User login
Taking a new obesity drug and birth control pills? Be careful
For women who are obese, daily life is wrought with landmines. Whether it’s the challenges of air travel because plane seats are too small, the need to shield themselves from the world’s discriminating eyes, or the great lengths many will go to achieve better health and the promise of longevity, navigating life as an obese person requires a thick skin.
So, it’s no wonder so many are willing to pay more than $1,000 a month out of pocket to get their hands on drugs like semaglutide (Ozempic and Wegovy) or tirzepatide (Mounjaro). The benefits of these drugs, which are part of a new class called glucagonlike peptide–1 (GLP-1) receptor agonists, include significant and rapid weight loss, blood sugar control, and improved life quality; they are unprecedented in a setting where surgery has long been considered the most effective long-term option.
On the flip side, the desire for rapid weight loss and better blood sugar control also comes with an unexpected cost. , making an unintended pregnancy more likely.
Neel Shah, MD, an endocrinologist and associate professor at the University of Texas Health Science Center at Houston, said he has had several patients become pregnant without intending to.
“It was when Mounjaro came out on the market when we started using it,” he said of the drug the Food and Drug Administration approved for type 2 diabetes in 2022. “It [the warning] was in the product insert, but clinically speaking, I don’t know if it was at the top of providers’ minds when they were prescribing Mounjaro.”
When asked if he believed that we were going to be seeing a significant increase in so-called Mounjaro babies, Dr. Shah was sure in his response.
“Absolutely. We will because the sheer volume [of patients] will increase,” he said.
It’s all in the gut
One of the ways that drugs like Mounjaro work is by delaying the time that it takes for food to move from the stomach to the small intestine. Although data are still evolving, it is believed that this process – delayed gastric emptying – may affect the absorption of birth control pills.
Dr. Shah said another theory is that vomiting, which is a common side effect of these types of drugs, also affects the pills’ ability to prevent pregnancy.
And “there’s a prolonged period of ramping up the dose because of the GI side effects,” said Pinar Kodaman, MD, PhD, a reproductive endocrinologist and assistant professor of gynecology at Yale University in New Haven, Conn.
“Initially, at the lowest dose, there may not be a lot of potential effect on absorption and gastric emptying. But as the dose goes up, it becomes more common, and it can cause diarrhea, which is another condition that can affect the absorption of any medication,” she said.
Unanticipated outcomes, extra prevention
Roughly 42% of women in the United States are obese, 40% of whom are between the ages of 20 and 39. Although these new drugs can improve fertility outcomes for women who are obese (especially those with polycystic ovary syndrome, or PCOS), only one – Mounjaro – currently carries a warning about birth control pill effectiveness on its label. Unfortunately, it appears that some doctors are unaware or not counseling patients about this risk, and the data are unclear about whether other drugs in this class, like Ozempic and Wegovy, have the same risks.
“To date, it hasn’t been a typical thing that we counsel about,” said Dr. Kodaman. “It’s all fairly new, but when we have patients on birth control pills, we do review other medications that they are on because some can affect efficacy, and it’s something to keep in mind.”
It’s also unclear if other forms of birth control – for example, birth control patches that deliver through the skin – might carry similar pregnancy risks. Dr. Shah said some of his patients who became pregnant without intending to were using these patches. This raises even more questions, since they deliver drugs through the skin directly into the bloodstream and not through the GI system.
What can women do to help ensure that they don’t become pregnant while using these drugs?
“I really think that if patients want to protect themselves from an unplanned pregnancy, that as soon as they start the GLP receptor agonists, it wouldn’t be a bad idea to use condoms, because the onset of action is pretty quick,” said Dr. Kodaman, noting also that “at the lowest dose there may not be a lot of potential effect on gastric emptying. But as the dose goes up, it becomes much more common or can cause diarrhea.”
Dr. Shah said that in his practice he’s “been telling patients to add barrier contraception” 4 weeks before they start their first dose “and at any dose adjustment.”
Zoobia Chaudhry, an obesity medicine doctor and assistant professor of medicine at Johns Hopkins University in Baltimore, recommends that “patients just make sure that the injection and medication that they take are at least 1 hour apart.”
“Most of the time, patients do take birth control before bedtime, so if the two are spaced, it should be OK,” she said.
Another option is for women to speak to their doctors about other contraceptive options like IUDs or implantable rods, where gastric absorption is not going to be an issue.
“There’s very little research on this class of drugs,” said Emily Goodstein, a 40-year-old small-business owner in Washington, who recently switched from Ozempic to Mounjaro. “Being a person who lives in a larger body is such a horrifying experience because of the way that the world discriminates against you.”
She appreciates the feeling of being proactive that these new drugs grant. It has “opened up a bunch of opportunities for me to be seen as a full individual by the medical establishment,” she said. “I was willing to take the risk, knowing that I would be on these drugs for the rest of my life.”
In addition to being what Dr. Goodstein refers to as a guinea pig, she said she made sure that her primary care doctor was aware that she was not trying or planning to become pregnant again. (She has a 3-year-old child.) Still, her doctor mentioned only the most common side effects linked to these drugs, like nausea, vomiting, and diarrhea, and did not mention the risk of pregnancy.
“Folks are really not talking about the reproductive implications,” she said, referring to members of a Facebook group on these drugs that she belongs to.
Like patients themselves, many doctors are just beginning to get their arms around these agents. “Awareness, education, provider involvement, and having a multidisciplinary team could help patients achieve the goals that they set out for themselves,” said Dr. Shah.
Clear conversations are key.
A version of this article first appeared on WebMD.com.
For women who are obese, daily life is wrought with landmines. Whether it’s the challenges of air travel because plane seats are too small, the need to shield themselves from the world’s discriminating eyes, or the great lengths many will go to achieve better health and the promise of longevity, navigating life as an obese person requires a thick skin.
So, it’s no wonder so many are willing to pay more than $1,000 a month out of pocket to get their hands on drugs like semaglutide (Ozempic and Wegovy) or tirzepatide (Mounjaro). The benefits of these drugs, which are part of a new class called glucagonlike peptide–1 (GLP-1) receptor agonists, include significant and rapid weight loss, blood sugar control, and improved life quality; they are unprecedented in a setting where surgery has long been considered the most effective long-term option.
On the flip side, the desire for rapid weight loss and better blood sugar control also comes with an unexpected cost. , making an unintended pregnancy more likely.
Neel Shah, MD, an endocrinologist and associate professor at the University of Texas Health Science Center at Houston, said he has had several patients become pregnant without intending to.
“It was when Mounjaro came out on the market when we started using it,” he said of the drug the Food and Drug Administration approved for type 2 diabetes in 2022. “It [the warning] was in the product insert, but clinically speaking, I don’t know if it was at the top of providers’ minds when they were prescribing Mounjaro.”
When asked if he believed that we were going to be seeing a significant increase in so-called Mounjaro babies, Dr. Shah was sure in his response.
“Absolutely. We will because the sheer volume [of patients] will increase,” he said.
It’s all in the gut
One of the ways that drugs like Mounjaro work is by delaying the time that it takes for food to move from the stomach to the small intestine. Although data are still evolving, it is believed that this process – delayed gastric emptying – may affect the absorption of birth control pills.
Dr. Shah said another theory is that vomiting, which is a common side effect of these types of drugs, also affects the pills’ ability to prevent pregnancy.
And “there’s a prolonged period of ramping up the dose because of the GI side effects,” said Pinar Kodaman, MD, PhD, a reproductive endocrinologist and assistant professor of gynecology at Yale University in New Haven, Conn.
“Initially, at the lowest dose, there may not be a lot of potential effect on absorption and gastric emptying. But as the dose goes up, it becomes more common, and it can cause diarrhea, which is another condition that can affect the absorption of any medication,” she said.
Unanticipated outcomes, extra prevention
Roughly 42% of women in the United States are obese, 40% of whom are between the ages of 20 and 39. Although these new drugs can improve fertility outcomes for women who are obese (especially those with polycystic ovary syndrome, or PCOS), only one – Mounjaro – currently carries a warning about birth control pill effectiveness on its label. Unfortunately, it appears that some doctors are unaware or not counseling patients about this risk, and the data are unclear about whether other drugs in this class, like Ozempic and Wegovy, have the same risks.
“To date, it hasn’t been a typical thing that we counsel about,” said Dr. Kodaman. “It’s all fairly new, but when we have patients on birth control pills, we do review other medications that they are on because some can affect efficacy, and it’s something to keep in mind.”
It’s also unclear if other forms of birth control – for example, birth control patches that deliver through the skin – might carry similar pregnancy risks. Dr. Shah said some of his patients who became pregnant without intending to were using these patches. This raises even more questions, since they deliver drugs through the skin directly into the bloodstream and not through the GI system.
What can women do to help ensure that they don’t become pregnant while using these drugs?
“I really think that if patients want to protect themselves from an unplanned pregnancy, that as soon as they start the GLP receptor agonists, it wouldn’t be a bad idea to use condoms, because the onset of action is pretty quick,” said Dr. Kodaman, noting also that “at the lowest dose there may not be a lot of potential effect on gastric emptying. But as the dose goes up, it becomes much more common or can cause diarrhea.”
Dr. Shah said that in his practice he’s “been telling patients to add barrier contraception” 4 weeks before they start their first dose “and at any dose adjustment.”
Zoobia Chaudhry, an obesity medicine doctor and assistant professor of medicine at Johns Hopkins University in Baltimore, recommends that “patients just make sure that the injection and medication that they take are at least 1 hour apart.”
“Most of the time, patients do take birth control before bedtime, so if the two are spaced, it should be OK,” she said.
Another option is for women to speak to their doctors about other contraceptive options like IUDs or implantable rods, where gastric absorption is not going to be an issue.
“There’s very little research on this class of drugs,” said Emily Goodstein, a 40-year-old small-business owner in Washington, who recently switched from Ozempic to Mounjaro. “Being a person who lives in a larger body is such a horrifying experience because of the way that the world discriminates against you.”
She appreciates the feeling of being proactive that these new drugs grant. It has “opened up a bunch of opportunities for me to be seen as a full individual by the medical establishment,” she said. “I was willing to take the risk, knowing that I would be on these drugs for the rest of my life.”
In addition to being what Dr. Goodstein refers to as a guinea pig, she said she made sure that her primary care doctor was aware that she was not trying or planning to become pregnant again. (She has a 3-year-old child.) Still, her doctor mentioned only the most common side effects linked to these drugs, like nausea, vomiting, and diarrhea, and did not mention the risk of pregnancy.
“Folks are really not talking about the reproductive implications,” she said, referring to members of a Facebook group on these drugs that she belongs to.
Like patients themselves, many doctors are just beginning to get their arms around these agents. “Awareness, education, provider involvement, and having a multidisciplinary team could help patients achieve the goals that they set out for themselves,” said Dr. Shah.
Clear conversations are key.
A version of this article first appeared on WebMD.com.
For women who are obese, daily life is wrought with landmines. Whether it’s the challenges of air travel because plane seats are too small, the need to shield themselves from the world’s discriminating eyes, or the great lengths many will go to achieve better health and the promise of longevity, navigating life as an obese person requires a thick skin.
So, it’s no wonder so many are willing to pay more than $1,000 a month out of pocket to get their hands on drugs like semaglutide (Ozempic and Wegovy) or tirzepatide (Mounjaro). The benefits of these drugs, which are part of a new class called glucagonlike peptide–1 (GLP-1) receptor agonists, include significant and rapid weight loss, blood sugar control, and improved life quality; they are unprecedented in a setting where surgery has long been considered the most effective long-term option.
On the flip side, the desire for rapid weight loss and better blood sugar control also comes with an unexpected cost. , making an unintended pregnancy more likely.
Neel Shah, MD, an endocrinologist and associate professor at the University of Texas Health Science Center at Houston, said he has had several patients become pregnant without intending to.
“It was when Mounjaro came out on the market when we started using it,” he said of the drug the Food and Drug Administration approved for type 2 diabetes in 2022. “It [the warning] was in the product insert, but clinically speaking, I don’t know if it was at the top of providers’ minds when they were prescribing Mounjaro.”
When asked if he believed that we were going to be seeing a significant increase in so-called Mounjaro babies, Dr. Shah was sure in his response.
“Absolutely. We will because the sheer volume [of patients] will increase,” he said.
It’s all in the gut
One of the ways that drugs like Mounjaro work is by delaying the time that it takes for food to move from the stomach to the small intestine. Although data are still evolving, it is believed that this process – delayed gastric emptying – may affect the absorption of birth control pills.
Dr. Shah said another theory is that vomiting, which is a common side effect of these types of drugs, also affects the pills’ ability to prevent pregnancy.
And “there’s a prolonged period of ramping up the dose because of the GI side effects,” said Pinar Kodaman, MD, PhD, a reproductive endocrinologist and assistant professor of gynecology at Yale University in New Haven, Conn.
“Initially, at the lowest dose, there may not be a lot of potential effect on absorption and gastric emptying. But as the dose goes up, it becomes more common, and it can cause diarrhea, which is another condition that can affect the absorption of any medication,” she said.
Unanticipated outcomes, extra prevention
Roughly 42% of women in the United States are obese, 40% of whom are between the ages of 20 and 39. Although these new drugs can improve fertility outcomes for women who are obese (especially those with polycystic ovary syndrome, or PCOS), only one – Mounjaro – currently carries a warning about birth control pill effectiveness on its label. Unfortunately, it appears that some doctors are unaware or not counseling patients about this risk, and the data are unclear about whether other drugs in this class, like Ozempic and Wegovy, have the same risks.
“To date, it hasn’t been a typical thing that we counsel about,” said Dr. Kodaman. “It’s all fairly new, but when we have patients on birth control pills, we do review other medications that they are on because some can affect efficacy, and it’s something to keep in mind.”
It’s also unclear if other forms of birth control – for example, birth control patches that deliver through the skin – might carry similar pregnancy risks. Dr. Shah said some of his patients who became pregnant without intending to were using these patches. This raises even more questions, since they deliver drugs through the skin directly into the bloodstream and not through the GI system.
What can women do to help ensure that they don’t become pregnant while using these drugs?
“I really think that if patients want to protect themselves from an unplanned pregnancy, that as soon as they start the GLP receptor agonists, it wouldn’t be a bad idea to use condoms, because the onset of action is pretty quick,” said Dr. Kodaman, noting also that “at the lowest dose there may not be a lot of potential effect on gastric emptying. But as the dose goes up, it becomes much more common or can cause diarrhea.”
Dr. Shah said that in his practice he’s “been telling patients to add barrier contraception” 4 weeks before they start their first dose “and at any dose adjustment.”
Zoobia Chaudhry, an obesity medicine doctor and assistant professor of medicine at Johns Hopkins University in Baltimore, recommends that “patients just make sure that the injection and medication that they take are at least 1 hour apart.”
“Most of the time, patients do take birth control before bedtime, so if the two are spaced, it should be OK,” she said.
Another option is for women to speak to their doctors about other contraceptive options like IUDs or implantable rods, where gastric absorption is not going to be an issue.
“There’s very little research on this class of drugs,” said Emily Goodstein, a 40-year-old small-business owner in Washington, who recently switched from Ozempic to Mounjaro. “Being a person who lives in a larger body is such a horrifying experience because of the way that the world discriminates against you.”
She appreciates the feeling of being proactive that these new drugs grant. It has “opened up a bunch of opportunities for me to be seen as a full individual by the medical establishment,” she said. “I was willing to take the risk, knowing that I would be on these drugs for the rest of my life.”
In addition to being what Dr. Goodstein refers to as a guinea pig, she said she made sure that her primary care doctor was aware that she was not trying or planning to become pregnant again. (She has a 3-year-old child.) Still, her doctor mentioned only the most common side effects linked to these drugs, like nausea, vomiting, and diarrhea, and did not mention the risk of pregnancy.
“Folks are really not talking about the reproductive implications,” she said, referring to members of a Facebook group on these drugs that she belongs to.
Like patients themselves, many doctors are just beginning to get their arms around these agents. “Awareness, education, provider involvement, and having a multidisciplinary team could help patients achieve the goals that they set out for themselves,” said Dr. Shah.
Clear conversations are key.
A version of this article first appeared on WebMD.com.
2023 Update on contraception
More US women are using IUDs than ever before. With more use comes the potential for complications and more requests related to non-contraceptive benefits. New information provides contemporary insight into rare IUD complications and the use of hormonal IUDs for treatment of HMB.
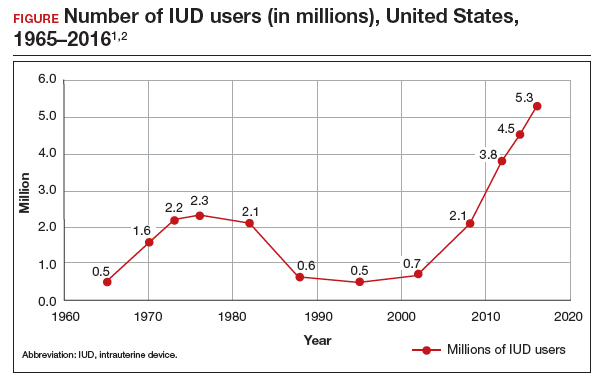
The first intrauterine device (IUD) to be approved in the United States, the Lippes Loop, became available in 1964. Sixty years later, more US women are using IUDs than ever before, and numbers are trending upward (FIGURE).1,2 Over the past year, contemporary information has become available to further inform IUD management when pregnancy occurs with an IUD in situ, as well as counseling about device breakage. Additionally, new data help clinicians expand which patients can use a levonorgestrel (LNG) 52-mg IUD for heavy menstrual bleeding (HMB) treatment.
As the total absolute number of IUD users increases, so do the absolute numbers of rare outcomes, such as pregnancy among IUD users. These highly effective contraceptives have a failure rate within the first year after placement ranging from 0.1% for the LNG 52-mg IUD to 0.8% for the copper 380-mm2 IUD.3 Although the possibility for extrauterine gestation is higher when pregnancy occurs while a patient is using an IUD as compared with most other contraceptive methods, most pregnancies that occur with an IUD in situ are intrauterine.4
The high contraceptive efficacy of IUDs make pregnancy with a retained IUD rare; therefore, it is difficult to perform a study with a large enough population to evaluate management of pregnancy complicated by an IUD in situ. Clinical management recommendations for these situations are 20 years old and are supported by limited data from case reports and series with fewer than 200 patients.5,6
Intrauterine device breakage is another rare event that is poorly understood due to the low absolute number of cases. Information about breakage has similarly been limited to case reports and case series.7,8 This past year, contemporary data were published to provide more insight into both intrauterine pregnancy with an IUD in situ and IUD breakage.
Beyond contraception, hormonal IUDs have become a popular and evidence-based treatment option for patients with HMB. The initial LNG 52-mg IUD (Mirena) regulatory approval studies for HMB treatment included data limited to parous patients and users with a body mass index (BMI) less than 35 kg/m2.9 Since that time, no studies have explored these populations. Although current practice has commonly extended use to include patients with these characteristics, we have lacked outcome data. New phase 3 data on the LNG 52-mg IUD (Liletta) included a broader range of participants and provide evidence to support this practice.
Removing retained copper 380-mm2 IUDs improves pregnancy outcomes
Panchal VR, Rau AR, Mandelbaum RS, et al. Pregnancy with retained intrauterine device: national-level assessment of characteristics and outcomes. Am J Obstet Gynecol MFM. 2023;5:101056. doi:10.1016/j.ajogmf.2023.101056
Karakuş SS, Karakuş R, Akalın EE, et al. Pregnancy outcomes with a copper 380 mm2 intrauterine device in place: a retrospective cohort study in Turkey, 2011-2021. Contraception. 2023;125:110090. doi:10.1016/j.contraception.2023.110090
To update our understanding of outcomes of pregnancy with an IUD in situ, Panchal and colleagues performed a cross-sectional study using the Healthcare Cost and Utilization Project’s National Inpatient Sample. This data set represents 85% of US hospital discharges. The population investigated included hospital deliveries from 2016 to 2020 with an ICD-10 (International Classification of Diseases, Tenth Revision) code of retained IUD. Those without the code were assigned to the comparison non-retained IUD group.
The primary outcome studied was the incidence rate of retained IUD, patient and pregnancy characteristics, and delivery outcomes including but not limited to gestational age at delivery, placental abnormalities, intrauterine fetal demise (IUFD), preterm premature rupture of membranes (PPROM), cesarean delivery, postpartum hemorrhage, and hysterectomy.
Outcomes were worse with retained IUD, regardless of IUD removal status
The authors found that an IUD in situ was reported in 1 out of 8,307 pregnancies and was associated with PPROM, fetal malpresentation, IUFD, placental abnormalities including abruption, accreta spectrum, retained placenta, and need for manual removal (TABLE 1). About three-quarters (76.3%) of patients had a term delivery (≥37 weeks).
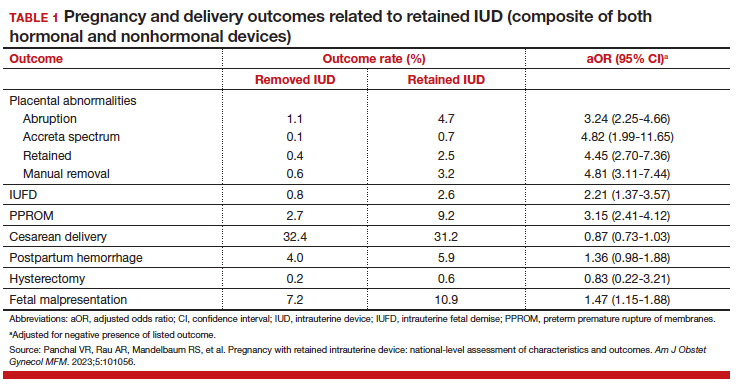
Retained IUD was associated with previable loss, defined as less than 22 weeks’ gestation (adjusted odds ratio [aOR], 5.49; 95% confidence interval [CI], 3.30–9.15) and periviable delivery, defined as 22 to 25 weeks’ gestation (aOR, 2.81; 95% CI, 1.63–4.85). Retained IUD was not associated with preterm delivery beyond 26 weeks’ gestation, cesarean delivery, postpartum hemorrhage, or hysterectomy.
Important limitations of this study are the lack of information on IUD type (copper vs hormonal) and the timing of removal or attempted removal in relation to measured pregnancy outcomes.
Continue to: Removal of copper IUD improves, but does not eliminate, poor pregnancy outcomes...
Removal of copper IUD improves, but does not eliminate, poor pregnancy outcomes
Karakus and colleagues conducted a retrospective cohort study of 233 patients in Turkey with pregnancies that occurred during copper 380-mm2 IUD use from 2011 to 2021. The authors reported that, at the time of first contact with the health system and diagnosis of retained IUD, 18.9% of the pregnancies were ectopic, 13.2% were first trimester losses, and 67.5% were ongoing pregnancies.
The authors assessed outcomes in patients with ongoing pregnancies based on whether or not the IUD was removed or retained. Outcomes included gestational age at delivery and adverse pregnancy outcomes, assessed as a composite of preterm delivery, PPROM, chorioamnionitis, placental abruption, and postpartum hemorrhage.
Of those with ongoing pregnancies, 13.3% chose to have an abortion, leaving 137 (86.7%) with continuing pregnancy. The IUD was able to be removed in 39.4% of the sample, with an average gestational age of 7 weeks at the time of removal.
Compared with those with a retained IUD, patients in the removal group had a lower rate of pregnancy loss (33.3% vs 61.4%; P<.001) and a lower rate of the composite adverse pregnancy outcomes (53.1% vs 27.8%; P=.03). TABLE 2 shows the approximate rate of ongoing pregnancy by gestational age in patients with retained and removed copper 380-mm2 IUDs. Notably, the largest change occurred periviably, with the proportion of patients with an ongoing pregnancy after 26 weeks reducing to about half for patients with a retained IUD as compared with patients with a removed IUD; this proportion of ongoing pregnancies held through the remainder of gestation.
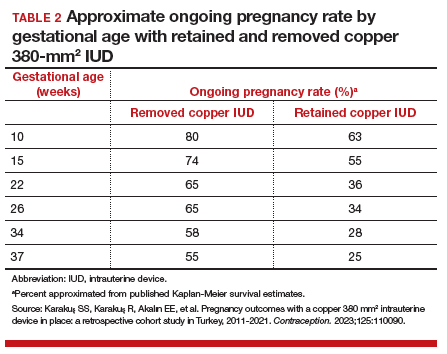
These studies confirm that a retained IUD is a rare outcome, occurring in about 1 in 8,000 pregnancies. Previous US national data from 2010 reported a similar incidence of 1 in 6,203 pregnancies (0.02%).10 Management and counseling depend on the patient’s desire to continue the pregnancy, gestational age, intrauterine IUD location, and ability to see the IUD strings. Contemporary data support management practices created from limited and outdated data, which include device removal (if able) and counseling those who desire to continue pregnancy about high-risk pregnancy complications. Those with a retained IUD should be counseled about increased risk of preterm or previable delivery, IUFD, and placental abnormalities (including accreta spectrum and retained placenta). Specifically, these contemporary data highlight that, beyond approximately 26 weeks’ gestation, the pregnancy loss rate is not different for those with a retained or removed IUD. Obstetric care providers should feel confident in using this more nuanced risk of extreme preterm delivery when counseling future patients. Implications for antepartum care and delivery timing with a retained IUD have not yet been defined.
Do national data reveal more breakage reports for copper 380-mm2 or LNG IUDs?
Latack KR, Nguyen BT. Trends in copper versus hormonal intrauterine device breakage reporting within the United States’ Food and Drug Administration Adverse Event Reporting System. Contraception. 2023;118:109909. doi:10.1016/j.contraception.2022.10.011
Latack and Nguyen reviewed postmarket surveillance data of IUD adverse events in the US Food and Drug Administration’s (FDA) Adverse Event Reporting System (FAERS) from 1998 to 2022. The FAERS is a voluntary, or passive, reporting system.
Study findings
Of the approximately 170,000 IUD-related adverse events reported to the agency during the 24-year timeframe, 25.4% were for copper IUDs and 74.6% were for hormonal IUDs. Slightly more than 4,000 reports were specific for device breakage, which the authors grouped into copper (copper 380-mm2)and hormonal (LNG 52 mg, 19.5 mg, and 13.5 mg) IUDs.
The copper 380-mm2 IUD was 6.19 times more likely to have a breakage report than hormonal IUDs (9.6% vs 1.7%; 95% CI, 5.87–6.53).
The overall proportion of IUD-related adverse events reported to the FDA was about 25% for copper and 75% for hormonal IUDs; this proportion is similar to sales figures, which show that about 15% of IUDs sold in the United States are copper and 85% are hormonal.11 However, the proportion of breakage events reported to the FDA is the inverse, with about 6 times more breakage reports with copper than with hormonal IUDs. Because these data come from a passive reporting system, the true incidence of IUD breakage cannot be assessed. However, these findings should remind clinicians to inform patients about this rare occurrence during counseling at the time of placement and, especially, when preparing for copper IUD removal. As the absolute number of IUD users increases, clinicians may be more likely to encounter this relatively rare event.
Management of IUD breakage is based on expert opinion, and recommendations are varied, ranging from observation to removal using an IUD hook, alligator forceps, manual vacuum aspiration, or hysteroscopy.7,10 Importantly, each individual patient situation will vary depending on the presence or absence of other symptoms and whether or not future pregnancy is desired.
Continue to: Data support the LNG 52-mg IUD for HMB in nulliparous and obese patients...
Data support the LNG 52-mg IUD for HMB in nulliparous and obese patients
Creinin MD, Barnhart KT, Gawron LM, et al. Heavy menstrual bleeding treatment with a levonorgestrel 52-mg intrauterine device. Obstet Gynecol. 2023;141:971-978. doi:10.1097AOG.0000000000005137
Creinin and colleagues conducted a study for US regulatory product approval of the LNG 52-mg IUD (Liletta) for HMB. This multicenter phase 3 open-label clinical trial recruited nonpregnant participants aged 18 to 50 years with HMB at 29 clinical sites in the United States. No BMI cutoff was used.
Baseline menstrual flow data were obtained over 2 to 3 screening cycles by collection of menstrual products and quantification of blood loss using alkaline hematin measurement. Patients with 2 cycles with a blood loss exceeding 80 mL had an IUD placement, with similar flow evaluations during the third and sixth postplacement cycles.
Treatment success was defined as a reduction in blood loss by more than 50% as compared with baseline (during screening) and measured blood loss of less than 80 mL. The enrolled population (n=105) included 28% nulliparous users, with 49% and 28% of participants having a BMI of 30 kg/m2 or higher and higher than 35 kg/m2, respectively.
Treatment highly successful in reducing blood loss
Participants in this trial had a 93% and a 98% reduction in blood loss at the third and sixth cycles of use, respectively. Additionally, during the sixth cycle of use, 19% of users had no bleeding. Treatment success occurred in about 80% of participants overall and occurred regardless of parity or BMI.
To assess a subjective measure of success, participants were asked to evaluate their menstrual bleeding and dysmenorrhea severity, acceptability, and overall impact on quality of life at 3 time points: during prior typical menses, cycle 3, and cycle 6. At cycle 6, all participants reported significantly improved acceptability of bleeding and uterine pain and, importantly, decreased overall menstrual interference with the ability to complete daily activities (TABLE 3).
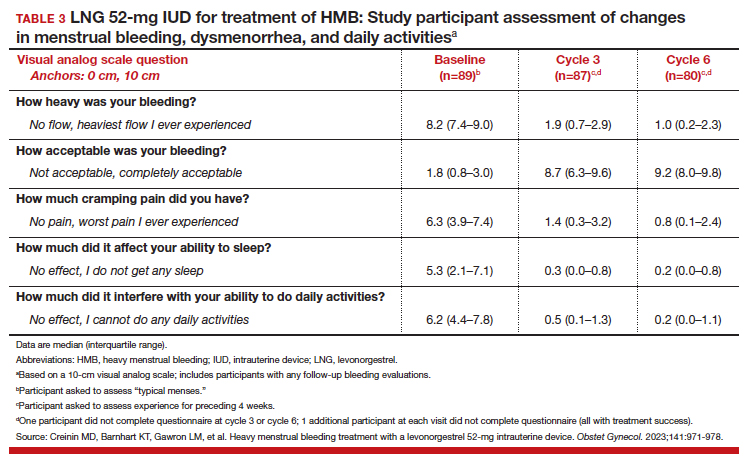
IUD expulsion and replacement rates
Although bleeding greatly decreased in all participants, 13% (n=14) discontinued before cycle 6 due to expulsion or IUD-related symptoms, with the majority citing bleeding irregularities. Expulsion occurred in 9% (n=5) of users, with the majority (2/3) occurring in the first 3 months of use and more commonly in obese and/or parous users. About half of participants with expulsion had the IUD replaced during the study. ●
Interestingly, both LNG 52-mg IUDs have been approved in most countries throughout the world for HMB treatment, and only in the United States was one of the products (Liletta) not approved until this past year. The FDA required more stringent trials than had been previously performed for approval outside of the United States. However, a benefit for clinicians is that this phase 3 study provided data in a contemporary US population. Clinicians can feel confident in counseling and offering the LNG 52-mg IUD as a first-line treatment option for patients with HMB, including those who have never been pregnant or have a BMI greater than 35 kg/m2.
Importantly, though, clinicians should be realistic with all patients that this treatment, although highly effective, is not successful for about 20% of patients by about 6 months of use. For those in whom the treatment is beneficial, the quality-of-life improvement is dramatic. Additionally, this study reminds us that expulsion risk in a population primarily using the IUD for HMB, especially if also obese and/or parous, is higher in the first 6 months of use than patients using the method for contraception. Expulsion occurs in 1.6% of contraception users through 6 months of use.12 These data highlight that IUD expulsion risk is not a fixed number, but instead is modified by patient characteristics. Patients should be counseled regarding the appropriate expulsion risk and that the IUD can be safely replaced should expulsion occur.
- Hubacher D, Kavanaugh M. Historical record-setting trends in IUD use in the United States. Contraception. 2018;98:467470. doi:10.1016/j.contraception.2018.05.016
- Kavanaugh ML, Pliskin E. Use of contraception among reproductive-aged women in the United States, 2014 and 2016. F S Rep. 2020;1:83-93. doi:10.1016/j.xfre.2020.06.006
- Jensen JT, Creinin MD. Speroff & Darney’s Clinical Guide to Contraception. 6th ed. Lippincott Williams & Wilkins; 2020:15.
- Jensen JT, Creinin MD. Speroff & Darney’s Clinical Guide to Contraception. 6th ed. Lippincott Williams & Wilkins; 2020:185.
- Ozgu-Erdinc AS, Tasdemir UG, Uygur D, et al. Outcome of intrauterine pregnancies with intrauterine device in place and effects of device location on prognosis. Contraception. 2014;89:426-430. doi:10.1016/j.contraception.2014.01.002
- Brahmi D, Steenland MW, Renner RM, et al. Pregnancy outcomes with an IUD in situ: a systematic review. Contraception. 2012;85:131-139. doi:10.1016/j.contraception . 2011.06.010
- Wilson S, Tan G, Baylson M, et al. Controversies in family planning: how to manage a fractured IUD. Contraception. 2013;88:599-603. doi:10.1016/j.contraception.2013.07.007
- Fulkerson Schaeffer S, Gimovsky AC, Aly H, et al. Pregnancy and delivery with an intrauterine device in situ: outcomes in the National Inpatient Sample Database. J Matern Fetal Neonatal Med. 2019;32:798-803. doi:10.1080/14767058.2017.1 391783
- Mirena. Prescribing information. Bayer HealthCare Pharmaceuticals. Accessed August 22, 2023. https://www .mirena-us.com/pi
- Myo MG, Nguyen BT. Intrauterine device complications and their management. Curr Obstet Gynecol Rep. 2023;12:88-95. doi.org/10.1007/s13669-023-00357-8
- National Center for Health Statistics (NCHS). 2017-2019 National Survey of Family Growth. Public-Use Data File Documentation. CDC National Center for Health Statistics. Accessed August 28, 2023. https://www.cdc.gov/nchs/data /nsfg/NSFG-2017-2019-UG-MainText-508.pdf
- Gilliam ML, Jensen JT, Eisenberg DL, et al. Relationship of parity and prior cesarean delivery to levonorgestrel 52 mg intrauterine system expulsion over 6 years. Contraception. 2021;103:444-449. doi: 10.1016/j.contraception.2021.02.013
More US women are using IUDs than ever before. With more use comes the potential for complications and more requests related to non-contraceptive benefits. New information provides contemporary insight into rare IUD complications and the use of hormonal IUDs for treatment of HMB.
The first intrauterine device (IUD) to be approved in the United States, the Lippes Loop, became available in 1964. Sixty years later, more US women are using IUDs than ever before, and numbers are trending upward (FIGURE).1,2 Over the past year, contemporary information has become available to further inform IUD management when pregnancy occurs with an IUD in situ, as well as counseling about device breakage. Additionally, new data help clinicians expand which patients can use a levonorgestrel (LNG) 52-mg IUD for heavy menstrual bleeding (HMB) treatment.

As the total absolute number of IUD users increases, so do the absolute numbers of rare outcomes, such as pregnancy among IUD users. These highly effective contraceptives have a failure rate within the first year after placement ranging from 0.1% for the LNG 52-mg IUD to 0.8% for the copper 380-mm2 IUD.3 Although the possibility for extrauterine gestation is higher when pregnancy occurs while a patient is using an IUD as compared with most other contraceptive methods, most pregnancies that occur with an IUD in situ are intrauterine.4
The high contraceptive efficacy of IUDs make pregnancy with a retained IUD rare; therefore, it is difficult to perform a study with a large enough population to evaluate management of pregnancy complicated by an IUD in situ. Clinical management recommendations for these situations are 20 years old and are supported by limited data from case reports and series with fewer than 200 patients.5,6
Intrauterine device breakage is another rare event that is poorly understood due to the low absolute number of cases. Information about breakage has similarly been limited to case reports and case series.7,8 This past year, contemporary data were published to provide more insight into both intrauterine pregnancy with an IUD in situ and IUD breakage.
Beyond contraception, hormonal IUDs have become a popular and evidence-based treatment option for patients with HMB. The initial LNG 52-mg IUD (Mirena) regulatory approval studies for HMB treatment included data limited to parous patients and users with a body mass index (BMI) less than 35 kg/m2.9 Since that time, no studies have explored these populations. Although current practice has commonly extended use to include patients with these characteristics, we have lacked outcome data. New phase 3 data on the LNG 52-mg IUD (Liletta) included a broader range of participants and provide evidence to support this practice.
Removing retained copper 380-mm2 IUDs improves pregnancy outcomes
Panchal VR, Rau AR, Mandelbaum RS, et al. Pregnancy with retained intrauterine device: national-level assessment of characteristics and outcomes. Am J Obstet Gynecol MFM. 2023;5:101056. doi:10.1016/j.ajogmf.2023.101056
Karakuş SS, Karakuş R, Akalın EE, et al. Pregnancy outcomes with a copper 380 mm2 intrauterine device in place: a retrospective cohort study in Turkey, 2011-2021. Contraception. 2023;125:110090. doi:10.1016/j.contraception.2023.110090
To update our understanding of outcomes of pregnancy with an IUD in situ, Panchal and colleagues performed a cross-sectional study using the Healthcare Cost and Utilization Project’s National Inpatient Sample. This data set represents 85% of US hospital discharges. The population investigated included hospital deliveries from 2016 to 2020 with an ICD-10 (International Classification of Diseases, Tenth Revision) code of retained IUD. Those without the code were assigned to the comparison non-retained IUD group.
The primary outcome studied was the incidence rate of retained IUD, patient and pregnancy characteristics, and delivery outcomes including but not limited to gestational age at delivery, placental abnormalities, intrauterine fetal demise (IUFD), preterm premature rupture of membranes (PPROM), cesarean delivery, postpartum hemorrhage, and hysterectomy.
Outcomes were worse with retained IUD, regardless of IUD removal status
The authors found that an IUD in situ was reported in 1 out of 8,307 pregnancies and was associated with PPROM, fetal malpresentation, IUFD, placental abnormalities including abruption, accreta spectrum, retained placenta, and need for manual removal (TABLE 1). About three-quarters (76.3%) of patients had a term delivery (≥37 weeks).

Retained IUD was associated with previable loss, defined as less than 22 weeks’ gestation (adjusted odds ratio [aOR], 5.49; 95% confidence interval [CI], 3.30–9.15) and periviable delivery, defined as 22 to 25 weeks’ gestation (aOR, 2.81; 95% CI, 1.63–4.85). Retained IUD was not associated with preterm delivery beyond 26 weeks’ gestation, cesarean delivery, postpartum hemorrhage, or hysterectomy.
Important limitations of this study are the lack of information on IUD type (copper vs hormonal) and the timing of removal or attempted removal in relation to measured pregnancy outcomes.
Continue to: Removal of copper IUD improves, but does not eliminate, poor pregnancy outcomes...
Removal of copper IUD improves, but does not eliminate, poor pregnancy outcomes
Karakus and colleagues conducted a retrospective cohort study of 233 patients in Turkey with pregnancies that occurred during copper 380-mm2 IUD use from 2011 to 2021. The authors reported that, at the time of first contact with the health system and diagnosis of retained IUD, 18.9% of the pregnancies were ectopic, 13.2% were first trimester losses, and 67.5% were ongoing pregnancies.
The authors assessed outcomes in patients with ongoing pregnancies based on whether or not the IUD was removed or retained. Outcomes included gestational age at delivery and adverse pregnancy outcomes, assessed as a composite of preterm delivery, PPROM, chorioamnionitis, placental abruption, and postpartum hemorrhage.
Of those with ongoing pregnancies, 13.3% chose to have an abortion, leaving 137 (86.7%) with continuing pregnancy. The IUD was able to be removed in 39.4% of the sample, with an average gestational age of 7 weeks at the time of removal.
Compared with those with a retained IUD, patients in the removal group had a lower rate of pregnancy loss (33.3% vs 61.4%; P<.001) and a lower rate of the composite adverse pregnancy outcomes (53.1% vs 27.8%; P=.03). TABLE 2 shows the approximate rate of ongoing pregnancy by gestational age in patients with retained and removed copper 380-mm2 IUDs. Notably, the largest change occurred periviably, with the proportion of patients with an ongoing pregnancy after 26 weeks reducing to about half for patients with a retained IUD as compared with patients with a removed IUD; this proportion of ongoing pregnancies held through the remainder of gestation.

These studies confirm that a retained IUD is a rare outcome, occurring in about 1 in 8,000 pregnancies. Previous US national data from 2010 reported a similar incidence of 1 in 6,203 pregnancies (0.02%).10 Management and counseling depend on the patient’s desire to continue the pregnancy, gestational age, intrauterine IUD location, and ability to see the IUD strings. Contemporary data support management practices created from limited and outdated data, which include device removal (if able) and counseling those who desire to continue pregnancy about high-risk pregnancy complications. Those with a retained IUD should be counseled about increased risk of preterm or previable delivery, IUFD, and placental abnormalities (including accreta spectrum and retained placenta). Specifically, these contemporary data highlight that, beyond approximately 26 weeks’ gestation, the pregnancy loss rate is not different for those with a retained or removed IUD. Obstetric care providers should feel confident in using this more nuanced risk of extreme preterm delivery when counseling future patients. Implications for antepartum care and delivery timing with a retained IUD have not yet been defined.
Do national data reveal more breakage reports for copper 380-mm2 or LNG IUDs?
Latack KR, Nguyen BT. Trends in copper versus hormonal intrauterine device breakage reporting within the United States’ Food and Drug Administration Adverse Event Reporting System. Contraception. 2023;118:109909. doi:10.1016/j.contraception.2022.10.011
Latack and Nguyen reviewed postmarket surveillance data of IUD adverse events in the US Food and Drug Administration’s (FDA) Adverse Event Reporting System (FAERS) from 1998 to 2022. The FAERS is a voluntary, or passive, reporting system.
Study findings
Of the approximately 170,000 IUD-related adverse events reported to the agency during the 24-year timeframe, 25.4% were for copper IUDs and 74.6% were for hormonal IUDs. Slightly more than 4,000 reports were specific for device breakage, which the authors grouped into copper (copper 380-mm2)and hormonal (LNG 52 mg, 19.5 mg, and 13.5 mg) IUDs.
The copper 380-mm2 IUD was 6.19 times more likely to have a breakage report than hormonal IUDs (9.6% vs 1.7%; 95% CI, 5.87–6.53).
The overall proportion of IUD-related adverse events reported to the FDA was about 25% for copper and 75% for hormonal IUDs; this proportion is similar to sales figures, which show that about 15% of IUDs sold in the United States are copper and 85% are hormonal.11 However, the proportion of breakage events reported to the FDA is the inverse, with about 6 times more breakage reports with copper than with hormonal IUDs. Because these data come from a passive reporting system, the true incidence of IUD breakage cannot be assessed. However, these findings should remind clinicians to inform patients about this rare occurrence during counseling at the time of placement and, especially, when preparing for copper IUD removal. As the absolute number of IUD users increases, clinicians may be more likely to encounter this relatively rare event.
Management of IUD breakage is based on expert opinion, and recommendations are varied, ranging from observation to removal using an IUD hook, alligator forceps, manual vacuum aspiration, or hysteroscopy.7,10 Importantly, each individual patient situation will vary depending on the presence or absence of other symptoms and whether or not future pregnancy is desired.
Continue to: Data support the LNG 52-mg IUD for HMB in nulliparous and obese patients...
Data support the LNG 52-mg IUD for HMB in nulliparous and obese patients
Creinin MD, Barnhart KT, Gawron LM, et al. Heavy menstrual bleeding treatment with a levonorgestrel 52-mg intrauterine device. Obstet Gynecol. 2023;141:971-978. doi:10.1097AOG.0000000000005137
Creinin and colleagues conducted a study for US regulatory product approval of the LNG 52-mg IUD (Liletta) for HMB. This multicenter phase 3 open-label clinical trial recruited nonpregnant participants aged 18 to 50 years with HMB at 29 clinical sites in the United States. No BMI cutoff was used.
Baseline menstrual flow data were obtained over 2 to 3 screening cycles by collection of menstrual products and quantification of blood loss using alkaline hematin measurement. Patients with 2 cycles with a blood loss exceeding 80 mL had an IUD placement, with similar flow evaluations during the third and sixth postplacement cycles.
Treatment success was defined as a reduction in blood loss by more than 50% as compared with baseline (during screening) and measured blood loss of less than 80 mL. The enrolled population (n=105) included 28% nulliparous users, with 49% and 28% of participants having a BMI of 30 kg/m2 or higher and higher than 35 kg/m2, respectively.
Treatment highly successful in reducing blood loss
Participants in this trial had a 93% and a 98% reduction in blood loss at the third and sixth cycles of use, respectively. Additionally, during the sixth cycle of use, 19% of users had no bleeding. Treatment success occurred in about 80% of participants overall and occurred regardless of parity or BMI.
To assess a subjective measure of success, participants were asked to evaluate their menstrual bleeding and dysmenorrhea severity, acceptability, and overall impact on quality of life at 3 time points: during prior typical menses, cycle 3, and cycle 6. At cycle 6, all participants reported significantly improved acceptability of bleeding and uterine pain and, importantly, decreased overall menstrual interference with the ability to complete daily activities (TABLE 3).

IUD expulsion and replacement rates
Although bleeding greatly decreased in all participants, 13% (n=14) discontinued before cycle 6 due to expulsion or IUD-related symptoms, with the majority citing bleeding irregularities. Expulsion occurred in 9% (n=5) of users, with the majority (2/3) occurring in the first 3 months of use and more commonly in obese and/or parous users. About half of participants with expulsion had the IUD replaced during the study. ●
Interestingly, both LNG 52-mg IUDs have been approved in most countries throughout the world for HMB treatment, and only in the United States was one of the products (Liletta) not approved until this past year. The FDA required more stringent trials than had been previously performed for approval outside of the United States. However, a benefit for clinicians is that this phase 3 study provided data in a contemporary US population. Clinicians can feel confident in counseling and offering the LNG 52-mg IUD as a first-line treatment option for patients with HMB, including those who have never been pregnant or have a BMI greater than 35 kg/m2.
Importantly, though, clinicians should be realistic with all patients that this treatment, although highly effective, is not successful for about 20% of patients by about 6 months of use. For those in whom the treatment is beneficial, the quality-of-life improvement is dramatic. Additionally, this study reminds us that expulsion risk in a population primarily using the IUD for HMB, especially if also obese and/or parous, is higher in the first 6 months of use than patients using the method for contraception. Expulsion occurs in 1.6% of contraception users through 6 months of use.12 These data highlight that IUD expulsion risk is not a fixed number, but instead is modified by patient characteristics. Patients should be counseled regarding the appropriate expulsion risk and that the IUD can be safely replaced should expulsion occur.
More US women are using IUDs than ever before. With more use comes the potential for complications and more requests related to non-contraceptive benefits. New information provides contemporary insight into rare IUD complications and the use of hormonal IUDs for treatment of HMB.
The first intrauterine device (IUD) to be approved in the United States, the Lippes Loop, became available in 1964. Sixty years later, more US women are using IUDs than ever before, and numbers are trending upward (FIGURE).1,2 Over the past year, contemporary information has become available to further inform IUD management when pregnancy occurs with an IUD in situ, as well as counseling about device breakage. Additionally, new data help clinicians expand which patients can use a levonorgestrel (LNG) 52-mg IUD for heavy menstrual bleeding (HMB) treatment.

As the total absolute number of IUD users increases, so do the absolute numbers of rare outcomes, such as pregnancy among IUD users. These highly effective contraceptives have a failure rate within the first year after placement ranging from 0.1% for the LNG 52-mg IUD to 0.8% for the copper 380-mm2 IUD.3 Although the possibility for extrauterine gestation is higher when pregnancy occurs while a patient is using an IUD as compared with most other contraceptive methods, most pregnancies that occur with an IUD in situ are intrauterine.4
The high contraceptive efficacy of IUDs make pregnancy with a retained IUD rare; therefore, it is difficult to perform a study with a large enough population to evaluate management of pregnancy complicated by an IUD in situ. Clinical management recommendations for these situations are 20 years old and are supported by limited data from case reports and series with fewer than 200 patients.5,6
Intrauterine device breakage is another rare event that is poorly understood due to the low absolute number of cases. Information about breakage has similarly been limited to case reports and case series.7,8 This past year, contemporary data were published to provide more insight into both intrauterine pregnancy with an IUD in situ and IUD breakage.
Beyond contraception, hormonal IUDs have become a popular and evidence-based treatment option for patients with HMB. The initial LNG 52-mg IUD (Mirena) regulatory approval studies for HMB treatment included data limited to parous patients and users with a body mass index (BMI) less than 35 kg/m2.9 Since that time, no studies have explored these populations. Although current practice has commonly extended use to include patients with these characteristics, we have lacked outcome data. New phase 3 data on the LNG 52-mg IUD (Liletta) included a broader range of participants and provide evidence to support this practice.
Removing retained copper 380-mm2 IUDs improves pregnancy outcomes
Panchal VR, Rau AR, Mandelbaum RS, et al. Pregnancy with retained intrauterine device: national-level assessment of characteristics and outcomes. Am J Obstet Gynecol MFM. 2023;5:101056. doi:10.1016/j.ajogmf.2023.101056
Karakuş SS, Karakuş R, Akalın EE, et al. Pregnancy outcomes with a copper 380 mm2 intrauterine device in place: a retrospective cohort study in Turkey, 2011-2021. Contraception. 2023;125:110090. doi:10.1016/j.contraception.2023.110090
To update our understanding of outcomes of pregnancy with an IUD in situ, Panchal and colleagues performed a cross-sectional study using the Healthcare Cost and Utilization Project’s National Inpatient Sample. This data set represents 85% of US hospital discharges. The population investigated included hospital deliveries from 2016 to 2020 with an ICD-10 (International Classification of Diseases, Tenth Revision) code of retained IUD. Those without the code were assigned to the comparison non-retained IUD group.
The primary outcome studied was the incidence rate of retained IUD, patient and pregnancy characteristics, and delivery outcomes including but not limited to gestational age at delivery, placental abnormalities, intrauterine fetal demise (IUFD), preterm premature rupture of membranes (PPROM), cesarean delivery, postpartum hemorrhage, and hysterectomy.
Outcomes were worse with retained IUD, regardless of IUD removal status
The authors found that an IUD in situ was reported in 1 out of 8,307 pregnancies and was associated with PPROM, fetal malpresentation, IUFD, placental abnormalities including abruption, accreta spectrum, retained placenta, and need for manual removal (TABLE 1). About three-quarters (76.3%) of patients had a term delivery (≥37 weeks).

Retained IUD was associated with previable loss, defined as less than 22 weeks’ gestation (adjusted odds ratio [aOR], 5.49; 95% confidence interval [CI], 3.30–9.15) and periviable delivery, defined as 22 to 25 weeks’ gestation (aOR, 2.81; 95% CI, 1.63–4.85). Retained IUD was not associated with preterm delivery beyond 26 weeks’ gestation, cesarean delivery, postpartum hemorrhage, or hysterectomy.
Important limitations of this study are the lack of information on IUD type (copper vs hormonal) and the timing of removal or attempted removal in relation to measured pregnancy outcomes.
Continue to: Removal of copper IUD improves, but does not eliminate, poor pregnancy outcomes...
Removal of copper IUD improves, but does not eliminate, poor pregnancy outcomes
Karakus and colleagues conducted a retrospective cohort study of 233 patients in Turkey with pregnancies that occurred during copper 380-mm2 IUD use from 2011 to 2021. The authors reported that, at the time of first contact with the health system and diagnosis of retained IUD, 18.9% of the pregnancies were ectopic, 13.2% were first trimester losses, and 67.5% were ongoing pregnancies.
The authors assessed outcomes in patients with ongoing pregnancies based on whether or not the IUD was removed or retained. Outcomes included gestational age at delivery and adverse pregnancy outcomes, assessed as a composite of preterm delivery, PPROM, chorioamnionitis, placental abruption, and postpartum hemorrhage.
Of those with ongoing pregnancies, 13.3% chose to have an abortion, leaving 137 (86.7%) with continuing pregnancy. The IUD was able to be removed in 39.4% of the sample, with an average gestational age of 7 weeks at the time of removal.
Compared with those with a retained IUD, patients in the removal group had a lower rate of pregnancy loss (33.3% vs 61.4%; P<.001) and a lower rate of the composite adverse pregnancy outcomes (53.1% vs 27.8%; P=.03). TABLE 2 shows the approximate rate of ongoing pregnancy by gestational age in patients with retained and removed copper 380-mm2 IUDs. Notably, the largest change occurred periviably, with the proportion of patients with an ongoing pregnancy after 26 weeks reducing to about half for patients with a retained IUD as compared with patients with a removed IUD; this proportion of ongoing pregnancies held through the remainder of gestation.

These studies confirm that a retained IUD is a rare outcome, occurring in about 1 in 8,000 pregnancies. Previous US national data from 2010 reported a similar incidence of 1 in 6,203 pregnancies (0.02%).10 Management and counseling depend on the patient’s desire to continue the pregnancy, gestational age, intrauterine IUD location, and ability to see the IUD strings. Contemporary data support management practices created from limited and outdated data, which include device removal (if able) and counseling those who desire to continue pregnancy about high-risk pregnancy complications. Those with a retained IUD should be counseled about increased risk of preterm or previable delivery, IUFD, and placental abnormalities (including accreta spectrum and retained placenta). Specifically, these contemporary data highlight that, beyond approximately 26 weeks’ gestation, the pregnancy loss rate is not different for those with a retained or removed IUD. Obstetric care providers should feel confident in using this more nuanced risk of extreme preterm delivery when counseling future patients. Implications for antepartum care and delivery timing with a retained IUD have not yet been defined.
Do national data reveal more breakage reports for copper 380-mm2 or LNG IUDs?
Latack KR, Nguyen BT. Trends in copper versus hormonal intrauterine device breakage reporting within the United States’ Food and Drug Administration Adverse Event Reporting System. Contraception. 2023;118:109909. doi:10.1016/j.contraception.2022.10.011
Latack and Nguyen reviewed postmarket surveillance data of IUD adverse events in the US Food and Drug Administration’s (FDA) Adverse Event Reporting System (FAERS) from 1998 to 2022. The FAERS is a voluntary, or passive, reporting system.
Study findings
Of the approximately 170,000 IUD-related adverse events reported to the agency during the 24-year timeframe, 25.4% were for copper IUDs and 74.6% were for hormonal IUDs. Slightly more than 4,000 reports were specific for device breakage, which the authors grouped into copper (copper 380-mm2)and hormonal (LNG 52 mg, 19.5 mg, and 13.5 mg) IUDs.
The copper 380-mm2 IUD was 6.19 times more likely to have a breakage report than hormonal IUDs (9.6% vs 1.7%; 95% CI, 5.87–6.53).
The overall proportion of IUD-related adverse events reported to the FDA was about 25% for copper and 75% for hormonal IUDs; this proportion is similar to sales figures, which show that about 15% of IUDs sold in the United States are copper and 85% are hormonal.11 However, the proportion of breakage events reported to the FDA is the inverse, with about 6 times more breakage reports with copper than with hormonal IUDs. Because these data come from a passive reporting system, the true incidence of IUD breakage cannot be assessed. However, these findings should remind clinicians to inform patients about this rare occurrence during counseling at the time of placement and, especially, when preparing for copper IUD removal. As the absolute number of IUD users increases, clinicians may be more likely to encounter this relatively rare event.
Management of IUD breakage is based on expert opinion, and recommendations are varied, ranging from observation to removal using an IUD hook, alligator forceps, manual vacuum aspiration, or hysteroscopy.7,10 Importantly, each individual patient situation will vary depending on the presence or absence of other symptoms and whether or not future pregnancy is desired.
Continue to: Data support the LNG 52-mg IUD for HMB in nulliparous and obese patients...
Data support the LNG 52-mg IUD for HMB in nulliparous and obese patients
Creinin MD, Barnhart KT, Gawron LM, et al. Heavy menstrual bleeding treatment with a levonorgestrel 52-mg intrauterine device. Obstet Gynecol. 2023;141:971-978. doi:10.1097AOG.0000000000005137
Creinin and colleagues conducted a study for US regulatory product approval of the LNG 52-mg IUD (Liletta) for HMB. This multicenter phase 3 open-label clinical trial recruited nonpregnant participants aged 18 to 50 years with HMB at 29 clinical sites in the United States. No BMI cutoff was used.
Baseline menstrual flow data were obtained over 2 to 3 screening cycles by collection of menstrual products and quantification of blood loss using alkaline hematin measurement. Patients with 2 cycles with a blood loss exceeding 80 mL had an IUD placement, with similar flow evaluations during the third and sixth postplacement cycles.
Treatment success was defined as a reduction in blood loss by more than 50% as compared with baseline (during screening) and measured blood loss of less than 80 mL. The enrolled population (n=105) included 28% nulliparous users, with 49% and 28% of participants having a BMI of 30 kg/m2 or higher and higher than 35 kg/m2, respectively.
Treatment highly successful in reducing blood loss
Participants in this trial had a 93% and a 98% reduction in blood loss at the third and sixth cycles of use, respectively. Additionally, during the sixth cycle of use, 19% of users had no bleeding. Treatment success occurred in about 80% of participants overall and occurred regardless of parity or BMI.
To assess a subjective measure of success, participants were asked to evaluate their menstrual bleeding and dysmenorrhea severity, acceptability, and overall impact on quality of life at 3 time points: during prior typical menses, cycle 3, and cycle 6. At cycle 6, all participants reported significantly improved acceptability of bleeding and uterine pain and, importantly, decreased overall menstrual interference with the ability to complete daily activities (TABLE 3).

IUD expulsion and replacement rates
Although bleeding greatly decreased in all participants, 13% (n=14) discontinued before cycle 6 due to expulsion or IUD-related symptoms, with the majority citing bleeding irregularities. Expulsion occurred in 9% (n=5) of users, with the majority (2/3) occurring in the first 3 months of use and more commonly in obese and/or parous users. About half of participants with expulsion had the IUD replaced during the study. ●
Interestingly, both LNG 52-mg IUDs have been approved in most countries throughout the world for HMB treatment, and only in the United States was one of the products (Liletta) not approved until this past year. The FDA required more stringent trials than had been previously performed for approval outside of the United States. However, a benefit for clinicians is that this phase 3 study provided data in a contemporary US population. Clinicians can feel confident in counseling and offering the LNG 52-mg IUD as a first-line treatment option for patients with HMB, including those who have never been pregnant or have a BMI greater than 35 kg/m2.
Importantly, though, clinicians should be realistic with all patients that this treatment, although highly effective, is not successful for about 20% of patients by about 6 months of use. For those in whom the treatment is beneficial, the quality-of-life improvement is dramatic. Additionally, this study reminds us that expulsion risk in a population primarily using the IUD for HMB, especially if also obese and/or parous, is higher in the first 6 months of use than patients using the method for contraception. Expulsion occurs in 1.6% of contraception users through 6 months of use.12 These data highlight that IUD expulsion risk is not a fixed number, but instead is modified by patient characteristics. Patients should be counseled regarding the appropriate expulsion risk and that the IUD can be safely replaced should expulsion occur.
- Hubacher D, Kavanaugh M. Historical record-setting trends in IUD use in the United States. Contraception. 2018;98:467470. doi:10.1016/j.contraception.2018.05.016
- Kavanaugh ML, Pliskin E. Use of contraception among reproductive-aged women in the United States, 2014 and 2016. F S Rep. 2020;1:83-93. doi:10.1016/j.xfre.2020.06.006
- Jensen JT, Creinin MD. Speroff & Darney’s Clinical Guide to Contraception. 6th ed. Lippincott Williams & Wilkins; 2020:15.
- Jensen JT, Creinin MD. Speroff & Darney’s Clinical Guide to Contraception. 6th ed. Lippincott Williams & Wilkins; 2020:185.
- Ozgu-Erdinc AS, Tasdemir UG, Uygur D, et al. Outcome of intrauterine pregnancies with intrauterine device in place and effects of device location on prognosis. Contraception. 2014;89:426-430. doi:10.1016/j.contraception.2014.01.002
- Brahmi D, Steenland MW, Renner RM, et al. Pregnancy outcomes with an IUD in situ: a systematic review. Contraception. 2012;85:131-139. doi:10.1016/j.contraception . 2011.06.010
- Wilson S, Tan G, Baylson M, et al. Controversies in family planning: how to manage a fractured IUD. Contraception. 2013;88:599-603. doi:10.1016/j.contraception.2013.07.007
- Fulkerson Schaeffer S, Gimovsky AC, Aly H, et al. Pregnancy and delivery with an intrauterine device in situ: outcomes in the National Inpatient Sample Database. J Matern Fetal Neonatal Med. 2019;32:798-803. doi:10.1080/14767058.2017.1 391783
- Mirena. Prescribing information. Bayer HealthCare Pharmaceuticals. Accessed August 22, 2023. https://www .mirena-us.com/pi
- Myo MG, Nguyen BT. Intrauterine device complications and their management. Curr Obstet Gynecol Rep. 2023;12:88-95. doi.org/10.1007/s13669-023-00357-8
- National Center for Health Statistics (NCHS). 2017-2019 National Survey of Family Growth. Public-Use Data File Documentation. CDC National Center for Health Statistics. Accessed August 28, 2023. https://www.cdc.gov/nchs/data /nsfg/NSFG-2017-2019-UG-MainText-508.pdf
- Gilliam ML, Jensen JT, Eisenberg DL, et al. Relationship of parity and prior cesarean delivery to levonorgestrel 52 mg intrauterine system expulsion over 6 years. Contraception. 2021;103:444-449. doi: 10.1016/j.contraception.2021.02.013
- Hubacher D, Kavanaugh M. Historical record-setting trends in IUD use in the United States. Contraception. 2018;98:467470. doi:10.1016/j.contraception.2018.05.016
- Kavanaugh ML, Pliskin E. Use of contraception among reproductive-aged women in the United States, 2014 and 2016. F S Rep. 2020;1:83-93. doi:10.1016/j.xfre.2020.06.006
- Jensen JT, Creinin MD. Speroff & Darney’s Clinical Guide to Contraception. 6th ed. Lippincott Williams & Wilkins; 2020:15.
- Jensen JT, Creinin MD. Speroff & Darney’s Clinical Guide to Contraception. 6th ed. Lippincott Williams & Wilkins; 2020:185.
- Ozgu-Erdinc AS, Tasdemir UG, Uygur D, et al. Outcome of intrauterine pregnancies with intrauterine device in place and effects of device location on prognosis. Contraception. 2014;89:426-430. doi:10.1016/j.contraception.2014.01.002
- Brahmi D, Steenland MW, Renner RM, et al. Pregnancy outcomes with an IUD in situ: a systematic review. Contraception. 2012;85:131-139. doi:10.1016/j.contraception . 2011.06.010
- Wilson S, Tan G, Baylson M, et al. Controversies in family planning: how to manage a fractured IUD. Contraception. 2013;88:599-603. doi:10.1016/j.contraception.2013.07.007
- Fulkerson Schaeffer S, Gimovsky AC, Aly H, et al. Pregnancy and delivery with an intrauterine device in situ: outcomes in the National Inpatient Sample Database. J Matern Fetal Neonatal Med. 2019;32:798-803. doi:10.1080/14767058.2017.1 391783
- Mirena. Prescribing information. Bayer HealthCare Pharmaceuticals. Accessed August 22, 2023. https://www .mirena-us.com/pi
- Myo MG, Nguyen BT. Intrauterine device complications and their management. Curr Obstet Gynecol Rep. 2023;12:88-95. doi.org/10.1007/s13669-023-00357-8
- National Center for Health Statistics (NCHS). 2017-2019 National Survey of Family Growth. Public-Use Data File Documentation. CDC National Center for Health Statistics. Accessed August 28, 2023. https://www.cdc.gov/nchs/data /nsfg/NSFG-2017-2019-UG-MainText-508.pdf
- Gilliam ML, Jensen JT, Eisenberg DL, et al. Relationship of parity and prior cesarean delivery to levonorgestrel 52 mg intrauterine system expulsion over 6 years. Contraception. 2021;103:444-449. doi: 10.1016/j.contraception.2021.02.013
Federal Health Care Data Trends 2023
Federal Health Care Data Trends (click to view the digital edition) is a special supplement to Federal Practitioner, highlighting the latest research and study outcomes related to the health of veteran and active-duty populations.
In this issue:
- Limb Loss and Prostheses
- Neurology
- Cardiology
- Mental Health
- Diabetes
- Rheumatoid Arthritis
- Respiratory illnesses
- Women's Health
- HPV and Related Cancers
Federal Health Care Data Trends (click to view the digital edition) is a special supplement to Federal Practitioner, highlighting the latest research and study outcomes related to the health of veteran and active-duty populations.
In this issue:
- Limb Loss and Prostheses
- Neurology
- Cardiology
- Mental Health
- Diabetes
- Rheumatoid Arthritis
- Respiratory illnesses
- Women's Health
- HPV and Related Cancers
Federal Health Care Data Trends (click to view the digital edition) is a special supplement to Federal Practitioner, highlighting the latest research and study outcomes related to the health of veteran and active-duty populations.
In this issue:
- Limb Loss and Prostheses
- Neurology
- Cardiology
- Mental Health
- Diabetes
- Rheumatoid Arthritis
- Respiratory illnesses
- Women's Health
- HPV and Related Cancers
Piroxicam boosts success of levonorgestrel for emergency contraception
Adding oral piroxicam to oral levonorgestrel significantly improved the efficacy of emergency contraception, based on data from 860 women.
Oral hormonal emergency contraception (EC) is the most widely used EC method worldwide, but the two currently available drugs, levonorgestrel and ulipristal acetate (UPA), are not effective when given after ovulation, wrote Raymond Hang Wun Li, MD, of the University of Hong Kong, and colleagues. Previous studies suggest that cyclo-oxygenase (COX) inhibitors may disrupt follicular rupture and prevent ovulation, but data on their use in combination with current oral ECs are lacking, the researchers said.
In a study published in The Lancet, the researchers randomized 430 women to receive a single oral dose of 1.5 mg levonorgestrel plus 40 mg of the COX-2 inhibitor piroxicam or 1.5 mg levonorgestrel plus a placebo. The study participants were women aged 18 years and older who requested EC within 72 hours of unprotected sex and who had regular menstrual cycles between 24 and 42 days long. The median age of the participants was 30 years; 97% were Chinese. The median time from intercourse to treatment was 18 hours for both groups.
The primary outcome was the percentage of pregnancies prevented, based on pregnancy status 1-2 weeks after treatment.
One pregnancy occurred in the piroxicam group, compared with seven pregnancies in the placebo group, which translated to a significant difference in the percentage of pregnancies prevented (94.7% vs. 63.4%, P < .0001).
No trend toward increased failure rates appeared based on the time elapsed between intercourse and EC use in either group, and no differences appeared in the return or delay of subsequent menstrual periods between the groups.
The most common adverse events (reported by more than 5% of participants in both groups) included fatigue or weakness, nausea, lower abdominal pain, dizziness, and headache.
The choice of piroxicam as the COX inhibitor in conjunction with levonorgestrel for the current study had several potential advantages, the researchers wrote in their discussion. These advantages include the widespread availability and long-acting characteristics of piroxicam, which is also true of levonorgestrel, they said.
The findings were limited by several factors including the generalizability to other settings and populations, the researchers noted. The efficacy of the levonorgestrel/piroxicam combination in women with a body mass index greater than 26 kg/m2 may be lower, but the current study population did not have enough women in this category to measure the potential effect, they said. The study also did not examine the effect of piroxicam in combination with ulipristal acetate.
However, the results are the first known to demonstrate the improved effectiveness of oral piroxicam coadministered with oral levonorgestrel for EC, they said.
“The strength of this recommendation and changes in clinical guidelines may be determined upon demonstration of reproducible results in further studies,” they added.
Pill combination shows potential and practicality
Oral emergency contraception on demand is an unmet need on a global level, Erica P. Cahill, MD, of the department of obstetrics and gynecology and division of family planning services at Stanford (Calif.) University, wrote in an accompanying editorial.
Dr. Cahill noted the longer half-life of piroxicam compared with other COX-2 inhibitors, which made it a practical choice. Although the study was not powered to evaluate secondary outcomes, bleeding patterns consistent with use of EC pills were observed. Documentation of these patterns is worthwhile, Dr. Cahill said, “because people using emergency contraceptive pills might also be using fertility awareness methods and need to know when they can be certain they are not pregnant.”
Overall, the study supports the addition of 40 mg piroxicam to 1.5 mg levonorgestrel as emergency contraception, said Dr. Cahill. Future studies can build on the current findings by evaluating repeat dosing of the piroxicam/levonorgestrel combination and by evaluating the combination of COX-2 inhibitors and ulipristal acetate to prevent pregnancy, she said.
The study received no outside funding. The researchers and Dr. Cahill had no financial conflicts to disclose.
Adding oral piroxicam to oral levonorgestrel significantly improved the efficacy of emergency contraception, based on data from 860 women.
Oral hormonal emergency contraception (EC) is the most widely used EC method worldwide, but the two currently available drugs, levonorgestrel and ulipristal acetate (UPA), are not effective when given after ovulation, wrote Raymond Hang Wun Li, MD, of the University of Hong Kong, and colleagues. Previous studies suggest that cyclo-oxygenase (COX) inhibitors may disrupt follicular rupture and prevent ovulation, but data on their use in combination with current oral ECs are lacking, the researchers said.
In a study published in The Lancet, the researchers randomized 430 women to receive a single oral dose of 1.5 mg levonorgestrel plus 40 mg of the COX-2 inhibitor piroxicam or 1.5 mg levonorgestrel plus a placebo. The study participants were women aged 18 years and older who requested EC within 72 hours of unprotected sex and who had regular menstrual cycles between 24 and 42 days long. The median age of the participants was 30 years; 97% were Chinese. The median time from intercourse to treatment was 18 hours for both groups.
The primary outcome was the percentage of pregnancies prevented, based on pregnancy status 1-2 weeks after treatment.
One pregnancy occurred in the piroxicam group, compared with seven pregnancies in the placebo group, which translated to a significant difference in the percentage of pregnancies prevented (94.7% vs. 63.4%, P < .0001).
No trend toward increased failure rates appeared based on the time elapsed between intercourse and EC use in either group, and no differences appeared in the return or delay of subsequent menstrual periods between the groups.
The most common adverse events (reported by more than 5% of participants in both groups) included fatigue or weakness, nausea, lower abdominal pain, dizziness, and headache.
The choice of piroxicam as the COX inhibitor in conjunction with levonorgestrel for the current study had several potential advantages, the researchers wrote in their discussion. These advantages include the widespread availability and long-acting characteristics of piroxicam, which is also true of levonorgestrel, they said.
The findings were limited by several factors including the generalizability to other settings and populations, the researchers noted. The efficacy of the levonorgestrel/piroxicam combination in women with a body mass index greater than 26 kg/m2 may be lower, but the current study population did not have enough women in this category to measure the potential effect, they said. The study also did not examine the effect of piroxicam in combination with ulipristal acetate.
However, the results are the first known to demonstrate the improved effectiveness of oral piroxicam coadministered with oral levonorgestrel for EC, they said.
“The strength of this recommendation and changes in clinical guidelines may be determined upon demonstration of reproducible results in further studies,” they added.
Pill combination shows potential and practicality
Oral emergency contraception on demand is an unmet need on a global level, Erica P. Cahill, MD, of the department of obstetrics and gynecology and division of family planning services at Stanford (Calif.) University, wrote in an accompanying editorial.
Dr. Cahill noted the longer half-life of piroxicam compared with other COX-2 inhibitors, which made it a practical choice. Although the study was not powered to evaluate secondary outcomes, bleeding patterns consistent with use of EC pills were observed. Documentation of these patterns is worthwhile, Dr. Cahill said, “because people using emergency contraceptive pills might also be using fertility awareness methods and need to know when they can be certain they are not pregnant.”
Overall, the study supports the addition of 40 mg piroxicam to 1.5 mg levonorgestrel as emergency contraception, said Dr. Cahill. Future studies can build on the current findings by evaluating repeat dosing of the piroxicam/levonorgestrel combination and by evaluating the combination of COX-2 inhibitors and ulipristal acetate to prevent pregnancy, she said.
The study received no outside funding. The researchers and Dr. Cahill had no financial conflicts to disclose.
Adding oral piroxicam to oral levonorgestrel significantly improved the efficacy of emergency contraception, based on data from 860 women.
Oral hormonal emergency contraception (EC) is the most widely used EC method worldwide, but the two currently available drugs, levonorgestrel and ulipristal acetate (UPA), are not effective when given after ovulation, wrote Raymond Hang Wun Li, MD, of the University of Hong Kong, and colleagues. Previous studies suggest that cyclo-oxygenase (COX) inhibitors may disrupt follicular rupture and prevent ovulation, but data on their use in combination with current oral ECs are lacking, the researchers said.
In a study published in The Lancet, the researchers randomized 430 women to receive a single oral dose of 1.5 mg levonorgestrel plus 40 mg of the COX-2 inhibitor piroxicam or 1.5 mg levonorgestrel plus a placebo. The study participants were women aged 18 years and older who requested EC within 72 hours of unprotected sex and who had regular menstrual cycles between 24 and 42 days long. The median age of the participants was 30 years; 97% were Chinese. The median time from intercourse to treatment was 18 hours for both groups.
The primary outcome was the percentage of pregnancies prevented, based on pregnancy status 1-2 weeks after treatment.
One pregnancy occurred in the piroxicam group, compared with seven pregnancies in the placebo group, which translated to a significant difference in the percentage of pregnancies prevented (94.7% vs. 63.4%, P < .0001).
No trend toward increased failure rates appeared based on the time elapsed between intercourse and EC use in either group, and no differences appeared in the return or delay of subsequent menstrual periods between the groups.
The most common adverse events (reported by more than 5% of participants in both groups) included fatigue or weakness, nausea, lower abdominal pain, dizziness, and headache.
The choice of piroxicam as the COX inhibitor in conjunction with levonorgestrel for the current study had several potential advantages, the researchers wrote in their discussion. These advantages include the widespread availability and long-acting characteristics of piroxicam, which is also true of levonorgestrel, they said.
The findings were limited by several factors including the generalizability to other settings and populations, the researchers noted. The efficacy of the levonorgestrel/piroxicam combination in women with a body mass index greater than 26 kg/m2 may be lower, but the current study population did not have enough women in this category to measure the potential effect, they said. The study also did not examine the effect of piroxicam in combination with ulipristal acetate.
However, the results are the first known to demonstrate the improved effectiveness of oral piroxicam coadministered with oral levonorgestrel for EC, they said.
“The strength of this recommendation and changes in clinical guidelines may be determined upon demonstration of reproducible results in further studies,” they added.
Pill combination shows potential and practicality
Oral emergency contraception on demand is an unmet need on a global level, Erica P. Cahill, MD, of the department of obstetrics and gynecology and division of family planning services at Stanford (Calif.) University, wrote in an accompanying editorial.
Dr. Cahill noted the longer half-life of piroxicam compared with other COX-2 inhibitors, which made it a practical choice. Although the study was not powered to evaluate secondary outcomes, bleeding patterns consistent with use of EC pills were observed. Documentation of these patterns is worthwhile, Dr. Cahill said, “because people using emergency contraceptive pills might also be using fertility awareness methods and need to know when they can be certain they are not pregnant.”
Overall, the study supports the addition of 40 mg piroxicam to 1.5 mg levonorgestrel as emergency contraception, said Dr. Cahill. Future studies can build on the current findings by evaluating repeat dosing of the piroxicam/levonorgestrel combination and by evaluating the combination of COX-2 inhibitors and ulipristal acetate to prevent pregnancy, she said.
The study received no outside funding. The researchers and Dr. Cahill had no financial conflicts to disclose.
FROM THE LANCET
News & Perspectives from Ob.Gyn. News
NEWS FROM THE FDA/CDC
FDA approves first over-the-counter birth control pill
The Food and Drug Administration’s approval today of the first birth control pill for women to be available without a prescription is being hailed by many as a long-needed development, but there remain questions to be resolved, including how much the drug will cost and how it will be used.
The drug, Opill, is expected to be available early next year, and its maker has yet to reveal a retail price. It is the same birth control pill that has been available by prescription for 50 years. But for the first time, women will be able to buy the contraception at a local pharmacy, other retail locations, or online without having to see a doctor first.
Likely to drive debate
Contraception in the United States is not without controversy. The FDA’s approval spurred reactions both for and against making hormonal birth control for women available without a prescription.
“It’s an exciting time, especially right now when reproductive rights are being curtailed in a lot of states. Giving people an additional option for contraception will change people’s lives,” said Beverly Gray, MD, division director of Women’s Community and Population Health at Duke University Medical Center in Durham, N.C.
https://www.mdedge.com/obgyn/news-fda/cdc
FEATURE
U.S. mammogram update sparks concern, reignites debates
A recent update to the U.S. recommendations for breast cancer screening is raising concerns about the costs associated with potential follow-up tests, while also renewing debates about the timing of these tests and the screening approaches used.
The U.S. Preventive Services Task Force is currently finalizing an update to its recommendations on breast cancer screening. In May, the task force released a proposed update that dropped the initial age for routine mammogram screening from 50 to 40.
The task force intends to give a “B” rating to this recommendation, which covers screening every other year up to age 74 for women deemed average risk for breast cancer.
The task force’s rating carries clout, A. Mark Fendrick, MD, director of the Value-Based Insurance Design at the University of Michigan, Ann Arbor, said in an interview.
For one, the Affordable Care Act requires that private insurers cover services that get top A or B marks from USPSTF without charging copays.
However, Dr. Fendrick noted, such coverage does not necessarily apply to follow-up testing when a routine mammogram comes back with a positive finding. The expense of follow-up testing may deter some women from seeking follow-up diagnostic imaging or biopsies after an abnormal result on a screen-ing mammogram.
A recent analysis in JAMA Network Open found that women facing higher anticipated out-of-pocket costs for breast cancer diagnostic tests, based on their health insurance plan, were less likely to get that follow-up screening. For instance, the use of breast MRI decreased by nearly 24% between patients undergoing subsequent diagnostic testing in plans with the lowest out-of-pocket costs vs. those with the highest.
https://www.mdedge.com/obgyn/article/264198/breast-cancer/us-mammogram-update-sparks-concern-reignites-debates
Continue to: GENDER-AFFIRMING GYNECOLOGY...
GENDER-AFFIRMING GYNECOLOGY
Updates on pregnancy outcomes in transgender men
Despite increased societal gains, transgender individuals are still a medically and socially underserved group. The historic rise of antitransgender legislation and the overturning of Roe v. Wade, further compound existing health care disparities, particularly in the realm of contraception and pregnancy. Obstetrician-gynecologistsand midwives are typically first-line providers when discussing family planning and fertility options for all patients assigned female at birth. Unfortunately, compared with the surgical, hormonal, and mental health aspects of gender-affirming care, fertility and pregnancy in transgender men is still a relatively new and under-researched topic.
Only individuals who are assigned female at birth and have a uterus are capable of pregnancy. This can include both cisgender women and nonbinary/transgender men. However, societal and medical institutions are struggling with this shift in perspective from a traditionally gendered role to a more inclusive one. Obstetrician-gynecologists and midwives can serve to bridge this gap between these patients and societal misconceptions surrounding transgender men who desire and experience pregnancy.
Providers need to remember that many transmasculine individuals will still retain their uterus and are therefore capable of getting pregnant. While testosterone causes amenorrhea, if patients are engaging in penile-vaginal intercourse, conception is still possible. If a patient does not desire pregnancy, all contraceptive options available for cisgender women, which also include combined oral contraceptives, should be offered.
https://www.mdedge.com/obgyn/gender-affirming-gynecology
REPRODUCTIVE ROUNDS
Affordable IVF—Are we there yet?
The price for an in vitro fertilization (IVF) cycle continues to increase annually by many clinics, particularly because of “add-ons” of dubious value.
The initial application of IVF was for tubal factor infertility. Over the decades since 1981, the year of the first successful live birth in the United States, indications for IVF have dramatically expanded—ovulation dysfunction, unexplained infertility, male factor, advanced stage endometriosis, unexplained infertility, embryo testing to avoid an inherited genetic disease from the intended parents carrying the same mutation, and family balancing for gender, along with fertility preservation, including before potentially gonadotoxic treatment and “elective” planned oocyte cryopreservation.
The cost of IVF remains a significant, and possibly leading, stumbling block for women, couples, and men who lack insurance coverage. From RESOLVE.org, the National Infertility Association: “As of June 2022, 20 states have passed fertility insurance coverage laws, 14 of those laws include IVF coverage, and 12 states have fertility preservation laws for iatrogenic (medically induced) infertility.” Consequently, “affordable IVF” is paramount to providing equal access for patients.
https://www.mdedge.com/obgyn/reproductive-rounds
CONFERENCE COVERAGE
‘Artificial pancreas’ for all type 1 diabetes pregnancies?
In the largest randomized controlled trial of an automated insulin delivery (AID) system (hybrid closed-loop) versus standard insulin delivery in pregnant women with type 1 diabetes, the automated CamAPS FX system prevailed.
The percentage of time spent in the pregnancy-specific target blood glucose range of 63-140 mg/dL (3.5-7.8 mmol/L) from 16 weeks’ gestation to delivery was significantly higher in women in the AID group.
Helen R. Murphy, MD, presented these topline findings from the Automated Insulin Delivery Amongst Pregnant Women With Type 1 Diabetes (AiDAPT) trial during an e-poster session at the annual scientific sessions of the American Diabetes Association.
The “hybrid closed-loop significantly improved maternal glucose and should be offered to all pregnant women with type 1 diabetes,” concluded Dr. Murphy, professor of medicine at the University of East Anglia and a clinician at Norfolk and Norwich University Hospital in the United Kingdom.
CamAPS FX is the only AID system approved in Europe and the United Kingdom for type 1 diabetes from age 1 and during pregnancy. The hybrid closed-loop system is not available in the United States but other systems are available and sometimes used off label in pregnancy. Such systems are sometimes known colloquially as an “artificial pancreas.”
The researchers said their findings provide evidence for the UK National Institute of Clinical Excellence (NICE) to recommend that all pregnant women with type 1 diabetes should be offered the CamAPS FX system.
NEWS FROM THE FDA/CDC
FDA approves first over-the-counter birth control pill
The Food and Drug Administration’s approval today of the first birth control pill for women to be available without a prescription is being hailed by many as a long-needed development, but there remain questions to be resolved, including how much the drug will cost and how it will be used.
The drug, Opill, is expected to be available early next year, and its maker has yet to reveal a retail price. It is the same birth control pill that has been available by prescription for 50 years. But for the first time, women will be able to buy the contraception at a local pharmacy, other retail locations, or online without having to see a doctor first.
Likely to drive debate
Contraception in the United States is not without controversy. The FDA’s approval spurred reactions both for and against making hormonal birth control for women available without a prescription.
“It’s an exciting time, especially right now when reproductive rights are being curtailed in a lot of states. Giving people an additional option for contraception will change people’s lives,” said Beverly Gray, MD, division director of Women’s Community and Population Health at Duke University Medical Center in Durham, N.C.
https://www.mdedge.com/obgyn/news-fda/cdc
FEATURE
U.S. mammogram update sparks concern, reignites debates
A recent update to the U.S. recommendations for breast cancer screening is raising concerns about the costs associated with potential follow-up tests, while also renewing debates about the timing of these tests and the screening approaches used.
The U.S. Preventive Services Task Force is currently finalizing an update to its recommendations on breast cancer screening. In May, the task force released a proposed update that dropped the initial age for routine mammogram screening from 50 to 40.
The task force intends to give a “B” rating to this recommendation, which covers screening every other year up to age 74 for women deemed average risk for breast cancer.
The task force’s rating carries clout, A. Mark Fendrick, MD, director of the Value-Based Insurance Design at the University of Michigan, Ann Arbor, said in an interview.
For one, the Affordable Care Act requires that private insurers cover services that get top A or B marks from USPSTF without charging copays.
However, Dr. Fendrick noted, such coverage does not necessarily apply to follow-up testing when a routine mammogram comes back with a positive finding. The expense of follow-up testing may deter some women from seeking follow-up diagnostic imaging or biopsies after an abnormal result on a screen-ing mammogram.
A recent analysis in JAMA Network Open found that women facing higher anticipated out-of-pocket costs for breast cancer diagnostic tests, based on their health insurance plan, were less likely to get that follow-up screening. For instance, the use of breast MRI decreased by nearly 24% between patients undergoing subsequent diagnostic testing in plans with the lowest out-of-pocket costs vs. those with the highest.
https://www.mdedge.com/obgyn/article/264198/breast-cancer/us-mammogram-update-sparks-concern-reignites-debates
Continue to: GENDER-AFFIRMING GYNECOLOGY...
GENDER-AFFIRMING GYNECOLOGY
Updates on pregnancy outcomes in transgender men
Despite increased societal gains, transgender individuals are still a medically and socially underserved group. The historic rise of antitransgender legislation and the overturning of Roe v. Wade, further compound existing health care disparities, particularly in the realm of contraception and pregnancy. Obstetrician-gynecologistsand midwives are typically first-line providers when discussing family planning and fertility options for all patients assigned female at birth. Unfortunately, compared with the surgical, hormonal, and mental health aspects of gender-affirming care, fertility and pregnancy in transgender men is still a relatively new and under-researched topic.
Only individuals who are assigned female at birth and have a uterus are capable of pregnancy. This can include both cisgender women and nonbinary/transgender men. However, societal and medical institutions are struggling with this shift in perspective from a traditionally gendered role to a more inclusive one. Obstetrician-gynecologists and midwives can serve to bridge this gap between these patients and societal misconceptions surrounding transgender men who desire and experience pregnancy.
Providers need to remember that many transmasculine individuals will still retain their uterus and are therefore capable of getting pregnant. While testosterone causes amenorrhea, if patients are engaging in penile-vaginal intercourse, conception is still possible. If a patient does not desire pregnancy, all contraceptive options available for cisgender women, which also include combined oral contraceptives, should be offered.
https://www.mdedge.com/obgyn/gender-affirming-gynecology
REPRODUCTIVE ROUNDS
Affordable IVF—Are we there yet?
The price for an in vitro fertilization (IVF) cycle continues to increase annually by many clinics, particularly because of “add-ons” of dubious value.
The initial application of IVF was for tubal factor infertility. Over the decades since 1981, the year of the first successful live birth in the United States, indications for IVF have dramatically expanded—ovulation dysfunction, unexplained infertility, male factor, advanced stage endometriosis, unexplained infertility, embryo testing to avoid an inherited genetic disease from the intended parents carrying the same mutation, and family balancing for gender, along with fertility preservation, including before potentially gonadotoxic treatment and “elective” planned oocyte cryopreservation.
The cost of IVF remains a significant, and possibly leading, stumbling block for women, couples, and men who lack insurance coverage. From RESOLVE.org, the National Infertility Association: “As of June 2022, 20 states have passed fertility insurance coverage laws, 14 of those laws include IVF coverage, and 12 states have fertility preservation laws for iatrogenic (medically induced) infertility.” Consequently, “affordable IVF” is paramount to providing equal access for patients.
https://www.mdedge.com/obgyn/reproductive-rounds
CONFERENCE COVERAGE
‘Artificial pancreas’ for all type 1 diabetes pregnancies?
In the largest randomized controlled trial of an automated insulin delivery (AID) system (hybrid closed-loop) versus standard insulin delivery in pregnant women with type 1 diabetes, the automated CamAPS FX system prevailed.
The percentage of time spent in the pregnancy-specific target blood glucose range of 63-140 mg/dL (3.5-7.8 mmol/L) from 16 weeks’ gestation to delivery was significantly higher in women in the AID group.
Helen R. Murphy, MD, presented these topline findings from the Automated Insulin Delivery Amongst Pregnant Women With Type 1 Diabetes (AiDAPT) trial during an e-poster session at the annual scientific sessions of the American Diabetes Association.
The “hybrid closed-loop significantly improved maternal glucose and should be offered to all pregnant women with type 1 diabetes,” concluded Dr. Murphy, professor of medicine at the University of East Anglia and a clinician at Norfolk and Norwich University Hospital in the United Kingdom.
CamAPS FX is the only AID system approved in Europe and the United Kingdom for type 1 diabetes from age 1 and during pregnancy. The hybrid closed-loop system is not available in the United States but other systems are available and sometimes used off label in pregnancy. Such systems are sometimes known colloquially as an “artificial pancreas.”
The researchers said their findings provide evidence for the UK National Institute of Clinical Excellence (NICE) to recommend that all pregnant women with type 1 diabetes should be offered the CamAPS FX system.
NEWS FROM THE FDA/CDC
FDA approves first over-the-counter birth control pill
The Food and Drug Administration’s approval today of the first birth control pill for women to be available without a prescription is being hailed by many as a long-needed development, but there remain questions to be resolved, including how much the drug will cost and how it will be used.
The drug, Opill, is expected to be available early next year, and its maker has yet to reveal a retail price. It is the same birth control pill that has been available by prescription for 50 years. But for the first time, women will be able to buy the contraception at a local pharmacy, other retail locations, or online without having to see a doctor first.
Likely to drive debate
Contraception in the United States is not without controversy. The FDA’s approval spurred reactions both for and against making hormonal birth control for women available without a prescription.
“It’s an exciting time, especially right now when reproductive rights are being curtailed in a lot of states. Giving people an additional option for contraception will change people’s lives,” said Beverly Gray, MD, division director of Women’s Community and Population Health at Duke University Medical Center in Durham, N.C.
https://www.mdedge.com/obgyn/news-fda/cdc
FEATURE
U.S. mammogram update sparks concern, reignites debates
A recent update to the U.S. recommendations for breast cancer screening is raising concerns about the costs associated with potential follow-up tests, while also renewing debates about the timing of these tests and the screening approaches used.
The U.S. Preventive Services Task Force is currently finalizing an update to its recommendations on breast cancer screening. In May, the task force released a proposed update that dropped the initial age for routine mammogram screening from 50 to 40.
The task force intends to give a “B” rating to this recommendation, which covers screening every other year up to age 74 for women deemed average risk for breast cancer.
The task force’s rating carries clout, A. Mark Fendrick, MD, director of the Value-Based Insurance Design at the University of Michigan, Ann Arbor, said in an interview.
For one, the Affordable Care Act requires that private insurers cover services that get top A or B marks from USPSTF without charging copays.
However, Dr. Fendrick noted, such coverage does not necessarily apply to follow-up testing when a routine mammogram comes back with a positive finding. The expense of follow-up testing may deter some women from seeking follow-up diagnostic imaging or biopsies after an abnormal result on a screen-ing mammogram.
A recent analysis in JAMA Network Open found that women facing higher anticipated out-of-pocket costs for breast cancer diagnostic tests, based on their health insurance plan, were less likely to get that follow-up screening. For instance, the use of breast MRI decreased by nearly 24% between patients undergoing subsequent diagnostic testing in plans with the lowest out-of-pocket costs vs. those with the highest.
https://www.mdedge.com/obgyn/article/264198/breast-cancer/us-mammogram-update-sparks-concern-reignites-debates
Continue to: GENDER-AFFIRMING GYNECOLOGY...
GENDER-AFFIRMING GYNECOLOGY
Updates on pregnancy outcomes in transgender men
Despite increased societal gains, transgender individuals are still a medically and socially underserved group. The historic rise of antitransgender legislation and the overturning of Roe v. Wade, further compound existing health care disparities, particularly in the realm of contraception and pregnancy. Obstetrician-gynecologistsand midwives are typically first-line providers when discussing family planning and fertility options for all patients assigned female at birth. Unfortunately, compared with the surgical, hormonal, and mental health aspects of gender-affirming care, fertility and pregnancy in transgender men is still a relatively new and under-researched topic.
Only individuals who are assigned female at birth and have a uterus are capable of pregnancy. This can include both cisgender women and nonbinary/transgender men. However, societal and medical institutions are struggling with this shift in perspective from a traditionally gendered role to a more inclusive one. Obstetrician-gynecologists and midwives can serve to bridge this gap between these patients and societal misconceptions surrounding transgender men who desire and experience pregnancy.
Providers need to remember that many transmasculine individuals will still retain their uterus and are therefore capable of getting pregnant. While testosterone causes amenorrhea, if patients are engaging in penile-vaginal intercourse, conception is still possible. If a patient does not desire pregnancy, all contraceptive options available for cisgender women, which also include combined oral contraceptives, should be offered.
https://www.mdedge.com/obgyn/gender-affirming-gynecology
REPRODUCTIVE ROUNDS
Affordable IVF—Are we there yet?
The price for an in vitro fertilization (IVF) cycle continues to increase annually by many clinics, particularly because of “add-ons” of dubious value.
The initial application of IVF was for tubal factor infertility. Over the decades since 1981, the year of the first successful live birth in the United States, indications for IVF have dramatically expanded—ovulation dysfunction, unexplained infertility, male factor, advanced stage endometriosis, unexplained infertility, embryo testing to avoid an inherited genetic disease from the intended parents carrying the same mutation, and family balancing for gender, along with fertility preservation, including before potentially gonadotoxic treatment and “elective” planned oocyte cryopreservation.
The cost of IVF remains a significant, and possibly leading, stumbling block for women, couples, and men who lack insurance coverage. From RESOLVE.org, the National Infertility Association: “As of June 2022, 20 states have passed fertility insurance coverage laws, 14 of those laws include IVF coverage, and 12 states have fertility preservation laws for iatrogenic (medically induced) infertility.” Consequently, “affordable IVF” is paramount to providing equal access for patients.
https://www.mdedge.com/obgyn/reproductive-rounds
CONFERENCE COVERAGE
‘Artificial pancreas’ for all type 1 diabetes pregnancies?
In the largest randomized controlled trial of an automated insulin delivery (AID) system (hybrid closed-loop) versus standard insulin delivery in pregnant women with type 1 diabetes, the automated CamAPS FX system prevailed.
The percentage of time spent in the pregnancy-specific target blood glucose range of 63-140 mg/dL (3.5-7.8 mmol/L) from 16 weeks’ gestation to delivery was significantly higher in women in the AID group.
Helen R. Murphy, MD, presented these topline findings from the Automated Insulin Delivery Amongst Pregnant Women With Type 1 Diabetes (AiDAPT) trial during an e-poster session at the annual scientific sessions of the American Diabetes Association.
The “hybrid closed-loop significantly improved maternal glucose and should be offered to all pregnant women with type 1 diabetes,” concluded Dr. Murphy, professor of medicine at the University of East Anglia and a clinician at Norfolk and Norwich University Hospital in the United Kingdom.
CamAPS FX is the only AID system approved in Europe and the United Kingdom for type 1 diabetes from age 1 and during pregnancy. The hybrid closed-loop system is not available in the United States but other systems are available and sometimes used off label in pregnancy. Such systems are sometimes known colloquially as an “artificial pancreas.”
The researchers said their findings provide evidence for the UK National Institute of Clinical Excellence (NICE) to recommend that all pregnant women with type 1 diabetes should be offered the CamAPS FX system.
Don’t skip contraception talk for women with complex health conditions
.
In an installment of the American College of Physicians’ In the Clinic series, Rachel Cannon, MD, Kelly Treder, MD, and Elisabeth J. Woodhams, MD, all of Boston Medical Center, presented an article on the complex topic of contraception for patients with chronic illness.
“Many patients with chronic illness or complex medical issues interact with a primary care provider on a frequent basis, which provides a great access point for contraceptive counseling with a provider they trust and know,” said Dr. Cannon and Dr. Treder in a joint interview. “We wanted to create a ‘go to’ resource for primary care physicians to review contraceptive options and counseling best practices for all of their patients. Contraceptive care is part of overall health care and should be included in the primary care encounter.”
The authors discussed the types of contraception, as well as risks and benefits, and offered guidance for choosing a contraceptive method for medically complex patients.
“In recent years, there has been a shift in contraceptive counseling toward shared decision-making, a counseling strategy that honors the patient as the expert in their body and their life experiences and emphasizes their autonomy and values,” the authors said. “For providers, this translates to understanding that contraceptive efficacy is not the only important characteristic to patients, and that many other important factors contribute to an individual’s decision to use a particular method or not use birth control at all,” they said.
Start the conversation
Start by assessing a patient’s interest in and readiness for pregnancy, if applicable, the authors said. One example of a screen, the PATH questionnaire (Parent/Pregnancy Attitudes, Timing, and How important), is designed for patients in any demographic, and includes questions about the timing and desire for pregnancy and feelings about birth control, as well as options for patients to express uncertainty or ambivalence about pregnancy and contraception.
Some patients may derive benefits from hormonal contraceptives beyond pregnancy prevention, the authors wrote. Combined hormonal contraceptives (CHCs) may improve menorrhagia, and data suggest that CHC use also may reduce risk for some cancer types, including endometrial and ovarian cancers, they said.
Overall, contraceptive counseling should include discussions of safety, efficacy, and the patient’s lived experience.
Clinical considerations and contraindications
Medically complex patients who desire contraception may consider hormonal or nonhormonal methods based on their preferences and medical conditions, but clinicians need to consider comorbidities and contraindications, the authors wrote.
When a woman of childbearing age with any complex medical issue starts a new medication or receives a new diagnosis, contraception and pregnancy planning should be part of the discussion, the authors said. Safe and successful pregnancies are possible for women with complex medical issues when underlying health concerns are identified and addressed in advance, they added. Alternatively, for patients seeking to avoid pregnancy permanently, options for sterilization can be part of an informed discussion.
The Centers for Disease Control and Prevention’s Medical Eligibility Criteria for Contraceptive Use offers clinicians detailed information about the risks of both contraceptives and pregnancy for patients with various medical conditions, according to the authors.
The CDC document lists medical conditions associated with an increased risk for adverse health events if the individual becomes pregnant. These conditions include breast cancer, complicated valvular heart disease, cystic fibrosis, diabetes, endometrial or ovarian cancer, epilepsy, hypertension, bariatric surgery within 2 years of the pregnancy, HIV, ischemic heart disease, severe cirrhosis, stroke, lupus, solid organ transplant within 2 years of the pregnancy, and tuberculosis. Women with these and other conditions associated with increased risk of adverse events if pregnancy occurs should be advised of the high failure rate of barrier and behavior-based contraceptive methods, and informed about options for long-acting contraceptives, according to the CDC.
Risks, benefits, and balance
“It is important to remember that the alternative to contraception for many patients is pregnancy – for many patients with complex medical conditions, pregnancy is far more dangerous than any contraceptive method,” Dr. Cannon and Dr. Treder said in an interview. “This is important to consider when thinking about relative contraindications to a certain method or when thinking about ‘less effective’ contraception methods. The most effective method is a method the patient will actually continue to use,” they said.
The recent approval of the over-the-counter minipill is “a huge win for reproductive health care,” said Dr. Cannon and Dr. Treder. The minipill has very few contraindications, and it is the most effective over-the-counter contraceptive now available, they said.
“An over-the-counter contraceptive pill can increase access to contraception without having to see a physician in the clinic, freeing patients from many of the challenges of navigating the health care system,” the authors added.
As for additional research, the establishment of a long-term safety record may help support other OTC contraceptive methods in the future, the authors said.
Contraceptive counseling is everyone’s specialty
In an accompanying editorial, Amy A. Sarma, MD, a cardiologist at Massachusetts General Hospital, Boston, shared an example of the importance of contraceptive discussions with medically complex patients outside of an ob.gyn. setting. A young woman with a family history of myocardial infarction had neglected her own primary care until an MI of her own sent her to the hospital. While hospitalized, the patient was diagnosed with diabetes, hypertension, and hyperlipidemia.
“Her cardiology care team made every effort to optimize her cardiac care, but no one considered that she was also a woman of childbearing potential despite the teratogenic potential of several of her prescribed medications,” Dr. Sarma wrote. When the patient visited Dr. Sarma to discuss prevention of future MIs, Dr. Sarma took the opportunity to discuss the cardiovascular risks of pregnancy and the risks for this patient not only because of her recent MI, but also because of her chronic health conditions.
As it happened, the woman did not want a high-risk pregnancy and was interested in contraceptive methods. Dr. Sarma pointed out that, had the woman been engaged in routine primary care, these issues would have arisen in that setting, but like many younger women with cardiovascular disease, she did not make her own primary care a priority, and had missed out on other opportunities to discuss contraception. “Her MI opened a window of opportunity to help prevent an unintended and high-risk pregnancy,” Dr. Sarma noted.
Dr. Sarma’s patient anecdote illustrated the point of the In the Clinic review: that any clinician can discuss pregnancy and contraception with patients of childbearing age who have medical comorbidities that could affect a pregnancy. “All clinicians who care for patients of reproductive potential should become comfortable discussing pregnancy intent, preconception risk assessment, and contraceptive counseling,” Dr. Sarma said.
The research for this article was funded by the American College of Physicians. The review authors had no financial conflicts to disclose. Dr. Sarma had no financial conflicts to disclose.
.
In an installment of the American College of Physicians’ In the Clinic series, Rachel Cannon, MD, Kelly Treder, MD, and Elisabeth J. Woodhams, MD, all of Boston Medical Center, presented an article on the complex topic of contraception for patients with chronic illness.
“Many patients with chronic illness or complex medical issues interact with a primary care provider on a frequent basis, which provides a great access point for contraceptive counseling with a provider they trust and know,” said Dr. Cannon and Dr. Treder in a joint interview. “We wanted to create a ‘go to’ resource for primary care physicians to review contraceptive options and counseling best practices for all of their patients. Contraceptive care is part of overall health care and should be included in the primary care encounter.”
The authors discussed the types of contraception, as well as risks and benefits, and offered guidance for choosing a contraceptive method for medically complex patients.
“In recent years, there has been a shift in contraceptive counseling toward shared decision-making, a counseling strategy that honors the patient as the expert in their body and their life experiences and emphasizes their autonomy and values,” the authors said. “For providers, this translates to understanding that contraceptive efficacy is not the only important characteristic to patients, and that many other important factors contribute to an individual’s decision to use a particular method or not use birth control at all,” they said.
Start the conversation
Start by assessing a patient’s interest in and readiness for pregnancy, if applicable, the authors said. One example of a screen, the PATH questionnaire (Parent/Pregnancy Attitudes, Timing, and How important), is designed for patients in any demographic, and includes questions about the timing and desire for pregnancy and feelings about birth control, as well as options for patients to express uncertainty or ambivalence about pregnancy and contraception.
Some patients may derive benefits from hormonal contraceptives beyond pregnancy prevention, the authors wrote. Combined hormonal contraceptives (CHCs) may improve menorrhagia, and data suggest that CHC use also may reduce risk for some cancer types, including endometrial and ovarian cancers, they said.
Overall, contraceptive counseling should include discussions of safety, efficacy, and the patient’s lived experience.
Clinical considerations and contraindications
Medically complex patients who desire contraception may consider hormonal or nonhormonal methods based on their preferences and medical conditions, but clinicians need to consider comorbidities and contraindications, the authors wrote.
When a woman of childbearing age with any complex medical issue starts a new medication or receives a new diagnosis, contraception and pregnancy planning should be part of the discussion, the authors said. Safe and successful pregnancies are possible for women with complex medical issues when underlying health concerns are identified and addressed in advance, they added. Alternatively, for patients seeking to avoid pregnancy permanently, options for sterilization can be part of an informed discussion.
The Centers for Disease Control and Prevention’s Medical Eligibility Criteria for Contraceptive Use offers clinicians detailed information about the risks of both contraceptives and pregnancy for patients with various medical conditions, according to the authors.
The CDC document lists medical conditions associated with an increased risk for adverse health events if the individual becomes pregnant. These conditions include breast cancer, complicated valvular heart disease, cystic fibrosis, diabetes, endometrial or ovarian cancer, epilepsy, hypertension, bariatric surgery within 2 years of the pregnancy, HIV, ischemic heart disease, severe cirrhosis, stroke, lupus, solid organ transplant within 2 years of the pregnancy, and tuberculosis. Women with these and other conditions associated with increased risk of adverse events if pregnancy occurs should be advised of the high failure rate of barrier and behavior-based contraceptive methods, and informed about options for long-acting contraceptives, according to the CDC.
Risks, benefits, and balance
“It is important to remember that the alternative to contraception for many patients is pregnancy – for many patients with complex medical conditions, pregnancy is far more dangerous than any contraceptive method,” Dr. Cannon and Dr. Treder said in an interview. “This is important to consider when thinking about relative contraindications to a certain method or when thinking about ‘less effective’ contraception methods. The most effective method is a method the patient will actually continue to use,” they said.
The recent approval of the over-the-counter minipill is “a huge win for reproductive health care,” said Dr. Cannon and Dr. Treder. The minipill has very few contraindications, and it is the most effective over-the-counter contraceptive now available, they said.
“An over-the-counter contraceptive pill can increase access to contraception without having to see a physician in the clinic, freeing patients from many of the challenges of navigating the health care system,” the authors added.
As for additional research, the establishment of a long-term safety record may help support other OTC contraceptive methods in the future, the authors said.
Contraceptive counseling is everyone’s specialty
In an accompanying editorial, Amy A. Sarma, MD, a cardiologist at Massachusetts General Hospital, Boston, shared an example of the importance of contraceptive discussions with medically complex patients outside of an ob.gyn. setting. A young woman with a family history of myocardial infarction had neglected her own primary care until an MI of her own sent her to the hospital. While hospitalized, the patient was diagnosed with diabetes, hypertension, and hyperlipidemia.
“Her cardiology care team made every effort to optimize her cardiac care, but no one considered that she was also a woman of childbearing potential despite the teratogenic potential of several of her prescribed medications,” Dr. Sarma wrote. When the patient visited Dr. Sarma to discuss prevention of future MIs, Dr. Sarma took the opportunity to discuss the cardiovascular risks of pregnancy and the risks for this patient not only because of her recent MI, but also because of her chronic health conditions.
As it happened, the woman did not want a high-risk pregnancy and was interested in contraceptive methods. Dr. Sarma pointed out that, had the woman been engaged in routine primary care, these issues would have arisen in that setting, but like many younger women with cardiovascular disease, she did not make her own primary care a priority, and had missed out on other opportunities to discuss contraception. “Her MI opened a window of opportunity to help prevent an unintended and high-risk pregnancy,” Dr. Sarma noted.
Dr. Sarma’s patient anecdote illustrated the point of the In the Clinic review: that any clinician can discuss pregnancy and contraception with patients of childbearing age who have medical comorbidities that could affect a pregnancy. “All clinicians who care for patients of reproductive potential should become comfortable discussing pregnancy intent, preconception risk assessment, and contraceptive counseling,” Dr. Sarma said.
The research for this article was funded by the American College of Physicians. The review authors had no financial conflicts to disclose. Dr. Sarma had no financial conflicts to disclose.
.
In an installment of the American College of Physicians’ In the Clinic series, Rachel Cannon, MD, Kelly Treder, MD, and Elisabeth J. Woodhams, MD, all of Boston Medical Center, presented an article on the complex topic of contraception for patients with chronic illness.
“Many patients with chronic illness or complex medical issues interact with a primary care provider on a frequent basis, which provides a great access point for contraceptive counseling with a provider they trust and know,” said Dr. Cannon and Dr. Treder in a joint interview. “We wanted to create a ‘go to’ resource for primary care physicians to review contraceptive options and counseling best practices for all of their patients. Contraceptive care is part of overall health care and should be included in the primary care encounter.”
The authors discussed the types of contraception, as well as risks and benefits, and offered guidance for choosing a contraceptive method for medically complex patients.
“In recent years, there has been a shift in contraceptive counseling toward shared decision-making, a counseling strategy that honors the patient as the expert in their body and their life experiences and emphasizes their autonomy and values,” the authors said. “For providers, this translates to understanding that contraceptive efficacy is not the only important characteristic to patients, and that many other important factors contribute to an individual’s decision to use a particular method or not use birth control at all,” they said.
Start the conversation
Start by assessing a patient’s interest in and readiness for pregnancy, if applicable, the authors said. One example of a screen, the PATH questionnaire (Parent/Pregnancy Attitudes, Timing, and How important), is designed for patients in any demographic, and includes questions about the timing and desire for pregnancy and feelings about birth control, as well as options for patients to express uncertainty or ambivalence about pregnancy and contraception.
Some patients may derive benefits from hormonal contraceptives beyond pregnancy prevention, the authors wrote. Combined hormonal contraceptives (CHCs) may improve menorrhagia, and data suggest that CHC use also may reduce risk for some cancer types, including endometrial and ovarian cancers, they said.
Overall, contraceptive counseling should include discussions of safety, efficacy, and the patient’s lived experience.
Clinical considerations and contraindications
Medically complex patients who desire contraception may consider hormonal or nonhormonal methods based on their preferences and medical conditions, but clinicians need to consider comorbidities and contraindications, the authors wrote.
When a woman of childbearing age with any complex medical issue starts a new medication or receives a new diagnosis, contraception and pregnancy planning should be part of the discussion, the authors said. Safe and successful pregnancies are possible for women with complex medical issues when underlying health concerns are identified and addressed in advance, they added. Alternatively, for patients seeking to avoid pregnancy permanently, options for sterilization can be part of an informed discussion.
The Centers for Disease Control and Prevention’s Medical Eligibility Criteria for Contraceptive Use offers clinicians detailed information about the risks of both contraceptives and pregnancy for patients with various medical conditions, according to the authors.
The CDC document lists medical conditions associated with an increased risk for adverse health events if the individual becomes pregnant. These conditions include breast cancer, complicated valvular heart disease, cystic fibrosis, diabetes, endometrial or ovarian cancer, epilepsy, hypertension, bariatric surgery within 2 years of the pregnancy, HIV, ischemic heart disease, severe cirrhosis, stroke, lupus, solid organ transplant within 2 years of the pregnancy, and tuberculosis. Women with these and other conditions associated with increased risk of adverse events if pregnancy occurs should be advised of the high failure rate of barrier and behavior-based contraceptive methods, and informed about options for long-acting contraceptives, according to the CDC.
Risks, benefits, and balance
“It is important to remember that the alternative to contraception for many patients is pregnancy – for many patients with complex medical conditions, pregnancy is far more dangerous than any contraceptive method,” Dr. Cannon and Dr. Treder said in an interview. “This is important to consider when thinking about relative contraindications to a certain method or when thinking about ‘less effective’ contraception methods. The most effective method is a method the patient will actually continue to use,” they said.
The recent approval of the over-the-counter minipill is “a huge win for reproductive health care,” said Dr. Cannon and Dr. Treder. The minipill has very few contraindications, and it is the most effective over-the-counter contraceptive now available, they said.
“An over-the-counter contraceptive pill can increase access to contraception without having to see a physician in the clinic, freeing patients from many of the challenges of navigating the health care system,” the authors added.
As for additional research, the establishment of a long-term safety record may help support other OTC contraceptive methods in the future, the authors said.
Contraceptive counseling is everyone’s specialty
In an accompanying editorial, Amy A. Sarma, MD, a cardiologist at Massachusetts General Hospital, Boston, shared an example of the importance of contraceptive discussions with medically complex patients outside of an ob.gyn. setting. A young woman with a family history of myocardial infarction had neglected her own primary care until an MI of her own sent her to the hospital. While hospitalized, the patient was diagnosed with diabetes, hypertension, and hyperlipidemia.
“Her cardiology care team made every effort to optimize her cardiac care, but no one considered that she was also a woman of childbearing potential despite the teratogenic potential of several of her prescribed medications,” Dr. Sarma wrote. When the patient visited Dr. Sarma to discuss prevention of future MIs, Dr. Sarma took the opportunity to discuss the cardiovascular risks of pregnancy and the risks for this patient not only because of her recent MI, but also because of her chronic health conditions.
As it happened, the woman did not want a high-risk pregnancy and was interested in contraceptive methods. Dr. Sarma pointed out that, had the woman been engaged in routine primary care, these issues would have arisen in that setting, but like many younger women with cardiovascular disease, she did not make her own primary care a priority, and had missed out on other opportunities to discuss contraception. “Her MI opened a window of opportunity to help prevent an unintended and high-risk pregnancy,” Dr. Sarma noted.
Dr. Sarma’s patient anecdote illustrated the point of the In the Clinic review: that any clinician can discuss pregnancy and contraception with patients of childbearing age who have medical comorbidities that could affect a pregnancy. “All clinicians who care for patients of reproductive potential should become comfortable discussing pregnancy intent, preconception risk assessment, and contraceptive counseling,” Dr. Sarma said.
The research for this article was funded by the American College of Physicians. The review authors had no financial conflicts to disclose. Dr. Sarma had no financial conflicts to disclose.
FROM THE ANNALS OF INTERNAL MEDICINE
FDA approves first over-the-counter birth control pill
The Food and Drug Administration’s approval today of the first birth control pill for women to be available without a prescription is being hailed by many as a long-needed development, but there remain questions to be resolved, including how much the drug will cost and how it will be used.
The drug, Opill, is expected to be available early next year, and its maker has yet to reveal a retail price. It is the same birth control pill that has been available by prescription for 50 years. But for the first time, women will be able to buy the contraception at a local pharmacy, other retail locations, or online without having to see a doctor first.
Likely to drive debate
Contraception in the United States is not without controversy. The FDA’s approval spurred reactions both for and against making hormonal birth control for women available without a prescription.
“It’s an exciting time, especially right now when reproductive rights are being curtailed in a lot of states. Giving people an additional option for contraception will change people’s lives,” said Beverly Gray, MD, division director of Women’s Community and Population Health at Duke University Medical Center in Durham, N.C.
“It’s a huge win for patients who need better access to contraception,” said Dr. Gray, who is also a spokesperson for the American College of Obstetricians and Gynecologists.
Women who want hormonal birth control but live in areas without convenient access to a doctor, women who cannot easily take time off of work to see a doctor and get a prescription filled, and women without insurance are examples of people who will benefit, she said.
The Catholic Medical Association, in contrast, expressed “deep concern and disappointment” after an FDA advisory committee’s unanimous vote on May 11 recommending the drug be available over the counter. In a statement after the vote, the group cited “extensive medical studies demonstrating the risks and adverse effects of hormonal contraceptives,” adding that “the social impact of [full approval] would be dramatic.”
But doctors largely disagreed.
“It is definitely a huge win for reproductive autonomy. I’m glad that the FDA is prioritizing patient safety and well-being over politics,” said Catherine Cansino, MD, MPH, an ob.gyn. and clinical professor in the University of California Davis department of obstetrics and gynecology. She said the FDA approved the over-the-counter version because the medication is safe.
While opponents like the Catholic Medical Association cite safety concerns and believe doctors should screen all women before prescribing hormonal contraception, Dr. Gray disagreed. “There’s a lot of evidence that patients can figure out if a progestin-only pill is right for them and safe for them. Medical professionals don’t have to be the gatekeepers for contraception,” she said.
Pricing unknown
Whether insurance companies will pay for Opill now that it will be available without a prescription remains unknown. For some medications, paying a copay through insurance can be less expensive than buying at a retail price.
“Although pricing issues will be relevant, the FDA’s decision will enhance women’s access to hormonal birth control,” said Andrew M. Kaunitz, MD, a professor and associate chairman in the department of obstetrics and gynecology at the University of Florida College of Medicine in Jacksonville.
The drugmaker, Perrigo, based in Ireland, has not yet announced how much the pill will cost. The price tag could affect how widely available this form of birth control is. The drug has been shown to be as much as 93% effective for pregnancy prevention. Perrigo says it plans to make the pill available at low or no cost to some women.
Caveats to consider
There are some women for whom hormonal contraceptives have always carried greater risks. For example, women who have breast cancer or a history of breast cancer should not use hormonal contraceptives, the FDA said in a news release announcing the approval. Women with other types of cancer should check with their doctors first, the agency noted.
Women who smoke, who take some medications to lower blood pressure, or who have migraines should also take caution, Dr. Cansino said. “People with migraines may not be suitable for over-the-counter oral contraceptives. But a simple screening through a provider can identify whether you are truly eligible or not.”
Irregular bleeding, headaches, dizziness, nausea, increased appetite, belly pain, cramps, or bloating are the most common side effects of Opill, the FDA said.
The Opill is a progestin-only birth control pill. Similar pills have been available in the United Kingdiom for about 2 years, often referred to as “mini pills” because they contain a single hormone. In contrast, prescription birth control pills in the United States and elsewhere contain more than one hormone, estrogen and progestin, to prevent pregnancy.
Prescription pill packs for combination contraception often feature a week of placebo pills without an active ingredient. While skipping a placebo pill might not make a difference in pregnancy prevention, Opill is different. Every pill in the packet will contain medication, Gray said. “So it’s important to take the pill the same time every day for it to be most effective.”
Even though this may mean one less visit to your doctor, Dr. Kaunitz hopes women will stay up to date on their other medical checkups. “One of our challenges as providers of care to women will be to encourage them to continue to receive important services, including cancer screening and vaccinations, even while they can initiate and continue hormonal contraception without contact with a provider.”
Just the beginning?
The American Medical Association hopes this approval signals more to come.
“While we applaud this move, the AMA continues to urge the FDA and HHS to consider a variety of oral contraceptive options for over-the-counter use,” the association, which has more than 250,000 doctor members, said in a statement. “It is important patients have options when choosing which type of birth control works best for them,”
The American College of Obstetricians and Gynecologists said the FDA’s decision will help many women. “We are glad that more patients will now be empowered to choose when and where they obtain a safe method of contraception without having to wait for a medical appointment or for a prescription to be filled,” Verda J. Hicks, MD, the group’s president, and Christopher M. Zahn, MD, interim chief executive officer, said in a statement.
“Allowing individuals to access birth control at their local pharmacy or drug store will eliminate some barriers,” they said.
A version of this article first appeared on WebMD.com.
This article was updated 7/13/23.
The Food and Drug Administration’s approval today of the first birth control pill for women to be available without a prescription is being hailed by many as a long-needed development, but there remain questions to be resolved, including how much the drug will cost and how it will be used.
The drug, Opill, is expected to be available early next year, and its maker has yet to reveal a retail price. It is the same birth control pill that has been available by prescription for 50 years. But for the first time, women will be able to buy the contraception at a local pharmacy, other retail locations, or online without having to see a doctor first.
Likely to drive debate
Contraception in the United States is not without controversy. The FDA’s approval spurred reactions both for and against making hormonal birth control for women available without a prescription.
“It’s an exciting time, especially right now when reproductive rights are being curtailed in a lot of states. Giving people an additional option for contraception will change people’s lives,” said Beverly Gray, MD, division director of Women’s Community and Population Health at Duke University Medical Center in Durham, N.C.
“It’s a huge win for patients who need better access to contraception,” said Dr. Gray, who is also a spokesperson for the American College of Obstetricians and Gynecologists.
Women who want hormonal birth control but live in areas without convenient access to a doctor, women who cannot easily take time off of work to see a doctor and get a prescription filled, and women without insurance are examples of people who will benefit, she said.
The Catholic Medical Association, in contrast, expressed “deep concern and disappointment” after an FDA advisory committee’s unanimous vote on May 11 recommending the drug be available over the counter. In a statement after the vote, the group cited “extensive medical studies demonstrating the risks and adverse effects of hormonal contraceptives,” adding that “the social impact of [full approval] would be dramatic.”
But doctors largely disagreed.
“It is definitely a huge win for reproductive autonomy. I’m glad that the FDA is prioritizing patient safety and well-being over politics,” said Catherine Cansino, MD, MPH, an ob.gyn. and clinical professor in the University of California Davis department of obstetrics and gynecology. She said the FDA approved the over-the-counter version because the medication is safe.
While opponents like the Catholic Medical Association cite safety concerns and believe doctors should screen all women before prescribing hormonal contraception, Dr. Gray disagreed. “There’s a lot of evidence that patients can figure out if a progestin-only pill is right for them and safe for them. Medical professionals don’t have to be the gatekeepers for contraception,” she said.
Pricing unknown
Whether insurance companies will pay for Opill now that it will be available without a prescription remains unknown. For some medications, paying a copay through insurance can be less expensive than buying at a retail price.
“Although pricing issues will be relevant, the FDA’s decision will enhance women’s access to hormonal birth control,” said Andrew M. Kaunitz, MD, a professor and associate chairman in the department of obstetrics and gynecology at the University of Florida College of Medicine in Jacksonville.
The drugmaker, Perrigo, based in Ireland, has not yet announced how much the pill will cost. The price tag could affect how widely available this form of birth control is. The drug has been shown to be as much as 93% effective for pregnancy prevention. Perrigo says it plans to make the pill available at low or no cost to some women.
Caveats to consider
There are some women for whom hormonal contraceptives have always carried greater risks. For example, women who have breast cancer or a history of breast cancer should not use hormonal contraceptives, the FDA said in a news release announcing the approval. Women with other types of cancer should check with their doctors first, the agency noted.
Women who smoke, who take some medications to lower blood pressure, or who have migraines should also take caution, Dr. Cansino said. “People with migraines may not be suitable for over-the-counter oral contraceptives. But a simple screening through a provider can identify whether you are truly eligible or not.”
Irregular bleeding, headaches, dizziness, nausea, increased appetite, belly pain, cramps, or bloating are the most common side effects of Opill, the FDA said.
The Opill is a progestin-only birth control pill. Similar pills have been available in the United Kingdiom for about 2 years, often referred to as “mini pills” because they contain a single hormone. In contrast, prescription birth control pills in the United States and elsewhere contain more than one hormone, estrogen and progestin, to prevent pregnancy.
Prescription pill packs for combination contraception often feature a week of placebo pills without an active ingredient. While skipping a placebo pill might not make a difference in pregnancy prevention, Opill is different. Every pill in the packet will contain medication, Gray said. “So it’s important to take the pill the same time every day for it to be most effective.”
Even though this may mean one less visit to your doctor, Dr. Kaunitz hopes women will stay up to date on their other medical checkups. “One of our challenges as providers of care to women will be to encourage them to continue to receive important services, including cancer screening and vaccinations, even while they can initiate and continue hormonal contraception without contact with a provider.”
Just the beginning?
The American Medical Association hopes this approval signals more to come.
“While we applaud this move, the AMA continues to urge the FDA and HHS to consider a variety of oral contraceptive options for over-the-counter use,” the association, which has more than 250,000 doctor members, said in a statement. “It is important patients have options when choosing which type of birth control works best for them,”
The American College of Obstetricians and Gynecologists said the FDA’s decision will help many women. “We are glad that more patients will now be empowered to choose when and where they obtain a safe method of contraception without having to wait for a medical appointment or for a prescription to be filled,” Verda J. Hicks, MD, the group’s president, and Christopher M. Zahn, MD, interim chief executive officer, said in a statement.
“Allowing individuals to access birth control at their local pharmacy or drug store will eliminate some barriers,” they said.
A version of this article first appeared on WebMD.com.
This article was updated 7/13/23.
The Food and Drug Administration’s approval today of the first birth control pill for women to be available without a prescription is being hailed by many as a long-needed development, but there remain questions to be resolved, including how much the drug will cost and how it will be used.
The drug, Opill, is expected to be available early next year, and its maker has yet to reveal a retail price. It is the same birth control pill that has been available by prescription for 50 years. But for the first time, women will be able to buy the contraception at a local pharmacy, other retail locations, or online without having to see a doctor first.
Likely to drive debate
Contraception in the United States is not without controversy. The FDA’s approval spurred reactions both for and against making hormonal birth control for women available without a prescription.
“It’s an exciting time, especially right now when reproductive rights are being curtailed in a lot of states. Giving people an additional option for contraception will change people’s lives,” said Beverly Gray, MD, division director of Women’s Community and Population Health at Duke University Medical Center in Durham, N.C.
“It’s a huge win for patients who need better access to contraception,” said Dr. Gray, who is also a spokesperson for the American College of Obstetricians and Gynecologists.
Women who want hormonal birth control but live in areas without convenient access to a doctor, women who cannot easily take time off of work to see a doctor and get a prescription filled, and women without insurance are examples of people who will benefit, she said.
The Catholic Medical Association, in contrast, expressed “deep concern and disappointment” after an FDA advisory committee’s unanimous vote on May 11 recommending the drug be available over the counter. In a statement after the vote, the group cited “extensive medical studies demonstrating the risks and adverse effects of hormonal contraceptives,” adding that “the social impact of [full approval] would be dramatic.”
But doctors largely disagreed.
“It is definitely a huge win for reproductive autonomy. I’m glad that the FDA is prioritizing patient safety and well-being over politics,” said Catherine Cansino, MD, MPH, an ob.gyn. and clinical professor in the University of California Davis department of obstetrics and gynecology. She said the FDA approved the over-the-counter version because the medication is safe.
While opponents like the Catholic Medical Association cite safety concerns and believe doctors should screen all women before prescribing hormonal contraception, Dr. Gray disagreed. “There’s a lot of evidence that patients can figure out if a progestin-only pill is right for them and safe for them. Medical professionals don’t have to be the gatekeepers for contraception,” she said.
Pricing unknown
Whether insurance companies will pay for Opill now that it will be available without a prescription remains unknown. For some medications, paying a copay through insurance can be less expensive than buying at a retail price.
“Although pricing issues will be relevant, the FDA’s decision will enhance women’s access to hormonal birth control,” said Andrew M. Kaunitz, MD, a professor and associate chairman in the department of obstetrics and gynecology at the University of Florida College of Medicine in Jacksonville.
The drugmaker, Perrigo, based in Ireland, has not yet announced how much the pill will cost. The price tag could affect how widely available this form of birth control is. The drug has been shown to be as much as 93% effective for pregnancy prevention. Perrigo says it plans to make the pill available at low or no cost to some women.
Caveats to consider
There are some women for whom hormonal contraceptives have always carried greater risks. For example, women who have breast cancer or a history of breast cancer should not use hormonal contraceptives, the FDA said in a news release announcing the approval. Women with other types of cancer should check with their doctors first, the agency noted.
Women who smoke, who take some medications to lower blood pressure, or who have migraines should also take caution, Dr. Cansino said. “People with migraines may not be suitable for over-the-counter oral contraceptives. But a simple screening through a provider can identify whether you are truly eligible or not.”
Irregular bleeding, headaches, dizziness, nausea, increased appetite, belly pain, cramps, or bloating are the most common side effects of Opill, the FDA said.
The Opill is a progestin-only birth control pill. Similar pills have been available in the United Kingdiom for about 2 years, often referred to as “mini pills” because they contain a single hormone. In contrast, prescription birth control pills in the United States and elsewhere contain more than one hormone, estrogen and progestin, to prevent pregnancy.
Prescription pill packs for combination contraception often feature a week of placebo pills without an active ingredient. While skipping a placebo pill might not make a difference in pregnancy prevention, Opill is different. Every pill in the packet will contain medication, Gray said. “So it’s important to take the pill the same time every day for it to be most effective.”
Even though this may mean one less visit to your doctor, Dr. Kaunitz hopes women will stay up to date on their other medical checkups. “One of our challenges as providers of care to women will be to encourage them to continue to receive important services, including cancer screening and vaccinations, even while they can initiate and continue hormonal contraception without contact with a provider.”
Just the beginning?
The American Medical Association hopes this approval signals more to come.
“While we applaud this move, the AMA continues to urge the FDA and HHS to consider a variety of oral contraceptive options for over-the-counter use,” the association, which has more than 250,000 doctor members, said in a statement. “It is important patients have options when choosing which type of birth control works best for them,”
The American College of Obstetricians and Gynecologists said the FDA’s decision will help many women. “We are glad that more patients will now be empowered to choose when and where they obtain a safe method of contraception without having to wait for a medical appointment or for a prescription to be filled,” Verda J. Hicks, MD, the group’s president, and Christopher M. Zahn, MD, interim chief executive officer, said in a statement.
“Allowing individuals to access birth control at their local pharmacy or drug store will eliminate some barriers,” they said.
A version of this article first appeared on WebMD.com.
This article was updated 7/13/23.
Do oral contraceptives increase depression risk?
In addition, OC use in adolescence has been tied to an increased risk for depression later in life. However, some experts believe the study’s methodology may be flawed.
The investigators tracked more than 250,000 women from birth to menopause, gathering information about their use of combined contraceptive pills (progesterone and estrogen), the timing of the initial depression diagnosis, and the onset of depressive symptoms that were not formally diagnosed.
Women who began using these OCs before or at the age of 20 experienced a 130% higher incidence of depressive symptoms, whereas adult users saw a 92% increase. But the higher occurrence of depression tended to decline after the first 2 years of use, except in teenagers, who maintained an increased incidence of depression even after discontinuation.
This effect remained, even after analysis of potential familial confounding.
“Our findings suggest that the use of OCs, particularly during the first 2 years, increases the risk of depression. Additionally, OC use during adolescence might increase the risk of depression later in life,” Therese Johansson, of the department of immunology, genetics, and pathology, Science for Life Laboratory, Uppsala (Sweden) University, and colleagues wrote.
The study was published online in Epidemiology and Psychiatric Sciences.
Inconsistent findings
Previous studies suggest an association between adolescent use of hormonal contraceptives (HCs) and increased depression risk, but it’s “less clear” whether these effects are similar in adults, the authors wrote. Randomized clinical trials have “shown little or no effect” of HCs on mood. However, most of these studies didn’t consider previous use of HC.
The researchers wanted to estimate the incidence rate of depression associated with first initiation of OC use as well as the lifetime risk associated with use.
They studied 264,557 female participants in the UK Biobank (aged 37-71 years), collecting data from questionnaires, interviews, physical health measures, biological samples, imaging, and linked health records.
Most participants taking OCs had initiated use during the 1970s/early 1980s when second-generation OCs were predominantly used, consisting of levonorgestrel and ethinyl estradiol.
The researchers conducted a secondary outcome analysis on women who completed the UK Biobank Mental Health Questionnaire (MHQ) to evaluate depressive symptoms.
They estimated the associated risk for depression within 2 years after starting OCs in all women, as well as in groups stratified by age at initiation: before age 20 (adolescents) and age 20 and older (adults). In addition, the investigators estimated the lifetime risk for depression.
Time-dependent analysis compared the effect of OC use at initiation to the effect during the remaining years of use in recent and previous users.
They analyzed a subcohort of female siblings, utilizing “inference about causation from examination of familial confounding,” defined by the authors as a “regression-based approach for determining causality through the use of paired observational data collected from related individuals.”
Adolescents at highest risk
Of the participants, 80.6% had used OCs at some point.
The first 2 years of use were associated with a higher rate of depression among users, compared with never-users (hazard ration, 1.79; 95% confidence interval, 1.63-1.96). Although the risk became less pronounced after that, ever-use was still associated with increased lifetime risk for depression (HR, 1.05; 95% CI, 1.01-1.09).
Adolescents and adult OC users both experienced higher rates of depression during the first 2 years, with a more marked effect in adolescents than in adults (HR, 1.95; 95% CI, 1.64-2.32; and HR, 1.74; 95% CI, 1.54-1.95, respectively).
Previous users of OCs had a higher lifetime risk for depression, compared with never-users (HR, 1.05; 95% CI, 1.01-1.09).
Of the subcohort of women who completed the MHQ (n = 82,232), about half reported experiencing at least one of the core depressive symptoms.
OC initiation was associated with an increased risk for depressive symptoms during the first 2 years in ever- versus never-users (HR, 2.00; 95% CI, 1.91-2.10).
Those who began using OCs during adolescence had a dramatically higher rate of depressive symptoms, compared with never-users (HR, 2.30; 95% CI, 2.11-2.51), as did adult initiators (HR, 1.92; 95% CI, 2.11-2.51).
In the analysis of 7,354 first-degree sister pairs, 81% had initiated OCs. A sibling’s OC use was positively associated with a depression diagnosis, and the cosibling’s OC use was also associated with the sibling’s depression diagnosis. “These results support the hypothesis of a causal relationship between OC use and depression, such that OC use increases the risk of depression,” the authors wrote.
The main limitation is the potential for recall bias in the self-reported data, and that the UK Biobank sample consists of a healthier population than the overall U.K. population, which “hampers the generalizability” of the findings, the authors stated.
Flawed study
In a comment, Natalie Rasgon, MD, founder and director of the Stanford (Calif.) Center for Neuroscience in Women’s Health, said the study was “well researched” and “well written” but had “methodological issues.”
She questioned the sibling component, “which the researchers regard as confirming causality.” The effect may be “important but not causative.” Causality in people who are recalling retrospectively “is highly questionable by any adept researcher because it’s subject to memory. Different siblings may have different recall.”
The authors also didn’t study the indication for OC use. Several medical conditions are treated with OCs, including premenstrual dysphoric disorder, the “number one mood disorder among women of reproductive age.” Including this “could have made a huge difference in outcome data,” said Dr. Rasgon, who was not involved with the study.
Anne-Marie Amies Oelschlager, MD, professor of obstetrics and gynecology, University of Washington, Seattle, noted participants were asked to recall depressive symptoms and OC use as far back as 20-30 years ago, which lends itself to inaccurate recall.
And the researchers didn’t ascertain whether the contraceptives had been used continuously or had been started, stopped, and restarted. Nor did they look at different formulations and doses. And the observational nature of the study “limits the ability to infer causation,” continued Dr. Oelschlager, chair of the American College of Obstetrics and Gynecology Clinical Consensus Gynecology Committee. She was not involved with the study.
“This study is too flawed to use meaningfully in clinical practice,” Dr. Oelschlager concluded.
The study was primarily funded by the Swedish Research Council, the Swedish Brain Foundation, and the Uppsala University Center for Women ‘s Mental Health during the Reproductive Lifespan. The authors, Dr. Rasgon, and Dr. Oelschlager declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In addition, OC use in adolescence has been tied to an increased risk for depression later in life. However, some experts believe the study’s methodology may be flawed.
The investigators tracked more than 250,000 women from birth to menopause, gathering information about their use of combined contraceptive pills (progesterone and estrogen), the timing of the initial depression diagnosis, and the onset of depressive symptoms that were not formally diagnosed.
Women who began using these OCs before or at the age of 20 experienced a 130% higher incidence of depressive symptoms, whereas adult users saw a 92% increase. But the higher occurrence of depression tended to decline after the first 2 years of use, except in teenagers, who maintained an increased incidence of depression even after discontinuation.
This effect remained, even after analysis of potential familial confounding.
“Our findings suggest that the use of OCs, particularly during the first 2 years, increases the risk of depression. Additionally, OC use during adolescence might increase the risk of depression later in life,” Therese Johansson, of the department of immunology, genetics, and pathology, Science for Life Laboratory, Uppsala (Sweden) University, and colleagues wrote.
The study was published online in Epidemiology and Psychiatric Sciences.
Inconsistent findings
Previous studies suggest an association between adolescent use of hormonal contraceptives (HCs) and increased depression risk, but it’s “less clear” whether these effects are similar in adults, the authors wrote. Randomized clinical trials have “shown little or no effect” of HCs on mood. However, most of these studies didn’t consider previous use of HC.
The researchers wanted to estimate the incidence rate of depression associated with first initiation of OC use as well as the lifetime risk associated with use.
They studied 264,557 female participants in the UK Biobank (aged 37-71 years), collecting data from questionnaires, interviews, physical health measures, biological samples, imaging, and linked health records.
Most participants taking OCs had initiated use during the 1970s/early 1980s when second-generation OCs were predominantly used, consisting of levonorgestrel and ethinyl estradiol.
The researchers conducted a secondary outcome analysis on women who completed the UK Biobank Mental Health Questionnaire (MHQ) to evaluate depressive symptoms.
They estimated the associated risk for depression within 2 years after starting OCs in all women, as well as in groups stratified by age at initiation: before age 20 (adolescents) and age 20 and older (adults). In addition, the investigators estimated the lifetime risk for depression.
Time-dependent analysis compared the effect of OC use at initiation to the effect during the remaining years of use in recent and previous users.
They analyzed a subcohort of female siblings, utilizing “inference about causation from examination of familial confounding,” defined by the authors as a “regression-based approach for determining causality through the use of paired observational data collected from related individuals.”
Adolescents at highest risk
Of the participants, 80.6% had used OCs at some point.
The first 2 years of use were associated with a higher rate of depression among users, compared with never-users (hazard ration, 1.79; 95% confidence interval, 1.63-1.96). Although the risk became less pronounced after that, ever-use was still associated with increased lifetime risk for depression (HR, 1.05; 95% CI, 1.01-1.09).
Adolescents and adult OC users both experienced higher rates of depression during the first 2 years, with a more marked effect in adolescents than in adults (HR, 1.95; 95% CI, 1.64-2.32; and HR, 1.74; 95% CI, 1.54-1.95, respectively).
Previous users of OCs had a higher lifetime risk for depression, compared with never-users (HR, 1.05; 95% CI, 1.01-1.09).
Of the subcohort of women who completed the MHQ (n = 82,232), about half reported experiencing at least one of the core depressive symptoms.
OC initiation was associated with an increased risk for depressive symptoms during the first 2 years in ever- versus never-users (HR, 2.00; 95% CI, 1.91-2.10).
Those who began using OCs during adolescence had a dramatically higher rate of depressive symptoms, compared with never-users (HR, 2.30; 95% CI, 2.11-2.51), as did adult initiators (HR, 1.92; 95% CI, 2.11-2.51).
In the analysis of 7,354 first-degree sister pairs, 81% had initiated OCs. A sibling’s OC use was positively associated with a depression diagnosis, and the cosibling’s OC use was also associated with the sibling’s depression diagnosis. “These results support the hypothesis of a causal relationship between OC use and depression, such that OC use increases the risk of depression,” the authors wrote.
The main limitation is the potential for recall bias in the self-reported data, and that the UK Biobank sample consists of a healthier population than the overall U.K. population, which “hampers the generalizability” of the findings, the authors stated.
Flawed study
In a comment, Natalie Rasgon, MD, founder and director of the Stanford (Calif.) Center for Neuroscience in Women’s Health, said the study was “well researched” and “well written” but had “methodological issues.”
She questioned the sibling component, “which the researchers regard as confirming causality.” The effect may be “important but not causative.” Causality in people who are recalling retrospectively “is highly questionable by any adept researcher because it’s subject to memory. Different siblings may have different recall.”
The authors also didn’t study the indication for OC use. Several medical conditions are treated with OCs, including premenstrual dysphoric disorder, the “number one mood disorder among women of reproductive age.” Including this “could have made a huge difference in outcome data,” said Dr. Rasgon, who was not involved with the study.
Anne-Marie Amies Oelschlager, MD, professor of obstetrics and gynecology, University of Washington, Seattle, noted participants were asked to recall depressive symptoms and OC use as far back as 20-30 years ago, which lends itself to inaccurate recall.
And the researchers didn’t ascertain whether the contraceptives had been used continuously or had been started, stopped, and restarted. Nor did they look at different formulations and doses. And the observational nature of the study “limits the ability to infer causation,” continued Dr. Oelschlager, chair of the American College of Obstetrics and Gynecology Clinical Consensus Gynecology Committee. She was not involved with the study.
“This study is too flawed to use meaningfully in clinical practice,” Dr. Oelschlager concluded.
The study was primarily funded by the Swedish Research Council, the Swedish Brain Foundation, and the Uppsala University Center for Women ‘s Mental Health during the Reproductive Lifespan. The authors, Dr. Rasgon, and Dr. Oelschlager declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In addition, OC use in adolescence has been tied to an increased risk for depression later in life. However, some experts believe the study’s methodology may be flawed.
The investigators tracked more than 250,000 women from birth to menopause, gathering information about their use of combined contraceptive pills (progesterone and estrogen), the timing of the initial depression diagnosis, and the onset of depressive symptoms that were not formally diagnosed.
Women who began using these OCs before or at the age of 20 experienced a 130% higher incidence of depressive symptoms, whereas adult users saw a 92% increase. But the higher occurrence of depression tended to decline after the first 2 years of use, except in teenagers, who maintained an increased incidence of depression even after discontinuation.
This effect remained, even after analysis of potential familial confounding.
“Our findings suggest that the use of OCs, particularly during the first 2 years, increases the risk of depression. Additionally, OC use during adolescence might increase the risk of depression later in life,” Therese Johansson, of the department of immunology, genetics, and pathology, Science for Life Laboratory, Uppsala (Sweden) University, and colleagues wrote.
The study was published online in Epidemiology and Psychiatric Sciences.
Inconsistent findings
Previous studies suggest an association between adolescent use of hormonal contraceptives (HCs) and increased depression risk, but it’s “less clear” whether these effects are similar in adults, the authors wrote. Randomized clinical trials have “shown little or no effect” of HCs on mood. However, most of these studies didn’t consider previous use of HC.
The researchers wanted to estimate the incidence rate of depression associated with first initiation of OC use as well as the lifetime risk associated with use.
They studied 264,557 female participants in the UK Biobank (aged 37-71 years), collecting data from questionnaires, interviews, physical health measures, biological samples, imaging, and linked health records.
Most participants taking OCs had initiated use during the 1970s/early 1980s when second-generation OCs were predominantly used, consisting of levonorgestrel and ethinyl estradiol.
The researchers conducted a secondary outcome analysis on women who completed the UK Biobank Mental Health Questionnaire (MHQ) to evaluate depressive symptoms.
They estimated the associated risk for depression within 2 years after starting OCs in all women, as well as in groups stratified by age at initiation: before age 20 (adolescents) and age 20 and older (adults). In addition, the investigators estimated the lifetime risk for depression.
Time-dependent analysis compared the effect of OC use at initiation to the effect during the remaining years of use in recent and previous users.
They analyzed a subcohort of female siblings, utilizing “inference about causation from examination of familial confounding,” defined by the authors as a “regression-based approach for determining causality through the use of paired observational data collected from related individuals.”
Adolescents at highest risk
Of the participants, 80.6% had used OCs at some point.
The first 2 years of use were associated with a higher rate of depression among users, compared with never-users (hazard ration, 1.79; 95% confidence interval, 1.63-1.96). Although the risk became less pronounced after that, ever-use was still associated with increased lifetime risk for depression (HR, 1.05; 95% CI, 1.01-1.09).
Adolescents and adult OC users both experienced higher rates of depression during the first 2 years, with a more marked effect in adolescents than in adults (HR, 1.95; 95% CI, 1.64-2.32; and HR, 1.74; 95% CI, 1.54-1.95, respectively).
Previous users of OCs had a higher lifetime risk for depression, compared with never-users (HR, 1.05; 95% CI, 1.01-1.09).
Of the subcohort of women who completed the MHQ (n = 82,232), about half reported experiencing at least one of the core depressive symptoms.
OC initiation was associated with an increased risk for depressive symptoms during the first 2 years in ever- versus never-users (HR, 2.00; 95% CI, 1.91-2.10).
Those who began using OCs during adolescence had a dramatically higher rate of depressive symptoms, compared with never-users (HR, 2.30; 95% CI, 2.11-2.51), as did adult initiators (HR, 1.92; 95% CI, 2.11-2.51).
In the analysis of 7,354 first-degree sister pairs, 81% had initiated OCs. A sibling’s OC use was positively associated with a depression diagnosis, and the cosibling’s OC use was also associated with the sibling’s depression diagnosis. “These results support the hypothesis of a causal relationship between OC use and depression, such that OC use increases the risk of depression,” the authors wrote.
The main limitation is the potential for recall bias in the self-reported data, and that the UK Biobank sample consists of a healthier population than the overall U.K. population, which “hampers the generalizability” of the findings, the authors stated.
Flawed study
In a comment, Natalie Rasgon, MD, founder and director of the Stanford (Calif.) Center for Neuroscience in Women’s Health, said the study was “well researched” and “well written” but had “methodological issues.”
She questioned the sibling component, “which the researchers regard as confirming causality.” The effect may be “important but not causative.” Causality in people who are recalling retrospectively “is highly questionable by any adept researcher because it’s subject to memory. Different siblings may have different recall.”
The authors also didn’t study the indication for OC use. Several medical conditions are treated with OCs, including premenstrual dysphoric disorder, the “number one mood disorder among women of reproductive age.” Including this “could have made a huge difference in outcome data,” said Dr. Rasgon, who was not involved with the study.
Anne-Marie Amies Oelschlager, MD, professor of obstetrics and gynecology, University of Washington, Seattle, noted participants were asked to recall depressive symptoms and OC use as far back as 20-30 years ago, which lends itself to inaccurate recall.
And the researchers didn’t ascertain whether the contraceptives had been used continuously or had been started, stopped, and restarted. Nor did they look at different formulations and doses. And the observational nature of the study “limits the ability to infer causation,” continued Dr. Oelschlager, chair of the American College of Obstetrics and Gynecology Clinical Consensus Gynecology Committee. She was not involved with the study.
“This study is too flawed to use meaningfully in clinical practice,” Dr. Oelschlager concluded.
The study was primarily funded by the Swedish Research Council, the Swedish Brain Foundation, and the Uppsala University Center for Women ‘s Mental Health during the Reproductive Lifespan. The authors, Dr. Rasgon, and Dr. Oelschlager declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM EPIDEMIOLOGY AND PSYCHIATRIC SCIENCES
Postpartum IUD insertion: Best practices
CASE 1 Multiparous female with short-interval pregnancies desires contraception
A 24-year-old woman (G4P3) presents for a routine prenatal visit in the third trimester. Her last 2 pregnancies have occurred within 3 months of her prior birth. She endorses feeling overwhelmed with having 4 children under the age of 5 years, and she specifies that she would like to avoid another pregnancy for several years. She plans to breast and bottle feed, and she notes that she tends to forget to take pills. When you look back at her prior charts, you note that she did not return for her last 2 postpartum visits. What can you offer her? What would be a safe contraceptive option for her?
Intrauterine devices (IUDs) are safe, effective, and reported by patients to be satisfactory methods of contraception precisely because they are prone to less user error. The Contraceptive Choice Project demonstrated that patients are more apt to choose them when barriers such as cost and access are removed and nondirective counseling is provided.1 Given that unintended pregnancy rates hover around 48%, the American College of Obstetricians and Gynecologists (ACOG) recommends them as first-line methods for pregnancy prevention.2,3
For repeat pregnancies, the postpartum period is an especially vulnerable time—non-breastfeeding women will ovulate as soon as 25 days after birth, and by 8 weeks 30% will have ovulated.4 Approximately 40% to 57% of women report having unprotected intercourse before 6 weeks postpartum, and nearly 70% of all pregnancies in the first year postpartum are unintended.3,5 Furthermore, patients at highest risk for short-interval pregnancy, such as adolescents, are less likely to return for a postpartum visit.3
Short-interval pregnancies confer greater fetal risk, including risks of low-birth weight, preterm birth, small for gestational age and increased risk of neonatal intensive care unit admission.6 Additionally, maternal health may be compromised during a short-interval pregnancy, particularly in medically complex patients due to increased risks of adverse pregnancy outcomes, such as postpartum bleeding or uterine rupture and disease progression.7 A 2006 meta-analysis by Conde-Agudelo and colleagues found that waiting at least 18 months between pregnancies was optimal for reducing these risks.6
Thus, the immediate postpartum period is an optimal time for addressing contraceptive needs and for preventing short-interval and unintended pregnancy. This article aims to provide evidence supporting the use of immediate postpartum IUDs, as well as their associated risks and barriers to use.
IUD types and routes for immediate postpartum insertion
There are several randomized controlled trials (RCTs) that examine the immediate postpartum use of copper IUDs and levonorgestrel-releasing (LNG) IUDs.8-11 In 2010, Chen and colleagues compared placement of the immediate postpartum IUD following vaginal delivery with interval placement at 6–8 weeks postpartum. Of 51 patients enrolled in each arm, 98% received an IUD immediately postpartum, and 90% received one during their postpartum visit. There were 12 expulsions (24%) in the immediate postpartum IUD group, compared with 2 (4.4%) in the interval group. Expelled IUDs were replaced, and at 6 months both groups had similar rates of IUD use.8
Whitaker and colleagues demonstrated similar findings after randomizing a small group of women who had a cesarean delivery (CD) to interval or immediate placement. There were significantly more expulsions in the post-placental group (20%) than the interval group (0%), but there were more users of the IUD in the post-placental group than in the interval group at 12 months.9
Two RCTs, by Lester and colleagues and Levi et al, demonstrated successful placement of the copper IUD or LNG-IUD following CD, with few expulsions (0% and 8%, respectively). Patients who were randomized to immediate postpartum IUD placement were more likely to receive an IUD than those who were randomized to interval insertion, mostly due to lack of postpartum follow up. Both studies followed patients out to 6 months, and rates of IUD continuation and satisfaction were higher at this time in the immediate postpartum IUD groups.10,11
Continue to: Risks, contraindications, and breastfeeding impact...
Risks, contraindications, and breastfeeding impact
What are the risks of immediate postpartum IUD placement? The highest risk of IUD placement in the immediate postpartum period appears to be expulsion (TABLE 1). In a meta-analysis conducted in 2022, which looked at 11 studies of immediate IUD insertion, the rates of expulsion were between 5% and 27%.3,8,12,13 Results of a study by Cohen and colleagues demonstrated that most expulsions occurred within the first 12 weeks following delivery; of those expulsions that occurred, only 11% went unrecognized.13 Immediate postpartum IUD insertion does not increase the IUD-associated risks of perforation, infection, or immediate postpartum bleeding (although prolonged bleeding may be more common).12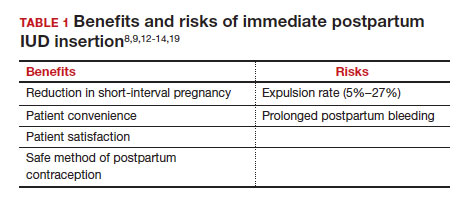
Are there contraindications to placing an IUD immediately postpartum? The main contraindication to immediate postpartum IUD use is peripartum infection, including Triple I, endomyometritis, and puerperal sepsis. Other contraindications include retained placenta requiring manual or surgical removal, uterine anomalies, and other medical contraindications to IUD use as recommended by the US Medical Eligibility Criteria.14
Does immediate IUD placement affect breastfeeding? There is theoretical risk of decreased milk supply or difficulty breastfeeding with initiation of progestin-only methods of contraception in the immediate postpartum period, as the rapid fall in progesterone levels initiates lactogenesis. However, progestin-only methods appear to have limited effect on initiation and continuation of breastfeeding in the immediate postpartum period.15
There were 2 secondary analyses of a pair of RCTs comparing immediate and delayed postpartum IUD use. Results from Levi and colleagues demonstrated no difference between immediate and interval IUD placement groups in the proportion of women who were breastfeeding at 6, 12, and 24 weeks.16 Chen and colleagues’ study was smaller; researchers found that women with interval IUD placement were more likely to be exclusively breastfeeding and continuing to breastfeed at 6 months compared with the immediate postpartum group.17
To better characterize the impact of progestin implants, in a recent meta-analysis, authors examined the use of subcutaneous levonorgestrel rods and found no difference in breastfeeding initiation and continuation rates between women who had them placed immediately versus 6 ̶ 8 weeks postpartum.12
Benefits of immediate postpartum IUD placement
One benefit of immediate postpartum IUD insertion is a reduction in short-interval pregnancies. In a study by Cohen and colleagues13 of young women aged 13 to 22 years choosing immediate postpartum IUDs (82) or implants (162), the authors found that 61% of women retained their IUDs at 12 months postpartum. Because few requested IUD removal over that time frame, the discontinuation rate at 1 year was primarily due to expulsions. Pregnancy rates at 1 year were 7.6% in the IUD group and 1.5% in the implant group. However, the 7.6% rate in the IUD group was lower than in previously studied adolescent control groups: 18.6% of control adolescents (38 of 204) using a contraceptive form other than a postpartum etonogestrel implant had repeat pregnancy at 1 year.13,18
Not only are patients who receive immediate postpartum IUDs more likely to receive them and continue their use, but they are also satisfied with the experience of receiving the IUD and with the method of contraception. A small mixed methods study of 66 patients demonstrated that women were interested in obtaining immediate postpartum contraception to avoid some of the logistical and financial challenges of returning for a postpartum visit. They also felt that the IUD placement was less painful than expected, and they didn’t feel that the insertion process imposed on their birth experience. Many described relief to know that they had a safe and effective contraceptive method upon leaving the hospital.19 Other studies have shown that even among women who expel an IUD following immediate postpartum placement, many choose to replace it in order to continue it as a contraceptive method.8,9,13
Continue to: Instructions for placement...
Instructions for placement
1. Counsel appropriately. Thoroughly counsel patients regarding their options for postpartum contraception, with emphasis on the benefits, risks, and contraindications. Current recommendations to reduce the risk of expulsion are to place the IUD in the delivery room or operating room within 10 minutes of placental delivery.
2. Post ̶ vaginal delivery. Following vaginal delivery, remove the IUD from the inserter, cut the strings to 10 cm and, using either fingers to grasp the wings of the IUD or ring forceps, advance the IUD to the fundus. Ultrasound guidance may be used, but it does not appear to be helpful in preventing expulsion.20
3. Post ̶ cesarean delivery. Once the placenta is delivered, place the IUD using the inserter or a ring forceps at the fundus and guide the strings into the cervix, then close the hysterotomy.
ACOG does recommend formal trainingbefore placing postpartum IUDs. One resource they provide is a free online webinar (https://www.acog.org/education-and-events/webinars/long-acting-reversible-contra ception-overview-and-hands-on-practice-for-residents).3
CASE 1 Resolved
The patient was counseled in the office about her options, and she was most interested in immediate postpartum LNG-IUD placement. She went on to deliver a healthy baby vaginally at 39 weeks. Within 10 minutes of placental delivery, she received an LNG-IUD. She returned to the office 3 months later for STI screening; her examination revealed correct placement and no evidence of expulsion. She expressed that she was happy with her IUD and thankful that she was able to receive it immediately after the birth of her baby.
CASE 2 Nulliparous woman desires IUD for postpartum contraception
A 33-year-old nulliparous woman presents in the third trimester for a routine prenatal visit. She had used the LNG-IUD prior to getting pregnant and reports that she was very happy with it. She knows she wants to wait at least 2 years before trying to get pregnant again, and she would like to resume contraception as soon as it is reasonably safe to do so. She has read that it is possible to get an IUD immediately postpartum and asks about it as a possible option.
What barriers will she face in obtaining an immediate postpartum IUD?
There are many barriers for patients who may be good candidates for immediate postpartum contraception (TABLE 2). Many patients are unaware that it is a safe option, and they often have concerns about such risks as infection, perforation, and effects on breastfeeding. Additionally, providers may not prioritize adequate counseling about postpartum contraception when they face time constraints and a need to counsel about other pregnancy-related topics during the prenatal visit schedule.7,21
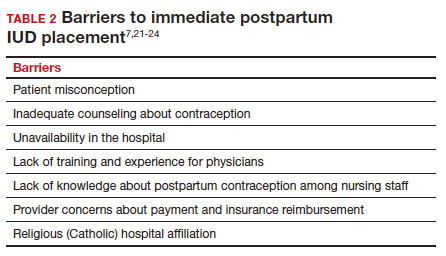
System, hospital, and clinician barriers to immediate postpartum IUD use
Hospital implementation of a successful postpartum IUD program requires pharmacy, intrapartum and postpartum nursing staff, physicians, administration, and billing to be aligned. Hospital administration and pharmacists must stock IUDs in the pharmacy. Hospital nursing staff attitudes toward and knowledge of postpartum contraception can have profound influence on how they discuss safe and effective methods of postpartum contraception with patients who may not have received counseling during prenatal care.22 In a survey of 108 ACOG fellows, nearly 75% of ObGyn physicians did not offer immediate postpartum IUDs; lack of provider training, lack of IUD availability, and concern about cost and payment were found to be common reasons why.21 Additionally, Catholic-affiliated and rural institutions are less likely to offer it, whereas more urban, teaching hospitals are more likely to have programs in place.23 Prior to 2012, immediate postpartum IUD insertions and device costs were part of the global Medicaid obstetric fee in most states, and both hospital systems and individual providers were concerned about loss of revenue.23
In 2015, Washington and colleagues published a decision analysis that examined the cost-effectiveness and cost savings associated with immediate postpartum IUD use. Accounting for expulsion rates, they found that immediate postpartum IUD placement can save $282,540 per 1,000 women over 2 years; additionally, immediate postpartum IUD use can prevent 88 unintended pregnancies per 1,000 women over 2 years.24 Not only do immediate postpartum IUDs have great potential to prevent individual patients from undesired short-interval pregnancies (FIGURE 1), but they can also save the system substantial health care dollars (FIGURE 2).
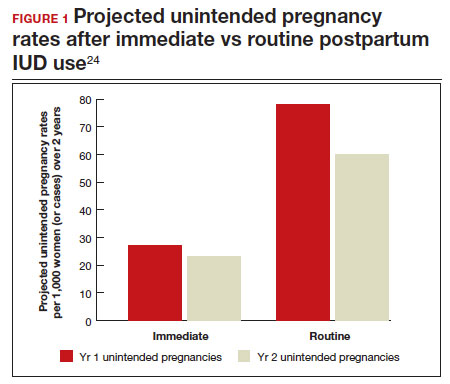
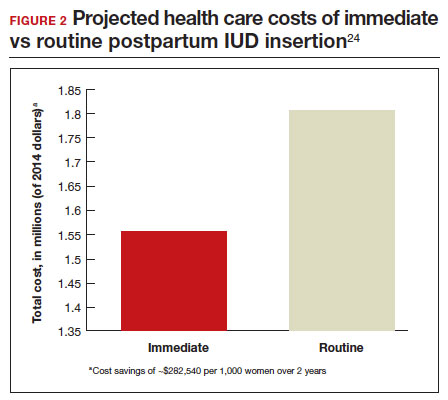
Overcoming barriers
Immediate postpartum IUD implementation is attainable with practice, policy, and institutional changes. Education and training programs geared toward providers and nursing staff can improve understanding of the benefits and risks of immediate postpartum IUD placement. Additionally, clinicians must provide comprehensive, nondirective counseling during the antepartum period, informing patients of all safe and effective options. Expulsion risks should be disclosed, as well as the benefit of not needing to return for a separate postpartum contraception appointment.
Since 2012, many state Medicaid agencies have decoupled reimbursement for inpatient postpartum IUD insertion from the delivery fee. By 2018, more than half of states adopted this practice. Commercial insurers have followed suit in some cases, and as such, both Medicaid and commercially insured patients have had increased access to immediate postpartum IUDs.23 This has translated into increased uptake of immediate postpartum IUDs among both Medicaid and commercially insured patients. Koch et al conducted a retrospective cohort study comparing IUD use in patients 1 year before and 1 year after the policy changes, and they found a 10-fold increase in use of immediate postpartum IUDs.25
While education, counseling, access, and changes in reimbursement may increase access in many hospital systems, some barriers, such as religious affiliation of the hospital system, may be impossible to overcome. A viable alternative to immediate postpartum IUD placement may be early postpartum IUD placement, which could allow patients to coordinate this procedure with 1- or 2-week return routine postpartum visits for CD recovery, mental health screenings, and/or well-baby visits. More data are necessary before recommending this universally, but Averbach and colleagues published a promising meta-analysis that demonstrated no complete expulsions in studies in which IUDs were placed between 2 and 4 weeks postpartum, and only a pooled partial expulsion rate (of immediate postpartum, early inpatient, early outpatient, and interval placement) of 3.7%.4
CASE 2 Resolved
Although the patient was interested in receiving a postpartum LNG-IUD immediately after her vaginal birth, she had to wait until her 6-week postpartum visit. The hospital did not stock IUDs for immediate postpartum IUD use, and her provider, having not been trained on immediate postpartum insertion, did not feel comfortable trying to place it in the immediate postpartum time frame. ●
- Immediate postpartum IUD insertion is a safe and effective method for postpartum contraception for many postpartum women.
- Immediate postpartum IUD insertion can result in increased uptake of postpartum contraception, a reduction in short interval pregnancies, and the opportunity for patients to plan their ideal family size.
- Patients should be thoroughly counseled about the safety of IUD placement and risks of expulsion associated with immediate postpartum placement.
- Successful programs for immediate postpartum IUD insertion incorporate training for providers on proper insertion techniques, education for nursing staff about safety and counseling, on-site IUD supply, and reimbursement that is decoupled from the payment for delivery.
- Winner B, Peipert JF, Zhao Q, et al. Effectiveness of longacting reversible contraception. N Engl J Med. 2012;366:19982007. doi: 10.1056/NEJMoa1110855.
- Bearak J, Popinchalk A, Ganatra B, et al. Unintended pregnancy and abortion by income, region, and the legal status of abortion: estimates from a comprehensive model for 1990-2019. Lancet Glob Health. 2020;8:e1152-e1161. doi: 10.1016/S2214-109X(20)30315-6.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice. Committee Opinion No. 670: Immediate postpartum long-acting reversible contraception. Obstet Gynecol. 2016;128:e32-e37. doi: 10.1097/AOG.0000000000001587.
- Averbach SH, Ermias Y, Jeng G, et al. Expulsion of intrauterine devices after postpartum placement by timing of placement, delivery type, and intrauterine device type: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;223:177188. doi: 10.1016/j.ajog.2020.02.045.
- Connolly A, Thorp J, Pahel L. Effects of pregnancy and childbirth on postpartum sexual function: a longitudinal prospective study. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:263-267. doi: 10.1007/s00192-005-1293-6.
- Conde-Agudelo A, Rosas-Bermúdez A, Kafury-Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a meta-analysis. JAMA. 2006;295:1809-1823. doi: 10.1001 /jama.295.15.1809.
- Vricella LK, Gawron LM, Louis JM. Society for MaternalFetal Medicine (SMFM) Consult Series #48: Immediate postpartum long-acting reversible contraception for women at high risk for medical complications. Am J Obstet Gynecol. 2019;220:B2-B12. doi: 10.1016/j.ajog.2019.02.011.
- Chen BA, Reeves MF, Hayes JL, et al. Postplacental or delayed insertion of the levonorgestrel intrauterine device after vaginal delivery: a randomized controlled trial. Obstet Gynecol. 2010;116:1079-1087. doi: 10.1097/AOG.0b013e3181f73fac.
- Whitaker AK, Endres LK, Mistretta SQ, et al. Postplacental insertion of the levonorgestrel intrauterine device after cesarean delivery vs. delayed insertion: a randomized controlled trial. Contraception. 2014;89:534-539. doi: 10.1016/j.contraception.2013.12.007.
- Lester F, Kakaire O, Byamugisha J, et al. Intracesarean insertion of the Copper T380A versus 6 weeks postcesarean: a randomized clinical trial. Contraception. 2015;91:198-203. doi: 10.1016/j.contraception.2014.12.002.
- Levi EE, Stuart GS, Zerden ML, et al. Intrauterine device placement during cesarean delivery and continued use 6 months postpartum: a randomized controlled trial. Obstet Gynecol. 2015;126:5-11. doi: 10.1097/AOG.0000000000000882.
- Sothornwit J, Kaewrudee S, Lumbiganon P, et al. Immediate versus delayed postpartum insertion of contraceptive implant and IUD for contraception. Cochrane Database Syst Rev. 2022;10:CD011913. doi: 10.1002/14651858.CD011913.pub3.
- Cohen R, Sheeder J, Arango N, et al. Twelve-month contraceptive continuation and repeat pregnancy among young mothers choosing postdelivery contraceptive implants or postplacental intrauterine devices. Contraception. 2016;93:178-183. doi: 10.1016/j.contraception.2015.10.001.
- Centers for Disease Control and Prevention (CDC). US Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep. 2010;59(RR-4):1-86.
- Kapp N, Curtis K, Nanda K. Progestogen-only contraceptive use among breastfeeding women: a systematic review. Contraception. 2010;82:17-37. doi: 10.1016 /j.contraception.2010.02.002.
- Levi EE, Findley MK, Avila K, et al. Placement of levonorgestrel intrauterine device at the time of cesarean delivery and the effect on breastfeeding duration. Breastfeed Med. 2018;13:674679. doi: 10.1089/bfm.2018.0060.
- Chen BA, Reeves MF, Creinin MD, et al. Postplacental or delayed levonorgestrel intrauterine device insertion and breast-feeding duration. Contraception. 2011;84:499-504. doi: 10.1016/j.contraception.2011.01.022.
- Tocce KM, Sheeder JL, Teal SB. Rapid repeat pregnancy in adolescents: do immediate postpartum contraceptive implants make a difference? Am J Obstet Gynecol. 2012;206:481.e1-7. doi: 10.1016/j.ajog.2012.04.015.
- Carr SL, Singh RH, Sussman AL, et al. Women’s experiences with immediate postpartum intrauterine device insertion: a mixed-methods study. Contraception. 2018;97:219-226. doi: 10.1016/j.contraception.2017.10.008.
- Martinez OP, Wilder L, Seal P. Ultrasound-guided compared with non-ultrasound-Guided placement of immediate postpartum intrauterine contraceptive devices. Obstet Gynecol. 2022;140:91-93. doi: 10.1097/AOG.0000000000004828.
- Holden EC, Lai E, Morelli SS, et al. Ongoing barriers to immediate postpartum long-acting reversible contraception: a physician survey. Contracept Reprod Med. 2018;3:23. doi: 10.1186/s40834-018-0078-5.
- Benfield N, Hawkins F, Ray L, et al. Exposure to routine availability of immediate postpartum LARC: effect on attitudes and practices of labor and delivery and postpartum nurses. Contraception. 2018;97:411-414. doi: 10.1016 /j.contraception.2018.01.017.
- Steenland MW, Vatsa R, Pace LE, et al. Immediate postpartum long-acting reversible contraceptive use following statespecific changes in hospital Medicaid reimbursement. JAMA Netw Open. 2022;5:e2237918. doi: 10.1001 /jamanetworkopen.2022.37918.
- Washington CI, Jamshidi R, Thung SF, et al. Timing of postpartum intrauterine device placement: a costeffectiveness analysis. Fertil Steril. 2015;103:131-137. doi: 10.1016/j.fertnstert.2014.09.032
CASE 1 Multiparous female with short-interval pregnancies desires contraception
A 24-year-old woman (G4P3) presents for a routine prenatal visit in the third trimester. Her last 2 pregnancies have occurred within 3 months of her prior birth. She endorses feeling overwhelmed with having 4 children under the age of 5 years, and she specifies that she would like to avoid another pregnancy for several years. She plans to breast and bottle feed, and she notes that she tends to forget to take pills. When you look back at her prior charts, you note that she did not return for her last 2 postpartum visits. What can you offer her? What would be a safe contraceptive option for her?
Intrauterine devices (IUDs) are safe, effective, and reported by patients to be satisfactory methods of contraception precisely because they are prone to less user error. The Contraceptive Choice Project demonstrated that patients are more apt to choose them when barriers such as cost and access are removed and nondirective counseling is provided.1 Given that unintended pregnancy rates hover around 48%, the American College of Obstetricians and Gynecologists (ACOG) recommends them as first-line methods for pregnancy prevention.2,3
For repeat pregnancies, the postpartum period is an especially vulnerable time—non-breastfeeding women will ovulate as soon as 25 days after birth, and by 8 weeks 30% will have ovulated.4 Approximately 40% to 57% of women report having unprotected intercourse before 6 weeks postpartum, and nearly 70% of all pregnancies in the first year postpartum are unintended.3,5 Furthermore, patients at highest risk for short-interval pregnancy, such as adolescents, are less likely to return for a postpartum visit.3
Short-interval pregnancies confer greater fetal risk, including risks of low-birth weight, preterm birth, small for gestational age and increased risk of neonatal intensive care unit admission.6 Additionally, maternal health may be compromised during a short-interval pregnancy, particularly in medically complex patients due to increased risks of adverse pregnancy outcomes, such as postpartum bleeding or uterine rupture and disease progression.7 A 2006 meta-analysis by Conde-Agudelo and colleagues found that waiting at least 18 months between pregnancies was optimal for reducing these risks.6
Thus, the immediate postpartum period is an optimal time for addressing contraceptive needs and for preventing short-interval and unintended pregnancy. This article aims to provide evidence supporting the use of immediate postpartum IUDs, as well as their associated risks and barriers to use.
IUD types and routes for immediate postpartum insertion
There are several randomized controlled trials (RCTs) that examine the immediate postpartum use of copper IUDs and levonorgestrel-releasing (LNG) IUDs.8-11 In 2010, Chen and colleagues compared placement of the immediate postpartum IUD following vaginal delivery with interval placement at 6–8 weeks postpartum. Of 51 patients enrolled in each arm, 98% received an IUD immediately postpartum, and 90% received one during their postpartum visit. There were 12 expulsions (24%) in the immediate postpartum IUD group, compared with 2 (4.4%) in the interval group. Expelled IUDs were replaced, and at 6 months both groups had similar rates of IUD use.8
Whitaker and colleagues demonstrated similar findings after randomizing a small group of women who had a cesarean delivery (CD) to interval or immediate placement. There were significantly more expulsions in the post-placental group (20%) than the interval group (0%), but there were more users of the IUD in the post-placental group than in the interval group at 12 months.9
Two RCTs, by Lester and colleagues and Levi et al, demonstrated successful placement of the copper IUD or LNG-IUD following CD, with few expulsions (0% and 8%, respectively). Patients who were randomized to immediate postpartum IUD placement were more likely to receive an IUD than those who were randomized to interval insertion, mostly due to lack of postpartum follow up. Both studies followed patients out to 6 months, and rates of IUD continuation and satisfaction were higher at this time in the immediate postpartum IUD groups.10,11
Continue to: Risks, contraindications, and breastfeeding impact...
Risks, contraindications, and breastfeeding impact
What are the risks of immediate postpartum IUD placement? The highest risk of IUD placement in the immediate postpartum period appears to be expulsion (TABLE 1). In a meta-analysis conducted in 2022, which looked at 11 studies of immediate IUD insertion, the rates of expulsion were between 5% and 27%.3,8,12,13 Results of a study by Cohen and colleagues demonstrated that most expulsions occurred within the first 12 weeks following delivery; of those expulsions that occurred, only 11% went unrecognized.13 Immediate postpartum IUD insertion does not increase the IUD-associated risks of perforation, infection, or immediate postpartum bleeding (although prolonged bleeding may be more common).12
Are there contraindications to placing an IUD immediately postpartum? The main contraindication to immediate postpartum IUD use is peripartum infection, including Triple I, endomyometritis, and puerperal sepsis. Other contraindications include retained placenta requiring manual or surgical removal, uterine anomalies, and other medical contraindications to IUD use as recommended by the US Medical Eligibility Criteria.14
Does immediate IUD placement affect breastfeeding? There is theoretical risk of decreased milk supply or difficulty breastfeeding with initiation of progestin-only methods of contraception in the immediate postpartum period, as the rapid fall in progesterone levels initiates lactogenesis. However, progestin-only methods appear to have limited effect on initiation and continuation of breastfeeding in the immediate postpartum period.15
There were 2 secondary analyses of a pair of RCTs comparing immediate and delayed postpartum IUD use. Results from Levi and colleagues demonstrated no difference between immediate and interval IUD placement groups in the proportion of women who were breastfeeding at 6, 12, and 24 weeks.16 Chen and colleagues’ study was smaller; researchers found that women with interval IUD placement were more likely to be exclusively breastfeeding and continuing to breastfeed at 6 months compared with the immediate postpartum group.17
To better characterize the impact of progestin implants, in a recent meta-analysis, authors examined the use of subcutaneous levonorgestrel rods and found no difference in breastfeeding initiation and continuation rates between women who had them placed immediately versus 6 ̶ 8 weeks postpartum.12
Benefits of immediate postpartum IUD placement
One benefit of immediate postpartum IUD insertion is a reduction in short-interval pregnancies. In a study by Cohen and colleagues13 of young women aged 13 to 22 years choosing immediate postpartum IUDs (82) or implants (162), the authors found that 61% of women retained their IUDs at 12 months postpartum. Because few requested IUD removal over that time frame, the discontinuation rate at 1 year was primarily due to expulsions. Pregnancy rates at 1 year were 7.6% in the IUD group and 1.5% in the implant group. However, the 7.6% rate in the IUD group was lower than in previously studied adolescent control groups: 18.6% of control adolescents (38 of 204) using a contraceptive form other than a postpartum etonogestrel implant had repeat pregnancy at 1 year.13,18
Not only are patients who receive immediate postpartum IUDs more likely to receive them and continue their use, but they are also satisfied with the experience of receiving the IUD and with the method of contraception. A small mixed methods study of 66 patients demonstrated that women were interested in obtaining immediate postpartum contraception to avoid some of the logistical and financial challenges of returning for a postpartum visit. They also felt that the IUD placement was less painful than expected, and they didn’t feel that the insertion process imposed on their birth experience. Many described relief to know that they had a safe and effective contraceptive method upon leaving the hospital.19 Other studies have shown that even among women who expel an IUD following immediate postpartum placement, many choose to replace it in order to continue it as a contraceptive method.8,9,13
Continue to: Instructions for placement...
Instructions for placement
1. Counsel appropriately. Thoroughly counsel patients regarding their options for postpartum contraception, with emphasis on the benefits, risks, and contraindications. Current recommendations to reduce the risk of expulsion are to place the IUD in the delivery room or operating room within 10 minutes of placental delivery.
2. Post ̶ vaginal delivery. Following vaginal delivery, remove the IUD from the inserter, cut the strings to 10 cm and, using either fingers to grasp the wings of the IUD or ring forceps, advance the IUD to the fundus. Ultrasound guidance may be used, but it does not appear to be helpful in preventing expulsion.20
3. Post ̶ cesarean delivery. Once the placenta is delivered, place the IUD using the inserter or a ring forceps at the fundus and guide the strings into the cervix, then close the hysterotomy.
ACOG does recommend formal trainingbefore placing postpartum IUDs. One resource they provide is a free online webinar (https://www.acog.org/education-and-events/webinars/long-acting-reversible-contra ception-overview-and-hands-on-practice-for-residents).3
CASE 1 Resolved
The patient was counseled in the office about her options, and she was most interested in immediate postpartum LNG-IUD placement. She went on to deliver a healthy baby vaginally at 39 weeks. Within 10 minutes of placental delivery, she received an LNG-IUD. She returned to the office 3 months later for STI screening; her examination revealed correct placement and no evidence of expulsion. She expressed that she was happy with her IUD and thankful that she was able to receive it immediately after the birth of her baby.
CASE 2 Nulliparous woman desires IUD for postpartum contraception
A 33-year-old nulliparous woman presents in the third trimester for a routine prenatal visit. She had used the LNG-IUD prior to getting pregnant and reports that she was very happy with it. She knows she wants to wait at least 2 years before trying to get pregnant again, and she would like to resume contraception as soon as it is reasonably safe to do so. She has read that it is possible to get an IUD immediately postpartum and asks about it as a possible option.
What barriers will she face in obtaining an immediate postpartum IUD?
There are many barriers for patients who may be good candidates for immediate postpartum contraception (TABLE 2). Many patients are unaware that it is a safe option, and they often have concerns about such risks as infection, perforation, and effects on breastfeeding. Additionally, providers may not prioritize adequate counseling about postpartum contraception when they face time constraints and a need to counsel about other pregnancy-related topics during the prenatal visit schedule.7,21

System, hospital, and clinician barriers to immediate postpartum IUD use
Hospital implementation of a successful postpartum IUD program requires pharmacy, intrapartum and postpartum nursing staff, physicians, administration, and billing to be aligned. Hospital administration and pharmacists must stock IUDs in the pharmacy. Hospital nursing staff attitudes toward and knowledge of postpartum contraception can have profound influence on how they discuss safe and effective methods of postpartum contraception with patients who may not have received counseling during prenatal care.22 In a survey of 108 ACOG fellows, nearly 75% of ObGyn physicians did not offer immediate postpartum IUDs; lack of provider training, lack of IUD availability, and concern about cost and payment were found to be common reasons why.21 Additionally, Catholic-affiliated and rural institutions are less likely to offer it, whereas more urban, teaching hospitals are more likely to have programs in place.23 Prior to 2012, immediate postpartum IUD insertions and device costs were part of the global Medicaid obstetric fee in most states, and both hospital systems and individual providers were concerned about loss of revenue.23
In 2015, Washington and colleagues published a decision analysis that examined the cost-effectiveness and cost savings associated with immediate postpartum IUD use. Accounting for expulsion rates, they found that immediate postpartum IUD placement can save $282,540 per 1,000 women over 2 years; additionally, immediate postpartum IUD use can prevent 88 unintended pregnancies per 1,000 women over 2 years.24 Not only do immediate postpartum IUDs have great potential to prevent individual patients from undesired short-interval pregnancies (FIGURE 1), but they can also save the system substantial health care dollars (FIGURE 2).


Overcoming barriers
Immediate postpartum IUD implementation is attainable with practice, policy, and institutional changes. Education and training programs geared toward providers and nursing staff can improve understanding of the benefits and risks of immediate postpartum IUD placement. Additionally, clinicians must provide comprehensive, nondirective counseling during the antepartum period, informing patients of all safe and effective options. Expulsion risks should be disclosed, as well as the benefit of not needing to return for a separate postpartum contraception appointment.
Since 2012, many state Medicaid agencies have decoupled reimbursement for inpatient postpartum IUD insertion from the delivery fee. By 2018, more than half of states adopted this practice. Commercial insurers have followed suit in some cases, and as such, both Medicaid and commercially insured patients have had increased access to immediate postpartum IUDs.23 This has translated into increased uptake of immediate postpartum IUDs among both Medicaid and commercially insured patients. Koch et al conducted a retrospective cohort study comparing IUD use in patients 1 year before and 1 year after the policy changes, and they found a 10-fold increase in use of immediate postpartum IUDs.25
While education, counseling, access, and changes in reimbursement may increase access in many hospital systems, some barriers, such as religious affiliation of the hospital system, may be impossible to overcome. A viable alternative to immediate postpartum IUD placement may be early postpartum IUD placement, which could allow patients to coordinate this procedure with 1- or 2-week return routine postpartum visits for CD recovery, mental health screenings, and/or well-baby visits. More data are necessary before recommending this universally, but Averbach and colleagues published a promising meta-analysis that demonstrated no complete expulsions in studies in which IUDs were placed between 2 and 4 weeks postpartum, and only a pooled partial expulsion rate (of immediate postpartum, early inpatient, early outpatient, and interval placement) of 3.7%.4
CASE 2 Resolved
Although the patient was interested in receiving a postpartum LNG-IUD immediately after her vaginal birth, she had to wait until her 6-week postpartum visit. The hospital did not stock IUDs for immediate postpartum IUD use, and her provider, having not been trained on immediate postpartum insertion, did not feel comfortable trying to place it in the immediate postpartum time frame. ●
- Immediate postpartum IUD insertion is a safe and effective method for postpartum contraception for many postpartum women.
- Immediate postpartum IUD insertion can result in increased uptake of postpartum contraception, a reduction in short interval pregnancies, and the opportunity for patients to plan their ideal family size.
- Patients should be thoroughly counseled about the safety of IUD placement and risks of expulsion associated with immediate postpartum placement.
- Successful programs for immediate postpartum IUD insertion incorporate training for providers on proper insertion techniques, education for nursing staff about safety and counseling, on-site IUD supply, and reimbursement that is decoupled from the payment for delivery.
CASE 1 Multiparous female with short-interval pregnancies desires contraception
A 24-year-old woman (G4P3) presents for a routine prenatal visit in the third trimester. Her last 2 pregnancies have occurred within 3 months of her prior birth. She endorses feeling overwhelmed with having 4 children under the age of 5 years, and she specifies that she would like to avoid another pregnancy for several years. She plans to breast and bottle feed, and she notes that she tends to forget to take pills. When you look back at her prior charts, you note that she did not return for her last 2 postpartum visits. What can you offer her? What would be a safe contraceptive option for her?
Intrauterine devices (IUDs) are safe, effective, and reported by patients to be satisfactory methods of contraception precisely because they are prone to less user error. The Contraceptive Choice Project demonstrated that patients are more apt to choose them when barriers such as cost and access are removed and nondirective counseling is provided.1 Given that unintended pregnancy rates hover around 48%, the American College of Obstetricians and Gynecologists (ACOG) recommends them as first-line methods for pregnancy prevention.2,3
For repeat pregnancies, the postpartum period is an especially vulnerable time—non-breastfeeding women will ovulate as soon as 25 days after birth, and by 8 weeks 30% will have ovulated.4 Approximately 40% to 57% of women report having unprotected intercourse before 6 weeks postpartum, and nearly 70% of all pregnancies in the first year postpartum are unintended.3,5 Furthermore, patients at highest risk for short-interval pregnancy, such as adolescents, are less likely to return for a postpartum visit.3
Short-interval pregnancies confer greater fetal risk, including risks of low-birth weight, preterm birth, small for gestational age and increased risk of neonatal intensive care unit admission.6 Additionally, maternal health may be compromised during a short-interval pregnancy, particularly in medically complex patients due to increased risks of adverse pregnancy outcomes, such as postpartum bleeding or uterine rupture and disease progression.7 A 2006 meta-analysis by Conde-Agudelo and colleagues found that waiting at least 18 months between pregnancies was optimal for reducing these risks.6
Thus, the immediate postpartum period is an optimal time for addressing contraceptive needs and for preventing short-interval and unintended pregnancy. This article aims to provide evidence supporting the use of immediate postpartum IUDs, as well as their associated risks and barriers to use.
IUD types and routes for immediate postpartum insertion
There are several randomized controlled trials (RCTs) that examine the immediate postpartum use of copper IUDs and levonorgestrel-releasing (LNG) IUDs.8-11 In 2010, Chen and colleagues compared placement of the immediate postpartum IUD following vaginal delivery with interval placement at 6–8 weeks postpartum. Of 51 patients enrolled in each arm, 98% received an IUD immediately postpartum, and 90% received one during their postpartum visit. There were 12 expulsions (24%) in the immediate postpartum IUD group, compared with 2 (4.4%) in the interval group. Expelled IUDs were replaced, and at 6 months both groups had similar rates of IUD use.8
Whitaker and colleagues demonstrated similar findings after randomizing a small group of women who had a cesarean delivery (CD) to interval or immediate placement. There were significantly more expulsions in the post-placental group (20%) than the interval group (0%), but there were more users of the IUD in the post-placental group than in the interval group at 12 months.9
Two RCTs, by Lester and colleagues and Levi et al, demonstrated successful placement of the copper IUD or LNG-IUD following CD, with few expulsions (0% and 8%, respectively). Patients who were randomized to immediate postpartum IUD placement were more likely to receive an IUD than those who were randomized to interval insertion, mostly due to lack of postpartum follow up. Both studies followed patients out to 6 months, and rates of IUD continuation and satisfaction were higher at this time in the immediate postpartum IUD groups.10,11
Continue to: Risks, contraindications, and breastfeeding impact...
Risks, contraindications, and breastfeeding impact
What are the risks of immediate postpartum IUD placement? The highest risk of IUD placement in the immediate postpartum period appears to be expulsion (TABLE 1). In a meta-analysis conducted in 2022, which looked at 11 studies of immediate IUD insertion, the rates of expulsion were between 5% and 27%.3,8,12,13 Results of a study by Cohen and colleagues demonstrated that most expulsions occurred within the first 12 weeks following delivery; of those expulsions that occurred, only 11% went unrecognized.13 Immediate postpartum IUD insertion does not increase the IUD-associated risks of perforation, infection, or immediate postpartum bleeding (although prolonged bleeding may be more common).12
Are there contraindications to placing an IUD immediately postpartum? The main contraindication to immediate postpartum IUD use is peripartum infection, including Triple I, endomyometritis, and puerperal sepsis. Other contraindications include retained placenta requiring manual or surgical removal, uterine anomalies, and other medical contraindications to IUD use as recommended by the US Medical Eligibility Criteria.14
Does immediate IUD placement affect breastfeeding? There is theoretical risk of decreased milk supply or difficulty breastfeeding with initiation of progestin-only methods of contraception in the immediate postpartum period, as the rapid fall in progesterone levels initiates lactogenesis. However, progestin-only methods appear to have limited effect on initiation and continuation of breastfeeding in the immediate postpartum period.15
There were 2 secondary analyses of a pair of RCTs comparing immediate and delayed postpartum IUD use. Results from Levi and colleagues demonstrated no difference between immediate and interval IUD placement groups in the proportion of women who were breastfeeding at 6, 12, and 24 weeks.16 Chen and colleagues’ study was smaller; researchers found that women with interval IUD placement were more likely to be exclusively breastfeeding and continuing to breastfeed at 6 months compared with the immediate postpartum group.17
To better characterize the impact of progestin implants, in a recent meta-analysis, authors examined the use of subcutaneous levonorgestrel rods and found no difference in breastfeeding initiation and continuation rates between women who had them placed immediately versus 6 ̶ 8 weeks postpartum.12
Benefits of immediate postpartum IUD placement
One benefit of immediate postpartum IUD insertion is a reduction in short-interval pregnancies. In a study by Cohen and colleagues13 of young women aged 13 to 22 years choosing immediate postpartum IUDs (82) or implants (162), the authors found that 61% of women retained their IUDs at 12 months postpartum. Because few requested IUD removal over that time frame, the discontinuation rate at 1 year was primarily due to expulsions. Pregnancy rates at 1 year were 7.6% in the IUD group and 1.5% in the implant group. However, the 7.6% rate in the IUD group was lower than in previously studied adolescent control groups: 18.6% of control adolescents (38 of 204) using a contraceptive form other than a postpartum etonogestrel implant had repeat pregnancy at 1 year.13,18
Not only are patients who receive immediate postpartum IUDs more likely to receive them and continue their use, but they are also satisfied with the experience of receiving the IUD and with the method of contraception. A small mixed methods study of 66 patients demonstrated that women were interested in obtaining immediate postpartum contraception to avoid some of the logistical and financial challenges of returning for a postpartum visit. They also felt that the IUD placement was less painful than expected, and they didn’t feel that the insertion process imposed on their birth experience. Many described relief to know that they had a safe and effective contraceptive method upon leaving the hospital.19 Other studies have shown that even among women who expel an IUD following immediate postpartum placement, many choose to replace it in order to continue it as a contraceptive method.8,9,13
Continue to: Instructions for placement...
Instructions for placement
1. Counsel appropriately. Thoroughly counsel patients regarding their options for postpartum contraception, with emphasis on the benefits, risks, and contraindications. Current recommendations to reduce the risk of expulsion are to place the IUD in the delivery room or operating room within 10 minutes of placental delivery.
2. Post ̶ vaginal delivery. Following vaginal delivery, remove the IUD from the inserter, cut the strings to 10 cm and, using either fingers to grasp the wings of the IUD or ring forceps, advance the IUD to the fundus. Ultrasound guidance may be used, but it does not appear to be helpful in preventing expulsion.20
3. Post ̶ cesarean delivery. Once the placenta is delivered, place the IUD using the inserter or a ring forceps at the fundus and guide the strings into the cervix, then close the hysterotomy.
ACOG does recommend formal trainingbefore placing postpartum IUDs. One resource they provide is a free online webinar (https://www.acog.org/education-and-events/webinars/long-acting-reversible-contra ception-overview-and-hands-on-practice-for-residents).3
CASE 1 Resolved
The patient was counseled in the office about her options, and she was most interested in immediate postpartum LNG-IUD placement. She went on to deliver a healthy baby vaginally at 39 weeks. Within 10 minutes of placental delivery, she received an LNG-IUD. She returned to the office 3 months later for STI screening; her examination revealed correct placement and no evidence of expulsion. She expressed that she was happy with her IUD and thankful that she was able to receive it immediately after the birth of her baby.
CASE 2 Nulliparous woman desires IUD for postpartum contraception
A 33-year-old nulliparous woman presents in the third trimester for a routine prenatal visit. She had used the LNG-IUD prior to getting pregnant and reports that she was very happy with it. She knows she wants to wait at least 2 years before trying to get pregnant again, and she would like to resume contraception as soon as it is reasonably safe to do so. She has read that it is possible to get an IUD immediately postpartum and asks about it as a possible option.
What barriers will she face in obtaining an immediate postpartum IUD?
There are many barriers for patients who may be good candidates for immediate postpartum contraception (TABLE 2). Many patients are unaware that it is a safe option, and they often have concerns about such risks as infection, perforation, and effects on breastfeeding. Additionally, providers may not prioritize adequate counseling about postpartum contraception when they face time constraints and a need to counsel about other pregnancy-related topics during the prenatal visit schedule.7,21

System, hospital, and clinician barriers to immediate postpartum IUD use
Hospital implementation of a successful postpartum IUD program requires pharmacy, intrapartum and postpartum nursing staff, physicians, administration, and billing to be aligned. Hospital administration and pharmacists must stock IUDs in the pharmacy. Hospital nursing staff attitudes toward and knowledge of postpartum contraception can have profound influence on how they discuss safe and effective methods of postpartum contraception with patients who may not have received counseling during prenatal care.22 In a survey of 108 ACOG fellows, nearly 75% of ObGyn physicians did not offer immediate postpartum IUDs; lack of provider training, lack of IUD availability, and concern about cost and payment were found to be common reasons why.21 Additionally, Catholic-affiliated and rural institutions are less likely to offer it, whereas more urban, teaching hospitals are more likely to have programs in place.23 Prior to 2012, immediate postpartum IUD insertions and device costs were part of the global Medicaid obstetric fee in most states, and both hospital systems and individual providers were concerned about loss of revenue.23
In 2015, Washington and colleagues published a decision analysis that examined the cost-effectiveness and cost savings associated with immediate postpartum IUD use. Accounting for expulsion rates, they found that immediate postpartum IUD placement can save $282,540 per 1,000 women over 2 years; additionally, immediate postpartum IUD use can prevent 88 unintended pregnancies per 1,000 women over 2 years.24 Not only do immediate postpartum IUDs have great potential to prevent individual patients from undesired short-interval pregnancies (FIGURE 1), but they can also save the system substantial health care dollars (FIGURE 2).


Overcoming barriers
Immediate postpartum IUD implementation is attainable with practice, policy, and institutional changes. Education and training programs geared toward providers and nursing staff can improve understanding of the benefits and risks of immediate postpartum IUD placement. Additionally, clinicians must provide comprehensive, nondirective counseling during the antepartum period, informing patients of all safe and effective options. Expulsion risks should be disclosed, as well as the benefit of not needing to return for a separate postpartum contraception appointment.
Since 2012, many state Medicaid agencies have decoupled reimbursement for inpatient postpartum IUD insertion from the delivery fee. By 2018, more than half of states adopted this practice. Commercial insurers have followed suit in some cases, and as such, both Medicaid and commercially insured patients have had increased access to immediate postpartum IUDs.23 This has translated into increased uptake of immediate postpartum IUDs among both Medicaid and commercially insured patients. Koch et al conducted a retrospective cohort study comparing IUD use in patients 1 year before and 1 year after the policy changes, and they found a 10-fold increase in use of immediate postpartum IUDs.25
While education, counseling, access, and changes in reimbursement may increase access in many hospital systems, some barriers, such as religious affiliation of the hospital system, may be impossible to overcome. A viable alternative to immediate postpartum IUD placement may be early postpartum IUD placement, which could allow patients to coordinate this procedure with 1- or 2-week return routine postpartum visits for CD recovery, mental health screenings, and/or well-baby visits. More data are necessary before recommending this universally, but Averbach and colleagues published a promising meta-analysis that demonstrated no complete expulsions in studies in which IUDs were placed between 2 and 4 weeks postpartum, and only a pooled partial expulsion rate (of immediate postpartum, early inpatient, early outpatient, and interval placement) of 3.7%.4
CASE 2 Resolved
Although the patient was interested in receiving a postpartum LNG-IUD immediately after her vaginal birth, she had to wait until her 6-week postpartum visit. The hospital did not stock IUDs for immediate postpartum IUD use, and her provider, having not been trained on immediate postpartum insertion, did not feel comfortable trying to place it in the immediate postpartum time frame. ●
- Immediate postpartum IUD insertion is a safe and effective method for postpartum contraception for many postpartum women.
- Immediate postpartum IUD insertion can result in increased uptake of postpartum contraception, a reduction in short interval pregnancies, and the opportunity for patients to plan their ideal family size.
- Patients should be thoroughly counseled about the safety of IUD placement and risks of expulsion associated with immediate postpartum placement.
- Successful programs for immediate postpartum IUD insertion incorporate training for providers on proper insertion techniques, education for nursing staff about safety and counseling, on-site IUD supply, and reimbursement that is decoupled from the payment for delivery.
- Winner B, Peipert JF, Zhao Q, et al. Effectiveness of longacting reversible contraception. N Engl J Med. 2012;366:19982007. doi: 10.1056/NEJMoa1110855.
- Bearak J, Popinchalk A, Ganatra B, et al. Unintended pregnancy and abortion by income, region, and the legal status of abortion: estimates from a comprehensive model for 1990-2019. Lancet Glob Health. 2020;8:e1152-e1161. doi: 10.1016/S2214-109X(20)30315-6.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice. Committee Opinion No. 670: Immediate postpartum long-acting reversible contraception. Obstet Gynecol. 2016;128:e32-e37. doi: 10.1097/AOG.0000000000001587.
- Averbach SH, Ermias Y, Jeng G, et al. Expulsion of intrauterine devices after postpartum placement by timing of placement, delivery type, and intrauterine device type: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;223:177188. doi: 10.1016/j.ajog.2020.02.045.
- Connolly A, Thorp J, Pahel L. Effects of pregnancy and childbirth on postpartum sexual function: a longitudinal prospective study. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:263-267. doi: 10.1007/s00192-005-1293-6.
- Conde-Agudelo A, Rosas-Bermúdez A, Kafury-Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a meta-analysis. JAMA. 2006;295:1809-1823. doi: 10.1001 /jama.295.15.1809.
- Vricella LK, Gawron LM, Louis JM. Society for MaternalFetal Medicine (SMFM) Consult Series #48: Immediate postpartum long-acting reversible contraception for women at high risk for medical complications. Am J Obstet Gynecol. 2019;220:B2-B12. doi: 10.1016/j.ajog.2019.02.011.
- Chen BA, Reeves MF, Hayes JL, et al. Postplacental or delayed insertion of the levonorgestrel intrauterine device after vaginal delivery: a randomized controlled trial. Obstet Gynecol. 2010;116:1079-1087. doi: 10.1097/AOG.0b013e3181f73fac.
- Whitaker AK, Endres LK, Mistretta SQ, et al. Postplacental insertion of the levonorgestrel intrauterine device after cesarean delivery vs. delayed insertion: a randomized controlled trial. Contraception. 2014;89:534-539. doi: 10.1016/j.contraception.2013.12.007.
- Lester F, Kakaire O, Byamugisha J, et al. Intracesarean insertion of the Copper T380A versus 6 weeks postcesarean: a randomized clinical trial. Contraception. 2015;91:198-203. doi: 10.1016/j.contraception.2014.12.002.
- Levi EE, Stuart GS, Zerden ML, et al. Intrauterine device placement during cesarean delivery and continued use 6 months postpartum: a randomized controlled trial. Obstet Gynecol. 2015;126:5-11. doi: 10.1097/AOG.0000000000000882.
- Sothornwit J, Kaewrudee S, Lumbiganon P, et al. Immediate versus delayed postpartum insertion of contraceptive implant and IUD for contraception. Cochrane Database Syst Rev. 2022;10:CD011913. doi: 10.1002/14651858.CD011913.pub3.
- Cohen R, Sheeder J, Arango N, et al. Twelve-month contraceptive continuation and repeat pregnancy among young mothers choosing postdelivery contraceptive implants or postplacental intrauterine devices. Contraception. 2016;93:178-183. doi: 10.1016/j.contraception.2015.10.001.
- Centers for Disease Control and Prevention (CDC). US Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep. 2010;59(RR-4):1-86.
- Kapp N, Curtis K, Nanda K. Progestogen-only contraceptive use among breastfeeding women: a systematic review. Contraception. 2010;82:17-37. doi: 10.1016 /j.contraception.2010.02.002.
- Levi EE, Findley MK, Avila K, et al. Placement of levonorgestrel intrauterine device at the time of cesarean delivery and the effect on breastfeeding duration. Breastfeed Med. 2018;13:674679. doi: 10.1089/bfm.2018.0060.
- Chen BA, Reeves MF, Creinin MD, et al. Postplacental or delayed levonorgestrel intrauterine device insertion and breast-feeding duration. Contraception. 2011;84:499-504. doi: 10.1016/j.contraception.2011.01.022.
- Tocce KM, Sheeder JL, Teal SB. Rapid repeat pregnancy in adolescents: do immediate postpartum contraceptive implants make a difference? Am J Obstet Gynecol. 2012;206:481.e1-7. doi: 10.1016/j.ajog.2012.04.015.
- Carr SL, Singh RH, Sussman AL, et al. Women’s experiences with immediate postpartum intrauterine device insertion: a mixed-methods study. Contraception. 2018;97:219-226. doi: 10.1016/j.contraception.2017.10.008.
- Martinez OP, Wilder L, Seal P. Ultrasound-guided compared with non-ultrasound-Guided placement of immediate postpartum intrauterine contraceptive devices. Obstet Gynecol. 2022;140:91-93. doi: 10.1097/AOG.0000000000004828.
- Holden EC, Lai E, Morelli SS, et al. Ongoing barriers to immediate postpartum long-acting reversible contraception: a physician survey. Contracept Reprod Med. 2018;3:23. doi: 10.1186/s40834-018-0078-5.
- Benfield N, Hawkins F, Ray L, et al. Exposure to routine availability of immediate postpartum LARC: effect on attitudes and practices of labor and delivery and postpartum nurses. Contraception. 2018;97:411-414. doi: 10.1016 /j.contraception.2018.01.017.
- Steenland MW, Vatsa R, Pace LE, et al. Immediate postpartum long-acting reversible contraceptive use following statespecific changes in hospital Medicaid reimbursement. JAMA Netw Open. 2022;5:e2237918. doi: 10.1001 /jamanetworkopen.2022.37918.
- Washington CI, Jamshidi R, Thung SF, et al. Timing of postpartum intrauterine device placement: a costeffectiveness analysis. Fertil Steril. 2015;103:131-137. doi: 10.1016/j.fertnstert.2014.09.032
- Winner B, Peipert JF, Zhao Q, et al. Effectiveness of longacting reversible contraception. N Engl J Med. 2012;366:19982007. doi: 10.1056/NEJMoa1110855.
- Bearak J, Popinchalk A, Ganatra B, et al. Unintended pregnancy and abortion by income, region, and the legal status of abortion: estimates from a comprehensive model for 1990-2019. Lancet Glob Health. 2020;8:e1152-e1161. doi: 10.1016/S2214-109X(20)30315-6.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice. Committee Opinion No. 670: Immediate postpartum long-acting reversible contraception. Obstet Gynecol. 2016;128:e32-e37. doi: 10.1097/AOG.0000000000001587.
- Averbach SH, Ermias Y, Jeng G, et al. Expulsion of intrauterine devices after postpartum placement by timing of placement, delivery type, and intrauterine device type: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;223:177188. doi: 10.1016/j.ajog.2020.02.045.
- Connolly A, Thorp J, Pahel L. Effects of pregnancy and childbirth on postpartum sexual function: a longitudinal prospective study. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:263-267. doi: 10.1007/s00192-005-1293-6.
- Conde-Agudelo A, Rosas-Bermúdez A, Kafury-Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a meta-analysis. JAMA. 2006;295:1809-1823. doi: 10.1001 /jama.295.15.1809.
- Vricella LK, Gawron LM, Louis JM. Society for MaternalFetal Medicine (SMFM) Consult Series #48: Immediate postpartum long-acting reversible contraception for women at high risk for medical complications. Am J Obstet Gynecol. 2019;220:B2-B12. doi: 10.1016/j.ajog.2019.02.011.
- Chen BA, Reeves MF, Hayes JL, et al. Postplacental or delayed insertion of the levonorgestrel intrauterine device after vaginal delivery: a randomized controlled trial. Obstet Gynecol. 2010;116:1079-1087. doi: 10.1097/AOG.0b013e3181f73fac.
- Whitaker AK, Endres LK, Mistretta SQ, et al. Postplacental insertion of the levonorgestrel intrauterine device after cesarean delivery vs. delayed insertion: a randomized controlled trial. Contraception. 2014;89:534-539. doi: 10.1016/j.contraception.2013.12.007.
- Lester F, Kakaire O, Byamugisha J, et al. Intracesarean insertion of the Copper T380A versus 6 weeks postcesarean: a randomized clinical trial. Contraception. 2015;91:198-203. doi: 10.1016/j.contraception.2014.12.002.
- Levi EE, Stuart GS, Zerden ML, et al. Intrauterine device placement during cesarean delivery and continued use 6 months postpartum: a randomized controlled trial. Obstet Gynecol. 2015;126:5-11. doi: 10.1097/AOG.0000000000000882.
- Sothornwit J, Kaewrudee S, Lumbiganon P, et al. Immediate versus delayed postpartum insertion of contraceptive implant and IUD for contraception. Cochrane Database Syst Rev. 2022;10:CD011913. doi: 10.1002/14651858.CD011913.pub3.
- Cohen R, Sheeder J, Arango N, et al. Twelve-month contraceptive continuation and repeat pregnancy among young mothers choosing postdelivery contraceptive implants or postplacental intrauterine devices. Contraception. 2016;93:178-183. doi: 10.1016/j.contraception.2015.10.001.
- Centers for Disease Control and Prevention (CDC). US Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep. 2010;59(RR-4):1-86.
- Kapp N, Curtis K, Nanda K. Progestogen-only contraceptive use among breastfeeding women: a systematic review. Contraception. 2010;82:17-37. doi: 10.1016 /j.contraception.2010.02.002.
- Levi EE, Findley MK, Avila K, et al. Placement of levonorgestrel intrauterine device at the time of cesarean delivery and the effect on breastfeeding duration. Breastfeed Med. 2018;13:674679. doi: 10.1089/bfm.2018.0060.
- Chen BA, Reeves MF, Creinin MD, et al. Postplacental or delayed levonorgestrel intrauterine device insertion and breast-feeding duration. Contraception. 2011;84:499-504. doi: 10.1016/j.contraception.2011.01.022.
- Tocce KM, Sheeder JL, Teal SB. Rapid repeat pregnancy in adolescents: do immediate postpartum contraceptive implants make a difference? Am J Obstet Gynecol. 2012;206:481.e1-7. doi: 10.1016/j.ajog.2012.04.015.
- Carr SL, Singh RH, Sussman AL, et al. Women’s experiences with immediate postpartum intrauterine device insertion: a mixed-methods study. Contraception. 2018;97:219-226. doi: 10.1016/j.contraception.2017.10.008.
- Martinez OP, Wilder L, Seal P. Ultrasound-guided compared with non-ultrasound-Guided placement of immediate postpartum intrauterine contraceptive devices. Obstet Gynecol. 2022;140:91-93. doi: 10.1097/AOG.0000000000004828.
- Holden EC, Lai E, Morelli SS, et al. Ongoing barriers to immediate postpartum long-acting reversible contraception: a physician survey. Contracept Reprod Med. 2018;3:23. doi: 10.1186/s40834-018-0078-5.
- Benfield N, Hawkins F, Ray L, et al. Exposure to routine availability of immediate postpartum LARC: effect on attitudes and practices of labor and delivery and postpartum nurses. Contraception. 2018;97:411-414. doi: 10.1016 /j.contraception.2018.01.017.
- Steenland MW, Vatsa R, Pace LE, et al. Immediate postpartum long-acting reversible contraceptive use following statespecific changes in hospital Medicaid reimbursement. JAMA Netw Open. 2022;5:e2237918. doi: 10.1001 /jamanetworkopen.2022.37918.
- Washington CI, Jamshidi R, Thung SF, et al. Timing of postpartum intrauterine device placement: a costeffectiveness analysis. Fertil Steril. 2015;103:131-137. doi: 10.1016/j.fertnstert.2014.09.032
The perimenopausal period and the benefits of progestin IUDs
Intrauterine devices (IUDs) are now used by more than 15% of US contraceptors. The majority of these IUDs release the progestin levonorgestrel, and with now longer extended use of the IUDs approved by the US Food and Drug Administration (FDA),1-3 they become even more attractive for use for contraception,control of menorrhagia or heavy menstrual bleeding (HMB) during reproductive years and perimenopause, and potentially, although not FDA approved for this purpose, postmenopause for endometrial protection in estrogen users. In this roundtable discussion, we will look at some of the benefits of the IUD for contraception effectiveness and control of bleeding, as well as the potential risks if used for postmenopausal women.
Progestin IUDs and contraception
JoAnn V. Pinkerton, MD, NCMP: Dr. Kaunitz, what are the contraceptive benefits of progestin IUDs during perimenopause?
Andrew M. Kaunitz, MD, NCMP: We know fertility declines as women approach menopause. However, when pregnancy occurs in older reproductive-age women, the pregnancies are often unintended, as reflected by high rates of induced abortion in this population. In addition, the prevalence of maternal comorbidities (during pregnancy and delivery) is higher in older reproductive-age women, with the maternal mortality rate more than 5 times higher compared with that of younger women.4 Two recently published clinical trials assessed the extended use of full-size IUDs containing 52 mg of levonor-gestrel (LNG), with the brand names Mirena and Liletta.1,2 The data from these trials confirmed that both IUDs remain highly effective for up to 8 years of use, and currently, both devices are approved for up to 8 years of use. One caveat is that, in the unusual occurrence of a pregnancy being diagnosed in a woman using an IUD, we as clinicians, must be alert to the high prevalence of ectopic pregnancies in this setting.
Progestin IUDs and HMB
Dr. Pinkerton: Dr. Goldstein, can you comment on how well progestin IUDs work for HMB?
Steven R. Goldstein, MD, NCMP, CCD: Many women who need contraception will use these devices for suppressing HMB, and they can be quite effective, if the diagnosis truly is HMB, at reducing bleeding.5 But that efficacy in bleeding reduction may not be quite as long as the efficacy in pregnancy prevention.6 In my experience, among women using IUDs specifically for their HMB, good bleeding control may require changing the IUD at 3 to 5 years.
Barbara S. Levy, MD: When inserting a LNG-IUD for menorrhagia in the perimenopausal time frame, sometimes I will do a progestin withdrawal first, which will thin the endometrium and induce withdrawal bleeding because, in my experience, if you place an IUD in someone with perimenopausal bleeding, you may end up with a lot of breakthrough bleeding.
Perimenopause and hot flashes
Dr. Pinkerton: Dr. Kaunitz, we have learned that hot flashes often occur and become bothersome to women during perimenopause. Many women have IUDs placed during perimenopause for bleeding. Can you comment about IUD use during perimenopause and postmenopause?
Dr. Kaunitz: In older reproductive-age women who already have a progestin-releasing IUD placed, as they get closer to menopause when vasomotor symptoms (VMS) might occur, if these symptoms are bothersome, the presence or placement of a progestin-releasing IUD can facilitate treatment of perimenopausal VMS with estrogen therapy.
Progestin IUDs cause profound endometrial suppression, reduce bleeding and often, over time, cause users to become amenorrheic.7
The Mirena package insert states, “Amenorrhea develops in about 20% of users by one year.”2 By year 3 and continuing through year 8, the prevalence of amenorrhea with the 52-mg LNG-IUD is 35% to 40%.8 From a study by Nanette Santoro, MD, and colleagues, we know that, in perimenopausal women with a progestin-releasing IUD in place, who are experiencing bothersome VMS, adding transdermal estrogen is very effective in treating and suppressing those hot flashes. In her small clinical trial, among participants with perimenopausal bothersome VMS with an IUD in place, half were randomized to use of transdermal estradiol and then compared with women who did not get the estradiol patch. There was excellent relief of perimenopausal hot flashes with the combination of the progestin IUD for endometrial suppression and transdermal estrogen to relieve hot flashes.9
Dr. Pinkerton: Which women would not be good candidates for the use of this combination?
Dr. Kaunitz: We know that, as women age, the prevalence of conditions that are contraindications to combination contraceptives (estrogen-progestin pills, patches, or rings) starts to increase. Specifically, we see more: hypertension, diabetes, and high body mass index (BMI), or obesity. We also know that migraine headaches in women older than age 35 years is another condition in which ACOG and the Centers for Disease Control and Prevention (CDC) would not recommend use of combination contraceptives.10,11 These older perimenopausal women may be excellent candidates for a progestin-only releasing IUD combined with use of transdermal menopausal doses of estradiol if needed for VMS.
Dr. Goldstein: I do want to add that, in those patients who don’t have these comorbidities, combination estrogen-progestin contraceptives do a very nice job of ovarian suppression and will prevent the erratic production of estradiol, which, in my experience, often results in not only irregular bleeding but also possible exacerbation of perimenopausal mood symptoms.
Dr. Kaunitz: I agree, Steve. The ideal older reproductive-age candidate for combination pills, patch, or ring would be a slender, healthy, nonsmoking woman with normal blood pressure. Such women would be a fairly small subgroup of my practice, but they can safely continue combination contraceptives right through menopause. Consistent with CDC and ACOG guidance, rather than checking gonadotropins to “determine when menopause has occurred,” (which is, in fact, not an evidence-based approach to diagnosing menopause in this setting), such women can continue the combination contraceptive right up until age 55—the likelihood that women are still going to be ovulating or at risk for pregnancy becomes vanishingly small at that age.11,12 Women in their mid-50s can either seamlessly transition to use of systemic estrogen-progestin menopausal therapy or go off hormones completely.
Continue to: The IUD and HMB...
The IUD and HMB
Dr. Pinkerton: Dr. Goldstein, there’s been some good literature on the best management options for women with HMB. What is the most current evidence?
Dr. Goldstein: I think that the retiring of the terms menorrhagia and metrorrhagia may have been premature because HMB implies cyclical bleeding, and this population of women with HMB will typically do quite well. Women who have what we used to call metrorrhagia or irregular bleeding, by definition, need endometrial evaluation to be sure they don’t have some sort of organic pathology. It would be a mistake for clinicians to use an LNG-IUD in patients with abnormal uterine bleeding (AUB) that has not been appropriately evaluated.
If we understand that we are discussing HMB, a Cochrane Review from 202213 suggests that an LNG intrauterine system is the best first-line treatment for reducing menstrual blood loss in perimenopausal women with HMB. Antifibrinolytics appeared second best, while long-cycle progestogens came in third place. Evidence on perception of improvement in satisfaction was ranked as low certainty. That same review found that hysterectomy was the best treatment for reducing bleeding, obviously, followed by resectoscopic endometrial ablation or a nonresectoscopic global endometrial ablation.
The evidence rating was low certainty regarding the likelihood that placing an LNG-IUD in women with HMB will result in amenorrhea, and I think that’s a very important point. The expectation of patients should be reduced or a significantly reduced amount of their HMB, not necessarily amenorrhea. Certainly, minimally invasive hysterectomy will result in total amenorrhea and may have a larger increase in satisfaction, but it has its own set of other kinds of possible complications.
Dr. Kaunitz: In an industry-funded, international multicenter trial,14 women with documented HMB (hemoglobin was eluted from soiled sanitary products), with menstrual blood loss of 80 mL or more per cycle, were randomized to placement of an LNG 52-mg IUD (Mirena) or cyclical medroxyprogesterone acetate (MPA)—oral progestin use.
Although menstrual blood loss declined in both groups, it declined dramatically more in women with an IUD placed, and specifically with the IUD, menstrual blood loss declined by 129 mL on average, whereas the decline in menstrual blood loss with cyclical MPA was 18 mL. This data, along with earlier European data,15 which showed similar findings in women with HMB led to the approval of the Mirena progestin IUD for a second indication to treat HMB in 2009.
I also want to point out that, in the May 2023 issue of Obstetrics & Gynecology, Creinin and colleagues published a similar trial in women with HMB showing, once again, that progestin IUDs (52-mg LNG-IUD, Liletta) are extremely effective in reducing HMB.16 There is crystal clear evidence from randomized trials that both 52-mg LNG-IUDs, Mirena and Liletta, are very effective in reducing HMB and, in fact, are contributing to many women who in the past would have proceeded with surgery, such as ablation or hysterectomy, to control their HMB.
Oral contraception
Dr. Pinkerton: What about using low-dose continuous oral contraceptives noncyclically for women with HMB?
Dr. Goldstein: I do that all the time. It is interesting that Dr. Kaunitz mentions his patient population. It’s why we understand that one size does not fit all. You need to see patients one at a time, and if they are good candidates for a combined estrogen-progestin contraception, whether it’s pills, patches, or rings, giving that continuously does a very nice job in reducing HMB and straightening out some of the other symptoms that these perimenopausal women will have.
IUD risks
Dr. Pinkerton: We all know about use of low-dose oral contraceptives for management of AUB, and we use them, although we worry a little bit about breast cancer risk. Dr. Levy, please comment on the risks with IUDs of expulsions and perforations. What are the downsides of IUDs?
Dr. Levy: Beyond the cost, although it is a minimally invasive procedure, IUD insertion can be an invasive procedure for a patient to undergo; expulsions can occur.17 We know that a substantial percentage of perimenopausal women will have fibroids. Although many fibroids are not located in the uterine cavity, the expulsion rate with HMB for an LNG-IUD can be higher,13,16,18,19 perhaps because of local prostaglandin release with an increase in uterine contractility. There is a low incidence of perforations, but they do happen, particularly among women with scars in the uterus or who have a severely anteflexed or retroflexed uterus, and women with cervical stenosis, for example, if they have had a LEEP procedure, etc. Even though progestin IUDs are outstanding tools in our toolbox, they are invasive to some extent, and they do have the possibility of complications.
Dr. Kaunitz: As Dr. Levy points out, although placement of an IUD may be considered an invasive procedure, it is also an office-based procedure, so women can drive home or drive back to work afterwards without the disruption in their life and the potential complications associated with surgery and anesthesia.
Continue to: Concerns with malpositioning...
Concerns with malpositioning
Dr. Pinkerton: After placement of an IUD, during a follow-up visit, sometimes you can’t visualize the string. The ultrasonography report may reveal, “IUD appears to be in the right place within the endometrium.” Dr. Goldstein, can you comment on how we should use ultrasound when we can’t visualize or find the IUD string, or if the patient complains of abdominal pain, lower abdominal discomfort, or irregular bleeding or spotting and we become concerned about IUD malposition?
Dr. Goldstein: Ultrasound is not really required after an uncomplicated placement of an IUD or during routine management of women who have no problems who are using an IUD. In patients who present with pain or some abnormal bleeding, however, sometimes it is the IUD being malpositioned. A very interesting study by the late great Beryl Benacerraf20 showed that there was a statistically significant higher incidence of the IUD being poorly positioned when patients have pain or bleeding (FIGURE 1). It was not always apparent on 2D ultrasonography. Using a standard transvaginal ultrasound of the long access plane, the IUD may appear to be very centrally located. However, if you do a 3D coronal section, not infrequently in these patients with any pain or bleeding, one of the arms has pierced the myometrium (FIGURE 2). This can actually be a source of pain and bleeding.
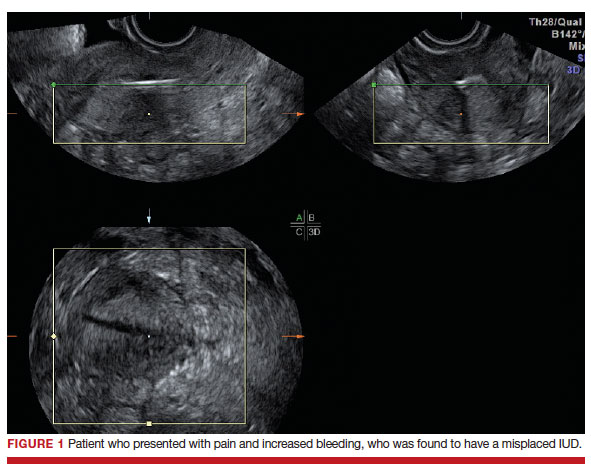
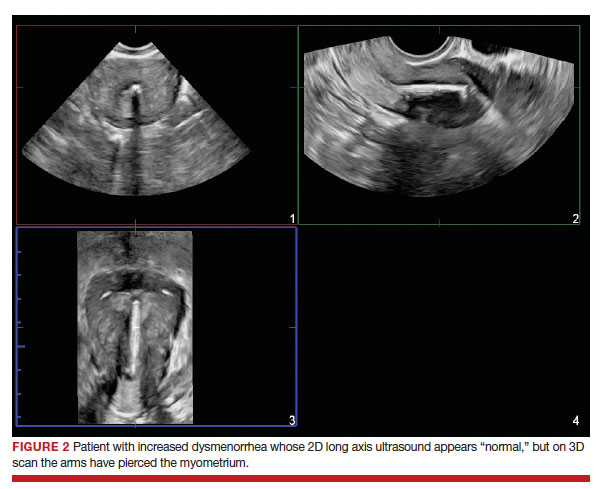
It’s also very interesting when you talk about perforation. I became aware of a big to-do in the medical/legal world about the possibility of the IUD migrating through the uterine cavity.21 This just does not exist, as was already pointed out. If the IUD is really going to go anywhere, if it’s properly placed, it’s going to be expelled through an open cervix. I do believe that, if you have pierced the myometrium through uterine contractility over time, some of these IUDs could work their way through the myometrium and somehow come out of the uterus either totally or partially. I think ultrasound is invaluable in patients with pain and bleeding, but I think you need to have an ultrasound lab capable of doing a 3D coronal section.
Progestin IUDs for HT replacement: Benefits/risks
Dr. Pinkerton: Many clinicians are excited that they can use essentially estrogen alone for women who have a progestin IUD in place. What about the possible off-label use of the progestin IUD to replace oral progestogen for hormone therapy (HT)? Dr. Kaunitz, are there any studies using this for postmenopausal HT (with a reminder that the IUD is not FDA approved for this purpose)?
Dr. Kaunitz: We have data from Europe indicating that, in menopausal women using systemic estrogen, the full-size LNG 52 IUD—Mirena or Liletta—provides excellent endometrial suppression.22 Where we don’t have data is with the smaller IUDs, which would be Kyleena and Skyla, which release smaller amounts of progestin each day into the endometrial cavity.
I have a number of patients, most of them women who started use of a progestin IUD as older reproductive-age women and then started systemic estrogen for treatment of perimenopausal hot flashes and then continued the use of their IUD plus systemic estrogen in treating postmenopausal hot flashes. The IUD is very useful in this setting, but as you pointed out, Dr. Pinkerton, this does represent off-label use.
Dr. Pinkerton: I know this use does not affect plasma lipids or cardiovascular risk markers, although users seem to report that the IUD has improved their quality of life. The question comes up, what are the benefits on cancer risk for using an IUD?
Dr. Levy: It’s such a great question because, as we talk about the balance of risks and benefits for anything that we are offering to our patients, it is really important to focus on some of the benefits. For both the copper and the LNG-IUD, there is a reduction in endometrial cancer,22 as well as pretty good data with the copper IUD about a reduction in cervical cancer.23 Those data are a little bit less clear for the LNG-IUD.
Interestingly, at least one meta-analysis published in 2020 shows about a 30% reduction in ovarian cancer risk with the LNG-IUD.24 We need to focus our patients on these other benefits. They tend to focus on the risks, and, of course, the media blows up the risks, but the benefits are quite substantial beyond just reducing HMB and providing contraception.
Dr. Pinkerton: As Dr. Kaunitz said, when you use this IUD, with its primarily local uterine progestin effects, it’s more like using estrogen alone without as much systemic progestin. Recently I wrote an editorial on the benefits of estrogen alone on the risk of breast cancer, primarily based on the Women’s Health Initiative (WHI) observational long-term 18-year cumulative follow-up. When estrogen alone was prescribed to women after a hysterectomy, estrogen therapy used at menopause did not increase the risk of invasive breast cancer, and was associated with decreased mortality.25 However, the nurse’s health study has suggested that longer-term use may be increased with estrogen alone.26 For women in the WHI with an intact uterus who used estrogen, oral MPA slightly increased the risk for breast cancer, and this elevated risk persisted even after discontinuation. This leads us to the question, what are the risks of breast cancer with progestin IUD use?
I recently reviewed the literature, and the answer is, it’s mixed. The FDA has put language into the package label that acknowledges a potential breast cancer risk for women who use a progestin IUD,27 and that warning states, “Women who currently have or have had breast cancer or suspect breast cancer should not use hormonal contraception because some breast cancers are hormone sensitive.” The label goes on to say, “Observational studies of the risk of breast cancer with the use of a levonorgestrel-releasing IUS don’t provide conclusive evidence of increased risk.” Thus, there is no conclusive answer as to whether there is a possible link of progestin IUDs to breast cancer.
What I tell my patients is that research is inconclusive. However, it’s unlikely for a 52-mg LNG-IUD to significantly increase a woman’s breast cancer risk, except possibly in those already at high risk from other risk factors. I tell them that breast cancer is listed in the package insert as a potential risk. I could not find any data on whether adding a low-dose estradiol patch would further increase that risk. So I counsel women about potential risk, but tell them that I don’t have any strong evidence of risk.
Continue to: Dr. Goldstein...
Dr. Goldstein: If you look in the package insert for Mirena,2 similar to Liletta, certainly the serum levels of LNG are lower than that for combination oral contraceptives. For the IUD progestins, they are not localized only to the uterus, and LNG levels range from about 150 to 200 µg/mL up to 60 months. It’s greater at 12 months, at about 180 µg/mL,at 24 months it was 192 µg/mL, and by 60 months it was 159 µg/mL. It’s important to realize that there is some systemic absorption of progestin with progestin IUDs, and it is not completely a local effect.
JoAnn, you mentioned the WHI data,25 and just to specify, it was not the estrogen-only arm, it was the conjugated equine estrogen-only arm of the WHI. I don’t think that estradiol alone increases breast cancer risk (although there are no good prospective, follow-through, 18-year study data, like the WHI), but I think readers need to understand the difference in the estrogen type.
Endometrial evaluation. My question for the panel is as follows. I agree that the use of the progestin-releasing IUD is very nice for that transition to menopause. I do believe it provides endometrial protection, but we know from other studies that, when we give continuous combined HT, about 21% to 26% of patients will experience some bleeding/staining, responding in the first 4-week cycles, and it can be as high as 9% at 1 year. If I have a patient who bleeds on continuous combined HT, I will evaluate her endometrium, usually just with a simple transvaginal ultrasound. If an IUD is in place, and the patient now begins to have some irregular bleeding, how do you evaluate her with the IUD in place?
Dr. Levy: That is a huge challenge. We know from a recent paper,28 that the endometrial thickness, while an excellent measure for Caucasian and European women, may be a poor marker for endometrial pathology in African-American women. What we thought we knew, which was, if the stripe is 4 mL or less, we can forget about it, I think in our more recent research that is not so true. So you bring up a great point, what do you do? The most reliable evaluation will be with an office hysteroscopy, where you can really look at the entire cavity and for tiny, little polyps and other things. But then we are off label because the use of hysteroscopy with an IUD in place is off label. So we are really in a conundrum.
Dr. Pinkerton: Also, if you do an endometrial biopsy, you might dislodge the IUD. If you think that you are going to take the IUD out, it may not matter if you dislodge it. I will often obtain a transvaginal ultrasound to help me figure out the next step, and maybe look at the dosing of the estrogen and progestin—but you can’t monitor an IUD with blood levels. You are in a vacuum of trying to figure out the best thing to do.
Dr. Kaunitz: One of the hats I wear here in Jacksonville is Director of GYN Ultrasound. I have a fair amount of experience doing endometrial biopsies in women with progestin IUDs in place under abdominal ultrasound guidance and keeping a close eye on the position of the IUD. In the first dozen or so such procedures I did, I was quite concerned about dislodging the IUD. It hasn’t happened yet, and it gives me some reassurance to be able to image the IUD and your endometrial suction curette inside the cavity as you are obtaining endometrial sampling. I have substantial experience now doing that, and so far, no problems. I do counsel all such women in advance that there is some chance I could dislodge their IUD.
Dr. Goldstein: In addition to dislodging the IUD, are you not concerned that, if the pathology is not global, that a blind endometrial sampling may be fraught with some error?
Dr. Kaunitz: The endometrium in women with a progestin-releasing IUD in place tends to be very well suppressed. Although one might occasionally find, for instance, a polyp in that setting, I have not run into, and I don’t expect to encounter going forward, endometrial hyperplasia or cancer in women with current use of a progestin IUD. It’s possible but unlikely.
Dr. Levy: The progestin IUD will counterbalance a type-1 endometrial cancer—an endometrial cancer related to hyperstimulation by estrogen. It will not do anything, to my knowledge, to counterbalance a type 2. I think the art of medicine is, you do the best you can with the first episode of bleeding, and if she persists in her bleeding, we have to persevere and continue to evaluate her.
Dr. Goldstein: I agree 100%.
Dr. Pinkerton: We all agree with you. That’s a really good point.
Continue to: Case examinations...
Case examinations
CASE 1 Woman with intramural fibroids
Dr. Pinkerton: Dr. Goldstein, you have a 48-year-old Black woman who has heavy but regular menstrual bleeding with multiple fibroids (the largest is about 4 to 5 cm, they look intramural, with some distortion of the cavity but not a submucous myoma, and the endometrial depth is 9 cm). Would you insert an IUD, and would you recommend an endometrial biopsy first?
Dr. Goldstein: I am not a huge fan of blind endometrial sampling, and I do think that we use the “biopsy” somewhat inappropriately since sampling is not a directed biopsy. This became obvious in the landmark paper by Guido et al in 1995 and was adopted by ACOG only in 2012.29 Cancers that occupy less than 50% of the endometrial surface area are often missed with such blind sampling. Thus I would not perform an endometrial biopsy first, but would rather rely on properly timed and performed transvaginal ultrasound to rule out any concurrent endometrial disease. I think a lot of patients who have HMB, not only because of their fibroids but also often just due to the surface area of their uterine cavity being increased—so essentially there is more blood volume when they bleed. However, you said that in this case the patient has regular menstrual bleeding, so I am assuming that she is still ovulatory. She may have some adenomyosis. She may have a large uterine cavity. I think she is an excellent candidate for an LNG-releasing IUD to reduce menstrual blood flow significantly. It will not necessarily give her amenorrhea, and it may give her some irregular bleeding. Then at some distant point, say in 5 or 6 months if she does have some irregular staining or bleeding, I would feel much better about the fact that nothing has developed as long as I knew that the endometrium was devoid of pathology when I started.
CASE 2 Woman with family history of breast cancer
Dr. Pinkerton: Dr. Levy, a 44-year-old woman has a family history of breast cancer in her mother at age 72, but she still needs contraceptionbecause of that unintended pregnancy risk in the 40s, and she wants something that is not going to increase her risk of breast cancer. What would you use, and how would you counsel her if you decided to use a progestin IUD?
Dr. Levy: The data are mixed,30-33 but whatever the risk, it is miniscule, and I would bring up the CDC Medical Eligibility Criteria.11 For a patient with a family history of breast cancer, for use of the progestin IUD, it is a 1—no contraindications. What I tend to tell my patients is, if you are worried about breast cancer, watch how much alcohol you are drinking and maintain regular exercise. There are so many preventive things that we can do to reduce risk of breast cancer when she needs contraception. If there is any increase in risk, it is so miniscule that I would very strongly recommend a progestin IUD for her.
Dr. Pinkerton: In addition, in recognizing the different densities of breast, dense breast density could lead to supplemental screening, which also could give her some reassurance that we are adequately screening for breast cancer.
CASE 3 Woman with IUD and VMS
Dr. Pinkerton: Dr. Kaunitz, you have a 52-year-old overweight female. She has been using a progestin IUD for 4 years, is amenorrheic, but now she is having moderate to severe vasomotor symptoms despite the IUD in place. You have talked to her about risks and benefits of HT, and she is interested in starting it. I know we talked about the studies, but I want to know what you are going to tell her. How do you counsel her about off-label use?
Dr. Kaunitz: The most important issue related to treating vasomotor symptoms in this patient is the route of systemic estrogen. Understandably, women’s biggest concern regarding the risks of systemic estrogen-progestin therapy is breast cancer. However, statistically, by far the biggest risk associated with oral estrogen-progestogen therapy, is elevated risk of venous thrombosis and pulmonary embolism. We have seen this, with a number of studies, and the WHI made it crystal clear with risks of oral conjugated equine estrogen at the dose of 0.625 mg daily. Oral estradiol 1 mg daily is also associated with a similar elevated risk of venous thrombosis. We also know that age and BMI are both independent risk factors for thrombosis. So, for a woman in her 50s who has a BMI > 30 mg/kg2, I don’t want to further elevate her risk of thrombosis by giving her oral estrogen, whether it is estradiol or conjugated equine estrogen. This is a patient in whom I would be comfortable using transdermal (patch) estradiol, perhaps starting with a standard dose of 0.05 mg weekly or twice weekly patch, keeping in mind that 0.05 mg in the setting of transdermal estrogen refer to the daily or to the 24-hour release rate. The 1.0 mg of oral estradiol and 0.625 mg of conjugated equine estrogen refers to the mg quantity of estrogen in each tablet. This is a source of great confusion for clinicians.
If, during follow-up, the 0.05 mg estradiol patch is not sufficient to substantially reduce symptoms, we could go up, for instance, to a 0.075 mg estradiol patch. We know very clearly from a variety of observational studies, including a very large UK study,34 that in contrast with oral estrogen, transdermal estradiol is safer from the perspective of thrombosis.
Insurance coverage for IUDs
Dr. Pinkerton: Dr. Levy: Can you discuss IUDs and the Affordable Care Act’s requirement to cover contraceptive services?
Dr. Levy: Unfortunately, we do not know whether this benefit will continue based on a very recent finding from a judge in Texas that ruled the preventive benefits of the ACA were illegal.35 We don’t know what will happen going forward. What I will say is that, unfortunately, many insurance companies have not preserved the meaning of “cover all things,” so what we are finding is that, for example, they only have to cover one type in a class. The FDA defined 18 classes of contraceptives, and a hormonal IUD is one class, so they can decide that they are only going to cover one of the four IUDS. And then women don’t have access to the other three, some of which might be more appropriate for them than another.
The other thing very relevant to this conversation is that, if you use an ICD-10 code for menorrhagia, for HMB, it no longer lives within that ACA preventive care requirement of coverage for contraceptives, and now she is going to owe a big deductible or a copay. If you are practicing in an institution that does not allow the use of IUDs for contraception, like a Catholic institution where I used to practice, you will want to use that ICD-10 code for HMB. But if you want it offered with no out-of-pocket cost for the patient, you need to use the preventive medicine codes and the contraception code. These little nuances for us can make a huge difference for our patients.
Dr. Pinkerton: Thank you for that reminder. I want to thank our panelists, Dr. Levy, Dr. Goldstein, and Dr. Kaunitz, for providing us with such a great mix of evidence and expert opinion and also giving a benefit of their vast experience as award-winning gynecologists. Hopefully, today you have learned the benefits of the progestin IUD not only for contraception in reproductive years and perimenopause but also for treatment of HMB, and the potential benefit due to the more prolonged effectiveness of the IUDs for endometrial protection in postmenopause. This allows less progestin risk, essentially estrogen alone for postmenopausal HT. Unsolved questions remain about whether there is a risk of breast cancer with their use, but there is a clear benefit of protecting against pregnancy and endometrial cancer. ●
- Liletta [package insert]. Allergan; Irvine, California. November 2022.
- Mirena [package insert]. Bayer; Whippany, New Jersey. 2000.
- Kaunitz AM. Safe extended use of levonorgestrel 52-mg IUDs. November 11, 2022. https://www.medscape.com/ viewarticle/983680. Accessed May 8, 2023.
- Kaunitz AM. Clinical practice. Hormonal contraception in women of older reproductive age. N Engl J Med. 2008;358:1262-1270. doi: 10.1056/NEJMcp0708481.
- Tucker ME. IUD-released levonorgestrel eases heavy menstrual periods. Medscape. April 10, 2023. https://www .medscape.com/viewarticle/777406. Accessed May 2, 2023.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice; Long-Acting Reversible Contraception Working Group. ACOG Committee Opinion No. 450: Increasing use of contraceptive implants and intrauterine devices to reduce unintended pregnancy. Obstet Gynecol. 2009;114:1434-1438.
- Critchley HO, Wang H, Jones RL, et al. Morphological and functional features of endometrial decidualization following long-term intrauterine levonorgestrel delivery. Hum Reprod. 1998;13:1218-1224. doi:10.1093/humrep/13.5.1218.
- Creinin MD, Schreiber CA, Turok DK, et al. Levonorgestrel 52 mg intrauterine system efficacy and safety through 8 years of use. Am J Obstet Gynecol. 2022;227:871.e1-871.e7. doi: 10.1016/j.ajog.2022.05.022.
- Santoro N, Teal S, Gavito C, et al. Use of a levonorgestrelcontaining intrauterine system with supplemental estrogen improves symptoms in perimenopausal women: a pilot study. Menopause. 2015;22:1301-1307. doi: 10.1097 /GME.0000000000000557.
- ACOG Committee on Practice Bulletins-Gynecology ACOG Practice Bulletin. The use of hormonal contraception in women with coexisting medical conditions. Number 18, July 2000. Int J Gynaecol Obstet. 2001;75:93-106. doi: 10.1016 /s0020-7292(01)00520-3.
- Curtis KM, Tepper NK, Jatlaoui TC, Berry-Bibee E, Horton LG, Zapata LB, Simmons KB, Pagano HP, Jamieson DJ, Whiteman MK. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-103. doi: 10.15585 /mmwr.rr6503a1.
- ACOG Practice Bulletin No. 206: use of hormonal contraception in women with coexisting medical conditions [published correction appears in: Obstet Gynecol. 2019;133:1288.] Obstet Gynecol. 2019;133:e128-e150. doi:10.1097/AOG.0000000000003072.
- Bofill Rodriguez M, Dias S, Jordan V, et al. Interventions for heavy menstrual bleeding; overview of Cochrane reviews and network meta-analysis. Cochrane Database Syst Rev. 2022;5:CD013180. doi: 10.1002/14651858.CD013180.pub2.
- Kaunitz AM, Bissonnette F, Monteiro I, et al. Levonorgestrelreleasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial [published correction appears in: Obstet Gynecol. 2010;116:999]. Obstet Gynecol. 2010;116:625-632. doi: 10.1097 /AOG.0b013e3181ec622b.
- Milsom I, Andersson K, Andersch B, et al. A comparison of flurbiprofen, tranexamic acid, and a levonorgestrel-releasing intrauterine contraceptive device in the treatment of idiopathic menorrhagia. Am J Obstet Gynecol. 1991;164:879883. doi: 10.1016/s0002-9378(11)90533-x.
- Creinin MD, Barnhart KT, Gawron LM, et al. Heavy menstrual bleeding treatment with a levonorgestrel 52-mg intrauterine device. Obstet Gynecol. 2023;141:971-978. doi: 10.1097 /AOG.0000000000005137.
- 1Madden T. Association of age and parity with intrauterine device expulsion. Obstet Gynecol. 2014:718-726. doi:10.1097 /aog.0000000000000475.
- Kaunitz AM, Stern L, Doyle J, et al. Use of the levonorgestrelIUD in the treatment of menorrhagia: improving patient outcomes while reducing the need for surgical management. Manag Care Interface. 2007;20:47-50.
- Getahun D, Fassett MJ, Gatz J, et al. Association between menorrhagia and risk of intrauterine device-related uterine perforation and device expulsion: results from the Association of Uterine Perforation and Expulsion of Intrauterine Device study. Am J Obstet Gynecol. 2022;227:59.e1-59.e9.
- Benacerraf BR, Shipp TD, Bromley B. Three-dimensional ultrasound detection of abnormally located intrauterine contraceptive devices that are a source of pelvic pain and abnormal bleeding. Ultrasound Obstet Gynecol. 2009;34:110115.
- Shipp TD, Bromley B, Benacerraf BR. The width of the uterine cavity is narrower in patients with an embedded intrauterine device (IUD) compared to a normally positioned IUD. J Ultrasound Med. 2010;29:1453-1456.
- Depypere H, Inki P. The levonorgestrel-releasing intrauterine system for endometrial protection during estrogen replacement therapy: a clinical review. Climacteric. 2015;18:470-482.
- Minalt N, Caldwell A, Yedlicka GM, et al. Association of intrauterine device use and endometrial, cervical, and ovarian cancer: an expert review. Am J Obstet Gynecol. 2023:S0002-9378(23)00224-7.
- Balayla J, Gil Y, Lasry A, et al. Ever-use of the intra-uterine device and the risk of ovarian cancer. J Obstet Gynaecol. 2021;41:848-853. doi: 10.1080/01443615.2020.1789960.
- Manson JE, Aragaki AK, Rossouw JE, et al. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318:927-938. doi:10.1001/jama.2017.11217.
- Chen WY, Manson JE, Hankinson SE, et al. Unopposed estrogen therapy and the risk of invasive breast cancer. Arch Intern Med. 2006;166:1027-1032. doi: 10.1001 /archinte.166.9.1027.
- Pinkerton JV, Wilson CS, Kaunitz AM. Reassuring data regarding the use of hormone therapy at menopause and risk of breast cancer. Menopause. 2022;29:1001-1004.doi:10.1097 /GME.0000000000002057.
- Romano SS, Doll KM. The impact of fibroids and histologic subtype on the performance of US clinical guidelines for the diagnosis of endometrial cancer among Black women. Ethn Dis. 2020;30:543-552. doi: 10.18865/ed.30.4.543.
- ACOG Committee on Practice Bulletins—Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206. doi: 10.1097/AOG.0b013e318262e320.
- Backman T, Rauramo I, Jaakkola Kimmo, et al. Use of the levonorgestrel-releasing intrauterine system and breast cancer. Obstet Gynecol. 2005;106:813-817.
- Conz L, Mota BS, Bahamondes L, et al. Levonorgestrelreleasing intrauterine system and breast cancer risk: A systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2020;99:970-982.
- Al Kiyumi MH, Al Battashi K, Al-Riyami HA. Levonorgestrelreleasing intrauterine system and breast cancer. Is there an association? Acta Obstet Gynecol Scand. 2021;100:1749.
- Marsden J. Hormonal contraception and breast cancer, what more do we need to know? Post Reprod Health. 2017;23:116127. doi: 10.1177/2053369117715370.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810 doi:10.1136/bmj.k4810.
- Levitt L, Cox C, Dawson L. Q&A: implications of the ruling on the ACA’s preventive services requirement. KFF.org. https://www .kff.org/policy-watch/qa-implications-of-the-ruling-on -the-acas-preventive-services-requirement/#:~:text=On%20 March%2030%2C%202023%2C%20a,cost%2Dsharing%20 for%20their%20enrollees. Accessed May 2, 2023.

Intrauterine devices (IUDs) are now used by more than 15% of US contraceptors. The majority of these IUDs release the progestin levonorgestrel, and with now longer extended use of the IUDs approved by the US Food and Drug Administration (FDA),1-3 they become even more attractive for use for contraception,control of menorrhagia or heavy menstrual bleeding (HMB) during reproductive years and perimenopause, and potentially, although not FDA approved for this purpose, postmenopause for endometrial protection in estrogen users. In this roundtable discussion, we will look at some of the benefits of the IUD for contraception effectiveness and control of bleeding, as well as the potential risks if used for postmenopausal women.
Progestin IUDs and contraception
JoAnn V. Pinkerton, MD, NCMP: Dr. Kaunitz, what are the contraceptive benefits of progestin IUDs during perimenopause?
Andrew M. Kaunitz, MD, NCMP: We know fertility declines as women approach menopause. However, when pregnancy occurs in older reproductive-age women, the pregnancies are often unintended, as reflected by high rates of induced abortion in this population. In addition, the prevalence of maternal comorbidities (during pregnancy and delivery) is higher in older reproductive-age women, with the maternal mortality rate more than 5 times higher compared with that of younger women.4 Two recently published clinical trials assessed the extended use of full-size IUDs containing 52 mg of levonor-gestrel (LNG), with the brand names Mirena and Liletta.1,2 The data from these trials confirmed that both IUDs remain highly effective for up to 8 years of use, and currently, both devices are approved for up to 8 years of use. One caveat is that, in the unusual occurrence of a pregnancy being diagnosed in a woman using an IUD, we as clinicians, must be alert to the high prevalence of ectopic pregnancies in this setting.
Progestin IUDs and HMB
Dr. Pinkerton: Dr. Goldstein, can you comment on how well progestin IUDs work for HMB?
Steven R. Goldstein, MD, NCMP, CCD: Many women who need contraception will use these devices for suppressing HMB, and they can be quite effective, if the diagnosis truly is HMB, at reducing bleeding.5 But that efficacy in bleeding reduction may not be quite as long as the efficacy in pregnancy prevention.6 In my experience, among women using IUDs specifically for their HMB, good bleeding control may require changing the IUD at 3 to 5 years.
Barbara S. Levy, MD: When inserting a LNG-IUD for menorrhagia in the perimenopausal time frame, sometimes I will do a progestin withdrawal first, which will thin the endometrium and induce withdrawal bleeding because, in my experience, if you place an IUD in someone with perimenopausal bleeding, you may end up with a lot of breakthrough bleeding.
Perimenopause and hot flashes
Dr. Pinkerton: Dr. Kaunitz, we have learned that hot flashes often occur and become bothersome to women during perimenopause. Many women have IUDs placed during perimenopause for bleeding. Can you comment about IUD use during perimenopause and postmenopause?
Dr. Kaunitz: In older reproductive-age women who already have a progestin-releasing IUD placed, as they get closer to menopause when vasomotor symptoms (VMS) might occur, if these symptoms are bothersome, the presence or placement of a progestin-releasing IUD can facilitate treatment of perimenopausal VMS with estrogen therapy.
Progestin IUDs cause profound endometrial suppression, reduce bleeding and often, over time, cause users to become amenorrheic.7
The Mirena package insert states, “Amenorrhea develops in about 20% of users by one year.”2 By year 3 and continuing through year 8, the prevalence of amenorrhea with the 52-mg LNG-IUD is 35% to 40%.8 From a study by Nanette Santoro, MD, and colleagues, we know that, in perimenopausal women with a progestin-releasing IUD in place, who are experiencing bothersome VMS, adding transdermal estrogen is very effective in treating and suppressing those hot flashes. In her small clinical trial, among participants with perimenopausal bothersome VMS with an IUD in place, half were randomized to use of transdermal estradiol and then compared with women who did not get the estradiol patch. There was excellent relief of perimenopausal hot flashes with the combination of the progestin IUD for endometrial suppression and transdermal estrogen to relieve hot flashes.9
Dr. Pinkerton: Which women would not be good candidates for the use of this combination?
Dr. Kaunitz: We know that, as women age, the prevalence of conditions that are contraindications to combination contraceptives (estrogen-progestin pills, patches, or rings) starts to increase. Specifically, we see more: hypertension, diabetes, and high body mass index (BMI), or obesity. We also know that migraine headaches in women older than age 35 years is another condition in which ACOG and the Centers for Disease Control and Prevention (CDC) would not recommend use of combination contraceptives.10,11 These older perimenopausal women may be excellent candidates for a progestin-only releasing IUD combined with use of transdermal menopausal doses of estradiol if needed for VMS.
Dr. Goldstein: I do want to add that, in those patients who don’t have these comorbidities, combination estrogen-progestin contraceptives do a very nice job of ovarian suppression and will prevent the erratic production of estradiol, which, in my experience, often results in not only irregular bleeding but also possible exacerbation of perimenopausal mood symptoms.
Dr. Kaunitz: I agree, Steve. The ideal older reproductive-age candidate for combination pills, patch, or ring would be a slender, healthy, nonsmoking woman with normal blood pressure. Such women would be a fairly small subgroup of my practice, but they can safely continue combination contraceptives right through menopause. Consistent with CDC and ACOG guidance, rather than checking gonadotropins to “determine when menopause has occurred,” (which is, in fact, not an evidence-based approach to diagnosing menopause in this setting), such women can continue the combination contraceptive right up until age 55—the likelihood that women are still going to be ovulating or at risk for pregnancy becomes vanishingly small at that age.11,12 Women in their mid-50s can either seamlessly transition to use of systemic estrogen-progestin menopausal therapy or go off hormones completely.
Continue to: The IUD and HMB...
The IUD and HMB
Dr. Pinkerton: Dr. Goldstein, there’s been some good literature on the best management options for women with HMB. What is the most current evidence?
Dr. Goldstein: I think that the retiring of the terms menorrhagia and metrorrhagia may have been premature because HMB implies cyclical bleeding, and this population of women with HMB will typically do quite well. Women who have what we used to call metrorrhagia or irregular bleeding, by definition, need endometrial evaluation to be sure they don’t have some sort of organic pathology. It would be a mistake for clinicians to use an LNG-IUD in patients with abnormal uterine bleeding (AUB) that has not been appropriately evaluated.
If we understand that we are discussing HMB, a Cochrane Review from 202213 suggests that an LNG intrauterine system is the best first-line treatment for reducing menstrual blood loss in perimenopausal women with HMB. Antifibrinolytics appeared second best, while long-cycle progestogens came in third place. Evidence on perception of improvement in satisfaction was ranked as low certainty. That same review found that hysterectomy was the best treatment for reducing bleeding, obviously, followed by resectoscopic endometrial ablation or a nonresectoscopic global endometrial ablation.
The evidence rating was low certainty regarding the likelihood that placing an LNG-IUD in women with HMB will result in amenorrhea, and I think that’s a very important point. The expectation of patients should be reduced or a significantly reduced amount of their HMB, not necessarily amenorrhea. Certainly, minimally invasive hysterectomy will result in total amenorrhea and may have a larger increase in satisfaction, but it has its own set of other kinds of possible complications.
Dr. Kaunitz: In an industry-funded, international multicenter trial,14 women with documented HMB (hemoglobin was eluted from soiled sanitary products), with menstrual blood loss of 80 mL or more per cycle, were randomized to placement of an LNG 52-mg IUD (Mirena) or cyclical medroxyprogesterone acetate (MPA)—oral progestin use.
Although menstrual blood loss declined in both groups, it declined dramatically more in women with an IUD placed, and specifically with the IUD, menstrual blood loss declined by 129 mL on average, whereas the decline in menstrual blood loss with cyclical MPA was 18 mL. This data, along with earlier European data,15 which showed similar findings in women with HMB led to the approval of the Mirena progestin IUD for a second indication to treat HMB in 2009.
I also want to point out that, in the May 2023 issue of Obstetrics & Gynecology, Creinin and colleagues published a similar trial in women with HMB showing, once again, that progestin IUDs (52-mg LNG-IUD, Liletta) are extremely effective in reducing HMB.16 There is crystal clear evidence from randomized trials that both 52-mg LNG-IUDs, Mirena and Liletta, are very effective in reducing HMB and, in fact, are contributing to many women who in the past would have proceeded with surgery, such as ablation or hysterectomy, to control their HMB.
Oral contraception
Dr. Pinkerton: What about using low-dose continuous oral contraceptives noncyclically for women with HMB?
Dr. Goldstein: I do that all the time. It is interesting that Dr. Kaunitz mentions his patient population. It’s why we understand that one size does not fit all. You need to see patients one at a time, and if they are good candidates for a combined estrogen-progestin contraception, whether it’s pills, patches, or rings, giving that continuously does a very nice job in reducing HMB and straightening out some of the other symptoms that these perimenopausal women will have.
IUD risks
Dr. Pinkerton: We all know about use of low-dose oral contraceptives for management of AUB, and we use them, although we worry a little bit about breast cancer risk. Dr. Levy, please comment on the risks with IUDs of expulsions and perforations. What are the downsides of IUDs?
Dr. Levy: Beyond the cost, although it is a minimally invasive procedure, IUD insertion can be an invasive procedure for a patient to undergo; expulsions can occur.17 We know that a substantial percentage of perimenopausal women will have fibroids. Although many fibroids are not located in the uterine cavity, the expulsion rate with HMB for an LNG-IUD can be higher,13,16,18,19 perhaps because of local prostaglandin release with an increase in uterine contractility. There is a low incidence of perforations, but they do happen, particularly among women with scars in the uterus or who have a severely anteflexed or retroflexed uterus, and women with cervical stenosis, for example, if they have had a LEEP procedure, etc. Even though progestin IUDs are outstanding tools in our toolbox, they are invasive to some extent, and they do have the possibility of complications.
Dr. Kaunitz: As Dr. Levy points out, although placement of an IUD may be considered an invasive procedure, it is also an office-based procedure, so women can drive home or drive back to work afterwards without the disruption in their life and the potential complications associated with surgery and anesthesia.
Continue to: Concerns with malpositioning...
Concerns with malpositioning
Dr. Pinkerton: After placement of an IUD, during a follow-up visit, sometimes you can’t visualize the string. The ultrasonography report may reveal, “IUD appears to be in the right place within the endometrium.” Dr. Goldstein, can you comment on how we should use ultrasound when we can’t visualize or find the IUD string, or if the patient complains of abdominal pain, lower abdominal discomfort, or irregular bleeding or spotting and we become concerned about IUD malposition?
Dr. Goldstein: Ultrasound is not really required after an uncomplicated placement of an IUD or during routine management of women who have no problems who are using an IUD. In patients who present with pain or some abnormal bleeding, however, sometimes it is the IUD being malpositioned. A very interesting study by the late great Beryl Benacerraf20 showed that there was a statistically significant higher incidence of the IUD being poorly positioned when patients have pain or bleeding (FIGURE 1). It was not always apparent on 2D ultrasonography. Using a standard transvaginal ultrasound of the long access plane, the IUD may appear to be very centrally located. However, if you do a 3D coronal section, not infrequently in these patients with any pain or bleeding, one of the arms has pierced the myometrium (FIGURE 2). This can actually be a source of pain and bleeding.


It’s also very interesting when you talk about perforation. I became aware of a big to-do in the medical/legal world about the possibility of the IUD migrating through the uterine cavity.21 This just does not exist, as was already pointed out. If the IUD is really going to go anywhere, if it’s properly placed, it’s going to be expelled through an open cervix. I do believe that, if you have pierced the myometrium through uterine contractility over time, some of these IUDs could work their way through the myometrium and somehow come out of the uterus either totally or partially. I think ultrasound is invaluable in patients with pain and bleeding, but I think you need to have an ultrasound lab capable of doing a 3D coronal section.
Progestin IUDs for HT replacement: Benefits/risks
Dr. Pinkerton: Many clinicians are excited that they can use essentially estrogen alone for women who have a progestin IUD in place. What about the possible off-label use of the progestin IUD to replace oral progestogen for hormone therapy (HT)? Dr. Kaunitz, are there any studies using this for postmenopausal HT (with a reminder that the IUD is not FDA approved for this purpose)?
Dr. Kaunitz: We have data from Europe indicating that, in menopausal women using systemic estrogen, the full-size LNG 52 IUD—Mirena or Liletta—provides excellent endometrial suppression.22 Where we don’t have data is with the smaller IUDs, which would be Kyleena and Skyla, which release smaller amounts of progestin each day into the endometrial cavity.
I have a number of patients, most of them women who started use of a progestin IUD as older reproductive-age women and then started systemic estrogen for treatment of perimenopausal hot flashes and then continued the use of their IUD plus systemic estrogen in treating postmenopausal hot flashes. The IUD is very useful in this setting, but as you pointed out, Dr. Pinkerton, this does represent off-label use.
Dr. Pinkerton: I know this use does not affect plasma lipids or cardiovascular risk markers, although users seem to report that the IUD has improved their quality of life. The question comes up, what are the benefits on cancer risk for using an IUD?
Dr. Levy: It’s such a great question because, as we talk about the balance of risks and benefits for anything that we are offering to our patients, it is really important to focus on some of the benefits. For both the copper and the LNG-IUD, there is a reduction in endometrial cancer,22 as well as pretty good data with the copper IUD about a reduction in cervical cancer.23 Those data are a little bit less clear for the LNG-IUD.
Interestingly, at least one meta-analysis published in 2020 shows about a 30% reduction in ovarian cancer risk with the LNG-IUD.24 We need to focus our patients on these other benefits. They tend to focus on the risks, and, of course, the media blows up the risks, but the benefits are quite substantial beyond just reducing HMB and providing contraception.
Dr. Pinkerton: As Dr. Kaunitz said, when you use this IUD, with its primarily local uterine progestin effects, it’s more like using estrogen alone without as much systemic progestin. Recently I wrote an editorial on the benefits of estrogen alone on the risk of breast cancer, primarily based on the Women’s Health Initiative (WHI) observational long-term 18-year cumulative follow-up. When estrogen alone was prescribed to women after a hysterectomy, estrogen therapy used at menopause did not increase the risk of invasive breast cancer, and was associated with decreased mortality.25 However, the nurse’s health study has suggested that longer-term use may be increased with estrogen alone.26 For women in the WHI with an intact uterus who used estrogen, oral MPA slightly increased the risk for breast cancer, and this elevated risk persisted even after discontinuation. This leads us to the question, what are the risks of breast cancer with progestin IUD use?
I recently reviewed the literature, and the answer is, it’s mixed. The FDA has put language into the package label that acknowledges a potential breast cancer risk for women who use a progestin IUD,27 and that warning states, “Women who currently have or have had breast cancer or suspect breast cancer should not use hormonal contraception because some breast cancers are hormone sensitive.” The label goes on to say, “Observational studies of the risk of breast cancer with the use of a levonorgestrel-releasing IUS don’t provide conclusive evidence of increased risk.” Thus, there is no conclusive answer as to whether there is a possible link of progestin IUDs to breast cancer.
What I tell my patients is that research is inconclusive. However, it’s unlikely for a 52-mg LNG-IUD to significantly increase a woman’s breast cancer risk, except possibly in those already at high risk from other risk factors. I tell them that breast cancer is listed in the package insert as a potential risk. I could not find any data on whether adding a low-dose estradiol patch would further increase that risk. So I counsel women about potential risk, but tell them that I don’t have any strong evidence of risk.
Continue to: Dr. Goldstein...
Dr. Goldstein: If you look in the package insert for Mirena,2 similar to Liletta, certainly the serum levels of LNG are lower than that for combination oral contraceptives. For the IUD progestins, they are not localized only to the uterus, and LNG levels range from about 150 to 200 µg/mL up to 60 months. It’s greater at 12 months, at about 180 µg/mL,at 24 months it was 192 µg/mL, and by 60 months it was 159 µg/mL. It’s important to realize that there is some systemic absorption of progestin with progestin IUDs, and it is not completely a local effect.
JoAnn, you mentioned the WHI data,25 and just to specify, it was not the estrogen-only arm, it was the conjugated equine estrogen-only arm of the WHI. I don’t think that estradiol alone increases breast cancer risk (although there are no good prospective, follow-through, 18-year study data, like the WHI), but I think readers need to understand the difference in the estrogen type.
Endometrial evaluation. My question for the panel is as follows. I agree that the use of the progestin-releasing IUD is very nice for that transition to menopause. I do believe it provides endometrial protection, but we know from other studies that, when we give continuous combined HT, about 21% to 26% of patients will experience some bleeding/staining, responding in the first 4-week cycles, and it can be as high as 9% at 1 year. If I have a patient who bleeds on continuous combined HT, I will evaluate her endometrium, usually just with a simple transvaginal ultrasound. If an IUD is in place, and the patient now begins to have some irregular bleeding, how do you evaluate her with the IUD in place?
Dr. Levy: That is a huge challenge. We know from a recent paper,28 that the endometrial thickness, while an excellent measure for Caucasian and European women, may be a poor marker for endometrial pathology in African-American women. What we thought we knew, which was, if the stripe is 4 mL or less, we can forget about it, I think in our more recent research that is not so true. So you bring up a great point, what do you do? The most reliable evaluation will be with an office hysteroscopy, where you can really look at the entire cavity and for tiny, little polyps and other things. But then we are off label because the use of hysteroscopy with an IUD in place is off label. So we are really in a conundrum.
Dr. Pinkerton: Also, if you do an endometrial biopsy, you might dislodge the IUD. If you think that you are going to take the IUD out, it may not matter if you dislodge it. I will often obtain a transvaginal ultrasound to help me figure out the next step, and maybe look at the dosing of the estrogen and progestin—but you can’t monitor an IUD with blood levels. You are in a vacuum of trying to figure out the best thing to do.
Dr. Kaunitz: One of the hats I wear here in Jacksonville is Director of GYN Ultrasound. I have a fair amount of experience doing endometrial biopsies in women with progestin IUDs in place under abdominal ultrasound guidance and keeping a close eye on the position of the IUD. In the first dozen or so such procedures I did, I was quite concerned about dislodging the IUD. It hasn’t happened yet, and it gives me some reassurance to be able to image the IUD and your endometrial suction curette inside the cavity as you are obtaining endometrial sampling. I have substantial experience now doing that, and so far, no problems. I do counsel all such women in advance that there is some chance I could dislodge their IUD.
Dr. Goldstein: In addition to dislodging the IUD, are you not concerned that, if the pathology is not global, that a blind endometrial sampling may be fraught with some error?
Dr. Kaunitz: The endometrium in women with a progestin-releasing IUD in place tends to be very well suppressed. Although one might occasionally find, for instance, a polyp in that setting, I have not run into, and I don’t expect to encounter going forward, endometrial hyperplasia or cancer in women with current use of a progestin IUD. It’s possible but unlikely.
Dr. Levy: The progestin IUD will counterbalance a type-1 endometrial cancer—an endometrial cancer related to hyperstimulation by estrogen. It will not do anything, to my knowledge, to counterbalance a type 2. I think the art of medicine is, you do the best you can with the first episode of bleeding, and if she persists in her bleeding, we have to persevere and continue to evaluate her.
Dr. Goldstein: I agree 100%.
Dr. Pinkerton: We all agree with you. That’s a really good point.
Continue to: Case examinations...
Case examinations
CASE 1 Woman with intramural fibroids
Dr. Pinkerton: Dr. Goldstein, you have a 48-year-old Black woman who has heavy but regular menstrual bleeding with multiple fibroids (the largest is about 4 to 5 cm, they look intramural, with some distortion of the cavity but not a submucous myoma, and the endometrial depth is 9 cm). Would you insert an IUD, and would you recommend an endometrial biopsy first?
Dr. Goldstein: I am not a huge fan of blind endometrial sampling, and I do think that we use the “biopsy” somewhat inappropriately since sampling is not a directed biopsy. This became obvious in the landmark paper by Guido et al in 1995 and was adopted by ACOG only in 2012.29 Cancers that occupy less than 50% of the endometrial surface area are often missed with such blind sampling. Thus I would not perform an endometrial biopsy first, but would rather rely on properly timed and performed transvaginal ultrasound to rule out any concurrent endometrial disease. I think a lot of patients who have HMB, not only because of their fibroids but also often just due to the surface area of their uterine cavity being increased—so essentially there is more blood volume when they bleed. However, you said that in this case the patient has regular menstrual bleeding, so I am assuming that she is still ovulatory. She may have some adenomyosis. She may have a large uterine cavity. I think she is an excellent candidate for an LNG-releasing IUD to reduce menstrual blood flow significantly. It will not necessarily give her amenorrhea, and it may give her some irregular bleeding. Then at some distant point, say in 5 or 6 months if she does have some irregular staining or bleeding, I would feel much better about the fact that nothing has developed as long as I knew that the endometrium was devoid of pathology when I started.
CASE 2 Woman with family history of breast cancer
Dr. Pinkerton: Dr. Levy, a 44-year-old woman has a family history of breast cancer in her mother at age 72, but she still needs contraceptionbecause of that unintended pregnancy risk in the 40s, and she wants something that is not going to increase her risk of breast cancer. What would you use, and how would you counsel her if you decided to use a progestin IUD?
Dr. Levy: The data are mixed,30-33 but whatever the risk, it is miniscule, and I would bring up the CDC Medical Eligibility Criteria.11 For a patient with a family history of breast cancer, for use of the progestin IUD, it is a 1—no contraindications. What I tend to tell my patients is, if you are worried about breast cancer, watch how much alcohol you are drinking and maintain regular exercise. There are so many preventive things that we can do to reduce risk of breast cancer when she needs contraception. If there is any increase in risk, it is so miniscule that I would very strongly recommend a progestin IUD for her.
Dr. Pinkerton: In addition, in recognizing the different densities of breast, dense breast density could lead to supplemental screening, which also could give her some reassurance that we are adequately screening for breast cancer.
CASE 3 Woman with IUD and VMS
Dr. Pinkerton: Dr. Kaunitz, you have a 52-year-old overweight female. She has been using a progestin IUD for 4 years, is amenorrheic, but now she is having moderate to severe vasomotor symptoms despite the IUD in place. You have talked to her about risks and benefits of HT, and she is interested in starting it. I know we talked about the studies, but I want to know what you are going to tell her. How do you counsel her about off-label use?
Dr. Kaunitz: The most important issue related to treating vasomotor symptoms in this patient is the route of systemic estrogen. Understandably, women’s biggest concern regarding the risks of systemic estrogen-progestin therapy is breast cancer. However, statistically, by far the biggest risk associated with oral estrogen-progestogen therapy, is elevated risk of venous thrombosis and pulmonary embolism. We have seen this, with a number of studies, and the WHI made it crystal clear with risks of oral conjugated equine estrogen at the dose of 0.625 mg daily. Oral estradiol 1 mg daily is also associated with a similar elevated risk of venous thrombosis. We also know that age and BMI are both independent risk factors for thrombosis. So, for a woman in her 50s who has a BMI > 30 mg/kg2, I don’t want to further elevate her risk of thrombosis by giving her oral estrogen, whether it is estradiol or conjugated equine estrogen. This is a patient in whom I would be comfortable using transdermal (patch) estradiol, perhaps starting with a standard dose of 0.05 mg weekly or twice weekly patch, keeping in mind that 0.05 mg in the setting of transdermal estrogen refer to the daily or to the 24-hour release rate. The 1.0 mg of oral estradiol and 0.625 mg of conjugated equine estrogen refers to the mg quantity of estrogen in each tablet. This is a source of great confusion for clinicians.
If, during follow-up, the 0.05 mg estradiol patch is not sufficient to substantially reduce symptoms, we could go up, for instance, to a 0.075 mg estradiol patch. We know very clearly from a variety of observational studies, including a very large UK study,34 that in contrast with oral estrogen, transdermal estradiol is safer from the perspective of thrombosis.
Insurance coverage for IUDs
Dr. Pinkerton: Dr. Levy: Can you discuss IUDs and the Affordable Care Act’s requirement to cover contraceptive services?
Dr. Levy: Unfortunately, we do not know whether this benefit will continue based on a very recent finding from a judge in Texas that ruled the preventive benefits of the ACA were illegal.35 We don’t know what will happen going forward. What I will say is that, unfortunately, many insurance companies have not preserved the meaning of “cover all things,” so what we are finding is that, for example, they only have to cover one type in a class. The FDA defined 18 classes of contraceptives, and a hormonal IUD is one class, so they can decide that they are only going to cover one of the four IUDS. And then women don’t have access to the other three, some of which might be more appropriate for them than another.
The other thing very relevant to this conversation is that, if you use an ICD-10 code for menorrhagia, for HMB, it no longer lives within that ACA preventive care requirement of coverage for contraceptives, and now she is going to owe a big deductible or a copay. If you are practicing in an institution that does not allow the use of IUDs for contraception, like a Catholic institution where I used to practice, you will want to use that ICD-10 code for HMB. But if you want it offered with no out-of-pocket cost for the patient, you need to use the preventive medicine codes and the contraception code. These little nuances for us can make a huge difference for our patients.
Dr. Pinkerton: Thank you for that reminder. I want to thank our panelists, Dr. Levy, Dr. Goldstein, and Dr. Kaunitz, for providing us with such a great mix of evidence and expert opinion and also giving a benefit of their vast experience as award-winning gynecologists. Hopefully, today you have learned the benefits of the progestin IUD not only for contraception in reproductive years and perimenopause but also for treatment of HMB, and the potential benefit due to the more prolonged effectiveness of the IUDs for endometrial protection in postmenopause. This allows less progestin risk, essentially estrogen alone for postmenopausal HT. Unsolved questions remain about whether there is a risk of breast cancer with their use, but there is a clear benefit of protecting against pregnancy and endometrial cancer. ●

Intrauterine devices (IUDs) are now used by more than 15% of US contraceptors. The majority of these IUDs release the progestin levonorgestrel, and with now longer extended use of the IUDs approved by the US Food and Drug Administration (FDA),1-3 they become even more attractive for use for contraception,control of menorrhagia or heavy menstrual bleeding (HMB) during reproductive years and perimenopause, and potentially, although not FDA approved for this purpose, postmenopause for endometrial protection in estrogen users. In this roundtable discussion, we will look at some of the benefits of the IUD for contraception effectiveness and control of bleeding, as well as the potential risks if used for postmenopausal women.
Progestin IUDs and contraception
JoAnn V. Pinkerton, MD, NCMP: Dr. Kaunitz, what are the contraceptive benefits of progestin IUDs during perimenopause?
Andrew M. Kaunitz, MD, NCMP: We know fertility declines as women approach menopause. However, when pregnancy occurs in older reproductive-age women, the pregnancies are often unintended, as reflected by high rates of induced abortion in this population. In addition, the prevalence of maternal comorbidities (during pregnancy and delivery) is higher in older reproductive-age women, with the maternal mortality rate more than 5 times higher compared with that of younger women.4 Two recently published clinical trials assessed the extended use of full-size IUDs containing 52 mg of levonor-gestrel (LNG), with the brand names Mirena and Liletta.1,2 The data from these trials confirmed that both IUDs remain highly effective for up to 8 years of use, and currently, both devices are approved for up to 8 years of use. One caveat is that, in the unusual occurrence of a pregnancy being diagnosed in a woman using an IUD, we as clinicians, must be alert to the high prevalence of ectopic pregnancies in this setting.
Progestin IUDs and HMB
Dr. Pinkerton: Dr. Goldstein, can you comment on how well progestin IUDs work for HMB?
Steven R. Goldstein, MD, NCMP, CCD: Many women who need contraception will use these devices for suppressing HMB, and they can be quite effective, if the diagnosis truly is HMB, at reducing bleeding.5 But that efficacy in bleeding reduction may not be quite as long as the efficacy in pregnancy prevention.6 In my experience, among women using IUDs specifically for their HMB, good bleeding control may require changing the IUD at 3 to 5 years.
Barbara S. Levy, MD: When inserting a LNG-IUD for menorrhagia in the perimenopausal time frame, sometimes I will do a progestin withdrawal first, which will thin the endometrium and induce withdrawal bleeding because, in my experience, if you place an IUD in someone with perimenopausal bleeding, you may end up with a lot of breakthrough bleeding.
Perimenopause and hot flashes
Dr. Pinkerton: Dr. Kaunitz, we have learned that hot flashes often occur and become bothersome to women during perimenopause. Many women have IUDs placed during perimenopause for bleeding. Can you comment about IUD use during perimenopause and postmenopause?
Dr. Kaunitz: In older reproductive-age women who already have a progestin-releasing IUD placed, as they get closer to menopause when vasomotor symptoms (VMS) might occur, if these symptoms are bothersome, the presence or placement of a progestin-releasing IUD can facilitate treatment of perimenopausal VMS with estrogen therapy.
Progestin IUDs cause profound endometrial suppression, reduce bleeding and often, over time, cause users to become amenorrheic.7
The Mirena package insert states, “Amenorrhea develops in about 20% of users by one year.”2 By year 3 and continuing through year 8, the prevalence of amenorrhea with the 52-mg LNG-IUD is 35% to 40%.8 From a study by Nanette Santoro, MD, and colleagues, we know that, in perimenopausal women with a progestin-releasing IUD in place, who are experiencing bothersome VMS, adding transdermal estrogen is very effective in treating and suppressing those hot flashes. In her small clinical trial, among participants with perimenopausal bothersome VMS with an IUD in place, half were randomized to use of transdermal estradiol and then compared with women who did not get the estradiol patch. There was excellent relief of perimenopausal hot flashes with the combination of the progestin IUD for endometrial suppression and transdermal estrogen to relieve hot flashes.9
Dr. Pinkerton: Which women would not be good candidates for the use of this combination?
Dr. Kaunitz: We know that, as women age, the prevalence of conditions that are contraindications to combination contraceptives (estrogen-progestin pills, patches, or rings) starts to increase. Specifically, we see more: hypertension, diabetes, and high body mass index (BMI), or obesity. We also know that migraine headaches in women older than age 35 years is another condition in which ACOG and the Centers for Disease Control and Prevention (CDC) would not recommend use of combination contraceptives.10,11 These older perimenopausal women may be excellent candidates for a progestin-only releasing IUD combined with use of transdermal menopausal doses of estradiol if needed for VMS.
Dr. Goldstein: I do want to add that, in those patients who don’t have these comorbidities, combination estrogen-progestin contraceptives do a very nice job of ovarian suppression and will prevent the erratic production of estradiol, which, in my experience, often results in not only irregular bleeding but also possible exacerbation of perimenopausal mood symptoms.
Dr. Kaunitz: I agree, Steve. The ideal older reproductive-age candidate for combination pills, patch, or ring would be a slender, healthy, nonsmoking woman with normal blood pressure. Such women would be a fairly small subgroup of my practice, but they can safely continue combination contraceptives right through menopause. Consistent with CDC and ACOG guidance, rather than checking gonadotropins to “determine when menopause has occurred,” (which is, in fact, not an evidence-based approach to diagnosing menopause in this setting), such women can continue the combination contraceptive right up until age 55—the likelihood that women are still going to be ovulating or at risk for pregnancy becomes vanishingly small at that age.11,12 Women in their mid-50s can either seamlessly transition to use of systemic estrogen-progestin menopausal therapy or go off hormones completely.
Continue to: The IUD and HMB...
The IUD and HMB
Dr. Pinkerton: Dr. Goldstein, there’s been some good literature on the best management options for women with HMB. What is the most current evidence?
Dr. Goldstein: I think that the retiring of the terms menorrhagia and metrorrhagia may have been premature because HMB implies cyclical bleeding, and this population of women with HMB will typically do quite well. Women who have what we used to call metrorrhagia or irregular bleeding, by definition, need endometrial evaluation to be sure they don’t have some sort of organic pathology. It would be a mistake for clinicians to use an LNG-IUD in patients with abnormal uterine bleeding (AUB) that has not been appropriately evaluated.
If we understand that we are discussing HMB, a Cochrane Review from 202213 suggests that an LNG intrauterine system is the best first-line treatment for reducing menstrual blood loss in perimenopausal women with HMB. Antifibrinolytics appeared second best, while long-cycle progestogens came in third place. Evidence on perception of improvement in satisfaction was ranked as low certainty. That same review found that hysterectomy was the best treatment for reducing bleeding, obviously, followed by resectoscopic endometrial ablation or a nonresectoscopic global endometrial ablation.
The evidence rating was low certainty regarding the likelihood that placing an LNG-IUD in women with HMB will result in amenorrhea, and I think that’s a very important point. The expectation of patients should be reduced or a significantly reduced amount of their HMB, not necessarily amenorrhea. Certainly, minimally invasive hysterectomy will result in total amenorrhea and may have a larger increase in satisfaction, but it has its own set of other kinds of possible complications.
Dr. Kaunitz: In an industry-funded, international multicenter trial,14 women with documented HMB (hemoglobin was eluted from soiled sanitary products), with menstrual blood loss of 80 mL or more per cycle, were randomized to placement of an LNG 52-mg IUD (Mirena) or cyclical medroxyprogesterone acetate (MPA)—oral progestin use.
Although menstrual blood loss declined in both groups, it declined dramatically more in women with an IUD placed, and specifically with the IUD, menstrual blood loss declined by 129 mL on average, whereas the decline in menstrual blood loss with cyclical MPA was 18 mL. This data, along with earlier European data,15 which showed similar findings in women with HMB led to the approval of the Mirena progestin IUD for a second indication to treat HMB in 2009.
I also want to point out that, in the May 2023 issue of Obstetrics & Gynecology, Creinin and colleagues published a similar trial in women with HMB showing, once again, that progestin IUDs (52-mg LNG-IUD, Liletta) are extremely effective in reducing HMB.16 There is crystal clear evidence from randomized trials that both 52-mg LNG-IUDs, Mirena and Liletta, are very effective in reducing HMB and, in fact, are contributing to many women who in the past would have proceeded with surgery, such as ablation or hysterectomy, to control their HMB.
Oral contraception
Dr. Pinkerton: What about using low-dose continuous oral contraceptives noncyclically for women with HMB?
Dr. Goldstein: I do that all the time. It is interesting that Dr. Kaunitz mentions his patient population. It’s why we understand that one size does not fit all. You need to see patients one at a time, and if they are good candidates for a combined estrogen-progestin contraception, whether it’s pills, patches, or rings, giving that continuously does a very nice job in reducing HMB and straightening out some of the other symptoms that these perimenopausal women will have.
IUD risks
Dr. Pinkerton: We all know about use of low-dose oral contraceptives for management of AUB, and we use them, although we worry a little bit about breast cancer risk. Dr. Levy, please comment on the risks with IUDs of expulsions and perforations. What are the downsides of IUDs?
Dr. Levy: Beyond the cost, although it is a minimally invasive procedure, IUD insertion can be an invasive procedure for a patient to undergo; expulsions can occur.17 We know that a substantial percentage of perimenopausal women will have fibroids. Although many fibroids are not located in the uterine cavity, the expulsion rate with HMB for an LNG-IUD can be higher,13,16,18,19 perhaps because of local prostaglandin release with an increase in uterine contractility. There is a low incidence of perforations, but they do happen, particularly among women with scars in the uterus or who have a severely anteflexed or retroflexed uterus, and women with cervical stenosis, for example, if they have had a LEEP procedure, etc. Even though progestin IUDs are outstanding tools in our toolbox, they are invasive to some extent, and they do have the possibility of complications.
Dr. Kaunitz: As Dr. Levy points out, although placement of an IUD may be considered an invasive procedure, it is also an office-based procedure, so women can drive home or drive back to work afterwards without the disruption in their life and the potential complications associated with surgery and anesthesia.
Continue to: Concerns with malpositioning...
Concerns with malpositioning
Dr. Pinkerton: After placement of an IUD, during a follow-up visit, sometimes you can’t visualize the string. The ultrasonography report may reveal, “IUD appears to be in the right place within the endometrium.” Dr. Goldstein, can you comment on how we should use ultrasound when we can’t visualize or find the IUD string, or if the patient complains of abdominal pain, lower abdominal discomfort, or irregular bleeding or spotting and we become concerned about IUD malposition?
Dr. Goldstein: Ultrasound is not really required after an uncomplicated placement of an IUD or during routine management of women who have no problems who are using an IUD. In patients who present with pain or some abnormal bleeding, however, sometimes it is the IUD being malpositioned. A very interesting study by the late great Beryl Benacerraf20 showed that there was a statistically significant higher incidence of the IUD being poorly positioned when patients have pain or bleeding (FIGURE 1). It was not always apparent on 2D ultrasonography. Using a standard transvaginal ultrasound of the long access plane, the IUD may appear to be very centrally located. However, if you do a 3D coronal section, not infrequently in these patients with any pain or bleeding, one of the arms has pierced the myometrium (FIGURE 2). This can actually be a source of pain and bleeding.


It’s also very interesting when you talk about perforation. I became aware of a big to-do in the medical/legal world about the possibility of the IUD migrating through the uterine cavity.21 This just does not exist, as was already pointed out. If the IUD is really going to go anywhere, if it’s properly placed, it’s going to be expelled through an open cervix. I do believe that, if you have pierced the myometrium through uterine contractility over time, some of these IUDs could work their way through the myometrium and somehow come out of the uterus either totally or partially. I think ultrasound is invaluable in patients with pain and bleeding, but I think you need to have an ultrasound lab capable of doing a 3D coronal section.
Progestin IUDs for HT replacement: Benefits/risks
Dr. Pinkerton: Many clinicians are excited that they can use essentially estrogen alone for women who have a progestin IUD in place. What about the possible off-label use of the progestin IUD to replace oral progestogen for hormone therapy (HT)? Dr. Kaunitz, are there any studies using this for postmenopausal HT (with a reminder that the IUD is not FDA approved for this purpose)?
Dr. Kaunitz: We have data from Europe indicating that, in menopausal women using systemic estrogen, the full-size LNG 52 IUD—Mirena or Liletta—provides excellent endometrial suppression.22 Where we don’t have data is with the smaller IUDs, which would be Kyleena and Skyla, which release smaller amounts of progestin each day into the endometrial cavity.
I have a number of patients, most of them women who started use of a progestin IUD as older reproductive-age women and then started systemic estrogen for treatment of perimenopausal hot flashes and then continued the use of their IUD plus systemic estrogen in treating postmenopausal hot flashes. The IUD is very useful in this setting, but as you pointed out, Dr. Pinkerton, this does represent off-label use.
Dr. Pinkerton: I know this use does not affect plasma lipids or cardiovascular risk markers, although users seem to report that the IUD has improved their quality of life. The question comes up, what are the benefits on cancer risk for using an IUD?
Dr. Levy: It’s such a great question because, as we talk about the balance of risks and benefits for anything that we are offering to our patients, it is really important to focus on some of the benefits. For both the copper and the LNG-IUD, there is a reduction in endometrial cancer,22 as well as pretty good data with the copper IUD about a reduction in cervical cancer.23 Those data are a little bit less clear for the LNG-IUD.
Interestingly, at least one meta-analysis published in 2020 shows about a 30% reduction in ovarian cancer risk with the LNG-IUD.24 We need to focus our patients on these other benefits. They tend to focus on the risks, and, of course, the media blows up the risks, but the benefits are quite substantial beyond just reducing HMB and providing contraception.
Dr. Pinkerton: As Dr. Kaunitz said, when you use this IUD, with its primarily local uterine progestin effects, it’s more like using estrogen alone without as much systemic progestin. Recently I wrote an editorial on the benefits of estrogen alone on the risk of breast cancer, primarily based on the Women’s Health Initiative (WHI) observational long-term 18-year cumulative follow-up. When estrogen alone was prescribed to women after a hysterectomy, estrogen therapy used at menopause did not increase the risk of invasive breast cancer, and was associated with decreased mortality.25 However, the nurse’s health study has suggested that longer-term use may be increased with estrogen alone.26 For women in the WHI with an intact uterus who used estrogen, oral MPA slightly increased the risk for breast cancer, and this elevated risk persisted even after discontinuation. This leads us to the question, what are the risks of breast cancer with progestin IUD use?
I recently reviewed the literature, and the answer is, it’s mixed. The FDA has put language into the package label that acknowledges a potential breast cancer risk for women who use a progestin IUD,27 and that warning states, “Women who currently have or have had breast cancer or suspect breast cancer should not use hormonal contraception because some breast cancers are hormone sensitive.” The label goes on to say, “Observational studies of the risk of breast cancer with the use of a levonorgestrel-releasing IUS don’t provide conclusive evidence of increased risk.” Thus, there is no conclusive answer as to whether there is a possible link of progestin IUDs to breast cancer.
What I tell my patients is that research is inconclusive. However, it’s unlikely for a 52-mg LNG-IUD to significantly increase a woman’s breast cancer risk, except possibly in those already at high risk from other risk factors. I tell them that breast cancer is listed in the package insert as a potential risk. I could not find any data on whether adding a low-dose estradiol patch would further increase that risk. So I counsel women about potential risk, but tell them that I don’t have any strong evidence of risk.
Continue to: Dr. Goldstein...
Dr. Goldstein: If you look in the package insert for Mirena,2 similar to Liletta, certainly the serum levels of LNG are lower than that for combination oral contraceptives. For the IUD progestins, they are not localized only to the uterus, and LNG levels range from about 150 to 200 µg/mL up to 60 months. It’s greater at 12 months, at about 180 µg/mL,at 24 months it was 192 µg/mL, and by 60 months it was 159 µg/mL. It’s important to realize that there is some systemic absorption of progestin with progestin IUDs, and it is not completely a local effect.
JoAnn, you mentioned the WHI data,25 and just to specify, it was not the estrogen-only arm, it was the conjugated equine estrogen-only arm of the WHI. I don’t think that estradiol alone increases breast cancer risk (although there are no good prospective, follow-through, 18-year study data, like the WHI), but I think readers need to understand the difference in the estrogen type.
Endometrial evaluation. My question for the panel is as follows. I agree that the use of the progestin-releasing IUD is very nice for that transition to menopause. I do believe it provides endometrial protection, but we know from other studies that, when we give continuous combined HT, about 21% to 26% of patients will experience some bleeding/staining, responding in the first 4-week cycles, and it can be as high as 9% at 1 year. If I have a patient who bleeds on continuous combined HT, I will evaluate her endometrium, usually just with a simple transvaginal ultrasound. If an IUD is in place, and the patient now begins to have some irregular bleeding, how do you evaluate her with the IUD in place?
Dr. Levy: That is a huge challenge. We know from a recent paper,28 that the endometrial thickness, while an excellent measure for Caucasian and European women, may be a poor marker for endometrial pathology in African-American women. What we thought we knew, which was, if the stripe is 4 mL or less, we can forget about it, I think in our more recent research that is not so true. So you bring up a great point, what do you do? The most reliable evaluation will be with an office hysteroscopy, where you can really look at the entire cavity and for tiny, little polyps and other things. But then we are off label because the use of hysteroscopy with an IUD in place is off label. So we are really in a conundrum.
Dr. Pinkerton: Also, if you do an endometrial biopsy, you might dislodge the IUD. If you think that you are going to take the IUD out, it may not matter if you dislodge it. I will often obtain a transvaginal ultrasound to help me figure out the next step, and maybe look at the dosing of the estrogen and progestin—but you can’t monitor an IUD with blood levels. You are in a vacuum of trying to figure out the best thing to do.
Dr. Kaunitz: One of the hats I wear here in Jacksonville is Director of GYN Ultrasound. I have a fair amount of experience doing endometrial biopsies in women with progestin IUDs in place under abdominal ultrasound guidance and keeping a close eye on the position of the IUD. In the first dozen or so such procedures I did, I was quite concerned about dislodging the IUD. It hasn’t happened yet, and it gives me some reassurance to be able to image the IUD and your endometrial suction curette inside the cavity as you are obtaining endometrial sampling. I have substantial experience now doing that, and so far, no problems. I do counsel all such women in advance that there is some chance I could dislodge their IUD.
Dr. Goldstein: In addition to dislodging the IUD, are you not concerned that, if the pathology is not global, that a blind endometrial sampling may be fraught with some error?
Dr. Kaunitz: The endometrium in women with a progestin-releasing IUD in place tends to be very well suppressed. Although one might occasionally find, for instance, a polyp in that setting, I have not run into, and I don’t expect to encounter going forward, endometrial hyperplasia or cancer in women with current use of a progestin IUD. It’s possible but unlikely.
Dr. Levy: The progestin IUD will counterbalance a type-1 endometrial cancer—an endometrial cancer related to hyperstimulation by estrogen. It will not do anything, to my knowledge, to counterbalance a type 2. I think the art of medicine is, you do the best you can with the first episode of bleeding, and if she persists in her bleeding, we have to persevere and continue to evaluate her.
Dr. Goldstein: I agree 100%.
Dr. Pinkerton: We all agree with you. That’s a really good point.
Continue to: Case examinations...
Case examinations
CASE 1 Woman with intramural fibroids
Dr. Pinkerton: Dr. Goldstein, you have a 48-year-old Black woman who has heavy but regular menstrual bleeding with multiple fibroids (the largest is about 4 to 5 cm, they look intramural, with some distortion of the cavity but not a submucous myoma, and the endometrial depth is 9 cm). Would you insert an IUD, and would you recommend an endometrial biopsy first?
Dr. Goldstein: I am not a huge fan of blind endometrial sampling, and I do think that we use the “biopsy” somewhat inappropriately since sampling is not a directed biopsy. This became obvious in the landmark paper by Guido et al in 1995 and was adopted by ACOG only in 2012.29 Cancers that occupy less than 50% of the endometrial surface area are often missed with such blind sampling. Thus I would not perform an endometrial biopsy first, but would rather rely on properly timed and performed transvaginal ultrasound to rule out any concurrent endometrial disease. I think a lot of patients who have HMB, not only because of their fibroids but also often just due to the surface area of their uterine cavity being increased—so essentially there is more blood volume when they bleed. However, you said that in this case the patient has regular menstrual bleeding, so I am assuming that she is still ovulatory. She may have some adenomyosis. She may have a large uterine cavity. I think she is an excellent candidate for an LNG-releasing IUD to reduce menstrual blood flow significantly. It will not necessarily give her amenorrhea, and it may give her some irregular bleeding. Then at some distant point, say in 5 or 6 months if she does have some irregular staining or bleeding, I would feel much better about the fact that nothing has developed as long as I knew that the endometrium was devoid of pathology when I started.
CASE 2 Woman with family history of breast cancer
Dr. Pinkerton: Dr. Levy, a 44-year-old woman has a family history of breast cancer in her mother at age 72, but she still needs contraceptionbecause of that unintended pregnancy risk in the 40s, and she wants something that is not going to increase her risk of breast cancer. What would you use, and how would you counsel her if you decided to use a progestin IUD?
Dr. Levy: The data are mixed,30-33 but whatever the risk, it is miniscule, and I would bring up the CDC Medical Eligibility Criteria.11 For a patient with a family history of breast cancer, for use of the progestin IUD, it is a 1—no contraindications. What I tend to tell my patients is, if you are worried about breast cancer, watch how much alcohol you are drinking and maintain regular exercise. There are so many preventive things that we can do to reduce risk of breast cancer when she needs contraception. If there is any increase in risk, it is so miniscule that I would very strongly recommend a progestin IUD for her.
Dr. Pinkerton: In addition, in recognizing the different densities of breast, dense breast density could lead to supplemental screening, which also could give her some reassurance that we are adequately screening for breast cancer.
CASE 3 Woman with IUD and VMS
Dr. Pinkerton: Dr. Kaunitz, you have a 52-year-old overweight female. She has been using a progestin IUD for 4 years, is amenorrheic, but now she is having moderate to severe vasomotor symptoms despite the IUD in place. You have talked to her about risks and benefits of HT, and she is interested in starting it. I know we talked about the studies, but I want to know what you are going to tell her. How do you counsel her about off-label use?
Dr. Kaunitz: The most important issue related to treating vasomotor symptoms in this patient is the route of systemic estrogen. Understandably, women’s biggest concern regarding the risks of systemic estrogen-progestin therapy is breast cancer. However, statistically, by far the biggest risk associated with oral estrogen-progestogen therapy, is elevated risk of venous thrombosis and pulmonary embolism. We have seen this, with a number of studies, and the WHI made it crystal clear with risks of oral conjugated equine estrogen at the dose of 0.625 mg daily. Oral estradiol 1 mg daily is also associated with a similar elevated risk of venous thrombosis. We also know that age and BMI are both independent risk factors for thrombosis. So, for a woman in her 50s who has a BMI > 30 mg/kg2, I don’t want to further elevate her risk of thrombosis by giving her oral estrogen, whether it is estradiol or conjugated equine estrogen. This is a patient in whom I would be comfortable using transdermal (patch) estradiol, perhaps starting with a standard dose of 0.05 mg weekly or twice weekly patch, keeping in mind that 0.05 mg in the setting of transdermal estrogen refer to the daily or to the 24-hour release rate. The 1.0 mg of oral estradiol and 0.625 mg of conjugated equine estrogen refers to the mg quantity of estrogen in each tablet. This is a source of great confusion for clinicians.
If, during follow-up, the 0.05 mg estradiol patch is not sufficient to substantially reduce symptoms, we could go up, for instance, to a 0.075 mg estradiol patch. We know very clearly from a variety of observational studies, including a very large UK study,34 that in contrast with oral estrogen, transdermal estradiol is safer from the perspective of thrombosis.
Insurance coverage for IUDs
Dr. Pinkerton: Dr. Levy: Can you discuss IUDs and the Affordable Care Act’s requirement to cover contraceptive services?
Dr. Levy: Unfortunately, we do not know whether this benefit will continue based on a very recent finding from a judge in Texas that ruled the preventive benefits of the ACA were illegal.35 We don’t know what will happen going forward. What I will say is that, unfortunately, many insurance companies have not preserved the meaning of “cover all things,” so what we are finding is that, for example, they only have to cover one type in a class. The FDA defined 18 classes of contraceptives, and a hormonal IUD is one class, so they can decide that they are only going to cover one of the four IUDS. And then women don’t have access to the other three, some of which might be more appropriate for them than another.
The other thing very relevant to this conversation is that, if you use an ICD-10 code for menorrhagia, for HMB, it no longer lives within that ACA preventive care requirement of coverage for contraceptives, and now she is going to owe a big deductible or a copay. If you are practicing in an institution that does not allow the use of IUDs for contraception, like a Catholic institution where I used to practice, you will want to use that ICD-10 code for HMB. But if you want it offered with no out-of-pocket cost for the patient, you need to use the preventive medicine codes and the contraception code. These little nuances for us can make a huge difference for our patients.
Dr. Pinkerton: Thank you for that reminder. I want to thank our panelists, Dr. Levy, Dr. Goldstein, and Dr. Kaunitz, for providing us with such a great mix of evidence and expert opinion and also giving a benefit of their vast experience as award-winning gynecologists. Hopefully, today you have learned the benefits of the progestin IUD not only for contraception in reproductive years and perimenopause but also for treatment of HMB, and the potential benefit due to the more prolonged effectiveness of the IUDs for endometrial protection in postmenopause. This allows less progestin risk, essentially estrogen alone for postmenopausal HT. Unsolved questions remain about whether there is a risk of breast cancer with their use, but there is a clear benefit of protecting against pregnancy and endometrial cancer. ●
- Liletta [package insert]. Allergan; Irvine, California. November 2022.
- Mirena [package insert]. Bayer; Whippany, New Jersey. 2000.
- Kaunitz AM. Safe extended use of levonorgestrel 52-mg IUDs. November 11, 2022. https://www.medscape.com/ viewarticle/983680. Accessed May 8, 2023.
- Kaunitz AM. Clinical practice. Hormonal contraception in women of older reproductive age. N Engl J Med. 2008;358:1262-1270. doi: 10.1056/NEJMcp0708481.
- Tucker ME. IUD-released levonorgestrel eases heavy menstrual periods. Medscape. April 10, 2023. https://www .medscape.com/viewarticle/777406. Accessed May 2, 2023.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice; Long-Acting Reversible Contraception Working Group. ACOG Committee Opinion No. 450: Increasing use of contraceptive implants and intrauterine devices to reduce unintended pregnancy. Obstet Gynecol. 2009;114:1434-1438.
- Critchley HO, Wang H, Jones RL, et al. Morphological and functional features of endometrial decidualization following long-term intrauterine levonorgestrel delivery. Hum Reprod. 1998;13:1218-1224. doi:10.1093/humrep/13.5.1218.
- Creinin MD, Schreiber CA, Turok DK, et al. Levonorgestrel 52 mg intrauterine system efficacy and safety through 8 years of use. Am J Obstet Gynecol. 2022;227:871.e1-871.e7. doi: 10.1016/j.ajog.2022.05.022.
- Santoro N, Teal S, Gavito C, et al. Use of a levonorgestrelcontaining intrauterine system with supplemental estrogen improves symptoms in perimenopausal women: a pilot study. Menopause. 2015;22:1301-1307. doi: 10.1097 /GME.0000000000000557.
- ACOG Committee on Practice Bulletins-Gynecology ACOG Practice Bulletin. The use of hormonal contraception in women with coexisting medical conditions. Number 18, July 2000. Int J Gynaecol Obstet. 2001;75:93-106. doi: 10.1016 /s0020-7292(01)00520-3.
- Curtis KM, Tepper NK, Jatlaoui TC, Berry-Bibee E, Horton LG, Zapata LB, Simmons KB, Pagano HP, Jamieson DJ, Whiteman MK. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-103. doi: 10.15585 /mmwr.rr6503a1.
- ACOG Practice Bulletin No. 206: use of hormonal contraception in women with coexisting medical conditions [published correction appears in: Obstet Gynecol. 2019;133:1288.] Obstet Gynecol. 2019;133:e128-e150. doi:10.1097/AOG.0000000000003072.
- Bofill Rodriguez M, Dias S, Jordan V, et al. Interventions for heavy menstrual bleeding; overview of Cochrane reviews and network meta-analysis. Cochrane Database Syst Rev. 2022;5:CD013180. doi: 10.1002/14651858.CD013180.pub2.
- Kaunitz AM, Bissonnette F, Monteiro I, et al. Levonorgestrelreleasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial [published correction appears in: Obstet Gynecol. 2010;116:999]. Obstet Gynecol. 2010;116:625-632. doi: 10.1097 /AOG.0b013e3181ec622b.
- Milsom I, Andersson K, Andersch B, et al. A comparison of flurbiprofen, tranexamic acid, and a levonorgestrel-releasing intrauterine contraceptive device in the treatment of idiopathic menorrhagia. Am J Obstet Gynecol. 1991;164:879883. doi: 10.1016/s0002-9378(11)90533-x.
- Creinin MD, Barnhart KT, Gawron LM, et al. Heavy menstrual bleeding treatment with a levonorgestrel 52-mg intrauterine device. Obstet Gynecol. 2023;141:971-978. doi: 10.1097 /AOG.0000000000005137.
- 1Madden T. Association of age and parity with intrauterine device expulsion. Obstet Gynecol. 2014:718-726. doi:10.1097 /aog.0000000000000475.
- Kaunitz AM, Stern L, Doyle J, et al. Use of the levonorgestrelIUD in the treatment of menorrhagia: improving patient outcomes while reducing the need for surgical management. Manag Care Interface. 2007;20:47-50.
- Getahun D, Fassett MJ, Gatz J, et al. Association between menorrhagia and risk of intrauterine device-related uterine perforation and device expulsion: results from the Association of Uterine Perforation and Expulsion of Intrauterine Device study. Am J Obstet Gynecol. 2022;227:59.e1-59.e9.
- Benacerraf BR, Shipp TD, Bromley B. Three-dimensional ultrasound detection of abnormally located intrauterine contraceptive devices that are a source of pelvic pain and abnormal bleeding. Ultrasound Obstet Gynecol. 2009;34:110115.
- Shipp TD, Bromley B, Benacerraf BR. The width of the uterine cavity is narrower in patients with an embedded intrauterine device (IUD) compared to a normally positioned IUD. J Ultrasound Med. 2010;29:1453-1456.
- Depypere H, Inki P. The levonorgestrel-releasing intrauterine system for endometrial protection during estrogen replacement therapy: a clinical review. Climacteric. 2015;18:470-482.
- Minalt N, Caldwell A, Yedlicka GM, et al. Association of intrauterine device use and endometrial, cervical, and ovarian cancer: an expert review. Am J Obstet Gynecol. 2023:S0002-9378(23)00224-7.
- Balayla J, Gil Y, Lasry A, et al. Ever-use of the intra-uterine device and the risk of ovarian cancer. J Obstet Gynaecol. 2021;41:848-853. doi: 10.1080/01443615.2020.1789960.
- Manson JE, Aragaki AK, Rossouw JE, et al. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318:927-938. doi:10.1001/jama.2017.11217.
- Chen WY, Manson JE, Hankinson SE, et al. Unopposed estrogen therapy and the risk of invasive breast cancer. Arch Intern Med. 2006;166:1027-1032. doi: 10.1001 /archinte.166.9.1027.
- Pinkerton JV, Wilson CS, Kaunitz AM. Reassuring data regarding the use of hormone therapy at menopause and risk of breast cancer. Menopause. 2022;29:1001-1004.doi:10.1097 /GME.0000000000002057.
- Romano SS, Doll KM. The impact of fibroids and histologic subtype on the performance of US clinical guidelines for the diagnosis of endometrial cancer among Black women. Ethn Dis. 2020;30:543-552. doi: 10.18865/ed.30.4.543.
- ACOG Committee on Practice Bulletins—Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206. doi: 10.1097/AOG.0b013e318262e320.
- Backman T, Rauramo I, Jaakkola Kimmo, et al. Use of the levonorgestrel-releasing intrauterine system and breast cancer. Obstet Gynecol. 2005;106:813-817.
- Conz L, Mota BS, Bahamondes L, et al. Levonorgestrelreleasing intrauterine system and breast cancer risk: A systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2020;99:970-982.
- Al Kiyumi MH, Al Battashi K, Al-Riyami HA. Levonorgestrelreleasing intrauterine system and breast cancer. Is there an association? Acta Obstet Gynecol Scand. 2021;100:1749.
- Marsden J. Hormonal contraception and breast cancer, what more do we need to know? Post Reprod Health. 2017;23:116127. doi: 10.1177/2053369117715370.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810 doi:10.1136/bmj.k4810.
- Levitt L, Cox C, Dawson L. Q&A: implications of the ruling on the ACA’s preventive services requirement. KFF.org. https://www .kff.org/policy-watch/qa-implications-of-the-ruling-on -the-acas-preventive-services-requirement/#:~:text=On%20 March%2030%2C%202023%2C%20a,cost%2Dsharing%20 for%20their%20enrollees. Accessed May 2, 2023.
- Liletta [package insert]. Allergan; Irvine, California. November 2022.
- Mirena [package insert]. Bayer; Whippany, New Jersey. 2000.
- Kaunitz AM. Safe extended use of levonorgestrel 52-mg IUDs. November 11, 2022. https://www.medscape.com/ viewarticle/983680. Accessed May 8, 2023.
- Kaunitz AM. Clinical practice. Hormonal contraception in women of older reproductive age. N Engl J Med. 2008;358:1262-1270. doi: 10.1056/NEJMcp0708481.
- Tucker ME. IUD-released levonorgestrel eases heavy menstrual periods. Medscape. April 10, 2023. https://www .medscape.com/viewarticle/777406. Accessed May 2, 2023.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice; Long-Acting Reversible Contraception Working Group. ACOG Committee Opinion No. 450: Increasing use of contraceptive implants and intrauterine devices to reduce unintended pregnancy. Obstet Gynecol. 2009;114:1434-1438.
- Critchley HO, Wang H, Jones RL, et al. Morphological and functional features of endometrial decidualization following long-term intrauterine levonorgestrel delivery. Hum Reprod. 1998;13:1218-1224. doi:10.1093/humrep/13.5.1218.
- Creinin MD, Schreiber CA, Turok DK, et al. Levonorgestrel 52 mg intrauterine system efficacy and safety through 8 years of use. Am J Obstet Gynecol. 2022;227:871.e1-871.e7. doi: 10.1016/j.ajog.2022.05.022.
- Santoro N, Teal S, Gavito C, et al. Use of a levonorgestrelcontaining intrauterine system with supplemental estrogen improves symptoms in perimenopausal women: a pilot study. Menopause. 2015;22:1301-1307. doi: 10.1097 /GME.0000000000000557.
- ACOG Committee on Practice Bulletins-Gynecology ACOG Practice Bulletin. The use of hormonal contraception in women with coexisting medical conditions. Number 18, July 2000. Int J Gynaecol Obstet. 2001;75:93-106. doi: 10.1016 /s0020-7292(01)00520-3.
- Curtis KM, Tepper NK, Jatlaoui TC, Berry-Bibee E, Horton LG, Zapata LB, Simmons KB, Pagano HP, Jamieson DJ, Whiteman MK. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-103. doi: 10.15585 /mmwr.rr6503a1.
- ACOG Practice Bulletin No. 206: use of hormonal contraception in women with coexisting medical conditions [published correction appears in: Obstet Gynecol. 2019;133:1288.] Obstet Gynecol. 2019;133:e128-e150. doi:10.1097/AOG.0000000000003072.
- Bofill Rodriguez M, Dias S, Jordan V, et al. Interventions for heavy menstrual bleeding; overview of Cochrane reviews and network meta-analysis. Cochrane Database Syst Rev. 2022;5:CD013180. doi: 10.1002/14651858.CD013180.pub2.
- Kaunitz AM, Bissonnette F, Monteiro I, et al. Levonorgestrelreleasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial [published correction appears in: Obstet Gynecol. 2010;116:999]. Obstet Gynecol. 2010;116:625-632. doi: 10.1097 /AOG.0b013e3181ec622b.
- Milsom I, Andersson K, Andersch B, et al. A comparison of flurbiprofen, tranexamic acid, and a levonorgestrel-releasing intrauterine contraceptive device in the treatment of idiopathic menorrhagia. Am J Obstet Gynecol. 1991;164:879883. doi: 10.1016/s0002-9378(11)90533-x.
- Creinin MD, Barnhart KT, Gawron LM, et al. Heavy menstrual bleeding treatment with a levonorgestrel 52-mg intrauterine device. Obstet Gynecol. 2023;141:971-978. doi: 10.1097 /AOG.0000000000005137.
- 1Madden T. Association of age and parity with intrauterine device expulsion. Obstet Gynecol. 2014:718-726. doi:10.1097 /aog.0000000000000475.
- Kaunitz AM, Stern L, Doyle J, et al. Use of the levonorgestrelIUD in the treatment of menorrhagia: improving patient outcomes while reducing the need for surgical management. Manag Care Interface. 2007;20:47-50.
- Getahun D, Fassett MJ, Gatz J, et al. Association between menorrhagia and risk of intrauterine device-related uterine perforation and device expulsion: results from the Association of Uterine Perforation and Expulsion of Intrauterine Device study. Am J Obstet Gynecol. 2022;227:59.e1-59.e9.
- Benacerraf BR, Shipp TD, Bromley B. Three-dimensional ultrasound detection of abnormally located intrauterine contraceptive devices that are a source of pelvic pain and abnormal bleeding. Ultrasound Obstet Gynecol. 2009;34:110115.
- Shipp TD, Bromley B, Benacerraf BR. The width of the uterine cavity is narrower in patients with an embedded intrauterine device (IUD) compared to a normally positioned IUD. J Ultrasound Med. 2010;29:1453-1456.
- Depypere H, Inki P. The levonorgestrel-releasing intrauterine system for endometrial protection during estrogen replacement therapy: a clinical review. Climacteric. 2015;18:470-482.
- Minalt N, Caldwell A, Yedlicka GM, et al. Association of intrauterine device use and endometrial, cervical, and ovarian cancer: an expert review. Am J Obstet Gynecol. 2023:S0002-9378(23)00224-7.
- Balayla J, Gil Y, Lasry A, et al. Ever-use of the intra-uterine device and the risk of ovarian cancer. J Obstet Gynaecol. 2021;41:848-853. doi: 10.1080/01443615.2020.1789960.
- Manson JE, Aragaki AK, Rossouw JE, et al. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318:927-938. doi:10.1001/jama.2017.11217.
- Chen WY, Manson JE, Hankinson SE, et al. Unopposed estrogen therapy and the risk of invasive breast cancer. Arch Intern Med. 2006;166:1027-1032. doi: 10.1001 /archinte.166.9.1027.
- Pinkerton JV, Wilson CS, Kaunitz AM. Reassuring data regarding the use of hormone therapy at menopause and risk of breast cancer. Menopause. 2022;29:1001-1004.doi:10.1097 /GME.0000000000002057.
- Romano SS, Doll KM. The impact of fibroids and histologic subtype on the performance of US clinical guidelines for the diagnosis of endometrial cancer among Black women. Ethn Dis. 2020;30:543-552. doi: 10.18865/ed.30.4.543.
- ACOG Committee on Practice Bulletins—Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206. doi: 10.1097/AOG.0b013e318262e320.
- Backman T, Rauramo I, Jaakkola Kimmo, et al. Use of the levonorgestrel-releasing intrauterine system and breast cancer. Obstet Gynecol. 2005;106:813-817.
- Conz L, Mota BS, Bahamondes L, et al. Levonorgestrelreleasing intrauterine system and breast cancer risk: A systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2020;99:970-982.
- Al Kiyumi MH, Al Battashi K, Al-Riyami HA. Levonorgestrelreleasing intrauterine system and breast cancer. Is there an association? Acta Obstet Gynecol Scand. 2021;100:1749.
- Marsden J. Hormonal contraception and breast cancer, what more do we need to know? Post Reprod Health. 2017;23:116127. doi: 10.1177/2053369117715370.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810 doi:10.1136/bmj.k4810.
- Levitt L, Cox C, Dawson L. Q&A: implications of the ruling on the ACA’s preventive services requirement. KFF.org. https://www .kff.org/policy-watch/qa-implications-of-the-ruling-on -the-acas-preventive-services-requirement/#:~:text=On%20 March%2030%2C%202023%2C%20a,cost%2Dsharing%20 for%20their%20enrollees. Accessed May 2, 2023.