User login
Review of pediatric data indicates link between vitamin D levels and atopic dermatitis severity
in the majority of studies, but evidence on whether supplementation can improve symptoms of the condition was inconsistent.
The data on the effect of vitamin D supplementation on AD severity “suggested potential benefit but were conflicting,” concluded Christina M. Huang, MD, of Queen’s University, Kingston, Ontario, and her coinvestigators from the department of dermatology, Hospital for Sick Children, Toronto. They reported the results of their systematic review of 21 studies published between 2008 and 2017, which included quantitative data on serum vitamin D levels or vitamin D supplementation and AD severity in patients aged 18 years or younger, in Pediatrics.
In the review, 16 studies explored the relationship between serum vitamin D status and disease severity (one was a randomized controlled trial; the rest were cohort, cross-sectional, or case control studies) in 1,847 children (average age, 5.6 years). Disease severity was measured with the SCORing Atopic Dermatitis (SCORAD) system. In 10 of the 16 studies, there was a significant inverse association between vitamin D levels and AD severity.
The studies that supported this association generally had larger sample sizes, which, the authors pointed out, suggested they were of higher quality and more reliable. However, the randomized controlled study of 89 children did not find a correlation, although in the study, vitamin D level and AD severity was a secondary outcome.
The randomized controlled trial of vitamin D supplementation used lower SCORAD cut-offs for the different severities of AD, which complicated interpretation the results, “as it may indicate that the severities reported in these articles were exaggerated as compared to other studies,” they wrote.
Six studies – four randomized controlled trials (including the study that was among the 16 studies on vitamin D and severity) and two cohort studies – with 354 participants (average age, 6.8 years) looked at the effects of oral vitamin D supplementation on the severity of AD, although dosage and duration of use varied across the studies. In four of the six studies, there were significant improvement in AD in patients given supplements, but the data were “conflicting,” partly because the largest study showing benefit used a different measure of disease severity, the Eczema Area and Severity Index (EASI), not SCORAD. “The inconsistency of tools used to measure outcomes makes it difficult to compare and understand results,” so the effects of vitamin D supplementation “are controversial and should be interpreted with caution, as certain patient populations may benefit more than others,” the authors wrote.
They also drew attention to previous research suggesting that vitamin D supplementation in the first year of life might actually increase the risk of AD in children. “Therefore, although there is a growing body of evidence supporting the beneficial effects of VD [vitamin D] supplementation, the age at which supplementation is given should be considered carefully.” The authors added that the inconclusive findings “may have been due to confounding factors that were not accounted for, such as age, season, latitude, dose, and duration. It is also possible that the lack of a true effect of VD may be contributing to the inconsistent results. Future large‐scale RCTs with consideration of these factors are needed.”
Funding and conflict of interest disclosures were not included in the study.
SOURCE: Huang C et al. Pediatr Dermatol. 2018;35: 754-60. doi: 10.1111/pde.13639.
in the majority of studies, but evidence on whether supplementation can improve symptoms of the condition was inconsistent.
The data on the effect of vitamin D supplementation on AD severity “suggested potential benefit but were conflicting,” concluded Christina M. Huang, MD, of Queen’s University, Kingston, Ontario, and her coinvestigators from the department of dermatology, Hospital for Sick Children, Toronto. They reported the results of their systematic review of 21 studies published between 2008 and 2017, which included quantitative data on serum vitamin D levels or vitamin D supplementation and AD severity in patients aged 18 years or younger, in Pediatrics.
In the review, 16 studies explored the relationship between serum vitamin D status and disease severity (one was a randomized controlled trial; the rest were cohort, cross-sectional, or case control studies) in 1,847 children (average age, 5.6 years). Disease severity was measured with the SCORing Atopic Dermatitis (SCORAD) system. In 10 of the 16 studies, there was a significant inverse association between vitamin D levels and AD severity.
The studies that supported this association generally had larger sample sizes, which, the authors pointed out, suggested they were of higher quality and more reliable. However, the randomized controlled study of 89 children did not find a correlation, although in the study, vitamin D level and AD severity was a secondary outcome.
The randomized controlled trial of vitamin D supplementation used lower SCORAD cut-offs for the different severities of AD, which complicated interpretation the results, “as it may indicate that the severities reported in these articles were exaggerated as compared to other studies,” they wrote.
Six studies – four randomized controlled trials (including the study that was among the 16 studies on vitamin D and severity) and two cohort studies – with 354 participants (average age, 6.8 years) looked at the effects of oral vitamin D supplementation on the severity of AD, although dosage and duration of use varied across the studies. In four of the six studies, there were significant improvement in AD in patients given supplements, but the data were “conflicting,” partly because the largest study showing benefit used a different measure of disease severity, the Eczema Area and Severity Index (EASI), not SCORAD. “The inconsistency of tools used to measure outcomes makes it difficult to compare and understand results,” so the effects of vitamin D supplementation “are controversial and should be interpreted with caution, as certain patient populations may benefit more than others,” the authors wrote.
They also drew attention to previous research suggesting that vitamin D supplementation in the first year of life might actually increase the risk of AD in children. “Therefore, although there is a growing body of evidence supporting the beneficial effects of VD [vitamin D] supplementation, the age at which supplementation is given should be considered carefully.” The authors added that the inconclusive findings “may have been due to confounding factors that were not accounted for, such as age, season, latitude, dose, and duration. It is also possible that the lack of a true effect of VD may be contributing to the inconsistent results. Future large‐scale RCTs with consideration of these factors are needed.”
Funding and conflict of interest disclosures were not included in the study.
SOURCE: Huang C et al. Pediatr Dermatol. 2018;35: 754-60. doi: 10.1111/pde.13639.
in the majority of studies, but evidence on whether supplementation can improve symptoms of the condition was inconsistent.
The data on the effect of vitamin D supplementation on AD severity “suggested potential benefit but were conflicting,” concluded Christina M. Huang, MD, of Queen’s University, Kingston, Ontario, and her coinvestigators from the department of dermatology, Hospital for Sick Children, Toronto. They reported the results of their systematic review of 21 studies published between 2008 and 2017, which included quantitative data on serum vitamin D levels or vitamin D supplementation and AD severity in patients aged 18 years or younger, in Pediatrics.
In the review, 16 studies explored the relationship between serum vitamin D status and disease severity (one was a randomized controlled trial; the rest were cohort, cross-sectional, or case control studies) in 1,847 children (average age, 5.6 years). Disease severity was measured with the SCORing Atopic Dermatitis (SCORAD) system. In 10 of the 16 studies, there was a significant inverse association between vitamin D levels and AD severity.
The studies that supported this association generally had larger sample sizes, which, the authors pointed out, suggested they were of higher quality and more reliable. However, the randomized controlled study of 89 children did not find a correlation, although in the study, vitamin D level and AD severity was a secondary outcome.
The randomized controlled trial of vitamin D supplementation used lower SCORAD cut-offs for the different severities of AD, which complicated interpretation the results, “as it may indicate that the severities reported in these articles were exaggerated as compared to other studies,” they wrote.
Six studies – four randomized controlled trials (including the study that was among the 16 studies on vitamin D and severity) and two cohort studies – with 354 participants (average age, 6.8 years) looked at the effects of oral vitamin D supplementation on the severity of AD, although dosage and duration of use varied across the studies. In four of the six studies, there were significant improvement in AD in patients given supplements, but the data were “conflicting,” partly because the largest study showing benefit used a different measure of disease severity, the Eczema Area and Severity Index (EASI), not SCORAD. “The inconsistency of tools used to measure outcomes makes it difficult to compare and understand results,” so the effects of vitamin D supplementation “are controversial and should be interpreted with caution, as certain patient populations may benefit more than others,” the authors wrote.
They also drew attention to previous research suggesting that vitamin D supplementation in the first year of life might actually increase the risk of AD in children. “Therefore, although there is a growing body of evidence supporting the beneficial effects of VD [vitamin D] supplementation, the age at which supplementation is given should be considered carefully.” The authors added that the inconclusive findings “may have been due to confounding factors that were not accounted for, such as age, season, latitude, dose, and duration. It is also possible that the lack of a true effect of VD may be contributing to the inconsistent results. Future large‐scale RCTs with consideration of these factors are needed.”
Funding and conflict of interest disclosures were not included in the study.
SOURCE: Huang C et al. Pediatr Dermatol. 2018;35: 754-60. doi: 10.1111/pde.13639.
FROM PEDIATRIC DERMATOLOGY
Key clinical point: There is evidence that vitamin D levels in children affect atopic dermatitis severity, but further study is needed.
Major finding: Serum vitamin D levels were significantly inversely correlated with AD severity in children in 10 of 16 studies.
Study details: A systematic review of 21 pediatric studies looking at the association of vitamin D levels or supplementation on AD severity.
Disclosures: No funding or conflicts of declarations interest were available.
Source: Huang C et al. Pediatr Dermatol. 2018;35: 754-60. doi: 10.1111/pde.13639.
Immunotherapy may hold the key to defeating virally associated cancers
Infection with certain viruses has been causally linked to the development of cancer. In recent years, an improved understanding of the unique pathology and molecular underpinnings of these virally associated cancers has prompted the development of more personalized treatment strategies, with a particular focus on immunotherapy. Here, we describe some of the latest developments.
The link between viruses and cancer
Suspicions about a possible role of viral infections in the development of cancer were first aroused in the early 1900s. The seminal discovery is traced back to Peyton Rous, who showed that a malignant tumor growing in a chicken could be transferred to a healthy bird by injecting it with tumor extracts that contained no actual tumor cells.1
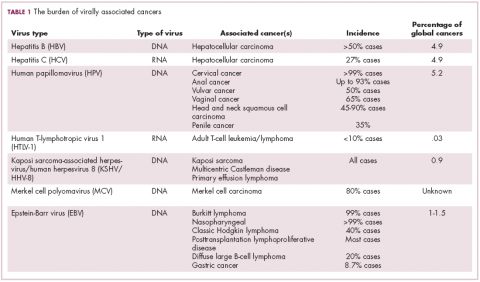
The infectious etiology of human cancer, however, remained controversial until many years later when the first cancer-causing virus, Epstein-Barr virus (EBV), was identified in cell cultures from patients with Burkitt lymphoma. Shortly afterward, the Rous sarcoma virus was unveiled as the oncogenic agent behind Rous’ observations.2Seven viruses have now been linked to the development of cancers and are thought to be responsible for around 12% of all cancer cases worldwide. The burden is likely to increase as technological advancements make it easier to establish a causal link between viruses and cancer development.3
In addition to making these links, researchers have also made significant headway in understanding how viruses cause cancer. Cancerous transformation of host cells occurs in only a minority of those who are infected with oncogenic viruses and often occurs in the setting of chronic infection.
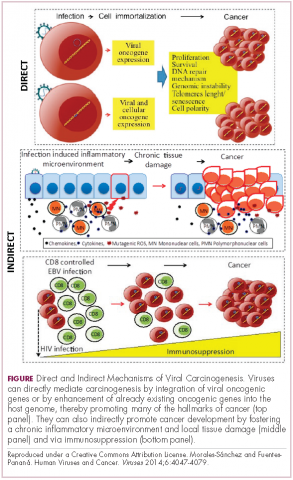
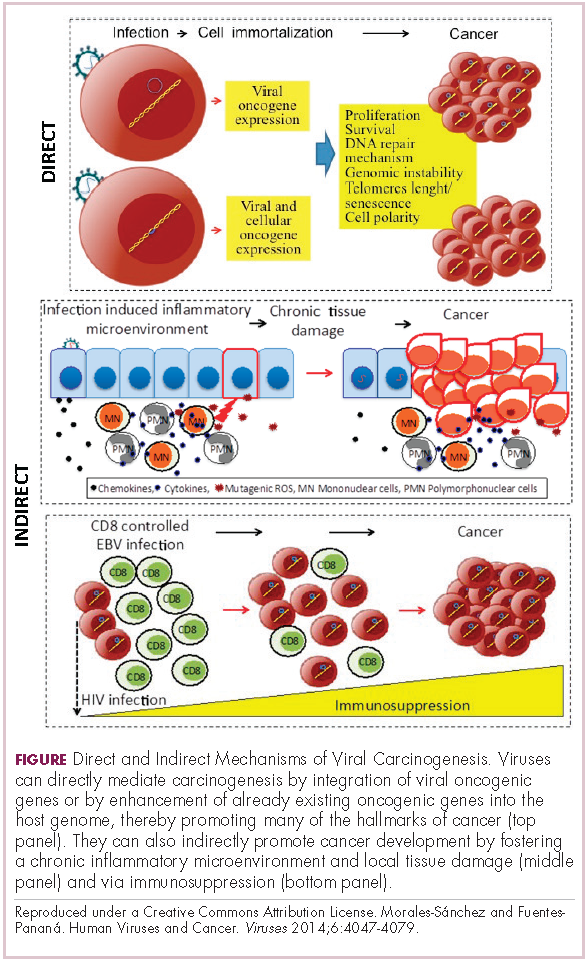
Viruses can mediate carcinogenesis by direct and/or indirect mechanisms (Figure 1). Many of the hallmarks of cancer, the key attributes that drive the transformation from a normal cell to a malignant one, are compatible with the virus’s needs, such as needing to avoid cell death, increasing cell proliferation, and avoiding detection by the immune system.
Viruses hijack the cellular machinery to meet those needs and they can do this either by producing viral proteins that have an oncogenic effect or by integrating their genetic material into the host cell genome. When the latter occurs, the process of integration can also cause damage to the DNA, which further increases the risk of cancer-promoting changes occurring in the host genome.
Viruses can indirectly contribute to carcinogenesis by fostering a microenvironment of chronic inflammation, causing oxidative stress and local tissue damage, and by suppressing the antitumor immune response.4,5
Screening and prevention efforts have helped to reduce the burden of several different virally associated cancers. However, for the substantial proportion of patients who are still affected by these cancers, there is a pressing need for new therapeutic options, particularly since genome sequencing studies have revealed that these cancers can often have distinct underlying molecular mechanisms.
Vaccines lead the charge in HPV-driven cancers
German virologist Harald zur Hausen received the Nobel Prize in 2008 for his discovery of the oncogenic role of human papillomaviruses (HPVs), a large family of more than 100 DNA viruses that infect the epithelial cells of the skin and mucous membranes. They are responsible for the largest number of virally associated cancer cases globally – around 5% (Table 1).
A number of different cancer types are linked to HPV infection, but it is best known as the cause of cervical cancer. The development of diagnostic blood tests and prophylactic vaccines for prevention and early intervention in HPV infection has helped to reduce the incidence of cervical cancer. Conversely, another type of HPV-associated cancer, head and neck squamous cell carcinoma (HNSCC), has seen increased incidence in recent years.
HPVs are categorized according to their oncogenic potential as high, intermediate, or low risk. The high-risk HPV16 and HPV18 strains are most commonly associated with cancer. They are thought to cause cancer predominantly through integration into the host genome. The HPV genome is composed of 8 genes encoding proteins that regulate viral replication and assembly. The E6 and E7 genes are the most highly oncogenic; as the HPV DNA is inserted into the host genome, the transcriptional regulator of E6/E7 is lost, leading to their increased expression. These genes have significant oncogenic potential because of their interaction with 2 tumor suppressor proteins, p53 and pRb.6,7
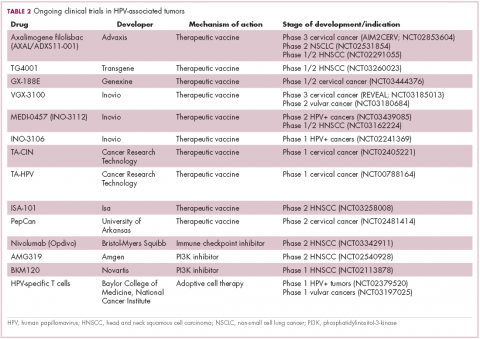
The largest investment in therapeutic development for HPV-positive cancers has been in the realm of immunotherapy in an effort to boost the anti-tumor immune response. In particular, there has been a focus on the development of therapeutic vaccines, designed to prime the anti-tumor immune response to recognize viral antigens. A variety of different types of vaccines are being developed, including live, attenuated and inactivated vaccines that are protein, DNA, or peptide based. Most developed to date target the E6/E7 proteins from the HPV16/18 strains (Table 2).8,9
Other immunotherapies are also being evaluated, including immune checkpoint inhibitors, antibodies designed to target one of the principal mechanisms of immune evasion exploited by cancer cells. The combination of immune checkpoint inhibitors with vaccines is a particularly promising strategy in HPV-associated cancers. At the European Society for Medical Oncology Congress in 2017, the results of a phase 2 trial of nivolumab in combination with ISA-101 were presented.
Among 24 patients with HPV-positive tumors, the majority oropharyngeal cancers, the combination elicited an overall response rate (ORR) of 33%, including 2 complete responses (CRs). Most adverse events (AEs) were mild to moderate in severity and included fever, injection site reactions, fatigue and nausea.14
Hepatocellular carcinoma: a tale of two viruses
The hepatitis viruses are a group of 5 unrelated viruses that causes inflammation of the liver. Hepatitis B (HBV), a DNA virus, and hepatitis C (HCV), an RNA virus, are also oncoviruses; HBV in particular is one of the main causes of hepatocellular carcinoma (HCC), the most common type of liver cancer.
The highly inflammatory environment fostered by HBV and HCV infection causes liver damage that often leads to cirrhosis. Continued infection can drive permanent damage to the hepatocytes, leading to genetic and epigenetic damage and driving oncogenesis. As an RNA virus, HCV doesn’t integrate into the genome and no confirmed viral oncoproteins have been identified to date, therefore it mostly drives cancer through these indirect mechanisms, which is also reflected in the fact that HCV-associated HCC predominantly occurs against a backdrop of liver cirrhosis.
HBV does integrate into the host genome. Genome sequencing studies revealed hundreds of integration sites, but most commonly they disrupted host genes involved in telomere stability and cell cycle regulation, providing some insight into the mechanisms by which HBV-associated HCC develops. In addition, HBV produces several oncoproteins, including HBx, which disrupts gene transcription, cell signaling pathways, cell cycle progress, apoptosis and other cellular processes.15,16
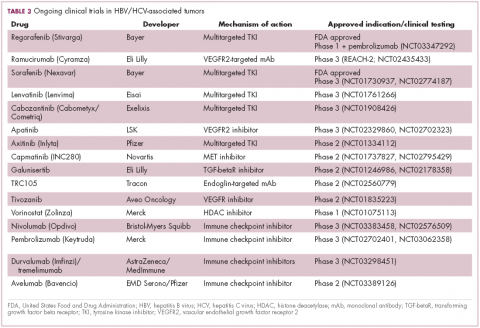
Multitargeted tyrosine kinase inhibitors (TKIs) have been the focal point of therapeutic development in HCC. However, following the approval of sorafenib in 2008, there was a dearth of effective new treatment options despite substantial efforts and numerous phase 3 trials. More recently, immunotherapy has also come to the forefront, especially immune checkpoint inhibitors.
Last year marked the first new drug approvals in nearly a decade – the TKI regorafenib (Stivarga) and immune checkpoint inhibitor nivolumab (Opdivo), both in the second-line setting after failure of sorafenib. Treatment options in this setting may continue to expand, with the TKIs cabozantinib and lenvatinib and the immune checkpoint inhibitor pembrolizumab and the combination of durvalumab and tremelimumab hot on their heels.17-20 Many of these drugs are also being evaluated in the front-line setting in comparison with sorafenib (Table 3).
At the current time, the treatment strategy for patients with HCC is independent of etiology, however, there are significant ongoing efforts to try to tease out the implications of infection for treatment efficacy. A recent meta-analysis of patients treated with sorafenib in 3 randomized phase 3 trials (n = 3,526) suggested that it improved overall survival (OS) among patients who were HCV-positive, but HBV-negative.21
Studies of the vascular endothelial growth factor receptor 2-targeting monoclonal antibody ramucirumab, on the other hand, suggested that it may have a greater OS benefit in patients with HBV, while regorafenib seemed to have a comparable OS benefit in both subgroups.22-25 The immune checkpoint inhibitors studied thus far seem to elicit responses irrespective of infection status.
A phase 2 trial of the immune checkpoint inhibitor tremelimumab was conducted specifically in patients with advanced HCC and chronic HCV infection. The disease control rate (DCR) was 76.4%, with 17.6% partial response (PR) rate. There was also a significant drop in viral load, suggesting that tremelimumab may have antiviral effects.26,27,28
Adoptive cell therapy promising in EBV-positive cancers
More than 90% of the global population is infected with EBV, making it one of the most common human viruses. It is a member of the herpesvirus family that is probably best known as the cause of infectious mononucleosis. On rare occasions, however, EBV can cause tumor development, though our understanding of its exact pathogenic role in cancer is still incomplete.
EBV is a DNA virus that doesn’t tend to integrate into the host genome, but instead remains in the nucleus in the form of episomes and produces several oncoproteins, including latent membrane protein-1. It is associated with a range of different cancer types, including Burkitt lymphoma and other B-cell malignancies. It also infects epithelial cells and can cause nasopharyngeal carcinoma and gastric cancer, however, much less is known about the molecular underpinnings of these EBV-positive cancer types.26,27Gastric cancers actually comprise the largest group of EBV-associated tumors because of the global incidence of this cancer type. The Cancer Genome Atlas Research Network recently characterized gastric cancer on a molecular level and identified an EBV-positive subgroup as a distinct clinical entity with unique molecular characteristics.29
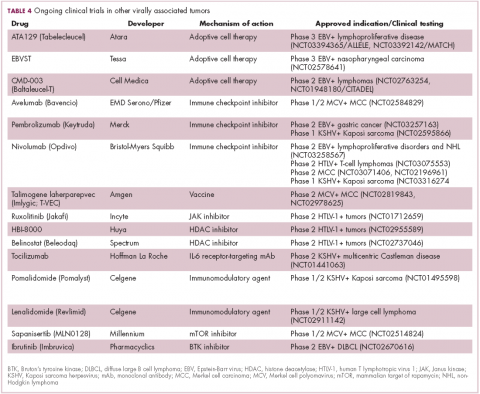
The focus of therapeutic development has again been on immunotherapy, however in this case the idea of collecting the patients T cells, engineering them to recognize EBV, and then reinfusing them into the patient – adoptive cell therapy – has gained the most traction (Table 4).
Two presentations at the American Society of Hematology annual meeting in 2017 detailed ongoing clinical trials of Atara Biotherapeutics’ ATA129 and Cell Medica’s CMD-003. ATA129 was associated with a high response rate and a low rate of serious AEs in patients with posttransplant lymphoproliferative disorder; ORR was 80% in 6 patients treated after hematopoietic stem cell transplantation, and 83% in 6 patients after solid organ transplant.30
CMD-003, meanwhile, demonstrated preliminary signs of activity and safety in patients with relapsed extranodal NK/T-cell lymphoma, according to early results from the phase 2 CITADEL trial. Among 6 evaluable patients, the ORR was 50% and the DCR was 67%.31
Newest oncovirus on the block
The most recently discovered cancer-associated virus is Merkel cell polyomavirus (MCV), a DNA virus that was identified in 2008. Like EBV, virtually the whole global adult population is infected with MCV. It is linked to the development of a highly aggressive and lethal, though rare, form of skin cancer – Merkel cell carcinoma.
MCV is found in around 80% of MCC cases and in fewer than 10% of melanomas and other skin cancers. Thus far, several direct mechanisms of oncogenesis have been described, including integration of MCV into the host genome and the production of viral oncogenes, though their precise function is as yet unclear.32-34
The American Cancer Society estimates that only 1500 cases of MCC are diagnosed each year in the United States.35 Its rarity makes it difficult to conduct clinical trials with sufficient power, yet some headway has still been made.
Around half of MCCs express the programmed cell death ligand 1 (PD-L1) on their surface, making them a logical candidate for immune checkpoint inhibition. In 2017, avelumab became the first FDA-approved drug for the treatment of MCC. Approval was based on the JAVELIN Merkel 200 study in which 88 patients received avelumab. After 1 year of follow-up the ORR was 31.8%, with a CR rate of 9%.36
Genome sequencing studies suggest that the mutational profile of MCV-positive tumors is quite different to those that are MCV-negative, which could have therapeutic implications. To date, these implications have not been delineated, given the challenge of small patient numbers, however an ongoing phase 1/2 trial is evaluating the combination of avelumab and radiation therapy or recombinant interferon beta, with or without MCV-specific cytotoxic T cells in patients with MCC and MCV infection.
The 2 other known cancer-causing viruses are human T-lymphotropic virus 1 (HTLV-1), a retrovirus associated with adult T-cell leukemia/lymphoma (ATL) and Kaposi sarcoma herpesvirus (KSHV). The latter is the causative agent of Kaposi sarcoma, often in combination with human immunodeficiency virus (HIV), a rare skin tumor that became renowned in the 1980s as an AIDS-defining illness.
The incidence of HTLV-1- and KSHV-positive tumors is substantially lower than the other virally associated cancers and, like MCC, this makes studying them and conducting clinical trials of novel therapeutic options a challenge. Nonetheless, several trials of targeted therapies and immunotherapies are underway.
1. Rous PA. Transmissible avain neoplasm. (Sarcoma of the common fowl). J Exp Med. 1910;12(5):696-705.
2. Epstein MA, Achong BG, Barr YM. Virus particles in cultured lymphoblasts from Burkitt's lymphoma. Lancet. 1964;1(7335):702-703.
3. Mesri Enrique A, Feitelson MA, Munger K. Human viral oncogenesis: a cancer hallmarks analysis. Cell Host & Microbe. 2014;15(3):266-282.
4. Santana-Davila R, Bhatia S, Chow LQ. Harnessing the immune system as a therapeutic tool in virus-associated cancers. JAMA Oncol. 2017;3(1):106-112.
5. Tashiro H, Brenner MK. Immunotherapy against cancer-related viruses. Cell Res. 2017;27(1):59-73.
6. Brianti P, De Flammineis E, Mercuri SR. Review of HPV-related diseases and cancers. New Microbiol. 2017;40(2):80-85.
7. Tulay P, Serakinci N. The route to HPV-associated neoplastic transformation: a review of the literature. Crit Rev Eukaryot Gene Expr. 2016;26(1):27-39.
8. Smola S. Immunopathogenesis of HPV-associated cancers and prospects for immunotherapy. Viruses. 2017;9(9).
9. Rosales R, Rosales C. Immune therapy for human papillomaviruses-related cancers. World Journal of Clinical Oncology. 2014;5(5):1002-1019.
10. Miles B, Safran HP, Monk BJ. Therapeutic options for treatment of human papillomavirus-associated cancers - novel immunologic vaccines: ADXS11-001. Gynecol Oncol Res Pract. 2017;4:10.
11. Miles BA, Monk BJ, Safran HP. Mechanistic insights into ADXS11-001 human papillomavirus-associated cancer immunotherapy. Gynecol Oncol Res Pract. 2017;4:9.
12. Huh W, Dizon D, Powell M, Landrum L, Leath C. A prospective phase II trial of the listeria-based human papillomavirus immunotherapy axalimogene filolisbac in second and third-line metastatic cervical cancer: A NRG oncology group trial. Paper presented at: Annual Meeting on Women's Cancer; March 12-15, 2017, 2017; National Harbor, MD.
13. Petit RG, Mehta A, Jain M, et al. ADXS11-001 immunotherapy targeting HPV-E7: final results from a Phase II study in Indian women with recurrent cervical cancer. Journal for Immunotherapy of Cancer. 2014;2(Suppl 3):P92-P92.
14. Glisson B, Massarelli E, William W, et al. Nivolumab and ISA 101 HPV vaccine in incurable HPV-16+ cancer. Ann Oncol. 2017;28(suppl_5):v403-v427.
15. Ding X-X, Zhu Q-G, Zhang S-M, et al. Precision medicine for hepatocellular carcinoma: driver mutations and targeted therapy. Oncotarget. 2017;8(33):55715-55730.
16. Ringehan M, McKeating JA, Protzer U. Viral hepatitis and liver cancer. Philosophical Transactions of the Royal Society B: Biological Sciences. 2017;372(1732):20160274.
17. Abou-Alfa G, Meyer T, Cheng AL, et al. Cabozantinib (C) versus placebo (P) in patients (pts) with advanced hepatocellular carcinoma (HCC) who have received prior sorafenib: results from the randomized phase III CELESTIAL trial. J Clin Oncol. 2017;36(Suppl 4S):abstr 207.
18. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018.
19. Zhu AX, Finn RS, Cattan S, et al. KEYNOTE-224: Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib. J Clin Oncol. 2018;36(Suppl 4S):Abstr 209.
20. Kelley RK, Abou-Alfa GK, Bendell JC, et al. Phase I/II study of durvalumab and tremelimumab in patients with unresectable hepatocellular carcinoma (HCC): Phase I safety and efficacy analyses. Journal of Clinical Oncology. 2017;35(15_suppl):4073-4073.
21. Jackson R, Psarelli E-E, Berhane S, Khan H, Johnson P. Impact of Viral Status on Survival in Patients Receiving Sorafenib for Advanced Hepatocellular Cancer: A Meta-Analysis of Randomized Phase III Trials. Journal of Clinical Oncology. 2017;35(6):622-628.
22. Kudo M. Molecular Targeted Agents for Hepatocellular Carcinoma: Current Status and Future Perspectives. Liver Cancer. 2017;6(2):101-112.
23. zur Hausen H, Meinhof W, Scheiber W, Bornkamm GW. Attempts to detect virus-secific DNA in human tumors. I. Nucleic acid hybridizations with complementary RNA of human wart virus. Int J Cancer. 1974;13(5):650-656.
24. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56-66.
25. Bruix J, Tak WY, Gasbarrini A, et al. Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: multicentre, open-label, phase II safety study. Eur J Cancer. 2013;49(16):3412-3419.
26. Neparidze N, Lacy J. Malignancies associated with epstein-barr virus: pathobiology, clinical features, and evolving treatments. Clin Adv Hematol Oncol. 2014;12(6):358-371.
27. Ozoya OO, Sokol L, Dalia S. EBV-Related Malignancies, Outcomes and Novel Prevention Strategies. Infect Disord Drug Targets. 2016;16(1):4-21.
28. Sangro B, Gomez-Martin C, de la Mata M, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59(1):81-88.
29. The Cancer Genome Atlas Research N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202.
30. Prockop S, Li A, Baiocchi R, et al. Efficacy and safety of ATA129, partially matched allogeneic third-party Epstein-Barr virus-targeted cytotoxic T lymphocytes in a multicenter study for post-transplant lymphoproliferative disorder. Paper presented at: 59th Annual Meeting of the American Society of Hematology; December 9-12, 2017, 2017; Atlanta, GA.
31. Kim W, Ardeshna K, Lin Y, et al. Autologous EBV-specific T cells (CMD-003): Early results from a multicenter, multinational Phase 2 trial for treatment of EBV-associated NK/T-cell lymphoma. Paper presented at: 59th Annual Meeting of the American Society of Hematology; December 9-12, 2017, 2017; Atlanta, GA.
32. Schadendorf D, Lebbé C, zur Hausen A, et al. Merkel cell carcinoma: Epidemiology, prognosis, therapy and unmet medical needs. European Journal of Cancer. 2017;71:53-69.
33. Spurgeon ME, Lambert PF. Merkel cell polyomavirus: a newly discovered human virus with oncogenic potential. Virology. 2013;435(1):118-130.
34. Tello TL, Coggshall K, Yom SS, Yu SS. Merkel cell carcinoma: An update and review: Current and future therapy. J Am Acad Dermatol. 2018;78(3):445-454.
35. American Cancer Society. Key Statistics for Merkel Cell Carcinoma. 2015; https://www.cancer.org/cancer/merkel-cell-skin-cancer/about/key-statistics.html#written_by. Accessed March 7th, 2017.
36. Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. The Lancet Oncology.17(10):1374-1385.
Infection with certain viruses has been causally linked to the development of cancer. In recent years, an improved understanding of the unique pathology and molecular underpinnings of these virally associated cancers has prompted the development of more personalized treatment strategies, with a particular focus on immunotherapy. Here, we describe some of the latest developments.
The link between viruses and cancer
Suspicions about a possible role of viral infections in the development of cancer were first aroused in the early 1900s. The seminal discovery is traced back to Peyton Rous, who showed that a malignant tumor growing in a chicken could be transferred to a healthy bird by injecting it with tumor extracts that contained no actual tumor cells.1
The infectious etiology of human cancer, however, remained controversial until many years later when the first cancer-causing virus, Epstein-Barr virus (EBV), was identified in cell cultures from patients with Burkitt lymphoma. Shortly afterward, the Rous sarcoma virus was unveiled as the oncogenic agent behind Rous’ observations.2Seven viruses have now been linked to the development of cancers and are thought to be responsible for around 12% of all cancer cases worldwide. The burden is likely to increase as technological advancements make it easier to establish a causal link between viruses and cancer development.3
In addition to making these links, researchers have also made significant headway in understanding how viruses cause cancer. Cancerous transformation of host cells occurs in only a minority of those who are infected with oncogenic viruses and often occurs in the setting of chronic infection.
Viruses can mediate carcinogenesis by direct and/or indirect mechanisms (Figure 1). Many of the hallmarks of cancer, the key attributes that drive the transformation from a normal cell to a malignant one, are compatible with the virus’s needs, such as needing to avoid cell death, increasing cell proliferation, and avoiding detection by the immune system.
Viruses hijack the cellular machinery to meet those needs and they can do this either by producing viral proteins that have an oncogenic effect or by integrating their genetic material into the host cell genome. When the latter occurs, the process of integration can also cause damage to the DNA, which further increases the risk of cancer-promoting changes occurring in the host genome.
Viruses can indirectly contribute to carcinogenesis by fostering a microenvironment of chronic inflammation, causing oxidative stress and local tissue damage, and by suppressing the antitumor immune response.4,5
Screening and prevention efforts have helped to reduce the burden of several different virally associated cancers. However, for the substantial proportion of patients who are still affected by these cancers, there is a pressing need for new therapeutic options, particularly since genome sequencing studies have revealed that these cancers can often have distinct underlying molecular mechanisms.
Vaccines lead the charge in HPV-driven cancers
German virologist Harald zur Hausen received the Nobel Prize in 2008 for his discovery of the oncogenic role of human papillomaviruses (HPVs), a large family of more than 100 DNA viruses that infect the epithelial cells of the skin and mucous membranes. They are responsible for the largest number of virally associated cancer cases globally – around 5% (Table 1).
A number of different cancer types are linked to HPV infection, but it is best known as the cause of cervical cancer. The development of diagnostic blood tests and prophylactic vaccines for prevention and early intervention in HPV infection has helped to reduce the incidence of cervical cancer. Conversely, another type of HPV-associated cancer, head and neck squamous cell carcinoma (HNSCC), has seen increased incidence in recent years.
HPVs are categorized according to their oncogenic potential as high, intermediate, or low risk. The high-risk HPV16 and HPV18 strains are most commonly associated with cancer. They are thought to cause cancer predominantly through integration into the host genome. The HPV genome is composed of 8 genes encoding proteins that regulate viral replication and assembly. The E6 and E7 genes are the most highly oncogenic; as the HPV DNA is inserted into the host genome, the transcriptional regulator of E6/E7 is lost, leading to their increased expression. These genes have significant oncogenic potential because of their interaction with 2 tumor suppressor proteins, p53 and pRb.6,7
The largest investment in therapeutic development for HPV-positive cancers has been in the realm of immunotherapy in an effort to boost the anti-tumor immune response. In particular, there has been a focus on the development of therapeutic vaccines, designed to prime the anti-tumor immune response to recognize viral antigens. A variety of different types of vaccines are being developed, including live, attenuated and inactivated vaccines that are protein, DNA, or peptide based. Most developed to date target the E6/E7 proteins from the HPV16/18 strains (Table 2).8,9
Other immunotherapies are also being evaluated, including immune checkpoint inhibitors, antibodies designed to target one of the principal mechanisms of immune evasion exploited by cancer cells. The combination of immune checkpoint inhibitors with vaccines is a particularly promising strategy in HPV-associated cancers. At the European Society for Medical Oncology Congress in 2017, the results of a phase 2 trial of nivolumab in combination with ISA-101 were presented.
Among 24 patients with HPV-positive tumors, the majority oropharyngeal cancers, the combination elicited an overall response rate (ORR) of 33%, including 2 complete responses (CRs). Most adverse events (AEs) were mild to moderate in severity and included fever, injection site reactions, fatigue and nausea.14
Hepatocellular carcinoma: a tale of two viruses
The hepatitis viruses are a group of 5 unrelated viruses that causes inflammation of the liver. Hepatitis B (HBV), a DNA virus, and hepatitis C (HCV), an RNA virus, are also oncoviruses; HBV in particular is one of the main causes of hepatocellular carcinoma (HCC), the most common type of liver cancer.
The highly inflammatory environment fostered by HBV and HCV infection causes liver damage that often leads to cirrhosis. Continued infection can drive permanent damage to the hepatocytes, leading to genetic and epigenetic damage and driving oncogenesis. As an RNA virus, HCV doesn’t integrate into the genome and no confirmed viral oncoproteins have been identified to date, therefore it mostly drives cancer through these indirect mechanisms, which is also reflected in the fact that HCV-associated HCC predominantly occurs against a backdrop of liver cirrhosis.
HBV does integrate into the host genome. Genome sequencing studies revealed hundreds of integration sites, but most commonly they disrupted host genes involved in telomere stability and cell cycle regulation, providing some insight into the mechanisms by which HBV-associated HCC develops. In addition, HBV produces several oncoproteins, including HBx, which disrupts gene transcription, cell signaling pathways, cell cycle progress, apoptosis and other cellular processes.15,16
Multitargeted tyrosine kinase inhibitors (TKIs) have been the focal point of therapeutic development in HCC. However, following the approval of sorafenib in 2008, there was a dearth of effective new treatment options despite substantial efforts and numerous phase 3 trials. More recently, immunotherapy has also come to the forefront, especially immune checkpoint inhibitors.
Last year marked the first new drug approvals in nearly a decade – the TKI regorafenib (Stivarga) and immune checkpoint inhibitor nivolumab (Opdivo), both in the second-line setting after failure of sorafenib. Treatment options in this setting may continue to expand, with the TKIs cabozantinib and lenvatinib and the immune checkpoint inhibitor pembrolizumab and the combination of durvalumab and tremelimumab hot on their heels.17-20 Many of these drugs are also being evaluated in the front-line setting in comparison with sorafenib (Table 3).
At the current time, the treatment strategy for patients with HCC is independent of etiology, however, there are significant ongoing efforts to try to tease out the implications of infection for treatment efficacy. A recent meta-analysis of patients treated with sorafenib in 3 randomized phase 3 trials (n = 3,526) suggested that it improved overall survival (OS) among patients who were HCV-positive, but HBV-negative.21
Studies of the vascular endothelial growth factor receptor 2-targeting monoclonal antibody ramucirumab, on the other hand, suggested that it may have a greater OS benefit in patients with HBV, while regorafenib seemed to have a comparable OS benefit in both subgroups.22-25 The immune checkpoint inhibitors studied thus far seem to elicit responses irrespective of infection status.
A phase 2 trial of the immune checkpoint inhibitor tremelimumab was conducted specifically in patients with advanced HCC and chronic HCV infection. The disease control rate (DCR) was 76.4%, with 17.6% partial response (PR) rate. There was also a significant drop in viral load, suggesting that tremelimumab may have antiviral effects.26,27,28
Adoptive cell therapy promising in EBV-positive cancers
More than 90% of the global population is infected with EBV, making it one of the most common human viruses. It is a member of the herpesvirus family that is probably best known as the cause of infectious mononucleosis. On rare occasions, however, EBV can cause tumor development, though our understanding of its exact pathogenic role in cancer is still incomplete.
EBV is a DNA virus that doesn’t tend to integrate into the host genome, but instead remains in the nucleus in the form of episomes and produces several oncoproteins, including latent membrane protein-1. It is associated with a range of different cancer types, including Burkitt lymphoma and other B-cell malignancies. It also infects epithelial cells and can cause nasopharyngeal carcinoma and gastric cancer, however, much less is known about the molecular underpinnings of these EBV-positive cancer types.26,27Gastric cancers actually comprise the largest group of EBV-associated tumors because of the global incidence of this cancer type. The Cancer Genome Atlas Research Network recently characterized gastric cancer on a molecular level and identified an EBV-positive subgroup as a distinct clinical entity with unique molecular characteristics.29
The focus of therapeutic development has again been on immunotherapy, however in this case the idea of collecting the patients T cells, engineering them to recognize EBV, and then reinfusing them into the patient – adoptive cell therapy – has gained the most traction (Table 4).
Two presentations at the American Society of Hematology annual meeting in 2017 detailed ongoing clinical trials of Atara Biotherapeutics’ ATA129 and Cell Medica’s CMD-003. ATA129 was associated with a high response rate and a low rate of serious AEs in patients with posttransplant lymphoproliferative disorder; ORR was 80% in 6 patients treated after hematopoietic stem cell transplantation, and 83% in 6 patients after solid organ transplant.30
CMD-003, meanwhile, demonstrated preliminary signs of activity and safety in patients with relapsed extranodal NK/T-cell lymphoma, according to early results from the phase 2 CITADEL trial. Among 6 evaluable patients, the ORR was 50% and the DCR was 67%.31
Newest oncovirus on the block
The most recently discovered cancer-associated virus is Merkel cell polyomavirus (MCV), a DNA virus that was identified in 2008. Like EBV, virtually the whole global adult population is infected with MCV. It is linked to the development of a highly aggressive and lethal, though rare, form of skin cancer – Merkel cell carcinoma.
MCV is found in around 80% of MCC cases and in fewer than 10% of melanomas and other skin cancers. Thus far, several direct mechanisms of oncogenesis have been described, including integration of MCV into the host genome and the production of viral oncogenes, though their precise function is as yet unclear.32-34
The American Cancer Society estimates that only 1500 cases of MCC are diagnosed each year in the United States.35 Its rarity makes it difficult to conduct clinical trials with sufficient power, yet some headway has still been made.
Around half of MCCs express the programmed cell death ligand 1 (PD-L1) on their surface, making them a logical candidate for immune checkpoint inhibition. In 2017, avelumab became the first FDA-approved drug for the treatment of MCC. Approval was based on the JAVELIN Merkel 200 study in which 88 patients received avelumab. After 1 year of follow-up the ORR was 31.8%, with a CR rate of 9%.36
Genome sequencing studies suggest that the mutational profile of MCV-positive tumors is quite different to those that are MCV-negative, which could have therapeutic implications. To date, these implications have not been delineated, given the challenge of small patient numbers, however an ongoing phase 1/2 trial is evaluating the combination of avelumab and radiation therapy or recombinant interferon beta, with or without MCV-specific cytotoxic T cells in patients with MCC and MCV infection.
The 2 other known cancer-causing viruses are human T-lymphotropic virus 1 (HTLV-1), a retrovirus associated with adult T-cell leukemia/lymphoma (ATL) and Kaposi sarcoma herpesvirus (KSHV). The latter is the causative agent of Kaposi sarcoma, often in combination with human immunodeficiency virus (HIV), a rare skin tumor that became renowned in the 1980s as an AIDS-defining illness.
The incidence of HTLV-1- and KSHV-positive tumors is substantially lower than the other virally associated cancers and, like MCC, this makes studying them and conducting clinical trials of novel therapeutic options a challenge. Nonetheless, several trials of targeted therapies and immunotherapies are underway.
Infection with certain viruses has been causally linked to the development of cancer. In recent years, an improved understanding of the unique pathology and molecular underpinnings of these virally associated cancers has prompted the development of more personalized treatment strategies, with a particular focus on immunotherapy. Here, we describe some of the latest developments.
The link between viruses and cancer
Suspicions about a possible role of viral infections in the development of cancer were first aroused in the early 1900s. The seminal discovery is traced back to Peyton Rous, who showed that a malignant tumor growing in a chicken could be transferred to a healthy bird by injecting it with tumor extracts that contained no actual tumor cells.1
The infectious etiology of human cancer, however, remained controversial until many years later when the first cancer-causing virus, Epstein-Barr virus (EBV), was identified in cell cultures from patients with Burkitt lymphoma. Shortly afterward, the Rous sarcoma virus was unveiled as the oncogenic agent behind Rous’ observations.2Seven viruses have now been linked to the development of cancers and are thought to be responsible for around 12% of all cancer cases worldwide. The burden is likely to increase as technological advancements make it easier to establish a causal link between viruses and cancer development.3
In addition to making these links, researchers have also made significant headway in understanding how viruses cause cancer. Cancerous transformation of host cells occurs in only a minority of those who are infected with oncogenic viruses and often occurs in the setting of chronic infection.
Viruses can mediate carcinogenesis by direct and/or indirect mechanisms (Figure 1). Many of the hallmarks of cancer, the key attributes that drive the transformation from a normal cell to a malignant one, are compatible with the virus’s needs, such as needing to avoid cell death, increasing cell proliferation, and avoiding detection by the immune system.
Viruses hijack the cellular machinery to meet those needs and they can do this either by producing viral proteins that have an oncogenic effect or by integrating their genetic material into the host cell genome. When the latter occurs, the process of integration can also cause damage to the DNA, which further increases the risk of cancer-promoting changes occurring in the host genome.
Viruses can indirectly contribute to carcinogenesis by fostering a microenvironment of chronic inflammation, causing oxidative stress and local tissue damage, and by suppressing the antitumor immune response.4,5
Screening and prevention efforts have helped to reduce the burden of several different virally associated cancers. However, for the substantial proportion of patients who are still affected by these cancers, there is a pressing need for new therapeutic options, particularly since genome sequencing studies have revealed that these cancers can often have distinct underlying molecular mechanisms.
Vaccines lead the charge in HPV-driven cancers
German virologist Harald zur Hausen received the Nobel Prize in 2008 for his discovery of the oncogenic role of human papillomaviruses (HPVs), a large family of more than 100 DNA viruses that infect the epithelial cells of the skin and mucous membranes. They are responsible for the largest number of virally associated cancer cases globally – around 5% (Table 1).
A number of different cancer types are linked to HPV infection, but it is best known as the cause of cervical cancer. The development of diagnostic blood tests and prophylactic vaccines for prevention and early intervention in HPV infection has helped to reduce the incidence of cervical cancer. Conversely, another type of HPV-associated cancer, head and neck squamous cell carcinoma (HNSCC), has seen increased incidence in recent years.
HPVs are categorized according to their oncogenic potential as high, intermediate, or low risk. The high-risk HPV16 and HPV18 strains are most commonly associated with cancer. They are thought to cause cancer predominantly through integration into the host genome. The HPV genome is composed of 8 genes encoding proteins that regulate viral replication and assembly. The E6 and E7 genes are the most highly oncogenic; as the HPV DNA is inserted into the host genome, the transcriptional regulator of E6/E7 is lost, leading to their increased expression. These genes have significant oncogenic potential because of their interaction with 2 tumor suppressor proteins, p53 and pRb.6,7
The largest investment in therapeutic development for HPV-positive cancers has been in the realm of immunotherapy in an effort to boost the anti-tumor immune response. In particular, there has been a focus on the development of therapeutic vaccines, designed to prime the anti-tumor immune response to recognize viral antigens. A variety of different types of vaccines are being developed, including live, attenuated and inactivated vaccines that are protein, DNA, or peptide based. Most developed to date target the E6/E7 proteins from the HPV16/18 strains (Table 2).8,9
Other immunotherapies are also being evaluated, including immune checkpoint inhibitors, antibodies designed to target one of the principal mechanisms of immune evasion exploited by cancer cells. The combination of immune checkpoint inhibitors with vaccines is a particularly promising strategy in HPV-associated cancers. At the European Society for Medical Oncology Congress in 2017, the results of a phase 2 trial of nivolumab in combination with ISA-101 were presented.
Among 24 patients with HPV-positive tumors, the majority oropharyngeal cancers, the combination elicited an overall response rate (ORR) of 33%, including 2 complete responses (CRs). Most adverse events (AEs) were mild to moderate in severity and included fever, injection site reactions, fatigue and nausea.14
Hepatocellular carcinoma: a tale of two viruses
The hepatitis viruses are a group of 5 unrelated viruses that causes inflammation of the liver. Hepatitis B (HBV), a DNA virus, and hepatitis C (HCV), an RNA virus, are also oncoviruses; HBV in particular is one of the main causes of hepatocellular carcinoma (HCC), the most common type of liver cancer.
The highly inflammatory environment fostered by HBV and HCV infection causes liver damage that often leads to cirrhosis. Continued infection can drive permanent damage to the hepatocytes, leading to genetic and epigenetic damage and driving oncogenesis. As an RNA virus, HCV doesn’t integrate into the genome and no confirmed viral oncoproteins have been identified to date, therefore it mostly drives cancer through these indirect mechanisms, which is also reflected in the fact that HCV-associated HCC predominantly occurs against a backdrop of liver cirrhosis.
HBV does integrate into the host genome. Genome sequencing studies revealed hundreds of integration sites, but most commonly they disrupted host genes involved in telomere stability and cell cycle regulation, providing some insight into the mechanisms by which HBV-associated HCC develops. In addition, HBV produces several oncoproteins, including HBx, which disrupts gene transcription, cell signaling pathways, cell cycle progress, apoptosis and other cellular processes.15,16
Multitargeted tyrosine kinase inhibitors (TKIs) have been the focal point of therapeutic development in HCC. However, following the approval of sorafenib in 2008, there was a dearth of effective new treatment options despite substantial efforts and numerous phase 3 trials. More recently, immunotherapy has also come to the forefront, especially immune checkpoint inhibitors.
Last year marked the first new drug approvals in nearly a decade – the TKI regorafenib (Stivarga) and immune checkpoint inhibitor nivolumab (Opdivo), both in the second-line setting after failure of sorafenib. Treatment options in this setting may continue to expand, with the TKIs cabozantinib and lenvatinib and the immune checkpoint inhibitor pembrolizumab and the combination of durvalumab and tremelimumab hot on their heels.17-20 Many of these drugs are also being evaluated in the front-line setting in comparison with sorafenib (Table 3).
At the current time, the treatment strategy for patients with HCC is independent of etiology, however, there are significant ongoing efforts to try to tease out the implications of infection for treatment efficacy. A recent meta-analysis of patients treated with sorafenib in 3 randomized phase 3 trials (n = 3,526) suggested that it improved overall survival (OS) among patients who were HCV-positive, but HBV-negative.21
Studies of the vascular endothelial growth factor receptor 2-targeting monoclonal antibody ramucirumab, on the other hand, suggested that it may have a greater OS benefit in patients with HBV, while regorafenib seemed to have a comparable OS benefit in both subgroups.22-25 The immune checkpoint inhibitors studied thus far seem to elicit responses irrespective of infection status.
A phase 2 trial of the immune checkpoint inhibitor tremelimumab was conducted specifically in patients with advanced HCC and chronic HCV infection. The disease control rate (DCR) was 76.4%, with 17.6% partial response (PR) rate. There was also a significant drop in viral load, suggesting that tremelimumab may have antiviral effects.26,27,28
Adoptive cell therapy promising in EBV-positive cancers
More than 90% of the global population is infected with EBV, making it one of the most common human viruses. It is a member of the herpesvirus family that is probably best known as the cause of infectious mononucleosis. On rare occasions, however, EBV can cause tumor development, though our understanding of its exact pathogenic role in cancer is still incomplete.
EBV is a DNA virus that doesn’t tend to integrate into the host genome, but instead remains in the nucleus in the form of episomes and produces several oncoproteins, including latent membrane protein-1. It is associated with a range of different cancer types, including Burkitt lymphoma and other B-cell malignancies. It also infects epithelial cells and can cause nasopharyngeal carcinoma and gastric cancer, however, much less is known about the molecular underpinnings of these EBV-positive cancer types.26,27Gastric cancers actually comprise the largest group of EBV-associated tumors because of the global incidence of this cancer type. The Cancer Genome Atlas Research Network recently characterized gastric cancer on a molecular level and identified an EBV-positive subgroup as a distinct clinical entity with unique molecular characteristics.29
The focus of therapeutic development has again been on immunotherapy, however in this case the idea of collecting the patients T cells, engineering them to recognize EBV, and then reinfusing them into the patient – adoptive cell therapy – has gained the most traction (Table 4).
Two presentations at the American Society of Hematology annual meeting in 2017 detailed ongoing clinical trials of Atara Biotherapeutics’ ATA129 and Cell Medica’s CMD-003. ATA129 was associated with a high response rate and a low rate of serious AEs in patients with posttransplant lymphoproliferative disorder; ORR was 80% in 6 patients treated after hematopoietic stem cell transplantation, and 83% in 6 patients after solid organ transplant.30
CMD-003, meanwhile, demonstrated preliminary signs of activity and safety in patients with relapsed extranodal NK/T-cell lymphoma, according to early results from the phase 2 CITADEL trial. Among 6 evaluable patients, the ORR was 50% and the DCR was 67%.31
Newest oncovirus on the block
The most recently discovered cancer-associated virus is Merkel cell polyomavirus (MCV), a DNA virus that was identified in 2008. Like EBV, virtually the whole global adult population is infected with MCV. It is linked to the development of a highly aggressive and lethal, though rare, form of skin cancer – Merkel cell carcinoma.
MCV is found in around 80% of MCC cases and in fewer than 10% of melanomas and other skin cancers. Thus far, several direct mechanisms of oncogenesis have been described, including integration of MCV into the host genome and the production of viral oncogenes, though their precise function is as yet unclear.32-34
The American Cancer Society estimates that only 1500 cases of MCC are diagnosed each year in the United States.35 Its rarity makes it difficult to conduct clinical trials with sufficient power, yet some headway has still been made.
Around half of MCCs express the programmed cell death ligand 1 (PD-L1) on their surface, making them a logical candidate for immune checkpoint inhibition. In 2017, avelumab became the first FDA-approved drug for the treatment of MCC. Approval was based on the JAVELIN Merkel 200 study in which 88 patients received avelumab. After 1 year of follow-up the ORR was 31.8%, with a CR rate of 9%.36
Genome sequencing studies suggest that the mutational profile of MCV-positive tumors is quite different to those that are MCV-negative, which could have therapeutic implications. To date, these implications have not been delineated, given the challenge of small patient numbers, however an ongoing phase 1/2 trial is evaluating the combination of avelumab and radiation therapy or recombinant interferon beta, with or without MCV-specific cytotoxic T cells in patients with MCC and MCV infection.
The 2 other known cancer-causing viruses are human T-lymphotropic virus 1 (HTLV-1), a retrovirus associated with adult T-cell leukemia/lymphoma (ATL) and Kaposi sarcoma herpesvirus (KSHV). The latter is the causative agent of Kaposi sarcoma, often in combination with human immunodeficiency virus (HIV), a rare skin tumor that became renowned in the 1980s as an AIDS-defining illness.
The incidence of HTLV-1- and KSHV-positive tumors is substantially lower than the other virally associated cancers and, like MCC, this makes studying them and conducting clinical trials of novel therapeutic options a challenge. Nonetheless, several trials of targeted therapies and immunotherapies are underway.
1. Rous PA. Transmissible avain neoplasm. (Sarcoma of the common fowl). J Exp Med. 1910;12(5):696-705.
2. Epstein MA, Achong BG, Barr YM. Virus particles in cultured lymphoblasts from Burkitt's lymphoma. Lancet. 1964;1(7335):702-703.
3. Mesri Enrique A, Feitelson MA, Munger K. Human viral oncogenesis: a cancer hallmarks analysis. Cell Host & Microbe. 2014;15(3):266-282.
4. Santana-Davila R, Bhatia S, Chow LQ. Harnessing the immune system as a therapeutic tool in virus-associated cancers. JAMA Oncol. 2017;3(1):106-112.
5. Tashiro H, Brenner MK. Immunotherapy against cancer-related viruses. Cell Res. 2017;27(1):59-73.
6. Brianti P, De Flammineis E, Mercuri SR. Review of HPV-related diseases and cancers. New Microbiol. 2017;40(2):80-85.
7. Tulay P, Serakinci N. The route to HPV-associated neoplastic transformation: a review of the literature. Crit Rev Eukaryot Gene Expr. 2016;26(1):27-39.
8. Smola S. Immunopathogenesis of HPV-associated cancers and prospects for immunotherapy. Viruses. 2017;9(9).
9. Rosales R, Rosales C. Immune therapy for human papillomaviruses-related cancers. World Journal of Clinical Oncology. 2014;5(5):1002-1019.
10. Miles B, Safran HP, Monk BJ. Therapeutic options for treatment of human papillomavirus-associated cancers - novel immunologic vaccines: ADXS11-001. Gynecol Oncol Res Pract. 2017;4:10.
11. Miles BA, Monk BJ, Safran HP. Mechanistic insights into ADXS11-001 human papillomavirus-associated cancer immunotherapy. Gynecol Oncol Res Pract. 2017;4:9.
12. Huh W, Dizon D, Powell M, Landrum L, Leath C. A prospective phase II trial of the listeria-based human papillomavirus immunotherapy axalimogene filolisbac in second and third-line metastatic cervical cancer: A NRG oncology group trial. Paper presented at: Annual Meeting on Women's Cancer; March 12-15, 2017, 2017; National Harbor, MD.
13. Petit RG, Mehta A, Jain M, et al. ADXS11-001 immunotherapy targeting HPV-E7: final results from a Phase II study in Indian women with recurrent cervical cancer. Journal for Immunotherapy of Cancer. 2014;2(Suppl 3):P92-P92.
14. Glisson B, Massarelli E, William W, et al. Nivolumab and ISA 101 HPV vaccine in incurable HPV-16+ cancer. Ann Oncol. 2017;28(suppl_5):v403-v427.
15. Ding X-X, Zhu Q-G, Zhang S-M, et al. Precision medicine for hepatocellular carcinoma: driver mutations and targeted therapy. Oncotarget. 2017;8(33):55715-55730.
16. Ringehan M, McKeating JA, Protzer U. Viral hepatitis and liver cancer. Philosophical Transactions of the Royal Society B: Biological Sciences. 2017;372(1732):20160274.
17. Abou-Alfa G, Meyer T, Cheng AL, et al. Cabozantinib (C) versus placebo (P) in patients (pts) with advanced hepatocellular carcinoma (HCC) who have received prior sorafenib: results from the randomized phase III CELESTIAL trial. J Clin Oncol. 2017;36(Suppl 4S):abstr 207.
18. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018.
19. Zhu AX, Finn RS, Cattan S, et al. KEYNOTE-224: Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib. J Clin Oncol. 2018;36(Suppl 4S):Abstr 209.
20. Kelley RK, Abou-Alfa GK, Bendell JC, et al. Phase I/II study of durvalumab and tremelimumab in patients with unresectable hepatocellular carcinoma (HCC): Phase I safety and efficacy analyses. Journal of Clinical Oncology. 2017;35(15_suppl):4073-4073.
21. Jackson R, Psarelli E-E, Berhane S, Khan H, Johnson P. Impact of Viral Status on Survival in Patients Receiving Sorafenib for Advanced Hepatocellular Cancer: A Meta-Analysis of Randomized Phase III Trials. Journal of Clinical Oncology. 2017;35(6):622-628.
22. Kudo M. Molecular Targeted Agents for Hepatocellular Carcinoma: Current Status and Future Perspectives. Liver Cancer. 2017;6(2):101-112.
23. zur Hausen H, Meinhof W, Scheiber W, Bornkamm GW. Attempts to detect virus-secific DNA in human tumors. I. Nucleic acid hybridizations with complementary RNA of human wart virus. Int J Cancer. 1974;13(5):650-656.
24. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56-66.
25. Bruix J, Tak WY, Gasbarrini A, et al. Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: multicentre, open-label, phase II safety study. Eur J Cancer. 2013;49(16):3412-3419.
26. Neparidze N, Lacy J. Malignancies associated with epstein-barr virus: pathobiology, clinical features, and evolving treatments. Clin Adv Hematol Oncol. 2014;12(6):358-371.
27. Ozoya OO, Sokol L, Dalia S. EBV-Related Malignancies, Outcomes and Novel Prevention Strategies. Infect Disord Drug Targets. 2016;16(1):4-21.
28. Sangro B, Gomez-Martin C, de la Mata M, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59(1):81-88.
29. The Cancer Genome Atlas Research N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202.
30. Prockop S, Li A, Baiocchi R, et al. Efficacy and safety of ATA129, partially matched allogeneic third-party Epstein-Barr virus-targeted cytotoxic T lymphocytes in a multicenter study for post-transplant lymphoproliferative disorder. Paper presented at: 59th Annual Meeting of the American Society of Hematology; December 9-12, 2017, 2017; Atlanta, GA.
31. Kim W, Ardeshna K, Lin Y, et al. Autologous EBV-specific T cells (CMD-003): Early results from a multicenter, multinational Phase 2 trial for treatment of EBV-associated NK/T-cell lymphoma. Paper presented at: 59th Annual Meeting of the American Society of Hematology; December 9-12, 2017, 2017; Atlanta, GA.
32. Schadendorf D, Lebbé C, zur Hausen A, et al. Merkel cell carcinoma: Epidemiology, prognosis, therapy and unmet medical needs. European Journal of Cancer. 2017;71:53-69.
33. Spurgeon ME, Lambert PF. Merkel cell polyomavirus: a newly discovered human virus with oncogenic potential. Virology. 2013;435(1):118-130.
34. Tello TL, Coggshall K, Yom SS, Yu SS. Merkel cell carcinoma: An update and review: Current and future therapy. J Am Acad Dermatol. 2018;78(3):445-454.
35. American Cancer Society. Key Statistics for Merkel Cell Carcinoma. 2015; https://www.cancer.org/cancer/merkel-cell-skin-cancer/about/key-statistics.html#written_by. Accessed March 7th, 2017.
36. Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. The Lancet Oncology.17(10):1374-1385.
1. Rous PA. Transmissible avain neoplasm. (Sarcoma of the common fowl). J Exp Med. 1910;12(5):696-705.
2. Epstein MA, Achong BG, Barr YM. Virus particles in cultured lymphoblasts from Burkitt's lymphoma. Lancet. 1964;1(7335):702-703.
3. Mesri Enrique A, Feitelson MA, Munger K. Human viral oncogenesis: a cancer hallmarks analysis. Cell Host & Microbe. 2014;15(3):266-282.
4. Santana-Davila R, Bhatia S, Chow LQ. Harnessing the immune system as a therapeutic tool in virus-associated cancers. JAMA Oncol. 2017;3(1):106-112.
5. Tashiro H, Brenner MK. Immunotherapy against cancer-related viruses. Cell Res. 2017;27(1):59-73.
6. Brianti P, De Flammineis E, Mercuri SR. Review of HPV-related diseases and cancers. New Microbiol. 2017;40(2):80-85.
7. Tulay P, Serakinci N. The route to HPV-associated neoplastic transformation: a review of the literature. Crit Rev Eukaryot Gene Expr. 2016;26(1):27-39.
8. Smola S. Immunopathogenesis of HPV-associated cancers and prospects for immunotherapy. Viruses. 2017;9(9).
9. Rosales R, Rosales C. Immune therapy for human papillomaviruses-related cancers. World Journal of Clinical Oncology. 2014;5(5):1002-1019.
10. Miles B, Safran HP, Monk BJ. Therapeutic options for treatment of human papillomavirus-associated cancers - novel immunologic vaccines: ADXS11-001. Gynecol Oncol Res Pract. 2017;4:10.
11. Miles BA, Monk BJ, Safran HP. Mechanistic insights into ADXS11-001 human papillomavirus-associated cancer immunotherapy. Gynecol Oncol Res Pract. 2017;4:9.
12. Huh W, Dizon D, Powell M, Landrum L, Leath C. A prospective phase II trial of the listeria-based human papillomavirus immunotherapy axalimogene filolisbac in second and third-line metastatic cervical cancer: A NRG oncology group trial. Paper presented at: Annual Meeting on Women's Cancer; March 12-15, 2017, 2017; National Harbor, MD.
13. Petit RG, Mehta A, Jain M, et al. ADXS11-001 immunotherapy targeting HPV-E7: final results from a Phase II study in Indian women with recurrent cervical cancer. Journal for Immunotherapy of Cancer. 2014;2(Suppl 3):P92-P92.
14. Glisson B, Massarelli E, William W, et al. Nivolumab and ISA 101 HPV vaccine in incurable HPV-16+ cancer. Ann Oncol. 2017;28(suppl_5):v403-v427.
15. Ding X-X, Zhu Q-G, Zhang S-M, et al. Precision medicine for hepatocellular carcinoma: driver mutations and targeted therapy. Oncotarget. 2017;8(33):55715-55730.
16. Ringehan M, McKeating JA, Protzer U. Viral hepatitis and liver cancer. Philosophical Transactions of the Royal Society B: Biological Sciences. 2017;372(1732):20160274.
17. Abou-Alfa G, Meyer T, Cheng AL, et al. Cabozantinib (C) versus placebo (P) in patients (pts) with advanced hepatocellular carcinoma (HCC) who have received prior sorafenib: results from the randomized phase III CELESTIAL trial. J Clin Oncol. 2017;36(Suppl 4S):abstr 207.
18. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018.
19. Zhu AX, Finn RS, Cattan S, et al. KEYNOTE-224: Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib. J Clin Oncol. 2018;36(Suppl 4S):Abstr 209.
20. Kelley RK, Abou-Alfa GK, Bendell JC, et al. Phase I/II study of durvalumab and tremelimumab in patients with unresectable hepatocellular carcinoma (HCC): Phase I safety and efficacy analyses. Journal of Clinical Oncology. 2017;35(15_suppl):4073-4073.
21. Jackson R, Psarelli E-E, Berhane S, Khan H, Johnson P. Impact of Viral Status on Survival in Patients Receiving Sorafenib for Advanced Hepatocellular Cancer: A Meta-Analysis of Randomized Phase III Trials. Journal of Clinical Oncology. 2017;35(6):622-628.
22. Kudo M. Molecular Targeted Agents for Hepatocellular Carcinoma: Current Status and Future Perspectives. Liver Cancer. 2017;6(2):101-112.
23. zur Hausen H, Meinhof W, Scheiber W, Bornkamm GW. Attempts to detect virus-secific DNA in human tumors. I. Nucleic acid hybridizations with complementary RNA of human wart virus. Int J Cancer. 1974;13(5):650-656.
24. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56-66.
25. Bruix J, Tak WY, Gasbarrini A, et al. Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: multicentre, open-label, phase II safety study. Eur J Cancer. 2013;49(16):3412-3419.
26. Neparidze N, Lacy J. Malignancies associated with epstein-barr virus: pathobiology, clinical features, and evolving treatments. Clin Adv Hematol Oncol. 2014;12(6):358-371.
27. Ozoya OO, Sokol L, Dalia S. EBV-Related Malignancies, Outcomes and Novel Prevention Strategies. Infect Disord Drug Targets. 2016;16(1):4-21.
28. Sangro B, Gomez-Martin C, de la Mata M, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59(1):81-88.
29. The Cancer Genome Atlas Research N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202.
30. Prockop S, Li A, Baiocchi R, et al. Efficacy and safety of ATA129, partially matched allogeneic third-party Epstein-Barr virus-targeted cytotoxic T lymphocytes in a multicenter study for post-transplant lymphoproliferative disorder. Paper presented at: 59th Annual Meeting of the American Society of Hematology; December 9-12, 2017, 2017; Atlanta, GA.
31. Kim W, Ardeshna K, Lin Y, et al. Autologous EBV-specific T cells (CMD-003): Early results from a multicenter, multinational Phase 2 trial for treatment of EBV-associated NK/T-cell lymphoma. Paper presented at: 59th Annual Meeting of the American Society of Hematology; December 9-12, 2017, 2017; Atlanta, GA.
32. Schadendorf D, Lebbé C, zur Hausen A, et al. Merkel cell carcinoma: Epidemiology, prognosis, therapy and unmet medical needs. European Journal of Cancer. 2017;71:53-69.
33. Spurgeon ME, Lambert PF. Merkel cell polyomavirus: a newly discovered human virus with oncogenic potential. Virology. 2013;435(1):118-130.
34. Tello TL, Coggshall K, Yom SS, Yu SS. Merkel cell carcinoma: An update and review: Current and future therapy. J Am Acad Dermatol. 2018;78(3):445-454.
35. American Cancer Society. Key Statistics for Merkel Cell Carcinoma. 2015; https://www.cancer.org/cancer/merkel-cell-skin-cancer/about/key-statistics.html#written_by. Accessed March 7th, 2017.
36. Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. The Lancet Oncology.17(10):1374-1385.
Expert Q&A: What’s new in alopecia areata research and treatment?
LAS VEGAS – Alopecia is hard to bear for patients and has been difficult to treat, but “,” according to Maria Hordinsky, MD.
Dr. Hordinsky, professor and chair of the department of dermatology, University of Minnesota, Minneapolis, discussed hair disorders in multiple presentations at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar. In an interview after her session on alopecia areata, she elaborated on the state of research and treatment for patients with this diagnosis.
DERMATOLOGY NEWS: What has changed in alopecia areata treatment over the last few years?
Dr. Hordinsky: There is still no Food and Drug Administration–approved treatment for this disease. But recent studies have helped us understand how alopecia areata occurs, how important interleukin-15 is, and how to target this cytokine as well as others. At the same time, expertise has developed at several centers across the United States with using the Janus kinase inhibitor tofacitinib [Xeljanz] at 5 milligrams twice a day. This has led to more off-label use. Concurrently, many pharmaceutical companies with interest in alopecia areata have begun to study different JAK inhibitors that target different cytokine receptors. Researchers also are exploring topical JAK inhibitors.
DN: What about the high costs of these drugs?
Dr. Hordinsky: Some insurers are covering tofacitinib, and patient assistance programs are very helpful and beneficial. In my own practice, most patients taking these drugs are using those programs.
DN: Are any treatments being used less than in the past?
Dr. Hordinsky: We’re still using tools that we have in the toolbox because we don’t have an approved treatment. We use topical steroids and intralesional steroids. We also use prednisone as needed, and we use contact sensitization therapy.
DN: In your presentation, you talked about alopecia areata in body areas outside of the scalp. What should dermatologists know about the eyebrows in patients with alopecia areata?
Dr. Hordinsky: Some patients don’t care as much about the scalp hair loss because they’ve figured out how to deal with it. What really bothers some patients is their eyebrow hair loss. You can think of situations where alopecia areata creates a circle in the middle of the eyebrow on the left side, but not the right, or you lose one eyebrow but not the other. We use techniques such as intralesional steroids. If there’s some hair growth present that’s lightly pigmented, we may apply topical minoxidil or Latisse [bimatoprost] to the brow area. Patients may also do microblading.
DN: Are there eyebrow prosthetics?
Dr. Hordinsky: Yes, there are. The National Alopecia Areata Foundation provides a lot of information to patients and providers about these devices. There are devices that you can tape on your brow area. Some don’t look great cosmetically, but some look fantastic. Microblading may create the most normal appearance.
DN: What about eyelashes?
Dr. Hordinsky: Eyelash loss is tough and really bothersome to patients if they don’t wear glasses because of the protection provided by eyelashes, such as when you blink against airborne dust. If there is some hair present in the eyebrow regions, one can try to regrow hair and use something that’s safe in that region, like topical Latisse. These treatments have to be tried for a couple of months before you say yea or nay, and you and the patient have to have reasonable expectations.
DN: What about men’s beards?
Dr. Hordinsky: You can treat those areas with topical or intralesional steroids. My own experience is that you have to use intralesional steroids, and overall, this form of alopecia areata may be one of the most difficult to manage successfully.
DN: Do men complain about missing chest hair?
Dr. Hordinsky: Chest hair doesn’t come up a lot for me. For men, it’s mainly the beard area. But people with alopecia areata may sometimes minimize or not bring up discussion of loss of hair in the underarms, the genital region, or the chest. It may be because they’ve figured out how to deal with it.
DN: How do you think treatments will improve over the next 5-10 years?
Dr. Hordinsky: We have a number of companies putting JAK inhibitors into clinical trials. A major step forward will be figuring out which one works the best, and then the next hurdle will be sustainability. There are very few studies in alopecia areata about how long a response to treatment can be maintained. Another big step will be the development of a topical agent that is able to penetrate through the skin to the level of the immune attack at the lower part of the hair follicle and provide the opportunity to possibly not only grow hair but also to maintain hair growth. So there’s a lot of evolution going on right now.
Dr. Hordinsky disclosed consulting work with Procter & Gamble, Concert, and Cassiopea, and grant/research support from Aclaris, National Alopecia Areata Foundation, Allergan.
SDEF and this news organization are owned by the same parent company.
LAS VEGAS – Alopecia is hard to bear for patients and has been difficult to treat, but “,” according to Maria Hordinsky, MD.
Dr. Hordinsky, professor and chair of the department of dermatology, University of Minnesota, Minneapolis, discussed hair disorders in multiple presentations at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar. In an interview after her session on alopecia areata, she elaborated on the state of research and treatment for patients with this diagnosis.
DERMATOLOGY NEWS: What has changed in alopecia areata treatment over the last few years?
Dr. Hordinsky: There is still no Food and Drug Administration–approved treatment for this disease. But recent studies have helped us understand how alopecia areata occurs, how important interleukin-15 is, and how to target this cytokine as well as others. At the same time, expertise has developed at several centers across the United States with using the Janus kinase inhibitor tofacitinib [Xeljanz] at 5 milligrams twice a day. This has led to more off-label use. Concurrently, many pharmaceutical companies with interest in alopecia areata have begun to study different JAK inhibitors that target different cytokine receptors. Researchers also are exploring topical JAK inhibitors.
DN: What about the high costs of these drugs?
Dr. Hordinsky: Some insurers are covering tofacitinib, and patient assistance programs are very helpful and beneficial. In my own practice, most patients taking these drugs are using those programs.
DN: Are any treatments being used less than in the past?
Dr. Hordinsky: We’re still using tools that we have in the toolbox because we don’t have an approved treatment. We use topical steroids and intralesional steroids. We also use prednisone as needed, and we use contact sensitization therapy.
DN: In your presentation, you talked about alopecia areata in body areas outside of the scalp. What should dermatologists know about the eyebrows in patients with alopecia areata?
Dr. Hordinsky: Some patients don’t care as much about the scalp hair loss because they’ve figured out how to deal with it. What really bothers some patients is their eyebrow hair loss. You can think of situations where alopecia areata creates a circle in the middle of the eyebrow on the left side, but not the right, or you lose one eyebrow but not the other. We use techniques such as intralesional steroids. If there’s some hair growth present that’s lightly pigmented, we may apply topical minoxidil or Latisse [bimatoprost] to the brow area. Patients may also do microblading.
DN: Are there eyebrow prosthetics?
Dr. Hordinsky: Yes, there are. The National Alopecia Areata Foundation provides a lot of information to patients and providers about these devices. There are devices that you can tape on your brow area. Some don’t look great cosmetically, but some look fantastic. Microblading may create the most normal appearance.
DN: What about eyelashes?
Dr. Hordinsky: Eyelash loss is tough and really bothersome to patients if they don’t wear glasses because of the protection provided by eyelashes, such as when you blink against airborne dust. If there is some hair present in the eyebrow regions, one can try to regrow hair and use something that’s safe in that region, like topical Latisse. These treatments have to be tried for a couple of months before you say yea or nay, and you and the patient have to have reasonable expectations.
DN: What about men’s beards?
Dr. Hordinsky: You can treat those areas with topical or intralesional steroids. My own experience is that you have to use intralesional steroids, and overall, this form of alopecia areata may be one of the most difficult to manage successfully.
DN: Do men complain about missing chest hair?
Dr. Hordinsky: Chest hair doesn’t come up a lot for me. For men, it’s mainly the beard area. But people with alopecia areata may sometimes minimize or not bring up discussion of loss of hair in the underarms, the genital region, or the chest. It may be because they’ve figured out how to deal with it.
DN: How do you think treatments will improve over the next 5-10 years?
Dr. Hordinsky: We have a number of companies putting JAK inhibitors into clinical trials. A major step forward will be figuring out which one works the best, and then the next hurdle will be sustainability. There are very few studies in alopecia areata about how long a response to treatment can be maintained. Another big step will be the development of a topical agent that is able to penetrate through the skin to the level of the immune attack at the lower part of the hair follicle and provide the opportunity to possibly not only grow hair but also to maintain hair growth. So there’s a lot of evolution going on right now.
Dr. Hordinsky disclosed consulting work with Procter & Gamble, Concert, and Cassiopea, and grant/research support from Aclaris, National Alopecia Areata Foundation, Allergan.
SDEF and this news organization are owned by the same parent company.
LAS VEGAS – Alopecia is hard to bear for patients and has been difficult to treat, but “,” according to Maria Hordinsky, MD.
Dr. Hordinsky, professor and chair of the department of dermatology, University of Minnesota, Minneapolis, discussed hair disorders in multiple presentations at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar. In an interview after her session on alopecia areata, she elaborated on the state of research and treatment for patients with this diagnosis.
DERMATOLOGY NEWS: What has changed in alopecia areata treatment over the last few years?
Dr. Hordinsky: There is still no Food and Drug Administration–approved treatment for this disease. But recent studies have helped us understand how alopecia areata occurs, how important interleukin-15 is, and how to target this cytokine as well as others. At the same time, expertise has developed at several centers across the United States with using the Janus kinase inhibitor tofacitinib [Xeljanz] at 5 milligrams twice a day. This has led to more off-label use. Concurrently, many pharmaceutical companies with interest in alopecia areata have begun to study different JAK inhibitors that target different cytokine receptors. Researchers also are exploring topical JAK inhibitors.
DN: What about the high costs of these drugs?
Dr. Hordinsky: Some insurers are covering tofacitinib, and patient assistance programs are very helpful and beneficial. In my own practice, most patients taking these drugs are using those programs.
DN: Are any treatments being used less than in the past?
Dr. Hordinsky: We’re still using tools that we have in the toolbox because we don’t have an approved treatment. We use topical steroids and intralesional steroids. We also use prednisone as needed, and we use contact sensitization therapy.
DN: In your presentation, you talked about alopecia areata in body areas outside of the scalp. What should dermatologists know about the eyebrows in patients with alopecia areata?
Dr. Hordinsky: Some patients don’t care as much about the scalp hair loss because they’ve figured out how to deal with it. What really bothers some patients is their eyebrow hair loss. You can think of situations where alopecia areata creates a circle in the middle of the eyebrow on the left side, but not the right, or you lose one eyebrow but not the other. We use techniques such as intralesional steroids. If there’s some hair growth present that’s lightly pigmented, we may apply topical minoxidil or Latisse [bimatoprost] to the brow area. Patients may also do microblading.
DN: Are there eyebrow prosthetics?
Dr. Hordinsky: Yes, there are. The National Alopecia Areata Foundation provides a lot of information to patients and providers about these devices. There are devices that you can tape on your brow area. Some don’t look great cosmetically, but some look fantastic. Microblading may create the most normal appearance.
DN: What about eyelashes?
Dr. Hordinsky: Eyelash loss is tough and really bothersome to patients if they don’t wear glasses because of the protection provided by eyelashes, such as when you blink against airborne dust. If there is some hair present in the eyebrow regions, one can try to regrow hair and use something that’s safe in that region, like topical Latisse. These treatments have to be tried for a couple of months before you say yea or nay, and you and the patient have to have reasonable expectations.
DN: What about men’s beards?
Dr. Hordinsky: You can treat those areas with topical or intralesional steroids. My own experience is that you have to use intralesional steroids, and overall, this form of alopecia areata may be one of the most difficult to manage successfully.
DN: Do men complain about missing chest hair?
Dr. Hordinsky: Chest hair doesn’t come up a lot for me. For men, it’s mainly the beard area. But people with alopecia areata may sometimes minimize or not bring up discussion of loss of hair in the underarms, the genital region, or the chest. It may be because they’ve figured out how to deal with it.
DN: How do you think treatments will improve over the next 5-10 years?
Dr. Hordinsky: We have a number of companies putting JAK inhibitors into clinical trials. A major step forward will be figuring out which one works the best, and then the next hurdle will be sustainability. There are very few studies in alopecia areata about how long a response to treatment can be maintained. Another big step will be the development of a topical agent that is able to penetrate through the skin to the level of the immune attack at the lower part of the hair follicle and provide the opportunity to possibly not only grow hair but also to maintain hair growth. So there’s a lot of evolution going on right now.
Dr. Hordinsky disclosed consulting work with Procter & Gamble, Concert, and Cassiopea, and grant/research support from Aclaris, National Alopecia Areata Foundation, Allergan.
SDEF and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF LAS VEGAS DERMATOLOGY SEMINAR
Mole near nose
The differential diagnosis for this lesion included a benign intradermal nevus and a basal cell carcinoma. So the FP recommended a shave biopsy to be sure that it was not cancer. (See the Watch & Learn video on “Shave biopsy.”)
After obtaining patient consent, he injected the 1% lidocaine with epinephrine and waited 5 minutes for the epinephrine to begin to work. He performed the shave with a Dermablade, and used a cotton-tipped applicator to apply aluminum chloride to the site. He used a twisting motion and pressure to achieve hemostasis. He dressed the lesion with petrolatum and some gauze.
Dermatopathology showed that this mole was a benign intradermal nevus. The FP reassured the patient and recommended that she be careful to avoid sun exposure.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Basal cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:989-998.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The differential diagnosis for this lesion included a benign intradermal nevus and a basal cell carcinoma. So the FP recommended a shave biopsy to be sure that it was not cancer. (See the Watch & Learn video on “Shave biopsy.”)
After obtaining patient consent, he injected the 1% lidocaine with epinephrine and waited 5 minutes for the epinephrine to begin to work. He performed the shave with a Dermablade, and used a cotton-tipped applicator to apply aluminum chloride to the site. He used a twisting motion and pressure to achieve hemostasis. He dressed the lesion with petrolatum and some gauze.
Dermatopathology showed that this mole was a benign intradermal nevus. The FP reassured the patient and recommended that she be careful to avoid sun exposure.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Basal cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:989-998.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The differential diagnosis for this lesion included a benign intradermal nevus and a basal cell carcinoma. So the FP recommended a shave biopsy to be sure that it was not cancer. (See the Watch & Learn video on “Shave biopsy.”)
After obtaining patient consent, he injected the 1% lidocaine with epinephrine and waited 5 minutes for the epinephrine to begin to work. He performed the shave with a Dermablade, and used a cotton-tipped applicator to apply aluminum chloride to the site. He used a twisting motion and pressure to achieve hemostasis. He dressed the lesion with petrolatum and some gauze.
Dermatopathology showed that this mole was a benign intradermal nevus. The FP reassured the patient and recommended that she be careful to avoid sun exposure.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Basal cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:989-998.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
Novel topical JAK inhibitor shows promise for atopic dermatitis
PARIS – A cream formulation of ruxolitinib, a selective inhibitor of Janus kinase (JAK) 1 and 2, outperformed triamcinolone cream 0.1% and vehicle control in a large, phase 2, dose-ranging, randomized trial in patients with atopic dermatitis (AD), Brian S. Kim, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
This novel topical JAK inhibitor not only modulates inflammatory cytokines involved in the pathogenesis of AD, including interleukin-4, -5, -13, and -31, but Dr. Kim and his coinvestigators also demonstrated that ruxolitinib has antipruritic effects achieved by acting directly on sensory nerve fibers.
“Ultimately, said Dr. Kim, a dermatologist and codirector of the Center for the Study of Itch at Washington University, St. Louis.
The trial included 307 adults, mean age 35 years, with a median 21-year disease history and a mean of 7.3 flares within the past 12 months. Dr. Kim characterized the study population as having AD of “high-moderate” severity, with a mean involved body surface area of 9.7%, half of patients having a baseline Eczema Area and Severity Index (EASI) score greater than 7, and having a mean itch numeric rating scale of 7. Two-thirds of patients had an Investigator’s Global Assessment (IGA) score of 3 and the rest had scores of 2.
Patients were randomized to one of six study arms entailing 8 weeks of double-blind therapy: ruxolitinib cream 1.5% once daily, 1.5% twice daily; 0.5% once daily; 0.15% once daily; twice-daily vehicle; or triamcinolone cream 0.1% twice a day for 4 weeks followed by 4 weeks of vehicle.
All the ruxolitinib regimens provided dose- and time-dependent efficacy, compared with vehicle. The best results were seen with ruxolitinib 1.5% twice daily, which outperformed triamcinolone cream.
The primary study endpoint was change in EASI score from baseline to week 4, but the week 2 and week 8 data were also informative. Key secondary endpoints included the proportion of subjects achieving an EASI-75 response and/or an IGA response, which required improvement to an IGA score of 0 or 1 with at least a 2-point reduction from baseline.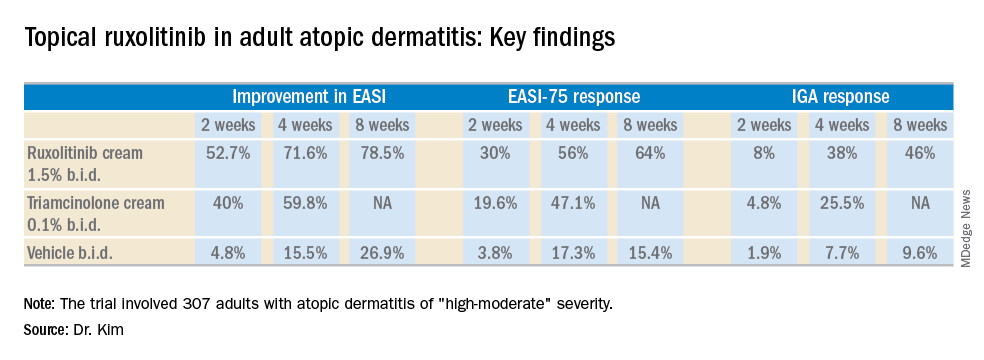
As for itch, ruxolitinib cream provided rapid and sustained improvement, said Dr. Kim. Indeed, within the first 2 days of the study, the ruxolitinib 1.5% twice-daily group had a mean 1.8-point reduction on the numeric rating scale, compared with a 0.2-point drop with vehicle and a 1-point drop with triamcinolone cream twice a day. By week 4, the twice-daily ruxolitinib 1.5% group had about a 4-point drop from baseline, the once-daily ruxolitinib 1.5% group had a 3.5-point drop, and the triamcinolone-treated patients had a 2.5-point drop.
Topical ruxolitinib was not associated with any significant safety or tolerability issues, and there were no clinically significant application site reactions, according to the dermatologist.
Session cochair Konstantine Buxtorf Friedli, MD, a Swiss dermatologist, commented that she could easily imagine this topical JAK inhibitor also being useful in other diseases with itch.
Dr. Kim reported serving as a consultant to and recipient of research funding from Incyte, which sponsored the study.
PARIS – A cream formulation of ruxolitinib, a selective inhibitor of Janus kinase (JAK) 1 and 2, outperformed triamcinolone cream 0.1% and vehicle control in a large, phase 2, dose-ranging, randomized trial in patients with atopic dermatitis (AD), Brian S. Kim, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
This novel topical JAK inhibitor not only modulates inflammatory cytokines involved in the pathogenesis of AD, including interleukin-4, -5, -13, and -31, but Dr. Kim and his coinvestigators also demonstrated that ruxolitinib has antipruritic effects achieved by acting directly on sensory nerve fibers.
“Ultimately, said Dr. Kim, a dermatologist and codirector of the Center for the Study of Itch at Washington University, St. Louis.
The trial included 307 adults, mean age 35 years, with a median 21-year disease history and a mean of 7.3 flares within the past 12 months. Dr. Kim characterized the study population as having AD of “high-moderate” severity, with a mean involved body surface area of 9.7%, half of patients having a baseline Eczema Area and Severity Index (EASI) score greater than 7, and having a mean itch numeric rating scale of 7. Two-thirds of patients had an Investigator’s Global Assessment (IGA) score of 3 and the rest had scores of 2.
Patients were randomized to one of six study arms entailing 8 weeks of double-blind therapy: ruxolitinib cream 1.5% once daily, 1.5% twice daily; 0.5% once daily; 0.15% once daily; twice-daily vehicle; or triamcinolone cream 0.1% twice a day for 4 weeks followed by 4 weeks of vehicle.
All the ruxolitinib regimens provided dose- and time-dependent efficacy, compared with vehicle. The best results were seen with ruxolitinib 1.5% twice daily, which outperformed triamcinolone cream.
The primary study endpoint was change in EASI score from baseline to week 4, but the week 2 and week 8 data were also informative. Key secondary endpoints included the proportion of subjects achieving an EASI-75 response and/or an IGA response, which required improvement to an IGA score of 0 or 1 with at least a 2-point reduction from baseline.
As for itch, ruxolitinib cream provided rapid and sustained improvement, said Dr. Kim. Indeed, within the first 2 days of the study, the ruxolitinib 1.5% twice-daily group had a mean 1.8-point reduction on the numeric rating scale, compared with a 0.2-point drop with vehicle and a 1-point drop with triamcinolone cream twice a day. By week 4, the twice-daily ruxolitinib 1.5% group had about a 4-point drop from baseline, the once-daily ruxolitinib 1.5% group had a 3.5-point drop, and the triamcinolone-treated patients had a 2.5-point drop.
Topical ruxolitinib was not associated with any significant safety or tolerability issues, and there were no clinically significant application site reactions, according to the dermatologist.
Session cochair Konstantine Buxtorf Friedli, MD, a Swiss dermatologist, commented that she could easily imagine this topical JAK inhibitor also being useful in other diseases with itch.
Dr. Kim reported serving as a consultant to and recipient of research funding from Incyte, which sponsored the study.
PARIS – A cream formulation of ruxolitinib, a selective inhibitor of Janus kinase (JAK) 1 and 2, outperformed triamcinolone cream 0.1% and vehicle control in a large, phase 2, dose-ranging, randomized trial in patients with atopic dermatitis (AD), Brian S. Kim, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
This novel topical JAK inhibitor not only modulates inflammatory cytokines involved in the pathogenesis of AD, including interleukin-4, -5, -13, and -31, but Dr. Kim and his coinvestigators also demonstrated that ruxolitinib has antipruritic effects achieved by acting directly on sensory nerve fibers.
“Ultimately, said Dr. Kim, a dermatologist and codirector of the Center for the Study of Itch at Washington University, St. Louis.
The trial included 307 adults, mean age 35 years, with a median 21-year disease history and a mean of 7.3 flares within the past 12 months. Dr. Kim characterized the study population as having AD of “high-moderate” severity, with a mean involved body surface area of 9.7%, half of patients having a baseline Eczema Area and Severity Index (EASI) score greater than 7, and having a mean itch numeric rating scale of 7. Two-thirds of patients had an Investigator’s Global Assessment (IGA) score of 3 and the rest had scores of 2.
Patients were randomized to one of six study arms entailing 8 weeks of double-blind therapy: ruxolitinib cream 1.5% once daily, 1.5% twice daily; 0.5% once daily; 0.15% once daily; twice-daily vehicle; or triamcinolone cream 0.1% twice a day for 4 weeks followed by 4 weeks of vehicle.
All the ruxolitinib regimens provided dose- and time-dependent efficacy, compared with vehicle. The best results were seen with ruxolitinib 1.5% twice daily, which outperformed triamcinolone cream.
The primary study endpoint was change in EASI score from baseline to week 4, but the week 2 and week 8 data were also informative. Key secondary endpoints included the proportion of subjects achieving an EASI-75 response and/or an IGA response, which required improvement to an IGA score of 0 or 1 with at least a 2-point reduction from baseline.
As for itch, ruxolitinib cream provided rapid and sustained improvement, said Dr. Kim. Indeed, within the first 2 days of the study, the ruxolitinib 1.5% twice-daily group had a mean 1.8-point reduction on the numeric rating scale, compared with a 0.2-point drop with vehicle and a 1-point drop with triamcinolone cream twice a day. By week 4, the twice-daily ruxolitinib 1.5% group had about a 4-point drop from baseline, the once-daily ruxolitinib 1.5% group had a 3.5-point drop, and the triamcinolone-treated patients had a 2.5-point drop.
Topical ruxolitinib was not associated with any significant safety or tolerability issues, and there were no clinically significant application site reactions, according to the dermatologist.
Session cochair Konstantine Buxtorf Friedli, MD, a Swiss dermatologist, commented that she could easily imagine this topical JAK inhibitor also being useful in other diseases with itch.
Dr. Kim reported serving as a consultant to and recipient of research funding from Incyte, which sponsored the study.
REPORTING FROM THE EADV CONGRESS
Key clinical point: A novel topical Janus kinase inhibitor may provide a valuable alternative to potent topical steroids in atopic dermatitis.
Major finding: At week 4, the mean improvement in Eczema Area and Severity Index score was 72% with ruxolitinib cream 1.5% twice a day, compared with 60% with triamcinolone cream 0.1% twice a day.
Study details: This 8-week, phase 2 clinical trial included 307 adult atopic dermatitis patients randomized to ruxolitinib cream, triamcinolone cream, or vehicle.
Disclosures: The study was sponsored by Incyte. The presenter reported serving as a consultant to and recipient of research funding from the company.
Second-melanoma risk higher with indoor tanning
than those who avoid indoor tanning, according to a retrospective study involving 434 melanoma patients.

The incidence of second melanomas over the entire 16-year course of the study was 25.2% among the tanning-bed users and 18.6% for nonusers. Among these study subjects – 27 with tanning-bed exposure and 61 without – median time to the second tumor was 225 days (0.62 years) for exposed patients and 1,280 days (3.50 years) for those with no exposure, the investigators reported.
This study, they wrote, is the first to show that “patients who had second primary melanoma diagnoses were more likely to have had” exposure to artificial UVR. The increased radiation intensity of tanning beds, “as opposed to UVR from ambient sunlight, in a physiologically vulnerable patient population [fair-skinned persons] at an early age contributes to our findings of decreased tumor lag time.”
SOURCE: Li Y et al. J Am Acad Dermatol. 2018;79(6):1101-8.
than those who avoid indoor tanning, according to a retrospective study involving 434 melanoma patients.

The incidence of second melanomas over the entire 16-year course of the study was 25.2% among the tanning-bed users and 18.6% for nonusers. Among these study subjects – 27 with tanning-bed exposure and 61 without – median time to the second tumor was 225 days (0.62 years) for exposed patients and 1,280 days (3.50 years) for those with no exposure, the investigators reported.
This study, they wrote, is the first to show that “patients who had second primary melanoma diagnoses were more likely to have had” exposure to artificial UVR. The increased radiation intensity of tanning beds, “as opposed to UVR from ambient sunlight, in a physiologically vulnerable patient population [fair-skinned persons] at an early age contributes to our findings of decreased tumor lag time.”
SOURCE: Li Y et al. J Am Acad Dermatol. 2018;79(6):1101-8.
than those who avoid indoor tanning, according to a retrospective study involving 434 melanoma patients.

The incidence of second melanomas over the entire 16-year course of the study was 25.2% among the tanning-bed users and 18.6% for nonusers. Among these study subjects – 27 with tanning-bed exposure and 61 without – median time to the second tumor was 225 days (0.62 years) for exposed patients and 1,280 days (3.50 years) for those with no exposure, the investigators reported.
This study, they wrote, is the first to show that “patients who had second primary melanoma diagnoses were more likely to have had” exposure to artificial UVR. The increased radiation intensity of tanning beds, “as opposed to UVR from ambient sunlight, in a physiologically vulnerable patient population [fair-skinned persons] at an early age contributes to our findings of decreased tumor lag time.”
SOURCE: Li Y et al. J Am Acad Dermatol. 2018;79(6):1101-8.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
AEDs strongly linked to rare serious skin reactions
Antiepileptic drugs were found to be linked with almost ninefold increased odds for two adverse skin reactions, Steven‐Johnson syndrome and toxic epidermal necrolysis, compared with non-AED medication classes in an analysis of adverse-event data from the Food and Drug Administration Adverse Event Reporting System.
Researchers at the University of Rhode Island College of Pharmacy in Kingston, who performed the retrospective study, also found that six drugs within the antiepileptic drug (AED) class had a reporting odds ratio estimate of more than 20, compared with other non-AEDs.
“Although several antiepileptic drugs have been associated with Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), the class effect and impact of other AEDs are not well described,” Eric P. Borrelli, PharmD, and his colleagues reported in Epilepsia.
The investigators examined rates of SJS and TEN for several AEDs using adverse event data from the Food and Drug Administration Adverse Event Reporting System between July 2014 and December 2017. The study investigators examined 198 adverse reaction reports related to AEDs, which was greater than any other drug class.
Overall, AEDs as a group had a reporting odds ratio risk estimate of 8.7 (95% confidence interval, 7.5-10.2), compared with non-AEDs. Similarly, the proportional reporting ratio was found to be 8.7 (95% CI, 7.5-10.2) in the AED group.
Within the class, the medications with the highest risk were zonisamide, rufinamide, and clorazepate, which had about 70-, 60-, and 56-fold higher odds for SJS and TEN, compared with all other medications. Other high-risk AEDs in the group included lamotrigine (reporting odds ratio, 53.0), carbamazepine (reporting OR, 24.5), and phenytoin (reporting OR, 26.3).
“Greater than 90% of SJS [and] TEN reactions associated with AEDs occur within the first 2 months of treatment initiation, although some AEDs have been associated with such reactions during long‐term use,” the researchers wrote.
The authors acknowledged that measures of prevalence and incidence could not be determined from these data since the number of patients taking AEDs is unknown.
“Increased awareness of this risk among both prescribers and patients, particularly variations in risk among different AEDs, along with education on early recognition of SJS [and] TEN signs [and] symptoms, may help mitigate the number and severity of these adverse events,” the researchers concluded.
The study was partially funded by the Department of Veterans Affairs. One coauthor reported receiving research funding from Pfizer, Merck (Cubist), and The Medicines Company.
SOURCE: Borrelli EP et al. Epilepsia. 2018 Nov 5. doi: 10.1111/epi.14591.
Antiepileptic drugs were found to be linked with almost ninefold increased odds for two adverse skin reactions, Steven‐Johnson syndrome and toxic epidermal necrolysis, compared with non-AED medication classes in an analysis of adverse-event data from the Food and Drug Administration Adverse Event Reporting System.
Researchers at the University of Rhode Island College of Pharmacy in Kingston, who performed the retrospective study, also found that six drugs within the antiepileptic drug (AED) class had a reporting odds ratio estimate of more than 20, compared with other non-AEDs.
“Although several antiepileptic drugs have been associated with Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), the class effect and impact of other AEDs are not well described,” Eric P. Borrelli, PharmD, and his colleagues reported in Epilepsia.
The investigators examined rates of SJS and TEN for several AEDs using adverse event data from the Food and Drug Administration Adverse Event Reporting System between July 2014 and December 2017. The study investigators examined 198 adverse reaction reports related to AEDs, which was greater than any other drug class.
Overall, AEDs as a group had a reporting odds ratio risk estimate of 8.7 (95% confidence interval, 7.5-10.2), compared with non-AEDs. Similarly, the proportional reporting ratio was found to be 8.7 (95% CI, 7.5-10.2) in the AED group.
Within the class, the medications with the highest risk were zonisamide, rufinamide, and clorazepate, which had about 70-, 60-, and 56-fold higher odds for SJS and TEN, compared with all other medications. Other high-risk AEDs in the group included lamotrigine (reporting odds ratio, 53.0), carbamazepine (reporting OR, 24.5), and phenytoin (reporting OR, 26.3).
“Greater than 90% of SJS [and] TEN reactions associated with AEDs occur within the first 2 months of treatment initiation, although some AEDs have been associated with such reactions during long‐term use,” the researchers wrote.
The authors acknowledged that measures of prevalence and incidence could not be determined from these data since the number of patients taking AEDs is unknown.
“Increased awareness of this risk among both prescribers and patients, particularly variations in risk among different AEDs, along with education on early recognition of SJS [and] TEN signs [and] symptoms, may help mitigate the number and severity of these adverse events,” the researchers concluded.
The study was partially funded by the Department of Veterans Affairs. One coauthor reported receiving research funding from Pfizer, Merck (Cubist), and The Medicines Company.
SOURCE: Borrelli EP et al. Epilepsia. 2018 Nov 5. doi: 10.1111/epi.14591.
Antiepileptic drugs were found to be linked with almost ninefold increased odds for two adverse skin reactions, Steven‐Johnson syndrome and toxic epidermal necrolysis, compared with non-AED medication classes in an analysis of adverse-event data from the Food and Drug Administration Adverse Event Reporting System.
Researchers at the University of Rhode Island College of Pharmacy in Kingston, who performed the retrospective study, also found that six drugs within the antiepileptic drug (AED) class had a reporting odds ratio estimate of more than 20, compared with other non-AEDs.
“Although several antiepileptic drugs have been associated with Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), the class effect and impact of other AEDs are not well described,” Eric P. Borrelli, PharmD, and his colleagues reported in Epilepsia.
The investigators examined rates of SJS and TEN for several AEDs using adverse event data from the Food and Drug Administration Adverse Event Reporting System between July 2014 and December 2017. The study investigators examined 198 adverse reaction reports related to AEDs, which was greater than any other drug class.
Overall, AEDs as a group had a reporting odds ratio risk estimate of 8.7 (95% confidence interval, 7.5-10.2), compared with non-AEDs. Similarly, the proportional reporting ratio was found to be 8.7 (95% CI, 7.5-10.2) in the AED group.
Within the class, the medications with the highest risk were zonisamide, rufinamide, and clorazepate, which had about 70-, 60-, and 56-fold higher odds for SJS and TEN, compared with all other medications. Other high-risk AEDs in the group included lamotrigine (reporting odds ratio, 53.0), carbamazepine (reporting OR, 24.5), and phenytoin (reporting OR, 26.3).
“Greater than 90% of SJS [and] TEN reactions associated with AEDs occur within the first 2 months of treatment initiation, although some AEDs have been associated with such reactions during long‐term use,” the researchers wrote.
The authors acknowledged that measures of prevalence and incidence could not be determined from these data since the number of patients taking AEDs is unknown.
“Increased awareness of this risk among both prescribers and patients, particularly variations in risk among different AEDs, along with education on early recognition of SJS [and] TEN signs [and] symptoms, may help mitigate the number and severity of these adverse events,” the researchers concluded.
The study was partially funded by the Department of Veterans Affairs. One coauthor reported receiving research funding from Pfizer, Merck (Cubist), and The Medicines Company.
SOURCE: Borrelli EP et al. Epilepsia. 2018 Nov 5. doi: 10.1111/epi.14591.
FROM EPILEPSIA
Key clinical point:
Major finding: The reporting odds ratio risk estimate for SJS and TEN in the AED group was 8.7 (95% CI, 7.5-10.2), compared with non-AEDs.
Study details: A retrospective analysis of 198 adverse reaction reports from the Food and Drug Administration Adverse Event Reporting System.
Disclosures: The study was partially funded by the Department of Veterans Affairs. One coauthor reported receiving research funding from Pfizer, Merck (Cubist), and The Medicines Company.
Source: Borrelli EP et al. Epilepsia. 2018 Nov 5. doi: 10.1111/epi.14591.
Multiple growths on face
Figure 1
While the differential diagnosis for these lesions (FIGURE 1A) included basal cell carcinoma, the FP had reason to suspect that these papules were actually sebaceous hyperplasia.
The FP saw a pattern of crown-like vessels and popcorn-like structures (FIGURE 1B) when he examined the patient with his dermatoscope. None of the vessels crossed the midline, and the popcorn-like structures were hyperplastic sebaceous glands. The FP photographed the largest lesion with a dermatoscope attached to his smart phone and showed the reassuring pattern to the anxious patient. He explained to her why this was not skin cancer and how hyperplasia of sebaceous glands is often normal for aging facial skin. He also offered her treatment if she thought that the lesions were cosmetically unappealing.
The patient said that she would be grateful to have the largest lesion treated because it did bother her when she looked in the mirror. The FP treated this lesion using electrosurgery with a blunt tipped electrode, on a setting of 2.1, without anesthesia. He warned the patient before he applied the activated electrode to the skin, and the patient tolerated the procedure well. The sebaceous glands melted easily with the current. The result appeared gray and was expected to heal with minimal to no scarring. At a future visit, the patient said that she was happy with the result and asked if additional lesions could be treated with the same electrosurgical approach.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Basal cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:989-998.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
Figure 1

While the differential diagnosis for these lesions (FIGURE 1A) included basal cell carcinoma, the FP had reason to suspect that these papules were actually sebaceous hyperplasia.
The FP saw a pattern of crown-like vessels and popcorn-like structures (FIGURE 1B) when he examined the patient with his dermatoscope. None of the vessels crossed the midline, and the popcorn-like structures were hyperplastic sebaceous glands. The FP photographed the largest lesion with a dermatoscope attached to his smart phone and showed the reassuring pattern to the anxious patient. He explained to her why this was not skin cancer and how hyperplasia of sebaceous glands is often normal for aging facial skin. He also offered her treatment if she thought that the lesions were cosmetically unappealing.
The patient said that she would be grateful to have the largest lesion treated because it did bother her when she looked in the mirror. The FP treated this lesion using electrosurgery with a blunt tipped electrode, on a setting of 2.1, without anesthesia. He warned the patient before he applied the activated electrode to the skin, and the patient tolerated the procedure well. The sebaceous glands melted easily with the current. The result appeared gray and was expected to heal with minimal to no scarring. At a future visit, the patient said that she was happy with the result and asked if additional lesions could be treated with the same electrosurgical approach.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Basal cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:989-998.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
Figure 1

While the differential diagnosis for these lesions (FIGURE 1A) included basal cell carcinoma, the FP had reason to suspect that these papules were actually sebaceous hyperplasia.
The FP saw a pattern of crown-like vessels and popcorn-like structures (FIGURE 1B) when he examined the patient with his dermatoscope. None of the vessels crossed the midline, and the popcorn-like structures were hyperplastic sebaceous glands. The FP photographed the largest lesion with a dermatoscope attached to his smart phone and showed the reassuring pattern to the anxious patient. He explained to her why this was not skin cancer and how hyperplasia of sebaceous glands is often normal for aging facial skin. He also offered her treatment if she thought that the lesions were cosmetically unappealing.
The patient said that she would be grateful to have the largest lesion treated because it did bother her when she looked in the mirror. The FP treated this lesion using electrosurgery with a blunt tipped electrode, on a setting of 2.1, without anesthesia. He warned the patient before he applied the activated electrode to the skin, and the patient tolerated the procedure well. The sebaceous glands melted easily with the current. The result appeared gray and was expected to heal with minimal to no scarring. At a future visit, the patient said that she was happy with the result and asked if additional lesions could be treated with the same electrosurgical approach.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Basal cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:989-998.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
Tofacitinib impresses in first trial for dermatomyositis
CHICAGO – Oral tofacitinib demonstrated strong clinical efficacy and good safety in the first-ever formal study of a Janus kinase inhibitor in patients with active, treatment-resistant dermatomyositis, Julie J. Paik, MD, reported at the annual meeting of the American College of Rheumatology.
Based upon the encouraging results of this small, open-label, proof-of-concept study, a larger randomized controlled trial is being planned, according to Dr. Paik, a rheumatologist at Johns Hopkins University in Baltimore.
The study included 10 dermatomyositis patients enrolled at the Johns Hopkins Myositis Clinic. All had previously failed at least two steroid-sparing drugs and/or high-dose steroids. After first going off all steroid-sparing agents and being limited to a maximum of 20 mg/day of prednisone, the participants were placed on 11 mg of open-label, extended-release tofacitinib (Xeljanz XR) once daily. Dr. Paik only reported results in 9 patients because the 10th hadn’t yet reached the 12-week mark.
The primary outcome was the rate of achievement of significant improvement at week 12 as defined using the validated International Myositis Assessment and Clinical Studies (IMACS) criteria, which require at least a 20% improvement in three of six core set measures coupled with no more than two measures worsening by 25% or more. By this standard, all nine patients met the primary endpoint. Five were rated as showing moderate improvement, and the other four demonstrated minimal improvement, based on the Total Improvement Score of the Myositis Response criteria. The median Total Improvement Score was 40, indicative of at least moderate improvement.
The mean CDASI (Cutaneous Dermatomyositis Disease Area and Severity Index) activity score – a key secondary endpoint – improved from 28 at baseline to 9.5 at week 12, which translates to a shift from moderate or severe disease to mild disease.
Levels of the alpha-chemokines CXCL9 and CXCL10, which are expressed in muscle affected by idiopathic inflammatory myopathies such as dermatomyositis, declined strongly over the course of 12 weeks of treatment to an extent that was just shy of statistical significance.
Myositis antibody titers didn’t change in response to tofacitinib therapy. Of note, six patients were positive for antitranscriptional intermediary factor 1-gamma, and five of the six were moderate responders to JAK inhibitor therapy.
Four patients were on 20 mg/day of prednisone at baseline; three of the four were able to go off steroids altogether over the course of 12 weeks.
The treatment was well tolerated, with no serious adverse events.
Asked if she thought 11 mg/day of tofacitinib was the right dose for this population of refractory dermatomyositis patients, Dr. Paik replied, “We’re not sure we have the right dose. We had one patient who didn’t have as robust a response, and I really wonder, if we gave her a higher dose, it would have made a difference.”
Extended-release tofacitinib at 11 mg/day is the approved dose for rheumatoid arthritis and psoriatic arthritis. However, 20 mg/day is approved for patients with ulcerative colitis, and Dr. Paik is lobbying for inclusion of a higher-dose arm in the randomized, controlled trial.
Prior to this formal proof-of-concept study, which was funded by Pfizer, the experience with tofacitinib in refractory dermatomyositis was limited to anecdotal case reports.
Dr. Paik reported having no financial conflicts.
SOURCE: Paik JJ et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract L02.
CHICAGO – Oral tofacitinib demonstrated strong clinical efficacy and good safety in the first-ever formal study of a Janus kinase inhibitor in patients with active, treatment-resistant dermatomyositis, Julie J. Paik, MD, reported at the annual meeting of the American College of Rheumatology.
Based upon the encouraging results of this small, open-label, proof-of-concept study, a larger randomized controlled trial is being planned, according to Dr. Paik, a rheumatologist at Johns Hopkins University in Baltimore.
The study included 10 dermatomyositis patients enrolled at the Johns Hopkins Myositis Clinic. All had previously failed at least two steroid-sparing drugs and/or high-dose steroids. After first going off all steroid-sparing agents and being limited to a maximum of 20 mg/day of prednisone, the participants were placed on 11 mg of open-label, extended-release tofacitinib (Xeljanz XR) once daily. Dr. Paik only reported results in 9 patients because the 10th hadn’t yet reached the 12-week mark.
The primary outcome was the rate of achievement of significant improvement at week 12 as defined using the validated International Myositis Assessment and Clinical Studies (IMACS) criteria, which require at least a 20% improvement in three of six core set measures coupled with no more than two measures worsening by 25% or more. By this standard, all nine patients met the primary endpoint. Five were rated as showing moderate improvement, and the other four demonstrated minimal improvement, based on the Total Improvement Score of the Myositis Response criteria. The median Total Improvement Score was 40, indicative of at least moderate improvement.
The mean CDASI (Cutaneous Dermatomyositis Disease Area and Severity Index) activity score – a key secondary endpoint – improved from 28 at baseline to 9.5 at week 12, which translates to a shift from moderate or severe disease to mild disease.
Levels of the alpha-chemokines CXCL9 and CXCL10, which are expressed in muscle affected by idiopathic inflammatory myopathies such as dermatomyositis, declined strongly over the course of 12 weeks of treatment to an extent that was just shy of statistical significance.
Myositis antibody titers didn’t change in response to tofacitinib therapy. Of note, six patients were positive for antitranscriptional intermediary factor 1-gamma, and five of the six were moderate responders to JAK inhibitor therapy.
Four patients were on 20 mg/day of prednisone at baseline; three of the four were able to go off steroids altogether over the course of 12 weeks.
The treatment was well tolerated, with no serious adverse events.
Asked if she thought 11 mg/day of tofacitinib was the right dose for this population of refractory dermatomyositis patients, Dr. Paik replied, “We’re not sure we have the right dose. We had one patient who didn’t have as robust a response, and I really wonder, if we gave her a higher dose, it would have made a difference.”
Extended-release tofacitinib at 11 mg/day is the approved dose for rheumatoid arthritis and psoriatic arthritis. However, 20 mg/day is approved for patients with ulcerative colitis, and Dr. Paik is lobbying for inclusion of a higher-dose arm in the randomized, controlled trial.
Prior to this formal proof-of-concept study, which was funded by Pfizer, the experience with tofacitinib in refractory dermatomyositis was limited to anecdotal case reports.
Dr. Paik reported having no financial conflicts.
SOURCE: Paik JJ et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract L02.
CHICAGO – Oral tofacitinib demonstrated strong clinical efficacy and good safety in the first-ever formal study of a Janus kinase inhibitor in patients with active, treatment-resistant dermatomyositis, Julie J. Paik, MD, reported at the annual meeting of the American College of Rheumatology.
Based upon the encouraging results of this small, open-label, proof-of-concept study, a larger randomized controlled trial is being planned, according to Dr. Paik, a rheumatologist at Johns Hopkins University in Baltimore.
The study included 10 dermatomyositis patients enrolled at the Johns Hopkins Myositis Clinic. All had previously failed at least two steroid-sparing drugs and/or high-dose steroids. After first going off all steroid-sparing agents and being limited to a maximum of 20 mg/day of prednisone, the participants were placed on 11 mg of open-label, extended-release tofacitinib (Xeljanz XR) once daily. Dr. Paik only reported results in 9 patients because the 10th hadn’t yet reached the 12-week mark.
The primary outcome was the rate of achievement of significant improvement at week 12 as defined using the validated International Myositis Assessment and Clinical Studies (IMACS) criteria, which require at least a 20% improvement in three of six core set measures coupled with no more than two measures worsening by 25% or more. By this standard, all nine patients met the primary endpoint. Five were rated as showing moderate improvement, and the other four demonstrated minimal improvement, based on the Total Improvement Score of the Myositis Response criteria. The median Total Improvement Score was 40, indicative of at least moderate improvement.
The mean CDASI (Cutaneous Dermatomyositis Disease Area and Severity Index) activity score – a key secondary endpoint – improved from 28 at baseline to 9.5 at week 12, which translates to a shift from moderate or severe disease to mild disease.
Levels of the alpha-chemokines CXCL9 and CXCL10, which are expressed in muscle affected by idiopathic inflammatory myopathies such as dermatomyositis, declined strongly over the course of 12 weeks of treatment to an extent that was just shy of statistical significance.
Myositis antibody titers didn’t change in response to tofacitinib therapy. Of note, six patients were positive for antitranscriptional intermediary factor 1-gamma, and five of the six were moderate responders to JAK inhibitor therapy.
Four patients were on 20 mg/day of prednisone at baseline; three of the four were able to go off steroids altogether over the course of 12 weeks.
The treatment was well tolerated, with no serious adverse events.
Asked if she thought 11 mg/day of tofacitinib was the right dose for this population of refractory dermatomyositis patients, Dr. Paik replied, “We’re not sure we have the right dose. We had one patient who didn’t have as robust a response, and I really wonder, if we gave her a higher dose, it would have made a difference.”
Extended-release tofacitinib at 11 mg/day is the approved dose for rheumatoid arthritis and psoriatic arthritis. However, 20 mg/day is approved for patients with ulcerative colitis, and Dr. Paik is lobbying for inclusion of a higher-dose arm in the randomized, controlled trial.
Prior to this formal proof-of-concept study, which was funded by Pfizer, the experience with tofacitinib in refractory dermatomyositis was limited to anecdotal case reports.
Dr. Paik reported having no financial conflicts.
SOURCE: Paik JJ et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract L02.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point:
Major finding: Among patients with refractory dermatomyositis, the mean Cutaneous Dermatomyositis Disease Area and Severity Index activity score improved from 28 at baseline to 9.5 after 12 weeks on oral tofacitinib.
Study details: This first-in-class, open-label, 12-week study included 10 patients with refractory dermatomyositis placed on extended-release tofacitinib at 11 mg once daily.
Disclosures: The presenter reported having no financial conflicts regarding the study, sponsored by Pfizer.
Source: Paik JJ et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract L02
New pediatric therapies show promise for influenza, multidrug-resistant pathogens
ORLANDO – John S. Bradley, MD, said at the annual meeting of the American Academy of Pediatrics.
Dr. Bradley, director of the division of infectious diseases at Rady Children’s Hospital–San Diego, discussed a therapy for influenza, baloxavir, which was recently approved as a fast-acting single-dose medication and currently is under study in children. Also, a recent double-blind, phase 3 trial in the New England Journal of Medicine recruited patients as young as 12 years old. In the study, patients in the intervention group resolved their fever in median 25 hours, compared with 42 hours in the placebo group. Baloxavir better reduced viral load at day 2, compared with oseltamivir and placebo, but there was a similar alleviation of symptoms between both groups. There was a greater incidence of nausea and vomiting among the oseltamivir group, while the baloxavir group had a higher rate of diarrhea (N Engl J Med 2018;379:913-23).
However, Dr. Bradley noted baloxavir is much more expensive than oseltamivir, which may not justify the better tolerance of the drug for influenza treatment.
You don’t get better with it faster, so I’m not going to be recommending you all run to baloxavir this flu season for kids 12 years of age and older,” Dr. Bradley said. “I think oseltamivir is still fine, unless we end up with oseltamivir resistance.”
Solithromycin, an intravenous and oral fluoroketolide, has shown promising results against gram-positive and gram-negative pathogens for community-acquired pneumonia and other infections. During the drug’s study period, Cempra sold solithromycin to Melinta. However, one trial showed elevated liver functions in a higher number of patients than expected, and the Food and Drug Administration asked Melinta to conduct additional studies. Investigations on solithromycin have currently stopped until Melinta secures funding. “Until they get better resources, this particular drug is on hold, but you’ll see it again, I’m sure,” said Dr. Bradley, who also is professor and chief of the division of infectious diseases at the University of California, San Diego.
Dr. Bradley also discussed the efficacy of tedizolid, a protein synthesis inhibitor similar to linezolid approved in adults for the treatment of skin infections. He noted tedizolid is more active than linezolid, but the treatment course is a shorter dose for a shorter amount of time. Compared with linezolid, which can cause thrombocytopenia or neutropenia if taken for more than 10 days to 14 days, there also are fewer side effects.
“The tedizolid is much, much safer,” Dr. Bradley said, who added that trials for efficacy of tedizolid are currently underway in pediatric patients. “We’re hoping that will end up being the pediatric oxazolidinone.”
Other investigative therapies approved for adults and under study for use in children include ceftazidime/avibactam for treatment of urinary tract and complicated intra-abdominal infections, which is effective against meropenem-resistant Enterobacteriaceae and resistant Escherichia coli with extended-spectrum beta-lactamases (ESBL); ceftolozane/tazobactam has also been approved for adults, is pending approval in pediatric patients, and is active against ESBLs such as Pseudomonas; and meropenem/vaborbactam, which is active against Klebsiella pneumoniae carbapenemase (KPC)–producing isolates. Plazomicin, an aminoglycoside similar to gentamicin used to treat KPC-producing isolates, is stable against enzymes that degrade gentamicin and tobramycin.
Therapies currently under study for adults and being considered for children include imipenem/relebactam for treatment against E. coli, Enterobacter species, and KPC-producing isolates, and cefiderocol, a siderophore cephalosporin antibiotic – commonly described as a “Trojan horse” antibiotic because it binds to iron and is actively transported into the organism – is effective against Pseudomonas and has finished phase 2 trials in adults, with researchers looking to do single-dose trials in children, Dr. Bradley noted.
More experimentally, phage therapy for multidrug-resistant Acinetobacter baumannii proved effective in a 68-year-old patient with necrotizing pancreatitis who continued to deteriorate over a 4-month period despite multiple courses of antibiotics and attempted drainage of a pancreatic pseudocyst. Researchers selected a phage-specific bacterium with specificity for A. baumannii and cured him. “This is like science fiction,” Dr. Bradley said.
Dr. Bradley reported no relevant conflicts of interest.
ORLANDO – John S. Bradley, MD, said at the annual meeting of the American Academy of Pediatrics.
Dr. Bradley, director of the division of infectious diseases at Rady Children’s Hospital–San Diego, discussed a therapy for influenza, baloxavir, which was recently approved as a fast-acting single-dose medication and currently is under study in children. Also, a recent double-blind, phase 3 trial in the New England Journal of Medicine recruited patients as young as 12 years old. In the study, patients in the intervention group resolved their fever in median 25 hours, compared with 42 hours in the placebo group. Baloxavir better reduced viral load at day 2, compared with oseltamivir and placebo, but there was a similar alleviation of symptoms between both groups. There was a greater incidence of nausea and vomiting among the oseltamivir group, while the baloxavir group had a higher rate of diarrhea (N Engl J Med 2018;379:913-23).
However, Dr. Bradley noted baloxavir is much more expensive than oseltamivir, which may not justify the better tolerance of the drug for influenza treatment.
You don’t get better with it faster, so I’m not going to be recommending you all run to baloxavir this flu season for kids 12 years of age and older,” Dr. Bradley said. “I think oseltamivir is still fine, unless we end up with oseltamivir resistance.”
Solithromycin, an intravenous and oral fluoroketolide, has shown promising results against gram-positive and gram-negative pathogens for community-acquired pneumonia and other infections. During the drug’s study period, Cempra sold solithromycin to Melinta. However, one trial showed elevated liver functions in a higher number of patients than expected, and the Food and Drug Administration asked Melinta to conduct additional studies. Investigations on solithromycin have currently stopped until Melinta secures funding. “Until they get better resources, this particular drug is on hold, but you’ll see it again, I’m sure,” said Dr. Bradley, who also is professor and chief of the division of infectious diseases at the University of California, San Diego.
Dr. Bradley also discussed the efficacy of tedizolid, a protein synthesis inhibitor similar to linezolid approved in adults for the treatment of skin infections. He noted tedizolid is more active than linezolid, but the treatment course is a shorter dose for a shorter amount of time. Compared with linezolid, which can cause thrombocytopenia or neutropenia if taken for more than 10 days to 14 days, there also are fewer side effects.
“The tedizolid is much, much safer,” Dr. Bradley said, who added that trials for efficacy of tedizolid are currently underway in pediatric patients. “We’re hoping that will end up being the pediatric oxazolidinone.”
Other investigative therapies approved for adults and under study for use in children include ceftazidime/avibactam for treatment of urinary tract and complicated intra-abdominal infections, which is effective against meropenem-resistant Enterobacteriaceae and resistant Escherichia coli with extended-spectrum beta-lactamases (ESBL); ceftolozane/tazobactam has also been approved for adults, is pending approval in pediatric patients, and is active against ESBLs such as Pseudomonas; and meropenem/vaborbactam, which is active against Klebsiella pneumoniae carbapenemase (KPC)–producing isolates. Plazomicin, an aminoglycoside similar to gentamicin used to treat KPC-producing isolates, is stable against enzymes that degrade gentamicin and tobramycin.
Therapies currently under study for adults and being considered for children include imipenem/relebactam for treatment against E. coli, Enterobacter species, and KPC-producing isolates, and cefiderocol, a siderophore cephalosporin antibiotic – commonly described as a “Trojan horse” antibiotic because it binds to iron and is actively transported into the organism – is effective against Pseudomonas and has finished phase 2 trials in adults, with researchers looking to do single-dose trials in children, Dr. Bradley noted.
More experimentally, phage therapy for multidrug-resistant Acinetobacter baumannii proved effective in a 68-year-old patient with necrotizing pancreatitis who continued to deteriorate over a 4-month period despite multiple courses of antibiotics and attempted drainage of a pancreatic pseudocyst. Researchers selected a phage-specific bacterium with specificity for A. baumannii and cured him. “This is like science fiction,” Dr. Bradley said.
Dr. Bradley reported no relevant conflicts of interest.
ORLANDO – John S. Bradley, MD, said at the annual meeting of the American Academy of Pediatrics.
Dr. Bradley, director of the division of infectious diseases at Rady Children’s Hospital–San Diego, discussed a therapy for influenza, baloxavir, which was recently approved as a fast-acting single-dose medication and currently is under study in children. Also, a recent double-blind, phase 3 trial in the New England Journal of Medicine recruited patients as young as 12 years old. In the study, patients in the intervention group resolved their fever in median 25 hours, compared with 42 hours in the placebo group. Baloxavir better reduced viral load at day 2, compared with oseltamivir and placebo, but there was a similar alleviation of symptoms between both groups. There was a greater incidence of nausea and vomiting among the oseltamivir group, while the baloxavir group had a higher rate of diarrhea (N Engl J Med 2018;379:913-23).
However, Dr. Bradley noted baloxavir is much more expensive than oseltamivir, which may not justify the better tolerance of the drug for influenza treatment.
You don’t get better with it faster, so I’m not going to be recommending you all run to baloxavir this flu season for kids 12 years of age and older,” Dr. Bradley said. “I think oseltamivir is still fine, unless we end up with oseltamivir resistance.”
Solithromycin, an intravenous and oral fluoroketolide, has shown promising results against gram-positive and gram-negative pathogens for community-acquired pneumonia and other infections. During the drug’s study period, Cempra sold solithromycin to Melinta. However, one trial showed elevated liver functions in a higher number of patients than expected, and the Food and Drug Administration asked Melinta to conduct additional studies. Investigations on solithromycin have currently stopped until Melinta secures funding. “Until they get better resources, this particular drug is on hold, but you’ll see it again, I’m sure,” said Dr. Bradley, who also is professor and chief of the division of infectious diseases at the University of California, San Diego.
Dr. Bradley also discussed the efficacy of tedizolid, a protein synthesis inhibitor similar to linezolid approved in adults for the treatment of skin infections. He noted tedizolid is more active than linezolid, but the treatment course is a shorter dose for a shorter amount of time. Compared with linezolid, which can cause thrombocytopenia or neutropenia if taken for more than 10 days to 14 days, there also are fewer side effects.
“The tedizolid is much, much safer,” Dr. Bradley said, who added that trials for efficacy of tedizolid are currently underway in pediatric patients. “We’re hoping that will end up being the pediatric oxazolidinone.”
Other investigative therapies approved for adults and under study for use in children include ceftazidime/avibactam for treatment of urinary tract and complicated intra-abdominal infections, which is effective against meropenem-resistant Enterobacteriaceae and resistant Escherichia coli with extended-spectrum beta-lactamases (ESBL); ceftolozane/tazobactam has also been approved for adults, is pending approval in pediatric patients, and is active against ESBLs such as Pseudomonas; and meropenem/vaborbactam, which is active against Klebsiella pneumoniae carbapenemase (KPC)–producing isolates. Plazomicin, an aminoglycoside similar to gentamicin used to treat KPC-producing isolates, is stable against enzymes that degrade gentamicin and tobramycin.
Therapies currently under study for adults and being considered for children include imipenem/relebactam for treatment against E. coli, Enterobacter species, and KPC-producing isolates, and cefiderocol, a siderophore cephalosporin antibiotic – commonly described as a “Trojan horse” antibiotic because it binds to iron and is actively transported into the organism – is effective against Pseudomonas and has finished phase 2 trials in adults, with researchers looking to do single-dose trials in children, Dr. Bradley noted.
More experimentally, phage therapy for multidrug-resistant Acinetobacter baumannii proved effective in a 68-year-old patient with necrotizing pancreatitis who continued to deteriorate over a 4-month period despite multiple courses of antibiotics and attempted drainage of a pancreatic pseudocyst. Researchers selected a phage-specific bacterium with specificity for A. baumannii and cured him. “This is like science fiction,” Dr. Bradley said.
Dr. Bradley reported no relevant conflicts of interest.
EXPERT ANALYSIS FROM AAP 18