User login
A pandemic silver lining? Dramatic drop in teen drug use
Illicit drug use among U.S. teenagers dropped sharply in 2021, likely because of stay-at-home orders and other restrictions on social activities due to the COVID-19 pandemic.
The latest findings, from the Monitoring the Future survey, represent the largest 1-year decrease in overall illicit drug use reported since the survey began in 1975.
“We have never seen such dramatic decreases in drug use among teens in just a 1-year period,” Nora Volkow, MD, director of the National Institute on Drug Abuse (NIDA), said in a news release.
“These data are unprecedented and highlight one unexpected potential consequence of the COVID-19 pandemic, which caused seismic shifts in the day-to-day lives of adolescents,” said Dr. Volkow.
The annual Monitoring the Future survey is conducted by researchers at the University of Michigan, Ann Arbor, and funded by NIDA, to assess drug and alcohol use and related attitudes among adolescent students across the United States.
This year’s self-reported survey included 32,260 students in grades 8, 10, and 12 across 319 public and private schools.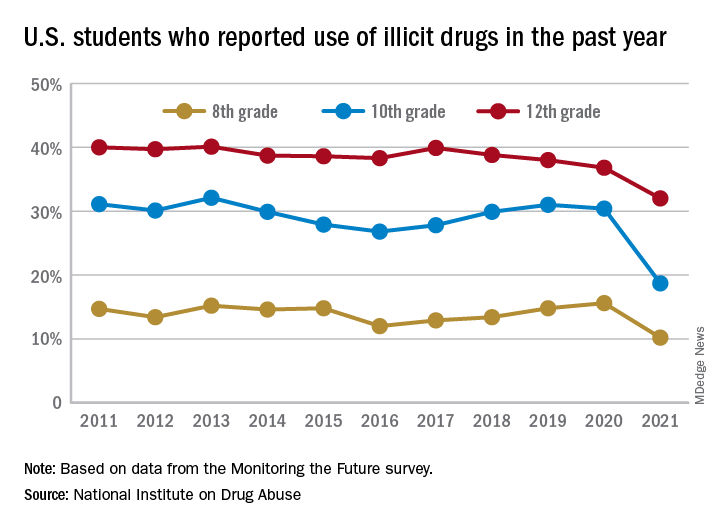
Compared with 2020, the percentage of students reporting any illicit drug use (other than marijuana) in 2021 decreased significantly for 8th graders (down 5.4%), 10th graders (down 11.7%), and 12th graders (down 4.8%).
For alcohol, about 47% of 12th graders and 29% of 10th graders said they drank alcohol in 2021, down significantly from 55% and 41%, respectively, in 2020. The percentage of 8th graders who said they drank alcohol remained stable (17% in 2021 and 20% in 2020).
For teen vaping, about 27% of 12th graders and 20% of 10th graders said they had vaped nicotine in 2021, down significantly from nearly 35% and 31%, respectively, in 2020. Fewer 8th graders also vaped nicotine in 2021 compared with 2020 (12% vs. 17%).
For marijuana, use dropped significantly for all three grades in 2021 compared with 2020. About 31% of 12th graders and 17% of 10th graders said they used marijuana in 2021, down from 35% and 28% in 2020. Among 8th graders, 7% used marijuana in 2021, down from 11% in 2020.
The latest survey also shows significant declines in use of a range of other drugs for many of the age cohorts, including cocaine, hallucinogens, and nonmedical use of amphetamines, tranquilizers, and prescription opioids.
“We knew that this year’s data would illuminate how the COVID-19 pandemic may have impacted substance use among young people, and in the coming years, we will find out whether those impacts are long-lasting as we continue tracking the drug use patterns of these unique cohorts of adolescents,” Richard A. Miech, PhD, who heads the Monitoring the Future study at the University of Michigan, said in the news release.
“Moving forward, it will be crucial to identify the pivotal elements of this past year that contributed to decreased drug use – whether related to drug availability, family involvement, differences in peer pressure, or other factors – and harness them to inform future prevention efforts,” Dr. Volkow added.
In 2021, students across all age groups reported moderate increases in feelings of boredom, anxiety, depression, loneliness, worry, difficulty sleeping, and other negative mental health indicators since the beginning of the pandemic.
A version of this article first appeared on Medscape.com.
Illicit drug use among U.S. teenagers dropped sharply in 2021, likely because of stay-at-home orders and other restrictions on social activities due to the COVID-19 pandemic.
The latest findings, from the Monitoring the Future survey, represent the largest 1-year decrease in overall illicit drug use reported since the survey began in 1975.
“We have never seen such dramatic decreases in drug use among teens in just a 1-year period,” Nora Volkow, MD, director of the National Institute on Drug Abuse (NIDA), said in a news release.
“These data are unprecedented and highlight one unexpected potential consequence of the COVID-19 pandemic, which caused seismic shifts in the day-to-day lives of adolescents,” said Dr. Volkow.
The annual Monitoring the Future survey is conducted by researchers at the University of Michigan, Ann Arbor, and funded by NIDA, to assess drug and alcohol use and related attitudes among adolescent students across the United States.
This year’s self-reported survey included 32,260 students in grades 8, 10, and 12 across 319 public and private schools.
Compared with 2020, the percentage of students reporting any illicit drug use (other than marijuana) in 2021 decreased significantly for 8th graders (down 5.4%), 10th graders (down 11.7%), and 12th graders (down 4.8%).
For alcohol, about 47% of 12th graders and 29% of 10th graders said they drank alcohol in 2021, down significantly from 55% and 41%, respectively, in 2020. The percentage of 8th graders who said they drank alcohol remained stable (17% in 2021 and 20% in 2020).
For teen vaping, about 27% of 12th graders and 20% of 10th graders said they had vaped nicotine in 2021, down significantly from nearly 35% and 31%, respectively, in 2020. Fewer 8th graders also vaped nicotine in 2021 compared with 2020 (12% vs. 17%).
For marijuana, use dropped significantly for all three grades in 2021 compared with 2020. About 31% of 12th graders and 17% of 10th graders said they used marijuana in 2021, down from 35% and 28% in 2020. Among 8th graders, 7% used marijuana in 2021, down from 11% in 2020.
The latest survey also shows significant declines in use of a range of other drugs for many of the age cohorts, including cocaine, hallucinogens, and nonmedical use of amphetamines, tranquilizers, and prescription opioids.
“We knew that this year’s data would illuminate how the COVID-19 pandemic may have impacted substance use among young people, and in the coming years, we will find out whether those impacts are long-lasting as we continue tracking the drug use patterns of these unique cohorts of adolescents,” Richard A. Miech, PhD, who heads the Monitoring the Future study at the University of Michigan, said in the news release.
“Moving forward, it will be crucial to identify the pivotal elements of this past year that contributed to decreased drug use – whether related to drug availability, family involvement, differences in peer pressure, or other factors – and harness them to inform future prevention efforts,” Dr. Volkow added.
In 2021, students across all age groups reported moderate increases in feelings of boredom, anxiety, depression, loneliness, worry, difficulty sleeping, and other negative mental health indicators since the beginning of the pandemic.
A version of this article first appeared on Medscape.com.
Illicit drug use among U.S. teenagers dropped sharply in 2021, likely because of stay-at-home orders and other restrictions on social activities due to the COVID-19 pandemic.
The latest findings, from the Monitoring the Future survey, represent the largest 1-year decrease in overall illicit drug use reported since the survey began in 1975.
“We have never seen such dramatic decreases in drug use among teens in just a 1-year period,” Nora Volkow, MD, director of the National Institute on Drug Abuse (NIDA), said in a news release.
“These data are unprecedented and highlight one unexpected potential consequence of the COVID-19 pandemic, which caused seismic shifts in the day-to-day lives of adolescents,” said Dr. Volkow.
The annual Monitoring the Future survey is conducted by researchers at the University of Michigan, Ann Arbor, and funded by NIDA, to assess drug and alcohol use and related attitudes among adolescent students across the United States.
This year’s self-reported survey included 32,260 students in grades 8, 10, and 12 across 319 public and private schools.
Compared with 2020, the percentage of students reporting any illicit drug use (other than marijuana) in 2021 decreased significantly for 8th graders (down 5.4%), 10th graders (down 11.7%), and 12th graders (down 4.8%).
For alcohol, about 47% of 12th graders and 29% of 10th graders said they drank alcohol in 2021, down significantly from 55% and 41%, respectively, in 2020. The percentage of 8th graders who said they drank alcohol remained stable (17% in 2021 and 20% in 2020).
For teen vaping, about 27% of 12th graders and 20% of 10th graders said they had vaped nicotine in 2021, down significantly from nearly 35% and 31%, respectively, in 2020. Fewer 8th graders also vaped nicotine in 2021 compared with 2020 (12% vs. 17%).
For marijuana, use dropped significantly for all three grades in 2021 compared with 2020. About 31% of 12th graders and 17% of 10th graders said they used marijuana in 2021, down from 35% and 28% in 2020. Among 8th graders, 7% used marijuana in 2021, down from 11% in 2020.
The latest survey also shows significant declines in use of a range of other drugs for many of the age cohorts, including cocaine, hallucinogens, and nonmedical use of amphetamines, tranquilizers, and prescription opioids.
“We knew that this year’s data would illuminate how the COVID-19 pandemic may have impacted substance use among young people, and in the coming years, we will find out whether those impacts are long-lasting as we continue tracking the drug use patterns of these unique cohorts of adolescents,” Richard A. Miech, PhD, who heads the Monitoring the Future study at the University of Michigan, said in the news release.
“Moving forward, it will be crucial to identify the pivotal elements of this past year that contributed to decreased drug use – whether related to drug availability, family involvement, differences in peer pressure, or other factors – and harness them to inform future prevention efforts,” Dr. Volkow added.
In 2021, students across all age groups reported moderate increases in feelings of boredom, anxiety, depression, loneliness, worry, difficulty sleeping, and other negative mental health indicators since the beginning of the pandemic.
A version of this article first appeared on Medscape.com.
Epilepsy linked to 1.5-fold higher COVID-19 mortality in hospital
according to a new study presented at the annual meeting of the American Epilepsy Society. While the findings are preliminary and not yet adjusted for various confounders, the authors say they are a warning sign that patients with epilepsy may face higher risks.
“These findings suggest that epilepsy may be a pre-existing condition that places patients at increased risk for death if hospitalized with a COVID-19 infection. It may offer neurologists guidance when counseling patients on critical preventative measures such as masking, social distancing, and most importantly, vaccination,” lead author Claire Ufongene, a student at Icahn School of Medicine at Mount Sinai, New York, said in an interview.
According to Ms. Ufongene, there’s sparse data about COVID-19 outcomes in patients with epilepsy, although she highlighted a 2021 meta-analysis of 13 studies that found a higher risk of severity (odds ratio, 1.69; 95% confidence interval, 1.11-2.59, P = .010) and mortality (OR, 1.71; 95% CI, 1.14-2.56, P = .010).
For the new study, researchers retrospectively tracked identified 334 patients with epilepsy and COVID-19 and 9,499 other patients with COVID-19 from March 15, 2020, to May 17, 2021. All were treated at hospitals within the New York–based Icahn School of Medicine at Mount Sinai.
The groups of patients with and without epilepsy were similar in some ways: 45% and 46%, respectively, were female (P = .674), and their ages were similar (average, 62 years and 65 years, respectively; P = .02). Racial makeup was also similar (non-Hispanic groups made up 27.8% of those with epilepsy and 24.5% of those without; the difference was not statistically significant).
“In addition, more of those with epilepsy were English speaking [83.2% vs. 77.9%] and had Medicaid insurance [50.9% vs. 38.9%], while fewer of those with epilepsy had private insurance [16.2% vs. 25.5%] or were Spanish speaking [14.0% vs. 9.3%],” study coauthor Nathalie Jette, MD, MSc, a neurologist at Icahn School of Medicine at Mount Sinai, said in an interview.
In terms of outcomes, patients with epilepsy were much more likely to need ventilator support (37.7% vs. 14.3%; P < .001), to be admitted to the ICU (39.2% vs. 17.7%; P < .001), and to die in the hospital (29.6% vs. 19.9%; P < .001).
“Most patients we follow in our practices with epilepsy who experienced COVID-19 in general have had symptoms similar to the general population,” Dr. Jette said. “There are rare instances where COVID-19 can result in an exacerbation of seizures in some with pre-existing epilepsy. This is not surprising as infections in particular can decrease the seizure threshold and result in breakthrough seizures in people living with epilepsy.”
Loss of seizure control
How might epilepsy be related to worse outcomes in COVID-19? Andrew Wilner, MD, a neurologist and internist at University of Tennessee Health Science Center, Memphis, who’s familiar with the study findings, said COVID-19 itself may not worsen epilepsy. “Evidence to suggest that COVID-19 directly affects the central nervous system is extremely limited. As such, one would not expect that a COVID-19 infection would cause epilepsy or exacerbate epilepsy,” he said. “However, patients with epilepsy who suffer from infections may be predisposed to decreased seizure control. Consequently, it would not be surprising if patients with epilepsy who also had COVID-19 had loss of seizure control and even status epilepticus, which could adversely affect their hospital course. However, there are no data on this potential phenomenon.”
Dr. Wilner suspected that comorbidities explain the higher mortality in patients with epilepsy. “The findings are probably most useful in that they call attention to the fact that epilepsy patients are more vulnerable to a host of comorbidities and resultant poorer outcomes due to any acute illness.”
As for treatment, Dr. Wilner urged colleagues to make sure that hospitalized patients with epilepsy “continue to receive their antiepileptic medications, which they may no longer be able to take orally. They may need to be switched temporarily to an intravenous formulation.”
In an interview, Selim Benbadis, MD, a neurologist from the University of South Florida, Tampa, suggested that antiseizure medications may play a role in the COVID-19 disease course because they can reduce the efficacy of other medications, although he noted that drug treatments for COVID-19 were limited early on. He recommended that neurologists “avoid old enzyme-inducing seizure medications, as is generally recommended.”
No study funding is reported. The study authors and Dr. Benbadis reported no relevant disclosures. Dr. Wilner is a medical adviser for the epilepsy disease management program for CVS/Health.
according to a new study presented at the annual meeting of the American Epilepsy Society. While the findings are preliminary and not yet adjusted for various confounders, the authors say they are a warning sign that patients with epilepsy may face higher risks.
“These findings suggest that epilepsy may be a pre-existing condition that places patients at increased risk for death if hospitalized with a COVID-19 infection. It may offer neurologists guidance when counseling patients on critical preventative measures such as masking, social distancing, and most importantly, vaccination,” lead author Claire Ufongene, a student at Icahn School of Medicine at Mount Sinai, New York, said in an interview.
According to Ms. Ufongene, there’s sparse data about COVID-19 outcomes in patients with epilepsy, although she highlighted a 2021 meta-analysis of 13 studies that found a higher risk of severity (odds ratio, 1.69; 95% confidence interval, 1.11-2.59, P = .010) and mortality (OR, 1.71; 95% CI, 1.14-2.56, P = .010).
For the new study, researchers retrospectively tracked identified 334 patients with epilepsy and COVID-19 and 9,499 other patients with COVID-19 from March 15, 2020, to May 17, 2021. All were treated at hospitals within the New York–based Icahn School of Medicine at Mount Sinai.
The groups of patients with and without epilepsy were similar in some ways: 45% and 46%, respectively, were female (P = .674), and their ages were similar (average, 62 years and 65 years, respectively; P = .02). Racial makeup was also similar (non-Hispanic groups made up 27.8% of those with epilepsy and 24.5% of those without; the difference was not statistically significant).
“In addition, more of those with epilepsy were English speaking [83.2% vs. 77.9%] and had Medicaid insurance [50.9% vs. 38.9%], while fewer of those with epilepsy had private insurance [16.2% vs. 25.5%] or were Spanish speaking [14.0% vs. 9.3%],” study coauthor Nathalie Jette, MD, MSc, a neurologist at Icahn School of Medicine at Mount Sinai, said in an interview.
In terms of outcomes, patients with epilepsy were much more likely to need ventilator support (37.7% vs. 14.3%; P < .001), to be admitted to the ICU (39.2% vs. 17.7%; P < .001), and to die in the hospital (29.6% vs. 19.9%; P < .001).
“Most patients we follow in our practices with epilepsy who experienced COVID-19 in general have had symptoms similar to the general population,” Dr. Jette said. “There are rare instances where COVID-19 can result in an exacerbation of seizures in some with pre-existing epilepsy. This is not surprising as infections in particular can decrease the seizure threshold and result in breakthrough seizures in people living with epilepsy.”
Loss of seizure control
How might epilepsy be related to worse outcomes in COVID-19? Andrew Wilner, MD, a neurologist and internist at University of Tennessee Health Science Center, Memphis, who’s familiar with the study findings, said COVID-19 itself may not worsen epilepsy. “Evidence to suggest that COVID-19 directly affects the central nervous system is extremely limited. As such, one would not expect that a COVID-19 infection would cause epilepsy or exacerbate epilepsy,” he said. “However, patients with epilepsy who suffer from infections may be predisposed to decreased seizure control. Consequently, it would not be surprising if patients with epilepsy who also had COVID-19 had loss of seizure control and even status epilepticus, which could adversely affect their hospital course. However, there are no data on this potential phenomenon.”
Dr. Wilner suspected that comorbidities explain the higher mortality in patients with epilepsy. “The findings are probably most useful in that they call attention to the fact that epilepsy patients are more vulnerable to a host of comorbidities and resultant poorer outcomes due to any acute illness.”
As for treatment, Dr. Wilner urged colleagues to make sure that hospitalized patients with epilepsy “continue to receive their antiepileptic medications, which they may no longer be able to take orally. They may need to be switched temporarily to an intravenous formulation.”
In an interview, Selim Benbadis, MD, a neurologist from the University of South Florida, Tampa, suggested that antiseizure medications may play a role in the COVID-19 disease course because they can reduce the efficacy of other medications, although he noted that drug treatments for COVID-19 were limited early on. He recommended that neurologists “avoid old enzyme-inducing seizure medications, as is generally recommended.”
No study funding is reported. The study authors and Dr. Benbadis reported no relevant disclosures. Dr. Wilner is a medical adviser for the epilepsy disease management program for CVS/Health.
according to a new study presented at the annual meeting of the American Epilepsy Society. While the findings are preliminary and not yet adjusted for various confounders, the authors say they are a warning sign that patients with epilepsy may face higher risks.
“These findings suggest that epilepsy may be a pre-existing condition that places patients at increased risk for death if hospitalized with a COVID-19 infection. It may offer neurologists guidance when counseling patients on critical preventative measures such as masking, social distancing, and most importantly, vaccination,” lead author Claire Ufongene, a student at Icahn School of Medicine at Mount Sinai, New York, said in an interview.
According to Ms. Ufongene, there’s sparse data about COVID-19 outcomes in patients with epilepsy, although she highlighted a 2021 meta-analysis of 13 studies that found a higher risk of severity (odds ratio, 1.69; 95% confidence interval, 1.11-2.59, P = .010) and mortality (OR, 1.71; 95% CI, 1.14-2.56, P = .010).
For the new study, researchers retrospectively tracked identified 334 patients with epilepsy and COVID-19 and 9,499 other patients with COVID-19 from March 15, 2020, to May 17, 2021. All were treated at hospitals within the New York–based Icahn School of Medicine at Mount Sinai.
The groups of patients with and without epilepsy were similar in some ways: 45% and 46%, respectively, were female (P = .674), and their ages were similar (average, 62 years and 65 years, respectively; P = .02). Racial makeup was also similar (non-Hispanic groups made up 27.8% of those with epilepsy and 24.5% of those without; the difference was not statistically significant).
“In addition, more of those with epilepsy were English speaking [83.2% vs. 77.9%] and had Medicaid insurance [50.9% vs. 38.9%], while fewer of those with epilepsy had private insurance [16.2% vs. 25.5%] or were Spanish speaking [14.0% vs. 9.3%],” study coauthor Nathalie Jette, MD, MSc, a neurologist at Icahn School of Medicine at Mount Sinai, said in an interview.
In terms of outcomes, patients with epilepsy were much more likely to need ventilator support (37.7% vs. 14.3%; P < .001), to be admitted to the ICU (39.2% vs. 17.7%; P < .001), and to die in the hospital (29.6% vs. 19.9%; P < .001).
“Most patients we follow in our practices with epilepsy who experienced COVID-19 in general have had symptoms similar to the general population,” Dr. Jette said. “There are rare instances where COVID-19 can result in an exacerbation of seizures in some with pre-existing epilepsy. This is not surprising as infections in particular can decrease the seizure threshold and result in breakthrough seizures in people living with epilepsy.”
Loss of seizure control
How might epilepsy be related to worse outcomes in COVID-19? Andrew Wilner, MD, a neurologist and internist at University of Tennessee Health Science Center, Memphis, who’s familiar with the study findings, said COVID-19 itself may not worsen epilepsy. “Evidence to suggest that COVID-19 directly affects the central nervous system is extremely limited. As such, one would not expect that a COVID-19 infection would cause epilepsy or exacerbate epilepsy,” he said. “However, patients with epilepsy who suffer from infections may be predisposed to decreased seizure control. Consequently, it would not be surprising if patients with epilepsy who also had COVID-19 had loss of seizure control and even status epilepticus, which could adversely affect their hospital course. However, there are no data on this potential phenomenon.”
Dr. Wilner suspected that comorbidities explain the higher mortality in patients with epilepsy. “The findings are probably most useful in that they call attention to the fact that epilepsy patients are more vulnerable to a host of comorbidities and resultant poorer outcomes due to any acute illness.”
As for treatment, Dr. Wilner urged colleagues to make sure that hospitalized patients with epilepsy “continue to receive their antiepileptic medications, which they may no longer be able to take orally. They may need to be switched temporarily to an intravenous formulation.”
In an interview, Selim Benbadis, MD, a neurologist from the University of South Florida, Tampa, suggested that antiseizure medications may play a role in the COVID-19 disease course because they can reduce the efficacy of other medications, although he noted that drug treatments for COVID-19 were limited early on. He recommended that neurologists “avoid old enzyme-inducing seizure medications, as is generally recommended.”
No study funding is reported. The study authors and Dr. Benbadis reported no relevant disclosures. Dr. Wilner is a medical adviser for the epilepsy disease management program for CVS/Health.
FROM AES 2021
What the Future May Hold for Covid-19 Survivors
What the Future May Hold for Covid-19 Survivors
More than 3 million Americans1 have been hospitalized with Covid-19, and
And these are just the patients with severe Covid: those who were never hospitalized are also showing deleterious effects from the effects of their illness.
Covid in the ICU
What we know is that prior to Covid, 10% of all patients were admitted to ICU with acute respiratory distress syndrome4 (ARDS), despite receiving such life-saving measures as mechanical ventilation, medication, and supportive nutrition. Those who do survive face a long journey.4 Besides the specific respiratory recovery needed in those with ARDS, patients who have spent time in the ICU can develop multiple non-respiratory complications, including muscle wasting, generalized weakness, and delirium. The physical, cognitive, and psychological impairments that follow an ICU stay are termed postintensive care syndrome (PICS). PICS is an underrecognized phenomenon that describes the immense complications of an ICU stay for any reason. Recognition of this entity, and education of patients, is particularly important now as we face an ongoing pandemic which is creating a burgeoning number of ICU graduates.
PICS
Cognitive dysfunction is one hallmark of PICS. Delirium is a common complication of any hospitalization, with critically ill patients particularly susceptible given the severity of their illness and their exposure to medications such as sedatives. However, persistent global cognitive impairment is unique to PICS. Up to
The second aspect of PICS is its psychological component. In the Hopkins study,5
The final component of PICS is physical impairment. Those who are critically ill commonly suffer intensive care unit
Covid in the ICU
Estimates of the incidence of PICS due to Covid are evolving. A report on 1700 Covid hospitalized patients in Wuhan, China demonstrated a large prevalence of residual symptoms at 6 months. The most common symptoms were fatigue and weakness (63%), insomnia (26%), and anxiety or depression (
As more centers track the progress of their ICU graduates over time, we can better understand the profound impact of critical illness on our Covid patients and better educate our patients and families on what to expect. One might be able to gain some clues from what is known regarding the prior coronavirus epidemics, severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS). In these diseases, a meta-analysis showed significant rates of
Additional issues
What is particularly unique to Covid is the prevalence of long-term symptoms in those who were never hospitalized for Covid. Recent estimates of non-hospitalized patients who had Covid are showing at least 25% of them have had long-lasting effects, including stomach pain and respiratory
Financially
Conclusion
The long-term effects of hospitalization from Covid argues further for continued work on increasing the vaccination rate of our population. Even with Delta variant, vaccines decrease the risk of hospitalization and death by more than a factor of
- CDC. Covid Data Tracker. https://covid.cdc.gov/covid-data-tracker/#new-hospital-admissions
- CDC. Covid Data Tracker. Trends total death. https://covid.cdc.gov/covid-data-tracker/#trends_totaldeaths_currenthospitaladmissions|tot_deaths|sum_inpatient_beds_used_covid_7DayAvg
- Johns Hopkins. Weekly hospital trends. https://coronavirus.jhu.edu/data/hospitalization-7-day-trend
- Bellani G, Laffey JG, Pham T, et al.; LUNG SAFE Investigators; ESICM Trials Group. Epidemiology, Patterns of Care, and Mortality for Patients with Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA. 2016 Feb 23;315(8):788-800
- Ramona O. Hopkins, Lindell K. Weaver, Dave Collingridge, et al. Two-Year Cognitive, Emotional, and Quality-of-Life Outcomes in Acute Respiratory Distress Syndrome. Amer J Resp Crit Care Med. 2005; (171):4.
- Stevens, Robert D, Marshall, Scott A, Cornblath, David R, et al. A framework for diagnosing and classifying intensive care unit-acquired weakness, Critic Care Medic. 2009; (37)10: S299-S308.
- Chaolin Huang, Lixue Huang, Yeming Wang, et al. 6-month consequences of COVID-19 in patients discharged from om hospital: a cohort study. The Lancet 2021; 397(10270): 220-232.
- Gamberini L, Mazzoli CA, Sintonen H, et al.; ICU-RER COVID-19 Collaboration. Quality of life of COVID-19 critically ill survivors after ICU discharge: 90 days follow-up. Qual Life Res. 2021 Oct;30(10):2805-2817.
- Chopra V, Flanders SA, O'Malley M, et al. Sixty-Day Outcomes Among Patients Hospitalized With COVID-19. Ann Intern Med. 2021 Apr;174(4):576-578.
- The Writing Committee for the COMEBAC Study Group. Four-Month Clinical Status of a Cohort of Patients After Hospitalization for COVID-19. JAMA. 2021;325(15):1525–1534.
- Ahmed H, Patel K, Greenwood DC, et al. Long-term clinical outcomes in survivors of severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus outbreaks after hospitalisation or ICU admission: A systematic review and meta-analysis. J Rehabil Med. 2020 May 31;52(5): jrm00063.
- Logue JK, Franko NM, McCulloch DJ, et al. Sequelae in Adults at 6 Months After COVID-19 Infection. JAMA Netw Open. 2021;4(2): e210830.
- Brenda Goodman. Major study will investigate long-haul Covid-19. WebMD News Brief. Sept. 15, 2021. https://www.webmd.com/lung/news/20210915/major-study-will-investigate-long-haul-covid
- Vineet Chopra, Scott A. Flanders, Megan O’Malley, et al. Sixty-Day Outcomes Among Patients Hospitalized With COVID-19. Ann Intern Med. Letters. 2021; Apr.
- Yuping Tsai, Tara M. Vogt, Fangjun Zhou. Patient Characteristics and Costs Associated With COVID-19–Related Medical Care Among Medicare Fee-for-Service Beneficiaries. Ann Intern Med. 2021; Aug.
- Lavery AM, Preston LE, Ko JY, et al. Characteristics of Hospitalized COVID-19 Patients Discharged and Experiencing Same-Hospital Readmission — United States, March–August 2020. MMWR Morb Mortal Wkly Rep 2020; 69:1695–1699.
- Scobie HM, Johnson AG, Suthar AB, et al. Monitoring Incidence of COVID-19 Cases, Hospitalizations, and Deaths, by Vaccination Status — 13 U.S. Jurisdictions, April 4–July 17, 2021. MMWR Morb Mortal Wkly Rep 2021; 70:1284–1290.
What the Future May Hold for Covid-19 Survivors
More than 3 million Americans1 have been hospitalized with Covid-19, and
And these are just the patients with severe Covid: those who were never hospitalized are also showing deleterious effects from the effects of their illness.
Covid in the ICU
What we know is that prior to Covid, 10% of all patients were admitted to ICU with acute respiratory distress syndrome4 (ARDS), despite receiving such life-saving measures as mechanical ventilation, medication, and supportive nutrition. Those who do survive face a long journey.4 Besides the specific respiratory recovery needed in those with ARDS, patients who have spent time in the ICU can develop multiple non-respiratory complications, including muscle wasting, generalized weakness, and delirium. The physical, cognitive, and psychological impairments that follow an ICU stay are termed postintensive care syndrome (PICS). PICS is an underrecognized phenomenon that describes the immense complications of an ICU stay for any reason. Recognition of this entity, and education of patients, is particularly important now as we face an ongoing pandemic which is creating a burgeoning number of ICU graduates.
PICS
Cognitive dysfunction is one hallmark of PICS. Delirium is a common complication of any hospitalization, with critically ill patients particularly susceptible given the severity of their illness and their exposure to medications such as sedatives. However, persistent global cognitive impairment is unique to PICS. Up to
The second aspect of PICS is its psychological component. In the Hopkins study,5
The final component of PICS is physical impairment. Those who are critically ill commonly suffer intensive care unit
Covid in the ICU
Estimates of the incidence of PICS due to Covid are evolving. A report on 1700 Covid hospitalized patients in Wuhan, China demonstrated a large prevalence of residual symptoms at 6 months. The most common symptoms were fatigue and weakness (63%), insomnia (26%), and anxiety or depression (
As more centers track the progress of their ICU graduates over time, we can better understand the profound impact of critical illness on our Covid patients and better educate our patients and families on what to expect. One might be able to gain some clues from what is known regarding the prior coronavirus epidemics, severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS). In these diseases, a meta-analysis showed significant rates of
Additional issues
What is particularly unique to Covid is the prevalence of long-term symptoms in those who were never hospitalized for Covid. Recent estimates of non-hospitalized patients who had Covid are showing at least 25% of them have had long-lasting effects, including stomach pain and respiratory
Financially
Conclusion
The long-term effects of hospitalization from Covid argues further for continued work on increasing the vaccination rate of our population. Even with Delta variant, vaccines decrease the risk of hospitalization and death by more than a factor of
What the Future May Hold for Covid-19 Survivors
More than 3 million Americans1 have been hospitalized with Covid-19, and
And these are just the patients with severe Covid: those who were never hospitalized are also showing deleterious effects from the effects of their illness.
Covid in the ICU
What we know is that prior to Covid, 10% of all patients were admitted to ICU with acute respiratory distress syndrome4 (ARDS), despite receiving such life-saving measures as mechanical ventilation, medication, and supportive nutrition. Those who do survive face a long journey.4 Besides the specific respiratory recovery needed in those with ARDS, patients who have spent time in the ICU can develop multiple non-respiratory complications, including muscle wasting, generalized weakness, and delirium. The physical, cognitive, and psychological impairments that follow an ICU stay are termed postintensive care syndrome (PICS). PICS is an underrecognized phenomenon that describes the immense complications of an ICU stay for any reason. Recognition of this entity, and education of patients, is particularly important now as we face an ongoing pandemic which is creating a burgeoning number of ICU graduates.
PICS
Cognitive dysfunction is one hallmark of PICS. Delirium is a common complication of any hospitalization, with critically ill patients particularly susceptible given the severity of their illness and their exposure to medications such as sedatives. However, persistent global cognitive impairment is unique to PICS. Up to
The second aspect of PICS is its psychological component. In the Hopkins study,5
The final component of PICS is physical impairment. Those who are critically ill commonly suffer intensive care unit
Covid in the ICU
Estimates of the incidence of PICS due to Covid are evolving. A report on 1700 Covid hospitalized patients in Wuhan, China demonstrated a large prevalence of residual symptoms at 6 months. The most common symptoms were fatigue and weakness (63%), insomnia (26%), and anxiety or depression (
As more centers track the progress of their ICU graduates over time, we can better understand the profound impact of critical illness on our Covid patients and better educate our patients and families on what to expect. One might be able to gain some clues from what is known regarding the prior coronavirus epidemics, severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS). In these diseases, a meta-analysis showed significant rates of
Additional issues
What is particularly unique to Covid is the prevalence of long-term symptoms in those who were never hospitalized for Covid. Recent estimates of non-hospitalized patients who had Covid are showing at least 25% of them have had long-lasting effects, including stomach pain and respiratory
Financially
Conclusion
The long-term effects of hospitalization from Covid argues further for continued work on increasing the vaccination rate of our population. Even with Delta variant, vaccines decrease the risk of hospitalization and death by more than a factor of
- CDC. Covid Data Tracker. https://covid.cdc.gov/covid-data-tracker/#new-hospital-admissions
- CDC. Covid Data Tracker. Trends total death. https://covid.cdc.gov/covid-data-tracker/#trends_totaldeaths_currenthospitaladmissions|tot_deaths|sum_inpatient_beds_used_covid_7DayAvg
- Johns Hopkins. Weekly hospital trends. https://coronavirus.jhu.edu/data/hospitalization-7-day-trend
- Bellani G, Laffey JG, Pham T, et al.; LUNG SAFE Investigators; ESICM Trials Group. Epidemiology, Patterns of Care, and Mortality for Patients with Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA. 2016 Feb 23;315(8):788-800
- Ramona O. Hopkins, Lindell K. Weaver, Dave Collingridge, et al. Two-Year Cognitive, Emotional, and Quality-of-Life Outcomes in Acute Respiratory Distress Syndrome. Amer J Resp Crit Care Med. 2005; (171):4.
- Stevens, Robert D, Marshall, Scott A, Cornblath, David R, et al. A framework for diagnosing and classifying intensive care unit-acquired weakness, Critic Care Medic. 2009; (37)10: S299-S308.
- Chaolin Huang, Lixue Huang, Yeming Wang, et al. 6-month consequences of COVID-19 in patients discharged from om hospital: a cohort study. The Lancet 2021; 397(10270): 220-232.
- Gamberini L, Mazzoli CA, Sintonen H, et al.; ICU-RER COVID-19 Collaboration. Quality of life of COVID-19 critically ill survivors after ICU discharge: 90 days follow-up. Qual Life Res. 2021 Oct;30(10):2805-2817.
- Chopra V, Flanders SA, O'Malley M, et al. Sixty-Day Outcomes Among Patients Hospitalized With COVID-19. Ann Intern Med. 2021 Apr;174(4):576-578.
- The Writing Committee for the COMEBAC Study Group. Four-Month Clinical Status of a Cohort of Patients After Hospitalization for COVID-19. JAMA. 2021;325(15):1525–1534.
- Ahmed H, Patel K, Greenwood DC, et al. Long-term clinical outcomes in survivors of severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus outbreaks after hospitalisation or ICU admission: A systematic review and meta-analysis. J Rehabil Med. 2020 May 31;52(5): jrm00063.
- Logue JK, Franko NM, McCulloch DJ, et al. Sequelae in Adults at 6 Months After COVID-19 Infection. JAMA Netw Open. 2021;4(2): e210830.
- Brenda Goodman. Major study will investigate long-haul Covid-19. WebMD News Brief. Sept. 15, 2021. https://www.webmd.com/lung/news/20210915/major-study-will-investigate-long-haul-covid
- Vineet Chopra, Scott A. Flanders, Megan O’Malley, et al. Sixty-Day Outcomes Among Patients Hospitalized With COVID-19. Ann Intern Med. Letters. 2021; Apr.
- Yuping Tsai, Tara M. Vogt, Fangjun Zhou. Patient Characteristics and Costs Associated With COVID-19–Related Medical Care Among Medicare Fee-for-Service Beneficiaries. Ann Intern Med. 2021; Aug.
- Lavery AM, Preston LE, Ko JY, et al. Characteristics of Hospitalized COVID-19 Patients Discharged and Experiencing Same-Hospital Readmission — United States, March–August 2020. MMWR Morb Mortal Wkly Rep 2020; 69:1695–1699.
- Scobie HM, Johnson AG, Suthar AB, et al. Monitoring Incidence of COVID-19 Cases, Hospitalizations, and Deaths, by Vaccination Status — 13 U.S. Jurisdictions, April 4–July 17, 2021. MMWR Morb Mortal Wkly Rep 2021; 70:1284–1290.
- CDC. Covid Data Tracker. https://covid.cdc.gov/covid-data-tracker/#new-hospital-admissions
- CDC. Covid Data Tracker. Trends total death. https://covid.cdc.gov/covid-data-tracker/#trends_totaldeaths_currenthospitaladmissions|tot_deaths|sum_inpatient_beds_used_covid_7DayAvg
- Johns Hopkins. Weekly hospital trends. https://coronavirus.jhu.edu/data/hospitalization-7-day-trend
- Bellani G, Laffey JG, Pham T, et al.; LUNG SAFE Investigators; ESICM Trials Group. Epidemiology, Patterns of Care, and Mortality for Patients with Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA. 2016 Feb 23;315(8):788-800
- Ramona O. Hopkins, Lindell K. Weaver, Dave Collingridge, et al. Two-Year Cognitive, Emotional, and Quality-of-Life Outcomes in Acute Respiratory Distress Syndrome. Amer J Resp Crit Care Med. 2005; (171):4.
- Stevens, Robert D, Marshall, Scott A, Cornblath, David R, et al. A framework for diagnosing and classifying intensive care unit-acquired weakness, Critic Care Medic. 2009; (37)10: S299-S308.
- Chaolin Huang, Lixue Huang, Yeming Wang, et al. 6-month consequences of COVID-19 in patients discharged from om hospital: a cohort study. The Lancet 2021; 397(10270): 220-232.
- Gamberini L, Mazzoli CA, Sintonen H, et al.; ICU-RER COVID-19 Collaboration. Quality of life of COVID-19 critically ill survivors after ICU discharge: 90 days follow-up. Qual Life Res. 2021 Oct;30(10):2805-2817.
- Chopra V, Flanders SA, O'Malley M, et al. Sixty-Day Outcomes Among Patients Hospitalized With COVID-19. Ann Intern Med. 2021 Apr;174(4):576-578.
- The Writing Committee for the COMEBAC Study Group. Four-Month Clinical Status of a Cohort of Patients After Hospitalization for COVID-19. JAMA. 2021;325(15):1525–1534.
- Ahmed H, Patel K, Greenwood DC, et al. Long-term clinical outcomes in survivors of severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus outbreaks after hospitalisation or ICU admission: A systematic review and meta-analysis. J Rehabil Med. 2020 May 31;52(5): jrm00063.
- Logue JK, Franko NM, McCulloch DJ, et al. Sequelae in Adults at 6 Months After COVID-19 Infection. JAMA Netw Open. 2021;4(2): e210830.
- Brenda Goodman. Major study will investigate long-haul Covid-19. WebMD News Brief. Sept. 15, 2021. https://www.webmd.com/lung/news/20210915/major-study-will-investigate-long-haul-covid
- Vineet Chopra, Scott A. Flanders, Megan O’Malley, et al. Sixty-Day Outcomes Among Patients Hospitalized With COVID-19. Ann Intern Med. Letters. 2021; Apr.
- Yuping Tsai, Tara M. Vogt, Fangjun Zhou. Patient Characteristics and Costs Associated With COVID-19–Related Medical Care Among Medicare Fee-for-Service Beneficiaries. Ann Intern Med. 2021; Aug.
- Lavery AM, Preston LE, Ko JY, et al. Characteristics of Hospitalized COVID-19 Patients Discharged and Experiencing Same-Hospital Readmission — United States, March–August 2020. MMWR Morb Mortal Wkly Rep 2020; 69:1695–1699.
- Scobie HM, Johnson AG, Suthar AB, et al. Monitoring Incidence of COVID-19 Cases, Hospitalizations, and Deaths, by Vaccination Status — 13 U.S. Jurisdictions, April 4–July 17, 2021. MMWR Morb Mortal Wkly Rep 2021; 70:1284–1290.
Risk for severe COVID-19 and death plummets with Pfizer booster
Both studies were completed before the advent of the Omicron variant.
In one study that included data on more than 4 million patients, led by Yinon M. Bar-On, MSc, of the Weizmann Institute of Science in Rehovot, Israel, the rate of confirmed SARS-CoV-2 infection was lower in the booster group than in the nonbooster group by a factor of about 10.
This was true across all five age groups studied (range among the groups [starting with age 16], 9.0-17.2).
The risk for severe COVID-19 in the primary analysis decreased in the booster group by a factor of 17.9 (95% confidence interval, 15.1-21.2), among those aged 60 years or older. Risk for severe illness in those ages 40-59 was lower by a factor of 21.7 (95% CI, 10.6-44.2).
Among the 60 and older age group, risk for death was also reduced by a factor of 14.7 (95% CI, 10.0-21.4).
Researchers analyzed data for the period from July 30 to Oct. 10, 2021, from the Israel Ministry of Health database on 4.69 million people at least 16 years old who had received two Pfizer doses at least 5 months earlier.
In the main analysis, the researchers compared the rates of confirmed COVID-19, severe disease, and death among those who had gotten a booster at least 12 days earlier with the rates in a nonbooster group.
The authors wrote: “Booster vaccination programs may provide a way to control transmission without costly social-distancing measures and quarantines. Our findings provide evidence for the short-term effectiveness of the booster dose against the currently dominant Delta variant in persons 16 years of age or older.”
Death risk down by 90%
A second study, led by Ronen Arbel, PhD, with the community medical services division, Clalit Health Services (CHS), Tel Aviv, which included more than 800,000 participants, also found mortality risk was greatly reduced among those who received the booster compared with those who didn’t get the booster.
Participants aged 50 years or older who received a booster at least 5 months after a second Pfizer dose had 90% lower mortality risk because of COVID-19 than participants who did not get the booster.
The adjusted hazard ratio for death as a result of COVID-19 in the booster group, as compared with the nonbooster group, was 0.10 (95% CI, 0.07-0.14; P < .001). Of the 843,208 eligible participants, 758,118 (90%) received the booster during the 54-day study period.
The study included all CHS members who were aged 50 years or older on the study start date and had received two Pfizer doses at least 5 months earlier. CHS covers about 52% of the Israeli population and is the largest of four health care organizations in Israel that provide mandatory health care.
The authors noted that, although the study period was only 54 days (Aug. 6–Sept. 29), during that time “the incidence of COVID-19 in Israel was one of the highest in the world.”
The authors of both original articles pointed out that the studies are limited by short time periods and that longer-term studies are needed to see how the booster shots stand up to known and future variants, such as Omicron.
None of the authors involved in both studies reported relevant financial relationships.
A version of this article first appeared on Medscape.com.
Both studies were completed before the advent of the Omicron variant.
In one study that included data on more than 4 million patients, led by Yinon M. Bar-On, MSc, of the Weizmann Institute of Science in Rehovot, Israel, the rate of confirmed SARS-CoV-2 infection was lower in the booster group than in the nonbooster group by a factor of about 10.
This was true across all five age groups studied (range among the groups [starting with age 16], 9.0-17.2).
The risk for severe COVID-19 in the primary analysis decreased in the booster group by a factor of 17.9 (95% confidence interval, 15.1-21.2), among those aged 60 years or older. Risk for severe illness in those ages 40-59 was lower by a factor of 21.7 (95% CI, 10.6-44.2).
Among the 60 and older age group, risk for death was also reduced by a factor of 14.7 (95% CI, 10.0-21.4).
Researchers analyzed data for the period from July 30 to Oct. 10, 2021, from the Israel Ministry of Health database on 4.69 million people at least 16 years old who had received two Pfizer doses at least 5 months earlier.
In the main analysis, the researchers compared the rates of confirmed COVID-19, severe disease, and death among those who had gotten a booster at least 12 days earlier with the rates in a nonbooster group.
The authors wrote: “Booster vaccination programs may provide a way to control transmission without costly social-distancing measures and quarantines. Our findings provide evidence for the short-term effectiveness of the booster dose against the currently dominant Delta variant in persons 16 years of age or older.”
Death risk down by 90%
A second study, led by Ronen Arbel, PhD, with the community medical services division, Clalit Health Services (CHS), Tel Aviv, which included more than 800,000 participants, also found mortality risk was greatly reduced among those who received the booster compared with those who didn’t get the booster.
Participants aged 50 years or older who received a booster at least 5 months after a second Pfizer dose had 90% lower mortality risk because of COVID-19 than participants who did not get the booster.
The adjusted hazard ratio for death as a result of COVID-19 in the booster group, as compared with the nonbooster group, was 0.10 (95% CI, 0.07-0.14; P < .001). Of the 843,208 eligible participants, 758,118 (90%) received the booster during the 54-day study period.
The study included all CHS members who were aged 50 years or older on the study start date and had received two Pfizer doses at least 5 months earlier. CHS covers about 52% of the Israeli population and is the largest of four health care organizations in Israel that provide mandatory health care.
The authors noted that, although the study period was only 54 days (Aug. 6–Sept. 29), during that time “the incidence of COVID-19 in Israel was one of the highest in the world.”
The authors of both original articles pointed out that the studies are limited by short time periods and that longer-term studies are needed to see how the booster shots stand up to known and future variants, such as Omicron.
None of the authors involved in both studies reported relevant financial relationships.
A version of this article first appeared on Medscape.com.
Both studies were completed before the advent of the Omicron variant.
In one study that included data on more than 4 million patients, led by Yinon M. Bar-On, MSc, of the Weizmann Institute of Science in Rehovot, Israel, the rate of confirmed SARS-CoV-2 infection was lower in the booster group than in the nonbooster group by a factor of about 10.
This was true across all five age groups studied (range among the groups [starting with age 16], 9.0-17.2).
The risk for severe COVID-19 in the primary analysis decreased in the booster group by a factor of 17.9 (95% confidence interval, 15.1-21.2), among those aged 60 years or older. Risk for severe illness in those ages 40-59 was lower by a factor of 21.7 (95% CI, 10.6-44.2).
Among the 60 and older age group, risk for death was also reduced by a factor of 14.7 (95% CI, 10.0-21.4).
Researchers analyzed data for the period from July 30 to Oct. 10, 2021, from the Israel Ministry of Health database on 4.69 million people at least 16 years old who had received two Pfizer doses at least 5 months earlier.
In the main analysis, the researchers compared the rates of confirmed COVID-19, severe disease, and death among those who had gotten a booster at least 12 days earlier with the rates in a nonbooster group.
The authors wrote: “Booster vaccination programs may provide a way to control transmission without costly social-distancing measures and quarantines. Our findings provide evidence for the short-term effectiveness of the booster dose against the currently dominant Delta variant in persons 16 years of age or older.”
Death risk down by 90%
A second study, led by Ronen Arbel, PhD, with the community medical services division, Clalit Health Services (CHS), Tel Aviv, which included more than 800,000 participants, also found mortality risk was greatly reduced among those who received the booster compared with those who didn’t get the booster.
Participants aged 50 years or older who received a booster at least 5 months after a second Pfizer dose had 90% lower mortality risk because of COVID-19 than participants who did not get the booster.
The adjusted hazard ratio for death as a result of COVID-19 in the booster group, as compared with the nonbooster group, was 0.10 (95% CI, 0.07-0.14; P < .001). Of the 843,208 eligible participants, 758,118 (90%) received the booster during the 54-day study period.
The study included all CHS members who were aged 50 years or older on the study start date and had received two Pfizer doses at least 5 months earlier. CHS covers about 52% of the Israeli population and is the largest of four health care organizations in Israel that provide mandatory health care.
The authors noted that, although the study period was only 54 days (Aug. 6–Sept. 29), during that time “the incidence of COVID-19 in Israel was one of the highest in the world.”
The authors of both original articles pointed out that the studies are limited by short time periods and that longer-term studies are needed to see how the booster shots stand up to known and future variants, such as Omicron.
None of the authors involved in both studies reported relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
CLL and COVID-19: Outcome trends and lessons learned
Retrospective but the data also highlight areas for further investigation, according to the researchers.
Specifically, “the data highlight opportunities for further investigation into optimal management of COVID-19, immune response after infection, and effective vaccination strategy for patients with CLL,” Lindsey E. Roeker, MD, a hematologic oncologist at Memorial Sloan Kettering Cancer Center, New York, and colleagues wrote in a Nov. 4, 2021, letter to the editor of Blood.
The researchers noted that recently reported COVID-19 case fatality rates from two large series of patients with CLL ranged from 31% to 33%, but trends over time were unclear.
“To understand change in outcomes over time, we present this follow-up study, which builds upon a previously reported cohort with extended follow up and addition of more recently diagnosed cases,” they wrote, explaining that “early data from a small series suggest that patients with CLL may not consistently generate anti–SARS-CoV-2 antibodies after infection.”
“This finding, along with previous reports of inadequate response to vaccines in patients with CLL, highlight significant questions regarding COVID-19 vaccine efficacy in this population,” they added.
Trends in outcomes
The review of outcomes in 374 CLL patients from 45 centers who were diagnosed with COVID-19 between Feb. 17, 2020, and Feb. 1, 2021, showed an overall case fatality rate (CFR) of 28%. Among the 278 patients (75%) admitted to the hospital, the CFR was 36%; among those not admitted, the CFR was 4.3%.
Independent predictors of poor survival were ages over 75 years (adjusted hazard ratio, 1.6) and Cumulative Illness Rating Scale–Geriatric (CIRS) scores greater than 6 (aHR, 1.6).
Updated data for 254 patients diagnosed from Feb. 17 to April 30, 2020, and 120 diagnosed from May 1, 2020, to Feb. 1, 2021, showed that more patients in the early versus later cohort were admitted to the hospital (85% vs. 55%) and more required ICU admission (32% vs. 11%).
The overall case fatality rates in the early and later cohorts were 35% and 11%, respectively (P < .001), and among those requiring hospitalization, the rates were 40% and 20% (P = .003).
“The proportion of hospitalized patients requiring ICU-level care was lower in the later cohort (37% vs. 29%), whereas the CFR remained high for the subset of patients who required ICU-level care (52% vs. 50%; P = .89),” the investigators wrote, noting that “[a] difference in management of BTKi[Bruton’s tyrosine kinase inhibitor]-treated patients was observed in the early versus the later cohort.”
“In the early cohort, 76% of patients receiving BTKi had their drug therapy suspended or discontinued. In the later cohort, only 20% of BTKi-treated patients had their therapy suspended or discontinued,” they added.
Univariate analyses showed significant associations between use of remdesivir and OS (HR, 0.48) and use of convalescent plasma and OS (HR, 0.50) in patients who were admitted, whereas admitted patients who received corticosteroids or hydroxychloroquine had an increased risk of death (HRs, 1.73 and 1.53, respectively).
“Corticosteroids were associated with increased risk of death when the data were adjusted for admission status (HR, 1.8) and the need for mechanical ventilation (HR, 2.0), although they were not significantly associated with survival when the data were adjusted for use of supplemental oxygen (HR, 1.4),” they wrote, also noting that admitted patients treated with corticosteroids in the later cohort did not experience an OS benefit (HR, 2.6).
The findings mirror population-based studies with decreasing CFR (35% in those diagnosed before May 1, 2020, versus 11% in those diagnosed after that date), they said, adding that “these trends suggest that patients in the later cohort experienced a less severe clinical course and that the observed difference in CFR over time may not just be due to more frequent testing and identification of less symptomatic patients.”
Of note, the outcomes observed for steroid-treated patients in the current cohort contrast with those from the RECOVERY trial as published in July 2020, which “may be an artifact of their use in patients with more severe disease,” they suggested.
They added that these data “are hypothesis generating and suggest that COVID-19 directed interventions, particularly immunomodulatory agents, require prospective study, specifically in immunocompromised populations.”
The investigators also noted that, consistent with a prior single-center study, 60% of patients with CLL developed positive anti–SARS-CoV-2 serology results after polymerase chain reaction diagnosis of COVID-19, adding further evidence of nonuniform antibody production after COVID-19 in patients with CLL.
Study is ongoing to gain understanding of the immune response to SARS-CoV-2 vaccination in patients with CLL, they said.
Changing the odds
In a related commentary also published in Blood, Yair Herishanu, MD, and Chava Perry, MD, PhD, of Tel Aviv Sourasky Medical Center called the reduction in mortality over time as reported by Dr. Roeker and colleagues “encouraging and intriguing.”
“One explanation is that the later cohort included a larger proportion of patients with mild symptoms who were diagnosed because of increased awareness of COVID-19 and more extensive screening to detect SARS-CoV-2 over time. That is supported by the lower hospitalization rates and lower rates of hospitalized patients requiring ICU care in the later cohort,” they wrote. “Another possibility is better patient management owing to increasing experience, expanding therapeutic options, and improved capacity of health systems to manage an influx of patients.”
The lower mortality in hospitalized patients over time may reflect better management of patients over time, but it also highlights the significance of “early introduction of various anti–COVID-19 therapies to prevent clinical deterioration to ICU-level care,” they added.
Also intriguing, according to Dr. Herishanu and Dr. Perry, was the finding of increased secondary infections and death rates among corticosteroid-treatment patients.
In the RECOVERY trial, the use of dexamethasone improved survival in patients hospitalized with COVID-19 who received respiratory support. Perhaps the impaired immune reactions in patients with CLL moderate the hyperinflammatory reactions to COVID-19, thus turning corticosteroids beneficial effects to somewhat redundant in this frail population,” they wrote.
Further, the finding that only 60% of patients with CLL seroconvert after the acute phase of SARS-CoV-2 infection suggests CLL patients may be at risk for reinfection, which “justifies vaccinating all patients with CLL who have recovered from COVID-19.”
“Likewise, patients with CLL may develop persistent COVID-19 infection,” they added, explaining that “prolonged shedding of infectious SARS-CoV-2 virus and within-host genomic evolution may eventually lead to emergence of new virus variants.”
Given the high risk of severe COVID-19 disease and impaired antibody-mediated immune response to the virus and its vaccine, a booster dose may be warranted in patients with CLL who fail to achieve seropositivity after 2 vaccine doses, they said.
The available data to date “call for early application of antiviral drugs, [monoclonal antibodies], and convalescent plasma as well as improved vaccination strategy, to improve the odds for patients with CLL confronting COVID-19,” they concluded, adding that large-scale prospective studies on the clinical disease course, outcomes, efficacy of treatments, and vaccination timing and schedule in patients with CLL and COVID-19 are still warranted.
The research was supported by a National Cancer Institute Cancer Center support grant. Dr. Roeker, Dr. Herishanu, and Dr. Perry reported having no financial disclosures.
Retrospective but the data also highlight areas for further investigation, according to the researchers.
Specifically, “the data highlight opportunities for further investigation into optimal management of COVID-19, immune response after infection, and effective vaccination strategy for patients with CLL,” Lindsey E. Roeker, MD, a hematologic oncologist at Memorial Sloan Kettering Cancer Center, New York, and colleagues wrote in a Nov. 4, 2021, letter to the editor of Blood.
The researchers noted that recently reported COVID-19 case fatality rates from two large series of patients with CLL ranged from 31% to 33%, but trends over time were unclear.
“To understand change in outcomes over time, we present this follow-up study, which builds upon a previously reported cohort with extended follow up and addition of more recently diagnosed cases,” they wrote, explaining that “early data from a small series suggest that patients with CLL may not consistently generate anti–SARS-CoV-2 antibodies after infection.”
“This finding, along with previous reports of inadequate response to vaccines in patients with CLL, highlight significant questions regarding COVID-19 vaccine efficacy in this population,” they added.
Trends in outcomes
The review of outcomes in 374 CLL patients from 45 centers who were diagnosed with COVID-19 between Feb. 17, 2020, and Feb. 1, 2021, showed an overall case fatality rate (CFR) of 28%. Among the 278 patients (75%) admitted to the hospital, the CFR was 36%; among those not admitted, the CFR was 4.3%.
Independent predictors of poor survival were ages over 75 years (adjusted hazard ratio, 1.6) and Cumulative Illness Rating Scale–Geriatric (CIRS) scores greater than 6 (aHR, 1.6).
Updated data for 254 patients diagnosed from Feb. 17 to April 30, 2020, and 120 diagnosed from May 1, 2020, to Feb. 1, 2021, showed that more patients in the early versus later cohort were admitted to the hospital (85% vs. 55%) and more required ICU admission (32% vs. 11%).
The overall case fatality rates in the early and later cohorts were 35% and 11%, respectively (P < .001), and among those requiring hospitalization, the rates were 40% and 20% (P = .003).
“The proportion of hospitalized patients requiring ICU-level care was lower in the later cohort (37% vs. 29%), whereas the CFR remained high for the subset of patients who required ICU-level care (52% vs. 50%; P = .89),” the investigators wrote, noting that “[a] difference in management of BTKi[Bruton’s tyrosine kinase inhibitor]-treated patients was observed in the early versus the later cohort.”
“In the early cohort, 76% of patients receiving BTKi had their drug therapy suspended or discontinued. In the later cohort, only 20% of BTKi-treated patients had their therapy suspended or discontinued,” they added.
Univariate analyses showed significant associations between use of remdesivir and OS (HR, 0.48) and use of convalescent plasma and OS (HR, 0.50) in patients who were admitted, whereas admitted patients who received corticosteroids or hydroxychloroquine had an increased risk of death (HRs, 1.73 and 1.53, respectively).
“Corticosteroids were associated with increased risk of death when the data were adjusted for admission status (HR, 1.8) and the need for mechanical ventilation (HR, 2.0), although they were not significantly associated with survival when the data were adjusted for use of supplemental oxygen (HR, 1.4),” they wrote, also noting that admitted patients treated with corticosteroids in the later cohort did not experience an OS benefit (HR, 2.6).
The findings mirror population-based studies with decreasing CFR (35% in those diagnosed before May 1, 2020, versus 11% in those diagnosed after that date), they said, adding that “these trends suggest that patients in the later cohort experienced a less severe clinical course and that the observed difference in CFR over time may not just be due to more frequent testing and identification of less symptomatic patients.”
Of note, the outcomes observed for steroid-treated patients in the current cohort contrast with those from the RECOVERY trial as published in July 2020, which “may be an artifact of their use in patients with more severe disease,” they suggested.
They added that these data “are hypothesis generating and suggest that COVID-19 directed interventions, particularly immunomodulatory agents, require prospective study, specifically in immunocompromised populations.”
The investigators also noted that, consistent with a prior single-center study, 60% of patients with CLL developed positive anti–SARS-CoV-2 serology results after polymerase chain reaction diagnosis of COVID-19, adding further evidence of nonuniform antibody production after COVID-19 in patients with CLL.
Study is ongoing to gain understanding of the immune response to SARS-CoV-2 vaccination in patients with CLL, they said.
Changing the odds
In a related commentary also published in Blood, Yair Herishanu, MD, and Chava Perry, MD, PhD, of Tel Aviv Sourasky Medical Center called the reduction in mortality over time as reported by Dr. Roeker and colleagues “encouraging and intriguing.”
“One explanation is that the later cohort included a larger proportion of patients with mild symptoms who were diagnosed because of increased awareness of COVID-19 and more extensive screening to detect SARS-CoV-2 over time. That is supported by the lower hospitalization rates and lower rates of hospitalized patients requiring ICU care in the later cohort,” they wrote. “Another possibility is better patient management owing to increasing experience, expanding therapeutic options, and improved capacity of health systems to manage an influx of patients.”
The lower mortality in hospitalized patients over time may reflect better management of patients over time, but it also highlights the significance of “early introduction of various anti–COVID-19 therapies to prevent clinical deterioration to ICU-level care,” they added.
Also intriguing, according to Dr. Herishanu and Dr. Perry, was the finding of increased secondary infections and death rates among corticosteroid-treatment patients.
In the RECOVERY trial, the use of dexamethasone improved survival in patients hospitalized with COVID-19 who received respiratory support. Perhaps the impaired immune reactions in patients with CLL moderate the hyperinflammatory reactions to COVID-19, thus turning corticosteroids beneficial effects to somewhat redundant in this frail population,” they wrote.
Further, the finding that only 60% of patients with CLL seroconvert after the acute phase of SARS-CoV-2 infection suggests CLL patients may be at risk for reinfection, which “justifies vaccinating all patients with CLL who have recovered from COVID-19.”
“Likewise, patients with CLL may develop persistent COVID-19 infection,” they added, explaining that “prolonged shedding of infectious SARS-CoV-2 virus and within-host genomic evolution may eventually lead to emergence of new virus variants.”
Given the high risk of severe COVID-19 disease and impaired antibody-mediated immune response to the virus and its vaccine, a booster dose may be warranted in patients with CLL who fail to achieve seropositivity after 2 vaccine doses, they said.
The available data to date “call for early application of antiviral drugs, [monoclonal antibodies], and convalescent plasma as well as improved vaccination strategy, to improve the odds for patients with CLL confronting COVID-19,” they concluded, adding that large-scale prospective studies on the clinical disease course, outcomes, efficacy of treatments, and vaccination timing and schedule in patients with CLL and COVID-19 are still warranted.
The research was supported by a National Cancer Institute Cancer Center support grant. Dr. Roeker, Dr. Herishanu, and Dr. Perry reported having no financial disclosures.
Retrospective but the data also highlight areas for further investigation, according to the researchers.
Specifically, “the data highlight opportunities for further investigation into optimal management of COVID-19, immune response after infection, and effective vaccination strategy for patients with CLL,” Lindsey E. Roeker, MD, a hematologic oncologist at Memorial Sloan Kettering Cancer Center, New York, and colleagues wrote in a Nov. 4, 2021, letter to the editor of Blood.
The researchers noted that recently reported COVID-19 case fatality rates from two large series of patients with CLL ranged from 31% to 33%, but trends over time were unclear.
“To understand change in outcomes over time, we present this follow-up study, which builds upon a previously reported cohort with extended follow up and addition of more recently diagnosed cases,” they wrote, explaining that “early data from a small series suggest that patients with CLL may not consistently generate anti–SARS-CoV-2 antibodies after infection.”
“This finding, along with previous reports of inadequate response to vaccines in patients with CLL, highlight significant questions regarding COVID-19 vaccine efficacy in this population,” they added.
Trends in outcomes
The review of outcomes in 374 CLL patients from 45 centers who were diagnosed with COVID-19 between Feb. 17, 2020, and Feb. 1, 2021, showed an overall case fatality rate (CFR) of 28%. Among the 278 patients (75%) admitted to the hospital, the CFR was 36%; among those not admitted, the CFR was 4.3%.
Independent predictors of poor survival were ages over 75 years (adjusted hazard ratio, 1.6) and Cumulative Illness Rating Scale–Geriatric (CIRS) scores greater than 6 (aHR, 1.6).
Updated data for 254 patients diagnosed from Feb. 17 to April 30, 2020, and 120 diagnosed from May 1, 2020, to Feb. 1, 2021, showed that more patients in the early versus later cohort were admitted to the hospital (85% vs. 55%) and more required ICU admission (32% vs. 11%).
The overall case fatality rates in the early and later cohorts were 35% and 11%, respectively (P < .001), and among those requiring hospitalization, the rates were 40% and 20% (P = .003).
“The proportion of hospitalized patients requiring ICU-level care was lower in the later cohort (37% vs. 29%), whereas the CFR remained high for the subset of patients who required ICU-level care (52% vs. 50%; P = .89),” the investigators wrote, noting that “[a] difference in management of BTKi[Bruton’s tyrosine kinase inhibitor]-treated patients was observed in the early versus the later cohort.”
“In the early cohort, 76% of patients receiving BTKi had their drug therapy suspended or discontinued. In the later cohort, only 20% of BTKi-treated patients had their therapy suspended or discontinued,” they added.
Univariate analyses showed significant associations between use of remdesivir and OS (HR, 0.48) and use of convalescent plasma and OS (HR, 0.50) in patients who were admitted, whereas admitted patients who received corticosteroids or hydroxychloroquine had an increased risk of death (HRs, 1.73 and 1.53, respectively).
“Corticosteroids were associated with increased risk of death when the data were adjusted for admission status (HR, 1.8) and the need for mechanical ventilation (HR, 2.0), although they were not significantly associated with survival when the data were adjusted for use of supplemental oxygen (HR, 1.4),” they wrote, also noting that admitted patients treated with corticosteroids in the later cohort did not experience an OS benefit (HR, 2.6).
The findings mirror population-based studies with decreasing CFR (35% in those diagnosed before May 1, 2020, versus 11% in those diagnosed after that date), they said, adding that “these trends suggest that patients in the later cohort experienced a less severe clinical course and that the observed difference in CFR over time may not just be due to more frequent testing and identification of less symptomatic patients.”
Of note, the outcomes observed for steroid-treated patients in the current cohort contrast with those from the RECOVERY trial as published in July 2020, which “may be an artifact of their use in patients with more severe disease,” they suggested.
They added that these data “are hypothesis generating and suggest that COVID-19 directed interventions, particularly immunomodulatory agents, require prospective study, specifically in immunocompromised populations.”
The investigators also noted that, consistent with a prior single-center study, 60% of patients with CLL developed positive anti–SARS-CoV-2 serology results after polymerase chain reaction diagnosis of COVID-19, adding further evidence of nonuniform antibody production after COVID-19 in patients with CLL.
Study is ongoing to gain understanding of the immune response to SARS-CoV-2 vaccination in patients with CLL, they said.
Changing the odds
In a related commentary also published in Blood, Yair Herishanu, MD, and Chava Perry, MD, PhD, of Tel Aviv Sourasky Medical Center called the reduction in mortality over time as reported by Dr. Roeker and colleagues “encouraging and intriguing.”
“One explanation is that the later cohort included a larger proportion of patients with mild symptoms who were diagnosed because of increased awareness of COVID-19 and more extensive screening to detect SARS-CoV-2 over time. That is supported by the lower hospitalization rates and lower rates of hospitalized patients requiring ICU care in the later cohort,” they wrote. “Another possibility is better patient management owing to increasing experience, expanding therapeutic options, and improved capacity of health systems to manage an influx of patients.”
The lower mortality in hospitalized patients over time may reflect better management of patients over time, but it also highlights the significance of “early introduction of various anti–COVID-19 therapies to prevent clinical deterioration to ICU-level care,” they added.
Also intriguing, according to Dr. Herishanu and Dr. Perry, was the finding of increased secondary infections and death rates among corticosteroid-treatment patients.
In the RECOVERY trial, the use of dexamethasone improved survival in patients hospitalized with COVID-19 who received respiratory support. Perhaps the impaired immune reactions in patients with CLL moderate the hyperinflammatory reactions to COVID-19, thus turning corticosteroids beneficial effects to somewhat redundant in this frail population,” they wrote.
Further, the finding that only 60% of patients with CLL seroconvert after the acute phase of SARS-CoV-2 infection suggests CLL patients may be at risk for reinfection, which “justifies vaccinating all patients with CLL who have recovered from COVID-19.”
“Likewise, patients with CLL may develop persistent COVID-19 infection,” they added, explaining that “prolonged shedding of infectious SARS-CoV-2 virus and within-host genomic evolution may eventually lead to emergence of new virus variants.”
Given the high risk of severe COVID-19 disease and impaired antibody-mediated immune response to the virus and its vaccine, a booster dose may be warranted in patients with CLL who fail to achieve seropositivity after 2 vaccine doses, they said.
The available data to date “call for early application of antiviral drugs, [monoclonal antibodies], and convalescent plasma as well as improved vaccination strategy, to improve the odds for patients with CLL confronting COVID-19,” they concluded, adding that large-scale prospective studies on the clinical disease course, outcomes, efficacy of treatments, and vaccination timing and schedule in patients with CLL and COVID-19 are still warranted.
The research was supported by a National Cancer Institute Cancer Center support grant. Dr. Roeker, Dr. Herishanu, and Dr. Perry reported having no financial disclosures.
FROM BLOOD
Intent to vaccinate kids against COVID higher among vaccinated parents
“Parental vaccine hesitancy is a major issue for schools resuming in-person instruction, potentially requiring regular testing, strict mask wearing, and physical distancing for safe operation,” wrote lead author Madhura S. Rane, PhD, from the City University of New York in New York City, and colleagues in their paper, published online in JAMA Pediatrics.
The survey was conducted in June 2021 of 1,162 parents with children ranging in age from 2 to 17 years. The majority of parents (74.4%) were already vaccinated/vaccine-willing ,while 25.6% were vaccine hesitant. The study cohort, including both 1,652 children and their parents, was part of the nationwide CHASING COVID.
Vaccinated parents overall were more willing to vaccinate or had already vaccinated their eligible children when compared with vaccine-hesitant parents: 64.9% vs. 8.3% for children 2-4 years of age; 77.6% vs. 12.1% for children 5-11 years of age; 81.3% vs. 13.9% for children 12-15 years of age; and 86.4% vs. 12.7% for children 16-17 years of age; P < .001.
The researchers found greater hesitancy among Black and Hispanic parents, compared with parents who were non-Hispanic White, women, younger, and did not have a college education. Parents of children who were currently attending school remotely or only partially, were found to be more willing to vaccinate their children when compared to parents of children who were attending school fully in person.
The authors also found that parents who knew someone who had died of COVID-19 or had experienced a prior COVID-19 infection, were more willing to vaccinate their children.
Hesitance in vaccinated parents
Interestingly, 10% of COVID-vaccinated parents said they were still hesitant to vaccinate their kids because of concern for long-term adverse effects of the vaccine.
“These data point out that vaccine concerns may exist even among vaccinated or vaccine-favorable parents, so we should ask any parent who has not vaccinated their child whether we can discuss their concerns and perhaps move their opinions,” said William T. Basco Jr, MD, MS, a professor of pediatrics at the Medical University of South Carolina, Charleston, and director of the division of general pediatrics.
In an interview, when asked whether recent approval of the vaccine for children aged 5-11 will likely aid in overcoming parental hesitancy, Dr. Basco replied: “Absolutely. As more children get the vaccine and people know a neighbor or nephew or cousin, etc., who received the vaccine and did fine, it will engender greater comfort and allow parents to feel better about having their own child receive the vaccine.”
Advice for clinicians from outside expert
“We can always start by asking parents if we can help them understand the vaccine and the need for it. The tidal wave of disinformation is huge, but we can, on a daily basis, offer to help families navigate this decision,” concluded Dr. Basco, who was not involved with the new paper.
Funding for this study was provided through grants from the National Institute of Allergy and Infectious Diseases, the CUNY Institute of Implementation Science in Population Health, and the COVID-19 Grant Program of the CUNY Graduate School of Public Health and Health Policy. The authors and Dr. Basco have disclosed no relevant financial relationships.
“Parental vaccine hesitancy is a major issue for schools resuming in-person instruction, potentially requiring regular testing, strict mask wearing, and physical distancing for safe operation,” wrote lead author Madhura S. Rane, PhD, from the City University of New York in New York City, and colleagues in their paper, published online in JAMA Pediatrics.
The survey was conducted in June 2021 of 1,162 parents with children ranging in age from 2 to 17 years. The majority of parents (74.4%) were already vaccinated/vaccine-willing ,while 25.6% were vaccine hesitant. The study cohort, including both 1,652 children and their parents, was part of the nationwide CHASING COVID.
Vaccinated parents overall were more willing to vaccinate or had already vaccinated their eligible children when compared with vaccine-hesitant parents: 64.9% vs. 8.3% for children 2-4 years of age; 77.6% vs. 12.1% for children 5-11 years of age; 81.3% vs. 13.9% for children 12-15 years of age; and 86.4% vs. 12.7% for children 16-17 years of age; P < .001.
The researchers found greater hesitancy among Black and Hispanic parents, compared with parents who were non-Hispanic White, women, younger, and did not have a college education. Parents of children who were currently attending school remotely or only partially, were found to be more willing to vaccinate their children when compared to parents of children who were attending school fully in person.
The authors also found that parents who knew someone who had died of COVID-19 or had experienced a prior COVID-19 infection, were more willing to vaccinate their children.
Hesitance in vaccinated parents
Interestingly, 10% of COVID-vaccinated parents said they were still hesitant to vaccinate their kids because of concern for long-term adverse effects of the vaccine.
“These data point out that vaccine concerns may exist even among vaccinated or vaccine-favorable parents, so we should ask any parent who has not vaccinated their child whether we can discuss their concerns and perhaps move their opinions,” said William T. Basco Jr, MD, MS, a professor of pediatrics at the Medical University of South Carolina, Charleston, and director of the division of general pediatrics.
In an interview, when asked whether recent approval of the vaccine for children aged 5-11 will likely aid in overcoming parental hesitancy, Dr. Basco replied: “Absolutely. As more children get the vaccine and people know a neighbor or nephew or cousin, etc., who received the vaccine and did fine, it will engender greater comfort and allow parents to feel better about having their own child receive the vaccine.”
Advice for clinicians from outside expert
“We can always start by asking parents if we can help them understand the vaccine and the need for it. The tidal wave of disinformation is huge, but we can, on a daily basis, offer to help families navigate this decision,” concluded Dr. Basco, who was not involved with the new paper.
Funding for this study was provided through grants from the National Institute of Allergy and Infectious Diseases, the CUNY Institute of Implementation Science in Population Health, and the COVID-19 Grant Program of the CUNY Graduate School of Public Health and Health Policy. The authors and Dr. Basco have disclosed no relevant financial relationships.
“Parental vaccine hesitancy is a major issue for schools resuming in-person instruction, potentially requiring regular testing, strict mask wearing, and physical distancing for safe operation,” wrote lead author Madhura S. Rane, PhD, from the City University of New York in New York City, and colleagues in their paper, published online in JAMA Pediatrics.
The survey was conducted in June 2021 of 1,162 parents with children ranging in age from 2 to 17 years. The majority of parents (74.4%) were already vaccinated/vaccine-willing ,while 25.6% were vaccine hesitant. The study cohort, including both 1,652 children and their parents, was part of the nationwide CHASING COVID.
Vaccinated parents overall were more willing to vaccinate or had already vaccinated their eligible children when compared with vaccine-hesitant parents: 64.9% vs. 8.3% for children 2-4 years of age; 77.6% vs. 12.1% for children 5-11 years of age; 81.3% vs. 13.9% for children 12-15 years of age; and 86.4% vs. 12.7% for children 16-17 years of age; P < .001.
The researchers found greater hesitancy among Black and Hispanic parents, compared with parents who were non-Hispanic White, women, younger, and did not have a college education. Parents of children who were currently attending school remotely or only partially, were found to be more willing to vaccinate their children when compared to parents of children who were attending school fully in person.
The authors also found that parents who knew someone who had died of COVID-19 or had experienced a prior COVID-19 infection, were more willing to vaccinate their children.
Hesitance in vaccinated parents
Interestingly, 10% of COVID-vaccinated parents said they were still hesitant to vaccinate their kids because of concern for long-term adverse effects of the vaccine.
“These data point out that vaccine concerns may exist even among vaccinated or vaccine-favorable parents, so we should ask any parent who has not vaccinated their child whether we can discuss their concerns and perhaps move their opinions,” said William T. Basco Jr, MD, MS, a professor of pediatrics at the Medical University of South Carolina, Charleston, and director of the division of general pediatrics.
In an interview, when asked whether recent approval of the vaccine for children aged 5-11 will likely aid in overcoming parental hesitancy, Dr. Basco replied: “Absolutely. As more children get the vaccine and people know a neighbor or nephew or cousin, etc., who received the vaccine and did fine, it will engender greater comfort and allow parents to feel better about having their own child receive the vaccine.”
Advice for clinicians from outside expert
“We can always start by asking parents if we can help them understand the vaccine and the need for it. The tidal wave of disinformation is huge, but we can, on a daily basis, offer to help families navigate this decision,” concluded Dr. Basco, who was not involved with the new paper.
Funding for this study was provided through grants from the National Institute of Allergy and Infectious Diseases, the CUNY Institute of Implementation Science in Population Health, and the COVID-19 Grant Program of the CUNY Graduate School of Public Health and Health Policy. The authors and Dr. Basco have disclosed no relevant financial relationships.
FROM JAMA PEDIATRICS
Clinical Edge Journal Scan Commentary: COVID-19 December 2021
A second study answered a question I am often asked about neurological sequalae such as Guillain Barre syndrome among patients with COVID-19 infection, compared to risk of the same from vaccines. Patone et al linked country wide data from English National Immunisation (NIMS) Database of COVID-19 vaccinations with patient level data to examine incidence of neurological adverse events such acute central nervous system (CNS) demyelinating events, encephalitis meningitis and myelitis, Guillain–Barré syndrome, Bell’s palsy, myasthenic disorders, hemorrhagic stroke and subarachnoid hemorrhage in the 28 days following either having a positive SARS-CoV-2 test, or neither ChAdOx1nCoV-19 or BNT162b2 vaccines. The study reported increased incidence risk ratios (IRR) of hospitalization or death related to all of the aforementioned neurological events in patients with SARS-CoV-2 infection, particularly in the time right after diagnosis. There was a small increase in IRR for Guillain-Barre syndrome (IRR, 2.90; 95% confidence interval (CI): 2.15–3.92 at 15–21 days after vaccination) and Bell’s palsy (IRR, 1.29; 95% CI: 1.08–1.56 at 15–21 days) with ChAdOx1nCoV-19. However, this was lower than what was seen after a positive SARS-CoV-2 test (Guillain–Barré syndrome (IRR, 5.25; 95% CI: 3.00–9.18) and Bell’s Palsy, (IRR, 1.34; 95% CI: 0.91–1.97). There was a slightly increased association seen between hemorrhagic stroke and the first dose of BNT162b2, with IRR at 1–7 days (IRR, 1.27; 95% CI: 1.02–1.59) and 15–21 days (IRR, 1.38; 95% CI: 1.12–1.71). However, this risk was dwarfed compared to risk for hemorrhagic stroke seen up to 7 days after a positive SARS-CoV-2 test (IRR, 12.42; 95% CI: 7.73–19.95 at day 0; IRR, 2.01; 95% CI: 1.29–3.15 at 1–7 days). The results highlight immense increase of neurological events after SARS-CoV-2 infection.
Lastly, the RECOVERY trial reported out results of colchicine treatment arm, where 5,610 patients were assigned to standard of care (SOC) with colchicine compared to 5,730 who just received standard of care. The ongoing RECOVERY trial has been an incredibly power tool in helping identify both some effective treatments as well as shedding light on the limited utility of others. No significant differences were seen between the treatment and SOC only arms in all-cause mortality (rate ratio [RR], 1.01; P = .77), the probability of being discharged alive within 28 days (RR, 0.98; P = .44), or the risk of progressing to invasive mechanical ventilation or death (RR, 1.02; P = .47). The large sample size as well as the well-controlled design provides good evidence that colchicine will not make the COVID-19 treatment arsenal.
A second study answered a question I am often asked about neurological sequalae such as Guillain Barre syndrome among patients with COVID-19 infection, compared to risk of the same from vaccines. Patone et al linked country wide data from English National Immunisation (NIMS) Database of COVID-19 vaccinations with patient level data to examine incidence of neurological adverse events such acute central nervous system (CNS) demyelinating events, encephalitis meningitis and myelitis, Guillain–Barré syndrome, Bell’s palsy, myasthenic disorders, hemorrhagic stroke and subarachnoid hemorrhage in the 28 days following either having a positive SARS-CoV-2 test, or neither ChAdOx1nCoV-19 or BNT162b2 vaccines. The study reported increased incidence risk ratios (IRR) of hospitalization or death related to all of the aforementioned neurological events in patients with SARS-CoV-2 infection, particularly in the time right after diagnosis. There was a small increase in IRR for Guillain-Barre syndrome (IRR, 2.90; 95% confidence interval (CI): 2.15–3.92 at 15–21 days after vaccination) and Bell’s palsy (IRR, 1.29; 95% CI: 1.08–1.56 at 15–21 days) with ChAdOx1nCoV-19. However, this was lower than what was seen after a positive SARS-CoV-2 test (Guillain–Barré syndrome (IRR, 5.25; 95% CI: 3.00–9.18) and Bell’s Palsy, (IRR, 1.34; 95% CI: 0.91–1.97). There was a slightly increased association seen between hemorrhagic stroke and the first dose of BNT162b2, with IRR at 1–7 days (IRR, 1.27; 95% CI: 1.02–1.59) and 15–21 days (IRR, 1.38; 95% CI: 1.12–1.71). However, this risk was dwarfed compared to risk for hemorrhagic stroke seen up to 7 days after a positive SARS-CoV-2 test (IRR, 12.42; 95% CI: 7.73–19.95 at day 0; IRR, 2.01; 95% CI: 1.29–3.15 at 1–7 days). The results highlight immense increase of neurological events after SARS-CoV-2 infection.
Lastly, the RECOVERY trial reported out results of colchicine treatment arm, where 5,610 patients were assigned to standard of care (SOC) with colchicine compared to 5,730 who just received standard of care. The ongoing RECOVERY trial has been an incredibly power tool in helping identify both some effective treatments as well as shedding light on the limited utility of others. No significant differences were seen between the treatment and SOC only arms in all-cause mortality (rate ratio [RR], 1.01; P = .77), the probability of being discharged alive within 28 days (RR, 0.98; P = .44), or the risk of progressing to invasive mechanical ventilation or death (RR, 1.02; P = .47). The large sample size as well as the well-controlled design provides good evidence that colchicine will not make the COVID-19 treatment arsenal.
A second study answered a question I am often asked about neurological sequalae such as Guillain Barre syndrome among patients with COVID-19 infection, compared to risk of the same from vaccines. Patone et al linked country wide data from English National Immunisation (NIMS) Database of COVID-19 vaccinations with patient level data to examine incidence of neurological adverse events such acute central nervous system (CNS) demyelinating events, encephalitis meningitis and myelitis, Guillain–Barré syndrome, Bell’s palsy, myasthenic disorders, hemorrhagic stroke and subarachnoid hemorrhage in the 28 days following either having a positive SARS-CoV-2 test, or neither ChAdOx1nCoV-19 or BNT162b2 vaccines. The study reported increased incidence risk ratios (IRR) of hospitalization or death related to all of the aforementioned neurological events in patients with SARS-CoV-2 infection, particularly in the time right after diagnosis. There was a small increase in IRR for Guillain-Barre syndrome (IRR, 2.90; 95% confidence interval (CI): 2.15–3.92 at 15–21 days after vaccination) and Bell’s palsy (IRR, 1.29; 95% CI: 1.08–1.56 at 15–21 days) with ChAdOx1nCoV-19. However, this was lower than what was seen after a positive SARS-CoV-2 test (Guillain–Barré syndrome (IRR, 5.25; 95% CI: 3.00–9.18) and Bell’s Palsy, (IRR, 1.34; 95% CI: 0.91–1.97). There was a slightly increased association seen between hemorrhagic stroke and the first dose of BNT162b2, with IRR at 1–7 days (IRR, 1.27; 95% CI: 1.02–1.59) and 15–21 days (IRR, 1.38; 95% CI: 1.12–1.71). However, this risk was dwarfed compared to risk for hemorrhagic stroke seen up to 7 days after a positive SARS-CoV-2 test (IRR, 12.42; 95% CI: 7.73–19.95 at day 0; IRR, 2.01; 95% CI: 1.29–3.15 at 1–7 days). The results highlight immense increase of neurological events after SARS-CoV-2 infection.
Lastly, the RECOVERY trial reported out results of colchicine treatment arm, where 5,610 patients were assigned to standard of care (SOC) with colchicine compared to 5,730 who just received standard of care. The ongoing RECOVERY trial has been an incredibly power tool in helping identify both some effective treatments as well as shedding light on the limited utility of others. No significant differences were seen between the treatment and SOC only arms in all-cause mortality (rate ratio [RR], 1.01; P = .77), the probability of being discharged alive within 28 days (RR, 0.98; P = .44), or the risk of progressing to invasive mechanical ventilation or death (RR, 1.02; P = .47). The large sample size as well as the well-controlled design provides good evidence that colchicine will not make the COVID-19 treatment arsenal.
It feels like COVID is closing in
Like so many of you, I have weathered COVID-19 for the last almost 2 years. We’ve dealt with anxiety in our patients and ourselves, ever conflicting directives over masks, and uncertainty and hope over vaccinations.
In the beginning, it seemed elsewhere. Wuhan, China, the state of Washington, New York City.
In the beginning, I awoke with rising anxiety every morning at 4 a.m.
Now, it is part of life. We know how to do this.
I work in a D.C. hospital that takes care of COVID-19 patients. I don’t intubate or come into direct contact with patients’ secretions.
I felt lucky.
Last summer, I felt relief, after being fully vaccinated. We thought we were almost over it. But the numbers abroad and in the United States keep rising.
We have developed protocols. We test every patient for COVID-19 before admitting them to psychiatry, which is now routine. COVID-19–positive patients with suicidal ideation go to our medicine-psychiatric unit. We are single-room occupancy. No visitors.
Now, it feels like COVID is closing in. Lots of my patients on consultation-liaison psychiatry had COVID-19 or do now. The number of patients with long COVID is increasing. My elderly mother-in-law picked it up from a hospital. My young, healthy adult son got it but is now doing relatively OK. We will see if his ADHD worsens.
I received contact tracing recently for going into a patient room with contact precautions. I had put on the gown and gloves, but did I wear my goggles? I keep them on my forehead but could not remember if I had slipped them over my eyes.
I get tested weekly. My nose runs inside my mask. I sneeze. Is this COVID?
Of course, I am vaccinated with a booster shot. But breakthrough infections occur.
I am lucky, I keep reminding myself. I have a job and income and good PPE.
So, we are learning how to manage this disease. But it still closes in. My brain screams: “I do not want to catch this disease. I do not want to get sick. I do not want to get long COVID.”
“Calm down, Cam,” I tell myself. “You can do this!” I have learned how to do all the PPE, including tying the plastic ties along the backs of the plastic gowns.
All psychiatry meetings are virtual now. I cannot do virtual with enthusiasm. I say I will, but then do not log on. I miss the camaraderie.
All appointments are mainly telehealth. That has its pros and cons.
So bottom line – I will keep keeping on.
But I really want others to get vaccinated and wear masks. More than that, how can we as a psychiatric community get us through this pandemic?
Here are a few suggestions, some of which I have made before:
- Focus on what we can control, especially exercise and sleep. Walk during times when the sun is shining. Rake the gorgeous autumn yellow and orange leaves.
- Give small (or large) gifts of kindness to others. Give to food banks, provide large tips to those who bring you takeout, help out at an animal shelter.
- Talk through established media about self-care and therapy for anxiety and depression.
- Clean out your closets. Give clothes to Afghan refugees.
- Read good books about trying times – such as World War II and the long wars in Afghanistan and Iraq.
- Take care of veterans and the elderly and homeless.
- Take care of yourself and your family.
Dr. Ritchie is chair of psychiatry at Medstar Washington Hospital Center. She has no conflicts of interest.
Like so many of you, I have weathered COVID-19 for the last almost 2 years. We’ve dealt with anxiety in our patients and ourselves, ever conflicting directives over masks, and uncertainty and hope over vaccinations.
In the beginning, it seemed elsewhere. Wuhan, China, the state of Washington, New York City.
In the beginning, I awoke with rising anxiety every morning at 4 a.m.
Now, it is part of life. We know how to do this.
I work in a D.C. hospital that takes care of COVID-19 patients. I don’t intubate or come into direct contact with patients’ secretions.
I felt lucky.
Last summer, I felt relief, after being fully vaccinated. We thought we were almost over it. But the numbers abroad and in the United States keep rising.
We have developed protocols. We test every patient for COVID-19 before admitting them to psychiatry, which is now routine. COVID-19–positive patients with suicidal ideation go to our medicine-psychiatric unit. We are single-room occupancy. No visitors.
Now, it feels like COVID is closing in. Lots of my patients on consultation-liaison psychiatry had COVID-19 or do now. The number of patients with long COVID is increasing. My elderly mother-in-law picked it up from a hospital. My young, healthy adult son got it but is now doing relatively OK. We will see if his ADHD worsens.
I received contact tracing recently for going into a patient room with contact precautions. I had put on the gown and gloves, but did I wear my goggles? I keep them on my forehead but could not remember if I had slipped them over my eyes.
I get tested weekly. My nose runs inside my mask. I sneeze. Is this COVID?
Of course, I am vaccinated with a booster shot. But breakthrough infections occur.
I am lucky, I keep reminding myself. I have a job and income and good PPE.
So, we are learning how to manage this disease. But it still closes in. My brain screams: “I do not want to catch this disease. I do not want to get sick. I do not want to get long COVID.”
“Calm down, Cam,” I tell myself. “You can do this!” I have learned how to do all the PPE, including tying the plastic ties along the backs of the plastic gowns.
All psychiatry meetings are virtual now. I cannot do virtual with enthusiasm. I say I will, but then do not log on. I miss the camaraderie.
All appointments are mainly telehealth. That has its pros and cons.
So bottom line – I will keep keeping on.
But I really want others to get vaccinated and wear masks. More than that, how can we as a psychiatric community get us through this pandemic?
Here are a few suggestions, some of which I have made before:
- Focus on what we can control, especially exercise and sleep. Walk during times when the sun is shining. Rake the gorgeous autumn yellow and orange leaves.
- Give small (or large) gifts of kindness to others. Give to food banks, provide large tips to those who bring you takeout, help out at an animal shelter.
- Talk through established media about self-care and therapy for anxiety and depression.
- Clean out your closets. Give clothes to Afghan refugees.
- Read good books about trying times – such as World War II and the long wars in Afghanistan and Iraq.
- Take care of veterans and the elderly and homeless.
- Take care of yourself and your family.
Dr. Ritchie is chair of psychiatry at Medstar Washington Hospital Center. She has no conflicts of interest.
Like so many of you, I have weathered COVID-19 for the last almost 2 years. We’ve dealt with anxiety in our patients and ourselves, ever conflicting directives over masks, and uncertainty and hope over vaccinations.
In the beginning, it seemed elsewhere. Wuhan, China, the state of Washington, New York City.
In the beginning, I awoke with rising anxiety every morning at 4 a.m.
Now, it is part of life. We know how to do this.
I work in a D.C. hospital that takes care of COVID-19 patients. I don’t intubate or come into direct contact with patients’ secretions.
I felt lucky.
Last summer, I felt relief, after being fully vaccinated. We thought we were almost over it. But the numbers abroad and in the United States keep rising.
We have developed protocols. We test every patient for COVID-19 before admitting them to psychiatry, which is now routine. COVID-19–positive patients with suicidal ideation go to our medicine-psychiatric unit. We are single-room occupancy. No visitors.
Now, it feels like COVID is closing in. Lots of my patients on consultation-liaison psychiatry had COVID-19 or do now. The number of patients with long COVID is increasing. My elderly mother-in-law picked it up from a hospital. My young, healthy adult son got it but is now doing relatively OK. We will see if his ADHD worsens.
I received contact tracing recently for going into a patient room with contact precautions. I had put on the gown and gloves, but did I wear my goggles? I keep them on my forehead but could not remember if I had slipped them over my eyes.
I get tested weekly. My nose runs inside my mask. I sneeze. Is this COVID?
Of course, I am vaccinated with a booster shot. But breakthrough infections occur.
I am lucky, I keep reminding myself. I have a job and income and good PPE.
So, we are learning how to manage this disease. But it still closes in. My brain screams: “I do not want to catch this disease. I do not want to get sick. I do not want to get long COVID.”
“Calm down, Cam,” I tell myself. “You can do this!” I have learned how to do all the PPE, including tying the plastic ties along the backs of the plastic gowns.
All psychiatry meetings are virtual now. I cannot do virtual with enthusiasm. I say I will, but then do not log on. I miss the camaraderie.
All appointments are mainly telehealth. That has its pros and cons.
So bottom line – I will keep keeping on.
But I really want others to get vaccinated and wear masks. More than that, how can we as a psychiatric community get us through this pandemic?
Here are a few suggestions, some of which I have made before:
- Focus on what we can control, especially exercise and sleep. Walk during times when the sun is shining. Rake the gorgeous autumn yellow and orange leaves.
- Give small (or large) gifts of kindness to others. Give to food banks, provide large tips to those who bring you takeout, help out at an animal shelter.
- Talk through established media about self-care and therapy for anxiety and depression.
- Clean out your closets. Give clothes to Afghan refugees.
- Read good books about trying times – such as World War II and the long wars in Afghanistan and Iraq.
- Take care of veterans and the elderly and homeless.
- Take care of yourself and your family.
Dr. Ritchie is chair of psychiatry at Medstar Washington Hospital Center. She has no conflicts of interest.
CDC unveils mental health protection plan for health care workers
Federal health officials have outlined a five-part plan to improve and protect the mental health and well-being of America’s health care workers (HCWs) and create sustainable change for the next generation of HCWs.
“It’s long past time for us to care for the people who care for all of us and address burnout in our health care workers,” U.S. Surgeon General Vivek H. Murthy, MD, MBA, said during a webinar hosted by the National Institute for Occupational Safety and Health, part of the U.S. Centers for Disease Control and Prevention.
“My hope is that, going forward, we will be able to embark on this journey together to create a health care system, a health care environment, a country where we can not only provide extraordinary care to all those who need it, but where we can take good care of those who have sacrificed so much and make sure that they are well,” Dr. Murthy said.
Burnout is not selective
There are 20 million HCWs in the United States, and no one is immune from burnout, said NIOSH Director John Howard, MD.
He noted that from June through Sept. of 2020 – the height of the COVID-19 pandemic – 93% of HCWs experienced some degree of stress, with 22% reporting moderate depression and post-traumatic stress disorder.
Looking at subsets of HCWs, a recent survey showed that one in five nurses contemplated leaving the profession because of insufficient staffing, intensity of workload, emotional and physical toll of the job, and lack of support, Dr. Howard noted.
Physician burnout was a significant issue even before the pandemic, with about 79% of physicians reporting burnout. , Dr. Howard said.
Women in health care jobs are especially vulnerable to burnout; 76% of health care jobs are held by women and 64% of physicians that feel burned-out are women, according to federal data.
“We have significant work to do in shoring up the safety and health of women in health care,” Dr. Howard said.
Mental health is also suffering among local and state public health workers. In a recent CDC survey of 26,000 of these workers, 53% reported symptoms of at least one mental health condition in the past 2 weeks.
“That is really an alarming proportion of public health workers who are as vital and essential as nurses and doctors are in our health care system,” Dr. Howard said.
Primary prevention approach
To tackle the burnout crisis, NIOSH plans to:
- Take a deep dive into understanding the personal, social, and economic burdens HCWs face on a daily basis.
- Assimilate the evidence and create a repository of best practices, resources, and interventions.
- Partner with key stakeholders, including the American Hospital Association, the American Nurses Association, National Nurses United, the Joint Commission.
- Identify and adapt tools for the health care workplace that emphasize stress reduction.
NIOSH also plans to “generate awareness through a national, multidimensional social marketing campaign to get the word out about stress so health care workers don’t feel so alone,” Dr. Howard said.
This five-part plan takes a primary prevention approach to identifying and eliminating risk factors for burnout and stress, he added.
Secondary prevention, “when damage has already been done and you’re trying to save a health care worker who is suffering from a mental health issue, that’s a lot harder than taking a good look at what you can do to organizational practices that lead to health care workers’ stress and burnout,” Dr. Howard said.
A version of this article first appeared on Medscape.com.
Federal health officials have outlined a five-part plan to improve and protect the mental health and well-being of America’s health care workers (HCWs) and create sustainable change for the next generation of HCWs.
“It’s long past time for us to care for the people who care for all of us and address burnout in our health care workers,” U.S. Surgeon General Vivek H. Murthy, MD, MBA, said during a webinar hosted by the National Institute for Occupational Safety and Health, part of the U.S. Centers for Disease Control and Prevention.
“My hope is that, going forward, we will be able to embark on this journey together to create a health care system, a health care environment, a country where we can not only provide extraordinary care to all those who need it, but where we can take good care of those who have sacrificed so much and make sure that they are well,” Dr. Murthy said.
Burnout is not selective
There are 20 million HCWs in the United States, and no one is immune from burnout, said NIOSH Director John Howard, MD.
He noted that from June through Sept. of 2020 – the height of the COVID-19 pandemic – 93% of HCWs experienced some degree of stress, with 22% reporting moderate depression and post-traumatic stress disorder.
Looking at subsets of HCWs, a recent survey showed that one in five nurses contemplated leaving the profession because of insufficient staffing, intensity of workload, emotional and physical toll of the job, and lack of support, Dr. Howard noted.
Physician burnout was a significant issue even before the pandemic, with about 79% of physicians reporting burnout. , Dr. Howard said.
Women in health care jobs are especially vulnerable to burnout; 76% of health care jobs are held by women and 64% of physicians that feel burned-out are women, according to federal data.
“We have significant work to do in shoring up the safety and health of women in health care,” Dr. Howard said.
Mental health is also suffering among local and state public health workers. In a recent CDC survey of 26,000 of these workers, 53% reported symptoms of at least one mental health condition in the past 2 weeks.
“That is really an alarming proportion of public health workers who are as vital and essential as nurses and doctors are in our health care system,” Dr. Howard said.
Primary prevention approach
To tackle the burnout crisis, NIOSH plans to:
- Take a deep dive into understanding the personal, social, and economic burdens HCWs face on a daily basis.
- Assimilate the evidence and create a repository of best practices, resources, and interventions.
- Partner with key stakeholders, including the American Hospital Association, the American Nurses Association, National Nurses United, the Joint Commission.
- Identify and adapt tools for the health care workplace that emphasize stress reduction.
NIOSH also plans to “generate awareness through a national, multidimensional social marketing campaign to get the word out about stress so health care workers don’t feel so alone,” Dr. Howard said.
This five-part plan takes a primary prevention approach to identifying and eliminating risk factors for burnout and stress, he added.
Secondary prevention, “when damage has already been done and you’re trying to save a health care worker who is suffering from a mental health issue, that’s a lot harder than taking a good look at what you can do to organizational practices that lead to health care workers’ stress and burnout,” Dr. Howard said.
A version of this article first appeared on Medscape.com.
Federal health officials have outlined a five-part plan to improve and protect the mental health and well-being of America’s health care workers (HCWs) and create sustainable change for the next generation of HCWs.
“It’s long past time for us to care for the people who care for all of us and address burnout in our health care workers,” U.S. Surgeon General Vivek H. Murthy, MD, MBA, said during a webinar hosted by the National Institute for Occupational Safety and Health, part of the U.S. Centers for Disease Control and Prevention.
“My hope is that, going forward, we will be able to embark on this journey together to create a health care system, a health care environment, a country where we can not only provide extraordinary care to all those who need it, but where we can take good care of those who have sacrificed so much and make sure that they are well,” Dr. Murthy said.
Burnout is not selective
There are 20 million HCWs in the United States, and no one is immune from burnout, said NIOSH Director John Howard, MD.
He noted that from June through Sept. of 2020 – the height of the COVID-19 pandemic – 93% of HCWs experienced some degree of stress, with 22% reporting moderate depression and post-traumatic stress disorder.
Looking at subsets of HCWs, a recent survey showed that one in five nurses contemplated leaving the profession because of insufficient staffing, intensity of workload, emotional and physical toll of the job, and lack of support, Dr. Howard noted.
Physician burnout was a significant issue even before the pandemic, with about 79% of physicians reporting burnout. , Dr. Howard said.
Women in health care jobs are especially vulnerable to burnout; 76% of health care jobs are held by women and 64% of physicians that feel burned-out are women, according to federal data.
“We have significant work to do in shoring up the safety and health of women in health care,” Dr. Howard said.
Mental health is also suffering among local and state public health workers. In a recent CDC survey of 26,000 of these workers, 53% reported symptoms of at least one mental health condition in the past 2 weeks.
“That is really an alarming proportion of public health workers who are as vital and essential as nurses and doctors are in our health care system,” Dr. Howard said.
Primary prevention approach
To tackle the burnout crisis, NIOSH plans to:
- Take a deep dive into understanding the personal, social, and economic burdens HCWs face on a daily basis.
- Assimilate the evidence and create a repository of best practices, resources, and interventions.
- Partner with key stakeholders, including the American Hospital Association, the American Nurses Association, National Nurses United, the Joint Commission.
- Identify and adapt tools for the health care workplace that emphasize stress reduction.
NIOSH also plans to “generate awareness through a national, multidimensional social marketing campaign to get the word out about stress so health care workers don’t feel so alone,” Dr. Howard said.
This five-part plan takes a primary prevention approach to identifying and eliminating risk factors for burnout and stress, he added.
Secondary prevention, “when damage has already been done and you’re trying to save a health care worker who is suffering from a mental health issue, that’s a lot harder than taking a good look at what you can do to organizational practices that lead to health care workers’ stress and burnout,” Dr. Howard said.
A version of this article first appeared on Medscape.com.
More tools for the COVID toolbox
I was recently asked to see a 16-year-old, unvaccinated (against COVID-19) adolescent with hypothyroidism and obesity (body mass index 37 kg/m2) seen in the pediatric emergency department with tachycardia, O2 saturation 96%, urinary tract infection, poor appetite, and nausea. Her chest x-ray had low lung volumes but no infiltrates. She was noted to be dehydrated. Testing for COVID-19 was PCR positive.1
She was observed overnight, tolerated oral rehydration, and was being readied for discharge. Pediatric Infectious Diseases was called about prescribing remdesivir.
Remdesivir was not indicated as its current use is limited to inpatients with oxygen desaturations less than 94%. Infectious Diseases Society of America guidelines do recommend the use of monoclonal antibodies against the SARS-CoV-2 spike protein for prevention of COVID disease progression in high-risk individuals. Specifically, the IDSA guidelines say, “Among ambulatory patients with mild to moderate COVID-19 at high risk for progression to severe disease, bamlanivimab/etesevimab, casirivimab/imdevimab, or sotrovimab rather than no neutralizing antibody treatment.”
The Food and Drug Administration’s Emergency Use Authorization (EUA) allowed use of specific monoclonal antibodies (casirivimab/imdevimab in combination, bamlanivimab/etesevimab in combination, and sotrovimab alone) for individuals 12 years and above with a minimum weight of 40 kg with high-risk conditions, describing the evidence as moderate certainty.2
Several questions have arisen regarding their use. Which children qualify under the EUA? Are the available monoclonal antibodies effective for SARS-CoV-2 variants? What adverse events were observed? Are there implementation hurdles?
Unlike the EUA for prophylactic use, which targeted unvaccinated individuals and those unlikely to have a good antibody response to vaccine, use of monoclonal antibody for prevention of progression does not have such restrictions. Effectiveness may vary by local variant susceptibility and should be considered in the choice of the most appropriate monoclonal antibody therapy. Reductions in hospitalization and progression to critical disease status were reported from phase 3 studies; reductions were also observed in mortality in some, but not all, studies. Enhanced viral clearance on day 7 was observed with few subjects having persistent high viral load.
Which children qualify under the EUA? Adolescents 12 years and older and over 40 kg are eligible if a high risk condition is present. High-risk conditions include body mass index at the 85th percentile or higher, immunosuppressive disease, or receipt of immunosuppressive therapies, or baseline (pre-COVID infection) medical-related technological dependence such as tracheostomy or positive pressure ventilation. Additional high-risk conditions are neurodevelopmental disorders, sickle cell disease, congenital or acquired heart disease, asthma, or reactive airway or other chronic respiratory disease that requires daily medication for control, diabetes, chronic kidney disease, or pregnancy.3
Are the available monoclonal antibodies effective for SARS-CoV-2 variants? Of course, this is a critical question and relies on knowledge of the dominant variant in a specific geographic location. The CDC data on which variants are susceptible to which monoclonal therapies were updated as of Oct. 21 online (see Table 1). Local departments of public health often will have current data on the dominant variant in the community. Currently, the dominant variant in the United States is Delta and it is anticipated to be susceptible to the three monoclonal treatments authorized under the EUA based on in vitro neutralizing assays.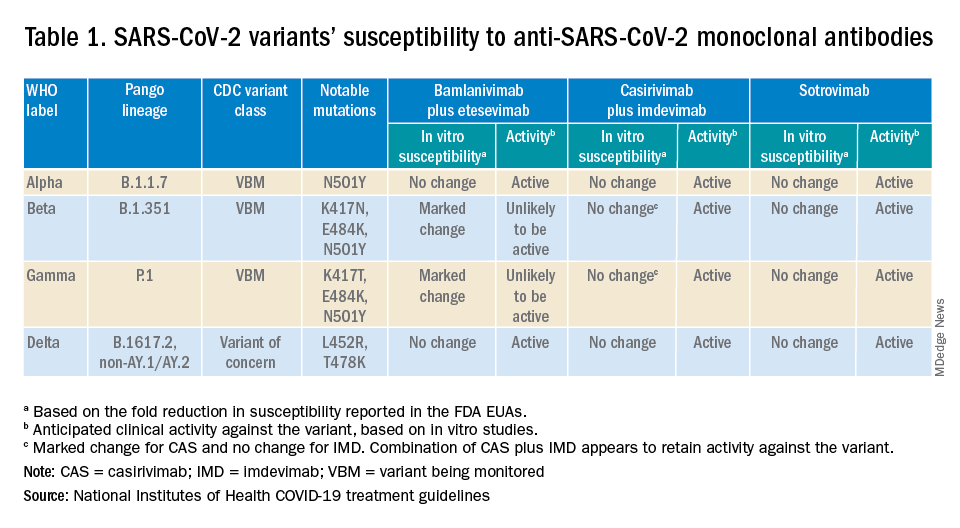
What adverse events were observed? Monoclonal antibody infusions are in general safe but anaphylaxis has been reported. Other infusion-related adverse events include urticaria, pruritis, flushing, pyrexia, shortness of breath, chest tightness, nausea, vomiting, and rash. Nearly all events were grade 1, mild, or grade 2, moderate. For nonsevere infusion-related reactions, consider slowing the infusion; if necessary, the infusion should be stopped.
Implementation challenges
The first challenge is finding a location to infuse the monoclonal antibodies. Although they can be given subcutaneously, the dose is large and little, if any, time is saved as the recommendation is for observation post administration for 1 hour. The challenge we and other centers may face is that the patients are COVID PCR+ and therefore our usual infusion program, which often is occupied by individuals already compromised and at high risk for severe COVID, is an undesirable location. We are planning to use the emergency department to accommodate such patients currently, but even that solution creates challenges for a busy, urban medical center.
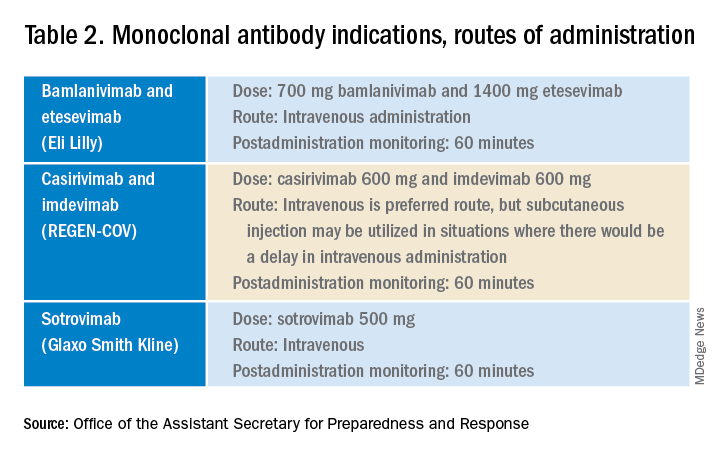
Summary
Anti–SARS-CoV-2 monoclonal antibodies are an important part of the therapeutic approach to minimizing disease severity. Clinicians should review high-risk conditions in adolescents who are PCR+ for SARS-CoV-2 and have mild to moderate symptoms. Medical care systems should implement programs to make monoclonal infusions available for such high-risk adolescents.4 Obesity and asthma reactive airways or requiring daily medication for control are the two most common conditions that place adolescents with COVID-19 at risk for progression to hospitalization and severe disease in addition to the more traditional immune-compromising conditions and medical fragility.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University schools of medicine and public health and senior attending physician in pediatric infectious diseases, Boston Medical Center. Email him at [email protected].
References
1. Federal Response to COVID-19: Monoclonal Antibody Clinical Implementation Guide. U.S. Department of Health and Human Services. 2021 Sep 2.
2. Bhimraj A et al. IDSA Guidelines on the Treatment and Management of Patients with COVID-19. Last updated 2021 Nov 9.
3. Anti-SARS-CoV-2 Monoclonal Antibodies. National Institutes of Health’s COVID 19 Treatment Guidelines. Last updated 2021 Oct 19.
4. Spreading the Word on the Benefits of Monoclonal Antibodies for COVID-19, by Hannah R. Buchdahl. CDC Foundation, 2021 Jul 2.
I was recently asked to see a 16-year-old, unvaccinated (against COVID-19) adolescent with hypothyroidism and obesity (body mass index 37 kg/m2) seen in the pediatric emergency department with tachycardia, O2 saturation 96%, urinary tract infection, poor appetite, and nausea. Her chest x-ray had low lung volumes but no infiltrates. She was noted to be dehydrated. Testing for COVID-19 was PCR positive.1
She was observed overnight, tolerated oral rehydration, and was being readied for discharge. Pediatric Infectious Diseases was called about prescribing remdesivir.
Remdesivir was not indicated as its current use is limited to inpatients with oxygen desaturations less than 94%. Infectious Diseases Society of America guidelines do recommend the use of monoclonal antibodies against the SARS-CoV-2 spike protein for prevention of COVID disease progression in high-risk individuals. Specifically, the IDSA guidelines say, “Among ambulatory patients with mild to moderate COVID-19 at high risk for progression to severe disease, bamlanivimab/etesevimab, casirivimab/imdevimab, or sotrovimab rather than no neutralizing antibody treatment.”
The Food and Drug Administration’s Emergency Use Authorization (EUA) allowed use of specific monoclonal antibodies (casirivimab/imdevimab in combination, bamlanivimab/etesevimab in combination, and sotrovimab alone) for individuals 12 years and above with a minimum weight of 40 kg with high-risk conditions, describing the evidence as moderate certainty.2
Several questions have arisen regarding their use. Which children qualify under the EUA? Are the available monoclonal antibodies effective for SARS-CoV-2 variants? What adverse events were observed? Are there implementation hurdles?
Unlike the EUA for prophylactic use, which targeted unvaccinated individuals and those unlikely to have a good antibody response to vaccine, use of monoclonal antibody for prevention of progression does not have such restrictions. Effectiveness may vary by local variant susceptibility and should be considered in the choice of the most appropriate monoclonal antibody therapy. Reductions in hospitalization and progression to critical disease status were reported from phase 3 studies; reductions were also observed in mortality in some, but not all, studies. Enhanced viral clearance on day 7 was observed with few subjects having persistent high viral load.
Which children qualify under the EUA? Adolescents 12 years and older and over 40 kg are eligible if a high risk condition is present. High-risk conditions include body mass index at the 85th percentile or higher, immunosuppressive disease, or receipt of immunosuppressive therapies, or baseline (pre-COVID infection) medical-related technological dependence such as tracheostomy or positive pressure ventilation. Additional high-risk conditions are neurodevelopmental disorders, sickle cell disease, congenital or acquired heart disease, asthma, or reactive airway or other chronic respiratory disease that requires daily medication for control, diabetes, chronic kidney disease, or pregnancy.3
Are the available monoclonal antibodies effective for SARS-CoV-2 variants? Of course, this is a critical question and relies on knowledge of the dominant variant in a specific geographic location. The CDC data on which variants are susceptible to which monoclonal therapies were updated as of Oct. 21 online (see Table 1). Local departments of public health often will have current data on the dominant variant in the community. Currently, the dominant variant in the United States is Delta and it is anticipated to be susceptible to the three monoclonal treatments authorized under the EUA based on in vitro neutralizing assays.
What adverse events were observed? Monoclonal antibody infusions are in general safe but anaphylaxis has been reported. Other infusion-related adverse events include urticaria, pruritis, flushing, pyrexia, shortness of breath, chest tightness, nausea, vomiting, and rash. Nearly all events were grade 1, mild, or grade 2, moderate. For nonsevere infusion-related reactions, consider slowing the infusion; if necessary, the infusion should be stopped.
Implementation challenges
The first challenge is finding a location to infuse the monoclonal antibodies. Although they can be given subcutaneously, the dose is large and little, if any, time is saved as the recommendation is for observation post administration for 1 hour. The challenge we and other centers may face is that the patients are COVID PCR+ and therefore our usual infusion program, which often is occupied by individuals already compromised and at high risk for severe COVID, is an undesirable location. We are planning to use the emergency department to accommodate such patients currently, but even that solution creates challenges for a busy, urban medical center.

Summary
Anti–SARS-CoV-2 monoclonal antibodies are an important part of the therapeutic approach to minimizing disease severity. Clinicians should review high-risk conditions in adolescents who are PCR+ for SARS-CoV-2 and have mild to moderate symptoms. Medical care systems should implement programs to make monoclonal infusions available for such high-risk adolescents.4 Obesity and asthma reactive airways or requiring daily medication for control are the two most common conditions that place adolescents with COVID-19 at risk for progression to hospitalization and severe disease in addition to the more traditional immune-compromising conditions and medical fragility.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University schools of medicine and public health and senior attending physician in pediatric infectious diseases, Boston Medical Center. Email him at [email protected].
References
1. Federal Response to COVID-19: Monoclonal Antibody Clinical Implementation Guide. U.S. Department of Health and Human Services. 2021 Sep 2.
2. Bhimraj A et al. IDSA Guidelines on the Treatment and Management of Patients with COVID-19. Last updated 2021 Nov 9.
3. Anti-SARS-CoV-2 Monoclonal Antibodies. National Institutes of Health’s COVID 19 Treatment Guidelines. Last updated 2021 Oct 19.
4. Spreading the Word on the Benefits of Monoclonal Antibodies for COVID-19, by Hannah R. Buchdahl. CDC Foundation, 2021 Jul 2.
I was recently asked to see a 16-year-old, unvaccinated (against COVID-19) adolescent with hypothyroidism and obesity (body mass index 37 kg/m2) seen in the pediatric emergency department with tachycardia, O2 saturation 96%, urinary tract infection, poor appetite, and nausea. Her chest x-ray had low lung volumes but no infiltrates. She was noted to be dehydrated. Testing for COVID-19 was PCR positive.1
She was observed overnight, tolerated oral rehydration, and was being readied for discharge. Pediatric Infectious Diseases was called about prescribing remdesivir.
Remdesivir was not indicated as its current use is limited to inpatients with oxygen desaturations less than 94%. Infectious Diseases Society of America guidelines do recommend the use of monoclonal antibodies against the SARS-CoV-2 spike protein for prevention of COVID disease progression in high-risk individuals. Specifically, the IDSA guidelines say, “Among ambulatory patients with mild to moderate COVID-19 at high risk for progression to severe disease, bamlanivimab/etesevimab, casirivimab/imdevimab, or sotrovimab rather than no neutralizing antibody treatment.”
The Food and Drug Administration’s Emergency Use Authorization (EUA) allowed use of specific monoclonal antibodies (casirivimab/imdevimab in combination, bamlanivimab/etesevimab in combination, and sotrovimab alone) for individuals 12 years and above with a minimum weight of 40 kg with high-risk conditions, describing the evidence as moderate certainty.2
Several questions have arisen regarding their use. Which children qualify under the EUA? Are the available monoclonal antibodies effective for SARS-CoV-2 variants? What adverse events were observed? Are there implementation hurdles?
Unlike the EUA for prophylactic use, which targeted unvaccinated individuals and those unlikely to have a good antibody response to vaccine, use of monoclonal antibody for prevention of progression does not have such restrictions. Effectiveness may vary by local variant susceptibility and should be considered in the choice of the most appropriate monoclonal antibody therapy. Reductions in hospitalization and progression to critical disease status were reported from phase 3 studies; reductions were also observed in mortality in some, but not all, studies. Enhanced viral clearance on day 7 was observed with few subjects having persistent high viral load.
Which children qualify under the EUA? Adolescents 12 years and older and over 40 kg are eligible if a high risk condition is present. High-risk conditions include body mass index at the 85th percentile or higher, immunosuppressive disease, or receipt of immunosuppressive therapies, or baseline (pre-COVID infection) medical-related technological dependence such as tracheostomy or positive pressure ventilation. Additional high-risk conditions are neurodevelopmental disorders, sickle cell disease, congenital or acquired heart disease, asthma, or reactive airway or other chronic respiratory disease that requires daily medication for control, diabetes, chronic kidney disease, or pregnancy.3
Are the available monoclonal antibodies effective for SARS-CoV-2 variants? Of course, this is a critical question and relies on knowledge of the dominant variant in a specific geographic location. The CDC data on which variants are susceptible to which monoclonal therapies were updated as of Oct. 21 online (see Table 1). Local departments of public health often will have current data on the dominant variant in the community. Currently, the dominant variant in the United States is Delta and it is anticipated to be susceptible to the three monoclonal treatments authorized under the EUA based on in vitro neutralizing assays.
What adverse events were observed? Monoclonal antibody infusions are in general safe but anaphylaxis has been reported. Other infusion-related adverse events include urticaria, pruritis, flushing, pyrexia, shortness of breath, chest tightness, nausea, vomiting, and rash. Nearly all events were grade 1, mild, or grade 2, moderate. For nonsevere infusion-related reactions, consider slowing the infusion; if necessary, the infusion should be stopped.
Implementation challenges
The first challenge is finding a location to infuse the monoclonal antibodies. Although they can be given subcutaneously, the dose is large and little, if any, time is saved as the recommendation is for observation post administration for 1 hour. The challenge we and other centers may face is that the patients are COVID PCR+ and therefore our usual infusion program, which often is occupied by individuals already compromised and at high risk for severe COVID, is an undesirable location. We are planning to use the emergency department to accommodate such patients currently, but even that solution creates challenges for a busy, urban medical center.

Summary
Anti–SARS-CoV-2 monoclonal antibodies are an important part of the therapeutic approach to minimizing disease severity. Clinicians should review high-risk conditions in adolescents who are PCR+ for SARS-CoV-2 and have mild to moderate symptoms. Medical care systems should implement programs to make monoclonal infusions available for such high-risk adolescents.4 Obesity and asthma reactive airways or requiring daily medication for control are the two most common conditions that place adolescents with COVID-19 at risk for progression to hospitalization and severe disease in addition to the more traditional immune-compromising conditions and medical fragility.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University schools of medicine and public health and senior attending physician in pediatric infectious diseases, Boston Medical Center. Email him at [email protected].
References
1. Federal Response to COVID-19: Monoclonal Antibody Clinical Implementation Guide. U.S. Department of Health and Human Services. 2021 Sep 2.
2. Bhimraj A et al. IDSA Guidelines on the Treatment and Management of Patients with COVID-19. Last updated 2021 Nov 9.
3. Anti-SARS-CoV-2 Monoclonal Antibodies. National Institutes of Health’s COVID 19 Treatment Guidelines. Last updated 2021 Oct 19.
4. Spreading the Word on the Benefits of Monoclonal Antibodies for COVID-19, by Hannah R. Buchdahl. CDC Foundation, 2021 Jul 2.









