User login
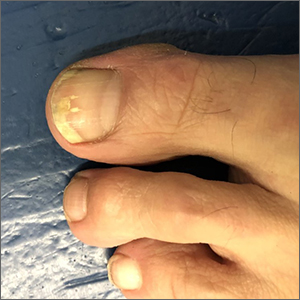
Toenail trauma
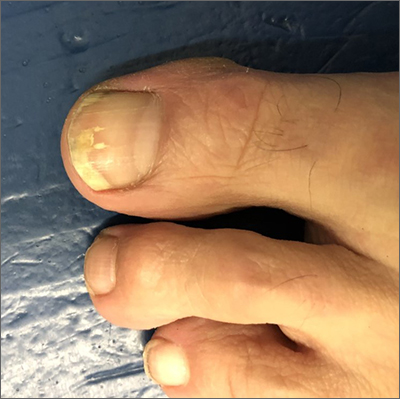
The patient’s initial injury was probably a subungual hematoma, which can take 12 to 18 months to resolve (the time it takes for a new toenail to grow). However, the precipitating trauma likely created an opportunity for fungal elements to invade the nail plate, resulting in the current complaint of superficial onychomycosis.
Onychomycosis is a frequently seen condition with increasing prevalence in older patients. It has several clinical presentations: Superficial onychomycosis manifests with chalky white changes on the surface of the nail. Distal subungual onychomycosis develops at the distal aspect of the nail with thickening and subungual debris. Proximal subungual onychomycosis occurs in the proximal aspect of the nail.
Although often asymptomatic, onychomycosis can cause thickening of the nails and development of subsequent deformity or pincer nails (which painfully “pinch” the underlying skin). It is especially concerning in patients with diabetes or peripheral neuropathy, in whom the abnormal thickness and shape of the nails can lead to microtrauma at the proximal and lateral attachments of the nail. These patients have an increased risk of secondary infection, possible complications, and even, for some, amputation.
If the patient is asymptomatic, and does not have diabetes, neuropathy, or other risk factors, treatment is not required. For those who would benefit from treatment, it is usually safe and inexpensive with the current generation of oral antifungal medications.
Some recommend confirmatory testing before treatment intiation,1 but the low adverse effect profile of terbinafine and its current cost below $10/month2 make empiric treatment safe and cost effective in most cases.3 If needed, and with access to microscopy, a potassium hydroxide (KOH) prep can be performed on scrapings from the affected portions of the nail. If that is not available, scrapings or clippings can be sent to the lab for KOH and periodic acid-Schiff staining.
The US Food and Drug Administration previously recommended follow-up liver enzyme tests if terbinafine is used for more than 6 weeks. (Fingernails require only 6 weeks of treatment, but toenails grow more slowly and require 12 weeks of treatment.) However, research has demonstrated that hepatotoxicity risk is extremely low and transaminase elevations are rare.4 In the rare cases that liver dysfunction has occurred, patients developed symptoms of jaundice, malaise, dark urine, or pruritis.4
This patient was counseled regarding the fungal nature of onychomycosis and the general safety of a 90-day course of oral terbinafine 250 mg/d—provided he did not have underlying liver or kidney disease or leukopenia. He reported that he had not had any blood work performed in the past year but was due for his annual wellness evaluation, at which he would discuss his overall health with his primary care provider, obtain baseline blood testing, and determine whether to proceed with treatment. He was advised that if, after starting treatment, he developed any symptoms of jaundice, dark urine, or other difficulties, he should report them to his care team.
Photo courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
- Frazier WT, Santiago-Delgado ZM, Stupka KC 2nd. Onychomycosis: rapid evidence review. Am Fam Physician. 2021;104:359-367.
- Terbinafine. GoodRx. Accessed August 9, 2022. https://www.goodrx.com/terbinafine
- Mikailov A, Cohen J, Joyce C, et al. Cost-effectiveness of confirmatory testing before treatment of onychomycosis. JAMA Dermatol. 2016;152:276-281. doi: 10.1001/jamadermatol.2015.4190
- Sun CW, Hsu S. Terbinafine: safety profile and monitoring in treatment of dermatophyte infections. Dermatol Ther. 2019;32:e13111. doi: 10.1111/dth.13111

The patient’s initial injury was probably a subungual hematoma, which can take 12 to 18 months to resolve (the time it takes for a new toenail to grow). However, the precipitating trauma likely created an opportunity for fungal elements to invade the nail plate, resulting in the current complaint of superficial onychomycosis.
Onychomycosis is a frequently seen condition with increasing prevalence in older patients. It has several clinical presentations: Superficial onychomycosis manifests with chalky white changes on the surface of the nail. Distal subungual onychomycosis develops at the distal aspect of the nail with thickening and subungual debris. Proximal subungual onychomycosis occurs in the proximal aspect of the nail.
Although often asymptomatic, onychomycosis can cause thickening of the nails and development of subsequent deformity or pincer nails (which painfully “pinch” the underlying skin). It is especially concerning in patients with diabetes or peripheral neuropathy, in whom the abnormal thickness and shape of the nails can lead to microtrauma at the proximal and lateral attachments of the nail. These patients have an increased risk of secondary infection, possible complications, and even, for some, amputation.
If the patient is asymptomatic, and does not have diabetes, neuropathy, or other risk factors, treatment is not required. For those who would benefit from treatment, it is usually safe and inexpensive with the current generation of oral antifungal medications.
Some recommend confirmatory testing before treatment intiation,1 but the low adverse effect profile of terbinafine and its current cost below $10/month2 make empiric treatment safe and cost effective in most cases.3 If needed, and with access to microscopy, a potassium hydroxide (KOH) prep can be performed on scrapings from the affected portions of the nail. If that is not available, scrapings or clippings can be sent to the lab for KOH and periodic acid-Schiff staining.
The US Food and Drug Administration previously recommended follow-up liver enzyme tests if terbinafine is used for more than 6 weeks. (Fingernails require only 6 weeks of treatment, but toenails grow more slowly and require 12 weeks of treatment.) However, research has demonstrated that hepatotoxicity risk is extremely low and transaminase elevations are rare.4 In the rare cases that liver dysfunction has occurred, patients developed symptoms of jaundice, malaise, dark urine, or pruritis.4
This patient was counseled regarding the fungal nature of onychomycosis and the general safety of a 90-day course of oral terbinafine 250 mg/d—provided he did not have underlying liver or kidney disease or leukopenia. He reported that he had not had any blood work performed in the past year but was due for his annual wellness evaluation, at which he would discuss his overall health with his primary care provider, obtain baseline blood testing, and determine whether to proceed with treatment. He was advised that if, after starting treatment, he developed any symptoms of jaundice, dark urine, or other difficulties, he should report them to his care team.
Photo courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

The patient’s initial injury was probably a subungual hematoma, which can take 12 to 18 months to resolve (the time it takes for a new toenail to grow). However, the precipitating trauma likely created an opportunity for fungal elements to invade the nail plate, resulting in the current complaint of superficial onychomycosis.
Onychomycosis is a frequently seen condition with increasing prevalence in older patients. It has several clinical presentations: Superficial onychomycosis manifests with chalky white changes on the surface of the nail. Distal subungual onychomycosis develops at the distal aspect of the nail with thickening and subungual debris. Proximal subungual onychomycosis occurs in the proximal aspect of the nail.
Although often asymptomatic, onychomycosis can cause thickening of the nails and development of subsequent deformity or pincer nails (which painfully “pinch” the underlying skin). It is especially concerning in patients with diabetes or peripheral neuropathy, in whom the abnormal thickness and shape of the nails can lead to microtrauma at the proximal and lateral attachments of the nail. These patients have an increased risk of secondary infection, possible complications, and even, for some, amputation.
If the patient is asymptomatic, and does not have diabetes, neuropathy, or other risk factors, treatment is not required. For those who would benefit from treatment, it is usually safe and inexpensive with the current generation of oral antifungal medications.
Some recommend confirmatory testing before treatment intiation,1 but the low adverse effect profile of terbinafine and its current cost below $10/month2 make empiric treatment safe and cost effective in most cases.3 If needed, and with access to microscopy, a potassium hydroxide (KOH) prep can be performed on scrapings from the affected portions of the nail. If that is not available, scrapings or clippings can be sent to the lab for KOH and periodic acid-Schiff staining.
The US Food and Drug Administration previously recommended follow-up liver enzyme tests if terbinafine is used for more than 6 weeks. (Fingernails require only 6 weeks of treatment, but toenails grow more slowly and require 12 weeks of treatment.) However, research has demonstrated that hepatotoxicity risk is extremely low and transaminase elevations are rare.4 In the rare cases that liver dysfunction has occurred, patients developed symptoms of jaundice, malaise, dark urine, or pruritis.4
This patient was counseled regarding the fungal nature of onychomycosis and the general safety of a 90-day course of oral terbinafine 250 mg/d—provided he did not have underlying liver or kidney disease or leukopenia. He reported that he had not had any blood work performed in the past year but was due for his annual wellness evaluation, at which he would discuss his overall health with his primary care provider, obtain baseline blood testing, and determine whether to proceed with treatment. He was advised that if, after starting treatment, he developed any symptoms of jaundice, dark urine, or other difficulties, he should report them to his care team.
Photo courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
- Frazier WT, Santiago-Delgado ZM, Stupka KC 2nd. Onychomycosis: rapid evidence review. Am Fam Physician. 2021;104:359-367.
- Terbinafine. GoodRx. Accessed August 9, 2022. https://www.goodrx.com/terbinafine
- Mikailov A, Cohen J, Joyce C, et al. Cost-effectiveness of confirmatory testing before treatment of onychomycosis. JAMA Dermatol. 2016;152:276-281. doi: 10.1001/jamadermatol.2015.4190
- Sun CW, Hsu S. Terbinafine: safety profile and monitoring in treatment of dermatophyte infections. Dermatol Ther. 2019;32:e13111. doi: 10.1111/dth.13111
- Frazier WT, Santiago-Delgado ZM, Stupka KC 2nd. Onychomycosis: rapid evidence review. Am Fam Physician. 2021;104:359-367.
- Terbinafine. GoodRx. Accessed August 9, 2022. https://www.goodrx.com/terbinafine
- Mikailov A, Cohen J, Joyce C, et al. Cost-effectiveness of confirmatory testing before treatment of onychomycosis. JAMA Dermatol. 2016;152:276-281. doi: 10.1001/jamadermatol.2015.4190
- Sun CW, Hsu S. Terbinafine: safety profile and monitoring in treatment of dermatophyte infections. Dermatol Ther. 2019;32:e13111. doi: 10.1111/dth.13111
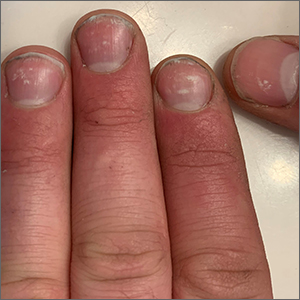
Spotted white fingernails
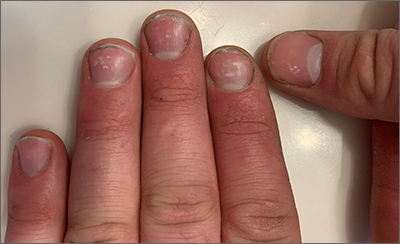
White nail changes are broadly called leukonychia: “leuko” meaning white and “nychia” referring to the nail. Scattered or single asymptomatic cloudy white nail lesions occurring without other associated skin or nail disorders are more specifically called punctate leukonychia.
Punctate leukonychia is theorized to be caused by trauma at the proximal nail matrix, affecting the developing nail.1 The trauma may result from aggressive nail care practices or damage to the cuticle. In many cases, there is no history of known trauma. For this patient with multiple lesions, who performed manual work, multiple small traumas may have induced the punctate leukonychia.
Other causes of leukonychia include superficial onychomycosis (in which discoloration may be whiter than the usual yellow-brown), renal disease, and arsenic toxicity.1 Arsenic toxicity causes transverse leukonychia in a band-like fashion, since it is a systemic insult to the growing nails. Longitudinal leukonychia is due to a more localized insult to the nail matrix, causing the white lines to grow out with the nail along the axis of the digit. Other than avoiding trauma, there is no treatment needed or recommended for punctate leukonychia.
The patient was counseled on the benign nature of his punctate leukonychia and assured that no treatment was necessary.
Photo courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Iorizzo M, Starace M, Pasch MC. Leukonychia: what can white nails tell us? Am J Clin Dermatol. 2022;23:177-193. doi: 10.1007/s40257-022-00671-6

White nail changes are broadly called leukonychia: “leuko” meaning white and “nychia” referring to the nail. Scattered or single asymptomatic cloudy white nail lesions occurring without other associated skin or nail disorders are more specifically called punctate leukonychia.
Punctate leukonychia is theorized to be caused by trauma at the proximal nail matrix, affecting the developing nail.1 The trauma may result from aggressive nail care practices or damage to the cuticle. In many cases, there is no history of known trauma. For this patient with multiple lesions, who performed manual work, multiple small traumas may have induced the punctate leukonychia.
Other causes of leukonychia include superficial onychomycosis (in which discoloration may be whiter than the usual yellow-brown), renal disease, and arsenic toxicity.1 Arsenic toxicity causes transverse leukonychia in a band-like fashion, since it is a systemic insult to the growing nails. Longitudinal leukonychia is due to a more localized insult to the nail matrix, causing the white lines to grow out with the nail along the axis of the digit. Other than avoiding trauma, there is no treatment needed or recommended for punctate leukonychia.
The patient was counseled on the benign nature of his punctate leukonychia and assured that no treatment was necessary.
Photo courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

White nail changes are broadly called leukonychia: “leuko” meaning white and “nychia” referring to the nail. Scattered or single asymptomatic cloudy white nail lesions occurring without other associated skin or nail disorders are more specifically called punctate leukonychia.
Punctate leukonychia is theorized to be caused by trauma at the proximal nail matrix, affecting the developing nail.1 The trauma may result from aggressive nail care practices or damage to the cuticle. In many cases, there is no history of known trauma. For this patient with multiple lesions, who performed manual work, multiple small traumas may have induced the punctate leukonychia.
Other causes of leukonychia include superficial onychomycosis (in which discoloration may be whiter than the usual yellow-brown), renal disease, and arsenic toxicity.1 Arsenic toxicity causes transverse leukonychia in a band-like fashion, since it is a systemic insult to the growing nails. Longitudinal leukonychia is due to a more localized insult to the nail matrix, causing the white lines to grow out with the nail along the axis of the digit. Other than avoiding trauma, there is no treatment needed or recommended for punctate leukonychia.
The patient was counseled on the benign nature of his punctate leukonychia and assured that no treatment was necessary.
Photo courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Iorizzo M, Starace M, Pasch MC. Leukonychia: what can white nails tell us? Am J Clin Dermatol. 2022;23:177-193. doi: 10.1007/s40257-022-00671-6
1. Iorizzo M, Starace M, Pasch MC. Leukonychia: what can white nails tell us? Am J Clin Dermatol. 2022;23:177-193. doi: 10.1007/s40257-022-00671-6
FDA approves adalimumab-bwwd biosimilar (Hadlima) in high-concentration form
The U.S. Food and Drug Administration today approved a citrate-free, high-concentration formulation of adalimumab-bwwd (Hadlima), the manufacturer, Samsung Bioepis, and its commercialization partner Organon said in an announcement.
Hadlima is a biosimilar of the tumor necrosis factor inhibitor reference product adalimumab (Humira).
Hadlima was first approved in July 2019 in a citrated, 50-mg/mL formulation. The new citrate-free, 100-mg/mL version will be available in prefilled syringe and autoinjector options.
The 100-mg/mL formulation is indicated for the same seven conditions as its 50-mg/mL counterpart: rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, plaque psoriasis, psoriatic arthritis, ankylosing spondylitis, adult and pediatric Crohn’s disease, and ulcerative colitis.
The approval was based on clinical data from a randomized, single-blind, two-arm, parallel group, single-dose study that compared the pharmacokinetics, safety, tolerability, and immunogenicity of the 100-mg/mL and 50-mg/mL formulations of Hadlima in healthy volunteers.
Both low- and high-concentration formulations of Humira are currently marketed in the United States. Organon said that it expects to market Hadlima in the United States on or after July 1, 2023, in accordance with a licensing agreement with AbbVie.
The prescribing information for Hadlima includes specific warnings and areas of concern. The drug should not be administered to individuals who are known to be hypersensitive to adalimumab. The drug may lower the ability of the immune system to fight infections and may increase risk of infections, including serious infections leading to hospitalization or death, such as tuberculosis, bacterial sepsis, invasive fungal infections (such as histoplasmosis), and infections attributable to other opportunistic pathogens.
A test for latent TB infection should be given before administration, and treatment of TB should begin before administration of Hadlima.
Patients taking Hadlima should not take a live vaccine.
The most common adverse effects (incidence > 10%) include infections (for example, upper respiratory infections, sinusitis), injection site reactions, headache, and rash.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration today approved a citrate-free, high-concentration formulation of adalimumab-bwwd (Hadlima), the manufacturer, Samsung Bioepis, and its commercialization partner Organon said in an announcement.
Hadlima is a biosimilar of the tumor necrosis factor inhibitor reference product adalimumab (Humira).
Hadlima was first approved in July 2019 in a citrated, 50-mg/mL formulation. The new citrate-free, 100-mg/mL version will be available in prefilled syringe and autoinjector options.
The 100-mg/mL formulation is indicated for the same seven conditions as its 50-mg/mL counterpart: rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, plaque psoriasis, psoriatic arthritis, ankylosing spondylitis, adult and pediatric Crohn’s disease, and ulcerative colitis.
The approval was based on clinical data from a randomized, single-blind, two-arm, parallel group, single-dose study that compared the pharmacokinetics, safety, tolerability, and immunogenicity of the 100-mg/mL and 50-mg/mL formulations of Hadlima in healthy volunteers.
Both low- and high-concentration formulations of Humira are currently marketed in the United States. Organon said that it expects to market Hadlima in the United States on or after July 1, 2023, in accordance with a licensing agreement with AbbVie.
The prescribing information for Hadlima includes specific warnings and areas of concern. The drug should not be administered to individuals who are known to be hypersensitive to adalimumab. The drug may lower the ability of the immune system to fight infections and may increase risk of infections, including serious infections leading to hospitalization or death, such as tuberculosis, bacterial sepsis, invasive fungal infections (such as histoplasmosis), and infections attributable to other opportunistic pathogens.
A test for latent TB infection should be given before administration, and treatment of TB should begin before administration of Hadlima.
Patients taking Hadlima should not take a live vaccine.
The most common adverse effects (incidence > 10%) include infections (for example, upper respiratory infections, sinusitis), injection site reactions, headache, and rash.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration today approved a citrate-free, high-concentration formulation of adalimumab-bwwd (Hadlima), the manufacturer, Samsung Bioepis, and its commercialization partner Organon said in an announcement.
Hadlima is a biosimilar of the tumor necrosis factor inhibitor reference product adalimumab (Humira).
Hadlima was first approved in July 2019 in a citrated, 50-mg/mL formulation. The new citrate-free, 100-mg/mL version will be available in prefilled syringe and autoinjector options.
The 100-mg/mL formulation is indicated for the same seven conditions as its 50-mg/mL counterpart: rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, plaque psoriasis, psoriatic arthritis, ankylosing spondylitis, adult and pediatric Crohn’s disease, and ulcerative colitis.
The approval was based on clinical data from a randomized, single-blind, two-arm, parallel group, single-dose study that compared the pharmacokinetics, safety, tolerability, and immunogenicity of the 100-mg/mL and 50-mg/mL formulations of Hadlima in healthy volunteers.
Both low- and high-concentration formulations of Humira are currently marketed in the United States. Organon said that it expects to market Hadlima in the United States on or after July 1, 2023, in accordance with a licensing agreement with AbbVie.
The prescribing information for Hadlima includes specific warnings and areas of concern. The drug should not be administered to individuals who are known to be hypersensitive to adalimumab. The drug may lower the ability of the immune system to fight infections and may increase risk of infections, including serious infections leading to hospitalization or death, such as tuberculosis, bacterial sepsis, invasive fungal infections (such as histoplasmosis), and infections attributable to other opportunistic pathogens.
A test for latent TB infection should be given before administration, and treatment of TB should begin before administration of Hadlima.
Patients taking Hadlima should not take a live vaccine.
The most common adverse effects (incidence > 10%) include infections (for example, upper respiratory infections, sinusitis), injection site reactions, headache, and rash.
A version of this article first appeared on Medscape.com.
New international dermatology registry tracks monkeypox cases
The American Academy of Dermatology and the International League of Dermatological Societies (ILDS) have created a new registry that now accepts reports from health care providers worldwide about monkeypox cases and monkeypox vaccine reactions.
Patient data such as names and dates of birth will not be collected.
“As with our joint COVID-19 registry, we will be doing real-time data analysis during the outbreak,” dermatologist Esther Freeman, MD, PhD, director of MGH Global Health Dermatology at Massachusetts General Hospital, Boston, and a member of the AAD’s monkeypox task force, said in an interview. “We will to try to feed information back to our front line in terms of clinical characteristics of cases, morphology, and any unexpected findings.”
According to Dr. Freeman, the principal investigator for the COVID-19 registry, this registry has allowed the quick gathering of information about dermatologic findings of COVID-19 from over 53 countries. “We have published over 15 papers, and we share data with outside investigators wishing to do their own analysis of registry-related data,” she said. “Our most-cited paper on COVID vaccine skin reactions has been cited almost 500 times since 2021. It has been used to educate the public on vaccine side effects and to combat vaccine hesitancy.”
The monkeypox registry “doesn’t belong to any one group or person,” Dr. Freeman said. “The idea with rapid data analysis is to be able to give back to the dermatologic community what is hard for us to see with any single case: Patterns and new findings that can be helpful to share with dermatologists and other physicians worldwide, all working together to stop an outbreak.”
The American Academy of Dermatology and the International League of Dermatological Societies (ILDS) have created a new registry that now accepts reports from health care providers worldwide about monkeypox cases and monkeypox vaccine reactions.
Patient data such as names and dates of birth will not be collected.
“As with our joint COVID-19 registry, we will be doing real-time data analysis during the outbreak,” dermatologist Esther Freeman, MD, PhD, director of MGH Global Health Dermatology at Massachusetts General Hospital, Boston, and a member of the AAD’s monkeypox task force, said in an interview. “We will to try to feed information back to our front line in terms of clinical characteristics of cases, morphology, and any unexpected findings.”
According to Dr. Freeman, the principal investigator for the COVID-19 registry, this registry has allowed the quick gathering of information about dermatologic findings of COVID-19 from over 53 countries. “We have published over 15 papers, and we share data with outside investigators wishing to do their own analysis of registry-related data,” she said. “Our most-cited paper on COVID vaccine skin reactions has been cited almost 500 times since 2021. It has been used to educate the public on vaccine side effects and to combat vaccine hesitancy.”
The monkeypox registry “doesn’t belong to any one group or person,” Dr. Freeman said. “The idea with rapid data analysis is to be able to give back to the dermatologic community what is hard for us to see with any single case: Patterns and new findings that can be helpful to share with dermatologists and other physicians worldwide, all working together to stop an outbreak.”
The American Academy of Dermatology and the International League of Dermatological Societies (ILDS) have created a new registry that now accepts reports from health care providers worldwide about monkeypox cases and monkeypox vaccine reactions.
Patient data such as names and dates of birth will not be collected.
“As with our joint COVID-19 registry, we will be doing real-time data analysis during the outbreak,” dermatologist Esther Freeman, MD, PhD, director of MGH Global Health Dermatology at Massachusetts General Hospital, Boston, and a member of the AAD’s monkeypox task force, said in an interview. “We will to try to feed information back to our front line in terms of clinical characteristics of cases, morphology, and any unexpected findings.”
According to Dr. Freeman, the principal investigator for the COVID-19 registry, this registry has allowed the quick gathering of information about dermatologic findings of COVID-19 from over 53 countries. “We have published over 15 papers, and we share data with outside investigators wishing to do their own analysis of registry-related data,” she said. “Our most-cited paper on COVID vaccine skin reactions has been cited almost 500 times since 2021. It has been used to educate the public on vaccine side effects and to combat vaccine hesitancy.”
The monkeypox registry “doesn’t belong to any one group or person,” Dr. Freeman said. “The idea with rapid data analysis is to be able to give back to the dermatologic community what is hard for us to see with any single case: Patterns and new findings that can be helpful to share with dermatologists and other physicians worldwide, all working together to stop an outbreak.”
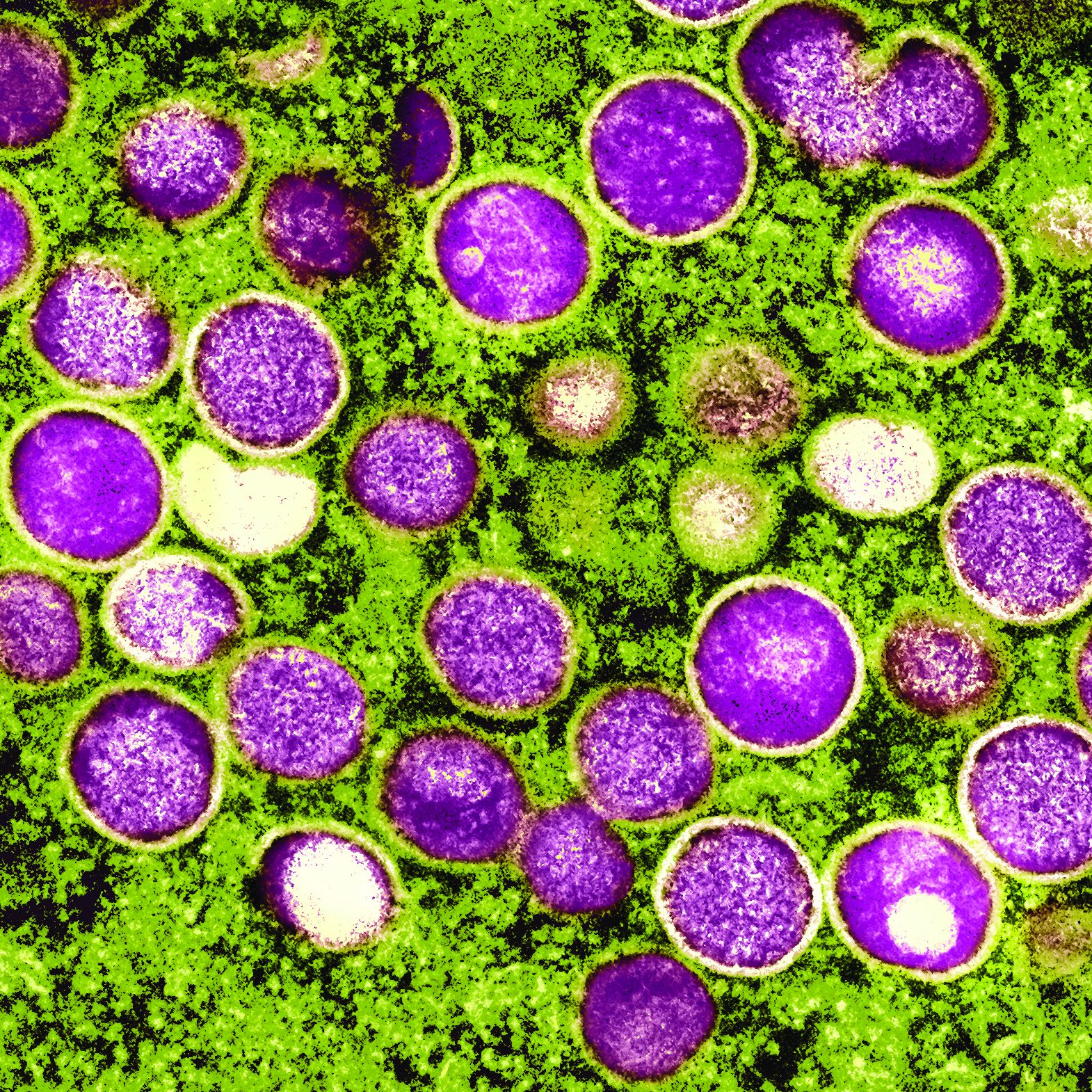
Dermatology and monkeypox: What you need to know
.
Diagnosing cases “can be hard and folks should keep a very open mind and consider monkeypox virus,” said Misha Rosenbach, MD, a University of Pennsylvania dermatologist and member of the American Academy of Dermatology’s ad hoc task force to develop monkeypox content.
Although it’s named after a primate, it turns out that monkeypox is quite the copycat. As dermatologists have learned, its lesions can look like those caused by a long list of other diseases including herpes, varicella, and syphilis. In small numbers, they can even appear to be insect bites.
To make things more complicated, a patient can have one or two lesions – or dozens. They often cluster in the anogenital area, likely reflecting transmission via sexual intercourse, unlike previous outbreaks in which lesions appeared all over the body. “We have to let go of some of our conceptions about what monkeypox might look like,” said dermatologist Esther Freeman, MD, PhD, associate professor of dermatology, Harvard University, Boston, and a member of the AAD task force.
To make things even more complicated, “the spectrum of illness that we are seeing has ranged from limited, subtle lesions to dramatic, widespread, ulcerative/necrotic lesions,” said Dr. Rosenbach, associate professor of dermatology at the University of Pennsylvania, Philadelphia.
But monkeypox has unique traits that can set it apart and pave the way toward a diagnosis, dermatologists say. And important patient data can help dermatologists gauge the likelihood of a case: Almost 99% of cases with data available have been in men, and among men with available information, 94% reported male-to-male sexual or close intimate contact during the 3 weeks before developing symptoms, according to a CDC report tracking cases from May through late July. So far, cases in women and children are extremely rare, although there have been some reported in the United States.
Are dermatologists likely to see monkeypox in the clinic? It’s unclear so far. Of four dermatologists interviewed for this article, only one has seen patients with monkeypox in person. But others say they’ve been sought for consultations. “I have been asked by infectious disease colleagues for advice remotely but have not seen it,” said dermatologist Howa Yeung, MD, MSc, assistant professor of dermatology, Emory University, Atlanta. “Most of the time, they’re catching all the symptomatic cases before any need for dermatology in-person referrals.”
Still, the rapid rate of growth of the outbreak – up from 3,487 in the United States on July 25 to 12,689 as of Aug.16 – suggests that more dermatologists will see cases, and consultations may become more common too.
Know your lesions
Lesions are the telltale signs of symptomatic monkeypox. According to a recent New England Journal of Medicine study of 528 monkeypox cases from 16 nations, diagnosed between April 27 and June 24, 2022, 95% had skin lesions (58% were vesiculopustular), most commonly in the anogenital area (73%), and on the trunk/arms/or legs (55%) and face (25%), and the palms/soles (10%).
However, “the current monkeypox outbreak often presents differently from the multiple classic vesiculopustules on the skin we see in textbooks,” Dr. Yeung said. “Sometimes people can present with throat pain or rectal pain, with isolated pharyngitis or proctitis. Sometimes there are so few lesions on the skin that it can be easily confused with a bug bite, folliculitis, herpes, dyshidrotic eczema, or other skin problems. This is where dermatologists will get consulted to clarify the diagnosis while the monkeypox PCR test is pending.”
Dr. Rosenbach, who has provided consultation services to other physicians about cases, said the lesions often appear to be vesicles or pustules, “but if you go to ‘pop’ it – e.g., for testing – it’s firm and without fluid. This is likely due to pox virus inclusion, similar to other diseases such as molluscum,” caused by another pox virus, he said. Molluscum lesions are “characteristically umbilicated, with a dimple in the center, and monkeypox lesions seem to be showing a roughly similar morphology with many bowl- or caldera-shaped lesions that are donut-like in appearance,” he added.
Over time, Dr. Rosenbach said, “lesions tend to evolve slowly from smaller flesh-colored or vaguely white firm papules to broader more umbilicated/donut-shaped lesions which may erode, ulcerate, develop a crust or scab, and then heal. The amount of scarring is not yet clear, but we anticipate it to be significant, especially in patients with more widespread or severe disease.”
Jon Peebles, MD, a dermatologist at Kaiser Permanente in Largo, Md., who has treated a few in-person monkeypox cases, said the lesions can be “exquisitely painful,” although he’s also seen patients with asymptomatic lesions. “Lesions are showing a predilection for the anogenital skin, though they can occur anywhere and not uncommonly involve the oral mucosa,” said Dr. Peebles, also a member of the AAD monkeypox task force.
Dr. Yeung said it’s important to ask patients about their sexual orientation, gender identity, and sexual behaviors. “That is the only way to know who your patients are and the only way to understand who else may be at risks and can benefit from contact tracing and additional prevention measures, such as vaccination for asymptomatic sex partners.” (The Jynneos smallpox vaccine is Food and Drug Administration–approved to prevent monkeypox, although its efficacy is not entirely clear, and there’s controversy over expanding its limited availability by administering the vaccine intradermally.)
It’s also important to keep in mind that sexually transmitted infections (STIs) are common in gay and bisexual men. “Just because the patient is diagnosed with gonorrhea or syphilis does not mean the patient cannot also have monkeypox,” Dr. Rosenbach said. Indeed, the NEJM study reported that of 377 patients screened, 29% had an STI other than HIV, mostly syphilis (9%) and gonorrhea (8%). Of all 528 patients in the study (all male or transgender/nonbinary), 41% were HIV-positive, and the median number of sex partners in the last 3 months was 5 (range, 3-15).
Testing is crucial to rule monkeypox in – or out
While monkeypox lesions can be confused for other diseases, Dr. Rosenbach said that a diagnosis can be confirmed through various tests. Varicella zoster virus (VZV) and herpes simplex virus (HSV) have distinct findings on Tzanck smears (nuclear molding, multinucleated cells), and have widely available fairly rapid tests (PCR, or in some places, DFA). “Staph and bacterial folliculitis can usually be cultured quickly,” he said. “If you have someone with no risk factors/exposure, and you test for VZV, HSV, folliculitis, and it’s negative – you should know within 24 hours in most places – then you can broaden your differential diagnosis and consider alternate explanations, including monkeypox.”
Quest Diagnostics and Labcorp, two of the largest commercial labs in the United States, are now offering monkeypox tests. Labcorp says its test has a 2- to 3-day turnaround time.
As for treatment, some physicians are prescribing off-label use of tecovirimat (also known as TPOXX or ST-246), a smallpox antiviral treatment. The CDC offers guidelines about its use. “It seems to work very fast, with patients improving in 24-72 hours,” Dr. Rosenbach said. However, “it is still very challenging to give and get. There’s a cumbersome system to prescribe it, and it needs to be shipped from the national stockpile. Dermatologists should be working with their state health department, infection control, and infectious disease doctors.”
It’s likely that dermatologists are not comfortable with the process to access the drug, he said, “but if we do not act quickly to control the current outbreak, we will all – unfortunately – need to learn to be comfortable prescribing it.”
In regard to pain control, an over-the-counter painkiller approach may be appropriate depending on comorbidities, Dr. Rosenbach said. “Some patients with very severe disease, such as perianal involvement and proctitis, have such severe pain they need to be hospitalized. This is less common.”
Recommendations pending on scarring prevention
There’s limited high-quality evidence about the prevention of scarring in diseases like monkeypox, Dr. Rosenbach noted. “Any recommendations are usually based on very small, limited, uncontrolled studies. In the case of monkeypox, truly we are off the edge of the map.”
He advises cleaning lesions with gentle soap and water – keeping in mind that contaminated towels may spread disease – and potentially using a topical ointment-based dressing such as a Vaseline/nonstick dressing or Vaseline-impregnated gauze. If there’s concern about superinfection, as can occur with staph infections, topical antibiotics such as mupirocin 2% ointment may be appropriate, he said.
“Some folks like to try silica gel sheets to prevent scarring,” Dr. Rosenbach said. “There’s not a lot of evidence to support that, but they’re unlikely to be harmful. I would personally consider them, but it really depends on the extent of disease, anatomic sites involved, and access to care.”
Emory University’s Dr. Yeung also suggested using silicone gel or sheets to optimize the scar appearance once the lesions have crusted over. “People have used lasers, microneedling, etc., to improve smallpox scar appearance,” he added, “and I’m sure dermatologists will be the ones to study what works best for treating monkeypox scars.”
As for the big picture, Dr. Yeung said that dermatologists are critical in the fight to control monkeypox: “We can help our colleagues and patients manage symptoms and wound care, advocate for vaccination and treatment, treat long-term scarring sequelae, and destigmatize LGBTQ health care.”
The dermatologists interviewed for this article report no disclosures.
.
Diagnosing cases “can be hard and folks should keep a very open mind and consider monkeypox virus,” said Misha Rosenbach, MD, a University of Pennsylvania dermatologist and member of the American Academy of Dermatology’s ad hoc task force to develop monkeypox content.
Although it’s named after a primate, it turns out that monkeypox is quite the copycat. As dermatologists have learned, its lesions can look like those caused by a long list of other diseases including herpes, varicella, and syphilis. In small numbers, they can even appear to be insect bites.
To make things more complicated, a patient can have one or two lesions – or dozens. They often cluster in the anogenital area, likely reflecting transmission via sexual intercourse, unlike previous outbreaks in which lesions appeared all over the body. “We have to let go of some of our conceptions about what monkeypox might look like,” said dermatologist Esther Freeman, MD, PhD, associate professor of dermatology, Harvard University, Boston, and a member of the AAD task force.
To make things even more complicated, “the spectrum of illness that we are seeing has ranged from limited, subtle lesions to dramatic, widespread, ulcerative/necrotic lesions,” said Dr. Rosenbach, associate professor of dermatology at the University of Pennsylvania, Philadelphia.
But monkeypox has unique traits that can set it apart and pave the way toward a diagnosis, dermatologists say. And important patient data can help dermatologists gauge the likelihood of a case: Almost 99% of cases with data available have been in men, and among men with available information, 94% reported male-to-male sexual or close intimate contact during the 3 weeks before developing symptoms, according to a CDC report tracking cases from May through late July. So far, cases in women and children are extremely rare, although there have been some reported in the United States.
Are dermatologists likely to see monkeypox in the clinic? It’s unclear so far. Of four dermatologists interviewed for this article, only one has seen patients with monkeypox in person. But others say they’ve been sought for consultations. “I have been asked by infectious disease colleagues for advice remotely but have not seen it,” said dermatologist Howa Yeung, MD, MSc, assistant professor of dermatology, Emory University, Atlanta. “Most of the time, they’re catching all the symptomatic cases before any need for dermatology in-person referrals.”
Still, the rapid rate of growth of the outbreak – up from 3,487 in the United States on July 25 to 12,689 as of Aug.16 – suggests that more dermatologists will see cases, and consultations may become more common too.
Know your lesions
Lesions are the telltale signs of symptomatic monkeypox. According to a recent New England Journal of Medicine study of 528 monkeypox cases from 16 nations, diagnosed between April 27 and June 24, 2022, 95% had skin lesions (58% were vesiculopustular), most commonly in the anogenital area (73%), and on the trunk/arms/or legs (55%) and face (25%), and the palms/soles (10%).
However, “the current monkeypox outbreak often presents differently from the multiple classic vesiculopustules on the skin we see in textbooks,” Dr. Yeung said. “Sometimes people can present with throat pain or rectal pain, with isolated pharyngitis or proctitis. Sometimes there are so few lesions on the skin that it can be easily confused with a bug bite, folliculitis, herpes, dyshidrotic eczema, or other skin problems. This is where dermatologists will get consulted to clarify the diagnosis while the monkeypox PCR test is pending.”
Dr. Rosenbach, who has provided consultation services to other physicians about cases, said the lesions often appear to be vesicles or pustules, “but if you go to ‘pop’ it – e.g., for testing – it’s firm and without fluid. This is likely due to pox virus inclusion, similar to other diseases such as molluscum,” caused by another pox virus, he said. Molluscum lesions are “characteristically umbilicated, with a dimple in the center, and monkeypox lesions seem to be showing a roughly similar morphology with many bowl- or caldera-shaped lesions that are donut-like in appearance,” he added.
Over time, Dr. Rosenbach said, “lesions tend to evolve slowly from smaller flesh-colored or vaguely white firm papules to broader more umbilicated/donut-shaped lesions which may erode, ulcerate, develop a crust or scab, and then heal. The amount of scarring is not yet clear, but we anticipate it to be significant, especially in patients with more widespread or severe disease.”
Jon Peebles, MD, a dermatologist at Kaiser Permanente in Largo, Md., who has treated a few in-person monkeypox cases, said the lesions can be “exquisitely painful,” although he’s also seen patients with asymptomatic lesions. “Lesions are showing a predilection for the anogenital skin, though they can occur anywhere and not uncommonly involve the oral mucosa,” said Dr. Peebles, also a member of the AAD monkeypox task force.
Dr. Yeung said it’s important to ask patients about their sexual orientation, gender identity, and sexual behaviors. “That is the only way to know who your patients are and the only way to understand who else may be at risks and can benefit from contact tracing and additional prevention measures, such as vaccination for asymptomatic sex partners.” (The Jynneos smallpox vaccine is Food and Drug Administration–approved to prevent monkeypox, although its efficacy is not entirely clear, and there’s controversy over expanding its limited availability by administering the vaccine intradermally.)
It’s also important to keep in mind that sexually transmitted infections (STIs) are common in gay and bisexual men. “Just because the patient is diagnosed with gonorrhea or syphilis does not mean the patient cannot also have monkeypox,” Dr. Rosenbach said. Indeed, the NEJM study reported that of 377 patients screened, 29% had an STI other than HIV, mostly syphilis (9%) and gonorrhea (8%). Of all 528 patients in the study (all male or transgender/nonbinary), 41% were HIV-positive, and the median number of sex partners in the last 3 months was 5 (range, 3-15).
Testing is crucial to rule monkeypox in – or out
While monkeypox lesions can be confused for other diseases, Dr. Rosenbach said that a diagnosis can be confirmed through various tests. Varicella zoster virus (VZV) and herpes simplex virus (HSV) have distinct findings on Tzanck smears (nuclear molding, multinucleated cells), and have widely available fairly rapid tests (PCR, or in some places, DFA). “Staph and bacterial folliculitis can usually be cultured quickly,” he said. “If you have someone with no risk factors/exposure, and you test for VZV, HSV, folliculitis, and it’s negative – you should know within 24 hours in most places – then you can broaden your differential diagnosis and consider alternate explanations, including monkeypox.”
Quest Diagnostics and Labcorp, two of the largest commercial labs in the United States, are now offering monkeypox tests. Labcorp says its test has a 2- to 3-day turnaround time.
As for treatment, some physicians are prescribing off-label use of tecovirimat (also known as TPOXX or ST-246), a smallpox antiviral treatment. The CDC offers guidelines about its use. “It seems to work very fast, with patients improving in 24-72 hours,” Dr. Rosenbach said. However, “it is still very challenging to give and get. There’s a cumbersome system to prescribe it, and it needs to be shipped from the national stockpile. Dermatologists should be working with their state health department, infection control, and infectious disease doctors.”
It’s likely that dermatologists are not comfortable with the process to access the drug, he said, “but if we do not act quickly to control the current outbreak, we will all – unfortunately – need to learn to be comfortable prescribing it.”
In regard to pain control, an over-the-counter painkiller approach may be appropriate depending on comorbidities, Dr. Rosenbach said. “Some patients with very severe disease, such as perianal involvement and proctitis, have such severe pain they need to be hospitalized. This is less common.”
Recommendations pending on scarring prevention
There’s limited high-quality evidence about the prevention of scarring in diseases like monkeypox, Dr. Rosenbach noted. “Any recommendations are usually based on very small, limited, uncontrolled studies. In the case of monkeypox, truly we are off the edge of the map.”
He advises cleaning lesions with gentle soap and water – keeping in mind that contaminated towels may spread disease – and potentially using a topical ointment-based dressing such as a Vaseline/nonstick dressing or Vaseline-impregnated gauze. If there’s concern about superinfection, as can occur with staph infections, topical antibiotics such as mupirocin 2% ointment may be appropriate, he said.
“Some folks like to try silica gel sheets to prevent scarring,” Dr. Rosenbach said. “There’s not a lot of evidence to support that, but they’re unlikely to be harmful. I would personally consider them, but it really depends on the extent of disease, anatomic sites involved, and access to care.”
Emory University’s Dr. Yeung also suggested using silicone gel or sheets to optimize the scar appearance once the lesions have crusted over. “People have used lasers, microneedling, etc., to improve smallpox scar appearance,” he added, “and I’m sure dermatologists will be the ones to study what works best for treating monkeypox scars.”
As for the big picture, Dr. Yeung said that dermatologists are critical in the fight to control monkeypox: “We can help our colleagues and patients manage symptoms and wound care, advocate for vaccination and treatment, treat long-term scarring sequelae, and destigmatize LGBTQ health care.”
The dermatologists interviewed for this article report no disclosures.
.
Diagnosing cases “can be hard and folks should keep a very open mind and consider monkeypox virus,” said Misha Rosenbach, MD, a University of Pennsylvania dermatologist and member of the American Academy of Dermatology’s ad hoc task force to develop monkeypox content.
Although it’s named after a primate, it turns out that monkeypox is quite the copycat. As dermatologists have learned, its lesions can look like those caused by a long list of other diseases including herpes, varicella, and syphilis. In small numbers, they can even appear to be insect bites.
To make things more complicated, a patient can have one or two lesions – or dozens. They often cluster in the anogenital area, likely reflecting transmission via sexual intercourse, unlike previous outbreaks in which lesions appeared all over the body. “We have to let go of some of our conceptions about what monkeypox might look like,” said dermatologist Esther Freeman, MD, PhD, associate professor of dermatology, Harvard University, Boston, and a member of the AAD task force.
To make things even more complicated, “the spectrum of illness that we are seeing has ranged from limited, subtle lesions to dramatic, widespread, ulcerative/necrotic lesions,” said Dr. Rosenbach, associate professor of dermatology at the University of Pennsylvania, Philadelphia.
But monkeypox has unique traits that can set it apart and pave the way toward a diagnosis, dermatologists say. And important patient data can help dermatologists gauge the likelihood of a case: Almost 99% of cases with data available have been in men, and among men with available information, 94% reported male-to-male sexual or close intimate contact during the 3 weeks before developing symptoms, according to a CDC report tracking cases from May through late July. So far, cases in women and children are extremely rare, although there have been some reported in the United States.
Are dermatologists likely to see monkeypox in the clinic? It’s unclear so far. Of four dermatologists interviewed for this article, only one has seen patients with monkeypox in person. But others say they’ve been sought for consultations. “I have been asked by infectious disease colleagues for advice remotely but have not seen it,” said dermatologist Howa Yeung, MD, MSc, assistant professor of dermatology, Emory University, Atlanta. “Most of the time, they’re catching all the symptomatic cases before any need for dermatology in-person referrals.”
Still, the rapid rate of growth of the outbreak – up from 3,487 in the United States on July 25 to 12,689 as of Aug.16 – suggests that more dermatologists will see cases, and consultations may become more common too.
Know your lesions
Lesions are the telltale signs of symptomatic monkeypox. According to a recent New England Journal of Medicine study of 528 monkeypox cases from 16 nations, diagnosed between April 27 and June 24, 2022, 95% had skin lesions (58% were vesiculopustular), most commonly in the anogenital area (73%), and on the trunk/arms/or legs (55%) and face (25%), and the palms/soles (10%).
However, “the current monkeypox outbreak often presents differently from the multiple classic vesiculopustules on the skin we see in textbooks,” Dr. Yeung said. “Sometimes people can present with throat pain or rectal pain, with isolated pharyngitis or proctitis. Sometimes there are so few lesions on the skin that it can be easily confused with a bug bite, folliculitis, herpes, dyshidrotic eczema, or other skin problems. This is where dermatologists will get consulted to clarify the diagnosis while the monkeypox PCR test is pending.”
Dr. Rosenbach, who has provided consultation services to other physicians about cases, said the lesions often appear to be vesicles or pustules, “but if you go to ‘pop’ it – e.g., for testing – it’s firm and without fluid. This is likely due to pox virus inclusion, similar to other diseases such as molluscum,” caused by another pox virus, he said. Molluscum lesions are “characteristically umbilicated, with a dimple in the center, and monkeypox lesions seem to be showing a roughly similar morphology with many bowl- or caldera-shaped lesions that are donut-like in appearance,” he added.
Over time, Dr. Rosenbach said, “lesions tend to evolve slowly from smaller flesh-colored or vaguely white firm papules to broader more umbilicated/donut-shaped lesions which may erode, ulcerate, develop a crust or scab, and then heal. The amount of scarring is not yet clear, but we anticipate it to be significant, especially in patients with more widespread or severe disease.”
Jon Peebles, MD, a dermatologist at Kaiser Permanente in Largo, Md., who has treated a few in-person monkeypox cases, said the lesions can be “exquisitely painful,” although he’s also seen patients with asymptomatic lesions. “Lesions are showing a predilection for the anogenital skin, though they can occur anywhere and not uncommonly involve the oral mucosa,” said Dr. Peebles, also a member of the AAD monkeypox task force.
Dr. Yeung said it’s important to ask patients about their sexual orientation, gender identity, and sexual behaviors. “That is the only way to know who your patients are and the only way to understand who else may be at risks and can benefit from contact tracing and additional prevention measures, such as vaccination for asymptomatic sex partners.” (The Jynneos smallpox vaccine is Food and Drug Administration–approved to prevent monkeypox, although its efficacy is not entirely clear, and there’s controversy over expanding its limited availability by administering the vaccine intradermally.)
It’s also important to keep in mind that sexually transmitted infections (STIs) are common in gay and bisexual men. “Just because the patient is diagnosed with gonorrhea or syphilis does not mean the patient cannot also have monkeypox,” Dr. Rosenbach said. Indeed, the NEJM study reported that of 377 patients screened, 29% had an STI other than HIV, mostly syphilis (9%) and gonorrhea (8%). Of all 528 patients in the study (all male or transgender/nonbinary), 41% were HIV-positive, and the median number of sex partners in the last 3 months was 5 (range, 3-15).
Testing is crucial to rule monkeypox in – or out
While monkeypox lesions can be confused for other diseases, Dr. Rosenbach said that a diagnosis can be confirmed through various tests. Varicella zoster virus (VZV) and herpes simplex virus (HSV) have distinct findings on Tzanck smears (nuclear molding, multinucleated cells), and have widely available fairly rapid tests (PCR, or in some places, DFA). “Staph and bacterial folliculitis can usually be cultured quickly,” he said. “If you have someone with no risk factors/exposure, and you test for VZV, HSV, folliculitis, and it’s negative – you should know within 24 hours in most places – then you can broaden your differential diagnosis and consider alternate explanations, including monkeypox.”
Quest Diagnostics and Labcorp, two of the largest commercial labs in the United States, are now offering monkeypox tests. Labcorp says its test has a 2- to 3-day turnaround time.
As for treatment, some physicians are prescribing off-label use of tecovirimat (also known as TPOXX or ST-246), a smallpox antiviral treatment. The CDC offers guidelines about its use. “It seems to work very fast, with patients improving in 24-72 hours,” Dr. Rosenbach said. However, “it is still very challenging to give and get. There’s a cumbersome system to prescribe it, and it needs to be shipped from the national stockpile. Dermatologists should be working with their state health department, infection control, and infectious disease doctors.”
It’s likely that dermatologists are not comfortable with the process to access the drug, he said, “but if we do not act quickly to control the current outbreak, we will all – unfortunately – need to learn to be comfortable prescribing it.”
In regard to pain control, an over-the-counter painkiller approach may be appropriate depending on comorbidities, Dr. Rosenbach said. “Some patients with very severe disease, such as perianal involvement and proctitis, have such severe pain they need to be hospitalized. This is less common.”
Recommendations pending on scarring prevention
There’s limited high-quality evidence about the prevention of scarring in diseases like monkeypox, Dr. Rosenbach noted. “Any recommendations are usually based on very small, limited, uncontrolled studies. In the case of monkeypox, truly we are off the edge of the map.”
He advises cleaning lesions with gentle soap and water – keeping in mind that contaminated towels may spread disease – and potentially using a topical ointment-based dressing such as a Vaseline/nonstick dressing or Vaseline-impregnated gauze. If there’s concern about superinfection, as can occur with staph infections, topical antibiotics such as mupirocin 2% ointment may be appropriate, he said.
“Some folks like to try silica gel sheets to prevent scarring,” Dr. Rosenbach said. “There’s not a lot of evidence to support that, but they’re unlikely to be harmful. I would personally consider them, but it really depends on the extent of disease, anatomic sites involved, and access to care.”
Emory University’s Dr. Yeung also suggested using silicone gel or sheets to optimize the scar appearance once the lesions have crusted over. “People have used lasers, microneedling, etc., to improve smallpox scar appearance,” he added, “and I’m sure dermatologists will be the ones to study what works best for treating monkeypox scars.”
As for the big picture, Dr. Yeung said that dermatologists are critical in the fight to control monkeypox: “We can help our colleagues and patients manage symptoms and wound care, advocate for vaccination and treatment, treat long-term scarring sequelae, and destigmatize LGBTQ health care.”
The dermatologists interviewed for this article report no disclosures.
Review cautions against influencer-promoted hair-growth remedies
One day in 2020, Ronda S. Farah, MD, was spending some downtime from her dermatology practice scrolling through social media. When she opened TikTok, she came across something that piqued her interest: A popular content creator was promoting the supplement biotin as a way to grow hair. Dr. Farah was immediately alarmed, because not only was the evidence that biotin increases hair growth shoddy, but the FDA had also warned that biotin supplements may interfere with lab tests for troponin.
Dr. Farah was moved to action and made a brief TikTok stating that use of biotin does not result in hair growth for most patients, which quickly shot up to over half a million views. She was flooded with messages from influencers and people desperate for an answer to their hair growth questions.
From that point on, Dr. Farah was immersed in the world of hairfluencers, the social media personalities who promote hair care trends, which formed the basis of a review, published in the Journal of Cosmetic Dermatology that she conducted with her colleagues at the University of Minnesota, Minneapolis. .
They reviewed five treatments that represent some of the most frequently discussed hair-growth trends on social media: rosemary, onion juice, rice water, castor oil, and aloe vera. For each, they evaluated recommendations on how the treatments were applied, possible harmful effects to the user, claims that weren’t totally based on scientific evidence, and the theoretical mechanism of action. “Overall,” they concluded, “there is little to no literature supporting these social media trends for hair growth.”
Of the five, rosemary, applied to the scalp or hair, has perhaps the most significant research behind it, according to Dr. Farah and coauthors. Methods of applying rosemary described on social media included use of prepackaged oil, boiling fresh rosemary leaves, adding leaves to oils and spraying it on or massaging it on the scalp, applying it in the hair, or using it as a rinse. Dr. Farah noted that the literature supporting the use of rosemary for hair growth does not represent the most robust science; the studies had small sample sizes and used nonstandardized methods of measuring hair growth.
“It didn’t really meet rigorous, strong study methods that a board-certified dermatologist with their expertise would consider a really solid study,” she said.
For the remaining methods, there was little research to support their use for hair growth. A few, the authors pointed out, can cause scalp burns (aloe vera), damage to hair follicles (rice water), contact dermatitis (aloe vera, onion juice), and, in the case of castor oil, hair felting..
Dr. Farah thinks social media can be a great tool to reach patients, but that people should be wary of what kind of information they’re consuming “and need to be aware of who their hairfluencer is,” she said. And, as she and her coauthors wrote: “We call on dermatologists, as hair and scalp disease experts, to serve as authorities on ‘hairfluencer’ trends and appropriately counsel patients.”
The study was independently supported. Dr. Farah reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
One day in 2020, Ronda S. Farah, MD, was spending some downtime from her dermatology practice scrolling through social media. When she opened TikTok, she came across something that piqued her interest: A popular content creator was promoting the supplement biotin as a way to grow hair. Dr. Farah was immediately alarmed, because not only was the evidence that biotin increases hair growth shoddy, but the FDA had also warned that biotin supplements may interfere with lab tests for troponin.
Dr. Farah was moved to action and made a brief TikTok stating that use of biotin does not result in hair growth for most patients, which quickly shot up to over half a million views. She was flooded with messages from influencers and people desperate for an answer to their hair growth questions.
From that point on, Dr. Farah was immersed in the world of hairfluencers, the social media personalities who promote hair care trends, which formed the basis of a review, published in the Journal of Cosmetic Dermatology that she conducted with her colleagues at the University of Minnesota, Minneapolis. .
They reviewed five treatments that represent some of the most frequently discussed hair-growth trends on social media: rosemary, onion juice, rice water, castor oil, and aloe vera. For each, they evaluated recommendations on how the treatments were applied, possible harmful effects to the user, claims that weren’t totally based on scientific evidence, and the theoretical mechanism of action. “Overall,” they concluded, “there is little to no literature supporting these social media trends for hair growth.”
Of the five, rosemary, applied to the scalp or hair, has perhaps the most significant research behind it, according to Dr. Farah and coauthors. Methods of applying rosemary described on social media included use of prepackaged oil, boiling fresh rosemary leaves, adding leaves to oils and spraying it on or massaging it on the scalp, applying it in the hair, or using it as a rinse. Dr. Farah noted that the literature supporting the use of rosemary for hair growth does not represent the most robust science; the studies had small sample sizes and used nonstandardized methods of measuring hair growth.
“It didn’t really meet rigorous, strong study methods that a board-certified dermatologist with their expertise would consider a really solid study,” she said.
For the remaining methods, there was little research to support their use for hair growth. A few, the authors pointed out, can cause scalp burns (aloe vera), damage to hair follicles (rice water), contact dermatitis (aloe vera, onion juice), and, in the case of castor oil, hair felting..
Dr. Farah thinks social media can be a great tool to reach patients, but that people should be wary of what kind of information they’re consuming “and need to be aware of who their hairfluencer is,” she said. And, as she and her coauthors wrote: “We call on dermatologists, as hair and scalp disease experts, to serve as authorities on ‘hairfluencer’ trends and appropriately counsel patients.”
The study was independently supported. Dr. Farah reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
One day in 2020, Ronda S. Farah, MD, was spending some downtime from her dermatology practice scrolling through social media. When she opened TikTok, she came across something that piqued her interest: A popular content creator was promoting the supplement biotin as a way to grow hair. Dr. Farah was immediately alarmed, because not only was the evidence that biotin increases hair growth shoddy, but the FDA had also warned that biotin supplements may interfere with lab tests for troponin.
Dr. Farah was moved to action and made a brief TikTok stating that use of biotin does not result in hair growth for most patients, which quickly shot up to over half a million views. She was flooded with messages from influencers and people desperate for an answer to their hair growth questions.
From that point on, Dr. Farah was immersed in the world of hairfluencers, the social media personalities who promote hair care trends, which formed the basis of a review, published in the Journal of Cosmetic Dermatology that she conducted with her colleagues at the University of Minnesota, Minneapolis. .
They reviewed five treatments that represent some of the most frequently discussed hair-growth trends on social media: rosemary, onion juice, rice water, castor oil, and aloe vera. For each, they evaluated recommendations on how the treatments were applied, possible harmful effects to the user, claims that weren’t totally based on scientific evidence, and the theoretical mechanism of action. “Overall,” they concluded, “there is little to no literature supporting these social media trends for hair growth.”
Of the five, rosemary, applied to the scalp or hair, has perhaps the most significant research behind it, according to Dr. Farah and coauthors. Methods of applying rosemary described on social media included use of prepackaged oil, boiling fresh rosemary leaves, adding leaves to oils and spraying it on or massaging it on the scalp, applying it in the hair, or using it as a rinse. Dr. Farah noted that the literature supporting the use of rosemary for hair growth does not represent the most robust science; the studies had small sample sizes and used nonstandardized methods of measuring hair growth.
“It didn’t really meet rigorous, strong study methods that a board-certified dermatologist with their expertise would consider a really solid study,” she said.
For the remaining methods, there was little research to support their use for hair growth. A few, the authors pointed out, can cause scalp burns (aloe vera), damage to hair follicles (rice water), contact dermatitis (aloe vera, onion juice), and, in the case of castor oil, hair felting..
Dr. Farah thinks social media can be a great tool to reach patients, but that people should be wary of what kind of information they’re consuming “and need to be aware of who their hairfluencer is,” she said. And, as she and her coauthors wrote: “We call on dermatologists, as hair and scalp disease experts, to serve as authorities on ‘hairfluencer’ trends and appropriately counsel patients.”
The study was independently supported. Dr. Farah reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF COSMETIC DERMATOLOGY
Dermatologists skeptical of calamine lotion TikTok trend
Though this may seem to work as a base layer for some people, dermatologists have concerns about this trend, particularly the risk of dryness.
As of Aug. 15, the #calaminelotion tag had more than 20.9 million views on TikTok, with hundreds of videos hailing the cream for its opaque pink tint and matte effect when used under foundation.
Calamine lotion has been used to treat itchy rashes, insect bites, and pain from chickenpox and poison ivy for years. It’s sold over the counter and is a common first-line treatment for skin discomfort that has been used for hundreds of years, says Doris Day, MD, a dermatologist who practices in New York City. It is also on the World Health Organization’s list of essential drugs, she points out in an interview.
“This is something that has been around for a long time. It’s recognized as a drug that has importance. So every now and then, I guess somebody comes across it” and says it’s a “new panacea” for something, “but it’s really not. It’s just an old-time simple product.”
Calamine lotion is made of ferric oxide and zinc oxide, which gives it its antiseptic and anti-itch properties, in addition to its characteristic pink color. Zinc oxide is also commonly used in mineral sunscreens, Dr. Day points out.
Although these ingredients are exceedingly safe with temporary, localized use, high concentrations and chronic use of calamine lotion can be irritating to the skin, says Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington.
At these high concentrations, calamine lotion can be drying, which may cause skin clumping and can be abrasive, says Dr. Sodha. She also cautions that the astringent properties of the zinc and the high pH may disrupt proteins on the skin, which breaks down the skin’s natural defenses. Using calamine lotion all over the face daily can “potentially damage your skin barrier to a point where you’re going to have to do a lot of extra work ... to bring it back,” says Dr. Sodha.
Dr. Day also worries about this trend resulting in dry skin among followers. Even in situations where using calamine lotion is appropriate, like treating poison ivy, its drying effects can sometimes irritate the skin.
And dry skin can be more than an aesthetic issue: It can lead to breaks in the skin, which can result in infections and scarring, she points out. Although this may not occur in someone with extremely oily skin, most people don’t have extremely oily skin, says Dr. Day, so this will be ineffective at best, and at worst, damaging.
If someone is looking for a good makeup base layer, Dr. Sodha recommends something that’s noncomedogenic and nonsensitizing, like silicon-based primers. “The great thing about these products is that they are noncomedogenic, so they won’t clog your pores. They’re synthetic, so they’re not going to cause some sort of allergy,” she says.
In general, both dermatologists warn their patients to be wary of the TikTok trends they see online, and they cautioned about possible effects with long term use of calamine lotion on the face, even if it appears to work with one-time use. “Consumers have to think about this like they do with any sort of product that they come across, just thinking about the long-term effects of something like this and how it works for their own skin,” says Dr. Sodha.
A version of this article first appeared on Medscape.com.
Though this may seem to work as a base layer for some people, dermatologists have concerns about this trend, particularly the risk of dryness.
As of Aug. 15, the #calaminelotion tag had more than 20.9 million views on TikTok, with hundreds of videos hailing the cream for its opaque pink tint and matte effect when used under foundation.
Calamine lotion has been used to treat itchy rashes, insect bites, and pain from chickenpox and poison ivy for years. It’s sold over the counter and is a common first-line treatment for skin discomfort that has been used for hundreds of years, says Doris Day, MD, a dermatologist who practices in New York City. It is also on the World Health Organization’s list of essential drugs, she points out in an interview.
“This is something that has been around for a long time. It’s recognized as a drug that has importance. So every now and then, I guess somebody comes across it” and says it’s a “new panacea” for something, “but it’s really not. It’s just an old-time simple product.”
Calamine lotion is made of ferric oxide and zinc oxide, which gives it its antiseptic and anti-itch properties, in addition to its characteristic pink color. Zinc oxide is also commonly used in mineral sunscreens, Dr. Day points out.
Although these ingredients are exceedingly safe with temporary, localized use, high concentrations and chronic use of calamine lotion can be irritating to the skin, says Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington.
At these high concentrations, calamine lotion can be drying, which may cause skin clumping and can be abrasive, says Dr. Sodha. She also cautions that the astringent properties of the zinc and the high pH may disrupt proteins on the skin, which breaks down the skin’s natural defenses. Using calamine lotion all over the face daily can “potentially damage your skin barrier to a point where you’re going to have to do a lot of extra work ... to bring it back,” says Dr. Sodha.
Dr. Day also worries about this trend resulting in dry skin among followers. Even in situations where using calamine lotion is appropriate, like treating poison ivy, its drying effects can sometimes irritate the skin.
And dry skin can be more than an aesthetic issue: It can lead to breaks in the skin, which can result in infections and scarring, she points out. Although this may not occur in someone with extremely oily skin, most people don’t have extremely oily skin, says Dr. Day, so this will be ineffective at best, and at worst, damaging.
If someone is looking for a good makeup base layer, Dr. Sodha recommends something that’s noncomedogenic and nonsensitizing, like silicon-based primers. “The great thing about these products is that they are noncomedogenic, so they won’t clog your pores. They’re synthetic, so they’re not going to cause some sort of allergy,” she says.
In general, both dermatologists warn their patients to be wary of the TikTok trends they see online, and they cautioned about possible effects with long term use of calamine lotion on the face, even if it appears to work with one-time use. “Consumers have to think about this like they do with any sort of product that they come across, just thinking about the long-term effects of something like this and how it works for their own skin,” says Dr. Sodha.
A version of this article first appeared on Medscape.com.
Though this may seem to work as a base layer for some people, dermatologists have concerns about this trend, particularly the risk of dryness.
As of Aug. 15, the #calaminelotion tag had more than 20.9 million views on TikTok, with hundreds of videos hailing the cream for its opaque pink tint and matte effect when used under foundation.
Calamine lotion has been used to treat itchy rashes, insect bites, and pain from chickenpox and poison ivy for years. It’s sold over the counter and is a common first-line treatment for skin discomfort that has been used for hundreds of years, says Doris Day, MD, a dermatologist who practices in New York City. It is also on the World Health Organization’s list of essential drugs, she points out in an interview.
“This is something that has been around for a long time. It’s recognized as a drug that has importance. So every now and then, I guess somebody comes across it” and says it’s a “new panacea” for something, “but it’s really not. It’s just an old-time simple product.”
Calamine lotion is made of ferric oxide and zinc oxide, which gives it its antiseptic and anti-itch properties, in addition to its characteristic pink color. Zinc oxide is also commonly used in mineral sunscreens, Dr. Day points out.
Although these ingredients are exceedingly safe with temporary, localized use, high concentrations and chronic use of calamine lotion can be irritating to the skin, says Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington.
At these high concentrations, calamine lotion can be drying, which may cause skin clumping and can be abrasive, says Dr. Sodha. She also cautions that the astringent properties of the zinc and the high pH may disrupt proteins on the skin, which breaks down the skin’s natural defenses. Using calamine lotion all over the face daily can “potentially damage your skin barrier to a point where you’re going to have to do a lot of extra work ... to bring it back,” says Dr. Sodha.
Dr. Day also worries about this trend resulting in dry skin among followers. Even in situations where using calamine lotion is appropriate, like treating poison ivy, its drying effects can sometimes irritate the skin.
And dry skin can be more than an aesthetic issue: It can lead to breaks in the skin, which can result in infections and scarring, she points out. Although this may not occur in someone with extremely oily skin, most people don’t have extremely oily skin, says Dr. Day, so this will be ineffective at best, and at worst, damaging.
If someone is looking for a good makeup base layer, Dr. Sodha recommends something that’s noncomedogenic and nonsensitizing, like silicon-based primers. “The great thing about these products is that they are noncomedogenic, so they won’t clog your pores. They’re synthetic, so they’re not going to cause some sort of allergy,” she says.
In general, both dermatologists warn their patients to be wary of the TikTok trends they see online, and they cautioned about possible effects with long term use of calamine lotion on the face, even if it appears to work with one-time use. “Consumers have to think about this like they do with any sort of product that they come across, just thinking about the long-term effects of something like this and how it works for their own skin,” says Dr. Sodha.
A version of this article first appeared on Medscape.com.
A 9-year-old girl was evaluated for a week-long history of rash on the feet
A complete body examination failed to reveal any other lesions suggestive of a fungal infection. A blood count and urinalysis were within normal limits. She had no lymphadenopathy or hepatosplenomegaly. She was diagnosed with cutaneous larva migrans (CLM) given the clinical appearance of the lesions and the recent travel history.
CLM is a zoonotic infection caused by several hookworms such as Ancylostoma braziliense, Ancylostoma caninum, and Uncinaria stenocephala, as well as human hookworms such as Ancylostoma duodenale and Necator americanus. The hookworms can be present in contaminated soils and sandy beaches on the coastal regions of South America, the Caribbean, the Southeastern United States, Southeast Asia, and Africa.1-5
It is a common disease in the tourist population visiting tropical countries because of exposure to the hookworms in the soil without use of proper foot protection.
The clinical features are of an erythematous linear serpiginous plaque that is pruritic and can progress from millimeters to centimeters in size within a few days to weeks. Vesicles and multiple tracks can also be seen. The most common locations are the feet, buttocks, and thighs.
The larvae in the soil come from eggs excreted in the feces of infected cats and dogs. The infection is caused by direct contact of the larvae with the stratum corneum of the skin creating a burrow and an inflammatory response that will cause erythema, edema, track formation, and pruritus.
Diagnosis is made clinically. Rarely, a skin biopsy is warranted. The differential diagnosis includes tinea pedis, granuloma annulare, larva currens, contact dermatitis, and herpes zoster.
Tinea pedis is a fungal infection of the skin of the feet, commonly localized on the web spaces. The risk factors are a hot and humid environment, prolonged wear of occlusive footwear, excess sweating, and prolonged exposure to water.6 Diagnosis is confirmed by microscopic evaluation of skin scrapings with potassium hydroxide or a fungal culture. The infection is treated with topical antifungal creams and, in severe cases, systemic antifungals. Granuloma annulare is a benign chronic skin condition that presents with annular-shaped lesions. Its etiology is unknown. The lesions may be asymptomatic or mildly pruritic. Localized granuloma annulare typically presents as reddish-brown papules or plaques on the fingers, hands, elbows, dorsal feet, or ankles. The feature distinguishing granuloma annulare from other annular lesions is its absence of scale.
Allergic contact dermatitis is caused by skin exposure to an allergen and a secondary inflammatory response to this material on the skin causing inflammation, vesiculation, and pruritus. Lesions are treated with topical corticosteroids and avoidance of the allergen.
Herpes zoster is caused by a viral infection of the latent varicella-zoster virus. Its reactivation causes the presence of vesicles with an erythematous base that have a dermatomal distribution. The lesions are usually tender. Treatment is recommended to be started within 72 hours of the eruption with antivirals such as acyclovir or valacyclovir.
Cutaneous larva currens is caused by the cutaneous infection with Strongyloides stercoralis. In comparison with CLM, the lesions progress faster, at up to a centimeter within hours.
CLM is usually self-limited. If the patient has multiple lesions or more severe disease, oral albendazole or ivermectin can be prescribed. Other treatments, though not preferred, include freezing and topical thiabendazole solutions.
As our patient had several lesions, oral ivermectin was chosen as treatment and the lesions cleared within a week. Also, she was recommended to always wear shoes when walking on the beach.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Dr. Valderrama is a pediatric dermatologist at Fundación Cardioinfantil, Bogota, Colombia.
References
1. Feldmeier H and Schuster A. Eur J Clin Microbiol Infect Dis. 2012 Jun;31(6):915-8.
2. Jacobson CC and Abel EA. J Am Acad Dermatol. 2007 Jun;56(6):1026-43.
3. Kincaid L et al. Travel Med Infect Dis. 2015 Sep-Oct;13(5):382-7.
4. Gill N et al. Adv Skin Wound Care. 2020 Jul;33(7):356-9.
5. Rodenas-Herranz T et al. Dermatol Ther. 2020 May;33(3):e13316.
6. Pramod K et al. In: StatPearls [Internet]. Treasure Island (Fla): StatPearls Publishing; 2022 Jan.
A complete body examination failed to reveal any other lesions suggestive of a fungal infection. A blood count and urinalysis were within normal limits. She had no lymphadenopathy or hepatosplenomegaly. She was diagnosed with cutaneous larva migrans (CLM) given the clinical appearance of the lesions and the recent travel history.
CLM is a zoonotic infection caused by several hookworms such as Ancylostoma braziliense, Ancylostoma caninum, and Uncinaria stenocephala, as well as human hookworms such as Ancylostoma duodenale and Necator americanus. The hookworms can be present in contaminated soils and sandy beaches on the coastal regions of South America, the Caribbean, the Southeastern United States, Southeast Asia, and Africa.1-5
It is a common disease in the tourist population visiting tropical countries because of exposure to the hookworms in the soil without use of proper foot protection.
The clinical features are of an erythematous linear serpiginous plaque that is pruritic and can progress from millimeters to centimeters in size within a few days to weeks. Vesicles and multiple tracks can also be seen. The most common locations are the feet, buttocks, and thighs.
The larvae in the soil come from eggs excreted in the feces of infected cats and dogs. The infection is caused by direct contact of the larvae with the stratum corneum of the skin creating a burrow and an inflammatory response that will cause erythema, edema, track formation, and pruritus.
Diagnosis is made clinically. Rarely, a skin biopsy is warranted. The differential diagnosis includes tinea pedis, granuloma annulare, larva currens, contact dermatitis, and herpes zoster.
Tinea pedis is a fungal infection of the skin of the feet, commonly localized on the web spaces. The risk factors are a hot and humid environment, prolonged wear of occlusive footwear, excess sweating, and prolonged exposure to water.6 Diagnosis is confirmed by microscopic evaluation of skin scrapings with potassium hydroxide or a fungal culture. The infection is treated with topical antifungal creams and, in severe cases, systemic antifungals. Granuloma annulare is a benign chronic skin condition that presents with annular-shaped lesions. Its etiology is unknown. The lesions may be asymptomatic or mildly pruritic. Localized granuloma annulare typically presents as reddish-brown papules or plaques on the fingers, hands, elbows, dorsal feet, or ankles. The feature distinguishing granuloma annulare from other annular lesions is its absence of scale.
Allergic contact dermatitis is caused by skin exposure to an allergen and a secondary inflammatory response to this material on the skin causing inflammation, vesiculation, and pruritus. Lesions are treated with topical corticosteroids and avoidance of the allergen.
Herpes zoster is caused by a viral infection of the latent varicella-zoster virus. Its reactivation causes the presence of vesicles with an erythematous base that have a dermatomal distribution. The lesions are usually tender. Treatment is recommended to be started within 72 hours of the eruption with antivirals such as acyclovir or valacyclovir.
Cutaneous larva currens is caused by the cutaneous infection with Strongyloides stercoralis. In comparison with CLM, the lesions progress faster, at up to a centimeter within hours.
CLM is usually self-limited. If the patient has multiple lesions or more severe disease, oral albendazole or ivermectin can be prescribed. Other treatments, though not preferred, include freezing and topical thiabendazole solutions.
As our patient had several lesions, oral ivermectin was chosen as treatment and the lesions cleared within a week. Also, she was recommended to always wear shoes when walking on the beach.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Dr. Valderrama is a pediatric dermatologist at Fundación Cardioinfantil, Bogota, Colombia.
References
1. Feldmeier H and Schuster A. Eur J Clin Microbiol Infect Dis. 2012 Jun;31(6):915-8.
2. Jacobson CC and Abel EA. J Am Acad Dermatol. 2007 Jun;56(6):1026-43.
3. Kincaid L et al. Travel Med Infect Dis. 2015 Sep-Oct;13(5):382-7.
4. Gill N et al. Adv Skin Wound Care. 2020 Jul;33(7):356-9.
5. Rodenas-Herranz T et al. Dermatol Ther. 2020 May;33(3):e13316.
6. Pramod K et al. In: StatPearls [Internet]. Treasure Island (Fla): StatPearls Publishing; 2022 Jan.
A complete body examination failed to reveal any other lesions suggestive of a fungal infection. A blood count and urinalysis were within normal limits. She had no lymphadenopathy or hepatosplenomegaly. She was diagnosed with cutaneous larva migrans (CLM) given the clinical appearance of the lesions and the recent travel history.
CLM is a zoonotic infection caused by several hookworms such as Ancylostoma braziliense, Ancylostoma caninum, and Uncinaria stenocephala, as well as human hookworms such as Ancylostoma duodenale and Necator americanus. The hookworms can be present in contaminated soils and sandy beaches on the coastal regions of South America, the Caribbean, the Southeastern United States, Southeast Asia, and Africa.1-5
It is a common disease in the tourist population visiting tropical countries because of exposure to the hookworms in the soil without use of proper foot protection.
The clinical features are of an erythematous linear serpiginous plaque that is pruritic and can progress from millimeters to centimeters in size within a few days to weeks. Vesicles and multiple tracks can also be seen. The most common locations are the feet, buttocks, and thighs.
The larvae in the soil come from eggs excreted in the feces of infected cats and dogs. The infection is caused by direct contact of the larvae with the stratum corneum of the skin creating a burrow and an inflammatory response that will cause erythema, edema, track formation, and pruritus.
Diagnosis is made clinically. Rarely, a skin biopsy is warranted. The differential diagnosis includes tinea pedis, granuloma annulare, larva currens, contact dermatitis, and herpes zoster.
Tinea pedis is a fungal infection of the skin of the feet, commonly localized on the web spaces. The risk factors are a hot and humid environment, prolonged wear of occlusive footwear, excess sweating, and prolonged exposure to water.6 Diagnosis is confirmed by microscopic evaluation of skin scrapings with potassium hydroxide or a fungal culture. The infection is treated with topical antifungal creams and, in severe cases, systemic antifungals. Granuloma annulare is a benign chronic skin condition that presents with annular-shaped lesions. Its etiology is unknown. The lesions may be asymptomatic or mildly pruritic. Localized granuloma annulare typically presents as reddish-brown papules or plaques on the fingers, hands, elbows, dorsal feet, or ankles. The feature distinguishing granuloma annulare from other annular lesions is its absence of scale.
Allergic contact dermatitis is caused by skin exposure to an allergen and a secondary inflammatory response to this material on the skin causing inflammation, vesiculation, and pruritus. Lesions are treated with topical corticosteroids and avoidance of the allergen.
Herpes zoster is caused by a viral infection of the latent varicella-zoster virus. Its reactivation causes the presence of vesicles with an erythematous base that have a dermatomal distribution. The lesions are usually tender. Treatment is recommended to be started within 72 hours of the eruption with antivirals such as acyclovir or valacyclovir.
Cutaneous larva currens is caused by the cutaneous infection with Strongyloides stercoralis. In comparison with CLM, the lesions progress faster, at up to a centimeter within hours.
CLM is usually self-limited. If the patient has multiple lesions or more severe disease, oral albendazole or ivermectin can be prescribed. Other treatments, though not preferred, include freezing and topical thiabendazole solutions.
As our patient had several lesions, oral ivermectin was chosen as treatment and the lesions cleared within a week. Also, she was recommended to always wear shoes when walking on the beach.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Dr. Valderrama is a pediatric dermatologist at Fundación Cardioinfantil, Bogota, Colombia.
References
1. Feldmeier H and Schuster A. Eur J Clin Microbiol Infect Dis. 2012 Jun;31(6):915-8.
2. Jacobson CC and Abel EA. J Am Acad Dermatol. 2007 Jun;56(6):1026-43.
3. Kincaid L et al. Travel Med Infect Dis. 2015 Sep-Oct;13(5):382-7.
4. Gill N et al. Adv Skin Wound Care. 2020 Jul;33(7):356-9.
5. Rodenas-Herranz T et al. Dermatol Ther. 2020 May;33(3):e13316.
6. Pramod K et al. In: StatPearls [Internet]. Treasure Island (Fla): StatPearls Publishing; 2022 Jan.
Her mother reported recent travel to a beachside city in Colombia. A review of systems was negative. She was not taking any other medications or vitamin supplements. There were no pets at home and no other affected family members. Physical exam was notable for an erythematous curvilinear plaque on the feet and a small vesicle.
Biosimilar-to-biosimilar switches deemed safe and effective, systematic review reveals
Switching from one biosimilar medication to another is safe and effective, a new systematic review indicates, even though this clinical practice is not governed by current health authority regulations or guidance.
“No reduction in effectiveness or increase in adverse events was detected in biosimilar-to-biosimilar switching studies conducted to date,” the review’s authors noted in their study, published online in BioDrugs.
“The possibility of multiple switches between biosimilars of the same reference biologic is already a reality, and these types of switches are expected to become more common in the future. ... Although it is not covered by current health authority regulations or guidance,” added the authors, led by Hillel P. Cohen, PhD, executive director of scientific affairs at Sandoz, a division of Novartis.
The researchers searched electronic databases through December 2021 and found 23 observational studies that met their search criteria, of which 13 were published in peer-reviewed journals; the remainder appeared in abstract form. The studies totaled 3,657 patients. The researchers did not identify any randomized clinical trials.
“The studies were heterogeneous in size, design, and endpoints, providing data on safety, effectiveness, immunogenicity, pharmacokinetics, patient retention, patient and physician perceptions, and drug-use patterns,” the authors wrote.
The authors found that the majority of studies evaluated switches between biosimilars of infliximab, but they also identified switches between biosimilars of adalimumab, etanercept, and rituximab.
“Some health care providers are hesitant to switch patients from one biosimilar to another biosimilar because of a perceived lack of clinical data on such switches,” Dr. Cohen said in an interview.
The review’s findings – that there were no clinically relevant differences when switching patients from one biosimilar to another – are consistent with the science, Dr. Cohen said. “Physicians should have confidence that the data demonstrate that safety and effectiveness are not impacted if patients switch from one biosimilar to another biosimilar of the same reference biologic,” he said.
Currently, the published data include biosimilars to only four reference biologics. “However, I anticipate additional biosimilar-to-biosimilar switching data will become available in the future,” Dr. Cohen said. “In fact, several new studies have been published in recent months, after the cut-off date for inclusion in our systematic review.”
Switching common in rheumatology, dermatology, and gastroenterology
Biosimilar-to-biosimilar switching was observed most commonly in rheumatology practice, but also was seen in the specialties of dermatology and gastroenterology.
Jeffrey Weinberg, MD, clinical professor of dermatology, Icahn School of Medicine at Mount Sinai, New York City, said in an interview that the study is among the best to date showing that switching biosimilars does not compromise efficacy or safety.
“I would hypothesize that the interchangeability would apply to psoriasis patients,” Dr. Weinberg said. However, “over the next few years, we will have an increasing number of biosimilars for an increasing number of different molecules. We will need to be vigilant to observe if similar behavior is observed with the biosimilars yet to come.”
Keith Choate, MD, PhD, professor of dermatology, pathology, and genetics, and associate dean for physician-scientist development at Yale University, New Haven, Conn., said that biosimilars have comparable efficacy to the branded medication they replace. “If response is lost to an individual agent, we would not typically then switch to a biosimilar, but would favor another class of therapy or a distinct therapeutic which targets the same pathway.”
When physicians prescribe a biosimilar for rheumatoid arthritis or psoriatic arthritis, in 9 out 10 people, “it’s going to work as well, and it’s not going to cause any more side effects,” said Stanford Shoor, MD, clinical professor of medicine and rheumatology, Stanford (Calif.) University.
The systematic review, even within its limitations, reinforces confidence in the antitumor necrosis factor biosimilars, said Jean-Frederic Colombel, MD, codirector of the Feinstein Inflammatory Bowel Disease Clinical Center at Mount Sinai, New York, and professor of medicine, division of gastroenterology, Icahn School of Medicine at Mount Sinai.
“Still, studies with longer follow-up are needed,” Dr. Colombel said, adding that the remaining questions relate to the efficacy and safety of switching multiple times, which will likely occur in the near future. There will be a “need to provide information to the patient regarding what originator or biosimilar(s) he has been exposed to during the course of his disease.”
Switching will increasingly become the norm, said Miguel Regueiro, MD, chair of the Digestive Disease & Surgery Institute, Cleveland Clinic. In his clinical practice, he has the most experience with Crohn’s disease and ulcerative colitis, and biosimilar-to-biosimilar infliximab switches. “Unless there are data that emerge, I have no concerns with this.”
He added that it’s an “interesting study that affirms my findings in clinical practice – that one can switch from a biosimilar to biosimilar (of the same reference product).”
The review’s results also make sense from an economic standpoint, said Rajat Bhatt, MD, owner of Prime Rheumatology in Richmond, Tex., and an adjunct faculty member at Caribbean Medical University, Willemstad, Curaçao. “Switching to biosimilars will result in cost savings for the health care system.” Patients on certain insurances also will save by switching to a biosimilar with a lower copay.
However, the review is limited by a relatively small number of studies that have provided primary data on this topic, and most of these were switching from infliximab to a biosimilar for inflammatory bowel disease, said Alfred Kim, MD, PhD, an adult rheumatologist at Barnes-Jewish Hospital, St. Louis, and assistant professor of medicine at Washington University in St. Louis.
As with any meta-analysis evaluating a small number of studies, “broad applicability to all conditions and reference/biosimilar pair can only be assumed. Also, many of the studies used for this meta-analysis are observational, which can introduce a variety of biases that can be difficult to adjust for,” Dr. Kim said. “Nevertheless, these analyses are an important first step in validating the [Food and Drug Administration’s] approach to evaluating biosimilars, as the clinical outcomes are consistent between different biosimilars.”
This systematic review is not enough to prove that all patients will do fine when switching from one biosimilar to another, said Florence Aslinia, MD, a gastroenterologist at the University of Kansas Health System in Kansas City. It’s possible that some patients may not do as well, she said, noting that, in one study of patients with inflammatory bowel disease, 10% of patients on a biosimilar infliximab needed to switch back to the originator infliximab (Remicade, Janssen) because of side effects attributed to the biosimilar. The same thing may or may not happen with biosimilar-to-biosimilar switching, and it requires further study.
The authors did not receive any funding for writing this review. Dr. Cohen is an employee of Sandoz, a division of Novartis. He may own stock in Novartis. Two coauthors are also employees of Sandoz. The other three coauthors reported having financial relationships with numerous pharmaceutical companies, including Sandoz and/or Novartis. Dr. Colombel reported financial relationships with many pharmaceutical companies, including Novartis and other manufacturers of biosimilars. Dr. Regueiro reports financial relationships with numerous pharmaceutical companies, including some manufacturers of biosimilars. Dr. Weinberg reported financial relationships with Celgene, AbbVie, Eli Lilly, and Novartis. Kim reports financial relationships with GlaxoSmithKline, Pfizer, and AstraZeneca. Dr. Aslinia, Dr. Shoor, Dr. Choate, and Dr. Bhatt reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Switching from one biosimilar medication to another is safe and effective, a new systematic review indicates, even though this clinical practice is not governed by current health authority regulations or guidance.
“No reduction in effectiveness or increase in adverse events was detected in biosimilar-to-biosimilar switching studies conducted to date,” the review’s authors noted in their study, published online in BioDrugs.
“The possibility of multiple switches between biosimilars of the same reference biologic is already a reality, and these types of switches are expected to become more common in the future. ... Although it is not covered by current health authority regulations or guidance,” added the authors, led by Hillel P. Cohen, PhD, executive director of scientific affairs at Sandoz, a division of Novartis.
The researchers searched electronic databases through December 2021 and found 23 observational studies that met their search criteria, of which 13 were published in peer-reviewed journals; the remainder appeared in abstract form. The studies totaled 3,657 patients. The researchers did not identify any randomized clinical trials.
“The studies were heterogeneous in size, design, and endpoints, providing data on safety, effectiveness, immunogenicity, pharmacokinetics, patient retention, patient and physician perceptions, and drug-use patterns,” the authors wrote.
The authors found that the majority of studies evaluated switches between biosimilars of infliximab, but they also identified switches between biosimilars of adalimumab, etanercept, and rituximab.
“Some health care providers are hesitant to switch patients from one biosimilar to another biosimilar because of a perceived lack of clinical data on such switches,” Dr. Cohen said in an interview.
The review’s findings – that there were no clinically relevant differences when switching patients from one biosimilar to another – are consistent with the science, Dr. Cohen said. “Physicians should have confidence that the data demonstrate that safety and effectiveness are not impacted if patients switch from one biosimilar to another biosimilar of the same reference biologic,” he said.
Currently, the published data include biosimilars to only four reference biologics. “However, I anticipate additional biosimilar-to-biosimilar switching data will become available in the future,” Dr. Cohen said. “In fact, several new studies have been published in recent months, after the cut-off date for inclusion in our systematic review.”
Switching common in rheumatology, dermatology, and gastroenterology
Biosimilar-to-biosimilar switching was observed most commonly in rheumatology practice, but also was seen in the specialties of dermatology and gastroenterology.
Jeffrey Weinberg, MD, clinical professor of dermatology, Icahn School of Medicine at Mount Sinai, New York City, said in an interview that the study is among the best to date showing that switching biosimilars does not compromise efficacy or safety.
“I would hypothesize that the interchangeability would apply to psoriasis patients,” Dr. Weinberg said. However, “over the next few years, we will have an increasing number of biosimilars for an increasing number of different molecules. We will need to be vigilant to observe if similar behavior is observed with the biosimilars yet to come.”
Keith Choate, MD, PhD, professor of dermatology, pathology, and genetics, and associate dean for physician-scientist development at Yale University, New Haven, Conn., said that biosimilars have comparable efficacy to the branded medication they replace. “If response is lost to an individual agent, we would not typically then switch to a biosimilar, but would favor another class of therapy or a distinct therapeutic which targets the same pathway.”
When physicians prescribe a biosimilar for rheumatoid arthritis or psoriatic arthritis, in 9 out 10 people, “it’s going to work as well, and it’s not going to cause any more side effects,” said Stanford Shoor, MD, clinical professor of medicine and rheumatology, Stanford (Calif.) University.
The systematic review, even within its limitations, reinforces confidence in the antitumor necrosis factor biosimilars, said Jean-Frederic Colombel, MD, codirector of the Feinstein Inflammatory Bowel Disease Clinical Center at Mount Sinai, New York, and professor of medicine, division of gastroenterology, Icahn School of Medicine at Mount Sinai.
“Still, studies with longer follow-up are needed,” Dr. Colombel said, adding that the remaining questions relate to the efficacy and safety of switching multiple times, which will likely occur in the near future. There will be a “need to provide information to the patient regarding what originator or biosimilar(s) he has been exposed to during the course of his disease.”
Switching will increasingly become the norm, said Miguel Regueiro, MD, chair of the Digestive Disease & Surgery Institute, Cleveland Clinic. In his clinical practice, he has the most experience with Crohn’s disease and ulcerative colitis, and biosimilar-to-biosimilar infliximab switches. “Unless there are data that emerge, I have no concerns with this.”
He added that it’s an “interesting study that affirms my findings in clinical practice – that one can switch from a biosimilar to biosimilar (of the same reference product).”
The review’s results also make sense from an economic standpoint, said Rajat Bhatt, MD, owner of Prime Rheumatology in Richmond, Tex., and an adjunct faculty member at Caribbean Medical University, Willemstad, Curaçao. “Switching to biosimilars will result in cost savings for the health care system.” Patients on certain insurances also will save by switching to a biosimilar with a lower copay.
However, the review is limited by a relatively small number of studies that have provided primary data on this topic, and most of these were switching from infliximab to a biosimilar for inflammatory bowel disease, said Alfred Kim, MD, PhD, an adult rheumatologist at Barnes-Jewish Hospital, St. Louis, and assistant professor of medicine at Washington University in St. Louis.
As with any meta-analysis evaluating a small number of studies, “broad applicability to all conditions and reference/biosimilar pair can only be assumed. Also, many of the studies used for this meta-analysis are observational, which can introduce a variety of biases that can be difficult to adjust for,” Dr. Kim said. “Nevertheless, these analyses are an important first step in validating the [Food and Drug Administration’s] approach to evaluating biosimilars, as the clinical outcomes are consistent between different biosimilars.”
This systematic review is not enough to prove that all patients will do fine when switching from one biosimilar to another, said Florence Aslinia, MD, a gastroenterologist at the University of Kansas Health System in Kansas City. It’s possible that some patients may not do as well, she said, noting that, in one study of patients with inflammatory bowel disease, 10% of patients on a biosimilar infliximab needed to switch back to the originator infliximab (Remicade, Janssen) because of side effects attributed to the biosimilar. The same thing may or may not happen with biosimilar-to-biosimilar switching, and it requires further study.
The authors did not receive any funding for writing this review. Dr. Cohen is an employee of Sandoz, a division of Novartis. He may own stock in Novartis. Two coauthors are also employees of Sandoz. The other three coauthors reported having financial relationships with numerous pharmaceutical companies, including Sandoz and/or Novartis. Dr. Colombel reported financial relationships with many pharmaceutical companies, including Novartis and other manufacturers of biosimilars. Dr. Regueiro reports financial relationships with numerous pharmaceutical companies, including some manufacturers of biosimilars. Dr. Weinberg reported financial relationships with Celgene, AbbVie, Eli Lilly, and Novartis. Kim reports financial relationships with GlaxoSmithKline, Pfizer, and AstraZeneca. Dr. Aslinia, Dr. Shoor, Dr. Choate, and Dr. Bhatt reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Switching from one biosimilar medication to another is safe and effective, a new systematic review indicates, even though this clinical practice is not governed by current health authority regulations or guidance.
“No reduction in effectiveness or increase in adverse events was detected in biosimilar-to-biosimilar switching studies conducted to date,” the review’s authors noted in their study, published online in BioDrugs.
“The possibility of multiple switches between biosimilars of the same reference biologic is already a reality, and these types of switches are expected to become more common in the future. ... Although it is not covered by current health authority regulations or guidance,” added the authors, led by Hillel P. Cohen, PhD, executive director of scientific affairs at Sandoz, a division of Novartis.
The researchers searched electronic databases through December 2021 and found 23 observational studies that met their search criteria, of which 13 were published in peer-reviewed journals; the remainder appeared in abstract form. The studies totaled 3,657 patients. The researchers did not identify any randomized clinical trials.
“The studies were heterogeneous in size, design, and endpoints, providing data on safety, effectiveness, immunogenicity, pharmacokinetics, patient retention, patient and physician perceptions, and drug-use patterns,” the authors wrote.
The authors found that the majority of studies evaluated switches between biosimilars of infliximab, but they also identified switches between biosimilars of adalimumab, etanercept, and rituximab.
“Some health care providers are hesitant to switch patients from one biosimilar to another biosimilar because of a perceived lack of clinical data on such switches,” Dr. Cohen said in an interview.
The review’s findings – that there were no clinically relevant differences when switching patients from one biosimilar to another – are consistent with the science, Dr. Cohen said. “Physicians should have confidence that the data demonstrate that safety and effectiveness are not impacted if patients switch from one biosimilar to another biosimilar of the same reference biologic,” he said.
Currently, the published data include biosimilars to only four reference biologics. “However, I anticipate additional biosimilar-to-biosimilar switching data will become available in the future,” Dr. Cohen said. “In fact, several new studies have been published in recent months, after the cut-off date for inclusion in our systematic review.”
Switching common in rheumatology, dermatology, and gastroenterology
Biosimilar-to-biosimilar switching was observed most commonly in rheumatology practice, but also was seen in the specialties of dermatology and gastroenterology.
Jeffrey Weinberg, MD, clinical professor of dermatology, Icahn School of Medicine at Mount Sinai, New York City, said in an interview that the study is among the best to date showing that switching biosimilars does not compromise efficacy or safety.
“I would hypothesize that the interchangeability would apply to psoriasis patients,” Dr. Weinberg said. However, “over the next few years, we will have an increasing number of biosimilars for an increasing number of different molecules. We will need to be vigilant to observe if similar behavior is observed with the biosimilars yet to come.”
Keith Choate, MD, PhD, professor of dermatology, pathology, and genetics, and associate dean for physician-scientist development at Yale University, New Haven, Conn., said that biosimilars have comparable efficacy to the branded medication they replace. “If response is lost to an individual agent, we would not typically then switch to a biosimilar, but would favor another class of therapy or a distinct therapeutic which targets the same pathway.”
When physicians prescribe a biosimilar for rheumatoid arthritis or psoriatic arthritis, in 9 out 10 people, “it’s going to work as well, and it’s not going to cause any more side effects,” said Stanford Shoor, MD, clinical professor of medicine and rheumatology, Stanford (Calif.) University.
The systematic review, even within its limitations, reinforces confidence in the antitumor necrosis factor biosimilars, said Jean-Frederic Colombel, MD, codirector of the Feinstein Inflammatory Bowel Disease Clinical Center at Mount Sinai, New York, and professor of medicine, division of gastroenterology, Icahn School of Medicine at Mount Sinai.
“Still, studies with longer follow-up are needed,” Dr. Colombel said, adding that the remaining questions relate to the efficacy and safety of switching multiple times, which will likely occur in the near future. There will be a “need to provide information to the patient regarding what originator or biosimilar(s) he has been exposed to during the course of his disease.”
Switching will increasingly become the norm, said Miguel Regueiro, MD, chair of the Digestive Disease & Surgery Institute, Cleveland Clinic. In his clinical practice, he has the most experience with Crohn’s disease and ulcerative colitis, and biosimilar-to-biosimilar infliximab switches. “Unless there are data that emerge, I have no concerns with this.”
He added that it’s an “interesting study that affirms my findings in clinical practice – that one can switch from a biosimilar to biosimilar (of the same reference product).”
The review’s results also make sense from an economic standpoint, said Rajat Bhatt, MD, owner of Prime Rheumatology in Richmond, Tex., and an adjunct faculty member at Caribbean Medical University, Willemstad, Curaçao. “Switching to biosimilars will result in cost savings for the health care system.” Patients on certain insurances also will save by switching to a biosimilar with a lower copay.
However, the review is limited by a relatively small number of studies that have provided primary data on this topic, and most of these were switching from infliximab to a biosimilar for inflammatory bowel disease, said Alfred Kim, MD, PhD, an adult rheumatologist at Barnes-Jewish Hospital, St. Louis, and assistant professor of medicine at Washington University in St. Louis.
As with any meta-analysis evaluating a small number of studies, “broad applicability to all conditions and reference/biosimilar pair can only be assumed. Also, many of the studies used for this meta-analysis are observational, which can introduce a variety of biases that can be difficult to adjust for,” Dr. Kim said. “Nevertheless, these analyses are an important first step in validating the [Food and Drug Administration’s] approach to evaluating biosimilars, as the clinical outcomes are consistent between different biosimilars.”
This systematic review is not enough to prove that all patients will do fine when switching from one biosimilar to another, said Florence Aslinia, MD, a gastroenterologist at the University of Kansas Health System in Kansas City. It’s possible that some patients may not do as well, she said, noting that, in one study of patients with inflammatory bowel disease, 10% of patients on a biosimilar infliximab needed to switch back to the originator infliximab (Remicade, Janssen) because of side effects attributed to the biosimilar. The same thing may or may not happen with biosimilar-to-biosimilar switching, and it requires further study.
The authors did not receive any funding for writing this review. Dr. Cohen is an employee of Sandoz, a division of Novartis. He may own stock in Novartis. Two coauthors are also employees of Sandoz. The other three coauthors reported having financial relationships with numerous pharmaceutical companies, including Sandoz and/or Novartis. Dr. Colombel reported financial relationships with many pharmaceutical companies, including Novartis and other manufacturers of biosimilars. Dr. Regueiro reports financial relationships with numerous pharmaceutical companies, including some manufacturers of biosimilars. Dr. Weinberg reported financial relationships with Celgene, AbbVie, Eli Lilly, and Novartis. Kim reports financial relationships with GlaxoSmithKline, Pfizer, and AstraZeneca. Dr. Aslinia, Dr. Shoor, Dr. Choate, and Dr. Bhatt reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM BIODRUGS
Dermatologists share vitiligo breakthrough news with patients
For the first time, patients with vitiligo who have long lived with patches of skin that are without pigment can now have even skin tones on their faces and other bodily regions with a Food and Drug Administration–approved, easy-to-use topical treatment.
In July, , the most common form of the disease.
Topical ruxolitinib was first approved in September 2021 for atopic dermatitis, and dermatologists are already writing prescriptions for its new vitiligo indication.
“The FDA approval of ruxolitinib for repigmentation of vitiligo is historic and groundbreaking,” Seemal R. Desai, MD, a dermatologist at the University of Texas Southwestern Medical Center, Dallas, told this news organization.
The news brings hope to patients 12 years and older who suffer from the psychosocial effects of the disease, which is estimated to affect 1.9 million to 2.8 million adults in the United States.
The announcement followed FDA approval a month earlier of another dermatologic milestone – an oral JAK inhibitor, baricitinib, which became the first treatment for patients with alopecia areata.
For Dr. Desai, the ruxolitinib news is personal. His brother, also a physician, has lived a lifetime with vitiligo. His family experience, Dr. Desai said, showed him “what a disease like this can do to a person psychologically.”
Dr. Desai said his early exposure helped lead to his own decision to dedicate his career to pigmentary diseases.
His brother won’t personally benefit from the cream because his skin has been completely depigmented and repigmentation is not of interest to him, Dr. Desai said. But both brothers are excited as physicians. “It’s really quite an emotional moment,” he said.
Getting the news to patients
As dermatologists introduce the topical treatment to patients, common questions center on why this cream is different and whether it is safe.
David Rosmarin, MD, vice chair of research and education, department of dermatology, Tufts Medical Center, Boston, led the Topical Ruxolitinib Evaluation in Vitiligo Study 1 and 2 (TruE-V1, TruE-V2), conducted in North America and Europe.
He summarized some key findings.
“If patients have involvement on the face, trunk, or extremities, the data show that about half the patients at 52 weeks will get half or more of their pigment back,” he said in an interview. Results for the face alone are even better. “Half the patients will get 75% or more pigment back in the face,” Dr. Rosmarin said.
In addition, analysis of subgroups shows benefit for all patients. “Patients seem to respond similarly well across all subgroups – across gender, sex, age, ethnicity, and race,” Dr. Rosmarin said.
However, anatomic region matters, he pointed out. Skin of the head and neck responds the best, followed by skin of the trunk and extremities. The hands and feet are the most difficult to repigment because there are few hair follicles, which help enable repigmentation.
He added that it’s important to understand patients’ goals, and dermatologists shouldn’t assume that all who have vitiligo will want to undergo repigmentation. They may be interested in the new treatment but may not want it for themselves, he explained.
Explaining risks
Patients may ask about the boxed warning on the label that lists risk of heart attack, stroke, cancer, infections, blood clots, and death. Dermatologists can explain that the warning pertains to the whole JAK class and was based on patients with rheumatoid arthritis, Dr. Rosmarin said.
He added, “We didn’t see a signal for heart attack and stroke for patients using the topical. But it’s still important to discuss the label as the FDA states it.”
There are two main side effects, Dr. Rosmarin said: acne (about 6% of treated patients get it, and it’s usually mild) and application-site reactions. “Luckily, the medication has a tendency not to sting or burn, which is not the case with some of our other treatments. It’s very well tolerated,” he said.
Patients should also know that repigmentation can take time, because initially, the immune system is directed to calm down with treatment, and then pigment must travel back to the affected sites.
Some patients may have a response in as early as 2-3 months, and others need more time, Dr. Rosmarin said.
Treatment responses among adolescents have been particularly good. Responses regarding the skin of the face have been similar to those of adults. “However, on the body, they respond even better,” Dr. Rosmarin said. “About 60% achieve 50% or more repigmentation on the whole body.”
It’s important that ruxolitinib has been approved for persons aged 12 years and older, he said, because “about half the patients will develop vitiligo by the age of 20.”
Approval and insurance coverage
FDA approval will help with reimbursement for the expensive treatment.
The label indicates that patients should not use more than one 60-g tube a week. Currently, the out-of-pocket cost for one tube can be close to $2,000, according to GoodRx.
Raj Chovatiya, MD, PhD, assistant professor of dermatology and director of the Center for Eczema and Itch at Northwestern University, Chicago, said that in recent years, vitiligo patients, aware that their condition could be treated by JAK inhibitors, have been paying out of pocket at compounding pharmacies, which take oral versions of the medication and compound them into topical formulations.
Unlike baricitinib, which is used to treat severe alopecia areata, and other oral JAK inhibitors, testing for TB and hepatitis is not required for initiating treatment with ruxolitinib, so no delay is necessary, Dr. Chovatiya said.
He noted, however, that patients with vitiligo may have given up on effective care after experiencing little or no improvement with topical corticosteroids, phototherapy, or topical calcineurin inhibitors.
“They end up losing steam, are less motivated on therapy, and are lost to care,” he said.
Dermatologists, he said, may need to proactively find these patients and tell them the good news. “Now that we have really good targeted therapeutic options, it’s really up to us to figure out how to bring these people back to the clinic and educate them,” Dr. Chovatiya said.
Unanswered questions to address
Some questions are still unanswered, lead study author Dr. Rosmarin said.
Two big questions are how long people will need to continue using ruxolitinib cream and whether depigmentation will recur if people stop using it.
Another aspect of therapy being studied is whether the cream will be even more effective in combination with other treatments.
“The main combination we think about is ruxolitinib with phototherapy – a light treatment – because light could stimulate those pigment cells,” Dr. Rosmarin said,
He noted that light therapy was included in phase 2 testing and that patients did respond. “What we need and what’s planned is a larger study looking at the combination to see whether it is synergistic or not. The longer patients use the cream, the more benefit we see,” Dr. Rosmarin said.
Dr. Desai has served as an investigator and/or consultant to several companies, including Incyte. Dr. Rosmarin has received honoraria as a consultant and has received research support from Incyte, and has served as a paid speaker for Incyte, as well as other companies.. Dr. Chovatiya has served as an advisory board member, consultant, and/or investigator for companies that include Incyte.
For the first time, patients with vitiligo who have long lived with patches of skin that are without pigment can now have even skin tones on their faces and other bodily regions with a Food and Drug Administration–approved, easy-to-use topical treatment.
In July, , the most common form of the disease.
Topical ruxolitinib was first approved in September 2021 for atopic dermatitis, and dermatologists are already writing prescriptions for its new vitiligo indication.
“The FDA approval of ruxolitinib for repigmentation of vitiligo is historic and groundbreaking,” Seemal R. Desai, MD, a dermatologist at the University of Texas Southwestern Medical Center, Dallas, told this news organization.
The news brings hope to patients 12 years and older who suffer from the psychosocial effects of the disease, which is estimated to affect 1.9 million to 2.8 million adults in the United States.
The announcement followed FDA approval a month earlier of another dermatologic milestone – an oral JAK inhibitor, baricitinib, which became the first treatment for patients with alopecia areata.
For Dr. Desai, the ruxolitinib news is personal. His brother, also a physician, has lived a lifetime with vitiligo. His family experience, Dr. Desai said, showed him “what a disease like this can do to a person psychologically.”
Dr. Desai said his early exposure helped lead to his own decision to dedicate his career to pigmentary diseases.
His brother won’t personally benefit from the cream because his skin has been completely depigmented and repigmentation is not of interest to him, Dr. Desai said. But both brothers are excited as physicians. “It’s really quite an emotional moment,” he said.
Getting the news to patients
As dermatologists introduce the topical treatment to patients, common questions center on why this cream is different and whether it is safe.
David Rosmarin, MD, vice chair of research and education, department of dermatology, Tufts Medical Center, Boston, led the Topical Ruxolitinib Evaluation in Vitiligo Study 1 and 2 (TruE-V1, TruE-V2), conducted in North America and Europe.
He summarized some key findings.
“If patients have involvement on the face, trunk, or extremities, the data show that about half the patients at 52 weeks will get half or more of their pigment back,” he said in an interview. Results for the face alone are even better. “Half the patients will get 75% or more pigment back in the face,” Dr. Rosmarin said.
In addition, analysis of subgroups shows benefit for all patients. “Patients seem to respond similarly well across all subgroups – across gender, sex, age, ethnicity, and race,” Dr. Rosmarin said.
However, anatomic region matters, he pointed out. Skin of the head and neck responds the best, followed by skin of the trunk and extremities. The hands and feet are the most difficult to repigment because there are few hair follicles, which help enable repigmentation.
He added that it’s important to understand patients’ goals, and dermatologists shouldn’t assume that all who have vitiligo will want to undergo repigmentation. They may be interested in the new treatment but may not want it for themselves, he explained.
Explaining risks
Patients may ask about the boxed warning on the label that lists risk of heart attack, stroke, cancer, infections, blood clots, and death. Dermatologists can explain that the warning pertains to the whole JAK class and was based on patients with rheumatoid arthritis, Dr. Rosmarin said.
He added, “We didn’t see a signal for heart attack and stroke for patients using the topical. But it’s still important to discuss the label as the FDA states it.”
There are two main side effects, Dr. Rosmarin said: acne (about 6% of treated patients get it, and it’s usually mild) and application-site reactions. “Luckily, the medication has a tendency not to sting or burn, which is not the case with some of our other treatments. It’s very well tolerated,” he said.
Patients should also know that repigmentation can take time, because initially, the immune system is directed to calm down with treatment, and then pigment must travel back to the affected sites.
Some patients may have a response in as early as 2-3 months, and others need more time, Dr. Rosmarin said.
Treatment responses among adolescents have been particularly good. Responses regarding the skin of the face have been similar to those of adults. “However, on the body, they respond even better,” Dr. Rosmarin said. “About 60% achieve 50% or more repigmentation on the whole body.”
It’s important that ruxolitinib has been approved for persons aged 12 years and older, he said, because “about half the patients will develop vitiligo by the age of 20.”
Approval and insurance coverage
FDA approval will help with reimbursement for the expensive treatment.
The label indicates that patients should not use more than one 60-g tube a week. Currently, the out-of-pocket cost for one tube can be close to $2,000, according to GoodRx.
Raj Chovatiya, MD, PhD, assistant professor of dermatology and director of the Center for Eczema and Itch at Northwestern University, Chicago, said that in recent years, vitiligo patients, aware that their condition could be treated by JAK inhibitors, have been paying out of pocket at compounding pharmacies, which take oral versions of the medication and compound them into topical formulations.
Unlike baricitinib, which is used to treat severe alopecia areata, and other oral JAK inhibitors, testing for TB and hepatitis is not required for initiating treatment with ruxolitinib, so no delay is necessary, Dr. Chovatiya said.
He noted, however, that patients with vitiligo may have given up on effective care after experiencing little or no improvement with topical corticosteroids, phototherapy, or topical calcineurin inhibitors.
“They end up losing steam, are less motivated on therapy, and are lost to care,” he said.
Dermatologists, he said, may need to proactively find these patients and tell them the good news. “Now that we have really good targeted therapeutic options, it’s really up to us to figure out how to bring these people back to the clinic and educate them,” Dr. Chovatiya said.
Unanswered questions to address
Some questions are still unanswered, lead study author Dr. Rosmarin said.
Two big questions are how long people will need to continue using ruxolitinib cream and whether depigmentation will recur if people stop using it.
Another aspect of therapy being studied is whether the cream will be even more effective in combination with other treatments.
“The main combination we think about is ruxolitinib with phototherapy – a light treatment – because light could stimulate those pigment cells,” Dr. Rosmarin said,
He noted that light therapy was included in phase 2 testing and that patients did respond. “What we need and what’s planned is a larger study looking at the combination to see whether it is synergistic or not. The longer patients use the cream, the more benefit we see,” Dr. Rosmarin said.
Dr. Desai has served as an investigator and/or consultant to several companies, including Incyte. Dr. Rosmarin has received honoraria as a consultant and has received research support from Incyte, and has served as a paid speaker for Incyte, as well as other companies.. Dr. Chovatiya has served as an advisory board member, consultant, and/or investigator for companies that include Incyte.
For the first time, patients with vitiligo who have long lived with patches of skin that are without pigment can now have even skin tones on their faces and other bodily regions with a Food and Drug Administration–approved, easy-to-use topical treatment.
In July, , the most common form of the disease.
Topical ruxolitinib was first approved in September 2021 for atopic dermatitis, and dermatologists are already writing prescriptions for its new vitiligo indication.
“The FDA approval of ruxolitinib for repigmentation of vitiligo is historic and groundbreaking,” Seemal R. Desai, MD, a dermatologist at the University of Texas Southwestern Medical Center, Dallas, told this news organization.
The news brings hope to patients 12 years and older who suffer from the psychosocial effects of the disease, which is estimated to affect 1.9 million to 2.8 million adults in the United States.
The announcement followed FDA approval a month earlier of another dermatologic milestone – an oral JAK inhibitor, baricitinib, which became the first treatment for patients with alopecia areata.
For Dr. Desai, the ruxolitinib news is personal. His brother, also a physician, has lived a lifetime with vitiligo. His family experience, Dr. Desai said, showed him “what a disease like this can do to a person psychologically.”
Dr. Desai said his early exposure helped lead to his own decision to dedicate his career to pigmentary diseases.
His brother won’t personally benefit from the cream because his skin has been completely depigmented and repigmentation is not of interest to him, Dr. Desai said. But both brothers are excited as physicians. “It’s really quite an emotional moment,” he said.
Getting the news to patients
As dermatologists introduce the topical treatment to patients, common questions center on why this cream is different and whether it is safe.
David Rosmarin, MD, vice chair of research and education, department of dermatology, Tufts Medical Center, Boston, led the Topical Ruxolitinib Evaluation in Vitiligo Study 1 and 2 (TruE-V1, TruE-V2), conducted in North America and Europe.
He summarized some key findings.
“If patients have involvement on the face, trunk, or extremities, the data show that about half the patients at 52 weeks will get half or more of their pigment back,” he said in an interview. Results for the face alone are even better. “Half the patients will get 75% or more pigment back in the face,” Dr. Rosmarin said.
In addition, analysis of subgroups shows benefit for all patients. “Patients seem to respond similarly well across all subgroups – across gender, sex, age, ethnicity, and race,” Dr. Rosmarin said.
However, anatomic region matters, he pointed out. Skin of the head and neck responds the best, followed by skin of the trunk and extremities. The hands and feet are the most difficult to repigment because there are few hair follicles, which help enable repigmentation.
He added that it’s important to understand patients’ goals, and dermatologists shouldn’t assume that all who have vitiligo will want to undergo repigmentation. They may be interested in the new treatment but may not want it for themselves, he explained.
Explaining risks
Patients may ask about the boxed warning on the label that lists risk of heart attack, stroke, cancer, infections, blood clots, and death. Dermatologists can explain that the warning pertains to the whole JAK class and was based on patients with rheumatoid arthritis, Dr. Rosmarin said.
He added, “We didn’t see a signal for heart attack and stroke for patients using the topical. But it’s still important to discuss the label as the FDA states it.”
There are two main side effects, Dr. Rosmarin said: acne (about 6% of treated patients get it, and it’s usually mild) and application-site reactions. “Luckily, the medication has a tendency not to sting or burn, which is not the case with some of our other treatments. It’s very well tolerated,” he said.
Patients should also know that repigmentation can take time, because initially, the immune system is directed to calm down with treatment, and then pigment must travel back to the affected sites.
Some patients may have a response in as early as 2-3 months, and others need more time, Dr. Rosmarin said.
Treatment responses among adolescents have been particularly good. Responses regarding the skin of the face have been similar to those of adults. “However, on the body, they respond even better,” Dr. Rosmarin said. “About 60% achieve 50% or more repigmentation on the whole body.”
It’s important that ruxolitinib has been approved for persons aged 12 years and older, he said, because “about half the patients will develop vitiligo by the age of 20.”
Approval and insurance coverage
FDA approval will help with reimbursement for the expensive treatment.
The label indicates that patients should not use more than one 60-g tube a week. Currently, the out-of-pocket cost for one tube can be close to $2,000, according to GoodRx.
Raj Chovatiya, MD, PhD, assistant professor of dermatology and director of the Center for Eczema and Itch at Northwestern University, Chicago, said that in recent years, vitiligo patients, aware that their condition could be treated by JAK inhibitors, have been paying out of pocket at compounding pharmacies, which take oral versions of the medication and compound them into topical formulations.
Unlike baricitinib, which is used to treat severe alopecia areata, and other oral JAK inhibitors, testing for TB and hepatitis is not required for initiating treatment with ruxolitinib, so no delay is necessary, Dr. Chovatiya said.
He noted, however, that patients with vitiligo may have given up on effective care after experiencing little or no improvement with topical corticosteroids, phototherapy, or topical calcineurin inhibitors.
“They end up losing steam, are less motivated on therapy, and are lost to care,” he said.
Dermatologists, he said, may need to proactively find these patients and tell them the good news. “Now that we have really good targeted therapeutic options, it’s really up to us to figure out how to bring these people back to the clinic and educate them,” Dr. Chovatiya said.
Unanswered questions to address
Some questions are still unanswered, lead study author Dr. Rosmarin said.
Two big questions are how long people will need to continue using ruxolitinib cream and whether depigmentation will recur if people stop using it.
Another aspect of therapy being studied is whether the cream will be even more effective in combination with other treatments.
“The main combination we think about is ruxolitinib with phototherapy – a light treatment – because light could stimulate those pigment cells,” Dr. Rosmarin said,
He noted that light therapy was included in phase 2 testing and that patients did respond. “What we need and what’s planned is a larger study looking at the combination to see whether it is synergistic or not. The longer patients use the cream, the more benefit we see,” Dr. Rosmarin said.
Dr. Desai has served as an investigator and/or consultant to several companies, including Incyte. Dr. Rosmarin has received honoraria as a consultant and has received research support from Incyte, and has served as a paid speaker for Incyte, as well as other companies.. Dr. Chovatiya has served as an advisory board member, consultant, and/or investigator for companies that include Incyte.