User login
December 2018
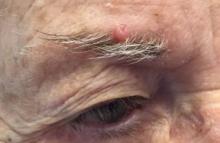
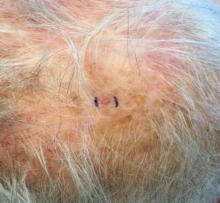
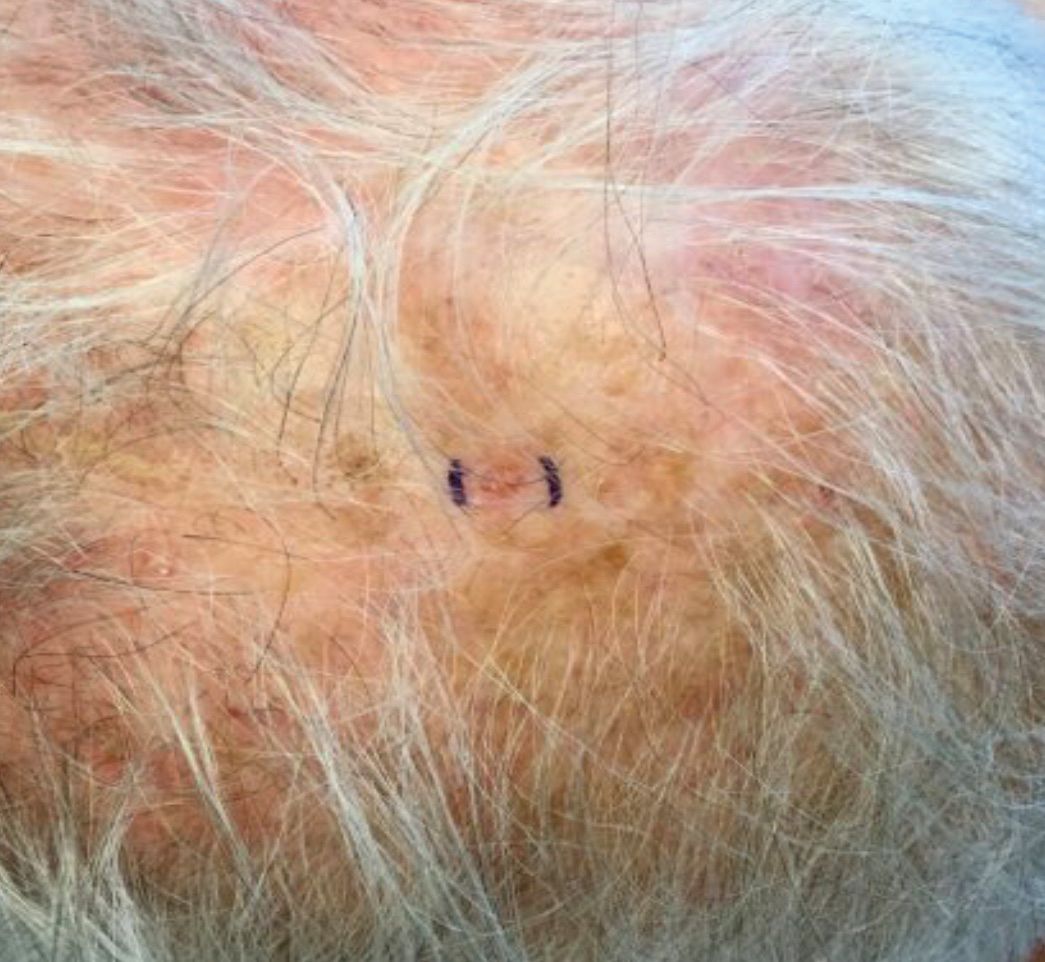
. White individuals over aged 50 years are more frequently affected. Both genders are equally affected, and 25% of cases occur on the covered areas (trunk or extremities) of younger patients. Clinically, lesions present as pink to red plaques or nodules that exhibit rapid growth. Ulceration or crusting may be present. Causes of AFX include ultraviolet radiation and ionizing radiation. AFX is considered a superficial variant of malignant fibrous histiocytoma (MFH), the most common soft tissue sarcoma of adults. Clinically, MFH involves deeper tissues than does AFX, often on the thighs or buttocks. MFH is a more aggressive malignancy that regularly metastasizes.
Histologically, the tumor occurs as a dermal proliferation of “bizarre” spindle cells, epithelioid cells, and atypical histiocytes. Vesicular changes may be present in the nucleus or cytoplasm of the spindle cells. Mitotic figures are present. Multinucleated giant cells may be present. Solar elastosis is often seen, as well. Vimentin and histiocyte stains are positive. Unlike melanoma, S-100 staining is minimal. Unlike squamous cell carcinoma, prekeratin staining is negative. CD34 is negative. AFX resembles MFH histologically.
Surgical excision by the Mohs procedure is preferred over wide excision as there is a risk of recurrence. AFX rarely metastasizes. This is more likely if inadequately excised or the patient is immunosuppressed. Sun protective practices, such as applying and reapplying sunscreen regularly, wearing sun protective clothing, and avoiding excessive UV exposure during peak hours is recommended.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to [email protected].
. White individuals over aged 50 years are more frequently affected. Both genders are equally affected, and 25% of cases occur on the covered areas (trunk or extremities) of younger patients. Clinically, lesions present as pink to red plaques or nodules that exhibit rapid growth. Ulceration or crusting may be present. Causes of AFX include ultraviolet radiation and ionizing radiation. AFX is considered a superficial variant of malignant fibrous histiocytoma (MFH), the most common soft tissue sarcoma of adults. Clinically, MFH involves deeper tissues than does AFX, often on the thighs or buttocks. MFH is a more aggressive malignancy that regularly metastasizes.
Histologically, the tumor occurs as a dermal proliferation of “bizarre” spindle cells, epithelioid cells, and atypical histiocytes. Vesicular changes may be present in the nucleus or cytoplasm of the spindle cells. Mitotic figures are present. Multinucleated giant cells may be present. Solar elastosis is often seen, as well. Vimentin and histiocyte stains are positive. Unlike melanoma, S-100 staining is minimal. Unlike squamous cell carcinoma, prekeratin staining is negative. CD34 is negative. AFX resembles MFH histologically.
Surgical excision by the Mohs procedure is preferred over wide excision as there is a risk of recurrence. AFX rarely metastasizes. This is more likely if inadequately excised or the patient is immunosuppressed. Sun protective practices, such as applying and reapplying sunscreen regularly, wearing sun protective clothing, and avoiding excessive UV exposure during peak hours is recommended.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to [email protected].
. White individuals over aged 50 years are more frequently affected. Both genders are equally affected, and 25% of cases occur on the covered areas (trunk or extremities) of younger patients. Clinically, lesions present as pink to red plaques or nodules that exhibit rapid growth. Ulceration or crusting may be present. Causes of AFX include ultraviolet radiation and ionizing radiation. AFX is considered a superficial variant of malignant fibrous histiocytoma (MFH), the most common soft tissue sarcoma of adults. Clinically, MFH involves deeper tissues than does AFX, often on the thighs or buttocks. MFH is a more aggressive malignancy that regularly metastasizes.
Histologically, the tumor occurs as a dermal proliferation of “bizarre” spindle cells, epithelioid cells, and atypical histiocytes. Vesicular changes may be present in the nucleus or cytoplasm of the spindle cells. Mitotic figures are present. Multinucleated giant cells may be present. Solar elastosis is often seen, as well. Vimentin and histiocyte stains are positive. Unlike melanoma, S-100 staining is minimal. Unlike squamous cell carcinoma, prekeratin staining is negative. CD34 is negative. AFX resembles MFH histologically.
Surgical excision by the Mohs procedure is preferred over wide excision as there is a risk of recurrence. AFX rarely metastasizes. This is more likely if inadequately excised or the patient is immunosuppressed. Sun protective practices, such as applying and reapplying sunscreen regularly, wearing sun protective clothing, and avoiding excessive UV exposure during peak hours is recommended.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to [email protected].
Atopic dermatitis hits mental health, quality of life
Atopic dermatitis (AD) places a considerable burden on mental health and quality of life for patients with disease of even moderate severity, according to a cross-sectional study of data from the Atopic Dermatitis in America survey.
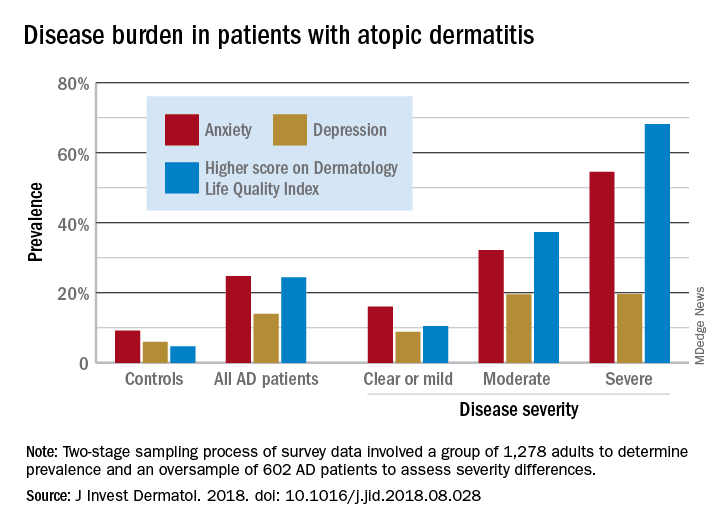
Among adults with severe AD, the mean score on the Dermatology Life Quality Index was 11.4, with a score of 6-30 representing a moderate to large effect on quality of life. The mean for those with moderate disease, 5.9, was just below that range, but 37% of that group did have scores between 6 and 30, Zelma C. Chiesa Fuxench, MD, of the University of Pennsylvania, Philadelphia, and her associates said in the Journal of Investigative Dermatology.
The mean on the Dermatology Life Quality Index for all AD patients was 4.1, with 24% falling into the moderate to large effect range, compared with 1% and 5% for controls. Results were similar on the mental health measure used, the Hospital Anxiety and Depression Scale (HADS). Mean HADS-anxiety scores were 7.0 for all AD patients and 4.7 for controls, and HADS-depression means were 5.8 for AD patients and 3.6 for controls, the investigators reported.
Analysis by disease severity found that 32% of those with moderate AD and almost 56% with severe AD had clinical anxiety (HADS-A score of 11-21), while somewhat lower prevalences were seen for clinical depression (HADS-D score of 11-21): 19.5% for those with moderate AD and 19.7% for patients with severe AD, Dr. Chiesa Fuxench and her associates said.
“An increasing number of studies provide evidence that AD is associated with marked [quality of life] impairment and increased health care costs with higher burden and costs in those with more severe disease. Additional studies should center on exploring those factors associated with AD, and AD disease severity, which lead to increased disease burden in this population,” they wrote.
Respondents to the Atopic Dermatitis in America survey were part of the GfK Knowledge Panel. The study involved a two-stage sampling process: one group of 1,278 adults determined prevalence ,and an oversample of 602 AD patients assessed severity differences.
Dr. Chiesa Fuxench has received research grants from Regeneron, Sanofi, Tioga, and Vanda for work related to atopic dermatitis and has received honoraria for CME work in atopic dermatitis sponsored by educational grants from Regeneron and Sanofi.
SOURCE: J Invest Dermatol. 2018. doi: 10.1016/j.jid.2018.08.028.
Atopic dermatitis (AD) places a considerable burden on mental health and quality of life for patients with disease of even moderate severity, according to a cross-sectional study of data from the Atopic Dermatitis in America survey.

Among adults with severe AD, the mean score on the Dermatology Life Quality Index was 11.4, with a score of 6-30 representing a moderate to large effect on quality of life. The mean for those with moderate disease, 5.9, was just below that range, but 37% of that group did have scores between 6 and 30, Zelma C. Chiesa Fuxench, MD, of the University of Pennsylvania, Philadelphia, and her associates said in the Journal of Investigative Dermatology.
The mean on the Dermatology Life Quality Index for all AD patients was 4.1, with 24% falling into the moderate to large effect range, compared with 1% and 5% for controls. Results were similar on the mental health measure used, the Hospital Anxiety and Depression Scale (HADS). Mean HADS-anxiety scores were 7.0 for all AD patients and 4.7 for controls, and HADS-depression means were 5.8 for AD patients and 3.6 for controls, the investigators reported.
Analysis by disease severity found that 32% of those with moderate AD and almost 56% with severe AD had clinical anxiety (HADS-A score of 11-21), while somewhat lower prevalences were seen for clinical depression (HADS-D score of 11-21): 19.5% for those with moderate AD and 19.7% for patients with severe AD, Dr. Chiesa Fuxench and her associates said.
“An increasing number of studies provide evidence that AD is associated with marked [quality of life] impairment and increased health care costs with higher burden and costs in those with more severe disease. Additional studies should center on exploring those factors associated with AD, and AD disease severity, which lead to increased disease burden in this population,” they wrote.
Respondents to the Atopic Dermatitis in America survey were part of the GfK Knowledge Panel. The study involved a two-stage sampling process: one group of 1,278 adults determined prevalence ,and an oversample of 602 AD patients assessed severity differences.
Dr. Chiesa Fuxench has received research grants from Regeneron, Sanofi, Tioga, and Vanda for work related to atopic dermatitis and has received honoraria for CME work in atopic dermatitis sponsored by educational grants from Regeneron and Sanofi.
SOURCE: J Invest Dermatol. 2018. doi: 10.1016/j.jid.2018.08.028.
Atopic dermatitis (AD) places a considerable burden on mental health and quality of life for patients with disease of even moderate severity, according to a cross-sectional study of data from the Atopic Dermatitis in America survey.

Among adults with severe AD, the mean score on the Dermatology Life Quality Index was 11.4, with a score of 6-30 representing a moderate to large effect on quality of life. The mean for those with moderate disease, 5.9, was just below that range, but 37% of that group did have scores between 6 and 30, Zelma C. Chiesa Fuxench, MD, of the University of Pennsylvania, Philadelphia, and her associates said in the Journal of Investigative Dermatology.
The mean on the Dermatology Life Quality Index for all AD patients was 4.1, with 24% falling into the moderate to large effect range, compared with 1% and 5% for controls. Results were similar on the mental health measure used, the Hospital Anxiety and Depression Scale (HADS). Mean HADS-anxiety scores were 7.0 for all AD patients and 4.7 for controls, and HADS-depression means were 5.8 for AD patients and 3.6 for controls, the investigators reported.
Analysis by disease severity found that 32% of those with moderate AD and almost 56% with severe AD had clinical anxiety (HADS-A score of 11-21), while somewhat lower prevalences were seen for clinical depression (HADS-D score of 11-21): 19.5% for those with moderate AD and 19.7% for patients with severe AD, Dr. Chiesa Fuxench and her associates said.
“An increasing number of studies provide evidence that AD is associated with marked [quality of life] impairment and increased health care costs with higher burden and costs in those with more severe disease. Additional studies should center on exploring those factors associated with AD, and AD disease severity, which lead to increased disease burden in this population,” they wrote.
Respondents to the Atopic Dermatitis in America survey were part of the GfK Knowledge Panel. The study involved a two-stage sampling process: one group of 1,278 adults determined prevalence ,and an oversample of 602 AD patients assessed severity differences.
Dr. Chiesa Fuxench has received research grants from Regeneron, Sanofi, Tioga, and Vanda for work related to atopic dermatitis and has received honoraria for CME work in atopic dermatitis sponsored by educational grants from Regeneron and Sanofi.
SOURCE: J Invest Dermatol. 2018. doi: 10.1016/j.jid.2018.08.028.
FROM THE JOURNAL OF INVESTIGATIVE DERMATOLOGY
Growing lesion on cheek
Figure 1
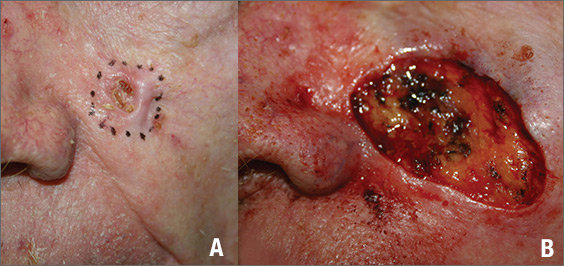
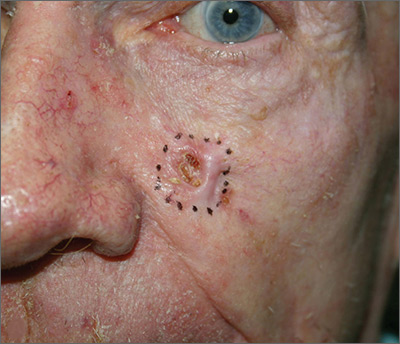
The FP suspected that this was a basal cell carcinoma (BCC) or squamous cell carcinoma. He leaned toward a BCC because of the pearly border on the edge, but knew that a biopsy diagnosis was needed before planning definitive treatment.
The FP recommended performing a shave biopsy that day. (See the Watch & Learn video on “Shave biopsy.”) After obtaining patient consent, he injected 1% lidocaine with epinephrine and waited for the epinephrine to work. He performed the shave biopsy with a Dermablade, and used a cotton-tipped applicator to vigorously apply aluminum chloride to the site. He used a twisting motion and pressure to achieve hemostasis. The bleeding stopped, and the FP dressed the lesion with petrolatum and some gauze. Dermatopathology revealed a sclerosing BCC.
The FP realized this was an aggressive tumor and referred the patient for Mohs surgery. The surgery required 4 excisions to get clean margins (FIGURE 1B). The usual 4- to 5-mm margins with an elliptical excision would not have removed the full tumor.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Basal cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:989-998.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
Figure 1

The FP suspected that this was a basal cell carcinoma (BCC) or squamous cell carcinoma. He leaned toward a BCC because of the pearly border on the edge, but knew that a biopsy diagnosis was needed before planning definitive treatment.
The FP recommended performing a shave biopsy that day. (See the Watch & Learn video on “Shave biopsy.”) After obtaining patient consent, he injected 1% lidocaine with epinephrine and waited for the epinephrine to work. He performed the shave biopsy with a Dermablade, and used a cotton-tipped applicator to vigorously apply aluminum chloride to the site. He used a twisting motion and pressure to achieve hemostasis. The bleeding stopped, and the FP dressed the lesion with petrolatum and some gauze. Dermatopathology revealed a sclerosing BCC.
The FP realized this was an aggressive tumor and referred the patient for Mohs surgery. The surgery required 4 excisions to get clean margins (FIGURE 1B). The usual 4- to 5-mm margins with an elliptical excision would not have removed the full tumor.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Basal cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:989-998.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
Figure 1

The FP suspected that this was a basal cell carcinoma (BCC) or squamous cell carcinoma. He leaned toward a BCC because of the pearly border on the edge, but knew that a biopsy diagnosis was needed before planning definitive treatment.
The FP recommended performing a shave biopsy that day. (See the Watch & Learn video on “Shave biopsy.”) After obtaining patient consent, he injected 1% lidocaine with epinephrine and waited for the epinephrine to work. He performed the shave biopsy with a Dermablade, and used a cotton-tipped applicator to vigorously apply aluminum chloride to the site. He used a twisting motion and pressure to achieve hemostasis. The bleeding stopped, and the FP dressed the lesion with petrolatum and some gauze. Dermatopathology revealed a sclerosing BCC.
The FP realized this was an aggressive tumor and referred the patient for Mohs surgery. The surgery required 4 excisions to get clean margins (FIGURE 1B). The usual 4- to 5-mm margins with an elliptical excision would not have removed the full tumor.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Basal cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:989-998.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
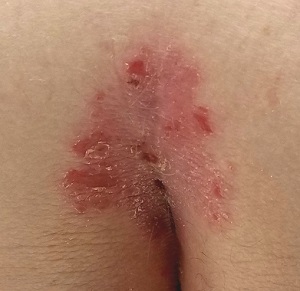
A Nuisance for the Newlyweds
Prompted by his new bride, who is concerned she might “catch something” from him, a 53-year-old man self-refers for evaluation of a slightly itchy intergluteal rash. He’s had it for years; it waxes and wanes but never fully resolves.
It has been previously diagnosed as a yeast infection, fungal infection, and even herpes. But none of the respective treatments have helped.
More history-taking reveals a family history of psoriasis (maternal grandmother), but the patient denies other areas of involvement or other skin changes. He also denies having arthritis.
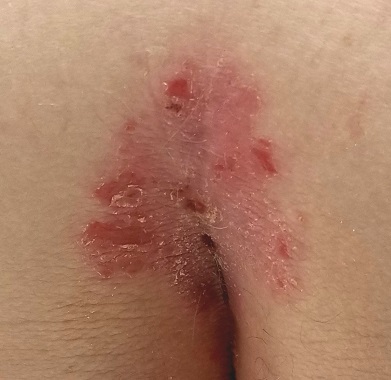
EXAMINATION
A salmon-pink, 7-cm, roughly round dry patch covered by white tenacious scale is located in the upper intergluteal/sacral interface. There is no increased warmth or tenderness on palpation.
A similar process is noted in the periumbilical area (a difficult area for this patient to see, due to his weight). Inspection of his fingernails reveals 3/10 with definite tiny pits.
What is the diagnosis?
DISCUSSION
The urge to call any and every rash occurring near the genitals a yeast infection is universally compelling among primary care providers. It is often so reflexive that even when anti-yeast medications fail, the provider hangs onto the diagnosis. This happens for one simple reason: Their differential is lacking.
This patient has psoriasis, albeit a somewhat unusual form, which demonstrates an important learning point: Psoriasis can present in any number of ways, not just in the standard “extensor surfaces of elbows and knees” distribution. It’s not unusual for psoriasis to zero in on one or two areas. I’ve seen it confined to the groin, the genitals, and the scalp. It can even involve the oral mucosa.
In these somewhat obscure cases, additional findings can be helpful to establish the diagnosis. The two areas of involvement in this case—the upper intergluteal area and the periumbilical region—may not fit the classic “knees and elbows” picture of psoriasis, but they are not atypical for the disease. Add the nail pits, the fixed nature of the problem, and the family history, and you’ve nailed the diagnosis.
Don’t forget that, occasionally, psoriatic arthropathy can precede the appearance of psoriasis, and that the severity of one does not predict the severity of the other.
Finally, in a fair number of cases, the diagnosis of psoriasis must be made by biopsy, which shows characteristic changes such as parakeratosis, epidermal thickening, and fusing of rete ridges. These “psoriasiform” changes seen microscopically must be corroborated by clinical findings, though, since many other papulosquamous diseases can exhibit similar changes.
TAKE-HOME LEARNING POINTS
- Psoriasis is one of the more common dermatoses in this country, which means you will see it with some frequency.
- Psoriasis can affect limited or atypical areas, but corroboration of the diagnosis can be sought in classic areas (nails, scalp, upper intergluteal and periumbilical areas).
- Strive to develop alternative diagnoses for similar rashes—in other words, build your differential for “yeast infection.”
Prompted by his new bride, who is concerned she might “catch something” from him, a 53-year-old man self-refers for evaluation of a slightly itchy intergluteal rash. He’s had it for years; it waxes and wanes but never fully resolves.
It has been previously diagnosed as a yeast infection, fungal infection, and even herpes. But none of the respective treatments have helped.
More history-taking reveals a family history of psoriasis (maternal grandmother), but the patient denies other areas of involvement or other skin changes. He also denies having arthritis.

EXAMINATION
A salmon-pink, 7-cm, roughly round dry patch covered by white tenacious scale is located in the upper intergluteal/sacral interface. There is no increased warmth or tenderness on palpation.
A similar process is noted in the periumbilical area (a difficult area for this patient to see, due to his weight). Inspection of his fingernails reveals 3/10 with definite tiny pits.
What is the diagnosis?
DISCUSSION
The urge to call any and every rash occurring near the genitals a yeast infection is universally compelling among primary care providers. It is often so reflexive that even when anti-yeast medications fail, the provider hangs onto the diagnosis. This happens for one simple reason: Their differential is lacking.
This patient has psoriasis, albeit a somewhat unusual form, which demonstrates an important learning point: Psoriasis can present in any number of ways, not just in the standard “extensor surfaces of elbows and knees” distribution. It’s not unusual for psoriasis to zero in on one or two areas. I’ve seen it confined to the groin, the genitals, and the scalp. It can even involve the oral mucosa.
In these somewhat obscure cases, additional findings can be helpful to establish the diagnosis. The two areas of involvement in this case—the upper intergluteal area and the periumbilical region—may not fit the classic “knees and elbows” picture of psoriasis, but they are not atypical for the disease. Add the nail pits, the fixed nature of the problem, and the family history, and you’ve nailed the diagnosis.
Don’t forget that, occasionally, psoriatic arthropathy can precede the appearance of psoriasis, and that the severity of one does not predict the severity of the other.
Finally, in a fair number of cases, the diagnosis of psoriasis must be made by biopsy, which shows characteristic changes such as parakeratosis, epidermal thickening, and fusing of rete ridges. These “psoriasiform” changes seen microscopically must be corroborated by clinical findings, though, since many other papulosquamous diseases can exhibit similar changes.
TAKE-HOME LEARNING POINTS
- Psoriasis is one of the more common dermatoses in this country, which means you will see it with some frequency.
- Psoriasis can affect limited or atypical areas, but corroboration of the diagnosis can be sought in classic areas (nails, scalp, upper intergluteal and periumbilical areas).
- Strive to develop alternative diagnoses for similar rashes—in other words, build your differential for “yeast infection.”
Prompted by his new bride, who is concerned she might “catch something” from him, a 53-year-old man self-refers for evaluation of a slightly itchy intergluteal rash. He’s had it for years; it waxes and wanes but never fully resolves.
It has been previously diagnosed as a yeast infection, fungal infection, and even herpes. But none of the respective treatments have helped.
More history-taking reveals a family history of psoriasis (maternal grandmother), but the patient denies other areas of involvement or other skin changes. He also denies having arthritis.

EXAMINATION
A salmon-pink, 7-cm, roughly round dry patch covered by white tenacious scale is located in the upper intergluteal/sacral interface. There is no increased warmth or tenderness on palpation.
A similar process is noted in the periumbilical area (a difficult area for this patient to see, due to his weight). Inspection of his fingernails reveals 3/10 with definite tiny pits.
What is the diagnosis?
DISCUSSION
The urge to call any and every rash occurring near the genitals a yeast infection is universally compelling among primary care providers. It is often so reflexive that even when anti-yeast medications fail, the provider hangs onto the diagnosis. This happens for one simple reason: Their differential is lacking.
This patient has psoriasis, albeit a somewhat unusual form, which demonstrates an important learning point: Psoriasis can present in any number of ways, not just in the standard “extensor surfaces of elbows and knees” distribution. It’s not unusual for psoriasis to zero in on one or two areas. I’ve seen it confined to the groin, the genitals, and the scalp. It can even involve the oral mucosa.
In these somewhat obscure cases, additional findings can be helpful to establish the diagnosis. The two areas of involvement in this case—the upper intergluteal area and the periumbilical region—may not fit the classic “knees and elbows” picture of psoriasis, but they are not atypical for the disease. Add the nail pits, the fixed nature of the problem, and the family history, and you’ve nailed the diagnosis.
Don’t forget that, occasionally, psoriatic arthropathy can precede the appearance of psoriasis, and that the severity of one does not predict the severity of the other.
Finally, in a fair number of cases, the diagnosis of psoriasis must be made by biopsy, which shows characteristic changes such as parakeratosis, epidermal thickening, and fusing of rete ridges. These “psoriasiform” changes seen microscopically must be corroborated by clinical findings, though, since many other papulosquamous diseases can exhibit similar changes.
TAKE-HOME LEARNING POINTS
- Psoriasis is one of the more common dermatoses in this country, which means you will see it with some frequency.
- Psoriasis can affect limited or atypical areas, but corroboration of the diagnosis can be sought in classic areas (nails, scalp, upper intergluteal and periumbilical areas).
- Strive to develop alternative diagnoses for similar rashes—in other words, build your differential for “yeast infection.”
FDA accepts priority review of dupilumab for adolescent atopic dermatitis
The Food and Drug Administration has accepted the who have not been well controlled with topical therapies or who are unable to use topical therapies.
In a statement, dupilumab manufacturers Regeneron and Sanofi announced that the target action data for an FDA decision on dupilumab for adolescents is March 11, 2019. “Currently, there are no FDA-approved systemic biologic medicines to treat adolescents with moderate to severe atopic dermatitis,” the companies said in the statement.
The sBLA for dupilumab use in teens is based on data from a phase 3 study presented at the annual congress of European Academy of Dermatology and Venereology in September 2018. In that study, the proportion of patients who achieved a 75% or greater improvement in the Eczema Area and Severity Index at 16 weeks was 38.1% with monthly dupilumab, 41.5% with dupilumab every 2 weeks, and 8.2% with placebo.
According to the companies, the most common adverse events included injection site reactions, oropharyngeal pain, and cold sores. Conjunctivitis has also been reported in some patients.
Dupilumab (Dupixent), which inhibits interleukin-4 and interleukin-13 signaling, is currently approved for treating uncontrolled moderate to severe AD in adults and, more recently, as an add-on maintenance treatment in patients with moderate to severe asthma aged 12 years and older with an eosinophilic phenotype or with oral corticosteroid–dependent asthma.
The FDA granted Breakthrough Therapy designation for dupilumab in 2016 for the treatment of moderate to severe AD in adolescents and severe AD in children aged 6 months to 11 years who are insufficiently controlled with topical medications.
The Food and Drug Administration has accepted the who have not been well controlled with topical therapies or who are unable to use topical therapies.
In a statement, dupilumab manufacturers Regeneron and Sanofi announced that the target action data for an FDA decision on dupilumab for adolescents is March 11, 2019. “Currently, there are no FDA-approved systemic biologic medicines to treat adolescents with moderate to severe atopic dermatitis,” the companies said in the statement.
The sBLA for dupilumab use in teens is based on data from a phase 3 study presented at the annual congress of European Academy of Dermatology and Venereology in September 2018. In that study, the proportion of patients who achieved a 75% or greater improvement in the Eczema Area and Severity Index at 16 weeks was 38.1% with monthly dupilumab, 41.5% with dupilumab every 2 weeks, and 8.2% with placebo.
According to the companies, the most common adverse events included injection site reactions, oropharyngeal pain, and cold sores. Conjunctivitis has also been reported in some patients.
Dupilumab (Dupixent), which inhibits interleukin-4 and interleukin-13 signaling, is currently approved for treating uncontrolled moderate to severe AD in adults and, more recently, as an add-on maintenance treatment in patients with moderate to severe asthma aged 12 years and older with an eosinophilic phenotype or with oral corticosteroid–dependent asthma.
The FDA granted Breakthrough Therapy designation for dupilumab in 2016 for the treatment of moderate to severe AD in adolescents and severe AD in children aged 6 months to 11 years who are insufficiently controlled with topical medications.
The Food and Drug Administration has accepted the who have not been well controlled with topical therapies or who are unable to use topical therapies.
In a statement, dupilumab manufacturers Regeneron and Sanofi announced that the target action data for an FDA decision on dupilumab for adolescents is March 11, 2019. “Currently, there are no FDA-approved systemic biologic medicines to treat adolescents with moderate to severe atopic dermatitis,” the companies said in the statement.
The sBLA for dupilumab use in teens is based on data from a phase 3 study presented at the annual congress of European Academy of Dermatology and Venereology in September 2018. In that study, the proportion of patients who achieved a 75% or greater improvement in the Eczema Area and Severity Index at 16 weeks was 38.1% with monthly dupilumab, 41.5% with dupilumab every 2 weeks, and 8.2% with placebo.
According to the companies, the most common adverse events included injection site reactions, oropharyngeal pain, and cold sores. Conjunctivitis has also been reported in some patients.
Dupilumab (Dupixent), which inhibits interleukin-4 and interleukin-13 signaling, is currently approved for treating uncontrolled moderate to severe AD in adults and, more recently, as an add-on maintenance treatment in patients with moderate to severe asthma aged 12 years and older with an eosinophilic phenotype or with oral corticosteroid–dependent asthma.
The FDA granted Breakthrough Therapy designation for dupilumab in 2016 for the treatment of moderate to severe AD in adolescents and severe AD in children aged 6 months to 11 years who are insufficiently controlled with topical medications.
Caffeinated coffee intake linked to lower rosacea risk
Caffeinated coffee intake is linked to a decreased incidence of rosacea, results of a large, observational study suggest.
Increased levels of caffeinated coffee consumption were associated with progressively lower levels of incident rosacea in a study of more than 82,000 participants representing more than 1.1 million person-years of follow-up.
By contrast, caffeine from other foods was not associated with rosacea incidence, reported Wen-Qing Li, PhD, of the department of dermatology at Brown University, Providence, R.I., and his coinvestigators. Those findings may have implications for the “causes and clinical approach” to rosacea.
“Our findings do not support limiting caffeine intake as a preventive strategy for rosacea,” they concluded in the study, published in JAMA Dermatology.
This is not the first study looking for potential links between rosacea and caffeine or coffee intake. However, previous studies didn’t distinguish between caffeinated coffee versus other beverages, and only one previous study made a distinction between the amounts of caffeine and coffee consumed, according to the authors.
Their research was based on data from the Nurses’ Health Study II, a prospective cohort study started in 1989. They looked specifically at 82,737 women who, in 2005, responded to the question about whether they had been diagnosed with rosacea. They identified 4,945 incident rosacea cases over the 1,120,051 person-years of follow-up.
A significant inverse association was found between caffeinated coffee intake and rosacea: Individuals who consumed four or more servings a day had a significantly lower risk of rosacea, compared with those who consumed one or fewer servings per month (hazard ratio, 0.77; 95% confidence interval, 0.69-0.87; P less than .001). They also found a dose-dependent effect, with the absolute risk of rosacea decreased by 131 per 100,000 person-years with at least four daily servings of caffeinated coffee, compared with under one serving a month.
By contrast, decaffeinated coffee was not associated with a reduced risk of rosacea, and in further analysis, the investigators found that there was no significant inverse association when they looked just at caffeine intake from sources other than coffee, such as chocolate, tea, and soda.
Caffeine could influence rosacea incidence by one of several mechanisms, including its effect on vascular contractility, the investigators hypothesized. “Increased caffeine intake may decrease vasodilation and consequently lead to diminution of rosacea symptoms.”
However, caffeine also has documented immunosuppressant effects that could possibly decrease rosacea-associated inflammation and has been shown to modulate hormone levels. “Hormonal factors have been implicated in the development of rosacea, and caffeine can modulate hormone levels,” they wrote.
Two study authors reported disclosures related to AbbVie, Amgen, Astellas Pharma, Janssen, Merck, Novartis, and Pfizer, among others. Funding for the study came from several sources, including National Institutes of Health grants for the Nurses’ Health Study II.
SOURCE: Li W-Q et al. JAMA Dermatol. 2018 Oct 17. doi: 10.1001/jamadermatol.2018.3301.
This study shows an inverse association between caffeine intake and incidence rosacea, which suggests that patients with rosacea need not avoid coffee, according to Mackenzie R. Wehner, MD, and Eleni Linos, MD, MPH.
For everyone else, the findings offer yet another reason to keep indulging in one of “life’s habitual pleasures,” they wrote. “We will raise an insulated travel mug to that.”
This latest study fits in with numerous studies suggesting coffee may be protective against a number of maladies, including cancer, cardiovascular disease, type 2 diabetes, and Parkinson’s disease, they wrote in their editorial published in JAMA Dermatology.
However, this is an observational study, not a rigorous, randomized trial that could more conclusively prove coffee actually provides an antirosacea benefit that cannot be explained by other factors, such as systematic differences between people who do and do not drink coffee. Enrollment of all women, mostly white, in the Nurses’ Health Study II is another limitation, they added.
Nevertheless, studies like this are the “next-best option” in lieu of randomized, controlled trials to evaluate these relationships, they wrote. “Importantly, the strength of the protective effect noted and the dose-response relationship with increasing coffee and caffeine intake are convincing.”
Dr. Wehner , is with the department of dermatology at the University of Pennsylvania, Philadelphia. Dr. Linos is with the department of dermatology at the University of California, San Francisco. Dr. Wehner reported support from a National Institute of Arthritis and Musculoskeletal and Skin Diseases/National Institutes of Health Dermatology Research Training grant. Dr. Linos reported support from the National Cancer Institute and the National Institute of Aging.
This study shows an inverse association between caffeine intake and incidence rosacea, which suggests that patients with rosacea need not avoid coffee, according to Mackenzie R. Wehner, MD, and Eleni Linos, MD, MPH.
For everyone else, the findings offer yet another reason to keep indulging in one of “life’s habitual pleasures,” they wrote. “We will raise an insulated travel mug to that.”
This latest study fits in with numerous studies suggesting coffee may be protective against a number of maladies, including cancer, cardiovascular disease, type 2 diabetes, and Parkinson’s disease, they wrote in their editorial published in JAMA Dermatology.
However, this is an observational study, not a rigorous, randomized trial that could more conclusively prove coffee actually provides an antirosacea benefit that cannot be explained by other factors, such as systematic differences between people who do and do not drink coffee. Enrollment of all women, mostly white, in the Nurses’ Health Study II is another limitation, they added.
Nevertheless, studies like this are the “next-best option” in lieu of randomized, controlled trials to evaluate these relationships, they wrote. “Importantly, the strength of the protective effect noted and the dose-response relationship with increasing coffee and caffeine intake are convincing.”
Dr. Wehner , is with the department of dermatology at the University of Pennsylvania, Philadelphia. Dr. Linos is with the department of dermatology at the University of California, San Francisco. Dr. Wehner reported support from a National Institute of Arthritis and Musculoskeletal and Skin Diseases/National Institutes of Health Dermatology Research Training grant. Dr. Linos reported support from the National Cancer Institute and the National Institute of Aging.
This study shows an inverse association between caffeine intake and incidence rosacea, which suggests that patients with rosacea need not avoid coffee, according to Mackenzie R. Wehner, MD, and Eleni Linos, MD, MPH.
For everyone else, the findings offer yet another reason to keep indulging in one of “life’s habitual pleasures,” they wrote. “We will raise an insulated travel mug to that.”
This latest study fits in with numerous studies suggesting coffee may be protective against a number of maladies, including cancer, cardiovascular disease, type 2 diabetes, and Parkinson’s disease, they wrote in their editorial published in JAMA Dermatology.
However, this is an observational study, not a rigorous, randomized trial that could more conclusively prove coffee actually provides an antirosacea benefit that cannot be explained by other factors, such as systematic differences between people who do and do not drink coffee. Enrollment of all women, mostly white, in the Nurses’ Health Study II is another limitation, they added.
Nevertheless, studies like this are the “next-best option” in lieu of randomized, controlled trials to evaluate these relationships, they wrote. “Importantly, the strength of the protective effect noted and the dose-response relationship with increasing coffee and caffeine intake are convincing.”
Dr. Wehner , is with the department of dermatology at the University of Pennsylvania, Philadelphia. Dr. Linos is with the department of dermatology at the University of California, San Francisco. Dr. Wehner reported support from a National Institute of Arthritis and Musculoskeletal and Skin Diseases/National Institutes of Health Dermatology Research Training grant. Dr. Linos reported support from the National Cancer Institute and the National Institute of Aging.
Caffeinated coffee intake is linked to a decreased incidence of rosacea, results of a large, observational study suggest.
Increased levels of caffeinated coffee consumption were associated with progressively lower levels of incident rosacea in a study of more than 82,000 participants representing more than 1.1 million person-years of follow-up.
By contrast, caffeine from other foods was not associated with rosacea incidence, reported Wen-Qing Li, PhD, of the department of dermatology at Brown University, Providence, R.I., and his coinvestigators. Those findings may have implications for the “causes and clinical approach” to rosacea.
“Our findings do not support limiting caffeine intake as a preventive strategy for rosacea,” they concluded in the study, published in JAMA Dermatology.
This is not the first study looking for potential links between rosacea and caffeine or coffee intake. However, previous studies didn’t distinguish between caffeinated coffee versus other beverages, and only one previous study made a distinction between the amounts of caffeine and coffee consumed, according to the authors.
Their research was based on data from the Nurses’ Health Study II, a prospective cohort study started in 1989. They looked specifically at 82,737 women who, in 2005, responded to the question about whether they had been diagnosed with rosacea. They identified 4,945 incident rosacea cases over the 1,120,051 person-years of follow-up.
A significant inverse association was found between caffeinated coffee intake and rosacea: Individuals who consumed four or more servings a day had a significantly lower risk of rosacea, compared with those who consumed one or fewer servings per month (hazard ratio, 0.77; 95% confidence interval, 0.69-0.87; P less than .001). They also found a dose-dependent effect, with the absolute risk of rosacea decreased by 131 per 100,000 person-years with at least four daily servings of caffeinated coffee, compared with under one serving a month.
By contrast, decaffeinated coffee was not associated with a reduced risk of rosacea, and in further analysis, the investigators found that there was no significant inverse association when they looked just at caffeine intake from sources other than coffee, such as chocolate, tea, and soda.
Caffeine could influence rosacea incidence by one of several mechanisms, including its effect on vascular contractility, the investigators hypothesized. “Increased caffeine intake may decrease vasodilation and consequently lead to diminution of rosacea symptoms.”
However, caffeine also has documented immunosuppressant effects that could possibly decrease rosacea-associated inflammation and has been shown to modulate hormone levels. “Hormonal factors have been implicated in the development of rosacea, and caffeine can modulate hormone levels,” they wrote.
Two study authors reported disclosures related to AbbVie, Amgen, Astellas Pharma, Janssen, Merck, Novartis, and Pfizer, among others. Funding for the study came from several sources, including National Institutes of Health grants for the Nurses’ Health Study II.
SOURCE: Li W-Q et al. JAMA Dermatol. 2018 Oct 17. doi: 10.1001/jamadermatol.2018.3301.
Caffeinated coffee intake is linked to a decreased incidence of rosacea, results of a large, observational study suggest.
Increased levels of caffeinated coffee consumption were associated with progressively lower levels of incident rosacea in a study of more than 82,000 participants representing more than 1.1 million person-years of follow-up.
By contrast, caffeine from other foods was not associated with rosacea incidence, reported Wen-Qing Li, PhD, of the department of dermatology at Brown University, Providence, R.I., and his coinvestigators. Those findings may have implications for the “causes and clinical approach” to rosacea.
“Our findings do not support limiting caffeine intake as a preventive strategy for rosacea,” they concluded in the study, published in JAMA Dermatology.
This is not the first study looking for potential links between rosacea and caffeine or coffee intake. However, previous studies didn’t distinguish between caffeinated coffee versus other beverages, and only one previous study made a distinction between the amounts of caffeine and coffee consumed, according to the authors.
Their research was based on data from the Nurses’ Health Study II, a prospective cohort study started in 1989. They looked specifically at 82,737 women who, in 2005, responded to the question about whether they had been diagnosed with rosacea. They identified 4,945 incident rosacea cases over the 1,120,051 person-years of follow-up.
A significant inverse association was found between caffeinated coffee intake and rosacea: Individuals who consumed four or more servings a day had a significantly lower risk of rosacea, compared with those who consumed one or fewer servings per month (hazard ratio, 0.77; 95% confidence interval, 0.69-0.87; P less than .001). They also found a dose-dependent effect, with the absolute risk of rosacea decreased by 131 per 100,000 person-years with at least four daily servings of caffeinated coffee, compared with under one serving a month.
By contrast, decaffeinated coffee was not associated with a reduced risk of rosacea, and in further analysis, the investigators found that there was no significant inverse association when they looked just at caffeine intake from sources other than coffee, such as chocolate, tea, and soda.
Caffeine could influence rosacea incidence by one of several mechanisms, including its effect on vascular contractility, the investigators hypothesized. “Increased caffeine intake may decrease vasodilation and consequently lead to diminution of rosacea symptoms.”
However, caffeine also has documented immunosuppressant effects that could possibly decrease rosacea-associated inflammation and has been shown to modulate hormone levels. “Hormonal factors have been implicated in the development of rosacea, and caffeine can modulate hormone levels,” they wrote.
Two study authors reported disclosures related to AbbVie, Amgen, Astellas Pharma, Janssen, Merck, Novartis, and Pfizer, among others. Funding for the study came from several sources, including National Institutes of Health grants for the Nurses’ Health Study II.
SOURCE: Li W-Q et al. JAMA Dermatol. 2018 Oct 17. doi: 10.1001/jamadermatol.2018.3301.
FROM JAMA DERMATOLOGY
Key clinical point: Caffeinated coffee intake was linked to decreased incidence of rosacea, while decaffeinated coffee and noncoffee sources of caffeine had no such effect.
Major finding: Consuming four or more servings of caffeinated coffee was associated with lower risk of rosacea versus one or fewer servings per month (hazard ratio, 0.77; P less than .001).
Study details: An analysis based on 82,737 participants in the Nurses’ Health Study II who responded to a question about rosacea.
Disclosures: Two study authors reported disclosures related to AbbVie, Amgen, Astellas Pharma, Janssen, Merck, Novartis, and Pfizer, among others. Funding for the study came from National Institutes of Health grants for the Nurses’ Health Study II and other sources.
Source: Li W-Q et al. JAMA Dermatol. 2018 Oct 17. doi: 10.1001/jamadermatol.2018.3301.
Ligelizumab outperformed omalizumab for refractory chronic spontaneous urticaria
.
“For sure, ligelizumab is the highlight of this year in urticariology,” Marcus Maurer, MD, declared at the annual congress of the European Academy of Dermatology and Venereology. An ongoing phase 3 trial will now compare more than 1,000 patients with CSU who will be randomized to ligelizumab, omalizumab, or placebo.
Like omalizumab (Xolair), which is approved in the United States and Europe for treatment of CSU, ligelizumab is a humanized anti-IgE monoclonal antibody. But the investigational agent binds to IgE with greater affinity than omalizumab, and this translated into greater therapeutic efficacy in the multicenter, double-blind, placebo-controlled clinical trial, explained Dr. Maurer, professor of dermatology and allergy at Charité University in Berlin.
Study participants, all refractory to histamine1 antihistamines and in many cases to leukotriene receptor antagonists as well, were randomized to omalizumab at 300 mg, placebo, or to ligelizumab at 24 mg, 72 mg, or 240 mg administered by subcutaneous injection every 4 weeks for 20 weeks. The study showed that the effective dose of ligelizumab lies somewhere between 72 and 240 mg; the 24-mg dose won’t be pursued in further studies.
“Three things are important in the comparison between ligelizumab and omalizumab: First, ligelizumab works faster – and omalizumab is a fast-working drug in urticaria. As early as week 4 after initiation of treatment, ligelizumab resulted in a significantly higher response rate,” he said.
Second, a complete response rate as defined by an Urticaria Activity Score over the past 7 days (UAS7) of 0 was achieved by more than 50% of patients on ligelizumab at 240 mg, a rate twice that seen in the omalizumab group. Indeed, more patients were symptom-free on ligelizumab at 72 mg or 240 mg than on omalizumab throughout the 20-week study.
And third, time to relapse after treatment discontinuation was markedly longer with ligelizumab.
“Once you stop the treatment, we expect patients to come back because we didn’t cure the disease, we blocked the signs and symptoms by blocking mast cell degranulation. Relapse after the last injection occurred at about 4 weeks with omalizumab versus 10 weeks for ligelizumab on average. That’s amazing,” Dr. Maurer said.
At week 20, the mean reductions from baseline in UAS7 scores were 13.6 points with placebo, 15.2 points with the lowest dose of ligelizumab, 18.2 points with omalizumab, 23.1 points with ligelizumab at 72 mg, and 22.5 points for ligelizumab at 240 mg.
The side effect profiles for both biologics were essentially the same as for placebo with the exception of a 5.9% rate of mild injection site reactions with ligelizumab at the 240-mg dose versus 2.3% with placebo.
Many clinicians have noticed a significant limitation of omalizumab: It is less effective in patients with more complex CSU having an autoimmune overlay, type 2b angioedema, and/or long disease duration.
“This does not seem to be the case with ligelizumab. Even for the difficult-to-treat subpopulations of CSU, ligelizumab appears to be a drug that can protect against mast cell degranulation. We see a reduction in angioedema activity; we see a reduction in wheal size and number; we see a reduction in the itch – so across all the symptoms in the difficult subpopulations, this is the better drug. Now we have to make it work in the phase 3 trials to bring it to clinical practice,” he said.
Dr. Maurer reported receiving research funding from and serving as an advisor to and paid speaker for Novartis, which markets omalizumab and is developing ligelizumab.
.
“For sure, ligelizumab is the highlight of this year in urticariology,” Marcus Maurer, MD, declared at the annual congress of the European Academy of Dermatology and Venereology. An ongoing phase 3 trial will now compare more than 1,000 patients with CSU who will be randomized to ligelizumab, omalizumab, or placebo.
Like omalizumab (Xolair), which is approved in the United States and Europe for treatment of CSU, ligelizumab is a humanized anti-IgE monoclonal antibody. But the investigational agent binds to IgE with greater affinity than omalizumab, and this translated into greater therapeutic efficacy in the multicenter, double-blind, placebo-controlled clinical trial, explained Dr. Maurer, professor of dermatology and allergy at Charité University in Berlin.
Study participants, all refractory to histamine1 antihistamines and in many cases to leukotriene receptor antagonists as well, were randomized to omalizumab at 300 mg, placebo, or to ligelizumab at 24 mg, 72 mg, or 240 mg administered by subcutaneous injection every 4 weeks for 20 weeks. The study showed that the effective dose of ligelizumab lies somewhere between 72 and 240 mg; the 24-mg dose won’t be pursued in further studies.
“Three things are important in the comparison between ligelizumab and omalizumab: First, ligelizumab works faster – and omalizumab is a fast-working drug in urticaria. As early as week 4 after initiation of treatment, ligelizumab resulted in a significantly higher response rate,” he said.
Second, a complete response rate as defined by an Urticaria Activity Score over the past 7 days (UAS7) of 0 was achieved by more than 50% of patients on ligelizumab at 240 mg, a rate twice that seen in the omalizumab group. Indeed, more patients were symptom-free on ligelizumab at 72 mg or 240 mg than on omalizumab throughout the 20-week study.
And third, time to relapse after treatment discontinuation was markedly longer with ligelizumab.
“Once you stop the treatment, we expect patients to come back because we didn’t cure the disease, we blocked the signs and symptoms by blocking mast cell degranulation. Relapse after the last injection occurred at about 4 weeks with omalizumab versus 10 weeks for ligelizumab on average. That’s amazing,” Dr. Maurer said.
At week 20, the mean reductions from baseline in UAS7 scores were 13.6 points with placebo, 15.2 points with the lowest dose of ligelizumab, 18.2 points with omalizumab, 23.1 points with ligelizumab at 72 mg, and 22.5 points for ligelizumab at 240 mg.
The side effect profiles for both biologics were essentially the same as for placebo with the exception of a 5.9% rate of mild injection site reactions with ligelizumab at the 240-mg dose versus 2.3% with placebo.
Many clinicians have noticed a significant limitation of omalizumab: It is less effective in patients with more complex CSU having an autoimmune overlay, type 2b angioedema, and/or long disease duration.
“This does not seem to be the case with ligelizumab. Even for the difficult-to-treat subpopulations of CSU, ligelizumab appears to be a drug that can protect against mast cell degranulation. We see a reduction in angioedema activity; we see a reduction in wheal size and number; we see a reduction in the itch – so across all the symptoms in the difficult subpopulations, this is the better drug. Now we have to make it work in the phase 3 trials to bring it to clinical practice,” he said.
Dr. Maurer reported receiving research funding from and serving as an advisor to and paid speaker for Novartis, which markets omalizumab and is developing ligelizumab.
.
“For sure, ligelizumab is the highlight of this year in urticariology,” Marcus Maurer, MD, declared at the annual congress of the European Academy of Dermatology and Venereology. An ongoing phase 3 trial will now compare more than 1,000 patients with CSU who will be randomized to ligelizumab, omalizumab, or placebo.
Like omalizumab (Xolair), which is approved in the United States and Europe for treatment of CSU, ligelizumab is a humanized anti-IgE monoclonal antibody. But the investigational agent binds to IgE with greater affinity than omalizumab, and this translated into greater therapeutic efficacy in the multicenter, double-blind, placebo-controlled clinical trial, explained Dr. Maurer, professor of dermatology and allergy at Charité University in Berlin.
Study participants, all refractory to histamine1 antihistamines and in many cases to leukotriene receptor antagonists as well, were randomized to omalizumab at 300 mg, placebo, or to ligelizumab at 24 mg, 72 mg, or 240 mg administered by subcutaneous injection every 4 weeks for 20 weeks. The study showed that the effective dose of ligelizumab lies somewhere between 72 and 240 mg; the 24-mg dose won’t be pursued in further studies.
“Three things are important in the comparison between ligelizumab and omalizumab: First, ligelizumab works faster – and omalizumab is a fast-working drug in urticaria. As early as week 4 after initiation of treatment, ligelizumab resulted in a significantly higher response rate,” he said.
Second, a complete response rate as defined by an Urticaria Activity Score over the past 7 days (UAS7) of 0 was achieved by more than 50% of patients on ligelizumab at 240 mg, a rate twice that seen in the omalizumab group. Indeed, more patients were symptom-free on ligelizumab at 72 mg or 240 mg than on omalizumab throughout the 20-week study.
And third, time to relapse after treatment discontinuation was markedly longer with ligelizumab.
“Once you stop the treatment, we expect patients to come back because we didn’t cure the disease, we blocked the signs and symptoms by blocking mast cell degranulation. Relapse after the last injection occurred at about 4 weeks with omalizumab versus 10 weeks for ligelizumab on average. That’s amazing,” Dr. Maurer said.
At week 20, the mean reductions from baseline in UAS7 scores were 13.6 points with placebo, 15.2 points with the lowest dose of ligelizumab, 18.2 points with omalizumab, 23.1 points with ligelizumab at 72 mg, and 22.5 points for ligelizumab at 240 mg.
The side effect profiles for both biologics were essentially the same as for placebo with the exception of a 5.9% rate of mild injection site reactions with ligelizumab at the 240-mg dose versus 2.3% with placebo.
Many clinicians have noticed a significant limitation of omalizumab: It is less effective in patients with more complex CSU having an autoimmune overlay, type 2b angioedema, and/or long disease duration.
“This does not seem to be the case with ligelizumab. Even for the difficult-to-treat subpopulations of CSU, ligelizumab appears to be a drug that can protect against mast cell degranulation. We see a reduction in angioedema activity; we see a reduction in wheal size and number; we see a reduction in the itch – so across all the symptoms in the difficult subpopulations, this is the better drug. Now we have to make it work in the phase 3 trials to bring it to clinical practice,” he said.
Dr. Maurer reported receiving research funding from and serving as an advisor to and paid speaker for Novartis, which markets omalizumab and is developing ligelizumab.
REPORTING FROM THE EADV CONGRESS
Key clinical point: Ligelizumab shows considerable promise for chronic spontaneous urticaria.
Major finding: The complete response rate was two times higher with ligelizumab than it was with omalizumab.
Study details: This phase 2b randomized, double-blind, multicenter, active- and placebo-controlled, 20-week study included 382 patients with chronic spontaneous urticaria.
Disclosures: The presenter reported receiving research funding from and serving as an advisor to and paid speaker for Novartis, which is developing ligelizumab.
Secukinumab shows promise in hidradenitis suppurativa
PARIS – David Rosmarin, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“It was especially notable that secukinumab was effective in five of the six patients who had previously failed anti-TNF [tumor necrosis factor] therapy,” said Dr. Rosmarin, a dermatologist at Tufts University, Boston.
At present, the sole medication approved for treatment of hidradenitis suppurativa (HS) is the TNF inhibitor adalimumab (Humira). The rationale for investigating secukinumab (Cosentyx), a biologic that blocks the interleukin-17A receptor and is approved for treatment of psoriasis, psoriatic arthritis, and ankylosing spondylitis, lies in the observation that HS lesions exhibit an increased ratio of Th17- to T-regulatory cells, compared with normal skin. Lesional skin also features elevated IL-17 levels. These abnormalities can be reversed by anti-TNF therapy, he explained.
Dr. Rosmarin presented a 28-week, open-label study in which 18 patients with moderate to severe HS received an induction regimen consisting of 300 mg secukinumab given subcutaneously once weekly for 5 weeks and were then randomized to the same dose of the biologic given either every 2 or 4 weeks until week 24.
The primary endpoint was achievement of a Hidradenitis Suppurativa Clinical Response (HiSCR) at 24 weeks. This requires no increase in the number of draining fistulae or abscesses, compared with baseline, plus at least a 50% reduction in total inflammatory nodules. Secondary endpoints were the mean change from baseline in the Sartorius Scale as well as on the Dermatology Life Quality Index (DLQI).
Participants were an average of 33 years and had a disease duration of 12 years; 14 patients were Hurley Stage II, the rest Stage III. Their mean baseline DLQI was 12.
“Patients with hidradenitis suppurativa have a horrible quality of life,” Dr. Rosmarin commented. “When we compare it to patients who have atopic dermatitis, psoriasis, or chronic idiopathic urticaria, oftentimes patients with hidradenitis suppurativa suffer the worst and have the most quality of life impairment.”
Of the 18 patients, 14 – 7 of 9 in each group – achieved HiSCR. This included five of the six patients who were previous nonresponders to adalimumab or another anti-TNF biologic.
“The other thing we noticed was the rapidity of response, which is important to a lot of our patients. It took an average of 7 weeks to achieve HiSCR, and eight patients achieved HiSCR during the induction phase of treatment,” the dermatologist said.
Mean scores on the Sartorius Scale dropped by 28%. Similarly, scores on the DLQI improved by a mean of 3.6 points, or 26%. Nine patients experienced a reduction of 5 points or more on the DLQI. “This happened largely in the first 1-2 months of therapy,” Dr. Rosmarin continued.
Secukinumab was well tolerated. There were no treatment discontinuations because of adverse events. Four patients, all in the biweekly dosing arm, developed Candida infections, all easily cured using topical ketoconazole.
The next step will be to conduct a large, placebo-controlled, randomized trial to firmly establish the efficacy of secukinumab for HS. Also, the optimal dosing of the biologic for induction and long-term maintenance therapy have yet to be determined. Over the long term, it will be important to see whether marked improvement in HS is accompanied by a reduction in the elevated cardiovascular risk associated with this inflammatory disease, he added.
In 2019, a trial will get underway to compare two doses of secukinumab for patients with HS. Based on a search of clinical trials at ClinicalTrials.gov, a wide range of monoclonal antibody therapies are being investigated for the treatment of HS.
The results of this preliminary study of secukinumab emphasize the importance of the Th17 pathway in HS and open the door to alternative strategies targeting this pathway. Dr. Rosmarin noted that he and his coinvestigators have collected a case series of positive responses to guselkumab (Tremfya), which targets the IL-23 p19 subunit, which also lies along the Th17 pathway.
The secukinumab study was sponsored by Novartis. Dr. Rosmarin reported serving as a consultant to or on speakers’ bureaus for that company and more than half a dozen other pharmaceutical companies.
PARIS – David Rosmarin, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“It was especially notable that secukinumab was effective in five of the six patients who had previously failed anti-TNF [tumor necrosis factor] therapy,” said Dr. Rosmarin, a dermatologist at Tufts University, Boston.
At present, the sole medication approved for treatment of hidradenitis suppurativa (HS) is the TNF inhibitor adalimumab (Humira). The rationale for investigating secukinumab (Cosentyx), a biologic that blocks the interleukin-17A receptor and is approved for treatment of psoriasis, psoriatic arthritis, and ankylosing spondylitis, lies in the observation that HS lesions exhibit an increased ratio of Th17- to T-regulatory cells, compared with normal skin. Lesional skin also features elevated IL-17 levels. These abnormalities can be reversed by anti-TNF therapy, he explained.
Dr. Rosmarin presented a 28-week, open-label study in which 18 patients with moderate to severe HS received an induction regimen consisting of 300 mg secukinumab given subcutaneously once weekly for 5 weeks and were then randomized to the same dose of the biologic given either every 2 or 4 weeks until week 24.
The primary endpoint was achievement of a Hidradenitis Suppurativa Clinical Response (HiSCR) at 24 weeks. This requires no increase in the number of draining fistulae or abscesses, compared with baseline, plus at least a 50% reduction in total inflammatory nodules. Secondary endpoints were the mean change from baseline in the Sartorius Scale as well as on the Dermatology Life Quality Index (DLQI).
Participants were an average of 33 years and had a disease duration of 12 years; 14 patients were Hurley Stage II, the rest Stage III. Their mean baseline DLQI was 12.
“Patients with hidradenitis suppurativa have a horrible quality of life,” Dr. Rosmarin commented. “When we compare it to patients who have atopic dermatitis, psoriasis, or chronic idiopathic urticaria, oftentimes patients with hidradenitis suppurativa suffer the worst and have the most quality of life impairment.”
Of the 18 patients, 14 – 7 of 9 in each group – achieved HiSCR. This included five of the six patients who were previous nonresponders to adalimumab or another anti-TNF biologic.
“The other thing we noticed was the rapidity of response, which is important to a lot of our patients. It took an average of 7 weeks to achieve HiSCR, and eight patients achieved HiSCR during the induction phase of treatment,” the dermatologist said.
Mean scores on the Sartorius Scale dropped by 28%. Similarly, scores on the DLQI improved by a mean of 3.6 points, or 26%. Nine patients experienced a reduction of 5 points or more on the DLQI. “This happened largely in the first 1-2 months of therapy,” Dr. Rosmarin continued.
Secukinumab was well tolerated. There were no treatment discontinuations because of adverse events. Four patients, all in the biweekly dosing arm, developed Candida infections, all easily cured using topical ketoconazole.
The next step will be to conduct a large, placebo-controlled, randomized trial to firmly establish the efficacy of secukinumab for HS. Also, the optimal dosing of the biologic for induction and long-term maintenance therapy have yet to be determined. Over the long term, it will be important to see whether marked improvement in HS is accompanied by a reduction in the elevated cardiovascular risk associated with this inflammatory disease, he added.
In 2019, a trial will get underway to compare two doses of secukinumab for patients with HS. Based on a search of clinical trials at ClinicalTrials.gov, a wide range of monoclonal antibody therapies are being investigated for the treatment of HS.
The results of this preliminary study of secukinumab emphasize the importance of the Th17 pathway in HS and open the door to alternative strategies targeting this pathway. Dr. Rosmarin noted that he and his coinvestigators have collected a case series of positive responses to guselkumab (Tremfya), which targets the IL-23 p19 subunit, which also lies along the Th17 pathway.
The secukinumab study was sponsored by Novartis. Dr. Rosmarin reported serving as a consultant to or on speakers’ bureaus for that company and more than half a dozen other pharmaceutical companies.
PARIS – David Rosmarin, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“It was especially notable that secukinumab was effective in five of the six patients who had previously failed anti-TNF [tumor necrosis factor] therapy,” said Dr. Rosmarin, a dermatologist at Tufts University, Boston.
At present, the sole medication approved for treatment of hidradenitis suppurativa (HS) is the TNF inhibitor adalimumab (Humira). The rationale for investigating secukinumab (Cosentyx), a biologic that blocks the interleukin-17A receptor and is approved for treatment of psoriasis, psoriatic arthritis, and ankylosing spondylitis, lies in the observation that HS lesions exhibit an increased ratio of Th17- to T-regulatory cells, compared with normal skin. Lesional skin also features elevated IL-17 levels. These abnormalities can be reversed by anti-TNF therapy, he explained.
Dr. Rosmarin presented a 28-week, open-label study in which 18 patients with moderate to severe HS received an induction regimen consisting of 300 mg secukinumab given subcutaneously once weekly for 5 weeks and were then randomized to the same dose of the biologic given either every 2 or 4 weeks until week 24.
The primary endpoint was achievement of a Hidradenitis Suppurativa Clinical Response (HiSCR) at 24 weeks. This requires no increase in the number of draining fistulae or abscesses, compared with baseline, plus at least a 50% reduction in total inflammatory nodules. Secondary endpoints were the mean change from baseline in the Sartorius Scale as well as on the Dermatology Life Quality Index (DLQI).
Participants were an average of 33 years and had a disease duration of 12 years; 14 patients were Hurley Stage II, the rest Stage III. Their mean baseline DLQI was 12.
“Patients with hidradenitis suppurativa have a horrible quality of life,” Dr. Rosmarin commented. “When we compare it to patients who have atopic dermatitis, psoriasis, or chronic idiopathic urticaria, oftentimes patients with hidradenitis suppurativa suffer the worst and have the most quality of life impairment.”
Of the 18 patients, 14 – 7 of 9 in each group – achieved HiSCR. This included five of the six patients who were previous nonresponders to adalimumab or another anti-TNF biologic.
“The other thing we noticed was the rapidity of response, which is important to a lot of our patients. It took an average of 7 weeks to achieve HiSCR, and eight patients achieved HiSCR during the induction phase of treatment,” the dermatologist said.
Mean scores on the Sartorius Scale dropped by 28%. Similarly, scores on the DLQI improved by a mean of 3.6 points, or 26%. Nine patients experienced a reduction of 5 points or more on the DLQI. “This happened largely in the first 1-2 months of therapy,” Dr. Rosmarin continued.
Secukinumab was well tolerated. There were no treatment discontinuations because of adverse events. Four patients, all in the biweekly dosing arm, developed Candida infections, all easily cured using topical ketoconazole.
The next step will be to conduct a large, placebo-controlled, randomized trial to firmly establish the efficacy of secukinumab for HS. Also, the optimal dosing of the biologic for induction and long-term maintenance therapy have yet to be determined. Over the long term, it will be important to see whether marked improvement in HS is accompanied by a reduction in the elevated cardiovascular risk associated with this inflammatory disease, he added.
In 2019, a trial will get underway to compare two doses of secukinumab for patients with HS. Based on a search of clinical trials at ClinicalTrials.gov, a wide range of monoclonal antibody therapies are being investigated for the treatment of HS.
The results of this preliminary study of secukinumab emphasize the importance of the Th17 pathway in HS and open the door to alternative strategies targeting this pathway. Dr. Rosmarin noted that he and his coinvestigators have collected a case series of positive responses to guselkumab (Tremfya), which targets the IL-23 p19 subunit, which also lies along the Th17 pathway.
The secukinumab study was sponsored by Novartis. Dr. Rosmarin reported serving as a consultant to or on speakers’ bureaus for that company and more than half a dozen other pharmaceutical companies.
REPORTING FROM THE EADV CONGRESS
Key clinical point: Secukinumab shows considerable promise for treatment of hidradenitis suppurativa.
Major finding: Hidradenitis suppurativa improved markedly in response to secukinumab in 14 of 18 patients.
Study details: This prospective, open-label, 28-week study included 18 patients with hidradenitis suppurativa who were randomized to one of two secukinumab dosing regimens.
Disclosures: The study was sponsored by Novartis. The presenter reported serving as a consultant to or on speakers’ bureaus for that company and more than half a dozen other pharmaceutical companies.
Skin-Colored Papules on the Chest
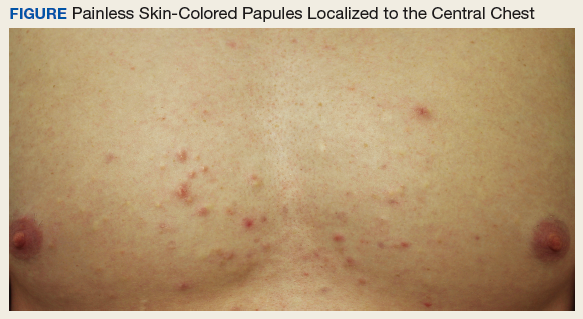
An otherwise healthy male presents with multiple smooth uniform painless cystic papules scattered across his central chest.
A 25-year-old man presented with multiple sternal cysts that he first noticed when he was aged 18 years and had persisted despite treatment with topical anti-acne agents, including tretinoin. No other medications were used. The patient was unable to express purulent material from the lesions and reported no infection or additional trauma to the affected area. He had no other significant past medical history and no family history of similar skin lesions.
A physical examination revealed an otherwise healthy-appearing male with multiple uniform painless cystic papules scattered across his central chest that were smooth and flesh-colored to slightly yellow-colored, measuring 2 mm to 6 mm in diameter (Figure). A ring of erythema surrounded the lesions that had been recently manipulated by the patient. There were no overlying central puncta, and the remainder of his body was spared.
Related: Mohs Micrographic Surgery in the VHA
- What is your diagnosis?
- How would you treat this patient?
Diagnosis
The patient was diagnosed with steatocystoma multiplex based on his poor response to topical anti-acne agents, the location of his lesions, and histopathology of a biopsy specimen. Steatocystoma multiplex, sometimes termed sebocystomatosis, typically presents between puberty and the third decade of life. Lesions are usually < 2 cm in diameter and occur as multiple smooth skin-colored or yellow-colored painless papules on areas with high concentrations of hormonally sensitive sebaceous glands, especially the chest. Lesions also can be found in the axillae and on the neck.1-3 Solitary lesions can occur and are termed steatocystoma simplex.
The timing and location of presentation can easily be mistaken for acne vulgaris, but steatocystoma lesions are true sebaceous cysts, which are rare, and spontaneous resolution with increasing age does not typically occur. The diagnosis of steatocystoma often goes unreported because the disease is usually asymptomatic and mimics more common benign skin conditions, so an accurate prevalence and incidence are both unknown.
First on the differential diagnosis is acne vulgaris, which also presents at puberty and affects nearly 85% of adolescents. However, acne is less common in people of Asian or African descent and may progress along a continuum of increasingly severe and larger lesions, including the primary comedones and papules followed by pustules, nodules, and pseudocysts. Painful lesions develop from inflammation of pilosebaceous units concentrated on the face, neck, trunk, upper arms, or buttocks and are typically worse in males. Resolution often occurs spontaneously by the third decade of life, but scarring can persist.4
Related: Using Dermoscopy to Identify Melanoma and Improve Diagnostic Discrimination
Eruptive vellus hair cysts present as dozens of skin-colored small (1-4 mm) painless dome-shaped papules, sometimes with erythema and crusting. Typically these appear on the head, trunk, or flexor surfaces of infants (familial cases) or adolescents (sporadic cases) without bias for gender or ethnicity. Although benign and potential mimickers of steatocystoma and acne, these lesions can also be associated with more serious syndromes, like ectodermal dysplasias and pachyonychia congenita.2,3
Epidermoid cysts are common benign solitary skin-colored subcutaneous dome-shaped nodules that contain a central punctum through which cheeselike keratinaceous material can be expressed.4 These benign lesions arising from the dermis can enlarge to several centimeters, and adults of both genders and most ethnicities tend to develop the lesions on the trunk or face, with small cysts on the face termed milia. Ruptured cysts can incite intense inflammation, and multiple epidermoid cysts should raise concern for Gardner syndrome.2,3
About This Condition
Steatocystoma lesions are benign and thought to arise from a mutation in keratin 17. The mutation can be inherited in an autosomal dominant pattern, but sporadic nonheritable cases are more common.5 There are no distinct associations with gender or ethnicity. The dermal cysts arise from the sebaceous ducts of the pilosebaceous unit, and histopathology typically shows numerous mature sebaceous cells encased by a thin wall of stratified squamous epithelium.2 Immunohistochemical staining for the defective keratin can help diagnose biopsy specimens, and histopathology confirmed the diagnosis in this case.
Related: Recurring Bilateral Rash Concomitant With Upper Respiratory Tract Infection in a Healthy Adult Male
Treatment
Steatocystoma is usually asymptomatic, so patients mainly present to physicians for cosmetic reasons. Puncturing the cyst wall within the dermis produces translucent sebum-containing fluid, and ruptured cysts can incite inflammation, pain, and scarring.2 However, prognosis is good, and treatment consists of excision, aspiration and curettage of the cyst wall, oral isotretinoin, or laser therapy. Our patient elected to forego treatment and will consider definitive removal in the future, since the lesions will persist and potentially enlarge. Accurate diagnosis of this rare cause of chest papules improves the timeliness and efficacy of appropriate treatment, favoring good cosmesis.
1. Zuber TJ. Minimal excision technique for epidermoid (sebaceous) cysts. Am Fam Physician. 2002;65(7):1409-1412.
2. du Vivier A. Atlas of Clinical Dermatology. 4th ed. Philadelphia, PA: Elsevier Saunders; 2012.
3. Brinster N, Liu V, Diwan AH, McKee PH. High Yield Pathology: Dermatopathology. 1st ed. Philadelphia, PA: Elsevier Saunders; 2011.
4. Wolff K, Johnson RA, Suurmond D. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 5th ed. New York: McGraw-Hill; 2005.
5. Gordon Spratt EA, Kaplan J, Patel RR, Kamino H, Ramachandran SM. Steatocystoma. Dermatol Online J. 2013;19(12):20721.
An otherwise healthy male presents with multiple smooth uniform painless cystic papules scattered across his central chest.
An otherwise healthy male presents with multiple smooth uniform painless cystic papules scattered across his central chest.
A 25-year-old man presented with multiple sternal cysts that he first noticed when he was aged 18 years and had persisted despite treatment with topical anti-acne agents, including tretinoin. No other medications were used. The patient was unable to express purulent material from the lesions and reported no infection or additional trauma to the affected area. He had no other significant past medical history and no family history of similar skin lesions.
A physical examination revealed an otherwise healthy-appearing male with multiple uniform painless cystic papules scattered across his central chest that were smooth and flesh-colored to slightly yellow-colored, measuring 2 mm to 6 mm in diameter (Figure). A ring of erythema surrounded the lesions that had been recently manipulated by the patient. There were no overlying central puncta, and the remainder of his body was spared.
Related: Mohs Micrographic Surgery in the VHA
- What is your diagnosis?
- How would you treat this patient?

Diagnosis
The patient was diagnosed with steatocystoma multiplex based on his poor response to topical anti-acne agents, the location of his lesions, and histopathology of a biopsy specimen. Steatocystoma multiplex, sometimes termed sebocystomatosis, typically presents between puberty and the third decade of life. Lesions are usually < 2 cm in diameter and occur as multiple smooth skin-colored or yellow-colored painless papules on areas with high concentrations of hormonally sensitive sebaceous glands, especially the chest. Lesions also can be found in the axillae and on the neck.1-3 Solitary lesions can occur and are termed steatocystoma simplex.
The timing and location of presentation can easily be mistaken for acne vulgaris, but steatocystoma lesions are true sebaceous cysts, which are rare, and spontaneous resolution with increasing age does not typically occur. The diagnosis of steatocystoma often goes unreported because the disease is usually asymptomatic and mimics more common benign skin conditions, so an accurate prevalence and incidence are both unknown.
First on the differential diagnosis is acne vulgaris, which also presents at puberty and affects nearly 85% of adolescents. However, acne is less common in people of Asian or African descent and may progress along a continuum of increasingly severe and larger lesions, including the primary comedones and papules followed by pustules, nodules, and pseudocysts. Painful lesions develop from inflammation of pilosebaceous units concentrated on the face, neck, trunk, upper arms, or buttocks and are typically worse in males. Resolution often occurs spontaneously by the third decade of life, but scarring can persist.4
Related: Using Dermoscopy to Identify Melanoma and Improve Diagnostic Discrimination
Eruptive vellus hair cysts present as dozens of skin-colored small (1-4 mm) painless dome-shaped papules, sometimes with erythema and crusting. Typically these appear on the head, trunk, or flexor surfaces of infants (familial cases) or adolescents (sporadic cases) without bias for gender or ethnicity. Although benign and potential mimickers of steatocystoma and acne, these lesions can also be associated with more serious syndromes, like ectodermal dysplasias and pachyonychia congenita.2,3
Epidermoid cysts are common benign solitary skin-colored subcutaneous dome-shaped nodules that contain a central punctum through which cheeselike keratinaceous material can be expressed.4 These benign lesions arising from the dermis can enlarge to several centimeters, and adults of both genders and most ethnicities tend to develop the lesions on the trunk or face, with small cysts on the face termed milia. Ruptured cysts can incite intense inflammation, and multiple epidermoid cysts should raise concern for Gardner syndrome.2,3
About This Condition
Steatocystoma lesions are benign and thought to arise from a mutation in keratin 17. The mutation can be inherited in an autosomal dominant pattern, but sporadic nonheritable cases are more common.5 There are no distinct associations with gender or ethnicity. The dermal cysts arise from the sebaceous ducts of the pilosebaceous unit, and histopathology typically shows numerous mature sebaceous cells encased by a thin wall of stratified squamous epithelium.2 Immunohistochemical staining for the defective keratin can help diagnose biopsy specimens, and histopathology confirmed the diagnosis in this case.
Related: Recurring Bilateral Rash Concomitant With Upper Respiratory Tract Infection in a Healthy Adult Male
Treatment
Steatocystoma is usually asymptomatic, so patients mainly present to physicians for cosmetic reasons. Puncturing the cyst wall within the dermis produces translucent sebum-containing fluid, and ruptured cysts can incite inflammation, pain, and scarring.2 However, prognosis is good, and treatment consists of excision, aspiration and curettage of the cyst wall, oral isotretinoin, or laser therapy. Our patient elected to forego treatment and will consider definitive removal in the future, since the lesions will persist and potentially enlarge. Accurate diagnosis of this rare cause of chest papules improves the timeliness and efficacy of appropriate treatment, favoring good cosmesis.
A 25-year-old man presented with multiple sternal cysts that he first noticed when he was aged 18 years and had persisted despite treatment with topical anti-acne agents, including tretinoin. No other medications were used. The patient was unable to express purulent material from the lesions and reported no infection or additional trauma to the affected area. He had no other significant past medical history and no family history of similar skin lesions.
A physical examination revealed an otherwise healthy-appearing male with multiple uniform painless cystic papules scattered across his central chest that were smooth and flesh-colored to slightly yellow-colored, measuring 2 mm to 6 mm in diameter (Figure). A ring of erythema surrounded the lesions that had been recently manipulated by the patient. There were no overlying central puncta, and the remainder of his body was spared.
Related: Mohs Micrographic Surgery in the VHA
- What is your diagnosis?
- How would you treat this patient?

Diagnosis
The patient was diagnosed with steatocystoma multiplex based on his poor response to topical anti-acne agents, the location of his lesions, and histopathology of a biopsy specimen. Steatocystoma multiplex, sometimes termed sebocystomatosis, typically presents between puberty and the third decade of life. Lesions are usually < 2 cm in diameter and occur as multiple smooth skin-colored or yellow-colored painless papules on areas with high concentrations of hormonally sensitive sebaceous glands, especially the chest. Lesions also can be found in the axillae and on the neck.1-3 Solitary lesions can occur and are termed steatocystoma simplex.
The timing and location of presentation can easily be mistaken for acne vulgaris, but steatocystoma lesions are true sebaceous cysts, which are rare, and spontaneous resolution with increasing age does not typically occur. The diagnosis of steatocystoma often goes unreported because the disease is usually asymptomatic and mimics more common benign skin conditions, so an accurate prevalence and incidence are both unknown.
First on the differential diagnosis is acne vulgaris, which also presents at puberty and affects nearly 85% of adolescents. However, acne is less common in people of Asian or African descent and may progress along a continuum of increasingly severe and larger lesions, including the primary comedones and papules followed by pustules, nodules, and pseudocysts. Painful lesions develop from inflammation of pilosebaceous units concentrated on the face, neck, trunk, upper arms, or buttocks and are typically worse in males. Resolution often occurs spontaneously by the third decade of life, but scarring can persist.4
Related: Using Dermoscopy to Identify Melanoma and Improve Diagnostic Discrimination
Eruptive vellus hair cysts present as dozens of skin-colored small (1-4 mm) painless dome-shaped papules, sometimes with erythema and crusting. Typically these appear on the head, trunk, or flexor surfaces of infants (familial cases) or adolescents (sporadic cases) without bias for gender or ethnicity. Although benign and potential mimickers of steatocystoma and acne, these lesions can also be associated with more serious syndromes, like ectodermal dysplasias and pachyonychia congenita.2,3
Epidermoid cysts are common benign solitary skin-colored subcutaneous dome-shaped nodules that contain a central punctum through which cheeselike keratinaceous material can be expressed.4 These benign lesions arising from the dermis can enlarge to several centimeters, and adults of both genders and most ethnicities tend to develop the lesions on the trunk or face, with small cysts on the face termed milia. Ruptured cysts can incite intense inflammation, and multiple epidermoid cysts should raise concern for Gardner syndrome.2,3
About This Condition
Steatocystoma lesions are benign and thought to arise from a mutation in keratin 17. The mutation can be inherited in an autosomal dominant pattern, but sporadic nonheritable cases are more common.5 There are no distinct associations with gender or ethnicity. The dermal cysts arise from the sebaceous ducts of the pilosebaceous unit, and histopathology typically shows numerous mature sebaceous cells encased by a thin wall of stratified squamous epithelium.2 Immunohistochemical staining for the defective keratin can help diagnose biopsy specimens, and histopathology confirmed the diagnosis in this case.
Related: Recurring Bilateral Rash Concomitant With Upper Respiratory Tract Infection in a Healthy Adult Male
Treatment
Steatocystoma is usually asymptomatic, so patients mainly present to physicians for cosmetic reasons. Puncturing the cyst wall within the dermis produces translucent sebum-containing fluid, and ruptured cysts can incite inflammation, pain, and scarring.2 However, prognosis is good, and treatment consists of excision, aspiration and curettage of the cyst wall, oral isotretinoin, or laser therapy. Our patient elected to forego treatment and will consider definitive removal in the future, since the lesions will persist and potentially enlarge. Accurate diagnosis of this rare cause of chest papules improves the timeliness and efficacy of appropriate treatment, favoring good cosmesis.
1. Zuber TJ. Minimal excision technique for epidermoid (sebaceous) cysts. Am Fam Physician. 2002;65(7):1409-1412.
2. du Vivier A. Atlas of Clinical Dermatology. 4th ed. Philadelphia, PA: Elsevier Saunders; 2012.
3. Brinster N, Liu V, Diwan AH, McKee PH. High Yield Pathology: Dermatopathology. 1st ed. Philadelphia, PA: Elsevier Saunders; 2011.
4. Wolff K, Johnson RA, Suurmond D. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 5th ed. New York: McGraw-Hill; 2005.
5. Gordon Spratt EA, Kaplan J, Patel RR, Kamino H, Ramachandran SM. Steatocystoma. Dermatol Online J. 2013;19(12):20721.
1. Zuber TJ. Minimal excision technique for epidermoid (sebaceous) cysts. Am Fam Physician. 2002;65(7):1409-1412.
2. du Vivier A. Atlas of Clinical Dermatology. 4th ed. Philadelphia, PA: Elsevier Saunders; 2012.
3. Brinster N, Liu V, Diwan AH, McKee PH. High Yield Pathology: Dermatopathology. 1st ed. Philadelphia, PA: Elsevier Saunders; 2011.
4. Wolff K, Johnson RA, Suurmond D. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 5th ed. New York: McGraw-Hill; 2005.
5. Gordon Spratt EA, Kaplan J, Patel RR, Kamino H, Ramachandran SM. Steatocystoma. Dermatol Online J. 2013;19(12):20721.
Think barrier repair for acne patients
LAS VEGAS – It’s time to start thinking about barrier repair in acne patients, Hilary E. Baldwin, MD, asserts.
“We think about barrier repair with our psoriasis patients, with our dermatitis patients, and our rosacea patients, but we don’t talk about it much with our acne patients,” Dr. Baldwin said at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar. “Now there is increasing evidence that acne pathophysiology may include a barrier defect.”
Both topical and systemic medications exacerbate the problem, because they are so drying, added Dr. Baldwin, medical director of the Acne Treatment & Research Center in Morristown, N.J. Benzoyl peroxide has been shown to increase transepidermal water loss (TEWL) and deplete tocopherol levels in the stratum corneum, while topical retinoids thin the stratum corneum, increase epidermal fragility, and increase TEWL.
The barrier defect may actually decrease medication use – patients are irritated and they stop taking their medication. “,” she said.
She described the results of an Internet-based survey of 200 acne patients aged 15-40 years who had been prescribed clindamycin/benzoyl peroxide 5% within the last 6 months (J Drugs Dermatol. 2011 Jun;10[6]:605-8). The researchers found that side effects lead to suboptimal use of the topical medication, as patients reported spot application, use only in flares, infrequent use, and discontinuation.
Further, 31% of patients complained to their doctors’ offices about dryness, 23% said their doctors did not understand the potential side effects, 21% lost confidence in their doctors, 11% said they were less likely to see their doctors again, and 41% reported using moisturizer to combat dryness and erythema.
“No acne visit is complete without a discussion of skin care,” Dr. Baldwin said. Quality moisturization is a must for acne patients and has been shown to improve TEWL, normalize ceramides, and repair the microbiome.
“I recommend moisturizers with ceramides, hyaluronic acid, and certainly moisturizers that have been shown to be noncomedogenic,” Dr. Baldwin said. “Most acne patients aren’t willing to use a cream moisturizer during the day – they just feel too greasy – so I have them use a cream in the evening when they are home and a lotion in the morning.”
While there has been one very specific study of using a moisturizer before topical medications, there is no good data for this practice, Dr. Baldwin said. “Clearly, though, using a moisturizer reduces irritation, so I prefer to have my patients put on the moisturizer at a different time, but if that doesn’t work, I have them put it on before the medication.”
Dr. Baldwin indicated that she is on the speakers bureau for LaRoche Posay, Galderma, and Valeant, and had received grant and/or contracted research support from Dermira and Valeant.
SDEF and this news organization are owned by the same parent company.
LAS VEGAS – It’s time to start thinking about barrier repair in acne patients, Hilary E. Baldwin, MD, asserts.
“We think about barrier repair with our psoriasis patients, with our dermatitis patients, and our rosacea patients, but we don’t talk about it much with our acne patients,” Dr. Baldwin said at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar. “Now there is increasing evidence that acne pathophysiology may include a barrier defect.”
Both topical and systemic medications exacerbate the problem, because they are so drying, added Dr. Baldwin, medical director of the Acne Treatment & Research Center in Morristown, N.J. Benzoyl peroxide has been shown to increase transepidermal water loss (TEWL) and deplete tocopherol levels in the stratum corneum, while topical retinoids thin the stratum corneum, increase epidermal fragility, and increase TEWL.
The barrier defect may actually decrease medication use – patients are irritated and they stop taking their medication. “,” she said.
She described the results of an Internet-based survey of 200 acne patients aged 15-40 years who had been prescribed clindamycin/benzoyl peroxide 5% within the last 6 months (J Drugs Dermatol. 2011 Jun;10[6]:605-8). The researchers found that side effects lead to suboptimal use of the topical medication, as patients reported spot application, use only in flares, infrequent use, and discontinuation.
Further, 31% of patients complained to their doctors’ offices about dryness, 23% said their doctors did not understand the potential side effects, 21% lost confidence in their doctors, 11% said they were less likely to see their doctors again, and 41% reported using moisturizer to combat dryness and erythema.
“No acne visit is complete without a discussion of skin care,” Dr. Baldwin said. Quality moisturization is a must for acne patients and has been shown to improve TEWL, normalize ceramides, and repair the microbiome.
“I recommend moisturizers with ceramides, hyaluronic acid, and certainly moisturizers that have been shown to be noncomedogenic,” Dr. Baldwin said. “Most acne patients aren’t willing to use a cream moisturizer during the day – they just feel too greasy – so I have them use a cream in the evening when they are home and a lotion in the morning.”
While there has been one very specific study of using a moisturizer before topical medications, there is no good data for this practice, Dr. Baldwin said. “Clearly, though, using a moisturizer reduces irritation, so I prefer to have my patients put on the moisturizer at a different time, but if that doesn’t work, I have them put it on before the medication.”
Dr. Baldwin indicated that she is on the speakers bureau for LaRoche Posay, Galderma, and Valeant, and had received grant and/or contracted research support from Dermira and Valeant.
SDEF and this news organization are owned by the same parent company.
LAS VEGAS – It’s time to start thinking about barrier repair in acne patients, Hilary E. Baldwin, MD, asserts.
“We think about barrier repair with our psoriasis patients, with our dermatitis patients, and our rosacea patients, but we don’t talk about it much with our acne patients,” Dr. Baldwin said at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar. “Now there is increasing evidence that acne pathophysiology may include a barrier defect.”
Both topical and systemic medications exacerbate the problem, because they are so drying, added Dr. Baldwin, medical director of the Acne Treatment & Research Center in Morristown, N.J. Benzoyl peroxide has been shown to increase transepidermal water loss (TEWL) and deplete tocopherol levels in the stratum corneum, while topical retinoids thin the stratum corneum, increase epidermal fragility, and increase TEWL.
The barrier defect may actually decrease medication use – patients are irritated and they stop taking their medication. “,” she said.
She described the results of an Internet-based survey of 200 acne patients aged 15-40 years who had been prescribed clindamycin/benzoyl peroxide 5% within the last 6 months (J Drugs Dermatol. 2011 Jun;10[6]:605-8). The researchers found that side effects lead to suboptimal use of the topical medication, as patients reported spot application, use only in flares, infrequent use, and discontinuation.
Further, 31% of patients complained to their doctors’ offices about dryness, 23% said their doctors did not understand the potential side effects, 21% lost confidence in their doctors, 11% said they were less likely to see their doctors again, and 41% reported using moisturizer to combat dryness and erythema.
“No acne visit is complete without a discussion of skin care,” Dr. Baldwin said. Quality moisturization is a must for acne patients and has been shown to improve TEWL, normalize ceramides, and repair the microbiome.
“I recommend moisturizers with ceramides, hyaluronic acid, and certainly moisturizers that have been shown to be noncomedogenic,” Dr. Baldwin said. “Most acne patients aren’t willing to use a cream moisturizer during the day – they just feel too greasy – so I have them use a cream in the evening when they are home and a lotion in the morning.”
While there has been one very specific study of using a moisturizer before topical medications, there is no good data for this practice, Dr. Baldwin said. “Clearly, though, using a moisturizer reduces irritation, so I prefer to have my patients put on the moisturizer at a different time, but if that doesn’t work, I have them put it on before the medication.”
Dr. Baldwin indicated that she is on the speakers bureau for LaRoche Posay, Galderma, and Valeant, and had received grant and/or contracted research support from Dermira and Valeant.
SDEF and this news organization are owned by the same parent company.
REPORTING FROM SDEF LAS VEGAS DERMATOLOGY SEMINAR