User login
Racial, ethnic minorities often don’t practice sun protective behaviors
Despite higher rates of skin cancer morbidity and mortality among racial and ethnic minorities, affected adults often are not recognizing their risks or taking preventive measures, said Costner McKenzie, BA, and Roopal V. Kundu, MD of Northwestern University, Chicago.
In a multivariable logistic regression analysis, Mr. Costner and Dr. Kundu sampled data of 33,672 adults included in the 2015 National Health Interview Survey. Data from the 2010 U.S. Census Bureau also were used to develop sample weights representative of the U.S. population. There was a survey of a smaller sample of adults who were determined to have sun-sensitive skin. The findings were published in the Journal of the American Academy of Dermatology.
Sun sensitivity was determined by skin reaction to 1 hour of unprotected sun exposure. Those who self-reported severe sunburn with blisters or moderate sunburn with peeling were determined to be sun sensitive.
The sample surveyed comprised 3,665 women (41%) and 5,287 men (59%). Of these, 82% were white non-Hispanic, 3% black non-Hispanic, 3% Asian non-Hispanic, 11% Hispanic, and 1% other non-Hispanic.
Mr. McKenzie and Dr. Kundu found that (adjusted odds ratio [aOR], 0.43, 0.54, and 0.70, respectively). Non-Hispanic blacks and Hispanics also were less likely to use sunscreen greater than SPF 15 (a0R, 0.39 and 0.64, respectively). Non-Hispanic blacks, non-Hispanic Asians, and Hispanics were less likely to have ever had a total body skin examination (aOR, 0.29, 0.21, and 0.39, respectively).
Yet these same three groups were more likely to wear long sleeves outside (non-Hispanic blacks aOR, 1.96, non-Hispanic Asians aOR, 2.09, and Hispanics aOR, 2.29). In addition, non-Hispanic Asians and Hispanics were more likely to shelter in the shade on warm, sunny days (aOR, 1.63 and 1.85, respectively).
Citing recent literature, the authors noted that although skin cancer is the most commonly diagnosed cancer, it is not typically thought of as a disease that afflicts minority populations, especially among minorities themselves, who do not generally recognize their own risk (Arch Dermatol. 2009;145[2]:207-8). In fact, morbidity and mortality from skin cancer actually are greater in racial and ethnic minorities (J Am Acad Dermatol. 2016;75[5]:983-91; J Am Acad Dermatol. 2006;55[5]:741-60), despite greater incidence of skin cancer among white adults.
“This study highlights the impact of race and ethnicity on sun protective behaviors,” said Mr. McKenzie and Dr. Kundu. Cultural beliefs, stigma, personal preferences, as well as a lack of “knowledge-based interventions” specifically intended for minorities could be responsible for the observed differences between population groups, they speculated.
The primary limitations of the study were its cross-sectional design and the use of self-reported data, the authors noted.
Additional research is needed to fully examine the reasons behind these differences as well as to identify appropriate interventions that promote sun protection, they added.
There was no external funding and the authors had no conflicts of interest to disclose.
SOURCE: McKenzie C and Kundu RV. J Am Acad Dermatol. 2019 Jun 19. doi: 10.1016/j.jaad.2019.06.1306.
Despite higher rates of skin cancer morbidity and mortality among racial and ethnic minorities, affected adults often are not recognizing their risks or taking preventive measures, said Costner McKenzie, BA, and Roopal V. Kundu, MD of Northwestern University, Chicago.
In a multivariable logistic regression analysis, Mr. Costner and Dr. Kundu sampled data of 33,672 adults included in the 2015 National Health Interview Survey. Data from the 2010 U.S. Census Bureau also were used to develop sample weights representative of the U.S. population. There was a survey of a smaller sample of adults who were determined to have sun-sensitive skin. The findings were published in the Journal of the American Academy of Dermatology.
Sun sensitivity was determined by skin reaction to 1 hour of unprotected sun exposure. Those who self-reported severe sunburn with blisters or moderate sunburn with peeling were determined to be sun sensitive.
The sample surveyed comprised 3,665 women (41%) and 5,287 men (59%). Of these, 82% were white non-Hispanic, 3% black non-Hispanic, 3% Asian non-Hispanic, 11% Hispanic, and 1% other non-Hispanic.
Mr. McKenzie and Dr. Kundu found that (adjusted odds ratio [aOR], 0.43, 0.54, and 0.70, respectively). Non-Hispanic blacks and Hispanics also were less likely to use sunscreen greater than SPF 15 (a0R, 0.39 and 0.64, respectively). Non-Hispanic blacks, non-Hispanic Asians, and Hispanics were less likely to have ever had a total body skin examination (aOR, 0.29, 0.21, and 0.39, respectively).
Yet these same three groups were more likely to wear long sleeves outside (non-Hispanic blacks aOR, 1.96, non-Hispanic Asians aOR, 2.09, and Hispanics aOR, 2.29). In addition, non-Hispanic Asians and Hispanics were more likely to shelter in the shade on warm, sunny days (aOR, 1.63 and 1.85, respectively).
Citing recent literature, the authors noted that although skin cancer is the most commonly diagnosed cancer, it is not typically thought of as a disease that afflicts minority populations, especially among minorities themselves, who do not generally recognize their own risk (Arch Dermatol. 2009;145[2]:207-8). In fact, morbidity and mortality from skin cancer actually are greater in racial and ethnic minorities (J Am Acad Dermatol. 2016;75[5]:983-91; J Am Acad Dermatol. 2006;55[5]:741-60), despite greater incidence of skin cancer among white adults.
“This study highlights the impact of race and ethnicity on sun protective behaviors,” said Mr. McKenzie and Dr. Kundu. Cultural beliefs, stigma, personal preferences, as well as a lack of “knowledge-based interventions” specifically intended for minorities could be responsible for the observed differences between population groups, they speculated.
The primary limitations of the study were its cross-sectional design and the use of self-reported data, the authors noted.
Additional research is needed to fully examine the reasons behind these differences as well as to identify appropriate interventions that promote sun protection, they added.
There was no external funding and the authors had no conflicts of interest to disclose.
SOURCE: McKenzie C and Kundu RV. J Am Acad Dermatol. 2019 Jun 19. doi: 10.1016/j.jaad.2019.06.1306.
Despite higher rates of skin cancer morbidity and mortality among racial and ethnic minorities, affected adults often are not recognizing their risks or taking preventive measures, said Costner McKenzie, BA, and Roopal V. Kundu, MD of Northwestern University, Chicago.
In a multivariable logistic regression analysis, Mr. Costner and Dr. Kundu sampled data of 33,672 adults included in the 2015 National Health Interview Survey. Data from the 2010 U.S. Census Bureau also were used to develop sample weights representative of the U.S. population. There was a survey of a smaller sample of adults who were determined to have sun-sensitive skin. The findings were published in the Journal of the American Academy of Dermatology.
Sun sensitivity was determined by skin reaction to 1 hour of unprotected sun exposure. Those who self-reported severe sunburn with blisters or moderate sunburn with peeling were determined to be sun sensitive.
The sample surveyed comprised 3,665 women (41%) and 5,287 men (59%). Of these, 82% were white non-Hispanic, 3% black non-Hispanic, 3% Asian non-Hispanic, 11% Hispanic, and 1% other non-Hispanic.
Mr. McKenzie and Dr. Kundu found that (adjusted odds ratio [aOR], 0.43, 0.54, and 0.70, respectively). Non-Hispanic blacks and Hispanics also were less likely to use sunscreen greater than SPF 15 (a0R, 0.39 and 0.64, respectively). Non-Hispanic blacks, non-Hispanic Asians, and Hispanics were less likely to have ever had a total body skin examination (aOR, 0.29, 0.21, and 0.39, respectively).
Yet these same three groups were more likely to wear long sleeves outside (non-Hispanic blacks aOR, 1.96, non-Hispanic Asians aOR, 2.09, and Hispanics aOR, 2.29). In addition, non-Hispanic Asians and Hispanics were more likely to shelter in the shade on warm, sunny days (aOR, 1.63 and 1.85, respectively).
Citing recent literature, the authors noted that although skin cancer is the most commonly diagnosed cancer, it is not typically thought of as a disease that afflicts minority populations, especially among minorities themselves, who do not generally recognize their own risk (Arch Dermatol. 2009;145[2]:207-8). In fact, morbidity and mortality from skin cancer actually are greater in racial and ethnic minorities (J Am Acad Dermatol. 2016;75[5]:983-91; J Am Acad Dermatol. 2006;55[5]:741-60), despite greater incidence of skin cancer among white adults.
“This study highlights the impact of race and ethnicity on sun protective behaviors,” said Mr. McKenzie and Dr. Kundu. Cultural beliefs, stigma, personal preferences, as well as a lack of “knowledge-based interventions” specifically intended for minorities could be responsible for the observed differences between population groups, they speculated.
The primary limitations of the study were its cross-sectional design and the use of self-reported data, the authors noted.
Additional research is needed to fully examine the reasons behind these differences as well as to identify appropriate interventions that promote sun protection, they added.
There was no external funding and the authors had no conflicts of interest to disclose.
SOURCE: McKenzie C and Kundu RV. J Am Acad Dermatol. 2019 Jun 19. doi: 10.1016/j.jaad.2019.06.1306.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Caution is key when pregnancy and psoriasis mix
NEWPORT BEACH, CALIF. – Psoriasis often clears in pregnant women, giving them a rare break from the skin disease. But
Data from 2011 found 45% of pregnancies in U.S. women aged 15-44 years were unintended (N Engl J Med. 2016 Mar 3;374[9]:843-52), cautioned Jashin J. Wu, MD, of Dermatology Research and Education Foundation, Irvine, Calif.
In a presentation at the Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar, Dr. Wu offered these tips about pregnancy and psoriasis:
Counsel patients before pregnancy
There’s conflicting data about the risks of psoriasis in pregnancy, Dr. Wu said. One 23-year-old study suggests a link to adverse outcomes such as preterm and low-birth-weight babies. But another more recent study found no sign of increased risk (Int J Dermatol. 1996;35:169-72; J Am Acad Dermatol. 2011;64:71-7).
Counseling can include information about risks such as hospitalization during pregnancy because of undertreatment of psoriasis, he said. Discuss lowering medication doses to the lowest effective dose, he recommended, and talk about alternatives to systemic medications.
Make adjustments to timing as needed
In patients with severe cases, it may be appropriate to recommend that they postpone pregnancy until their psoriasis is under better control. As for treatment of psoriasis, “you may want to consider timing medication to end around the first trimester to get the medication out of them during the greatest risk period for the baby,” Dr. Wu said.
Adjust steroids as necessary
There are no “good” studies about the use of steroids in pregnant women with psoriasis, Dr. Wu said. “We can probably assume they are safe overall. Weaker steroids may have less risk,” and some of the stronger steroids may raise concerns.
Dr. Wu made these recommendations: Limit mild-potency topical corticosteroids to less than 100 g/week, potent topical corticosteroids to less than 50 g/week, and superpotent topical corticosteroids to less than 30 g/week.
Some topical drugs appear to be OK
Vitamin D analogues have not been well-studied in pregnancy, he said, but “we consider topical use to be fairly safe.”
There’s no data on calcineurin inhibitors in pregnancy, he said, but topical use is considered to be safe because there’s limited systemic absorption.
Beware of certain drugs in pregnancyTazarotene is considered to be dangerous in pregnancy, Dr. Wu said, and females of childbearing age who take it should use effective contraception, and have a recent negative pregnancy test (within 2 weeks before treatment begins). “In general, I’d probably not use this,” he said. “We have so many other options.”
Data about pregnancy safety for three topical drugs – coal tar, anthralin, and salicylic acid – is limited or nonexistent, Dr. Wu said, and he recommends against their use in pregnancy.
Phototherapy is OK in pregnancy
Phototherapy is considered safe because UVB doesn’t penetrate the superficial layer of the skin, he said. But phototherapy brings a potential risk of lowered folic acid levels, and he urges folic acid supplementation in women undergoing the treatment who are considering pregnancy or who are in the first trimester.
Avoid certain systemic drugs
Dr. Wu offered these recommendations:
- Methotrexate: Do not take during pregnancy, or 3 months prior to conception.
- Acitretin (Soriatane): Avoid all use in women who may become pregnant.
- Cyclosporine: Be aware of reports of prematurity and low birth weight linked to the drug.
- Apremilast (Otezla): Animal studies have shown a risk in pregnancy. Stop the drug at least 2 days before conception.
Avoid monoclonal antibodies
These drugs “result in therapeutic levels in the fetus, which is not a good thing,” Dr. Wu said. “You obviously don’t want to have monoclonal antibodies in the baby.”
Nix the PUVA
While one study found no link between psoralen plus UVA (PUVA) and birth defects (Arch Dermatol. 1993 Mar;129[3]:320-3), there’s still a theoretical risk, Dr. Wu said. He recommended that the treatment be avoided during pregnancy.
Watch for waxing and waning
Dr. Wu pointed to a small 2005 study that suggested that psoriasis activity declines during pregnancy. The study used different measures, finding that psoriasis improved by 30% (based on at least a 3% change in body surface area) or 55% (based on patient self-reporting). But it flares after pregnancy as reported by 65% of women surveyed; a body surface area analysis found that psoriasis worsened in 41% (Arch Dermatol. 2005 May;141[5]:601-6).
Dr. Wu reports various relationships (research, consultation and speaking) with 15 pharmaceutical companies. SDEF and this news organization are owned by the same parent company.
NEWPORT BEACH, CALIF. – Psoriasis often clears in pregnant women, giving them a rare break from the skin disease. But
Data from 2011 found 45% of pregnancies in U.S. women aged 15-44 years were unintended (N Engl J Med. 2016 Mar 3;374[9]:843-52), cautioned Jashin J. Wu, MD, of Dermatology Research and Education Foundation, Irvine, Calif.
In a presentation at the Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar, Dr. Wu offered these tips about pregnancy and psoriasis:
Counsel patients before pregnancy
There’s conflicting data about the risks of psoriasis in pregnancy, Dr. Wu said. One 23-year-old study suggests a link to adverse outcomes such as preterm and low-birth-weight babies. But another more recent study found no sign of increased risk (Int J Dermatol. 1996;35:169-72; J Am Acad Dermatol. 2011;64:71-7).
Counseling can include information about risks such as hospitalization during pregnancy because of undertreatment of psoriasis, he said. Discuss lowering medication doses to the lowest effective dose, he recommended, and talk about alternatives to systemic medications.
Make adjustments to timing as needed
In patients with severe cases, it may be appropriate to recommend that they postpone pregnancy until their psoriasis is under better control. As for treatment of psoriasis, “you may want to consider timing medication to end around the first trimester to get the medication out of them during the greatest risk period for the baby,” Dr. Wu said.
Adjust steroids as necessary
There are no “good” studies about the use of steroids in pregnant women with psoriasis, Dr. Wu said. “We can probably assume they are safe overall. Weaker steroids may have less risk,” and some of the stronger steroids may raise concerns.
Dr. Wu made these recommendations: Limit mild-potency topical corticosteroids to less than 100 g/week, potent topical corticosteroids to less than 50 g/week, and superpotent topical corticosteroids to less than 30 g/week.
Some topical drugs appear to be OK
Vitamin D analogues have not been well-studied in pregnancy, he said, but “we consider topical use to be fairly safe.”
There’s no data on calcineurin inhibitors in pregnancy, he said, but topical use is considered to be safe because there’s limited systemic absorption.
Beware of certain drugs in pregnancyTazarotene is considered to be dangerous in pregnancy, Dr. Wu said, and females of childbearing age who take it should use effective contraception, and have a recent negative pregnancy test (within 2 weeks before treatment begins). “In general, I’d probably not use this,” he said. “We have so many other options.”
Data about pregnancy safety for three topical drugs – coal tar, anthralin, and salicylic acid – is limited or nonexistent, Dr. Wu said, and he recommends against their use in pregnancy.
Phototherapy is OK in pregnancy
Phototherapy is considered safe because UVB doesn’t penetrate the superficial layer of the skin, he said. But phototherapy brings a potential risk of lowered folic acid levels, and he urges folic acid supplementation in women undergoing the treatment who are considering pregnancy or who are in the first trimester.
Avoid certain systemic drugs
Dr. Wu offered these recommendations:
- Methotrexate: Do not take during pregnancy, or 3 months prior to conception.
- Acitretin (Soriatane): Avoid all use in women who may become pregnant.
- Cyclosporine: Be aware of reports of prematurity and low birth weight linked to the drug.
- Apremilast (Otezla): Animal studies have shown a risk in pregnancy. Stop the drug at least 2 days before conception.
Avoid monoclonal antibodies
These drugs “result in therapeutic levels in the fetus, which is not a good thing,” Dr. Wu said. “You obviously don’t want to have monoclonal antibodies in the baby.”
Nix the PUVA
While one study found no link between psoralen plus UVA (PUVA) and birth defects (Arch Dermatol. 1993 Mar;129[3]:320-3), there’s still a theoretical risk, Dr. Wu said. He recommended that the treatment be avoided during pregnancy.
Watch for waxing and waning
Dr. Wu pointed to a small 2005 study that suggested that psoriasis activity declines during pregnancy. The study used different measures, finding that psoriasis improved by 30% (based on at least a 3% change in body surface area) or 55% (based on patient self-reporting). But it flares after pregnancy as reported by 65% of women surveyed; a body surface area analysis found that psoriasis worsened in 41% (Arch Dermatol. 2005 May;141[5]:601-6).
Dr. Wu reports various relationships (research, consultation and speaking) with 15 pharmaceutical companies. SDEF and this news organization are owned by the same parent company.
NEWPORT BEACH, CALIF. – Psoriasis often clears in pregnant women, giving them a rare break from the skin disease. But
Data from 2011 found 45% of pregnancies in U.S. women aged 15-44 years were unintended (N Engl J Med. 2016 Mar 3;374[9]:843-52), cautioned Jashin J. Wu, MD, of Dermatology Research and Education Foundation, Irvine, Calif.
In a presentation at the Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar, Dr. Wu offered these tips about pregnancy and psoriasis:
Counsel patients before pregnancy
There’s conflicting data about the risks of psoriasis in pregnancy, Dr. Wu said. One 23-year-old study suggests a link to adverse outcomes such as preterm and low-birth-weight babies. But another more recent study found no sign of increased risk (Int J Dermatol. 1996;35:169-72; J Am Acad Dermatol. 2011;64:71-7).
Counseling can include information about risks such as hospitalization during pregnancy because of undertreatment of psoriasis, he said. Discuss lowering medication doses to the lowest effective dose, he recommended, and talk about alternatives to systemic medications.
Make adjustments to timing as needed
In patients with severe cases, it may be appropriate to recommend that they postpone pregnancy until their psoriasis is under better control. As for treatment of psoriasis, “you may want to consider timing medication to end around the first trimester to get the medication out of them during the greatest risk period for the baby,” Dr. Wu said.
Adjust steroids as necessary
There are no “good” studies about the use of steroids in pregnant women with psoriasis, Dr. Wu said. “We can probably assume they are safe overall. Weaker steroids may have less risk,” and some of the stronger steroids may raise concerns.
Dr. Wu made these recommendations: Limit mild-potency topical corticosteroids to less than 100 g/week, potent topical corticosteroids to less than 50 g/week, and superpotent topical corticosteroids to less than 30 g/week.
Some topical drugs appear to be OK
Vitamin D analogues have not been well-studied in pregnancy, he said, but “we consider topical use to be fairly safe.”
There’s no data on calcineurin inhibitors in pregnancy, he said, but topical use is considered to be safe because there’s limited systemic absorption.
Beware of certain drugs in pregnancyTazarotene is considered to be dangerous in pregnancy, Dr. Wu said, and females of childbearing age who take it should use effective contraception, and have a recent negative pregnancy test (within 2 weeks before treatment begins). “In general, I’d probably not use this,” he said. “We have so many other options.”
Data about pregnancy safety for three topical drugs – coal tar, anthralin, and salicylic acid – is limited or nonexistent, Dr. Wu said, and he recommends against their use in pregnancy.
Phototherapy is OK in pregnancy
Phototherapy is considered safe because UVB doesn’t penetrate the superficial layer of the skin, he said. But phototherapy brings a potential risk of lowered folic acid levels, and he urges folic acid supplementation in women undergoing the treatment who are considering pregnancy or who are in the first trimester.
Avoid certain systemic drugs
Dr. Wu offered these recommendations:
- Methotrexate: Do not take during pregnancy, or 3 months prior to conception.
- Acitretin (Soriatane): Avoid all use in women who may become pregnant.
- Cyclosporine: Be aware of reports of prematurity and low birth weight linked to the drug.
- Apremilast (Otezla): Animal studies have shown a risk in pregnancy. Stop the drug at least 2 days before conception.
Avoid monoclonal antibodies
These drugs “result in therapeutic levels in the fetus, which is not a good thing,” Dr. Wu said. “You obviously don’t want to have monoclonal antibodies in the baby.”
Nix the PUVA
While one study found no link between psoralen plus UVA (PUVA) and birth defects (Arch Dermatol. 1993 Mar;129[3]:320-3), there’s still a theoretical risk, Dr. Wu said. He recommended that the treatment be avoided during pregnancy.
Watch for waxing and waning
Dr. Wu pointed to a small 2005 study that suggested that psoriasis activity declines during pregnancy. The study used different measures, finding that psoriasis improved by 30% (based on at least a 3% change in body surface area) or 55% (based on patient self-reporting). But it flares after pregnancy as reported by 65% of women surveyed; a body surface area analysis found that psoriasis worsened in 41% (Arch Dermatol. 2005 May;141[5]:601-6).
Dr. Wu reports various relationships (research, consultation and speaking) with 15 pharmaceutical companies. SDEF and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR
In psoriasis, risankizumab outperforms adalimumab
Results of the IMMvent trial were published online ahead of print July 4 in the Lancet.
Risankizumab targets the p19 subunit of the cytokine IL-23. Selectivity for p19 has the potential to be safer than some other approaches that target the p40 subunit, because p19 is specific to IL-23, and many immune defense processes can function without IL-23. The p40 subunit is shared with IL-12, and blocking it can therefore lead to off-target effects.
Risankizumab was previously shown to have superior safety and efficacy over ustekinumab, which inhibits a subunit shared by IL-23 and IL-12 (Gordon KB et al. Lancet. 2018;392[10148]:650-61). Adalimumab is a TNF-alpha inhibitor that is frequently used to treat psoriasis, and which became available in biosimilar form in Europe in 2018.
The researchers randomized 605 adult patients from 66 sites in 11 countries to receive either risankizumab or adalimumab. The first phase (Part A) of the trial lasted up to 16 weeks, and tested the general superiority of risankizumab over adalimumab. The second phase (Part B), from week 16 to 44, evaluated the efficacy of risankizumab in participants who experienced an intermediate response, defined as Psoriasis Area and Severity Index (PASI) score of 50-90.
At the start of Part B, subjects initially receiving adalimumab who had at least a 90% improvement in PASI stayed on adalimumab (PASI 90), while those who had less than 50% improvement in PASI were switched to risankizumab. The remaining intermediate responders (PASI 50-90) were re-randomized to continue adalimumab or switch to risankizumab. All subjects initially randomized to risankizumab continued risankizumab during part B.
At the end of Part A, 72% of the risankizumab group achieved PASI 90, compared with 47% in the adalimumab group (p < 0.0001). A total of 84% in the risankizumab group had a static Physician’s Global Assessment (sPGA) score of 0 or 1 at the end of Part A, compared with 60% in the adalimumab group (p < 0.0001).
During Part B, among intermediate adalimumab responders, 66% of those switched to risankizumab achieved PASI 90, compared with 21% of continued on adalimumab (p < 0.0001).
In Part A, 56% of patients taking risankizumab experienced an adverse event, as did 57% of those taking adalimumab. Among adalimumab intermediate responders, 75% of those who switched to risankizumab during Part B had an adverse event, compared with 66% of those who continued adalimumab.
SOURCE: Reich K, et al. Lancet 2019, July 4 .
Until recently, TNF-alpha inhibitors have been the most commonly prescribed biologic agents for psoriasis. They are more targeted than small molecules like cyclosporine or methotrexate, but still are associated with immune side effects like infection and malignancy. Drugs that specifically target IL-23 home in on the pathogenicity of psoriasis, and they are not associated with infection and malignancy. The results of this study offer evidence that IL-23 inhibitors represent another effective and convenient option for the treatment of psoriasis. Physicians can select from IL-23 inhibitors, IL-17 inhibitors, and TNF-alpha inhibitors to determine the optimal treatment for patients based on patient weight, childbearing status, age, and comorbid conditions.
Mark Lebwohl, MD, is in the department of dermatology at Icahn School of Medicine at Mount Sinai, New York.
Until recently, TNF-alpha inhibitors have been the most commonly prescribed biologic agents for psoriasis. They are more targeted than small molecules like cyclosporine or methotrexate, but still are associated with immune side effects like infection and malignancy. Drugs that specifically target IL-23 home in on the pathogenicity of psoriasis, and they are not associated with infection and malignancy. The results of this study offer evidence that IL-23 inhibitors represent another effective and convenient option for the treatment of psoriasis. Physicians can select from IL-23 inhibitors, IL-17 inhibitors, and TNF-alpha inhibitors to determine the optimal treatment for patients based on patient weight, childbearing status, age, and comorbid conditions.
Mark Lebwohl, MD, is in the department of dermatology at Icahn School of Medicine at Mount Sinai, New York.
Until recently, TNF-alpha inhibitors have been the most commonly prescribed biologic agents for psoriasis. They are more targeted than small molecules like cyclosporine or methotrexate, but still are associated with immune side effects like infection and malignancy. Drugs that specifically target IL-23 home in on the pathogenicity of psoriasis, and they are not associated with infection and malignancy. The results of this study offer evidence that IL-23 inhibitors represent another effective and convenient option for the treatment of psoriasis. Physicians can select from IL-23 inhibitors, IL-17 inhibitors, and TNF-alpha inhibitors to determine the optimal treatment for patients based on patient weight, childbearing status, age, and comorbid conditions.
Mark Lebwohl, MD, is in the department of dermatology at Icahn School of Medicine at Mount Sinai, New York.
Results of the IMMvent trial were published online ahead of print July 4 in the Lancet.
Risankizumab targets the p19 subunit of the cytokine IL-23. Selectivity for p19 has the potential to be safer than some other approaches that target the p40 subunit, because p19 is specific to IL-23, and many immune defense processes can function without IL-23. The p40 subunit is shared with IL-12, and blocking it can therefore lead to off-target effects.
Risankizumab was previously shown to have superior safety and efficacy over ustekinumab, which inhibits a subunit shared by IL-23 and IL-12 (Gordon KB et al. Lancet. 2018;392[10148]:650-61). Adalimumab is a TNF-alpha inhibitor that is frequently used to treat psoriasis, and which became available in biosimilar form in Europe in 2018.
The researchers randomized 605 adult patients from 66 sites in 11 countries to receive either risankizumab or adalimumab. The first phase (Part A) of the trial lasted up to 16 weeks, and tested the general superiority of risankizumab over adalimumab. The second phase (Part B), from week 16 to 44, evaluated the efficacy of risankizumab in participants who experienced an intermediate response, defined as Psoriasis Area and Severity Index (PASI) score of 50-90.
At the start of Part B, subjects initially receiving adalimumab who had at least a 90% improvement in PASI stayed on adalimumab (PASI 90), while those who had less than 50% improvement in PASI were switched to risankizumab. The remaining intermediate responders (PASI 50-90) were re-randomized to continue adalimumab or switch to risankizumab. All subjects initially randomized to risankizumab continued risankizumab during part B.
At the end of Part A, 72% of the risankizumab group achieved PASI 90, compared with 47% in the adalimumab group (p < 0.0001). A total of 84% in the risankizumab group had a static Physician’s Global Assessment (sPGA) score of 0 or 1 at the end of Part A, compared with 60% in the adalimumab group (p < 0.0001).
During Part B, among intermediate adalimumab responders, 66% of those switched to risankizumab achieved PASI 90, compared with 21% of continued on adalimumab (p < 0.0001).
In Part A, 56% of patients taking risankizumab experienced an adverse event, as did 57% of those taking adalimumab. Among adalimumab intermediate responders, 75% of those who switched to risankizumab during Part B had an adverse event, compared with 66% of those who continued adalimumab.
SOURCE: Reich K, et al. Lancet 2019, July 4 .
Results of the IMMvent trial were published online ahead of print July 4 in the Lancet.
Risankizumab targets the p19 subunit of the cytokine IL-23. Selectivity for p19 has the potential to be safer than some other approaches that target the p40 subunit, because p19 is specific to IL-23, and many immune defense processes can function without IL-23. The p40 subunit is shared with IL-12, and blocking it can therefore lead to off-target effects.
Risankizumab was previously shown to have superior safety and efficacy over ustekinumab, which inhibits a subunit shared by IL-23 and IL-12 (Gordon KB et al. Lancet. 2018;392[10148]:650-61). Adalimumab is a TNF-alpha inhibitor that is frequently used to treat psoriasis, and which became available in biosimilar form in Europe in 2018.
The researchers randomized 605 adult patients from 66 sites in 11 countries to receive either risankizumab or adalimumab. The first phase (Part A) of the trial lasted up to 16 weeks, and tested the general superiority of risankizumab over adalimumab. The second phase (Part B), from week 16 to 44, evaluated the efficacy of risankizumab in participants who experienced an intermediate response, defined as Psoriasis Area and Severity Index (PASI) score of 50-90.
At the start of Part B, subjects initially receiving adalimumab who had at least a 90% improvement in PASI stayed on adalimumab (PASI 90), while those who had less than 50% improvement in PASI were switched to risankizumab. The remaining intermediate responders (PASI 50-90) were re-randomized to continue adalimumab or switch to risankizumab. All subjects initially randomized to risankizumab continued risankizumab during part B.
At the end of Part A, 72% of the risankizumab group achieved PASI 90, compared with 47% in the adalimumab group (p < 0.0001). A total of 84% in the risankizumab group had a static Physician’s Global Assessment (sPGA) score of 0 or 1 at the end of Part A, compared with 60% in the adalimumab group (p < 0.0001).
During Part B, among intermediate adalimumab responders, 66% of those switched to risankizumab achieved PASI 90, compared with 21% of continued on adalimumab (p < 0.0001).
In Part A, 56% of patients taking risankizumab experienced an adverse event, as did 57% of those taking adalimumab. Among adalimumab intermediate responders, 75% of those who switched to risankizumab during Part B had an adverse event, compared with 66% of those who continued adalimumab.
SOURCE: Reich K, et al. Lancet 2019, July 4 .
FROM THE LANCET
Topical calcineurin inhibitors are an effective treatment option for pediatric periorificial dermatitis
AUSTIN, TEX. – , results from a retrospective cohort study showed.
The mainstays of treatment for POD include topical and oral antibiotics. In an interview prior to the annual meeting of the Society for Pediatric Dermatology, Ayelet Ollech, MD, said that the most common systemic agents used include erythromycin, azithromycin, and, in patients older than 8-10 years of age, minocycline or doxycycline. Topical agents, which are often used as monotherapy in mild disease, include metronidazole, clindamycin, erythromycin, sodium sulfacetamide, and, less often, azelaic acid, topical retinoids, and ivermectin. “TCIs (pimecrolimus 1% cream and tacrolimus 0.03% or 0.1% ointment) are a good steroid sparing option for POD,” said Dr. Ollech, a pediatric dermatology fellow at Ann & Robert H. Lurie Children’s Hospital of Chicago. “In the adult population, two randomized controlled studies of pimecrolimus 1% cream showed good results. In the pediatric population, there are only a few case series and case reports of TCIs for the treatment of POD.”
In what is believed to be the largest study of its kind, Dr. Ollech, Anthony J. Mancini, MD, and colleagues assessed the clinical utility of TCI in 132 pediatric patients with POD who were treated in the division of dermatology at Children’s Hospital of Chicago between 2008 and 2018. The researchers made note of epidemiologic variables, personal and family medical histories, possible triggers, duration of illness, previous treatments, distribution (periocular, perinasal, perioral, extra facial regions), severity of POD, treatment(s) prescribed, duration of therapy, clinical response, recurrences, and side effects. In an effort to capture missing data, the researchers performed follow-up via telephone for all patients who lacked appropriate follow-up documentation in the medical record.
Of the 132 patients, the female: male ratio was 1.2:1 and the median age at diagnosis was 4.2 years. About one-third of patients (33%) had involvement of one region, 38% had involvement of two regions, 26% had involvement of three regions, and 3% patients had involvement of all regions. The most common disorders on medical history were atopic dermatitis and asthma (in 29% and 17% of patients, respectively).
Dr. Ollech reported that 72 of the 132 patients (55%) had evaluable follow up data via either medical record documentation or the phone questionnaire. Of these, 67% were treated with TCI alone, 19% were treated with a combination of TCI and topical metronidazole, and 10% were treated with a combination of TCI and a systemic antibiotic. The median duration of treatment was 60 days. The researchers observed complete response in 65% of patients treated with TCI alone, in 64% of those treated with TCI and metronidazole, and in 70% of those treated with TCI and a systemic antibiotic. Adverse events attributed to TCI were rare and mild in severity.
“We were surprised that there were almost no reported side effects from the usage of TCIs as it is known that these agents can cause a burning or stinging sensation,” Dr. Ollech said. “Only one case described this side effect. We found 30% of the patients to have associated atopic dermatitis (AD) as well as a few patients with irritant dermatitis. We were also surprised how convenient the TCI treatment was for a patient who had POD and concomitant facial AD or even irritant dermatitis as an agent that can treat both. This can be very helpful for the parents that apply the medication to have a single solution to more than one rash.”
The researchers noted recurrence of POD in 14% of patients overall, including 6% of patients treated with TCI alone, 29% of patients treated with TCI and metronidazole, and 30% of patients treated with TCI and a systemic antibiotic.
Dr. Ollech acknowledged certain limitations of the study, including its retrospective design and lack of a control group. She and her colleagues reported having no financial disclosures.
SOURCE: Ollech A et al. SPD 2019, poster 23.
AUSTIN, TEX. – , results from a retrospective cohort study showed.
The mainstays of treatment for POD include topical and oral antibiotics. In an interview prior to the annual meeting of the Society for Pediatric Dermatology, Ayelet Ollech, MD, said that the most common systemic agents used include erythromycin, azithromycin, and, in patients older than 8-10 years of age, minocycline or doxycycline. Topical agents, which are often used as monotherapy in mild disease, include metronidazole, clindamycin, erythromycin, sodium sulfacetamide, and, less often, azelaic acid, topical retinoids, and ivermectin. “TCIs (pimecrolimus 1% cream and tacrolimus 0.03% or 0.1% ointment) are a good steroid sparing option for POD,” said Dr. Ollech, a pediatric dermatology fellow at Ann & Robert H. Lurie Children’s Hospital of Chicago. “In the adult population, two randomized controlled studies of pimecrolimus 1% cream showed good results. In the pediatric population, there are only a few case series and case reports of TCIs for the treatment of POD.”
In what is believed to be the largest study of its kind, Dr. Ollech, Anthony J. Mancini, MD, and colleagues assessed the clinical utility of TCI in 132 pediatric patients with POD who were treated in the division of dermatology at Children’s Hospital of Chicago between 2008 and 2018. The researchers made note of epidemiologic variables, personal and family medical histories, possible triggers, duration of illness, previous treatments, distribution (periocular, perinasal, perioral, extra facial regions), severity of POD, treatment(s) prescribed, duration of therapy, clinical response, recurrences, and side effects. In an effort to capture missing data, the researchers performed follow-up via telephone for all patients who lacked appropriate follow-up documentation in the medical record.
Of the 132 patients, the female: male ratio was 1.2:1 and the median age at diagnosis was 4.2 years. About one-third of patients (33%) had involvement of one region, 38% had involvement of two regions, 26% had involvement of three regions, and 3% patients had involvement of all regions. The most common disorders on medical history were atopic dermatitis and asthma (in 29% and 17% of patients, respectively).
Dr. Ollech reported that 72 of the 132 patients (55%) had evaluable follow up data via either medical record documentation or the phone questionnaire. Of these, 67% were treated with TCI alone, 19% were treated with a combination of TCI and topical metronidazole, and 10% were treated with a combination of TCI and a systemic antibiotic. The median duration of treatment was 60 days. The researchers observed complete response in 65% of patients treated with TCI alone, in 64% of those treated with TCI and metronidazole, and in 70% of those treated with TCI and a systemic antibiotic. Adverse events attributed to TCI were rare and mild in severity.
“We were surprised that there were almost no reported side effects from the usage of TCIs as it is known that these agents can cause a burning or stinging sensation,” Dr. Ollech said. “Only one case described this side effect. We found 30% of the patients to have associated atopic dermatitis (AD) as well as a few patients with irritant dermatitis. We were also surprised how convenient the TCI treatment was for a patient who had POD and concomitant facial AD or even irritant dermatitis as an agent that can treat both. This can be very helpful for the parents that apply the medication to have a single solution to more than one rash.”
The researchers noted recurrence of POD in 14% of patients overall, including 6% of patients treated with TCI alone, 29% of patients treated with TCI and metronidazole, and 30% of patients treated with TCI and a systemic antibiotic.
Dr. Ollech acknowledged certain limitations of the study, including its retrospective design and lack of a control group. She and her colleagues reported having no financial disclosures.
SOURCE: Ollech A et al. SPD 2019, poster 23.
AUSTIN, TEX. – , results from a retrospective cohort study showed.
The mainstays of treatment for POD include topical and oral antibiotics. In an interview prior to the annual meeting of the Society for Pediatric Dermatology, Ayelet Ollech, MD, said that the most common systemic agents used include erythromycin, azithromycin, and, in patients older than 8-10 years of age, minocycline or doxycycline. Topical agents, which are often used as monotherapy in mild disease, include metronidazole, clindamycin, erythromycin, sodium sulfacetamide, and, less often, azelaic acid, topical retinoids, and ivermectin. “TCIs (pimecrolimus 1% cream and tacrolimus 0.03% or 0.1% ointment) are a good steroid sparing option for POD,” said Dr. Ollech, a pediatric dermatology fellow at Ann & Robert H. Lurie Children’s Hospital of Chicago. “In the adult population, two randomized controlled studies of pimecrolimus 1% cream showed good results. In the pediatric population, there are only a few case series and case reports of TCIs for the treatment of POD.”
In what is believed to be the largest study of its kind, Dr. Ollech, Anthony J. Mancini, MD, and colleagues assessed the clinical utility of TCI in 132 pediatric patients with POD who were treated in the division of dermatology at Children’s Hospital of Chicago between 2008 and 2018. The researchers made note of epidemiologic variables, personal and family medical histories, possible triggers, duration of illness, previous treatments, distribution (periocular, perinasal, perioral, extra facial regions), severity of POD, treatment(s) prescribed, duration of therapy, clinical response, recurrences, and side effects. In an effort to capture missing data, the researchers performed follow-up via telephone for all patients who lacked appropriate follow-up documentation in the medical record.
Of the 132 patients, the female: male ratio was 1.2:1 and the median age at diagnosis was 4.2 years. About one-third of patients (33%) had involvement of one region, 38% had involvement of two regions, 26% had involvement of three regions, and 3% patients had involvement of all regions. The most common disorders on medical history were atopic dermatitis and asthma (in 29% and 17% of patients, respectively).
Dr. Ollech reported that 72 of the 132 patients (55%) had evaluable follow up data via either medical record documentation or the phone questionnaire. Of these, 67% were treated with TCI alone, 19% were treated with a combination of TCI and topical metronidazole, and 10% were treated with a combination of TCI and a systemic antibiotic. The median duration of treatment was 60 days. The researchers observed complete response in 65% of patients treated with TCI alone, in 64% of those treated with TCI and metronidazole, and in 70% of those treated with TCI and a systemic antibiotic. Adverse events attributed to TCI were rare and mild in severity.
“We were surprised that there were almost no reported side effects from the usage of TCIs as it is known that these agents can cause a burning or stinging sensation,” Dr. Ollech said. “Only one case described this side effect. We found 30% of the patients to have associated atopic dermatitis (AD) as well as a few patients with irritant dermatitis. We were also surprised how convenient the TCI treatment was for a patient who had POD and concomitant facial AD or even irritant dermatitis as an agent that can treat both. This can be very helpful for the parents that apply the medication to have a single solution to more than one rash.”
The researchers noted recurrence of POD in 14% of patients overall, including 6% of patients treated with TCI alone, 29% of patients treated with TCI and metronidazole, and 30% of patients treated with TCI and a systemic antibiotic.
Dr. Ollech acknowledged certain limitations of the study, including its retrospective design and lack of a control group. She and her colleagues reported having no financial disclosures.
SOURCE: Ollech A et al. SPD 2019, poster 23.
REPORTING FROM SPD 2019
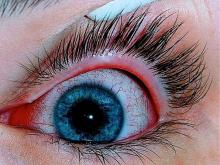
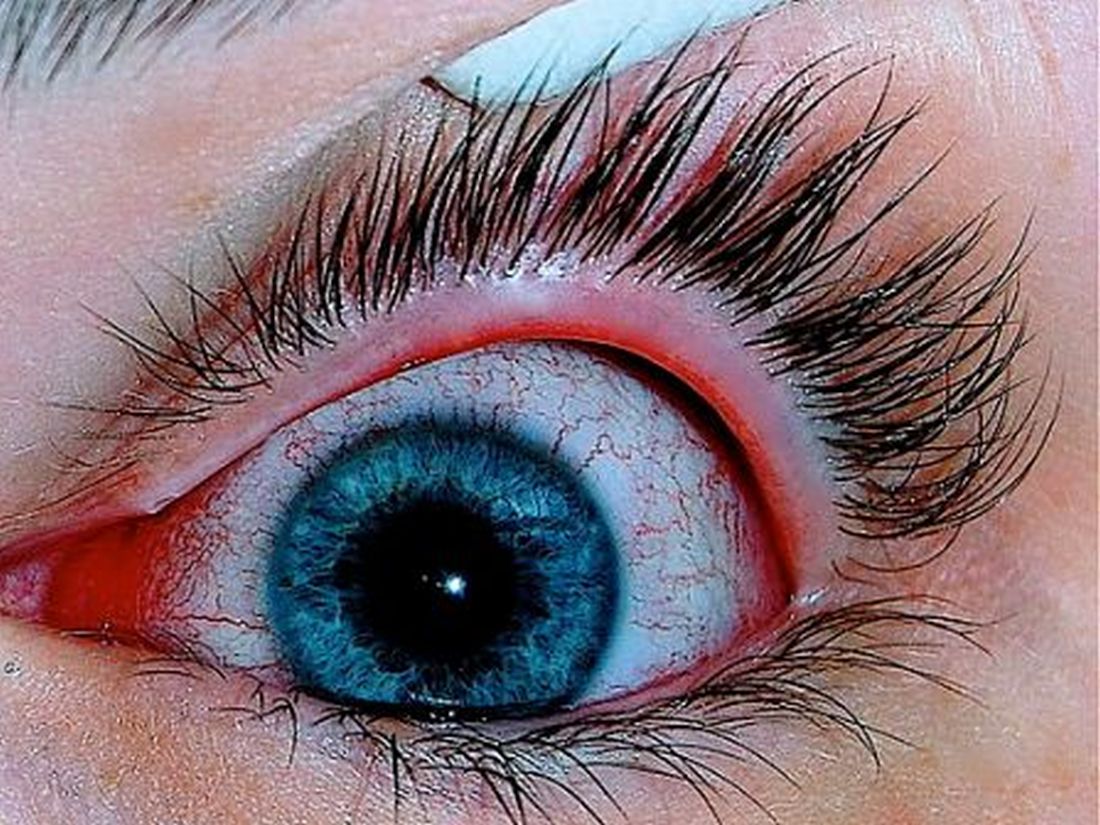
Patients with AD should routinely be asked about conjunctivitis
according to a recent position statement from the International Eczema Council.
Patients with AD who develop conjunctivitis during dupilumab treatment may experience “bilateral inflammation of the anterior conjunctiva and hyperaemia of the limbus, which may cause nodular swelling.”according to the statement, which pertains to conjunctivitis in AD patients, “with and without dupilumab therapy.” (J Eur Acad Dermatol Venereol. 2019 May 6. doi: 10.1111/jdv.15608). Currently, there are no predictive factors of conjunctivitis and no guidance in the literature on how to manage conjunctivitis associated with dupilumab, which in some cases can make it necessary to stop treatment, the authors wrote.
The International Eczema Council (IEC) polled 86 dermatologists in 22 countries who are experts in AD; 46 members responded from 19 countries, including dermatologists from Australia, Canada, Denmark, France, Germany, Japan, Korea, the Netherlands, the United Kingdom, and the United States. The questions centered on the diagnosis and management of conjunctivitis in AD patients, and whether to refer cases to an ophthalmologist. Consensus was achieved if less than 30% of participants disagreed with a statement. IEC members then met in person at a European Academy of Dermatology and Venereology meeting in Paris to discuss the results of the survey. Survey respondents noted they had seen dupilumab-associated conjunctivitis in both pediatric and adult patients.
The IEC members recommended that:
- Patients should be informed about the risks of conjunctivitis before being prescribed dupilumab.
- AD patients should be asked “routinely” about ocular complaints or symptoms.
- AD patients with conjunctivitis should be referred to an ophthalmologist for diagnosis and treatment.
- AD patients with new-onset conjunctivitis during dupilumab treatment always should be referred to an ophthalmologist, especially in more severe cases such as when they do not respond to treatment with antihistamine or artificial tears.
- Dermatologists also should rule out keratoconjunctivitis before treating with dupilumab, as it may cause keratitis and blindness.
- Patients who have had keratoconjunctivitis in the past should not be precluded from treatment with dupilumab, and those who develop conjunctivitis during treatment should be referred to an ophthalmologist – but should stay on treatment while waiting for the consult.
“It was stressed that at this moment there are also no reliable data on the course of atopic keratoconjunctivitis and vernal keratoconjunctivitis during dupilumab treatment,” according to Jacob P. Thyssen, MD, PhD, Herlev and Gentofte Hospital, Hellerup, Denmark, and coauthors. These patients “should be carefully monitored by an ophthalmologist before and during treatment with dupilumab.”
The recommendations also centered around which treatments should be initiated by dermatologists, and which should be referred to ophthalmologists. Those patients with conjunctivitis should receive eye drops, eye ointment, or oral antihistamines from their dermatologists before an ophthalmology referral, the IEC members said. Dermatologists also should perform, or refer patients for, skin prick testing or specific IgE testing for aeroallergens in patients with AD who have conjunctivitis, and patch testing with an “ophthalmologic series, and native eye drops/ointments to diagnose possible delayed type hypersensitivity reactions to topical ingredients,” they added.
Among the treatments for conjunctivitis best left to ophthalmologists are cyclosporine, tacrolimus, or corticosteroid eye drops.
“Despite the more limited experience with eye drops by dermatologists, rapid access to ophthalmological service may be difficult, sometimes warranting a short course of corticosteroid eye drops without ophthalmological consultations,” Dr. Thyssen and associates said. “However, persistent or recurrent conjunctivitis requiring repeated or prolonged use of corticosteroid, tacrolimus, and ciclosporin-containing eye drops, must be managed by an ophthalmologist, given the risk of glaucoma, cataract, and infections.”
“The AD severity, conjunctivitis severity, possible contraindications, possible effect of dupilumab therapy on concomitant asthma or other comorbidities, as well as other treatment options, should be considered on an individual patient basis,” the authors concluded.
The IEC survey was limited by the small survey response and reliance on expert opinion.
The authors reported personal and institutional relationships in the form of grants, corporate sponsorships, advisory board memberships, investigator appointments, speakers bureau positions, and consultancies for a variety of pharmaceutical companies, agencies, societies, and other organizations. No funding was obtained for the study.
This article was updated 7/17/19.
according to a recent position statement from the International Eczema Council.
Patients with AD who develop conjunctivitis during dupilumab treatment may experience “bilateral inflammation of the anterior conjunctiva and hyperaemia of the limbus, which may cause nodular swelling.”according to the statement, which pertains to conjunctivitis in AD patients, “with and without dupilumab therapy.” (J Eur Acad Dermatol Venereol. 2019 May 6. doi: 10.1111/jdv.15608). Currently, there are no predictive factors of conjunctivitis and no guidance in the literature on how to manage conjunctivitis associated with dupilumab, which in some cases can make it necessary to stop treatment, the authors wrote.
The International Eczema Council (IEC) polled 86 dermatologists in 22 countries who are experts in AD; 46 members responded from 19 countries, including dermatologists from Australia, Canada, Denmark, France, Germany, Japan, Korea, the Netherlands, the United Kingdom, and the United States. The questions centered on the diagnosis and management of conjunctivitis in AD patients, and whether to refer cases to an ophthalmologist. Consensus was achieved if less than 30% of participants disagreed with a statement. IEC members then met in person at a European Academy of Dermatology and Venereology meeting in Paris to discuss the results of the survey. Survey respondents noted they had seen dupilumab-associated conjunctivitis in both pediatric and adult patients.
The IEC members recommended that:
- Patients should be informed about the risks of conjunctivitis before being prescribed dupilumab.
- AD patients should be asked “routinely” about ocular complaints or symptoms.
- AD patients with conjunctivitis should be referred to an ophthalmologist for diagnosis and treatment.
- AD patients with new-onset conjunctivitis during dupilumab treatment always should be referred to an ophthalmologist, especially in more severe cases such as when they do not respond to treatment with antihistamine or artificial tears.
- Dermatologists also should rule out keratoconjunctivitis before treating with dupilumab, as it may cause keratitis and blindness.
- Patients who have had keratoconjunctivitis in the past should not be precluded from treatment with dupilumab, and those who develop conjunctivitis during treatment should be referred to an ophthalmologist – but should stay on treatment while waiting for the consult.
“It was stressed that at this moment there are also no reliable data on the course of atopic keratoconjunctivitis and vernal keratoconjunctivitis during dupilumab treatment,” according to Jacob P. Thyssen, MD, PhD, Herlev and Gentofte Hospital, Hellerup, Denmark, and coauthors. These patients “should be carefully monitored by an ophthalmologist before and during treatment with dupilumab.”
The recommendations also centered around which treatments should be initiated by dermatologists, and which should be referred to ophthalmologists. Those patients with conjunctivitis should receive eye drops, eye ointment, or oral antihistamines from their dermatologists before an ophthalmology referral, the IEC members said. Dermatologists also should perform, or refer patients for, skin prick testing or specific IgE testing for aeroallergens in patients with AD who have conjunctivitis, and patch testing with an “ophthalmologic series, and native eye drops/ointments to diagnose possible delayed type hypersensitivity reactions to topical ingredients,” they added.
Among the treatments for conjunctivitis best left to ophthalmologists are cyclosporine, tacrolimus, or corticosteroid eye drops.
“Despite the more limited experience with eye drops by dermatologists, rapid access to ophthalmological service may be difficult, sometimes warranting a short course of corticosteroid eye drops without ophthalmological consultations,” Dr. Thyssen and associates said. “However, persistent or recurrent conjunctivitis requiring repeated or prolonged use of corticosteroid, tacrolimus, and ciclosporin-containing eye drops, must be managed by an ophthalmologist, given the risk of glaucoma, cataract, and infections.”
“The AD severity, conjunctivitis severity, possible contraindications, possible effect of dupilumab therapy on concomitant asthma or other comorbidities, as well as other treatment options, should be considered on an individual patient basis,” the authors concluded.
The IEC survey was limited by the small survey response and reliance on expert opinion.
The authors reported personal and institutional relationships in the form of grants, corporate sponsorships, advisory board memberships, investigator appointments, speakers bureau positions, and consultancies for a variety of pharmaceutical companies, agencies, societies, and other organizations. No funding was obtained for the study.
This article was updated 7/17/19.
according to a recent position statement from the International Eczema Council.
Patients with AD who develop conjunctivitis during dupilumab treatment may experience “bilateral inflammation of the anterior conjunctiva and hyperaemia of the limbus, which may cause nodular swelling.”according to the statement, which pertains to conjunctivitis in AD patients, “with and without dupilumab therapy.” (J Eur Acad Dermatol Venereol. 2019 May 6. doi: 10.1111/jdv.15608). Currently, there are no predictive factors of conjunctivitis and no guidance in the literature on how to manage conjunctivitis associated with dupilumab, which in some cases can make it necessary to stop treatment, the authors wrote.
The International Eczema Council (IEC) polled 86 dermatologists in 22 countries who are experts in AD; 46 members responded from 19 countries, including dermatologists from Australia, Canada, Denmark, France, Germany, Japan, Korea, the Netherlands, the United Kingdom, and the United States. The questions centered on the diagnosis and management of conjunctivitis in AD patients, and whether to refer cases to an ophthalmologist. Consensus was achieved if less than 30% of participants disagreed with a statement. IEC members then met in person at a European Academy of Dermatology and Venereology meeting in Paris to discuss the results of the survey. Survey respondents noted they had seen dupilumab-associated conjunctivitis in both pediatric and adult patients.
The IEC members recommended that:
- Patients should be informed about the risks of conjunctivitis before being prescribed dupilumab.
- AD patients should be asked “routinely” about ocular complaints or symptoms.
- AD patients with conjunctivitis should be referred to an ophthalmologist for diagnosis and treatment.
- AD patients with new-onset conjunctivitis during dupilumab treatment always should be referred to an ophthalmologist, especially in more severe cases such as when they do not respond to treatment with antihistamine or artificial tears.
- Dermatologists also should rule out keratoconjunctivitis before treating with dupilumab, as it may cause keratitis and blindness.
- Patients who have had keratoconjunctivitis in the past should not be precluded from treatment with dupilumab, and those who develop conjunctivitis during treatment should be referred to an ophthalmologist – but should stay on treatment while waiting for the consult.
“It was stressed that at this moment there are also no reliable data on the course of atopic keratoconjunctivitis and vernal keratoconjunctivitis during dupilumab treatment,” according to Jacob P. Thyssen, MD, PhD, Herlev and Gentofte Hospital, Hellerup, Denmark, and coauthors. These patients “should be carefully monitored by an ophthalmologist before and during treatment with dupilumab.”
The recommendations also centered around which treatments should be initiated by dermatologists, and which should be referred to ophthalmologists. Those patients with conjunctivitis should receive eye drops, eye ointment, or oral antihistamines from their dermatologists before an ophthalmology referral, the IEC members said. Dermatologists also should perform, or refer patients for, skin prick testing or specific IgE testing for aeroallergens in patients with AD who have conjunctivitis, and patch testing with an “ophthalmologic series, and native eye drops/ointments to diagnose possible delayed type hypersensitivity reactions to topical ingredients,” they added.
Among the treatments for conjunctivitis best left to ophthalmologists are cyclosporine, tacrolimus, or corticosteroid eye drops.
“Despite the more limited experience with eye drops by dermatologists, rapid access to ophthalmological service may be difficult, sometimes warranting a short course of corticosteroid eye drops without ophthalmological consultations,” Dr. Thyssen and associates said. “However, persistent or recurrent conjunctivitis requiring repeated or prolonged use of corticosteroid, tacrolimus, and ciclosporin-containing eye drops, must be managed by an ophthalmologist, given the risk of glaucoma, cataract, and infections.”
“The AD severity, conjunctivitis severity, possible contraindications, possible effect of dupilumab therapy on concomitant asthma or other comorbidities, as well as other treatment options, should be considered on an individual patient basis,” the authors concluded.
The IEC survey was limited by the small survey response and reliance on expert opinion.
The authors reported personal and institutional relationships in the form of grants, corporate sponsorships, advisory board memberships, investigator appointments, speakers bureau positions, and consultancies for a variety of pharmaceutical companies, agencies, societies, and other organizations. No funding was obtained for the study.
This article was updated 7/17/19.
FROM THE JOURNAL OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Cellulitis ranks as top reason for skin-related pediatric inpatient admissions
AUSTIN, TEX. – showed.
“Skin conditions significantly affect pediatric inpatients, and dermatologists ought be accessible for consultation to enhance care and costs,” the study’s first author, Marcus L. Elias, said in an interview prior to the annual meeting of the Society for Pediatric Dermatology.
According to Mr. Elias, who is a 4th-year medical student at Rutgers New Jersey Medical School–Newark, few national studies on skin diseases for pediatric inpatients have been published in the medical literature. Earlier this year, researchers examined inpatient dermatologic conditions in patients aged 18 years and older (J Am Acad Dermatol 2019;80[2]:425-32), but Mr. Elias and associates set out to analyze the burden of inpatient pediatric dermatologic conditions on a national basis. “We wanted to see if the same conditions that were hospitalizing adults were also hospitalizing kids,” he said. “We found that this was indeed the case.”
The researchers queried the National Inpatient Sample database for all cases involving patients aged 18 years and younger during 2001-2013. The search yielded a sample of 16,837,857 patients. From this, the researchers analyzed diagnosis-related groups for dermatologic conditions denoting the principal diagnosis at discharge, which left a final sample of 84,090 patients. Frequency and chi-squared tests were used to analyze categorical variables.
More than half of patients (54%) were male, 36% were white, 48% had Medicaid insurance, and 43% had private insurance. Mr. Elias reported that the median length of stay for patients was 2 days and the median cost of care was $6,289.50 for each case. More than three-quarters of pediatric inpatients with dermatologic diagnoses were treated for “cellulitis” (66,147 cases, or 79%), with most cases involving the legs (16,875 cases, or 20%). Other pediatric inpatients were admitted for “minor skin disorder without complications” (5,458 cases, or 7%), and “minor skin disorder with complications” (2,822 cases, or 3%). A total of 64 patients died during the study period. Of these, 31 cases (50%) involved “skin graft and/or debridement of skin ulcer or cellulitis without complications,” the study found.
“We were surprised that the major cause of mortality for our patients was classified as ‘skin graft and/or debridement of skin ulcer or cellulitis without complications,’ as a similar diagnosis-related groupings exist denoting that complications did arise,” Mr. Elias said. “Still, it is not possible for us to determine if the mortality was from the skin graft/debridement or another cause entirely. It is possible that the procedure was without complications, only to have the patient succumb to an ancillary process.”
He acknowledged certain limitations of the study, including the fact that the function of dermatologic consults for hospitalized patients was not examined. “We also cannot draw conclusions as to whether improved outpatient therapy reduces the need for hospitalization,” he said. Mr. Elias reported having no financial disclosures.
AUSTIN, TEX. – showed.
“Skin conditions significantly affect pediatric inpatients, and dermatologists ought be accessible for consultation to enhance care and costs,” the study’s first author, Marcus L. Elias, said in an interview prior to the annual meeting of the Society for Pediatric Dermatology.
According to Mr. Elias, who is a 4th-year medical student at Rutgers New Jersey Medical School–Newark, few national studies on skin diseases for pediatric inpatients have been published in the medical literature. Earlier this year, researchers examined inpatient dermatologic conditions in patients aged 18 years and older (J Am Acad Dermatol 2019;80[2]:425-32), but Mr. Elias and associates set out to analyze the burden of inpatient pediatric dermatologic conditions on a national basis. “We wanted to see if the same conditions that were hospitalizing adults were also hospitalizing kids,” he said. “We found that this was indeed the case.”
The researchers queried the National Inpatient Sample database for all cases involving patients aged 18 years and younger during 2001-2013. The search yielded a sample of 16,837,857 patients. From this, the researchers analyzed diagnosis-related groups for dermatologic conditions denoting the principal diagnosis at discharge, which left a final sample of 84,090 patients. Frequency and chi-squared tests were used to analyze categorical variables.
More than half of patients (54%) were male, 36% were white, 48% had Medicaid insurance, and 43% had private insurance. Mr. Elias reported that the median length of stay for patients was 2 days and the median cost of care was $6,289.50 for each case. More than three-quarters of pediatric inpatients with dermatologic diagnoses were treated for “cellulitis” (66,147 cases, or 79%), with most cases involving the legs (16,875 cases, or 20%). Other pediatric inpatients were admitted for “minor skin disorder without complications” (5,458 cases, or 7%), and “minor skin disorder with complications” (2,822 cases, or 3%). A total of 64 patients died during the study period. Of these, 31 cases (50%) involved “skin graft and/or debridement of skin ulcer or cellulitis without complications,” the study found.
“We were surprised that the major cause of mortality for our patients was classified as ‘skin graft and/or debridement of skin ulcer or cellulitis without complications,’ as a similar diagnosis-related groupings exist denoting that complications did arise,” Mr. Elias said. “Still, it is not possible for us to determine if the mortality was from the skin graft/debridement or another cause entirely. It is possible that the procedure was without complications, only to have the patient succumb to an ancillary process.”
He acknowledged certain limitations of the study, including the fact that the function of dermatologic consults for hospitalized patients was not examined. “We also cannot draw conclusions as to whether improved outpatient therapy reduces the need for hospitalization,” he said. Mr. Elias reported having no financial disclosures.
AUSTIN, TEX. – showed.
“Skin conditions significantly affect pediatric inpatients, and dermatologists ought be accessible for consultation to enhance care and costs,” the study’s first author, Marcus L. Elias, said in an interview prior to the annual meeting of the Society for Pediatric Dermatology.
According to Mr. Elias, who is a 4th-year medical student at Rutgers New Jersey Medical School–Newark, few national studies on skin diseases for pediatric inpatients have been published in the medical literature. Earlier this year, researchers examined inpatient dermatologic conditions in patients aged 18 years and older (J Am Acad Dermatol 2019;80[2]:425-32), but Mr. Elias and associates set out to analyze the burden of inpatient pediatric dermatologic conditions on a national basis. “We wanted to see if the same conditions that were hospitalizing adults were also hospitalizing kids,” he said. “We found that this was indeed the case.”
The researchers queried the National Inpatient Sample database for all cases involving patients aged 18 years and younger during 2001-2013. The search yielded a sample of 16,837,857 patients. From this, the researchers analyzed diagnosis-related groups for dermatologic conditions denoting the principal diagnosis at discharge, which left a final sample of 84,090 patients. Frequency and chi-squared tests were used to analyze categorical variables.
More than half of patients (54%) were male, 36% were white, 48% had Medicaid insurance, and 43% had private insurance. Mr. Elias reported that the median length of stay for patients was 2 days and the median cost of care was $6,289.50 for each case. More than three-quarters of pediatric inpatients with dermatologic diagnoses were treated for “cellulitis” (66,147 cases, or 79%), with most cases involving the legs (16,875 cases, or 20%). Other pediatric inpatients were admitted for “minor skin disorder without complications” (5,458 cases, or 7%), and “minor skin disorder with complications” (2,822 cases, or 3%). A total of 64 patients died during the study period. Of these, 31 cases (50%) involved “skin graft and/or debridement of skin ulcer or cellulitis without complications,” the study found.
“We were surprised that the major cause of mortality for our patients was classified as ‘skin graft and/or debridement of skin ulcer or cellulitis without complications,’ as a similar diagnosis-related groupings exist denoting that complications did arise,” Mr. Elias said. “Still, it is not possible for us to determine if the mortality was from the skin graft/debridement or another cause entirely. It is possible that the procedure was without complications, only to have the patient succumb to an ancillary process.”
He acknowledged certain limitations of the study, including the fact that the function of dermatologic consults for hospitalized patients was not examined. “We also cannot draw conclusions as to whether improved outpatient therapy reduces the need for hospitalization,” he said. Mr. Elias reported having no financial disclosures.
REPORTING FROM SPD 2019
Key clinical point: Cellulitis is the cause of the majority of skin-related pediatric inpatient admissions in the United States.
Major finding: In all, 79% of pediatric inpatients with dermatologic diagnoses were treated for cellulitis.
Study details: An analysis of data from 84,090 patients younger than age 18 in the National Inpatient Sample.
Disclosures: The researchers reported having no financial disclosures.
Neonatal ICU stay found ‘protective’ against risk for developing atopic dermatitis
AUSTIN – The
“While more time in the NICU is associated with a lesser risk of developing atopic dermatitis, we certainly do not want to keep infants in the NICU longer in order to lower their risk of atopic dermatitis,” the study’s first author, Jennifer J. Schoch, MD, said in an interview prior to the annual meeting of the Society for Pediatric Dermatology. “Instead, we need to work on understanding the mechanisms behind this relationship. For example, are there certain exposures in the NICU that influence the cutaneous immunity to ultimately reduce the risk of atopic dermatitis?”
According to Dr. Schoch, a pediatric dermatologist at the University of Florida, Gainesville, the medical literature has been conflicted regarding the relationship between prematurity and eczema. A recent meta-analysis of 18 studies found an association between very preterm birth and a decreased risk of eczema, yet the risk became insignificant among children born moderately preterm (J Am Acad Dermatol. 2018;78[6]:1142-8). However, the factors contributing to this relationship are not well understood.
In an effort to explore the infant, maternal, and environmental factors of infants who developed AD, compared with infants who did not, Dr. Schoch and colleagues evaluated infants who were born at University of Florida Health from June 1, 2011, to April 30, 2017; had at least two well-child visits; and had at least one visit at 300 days old or later. The researchers included 4,016 mother-infant dyads in the study. Atopic dermatitis was diagnosed in 26.5% of the infants. Factors significantly associated with the incidence of AD were delivery mode (P = .0127), NICU stay (P = .0001), gestational age (P = .0006), and birth weight (P = .0020). Specifically, infants had a higher risk of developing AD if they were delivered vaginally, did not stay in the NICU, had a higher gestational age, or had a higher birth weight. Extremely preterm (less than 28 weeks’ gestation) and very preterm (28 to less than 32 weeks’ gestation) infants had the lowest rates of AD, at 10.9% and 19%, respectively.
When the researchers adjusted for other variables to their model, only length of stay in the NICU was related to the development of AD. Specifically, infants who spent more time in the NICU had a lower risk of developing atopic dermatitis (P = .0039).
“We were surprised to find that the length of stay in the neonatal intensive care unit was the strongest protective factor against the future development of eczema,” Dr. Schoch said. “Instead of this relationship being mediated by gestational age or birth weight, it was how much time the infants spent in the NICU that seemed to ‘protect’ from future eczema.”
She acknowledged certain limitations of the study, including its retrospective design with data gathered from electronic medical records. Also, “diagnosis was determined by ICD-9 or ICD-10 code, and not confirmed by dermatologists,” she said.
In their abstract, the researchers wrote that the finding highlights “the importance of early life interactions between the microbiome, developing cutaneous immunity, and the evolving skin barrier of the preterm infant. The skin microbiome of premature infants differs from full-term infants, in that the premature infant cutaneous microbiome is dominated by Staphylococcus species” (Microbiome. 2018;6[1]:98). They added that “the early presence of Staphylococcus on the skin may confer protection.”
Dr. Schoch reported having no relevant financial disclosures.
SOURCE: Schoch J et al. SPD 2019, Poster 2.
AUSTIN – The
“While more time in the NICU is associated with a lesser risk of developing atopic dermatitis, we certainly do not want to keep infants in the NICU longer in order to lower their risk of atopic dermatitis,” the study’s first author, Jennifer J. Schoch, MD, said in an interview prior to the annual meeting of the Society for Pediatric Dermatology. “Instead, we need to work on understanding the mechanisms behind this relationship. For example, are there certain exposures in the NICU that influence the cutaneous immunity to ultimately reduce the risk of atopic dermatitis?”
According to Dr. Schoch, a pediatric dermatologist at the University of Florida, Gainesville, the medical literature has been conflicted regarding the relationship between prematurity and eczema. A recent meta-analysis of 18 studies found an association between very preterm birth and a decreased risk of eczema, yet the risk became insignificant among children born moderately preterm (J Am Acad Dermatol. 2018;78[6]:1142-8). However, the factors contributing to this relationship are not well understood.
In an effort to explore the infant, maternal, and environmental factors of infants who developed AD, compared with infants who did not, Dr. Schoch and colleagues evaluated infants who were born at University of Florida Health from June 1, 2011, to April 30, 2017; had at least two well-child visits; and had at least one visit at 300 days old or later. The researchers included 4,016 mother-infant dyads in the study. Atopic dermatitis was diagnosed in 26.5% of the infants. Factors significantly associated with the incidence of AD were delivery mode (P = .0127), NICU stay (P = .0001), gestational age (P = .0006), and birth weight (P = .0020). Specifically, infants had a higher risk of developing AD if they were delivered vaginally, did not stay in the NICU, had a higher gestational age, or had a higher birth weight. Extremely preterm (less than 28 weeks’ gestation) and very preterm (28 to less than 32 weeks’ gestation) infants had the lowest rates of AD, at 10.9% and 19%, respectively.
When the researchers adjusted for other variables to their model, only length of stay in the NICU was related to the development of AD. Specifically, infants who spent more time in the NICU had a lower risk of developing atopic dermatitis (P = .0039).
“We were surprised to find that the length of stay in the neonatal intensive care unit was the strongest protective factor against the future development of eczema,” Dr. Schoch said. “Instead of this relationship being mediated by gestational age or birth weight, it was how much time the infants spent in the NICU that seemed to ‘protect’ from future eczema.”
She acknowledged certain limitations of the study, including its retrospective design with data gathered from electronic medical records. Also, “diagnosis was determined by ICD-9 or ICD-10 code, and not confirmed by dermatologists,” she said.
In their abstract, the researchers wrote that the finding highlights “the importance of early life interactions between the microbiome, developing cutaneous immunity, and the evolving skin barrier of the preterm infant. The skin microbiome of premature infants differs from full-term infants, in that the premature infant cutaneous microbiome is dominated by Staphylococcus species” (Microbiome. 2018;6[1]:98). They added that “the early presence of Staphylococcus on the skin may confer protection.”
Dr. Schoch reported having no relevant financial disclosures.
SOURCE: Schoch J et al. SPD 2019, Poster 2.
AUSTIN – The
“While more time in the NICU is associated with a lesser risk of developing atopic dermatitis, we certainly do not want to keep infants in the NICU longer in order to lower their risk of atopic dermatitis,” the study’s first author, Jennifer J. Schoch, MD, said in an interview prior to the annual meeting of the Society for Pediatric Dermatology. “Instead, we need to work on understanding the mechanisms behind this relationship. For example, are there certain exposures in the NICU that influence the cutaneous immunity to ultimately reduce the risk of atopic dermatitis?”
According to Dr. Schoch, a pediatric dermatologist at the University of Florida, Gainesville, the medical literature has been conflicted regarding the relationship between prematurity and eczema. A recent meta-analysis of 18 studies found an association between very preterm birth and a decreased risk of eczema, yet the risk became insignificant among children born moderately preterm (J Am Acad Dermatol. 2018;78[6]:1142-8). However, the factors contributing to this relationship are not well understood.
In an effort to explore the infant, maternal, and environmental factors of infants who developed AD, compared with infants who did not, Dr. Schoch and colleagues evaluated infants who were born at University of Florida Health from June 1, 2011, to April 30, 2017; had at least two well-child visits; and had at least one visit at 300 days old or later. The researchers included 4,016 mother-infant dyads in the study. Atopic dermatitis was diagnosed in 26.5% of the infants. Factors significantly associated with the incidence of AD were delivery mode (P = .0127), NICU stay (P = .0001), gestational age (P = .0006), and birth weight (P = .0020). Specifically, infants had a higher risk of developing AD if they were delivered vaginally, did not stay in the NICU, had a higher gestational age, or had a higher birth weight. Extremely preterm (less than 28 weeks’ gestation) and very preterm (28 to less than 32 weeks’ gestation) infants had the lowest rates of AD, at 10.9% and 19%, respectively.
When the researchers adjusted for other variables to their model, only length of stay in the NICU was related to the development of AD. Specifically, infants who spent more time in the NICU had a lower risk of developing atopic dermatitis (P = .0039).
“We were surprised to find that the length of stay in the neonatal intensive care unit was the strongest protective factor against the future development of eczema,” Dr. Schoch said. “Instead of this relationship being mediated by gestational age or birth weight, it was how much time the infants spent in the NICU that seemed to ‘protect’ from future eczema.”
She acknowledged certain limitations of the study, including its retrospective design with data gathered from electronic medical records. Also, “diagnosis was determined by ICD-9 or ICD-10 code, and not confirmed by dermatologists,” she said.
In their abstract, the researchers wrote that the finding highlights “the importance of early life interactions between the microbiome, developing cutaneous immunity, and the evolving skin barrier of the preterm infant. The skin microbiome of premature infants differs from full-term infants, in that the premature infant cutaneous microbiome is dominated by Staphylococcus species” (Microbiome. 2018;6[1]:98). They added that “the early presence of Staphylococcus on the skin may confer protection.”
Dr. Schoch reported having no relevant financial disclosures.
SOURCE: Schoch J et al. SPD 2019, Poster 2.
REPORTING FROM SPD 2019
Key clinical point: Preterm infants develop atopic dermatitis less often than full term infants.
Major finding: Infants that spent more time in the neonatal ICU had a lower risk of developing atopic dermatitis (P = .0039).
Study details: A single-center study of 4,016 mother-infant dyads.
Disclosures: Dr. Schoch reported having no relevant financial disclosures.
Source: Schoch J et al. SPD 2019, Poster 2.
Rash over homemade tattoo
Although the FP had never seen anything like this before, he was aware that dyes in tattoos could cause an allergic reaction. His research also suggested that sarcoidosis could occur in a tattoo, so this was part of his differential diagnosis written on the pathology form. He suggested a 4 mm punch biopsy to determine what was going on.
(See the Watch & Learn video on “Punch biopsy.”)
The pathology came back consistent with sarcoidosis. On the follow-up visit, the FP explained the diagnosis and suggested that the patient have a chest x-ray. The chest x-ray showed bilateral hilar adenopathy consistent with stage I sarcoidosis. The FP prescribed a 15-g tube of 0.05% clobetasol to treat the lesion and referred the patient to Dermatology and Pulmonology.
When the patient visited Dermatology 2 months later, most of the sarcoid plaques were flat but some remained raised and pruritic. The dermatologist offered intralesional triamcinolone for the stubborn plaques, and the patient consented. This intralesional steroid in conjunction with the topical steroid provided a good result that treated the itching and flattened the lesions. The remaining tattoo and color of the skin were not normal, but the patient was happy with the result. She had no pulmonary symptoms, and her pulmonary function tests showed a mild decrease in her diffusing capacity. She continued to see the dermatologist and pulmonologist for ongoing care of her sarcoidosis.
Photo courtesy of Amor Khachemoune, MD, and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Bae E, Bae Y, Sarabi K, et al. Sarcoidosis. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1153-1160.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

Although the FP had never seen anything like this before, he was aware that dyes in tattoos could cause an allergic reaction. His research also suggested that sarcoidosis could occur in a tattoo, so this was part of his differential diagnosis written on the pathology form. He suggested a 4 mm punch biopsy to determine what was going on.
(See the Watch & Learn video on “Punch biopsy.”)
The pathology came back consistent with sarcoidosis. On the follow-up visit, the FP explained the diagnosis and suggested that the patient have a chest x-ray. The chest x-ray showed bilateral hilar adenopathy consistent with stage I sarcoidosis. The FP prescribed a 15-g tube of 0.05% clobetasol to treat the lesion and referred the patient to Dermatology and Pulmonology.
When the patient visited Dermatology 2 months later, most of the sarcoid plaques were flat but some remained raised and pruritic. The dermatologist offered intralesional triamcinolone for the stubborn plaques, and the patient consented. This intralesional steroid in conjunction with the topical steroid provided a good result that treated the itching and flattened the lesions. The remaining tattoo and color of the skin were not normal, but the patient was happy with the result. She had no pulmonary symptoms, and her pulmonary function tests showed a mild decrease in her diffusing capacity. She continued to see the dermatologist and pulmonologist for ongoing care of her sarcoidosis.
Photo courtesy of Amor Khachemoune, MD, and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Bae E, Bae Y, Sarabi K, et al. Sarcoidosis. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1153-1160.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

Although the FP had never seen anything like this before, he was aware that dyes in tattoos could cause an allergic reaction. His research also suggested that sarcoidosis could occur in a tattoo, so this was part of his differential diagnosis written on the pathology form. He suggested a 4 mm punch biopsy to determine what was going on.
(See the Watch & Learn video on “Punch biopsy.”)
The pathology came back consistent with sarcoidosis. On the follow-up visit, the FP explained the diagnosis and suggested that the patient have a chest x-ray. The chest x-ray showed bilateral hilar adenopathy consistent with stage I sarcoidosis. The FP prescribed a 15-g tube of 0.05% clobetasol to treat the lesion and referred the patient to Dermatology and Pulmonology.
When the patient visited Dermatology 2 months later, most of the sarcoid plaques were flat but some remained raised and pruritic. The dermatologist offered intralesional triamcinolone for the stubborn plaques, and the patient consented. This intralesional steroid in conjunction with the topical steroid provided a good result that treated the itching and flattened the lesions. The remaining tattoo and color of the skin were not normal, but the patient was happy with the result. She had no pulmonary symptoms, and her pulmonary function tests showed a mild decrease in her diffusing capacity. She continued to see the dermatologist and pulmonologist for ongoing care of her sarcoidosis.
Photo courtesy of Amor Khachemoune, MD, and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Bae E, Bae Y, Sarabi K, et al. Sarcoidosis. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1153-1160.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
Novel topical psoriasis treatment targets nerve pathways
MILAN – A novel topical nonsteroidal treatment for psoriasis showed sufficient efficacy in phase 2b clinical trials to proceed to phase 3 studies, with improvements in severity, pain, and burning in adults with mild to moderate psoriasis.
At the end of 12 weeks of treatment, 29% of patients receiving the medication – which targets nerve pathways – experienced a decrease of at least 2 grades on the 5-point Investigator’s Global Assessment (IGA) scale, compared with 13% of those receiving the topical vehicle only (P = .036). A similar proportion of patients achieved 75% improvement on the Psoriasis Area and Severity Index (PASI-75)compared with those on vehicle alone (27% versus 13%; P = .045).
, said Paul F. Lizzul, MD, PhD, presenting the findings during a late-breaking abstract session at the World Congress of Dermatology.
Pruritus severity also dropped by about 60%, but the decrease did not differ significantly from the change seen with vehicle alone, said Dr. Lizzul, chief medical officer for Sienna Biopharmaceuticals, Westlake Village, Calif., which funded the study. He and his coinvestigators found this “interesting, surprising, and different from what we had seen previously,” he said. “We think a few things happened here,” including intensive querying on itch by means of daily diaries, a different approach than had been taken in the investigator’s earlier SNA-120 trials. “We think in this way we probably biased patients’ expectations, altering reporting on this subjective measure,” he added.
“There’s been really a lack of innovation in the topical world in developing nonsteroidal therapies for the majority of patients who are treated with topicals, said Dr. Lizzul. Keratinocytes within psoriatic plaques are known to have elevated levels of nerve growth factor (NGF), he explained. Together with tropomyosin receptor kinase A (TrkA), NGF is implicated in the pathogenesis of psoriasis; it stimulates keratinocyte hyperproliferation, is a factor in neurogenic inflammation, and contributes to pruritus. Upregulation of TrkA expression is seen in nerve fibers within pruritic psoriasis plaques as well, said Dr. Lizzul, senior author of the study. (The first author was Kristina Callis Duffin, MD, cochair of the dermatology department at the University of Utah, Salt Lake City.)
In fact, the pruritus that plagues many psoriasis patients, said Dr. Lizzul, may “serve as a clinical biomarker for elevated NGF/TrkA expression.” And certain clinical phenomena observed in psoriasis, such as the Koebner phenomenon and plaque resolution along the path of damaged nerves, provide other clues. “Clearly, astute clinicians going back many, many years have recognized the very important role that nerves and neuropeptides play in psoriasis,” he added.
SNA-120 targets NGF TrKA activity, and “achieves high local drug concentration in the skin, with low systemic availability,” he said.
The randomized, double-blind, vehicle-controlled study enrolled 208 adults with mild to moderate psoriasis (scores of 2 or 3 on the IGA), with pruritus of at least moderate intensity (5 or higher on a 10-point itch numeric rating scale, or I-NRS). The mean age of the patients was 50 years, and about half were male. Most (84%-90% across study arms) were white. At baseline, the mean I-NRS was 7.3-7.4, and the mean PASI score at baseline ranged from 5.9 to 6.5.
Patients were randomized to receive SNA-120 twice daily at either 0.05% (70 patients) or 0.5% (69 patients) in an ointment formulation, or vehicle alone twice daily (69 patients). Efficacy was tracked by measuring decrease in IGA by one or two grades, the number of patients achieving PASI-50 and PASI-75, reduction in itch, and a composite of a decrease of at least 2 grades on the IGA and having clear or almost clear skin.
The investigators also tracked reduction in burning and pain as measured on a 10-point numeric rating scale. Though itch scores didn’t differ significantly from reductions seen with the topical vehicle alone, pain and burning were both reduced significantly compared with vehicle by week 12 of the study (P = .033 for pain; P = .043 for burning).
All improvements were seen only with the lower dose, not the 0.5% dose of SNA-120, noted Dr. Lizzul, adding: “This is not necessarily surprising in the world of kinase inhibitors, where you can see these J-shaped or inverse dose-response curves.”
In addition to recording adverse events, the researchers assessed safety by obtaining laboratory values and electrocardiograms. Plasma SNA-120 levels at study weeks 2, 4, and 8 were obtained for pharmacokinetic analysis. Systemic uptake was virtually nil, and the safety profile overall was good, said Dr. Lizzul.
Next steps are phase 3 clinical trials that will evaluate global improvement as well as pain, burning, and itch in psoriasis, he noted.
Dr. Lizzul is an employee of Sienna Biopharmaceuticals, which is developing SNA-120.
MILAN – A novel topical nonsteroidal treatment for psoriasis showed sufficient efficacy in phase 2b clinical trials to proceed to phase 3 studies, with improvements in severity, pain, and burning in adults with mild to moderate psoriasis.
At the end of 12 weeks of treatment, 29% of patients receiving the medication – which targets nerve pathways – experienced a decrease of at least 2 grades on the 5-point Investigator’s Global Assessment (IGA) scale, compared with 13% of those receiving the topical vehicle only (P = .036). A similar proportion of patients achieved 75% improvement on the Psoriasis Area and Severity Index (PASI-75)compared with those on vehicle alone (27% versus 13%; P = .045).
, said Paul F. Lizzul, MD, PhD, presenting the findings during a late-breaking abstract session at the World Congress of Dermatology.
Pruritus severity also dropped by about 60%, but the decrease did not differ significantly from the change seen with vehicle alone, said Dr. Lizzul, chief medical officer for Sienna Biopharmaceuticals, Westlake Village, Calif., which funded the study. He and his coinvestigators found this “interesting, surprising, and different from what we had seen previously,” he said. “We think a few things happened here,” including intensive querying on itch by means of daily diaries, a different approach than had been taken in the investigator’s earlier SNA-120 trials. “We think in this way we probably biased patients’ expectations, altering reporting on this subjective measure,” he added.
“There’s been really a lack of innovation in the topical world in developing nonsteroidal therapies for the majority of patients who are treated with topicals, said Dr. Lizzul. Keratinocytes within psoriatic plaques are known to have elevated levels of nerve growth factor (NGF), he explained. Together with tropomyosin receptor kinase A (TrkA), NGF is implicated in the pathogenesis of psoriasis; it stimulates keratinocyte hyperproliferation, is a factor in neurogenic inflammation, and contributes to pruritus. Upregulation of TrkA expression is seen in nerve fibers within pruritic psoriasis plaques as well, said Dr. Lizzul, senior author of the study. (The first author was Kristina Callis Duffin, MD, cochair of the dermatology department at the University of Utah, Salt Lake City.)
In fact, the pruritus that plagues many psoriasis patients, said Dr. Lizzul, may “serve as a clinical biomarker for elevated NGF/TrkA expression.” And certain clinical phenomena observed in psoriasis, such as the Koebner phenomenon and plaque resolution along the path of damaged nerves, provide other clues. “Clearly, astute clinicians going back many, many years have recognized the very important role that nerves and neuropeptides play in psoriasis,” he added.
SNA-120 targets NGF TrKA activity, and “achieves high local drug concentration in the skin, with low systemic availability,” he said.
The randomized, double-blind, vehicle-controlled study enrolled 208 adults with mild to moderate psoriasis (scores of 2 or 3 on the IGA), with pruritus of at least moderate intensity (5 or higher on a 10-point itch numeric rating scale, or I-NRS). The mean age of the patients was 50 years, and about half were male. Most (84%-90% across study arms) were white. At baseline, the mean I-NRS was 7.3-7.4, and the mean PASI score at baseline ranged from 5.9 to 6.5.
Patients were randomized to receive SNA-120 twice daily at either 0.05% (70 patients) or 0.5% (69 patients) in an ointment formulation, or vehicle alone twice daily (69 patients). Efficacy was tracked by measuring decrease in IGA by one or two grades, the number of patients achieving PASI-50 and PASI-75, reduction in itch, and a composite of a decrease of at least 2 grades on the IGA and having clear or almost clear skin.
The investigators also tracked reduction in burning and pain as measured on a 10-point numeric rating scale. Though itch scores didn’t differ significantly from reductions seen with the topical vehicle alone, pain and burning were both reduced significantly compared with vehicle by week 12 of the study (P = .033 for pain; P = .043 for burning).
All improvements were seen only with the lower dose, not the 0.5% dose of SNA-120, noted Dr. Lizzul, adding: “This is not necessarily surprising in the world of kinase inhibitors, where you can see these J-shaped or inverse dose-response curves.”
In addition to recording adverse events, the researchers assessed safety by obtaining laboratory values and electrocardiograms. Plasma SNA-120 levels at study weeks 2, 4, and 8 were obtained for pharmacokinetic analysis. Systemic uptake was virtually nil, and the safety profile overall was good, said Dr. Lizzul.
Next steps are phase 3 clinical trials that will evaluate global improvement as well as pain, burning, and itch in psoriasis, he noted.
Dr. Lizzul is an employee of Sienna Biopharmaceuticals, which is developing SNA-120.
MILAN – A novel topical nonsteroidal treatment for psoriasis showed sufficient efficacy in phase 2b clinical trials to proceed to phase 3 studies, with improvements in severity, pain, and burning in adults with mild to moderate psoriasis.
At the end of 12 weeks of treatment, 29% of patients receiving the medication – which targets nerve pathways – experienced a decrease of at least 2 grades on the 5-point Investigator’s Global Assessment (IGA) scale, compared with 13% of those receiving the topical vehicle only (P = .036). A similar proportion of patients achieved 75% improvement on the Psoriasis Area and Severity Index (PASI-75)compared with those on vehicle alone (27% versus 13%; P = .045).
, said Paul F. Lizzul, MD, PhD, presenting the findings during a late-breaking abstract session at the World Congress of Dermatology.
Pruritus severity also dropped by about 60%, but the decrease did not differ significantly from the change seen with vehicle alone, said Dr. Lizzul, chief medical officer for Sienna Biopharmaceuticals, Westlake Village, Calif., which funded the study. He and his coinvestigators found this “interesting, surprising, and different from what we had seen previously,” he said. “We think a few things happened here,” including intensive querying on itch by means of daily diaries, a different approach than had been taken in the investigator’s earlier SNA-120 trials. “We think in this way we probably biased patients’ expectations, altering reporting on this subjective measure,” he added.
“There’s been really a lack of innovation in the topical world in developing nonsteroidal therapies for the majority of patients who are treated with topicals, said Dr. Lizzul. Keratinocytes within psoriatic plaques are known to have elevated levels of nerve growth factor (NGF), he explained. Together with tropomyosin receptor kinase A (TrkA), NGF is implicated in the pathogenesis of psoriasis; it stimulates keratinocyte hyperproliferation, is a factor in neurogenic inflammation, and contributes to pruritus. Upregulation of TrkA expression is seen in nerve fibers within pruritic psoriasis plaques as well, said Dr. Lizzul, senior author of the study. (The first author was Kristina Callis Duffin, MD, cochair of the dermatology department at the University of Utah, Salt Lake City.)
In fact, the pruritus that plagues many psoriasis patients, said Dr. Lizzul, may “serve as a clinical biomarker for elevated NGF/TrkA expression.” And certain clinical phenomena observed in psoriasis, such as the Koebner phenomenon and plaque resolution along the path of damaged nerves, provide other clues. “Clearly, astute clinicians going back many, many years have recognized the very important role that nerves and neuropeptides play in psoriasis,” he added.
SNA-120 targets NGF TrKA activity, and “achieves high local drug concentration in the skin, with low systemic availability,” he said.
The randomized, double-blind, vehicle-controlled study enrolled 208 adults with mild to moderate psoriasis (scores of 2 or 3 on the IGA), with pruritus of at least moderate intensity (5 or higher on a 10-point itch numeric rating scale, or I-NRS). The mean age of the patients was 50 years, and about half were male. Most (84%-90% across study arms) were white. At baseline, the mean I-NRS was 7.3-7.4, and the mean PASI score at baseline ranged from 5.9 to 6.5.
Patients were randomized to receive SNA-120 twice daily at either 0.05% (70 patients) or 0.5% (69 patients) in an ointment formulation, or vehicle alone twice daily (69 patients). Efficacy was tracked by measuring decrease in IGA by one or two grades, the number of patients achieving PASI-50 and PASI-75, reduction in itch, and a composite of a decrease of at least 2 grades on the IGA and having clear or almost clear skin.
The investigators also tracked reduction in burning and pain as measured on a 10-point numeric rating scale. Though itch scores didn’t differ significantly from reductions seen with the topical vehicle alone, pain and burning were both reduced significantly compared with vehicle by week 12 of the study (P = .033 for pain; P = .043 for burning).
All improvements were seen only with the lower dose, not the 0.5% dose of SNA-120, noted Dr. Lizzul, adding: “This is not necessarily surprising in the world of kinase inhibitors, where you can see these J-shaped or inverse dose-response curves.”
In addition to recording adverse events, the researchers assessed safety by obtaining laboratory values and electrocardiograms. Plasma SNA-120 levels at study weeks 2, 4, and 8 were obtained for pharmacokinetic analysis. Systemic uptake was virtually nil, and the safety profile overall was good, said Dr. Lizzul.
Next steps are phase 3 clinical trials that will evaluate global improvement as well as pain, burning, and itch in psoriasis, he noted.
Dr. Lizzul is an employee of Sienna Biopharmaceuticals, which is developing SNA-120.
REPORTING FROM WCD2019
Polyester. Plywood. Pizza. Skin allergens lurk in unusual places
NEWPORT BEACH, CALIF. – , according to dermatologist Jennifer H. Perryman, MD.
Here’s a closer look at the allergens highlighted by Dr. Perryman in a presentation at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar:
Formaldehyde: It’s everywhere
“In general, formaldehyde is found on everyone in this room in two different places: preservatives in skin care products and in a lot of our clothing,” said Dr. Perryman, who practices in Greeley and Fort Collins, Colo.
The preservative is used in an even wider variety of products, including fluids used in industry (such as metalworking) and topical medications. But people are especially likely to encounter it in clothing – via formaldehyde textile resins – as well as in cosmetics, soaps, and lotions.
On the clothing front, Dr. Perryman said, formaldehyde textile resins have been used since the 1930s. They’re used to treat blends of synthetic and cotton fibers and bed sheets. Beware of “wrinkle resistant” and “permanent press” clothing (although not all have been treated with this resin). “Newer formaldehyde textile resins have less formaldehyde release, but they may be more expensive, and some industries may not use them,” she said.
Avoiding formaldehyde textile resins isn’t a simple matter.” You have to go out of your way to stay away from a polyester-cotton blend,” she said. “And don’t forget bedsheets,” she added, noting that the packaging on some sheets include information about cotton count, “but when you flip over the label it says it’s ‘50% cotton and 50% polyester or other.’ ”
Some patients will bring their own bedsheets to hotels so they don’t experience flares from hotel bedsheets, she added.
Other products can trigger this skin allergy. Beware, Dr. Perryman said, of formaldehyde exposure from paper, cardboard, cigarette smoke, processed wood products like plywood, foam housing and industrial insulation, embalming fluid and tissue fixatives, and some paints and adhesives.
What are the signs that someone may have a case of formaldehyde allergy? It may cause patchy generalized dermatitis, erythroderma, and nummular dermatitis. It may spare the hands, feet, and face because those parts of the body have less exposure to clothing, and it’s likely to especially affect body areas where clothing is tight. And for unknown reasons, this allergy is more common in the elderly, Dr. Perryman said.
Textile dye: Beware polyester
This allergy is mainly triggered by synthetic fabrics like polyester, rayon, and acetate, she noted. Darker colors are more allergenic. Clothes made of natural fibers such as cotton, silk, linen, and wool are alternatives. These are not dyed with these dyes, she said, adding that a reaction to wool will be from irritation, not from the dye.
Paraphenylenediamine: Keep an eye out for this dye ingredient
Paraphenylenediamine, which can trigger allergic reactions, is found in leather dye, fur dye, and some (but not all) hair dyes. Be aware that it can cross-react with other allergens like sulfonamide medications.
If a patch test turns up a reaction to “Black-Rubber Mix,” which includes paraphenylenediamine, consider whether the patient has exposure to the rubber in tires. Car mechanics may be affected by this allergy, Dr. Perryman said.
Neomycin: A drop of trouble
Allergy to the antibiotic neomycin can be triggered by exposure to gentamicin and tobramycin eye drops. Patients may believe they have an infection, Dr. Perryman said, so consider getting a culture. In some cases, an allergic reaction to neomycin may be incorrectly diagnosed as cellulitis.
Nickel: Not just a jewelry hazard
Jewelry and coins can trigger nickel allergies, but be aware that systemic nickel allergy can also trigger skin problems from a patient’s diet. It may be necessary to put patients on a low-nickel diet that avoids foods such as healthy grains, greens (especially spinach), nuts, legumes, and chocolate. “I always feel bad” putting patients on a restrictive diet, Dr. Perryman said, but it can be helpful to take 500 mg of vitamin C three times a day since it binds to nickel.
Cobalt: Watch the chocolate and coffee
Jewelry with cobalt can cause an allergic reaction. Dr. Perryman tells patients to buy an inexpensive “spot test” product online that detects whether jewelry has nickel or cobalt. Cobalt allergy can also trigger symptoms in patients exposed to “hard metal” industrial tools, cement, and masonry. Workers in the plastics and dye industries may be exposed too.
Like nickel, Dr. Perryman said, systemic cobalt allergy related to diet is also possible. The list of foods that contain higher levels of cobalt is long, and includes apricots, beans, beer, chocolate, coffee, nuts, tea, and whole-grain flour.
Dr. Perryman also mentioned several other allergens to keep in mind:
- Chromate can trigger reactions in people who wear leather shoes (the metal can be used in tanning). It can also cause problems in workers exposed to it via cement, bricks, drywall, and metal plating.
- Chromium picolinate, an over-the-counter supplement, can cause systemic dermatitis.
- Gold in jewelry can trigger an allergic reaction. Talk to patients about replating their jewelry, Dr. Perryman said.
- Rubber can trigger reactions due to exposure to rubber bands, makeup sponges, and rubber gloves (even nitrile ones). Be aware that both rubber and latex allergies may coexist and consider a blood test for latex allergy.
- Systemic balsam allergy related to an individual’s diet is possible. Tomato is an especially big villain on this front, along with citrus fruits, spices, cola, chili, and chocolate.
Dr. Perryman disclosed consulting work for IntraDerm. SDEF and this news organization are owned by the same parent company.
NEWPORT BEACH, CALIF. – , according to dermatologist Jennifer H. Perryman, MD.
Here’s a closer look at the allergens highlighted by Dr. Perryman in a presentation at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar:
Formaldehyde: It’s everywhere
“In general, formaldehyde is found on everyone in this room in two different places: preservatives in skin care products and in a lot of our clothing,” said Dr. Perryman, who practices in Greeley and Fort Collins, Colo.
The preservative is used in an even wider variety of products, including fluids used in industry (such as metalworking) and topical medications. But people are especially likely to encounter it in clothing – via formaldehyde textile resins – as well as in cosmetics, soaps, and lotions.
On the clothing front, Dr. Perryman said, formaldehyde textile resins have been used since the 1930s. They’re used to treat blends of synthetic and cotton fibers and bed sheets. Beware of “wrinkle resistant” and “permanent press” clothing (although not all have been treated with this resin). “Newer formaldehyde textile resins have less formaldehyde release, but they may be more expensive, and some industries may not use them,” she said.
Avoiding formaldehyde textile resins isn’t a simple matter.” You have to go out of your way to stay away from a polyester-cotton blend,” she said. “And don’t forget bedsheets,” she added, noting that the packaging on some sheets include information about cotton count, “but when you flip over the label it says it’s ‘50% cotton and 50% polyester or other.’ ”
Some patients will bring their own bedsheets to hotels so they don’t experience flares from hotel bedsheets, she added.
Other products can trigger this skin allergy. Beware, Dr. Perryman said, of formaldehyde exposure from paper, cardboard, cigarette smoke, processed wood products like plywood, foam housing and industrial insulation, embalming fluid and tissue fixatives, and some paints and adhesives.
What are the signs that someone may have a case of formaldehyde allergy? It may cause patchy generalized dermatitis, erythroderma, and nummular dermatitis. It may spare the hands, feet, and face because those parts of the body have less exposure to clothing, and it’s likely to especially affect body areas where clothing is tight. And for unknown reasons, this allergy is more common in the elderly, Dr. Perryman said.
Textile dye: Beware polyester
This allergy is mainly triggered by synthetic fabrics like polyester, rayon, and acetate, she noted. Darker colors are more allergenic. Clothes made of natural fibers such as cotton, silk, linen, and wool are alternatives. These are not dyed with these dyes, she said, adding that a reaction to wool will be from irritation, not from the dye.
Paraphenylenediamine: Keep an eye out for this dye ingredient
Paraphenylenediamine, which can trigger allergic reactions, is found in leather dye, fur dye, and some (but not all) hair dyes. Be aware that it can cross-react with other allergens like sulfonamide medications.
If a patch test turns up a reaction to “Black-Rubber Mix,” which includes paraphenylenediamine, consider whether the patient has exposure to the rubber in tires. Car mechanics may be affected by this allergy, Dr. Perryman said.
Neomycin: A drop of trouble
Allergy to the antibiotic neomycin can be triggered by exposure to gentamicin and tobramycin eye drops. Patients may believe they have an infection, Dr. Perryman said, so consider getting a culture. In some cases, an allergic reaction to neomycin may be incorrectly diagnosed as cellulitis.
Nickel: Not just a jewelry hazard
Jewelry and coins can trigger nickel allergies, but be aware that systemic nickel allergy can also trigger skin problems from a patient’s diet. It may be necessary to put patients on a low-nickel diet that avoids foods such as healthy grains, greens (especially spinach), nuts, legumes, and chocolate. “I always feel bad” putting patients on a restrictive diet, Dr. Perryman said, but it can be helpful to take 500 mg of vitamin C three times a day since it binds to nickel.
Cobalt: Watch the chocolate and coffee
Jewelry with cobalt can cause an allergic reaction. Dr. Perryman tells patients to buy an inexpensive “spot test” product online that detects whether jewelry has nickel or cobalt. Cobalt allergy can also trigger symptoms in patients exposed to “hard metal” industrial tools, cement, and masonry. Workers in the plastics and dye industries may be exposed too.
Like nickel, Dr. Perryman said, systemic cobalt allergy related to diet is also possible. The list of foods that contain higher levels of cobalt is long, and includes apricots, beans, beer, chocolate, coffee, nuts, tea, and whole-grain flour.
Dr. Perryman also mentioned several other allergens to keep in mind:
- Chromate can trigger reactions in people who wear leather shoes (the metal can be used in tanning). It can also cause problems in workers exposed to it via cement, bricks, drywall, and metal plating.
- Chromium picolinate, an over-the-counter supplement, can cause systemic dermatitis.
- Gold in jewelry can trigger an allergic reaction. Talk to patients about replating their jewelry, Dr. Perryman said.
- Rubber can trigger reactions due to exposure to rubber bands, makeup sponges, and rubber gloves (even nitrile ones). Be aware that both rubber and latex allergies may coexist and consider a blood test for latex allergy.
- Systemic balsam allergy related to an individual’s diet is possible. Tomato is an especially big villain on this front, along with citrus fruits, spices, cola, chili, and chocolate.
Dr. Perryman disclosed consulting work for IntraDerm. SDEF and this news organization are owned by the same parent company.
NEWPORT BEACH, CALIF. – , according to dermatologist Jennifer H. Perryman, MD.
Here’s a closer look at the allergens highlighted by Dr. Perryman in a presentation at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar:
Formaldehyde: It’s everywhere
“In general, formaldehyde is found on everyone in this room in two different places: preservatives in skin care products and in a lot of our clothing,” said Dr. Perryman, who practices in Greeley and Fort Collins, Colo.
The preservative is used in an even wider variety of products, including fluids used in industry (such as metalworking) and topical medications. But people are especially likely to encounter it in clothing – via formaldehyde textile resins – as well as in cosmetics, soaps, and lotions.
On the clothing front, Dr. Perryman said, formaldehyde textile resins have been used since the 1930s. They’re used to treat blends of synthetic and cotton fibers and bed sheets. Beware of “wrinkle resistant” and “permanent press” clothing (although not all have been treated with this resin). “Newer formaldehyde textile resins have less formaldehyde release, but they may be more expensive, and some industries may not use them,” she said.
Avoiding formaldehyde textile resins isn’t a simple matter.” You have to go out of your way to stay away from a polyester-cotton blend,” she said. “And don’t forget bedsheets,” she added, noting that the packaging on some sheets include information about cotton count, “but when you flip over the label it says it’s ‘50% cotton and 50% polyester or other.’ ”
Some patients will bring their own bedsheets to hotels so they don’t experience flares from hotel bedsheets, she added.
Other products can trigger this skin allergy. Beware, Dr. Perryman said, of formaldehyde exposure from paper, cardboard, cigarette smoke, processed wood products like plywood, foam housing and industrial insulation, embalming fluid and tissue fixatives, and some paints and adhesives.
What are the signs that someone may have a case of formaldehyde allergy? It may cause patchy generalized dermatitis, erythroderma, and nummular dermatitis. It may spare the hands, feet, and face because those parts of the body have less exposure to clothing, and it’s likely to especially affect body areas where clothing is tight. And for unknown reasons, this allergy is more common in the elderly, Dr. Perryman said.
Textile dye: Beware polyester
This allergy is mainly triggered by synthetic fabrics like polyester, rayon, and acetate, she noted. Darker colors are more allergenic. Clothes made of natural fibers such as cotton, silk, linen, and wool are alternatives. These are not dyed with these dyes, she said, adding that a reaction to wool will be from irritation, not from the dye.
Paraphenylenediamine: Keep an eye out for this dye ingredient
Paraphenylenediamine, which can trigger allergic reactions, is found in leather dye, fur dye, and some (but not all) hair dyes. Be aware that it can cross-react with other allergens like sulfonamide medications.
If a patch test turns up a reaction to “Black-Rubber Mix,” which includes paraphenylenediamine, consider whether the patient has exposure to the rubber in tires. Car mechanics may be affected by this allergy, Dr. Perryman said.
Neomycin: A drop of trouble
Allergy to the antibiotic neomycin can be triggered by exposure to gentamicin and tobramycin eye drops. Patients may believe they have an infection, Dr. Perryman said, so consider getting a culture. In some cases, an allergic reaction to neomycin may be incorrectly diagnosed as cellulitis.
Nickel: Not just a jewelry hazard
Jewelry and coins can trigger nickel allergies, but be aware that systemic nickel allergy can also trigger skin problems from a patient’s diet. It may be necessary to put patients on a low-nickel diet that avoids foods such as healthy grains, greens (especially spinach), nuts, legumes, and chocolate. “I always feel bad” putting patients on a restrictive diet, Dr. Perryman said, but it can be helpful to take 500 mg of vitamin C three times a day since it binds to nickel.
Cobalt: Watch the chocolate and coffee
Jewelry with cobalt can cause an allergic reaction. Dr. Perryman tells patients to buy an inexpensive “spot test” product online that detects whether jewelry has nickel or cobalt. Cobalt allergy can also trigger symptoms in patients exposed to “hard metal” industrial tools, cement, and masonry. Workers in the plastics and dye industries may be exposed too.
Like nickel, Dr. Perryman said, systemic cobalt allergy related to diet is also possible. The list of foods that contain higher levels of cobalt is long, and includes apricots, beans, beer, chocolate, coffee, nuts, tea, and whole-grain flour.
Dr. Perryman also mentioned several other allergens to keep in mind:
- Chromate can trigger reactions in people who wear leather shoes (the metal can be used in tanning). It can also cause problems in workers exposed to it via cement, bricks, drywall, and metal plating.
- Chromium picolinate, an over-the-counter supplement, can cause systemic dermatitis.
- Gold in jewelry can trigger an allergic reaction. Talk to patients about replating their jewelry, Dr. Perryman said.
- Rubber can trigger reactions due to exposure to rubber bands, makeup sponges, and rubber gloves (even nitrile ones). Be aware that both rubber and latex allergies may coexist and consider a blood test for latex allergy.
- Systemic balsam allergy related to an individual’s diet is possible. Tomato is an especially big villain on this front, along with citrus fruits, spices, cola, chili, and chocolate.
Dr. Perryman disclosed consulting work for IntraDerm. SDEF and this news organization are owned by the same parent company.
REPORTING FROM SDEF WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR