User login
Dermatoporosis in Older Adults: A Condition That Requires Holistic, Creative Management
WASHINGTON — and conveys the skin’s vulnerability to serious medical complications, said Adam Friedman, MD, at the ElderDerm conference on dermatology in the older patient.
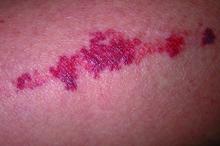
Key features of dermatoporosis include atrophic skin, solar purpura, white pseudoscars, easily acquired skin lacerations and tears, bruises, and delayed healing. “We’re going to see more of this, and it will more and more be a chief complaint of patients,” said Dr. Friedman, professor and chair of dermatology at George Washington University (GWU) in Washington, and co-chair of the meeting. GWU hosted the conference, describing it as a first-of-its-kind meeting dedicated to improving dermatologic care for older adults.
Dermatoporosis was described in the literature in 2007 by dermatologists at the University of Geneva in Switzerland. “It is not only a cosmetic problem,” Dr. Friedman said. “This is a medical problem ... which can absolutely lead to comorbidities [such as deep dissecting hematomas] that are a huge strain on the healthcare system.”
Dermatologists can meet the moment with holistic, creative combination treatment and counseling approaches aimed at improving the mechanical strength of skin and preventing potential complications in older patients, Dr. Friedman said at the meeting.
He described the case of a 76-year-old woman who presented with dermatoporosis on her arms involving pronounced skin atrophy, solar purpura, and a small covered laceration. “This was a patient who was both devastated by the appearance” and impacted by the pain and burden of dressing frequent wounds, said Dr. Friedman, who is also the director of the Residency Program, of Translational Research, and of Supportive Oncodermatology, all within the Department of Dermatology at GWU.
With 11 months of topical treatment that included daily application of calcipotriene 0.05% ointment and nightly application of tazarotene 0.045% lotion and oral supplementation with 1000-mg vitamin C twice daily and 1000-mg citrus bioflavonoid complex daily, as well as no changes to the medications she took for various comorbidities, the solar purpura improved significantly and “we made a huge difference in the integrity of her skin,” he said.
Dr. Friedman also described this case in a recently published article in the Journal of Drugs in Dermatology titled “What’s Old Is New: An Emerging Focus on Dermatoporosis”.
Likely Pathophysiology
Advancing age and chronic ultraviolet (UV) radiation exposure are the chief drivers of dermatoporosis. In addition to UVA and UVB light, other secondary drivers include genetic susceptibility, topical and systematic corticosteroid use, and anticoagulant treatment.
Its pathogenesis is not well described in the literature but is easy to envision, Dr. Friedman said. For one, both advancing age and exposure to UV light lead to a reduction in hygroscopic glycosaminoglycans, including hyaluronate (HA), and the impact of this diminishment is believed to go “beyond [the loss of] buoyancy,” he noted. Researchers have “been showing these are not just water-loving molecules, they also have some biologic properties” relating to keratinocyte production and epidermal turnover that appear to be intricately linked to the pathogenesis of dermatoporosis.
HAs have been shown to interact with the cell surface receptor CD44 to stimulate keratinocyte proliferation, and low levels of CD44 have been reported in skin with dermatoporosis compared with a younger control population. (A newly characterized organelle, the hyaluronosome, serves as an HA factory and contains CD44 and heparin-binding epidermal growth factor, Dr. Friedman noted. Inadequate functioning may be involved in skin atrophy.)
Advancing age also brings an increase in matrix metalloproteinases (MMPs)–1, –2, and –3, which are “the demolition workers of the skin,” and downregulation of a tissue inhibitor of MMPs, he said.
Adding insult to injury, dermis-penetrating UVA also activates MMPs, “obliterating collagen and elastin.” UVB generates DNA photoproducts, including oxidative stress and damaging skin cell DNA. “That UV light induces breakdown [of the skin] through different mechanisms and inhibits buildup is a simple concept I think our patients can understand,” Dr. Friedman said.
Multifaceted Treatment
For an older adult, “there is never a wrong time to start sun-protective measures” to prevent or try to halt the progression of dermatoporosis, Dr. Friedman said, noting that “UV radiation is an immunosuppressant, so there are many good reasons to start” if the adult is not already taking measures on a regular basis.
Potential treatments for the syndrome of dermatoporosis are backed by few clinical studies, but dermatologists are skilled at translating the use of products from one disease state to another based on understandings of pathophysiology and mechanistic pathways, Dr. Friedman commented in an interview after the meeting.
For instance, “from decades of research, we know what retinoids will do to the skin,” he said in the interview. “We know they will turn on collagen-1 and -3 genes in the skin, and that they will increase the production of glycosaminoglycans ... By understanding the biology, we can translate this to dermatoporosis.” These changes were demonstrated, for instance, in a small study of topical retinol in older adults.
Studies of topical alpha hydroxy acid (AHA), moreover, have demonstrated epidermal thickening and firmness, and “some studies show they can limit steroid-induced atrophy,” Dr. Friedman said at the meeting. “And things like lactic acid and urea are super accessible.”
Topical dehydroepiandrosterone is backed by even less data than retinoids or AHAs are, “but it’s still something to consider” as part of a multimechanistic approach to dermatoporosis, Dr. Friedman shared, noting that a small study demonstrated beneficial effects on epidermal atrophy in aging skin.
The use of vitamin D analogues such as calcipotriene, which is approved for the treatment of psoriasis, may also be promising. “One concept is that [vitamin D analogues] increase calcium concentrations in the epidermis, and calcium is so central to keratinocyte differentiation” and epidermal function that calcipotriene in combination with topical steroid therapy has been shown to limit skin atrophy, he noted.
Nutritionally, low protein intake is a known problem in the older population and is associated with increased skin fragility and poorer healing. From a prevention and treatment standpoint, therefore, patients can be counseled to be attentive to their diets, Dr. Friedman said. Experts have recommended a higher protein intake for older adults than for younger adults; in 2013, an international group recommended a protein intake of 1-1.5 g/kg/d for healthy older adults and more for those with acute or chronic illness.
“Patients love talking about diet and skin disease ... and they love over-the-counter nutraceuticals as well because they want something natural,” Dr. Friedman said. “I like using bioflavonoids in combination with vitamin C, which can be effective especially for solar purpura.”
A 6-week randomized, placebo-controlled, double-blind trial involving 67 patients with purpura associated with aging found a 50% reduction in purpura lesions among those took a particular citrus bioflavonoid blend twice daily. “I thought this was a pretty well-done study,” he said, noting that patient self-assessment and investigator global assessment were utilized.
Skin Injury and Wound Prevention
In addition to recommending gentle skin cleansers and daily moisturizing, dermatologists should talk to their older patients with dermatoporosis about their home environments. “What is it like? Is there furniture with sharp edges?” Dr. Friedman advised. If so, could they use sleeves or protectors on their arms or legs “to protect against injury?”
In a later meeting session about lower-extremity wounds on geriatric patients, Michael Stempel, DPM, assistant professor of medicine and surgery and chief of podiatry at GWU, said that he was happy to hear the term dermatoporosis being used because like diabetes, it’s a risk factor for developing lower-extremity wounds and poor wound healing.
He shared the case of an older woman with dermatoporosis who “tripped and skinned her knee against a step and then self-treated it for over a month by pouring hydrogen peroxide over it and letting air get to it.” The wound developed into “full-thickness tissue loss,” said Dr. Stempel, also medical director of the Wound Healing and Limb Preservation Center at GWU Hospital.
Misperceptions are common among older patients about how a simple wound should be managed; for instance, the adage “just let it get air” is not uncommon. This makes anticipatory guidance about basic wound care — such as the importance of a moist and occlusive environment and the safe use of hydrogen peroxide — especially important for patients with dermatoporosis, Dr. Friedman commented after the meeting.
Dermatoporosis is quantifiable, Dr. Friedman said during the meeting, with a scoring system having been developed by the researchers in Switzerland who originally coined the term. Its use in practice is unnecessary, but its existence is “nice to share with patients who feel bothered because oftentimes, patients feel it’s been dismissed by other providers,” he said. “Telling your patients there’s an actual name for their problem, and that there are ways to quantify and measure changes over time, is validating.”
Its recognition as a medical condition, Dr. Friedman added, also enables the dermatologist to bring it up and counsel appropriately — without a patient feeling shame — when it is identified in the context of a skin excision, treatment of a primary inflammatory skin disease, or management of another dermatologic problem.
Dr. Friedman disclosed that he is a consultant/advisory board member for L’Oréal, La Roche-Posay, Galderma, and other companies; a speaker for Regeneron/Sanofi, Incyte, BMD, and Janssen; and has grants from Pfizer, Lilly, Incyte, and other companies. Dr. Stempel reported no disclosures.
A version of this article first appeared on Medscape.com.
WASHINGTON — and conveys the skin’s vulnerability to serious medical complications, said Adam Friedman, MD, at the ElderDerm conference on dermatology in the older patient.
Key features of dermatoporosis include atrophic skin, solar purpura, white pseudoscars, easily acquired skin lacerations and tears, bruises, and delayed healing. “We’re going to see more of this, and it will more and more be a chief complaint of patients,” said Dr. Friedman, professor and chair of dermatology at George Washington University (GWU) in Washington, and co-chair of the meeting. GWU hosted the conference, describing it as a first-of-its-kind meeting dedicated to improving dermatologic care for older adults.
Dermatoporosis was described in the literature in 2007 by dermatologists at the University of Geneva in Switzerland. “It is not only a cosmetic problem,” Dr. Friedman said. “This is a medical problem ... which can absolutely lead to comorbidities [such as deep dissecting hematomas] that are a huge strain on the healthcare system.”
Dermatologists can meet the moment with holistic, creative combination treatment and counseling approaches aimed at improving the mechanical strength of skin and preventing potential complications in older patients, Dr. Friedman said at the meeting.
He described the case of a 76-year-old woman who presented with dermatoporosis on her arms involving pronounced skin atrophy, solar purpura, and a small covered laceration. “This was a patient who was both devastated by the appearance” and impacted by the pain and burden of dressing frequent wounds, said Dr. Friedman, who is also the director of the Residency Program, of Translational Research, and of Supportive Oncodermatology, all within the Department of Dermatology at GWU.
With 11 months of topical treatment that included daily application of calcipotriene 0.05% ointment and nightly application of tazarotene 0.045% lotion and oral supplementation with 1000-mg vitamin C twice daily and 1000-mg citrus bioflavonoid complex daily, as well as no changes to the medications she took for various comorbidities, the solar purpura improved significantly and “we made a huge difference in the integrity of her skin,” he said.
Dr. Friedman also described this case in a recently published article in the Journal of Drugs in Dermatology titled “What’s Old Is New: An Emerging Focus on Dermatoporosis”.
Likely Pathophysiology
Advancing age and chronic ultraviolet (UV) radiation exposure are the chief drivers of dermatoporosis. In addition to UVA and UVB light, other secondary drivers include genetic susceptibility, topical and systematic corticosteroid use, and anticoagulant treatment.
Its pathogenesis is not well described in the literature but is easy to envision, Dr. Friedman said. For one, both advancing age and exposure to UV light lead to a reduction in hygroscopic glycosaminoglycans, including hyaluronate (HA), and the impact of this diminishment is believed to go “beyond [the loss of] buoyancy,” he noted. Researchers have “been showing these are not just water-loving molecules, they also have some biologic properties” relating to keratinocyte production and epidermal turnover that appear to be intricately linked to the pathogenesis of dermatoporosis.
HAs have been shown to interact with the cell surface receptor CD44 to stimulate keratinocyte proliferation, and low levels of CD44 have been reported in skin with dermatoporosis compared with a younger control population. (A newly characterized organelle, the hyaluronosome, serves as an HA factory and contains CD44 and heparin-binding epidermal growth factor, Dr. Friedman noted. Inadequate functioning may be involved in skin atrophy.)
Advancing age also brings an increase in matrix metalloproteinases (MMPs)–1, –2, and –3, which are “the demolition workers of the skin,” and downregulation of a tissue inhibitor of MMPs, he said.
Adding insult to injury, dermis-penetrating UVA also activates MMPs, “obliterating collagen and elastin.” UVB generates DNA photoproducts, including oxidative stress and damaging skin cell DNA. “That UV light induces breakdown [of the skin] through different mechanisms and inhibits buildup is a simple concept I think our patients can understand,” Dr. Friedman said.
Multifaceted Treatment
For an older adult, “there is never a wrong time to start sun-protective measures” to prevent or try to halt the progression of dermatoporosis, Dr. Friedman said, noting that “UV radiation is an immunosuppressant, so there are many good reasons to start” if the adult is not already taking measures on a regular basis.
Potential treatments for the syndrome of dermatoporosis are backed by few clinical studies, but dermatologists are skilled at translating the use of products from one disease state to another based on understandings of pathophysiology and mechanistic pathways, Dr. Friedman commented in an interview after the meeting.
For instance, “from decades of research, we know what retinoids will do to the skin,” he said in the interview. “We know they will turn on collagen-1 and -3 genes in the skin, and that they will increase the production of glycosaminoglycans ... By understanding the biology, we can translate this to dermatoporosis.” These changes were demonstrated, for instance, in a small study of topical retinol in older adults.
Studies of topical alpha hydroxy acid (AHA), moreover, have demonstrated epidermal thickening and firmness, and “some studies show they can limit steroid-induced atrophy,” Dr. Friedman said at the meeting. “And things like lactic acid and urea are super accessible.”
Topical dehydroepiandrosterone is backed by even less data than retinoids or AHAs are, “but it’s still something to consider” as part of a multimechanistic approach to dermatoporosis, Dr. Friedman shared, noting that a small study demonstrated beneficial effects on epidermal atrophy in aging skin.
The use of vitamin D analogues such as calcipotriene, which is approved for the treatment of psoriasis, may also be promising. “One concept is that [vitamin D analogues] increase calcium concentrations in the epidermis, and calcium is so central to keratinocyte differentiation” and epidermal function that calcipotriene in combination with topical steroid therapy has been shown to limit skin atrophy, he noted.
Nutritionally, low protein intake is a known problem in the older population and is associated with increased skin fragility and poorer healing. From a prevention and treatment standpoint, therefore, patients can be counseled to be attentive to their diets, Dr. Friedman said. Experts have recommended a higher protein intake for older adults than for younger adults; in 2013, an international group recommended a protein intake of 1-1.5 g/kg/d for healthy older adults and more for those with acute or chronic illness.
“Patients love talking about diet and skin disease ... and they love over-the-counter nutraceuticals as well because they want something natural,” Dr. Friedman said. “I like using bioflavonoids in combination with vitamin C, which can be effective especially for solar purpura.”
A 6-week randomized, placebo-controlled, double-blind trial involving 67 patients with purpura associated with aging found a 50% reduction in purpura lesions among those took a particular citrus bioflavonoid blend twice daily. “I thought this was a pretty well-done study,” he said, noting that patient self-assessment and investigator global assessment were utilized.
Skin Injury and Wound Prevention
In addition to recommending gentle skin cleansers and daily moisturizing, dermatologists should talk to their older patients with dermatoporosis about their home environments. “What is it like? Is there furniture with sharp edges?” Dr. Friedman advised. If so, could they use sleeves or protectors on their arms or legs “to protect against injury?”
In a later meeting session about lower-extremity wounds on geriatric patients, Michael Stempel, DPM, assistant professor of medicine and surgery and chief of podiatry at GWU, said that he was happy to hear the term dermatoporosis being used because like diabetes, it’s a risk factor for developing lower-extremity wounds and poor wound healing.
He shared the case of an older woman with dermatoporosis who “tripped and skinned her knee against a step and then self-treated it for over a month by pouring hydrogen peroxide over it and letting air get to it.” The wound developed into “full-thickness tissue loss,” said Dr. Stempel, also medical director of the Wound Healing and Limb Preservation Center at GWU Hospital.
Misperceptions are common among older patients about how a simple wound should be managed; for instance, the adage “just let it get air” is not uncommon. This makes anticipatory guidance about basic wound care — such as the importance of a moist and occlusive environment and the safe use of hydrogen peroxide — especially important for patients with dermatoporosis, Dr. Friedman commented after the meeting.
Dermatoporosis is quantifiable, Dr. Friedman said during the meeting, with a scoring system having been developed by the researchers in Switzerland who originally coined the term. Its use in practice is unnecessary, but its existence is “nice to share with patients who feel bothered because oftentimes, patients feel it’s been dismissed by other providers,” he said. “Telling your patients there’s an actual name for their problem, and that there are ways to quantify and measure changes over time, is validating.”
Its recognition as a medical condition, Dr. Friedman added, also enables the dermatologist to bring it up and counsel appropriately — without a patient feeling shame — when it is identified in the context of a skin excision, treatment of a primary inflammatory skin disease, or management of another dermatologic problem.
Dr. Friedman disclosed that he is a consultant/advisory board member for L’Oréal, La Roche-Posay, Galderma, and other companies; a speaker for Regeneron/Sanofi, Incyte, BMD, and Janssen; and has grants from Pfizer, Lilly, Incyte, and other companies. Dr. Stempel reported no disclosures.
A version of this article first appeared on Medscape.com.
WASHINGTON — and conveys the skin’s vulnerability to serious medical complications, said Adam Friedman, MD, at the ElderDerm conference on dermatology in the older patient.
Key features of dermatoporosis include atrophic skin, solar purpura, white pseudoscars, easily acquired skin lacerations and tears, bruises, and delayed healing. “We’re going to see more of this, and it will more and more be a chief complaint of patients,” said Dr. Friedman, professor and chair of dermatology at George Washington University (GWU) in Washington, and co-chair of the meeting. GWU hosted the conference, describing it as a first-of-its-kind meeting dedicated to improving dermatologic care for older adults.
Dermatoporosis was described in the literature in 2007 by dermatologists at the University of Geneva in Switzerland. “It is not only a cosmetic problem,” Dr. Friedman said. “This is a medical problem ... which can absolutely lead to comorbidities [such as deep dissecting hematomas] that are a huge strain on the healthcare system.”
Dermatologists can meet the moment with holistic, creative combination treatment and counseling approaches aimed at improving the mechanical strength of skin and preventing potential complications in older patients, Dr. Friedman said at the meeting.
He described the case of a 76-year-old woman who presented with dermatoporosis on her arms involving pronounced skin atrophy, solar purpura, and a small covered laceration. “This was a patient who was both devastated by the appearance” and impacted by the pain and burden of dressing frequent wounds, said Dr. Friedman, who is also the director of the Residency Program, of Translational Research, and of Supportive Oncodermatology, all within the Department of Dermatology at GWU.
With 11 months of topical treatment that included daily application of calcipotriene 0.05% ointment and nightly application of tazarotene 0.045% lotion and oral supplementation with 1000-mg vitamin C twice daily and 1000-mg citrus bioflavonoid complex daily, as well as no changes to the medications she took for various comorbidities, the solar purpura improved significantly and “we made a huge difference in the integrity of her skin,” he said.
Dr. Friedman also described this case in a recently published article in the Journal of Drugs in Dermatology titled “What’s Old Is New: An Emerging Focus on Dermatoporosis”.
Likely Pathophysiology
Advancing age and chronic ultraviolet (UV) radiation exposure are the chief drivers of dermatoporosis. In addition to UVA and UVB light, other secondary drivers include genetic susceptibility, topical and systematic corticosteroid use, and anticoagulant treatment.
Its pathogenesis is not well described in the literature but is easy to envision, Dr. Friedman said. For one, both advancing age and exposure to UV light lead to a reduction in hygroscopic glycosaminoglycans, including hyaluronate (HA), and the impact of this diminishment is believed to go “beyond [the loss of] buoyancy,” he noted. Researchers have “been showing these are not just water-loving molecules, they also have some biologic properties” relating to keratinocyte production and epidermal turnover that appear to be intricately linked to the pathogenesis of dermatoporosis.
HAs have been shown to interact with the cell surface receptor CD44 to stimulate keratinocyte proliferation, and low levels of CD44 have been reported in skin with dermatoporosis compared with a younger control population. (A newly characterized organelle, the hyaluronosome, serves as an HA factory and contains CD44 and heparin-binding epidermal growth factor, Dr. Friedman noted. Inadequate functioning may be involved in skin atrophy.)
Advancing age also brings an increase in matrix metalloproteinases (MMPs)–1, –2, and –3, which are “the demolition workers of the skin,” and downregulation of a tissue inhibitor of MMPs, he said.
Adding insult to injury, dermis-penetrating UVA also activates MMPs, “obliterating collagen and elastin.” UVB generates DNA photoproducts, including oxidative stress and damaging skin cell DNA. “That UV light induces breakdown [of the skin] through different mechanisms and inhibits buildup is a simple concept I think our patients can understand,” Dr. Friedman said.
Multifaceted Treatment
For an older adult, “there is never a wrong time to start sun-protective measures” to prevent or try to halt the progression of dermatoporosis, Dr. Friedman said, noting that “UV radiation is an immunosuppressant, so there are many good reasons to start” if the adult is not already taking measures on a regular basis.
Potential treatments for the syndrome of dermatoporosis are backed by few clinical studies, but dermatologists are skilled at translating the use of products from one disease state to another based on understandings of pathophysiology and mechanistic pathways, Dr. Friedman commented in an interview after the meeting.
For instance, “from decades of research, we know what retinoids will do to the skin,” he said in the interview. “We know they will turn on collagen-1 and -3 genes in the skin, and that they will increase the production of glycosaminoglycans ... By understanding the biology, we can translate this to dermatoporosis.” These changes were demonstrated, for instance, in a small study of topical retinol in older adults.
Studies of topical alpha hydroxy acid (AHA), moreover, have demonstrated epidermal thickening and firmness, and “some studies show they can limit steroid-induced atrophy,” Dr. Friedman said at the meeting. “And things like lactic acid and urea are super accessible.”
Topical dehydroepiandrosterone is backed by even less data than retinoids or AHAs are, “but it’s still something to consider” as part of a multimechanistic approach to dermatoporosis, Dr. Friedman shared, noting that a small study demonstrated beneficial effects on epidermal atrophy in aging skin.
The use of vitamin D analogues such as calcipotriene, which is approved for the treatment of psoriasis, may also be promising. “One concept is that [vitamin D analogues] increase calcium concentrations in the epidermis, and calcium is so central to keratinocyte differentiation” and epidermal function that calcipotriene in combination with topical steroid therapy has been shown to limit skin atrophy, he noted.
Nutritionally, low protein intake is a known problem in the older population and is associated with increased skin fragility and poorer healing. From a prevention and treatment standpoint, therefore, patients can be counseled to be attentive to their diets, Dr. Friedman said. Experts have recommended a higher protein intake for older adults than for younger adults; in 2013, an international group recommended a protein intake of 1-1.5 g/kg/d for healthy older adults and more for those with acute or chronic illness.
“Patients love talking about diet and skin disease ... and they love over-the-counter nutraceuticals as well because they want something natural,” Dr. Friedman said. “I like using bioflavonoids in combination with vitamin C, which can be effective especially for solar purpura.”
A 6-week randomized, placebo-controlled, double-blind trial involving 67 patients with purpura associated with aging found a 50% reduction in purpura lesions among those took a particular citrus bioflavonoid blend twice daily. “I thought this was a pretty well-done study,” he said, noting that patient self-assessment and investigator global assessment were utilized.
Skin Injury and Wound Prevention
In addition to recommending gentle skin cleansers and daily moisturizing, dermatologists should talk to their older patients with dermatoporosis about their home environments. “What is it like? Is there furniture with sharp edges?” Dr. Friedman advised. If so, could they use sleeves or protectors on their arms or legs “to protect against injury?”
In a later meeting session about lower-extremity wounds on geriatric patients, Michael Stempel, DPM, assistant professor of medicine and surgery and chief of podiatry at GWU, said that he was happy to hear the term dermatoporosis being used because like diabetes, it’s a risk factor for developing lower-extremity wounds and poor wound healing.
He shared the case of an older woman with dermatoporosis who “tripped and skinned her knee against a step and then self-treated it for over a month by pouring hydrogen peroxide over it and letting air get to it.” The wound developed into “full-thickness tissue loss,” said Dr. Stempel, also medical director of the Wound Healing and Limb Preservation Center at GWU Hospital.
Misperceptions are common among older patients about how a simple wound should be managed; for instance, the adage “just let it get air” is not uncommon. This makes anticipatory guidance about basic wound care — such as the importance of a moist and occlusive environment and the safe use of hydrogen peroxide — especially important for patients with dermatoporosis, Dr. Friedman commented after the meeting.
Dermatoporosis is quantifiable, Dr. Friedman said during the meeting, with a scoring system having been developed by the researchers in Switzerland who originally coined the term. Its use in practice is unnecessary, but its existence is “nice to share with patients who feel bothered because oftentimes, patients feel it’s been dismissed by other providers,” he said. “Telling your patients there’s an actual name for their problem, and that there are ways to quantify and measure changes over time, is validating.”
Its recognition as a medical condition, Dr. Friedman added, also enables the dermatologist to bring it up and counsel appropriately — without a patient feeling shame — when it is identified in the context of a skin excision, treatment of a primary inflammatory skin disease, or management of another dermatologic problem.
Dr. Friedman disclosed that he is a consultant/advisory board member for L’Oréal, La Roche-Posay, Galderma, and other companies; a speaker for Regeneron/Sanofi, Incyte, BMD, and Janssen; and has grants from Pfizer, Lilly, Incyte, and other companies. Dr. Stempel reported no disclosures.
A version of this article first appeared on Medscape.com.
FROM ELDERDERM 2024
Managing Atopic Dermatitis in Older Adults: A Common, Unique Challenge
WASHINGTON, DC — Jonathan I. Silverberg, MD, PhD, MPH, said at the ElderDerm Conference on dermatology in the older patient hosted by the George Washington University School of Medicine and Health Sciences, Washington, DC.
“I walked out of residency under the impression that if it didn’t start in the first year or two of life, it’s not AD,” said Dr. Silverberg, professor of dermatology and director of clinical research at George Washington University. “The numbers tell us a very different story.”
The prevalence of AD in the United States fluctuates between 6% and 8% through adulthood, including age categories up to 81-85 years, according to 2012 National Health Interview Survey data. And while persistence of childhood-onset AD is common, a systematic review and meta-analysis published in 2018 concluded that one in four adults with AD report adult-onset disease.
The investigators, including Dr. Silverberg, identified 25 observational studies — studies conducted across 16 countries and published during 1956-2017 — that included an analysis of age of onset beyond 10 years of age, and other inclusion criteria. Of the 25 studies, 17 reported age of onset after 16 years of age and had sufficient data for the meta-analysis. Using random-effects weighting, the investigators found a pooled proportion of adult-onset AD of 26.1% (95% CI, 16.5%-37.2%).
The research demonstrates that “the age of onset is distributed well throughout the lifespan,” Dr. Silverberg said, with the data “indicating there are many elderly-onset cases of true AD as well.” (Thirteen of the studies analyzed an age of onset from age ≥ 65, and several looked beyond age 80).
A 2021 study of a primary care database in the United Kingdom of 3.85 million children and adults found a “fascinating” bimodal distribution of incidence across the lifespan, with peaks in both infancy and older adulthood, he said. Incidence in adulthood was relatively stable from ages 18-49 years, after which, “into the 50s, 60s and beyond, you started to see a steady climb again.”
Also intriguing, Dr. Silverberg continued, are findings from a study of outpatient healthcare utilization for AD in which he and his coinvestigator analyzed data from the National Ambulatory Medical Care Survey (NAMCS). In the article, published in 2023 covering data from the 1993-2015 NAMCS, they reported that AD visits were more common among children aged 0-4 years (32.0%) and 5-9 years of age (10.6%), then decreased in adolescents aged 10-19 years (11.6%), remained fairly steady in patients aged 20-89 years (1.0%-4.7%), and increased in patients aged > 90 years (20.7%).
“The peak usage for dermatologists, primary care physicians, etc., is happening in the first few years of life, partially because that’s when the disease is more common and more severe but also partially because that’s when parents and caregivers are first learning [about] the disease and trying to understand how to gain control,” Dr. Silverberg said at the meeting, presenting data from an expanded, unpublished analysis of NAMCS data showing these same outpatient utilization patterns.
“It’s fascinating — there’s a much greater utilization in the elderly population. Why? The short answer is, we don’t know,” he said.
Risk Factors, Immune Differences
People with adult-onset AD were more likely to be women, smokers in adulthood, and have a lower childhood socioeconomic status than those whose AD started in childhood in a longitudinal study of two large birth cohorts from the United Kingdom , Dr. Silverberg pointed out.
Patients with childhood-onset AD, meanwhile, were more likely to have asthma, allergen-specific immunoglobulin E (IgE), and known genetic polymorphisms previously associated with AD. (Each cohort — the 1958 British Cohort Study and the 1970 British Cohort Study — had more than 17,000 participants who were followed from birth through middle age.)
Data is limited, but “mechanistically,” AD in much older adults appears to have a unique serum cytokine pattern, Dr. Silverberg said. He pointed to a cross-sectional study in China of 1312 children and adults with AD in which researchers analyzed clinical features, serum samples, and skin biopsy samples.
Adults aged > 60 years showed more lesions on the trunk and extensor sites of the extremities and lower levels of serum IgE and peripheral eosinophil counts than those in younger age groups. And “interestingly,” compared with healthy controls, older patients with AD had “higher levels of serum expression of a variety of cytokines, including IL [interleukin]-4 but also high TARC levels ... and a variety of cytokines related to the Th17, TH1 axes, etc.,” he said.
“So, we’re seeing a fascinating new profile that may be a little different than younger-onset cases,” he said, noting that TARC (thymus and activation-regulated chemokine) is regarded as a “decent biomarker” for AD.
In addition to higher levels of IL-4 and TARC, the study investigators reported significantly higher levels of IL-17A, IL-6, IL-22, IL-33, and thymic stromal lymphopoietin in older patients, compared with healthy controls.
Research also suggests that air pollution may play a role in the onset of AD in older age, Dr. Silverberg said, referencing a 2023 study that explored the association of air pollution and genetic risk with the onset of AD after age 50. The study analyzed 337,910 participants from the UK Biobank, with a median 12-year follow-up. Genetic risks were assessed as low, intermediate, and high, based on tertiles of polygenic risk scores. Exposure to various air pollutants was assessed using weighted quantile sum and also categorized into tertiles.
The incidence of older adult-onset AD was associated with medium and high air pollution compared with low air pollution, with hazard ratios (HRs) of 1.182 (P = .003) and 1.359 (P < .001), respectively. And “to a lesser extent,” Dr. Silverberg said, incidence was associated with medium and high genetic susceptibility, with HRs of 1.065 (P = .249) and 1.153 (P = .008).
The researchers calculated a greater population-attributable fraction of air pollution (15.5%) than genetic risk (6.4%). “This means that yes, genetics can contribute even to later-onset disease ... but environment may play an even more important role,” Dr. Silverberg said.
In the Clinic
In all patients, and especially in older adults, sleep disturbance associated with AD is a consideration for care. Data collected at the eczema clinic of Northwestern University, Chicago, Illinois, between 2014 and 2019 through previsit, self-administered questionnaires show that patients ≥ 65 years of age have more profound sleep disturbance (especially trouble staying asleep) than patients aged 18-64 years, despite having similar AD severity, said Dr. Silverberg, a coinvestigator of the study.
Older age was associated with having an increased number of nights of sleep disturbance (3-7 nights in the previous week) because of eczema (adjusted odds ratio [aOR], 2.14; 95% CI, 1.16-3.92). It was also associated with itching-attributed delays in falling asleep and nighttime awakenings in the prior 2 weeks (aOR, 1.88; 95% CI, 1.05-3.39).
“The aging population has dysregulated sleep patterns and altered circadian rhythms, so some of this is just natural predisposition,” Dr. Silverberg said. “But it’s amplified [with AD and itching], and it becomes a big clinical problem when we get into treatment because it’s our natural inclination to prescribe antihistamines for their sedative properties.”
Antihistamines can cause more profound sedation, more forgetfulness, and more anticholinergic side effects, he said, noting that “there’s some evidence that high-dose antihistamines may exacerbate dementia.”
Medication side effects and medication interactions, comorbidities, and decreased renal and hepatic clearance all can complicate treatment of AD in older adults. So can mobility, the extent of social/caregiving support, and other aspects of aging. For example, “I’m a big fan of ‘soak and smears’ ... but you have to ask, can you get out of a bathtub safely?” Dr. Silverberg said. “And you have to ask, can you reach the areas you need to [in order] to apply topicals?”
With oral Janus kinase inhibitors and other systemic medications, as with other drugs, “our older population is the most vulnerable from a safety perspective,” he said. A recently published post hoc analysis of four randomized trials of dupilumab in adults ≥ 60 years of age with moderate to severe AD demonstrated efficacy comparable with that in younger patients and “a really clean safety profile,” said Dr. Silverberg, the lead author. “We really need more of these types of post hocs to have some relative contextualization” for older adults.
Dr. Silverberg reported being a speaker for AbbVie, Eli Lilly, Leo Pharma, Pfizer, Regeneron, and Sanofi-Genzyme; a consultant and/or advisory board member for Regeneron, Sanofi-Genzyme, and other companies; and an investigator for several companies.
A version of this article first appeared on Medscape.com.
WASHINGTON, DC — Jonathan I. Silverberg, MD, PhD, MPH, said at the ElderDerm Conference on dermatology in the older patient hosted by the George Washington University School of Medicine and Health Sciences, Washington, DC.
“I walked out of residency under the impression that if it didn’t start in the first year or two of life, it’s not AD,” said Dr. Silverberg, professor of dermatology and director of clinical research at George Washington University. “The numbers tell us a very different story.”
The prevalence of AD in the United States fluctuates between 6% and 8% through adulthood, including age categories up to 81-85 years, according to 2012 National Health Interview Survey data. And while persistence of childhood-onset AD is common, a systematic review and meta-analysis published in 2018 concluded that one in four adults with AD report adult-onset disease.
The investigators, including Dr. Silverberg, identified 25 observational studies — studies conducted across 16 countries and published during 1956-2017 — that included an analysis of age of onset beyond 10 years of age, and other inclusion criteria. Of the 25 studies, 17 reported age of onset after 16 years of age and had sufficient data for the meta-analysis. Using random-effects weighting, the investigators found a pooled proportion of adult-onset AD of 26.1% (95% CI, 16.5%-37.2%).
The research demonstrates that “the age of onset is distributed well throughout the lifespan,” Dr. Silverberg said, with the data “indicating there are many elderly-onset cases of true AD as well.” (Thirteen of the studies analyzed an age of onset from age ≥ 65, and several looked beyond age 80).
A 2021 study of a primary care database in the United Kingdom of 3.85 million children and adults found a “fascinating” bimodal distribution of incidence across the lifespan, with peaks in both infancy and older adulthood, he said. Incidence in adulthood was relatively stable from ages 18-49 years, after which, “into the 50s, 60s and beyond, you started to see a steady climb again.”
Also intriguing, Dr. Silverberg continued, are findings from a study of outpatient healthcare utilization for AD in which he and his coinvestigator analyzed data from the National Ambulatory Medical Care Survey (NAMCS). In the article, published in 2023 covering data from the 1993-2015 NAMCS, they reported that AD visits were more common among children aged 0-4 years (32.0%) and 5-9 years of age (10.6%), then decreased in adolescents aged 10-19 years (11.6%), remained fairly steady in patients aged 20-89 years (1.0%-4.7%), and increased in patients aged > 90 years (20.7%).
“The peak usage for dermatologists, primary care physicians, etc., is happening in the first few years of life, partially because that’s when the disease is more common and more severe but also partially because that’s when parents and caregivers are first learning [about] the disease and trying to understand how to gain control,” Dr. Silverberg said at the meeting, presenting data from an expanded, unpublished analysis of NAMCS data showing these same outpatient utilization patterns.
“It’s fascinating — there’s a much greater utilization in the elderly population. Why? The short answer is, we don’t know,” he said.
Risk Factors, Immune Differences
People with adult-onset AD were more likely to be women, smokers in adulthood, and have a lower childhood socioeconomic status than those whose AD started in childhood in a longitudinal study of two large birth cohorts from the United Kingdom , Dr. Silverberg pointed out.
Patients with childhood-onset AD, meanwhile, were more likely to have asthma, allergen-specific immunoglobulin E (IgE), and known genetic polymorphisms previously associated with AD. (Each cohort — the 1958 British Cohort Study and the 1970 British Cohort Study — had more than 17,000 participants who were followed from birth through middle age.)
Data is limited, but “mechanistically,” AD in much older adults appears to have a unique serum cytokine pattern, Dr. Silverberg said. He pointed to a cross-sectional study in China of 1312 children and adults with AD in which researchers analyzed clinical features, serum samples, and skin biopsy samples.
Adults aged > 60 years showed more lesions on the trunk and extensor sites of the extremities and lower levels of serum IgE and peripheral eosinophil counts than those in younger age groups. And “interestingly,” compared with healthy controls, older patients with AD had “higher levels of serum expression of a variety of cytokines, including IL [interleukin]-4 but also high TARC levels ... and a variety of cytokines related to the Th17, TH1 axes, etc.,” he said.
“So, we’re seeing a fascinating new profile that may be a little different than younger-onset cases,” he said, noting that TARC (thymus and activation-regulated chemokine) is regarded as a “decent biomarker” for AD.
In addition to higher levels of IL-4 and TARC, the study investigators reported significantly higher levels of IL-17A, IL-6, IL-22, IL-33, and thymic stromal lymphopoietin in older patients, compared with healthy controls.
Research also suggests that air pollution may play a role in the onset of AD in older age, Dr. Silverberg said, referencing a 2023 study that explored the association of air pollution and genetic risk with the onset of AD after age 50. The study analyzed 337,910 participants from the UK Biobank, with a median 12-year follow-up. Genetic risks were assessed as low, intermediate, and high, based on tertiles of polygenic risk scores. Exposure to various air pollutants was assessed using weighted quantile sum and also categorized into tertiles.
The incidence of older adult-onset AD was associated with medium and high air pollution compared with low air pollution, with hazard ratios (HRs) of 1.182 (P = .003) and 1.359 (P < .001), respectively. And “to a lesser extent,” Dr. Silverberg said, incidence was associated with medium and high genetic susceptibility, with HRs of 1.065 (P = .249) and 1.153 (P = .008).
The researchers calculated a greater population-attributable fraction of air pollution (15.5%) than genetic risk (6.4%). “This means that yes, genetics can contribute even to later-onset disease ... but environment may play an even more important role,” Dr. Silverberg said.
In the Clinic
In all patients, and especially in older adults, sleep disturbance associated with AD is a consideration for care. Data collected at the eczema clinic of Northwestern University, Chicago, Illinois, between 2014 and 2019 through previsit, self-administered questionnaires show that patients ≥ 65 years of age have more profound sleep disturbance (especially trouble staying asleep) than patients aged 18-64 years, despite having similar AD severity, said Dr. Silverberg, a coinvestigator of the study.
Older age was associated with having an increased number of nights of sleep disturbance (3-7 nights in the previous week) because of eczema (adjusted odds ratio [aOR], 2.14; 95% CI, 1.16-3.92). It was also associated with itching-attributed delays in falling asleep and nighttime awakenings in the prior 2 weeks (aOR, 1.88; 95% CI, 1.05-3.39).
“The aging population has dysregulated sleep patterns and altered circadian rhythms, so some of this is just natural predisposition,” Dr. Silverberg said. “But it’s amplified [with AD and itching], and it becomes a big clinical problem when we get into treatment because it’s our natural inclination to prescribe antihistamines for their sedative properties.”
Antihistamines can cause more profound sedation, more forgetfulness, and more anticholinergic side effects, he said, noting that “there’s some evidence that high-dose antihistamines may exacerbate dementia.”
Medication side effects and medication interactions, comorbidities, and decreased renal and hepatic clearance all can complicate treatment of AD in older adults. So can mobility, the extent of social/caregiving support, and other aspects of aging. For example, “I’m a big fan of ‘soak and smears’ ... but you have to ask, can you get out of a bathtub safely?” Dr. Silverberg said. “And you have to ask, can you reach the areas you need to [in order] to apply topicals?”
With oral Janus kinase inhibitors and other systemic medications, as with other drugs, “our older population is the most vulnerable from a safety perspective,” he said. A recently published post hoc analysis of four randomized trials of dupilumab in adults ≥ 60 years of age with moderate to severe AD demonstrated efficacy comparable with that in younger patients and “a really clean safety profile,” said Dr. Silverberg, the lead author. “We really need more of these types of post hocs to have some relative contextualization” for older adults.
Dr. Silverberg reported being a speaker for AbbVie, Eli Lilly, Leo Pharma, Pfizer, Regeneron, and Sanofi-Genzyme; a consultant and/or advisory board member for Regeneron, Sanofi-Genzyme, and other companies; and an investigator for several companies.
A version of this article first appeared on Medscape.com.
WASHINGTON, DC — Jonathan I. Silverberg, MD, PhD, MPH, said at the ElderDerm Conference on dermatology in the older patient hosted by the George Washington University School of Medicine and Health Sciences, Washington, DC.
“I walked out of residency under the impression that if it didn’t start in the first year or two of life, it’s not AD,” said Dr. Silverberg, professor of dermatology and director of clinical research at George Washington University. “The numbers tell us a very different story.”
The prevalence of AD in the United States fluctuates between 6% and 8% through adulthood, including age categories up to 81-85 years, according to 2012 National Health Interview Survey data. And while persistence of childhood-onset AD is common, a systematic review and meta-analysis published in 2018 concluded that one in four adults with AD report adult-onset disease.
The investigators, including Dr. Silverberg, identified 25 observational studies — studies conducted across 16 countries and published during 1956-2017 — that included an analysis of age of onset beyond 10 years of age, and other inclusion criteria. Of the 25 studies, 17 reported age of onset after 16 years of age and had sufficient data for the meta-analysis. Using random-effects weighting, the investigators found a pooled proportion of adult-onset AD of 26.1% (95% CI, 16.5%-37.2%).
The research demonstrates that “the age of onset is distributed well throughout the lifespan,” Dr. Silverberg said, with the data “indicating there are many elderly-onset cases of true AD as well.” (Thirteen of the studies analyzed an age of onset from age ≥ 65, and several looked beyond age 80).
A 2021 study of a primary care database in the United Kingdom of 3.85 million children and adults found a “fascinating” bimodal distribution of incidence across the lifespan, with peaks in both infancy and older adulthood, he said. Incidence in adulthood was relatively stable from ages 18-49 years, after which, “into the 50s, 60s and beyond, you started to see a steady climb again.”
Also intriguing, Dr. Silverberg continued, are findings from a study of outpatient healthcare utilization for AD in which he and his coinvestigator analyzed data from the National Ambulatory Medical Care Survey (NAMCS). In the article, published in 2023 covering data from the 1993-2015 NAMCS, they reported that AD visits were more common among children aged 0-4 years (32.0%) and 5-9 years of age (10.6%), then decreased in adolescents aged 10-19 years (11.6%), remained fairly steady in patients aged 20-89 years (1.0%-4.7%), and increased in patients aged > 90 years (20.7%).
“The peak usage for dermatologists, primary care physicians, etc., is happening in the first few years of life, partially because that’s when the disease is more common and more severe but also partially because that’s when parents and caregivers are first learning [about] the disease and trying to understand how to gain control,” Dr. Silverberg said at the meeting, presenting data from an expanded, unpublished analysis of NAMCS data showing these same outpatient utilization patterns.
“It’s fascinating — there’s a much greater utilization in the elderly population. Why? The short answer is, we don’t know,” he said.
Risk Factors, Immune Differences
People with adult-onset AD were more likely to be women, smokers in adulthood, and have a lower childhood socioeconomic status than those whose AD started in childhood in a longitudinal study of two large birth cohorts from the United Kingdom , Dr. Silverberg pointed out.
Patients with childhood-onset AD, meanwhile, were more likely to have asthma, allergen-specific immunoglobulin E (IgE), and known genetic polymorphisms previously associated with AD. (Each cohort — the 1958 British Cohort Study and the 1970 British Cohort Study — had more than 17,000 participants who were followed from birth through middle age.)
Data is limited, but “mechanistically,” AD in much older adults appears to have a unique serum cytokine pattern, Dr. Silverberg said. He pointed to a cross-sectional study in China of 1312 children and adults with AD in which researchers analyzed clinical features, serum samples, and skin biopsy samples.
Adults aged > 60 years showed more lesions on the trunk and extensor sites of the extremities and lower levels of serum IgE and peripheral eosinophil counts than those in younger age groups. And “interestingly,” compared with healthy controls, older patients with AD had “higher levels of serum expression of a variety of cytokines, including IL [interleukin]-4 but also high TARC levels ... and a variety of cytokines related to the Th17, TH1 axes, etc.,” he said.
“So, we’re seeing a fascinating new profile that may be a little different than younger-onset cases,” he said, noting that TARC (thymus and activation-regulated chemokine) is regarded as a “decent biomarker” for AD.
In addition to higher levels of IL-4 and TARC, the study investigators reported significantly higher levels of IL-17A, IL-6, IL-22, IL-33, and thymic stromal lymphopoietin in older patients, compared with healthy controls.
Research also suggests that air pollution may play a role in the onset of AD in older age, Dr. Silverberg said, referencing a 2023 study that explored the association of air pollution and genetic risk with the onset of AD after age 50. The study analyzed 337,910 participants from the UK Biobank, with a median 12-year follow-up. Genetic risks were assessed as low, intermediate, and high, based on tertiles of polygenic risk scores. Exposure to various air pollutants was assessed using weighted quantile sum and also categorized into tertiles.
The incidence of older adult-onset AD was associated with medium and high air pollution compared with low air pollution, with hazard ratios (HRs) of 1.182 (P = .003) and 1.359 (P < .001), respectively. And “to a lesser extent,” Dr. Silverberg said, incidence was associated with medium and high genetic susceptibility, with HRs of 1.065 (P = .249) and 1.153 (P = .008).
The researchers calculated a greater population-attributable fraction of air pollution (15.5%) than genetic risk (6.4%). “This means that yes, genetics can contribute even to later-onset disease ... but environment may play an even more important role,” Dr. Silverberg said.
In the Clinic
In all patients, and especially in older adults, sleep disturbance associated with AD is a consideration for care. Data collected at the eczema clinic of Northwestern University, Chicago, Illinois, between 2014 and 2019 through previsit, self-administered questionnaires show that patients ≥ 65 years of age have more profound sleep disturbance (especially trouble staying asleep) than patients aged 18-64 years, despite having similar AD severity, said Dr. Silverberg, a coinvestigator of the study.
Older age was associated with having an increased number of nights of sleep disturbance (3-7 nights in the previous week) because of eczema (adjusted odds ratio [aOR], 2.14; 95% CI, 1.16-3.92). It was also associated with itching-attributed delays in falling asleep and nighttime awakenings in the prior 2 weeks (aOR, 1.88; 95% CI, 1.05-3.39).
“The aging population has dysregulated sleep patterns and altered circadian rhythms, so some of this is just natural predisposition,” Dr. Silverberg said. “But it’s amplified [with AD and itching], and it becomes a big clinical problem when we get into treatment because it’s our natural inclination to prescribe antihistamines for their sedative properties.”
Antihistamines can cause more profound sedation, more forgetfulness, and more anticholinergic side effects, he said, noting that “there’s some evidence that high-dose antihistamines may exacerbate dementia.”
Medication side effects and medication interactions, comorbidities, and decreased renal and hepatic clearance all can complicate treatment of AD in older adults. So can mobility, the extent of social/caregiving support, and other aspects of aging. For example, “I’m a big fan of ‘soak and smears’ ... but you have to ask, can you get out of a bathtub safely?” Dr. Silverberg said. “And you have to ask, can you reach the areas you need to [in order] to apply topicals?”
With oral Janus kinase inhibitors and other systemic medications, as with other drugs, “our older population is the most vulnerable from a safety perspective,” he said. A recently published post hoc analysis of four randomized trials of dupilumab in adults ≥ 60 years of age with moderate to severe AD demonstrated efficacy comparable with that in younger patients and “a really clean safety profile,” said Dr. Silverberg, the lead author. “We really need more of these types of post hocs to have some relative contextualization” for older adults.
Dr. Silverberg reported being a speaker for AbbVie, Eli Lilly, Leo Pharma, Pfizer, Regeneron, and Sanofi-Genzyme; a consultant and/or advisory board member for Regeneron, Sanofi-Genzyme, and other companies; and an investigator for several companies.
A version of this article first appeared on Medscape.com.
FROM ELDERDERM 2024
Debate: Should Dermatologists or Rheumatologists Manage Musculoskeletal Symptoms in Patients With Psoriasis?
SEATTLE — That was the subject of a debate between a dermatologist and a rheumatologist at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
Fabian Proft, MD, the rheumatologist, spoke first and emphasized the potential that MSK symptoms are a sign of psoriatic arthritis (PsA) and therefore should be managed by a rheumatologist.
“Obviously, the rheumatologist perspective [is that] I should be in the driver’s seat when taking care of patient with psoriasis and MSK symptoms, but I will still need to have a copilot there: [The dermatologist] will have a slot,” said Dr. Proft, who is a rheumatologist at Charité — Universitätsmedizin Berlin.
“It’s so important that we make the correct and early diagnosis of [psoriatic arthritis and psoriasis] symptoms,” said Dr. Proft. He specifically called out cases where patients have symptoms that are difficult to determine, whether the cause is inflammatory, and when experience with imaging can be a key factor in the diagnosis.
It’s important not to overdiagnose or overtreat patients, he said, providing an example of a patient with psoriasis who had been training for a marathon. The MRI image suggested that his Achilles tendonitis pain was related to his athletic training, not PsA-associated inflammation. “So I think this is very important that you have the knowledge to read MRIs, and especially also carefully assessing them so as not to overdiagnose patients,” said Dr. Proft.
Dermatologist Rebuttal
In her rebuttal, Laura Savage, MD, PhD, emphasized the need for more of a coequal partnership between the two specialties because of the ability of dermatologists to intervene early in the treatment and prevention of PsA.
“Traditionally, I agree rheumatologists would solely be responsible for the assessment and the management of psoriatic arthritis, but I think that paradigm has shifted in part due to the increased recognition of the need for earlier intervention to limit disease progression and to reduce or even prevent functional limitation,” said Dr. Savage, who is a consultant dermatologist at Leeds Teaching Hospitals NHS Trust and a senior lecturer at the University of Leeds, Leeds, England.
Ideally, molecular biomarkers would be available to predict the development of PsA, but there aren’t any. Still, “we have a huge biomarker in the form of the skin, and it’s recognized that the majority of patients who will develop psoriatic arthritis will have antecedent psoriasis in about 70% of cases,” Dr. Savage said. “There’s a typical time delay of around 7-12 years between the onset of the skin [disease] and the patients developing psoriatic arthritis, and so many of them are going to be into the care of other healthcare practitioners, and particularly the care of dermatologists.”
Dermatologists may also be able to play a role in the prevention of PsA, according to Dr. Savage. In one retrospective study, treatment of skin lesions with biologics was associated with a reduced frequency of progression to PsA (11.1% vs 16.4%) over 10 years (P = .0006). Studies with tumor necrosis factor inhibitors and other interventions have shown similar results.
Such findings have led to the treat intercept strategy, which targets patients with psoriasis who have risk factors for transition to PsA — such as nail pitting, gluteal cleft disease, scalp disease, type 2 diabetes, obesity, and a first-degree relative with PsA — as well as symptoms of prodromal PSA, such as arthralgia and fatigue.
“I think dermatologists are aware of the need to not leave our patients languishing on these therapies and actually escalating them onto effective treatments that may also be able to treat early psoriatic arthritis. We could be more mindful about our choice of treatments for these patients, going on to thinking about their increased risk of PSA and trying to intercept,” Dr. Savage said. “What we don’t want is our patients to be developing these musculoskeletal symptoms of pain and stiffness and functional limitation and disability. We want to be treating the patients with musculoskeletal symptoms of that earlier prodromal phase when they’re developing arthralgia and fatigue.”
She conceded that more complicated patients are good candidates for care by the rheumatologist. “You can do your fancy imaging, and we’ll leave that to you, and the difficult-to-treat patients to [the rheumatologist], but actually we need to just get on and treat them,” she said. “One could argue as well that as a dermatologist, I’m likely to broaden my horizons in terms of choice of therapy and treat all of the domains of the patient. So I would argue that actually it should be the dermatologist who is in that driving seat, particularly when it comes to the management of early psoriatic arthritis, and actually what we should be doing is driving our patients and steering them to earlier intervention and better control for all domains of disease.”
Collaborative Care
During the follow-up discussion, both Dr. Proft and Dr. Savage agreed that dermatologists and rheumatologists should be working together in managing patients. “What we need to do is steer our patients toward collaborative care with our rheumatologists by trying to minimize delays to treatment, by working together in parallel clinics, combined clinics, and on virtual [multidisciplinary teams],” said Dr. Savage.
Dr. Proft agreed. “We should join forces and make decisions together.”
Dr. Savage and Dr. Proft did not provide any financial disclosures.
A version of this article appeared on Medscape.com.
SEATTLE — That was the subject of a debate between a dermatologist and a rheumatologist at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
Fabian Proft, MD, the rheumatologist, spoke first and emphasized the potential that MSK symptoms are a sign of psoriatic arthritis (PsA) and therefore should be managed by a rheumatologist.
“Obviously, the rheumatologist perspective [is that] I should be in the driver’s seat when taking care of patient with psoriasis and MSK symptoms, but I will still need to have a copilot there: [The dermatologist] will have a slot,” said Dr. Proft, who is a rheumatologist at Charité — Universitätsmedizin Berlin.
“It’s so important that we make the correct and early diagnosis of [psoriatic arthritis and psoriasis] symptoms,” said Dr. Proft. He specifically called out cases where patients have symptoms that are difficult to determine, whether the cause is inflammatory, and when experience with imaging can be a key factor in the diagnosis.
It’s important not to overdiagnose or overtreat patients, he said, providing an example of a patient with psoriasis who had been training for a marathon. The MRI image suggested that his Achilles tendonitis pain was related to his athletic training, not PsA-associated inflammation. “So I think this is very important that you have the knowledge to read MRIs, and especially also carefully assessing them so as not to overdiagnose patients,” said Dr. Proft.
Dermatologist Rebuttal
In her rebuttal, Laura Savage, MD, PhD, emphasized the need for more of a coequal partnership between the two specialties because of the ability of dermatologists to intervene early in the treatment and prevention of PsA.
“Traditionally, I agree rheumatologists would solely be responsible for the assessment and the management of psoriatic arthritis, but I think that paradigm has shifted in part due to the increased recognition of the need for earlier intervention to limit disease progression and to reduce or even prevent functional limitation,” said Dr. Savage, who is a consultant dermatologist at Leeds Teaching Hospitals NHS Trust and a senior lecturer at the University of Leeds, Leeds, England.
Ideally, molecular biomarkers would be available to predict the development of PsA, but there aren’t any. Still, “we have a huge biomarker in the form of the skin, and it’s recognized that the majority of patients who will develop psoriatic arthritis will have antecedent psoriasis in about 70% of cases,” Dr. Savage said. “There’s a typical time delay of around 7-12 years between the onset of the skin [disease] and the patients developing psoriatic arthritis, and so many of them are going to be into the care of other healthcare practitioners, and particularly the care of dermatologists.”
Dermatologists may also be able to play a role in the prevention of PsA, according to Dr. Savage. In one retrospective study, treatment of skin lesions with biologics was associated with a reduced frequency of progression to PsA (11.1% vs 16.4%) over 10 years (P = .0006). Studies with tumor necrosis factor inhibitors and other interventions have shown similar results.
Such findings have led to the treat intercept strategy, which targets patients with psoriasis who have risk factors for transition to PsA — such as nail pitting, gluteal cleft disease, scalp disease, type 2 diabetes, obesity, and a first-degree relative with PsA — as well as symptoms of prodromal PSA, such as arthralgia and fatigue.
“I think dermatologists are aware of the need to not leave our patients languishing on these therapies and actually escalating them onto effective treatments that may also be able to treat early psoriatic arthritis. We could be more mindful about our choice of treatments for these patients, going on to thinking about their increased risk of PSA and trying to intercept,” Dr. Savage said. “What we don’t want is our patients to be developing these musculoskeletal symptoms of pain and stiffness and functional limitation and disability. We want to be treating the patients with musculoskeletal symptoms of that earlier prodromal phase when they’re developing arthralgia and fatigue.”
She conceded that more complicated patients are good candidates for care by the rheumatologist. “You can do your fancy imaging, and we’ll leave that to you, and the difficult-to-treat patients to [the rheumatologist], but actually we need to just get on and treat them,” she said. “One could argue as well that as a dermatologist, I’m likely to broaden my horizons in terms of choice of therapy and treat all of the domains of the patient. So I would argue that actually it should be the dermatologist who is in that driving seat, particularly when it comes to the management of early psoriatic arthritis, and actually what we should be doing is driving our patients and steering them to earlier intervention and better control for all domains of disease.”
Collaborative Care
During the follow-up discussion, both Dr. Proft and Dr. Savage agreed that dermatologists and rheumatologists should be working together in managing patients. “What we need to do is steer our patients toward collaborative care with our rheumatologists by trying to minimize delays to treatment, by working together in parallel clinics, combined clinics, and on virtual [multidisciplinary teams],” said Dr. Savage.
Dr. Proft agreed. “We should join forces and make decisions together.”
Dr. Savage and Dr. Proft did not provide any financial disclosures.
A version of this article appeared on Medscape.com.
SEATTLE — That was the subject of a debate between a dermatologist and a rheumatologist at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
Fabian Proft, MD, the rheumatologist, spoke first and emphasized the potential that MSK symptoms are a sign of psoriatic arthritis (PsA) and therefore should be managed by a rheumatologist.
“Obviously, the rheumatologist perspective [is that] I should be in the driver’s seat when taking care of patient with psoriasis and MSK symptoms, but I will still need to have a copilot there: [The dermatologist] will have a slot,” said Dr. Proft, who is a rheumatologist at Charité — Universitätsmedizin Berlin.
“It’s so important that we make the correct and early diagnosis of [psoriatic arthritis and psoriasis] symptoms,” said Dr. Proft. He specifically called out cases where patients have symptoms that are difficult to determine, whether the cause is inflammatory, and when experience with imaging can be a key factor in the diagnosis.
It’s important not to overdiagnose or overtreat patients, he said, providing an example of a patient with psoriasis who had been training for a marathon. The MRI image suggested that his Achilles tendonitis pain was related to his athletic training, not PsA-associated inflammation. “So I think this is very important that you have the knowledge to read MRIs, and especially also carefully assessing them so as not to overdiagnose patients,” said Dr. Proft.
Dermatologist Rebuttal
In her rebuttal, Laura Savage, MD, PhD, emphasized the need for more of a coequal partnership between the two specialties because of the ability of dermatologists to intervene early in the treatment and prevention of PsA.
“Traditionally, I agree rheumatologists would solely be responsible for the assessment and the management of psoriatic arthritis, but I think that paradigm has shifted in part due to the increased recognition of the need for earlier intervention to limit disease progression and to reduce or even prevent functional limitation,” said Dr. Savage, who is a consultant dermatologist at Leeds Teaching Hospitals NHS Trust and a senior lecturer at the University of Leeds, Leeds, England.
Ideally, molecular biomarkers would be available to predict the development of PsA, but there aren’t any. Still, “we have a huge biomarker in the form of the skin, and it’s recognized that the majority of patients who will develop psoriatic arthritis will have antecedent psoriasis in about 70% of cases,” Dr. Savage said. “There’s a typical time delay of around 7-12 years between the onset of the skin [disease] and the patients developing psoriatic arthritis, and so many of them are going to be into the care of other healthcare practitioners, and particularly the care of dermatologists.”
Dermatologists may also be able to play a role in the prevention of PsA, according to Dr. Savage. In one retrospective study, treatment of skin lesions with biologics was associated with a reduced frequency of progression to PsA (11.1% vs 16.4%) over 10 years (P = .0006). Studies with tumor necrosis factor inhibitors and other interventions have shown similar results.
Such findings have led to the treat intercept strategy, which targets patients with psoriasis who have risk factors for transition to PsA — such as nail pitting, gluteal cleft disease, scalp disease, type 2 diabetes, obesity, and a first-degree relative with PsA — as well as symptoms of prodromal PSA, such as arthralgia and fatigue.
“I think dermatologists are aware of the need to not leave our patients languishing on these therapies and actually escalating them onto effective treatments that may also be able to treat early psoriatic arthritis. We could be more mindful about our choice of treatments for these patients, going on to thinking about their increased risk of PSA and trying to intercept,” Dr. Savage said. “What we don’t want is our patients to be developing these musculoskeletal symptoms of pain and stiffness and functional limitation and disability. We want to be treating the patients with musculoskeletal symptoms of that earlier prodromal phase when they’re developing arthralgia and fatigue.”
She conceded that more complicated patients are good candidates for care by the rheumatologist. “You can do your fancy imaging, and we’ll leave that to you, and the difficult-to-treat patients to [the rheumatologist], but actually we need to just get on and treat them,” she said. “One could argue as well that as a dermatologist, I’m likely to broaden my horizons in terms of choice of therapy and treat all of the domains of the patient. So I would argue that actually it should be the dermatologist who is in that driving seat, particularly when it comes to the management of early psoriatic arthritis, and actually what we should be doing is driving our patients and steering them to earlier intervention and better control for all domains of disease.”
Collaborative Care
During the follow-up discussion, both Dr. Proft and Dr. Savage agreed that dermatologists and rheumatologists should be working together in managing patients. “What we need to do is steer our patients toward collaborative care with our rheumatologists by trying to minimize delays to treatment, by working together in parallel clinics, combined clinics, and on virtual [multidisciplinary teams],” said Dr. Savage.
Dr. Proft agreed. “We should join forces and make decisions together.”
Dr. Savage and Dr. Proft did not provide any financial disclosures.
A version of this article appeared on Medscape.com.
FROM GRAPPA 2024
Two New Studies on Benzoyl Peroxide Provide Reassuring Data on Safety
Two .
Earlier this year, controversy erupted after an independent lab Valisure petitioned the US Food and Drug Administration (FDA) to recall acne products with BP because it found extremely high levels of the carcinogen benzene. In the research, the lab directors contended that the products can form over 800 times the “conditionally restricted” FDA concentration limit of 2 parts per million (ppm) of benzene, with both prescription and over-the-counter (OTC) products affected. The issue, according to the lab’s report, is one of degradation, not contamination; BP can decompose into benzene. Exposures to benzene have been linked with a higher risk for leukemia and other blood cancers.
(“Conditionally restricted” means that the maximum of 2 ppm only applies to a drug product in which the use of benzene is unavoidable in order to produce a drug product with a significant therapeutic advance, according to FDA guidance.)
Critics of the report questioned the method used to test the products, calling for more “real-world” use data, and said the temperature used may not be what is expected with everyday use.
Now, both new studies are reassuring about the safety of the products, John Barbieri, MD, MBA, assistant professor of dermatology at Harvard Medical School and director of the Advanced Acne Therapeutics Clinic at Brigham and Women’s Hospital, Boston, said in a telephone interview. He was a coauthor of both studies. A leading dermatologist not involved in the new research reviewed the findings and agreed.
One study using data from the National Health and Nutrition Examination Survey compared blood levels of benzene between 14 people who had used BP products and 65 people without a history of BP product use, finding no difference between the groups .
The other, much larger study analyzed electronic health records of more than 27,000 patients with acne using BP products, comparing them with more than 27,000 controls who did not use the products. The patients were followed for 10 years after the use of BP products began, and no increased risk for cancer, either blood cancers or solid tumors, was found.
The studies were recently published in the Journal of the American Academy of Dermatology.
“Both studies are well done,” said Henry W. Lim, MD, former chair of the Department of Dermatology and senior vice president for academic affairs at Henry Ford Health, Detroit. Dr. Lim, a former president of the American Academy of Dermatology, reviewed the results of both studies.
“These studies indicate that [a] report of detection of benzene in [BP] products exposed to high temperature does not have any relevant clinical significance, both in terms of blood levels and in terms of internal cancer,” Dr. Lim said. “This is consistent with the clinical experience of practicing dermatologists; no internal side effects have been observed in patients using [BP products].”
Further Details
Under high temperatures, or over a long period, BP can decompose to benzene, a colorless, flammable liquid with a sweet odor. Benzene is formed from natural processes such as forest fires and volcanoes, according to the American Cancer Society, and is found in the air, cigarette smoke, some foods (at low levels), and contaminated drinking water. It’s one of the 20 widely used chemicals involved in making plastics, resins, detergents, and pesticides, among other products.
In the study evaluating blood levels, the researchers matched 14 people who used BP products currently with 65 controls who did not. Five (36%) of those using the products had detectable blood levels; 21 (32%) of those who did not use them did. There was no association between BP exposure and detectable blood benzene levels (odds ratio, 1.12; P = .80).
In the larger study, the researchers used the TriNetX US Collaborative Network database, comparing more than 27,000 patients treated with BP products for acne with more than 27,000 patients aged 12-40 years who had a diagnosis of nevus or seborrheic keratosis with no exposure to prescribed BP or any diagnosis of acne, hidradenitis suppurativa, or rosacea. The researchers looked at the database over the subsequent 10 years to determine the risk for either blood cancers or internal malignancies.
Compared with patients diagnosed with nevus or seborrheic keratosis, those with acne treated with BP had no significant difference in the risk for lymphoma (hazard ratio [HR], 1.00), leukemia (HR, 0.91), any lymphoma or leukemia (HR, 1.04), and internal malignancies (HR, 0.93).
The findings suggest no increased risk for malignancy, the researchers said, although they acknowledged study limitations, such as possible misclassification of BP exposure due to OTC availability and other issues.
Value of BP Treatments
BP is the “go-to” acne treatment, as Dr. Barbieri pointed out. “It’s probably the number one treatment for acne,” and there’s no substitute for it and it’s one of the most effective topical acne treatments, he noted.
Despite the reassuring findings, Dr. Barbieri repeated advice he gave soon after the Valisure report was released. Use common sense and don’t store BP-containing products in hot cars or other hot environments. In warmer climates, refrigeration could be considered, he said. Discard old products. Manufacturers should use cold-chain storage from the manufacturing site to retail or pharmacy sale sites, he added.
FDA and Citizen Petition Status
Asked about the status of the petition from Valisure, an FDA spokesperson said: “The FDA does not comment on the status of pending petitions.”
Dr. Barbieri and Dr. Lim had no relevant disclosures. There were no funding sources for either of the two studies.
A version of this article first appeared on Medscape.com.
Two .
Earlier this year, controversy erupted after an independent lab Valisure petitioned the US Food and Drug Administration (FDA) to recall acne products with BP because it found extremely high levels of the carcinogen benzene. In the research, the lab directors contended that the products can form over 800 times the “conditionally restricted” FDA concentration limit of 2 parts per million (ppm) of benzene, with both prescription and over-the-counter (OTC) products affected. The issue, according to the lab’s report, is one of degradation, not contamination; BP can decompose into benzene. Exposures to benzene have been linked with a higher risk for leukemia and other blood cancers.
(“Conditionally restricted” means that the maximum of 2 ppm only applies to a drug product in which the use of benzene is unavoidable in order to produce a drug product with a significant therapeutic advance, according to FDA guidance.)
Critics of the report questioned the method used to test the products, calling for more “real-world” use data, and said the temperature used may not be what is expected with everyday use.
Now, both new studies are reassuring about the safety of the products, John Barbieri, MD, MBA, assistant professor of dermatology at Harvard Medical School and director of the Advanced Acne Therapeutics Clinic at Brigham and Women’s Hospital, Boston, said in a telephone interview. He was a coauthor of both studies. A leading dermatologist not involved in the new research reviewed the findings and agreed.
One study using data from the National Health and Nutrition Examination Survey compared blood levels of benzene between 14 people who had used BP products and 65 people without a history of BP product use, finding no difference between the groups .
The other, much larger study analyzed electronic health records of more than 27,000 patients with acne using BP products, comparing them with more than 27,000 controls who did not use the products. The patients were followed for 10 years after the use of BP products began, and no increased risk for cancer, either blood cancers or solid tumors, was found.
The studies were recently published in the Journal of the American Academy of Dermatology.
“Both studies are well done,” said Henry W. Lim, MD, former chair of the Department of Dermatology and senior vice president for academic affairs at Henry Ford Health, Detroit. Dr. Lim, a former president of the American Academy of Dermatology, reviewed the results of both studies.
“These studies indicate that [a] report of detection of benzene in [BP] products exposed to high temperature does not have any relevant clinical significance, both in terms of blood levels and in terms of internal cancer,” Dr. Lim said. “This is consistent with the clinical experience of practicing dermatologists; no internal side effects have been observed in patients using [BP products].”
Further Details
Under high temperatures, or over a long period, BP can decompose to benzene, a colorless, flammable liquid with a sweet odor. Benzene is formed from natural processes such as forest fires and volcanoes, according to the American Cancer Society, and is found in the air, cigarette smoke, some foods (at low levels), and contaminated drinking water. It’s one of the 20 widely used chemicals involved in making plastics, resins, detergents, and pesticides, among other products.
In the study evaluating blood levels, the researchers matched 14 people who used BP products currently with 65 controls who did not. Five (36%) of those using the products had detectable blood levels; 21 (32%) of those who did not use them did. There was no association between BP exposure and detectable blood benzene levels (odds ratio, 1.12; P = .80).
In the larger study, the researchers used the TriNetX US Collaborative Network database, comparing more than 27,000 patients treated with BP products for acne with more than 27,000 patients aged 12-40 years who had a diagnosis of nevus or seborrheic keratosis with no exposure to prescribed BP or any diagnosis of acne, hidradenitis suppurativa, or rosacea. The researchers looked at the database over the subsequent 10 years to determine the risk for either blood cancers or internal malignancies.
Compared with patients diagnosed with nevus or seborrheic keratosis, those with acne treated with BP had no significant difference in the risk for lymphoma (hazard ratio [HR], 1.00), leukemia (HR, 0.91), any lymphoma or leukemia (HR, 1.04), and internal malignancies (HR, 0.93).
The findings suggest no increased risk for malignancy, the researchers said, although they acknowledged study limitations, such as possible misclassification of BP exposure due to OTC availability and other issues.
Value of BP Treatments
BP is the “go-to” acne treatment, as Dr. Barbieri pointed out. “It’s probably the number one treatment for acne,” and there’s no substitute for it and it’s one of the most effective topical acne treatments, he noted.
Despite the reassuring findings, Dr. Barbieri repeated advice he gave soon after the Valisure report was released. Use common sense and don’t store BP-containing products in hot cars or other hot environments. In warmer climates, refrigeration could be considered, he said. Discard old products. Manufacturers should use cold-chain storage from the manufacturing site to retail or pharmacy sale sites, he added.
FDA and Citizen Petition Status
Asked about the status of the petition from Valisure, an FDA spokesperson said: “The FDA does not comment on the status of pending petitions.”
Dr. Barbieri and Dr. Lim had no relevant disclosures. There were no funding sources for either of the two studies.
A version of this article first appeared on Medscape.com.
Two .
Earlier this year, controversy erupted after an independent lab Valisure petitioned the US Food and Drug Administration (FDA) to recall acne products with BP because it found extremely high levels of the carcinogen benzene. In the research, the lab directors contended that the products can form over 800 times the “conditionally restricted” FDA concentration limit of 2 parts per million (ppm) of benzene, with both prescription and over-the-counter (OTC) products affected. The issue, according to the lab’s report, is one of degradation, not contamination; BP can decompose into benzene. Exposures to benzene have been linked with a higher risk for leukemia and other blood cancers.
(“Conditionally restricted” means that the maximum of 2 ppm only applies to a drug product in which the use of benzene is unavoidable in order to produce a drug product with a significant therapeutic advance, according to FDA guidance.)
Critics of the report questioned the method used to test the products, calling for more “real-world” use data, and said the temperature used may not be what is expected with everyday use.
Now, both new studies are reassuring about the safety of the products, John Barbieri, MD, MBA, assistant professor of dermatology at Harvard Medical School and director of the Advanced Acne Therapeutics Clinic at Brigham and Women’s Hospital, Boston, said in a telephone interview. He was a coauthor of both studies. A leading dermatologist not involved in the new research reviewed the findings and agreed.
One study using data from the National Health and Nutrition Examination Survey compared blood levels of benzene between 14 people who had used BP products and 65 people without a history of BP product use, finding no difference between the groups .
The other, much larger study analyzed electronic health records of more than 27,000 patients with acne using BP products, comparing them with more than 27,000 controls who did not use the products. The patients were followed for 10 years after the use of BP products began, and no increased risk for cancer, either blood cancers or solid tumors, was found.
The studies were recently published in the Journal of the American Academy of Dermatology.
“Both studies are well done,” said Henry W. Lim, MD, former chair of the Department of Dermatology and senior vice president for academic affairs at Henry Ford Health, Detroit. Dr. Lim, a former president of the American Academy of Dermatology, reviewed the results of both studies.
“These studies indicate that [a] report of detection of benzene in [BP] products exposed to high temperature does not have any relevant clinical significance, both in terms of blood levels and in terms of internal cancer,” Dr. Lim said. “This is consistent with the clinical experience of practicing dermatologists; no internal side effects have been observed in patients using [BP products].”
Further Details
Under high temperatures, or over a long period, BP can decompose to benzene, a colorless, flammable liquid with a sweet odor. Benzene is formed from natural processes such as forest fires and volcanoes, according to the American Cancer Society, and is found in the air, cigarette smoke, some foods (at low levels), and contaminated drinking water. It’s one of the 20 widely used chemicals involved in making plastics, resins, detergents, and pesticides, among other products.
In the study evaluating blood levels, the researchers matched 14 people who used BP products currently with 65 controls who did not. Five (36%) of those using the products had detectable blood levels; 21 (32%) of those who did not use them did. There was no association between BP exposure and detectable blood benzene levels (odds ratio, 1.12; P = .80).
In the larger study, the researchers used the TriNetX US Collaborative Network database, comparing more than 27,000 patients treated with BP products for acne with more than 27,000 patients aged 12-40 years who had a diagnosis of nevus or seborrheic keratosis with no exposure to prescribed BP or any diagnosis of acne, hidradenitis suppurativa, or rosacea. The researchers looked at the database over the subsequent 10 years to determine the risk for either blood cancers or internal malignancies.
Compared with patients diagnosed with nevus or seborrheic keratosis, those with acne treated with BP had no significant difference in the risk for lymphoma (hazard ratio [HR], 1.00), leukemia (HR, 0.91), any lymphoma or leukemia (HR, 1.04), and internal malignancies (HR, 0.93).
The findings suggest no increased risk for malignancy, the researchers said, although they acknowledged study limitations, such as possible misclassification of BP exposure due to OTC availability and other issues.
Value of BP Treatments
BP is the “go-to” acne treatment, as Dr. Barbieri pointed out. “It’s probably the number one treatment for acne,” and there’s no substitute for it and it’s one of the most effective topical acne treatments, he noted.
Despite the reassuring findings, Dr. Barbieri repeated advice he gave soon after the Valisure report was released. Use common sense and don’t store BP-containing products in hot cars or other hot environments. In warmer climates, refrigeration could be considered, he said. Discard old products. Manufacturers should use cold-chain storage from the manufacturing site to retail or pharmacy sale sites, he added.
FDA and Citizen Petition Status
Asked about the status of the petition from Valisure, an FDA spokesperson said: “The FDA does not comment on the status of pending petitions.”
Dr. Barbieri and Dr. Lim had no relevant disclosures. There were no funding sources for either of the two studies.
A version of this article first appeared on Medscape.com.
Munchausen Syndrome by Proxy: Be Aware of Cutaneous Signs
TORONTO — Be suspicious if a child with a severe dermatologic condition is unresponsive to treatment, especially if their parent or caregiver exhibits deceptive behavior.
These could be red flags for Munchausen syndrome by proxy (MSBP), also known as factitious disorder.
“The No. 1 thing dermatologists can do in situations like this is be open to thinking outside the box and ask themselves the difficult question: Could this be something the parent is inflicting on the child,” Kelly Frasier, DO, a dermatology clinical trials and epidemiology research fellow at Northwell Health, Poughkeepsie, New York, said in an interview.
She provided a review on advancing the understanding of the dermatologic manifestations of MSBP during a poster session at the annual meeting of the Society for Pediatric Dermatology (SPD). Dr. Frasier has a particular interest in psychodermatology — she was a mental health therapist before going to medical school.
MSBP is a type of abuse intentionally inflicted by a caregiver typically on their child “for some ulterior motive,” usually to seek attention or sympathy and not for material or financial gain, explained Dr. Frasier. People with MSBP seek medical help for exaggerated or fabricated symptoms in their child. They may alter medical tests, falsify medical records, or induce symptoms in their child.
To do this, these abusers may apply any number of caustic household products, including glue, directly to the child’s skin or even in formula. Dr. Frasier shared a picture of a baby whose formula had been doctored with a caustic substance that had dripped onto his neck and face, causing a rash with blisters.
In addition to blistering, cutaneous manifestations of MSBP can include severe bruising. Or the child may present with signs similar to those of granuloma annulare (a benign condition characterized by small, raised bumps) or cicatricial pemphigoid (a rare, chronic autoimmune blistering disorder) or may have recurrent nail avulsion, purpura, or coagulopathy, said Dr. Frasier.
In almost all cases of MSBP (an estimated 96%), the abuse is inflicted by the mother, who may have a preexisting mental illness. “Usually, a psychological disorder is at play, such as depression or anxiety,” said Dr. Frasier.
Some evidence suggests that, in cases of MSBP, the caregiver may have a personality disorder such as borderline or histrionic personality disorder — or may have suffered abuse or neglect as a child or is experiencing major stress, which some evidence suggests can trigger MSPB, she added.
This type of abuse is rarely seen in children older than 6 years, likely because they get wise to what’s going on and are better able to fight back or resist as they get older, Dr. Fraser noted.
High Mortality Rate
It’s critical that cases of MSBP are identified early. While a small proportion of child abuse cases involve MSBP, the mortality rate is extremely high, about 10%, research suggests, said Dr. Frasier.
Dermatologists should be skeptical if the child’s condition hasn’t improved despite trying numerous treatments that normally would have some effect. “If you’re doing everything you can to treat something that’s usually pretty simple in terms of what you normally see clinically and how you treat it, and you’re not seeing any improvement or things continue to get worse, that’s definitely a sign something else may be going on,” Dr. Frasier said.
Another suspicious sign is inflammation that continues “for weeks or months” and “doesn’t match up with actual lab markers and lab values,” said Dr. Frasier.
Other signs of possible MSBP include evidence of chemicals in the child’s blood, stool, or urine, or the child’s condition improves while in the hospital, but symptoms return after returning home.
Also be aware of the interaction between the parent and child, said Dr. Frasier. “See if you can pick up that something else might be going on, especially if the symptoms aren’t lining up very well with what you’re physically seeing and what your clinical impression is.”
And be suspicious of a parent’s inappropriate behavior; for example, they seem to be deliberately making symptoms worse or appear overly distraught. The seemingly caring parent could be overcompensating for what she’s doing at home, “and she wants to make sure it doesn’t appear that way,” said Dr. Frasier.
To help determine if some sort of trauma is occurring at home, the child would ideally be separated from the caregiver, perhaps with a nurse or other member of the interdisciplinary medical team, Dr. Frasier said.
It appears that pediatric dermatologists are already aware of the importance of protecting children from abuse. During a presentation at the meeting on child abuse and maltreatment in dermatology, not specifically on MSBP, Romy Cho, MD, assistant professor, Department of Pediatrics, University of Toronto, who is involved with the SCAN Program at The Hospital for Sick Children, Toronto, Canada, polled the audience on whether they had ever contacted child protective services (CPS). Almost 80% said they had.
That’s good news for Dr. Frasier. “We have to be willing to contact CPS if we think there’s something going on, and be more open to that because it’s better to be safe than sorry, especially in cases involving children.”
Dr. Frasier and Dr. Cho had no relevant disclosures.
A version of this article first appeared on Medscape.com.
TORONTO — Be suspicious if a child with a severe dermatologic condition is unresponsive to treatment, especially if their parent or caregiver exhibits deceptive behavior.
These could be red flags for Munchausen syndrome by proxy (MSBP), also known as factitious disorder.
“The No. 1 thing dermatologists can do in situations like this is be open to thinking outside the box and ask themselves the difficult question: Could this be something the parent is inflicting on the child,” Kelly Frasier, DO, a dermatology clinical trials and epidemiology research fellow at Northwell Health, Poughkeepsie, New York, said in an interview.
She provided a review on advancing the understanding of the dermatologic manifestations of MSBP during a poster session at the annual meeting of the Society for Pediatric Dermatology (SPD). Dr. Frasier has a particular interest in psychodermatology — she was a mental health therapist before going to medical school.
MSBP is a type of abuse intentionally inflicted by a caregiver typically on their child “for some ulterior motive,” usually to seek attention or sympathy and not for material or financial gain, explained Dr. Frasier. People with MSBP seek medical help for exaggerated or fabricated symptoms in their child. They may alter medical tests, falsify medical records, or induce symptoms in their child.
To do this, these abusers may apply any number of caustic household products, including glue, directly to the child’s skin or even in formula. Dr. Frasier shared a picture of a baby whose formula had been doctored with a caustic substance that had dripped onto his neck and face, causing a rash with blisters.
In addition to blistering, cutaneous manifestations of MSBP can include severe bruising. Or the child may present with signs similar to those of granuloma annulare (a benign condition characterized by small, raised bumps) or cicatricial pemphigoid (a rare, chronic autoimmune blistering disorder) or may have recurrent nail avulsion, purpura, or coagulopathy, said Dr. Frasier.
In almost all cases of MSBP (an estimated 96%), the abuse is inflicted by the mother, who may have a preexisting mental illness. “Usually, a psychological disorder is at play, such as depression or anxiety,” said Dr. Frasier.
Some evidence suggests that, in cases of MSBP, the caregiver may have a personality disorder such as borderline or histrionic personality disorder — or may have suffered abuse or neglect as a child or is experiencing major stress, which some evidence suggests can trigger MSPB, she added.
This type of abuse is rarely seen in children older than 6 years, likely because they get wise to what’s going on and are better able to fight back or resist as they get older, Dr. Fraser noted.
High Mortality Rate
It’s critical that cases of MSBP are identified early. While a small proportion of child abuse cases involve MSBP, the mortality rate is extremely high, about 10%, research suggests, said Dr. Frasier.
Dermatologists should be skeptical if the child’s condition hasn’t improved despite trying numerous treatments that normally would have some effect. “If you’re doing everything you can to treat something that’s usually pretty simple in terms of what you normally see clinically and how you treat it, and you’re not seeing any improvement or things continue to get worse, that’s definitely a sign something else may be going on,” Dr. Frasier said.
Another suspicious sign is inflammation that continues “for weeks or months” and “doesn’t match up with actual lab markers and lab values,” said Dr. Frasier.
Other signs of possible MSBP include evidence of chemicals in the child’s blood, stool, or urine, or the child’s condition improves while in the hospital, but symptoms return after returning home.
Also be aware of the interaction between the parent and child, said Dr. Frasier. “See if you can pick up that something else might be going on, especially if the symptoms aren’t lining up very well with what you’re physically seeing and what your clinical impression is.”
And be suspicious of a parent’s inappropriate behavior; for example, they seem to be deliberately making symptoms worse or appear overly distraught. The seemingly caring parent could be overcompensating for what she’s doing at home, “and she wants to make sure it doesn’t appear that way,” said Dr. Frasier.
To help determine if some sort of trauma is occurring at home, the child would ideally be separated from the caregiver, perhaps with a nurse or other member of the interdisciplinary medical team, Dr. Frasier said.
It appears that pediatric dermatologists are already aware of the importance of protecting children from abuse. During a presentation at the meeting on child abuse and maltreatment in dermatology, not specifically on MSBP, Romy Cho, MD, assistant professor, Department of Pediatrics, University of Toronto, who is involved with the SCAN Program at The Hospital for Sick Children, Toronto, Canada, polled the audience on whether they had ever contacted child protective services (CPS). Almost 80% said they had.
That’s good news for Dr. Frasier. “We have to be willing to contact CPS if we think there’s something going on, and be more open to that because it’s better to be safe than sorry, especially in cases involving children.”
Dr. Frasier and Dr. Cho had no relevant disclosures.
A version of this article first appeared on Medscape.com.
TORONTO — Be suspicious if a child with a severe dermatologic condition is unresponsive to treatment, especially if their parent or caregiver exhibits deceptive behavior.
These could be red flags for Munchausen syndrome by proxy (MSBP), also known as factitious disorder.
“The No. 1 thing dermatologists can do in situations like this is be open to thinking outside the box and ask themselves the difficult question: Could this be something the parent is inflicting on the child,” Kelly Frasier, DO, a dermatology clinical trials and epidemiology research fellow at Northwell Health, Poughkeepsie, New York, said in an interview.
She provided a review on advancing the understanding of the dermatologic manifestations of MSBP during a poster session at the annual meeting of the Society for Pediatric Dermatology (SPD). Dr. Frasier has a particular interest in psychodermatology — she was a mental health therapist before going to medical school.
MSBP is a type of abuse intentionally inflicted by a caregiver typically on their child “for some ulterior motive,” usually to seek attention or sympathy and not for material or financial gain, explained Dr. Frasier. People with MSBP seek medical help for exaggerated or fabricated symptoms in their child. They may alter medical tests, falsify medical records, or induce symptoms in their child.
To do this, these abusers may apply any number of caustic household products, including glue, directly to the child’s skin or even in formula. Dr. Frasier shared a picture of a baby whose formula had been doctored with a caustic substance that had dripped onto his neck and face, causing a rash with blisters.
In addition to blistering, cutaneous manifestations of MSBP can include severe bruising. Or the child may present with signs similar to those of granuloma annulare (a benign condition characterized by small, raised bumps) or cicatricial pemphigoid (a rare, chronic autoimmune blistering disorder) or may have recurrent nail avulsion, purpura, or coagulopathy, said Dr. Frasier.
In almost all cases of MSBP (an estimated 96%), the abuse is inflicted by the mother, who may have a preexisting mental illness. “Usually, a psychological disorder is at play, such as depression or anxiety,” said Dr. Frasier.
Some evidence suggests that, in cases of MSBP, the caregiver may have a personality disorder such as borderline or histrionic personality disorder — or may have suffered abuse or neglect as a child or is experiencing major stress, which some evidence suggests can trigger MSPB, she added.
This type of abuse is rarely seen in children older than 6 years, likely because they get wise to what’s going on and are better able to fight back or resist as they get older, Dr. Fraser noted.
High Mortality Rate
It’s critical that cases of MSBP are identified early. While a small proportion of child abuse cases involve MSBP, the mortality rate is extremely high, about 10%, research suggests, said Dr. Frasier.
Dermatologists should be skeptical if the child’s condition hasn’t improved despite trying numerous treatments that normally would have some effect. “If you’re doing everything you can to treat something that’s usually pretty simple in terms of what you normally see clinically and how you treat it, and you’re not seeing any improvement or things continue to get worse, that’s definitely a sign something else may be going on,” Dr. Frasier said.
Another suspicious sign is inflammation that continues “for weeks or months” and “doesn’t match up with actual lab markers and lab values,” said Dr. Frasier.
Other signs of possible MSBP include evidence of chemicals in the child’s blood, stool, or urine, or the child’s condition improves while in the hospital, but symptoms return after returning home.
Also be aware of the interaction between the parent and child, said Dr. Frasier. “See if you can pick up that something else might be going on, especially if the symptoms aren’t lining up very well with what you’re physically seeing and what your clinical impression is.”
And be suspicious of a parent’s inappropriate behavior; for example, they seem to be deliberately making symptoms worse or appear overly distraught. The seemingly caring parent could be overcompensating for what she’s doing at home, “and she wants to make sure it doesn’t appear that way,” said Dr. Frasier.
To help determine if some sort of trauma is occurring at home, the child would ideally be separated from the caregiver, perhaps with a nurse or other member of the interdisciplinary medical team, Dr. Frasier said.
It appears that pediatric dermatologists are already aware of the importance of protecting children from abuse. During a presentation at the meeting on child abuse and maltreatment in dermatology, not specifically on MSBP, Romy Cho, MD, assistant professor, Department of Pediatrics, University of Toronto, who is involved with the SCAN Program at The Hospital for Sick Children, Toronto, Canada, polled the audience on whether they had ever contacted child protective services (CPS). Almost 80% said they had.
That’s good news for Dr. Frasier. “We have to be willing to contact CPS if we think there’s something going on, and be more open to that because it’s better to be safe than sorry, especially in cases involving children.”
Dr. Frasier and Dr. Cho had no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM SPD 2024
More Illnesses Possible Related Linked to Counterfeit Botulinum Toxin Reported
— two in the intensive care unit. None of the cases required intubation, according to an announcement of an investigation into these reports in by the Centers for Disease Control and Prevention (CDC).
The report, published online in the Morbidity and Mortality Weekly Report, notes that the four patients in Tennessee received counterfeit BoNT, while product information was not available for the three cases in New York City. “However, one person reported paying less than US wholesale acquisition cost for the administered product, and another reported that the product had been purchased overseas,” the authors of the report wrote. The development underscores that BoNT injections “should be administered only by licensed and trained providers using recommended doses of FDA [Food and Drug Admininstration]-approved products.”
This report follows a CDC advisory published in April 2024 of at least 22 people from 11 states who reported serious reactions after receiving botulinum toxin injections from unlicensed or untrained individuals or in nonhealthcare settings, such as homes and spas.
The median age of the women in the July report was 48 years, and signs and symptoms included ptosis, dry mouth, dysphagia, shortness of breath, and weakness. Onset occurred between February 23 and March 7, 2024.
“This investigation did not determine why these illnesses occurred after cosmetic BoNT injections; potential reasons might include use of counterfeit BoNT, which might be more potent or contain harmful additional ingredients or higher susceptibility to BoNT effects among some persons,” the investigators wrote. They recommended further studies to describe the clinical spectrum of cosmetic BoNT injection effects such as severity of signs and symptoms.
For cases of suspected systemic botulism, the CDC recommends calling the local or state health department for consultation and antitoxin release (as well as information on reporting adverse events). Alternatively, the 24/7 phone number for the CDC clinical botulism service is 770-488-7100.
A version of this article first appeared on Medscape.com.
— two in the intensive care unit. None of the cases required intubation, according to an announcement of an investigation into these reports in by the Centers for Disease Control and Prevention (CDC).
The report, published online in the Morbidity and Mortality Weekly Report, notes that the four patients in Tennessee received counterfeit BoNT, while product information was not available for the three cases in New York City. “However, one person reported paying less than US wholesale acquisition cost for the administered product, and another reported that the product had been purchased overseas,” the authors of the report wrote. The development underscores that BoNT injections “should be administered only by licensed and trained providers using recommended doses of FDA [Food and Drug Admininstration]-approved products.”
This report follows a CDC advisory published in April 2024 of at least 22 people from 11 states who reported serious reactions after receiving botulinum toxin injections from unlicensed or untrained individuals or in nonhealthcare settings, such as homes and spas.
The median age of the women in the July report was 48 years, and signs and symptoms included ptosis, dry mouth, dysphagia, shortness of breath, and weakness. Onset occurred between February 23 and March 7, 2024.
“This investigation did not determine why these illnesses occurred after cosmetic BoNT injections; potential reasons might include use of counterfeit BoNT, which might be more potent or contain harmful additional ingredients or higher susceptibility to BoNT effects among some persons,” the investigators wrote. They recommended further studies to describe the clinical spectrum of cosmetic BoNT injection effects such as severity of signs and symptoms.
For cases of suspected systemic botulism, the CDC recommends calling the local or state health department for consultation and antitoxin release (as well as information on reporting adverse events). Alternatively, the 24/7 phone number for the CDC clinical botulism service is 770-488-7100.
A version of this article first appeared on Medscape.com.
— two in the intensive care unit. None of the cases required intubation, according to an announcement of an investigation into these reports in by the Centers for Disease Control and Prevention (CDC).
The report, published online in the Morbidity and Mortality Weekly Report, notes that the four patients in Tennessee received counterfeit BoNT, while product information was not available for the three cases in New York City. “However, one person reported paying less than US wholesale acquisition cost for the administered product, and another reported that the product had been purchased overseas,” the authors of the report wrote. The development underscores that BoNT injections “should be administered only by licensed and trained providers using recommended doses of FDA [Food and Drug Admininstration]-approved products.”
This report follows a CDC advisory published in April 2024 of at least 22 people from 11 states who reported serious reactions after receiving botulinum toxin injections from unlicensed or untrained individuals or in nonhealthcare settings, such as homes and spas.
The median age of the women in the July report was 48 years, and signs and symptoms included ptosis, dry mouth, dysphagia, shortness of breath, and weakness. Onset occurred between February 23 and March 7, 2024.
“This investigation did not determine why these illnesses occurred after cosmetic BoNT injections; potential reasons might include use of counterfeit BoNT, which might be more potent or contain harmful additional ingredients or higher susceptibility to BoNT effects among some persons,” the investigators wrote. They recommended further studies to describe the clinical spectrum of cosmetic BoNT injection effects such as severity of signs and symptoms.
For cases of suspected systemic botulism, the CDC recommends calling the local or state health department for consultation and antitoxin release (as well as information on reporting adverse events). Alternatively, the 24/7 phone number for the CDC clinical botulism service is 770-488-7100.
A version of this article first appeared on Medscape.com.
FROM THE MMWR
Study Finds Potential benefits of Spironolactone for Women with HS
TOPLINE:
according to the results of a single-center retrospective study.
METHODOLOGY:
- This retrospective study included 157 women (median age, 36.5 years) with HS who received spironolactone for at least 3 months between 2000 and 2021 at Michigan Medicine outpatient dermatology clinics. The majority of patients were White (59%) or Black (37%) individuals.
- The median prescribed dose was 100 mg/d, the most common dose was 50-100 mg/d, and the median time spironolactone was initiated was 8.8 years after HS was diagnosed.
- Improvement status was classified on the basis of objective clinician assessments, including documented reductions in the lesion count, pain, and symptoms.
TAKEAWAY:
- Overall, 31 patients (20%) showed improvements with spironolactone treatment.
- A shorter duration between the diagnosis of HS and the initiation of spironolactone was associated with improvement (P = .047).
- Axillary involvement (P = .003), the use of intralesional steroids (P = .015), previous treatments (P = .023), and previous treatment failures (P = .030) were linked to a lack of improvement with spironolactone.
- Patients with Hurley stage III were 85% less likely to experience improvement with spironolactone (P = .036).
IN PRACTICE:
Spironolactone, which has antiandrogenic properties, “may be beneficial for patients with mild HS, notably those at Hurley stage I if implemented early as a primary or ancillary treatment,” the authors concluded, adding that prospective, multicenter studies are needed to “elucidate further the safety and efficacy of spironolactone for treating HS.”
SOURCE:
The study was led by Suma V. Gangidi, BS, Carle Illinois College of Medicine, University of Illinois Urbana-Champaign, and was published online in the International Journal of Women’s Dermatology.
LIMITATIONS:
Retrospective design can introduce inherent biases, including the potential for missing or misclassified data. Additionally, the study was conducted at a single center, which may limit the generalizability, and findings were also limited by lack of standardized objective measures for assessing treatment improvement, presence of confounding variables from concomitant treatments, small sample size, and potential multicollinearity.
DISCLOSURES:
This study did not receive any funding. One author declared ties with various pharmaceutical companies. The other authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
according to the results of a single-center retrospective study.
METHODOLOGY:
- This retrospective study included 157 women (median age, 36.5 years) with HS who received spironolactone for at least 3 months between 2000 and 2021 at Michigan Medicine outpatient dermatology clinics. The majority of patients were White (59%) or Black (37%) individuals.
- The median prescribed dose was 100 mg/d, the most common dose was 50-100 mg/d, and the median time spironolactone was initiated was 8.8 years after HS was diagnosed.
- Improvement status was classified on the basis of objective clinician assessments, including documented reductions in the lesion count, pain, and symptoms.
TAKEAWAY:
- Overall, 31 patients (20%) showed improvements with spironolactone treatment.
- A shorter duration between the diagnosis of HS and the initiation of spironolactone was associated with improvement (P = .047).
- Axillary involvement (P = .003), the use of intralesional steroids (P = .015), previous treatments (P = .023), and previous treatment failures (P = .030) were linked to a lack of improvement with spironolactone.
- Patients with Hurley stage III were 85% less likely to experience improvement with spironolactone (P = .036).
IN PRACTICE:
Spironolactone, which has antiandrogenic properties, “may be beneficial for patients with mild HS, notably those at Hurley stage I if implemented early as a primary or ancillary treatment,” the authors concluded, adding that prospective, multicenter studies are needed to “elucidate further the safety and efficacy of spironolactone for treating HS.”
SOURCE:
The study was led by Suma V. Gangidi, BS, Carle Illinois College of Medicine, University of Illinois Urbana-Champaign, and was published online in the International Journal of Women’s Dermatology.
LIMITATIONS:
Retrospective design can introduce inherent biases, including the potential for missing or misclassified data. Additionally, the study was conducted at a single center, which may limit the generalizability, and findings were also limited by lack of standardized objective measures for assessing treatment improvement, presence of confounding variables from concomitant treatments, small sample size, and potential multicollinearity.
DISCLOSURES:
This study did not receive any funding. One author declared ties with various pharmaceutical companies. The other authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
according to the results of a single-center retrospective study.
METHODOLOGY:
- This retrospective study included 157 women (median age, 36.5 years) with HS who received spironolactone for at least 3 months between 2000 and 2021 at Michigan Medicine outpatient dermatology clinics. The majority of patients were White (59%) or Black (37%) individuals.
- The median prescribed dose was 100 mg/d, the most common dose was 50-100 mg/d, and the median time spironolactone was initiated was 8.8 years after HS was diagnosed.
- Improvement status was classified on the basis of objective clinician assessments, including documented reductions in the lesion count, pain, and symptoms.
TAKEAWAY:
- Overall, 31 patients (20%) showed improvements with spironolactone treatment.
- A shorter duration between the diagnosis of HS and the initiation of spironolactone was associated with improvement (P = .047).
- Axillary involvement (P = .003), the use of intralesional steroids (P = .015), previous treatments (P = .023), and previous treatment failures (P = .030) were linked to a lack of improvement with spironolactone.
- Patients with Hurley stage III were 85% less likely to experience improvement with spironolactone (P = .036).
IN PRACTICE:
Spironolactone, which has antiandrogenic properties, “may be beneficial for patients with mild HS, notably those at Hurley stage I if implemented early as a primary or ancillary treatment,” the authors concluded, adding that prospective, multicenter studies are needed to “elucidate further the safety and efficacy of spironolactone for treating HS.”
SOURCE:
The study was led by Suma V. Gangidi, BS, Carle Illinois College of Medicine, University of Illinois Urbana-Champaign, and was published online in the International Journal of Women’s Dermatology.
LIMITATIONS:
Retrospective design can introduce inherent biases, including the potential for missing or misclassified data. Additionally, the study was conducted at a single center, which may limit the generalizability, and findings were also limited by lack of standardized objective measures for assessing treatment improvement, presence of confounding variables from concomitant treatments, small sample size, and potential multicollinearity.
DISCLOSURES:
This study did not receive any funding. One author declared ties with various pharmaceutical companies. The other authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Studies Show Dupilumab Effects In Children with Both Atopic Dermatitis and Alopecia
TOPLINE:
(AA) in a review.
METHODOLOGY:
- Researchers conducted a scoping review of seven studies, a result of a MEDLINE and Embase search on March 1, 2024, which included 31 patients aged 4-17 years with both AD and AA (average age, 11.4 years; 64.5% women).
- The review included four case reports, two case series, and one retrospective chart review.
- Patients had an average duration of AA and AD of 3.31 years and 5.33 years, respectively, before starting dupilumab.
- The type of AA was listed in 22 patients; among these patients, alopecia universalis was the most common (50%), followed by alopecia ophiasis (22.7%), patchy alopecia (18.2%), and alopecia totalis (9.09%).
TAKEAWAY:
- Overall, 77.4% of patients in the trials achieved hair regrowth with dupilumab treatment with a mean 42.6 reduction in SALT score (measuring scalp hair loss on a scale of 0-100) over an average of 3.21 months (P < .01).
- Severity of AD was reduced by an average of 2.14 units to an average of 0.857 (clear or almost clear AD; P < .01) on the AD Investigator Global Assessment dropping from an average of 3 (severe disease) before treatment.
- There were no characteristics that significantly distinguished patients with AA who responded to treatment from those who did not.
- Four patients reported worsening of preexisting AA after starting dupilumab; two of these continued dupilumab and showed improvement at subsequent follow-ups.
IN PRACTICE:
“Our review highlights the efficacy of dupilumab in pediatric AA with concurrent AD,” wrote the authors, noting that “the exact mechanism for this efficacy remains speculative.” Although there have been reports of new or worsening AA with dupilumab, they added, its “favorable safety profile in pediatrics enhances its appeal for AA treatment, as monotherapy or in combination with other AA medications.”
SOURCE:
The study was led by Dea Metko, Michael G. DeGroote School of Medicine in Hamilton, Ontario, Canada. It was published online on July 4, 2024, in Pediatric Dermatology.
LIMITATIONS:
Potential publication bias, inconsistent data reporting, the small number of patients, and short follow-up duration were the main limitations of this study.
DISCLOSURES:
The study funding source was not disclosed. One author received honoraria outside this work. Other authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
(AA) in a review.
METHODOLOGY:
- Researchers conducted a scoping review of seven studies, a result of a MEDLINE and Embase search on March 1, 2024, which included 31 patients aged 4-17 years with both AD and AA (average age, 11.4 years; 64.5% women).
- The review included four case reports, two case series, and one retrospective chart review.
- Patients had an average duration of AA and AD of 3.31 years and 5.33 years, respectively, before starting dupilumab.
- The type of AA was listed in 22 patients; among these patients, alopecia universalis was the most common (50%), followed by alopecia ophiasis (22.7%), patchy alopecia (18.2%), and alopecia totalis (9.09%).
TAKEAWAY:
- Overall, 77.4% of patients in the trials achieved hair regrowth with dupilumab treatment with a mean 42.6 reduction in SALT score (measuring scalp hair loss on a scale of 0-100) over an average of 3.21 months (P < .01).
- Severity of AD was reduced by an average of 2.14 units to an average of 0.857 (clear or almost clear AD; P < .01) on the AD Investigator Global Assessment dropping from an average of 3 (severe disease) before treatment.
- There were no characteristics that significantly distinguished patients with AA who responded to treatment from those who did not.
- Four patients reported worsening of preexisting AA after starting dupilumab; two of these continued dupilumab and showed improvement at subsequent follow-ups.
IN PRACTICE:
“Our review highlights the efficacy of dupilumab in pediatric AA with concurrent AD,” wrote the authors, noting that “the exact mechanism for this efficacy remains speculative.” Although there have been reports of new or worsening AA with dupilumab, they added, its “favorable safety profile in pediatrics enhances its appeal for AA treatment, as monotherapy or in combination with other AA medications.”
SOURCE:
The study was led by Dea Metko, Michael G. DeGroote School of Medicine in Hamilton, Ontario, Canada. It was published online on July 4, 2024, in Pediatric Dermatology.
LIMITATIONS:
Potential publication bias, inconsistent data reporting, the small number of patients, and short follow-up duration were the main limitations of this study.
DISCLOSURES:
The study funding source was not disclosed. One author received honoraria outside this work. Other authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
(AA) in a review.
METHODOLOGY:
- Researchers conducted a scoping review of seven studies, a result of a MEDLINE and Embase search on March 1, 2024, which included 31 patients aged 4-17 years with both AD and AA (average age, 11.4 years; 64.5% women).
- The review included four case reports, two case series, and one retrospective chart review.
- Patients had an average duration of AA and AD of 3.31 years and 5.33 years, respectively, before starting dupilumab.
- The type of AA was listed in 22 patients; among these patients, alopecia universalis was the most common (50%), followed by alopecia ophiasis (22.7%), patchy alopecia (18.2%), and alopecia totalis (9.09%).
TAKEAWAY:
- Overall, 77.4% of patients in the trials achieved hair regrowth with dupilumab treatment with a mean 42.6 reduction in SALT score (measuring scalp hair loss on a scale of 0-100) over an average of 3.21 months (P < .01).
- Severity of AD was reduced by an average of 2.14 units to an average of 0.857 (clear or almost clear AD; P < .01) on the AD Investigator Global Assessment dropping from an average of 3 (severe disease) before treatment.
- There were no characteristics that significantly distinguished patients with AA who responded to treatment from those who did not.
- Four patients reported worsening of preexisting AA after starting dupilumab; two of these continued dupilumab and showed improvement at subsequent follow-ups.
IN PRACTICE:
“Our review highlights the efficacy of dupilumab in pediatric AA with concurrent AD,” wrote the authors, noting that “the exact mechanism for this efficacy remains speculative.” Although there have been reports of new or worsening AA with dupilumab, they added, its “favorable safety profile in pediatrics enhances its appeal for AA treatment, as monotherapy or in combination with other AA medications.”
SOURCE:
The study was led by Dea Metko, Michael G. DeGroote School of Medicine in Hamilton, Ontario, Canada. It was published online on July 4, 2024, in Pediatric Dermatology.
LIMITATIONS:
Potential publication bias, inconsistent data reporting, the small number of patients, and short follow-up duration were the main limitations of this study.
DISCLOSURES:
The study funding source was not disclosed. One author received honoraria outside this work. Other authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Study Estimates Global Prevalence of Seborrheic Dermatitis
TOPLINE:
, according to a meta-analysis that also found a higher prevalence in adults than in children.
METHODOLOGY:
- Researchers conducted a meta-analysis of 121 studies, which included 1,260,163 people with clinician-diagnosed seborrheic dermatitis.
- The included studies represented nine countries; most were from India (n = 18), Turkey (n = 13), and the United States (n = 8).
- The primary outcome was the pooled prevalence of seborrheic dermatitis.
TAKEAWAY:
- The overall pooled prevalence of seborrheic dermatitis was 4.38%, 4.08% in clinical settings, and 4.71% in the studies conducted in the general population.
- The prevalence of seborrheic dermatitis was higher among adults (5.64%) than in children (3.7%) and neonates (0.23%).
- A significant variation was observed across countries, with South Africa having the highest prevalence at 8.82%, followed by the United States at 5.86% and Turkey at 3.74%, while India had the lowest prevalence at 2.62%.
IN PRACTICE:
The global prevalence in this meta-analysis was “higher than previous large-scale global estimates, with notable geographic and sociodemographic variability, highlighting the potential impact of environmental factors and cultural practices,” the authors wrote.
SOURCE:
The study was led by Meredith Tyree Polaskey, MS, Chicago Medical School, Rosalind Franklin University of Medicine and Science, North Chicago, Illinois, and was published online on July 3, 2024, in the JAMA Dermatology.
LIMITATIONS:
Interpretation of the findings is limited by research gaps in Central Asia, much of Sub-Saharan Africa, Eastern Europe, Southeast Asia, Latin America (excluding Brazil), and the Caribbean, along with potential underreporting in regions with restricted healthcare access and significant heterogeneity across studies.
DISCLOSURES:
Funding information was not available. One author reported serving as an advisor, consultant, speaker, and/or investigator for multiple pharmaceutical companies, including AbbVie, Amgen, and Pfizer.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
, according to a meta-analysis that also found a higher prevalence in adults than in children.
METHODOLOGY:
- Researchers conducted a meta-analysis of 121 studies, which included 1,260,163 people with clinician-diagnosed seborrheic dermatitis.
- The included studies represented nine countries; most were from India (n = 18), Turkey (n = 13), and the United States (n = 8).
- The primary outcome was the pooled prevalence of seborrheic dermatitis.
TAKEAWAY:
- The overall pooled prevalence of seborrheic dermatitis was 4.38%, 4.08% in clinical settings, and 4.71% in the studies conducted in the general population.
- The prevalence of seborrheic dermatitis was higher among adults (5.64%) than in children (3.7%) and neonates (0.23%).
- A significant variation was observed across countries, with South Africa having the highest prevalence at 8.82%, followed by the United States at 5.86% and Turkey at 3.74%, while India had the lowest prevalence at 2.62%.
IN PRACTICE:
The global prevalence in this meta-analysis was “higher than previous large-scale global estimates, with notable geographic and sociodemographic variability, highlighting the potential impact of environmental factors and cultural practices,” the authors wrote.
SOURCE:
The study was led by Meredith Tyree Polaskey, MS, Chicago Medical School, Rosalind Franklin University of Medicine and Science, North Chicago, Illinois, and was published online on July 3, 2024, in the JAMA Dermatology.
LIMITATIONS:
Interpretation of the findings is limited by research gaps in Central Asia, much of Sub-Saharan Africa, Eastern Europe, Southeast Asia, Latin America (excluding Brazil), and the Caribbean, along with potential underreporting in regions with restricted healthcare access and significant heterogeneity across studies.
DISCLOSURES:
Funding information was not available. One author reported serving as an advisor, consultant, speaker, and/or investigator for multiple pharmaceutical companies, including AbbVie, Amgen, and Pfizer.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
, according to a meta-analysis that also found a higher prevalence in adults than in children.
METHODOLOGY:
- Researchers conducted a meta-analysis of 121 studies, which included 1,260,163 people with clinician-diagnosed seborrheic dermatitis.
- The included studies represented nine countries; most were from India (n = 18), Turkey (n = 13), and the United States (n = 8).
- The primary outcome was the pooled prevalence of seborrheic dermatitis.
TAKEAWAY:
- The overall pooled prevalence of seborrheic dermatitis was 4.38%, 4.08% in clinical settings, and 4.71% in the studies conducted in the general population.
- The prevalence of seborrheic dermatitis was higher among adults (5.64%) than in children (3.7%) and neonates (0.23%).
- A significant variation was observed across countries, with South Africa having the highest prevalence at 8.82%, followed by the United States at 5.86% and Turkey at 3.74%, while India had the lowest prevalence at 2.62%.
IN PRACTICE:
The global prevalence in this meta-analysis was “higher than previous large-scale global estimates, with notable geographic and sociodemographic variability, highlighting the potential impact of environmental factors and cultural practices,” the authors wrote.
SOURCE:
The study was led by Meredith Tyree Polaskey, MS, Chicago Medical School, Rosalind Franklin University of Medicine and Science, North Chicago, Illinois, and was published online on July 3, 2024, in the JAMA Dermatology.
LIMITATIONS:
Interpretation of the findings is limited by research gaps in Central Asia, much of Sub-Saharan Africa, Eastern Europe, Southeast Asia, Latin America (excluding Brazil), and the Caribbean, along with potential underreporting in regions with restricted healthcare access and significant heterogeneity across studies.
DISCLOSURES:
Funding information was not available. One author reported serving as an advisor, consultant, speaker, and/or investigator for multiple pharmaceutical companies, including AbbVie, Amgen, and Pfizer.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
How Common Are Life-Threatening Infections In Infants with Pustules, Vesicles?
TOPLINE:
, according to the findings from a retrospective study.
METHODOLOGY:
- Researchers reviewed the electronic medical records of infants aged ≤ 60 days who received a pediatric dermatology consultation at six US academic institutions between September 2013 and August 2019.
- Among 879 consults, 183 afebrile infants were identified as having presented with pustules, vesicles, and/or bullae.
- Infectious disease workups included blood cultures, urine cultures, lumbar punctures, and HSV testing using viral skin culture, direct immunofluorescence assay, and/or polymerase chain reaction.
- Patients were categorized by gestational age as preterm (< 37 weeks), full-term (37-42 weeks), and post-term (≥ 42 weeks).
- Overall, 67.8% of infants had pustules, 31.1% had vesicles, and 10.4% had bullae.
TAKEAWAY:
- None of the cases showed positive cerebrospinal fluid or pathogenic blood cultures. In 122 of the cases (66.6%), a noninfectious cause was diagnosed, and an infectious cause was diagnosed in 71 cases (38.8%; some patients had more than one diagnosis).
- Of the 127 newborns evaluated for HSV infection, nine (7.1%) tested positive, of whom seven (5.5%) had disease affecting the skin, eye, and mouth and were full- term infants, and two (1.6%) had disseminated HSV and were preterm infants.
- Angioinvasive fungal infection was diagnosed in five infants (2.7%), all of whom were preterm infants (< 28 weeks gestational age).
- The risk for life-threatening disease was higher in preterm infants born before 32 weeks of gestational age (P < .01) compared with those born after 32 weeks.
IN PRACTICE:
“Full-term, well-appearing, afebrile infants ≤ 60 days of age presenting with pustules or vesicles may not require full SBI [serious bacterial infection] work-up, although larger studies are needed,” the authors concluded. Testing for HSV, they added, “is recommended in all infants with vesicles, grouped pustules, or pustules accompanied by punched out or grouped erosions,” and preterm infants “should be assessed for disseminated fungal infection and HSV in the setting of fluid-filled skin lesions.”
SOURCE:
The study was led by Sonora Yun, BA, Columbia University, New York City, and was published online in Pediatrics.
LIMITATIONS:
The data were limited by the sample size and very low incidence of serious infections. Infants probably had atypical or severe presentations that warranted pediatric dermatology consultation, which may have led to overrepresentation of infectious disease rates. The study inclusion was restricted to those who received a dermatology consult; therefore, the findings may not be generalizable to outpatient primary care.
DISCLOSURES:
This study did not receive any external funding. The authors declared that they had no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
, according to the findings from a retrospective study.
METHODOLOGY:
- Researchers reviewed the electronic medical records of infants aged ≤ 60 days who received a pediatric dermatology consultation at six US academic institutions between September 2013 and August 2019.
- Among 879 consults, 183 afebrile infants were identified as having presented with pustules, vesicles, and/or bullae.
- Infectious disease workups included blood cultures, urine cultures, lumbar punctures, and HSV testing using viral skin culture, direct immunofluorescence assay, and/or polymerase chain reaction.
- Patients were categorized by gestational age as preterm (< 37 weeks), full-term (37-42 weeks), and post-term (≥ 42 weeks).
- Overall, 67.8% of infants had pustules, 31.1% had vesicles, and 10.4% had bullae.
TAKEAWAY:
- None of the cases showed positive cerebrospinal fluid or pathogenic blood cultures. In 122 of the cases (66.6%), a noninfectious cause was diagnosed, and an infectious cause was diagnosed in 71 cases (38.8%; some patients had more than one diagnosis).
- Of the 127 newborns evaluated for HSV infection, nine (7.1%) tested positive, of whom seven (5.5%) had disease affecting the skin, eye, and mouth and were full- term infants, and two (1.6%) had disseminated HSV and were preterm infants.
- Angioinvasive fungal infection was diagnosed in five infants (2.7%), all of whom were preterm infants (< 28 weeks gestational age).
- The risk for life-threatening disease was higher in preterm infants born before 32 weeks of gestational age (P < .01) compared with those born after 32 weeks.
IN PRACTICE:
“Full-term, well-appearing, afebrile infants ≤ 60 days of age presenting with pustules or vesicles may not require full SBI [serious bacterial infection] work-up, although larger studies are needed,” the authors concluded. Testing for HSV, they added, “is recommended in all infants with vesicles, grouped pustules, or pustules accompanied by punched out or grouped erosions,” and preterm infants “should be assessed for disseminated fungal infection and HSV in the setting of fluid-filled skin lesions.”
SOURCE:
The study was led by Sonora Yun, BA, Columbia University, New York City, and was published online in Pediatrics.
LIMITATIONS:
The data were limited by the sample size and very low incidence of serious infections. Infants probably had atypical or severe presentations that warranted pediatric dermatology consultation, which may have led to overrepresentation of infectious disease rates. The study inclusion was restricted to those who received a dermatology consult; therefore, the findings may not be generalizable to outpatient primary care.
DISCLOSURES:
This study did not receive any external funding. The authors declared that they had no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
, according to the findings from a retrospective study.
METHODOLOGY:
- Researchers reviewed the electronic medical records of infants aged ≤ 60 days who received a pediatric dermatology consultation at six US academic institutions between September 2013 and August 2019.
- Among 879 consults, 183 afebrile infants were identified as having presented with pustules, vesicles, and/or bullae.
- Infectious disease workups included blood cultures, urine cultures, lumbar punctures, and HSV testing using viral skin culture, direct immunofluorescence assay, and/or polymerase chain reaction.
- Patients were categorized by gestational age as preterm (< 37 weeks), full-term (37-42 weeks), and post-term (≥ 42 weeks).
- Overall, 67.8% of infants had pustules, 31.1% had vesicles, and 10.4% had bullae.
TAKEAWAY:
- None of the cases showed positive cerebrospinal fluid or pathogenic blood cultures. In 122 of the cases (66.6%), a noninfectious cause was diagnosed, and an infectious cause was diagnosed in 71 cases (38.8%; some patients had more than one diagnosis).
- Of the 127 newborns evaluated for HSV infection, nine (7.1%) tested positive, of whom seven (5.5%) had disease affecting the skin, eye, and mouth and were full- term infants, and two (1.6%) had disseminated HSV and were preterm infants.
- Angioinvasive fungal infection was diagnosed in five infants (2.7%), all of whom were preterm infants (< 28 weeks gestational age).
- The risk for life-threatening disease was higher in preterm infants born before 32 weeks of gestational age (P < .01) compared with those born after 32 weeks.
IN PRACTICE:
“Full-term, well-appearing, afebrile infants ≤ 60 days of age presenting with pustules or vesicles may not require full SBI [serious bacterial infection] work-up, although larger studies are needed,” the authors concluded. Testing for HSV, they added, “is recommended in all infants with vesicles, grouped pustules, or pustules accompanied by punched out or grouped erosions,” and preterm infants “should be assessed for disseminated fungal infection and HSV in the setting of fluid-filled skin lesions.”
SOURCE:
The study was led by Sonora Yun, BA, Columbia University, New York City, and was published online in Pediatrics.
LIMITATIONS:
The data were limited by the sample size and very low incidence of serious infections. Infants probably had atypical or severe presentations that warranted pediatric dermatology consultation, which may have led to overrepresentation of infectious disease rates. The study inclusion was restricted to those who received a dermatology consult; therefore, the findings may not be generalizable to outpatient primary care.
DISCLOSURES:
This study did not receive any external funding. The authors declared that they had no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.