User login
Topical PDE4 Inhibitor Now Approved for Atopic Dermatitis in Children, Adults
On July 9, the aged 6 years or older.
Roflumilast cream 0.15%, which has been developed by Arcutis Biotherapeutics and is marketed under the brand name Zoryve, is a steroid-free topical phosphodiesterase-4 inhibitor that was previously approved in a higher concentration to treat seborrheic dermatitis and plaque psoriasis.
According to a press release from Arcutis, approval for AD was supported by positive results from three phase 3 studies, a phase 2 dose-ranging study, and two phase 1 pharmacokinetic trials. In two identical phase 3 studies known as INTEGUMENT-1 and INTEGUMENT-2, about 40% of children and adults treated with roflumilast cream 0.15% achieved a Validated Investigator Global Assessment for Atopic Dermatitis score of clear (0) or almost clear (1) at week 4 (INTEGUMENT-1: 41.5% vs 25.2%; P < .0001; INTEGUMENT-2: 39% vs 16.9%; P < .0001), with significant improvement as early as week 1 (P < .0001).
Among children and adults who participated in the INTEGUMENT studies for 28 and 56 weeks, 61.3% and 65.7% achieved a 75% reduction in their Eczema Area and Severity Index scores, respectively. According to the company, there were no adverse reactions in the combined phase 3 pivotal trials that occurred in more than 2.9% of participants in either arm. The most common adverse reactions included headache (2.9%), nausea (1.9%), application-site pain (1.5%), diarrhea (1.5%), and vomiting (1.5%).
The product is expected to be available commercially at the end of July 2024, according to Arcutis. Roflumilast cream 0.3% is indicated for topical treatment of plaque psoriasis, including intertriginous areas, in adult and pediatric patients aged 6 years or older; roflumilast foam 0.3% is indicated for the treatment of seborrheic dermatitis in adult and pediatric patients aged 9 years or older.
A version of this article first appeared on Medscape.com.
On July 9, the aged 6 years or older.
Roflumilast cream 0.15%, which has been developed by Arcutis Biotherapeutics and is marketed under the brand name Zoryve, is a steroid-free topical phosphodiesterase-4 inhibitor that was previously approved in a higher concentration to treat seborrheic dermatitis and plaque psoriasis.
According to a press release from Arcutis, approval for AD was supported by positive results from three phase 3 studies, a phase 2 dose-ranging study, and two phase 1 pharmacokinetic trials. In two identical phase 3 studies known as INTEGUMENT-1 and INTEGUMENT-2, about 40% of children and adults treated with roflumilast cream 0.15% achieved a Validated Investigator Global Assessment for Atopic Dermatitis score of clear (0) or almost clear (1) at week 4 (INTEGUMENT-1: 41.5% vs 25.2%; P < .0001; INTEGUMENT-2: 39% vs 16.9%; P < .0001), with significant improvement as early as week 1 (P < .0001).
Among children and adults who participated in the INTEGUMENT studies for 28 and 56 weeks, 61.3% and 65.7% achieved a 75% reduction in their Eczema Area and Severity Index scores, respectively. According to the company, there were no adverse reactions in the combined phase 3 pivotal trials that occurred in more than 2.9% of participants in either arm. The most common adverse reactions included headache (2.9%), nausea (1.9%), application-site pain (1.5%), diarrhea (1.5%), and vomiting (1.5%).
The product is expected to be available commercially at the end of July 2024, according to Arcutis. Roflumilast cream 0.3% is indicated for topical treatment of plaque psoriasis, including intertriginous areas, in adult and pediatric patients aged 6 years or older; roflumilast foam 0.3% is indicated for the treatment of seborrheic dermatitis in adult and pediatric patients aged 9 years or older.
A version of this article first appeared on Medscape.com.
On July 9, the aged 6 years or older.
Roflumilast cream 0.15%, which has been developed by Arcutis Biotherapeutics and is marketed under the brand name Zoryve, is a steroid-free topical phosphodiesterase-4 inhibitor that was previously approved in a higher concentration to treat seborrheic dermatitis and plaque psoriasis.
According to a press release from Arcutis, approval for AD was supported by positive results from three phase 3 studies, a phase 2 dose-ranging study, and two phase 1 pharmacokinetic trials. In two identical phase 3 studies known as INTEGUMENT-1 and INTEGUMENT-2, about 40% of children and adults treated with roflumilast cream 0.15% achieved a Validated Investigator Global Assessment for Atopic Dermatitis score of clear (0) or almost clear (1) at week 4 (INTEGUMENT-1: 41.5% vs 25.2%; P < .0001; INTEGUMENT-2: 39% vs 16.9%; P < .0001), with significant improvement as early as week 1 (P < .0001).
Among children and adults who participated in the INTEGUMENT studies for 28 and 56 weeks, 61.3% and 65.7% achieved a 75% reduction in their Eczema Area and Severity Index scores, respectively. According to the company, there were no adverse reactions in the combined phase 3 pivotal trials that occurred in more than 2.9% of participants in either arm. The most common adverse reactions included headache (2.9%), nausea (1.9%), application-site pain (1.5%), diarrhea (1.5%), and vomiting (1.5%).
The product is expected to be available commercially at the end of July 2024, according to Arcutis. Roflumilast cream 0.3% is indicated for topical treatment of plaque psoriasis, including intertriginous areas, in adult and pediatric patients aged 6 years or older; roflumilast foam 0.3% is indicated for the treatment of seborrheic dermatitis in adult and pediatric patients aged 9 years or older.
A version of this article first appeared on Medscape.com.
Dupilumab Safe, Effective Over 5 Years in Moderate to Severe Atopic Dermatitis
TOPLINE:
Over 5 years, dupilumab demonstrated acceptable safety and sustained efficacy, with significant improvements in the signs and symptoms of AD, in the treatment of moderate to severe atopic dermatitis (AD).
METHODOLOGY:
- The phase 3 multinational LIBERTY AD open-label extension study evaluated the safety and efficacy of dupilumab in 2677 adults with moderate to severe AD who had previously participated in dupilumab trials over 5 years; 334 patients (12.5%) completed treatment up to 5 years.
- Patients started with subcutaneous dupilumab, initially dosed weekly after a loading dose, then every 2 weeks in 2019.
- The primary outcomes were the incidence and rate of treatment-emergent adverse events (TEAEs).
TAKEAWAY:
- Overall, 14,717 TEAEs were reported over 5 years. The exposure-adjusted incidence rate decreased over time and was 252.48 events per 100 patient-years.
- The most common TEAEs were nasopharyngitis (28.9%), worsening AD (16.7%), upper respiratory tract infection (13.6%), conjunctivitis (10.3%), allergic conjunctivitis (9%), headache (8.1%), oral herpes (7.5%), and injection-site reactions (5.2%).
- Serious and severe TEAE rates were 10.6% and 10.0%, respectively. Exposure-adjusted incidence rates were 6.66 and 6.71 events per 100 patient-years, respectively.
- At week 260, 67.5% of patients had achieved clear or almost clear skin according to the Investigator’s Global Assessment, and 88.9% experienced a 75% or greater improvement in the Eczema Area and Severity Index.
IN PRACTICE:
“Safety and efficacy results from up to 5 years of dupilumab treatment in the LIBERTY AD open-label extension study support dupilumab as a continuous long-term treatment for adults with moderate to severe AD,” the authors concluded.
SOURCE:
The study was led by Lisa A. Beck, MD, University of Rochester, Rochester, New York, and was published online in JAMA Dermatology.
LIMITATIONS:
Study limitations included the absence of a placebo arm and treatment interruptions stemming from protocol changes. The number of patients who received biweekly doses was small. The early conclusion of the trial by the sponsor because of regulatory approval also resulted in a lower number of patients at later stages.
DISCLOSURES:
This study was funded by dupilumab manufacturers Sanofi and Regeneron Pharmaceuticals. Several authors declared ties with various pharmaceutical companies including Sanofi and Regeneron, and several authors were employees of Sanofi or Regeneron. No disclosures were reported by other authors.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Over 5 years, dupilumab demonstrated acceptable safety and sustained efficacy, with significant improvements in the signs and symptoms of AD, in the treatment of moderate to severe atopic dermatitis (AD).
METHODOLOGY:
- The phase 3 multinational LIBERTY AD open-label extension study evaluated the safety and efficacy of dupilumab in 2677 adults with moderate to severe AD who had previously participated in dupilumab trials over 5 years; 334 patients (12.5%) completed treatment up to 5 years.
- Patients started with subcutaneous dupilumab, initially dosed weekly after a loading dose, then every 2 weeks in 2019.
- The primary outcomes were the incidence and rate of treatment-emergent adverse events (TEAEs).
TAKEAWAY:
- Overall, 14,717 TEAEs were reported over 5 years. The exposure-adjusted incidence rate decreased over time and was 252.48 events per 100 patient-years.
- The most common TEAEs were nasopharyngitis (28.9%), worsening AD (16.7%), upper respiratory tract infection (13.6%), conjunctivitis (10.3%), allergic conjunctivitis (9%), headache (8.1%), oral herpes (7.5%), and injection-site reactions (5.2%).
- Serious and severe TEAE rates were 10.6% and 10.0%, respectively. Exposure-adjusted incidence rates were 6.66 and 6.71 events per 100 patient-years, respectively.
- At week 260, 67.5% of patients had achieved clear or almost clear skin according to the Investigator’s Global Assessment, and 88.9% experienced a 75% or greater improvement in the Eczema Area and Severity Index.
IN PRACTICE:
“Safety and efficacy results from up to 5 years of dupilumab treatment in the LIBERTY AD open-label extension study support dupilumab as a continuous long-term treatment for adults with moderate to severe AD,” the authors concluded.
SOURCE:
The study was led by Lisa A. Beck, MD, University of Rochester, Rochester, New York, and was published online in JAMA Dermatology.
LIMITATIONS:
Study limitations included the absence of a placebo arm and treatment interruptions stemming from protocol changes. The number of patients who received biweekly doses was small. The early conclusion of the trial by the sponsor because of regulatory approval also resulted in a lower number of patients at later stages.
DISCLOSURES:
This study was funded by dupilumab manufacturers Sanofi and Regeneron Pharmaceuticals. Several authors declared ties with various pharmaceutical companies including Sanofi and Regeneron, and several authors were employees of Sanofi or Regeneron. No disclosures were reported by other authors.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Over 5 years, dupilumab demonstrated acceptable safety and sustained efficacy, with significant improvements in the signs and symptoms of AD, in the treatment of moderate to severe atopic dermatitis (AD).
METHODOLOGY:
- The phase 3 multinational LIBERTY AD open-label extension study evaluated the safety and efficacy of dupilumab in 2677 adults with moderate to severe AD who had previously participated in dupilumab trials over 5 years; 334 patients (12.5%) completed treatment up to 5 years.
- Patients started with subcutaneous dupilumab, initially dosed weekly after a loading dose, then every 2 weeks in 2019.
- The primary outcomes were the incidence and rate of treatment-emergent adverse events (TEAEs).
TAKEAWAY:
- Overall, 14,717 TEAEs were reported over 5 years. The exposure-adjusted incidence rate decreased over time and was 252.48 events per 100 patient-years.
- The most common TEAEs were nasopharyngitis (28.9%), worsening AD (16.7%), upper respiratory tract infection (13.6%), conjunctivitis (10.3%), allergic conjunctivitis (9%), headache (8.1%), oral herpes (7.5%), and injection-site reactions (5.2%).
- Serious and severe TEAE rates were 10.6% and 10.0%, respectively. Exposure-adjusted incidence rates were 6.66 and 6.71 events per 100 patient-years, respectively.
- At week 260, 67.5% of patients had achieved clear or almost clear skin according to the Investigator’s Global Assessment, and 88.9% experienced a 75% or greater improvement in the Eczema Area and Severity Index.
IN PRACTICE:
“Safety and efficacy results from up to 5 years of dupilumab treatment in the LIBERTY AD open-label extension study support dupilumab as a continuous long-term treatment for adults with moderate to severe AD,” the authors concluded.
SOURCE:
The study was led by Lisa A. Beck, MD, University of Rochester, Rochester, New York, and was published online in JAMA Dermatology.
LIMITATIONS:
Study limitations included the absence of a placebo arm and treatment interruptions stemming from protocol changes. The number of patients who received biweekly doses was small. The early conclusion of the trial by the sponsor because of regulatory approval also resulted in a lower number of patients at later stages.
DISCLOSURES:
This study was funded by dupilumab manufacturers Sanofi and Regeneron Pharmaceuticals. Several authors declared ties with various pharmaceutical companies including Sanofi and Regeneron, and several authors were employees of Sanofi or Regeneron. No disclosures were reported by other authors.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
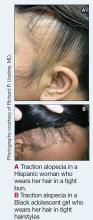
Act Fast With Traction Alopecia to Avoid Permanent Hair Loss
Traction alopecia (TA) is a common type of alopecia that ultimately can result in permanent hair loss. It often is caused or worsened by repetitive and prolonged hairstyling practices such as tight ponytails, braids, or locs, or use of wigs or weaves.1 Use of headwear, as in certain religious or ethnic groups, also can be contributory.2 Individuals participating in or training for occupations involving military service or ballet are at risk for TA due to hairstyling-specific policies. Early stages of TA are reversible with proper treatment and avoidance of exacerbating factors, emphasizing the importance of prompt recognition.3
Epidemiology
Data on the true prevalence of TA are lacking. It can occur in individuals of any race or any hair type. However, it is most common in women of African descent, affecting approximately one-third of this population.4 Other commonly affected groups include ballerinas and active-duty service members due to tight ponytails and buns, as well as the Sikh population due to the use of turbans as a part of their religious practice.2,5,6
Traction alopecia also impacts children, particularly those of African descent. A 2007 study of schoolchildren in South Africa determined that more than 17% of young African girls had evidence of TA—even some as young as 6 years of age.7
Traction alopecia can be caused or exacerbated by the use of hair clips and bobby pins that aid holding styles in place.8 Hair shaft morphology may contribute to the risk for TA, with more tightly coiled hair types being more susceptible.8 Variables such as use of chemical relaxers also increase the risk for disease, especially when combined with high-tension styling methods such as braids.9
Key clinical features
Patients with TA clinically present with hair loss and breakage in areas with tension, most commonly the marginal areas of the scalp as well as the frontal hairline and temporal scalp. Hair loss can result in a “fringe sign,” in which a patient may have preservation of a thin line of hairs at the frontal aspect of the hairline with a band of hair loss behind.10 This presentation may be used to differentiate TA from other forms of alopecia, including frontal fibrosing alopecia and female pattern hair loss. When the hair loss is not marginal, it may mimic other forms of patchy hair loss including alopecia areata and trichotillomania. Other clinical findings in TA may include broken hairs, pustules, and follicular papules.10 Patients also may describe symptoms such as scalp tenderness with specific hairstyles or headaches,11 or they may be completely asymptomatic.
Trichoscopy can be helpful in guiding diagnosis and treatment. Patients with TA often have perifollicular erythema and hair casts (cylindrical structures that encircle the proximal hair shafts) in the earlier stages of the disease, with eventual loss of follicular ostia in the later stages.10,12 Hair casts also may indicate ongoing traction.12 The flambeau sign—white tracks seen on trichoscopy in the direction the hair is pulled—resembles a lit torch.13
Worth noting
Early-stage TA can be reversed by avoiding hair tension. However, patients may not be amenable to this due to personal hairstyling preferences, job duties, or religious practices. Treatment with topical or intralesional steroids or even oral antibiotics such as doxycycline for its anti-inflammatory ability may result in regrowth of lost hair if the follicles are not permanently lost and exacerbating factors are avoided.3,14 Both topical and oral minoxidil have been used with success, with minoxidil thought to increase hair density by extending the anagen (growth) phase of hair follicles.3,15 Culturally sensitive patient counseling on the condition and potential exacerbating factors is critical.16
At later stages of the disease—after loss of follicular ostia has occurred—surgical interventions should be considered,17 such as hair transplantation, which can be successful but remains a technical challenge due to variability in hair shaft curvature.18 Additionally, the cost of the procedure can limit use, and some patients may not be optimal candidates due to the extent of their hair loss. Traction alopecia may not be the only hair loss condition present. Examining the scalp is important even if the chief area of concern is the marginal scalp.
Health disparity highlight
Prevention, early identification, and treatment initiated in a timely fashion are crucial to prevent permanent hair loss. There are added societal and cultural pressures that impact hairstyle and hair care practices, especially for those with tightly coiled hair.19 Historically, tightly coiled hair has been unfairly viewed as “unprofessional,” “unkempt,” and a challenge to “manage” by some. Thus, heat, chemical relaxers, and tight hairstyles holding hair in one position have been used to straighten the hair permanently or temporarily or to keep it maintained in a style that did not necessitate excessive manipulation—often contributing to further tension on the hair.
Military service branches have evaluated and changed some hair-related policies to reflect the diverse hair types of military personnel.20 The CROWN Act (www.thecrownact.com/about)—“Creating a Respectful and Open World for Natural Hair”—is a model law passed by 26 states that prohibits race-based hair discrimination, which is the denial of employment and educational opportunities because of hair texture. Although the law has not been passed in every state, it may help individuals with tightly coiled hair to embrace natural hairstyles. However, even hairstyles with one’s own natural curl pattern can contribute to tension and thus potential development of TA.
1. Larrondo J, McMichael AJ. Traction alopecia. JAMA Dermatol. 2023;159:676. doi:10.1001/jamadermatol.2022.6298
2. James J, Saladi RN, Fox JL. Traction alopecia in Sikh male patients. J Am Board Fam Med. 2007;20:497-498. doi:10.3122/jabfm.2007.05.070076
3. Callender VD, McMichael AJ, Cohen GF. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17:164-176.
4. Loussouarn G, El Rawadi C, Genain G. Diversity of hair growth profiles. Int J Dermatol. 2005;44(suppl 1):6-9.
5. Samrao AChen CZedek Det al. Traction alopecia in a ballerina: clinicopathologic features. Arch Dermatol. 2010;146:918-935. doi:10.1001/archdermatol.2010.183
6. Korona-Bailey J, Banaag A, Nguyen DR, et al. Free the bun: prevalence of alopecia among active duty service women, fiscal years 2010-2019. Mil Med. 2023;188:e492-e496. doi:10.1093/milmed/usab274
7. Khumalo NP, Jessop S, Gumedze F, et al. Hairdressing is associated with scalp disease in African schoolchildren. Br J Dermatol. 2007;157:106-110. doi:10.1111/j.1365-2133.2007.07987.x
8. Billero V, Miteva M. Traction alopecia: the root of the problem. Clin Cosmet Investig Dermatol. 2018;11:149-159. doi:10.2147/CCID.S137296
9. Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia (TA). J Am Acad Dermatol. 2016;75:606-611. doi:10.1016/j.jaad.2016.02.1162
10. Samrao A, Price VH, Zedek D, et al. The “fringe sign”—a useful clinical finding in traction alopecia of the marginal hair line. Dermatol Online J. 2011;17:1.
11. Kararizou E, Bougea AM, Giotopoulou D, et al. An update on the less-known group of other primary headaches—a review. Eur Neurol Rev. 2014;9:71-77. doi:10.17925/ENR.2014.09.01.71
12. Tosti A, Miteva M, Torres F, et al. Hair casts are a dermoscopic clue for the diagnosis of traction alopecia. Br J Dermatol. 2010;163:1353-1355.
13. Agrawal S, Daruwalla SB, Dhurat RS. The flambeau sign—a new dermoscopy finding in a case of marginal traction alopecia. Australas J Dermatol. 2020;61:49-50. doi:10.1111/ajd.13187
14. Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3:S21-S37.
15. Awad A, Chim I, Sharma P, et al. Low-dose oral minoxidil improves hair density in traction alopecia. J Am Acad Dermatol. 2023;89:157-159. doi:10.1016/j.jaad.2023.02.024
16. Grayson C, Heath CR. Counseling about traction alopecia: a “compliment, discuss, and suggest” method. Cutis. 2021;108:20-22.
17. Ozçelik D. Extensive traction alopecia attributable to ponytail hairstyle and its treatment with hair transplantation. Aesthetic Plast Surg. 2005;29:325-327. doi:10.1007/s00266-005-0004-5
18. Singh MK, Avram MR. Technical considerations for follicular unit extraction in African-American hair. Dermatol Surg. 2013;39:1282-1284. doi:10.1111/dsu.12229
19. Jones NL, Heath CR. Hair at the intersection of dermatology and anthropology: a conversation on race and relationships. Pediatr Dermatol. 2021;38(suppl 2):158-160.
20. Franklin JMM, Wohltmann WE, Wong EB. From buns to braids and ponytails: entering a new era of female military hair-grooming standards. Cutis. 2021;108:31-35. doi:10.12788/cutis.0296
Traction alopecia (TA) is a common type of alopecia that ultimately can result in permanent hair loss. It often is caused or worsened by repetitive and prolonged hairstyling practices such as tight ponytails, braids, or locs, or use of wigs or weaves.1 Use of headwear, as in certain religious or ethnic groups, also can be contributory.2 Individuals participating in or training for occupations involving military service or ballet are at risk for TA due to hairstyling-specific policies. Early stages of TA are reversible with proper treatment and avoidance of exacerbating factors, emphasizing the importance of prompt recognition.3
Epidemiology
Data on the true prevalence of TA are lacking. It can occur in individuals of any race or any hair type. However, it is most common in women of African descent, affecting approximately one-third of this population.4 Other commonly affected groups include ballerinas and active-duty service members due to tight ponytails and buns, as well as the Sikh population due to the use of turbans as a part of their religious practice.2,5,6
Traction alopecia also impacts children, particularly those of African descent. A 2007 study of schoolchildren in South Africa determined that more than 17% of young African girls had evidence of TA—even some as young as 6 years of age.7
Traction alopecia can be caused or exacerbated by the use of hair clips and bobby pins that aid holding styles in place.8 Hair shaft morphology may contribute to the risk for TA, with more tightly coiled hair types being more susceptible.8 Variables such as use of chemical relaxers also increase the risk for disease, especially when combined with high-tension styling methods such as braids.9
Key clinical features
Patients with TA clinically present with hair loss and breakage in areas with tension, most commonly the marginal areas of the scalp as well as the frontal hairline and temporal scalp. Hair loss can result in a “fringe sign,” in which a patient may have preservation of a thin line of hairs at the frontal aspect of the hairline with a band of hair loss behind.10 This presentation may be used to differentiate TA from other forms of alopecia, including frontal fibrosing alopecia and female pattern hair loss. When the hair loss is not marginal, it may mimic other forms of patchy hair loss including alopecia areata and trichotillomania. Other clinical findings in TA may include broken hairs, pustules, and follicular papules.10 Patients also may describe symptoms such as scalp tenderness with specific hairstyles or headaches,11 or they may be completely asymptomatic.
Trichoscopy can be helpful in guiding diagnosis and treatment. Patients with TA often have perifollicular erythema and hair casts (cylindrical structures that encircle the proximal hair shafts) in the earlier stages of the disease, with eventual loss of follicular ostia in the later stages.10,12 Hair casts also may indicate ongoing traction.12 The flambeau sign—white tracks seen on trichoscopy in the direction the hair is pulled—resembles a lit torch.13
Worth noting
Early-stage TA can be reversed by avoiding hair tension. However, patients may not be amenable to this due to personal hairstyling preferences, job duties, or religious practices. Treatment with topical or intralesional steroids or even oral antibiotics such as doxycycline for its anti-inflammatory ability may result in regrowth of lost hair if the follicles are not permanently lost and exacerbating factors are avoided.3,14 Both topical and oral minoxidil have been used with success, with minoxidil thought to increase hair density by extending the anagen (growth) phase of hair follicles.3,15 Culturally sensitive patient counseling on the condition and potential exacerbating factors is critical.16
At later stages of the disease—after loss of follicular ostia has occurred—surgical interventions should be considered,17 such as hair transplantation, which can be successful but remains a technical challenge due to variability in hair shaft curvature.18 Additionally, the cost of the procedure can limit use, and some patients may not be optimal candidates due to the extent of their hair loss. Traction alopecia may not be the only hair loss condition present. Examining the scalp is important even if the chief area of concern is the marginal scalp.
Health disparity highlight
Prevention, early identification, and treatment initiated in a timely fashion are crucial to prevent permanent hair loss. There are added societal and cultural pressures that impact hairstyle and hair care practices, especially for those with tightly coiled hair.19 Historically, tightly coiled hair has been unfairly viewed as “unprofessional,” “unkempt,” and a challenge to “manage” by some. Thus, heat, chemical relaxers, and tight hairstyles holding hair in one position have been used to straighten the hair permanently or temporarily or to keep it maintained in a style that did not necessitate excessive manipulation—often contributing to further tension on the hair.
Military service branches have evaluated and changed some hair-related policies to reflect the diverse hair types of military personnel.20 The CROWN Act (www.thecrownact.com/about)—“Creating a Respectful and Open World for Natural Hair”—is a model law passed by 26 states that prohibits race-based hair discrimination, which is the denial of employment and educational opportunities because of hair texture. Although the law has not been passed in every state, it may help individuals with tightly coiled hair to embrace natural hairstyles. However, even hairstyles with one’s own natural curl pattern can contribute to tension and thus potential development of TA.
Traction alopecia (TA) is a common type of alopecia that ultimately can result in permanent hair loss. It often is caused or worsened by repetitive and prolonged hairstyling practices such as tight ponytails, braids, or locs, or use of wigs or weaves.1 Use of headwear, as in certain religious or ethnic groups, also can be contributory.2 Individuals participating in or training for occupations involving military service or ballet are at risk for TA due to hairstyling-specific policies. Early stages of TA are reversible with proper treatment and avoidance of exacerbating factors, emphasizing the importance of prompt recognition.3
Epidemiology
Data on the true prevalence of TA are lacking. It can occur in individuals of any race or any hair type. However, it is most common in women of African descent, affecting approximately one-third of this population.4 Other commonly affected groups include ballerinas and active-duty service members due to tight ponytails and buns, as well as the Sikh population due to the use of turbans as a part of their religious practice.2,5,6
Traction alopecia also impacts children, particularly those of African descent. A 2007 study of schoolchildren in South Africa determined that more than 17% of young African girls had evidence of TA—even some as young as 6 years of age.7
Traction alopecia can be caused or exacerbated by the use of hair clips and bobby pins that aid holding styles in place.8 Hair shaft morphology may contribute to the risk for TA, with more tightly coiled hair types being more susceptible.8 Variables such as use of chemical relaxers also increase the risk for disease, especially when combined with high-tension styling methods such as braids.9
Key clinical features
Patients with TA clinically present with hair loss and breakage in areas with tension, most commonly the marginal areas of the scalp as well as the frontal hairline and temporal scalp. Hair loss can result in a “fringe sign,” in which a patient may have preservation of a thin line of hairs at the frontal aspect of the hairline with a band of hair loss behind.10 This presentation may be used to differentiate TA from other forms of alopecia, including frontal fibrosing alopecia and female pattern hair loss. When the hair loss is not marginal, it may mimic other forms of patchy hair loss including alopecia areata and trichotillomania. Other clinical findings in TA may include broken hairs, pustules, and follicular papules.10 Patients also may describe symptoms such as scalp tenderness with specific hairstyles or headaches,11 or they may be completely asymptomatic.
Trichoscopy can be helpful in guiding diagnosis and treatment. Patients with TA often have perifollicular erythema and hair casts (cylindrical structures that encircle the proximal hair shafts) in the earlier stages of the disease, with eventual loss of follicular ostia in the later stages.10,12 Hair casts also may indicate ongoing traction.12 The flambeau sign—white tracks seen on trichoscopy in the direction the hair is pulled—resembles a lit torch.13
Worth noting
Early-stage TA can be reversed by avoiding hair tension. However, patients may not be amenable to this due to personal hairstyling preferences, job duties, or religious practices. Treatment with topical or intralesional steroids or even oral antibiotics such as doxycycline for its anti-inflammatory ability may result in regrowth of lost hair if the follicles are not permanently lost and exacerbating factors are avoided.3,14 Both topical and oral minoxidil have been used with success, with minoxidil thought to increase hair density by extending the anagen (growth) phase of hair follicles.3,15 Culturally sensitive patient counseling on the condition and potential exacerbating factors is critical.16
At later stages of the disease—after loss of follicular ostia has occurred—surgical interventions should be considered,17 such as hair transplantation, which can be successful but remains a technical challenge due to variability in hair shaft curvature.18 Additionally, the cost of the procedure can limit use, and some patients may not be optimal candidates due to the extent of their hair loss. Traction alopecia may not be the only hair loss condition present. Examining the scalp is important even if the chief area of concern is the marginal scalp.
Health disparity highlight
Prevention, early identification, and treatment initiated in a timely fashion are crucial to prevent permanent hair loss. There are added societal and cultural pressures that impact hairstyle and hair care practices, especially for those with tightly coiled hair.19 Historically, tightly coiled hair has been unfairly viewed as “unprofessional,” “unkempt,” and a challenge to “manage” by some. Thus, heat, chemical relaxers, and tight hairstyles holding hair in one position have been used to straighten the hair permanently or temporarily or to keep it maintained in a style that did not necessitate excessive manipulation—often contributing to further tension on the hair.
Military service branches have evaluated and changed some hair-related policies to reflect the diverse hair types of military personnel.20 The CROWN Act (www.thecrownact.com/about)—“Creating a Respectful and Open World for Natural Hair”—is a model law passed by 26 states that prohibits race-based hair discrimination, which is the denial of employment and educational opportunities because of hair texture. Although the law has not been passed in every state, it may help individuals with tightly coiled hair to embrace natural hairstyles. However, even hairstyles with one’s own natural curl pattern can contribute to tension and thus potential development of TA.
1. Larrondo J, McMichael AJ. Traction alopecia. JAMA Dermatol. 2023;159:676. doi:10.1001/jamadermatol.2022.6298
2. James J, Saladi RN, Fox JL. Traction alopecia in Sikh male patients. J Am Board Fam Med. 2007;20:497-498. doi:10.3122/jabfm.2007.05.070076
3. Callender VD, McMichael AJ, Cohen GF. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17:164-176.
4. Loussouarn G, El Rawadi C, Genain G. Diversity of hair growth profiles. Int J Dermatol. 2005;44(suppl 1):6-9.
5. Samrao AChen CZedek Det al. Traction alopecia in a ballerina: clinicopathologic features. Arch Dermatol. 2010;146:918-935. doi:10.1001/archdermatol.2010.183
6. Korona-Bailey J, Banaag A, Nguyen DR, et al. Free the bun: prevalence of alopecia among active duty service women, fiscal years 2010-2019. Mil Med. 2023;188:e492-e496. doi:10.1093/milmed/usab274
7. Khumalo NP, Jessop S, Gumedze F, et al. Hairdressing is associated with scalp disease in African schoolchildren. Br J Dermatol. 2007;157:106-110. doi:10.1111/j.1365-2133.2007.07987.x
8. Billero V, Miteva M. Traction alopecia: the root of the problem. Clin Cosmet Investig Dermatol. 2018;11:149-159. doi:10.2147/CCID.S137296
9. Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia (TA). J Am Acad Dermatol. 2016;75:606-611. doi:10.1016/j.jaad.2016.02.1162
10. Samrao A, Price VH, Zedek D, et al. The “fringe sign”—a useful clinical finding in traction alopecia of the marginal hair line. Dermatol Online J. 2011;17:1.
11. Kararizou E, Bougea AM, Giotopoulou D, et al. An update on the less-known group of other primary headaches—a review. Eur Neurol Rev. 2014;9:71-77. doi:10.17925/ENR.2014.09.01.71
12. Tosti A, Miteva M, Torres F, et al. Hair casts are a dermoscopic clue for the diagnosis of traction alopecia. Br J Dermatol. 2010;163:1353-1355.
13. Agrawal S, Daruwalla SB, Dhurat RS. The flambeau sign—a new dermoscopy finding in a case of marginal traction alopecia. Australas J Dermatol. 2020;61:49-50. doi:10.1111/ajd.13187
14. Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3:S21-S37.
15. Awad A, Chim I, Sharma P, et al. Low-dose oral minoxidil improves hair density in traction alopecia. J Am Acad Dermatol. 2023;89:157-159. doi:10.1016/j.jaad.2023.02.024
16. Grayson C, Heath CR. Counseling about traction alopecia: a “compliment, discuss, and suggest” method. Cutis. 2021;108:20-22.
17. Ozçelik D. Extensive traction alopecia attributable to ponytail hairstyle and its treatment with hair transplantation. Aesthetic Plast Surg. 2005;29:325-327. doi:10.1007/s00266-005-0004-5
18. Singh MK, Avram MR. Technical considerations for follicular unit extraction in African-American hair. Dermatol Surg. 2013;39:1282-1284. doi:10.1111/dsu.12229
19. Jones NL, Heath CR. Hair at the intersection of dermatology and anthropology: a conversation on race and relationships. Pediatr Dermatol. 2021;38(suppl 2):158-160.
20. Franklin JMM, Wohltmann WE, Wong EB. From buns to braids and ponytails: entering a new era of female military hair-grooming standards. Cutis. 2021;108:31-35. doi:10.12788/cutis.0296
1. Larrondo J, McMichael AJ. Traction alopecia. JAMA Dermatol. 2023;159:676. doi:10.1001/jamadermatol.2022.6298
2. James J, Saladi RN, Fox JL. Traction alopecia in Sikh male patients. J Am Board Fam Med. 2007;20:497-498. doi:10.3122/jabfm.2007.05.070076
3. Callender VD, McMichael AJ, Cohen GF. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17:164-176.
4. Loussouarn G, El Rawadi C, Genain G. Diversity of hair growth profiles. Int J Dermatol. 2005;44(suppl 1):6-9.
5. Samrao AChen CZedek Det al. Traction alopecia in a ballerina: clinicopathologic features. Arch Dermatol. 2010;146:918-935. doi:10.1001/archdermatol.2010.183
6. Korona-Bailey J, Banaag A, Nguyen DR, et al. Free the bun: prevalence of alopecia among active duty service women, fiscal years 2010-2019. Mil Med. 2023;188:e492-e496. doi:10.1093/milmed/usab274
7. Khumalo NP, Jessop S, Gumedze F, et al. Hairdressing is associated with scalp disease in African schoolchildren. Br J Dermatol. 2007;157:106-110. doi:10.1111/j.1365-2133.2007.07987.x
8. Billero V, Miteva M. Traction alopecia: the root of the problem. Clin Cosmet Investig Dermatol. 2018;11:149-159. doi:10.2147/CCID.S137296
9. Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia (TA). J Am Acad Dermatol. 2016;75:606-611. doi:10.1016/j.jaad.2016.02.1162
10. Samrao A, Price VH, Zedek D, et al. The “fringe sign”—a useful clinical finding in traction alopecia of the marginal hair line. Dermatol Online J. 2011;17:1.
11. Kararizou E, Bougea AM, Giotopoulou D, et al. An update on the less-known group of other primary headaches—a review. Eur Neurol Rev. 2014;9:71-77. doi:10.17925/ENR.2014.09.01.71
12. Tosti A, Miteva M, Torres F, et al. Hair casts are a dermoscopic clue for the diagnosis of traction alopecia. Br J Dermatol. 2010;163:1353-1355.
13. Agrawal S, Daruwalla SB, Dhurat RS. The flambeau sign—a new dermoscopy finding in a case of marginal traction alopecia. Australas J Dermatol. 2020;61:49-50. doi:10.1111/ajd.13187
14. Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3:S21-S37.
15. Awad A, Chim I, Sharma P, et al. Low-dose oral minoxidil improves hair density in traction alopecia. J Am Acad Dermatol. 2023;89:157-159. doi:10.1016/j.jaad.2023.02.024
16. Grayson C, Heath CR. Counseling about traction alopecia: a “compliment, discuss, and suggest” method. Cutis. 2021;108:20-22.
17. Ozçelik D. Extensive traction alopecia attributable to ponytail hairstyle and its treatment with hair transplantation. Aesthetic Plast Surg. 2005;29:325-327. doi:10.1007/s00266-005-0004-5
18. Singh MK, Avram MR. Technical considerations for follicular unit extraction in African-American hair. Dermatol Surg. 2013;39:1282-1284. doi:10.1111/dsu.12229
19. Jones NL, Heath CR. Hair at the intersection of dermatology and anthropology: a conversation on race and relationships. Pediatr Dermatol. 2021;38(suppl 2):158-160.
20. Franklin JMM, Wohltmann WE, Wong EB. From buns to braids and ponytails: entering a new era of female military hair-grooming standards. Cutis. 2021;108:31-35. doi:10.12788/cutis.0296
Cosmetic Botulinum Toxin A Doses May Differ in Sunny Climates
findings from a comparative cohort study suggested.
“Botulinum toxin A to the glabella is a popular cosmetic intervention,” researchers led by Kim L. Borsky, MD, MBBS, of the Department of Plastic and Reconstructive Surgery at Stoke Mandeville Hospital, Aylesbury, England, and colleagues wrote in their study, which was published in Plastic and Reconstructive Surgery. “Functional musculature differences may arise from chronic behavioral adjustment to high sun exposure levels, requiring greater doses. This could affect clinical practice globally.”
To investigate the effect of climate on real-world doses of the product, the researchers enrolled 523 women aged 35-60 years who received glabellar botulinum toxin treatment at two centers between 2012 and 2019: one in the United Kingdom and one in Malta. They evaluated data on 292 patients treated during the summer months at the Malta center (classified as the high sun-exposure group), and 231 patients treated during the winter months at the UK center (classified as the low sun-exposure group). The primary outcomes of interest were the required top-up doses and the total dose to achieve full paralysis. Smokers were excluded from the analysis, as were those who did not seek maximal paralysis, those documented as not compliant with posttreatment advice, and those with colds or fevers. They used univariable and multivariable analyses to compare the high vs low sun-exposure groups.
The researchers found that 68.5% of women in the high-sun group required a top-up dose to achieve full paralysis, compared with 61.5% in the low-sun group, a difference that did not reach statistical significance (P = .1032). All patients achieved full paralysis with the treatment protocol used. However, in the high-sun group, the mean top-up dose was significantly higher than that in the low-sun group (a mean of 9.30 vs 7.06 units, respectively; P = .0009), as was the mean total dose (a mean of 29.23 vs 27.25 units; P = .0031).
“Patients subject to less sun exposure require a lower dose than patients with high sun exposure, and this was present and persisted when controlling for potential confounders,” the researchers wrote. “Although robustly demonstrated, the difference in doses seen here was small, and so may not directly impact at a health economic level, as the difference would not necessarily change the number of vials used. However, it may be of relevance to training and protocolization of treatments. Rigid protocols about doses and distributions may lead to undertreatment if applied in sunnier climates.”
They acknowledged certain limitations of their study, including its unblinded design and the fact that they did not evaluate or control for ethnicity. They also characterized the population of Malta as “very homogeneous, mainly made up of Maltese with less than 5% foreigners,” while the demographics of the United Kingdom and especially London, where the injections were performed, “are much more diverse.”
Asked to comment on the results, Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington, DC, said that the study highlights the importance of tailoring neuromodulator treatment to the individual patient based not just on gender but also on lifestyle and climate. “The conclusion [of the study] is logical, but it’s encouraging that the data supports this,” Dr. Sodha told this news organization. “The potential confounders, such as injection technique (5 point vs 3 point), nonblinding of the evaluator, history of prior treatments, and variation in treatment effect by different botulinum toxin products may be important as well in how we consider this data in practice.”
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Neither the researchers nor Dr. Sodha reported having financial disclosures.
A version of this article appeared on Medscape.com.
findings from a comparative cohort study suggested.
“Botulinum toxin A to the glabella is a popular cosmetic intervention,” researchers led by Kim L. Borsky, MD, MBBS, of the Department of Plastic and Reconstructive Surgery at Stoke Mandeville Hospital, Aylesbury, England, and colleagues wrote in their study, which was published in Plastic and Reconstructive Surgery. “Functional musculature differences may arise from chronic behavioral adjustment to high sun exposure levels, requiring greater doses. This could affect clinical practice globally.”
To investigate the effect of climate on real-world doses of the product, the researchers enrolled 523 women aged 35-60 years who received glabellar botulinum toxin treatment at two centers between 2012 and 2019: one in the United Kingdom and one in Malta. They evaluated data on 292 patients treated during the summer months at the Malta center (classified as the high sun-exposure group), and 231 patients treated during the winter months at the UK center (classified as the low sun-exposure group). The primary outcomes of interest were the required top-up doses and the total dose to achieve full paralysis. Smokers were excluded from the analysis, as were those who did not seek maximal paralysis, those documented as not compliant with posttreatment advice, and those with colds or fevers. They used univariable and multivariable analyses to compare the high vs low sun-exposure groups.
The researchers found that 68.5% of women in the high-sun group required a top-up dose to achieve full paralysis, compared with 61.5% in the low-sun group, a difference that did not reach statistical significance (P = .1032). All patients achieved full paralysis with the treatment protocol used. However, in the high-sun group, the mean top-up dose was significantly higher than that in the low-sun group (a mean of 9.30 vs 7.06 units, respectively; P = .0009), as was the mean total dose (a mean of 29.23 vs 27.25 units; P = .0031).
“Patients subject to less sun exposure require a lower dose than patients with high sun exposure, and this was present and persisted when controlling for potential confounders,” the researchers wrote. “Although robustly demonstrated, the difference in doses seen here was small, and so may not directly impact at a health economic level, as the difference would not necessarily change the number of vials used. However, it may be of relevance to training and protocolization of treatments. Rigid protocols about doses and distributions may lead to undertreatment if applied in sunnier climates.”
They acknowledged certain limitations of their study, including its unblinded design and the fact that they did not evaluate or control for ethnicity. They also characterized the population of Malta as “very homogeneous, mainly made up of Maltese with less than 5% foreigners,” while the demographics of the United Kingdom and especially London, where the injections were performed, “are much more diverse.”
Asked to comment on the results, Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington, DC, said that the study highlights the importance of tailoring neuromodulator treatment to the individual patient based not just on gender but also on lifestyle and climate. “The conclusion [of the study] is logical, but it’s encouraging that the data supports this,” Dr. Sodha told this news organization. “The potential confounders, such as injection technique (5 point vs 3 point), nonblinding of the evaluator, history of prior treatments, and variation in treatment effect by different botulinum toxin products may be important as well in how we consider this data in practice.”
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Neither the researchers nor Dr. Sodha reported having financial disclosures.
A version of this article appeared on Medscape.com.
findings from a comparative cohort study suggested.
“Botulinum toxin A to the glabella is a popular cosmetic intervention,” researchers led by Kim L. Borsky, MD, MBBS, of the Department of Plastic and Reconstructive Surgery at Stoke Mandeville Hospital, Aylesbury, England, and colleagues wrote in their study, which was published in Plastic and Reconstructive Surgery. “Functional musculature differences may arise from chronic behavioral adjustment to high sun exposure levels, requiring greater doses. This could affect clinical practice globally.”
To investigate the effect of climate on real-world doses of the product, the researchers enrolled 523 women aged 35-60 years who received glabellar botulinum toxin treatment at two centers between 2012 and 2019: one in the United Kingdom and one in Malta. They evaluated data on 292 patients treated during the summer months at the Malta center (classified as the high sun-exposure group), and 231 patients treated during the winter months at the UK center (classified as the low sun-exposure group). The primary outcomes of interest were the required top-up doses and the total dose to achieve full paralysis. Smokers were excluded from the analysis, as were those who did not seek maximal paralysis, those documented as not compliant with posttreatment advice, and those with colds or fevers. They used univariable and multivariable analyses to compare the high vs low sun-exposure groups.
The researchers found that 68.5% of women in the high-sun group required a top-up dose to achieve full paralysis, compared with 61.5% in the low-sun group, a difference that did not reach statistical significance (P = .1032). All patients achieved full paralysis with the treatment protocol used. However, in the high-sun group, the mean top-up dose was significantly higher than that in the low-sun group (a mean of 9.30 vs 7.06 units, respectively; P = .0009), as was the mean total dose (a mean of 29.23 vs 27.25 units; P = .0031).
“Patients subject to less sun exposure require a lower dose than patients with high sun exposure, and this was present and persisted when controlling for potential confounders,” the researchers wrote. “Although robustly demonstrated, the difference in doses seen here was small, and so may not directly impact at a health economic level, as the difference would not necessarily change the number of vials used. However, it may be of relevance to training and protocolization of treatments. Rigid protocols about doses and distributions may lead to undertreatment if applied in sunnier climates.”
They acknowledged certain limitations of their study, including its unblinded design and the fact that they did not evaluate or control for ethnicity. They also characterized the population of Malta as “very homogeneous, mainly made up of Maltese with less than 5% foreigners,” while the demographics of the United Kingdom and especially London, where the injections were performed, “are much more diverse.”
Asked to comment on the results, Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington, DC, said that the study highlights the importance of tailoring neuromodulator treatment to the individual patient based not just on gender but also on lifestyle and climate. “The conclusion [of the study] is logical, but it’s encouraging that the data supports this,” Dr. Sodha told this news organization. “The potential confounders, such as injection technique (5 point vs 3 point), nonblinding of the evaluator, history of prior treatments, and variation in treatment effect by different botulinum toxin products may be important as well in how we consider this data in practice.”
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Neither the researchers nor Dr. Sodha reported having financial disclosures.
A version of this article appeared on Medscape.com.
FROM PLASTIC AND RECONSTRUCTIVE SURGERY
Urticaria Linked to Higher Cancer Risk, Study Finds
TOPLINE:
which decreased to 6% in subsequent years, in a cohort study using Danish healthcare databases.
METHODOLOGY:
- Researchers conducted a retrospective cohort study using data from Danish healthcare registries and compared the incident cancer risk between patients with urticaria and the risk in the general population.
- They identified 87,507 patients (58% women) with a primary or secondary first-time hospital outpatient clinic, emergency room, or inpatient diagnosis of urticaria between 1980 and 2022, who were followed for a median of 10.1 years.
- Incident cancers, including nonmelanoma skin cancer, were identified using the Danish Cancer Registry and classified by the extent of spread at the time of diagnosis.
- This study computed the absolute cancer risk within the first year of an urticaria diagnosis and standardized incidence ratios (SIRs), with 95% CIs standardized to Danish national cancer rates.
TAKEAWAY:
- For the first year of follow-up, the absolute risk for all cancer types was 0.7%, and it was 29.5% for subsequent years. The overall SIR for all types of cancer was 1.09 (95% CI, 1.06-1.11), which was based on 7788 observed cancer cases compared with 7161 cases expected over the entire follow-up period.
- Within the first year of follow-up, 588 patients with urticaria were diagnosed with cancer, for an SIR of 1.49 (95% CI, 1.38-1.62) for all cancer types.
- After the first year, the SIR for all cancer sites decreased and stabilized at 1.06 (95% CI, 1.04-1.09), with 7200 observed cancer cases.
- The risk was highest for hematological cancers in the first year, particularly Hodgkin lymphoma (SIR, 5.35; 95% CI, 2.56-9.85).
IN PRACTICE:
“Our study suggests that urticaria may be a marker of occult cancer and that it is associated with a slightly increased long-term cancer risk,” the authors wrote.
SOURCE:
The study was led by Sissel B.T. Sørensen, departments of dermatology and rheumatology, Aarhus University Hospital, Aarhus, Denmark. It was published online on June 27, 2024, in the British Journal of Dermatology.
LIMITATIONS:
The study is limited by its observational design and reliance on registry data, which may be subject to misclassification or incomplete information. In addition, the study could not assess individual patient factors such as lifestyle or genetic predispositions that may influence cancer risk, and the results may not be generalizable to other populations. Finally, the exact biologic mechanisms linking urticaria and cancer remain unclear, warranting further investigation.
DISCLOSURES:
The study did not receive any funding. The authors reported that they had no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
which decreased to 6% in subsequent years, in a cohort study using Danish healthcare databases.
METHODOLOGY:
- Researchers conducted a retrospective cohort study using data from Danish healthcare registries and compared the incident cancer risk between patients with urticaria and the risk in the general population.
- They identified 87,507 patients (58% women) with a primary or secondary first-time hospital outpatient clinic, emergency room, or inpatient diagnosis of urticaria between 1980 and 2022, who were followed for a median of 10.1 years.
- Incident cancers, including nonmelanoma skin cancer, were identified using the Danish Cancer Registry and classified by the extent of spread at the time of diagnosis.
- This study computed the absolute cancer risk within the first year of an urticaria diagnosis and standardized incidence ratios (SIRs), with 95% CIs standardized to Danish national cancer rates.
TAKEAWAY:
- For the first year of follow-up, the absolute risk for all cancer types was 0.7%, and it was 29.5% for subsequent years. The overall SIR for all types of cancer was 1.09 (95% CI, 1.06-1.11), which was based on 7788 observed cancer cases compared with 7161 cases expected over the entire follow-up period.
- Within the first year of follow-up, 588 patients with urticaria were diagnosed with cancer, for an SIR of 1.49 (95% CI, 1.38-1.62) for all cancer types.
- After the first year, the SIR for all cancer sites decreased and stabilized at 1.06 (95% CI, 1.04-1.09), with 7200 observed cancer cases.
- The risk was highest for hematological cancers in the first year, particularly Hodgkin lymphoma (SIR, 5.35; 95% CI, 2.56-9.85).
IN PRACTICE:
“Our study suggests that urticaria may be a marker of occult cancer and that it is associated with a slightly increased long-term cancer risk,” the authors wrote.
SOURCE:
The study was led by Sissel B.T. Sørensen, departments of dermatology and rheumatology, Aarhus University Hospital, Aarhus, Denmark. It was published online on June 27, 2024, in the British Journal of Dermatology.
LIMITATIONS:
The study is limited by its observational design and reliance on registry data, which may be subject to misclassification or incomplete information. In addition, the study could not assess individual patient factors such as lifestyle or genetic predispositions that may influence cancer risk, and the results may not be generalizable to other populations. Finally, the exact biologic mechanisms linking urticaria and cancer remain unclear, warranting further investigation.
DISCLOSURES:
The study did not receive any funding. The authors reported that they had no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
which decreased to 6% in subsequent years, in a cohort study using Danish healthcare databases.
METHODOLOGY:
- Researchers conducted a retrospective cohort study using data from Danish healthcare registries and compared the incident cancer risk between patients with urticaria and the risk in the general population.
- They identified 87,507 patients (58% women) with a primary or secondary first-time hospital outpatient clinic, emergency room, or inpatient diagnosis of urticaria between 1980 and 2022, who were followed for a median of 10.1 years.
- Incident cancers, including nonmelanoma skin cancer, were identified using the Danish Cancer Registry and classified by the extent of spread at the time of diagnosis.
- This study computed the absolute cancer risk within the first year of an urticaria diagnosis and standardized incidence ratios (SIRs), with 95% CIs standardized to Danish national cancer rates.
TAKEAWAY:
- For the first year of follow-up, the absolute risk for all cancer types was 0.7%, and it was 29.5% for subsequent years. The overall SIR for all types of cancer was 1.09 (95% CI, 1.06-1.11), which was based on 7788 observed cancer cases compared with 7161 cases expected over the entire follow-up period.
- Within the first year of follow-up, 588 patients with urticaria were diagnosed with cancer, for an SIR of 1.49 (95% CI, 1.38-1.62) for all cancer types.
- After the first year, the SIR for all cancer sites decreased and stabilized at 1.06 (95% CI, 1.04-1.09), with 7200 observed cancer cases.
- The risk was highest for hematological cancers in the first year, particularly Hodgkin lymphoma (SIR, 5.35; 95% CI, 2.56-9.85).
IN PRACTICE:
“Our study suggests that urticaria may be a marker of occult cancer and that it is associated with a slightly increased long-term cancer risk,” the authors wrote.
SOURCE:
The study was led by Sissel B.T. Sørensen, departments of dermatology and rheumatology, Aarhus University Hospital, Aarhus, Denmark. It was published online on June 27, 2024, in the British Journal of Dermatology.
LIMITATIONS:
The study is limited by its observational design and reliance on registry data, which may be subject to misclassification or incomplete information. In addition, the study could not assess individual patient factors such as lifestyle or genetic predispositions that may influence cancer risk, and the results may not be generalizable to other populations. Finally, the exact biologic mechanisms linking urticaria and cancer remain unclear, warranting further investigation.
DISCLOSURES:
The study did not receive any funding. The authors reported that they had no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Survey Highlights Real-World Use of Upadacitinib in Adults With Atopic Dermatitis
, while 7.8% rated their itch as minimally improved.
Also, 27.5% reported itch improvement within one day of taking upadacitinib (Rinvoq), an oral Janus kinase inhibitor that was approved to treat moderate to severe AD in adults and children aged ≥ 12 years in January 2022.
“We have a lot of data about upadacitinib from clinical trials, but sometimes there’s a concern that when you start using a medication in the real world, the effectiveness doesn’t match up with the efficacy observed in clinical trials,” the study’s first author, Jonathan I. Silverberg, MD, PhD, professor of dermatology at George Washington University, Washington, said in an interview after the Revolutionizing Atopic Dermatitis conference, where the study was presented during a late-breaking abstract session. “We always want to confirm or reaffirm clinical trial results with real-world data.”
In SCALE-UP, 6191 adults with moderate to severe AD participating in the patient support program for upadacitinib in the United States were invited to complete a one-time online survey about their experience with upadacitinib, including the degree of and time to itch improvement and skin clearance. The researchers reported on 204 patients who completed the survey questions, for a response rate of 3.3%. The mean age of respondents was 45.3 years, their mean age when diagnosed with AD was 30.3 years; 70.1% were women, and 37% were using topical corticosteroids. In addition, 68.6% were White individuals, 12.3% were Black individuals, 8.8% were Asian individuals or Pacific Islanders, and 0.5% were Native Americans/Alaska Natives.
Duration of upadacitinib treatment was 2-6 months for 50.5% of the patients and 7-12 months for the remaining patients. Starting upadacitinib dose was 15 mg for about 95% of patients and 30 mg for nearly 4% of patients. At the time of the survey, 79.4% of patients were receiving upadacitinib 15 mg once a day, and 19.6% were receiving upadacitinib 30 mg once a day.
Improvements in Itch, Skin Clearance
Nearly all experienced improvements in itch, with 86.8% reporting “very much” or “much” improved itch. Relief was rapid, with 87% noticing improvement in itch within 7 days and 27.5% noticing improvement within 1 day. “This is something I have clinically seen,” Dr. Silverberg said.
After receiving upadacitinib, 87% and 86% of patients indicated they were “extremely” or “very” satisfied with the degree and speed of itch improvement, respectively.
In findings related to skin clearance, 90.7% of respondents reported clearer skin after initiating upadacitinib, with 81.4% reporting “very much” or “much” clearer skin. Skin clearance occurred rapidly, with 30.8% of patients noticing clearer skin within 3 days of starting upadacitinib and 89.2% of patients noticing clearer skin within 14 days. The proportions of patients who were “extremely” or “very” satisfied with the degree and speed of skin clearance were 83.8% and 83.2%, respectively.
“What we’re seeing is that the real-world effectiveness [of upadacitinib] aligns with the clinical trial efficacy,” Dr. Silverberg told this news organization. “This study adds even more data to help inform shared decision-making discussion with our patients in trying to decide what medication is best for them.”
He acknowledged certain limitations of the survey, including the lack of a control group of other treatments for comparison, a low response rate, and the potential for response bias. “That said, I think the results remain important, but we value having even more real-world data in the future from prospective registries,” he said. “Those kinds of studies are ongoing, and we look forward to getting more real-world data readouts.”
AbbVie, the manufacturer of upadacitinib, funded the study. Dr. Silverberg reported having served as an advisor, consultant, speaker, and/or investigator for several pharmaceutical companies, including AbbVie. Two authors are AbbVie employees.
A version of this article appeared on Medscape.com.
, while 7.8% rated their itch as minimally improved.
Also, 27.5% reported itch improvement within one day of taking upadacitinib (Rinvoq), an oral Janus kinase inhibitor that was approved to treat moderate to severe AD in adults and children aged ≥ 12 years in January 2022.
“We have a lot of data about upadacitinib from clinical trials, but sometimes there’s a concern that when you start using a medication in the real world, the effectiveness doesn’t match up with the efficacy observed in clinical trials,” the study’s first author, Jonathan I. Silverberg, MD, PhD, professor of dermatology at George Washington University, Washington, said in an interview after the Revolutionizing Atopic Dermatitis conference, where the study was presented during a late-breaking abstract session. “We always want to confirm or reaffirm clinical trial results with real-world data.”
In SCALE-UP, 6191 adults with moderate to severe AD participating in the patient support program for upadacitinib in the United States were invited to complete a one-time online survey about their experience with upadacitinib, including the degree of and time to itch improvement and skin clearance. The researchers reported on 204 patients who completed the survey questions, for a response rate of 3.3%. The mean age of respondents was 45.3 years, their mean age when diagnosed with AD was 30.3 years; 70.1% were women, and 37% were using topical corticosteroids. In addition, 68.6% were White individuals, 12.3% were Black individuals, 8.8% were Asian individuals or Pacific Islanders, and 0.5% were Native Americans/Alaska Natives.
Duration of upadacitinib treatment was 2-6 months for 50.5% of the patients and 7-12 months for the remaining patients. Starting upadacitinib dose was 15 mg for about 95% of patients and 30 mg for nearly 4% of patients. At the time of the survey, 79.4% of patients were receiving upadacitinib 15 mg once a day, and 19.6% were receiving upadacitinib 30 mg once a day.
Improvements in Itch, Skin Clearance
Nearly all experienced improvements in itch, with 86.8% reporting “very much” or “much” improved itch. Relief was rapid, with 87% noticing improvement in itch within 7 days and 27.5% noticing improvement within 1 day. “This is something I have clinically seen,” Dr. Silverberg said.
After receiving upadacitinib, 87% and 86% of patients indicated they were “extremely” or “very” satisfied with the degree and speed of itch improvement, respectively.
In findings related to skin clearance, 90.7% of respondents reported clearer skin after initiating upadacitinib, with 81.4% reporting “very much” or “much” clearer skin. Skin clearance occurred rapidly, with 30.8% of patients noticing clearer skin within 3 days of starting upadacitinib and 89.2% of patients noticing clearer skin within 14 days. The proportions of patients who were “extremely” or “very” satisfied with the degree and speed of skin clearance were 83.8% and 83.2%, respectively.
“What we’re seeing is that the real-world effectiveness [of upadacitinib] aligns with the clinical trial efficacy,” Dr. Silverberg told this news organization. “This study adds even more data to help inform shared decision-making discussion with our patients in trying to decide what medication is best for them.”
He acknowledged certain limitations of the survey, including the lack of a control group of other treatments for comparison, a low response rate, and the potential for response bias. “That said, I think the results remain important, but we value having even more real-world data in the future from prospective registries,” he said. “Those kinds of studies are ongoing, and we look forward to getting more real-world data readouts.”
AbbVie, the manufacturer of upadacitinib, funded the study. Dr. Silverberg reported having served as an advisor, consultant, speaker, and/or investigator for several pharmaceutical companies, including AbbVie. Two authors are AbbVie employees.
A version of this article appeared on Medscape.com.
, while 7.8% rated their itch as minimally improved.
Also, 27.5% reported itch improvement within one day of taking upadacitinib (Rinvoq), an oral Janus kinase inhibitor that was approved to treat moderate to severe AD in adults and children aged ≥ 12 years in January 2022.
“We have a lot of data about upadacitinib from clinical trials, but sometimes there’s a concern that when you start using a medication in the real world, the effectiveness doesn’t match up with the efficacy observed in clinical trials,” the study’s first author, Jonathan I. Silverberg, MD, PhD, professor of dermatology at George Washington University, Washington, said in an interview after the Revolutionizing Atopic Dermatitis conference, where the study was presented during a late-breaking abstract session. “We always want to confirm or reaffirm clinical trial results with real-world data.”
In SCALE-UP, 6191 adults with moderate to severe AD participating in the patient support program for upadacitinib in the United States were invited to complete a one-time online survey about their experience with upadacitinib, including the degree of and time to itch improvement and skin clearance. The researchers reported on 204 patients who completed the survey questions, for a response rate of 3.3%. The mean age of respondents was 45.3 years, their mean age when diagnosed with AD was 30.3 years; 70.1% were women, and 37% were using topical corticosteroids. In addition, 68.6% were White individuals, 12.3% were Black individuals, 8.8% were Asian individuals or Pacific Islanders, and 0.5% were Native Americans/Alaska Natives.
Duration of upadacitinib treatment was 2-6 months for 50.5% of the patients and 7-12 months for the remaining patients. Starting upadacitinib dose was 15 mg for about 95% of patients and 30 mg for nearly 4% of patients. At the time of the survey, 79.4% of patients were receiving upadacitinib 15 mg once a day, and 19.6% were receiving upadacitinib 30 mg once a day.
Improvements in Itch, Skin Clearance
Nearly all experienced improvements in itch, with 86.8% reporting “very much” or “much” improved itch. Relief was rapid, with 87% noticing improvement in itch within 7 days and 27.5% noticing improvement within 1 day. “This is something I have clinically seen,” Dr. Silverberg said.
After receiving upadacitinib, 87% and 86% of patients indicated they were “extremely” or “very” satisfied with the degree and speed of itch improvement, respectively.
In findings related to skin clearance, 90.7% of respondents reported clearer skin after initiating upadacitinib, with 81.4% reporting “very much” or “much” clearer skin. Skin clearance occurred rapidly, with 30.8% of patients noticing clearer skin within 3 days of starting upadacitinib and 89.2% of patients noticing clearer skin within 14 days. The proportions of patients who were “extremely” or “very” satisfied with the degree and speed of skin clearance were 83.8% and 83.2%, respectively.
“What we’re seeing is that the real-world effectiveness [of upadacitinib] aligns with the clinical trial efficacy,” Dr. Silverberg told this news organization. “This study adds even more data to help inform shared decision-making discussion with our patients in trying to decide what medication is best for them.”
He acknowledged certain limitations of the survey, including the lack of a control group of other treatments for comparison, a low response rate, and the potential for response bias. “That said, I think the results remain important, but we value having even more real-world data in the future from prospective registries,” he said. “Those kinds of studies are ongoing, and we look forward to getting more real-world data readouts.”
AbbVie, the manufacturer of upadacitinib, funded the study. Dr. Silverberg reported having served as an advisor, consultant, speaker, and/or investigator for several pharmaceutical companies, including AbbVie. Two authors are AbbVie employees.
A version of this article appeared on Medscape.com.
Small Melanoma In Situ: Single Center Study Finds Recurrence Low With 5-mm Margin Excisions
. This approach has the potential to reduce morbidity and cost associated with treatment “without compromising patient outcomes in a selected population of lesions,” the authors say.
“Currently, there is uncertainty regarding the optimal excision margin for MIS, with different guidelines recommending a range between 5 and 10 mm,” corresponding author Cong Sun, MD, of Mater Hospital Brisbane Raymond Terrace, South Brisbane, Queensland, Australia, and colleagues wrote in the study, which was published in JAMA Dermatology. “In addition, studies using the Mohs micrographic surgery technique have suggested that wider margins, up to 18 mm, may be required for MIS in some settings.”
To further examine the use of 5-mm margins for excision of small MIS on low-risk sites, the researchers retrospectively evaluated 351 MIS lesions diagnosed in 292 patients between January 1, 2011, and November 30, 2018. Lesions were eligible for analysis if a 5-mm excisional margin was documented on the operation report and if there was more than 5 years of site-specific follow-up after wide local excision. Lesions with undocumented margins were excluded from analysis, as were those with fewer than 5 years of follow-up, and those that required more than one wide local excision.
The mean age of patients was 60.3 years, 55.5% were female, and the mean dimensions of the lesions was 6 × 5 mm. The most common subtype of melanoma diagnosed was superficial spreading melanoma (50.4% of lesions), followed by lentigo maligna (30.5%) and lentiginous MIS (19.1%). Nearly half of the lesions were on the trunk (47.9%), followed by the upper limb (27.4%), lower limb (16.8%), neck (4%), face (3.4%), and scalp (0.6%). As for the size of lesions, 78.1% were < 10 mm long and 88.9% were < 10 mm wide.
Nearly 71% (248) of the lesions were treated with an initial excisional biopsy, and 29.3% (103) underwent an initial shave excision. Median follow-up was 7 years.
Only three of the 351 lesions (0.9%) had a local recurrence, with no regional recurrence or metastatic spread, and 99.1% had no recurrence. The recurrences were reexcised “with clear margins” and after at least 5 years of follow-up, no further recurrences were reported, the authors said.
In Mohs surgery studies, reported recurrence rates for MIS have been “between 0.26% and 1.1%, with excisional margins between 6 and 12 mm required,” the authors noted. “This study demonstrated a comparable 0.9% recurrence rate achieved with a conservative 5-mm excisional margin. This shows that using a 5-mm margin for MIS of smaller size (< 10 mm) may reduce morbidity and cost associated with treatment without compromising patient outcomes in a selected population of lesions.”
The researchers recommended additional studies to confirm their findings and acknowledged certain limitations of their analysis, including its retrospective, single-center design and the predominantly small sizes of the lesions.
In an accompanying editorial, John A. Zitelli, MD, of the University of Pittsburgh, Pittsburgh, Pennsylvania, said that the margin measurement used by the researchers was another limitation. “Before the excision with a 5-mm margin was performed, the diagnosis of MIS was obtained by shave biopsy or excisional biopsy with a 2- to 3-mm margin of clinically normal skin,” Dr. Zitelli wrote. “Therefore, in patients without a 2- to 3-mm biopsy margin, a minimum surgical margin of 7-8 mm would be required to achieve a similar true negative excision margin.”
Also, he continued, the exclusion of lesions with wide subclinical extension that required wider margins “weakens the conclusion that 5 mm would be an effective treatment for all MIS.”
Hugh Greenway, MD, head of Mohs micrographic surgery and director of cutaneous oncology at Scripps Cancer Center, San Diego, who was asked to comment on the study, said that clinicians continue to search for the optimum smaller surgical margin for MIS. “This can be challenging with the variability of MIS based on location and other factors,” Dr. Greenway told this news organization. “This Australian retrospective study notes that for selected, well-defined 6 × 5 mm lesions of low-risk body sites (mainly torso and limbs), a 5-mm surgical margin can provide a high cure rate. The authors note further studies are indicated. Thus, for selected lesions in selected locations, the 5-mm surgical margin may be appropriate for MIS.”
The study authors, Dr. Zitelli, and Dr. Greenway reported no financial disclosures.
A version of this article appeared on Medscape.com.
. This approach has the potential to reduce morbidity and cost associated with treatment “without compromising patient outcomes in a selected population of lesions,” the authors say.
“Currently, there is uncertainty regarding the optimal excision margin for MIS, with different guidelines recommending a range between 5 and 10 mm,” corresponding author Cong Sun, MD, of Mater Hospital Brisbane Raymond Terrace, South Brisbane, Queensland, Australia, and colleagues wrote in the study, which was published in JAMA Dermatology. “In addition, studies using the Mohs micrographic surgery technique have suggested that wider margins, up to 18 mm, may be required for MIS in some settings.”
To further examine the use of 5-mm margins for excision of small MIS on low-risk sites, the researchers retrospectively evaluated 351 MIS lesions diagnosed in 292 patients between January 1, 2011, and November 30, 2018. Lesions were eligible for analysis if a 5-mm excisional margin was documented on the operation report and if there was more than 5 years of site-specific follow-up after wide local excision. Lesions with undocumented margins were excluded from analysis, as were those with fewer than 5 years of follow-up, and those that required more than one wide local excision.
The mean age of patients was 60.3 years, 55.5% were female, and the mean dimensions of the lesions was 6 × 5 mm. The most common subtype of melanoma diagnosed was superficial spreading melanoma (50.4% of lesions), followed by lentigo maligna (30.5%) and lentiginous MIS (19.1%). Nearly half of the lesions were on the trunk (47.9%), followed by the upper limb (27.4%), lower limb (16.8%), neck (4%), face (3.4%), and scalp (0.6%). As for the size of lesions, 78.1% were < 10 mm long and 88.9% were < 10 mm wide.
Nearly 71% (248) of the lesions were treated with an initial excisional biopsy, and 29.3% (103) underwent an initial shave excision. Median follow-up was 7 years.
Only three of the 351 lesions (0.9%) had a local recurrence, with no regional recurrence or metastatic spread, and 99.1% had no recurrence. The recurrences were reexcised “with clear margins” and after at least 5 years of follow-up, no further recurrences were reported, the authors said.
In Mohs surgery studies, reported recurrence rates for MIS have been “between 0.26% and 1.1%, with excisional margins between 6 and 12 mm required,” the authors noted. “This study demonstrated a comparable 0.9% recurrence rate achieved with a conservative 5-mm excisional margin. This shows that using a 5-mm margin for MIS of smaller size (< 10 mm) may reduce morbidity and cost associated with treatment without compromising patient outcomes in a selected population of lesions.”
The researchers recommended additional studies to confirm their findings and acknowledged certain limitations of their analysis, including its retrospective, single-center design and the predominantly small sizes of the lesions.
In an accompanying editorial, John A. Zitelli, MD, of the University of Pittsburgh, Pittsburgh, Pennsylvania, said that the margin measurement used by the researchers was another limitation. “Before the excision with a 5-mm margin was performed, the diagnosis of MIS was obtained by shave biopsy or excisional biopsy with a 2- to 3-mm margin of clinically normal skin,” Dr. Zitelli wrote. “Therefore, in patients without a 2- to 3-mm biopsy margin, a minimum surgical margin of 7-8 mm would be required to achieve a similar true negative excision margin.”
Also, he continued, the exclusion of lesions with wide subclinical extension that required wider margins “weakens the conclusion that 5 mm would be an effective treatment for all MIS.”
Hugh Greenway, MD, head of Mohs micrographic surgery and director of cutaneous oncology at Scripps Cancer Center, San Diego, who was asked to comment on the study, said that clinicians continue to search for the optimum smaller surgical margin for MIS. “This can be challenging with the variability of MIS based on location and other factors,” Dr. Greenway told this news organization. “This Australian retrospective study notes that for selected, well-defined 6 × 5 mm lesions of low-risk body sites (mainly torso and limbs), a 5-mm surgical margin can provide a high cure rate. The authors note further studies are indicated. Thus, for selected lesions in selected locations, the 5-mm surgical margin may be appropriate for MIS.”
The study authors, Dr. Zitelli, and Dr. Greenway reported no financial disclosures.
A version of this article appeared on Medscape.com.
. This approach has the potential to reduce morbidity and cost associated with treatment “without compromising patient outcomes in a selected population of lesions,” the authors say.
“Currently, there is uncertainty regarding the optimal excision margin for MIS, with different guidelines recommending a range between 5 and 10 mm,” corresponding author Cong Sun, MD, of Mater Hospital Brisbane Raymond Terrace, South Brisbane, Queensland, Australia, and colleagues wrote in the study, which was published in JAMA Dermatology. “In addition, studies using the Mohs micrographic surgery technique have suggested that wider margins, up to 18 mm, may be required for MIS in some settings.”
To further examine the use of 5-mm margins for excision of small MIS on low-risk sites, the researchers retrospectively evaluated 351 MIS lesions diagnosed in 292 patients between January 1, 2011, and November 30, 2018. Lesions were eligible for analysis if a 5-mm excisional margin was documented on the operation report and if there was more than 5 years of site-specific follow-up after wide local excision. Lesions with undocumented margins were excluded from analysis, as were those with fewer than 5 years of follow-up, and those that required more than one wide local excision.
The mean age of patients was 60.3 years, 55.5% were female, and the mean dimensions of the lesions was 6 × 5 mm. The most common subtype of melanoma diagnosed was superficial spreading melanoma (50.4% of lesions), followed by lentigo maligna (30.5%) and lentiginous MIS (19.1%). Nearly half of the lesions were on the trunk (47.9%), followed by the upper limb (27.4%), lower limb (16.8%), neck (4%), face (3.4%), and scalp (0.6%). As for the size of lesions, 78.1% were < 10 mm long and 88.9% were < 10 mm wide.
Nearly 71% (248) of the lesions were treated with an initial excisional biopsy, and 29.3% (103) underwent an initial shave excision. Median follow-up was 7 years.
Only three of the 351 lesions (0.9%) had a local recurrence, with no regional recurrence or metastatic spread, and 99.1% had no recurrence. The recurrences were reexcised “with clear margins” and after at least 5 years of follow-up, no further recurrences were reported, the authors said.
In Mohs surgery studies, reported recurrence rates for MIS have been “between 0.26% and 1.1%, with excisional margins between 6 and 12 mm required,” the authors noted. “This study demonstrated a comparable 0.9% recurrence rate achieved with a conservative 5-mm excisional margin. This shows that using a 5-mm margin for MIS of smaller size (< 10 mm) may reduce morbidity and cost associated with treatment without compromising patient outcomes in a selected population of lesions.”
The researchers recommended additional studies to confirm their findings and acknowledged certain limitations of their analysis, including its retrospective, single-center design and the predominantly small sizes of the lesions.
In an accompanying editorial, John A. Zitelli, MD, of the University of Pittsburgh, Pittsburgh, Pennsylvania, said that the margin measurement used by the researchers was another limitation. “Before the excision with a 5-mm margin was performed, the diagnosis of MIS was obtained by shave biopsy or excisional biopsy with a 2- to 3-mm margin of clinically normal skin,” Dr. Zitelli wrote. “Therefore, in patients without a 2- to 3-mm biopsy margin, a minimum surgical margin of 7-8 mm would be required to achieve a similar true negative excision margin.”
Also, he continued, the exclusion of lesions with wide subclinical extension that required wider margins “weakens the conclusion that 5 mm would be an effective treatment for all MIS.”
Hugh Greenway, MD, head of Mohs micrographic surgery and director of cutaneous oncology at Scripps Cancer Center, San Diego, who was asked to comment on the study, said that clinicians continue to search for the optimum smaller surgical margin for MIS. “This can be challenging with the variability of MIS based on location and other factors,” Dr. Greenway told this news organization. “This Australian retrospective study notes that for selected, well-defined 6 × 5 mm lesions of low-risk body sites (mainly torso and limbs), a 5-mm surgical margin can provide a high cure rate. The authors note further studies are indicated. Thus, for selected lesions in selected locations, the 5-mm surgical margin may be appropriate for MIS.”
The study authors, Dr. Zitelli, and Dr. Greenway reported no financial disclosures.
A version of this article appeared on Medscape.com.
Study Finds Variations in Pediatric Dermatologists Who Accept Medicaid
TOPLINE:
METHODOLOGY:
- Researchers identified 352 actively practicing board-certified pediatric dermatologists using the Society for Pediatric Dermatology database and determined Medicaid acceptance status.
- They collected physician and practice characteristics from the US Census American Community Survey data and a web search.
TAKEAWAY:
- A total of 275 (78.1%) board-certified pediatric dermatologists accepted Medicaid.
- Academic practices had the highest Medicaid acceptance rate (98.7%), while private practices had the lowest (43.1%), a significant difference (P < .001).
- Acceptance rates were significantly higher in the Midwest (90.9%) than in the Northeast (71.8%) or West (71.4%; P = .005). Regional differences persisted after controlling for practice type: Midwest practice locations had greater odds of Medicaid acceptance than those in the Northeast (odds ratio [OR], 5.25; 95% confidence interval [CI], 1.76-15.65) or West (OR, 5.26; 95% CI, 1.88-14.66).
- Practices in counties with lower median household incomes and greater densities of pediatric dermatologists were associated with higher Medicaid acceptance (P = .001).
IN PRACTICE:
“While most pediatric dermatologists accept Medicaid, this study revealed differential access to care based on practice type, geographic location, and density of pediatric dermatologists per county,” the authors wrote. More research is needed on “the impact on health outcomes when specialty services are unavailable” and on “the role of administrative and reimbursement barriers limiting Medicaid acceptance among pediatric dermatologists,” they added.
SOURCE:
The study was led by Madeleine Tessier-Kay, MPH, Department of Dermatology, at the University of Connecticut Health Center in Farmington, Connecticut. It was published online in Pediatric Dermatology.
LIMITATIONS:
Limitations include potential incomplete capture of board-certified physicians, as not all board-certified pediatric dermatologists may be members of the Society for Pediatric Dermatology, and potential inaccurate capture of physician characteristics and Medicaid acceptance status.
DISCLOSURES:
The study funding source was not disclosed. One author was a consultant for AbbVie. Other authors declared no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers identified 352 actively practicing board-certified pediatric dermatologists using the Society for Pediatric Dermatology database and determined Medicaid acceptance status.
- They collected physician and practice characteristics from the US Census American Community Survey data and a web search.
TAKEAWAY:
- A total of 275 (78.1%) board-certified pediatric dermatologists accepted Medicaid.
- Academic practices had the highest Medicaid acceptance rate (98.7%), while private practices had the lowest (43.1%), a significant difference (P < .001).
- Acceptance rates were significantly higher in the Midwest (90.9%) than in the Northeast (71.8%) or West (71.4%; P = .005). Regional differences persisted after controlling for practice type: Midwest practice locations had greater odds of Medicaid acceptance than those in the Northeast (odds ratio [OR], 5.25; 95% confidence interval [CI], 1.76-15.65) or West (OR, 5.26; 95% CI, 1.88-14.66).
- Practices in counties with lower median household incomes and greater densities of pediatric dermatologists were associated with higher Medicaid acceptance (P = .001).
IN PRACTICE:
“While most pediatric dermatologists accept Medicaid, this study revealed differential access to care based on practice type, geographic location, and density of pediatric dermatologists per county,” the authors wrote. More research is needed on “the impact on health outcomes when specialty services are unavailable” and on “the role of administrative and reimbursement barriers limiting Medicaid acceptance among pediatric dermatologists,” they added.
SOURCE:
The study was led by Madeleine Tessier-Kay, MPH, Department of Dermatology, at the University of Connecticut Health Center in Farmington, Connecticut. It was published online in Pediatric Dermatology.
LIMITATIONS:
Limitations include potential incomplete capture of board-certified physicians, as not all board-certified pediatric dermatologists may be members of the Society for Pediatric Dermatology, and potential inaccurate capture of physician characteristics and Medicaid acceptance status.
DISCLOSURES:
The study funding source was not disclosed. One author was a consultant for AbbVie. Other authors declared no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers identified 352 actively practicing board-certified pediatric dermatologists using the Society for Pediatric Dermatology database and determined Medicaid acceptance status.
- They collected physician and practice characteristics from the US Census American Community Survey data and a web search.
TAKEAWAY:
- A total of 275 (78.1%) board-certified pediatric dermatologists accepted Medicaid.
- Academic practices had the highest Medicaid acceptance rate (98.7%), while private practices had the lowest (43.1%), a significant difference (P < .001).
- Acceptance rates were significantly higher in the Midwest (90.9%) than in the Northeast (71.8%) or West (71.4%; P = .005). Regional differences persisted after controlling for practice type: Midwest practice locations had greater odds of Medicaid acceptance than those in the Northeast (odds ratio [OR], 5.25; 95% confidence interval [CI], 1.76-15.65) or West (OR, 5.26; 95% CI, 1.88-14.66).
- Practices in counties with lower median household incomes and greater densities of pediatric dermatologists were associated with higher Medicaid acceptance (P = .001).
IN PRACTICE:
“While most pediatric dermatologists accept Medicaid, this study revealed differential access to care based on practice type, geographic location, and density of pediatric dermatologists per county,” the authors wrote. More research is needed on “the impact on health outcomes when specialty services are unavailable” and on “the role of administrative and reimbursement barriers limiting Medicaid acceptance among pediatric dermatologists,” they added.
SOURCE:
The study was led by Madeleine Tessier-Kay, MPH, Department of Dermatology, at the University of Connecticut Health Center in Farmington, Connecticut. It was published online in Pediatric Dermatology.
LIMITATIONS:
Limitations include potential incomplete capture of board-certified physicians, as not all board-certified pediatric dermatologists may be members of the Society for Pediatric Dermatology, and potential inaccurate capture of physician characteristics and Medicaid acceptance status.
DISCLOSURES:
The study funding source was not disclosed. One author was a consultant for AbbVie. Other authors declared no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Dermatofibrosarcoma Protuberans More Common In Black Patients, Analysis Finds
TOPLINE:
that also found that larger tumor size and older age were associated with survival outcomes.
METHODOLOGY:
- Researchers used the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) registry from 2000 through 2018 to provide a comprehensive report on the incidence of DFSP, a rare, low-grade cutaneous soft tissue sarcoma, and factors associated with metastatic progression, overall survival (OS), and cancer-specific survival.
- A total of 7748 patients (mean age, 43.5 years; 53.3% women; 52% non-Hispanic White) were diagnosed with histologically confirmed DFSP of the skin and connective tissue and were included in the study.
- DFSP incidence was reported as cases per million person-years and age-adjusted to the 2000 US Standard Population, and factors influencing metastasis were assessed.
TAKEAWAY:
- The overall DFSP incidence rate was 6.25 cases per million person-years, with a higher incidence in Black individuals than in White individuals (8.74 vs 4.53).
- The 5-year OS rate was 95.8%. Older age (≥ 60 years; hazard ratio [HR], 6.66), male gender assigned at birth (HR, 1.79), and larger tumor size (≥ 3 cm; HR, 2.02) were associated with poorer OS (P < .001 for all).
- The 1-year and 5-year DFSP-specific survival rates were 99.9% and 99.2%, respectively. Older age (HR, 3.47; P < .001) and larger tumor size (≥ 3 cm; HR, 5.34; P = .002) were associated with significantly worse cancer-specific survival.
- Large tumor size (odds ratio [OR], 2.24) and DFSP located on the head and neck (OR, 4.88), or genitalia (OR, 3.16) were significantly associated with increased metastasis risk. Higher socioeconomic status was linked to a lower risk for metastasis.
IN PRACTICE:
“Our findings highlight the increased incidence rates of DFSP among Black patients. We demonstrate the interplay between patient demographics and clinical factors in influencing DFSP metastasis, OS, and cancer-specific survival,” the authors wrote. The results, they added, “may be useful for further evaluation of proposed causes, which will ultimately lead to further understanding and prevention of this disease.”
SOURCE:
The study was led by Jalal Maghfour, MD, Department of Dermatology, Henry Ford Health, Detroit, and was published online on June 20 in the Journal of the American Academy of Dermatology.
LIMITATIONS:
Details on specific cases in the SEER registry are limited. For 1752 patients, tumor size was not included, increasing the risk for misclassification bias. Because specific pathology reports were not available, the analysis did not address histologic grade.
DISCLOSURES:
The study did not receive any funding support. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
that also found that larger tumor size and older age were associated with survival outcomes.
METHODOLOGY:
- Researchers used the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) registry from 2000 through 2018 to provide a comprehensive report on the incidence of DFSP, a rare, low-grade cutaneous soft tissue sarcoma, and factors associated with metastatic progression, overall survival (OS), and cancer-specific survival.
- A total of 7748 patients (mean age, 43.5 years; 53.3% women; 52% non-Hispanic White) were diagnosed with histologically confirmed DFSP of the skin and connective tissue and were included in the study.
- DFSP incidence was reported as cases per million person-years and age-adjusted to the 2000 US Standard Population, and factors influencing metastasis were assessed.
TAKEAWAY:
- The overall DFSP incidence rate was 6.25 cases per million person-years, with a higher incidence in Black individuals than in White individuals (8.74 vs 4.53).
- The 5-year OS rate was 95.8%. Older age (≥ 60 years; hazard ratio [HR], 6.66), male gender assigned at birth (HR, 1.79), and larger tumor size (≥ 3 cm; HR, 2.02) were associated with poorer OS (P < .001 for all).
- The 1-year and 5-year DFSP-specific survival rates were 99.9% and 99.2%, respectively. Older age (HR, 3.47; P < .001) and larger tumor size (≥ 3 cm; HR, 5.34; P = .002) were associated with significantly worse cancer-specific survival.
- Large tumor size (odds ratio [OR], 2.24) and DFSP located on the head and neck (OR, 4.88), or genitalia (OR, 3.16) were significantly associated with increased metastasis risk. Higher socioeconomic status was linked to a lower risk for metastasis.
IN PRACTICE:
“Our findings highlight the increased incidence rates of DFSP among Black patients. We demonstrate the interplay between patient demographics and clinical factors in influencing DFSP metastasis, OS, and cancer-specific survival,” the authors wrote. The results, they added, “may be useful for further evaluation of proposed causes, which will ultimately lead to further understanding and prevention of this disease.”
SOURCE:
The study was led by Jalal Maghfour, MD, Department of Dermatology, Henry Ford Health, Detroit, and was published online on June 20 in the Journal of the American Academy of Dermatology.
LIMITATIONS:
Details on specific cases in the SEER registry are limited. For 1752 patients, tumor size was not included, increasing the risk for misclassification bias. Because specific pathology reports were not available, the analysis did not address histologic grade.
DISCLOSURES:
The study did not receive any funding support. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
that also found that larger tumor size and older age were associated with survival outcomes.
METHODOLOGY:
- Researchers used the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) registry from 2000 through 2018 to provide a comprehensive report on the incidence of DFSP, a rare, low-grade cutaneous soft tissue sarcoma, and factors associated with metastatic progression, overall survival (OS), and cancer-specific survival.
- A total of 7748 patients (mean age, 43.5 years; 53.3% women; 52% non-Hispanic White) were diagnosed with histologically confirmed DFSP of the skin and connective tissue and were included in the study.
- DFSP incidence was reported as cases per million person-years and age-adjusted to the 2000 US Standard Population, and factors influencing metastasis were assessed.
TAKEAWAY:
- The overall DFSP incidence rate was 6.25 cases per million person-years, with a higher incidence in Black individuals than in White individuals (8.74 vs 4.53).
- The 5-year OS rate was 95.8%. Older age (≥ 60 years; hazard ratio [HR], 6.66), male gender assigned at birth (HR, 1.79), and larger tumor size (≥ 3 cm; HR, 2.02) were associated with poorer OS (P < .001 for all).
- The 1-year and 5-year DFSP-specific survival rates were 99.9% and 99.2%, respectively. Older age (HR, 3.47; P < .001) and larger tumor size (≥ 3 cm; HR, 5.34; P = .002) were associated with significantly worse cancer-specific survival.
- Large tumor size (odds ratio [OR], 2.24) and DFSP located on the head and neck (OR, 4.88), or genitalia (OR, 3.16) were significantly associated with increased metastasis risk. Higher socioeconomic status was linked to a lower risk for metastasis.
IN PRACTICE:
“Our findings highlight the increased incidence rates of DFSP among Black patients. We demonstrate the interplay between patient demographics and clinical factors in influencing DFSP metastasis, OS, and cancer-specific survival,” the authors wrote. The results, they added, “may be useful for further evaluation of proposed causes, which will ultimately lead to further understanding and prevention of this disease.”
SOURCE:
The study was led by Jalal Maghfour, MD, Department of Dermatology, Henry Ford Health, Detroit, and was published online on June 20 in the Journal of the American Academy of Dermatology.
LIMITATIONS:
Details on specific cases in the SEER registry are limited. For 1752 patients, tumor size was not included, increasing the risk for misclassification bias. Because specific pathology reports were not available, the analysis did not address histologic grade.
DISCLOSURES:
The study did not receive any funding support. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Pyzchiva Receives FDA Approval as Third Ustekinumab Biosimilar
The Food and Drug Administration has approved ustekinumab-ttwe (Pyzchiva) as a biosimilar to ustekinumab (Stelara) for the treatment of multiple inflammatory conditions.
In addition, the agency “provisionally determined” that the medication would be interchangeable with the reference product but that designation would not take hold until the interchangeability exclusivity period for the first approved biosimilar ustekinumab-auub (Wezlana) expires, according to a press release. This designation would, depending on state law, allow a pharmacist to substitute the biosimilar for the reference product without involving the prescribing clinician. It’s unclear when ustekinumab-auub’s interchangeability exclusivity ends.
Ustekinumab-ttwe, a human interleukin (IL)-12 and IL-23 antagonist, is indicated for the treatment of:
- Moderate to severe plaque psoriasis in adults and pediatric patients aged 6 years or older who are candidates for phototherapy or systemic therapy
- Active psoriatic arthritis in adults and pediatric patients aged 6 years or older with moderately to severely active Crohn’s disease or ulcerative colitis
It is administered via subcutaneous injection in 45 mg/0.5 mL and 90 mg/mL prefilled syringes or via intravenous infusion in 130 mg/26 mL (5 mg/mL) single-dose vial.
Developed by Samsung Bioepis, ustekinumab-ttwe will be commercialized by Sandoz in the United States. Besides ustekinumab-auub, the other ustekinumab biosimilar is ustekinumab-aekn (Selarsdi).
Ustekinumab-ttwe is expected to launch in February 2025 “in accordance with the settlement and license agreement with Janssen Biotech,” which manufacturers the reference product, Sandoz said. The other approved ustekinumab biosimilars will launch within a similar time frame.
A version of this article appeared on Medscape.com.
The Food and Drug Administration has approved ustekinumab-ttwe (Pyzchiva) as a biosimilar to ustekinumab (Stelara) for the treatment of multiple inflammatory conditions.
In addition, the agency “provisionally determined” that the medication would be interchangeable with the reference product but that designation would not take hold until the interchangeability exclusivity period for the first approved biosimilar ustekinumab-auub (Wezlana) expires, according to a press release. This designation would, depending on state law, allow a pharmacist to substitute the biosimilar for the reference product without involving the prescribing clinician. It’s unclear when ustekinumab-auub’s interchangeability exclusivity ends.
Ustekinumab-ttwe, a human interleukin (IL)-12 and IL-23 antagonist, is indicated for the treatment of:
- Moderate to severe plaque psoriasis in adults and pediatric patients aged 6 years or older who are candidates for phototherapy or systemic therapy
- Active psoriatic arthritis in adults and pediatric patients aged 6 years or older with moderately to severely active Crohn’s disease or ulcerative colitis
It is administered via subcutaneous injection in 45 mg/0.5 mL and 90 mg/mL prefilled syringes or via intravenous infusion in 130 mg/26 mL (5 mg/mL) single-dose vial.
Developed by Samsung Bioepis, ustekinumab-ttwe will be commercialized by Sandoz in the United States. Besides ustekinumab-auub, the other ustekinumab biosimilar is ustekinumab-aekn (Selarsdi).
Ustekinumab-ttwe is expected to launch in February 2025 “in accordance with the settlement and license agreement with Janssen Biotech,” which manufacturers the reference product, Sandoz said. The other approved ustekinumab biosimilars will launch within a similar time frame.
A version of this article appeared on Medscape.com.
The Food and Drug Administration has approved ustekinumab-ttwe (Pyzchiva) as a biosimilar to ustekinumab (Stelara) for the treatment of multiple inflammatory conditions.
In addition, the agency “provisionally determined” that the medication would be interchangeable with the reference product but that designation would not take hold until the interchangeability exclusivity period for the first approved biosimilar ustekinumab-auub (Wezlana) expires, according to a press release. This designation would, depending on state law, allow a pharmacist to substitute the biosimilar for the reference product without involving the prescribing clinician. It’s unclear when ustekinumab-auub’s interchangeability exclusivity ends.
Ustekinumab-ttwe, a human interleukin (IL)-12 and IL-23 antagonist, is indicated for the treatment of:
- Moderate to severe plaque psoriasis in adults and pediatric patients aged 6 years or older who are candidates for phototherapy or systemic therapy
- Active psoriatic arthritis in adults and pediatric patients aged 6 years or older with moderately to severely active Crohn’s disease or ulcerative colitis
It is administered via subcutaneous injection in 45 mg/0.5 mL and 90 mg/mL prefilled syringes or via intravenous infusion in 130 mg/26 mL (5 mg/mL) single-dose vial.
Developed by Samsung Bioepis, ustekinumab-ttwe will be commercialized by Sandoz in the United States. Besides ustekinumab-auub, the other ustekinumab biosimilar is ustekinumab-aekn (Selarsdi).
Ustekinumab-ttwe is expected to launch in February 2025 “in accordance with the settlement and license agreement with Janssen Biotech,” which manufacturers the reference product, Sandoz said. The other approved ustekinumab biosimilars will launch within a similar time frame.
A version of this article appeared on Medscape.com.