User login
Expert shares contact dermatitis trends
AUSTIN – Not long ago, Rajani Katta, MD, received a text message from a friend who expressed concern about a rash that developed in the underarm of her teenage daughter.
The culprit turned out to be the lavender essential oil contained in an “all natural” deodorant that her daughter had recently switched to – a storyline that Dr. Katta encounters with increasing frequency in her role as clinical professor of dermatology at the University of Texas Health Science Center at Houston.
Dr. Katta said at the annual meeting of the Society for Pediatric Dermatology. “When you talk about a natural allergy, it is more likely to occur if your skin barrier is compromised, so I think that’s why we’re seeing it, especially in young girls in the underarm area. If you shave the underarm, you impair that skin barrier and you’re more likely to develop a reaction to something you’re using over it.”
Her list of recommended deodorants includes Almay Roll-On Antiperspirant & Deodorant, Crystal Body Deodorant Stick, Crystal Roll-On Body Deodorant, Vanicream Deodorant for Sensitive Skin (aluminum-free), Vanicream Antiperspirant/Deodorant, and CertainDri Clinical Strength Roll-On. They are fragrance-free and lack propylene glycol, which is a common allergen.
Increasingly, essential oils are being added to lip balms and toothpastes, said Dr. Katta, who is also author of the 2018 book “Glow: The Dermatologist’s Guide to a Whole Foods Younger Skin Diet.” She recalled one patient who presented with chronic chapped lips. “It doesn’t matter how many lip glosses I try; it just keeps getting worse,” the patient told her. The likely culprit turned out to be ingredients contained in flavored lip balm from EOS. Reports of blistering and cracking of the lips from use of the products prompted a class action lawsuit and a notice to consumers from the Food and Drug Administration.
Another patient presented with cracked lips after switching to an “all natural” toothpaste that was labeled “gluten free.”
“It looked great,” Dr. Katta recalled. “Unfortunately it was not flavoring free. She reacted to multiple essential oils, including tea tree oil, contained in the toothpaste. This is being added to a number of toothpastes, and I think we’re going to see more of these types of reactions.”
Other toothpastes contain balsam of Peru, “which is consistently one of the top allergens in patch test clinics,” she said. “One of the components of balsam of Peru is a cinnamon compound, which can be an issue.”
Dr. Katta advises her patients to use Vaseline petroleum jelly as a lip balm and recommends Tom’s of Maine Silly Strawberry Flavor (this flavor only) toothpaste for children.
A few years ago, a teenager presented to Dr. Katta with intense bullae on the dorsum of the foot after wearing shoes without socks. “She was wearing white canvas Keds, which looked very innocuous,” she said. Patch testing revealed that the teen reacted to four different rubber accelerators. “When we contacted the company, they [acknowledged] using rubber cement to make the canvas Keds,” Dr. Katta said. “Rubber cement is an adhesive and it does contain rubber accelerators. Later, I saw two cases of children who had walked around all day at the amusement park wearing their Sperry Topsiders without any socks. We couldn’t get any information from that manufacturer, but I suspect that they also use a rubber-based glue to make those shoes.” She characterized shoes as “a real setup for a foot allergy because you have friction, sweat that’s pulling allergen out of an object, and sweat is carrying it over, especially to the dorsum of the foot.”
Dr. Katta has also noticed an uptick in the number of young patients who develop allergic reactions to dyes used to make workout clothing. “If you ever see rashes that do not involve the axillary vault but do have peraxillary accentuation, think textile allergy,” she said. “We’re seeing a lot of reactions to disperse blue clothing dyes. When you think about textile allergy from the dyes, it tends to be the blue and black clothing. It’s more likely in the setting of synthetic fabrics because they leach out dyes more easily, and it’s more likely in the setting of sweat because sweat helps pull allergen out. I’m seeing it a lot from sports uniforms and tight black leggings and tight sports bras that people are wearing. I’m also seeing some from bathing suits and swim shirts.”
Exposure to products containing the preservative methylisothiazolinone (MI) is also on the rise. It ranks as the second most frequent allergen for which the North American Contact Dermatitis Group is seeing positive results on patch testing, with rates of 13.4%. MI can be found in many skin care products and “probably about half of school glues, fabric glues, and craft glues,” Dr. Katta said. “Stick versus liquid doesn’t make a difference.” Children and teens often use craft glues, laundry detergents, and other products to create “slime” as a way to learn about viscosity, polymers, and chemical reactions. “Sometimes these children have sensitive skin, or they’re using it with prolonged contact, so they may be sensitizing themselves to the MI,” she said.
She concluded her remarks by noting that an increasing number of young patients are developing reactions to wearable medical devices such as insulin pumps and glucose monitors. “With this, the first thing to think about is frictional irritant dermatitis,” she said. “You can put Scanpor medical paper tape on people’s back for 48 hours straight to patch test them. Some people are incredibly reactive to the friction of just that tape. You also have to think about trapped allergen. One of my patients reacted to colophony, fragrance mix, and propylene glycol, all of which were contained in his skin care products. Some people are getting advice from other patients to use Mastisol liquid adhesive to help their glucose monitors stick better. Mastisol has a high rate of cross-reactivity with balsam of Peru, so it’s a fragrance allergen. That’s the first thing you want to ask patients about: what products they’re using.”
One of her patients thought she was reacting to adhesive tape on her skin, but in fact she was reacting to two different acrylates: ethylene glycol dimethacrylate (EGDMA) and hydroxyethyl methacrylate (HEMA). “I know about these allergens because I see reactions from butterfly needles in dialysis patients,” Dr. Katta explained. “What happens is, these acrylates are glues or plastics used somewhere else on the device, and they can migrate through barriers.”
In one published case, a 9-year-old boy developed a reaction to ethyl cyanoacrylate contained in a glucose sensor adhesive (Dermatitis. 2017; 28[4]:289-91). It never touched the boy’s skin directly but was presumed to migrate through that tape. “The bottom line is that acrylates may induce contact dermatitis even through perceived barriers,” she said. “Their use anywhere in medical devices may prove problematic.”
Dr. Katta reported that she is a member of the advisory board for Vichy Laboratories.
AUSTIN – Not long ago, Rajani Katta, MD, received a text message from a friend who expressed concern about a rash that developed in the underarm of her teenage daughter.
The culprit turned out to be the lavender essential oil contained in an “all natural” deodorant that her daughter had recently switched to – a storyline that Dr. Katta encounters with increasing frequency in her role as clinical professor of dermatology at the University of Texas Health Science Center at Houston.
Dr. Katta said at the annual meeting of the Society for Pediatric Dermatology. “When you talk about a natural allergy, it is more likely to occur if your skin barrier is compromised, so I think that’s why we’re seeing it, especially in young girls in the underarm area. If you shave the underarm, you impair that skin barrier and you’re more likely to develop a reaction to something you’re using over it.”
Her list of recommended deodorants includes Almay Roll-On Antiperspirant & Deodorant, Crystal Body Deodorant Stick, Crystal Roll-On Body Deodorant, Vanicream Deodorant for Sensitive Skin (aluminum-free), Vanicream Antiperspirant/Deodorant, and CertainDri Clinical Strength Roll-On. They are fragrance-free and lack propylene glycol, which is a common allergen.
Increasingly, essential oils are being added to lip balms and toothpastes, said Dr. Katta, who is also author of the 2018 book “Glow: The Dermatologist’s Guide to a Whole Foods Younger Skin Diet.” She recalled one patient who presented with chronic chapped lips. “It doesn’t matter how many lip glosses I try; it just keeps getting worse,” the patient told her. The likely culprit turned out to be ingredients contained in flavored lip balm from EOS. Reports of blistering and cracking of the lips from use of the products prompted a class action lawsuit and a notice to consumers from the Food and Drug Administration.
Another patient presented with cracked lips after switching to an “all natural” toothpaste that was labeled “gluten free.”
“It looked great,” Dr. Katta recalled. “Unfortunately it was not flavoring free. She reacted to multiple essential oils, including tea tree oil, contained in the toothpaste. This is being added to a number of toothpastes, and I think we’re going to see more of these types of reactions.”
Other toothpastes contain balsam of Peru, “which is consistently one of the top allergens in patch test clinics,” she said. “One of the components of balsam of Peru is a cinnamon compound, which can be an issue.”
Dr. Katta advises her patients to use Vaseline petroleum jelly as a lip balm and recommends Tom’s of Maine Silly Strawberry Flavor (this flavor only) toothpaste for children.
A few years ago, a teenager presented to Dr. Katta with intense bullae on the dorsum of the foot after wearing shoes without socks. “She was wearing white canvas Keds, which looked very innocuous,” she said. Patch testing revealed that the teen reacted to four different rubber accelerators. “When we contacted the company, they [acknowledged] using rubber cement to make the canvas Keds,” Dr. Katta said. “Rubber cement is an adhesive and it does contain rubber accelerators. Later, I saw two cases of children who had walked around all day at the amusement park wearing their Sperry Topsiders without any socks. We couldn’t get any information from that manufacturer, but I suspect that they also use a rubber-based glue to make those shoes.” She characterized shoes as “a real setup for a foot allergy because you have friction, sweat that’s pulling allergen out of an object, and sweat is carrying it over, especially to the dorsum of the foot.”
Dr. Katta has also noticed an uptick in the number of young patients who develop allergic reactions to dyes used to make workout clothing. “If you ever see rashes that do not involve the axillary vault but do have peraxillary accentuation, think textile allergy,” she said. “We’re seeing a lot of reactions to disperse blue clothing dyes. When you think about textile allergy from the dyes, it tends to be the blue and black clothing. It’s more likely in the setting of synthetic fabrics because they leach out dyes more easily, and it’s more likely in the setting of sweat because sweat helps pull allergen out. I’m seeing it a lot from sports uniforms and tight black leggings and tight sports bras that people are wearing. I’m also seeing some from bathing suits and swim shirts.”
Exposure to products containing the preservative methylisothiazolinone (MI) is also on the rise. It ranks as the second most frequent allergen for which the North American Contact Dermatitis Group is seeing positive results on patch testing, with rates of 13.4%. MI can be found in many skin care products and “probably about half of school glues, fabric glues, and craft glues,” Dr. Katta said. “Stick versus liquid doesn’t make a difference.” Children and teens often use craft glues, laundry detergents, and other products to create “slime” as a way to learn about viscosity, polymers, and chemical reactions. “Sometimes these children have sensitive skin, or they’re using it with prolonged contact, so they may be sensitizing themselves to the MI,” she said.
She concluded her remarks by noting that an increasing number of young patients are developing reactions to wearable medical devices such as insulin pumps and glucose monitors. “With this, the first thing to think about is frictional irritant dermatitis,” she said. “You can put Scanpor medical paper tape on people’s back for 48 hours straight to patch test them. Some people are incredibly reactive to the friction of just that tape. You also have to think about trapped allergen. One of my patients reacted to colophony, fragrance mix, and propylene glycol, all of which were contained in his skin care products. Some people are getting advice from other patients to use Mastisol liquid adhesive to help their glucose monitors stick better. Mastisol has a high rate of cross-reactivity with balsam of Peru, so it’s a fragrance allergen. That’s the first thing you want to ask patients about: what products they’re using.”
One of her patients thought she was reacting to adhesive tape on her skin, but in fact she was reacting to two different acrylates: ethylene glycol dimethacrylate (EGDMA) and hydroxyethyl methacrylate (HEMA). “I know about these allergens because I see reactions from butterfly needles in dialysis patients,” Dr. Katta explained. “What happens is, these acrylates are glues or plastics used somewhere else on the device, and they can migrate through barriers.”
In one published case, a 9-year-old boy developed a reaction to ethyl cyanoacrylate contained in a glucose sensor adhesive (Dermatitis. 2017; 28[4]:289-91). It never touched the boy’s skin directly but was presumed to migrate through that tape. “The bottom line is that acrylates may induce contact dermatitis even through perceived barriers,” she said. “Their use anywhere in medical devices may prove problematic.”
Dr. Katta reported that she is a member of the advisory board for Vichy Laboratories.
AUSTIN – Not long ago, Rajani Katta, MD, received a text message from a friend who expressed concern about a rash that developed in the underarm of her teenage daughter.
The culprit turned out to be the lavender essential oil contained in an “all natural” deodorant that her daughter had recently switched to – a storyline that Dr. Katta encounters with increasing frequency in her role as clinical professor of dermatology at the University of Texas Health Science Center at Houston.
Dr. Katta said at the annual meeting of the Society for Pediatric Dermatology. “When you talk about a natural allergy, it is more likely to occur if your skin barrier is compromised, so I think that’s why we’re seeing it, especially in young girls in the underarm area. If you shave the underarm, you impair that skin barrier and you’re more likely to develop a reaction to something you’re using over it.”
Her list of recommended deodorants includes Almay Roll-On Antiperspirant & Deodorant, Crystal Body Deodorant Stick, Crystal Roll-On Body Deodorant, Vanicream Deodorant for Sensitive Skin (aluminum-free), Vanicream Antiperspirant/Deodorant, and CertainDri Clinical Strength Roll-On. They are fragrance-free and lack propylene glycol, which is a common allergen.
Increasingly, essential oils are being added to lip balms and toothpastes, said Dr. Katta, who is also author of the 2018 book “Glow: The Dermatologist’s Guide to a Whole Foods Younger Skin Diet.” She recalled one patient who presented with chronic chapped lips. “It doesn’t matter how many lip glosses I try; it just keeps getting worse,” the patient told her. The likely culprit turned out to be ingredients contained in flavored lip balm from EOS. Reports of blistering and cracking of the lips from use of the products prompted a class action lawsuit and a notice to consumers from the Food and Drug Administration.
Another patient presented with cracked lips after switching to an “all natural” toothpaste that was labeled “gluten free.”
“It looked great,” Dr. Katta recalled. “Unfortunately it was not flavoring free. She reacted to multiple essential oils, including tea tree oil, contained in the toothpaste. This is being added to a number of toothpastes, and I think we’re going to see more of these types of reactions.”
Other toothpastes contain balsam of Peru, “which is consistently one of the top allergens in patch test clinics,” she said. “One of the components of balsam of Peru is a cinnamon compound, which can be an issue.”
Dr. Katta advises her patients to use Vaseline petroleum jelly as a lip balm and recommends Tom’s of Maine Silly Strawberry Flavor (this flavor only) toothpaste for children.
A few years ago, a teenager presented to Dr. Katta with intense bullae on the dorsum of the foot after wearing shoes without socks. “She was wearing white canvas Keds, which looked very innocuous,” she said. Patch testing revealed that the teen reacted to four different rubber accelerators. “When we contacted the company, they [acknowledged] using rubber cement to make the canvas Keds,” Dr. Katta said. “Rubber cement is an adhesive and it does contain rubber accelerators. Later, I saw two cases of children who had walked around all day at the amusement park wearing their Sperry Topsiders without any socks. We couldn’t get any information from that manufacturer, but I suspect that they also use a rubber-based glue to make those shoes.” She characterized shoes as “a real setup for a foot allergy because you have friction, sweat that’s pulling allergen out of an object, and sweat is carrying it over, especially to the dorsum of the foot.”
Dr. Katta has also noticed an uptick in the number of young patients who develop allergic reactions to dyes used to make workout clothing. “If you ever see rashes that do not involve the axillary vault but do have peraxillary accentuation, think textile allergy,” she said. “We’re seeing a lot of reactions to disperse blue clothing dyes. When you think about textile allergy from the dyes, it tends to be the blue and black clothing. It’s more likely in the setting of synthetic fabrics because they leach out dyes more easily, and it’s more likely in the setting of sweat because sweat helps pull allergen out. I’m seeing it a lot from sports uniforms and tight black leggings and tight sports bras that people are wearing. I’m also seeing some from bathing suits and swim shirts.”
Exposure to products containing the preservative methylisothiazolinone (MI) is also on the rise. It ranks as the second most frequent allergen for which the North American Contact Dermatitis Group is seeing positive results on patch testing, with rates of 13.4%. MI can be found in many skin care products and “probably about half of school glues, fabric glues, and craft glues,” Dr. Katta said. “Stick versus liquid doesn’t make a difference.” Children and teens often use craft glues, laundry detergents, and other products to create “slime” as a way to learn about viscosity, polymers, and chemical reactions. “Sometimes these children have sensitive skin, or they’re using it with prolonged contact, so they may be sensitizing themselves to the MI,” she said.
She concluded her remarks by noting that an increasing number of young patients are developing reactions to wearable medical devices such as insulin pumps and glucose monitors. “With this, the first thing to think about is frictional irritant dermatitis,” she said. “You can put Scanpor medical paper tape on people’s back for 48 hours straight to patch test them. Some people are incredibly reactive to the friction of just that tape. You also have to think about trapped allergen. One of my patients reacted to colophony, fragrance mix, and propylene glycol, all of which were contained in his skin care products. Some people are getting advice from other patients to use Mastisol liquid adhesive to help their glucose monitors stick better. Mastisol has a high rate of cross-reactivity with balsam of Peru, so it’s a fragrance allergen. That’s the first thing you want to ask patients about: what products they’re using.”
One of her patients thought she was reacting to adhesive tape on her skin, but in fact she was reacting to two different acrylates: ethylene glycol dimethacrylate (EGDMA) and hydroxyethyl methacrylate (HEMA). “I know about these allergens because I see reactions from butterfly needles in dialysis patients,” Dr. Katta explained. “What happens is, these acrylates are glues or plastics used somewhere else on the device, and they can migrate through barriers.”
In one published case, a 9-year-old boy developed a reaction to ethyl cyanoacrylate contained in a glucose sensor adhesive (Dermatitis. 2017; 28[4]:289-91). It never touched the boy’s skin directly but was presumed to migrate through that tape. “The bottom line is that acrylates may induce contact dermatitis even through perceived barriers,” she said. “Their use anywhere in medical devices may prove problematic.”
Dr. Katta reported that she is a member of the advisory board for Vichy Laboratories.
EXPERT ANALYSIS FROM SPD 2019
Cases of pediatric invasive melanoma have declined since 2002, study finds
AUSTIN – The compared with females. The risk of death is also significantly increased in black patients, other nonwhite patients, and in cases where surgery was not performed.
Those are key findings from a study that set out to investigate the incidence of pediatric melanoma over the last 2 decades and factors influencing survival. At the annual meeting of the Society for Pediatric Dermatology, one of the study authors, Spandana Maddukuri, said that pediatric melanoma is the most common skin cancer in the pediatric population, accounting for 1-3% of all pediatric malignancies and 1%-4% of all cases of melanoma (Pediatr Surg. 2013;48[11]:2207-13).
“Nonmodifiable risk factors are similar to those in adult melanoma and include fair skin, light hair and eye color, increased number of congenital nevi, and family history of melanoma,” said Ms. Maddukuri, a third-year student at New Jersey Medical School, Newark. “Environmental risk factors are similar to those in adult melanoma and include exposure to UV radiation. About 60% of children do not meet standard ABCDE [asymmetrical, border, color, diameter, evolving] diagnosis criteria, which often leads to delayed diagnosis.”
Some of the characteristics that are more commonly found in pediatric lesions include amelanosis, bleeding, uniform color, and variable diameter (J Am Acad Dermatol. 2013; 68[6]:913-25).
Ms. Maddukuri and colleagues queried the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) database for cases of malignant melanoma that were diagnosed in individuals aged younger than 20 years between 2002 and 2015. After excluding all cases of adult melanoma and all cases of in situ melanoma, they included 1,620 patients in the final analysis and divided them into five age groups: less than 1 year, 1-4 years, 5-9 years, 10-14 years, and 15-19 years. They calculated the overall incidence rate per 100,000 population of pediatric melanoma based on data from the 2000 U.S. Census. Age-, sex-, and race-specific incidence rates were also calculated. Kaplan-Meier and Cox regression analyses to investigate disease-specific survival and risk factors.
With each successive age group, the investigators observed that incidence rate was significantly higher than that of the previous age group (P less than .005). “However, the most striking increase in incidence occurs between the age group of 10-14 and 15-19,” she said. “Sex also influenced incidence rates. Males had an incidence rate of 0.396 per 100,000 population while females had an incidence rate of 0.579 per 100,000 population.”
Race also influenced incidence rates. White patients had the highest incidence rate of 0.605 per 100,000 population, while blacks had the lowest incident rate at 0.042 per 100,000 population. American Indian and Alaska Native patients had incidence rates of 0.046 per 100,000 population, while Asians and Pacific Islanders had an incidence rate of 0.127 per 100,000 population.
The researchers found that increased survival was associated with white race, female sex, treatment with surgical intervention, and age older than 5 years. No differences in survival were observed regarding the primary anatomic location or extent of disease. The hazard ratio of death from invasive melanoma was significantly increased in males (HR, 2.34), black patients (HR, 3.96), other nonwhite patients (HR, 3.64), and in cases where surgery was not performed (HR, 6.04).
“It is surprising that, although incidence is significantly higher in white patients and females, compared to black patients and males, respectively, the risk of dying from melanoma is much higher in black patients and males,” Ms. Maddukuri said in an interview at the meeting. “Overall, the dermatologic community is on the right track in screening and diagnosing pediatric melanoma, as seen by the decreased incidence over the last 2 decades. However, increased awareness regarding pediatric melanoma is still encouraged. I believe we were able to identify certain populations that need more attention in terms of screening, diagnosis, and treatment, which are patients less than 5 years old, black and other nonwhite patients, and males.”
She acknowledged certain shortcomings of the study, including a limited clinical history of the patient population because of the nature of the database. She also said that further studies are required to investigate the contributing factors to decreasing incidence and to evaluate the relationship of the favorable prognostic factors to increased survival. The researchers are currently working on correlating incidence rates with UV exposure and geographical location.
They reported having no financial disclosures.
AUSTIN – The compared with females. The risk of death is also significantly increased in black patients, other nonwhite patients, and in cases where surgery was not performed.
Those are key findings from a study that set out to investigate the incidence of pediatric melanoma over the last 2 decades and factors influencing survival. At the annual meeting of the Society for Pediatric Dermatology, one of the study authors, Spandana Maddukuri, said that pediatric melanoma is the most common skin cancer in the pediatric population, accounting for 1-3% of all pediatric malignancies and 1%-4% of all cases of melanoma (Pediatr Surg. 2013;48[11]:2207-13).
“Nonmodifiable risk factors are similar to those in adult melanoma and include fair skin, light hair and eye color, increased number of congenital nevi, and family history of melanoma,” said Ms. Maddukuri, a third-year student at New Jersey Medical School, Newark. “Environmental risk factors are similar to those in adult melanoma and include exposure to UV radiation. About 60% of children do not meet standard ABCDE [asymmetrical, border, color, diameter, evolving] diagnosis criteria, which often leads to delayed diagnosis.”
Some of the characteristics that are more commonly found in pediatric lesions include amelanosis, bleeding, uniform color, and variable diameter (J Am Acad Dermatol. 2013; 68[6]:913-25).
Ms. Maddukuri and colleagues queried the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) database for cases of malignant melanoma that were diagnosed in individuals aged younger than 20 years between 2002 and 2015. After excluding all cases of adult melanoma and all cases of in situ melanoma, they included 1,620 patients in the final analysis and divided them into five age groups: less than 1 year, 1-4 years, 5-9 years, 10-14 years, and 15-19 years. They calculated the overall incidence rate per 100,000 population of pediatric melanoma based on data from the 2000 U.S. Census. Age-, sex-, and race-specific incidence rates were also calculated. Kaplan-Meier and Cox regression analyses to investigate disease-specific survival and risk factors.
With each successive age group, the investigators observed that incidence rate was significantly higher than that of the previous age group (P less than .005). “However, the most striking increase in incidence occurs between the age group of 10-14 and 15-19,” she said. “Sex also influenced incidence rates. Males had an incidence rate of 0.396 per 100,000 population while females had an incidence rate of 0.579 per 100,000 population.”
Race also influenced incidence rates. White patients had the highest incidence rate of 0.605 per 100,000 population, while blacks had the lowest incident rate at 0.042 per 100,000 population. American Indian and Alaska Native patients had incidence rates of 0.046 per 100,000 population, while Asians and Pacific Islanders had an incidence rate of 0.127 per 100,000 population.
The researchers found that increased survival was associated with white race, female sex, treatment with surgical intervention, and age older than 5 years. No differences in survival were observed regarding the primary anatomic location or extent of disease. The hazard ratio of death from invasive melanoma was significantly increased in males (HR, 2.34), black patients (HR, 3.96), other nonwhite patients (HR, 3.64), and in cases where surgery was not performed (HR, 6.04).
“It is surprising that, although incidence is significantly higher in white patients and females, compared to black patients and males, respectively, the risk of dying from melanoma is much higher in black patients and males,” Ms. Maddukuri said in an interview at the meeting. “Overall, the dermatologic community is on the right track in screening and diagnosing pediatric melanoma, as seen by the decreased incidence over the last 2 decades. However, increased awareness regarding pediatric melanoma is still encouraged. I believe we were able to identify certain populations that need more attention in terms of screening, diagnosis, and treatment, which are patients less than 5 years old, black and other nonwhite patients, and males.”
She acknowledged certain shortcomings of the study, including a limited clinical history of the patient population because of the nature of the database. She also said that further studies are required to investigate the contributing factors to decreasing incidence and to evaluate the relationship of the favorable prognostic factors to increased survival. The researchers are currently working on correlating incidence rates with UV exposure and geographical location.
They reported having no financial disclosures.
AUSTIN – The compared with females. The risk of death is also significantly increased in black patients, other nonwhite patients, and in cases where surgery was not performed.
Those are key findings from a study that set out to investigate the incidence of pediatric melanoma over the last 2 decades and factors influencing survival. At the annual meeting of the Society for Pediatric Dermatology, one of the study authors, Spandana Maddukuri, said that pediatric melanoma is the most common skin cancer in the pediatric population, accounting for 1-3% of all pediatric malignancies and 1%-4% of all cases of melanoma (Pediatr Surg. 2013;48[11]:2207-13).
“Nonmodifiable risk factors are similar to those in adult melanoma and include fair skin, light hair and eye color, increased number of congenital nevi, and family history of melanoma,” said Ms. Maddukuri, a third-year student at New Jersey Medical School, Newark. “Environmental risk factors are similar to those in adult melanoma and include exposure to UV radiation. About 60% of children do not meet standard ABCDE [asymmetrical, border, color, diameter, evolving] diagnosis criteria, which often leads to delayed diagnosis.”
Some of the characteristics that are more commonly found in pediatric lesions include amelanosis, bleeding, uniform color, and variable diameter (J Am Acad Dermatol. 2013; 68[6]:913-25).
Ms. Maddukuri and colleagues queried the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) database for cases of malignant melanoma that were diagnosed in individuals aged younger than 20 years between 2002 and 2015. After excluding all cases of adult melanoma and all cases of in situ melanoma, they included 1,620 patients in the final analysis and divided them into five age groups: less than 1 year, 1-4 years, 5-9 years, 10-14 years, and 15-19 years. They calculated the overall incidence rate per 100,000 population of pediatric melanoma based on data from the 2000 U.S. Census. Age-, sex-, and race-specific incidence rates were also calculated. Kaplan-Meier and Cox regression analyses to investigate disease-specific survival and risk factors.
With each successive age group, the investigators observed that incidence rate was significantly higher than that of the previous age group (P less than .005). “However, the most striking increase in incidence occurs between the age group of 10-14 and 15-19,” she said. “Sex also influenced incidence rates. Males had an incidence rate of 0.396 per 100,000 population while females had an incidence rate of 0.579 per 100,000 population.”
Race also influenced incidence rates. White patients had the highest incidence rate of 0.605 per 100,000 population, while blacks had the lowest incident rate at 0.042 per 100,000 population. American Indian and Alaska Native patients had incidence rates of 0.046 per 100,000 population, while Asians and Pacific Islanders had an incidence rate of 0.127 per 100,000 population.
The researchers found that increased survival was associated with white race, female sex, treatment with surgical intervention, and age older than 5 years. No differences in survival were observed regarding the primary anatomic location or extent of disease. The hazard ratio of death from invasive melanoma was significantly increased in males (HR, 2.34), black patients (HR, 3.96), other nonwhite patients (HR, 3.64), and in cases where surgery was not performed (HR, 6.04).
“It is surprising that, although incidence is significantly higher in white patients and females, compared to black patients and males, respectively, the risk of dying from melanoma is much higher in black patients and males,” Ms. Maddukuri said in an interview at the meeting. “Overall, the dermatologic community is on the right track in screening and diagnosing pediatric melanoma, as seen by the decreased incidence over the last 2 decades. However, increased awareness regarding pediatric melanoma is still encouraged. I believe we were able to identify certain populations that need more attention in terms of screening, diagnosis, and treatment, which are patients less than 5 years old, black and other nonwhite patients, and males.”
She acknowledged certain shortcomings of the study, including a limited clinical history of the patient population because of the nature of the database. She also said that further studies are required to investigate the contributing factors to decreasing incidence and to evaluate the relationship of the favorable prognostic factors to increased survival. The researchers are currently working on correlating incidence rates with UV exposure and geographical location.
They reported having no financial disclosures.
REPORTING FROM SPD 2019
Online ped-derm searches: What are folks looking for?
AUSTIN – After searching online for information about a suspected pediatric dermatologic condition, one in five parents and/or pediatric patients make dermatology appointments sooner than they normally would, results from a novel survey showed.
In an interview at the annual meeting of the Society for Pediatric Dermatology, study author Jamie P. Schlarbaum noted that about one-third of Americans use the Internet to research their condition or symptoms prior to visiting a physician, mostly through Google. “While nearly 50% of parents look up health care information online for their children, rashes were the most common search in pediatrics in 2011,” said Mr. Schlarbaum, who is a fourth-year medical student at the University of Minnesota, Minneapolis. “However, no studies have examined the characteristics and implications of these searches; our study is the first in pediatric dermatology and also adds a new dimension to concern in an online era: How these searches influence health care behaviors.”
During February 2018–February 2019, Kristen Hook, MD, a pediatric dermatologist in Minneapolis and the study’s principal investigator, and Mr. Schlarbaum administered a survey to 220 parents/guardians and pediatric patients who had appointments in pediatric dermatology at a University of Minnesota clinic. The survey consisted of questions about demographics, search tools, search terms, and health care decisions based on this information.
Of the 220 respondents, more than half (59%) did not use an online search engine/tool prior to their appointment. Compared with parents who did not use an online search tool, those who did were slightly younger (34 vs. 36 years, respectively), more likely to be college educated (68% vs. 48%), and less likely to have the patient in question be their first child (37% vs. 52%).
Google ranked as the most common search engine used by the survey respondents (92%), followed distantly by WebMD (18%). About 15% of respondents became more concerned about the pediatric skin condition after searching online, and 20% made appointments sooner because of the information they gleaned from their searches. “Online dermatology clearly has an influence on care today,” Mr. Schlarbaum said. “As we become an even more technologically advanced and dependent society, we anticipate that both of these numbers will grow.”
The researchers also found that (33%), moles (15%), and infections (11%). “The big takeaway [from this study] is to ask your parents and teenagers if they’ve looked up information online,” Mr. Schlarbaum said. “Whether it’s photos of the ‘worst cases’ or concerning differentials that might pop up, it’s worth it to take a few seconds to ask what they’re worried about and why.”
He acknowledged certain limitations of the study, including its small sample size and single-center design. The researchers reported having no financial disclosures.
AUSTIN – After searching online for information about a suspected pediatric dermatologic condition, one in five parents and/or pediatric patients make dermatology appointments sooner than they normally would, results from a novel survey showed.
In an interview at the annual meeting of the Society for Pediatric Dermatology, study author Jamie P. Schlarbaum noted that about one-third of Americans use the Internet to research their condition or symptoms prior to visiting a physician, mostly through Google. “While nearly 50% of parents look up health care information online for their children, rashes were the most common search in pediatrics in 2011,” said Mr. Schlarbaum, who is a fourth-year medical student at the University of Minnesota, Minneapolis. “However, no studies have examined the characteristics and implications of these searches; our study is the first in pediatric dermatology and also adds a new dimension to concern in an online era: How these searches influence health care behaviors.”
During February 2018–February 2019, Kristen Hook, MD, a pediatric dermatologist in Minneapolis and the study’s principal investigator, and Mr. Schlarbaum administered a survey to 220 parents/guardians and pediatric patients who had appointments in pediatric dermatology at a University of Minnesota clinic. The survey consisted of questions about demographics, search tools, search terms, and health care decisions based on this information.
Of the 220 respondents, more than half (59%) did not use an online search engine/tool prior to their appointment. Compared with parents who did not use an online search tool, those who did were slightly younger (34 vs. 36 years, respectively), more likely to be college educated (68% vs. 48%), and less likely to have the patient in question be their first child (37% vs. 52%).
Google ranked as the most common search engine used by the survey respondents (92%), followed distantly by WebMD (18%). About 15% of respondents became more concerned about the pediatric skin condition after searching online, and 20% made appointments sooner because of the information they gleaned from their searches. “Online dermatology clearly has an influence on care today,” Mr. Schlarbaum said. “As we become an even more technologically advanced and dependent society, we anticipate that both of these numbers will grow.”
The researchers also found that (33%), moles (15%), and infections (11%). “The big takeaway [from this study] is to ask your parents and teenagers if they’ve looked up information online,” Mr. Schlarbaum said. “Whether it’s photos of the ‘worst cases’ or concerning differentials that might pop up, it’s worth it to take a few seconds to ask what they’re worried about and why.”
He acknowledged certain limitations of the study, including its small sample size and single-center design. The researchers reported having no financial disclosures.
AUSTIN – After searching online for information about a suspected pediatric dermatologic condition, one in five parents and/or pediatric patients make dermatology appointments sooner than they normally would, results from a novel survey showed.
In an interview at the annual meeting of the Society for Pediatric Dermatology, study author Jamie P. Schlarbaum noted that about one-third of Americans use the Internet to research their condition or symptoms prior to visiting a physician, mostly through Google. “While nearly 50% of parents look up health care information online for their children, rashes were the most common search in pediatrics in 2011,” said Mr. Schlarbaum, who is a fourth-year medical student at the University of Minnesota, Minneapolis. “However, no studies have examined the characteristics and implications of these searches; our study is the first in pediatric dermatology and also adds a new dimension to concern in an online era: How these searches influence health care behaviors.”
During February 2018–February 2019, Kristen Hook, MD, a pediatric dermatologist in Minneapolis and the study’s principal investigator, and Mr. Schlarbaum administered a survey to 220 parents/guardians and pediatric patients who had appointments in pediatric dermatology at a University of Minnesota clinic. The survey consisted of questions about demographics, search tools, search terms, and health care decisions based on this information.
Of the 220 respondents, more than half (59%) did not use an online search engine/tool prior to their appointment. Compared with parents who did not use an online search tool, those who did were slightly younger (34 vs. 36 years, respectively), more likely to be college educated (68% vs. 48%), and less likely to have the patient in question be their first child (37% vs. 52%).
Google ranked as the most common search engine used by the survey respondents (92%), followed distantly by WebMD (18%). About 15% of respondents became more concerned about the pediatric skin condition after searching online, and 20% made appointments sooner because of the information they gleaned from their searches. “Online dermatology clearly has an influence on care today,” Mr. Schlarbaum said. “As we become an even more technologically advanced and dependent society, we anticipate that both of these numbers will grow.”
The researchers also found that (33%), moles (15%), and infections (11%). “The big takeaway [from this study] is to ask your parents and teenagers if they’ve looked up information online,” Mr. Schlarbaum said. “Whether it’s photos of the ‘worst cases’ or concerning differentials that might pop up, it’s worth it to take a few seconds to ask what they’re worried about and why.”
He acknowledged certain limitations of the study, including its small sample size and single-center design. The researchers reported having no financial disclosures.
REPORTING FROM SPD 2019
Collagen powder deemed noninferior to primary closure for punch-biopsy healing
Collagen powder may be noninferior to primary closure for healing punch biopsy–induced wounds and possibly leads to improved early cosmetic outcomes and accelerated wound maturation, according to Azam Qureshi of the University of Maryland, Baltimore, and associates.
In a small pilot study published in Journal of Drugs in Dermatology, eight volunteers (mean age, 37 years) received a 4-mm punch biopsy on each thigh. One wound was managed with primary closure, the other with daily application of collagen powder. The wounds were biopsied at 4 weeks for histopathologic analysis, and the study subjects rated pain, itch, and treatment preferences at 1, 2, 4, 6, and 12 weeks.
The size of wounds treated with collagen was reduced by 28.95% at 1 week, 55.76% at 2 weeks, and 95.94% at 4 weeks; six of the eight collagen-treated wounds were completely healed at 4 weeks. Wound size was reduced by 75.71% 1 week after the second biopsy, much faster than the initial healing. In addition to collagen, one patient required hyfrecation for hemostasis, which did not affect results; three of the eight subjects rated the collagen treatment as “annoying,” but no one rated it as “difficult,” and patients generally regarded collagen treatment as more time consuming.
The histopathologic analysis showed epidermal reepithelialization in collagen-treated wounds and wounds managed with primary closure, with more organized granulation tissue in the collagen-treated wounds. Similar pain and itch ratings were reported between wound types, and both patients and blinded dermatologists observing the study preferred the appearance of collagen-treated wounds.
“Future research elucidating the optimal duration of collagen therapy is needed, as less than 4 weeks may be sufficient. Shortened treatment courses would decrease the cost and effort required by patients. Future studies should also investigate the efficacy of collagen powder in healing larger wounds and in comparison to healing by secondary intention,” the investigators wrote.
CPN Biosciences funded the study. No authors had relevant financial disclosures.
SOURCE: Qureshi A et al. J Drug Dermatol. 2019;18(7):667-73
Collagen powder may be noninferior to primary closure for healing punch biopsy–induced wounds and possibly leads to improved early cosmetic outcomes and accelerated wound maturation, according to Azam Qureshi of the University of Maryland, Baltimore, and associates.
In a small pilot study published in Journal of Drugs in Dermatology, eight volunteers (mean age, 37 years) received a 4-mm punch biopsy on each thigh. One wound was managed with primary closure, the other with daily application of collagen powder. The wounds were biopsied at 4 weeks for histopathologic analysis, and the study subjects rated pain, itch, and treatment preferences at 1, 2, 4, 6, and 12 weeks.
The size of wounds treated with collagen was reduced by 28.95% at 1 week, 55.76% at 2 weeks, and 95.94% at 4 weeks; six of the eight collagen-treated wounds were completely healed at 4 weeks. Wound size was reduced by 75.71% 1 week after the second biopsy, much faster than the initial healing. In addition to collagen, one patient required hyfrecation for hemostasis, which did not affect results; three of the eight subjects rated the collagen treatment as “annoying,” but no one rated it as “difficult,” and patients generally regarded collagen treatment as more time consuming.
The histopathologic analysis showed epidermal reepithelialization in collagen-treated wounds and wounds managed with primary closure, with more organized granulation tissue in the collagen-treated wounds. Similar pain and itch ratings were reported between wound types, and both patients and blinded dermatologists observing the study preferred the appearance of collagen-treated wounds.
“Future research elucidating the optimal duration of collagen therapy is needed, as less than 4 weeks may be sufficient. Shortened treatment courses would decrease the cost and effort required by patients. Future studies should also investigate the efficacy of collagen powder in healing larger wounds and in comparison to healing by secondary intention,” the investigators wrote.
CPN Biosciences funded the study. No authors had relevant financial disclosures.
SOURCE: Qureshi A et al. J Drug Dermatol. 2019;18(7):667-73
Collagen powder may be noninferior to primary closure for healing punch biopsy–induced wounds and possibly leads to improved early cosmetic outcomes and accelerated wound maturation, according to Azam Qureshi of the University of Maryland, Baltimore, and associates.
In a small pilot study published in Journal of Drugs in Dermatology, eight volunteers (mean age, 37 years) received a 4-mm punch biopsy on each thigh. One wound was managed with primary closure, the other with daily application of collagen powder. The wounds were biopsied at 4 weeks for histopathologic analysis, and the study subjects rated pain, itch, and treatment preferences at 1, 2, 4, 6, and 12 weeks.
The size of wounds treated with collagen was reduced by 28.95% at 1 week, 55.76% at 2 weeks, and 95.94% at 4 weeks; six of the eight collagen-treated wounds were completely healed at 4 weeks. Wound size was reduced by 75.71% 1 week after the second biopsy, much faster than the initial healing. In addition to collagen, one patient required hyfrecation for hemostasis, which did not affect results; three of the eight subjects rated the collagen treatment as “annoying,” but no one rated it as “difficult,” and patients generally regarded collagen treatment as more time consuming.
The histopathologic analysis showed epidermal reepithelialization in collagen-treated wounds and wounds managed with primary closure, with more organized granulation tissue in the collagen-treated wounds. Similar pain and itch ratings were reported between wound types, and both patients and blinded dermatologists observing the study preferred the appearance of collagen-treated wounds.
“Future research elucidating the optimal duration of collagen therapy is needed, as less than 4 weeks may be sufficient. Shortened treatment courses would decrease the cost and effort required by patients. Future studies should also investigate the efficacy of collagen powder in healing larger wounds and in comparison to healing by secondary intention,” the investigators wrote.
CPN Biosciences funded the study. No authors had relevant financial disclosures.
SOURCE: Qureshi A et al. J Drug Dermatol. 2019;18(7):667-73
FROM JOURNAL OF DRUGS IN DERMATOLOGY
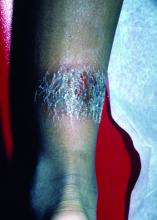
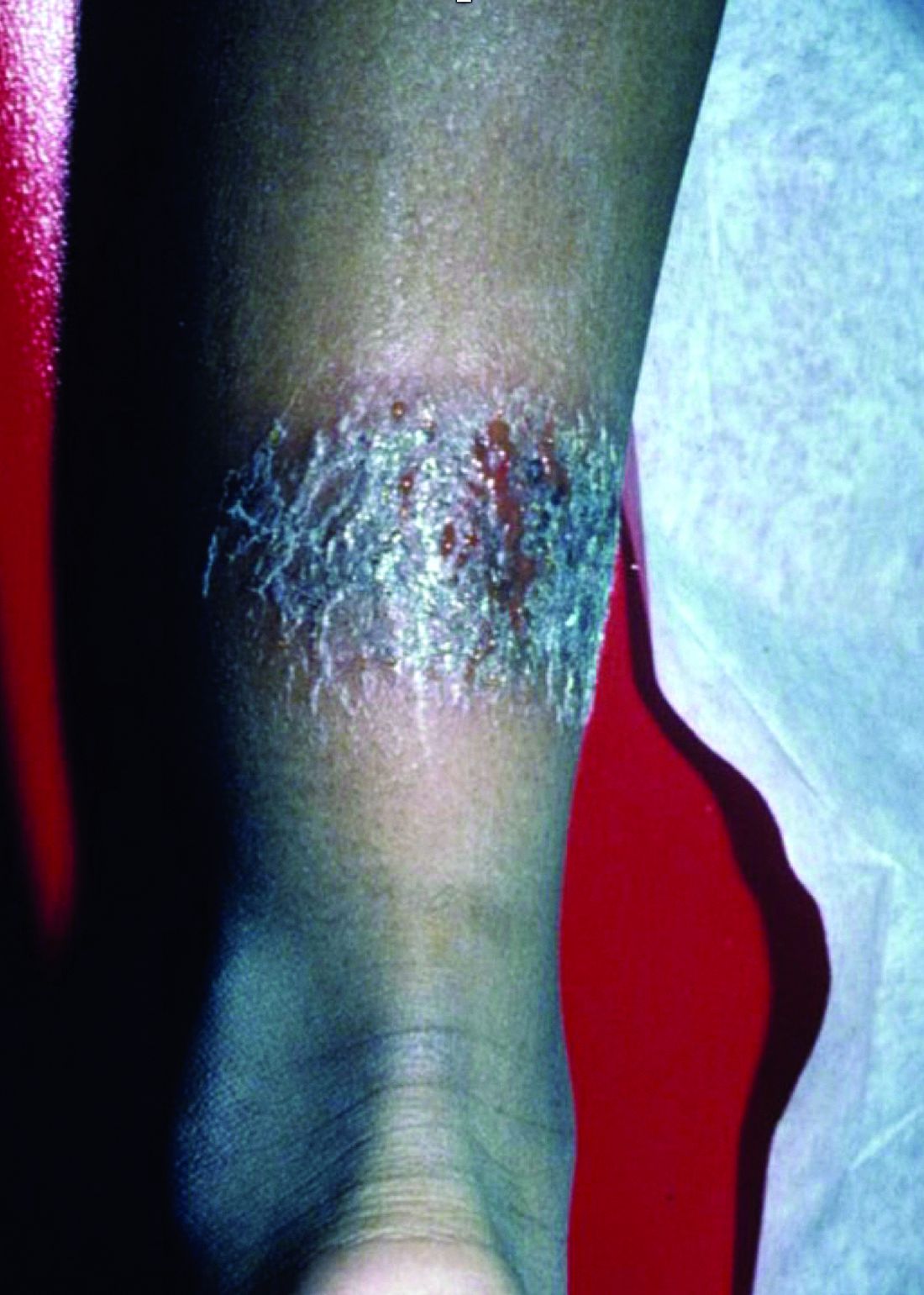
A 3-year-old is brought to the clinic for evaluation of a localized, scaling inflamed lesion on the left leg
Nummular dermatitis, or nummular eczema, is an inflammatory skin condition that is considered to be a distinctive form of idiopathic eczema, while the term also is used to describe lesional morphology associated with other conditions.
The term nummular derives from the Latin word for “coin,” as lesions are commonly annular plaques. Lesions of nummular dermatitis can be single or multiple. The typical distribution involves the extremities and, although less common, it can affect the trunk as well.
Nummular dermatitis may be associated with atopic dermatitis, or it can be an isolated condition.1 While the pathogenesis is uncertain, instigating factors include xerotic skin, insect bites, or scratches or scrapes.1Staphylococcus infection or colonization, contact allergies to metals such as nickel and less commonly mercury, sensitivity to formaldehyde or medicines such as neomycin, and sensitization to an environmental aeroallergen (such as Candida albicans, dust mites) are considered risk factors.2
The diagnosis of nummular dermatitis is clinical. Laboratory testing and/or biopsy generally are not necessary, although a bacterial culture can be considered in patients with exudative and/or crusted lesions to rule out impetigo as a primary process of secondary infection. In some cases, patch testing for allergic contact dermatitis may be useful.
The differential diagnosis of nummular dermatitis includes tinea corporis (ringworm), atopic dermatitis, allergic contact dermatitis, impetigo, and psoriasis. Tinea corporis usually presents as annular lesions with a distinct peripheral scaling, rather than the diffuse induration of nummular dermatitis. Potassium hydroxide preparation or fungal culture can identify tinea species. Nummular dermatitis may be seen in patients with atopic dermatitis, who should have typical history, morphology, and course consistent with standard diagnostic criteria. Allergic contact dermatitis can present with regional, localized eczematous plaques in areas exposed to contact allergens. Patterns of lesions in areas of contact and worsening with repeat exposures can be clues to this diagnosis. Impetigo can present with honey-colored crusted lesions and/or superficial erosions, or purulent pyoderma. Lesions can be single or multiple and generally appear less inflammatory than nummular dermatitis. Psoriasis lesions may be annular, are more common on extensor surfaces, and usually have more prominent overlying pinkish, silvery white or micaceous scale.
Management of nummular dermatitis requires strong anti-inflammatory medications, usually mid-potency or higher topical corticosteroids, along with moisturizers and limiting exposure to skin irritants. “Wet wraps,” with application of topical corticosteroids to wet skin with occlusive wet dressings can enhance response. Transition from higher strength topical corticosteroids to lower strength agents used intermittently can help achieve remission or cure. Management practices include less frequent bathing with lukewarm water, using hypoallergenic cleansers and detergents, and applying moisturizers frequently. If plaques do recur, they tend to do so in the same location and in some patients resolution may result in hyper or hypopigmentation. Refractory disease may be managed with intralesional steroid injections, or systemic medications such as methotrexate.3
Dr. Tracy is a research fellow in pediatric dermatology at Rady Children’s Hospital–San Diego and the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Neither Dr. Tracy nor Dr. Eichenfield have any relevant financial disclosures. Email them at [email protected].
References
1. Pediatr Dermatol. 2012 Sep-Oct;29(5):580-3.
2. American Academy of Dermatology. Nummular Dermatitis Overview
3. Pediatr Dermatol. 2018 Sep;35(5):611-5.
Nummular dermatitis, or nummular eczema, is an inflammatory skin condition that is considered to be a distinctive form of idiopathic eczema, while the term also is used to describe lesional morphology associated with other conditions.
The term nummular derives from the Latin word for “coin,” as lesions are commonly annular plaques. Lesions of nummular dermatitis can be single or multiple. The typical distribution involves the extremities and, although less common, it can affect the trunk as well.
Nummular dermatitis may be associated with atopic dermatitis, or it can be an isolated condition.1 While the pathogenesis is uncertain, instigating factors include xerotic skin, insect bites, or scratches or scrapes.1Staphylococcus infection or colonization, contact allergies to metals such as nickel and less commonly mercury, sensitivity to formaldehyde or medicines such as neomycin, and sensitization to an environmental aeroallergen (such as Candida albicans, dust mites) are considered risk factors.2
The diagnosis of nummular dermatitis is clinical. Laboratory testing and/or biopsy generally are not necessary, although a bacterial culture can be considered in patients with exudative and/or crusted lesions to rule out impetigo as a primary process of secondary infection. In some cases, patch testing for allergic contact dermatitis may be useful.
The differential diagnosis of nummular dermatitis includes tinea corporis (ringworm), atopic dermatitis, allergic contact dermatitis, impetigo, and psoriasis. Tinea corporis usually presents as annular lesions with a distinct peripheral scaling, rather than the diffuse induration of nummular dermatitis. Potassium hydroxide preparation or fungal culture can identify tinea species. Nummular dermatitis may be seen in patients with atopic dermatitis, who should have typical history, morphology, and course consistent with standard diagnostic criteria. Allergic contact dermatitis can present with regional, localized eczematous plaques in areas exposed to contact allergens. Patterns of lesions in areas of contact and worsening with repeat exposures can be clues to this diagnosis. Impetigo can present with honey-colored crusted lesions and/or superficial erosions, or purulent pyoderma. Lesions can be single or multiple and generally appear less inflammatory than nummular dermatitis. Psoriasis lesions may be annular, are more common on extensor surfaces, and usually have more prominent overlying pinkish, silvery white or micaceous scale.
Management of nummular dermatitis requires strong anti-inflammatory medications, usually mid-potency or higher topical corticosteroids, along with moisturizers and limiting exposure to skin irritants. “Wet wraps,” with application of topical corticosteroids to wet skin with occlusive wet dressings can enhance response. Transition from higher strength topical corticosteroids to lower strength agents used intermittently can help achieve remission or cure. Management practices include less frequent bathing with lukewarm water, using hypoallergenic cleansers and detergents, and applying moisturizers frequently. If plaques do recur, they tend to do so in the same location and in some patients resolution may result in hyper or hypopigmentation. Refractory disease may be managed with intralesional steroid injections, or systemic medications such as methotrexate.3
Dr. Tracy is a research fellow in pediatric dermatology at Rady Children’s Hospital–San Diego and the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Neither Dr. Tracy nor Dr. Eichenfield have any relevant financial disclosures. Email them at [email protected].
References
1. Pediatr Dermatol. 2012 Sep-Oct;29(5):580-3.
2. American Academy of Dermatology. Nummular Dermatitis Overview
3. Pediatr Dermatol. 2018 Sep;35(5):611-5.
Nummular dermatitis, or nummular eczema, is an inflammatory skin condition that is considered to be a distinctive form of idiopathic eczema, while the term also is used to describe lesional morphology associated with other conditions.
The term nummular derives from the Latin word for “coin,” as lesions are commonly annular plaques. Lesions of nummular dermatitis can be single or multiple. The typical distribution involves the extremities and, although less common, it can affect the trunk as well.
Nummular dermatitis may be associated with atopic dermatitis, or it can be an isolated condition.1 While the pathogenesis is uncertain, instigating factors include xerotic skin, insect bites, or scratches or scrapes.1Staphylococcus infection or colonization, contact allergies to metals such as nickel and less commonly mercury, sensitivity to formaldehyde or medicines such as neomycin, and sensitization to an environmental aeroallergen (such as Candida albicans, dust mites) are considered risk factors.2
The diagnosis of nummular dermatitis is clinical. Laboratory testing and/or biopsy generally are not necessary, although a bacterial culture can be considered in patients with exudative and/or crusted lesions to rule out impetigo as a primary process of secondary infection. In some cases, patch testing for allergic contact dermatitis may be useful.
The differential diagnosis of nummular dermatitis includes tinea corporis (ringworm), atopic dermatitis, allergic contact dermatitis, impetigo, and psoriasis. Tinea corporis usually presents as annular lesions with a distinct peripheral scaling, rather than the diffuse induration of nummular dermatitis. Potassium hydroxide preparation or fungal culture can identify tinea species. Nummular dermatitis may be seen in patients with atopic dermatitis, who should have typical history, morphology, and course consistent with standard diagnostic criteria. Allergic contact dermatitis can present with regional, localized eczematous plaques in areas exposed to contact allergens. Patterns of lesions in areas of contact and worsening with repeat exposures can be clues to this diagnosis. Impetigo can present with honey-colored crusted lesions and/or superficial erosions, or purulent pyoderma. Lesions can be single or multiple and generally appear less inflammatory than nummular dermatitis. Psoriasis lesions may be annular, are more common on extensor surfaces, and usually have more prominent overlying pinkish, silvery white or micaceous scale.
Management of nummular dermatitis requires strong anti-inflammatory medications, usually mid-potency or higher topical corticosteroids, along with moisturizers and limiting exposure to skin irritants. “Wet wraps,” with application of topical corticosteroids to wet skin with occlusive wet dressings can enhance response. Transition from higher strength topical corticosteroids to lower strength agents used intermittently can help achieve remission or cure. Management practices include less frequent bathing with lukewarm water, using hypoallergenic cleansers and detergents, and applying moisturizers frequently. If plaques do recur, they tend to do so in the same location and in some patients resolution may result in hyper or hypopigmentation. Refractory disease may be managed with intralesional steroid injections, or systemic medications such as methotrexate.3
Dr. Tracy is a research fellow in pediatric dermatology at Rady Children’s Hospital–San Diego and the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Neither Dr. Tracy nor Dr. Eichenfield have any relevant financial disclosures. Email them at [email protected].
References
1. Pediatr Dermatol. 2012 Sep-Oct;29(5):580-3.
2. American Academy of Dermatology. Nummular Dermatitis Overview
3. Pediatr Dermatol. 2018 Sep;35(5):611-5.
On physical exam, he is noted to have a localized eczematous plaque with erythema and edema. Also, he is noted to have diffuse, fine xerosis of the bilateral lower extremities. His skin is otherwise nonremarkable.
iPledge: Fetal exposure to isotretinoin continues
but pregnancy, abortions, and fetal defects associated with isotretinoin exposure are still occurring in women of reproductive age, according to a retrospective study published in JAMA Dermatology.
In 2006, the Food and Drug Administration implemented the iPledge program, with requirements that include women of childbearing age having a negative pregnancy test and evidence of using two forms of contraception monthly to use isotretinoin, a teratogen. “Although the number of pregnancy-related adverse events for patients taking isotretinoin has decreased since 2006, pregnancies, abortions, and fetal defects associated with isotretinoin exposure continue to be a problem,” Elizabeth Tkachenko, BS, from the University of Massachusetts Medical School, Worcester, and coauthors concluded. “Further research is required to determine the most efficacious system to reduce complications for patients and administrative requirements for physicians while at the same time maintaining access to this important drug.” (iPledge followed other Risk Evaluation and Mitigation Strategy systems for isotretinoin.)
She and her colleagues performed a retrospective evaluation of pregnancy-related adverse events related to isotretinoin that had occurred between January 1997 and December 2017 using the FDA Adverse Event Reporting System (FAERS), which receives reports from prescribers, consumers, and pharmaceutical manufacturers. While there could be many different classification terms for each individual, any number of adverse events reported by an individual was counted as one pregnancy. Ms. Tkachenko and colleagues classified abortions, pregnancies during contraception use, and pregnancy-related defects into separate subgroups for analysis.
From 1997 to 2017, there were 6,740 pregnancies among women (mean age, 24.6 years) during treatment with isotretinoin reported to FAERS, with 7 reports in 1997, and a peak of 768 pregnancies in 2006. Almost 70% (4,647) of the pregnancies were reported after iPledge was introduced. Between 2011 and 2017, there were 218-310 pregnancy reports each year.
Of the total number of pregnancy reports during the study period, 1,896 were abortions (28.1% of the total); 10.9% of the total number of pregnancy reports were spontaneous abortions (733). The number of abortions peaked in 2008, with 291 reports, of which 85% were therapeutic abortions. Also peaking in 2008 was the number of reports of pregnancies while taking a contraceptive (64). After 2008, pregnancies and abortions dropped.
Fetal defects peaked in 2000, with 34 cases reported, and dropped to four or fewer reports annually after 2008.
“Our findings demonstrate that reports of pregnancy among women taking isotretinoin are concentrated among those aged 20 to 29 years, peaked in 2006, and have been consistent since 2011,” the authors wrote.
Limitations of the study, they noted, include limitations of FAERS data and possible reporting fatigue among doctors and patients. The total number of isotretinoin courses prescribed to this patient population is also unknown, which affected their ability to determine the true rate of pregnancy-related adverse events, they noted.
The other authors for this study were from Harvard Medical School and the departments of dermatology at Brigham and Women’s Hospital, both in Boston, as well as the University of Pennsylvania, Philadelphia. One author reported support from an award by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health and salary support from a Pfizer Fellowship in Dermatology Patient Oriented Research grant to the trustees of the University of Pennsylvania. The other authors reported no relevant conflicts of interest.
SOURCE: Tkachenko E et al. JAMA Dermatol. 2019. doi: 10.1001/jamadermatol.2019.1388.
The rate of fetal exposure to isotretinoin has generally decreased since the implementation of the iPledge program, but rates have plateaued since 2011, and it is unclear why the exposure rate does not continue to decrease, Arielle R. Nagler, MD, wrote in a related editorial.
As noted by Tkachenko et al., it is not possible to infer that iPledge resulted in declines in fetal exposure, abortions, and pregnancy-related complications. Use of long-acting reversible contraception, education about contraception use, and reporting fatigue could be factors in the decline, Dr. Nagler noted. “The inability to clearly demonstrate causality, combined with the unexplained delay and plateau in the number of fetal exposures to isotretinoin after the implementation of iPledge, makes it difficult to draw firm conclusions about the role of iPledge in this reported trend,” she said.
The decrease in fetal exposure could also potentially be explained by effects of iPledge on the availability of isotretinoin for women of childbearing age. Indeed, studies have shown a significant decrease in isotretinoin prescriptions in this patient population after iPledge was implemented.
Despite lack of data, there is still too much fetal exposure to isotretinoin, wrote Dr. Nagler, which calls into question the efficacy of the iPledge program. “We can all agree that 1 fetal exposure to isotretinoin should be too many, but without taking isotretinoin off the market, we will never achieve zero fetal exposures to isotretinoin. Still, we can – and should – expect more from a REMS [Risk Evaluation and Mitigation Strategy] program,” Dr. Nagler concluded.
Dr. Nagler is with the department of dermatology at New York University. She reported no relevant conflicts of interest.
The rate of fetal exposure to isotretinoin has generally decreased since the implementation of the iPledge program, but rates have plateaued since 2011, and it is unclear why the exposure rate does not continue to decrease, Arielle R. Nagler, MD, wrote in a related editorial.
As noted by Tkachenko et al., it is not possible to infer that iPledge resulted in declines in fetal exposure, abortions, and pregnancy-related complications. Use of long-acting reversible contraception, education about contraception use, and reporting fatigue could be factors in the decline, Dr. Nagler noted. “The inability to clearly demonstrate causality, combined with the unexplained delay and plateau in the number of fetal exposures to isotretinoin after the implementation of iPledge, makes it difficult to draw firm conclusions about the role of iPledge in this reported trend,” she said.
The decrease in fetal exposure could also potentially be explained by effects of iPledge on the availability of isotretinoin for women of childbearing age. Indeed, studies have shown a significant decrease in isotretinoin prescriptions in this patient population after iPledge was implemented.
Despite lack of data, there is still too much fetal exposure to isotretinoin, wrote Dr. Nagler, which calls into question the efficacy of the iPledge program. “We can all agree that 1 fetal exposure to isotretinoin should be too many, but without taking isotretinoin off the market, we will never achieve zero fetal exposures to isotretinoin. Still, we can – and should – expect more from a REMS [Risk Evaluation and Mitigation Strategy] program,” Dr. Nagler concluded.
Dr. Nagler is with the department of dermatology at New York University. She reported no relevant conflicts of interest.
The rate of fetal exposure to isotretinoin has generally decreased since the implementation of the iPledge program, but rates have plateaued since 2011, and it is unclear why the exposure rate does not continue to decrease, Arielle R. Nagler, MD, wrote in a related editorial.
As noted by Tkachenko et al., it is not possible to infer that iPledge resulted in declines in fetal exposure, abortions, and pregnancy-related complications. Use of long-acting reversible contraception, education about contraception use, and reporting fatigue could be factors in the decline, Dr. Nagler noted. “The inability to clearly demonstrate causality, combined with the unexplained delay and plateau in the number of fetal exposures to isotretinoin after the implementation of iPledge, makes it difficult to draw firm conclusions about the role of iPledge in this reported trend,” she said.
The decrease in fetal exposure could also potentially be explained by effects of iPledge on the availability of isotretinoin for women of childbearing age. Indeed, studies have shown a significant decrease in isotretinoin prescriptions in this patient population after iPledge was implemented.
Despite lack of data, there is still too much fetal exposure to isotretinoin, wrote Dr. Nagler, which calls into question the efficacy of the iPledge program. “We can all agree that 1 fetal exposure to isotretinoin should be too many, but without taking isotretinoin off the market, we will never achieve zero fetal exposures to isotretinoin. Still, we can – and should – expect more from a REMS [Risk Evaluation and Mitigation Strategy] program,” Dr. Nagler concluded.
Dr. Nagler is with the department of dermatology at New York University. She reported no relevant conflicts of interest.
but pregnancy, abortions, and fetal defects associated with isotretinoin exposure are still occurring in women of reproductive age, according to a retrospective study published in JAMA Dermatology.
In 2006, the Food and Drug Administration implemented the iPledge program, with requirements that include women of childbearing age having a negative pregnancy test and evidence of using two forms of contraception monthly to use isotretinoin, a teratogen. “Although the number of pregnancy-related adverse events for patients taking isotretinoin has decreased since 2006, pregnancies, abortions, and fetal defects associated with isotretinoin exposure continue to be a problem,” Elizabeth Tkachenko, BS, from the University of Massachusetts Medical School, Worcester, and coauthors concluded. “Further research is required to determine the most efficacious system to reduce complications for patients and administrative requirements for physicians while at the same time maintaining access to this important drug.” (iPledge followed other Risk Evaluation and Mitigation Strategy systems for isotretinoin.)
She and her colleagues performed a retrospective evaluation of pregnancy-related adverse events related to isotretinoin that had occurred between January 1997 and December 2017 using the FDA Adverse Event Reporting System (FAERS), which receives reports from prescribers, consumers, and pharmaceutical manufacturers. While there could be many different classification terms for each individual, any number of adverse events reported by an individual was counted as one pregnancy. Ms. Tkachenko and colleagues classified abortions, pregnancies during contraception use, and pregnancy-related defects into separate subgroups for analysis.
From 1997 to 2017, there were 6,740 pregnancies among women (mean age, 24.6 years) during treatment with isotretinoin reported to FAERS, with 7 reports in 1997, and a peak of 768 pregnancies in 2006. Almost 70% (4,647) of the pregnancies were reported after iPledge was introduced. Between 2011 and 2017, there were 218-310 pregnancy reports each year.
Of the total number of pregnancy reports during the study period, 1,896 were abortions (28.1% of the total); 10.9% of the total number of pregnancy reports were spontaneous abortions (733). The number of abortions peaked in 2008, with 291 reports, of which 85% were therapeutic abortions. Also peaking in 2008 was the number of reports of pregnancies while taking a contraceptive (64). After 2008, pregnancies and abortions dropped.
Fetal defects peaked in 2000, with 34 cases reported, and dropped to four or fewer reports annually after 2008.
“Our findings demonstrate that reports of pregnancy among women taking isotretinoin are concentrated among those aged 20 to 29 years, peaked in 2006, and have been consistent since 2011,” the authors wrote.
Limitations of the study, they noted, include limitations of FAERS data and possible reporting fatigue among doctors and patients. The total number of isotretinoin courses prescribed to this patient population is also unknown, which affected their ability to determine the true rate of pregnancy-related adverse events, they noted.
The other authors for this study were from Harvard Medical School and the departments of dermatology at Brigham and Women’s Hospital, both in Boston, as well as the University of Pennsylvania, Philadelphia. One author reported support from an award by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health and salary support from a Pfizer Fellowship in Dermatology Patient Oriented Research grant to the trustees of the University of Pennsylvania. The other authors reported no relevant conflicts of interest.
SOURCE: Tkachenko E et al. JAMA Dermatol. 2019. doi: 10.1001/jamadermatol.2019.1388.
but pregnancy, abortions, and fetal defects associated with isotretinoin exposure are still occurring in women of reproductive age, according to a retrospective study published in JAMA Dermatology.
In 2006, the Food and Drug Administration implemented the iPledge program, with requirements that include women of childbearing age having a negative pregnancy test and evidence of using two forms of contraception monthly to use isotretinoin, a teratogen. “Although the number of pregnancy-related adverse events for patients taking isotretinoin has decreased since 2006, pregnancies, abortions, and fetal defects associated with isotretinoin exposure continue to be a problem,” Elizabeth Tkachenko, BS, from the University of Massachusetts Medical School, Worcester, and coauthors concluded. “Further research is required to determine the most efficacious system to reduce complications for patients and administrative requirements for physicians while at the same time maintaining access to this important drug.” (iPledge followed other Risk Evaluation and Mitigation Strategy systems for isotretinoin.)
She and her colleagues performed a retrospective evaluation of pregnancy-related adverse events related to isotretinoin that had occurred between January 1997 and December 2017 using the FDA Adverse Event Reporting System (FAERS), which receives reports from prescribers, consumers, and pharmaceutical manufacturers. While there could be many different classification terms for each individual, any number of adverse events reported by an individual was counted as one pregnancy. Ms. Tkachenko and colleagues classified abortions, pregnancies during contraception use, and pregnancy-related defects into separate subgroups for analysis.
From 1997 to 2017, there were 6,740 pregnancies among women (mean age, 24.6 years) during treatment with isotretinoin reported to FAERS, with 7 reports in 1997, and a peak of 768 pregnancies in 2006. Almost 70% (4,647) of the pregnancies were reported after iPledge was introduced. Between 2011 and 2017, there were 218-310 pregnancy reports each year.
Of the total number of pregnancy reports during the study period, 1,896 were abortions (28.1% of the total); 10.9% of the total number of pregnancy reports were spontaneous abortions (733). The number of abortions peaked in 2008, with 291 reports, of which 85% were therapeutic abortions. Also peaking in 2008 was the number of reports of pregnancies while taking a contraceptive (64). After 2008, pregnancies and abortions dropped.
Fetal defects peaked in 2000, with 34 cases reported, and dropped to four or fewer reports annually after 2008.
“Our findings demonstrate that reports of pregnancy among women taking isotretinoin are concentrated among those aged 20 to 29 years, peaked in 2006, and have been consistent since 2011,” the authors wrote.
Limitations of the study, they noted, include limitations of FAERS data and possible reporting fatigue among doctors and patients. The total number of isotretinoin courses prescribed to this patient population is also unknown, which affected their ability to determine the true rate of pregnancy-related adverse events, they noted.
The other authors for this study were from Harvard Medical School and the departments of dermatology at Brigham and Women’s Hospital, both in Boston, as well as the University of Pennsylvania, Philadelphia. One author reported support from an award by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health and salary support from a Pfizer Fellowship in Dermatology Patient Oriented Research grant to the trustees of the University of Pennsylvania. The other authors reported no relevant conflicts of interest.
SOURCE: Tkachenko E et al. JAMA Dermatol. 2019. doi: 10.1001/jamadermatol.2019.1388.
FROM JAMA DERMATOLOGY
Baby’s Rash Causes Family Feud
Since birth, this 5-month-old boy has had a facial rash that comes and goes. At times severe, it is the source of much familial disagreement about its cause: Some say the problem is related to food, while others are sure it represents infection.
Several medications, including triple-antibiotic ointment and nystatin cream, have been tried. None have had much effect.
The child is well in all other respects—gaining weight as expected and experiencing normal growth and development. The rash does not appear to bother him as much as it bothers his family to see.
Further questioning reveals a strong family history of seasonal allergies, eczema, and asthma. Notably, all affected individuals have long since outgrown those problems.
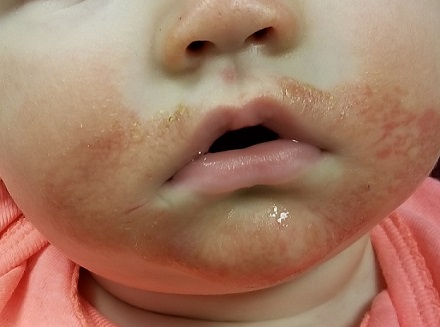
EXAMINATION
The child is in no apparent distress but is noted to have nasal congestion, with continual mouth breathing. Overall, his skin is quite dry and fair.
The rash itself is rather florid, affecting the perioral area and spreading onto the cheeks in a symmetrical configuration. The skin in these areas is focally erythematous, though not swollen. It is also quite scaly in places, giving the appearance of, as his parents note, “chapped” skin. Examination of the diaper area reveals a similar look focally.
What’s the diagnosis?
DISCUSSION
This case is typical of those seen multiple times daily in primary care and dermatology offices—hardly surprising, since atopic dermatitis (AD) affects about 20% of all newborns in this and other developed countries. In very young children, AD primarily affects the face and diaper area, as well as the trunk. About 50% of affected patients will have cradle cap—as did this child in his first month of life, we subsequently learned.
The tendency to develop AD is inherited. It is not related to food, although children with AD could develop a food allergy. However, it would more likely manifest with gastrointestinal symptoms. Another myth embedded in Western culture is that AD is caused by exposure to a particular laundry detergent.
Rather, this child and others like him have inherited dry, thin, overreactive skin that is bathed early on with nasal secretions, bacteria, and saliva. Later, their eczema will migrate to areas that stay moist, such as the antecubital and popliteal folds, or to the area around the neck, where the itching can be intense—which of course causes the child to scratch, in turn worsening the problem.
Patient/parent education is the key to dealing with AD and can be bolstered by handouts or direction to reliable websites. Besides objectifying the problem, these resources detail its nature and outline the need for daily bathing with mild cleansers, generous application of heavy moisturizers, careful use of topical corticosteroid creams or ointments (eg, 2.5% hydrocortisone), and avoidance of woolen clothing or bedding.
Another important component of patient/parent education is the reassurance that eczema will not scar the patient. It does occasionally become severe enough to require a short course of oral antibiotics (eg, cephalexin) or even the use of oral prednisolone. Since AD is not a histamine-driven process, antihistamines are ineffective for eczema.
TAKE-HOME LEARNING POINTS
- Atopic dermatitis (AD) is extremely common, affecting 20% of all newborns in developed countries.
- Infantile eczema typically centers on the face, particularly the perioral area.
- Later, it begins to involve the antecubital and popliteal areas, which stay moist a good part of the time.
- Most children outgrow the worst of the problem—and go on to have children who develop it.
Since birth, this 5-month-old boy has had a facial rash that comes and goes. At times severe, it is the source of much familial disagreement about its cause: Some say the problem is related to food, while others are sure it represents infection.
Several medications, including triple-antibiotic ointment and nystatin cream, have been tried. None have had much effect.
The child is well in all other respects—gaining weight as expected and experiencing normal growth and development. The rash does not appear to bother him as much as it bothers his family to see.
Further questioning reveals a strong family history of seasonal allergies, eczema, and asthma. Notably, all affected individuals have long since outgrown those problems.

EXAMINATION
The child is in no apparent distress but is noted to have nasal congestion, with continual mouth breathing. Overall, his skin is quite dry and fair.
The rash itself is rather florid, affecting the perioral area and spreading onto the cheeks in a symmetrical configuration. The skin in these areas is focally erythematous, though not swollen. It is also quite scaly in places, giving the appearance of, as his parents note, “chapped” skin. Examination of the diaper area reveals a similar look focally.
What’s the diagnosis?
DISCUSSION
This case is typical of those seen multiple times daily in primary care and dermatology offices—hardly surprising, since atopic dermatitis (AD) affects about 20% of all newborns in this and other developed countries. In very young children, AD primarily affects the face and diaper area, as well as the trunk. About 50% of affected patients will have cradle cap—as did this child in his first month of life, we subsequently learned.
The tendency to develop AD is inherited. It is not related to food, although children with AD could develop a food allergy. However, it would more likely manifest with gastrointestinal symptoms. Another myth embedded in Western culture is that AD is caused by exposure to a particular laundry detergent.
Rather, this child and others like him have inherited dry, thin, overreactive skin that is bathed early on with nasal secretions, bacteria, and saliva. Later, their eczema will migrate to areas that stay moist, such as the antecubital and popliteal folds, or to the area around the neck, where the itching can be intense—which of course causes the child to scratch, in turn worsening the problem.
Patient/parent education is the key to dealing with AD and can be bolstered by handouts or direction to reliable websites. Besides objectifying the problem, these resources detail its nature and outline the need for daily bathing with mild cleansers, generous application of heavy moisturizers, careful use of topical corticosteroid creams or ointments (eg, 2.5% hydrocortisone), and avoidance of woolen clothing or bedding.
Another important component of patient/parent education is the reassurance that eczema will not scar the patient. It does occasionally become severe enough to require a short course of oral antibiotics (eg, cephalexin) or even the use of oral prednisolone. Since AD is not a histamine-driven process, antihistamines are ineffective for eczema.
TAKE-HOME LEARNING POINTS
- Atopic dermatitis (AD) is extremely common, affecting 20% of all newborns in developed countries.
- Infantile eczema typically centers on the face, particularly the perioral area.
- Later, it begins to involve the antecubital and popliteal areas, which stay moist a good part of the time.
- Most children outgrow the worst of the problem—and go on to have children who develop it.
Since birth, this 5-month-old boy has had a facial rash that comes and goes. At times severe, it is the source of much familial disagreement about its cause: Some say the problem is related to food, while others are sure it represents infection.
Several medications, including triple-antibiotic ointment and nystatin cream, have been tried. None have had much effect.
The child is well in all other respects—gaining weight as expected and experiencing normal growth and development. The rash does not appear to bother him as much as it bothers his family to see.
Further questioning reveals a strong family history of seasonal allergies, eczema, and asthma. Notably, all affected individuals have long since outgrown those problems.

EXAMINATION
The child is in no apparent distress but is noted to have nasal congestion, with continual mouth breathing. Overall, his skin is quite dry and fair.
The rash itself is rather florid, affecting the perioral area and spreading onto the cheeks in a symmetrical configuration. The skin in these areas is focally erythematous, though not swollen. It is also quite scaly in places, giving the appearance of, as his parents note, “chapped” skin. Examination of the diaper area reveals a similar look focally.
What’s the diagnosis?
DISCUSSION
This case is typical of those seen multiple times daily in primary care and dermatology offices—hardly surprising, since atopic dermatitis (AD) affects about 20% of all newborns in this and other developed countries. In very young children, AD primarily affects the face and diaper area, as well as the trunk. About 50% of affected patients will have cradle cap—as did this child in his first month of life, we subsequently learned.
The tendency to develop AD is inherited. It is not related to food, although children with AD could develop a food allergy. However, it would more likely manifest with gastrointestinal symptoms. Another myth embedded in Western culture is that AD is caused by exposure to a particular laundry detergent.
Rather, this child and others like him have inherited dry, thin, overreactive skin that is bathed early on with nasal secretions, bacteria, and saliva. Later, their eczema will migrate to areas that stay moist, such as the antecubital and popliteal folds, or to the area around the neck, where the itching can be intense—which of course causes the child to scratch, in turn worsening the problem.
Patient/parent education is the key to dealing with AD and can be bolstered by handouts or direction to reliable websites. Besides objectifying the problem, these resources detail its nature and outline the need for daily bathing with mild cleansers, generous application of heavy moisturizers, careful use of topical corticosteroid creams or ointments (eg, 2.5% hydrocortisone), and avoidance of woolen clothing or bedding.
Another important component of patient/parent education is the reassurance that eczema will not scar the patient. It does occasionally become severe enough to require a short course of oral antibiotics (eg, cephalexin) or even the use of oral prednisolone. Since AD is not a histamine-driven process, antihistamines are ineffective for eczema.
TAKE-HOME LEARNING POINTS
- Atopic dermatitis (AD) is extremely common, affecting 20% of all newborns in developed countries.
- Infantile eczema typically centers on the face, particularly the perioral area.
- Later, it begins to involve the antecubital and popliteal areas, which stay moist a good part of the time.
- Most children outgrow the worst of the problem—and go on to have children who develop it.
Rash on elbows and hands
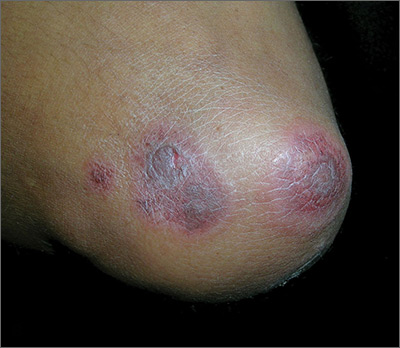
Based on the target lesions with central epithelial disruption, the FP diagnosed erythema multiforme (EM) in this patient. He also diagnosed genital herpes simplex in the crusting stage and suspected that it was the inciting event for the EM.
EM is considered a hypersensitivity reaction that is often secondary to infections or medications. Herpes simplex viruses (HSVI and HSV2) are the most common causative agents and have been implicated in at least 60% of cases.
The patient did not know that she had genital herpes simplex but remembered having sores in the genital area that preceded the similar rash a year earlier. This was a case of recurrent EM responding to recurrent herpes simplex.
The patient suspected that she had genital herpes simplex on and off for the past 10 years but lacked health insurance, so she never visited a doctor for treatment. She recently obtained health insurance and was willing to be tested for other sexually transmitted diseases. She also wanted to have a test done to confirm this was herpes simplex. The FP explained that it was unlikely that an antiviral agent would help the current case of herpes simplex and the associated EM because of the late timing, but antiviral medication could help to prevent further outbreaks and decrease transmission to her husband.
The patient was enthusiastic to start valacyclovir 500 mg/d for prophylaxis. For symptomatic relief, the FP prescribed a 30-g tube of 0.1% triamcinolone appointment and instructed the patient to apply it to the EM twice daily. The patient was sent for blood tests for syphilis and HIV; fortunately, both came back negative. The herpes simplex PCR test was positive for HSV2.
On a 1-week follow-up visit, the skin lesions were resolving, and the patient had no further symptoms. The patient said she understood that she should refrain from sexual intercourse if she developed lesions while on prophylaxis.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Milana C, Smith M. Erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1161-1168.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

Based on the target lesions with central epithelial disruption, the FP diagnosed erythema multiforme (EM) in this patient. He also diagnosed genital herpes simplex in the crusting stage and suspected that it was the inciting event for the EM.
EM is considered a hypersensitivity reaction that is often secondary to infections or medications. Herpes simplex viruses (HSVI and HSV2) are the most common causative agents and have been implicated in at least 60% of cases.
The patient did not know that she had genital herpes simplex but remembered having sores in the genital area that preceded the similar rash a year earlier. This was a case of recurrent EM responding to recurrent herpes simplex.
The patient suspected that she had genital herpes simplex on and off for the past 10 years but lacked health insurance, so she never visited a doctor for treatment. She recently obtained health insurance and was willing to be tested for other sexually transmitted diseases. She also wanted to have a test done to confirm this was herpes simplex. The FP explained that it was unlikely that an antiviral agent would help the current case of herpes simplex and the associated EM because of the late timing, but antiviral medication could help to prevent further outbreaks and decrease transmission to her husband.
The patient was enthusiastic to start valacyclovir 500 mg/d for prophylaxis. For symptomatic relief, the FP prescribed a 30-g tube of 0.1% triamcinolone appointment and instructed the patient to apply it to the EM twice daily. The patient was sent for blood tests for syphilis and HIV; fortunately, both came back negative. The herpes simplex PCR test was positive for HSV2.
On a 1-week follow-up visit, the skin lesions were resolving, and the patient had no further symptoms. The patient said she understood that she should refrain from sexual intercourse if she developed lesions while on prophylaxis.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Milana C, Smith M. Erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1161-1168.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

Based on the target lesions with central epithelial disruption, the FP diagnosed erythema multiforme (EM) in this patient. He also diagnosed genital herpes simplex in the crusting stage and suspected that it was the inciting event for the EM.
EM is considered a hypersensitivity reaction that is often secondary to infections or medications. Herpes simplex viruses (HSVI and HSV2) are the most common causative agents and have been implicated in at least 60% of cases.
The patient did not know that she had genital herpes simplex but remembered having sores in the genital area that preceded the similar rash a year earlier. This was a case of recurrent EM responding to recurrent herpes simplex.
The patient suspected that she had genital herpes simplex on and off for the past 10 years but lacked health insurance, so she never visited a doctor for treatment. She recently obtained health insurance and was willing to be tested for other sexually transmitted diseases. She also wanted to have a test done to confirm this was herpes simplex. The FP explained that it was unlikely that an antiviral agent would help the current case of herpes simplex and the associated EM because of the late timing, but antiviral medication could help to prevent further outbreaks and decrease transmission to her husband.
The patient was enthusiastic to start valacyclovir 500 mg/d for prophylaxis. For symptomatic relief, the FP prescribed a 30-g tube of 0.1% triamcinolone appointment and instructed the patient to apply it to the EM twice daily. The patient was sent for blood tests for syphilis and HIV; fortunately, both came back negative. The herpes simplex PCR test was positive for HSV2.
On a 1-week follow-up visit, the skin lesions were resolving, and the patient had no further symptoms. The patient said she understood that she should refrain from sexual intercourse if she developed lesions while on prophylaxis.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Milana C, Smith M. Erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1161-1168.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
Acne before puberty: When to treat, when to worry
NEWPORT BEACH, CALIF. – according to Sheila Fallon Friedlander, MD.
“This is something you are going to see in your practice,” said Dr. Friedlander, a pediatric dermatologists at Rady Children’s Hospital–San Diego. It’s important to know when it’s time to be concerned and when another condition may be masquerading as acne, she said at the at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
Dr. Friedlander, who is professor of dermatology and pediatrics at the University of California, San Diego, talked about treating acne in the following prepubertal age groups:
Neonatal acne (ages birth to 4 weeks)
Acne appears in this population up to 20% of the time, according to research, and it is much more common in males than in females, at a ratio of five to one.
The cause is “most likely the relationship between placental androgens and the baby’s adrenal glands,” Dr. Friedlander said. However, something more serious could be going on. “Look at the child and see if he’s sick. If he looks sick, then we need to worry.”
Hormonal abnormalities also could be a cause, she said. Refer a baby to a specialist if there are other signs of hyperandrogenism. However, “the likelihood is very low,” and she’s never needed to refer a neonate with acne for evaluation.
As for treatment, she said, “Mainly, I’m using tincture of time.” However, “many of my mothers have told me that topical yogurt application will work.” Why yogurt? It’s possible that its bacteria could play a role in combating acne, she said.
Masquerader alert! Beware of neonatal cephalic pustulosis, Dr. Friedlander cautioned, which may be an inflammatory response to yeast. Ketoconazole cream may be helpful.
Infantile acne (ages 0-12 months)
This form of acne is more common in males and may hint at the future development of severe adolescent acne. It does resolve but it may take months or years, Dr. Friedlander said.
In general, this acne isn’t a sign of something more serious. “You do not need to go crazy with the work-up,” she said. “With mild to moderate disease, with nothing else suspicious, I don’t do a big work-up.”
However, do consider whether the child is undergoing precocious puberty, Dr. Friedlander said. Signs include axillary hair, pubic hair, and body odor.
As for treatment of infantile acne, “start out topically” and consider options such as Bactrim (sulfamethoxazole/trimethoprim) and erythromycin.
Masquerader alert! Idiopathic facial aseptic granuloma can be mistaken for acne and abscess, and ultrasound is helpful to confirm it. “It’s not so easy to treat,” she said. “Ivermectin may be helpful. Sometimes you do cultures and make sure something else isn’t going on.”
Midchildhood (ages 1-7 years)
“It’s not as common to have acne develop in this age group, but when it develops you need to be concerned,” Dr. Friedlander said. “This is the age period when there is more often something really wrong.”
Be on the lookout for a family history of hormonal abnormalities, and check if the child is on medication. “You need to look carefully,” she said, adding that it’s important to check for signs of premature puberty such as giant spikes in growth, abnormally large hands and feet, genital changes, and body odor. Check blood pressure if you’re worried about an adrenal tumor.
It’s possible for children to develop precocious puberty – with acne – because of exposure to testosterone gel used by a father. Dehydroepiandrosterone (DHEA) creams also may cause the condition. “The more creams out there with androgenic effects, the more we may see it,” Dr. Friedlander said. “This is something to ask about because families may not be forthcoming.”
Masquerader alert! Perioral dermatitis may look like acne, and it may be linked to inhaled or topical steroids, she said.
Other masqueraders include demodex folliculitis, angiofibromas (think tuberous sclerosis), and keratosis pilaris (the most common type of bump on a children aged 1-7 years). The latter condition “is not the end of the world,” said Dr. Friedlander, who added that “I’ve never cured anyone of it.”
Prepubertal acne (ages 7 years to puberty)
Acne in this group is generally not worrisome, Dr. Friedlander said, but investigate further if there’s significant inflammation and signs of early sexual development or virilization.
Benzoyl peroxide wash may be enough to help the condition initially, and consider topical clindamycin or a combination product. “Start out slow,” she said. Twice a week to start might be appropriate. Moisturizers can be helpful, as can topical adapalene.
Also, keep in mind that even mild acne can be emotionally devastating to a child in this age group and worthy of treatment. “Your assessment may be very different than hers,” she said. It’s possible that “she has a few lesions, but she feels like an outcast.”
Dr. Friedlander reported no relevant financial disclosures. SDEF and this news organization are owned by the same parent company.
NEWPORT BEACH, CALIF. – according to Sheila Fallon Friedlander, MD.
“This is something you are going to see in your practice,” said Dr. Friedlander, a pediatric dermatologists at Rady Children’s Hospital–San Diego. It’s important to know when it’s time to be concerned and when another condition may be masquerading as acne, she said at the at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
Dr. Friedlander, who is professor of dermatology and pediatrics at the University of California, San Diego, talked about treating acne in the following prepubertal age groups:
Neonatal acne (ages birth to 4 weeks)
Acne appears in this population up to 20% of the time, according to research, and it is much more common in males than in females, at a ratio of five to one.
The cause is “most likely the relationship between placental androgens and the baby’s adrenal glands,” Dr. Friedlander said. However, something more serious could be going on. “Look at the child and see if he’s sick. If he looks sick, then we need to worry.”
Hormonal abnormalities also could be a cause, she said. Refer a baby to a specialist if there are other signs of hyperandrogenism. However, “the likelihood is very low,” and she’s never needed to refer a neonate with acne for evaluation.
As for treatment, she said, “Mainly, I’m using tincture of time.” However, “many of my mothers have told me that topical yogurt application will work.” Why yogurt? It’s possible that its bacteria could play a role in combating acne, she said.
Masquerader alert! Beware of neonatal cephalic pustulosis, Dr. Friedlander cautioned, which may be an inflammatory response to yeast. Ketoconazole cream may be helpful.
Infantile acne (ages 0-12 months)
This form of acne is more common in males and may hint at the future development of severe adolescent acne. It does resolve but it may take months or years, Dr. Friedlander said.
In general, this acne isn’t a sign of something more serious. “You do not need to go crazy with the work-up,” she said. “With mild to moderate disease, with nothing else suspicious, I don’t do a big work-up.”
However, do consider whether the child is undergoing precocious puberty, Dr. Friedlander said. Signs include axillary hair, pubic hair, and body odor.
As for treatment of infantile acne, “start out topically” and consider options such as Bactrim (sulfamethoxazole/trimethoprim) and erythromycin.
Masquerader alert! Idiopathic facial aseptic granuloma can be mistaken for acne and abscess, and ultrasound is helpful to confirm it. “It’s not so easy to treat,” she said. “Ivermectin may be helpful. Sometimes you do cultures and make sure something else isn’t going on.”
Midchildhood (ages 1-7 years)
“It’s not as common to have acne develop in this age group, but when it develops you need to be concerned,” Dr. Friedlander said. “This is the age period when there is more often something really wrong.”
Be on the lookout for a family history of hormonal abnormalities, and check if the child is on medication. “You need to look carefully,” she said, adding that it’s important to check for signs of premature puberty such as giant spikes in growth, abnormally large hands and feet, genital changes, and body odor. Check blood pressure if you’re worried about an adrenal tumor.
It’s possible for children to develop precocious puberty – with acne – because of exposure to testosterone gel used by a father. Dehydroepiandrosterone (DHEA) creams also may cause the condition. “The more creams out there with androgenic effects, the more we may see it,” Dr. Friedlander said. “This is something to ask about because families may not be forthcoming.”
Masquerader alert! Perioral dermatitis may look like acne, and it may be linked to inhaled or topical steroids, she said.
Other masqueraders include demodex folliculitis, angiofibromas (think tuberous sclerosis), and keratosis pilaris (the most common type of bump on a children aged 1-7 years). The latter condition “is not the end of the world,” said Dr. Friedlander, who added that “I’ve never cured anyone of it.”
Prepubertal acne (ages 7 years to puberty)
Acne in this group is generally not worrisome, Dr. Friedlander said, but investigate further if there’s significant inflammation and signs of early sexual development or virilization.
Benzoyl peroxide wash may be enough to help the condition initially, and consider topical clindamycin or a combination product. “Start out slow,” she said. Twice a week to start might be appropriate. Moisturizers can be helpful, as can topical adapalene.
Also, keep in mind that even mild acne can be emotionally devastating to a child in this age group and worthy of treatment. “Your assessment may be very different than hers,” she said. It’s possible that “she has a few lesions, but she feels like an outcast.”
Dr. Friedlander reported no relevant financial disclosures. SDEF and this news organization are owned by the same parent company.
NEWPORT BEACH, CALIF. – according to Sheila Fallon Friedlander, MD.
“This is something you are going to see in your practice,” said Dr. Friedlander, a pediatric dermatologists at Rady Children’s Hospital–San Diego. It’s important to know when it’s time to be concerned and when another condition may be masquerading as acne, she said at the at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
Dr. Friedlander, who is professor of dermatology and pediatrics at the University of California, San Diego, talked about treating acne in the following prepubertal age groups:
Neonatal acne (ages birth to 4 weeks)
Acne appears in this population up to 20% of the time, according to research, and it is much more common in males than in females, at a ratio of five to one.
The cause is “most likely the relationship between placental androgens and the baby’s adrenal glands,” Dr. Friedlander said. However, something more serious could be going on. “Look at the child and see if he’s sick. If he looks sick, then we need to worry.”
Hormonal abnormalities also could be a cause, she said. Refer a baby to a specialist if there are other signs of hyperandrogenism. However, “the likelihood is very low,” and she’s never needed to refer a neonate with acne for evaluation.
As for treatment, she said, “Mainly, I’m using tincture of time.” However, “many of my mothers have told me that topical yogurt application will work.” Why yogurt? It’s possible that its bacteria could play a role in combating acne, she said.
Masquerader alert! Beware of neonatal cephalic pustulosis, Dr. Friedlander cautioned, which may be an inflammatory response to yeast. Ketoconazole cream may be helpful.
Infantile acne (ages 0-12 months)
This form of acne is more common in males and may hint at the future development of severe adolescent acne. It does resolve but it may take months or years, Dr. Friedlander said.
In general, this acne isn’t a sign of something more serious. “You do not need to go crazy with the work-up,” she said. “With mild to moderate disease, with nothing else suspicious, I don’t do a big work-up.”
However, do consider whether the child is undergoing precocious puberty, Dr. Friedlander said. Signs include axillary hair, pubic hair, and body odor.
As for treatment of infantile acne, “start out topically” and consider options such as Bactrim (sulfamethoxazole/trimethoprim) and erythromycin.
Masquerader alert! Idiopathic facial aseptic granuloma can be mistaken for acne and abscess, and ultrasound is helpful to confirm it. “It’s not so easy to treat,” she said. “Ivermectin may be helpful. Sometimes you do cultures and make sure something else isn’t going on.”
Midchildhood (ages 1-7 years)
“It’s not as common to have acne develop in this age group, but when it develops you need to be concerned,” Dr. Friedlander said. “This is the age period when there is more often something really wrong.”
Be on the lookout for a family history of hormonal abnormalities, and check if the child is on medication. “You need to look carefully,” she said, adding that it’s important to check for signs of premature puberty such as giant spikes in growth, abnormally large hands and feet, genital changes, and body odor. Check blood pressure if you’re worried about an adrenal tumor.
It’s possible for children to develop precocious puberty – with acne – because of exposure to testosterone gel used by a father. Dehydroepiandrosterone (DHEA) creams also may cause the condition. “The more creams out there with androgenic effects, the more we may see it,” Dr. Friedlander said. “This is something to ask about because families may not be forthcoming.”
Masquerader alert! Perioral dermatitis may look like acne, and it may be linked to inhaled or topical steroids, she said.
Other masqueraders include demodex folliculitis, angiofibromas (think tuberous sclerosis), and keratosis pilaris (the most common type of bump on a children aged 1-7 years). The latter condition “is not the end of the world,” said Dr. Friedlander, who added that “I’ve never cured anyone of it.”
Prepubertal acne (ages 7 years to puberty)
Acne in this group is generally not worrisome, Dr. Friedlander said, but investigate further if there’s significant inflammation and signs of early sexual development or virilization.
Benzoyl peroxide wash may be enough to help the condition initially, and consider topical clindamycin or a combination product. “Start out slow,” she said. Twice a week to start might be appropriate. Moisturizers can be helpful, as can topical adapalene.
Also, keep in mind that even mild acne can be emotionally devastating to a child in this age group and worthy of treatment. “Your assessment may be very different than hers,” she said. It’s possible that “she has a few lesions, but she feels like an outcast.”
Dr. Friedlander reported no relevant financial disclosures. SDEF and this news organization are owned by the same parent company.
REPORTING FROM SDEF WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR
Alopecia areata: Study finds racial disparities
, according to a new study involving registry data for more than 11,000 individuals.
These new findings “raise a different perspective from the conventional view that AA does not differ by race/ethnicity,” said Hemin Lee, MD, MPH, of the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital, Boston, and associates.
Multivariate-adjusted odds ratios for ever-diagnosis of AA were 1.77 for African Americans and 0.4 for Asians when whites were the referent group. Hispanics/Latinos were similar to whites, with an odds ratio of 0.9, and the group of other races/ethnicities (including American Indians and Pacific Islanders) was higher at 1.27, the investigators noted. The report is in the Journal of the American Academy of Dermatology.
The odds played out in a similar fashion when broken down by AA subtype. With whites as the referent at 1.0, blacks were most likely to have been diagnosed with AA transient/persistent at 1.93 and Asians were lowest at 0.46. For AA totalis/universalis, odds ratios were 1.57 for blacks and 0.32 for Asians, they said, based on 2000-2016 data from the National Alopecia Areata Registry.
“An intricate interplay between genetic and environmental factors may account for the racial differences. Pathogenesis of AA is at times linked with autoimmunity by its strong association with HLA class II alleles,” Dr. Lee and associates wrote.
The study involved 11,404 participants from the registry: 9,340 had reported at least one episode of AA and 2,064 had no history of lifetime alopecia. The multivariate analysis was based on the same group of noncases but a subgroup of 1,970 AA patients who had been enrolled in the registry “through academic institutions after dermatologist-confirmed diagnosis,” they said.
There was no funding source to report. One of Dr. Lee’s associates has received honoraria from Abbvie, Amgen, the Centers for Disease Control and Prevention, Janssen, Merck, Novartis, Pfizer, and Amgen, which have been donated to charity, and is an investigator for Sanofi/Regeneron with no financial compensation. All other authors have no conflicts of interest.
SOURCE: Lee H et al. J Am Acad Dermatol. 2019 Jul 3. doi: 10.1016/j.jaad.2019.06.1300.
, according to a new study involving registry data for more than 11,000 individuals.

These new findings “raise a different perspective from the conventional view that AA does not differ by race/ethnicity,” said Hemin Lee, MD, MPH, of the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital, Boston, and associates.
Multivariate-adjusted odds ratios for ever-diagnosis of AA were 1.77 for African Americans and 0.4 for Asians when whites were the referent group. Hispanics/Latinos were similar to whites, with an odds ratio of 0.9, and the group of other races/ethnicities (including American Indians and Pacific Islanders) was higher at 1.27, the investigators noted. The report is in the Journal of the American Academy of Dermatology.
The odds played out in a similar fashion when broken down by AA subtype. With whites as the referent at 1.0, blacks were most likely to have been diagnosed with AA transient/persistent at 1.93 and Asians were lowest at 0.46. For AA totalis/universalis, odds ratios were 1.57 for blacks and 0.32 for Asians, they said, based on 2000-2016 data from the National Alopecia Areata Registry.
“An intricate interplay between genetic and environmental factors may account for the racial differences. Pathogenesis of AA is at times linked with autoimmunity by its strong association with HLA class II alleles,” Dr. Lee and associates wrote.
The study involved 11,404 participants from the registry: 9,340 had reported at least one episode of AA and 2,064 had no history of lifetime alopecia. The multivariate analysis was based on the same group of noncases but a subgroup of 1,970 AA patients who had been enrolled in the registry “through academic institutions after dermatologist-confirmed diagnosis,” they said.
There was no funding source to report. One of Dr. Lee’s associates has received honoraria from Abbvie, Amgen, the Centers for Disease Control and Prevention, Janssen, Merck, Novartis, Pfizer, and Amgen, which have been donated to charity, and is an investigator for Sanofi/Regeneron with no financial compensation. All other authors have no conflicts of interest.
SOURCE: Lee H et al. J Am Acad Dermatol. 2019 Jul 3. doi: 10.1016/j.jaad.2019.06.1300.
, according to a new study involving registry data for more than 11,000 individuals.

These new findings “raise a different perspective from the conventional view that AA does not differ by race/ethnicity,” said Hemin Lee, MD, MPH, of the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital, Boston, and associates.
Multivariate-adjusted odds ratios for ever-diagnosis of AA were 1.77 for African Americans and 0.4 for Asians when whites were the referent group. Hispanics/Latinos were similar to whites, with an odds ratio of 0.9, and the group of other races/ethnicities (including American Indians and Pacific Islanders) was higher at 1.27, the investigators noted. The report is in the Journal of the American Academy of Dermatology.
The odds played out in a similar fashion when broken down by AA subtype. With whites as the referent at 1.0, blacks were most likely to have been diagnosed with AA transient/persistent at 1.93 and Asians were lowest at 0.46. For AA totalis/universalis, odds ratios were 1.57 for blacks and 0.32 for Asians, they said, based on 2000-2016 data from the National Alopecia Areata Registry.
“An intricate interplay between genetic and environmental factors may account for the racial differences. Pathogenesis of AA is at times linked with autoimmunity by its strong association with HLA class II alleles,” Dr. Lee and associates wrote.
The study involved 11,404 participants from the registry: 9,340 had reported at least one episode of AA and 2,064 had no history of lifetime alopecia. The multivariate analysis was based on the same group of noncases but a subgroup of 1,970 AA patients who had been enrolled in the registry “through academic institutions after dermatologist-confirmed diagnosis,” they said.
There was no funding source to report. One of Dr. Lee’s associates has received honoraria from Abbvie, Amgen, the Centers for Disease Control and Prevention, Janssen, Merck, Novartis, Pfizer, and Amgen, which have been donated to charity, and is an investigator for Sanofi/Regeneron with no financial compensation. All other authors have no conflicts of interest.
SOURCE: Lee H et al. J Am Acad Dermatol. 2019 Jul 3. doi: 10.1016/j.jaad.2019.06.1300.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY