User login
Inflammation, thrombosis biomarkers tied to COVID-19 deaths
Their prospective cohort study of 1150 patients hospitalized with the disease in New York City also revealed a high proportion of racial and ethnic minorities, and confirmed high rates of critical illness and mortality.
“Of particular interest is the finding that over three quarters of critically ill patients required a ventilator and almost one third required renal dialysis support,” Max O’Donnell, MD, MPH, assistant professor of medicine and epidemiology at Columbia University in New York City, said in a press release.
O’Donnell and colleagues published the results of their study online today in The Lancet. It is the largest prospective cohort study published in the United States, they said.
“Although the clinical spectrum of disease has been characterised in reports from China and Italy, until now, detailed understanding of how the virus is affecting critically ill patients in the US has been limited to reports from a small number of cases,” said Natalie Yip, MD, assistant professor of medicine at Columbia University.
In the cohort, drawn from two NewYork-Presbyterian hospitals, the researchers focused on the 257 (22%) patients who required intensive care. When they estimated inflammation through interleukin-6 (IL-6) concentrations and thrombosis through D-dimer concentrations, they found a 10% increased risk for death with every 10% increase of IL-6 (adjusted hazard ratio [aHR], 1.11; 95% confidence interval [CI], 1.02–1.20) or D-dimer concentration (aHR, 1.10; 95% CI, 1.01–1.19).
“The association of mortality with higher concentrations of IL-6 and d-dimer is particularly relevant for two reasons,” write Giacomo Grasselli, from the Fondazione IRCCS Ca’ Granda Ospediale Maggiore Policlinico, and Alberto Zanella, from the University of Milan, Italy, in an accompanying commentary.
“First, it confirms the key pathogenic role played by the activation of systemic inflammation and endothelial-vascular damage in the development of organ dysfunction,” they write. “Second, it provides the rationale for the design of clinical trials for measuring the efficacy of treatment with immunomodulating and anticoagulant drugs.”
Seventeen percent of patients received interleukin receptor antagonists and 26% received corticosteroids, but the authors did not report any data on the effects of these treatments, or any data about anticoagulant therapies administered.
Severe disease common
The study also highlighted a high proportion of ethnic and racial minorities. Sixty-two percent of the critically ill patients were Hispanic or Latinx, 19% Black, 32% White, and 3% Asian.
Their median age was 62 years and 67% were men. Eighty-two percent had at least one chronic illness, most commonly hypertension (63%), followed by diabetes (36%). Forty-six percent were obese.
As of April 28, 2020, 101 (39%) of the critically ill patients had died following a median of 9 days (interquartile range (IQR), 5–15) in the hospital and 94 (37%) remained hospitalized. Of the 203 patients who received invasive mechanical ventilation, 84 (41%) had died.
The poor prognosis of patients requiring ventilation is consistent with data from a report on patients treated in National Health Service intensive care units in England, Wales, and Northern Ireland through May 15. Overall, 11,292 patients with COVID-19 required critical care, and 4855 needed advanced respiratory support. Approximately half of the patients receiving mechanical ventilation had died 30 days after starting critical care.
In the New York study, patients spent an average of 18 days on a ventilator (IQR, 9–28 days). This is a longer period than reported in smaller studies of cases from Washington state, but corresponds with a recent report from Italy, the researchers said.
Remarkably, O’Donnell and colleagues report that almost a third (31%) of critically ill patients developed severe kidney damage and required dialysis.
Mortality was associated with several baseline factors, including older age (aHR, 1.31 [95% CI, 1.09–1.57] per 10-year increase), chronic cardiac disease (aHR, 1.76; 95% CI, 1.08–2·86), and chronic pulmonary disease (aHR, 2.94; 95% CI, 1.48–5.84).
Authors of the New York study reported financial relationships to ICE Neurosystems, ALung Technologies, Baxter, BREETHE, Xenios, Hemovent, Gilead Sciences, Amazon, and Karyopharm Therapeutics. Grasselli reports personal fees from Biotest, Draeger, Fisher & Paykel, Maquet, Merck Sharp & Dohme, and Pfizer, all outside the area of work commented on here. Zanella has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Their prospective cohort study of 1150 patients hospitalized with the disease in New York City also revealed a high proportion of racial and ethnic minorities, and confirmed high rates of critical illness and mortality.
“Of particular interest is the finding that over three quarters of critically ill patients required a ventilator and almost one third required renal dialysis support,” Max O’Donnell, MD, MPH, assistant professor of medicine and epidemiology at Columbia University in New York City, said in a press release.
O’Donnell and colleagues published the results of their study online today in The Lancet. It is the largest prospective cohort study published in the United States, they said.
“Although the clinical spectrum of disease has been characterised in reports from China and Italy, until now, detailed understanding of how the virus is affecting critically ill patients in the US has been limited to reports from a small number of cases,” said Natalie Yip, MD, assistant professor of medicine at Columbia University.
In the cohort, drawn from two NewYork-Presbyterian hospitals, the researchers focused on the 257 (22%) patients who required intensive care. When they estimated inflammation through interleukin-6 (IL-6) concentrations and thrombosis through D-dimer concentrations, they found a 10% increased risk for death with every 10% increase of IL-6 (adjusted hazard ratio [aHR], 1.11; 95% confidence interval [CI], 1.02–1.20) or D-dimer concentration (aHR, 1.10; 95% CI, 1.01–1.19).
“The association of mortality with higher concentrations of IL-6 and d-dimer is particularly relevant for two reasons,” write Giacomo Grasselli, from the Fondazione IRCCS Ca’ Granda Ospediale Maggiore Policlinico, and Alberto Zanella, from the University of Milan, Italy, in an accompanying commentary.
“First, it confirms the key pathogenic role played by the activation of systemic inflammation and endothelial-vascular damage in the development of organ dysfunction,” they write. “Second, it provides the rationale for the design of clinical trials for measuring the efficacy of treatment with immunomodulating and anticoagulant drugs.”
Seventeen percent of patients received interleukin receptor antagonists and 26% received corticosteroids, but the authors did not report any data on the effects of these treatments, or any data about anticoagulant therapies administered.
Severe disease common
The study also highlighted a high proportion of ethnic and racial minorities. Sixty-two percent of the critically ill patients were Hispanic or Latinx, 19% Black, 32% White, and 3% Asian.
Their median age was 62 years and 67% were men. Eighty-two percent had at least one chronic illness, most commonly hypertension (63%), followed by diabetes (36%). Forty-six percent were obese.
As of April 28, 2020, 101 (39%) of the critically ill patients had died following a median of 9 days (interquartile range (IQR), 5–15) in the hospital and 94 (37%) remained hospitalized. Of the 203 patients who received invasive mechanical ventilation, 84 (41%) had died.
The poor prognosis of patients requiring ventilation is consistent with data from a report on patients treated in National Health Service intensive care units in England, Wales, and Northern Ireland through May 15. Overall, 11,292 patients with COVID-19 required critical care, and 4855 needed advanced respiratory support. Approximately half of the patients receiving mechanical ventilation had died 30 days after starting critical care.
In the New York study, patients spent an average of 18 days on a ventilator (IQR, 9–28 days). This is a longer period than reported in smaller studies of cases from Washington state, but corresponds with a recent report from Italy, the researchers said.
Remarkably, O’Donnell and colleagues report that almost a third (31%) of critically ill patients developed severe kidney damage and required dialysis.
Mortality was associated with several baseline factors, including older age (aHR, 1.31 [95% CI, 1.09–1.57] per 10-year increase), chronic cardiac disease (aHR, 1.76; 95% CI, 1.08–2·86), and chronic pulmonary disease (aHR, 2.94; 95% CI, 1.48–5.84).
Authors of the New York study reported financial relationships to ICE Neurosystems, ALung Technologies, Baxter, BREETHE, Xenios, Hemovent, Gilead Sciences, Amazon, and Karyopharm Therapeutics. Grasselli reports personal fees from Biotest, Draeger, Fisher & Paykel, Maquet, Merck Sharp & Dohme, and Pfizer, all outside the area of work commented on here. Zanella has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Their prospective cohort study of 1150 patients hospitalized with the disease in New York City also revealed a high proportion of racial and ethnic minorities, and confirmed high rates of critical illness and mortality.
“Of particular interest is the finding that over three quarters of critically ill patients required a ventilator and almost one third required renal dialysis support,” Max O’Donnell, MD, MPH, assistant professor of medicine and epidemiology at Columbia University in New York City, said in a press release.
O’Donnell and colleagues published the results of their study online today in The Lancet. It is the largest prospective cohort study published in the United States, they said.
“Although the clinical spectrum of disease has been characterised in reports from China and Italy, until now, detailed understanding of how the virus is affecting critically ill patients in the US has been limited to reports from a small number of cases,” said Natalie Yip, MD, assistant professor of medicine at Columbia University.
In the cohort, drawn from two NewYork-Presbyterian hospitals, the researchers focused on the 257 (22%) patients who required intensive care. When they estimated inflammation through interleukin-6 (IL-6) concentrations and thrombosis through D-dimer concentrations, they found a 10% increased risk for death with every 10% increase of IL-6 (adjusted hazard ratio [aHR], 1.11; 95% confidence interval [CI], 1.02–1.20) or D-dimer concentration (aHR, 1.10; 95% CI, 1.01–1.19).
“The association of mortality with higher concentrations of IL-6 and d-dimer is particularly relevant for two reasons,” write Giacomo Grasselli, from the Fondazione IRCCS Ca’ Granda Ospediale Maggiore Policlinico, and Alberto Zanella, from the University of Milan, Italy, in an accompanying commentary.
“First, it confirms the key pathogenic role played by the activation of systemic inflammation and endothelial-vascular damage in the development of organ dysfunction,” they write. “Second, it provides the rationale for the design of clinical trials for measuring the efficacy of treatment with immunomodulating and anticoagulant drugs.”
Seventeen percent of patients received interleukin receptor antagonists and 26% received corticosteroids, but the authors did not report any data on the effects of these treatments, or any data about anticoagulant therapies administered.
Severe disease common
The study also highlighted a high proportion of ethnic and racial minorities. Sixty-two percent of the critically ill patients were Hispanic or Latinx, 19% Black, 32% White, and 3% Asian.
Their median age was 62 years and 67% were men. Eighty-two percent had at least one chronic illness, most commonly hypertension (63%), followed by diabetes (36%). Forty-six percent were obese.
As of April 28, 2020, 101 (39%) of the critically ill patients had died following a median of 9 days (interquartile range (IQR), 5–15) in the hospital and 94 (37%) remained hospitalized. Of the 203 patients who received invasive mechanical ventilation, 84 (41%) had died.
The poor prognosis of patients requiring ventilation is consistent with data from a report on patients treated in National Health Service intensive care units in England, Wales, and Northern Ireland through May 15. Overall, 11,292 patients with COVID-19 required critical care, and 4855 needed advanced respiratory support. Approximately half of the patients receiving mechanical ventilation had died 30 days after starting critical care.
In the New York study, patients spent an average of 18 days on a ventilator (IQR, 9–28 days). This is a longer period than reported in smaller studies of cases from Washington state, but corresponds with a recent report from Italy, the researchers said.
Remarkably, O’Donnell and colleagues report that almost a third (31%) of critically ill patients developed severe kidney damage and required dialysis.
Mortality was associated with several baseline factors, including older age (aHR, 1.31 [95% CI, 1.09–1.57] per 10-year increase), chronic cardiac disease (aHR, 1.76; 95% CI, 1.08–2·86), and chronic pulmonary disease (aHR, 2.94; 95% CI, 1.48–5.84).
Authors of the New York study reported financial relationships to ICE Neurosystems, ALung Technologies, Baxter, BREETHE, Xenios, Hemovent, Gilead Sciences, Amazon, and Karyopharm Therapeutics. Grasselli reports personal fees from Biotest, Draeger, Fisher & Paykel, Maquet, Merck Sharp & Dohme, and Pfizer, all outside the area of work commented on here. Zanella has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
ASCO goes ahead online, as conference center is used as hospital
Traditionally at this time of year, everyone working in cancer turns their attention toward Chicago, and 40,000 or so travel to the city for the annual meeting of the American Society of Clinical Oncology (ASCO).
Not this year.
The McCormick Place convention center has been converted to a field hospital to cope with the ongoing COVID-19 pandemic. The cavernous meeting halls have been filled with makeshift wards with 750 acute care beds, as shown in a tweet from Toni Choueiri, MD, chief of genitourinary oncology at the Dana Farber Cancer Center in Boston.
But the annual meeting is still going ahead, having been transferred online.
“We have to remember that even though there’s a pandemic going on and people are dying every day from coronavirus, people are still dying every day from cancer,” Richard Schilsky, MD, PhD, chief medical officer at ASCO, told Medscape Medical News.
“This pandemic will end, but cancer will continue, and we need to be able to continue to get the most cutting edge scientific results out there to our members and our constituents so they can act on those results on behalf of their patients,” he said.
The ASCO Virtual Scientific Program will take place over the weekend of May 30-31.
“We’re certainly hoping that we’re going to deliver a program that features all of the most important science that would have been presented in person in Chicago,” Schilsky commented in an interview.
Most of the presentations will be prerecorded and then streamed, which “we hope will mitigate any of the technical glitches that could come from trying to do a live broadcast of the meeting,” he said.
There will be 250 oral and 2500 poster presentations in 24 disease-based and specialty tracks.
The majority of the abstracts will be released online on May 13. The majority of the on-demand content will be released on May 29. Some of the abstracts will be highlighted at ASCO press briefings and released on those two dates.
But some of the material will be made available only on the weekend of the meeting. The opening session, plenaries featuring late-breaking abstracts, special highlights sessions, and other clinical science symposia will be broadcast on Saturday, May 30, and Sunday, May 31 (the schedule for the weekend program is available on the ASCO meeting website).
Among the plenary presentations are some clinical results that are likely to change practice immediately, Schilsky predicted. These include data to be presented in the following abstracts:
- Abstract LBA4 on the KEYNOTE-177 study comparing immunotherapy using pembrolizumab (Keytruda, Merck & Co) with chemotherapy in patients with metastatic colorectal cancer whose tumors show microsatellite instability or mismatch repair deficiency;
- Abstract LBA5 on the ADAURA study exploring osimertinib (Tagrisso, AstraZeneca) as adjuvant therapy after complete tumor reseaction in patients with early-stage non–small cell lung cancer whose tumors are EGFR mutation positive;
- Abstract LBA1 on the JAVELIN Bladder 100 study exploring maintenance avelumab (Bavencio, Merck and Pfizer) with best supportive care after platinum-based first-line chemotherapy in patients with advanced urothelial carcinoma.
However, some of the material that would have been part of the annual meeting, which includes mostly educational sessions and invited talks, has been moved to another event, the ASCO Educational Program, to be held in August 2020.
“So I suppose, in the grand scheme of things, the meeting is going to be compressed a little bit,” Schilsky commented. “Obviously, we can’t deliver all the interactions that happen in the hallways and everywhere else at the meeting that really gives so much energy to the meeting, but, at this moment in our history, probably getting the science out there is what’s most important.”
Virtual exhibition hall
There will also be a virtual exhibition hall, which will open on May 29.
“Just as there is a typical exhibit hall in the convention center,” Schilsky commented, most of the companies that were planning to be in Chicago have “now transitioned to creating a virtual booth that people who are participating in the virtual meeting can visit.
“I don’t know exactly how each company is going to use their time and their virtual space, and that’s part of the whole learning process here to see how this whole experiment is going to work out,” he added.
Unlike some of the other conferences that have gone virtual, in which access has been made available to everyone for free, registration is still required for the ASCO meeting. But the society notes that the registration fee has been discounted for nonmembers and has been waived for ASCO members. Also, the fee covers both the Virtual Scientific Program in May and the ASCO Educational Program in August.
Registrants will have access to video and slide presentations, as well as discussant commentaries, for 180 days.
The article first appeared on Medscape.com.
Traditionally at this time of year, everyone working in cancer turns their attention toward Chicago, and 40,000 or so travel to the city for the annual meeting of the American Society of Clinical Oncology (ASCO).
Not this year.
The McCormick Place convention center has been converted to a field hospital to cope with the ongoing COVID-19 pandemic. The cavernous meeting halls have been filled with makeshift wards with 750 acute care beds, as shown in a tweet from Toni Choueiri, MD, chief of genitourinary oncology at the Dana Farber Cancer Center in Boston.
But the annual meeting is still going ahead, having been transferred online.
“We have to remember that even though there’s a pandemic going on and people are dying every day from coronavirus, people are still dying every day from cancer,” Richard Schilsky, MD, PhD, chief medical officer at ASCO, told Medscape Medical News.
“This pandemic will end, but cancer will continue, and we need to be able to continue to get the most cutting edge scientific results out there to our members and our constituents so they can act on those results on behalf of their patients,” he said.
The ASCO Virtual Scientific Program will take place over the weekend of May 30-31.
“We’re certainly hoping that we’re going to deliver a program that features all of the most important science that would have been presented in person in Chicago,” Schilsky commented in an interview.
Most of the presentations will be prerecorded and then streamed, which “we hope will mitigate any of the technical glitches that could come from trying to do a live broadcast of the meeting,” he said.
There will be 250 oral and 2500 poster presentations in 24 disease-based and specialty tracks.
The majority of the abstracts will be released online on May 13. The majority of the on-demand content will be released on May 29. Some of the abstracts will be highlighted at ASCO press briefings and released on those two dates.
But some of the material will be made available only on the weekend of the meeting. The opening session, plenaries featuring late-breaking abstracts, special highlights sessions, and other clinical science symposia will be broadcast on Saturday, May 30, and Sunday, May 31 (the schedule for the weekend program is available on the ASCO meeting website).
Among the plenary presentations are some clinical results that are likely to change practice immediately, Schilsky predicted. These include data to be presented in the following abstracts:
- Abstract LBA4 on the KEYNOTE-177 study comparing immunotherapy using pembrolizumab (Keytruda, Merck & Co) with chemotherapy in patients with metastatic colorectal cancer whose tumors show microsatellite instability or mismatch repair deficiency;
- Abstract LBA5 on the ADAURA study exploring osimertinib (Tagrisso, AstraZeneca) as adjuvant therapy after complete tumor reseaction in patients with early-stage non–small cell lung cancer whose tumors are EGFR mutation positive;
- Abstract LBA1 on the JAVELIN Bladder 100 study exploring maintenance avelumab (Bavencio, Merck and Pfizer) with best supportive care after platinum-based first-line chemotherapy in patients with advanced urothelial carcinoma.
However, some of the material that would have been part of the annual meeting, which includes mostly educational sessions and invited talks, has been moved to another event, the ASCO Educational Program, to be held in August 2020.
“So I suppose, in the grand scheme of things, the meeting is going to be compressed a little bit,” Schilsky commented. “Obviously, we can’t deliver all the interactions that happen in the hallways and everywhere else at the meeting that really gives so much energy to the meeting, but, at this moment in our history, probably getting the science out there is what’s most important.”
Virtual exhibition hall
There will also be a virtual exhibition hall, which will open on May 29.
“Just as there is a typical exhibit hall in the convention center,” Schilsky commented, most of the companies that were planning to be in Chicago have “now transitioned to creating a virtual booth that people who are participating in the virtual meeting can visit.
“I don’t know exactly how each company is going to use their time and their virtual space, and that’s part of the whole learning process here to see how this whole experiment is going to work out,” he added.
Unlike some of the other conferences that have gone virtual, in which access has been made available to everyone for free, registration is still required for the ASCO meeting. But the society notes that the registration fee has been discounted for nonmembers and has been waived for ASCO members. Also, the fee covers both the Virtual Scientific Program in May and the ASCO Educational Program in August.
Registrants will have access to video and slide presentations, as well as discussant commentaries, for 180 days.
The article first appeared on Medscape.com.
Traditionally at this time of year, everyone working in cancer turns their attention toward Chicago, and 40,000 or so travel to the city for the annual meeting of the American Society of Clinical Oncology (ASCO).
Not this year.
The McCormick Place convention center has been converted to a field hospital to cope with the ongoing COVID-19 pandemic. The cavernous meeting halls have been filled with makeshift wards with 750 acute care beds, as shown in a tweet from Toni Choueiri, MD, chief of genitourinary oncology at the Dana Farber Cancer Center in Boston.
But the annual meeting is still going ahead, having been transferred online.
“We have to remember that even though there’s a pandemic going on and people are dying every day from coronavirus, people are still dying every day from cancer,” Richard Schilsky, MD, PhD, chief medical officer at ASCO, told Medscape Medical News.
“This pandemic will end, but cancer will continue, and we need to be able to continue to get the most cutting edge scientific results out there to our members and our constituents so they can act on those results on behalf of their patients,” he said.
The ASCO Virtual Scientific Program will take place over the weekend of May 30-31.
“We’re certainly hoping that we’re going to deliver a program that features all of the most important science that would have been presented in person in Chicago,” Schilsky commented in an interview.
Most of the presentations will be prerecorded and then streamed, which “we hope will mitigate any of the technical glitches that could come from trying to do a live broadcast of the meeting,” he said.
There will be 250 oral and 2500 poster presentations in 24 disease-based and specialty tracks.
The majority of the abstracts will be released online on May 13. The majority of the on-demand content will be released on May 29. Some of the abstracts will be highlighted at ASCO press briefings and released on those two dates.
But some of the material will be made available only on the weekend of the meeting. The opening session, plenaries featuring late-breaking abstracts, special highlights sessions, and other clinical science symposia will be broadcast on Saturday, May 30, and Sunday, May 31 (the schedule for the weekend program is available on the ASCO meeting website).
Among the plenary presentations are some clinical results that are likely to change practice immediately, Schilsky predicted. These include data to be presented in the following abstracts:
- Abstract LBA4 on the KEYNOTE-177 study comparing immunotherapy using pembrolizumab (Keytruda, Merck & Co) with chemotherapy in patients with metastatic colorectal cancer whose tumors show microsatellite instability or mismatch repair deficiency;
- Abstract LBA5 on the ADAURA study exploring osimertinib (Tagrisso, AstraZeneca) as adjuvant therapy after complete tumor reseaction in patients with early-stage non–small cell lung cancer whose tumors are EGFR mutation positive;
- Abstract LBA1 on the JAVELIN Bladder 100 study exploring maintenance avelumab (Bavencio, Merck and Pfizer) with best supportive care after platinum-based first-line chemotherapy in patients with advanced urothelial carcinoma.
However, some of the material that would have been part of the annual meeting, which includes mostly educational sessions and invited talks, has been moved to another event, the ASCO Educational Program, to be held in August 2020.
“So I suppose, in the grand scheme of things, the meeting is going to be compressed a little bit,” Schilsky commented. “Obviously, we can’t deliver all the interactions that happen in the hallways and everywhere else at the meeting that really gives so much energy to the meeting, but, at this moment in our history, probably getting the science out there is what’s most important.”
Virtual exhibition hall
There will also be a virtual exhibition hall, which will open on May 29.
“Just as there is a typical exhibit hall in the convention center,” Schilsky commented, most of the companies that were planning to be in Chicago have “now transitioned to creating a virtual booth that people who are participating in the virtual meeting can visit.
“I don’t know exactly how each company is going to use their time and their virtual space, and that’s part of the whole learning process here to see how this whole experiment is going to work out,” he added.
Unlike some of the other conferences that have gone virtual, in which access has been made available to everyone for free, registration is still required for the ASCO meeting. But the society notes that the registration fee has been discounted for nonmembers and has been waived for ASCO members. Also, the fee covers both the Virtual Scientific Program in May and the ASCO Educational Program in August.
Registrants will have access to video and slide presentations, as well as discussant commentaries, for 180 days.
The article first appeared on Medscape.com.
Androgens may explain male vulnerability to COVID-19
As the COVID-19 pandemic has swept across the world, a striking difference has been seen between the sexes. But why are men so much more susceptible to severe outcomes from COVID-19 than women?
Suspicions naturally turn to the sex hormones, and there have been suggestions that estrogen may be protective against COVID-19 in females and/or that androgens worsen COVID-19 outcomes in males.
New data supporting the androgen theory come from a study in Italy.
These researchers found that patients with prostate cancer being treated with androgen deprivation therapy (ADT) were less likely to become infected with COVID-19 and die from the disease than other groups, including other patients with cancer.
The findings suggest that androgens somehow make the virus more virulent and that this exacerbates the severity of disease in men, they say. They also speculate that ADT may be protective against COVID-19.
The study was published online May 7 in Annals of Oncology.
The team analyzed data from 68 hospitals in the Veneto region, one of the areas in Italy most severely affected by the COVID-19 pandemic.
They found data on 9280 patients with laboratory-confirmed SARS-CoV-2 infection — of whom 4532 were males.
Women in the region were actually slightly more likely to be infected with COVID-19 than men, 56% vs 44%, the researchers point out.
However, men were more prone to develop more severe forms of the disease: 60% of men vs 40% of women required hospitalization, rising to 78% of men vs 22% of women who required intensive care. Also, more men died than women (62% vs 38%).
The team then turned their focus onto patients with cancer.
Of the entire male population of Veneto, those with cancer had an almost twofold higher risk of becoming infected with COVID-19 than men without cancer (P < .0001).
However, when the team looked specifically at men with prostate cancer in the region, they found “strikingly, only 4 out of 5273 patients receiving ADT developed SARS-CoV-2 infection and none of these patients died.”
This compared to 37,161 men with prostate cancer who were not receiving ADT, among whom 114 men developed COVID-19 and 18 died.
Among another 79,661 patients in the Veneto region with cancer other than prostate cancer, 312 developed COVID-19 and 57 died.
“This is the first paper to suggest a link between ADT and COVID-19,” commented lead author Andrea Alimonti, MD, PhD, Università della Svizzera Italiana in Lugano, Switzerland.
“Patients with prostate cancer receiving ADT had a significant fourfold reduced risk of COVID-19 infections compared to patients who did not receive ADT. An even greater difference (fivefold reduction in risk) was found when we compared prostate cancer patients receiving ADT to patients with any other type of cancer,” he said.
The finding raises “the hypothesis that androgen levels can facilitate coronavirus infections and increase the severity of symptoms, as has been seen in male patients,” he said.
“These data are very interesting and raise a fascinating hypothesis,” said Richard Martin, PhD, professor of clinical epidemiology at the University of Bristol, UK, commenting about the study. “But they do need independent validation in other large population-wide datasets...with appropriate statistical analysis including adjustment for important risk factors for SARS-CoV-2.”
He noted that the Italian study results were not adjusted for potential confounders, for example, age, body mass index, and cardiometabolic comorbidities, that are strong risk factors for SARS-CoV-2. In addition, men taking ADT may have been more likely to self-isolate and so be at reduced risk of getting the infection, he suggested.
How Do Androgens Interact With the Virus?
Alimonti and colleagues offer a mechanistic explanation of how androgens interact with the virus.
Coronavirus gains entry into the human cell by binding its viral spike (S) proteins to ACE2 and on S protein priming by TMPRSS2. TMPRSS2 is a member of a family of proteins called type II transmembrane serine proteases, which are involved in a number of processes including cancer and viral infections, they explain.
“Intriguingly, TMPRSS2 is an androgen-regulated gene that is upregulated in prostate cancer where it supports tumor progression,” they point out.
There is also evidence that the same androgen receptor regulates TMPRSS2 expression in nonprostatic tissues, including the lungs.
“[This] may explain the increased susceptibility of men to develop SARS-CoV-2 severe infections when compared to women,” the authors speculate.
Because ADT is known to decrease TMPRSS2 levels, they suggest that androgen receptor antagonists “could be used to block or decrease the severity of SARS-CoV-2 infection in male patients.”
They go even further and suggest that men without prostate cancer at high risk for COVID-19 could take ADT to prevent infection.
For men who do become infected with COVID-19, ADT might also help reduce symptom severity, they add.
Given that the effects of androgen receptor antagonists are reversible, “they could be used transiently (eg, 1 month) in patients affected by SARS-CoV-2, thereby reducing the risk of side effects due to long-term administration,” the authors suggest.
Another Theory: Is Estrogen Protective?
Another theory to explain the male/female difference for severe COVID-19 is that the female hormone estrogen may be protective.
“People have to stop putting estrogen in that ‘female hormone box’ because it’s a molecule that we all use as humans, it’s just not women,” Sharon Nachman, MD, told Medscape Medical News.
“Looking at estrogen as having potentially important immune effects is part of thinking outside the box,” she said.
Nachman is associate dean for research at the Renaissance School of Medicine, Stony Brook University in New York, and is working together with Antonios Gasparis, MD, professor of surgery at the same center.
They are exploring the use of a transdermal estrogen patch in patients with COVID-19 in a randomized trial with a placebo-controlled arm. They are recruiting patients who present to their emergency department with signs and symptoms of COVID-19, and enroll them into the trial if they are interested.
“We are testing everyone as well, but we are starting patients on the medication at the time of entry as opposed to waiting until we have a test result back,” Nachman explained.
The primary objective of the study is to evaluate whether the transdermal patch, applied to the skin for 7 days, might reduce the need for intubation in men and women infected with COVID-19 versus standard of care.
The product is the same single-use transdermal estradiol patch (Climara, 25 cm2, Bayer) prescribed for postmenopausal women and will be used at the same dose, which is known to be safe.
After the patch is removed, patients will be carefully tracked for symptoms over the next 45 days to see if the patch reduced symptom severity, and if so, in which patients.
Nachman would have preferred to enroll patients before they had overt symptoms, but this simply isn’t possible in a medical center where symptomatic patients present, she told Medscape Medical News.
However, she does know that even at their own medical center, the odds are stacked against male COVID-19 patients — and something is needed to mitigate its severity in this patient group.
As they were developing the protocol for the current study, the team decided to see who was in their ICU during a single study day.
The answer: mostly males. Intubation and death rates in men in their ICU for that single day was approximately 80% compared with only 20% among women.
“We have a new horrific pathogen that is pandemic and we’re all probably going to get it, it’s just a question of when and how sick we’ll be from it,” Nachman said.
Alimonti and coauthors have reported no relevant financial relationships, as did Goulder and Nachman.
This article first appeared on Medscape.com.
As the COVID-19 pandemic has swept across the world, a striking difference has been seen between the sexes. But why are men so much more susceptible to severe outcomes from COVID-19 than women?
Suspicions naturally turn to the sex hormones, and there have been suggestions that estrogen may be protective against COVID-19 in females and/or that androgens worsen COVID-19 outcomes in males.
New data supporting the androgen theory come from a study in Italy.
These researchers found that patients with prostate cancer being treated with androgen deprivation therapy (ADT) were less likely to become infected with COVID-19 and die from the disease than other groups, including other patients with cancer.
The findings suggest that androgens somehow make the virus more virulent and that this exacerbates the severity of disease in men, they say. They also speculate that ADT may be protective against COVID-19.
The study was published online May 7 in Annals of Oncology.
The team analyzed data from 68 hospitals in the Veneto region, one of the areas in Italy most severely affected by the COVID-19 pandemic.
They found data on 9280 patients with laboratory-confirmed SARS-CoV-2 infection — of whom 4532 were males.
Women in the region were actually slightly more likely to be infected with COVID-19 than men, 56% vs 44%, the researchers point out.
However, men were more prone to develop more severe forms of the disease: 60% of men vs 40% of women required hospitalization, rising to 78% of men vs 22% of women who required intensive care. Also, more men died than women (62% vs 38%).
The team then turned their focus onto patients with cancer.
Of the entire male population of Veneto, those with cancer had an almost twofold higher risk of becoming infected with COVID-19 than men without cancer (P < .0001).
However, when the team looked specifically at men with prostate cancer in the region, they found “strikingly, only 4 out of 5273 patients receiving ADT developed SARS-CoV-2 infection and none of these patients died.”
This compared to 37,161 men with prostate cancer who were not receiving ADT, among whom 114 men developed COVID-19 and 18 died.
Among another 79,661 patients in the Veneto region with cancer other than prostate cancer, 312 developed COVID-19 and 57 died.
“This is the first paper to suggest a link between ADT and COVID-19,” commented lead author Andrea Alimonti, MD, PhD, Università della Svizzera Italiana in Lugano, Switzerland.
“Patients with prostate cancer receiving ADT had a significant fourfold reduced risk of COVID-19 infections compared to patients who did not receive ADT. An even greater difference (fivefold reduction in risk) was found when we compared prostate cancer patients receiving ADT to patients with any other type of cancer,” he said.
The finding raises “the hypothesis that androgen levels can facilitate coronavirus infections and increase the severity of symptoms, as has been seen in male patients,” he said.
“These data are very interesting and raise a fascinating hypothesis,” said Richard Martin, PhD, professor of clinical epidemiology at the University of Bristol, UK, commenting about the study. “But they do need independent validation in other large population-wide datasets...with appropriate statistical analysis including adjustment for important risk factors for SARS-CoV-2.”
He noted that the Italian study results were not adjusted for potential confounders, for example, age, body mass index, and cardiometabolic comorbidities, that are strong risk factors for SARS-CoV-2. In addition, men taking ADT may have been more likely to self-isolate and so be at reduced risk of getting the infection, he suggested.
How Do Androgens Interact With the Virus?
Alimonti and colleagues offer a mechanistic explanation of how androgens interact with the virus.
Coronavirus gains entry into the human cell by binding its viral spike (S) proteins to ACE2 and on S protein priming by TMPRSS2. TMPRSS2 is a member of a family of proteins called type II transmembrane serine proteases, which are involved in a number of processes including cancer and viral infections, they explain.
“Intriguingly, TMPRSS2 is an androgen-regulated gene that is upregulated in prostate cancer where it supports tumor progression,” they point out.
There is also evidence that the same androgen receptor regulates TMPRSS2 expression in nonprostatic tissues, including the lungs.
“[This] may explain the increased susceptibility of men to develop SARS-CoV-2 severe infections when compared to women,” the authors speculate.
Because ADT is known to decrease TMPRSS2 levels, they suggest that androgen receptor antagonists “could be used to block or decrease the severity of SARS-CoV-2 infection in male patients.”
They go even further and suggest that men without prostate cancer at high risk for COVID-19 could take ADT to prevent infection.
For men who do become infected with COVID-19, ADT might also help reduce symptom severity, they add.
Given that the effects of androgen receptor antagonists are reversible, “they could be used transiently (eg, 1 month) in patients affected by SARS-CoV-2, thereby reducing the risk of side effects due to long-term administration,” the authors suggest.
Another Theory: Is Estrogen Protective?
Another theory to explain the male/female difference for severe COVID-19 is that the female hormone estrogen may be protective.
“People have to stop putting estrogen in that ‘female hormone box’ because it’s a molecule that we all use as humans, it’s just not women,” Sharon Nachman, MD, told Medscape Medical News.
“Looking at estrogen as having potentially important immune effects is part of thinking outside the box,” she said.
Nachman is associate dean for research at the Renaissance School of Medicine, Stony Brook University in New York, and is working together with Antonios Gasparis, MD, professor of surgery at the same center.
They are exploring the use of a transdermal estrogen patch in patients with COVID-19 in a randomized trial with a placebo-controlled arm. They are recruiting patients who present to their emergency department with signs and symptoms of COVID-19, and enroll them into the trial if they are interested.
“We are testing everyone as well, but we are starting patients on the medication at the time of entry as opposed to waiting until we have a test result back,” Nachman explained.
The primary objective of the study is to evaluate whether the transdermal patch, applied to the skin for 7 days, might reduce the need for intubation in men and women infected with COVID-19 versus standard of care.
The product is the same single-use transdermal estradiol patch (Climara, 25 cm2, Bayer) prescribed for postmenopausal women and will be used at the same dose, which is known to be safe.
After the patch is removed, patients will be carefully tracked for symptoms over the next 45 days to see if the patch reduced symptom severity, and if so, in which patients.
Nachman would have preferred to enroll patients before they had overt symptoms, but this simply isn’t possible in a medical center where symptomatic patients present, she told Medscape Medical News.
However, she does know that even at their own medical center, the odds are stacked against male COVID-19 patients — and something is needed to mitigate its severity in this patient group.
As they were developing the protocol for the current study, the team decided to see who was in their ICU during a single study day.
The answer: mostly males. Intubation and death rates in men in their ICU for that single day was approximately 80% compared with only 20% among women.
“We have a new horrific pathogen that is pandemic and we’re all probably going to get it, it’s just a question of when and how sick we’ll be from it,” Nachman said.
Alimonti and coauthors have reported no relevant financial relationships, as did Goulder and Nachman.
This article first appeared on Medscape.com.
As the COVID-19 pandemic has swept across the world, a striking difference has been seen between the sexes. But why are men so much more susceptible to severe outcomes from COVID-19 than women?
Suspicions naturally turn to the sex hormones, and there have been suggestions that estrogen may be protective against COVID-19 in females and/or that androgens worsen COVID-19 outcomes in males.
New data supporting the androgen theory come from a study in Italy.
These researchers found that patients with prostate cancer being treated with androgen deprivation therapy (ADT) were less likely to become infected with COVID-19 and die from the disease than other groups, including other patients with cancer.
The findings suggest that androgens somehow make the virus more virulent and that this exacerbates the severity of disease in men, they say. They also speculate that ADT may be protective against COVID-19.
The study was published online May 7 in Annals of Oncology.
The team analyzed data from 68 hospitals in the Veneto region, one of the areas in Italy most severely affected by the COVID-19 pandemic.
They found data on 9280 patients with laboratory-confirmed SARS-CoV-2 infection — of whom 4532 were males.
Women in the region were actually slightly more likely to be infected with COVID-19 than men, 56% vs 44%, the researchers point out.
However, men were more prone to develop more severe forms of the disease: 60% of men vs 40% of women required hospitalization, rising to 78% of men vs 22% of women who required intensive care. Also, more men died than women (62% vs 38%).
The team then turned their focus onto patients with cancer.
Of the entire male population of Veneto, those with cancer had an almost twofold higher risk of becoming infected with COVID-19 than men without cancer (P < .0001).
However, when the team looked specifically at men with prostate cancer in the region, they found “strikingly, only 4 out of 5273 patients receiving ADT developed SARS-CoV-2 infection and none of these patients died.”
This compared to 37,161 men with prostate cancer who were not receiving ADT, among whom 114 men developed COVID-19 and 18 died.
Among another 79,661 patients in the Veneto region with cancer other than prostate cancer, 312 developed COVID-19 and 57 died.
“This is the first paper to suggest a link between ADT and COVID-19,” commented lead author Andrea Alimonti, MD, PhD, Università della Svizzera Italiana in Lugano, Switzerland.
“Patients with prostate cancer receiving ADT had a significant fourfold reduced risk of COVID-19 infections compared to patients who did not receive ADT. An even greater difference (fivefold reduction in risk) was found when we compared prostate cancer patients receiving ADT to patients with any other type of cancer,” he said.
The finding raises “the hypothesis that androgen levels can facilitate coronavirus infections and increase the severity of symptoms, as has been seen in male patients,” he said.
“These data are very interesting and raise a fascinating hypothesis,” said Richard Martin, PhD, professor of clinical epidemiology at the University of Bristol, UK, commenting about the study. “But they do need independent validation in other large population-wide datasets...with appropriate statistical analysis including adjustment for important risk factors for SARS-CoV-2.”
He noted that the Italian study results were not adjusted for potential confounders, for example, age, body mass index, and cardiometabolic comorbidities, that are strong risk factors for SARS-CoV-2. In addition, men taking ADT may have been more likely to self-isolate and so be at reduced risk of getting the infection, he suggested.
How Do Androgens Interact With the Virus?
Alimonti and colleagues offer a mechanistic explanation of how androgens interact with the virus.
Coronavirus gains entry into the human cell by binding its viral spike (S) proteins to ACE2 and on S protein priming by TMPRSS2. TMPRSS2 is a member of a family of proteins called type II transmembrane serine proteases, which are involved in a number of processes including cancer and viral infections, they explain.
“Intriguingly, TMPRSS2 is an androgen-regulated gene that is upregulated in prostate cancer where it supports tumor progression,” they point out.
There is also evidence that the same androgen receptor regulates TMPRSS2 expression in nonprostatic tissues, including the lungs.
“[This] may explain the increased susceptibility of men to develop SARS-CoV-2 severe infections when compared to women,” the authors speculate.
Because ADT is known to decrease TMPRSS2 levels, they suggest that androgen receptor antagonists “could be used to block or decrease the severity of SARS-CoV-2 infection in male patients.”
They go even further and suggest that men without prostate cancer at high risk for COVID-19 could take ADT to prevent infection.
For men who do become infected with COVID-19, ADT might also help reduce symptom severity, they add.
Given that the effects of androgen receptor antagonists are reversible, “they could be used transiently (eg, 1 month) in patients affected by SARS-CoV-2, thereby reducing the risk of side effects due to long-term administration,” the authors suggest.
Another Theory: Is Estrogen Protective?
Another theory to explain the male/female difference for severe COVID-19 is that the female hormone estrogen may be protective.
“People have to stop putting estrogen in that ‘female hormone box’ because it’s a molecule that we all use as humans, it’s just not women,” Sharon Nachman, MD, told Medscape Medical News.
“Looking at estrogen as having potentially important immune effects is part of thinking outside the box,” she said.
Nachman is associate dean for research at the Renaissance School of Medicine, Stony Brook University in New York, and is working together with Antonios Gasparis, MD, professor of surgery at the same center.
They are exploring the use of a transdermal estrogen patch in patients with COVID-19 in a randomized trial with a placebo-controlled arm. They are recruiting patients who present to their emergency department with signs and symptoms of COVID-19, and enroll them into the trial if they are interested.
“We are testing everyone as well, but we are starting patients on the medication at the time of entry as opposed to waiting until we have a test result back,” Nachman explained.
The primary objective of the study is to evaluate whether the transdermal patch, applied to the skin for 7 days, might reduce the need for intubation in men and women infected with COVID-19 versus standard of care.
The product is the same single-use transdermal estradiol patch (Climara, 25 cm2, Bayer) prescribed for postmenopausal women and will be used at the same dose, which is known to be safe.
After the patch is removed, patients will be carefully tracked for symptoms over the next 45 days to see if the patch reduced symptom severity, and if so, in which patients.
Nachman would have preferred to enroll patients before they had overt symptoms, but this simply isn’t possible in a medical center where symptomatic patients present, she told Medscape Medical News.
However, she does know that even at their own medical center, the odds are stacked against male COVID-19 patients — and something is needed to mitigate its severity in this patient group.
As they were developing the protocol for the current study, the team decided to see who was in their ICU during a single study day.
The answer: mostly males. Intubation and death rates in men in their ICU for that single day was approximately 80% compared with only 20% among women.
“We have a new horrific pathogen that is pandemic and we’re all probably going to get it, it’s just a question of when and how sick we’ll be from it,” Nachman said.
Alimonti and coauthors have reported no relevant financial relationships, as did Goulder and Nachman.
This article first appeared on Medscape.com.
COVID-19 death rate was twice as high in cancer patients in NYC study
COVID-19 patients with cancer had double the fatality rate of COVID-19 patients without cancer treated in an urban New York hospital system, according to data from a retrospective study.
with COVID-19 treated during the same time period in the same hospital system.
Vikas Mehta, MD, of Montefiore Medical Center, New York, and colleagues reported these results in Cancer Discovery.
“As New York has emerged as the current epicenter of the pandemic, we sought to investigate the risk posed by COVID-19 to our cancer population,” the authors wrote.
They identified 218 cancer patients treated for COVID-19 in the Montefiore Health System between March 18 and April 8, 2020. Three-quarters of patients had solid tumors, and 25% had hematologic malignancies. Most patients were adults (98.6%), their median age was 69 years (range, 10-92 years), and 58% were men.
In all, 28% of the cancer patients (61/218) died from COVID-19, including 25% (41/164) of those with solid tumors and 37% (20/54) of those with hematologic malignancies.
Deaths by cancer type
Among the 164 patients with solid tumors, case fatality rates were as follows:
- Pancreatic – 67% (2/3)
- Lung – 55% (6/11)
- Colorectal – 38% (8/21)
- Upper gastrointestinal – 38% (3/8)
- Gynecologic – 38% (5/13)
- Skin – 33% (1/3)
- Hepatobiliary – 29% (2/7)
- Bone/soft tissue – 20% (1/5)
- Genitourinary – 15% (7/46)
- Breast – 14% (4/28)
- Neurologic – 13% (1/8)
- Head and neck – 13% (1/8).
None of the three patients with neuroendocrine tumors died.
Among the 54 patients with hematologic malignancies, case fatality rates were as follows:
- Chronic myeloid leukemia – 100% (1/1)
- Hodgkin lymphoma – 60% (3/5)
- Myelodysplastic syndromes – 60% (3/5)
- Multiple myeloma – 38% (5/13)
- Non-Hodgkin lymphoma – 33% (5/15)
- Chronic lymphocytic leukemia – 33% (1/3)
- Myeloproliferative neoplasms – 29% (2/7).
None of the four patients with acute lymphoblastic leukemia died, and there was one patient with acute myeloid leukemia who did not die.
Factors associated with increased mortality
The researchers compared the 218 cancer patients with COVID-19 with 1,090 age- and sex-matched noncancer patients with COVID-19 treated in the Montefiore Health System between March 18 and April 8, 2020.
Case fatality rates in cancer patients with COVID-19 were significantly increased in all age groups, but older age was associated with higher mortality.
“We observed case fatality rates were elevated in all age cohorts in cancer patients and achieved statistical significance in the age groups 45-64 and in patients older than 75 years of age,” the authors reported.
Other factors significantly associated with higher mortality in a multivariable analysis included the presence of multiple comorbidities; the need for ICU support; and increased levels of d-dimer, lactate, and lactate dehydrogenase.
Additional factors, such as socioeconomic and health disparities, may also be significant predictors of mortality, according to the authors. They noted that this cohort largely consisted of patients from a socioeconomically underprivileged community where mortality because of COVID-19 is reportedly higher.
Proactive strategies moving forward
“We have been addressing the significant burden of the COVID-19 pandemic on our vulnerable cancer patients through a variety of ways,” said study author Balazs Halmos, MD, of Montefiore Medical Center.
The center set up a separate infusion unit exclusively for COVID-positive patients and established separate inpatient areas. Dr. Halmos and colleagues are also providing telemedicine, virtual supportive care services, telephonic counseling, and bilingual peer-support programs.
“Many questions remain as we continue to establish new practices for our cancer patients,” Dr. Halmos said. “We will find answers to these questions as we continue to focus on adaptation and not acceptance in response to the COVID crisis. Our patients deserve nothing less.”
The Albert Einstein Cancer Center supported this study. The authors reported having no conflicts of interest.
SOURCE: Mehta V et al. Cancer Discov. 2020 May 1. doi: 10.1158/2159-8290.CD-20-0516.
COVID-19 patients with cancer had double the fatality rate of COVID-19 patients without cancer treated in an urban New York hospital system, according to data from a retrospective study.
with COVID-19 treated during the same time period in the same hospital system.
Vikas Mehta, MD, of Montefiore Medical Center, New York, and colleagues reported these results in Cancer Discovery.
“As New York has emerged as the current epicenter of the pandemic, we sought to investigate the risk posed by COVID-19 to our cancer population,” the authors wrote.
They identified 218 cancer patients treated for COVID-19 in the Montefiore Health System between March 18 and April 8, 2020. Three-quarters of patients had solid tumors, and 25% had hematologic malignancies. Most patients were adults (98.6%), their median age was 69 years (range, 10-92 years), and 58% were men.
In all, 28% of the cancer patients (61/218) died from COVID-19, including 25% (41/164) of those with solid tumors and 37% (20/54) of those with hematologic malignancies.
Deaths by cancer type
Among the 164 patients with solid tumors, case fatality rates were as follows:
- Pancreatic – 67% (2/3)
- Lung – 55% (6/11)
- Colorectal – 38% (8/21)
- Upper gastrointestinal – 38% (3/8)
- Gynecologic – 38% (5/13)
- Skin – 33% (1/3)
- Hepatobiliary – 29% (2/7)
- Bone/soft tissue – 20% (1/5)
- Genitourinary – 15% (7/46)
- Breast – 14% (4/28)
- Neurologic – 13% (1/8)
- Head and neck – 13% (1/8).
None of the three patients with neuroendocrine tumors died.
Among the 54 patients with hematologic malignancies, case fatality rates were as follows:
- Chronic myeloid leukemia – 100% (1/1)
- Hodgkin lymphoma – 60% (3/5)
- Myelodysplastic syndromes – 60% (3/5)
- Multiple myeloma – 38% (5/13)
- Non-Hodgkin lymphoma – 33% (5/15)
- Chronic lymphocytic leukemia – 33% (1/3)
- Myeloproliferative neoplasms – 29% (2/7).
None of the four patients with acute lymphoblastic leukemia died, and there was one patient with acute myeloid leukemia who did not die.
Factors associated with increased mortality
The researchers compared the 218 cancer patients with COVID-19 with 1,090 age- and sex-matched noncancer patients with COVID-19 treated in the Montefiore Health System between March 18 and April 8, 2020.
Case fatality rates in cancer patients with COVID-19 were significantly increased in all age groups, but older age was associated with higher mortality.
“We observed case fatality rates were elevated in all age cohorts in cancer patients and achieved statistical significance in the age groups 45-64 and in patients older than 75 years of age,” the authors reported.
Other factors significantly associated with higher mortality in a multivariable analysis included the presence of multiple comorbidities; the need for ICU support; and increased levels of d-dimer, lactate, and lactate dehydrogenase.
Additional factors, such as socioeconomic and health disparities, may also be significant predictors of mortality, according to the authors. They noted that this cohort largely consisted of patients from a socioeconomically underprivileged community where mortality because of COVID-19 is reportedly higher.
Proactive strategies moving forward
“We have been addressing the significant burden of the COVID-19 pandemic on our vulnerable cancer patients through a variety of ways,” said study author Balazs Halmos, MD, of Montefiore Medical Center.
The center set up a separate infusion unit exclusively for COVID-positive patients and established separate inpatient areas. Dr. Halmos and colleagues are also providing telemedicine, virtual supportive care services, telephonic counseling, and bilingual peer-support programs.
“Many questions remain as we continue to establish new practices for our cancer patients,” Dr. Halmos said. “We will find answers to these questions as we continue to focus on adaptation and not acceptance in response to the COVID crisis. Our patients deserve nothing less.”
The Albert Einstein Cancer Center supported this study. The authors reported having no conflicts of interest.
SOURCE: Mehta V et al. Cancer Discov. 2020 May 1. doi: 10.1158/2159-8290.CD-20-0516.
COVID-19 patients with cancer had double the fatality rate of COVID-19 patients without cancer treated in an urban New York hospital system, according to data from a retrospective study.
with COVID-19 treated during the same time period in the same hospital system.
Vikas Mehta, MD, of Montefiore Medical Center, New York, and colleagues reported these results in Cancer Discovery.
“As New York has emerged as the current epicenter of the pandemic, we sought to investigate the risk posed by COVID-19 to our cancer population,” the authors wrote.
They identified 218 cancer patients treated for COVID-19 in the Montefiore Health System between March 18 and April 8, 2020. Three-quarters of patients had solid tumors, and 25% had hematologic malignancies. Most patients were adults (98.6%), their median age was 69 years (range, 10-92 years), and 58% were men.
In all, 28% of the cancer patients (61/218) died from COVID-19, including 25% (41/164) of those with solid tumors and 37% (20/54) of those with hematologic malignancies.
Deaths by cancer type
Among the 164 patients with solid tumors, case fatality rates were as follows:
- Pancreatic – 67% (2/3)
- Lung – 55% (6/11)
- Colorectal – 38% (8/21)
- Upper gastrointestinal – 38% (3/8)
- Gynecologic – 38% (5/13)
- Skin – 33% (1/3)
- Hepatobiliary – 29% (2/7)
- Bone/soft tissue – 20% (1/5)
- Genitourinary – 15% (7/46)
- Breast – 14% (4/28)
- Neurologic – 13% (1/8)
- Head and neck – 13% (1/8).
None of the three patients with neuroendocrine tumors died.
Among the 54 patients with hematologic malignancies, case fatality rates were as follows:
- Chronic myeloid leukemia – 100% (1/1)
- Hodgkin lymphoma – 60% (3/5)
- Myelodysplastic syndromes – 60% (3/5)
- Multiple myeloma – 38% (5/13)
- Non-Hodgkin lymphoma – 33% (5/15)
- Chronic lymphocytic leukemia – 33% (1/3)
- Myeloproliferative neoplasms – 29% (2/7).
None of the four patients with acute lymphoblastic leukemia died, and there was one patient with acute myeloid leukemia who did not die.
Factors associated with increased mortality
The researchers compared the 218 cancer patients with COVID-19 with 1,090 age- and sex-matched noncancer patients with COVID-19 treated in the Montefiore Health System between March 18 and April 8, 2020.
Case fatality rates in cancer patients with COVID-19 were significantly increased in all age groups, but older age was associated with higher mortality.
“We observed case fatality rates were elevated in all age cohorts in cancer patients and achieved statistical significance in the age groups 45-64 and in patients older than 75 years of age,” the authors reported.
Other factors significantly associated with higher mortality in a multivariable analysis included the presence of multiple comorbidities; the need for ICU support; and increased levels of d-dimer, lactate, and lactate dehydrogenase.
Additional factors, such as socioeconomic and health disparities, may also be significant predictors of mortality, according to the authors. They noted that this cohort largely consisted of patients from a socioeconomically underprivileged community where mortality because of COVID-19 is reportedly higher.
Proactive strategies moving forward
“We have been addressing the significant burden of the COVID-19 pandemic on our vulnerable cancer patients through a variety of ways,” said study author Balazs Halmos, MD, of Montefiore Medical Center.
The center set up a separate infusion unit exclusively for COVID-positive patients and established separate inpatient areas. Dr. Halmos and colleagues are also providing telemedicine, virtual supportive care services, telephonic counseling, and bilingual peer-support programs.
“Many questions remain as we continue to establish new practices for our cancer patients,” Dr. Halmos said. “We will find answers to these questions as we continue to focus on adaptation and not acceptance in response to the COVID crisis. Our patients deserve nothing less.”
The Albert Einstein Cancer Center supported this study. The authors reported having no conflicts of interest.
SOURCE: Mehta V et al. Cancer Discov. 2020 May 1. doi: 10.1158/2159-8290.CD-20-0516.
FROM CANCER DISCOVERY
Three months of COVID-19 may mean 80,000 missed cancer diagnoses
, according to a report by the IQVIA Institute for Human Data Science looking at trends in the United States.
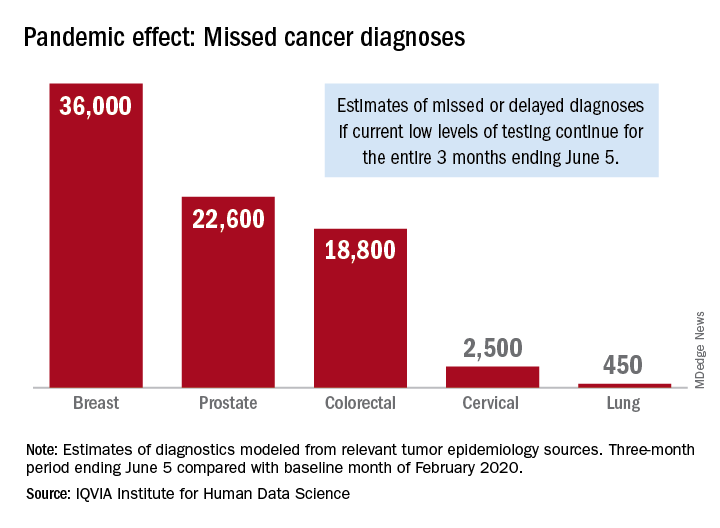
Screening and monitoring tests for breast, prostate, colorectal, cervical, and lung cancer were down 39%-90% in early April, compared with the baseline month of February, according to report authors Murray Aitken and Michael Kleinrock, both of IQVIA.
These findings are based on data from IQVIA’s medical claims database, which includes more than 205 million patients, over 1.7 billion claims, and 3 billion service records obtained annually.
The data suggest that, at current positivity rates, there could be 36,000 missed or delayed diagnoses of breast cancer during the 3-month period from early March through early June. Estimates for missed diagnoses of the four other cancers analyzed include 450 for lung cancer, 2,500 for cervical cancer, 18,800 for colorectal cancer, and 22,600 for prostate cancer.
The authors project a total of 22 million canceled or delayed tests for the five cancers over the 3-month period ending June 5, based on a comparison of claims data for early April with the February baseline. Catching up on this backlog will be problematic, according to the authors.
“Current excess health care capacity ... would require providers to shift priorities to make time and space in schedules and facilities as well as the cooperation of patients to return to health care providers,” the authors wrote. “Both of these could be further disrupted by economic factors or reintroduction of social distancing in a reemergence of the outbreak.”
The report was produced by the IQVIA Institute for Human Data Science without industry or government funding.
SOURCE: Murray A and Kleinrock M. Shifts in healthcare demand, delivery and care during the COVID-19 era. IQVIA Institute for Human Data Science. April 2020.
, according to a report by the IQVIA Institute for Human Data Science looking at trends in the United States.

Screening and monitoring tests for breast, prostate, colorectal, cervical, and lung cancer were down 39%-90% in early April, compared with the baseline month of February, according to report authors Murray Aitken and Michael Kleinrock, both of IQVIA.
These findings are based on data from IQVIA’s medical claims database, which includes more than 205 million patients, over 1.7 billion claims, and 3 billion service records obtained annually.
The data suggest that, at current positivity rates, there could be 36,000 missed or delayed diagnoses of breast cancer during the 3-month period from early March through early June. Estimates for missed diagnoses of the four other cancers analyzed include 450 for lung cancer, 2,500 for cervical cancer, 18,800 for colorectal cancer, and 22,600 for prostate cancer.
The authors project a total of 22 million canceled or delayed tests for the five cancers over the 3-month period ending June 5, based on a comparison of claims data for early April with the February baseline. Catching up on this backlog will be problematic, according to the authors.
“Current excess health care capacity ... would require providers to shift priorities to make time and space in schedules and facilities as well as the cooperation of patients to return to health care providers,” the authors wrote. “Both of these could be further disrupted by economic factors or reintroduction of social distancing in a reemergence of the outbreak.”
The report was produced by the IQVIA Institute for Human Data Science without industry or government funding.
SOURCE: Murray A and Kleinrock M. Shifts in healthcare demand, delivery and care during the COVID-19 era. IQVIA Institute for Human Data Science. April 2020.
, according to a report by the IQVIA Institute for Human Data Science looking at trends in the United States.

Screening and monitoring tests for breast, prostate, colorectal, cervical, and lung cancer were down 39%-90% in early April, compared with the baseline month of February, according to report authors Murray Aitken and Michael Kleinrock, both of IQVIA.
These findings are based on data from IQVIA’s medical claims database, which includes more than 205 million patients, over 1.7 billion claims, and 3 billion service records obtained annually.
The data suggest that, at current positivity rates, there could be 36,000 missed or delayed diagnoses of breast cancer during the 3-month period from early March through early June. Estimates for missed diagnoses of the four other cancers analyzed include 450 for lung cancer, 2,500 for cervical cancer, 18,800 for colorectal cancer, and 22,600 for prostate cancer.
The authors project a total of 22 million canceled or delayed tests for the five cancers over the 3-month period ending June 5, based on a comparison of claims data for early April with the February baseline. Catching up on this backlog will be problematic, according to the authors.
“Current excess health care capacity ... would require providers to shift priorities to make time and space in schedules and facilities as well as the cooperation of patients to return to health care providers,” the authors wrote. “Both of these could be further disrupted by economic factors or reintroduction of social distancing in a reemergence of the outbreak.”
The report was produced by the IQVIA Institute for Human Data Science without industry or government funding.
SOURCE: Murray A and Kleinrock M. Shifts in healthcare demand, delivery and care during the COVID-19 era. IQVIA Institute for Human Data Science. April 2020.
Cancer screening, monitoring down during pandemic
according to a report by the IQVIA Institute for Human Data Science.
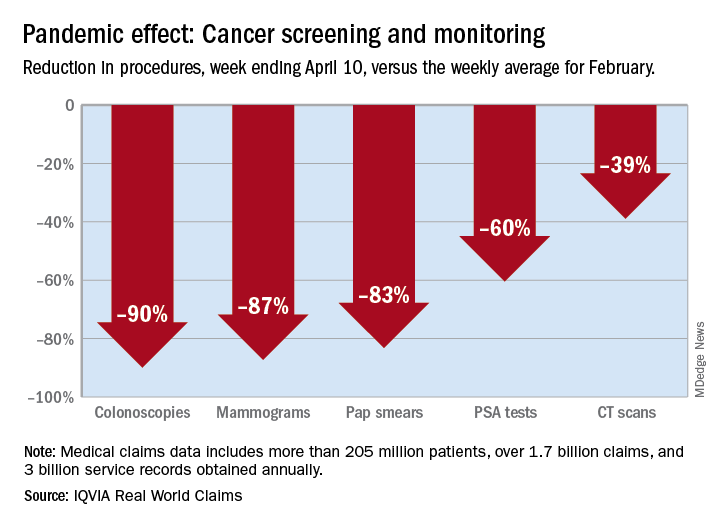
There were 90% fewer colonoscopies ordered during the week ending April 10, compared with the weekly average for Feb. 1-28, based on claims data analyzed by IQVIA.
IQVIA’s medical claims database includes more than 205 million patients, over 1.7 billion claims, and 3 billion service records obtained annually.
The data also showed an 87% reduction in mammograms and an 83% reduction in Pap smears during the week ending April 10. Prostate-specific antigen tests for prostate cancer decreased by 60%, and CT scans for lung cancer decreased by 39%.
The smaller decrease in CT scans for lung cancer “may reflect the generally more serious nature of those tumors or be due to concerns about ruling out COVID-related issues in some patients,” according to report authors Murray Aitken and Michael Kleinrock, both of IQVIA.
The report also showed that overall patient interactions with oncologists were down by 20% through April 3, based on medical and pharmacy claims processed since February, but there was variation by tumor type.
The authors noted “little or no disruption” in oncologist visits in March for patients with aggressive tumors or those diagnosed at advanced stages, compared with February. However, for patients with skin cancer or prostate cancer, visit rates were down by 20%-50% in March.
“This may reflect that oncologists who are providing care across multiple tumor types are prioritizing their time and efforts to those patients with more advanced or aggressive tumors,” the authors wrote.
This report was produced by the IQVIA Institute for Human Data Science without industry or government funding.
SOURCE: Murray A and Kleinrock M. Shifts in healthcare demand, delivery and care during the COVID-19 era. IQVIA Institute for Human Data Science. April 2020.
according to a report by the IQVIA Institute for Human Data Science.

There were 90% fewer colonoscopies ordered during the week ending April 10, compared with the weekly average for Feb. 1-28, based on claims data analyzed by IQVIA.
IQVIA’s medical claims database includes more than 205 million patients, over 1.7 billion claims, and 3 billion service records obtained annually.
The data also showed an 87% reduction in mammograms and an 83% reduction in Pap smears during the week ending April 10. Prostate-specific antigen tests for prostate cancer decreased by 60%, and CT scans for lung cancer decreased by 39%.
The smaller decrease in CT scans for lung cancer “may reflect the generally more serious nature of those tumors or be due to concerns about ruling out COVID-related issues in some patients,” according to report authors Murray Aitken and Michael Kleinrock, both of IQVIA.
The report also showed that overall patient interactions with oncologists were down by 20% through April 3, based on medical and pharmacy claims processed since February, but there was variation by tumor type.
The authors noted “little or no disruption” in oncologist visits in March for patients with aggressive tumors or those diagnosed at advanced stages, compared with February. However, for patients with skin cancer or prostate cancer, visit rates were down by 20%-50% in March.
“This may reflect that oncologists who are providing care across multiple tumor types are prioritizing their time and efforts to those patients with more advanced or aggressive tumors,” the authors wrote.
This report was produced by the IQVIA Institute for Human Data Science without industry or government funding.
SOURCE: Murray A and Kleinrock M. Shifts in healthcare demand, delivery and care during the COVID-19 era. IQVIA Institute for Human Data Science. April 2020.
according to a report by the IQVIA Institute for Human Data Science.

There were 90% fewer colonoscopies ordered during the week ending April 10, compared with the weekly average for Feb. 1-28, based on claims data analyzed by IQVIA.
IQVIA’s medical claims database includes more than 205 million patients, over 1.7 billion claims, and 3 billion service records obtained annually.
The data also showed an 87% reduction in mammograms and an 83% reduction in Pap smears during the week ending April 10. Prostate-specific antigen tests for prostate cancer decreased by 60%, and CT scans for lung cancer decreased by 39%.
The smaller decrease in CT scans for lung cancer “may reflect the generally more serious nature of those tumors or be due to concerns about ruling out COVID-related issues in some patients,” according to report authors Murray Aitken and Michael Kleinrock, both of IQVIA.
The report also showed that overall patient interactions with oncologists were down by 20% through April 3, based on medical and pharmacy claims processed since February, but there was variation by tumor type.
The authors noted “little or no disruption” in oncologist visits in March for patients with aggressive tumors or those diagnosed at advanced stages, compared with February. However, for patients with skin cancer or prostate cancer, visit rates were down by 20%-50% in March.
“This may reflect that oncologists who are providing care across multiple tumor types are prioritizing their time and efforts to those patients with more advanced or aggressive tumors,” the authors wrote.
This report was produced by the IQVIA Institute for Human Data Science without industry or government funding.
SOURCE: Murray A and Kleinrock M. Shifts in healthcare demand, delivery and care during the COVID-19 era. IQVIA Institute for Human Data Science. April 2020.
Excess cancer deaths predicted as care is disrupted by COVID-19
The majority of patients who have cancer or are suspected of having cancer are not accessing healthcare services in the United Kingdom or the United States because of the COVID-19 pandemic, the first report of its kind estimates.
As a result, there will be an excess of deaths among patients who have cancer and multiple comorbidities in both countries during the current coronavirus emergency, the report warns.
The authors calculate that there will be 6,270 excess deaths among cancer patients 1 year from now in England and 33,890 excess deaths among cancer patients in the United States. (In the United States, the estimated excess number of deaths applies only to patients older than 40 years, they note.)
“The recorded underlying cause of these excess deaths may be cancer, COVID-19, or comorbidity (such as myocardial infarction),” Alvina Lai, PhD, University College London, United Kingdom, and colleagues observe.
“Our data have highlighted how cancer patients with multimorbidity are a particularly at-risk group during the current pandemic,” they emphasize.
The study was published on ResearchGate as a preprint and has not undergone peer review.
Commenting on the study on the UK Science Media Center, several experts emphasized the lack of peer review, noting that interpretation of these data needs to be further refined on the basis of that input. One expert suggested that there are “substantial uncertainties that this paper does not adequately communicate.” But others argued that this topic was important enough to warrant early release of the data.
Chris Bunce, PhD, University of Birmingham, United Kingdom, said this study represents “a highly valuable contribution.”
“It is universally accepted that early diagnosis and treatment and adherence to treatment regimens saves lives,” he pointed out.
“Therefore, these COVID-19-related impacts will cost lives,” Bunce said.
“And if this information is to influence cancer care and guide policy during the COVID-19 crisis, then it is important that the findings are disseminated and discussed immediately, warranting their release ahead of peer view,” he added.
In a Medscape UK commentary, oncologist Karol Sikora, MD, PhD, argues that “restarting cancer services can’t come soon enough.”
“Resonably Argued Numerical Estimate”
“It’s well known that there have been considerable changes in the provision of health care for many conditions, including cancers, as a result of all the measures to deal with the COVID-19 crisis,” said Kevin McConway, PhD, professor emeritus of applied statistics, the Open University, Milton Keynes, United Kingdom.
“It seems inevitable that there will be increased deaths in cancer patients if they are infected with the virus or because of changes in the health services available to them, and quite possibly also from socio-economic effects of the responses to the crisis,” he continued.
“This study is the first that I have seen that produces a reasonably argued numerical estimate of the number of excess deaths of people with cancer arising from these factors in the UK and the USA,” he added.
Declines in Urgent Referrals and Chemo Attendance
For the study, the team used DATA-CAN, the UK National Health Data Research Hub for Cancer, to assess weekly returns for urgent cancer referrals for early diagnosis and also chemotherapy attendances for hospitals in Leeds, London, and Northern Ireland going back to 2018.
The data revealed that there have been major declines in chemotherapy attendances. There has been, on average, a 60% decrease from prepandemic levels in eight hospitals in the three regions that were assessed.
Urgent cancer referrals have dropped by an average of 76% compared to prepandemic levels in the three regions.
On the conservative assumption that the COVID-19 pandemic will only affect patients with newly diagnosed cancer (incident cases), the researchers estimate that the proportion of the population affected by the emergency (PAE) is 40% and that the relative impact of the emergency (RIE) is 1.5.
PAE is a summary measure of exposure to the adverse health consequences of the emergency; RIE is a summary measure of the combined impact on mortality of infection, health service change, physical distancing, and economic downturn, the authors explain.
Comorbidities Common
“Comorbidities were common in people with cancer,” the study authors note. For example, more than one quarter of the study population had at least one comorbidity; more than 14% had two.
For incident cancers, the number of excess deaths steadily increased in conjunction with an increase in the number of comorbidities, such that more than 80% of deaths occurred in patients with one or more comorbidities.
“When considering both prevalent and incident cancers together with a COVID-19 PAE of 40%, we estimated 17,991 excess deaths at a RIE of 1.5; 78.1% of these deaths occur in patients with ≥1 comorbidities,” the authors report.
“The excess risk of death in people living with cancer during the COVID-19 emergency may be due not only to COVID-19 infection, but also to the unintended health consequences of changes in health service provision, the physical or psychological effects of social distancing, and economic upheaval,” they state.
“This is the first study demonstrating profound recent changes in cancer care delivery in multiple centers,” the authors observe.
Lai has disclosed no relevant financial relationships. Several coauthors have various relationships with industry, as listed in their article. The commentators have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The majority of patients who have cancer or are suspected of having cancer are not accessing healthcare services in the United Kingdom or the United States because of the COVID-19 pandemic, the first report of its kind estimates.
As a result, there will be an excess of deaths among patients who have cancer and multiple comorbidities in both countries during the current coronavirus emergency, the report warns.
The authors calculate that there will be 6,270 excess deaths among cancer patients 1 year from now in England and 33,890 excess deaths among cancer patients in the United States. (In the United States, the estimated excess number of deaths applies only to patients older than 40 years, they note.)
“The recorded underlying cause of these excess deaths may be cancer, COVID-19, or comorbidity (such as myocardial infarction),” Alvina Lai, PhD, University College London, United Kingdom, and colleagues observe.
“Our data have highlighted how cancer patients with multimorbidity are a particularly at-risk group during the current pandemic,” they emphasize.
The study was published on ResearchGate as a preprint and has not undergone peer review.
Commenting on the study on the UK Science Media Center, several experts emphasized the lack of peer review, noting that interpretation of these data needs to be further refined on the basis of that input. One expert suggested that there are “substantial uncertainties that this paper does not adequately communicate.” But others argued that this topic was important enough to warrant early release of the data.
Chris Bunce, PhD, University of Birmingham, United Kingdom, said this study represents “a highly valuable contribution.”
“It is universally accepted that early diagnosis and treatment and adherence to treatment regimens saves lives,” he pointed out.
“Therefore, these COVID-19-related impacts will cost lives,” Bunce said.
“And if this information is to influence cancer care and guide policy during the COVID-19 crisis, then it is important that the findings are disseminated and discussed immediately, warranting their release ahead of peer view,” he added.
In a Medscape UK commentary, oncologist Karol Sikora, MD, PhD, argues that “restarting cancer services can’t come soon enough.”
“Resonably Argued Numerical Estimate”
“It’s well known that there have been considerable changes in the provision of health care for many conditions, including cancers, as a result of all the measures to deal with the COVID-19 crisis,” said Kevin McConway, PhD, professor emeritus of applied statistics, the Open University, Milton Keynes, United Kingdom.
“It seems inevitable that there will be increased deaths in cancer patients if they are infected with the virus or because of changes in the health services available to them, and quite possibly also from socio-economic effects of the responses to the crisis,” he continued.
“This study is the first that I have seen that produces a reasonably argued numerical estimate of the number of excess deaths of people with cancer arising from these factors in the UK and the USA,” he added.
Declines in Urgent Referrals and Chemo Attendance
For the study, the team used DATA-CAN, the UK National Health Data Research Hub for Cancer, to assess weekly returns for urgent cancer referrals for early diagnosis and also chemotherapy attendances for hospitals in Leeds, London, and Northern Ireland going back to 2018.
The data revealed that there have been major declines in chemotherapy attendances. There has been, on average, a 60% decrease from prepandemic levels in eight hospitals in the three regions that were assessed.
Urgent cancer referrals have dropped by an average of 76% compared to prepandemic levels in the three regions.
On the conservative assumption that the COVID-19 pandemic will only affect patients with newly diagnosed cancer (incident cases), the researchers estimate that the proportion of the population affected by the emergency (PAE) is 40% and that the relative impact of the emergency (RIE) is 1.5.
PAE is a summary measure of exposure to the adverse health consequences of the emergency; RIE is a summary measure of the combined impact on mortality of infection, health service change, physical distancing, and economic downturn, the authors explain.
Comorbidities Common
“Comorbidities were common in people with cancer,” the study authors note. For example, more than one quarter of the study population had at least one comorbidity; more than 14% had two.
For incident cancers, the number of excess deaths steadily increased in conjunction with an increase in the number of comorbidities, such that more than 80% of deaths occurred in patients with one or more comorbidities.
“When considering both prevalent and incident cancers together with a COVID-19 PAE of 40%, we estimated 17,991 excess deaths at a RIE of 1.5; 78.1% of these deaths occur in patients with ≥1 comorbidities,” the authors report.
“The excess risk of death in people living with cancer during the COVID-19 emergency may be due not only to COVID-19 infection, but also to the unintended health consequences of changes in health service provision, the physical or psychological effects of social distancing, and economic upheaval,” they state.
“This is the first study demonstrating profound recent changes in cancer care delivery in multiple centers,” the authors observe.
Lai has disclosed no relevant financial relationships. Several coauthors have various relationships with industry, as listed in their article. The commentators have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The majority of patients who have cancer or are suspected of having cancer are not accessing healthcare services in the United Kingdom or the United States because of the COVID-19 pandemic, the first report of its kind estimates.
As a result, there will be an excess of deaths among patients who have cancer and multiple comorbidities in both countries during the current coronavirus emergency, the report warns.
The authors calculate that there will be 6,270 excess deaths among cancer patients 1 year from now in England and 33,890 excess deaths among cancer patients in the United States. (In the United States, the estimated excess number of deaths applies only to patients older than 40 years, they note.)
“The recorded underlying cause of these excess deaths may be cancer, COVID-19, or comorbidity (such as myocardial infarction),” Alvina Lai, PhD, University College London, United Kingdom, and colleagues observe.
“Our data have highlighted how cancer patients with multimorbidity are a particularly at-risk group during the current pandemic,” they emphasize.
The study was published on ResearchGate as a preprint and has not undergone peer review.
Commenting on the study on the UK Science Media Center, several experts emphasized the lack of peer review, noting that interpretation of these data needs to be further refined on the basis of that input. One expert suggested that there are “substantial uncertainties that this paper does not adequately communicate.” But others argued that this topic was important enough to warrant early release of the data.
Chris Bunce, PhD, University of Birmingham, United Kingdom, said this study represents “a highly valuable contribution.”
“It is universally accepted that early diagnosis and treatment and adherence to treatment regimens saves lives,” he pointed out.
“Therefore, these COVID-19-related impacts will cost lives,” Bunce said.
“And if this information is to influence cancer care and guide policy during the COVID-19 crisis, then it is important that the findings are disseminated and discussed immediately, warranting their release ahead of peer view,” he added.
In a Medscape UK commentary, oncologist Karol Sikora, MD, PhD, argues that “restarting cancer services can’t come soon enough.”
“Resonably Argued Numerical Estimate”
“It’s well known that there have been considerable changes in the provision of health care for many conditions, including cancers, as a result of all the measures to deal with the COVID-19 crisis,” said Kevin McConway, PhD, professor emeritus of applied statistics, the Open University, Milton Keynes, United Kingdom.
“It seems inevitable that there will be increased deaths in cancer patients if they are infected with the virus or because of changes in the health services available to them, and quite possibly also from socio-economic effects of the responses to the crisis,” he continued.
“This study is the first that I have seen that produces a reasonably argued numerical estimate of the number of excess deaths of people with cancer arising from these factors in the UK and the USA,” he added.
Declines in Urgent Referrals and Chemo Attendance
For the study, the team used DATA-CAN, the UK National Health Data Research Hub for Cancer, to assess weekly returns for urgent cancer referrals for early diagnosis and also chemotherapy attendances for hospitals in Leeds, London, and Northern Ireland going back to 2018.
The data revealed that there have been major declines in chemotherapy attendances. There has been, on average, a 60% decrease from prepandemic levels in eight hospitals in the three regions that were assessed.
Urgent cancer referrals have dropped by an average of 76% compared to prepandemic levels in the three regions.
On the conservative assumption that the COVID-19 pandemic will only affect patients with newly diagnosed cancer (incident cases), the researchers estimate that the proportion of the population affected by the emergency (PAE) is 40% and that the relative impact of the emergency (RIE) is 1.5.
PAE is a summary measure of exposure to the adverse health consequences of the emergency; RIE is a summary measure of the combined impact on mortality of infection, health service change, physical distancing, and economic downturn, the authors explain.
Comorbidities Common
“Comorbidities were common in people with cancer,” the study authors note. For example, more than one quarter of the study population had at least one comorbidity; more than 14% had two.
For incident cancers, the number of excess deaths steadily increased in conjunction with an increase in the number of comorbidities, such that more than 80% of deaths occurred in patients with one or more comorbidities.
“When considering both prevalent and incident cancers together with a COVID-19 PAE of 40%, we estimated 17,991 excess deaths at a RIE of 1.5; 78.1% of these deaths occur in patients with ≥1 comorbidities,” the authors report.
“The excess risk of death in people living with cancer during the COVID-19 emergency may be due not only to COVID-19 infection, but also to the unintended health consequences of changes in health service provision, the physical or psychological effects of social distancing, and economic upheaval,” they state.
“This is the first study demonstrating profound recent changes in cancer care delivery in multiple centers,” the authors observe.
Lai has disclosed no relevant financial relationships. Several coauthors have various relationships with industry, as listed in their article. The commentators have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Antitumor treatment may increase risk of severe events in COVID-19 patients
Cancer patients who received antitumor treatment within 14 days of COVID-19 diagnosis had an increased risk of severe events, according to data from three hospitals in Wuhan.
Patients with patchy consolidation at hospital admission also had an increased risk of severe events, defined as ICU admission, mechanical ventilation, or death.
However, these findings are limited by the small number of patients studied and the retrospective nature of the analysis, according to researchers.
Li Zhang, MD, PhD, of Tongji Hospital in Wuhan, China, presented this research at the AACR virtual meeting I. Some of the data were previously published in Annals of Oncology.
The researchers studied 28 patients with cancer among 1,276 patients with COVID-19 treated at three hospitals in Wuhan. The most common cancer types were lung (n = 7), esophageal (n = 4), and breast (n = 3). Patients had other gastrointestinal, gynecologic, genitourinary, and head and neck cancers as well.
The patients’ median age was 65 years (range, 56-70 years), 60.9% were men, 35.7% had stage IV cancer, and 28.6% had hospital-acquired COVID-19. Antitumor treatments included chemotherapy (n = 22), surgery (n = 21), radiotherapy (n = 21), targeted therapy (n = 5), and immune checkpoint inhibitors (n = 2).
COVID-19 treatment
Most patients (n = 22) received oxygen as their only respiratory intervention, although 10 received mechanical ventilation.
For systemic therapy, patients received antibiotic treatment (n = 23), corticosteroids (n = 15), intravenous immunoglobulin (n = 10), and tocilizumab (n = 1).
Antiviral treatments included umifenovir (n = 14), lopinavir/ritonavir (n = 10), ganciclovir (n = 9), ribavirin (n = 1), or a combination of antiviral drugs (n = 9).
“No cancer patients were enrolled in clinical trials, so no one received hydroxychloroquine or remdesivir,” Dr. Zhang noted.
Outcomes
In all, 15 patients (53.6%) had severe events. The median time from COVID-19 diagnosis to severe events was 7 days (range, 5-15 days).
A total of eight patients (28.6%) died – three with lung cancer, two with prostate cancer, one with liver cancer, one with rectal cancer, and one with testicular cancer.
Causes of death were acute respiratory distress syndrome (n = 5), septic shock (n = 1), suspected pulmonary embolism (n = 1), and acute myocardial infarction (n = 1).
By April 4, 14 patients had been discharged from the hospital, and 6 were still hospitalized. The median duration of hospitalization was 18.4 days for discharged patients and 29.4 days for patients still in hospital.
Follow-up CT scans showed improvement in 13 patients, no changes in 5 patients, and deterioration in 6 patients.
Factors associated with severe events
In a multivariable analysis, receiving antitumor treatment within 14 days of COVID-19 diagnosis was associated with severe events (hazard ratio, 4.079; P = .037).
However, only seven patients received antitumor treatments within 14 days of COVID-19 diagnosis – three chemotherapy, two targeted therapy, one radiotherapy, and one immune checkpoint inhibitor. Five of these seven patients had severe events.
Another factor associated with severe events in multivariable analysis was patchy consolidation on CT scan at admission (HR, 5.438; P = .01). Age and gender were not significantly associated with severe events.
Immune checkpoint inhibitors
Dr. Zhang and colleagues also analyzed a second group of cancer patients and their family members to determine if patients on immune checkpoint inhibitors have an increased risk of COVID-19.
This group included 124 cancer patients treated with immune checkpoint inhibitors for at least 2 months. The patients had a median age of 59 years (range, 54-65 years), and 61.8% were men. Most patients (95.2%) had stage IV cancer, and the most common cancers were lung (54.0%), esophageal (18.6%), and head and neck (10.7%).
In this group, only one cancer patient developed COVID-19 (via nosocomial infection). In another case, a patient’s spouse developed COVID-19, but the patient did not.
Dr. Zhang said this “limited information did not suggest cancer patients treated with immune checkpoint inhibitors were more vulnerable to COVID infection.”
Dr. Zhang and colleagues reported no conflicts of interest. This research was funded by the National Natural Science Foundation of China and Huazhong University of Science and Technology COVID-19 Rapid Response Call China.
SOURCE: Zhang L et al. Ann Oncol. 2020 Mar 26. doi: 10.1016/j.annonc.2020.03.296.
Cancer patients who received antitumor treatment within 14 days of COVID-19 diagnosis had an increased risk of severe events, according to data from three hospitals in Wuhan.
Patients with patchy consolidation at hospital admission also had an increased risk of severe events, defined as ICU admission, mechanical ventilation, or death.
However, these findings are limited by the small number of patients studied and the retrospective nature of the analysis, according to researchers.
Li Zhang, MD, PhD, of Tongji Hospital in Wuhan, China, presented this research at the AACR virtual meeting I. Some of the data were previously published in Annals of Oncology.
The researchers studied 28 patients with cancer among 1,276 patients with COVID-19 treated at three hospitals in Wuhan. The most common cancer types were lung (n = 7), esophageal (n = 4), and breast (n = 3). Patients had other gastrointestinal, gynecologic, genitourinary, and head and neck cancers as well.
The patients’ median age was 65 years (range, 56-70 years), 60.9% were men, 35.7% had stage IV cancer, and 28.6% had hospital-acquired COVID-19. Antitumor treatments included chemotherapy (n = 22), surgery (n = 21), radiotherapy (n = 21), targeted therapy (n = 5), and immune checkpoint inhibitors (n = 2).
COVID-19 treatment
Most patients (n = 22) received oxygen as their only respiratory intervention, although 10 received mechanical ventilation.
For systemic therapy, patients received antibiotic treatment (n = 23), corticosteroids (n = 15), intravenous immunoglobulin (n = 10), and tocilizumab (n = 1).
Antiviral treatments included umifenovir (n = 14), lopinavir/ritonavir (n = 10), ganciclovir (n = 9), ribavirin (n = 1), or a combination of antiviral drugs (n = 9).
“No cancer patients were enrolled in clinical trials, so no one received hydroxychloroquine or remdesivir,” Dr. Zhang noted.
Outcomes
In all, 15 patients (53.6%) had severe events. The median time from COVID-19 diagnosis to severe events was 7 days (range, 5-15 days).
A total of eight patients (28.6%) died – three with lung cancer, two with prostate cancer, one with liver cancer, one with rectal cancer, and one with testicular cancer.
Causes of death were acute respiratory distress syndrome (n = 5), septic shock (n = 1), suspected pulmonary embolism (n = 1), and acute myocardial infarction (n = 1).
By April 4, 14 patients had been discharged from the hospital, and 6 were still hospitalized. The median duration of hospitalization was 18.4 days for discharged patients and 29.4 days for patients still in hospital.
Follow-up CT scans showed improvement in 13 patients, no changes in 5 patients, and deterioration in 6 patients.
Factors associated with severe events
In a multivariable analysis, receiving antitumor treatment within 14 days of COVID-19 diagnosis was associated with severe events (hazard ratio, 4.079; P = .037).
However, only seven patients received antitumor treatments within 14 days of COVID-19 diagnosis – three chemotherapy, two targeted therapy, one radiotherapy, and one immune checkpoint inhibitor. Five of these seven patients had severe events.
Another factor associated with severe events in multivariable analysis was patchy consolidation on CT scan at admission (HR, 5.438; P = .01). Age and gender were not significantly associated with severe events.
Immune checkpoint inhibitors
Dr. Zhang and colleagues also analyzed a second group of cancer patients and their family members to determine if patients on immune checkpoint inhibitors have an increased risk of COVID-19.
This group included 124 cancer patients treated with immune checkpoint inhibitors for at least 2 months. The patients had a median age of 59 years (range, 54-65 years), and 61.8% were men. Most patients (95.2%) had stage IV cancer, and the most common cancers were lung (54.0%), esophageal (18.6%), and head and neck (10.7%).
In this group, only one cancer patient developed COVID-19 (via nosocomial infection). In another case, a patient’s spouse developed COVID-19, but the patient did not.
Dr. Zhang said this “limited information did not suggest cancer patients treated with immune checkpoint inhibitors were more vulnerable to COVID infection.”
Dr. Zhang and colleagues reported no conflicts of interest. This research was funded by the National Natural Science Foundation of China and Huazhong University of Science and Technology COVID-19 Rapid Response Call China.
SOURCE: Zhang L et al. Ann Oncol. 2020 Mar 26. doi: 10.1016/j.annonc.2020.03.296.
Cancer patients who received antitumor treatment within 14 days of COVID-19 diagnosis had an increased risk of severe events, according to data from three hospitals in Wuhan.
Patients with patchy consolidation at hospital admission also had an increased risk of severe events, defined as ICU admission, mechanical ventilation, or death.
However, these findings are limited by the small number of patients studied and the retrospective nature of the analysis, according to researchers.
Li Zhang, MD, PhD, of Tongji Hospital in Wuhan, China, presented this research at the AACR virtual meeting I. Some of the data were previously published in Annals of Oncology.
The researchers studied 28 patients with cancer among 1,276 patients with COVID-19 treated at three hospitals in Wuhan. The most common cancer types were lung (n = 7), esophageal (n = 4), and breast (n = 3). Patients had other gastrointestinal, gynecologic, genitourinary, and head and neck cancers as well.
The patients’ median age was 65 years (range, 56-70 years), 60.9% were men, 35.7% had stage IV cancer, and 28.6% had hospital-acquired COVID-19. Antitumor treatments included chemotherapy (n = 22), surgery (n = 21), radiotherapy (n = 21), targeted therapy (n = 5), and immune checkpoint inhibitors (n = 2).
COVID-19 treatment
Most patients (n = 22) received oxygen as their only respiratory intervention, although 10 received mechanical ventilation.
For systemic therapy, patients received antibiotic treatment (n = 23), corticosteroids (n = 15), intravenous immunoglobulin (n = 10), and tocilizumab (n = 1).
Antiviral treatments included umifenovir (n = 14), lopinavir/ritonavir (n = 10), ganciclovir (n = 9), ribavirin (n = 1), or a combination of antiviral drugs (n = 9).
“No cancer patients were enrolled in clinical trials, so no one received hydroxychloroquine or remdesivir,” Dr. Zhang noted.
Outcomes
In all, 15 patients (53.6%) had severe events. The median time from COVID-19 diagnosis to severe events was 7 days (range, 5-15 days).
A total of eight patients (28.6%) died – three with lung cancer, two with prostate cancer, one with liver cancer, one with rectal cancer, and one with testicular cancer.
Causes of death were acute respiratory distress syndrome (n = 5), septic shock (n = 1), suspected pulmonary embolism (n = 1), and acute myocardial infarction (n = 1).
By April 4, 14 patients had been discharged from the hospital, and 6 were still hospitalized. The median duration of hospitalization was 18.4 days for discharged patients and 29.4 days for patients still in hospital.
Follow-up CT scans showed improvement in 13 patients, no changes in 5 patients, and deterioration in 6 patients.
Factors associated with severe events
In a multivariable analysis, receiving antitumor treatment within 14 days of COVID-19 diagnosis was associated with severe events (hazard ratio, 4.079; P = .037).
However, only seven patients received antitumor treatments within 14 days of COVID-19 diagnosis – three chemotherapy, two targeted therapy, one radiotherapy, and one immune checkpoint inhibitor. Five of these seven patients had severe events.
Another factor associated with severe events in multivariable analysis was patchy consolidation on CT scan at admission (HR, 5.438; P = .01). Age and gender were not significantly associated with severe events.
Immune checkpoint inhibitors
Dr. Zhang and colleagues also analyzed a second group of cancer patients and their family members to determine if patients on immune checkpoint inhibitors have an increased risk of COVID-19.
This group included 124 cancer patients treated with immune checkpoint inhibitors for at least 2 months. The patients had a median age of 59 years (range, 54-65 years), and 61.8% were men. Most patients (95.2%) had stage IV cancer, and the most common cancers were lung (54.0%), esophageal (18.6%), and head and neck (10.7%).
In this group, only one cancer patient developed COVID-19 (via nosocomial infection). In another case, a patient’s spouse developed COVID-19, but the patient did not.
Dr. Zhang said this “limited information did not suggest cancer patients treated with immune checkpoint inhibitors were more vulnerable to COVID infection.”
Dr. Zhang and colleagues reported no conflicts of interest. This research was funded by the National Natural Science Foundation of China and Huazhong University of Science and Technology COVID-19 Rapid Response Call China.
SOURCE: Zhang L et al. Ann Oncol. 2020 Mar 26. doi: 10.1016/j.annonc.2020.03.296.
FROM AACR 2020
TERAVOLT data suggest high death rate in lung cancer patients with COVID-19
Registry data suggest an “unexpectedly high” mortality rate among patients with thoracic cancers who develop COVID-19, according to a presenter at the AACR virtual meeting I.
Data from the TERAVOLT registry showed a 34.6% mortality rate among 200 patients with COVID-19 and thoracic cancer, according to Marina Chiara Garassino, MD, of Fondazione IRCCS Instituto Nazionale dei Tumor in Milan, Italy, who presented the data at the meeting in a session on cancer and COVID-19.
Cancer patients infected with COVID-19 have been reported to be at increased risk of death, but the magnitude of increase is uncertain (Lancet Oncol. 2020 Mar;21[3]:335-7; JAMA. 2020 Mar 23. doi: 10.1001/jama.2020.4683).
Patients with thoracic cancer may be particularly vulnerable because of older age, tobacco use, preexisting cardiopulmonary comorbidities, and the immunosuppressive effects of treatment.
The global TERAVOLT registry was begun in late March 2020 to provide outcome data for coronavirus infections in thoracic cancer patients specifically. It is hoped that the data collected will guide patient management and define factors influencing morbidity and mortality.
Dr. Garassino said institutions from 21 countries have joined the TERAVOLT registry thus far. Currently, about 17 new patients with thoracic cancer and laboratory confirmed or clinically suspected COVID-19 are added to the registry each week.
As of April 12, 2020, there were 200 patients included in the registry. Their median age was 68 years, and 70.5% were men. Non–small cell lung cancer was the histology in 75.5% and small-cell lung cancer in 14.5% of patients. Most patients (73.5%) had stage IV disease. Approximately 27% of patients had at least three comorbid conditions.
About 74% of patients were on current cancer treatment, with 19% on tyrosine kinase inhibitors alone, 32.7% on chemotherapy alone, 23.1% on immunotherapy alone, and 13.6% on chemotherapy plus immunotherapy.
In all, 152 patients (76.0%) were hospitalized. However, 91.2% of patients were not admitted to the ICU, either because of a shortage of equipment or institutional policy.
The most common complications were pneumonia/pneumonitis (79.6%), acute respiratory distress syndrome (26.8%), multiorgan failure (7.6%), and sepsis (5.1%).
A total of 66 patients (34.6%) died. Most deaths were attributed to COVID-19 and not the underlying cancer, Dr. Garassino said.
A univariate analysis showed no association between cancer treatment and an increased risk of hospitalization or death. However, Dr. Garassino and colleagues are collecting more data to confirm these results.
In a multivariate analysis, no factors were associated with the risk of death, although data from a larger number of patients may shed more light on that issue.
TERAVOLT will continue to collect and provide data to identify characteristics associated with severe COVID-19–related illness, to guide physicians with information applicable to patients with thoracic malignancies, tailored to individual risk.
Like the COVID-19 and Cancer Consortium and the ESMO CoCare registry, TERAVOLT represents a way for the patient care and translational science communities to share lessons from the COVID-19 pandemic.
AACR plans to help share those lessons as well, in another session on COVID-19 and cancer at the AACR virtual meeting II in June and at a conference on COVID-19 and cancer in July, according to session moderator Antoni Ribas, MD, PhD, of the University of California, Los Angeles.
Dr. Garassino disclosed relationships with AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, and other companies.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Registry data suggest an “unexpectedly high” mortality rate among patients with thoracic cancers who develop COVID-19, according to a presenter at the AACR virtual meeting I.
Data from the TERAVOLT registry showed a 34.6% mortality rate among 200 patients with COVID-19 and thoracic cancer, according to Marina Chiara Garassino, MD, of Fondazione IRCCS Instituto Nazionale dei Tumor in Milan, Italy, who presented the data at the meeting in a session on cancer and COVID-19.
Cancer patients infected with COVID-19 have been reported to be at increased risk of death, but the magnitude of increase is uncertain (Lancet Oncol. 2020 Mar;21[3]:335-7; JAMA. 2020 Mar 23. doi: 10.1001/jama.2020.4683).
Patients with thoracic cancer may be particularly vulnerable because of older age, tobacco use, preexisting cardiopulmonary comorbidities, and the immunosuppressive effects of treatment.
The global TERAVOLT registry was begun in late March 2020 to provide outcome data for coronavirus infections in thoracic cancer patients specifically. It is hoped that the data collected will guide patient management and define factors influencing morbidity and mortality.
Dr. Garassino said institutions from 21 countries have joined the TERAVOLT registry thus far. Currently, about 17 new patients with thoracic cancer and laboratory confirmed or clinically suspected COVID-19 are added to the registry each week.
As of April 12, 2020, there were 200 patients included in the registry. Their median age was 68 years, and 70.5% were men. Non–small cell lung cancer was the histology in 75.5% and small-cell lung cancer in 14.5% of patients. Most patients (73.5%) had stage IV disease. Approximately 27% of patients had at least three comorbid conditions.
About 74% of patients were on current cancer treatment, with 19% on tyrosine kinase inhibitors alone, 32.7% on chemotherapy alone, 23.1% on immunotherapy alone, and 13.6% on chemotherapy plus immunotherapy.
In all, 152 patients (76.0%) were hospitalized. However, 91.2% of patients were not admitted to the ICU, either because of a shortage of equipment or institutional policy.
The most common complications were pneumonia/pneumonitis (79.6%), acute respiratory distress syndrome (26.8%), multiorgan failure (7.6%), and sepsis (5.1%).
A total of 66 patients (34.6%) died. Most deaths were attributed to COVID-19 and not the underlying cancer, Dr. Garassino said.
A univariate analysis showed no association between cancer treatment and an increased risk of hospitalization or death. However, Dr. Garassino and colleagues are collecting more data to confirm these results.
In a multivariate analysis, no factors were associated with the risk of death, although data from a larger number of patients may shed more light on that issue.
TERAVOLT will continue to collect and provide data to identify characteristics associated with severe COVID-19–related illness, to guide physicians with information applicable to patients with thoracic malignancies, tailored to individual risk.
Like the COVID-19 and Cancer Consortium and the ESMO CoCare registry, TERAVOLT represents a way for the patient care and translational science communities to share lessons from the COVID-19 pandemic.
AACR plans to help share those lessons as well, in another session on COVID-19 and cancer at the AACR virtual meeting II in June and at a conference on COVID-19 and cancer in July, according to session moderator Antoni Ribas, MD, PhD, of the University of California, Los Angeles.
Dr. Garassino disclosed relationships with AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, and other companies.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Registry data suggest an “unexpectedly high” mortality rate among patients with thoracic cancers who develop COVID-19, according to a presenter at the AACR virtual meeting I.
Data from the TERAVOLT registry showed a 34.6% mortality rate among 200 patients with COVID-19 and thoracic cancer, according to Marina Chiara Garassino, MD, of Fondazione IRCCS Instituto Nazionale dei Tumor in Milan, Italy, who presented the data at the meeting in a session on cancer and COVID-19.
Cancer patients infected with COVID-19 have been reported to be at increased risk of death, but the magnitude of increase is uncertain (Lancet Oncol. 2020 Mar;21[3]:335-7; JAMA. 2020 Mar 23. doi: 10.1001/jama.2020.4683).
Patients with thoracic cancer may be particularly vulnerable because of older age, tobacco use, preexisting cardiopulmonary comorbidities, and the immunosuppressive effects of treatment.
The global TERAVOLT registry was begun in late March 2020 to provide outcome data for coronavirus infections in thoracic cancer patients specifically. It is hoped that the data collected will guide patient management and define factors influencing morbidity and mortality.
Dr. Garassino said institutions from 21 countries have joined the TERAVOLT registry thus far. Currently, about 17 new patients with thoracic cancer and laboratory confirmed or clinically suspected COVID-19 are added to the registry each week.
As of April 12, 2020, there were 200 patients included in the registry. Their median age was 68 years, and 70.5% were men. Non–small cell lung cancer was the histology in 75.5% and small-cell lung cancer in 14.5% of patients. Most patients (73.5%) had stage IV disease. Approximately 27% of patients had at least three comorbid conditions.
About 74% of patients were on current cancer treatment, with 19% on tyrosine kinase inhibitors alone, 32.7% on chemotherapy alone, 23.1% on immunotherapy alone, and 13.6% on chemotherapy plus immunotherapy.
In all, 152 patients (76.0%) were hospitalized. However, 91.2% of patients were not admitted to the ICU, either because of a shortage of equipment or institutional policy.
The most common complications were pneumonia/pneumonitis (79.6%), acute respiratory distress syndrome (26.8%), multiorgan failure (7.6%), and sepsis (5.1%).
A total of 66 patients (34.6%) died. Most deaths were attributed to COVID-19 and not the underlying cancer, Dr. Garassino said.
A univariate analysis showed no association between cancer treatment and an increased risk of hospitalization or death. However, Dr. Garassino and colleagues are collecting more data to confirm these results.
In a multivariate analysis, no factors were associated with the risk of death, although data from a larger number of patients may shed more light on that issue.
TERAVOLT will continue to collect and provide data to identify characteristics associated with severe COVID-19–related illness, to guide physicians with information applicable to patients with thoracic malignancies, tailored to individual risk.
Like the COVID-19 and Cancer Consortium and the ESMO CoCare registry, TERAVOLT represents a way for the patient care and translational science communities to share lessons from the COVID-19 pandemic.
AACR plans to help share those lessons as well, in another session on COVID-19 and cancer at the AACR virtual meeting II in June and at a conference on COVID-19 and cancer in July, according to session moderator Antoni Ribas, MD, PhD, of the University of California, Los Angeles.
Dr. Garassino disclosed relationships with AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, and other companies.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
FROM AACR 2020
Metastatic cancer linked to worse outcomes of COVID-19
Cancer type, stage, and recent treatment may affect outcomes of COVID-19 in cancer patients, according to a study of patients from China.
The data showed that patients with hematologic malignancies and those with metastatic cancers had higher risks of developing severe or critical COVID-19 symptoms, being admitted to the ICU, requiring ventilation, and dying.
On the other hand, patients with nonmetastatic cancer had outcomes comparable to those of noncancer patients with COVID-19.
Similarly, cancer patients who had recently undergone surgery or received immunotherapy were more likely to have poor outcomes, whereas cancer patients treated with radiotherapy had outcomes similar to those of noncancer COVID-19 patients.
Hongbing Cai, MD, of Zhongnan Hospital of Wuhan University in China, presented these results at the AACR virtual meeting I. The results also were published in Cancer Discovery.
Cancer vs. noncancer patients
The study included 105 cancer patients with COVID-19 who were treated from Jan. 1 to Feb. 24, 2020, at 14 hospitals in Wuhan, China. Patients had lung (20.95%), gastrointestinal (12.38%), breast (10.48%), and thyroid cancers (10.48%) as well as hematologic malignancies (8.57%). Dr. Cai and colleagues matched the COVID-19 cancer patients to 536 COVID-19 patients without cancer. Patients were matched by hospital, duration of hospitalization, and age.
“COVID-19 patients with cancer had higher risks of all severe outcomes,” Dr. Cai noted.
Compared with noncancer patients, the cancer patients had a higher risk of:
- Severe or critical COVID-19 symptoms – odds ratio, 2.79 (P < .01).
- Being admitted to the ICU – OR, 2.84 (P < .01).
- Requiring invasive mechanical ventilation – OR, 14 (P < .01).
- Death – OR, 2.34 (P = .03).
Cancer type and stage
Dr. Cai noted that outcomes were the worst among patients with hematologic malignancies and those with metastatic cancer (stage IV).
Compared with patients without cancer, those with hematologic malignancies had a higher risk of:
- Severe/critical symptoms – OR, 10.61 (P < .01).
- ICU admission – OR, 9.66 (P < .01).
- Invasive mechanical ventilation – OR, 38 (P < .01).
- Death – OR, 9.07 (P = .01).
Compared with patients without cancer, those with metastatic cancer had a higher risk of:
- Severe/critical symptoms – OR, 5.97 (P < .01).
- ICU admission – OR, 6.59 (P < 0.01).
- Invasive mechanical ventilation – OR, 55.42 (P < .01).
- Death – OR, 5.58 (P = .01).
On the other hand, outcomes in patients with nonmetastatic cancer were not significantly different from outcomes in patients without cancer (P > .05 for all outcomes).
Cancer treatment
The treatments cancer patients received within 40 days before the onset of COVID-19 symptoms were radiotherapy (12.26%), chemotherapy (14.15%), surgery (7.62%), targeted therapies (3.81%), and immunotherapy (5.71%).
Compared with patients without cancer, those who received immunotherapy had a higher risk of:
- Severe/critical symptoms – OR, 10.61 (P < .01).
- Death – OR, 9.07 (P = .04).
Patients who underwent surgery had a higher risk of:
- Severe/critical symptoms – OR, 8.84 (P < .01).
- ICU admission – OR, 7.24 (P = .02).
- Invasive mechanical ventilation – OR, 44.33 (P < .01).
Conversely, outcomes in cancer patients who received radiotherapy were not significantly different from outcomes in patients without cancer (P > .10 for all).
These results suggest that “postponing surgery should be considered in outbreak areas,” Dr. Cai said, adding that scheduled radiotherapy can go ahead but with “intensive protection and surveillance.”
Dr. Cai said it remains to be seen whether patients with early-stage cancer need to postpone their treatments during the COVID-19 pandemic or whether immunotherapy aggravates severe outcomes in cancer patients with COVID-19. For now, she said, cancer patients should have individualized treatment plans based on their tumor type and stage.
Dr. Cai disclosed no conflicts of interest. This study was supported by the National Natural Science Foundation of China, the Singapore Ministry of Health’s National Medical Research Council, the National Institutes of Health/National Heart, Lung, and Blood Institute, and the Xiu Research Fund.
SOURCE: Cai H. AACR 2020. Patients with cancer appear more vulnerable to SARS-COV-2: A multicenter study during the COVID-19 outbreak; Dai M et al. Cancer Discov. 2020 Apr 28. doi: 10.1158/2159-8290.CD-20-0422.
Cancer type, stage, and recent treatment may affect outcomes of COVID-19 in cancer patients, according to a study of patients from China.
The data showed that patients with hematologic malignancies and those with metastatic cancers had higher risks of developing severe or critical COVID-19 symptoms, being admitted to the ICU, requiring ventilation, and dying.
On the other hand, patients with nonmetastatic cancer had outcomes comparable to those of noncancer patients with COVID-19.
Similarly, cancer patients who had recently undergone surgery or received immunotherapy were more likely to have poor outcomes, whereas cancer patients treated with radiotherapy had outcomes similar to those of noncancer COVID-19 patients.
Hongbing Cai, MD, of Zhongnan Hospital of Wuhan University in China, presented these results at the AACR virtual meeting I. The results also were published in Cancer Discovery.
Cancer vs. noncancer patients
The study included 105 cancer patients with COVID-19 who were treated from Jan. 1 to Feb. 24, 2020, at 14 hospitals in Wuhan, China. Patients had lung (20.95%), gastrointestinal (12.38%), breast (10.48%), and thyroid cancers (10.48%) as well as hematologic malignancies (8.57%). Dr. Cai and colleagues matched the COVID-19 cancer patients to 536 COVID-19 patients without cancer. Patients were matched by hospital, duration of hospitalization, and age.
“COVID-19 patients with cancer had higher risks of all severe outcomes,” Dr. Cai noted.
Compared with noncancer patients, the cancer patients had a higher risk of:
- Severe or critical COVID-19 symptoms – odds ratio, 2.79 (P < .01).
- Being admitted to the ICU – OR, 2.84 (P < .01).
- Requiring invasive mechanical ventilation – OR, 14 (P < .01).
- Death – OR, 2.34 (P = .03).
Cancer type and stage
Dr. Cai noted that outcomes were the worst among patients with hematologic malignancies and those with metastatic cancer (stage IV).
Compared with patients without cancer, those with hematologic malignancies had a higher risk of:
- Severe/critical symptoms – OR, 10.61 (P < .01).
- ICU admission – OR, 9.66 (P < .01).
- Invasive mechanical ventilation – OR, 38 (P < .01).
- Death – OR, 9.07 (P = .01).
Compared with patients without cancer, those with metastatic cancer had a higher risk of:
- Severe/critical symptoms – OR, 5.97 (P < .01).
- ICU admission – OR, 6.59 (P < 0.01).
- Invasive mechanical ventilation – OR, 55.42 (P < .01).
- Death – OR, 5.58 (P = .01).
On the other hand, outcomes in patients with nonmetastatic cancer were not significantly different from outcomes in patients without cancer (P > .05 for all outcomes).
Cancer treatment
The treatments cancer patients received within 40 days before the onset of COVID-19 symptoms were radiotherapy (12.26%), chemotherapy (14.15%), surgery (7.62%), targeted therapies (3.81%), and immunotherapy (5.71%).
Compared with patients without cancer, those who received immunotherapy had a higher risk of:
- Severe/critical symptoms – OR, 10.61 (P < .01).
- Death – OR, 9.07 (P = .04).
Patients who underwent surgery had a higher risk of:
- Severe/critical symptoms – OR, 8.84 (P < .01).
- ICU admission – OR, 7.24 (P = .02).
- Invasive mechanical ventilation – OR, 44.33 (P < .01).
Conversely, outcomes in cancer patients who received radiotherapy were not significantly different from outcomes in patients without cancer (P > .10 for all).
These results suggest that “postponing surgery should be considered in outbreak areas,” Dr. Cai said, adding that scheduled radiotherapy can go ahead but with “intensive protection and surveillance.”
Dr. Cai said it remains to be seen whether patients with early-stage cancer need to postpone their treatments during the COVID-19 pandemic or whether immunotherapy aggravates severe outcomes in cancer patients with COVID-19. For now, she said, cancer patients should have individualized treatment plans based on their tumor type and stage.
Dr. Cai disclosed no conflicts of interest. This study was supported by the National Natural Science Foundation of China, the Singapore Ministry of Health’s National Medical Research Council, the National Institutes of Health/National Heart, Lung, and Blood Institute, and the Xiu Research Fund.
SOURCE: Cai H. AACR 2020. Patients with cancer appear more vulnerable to SARS-COV-2: A multicenter study during the COVID-19 outbreak; Dai M et al. Cancer Discov. 2020 Apr 28. doi: 10.1158/2159-8290.CD-20-0422.
Cancer type, stage, and recent treatment may affect outcomes of COVID-19 in cancer patients, according to a study of patients from China.
The data showed that patients with hematologic malignancies and those with metastatic cancers had higher risks of developing severe or critical COVID-19 symptoms, being admitted to the ICU, requiring ventilation, and dying.
On the other hand, patients with nonmetastatic cancer had outcomes comparable to those of noncancer patients with COVID-19.
Similarly, cancer patients who had recently undergone surgery or received immunotherapy were more likely to have poor outcomes, whereas cancer patients treated with radiotherapy had outcomes similar to those of noncancer COVID-19 patients.
Hongbing Cai, MD, of Zhongnan Hospital of Wuhan University in China, presented these results at the AACR virtual meeting I. The results also were published in Cancer Discovery.
Cancer vs. noncancer patients
The study included 105 cancer patients with COVID-19 who were treated from Jan. 1 to Feb. 24, 2020, at 14 hospitals in Wuhan, China. Patients had lung (20.95%), gastrointestinal (12.38%), breast (10.48%), and thyroid cancers (10.48%) as well as hematologic malignancies (8.57%). Dr. Cai and colleagues matched the COVID-19 cancer patients to 536 COVID-19 patients without cancer. Patients were matched by hospital, duration of hospitalization, and age.
“COVID-19 patients with cancer had higher risks of all severe outcomes,” Dr. Cai noted.
Compared with noncancer patients, the cancer patients had a higher risk of:
- Severe or critical COVID-19 symptoms – odds ratio, 2.79 (P < .01).
- Being admitted to the ICU – OR, 2.84 (P < .01).
- Requiring invasive mechanical ventilation – OR, 14 (P < .01).
- Death – OR, 2.34 (P = .03).
Cancer type and stage
Dr. Cai noted that outcomes were the worst among patients with hematologic malignancies and those with metastatic cancer (stage IV).
Compared with patients without cancer, those with hematologic malignancies had a higher risk of:
- Severe/critical symptoms – OR, 10.61 (P < .01).
- ICU admission – OR, 9.66 (P < .01).
- Invasive mechanical ventilation – OR, 38 (P < .01).
- Death – OR, 9.07 (P = .01).
Compared with patients without cancer, those with metastatic cancer had a higher risk of:
- Severe/critical symptoms – OR, 5.97 (P < .01).
- ICU admission – OR, 6.59 (P < 0.01).
- Invasive mechanical ventilation – OR, 55.42 (P < .01).
- Death – OR, 5.58 (P = .01).
On the other hand, outcomes in patients with nonmetastatic cancer were not significantly different from outcomes in patients without cancer (P > .05 for all outcomes).
Cancer treatment
The treatments cancer patients received within 40 days before the onset of COVID-19 symptoms were radiotherapy (12.26%), chemotherapy (14.15%), surgery (7.62%), targeted therapies (3.81%), and immunotherapy (5.71%).
Compared with patients without cancer, those who received immunotherapy had a higher risk of:
- Severe/critical symptoms – OR, 10.61 (P < .01).
- Death – OR, 9.07 (P = .04).
Patients who underwent surgery had a higher risk of:
- Severe/critical symptoms – OR, 8.84 (P < .01).
- ICU admission – OR, 7.24 (P = .02).
- Invasive mechanical ventilation – OR, 44.33 (P < .01).
Conversely, outcomes in cancer patients who received radiotherapy were not significantly different from outcomes in patients without cancer (P > .10 for all).
These results suggest that “postponing surgery should be considered in outbreak areas,” Dr. Cai said, adding that scheduled radiotherapy can go ahead but with “intensive protection and surveillance.”
Dr. Cai said it remains to be seen whether patients with early-stage cancer need to postpone their treatments during the COVID-19 pandemic or whether immunotherapy aggravates severe outcomes in cancer patients with COVID-19. For now, she said, cancer patients should have individualized treatment plans based on their tumor type and stage.
Dr. Cai disclosed no conflicts of interest. This study was supported by the National Natural Science Foundation of China, the Singapore Ministry of Health’s National Medical Research Council, the National Institutes of Health/National Heart, Lung, and Blood Institute, and the Xiu Research Fund.
SOURCE: Cai H. AACR 2020. Patients with cancer appear more vulnerable to SARS-COV-2: A multicenter study during the COVID-19 outbreak; Dai M et al. Cancer Discov. 2020 Apr 28. doi: 10.1158/2159-8290.CD-20-0422.
FROM AACR 2020