User login
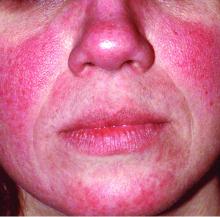
In rosacea, a single treatment may not be enough
MONTEREY, CALIF. – , a dermatologist said at the annual Coastal Dermatology Symposium. Don’t assume you can just prescribe one drug like you might with acne, she advised.
“Treat everything that you see,” said dermatologist Julie C. Harper, MD, of Birmingham, Ala. “That may mean a laser or something you’re using off-label. Different lesions and signs of rosacea will require multiple modes of treatment.”
Dr. Harper offered these other pearls to consider when treating rosacea:
- Don’t get hung up on subtypes.
The four subtypes of rosacea should be used to classify lesions, not people, she said. That’s because patients can fall into more than one of the four categories – erythematotelangiectatic rosacea, papulopustular rosacea, phymatous rosacea, and ocular rosacea, she noted.
“Document the redness you see and ask them what’s bothering them the most,” she said. And ask yourself, she added, “Do I have them on everything that I should have them on?”
- Talk to patients about triggers.
For the first visit, “we have to talk to patients about skin care and triggers,” Dr. Harper noted. According to the American Academy of Dermatology, common rosacea triggers include sunlight, hairspray, heat, stress, alcohol, and spicy foods.
- Consider an ivermectin-brimonidine combination.
“Targeting inflammation in papules and pustules doesn’t necessarily translate to less background erythema,” Dr. Harper said. What to do? She pointed to a 2017 study that examined a combination treatment of ivermectin 1% topical cream (Soolantra) and brimonidine 0.33% topical gel (Mirvaso) for patients with rosacea with moderate to severe persistent erythema and inflammatory lesions. Ivermectin is indicated for inflammatory lesions, while brimonidine treats persistent erythema.
At week 12, the proportion of patients who achieved investigator global assessment of clear or almost clear was 55.8% in the combination group, versus 36.8% of those in the vehicle group (P = .007), according to the study (J Drugs Dermatol. 2017 Sep 1;16[9]:909-16). Dr. Harper highlighted the effect of brimonidine when added to ivermectin. “In a period of 3 hours,” she said, “we had twice as many people fall into clear or almost clear.”
- Consider adding botulinum toxin to your toolbox.
This “really does work,” Dr. Harper said. She pointed to a 2015 report of botulinum toxin use in two cases of refractory flushing and erythema and a 2012 report of 13 cases in patients with the same symptoms (Dermatology. 2015;230:299-301; J Drugs Dermatol. 2012 Dec;11[12]:e76-9). Dr. Harper said that she usually uses the full 50-unit dose of Botox.
- Consider a beta-blocker.
According to a 2018 report, the beta-blocker carvedilol (Coreg) showed benefit when added to other treatments in five patients with facial flushing and persistent erythema.
- Keep isotretinoin in mind.
A 2016 report suggested low-dose isotretinoin had value for difficult-to-treat papulopustular rosacea. As Dr. Harper noted, 57% of those who took isotretinoin reached the primary endpoint, versus 10% of those taking the placebo. However, relapses over 4 months were common, which is a sign that it may be wise to prescribe low doses over the long term, but not in females of child-bearing potential, she said.
The Coastal Dermatology Symposium is jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical Communications.
Dr. Harper disclosed speaker/advisor relationships with Allergan, Bayer, BioPharmX, Galderma, LaRoche Posay, and Ortho and has served as investigator for Bayer.
MONTEREY, CALIF. – , a dermatologist said at the annual Coastal Dermatology Symposium. Don’t assume you can just prescribe one drug like you might with acne, she advised.
“Treat everything that you see,” said dermatologist Julie C. Harper, MD, of Birmingham, Ala. “That may mean a laser or something you’re using off-label. Different lesions and signs of rosacea will require multiple modes of treatment.”
Dr. Harper offered these other pearls to consider when treating rosacea:
- Don’t get hung up on subtypes.
The four subtypes of rosacea should be used to classify lesions, not people, she said. That’s because patients can fall into more than one of the four categories – erythematotelangiectatic rosacea, papulopustular rosacea, phymatous rosacea, and ocular rosacea, she noted.
“Document the redness you see and ask them what’s bothering them the most,” she said. And ask yourself, she added, “Do I have them on everything that I should have them on?”
- Talk to patients about triggers.
For the first visit, “we have to talk to patients about skin care and triggers,” Dr. Harper noted. According to the American Academy of Dermatology, common rosacea triggers include sunlight, hairspray, heat, stress, alcohol, and spicy foods.
- Consider an ivermectin-brimonidine combination.
“Targeting inflammation in papules and pustules doesn’t necessarily translate to less background erythema,” Dr. Harper said. What to do? She pointed to a 2017 study that examined a combination treatment of ivermectin 1% topical cream (Soolantra) and brimonidine 0.33% topical gel (Mirvaso) for patients with rosacea with moderate to severe persistent erythema and inflammatory lesions. Ivermectin is indicated for inflammatory lesions, while brimonidine treats persistent erythema.
At week 12, the proportion of patients who achieved investigator global assessment of clear or almost clear was 55.8% in the combination group, versus 36.8% of those in the vehicle group (P = .007), according to the study (J Drugs Dermatol. 2017 Sep 1;16[9]:909-16). Dr. Harper highlighted the effect of brimonidine when added to ivermectin. “In a period of 3 hours,” she said, “we had twice as many people fall into clear or almost clear.”
- Consider adding botulinum toxin to your toolbox.
This “really does work,” Dr. Harper said. She pointed to a 2015 report of botulinum toxin use in two cases of refractory flushing and erythema and a 2012 report of 13 cases in patients with the same symptoms (Dermatology. 2015;230:299-301; J Drugs Dermatol. 2012 Dec;11[12]:e76-9). Dr. Harper said that she usually uses the full 50-unit dose of Botox.
- Consider a beta-blocker.
According to a 2018 report, the beta-blocker carvedilol (Coreg) showed benefit when added to other treatments in five patients with facial flushing and persistent erythema.
- Keep isotretinoin in mind.
A 2016 report suggested low-dose isotretinoin had value for difficult-to-treat papulopustular rosacea. As Dr. Harper noted, 57% of those who took isotretinoin reached the primary endpoint, versus 10% of those taking the placebo. However, relapses over 4 months were common, which is a sign that it may be wise to prescribe low doses over the long term, but not in females of child-bearing potential, she said.
The Coastal Dermatology Symposium is jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical Communications.
Dr. Harper disclosed speaker/advisor relationships with Allergan, Bayer, BioPharmX, Galderma, LaRoche Posay, and Ortho and has served as investigator for Bayer.
MONTEREY, CALIF. – , a dermatologist said at the annual Coastal Dermatology Symposium. Don’t assume you can just prescribe one drug like you might with acne, she advised.
“Treat everything that you see,” said dermatologist Julie C. Harper, MD, of Birmingham, Ala. “That may mean a laser or something you’re using off-label. Different lesions and signs of rosacea will require multiple modes of treatment.”
Dr. Harper offered these other pearls to consider when treating rosacea:
- Don’t get hung up on subtypes.
The four subtypes of rosacea should be used to classify lesions, not people, she said. That’s because patients can fall into more than one of the four categories – erythematotelangiectatic rosacea, papulopustular rosacea, phymatous rosacea, and ocular rosacea, she noted.
“Document the redness you see and ask them what’s bothering them the most,” she said. And ask yourself, she added, “Do I have them on everything that I should have them on?”
- Talk to patients about triggers.
For the first visit, “we have to talk to patients about skin care and triggers,” Dr. Harper noted. According to the American Academy of Dermatology, common rosacea triggers include sunlight, hairspray, heat, stress, alcohol, and spicy foods.
- Consider an ivermectin-brimonidine combination.
“Targeting inflammation in papules and pustules doesn’t necessarily translate to less background erythema,” Dr. Harper said. What to do? She pointed to a 2017 study that examined a combination treatment of ivermectin 1% topical cream (Soolantra) and brimonidine 0.33% topical gel (Mirvaso) for patients with rosacea with moderate to severe persistent erythema and inflammatory lesions. Ivermectin is indicated for inflammatory lesions, while brimonidine treats persistent erythema.
At week 12, the proportion of patients who achieved investigator global assessment of clear or almost clear was 55.8% in the combination group, versus 36.8% of those in the vehicle group (P = .007), according to the study (J Drugs Dermatol. 2017 Sep 1;16[9]:909-16). Dr. Harper highlighted the effect of brimonidine when added to ivermectin. “In a period of 3 hours,” she said, “we had twice as many people fall into clear or almost clear.”
- Consider adding botulinum toxin to your toolbox.
This “really does work,” Dr. Harper said. She pointed to a 2015 report of botulinum toxin use in two cases of refractory flushing and erythema and a 2012 report of 13 cases in patients with the same symptoms (Dermatology. 2015;230:299-301; J Drugs Dermatol. 2012 Dec;11[12]:e76-9). Dr. Harper said that she usually uses the full 50-unit dose of Botox.
- Consider a beta-blocker.
According to a 2018 report, the beta-blocker carvedilol (Coreg) showed benefit when added to other treatments in five patients with facial flushing and persistent erythema.
- Keep isotretinoin in mind.
A 2016 report suggested low-dose isotretinoin had value for difficult-to-treat papulopustular rosacea. As Dr. Harper noted, 57% of those who took isotretinoin reached the primary endpoint, versus 10% of those taking the placebo. However, relapses over 4 months were common, which is a sign that it may be wise to prescribe low doses over the long term, but not in females of child-bearing potential, she said.
The Coastal Dermatology Symposium is jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical Communications.
Dr. Harper disclosed speaker/advisor relationships with Allergan, Bayer, BioPharmX, Galderma, LaRoche Posay, and Ortho and has served as investigator for Bayer.
EXPERT ANALYSIS FROM THE COASTAL DERMATOLOGY SYMPOSIUM
Sunscreens: Misleading labels, poor performance, and hype about their risks
MONTEREY, CALIF. – Heads up! “Natural” mineral-based sunscreens don’t provide the protection of their rivals. Patients may get burned by scary hype about the supposed dangers of sunscreen. And sunscreen spray is great for the scalp of people whose hair is thinning.
In a presentation on sunscreens at the annual Coastal Dermatology Symposium, Vincent DeLeo, MD, of the University of Southern California, Los Angeles, offered the following tips on sunscreen and more.
Here’s a roundup of his pearls:
Sunscreens are getting better and are faring poorly, too.
Dr. DeLeo said. A 2013 comparison of sunscreens in 1997 and 2009 found that, among available sunscreens, the percentage of those with low SPF (under 15) fell from 27% to 6% during that time. (The Food and Drug Administration declared in 2011 that manufacturers must tell consumers that low SPF and/or non–broad spectrum sunscreens protect only against sunburn, not against skin cancer or skin aging.) The study also found that the percentage of sunscreens with UVA-1 (such as avobenzone or zinc oxide) filters grew from 5% to 70% (Photochem Photobiol Sci. 2013 Jan;12[1]:197-202).
But the label of sunscreens may not always be accurate. Earlier this year, Consumer Reports wrote that 36 of 73 sunscreens tested failed to correctly list their SPF protection level; 23 sunscreens missed their listed SPF levels by more than half. “Natural” or “mineral-only” sunscreens, which rely on such blockers as zinc oxide or titanium dioxide, performed the worst. Some patients prefer to use these sunscreens because they aren’t chemical based, and “may want to have a more natural sunscreen,” Dr. DeLeo said. “But they should be aware the sunscreens don’t always live up to the SPF level on the label.”
Beware of warnings about sunscreens.
Reports have warned Americans about supposed risks of sunscreen use such as low vitamin D levels from the lack of sun exposure, the exposure to titanium dioxide and zinc oxide nanoparticles, and the exposure to retinyl palmitate in sunscreen. Hawaiian officials, meanwhile, are banning some types of sunscreen chemicals in order to protect coral reefs.
Typical use of sunscreen will not dangerously lower vitamin D levels, Dr. DeLeo said, but people who use it every day may want to be cautious. He dismissed the concerns about nanoparticles and retinyl palmitate.
Dr. DeLeo said two sunscreen risks are real; sunscreens can trigger irritation, at a rate as high as 20%, and, rarely, allergic reactions, as well.
American sunscreens don’t stack up worldwide.
Simplicity often is a virtue. But, Dr. DeLeo said, it’s not helpful when it comes to the components of American sunscreens.
U.S. regulations only allow 16 ingredients in sunscreen while several more are allowed in Europe, he said. According to him, this helps explain why European sunscreens do a better job. European sunscreens “are much more absorbent, much better at absorbing radiation than the U.S. sunscreens,” he said. “It’s because we don’t have the same products as they have in Europe.”
The good news, he said, is that the FDA is considering expanding the number of ingredients allowed in sunscreen. The Sunscreen Innovation Act of 2014, a law passed by Congress, allows the FDA to use efficacy and safety data from Europe without requiring manufacturers to launch new, multimillion dollar tests, he said.
That’s good news for companies that want to improve U.S. sunscreens by selling a wider variety of types. “Sooner or later,” he said, “we will probably get these.”
Sunscreen sprays are tops at scalp protection.
Sunscreen sprays shouldn’t be applied to the face in children, Dr. DeLeo said, but they’re great for solo people because they facilitate protecting the back when there isn’t someone around to help them apply topical sunscreen.
How much spray should people use? A lot, he said. He added that sunscreen sprays are especially useful for the scalps of people with thinning hair.
Dr. DeLeo disclosed consulting work for Estée Lauder.
The meeting is jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical Communications.
MONTEREY, CALIF. – Heads up! “Natural” mineral-based sunscreens don’t provide the protection of their rivals. Patients may get burned by scary hype about the supposed dangers of sunscreen. And sunscreen spray is great for the scalp of people whose hair is thinning.
In a presentation on sunscreens at the annual Coastal Dermatology Symposium, Vincent DeLeo, MD, of the University of Southern California, Los Angeles, offered the following tips on sunscreen and more.
Here’s a roundup of his pearls:
Sunscreens are getting better and are faring poorly, too.
Dr. DeLeo said. A 2013 comparison of sunscreens in 1997 and 2009 found that, among available sunscreens, the percentage of those with low SPF (under 15) fell from 27% to 6% during that time. (The Food and Drug Administration declared in 2011 that manufacturers must tell consumers that low SPF and/or non–broad spectrum sunscreens protect only against sunburn, not against skin cancer or skin aging.) The study also found that the percentage of sunscreens with UVA-1 (such as avobenzone or zinc oxide) filters grew from 5% to 70% (Photochem Photobiol Sci. 2013 Jan;12[1]:197-202).
But the label of sunscreens may not always be accurate. Earlier this year, Consumer Reports wrote that 36 of 73 sunscreens tested failed to correctly list their SPF protection level; 23 sunscreens missed their listed SPF levels by more than half. “Natural” or “mineral-only” sunscreens, which rely on such blockers as zinc oxide or titanium dioxide, performed the worst. Some patients prefer to use these sunscreens because they aren’t chemical based, and “may want to have a more natural sunscreen,” Dr. DeLeo said. “But they should be aware the sunscreens don’t always live up to the SPF level on the label.”
Beware of warnings about sunscreens.
Reports have warned Americans about supposed risks of sunscreen use such as low vitamin D levels from the lack of sun exposure, the exposure to titanium dioxide and zinc oxide nanoparticles, and the exposure to retinyl palmitate in sunscreen. Hawaiian officials, meanwhile, are banning some types of sunscreen chemicals in order to protect coral reefs.
Typical use of sunscreen will not dangerously lower vitamin D levels, Dr. DeLeo said, but people who use it every day may want to be cautious. He dismissed the concerns about nanoparticles and retinyl palmitate.
Dr. DeLeo said two sunscreen risks are real; sunscreens can trigger irritation, at a rate as high as 20%, and, rarely, allergic reactions, as well.
American sunscreens don’t stack up worldwide.
Simplicity often is a virtue. But, Dr. DeLeo said, it’s not helpful when it comes to the components of American sunscreens.
U.S. regulations only allow 16 ingredients in sunscreen while several more are allowed in Europe, he said. According to him, this helps explain why European sunscreens do a better job. European sunscreens “are much more absorbent, much better at absorbing radiation than the U.S. sunscreens,” he said. “It’s because we don’t have the same products as they have in Europe.”
The good news, he said, is that the FDA is considering expanding the number of ingredients allowed in sunscreen. The Sunscreen Innovation Act of 2014, a law passed by Congress, allows the FDA to use efficacy and safety data from Europe without requiring manufacturers to launch new, multimillion dollar tests, he said.
That’s good news for companies that want to improve U.S. sunscreens by selling a wider variety of types. “Sooner or later,” he said, “we will probably get these.”
Sunscreen sprays are tops at scalp protection.
Sunscreen sprays shouldn’t be applied to the face in children, Dr. DeLeo said, but they’re great for solo people because they facilitate protecting the back when there isn’t someone around to help them apply topical sunscreen.
How much spray should people use? A lot, he said. He added that sunscreen sprays are especially useful for the scalps of people with thinning hair.
Dr. DeLeo disclosed consulting work for Estée Lauder.
The meeting is jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical Communications.
MONTEREY, CALIF. – Heads up! “Natural” mineral-based sunscreens don’t provide the protection of their rivals. Patients may get burned by scary hype about the supposed dangers of sunscreen. And sunscreen spray is great for the scalp of people whose hair is thinning.
In a presentation on sunscreens at the annual Coastal Dermatology Symposium, Vincent DeLeo, MD, of the University of Southern California, Los Angeles, offered the following tips on sunscreen and more.
Here’s a roundup of his pearls:
Sunscreens are getting better and are faring poorly, too.
Dr. DeLeo said. A 2013 comparison of sunscreens in 1997 and 2009 found that, among available sunscreens, the percentage of those with low SPF (under 15) fell from 27% to 6% during that time. (The Food and Drug Administration declared in 2011 that manufacturers must tell consumers that low SPF and/or non–broad spectrum sunscreens protect only against sunburn, not against skin cancer or skin aging.) The study also found that the percentage of sunscreens with UVA-1 (such as avobenzone or zinc oxide) filters grew from 5% to 70% (Photochem Photobiol Sci. 2013 Jan;12[1]:197-202).
But the label of sunscreens may not always be accurate. Earlier this year, Consumer Reports wrote that 36 of 73 sunscreens tested failed to correctly list their SPF protection level; 23 sunscreens missed their listed SPF levels by more than half. “Natural” or “mineral-only” sunscreens, which rely on such blockers as zinc oxide or titanium dioxide, performed the worst. Some patients prefer to use these sunscreens because they aren’t chemical based, and “may want to have a more natural sunscreen,” Dr. DeLeo said. “But they should be aware the sunscreens don’t always live up to the SPF level on the label.”
Beware of warnings about sunscreens.
Reports have warned Americans about supposed risks of sunscreen use such as low vitamin D levels from the lack of sun exposure, the exposure to titanium dioxide and zinc oxide nanoparticles, and the exposure to retinyl palmitate in sunscreen. Hawaiian officials, meanwhile, are banning some types of sunscreen chemicals in order to protect coral reefs.
Typical use of sunscreen will not dangerously lower vitamin D levels, Dr. DeLeo said, but people who use it every day may want to be cautious. He dismissed the concerns about nanoparticles and retinyl palmitate.
Dr. DeLeo said two sunscreen risks are real; sunscreens can trigger irritation, at a rate as high as 20%, and, rarely, allergic reactions, as well.
American sunscreens don’t stack up worldwide.
Simplicity often is a virtue. But, Dr. DeLeo said, it’s not helpful when it comes to the components of American sunscreens.
U.S. regulations only allow 16 ingredients in sunscreen while several more are allowed in Europe, he said. According to him, this helps explain why European sunscreens do a better job. European sunscreens “are much more absorbent, much better at absorbing radiation than the U.S. sunscreens,” he said. “It’s because we don’t have the same products as they have in Europe.”
The good news, he said, is that the FDA is considering expanding the number of ingredients allowed in sunscreen. The Sunscreen Innovation Act of 2014, a law passed by Congress, allows the FDA to use efficacy and safety data from Europe without requiring manufacturers to launch new, multimillion dollar tests, he said.
That’s good news for companies that want to improve U.S. sunscreens by selling a wider variety of types. “Sooner or later,” he said, “we will probably get these.”
Sunscreen sprays are tops at scalp protection.
Sunscreen sprays shouldn’t be applied to the face in children, Dr. DeLeo said, but they’re great for solo people because they facilitate protecting the back when there isn’t someone around to help them apply topical sunscreen.
How much spray should people use? A lot, he said. He added that sunscreen sprays are especially useful for the scalps of people with thinning hair.
Dr. DeLeo disclosed consulting work for Estée Lauder.
The meeting is jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical Communications.
REPORTING FROM THE COASTAL DERMATOLOGY SYMPOSIUM
Allergen of the year may be nearer than you think
MONTEREY, CALIF. – It’s only found in 2%-3% of allergy cases. So Because, a dermatologist told colleagues, it’s so common.
“If you’re allergic to it, it’s tough to stay away from it,” said Joseph F. Fowler Jr., MD, clinical professor of dermatology at the University of Louisville (Ky.) in a presentation about contact dermatitis at the annual Coastal Dermatology Symposium.
Indeed, the synthetic compound PG is found in skin care products and cosmetics, coated pills, topical medications such as corticosteroids, foods (including bread, food coloring, and such flavorings as vanilla extracts). “It’s in every topical acne product I know of,” and is even in brake fluid and so-called nontoxic antifreeze, he said. (Propylene glycol shouldn’t be confused with the poisonous toxin ethylene glycol, which also is found in antifreeze.)
Patients can be tested for allergy to PG, Dr. Fowler pointed out, but it’s important to understand that it can trigger an irritation reaction that can be mistaken for an allergic reaction.
Dr. Fowler offered the following tips related to contact dermatitis and allergens. Be aware that metals, topical antibiotics, fragrances, and preservatives are most likely to cause allergic contact dermatitis. According to 2016 figures on allergen prevalence from the North American Contact Dermatitis Group (NACDG), allergy to the metal nickel is the most common (16%); followed by neomycin (9%); fragrance mix I, a mixture of fragrances used in allergen testing (9%); bacitracin (8%); and myroxylon, also known as balsam of Peru, which is used for a variety of purposes in food, medicines, and fragrances (7%).
These are followed by the metal cobalt (6%); the preservatives quaternium 15 and formaldehyde (both 6%); para-phenylenediamine, also known as PPD, which is used in hair dye (5%); and the fragrance mix II (5%), another mix of fragrances used in allergen testing.
Dr. Fowler cautioned that nickel can trigger an intense body-wide allergic reaction in children with atopic dermatitis. “In this situation, it’s really good to be compulsive and tell parents to absolutely keep that person away from nickel as much as humanly possible,” he said.
Keep an eye out for allergens that aren’t on the NACDG list, which includes 70 items. According to Dr. Fowler, more than 20% of his patients were positive to allergens not on the NACDG list.
Contact dermatitis is as common in children as in adults and can even be more common in children. An Italian study published in 2012 found that 70% of children aged 1-15 years tested via patch test were allergic to at least one allergen, a number that’s similar in adults (Dermatitis. 2012 Nov-Dec;23[6]:275-80). There are wide disparities in reported levels of children who are allergic to nickel, cobalt, and myroxylon, Dr. Fowler said.
The T.R.U.E. Test patch test system has value, compared with standard patch tests, but beware of its limitations, he advised. T.R.U.E. is easy to use and requires no prep time, he said, but the number of allergens is limited. By contrast, his clinic mostly uses the Finn Chambers on Scanpor tape system, which can test for many more allergens and is cheaper if used at least 5-10 times a month.
He cautioned that T.R.U.E. could miss the cause of contact dermatitis as often as 39% of the time, as demonstrated in one study of children undergoing patch testing (Arch Dermatol. 2008 Oct;144[10]:1329-36). However, he said, the T.R.U.E test has value in detecting allergies to nickel, methylchloroisothiazolinone/methylisothiazolinone (MCI/MI), and neomycin (J Am Acad Dermatol. 2001 Dec;45[6]:836-9).
Consider patch testing in a child with eczema if the eczema is not in normal atopic areas, it spreads beyond normal areas, it doesn’t respond to usual treatments, or it begins later than 5 years of age.
And, Dr. Fowler added, it’s fine to perform patch testing on patients who are taking antihistamines, tumor necrosis factor–alpha inhibitors, NSAIDs, or methotrexate.
Dr. Fowler disclosed consulting for IntraDerm, serving on speakers bureaus for SmartPractice and Regeneron/Sanofi, and serving as an investigator for companies that include AbbVie, Allergan, Bayer, Dow, Galderma, Johnson & Johnson, Eli Lilly, Merck, Regeneron, SmartPractice, and Valeant (now Bausch).
The meeting was jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical Communications.
MONTEREY, CALIF. – It’s only found in 2%-3% of allergy cases. So Because, a dermatologist told colleagues, it’s so common.
“If you’re allergic to it, it’s tough to stay away from it,” said Joseph F. Fowler Jr., MD, clinical professor of dermatology at the University of Louisville (Ky.) in a presentation about contact dermatitis at the annual Coastal Dermatology Symposium.
Indeed, the synthetic compound PG is found in skin care products and cosmetics, coated pills, topical medications such as corticosteroids, foods (including bread, food coloring, and such flavorings as vanilla extracts). “It’s in every topical acne product I know of,” and is even in brake fluid and so-called nontoxic antifreeze, he said. (Propylene glycol shouldn’t be confused with the poisonous toxin ethylene glycol, which also is found in antifreeze.)
Patients can be tested for allergy to PG, Dr. Fowler pointed out, but it’s important to understand that it can trigger an irritation reaction that can be mistaken for an allergic reaction.
Dr. Fowler offered the following tips related to contact dermatitis and allergens. Be aware that metals, topical antibiotics, fragrances, and preservatives are most likely to cause allergic contact dermatitis. According to 2016 figures on allergen prevalence from the North American Contact Dermatitis Group (NACDG), allergy to the metal nickel is the most common (16%); followed by neomycin (9%); fragrance mix I, a mixture of fragrances used in allergen testing (9%); bacitracin (8%); and myroxylon, also known as balsam of Peru, which is used for a variety of purposes in food, medicines, and fragrances (7%).
These are followed by the metal cobalt (6%); the preservatives quaternium 15 and formaldehyde (both 6%); para-phenylenediamine, also known as PPD, which is used in hair dye (5%); and the fragrance mix II (5%), another mix of fragrances used in allergen testing.
Dr. Fowler cautioned that nickel can trigger an intense body-wide allergic reaction in children with atopic dermatitis. “In this situation, it’s really good to be compulsive and tell parents to absolutely keep that person away from nickel as much as humanly possible,” he said.
Keep an eye out for allergens that aren’t on the NACDG list, which includes 70 items. According to Dr. Fowler, more than 20% of his patients were positive to allergens not on the NACDG list.
Contact dermatitis is as common in children as in adults and can even be more common in children. An Italian study published in 2012 found that 70% of children aged 1-15 years tested via patch test were allergic to at least one allergen, a number that’s similar in adults (Dermatitis. 2012 Nov-Dec;23[6]:275-80). There are wide disparities in reported levels of children who are allergic to nickel, cobalt, and myroxylon, Dr. Fowler said.
The T.R.U.E. Test patch test system has value, compared with standard patch tests, but beware of its limitations, he advised. T.R.U.E. is easy to use and requires no prep time, he said, but the number of allergens is limited. By contrast, his clinic mostly uses the Finn Chambers on Scanpor tape system, which can test for many more allergens and is cheaper if used at least 5-10 times a month.
He cautioned that T.R.U.E. could miss the cause of contact dermatitis as often as 39% of the time, as demonstrated in one study of children undergoing patch testing (Arch Dermatol. 2008 Oct;144[10]:1329-36). However, he said, the T.R.U.E test has value in detecting allergies to nickel, methylchloroisothiazolinone/methylisothiazolinone (MCI/MI), and neomycin (J Am Acad Dermatol. 2001 Dec;45[6]:836-9).
Consider patch testing in a child with eczema if the eczema is not in normal atopic areas, it spreads beyond normal areas, it doesn’t respond to usual treatments, or it begins later than 5 years of age.
And, Dr. Fowler added, it’s fine to perform patch testing on patients who are taking antihistamines, tumor necrosis factor–alpha inhibitors, NSAIDs, or methotrexate.
Dr. Fowler disclosed consulting for IntraDerm, serving on speakers bureaus for SmartPractice and Regeneron/Sanofi, and serving as an investigator for companies that include AbbVie, Allergan, Bayer, Dow, Galderma, Johnson & Johnson, Eli Lilly, Merck, Regeneron, SmartPractice, and Valeant (now Bausch).
The meeting was jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical Communications.
MONTEREY, CALIF. – It’s only found in 2%-3% of allergy cases. So Because, a dermatologist told colleagues, it’s so common.
“If you’re allergic to it, it’s tough to stay away from it,” said Joseph F. Fowler Jr., MD, clinical professor of dermatology at the University of Louisville (Ky.) in a presentation about contact dermatitis at the annual Coastal Dermatology Symposium.
Indeed, the synthetic compound PG is found in skin care products and cosmetics, coated pills, topical medications such as corticosteroids, foods (including bread, food coloring, and such flavorings as vanilla extracts). “It’s in every topical acne product I know of,” and is even in brake fluid and so-called nontoxic antifreeze, he said. (Propylene glycol shouldn’t be confused with the poisonous toxin ethylene glycol, which also is found in antifreeze.)
Patients can be tested for allergy to PG, Dr. Fowler pointed out, but it’s important to understand that it can trigger an irritation reaction that can be mistaken for an allergic reaction.
Dr. Fowler offered the following tips related to contact dermatitis and allergens. Be aware that metals, topical antibiotics, fragrances, and preservatives are most likely to cause allergic contact dermatitis. According to 2016 figures on allergen prevalence from the North American Contact Dermatitis Group (NACDG), allergy to the metal nickel is the most common (16%); followed by neomycin (9%); fragrance mix I, a mixture of fragrances used in allergen testing (9%); bacitracin (8%); and myroxylon, also known as balsam of Peru, which is used for a variety of purposes in food, medicines, and fragrances (7%).
These are followed by the metal cobalt (6%); the preservatives quaternium 15 and formaldehyde (both 6%); para-phenylenediamine, also known as PPD, which is used in hair dye (5%); and the fragrance mix II (5%), another mix of fragrances used in allergen testing.
Dr. Fowler cautioned that nickel can trigger an intense body-wide allergic reaction in children with atopic dermatitis. “In this situation, it’s really good to be compulsive and tell parents to absolutely keep that person away from nickel as much as humanly possible,” he said.
Keep an eye out for allergens that aren’t on the NACDG list, which includes 70 items. According to Dr. Fowler, more than 20% of his patients were positive to allergens not on the NACDG list.
Contact dermatitis is as common in children as in adults and can even be more common in children. An Italian study published in 2012 found that 70% of children aged 1-15 years tested via patch test were allergic to at least one allergen, a number that’s similar in adults (Dermatitis. 2012 Nov-Dec;23[6]:275-80). There are wide disparities in reported levels of children who are allergic to nickel, cobalt, and myroxylon, Dr. Fowler said.
The T.R.U.E. Test patch test system has value, compared with standard patch tests, but beware of its limitations, he advised. T.R.U.E. is easy to use and requires no prep time, he said, but the number of allergens is limited. By contrast, his clinic mostly uses the Finn Chambers on Scanpor tape system, which can test for many more allergens and is cheaper if used at least 5-10 times a month.
He cautioned that T.R.U.E. could miss the cause of contact dermatitis as often as 39% of the time, as demonstrated in one study of children undergoing patch testing (Arch Dermatol. 2008 Oct;144[10]:1329-36). However, he said, the T.R.U.E test has value in detecting allergies to nickel, methylchloroisothiazolinone/methylisothiazolinone (MCI/MI), and neomycin (J Am Acad Dermatol. 2001 Dec;45[6]:836-9).
Consider patch testing in a child with eczema if the eczema is not in normal atopic areas, it spreads beyond normal areas, it doesn’t respond to usual treatments, or it begins later than 5 years of age.
And, Dr. Fowler added, it’s fine to perform patch testing on patients who are taking antihistamines, tumor necrosis factor–alpha inhibitors, NSAIDs, or methotrexate.
Dr. Fowler disclosed consulting for IntraDerm, serving on speakers bureaus for SmartPractice and Regeneron/Sanofi, and serving as an investigator for companies that include AbbVie, Allergan, Bayer, Dow, Galderma, Johnson & Johnson, Eli Lilly, Merck, Regeneron, SmartPractice, and Valeant (now Bausch).
The meeting was jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical Communications.
EXPERT ANALYSIS FROM THE COASTAL DERMATOLOGY SYMPOSIUM
FDA approves omadacycline for pneumonia and skin infections
The, for treating community-acquired bacterial pneumonia (CABP) and acute bacterial skin and skin structure infections (ABSSSI) in adults, the manufacturer, Paratek, announced in a press release.
The company expects that omadacycline will be available in the first quarter of 2019. Administered once-daily in either oral or IV formulations, the antibiotic was effective and well tolerated across multiple trials, which altogether included almost 2,000 patients, according to Paratek. As part of the approval, the company has agreed to conduct postmarketing studies, specifically, more studies in CABP and in pediatric populations. “To reduce the development of drug-resistant bacteria and maintain the effectiveness of Nuzyra and other antibacterial drugs, Nuzyra should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria,” according to a statement in the indications section of the prescribing information.
Omadacycline is contraindicated for patients with a known hypersensitivity to the drug or any members of the tetracycline class of antibacterial drugs; hypersensitivity reactions have been observed, so use should be discontinued if one is suspected. Use of this drug during later stages of pregnancy can lead to irreversible discoloration of the infant’s teeth and inhibition of bone growth; it should also not be used during breastfeeding.
Because omadacycline is structurally similar to tetracycline class drugs, some adverse reactions to those drugs may be seen with this one, such as photosensitivity, pseudotumor cerebri, and antianabolic action. Adverse reactions known to have an association with omadacycline include nausea, vomiting, hypertension, insomnia, diarrhea, constipation, and increases of alanine aminotransferase, aspartate aminotransferase, and/or gamma-glutamyl transferase.
Drug interactions may occur with anticoagulants, so dosage of those drugs may need to be reduced while treating with omadacycline. Antacids also are believed to have a drug interaction – specifically, impairing absorption of omadacycline
The, for treating community-acquired bacterial pneumonia (CABP) and acute bacterial skin and skin structure infections (ABSSSI) in adults, the manufacturer, Paratek, announced in a press release.
The company expects that omadacycline will be available in the first quarter of 2019. Administered once-daily in either oral or IV formulations, the antibiotic was effective and well tolerated across multiple trials, which altogether included almost 2,000 patients, according to Paratek. As part of the approval, the company has agreed to conduct postmarketing studies, specifically, more studies in CABP and in pediatric populations. “To reduce the development of drug-resistant bacteria and maintain the effectiveness of Nuzyra and other antibacterial drugs, Nuzyra should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria,” according to a statement in the indications section of the prescribing information.
Omadacycline is contraindicated for patients with a known hypersensitivity to the drug or any members of the tetracycline class of antibacterial drugs; hypersensitivity reactions have been observed, so use should be discontinued if one is suspected. Use of this drug during later stages of pregnancy can lead to irreversible discoloration of the infant’s teeth and inhibition of bone growth; it should also not be used during breastfeeding.
Because omadacycline is structurally similar to tetracycline class drugs, some adverse reactions to those drugs may be seen with this one, such as photosensitivity, pseudotumor cerebri, and antianabolic action. Adverse reactions known to have an association with omadacycline include nausea, vomiting, hypertension, insomnia, diarrhea, constipation, and increases of alanine aminotransferase, aspartate aminotransferase, and/or gamma-glutamyl transferase.
Drug interactions may occur with anticoagulants, so dosage of those drugs may need to be reduced while treating with omadacycline. Antacids also are believed to have a drug interaction – specifically, impairing absorption of omadacycline
The, for treating community-acquired bacterial pneumonia (CABP) and acute bacterial skin and skin structure infections (ABSSSI) in adults, the manufacturer, Paratek, announced in a press release.
The company expects that omadacycline will be available in the first quarter of 2019. Administered once-daily in either oral or IV formulations, the antibiotic was effective and well tolerated across multiple trials, which altogether included almost 2,000 patients, according to Paratek. As part of the approval, the company has agreed to conduct postmarketing studies, specifically, more studies in CABP and in pediatric populations. “To reduce the development of drug-resistant bacteria and maintain the effectiveness of Nuzyra and other antibacterial drugs, Nuzyra should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria,” according to a statement in the indications section of the prescribing information.
Omadacycline is contraindicated for patients with a known hypersensitivity to the drug or any members of the tetracycline class of antibacterial drugs; hypersensitivity reactions have been observed, so use should be discontinued if one is suspected. Use of this drug during later stages of pregnancy can lead to irreversible discoloration of the infant’s teeth and inhibition of bone growth; it should also not be used during breastfeeding.
Because omadacycline is structurally similar to tetracycline class drugs, some adverse reactions to those drugs may be seen with this one, such as photosensitivity, pseudotumor cerebri, and antianabolic action. Adverse reactions known to have an association with omadacycline include nausea, vomiting, hypertension, insomnia, diarrhea, constipation, and increases of alanine aminotransferase, aspartate aminotransferase, and/or gamma-glutamyl transferase.
Drug interactions may occur with anticoagulants, so dosage of those drugs may need to be reduced while treating with omadacycline. Antacids also are believed to have a drug interaction – specifically, impairing absorption of omadacycline
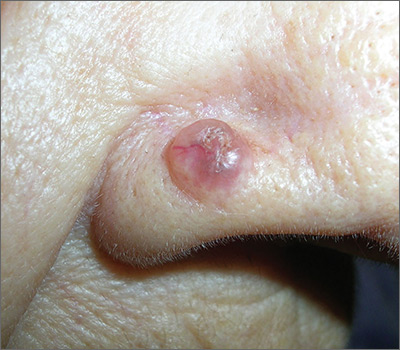
Growth on nose
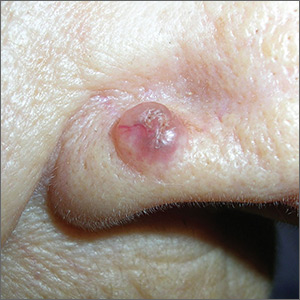
The FP made the presumptive diagnosis of a nodular basal cell carcinoma.
He explained the importance of performing a biopsy and obtained informed consent. On the same day of the patient’s visit, he injected 1% lidocaine with epinephrine under the lesion with a single stick of a 30 gauge needle. He knew that it was safe to use epinephrine on the nose, and that it would prevent excessive bleeding during the biopsy. Contrary to the myth frequently taught in medical school, the nose is a very vascular area. The physician performed a shave biopsy that removed the top of the lesion flush with the skin around it. (See the Watch & Learn video on “Shave biopsy.”)
The pathology report came back as a nodular basal cell carcinoma. On the following visit, the physician recommended Mohs surgery as a way to preserve the vital anatomy of the nasal ala and achieve the highest cure rate.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Basal cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:989-998.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The FP made the presumptive diagnosis of a nodular basal cell carcinoma.
He explained the importance of performing a biopsy and obtained informed consent. On the same day of the patient’s visit, he injected 1% lidocaine with epinephrine under the lesion with a single stick of a 30 gauge needle. He knew that it was safe to use epinephrine on the nose, and that it would prevent excessive bleeding during the biopsy. Contrary to the myth frequently taught in medical school, the nose is a very vascular area. The physician performed a shave biopsy that removed the top of the lesion flush with the skin around it. (See the Watch & Learn video on “Shave biopsy.”)
The pathology report came back as a nodular basal cell carcinoma. On the following visit, the physician recommended Mohs surgery as a way to preserve the vital anatomy of the nasal ala and achieve the highest cure rate.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Basal cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:989-998.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The FP made the presumptive diagnosis of a nodular basal cell carcinoma.
He explained the importance of performing a biopsy and obtained informed consent. On the same day of the patient’s visit, he injected 1% lidocaine with epinephrine under the lesion with a single stick of a 30 gauge needle. He knew that it was safe to use epinephrine on the nose, and that it would prevent excessive bleeding during the biopsy. Contrary to the myth frequently taught in medical school, the nose is a very vascular area. The physician performed a shave biopsy that removed the top of the lesion flush with the skin around it. (See the Watch & Learn video on “Shave biopsy.”)
The pathology report came back as a nodular basal cell carcinoma. On the following visit, the physician recommended Mohs surgery as a way to preserve the vital anatomy of the nasal ala and achieve the highest cure rate.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Basal cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:989-998.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
Adalimumab safety update finds no new signals
not included in the previous 2009 analysis; their evaluation of data from these 18 trials found no new safety signals, they reported in the British Journal of Dermatology.
Adverse event incidence rates were expressed as events per 100 patient-years of exposure to adalimumab and, among the 3,727 patients who were aged 18 years or older and had moderate to severe plaque psoriasis for at least 6 months, there were 5,430 patient-years of cumulative exposure at the December 2015 cutoff date.
There were 3,798 treatment-related events altogether (70 events/100 patient-years); 269 events (5 events/100 patient-years ) led to discontinuation of treatment. The rates for serious adverse events and serious infections were 8.4 and 1.8 events per 100 patient-years, respectively; the most common types of serious infections were pneumonia and cellulitis.
The rates of the most frequently reported adverse events were comparable with those in the 2009 data set, with the most common being nasopharyngitis, upper respiratory tract infection, and headache. Furthermore, the rates of serious adverse events, serious infections, and malignancies were also stable, even with the increasing adalimumab exposure, and these were mostly consistent with what has been seen in large real-world registries.
The researchers did note that the rates of melanoma and nonmelanoma skin cancer were higher than would be expected in the general population, but they suspected this was at least partly because these psoriasis patients were receiving more frequent skin examinations and more skin cancers were being detected. (Incidence rates for these two cancers were stable during 2009-2015).
The analysis had certain limitations, such as a lack of a long-term comparator group. Also, while some patients continue to receive adalimumab for more than 10 years, the maximum duration of treatment in this analysis was only 5.5 years. Finally, the population in these clinical trials may differ from that seen in general practice settings because of the inclusion/exclusion criteria.
Six authors of the study reported multiple disclosures with pharmaceutical companies, including serving as a consultant, speaker, and/or adviser for, receiving honoraria from, and/or receiving grant/research support from AbbVie, which developed adalimumab and funded/advised this study; two authors are AbbVie employees, one is a former employee.
SOURCE: Leonardi C et al. Br J Dermatol. 2018 Aug 31. doi: 10.1111/bjd.17084.
not included in the previous 2009 analysis; their evaluation of data from these 18 trials found no new safety signals, they reported in the British Journal of Dermatology.
Adverse event incidence rates were expressed as events per 100 patient-years of exposure to adalimumab and, among the 3,727 patients who were aged 18 years or older and had moderate to severe plaque psoriasis for at least 6 months, there were 5,430 patient-years of cumulative exposure at the December 2015 cutoff date.
There were 3,798 treatment-related events altogether (70 events/100 patient-years); 269 events (5 events/100 patient-years ) led to discontinuation of treatment. The rates for serious adverse events and serious infections were 8.4 and 1.8 events per 100 patient-years, respectively; the most common types of serious infections were pneumonia and cellulitis.
The rates of the most frequently reported adverse events were comparable with those in the 2009 data set, with the most common being nasopharyngitis, upper respiratory tract infection, and headache. Furthermore, the rates of serious adverse events, serious infections, and malignancies were also stable, even with the increasing adalimumab exposure, and these were mostly consistent with what has been seen in large real-world registries.
The researchers did note that the rates of melanoma and nonmelanoma skin cancer were higher than would be expected in the general population, but they suspected this was at least partly because these psoriasis patients were receiving more frequent skin examinations and more skin cancers were being detected. (Incidence rates for these two cancers were stable during 2009-2015).
The analysis had certain limitations, such as a lack of a long-term comparator group. Also, while some patients continue to receive adalimumab for more than 10 years, the maximum duration of treatment in this analysis was only 5.5 years. Finally, the population in these clinical trials may differ from that seen in general practice settings because of the inclusion/exclusion criteria.
Six authors of the study reported multiple disclosures with pharmaceutical companies, including serving as a consultant, speaker, and/or adviser for, receiving honoraria from, and/or receiving grant/research support from AbbVie, which developed adalimumab and funded/advised this study; two authors are AbbVie employees, one is a former employee.
SOURCE: Leonardi C et al. Br J Dermatol. 2018 Aug 31. doi: 10.1111/bjd.17084.
not included in the previous 2009 analysis; their evaluation of data from these 18 trials found no new safety signals, they reported in the British Journal of Dermatology.
Adverse event incidence rates were expressed as events per 100 patient-years of exposure to adalimumab and, among the 3,727 patients who were aged 18 years or older and had moderate to severe plaque psoriasis for at least 6 months, there were 5,430 patient-years of cumulative exposure at the December 2015 cutoff date.
There were 3,798 treatment-related events altogether (70 events/100 patient-years); 269 events (5 events/100 patient-years ) led to discontinuation of treatment. The rates for serious adverse events and serious infections were 8.4 and 1.8 events per 100 patient-years, respectively; the most common types of serious infections were pneumonia and cellulitis.
The rates of the most frequently reported adverse events were comparable with those in the 2009 data set, with the most common being nasopharyngitis, upper respiratory tract infection, and headache. Furthermore, the rates of serious adverse events, serious infections, and malignancies were also stable, even with the increasing adalimumab exposure, and these were mostly consistent with what has been seen in large real-world registries.
The researchers did note that the rates of melanoma and nonmelanoma skin cancer were higher than would be expected in the general population, but they suspected this was at least partly because these psoriasis patients were receiving more frequent skin examinations and more skin cancers were being detected. (Incidence rates for these two cancers were stable during 2009-2015).
The analysis had certain limitations, such as a lack of a long-term comparator group. Also, while some patients continue to receive adalimumab for more than 10 years, the maximum duration of treatment in this analysis was only 5.5 years. Finally, the population in these clinical trials may differ from that seen in general practice settings because of the inclusion/exclusion criteria.
Six authors of the study reported multiple disclosures with pharmaceutical companies, including serving as a consultant, speaker, and/or adviser for, receiving honoraria from, and/or receiving grant/research support from AbbVie, which developed adalimumab and funded/advised this study; two authors are AbbVie employees, one is a former employee.
SOURCE: Leonardi C et al. Br J Dermatol. 2018 Aug 31. doi: 10.1111/bjd.17084.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Anti-TNF agents preferred for severe psoriasis in pregnancy
CHICAGO – according to Kenneth B. Gordon, MD, professor and chair of dermatology at Medical College of Wisconsin in Milwaukee.
“We always had this concept that psoriasis gets better during pregnancy, that you might have 20% or 30% of patients who might have a little bit of a flare or maintain, but most keep on getting better,” Dr. Gordon told attendees at the American Academy of Dermatology summer meeting.
But the majority doesn’t mean everyone. He shared the case of one pregnant woman who came to him with severe psoriasis, covering the whole of her inner thigh, to underscore that severe cases do happen in pregnancy.
“These are real situations, and when you talk about maternal health, this woman is uncomfortable, she can’t sleep, and she’s having huge stressors that are not only going to impact her and her pregnancy but also that impact her child,” Dr. Gordon said.
Dr. Gordon clarified that he is not referring to patients with limited psoriasis or those who respond to topicals or phototherapy. But because methotrexate or acitretin are “hands-off during pregnancy,” he said, the only systemic therapy available for serious cases besides biologics is cyclosporine, which has its own risks. “We know that [cyclosporine] is associated with preterm labor and preterm birth and significant low birth weight, so even in the best scenario, when we have someone with persistent severe psoriasis in pregnancy, our best agent has a lot of downsides.”
Too few data exist on anti–interleukin (IL)-17 or anti-IL-23 therapies to draw conclusions about their use, he said, and but gastroenterology and rheumatology have a fair amount of evidence on anti–tumor necrosis factor (TNF) therapies during pregnancy because it’s usually too risky to stop treating conditions such as Crohn’s with these drugs. Still, Dr. Gordon cautioned, much of the data on biologics in pregnancy are conflicting.
The question of what medications to use, and in whom, centers on balancing risks to the fetus from the medication versus risks from the condition.
“There are impacts on the fetus of having severe psoriasis, and it varies with severity of disease,” Dr. Gordon said. For example, data suggest an increased likelihood of low birth weight in children born to mothers with severe psoriasis, and that risk may extend to preterm birth as well, although “we don’t know exactly the magnitude of that effect.”
Meanwhile, the consensus from the literature throughout dermatology, rheumatology, and gastroenterology is that anti-TNF agents do not cause birth defects or affect risk of preterm birth or low birth weight.
“The bigger question is what’s the impact on the immune system of the child,” Dr. Gordon said. Data from a small Scandinavian study suggested no increased risk of allergies, infections, or similar immunologic outcomes, but evidence remains limited.
Research has shown that infants’ exposure to anti-TNF medications persists for 3-6 months after delivery, and the American Academy of Pediatrics recommends delaying immunization in children exposed to anti-TNF agents in pregnancy. But actual evidence on immunization outcomes shows no reduced immunogenicity in such children.
“Clearly there is persistence of drug in the child, but in fact you have normal responses to immunization,” Dr. Gordon said. “The pediatricians’ argument is not based on data of what actually happens in immunization; it’s based on the fact that the drug is there.”
So what’s the bottom line?
The National Psoriasis Foundation recommends moisturizers and topical corticosteroids as first-line therapy in pregnant women with psoriasis, followed by phototherapy for second-line treatment.
But some patients will need systemic therapy during pregnancy, although it’s “best not to introduce more medications than needed in pregnancy,” Dr. Gordon said. For women with a significant flare-up or very persistent volatile disease, NPF first recommends cyclosporine, but Dr. Gordon disagrees and would go with anti-TNF agents before cyclosporine.
Data show that certolizumab is not actively transported across the placenta therefore reducing fetal exposure, so Dr. Gordon would specifically use certolizumab first, all other things being equal.
“But if the patient has been on another anti-TNF that’s been working, I don’t really have an issue with staying with it,” he added.
Existing evidence so far shows no impact in terms of genetic abnormalities, birth weight, premature birth, or even infant immunizations from anti-TNF agents. But beyond those, “there is simply not enough information on pregnancy with other forms of biologic therapy to draw conclusions.” Dr. Gordon said.
Dr. Gordon disclosed that he has received grant support and/or honoraria from Abbvie, Amgen, Almirall, and Boehringer Ingelheim.
CHICAGO – according to Kenneth B. Gordon, MD, professor and chair of dermatology at Medical College of Wisconsin in Milwaukee.
“We always had this concept that psoriasis gets better during pregnancy, that you might have 20% or 30% of patients who might have a little bit of a flare or maintain, but most keep on getting better,” Dr. Gordon told attendees at the American Academy of Dermatology summer meeting.
But the majority doesn’t mean everyone. He shared the case of one pregnant woman who came to him with severe psoriasis, covering the whole of her inner thigh, to underscore that severe cases do happen in pregnancy.
“These are real situations, and when you talk about maternal health, this woman is uncomfortable, she can’t sleep, and she’s having huge stressors that are not only going to impact her and her pregnancy but also that impact her child,” Dr. Gordon said.
Dr. Gordon clarified that he is not referring to patients with limited psoriasis or those who respond to topicals or phototherapy. But because methotrexate or acitretin are “hands-off during pregnancy,” he said, the only systemic therapy available for serious cases besides biologics is cyclosporine, which has its own risks. “We know that [cyclosporine] is associated with preterm labor and preterm birth and significant low birth weight, so even in the best scenario, when we have someone with persistent severe psoriasis in pregnancy, our best agent has a lot of downsides.”
Too few data exist on anti–interleukin (IL)-17 or anti-IL-23 therapies to draw conclusions about their use, he said, and but gastroenterology and rheumatology have a fair amount of evidence on anti–tumor necrosis factor (TNF) therapies during pregnancy because it’s usually too risky to stop treating conditions such as Crohn’s with these drugs. Still, Dr. Gordon cautioned, much of the data on biologics in pregnancy are conflicting.
The question of what medications to use, and in whom, centers on balancing risks to the fetus from the medication versus risks from the condition.
“There are impacts on the fetus of having severe psoriasis, and it varies with severity of disease,” Dr. Gordon said. For example, data suggest an increased likelihood of low birth weight in children born to mothers with severe psoriasis, and that risk may extend to preterm birth as well, although “we don’t know exactly the magnitude of that effect.”
Meanwhile, the consensus from the literature throughout dermatology, rheumatology, and gastroenterology is that anti-TNF agents do not cause birth defects or affect risk of preterm birth or low birth weight.
“The bigger question is what’s the impact on the immune system of the child,” Dr. Gordon said. Data from a small Scandinavian study suggested no increased risk of allergies, infections, or similar immunologic outcomes, but evidence remains limited.
Research has shown that infants’ exposure to anti-TNF medications persists for 3-6 months after delivery, and the American Academy of Pediatrics recommends delaying immunization in children exposed to anti-TNF agents in pregnancy. But actual evidence on immunization outcomes shows no reduced immunogenicity in such children.
“Clearly there is persistence of drug in the child, but in fact you have normal responses to immunization,” Dr. Gordon said. “The pediatricians’ argument is not based on data of what actually happens in immunization; it’s based on the fact that the drug is there.”
So what’s the bottom line?
The National Psoriasis Foundation recommends moisturizers and topical corticosteroids as first-line therapy in pregnant women with psoriasis, followed by phototherapy for second-line treatment.
But some patients will need systemic therapy during pregnancy, although it’s “best not to introduce more medications than needed in pregnancy,” Dr. Gordon said. For women with a significant flare-up or very persistent volatile disease, NPF first recommends cyclosporine, but Dr. Gordon disagrees and would go with anti-TNF agents before cyclosporine.
Data show that certolizumab is not actively transported across the placenta therefore reducing fetal exposure, so Dr. Gordon would specifically use certolizumab first, all other things being equal.
“But if the patient has been on another anti-TNF that’s been working, I don’t really have an issue with staying with it,” he added.
Existing evidence so far shows no impact in terms of genetic abnormalities, birth weight, premature birth, or even infant immunizations from anti-TNF agents. But beyond those, “there is simply not enough information on pregnancy with other forms of biologic therapy to draw conclusions.” Dr. Gordon said.
Dr. Gordon disclosed that he has received grant support and/or honoraria from Abbvie, Amgen, Almirall, and Boehringer Ingelheim.
CHICAGO – according to Kenneth B. Gordon, MD, professor and chair of dermatology at Medical College of Wisconsin in Milwaukee.
“We always had this concept that psoriasis gets better during pregnancy, that you might have 20% or 30% of patients who might have a little bit of a flare or maintain, but most keep on getting better,” Dr. Gordon told attendees at the American Academy of Dermatology summer meeting.
But the majority doesn’t mean everyone. He shared the case of one pregnant woman who came to him with severe psoriasis, covering the whole of her inner thigh, to underscore that severe cases do happen in pregnancy.
“These are real situations, and when you talk about maternal health, this woman is uncomfortable, she can’t sleep, and she’s having huge stressors that are not only going to impact her and her pregnancy but also that impact her child,” Dr. Gordon said.
Dr. Gordon clarified that he is not referring to patients with limited psoriasis or those who respond to topicals or phototherapy. But because methotrexate or acitretin are “hands-off during pregnancy,” he said, the only systemic therapy available for serious cases besides biologics is cyclosporine, which has its own risks. “We know that [cyclosporine] is associated with preterm labor and preterm birth and significant low birth weight, so even in the best scenario, when we have someone with persistent severe psoriasis in pregnancy, our best agent has a lot of downsides.”
Too few data exist on anti–interleukin (IL)-17 or anti-IL-23 therapies to draw conclusions about their use, he said, and but gastroenterology and rheumatology have a fair amount of evidence on anti–tumor necrosis factor (TNF) therapies during pregnancy because it’s usually too risky to stop treating conditions such as Crohn’s with these drugs. Still, Dr. Gordon cautioned, much of the data on biologics in pregnancy are conflicting.
The question of what medications to use, and in whom, centers on balancing risks to the fetus from the medication versus risks from the condition.
“There are impacts on the fetus of having severe psoriasis, and it varies with severity of disease,” Dr. Gordon said. For example, data suggest an increased likelihood of low birth weight in children born to mothers with severe psoriasis, and that risk may extend to preterm birth as well, although “we don’t know exactly the magnitude of that effect.”
Meanwhile, the consensus from the literature throughout dermatology, rheumatology, and gastroenterology is that anti-TNF agents do not cause birth defects or affect risk of preterm birth or low birth weight.
“The bigger question is what’s the impact on the immune system of the child,” Dr. Gordon said. Data from a small Scandinavian study suggested no increased risk of allergies, infections, or similar immunologic outcomes, but evidence remains limited.
Research has shown that infants’ exposure to anti-TNF medications persists for 3-6 months after delivery, and the American Academy of Pediatrics recommends delaying immunization in children exposed to anti-TNF agents in pregnancy. But actual evidence on immunization outcomes shows no reduced immunogenicity in such children.
“Clearly there is persistence of drug in the child, but in fact you have normal responses to immunization,” Dr. Gordon said. “The pediatricians’ argument is not based on data of what actually happens in immunization; it’s based on the fact that the drug is there.”
So what’s the bottom line?
The National Psoriasis Foundation recommends moisturizers and topical corticosteroids as first-line therapy in pregnant women with psoriasis, followed by phototherapy for second-line treatment.
But some patients will need systemic therapy during pregnancy, although it’s “best not to introduce more medications than needed in pregnancy,” Dr. Gordon said. For women with a significant flare-up or very persistent volatile disease, NPF first recommends cyclosporine, but Dr. Gordon disagrees and would go with anti-TNF agents before cyclosporine.
Data show that certolizumab is not actively transported across the placenta therefore reducing fetal exposure, so Dr. Gordon would specifically use certolizumab first, all other things being equal.
“But if the patient has been on another anti-TNF that’s been working, I don’t really have an issue with staying with it,” he added.
Existing evidence so far shows no impact in terms of genetic abnormalities, birth weight, premature birth, or even infant immunizations from anti-TNF agents. But beyond those, “there is simply not enough information on pregnancy with other forms of biologic therapy to draw conclusions.” Dr. Gordon said.
Dr. Gordon disclosed that he has received grant support and/or honoraria from Abbvie, Amgen, Almirall, and Boehringer Ingelheim.
EXPERT ANALYSIS FROM SUMMER AAD 2018
Adalimumab safety profile similar in children and adults
The safety profile for adalimumab in children is similar to that of adults, according to findings published in the Journal of Pediatrics.
In an analysis of data from seven clinical trials from 2002-2015, the most common adverse events across indications were upper respiratory tract infection (24 events per 100 patient-years), nasopharyngitis (17 events per 100 PY), and headache (20 events per 100 PY). Serious infections were the most frequent adverse events across indications (8% of all patients; 4 events per 100 PY), reported Gerd Horneff, MD, of the department of general pediatrics at Asklepios Klinik Sankt Augustin (Germany), and his coauthors.
All of the clinical trials were funded by AbbVie, and included 577 pediatric patients with juvenile idiopathic arthritis (JIA), psoriasis, or Crohn’s disease. Patients received subcutaneous injection of adalimumab at a dosage of either 40 mg/0.8 mL or 20 mg/0.4 mL.
Adverse events that occurred after the first adalimumab dose and up to 70 days after the last dose were included. Serious adverse events were defined as “events that were fatal or immediately life-threatening; required inpatient or prolonged hospitalization; resulted in persistent or significant disability/incapacity, congenital anomaly, or spontaneous or elective abortion; or required medical or surgical intervention to prevent a serious outcome,” the authors said.
Infections occurred in 82% of JIA patients (151 events per 100 PY), 74% of patients with psoriasis (169 events per 100 PY), and 76% of patients with CD (132 events per 100 PY). The most common events for JIA, psoriasis, and Crohn’s were injection-site pain (22% of patients; 75 events per 100 PY), headache (30% of patients; 47 events per 100 PY), and worsening of Crohn’s disease (55% of patients; 37 events per 100 PY), respectively.
Serious adverse events occurred in 29% of patients. Rates for JIA, psoriasis, and Crohn’s were 14, 7, and 32 events per 100 PY, respectively. Serious infections were the most common serious adverse event, with rates of 3, 1, and 7 events per 100 PY for JIA, psoriasis, and Crohn’s disease, respectively. Pneumonia was the most commonly reported serious infection (1% of patients; 1 event per 100 PY). One death, due to an accidental fall, occurred in an adolescent patient with psoriasis.
The study findings add to “a more complete understanding of the established safety profile of adalimumab,” and suggest that in pediatric patients, “the overall safety profile was comparable and consistent with that in adults,” Dr. Horneff and his associates added.
AbbVie funded the study. Dr. Horneff has received grants from AbbVie, Chugai, Novartis, Pfizer, and Roche. Seven of the investigators are or were employees of AbbVie and may own AbbVie stock and stock options. Two of the investigators disclosed ties with a number of pharmaceutical companies.
SOURCE: Horneff G et al. J Pediatr. 2018 Oct. doi: 10.1016/j.jpeds.2018.05.042.
The findings of this study underscore the importance of being “aware of the safety profile of this widely used biologic medication,” Philip J. Hashkes, MD, MSc, wrote in an accompanying editorial.
“The major finding was that the safety profile is similar to that seen in adults,” he added. “Although almost all patients developed adverse effects, especially infections, most were usual pediatric infections (including the serious infections) with very few opportunistic infections.” Patients with Crohn’s disease had more serious adverse effects and infections.
Future research should go a step further and focus on “post-marketing surveillance in ‘real life’ settings,” he concluded.
Dr. Hashkes is a pediatric rheumatologist at the Cleveland Clinic. His editorial in response to the article by Horneff et al. appeared in the Journal of Pediatrics (J Pediatr. 2018 Oct;201:2-3).
The findings of this study underscore the importance of being “aware of the safety profile of this widely used biologic medication,” Philip J. Hashkes, MD, MSc, wrote in an accompanying editorial.
“The major finding was that the safety profile is similar to that seen in adults,” he added. “Although almost all patients developed adverse effects, especially infections, most were usual pediatric infections (including the serious infections) with very few opportunistic infections.” Patients with Crohn’s disease had more serious adverse effects and infections.
Future research should go a step further and focus on “post-marketing surveillance in ‘real life’ settings,” he concluded.
Dr. Hashkes is a pediatric rheumatologist at the Cleveland Clinic. His editorial in response to the article by Horneff et al. appeared in the Journal of Pediatrics (J Pediatr. 2018 Oct;201:2-3).
The findings of this study underscore the importance of being “aware of the safety profile of this widely used biologic medication,” Philip J. Hashkes, MD, MSc, wrote in an accompanying editorial.
“The major finding was that the safety profile is similar to that seen in adults,” he added. “Although almost all patients developed adverse effects, especially infections, most were usual pediatric infections (including the serious infections) with very few opportunistic infections.” Patients with Crohn’s disease had more serious adverse effects and infections.
Future research should go a step further and focus on “post-marketing surveillance in ‘real life’ settings,” he concluded.
Dr. Hashkes is a pediatric rheumatologist at the Cleveland Clinic. His editorial in response to the article by Horneff et al. appeared in the Journal of Pediatrics (J Pediatr. 2018 Oct;201:2-3).
The safety profile for adalimumab in children is similar to that of adults, according to findings published in the Journal of Pediatrics.
In an analysis of data from seven clinical trials from 2002-2015, the most common adverse events across indications were upper respiratory tract infection (24 events per 100 patient-years), nasopharyngitis (17 events per 100 PY), and headache (20 events per 100 PY). Serious infections were the most frequent adverse events across indications (8% of all patients; 4 events per 100 PY), reported Gerd Horneff, MD, of the department of general pediatrics at Asklepios Klinik Sankt Augustin (Germany), and his coauthors.
All of the clinical trials were funded by AbbVie, and included 577 pediatric patients with juvenile idiopathic arthritis (JIA), psoriasis, or Crohn’s disease. Patients received subcutaneous injection of adalimumab at a dosage of either 40 mg/0.8 mL or 20 mg/0.4 mL.
Adverse events that occurred after the first adalimumab dose and up to 70 days after the last dose were included. Serious adverse events were defined as “events that were fatal or immediately life-threatening; required inpatient or prolonged hospitalization; resulted in persistent or significant disability/incapacity, congenital anomaly, or spontaneous or elective abortion; or required medical or surgical intervention to prevent a serious outcome,” the authors said.
Infections occurred in 82% of JIA patients (151 events per 100 PY), 74% of patients with psoriasis (169 events per 100 PY), and 76% of patients with CD (132 events per 100 PY). The most common events for JIA, psoriasis, and Crohn’s were injection-site pain (22% of patients; 75 events per 100 PY), headache (30% of patients; 47 events per 100 PY), and worsening of Crohn’s disease (55% of patients; 37 events per 100 PY), respectively.
Serious adverse events occurred in 29% of patients. Rates for JIA, psoriasis, and Crohn’s were 14, 7, and 32 events per 100 PY, respectively. Serious infections were the most common serious adverse event, with rates of 3, 1, and 7 events per 100 PY for JIA, psoriasis, and Crohn’s disease, respectively. Pneumonia was the most commonly reported serious infection (1% of patients; 1 event per 100 PY). One death, due to an accidental fall, occurred in an adolescent patient with psoriasis.
The study findings add to “a more complete understanding of the established safety profile of adalimumab,” and suggest that in pediatric patients, “the overall safety profile was comparable and consistent with that in adults,” Dr. Horneff and his associates added.
AbbVie funded the study. Dr. Horneff has received grants from AbbVie, Chugai, Novartis, Pfizer, and Roche. Seven of the investigators are or were employees of AbbVie and may own AbbVie stock and stock options. Two of the investigators disclosed ties with a number of pharmaceutical companies.
SOURCE: Horneff G et al. J Pediatr. 2018 Oct. doi: 10.1016/j.jpeds.2018.05.042.
The safety profile for adalimumab in children is similar to that of adults, according to findings published in the Journal of Pediatrics.
In an analysis of data from seven clinical trials from 2002-2015, the most common adverse events across indications were upper respiratory tract infection (24 events per 100 patient-years), nasopharyngitis (17 events per 100 PY), and headache (20 events per 100 PY). Serious infections were the most frequent adverse events across indications (8% of all patients; 4 events per 100 PY), reported Gerd Horneff, MD, of the department of general pediatrics at Asklepios Klinik Sankt Augustin (Germany), and his coauthors.
All of the clinical trials were funded by AbbVie, and included 577 pediatric patients with juvenile idiopathic arthritis (JIA), psoriasis, or Crohn’s disease. Patients received subcutaneous injection of adalimumab at a dosage of either 40 mg/0.8 mL or 20 mg/0.4 mL.
Adverse events that occurred after the first adalimumab dose and up to 70 days after the last dose were included. Serious adverse events were defined as “events that were fatal or immediately life-threatening; required inpatient or prolonged hospitalization; resulted in persistent or significant disability/incapacity, congenital anomaly, or spontaneous or elective abortion; or required medical or surgical intervention to prevent a serious outcome,” the authors said.
Infections occurred in 82% of JIA patients (151 events per 100 PY), 74% of patients with psoriasis (169 events per 100 PY), and 76% of patients with CD (132 events per 100 PY). The most common events for JIA, psoriasis, and Crohn’s were injection-site pain (22% of patients; 75 events per 100 PY), headache (30% of patients; 47 events per 100 PY), and worsening of Crohn’s disease (55% of patients; 37 events per 100 PY), respectively.
Serious adverse events occurred in 29% of patients. Rates for JIA, psoriasis, and Crohn’s were 14, 7, and 32 events per 100 PY, respectively. Serious infections were the most common serious adverse event, with rates of 3, 1, and 7 events per 100 PY for JIA, psoriasis, and Crohn’s disease, respectively. Pneumonia was the most commonly reported serious infection (1% of patients; 1 event per 100 PY). One death, due to an accidental fall, occurred in an adolescent patient with psoriasis.
The study findings add to “a more complete understanding of the established safety profile of adalimumab,” and suggest that in pediatric patients, “the overall safety profile was comparable and consistent with that in adults,” Dr. Horneff and his associates added.
AbbVie funded the study. Dr. Horneff has received grants from AbbVie, Chugai, Novartis, Pfizer, and Roche. Seven of the investigators are or were employees of AbbVie and may own AbbVie stock and stock options. Two of the investigators disclosed ties with a number of pharmaceutical companies.
SOURCE: Horneff G et al. J Pediatr. 2018 Oct. doi: 10.1016/j.jpeds.2018.05.042.
FROM THE JOURNAL OF PEDIATRICS
Key clinical point:
Major finding: The most common adverse events across indications were upper respiratory tract infection (24 events per 100 patient-years), nasopharyngitis (17 events per 100 PY), and headache (20 events per 100 PY).
Study details: An analysis of data for 577 pediatric patients from seven clinical trials between September 2002 and December 2015.
Disclosures: AbbVie funded the study. Dr. Horneff has received grants from AbbVie, Chugai, Novartis, Pfizer, and Roche. Seven of the investigators are or were employees of AbbVie and may own AbbVie stock and stock options. Two of the investigators disclosed ties with a number of pharmaceutical companies.
Source: Horneff G et al. J Pediatr. 2018 Oct. doi: 10.1016/j.jpeds.2018.05.042.
Dual-frequency ultrasound promising for refractory rosacea
, according to a new study in Dermatologic Surgery.
In the study, a retrospective medical record analysis of 42 rosacea patients, DFU improved symptoms, including erythema index (EI) and transepithelial water loss (TEWL), and also improved outcomes on the patient self-assessment (PSA), reported Jun Yeong Park, MD, and coauthors, from the department of dermatology, Hallym University Sacred Heart Hospital in Anyang, South Korea.
Of the 42 patients, 26 had erythematotelangiectatic rosacea, 14 had papulopustular rosacea, and 2 had mixed disease; their mean age was 48 years, and they had had rosacea for a mean of 2 years. Patients had started DFU treatment between September 2016 and December 2016, and were not taking oral medication (besides antihistamines), topical ointments, or other laser treatments at the time. Most had been treated with various systemic therapies, topical therapies, or lasers, but had not had adequate improvement of flushing and erythema.
Patients received DFU treatment of the entire face twice per week for the first week, followed by one-week intervals, for a total of 12 weeks. Each treatment session lasted 10 minutes, and included DFU frequencies of 3/4.5 MHz at an ultrasound intensity of 2.0W/cm2 for 5 minutes, followed by 4.5 MHz at an intensity of 2.0W/cm2. Responses to treatment were based on EI, TEWL values (measured on three different sites on each cheek according to guidelines established by the European Group for Efficacy Measurements on Cosmetics and Other Topical Products), and PSA. PSA was completed on a scale of 0 (absent) to 4 (severe) for erythema, itching sensation, and burning sensation.
At 12 weeks follow-up, the mean EI dropped from 16.3 at baseline to 12.7 at 12 weeks (P less than .01). Mean TEWL values dropped from a baseline of 35.8 g m–1 h–1 to 22.8 g m–1 h–1 at 12 weeks (P less than .01).
When evaluated by rosacea subtype, a slightly higher reduction in the group with papulopustular rosacea was seen for EI and TEWL, compared with those with the erythematotelangiectatic subtype, but the differences were not statistically significant for either, the authors reported.
Between baseline and 12 weeks, the PSA values for erythema decreased from 2.6 to 1.1. Itching and burning grades also decreased, from 2.4 to 0.4 and from 2.4 to 0.3, respectively.
The findings verify that there were “improvements in the barrier function of patients with refractory rosacea, based on the TEWL level before and after treatment,” the authors noted. “Therefore, DFU may be an additional treatment option for rosacea that is resistant to other treatments.”
This study is the first to evaluate DFU in patients with refractory rosacea “who did not show signs of recovery after undergoing previous therapies,” they noted.
No disclosures were reported.
SOURCE: Park J et al. Dermatol Surg 2018 Sep;44(9):1209-15. doi: 10.1097/DSS.0000000000001552.
, according to a new study in Dermatologic Surgery.
In the study, a retrospective medical record analysis of 42 rosacea patients, DFU improved symptoms, including erythema index (EI) and transepithelial water loss (TEWL), and also improved outcomes on the patient self-assessment (PSA), reported Jun Yeong Park, MD, and coauthors, from the department of dermatology, Hallym University Sacred Heart Hospital in Anyang, South Korea.
Of the 42 patients, 26 had erythematotelangiectatic rosacea, 14 had papulopustular rosacea, and 2 had mixed disease; their mean age was 48 years, and they had had rosacea for a mean of 2 years. Patients had started DFU treatment between September 2016 and December 2016, and were not taking oral medication (besides antihistamines), topical ointments, or other laser treatments at the time. Most had been treated with various systemic therapies, topical therapies, or lasers, but had not had adequate improvement of flushing and erythema.
Patients received DFU treatment of the entire face twice per week for the first week, followed by one-week intervals, for a total of 12 weeks. Each treatment session lasted 10 minutes, and included DFU frequencies of 3/4.5 MHz at an ultrasound intensity of 2.0W/cm2 for 5 minutes, followed by 4.5 MHz at an intensity of 2.0W/cm2. Responses to treatment were based on EI, TEWL values (measured on three different sites on each cheek according to guidelines established by the European Group for Efficacy Measurements on Cosmetics and Other Topical Products), and PSA. PSA was completed on a scale of 0 (absent) to 4 (severe) for erythema, itching sensation, and burning sensation.
At 12 weeks follow-up, the mean EI dropped from 16.3 at baseline to 12.7 at 12 weeks (P less than .01). Mean TEWL values dropped from a baseline of 35.8 g m–1 h–1 to 22.8 g m–1 h–1 at 12 weeks (P less than .01).
When evaluated by rosacea subtype, a slightly higher reduction in the group with papulopustular rosacea was seen for EI and TEWL, compared with those with the erythematotelangiectatic subtype, but the differences were not statistically significant for either, the authors reported.
Between baseline and 12 weeks, the PSA values for erythema decreased from 2.6 to 1.1. Itching and burning grades also decreased, from 2.4 to 0.4 and from 2.4 to 0.3, respectively.
The findings verify that there were “improvements in the barrier function of patients with refractory rosacea, based on the TEWL level before and after treatment,” the authors noted. “Therefore, DFU may be an additional treatment option for rosacea that is resistant to other treatments.”
This study is the first to evaluate DFU in patients with refractory rosacea “who did not show signs of recovery after undergoing previous therapies,” they noted.
No disclosures were reported.
SOURCE: Park J et al. Dermatol Surg 2018 Sep;44(9):1209-15. doi: 10.1097/DSS.0000000000001552.
, according to a new study in Dermatologic Surgery.
In the study, a retrospective medical record analysis of 42 rosacea patients, DFU improved symptoms, including erythema index (EI) and transepithelial water loss (TEWL), and also improved outcomes on the patient self-assessment (PSA), reported Jun Yeong Park, MD, and coauthors, from the department of dermatology, Hallym University Sacred Heart Hospital in Anyang, South Korea.
Of the 42 patients, 26 had erythematotelangiectatic rosacea, 14 had papulopustular rosacea, and 2 had mixed disease; their mean age was 48 years, and they had had rosacea for a mean of 2 years. Patients had started DFU treatment between September 2016 and December 2016, and were not taking oral medication (besides antihistamines), topical ointments, or other laser treatments at the time. Most had been treated with various systemic therapies, topical therapies, or lasers, but had not had adequate improvement of flushing and erythema.
Patients received DFU treatment of the entire face twice per week for the first week, followed by one-week intervals, for a total of 12 weeks. Each treatment session lasted 10 minutes, and included DFU frequencies of 3/4.5 MHz at an ultrasound intensity of 2.0W/cm2 for 5 minutes, followed by 4.5 MHz at an intensity of 2.0W/cm2. Responses to treatment were based on EI, TEWL values (measured on three different sites on each cheek according to guidelines established by the European Group for Efficacy Measurements on Cosmetics and Other Topical Products), and PSA. PSA was completed on a scale of 0 (absent) to 4 (severe) for erythema, itching sensation, and burning sensation.
At 12 weeks follow-up, the mean EI dropped from 16.3 at baseline to 12.7 at 12 weeks (P less than .01). Mean TEWL values dropped from a baseline of 35.8 g m–1 h–1 to 22.8 g m–1 h–1 at 12 weeks (P less than .01).
When evaluated by rosacea subtype, a slightly higher reduction in the group with papulopustular rosacea was seen for EI and TEWL, compared with those with the erythematotelangiectatic subtype, but the differences were not statistically significant for either, the authors reported.
Between baseline and 12 weeks, the PSA values for erythema decreased from 2.6 to 1.1. Itching and burning grades also decreased, from 2.4 to 0.4 and from 2.4 to 0.3, respectively.
The findings verify that there were “improvements in the barrier function of patients with refractory rosacea, based on the TEWL level before and after treatment,” the authors noted. “Therefore, DFU may be an additional treatment option for rosacea that is resistant to other treatments.”
This study is the first to evaluate DFU in patients with refractory rosacea “who did not show signs of recovery after undergoing previous therapies,” they noted.
No disclosures were reported.
SOURCE: Park J et al. Dermatol Surg 2018 Sep;44(9):1209-15. doi: 10.1097/DSS.0000000000001552.
Key clinical point: Dual-frequency ultrasound may be an effective option for treatment-resistant rosacea.
Major finding: In 12 weeks, the erythema index dropped from 16.3 to 12.7 (P less than .01), along with drops in patient self assessment measures for erythema, itching, and burning.
Study details: A retrospective electronic medical records analysis of 42 rosacea patients.
Disclosures: No disclosures were reported.
Source: Park J et al. Dermatol Surg 2018 Sep;44(9):1209-15. doi: 10.1097/DSS.0000000000001552.
FDA approves Seysara for treatment of moderate to severe acne
vulgaris for people aged 9 years and older, according to a press release from Paratek, the drug’s manufacturer.
FDA approval is based on results from two phase 3 clinical trials (NCT02320149 and NCT02322866), in which patients received either sarecycline at 1.5 mg/kg per day or placebo for 12 weeks. Patients who received sarecycline were significantly more likely to reach the primary endpoint of improved acne severity based on absolute change in facial lesion counts and percentage of participants with Investigator Global Assessment success.
Common adverse events reported in the sarecycline group were nausea (3.2%), nasopharyngitis (2.8%), and headache (2.8%). The discontinuation rate due to adverse events among sarecycline-treated patients in both studies combined was 1.4%.
Sarecycline is an oral, narrow spectrum tetracycline-derived antibiotic with anti-inflammatory properties, and is approved for once-daily use, according to Paratek. Seysara will be marketed in the United States by Almirall SA.
vulgaris for people aged 9 years and older, according to a press release from Paratek, the drug’s manufacturer.
FDA approval is based on results from two phase 3 clinical trials (NCT02320149 and NCT02322866), in which patients received either sarecycline at 1.5 mg/kg per day or placebo for 12 weeks. Patients who received sarecycline were significantly more likely to reach the primary endpoint of improved acne severity based on absolute change in facial lesion counts and percentage of participants with Investigator Global Assessment success.
Common adverse events reported in the sarecycline group were nausea (3.2%), nasopharyngitis (2.8%), and headache (2.8%). The discontinuation rate due to adverse events among sarecycline-treated patients in both studies combined was 1.4%.
Sarecycline is an oral, narrow spectrum tetracycline-derived antibiotic with anti-inflammatory properties, and is approved for once-daily use, according to Paratek. Seysara will be marketed in the United States by Almirall SA.
vulgaris for people aged 9 years and older, according to a press release from Paratek, the drug’s manufacturer.
FDA approval is based on results from two phase 3 clinical trials (NCT02320149 and NCT02322866), in which patients received either sarecycline at 1.5 mg/kg per day or placebo for 12 weeks. Patients who received sarecycline were significantly more likely to reach the primary endpoint of improved acne severity based on absolute change in facial lesion counts and percentage of participants with Investigator Global Assessment success.
Common adverse events reported in the sarecycline group were nausea (3.2%), nasopharyngitis (2.8%), and headache (2.8%). The discontinuation rate due to adverse events among sarecycline-treated patients in both studies combined was 1.4%.
Sarecycline is an oral, narrow spectrum tetracycline-derived antibiotic with anti-inflammatory properties, and is approved for once-daily use, according to Paratek. Seysara will be marketed in the United States by Almirall SA.