dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:1-10. doi:10.1016/j.jaad.2009.08.060
- Forrester MB. Megalopyge opercularis caterpillar stings reported to Texas poison centers. Wilderness Environ Med. 2018;29:215-220. doi:10.1016/j.wem.2018.02.002
- Hossler EW. Lepidopterism: skin disorders secondary to caterpillars and moths. UpToDate website. Published October 20, 2021. Accessed November 18, 2021. https://www.uptodate.com/contents/lepidopterism-skin-disorders-secondary-to-caterpillars-and-moths
- Villas-Boas IM, Bonfá G, Tambourgi DV. Venomous caterpillars: from inoculation apparatus to venom composition and envenomation. Toxicon. 2018;153:39-52. doi:10.1016/j.toxicon.2018.08.007
- Hossler EW. Caterpillars and moths: part II. dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:13-28. doi:10.1016/j.jaad.2009.08.061
- Yao Z, Kamau PM, Han Y, et al. The Latoia consocia caterpillar induces pain by targeting nociceptive ion channel TRPV1. Toxins (Basel). 2019;11:695. doi:10.3390/toxins11120695
- Paniz-Mondolfi AE, Pérez-Alvarez AM, Lundberg U, et al. Cutaneous lepidopterism: dermatitis from contact with moths of Hylesia metabus (Cramer 1775) (Lepidoptera: Saturniidae), the causative agent of caripito itch. Int J Dermatol. 2011;50:535-541. doi:10.1111/j.1365-4632.2010.04683.x
- Santos-Magadán S, González de Olano D, Bartolomé-Zavala B, et al. Adverse reactions to the processionary caterpillar: irritant or allergic mechanism? Contact Dermatitis. 2009;60:109-110. doi:10.1111/j.1600-0536.2008.01464.x
- González-Martín-Moro J, Contreras-Martín I, Castro-Rebollo M, et al. Focal cortical cataract due to caterpillar hair migration. Clin Exp Optom. 2019;102:89-90. doi:10.1111/cxo.12809
- Singh A, Behera UC, Agrawal H. Intra-lenticular caterpillar seta in ophthalmia nodosa. Eur J Ophthalmol. 2021;31:NP109-NP111. doi:10.1177/1120672119858899
- Schmitberger PA, Fernandes TC, Santos RC, et al. Probable chronic renal failure caused by Lonomia caterpillar envenomation. J Venom Anim Toxins Incl Trop Dis. 2013;19:14. doi:10.1186/1678-9199-19-14
- Moraes JA, Rodrigues G, Nascimento-Silva V, et al. Effects of Lonomia obliqua venom on vascular smooth muscle cells: contribution of NADPH oxidase-derived reactive oxygen species. Toxins (Basel). 2017;9:360. doi:10.3390/toxins9110360
- Bernardi L, Pinto AFM, Mendes E, et al. Lonomia obliqua bristle extract modulates Rac1 activation, membrane dynamics and cell adhesion properties. Toxicon. 2019;162:32-39. doi:10.1016/j.toxicon.2019.02.019
- Cabrera G, Lundberg U, Rodríguez-Ulloa A, et al. Protein content of the Hylesia metabus egg nest setae (Cramer [1775]) (Lepidoptera: Saturniidae) and its association with the parental investment for the reproductive success and lepidopterism. J Proteomics. 2017;150:183-200. doi:10.1016/j.jprot.2016.08.010
- Greene SC, Carey JM. Puss caterpillar envenomation: erucism mimicking appendicitis in a young child. Pediatr Emerg Care. 2020;36:E732-E734. doi:10.1097/PEC.0000000000001514
- Berger M, de Moraes JA, Beys-da-Silva WO, et al. Renal and vascular effects of kallikrein inhibition in a model of Lonomia obliqua venom-induced acute kidney injury. PLoS Negl Trop Dis. 2019;13:e0007197. doi:10.1371/journal.pntd.0007197
Causes of Lepidopterism
Caterpillars are wormlike organisms that serve as the larval stage of moths and butterflies, which belong to the order Lepidoptera. There are almost 165,000 discovered species, with 13,000 found in the United States.1,2 Roughly 150 species are known to have the potential to cause an adverse reaction in humans, with 50 of these in the United States.1Lepidopterism describes systemic and cutaneous reactions to moths, butterflies, and caterpillars; erucism describes strictly cutaneous reactions.1
Although the rate of lepidopterism is thought to be underreported because it often is self-limited and of a mild nature, a review found caterpillars to be the cause of roughly 2.2% of reported bites and stings annually.2 Cases increase in number with seasonal increases in caterpillars, which vary by region and species. For example, the Megalopyge opercularis (southern flannel moth) caterpillar was noted to have 2 peaks in a Texas-based study: 12% of reported stings occurred in July; 59% from October through November.3 In general, the likelihood of exposure increases during warmer months, and exposure is more common in people who work outdoors in a rural area or in a suburban area where there are many caterpillar-infested trees.4
Most cases of lepidopterism are caused by caterpillars, not by adult butterflies and moths, because the former have many tubular, or porous, hairlike structures called setae that are embedded in the integument. Setae were once thought to be connected to poison-secreting glandular cells, but current belief is that venomous caterpillars lack specialized gland cells and instead produce venom through secretory epithelial cells located above the integument.1 Venom accumulates in the hemolymph and is stored in the setae or other types of bristles, such as scoli (wartlike bumps that bear setae) or spines.5 With a large amount of chitin, bristles have a tendency to fracture and release venom upon contact.1 It is thought that some species of caterpillars formulate venom by ingesting toxins or toxin precursors from plants; for example, the tiger moth (family Arctiidae) is known to produce venom containing biogenic amines, pyrrolizidine, alkaloids, and cardiac glycosides obtained through food sources.5
Even if a caterpillar does not produce venom, its setae might embed into skin or mucous membranes and cause an adverse irritant reaction.1 Setae also might dislodge and be transported in the air to embed in objects—some remaining stable in the environment for longer than a year.2 In contrast to setae, spines are permanently fixed into the integument; for that reason, only direct contact with the caterpillar can result in an adverse reaction. Although it is mostly caterpillars that contain setae and spines, certain species of moths also might contain these structures or might acquire them as they emerge from the cocoon, which often contains incorporated setae.2
Reactions in Humans
Lepidopterism encompasses 3 principal reactions in humans: sting reaction, hypersensitivity reaction, and lonomism (a hemorrhagic diathesis produced by Lonomia caterpillars). The type and severity of the reaction depends on (1) the species of caterpillar or moth and (2) the individual patient.2 There are approximately 12 families of caterpillars, mainly of the moth variety, that can cause an adverse reaction in humans.1 Tables 1 and 2 list examples of species that cause each type of reaction.6



Chemicals and toxins contained in the poison of setae and spines vary by species of caterpillar. Numerous kinds have been isolated from different venoms,1,2 including several peptides, histamine, histamine-releasing substances, acetylcholine, phospholipase A, hyaluronidase, formic acid, proteins with trypsinlike activity, serine proteases such as kallikrein, and other enzymes with vasodegenerative and fibrinolytic properties

Stings: An Immediate Adverse Reaction—Depending on the venom, a sting might result in mild to severe burning pain, accompanied by welts, vesicles, and red papules or plaques.2 Figure 1 demonstrates a particularly mild sting from a caterpillar of the family Automeris, examples of which are seen in Figures 2 and 3 and eFigure 1. Components of the venom determine the mechanism of the sting and the pain that accompanies it. For example, a recent study demonstrated that the venom of the Latoia consocia caterpillar induces pain through the ion-channel receptor known as transient receptor potential vanilloid 1, which integrates and sends painful stimuli from the peripheral nervous system to the central nervous system.7 It is thought that a variety of ion channels are targets of the venom of caterpillars.




One of the most characteristic sting patterns is that of the caterpillar of family Megalopygidae (flannel moth)(eFigures 2 and 3). The stings of these caterpillars create a unique tram-track pattern of hemorrhagic macules or papules (Figure 4).4 A study found that 90% of reported M opercularis envenomations consist primarily of cutaneous symptoms, with 84% of those symptoms being irritation or pain; 45% a puncture or wound; 29% erythema; and 15% edema.3 Systemic findings can include headache, fever, adenopathy, nausea, vomiting, abdominal pain, and chest pain.4 Symptoms normally are self-limited, though they can last minutes or hours.



Hypersensitivity Reaction—Studies demonstrate that the symptoms of this reaction are a mixture of type I hypersensitivity, type IV hypersensitivity, and a foreign-body response.2 The specific hypersensitivity reaction depends on the venom and the exposed individual—most commonly including a combination of pruritic papules, urticarial wheals, flares, and dermatitis.2 A reaction that is a result of direct contact with the caterpillar or moth will appear on exposed areas; however, because setae embed in linens and clothing, they might cause a reaction anywhere on the body. Although usually self-limited, a hypersensitivity reaction might develop within minutes and can last for days or weeks.
Stings and hypersensitivity reactions to caterpillars and moths tend to lead to a nonspecific histologic presentation characterized by epidermal edema and a superficial perivascular lymphocytic infiltrate, often with eosinophils.6 After approximately 1 week, a foreign-body response to setae can lead to tuberculoid granulomas accompanied by neutrophils in the dermis and occasionally in subcutaneous tissues (Figures 5 and 6).8 If setae have not yet been removed, they also might be visible in skin scrapings.


Additional complications can accompany the hypersensitivity reaction to setae or spines. Type I hypersensitivity reactions can lead to severe reactions on second contact due to previously sensitized IgE antibodies. Although the first reaction appears mild, second contact might result in angioedema, wheezing, dyspnea, or anaphylaxis, or a combination of these findings.9 In addition, some patients who come in contact with Dendrolimus caterpillars might develop a condition known as dendrolimiasis, characterized by dermatitis in addition to arthritis or chondritis.6 The arthritis is normally monoarticular and can result in complete destruction of the joint. Pararamose, a condition with a similar presentation, is caused by the Brazilian moth Premolis semirufa.6
Contact of setae or spines with mucous membranes or inhalation of setae also might result in edema, dysphagia, dyspnea, drooling, rhinitis, or conjunctivitis, or a combination of these findings.6 In addition, setae can embed in the eye and cause an inflammatory reaction—ophthalmia nodosa—most commonly caused by caterpillars of the pine processionary moth (Thaumetopoea pityocampa) and characterized by immediate chemosis, which can progress to liquefactive necrosis and hypopyon, later developing into a granulomatous foreign-body response.2,10 The process is thought to be the result of a combination of the thaumetopoein toxin in the setae and an IgE-mediated response to other proteins.10
Due to their harpoon shape and forward-only motion, setae might migrate deeper, potentially even to the optic nerve.11 Because migration might take years and the barbed shape of setae does not always allow removal, some patients require lifetime monitoring with slit-lamp examination.Chronic problems, such as cataracts and persistent posterior uveitis, have been reported.10,11
Lonomism—One of the most serious (though rarest) reactions to caterpillars is lonomism, a condition caused by the caterpillars of Lonomia achelous and Lonomia obliqua moths. These caterpillars have a unique combination of toxins filling their branched spines, which ultimately leads to the same outcome: a hemorrhagic diathesis.
The toxin of L achelous comprises several proteases that degrade fibrin, fibrinogen, and factor XIII while activating prothrombin. In contrast, L obliqua poison causes a hemorrhagic diathesis by promoting a consumptive coagulopathy through enzymes that activate factor X and prothrombin.
With initial contact with either of these Lonomia caterpillars, the patient experiences severe pain accompanied by systemic symptoms, including headache, nausea, and vomiting. Shortly afterward, symptoms of a hemorrhagic diathesis manifest, including bleeding gums, hematuria, bleeding from prior wounds, and epistaxis.5 Serious complications of the hemorrhagic diathesis, such as hemorrhage of major organs, leads to death in 4% of patients.5 A reported case of a patient whose Lonomia caterpillar sting went unrecognized until a week after the accident ended with progression to stage V chronic renal disease.12
Recent research has focused on the specific mechanism of injury caused by Lonomia species. A study found that the venom of L obliqua causes cytoskeleton rearrangement and migration in vascular smooth muscle cells (VSMCs) by inducing formation of reactive oxygen species through activation of nicotinamide adenine dinucleotide phosphate oxidase.13 Thus, the venom directly contributes to the proinflammatory phenotype of endothelial cells seen following envenomation. The same study also demonstrated that elevated reactive oxygen species trigger extracellular signal-regulated kinase pathway activation in VSMCs, leading to cell proliferation, re-stenosis, and ischemia.13 This finding was confirmed by another study,14 which demonstrated an increase in Rac1, a signaling protein involved in the extracellular signal-regulated kinase pathway, in VSMCs upon exposure to L obliqua venom. These studies propose potential new targets for treatment to prevent vascular damage.
Reactions to Adult Organisms—Although it is more common for the caterpillar form of these organisms to cause an adverse reaction, the adult moth also might be capable of causing a similar reaction by retaining setae from the cocoon or by their own spines. The most notable example of this is female moths of the genus Hylesia, which possess spines attached to glands on the abdomen. The poison in these spines—a mixture of proteases and chitinase—causes a dermatitis known as Caripito itch—the name derived from a river port in Venezuela where this moth caused a memorable epidemic of moth-induced dermatitis.7,15 Caripito itch is known for intense pruritus that most commonly lasts days or weeks, possibly longer than 1 year.
Diagnostic Difficulties
The challenge of diagnosing a caterpillar- or moth-induced reaction in humans arises from (1) the lack of clinical history (the caterpillar might not be seen at all by the patient or the examiner) and (2) the similarity of these reactions to those with more common triggers.
When setae remain embedded in the skin or mucous membranes, skin scrapings allow accelerated diagnosis. On a skin scraping prepared with 20% potassium hydroxide, setae appear as tapered and barbed hairlike structures, which allows them to be distinguished from other similar-appearing but differently shaped structures, such as glass fibers.
When setae do not remain embedded in the skin or when the cause of the reaction is due to spines, the physician is left with a nonspecific histologic picture and a large differential diagnosis to be narrowed down based on the history and occasionally the pattern of the skin lesion.
A challenge in sting diagnosis is differentiating a caterpillar or moth sting from that of another organism. In certain cases, such as those of the family Megalopygidae, specific patterns of stings might assist in making the diagnosis. Hypersensitivity reactions are associated with a wider differential diagnosis, including irritant or allergic dermatitis from other causes, scabies, eczema, lichen planus, lichen simplex chronicus, seborrheic dermatitis, and tinea corporis, to name a few.6 Skin scrapings can be examined for other features, such as burrows in the case of scabies, to further narrow the differential.
Stings and hypersensitivity reactions lacking a proper history and associated with more severe systemic symptoms have caused misdiagnosis or led to a workup for the wrong condition; for example, the picture of abdominal pain, nausea, vomiting, tachycardia, leukocytosis, hypokalemia, and metabolic acidosis can simulate appendicitis.16 Upon discovery of a puss caterpillar sting in a patient, her symptoms resolved after treatment with ondansetron, morphine, and intravenous fluids.16
In lonomism, the diagnosis must be established by laboratory measurement of the fibrinogen level, clotting factors, prothrombin time, and activated partial thromboplastin time.4 The differential diagnosis associated with lonomism includes disseminated intravascular coagulation (DIC), snakebite, and a hereditary bleeding disorder.4 The combination of laboratory tests and an extensive medical history allows a diagnosis. Absence of a personal or family history of bleeding excludes a diagnosis of hereditary bleeding disorder, whereas the absence of known causes of DIC or thrombocytopenia allows DIC to be excluded from the differential.
Treatment Options and Prevention
Treatment—The first step is to remove any embedded setae from the skin or mucous membranes. The stepwise recommendation is to remove any constricted clothing, detach setae with adhesive tape, wash with soap and water, and dry without touching the skin.1 Any remaining setae can be removed with additional tape or forceps; setae tend to be fragile and are difficult to remove in their entirety.
Other than removal of the setae, skin reactions are treated symptomatically. Ice packs and isopropyl alcohol have been utilized to cool burning or stinging areas. Pain, pruritus, and inflammation have been alleviated with antihistamines and topical corticosteroids.1 When pain is severe, oral codeine or local injection of anesthetic can be used. For severe and persistent skin lesions, a course of an oral glucocorticoid can be administered. Intramuscular triamcinolone acetonide has been shown to treat pain, dermatitis, and subcutaneous nodules otherwise refractory to treatment.8
Antivenin specific for L obliqua exists to treat lonomism and is therefore effective only when lonomism is caused by that species. Lonomism caused by L achelous is treated with cryoprecipitate, purified fibrinogen, and antifibrinolytic drugs, such as aprotinin.6 Whole blood and fresh-frozen plasma have been noted to make hemorrhage worse when utilized to treat lonomism. Because the mechanism of action of the venom of Lonomia species is based, in part, on inducing a proinflammatory profile in endothelial cells, studies have demonstrated that inhibition of kallikrein might prevent vascular injury and thus prevent serious adverse effects, such as renal failure.17
Prevention—People should wear proper protective clothing when outdoors in potentially infested areas. Measures should be taken to ensure that linens and clothing are not left outside in areas where setae might be carried on the wind. Infestation control is necessary if the population of caterpillars reaches a high enough level.
Conclusion
Several species of caterpillars and moths cause adverse reactions in humans: stings, hypersensitivity reactions, and lonomism. Although most reactions are self-limited, some might have more serious effects, including organ failure and death. Mechanisms of injury vary by species of caterpillar, moth, and butterfly; current research is focused on further defining venom components and signaling pathways to isolate potential targets to aid in the diagnosis and treatment of lepidopterism.
Causes of Lepidopterism
Caterpillars are wormlike organisms that serve as the larval stage of moths and butterflies, which belong to the order Lepidoptera. There are almost 165,000 discovered species, with 13,000 found in the United States.1,2 Roughly 150 species are known to have the potential to cause an adverse reaction in humans, with 50 of these in the United States.1Lepidopterism describes systemic and cutaneous reactions to moths, butterflies, and caterpillars; erucism describes strictly cutaneous reactions.1
Although the rate of lepidopterism is thought to be underreported because it often is self-limited and of a mild nature, a review found caterpillars to be the cause of roughly 2.2% of reported bites and stings annually.2 Cases increase in number with seasonal increases in caterpillars, which vary by region and species. For example, the Megalopyge opercularis (southern flannel moth) caterpillar was noted to have 2 peaks in a Texas-based study: 12% of reported stings occurred in July; 59% from October through November.3 In general, the likelihood of exposure increases during warmer months, and exposure is more common in people who work outdoors in a rural area or in a suburban area where there are many caterpillar-infested trees.4
Most cases of lepidopterism are caused by caterpillars, not by adult butterflies and moths, because the former have many tubular, or porous, hairlike structures called setae that are embedded in the integument. Setae were once thought to be connected to poison-secreting glandular cells, but current belief is that venomous caterpillars lack specialized gland cells and instead produce venom through secretory epithelial cells located above the integument.1 Venom accumulates in the hemolymph and is stored in the setae or other types of bristles, such as scoli (wartlike bumps that bear setae) or spines.5 With a large amount of chitin, bristles have a tendency to fracture and release venom upon contact.1 It is thought that some species of caterpillars formulate venom by ingesting toxins or toxin precursors from plants; for example, the tiger moth (family Arctiidae) is known to produce venom containing biogenic amines, pyrrolizidine, alkaloids, and cardiac glycosides obtained through food sources.5
Even if a caterpillar does not produce venom, its setae might embed into skin or mucous membranes and cause an adverse irritant reaction.1 Setae also might dislodge and be transported in the air to embed in objects—some remaining stable in the environment for longer than a year.2 In contrast to setae, spines are permanently fixed into the integument; for that reason, only direct contact with the caterpillar can result in an adverse reaction. Although it is mostly caterpillars that contain setae and spines, certain species of moths also might contain these structures or might acquire them as they emerge from the cocoon, which often contains incorporated setae.2
Reactions in Humans
Lepidopterism encompasses 3 principal reactions in humans: sting reaction, hypersensitivity reaction, and lonomism (a hemorrhagic diathesis produced by Lonomia caterpillars). The type and severity of the reaction depends on (1) the species of caterpillar or moth and (2) the individual patient.2 There are approximately 12 families of caterpillars, mainly of the moth variety, that can cause an adverse reaction in humans.1 Tables 1 and 2 list examples of species that cause each type of reaction.6



Chemicals and toxins contained in the poison of setae and spines vary by species of caterpillar. Numerous kinds have been isolated from different venoms,1,2 including several peptides, histamine, histamine-releasing substances, acetylcholine, phospholipase A, hyaluronidase, formic acid, proteins with trypsinlike activity, serine proteases such as kallikrein, and other enzymes with vasodegenerative and fibrinolytic properties

Stings: An Immediate Adverse Reaction—Depending on the venom, a sting might result in mild to severe burning pain, accompanied by welts, vesicles, and red papules or plaques.2 Figure 1 demonstrates a particularly mild sting from a caterpillar of the family Automeris, examples of which are seen in Figures 2 and 3 and eFigure 1. Components of the venom determine the mechanism of the sting and the pain that accompanies it. For example, a recent study demonstrated that the venom of the Latoia consocia caterpillar induces pain through the ion-channel receptor known as transient receptor potential vanilloid 1, which integrates and sends painful stimuli from the peripheral nervous system to the central nervous system.7 It is thought that a variety of ion channels are targets of the venom of caterpillars.




One of the most characteristic sting patterns is that of the caterpillar of family Megalopygidae (flannel moth)(eFigures 2 and 3). The stings of these caterpillars create a unique tram-track pattern of hemorrhagic macules or papules (Figure 4).4 A study found that 90% of reported M opercularis envenomations consist primarily of cutaneous symptoms, with 84% of those symptoms being irritation or pain; 45% a puncture or wound; 29% erythema; and 15% edema.3 Systemic findings can include headache, fever, adenopathy, nausea, vomiting, abdominal pain, and chest pain.4 Symptoms normally are self-limited, though they can last minutes or hours.



Hypersensitivity Reaction—Studies demonstrate that the symptoms of this reaction are a mixture of type I hypersensitivity, type IV hypersensitivity, and a foreign-body response.2 The specific hypersensitivity reaction depends on the venom and the exposed individual—most commonly including a combination of pruritic papules, urticarial wheals, flares, and dermatitis.2 A reaction that is a result of direct contact with the caterpillar or moth will appear on exposed areas; however, because setae embed in linens and clothing, they might cause a reaction anywhere on the body. Although usually self-limited, a hypersensitivity reaction might develop within minutes and can last for days or weeks.
Stings and hypersensitivity reactions to caterpillars and moths tend to lead to a nonspecific histologic presentation characterized by epidermal edema and a superficial perivascular lymphocytic infiltrate, often with eosinophils.6 After approximately 1 week, a foreign-body response to setae can lead to tuberculoid granulomas accompanied by neutrophils in the dermis and occasionally in subcutaneous tissues (Figures 5 and 6).8 If setae have not yet been removed, they also might be visible in skin scrapings.


Additional complications can accompany the hypersensitivity reaction to setae or spines. Type I hypersensitivity reactions can lead to severe reactions on second contact due to previously sensitized IgE antibodies. Although the first reaction appears mild, second contact might result in angioedema, wheezing, dyspnea, or anaphylaxis, or a combination of these findings.9 In addition, some patients who come in contact with Dendrolimus caterpillars might develop a condition known as dendrolimiasis, characterized by dermatitis in addition to arthritis or chondritis.6 The arthritis is normally monoarticular and can result in complete destruction of the joint. Pararamose, a condition with a similar presentation, is caused by the Brazilian moth Premolis semirufa.6
Contact of setae or spines with mucous membranes or inhalation of setae also might result in edema, dysphagia, dyspnea, drooling, rhinitis, or conjunctivitis, or a combination of these findings.6 In addition, setae can embed in the eye and cause an inflammatory reaction—ophthalmia nodosa—most commonly caused by caterpillars of the pine processionary moth (Thaumetopoea pityocampa) and characterized by immediate chemosis, which can progress to liquefactive necrosis and hypopyon, later developing into a granulomatous foreign-body response.2,10 The process is thought to be the result of a combination of the thaumetopoein toxin in the setae and an IgE-mediated response to other proteins.10
Due to their harpoon shape and forward-only motion, setae might migrate deeper, potentially even to the optic nerve.11 Because migration might take years and the barbed shape of setae does not always allow removal, some patients require lifetime monitoring with slit-lamp examination.Chronic problems, such as cataracts and persistent posterior uveitis, have been reported.10,11
Lonomism—One of the most serious (though rarest) reactions to caterpillars is lonomism, a condition caused by the caterpillars of Lonomia achelous and Lonomia obliqua moths. These caterpillars have a unique combination of toxins filling their branched spines, which ultimately leads to the same outcome: a hemorrhagic diathesis.
The toxin of L achelous comprises several proteases that degrade fibrin, fibrinogen, and factor XIII while activating prothrombin. In contrast, L obliqua poison causes a hemorrhagic diathesis by promoting a consumptive coagulopathy through enzymes that activate factor X and prothrombin.
With initial contact with either of these Lonomia caterpillars, the patient experiences severe pain accompanied by systemic symptoms, including headache, nausea, and vomiting. Shortly afterward, symptoms of a hemorrhagic diathesis manifest, including bleeding gums, hematuria, bleeding from prior wounds, and epistaxis.5 Serious complications of the hemorrhagic diathesis, such as hemorrhage of major organs, leads to death in 4% of patients.5 A reported case of a patient whose Lonomia caterpillar sting went unrecognized until a week after the accident ended with progression to stage V chronic renal disease.12
Recent research has focused on the specific mechanism of injury caused by Lonomia species. A study found that the venom of L obliqua causes cytoskeleton rearrangement and migration in vascular smooth muscle cells (VSMCs) by inducing formation of reactive oxygen species through activation of nicotinamide adenine dinucleotide phosphate oxidase.13 Thus, the venom directly contributes to the proinflammatory phenotype of endothelial cells seen following envenomation. The same study also demonstrated that elevated reactive oxygen species trigger extracellular signal-regulated kinase pathway activation in VSMCs, leading to cell proliferation, re-stenosis, and ischemia.13 This finding was confirmed by another study,14 which demonstrated an increase in Rac1, a signaling protein involved in the extracellular signal-regulated kinase pathway, in VSMCs upon exposure to L obliqua venom. These studies propose potential new targets for treatment to prevent vascular damage.
Reactions to Adult Organisms—Although it is more common for the caterpillar form of these organisms to cause an adverse reaction, the adult moth also might be capable of causing a similar reaction by retaining setae from the cocoon or by their own spines. The most notable example of this is female moths of the genus Hylesia, which possess spines attached to glands on the abdomen. The poison in these spines—a mixture of proteases and chitinase—causes a dermatitis known as Caripito itch—the name derived from a river port in Venezuela where this moth caused a memorable epidemic of moth-induced dermatitis.7,15 Caripito itch is known for intense pruritus that most commonly lasts days or weeks, possibly longer than 1 year.
Diagnostic Difficulties
The challenge of diagnosing a caterpillar- or moth-induced reaction in humans arises from (1) the lack of clinical history (the caterpillar might not be seen at all by the patient or the examiner) and (2) the similarity of these reactions to those with more common triggers.
When setae remain embedded in the skin or mucous membranes, skin scrapings allow accelerated diagnosis. On a skin scraping prepared with 20% potassium hydroxide, setae appear as tapered and barbed hairlike structures, which allows them to be distinguished from other similar-appearing but differently shaped structures, such as glass fibers.
When setae do not remain embedded in the skin or when the cause of the reaction is due to spines, the physician is left with a nonspecific histologic picture and a large differential diagnosis to be narrowed down based on the history and occasionally the pattern of the skin lesion.
A challenge in sting diagnosis is differentiating a caterpillar or moth sting from that of another organism. In certain cases, such as those of the family Megalopygidae, specific patterns of stings might assist in making the diagnosis. Hypersensitivity reactions are associated with a wider differential diagnosis, including irritant or allergic dermatitis from other causes, scabies, eczema, lichen planus, lichen simplex chronicus, seborrheic dermatitis, and tinea corporis, to name a few.6 Skin scrapings can be examined for other features, such as burrows in the case of scabies, to further narrow the differential.
Stings and hypersensitivity reactions lacking a proper history and associated with more severe systemic symptoms have caused misdiagnosis or led to a workup for the wrong condition; for example, the picture of abdominal pain, nausea, vomiting, tachycardia, leukocytosis, hypokalemia, and metabolic acidosis can simulate appendicitis.16 Upon discovery of a puss caterpillar sting in a patient, her symptoms resolved after treatment with ondansetron, morphine, and intravenous fluids.16
In lonomism, the diagnosis must be established by laboratory measurement of the fibrinogen level, clotting factors, prothrombin time, and activated partial thromboplastin time.4 The differential diagnosis associated with lonomism includes disseminated intravascular coagulation (DIC), snakebite, and a hereditary bleeding disorder.4 The combination of laboratory tests and an extensive medical history allows a diagnosis. Absence of a personal or family history of bleeding excludes a diagnosis of hereditary bleeding disorder, whereas the absence of known causes of DIC or thrombocytopenia allows DIC to be excluded from the differential.
Treatment Options and Prevention
Treatment—The first step is to remove any embedded setae from the skin or mucous membranes. The stepwise recommendation is to remove any constricted clothing, detach setae with adhesive tape, wash with soap and water, and dry without touching the skin.1 Any remaining setae can be removed with additional tape or forceps; setae tend to be fragile and are difficult to remove in their entirety.
Other than removal of the setae, skin reactions are treated symptomatically. Ice packs and isopropyl alcohol have been utilized to cool burning or stinging areas. Pain, pruritus, and inflammation have been alleviated with antihistamines and topical corticosteroids.1 When pain is severe, oral codeine or local injection of anesthetic can be used. For severe and persistent skin lesions, a course of an oral glucocorticoid can be administered. Intramuscular triamcinolone acetonide has been shown to treat pain, dermatitis, and subcutaneous nodules otherwise refractory to treatment.8
Antivenin specific for L obliqua exists to treat lonomism and is therefore effective only when lonomism is caused by that species. Lonomism caused by L achelous is treated with cryoprecipitate, purified fibrinogen, and antifibrinolytic drugs, such as aprotinin.6 Whole blood and fresh-frozen plasma have been noted to make hemorrhage worse when utilized to treat lonomism. Because the mechanism of action of the venom of Lonomia species is based, in part, on inducing a proinflammatory profile in endothelial cells, studies have demonstrated that inhibition of kallikrein might prevent vascular injury and thus prevent serious adverse effects, such as renal failure.17
Prevention—People should wear proper protective clothing when outdoors in potentially infested areas. Measures should be taken to ensure that linens and clothing are not left outside in areas where setae might be carried on the wind. Infestation control is necessary if the population of caterpillars reaches a high enough level.
Conclusion
Several species of caterpillars and moths cause adverse reactions in humans: stings, hypersensitivity reactions, and lonomism. Although most reactions are self-limited, some might have more serious effects, including organ failure and death. Mechanisms of injury vary by species of caterpillar, moth, and butterfly; current research is focused on further defining venom components and signaling pathways to isolate potential targets to aid in the diagnosis and treatment of lepidopterism.
- Goldman BS, Bragg BN. Caterpillar and moth bites. Stat Pearls [Internet]. StatPearls Publishing. Updated August 3, 2021. Accessed November 4, 2021. https://www.ncbi.nlm.nih.gov/books/NBK539851/
- Hossler EW. Caterpillars and moths: part I. Dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:1-10. doi:10.1016/j.jaad.2009.08.060
- Forrester MB. Megalopyge opercularis caterpillar stings reported to Texas poison centers. Wilderness Environ Med. 2018;29:215-220. doi:10.1016/j.wem.2018.02.002
- Hossler EW. Lepidopterism: skin disorders secondary to caterpillars and moths. UpToDate website. Published October 20, 2021. Accessed November 18, 2021. https://www.uptodate.com/contents/lepidopterism-skin-disorders-secondary-to-caterpillars-and-moths
- Villas-Boas IM, Bonfá G, Tambourgi DV. Venomous caterpillars: from inoculation apparatus to venom composition and envenomation. Toxicon. 2018;153:39-52. doi:10.1016/j.toxicon.2018.08.007
- Hossler EW. Caterpillars and moths: part II. dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:13-28. doi:10.1016/j.jaad.2009.08.061
- Yao Z, Kamau PM, Han Y, et al. The Latoia consocia caterpillar induces pain by targeting nociceptive ion channel TRPV1. Toxins (Basel). 2019;11:695. doi:10.3390/toxins11120695
- Paniz-Mondolfi AE, Pérez-Alvarez AM, Lundberg U, et al. Cutaneous lepidopterism: dermatitis from contact with moths of Hylesia metabus (Cramer 1775) (Lepidoptera: Saturniidae), the causative agent of caripito itch. Int J Dermatol. 2011;50:535-541. doi:10.1111/j.1365-4632.2010.04683.x
- Santos-Magadán S, González de Olano D, Bartolomé-Zavala B, et al. Adverse reactions to the processionary caterpillar: irritant or allergic mechanism? Contact Dermatitis. 2009;60:109-110. doi:10.1111/j.1600-0536.2008.01464.x
- González-Martín-Moro J, Contreras-Martín I, Castro-Rebollo M, et al. Focal cortical cataract due to caterpillar hair migration. Clin Exp Optom. 2019;102:89-90. doi:10.1111/cxo.12809
- Singh A, Behera UC, Agrawal H. Intra-lenticular caterpillar seta in ophthalmia nodosa. Eur J Ophthalmol. 2021;31:NP109-NP111. doi:10.1177/1120672119858899
- Schmitberger PA, Fernandes TC, Santos RC, et al. Probable chronic renal failure caused by Lonomia caterpillar envenomation. J Venom Anim Toxins Incl Trop Dis. 2013;19:14. doi:10.1186/1678-9199-19-14
- Moraes JA, Rodrigues G, Nascimento-Silva V, et al. Effects of Lonomia obliqua venom on vascular smooth muscle cells: contribution of NADPH oxidase-derived reactive oxygen species. Toxins (Basel). 2017;9:360. doi:10.3390/toxins9110360
- Bernardi L, Pinto AFM, Mendes E, et al. Lonomia obliqua bristle extract modulates Rac1 activation, membrane dynamics and cell adhesion properties. Toxicon. 2019;162:32-39. doi:10.1016/j.toxicon.2019.02.019
- Cabrera G, Lundberg U, Rodríguez-Ulloa A, et al. Protein content of the Hylesia metabus egg nest setae (Cramer [1775]) (Lepidoptera: Saturniidae) and its association with the parental investment for the reproductive success and lepidopterism. J Proteomics. 2017;150:183-200. doi:10.1016/j.jprot.2016.08.010
- Greene SC, Carey JM. Puss caterpillar envenomation: erucism mimicking appendicitis in a young child. Pediatr Emerg Care. 2020;36:E732-E734. doi:10.1097/PEC.0000000000001514
- Berger M, de Moraes JA, Beys-da-Silva WO, et al. Renal and vascular effects of kallikrein inhibition in a model of Lonomia obliqua venom-induced acute kidney injury. PLoS Negl Trop Dis. 2019;13:e0007197. doi:10.1371/journal.pntd.0007197
- Goldman BS, Bragg BN. Caterpillar and moth bites. Stat Pearls [Internet]. StatPearls Publishing. Updated August 3, 2021. Accessed November 4, 2021. https://www.ncbi.nlm.nih.gov/books/NBK539851/
- Hossler EW. Caterpillars and moths: part I. Dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:1-10. doi:10.1016/j.jaad.2009.08.060
- Forrester MB. Megalopyge opercularis caterpillar stings reported to Texas poison centers. Wilderness Environ Med. 2018;29:215-220. doi:10.1016/j.wem.2018.02.002
- Hossler EW. Lepidopterism: skin disorders secondary to caterpillars and moths. UpToDate website. Published October 20, 2021. Accessed November 18, 2021. https://www.uptodate.com/contents/lepidopterism-skin-disorders-secondary-to-caterpillars-and-moths
- Villas-Boas IM, Bonfá G, Tambourgi DV. Venomous caterpillars: from inoculation apparatus to venom composition and envenomation. Toxicon. 2018;153:39-52. doi:10.1016/j.toxicon.2018.08.007
- Hossler EW. Caterpillars and moths: part II. dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:13-28. doi:10.1016/j.jaad.2009.08.061
- Yao Z, Kamau PM, Han Y, et al. The Latoia consocia caterpillar induces pain by targeting nociceptive ion channel TRPV1. Toxins (Basel). 2019;11:695. doi:10.3390/toxins11120695
- Paniz-Mondolfi AE, Pérez-Alvarez AM, Lundberg U, et al. Cutaneous lepidopterism: dermatitis from contact with moths of Hylesia metabus (Cramer 1775) (Lepidoptera: Saturniidae), the causative agent of caripito itch. Int J Dermatol. 2011;50:535-541. doi:10.1111/j.1365-4632.2010.04683.x
- Santos-Magadán S, González de Olano D, Bartolomé-Zavala B, et al. Adverse reactions to the processionary caterpillar: irritant or allergic mechanism? Contact Dermatitis. 2009;60:109-110. doi:10.1111/j.1600-0536.2008.01464.x
- González-Martín-Moro J, Contreras-Martín I, Castro-Rebollo M, et al. Focal cortical cataract due to caterpillar hair migration. Clin Exp Optom. 2019;102:89-90. doi:10.1111/cxo.12809
- Singh A, Behera UC, Agrawal H. Intra-lenticular caterpillar seta in ophthalmia nodosa. Eur J Ophthalmol. 2021;31:NP109-NP111. doi:10.1177/1120672119858899
- Schmitberger PA, Fernandes TC, Santos RC, et al. Probable chronic renal failure caused by Lonomia caterpillar envenomation. J Venom Anim Toxins Incl Trop Dis. 2013;19:14. doi:10.1186/1678-9199-19-14
- Moraes JA, Rodrigues G, Nascimento-Silva V, et al. Effects of Lonomia obliqua venom on vascular smooth muscle cells: contribution of NADPH oxidase-derived reactive oxygen species. Toxins (Basel). 2017;9:360. doi:10.3390/toxins9110360
- Bernardi L, Pinto AFM, Mendes E, et al. Lonomia obliqua bristle extract modulates Rac1 activation, membrane dynamics and cell adhesion properties. Toxicon. 2019;162:32-39. doi:10.1016/j.toxicon.2019.02.019
- Cabrera G, Lundberg U, Rodríguez-Ulloa A, et al. Protein content of the Hylesia metabus egg nest setae (Cramer [1775]) (Lepidoptera: Saturniidae) and its association with the parental investment for the reproductive success and lepidopterism. J Proteomics. 2017;150:183-200. doi:10.1016/j.jprot.2016.08.010
- Greene SC, Carey JM. Puss caterpillar envenomation: erucism mimicking appendicitis in a young child. Pediatr Emerg Care. 2020;36:E732-E734. doi:10.1097/PEC.0000000000001514
- Berger M, de Moraes JA, Beys-da-Silva WO, et al. Renal and vascular effects of kallikrein inhibition in a model of Lonomia obliqua venom-induced acute kidney injury. PLoS Negl Trop Dis. 2019;13:e0007197. doi:10.1371/journal.pntd.0007197
Practice Points
- Lepidopterism describes adverse reactions caused by the stings, hypersensitivity reactions, and lonomism (a hemorrhagic diathesis) of caterpillars, moths, and butterflies.
- Caterpillars can induce an adverse reaction by injecting venom stored in their bristles, inducing a foreign-body reaction to embedded bristles, or a combination of these mechanisms.
- A thorough history, skin scrapings, relevant examination of affected body parts (such as slit-lamp examination, in the case of eyes), and laboratory testing should be conducted to narrow the wide differential diagnosis associated with lepidopterism.
Contact allergens in medical devices: A cause for concern?
Despite the clinical value of medical devices, there is a potential for these products to cause adverse skin reactions in some patients. highlighting the possibility of a high prevalence of contact allergens in these devices.
“We found it important to publish these findings, because up until now no clear figures have been reported regarding this particular clinical problem,” said study author Olivier Aerts, MD, a researcher in the contact allergy unit at the University Hospital Antwerp, Belgium, in an interview with this news organization.
For the study, Dr. Aerts and colleagues conducted a retrospective analysis of medical device users with suspected allergic contact dermatitis. All patients had been patch tested at a tertiary European clinic between 2018 and 2020.
The cohort included patients who experienced suspected contact allergy from medical adhesives (n = 57), gloves (n = 38), topical and surface medical devices (n = 38), glucose sensors and insulin pumps (n = 74), and prostheses (n = 75). Other medical products associated with contact allergy in another 44 patients included surgical glues, face masks, compression stockings, condoms, and suture materials.
Overall, 326 patients had been patch-tested during the 30-month study period. Approximately 25.8% of all patients – including 299 adults and 27 children – were referred for contact allergy associated with medical devices.
Acrylates were the most frequently encountered contact allergens and were found in diabetes devices and medical adhesives. Potential skin sensitizers included colophonium-related substances, D-limonene, isothiazolinone derivatives, salicylates, and sulphites, all of which were identified across most products.
According to the investigators, many of the labels for the medical devices made no mention of the potential skin sensitizers, except in the cases of some topical and surface disinfectants. And many topical products are often marketed as medical devices rather than cosmetics, further complicating labeling issues, according to Dr. Aerts.
“What should be done to help any patient suffering from allergic contact due to medical devices is that these devices should be labeled with all their components, or at the very least with the potential skin sensitizers these may contain,” Dr. Aerts explained. He added that manufacturers should “establish more cooperation with physicians/dermatologists who evaluate such patients,” a cooperation that often exists with cosmetic companies.
Dr. Aerts noted that while it’s important for patch testers and dermatologists to be aware of the prevalence of allergic contact dermatitis in medical device users, companies producing these devices should also be aware of these potential issues. “Additionally, legislators/regulators should perhaps focus some more on the cutaneous side effects these products may provoke,” he said, “as this awareness may hopefully also serve as a stimulant to perform more clinical allergy research in this field.”
Leonard Bielory, MD, an allergist at Robert Wood Johnson University Hospital in Rahway, New Jersey, told this news organization that the findings are “alarming” and should heighten clinicians’ awareness of the possibility of allergic contact dermatitis among medical device users.
Dr. Bielory, who wasn’t involved in the research, noted that the findings from this study may not be entirely generalizable to the U.S., given the study was performed in Europe. “In contrast to other countries, the U.S. is very conscientious about allergic responses to items being used in hospitals,” he added, “or such that the issue here is that many of these things would be an adverse reaction, which you have to report.” He suggested that further research in this field is needed to determine the prevalence of possible skin sensitizers in products specifically developed and marketed in the U.S.
The study had no specific funding. Dr. Aerts and Dr. Bielory have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Despite the clinical value of medical devices, there is a potential for these products to cause adverse skin reactions in some patients. highlighting the possibility of a high prevalence of contact allergens in these devices.
“We found it important to publish these findings, because up until now no clear figures have been reported regarding this particular clinical problem,” said study author Olivier Aerts, MD, a researcher in the contact allergy unit at the University Hospital Antwerp, Belgium, in an interview with this news organization.
For the study, Dr. Aerts and colleagues conducted a retrospective analysis of medical device users with suspected allergic contact dermatitis. All patients had been patch tested at a tertiary European clinic between 2018 and 2020.
The cohort included patients who experienced suspected contact allergy from medical adhesives (n = 57), gloves (n = 38), topical and surface medical devices (n = 38), glucose sensors and insulin pumps (n = 74), and prostheses (n = 75). Other medical products associated with contact allergy in another 44 patients included surgical glues, face masks, compression stockings, condoms, and suture materials.
Overall, 326 patients had been patch-tested during the 30-month study period. Approximately 25.8% of all patients – including 299 adults and 27 children – were referred for contact allergy associated with medical devices.
Acrylates were the most frequently encountered contact allergens and were found in diabetes devices and medical adhesives. Potential skin sensitizers included colophonium-related substances, D-limonene, isothiazolinone derivatives, salicylates, and sulphites, all of which were identified across most products.
According to the investigators, many of the labels for the medical devices made no mention of the potential skin sensitizers, except in the cases of some topical and surface disinfectants. And many topical products are often marketed as medical devices rather than cosmetics, further complicating labeling issues, according to Dr. Aerts.
“What should be done to help any patient suffering from allergic contact due to medical devices is that these devices should be labeled with all their components, or at the very least with the potential skin sensitizers these may contain,” Dr. Aerts explained. He added that manufacturers should “establish more cooperation with physicians/dermatologists who evaluate such patients,” a cooperation that often exists with cosmetic companies.
Dr. Aerts noted that while it’s important for patch testers and dermatologists to be aware of the prevalence of allergic contact dermatitis in medical device users, companies producing these devices should also be aware of these potential issues. “Additionally, legislators/regulators should perhaps focus some more on the cutaneous side effects these products may provoke,” he said, “as this awareness may hopefully also serve as a stimulant to perform more clinical allergy research in this field.”
Leonard Bielory, MD, an allergist at Robert Wood Johnson University Hospital in Rahway, New Jersey, told this news organization that the findings are “alarming” and should heighten clinicians’ awareness of the possibility of allergic contact dermatitis among medical device users.
Dr. Bielory, who wasn’t involved in the research, noted that the findings from this study may not be entirely generalizable to the U.S., given the study was performed in Europe. “In contrast to other countries, the U.S. is very conscientious about allergic responses to items being used in hospitals,” he added, “or such that the issue here is that many of these things would be an adverse reaction, which you have to report.” He suggested that further research in this field is needed to determine the prevalence of possible skin sensitizers in products specifically developed and marketed in the U.S.
The study had no specific funding. Dr. Aerts and Dr. Bielory have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Despite the clinical value of medical devices, there is a potential for these products to cause adverse skin reactions in some patients. highlighting the possibility of a high prevalence of contact allergens in these devices.
“We found it important to publish these findings, because up until now no clear figures have been reported regarding this particular clinical problem,” said study author Olivier Aerts, MD, a researcher in the contact allergy unit at the University Hospital Antwerp, Belgium, in an interview with this news organization.
For the study, Dr. Aerts and colleagues conducted a retrospective analysis of medical device users with suspected allergic contact dermatitis. All patients had been patch tested at a tertiary European clinic between 2018 and 2020.
The cohort included patients who experienced suspected contact allergy from medical adhesives (n = 57), gloves (n = 38), topical and surface medical devices (n = 38), glucose sensors and insulin pumps (n = 74), and prostheses (n = 75). Other medical products associated with contact allergy in another 44 patients included surgical glues, face masks, compression stockings, condoms, and suture materials.
Overall, 326 patients had been patch-tested during the 30-month study period. Approximately 25.8% of all patients – including 299 adults and 27 children – were referred for contact allergy associated with medical devices.
Acrylates were the most frequently encountered contact allergens and were found in diabetes devices and medical adhesives. Potential skin sensitizers included colophonium-related substances, D-limonene, isothiazolinone derivatives, salicylates, and sulphites, all of which were identified across most products.
According to the investigators, many of the labels for the medical devices made no mention of the potential skin sensitizers, except in the cases of some topical and surface disinfectants. And many topical products are often marketed as medical devices rather than cosmetics, further complicating labeling issues, according to Dr. Aerts.
“What should be done to help any patient suffering from allergic contact due to medical devices is that these devices should be labeled with all their components, or at the very least with the potential skin sensitizers these may contain,” Dr. Aerts explained. He added that manufacturers should “establish more cooperation with physicians/dermatologists who evaluate such patients,” a cooperation that often exists with cosmetic companies.
Dr. Aerts noted that while it’s important for patch testers and dermatologists to be aware of the prevalence of allergic contact dermatitis in medical device users, companies producing these devices should also be aware of these potential issues. “Additionally, legislators/regulators should perhaps focus some more on the cutaneous side effects these products may provoke,” he said, “as this awareness may hopefully also serve as a stimulant to perform more clinical allergy research in this field.”
Leonard Bielory, MD, an allergist at Robert Wood Johnson University Hospital in Rahway, New Jersey, told this news organization that the findings are “alarming” and should heighten clinicians’ awareness of the possibility of allergic contact dermatitis among medical device users.
Dr. Bielory, who wasn’t involved in the research, noted that the findings from this study may not be entirely generalizable to the U.S., given the study was performed in Europe. “In contrast to other countries, the U.S. is very conscientious about allergic responses to items being used in hospitals,” he added, “or such that the issue here is that many of these things would be an adverse reaction, which you have to report.” He suggested that further research in this field is needed to determine the prevalence of possible skin sensitizers in products specifically developed and marketed in the U.S.
The study had no specific funding. Dr. Aerts and Dr. Bielory have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Contact Allergy to Topical Medicaments, Part 1: A Double-edged Sword
Topical medications frequently are prescribed in dermatology and provide the advantages of direct skin penetration and targeted application while typically sparing patients from systemic effects. Adverse cutaneous effects include allergic contact dermatitis (ACD), irritant contact dermatitis (ICD), photosensitivity, urticaria, hyperpigmentation or hypopigmentation, atrophy, periorificial dermatitis, and acneform eruptions. Allergic contact dermatitis can develop from the active drug or vehicle components.
Patients with medicament ACD often present with symptoms of pruritus and dermatitis at the site of topical application. They may express concern that the medication is no longer working or seems to be making things worse. Certain sites are more prone to developing medicament dermatitis, including the face, groin, and lower legs. Older adults may be more at risk. Other risk factors include pre-existing skin diseases such as stasis dermatitis, acne, psoriasis, atopic dermatitis, and genital dermatoses.1 A review of 14,911 patch-tested patients from a single referral clinic revealed that 17.4% had iatrogenic contact dermatitis, with the most common culprits being topical antibiotics, antiseptics, and steroids.2
In this 2-part series, we will focus on the active drug as a source of ACD. Part 1 explores ACD associated with acne and rosacea medications, antimicrobials, antihistamines, and topical pain preparations.
Acne and Rosacea Medications
Retinoids—Topical retinoids are first-line acne treatments that help normalize skin keratinization. Irritant contact dermatitis from retinoids is a well-known and common side effect. Although far less common than ICD, ACD from topical retinoid use has been reported.3,4 Reactions to tretinoin are most frequently reported in the literature compared to adapalene gel5 and tazarotene foam, which have lower potential for sensitization.6 Allergic contact dermatitis also has been reported from retinyl palmitate7,8 in cosmetic creams and from occupational exposure in settings of industrial vitamin A production.9 Both ICD and ACD from topical retinoids can present with pruritus, erythema, and scaling. Given this clinical overlap between ACD and ICD, patch testing is crucial in differentiating the underlying etiology of the dermatitis.
Benzoyl Peroxide—Benzoyl peroxide (BP) is another popular topical acne treatment that targets Cutibacterium acnes, a bacterium often implicated in the pathogenesis of acne vulgaris. Similar to retinoids, ICD is more common than ACD. Several cases of ACD to BP have been reported.10-14 Occasionally, honey-colored crusting associated with ACD to BP can mimic impetigo.10 Aside from use of BP as an acne treatment, other potential exposures to BP include bleached flour13 and orthopedic bone cement. Occupations at risk for potential BP exposure include dental technicians15 and those working in plastic manufacturing.
Brimonidine—Brimonidine tartrate is a selective α2-adrenergic agonist initially used to treat open-angle glaucoma and also is used as a topical treatment for rosacea. Allergic reactions to brimonidine eye drops may present with periorbital hyperpigmentation and pruritic bullous lesions.16 Case reports of topical brimonidine ACD have demonstrated mixed patch test results, with positive patch tests to Mirvaso (Galderma) as is but negative patch tests to pure brimonidine tartrate 0.33%.17,18 Ringuet and Houle19 reported the first known positive patch test reaction to pure topical brimonidine, testing with brimonidine tartrate 1% in petrolatum.20,21 Clinicians should be attuned to ACD to topical brimonidine in patients previously treated for glaucoma, as prior use of ophthalmic preparations may result in sensitization.18,20
Antimicrobials
Clindamycin—Clindamycin targets bacterial protein synthesis and is an effective adjunct in the treatment of acne. Despite its widespread and often long-term use, topical clindamycin is a weak sensitizer.22 To date, limited case reports on ACD to topical clindamycin exist.23-28 Rare clinical patterns of ACD to clindamycin include mimickers of irritant retinoid dermatitis, erythema multiforme, or pustular rosacea.25,26,29
Metronidazole—Metronidazole is a bactericidal agent that disrupts nucleic acid synthesis with additional anti-inflammatory properties used in the treatment of rosacea. Allergic contact dermatitis to topical metronidazole has been reported.30-34 In 2006, Beutner at al35 patch tested 215 patients using metronidazole gel 1%, which revealed no positive reactions to indicate contact sensitization. Similarly, Jappe et al36 found no positive reactions to metronidazole 2% in petrolatum in their prospective analysis of 78 rosacea patients, further highlighting the exceptionally low incidence of ACD. Cross-reaction with isothiazolinone, which shares structurally similar properties to metronidazole, has been speculated.31,34 One patient developed an acute reaction to metronidazole gel 0.75% within 24 hours of application, suggesting that isothiazolinone may act as a sensitizer, though this relationship has not been proven.31
Neomycin—Neomycin blocks bacterial protein synthesis and is available in both prescription and over-the-counter (OTC) formulations. It commonly is used to treat and prevent superficial wound infections as an OTC antibiotic and also has otic, ophthalmologic, gastroenterologic, urologic, and peritoneal formulations. It also can be used in the dental and veterinary fields and is present in some animal feeds and in trace amounts in some vaccines for humans. Neomycin is a common antibiotic contact allergen, and the most recently reported 2017-2018 North American Contact Dermatitis Group data cycle placed it at number 12 with 5.4% positivity.37 Co-reactions with bacitracin can occur, substantially limiting OTC topical antibiotic options for allergic patients. A safe alternative for patients with neomycin (and bacitracin and polymyxin) contact allergy is prescription mupirocin.
Bacitracin—Bacitracin interferes with peptidoglycan and cell-wall synthesis to treat superficial cutaneous infections. Similar to neomycin, it also can be found in OTC antibiotic ointments as well as in antibacterial bandages. There are several case reports of patients with both type IV delayed hypersensitivity (contact dermatitis) and type I anaphylactic reactions to bacitracin38-40; patch testers should be aware of this rare association. Bacitracin was positive in 5.5% of patch tested patients in the 2017-2018 North American Contact Dermatitis Group data cycle,37 and as with neomycin, bacitracin also is commonly patch tested in most screening patch test series.
Polymyxin—Polymyxin is a polypeptide topical antibiotic that is used to treat superficial wound infections and can be used in combination with neomycin and/or bacitracin. Historically, it is a less common antibiotic allergen; however, it is now frequently included in comprehensive patch test series, as the frequency of positive reactions seems to be increasing, probably due to polysensitization with neomycin and bacitracin.
Nystatin—Nystatin is an antifungal that binds to ergosterol and disrupts the cell wall. Cases exist of ACD to topical nystatin as well as systemic ACD from oral exposure, though both are quite rare. Authors have surmised that the overall low rates of ACD may be due to poor skin absorption of nystatin, which also can confound patch testing.41,42 For patients with suspected ACD to nystatin, repeat open application testing also can be performed to confirm allergy.
Imidazole Antifungals—Similar to nystatins, imidazole antifungals also work by disrupting the fungal cell wall. Imidazole antifungal preparations that have been reported to cause ACD include clotrimazole, miconazole, econazole, and isoconazole, and although cross-reactivity patterns have been described, they are not always reproducible with patch testing.43 In one reported case, tioconazole found in an antifungal nail lacquer triggered ACD involving not only the fingers and toes but also the trunk.44 Erythema multiforme–like reactions also have been described from topical use.45 Commercial patch test preparations of the most common imidazole allergens do exist. Nonimidazole antifungals remain a safe option for allergic patients.
Antihistamines
Antihistamines, or H1-receptor antagonists, are marketed to be applied topically for relief of pruritus associated with allergic cutaneous reactions. Ironically, they are known to be potent sensitizers themselves. There are 6 main chemical classes of antihistamines: phenothiazines, ethylenediamines, ethanolamines, alkylamines, piperazines, and piperidines. Goossens and Linsen46 patch tested 12,460 patients from 1978 to 1997 and found the most positive reactions to promethazine (phenothiazine)(n=12), followed by diphenhydramine (ethanolamine)(n=8) and clemizole (benzimidazole)(n=6). The authors also noted cross-reactions between diphenhydramine derivatives and between promethazine and chlorpromazine.46
Doxepin is a tricyclic antidepressant with antihistamine activity and is a well-documented sensitizer.47-52 Taylor et al47 evaluated 97 patients with chronic dermatoses, and patch testing revealed 17 (17.5%) positive reactions to doxepin cream, 13 (76.5%) of which were positive reactions to both the commercial cream and the active ingredient. Patch testing using doxepin dilution as low as 0.5% in petrolatum is sufficient to provoke a strong (++) allergic reaction.50,51 Early-onset ACD following the use of doxepin cream suggests the possibility of prior sensitization, perhaps with a structurally similar phenothiazine drug.51 A keen suspicion for ACD in patients using doxepin cream for longer than the recommended duration can help make the diagnosis.49,52
Topical Analgesics
Nonsteroidal Anti-inflammatory Drugs—Ketoprofen is one of the most frequent culprits of photoallergic contact dermatitis. Pruritic, papulovesicular, and bullous lesions typically develop acutely weeks after exposure. Prolonged photosensitivity is common and can last years after discontinuation of the nonsteroidal anti-inflammatory drug.53 Cases of cross-reactions and co-sensitization to structurally similar substances have been reported, including to benzophenone-related chemicals in sunscreen and aldehyde groups in fragrance mix.53,54
Diclofenac gel generally is well tolerated in the topical treatment of joint pain and inflammation. In the setting of ACD, patients typically present with dermatitis localized to the area of application.55 Immediate cessation and avoidance of topical diclofenac are crucial components of management. Although systemic contact dermatitis has been reported with oral diclofenac use,56 a recent report suggested that oral diclofenac may be well tolerated for some patients with topical ACD.57
Publications on bufexamac-induced ACD mainly consist of international reports, as this medication has been discontinued in the United States. Bufexamac is a highly sensitizing agent that can lead to severe polymorphic eruptions requiring treatment with prednisolone and even hospitalization.58 In one Australian case report, a mother developed an edematous, erythematous, papulovesicular eruption on the breast while breastfeeding her baby, who was being treated with bufexamac cream 5% for infantile eczema.59 Carprofen-induced photoallergic contact dermatitis is associated with occupational exposure in pharmaceutical workers.60,61 A few case reports on other nonsteroidal anti-inflammatory drugs, including etofenamate and aceclofenac, have been published.62,63
Compounded Medications—Compounded topical analgesics, which help to control pain via multiple combined effects, have gained increasing popularity in the management of chronic neuropathic pain disorders. Only a few recent retrospective studies assessing the efficacy and safety of these medications have mentioned suspected allergic cutaneous reactions.62,63 In 2015, Turrentine et al64 reported a case of ACD to cyclobenzaprine in a compound containing ketamine 10%, diclofenac 5%, baclofen 2%, bupivacaine 1%, cyclobenzaprine 2%, gabapentin 6%, ibuprofen 3%, and pentoxifylline 3% in a proprietary cream base. When patients present with suspected ACD to a compounded pain medication, obtaining individual components for patch testing is key to determining the allergic ingredient(s). We suspect that we will see a rise in reports of ACD as these topical compounds become readily adopted in clinical practices.
Patch Testing for Diagnosis
When patients present with symptoms concerning for ACD to medicaments, the astute clinician should promptly stop the suspected topical medication and consider patch testing. For common allergens such as neomycin, bacitracin, or ethylenediamine, commercial patch test preparations exist and should be used; however, for drugs that do not have a commercial patch test preparation, the patient’s product can be applied as is, keeping in mind that certain preparations (such as retinoids) can cause irritant patch test reactions, which may confound the reading. Alternatively, individual ingredients in the medication’s formulation can be requested from the manufacturer or a compounding pharmacy for targeted testing. Suggested concentrations for patch testing based on the literature and expert reference are listed in the Table. The authors (M.R., A.R.A.) frequently rely on an expert reference66 to determine ideal concentrations for patch testing. Referral to a specialized patch test clinic may be appropriate.
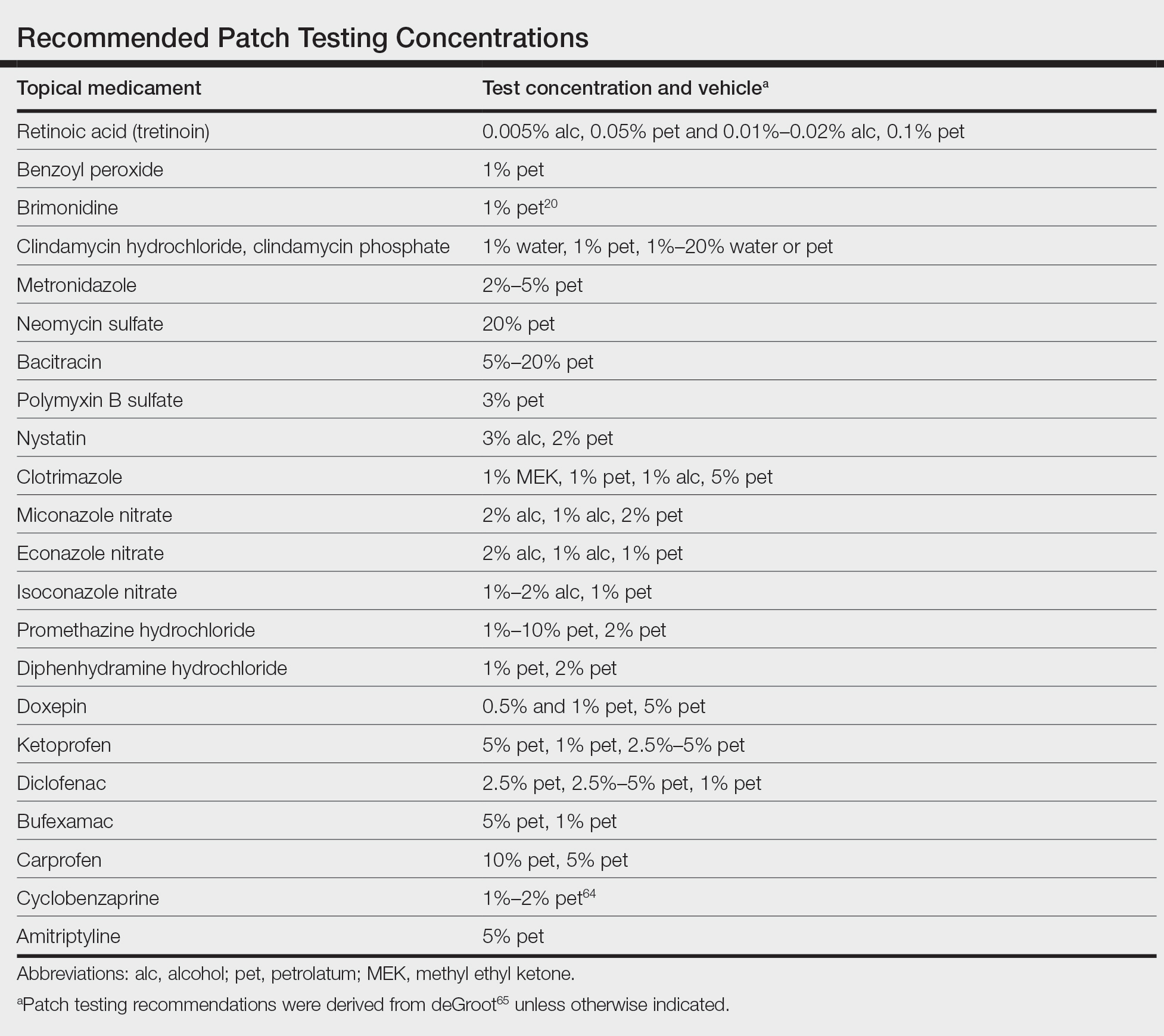
Final Interpretation
Although their intent is to heal, topical medicaments also can be a source of ACD. The astute clinician should consider ACD when topicals either no longer seem to help the patient or trigger new-onset dermatitis. Patch testing directly with the culprit medicament, or individual medication ingredients when needed, can lead to the diagnosis, though caution is advised. Stay tuned for part 2 of this series in which we will discuss ACD to topical steroids, immunomodulators, and anesthetic medications.
- Davis MD. Unusual patterns in contact dermatitis: medicaments. Dermatol Clin. 2009;27:289-297, vi. doi:10.1016/j.det.2009.05.003
- Gilissen L, Goossens A. Frequency and trends of contact allergy to and iatrogenic contact dermatitis caused by topical drugs over a 25-year period. Contact Dermatitis. 2016;75:290-302. doi:10.1111/cod.12621
- Balato N, Patruno C, Lembo G, et al. Allergic contact dermatitis from retinoic acid. Contact Dermatitis. 1995;32:51. doi:10.1111/j.1600-0536.1995.tb00846.x
- Berg JE, Bowman JP, Saenz AB. Cumulative irritation potential and contact sensitization potential of tazarotene foam 0.1% in 2 phase 1 patch studies. Cutis. 2012;90:206-211.
- Numata T, Jo R, Kobayashi Y, et al. Allergic contact dermatitis caused by adapalene. Contact Dermatitis. 2015;73:187-188. doi:10.1111/cod.12410
- Anderson A, Gebauer K. Periorbital allergic contact dermatitis resulting from topical retinoic acid use. Australas J Dermatol. 2014;55:152-153. doi:10.1111/ajd.12041
- Blondeel A. Contact allergy to vitamin A. Contact Dermatitis. 1984;11:191-192. doi:10.1111/j.1600-0536.1984.tb00976.x
- Manzano D, Aguirre A, Gardeazabal J, et al. Allergic contact dermatitis from tocopheryl acetate (vitamin E) and retinol palmitate (vitamin A) in a moisturizing cream. Contact Dermatitis. 1994;31:324. doi:10.1111/j.1600-0536.1994.tb02030.x
- Heidenheim M, Jemec GB. Occupational allergic contact dermatitis from vitamin A acetate. Contact Dermatitis. 1995;33:439. doi:10.1111/j.1600-0536.1995.tb02091.x
- Kim C, Craiglow BG, Watsky KL, et al. Allergic contact dermatitis to benzoyl peroxide resembling impetigo. Pediatr Dermatol. 2015;32:E161-E162. doi:10.1111/pde.12585
- Sandre M, Skotnicki-Grant S. A case of a paediatric patient with allergic contact dermatitis to benzoyl peroxide. J Cutan Med Surg. 2018;22:226-228. doi:10.1177/1203475417733462
- Corazza M, Amendolagine G, Musmeci D, et al. Sometimes even Dr Google is wrong: an unusual contact dermatitis caused by benzoyl peroxide. Contact Dermatitis. 2018;79:380-381. doi:10.1111/cod.13086
- Adelman M, Mohammad T, Kerr H. Allergic contact dermatitis due to benzoyl peroxide from an unlikely source. Dermatitis. 2019;30:230-231. doi:10.1097/DER.0000000000000470
- Gatica-Ortega ME, Pastor-Nieto MA. Allergic contact dermatitis to Glycyrrhiza inflata root extract in an anti-acne cosmetic product [published online April 28, 2021]. Contact Dermatitis. doi:10.1111/cod.13872
- Ockenfels HM, Uter W, Lessmann H, et al. Patch testing with benzoyl peroxide: reaction profile and interpretation of positive patch test reactions. Contact Dermatitis. 2009;61:209-216. doi:10.1111/j.1600-0536.2009.01603.x
- Sodhi PK, Verma L, Ratan J. Dermatological side effects of brimonidine: a report of three cases. J Dermatol. 2003;30:697-700. doi:10.1111/j.1346-8138.2003.tb00461.x
- Swanson LA, Warshaw EM. Allergic contact dermatitis to topical brimonidine tartrate gel 0.33% for treatment of rosacea. J Am Acad Dermatol. 2014;71:832-833. doi:10.1016/j.jaad.2014.05.073
- Bangsgaard N, Fischer LA, Zachariae C. Sensitization to and allergic contact dermatitis caused by Mirvaso(®)(brimonidine tartrate) for treatment of rosacea—2 cases. Contact Dermatitis. 2016;74:378-379. doi:10.1111/cod.12547
- Ringuet J, Houle MC. Case report: allergic contact dermatitis to topical brimonidine demonstrated with patch testing: insights on evaluation of brimonidine sensitization. J Cutan Med Surg. 2018;22:636-638. doi:10.1177/1203475418789020
- Cookson H, McFadden J, White J, et al. Allergic contact dermatitis caused by Mirvaso®, brimonidine tartrate gel 0.33%, a new topical treatment for rosaceal erythema. Contact Dermatitis. 2015;73:366-367. doi:10.1111/cod.12476
- Rajagopalan A, Rajagopalan B. Allergic contact dermatitis to topical brimonidine. Australas J Dermatol. 2015;56:235. doi:10.1111/ajd.12299
- Veraldi S, Brena M, Barbareschi M. Allergic contact dermatitis caused by topical antiacne drugs. Expert Rev Clin Pharmacol. 2015;8:377-381. doi:10.1586/17512433.2015.1046839
- Vejlstrup E, Menné T. Contact dermatitis from clindamycin. Contact Dermatitis. 1995;32:110. doi:10.1111/j.1600-0536.1995.tb00759.x
- García R, Galindo PA, Feo F, et al. Delayed allergic reactions to amoxycillin and clindamycin. Contact Dermatitis. 1996;35:116-117. doi:10.1111/j.1600-0536.1996.tb02312.x
- Muñoz D, Del Pozo MD, Audicana M, et al. Erythema-multiforme-like eruption from antibiotics of 3 different groups. Contact Dermatitis. 1996;34:227-228. doi:10.1111/j.1600-0536.1996.tb02187.x
- Romita P, Ettorre G, Corazza M, et al. Allergic contact dermatitis caused by clindamycin mimicking ‘retinoid flare.’ Contact Dermatitis. 2017;77:181-182. doi:10.1111/cod.12784
- Veraldi S, Guanziroli E, Ferrucci S, et al. Allergic contact dermatitis caused by clindamycin. Contact Dermatitis. 2019;80:68-69. doi:10.1111/cod.13133
- Voller LM, Kullberg SA, Warshaw EM. Axillary allergic contact dermatitis to topical clindamycin. Contact Dermatitis. 2020;82:313-314. doi:10.1111/cod.13465
- de Kort WJ, de Groot AC. Clindamycin allergy presenting as rosacea. Contact Dermatitis. 1989;20:72-73. doi:10.1111/j.1600-0536.1989.tb03108.x
- Vincenzi C, Lucente P, Ricci C, et al. Facial contact dermatitis due to metronidazole. Contact Dermatitis. 1997;36:116-117. doi:10.1111/j.1600-0536.1997.tb00434.x
- Wolf R, Orion E, Matz H. Co-existing sensitivity to metronidazole and isothiazolinone. Clin Exp Dermatol. 2003;28:506-507. doi:10.1046/j.1365-2230.2003.01364.x
- Madsen JT, Thormann J, Kerre S, et al. Allergic contact dermatitis to topical metronidazole—3 cases. Contact Dermatitis. 2007;56:364-366. doi:10.1111/j.1600-0536.2006.01064.x
- Fernández-Jorge B, Goday Buján J, Fernández-Torres R, et al. Concomitant allergic contact dermatitis from diphenhydramine and metronidazole. Contact Dermatitis. 2008;59:115-116. doi:10.1111/j.1600-0536.2008.01332.x
- Madsen JT, Lorentzen HF, Paulsen E. Contact sensitization to metronidazole from possible occupational exposure. Contact Dermatitis. 2009;60:117-118. doi:10.1111/j.1600-0536.2008.01490.x
- Beutner KR, Lemke S, Calvarese B. A look at the safety of metronidazole 1% gel: cumulative irritation, contact sensitization, phototoxicity, and photoallergy potential. Cutis. 2006;77(4 suppl):12-17.
- Jappe U, Schäfer T, Schnuch A, et al. Contact allergy in patients with rosacea: a clinic-based, prospective epidemiological study. J Eur Acad Dermatol Venereol. 2008;22:1208-1214. doi:10.1111/j.1468-3083.2008.02778.x
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group Patch Test Results: 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Comaish JS, Cunliffe WJ. Absorption of drugs from varicose ulcers: a cause of anaphylaxis. Br J Clin Pract. 1967;21:97-98.
- Roupe G, Strannegård O. Anaphylactic shock elicited by topical administration of bacitracin. Arch Dermatol. 1969;100:450-452.
- Farley M, Pak H, Carregal V, et al. Anaphylaxis to topically applied bacitracin. Am J Contact Dermat. 1995;6:28-31.
- Barranco R, Tornero P, de Barrio M, et al. Type IV hypersensitivity to oral nystatin. Contact Dermatitis. 2001;45:60. doi:10.1034/j.1600-0536.2001.045001060.x
- Cooper SM, Shaw S. Contact allergy to nystatin: an unusual allergen. Contact Dermatitis. 1999;41:120. doi:10.1111/j.1600-0536.1999.tb06254.x
- Dooms-Goossens A, Matura M, Drieghe J, et al. Contact allergy to imidazoles used as antimycotic agents. Contact Dermatitis. 1995;33:73-77. doi:10.1111/j.1600-0536.1995.tb00504.x
- Pérez-Mesonero R, Schneller-Pavelescu L, Ochando-Ibernón G, et al. Is tioconazole contact dermatitis still a concern? bringing allergic contact dermatitis caused by topical tioconazole back into the spotlight. Contact Dermatitis. 2019;80:168-169.
- Tang MM, Corti MA, Stirnimann R, et al. Severe cutaneous allergic reactions following topical antifungal therapy. Contact Dermatitis. 2013;68:56-57.
- Goossens A, Linsen G. Contact allergy to antihistamines is not common. Contact Dermatitis. 1998;39:38. doi:10.1111/j.1600-0536.1998.tb05817.x
- Taylor JS, Praditsuwan P, Handel D, et al. Allergic contact dermatitis from doxepin cream. one-year patch test clinic experience. Arch Dermatol. 1996;132:515-518.
- Bilbao I, Aguirre A, Vicente JM, et al. Allergic contact dermatitis due to 5% doxepin cream. Contact Dermatitis. 1996;35:254-255. doi:10.1111/j.1600-0536.1996.tb02374.x
- Shelley WB, Shelley ED, Talanin NY. Self-potentiating allergic contact dermatitis caused by doxepin hydrochloride cream. J Am Acad Dermatol. 1996;34:143-144. doi:10.1016/s0190-9622(96)90864-6
- Wakelin SH, Rycroft RJ. Allergic contact dermatitis from doxepin. Contact Dermatitis. 1999;40:214. doi:10.1111/j.1600-0536.1999.tb06037.x
- Horn HM, Tidman MJ, Aldridge RD. Allergic contact dermatitis due to doxepin cream in a patient with dystrophic epidermolysis bullosa. Contact Dermatitis. 2001;45:115. doi:10.1034/j.1600-0536.2001.045002115.x
- Bonnel RA, La Grenade L, Karwoski CB, et al. Allergic contact dermatitis from topical doxepin: Food and Drug Administration’s postmarketing surveillance experience. J Am Acad Dermatol. 2003;48:294-296. doi:10.1067/mjd.2003.46
- Devleeschouwer V, Roelandts R, Garmyn M, et al. Allergic and photoallergic contact dermatitis from ketoprofen: results of (photo) patch testing and follow-up of 42 patients. Contact Dermatitis. 2008;58:159-166. doi:10.1111/j.1600-0536.2007.01296.x
- Foti C, Bonamonte D, Conserva A, et al. Allergic and photoallergic contact dermatitis from ketoprofen: evaluation of cross-reactivities by a combination of photopatch testing and computerized conformational analysis. Curr Pharm Des. 2008;14:2833-2839. doi:10.2174/138161208786369696
- Gulin SJ, Chiriac A. Diclofenac-induced allergic contact dermatitis: a series of four patients. Drug Saf Case Rep. 2016;3:15. doi:10.1007/s40800-016-0039-3
- Lakshmi C, Srinivas CR. Systemic (allergic) contact dermatitis to diclofenac. Indian J Dermatol Venereol Leprol. 2011;77:536. doi:10.4103/0378-6323.82424
- Beutner C, Forkel S, Kreipe K, et al. Contact allergy to topical diclofenac with systemic tolerance [published online August 22, 2021]. Contact Dermatitis. doi:10.1111/cod.13961
- Pan Y, Nixon R. Allergic contact dermatitis to topical preparations of bufexamac. Australas J Dermatol. 2012;53:207-210. doi:10.1111/j.1440-0960.2012.00876.x
- Nakada T, Matsuzawa Y. Allergic contact dermatitis syndrome from bufexamac for nursing infant. Dermatitis. 2012;23:185-186. doi:10.1097/DER.0b013e318260d774
- Kerr AC, Muller F, Ferguson J, et al. Occupational carprofen photoallergic contact dermatitis. Br J Dermatol. 2008;159:1303-1308. doi:10.1111/j.1365-2133.2008.08847.x
- Kiely C, Murphy G. Photoallergic contact dermatitis caused by occupational exposure to the canine non-steroidal anti-inflammatory drug carprofen. Contact Dermatitis. 2010;63:364-365. doi:10.1111/j.1600-0536.2010.01820.x
- Somberg J, Molnar J. Retrospective evaluation on the analgesic activities of 2 compounded topical creams and voltaren gel in chronic noncancer pain. Am J Ther. 2015;22:342-349. doi:10.1097/MJT.0000000000000275
- Lee HG, Grossman SK, Valdes-Rodriguez R, et al. Topical ketamine-amitriptyline-lidocaine for chronic pruritus: a retrospective study assessing efficacy and tolerability. J Am Acad Dermatol. 2017;76:760-761. doi:10.1016/j.jaad.2016.10.030
- Turrentine JE, Marrazzo G, Cruz PD Jr. Novel use of patch testing in the first report of allergic contact dermatitis to cyclobenzaprine. Dermatitis. 2015;26:60-61. doi:10.1097/DER.0000000000000099
- de Groot A. Patch Testing. 3rd ed. acdegroot publishing; 2008.
- de Groot A. Patch Testing. 4th ed. acdegroot publishing; 2018.
Topical medications frequently are prescribed in dermatology and provide the advantages of direct skin penetration and targeted application while typically sparing patients from systemic effects. Adverse cutaneous effects include allergic contact dermatitis (ACD), irritant contact dermatitis (ICD), photosensitivity, urticaria, hyperpigmentation or hypopigmentation, atrophy, periorificial dermatitis, and acneform eruptions. Allergic contact dermatitis can develop from the active drug or vehicle components.
Patients with medicament ACD often present with symptoms of pruritus and dermatitis at the site of topical application. They may express concern that the medication is no longer working or seems to be making things worse. Certain sites are more prone to developing medicament dermatitis, including the face, groin, and lower legs. Older adults may be more at risk. Other risk factors include pre-existing skin diseases such as stasis dermatitis, acne, psoriasis, atopic dermatitis, and genital dermatoses.1 A review of 14,911 patch-tested patients from a single referral clinic revealed that 17.4% had iatrogenic contact dermatitis, with the most common culprits being topical antibiotics, antiseptics, and steroids.2
In this 2-part series, we will focus on the active drug as a source of ACD. Part 1 explores ACD associated with acne and rosacea medications, antimicrobials, antihistamines, and topical pain preparations.
Acne and Rosacea Medications
Retinoids—Topical retinoids are first-line acne treatments that help normalize skin keratinization. Irritant contact dermatitis from retinoids is a well-known and common side effect. Although far less common than ICD, ACD from topical retinoid use has been reported.3,4 Reactions to tretinoin are most frequently reported in the literature compared to adapalene gel5 and tazarotene foam, which have lower potential for sensitization.6 Allergic contact dermatitis also has been reported from retinyl palmitate7,8 in cosmetic creams and from occupational exposure in settings of industrial vitamin A production.9 Both ICD and ACD from topical retinoids can present with pruritus, erythema, and scaling. Given this clinical overlap between ACD and ICD, patch testing is crucial in differentiating the underlying etiology of the dermatitis.
Benzoyl Peroxide—Benzoyl peroxide (BP) is another popular topical acne treatment that targets Cutibacterium acnes, a bacterium often implicated in the pathogenesis of acne vulgaris. Similar to retinoids, ICD is more common than ACD. Several cases of ACD to BP have been reported.10-14 Occasionally, honey-colored crusting associated with ACD to BP can mimic impetigo.10 Aside from use of BP as an acne treatment, other potential exposures to BP include bleached flour13 and orthopedic bone cement. Occupations at risk for potential BP exposure include dental technicians15 and those working in plastic manufacturing.
Brimonidine—Brimonidine tartrate is a selective α2-adrenergic agonist initially used to treat open-angle glaucoma and also is used as a topical treatment for rosacea. Allergic reactions to brimonidine eye drops may present with periorbital hyperpigmentation and pruritic bullous lesions.16 Case reports of topical brimonidine ACD have demonstrated mixed patch test results, with positive patch tests to Mirvaso (Galderma) as is but negative patch tests to pure brimonidine tartrate 0.33%.17,18 Ringuet and Houle19 reported the first known positive patch test reaction to pure topical brimonidine, testing with brimonidine tartrate 1% in petrolatum.20,21 Clinicians should be attuned to ACD to topical brimonidine in patients previously treated for glaucoma, as prior use of ophthalmic preparations may result in sensitization.18,20
Antimicrobials
Clindamycin—Clindamycin targets bacterial protein synthesis and is an effective adjunct in the treatment of acne. Despite its widespread and often long-term use, topical clindamycin is a weak sensitizer.22 To date, limited case reports on ACD to topical clindamycin exist.23-28 Rare clinical patterns of ACD to clindamycin include mimickers of irritant retinoid dermatitis, erythema multiforme, or pustular rosacea.25,26,29
Metronidazole—Metronidazole is a bactericidal agent that disrupts nucleic acid synthesis with additional anti-inflammatory properties used in the treatment of rosacea. Allergic contact dermatitis to topical metronidazole has been reported.30-34 In 2006, Beutner at al35 patch tested 215 patients using metronidazole gel 1%, which revealed no positive reactions to indicate contact sensitization. Similarly, Jappe et al36 found no positive reactions to metronidazole 2% in petrolatum in their prospective analysis of 78 rosacea patients, further highlighting the exceptionally low incidence of ACD. Cross-reaction with isothiazolinone, which shares structurally similar properties to metronidazole, has been speculated.31,34 One patient developed an acute reaction to metronidazole gel 0.75% within 24 hours of application, suggesting that isothiazolinone may act as a sensitizer, though this relationship has not been proven.31
Neomycin—Neomycin blocks bacterial protein synthesis and is available in both prescription and over-the-counter (OTC) formulations. It commonly is used to treat and prevent superficial wound infections as an OTC antibiotic and also has otic, ophthalmologic, gastroenterologic, urologic, and peritoneal formulations. It also can be used in the dental and veterinary fields and is present in some animal feeds and in trace amounts in some vaccines for humans. Neomycin is a common antibiotic contact allergen, and the most recently reported 2017-2018 North American Contact Dermatitis Group data cycle placed it at number 12 with 5.4% positivity.37 Co-reactions with bacitracin can occur, substantially limiting OTC topical antibiotic options for allergic patients. A safe alternative for patients with neomycin (and bacitracin and polymyxin) contact allergy is prescription mupirocin.
Bacitracin—Bacitracin interferes with peptidoglycan and cell-wall synthesis to treat superficial cutaneous infections. Similar to neomycin, it also can be found in OTC antibiotic ointments as well as in antibacterial bandages. There are several case reports of patients with both type IV delayed hypersensitivity (contact dermatitis) and type I anaphylactic reactions to bacitracin38-40; patch testers should be aware of this rare association. Bacitracin was positive in 5.5% of patch tested patients in the 2017-2018 North American Contact Dermatitis Group data cycle,37 and as with neomycin, bacitracin also is commonly patch tested in most screening patch test series.
Polymyxin—Polymyxin is a polypeptide topical antibiotic that is used to treat superficial wound infections and can be used in combination with neomycin and/or bacitracin. Historically, it is a less common antibiotic allergen; however, it is now frequently included in comprehensive patch test series, as the frequency of positive reactions seems to be increasing, probably due to polysensitization with neomycin and bacitracin.
Nystatin—Nystatin is an antifungal that binds to ergosterol and disrupts the cell wall. Cases exist of ACD to topical nystatin as well as systemic ACD from oral exposure, though both are quite rare. Authors have surmised that the overall low rates of ACD may be due to poor skin absorption of nystatin, which also can confound patch testing.41,42 For patients with suspected ACD to nystatin, repeat open application testing also can be performed to confirm allergy.
Imidazole Antifungals—Similar to nystatins, imidazole antifungals also work by disrupting the fungal cell wall. Imidazole antifungal preparations that have been reported to cause ACD include clotrimazole, miconazole, econazole, and isoconazole, and although cross-reactivity patterns have been described, they are not always reproducible with patch testing.43 In one reported case, tioconazole found in an antifungal nail lacquer triggered ACD involving not only the fingers and toes but also the trunk.44 Erythema multiforme–like reactions also have been described from topical use.45 Commercial patch test preparations of the most common imidazole allergens do exist. Nonimidazole antifungals remain a safe option for allergic patients.
Antihistamines
Antihistamines, or H1-receptor antagonists, are marketed to be applied topically for relief of pruritus associated with allergic cutaneous reactions. Ironically, they are known to be potent sensitizers themselves. There are 6 main chemical classes of antihistamines: phenothiazines, ethylenediamines, ethanolamines, alkylamines, piperazines, and piperidines. Goossens and Linsen46 patch tested 12,460 patients from 1978 to 1997 and found the most positive reactions to promethazine (phenothiazine)(n=12), followed by diphenhydramine (ethanolamine)(n=8) and clemizole (benzimidazole)(n=6). The authors also noted cross-reactions between diphenhydramine derivatives and between promethazine and chlorpromazine.46
Doxepin is a tricyclic antidepressant with antihistamine activity and is a well-documented sensitizer.47-52 Taylor et al47 evaluated 97 patients with chronic dermatoses, and patch testing revealed 17 (17.5%) positive reactions to doxepin cream, 13 (76.5%) of which were positive reactions to both the commercial cream and the active ingredient. Patch testing using doxepin dilution as low as 0.5% in petrolatum is sufficient to provoke a strong (++) allergic reaction.50,51 Early-onset ACD following the use of doxepin cream suggests the possibility of prior sensitization, perhaps with a structurally similar phenothiazine drug.51 A keen suspicion for ACD in patients using doxepin cream for longer than the recommended duration can help make the diagnosis.49,52
Topical Analgesics
Nonsteroidal Anti-inflammatory Drugs—Ketoprofen is one of the most frequent culprits of photoallergic contact dermatitis. Pruritic, papulovesicular, and bullous lesions typically develop acutely weeks after exposure. Prolonged photosensitivity is common and can last years after discontinuation of the nonsteroidal anti-inflammatory drug.53 Cases of cross-reactions and co-sensitization to structurally similar substances have been reported, including to benzophenone-related chemicals in sunscreen and aldehyde groups in fragrance mix.53,54
Diclofenac gel generally is well tolerated in the topical treatment of joint pain and inflammation. In the setting of ACD, patients typically present with dermatitis localized to the area of application.55 Immediate cessation and avoidance of topical diclofenac are crucial components of management. Although systemic contact dermatitis has been reported with oral diclofenac use,56 a recent report suggested that oral diclofenac may be well tolerated for some patients with topical ACD.57
Publications on bufexamac-induced ACD mainly consist of international reports, as this medication has been discontinued in the United States. Bufexamac is a highly sensitizing agent that can lead to severe polymorphic eruptions requiring treatment with prednisolone and even hospitalization.58 In one Australian case report, a mother developed an edematous, erythematous, papulovesicular eruption on the breast while breastfeeding her baby, who was being treated with bufexamac cream 5% for infantile eczema.59 Carprofen-induced photoallergic contact dermatitis is associated with occupational exposure in pharmaceutical workers.60,61 A few case reports on other nonsteroidal anti-inflammatory drugs, including etofenamate and aceclofenac, have been published.62,63
Compounded Medications—Compounded topical analgesics, which help to control pain via multiple combined effects, have gained increasing popularity in the management of chronic neuropathic pain disorders. Only a few recent retrospective studies assessing the efficacy and safety of these medications have mentioned suspected allergic cutaneous reactions.62,63 In 2015, Turrentine et al64 reported a case of ACD to cyclobenzaprine in a compound containing ketamine 10%, diclofenac 5%, baclofen 2%, bupivacaine 1%, cyclobenzaprine 2%, gabapentin 6%, ibuprofen 3%, and pentoxifylline 3% in a proprietary cream base. When patients present with suspected ACD to a compounded pain medication, obtaining individual components for patch testing is key to determining the allergic ingredient(s). We suspect that we will see a rise in reports of ACD as these topical compounds become readily adopted in clinical practices.
Patch Testing for Diagnosis
When patients present with symptoms concerning for ACD to medicaments, the astute clinician should promptly stop the suspected topical medication and consider patch testing. For common allergens such as neomycin, bacitracin, or ethylenediamine, commercial patch test preparations exist and should be used; however, for drugs that do not have a commercial patch test preparation, the patient’s product can be applied as is, keeping in mind that certain preparations (such as retinoids) can cause irritant patch test reactions, which may confound the reading. Alternatively, individual ingredients in the medication’s formulation can be requested from the manufacturer or a compounding pharmacy for targeted testing. Suggested concentrations for patch testing based on the literature and expert reference are listed in the Table. The authors (M.R., A.R.A.) frequently rely on an expert reference66 to determine ideal concentrations for patch testing. Referral to a specialized patch test clinic may be appropriate.

Final Interpretation
Although their intent is to heal, topical medicaments also can be a source of ACD. The astute clinician should consider ACD when topicals either no longer seem to help the patient or trigger new-onset dermatitis. Patch testing directly with the culprit medicament, or individual medication ingredients when needed, can lead to the diagnosis, though caution is advised. Stay tuned for part 2 of this series in which we will discuss ACD to topical steroids, immunomodulators, and anesthetic medications.
Topical medications frequently are prescribed in dermatology and provide the advantages of direct skin penetration and targeted application while typically sparing patients from systemic effects. Adverse cutaneous effects include allergic contact dermatitis (ACD), irritant contact dermatitis (ICD), photosensitivity, urticaria, hyperpigmentation or hypopigmentation, atrophy, periorificial dermatitis, and acneform eruptions. Allergic contact dermatitis can develop from the active drug or vehicle components.
Patients with medicament ACD often present with symptoms of pruritus and dermatitis at the site of topical application. They may express concern that the medication is no longer working or seems to be making things worse. Certain sites are more prone to developing medicament dermatitis, including the face, groin, and lower legs. Older adults may be more at risk. Other risk factors include pre-existing skin diseases such as stasis dermatitis, acne, psoriasis, atopic dermatitis, and genital dermatoses.1 A review of 14,911 patch-tested patients from a single referral clinic revealed that 17.4% had iatrogenic contact dermatitis, with the most common culprits being topical antibiotics, antiseptics, and steroids.2
In this 2-part series, we will focus on the active drug as a source of ACD. Part 1 explores ACD associated with acne and rosacea medications, antimicrobials, antihistamines, and topical pain preparations.
Acne and Rosacea Medications
Retinoids—Topical retinoids are first-line acne treatments that help normalize skin keratinization. Irritant contact dermatitis from retinoids is a well-known and common side effect. Although far less common than ICD, ACD from topical retinoid use has been reported.3,4 Reactions to tretinoin are most frequently reported in the literature compared to adapalene gel5 and tazarotene foam, which have lower potential for sensitization.6 Allergic contact dermatitis also has been reported from retinyl palmitate7,8 in cosmetic creams and from occupational exposure in settings of industrial vitamin A production.9 Both ICD and ACD from topical retinoids can present with pruritus, erythema, and scaling. Given this clinical overlap between ACD and ICD, patch testing is crucial in differentiating the underlying etiology of the dermatitis.
Benzoyl Peroxide—Benzoyl peroxide (BP) is another popular topical acne treatment that targets Cutibacterium acnes, a bacterium often implicated in the pathogenesis of acne vulgaris. Similar to retinoids, ICD is more common than ACD. Several cases of ACD to BP have been reported.10-14 Occasionally, honey-colored crusting associated with ACD to BP can mimic impetigo.10 Aside from use of BP as an acne treatment, other potential exposures to BP include bleached flour13 and orthopedic bone cement. Occupations at risk for potential BP exposure include dental technicians15 and those working in plastic manufacturing.
Brimonidine—Brimonidine tartrate is a selective α2-adrenergic agonist initially used to treat open-angle glaucoma and also is used as a topical treatment for rosacea. Allergic reactions to brimonidine eye drops may present with periorbital hyperpigmentation and pruritic bullous lesions.16 Case reports of topical brimonidine ACD have demonstrated mixed patch test results, with positive patch tests to Mirvaso (Galderma) as is but negative patch tests to pure brimonidine tartrate 0.33%.17,18 Ringuet and Houle19 reported the first known positive patch test reaction to pure topical brimonidine, testing with brimonidine tartrate 1% in petrolatum.20,21 Clinicians should be attuned to ACD to topical brimonidine in patients previously treated for glaucoma, as prior use of ophthalmic preparations may result in sensitization.18,20
Antimicrobials
Clindamycin—Clindamycin targets bacterial protein synthesis and is an effective adjunct in the treatment of acne. Despite its widespread and often long-term use, topical clindamycin is a weak sensitizer.22 To date, limited case reports on ACD to topical clindamycin exist.23-28 Rare clinical patterns of ACD to clindamycin include mimickers of irritant retinoid dermatitis, erythema multiforme, or pustular rosacea.25,26,29
Metronidazole—Metronidazole is a bactericidal agent that disrupts nucleic acid synthesis with additional anti-inflammatory properties used in the treatment of rosacea. Allergic contact dermatitis to topical metronidazole has been reported.30-34 In 2006, Beutner at al35 patch tested 215 patients using metronidazole gel 1%, which revealed no positive reactions to indicate contact sensitization. Similarly, Jappe et al36 found no positive reactions to metronidazole 2% in petrolatum in their prospective analysis of 78 rosacea patients, further highlighting the exceptionally low incidence of ACD. Cross-reaction with isothiazolinone, which shares structurally similar properties to metronidazole, has been speculated.31,34 One patient developed an acute reaction to metronidazole gel 0.75% within 24 hours of application, suggesting that isothiazolinone may act as a sensitizer, though this relationship has not been proven.31
Neomycin—Neomycin blocks bacterial protein synthesis and is available in both prescription and over-the-counter (OTC) formulations. It commonly is used to treat and prevent superficial wound infections as an OTC antibiotic and also has otic, ophthalmologic, gastroenterologic, urologic, and peritoneal formulations. It also can be used in the dental and veterinary fields and is present in some animal feeds and in trace amounts in some vaccines for humans. Neomycin is a common antibiotic contact allergen, and the most recently reported 2017-2018 North American Contact Dermatitis Group data cycle placed it at number 12 with 5.4% positivity.37 Co-reactions with bacitracin can occur, substantially limiting OTC topical antibiotic options for allergic patients. A safe alternative for patients with neomycin (and bacitracin and polymyxin) contact allergy is prescription mupirocin.
Bacitracin—Bacitracin interferes with peptidoglycan and cell-wall synthesis to treat superficial cutaneous infections. Similar to neomycin, it also can be found in OTC antibiotic ointments as well as in antibacterial bandages. There are several case reports of patients with both type IV delayed hypersensitivity (contact dermatitis) and type I anaphylactic reactions to bacitracin38-40; patch testers should be aware of this rare association. Bacitracin was positive in 5.5% of patch tested patients in the 2017-2018 North American Contact Dermatitis Group data cycle,37 and as with neomycin, bacitracin also is commonly patch tested in most screening patch test series.
Polymyxin—Polymyxin is a polypeptide topical antibiotic that is used to treat superficial wound infections and can be used in combination with neomycin and/or bacitracin. Historically, it is a less common antibiotic allergen; however, it is now frequently included in comprehensive patch test series, as the frequency of positive reactions seems to be increasing, probably due to polysensitization with neomycin and bacitracin.
Nystatin—Nystatin is an antifungal that binds to ergosterol and disrupts the cell wall. Cases exist of ACD to topical nystatin as well as systemic ACD from oral exposure, though both are quite rare. Authors have surmised that the overall low rates of ACD may be due to poor skin absorption of nystatin, which also can confound patch testing.41,42 For patients with suspected ACD to nystatin, repeat open application testing also can be performed to confirm allergy.
Imidazole Antifungals—Similar to nystatins, imidazole antifungals also work by disrupting the fungal cell wall. Imidazole antifungal preparations that have been reported to cause ACD include clotrimazole, miconazole, econazole, and isoconazole, and although cross-reactivity patterns have been described, they are not always reproducible with patch testing.43 In one reported case, tioconazole found in an antifungal nail lacquer triggered ACD involving not only the fingers and toes but also the trunk.44 Erythema multiforme–like reactions also have been described from topical use.45 Commercial patch test preparations of the most common imidazole allergens do exist. Nonimidazole antifungals remain a safe option for allergic patients.
Antihistamines
Antihistamines, or H1-receptor antagonists, are marketed to be applied topically for relief of pruritus associated with allergic cutaneous reactions. Ironically, they are known to be potent sensitizers themselves. There are 6 main chemical classes of antihistamines: phenothiazines, ethylenediamines, ethanolamines, alkylamines, piperazines, and piperidines. Goossens and Linsen46 patch tested 12,460 patients from 1978 to 1997 and found the most positive reactions to promethazine (phenothiazine)(n=12), followed by diphenhydramine (ethanolamine)(n=8) and clemizole (benzimidazole)(n=6). The authors also noted cross-reactions between diphenhydramine derivatives and between promethazine and chlorpromazine.46
Doxepin is a tricyclic antidepressant with antihistamine activity and is a well-documented sensitizer.47-52 Taylor et al47 evaluated 97 patients with chronic dermatoses, and patch testing revealed 17 (17.5%) positive reactions to doxepin cream, 13 (76.5%) of which were positive reactions to both the commercial cream and the active ingredient. Patch testing using doxepin dilution as low as 0.5% in petrolatum is sufficient to provoke a strong (++) allergic reaction.50,51 Early-onset ACD following the use of doxepin cream suggests the possibility of prior sensitization, perhaps with a structurally similar phenothiazine drug.51 A keen suspicion for ACD in patients using doxepin cream for longer than the recommended duration can help make the diagnosis.49,52
Topical Analgesics
Nonsteroidal Anti-inflammatory Drugs—Ketoprofen is one of the most frequent culprits of photoallergic contact dermatitis. Pruritic, papulovesicular, and bullous lesions typically develop acutely weeks after exposure. Prolonged photosensitivity is common and can last years after discontinuation of the nonsteroidal anti-inflammatory drug.53 Cases of cross-reactions and co-sensitization to structurally similar substances have been reported, including to benzophenone-related chemicals in sunscreen and aldehyde groups in fragrance mix.53,54
Diclofenac gel generally is well tolerated in the topical treatment of joint pain and inflammation. In the setting of ACD, patients typically present with dermatitis localized to the area of application.55 Immediate cessation and avoidance of topical diclofenac are crucial components of management. Although systemic contact dermatitis has been reported with oral diclofenac use,56 a recent report suggested that oral diclofenac may be well tolerated for some patients with topical ACD.57
Publications on bufexamac-induced ACD mainly consist of international reports, as this medication has been discontinued in the United States. Bufexamac is a highly sensitizing agent that can lead to severe polymorphic eruptions requiring treatment with prednisolone and even hospitalization.58 In one Australian case report, a mother developed an edematous, erythematous, papulovesicular eruption on the breast while breastfeeding her baby, who was being treated with bufexamac cream 5% for infantile eczema.59 Carprofen-induced photoallergic contact dermatitis is associated with occupational exposure in pharmaceutical workers.60,61 A few case reports on other nonsteroidal anti-inflammatory drugs, including etofenamate and aceclofenac, have been published.62,63
Compounded Medications—Compounded topical analgesics, which help to control pain via multiple combined effects, have gained increasing popularity in the management of chronic neuropathic pain disorders. Only a few recent retrospective studies assessing the efficacy and safety of these medications have mentioned suspected allergic cutaneous reactions.62,63 In 2015, Turrentine et al64 reported a case of ACD to cyclobenzaprine in a compound containing ketamine 10%, diclofenac 5%, baclofen 2%, bupivacaine 1%, cyclobenzaprine 2%, gabapentin 6%, ibuprofen 3%, and pentoxifylline 3% in a proprietary cream base. When patients present with suspected ACD to a compounded pain medication, obtaining individual components for patch testing is key to determining the allergic ingredient(s). We suspect that we will see a rise in reports of ACD as these topical compounds become readily adopted in clinical practices.
Patch Testing for Diagnosis
When patients present with symptoms concerning for ACD to medicaments, the astute clinician should promptly stop the suspected topical medication and consider patch testing. For common allergens such as neomycin, bacitracin, or ethylenediamine, commercial patch test preparations exist and should be used; however, for drugs that do not have a commercial patch test preparation, the patient’s product can be applied as is, keeping in mind that certain preparations (such as retinoids) can cause irritant patch test reactions, which may confound the reading. Alternatively, individual ingredients in the medication’s formulation can be requested from the manufacturer or a compounding pharmacy for targeted testing. Suggested concentrations for patch testing based on the literature and expert reference are listed in the Table. The authors (M.R., A.R.A.) frequently rely on an expert reference66 to determine ideal concentrations for patch testing. Referral to a specialized patch test clinic may be appropriate.

Final Interpretation
Although their intent is to heal, topical medicaments also can be a source of ACD. The astute clinician should consider ACD when topicals either no longer seem to help the patient or trigger new-onset dermatitis. Patch testing directly with the culprit medicament, or individual medication ingredients when needed, can lead to the diagnosis, though caution is advised. Stay tuned for part 2 of this series in which we will discuss ACD to topical steroids, immunomodulators, and anesthetic medications.
- Davis MD. Unusual patterns in contact dermatitis: medicaments. Dermatol Clin. 2009;27:289-297, vi. doi:10.1016/j.det.2009.05.003
- Gilissen L, Goossens A. Frequency and trends of contact allergy to and iatrogenic contact dermatitis caused by topical drugs over a 25-year period. Contact Dermatitis. 2016;75:290-302. doi:10.1111/cod.12621
- Balato N, Patruno C, Lembo G, et al. Allergic contact dermatitis from retinoic acid. Contact Dermatitis. 1995;32:51. doi:10.1111/j.1600-0536.1995.tb00846.x
- Berg JE, Bowman JP, Saenz AB. Cumulative irritation potential and contact sensitization potential of tazarotene foam 0.1% in 2 phase 1 patch studies. Cutis. 2012;90:206-211.
- Numata T, Jo R, Kobayashi Y, et al. Allergic contact dermatitis caused by adapalene. Contact Dermatitis. 2015;73:187-188. doi:10.1111/cod.12410
- Anderson A, Gebauer K. Periorbital allergic contact dermatitis resulting from topical retinoic acid use. Australas J Dermatol. 2014;55:152-153. doi:10.1111/ajd.12041
- Blondeel A. Contact allergy to vitamin A. Contact Dermatitis. 1984;11:191-192. doi:10.1111/j.1600-0536.1984.tb00976.x
- Manzano D, Aguirre A, Gardeazabal J, et al. Allergic contact dermatitis from tocopheryl acetate (vitamin E) and retinol palmitate (vitamin A) in a moisturizing cream. Contact Dermatitis. 1994;31:324. doi:10.1111/j.1600-0536.1994.tb02030.x
- Heidenheim M, Jemec GB. Occupational allergic contact dermatitis from vitamin A acetate. Contact Dermatitis. 1995;33:439. doi:10.1111/j.1600-0536.1995.tb02091.x
- Kim C, Craiglow BG, Watsky KL, et al. Allergic contact dermatitis to benzoyl peroxide resembling impetigo. Pediatr Dermatol. 2015;32:E161-E162. doi:10.1111/pde.12585
- Sandre M, Skotnicki-Grant S. A case of a paediatric patient with allergic contact dermatitis to benzoyl peroxide. J Cutan Med Surg. 2018;22:226-228. doi:10.1177/1203475417733462
- Corazza M, Amendolagine G, Musmeci D, et al. Sometimes even Dr Google is wrong: an unusual contact dermatitis caused by benzoyl peroxide. Contact Dermatitis. 2018;79:380-381. doi:10.1111/cod.13086
- Adelman M, Mohammad T, Kerr H. Allergic contact dermatitis due to benzoyl peroxide from an unlikely source. Dermatitis. 2019;30:230-231. doi:10.1097/DER.0000000000000470
- Gatica-Ortega ME, Pastor-Nieto MA. Allergic contact dermatitis to Glycyrrhiza inflata root extract in an anti-acne cosmetic product [published online April 28, 2021]. Contact Dermatitis. doi:10.1111/cod.13872
- Ockenfels HM, Uter W, Lessmann H, et al. Patch testing with benzoyl peroxide: reaction profile and interpretation of positive patch test reactions. Contact Dermatitis. 2009;61:209-216. doi:10.1111/j.1600-0536.2009.01603.x
- Sodhi PK, Verma L, Ratan J. Dermatological side effects of brimonidine: a report of three cases. J Dermatol. 2003;30:697-700. doi:10.1111/j.1346-8138.2003.tb00461.x
- Swanson LA, Warshaw EM. Allergic contact dermatitis to topical brimonidine tartrate gel 0.33% for treatment of rosacea. J Am Acad Dermatol. 2014;71:832-833. doi:10.1016/j.jaad.2014.05.073
- Bangsgaard N, Fischer LA, Zachariae C. Sensitization to and allergic contact dermatitis caused by Mirvaso(®)(brimonidine tartrate) for treatment of rosacea—2 cases. Contact Dermatitis. 2016;74:378-379. doi:10.1111/cod.12547
- Ringuet J, Houle MC. Case report: allergic contact dermatitis to topical brimonidine demonstrated with patch testing: insights on evaluation of brimonidine sensitization. J Cutan Med Surg. 2018;22:636-638. doi:10.1177/1203475418789020
- Cookson H, McFadden J, White J, et al. Allergic contact dermatitis caused by Mirvaso®, brimonidine tartrate gel 0.33%, a new topical treatment for rosaceal erythema. Contact Dermatitis. 2015;73:366-367. doi:10.1111/cod.12476
- Rajagopalan A, Rajagopalan B. Allergic contact dermatitis to topical brimonidine. Australas J Dermatol. 2015;56:235. doi:10.1111/ajd.12299
- Veraldi S, Brena M, Barbareschi M. Allergic contact dermatitis caused by topical antiacne drugs. Expert Rev Clin Pharmacol. 2015;8:377-381. doi:10.1586/17512433.2015.1046839
- Vejlstrup E, Menné T. Contact dermatitis from clindamycin. Contact Dermatitis. 1995;32:110. doi:10.1111/j.1600-0536.1995.tb00759.x
- García R, Galindo PA, Feo F, et al. Delayed allergic reactions to amoxycillin and clindamycin. Contact Dermatitis. 1996;35:116-117. doi:10.1111/j.1600-0536.1996.tb02312.x
- Muñoz D, Del Pozo MD, Audicana M, et al. Erythema-multiforme-like eruption from antibiotics of 3 different groups. Contact Dermatitis. 1996;34:227-228. doi:10.1111/j.1600-0536.1996.tb02187.x
- Romita P, Ettorre G, Corazza M, et al. Allergic contact dermatitis caused by clindamycin mimicking ‘retinoid flare.’ Contact Dermatitis. 2017;77:181-182. doi:10.1111/cod.12784
- Veraldi S, Guanziroli E, Ferrucci S, et al. Allergic contact dermatitis caused by clindamycin. Contact Dermatitis. 2019;80:68-69. doi:10.1111/cod.13133
- Voller LM, Kullberg SA, Warshaw EM. Axillary allergic contact dermatitis to topical clindamycin. Contact Dermatitis. 2020;82:313-314. doi:10.1111/cod.13465
- de Kort WJ, de Groot AC. Clindamycin allergy presenting as rosacea. Contact Dermatitis. 1989;20:72-73. doi:10.1111/j.1600-0536.1989.tb03108.x
- Vincenzi C, Lucente P, Ricci C, et al. Facial contact dermatitis due to metronidazole. Contact Dermatitis. 1997;36:116-117. doi:10.1111/j.1600-0536.1997.tb00434.x
- Wolf R, Orion E, Matz H. Co-existing sensitivity to metronidazole and isothiazolinone. Clin Exp Dermatol. 2003;28:506-507. doi:10.1046/j.1365-2230.2003.01364.x
- Madsen JT, Thormann J, Kerre S, et al. Allergic contact dermatitis to topical metronidazole—3 cases. Contact Dermatitis. 2007;56:364-366. doi:10.1111/j.1600-0536.2006.01064.x
- Fernández-Jorge B, Goday Buján J, Fernández-Torres R, et al. Concomitant allergic contact dermatitis from diphenhydramine and metronidazole. Contact Dermatitis. 2008;59:115-116. doi:10.1111/j.1600-0536.2008.01332.x
- Madsen JT, Lorentzen HF, Paulsen E. Contact sensitization to metronidazole from possible occupational exposure. Contact Dermatitis. 2009;60:117-118. doi:10.1111/j.1600-0536.2008.01490.x
- Beutner KR, Lemke S, Calvarese B. A look at the safety of metronidazole 1% gel: cumulative irritation, contact sensitization, phototoxicity, and photoallergy potential. Cutis. 2006;77(4 suppl):12-17.
- Jappe U, Schäfer T, Schnuch A, et al. Contact allergy in patients with rosacea: a clinic-based, prospective epidemiological study. J Eur Acad Dermatol Venereol. 2008;22:1208-1214. doi:10.1111/j.1468-3083.2008.02778.x
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group Patch Test Results: 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Comaish JS, Cunliffe WJ. Absorption of drugs from varicose ulcers: a cause of anaphylaxis. Br J Clin Pract. 1967;21:97-98.
- Roupe G, Strannegård O. Anaphylactic shock elicited by topical administration of bacitracin. Arch Dermatol. 1969;100:450-452.
- Farley M, Pak H, Carregal V, et al. Anaphylaxis to topically applied bacitracin. Am J Contact Dermat. 1995;6:28-31.
- Barranco R, Tornero P, de Barrio M, et al. Type IV hypersensitivity to oral nystatin. Contact Dermatitis. 2001;45:60. doi:10.1034/j.1600-0536.2001.045001060.x
- Cooper SM, Shaw S. Contact allergy to nystatin: an unusual allergen. Contact Dermatitis. 1999;41:120. doi:10.1111/j.1600-0536.1999.tb06254.x
- Dooms-Goossens A, Matura M, Drieghe J, et al. Contact allergy to imidazoles used as antimycotic agents. Contact Dermatitis. 1995;33:73-77. doi:10.1111/j.1600-0536.1995.tb00504.x
- Pérez-Mesonero R, Schneller-Pavelescu L, Ochando-Ibernón G, et al. Is tioconazole contact dermatitis still a concern? bringing allergic contact dermatitis caused by topical tioconazole back into the spotlight. Contact Dermatitis. 2019;80:168-169.
- Tang MM, Corti MA, Stirnimann R, et al. Severe cutaneous allergic reactions following topical antifungal therapy. Contact Dermatitis. 2013;68:56-57.
- Goossens A, Linsen G. Contact allergy to antihistamines is not common. Contact Dermatitis. 1998;39:38. doi:10.1111/j.1600-0536.1998.tb05817.x
- Taylor JS, Praditsuwan P, Handel D, et al. Allergic contact dermatitis from doxepin cream. one-year patch test clinic experience. Arch Dermatol. 1996;132:515-518.
- Bilbao I, Aguirre A, Vicente JM, et al. Allergic contact dermatitis due to 5% doxepin cream. Contact Dermatitis. 1996;35:254-255. doi:10.1111/j.1600-0536.1996.tb02374.x
- Shelley WB, Shelley ED, Talanin NY. Self-potentiating allergic contact dermatitis caused by doxepin hydrochloride cream. J Am Acad Dermatol. 1996;34:143-144. doi:10.1016/s0190-9622(96)90864-6
- Wakelin SH, Rycroft RJ. Allergic contact dermatitis from doxepin. Contact Dermatitis. 1999;40:214. doi:10.1111/j.1600-0536.1999.tb06037.x
- Horn HM, Tidman MJ, Aldridge RD. Allergic contact dermatitis due to doxepin cream in a patient with dystrophic epidermolysis bullosa. Contact Dermatitis. 2001;45:115. doi:10.1034/j.1600-0536.2001.045002115.x
- Bonnel RA, La Grenade L, Karwoski CB, et al. Allergic contact dermatitis from topical doxepin: Food and Drug Administration’s postmarketing surveillance experience. J Am Acad Dermatol. 2003;48:294-296. doi:10.1067/mjd.2003.46
- Devleeschouwer V, Roelandts R, Garmyn M, et al. Allergic and photoallergic contact dermatitis from ketoprofen: results of (photo) patch testing and follow-up of 42 patients. Contact Dermatitis. 2008;58:159-166. doi:10.1111/j.1600-0536.2007.01296.x
- Foti C, Bonamonte D, Conserva A, et al. Allergic and photoallergic contact dermatitis from ketoprofen: evaluation of cross-reactivities by a combination of photopatch testing and computerized conformational analysis. Curr Pharm Des. 2008;14:2833-2839. doi:10.2174/138161208786369696
- Gulin SJ, Chiriac A. Diclofenac-induced allergic contact dermatitis: a series of four patients. Drug Saf Case Rep. 2016;3:15. doi:10.1007/s40800-016-0039-3
- Lakshmi C, Srinivas CR. Systemic (allergic) contact dermatitis to diclofenac. Indian J Dermatol Venereol Leprol. 2011;77:536. doi:10.4103/0378-6323.82424
- Beutner C, Forkel S, Kreipe K, et al. Contact allergy to topical diclofenac with systemic tolerance [published online August 22, 2021]. Contact Dermatitis. doi:10.1111/cod.13961
- Pan Y, Nixon R. Allergic contact dermatitis to topical preparations of bufexamac. Australas J Dermatol. 2012;53:207-210. doi:10.1111/j.1440-0960.2012.00876.x
- Nakada T, Matsuzawa Y. Allergic contact dermatitis syndrome from bufexamac for nursing infant. Dermatitis. 2012;23:185-186. doi:10.1097/DER.0b013e318260d774
- Kerr AC, Muller F, Ferguson J, et al. Occupational carprofen photoallergic contact dermatitis. Br J Dermatol. 2008;159:1303-1308. doi:10.1111/j.1365-2133.2008.08847.x
- Kiely C, Murphy G. Photoallergic contact dermatitis caused by occupational exposure to the canine non-steroidal anti-inflammatory drug carprofen. Contact Dermatitis. 2010;63:364-365. doi:10.1111/j.1600-0536.2010.01820.x
- Somberg J, Molnar J. Retrospective evaluation on the analgesic activities of 2 compounded topical creams and voltaren gel in chronic noncancer pain. Am J Ther. 2015;22:342-349. doi:10.1097/MJT.0000000000000275
- Lee HG, Grossman SK, Valdes-Rodriguez R, et al. Topical ketamine-amitriptyline-lidocaine for chronic pruritus: a retrospective study assessing efficacy and tolerability. J Am Acad Dermatol. 2017;76:760-761. doi:10.1016/j.jaad.2016.10.030
- Turrentine JE, Marrazzo G, Cruz PD Jr. Novel use of patch testing in the first report of allergic contact dermatitis to cyclobenzaprine. Dermatitis. 2015;26:60-61. doi:10.1097/DER.0000000000000099
- de Groot A. Patch Testing. 3rd ed. acdegroot publishing; 2008.
- de Groot A. Patch Testing. 4th ed. acdegroot publishing; 2018.
- Davis MD. Unusual patterns in contact dermatitis: medicaments. Dermatol Clin. 2009;27:289-297, vi. doi:10.1016/j.det.2009.05.003
- Gilissen L, Goossens A. Frequency and trends of contact allergy to and iatrogenic contact dermatitis caused by topical drugs over a 25-year period. Contact Dermatitis. 2016;75:290-302. doi:10.1111/cod.12621
- Balato N, Patruno C, Lembo G, et al. Allergic contact dermatitis from retinoic acid. Contact Dermatitis. 1995;32:51. doi:10.1111/j.1600-0536.1995.tb00846.x
- Berg JE, Bowman JP, Saenz AB. Cumulative irritation potential and contact sensitization potential of tazarotene foam 0.1% in 2 phase 1 patch studies. Cutis. 2012;90:206-211.
- Numata T, Jo R, Kobayashi Y, et al. Allergic contact dermatitis caused by adapalene. Contact Dermatitis. 2015;73:187-188. doi:10.1111/cod.12410
- Anderson A, Gebauer K. Periorbital allergic contact dermatitis resulting from topical retinoic acid use. Australas J Dermatol. 2014;55:152-153. doi:10.1111/ajd.12041
- Blondeel A. Contact allergy to vitamin A. Contact Dermatitis. 1984;11:191-192. doi:10.1111/j.1600-0536.1984.tb00976.x
- Manzano D, Aguirre A, Gardeazabal J, et al. Allergic contact dermatitis from tocopheryl acetate (vitamin E) and retinol palmitate (vitamin A) in a moisturizing cream. Contact Dermatitis. 1994;31:324. doi:10.1111/j.1600-0536.1994.tb02030.x
- Heidenheim M, Jemec GB. Occupational allergic contact dermatitis from vitamin A acetate. Contact Dermatitis. 1995;33:439. doi:10.1111/j.1600-0536.1995.tb02091.x
- Kim C, Craiglow BG, Watsky KL, et al. Allergic contact dermatitis to benzoyl peroxide resembling impetigo. Pediatr Dermatol. 2015;32:E161-E162. doi:10.1111/pde.12585
- Sandre M, Skotnicki-Grant S. A case of a paediatric patient with allergic contact dermatitis to benzoyl peroxide. J Cutan Med Surg. 2018;22:226-228. doi:10.1177/1203475417733462
- Corazza M, Amendolagine G, Musmeci D, et al. Sometimes even Dr Google is wrong: an unusual contact dermatitis caused by benzoyl peroxide. Contact Dermatitis. 2018;79:380-381. doi:10.1111/cod.13086
- Adelman M, Mohammad T, Kerr H. Allergic contact dermatitis due to benzoyl peroxide from an unlikely source. Dermatitis. 2019;30:230-231. doi:10.1097/DER.0000000000000470
- Gatica-Ortega ME, Pastor-Nieto MA. Allergic contact dermatitis to Glycyrrhiza inflata root extract in an anti-acne cosmetic product [published online April 28, 2021]. Contact Dermatitis. doi:10.1111/cod.13872
- Ockenfels HM, Uter W, Lessmann H, et al. Patch testing with benzoyl peroxide: reaction profile and interpretation of positive patch test reactions. Contact Dermatitis. 2009;61:209-216. doi:10.1111/j.1600-0536.2009.01603.x
- Sodhi PK, Verma L, Ratan J. Dermatological side effects of brimonidine: a report of three cases. J Dermatol. 2003;30:697-700. doi:10.1111/j.1346-8138.2003.tb00461.x
- Swanson LA, Warshaw EM. Allergic contact dermatitis to topical brimonidine tartrate gel 0.33% for treatment of rosacea. J Am Acad Dermatol. 2014;71:832-833. doi:10.1016/j.jaad.2014.05.073
- Bangsgaard N, Fischer LA, Zachariae C. Sensitization to and allergic contact dermatitis caused by Mirvaso(®)(brimonidine tartrate) for treatment of rosacea—2 cases. Contact Dermatitis. 2016;74:378-379. doi:10.1111/cod.12547
- Ringuet J, Houle MC. Case report: allergic contact dermatitis to topical brimonidine demonstrated with patch testing: insights on evaluation of brimonidine sensitization. J Cutan Med Surg. 2018;22:636-638. doi:10.1177/1203475418789020
- Cookson H, McFadden J, White J, et al. Allergic contact dermatitis caused by Mirvaso®, brimonidine tartrate gel 0.33%, a new topical treatment for rosaceal erythema. Contact Dermatitis. 2015;73:366-367. doi:10.1111/cod.12476
- Rajagopalan A, Rajagopalan B. Allergic contact dermatitis to topical brimonidine. Australas J Dermatol. 2015;56:235. doi:10.1111/ajd.12299
- Veraldi S, Brena M, Barbareschi M. Allergic contact dermatitis caused by topical antiacne drugs. Expert Rev Clin Pharmacol. 2015;8:377-381. doi:10.1586/17512433.2015.1046839
- Vejlstrup E, Menné T. Contact dermatitis from clindamycin. Contact Dermatitis. 1995;32:110. doi:10.1111/j.1600-0536.1995.tb00759.x
- García R, Galindo PA, Feo F, et al. Delayed allergic reactions to amoxycillin and clindamycin. Contact Dermatitis. 1996;35:116-117. doi:10.1111/j.1600-0536.1996.tb02312.x
- Muñoz D, Del Pozo MD, Audicana M, et al. Erythema-multiforme-like eruption from antibiotics of 3 different groups. Contact Dermatitis. 1996;34:227-228. doi:10.1111/j.1600-0536.1996.tb02187.x
- Romita P, Ettorre G, Corazza M, et al. Allergic contact dermatitis caused by clindamycin mimicking ‘retinoid flare.’ Contact Dermatitis. 2017;77:181-182. doi:10.1111/cod.12784
- Veraldi S, Guanziroli E, Ferrucci S, et al. Allergic contact dermatitis caused by clindamycin. Contact Dermatitis. 2019;80:68-69. doi:10.1111/cod.13133
- Voller LM, Kullberg SA, Warshaw EM. Axillary allergic contact dermatitis to topical clindamycin. Contact Dermatitis. 2020;82:313-314. doi:10.1111/cod.13465
- de Kort WJ, de Groot AC. Clindamycin allergy presenting as rosacea. Contact Dermatitis. 1989;20:72-73. doi:10.1111/j.1600-0536.1989.tb03108.x
- Vincenzi C, Lucente P, Ricci C, et al. Facial contact dermatitis due to metronidazole. Contact Dermatitis. 1997;36:116-117. doi:10.1111/j.1600-0536.1997.tb00434.x
- Wolf R, Orion E, Matz H. Co-existing sensitivity to metronidazole and isothiazolinone. Clin Exp Dermatol. 2003;28:506-507. doi:10.1046/j.1365-2230.2003.01364.x
- Madsen JT, Thormann J, Kerre S, et al. Allergic contact dermatitis to topical metronidazole—3 cases. Contact Dermatitis. 2007;56:364-366. doi:10.1111/j.1600-0536.2006.01064.x
- Fernández-Jorge B, Goday Buján J, Fernández-Torres R, et al. Concomitant allergic contact dermatitis from diphenhydramine and metronidazole. Contact Dermatitis. 2008;59:115-116. doi:10.1111/j.1600-0536.2008.01332.x
- Madsen JT, Lorentzen HF, Paulsen E. Contact sensitization to metronidazole from possible occupational exposure. Contact Dermatitis. 2009;60:117-118. doi:10.1111/j.1600-0536.2008.01490.x
- Beutner KR, Lemke S, Calvarese B. A look at the safety of metronidazole 1% gel: cumulative irritation, contact sensitization, phototoxicity, and photoallergy potential. Cutis. 2006;77(4 suppl):12-17.
- Jappe U, Schäfer T, Schnuch A, et al. Contact allergy in patients with rosacea: a clinic-based, prospective epidemiological study. J Eur Acad Dermatol Venereol. 2008;22:1208-1214. doi:10.1111/j.1468-3083.2008.02778.x
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group Patch Test Results: 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Comaish JS, Cunliffe WJ. Absorption of drugs from varicose ulcers: a cause of anaphylaxis. Br J Clin Pract. 1967;21:97-98.
- Roupe G, Strannegård O. Anaphylactic shock elicited by topical administration of bacitracin. Arch Dermatol. 1969;100:450-452.
- Farley M, Pak H, Carregal V, et al. Anaphylaxis to topically applied bacitracin. Am J Contact Dermat. 1995;6:28-31.
- Barranco R, Tornero P, de Barrio M, et al. Type IV hypersensitivity to oral nystatin. Contact Dermatitis. 2001;45:60. doi:10.1034/j.1600-0536.2001.045001060.x
- Cooper SM, Shaw S. Contact allergy to nystatin: an unusual allergen. Contact Dermatitis. 1999;41:120. doi:10.1111/j.1600-0536.1999.tb06254.x
- Dooms-Goossens A, Matura M, Drieghe J, et al. Contact allergy to imidazoles used as antimycotic agents. Contact Dermatitis. 1995;33:73-77. doi:10.1111/j.1600-0536.1995.tb00504.x
- Pérez-Mesonero R, Schneller-Pavelescu L, Ochando-Ibernón G, et al. Is tioconazole contact dermatitis still a concern? bringing allergic contact dermatitis caused by topical tioconazole back into the spotlight. Contact Dermatitis. 2019;80:168-169.
- Tang MM, Corti MA, Stirnimann R, et al. Severe cutaneous allergic reactions following topical antifungal therapy. Contact Dermatitis. 2013;68:56-57.
- Goossens A, Linsen G. Contact allergy to antihistamines is not common. Contact Dermatitis. 1998;39:38. doi:10.1111/j.1600-0536.1998.tb05817.x
- Taylor JS, Praditsuwan P, Handel D, et al. Allergic contact dermatitis from doxepin cream. one-year patch test clinic experience. Arch Dermatol. 1996;132:515-518.
- Bilbao I, Aguirre A, Vicente JM, et al. Allergic contact dermatitis due to 5% doxepin cream. Contact Dermatitis. 1996;35:254-255. doi:10.1111/j.1600-0536.1996.tb02374.x
- Shelley WB, Shelley ED, Talanin NY. Self-potentiating allergic contact dermatitis caused by doxepin hydrochloride cream. J Am Acad Dermatol. 1996;34:143-144. doi:10.1016/s0190-9622(96)90864-6
- Wakelin SH, Rycroft RJ. Allergic contact dermatitis from doxepin. Contact Dermatitis. 1999;40:214. doi:10.1111/j.1600-0536.1999.tb06037.x
- Horn HM, Tidman MJ, Aldridge RD. Allergic contact dermatitis due to doxepin cream in a patient with dystrophic epidermolysis bullosa. Contact Dermatitis. 2001;45:115. doi:10.1034/j.1600-0536.2001.045002115.x
- Bonnel RA, La Grenade L, Karwoski CB, et al. Allergic contact dermatitis from topical doxepin: Food and Drug Administration’s postmarketing surveillance experience. J Am Acad Dermatol. 2003;48:294-296. doi:10.1067/mjd.2003.46
- Devleeschouwer V, Roelandts R, Garmyn M, et al. Allergic and photoallergic contact dermatitis from ketoprofen: results of (photo) patch testing and follow-up of 42 patients. Contact Dermatitis. 2008;58:159-166. doi:10.1111/j.1600-0536.2007.01296.x
- Foti C, Bonamonte D, Conserva A, et al. Allergic and photoallergic contact dermatitis from ketoprofen: evaluation of cross-reactivities by a combination of photopatch testing and computerized conformational analysis. Curr Pharm Des. 2008;14:2833-2839. doi:10.2174/138161208786369696
- Gulin SJ, Chiriac A. Diclofenac-induced allergic contact dermatitis: a series of four patients. Drug Saf Case Rep. 2016;3:15. doi:10.1007/s40800-016-0039-3
- Lakshmi C, Srinivas CR. Systemic (allergic) contact dermatitis to diclofenac. Indian J Dermatol Venereol Leprol. 2011;77:536. doi:10.4103/0378-6323.82424
- Beutner C, Forkel S, Kreipe K, et al. Contact allergy to topical diclofenac with systemic tolerance [published online August 22, 2021]. Contact Dermatitis. doi:10.1111/cod.13961
- Pan Y, Nixon R. Allergic contact dermatitis to topical preparations of bufexamac. Australas J Dermatol. 2012;53:207-210. doi:10.1111/j.1440-0960.2012.00876.x
- Nakada T, Matsuzawa Y. Allergic contact dermatitis syndrome from bufexamac for nursing infant. Dermatitis. 2012;23:185-186. doi:10.1097/DER.0b013e318260d774
- Kerr AC, Muller F, Ferguson J, et al. Occupational carprofen photoallergic contact dermatitis. Br J Dermatol. 2008;159:1303-1308. doi:10.1111/j.1365-2133.2008.08847.x
- Kiely C, Murphy G. Photoallergic contact dermatitis caused by occupational exposure to the canine non-steroidal anti-inflammatory drug carprofen. Contact Dermatitis. 2010;63:364-365. doi:10.1111/j.1600-0536.2010.01820.x
- Somberg J, Molnar J. Retrospective evaluation on the analgesic activities of 2 compounded topical creams and voltaren gel in chronic noncancer pain. Am J Ther. 2015;22:342-349. doi:10.1097/MJT.0000000000000275
- Lee HG, Grossman SK, Valdes-Rodriguez R, et al. Topical ketamine-amitriptyline-lidocaine for chronic pruritus: a retrospective study assessing efficacy and tolerability. J Am Acad Dermatol. 2017;76:760-761. doi:10.1016/j.jaad.2016.10.030
- Turrentine JE, Marrazzo G, Cruz PD Jr. Novel use of patch testing in the first report of allergic contact dermatitis to cyclobenzaprine. Dermatitis. 2015;26:60-61. doi:10.1097/DER.0000000000000099
- de Groot A. Patch Testing. 3rd ed. acdegroot publishing; 2008.
- de Groot A. Patch Testing. 4th ed. acdegroot publishing; 2018.
Practice Points
- Allergic contact dermatitis should be suspected in patients with persistent or worsening dermatitis after use of topical medications.
- Prior sensitization is not always apparent, and cross-reactions may occur between structurally similar compounds.
- Although most medicaments can be patch tested as is, patch testing to the individual components may be necessary to identify the causative allergen.
Botanical Briefs: Phytophotodermatitis Caused by Giant Hogweed (Heracleum mantegazzianum)
Giant hogweed (Heracleum mantegazzianum) is an invasive flowering weed of the family Apiaceae that typically reaches a height of 13 feet, with thick stems; large green leaves; and umbrella-shaped, flat-topped, radial clusters (umbels) of small individual white flowers1 (Figure 1). Because of the size and beauty of giant hogweed, it was widely planted in 19th century ornamental gardens in the United Kingdom and has since naturalized and spread throughout central Europe, Canada, and the United States.1,2 The plant most commonly is found in shady areas near rivers and woodlands.1

Due to the invasive nature of the giant hogweed, its prevalence continues to grow, its eradication remains difficult, and reports of phytophotodermatitis are increasing in number and distribution. In fact, there has been widespread media coverage of the dangers of giant hogweed in the United Kingdom since 20161 and in the United States in 2018 and 2019.3-6
Transmission
Phytophotodermatitis is a type of nonimmunologic dermatitis caused by UV light reacting with a plant-based photosensitizing agent. In the case of giant hogweed, sap from the plant’s fruits, leaves, and stem contain furocoumarins or psoralens.7 Upon activation by UVA radiation, furan rings of these compounds create reactive oxygen species and intercalate with DNA pyrimidine bases, which results in cellular death, damage to successive skin layers, and reduced wound healing at the cellular level.8 This effect is intensified with increased percutaneous absorption of furocoumarin, which can result from high temperature, humidity, skin infection, lack of protective clothing, and moist conditions.9
The highest concentration of phototoxic compounds is found in giant hogweed from June through August,7 which, in combination with people increasing their outdoor activity in the summer, results in a greater prevalence and severity of H mantegazzianum phytophotodermatitis during summer months.
Presentation
Phytophotodermatitis caused by giant hogweed can range from burning and erythema to full-thickness chemical burns that require surgical debridement and skin grafting.8 After exposure to the offending agent, a harmful skin reaction can start within 15 minutes. After a latent period of approximately 24 hours, erythema, edema, and bullae can appear and generally peak by 72 hours.10 In addition to cutaneous injury, inhalation of giant hogweed traces can result in obstructive pulmonary symptoms. Eye contact can result in blindness.9
In addition to the rash caused by giant hogweed, a “weed-wacker dermatitis” or “strimmer rash” can be caused by the similar-appearing but smaller common hogweed (Heracleum sphondylium). Common hogweed is highly prevalent in the United States and often is confused with the larger giant hogweed because of tall stems and white, flat-topped flowers.
Management
Following contact with giant hogweed, a person should immediately avoid UV exposure and rinse the area with soap and water. UV radiation must be avoided for at least 48 hours. If erythema occurs, a topical steroid can be applied to the affected area; pain can be alleviated by a nonsteroidal anti-inflammatory drug.9
Further treatment might be required if bullous lesions are present. Small blisters can be punctured and drained; however, large blisters, extensive epidermal-dermal separation, and large areas of detached epidermis should simply be cleansed and dressed. An oral steroid also can be used to reduce inflammation in moderate and severe cases. Full-thickness injury might require surgical intervention.8
Clinical Case
A 27-year-old male landscaper presented to the emergency department with an increasingly painful blistering rash on the arms and neck of 1 day’s duration. He noticed bright red skin and blisters 18 to 24 hours after trimming what he identified as shoulder-high giant hogweed plants. Neither he nor his coworkers were wearing sunscreen or protective clothing as they cleared the plants for several hours. His coworkers developed similar rashes, but his rash was the most severe, requiring treatment in the emergency department.
Physical examination showed innumerable 2- to 10-mm, tense vesicles and bullae on a background of blanching erythema in a striking photodistribution along the neck (Figure 2) and arms (Figure 3). He had notable edema of both arms and several large 3- to 4-cm bullae on the ventral aspects of the forearms.
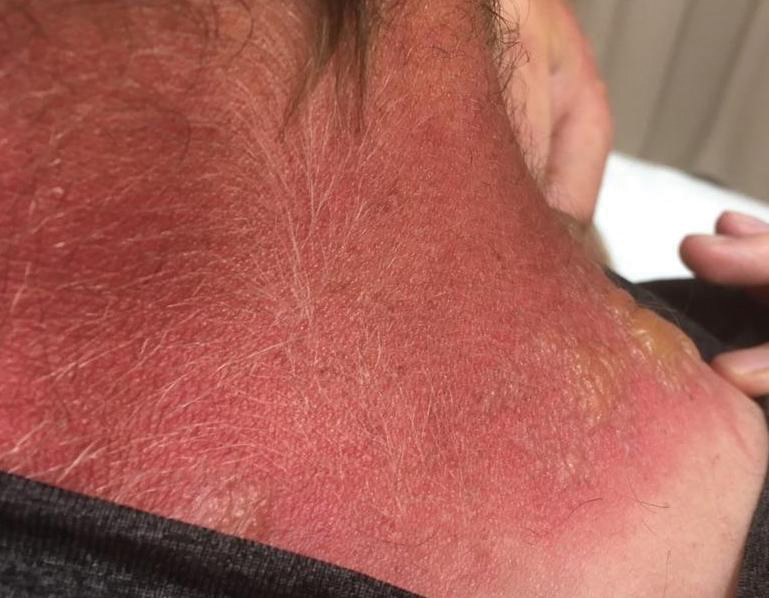
The patient was diagnosed with severe phytophotodermatitis secondary to contact with H mantegazzianum and was started on oral prednisone 70 mg daily (1 mg/kg/d), which was decreased by 10 mg every 3 days until the course of treatment was complete. He also was instructed to apply mupirocin ointment to open areas and petroleum jelly to intact skin. Additionally, he was advised to practice strict photoprotection for the near and distant future.
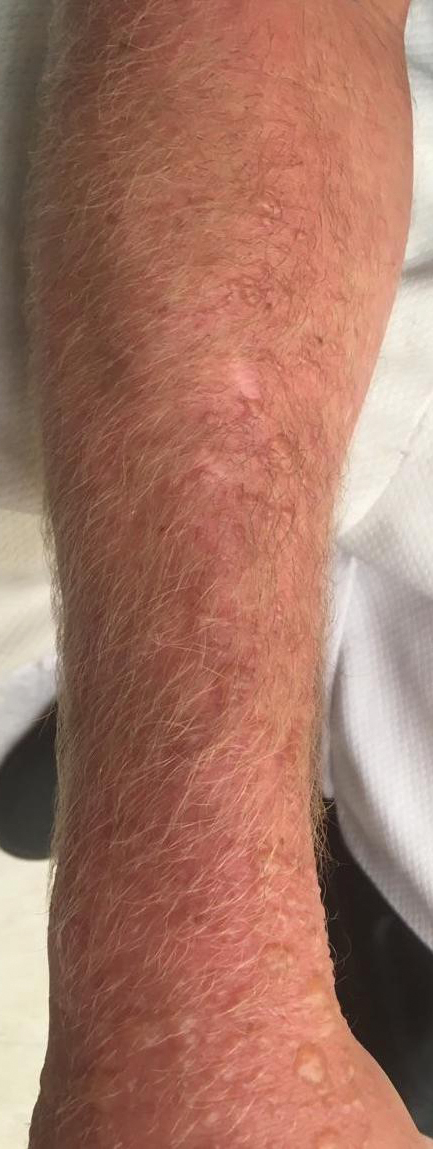
Within several days after treatment began, the phytophotodermatitis dramatically improved, with complete resolution in 1 week. Postinflammatory hyperpigmentation resolved after several weeks.
- Baker B, Bedford J, Kanitkar S. Keeping pace with the media; giant hogweed burns—a case series and comprehensive review [published online December 26, 2016]. Burns. 2017;13:933-938. doi:10.1016/j.burns.2016.10.018
- Klimaszyk P, Klimaszyk D, Piotrowiak M, et al. Unusual complications after occupational exposure to giant hogweed (Heracleum mantegazzianum): a case report. Int J Occup Med Environ Health. 2014;27:141-144. doi:10.2478/s13382-014-0238-z
- Zaveria M, Hauser C. Giant hogweed: a plant that can burn and blind you. but don’t panic. New York Times. July 2, 2018. Accessed October 18, 2021. https://www.nytimes.com/2018/07/02/us/giant-hogweed-nyt.html
- Hignett K. Giant hogweed: New York officials warn residents about dangerous plant that causes serious burns, blisters and scars. Newsweek. June 25, 2019. Accessed October 18, 2021. https://www.newsweek.com/giant-hogweed-new-york-dangerous-plant-burns-skin-sunlight-1445785
- Eastman J. Toxic giant hogweed sap that burns, blisters skin found in Clark County. The Oregonian. Updated July 16, 2019. Accessed October 18, 2021. https://www.oregonlive.com/news/2019/07/toxic-giant-hogweed-plant-that-burns-blisters-skin-found-in-clark-county.html
- O’Kane C. Giant hogweed, plant that causes blindness and third-degree burns, discovered in Virginia. CBS News. June 18, 2018. Accessed October 18, 2021. https://www.cbsnews.com/news/giant-hogweed-plant-causes-blindness-third-degree-burns-discovered-in-virginia-otherstates/
- Pira E, Romano C, Sulotto F, et al. Heracleum mantegazzianum growth phases and furocoumarin content. Contact Dermatitis. 1989;21:300-303. doi:10.1111/j.1600-0536.1989.tb04747.x
- Chan JCY, Sullivan PJ, O’Sullivan MJ, et al. Full thickness burn caused by exposure to giant hogweed: delayed presentation, histological features and surgical management. J Plast Reconstr Aesthet Surg. 2011;64:128-130. doi:10.1016/j.bjps.2010.03.030
- Pfurtscheller K, Trop M. Phototoxic plant burns: report of a case and review of topical wound treatment in children. Pediatr Dermatol. 2014;31:E156-E159. doi:10.1111/pde.12396
- Kavli G, Volden G: Phytophotodermatitis. Photodermatol. 1984;1:65-75.
Giant hogweed (Heracleum mantegazzianum) is an invasive flowering weed of the family Apiaceae that typically reaches a height of 13 feet, with thick stems; large green leaves; and umbrella-shaped, flat-topped, radial clusters (umbels) of small individual white flowers1 (Figure 1). Because of the size and beauty of giant hogweed, it was widely planted in 19th century ornamental gardens in the United Kingdom and has since naturalized and spread throughout central Europe, Canada, and the United States.1,2 The plant most commonly is found in shady areas near rivers and woodlands.1

Due to the invasive nature of the giant hogweed, its prevalence continues to grow, its eradication remains difficult, and reports of phytophotodermatitis are increasing in number and distribution. In fact, there has been widespread media coverage of the dangers of giant hogweed in the United Kingdom since 20161 and in the United States in 2018 and 2019.3-6
Transmission
Phytophotodermatitis is a type of nonimmunologic dermatitis caused by UV light reacting with a plant-based photosensitizing agent. In the case of giant hogweed, sap from the plant’s fruits, leaves, and stem contain furocoumarins or psoralens.7 Upon activation by UVA radiation, furan rings of these compounds create reactive oxygen species and intercalate with DNA pyrimidine bases, which results in cellular death, damage to successive skin layers, and reduced wound healing at the cellular level.8 This effect is intensified with increased percutaneous absorption of furocoumarin, which can result from high temperature, humidity, skin infection, lack of protective clothing, and moist conditions.9
The highest concentration of phototoxic compounds is found in giant hogweed from June through August,7 which, in combination with people increasing their outdoor activity in the summer, results in a greater prevalence and severity of H mantegazzianum phytophotodermatitis during summer months.
Presentation
Phytophotodermatitis caused by giant hogweed can range from burning and erythema to full-thickness chemical burns that require surgical debridement and skin grafting.8 After exposure to the offending agent, a harmful skin reaction can start within 15 minutes. After a latent period of approximately 24 hours, erythema, edema, and bullae can appear and generally peak by 72 hours.10 In addition to cutaneous injury, inhalation of giant hogweed traces can result in obstructive pulmonary symptoms. Eye contact can result in blindness.9
In addition to the rash caused by giant hogweed, a “weed-wacker dermatitis” or “strimmer rash” can be caused by the similar-appearing but smaller common hogweed (Heracleum sphondylium). Common hogweed is highly prevalent in the United States and often is confused with the larger giant hogweed because of tall stems and white, flat-topped flowers.
Management
Following contact with giant hogweed, a person should immediately avoid UV exposure and rinse the area with soap and water. UV radiation must be avoided for at least 48 hours. If erythema occurs, a topical steroid can be applied to the affected area; pain can be alleviated by a nonsteroidal anti-inflammatory drug.9
Further treatment might be required if bullous lesions are present. Small blisters can be punctured and drained; however, large blisters, extensive epidermal-dermal separation, and large areas of detached epidermis should simply be cleansed and dressed. An oral steroid also can be used to reduce inflammation in moderate and severe cases. Full-thickness injury might require surgical intervention.8
Clinical Case
A 27-year-old male landscaper presented to the emergency department with an increasingly painful blistering rash on the arms and neck of 1 day’s duration. He noticed bright red skin and blisters 18 to 24 hours after trimming what he identified as shoulder-high giant hogweed plants. Neither he nor his coworkers were wearing sunscreen or protective clothing as they cleared the plants for several hours. His coworkers developed similar rashes, but his rash was the most severe, requiring treatment in the emergency department.
Physical examination showed innumerable 2- to 10-mm, tense vesicles and bullae on a background of blanching erythema in a striking photodistribution along the neck (Figure 2) and arms (Figure 3). He had notable edema of both arms and several large 3- to 4-cm bullae on the ventral aspects of the forearms.

The patient was diagnosed with severe phytophotodermatitis secondary to contact with H mantegazzianum and was started on oral prednisone 70 mg daily (1 mg/kg/d), which was decreased by 10 mg every 3 days until the course of treatment was complete. He also was instructed to apply mupirocin ointment to open areas and petroleum jelly to intact skin. Additionally, he was advised to practice strict photoprotection for the near and distant future.

Within several days after treatment began, the phytophotodermatitis dramatically improved, with complete resolution in 1 week. Postinflammatory hyperpigmentation resolved after several weeks.
Giant hogweed (Heracleum mantegazzianum) is an invasive flowering weed of the family Apiaceae that typically reaches a height of 13 feet, with thick stems; large green leaves; and umbrella-shaped, flat-topped, radial clusters (umbels) of small individual white flowers1 (Figure 1). Because of the size and beauty of giant hogweed, it was widely planted in 19th century ornamental gardens in the United Kingdom and has since naturalized and spread throughout central Europe, Canada, and the United States.1,2 The plant most commonly is found in shady areas near rivers and woodlands.1

Due to the invasive nature of the giant hogweed, its prevalence continues to grow, its eradication remains difficult, and reports of phytophotodermatitis are increasing in number and distribution. In fact, there has been widespread media coverage of the dangers of giant hogweed in the United Kingdom since 20161 and in the United States in 2018 and 2019.3-6
Transmission
Phytophotodermatitis is a type of nonimmunologic dermatitis caused by UV light reacting with a plant-based photosensitizing agent. In the case of giant hogweed, sap from the plant’s fruits, leaves, and stem contain furocoumarins or psoralens.7 Upon activation by UVA radiation, furan rings of these compounds create reactive oxygen species and intercalate with DNA pyrimidine bases, which results in cellular death, damage to successive skin layers, and reduced wound healing at the cellular level.8 This effect is intensified with increased percutaneous absorption of furocoumarin, which can result from high temperature, humidity, skin infection, lack of protective clothing, and moist conditions.9
The highest concentration of phototoxic compounds is found in giant hogweed from June through August,7 which, in combination with people increasing their outdoor activity in the summer, results in a greater prevalence and severity of H mantegazzianum phytophotodermatitis during summer months.
Presentation
Phytophotodermatitis caused by giant hogweed can range from burning and erythema to full-thickness chemical burns that require surgical debridement and skin grafting.8 After exposure to the offending agent, a harmful skin reaction can start within 15 minutes. After a latent period of approximately 24 hours, erythema, edema, and bullae can appear and generally peak by 72 hours.10 In addition to cutaneous injury, inhalation of giant hogweed traces can result in obstructive pulmonary symptoms. Eye contact can result in blindness.9
In addition to the rash caused by giant hogweed, a “weed-wacker dermatitis” or “strimmer rash” can be caused by the similar-appearing but smaller common hogweed (Heracleum sphondylium). Common hogweed is highly prevalent in the United States and often is confused with the larger giant hogweed because of tall stems and white, flat-topped flowers.
Management
Following contact with giant hogweed, a person should immediately avoid UV exposure and rinse the area with soap and water. UV radiation must be avoided for at least 48 hours. If erythema occurs, a topical steroid can be applied to the affected area; pain can be alleviated by a nonsteroidal anti-inflammatory drug.9
Further treatment might be required if bullous lesions are present. Small blisters can be punctured and drained; however, large blisters, extensive epidermal-dermal separation, and large areas of detached epidermis should simply be cleansed and dressed. An oral steroid also can be used to reduce inflammation in moderate and severe cases. Full-thickness injury might require surgical intervention.8
Clinical Case
A 27-year-old male landscaper presented to the emergency department with an increasingly painful blistering rash on the arms and neck of 1 day’s duration. He noticed bright red skin and blisters 18 to 24 hours after trimming what he identified as shoulder-high giant hogweed plants. Neither he nor his coworkers were wearing sunscreen or protective clothing as they cleared the plants for several hours. His coworkers developed similar rashes, but his rash was the most severe, requiring treatment in the emergency department.
Physical examination showed innumerable 2- to 10-mm, tense vesicles and bullae on a background of blanching erythema in a striking photodistribution along the neck (Figure 2) and arms (Figure 3). He had notable edema of both arms and several large 3- to 4-cm bullae on the ventral aspects of the forearms.

The patient was diagnosed with severe phytophotodermatitis secondary to contact with H mantegazzianum and was started on oral prednisone 70 mg daily (1 mg/kg/d), which was decreased by 10 mg every 3 days until the course of treatment was complete. He also was instructed to apply mupirocin ointment to open areas and petroleum jelly to intact skin. Additionally, he was advised to practice strict photoprotection for the near and distant future.

Within several days after treatment began, the phytophotodermatitis dramatically improved, with complete resolution in 1 week. Postinflammatory hyperpigmentation resolved after several weeks.
- Baker B, Bedford J, Kanitkar S. Keeping pace with the media; giant hogweed burns—a case series and comprehensive review [published online December 26, 2016]. Burns. 2017;13:933-938. doi:10.1016/j.burns.2016.10.018
- Klimaszyk P, Klimaszyk D, Piotrowiak M, et al. Unusual complications after occupational exposure to giant hogweed (Heracleum mantegazzianum): a case report. Int J Occup Med Environ Health. 2014;27:141-144. doi:10.2478/s13382-014-0238-z
- Zaveria M, Hauser C. Giant hogweed: a plant that can burn and blind you. but don’t panic. New York Times. July 2, 2018. Accessed October 18, 2021. https://www.nytimes.com/2018/07/02/us/giant-hogweed-nyt.html
- Hignett K. Giant hogweed: New York officials warn residents about dangerous plant that causes serious burns, blisters and scars. Newsweek. June 25, 2019. Accessed October 18, 2021. https://www.newsweek.com/giant-hogweed-new-york-dangerous-plant-burns-skin-sunlight-1445785
- Eastman J. Toxic giant hogweed sap that burns, blisters skin found in Clark County. The Oregonian. Updated July 16, 2019. Accessed October 18, 2021. https://www.oregonlive.com/news/2019/07/toxic-giant-hogweed-plant-that-burns-blisters-skin-found-in-clark-county.html
- O’Kane C. Giant hogweed, plant that causes blindness and third-degree burns, discovered in Virginia. CBS News. June 18, 2018. Accessed October 18, 2021. https://www.cbsnews.com/news/giant-hogweed-plant-causes-blindness-third-degree-burns-discovered-in-virginia-otherstates/
- Pira E, Romano C, Sulotto F, et al. Heracleum mantegazzianum growth phases and furocoumarin content. Contact Dermatitis. 1989;21:300-303. doi:10.1111/j.1600-0536.1989.tb04747.x
- Chan JCY, Sullivan PJ, O’Sullivan MJ, et al. Full thickness burn caused by exposure to giant hogweed: delayed presentation, histological features and surgical management. J Plast Reconstr Aesthet Surg. 2011;64:128-130. doi:10.1016/j.bjps.2010.03.030
- Pfurtscheller K, Trop M. Phototoxic plant burns: report of a case and review of topical wound treatment in children. Pediatr Dermatol. 2014;31:E156-E159. doi:10.1111/pde.12396
- Kavli G, Volden G: Phytophotodermatitis. Photodermatol. 1984;1:65-75.
- Baker B, Bedford J, Kanitkar S. Keeping pace with the media; giant hogweed burns—a case series and comprehensive review [published online December 26, 2016]. Burns. 2017;13:933-938. doi:10.1016/j.burns.2016.10.018
- Klimaszyk P, Klimaszyk D, Piotrowiak M, et al. Unusual complications after occupational exposure to giant hogweed (Heracleum mantegazzianum): a case report. Int J Occup Med Environ Health. 2014;27:141-144. doi:10.2478/s13382-014-0238-z
- Zaveria M, Hauser C. Giant hogweed: a plant that can burn and blind you. but don’t panic. New York Times. July 2, 2018. Accessed October 18, 2021. https://www.nytimes.com/2018/07/02/us/giant-hogweed-nyt.html
- Hignett K. Giant hogweed: New York officials warn residents about dangerous plant that causes serious burns, blisters and scars. Newsweek. June 25, 2019. Accessed October 18, 2021. https://www.newsweek.com/giant-hogweed-new-york-dangerous-plant-burns-skin-sunlight-1445785
- Eastman J. Toxic giant hogweed sap that burns, blisters skin found in Clark County. The Oregonian. Updated July 16, 2019. Accessed October 18, 2021. https://www.oregonlive.com/news/2019/07/toxic-giant-hogweed-plant-that-burns-blisters-skin-found-in-clark-county.html
- O’Kane C. Giant hogweed, plant that causes blindness and third-degree burns, discovered in Virginia. CBS News. June 18, 2018. Accessed October 18, 2021. https://www.cbsnews.com/news/giant-hogweed-plant-causes-blindness-third-degree-burns-discovered-in-virginia-otherstates/
- Pira E, Romano C, Sulotto F, et al. Heracleum mantegazzianum growth phases and furocoumarin content. Contact Dermatitis. 1989;21:300-303. doi:10.1111/j.1600-0536.1989.tb04747.x
- Chan JCY, Sullivan PJ, O’Sullivan MJ, et al. Full thickness burn caused by exposure to giant hogweed: delayed presentation, histological features and surgical management. J Plast Reconstr Aesthet Surg. 2011;64:128-130. doi:10.1016/j.bjps.2010.03.030
- Pfurtscheller K, Trop M. Phototoxic plant burns: report of a case and review of topical wound treatment in children. Pediatr Dermatol. 2014;31:E156-E159. doi:10.1111/pde.12396
- Kavli G, Volden G: Phytophotodermatitis. Photodermatol. 1984;1:65-75.
PRACTICE POINTS
- The public should be educated, especially during summer months, about how to identify giant hogweed, reduce exposure to the plant’s phototoxin, and thus reduce the risk for severe phytophotodermatitis.
- Phytophotodermatitis should be included in the differential diagnosis when a patient presents with acute erythema and bullae in sun-exposed areas.
- Phytophotodermatitis can be treated by promptly washing the skin with soap and water, protecting the skin from exposure to UV light, and utilizing topical and oral steroids.
Atypical Presentation of Pityriasis Rubra Pilaris: Challenges in Diagnosis and Management
To the Editor:
Pityriasis rubra pilaris (PRP) is a rare inflammatory dermatosis of unknown etiology characterized by erythematosquamous salmon-colored plaques with well-demarcated islands of unaffected skin and hyperkeratotic follicles.1 In the United States, an incidence of 1 in 3500to 5000 patients presenting to dermatology clinics has been reported.2 Pityriasis rubra pilaris has several subtypes and variability in presentation that can make accurate and timely diagnosis challenging.3-5 Herein, we present a case of PRP with complex diagnostic and therapeutic challenges.
A 22-year-old woman presented with symmetrical, well-demarcated, hyperkeratotic, erythematous plaques with a carnauba wax–like appearance on the palms (Figure 1), soles, elbows, and trunk covering approximately 5% of the body surface area. Two weeks prior to presentation, she experienced an upper respiratory tract infection without any treatment and subsequently developed redness on the palms, which became very hard and scaly. The redness then spread to the elbows, soles, and trunk. She reported itching as well as pain in areas of fissuring. Hand mobility became restricted due to thick scale.
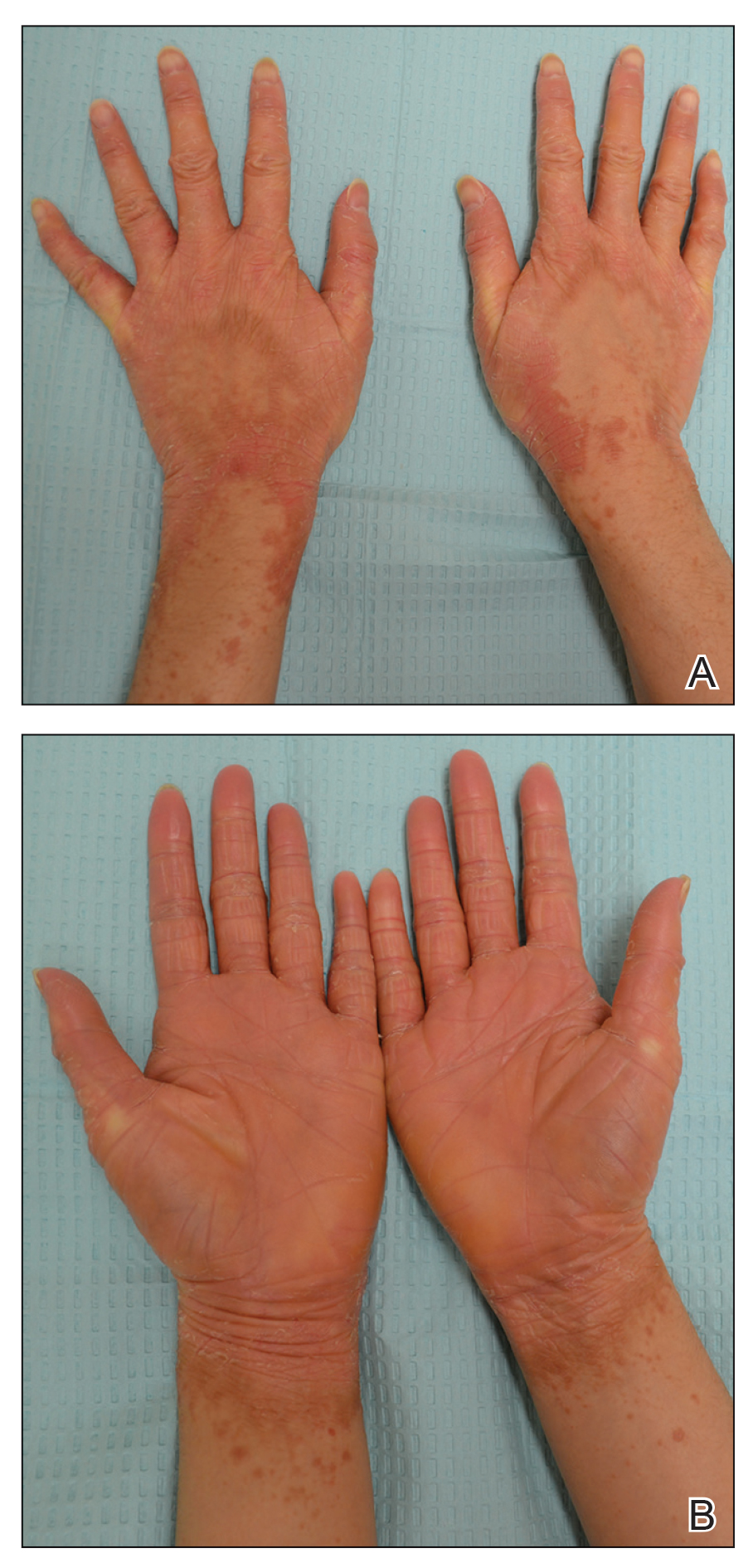
The patient’s medical history was notable for suspected psoriasis 9 years prior, but there were no records or biopsy reports that could be obtained to confirm the diagnosis. She also reported a similar skin condition in her father, which also was diagnosed as psoriasis, but this diagnosis could not be verified.
Although the morphology of the lesions was most consistent with localized PRP, atypical psoriasis, palmoplantar keratoderma (PPK), and erythroderma progressive symmetrica (EPS) also were considered given the personal and family history of suspected psoriasis. A biopsy could not be obtained due to an insurance issue. She was started on clobetasol cream 0.05% and ointment. At 2-week follow-up, her condition remained unchanged. Empiric systemic treatment was discussed, which would potentially work for diagnoses of both PRP and psoriasis. Due to the history of psoriasis and level of discomfort, cyclosporine 300 mg once daily was started to gain rapid control of the disease. Methotrexate also was considered due to its efficacy and economic considerations but was not selected due to patient concerns about the medication.
After 10 weeks of cyclosporine treatment, our patient showed some improvement of the skin with decreased scale and flattening of plaques but not complete resolution. At this point, a biopsy was able to be obtained with prior authorization. A 4-mm punch biopsy of the right flank demonstrated a psoriasiform and papillated epidermis with multifocally capped, compact parakeratosis and minimal lymphocytic infiltrate consistent with PRP. Although EPS also was on the histologic differential, clinical history was more consistent with a diagnosis of PRP. There was some minimal improvement with cyclosporine, but with the diagnosis of PRP confirmed, a systemic retinoid became the treatment of choice. Although acitretin is the preferred treatment for PRP, given that pregnancy would be contraindicated during and for 3 years following acitretin therapy, a trial of isotretinoin 40 mg once daily was started due to its shorter half-life compared to acitretin and was continued for 3 months (Figure 2).6,7
The diagnosis of PRP often can be challenging given the variety of clinical presentations. This case was an atypical presentation of PRP with several learning points, as our patient’s condition did not fit perfectly into any of the 6 types of PRP. The age of onset was atypical at 22 years old. Pityriasis rubra pilaris typically presents with a bimodal age distribution, appearing either in the first decade or the fifth to sixth decades of life.3,8 Her clinical presentation was atypical for adult-onset types I and II, which typically present with cephalocaudal progression or ichthyosiform dermatitis, respectively. Her presentation also was atypical for juvenile onset in types III, IV, and V, which tend to present in younger children and with different physical examination findings.3,8
The morphology of our patient’s lesions also was atypical for PRP, PPK, EPS, and psoriasis. The clinical presentation had features of these entities with erythema, fissuring, xerosis, carnauba wax–like appearance, symmetric scale, and well-demarcated plaques. Although these findings are not mutually exclusive, their combined presentation is atypical. Coupled with the ambiguous family history of similar skin disease in the patient’s father, the discussion of genodermatoses, particularly PPK, further confounded the diagnosis.4,9 When evaluating for PRP, especially with any family history of skin conditions, genodermatoses should be considered. Furthermore, our patient’s remote and unverifiable history of psoriasis serves as a cautionary reminder that prior diagnoses and medical history always should be reasonably scrutinized. Additionally, a drug-induced PRP eruption also should be considered. Although our patient received no medical treatment for the upper respiratory tract infection prior to the onset of PRP, there have been several reports of drug-induced PRP.10-12
The therapeutic challenge in this case is one that often is encountered in clinical practice. The health care system often may pose a barrier to diagnosis by inhibiting particular services required for adequate patient care. For our patient, diagnosis was delayed by several weeks due to difficulties obtaining a diagnostic skin biopsy. When faced with challenges from health care infrastructure, creativity with treatment options, such as finding an empiric treatment option (cyclosporine in this case), must be considered.
Systemic retinoids have been found to be efficacious treatment options for PRP, but when dealing with a woman of reproductive age, reproductive preferences must be discussed before identifying an appropriate treatment regimen.1,13-15 The half-life of acitretin compared to isotretinoin is 2 days vs 22 hours.6,16 With alcohol consumption, acitretin can be metabolized to etretinate, which has a half-life of 120 days.17 In our patient, isotretinoin was a more manageable option to allow for greater reproductive freedom upon treatment completion.
- Klein A, Landthaler M, Karrer S. Pityriasis rubra pilaris: a review of diagnosis and treatment. Am J Clin Dermatol. 2010;11:157-170.
- Shenefelt PD. Pityriasis rubra pilaris. Medscape website. Updated September 11, 2020. Accessed September 28, 2021. https://reference.medscape.com/article/1107742-overview
- Griffiths WA. Pityriasis rubra pilaris. Clin Exp Dermatol. 1980;5:105-112.
- Itin PH, Lautenschlager S. Palmoplantar keratoderma and associated syndromes. Semin Dermatol. 1995;14:152-161.
- Guidelines of care for psoriasis. Committee on Guidelines of Care. Task Force on Psoriasis. J Am Acad Dermatol. 1993;28:632-637.
- Larsen FG, Jakobsen P, Eriksen H, et al. The pharmacokinetics of acitretin and its 13-cis-metabolite in psoriatic patients. J Clin Pharmacol. 1991;31:477-483.
- Layton A. The use of isotretinoin in acne. Dermatoendocrinol. 2009;1:162-169.
- Sørensen KB, Thestrup-Pedersen K. Pityriasis rubra pilaris: a retrospective analysis of 43 patients. Acta Derm Venereol. 1999;79:405-406.
- Lucker GP, Van de Kerkhof PC, Steijlen PM. The hereditary palmoplantar keratoses: an updated review and classification. Br J Dermatol. 1994;131:1-14.
- Cutaneous reactions to labetalol. Br Med J. 1978;1:987.
- Plana A, Carrascosa JM, Vilavella M. Pityriasis rubra pilaris‐like reaction induced by imatinib. Clin Exp Dermatol. 2013;38:520-522.
- Gajinov ZT, Matc´ MB, Duran VD, et al. Drug-related pityriasis rubra pilaris with acantholysis. Vojnosanit Pregl. 2013;70:871-873.
- Clayton BD, Jorizzo JL, Hitchcock MG, et al. Adult pityriasis rubra pilaris: a 10-year case series. J Am Acad Dermatol. 1997;36:959-964.
- Cohen PR, Prystowsky JH. Pityriasis rubra pilaris: a review of diagnosis and treatment. J Am Acad Dermatol. 1989;20:801-807.
- Dicken CH. Isotretinoin treatment of pityriasis rubra pilaris. J Am Acad Dermatol. 1987;16(2 pt 1):297-301.
- Layton A. The use of isotretinoin in acne. Dermatoendocrinol. 2009;1:162-169.
- Grønhøj Larsen F, Steinkjer B, Jakobsen P, et al. Acitretin is converted to etretinate only during concomitant alcohol intake. Br J Dermatol. 2000;143:1164-1169.
To the Editor:
Pityriasis rubra pilaris (PRP) is a rare inflammatory dermatosis of unknown etiology characterized by erythematosquamous salmon-colored plaques with well-demarcated islands of unaffected skin and hyperkeratotic follicles.1 In the United States, an incidence of 1 in 3500to 5000 patients presenting to dermatology clinics has been reported.2 Pityriasis rubra pilaris has several subtypes and variability in presentation that can make accurate and timely diagnosis challenging.3-5 Herein, we present a case of PRP with complex diagnostic and therapeutic challenges.
A 22-year-old woman presented with symmetrical, well-demarcated, hyperkeratotic, erythematous plaques with a carnauba wax–like appearance on the palms (Figure 1), soles, elbows, and trunk covering approximately 5% of the body surface area. Two weeks prior to presentation, she experienced an upper respiratory tract infection without any treatment and subsequently developed redness on the palms, which became very hard and scaly. The redness then spread to the elbows, soles, and trunk. She reported itching as well as pain in areas of fissuring. Hand mobility became restricted due to thick scale.

The patient’s medical history was notable for suspected psoriasis 9 years prior, but there were no records or biopsy reports that could be obtained to confirm the diagnosis. She also reported a similar skin condition in her father, which also was diagnosed as psoriasis, but this diagnosis could not be verified.
Although the morphology of the lesions was most consistent with localized PRP, atypical psoriasis, palmoplantar keratoderma (PPK), and erythroderma progressive symmetrica (EPS) also were considered given the personal and family history of suspected psoriasis. A biopsy could not be obtained due to an insurance issue. She was started on clobetasol cream 0.05% and ointment. At 2-week follow-up, her condition remained unchanged. Empiric systemic treatment was discussed, which would potentially work for diagnoses of both PRP and psoriasis. Due to the history of psoriasis and level of discomfort, cyclosporine 300 mg once daily was started to gain rapid control of the disease. Methotrexate also was considered due to its efficacy and economic considerations but was not selected due to patient concerns about the medication.
After 10 weeks of cyclosporine treatment, our patient showed some improvement of the skin with decreased scale and flattening of plaques but not complete resolution. At this point, a biopsy was able to be obtained with prior authorization. A 4-mm punch biopsy of the right flank demonstrated a psoriasiform and papillated epidermis with multifocally capped, compact parakeratosis and minimal lymphocytic infiltrate consistent with PRP. Although EPS also was on the histologic differential, clinical history was more consistent with a diagnosis of PRP. There was some minimal improvement with cyclosporine, but with the diagnosis of PRP confirmed, a systemic retinoid became the treatment of choice. Although acitretin is the preferred treatment for PRP, given that pregnancy would be contraindicated during and for 3 years following acitretin therapy, a trial of isotretinoin 40 mg once daily was started due to its shorter half-life compared to acitretin and was continued for 3 months (Figure 2).6,7

The diagnosis of PRP often can be challenging given the variety of clinical presentations. This case was an atypical presentation of PRP with several learning points, as our patient’s condition did not fit perfectly into any of the 6 types of PRP. The age of onset was atypical at 22 years old. Pityriasis rubra pilaris typically presents with a bimodal age distribution, appearing either in the first decade or the fifth to sixth decades of life.3,8 Her clinical presentation was atypical for adult-onset types I and II, which typically present with cephalocaudal progression or ichthyosiform dermatitis, respectively. Her presentation also was atypical for juvenile onset in types III, IV, and V, which tend to present in younger children and with different physical examination findings.3,8
The morphology of our patient’s lesions also was atypical for PRP, PPK, EPS, and psoriasis. The clinical presentation had features of these entities with erythema, fissuring, xerosis, carnauba wax–like appearance, symmetric scale, and well-demarcated plaques. Although these findings are not mutually exclusive, their combined presentation is atypical. Coupled with the ambiguous family history of similar skin disease in the patient’s father, the discussion of genodermatoses, particularly PPK, further confounded the diagnosis.4,9 When evaluating for PRP, especially with any family history of skin conditions, genodermatoses should be considered. Furthermore, our patient’s remote and unverifiable history of psoriasis serves as a cautionary reminder that prior diagnoses and medical history always should be reasonably scrutinized. Additionally, a drug-induced PRP eruption also should be considered. Although our patient received no medical treatment for the upper respiratory tract infection prior to the onset of PRP, there have been several reports of drug-induced PRP.10-12
The therapeutic challenge in this case is one that often is encountered in clinical practice. The health care system often may pose a barrier to diagnosis by inhibiting particular services required for adequate patient care. For our patient, diagnosis was delayed by several weeks due to difficulties obtaining a diagnostic skin biopsy. When faced with challenges from health care infrastructure, creativity with treatment options, such as finding an empiric treatment option (cyclosporine in this case), must be considered.
Systemic retinoids have been found to be efficacious treatment options for PRP, but when dealing with a woman of reproductive age, reproductive preferences must be discussed before identifying an appropriate treatment regimen.1,13-15 The half-life of acitretin compared to isotretinoin is 2 days vs 22 hours.6,16 With alcohol consumption, acitretin can be metabolized to etretinate, which has a half-life of 120 days.17 In our patient, isotretinoin was a more manageable option to allow for greater reproductive freedom upon treatment completion.
To the Editor:
Pityriasis rubra pilaris (PRP) is a rare inflammatory dermatosis of unknown etiology characterized by erythematosquamous salmon-colored plaques with well-demarcated islands of unaffected skin and hyperkeratotic follicles.1 In the United States, an incidence of 1 in 3500to 5000 patients presenting to dermatology clinics has been reported.2 Pityriasis rubra pilaris has several subtypes and variability in presentation that can make accurate and timely diagnosis challenging.3-5 Herein, we present a case of PRP with complex diagnostic and therapeutic challenges.
A 22-year-old woman presented with symmetrical, well-demarcated, hyperkeratotic, erythematous plaques with a carnauba wax–like appearance on the palms (Figure 1), soles, elbows, and trunk covering approximately 5% of the body surface area. Two weeks prior to presentation, she experienced an upper respiratory tract infection without any treatment and subsequently developed redness on the palms, which became very hard and scaly. The redness then spread to the elbows, soles, and trunk. She reported itching as well as pain in areas of fissuring. Hand mobility became restricted due to thick scale.

The patient’s medical history was notable for suspected psoriasis 9 years prior, but there were no records or biopsy reports that could be obtained to confirm the diagnosis. She also reported a similar skin condition in her father, which also was diagnosed as psoriasis, but this diagnosis could not be verified.
Although the morphology of the lesions was most consistent with localized PRP, atypical psoriasis, palmoplantar keratoderma (PPK), and erythroderma progressive symmetrica (EPS) also were considered given the personal and family history of suspected psoriasis. A biopsy could not be obtained due to an insurance issue. She was started on clobetasol cream 0.05% and ointment. At 2-week follow-up, her condition remained unchanged. Empiric systemic treatment was discussed, which would potentially work for diagnoses of both PRP and psoriasis. Due to the history of psoriasis and level of discomfort, cyclosporine 300 mg once daily was started to gain rapid control of the disease. Methotrexate also was considered due to its efficacy and economic considerations but was not selected due to patient concerns about the medication.
After 10 weeks of cyclosporine treatment, our patient showed some improvement of the skin with decreased scale and flattening of plaques but not complete resolution. At this point, a biopsy was able to be obtained with prior authorization. A 4-mm punch biopsy of the right flank demonstrated a psoriasiform and papillated epidermis with multifocally capped, compact parakeratosis and minimal lymphocytic infiltrate consistent with PRP. Although EPS also was on the histologic differential, clinical history was more consistent with a diagnosis of PRP. There was some minimal improvement with cyclosporine, but with the diagnosis of PRP confirmed, a systemic retinoid became the treatment of choice. Although acitretin is the preferred treatment for PRP, given that pregnancy would be contraindicated during and for 3 years following acitretin therapy, a trial of isotretinoin 40 mg once daily was started due to its shorter half-life compared to acitretin and was continued for 3 months (Figure 2).6,7

The diagnosis of PRP often can be challenging given the variety of clinical presentations. This case was an atypical presentation of PRP with several learning points, as our patient’s condition did not fit perfectly into any of the 6 types of PRP. The age of onset was atypical at 22 years old. Pityriasis rubra pilaris typically presents with a bimodal age distribution, appearing either in the first decade or the fifth to sixth decades of life.3,8 Her clinical presentation was atypical for adult-onset types I and II, which typically present with cephalocaudal progression or ichthyosiform dermatitis, respectively. Her presentation also was atypical for juvenile onset in types III, IV, and V, which tend to present in younger children and with different physical examination findings.3,8
The morphology of our patient’s lesions also was atypical for PRP, PPK, EPS, and psoriasis. The clinical presentation had features of these entities with erythema, fissuring, xerosis, carnauba wax–like appearance, symmetric scale, and well-demarcated plaques. Although these findings are not mutually exclusive, their combined presentation is atypical. Coupled with the ambiguous family history of similar skin disease in the patient’s father, the discussion of genodermatoses, particularly PPK, further confounded the diagnosis.4,9 When evaluating for PRP, especially with any family history of skin conditions, genodermatoses should be considered. Furthermore, our patient’s remote and unverifiable history of psoriasis serves as a cautionary reminder that prior diagnoses and medical history always should be reasonably scrutinized. Additionally, a drug-induced PRP eruption also should be considered. Although our patient received no medical treatment for the upper respiratory tract infection prior to the onset of PRP, there have been several reports of drug-induced PRP.10-12
The therapeutic challenge in this case is one that often is encountered in clinical practice. The health care system often may pose a barrier to diagnosis by inhibiting particular services required for adequate patient care. For our patient, diagnosis was delayed by several weeks due to difficulties obtaining a diagnostic skin biopsy. When faced with challenges from health care infrastructure, creativity with treatment options, such as finding an empiric treatment option (cyclosporine in this case), must be considered.
Systemic retinoids have been found to be efficacious treatment options for PRP, but when dealing with a woman of reproductive age, reproductive preferences must be discussed before identifying an appropriate treatment regimen.1,13-15 The half-life of acitretin compared to isotretinoin is 2 days vs 22 hours.6,16 With alcohol consumption, acitretin can be metabolized to etretinate, which has a half-life of 120 days.17 In our patient, isotretinoin was a more manageable option to allow for greater reproductive freedom upon treatment completion.
- Klein A, Landthaler M, Karrer S. Pityriasis rubra pilaris: a review of diagnosis and treatment. Am J Clin Dermatol. 2010;11:157-170.
- Shenefelt PD. Pityriasis rubra pilaris. Medscape website. Updated September 11, 2020. Accessed September 28, 2021. https://reference.medscape.com/article/1107742-overview
- Griffiths WA. Pityriasis rubra pilaris. Clin Exp Dermatol. 1980;5:105-112.
- Itin PH, Lautenschlager S. Palmoplantar keratoderma and associated syndromes. Semin Dermatol. 1995;14:152-161.
- Guidelines of care for psoriasis. Committee on Guidelines of Care. Task Force on Psoriasis. J Am Acad Dermatol. 1993;28:632-637.
- Larsen FG, Jakobsen P, Eriksen H, et al. The pharmacokinetics of acitretin and its 13-cis-metabolite in psoriatic patients. J Clin Pharmacol. 1991;31:477-483.
- Layton A. The use of isotretinoin in acne. Dermatoendocrinol. 2009;1:162-169.
- Sørensen KB, Thestrup-Pedersen K. Pityriasis rubra pilaris: a retrospective analysis of 43 patients. Acta Derm Venereol. 1999;79:405-406.
- Lucker GP, Van de Kerkhof PC, Steijlen PM. The hereditary palmoplantar keratoses: an updated review and classification. Br J Dermatol. 1994;131:1-14.
- Cutaneous reactions to labetalol. Br Med J. 1978;1:987.
- Plana A, Carrascosa JM, Vilavella M. Pityriasis rubra pilaris‐like reaction induced by imatinib. Clin Exp Dermatol. 2013;38:520-522.
- Gajinov ZT, Matc´ MB, Duran VD, et al. Drug-related pityriasis rubra pilaris with acantholysis. Vojnosanit Pregl. 2013;70:871-873.
- Clayton BD, Jorizzo JL, Hitchcock MG, et al. Adult pityriasis rubra pilaris: a 10-year case series. J Am Acad Dermatol. 1997;36:959-964.
- Cohen PR, Prystowsky JH. Pityriasis rubra pilaris: a review of diagnosis and treatment. J Am Acad Dermatol. 1989;20:801-807.
- Dicken CH. Isotretinoin treatment of pityriasis rubra pilaris. J Am Acad Dermatol. 1987;16(2 pt 1):297-301.
- Layton A. The use of isotretinoin in acne. Dermatoendocrinol. 2009;1:162-169.
- Grønhøj Larsen F, Steinkjer B, Jakobsen P, et al. Acitretin is converted to etretinate only during concomitant alcohol intake. Br J Dermatol. 2000;143:1164-1169.
- Klein A, Landthaler M, Karrer S. Pityriasis rubra pilaris: a review of diagnosis and treatment. Am J Clin Dermatol. 2010;11:157-170.
- Shenefelt PD. Pityriasis rubra pilaris. Medscape website. Updated September 11, 2020. Accessed September 28, 2021. https://reference.medscape.com/article/1107742-overview
- Griffiths WA. Pityriasis rubra pilaris. Clin Exp Dermatol. 1980;5:105-112.
- Itin PH, Lautenschlager S. Palmoplantar keratoderma and associated syndromes. Semin Dermatol. 1995;14:152-161.
- Guidelines of care for psoriasis. Committee on Guidelines of Care. Task Force on Psoriasis. J Am Acad Dermatol. 1993;28:632-637.
- Larsen FG, Jakobsen P, Eriksen H, et al. The pharmacokinetics of acitretin and its 13-cis-metabolite in psoriatic patients. J Clin Pharmacol. 1991;31:477-483.
- Layton A. The use of isotretinoin in acne. Dermatoendocrinol. 2009;1:162-169.
- Sørensen KB, Thestrup-Pedersen K. Pityriasis rubra pilaris: a retrospective analysis of 43 patients. Acta Derm Venereol. 1999;79:405-406.
- Lucker GP, Van de Kerkhof PC, Steijlen PM. The hereditary palmoplantar keratoses: an updated review and classification. Br J Dermatol. 1994;131:1-14.
- Cutaneous reactions to labetalol. Br Med J. 1978;1:987.
- Plana A, Carrascosa JM, Vilavella M. Pityriasis rubra pilaris‐like reaction induced by imatinib. Clin Exp Dermatol. 2013;38:520-522.
- Gajinov ZT, Matc´ MB, Duran VD, et al. Drug-related pityriasis rubra pilaris with acantholysis. Vojnosanit Pregl. 2013;70:871-873.
- Clayton BD, Jorizzo JL, Hitchcock MG, et al. Adult pityriasis rubra pilaris: a 10-year case series. J Am Acad Dermatol. 1997;36:959-964.
- Cohen PR, Prystowsky JH. Pityriasis rubra pilaris: a review of diagnosis and treatment. J Am Acad Dermatol. 1989;20:801-807.
- Dicken CH. Isotretinoin treatment of pityriasis rubra pilaris. J Am Acad Dermatol. 1987;16(2 pt 1):297-301.
- Layton A. The use of isotretinoin in acne. Dermatoendocrinol. 2009;1:162-169.
- Grønhøj Larsen F, Steinkjer B, Jakobsen P, et al. Acitretin is converted to etretinate only during concomitant alcohol intake. Br J Dermatol. 2000;143:1164-1169.
Practice Points
- Pityriasis rubra pilaris (PRP) is a rare inflammatory dermatosis of unknown etiology characterized by erythematosquamous salmon-colored plaques with well-demarcated islands of unaffected skin and hyperkeratotic follicles.
- The diagnosis of PRP often can be challenging given the variety of clinical presentations.
Lessons from an ethnic skin center: Awareness and respect for diversity
With the strong likelihood that , according to a dermatologist with expertise in these types of cases who spoke at the Skin of Color Update 2021.
“Instead of avoiding the discussion of cultural practices, we should discuss them and be open about them. It fosters a comfortable environment, trust, and better compliance,” reported Neelam Ajit Vashi, MD, founding director of the Boston University Center for Ethnic Skin.
Out of fear of causing offense, a desire to be discreet, or of personal discomfort with foreign cultural practices, some clinicians might elect to limit themselves to the information that the patient volunteers, which is a mistake, according to Dr. Vashi.
“The avoidance of topics around culture actually limits the ability to have a successful relationship,” she maintained.
Successful encounters are not just based on a willingness to listen, Dr. Vashi said. Clinicians should be seeking a base of knowledge. With growing globalization and widespread immigration, “it is increasingly important for dermatologists in the U.S. to understand the role of cultural practices [in creating skin problems] and recognize the sequelae,” Dr. Vashi said.
Taking some common examples of dermatologic complaints created by cosmetic practices originating elsewhere, Dr. Vashi described key clinical points in addressing complications related to henna, hair removal through threading, and placement of decorative adornments on the forehead, called bindi. In addition, she pointed out common issues with facial and body marking created with kumkum powder, hair oils, and skin lightening agents.
Black henna
For cosmetic enhancement, henna is relatively benign. It is also no longer confined to the south Asian communities where it originated. However, Dr. Vashi pointed out that patients of south Asian origin or descent might be more likely to use black henna, a variety with more risks.
Black henna contains additives, such as diaminobenzenes and p-phenylenediamine (PPD), to darken the tone of the product as well as provide other desired characteristics, such as an accelerated drying time. While some patients do develop reactions to conventional henna, the risks of black henna are greater.
“The acute contact dermatitis reactions can include dyspigmentation, leukoderma, and keloids,” Dr. Vashi said. Other complications include erythema multiforme, temporary hypertrichosis, and systemic allergic reactions, such as angioedema.
While those who have had a reaction to henna should avoid further contact, Dr. Vashi warned that sequelae can include cross reactions with latex and rubber as well as some pharmaceutical agents, such as sulfonamides. When taking a patient history, she noted, be aware that risks of henna extend to the hairdressers and cosmeticians who sometimes apply these products on others.
Hair threading, bindi, and kumkum
Hair threading, another practice popularized in south Asia and now growing in popularity globally, involves capturing hairs between cotton threads for removal of both the hair and its follicle. It is a relatively rapid and efficient method of permanent depilation. In addition to pain and erythema, Dr. Vashi reported that the complications associated with hair threading include pigmentary changes, infections such as bullous impetigo, and lesions of koebnerization – such as vitiligo and lichen planus.
Bindi, a Hindi tradition that involves placing adornments between the eyebrows, and kumkum, a powder typically made from turmeric to be employed for decorative markings, have also spread to use outside of their cultural context, according to Dr. Vashi. She said that the complications of these two cosmetic practices are shared, and stem largely from contact dermatitis.
In the case of bindi, para-tertiary-butylphenol in adhesives is one source of reactions, whereas kumkum itself can be an irritant. As these are typically local to the site of application, the diagnosis is not difficult, but treatment can be more challenging for patients unwilling to abandon the practice.
Hair oils, skin-lightening agents
Culturally-linked hair oils among patients from south Asia or Africa – or descendants from these areas – can damage hair in a variety of ways as well as cause contact dermatitis. The oils can also exacerbate existing skin diseases.
“Oils with high oleic acid, such as coconut or olive oils or shea butter, can worsen seborrheic dermatitis,” Dr. Vashi cautioned.
Of this list of dermatologic issues induced by culturally linked cosmetic practices, skin lightening agents might pose the most risk for permanent and irreversible complications. Dr. Vashi said that up to 70% of patients using lighteners develop complications, and there is a relationship between the severity of side effects as duration of use increases.
“The problem is that ingredients of many of these products, which are imported illegally and sold on the black market, are often not disclosed,” Dr. Vashi said. Some contain a high content of metals such as lead, copper, and iron, whether they are added intentionally or end up in the product because of poor quality control. For those developing adverse events associated with the products, the obvious treatment is discontinuation.
When patients are unwilling to discontinue any of the products that have led to dermatologic issues, Dr. Vashi encouraged physicians “to take a middle ground.” Simple avoidance can be challenging for practices that are culturally meaningful. In respecting cultural differences, she encouraged tolerance and compromise.
“Often these patients will be doing an alternative medication or intervention, but this does not mean that they are not accepting what we have to offer,” she said. She indicated that mutual respect will lead to better solutions.
The awareness of common cultural practices that can have a harmful impact on the skin is an area of practice that deserves more attention, Andrew F. Alexis, MD, vice-chair for diversity and inclusion in the department of dermatology at Weill Cornell Medical Center, New York, said in an interview.
He said that he agreed with Dr. Vashi that understanding the role of cultural practices leading to dermatoses is not enough.
“Advising patients to alter or discontinue a specific cultural practice due to a dermatologic complication should be done with respect, humility, and understanding that may be challenging,” said Dr. Alexis.
While being aware of the specific cultural practices that might be causing or exacerbating dermatoses is important for accurate diagnosis, he said he believes that “partnering with the patient to modify the cultural practices in question” is important for a clinical outcome acceptable to the patient.
“Educational resources to inform clinicians of dermatoses associated with cultural practices are available and can be helpful for dermatologists in any practice setting,” he said.
Dr. Vashi reports that she has no relevant financial relationships to disclose. Dr. Alexis reports financial relationships with Abbvie, Allergan, Almirall, Amgen, Arcutis, AstraZeneca, Bristol-Myers Squibb, Cara, Galderma, Genzyme, Janssen, Leo, Menlo, Novartis, Regeneron, Sanofi, and Valeant.
With the strong likelihood that , according to a dermatologist with expertise in these types of cases who spoke at the Skin of Color Update 2021.
“Instead of avoiding the discussion of cultural practices, we should discuss them and be open about them. It fosters a comfortable environment, trust, and better compliance,” reported Neelam Ajit Vashi, MD, founding director of the Boston University Center for Ethnic Skin.
Out of fear of causing offense, a desire to be discreet, or of personal discomfort with foreign cultural practices, some clinicians might elect to limit themselves to the information that the patient volunteers, which is a mistake, according to Dr. Vashi.
“The avoidance of topics around culture actually limits the ability to have a successful relationship,” she maintained.
Successful encounters are not just based on a willingness to listen, Dr. Vashi said. Clinicians should be seeking a base of knowledge. With growing globalization and widespread immigration, “it is increasingly important for dermatologists in the U.S. to understand the role of cultural practices [in creating skin problems] and recognize the sequelae,” Dr. Vashi said.
Taking some common examples of dermatologic complaints created by cosmetic practices originating elsewhere, Dr. Vashi described key clinical points in addressing complications related to henna, hair removal through threading, and placement of decorative adornments on the forehead, called bindi. In addition, she pointed out common issues with facial and body marking created with kumkum powder, hair oils, and skin lightening agents.
Black henna
For cosmetic enhancement, henna is relatively benign. It is also no longer confined to the south Asian communities where it originated. However, Dr. Vashi pointed out that patients of south Asian origin or descent might be more likely to use black henna, a variety with more risks.
Black henna contains additives, such as diaminobenzenes and p-phenylenediamine (PPD), to darken the tone of the product as well as provide other desired characteristics, such as an accelerated drying time. While some patients do develop reactions to conventional henna, the risks of black henna are greater.
“The acute contact dermatitis reactions can include dyspigmentation, leukoderma, and keloids,” Dr. Vashi said. Other complications include erythema multiforme, temporary hypertrichosis, and systemic allergic reactions, such as angioedema.
While those who have had a reaction to henna should avoid further contact, Dr. Vashi warned that sequelae can include cross reactions with latex and rubber as well as some pharmaceutical agents, such as sulfonamides. When taking a patient history, she noted, be aware that risks of henna extend to the hairdressers and cosmeticians who sometimes apply these products on others.
Hair threading, bindi, and kumkum
Hair threading, another practice popularized in south Asia and now growing in popularity globally, involves capturing hairs between cotton threads for removal of both the hair and its follicle. It is a relatively rapid and efficient method of permanent depilation. In addition to pain and erythema, Dr. Vashi reported that the complications associated with hair threading include pigmentary changes, infections such as bullous impetigo, and lesions of koebnerization – such as vitiligo and lichen planus.
Bindi, a Hindi tradition that involves placing adornments between the eyebrows, and kumkum, a powder typically made from turmeric to be employed for decorative markings, have also spread to use outside of their cultural context, according to Dr. Vashi. She said that the complications of these two cosmetic practices are shared, and stem largely from contact dermatitis.
In the case of bindi, para-tertiary-butylphenol in adhesives is one source of reactions, whereas kumkum itself can be an irritant. As these are typically local to the site of application, the diagnosis is not difficult, but treatment can be more challenging for patients unwilling to abandon the practice.
Hair oils, skin-lightening agents
Culturally-linked hair oils among patients from south Asia or Africa – or descendants from these areas – can damage hair in a variety of ways as well as cause contact dermatitis. The oils can also exacerbate existing skin diseases.
“Oils with high oleic acid, such as coconut or olive oils or shea butter, can worsen seborrheic dermatitis,” Dr. Vashi cautioned.
Of this list of dermatologic issues induced by culturally linked cosmetic practices, skin lightening agents might pose the most risk for permanent and irreversible complications. Dr. Vashi said that up to 70% of patients using lighteners develop complications, and there is a relationship between the severity of side effects as duration of use increases.
“The problem is that ingredients of many of these products, which are imported illegally and sold on the black market, are often not disclosed,” Dr. Vashi said. Some contain a high content of metals such as lead, copper, and iron, whether they are added intentionally or end up in the product because of poor quality control. For those developing adverse events associated with the products, the obvious treatment is discontinuation.
When patients are unwilling to discontinue any of the products that have led to dermatologic issues, Dr. Vashi encouraged physicians “to take a middle ground.” Simple avoidance can be challenging for practices that are culturally meaningful. In respecting cultural differences, she encouraged tolerance and compromise.
“Often these patients will be doing an alternative medication or intervention, but this does not mean that they are not accepting what we have to offer,” she said. She indicated that mutual respect will lead to better solutions.
The awareness of common cultural practices that can have a harmful impact on the skin is an area of practice that deserves more attention, Andrew F. Alexis, MD, vice-chair for diversity and inclusion in the department of dermatology at Weill Cornell Medical Center, New York, said in an interview.
He said that he agreed with Dr. Vashi that understanding the role of cultural practices leading to dermatoses is not enough.
“Advising patients to alter or discontinue a specific cultural practice due to a dermatologic complication should be done with respect, humility, and understanding that may be challenging,” said Dr. Alexis.
While being aware of the specific cultural practices that might be causing or exacerbating dermatoses is important for accurate diagnosis, he said he believes that “partnering with the patient to modify the cultural practices in question” is important for a clinical outcome acceptable to the patient.
“Educational resources to inform clinicians of dermatoses associated with cultural practices are available and can be helpful for dermatologists in any practice setting,” he said.
Dr. Vashi reports that she has no relevant financial relationships to disclose. Dr. Alexis reports financial relationships with Abbvie, Allergan, Almirall, Amgen, Arcutis, AstraZeneca, Bristol-Myers Squibb, Cara, Galderma, Genzyme, Janssen, Leo, Menlo, Novartis, Regeneron, Sanofi, and Valeant.
With the strong likelihood that , according to a dermatologist with expertise in these types of cases who spoke at the Skin of Color Update 2021.
“Instead of avoiding the discussion of cultural practices, we should discuss them and be open about them. It fosters a comfortable environment, trust, and better compliance,” reported Neelam Ajit Vashi, MD, founding director of the Boston University Center for Ethnic Skin.
Out of fear of causing offense, a desire to be discreet, or of personal discomfort with foreign cultural practices, some clinicians might elect to limit themselves to the information that the patient volunteers, which is a mistake, according to Dr. Vashi.
“The avoidance of topics around culture actually limits the ability to have a successful relationship,” she maintained.
Successful encounters are not just based on a willingness to listen, Dr. Vashi said. Clinicians should be seeking a base of knowledge. With growing globalization and widespread immigration, “it is increasingly important for dermatologists in the U.S. to understand the role of cultural practices [in creating skin problems] and recognize the sequelae,” Dr. Vashi said.
Taking some common examples of dermatologic complaints created by cosmetic practices originating elsewhere, Dr. Vashi described key clinical points in addressing complications related to henna, hair removal through threading, and placement of decorative adornments on the forehead, called bindi. In addition, she pointed out common issues with facial and body marking created with kumkum powder, hair oils, and skin lightening agents.
Black henna
For cosmetic enhancement, henna is relatively benign. It is also no longer confined to the south Asian communities where it originated. However, Dr. Vashi pointed out that patients of south Asian origin or descent might be more likely to use black henna, a variety with more risks.
Black henna contains additives, such as diaminobenzenes and p-phenylenediamine (PPD), to darken the tone of the product as well as provide other desired characteristics, such as an accelerated drying time. While some patients do develop reactions to conventional henna, the risks of black henna are greater.
“The acute contact dermatitis reactions can include dyspigmentation, leukoderma, and keloids,” Dr. Vashi said. Other complications include erythema multiforme, temporary hypertrichosis, and systemic allergic reactions, such as angioedema.
While those who have had a reaction to henna should avoid further contact, Dr. Vashi warned that sequelae can include cross reactions with latex and rubber as well as some pharmaceutical agents, such as sulfonamides. When taking a patient history, she noted, be aware that risks of henna extend to the hairdressers and cosmeticians who sometimes apply these products on others.
Hair threading, bindi, and kumkum
Hair threading, another practice popularized in south Asia and now growing in popularity globally, involves capturing hairs between cotton threads for removal of both the hair and its follicle. It is a relatively rapid and efficient method of permanent depilation. In addition to pain and erythema, Dr. Vashi reported that the complications associated with hair threading include pigmentary changes, infections such as bullous impetigo, and lesions of koebnerization – such as vitiligo and lichen planus.
Bindi, a Hindi tradition that involves placing adornments between the eyebrows, and kumkum, a powder typically made from turmeric to be employed for decorative markings, have also spread to use outside of their cultural context, according to Dr. Vashi. She said that the complications of these two cosmetic practices are shared, and stem largely from contact dermatitis.
In the case of bindi, para-tertiary-butylphenol in adhesives is one source of reactions, whereas kumkum itself can be an irritant. As these are typically local to the site of application, the diagnosis is not difficult, but treatment can be more challenging for patients unwilling to abandon the practice.
Hair oils, skin-lightening agents
Culturally-linked hair oils among patients from south Asia or Africa – or descendants from these areas – can damage hair in a variety of ways as well as cause contact dermatitis. The oils can also exacerbate existing skin diseases.
“Oils with high oleic acid, such as coconut or olive oils or shea butter, can worsen seborrheic dermatitis,” Dr. Vashi cautioned.
Of this list of dermatologic issues induced by culturally linked cosmetic practices, skin lightening agents might pose the most risk for permanent and irreversible complications. Dr. Vashi said that up to 70% of patients using lighteners develop complications, and there is a relationship between the severity of side effects as duration of use increases.
“The problem is that ingredients of many of these products, which are imported illegally and sold on the black market, are often not disclosed,” Dr. Vashi said. Some contain a high content of metals such as lead, copper, and iron, whether they are added intentionally or end up in the product because of poor quality control. For those developing adverse events associated with the products, the obvious treatment is discontinuation.
When patients are unwilling to discontinue any of the products that have led to dermatologic issues, Dr. Vashi encouraged physicians “to take a middle ground.” Simple avoidance can be challenging for practices that are culturally meaningful. In respecting cultural differences, she encouraged tolerance and compromise.
“Often these patients will be doing an alternative medication or intervention, but this does not mean that they are not accepting what we have to offer,” she said. She indicated that mutual respect will lead to better solutions.
The awareness of common cultural practices that can have a harmful impact on the skin is an area of practice that deserves more attention, Andrew F. Alexis, MD, vice-chair for diversity and inclusion in the department of dermatology at Weill Cornell Medical Center, New York, said in an interview.
He said that he agreed with Dr. Vashi that understanding the role of cultural practices leading to dermatoses is not enough.
“Advising patients to alter or discontinue a specific cultural practice due to a dermatologic complication should be done with respect, humility, and understanding that may be challenging,” said Dr. Alexis.
While being aware of the specific cultural practices that might be causing or exacerbating dermatoses is important for accurate diagnosis, he said he believes that “partnering with the patient to modify the cultural practices in question” is important for a clinical outcome acceptable to the patient.
“Educational resources to inform clinicians of dermatoses associated with cultural practices are available and can be helpful for dermatologists in any practice setting,” he said.
Dr. Vashi reports that she has no relevant financial relationships to disclose. Dr. Alexis reports financial relationships with Abbvie, Allergan, Almirall, Amgen, Arcutis, AstraZeneca, Bristol-Myers Squibb, Cara, Galderma, Genzyme, Janssen, Leo, Menlo, Novartis, Regeneron, Sanofi, and Valeant.
FROM SOC 2021