User login
Federal Health Care Data Trends 2022
Federal Health Care Data Trends (click to view the digital edition) is a special supplement to Federal Practitioner highlighting the latest research and study outcomes related to the health of veteran and active-duty populations.
In this issue:
- Vaccinations
- Mental Health and Related Disorders
- LGBTQ+ Veterans
- Military Sexual Trauma
- Sleep Disorders
- Respiratory Illnesses
- HIV Care in the VA
- Rheumatologic Diseases
- The Cancer-Obesity Connection
- Skin Health for Active-Duty Personnel
- Contraception
- Chronic Kidney Disease
- Cardiovascular Diseases
- Neurologic Disorders
- Hearing, Vision, and Balance
Federal Practitioner would like to thank the following experts for their review of content and helpful guidance in developing this issue:
Kelvin N.V. Bush, MD, FACC, CCDS; Sonya Borrero, MD, MS; Kenneth L. Cameron, PhD, MPH, ATC, FNATA; Jason DeViva, PhD; Ellen Lockard Edens, MD; Leonard E. Egede, MD, MS; Amy Justice, MD, PhD; Stephanie Knudson, MD; Willis H. Lyford, MD; Sarah O. Meadows, PhD; Tamara Schult, PhD, MPH; Eric L. Singman, MD, PhD; Art Wallace, MD, PhD; Elizabeth Waterhouse, MD, FAAN
Federal Health Care Data Trends (click to view the digital edition) is a special supplement to Federal Practitioner highlighting the latest research and study outcomes related to the health of veteran and active-duty populations.
In this issue:
- Vaccinations
- Mental Health and Related Disorders
- LGBTQ+ Veterans
- Military Sexual Trauma
- Sleep Disorders
- Respiratory Illnesses
- HIV Care in the VA
- Rheumatologic Diseases
- The Cancer-Obesity Connection
- Skin Health for Active-Duty Personnel
- Contraception
- Chronic Kidney Disease
- Cardiovascular Diseases
- Neurologic Disorders
- Hearing, Vision, and Balance
Federal Practitioner would like to thank the following experts for their review of content and helpful guidance in developing this issue:
Kelvin N.V. Bush, MD, FACC, CCDS; Sonya Borrero, MD, MS; Kenneth L. Cameron, PhD, MPH, ATC, FNATA; Jason DeViva, PhD; Ellen Lockard Edens, MD; Leonard E. Egede, MD, MS; Amy Justice, MD, PhD; Stephanie Knudson, MD; Willis H. Lyford, MD; Sarah O. Meadows, PhD; Tamara Schult, PhD, MPH; Eric L. Singman, MD, PhD; Art Wallace, MD, PhD; Elizabeth Waterhouse, MD, FAAN
Federal Health Care Data Trends (click to view the digital edition) is a special supplement to Federal Practitioner highlighting the latest research and study outcomes related to the health of veteran and active-duty populations.
In this issue:
- Vaccinations
- Mental Health and Related Disorders
- LGBTQ+ Veterans
- Military Sexual Trauma
- Sleep Disorders
- Respiratory Illnesses
- HIV Care in the VA
- Rheumatologic Diseases
- The Cancer-Obesity Connection
- Skin Health for Active-Duty Personnel
- Contraception
- Chronic Kidney Disease
- Cardiovascular Diseases
- Neurologic Disorders
- Hearing, Vision, and Balance
Federal Practitioner would like to thank the following experts for their review of content and helpful guidance in developing this issue:
Kelvin N.V. Bush, MD, FACC, CCDS; Sonya Borrero, MD, MS; Kenneth L. Cameron, PhD, MPH, ATC, FNATA; Jason DeViva, PhD; Ellen Lockard Edens, MD; Leonard E. Egede, MD, MS; Amy Justice, MD, PhD; Stephanie Knudson, MD; Willis H. Lyford, MD; Sarah O. Meadows, PhD; Tamara Schult, PhD, MPH; Eric L. Singman, MD, PhD; Art Wallace, MD, PhD; Elizabeth Waterhouse, MD, FAAN
In California, abortion could become a constitutional right. So could birth control.
SACRAMENTO, CALIF. –
If they vote “yes” on Proposition 1, they will also lock in a right that has gotten less attention: The right to birth control.
Should the measure succeed, California would become one of the first states – if not the first – to create explicit constitutional rights to both abortion and contraception.
The lawmakers and activists behind the constitutional amendment said they hope to score a one-two punch: Protect abortion in California after the U.S. Supreme Court ended the federal constitutional right to abortion under Roe v. Wade, and get ahead of what they see as the next front in the reproductive rights fight: Birth control.
“The United States Supreme Court said that the privacy and liberty protections in the United States Constitution did not extend to abortion,” said UCLA law professor Cary Franklin, an expert in constitutional law and reproductive rights who has testified before the California legislature in support of the amendment. “If they said ‘no’ on abortion, they’re probably going to say ‘no’ on birth control because that has a similar history.”
In June, the U.S. Supreme Court’s decision in Dobbs v. Jackson Women’s Health Organization ended the federal right to abortion and left states to regulate the service. In his concurring opinion, Justice Clarence Thomas said the court should revisit other cases that have created protections for Americans based on an implicit right to privacy in the U.S. Constitution, such as the 1965 case Griswold v. Connecticut, which established a federal right to contraception for married people, and which was later extended to unmarried people.
Some congressional Democrats are now trying to codify the right to contraception in federal law. In July, the U.S. House of Representatives passed the Right to Contraception Act, which would give patients the right to access and use contraception and providers the right to furnish it. But the bill has little chance of success in the U.S. Senate, where Republicans have already blocked it once.
Protecting access to contraception is popular with voters. A national poll from Morning Consult and Politico conducted in late July found that 75% of registered voters support a federal law that protects a right to birth control access.
California isn’t the only state where voters are considering reproductive rights in their constitutions.
On Aug. 2, Kansas voters decisively rejected a constitutional amendment that would have allowed state lawmakers to ban or dramatically restrict abortion. It failed by nearly 18 percentage points.
Kentucky voters will face a similar decision in November with a proposed constitutional amendment that would declare that the state’s constitutional right to privacy does not cover abortion.
Vermont is going in the opposite direction. Voters there will weigh a ballot measure in November that would add a right to “personal reproductive autonomy” to the state constitution, though it does not explicitly mention abortion or contraception. In Michigan, a proposed constitutional amendment that would guarantee a right to both abortion and contraception is expected to qualify for the November ballot.
In California, Proposition 1 would prevent the state from denying or interfering with “an individual’s reproductive freedom in their most intimate decisions, which includes their fundamental right to choose to have an abortion and their fundamental right to choose or refuse contraceptives.”
The proposed constitutional amendment doesn’t go into detail about what enshrining the right to contraception in the state constitution would mean.
California already has some of the strongest contraceptive-access laws in the country – and lawmakers are considering more proposals this year. For instance, state-regulated health plans must cover all Food and Drug Administration–approved contraception; pharmacists must dispense emergency contraception to anyone with a prescription, regardless of age; and pharmacists can prescribe birth control pills on the spot. State courts have also interpreted California’s constitution to include a right to privacy that covers reproductive health decisions.
The amendment, if adopted, could provide a new legal pathway for people to sue when they’re denied contraceptives, said Michele Goodwin, chancellor’s professor of law at the University of California, Irvine.
If a pharmacist refused to fill a birth control prescription or a cashier declined to ring up condoms, she said, customers could make a case that their rights had been violated.
Making the rights to abortion and contraception explicit in the state constitution – instead of relying on a right to privacy – would also protect against shifting political winds, said state Senate leader Toni Atkins (D–San Diego), who was the director of a women’s health clinic in the 1980s. Although California’s lawmakers and executive officers are solid supporters of abortion rights, she said, the composition of the legislature and courts’ interpretation of laws could change.
“I want to know for sure that that right is protected,” Ms. Atkins said at a legislative hearing in June. “We are protecting ourselves from future courts and future politicians.”
The amendment would solidify California’s role as a reproductive rights sanctuary as much of the country chips away at birth control availability, Ms. Goodwin added.
Experts said two forms of birth control that are vulnerable to restrictions in other states are intrauterine devices, or IUDs, and emergency contraception such as Plan B. These methods are often incorrectly conflated with abortion pills, which end a pregnancy instead of preventing it.
Nine states have laws that restrict emergency contraception – for example, by allowing pharmacies to refuse to dispense it or excluding it from state family planning programs – according to the Guttmacher Institute, a research organization that supports abortion rights. In Alabama and Louisiana this year, abortion opponents introduced legislation that would restrict or ban abortion, and would also apply to emergency contraception.
“We’re seeing an erosion of abortion access that is playing out in statehouses across the country that have and will continue to target contraceptive care as well,” said Audrey Sandusky, senior director of policy and communications for the National Family Planning and Reproductive Health Association.
Susan Arnall, vice president of California’s Right to Life League, said the proposed amendment is symbolic and merely echoes current laws. Ms. Arnall said the campaign is mostly about Democratic politicians trying to score political points.
“It just allows the pro-abort legislators to trumpet and give them talking points about how they’re doing something about the overturn of Roe v. Wade,” she said. “It is political virtue signaling. I don’t think it does much of anything else.”
Ms. Goodwin argues that the measure’s symbolism is significant and overdue. She pointed to the Civil War era, when enslaved people in Southern states could look to free states for spiritual hope and material help. “Symbolically, what that meant is a kind of beacon of hope, that those places did exist, where one’s humanity could be regarded,” Ms. Goodwin said.
But California’s reputation as a haven for contraceptive availability may not be fully warranted, said Dima Qato, PharmD, PhD, an associate professor at the University of Southern California School of Pharmacy. In her 2020 study of contraceptive access in Los Angeles County, which has some of the highest rates of teen and unintended pregnancy in the country, Dr. Qato found that only 10% of pharmacies surveyed offered pharmacist-prescribed birth control. Pharmacies in low-income and minority communities were the least likely to offer the service, Dr. Qato said, worsening disparities instead of solving them.
Dr. Qato supports the constitutional amendment but said California should focus on improving and enforcing the laws it already has.
“We don’t need more laws when we don’t address the root cause of a lack of effectiveness of these laws in these communities,” she said. “Lack of enforcement and accountability disproportionately impacts communities of color.”
This story was produced by KHN, which publishes California Healthline, an editorially independent service of the California Health Care Foundation. Rachel Bluth is a correspondent for California Healthline. KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
SACRAMENTO, CALIF. –
If they vote “yes” on Proposition 1, they will also lock in a right that has gotten less attention: The right to birth control.
Should the measure succeed, California would become one of the first states – if not the first – to create explicit constitutional rights to both abortion and contraception.
The lawmakers and activists behind the constitutional amendment said they hope to score a one-two punch: Protect abortion in California after the U.S. Supreme Court ended the federal constitutional right to abortion under Roe v. Wade, and get ahead of what they see as the next front in the reproductive rights fight: Birth control.
“The United States Supreme Court said that the privacy and liberty protections in the United States Constitution did not extend to abortion,” said UCLA law professor Cary Franklin, an expert in constitutional law and reproductive rights who has testified before the California legislature in support of the amendment. “If they said ‘no’ on abortion, they’re probably going to say ‘no’ on birth control because that has a similar history.”
In June, the U.S. Supreme Court’s decision in Dobbs v. Jackson Women’s Health Organization ended the federal right to abortion and left states to regulate the service. In his concurring opinion, Justice Clarence Thomas said the court should revisit other cases that have created protections for Americans based on an implicit right to privacy in the U.S. Constitution, such as the 1965 case Griswold v. Connecticut, which established a federal right to contraception for married people, and which was later extended to unmarried people.
Some congressional Democrats are now trying to codify the right to contraception in federal law. In July, the U.S. House of Representatives passed the Right to Contraception Act, which would give patients the right to access and use contraception and providers the right to furnish it. But the bill has little chance of success in the U.S. Senate, where Republicans have already blocked it once.
Protecting access to contraception is popular with voters. A national poll from Morning Consult and Politico conducted in late July found that 75% of registered voters support a federal law that protects a right to birth control access.
California isn’t the only state where voters are considering reproductive rights in their constitutions.
On Aug. 2, Kansas voters decisively rejected a constitutional amendment that would have allowed state lawmakers to ban or dramatically restrict abortion. It failed by nearly 18 percentage points.
Kentucky voters will face a similar decision in November with a proposed constitutional amendment that would declare that the state’s constitutional right to privacy does not cover abortion.
Vermont is going in the opposite direction. Voters there will weigh a ballot measure in November that would add a right to “personal reproductive autonomy” to the state constitution, though it does not explicitly mention abortion or contraception. In Michigan, a proposed constitutional amendment that would guarantee a right to both abortion and contraception is expected to qualify for the November ballot.
In California, Proposition 1 would prevent the state from denying or interfering with “an individual’s reproductive freedom in their most intimate decisions, which includes their fundamental right to choose to have an abortion and their fundamental right to choose or refuse contraceptives.”
The proposed constitutional amendment doesn’t go into detail about what enshrining the right to contraception in the state constitution would mean.
California already has some of the strongest contraceptive-access laws in the country – and lawmakers are considering more proposals this year. For instance, state-regulated health plans must cover all Food and Drug Administration–approved contraception; pharmacists must dispense emergency contraception to anyone with a prescription, regardless of age; and pharmacists can prescribe birth control pills on the spot. State courts have also interpreted California’s constitution to include a right to privacy that covers reproductive health decisions.
The amendment, if adopted, could provide a new legal pathway for people to sue when they’re denied contraceptives, said Michele Goodwin, chancellor’s professor of law at the University of California, Irvine.
If a pharmacist refused to fill a birth control prescription or a cashier declined to ring up condoms, she said, customers could make a case that their rights had been violated.
Making the rights to abortion and contraception explicit in the state constitution – instead of relying on a right to privacy – would also protect against shifting political winds, said state Senate leader Toni Atkins (D–San Diego), who was the director of a women’s health clinic in the 1980s. Although California’s lawmakers and executive officers are solid supporters of abortion rights, she said, the composition of the legislature and courts’ interpretation of laws could change.
“I want to know for sure that that right is protected,” Ms. Atkins said at a legislative hearing in June. “We are protecting ourselves from future courts and future politicians.”
The amendment would solidify California’s role as a reproductive rights sanctuary as much of the country chips away at birth control availability, Ms. Goodwin added.
Experts said two forms of birth control that are vulnerable to restrictions in other states are intrauterine devices, or IUDs, and emergency contraception such as Plan B. These methods are often incorrectly conflated with abortion pills, which end a pregnancy instead of preventing it.
Nine states have laws that restrict emergency contraception – for example, by allowing pharmacies to refuse to dispense it or excluding it from state family planning programs – according to the Guttmacher Institute, a research organization that supports abortion rights. In Alabama and Louisiana this year, abortion opponents introduced legislation that would restrict or ban abortion, and would also apply to emergency contraception.
“We’re seeing an erosion of abortion access that is playing out in statehouses across the country that have and will continue to target contraceptive care as well,” said Audrey Sandusky, senior director of policy and communications for the National Family Planning and Reproductive Health Association.
Susan Arnall, vice president of California’s Right to Life League, said the proposed amendment is symbolic and merely echoes current laws. Ms. Arnall said the campaign is mostly about Democratic politicians trying to score political points.
“It just allows the pro-abort legislators to trumpet and give them talking points about how they’re doing something about the overturn of Roe v. Wade,” she said. “It is political virtue signaling. I don’t think it does much of anything else.”
Ms. Goodwin argues that the measure’s symbolism is significant and overdue. She pointed to the Civil War era, when enslaved people in Southern states could look to free states for spiritual hope and material help. “Symbolically, what that meant is a kind of beacon of hope, that those places did exist, where one’s humanity could be regarded,” Ms. Goodwin said.
But California’s reputation as a haven for contraceptive availability may not be fully warranted, said Dima Qato, PharmD, PhD, an associate professor at the University of Southern California School of Pharmacy. In her 2020 study of contraceptive access in Los Angeles County, which has some of the highest rates of teen and unintended pregnancy in the country, Dr. Qato found that only 10% of pharmacies surveyed offered pharmacist-prescribed birth control. Pharmacies in low-income and minority communities were the least likely to offer the service, Dr. Qato said, worsening disparities instead of solving them.
Dr. Qato supports the constitutional amendment but said California should focus on improving and enforcing the laws it already has.
“We don’t need more laws when we don’t address the root cause of a lack of effectiveness of these laws in these communities,” she said. “Lack of enforcement and accountability disproportionately impacts communities of color.”
This story was produced by KHN, which publishes California Healthline, an editorially independent service of the California Health Care Foundation. Rachel Bluth is a correspondent for California Healthline. KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
SACRAMENTO, CALIF. –
If they vote “yes” on Proposition 1, they will also lock in a right that has gotten less attention: The right to birth control.
Should the measure succeed, California would become one of the first states – if not the first – to create explicit constitutional rights to both abortion and contraception.
The lawmakers and activists behind the constitutional amendment said they hope to score a one-two punch: Protect abortion in California after the U.S. Supreme Court ended the federal constitutional right to abortion under Roe v. Wade, and get ahead of what they see as the next front in the reproductive rights fight: Birth control.
“The United States Supreme Court said that the privacy and liberty protections in the United States Constitution did not extend to abortion,” said UCLA law professor Cary Franklin, an expert in constitutional law and reproductive rights who has testified before the California legislature in support of the amendment. “If they said ‘no’ on abortion, they’re probably going to say ‘no’ on birth control because that has a similar history.”
In June, the U.S. Supreme Court’s decision in Dobbs v. Jackson Women’s Health Organization ended the federal right to abortion and left states to regulate the service. In his concurring opinion, Justice Clarence Thomas said the court should revisit other cases that have created protections for Americans based on an implicit right to privacy in the U.S. Constitution, such as the 1965 case Griswold v. Connecticut, which established a federal right to contraception for married people, and which was later extended to unmarried people.
Some congressional Democrats are now trying to codify the right to contraception in federal law. In July, the U.S. House of Representatives passed the Right to Contraception Act, which would give patients the right to access and use contraception and providers the right to furnish it. But the bill has little chance of success in the U.S. Senate, where Republicans have already blocked it once.
Protecting access to contraception is popular with voters. A national poll from Morning Consult and Politico conducted in late July found that 75% of registered voters support a federal law that protects a right to birth control access.
California isn’t the only state where voters are considering reproductive rights in their constitutions.
On Aug. 2, Kansas voters decisively rejected a constitutional amendment that would have allowed state lawmakers to ban or dramatically restrict abortion. It failed by nearly 18 percentage points.
Kentucky voters will face a similar decision in November with a proposed constitutional amendment that would declare that the state’s constitutional right to privacy does not cover abortion.
Vermont is going in the opposite direction. Voters there will weigh a ballot measure in November that would add a right to “personal reproductive autonomy” to the state constitution, though it does not explicitly mention abortion or contraception. In Michigan, a proposed constitutional amendment that would guarantee a right to both abortion and contraception is expected to qualify for the November ballot.
In California, Proposition 1 would prevent the state from denying or interfering with “an individual’s reproductive freedom in their most intimate decisions, which includes their fundamental right to choose to have an abortion and their fundamental right to choose or refuse contraceptives.”
The proposed constitutional amendment doesn’t go into detail about what enshrining the right to contraception in the state constitution would mean.
California already has some of the strongest contraceptive-access laws in the country – and lawmakers are considering more proposals this year. For instance, state-regulated health plans must cover all Food and Drug Administration–approved contraception; pharmacists must dispense emergency contraception to anyone with a prescription, regardless of age; and pharmacists can prescribe birth control pills on the spot. State courts have also interpreted California’s constitution to include a right to privacy that covers reproductive health decisions.
The amendment, if adopted, could provide a new legal pathway for people to sue when they’re denied contraceptives, said Michele Goodwin, chancellor’s professor of law at the University of California, Irvine.
If a pharmacist refused to fill a birth control prescription or a cashier declined to ring up condoms, she said, customers could make a case that their rights had been violated.
Making the rights to abortion and contraception explicit in the state constitution – instead of relying on a right to privacy – would also protect against shifting political winds, said state Senate leader Toni Atkins (D–San Diego), who was the director of a women’s health clinic in the 1980s. Although California’s lawmakers and executive officers are solid supporters of abortion rights, she said, the composition of the legislature and courts’ interpretation of laws could change.
“I want to know for sure that that right is protected,” Ms. Atkins said at a legislative hearing in June. “We are protecting ourselves from future courts and future politicians.”
The amendment would solidify California’s role as a reproductive rights sanctuary as much of the country chips away at birth control availability, Ms. Goodwin added.
Experts said two forms of birth control that are vulnerable to restrictions in other states are intrauterine devices, or IUDs, and emergency contraception such as Plan B. These methods are often incorrectly conflated with abortion pills, which end a pregnancy instead of preventing it.
Nine states have laws that restrict emergency contraception – for example, by allowing pharmacies to refuse to dispense it or excluding it from state family planning programs – according to the Guttmacher Institute, a research organization that supports abortion rights. In Alabama and Louisiana this year, abortion opponents introduced legislation that would restrict or ban abortion, and would also apply to emergency contraception.
“We’re seeing an erosion of abortion access that is playing out in statehouses across the country that have and will continue to target contraceptive care as well,” said Audrey Sandusky, senior director of policy and communications for the National Family Planning and Reproductive Health Association.
Susan Arnall, vice president of California’s Right to Life League, said the proposed amendment is symbolic and merely echoes current laws. Ms. Arnall said the campaign is mostly about Democratic politicians trying to score political points.
“It just allows the pro-abort legislators to trumpet and give them talking points about how they’re doing something about the overturn of Roe v. Wade,” she said. “It is political virtue signaling. I don’t think it does much of anything else.”
Ms. Goodwin argues that the measure’s symbolism is significant and overdue. She pointed to the Civil War era, when enslaved people in Southern states could look to free states for spiritual hope and material help. “Symbolically, what that meant is a kind of beacon of hope, that those places did exist, where one’s humanity could be regarded,” Ms. Goodwin said.
But California’s reputation as a haven for contraceptive availability may not be fully warranted, said Dima Qato, PharmD, PhD, an associate professor at the University of Southern California School of Pharmacy. In her 2020 study of contraceptive access in Los Angeles County, which has some of the highest rates of teen and unintended pregnancy in the country, Dr. Qato found that only 10% of pharmacies surveyed offered pharmacist-prescribed birth control. Pharmacies in low-income and minority communities were the least likely to offer the service, Dr. Qato said, worsening disparities instead of solving them.
Dr. Qato supports the constitutional amendment but said California should focus on improving and enforcing the laws it already has.
“We don’t need more laws when we don’t address the root cause of a lack of effectiveness of these laws in these communities,” she said. “Lack of enforcement and accountability disproportionately impacts communities of color.”
This story was produced by KHN, which publishes California Healthline, an editorially independent service of the California Health Care Foundation. Rachel Bluth is a correspondent for California Healthline. KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Best practices for evaluating pelvic pain in patients with Essure tubal occlusion devices
The evaluation and management of chronic pelvic pain in patients with a history of Essure device (Bayer HealthCare Pharmaceuticals Inc, Whippany, New Jersey) insertion have posed many challenges for both clinicians and patients. The availability of high-quality, evidence-based clinical guidance has been limited. We have reviewed the currently available published data, and here provide an overview of takeaways, as well as share our perspective and approach on evaluating and managing chronic pelvic pain in this unique patient population.
The device
The Essure microinsert is a hysteroscopically placed device that facilitates permanent sterilization by occluding the bilateral proximal fallopian tubes. The microinsert has an inner and outer nitinol coil that attaches the device to the proximal fallopian tube to ensure retention. The inner coil releases polyethylene terephthalate fibers that cause tubal fiber proliferation to occlude the lumen of the fallopian tube and achieve sterilization.
The device was first approved by the US Food and Drug Administration (FDA) in 2002. In subsequent years, the device was well received and widely used, with approximately 750,000 women worldwide undergoing Essure placement.1,2 Shortly after approval, many adverse events (AEs), including pelvic pain and abnormal uterine bleeding, were reported, resulting in a public meeting of the FDA Obstetrics and Gynecology Devices Panel in September 2015. A postmarket surveillance study on the device ensued to assess complication rates including unplanned pregnancy, pelvic pain, and surgery for removal. In February 2016, the FDA issued a black box warning and a patient decision checklist.3,4 In December 2018, Bayer stopped selling and distributing Essure in the United States.5 A 4-year follow-up surveillance study on Essure was submitted to the FDA in March 2020.
Adverse outcomes
Common AEs related to the Essure device include heavy uterine bleeding, pelvic pain, and other quality-of-life symptoms such as fatigue and weight gain.6-8 The main safety endpoints for the mandated FDA postmarket 522 surveillance studies were chronic lower abdominal and pelvic pain; abnormal uterine bleeding; hypersensitivity; allergic reaction, as well as autoimmune disorders incorporating inflammatory markers and human leukocyte antigen; and gynecologic surgery for device removal.9 Postmarket surveillence has shown that most AEs are related to placement complications or pelvic pain after Essure insertion. However, there have been several reports of autoimmune diseases categorized as serious AEs, such as new-onset systemic lupus erythematosus, rheumatoid arthritis, and worsening ulcerative colitis, after Essure insertion.5
Evaluation of symptoms
Prevalence of pelvic pain following device placement
We conducted a PubMed and MEDLINE search from January 2000 to May 2020, which identified 43 studies citing AEs related to device placement, including pelvic or abdominal pain, abnormal uterine bleeding, hypersensitivity, and autoimmune disorders. A particularly debilitating and frequently cited AE was new-onset pelvic pain or worsening of preexisting pelvic pain. Perforation of the uterus or fallopian tube, resulting in displacement of the device into the peritoneal cavity, or fragmentation of the microinsert was reported as a serious AE that occurred after device placement. However, due to the complexity of chronic pelvic pain pathogenesis, the effect of the insert on patients with existing chronic pelvic pain remains unknown.
Authors of a large retrospective study found that approximately 2.7% of 1,430 patients developed new-onset or worsening pelvic pain after device placement. New-onset pelvic pain in 1% of patients was thought to be secondary to device placement, without a coexisting pathology or diagnosis.10
In a retrospective study by Clark and colleagues, 22 of 50 women (44%) with pelvic pain after microinsert placement were found to have at least one other cause of pelvic pain. The most common alternative diagnoses were endometriosis, adenomyosis, salpingitis, and adhesive disease. Nine of the 50 patients (18%) were found to have endometriosis upon surgical removal of the microinsert.7
Another case series examined outcomes in 29 patients undergoing laparoscopic device removal due to new-onset pelvic pain. Intraoperative findings included endometriosis in 5 patients (17.2%) and pelvic adhesions in 3 (10.3%).2 Chronic pelvic pain secondary to endometriosis may be exacerbated with Essure insertion due to discontinuation of hormonal birth control after device placement,7 and this diagnosis along with adenomyosis should be strongly considered in patients whose pelvic pain began when hormonal contraception was discontinued after placement of the device.
Continue to: Risk factors...
Risk factors
Authors of a retrospective cohort study found that patients with prior diagnosis of a chronic pain syndrome, low back pain, headaches, or fibromyalgia were 5 to 6 times more likely to report acute and chronic pain after hysteroscopic sterilization with Essure.11 Since chronic pain is often thought to be driven by a hyperalgesic state of the central nervous system, as previously shown in patients with conditions such as vulvodynia, interstitial cystitis, and fibromyalgia,12 a hyperalgesic state can potentially explain why some patients are more susceptible to developing worsening pain.
Van Limburg and colleagues conducted a retrospective cohort study with prospective follow-up on 284 women who underwent Essure sterilization. Among these patients, 48% reported negative AEs; risk factors included young age at placement, increasing gravidity, and no prior abdominal surgery.13
Onset of pain
The timing and onset of pelvic pain vary widely, suggesting there is no particular time frame for this AE after device placement.2,6,14-18 A case series by Arjona and colleagues analyzed the incidence of chronic pelvic pain in 4,274 patients after Essure sterilization. Seven patients (0.16%) reported chronic pelvic pain that necessitated device removal. In 6 of the women, the pelvic pain began within 1 week of device placement. In 3 of the 6 cases, the surgeon reported the removal procedures as “difficult.” In all 6 cases, the level of pelvic pain increased with time and was not alleviated with standard analgesic medications.6
In another case series of 26 patients, the authors evaluated patients undergoing laparoscopic removal of Essure secondary to pelvic pain and reported that the time range for symptom presentation was immediate to 85 months. Thirteen of 26 patients (50%) reported pain onset within less than 1 month of device placement, 5 of 26 patients (19.2%) reported pain between 1 and 12 months after device placement, and 8 of 26 patients (30.8%) reported pain onset more than 12 months after microinsert placement.2 In this study, 17.2% of operative reports indicated difficulty with device placement. It is unclear whether difficulty with placement was associated with development of subsequent abdominal or pelvic pain; however, the relevance of initial insertion difficulty diminished with longer follow-up.
Workup and evaluation
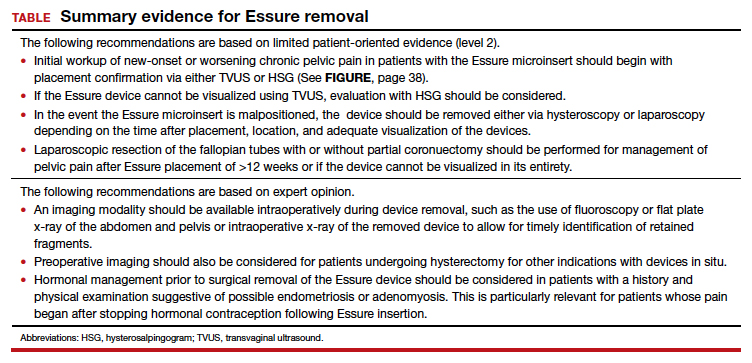
We found 5 studies that provided some framework for evaluating a patient with new-onset or worsening pelvic pain after microinsert placement. Overall, correct placement and functionality of the device should be confirmed by either hysterosalpingogram (HSG) or transvaginal ultrasonography (TVUS). The gold standard to determine tubal occlusion is the HSG. However, TVUS may be a dependable alternative, and either test can accurately demonstrate Essure location.19 Patients often prefer TVUS over HSG due to the low cost, minimal discomfort, and short examination time.1 TVUS is a noninvasive and reasonable test to start the initial assessment. The Essure devices are highly echogenic on pelvic ultrasound and easily identifiable by the proximity of the device to the uterotubal junction and its relationship with the surrounding soft tissue. If the device perforates the peritoneal cavity, then the echogenic bowel can impede adequate visualization of the Essure microinsert. If the Essure insert is not visualized on TVUS, an HSG will not only confirm placement but also test insert functionality. After confirming correct placement of the device, the provider can proceed with standard workup for chronic pelvic pain.
If one or more of the devices are malpositioned, the devices are generally presumed to be the etiology of the new pain. Multiple case reports demonstrate pain due to Essure misconfiguration or perforation with subsequent resolution of symptoms after device removal.18,20,21 A case study by Alcantara and colleagues described a patient with chronic pelvic pain and an Essure coil that was curved in an elliptical shape, not adhering to the anatomic course of the fallopian tube. The patient reported pain resolution after laparoscopic removal of the device.20 Another case report by Mahmoud et al described a subserosal malpositioned device that caused acute pelvic pain 4 months after sterilization. The patient reported resolution of pain after the microinsert was removed via laparoscopy.21 These reports highlight the importance of considering malpositioned devices as the etiology of new pelvic pain after Essure placement.
Continue to: Device removal and patient outcomes...
Device removal and patient outcomes
Removal
Several studies that we evaluated included a discussion on the methods for Essure removal. which are divided into 2 general categories: hysteroscopy and laparoscopy.
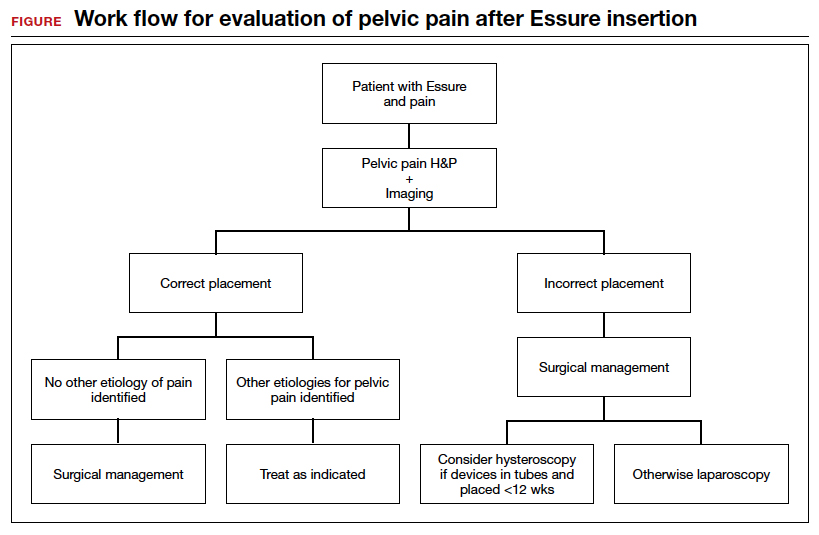
Hysteroscopic removal is generally used when the device was placed less than 12 weeks prior to removal.7,19 After 12 weeks, removal is more difficult due to fibrosis within the fallopian tubes. A risk with hysteroscopic removal is failure to remove all fibers, which allows inflammation and fibrosis to continue.7 This risk is mitigated via laparoscopic hysterectomy or mini-cornuectomy with bilateral salpingectomy, where the devices can be removed en bloc and without excessive traction.
Laparoscopic Essure removal procedures described in the literature include salpingostomy and traction on the device, salpingectomy, and salpingectomy with mini-cornuectomy. The incision and traction method is typically performed via a 2- to 3-cm incision on the antimesial edge of the fallopian tube along with a circumferential incision to surround the interstitial tubal area. The implant is carefully extracted from the fallopian tube and cornua, and a salpingectomy is then performed.22 The implant is removed prior to the salpingectomy to ensure that the Essure device is removed in its entirety prior to performing a salpingectomy.
A prospective observational study evaluated laparoscopic removal of Essure devices in 80 women with or without cornual excision. Results suggest that the incision and traction method poses more technical difficulties than the cornuectomy approach.23 Surgeons reported significant difficulty controlling the tensile pressure with traction, whereas use of the cornuectomy approach eliminated this risk and decreased the risk of fragmentation and incomplete removal.23,24
Charavil and colleagues demonstrated in a prospective observational study that a vaginal hysterectomy with bilateral salpingectomy is a feasible approach to Essure removal. Twenty-six vaginal hysterectomies with bilateral salpingectomy and Essure removal were performed without conversion to laparoscopy or laparotomy. The surgeons performed an en bloc removal of each hemiuterus along with the ipsilateral tube, which ensured complete removal of the Essure device. Each case was confirmed with an x-ray of the surgical specimen.25
If device fragmentation occurs, there are different methods recommended for locating fragments. A case report of bilateral uterine perforation after uncomplicated Essure placement used a preoperative computed tomography (CT) scan to locate the Essure fragments, but no intraoperative imaging was performed to confirm complete fragment removal.26 The patient continued reporting chronic pelvic pain and ultimately underwent exploratory laparotomy with intraoperative fluoroscopy. Using fluoroscopy, investigators identified omental fragments that were missed on preoperative CT imaging. Fluoroscopy is not commonly used intraoperatively, but it may have added benefit for localizing retained fragments.
A retrospective cohort study reviewed the use of intraoperative x-ray of the removed specimen to confirm complete Essure removal.27 If an x-ray of the removed specimen showed incomplete removal, an intraoperative pelvic x-ray was performed to locate missing fragments. X-ray of the removed devices confirmed complete removal in 63 of 72 patients (87.5%). Six of 9 women with an unsatisfactory specimen x-ray had no residual fragments identified during pelvic x-ray, and the device removal was deemed adequate. The remaining 3 women had radiologic evidence of incomplete device removal and required additional dissection for complete removal. Overall, use of x-ray or fluoroscopy is a relatively safe and accessible way to ensure complete removal of the Essure device and is worth consideration, especially when retained device fragments are suspected.
Symptom resolution
We reviewed 5 studies that examined pain outcomes after removal of the Essure devices. Casey et al found that 23 of 26 patients (88.5%) reported significant pain relief at the postoperative visit, while 3 of 26 (11.5%) reported persistent pelvic pain.2 Two of 3 case series examined other outcomes in addition to postoperative pelvic pain, including sexual function and activities of daily living.7,14 In the first case series by Brito and colleagues, 8 of 11 patients (72.7%) reported an improvement in pelvic pain, ability to perform daily activities, sexual life, and overall quality of life after Essure removal. For the remaining 3 patients with persistent pelvic pain after surgical removal of the device, 2 patients reported worsening pain symptoms and dyspareunia.14 In this study, 5 of 11 patients reported a history of chronic pelvic pain at baseline. In a retrospective case series by Clark et al, 28 of 32 women (87.5%) reported some improvement in all domains, with 24 of 32 patients (75%) reporting almost total or complete improvement in quality of life, sexual life, pelvic pain, and scores related to activities of daily living. Pain and quality-of-life scores were similar for women who underwent uterine-preserving surgery and for those who underwent hysterectomy. Ten of 32 women (31.3%) reported persistent or worsening symptoms after the Essure removal surgery. In these patients, the authors recommended consideration of other autoimmune and hypersensitivity etiologies.7
In a retrospective cohort study by Kamencic et al from 2002 to 2013 of 1,430 patients who underwent Essure placement with postplacement imaging, 62 patients (4.3%) required a second surgery after Essure placement due to pelvic pain.10 This study also found that 4 of 62 patients (0.3%) had no other obvious cause for the pelvic pain. All 4 of these women had complete resolution of their pain with removal of the Essure microinsert device. A prospective observational study by Chene e
Summary
Although Essure products were withdrawn from the market in the United States in 2018, many patients still experience significant AEs associated with the device. The goal of the perspectives and data presented here is to assist clinicians in addressing and managing the pain experienced by patients after device insertion. ●
- Connor VF. Essure: a review six years later. J Minim Invasive Gynecol. 2009;16:282-290. doi:10.1016/j.jmig.2009.02.009.
- Casey J, Aguirre F, Yunker A. Outcomes of laparoscopic removal of the Essure sterilization device for pelvic pain: a case series. Contraception. 2016;94:190-192. doi:10.1016/j.contraception.2016.03.017.
- Jackson I. Essure device removed entirely from market, with 99% of unused birth control implants retrieved: FDA. AboutLawsuits.com. January 13, 2020. https://www.aboutlawsuits.com/Essure-removal-update-166509. Accessed June 7, 2022.
- US Food and Drug Administration. Labeling for permanent hysteroscopically-placed tubal implants intended for sterilization. October 31, 2016. https://www.fda.gov/media/96315/download. Accessed June 7, 2022.
- US Food and Drug Administration. FDA activities related to Essure. March 14, 2022. https://www.fda.gov/medical-devices/essure-permanent-birth-control/fda-activities-related-essure. Accessed June 8, 2022.
- Arjona Berral JE, Rodríguez Jiménez B, Velasco Sánchez E, et al. Essure and chronic pelvic pain: a population-based cohort. J Obstet Gynaecol. 2014;34:712-713. doi:10.3109/01443615.2014.92075.
- Clark NV, Rademaker D, Mushinski AA, et al. Essure removal for the treatment of device-attributed symptoms: an expanded case series and follow-up survey. J Minim Invasive Gynecol. 2017;24:971-976. doi:10.1016/j.jmig.2017.05.015.
- Sills ES, Rickers NS, Li X. Surgical management after hysteroscopic sterilization: minimally invasive approach incorporating intraoperative fluoroscopy for symptomatic patients with >2 Essure devices. Surg Technol Int. 2018;32:156-161.
- Administration USF and D. 522 Postmarket Surveillance Studies. Center for Devices and Radiological Health; 2020.
- Kamencic H, Thiel L, Karreman E, et al. Does Essure cause significant de novo pain? A retrospective review of indications for second surgeries after Essure placement. J Minim Invasive Gynecol. 2016;23:1158-1162. doi:10.1016/j.jmig.2016.08.823.
- Yunker AC, Ritch JM, Robinson EF, et al. Incidence and risk factors for chronic pelvic pain after hysteroscopic sterilization. J Minim Invasive Gynecol. 2015;22:390-994. doi:10.1016/j.jmig.2014.06.007.
- Phillips K, Clauw DJ. Central pain mechanisms in chronic pain states--maybe it is all in their head. Best Pract Res Clin Rheumatol. 2011;25:141-154. doi:10.1016/j.berh.2011.02.005.
- van Limburg Stirum EVJ, Clark NV, Lindsey A, et al. Factors associated with negative patient experiences with Essure sterilization. JSLS. 2020;24(1):e2019.00065. doi:10.4293/JSLS.2019.00065.
- Brito LG, Cohen SL, Goggins ER, et al. Essure surgical removal and subsequent symptom resolution: case series and follow-up survey. J Minim Invasive Gynecol. 2015;22:910-913. doi:10.1016/j.jmig.2015.03.018.
- Maassen LW, van Gastel DM, Haveman I, et al. Removal of Essure sterilization devices: a retrospective cohort study in the Netherlands. J Minim Invasive Gynecol. 2019;26:1056-1062. doi:10.1016/j.jmig.2018.10.009.
- Sills ES, Palermo GD. Surgical excision of Essure devices with ESHRE class IIb uterine malformation: sequential hysteroscopic-laparoscopic approach to the septate uterus. Facts Views Vis Obgyn. 2016;8:49-52.
- Ricci G, Restaino S, Di Lorenzo G, et al. Risk of Essure microinsert abdominal migration: case report and review of literature. Ther Clin Risk Manag. 2014;10:963-968. doi:10.2147/TCRM.S65634.
- Borley J, Shabajee N, Tan TL. A kink is not always a perforation: assessing Essure hysteroscopic sterilization placement. Fertil Steril. 2011;95:2429.e15-7. doi:10.1016/j.fertnstert.2011.02.006.
- Djeffal H, Blouet M, Pizzoferato AC, et al. Imaging findings in Essure-related complications: a pictorial review.7Br J Radiol. 2018;91(1090):20170686. doi:10.1259/bjr.20170686.
- Lora Alcantara I, Rezai S, Kirby C, et al. Essure surgical removal and subsequent resolution of chronic pelvic pain: a case report and review of the literature. Case Rep Obstet Gynecol. 2016;2016:6961202. doi:10.1155/2016/6961202.
- Mahmoud MS, Fridman D, Merhi ZO. Subserosal misplacement of Essure device manifested by late-onset acute pelvic pain. Fertil Steril. 2009;92:2038.e1-3. doi:10.1016/j.fertnstert.2009.07.1677.
- Tissot M, Petry S, Lecointre L, et al. Two surgical techniques for Essure device ablation: the hysteroscopic way and the laparoscopic way by salpingectomy with tubal interstitial resection. J Minim Invasive Gynecol. 2019;26(4):603. doi:10.1016/j.jmig.2018.07.017.
- Chene G, Cerruto E, Moret S, et al. Quality of life after laparoscopic removal of Essure sterilization devices. Eur J Obstet Gynecol Reprod Biol X. 2019;3:100054. doi:10.1016/j.eurox.2019.100054.
- Thiel L, Rattray D, Thiel J. Laparoscopic cornuectomy as a technique for removal of Essure microinserts. J Minim Invasive Gynecol. 2017;24(1):10. doi:10.1016/j.jmig.2016.07.004.
- Charavil A, Agostini A, Rambeaud C, et al. Vaginal hysterectomy with salpingectomy for Essure insert removal. J Minim Invasive Gynecol. 2019;2:695-701. doi:10.1016/j.jmig.2018.07.019.
- Howard DL, Christenson PJ, Strickland JL. Use of intraoperative fluoroscopy during laparotomy to identify fragments of retained Essure microinserts: case report. J Minim Invasive Gynecol. 2012;19:667-670. doi:10.1016/j.jmig.2012.04.007.
- Miquel L, Crochet P, Francini S, et al. Laparoscopic Essure device removal by en bloc salpingectomy-cornuectomy with intraoperative x-ray checking: a retrospective cohort study. J Minim Invasive Gynecol. 2020;27:697-703. doi:10.1016/j. jmig.2019.06.006.
The evaluation and management of chronic pelvic pain in patients with a history of Essure device (Bayer HealthCare Pharmaceuticals Inc, Whippany, New Jersey) insertion have posed many challenges for both clinicians and patients. The availability of high-quality, evidence-based clinical guidance has been limited. We have reviewed the currently available published data, and here provide an overview of takeaways, as well as share our perspective and approach on evaluating and managing chronic pelvic pain in this unique patient population.
The device
The Essure microinsert is a hysteroscopically placed device that facilitates permanent sterilization by occluding the bilateral proximal fallopian tubes. The microinsert has an inner and outer nitinol coil that attaches the device to the proximal fallopian tube to ensure retention. The inner coil releases polyethylene terephthalate fibers that cause tubal fiber proliferation to occlude the lumen of the fallopian tube and achieve sterilization.
The device was first approved by the US Food and Drug Administration (FDA) in 2002. In subsequent years, the device was well received and widely used, with approximately 750,000 women worldwide undergoing Essure placement.1,2 Shortly after approval, many adverse events (AEs), including pelvic pain and abnormal uterine bleeding, were reported, resulting in a public meeting of the FDA Obstetrics and Gynecology Devices Panel in September 2015. A postmarket surveillance study on the device ensued to assess complication rates including unplanned pregnancy, pelvic pain, and surgery for removal. In February 2016, the FDA issued a black box warning and a patient decision checklist.3,4 In December 2018, Bayer stopped selling and distributing Essure in the United States.5 A 4-year follow-up surveillance study on Essure was submitted to the FDA in March 2020.
Adverse outcomes
Common AEs related to the Essure device include heavy uterine bleeding, pelvic pain, and other quality-of-life symptoms such as fatigue and weight gain.6-8 The main safety endpoints for the mandated FDA postmarket 522 surveillance studies were chronic lower abdominal and pelvic pain; abnormal uterine bleeding; hypersensitivity; allergic reaction, as well as autoimmune disorders incorporating inflammatory markers and human leukocyte antigen; and gynecologic surgery for device removal.9 Postmarket surveillence has shown that most AEs are related to placement complications or pelvic pain after Essure insertion. However, there have been several reports of autoimmune diseases categorized as serious AEs, such as new-onset systemic lupus erythematosus, rheumatoid arthritis, and worsening ulcerative colitis, after Essure insertion.5
Evaluation of symptoms
Prevalence of pelvic pain following device placement
We conducted a PubMed and MEDLINE search from January 2000 to May 2020, which identified 43 studies citing AEs related to device placement, including pelvic or abdominal pain, abnormal uterine bleeding, hypersensitivity, and autoimmune disorders. A particularly debilitating and frequently cited AE was new-onset pelvic pain or worsening of preexisting pelvic pain. Perforation of the uterus or fallopian tube, resulting in displacement of the device into the peritoneal cavity, or fragmentation of the microinsert was reported as a serious AE that occurred after device placement. However, due to the complexity of chronic pelvic pain pathogenesis, the effect of the insert on patients with existing chronic pelvic pain remains unknown.
Authors of a large retrospective study found that approximately 2.7% of 1,430 patients developed new-onset or worsening pelvic pain after device placement. New-onset pelvic pain in 1% of patients was thought to be secondary to device placement, without a coexisting pathology or diagnosis.10
In a retrospective study by Clark and colleagues, 22 of 50 women (44%) with pelvic pain after microinsert placement were found to have at least one other cause of pelvic pain. The most common alternative diagnoses were endometriosis, adenomyosis, salpingitis, and adhesive disease. Nine of the 50 patients (18%) were found to have endometriosis upon surgical removal of the microinsert.7
Another case series examined outcomes in 29 patients undergoing laparoscopic device removal due to new-onset pelvic pain. Intraoperative findings included endometriosis in 5 patients (17.2%) and pelvic adhesions in 3 (10.3%).2 Chronic pelvic pain secondary to endometriosis may be exacerbated with Essure insertion due to discontinuation of hormonal birth control after device placement,7 and this diagnosis along with adenomyosis should be strongly considered in patients whose pelvic pain began when hormonal contraception was discontinued after placement of the device.
Continue to: Risk factors...
Risk factors
Authors of a retrospective cohort study found that patients with prior diagnosis of a chronic pain syndrome, low back pain, headaches, or fibromyalgia were 5 to 6 times more likely to report acute and chronic pain after hysteroscopic sterilization with Essure.11 Since chronic pain is often thought to be driven by a hyperalgesic state of the central nervous system, as previously shown in patients with conditions such as vulvodynia, interstitial cystitis, and fibromyalgia,12 a hyperalgesic state can potentially explain why some patients are more susceptible to developing worsening pain.
Van Limburg and colleagues conducted a retrospective cohort study with prospective follow-up on 284 women who underwent Essure sterilization. Among these patients, 48% reported negative AEs; risk factors included young age at placement, increasing gravidity, and no prior abdominal surgery.13
Onset of pain
The timing and onset of pelvic pain vary widely, suggesting there is no particular time frame for this AE after device placement.2,6,14-18 A case series by Arjona and colleagues analyzed the incidence of chronic pelvic pain in 4,274 patients after Essure sterilization. Seven patients (0.16%) reported chronic pelvic pain that necessitated device removal. In 6 of the women, the pelvic pain began within 1 week of device placement. In 3 of the 6 cases, the surgeon reported the removal procedures as “difficult.” In all 6 cases, the level of pelvic pain increased with time and was not alleviated with standard analgesic medications.6
In another case series of 26 patients, the authors evaluated patients undergoing laparoscopic removal of Essure secondary to pelvic pain and reported that the time range for symptom presentation was immediate to 85 months. Thirteen of 26 patients (50%) reported pain onset within less than 1 month of device placement, 5 of 26 patients (19.2%) reported pain between 1 and 12 months after device placement, and 8 of 26 patients (30.8%) reported pain onset more than 12 months after microinsert placement.2 In this study, 17.2% of operative reports indicated difficulty with device placement. It is unclear whether difficulty with placement was associated with development of subsequent abdominal or pelvic pain; however, the relevance of initial insertion difficulty diminished with longer follow-up.
Workup and evaluation
We found 5 studies that provided some framework for evaluating a patient with new-onset or worsening pelvic pain after microinsert placement. Overall, correct placement and functionality of the device should be confirmed by either hysterosalpingogram (HSG) or transvaginal ultrasonography (TVUS). The gold standard to determine tubal occlusion is the HSG. However, TVUS may be a dependable alternative, and either test can accurately demonstrate Essure location.19 Patients often prefer TVUS over HSG due to the low cost, minimal discomfort, and short examination time.1 TVUS is a noninvasive and reasonable test to start the initial assessment. The Essure devices are highly echogenic on pelvic ultrasound and easily identifiable by the proximity of the device to the uterotubal junction and its relationship with the surrounding soft tissue. If the device perforates the peritoneal cavity, then the echogenic bowel can impede adequate visualization of the Essure microinsert. If the Essure insert is not visualized on TVUS, an HSG will not only confirm placement but also test insert functionality. After confirming correct placement of the device, the provider can proceed with standard workup for chronic pelvic pain.
If one or more of the devices are malpositioned, the devices are generally presumed to be the etiology of the new pain. Multiple case reports demonstrate pain due to Essure misconfiguration or perforation with subsequent resolution of symptoms after device removal.18,20,21 A case study by Alcantara and colleagues described a patient with chronic pelvic pain and an Essure coil that was curved in an elliptical shape, not adhering to the anatomic course of the fallopian tube. The patient reported pain resolution after laparoscopic removal of the device.20 Another case report by Mahmoud et al described a subserosal malpositioned device that caused acute pelvic pain 4 months after sterilization. The patient reported resolution of pain after the microinsert was removed via laparoscopy.21 These reports highlight the importance of considering malpositioned devices as the etiology of new pelvic pain after Essure placement.

Continue to: Device removal and patient outcomes...
Device removal and patient outcomes
Removal
Several studies that we evaluated included a discussion on the methods for Essure removal. which are divided into 2 general categories: hysteroscopy and laparoscopy.
Hysteroscopic removal is generally used when the device was placed less than 12 weeks prior to removal.7,19 After 12 weeks, removal is more difficult due to fibrosis within the fallopian tubes. A risk with hysteroscopic removal is failure to remove all fibers, which allows inflammation and fibrosis to continue.7 This risk is mitigated via laparoscopic hysterectomy or mini-cornuectomy with bilateral salpingectomy, where the devices can be removed en bloc and without excessive traction.
Laparoscopic Essure removal procedures described in the literature include salpingostomy and traction on the device, salpingectomy, and salpingectomy with mini-cornuectomy. The incision and traction method is typically performed via a 2- to 3-cm incision on the antimesial edge of the fallopian tube along with a circumferential incision to surround the interstitial tubal area. The implant is carefully extracted from the fallopian tube and cornua, and a salpingectomy is then performed.22 The implant is removed prior to the salpingectomy to ensure that the Essure device is removed in its entirety prior to performing a salpingectomy.
A prospective observational study evaluated laparoscopic removal of Essure devices in 80 women with or without cornual excision. Results suggest that the incision and traction method poses more technical difficulties than the cornuectomy approach.23 Surgeons reported significant difficulty controlling the tensile pressure with traction, whereas use of the cornuectomy approach eliminated this risk and decreased the risk of fragmentation and incomplete removal.23,24
Charavil and colleagues demonstrated in a prospective observational study that a vaginal hysterectomy with bilateral salpingectomy is a feasible approach to Essure removal. Twenty-six vaginal hysterectomies with bilateral salpingectomy and Essure removal were performed without conversion to laparoscopy or laparotomy. The surgeons performed an en bloc removal of each hemiuterus along with the ipsilateral tube, which ensured complete removal of the Essure device. Each case was confirmed with an x-ray of the surgical specimen.25
If device fragmentation occurs, there are different methods recommended for locating fragments. A case report of bilateral uterine perforation after uncomplicated Essure placement used a preoperative computed tomography (CT) scan to locate the Essure fragments, but no intraoperative imaging was performed to confirm complete fragment removal.26 The patient continued reporting chronic pelvic pain and ultimately underwent exploratory laparotomy with intraoperative fluoroscopy. Using fluoroscopy, investigators identified omental fragments that were missed on preoperative CT imaging. Fluoroscopy is not commonly used intraoperatively, but it may have added benefit for localizing retained fragments.
A retrospective cohort study reviewed the use of intraoperative x-ray of the removed specimen to confirm complete Essure removal.27 If an x-ray of the removed specimen showed incomplete removal, an intraoperative pelvic x-ray was performed to locate missing fragments. X-ray of the removed devices confirmed complete removal in 63 of 72 patients (87.5%). Six of 9 women with an unsatisfactory specimen x-ray had no residual fragments identified during pelvic x-ray, and the device removal was deemed adequate. The remaining 3 women had radiologic evidence of incomplete device removal and required additional dissection for complete removal. Overall, use of x-ray or fluoroscopy is a relatively safe and accessible way to ensure complete removal of the Essure device and is worth consideration, especially when retained device fragments are suspected.

Symptom resolution
We reviewed 5 studies that examined pain outcomes after removal of the Essure devices. Casey et al found that 23 of 26 patients (88.5%) reported significant pain relief at the postoperative visit, while 3 of 26 (11.5%) reported persistent pelvic pain.2 Two of 3 case series examined other outcomes in addition to postoperative pelvic pain, including sexual function and activities of daily living.7,14 In the first case series by Brito and colleagues, 8 of 11 patients (72.7%) reported an improvement in pelvic pain, ability to perform daily activities, sexual life, and overall quality of life after Essure removal. For the remaining 3 patients with persistent pelvic pain after surgical removal of the device, 2 patients reported worsening pain symptoms and dyspareunia.14 In this study, 5 of 11 patients reported a history of chronic pelvic pain at baseline. In a retrospective case series by Clark et al, 28 of 32 women (87.5%) reported some improvement in all domains, with 24 of 32 patients (75%) reporting almost total or complete improvement in quality of life, sexual life, pelvic pain, and scores related to activities of daily living. Pain and quality-of-life scores were similar for women who underwent uterine-preserving surgery and for those who underwent hysterectomy. Ten of 32 women (31.3%) reported persistent or worsening symptoms after the Essure removal surgery. In these patients, the authors recommended consideration of other autoimmune and hypersensitivity etiologies.7
In a retrospective cohort study by Kamencic et al from 2002 to 2013 of 1,430 patients who underwent Essure placement with postplacement imaging, 62 patients (4.3%) required a second surgery after Essure placement due to pelvic pain.10 This study also found that 4 of 62 patients (0.3%) had no other obvious cause for the pelvic pain. All 4 of these women had complete resolution of their pain with removal of the Essure microinsert device. A prospective observational study by Chene e
Summary
Although Essure products were withdrawn from the market in the United States in 2018, many patients still experience significant AEs associated with the device. The goal of the perspectives and data presented here is to assist clinicians in addressing and managing the pain experienced by patients after device insertion. ●
The evaluation and management of chronic pelvic pain in patients with a history of Essure device (Bayer HealthCare Pharmaceuticals Inc, Whippany, New Jersey) insertion have posed many challenges for both clinicians and patients. The availability of high-quality, evidence-based clinical guidance has been limited. We have reviewed the currently available published data, and here provide an overview of takeaways, as well as share our perspective and approach on evaluating and managing chronic pelvic pain in this unique patient population.
The device
The Essure microinsert is a hysteroscopically placed device that facilitates permanent sterilization by occluding the bilateral proximal fallopian tubes. The microinsert has an inner and outer nitinol coil that attaches the device to the proximal fallopian tube to ensure retention. The inner coil releases polyethylene terephthalate fibers that cause tubal fiber proliferation to occlude the lumen of the fallopian tube and achieve sterilization.
The device was first approved by the US Food and Drug Administration (FDA) in 2002. In subsequent years, the device was well received and widely used, with approximately 750,000 women worldwide undergoing Essure placement.1,2 Shortly after approval, many adverse events (AEs), including pelvic pain and abnormal uterine bleeding, were reported, resulting in a public meeting of the FDA Obstetrics and Gynecology Devices Panel in September 2015. A postmarket surveillance study on the device ensued to assess complication rates including unplanned pregnancy, pelvic pain, and surgery for removal. In February 2016, the FDA issued a black box warning and a patient decision checklist.3,4 In December 2018, Bayer stopped selling and distributing Essure in the United States.5 A 4-year follow-up surveillance study on Essure was submitted to the FDA in March 2020.
Adverse outcomes
Common AEs related to the Essure device include heavy uterine bleeding, pelvic pain, and other quality-of-life symptoms such as fatigue and weight gain.6-8 The main safety endpoints for the mandated FDA postmarket 522 surveillance studies were chronic lower abdominal and pelvic pain; abnormal uterine bleeding; hypersensitivity; allergic reaction, as well as autoimmune disorders incorporating inflammatory markers and human leukocyte antigen; and gynecologic surgery for device removal.9 Postmarket surveillence has shown that most AEs are related to placement complications or pelvic pain after Essure insertion. However, there have been several reports of autoimmune diseases categorized as serious AEs, such as new-onset systemic lupus erythematosus, rheumatoid arthritis, and worsening ulcerative colitis, after Essure insertion.5
Evaluation of symptoms
Prevalence of pelvic pain following device placement
We conducted a PubMed and MEDLINE search from January 2000 to May 2020, which identified 43 studies citing AEs related to device placement, including pelvic or abdominal pain, abnormal uterine bleeding, hypersensitivity, and autoimmune disorders. A particularly debilitating and frequently cited AE was new-onset pelvic pain or worsening of preexisting pelvic pain. Perforation of the uterus or fallopian tube, resulting in displacement of the device into the peritoneal cavity, or fragmentation of the microinsert was reported as a serious AE that occurred after device placement. However, due to the complexity of chronic pelvic pain pathogenesis, the effect of the insert on patients with existing chronic pelvic pain remains unknown.
Authors of a large retrospective study found that approximately 2.7% of 1,430 patients developed new-onset or worsening pelvic pain after device placement. New-onset pelvic pain in 1% of patients was thought to be secondary to device placement, without a coexisting pathology or diagnosis.10
In a retrospective study by Clark and colleagues, 22 of 50 women (44%) with pelvic pain after microinsert placement were found to have at least one other cause of pelvic pain. The most common alternative diagnoses were endometriosis, adenomyosis, salpingitis, and adhesive disease. Nine of the 50 patients (18%) were found to have endometriosis upon surgical removal of the microinsert.7
Another case series examined outcomes in 29 patients undergoing laparoscopic device removal due to new-onset pelvic pain. Intraoperative findings included endometriosis in 5 patients (17.2%) and pelvic adhesions in 3 (10.3%).2 Chronic pelvic pain secondary to endometriosis may be exacerbated with Essure insertion due to discontinuation of hormonal birth control after device placement,7 and this diagnosis along with adenomyosis should be strongly considered in patients whose pelvic pain began when hormonal contraception was discontinued after placement of the device.
Continue to: Risk factors...
Risk factors
Authors of a retrospective cohort study found that patients with prior diagnosis of a chronic pain syndrome, low back pain, headaches, or fibromyalgia were 5 to 6 times more likely to report acute and chronic pain after hysteroscopic sterilization with Essure.11 Since chronic pain is often thought to be driven by a hyperalgesic state of the central nervous system, as previously shown in patients with conditions such as vulvodynia, interstitial cystitis, and fibromyalgia,12 a hyperalgesic state can potentially explain why some patients are more susceptible to developing worsening pain.
Van Limburg and colleagues conducted a retrospective cohort study with prospective follow-up on 284 women who underwent Essure sterilization. Among these patients, 48% reported negative AEs; risk factors included young age at placement, increasing gravidity, and no prior abdominal surgery.13
Onset of pain
The timing and onset of pelvic pain vary widely, suggesting there is no particular time frame for this AE after device placement.2,6,14-18 A case series by Arjona and colleagues analyzed the incidence of chronic pelvic pain in 4,274 patients after Essure sterilization. Seven patients (0.16%) reported chronic pelvic pain that necessitated device removal. In 6 of the women, the pelvic pain began within 1 week of device placement. In 3 of the 6 cases, the surgeon reported the removal procedures as “difficult.” In all 6 cases, the level of pelvic pain increased with time and was not alleviated with standard analgesic medications.6
In another case series of 26 patients, the authors evaluated patients undergoing laparoscopic removal of Essure secondary to pelvic pain and reported that the time range for symptom presentation was immediate to 85 months. Thirteen of 26 patients (50%) reported pain onset within less than 1 month of device placement, 5 of 26 patients (19.2%) reported pain between 1 and 12 months after device placement, and 8 of 26 patients (30.8%) reported pain onset more than 12 months after microinsert placement.2 In this study, 17.2% of operative reports indicated difficulty with device placement. It is unclear whether difficulty with placement was associated with development of subsequent abdominal or pelvic pain; however, the relevance of initial insertion difficulty diminished with longer follow-up.
Workup and evaluation
We found 5 studies that provided some framework for evaluating a patient with new-onset or worsening pelvic pain after microinsert placement. Overall, correct placement and functionality of the device should be confirmed by either hysterosalpingogram (HSG) or transvaginal ultrasonography (TVUS). The gold standard to determine tubal occlusion is the HSG. However, TVUS may be a dependable alternative, and either test can accurately demonstrate Essure location.19 Patients often prefer TVUS over HSG due to the low cost, minimal discomfort, and short examination time.1 TVUS is a noninvasive and reasonable test to start the initial assessment. The Essure devices are highly echogenic on pelvic ultrasound and easily identifiable by the proximity of the device to the uterotubal junction and its relationship with the surrounding soft tissue. If the device perforates the peritoneal cavity, then the echogenic bowel can impede adequate visualization of the Essure microinsert. If the Essure insert is not visualized on TVUS, an HSG will not only confirm placement but also test insert functionality. After confirming correct placement of the device, the provider can proceed with standard workup for chronic pelvic pain.
If one or more of the devices are malpositioned, the devices are generally presumed to be the etiology of the new pain. Multiple case reports demonstrate pain due to Essure misconfiguration or perforation with subsequent resolution of symptoms after device removal.18,20,21 A case study by Alcantara and colleagues described a patient with chronic pelvic pain and an Essure coil that was curved in an elliptical shape, not adhering to the anatomic course of the fallopian tube. The patient reported pain resolution after laparoscopic removal of the device.20 Another case report by Mahmoud et al described a subserosal malpositioned device that caused acute pelvic pain 4 months after sterilization. The patient reported resolution of pain after the microinsert was removed via laparoscopy.21 These reports highlight the importance of considering malpositioned devices as the etiology of new pelvic pain after Essure placement.

Continue to: Device removal and patient outcomes...
Device removal and patient outcomes
Removal
Several studies that we evaluated included a discussion on the methods for Essure removal. which are divided into 2 general categories: hysteroscopy and laparoscopy.
Hysteroscopic removal is generally used when the device was placed less than 12 weeks prior to removal.7,19 After 12 weeks, removal is more difficult due to fibrosis within the fallopian tubes. A risk with hysteroscopic removal is failure to remove all fibers, which allows inflammation and fibrosis to continue.7 This risk is mitigated via laparoscopic hysterectomy or mini-cornuectomy with bilateral salpingectomy, where the devices can be removed en bloc and without excessive traction.
Laparoscopic Essure removal procedures described in the literature include salpingostomy and traction on the device, salpingectomy, and salpingectomy with mini-cornuectomy. The incision and traction method is typically performed via a 2- to 3-cm incision on the antimesial edge of the fallopian tube along with a circumferential incision to surround the interstitial tubal area. The implant is carefully extracted from the fallopian tube and cornua, and a salpingectomy is then performed.22 The implant is removed prior to the salpingectomy to ensure that the Essure device is removed in its entirety prior to performing a salpingectomy.
A prospective observational study evaluated laparoscopic removal of Essure devices in 80 women with or without cornual excision. Results suggest that the incision and traction method poses more technical difficulties than the cornuectomy approach.23 Surgeons reported significant difficulty controlling the tensile pressure with traction, whereas use of the cornuectomy approach eliminated this risk and decreased the risk of fragmentation and incomplete removal.23,24
Charavil and colleagues demonstrated in a prospective observational study that a vaginal hysterectomy with bilateral salpingectomy is a feasible approach to Essure removal. Twenty-six vaginal hysterectomies with bilateral salpingectomy and Essure removal were performed without conversion to laparoscopy or laparotomy. The surgeons performed an en bloc removal of each hemiuterus along with the ipsilateral tube, which ensured complete removal of the Essure device. Each case was confirmed with an x-ray of the surgical specimen.25
If device fragmentation occurs, there are different methods recommended for locating fragments. A case report of bilateral uterine perforation after uncomplicated Essure placement used a preoperative computed tomography (CT) scan to locate the Essure fragments, but no intraoperative imaging was performed to confirm complete fragment removal.26 The patient continued reporting chronic pelvic pain and ultimately underwent exploratory laparotomy with intraoperative fluoroscopy. Using fluoroscopy, investigators identified omental fragments that were missed on preoperative CT imaging. Fluoroscopy is not commonly used intraoperatively, but it may have added benefit for localizing retained fragments.
A retrospective cohort study reviewed the use of intraoperative x-ray of the removed specimen to confirm complete Essure removal.27 If an x-ray of the removed specimen showed incomplete removal, an intraoperative pelvic x-ray was performed to locate missing fragments. X-ray of the removed devices confirmed complete removal in 63 of 72 patients (87.5%). Six of 9 women with an unsatisfactory specimen x-ray had no residual fragments identified during pelvic x-ray, and the device removal was deemed adequate. The remaining 3 women had radiologic evidence of incomplete device removal and required additional dissection for complete removal. Overall, use of x-ray or fluoroscopy is a relatively safe and accessible way to ensure complete removal of the Essure device and is worth consideration, especially when retained device fragments are suspected.

Symptom resolution
We reviewed 5 studies that examined pain outcomes after removal of the Essure devices. Casey et al found that 23 of 26 patients (88.5%) reported significant pain relief at the postoperative visit, while 3 of 26 (11.5%) reported persistent pelvic pain.2 Two of 3 case series examined other outcomes in addition to postoperative pelvic pain, including sexual function and activities of daily living.7,14 In the first case series by Brito and colleagues, 8 of 11 patients (72.7%) reported an improvement in pelvic pain, ability to perform daily activities, sexual life, and overall quality of life after Essure removal. For the remaining 3 patients with persistent pelvic pain after surgical removal of the device, 2 patients reported worsening pain symptoms and dyspareunia.14 In this study, 5 of 11 patients reported a history of chronic pelvic pain at baseline. In a retrospective case series by Clark et al, 28 of 32 women (87.5%) reported some improvement in all domains, with 24 of 32 patients (75%) reporting almost total or complete improvement in quality of life, sexual life, pelvic pain, and scores related to activities of daily living. Pain and quality-of-life scores were similar for women who underwent uterine-preserving surgery and for those who underwent hysterectomy. Ten of 32 women (31.3%) reported persistent or worsening symptoms after the Essure removal surgery. In these patients, the authors recommended consideration of other autoimmune and hypersensitivity etiologies.7
In a retrospective cohort study by Kamencic et al from 2002 to 2013 of 1,430 patients who underwent Essure placement with postplacement imaging, 62 patients (4.3%) required a second surgery after Essure placement due to pelvic pain.10 This study also found that 4 of 62 patients (0.3%) had no other obvious cause for the pelvic pain. All 4 of these women had complete resolution of their pain with removal of the Essure microinsert device. A prospective observational study by Chene e
Summary
Although Essure products were withdrawn from the market in the United States in 2018, many patients still experience significant AEs associated with the device. The goal of the perspectives and data presented here is to assist clinicians in addressing and managing the pain experienced by patients after device insertion. ●
- Connor VF. Essure: a review six years later. J Minim Invasive Gynecol. 2009;16:282-290. doi:10.1016/j.jmig.2009.02.009.
- Casey J, Aguirre F, Yunker A. Outcomes of laparoscopic removal of the Essure sterilization device for pelvic pain: a case series. Contraception. 2016;94:190-192. doi:10.1016/j.contraception.2016.03.017.
- Jackson I. Essure device removed entirely from market, with 99% of unused birth control implants retrieved: FDA. AboutLawsuits.com. January 13, 2020. https://www.aboutlawsuits.com/Essure-removal-update-166509. Accessed June 7, 2022.
- US Food and Drug Administration. Labeling for permanent hysteroscopically-placed tubal implants intended for sterilization. October 31, 2016. https://www.fda.gov/media/96315/download. Accessed June 7, 2022.
- US Food and Drug Administration. FDA activities related to Essure. March 14, 2022. https://www.fda.gov/medical-devices/essure-permanent-birth-control/fda-activities-related-essure. Accessed June 8, 2022.
- Arjona Berral JE, Rodríguez Jiménez B, Velasco Sánchez E, et al. Essure and chronic pelvic pain: a population-based cohort. J Obstet Gynaecol. 2014;34:712-713. doi:10.3109/01443615.2014.92075.
- Clark NV, Rademaker D, Mushinski AA, et al. Essure removal for the treatment of device-attributed symptoms: an expanded case series and follow-up survey. J Minim Invasive Gynecol. 2017;24:971-976. doi:10.1016/j.jmig.2017.05.015.
- Sills ES, Rickers NS, Li X. Surgical management after hysteroscopic sterilization: minimally invasive approach incorporating intraoperative fluoroscopy for symptomatic patients with >2 Essure devices. Surg Technol Int. 2018;32:156-161.
- Administration USF and D. 522 Postmarket Surveillance Studies. Center for Devices and Radiological Health; 2020.
- Kamencic H, Thiel L, Karreman E, et al. Does Essure cause significant de novo pain? A retrospective review of indications for second surgeries after Essure placement. J Minim Invasive Gynecol. 2016;23:1158-1162. doi:10.1016/j.jmig.2016.08.823.
- Yunker AC, Ritch JM, Robinson EF, et al. Incidence and risk factors for chronic pelvic pain after hysteroscopic sterilization. J Minim Invasive Gynecol. 2015;22:390-994. doi:10.1016/j.jmig.2014.06.007.
- Phillips K, Clauw DJ. Central pain mechanisms in chronic pain states--maybe it is all in their head. Best Pract Res Clin Rheumatol. 2011;25:141-154. doi:10.1016/j.berh.2011.02.005.
- van Limburg Stirum EVJ, Clark NV, Lindsey A, et al. Factors associated with negative patient experiences with Essure sterilization. JSLS. 2020;24(1):e2019.00065. doi:10.4293/JSLS.2019.00065.
- Brito LG, Cohen SL, Goggins ER, et al. Essure surgical removal and subsequent symptom resolution: case series and follow-up survey. J Minim Invasive Gynecol. 2015;22:910-913. doi:10.1016/j.jmig.2015.03.018.
- Maassen LW, van Gastel DM, Haveman I, et al. Removal of Essure sterilization devices: a retrospective cohort study in the Netherlands. J Minim Invasive Gynecol. 2019;26:1056-1062. doi:10.1016/j.jmig.2018.10.009.
- Sills ES, Palermo GD. Surgical excision of Essure devices with ESHRE class IIb uterine malformation: sequential hysteroscopic-laparoscopic approach to the septate uterus. Facts Views Vis Obgyn. 2016;8:49-52.
- Ricci G, Restaino S, Di Lorenzo G, et al. Risk of Essure microinsert abdominal migration: case report and review of literature. Ther Clin Risk Manag. 2014;10:963-968. doi:10.2147/TCRM.S65634.
- Borley J, Shabajee N, Tan TL. A kink is not always a perforation: assessing Essure hysteroscopic sterilization placement. Fertil Steril. 2011;95:2429.e15-7. doi:10.1016/j.fertnstert.2011.02.006.
- Djeffal H, Blouet M, Pizzoferato AC, et al. Imaging findings in Essure-related complications: a pictorial review.7Br J Radiol. 2018;91(1090):20170686. doi:10.1259/bjr.20170686.
- Lora Alcantara I, Rezai S, Kirby C, et al. Essure surgical removal and subsequent resolution of chronic pelvic pain: a case report and review of the literature. Case Rep Obstet Gynecol. 2016;2016:6961202. doi:10.1155/2016/6961202.
- Mahmoud MS, Fridman D, Merhi ZO. Subserosal misplacement of Essure device manifested by late-onset acute pelvic pain. Fertil Steril. 2009;92:2038.e1-3. doi:10.1016/j.fertnstert.2009.07.1677.
- Tissot M, Petry S, Lecointre L, et al. Two surgical techniques for Essure device ablation: the hysteroscopic way and the laparoscopic way by salpingectomy with tubal interstitial resection. J Minim Invasive Gynecol. 2019;26(4):603. doi:10.1016/j.jmig.2018.07.017.
- Chene G, Cerruto E, Moret S, et al. Quality of life after laparoscopic removal of Essure sterilization devices. Eur J Obstet Gynecol Reprod Biol X. 2019;3:100054. doi:10.1016/j.eurox.2019.100054.
- Thiel L, Rattray D, Thiel J. Laparoscopic cornuectomy as a technique for removal of Essure microinserts. J Minim Invasive Gynecol. 2017;24(1):10. doi:10.1016/j.jmig.2016.07.004.
- Charavil A, Agostini A, Rambeaud C, et al. Vaginal hysterectomy with salpingectomy for Essure insert removal. J Minim Invasive Gynecol. 2019;2:695-701. doi:10.1016/j.jmig.2018.07.019.
- Howard DL, Christenson PJ, Strickland JL. Use of intraoperative fluoroscopy during laparotomy to identify fragments of retained Essure microinserts: case report. J Minim Invasive Gynecol. 2012;19:667-670. doi:10.1016/j.jmig.2012.04.007.
- Miquel L, Crochet P, Francini S, et al. Laparoscopic Essure device removal by en bloc salpingectomy-cornuectomy with intraoperative x-ray checking: a retrospective cohort study. J Minim Invasive Gynecol. 2020;27:697-703. doi:10.1016/j. jmig.2019.06.006.
- Connor VF. Essure: a review six years later. J Minim Invasive Gynecol. 2009;16:282-290. doi:10.1016/j.jmig.2009.02.009.
- Casey J, Aguirre F, Yunker A. Outcomes of laparoscopic removal of the Essure sterilization device for pelvic pain: a case series. Contraception. 2016;94:190-192. doi:10.1016/j.contraception.2016.03.017.
- Jackson I. Essure device removed entirely from market, with 99% of unused birth control implants retrieved: FDA. AboutLawsuits.com. January 13, 2020. https://www.aboutlawsuits.com/Essure-removal-update-166509. Accessed June 7, 2022.
- US Food and Drug Administration. Labeling for permanent hysteroscopically-placed tubal implants intended for sterilization. October 31, 2016. https://www.fda.gov/media/96315/download. Accessed June 7, 2022.
- US Food and Drug Administration. FDA activities related to Essure. March 14, 2022. https://www.fda.gov/medical-devices/essure-permanent-birth-control/fda-activities-related-essure. Accessed June 8, 2022.
- Arjona Berral JE, Rodríguez Jiménez B, Velasco Sánchez E, et al. Essure and chronic pelvic pain: a population-based cohort. J Obstet Gynaecol. 2014;34:712-713. doi:10.3109/01443615.2014.92075.
- Clark NV, Rademaker D, Mushinski AA, et al. Essure removal for the treatment of device-attributed symptoms: an expanded case series and follow-up survey. J Minim Invasive Gynecol. 2017;24:971-976. doi:10.1016/j.jmig.2017.05.015.
- Sills ES, Rickers NS, Li X. Surgical management after hysteroscopic sterilization: minimally invasive approach incorporating intraoperative fluoroscopy for symptomatic patients with >2 Essure devices. Surg Technol Int. 2018;32:156-161.
- Administration USF and D. 522 Postmarket Surveillance Studies. Center for Devices and Radiological Health; 2020.
- Kamencic H, Thiel L, Karreman E, et al. Does Essure cause significant de novo pain? A retrospective review of indications for second surgeries after Essure placement. J Minim Invasive Gynecol. 2016;23:1158-1162. doi:10.1016/j.jmig.2016.08.823.
- Yunker AC, Ritch JM, Robinson EF, et al. Incidence and risk factors for chronic pelvic pain after hysteroscopic sterilization. J Minim Invasive Gynecol. 2015;22:390-994. doi:10.1016/j.jmig.2014.06.007.
- Phillips K, Clauw DJ. Central pain mechanisms in chronic pain states--maybe it is all in their head. Best Pract Res Clin Rheumatol. 2011;25:141-154. doi:10.1016/j.berh.2011.02.005.
- van Limburg Stirum EVJ, Clark NV, Lindsey A, et al. Factors associated with negative patient experiences with Essure sterilization. JSLS. 2020;24(1):e2019.00065. doi:10.4293/JSLS.2019.00065.
- Brito LG, Cohen SL, Goggins ER, et al. Essure surgical removal and subsequent symptom resolution: case series and follow-up survey. J Minim Invasive Gynecol. 2015;22:910-913. doi:10.1016/j.jmig.2015.03.018.
- Maassen LW, van Gastel DM, Haveman I, et al. Removal of Essure sterilization devices: a retrospective cohort study in the Netherlands. J Minim Invasive Gynecol. 2019;26:1056-1062. doi:10.1016/j.jmig.2018.10.009.
- Sills ES, Palermo GD. Surgical excision of Essure devices with ESHRE class IIb uterine malformation: sequential hysteroscopic-laparoscopic approach to the septate uterus. Facts Views Vis Obgyn. 2016;8:49-52.
- Ricci G, Restaino S, Di Lorenzo G, et al. Risk of Essure microinsert abdominal migration: case report and review of literature. Ther Clin Risk Manag. 2014;10:963-968. doi:10.2147/TCRM.S65634.
- Borley J, Shabajee N, Tan TL. A kink is not always a perforation: assessing Essure hysteroscopic sterilization placement. Fertil Steril. 2011;95:2429.e15-7. doi:10.1016/j.fertnstert.2011.02.006.
- Djeffal H, Blouet M, Pizzoferato AC, et al. Imaging findings in Essure-related complications: a pictorial review.7Br J Radiol. 2018;91(1090):20170686. doi:10.1259/bjr.20170686.
- Lora Alcantara I, Rezai S, Kirby C, et al. Essure surgical removal and subsequent resolution of chronic pelvic pain: a case report and review of the literature. Case Rep Obstet Gynecol. 2016;2016:6961202. doi:10.1155/2016/6961202.
- Mahmoud MS, Fridman D, Merhi ZO. Subserosal misplacement of Essure device manifested by late-onset acute pelvic pain. Fertil Steril. 2009;92:2038.e1-3. doi:10.1016/j.fertnstert.2009.07.1677.
- Tissot M, Petry S, Lecointre L, et al. Two surgical techniques for Essure device ablation: the hysteroscopic way and the laparoscopic way by salpingectomy with tubal interstitial resection. J Minim Invasive Gynecol. 2019;26(4):603. doi:10.1016/j.jmig.2018.07.017.
- Chene G, Cerruto E, Moret S, et al. Quality of life after laparoscopic removal of Essure sterilization devices. Eur J Obstet Gynecol Reprod Biol X. 2019;3:100054. doi:10.1016/j.eurox.2019.100054.
- Thiel L, Rattray D, Thiel J. Laparoscopic cornuectomy as a technique for removal of Essure microinserts. J Minim Invasive Gynecol. 2017;24(1):10. doi:10.1016/j.jmig.2016.07.004.
- Charavil A, Agostini A, Rambeaud C, et al. Vaginal hysterectomy with salpingectomy for Essure insert removal. J Minim Invasive Gynecol. 2019;2:695-701. doi:10.1016/j.jmig.2018.07.019.
- Howard DL, Christenson PJ, Strickland JL. Use of intraoperative fluoroscopy during laparotomy to identify fragments of retained Essure microinserts: case report. J Minim Invasive Gynecol. 2012;19:667-670. doi:10.1016/j.jmig.2012.04.007.
- Miquel L, Crochet P, Francini S, et al. Laparoscopic Essure device removal by en bloc salpingectomy-cornuectomy with intraoperative x-ray checking: a retrospective cohort study. J Minim Invasive Gynecol. 2020;27:697-703. doi:10.1016/j. jmig.2019.06.006.
No more ‘escape hatch’: Post Roe, new worries about meds linked to birth defects
As states ban or limit abortion in the wake of the demise of Roe v. Wade, physicians are turning their attention to widely-used drugs that can cause birth defects. At issue: Should these drugs still be prescribed to women of childbearing age if they don’t have the option of terminating their pregnancies?
“Doctors are going to understandably be terrified that a patient may become pregnant using a teratogen that they have prescribed,” said University of Pittsburgh rheumatologist Mehret Birru Talabi, MD, PhD, who works in a state where the future of abortion rights is uncertain. “While this was a feared outcome before Roe v. Wade was overturned, abortion provided an escape hatch by which women could avoid having to continue a pregnancy and potentially raise a child with congenital anomalies. I believe that prescribing is going to become much more defensive and conservative. Some clinicians may choose not to prescribe these medications to patients who have childbearing potential, even if they don’t have much risk for pregnancy.”
Other physicians expressed similar concerns in interviews. Duke University, Durham, N.C., rheumatologist Megan E. B. Clowse, MD, MPH, fears that physicians will be wary of prescribing a variety of medications – including new ones for which there are few pregnancy data – if abortion is unavailable. “Women who receive these new or teratogenic medications will likely lose their reproductive autonomy and be forced to choose between having sexual relationships with men, obtaining procedures that make them permanently sterile, or using contraception that may cause intolerable side effects,” she said. “I am very concerned that young women with rheumatic disease will now be left with active disease resulting in joint damage and renal failure.”
Abortion is now banned in at least six states, according to The New York Times. That number may rise to 16 as more restrictions become law. Another five states aren’t expected to ban abortion soon but have implemented gestational age limits on abortion or are expected to adopt them. In another nine states, courts or lawmakers will decide whether abortion remains legal.
Only 20 states and the District of Columbia have firm abortion protections in place.
Numerous drugs are considered teratogens, which means they may cause birth defects. Thalidomide is the most infamous, but there are many more, including several used in rheumatology, dermatology, and gastroenterology. Among the most widely used teratogenic medications are the acne drugs isotretinoin and methotrexate, which are used to treat a variety of conditions, such as cancer, rheumatoid arthritis, and psoriasis.
Dr. Clowse, who helps manage an industry-supported website devoted to reproductive care for women with lupus (www.LupusPregnancy.org), noted that several drugs linked to birth defects and pregnancy loss are commonly prescribed in rheumatology.
“Methotrexate is the most common medication and has been the cornerstone of rheumatoid arthritis [treatment] for at least two decades,” she said. “Mycophenolate is our best medication to treat lupus nephritis, which is inflammation in the kidneys caused by lupus. This is a common complication for young women with lupus, and all of our guideline-recommended treatment regimens include a medication that causes pregnancy loss and birth defects, either mycophenolate or cyclophosphamide.”
Rheumatologists also prescribe a large number of new drugs for which there are few data about pregnancy risks. “It typically takes about two decades to have sufficient data about the safety of our medications,” she said.
Reflecting the sensitivity of the topic, Dr. Clowse made clear that her opinions don’t represent the views of her institution. She works in North Carolina, where the fate of abortion rights is uncertain, according to The New York Times.
What about alternatives? “The short answer is that some of these medications work really well and sometimes much better than the nonteratogenic alternatives,” said Dr. Birru Talabi. “I’m worried about methotrexate. It has been used to induce abortions but is primarily used in the United States as a highly effective treatment for cancer as well as a myriad of rheumatic diseases. If legislators try to restrict access to methotrexate, we may see increasing disability and even death among people who need this medication but cannot access it.”
Rheumatologists aren’t the only physicians who are worrying about the fates of their patients in a new era of abortion restrictions. Gastroenterologist Sunanda Kane, MD, MSPH, of the Mayo Clinic, Rochester, Minn., said several teratogenic medications are used in her field to treat constipation, viral hepatitis, and inflammatory bowel disease.
“When treating women of childbearing age, there are usually alternatives. If we do prescribe a medication with a high teratogenic potential, we counsel and document that we have discussed two forms of birth control to avoid pregnancy. We usually do not prescribe a drug with teratogenic potential with the ‘out’ being an abortion if a pregnancy does occur,” she said. However, “if abortion is not even on the table as an option, we may be much less likely to prescribe these medications. This will be particularly true in patients who clearly do not have the means to travel to have an abortion in any situation.”
Abortion is expected to remain legal in Minnesota, where Dr. Kane practices, but it may be restricted or banned in nearby Wisconsin, depending on the state legislature. None of her patients have had abortions after becoming pregnant while taking the medications, she said, although she “did have a patient who because of her religious faith did not have an abortion after exposure and ended up with a stillbirth.”
The crackdown on abortion won’t just pose risks to patients who take potentially dangerous medications, physicians said. Dr. Kane said pregnancy itself is a significant risk for patients with “very active, uncontrolled gastrointestinal conditions where a pregnancy could be harmful to the mother’s health or result in offspring that are very unhealthy.” These include decompensated cirrhosis, uncontrolled Crohn’s disease or ulcerative colitis, refractory gastroparesis, uncontrolled celiac sprue, and chronic pancreatitis, she said.
“There have been times when after shared decisionmaking, a patient with very active inflammatory bowel disease has decided to terminate the pregnancy because of her own ongoing health issues,” she said. “Not having this option will potentially lead to disastrous results.”
Dr. Clowse, the Duke University rheumatologist, echoed Dr. Kane’s concerns about women who are too sick to bear children. “The removal of abortion rights puts the lives and quality of life for women with rheumatic disease at risk. For patients with lupus and other systemic rheumatic disease, pregnancy can be medically catastrophic, leading to permanent harm and even death to the woman and her offspring. I am worried that women in these conditions will die without lifesaving pregnancy terminations, due to worries about the legal consequences for their physicians.”
The U.S. Supreme Court’s ruling that overturned Roe v. Wade has also raised the prospect that the court could ultimately allow birth control to be restricted or outlawed.
While the ruling states that “nothing in this opinion should be understood to cast doubt on precedents that do not concern abortion,” Justice Clarence Thomas wrote a concurrence in which he said that the court should reconsider a 1960s ruling that forbids the banning of contraceptives. Republicans have dismissed concerns about bans being allowed, although Democrats, including the president and vice president, starkly warn that they could happen.
“If we as providers have to be concerned that there will be an unplanned pregnancy because of the lack of access to contraception,” Dr. Kane said, “this will have significant downstream consequences to the kind of care we can provide and might just drive some providers to not give care to female patients at all given this concern.”
The physicians quoted in this article report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
As states ban or limit abortion in the wake of the demise of Roe v. Wade, physicians are turning their attention to widely-used drugs that can cause birth defects. At issue: Should these drugs still be prescribed to women of childbearing age if they don’t have the option of terminating their pregnancies?
“Doctors are going to understandably be terrified that a patient may become pregnant using a teratogen that they have prescribed,” said University of Pittsburgh rheumatologist Mehret Birru Talabi, MD, PhD, who works in a state where the future of abortion rights is uncertain. “While this was a feared outcome before Roe v. Wade was overturned, abortion provided an escape hatch by which women could avoid having to continue a pregnancy and potentially raise a child with congenital anomalies. I believe that prescribing is going to become much more defensive and conservative. Some clinicians may choose not to prescribe these medications to patients who have childbearing potential, even if they don’t have much risk for pregnancy.”
Other physicians expressed similar concerns in interviews. Duke University, Durham, N.C., rheumatologist Megan E. B. Clowse, MD, MPH, fears that physicians will be wary of prescribing a variety of medications – including new ones for which there are few pregnancy data – if abortion is unavailable. “Women who receive these new or teratogenic medications will likely lose their reproductive autonomy and be forced to choose between having sexual relationships with men, obtaining procedures that make them permanently sterile, or using contraception that may cause intolerable side effects,” she said. “I am very concerned that young women with rheumatic disease will now be left with active disease resulting in joint damage and renal failure.”
Abortion is now banned in at least six states, according to The New York Times. That number may rise to 16 as more restrictions become law. Another five states aren’t expected to ban abortion soon but have implemented gestational age limits on abortion or are expected to adopt them. In another nine states, courts or lawmakers will decide whether abortion remains legal.
Only 20 states and the District of Columbia have firm abortion protections in place.
Numerous drugs are considered teratogens, which means they may cause birth defects. Thalidomide is the most infamous, but there are many more, including several used in rheumatology, dermatology, and gastroenterology. Among the most widely used teratogenic medications are the acne drugs isotretinoin and methotrexate, which are used to treat a variety of conditions, such as cancer, rheumatoid arthritis, and psoriasis.
Dr. Clowse, who helps manage an industry-supported website devoted to reproductive care for women with lupus (www.LupusPregnancy.org), noted that several drugs linked to birth defects and pregnancy loss are commonly prescribed in rheumatology.
“Methotrexate is the most common medication and has been the cornerstone of rheumatoid arthritis [treatment] for at least two decades,” she said. “Mycophenolate is our best medication to treat lupus nephritis, which is inflammation in the kidneys caused by lupus. This is a common complication for young women with lupus, and all of our guideline-recommended treatment regimens include a medication that causes pregnancy loss and birth defects, either mycophenolate or cyclophosphamide.”
Rheumatologists also prescribe a large number of new drugs for which there are few data about pregnancy risks. “It typically takes about two decades to have sufficient data about the safety of our medications,” she said.
Reflecting the sensitivity of the topic, Dr. Clowse made clear that her opinions don’t represent the views of her institution. She works in North Carolina, where the fate of abortion rights is uncertain, according to The New York Times.
What about alternatives? “The short answer is that some of these medications work really well and sometimes much better than the nonteratogenic alternatives,” said Dr. Birru Talabi. “I’m worried about methotrexate. It has been used to induce abortions but is primarily used in the United States as a highly effective treatment for cancer as well as a myriad of rheumatic diseases. If legislators try to restrict access to methotrexate, we may see increasing disability and even death among people who need this medication but cannot access it.”
Rheumatologists aren’t the only physicians who are worrying about the fates of their patients in a new era of abortion restrictions. Gastroenterologist Sunanda Kane, MD, MSPH, of the Mayo Clinic, Rochester, Minn., said several teratogenic medications are used in her field to treat constipation, viral hepatitis, and inflammatory bowel disease.
“When treating women of childbearing age, there are usually alternatives. If we do prescribe a medication with a high teratogenic potential, we counsel and document that we have discussed two forms of birth control to avoid pregnancy. We usually do not prescribe a drug with teratogenic potential with the ‘out’ being an abortion if a pregnancy does occur,” she said. However, “if abortion is not even on the table as an option, we may be much less likely to prescribe these medications. This will be particularly true in patients who clearly do not have the means to travel to have an abortion in any situation.”
Abortion is expected to remain legal in Minnesota, where Dr. Kane practices, but it may be restricted or banned in nearby Wisconsin, depending on the state legislature. None of her patients have had abortions after becoming pregnant while taking the medications, she said, although she “did have a patient who because of her religious faith did not have an abortion after exposure and ended up with a stillbirth.”
The crackdown on abortion won’t just pose risks to patients who take potentially dangerous medications, physicians said. Dr. Kane said pregnancy itself is a significant risk for patients with “very active, uncontrolled gastrointestinal conditions where a pregnancy could be harmful to the mother’s health or result in offspring that are very unhealthy.” These include decompensated cirrhosis, uncontrolled Crohn’s disease or ulcerative colitis, refractory gastroparesis, uncontrolled celiac sprue, and chronic pancreatitis, she said.
“There have been times when after shared decisionmaking, a patient with very active inflammatory bowel disease has decided to terminate the pregnancy because of her own ongoing health issues,” she said. “Not having this option will potentially lead to disastrous results.”
Dr. Clowse, the Duke University rheumatologist, echoed Dr. Kane’s concerns about women who are too sick to bear children. “The removal of abortion rights puts the lives and quality of life for women with rheumatic disease at risk. For patients with lupus and other systemic rheumatic disease, pregnancy can be medically catastrophic, leading to permanent harm and even death to the woman and her offspring. I am worried that women in these conditions will die without lifesaving pregnancy terminations, due to worries about the legal consequences for their physicians.”
The U.S. Supreme Court’s ruling that overturned Roe v. Wade has also raised the prospect that the court could ultimately allow birth control to be restricted or outlawed.
While the ruling states that “nothing in this opinion should be understood to cast doubt on precedents that do not concern abortion,” Justice Clarence Thomas wrote a concurrence in which he said that the court should reconsider a 1960s ruling that forbids the banning of contraceptives. Republicans have dismissed concerns about bans being allowed, although Democrats, including the president and vice president, starkly warn that they could happen.
“If we as providers have to be concerned that there will be an unplanned pregnancy because of the lack of access to contraception,” Dr. Kane said, “this will have significant downstream consequences to the kind of care we can provide and might just drive some providers to not give care to female patients at all given this concern.”
The physicians quoted in this article report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
As states ban or limit abortion in the wake of the demise of Roe v. Wade, physicians are turning their attention to widely-used drugs that can cause birth defects. At issue: Should these drugs still be prescribed to women of childbearing age if they don’t have the option of terminating their pregnancies?
“Doctors are going to understandably be terrified that a patient may become pregnant using a teratogen that they have prescribed,” said University of Pittsburgh rheumatologist Mehret Birru Talabi, MD, PhD, who works in a state where the future of abortion rights is uncertain. “While this was a feared outcome before Roe v. Wade was overturned, abortion provided an escape hatch by which women could avoid having to continue a pregnancy and potentially raise a child with congenital anomalies. I believe that prescribing is going to become much more defensive and conservative. Some clinicians may choose not to prescribe these medications to patients who have childbearing potential, even if they don’t have much risk for pregnancy.”
Other physicians expressed similar concerns in interviews. Duke University, Durham, N.C., rheumatologist Megan E. B. Clowse, MD, MPH, fears that physicians will be wary of prescribing a variety of medications – including new ones for which there are few pregnancy data – if abortion is unavailable. “Women who receive these new or teratogenic medications will likely lose their reproductive autonomy and be forced to choose between having sexual relationships with men, obtaining procedures that make them permanently sterile, or using contraception that may cause intolerable side effects,” she said. “I am very concerned that young women with rheumatic disease will now be left with active disease resulting in joint damage and renal failure.”
Abortion is now banned in at least six states, according to The New York Times. That number may rise to 16 as more restrictions become law. Another five states aren’t expected to ban abortion soon but have implemented gestational age limits on abortion or are expected to adopt them. In another nine states, courts or lawmakers will decide whether abortion remains legal.
Only 20 states and the District of Columbia have firm abortion protections in place.
Numerous drugs are considered teratogens, which means they may cause birth defects. Thalidomide is the most infamous, but there are many more, including several used in rheumatology, dermatology, and gastroenterology. Among the most widely used teratogenic medications are the acne drugs isotretinoin and methotrexate, which are used to treat a variety of conditions, such as cancer, rheumatoid arthritis, and psoriasis.
Dr. Clowse, who helps manage an industry-supported website devoted to reproductive care for women with lupus (www.LupusPregnancy.org), noted that several drugs linked to birth defects and pregnancy loss are commonly prescribed in rheumatology.
“Methotrexate is the most common medication and has been the cornerstone of rheumatoid arthritis [treatment] for at least two decades,” she said. “Mycophenolate is our best medication to treat lupus nephritis, which is inflammation in the kidneys caused by lupus. This is a common complication for young women with lupus, and all of our guideline-recommended treatment regimens include a medication that causes pregnancy loss and birth defects, either mycophenolate or cyclophosphamide.”
Rheumatologists also prescribe a large number of new drugs for which there are few data about pregnancy risks. “It typically takes about two decades to have sufficient data about the safety of our medications,” she said.
Reflecting the sensitivity of the topic, Dr. Clowse made clear that her opinions don’t represent the views of her institution. She works in North Carolina, where the fate of abortion rights is uncertain, according to The New York Times.
What about alternatives? “The short answer is that some of these medications work really well and sometimes much better than the nonteratogenic alternatives,” said Dr. Birru Talabi. “I’m worried about methotrexate. It has been used to induce abortions but is primarily used in the United States as a highly effective treatment for cancer as well as a myriad of rheumatic diseases. If legislators try to restrict access to methotrexate, we may see increasing disability and even death among people who need this medication but cannot access it.”
Rheumatologists aren’t the only physicians who are worrying about the fates of their patients in a new era of abortion restrictions. Gastroenterologist Sunanda Kane, MD, MSPH, of the Mayo Clinic, Rochester, Minn., said several teratogenic medications are used in her field to treat constipation, viral hepatitis, and inflammatory bowel disease.
“When treating women of childbearing age, there are usually alternatives. If we do prescribe a medication with a high teratogenic potential, we counsel and document that we have discussed two forms of birth control to avoid pregnancy. We usually do not prescribe a drug with teratogenic potential with the ‘out’ being an abortion if a pregnancy does occur,” she said. However, “if abortion is not even on the table as an option, we may be much less likely to prescribe these medications. This will be particularly true in patients who clearly do not have the means to travel to have an abortion in any situation.”
Abortion is expected to remain legal in Minnesota, where Dr. Kane practices, but it may be restricted or banned in nearby Wisconsin, depending on the state legislature. None of her patients have had abortions after becoming pregnant while taking the medications, she said, although she “did have a patient who because of her religious faith did not have an abortion after exposure and ended up with a stillbirth.”
The crackdown on abortion won’t just pose risks to patients who take potentially dangerous medications, physicians said. Dr. Kane said pregnancy itself is a significant risk for patients with “very active, uncontrolled gastrointestinal conditions where a pregnancy could be harmful to the mother’s health or result in offspring that are very unhealthy.” These include decompensated cirrhosis, uncontrolled Crohn’s disease or ulcerative colitis, refractory gastroparesis, uncontrolled celiac sprue, and chronic pancreatitis, she said.
“There have been times when after shared decisionmaking, a patient with very active inflammatory bowel disease has decided to terminate the pregnancy because of her own ongoing health issues,” she said. “Not having this option will potentially lead to disastrous results.”
Dr. Clowse, the Duke University rheumatologist, echoed Dr. Kane’s concerns about women who are too sick to bear children. “The removal of abortion rights puts the lives and quality of life for women with rheumatic disease at risk. For patients with lupus and other systemic rheumatic disease, pregnancy can be medically catastrophic, leading to permanent harm and even death to the woman and her offspring. I am worried that women in these conditions will die without lifesaving pregnancy terminations, due to worries about the legal consequences for their physicians.”
The U.S. Supreme Court’s ruling that overturned Roe v. Wade has also raised the prospect that the court could ultimately allow birth control to be restricted or outlawed.
While the ruling states that “nothing in this opinion should be understood to cast doubt on precedents that do not concern abortion,” Justice Clarence Thomas wrote a concurrence in which he said that the court should reconsider a 1960s ruling that forbids the banning of contraceptives. Republicans have dismissed concerns about bans being allowed, although Democrats, including the president and vice president, starkly warn that they could happen.
“If we as providers have to be concerned that there will be an unplanned pregnancy because of the lack of access to contraception,” Dr. Kane said, “this will have significant downstream consequences to the kind of care we can provide and might just drive some providers to not give care to female patients at all given this concern.”
The physicians quoted in this article report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
IUD injury risk rises shortly after women give birth
Women for whom an intrauterine device is inserted from 4 days to 6 weeks after giving birth, as well as those who are breastfeeding, are at higher risk of the contraceptive device puncturing their uterus, new research shows.
The risk of perforation was nearly seven times higher for patients who received an IUD within that window than for those with an IUD who’d never given birth or who were more than a year out from delivery, the researchers found. Health care providers should make patients aware of the heightened risk and should monitor these patients more closely, according to Susan Reed, MD, an ob.gyn. at the University of Washington, Seattle, lead author of the new study.
“I’m a surgeon, and I like to be able to give people good information and good data about risks and benefits for their choices,” Dr. Reed told this news organization. “Uterine perforations related to IUDs are exceedingly rare, and to get good data or known risk factors, you need huge studies. This was the largest study done that really provided accurate information for patients and providers.” The new study, which appears in a recent issue of The Lancet, also found that the risk of uterine perforation was lower if an IUD had been inserted immediately after delivery.
Dr. Reed and colleagues analyzed data from the health records of 326,658 women younger than 50 years for whom an IUD was inserted between 2001 and 2018 at four health care systems. Nearly 30% of these patients received an IUD after giving birth.
The researchers identified a total of 1,008 uterine perforations, for a cumulative incidence at 5 years of 0.6%. The cumulative incidence of uterine perforations was lowest in the group of women who were considered “nonpostpartum”; these women either received an IUD a full year after giving birth or had not given birth during the study period (0.29%; 95% confidence interval, 0.26-0.34).
Women who received an IUD during the 3 days after delivery had a nearly threefold increased risk of an IUD perforation over nonpostpartum women.
In addition, the cumulative incidence of perforation was almost double among breastfeeding women, compared with women who were not breastfeeding. However, Dr. Reed and coauthors noted that breastfeeding is highly beneficial for babies and that the risk of IUD perforation is relatively small.
Among the women who received an IUD following birth, Dr. Reed’s group found that 673 uterine perforations – of which 62% were complete – occurred in breastfeeding individuals, 37% more than for those who did not breastfeed.
Dr. Reed said the study provided some clarity on previous notions that women who’d never given birth were possibly at higher risk for uterine perforation because of smaller uteruses.
“We used to be concerned that women who had never had a pregnancy at all might be at higher risk because their uterus was smaller, the cervix was tighter, and therefore perhaps they might have greater risks,” Dr. Reed said in an interview. “As a clinician and as a provider, it’s pretty exciting to me to be able to tell our younger women who have never had a pregnancy that indeed their risk is lower than anybody else’s.”
The findings help women to make informed decisions, but overall, the benefits of IUDs outweigh the risks, said Monica V. Dragoman, MD, assistant professor of obstetrics, gynecology, and reproductive science at the Icahn School of Medicine at Mount Sinai, New York.
“The likelihood of anyone experiencing these types of complications of that population level remains really low,” Dr. Dragoman said.
The findings also provide guidance for providers as to which patients should come in for additional follow-up visits following insertion, Dr. Reed said.
“These are small risks, but it does tell us where we need to consider if there’s a challenging insertion,” Dr. Reed said. “You’re going to look with the ultrasound and make sure the placement looks right. You’re going to give instructions that if the woman has pain or a change in her bleeding pattern, you want to see her back.”
Patients should be aware of the symptoms of uterine perforation – an abrupt change in bleeding pattern and pelvic pain. Perforation correction typically consists of a minimally invasive surgical procedure.
The study was conducted as a result of an order from the Food and Drug Administration to Bayer Pharmaceuticals to evaluate risks of uterine perforation for women who’d received the company’s Mirena IUD. The findings led the company to update the language on the packaging of the device so as to specify the main symptoms of uterine perforations.
The study authors received research funding from Bayer. Multiple authors are employees of Bayer. One study author has in the past received funding from CooperSurgical, Bayer Healthcare Pharmaceutical, and Merck. Bayer was provided the opportunity to review the manuscript before submission, and comments were advisory only.
A version of this article first appeared on Medscape.com.
Women for whom an intrauterine device is inserted from 4 days to 6 weeks after giving birth, as well as those who are breastfeeding, are at higher risk of the contraceptive device puncturing their uterus, new research shows.
The risk of perforation was nearly seven times higher for patients who received an IUD within that window than for those with an IUD who’d never given birth or who were more than a year out from delivery, the researchers found. Health care providers should make patients aware of the heightened risk and should monitor these patients more closely, according to Susan Reed, MD, an ob.gyn. at the University of Washington, Seattle, lead author of the new study.
“I’m a surgeon, and I like to be able to give people good information and good data about risks and benefits for their choices,” Dr. Reed told this news organization. “Uterine perforations related to IUDs are exceedingly rare, and to get good data or known risk factors, you need huge studies. This was the largest study done that really provided accurate information for patients and providers.” The new study, which appears in a recent issue of The Lancet, also found that the risk of uterine perforation was lower if an IUD had been inserted immediately after delivery.
Dr. Reed and colleagues analyzed data from the health records of 326,658 women younger than 50 years for whom an IUD was inserted between 2001 and 2018 at four health care systems. Nearly 30% of these patients received an IUD after giving birth.
The researchers identified a total of 1,008 uterine perforations, for a cumulative incidence at 5 years of 0.6%. The cumulative incidence of uterine perforations was lowest in the group of women who were considered “nonpostpartum”; these women either received an IUD a full year after giving birth or had not given birth during the study period (0.29%; 95% confidence interval, 0.26-0.34).
Women who received an IUD during the 3 days after delivery had a nearly threefold increased risk of an IUD perforation over nonpostpartum women.
In addition, the cumulative incidence of perforation was almost double among breastfeeding women, compared with women who were not breastfeeding. However, Dr. Reed and coauthors noted that breastfeeding is highly beneficial for babies and that the risk of IUD perforation is relatively small.
Among the women who received an IUD following birth, Dr. Reed’s group found that 673 uterine perforations – of which 62% were complete – occurred in breastfeeding individuals, 37% more than for those who did not breastfeed.
Dr. Reed said the study provided some clarity on previous notions that women who’d never given birth were possibly at higher risk for uterine perforation because of smaller uteruses.
“We used to be concerned that women who had never had a pregnancy at all might be at higher risk because their uterus was smaller, the cervix was tighter, and therefore perhaps they might have greater risks,” Dr. Reed said in an interview. “As a clinician and as a provider, it’s pretty exciting to me to be able to tell our younger women who have never had a pregnancy that indeed their risk is lower than anybody else’s.”
The findings help women to make informed decisions, but overall, the benefits of IUDs outweigh the risks, said Monica V. Dragoman, MD, assistant professor of obstetrics, gynecology, and reproductive science at the Icahn School of Medicine at Mount Sinai, New York.
“The likelihood of anyone experiencing these types of complications of that population level remains really low,” Dr. Dragoman said.
The findings also provide guidance for providers as to which patients should come in for additional follow-up visits following insertion, Dr. Reed said.
“These are small risks, but it does tell us where we need to consider if there’s a challenging insertion,” Dr. Reed said. “You’re going to look with the ultrasound and make sure the placement looks right. You’re going to give instructions that if the woman has pain or a change in her bleeding pattern, you want to see her back.”
Patients should be aware of the symptoms of uterine perforation – an abrupt change in bleeding pattern and pelvic pain. Perforation correction typically consists of a minimally invasive surgical procedure.
The study was conducted as a result of an order from the Food and Drug Administration to Bayer Pharmaceuticals to evaluate risks of uterine perforation for women who’d received the company’s Mirena IUD. The findings led the company to update the language on the packaging of the device so as to specify the main symptoms of uterine perforations.
The study authors received research funding from Bayer. Multiple authors are employees of Bayer. One study author has in the past received funding from CooperSurgical, Bayer Healthcare Pharmaceutical, and Merck. Bayer was provided the opportunity to review the manuscript before submission, and comments were advisory only.
A version of this article first appeared on Medscape.com.
Women for whom an intrauterine device is inserted from 4 days to 6 weeks after giving birth, as well as those who are breastfeeding, are at higher risk of the contraceptive device puncturing their uterus, new research shows.
The risk of perforation was nearly seven times higher for patients who received an IUD within that window than for those with an IUD who’d never given birth or who were more than a year out from delivery, the researchers found. Health care providers should make patients aware of the heightened risk and should monitor these patients more closely, according to Susan Reed, MD, an ob.gyn. at the University of Washington, Seattle, lead author of the new study.
“I’m a surgeon, and I like to be able to give people good information and good data about risks and benefits for their choices,” Dr. Reed told this news organization. “Uterine perforations related to IUDs are exceedingly rare, and to get good data or known risk factors, you need huge studies. This was the largest study done that really provided accurate information for patients and providers.” The new study, which appears in a recent issue of The Lancet, also found that the risk of uterine perforation was lower if an IUD had been inserted immediately after delivery.
Dr. Reed and colleagues analyzed data from the health records of 326,658 women younger than 50 years for whom an IUD was inserted between 2001 and 2018 at four health care systems. Nearly 30% of these patients received an IUD after giving birth.
The researchers identified a total of 1,008 uterine perforations, for a cumulative incidence at 5 years of 0.6%. The cumulative incidence of uterine perforations was lowest in the group of women who were considered “nonpostpartum”; these women either received an IUD a full year after giving birth or had not given birth during the study period (0.29%; 95% confidence interval, 0.26-0.34).
Women who received an IUD during the 3 days after delivery had a nearly threefold increased risk of an IUD perforation over nonpostpartum women.
In addition, the cumulative incidence of perforation was almost double among breastfeeding women, compared with women who were not breastfeeding. However, Dr. Reed and coauthors noted that breastfeeding is highly beneficial for babies and that the risk of IUD perforation is relatively small.
Among the women who received an IUD following birth, Dr. Reed’s group found that 673 uterine perforations – of which 62% were complete – occurred in breastfeeding individuals, 37% more than for those who did not breastfeed.
Dr. Reed said the study provided some clarity on previous notions that women who’d never given birth were possibly at higher risk for uterine perforation because of smaller uteruses.
“We used to be concerned that women who had never had a pregnancy at all might be at higher risk because their uterus was smaller, the cervix was tighter, and therefore perhaps they might have greater risks,” Dr. Reed said in an interview. “As a clinician and as a provider, it’s pretty exciting to me to be able to tell our younger women who have never had a pregnancy that indeed their risk is lower than anybody else’s.”
The findings help women to make informed decisions, but overall, the benefits of IUDs outweigh the risks, said Monica V. Dragoman, MD, assistant professor of obstetrics, gynecology, and reproductive science at the Icahn School of Medicine at Mount Sinai, New York.
“The likelihood of anyone experiencing these types of complications of that population level remains really low,” Dr. Dragoman said.
The findings also provide guidance for providers as to which patients should come in for additional follow-up visits following insertion, Dr. Reed said.
“These are small risks, but it does tell us where we need to consider if there’s a challenging insertion,” Dr. Reed said. “You’re going to look with the ultrasound and make sure the placement looks right. You’re going to give instructions that if the woman has pain or a change in her bleeding pattern, you want to see her back.”
Patients should be aware of the symptoms of uterine perforation – an abrupt change in bleeding pattern and pelvic pain. Perforation correction typically consists of a minimally invasive surgical procedure.
The study was conducted as a result of an order from the Food and Drug Administration to Bayer Pharmaceuticals to evaluate risks of uterine perforation for women who’d received the company’s Mirena IUD. The findings led the company to update the language on the packaging of the device so as to specify the main symptoms of uterine perforations.
The study authors received research funding from Bayer. Multiple authors are employees of Bayer. One study author has in the past received funding from CooperSurgical, Bayer Healthcare Pharmaceutical, and Merck. Bayer was provided the opportunity to review the manuscript before submission, and comments were advisory only.
A version of this article first appeared on Medscape.com.
FROM THE LANCET
Hormonal contraceptives protective against suicide?
Contrary to previous analyses, new research suggests.
In a study of more than 800 women younger than age 50 who attempted suicide and more than 3,000 age-matched peers, results showed those who took hormonal contraceptives had a 27% reduced risk for attempted suicide.
Further analysis showed this was confined to women without a history of psychiatric illness and the reduction in risk rose to 43% among those who took combined hormonal contraceptives rather than progestin-only versions.
The protective effect against attempted suicide increased further to 46% if ethinyl estradiol (EE)–containing preparations were used. Moreover, the beneficial effect of contraceptive use increased over time.
The main message is the “current use of hormonal contraceptives is not associated with an increased risk of attempted suicide in our population,” study presenter Elena Toffol, MD, PhD, department of public health, University of Helsinki, told meeting attendees at the European Psychiatric Association 2022 Congress.
Age range differences
Dr. Toffol said there could be “several reasons” why the results are different from those in previous studies, including that the researchers included a “larger age range.” She noted it is known that “older women have a lower rate of attempted suicide and use different types of contraceptives.”
Dr. Toffol said in an interview that, although it’s “hard to estimate any causality” because this is an observational study, it is “tempting to speculate, and it is plausible, that hormones partly play a role with some, but not all, women being more sensitive to hormonal influences.”
However, the results “may also reflect life choices or a protective life status; for example, more stable relationships or more conscious and health-focused behaviors,” she said.
“It may also be that the underlying characteristics of women who are prescribed or opt for certain types of contraceptives are somehow related to their suicidal risk,” she added.
In 2019, the global age-standardized suicide rate was 9.0 per 100,000, which translates into more than 700,000 deaths every year, Dr. Toffol noted.
However, she emphasized the World Health Organization has calculated that, for every adult who dies by suicide, more than 20 people attempt suicide. In addition, data from the U.S. Centers for Disease Control and Prevention indicate that attempted suicides are three times more common among young women than in men.
“What are the reasons for this gender gap?” Dr. Toffol asked during her presentation.
“It is known that the major risk factor for suicidal behavior is a psychiatric disorder, and in particular depression and mood disorders. And depression and mood disorders are more common in women than in men,” she said.
However, there is also “growing interest into the role of biological factors” in the risk for suicide, including hormones and hormonal contraception. Some studies have also suggested that there is an increased risk for depression and “both completed and attempted suicide” after starting hormonal contraception.
Dr. Toffol added that about 70% of European women use some form of contraception and, among Finnish women, 40% choose a hormonal contraceptive.
Nested analysis
The researchers conducted a nested case-control analysis combining 2017 national prescription data on 587,823 women aged 15-49 years with information from general and primary healthcare registers for the years 2018 to 2019.
They were able to identify 818 cases of attempted suicide among the women. These were matched 4:1 with 3,272 age-matched healthy women who acted as the control group. Use of hormonal contraceptives in the previous 180 days was determined for the whole cohort.
Among users of hormonal contraceptives, there were 344 attempted suicides in 2017, at an incidence rate of 0.59 per 1,000 person-years. This compared with 474 attempted suicides among nonusers, at an incidence rate of 0.81 per 1000 person-years.
Kaplan-Meier analysis showed there was a significant difference in rates for attempted suicide among hormonal contraceptive users versus nonusers, at an incidence rate ratio of 0.73 (P < .0001) – and the difference increased over time.
In addition, the incidence of attempted suicide decreased with increasing age, with the highest incidence rate in women aged 15-19 years (1.62 per 1,000 person-years).
Conditional logistic regression analysis that controlled for education, marital status, chronic disease, recent psychiatric hospitalization, and current use of psychotropic medication showed hormonal contraceptive use was not linked to an increased risk of attempted suicide overall, at an odds ratio of 0.79 (95% confidence interval, 0.56-1.11).
However, when they looked specifically at women without a history of psychiatric illness, the association became significant, at an OR of 0.73 for attempted suicide among hormonal contraceptive users (95% CI, 0.58-0.91), while the relationship remained nonsignificant in women with a history of psychiatric disorders.
Further analysis suggested the significant association was confined to women taking combined hormonal contraceptives, at an OR of 0.57 for suicide attempt versus nonusers (95% CI, 0.44-0.75), and those use EE-containing preparations (OR, 0.54; 95% CI, 0.40-0.73).
There was a suggestion in the data that hormonal contraceptives containing desogestrel or drospirenone alongside EE may offer the greatest reduction in attempted suicide risk, but that did not survive multivariate analysis.
Dr. Toffol also noted that they were not able to capture data on use of intrauterine devices in their analysis.
“There is a growing number of municipalities in Finland that are providing free-of-charge contraception to young women” that is often an intrauterine device, she said. The researchers hope to include these women in a future analysis.
‘Age matters’
Commenting on the findings, Alexis C. Edwards, PhD, Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond, said the current study’s findings “made a lot of sense.” Dr. Edwards wasn’t involved with this study but conducted a previous study of 216,702 Swedish women aged 15-22 years that showed use of combination or progestin-only oral contraceptives was associated with an increased risk for suicidal behavior.
She agreed with Dr. Toffol that the “much larger age range” in the new study may have played a role in showing the opposite result.
“The trajectory that we saw if we had been able to continue following the women for longer – which we couldn’t, due to limitations of the registries – [was that] using hormonal contraceptives was going to end up being protective, so I do think that it matters what age you’re looking at,” she said.
Dr. Edwards noted the takeaway from both studies “is that, even if there is a slight increase in risk from using hormonal contraceptives, it’s short lived and it’s probably specific to young women, which is important.”
She suggested the hormonal benefit from extended contraceptive use could come from the regulation of mood, as it offers a “more stable hormonal course than what their body might be putting them through in the absence of using the pill.”
Overall, it is “really lovely to see very well-executed studies on this, providing more empirical evidence on this question, because it is something that’s relevant to anyone who’s potentially going to be using hormonal contraception,” Dr. Edwards said.
Clinical implications?
Andrea Fiorillo, MD, PhD, department of psychiatry, University of Campania “Luigi Vanvitelli,” Naples, Italy, said in a press release that the “striking” findings of the current study need “careful evaluation.”
They also need to be replicated in “different cohorts of women and controlled for the impact of several psychosocial stressors, such as economic upheavals, social insecurity, and uncertainty due to the COVID pandemic,” said Dr. Fiorillo, who was not involved with the research.
Nevertheless, she believes the “clinical implications of the study are obvious and may help to destigmatize the use of hormonal contraceptives.”
The study was funded by the Jane and Aatos Erkko Foundation, the Avohoidon Tsukimis äätiö (Foundation for Primary Care Research), the Yrj ö Jahnsson Foundation, and the Finnish Cultural Foundation. No relevant financial relationships were reported.
A version of this article first appeared on Medscape.com.
Contrary to previous analyses, new research suggests.
In a study of more than 800 women younger than age 50 who attempted suicide and more than 3,000 age-matched peers, results showed those who took hormonal contraceptives had a 27% reduced risk for attempted suicide.
Further analysis showed this was confined to women without a history of psychiatric illness and the reduction in risk rose to 43% among those who took combined hormonal contraceptives rather than progestin-only versions.
The protective effect against attempted suicide increased further to 46% if ethinyl estradiol (EE)–containing preparations were used. Moreover, the beneficial effect of contraceptive use increased over time.
The main message is the “current use of hormonal contraceptives is not associated with an increased risk of attempted suicide in our population,” study presenter Elena Toffol, MD, PhD, department of public health, University of Helsinki, told meeting attendees at the European Psychiatric Association 2022 Congress.
Age range differences
Dr. Toffol said there could be “several reasons” why the results are different from those in previous studies, including that the researchers included a “larger age range.” She noted it is known that “older women have a lower rate of attempted suicide and use different types of contraceptives.”
Dr. Toffol said in an interview that, although it’s “hard to estimate any causality” because this is an observational study, it is “tempting to speculate, and it is plausible, that hormones partly play a role with some, but not all, women being more sensitive to hormonal influences.”
However, the results “may also reflect life choices or a protective life status; for example, more stable relationships or more conscious and health-focused behaviors,” she said.
“It may also be that the underlying characteristics of women who are prescribed or opt for certain types of contraceptives are somehow related to their suicidal risk,” she added.
In 2019, the global age-standardized suicide rate was 9.0 per 100,000, which translates into more than 700,000 deaths every year, Dr. Toffol noted.
However, she emphasized the World Health Organization has calculated that, for every adult who dies by suicide, more than 20 people attempt suicide. In addition, data from the U.S. Centers for Disease Control and Prevention indicate that attempted suicides are three times more common among young women than in men.
“What are the reasons for this gender gap?” Dr. Toffol asked during her presentation.
“It is known that the major risk factor for suicidal behavior is a psychiatric disorder, and in particular depression and mood disorders. And depression and mood disorders are more common in women than in men,” she said.
However, there is also “growing interest into the role of biological factors” in the risk for suicide, including hormones and hormonal contraception. Some studies have also suggested that there is an increased risk for depression and “both completed and attempted suicide” after starting hormonal contraception.
Dr. Toffol added that about 70% of European women use some form of contraception and, among Finnish women, 40% choose a hormonal contraceptive.
Nested analysis
The researchers conducted a nested case-control analysis combining 2017 national prescription data on 587,823 women aged 15-49 years with information from general and primary healthcare registers for the years 2018 to 2019.
They were able to identify 818 cases of attempted suicide among the women. These were matched 4:1 with 3,272 age-matched healthy women who acted as the control group. Use of hormonal contraceptives in the previous 180 days was determined for the whole cohort.
Among users of hormonal contraceptives, there were 344 attempted suicides in 2017, at an incidence rate of 0.59 per 1,000 person-years. This compared with 474 attempted suicides among nonusers, at an incidence rate of 0.81 per 1000 person-years.
Kaplan-Meier analysis showed there was a significant difference in rates for attempted suicide among hormonal contraceptive users versus nonusers, at an incidence rate ratio of 0.73 (P < .0001) – and the difference increased over time.
In addition, the incidence of attempted suicide decreased with increasing age, with the highest incidence rate in women aged 15-19 years (1.62 per 1,000 person-years).
Conditional logistic regression analysis that controlled for education, marital status, chronic disease, recent psychiatric hospitalization, and current use of psychotropic medication showed hormonal contraceptive use was not linked to an increased risk of attempted suicide overall, at an odds ratio of 0.79 (95% confidence interval, 0.56-1.11).
However, when they looked specifically at women without a history of psychiatric illness, the association became significant, at an OR of 0.73 for attempted suicide among hormonal contraceptive users (95% CI, 0.58-0.91), while the relationship remained nonsignificant in women with a history of psychiatric disorders.
Further analysis suggested the significant association was confined to women taking combined hormonal contraceptives, at an OR of 0.57 for suicide attempt versus nonusers (95% CI, 0.44-0.75), and those use EE-containing preparations (OR, 0.54; 95% CI, 0.40-0.73).
There was a suggestion in the data that hormonal contraceptives containing desogestrel or drospirenone alongside EE may offer the greatest reduction in attempted suicide risk, but that did not survive multivariate analysis.
Dr. Toffol also noted that they were not able to capture data on use of intrauterine devices in their analysis.
“There is a growing number of municipalities in Finland that are providing free-of-charge contraception to young women” that is often an intrauterine device, she said. The researchers hope to include these women in a future analysis.
‘Age matters’
Commenting on the findings, Alexis C. Edwards, PhD, Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond, said the current study’s findings “made a lot of sense.” Dr. Edwards wasn’t involved with this study but conducted a previous study of 216,702 Swedish women aged 15-22 years that showed use of combination or progestin-only oral contraceptives was associated with an increased risk for suicidal behavior.
She agreed with Dr. Toffol that the “much larger age range” in the new study may have played a role in showing the opposite result.
“The trajectory that we saw if we had been able to continue following the women for longer – which we couldn’t, due to limitations of the registries – [was that] using hormonal contraceptives was going to end up being protective, so I do think that it matters what age you’re looking at,” she said.
Dr. Edwards noted the takeaway from both studies “is that, even if there is a slight increase in risk from using hormonal contraceptives, it’s short lived and it’s probably specific to young women, which is important.”
She suggested the hormonal benefit from extended contraceptive use could come from the regulation of mood, as it offers a “more stable hormonal course than what their body might be putting them through in the absence of using the pill.”
Overall, it is “really lovely to see very well-executed studies on this, providing more empirical evidence on this question, because it is something that’s relevant to anyone who’s potentially going to be using hormonal contraception,” Dr. Edwards said.
Clinical implications?
Andrea Fiorillo, MD, PhD, department of psychiatry, University of Campania “Luigi Vanvitelli,” Naples, Italy, said in a press release that the “striking” findings of the current study need “careful evaluation.”
They also need to be replicated in “different cohorts of women and controlled for the impact of several psychosocial stressors, such as economic upheavals, social insecurity, and uncertainty due to the COVID pandemic,” said Dr. Fiorillo, who was not involved with the research.
Nevertheless, she believes the “clinical implications of the study are obvious and may help to destigmatize the use of hormonal contraceptives.”
The study was funded by the Jane and Aatos Erkko Foundation, the Avohoidon Tsukimis äätiö (Foundation for Primary Care Research), the Yrj ö Jahnsson Foundation, and the Finnish Cultural Foundation. No relevant financial relationships were reported.
A version of this article first appeared on Medscape.com.
Contrary to previous analyses, new research suggests.
In a study of more than 800 women younger than age 50 who attempted suicide and more than 3,000 age-matched peers, results showed those who took hormonal contraceptives had a 27% reduced risk for attempted suicide.
Further analysis showed this was confined to women without a history of psychiatric illness and the reduction in risk rose to 43% among those who took combined hormonal contraceptives rather than progestin-only versions.
The protective effect against attempted suicide increased further to 46% if ethinyl estradiol (EE)–containing preparations were used. Moreover, the beneficial effect of contraceptive use increased over time.
The main message is the “current use of hormonal contraceptives is not associated with an increased risk of attempted suicide in our population,” study presenter Elena Toffol, MD, PhD, department of public health, University of Helsinki, told meeting attendees at the European Psychiatric Association 2022 Congress.
Age range differences
Dr. Toffol said there could be “several reasons” why the results are different from those in previous studies, including that the researchers included a “larger age range.” She noted it is known that “older women have a lower rate of attempted suicide and use different types of contraceptives.”
Dr. Toffol said in an interview that, although it’s “hard to estimate any causality” because this is an observational study, it is “tempting to speculate, and it is plausible, that hormones partly play a role with some, but not all, women being more sensitive to hormonal influences.”
However, the results “may also reflect life choices or a protective life status; for example, more stable relationships or more conscious and health-focused behaviors,” she said.
“It may also be that the underlying characteristics of women who are prescribed or opt for certain types of contraceptives are somehow related to their suicidal risk,” she added.
In 2019, the global age-standardized suicide rate was 9.0 per 100,000, which translates into more than 700,000 deaths every year, Dr. Toffol noted.
However, she emphasized the World Health Organization has calculated that, for every adult who dies by suicide, more than 20 people attempt suicide. In addition, data from the U.S. Centers for Disease Control and Prevention indicate that attempted suicides are three times more common among young women than in men.
“What are the reasons for this gender gap?” Dr. Toffol asked during her presentation.
“It is known that the major risk factor for suicidal behavior is a psychiatric disorder, and in particular depression and mood disorders. And depression and mood disorders are more common in women than in men,” she said.
However, there is also “growing interest into the role of biological factors” in the risk for suicide, including hormones and hormonal contraception. Some studies have also suggested that there is an increased risk for depression and “both completed and attempted suicide” after starting hormonal contraception.
Dr. Toffol added that about 70% of European women use some form of contraception and, among Finnish women, 40% choose a hormonal contraceptive.
Nested analysis
The researchers conducted a nested case-control analysis combining 2017 national prescription data on 587,823 women aged 15-49 years with information from general and primary healthcare registers for the years 2018 to 2019.
They were able to identify 818 cases of attempted suicide among the women. These were matched 4:1 with 3,272 age-matched healthy women who acted as the control group. Use of hormonal contraceptives in the previous 180 days was determined for the whole cohort.
Among users of hormonal contraceptives, there were 344 attempted suicides in 2017, at an incidence rate of 0.59 per 1,000 person-years. This compared with 474 attempted suicides among nonusers, at an incidence rate of 0.81 per 1000 person-years.
Kaplan-Meier analysis showed there was a significant difference in rates for attempted suicide among hormonal contraceptive users versus nonusers, at an incidence rate ratio of 0.73 (P < .0001) – and the difference increased over time.
In addition, the incidence of attempted suicide decreased with increasing age, with the highest incidence rate in women aged 15-19 years (1.62 per 1,000 person-years).
Conditional logistic regression analysis that controlled for education, marital status, chronic disease, recent psychiatric hospitalization, and current use of psychotropic medication showed hormonal contraceptive use was not linked to an increased risk of attempted suicide overall, at an odds ratio of 0.79 (95% confidence interval, 0.56-1.11).
However, when they looked specifically at women without a history of psychiatric illness, the association became significant, at an OR of 0.73 for attempted suicide among hormonal contraceptive users (95% CI, 0.58-0.91), while the relationship remained nonsignificant in women with a history of psychiatric disorders.
Further analysis suggested the significant association was confined to women taking combined hormonal contraceptives, at an OR of 0.57 for suicide attempt versus nonusers (95% CI, 0.44-0.75), and those use EE-containing preparations (OR, 0.54; 95% CI, 0.40-0.73).
There was a suggestion in the data that hormonal contraceptives containing desogestrel or drospirenone alongside EE may offer the greatest reduction in attempted suicide risk, but that did not survive multivariate analysis.
Dr. Toffol also noted that they were not able to capture data on use of intrauterine devices in their analysis.
“There is a growing number of municipalities in Finland that are providing free-of-charge contraception to young women” that is often an intrauterine device, she said. The researchers hope to include these women in a future analysis.
‘Age matters’
Commenting on the findings, Alexis C. Edwards, PhD, Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond, said the current study’s findings “made a lot of sense.” Dr. Edwards wasn’t involved with this study but conducted a previous study of 216,702 Swedish women aged 15-22 years that showed use of combination or progestin-only oral contraceptives was associated with an increased risk for suicidal behavior.
She agreed with Dr. Toffol that the “much larger age range” in the new study may have played a role in showing the opposite result.
“The trajectory that we saw if we had been able to continue following the women for longer – which we couldn’t, due to limitations of the registries – [was that] using hormonal contraceptives was going to end up being protective, so I do think that it matters what age you’re looking at,” she said.
Dr. Edwards noted the takeaway from both studies “is that, even if there is a slight increase in risk from using hormonal contraceptives, it’s short lived and it’s probably specific to young women, which is important.”
She suggested the hormonal benefit from extended contraceptive use could come from the regulation of mood, as it offers a “more stable hormonal course than what their body might be putting them through in the absence of using the pill.”
Overall, it is “really lovely to see very well-executed studies on this, providing more empirical evidence on this question, because it is something that’s relevant to anyone who’s potentially going to be using hormonal contraception,” Dr. Edwards said.
Clinical implications?
Andrea Fiorillo, MD, PhD, department of psychiatry, University of Campania “Luigi Vanvitelli,” Naples, Italy, said in a press release that the “striking” findings of the current study need “careful evaluation.”
They also need to be replicated in “different cohorts of women and controlled for the impact of several psychosocial stressors, such as economic upheavals, social insecurity, and uncertainty due to the COVID pandemic,” said Dr. Fiorillo, who was not involved with the research.
Nevertheless, she believes the “clinical implications of the study are obvious and may help to destigmatize the use of hormonal contraceptives.”
The study was funded by the Jane and Aatos Erkko Foundation, the Avohoidon Tsukimis äätiö (Foundation for Primary Care Research), the Yrj ö Jahnsson Foundation, and the Finnish Cultural Foundation. No relevant financial relationships were reported.
A version of this article first appeared on Medscape.com.
FROM EPA 2022
Male contraceptive pill appears feasible in very early trials
ATLANTA – Potential once-daily male oral contraceptives have passed a first clinical hurdle, showing a degree of testosterone suppression that should be sufficient for a contraceptive effect without causing symptomatic hypogonadism, according to phase 1 study results to be presented at the annual meeting of the Endocrine Society.

Credit: Flickr/Marco Verch Professional Photographer/CC by 2.0
There are two pills in development and the studies so far suggest that both or a combination might be able to provide an acceptable balance of efficacy and tolerability, according to Tamar Jacobsohn, a researcher in the Contraceptive Development Program, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Md.
The two drugs evaluated in this study are dimethandrolone undecanoate (DMAU) and 11b-methyl-19-nortestosterone-17b-dodecylcarbonate (11b-MNTDC). Both are bifunctional prodrugs with androgenic and progestogenic effects. The prodrugs are designed to be cleaved after ingestion so that the active hormones are released over 24 hours, permitting once-daily dosing.
“As potent androgens, these steroids suppress gonadotropin secretion, leading to markedly decreased serum testosterone production,” explained Ms. Jacobsohn in an interview.
However, she noted that there is still a long way to go on this research path. While the phase 1 studies have shown tolerability, the biology involved in suppressing sperm production suggests that men would need to take these pills daily for about 3 months at the very beginning of contraceptive treatment, until adequate sperm suppression is achieved to prevent pregnancy.
“We are working toward a phase 2 trial that will include a contraceptive efficacy endpoint, but there are lots of steps to get there, including more early phase studies,” she noted.
“There is a huge unmet need in terms of male contraceptive methods,” said Arthi Thirumalai, MBBS, an endocrinologist and assistant professor of medicine at the University of Washington School of Medicine in Seattle.
Senior author of a 2020 review article on male contraception, Dr. Thirumalai said in an interview that prodrugs and other hormonal methods to lower testosterone and suppress sperm production are attractive because of convenience, efficacy, and reversibility,
“We hope that oral formulations can be used to address this need,” said Dr. Thirumalai, who has participated in several experimental and clinical studies of male contraception methods. She is, in fact, one of the many coauthors of the data presented by Ms. Jacobsohn.
Ms. Jacobsohn emphasized: “Development of an effective, reversible male contraceptive method will improve reproductive options for men and women, have a major impact on public health by decreasing unintended pregnancy, and allow men to have an increasingly active role in family planning.”
Phase 1 results with DMAU and MNTDC
The work that led to phase 1 studies suggested that each of the drugs — DMAU and MNTDC — might provide adequate hormone suppression to reduce sperm counts without inducing unacceptable symptoms of hypogonadism. To test this potential, dose-ranging phase 1 studies with an endpoint of testosterone suppression were conducted with each one.
In the two placebo-controlled phase 1a studies, which are to be presented in a poster on June 13, healthy male subjects were randomly assigned to two pills of active therapy, four pills of active therapy, or placebo. In the two studies combined, 39 subjects received DMAU, 30 received 11b-MNTDC, and 28 received placebo.
Efficacy was evaluated by measuring testosterone levels. Tolerability was largely based on patient questionnaires.
At the end of 7 days, testosterone levels remained at reference levels (400 to 600 ng/dL) in those who received placebo. The levels fell to less than 100 ng/dL in all subjects assigned to an active agent regardless of which agent or dose was used.
From day 7 to 28, there was less median suppression of testosterone on 200 mg than 400 mg daily (92.7 ng/dL vs. 49.6 ng/dL; P < .001), but both remained below the target of 100 ng/dL, Ms. Jacobsohn reported.
The difference in degree of testosterone suppression did not appear to influence tolerability.
Subjects on four vs. two daily pills “did not report a significant difference in general satisfaction or their willingness to use the pills in the future or recommend them to other men,” said Ms. Jacobson, presenting P values for these outcomes among subjects on active therapy relative to placebo that were not significant, ranging from 0.48 to 0.85.
Overall, there were no serious adverse events. Mild side effects associated with hypogonadism did occur, but “all resolved by the end of the study,” she said.
Zero sperm production is not the goal. Lowering it sufficiently is
Dr. Thirumalai said the need for a male contraceptive is strong. While condoms have a substantial failure rate, vasectomy is not reliably reversible even though the majority of men agree that the responsibility for preventing pregnancy should be shared, she said.
Dr. Thirumalai’s earlier review article found that clinical trials of hormonal suppression to provide male contraception have been conducted for at least 30 years. The challenge has been finding an effective therapy that is well tolerated.
Drugs that combine both androgenic and progestogenic activity might be the answer. By manipulating hormones that lower testosterone, sperm production is reduced without eliminating a man’s ability to ejaculate. Zero sperm production is not the goal, according to data in Dr. Thirumalai’s review article.
Rather, studies suggest that when ejaculate contains less than 1 million sperm per mL (levels typically range from 15 to 200 million sperm/mL), the antipregnancy efficacy is similar to that achieved with female oral contraceptives.
However, clinical trials to demonstrate that this can be achieved safely have yet to be conducted.
Ms. Jacobsohn said that sperm half-life is about 3 months. This means that patients would need to be on hormonal therapy for a period of about this duration before reliable contraception is achieved.
In other words, the efficacy endpoint used in this current study [of 28 days duration] does not ensure effective contraception, but Ms. Jacobsohn suggested this is nevertheless an important step forward in clinical development.
Ms. Jacobsohn and Dr. Thirumalai report no relevant financial relationships.
A version of this article first appeared on Medscape.com .
ATLANTA – Potential once-daily male oral contraceptives have passed a first clinical hurdle, showing a degree of testosterone suppression that should be sufficient for a contraceptive effect without causing symptomatic hypogonadism, according to phase 1 study results to be presented at the annual meeting of the Endocrine Society.

Credit: Flickr/Marco Verch Professional Photographer/CC by 2.0
There are two pills in development and the studies so far suggest that both or a combination might be able to provide an acceptable balance of efficacy and tolerability, according to Tamar Jacobsohn, a researcher in the Contraceptive Development Program, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Md.
The two drugs evaluated in this study are dimethandrolone undecanoate (DMAU) and 11b-methyl-19-nortestosterone-17b-dodecylcarbonate (11b-MNTDC). Both are bifunctional prodrugs with androgenic and progestogenic effects. The prodrugs are designed to be cleaved after ingestion so that the active hormones are released over 24 hours, permitting once-daily dosing.
“As potent androgens, these steroids suppress gonadotropin secretion, leading to markedly decreased serum testosterone production,” explained Ms. Jacobsohn in an interview.
However, she noted that there is still a long way to go on this research path. While the phase 1 studies have shown tolerability, the biology involved in suppressing sperm production suggests that men would need to take these pills daily for about 3 months at the very beginning of contraceptive treatment, until adequate sperm suppression is achieved to prevent pregnancy.
“We are working toward a phase 2 trial that will include a contraceptive efficacy endpoint, but there are lots of steps to get there, including more early phase studies,” she noted.
“There is a huge unmet need in terms of male contraceptive methods,” said Arthi Thirumalai, MBBS, an endocrinologist and assistant professor of medicine at the University of Washington School of Medicine in Seattle.
Senior author of a 2020 review article on male contraception, Dr. Thirumalai said in an interview that prodrugs and other hormonal methods to lower testosterone and suppress sperm production are attractive because of convenience, efficacy, and reversibility,
“We hope that oral formulations can be used to address this need,” said Dr. Thirumalai, who has participated in several experimental and clinical studies of male contraception methods. She is, in fact, one of the many coauthors of the data presented by Ms. Jacobsohn.
Ms. Jacobsohn emphasized: “Development of an effective, reversible male contraceptive method will improve reproductive options for men and women, have a major impact on public health by decreasing unintended pregnancy, and allow men to have an increasingly active role in family planning.”
Phase 1 results with DMAU and MNTDC
The work that led to phase 1 studies suggested that each of the drugs — DMAU and MNTDC — might provide adequate hormone suppression to reduce sperm counts without inducing unacceptable symptoms of hypogonadism. To test this potential, dose-ranging phase 1 studies with an endpoint of testosterone suppression were conducted with each one.
In the two placebo-controlled phase 1a studies, which are to be presented in a poster on June 13, healthy male subjects were randomly assigned to two pills of active therapy, four pills of active therapy, or placebo. In the two studies combined, 39 subjects received DMAU, 30 received 11b-MNTDC, and 28 received placebo.
Efficacy was evaluated by measuring testosterone levels. Tolerability was largely based on patient questionnaires.
At the end of 7 days, testosterone levels remained at reference levels (400 to 600 ng/dL) in those who received placebo. The levels fell to less than 100 ng/dL in all subjects assigned to an active agent regardless of which agent or dose was used.
From day 7 to 28, there was less median suppression of testosterone on 200 mg than 400 mg daily (92.7 ng/dL vs. 49.6 ng/dL; P < .001), but both remained below the target of 100 ng/dL, Ms. Jacobsohn reported.
The difference in degree of testosterone suppression did not appear to influence tolerability.
Subjects on four vs. two daily pills “did not report a significant difference in general satisfaction or their willingness to use the pills in the future or recommend them to other men,” said Ms. Jacobson, presenting P values for these outcomes among subjects on active therapy relative to placebo that were not significant, ranging from 0.48 to 0.85.
Overall, there were no serious adverse events. Mild side effects associated with hypogonadism did occur, but “all resolved by the end of the study,” she said.
Zero sperm production is not the goal. Lowering it sufficiently is
Dr. Thirumalai said the need for a male contraceptive is strong. While condoms have a substantial failure rate, vasectomy is not reliably reversible even though the majority of men agree that the responsibility for preventing pregnancy should be shared, she said.
Dr. Thirumalai’s earlier review article found that clinical trials of hormonal suppression to provide male contraception have been conducted for at least 30 years. The challenge has been finding an effective therapy that is well tolerated.
Drugs that combine both androgenic and progestogenic activity might be the answer. By manipulating hormones that lower testosterone, sperm production is reduced without eliminating a man’s ability to ejaculate. Zero sperm production is not the goal, according to data in Dr. Thirumalai’s review article.
Rather, studies suggest that when ejaculate contains less than 1 million sperm per mL (levels typically range from 15 to 200 million sperm/mL), the antipregnancy efficacy is similar to that achieved with female oral contraceptives.
However, clinical trials to demonstrate that this can be achieved safely have yet to be conducted.
Ms. Jacobsohn said that sperm half-life is about 3 months. This means that patients would need to be on hormonal therapy for a period of about this duration before reliable contraception is achieved.
In other words, the efficacy endpoint used in this current study [of 28 days duration] does not ensure effective contraception, but Ms. Jacobsohn suggested this is nevertheless an important step forward in clinical development.
Ms. Jacobsohn and Dr. Thirumalai report no relevant financial relationships.
A version of this article first appeared on Medscape.com .
ATLANTA – Potential once-daily male oral contraceptives have passed a first clinical hurdle, showing a degree of testosterone suppression that should be sufficient for a contraceptive effect without causing symptomatic hypogonadism, according to phase 1 study results to be presented at the annual meeting of the Endocrine Society.

Credit: Flickr/Marco Verch Professional Photographer/CC by 2.0
There are two pills in development and the studies so far suggest that both or a combination might be able to provide an acceptable balance of efficacy and tolerability, according to Tamar Jacobsohn, a researcher in the Contraceptive Development Program, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Md.
The two drugs evaluated in this study are dimethandrolone undecanoate (DMAU) and 11b-methyl-19-nortestosterone-17b-dodecylcarbonate (11b-MNTDC). Both are bifunctional prodrugs with androgenic and progestogenic effects. The prodrugs are designed to be cleaved after ingestion so that the active hormones are released over 24 hours, permitting once-daily dosing.
“As potent androgens, these steroids suppress gonadotropin secretion, leading to markedly decreased serum testosterone production,” explained Ms. Jacobsohn in an interview.
However, she noted that there is still a long way to go on this research path. While the phase 1 studies have shown tolerability, the biology involved in suppressing sperm production suggests that men would need to take these pills daily for about 3 months at the very beginning of contraceptive treatment, until adequate sperm suppression is achieved to prevent pregnancy.
“We are working toward a phase 2 trial that will include a contraceptive efficacy endpoint, but there are lots of steps to get there, including more early phase studies,” she noted.
“There is a huge unmet need in terms of male contraceptive methods,” said Arthi Thirumalai, MBBS, an endocrinologist and assistant professor of medicine at the University of Washington School of Medicine in Seattle.
Senior author of a 2020 review article on male contraception, Dr. Thirumalai said in an interview that prodrugs and other hormonal methods to lower testosterone and suppress sperm production are attractive because of convenience, efficacy, and reversibility,
“We hope that oral formulations can be used to address this need,” said Dr. Thirumalai, who has participated in several experimental and clinical studies of male contraception methods. She is, in fact, one of the many coauthors of the data presented by Ms. Jacobsohn.
Ms. Jacobsohn emphasized: “Development of an effective, reversible male contraceptive method will improve reproductive options for men and women, have a major impact on public health by decreasing unintended pregnancy, and allow men to have an increasingly active role in family planning.”
Phase 1 results with DMAU and MNTDC
The work that led to phase 1 studies suggested that each of the drugs — DMAU and MNTDC — might provide adequate hormone suppression to reduce sperm counts without inducing unacceptable symptoms of hypogonadism. To test this potential, dose-ranging phase 1 studies with an endpoint of testosterone suppression were conducted with each one.
In the two placebo-controlled phase 1a studies, which are to be presented in a poster on June 13, healthy male subjects were randomly assigned to two pills of active therapy, four pills of active therapy, or placebo. In the two studies combined, 39 subjects received DMAU, 30 received 11b-MNTDC, and 28 received placebo.
Efficacy was evaluated by measuring testosterone levels. Tolerability was largely based on patient questionnaires.
At the end of 7 days, testosterone levels remained at reference levels (400 to 600 ng/dL) in those who received placebo. The levels fell to less than 100 ng/dL in all subjects assigned to an active agent regardless of which agent or dose was used.
From day 7 to 28, there was less median suppression of testosterone on 200 mg than 400 mg daily (92.7 ng/dL vs. 49.6 ng/dL; P < .001), but both remained below the target of 100 ng/dL, Ms. Jacobsohn reported.
The difference in degree of testosterone suppression did not appear to influence tolerability.
Subjects on four vs. two daily pills “did not report a significant difference in general satisfaction or their willingness to use the pills in the future or recommend them to other men,” said Ms. Jacobson, presenting P values for these outcomes among subjects on active therapy relative to placebo that were not significant, ranging from 0.48 to 0.85.
Overall, there were no serious adverse events. Mild side effects associated with hypogonadism did occur, but “all resolved by the end of the study,” she said.
Zero sperm production is not the goal. Lowering it sufficiently is
Dr. Thirumalai said the need for a male contraceptive is strong. While condoms have a substantial failure rate, vasectomy is not reliably reversible even though the majority of men agree that the responsibility for preventing pregnancy should be shared, she said.
Dr. Thirumalai’s earlier review article found that clinical trials of hormonal suppression to provide male contraception have been conducted for at least 30 years. The challenge has been finding an effective therapy that is well tolerated.
Drugs that combine both androgenic and progestogenic activity might be the answer. By manipulating hormones that lower testosterone, sperm production is reduced without eliminating a man’s ability to ejaculate. Zero sperm production is not the goal, according to data in Dr. Thirumalai’s review article.
Rather, studies suggest that when ejaculate contains less than 1 million sperm per mL (levels typically range from 15 to 200 million sperm/mL), the antipregnancy efficacy is similar to that achieved with female oral contraceptives.
However, clinical trials to demonstrate that this can be achieved safely have yet to be conducted.
Ms. Jacobsohn said that sperm half-life is about 3 months. This means that patients would need to be on hormonal therapy for a period of about this duration before reliable contraception is achieved.
In other words, the efficacy endpoint used in this current study [of 28 days duration] does not ensure effective contraception, but Ms. Jacobsohn suggested this is nevertheless an important step forward in clinical development.
Ms. Jacobsohn and Dr. Thirumalai report no relevant financial relationships.
A version of this article first appeared on Medscape.com .
AT ENDO 2022
Double morning-after pill dose for women with obesity not effective
Emergency contraception is more likely to fail in women with obesity, but simply doubling the dose of levonorgestrel (LNG)-based contraception does not appear to be effective according to the results of a randomized, controlled trial.
Alison B. Edelman, MD, MPH, of the department of obstetrics & gynecology at Oregon Health & Science University, Portland, led the study published online in Obstetrics & Gynecology.
The researchers included healthy women ages 18-35 with regular menstrual cycles, body mass index (BMI) higher than 30 kg/m2, and weight at least 176 pounds in a randomized study.
After confirming ovulation, researchers monitored participants with transvaginal ultrasonography and blood sampling for progesterone, luteinizing hormone, and estradiol every other day until a dominant follicle 15 mm or greater was seen.
At that point the women received either LNG 1.5 mg or 3 mg and returned for daily monitoring up to 7 days.
Emergency contraception with LNG works by preventing the luteinizing hormone surge, blocking follicle rupture. The researchers had hypothesized that women with obesity might not be getting enough LNG to block the surge after oral dosing.
Previous trials had shown women with obesity had a fourfold higher risk of pregnancy, compared with women with normal BMI taking emergency contraception.
The primary outcome in this trial was whether women had follicle rupture 5 days after dosing.
The authors wrote: “The study had 80% power to detect a 30% difference in the proportion of cycles with at least a 5-day delay in follicle rupture (50% decrease).”
A total of 70 women completed study procedures. The two groups (35 women in each) had similar demographics (mean age, 28 years; BMI, 38).
No differences found between groups
“We found no difference between groups in the proportion of participants without follicle rupture,” the researchers wrote.
More than 5 days after dosing, 51.4% in the lower-dose group did not experience follicle rupture. In the double-dose group 68.6% did not experience rupture but the difference was not significant (P = .14).
Among participants with follicle rupture before 5 days, the time to rupture – the secondary endpoint – also did not differ between groups.
The researchers concluded that more research on the failures of hormonal emergency contraception in women with obesity is needed.
Eve Espey, MD, MPH, distinguished professor and chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque, said in an interview that the study was well designed and the results “form a strong basis for clinical recommendations.”
“Providers should not recommend a higher dose of LNG emergency contraception for patients who are overweight or obese, but rather should counsel patients on the superior effectiveness of ulipristal acetate for those seeking oral emergency contraception as well as the longer time period after unprotected sex – 5 days – that ulipristal maintains its effectiveness.”
“Providers should also counsel patients on the most effective emergency contraception methods, the copper or LNG intrauterine device,” she said.
She said the unique study design of a pharmacodynamic randomized controlled trial adds weight to the findings.
She and the authors noted a limitation is the use of a surrogate outcome, ovulation delay, for ethical and feasibility reasons, instead of the outcome of interest, pregnancy.
The trial was conducted at Oregon Health & Science University and Eastern Virginia Medical School, Norfolk, from June 2017 to February 2021.
Study enrollees were compensated for their time. They were required not to be at risk for pregnancy (abstinent or using a nonhormonal method of contraception).
Dr. Edelman reported receiving honoraria and travel reimbursement from the American College of Obstetricians and Gynecologists, the World Health Organization, and Gynuity for committee activities and honoraria for peer review from the Karolinska Institute. She receives royalties from UpToDate. Several coauthors have received payments for consulting from multiple pharmaceutical companies. These companies and organizations may have a commercial or financial interest in the results of this research and technology. Another was involved in this study as a private consultant and is employed by Gilead Sciences, which was not involved in this research.
Emergency contraception is more likely to fail in women with obesity, but simply doubling the dose of levonorgestrel (LNG)-based contraception does not appear to be effective according to the results of a randomized, controlled trial.
Alison B. Edelman, MD, MPH, of the department of obstetrics & gynecology at Oregon Health & Science University, Portland, led the study published online in Obstetrics & Gynecology.
The researchers included healthy women ages 18-35 with regular menstrual cycles, body mass index (BMI) higher than 30 kg/m2, and weight at least 176 pounds in a randomized study.
After confirming ovulation, researchers monitored participants with transvaginal ultrasonography and blood sampling for progesterone, luteinizing hormone, and estradiol every other day until a dominant follicle 15 mm or greater was seen.
At that point the women received either LNG 1.5 mg or 3 mg and returned for daily monitoring up to 7 days.
Emergency contraception with LNG works by preventing the luteinizing hormone surge, blocking follicle rupture. The researchers had hypothesized that women with obesity might not be getting enough LNG to block the surge after oral dosing.
Previous trials had shown women with obesity had a fourfold higher risk of pregnancy, compared with women with normal BMI taking emergency contraception.
The primary outcome in this trial was whether women had follicle rupture 5 days after dosing.
The authors wrote: “The study had 80% power to detect a 30% difference in the proportion of cycles with at least a 5-day delay in follicle rupture (50% decrease).”
A total of 70 women completed study procedures. The two groups (35 women in each) had similar demographics (mean age, 28 years; BMI, 38).
No differences found between groups
“We found no difference between groups in the proportion of participants without follicle rupture,” the researchers wrote.
More than 5 days after dosing, 51.4% in the lower-dose group did not experience follicle rupture. In the double-dose group 68.6% did not experience rupture but the difference was not significant (P = .14).
Among participants with follicle rupture before 5 days, the time to rupture – the secondary endpoint – also did not differ between groups.
The researchers concluded that more research on the failures of hormonal emergency contraception in women with obesity is needed.
Eve Espey, MD, MPH, distinguished professor and chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque, said in an interview that the study was well designed and the results “form a strong basis for clinical recommendations.”
“Providers should not recommend a higher dose of LNG emergency contraception for patients who are overweight or obese, but rather should counsel patients on the superior effectiveness of ulipristal acetate for those seeking oral emergency contraception as well as the longer time period after unprotected sex – 5 days – that ulipristal maintains its effectiveness.”
“Providers should also counsel patients on the most effective emergency contraception methods, the copper or LNG intrauterine device,” she said.
She said the unique study design of a pharmacodynamic randomized controlled trial adds weight to the findings.
She and the authors noted a limitation is the use of a surrogate outcome, ovulation delay, for ethical and feasibility reasons, instead of the outcome of interest, pregnancy.
The trial was conducted at Oregon Health & Science University and Eastern Virginia Medical School, Norfolk, from June 2017 to February 2021.
Study enrollees were compensated for their time. They were required not to be at risk for pregnancy (abstinent or using a nonhormonal method of contraception).
Dr. Edelman reported receiving honoraria and travel reimbursement from the American College of Obstetricians and Gynecologists, the World Health Organization, and Gynuity for committee activities and honoraria for peer review from the Karolinska Institute. She receives royalties from UpToDate. Several coauthors have received payments for consulting from multiple pharmaceutical companies. These companies and organizations may have a commercial or financial interest in the results of this research and technology. Another was involved in this study as a private consultant and is employed by Gilead Sciences, which was not involved in this research.
Emergency contraception is more likely to fail in women with obesity, but simply doubling the dose of levonorgestrel (LNG)-based contraception does not appear to be effective according to the results of a randomized, controlled trial.
Alison B. Edelman, MD, MPH, of the department of obstetrics & gynecology at Oregon Health & Science University, Portland, led the study published online in Obstetrics & Gynecology.
The researchers included healthy women ages 18-35 with regular menstrual cycles, body mass index (BMI) higher than 30 kg/m2, and weight at least 176 pounds in a randomized study.
After confirming ovulation, researchers monitored participants with transvaginal ultrasonography and blood sampling for progesterone, luteinizing hormone, and estradiol every other day until a dominant follicle 15 mm or greater was seen.
At that point the women received either LNG 1.5 mg or 3 mg and returned for daily monitoring up to 7 days.
Emergency contraception with LNG works by preventing the luteinizing hormone surge, blocking follicle rupture. The researchers had hypothesized that women with obesity might not be getting enough LNG to block the surge after oral dosing.
Previous trials had shown women with obesity had a fourfold higher risk of pregnancy, compared with women with normal BMI taking emergency contraception.
The primary outcome in this trial was whether women had follicle rupture 5 days after dosing.
The authors wrote: “The study had 80% power to detect a 30% difference in the proportion of cycles with at least a 5-day delay in follicle rupture (50% decrease).”
A total of 70 women completed study procedures. The two groups (35 women in each) had similar demographics (mean age, 28 years; BMI, 38).
No differences found between groups
“We found no difference between groups in the proportion of participants without follicle rupture,” the researchers wrote.
More than 5 days after dosing, 51.4% in the lower-dose group did not experience follicle rupture. In the double-dose group 68.6% did not experience rupture but the difference was not significant (P = .14).
Among participants with follicle rupture before 5 days, the time to rupture – the secondary endpoint – also did not differ between groups.
The researchers concluded that more research on the failures of hormonal emergency contraception in women with obesity is needed.
Eve Espey, MD, MPH, distinguished professor and chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque, said in an interview that the study was well designed and the results “form a strong basis for clinical recommendations.”
“Providers should not recommend a higher dose of LNG emergency contraception for patients who are overweight or obese, but rather should counsel patients on the superior effectiveness of ulipristal acetate for those seeking oral emergency contraception as well as the longer time period after unprotected sex – 5 days – that ulipristal maintains its effectiveness.”
“Providers should also counsel patients on the most effective emergency contraception methods, the copper or LNG intrauterine device,” she said.
She said the unique study design of a pharmacodynamic randomized controlled trial adds weight to the findings.
She and the authors noted a limitation is the use of a surrogate outcome, ovulation delay, for ethical and feasibility reasons, instead of the outcome of interest, pregnancy.
The trial was conducted at Oregon Health & Science University and Eastern Virginia Medical School, Norfolk, from June 2017 to February 2021.
Study enrollees were compensated for their time. They were required not to be at risk for pregnancy (abstinent or using a nonhormonal method of contraception).
Dr. Edelman reported receiving honoraria and travel reimbursement from the American College of Obstetricians and Gynecologists, the World Health Organization, and Gynuity for committee activities and honoraria for peer review from the Karolinska Institute. She receives royalties from UpToDate. Several coauthors have received payments for consulting from multiple pharmaceutical companies. These companies and organizations may have a commercial or financial interest in the results of this research and technology. Another was involved in this study as a private consultant and is employed by Gilead Sciences, which was not involved in this research.
FROM OBSTETRICS & GYNECOLOGY
Women are not being warned that anesthetic may reduce birth pill efficacy
The effectiveness of hormonal contraceptives, including the pill and mini-pill, may be compromised by sugammadex, a drug widely used in anesthesia for reversing neuromuscular blockade induced by rocuronium or vecuronium.
Yet women are not routinely informed that the drug may make their contraception less effective, delegates at Euroanaesthesia, the annual meeting of the European Society of Anaesthesiology and Intensive Care in Milan were told.
New research presented at the meeting supports the authors’ experience that “robust methods for identifying at-risk patients and informing them of the associated risk of contraceptive failures is not common practice across anesthetic departments within the United Kingdom, and likely further afield.”
This is according to a survey of almost 150 anesthetic professionals, including consultants, junior doctors, and physician assistants, working at University College London Hospitals NHS Foundation Trust.
Dr. Neha Passi, Dr. Matt Oliver, and colleagues at the trust’s department of anesthesiology sent out a seven-question survey to their 150 colleagues and received 82 responses, 94% of which claimed awareness of the risk of contraceptive failure with sugammadex. However, 70% of the respondents admitted that they do not routinely discuss this with patients who have received the drug.
Risk with all forms of hormonal contraceptive
Yet current guidance is to inform women of child-bearing age that they have received the drug and, because of increased risk of contraceptive failure, advise those taking oral hormonal contraceptives to follow the missed pill advice in the leaflet that comes with their contraceptives. It also counsels that clinicians should advise women using other types of hormonal contraceptive to use an additional nonhormonal means of contraception for 7 days.
The study authors also carried out a retrospective audit of sugammadex use in the trust and reported that during the 6 weeks covered by the audit, 234 patients were administered sugammadex of whom 65 (28%) were women of childbearing age. Of these, 17 had a medical history that meant they weren’t at risk of pregnancy, but the other 48 should have received advice on the risks of contraceptive failure – however there was no record in the medical notes of such advice having been given for any of the at-risk 48 women.
While sugammadex is the only anesthetic drug known to have this effect, it is recognized to interact with progesterone and so may reduce the effectiveness of hormonal contraceptives, including the progesterone-only pill, combined pill, vaginal rings, implants, and intrauterine devices.
Dr. Passi said: “It is concerning that we are so seldom informing patients of the risk of contraceptive failure following sugammadex use.
“Use of sugammadex is expected to rise as it becomes cheaper in the future, and ensuring that women receiving this medicine are aware it may increase their risk of unwanted pregnancy must be a priority.”
She added: “It is important to note, however, that most patients receiving an anesthetic do not need a muscle relaxant and that sugammadex is one of several drugs available to reverse muscle relaxation.”
Dr. Oliver said: “We only studied one hospital trust but we expect the results to be similar in elsewhere in the U.K.”
In response to their findings, the study’s authors have created patient information leaflets and letters and programmed the trust’s electronic patient record system to identify “at-risk” patients and deliver electronic prompts to the anesthetists caring for them in the perioperative period.
A version of this article first appeared on Medscape UK.
The effectiveness of hormonal contraceptives, including the pill and mini-pill, may be compromised by sugammadex, a drug widely used in anesthesia for reversing neuromuscular blockade induced by rocuronium or vecuronium.
Yet women are not routinely informed that the drug may make their contraception less effective, delegates at Euroanaesthesia, the annual meeting of the European Society of Anaesthesiology and Intensive Care in Milan were told.
New research presented at the meeting supports the authors’ experience that “robust methods for identifying at-risk patients and informing them of the associated risk of contraceptive failures is not common practice across anesthetic departments within the United Kingdom, and likely further afield.”
This is according to a survey of almost 150 anesthetic professionals, including consultants, junior doctors, and physician assistants, working at University College London Hospitals NHS Foundation Trust.
Dr. Neha Passi, Dr. Matt Oliver, and colleagues at the trust’s department of anesthesiology sent out a seven-question survey to their 150 colleagues and received 82 responses, 94% of which claimed awareness of the risk of contraceptive failure with sugammadex. However, 70% of the respondents admitted that they do not routinely discuss this with patients who have received the drug.
Risk with all forms of hormonal contraceptive
Yet current guidance is to inform women of child-bearing age that they have received the drug and, because of increased risk of contraceptive failure, advise those taking oral hormonal contraceptives to follow the missed pill advice in the leaflet that comes with their contraceptives. It also counsels that clinicians should advise women using other types of hormonal contraceptive to use an additional nonhormonal means of contraception for 7 days.
The study authors also carried out a retrospective audit of sugammadex use in the trust and reported that during the 6 weeks covered by the audit, 234 patients were administered sugammadex of whom 65 (28%) were women of childbearing age. Of these, 17 had a medical history that meant they weren’t at risk of pregnancy, but the other 48 should have received advice on the risks of contraceptive failure – however there was no record in the medical notes of such advice having been given for any of the at-risk 48 women.
While sugammadex is the only anesthetic drug known to have this effect, it is recognized to interact with progesterone and so may reduce the effectiveness of hormonal contraceptives, including the progesterone-only pill, combined pill, vaginal rings, implants, and intrauterine devices.
Dr. Passi said: “It is concerning that we are so seldom informing patients of the risk of contraceptive failure following sugammadex use.
“Use of sugammadex is expected to rise as it becomes cheaper in the future, and ensuring that women receiving this medicine are aware it may increase their risk of unwanted pregnancy must be a priority.”
She added: “It is important to note, however, that most patients receiving an anesthetic do not need a muscle relaxant and that sugammadex is one of several drugs available to reverse muscle relaxation.”
Dr. Oliver said: “We only studied one hospital trust but we expect the results to be similar in elsewhere in the U.K.”
In response to their findings, the study’s authors have created patient information leaflets and letters and programmed the trust’s electronic patient record system to identify “at-risk” patients and deliver electronic prompts to the anesthetists caring for them in the perioperative period.
A version of this article first appeared on Medscape UK.
The effectiveness of hormonal contraceptives, including the pill and mini-pill, may be compromised by sugammadex, a drug widely used in anesthesia for reversing neuromuscular blockade induced by rocuronium or vecuronium.
Yet women are not routinely informed that the drug may make their contraception less effective, delegates at Euroanaesthesia, the annual meeting of the European Society of Anaesthesiology and Intensive Care in Milan were told.
New research presented at the meeting supports the authors’ experience that “robust methods for identifying at-risk patients and informing them of the associated risk of contraceptive failures is not common practice across anesthetic departments within the United Kingdom, and likely further afield.”
This is according to a survey of almost 150 anesthetic professionals, including consultants, junior doctors, and physician assistants, working at University College London Hospitals NHS Foundation Trust.
Dr. Neha Passi, Dr. Matt Oliver, and colleagues at the trust’s department of anesthesiology sent out a seven-question survey to their 150 colleagues and received 82 responses, 94% of which claimed awareness of the risk of contraceptive failure with sugammadex. However, 70% of the respondents admitted that they do not routinely discuss this with patients who have received the drug.
Risk with all forms of hormonal contraceptive
Yet current guidance is to inform women of child-bearing age that they have received the drug and, because of increased risk of contraceptive failure, advise those taking oral hormonal contraceptives to follow the missed pill advice in the leaflet that comes with their contraceptives. It also counsels that clinicians should advise women using other types of hormonal contraceptive to use an additional nonhormonal means of contraception for 7 days.
The study authors also carried out a retrospective audit of sugammadex use in the trust and reported that during the 6 weeks covered by the audit, 234 patients were administered sugammadex of whom 65 (28%) were women of childbearing age. Of these, 17 had a medical history that meant they weren’t at risk of pregnancy, but the other 48 should have received advice on the risks of contraceptive failure – however there was no record in the medical notes of such advice having been given for any of the at-risk 48 women.
While sugammadex is the only anesthetic drug known to have this effect, it is recognized to interact with progesterone and so may reduce the effectiveness of hormonal contraceptives, including the progesterone-only pill, combined pill, vaginal rings, implants, and intrauterine devices.
Dr. Passi said: “It is concerning that we are so seldom informing patients of the risk of contraceptive failure following sugammadex use.
“Use of sugammadex is expected to rise as it becomes cheaper in the future, and ensuring that women receiving this medicine are aware it may increase their risk of unwanted pregnancy must be a priority.”
She added: “It is important to note, however, that most patients receiving an anesthetic do not need a muscle relaxant and that sugammadex is one of several drugs available to reverse muscle relaxation.”
Dr. Oliver said: “We only studied one hospital trust but we expect the results to be similar in elsewhere in the U.K.”
In response to their findings, the study’s authors have created patient information leaflets and letters and programmed the trust’s electronic patient record system to identify “at-risk” patients and deliver electronic prompts to the anesthetists caring for them in the perioperative period.
A version of this article first appeared on Medscape UK.
FROM EUROANAESTHESIA
Tin in permanent contraception implants causes toxicity
Essure implants arrived on the market in 2002 as permanent contraception for women older than age 45 years with children. They were recalled in 2017. Presented as an alternative to laparoscopic tubal ligation, this medical device resulted in rare side effects affecting thousands of women, most notably the nervous system, cardiovascular system, endocrine system, and musculoskeletal system.
Implant analysis protocol
“My research focuses on a variety of medical devices, mostly joint replacements, and more specifically, hip replacements. I look at how these materials behave in humans and how the wear debris affects the body,” explained Ana Maria Trunfio-Sfarghiu, bioengineering expert and research associate with the French National Center for Scientific Research at the Lyon National Institute of Applied Sciences’ Contact and Structure Mechanics Laboratory.
“The problems with Essure implants started with a woman who had been using one for about 10 years and was experiencing side effects such as trouble concentrating and focusing, significant vaginal bleeding, extreme tiredness, hair loss, etc. She had the implant removed, and we retrieved it from her gynecologist and analyzed it alongside other implants,” said Ms. Trunfio-Sfarghiu.
“Together with the hospital, we set up an implant analysis protocol. We visited hospital teams to demonstrate how to prepare the biopsies, embedded in paraffin blocks, before sending them to us for analysis. We gave the same specimen preparation instructions for all subjects,” Ms. Trunfio-Sfarghiu explained.
After a year of clinical analysis, the Journal of Trace Elements in Medicine and Biology published an article about 18 cases.
Implant weld corrosion
The Essure implant measures a few centimeters long and resembles a small spring. Once it is released inside the fallopian tube, its goal is to create inflammation and block the tube. It triggers fibrosis, which prevents the sperm from reaching the egg. Premarketing tests had shown that the fibrosis surrounding the implant would keep it from moving. However, the pharmaceutical company hadn’t assessed the mechanical integrity of the spring weld, which was made of silver-tin.
During their analysis in collaboration with the Minapath laboratory, Ms. Trunfio-Sfarghiu’s team found that the weld had corroded and that tin particles had been released into the subjects’ bodies. “The study included about 40 women, and we found tin in all of them,” said Ms. Trunfio-Sfarghiu.
This weld corrosion has several possible consequences. “When the implant degrades, it can travel anywhere in the pelvis, like a needle moving through the body with no apparent destination. The surgeons who operate to remove it describe similar surgeries in military medicine when the patient has been hit by a bullet!”
Organotin toxicity
Although tin is not especially toxic for the body when ingested, it can bind to organic compounds if it passes through to the blood. “When tin binds to a carbon atom, it becomes organotin, a neurotoxin,” said Ms. Trunfio-Sfarghiu.
She said that this organotin can travel to the brain and trigger symptoms like those found in patients with Essure implants. “For the time being, there is insufficient data to assert that we found organotin in all subjects. Another more in-depth study would be needed to assess migration to the brain. For the past 2 years, we have tried to obtain academic funding to continue our research, so far without success. Academic and political authorities seem to be a bit scared of what we’ve found,” said Ms. Trunfio-Sfarghiu.
For her, “it’s how the implant was marketed that is problematic. The implant was designed to create local inflammation, inflammation in itself being difficult to control. Some women need to have their entire uterus and ovaries removed to resolve problems caused by the implant.”
Harm in the United States
Ms. Trunfio-Sfarghiu’s research has helped American victims obtain acknowledgment of their suffering in the United States. “But the harm caused to women by defective implants has yet to be acknowledged in France,” she added.
She explained that Essure was recalled in 2017 because sales were poor, not because it was deemed dangerous. Her conclusion? “No implant that creates inflammation should be authorized, especially if there is a surgical alternative, which there is here: tubal ligation.”
A version of this article appeared on Medscape.com. This article was translated from the Medscape French edition.
Essure implants arrived on the market in 2002 as permanent contraception for women older than age 45 years with children. They were recalled in 2017. Presented as an alternative to laparoscopic tubal ligation, this medical device resulted in rare side effects affecting thousands of women, most notably the nervous system, cardiovascular system, endocrine system, and musculoskeletal system.
Implant analysis protocol
“My research focuses on a variety of medical devices, mostly joint replacements, and more specifically, hip replacements. I look at how these materials behave in humans and how the wear debris affects the body,” explained Ana Maria Trunfio-Sfarghiu, bioengineering expert and research associate with the French National Center for Scientific Research at the Lyon National Institute of Applied Sciences’ Contact and Structure Mechanics Laboratory.
“The problems with Essure implants started with a woman who had been using one for about 10 years and was experiencing side effects such as trouble concentrating and focusing, significant vaginal bleeding, extreme tiredness, hair loss, etc. She had the implant removed, and we retrieved it from her gynecologist and analyzed it alongside other implants,” said Ms. Trunfio-Sfarghiu.
“Together with the hospital, we set up an implant analysis protocol. We visited hospital teams to demonstrate how to prepare the biopsies, embedded in paraffin blocks, before sending them to us for analysis. We gave the same specimen preparation instructions for all subjects,” Ms. Trunfio-Sfarghiu explained.
After a year of clinical analysis, the Journal of Trace Elements in Medicine and Biology published an article about 18 cases.
Implant weld corrosion
The Essure implant measures a few centimeters long and resembles a small spring. Once it is released inside the fallopian tube, its goal is to create inflammation and block the tube. It triggers fibrosis, which prevents the sperm from reaching the egg. Premarketing tests had shown that the fibrosis surrounding the implant would keep it from moving. However, the pharmaceutical company hadn’t assessed the mechanical integrity of the spring weld, which was made of silver-tin.
During their analysis in collaboration with the Minapath laboratory, Ms. Trunfio-Sfarghiu’s team found that the weld had corroded and that tin particles had been released into the subjects’ bodies. “The study included about 40 women, and we found tin in all of them,” said Ms. Trunfio-Sfarghiu.
This weld corrosion has several possible consequences. “When the implant degrades, it can travel anywhere in the pelvis, like a needle moving through the body with no apparent destination. The surgeons who operate to remove it describe similar surgeries in military medicine when the patient has been hit by a bullet!”
Organotin toxicity
Although tin is not especially toxic for the body when ingested, it can bind to organic compounds if it passes through to the blood. “When tin binds to a carbon atom, it becomes organotin, a neurotoxin,” said Ms. Trunfio-Sfarghiu.
She said that this organotin can travel to the brain and trigger symptoms like those found in patients with Essure implants. “For the time being, there is insufficient data to assert that we found organotin in all subjects. Another more in-depth study would be needed to assess migration to the brain. For the past 2 years, we have tried to obtain academic funding to continue our research, so far without success. Academic and political authorities seem to be a bit scared of what we’ve found,” said Ms. Trunfio-Sfarghiu.
For her, “it’s how the implant was marketed that is problematic. The implant was designed to create local inflammation, inflammation in itself being difficult to control. Some women need to have their entire uterus and ovaries removed to resolve problems caused by the implant.”
Harm in the United States
Ms. Trunfio-Sfarghiu’s research has helped American victims obtain acknowledgment of their suffering in the United States. “But the harm caused to women by defective implants has yet to be acknowledged in France,” she added.
She explained that Essure was recalled in 2017 because sales were poor, not because it was deemed dangerous. Her conclusion? “No implant that creates inflammation should be authorized, especially if there is a surgical alternative, which there is here: tubal ligation.”
A version of this article appeared on Medscape.com. This article was translated from the Medscape French edition.
Essure implants arrived on the market in 2002 as permanent contraception for women older than age 45 years with children. They were recalled in 2017. Presented as an alternative to laparoscopic tubal ligation, this medical device resulted in rare side effects affecting thousands of women, most notably the nervous system, cardiovascular system, endocrine system, and musculoskeletal system.
Implant analysis protocol
“My research focuses on a variety of medical devices, mostly joint replacements, and more specifically, hip replacements. I look at how these materials behave in humans and how the wear debris affects the body,” explained Ana Maria Trunfio-Sfarghiu, bioengineering expert and research associate with the French National Center for Scientific Research at the Lyon National Institute of Applied Sciences’ Contact and Structure Mechanics Laboratory.
“The problems with Essure implants started with a woman who had been using one for about 10 years and was experiencing side effects such as trouble concentrating and focusing, significant vaginal bleeding, extreme tiredness, hair loss, etc. She had the implant removed, and we retrieved it from her gynecologist and analyzed it alongside other implants,” said Ms. Trunfio-Sfarghiu.
“Together with the hospital, we set up an implant analysis protocol. We visited hospital teams to demonstrate how to prepare the biopsies, embedded in paraffin blocks, before sending them to us for analysis. We gave the same specimen preparation instructions for all subjects,” Ms. Trunfio-Sfarghiu explained.
After a year of clinical analysis, the Journal of Trace Elements in Medicine and Biology published an article about 18 cases.
Implant weld corrosion
The Essure implant measures a few centimeters long and resembles a small spring. Once it is released inside the fallopian tube, its goal is to create inflammation and block the tube. It triggers fibrosis, which prevents the sperm from reaching the egg. Premarketing tests had shown that the fibrosis surrounding the implant would keep it from moving. However, the pharmaceutical company hadn’t assessed the mechanical integrity of the spring weld, which was made of silver-tin.
During their analysis in collaboration with the Minapath laboratory, Ms. Trunfio-Sfarghiu’s team found that the weld had corroded and that tin particles had been released into the subjects’ bodies. “The study included about 40 women, and we found tin in all of them,” said Ms. Trunfio-Sfarghiu.
This weld corrosion has several possible consequences. “When the implant degrades, it can travel anywhere in the pelvis, like a needle moving through the body with no apparent destination. The surgeons who operate to remove it describe similar surgeries in military medicine when the patient has been hit by a bullet!”
Organotin toxicity
Although tin is not especially toxic for the body when ingested, it can bind to organic compounds if it passes through to the blood. “When tin binds to a carbon atom, it becomes organotin, a neurotoxin,” said Ms. Trunfio-Sfarghiu.
She said that this organotin can travel to the brain and trigger symptoms like those found in patients with Essure implants. “For the time being, there is insufficient data to assert that we found organotin in all subjects. Another more in-depth study would be needed to assess migration to the brain. For the past 2 years, we have tried to obtain academic funding to continue our research, so far without success. Academic and political authorities seem to be a bit scared of what we’ve found,” said Ms. Trunfio-Sfarghiu.
For her, “it’s how the implant was marketed that is problematic. The implant was designed to create local inflammation, inflammation in itself being difficult to control. Some women need to have their entire uterus and ovaries removed to resolve problems caused by the implant.”
Harm in the United States
Ms. Trunfio-Sfarghiu’s research has helped American victims obtain acknowledgment of their suffering in the United States. “But the harm caused to women by defective implants has yet to be acknowledged in France,” she added.
She explained that Essure was recalled in 2017 because sales were poor, not because it was deemed dangerous. Her conclusion? “No implant that creates inflammation should be authorized, especially if there is a surgical alternative, which there is here: tubal ligation.”
A version of this article appeared on Medscape.com. This article was translated from the Medscape French edition.








