User login
Study suggests psoriasis and PsA are underdiagnosed in underserved groups
, a study based on national registry data suggests.
“Using the All of Us dataset, we identified lower rates of psoriasis and psoriatic arthritis in participants with skin of color, lower education levels, and no health insurance,” lead author Megan M. Tran said in her oral presentation at the annual meeting of the Society for Investigative Dermatology.
“This suggests psoriasis and psoriatic arthritis underdiagnosis in these underserved populations, possibly due to limited dermatologic care access,” added Ms. Tran, a second-year medical student at Brown University in Providence, R.I.
Ms. Tran and colleagues used the ongoing National Institutes of Health All of Us Research Program registry that contains a large proportion of participants from groups in the United States who have historically been underrepresented in biomedical research, she said in her talk.
Of the 329,038 participants with data in version 5 (released this past March) of the All of Us database, 150,158 (45.6%) had skin of color, and 251,597 (76.5%) had available electronic health records (EHRs).
Underserved groups need better access to health care
Linking data from EHRs, surveys, and physical measurements at enrollment, the researchers used several variables to estimate psoriasis and psoriatic arthritis (PsA) prevalence, and they used multivariate logistic regression to adjust for the variables. They found:
- Twenty-two percent of patients with psoriasis had PsA. Odds of psoriasis and PsA were lower among Black (psoriasis odds ratio [OR], 0.32, 95% confidence interval [CI], 0.28-0.36; PsA OR, 0.20, 95% CI, 0.15-0.26) and Hispanic participants (psoriasis OR, 0.77, 95% CI, 0.71-0.84; PsA OR, 0.74, 95% CI, 0.61-0.89) compared with White participants.
- Psoriasis prevalence increased linearly with age (topping off at age 70 and older [OR, 3.35, 95% CI, 2.91-3.88], with 18-29 years as the reference). The same trend was found with PsA (70 years and above [OR, 4.41, 95% CI, 3.07-6.55] compared with those aged 18-29 years).
- Psoriasis prevalence increased linearly with body mass index (BMI 40 and above [OR, 1.71, 95% CI, 1.54-1.90], with 20-24.9 as the reference). The same trend was found with PsA (BMI 40 and above [OR, 2.09, 95% CI, 1.68-2.59], with 20-24.9 as the reference).
- Former smokers were at increased risk for disease, compared with people who had never smoked (psoriasis OR, 1.30, 95% CI, 1.22-1.39; PsA OR, 2.15, 95% CI, 1.33-3.78).
- Lower odds were found in uninsured adults (psoriasis OR, 0.43, 95% CI, 0.35-0.52; PsA OR, 0.37, 95% CI, 0.22-0.58) compared with those who were insured, and in those with less than a high school degree (psoriasis OR, 0.72, 95% CI, 0.63-0.82; PsA OR, 0.65, 95% CI, 0.47-0.87) compared with those with a college degree.
“The All of Us research program has demonstrated to be a valuable resource to gain unique dermatologic insights on diverse participant populations,” Ms. Tran said.
“There needs to be improvement in access to quality dermatologic care, as this may help to reduce underdiagnosis of psoriasis and psoriatic arthritis,” she added. Access can be increased in various ways, including “outreach to underserved communities, equitable distribution of resources, and increased awareness of clinical variations in skin of color.”
Laura Korb Ferris, MD, PhD, professor of dermatology and director of clinical trials for the department of dermatology at University of Pittsburgh Medical Center, said the study is interesting.
“Because All of Us uses electronic health records to identify cases, while these findings could suggest that these patients are less likely to develop psoriasis and psoriatic arthritis, it more likely shows that they are less likely to receive care for these conditions,” she told this news organization.
“This is concerning, as psoriasis is associated with other comorbidities such as cardiovascular disease and depression, and psoriatic arthritis if left untreated can cause irreversible joint damage that limits function,” she explained in an email. “Both conditions profoundly impact a patient’s quality of life.
“It is important to know whether the diagnoses are simply being missed in these patients or are being neglected,” noted Dr. Ferris, who was not involved in the study and was asked to comment on the results. “It is also important to find strategies to improve diagnosis and treatment, improve quality of life, and allow for interventions to improve long-term sequelae of these diseases and their comorbid conditions.”
The NIH All of Us Research Program, which aims to build a diverse database from at least 1 million adult participants in the United States as a part of the agency’s precision medicine initiative, is open to researchers and to the public. Researchers can access All of Us data and tools to conduct studies at the All of Us Research Hub, and adults who live in the United States can contribute their health data at the All of Us Research Program website and at participating health care provider organizations.
Ms. Tran, study coauthors, and Dr. Ferris reported no relevant relationships. The All of Us Research Program is supported by the National Institutes of Health.
A version of this article first appeared on Medscape.com.
, a study based on national registry data suggests.
“Using the All of Us dataset, we identified lower rates of psoriasis and psoriatic arthritis in participants with skin of color, lower education levels, and no health insurance,” lead author Megan M. Tran said in her oral presentation at the annual meeting of the Society for Investigative Dermatology.
“This suggests psoriasis and psoriatic arthritis underdiagnosis in these underserved populations, possibly due to limited dermatologic care access,” added Ms. Tran, a second-year medical student at Brown University in Providence, R.I.
Ms. Tran and colleagues used the ongoing National Institutes of Health All of Us Research Program registry that contains a large proportion of participants from groups in the United States who have historically been underrepresented in biomedical research, she said in her talk.
Of the 329,038 participants with data in version 5 (released this past March) of the All of Us database, 150,158 (45.6%) had skin of color, and 251,597 (76.5%) had available electronic health records (EHRs).
Underserved groups need better access to health care
Linking data from EHRs, surveys, and physical measurements at enrollment, the researchers used several variables to estimate psoriasis and psoriatic arthritis (PsA) prevalence, and they used multivariate logistic regression to adjust for the variables. They found:
- Twenty-two percent of patients with psoriasis had PsA. Odds of psoriasis and PsA were lower among Black (psoriasis odds ratio [OR], 0.32, 95% confidence interval [CI], 0.28-0.36; PsA OR, 0.20, 95% CI, 0.15-0.26) and Hispanic participants (psoriasis OR, 0.77, 95% CI, 0.71-0.84; PsA OR, 0.74, 95% CI, 0.61-0.89) compared with White participants.
- Psoriasis prevalence increased linearly with age (topping off at age 70 and older [OR, 3.35, 95% CI, 2.91-3.88], with 18-29 years as the reference). The same trend was found with PsA (70 years and above [OR, 4.41, 95% CI, 3.07-6.55] compared with those aged 18-29 years).
- Psoriasis prevalence increased linearly with body mass index (BMI 40 and above [OR, 1.71, 95% CI, 1.54-1.90], with 20-24.9 as the reference). The same trend was found with PsA (BMI 40 and above [OR, 2.09, 95% CI, 1.68-2.59], with 20-24.9 as the reference).
- Former smokers were at increased risk for disease, compared with people who had never smoked (psoriasis OR, 1.30, 95% CI, 1.22-1.39; PsA OR, 2.15, 95% CI, 1.33-3.78).
- Lower odds were found in uninsured adults (psoriasis OR, 0.43, 95% CI, 0.35-0.52; PsA OR, 0.37, 95% CI, 0.22-0.58) compared with those who were insured, and in those with less than a high school degree (psoriasis OR, 0.72, 95% CI, 0.63-0.82; PsA OR, 0.65, 95% CI, 0.47-0.87) compared with those with a college degree.
“The All of Us research program has demonstrated to be a valuable resource to gain unique dermatologic insights on diverse participant populations,” Ms. Tran said.
“There needs to be improvement in access to quality dermatologic care, as this may help to reduce underdiagnosis of psoriasis and psoriatic arthritis,” she added. Access can be increased in various ways, including “outreach to underserved communities, equitable distribution of resources, and increased awareness of clinical variations in skin of color.”
Laura Korb Ferris, MD, PhD, professor of dermatology and director of clinical trials for the department of dermatology at University of Pittsburgh Medical Center, said the study is interesting.
“Because All of Us uses electronic health records to identify cases, while these findings could suggest that these patients are less likely to develop psoriasis and psoriatic arthritis, it more likely shows that they are less likely to receive care for these conditions,” she told this news organization.
“This is concerning, as psoriasis is associated with other comorbidities such as cardiovascular disease and depression, and psoriatic arthritis if left untreated can cause irreversible joint damage that limits function,” she explained in an email. “Both conditions profoundly impact a patient’s quality of life.
“It is important to know whether the diagnoses are simply being missed in these patients or are being neglected,” noted Dr. Ferris, who was not involved in the study and was asked to comment on the results. “It is also important to find strategies to improve diagnosis and treatment, improve quality of life, and allow for interventions to improve long-term sequelae of these diseases and their comorbid conditions.”
The NIH All of Us Research Program, which aims to build a diverse database from at least 1 million adult participants in the United States as a part of the agency’s precision medicine initiative, is open to researchers and to the public. Researchers can access All of Us data and tools to conduct studies at the All of Us Research Hub, and adults who live in the United States can contribute their health data at the All of Us Research Program website and at participating health care provider organizations.
Ms. Tran, study coauthors, and Dr. Ferris reported no relevant relationships. The All of Us Research Program is supported by the National Institutes of Health.
A version of this article first appeared on Medscape.com.
, a study based on national registry data suggests.
“Using the All of Us dataset, we identified lower rates of psoriasis and psoriatic arthritis in participants with skin of color, lower education levels, and no health insurance,” lead author Megan M. Tran said in her oral presentation at the annual meeting of the Society for Investigative Dermatology.
“This suggests psoriasis and psoriatic arthritis underdiagnosis in these underserved populations, possibly due to limited dermatologic care access,” added Ms. Tran, a second-year medical student at Brown University in Providence, R.I.
Ms. Tran and colleagues used the ongoing National Institutes of Health All of Us Research Program registry that contains a large proportion of participants from groups in the United States who have historically been underrepresented in biomedical research, she said in her talk.
Of the 329,038 participants with data in version 5 (released this past March) of the All of Us database, 150,158 (45.6%) had skin of color, and 251,597 (76.5%) had available electronic health records (EHRs).
Underserved groups need better access to health care
Linking data from EHRs, surveys, and physical measurements at enrollment, the researchers used several variables to estimate psoriasis and psoriatic arthritis (PsA) prevalence, and they used multivariate logistic regression to adjust for the variables. They found:
- Twenty-two percent of patients with psoriasis had PsA. Odds of psoriasis and PsA were lower among Black (psoriasis odds ratio [OR], 0.32, 95% confidence interval [CI], 0.28-0.36; PsA OR, 0.20, 95% CI, 0.15-0.26) and Hispanic participants (psoriasis OR, 0.77, 95% CI, 0.71-0.84; PsA OR, 0.74, 95% CI, 0.61-0.89) compared with White participants.
- Psoriasis prevalence increased linearly with age (topping off at age 70 and older [OR, 3.35, 95% CI, 2.91-3.88], with 18-29 years as the reference). The same trend was found with PsA (70 years and above [OR, 4.41, 95% CI, 3.07-6.55] compared with those aged 18-29 years).
- Psoriasis prevalence increased linearly with body mass index (BMI 40 and above [OR, 1.71, 95% CI, 1.54-1.90], with 20-24.9 as the reference). The same trend was found with PsA (BMI 40 and above [OR, 2.09, 95% CI, 1.68-2.59], with 20-24.9 as the reference).
- Former smokers were at increased risk for disease, compared with people who had never smoked (psoriasis OR, 1.30, 95% CI, 1.22-1.39; PsA OR, 2.15, 95% CI, 1.33-3.78).
- Lower odds were found in uninsured adults (psoriasis OR, 0.43, 95% CI, 0.35-0.52; PsA OR, 0.37, 95% CI, 0.22-0.58) compared with those who were insured, and in those with less than a high school degree (psoriasis OR, 0.72, 95% CI, 0.63-0.82; PsA OR, 0.65, 95% CI, 0.47-0.87) compared with those with a college degree.
“The All of Us research program has demonstrated to be a valuable resource to gain unique dermatologic insights on diverse participant populations,” Ms. Tran said.
“There needs to be improvement in access to quality dermatologic care, as this may help to reduce underdiagnosis of psoriasis and psoriatic arthritis,” she added. Access can be increased in various ways, including “outreach to underserved communities, equitable distribution of resources, and increased awareness of clinical variations in skin of color.”
Laura Korb Ferris, MD, PhD, professor of dermatology and director of clinical trials for the department of dermatology at University of Pittsburgh Medical Center, said the study is interesting.
“Because All of Us uses electronic health records to identify cases, while these findings could suggest that these patients are less likely to develop psoriasis and psoriatic arthritis, it more likely shows that they are less likely to receive care for these conditions,” she told this news organization.
“This is concerning, as psoriasis is associated with other comorbidities such as cardiovascular disease and depression, and psoriatic arthritis if left untreated can cause irreversible joint damage that limits function,” she explained in an email. “Both conditions profoundly impact a patient’s quality of life.
“It is important to know whether the diagnoses are simply being missed in these patients or are being neglected,” noted Dr. Ferris, who was not involved in the study and was asked to comment on the results. “It is also important to find strategies to improve diagnosis and treatment, improve quality of life, and allow for interventions to improve long-term sequelae of these diseases and their comorbid conditions.”
The NIH All of Us Research Program, which aims to build a diverse database from at least 1 million adult participants in the United States as a part of the agency’s precision medicine initiative, is open to researchers and to the public. Researchers can access All of Us data and tools to conduct studies at the All of Us Research Hub, and adults who live in the United States can contribute their health data at the All of Us Research Program website and at participating health care provider organizations.
Ms. Tran, study coauthors, and Dr. Ferris reported no relevant relationships. The All of Us Research Program is supported by the National Institutes of Health.
A version of this article first appeared on Medscape.com.
FROM SID 2022
Low-level light therapy cap shows subtle effects on CCCA
though the treatment effects from a small prospective trial appear to be subtle.
Central centrifugal cicatricial alopecia (CCCA) is a form of scarring hair loss with unknown etiology and no known cure that affects mainly women of African descent.
“The low-level light therapy (LLLT) cap does indeed seem to help with symptoms and mild regrowth in CCCA,” senior study author Amy J. McMichael, MD, told this news organization. “The dual-wavelength cap we used appears to have anti-inflammatory properties, and that makes sense for a primarily inflammatory scarring from of alopecia.
“Quality of life improved with the treatment and there were no reported side effects,” added Dr. McMichael, professor of dermatology at Wake Forest University, Winston-Salem, N.C.
The results of the study were presented in a poster at the annual meeting of the Society for Investigative Dermatology.
The REVIAN RED cap (REVIAN Inc.) used in the study contains 119 light-emitting diodes (LEDs) arrayed on the cap’s interior surface that emit orange (620 nm) and red (660 nm) light.
The hypothesis for how the dual-wavelength lights work is that light is absorbed by the chromophore cytochrome c oxidase in the mitochondrial membrane. This induces the release of nitric oxide and the production of adenosine triphosphate (ATP), which leads to vasodilation, cytokine regulation, and increased transcription and release of growth factors.
LLLT is approved to treat androgenetic alopecia, the authors wrote, but has not been studied as a treatment for CCCA.
To assess the effects of LLLT on CCCA, Dr. McMichael and her colleagues at Wake Forest followed the condition’s progress in five Black women over their 6-month course of treatment. Four participants completed the study.
At baseline, all participants had been on individual stable CCCA treatment regimens for at least 3 months. They continued those treatments along with LLLT therapy throughout the study. The women ranged in age from 38 to 69 years, had had CCCA for an average of 12 years, and their disease severity ranged from stage IIB to IVA.
They were instructed to wear the REVIAN RED cap with the LEDs activated for 10 minutes each day.
At 2, 4, and 6 months, participants self-assessed their symptoms, a clinician evaluated the condition’s severity, and digital photographs were taken.
At 6 months:
- Three patients showed improved Dermatology Life Quality Index (DLQI).
- Three patients showed decreased loss of follicular openings and breakage.
- A dermoscopic image of the scalp of one patient revealed short, regrowing vellus hairs and minimal interfollicular and perifollicular scale.
- No patients reported side effects.
Small study raises big questions
“I hope this study will lead to a larger study that will look at the long-term outcomes of CCCA,” Dr. McMichael said. “This is a nice treatment that does not require application of something to the scalp that may affect hair styling, and it has no systemic side effects.”
Dr. McMichael acknowledges that the small sample size, participants continuing with their individual stable treatments while also undergoing light therapy, and the lack of patients with stage I disease, are weaknesses in the study.
“However, the strength is that none of the patients had side effects or stopped using the treatment due to difficulty with the system,” she added.
Dr. McMichael said she would like to investigate the effects of longer use of the cap and whether the cap can be used to prevent CCCA.
Chesahna Kindred, MD, assistant professor of dermatology at Howard University, Washington, D.C., and founder of Kindred Hair & Skin Center in Columbia, Md., told this news organization that she uses LLLT in her practice.
“I find that LLLT is mildly helpful, or at least does not worsen, androgenetic alopecia,” she said.
“Interestingly, while all four patients had stable disease upon initiating the study, it appears as though two of the four worsened after the use of LLLT, one improved, and one remained relatively stable,” noted Dr. Kindred, who was not involved in the study. “This is important because once there is complete destruction of the follicle, CCCA is difficult to improve.
“Given that there are several options to address inflammation and follicular damage in CCCA, more studies are needed before I would incorporate LLLT into my regular treatment algorithms,” she added.
“Studies like this are important and remind us to not lump all forms of hair loss together,” she said.
REVIAN Inc. provided the caps, but the study received no additional funding. Dr. McMichael and Dr. Kindred report relevant financial relationships with the pharmaceutical industry. Study coauthors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
though the treatment effects from a small prospective trial appear to be subtle.
Central centrifugal cicatricial alopecia (CCCA) is a form of scarring hair loss with unknown etiology and no known cure that affects mainly women of African descent.
“The low-level light therapy (LLLT) cap does indeed seem to help with symptoms and mild regrowth in CCCA,” senior study author Amy J. McMichael, MD, told this news organization. “The dual-wavelength cap we used appears to have anti-inflammatory properties, and that makes sense for a primarily inflammatory scarring from of alopecia.
“Quality of life improved with the treatment and there were no reported side effects,” added Dr. McMichael, professor of dermatology at Wake Forest University, Winston-Salem, N.C.
The results of the study were presented in a poster at the annual meeting of the Society for Investigative Dermatology.
The REVIAN RED cap (REVIAN Inc.) used in the study contains 119 light-emitting diodes (LEDs) arrayed on the cap’s interior surface that emit orange (620 nm) and red (660 nm) light.
The hypothesis for how the dual-wavelength lights work is that light is absorbed by the chromophore cytochrome c oxidase in the mitochondrial membrane. This induces the release of nitric oxide and the production of adenosine triphosphate (ATP), which leads to vasodilation, cytokine regulation, and increased transcription and release of growth factors.
LLLT is approved to treat androgenetic alopecia, the authors wrote, but has not been studied as a treatment for CCCA.
To assess the effects of LLLT on CCCA, Dr. McMichael and her colleagues at Wake Forest followed the condition’s progress in five Black women over their 6-month course of treatment. Four participants completed the study.
At baseline, all participants had been on individual stable CCCA treatment regimens for at least 3 months. They continued those treatments along with LLLT therapy throughout the study. The women ranged in age from 38 to 69 years, had had CCCA for an average of 12 years, and their disease severity ranged from stage IIB to IVA.
They were instructed to wear the REVIAN RED cap with the LEDs activated for 10 minutes each day.
At 2, 4, and 6 months, participants self-assessed their symptoms, a clinician evaluated the condition’s severity, and digital photographs were taken.
At 6 months:
- Three patients showed improved Dermatology Life Quality Index (DLQI).
- Three patients showed decreased loss of follicular openings and breakage.
- A dermoscopic image of the scalp of one patient revealed short, regrowing vellus hairs and minimal interfollicular and perifollicular scale.
- No patients reported side effects.
Small study raises big questions
“I hope this study will lead to a larger study that will look at the long-term outcomes of CCCA,” Dr. McMichael said. “This is a nice treatment that does not require application of something to the scalp that may affect hair styling, and it has no systemic side effects.”
Dr. McMichael acknowledges that the small sample size, participants continuing with their individual stable treatments while also undergoing light therapy, and the lack of patients with stage I disease, are weaknesses in the study.
“However, the strength is that none of the patients had side effects or stopped using the treatment due to difficulty with the system,” she added.
Dr. McMichael said she would like to investigate the effects of longer use of the cap and whether the cap can be used to prevent CCCA.
Chesahna Kindred, MD, assistant professor of dermatology at Howard University, Washington, D.C., and founder of Kindred Hair & Skin Center in Columbia, Md., told this news organization that she uses LLLT in her practice.
“I find that LLLT is mildly helpful, or at least does not worsen, androgenetic alopecia,” she said.
“Interestingly, while all four patients had stable disease upon initiating the study, it appears as though two of the four worsened after the use of LLLT, one improved, and one remained relatively stable,” noted Dr. Kindred, who was not involved in the study. “This is important because once there is complete destruction of the follicle, CCCA is difficult to improve.
“Given that there are several options to address inflammation and follicular damage in CCCA, more studies are needed before I would incorporate LLLT into my regular treatment algorithms,” she added.
“Studies like this are important and remind us to not lump all forms of hair loss together,” she said.
REVIAN Inc. provided the caps, but the study received no additional funding. Dr. McMichael and Dr. Kindred report relevant financial relationships with the pharmaceutical industry. Study coauthors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
though the treatment effects from a small prospective trial appear to be subtle.
Central centrifugal cicatricial alopecia (CCCA) is a form of scarring hair loss with unknown etiology and no known cure that affects mainly women of African descent.
“The low-level light therapy (LLLT) cap does indeed seem to help with symptoms and mild regrowth in CCCA,” senior study author Amy J. McMichael, MD, told this news organization. “The dual-wavelength cap we used appears to have anti-inflammatory properties, and that makes sense for a primarily inflammatory scarring from of alopecia.
“Quality of life improved with the treatment and there were no reported side effects,” added Dr. McMichael, professor of dermatology at Wake Forest University, Winston-Salem, N.C.
The results of the study were presented in a poster at the annual meeting of the Society for Investigative Dermatology.
The REVIAN RED cap (REVIAN Inc.) used in the study contains 119 light-emitting diodes (LEDs) arrayed on the cap’s interior surface that emit orange (620 nm) and red (660 nm) light.
The hypothesis for how the dual-wavelength lights work is that light is absorbed by the chromophore cytochrome c oxidase in the mitochondrial membrane. This induces the release of nitric oxide and the production of adenosine triphosphate (ATP), which leads to vasodilation, cytokine regulation, and increased transcription and release of growth factors.
LLLT is approved to treat androgenetic alopecia, the authors wrote, but has not been studied as a treatment for CCCA.
To assess the effects of LLLT on CCCA, Dr. McMichael and her colleagues at Wake Forest followed the condition’s progress in five Black women over their 6-month course of treatment. Four participants completed the study.
At baseline, all participants had been on individual stable CCCA treatment regimens for at least 3 months. They continued those treatments along with LLLT therapy throughout the study. The women ranged in age from 38 to 69 years, had had CCCA for an average of 12 years, and their disease severity ranged from stage IIB to IVA.
They were instructed to wear the REVIAN RED cap with the LEDs activated for 10 minutes each day.
At 2, 4, and 6 months, participants self-assessed their symptoms, a clinician evaluated the condition’s severity, and digital photographs were taken.
At 6 months:
- Three patients showed improved Dermatology Life Quality Index (DLQI).
- Three patients showed decreased loss of follicular openings and breakage.
- A dermoscopic image of the scalp of one patient revealed short, regrowing vellus hairs and minimal interfollicular and perifollicular scale.
- No patients reported side effects.
Small study raises big questions
“I hope this study will lead to a larger study that will look at the long-term outcomes of CCCA,” Dr. McMichael said. “This is a nice treatment that does not require application of something to the scalp that may affect hair styling, and it has no systemic side effects.”
Dr. McMichael acknowledges that the small sample size, participants continuing with their individual stable treatments while also undergoing light therapy, and the lack of patients with stage I disease, are weaknesses in the study.
“However, the strength is that none of the patients had side effects or stopped using the treatment due to difficulty with the system,” she added.
Dr. McMichael said she would like to investigate the effects of longer use of the cap and whether the cap can be used to prevent CCCA.
Chesahna Kindred, MD, assistant professor of dermatology at Howard University, Washington, D.C., and founder of Kindred Hair & Skin Center in Columbia, Md., told this news organization that she uses LLLT in her practice.
“I find that LLLT is mildly helpful, or at least does not worsen, androgenetic alopecia,” she said.
“Interestingly, while all four patients had stable disease upon initiating the study, it appears as though two of the four worsened after the use of LLLT, one improved, and one remained relatively stable,” noted Dr. Kindred, who was not involved in the study. “This is important because once there is complete destruction of the follicle, CCCA is difficult to improve.
“Given that there are several options to address inflammation and follicular damage in CCCA, more studies are needed before I would incorporate LLLT into my regular treatment algorithms,” she added.
“Studies like this are important and remind us to not lump all forms of hair loss together,” she said.
REVIAN Inc. provided the caps, but the study received no additional funding. Dr. McMichael and Dr. Kindred report relevant financial relationships with the pharmaceutical industry. Study coauthors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM SID 2022
Vitamin D supplements during pregnancy may protect infants from atopic eczema
according to results of a clinical trial.
“Our data provide the first randomized controlled trial evidence of a protective effect of antenatal cholecalciferol supplementation on risk of infantile atopic eczema, with the effect only seen in infants that were breastfed for more than 1 month,” lead study author Sarah El-Heis, MRCP, DM, and colleagues wrote.
“The findings support a developmental influence on infantile atopic eczema and point to gestational cholecalciferol supplementation as a preventive strategy to reduce the burden of atopic eczema during infancy,” Dr. El-Heis, an academic clinical lecturer in dermatology at the Medical Research Council Lifecourse Epidemiology Center of the University of Southampton (England), said in a presentation at the annual meeting of the Society for Investigative Dermatology.
The study also was published in the British Journal of Dermatology.
Dr. El-Heis and colleagues analyzed data from one of the three U.K. study sites involved in the double-blind Maternal Vitamin D Osteoporosis Study (MAVIDOS), which enrolled participants between 2008 and 2014.
The women enrolled at the University of Southampton site were of age 18 or older, and had a singleton pregnancy. Serum 25-hydroxy vitamin D (25[OH]D) levels were 25-100 nmol/L, and calcium levels were less than 2.75 mmol/L.
Those who had metabolic bone disease, kidney stones, hyperparathyroidism, or hypercalciuria or who were taking more than 400 IU/day of vitamin D supplements or medication known to interfere with fetal growth or whose fetus had a major anomaly were excluded.
The study included 1,134 women. Half of the participants were randomly assigned to receive cholecalciferol 1,000 IU/day from around 14 weeks’ gestation until delivery, and half were assigned to receive placebo. Their babies were assessed for atopic eczema at 12, 24, and 48 months of age.
The maternal and infant characteristics were similar in both groups, but the treatment group tended to breastfeed longer.
Infants appear to be protected up to 1 year of age
Using logistic regression, the researchers analyzed links between maternal cholecalciferol 1,000 IU/day supplements or placebo and atopic eczema risk in their offspring.
After adjustments for breastfeeding duration, among the 636 infants assessed at 12 months, those whose mothers received cholecalciferol had lower odds ratios of atopic eczema than those whose mothers received placebo (OR, 0.55; 95% confidence interval, 0.32-0.97).
The risk of atopic eczema at 12 months was reduced only for children in the treatment group who were breastfed longer than 1 month (OR, 0.48; 95% CI, 0.24-0.94), further analysis showed. Those who were breastfed for less than 1 month showed no reduced risk.
The combined effect of vitamin D and breastfeeding for longer than 1 month weakened after 1 year and was not statistically significant among the 611 children assessed at 24 months and the 450 children assessed at 48 months. The ORs of atopic eczema in the treatment group and in the control group increased to 0.76 (95% CI, 0.47-1.23) and 0.75 (95% CI, 0.37-1.52), respectively.
At baseline, the mean maternal serum 25(OH)D levels in the treatment group (46.0 nmol/L) and in the control group (44.7 nmol/L) were similar. But by late pregnancy, maternal serum 25(OH)D levels in the treatment group were higher (67.4 nmol/L) than in the control group (42.4 nmol/L).
The authors note that strengths of the study include its design, the uniformity of criteria used to diagnose atopic eczema, and the similarity of both pregnant groups in their intake of vitamin D during the study.
Limitations included the lack of ultraviolet B light exposure data, the lack of non-White women in the study, the lack of measurement of cord blood and offspring 25(OH)D levels, and the exclusion of women with baseline 25(OH)D concentrations less than 25 nmol/L.
“This is an interesting study that brings up the possibility that maternal factors during pregnancy may impact atopic dermatitis,” Kalyani S. Marathe, MD, MPH, the director of the division of dermatology at Cincinnati Children’s Hospital Medical Center, told this news organization.
The results are mixed, though, she noted.
“While some impact on the risk of eczema is seen at 1 year of age, that protective effect is gone by 2 years and 4 years,” Dr. Marathe, who was not involved in the study, said in an email. “So if maternal supplementation does improve eczema, the effect is not long-lasting.
“The other complicating factor is that the babies who showed reduction in eczema were also the ones who were breastfed longer than 1 month,” she added. “We know that breastfeeding is associated with several factors, including socioeconomic status, so it is difficult to tease out the relationships here.
“Vitamin D has become a very hot topic lately and seems to have protective effects in many areas of health care,” Dr. Marathe said. “These results may motivate pregnant women to be compliant with their prenatal vitamins that contain the amount of vitamin D studied here.”
The study received grant support. Several authors disclosed financial relationships with pharmaceutical and nutritional products industries. Dr. El-Heis and Dr. Marathe reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to results of a clinical trial.
“Our data provide the first randomized controlled trial evidence of a protective effect of antenatal cholecalciferol supplementation on risk of infantile atopic eczema, with the effect only seen in infants that were breastfed for more than 1 month,” lead study author Sarah El-Heis, MRCP, DM, and colleagues wrote.
“The findings support a developmental influence on infantile atopic eczema and point to gestational cholecalciferol supplementation as a preventive strategy to reduce the burden of atopic eczema during infancy,” Dr. El-Heis, an academic clinical lecturer in dermatology at the Medical Research Council Lifecourse Epidemiology Center of the University of Southampton (England), said in a presentation at the annual meeting of the Society for Investigative Dermatology.
The study also was published in the British Journal of Dermatology.
Dr. El-Heis and colleagues analyzed data from one of the three U.K. study sites involved in the double-blind Maternal Vitamin D Osteoporosis Study (MAVIDOS), which enrolled participants between 2008 and 2014.
The women enrolled at the University of Southampton site were of age 18 or older, and had a singleton pregnancy. Serum 25-hydroxy vitamin D (25[OH]D) levels were 25-100 nmol/L, and calcium levels were less than 2.75 mmol/L.
Those who had metabolic bone disease, kidney stones, hyperparathyroidism, or hypercalciuria or who were taking more than 400 IU/day of vitamin D supplements or medication known to interfere with fetal growth or whose fetus had a major anomaly were excluded.
The study included 1,134 women. Half of the participants were randomly assigned to receive cholecalciferol 1,000 IU/day from around 14 weeks’ gestation until delivery, and half were assigned to receive placebo. Their babies were assessed for atopic eczema at 12, 24, and 48 months of age.
The maternal and infant characteristics were similar in both groups, but the treatment group tended to breastfeed longer.
Infants appear to be protected up to 1 year of age
Using logistic regression, the researchers analyzed links between maternal cholecalciferol 1,000 IU/day supplements or placebo and atopic eczema risk in their offspring.
After adjustments for breastfeeding duration, among the 636 infants assessed at 12 months, those whose mothers received cholecalciferol had lower odds ratios of atopic eczema than those whose mothers received placebo (OR, 0.55; 95% confidence interval, 0.32-0.97).
The risk of atopic eczema at 12 months was reduced only for children in the treatment group who were breastfed longer than 1 month (OR, 0.48; 95% CI, 0.24-0.94), further analysis showed. Those who were breastfed for less than 1 month showed no reduced risk.
The combined effect of vitamin D and breastfeeding for longer than 1 month weakened after 1 year and was not statistically significant among the 611 children assessed at 24 months and the 450 children assessed at 48 months. The ORs of atopic eczema in the treatment group and in the control group increased to 0.76 (95% CI, 0.47-1.23) and 0.75 (95% CI, 0.37-1.52), respectively.
At baseline, the mean maternal serum 25(OH)D levels in the treatment group (46.0 nmol/L) and in the control group (44.7 nmol/L) were similar. But by late pregnancy, maternal serum 25(OH)D levels in the treatment group were higher (67.4 nmol/L) than in the control group (42.4 nmol/L).
The authors note that strengths of the study include its design, the uniformity of criteria used to diagnose atopic eczema, and the similarity of both pregnant groups in their intake of vitamin D during the study.
Limitations included the lack of ultraviolet B light exposure data, the lack of non-White women in the study, the lack of measurement of cord blood and offspring 25(OH)D levels, and the exclusion of women with baseline 25(OH)D concentrations less than 25 nmol/L.
“This is an interesting study that brings up the possibility that maternal factors during pregnancy may impact atopic dermatitis,” Kalyani S. Marathe, MD, MPH, the director of the division of dermatology at Cincinnati Children’s Hospital Medical Center, told this news organization.
The results are mixed, though, she noted.
“While some impact on the risk of eczema is seen at 1 year of age, that protective effect is gone by 2 years and 4 years,” Dr. Marathe, who was not involved in the study, said in an email. “So if maternal supplementation does improve eczema, the effect is not long-lasting.
“The other complicating factor is that the babies who showed reduction in eczema were also the ones who were breastfed longer than 1 month,” she added. “We know that breastfeeding is associated with several factors, including socioeconomic status, so it is difficult to tease out the relationships here.
“Vitamin D has become a very hot topic lately and seems to have protective effects in many areas of health care,” Dr. Marathe said. “These results may motivate pregnant women to be compliant with their prenatal vitamins that contain the amount of vitamin D studied here.”
The study received grant support. Several authors disclosed financial relationships with pharmaceutical and nutritional products industries. Dr. El-Heis and Dr. Marathe reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to results of a clinical trial.
“Our data provide the first randomized controlled trial evidence of a protective effect of antenatal cholecalciferol supplementation on risk of infantile atopic eczema, with the effect only seen in infants that were breastfed for more than 1 month,” lead study author Sarah El-Heis, MRCP, DM, and colleagues wrote.
“The findings support a developmental influence on infantile atopic eczema and point to gestational cholecalciferol supplementation as a preventive strategy to reduce the burden of atopic eczema during infancy,” Dr. El-Heis, an academic clinical lecturer in dermatology at the Medical Research Council Lifecourse Epidemiology Center of the University of Southampton (England), said in a presentation at the annual meeting of the Society for Investigative Dermatology.
The study also was published in the British Journal of Dermatology.
Dr. El-Heis and colleagues analyzed data from one of the three U.K. study sites involved in the double-blind Maternal Vitamin D Osteoporosis Study (MAVIDOS), which enrolled participants between 2008 and 2014.
The women enrolled at the University of Southampton site were of age 18 or older, and had a singleton pregnancy. Serum 25-hydroxy vitamin D (25[OH]D) levels were 25-100 nmol/L, and calcium levels were less than 2.75 mmol/L.
Those who had metabolic bone disease, kidney stones, hyperparathyroidism, or hypercalciuria or who were taking more than 400 IU/day of vitamin D supplements or medication known to interfere with fetal growth or whose fetus had a major anomaly were excluded.
The study included 1,134 women. Half of the participants were randomly assigned to receive cholecalciferol 1,000 IU/day from around 14 weeks’ gestation until delivery, and half were assigned to receive placebo. Their babies were assessed for atopic eczema at 12, 24, and 48 months of age.
The maternal and infant characteristics were similar in both groups, but the treatment group tended to breastfeed longer.
Infants appear to be protected up to 1 year of age
Using logistic regression, the researchers analyzed links between maternal cholecalciferol 1,000 IU/day supplements or placebo and atopic eczema risk in their offspring.
After adjustments for breastfeeding duration, among the 636 infants assessed at 12 months, those whose mothers received cholecalciferol had lower odds ratios of atopic eczema than those whose mothers received placebo (OR, 0.55; 95% confidence interval, 0.32-0.97).
The risk of atopic eczema at 12 months was reduced only for children in the treatment group who were breastfed longer than 1 month (OR, 0.48; 95% CI, 0.24-0.94), further analysis showed. Those who were breastfed for less than 1 month showed no reduced risk.
The combined effect of vitamin D and breastfeeding for longer than 1 month weakened after 1 year and was not statistically significant among the 611 children assessed at 24 months and the 450 children assessed at 48 months. The ORs of atopic eczema in the treatment group and in the control group increased to 0.76 (95% CI, 0.47-1.23) and 0.75 (95% CI, 0.37-1.52), respectively.
At baseline, the mean maternal serum 25(OH)D levels in the treatment group (46.0 nmol/L) and in the control group (44.7 nmol/L) were similar. But by late pregnancy, maternal serum 25(OH)D levels in the treatment group were higher (67.4 nmol/L) than in the control group (42.4 nmol/L).
The authors note that strengths of the study include its design, the uniformity of criteria used to diagnose atopic eczema, and the similarity of both pregnant groups in their intake of vitamin D during the study.
Limitations included the lack of ultraviolet B light exposure data, the lack of non-White women in the study, the lack of measurement of cord blood and offspring 25(OH)D levels, and the exclusion of women with baseline 25(OH)D concentrations less than 25 nmol/L.
“This is an interesting study that brings up the possibility that maternal factors during pregnancy may impact atopic dermatitis,” Kalyani S. Marathe, MD, MPH, the director of the division of dermatology at Cincinnati Children’s Hospital Medical Center, told this news organization.
The results are mixed, though, she noted.
“While some impact on the risk of eczema is seen at 1 year of age, that protective effect is gone by 2 years and 4 years,” Dr. Marathe, who was not involved in the study, said in an email. “So if maternal supplementation does improve eczema, the effect is not long-lasting.
“The other complicating factor is that the babies who showed reduction in eczema were also the ones who were breastfed longer than 1 month,” she added. “We know that breastfeeding is associated with several factors, including socioeconomic status, so it is difficult to tease out the relationships here.
“Vitamin D has become a very hot topic lately and seems to have protective effects in many areas of health care,” Dr. Marathe said. “These results may motivate pregnant women to be compliant with their prenatal vitamins that contain the amount of vitamin D studied here.”
The study received grant support. Several authors disclosed financial relationships with pharmaceutical and nutritional products industries. Dr. El-Heis and Dr. Marathe reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM SID 2022
Prolonged Drug-Induced Hypersensitivity Syndrome/DRESS With Alopecia Areata and Autoimmune Thyroiditis
Drug-induced hypersensitivity syndrome (DIHS), also called drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, is a potentially fatal drug-induced hypersensitivity reaction that is characterized by a cutaneous eruption, multiorgan involvement, viral reactivation, and hematologic abnormalities. As the nomenclature of this disease advances, consensus groups have adopted DIHS/DRESS to underscore that both names refer to the same clinical phenomenon.1 Autoimmune sequelae have been reported after DIHS/DRESS that include vitiligo, thyroid disease, and type 1 diabetes mellitus (T1DM). We present a case of lamotrigine-associated DIHS/DRESS complicated by an unusually prolonged course requiring oral corticosteroids and narrow-band ultraviolet B (UVB) treatment and with development of extensive alopecia areata and autoimmune thyroiditis.
Case Presentation
A 35-year-old female Filipino patient was prescribed lamotrigine 25 mg daily for bipolar II disorder and titrated to 100 mg twice daily after 1 month. One week after the increase, the patient developed a diffuse morbilliform rash covering their entire body along with facial swelling and generalized pruritus. Lamotrigine was discontinued after lamotrigine allergy was diagnosed. The patient improved following a 9-day oral prednisone taper and was placed on oxcarbazepine 300 mg twice daily to manage their bipolar disorder. One day after completing the taper, the patient presented again with worsening rash, swelling, and cervical lymphadenopathy. Oxcarbazepine was discontinued, and oral prednisone 60 mg was reinstituted for an additional 11 days.
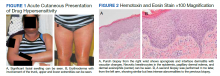
Dermatology evaluated the patient 10 days after completion of the second oral steroid taper (1 month after cessation of lamotrigine). The patient had erythroderma along with malaise, fevers, chills, and fatigue and a diffuse burning sensation (Figure 1). The patient was hypotensive and tachycardic with significant eosinophilia (42%; reference range, 0%-8%), transaminitis, and renal insufficiency. The patient was diagnosed with DIHS/DRESS based on their clinical presentation and calculated RegiSCAR score of 7 (score > 5 corresponds with definite DIHS/DRESS and points were given for fever, enlarged lymph nodes, eosinophilia ≥ 20%, skin rash extending > 50% of their body, edema and scaling, and 2 organs involved).2 A punch biopsy was confirmatory (Figure 2A).3 The patient was started on prednisone 80 mg once daily along with topical fluocinonide 0.05% ointment. However, the patient’s clinical status deteriorated, requiring hospital admission for heart failure evaluation. The echocardiogram revealed hyperdynamic circulation but was otherwise unremarkable.
The patient was maintained on prednisone 70 to 80 mg daily for 2 months before improvement of the rash and pruritus. The prednisone was slowly tapered over a 6-week period and then discontinued. Shortly after discontinuation, the patient redeveloped erythroderma. Skin biopsy and complete blood count (17.3% eosinophilia) confirmed the suspected DIHS/DRESS relapse (Figure 2B). In addition, the patient reported upper respiratory tract symptoms and concurrently tested positive for human herpesvirus 6 (HHV-6). The patient was restarted on prednisone and low-dose narrow-band UVB (nbUVB) therapy was added. Over the following 2 months, they responded well to low-dose nbUVB therapy. By the end of nbUVB treatment, about 5 months after initial presentation, the patient’s erythroderma improved, eosinophilia resolved, and they were able to tolerate prednisone taper. Ten months after cessation of lamotrigine, prednisone was finally discontinued. Two weeks later, the patient was screened for adrenal insufficiency (AI) given the prolonged steroid course. Their serum morning cortisol level was within normal limits.
Four months after DIHS/DRESS resolution and cessation of steroids, the patient noted significant patches of smooth alopecia on their posterior scalp and was diagnosed with alopecia areata. Treatment with intralesional triamcinolone over 2 months resulted in regrowth of hair (Figure 3). A month later, the patient reported increasing fatigue and anorexia. The patient was evaluated once more for AI, this time with low morning cortisol and low adrenocorticotrophic hormone (ACTH) levels—consistent with AI secondary to prolonged glucocorticoid therapy. The patient also was concomitantly evaluated for hypothyroidism with significantly elevated thyroperoxidase antibodies—confirming the diagnosis of Hashimoto thyroiditis.
Discussion
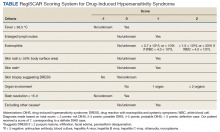
DIHS/DRESS syndrome is a rare, but potentially life-threatening hypersensitivity to a medication, often beginning 2 to 6 weeks after exposure to the causative agent. The incidence of DIHS/DRESS in the general population is about 2 per 100,000.3 Our patient presented with DIHS/DRESS 33 days after starting lamotrigine, which corresponds with the published mean onset of anticonvulsant-induced DIHS/DRESS (29.7-33.3 days).4 Recent evidence shows that time from drug exposure to DIHS/DRESS symptoms may vary by drug class, with antibiotics implicated as precipitating DIHS/DRESS in < 15 days.3 The diagnosis of DIHS/DRESS may be complicated for many reasons. The accompanying rash may be morbilliform, erythroderma, or exfoliative dermatitis with multiple anatomic regions affected.5 Systemic involvement with various internal organs occurs in > 90% of cases, with the liver and kidney involved most frequently.5 Overall mortality rate may be as high as 10% most commonly due to acute liver failure.5 Biopsy may be helpful in the diagnosis but is not always specific.5 Diagnostic criteria include RegiSCAR and J-SCAR scores; our patient met criteria for both (Table).5
The pathogenesis of DIHS/DRESS remains unclear. Proposed mechanisms include genetic predisposition with human leukocyte antigen (HLA) haplotypes, autoimmune with a delayed cell-mediated immune response associated with herpesviruses, and abnormal enzymatic pathways that metabolize medications.2 Although no HLA has been identified between lamotrigine and DIHS, HLA-A*02:07 and HLA-B*15:02 have been associated with lamotrigine-induced cutaneous drug reactions in patients of Thai ancestry.6 Immunosuppression also is a risk factor, especially when accompanied by a primary or reactivated HHV-6 infection, as seen in our patient.2 Additionally, HHV-6 infection may be a common link between DIHS/DRESS and autoimmune thyroiditis but is believed to involve elevated levels of interferon-γ-induced protein-10 (IP-10) that may lead to excessive recruitment of cytotoxic T cells into target tissues.7 Elevated levels of IP-10 are seen in many autoimmune conditions, such as autoimmune thyroiditis, Sjögren syndrome, and Graves disease.8
DIHS/DRESS syndrome has been associated with development of autoimmune diseases as long-term sequelae. The most commonly affected organs are the thyroid and pancreas; approximately 4.8% of patients develop autoimmune thyroiditis and 3.5% develop fulminant T1DM.9 The time from onset of DIHS/DRESS to development of autoimmune thyroiditis can range from 2 months to 2 years, whereas the range from DIHS/DRESS onset to fulminant T1DM is about 40 days.9 Alopecia had been reported in 1, occurring 4 months after DIHS/DRESS onset. Our patient’s alopecia areata and Hashimoto thyroiditis occurred 14 and 15 months after DIHS/DRESS presentation, respectively.
Treatment
For management, early recognition and discontinuation of the offending agent is paramount. Systemic corticosteroids are the accepted treatment standard. Symptoms of DIHS/DRESS usually resolve between 3 and 18 weeks, with the mean resolution time at 7 weeks.10 Our patient developed a prolonged course with persistent eosinophilia for 20 weeks and cutaneous symptoms for 32 weeks—requiring 40 weeks of oral prednisone. The most significant clinical improvement occurred during the 8-week period low-dose nbUVB was used (Figure 4). There also are reports outlining the successful use of intravenous immunoglobulin, cyclosporine, cyclophosphamide, rituximab, or plasma exchange in cases refractory to oral corticosteroids.11
A recent retrospective case control study showed that treatment of DIHS/DRESS with cyclosporine in patients who had a contraindication to steroids resulted in faster resolution of symptoms, shorter treatment durations, and shorter hospitalizations than did those treated with corticosteroids.12 However, the data are limited by a significantly smaller number of patients treated with cyclosporine than steroids and the cyclosporine treatment group having milder cases of DIHS/DRESS.12
The risk of AI is increased for patients who have taken > 20 mg of prednisone daily ≥ 3 weeks, an evening dose ≥ 5 mg for a few weeks, or have a Cushingoid appearance.13 Patients may not regain full adrenal function for 12 to 18 months.14 Our patient had a normal basal serum cortisol level 2 weeks after prednisone cessation and then presented 5 months later with AI. While the reason for this period of normality is unclear, it may partly be due to the variable length of hypothalamic-pituitary-adrenal axis recovery time. Thus, ACTH stimulation tests in addition to serum cortisol may be done in patients with suspected AI for higher diagnostic certainty.10
Conclusions
DIHS/DRESS is a severe cutaneous adverse reaction that may require a prolonged treatment course until symptom resolution (40 weeks of oral prednisone in our patient). Oral corticosteroids are the mainstay of treatment, but long-term use is associated with significant adverse effects, such as AI in our patient. Alternative therapies, such as cyclosporine, look promising, but further studies are needed to determine safety profile and efficacy.12 Additionally, patients with DIHS/DRESS should be educated and followed for potential autoimmune sequelae; in our patient alopecia areata and autoimmune thyroiditis were late sequelae, occurring 14 and 15 months, respectively, after onset of DIHS/DRESS.
1. RegiSCAR. Accessed June 3, 2022. http://www.regiscar.org
2. Shiohara T, Mizukawa Y. Drug-induced hypersensitivity syndrome (DiHS)/drug reaction with eosinophilia and systemic symptoms (DRESS): an update in 2019. Allergol Int. 2019;68(3):301-308. doi:10.1016/j.alit.2019.03.006
3. Wolfson AR, Zhou L, Li Y, Phadke NA, Chow OA, Blumenthal KG. Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome identified in the electronic health record allergy module. J Allergy Clin Immunol Pract. 2019;7(2):633-640. doi:10.1016/j.jaip.2018.08.013
4. Sasidharanpillai S, Govindan A, Riyaz N, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): a histopathology based analysis. Indian J Dermatol Venereol Leprol. 2016;82(1):28. doi:10.4103/0378-6323.168934
5. Kardaun SH, Sekula P, Valeyrie‐Allanore L, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol. 2013;169(5):1071-1080. doi:10.1111/bjd.12501
6. Koomdee N, Pratoomwun J, Jantararoungtong T, et al. Association of HLA-A and HLA-B alleles with lamotrigine-induced cutaneous adverse drug reactions in the Thai population. Front Pharmacol. 2017;8. doi:10.3389/fphar.2017.00879
7. Yang C-W, Cho Y-T, Hsieh Y-C, Hsu S-H, Chen K-L, Chu C-Y. The interferon-γ-induced protein 10/CXCR3 axis is associated with human herpesvirus-6 reactivation and the development of sequelae in drug reaction with eosinophilia and systemic symptoms. Br J Dermatol. 2020;183(5):909-919. doi:10.1111/bjd.18942
8. Ruffilli I, Ferrari SM, Colaci M, Ferri C, Fallahi P, Antonelli A. IP-10 in autoimmune thyroiditis. Horm Metab Res. 2014;46(9):597-602. doi:10.1055/s-0034-1382053
9. Kano Y, Tohyama M, Aihara M, et al. Sequelae in 145 patients with drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms: survey conducted by the Asian Research Committee on Severe Cutaneous Adverse Reactions (ASCAR). J Dermatol. 2015;42(3):276-282. doi:10.1111/1346-8138.12770
10. Cacoub P, Musette P, Descamps V, et al. The DRESS syndrome: a literature review. Am J Med. 2011;124(7):588-597. doi:10.1016/j.amjmed.2011.01.017
11. Bommersbach TJ, Lapid MI, Leung JG, Cunningham JL, Rummans TA, Kung S. Management of psychotropic drug-induced dress syndrome: a systematic review. Mayo Clin Proc. 2016;91(6):787-801. doi:10.1016/j.mayocp.2016.03.006
12. Nguyen E, Yanes D, Imadojemu S, Kroshinsky D. Evaluation of cyclosporine for the treatment of DRESS syndrome. JAMA Dermatol. 2020;156(6):704-706. doi:10.1001/jamadermatol.2020.0048
13. Joseph RM, Hunter AL, Ray DW, Dixon WG. Systemic glucocorticoid therapy and adrenal insufficiency in adults: a systematic review. Semin Arthritis Rheum. 2016;46(1):133-141. doi:10.1016/j.semarthrit.2016.03.001
14. Jamilloux Y, Liozon E, Pugnet G, et al. Recovery of adrenal function after long-term glucocorticoid therapy for giant cell arteritis: a cohort study. PLoS ONE. 2013;8(7):e68713. doi:10.1371/journal.pone.0068713
Drug-induced hypersensitivity syndrome (DIHS), also called drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, is a potentially fatal drug-induced hypersensitivity reaction that is characterized by a cutaneous eruption, multiorgan involvement, viral reactivation, and hematologic abnormalities. As the nomenclature of this disease advances, consensus groups have adopted DIHS/DRESS to underscore that both names refer to the same clinical phenomenon.1 Autoimmune sequelae have been reported after DIHS/DRESS that include vitiligo, thyroid disease, and type 1 diabetes mellitus (T1DM). We present a case of lamotrigine-associated DIHS/DRESS complicated by an unusually prolonged course requiring oral corticosteroids and narrow-band ultraviolet B (UVB) treatment and with development of extensive alopecia areata and autoimmune thyroiditis.
Case Presentation
A 35-year-old female Filipino patient was prescribed lamotrigine 25 mg daily for bipolar II disorder and titrated to 100 mg twice daily after 1 month. One week after the increase, the patient developed a diffuse morbilliform rash covering their entire body along with facial swelling and generalized pruritus. Lamotrigine was discontinued after lamotrigine allergy was diagnosed. The patient improved following a 9-day oral prednisone taper and was placed on oxcarbazepine 300 mg twice daily to manage their bipolar disorder. One day after completing the taper, the patient presented again with worsening rash, swelling, and cervical lymphadenopathy. Oxcarbazepine was discontinued, and oral prednisone 60 mg was reinstituted for an additional 11 days.
Dermatology evaluated the patient 10 days after completion of the second oral steroid taper (1 month after cessation of lamotrigine). The patient had erythroderma along with malaise, fevers, chills, and fatigue and a diffuse burning sensation (Figure 1). The patient was hypotensive and tachycardic with significant eosinophilia (42%; reference range, 0%-8%), transaminitis, and renal insufficiency. The patient was diagnosed with DIHS/DRESS based on their clinical presentation and calculated RegiSCAR score of 7 (score > 5 corresponds with definite DIHS/DRESS and points were given for fever, enlarged lymph nodes, eosinophilia ≥ 20%, skin rash extending > 50% of their body, edema and scaling, and 2 organs involved).2 A punch biopsy was confirmatory (Figure 2A).3 The patient was started on prednisone 80 mg once daily along with topical fluocinonide 0.05% ointment. However, the patient’s clinical status deteriorated, requiring hospital admission for heart failure evaluation. The echocardiogram revealed hyperdynamic circulation but was otherwise unremarkable.
The patient was maintained on prednisone 70 to 80 mg daily for 2 months before improvement of the rash and pruritus. The prednisone was slowly tapered over a 6-week period and then discontinued. Shortly after discontinuation, the patient redeveloped erythroderma. Skin biopsy and complete blood count (17.3% eosinophilia) confirmed the suspected DIHS/DRESS relapse (Figure 2B). In addition, the patient reported upper respiratory tract symptoms and concurrently tested positive for human herpesvirus 6 (HHV-6). The patient was restarted on prednisone and low-dose narrow-band UVB (nbUVB) therapy was added. Over the following 2 months, they responded well to low-dose nbUVB therapy. By the end of nbUVB treatment, about 5 months after initial presentation, the patient’s erythroderma improved, eosinophilia resolved, and they were able to tolerate prednisone taper. Ten months after cessation of lamotrigine, prednisone was finally discontinued. Two weeks later, the patient was screened for adrenal insufficiency (AI) given the prolonged steroid course. Their serum morning cortisol level was within normal limits.
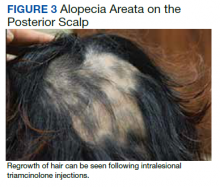
Four months after DIHS/DRESS resolution and cessation of steroids, the patient noted significant patches of smooth alopecia on their posterior scalp and was diagnosed with alopecia areata. Treatment with intralesional triamcinolone over 2 months resulted in regrowth of hair (Figure 3). A month later, the patient reported increasing fatigue and anorexia. The patient was evaluated once more for AI, this time with low morning cortisol and low adrenocorticotrophic hormone (ACTH) levels—consistent with AI secondary to prolonged glucocorticoid therapy. The patient also was concomitantly evaluated for hypothyroidism with significantly elevated thyroperoxidase antibodies—confirming the diagnosis of Hashimoto thyroiditis.
Discussion
DIHS/DRESS syndrome is a rare, but potentially life-threatening hypersensitivity to a medication, often beginning 2 to 6 weeks after exposure to the causative agent. The incidence of DIHS/DRESS in the general population is about 2 per 100,000.3 Our patient presented with DIHS/DRESS 33 days after starting lamotrigine, which corresponds with the published mean onset of anticonvulsant-induced DIHS/DRESS (29.7-33.3 days).4 Recent evidence shows that time from drug exposure to DIHS/DRESS symptoms may vary by drug class, with antibiotics implicated as precipitating DIHS/DRESS in < 15 days.3 The diagnosis of DIHS/DRESS may be complicated for many reasons. The accompanying rash may be morbilliform, erythroderma, or exfoliative dermatitis with multiple anatomic regions affected.5 Systemic involvement with various internal organs occurs in > 90% of cases, with the liver and kidney involved most frequently.5 Overall mortality rate may be as high as 10% most commonly due to acute liver failure.5 Biopsy may be helpful in the diagnosis but is not always specific.5 Diagnostic criteria include RegiSCAR and J-SCAR scores; our patient met criteria for both (Table).5
The pathogenesis of DIHS/DRESS remains unclear. Proposed mechanisms include genetic predisposition with human leukocyte antigen (HLA) haplotypes, autoimmune with a delayed cell-mediated immune response associated with herpesviruses, and abnormal enzymatic pathways that metabolize medications.2 Although no HLA has been identified between lamotrigine and DIHS, HLA-A*02:07 and HLA-B*15:02 have been associated with lamotrigine-induced cutaneous drug reactions in patients of Thai ancestry.6 Immunosuppression also is a risk factor, especially when accompanied by a primary or reactivated HHV-6 infection, as seen in our patient.2 Additionally, HHV-6 infection may be a common link between DIHS/DRESS and autoimmune thyroiditis but is believed to involve elevated levels of interferon-γ-induced protein-10 (IP-10) that may lead to excessive recruitment of cytotoxic T cells into target tissues.7 Elevated levels of IP-10 are seen in many autoimmune conditions, such as autoimmune thyroiditis, Sjögren syndrome, and Graves disease.8
DIHS/DRESS syndrome has been associated with development of autoimmune diseases as long-term sequelae. The most commonly affected organs are the thyroid and pancreas; approximately 4.8% of patients develop autoimmune thyroiditis and 3.5% develop fulminant T1DM.9 The time from onset of DIHS/DRESS to development of autoimmune thyroiditis can range from 2 months to 2 years, whereas the range from DIHS/DRESS onset to fulminant T1DM is about 40 days.9 Alopecia had been reported in 1, occurring 4 months after DIHS/DRESS onset. Our patient’s alopecia areata and Hashimoto thyroiditis occurred 14 and 15 months after DIHS/DRESS presentation, respectively.
Treatment
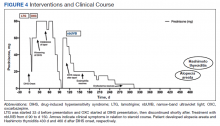
For management, early recognition and discontinuation of the offending agent is paramount. Systemic corticosteroids are the accepted treatment standard. Symptoms of DIHS/DRESS usually resolve between 3 and 18 weeks, with the mean resolution time at 7 weeks.10 Our patient developed a prolonged course with persistent eosinophilia for 20 weeks and cutaneous symptoms for 32 weeks—requiring 40 weeks of oral prednisone. The most significant clinical improvement occurred during the 8-week period low-dose nbUVB was used (Figure 4). There also are reports outlining the successful use of intravenous immunoglobulin, cyclosporine, cyclophosphamide, rituximab, or plasma exchange in cases refractory to oral corticosteroids.11
A recent retrospective case control study showed that treatment of DIHS/DRESS with cyclosporine in patients who had a contraindication to steroids resulted in faster resolution of symptoms, shorter treatment durations, and shorter hospitalizations than did those treated with corticosteroids.12 However, the data are limited by a significantly smaller number of patients treated with cyclosporine than steroids and the cyclosporine treatment group having milder cases of DIHS/DRESS.12
The risk of AI is increased for patients who have taken > 20 mg of prednisone daily ≥ 3 weeks, an evening dose ≥ 5 mg for a few weeks, or have a Cushingoid appearance.13 Patients may not regain full adrenal function for 12 to 18 months.14 Our patient had a normal basal serum cortisol level 2 weeks after prednisone cessation and then presented 5 months later with AI. While the reason for this period of normality is unclear, it may partly be due to the variable length of hypothalamic-pituitary-adrenal axis recovery time. Thus, ACTH stimulation tests in addition to serum cortisol may be done in patients with suspected AI for higher diagnostic certainty.10
Conclusions
DIHS/DRESS is a severe cutaneous adverse reaction that may require a prolonged treatment course until symptom resolution (40 weeks of oral prednisone in our patient). Oral corticosteroids are the mainstay of treatment, but long-term use is associated with significant adverse effects, such as AI in our patient. Alternative therapies, such as cyclosporine, look promising, but further studies are needed to determine safety profile and efficacy.12 Additionally, patients with DIHS/DRESS should be educated and followed for potential autoimmune sequelae; in our patient alopecia areata and autoimmune thyroiditis were late sequelae, occurring 14 and 15 months, respectively, after onset of DIHS/DRESS.
Drug-induced hypersensitivity syndrome (DIHS), also called drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, is a potentially fatal drug-induced hypersensitivity reaction that is characterized by a cutaneous eruption, multiorgan involvement, viral reactivation, and hematologic abnormalities. As the nomenclature of this disease advances, consensus groups have adopted DIHS/DRESS to underscore that both names refer to the same clinical phenomenon.1 Autoimmune sequelae have been reported after DIHS/DRESS that include vitiligo, thyroid disease, and type 1 diabetes mellitus (T1DM). We present a case of lamotrigine-associated DIHS/DRESS complicated by an unusually prolonged course requiring oral corticosteroids and narrow-band ultraviolet B (UVB) treatment and with development of extensive alopecia areata and autoimmune thyroiditis.
Case Presentation
A 35-year-old female Filipino patient was prescribed lamotrigine 25 mg daily for bipolar II disorder and titrated to 100 mg twice daily after 1 month. One week after the increase, the patient developed a diffuse morbilliform rash covering their entire body along with facial swelling and generalized pruritus. Lamotrigine was discontinued after lamotrigine allergy was diagnosed. The patient improved following a 9-day oral prednisone taper and was placed on oxcarbazepine 300 mg twice daily to manage their bipolar disorder. One day after completing the taper, the patient presented again with worsening rash, swelling, and cervical lymphadenopathy. Oxcarbazepine was discontinued, and oral prednisone 60 mg was reinstituted for an additional 11 days.
Dermatology evaluated the patient 10 days after completion of the second oral steroid taper (1 month after cessation of lamotrigine). The patient had erythroderma along with malaise, fevers, chills, and fatigue and a diffuse burning sensation (Figure 1). The patient was hypotensive and tachycardic with significant eosinophilia (42%; reference range, 0%-8%), transaminitis, and renal insufficiency. The patient was diagnosed with DIHS/DRESS based on their clinical presentation and calculated RegiSCAR score of 7 (score > 5 corresponds with definite DIHS/DRESS and points were given for fever, enlarged lymph nodes, eosinophilia ≥ 20%, skin rash extending > 50% of their body, edema and scaling, and 2 organs involved).2 A punch biopsy was confirmatory (Figure 2A).3 The patient was started on prednisone 80 mg once daily along with topical fluocinonide 0.05% ointment. However, the patient’s clinical status deteriorated, requiring hospital admission for heart failure evaluation. The echocardiogram revealed hyperdynamic circulation but was otherwise unremarkable.
The patient was maintained on prednisone 70 to 80 mg daily for 2 months before improvement of the rash and pruritus. The prednisone was slowly tapered over a 6-week period and then discontinued. Shortly after discontinuation, the patient redeveloped erythroderma. Skin biopsy and complete blood count (17.3% eosinophilia) confirmed the suspected DIHS/DRESS relapse (Figure 2B). In addition, the patient reported upper respiratory tract symptoms and concurrently tested positive for human herpesvirus 6 (HHV-6). The patient was restarted on prednisone and low-dose narrow-band UVB (nbUVB) therapy was added. Over the following 2 months, they responded well to low-dose nbUVB therapy. By the end of nbUVB treatment, about 5 months after initial presentation, the patient’s erythroderma improved, eosinophilia resolved, and they were able to tolerate prednisone taper. Ten months after cessation of lamotrigine, prednisone was finally discontinued. Two weeks later, the patient was screened for adrenal insufficiency (AI) given the prolonged steroid course. Their serum morning cortisol level was within normal limits.
Four months after DIHS/DRESS resolution and cessation of steroids, the patient noted significant patches of smooth alopecia on their posterior scalp and was diagnosed with alopecia areata. Treatment with intralesional triamcinolone over 2 months resulted in regrowth of hair (Figure 3). A month later, the patient reported increasing fatigue and anorexia. The patient was evaluated once more for AI, this time with low morning cortisol and low adrenocorticotrophic hormone (ACTH) levels—consistent with AI secondary to prolonged glucocorticoid therapy. The patient also was concomitantly evaluated for hypothyroidism with significantly elevated thyroperoxidase antibodies—confirming the diagnosis of Hashimoto thyroiditis.
Discussion
DIHS/DRESS syndrome is a rare, but potentially life-threatening hypersensitivity to a medication, often beginning 2 to 6 weeks after exposure to the causative agent. The incidence of DIHS/DRESS in the general population is about 2 per 100,000.3 Our patient presented with DIHS/DRESS 33 days after starting lamotrigine, which corresponds with the published mean onset of anticonvulsant-induced DIHS/DRESS (29.7-33.3 days).4 Recent evidence shows that time from drug exposure to DIHS/DRESS symptoms may vary by drug class, with antibiotics implicated as precipitating DIHS/DRESS in < 15 days.3 The diagnosis of DIHS/DRESS may be complicated for many reasons. The accompanying rash may be morbilliform, erythroderma, or exfoliative dermatitis with multiple anatomic regions affected.5 Systemic involvement with various internal organs occurs in > 90% of cases, with the liver and kidney involved most frequently.5 Overall mortality rate may be as high as 10% most commonly due to acute liver failure.5 Biopsy may be helpful in the diagnosis but is not always specific.5 Diagnostic criteria include RegiSCAR and J-SCAR scores; our patient met criteria for both (Table).5
The pathogenesis of DIHS/DRESS remains unclear. Proposed mechanisms include genetic predisposition with human leukocyte antigen (HLA) haplotypes, autoimmune with a delayed cell-mediated immune response associated with herpesviruses, and abnormal enzymatic pathways that metabolize medications.2 Although no HLA has been identified between lamotrigine and DIHS, HLA-A*02:07 and HLA-B*15:02 have been associated with lamotrigine-induced cutaneous drug reactions in patients of Thai ancestry.6 Immunosuppression also is a risk factor, especially when accompanied by a primary or reactivated HHV-6 infection, as seen in our patient.2 Additionally, HHV-6 infection may be a common link between DIHS/DRESS and autoimmune thyroiditis but is believed to involve elevated levels of interferon-γ-induced protein-10 (IP-10) that may lead to excessive recruitment of cytotoxic T cells into target tissues.7 Elevated levels of IP-10 are seen in many autoimmune conditions, such as autoimmune thyroiditis, Sjögren syndrome, and Graves disease.8
DIHS/DRESS syndrome has been associated with development of autoimmune diseases as long-term sequelae. The most commonly affected organs are the thyroid and pancreas; approximately 4.8% of patients develop autoimmune thyroiditis and 3.5% develop fulminant T1DM.9 The time from onset of DIHS/DRESS to development of autoimmune thyroiditis can range from 2 months to 2 years, whereas the range from DIHS/DRESS onset to fulminant T1DM is about 40 days.9 Alopecia had been reported in 1, occurring 4 months after DIHS/DRESS onset. Our patient’s alopecia areata and Hashimoto thyroiditis occurred 14 and 15 months after DIHS/DRESS presentation, respectively.
Treatment
For management, early recognition and discontinuation of the offending agent is paramount. Systemic corticosteroids are the accepted treatment standard. Symptoms of DIHS/DRESS usually resolve between 3 and 18 weeks, with the mean resolution time at 7 weeks.10 Our patient developed a prolonged course with persistent eosinophilia for 20 weeks and cutaneous symptoms for 32 weeks—requiring 40 weeks of oral prednisone. The most significant clinical improvement occurred during the 8-week period low-dose nbUVB was used (Figure 4). There also are reports outlining the successful use of intravenous immunoglobulin, cyclosporine, cyclophosphamide, rituximab, or plasma exchange in cases refractory to oral corticosteroids.11
A recent retrospective case control study showed that treatment of DIHS/DRESS with cyclosporine in patients who had a contraindication to steroids resulted in faster resolution of symptoms, shorter treatment durations, and shorter hospitalizations than did those treated with corticosteroids.12 However, the data are limited by a significantly smaller number of patients treated with cyclosporine than steroids and the cyclosporine treatment group having milder cases of DIHS/DRESS.12
The risk of AI is increased for patients who have taken > 20 mg of prednisone daily ≥ 3 weeks, an evening dose ≥ 5 mg for a few weeks, or have a Cushingoid appearance.13 Patients may not regain full adrenal function for 12 to 18 months.14 Our patient had a normal basal serum cortisol level 2 weeks after prednisone cessation and then presented 5 months later with AI. While the reason for this period of normality is unclear, it may partly be due to the variable length of hypothalamic-pituitary-adrenal axis recovery time. Thus, ACTH stimulation tests in addition to serum cortisol may be done in patients with suspected AI for higher diagnostic certainty.10
Conclusions
DIHS/DRESS is a severe cutaneous adverse reaction that may require a prolonged treatment course until symptom resolution (40 weeks of oral prednisone in our patient). Oral corticosteroids are the mainstay of treatment, but long-term use is associated with significant adverse effects, such as AI in our patient. Alternative therapies, such as cyclosporine, look promising, but further studies are needed to determine safety profile and efficacy.12 Additionally, patients with DIHS/DRESS should be educated and followed for potential autoimmune sequelae; in our patient alopecia areata and autoimmune thyroiditis were late sequelae, occurring 14 and 15 months, respectively, after onset of DIHS/DRESS.
1. RegiSCAR. Accessed June 3, 2022. http://www.regiscar.org
2. Shiohara T, Mizukawa Y. Drug-induced hypersensitivity syndrome (DiHS)/drug reaction with eosinophilia and systemic symptoms (DRESS): an update in 2019. Allergol Int. 2019;68(3):301-308. doi:10.1016/j.alit.2019.03.006
3. Wolfson AR, Zhou L, Li Y, Phadke NA, Chow OA, Blumenthal KG. Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome identified in the electronic health record allergy module. J Allergy Clin Immunol Pract. 2019;7(2):633-640. doi:10.1016/j.jaip.2018.08.013
4. Sasidharanpillai S, Govindan A, Riyaz N, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): a histopathology based analysis. Indian J Dermatol Venereol Leprol. 2016;82(1):28. doi:10.4103/0378-6323.168934
5. Kardaun SH, Sekula P, Valeyrie‐Allanore L, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol. 2013;169(5):1071-1080. doi:10.1111/bjd.12501
6. Koomdee N, Pratoomwun J, Jantararoungtong T, et al. Association of HLA-A and HLA-B alleles with lamotrigine-induced cutaneous adverse drug reactions in the Thai population. Front Pharmacol. 2017;8. doi:10.3389/fphar.2017.00879
7. Yang C-W, Cho Y-T, Hsieh Y-C, Hsu S-H, Chen K-L, Chu C-Y. The interferon-γ-induced protein 10/CXCR3 axis is associated with human herpesvirus-6 reactivation and the development of sequelae in drug reaction with eosinophilia and systemic symptoms. Br J Dermatol. 2020;183(5):909-919. doi:10.1111/bjd.18942
8. Ruffilli I, Ferrari SM, Colaci M, Ferri C, Fallahi P, Antonelli A. IP-10 in autoimmune thyroiditis. Horm Metab Res. 2014;46(9):597-602. doi:10.1055/s-0034-1382053
9. Kano Y, Tohyama M, Aihara M, et al. Sequelae in 145 patients with drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms: survey conducted by the Asian Research Committee on Severe Cutaneous Adverse Reactions (ASCAR). J Dermatol. 2015;42(3):276-282. doi:10.1111/1346-8138.12770
10. Cacoub P, Musette P, Descamps V, et al. The DRESS syndrome: a literature review. Am J Med. 2011;124(7):588-597. doi:10.1016/j.amjmed.2011.01.017
11. Bommersbach TJ, Lapid MI, Leung JG, Cunningham JL, Rummans TA, Kung S. Management of psychotropic drug-induced dress syndrome: a systematic review. Mayo Clin Proc. 2016;91(6):787-801. doi:10.1016/j.mayocp.2016.03.006
12. Nguyen E, Yanes D, Imadojemu S, Kroshinsky D. Evaluation of cyclosporine for the treatment of DRESS syndrome. JAMA Dermatol. 2020;156(6):704-706. doi:10.1001/jamadermatol.2020.0048
13. Joseph RM, Hunter AL, Ray DW, Dixon WG. Systemic glucocorticoid therapy and adrenal insufficiency in adults: a systematic review. Semin Arthritis Rheum. 2016;46(1):133-141. doi:10.1016/j.semarthrit.2016.03.001
14. Jamilloux Y, Liozon E, Pugnet G, et al. Recovery of adrenal function after long-term glucocorticoid therapy for giant cell arteritis: a cohort study. PLoS ONE. 2013;8(7):e68713. doi:10.1371/journal.pone.0068713
1. RegiSCAR. Accessed June 3, 2022. http://www.regiscar.org
2. Shiohara T, Mizukawa Y. Drug-induced hypersensitivity syndrome (DiHS)/drug reaction with eosinophilia and systemic symptoms (DRESS): an update in 2019. Allergol Int. 2019;68(3):301-308. doi:10.1016/j.alit.2019.03.006
3. Wolfson AR, Zhou L, Li Y, Phadke NA, Chow OA, Blumenthal KG. Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome identified in the electronic health record allergy module. J Allergy Clin Immunol Pract. 2019;7(2):633-640. doi:10.1016/j.jaip.2018.08.013
4. Sasidharanpillai S, Govindan A, Riyaz N, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): a histopathology based analysis. Indian J Dermatol Venereol Leprol. 2016;82(1):28. doi:10.4103/0378-6323.168934
5. Kardaun SH, Sekula P, Valeyrie‐Allanore L, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol. 2013;169(5):1071-1080. doi:10.1111/bjd.12501
6. Koomdee N, Pratoomwun J, Jantararoungtong T, et al. Association of HLA-A and HLA-B alleles with lamotrigine-induced cutaneous adverse drug reactions in the Thai population. Front Pharmacol. 2017;8. doi:10.3389/fphar.2017.00879
7. Yang C-W, Cho Y-T, Hsieh Y-C, Hsu S-H, Chen K-L, Chu C-Y. The interferon-γ-induced protein 10/CXCR3 axis is associated with human herpesvirus-6 reactivation and the development of sequelae in drug reaction with eosinophilia and systemic symptoms. Br J Dermatol. 2020;183(5):909-919. doi:10.1111/bjd.18942
8. Ruffilli I, Ferrari SM, Colaci M, Ferri C, Fallahi P, Antonelli A. IP-10 in autoimmune thyroiditis. Horm Metab Res. 2014;46(9):597-602. doi:10.1055/s-0034-1382053
9. Kano Y, Tohyama M, Aihara M, et al. Sequelae in 145 patients with drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms: survey conducted by the Asian Research Committee on Severe Cutaneous Adverse Reactions (ASCAR). J Dermatol. 2015;42(3):276-282. doi:10.1111/1346-8138.12770
10. Cacoub P, Musette P, Descamps V, et al. The DRESS syndrome: a literature review. Am J Med. 2011;124(7):588-597. doi:10.1016/j.amjmed.2011.01.017
11. Bommersbach TJ, Lapid MI, Leung JG, Cunningham JL, Rummans TA, Kung S. Management of psychotropic drug-induced dress syndrome: a systematic review. Mayo Clin Proc. 2016;91(6):787-801. doi:10.1016/j.mayocp.2016.03.006
12. Nguyen E, Yanes D, Imadojemu S, Kroshinsky D. Evaluation of cyclosporine for the treatment of DRESS syndrome. JAMA Dermatol. 2020;156(6):704-706. doi:10.1001/jamadermatol.2020.0048
13. Joseph RM, Hunter AL, Ray DW, Dixon WG. Systemic glucocorticoid therapy and adrenal insufficiency in adults: a systematic review. Semin Arthritis Rheum. 2016;46(1):133-141. doi:10.1016/j.semarthrit.2016.03.001
14. Jamilloux Y, Liozon E, Pugnet G, et al. Recovery of adrenal function after long-term glucocorticoid therapy for giant cell arteritis: a cohort study. PLoS ONE. 2013;8(7):e68713. doi:10.1371/journal.pone.0068713
Experts: EPA should assess risk of sunscreens’ UV filters
The , an expert panel of the National Academies of Sciences, Engineering, and Medicine (NAS) said on Aug. 9.
The assessment is urgently needed, the experts said, and the results should be shared with the Food and Drug Administration, which oversees sunscreens.
In its 400-page report, titled the Review of Fate, Exposure, and Effects of Sunscreens in Aquatic Environments and Implications for Sunscreen Usage and Human Health, the panel does not make recommendations but suggests that such an EPA risk assessment should highlight gaps in knowledge.
“We are teeing up the critical information that will be used to take on the challenge of risk assessment,” Charles A. Menzie, PhD, chair of the committee that wrote the report, said at a media briefing Aug. 9 when the report was released. Dr. Menzie is a principal at Exponent, Inc., an engineering and scientific consulting firm. He is former executive director of the Society of Environmental Toxicology and Chemistry.
The EPA sponsored the study, which was conducted by a committee of the National Academy of Sciences, a nonprofit, nongovernmental organization authorized by Congress that studies issues related to science, technology, and medicine.
Balancing aquatic, human health concerns
Such an EPA assessment, Dr. Menzie said in a statement, will help inform efforts to understand the environmental effects of UV filters as well as clarify a path forward for managing sunscreens. For years, concerns have been raised about the potential toxicity of sunscreens regarding many marine and freshwater aquatic organisms, especially coral. That concern, however, must be balanced against the benefits of sunscreens, which are known to protect against skin cancer. A low percentage of people use sunscreen regularly, Dr. Menzie and other panel members said.
“Only about a third of the U.S. population regularly uses sunscreen,” Mark Cullen, MD, vice chair of the NAS committee and former director of the Center for Population Health Sciences, Stanford (Calif.) University, said at the briefing. About 70% or 80% of people use it at the beach or outdoors, he said.
Report background, details
UV filters are the active ingredients in physical as well as chemical sunscreen products. They decrease the amount of UV radiation that reaches the skin. They have been found in water, sediments, and marine organisms, both saltwater and freshwater.
Currently, 17 UV filters are used in U.S. sunscreens; 15 of those are organic, such as oxybenzone and avobenzone, and are used in chemical sunscreens. They work by absorbing the rays before they damage the skin. In addition, two inorganic filters, which are used in physical sunscreens, sit on the skin and as a shield to block the rays.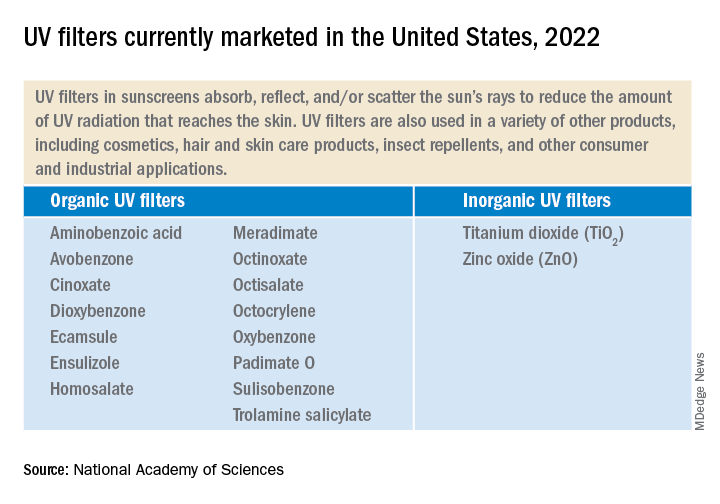
UV filters enter bodies of water by direct release, as when sunscreens rinse off people while swimming or while engaging in other water activities. They also enter bodies of water in storm water runoff and wastewater.
Lab toxicity tests, which are the most widely used, provide effects data for ecologic risk assessment. The tests are more often used in the study of short-term, not long-term exposure. Test results have shown that in high enough concentrations, some UV filters can be toxic to algal, invertebrate, and fish species.
But much information is lacking, the experts said. Toxicity data for many species, for instance, are limited. There are few studies on the longer-term environmental effects of UV filter exposure. Not enough is known about the rate at which the filters degrade in the environment. The filters accumulate in higher amounts in different areas. Recreational water areas have higher concentrations.
The recommendations
The panel is urging the EPA to complete a formal risk assessment of the UV filters “with some urgency,” Dr. Cullen said. That will enable decisions to be made about the use of the products. The risks to aquatic life must be balanced against the need for sun protection to reduce skin cancer risk.
The experts made two recommendations:
- The EPA should conduct ecologic risk assessments for all the UV filters now marketed and for all new ones. The assessment should evaluate the filters individually as well as the risk from co-occurring filters. The assessments should take into account the different exposure scenarios.
- The EPA, along with partner agencies, and sunscreen and UV filter manufacturers should fund, support, and conduct research and share data. Research should include study of human health outcomes if usage and availability of sunscreens change.
Dermatologists should “continue to emphasize the importance of protection from UV radiation in every way that can be done,” Dr. Cullen said, including the use of sunscreen as well as other protective practices, such as wearing long sleeves and hats, seeking shade, and avoiding the sun during peak hours.
A dermatologist’s perspective
“I applaud their scientific curiosity to know one way or the other whether this is an issue,” said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, DC. “I welcome this investigation.”
The multitude of studies, Dr. Friedman said, don’t always agree about whether the filters pose dangers. He noted that the concentration of UV filters detected in water is often lower than the concentrations found to be harmful in a lab setting to marine life, specifically coral.
However, he said, “these studies are snapshots.” For that reason, calling for more assessment of risk is desirable, Dr. Friedman said, but “I want to be sure the call to do more research is not an admission of guilt. It’s very easy to vilify sunscreens – but the facts we know are that UV light causes skin cancer and aging, and sunscreen protects us against this.”
Dr. Friedman has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The , an expert panel of the National Academies of Sciences, Engineering, and Medicine (NAS) said on Aug. 9.
The assessment is urgently needed, the experts said, and the results should be shared with the Food and Drug Administration, which oversees sunscreens.
In its 400-page report, titled the Review of Fate, Exposure, and Effects of Sunscreens in Aquatic Environments and Implications for Sunscreen Usage and Human Health, the panel does not make recommendations but suggests that such an EPA risk assessment should highlight gaps in knowledge.
“We are teeing up the critical information that will be used to take on the challenge of risk assessment,” Charles A. Menzie, PhD, chair of the committee that wrote the report, said at a media briefing Aug. 9 when the report was released. Dr. Menzie is a principal at Exponent, Inc., an engineering and scientific consulting firm. He is former executive director of the Society of Environmental Toxicology and Chemistry.
The EPA sponsored the study, which was conducted by a committee of the National Academy of Sciences, a nonprofit, nongovernmental organization authorized by Congress that studies issues related to science, technology, and medicine.
Balancing aquatic, human health concerns
Such an EPA assessment, Dr. Menzie said in a statement, will help inform efforts to understand the environmental effects of UV filters as well as clarify a path forward for managing sunscreens. For years, concerns have been raised about the potential toxicity of sunscreens regarding many marine and freshwater aquatic organisms, especially coral. That concern, however, must be balanced against the benefits of sunscreens, which are known to protect against skin cancer. A low percentage of people use sunscreen regularly, Dr. Menzie and other panel members said.
“Only about a third of the U.S. population regularly uses sunscreen,” Mark Cullen, MD, vice chair of the NAS committee and former director of the Center for Population Health Sciences, Stanford (Calif.) University, said at the briefing. About 70% or 80% of people use it at the beach or outdoors, he said.
Report background, details
UV filters are the active ingredients in physical as well as chemical sunscreen products. They decrease the amount of UV radiation that reaches the skin. They have been found in water, sediments, and marine organisms, both saltwater and freshwater.
Currently, 17 UV filters are used in U.S. sunscreens; 15 of those are organic, such as oxybenzone and avobenzone, and are used in chemical sunscreens. They work by absorbing the rays before they damage the skin. In addition, two inorganic filters, which are used in physical sunscreens, sit on the skin and as a shield to block the rays.
UV filters enter bodies of water by direct release, as when sunscreens rinse off people while swimming or while engaging in other water activities. They also enter bodies of water in storm water runoff and wastewater.
Lab toxicity tests, which are the most widely used, provide effects data for ecologic risk assessment. The tests are more often used in the study of short-term, not long-term exposure. Test results have shown that in high enough concentrations, some UV filters can be toxic to algal, invertebrate, and fish species.
But much information is lacking, the experts said. Toxicity data for many species, for instance, are limited. There are few studies on the longer-term environmental effects of UV filter exposure. Not enough is known about the rate at which the filters degrade in the environment. The filters accumulate in higher amounts in different areas. Recreational water areas have higher concentrations.
The recommendations
The panel is urging the EPA to complete a formal risk assessment of the UV filters “with some urgency,” Dr. Cullen said. That will enable decisions to be made about the use of the products. The risks to aquatic life must be balanced against the need for sun protection to reduce skin cancer risk.
The experts made two recommendations:
- The EPA should conduct ecologic risk assessments for all the UV filters now marketed and for all new ones. The assessment should evaluate the filters individually as well as the risk from co-occurring filters. The assessments should take into account the different exposure scenarios.
- The EPA, along with partner agencies, and sunscreen and UV filter manufacturers should fund, support, and conduct research and share data. Research should include study of human health outcomes if usage and availability of sunscreens change.
Dermatologists should “continue to emphasize the importance of protection from UV radiation in every way that can be done,” Dr. Cullen said, including the use of sunscreen as well as other protective practices, such as wearing long sleeves and hats, seeking shade, and avoiding the sun during peak hours.
A dermatologist’s perspective
“I applaud their scientific curiosity to know one way or the other whether this is an issue,” said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, DC. “I welcome this investigation.”
The multitude of studies, Dr. Friedman said, don’t always agree about whether the filters pose dangers. He noted that the concentration of UV filters detected in water is often lower than the concentrations found to be harmful in a lab setting to marine life, specifically coral.
However, he said, “these studies are snapshots.” For that reason, calling for more assessment of risk is desirable, Dr. Friedman said, but “I want to be sure the call to do more research is not an admission of guilt. It’s very easy to vilify sunscreens – but the facts we know are that UV light causes skin cancer and aging, and sunscreen protects us against this.”
Dr. Friedman has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The , an expert panel of the National Academies of Sciences, Engineering, and Medicine (NAS) said on Aug. 9.
The assessment is urgently needed, the experts said, and the results should be shared with the Food and Drug Administration, which oversees sunscreens.
In its 400-page report, titled the Review of Fate, Exposure, and Effects of Sunscreens in Aquatic Environments and Implications for Sunscreen Usage and Human Health, the panel does not make recommendations but suggests that such an EPA risk assessment should highlight gaps in knowledge.
“We are teeing up the critical information that will be used to take on the challenge of risk assessment,” Charles A. Menzie, PhD, chair of the committee that wrote the report, said at a media briefing Aug. 9 when the report was released. Dr. Menzie is a principal at Exponent, Inc., an engineering and scientific consulting firm. He is former executive director of the Society of Environmental Toxicology and Chemistry.
The EPA sponsored the study, which was conducted by a committee of the National Academy of Sciences, a nonprofit, nongovernmental organization authorized by Congress that studies issues related to science, technology, and medicine.
Balancing aquatic, human health concerns
Such an EPA assessment, Dr. Menzie said in a statement, will help inform efforts to understand the environmental effects of UV filters as well as clarify a path forward for managing sunscreens. For years, concerns have been raised about the potential toxicity of sunscreens regarding many marine and freshwater aquatic organisms, especially coral. That concern, however, must be balanced against the benefits of sunscreens, which are known to protect against skin cancer. A low percentage of people use sunscreen regularly, Dr. Menzie and other panel members said.
“Only about a third of the U.S. population regularly uses sunscreen,” Mark Cullen, MD, vice chair of the NAS committee and former director of the Center for Population Health Sciences, Stanford (Calif.) University, said at the briefing. About 70% or 80% of people use it at the beach or outdoors, he said.
Report background, details
UV filters are the active ingredients in physical as well as chemical sunscreen products. They decrease the amount of UV radiation that reaches the skin. They have been found in water, sediments, and marine organisms, both saltwater and freshwater.
Currently, 17 UV filters are used in U.S. sunscreens; 15 of those are organic, such as oxybenzone and avobenzone, and are used in chemical sunscreens. They work by absorbing the rays before they damage the skin. In addition, two inorganic filters, which are used in physical sunscreens, sit on the skin and as a shield to block the rays.
UV filters enter bodies of water by direct release, as when sunscreens rinse off people while swimming or while engaging in other water activities. They also enter bodies of water in storm water runoff and wastewater.
Lab toxicity tests, which are the most widely used, provide effects data for ecologic risk assessment. The tests are more often used in the study of short-term, not long-term exposure. Test results have shown that in high enough concentrations, some UV filters can be toxic to algal, invertebrate, and fish species.
But much information is lacking, the experts said. Toxicity data for many species, for instance, are limited. There are few studies on the longer-term environmental effects of UV filter exposure. Not enough is known about the rate at which the filters degrade in the environment. The filters accumulate in higher amounts in different areas. Recreational water areas have higher concentrations.
The recommendations
The panel is urging the EPA to complete a formal risk assessment of the UV filters “with some urgency,” Dr. Cullen said. That will enable decisions to be made about the use of the products. The risks to aquatic life must be balanced against the need for sun protection to reduce skin cancer risk.
The experts made two recommendations:
- The EPA should conduct ecologic risk assessments for all the UV filters now marketed and for all new ones. The assessment should evaluate the filters individually as well as the risk from co-occurring filters. The assessments should take into account the different exposure scenarios.
- The EPA, along with partner agencies, and sunscreen and UV filter manufacturers should fund, support, and conduct research and share data. Research should include study of human health outcomes if usage and availability of sunscreens change.
Dermatologists should “continue to emphasize the importance of protection from UV radiation in every way that can be done,” Dr. Cullen said, including the use of sunscreen as well as other protective practices, such as wearing long sleeves and hats, seeking shade, and avoiding the sun during peak hours.
A dermatologist’s perspective
“I applaud their scientific curiosity to know one way or the other whether this is an issue,” said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, DC. “I welcome this investigation.”
The multitude of studies, Dr. Friedman said, don’t always agree about whether the filters pose dangers. He noted that the concentration of UV filters detected in water is often lower than the concentrations found to be harmful in a lab setting to marine life, specifically coral.
However, he said, “these studies are snapshots.” For that reason, calling for more assessment of risk is desirable, Dr. Friedman said, but “I want to be sure the call to do more research is not an admission of guilt. It’s very easy to vilify sunscreens – but the facts we know are that UV light causes skin cancer and aging, and sunscreen protects us against this.”
Dr. Friedman has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Federal Health Care Data Trends 2022
Federal Health Care Data Trends (click to view the digital edition) is a special supplement to Federal Practitioner highlighting the latest research and study outcomes related to the health of veteran and active-duty populations.
In this issue:
- Vaccinations
- Mental Health and Related Disorders
- LGBTQ+ Veterans
- Military Sexual Trauma
- Sleep Disorders
- Respiratory Illnesses
- HIV Care in the VA
- Rheumatologic Diseases
- The Cancer-Obesity Connection
- Skin Health for Active-Duty Personnel
- Contraception
- Chronic Kidney Disease
- Cardiovascular Diseases
- Neurologic Disorders
- Hearing, Vision, and Balance
Federal Practitioner would like to thank the following experts for their review of content and helpful guidance in developing this issue:
Kelvin N.V. Bush, MD, FACC, CCDS; Sonya Borrero, MD, MS; Kenneth L. Cameron, PhD, MPH, ATC, FNATA; Jason DeViva, PhD; Ellen Lockard Edens, MD; Leonard E. Egede, MD, MS; Amy Justice, MD, PhD; Stephanie Knudson, MD; Willis H. Lyford, MD; Sarah O. Meadows, PhD; Tamara Schult, PhD, MPH; Eric L. Singman, MD, PhD; Art Wallace, MD, PhD; Elizabeth Waterhouse, MD, FAAN
Federal Health Care Data Trends (click to view the digital edition) is a special supplement to Federal Practitioner highlighting the latest research and study outcomes related to the health of veteran and active-duty populations.
In this issue:
- Vaccinations
- Mental Health and Related Disorders
- LGBTQ+ Veterans
- Military Sexual Trauma
- Sleep Disorders
- Respiratory Illnesses
- HIV Care in the VA
- Rheumatologic Diseases
- The Cancer-Obesity Connection
- Skin Health for Active-Duty Personnel
- Contraception
- Chronic Kidney Disease
- Cardiovascular Diseases
- Neurologic Disorders
- Hearing, Vision, and Balance
Federal Practitioner would like to thank the following experts for their review of content and helpful guidance in developing this issue:
Kelvin N.V. Bush, MD, FACC, CCDS; Sonya Borrero, MD, MS; Kenneth L. Cameron, PhD, MPH, ATC, FNATA; Jason DeViva, PhD; Ellen Lockard Edens, MD; Leonard E. Egede, MD, MS; Amy Justice, MD, PhD; Stephanie Knudson, MD; Willis H. Lyford, MD; Sarah O. Meadows, PhD; Tamara Schult, PhD, MPH; Eric L. Singman, MD, PhD; Art Wallace, MD, PhD; Elizabeth Waterhouse, MD, FAAN
Federal Health Care Data Trends (click to view the digital edition) is a special supplement to Federal Practitioner highlighting the latest research and study outcomes related to the health of veteran and active-duty populations.
In this issue:
- Vaccinations
- Mental Health and Related Disorders
- LGBTQ+ Veterans
- Military Sexual Trauma
- Sleep Disorders
- Respiratory Illnesses
- HIV Care in the VA
- Rheumatologic Diseases
- The Cancer-Obesity Connection
- Skin Health for Active-Duty Personnel
- Contraception
- Chronic Kidney Disease
- Cardiovascular Diseases
- Neurologic Disorders
- Hearing, Vision, and Balance
Federal Practitioner would like to thank the following experts for their review of content and helpful guidance in developing this issue:
Kelvin N.V. Bush, MD, FACC, CCDS; Sonya Borrero, MD, MS; Kenneth L. Cameron, PhD, MPH, ATC, FNATA; Jason DeViva, PhD; Ellen Lockard Edens, MD; Leonard E. Egede, MD, MS; Amy Justice, MD, PhD; Stephanie Knudson, MD; Willis H. Lyford, MD; Sarah O. Meadows, PhD; Tamara Schult, PhD, MPH; Eric L. Singman, MD, PhD; Art Wallace, MD, PhD; Elizabeth Waterhouse, MD, FAAN
Does hidradenitis suppurativa worsen during pregnancy?
PORTLAND, ORE. – The recurrent boils, abscesses, and nodules of the chronic inflammatory skin condition hidradenitis suppurativa (HS) may improve during pregnancy for a subset of women, but for many, pregnancy does not change the disease course and may worsen symptoms.
In addition, HS appears to be a risk factor for adverse pregnancy and maternal outcomes.
“This is relevant, because in the United States, HS disproportionately impacts women compared with men by a ratio of about 3:1,” Jennifer Hsiao, MD, said at the annual meeting of the Pacific Dermatologic Association.
“Also, the highest prevalence of HS is among people in their 20s and 30s, so in their practice, clinicians will encounter female patients with HS who are either pregnant or actively thinking about getting pregnant,” she said.
During a wide-ranging presentation, Dr. Hsiao of the department of dermatology at the University of Southern California, Los Angeles, described the impact of pregnancy on HS, identified appropriate treatment options for this population of patients, and discussed HS comorbidities that may be exacerbated during pregnancy.
She began by noting that levels of progesterone and estrogen both rise during pregnancy. Progesterone is known to suppress development and function of Th1 and Th17 T cells, but the effect of estrogen on inflammation is less well known. At the same time, serum levels of interleukin (IL)-1 receptor antagonist and soluble TNF-alpha receptor both increase during pregnancy.
“This would lead to serum IL-1 and TNF-alpha falling, sort of like the way that we give anti–IL-1 and TNF blockers as HS treatments,” she explained. “So, presumably that might be helpful during HS in pregnancy. On the flip side, pregnancy weight gain can exacerbate HS, with increased friction between skin folds. In addition, just having more adipocytes can promote secretion of proinflammatory cytokines like TNF-alpha.”
To better understand the effect of pregnancy on patients with HS, Dr. Hsiao and colleagues conducted a systematic review and meta-analysis on the topic published in Dermatology. They included eight studies in which a total of 672 patients self-reported their HS disease course during pregnancy and 164 self-reported whether they had a postpartum HS flare or not. On pooled analyses, HS improved in 24% of patients but worsened in 20%. In addition, 60% of patients experienced a postpartum flare.
“So, at this point in time, based on the literature, it would be fair to tell your patient that during pregnancy, HS has a mixed response,” Dr. Hsiao said. “About 25% may have improvement, but for the rest, HS symptoms may be unchanged or even worsen. That’s why it’s so important to be in contact with your pregnant patients, because not only may they have to stay on treatment, but they might also have to escalate [their treatment] during pregnancy.”
Lifestyle modifications to discuss with pregnant HS patients include appropriate weight gain during pregnancy, smoking cessation, and avoidance of tight-fitting clothing, “since friction can make things worse,” she said. Topical antibiotics safe to use during pregnancy for patients with mild HS include clindamycin 1%, erythromycin 2%, and metronidazole 0.75% applied twice per day to active lesions, she continued.
As for systemic therapies, some data exist to support the use of metformin 500 mg once daily, titrating up to twice or – if needed and tolerated – three times daily for patients with mild to moderate HS, she said, referencing a paper published in the Journal of the European Academy of Dermatology and Venereology.
Zinc gluconate is another potential option. Of 22 nonpregnant HS patients with Hurley stage I-II disease who were treated with zinc gluconate 90 mg daily, 8 had a complete remission of HS and 14 had partial remission, according to a report in Dermatology.
“Zinc supplementation of up to 50 mg daily has shown no effect on neonatal or maternal outcomes at birth based on existing medical literature,” Dr. Hsiao added.
Among antibiotics, injections of intralesional Kenalog 5-10 mg/mL have been shown to decrease pain and inflammation in acute HS lesions and are unlikely to pose significant risks during pregnancy, but a course of systemic antibiotics may be warranted in moderate to severe disease, she said. These include, but are not limited to, clindamycin, erythromycin base, cephalexin, or metronidazole.
“In addition, some of my HS colleagues and I will also use other antibiotics such as Augmentin [amoxicillin/clavulanate] or cefdinir for HS and these are also generally considered safe to use in pregnancy,” she said. “Caution is advised with using rifampin, dapsone, and moxifloxacin during pregnancy.”
As for biologic agents, the first-line option is adalimumab, which is currently the only Food and Drug Administration–approved treatment for HS.
“There is also good efficacy data for infliximab,” she said. “Etanercept has less placental transfer than adalimumab or infliximab so it’s safer to use in pregnancy, but it has inconsistent data for efficacy in HS, so I would generally avoid using it to treat HS and reach for adalimumab or infliximab instead.”
Data on TNF-alpha inhibitors from the GI and rheumatology literature have demonstrated that there is minimal placental transport of maternal antibodies during the first two trimesters of pregnancy.
“It’s at the beginning of the third trimester that the placental transfer of antibodies picks up,” she said. “At that point in time, you can have a discussion with the patient: do you want to stay on treatment and treat through, or do you want to consider being taken off the medication? I think this is a discussion that needs to be had, because let’s say you peel off adalimumab or infliximab and they have severe HS flares. I’m not sure that leads to a better outcome. I usually treat through for my pregnant patients.”
To better understand clinician practice patterns on the management of HS in pregnancy, Dr. Hsiao and Erin Collier, MD, MPH, of University of California, Los Angeles, and colleagues distributed an online survey to HS specialists in North America. They reported the findings in the International Journal of Women’s Dermatology.
Of the 49 respondents, 36 (73%) directed an HS specialty clinic and 29 (59%) reported having prescribed or continued a biologic agent in a pregnant HS patient. The top three biologics prescribed were adalimumab (90%), infliximab (41%), and certolizumab pegol (34%). Dr. Hsiao noted that certolizumab pegol is a pegylated anti-TNF, so it lacks an Fc region on the medication.
“This means that it cannot be actively transported by the neonatal Fc receptor on the placenta, thus resulting in minimal placental transmission,” she said. “The main issue is that there is little data on its efficacy in HS, but it’s a reasonable option to consider in a pregnant patient, especially in a patient with severe HS who asks, ‘what’s the safest biologic that I can go on?’ But you’d have to discuss with the patient that in terms of efficacy data, there is much less in the literature compared to adalimumab or infliximab.”
Breastfeeding while on anti–TNF-alpha biologics is considered safe. “There are minimal amounts of medication in breast milk,” she said. “If any gets through, infant gastric digestion is thought to take care of the rest. Of note, babies born to mothers who are continually treated with biologic agents should not be given live vaccinations for 6 months after birth.”
In a single-center study, Dr. Hsiao and colleagues retrospectively examined pregnancy complications, pregnancy outcomes, and neonatal outcomes in patients with HS. The study population included 202 pregnancies in 127 HS patients. Of 134 babies born to mothers with HS, 74% were breastfed and 24% were bottle-fed, and presence of HS lesions on the breast was significantly associated with not breastfeeding.
“So, when we see these patients, if moms decide to breastfeed and they have lesions on the breast, it would be helpful to discuss expectations and perhaps treat HS breast lesions early, so the breastfeeding process may go more smoothly for them after they deliver,” said Dr. Hsiao, who is one of the editors of the textbook “A Comprehensive Guide to Hidradenitis Suppurativa” (Elsevier, 2021). Safety-related resources that she recommends for clinicians include Mother to Baby and the Drugs and Lactation Database (LactMed).
Dr. Hsiao concluded her presentation by spotlighting the influence of pregnancy on HS comorbidities. Patients with HS already have a higher prevalence of depression and anxiety compared to controls. “Pregnancy can exacerbate underlying mood disorders in patients,” she said. “That’s why monitoring the patient’s mood and coordinating mental health care with the patient’s primary care physician and ob.gyn. is important.”
In addition, pregnancy-related changes in body mass index, blood pressure, lipid metabolism, and glucose tolerance trend toward changes seen in metabolic syndrome, she said, and HS patients are already at higher risk of metabolic syndrome compared with the general population.
HS may also compromise a patient’s ability to have a healthy pregnancy. Dr. Hsiao worked with Amit Garg, MD, and colleagues on a study that drew from the IBM MarketScan Commercial Claims Database to evaluate adverse pregnancy and maternal outcomes in women with HS between Jan. 1, 2011, and Sept. 30, 2015.
After the researchers adjusted for age, race, smoking status, and other comorbidities, they found that HS pregnancies were independently associated with spontaneous abortion (odds ratio, 1.20), gestational diabetes (OR, 1.26), and cesarean section (OR, 1.09). The findings were published in the Journal of the American Academy of Dermatology.
A separate study that used the same database found comparable results, also published in the Journal of the American Academy of Dermatology. “What I say to patients right now is, ‘there are many women with HS who have healthy pregnancies and deliver healthy babies, but HS could be a risk factor for a higher-risk pregnancy.’ It’s important that these patients are established with an ob.gyn. and are closely monitored to make sure that we optimize their care and give them the best outcome possible for mom and baby.”
Dr. Hsiao disclosed that she is on the board of directors for the Hidradenitis Suppurativa Foundation. She has also served as an advisor for Novartis, UCB, and Boehringer Ingelheim and as a speaker and advisor for AbbVie.
PORTLAND, ORE. – The recurrent boils, abscesses, and nodules of the chronic inflammatory skin condition hidradenitis suppurativa (HS) may improve during pregnancy for a subset of women, but for many, pregnancy does not change the disease course and may worsen symptoms.
In addition, HS appears to be a risk factor for adverse pregnancy and maternal outcomes.
“This is relevant, because in the United States, HS disproportionately impacts women compared with men by a ratio of about 3:1,” Jennifer Hsiao, MD, said at the annual meeting of the Pacific Dermatologic Association.
“Also, the highest prevalence of HS is among people in their 20s and 30s, so in their practice, clinicians will encounter female patients with HS who are either pregnant or actively thinking about getting pregnant,” she said.
During a wide-ranging presentation, Dr. Hsiao of the department of dermatology at the University of Southern California, Los Angeles, described the impact of pregnancy on HS, identified appropriate treatment options for this population of patients, and discussed HS comorbidities that may be exacerbated during pregnancy.
She began by noting that levels of progesterone and estrogen both rise during pregnancy. Progesterone is known to suppress development and function of Th1 and Th17 T cells, but the effect of estrogen on inflammation is less well known. At the same time, serum levels of interleukin (IL)-1 receptor antagonist and soluble TNF-alpha receptor both increase during pregnancy.
“This would lead to serum IL-1 and TNF-alpha falling, sort of like the way that we give anti–IL-1 and TNF blockers as HS treatments,” she explained. “So, presumably that might be helpful during HS in pregnancy. On the flip side, pregnancy weight gain can exacerbate HS, with increased friction between skin folds. In addition, just having more adipocytes can promote secretion of proinflammatory cytokines like TNF-alpha.”
To better understand the effect of pregnancy on patients with HS, Dr. Hsiao and colleagues conducted a systematic review and meta-analysis on the topic published in Dermatology. They included eight studies in which a total of 672 patients self-reported their HS disease course during pregnancy and 164 self-reported whether they had a postpartum HS flare or not. On pooled analyses, HS improved in 24% of patients but worsened in 20%. In addition, 60% of patients experienced a postpartum flare.
“So, at this point in time, based on the literature, it would be fair to tell your patient that during pregnancy, HS has a mixed response,” Dr. Hsiao said. “About 25% may have improvement, but for the rest, HS symptoms may be unchanged or even worsen. That’s why it’s so important to be in contact with your pregnant patients, because not only may they have to stay on treatment, but they might also have to escalate [their treatment] during pregnancy.”
Lifestyle modifications to discuss with pregnant HS patients include appropriate weight gain during pregnancy, smoking cessation, and avoidance of tight-fitting clothing, “since friction can make things worse,” she said. Topical antibiotics safe to use during pregnancy for patients with mild HS include clindamycin 1%, erythromycin 2%, and metronidazole 0.75% applied twice per day to active lesions, she continued.
As for systemic therapies, some data exist to support the use of metformin 500 mg once daily, titrating up to twice or – if needed and tolerated – three times daily for patients with mild to moderate HS, she said, referencing a paper published in the Journal of the European Academy of Dermatology and Venereology.
Zinc gluconate is another potential option. Of 22 nonpregnant HS patients with Hurley stage I-II disease who were treated with zinc gluconate 90 mg daily, 8 had a complete remission of HS and 14 had partial remission, according to a report in Dermatology.
“Zinc supplementation of up to 50 mg daily has shown no effect on neonatal or maternal outcomes at birth based on existing medical literature,” Dr. Hsiao added.
Among antibiotics, injections of intralesional Kenalog 5-10 mg/mL have been shown to decrease pain and inflammation in acute HS lesions and are unlikely to pose significant risks during pregnancy, but a course of systemic antibiotics may be warranted in moderate to severe disease, she said. These include, but are not limited to, clindamycin, erythromycin base, cephalexin, or metronidazole.
“In addition, some of my HS colleagues and I will also use other antibiotics such as Augmentin [amoxicillin/clavulanate] or cefdinir for HS and these are also generally considered safe to use in pregnancy,” she said. “Caution is advised with using rifampin, dapsone, and moxifloxacin during pregnancy.”
As for biologic agents, the first-line option is adalimumab, which is currently the only Food and Drug Administration–approved treatment for HS.
“There is also good efficacy data for infliximab,” she said. “Etanercept has less placental transfer than adalimumab or infliximab so it’s safer to use in pregnancy, but it has inconsistent data for efficacy in HS, so I would generally avoid using it to treat HS and reach for adalimumab or infliximab instead.”
Data on TNF-alpha inhibitors from the GI and rheumatology literature have demonstrated that there is minimal placental transport of maternal antibodies during the first two trimesters of pregnancy.
“It’s at the beginning of the third trimester that the placental transfer of antibodies picks up,” she said. “At that point in time, you can have a discussion with the patient: do you want to stay on treatment and treat through, or do you want to consider being taken off the medication? I think this is a discussion that needs to be had, because let’s say you peel off adalimumab or infliximab and they have severe HS flares. I’m not sure that leads to a better outcome. I usually treat through for my pregnant patients.”
To better understand clinician practice patterns on the management of HS in pregnancy, Dr. Hsiao and Erin Collier, MD, MPH, of University of California, Los Angeles, and colleagues distributed an online survey to HS specialists in North America. They reported the findings in the International Journal of Women’s Dermatology.
Of the 49 respondents, 36 (73%) directed an HS specialty clinic and 29 (59%) reported having prescribed or continued a biologic agent in a pregnant HS patient. The top three biologics prescribed were adalimumab (90%), infliximab (41%), and certolizumab pegol (34%). Dr. Hsiao noted that certolizumab pegol is a pegylated anti-TNF, so it lacks an Fc region on the medication.
“This means that it cannot be actively transported by the neonatal Fc receptor on the placenta, thus resulting in minimal placental transmission,” she said. “The main issue is that there is little data on its efficacy in HS, but it’s a reasonable option to consider in a pregnant patient, especially in a patient with severe HS who asks, ‘what’s the safest biologic that I can go on?’ But you’d have to discuss with the patient that in terms of efficacy data, there is much less in the literature compared to adalimumab or infliximab.”
Breastfeeding while on anti–TNF-alpha biologics is considered safe. “There are minimal amounts of medication in breast milk,” she said. “If any gets through, infant gastric digestion is thought to take care of the rest. Of note, babies born to mothers who are continually treated with biologic agents should not be given live vaccinations for 6 months after birth.”
In a single-center study, Dr. Hsiao and colleagues retrospectively examined pregnancy complications, pregnancy outcomes, and neonatal outcomes in patients with HS. The study population included 202 pregnancies in 127 HS patients. Of 134 babies born to mothers with HS, 74% were breastfed and 24% were bottle-fed, and presence of HS lesions on the breast was significantly associated with not breastfeeding.
“So, when we see these patients, if moms decide to breastfeed and they have lesions on the breast, it would be helpful to discuss expectations and perhaps treat HS breast lesions early, so the breastfeeding process may go more smoothly for them after they deliver,” said Dr. Hsiao, who is one of the editors of the textbook “A Comprehensive Guide to Hidradenitis Suppurativa” (Elsevier, 2021). Safety-related resources that she recommends for clinicians include Mother to Baby and the Drugs and Lactation Database (LactMed).
Dr. Hsiao concluded her presentation by spotlighting the influence of pregnancy on HS comorbidities. Patients with HS already have a higher prevalence of depression and anxiety compared to controls. “Pregnancy can exacerbate underlying mood disorders in patients,” she said. “That’s why monitoring the patient’s mood and coordinating mental health care with the patient’s primary care physician and ob.gyn. is important.”
In addition, pregnancy-related changes in body mass index, blood pressure, lipid metabolism, and glucose tolerance trend toward changes seen in metabolic syndrome, she said, and HS patients are already at higher risk of metabolic syndrome compared with the general population.
HS may also compromise a patient’s ability to have a healthy pregnancy. Dr. Hsiao worked with Amit Garg, MD, and colleagues on a study that drew from the IBM MarketScan Commercial Claims Database to evaluate adverse pregnancy and maternal outcomes in women with HS between Jan. 1, 2011, and Sept. 30, 2015.
After the researchers adjusted for age, race, smoking status, and other comorbidities, they found that HS pregnancies were independently associated with spontaneous abortion (odds ratio, 1.20), gestational diabetes (OR, 1.26), and cesarean section (OR, 1.09). The findings were published in the Journal of the American Academy of Dermatology.
A separate study that used the same database found comparable results, also published in the Journal of the American Academy of Dermatology. “What I say to patients right now is, ‘there are many women with HS who have healthy pregnancies and deliver healthy babies, but HS could be a risk factor for a higher-risk pregnancy.’ It’s important that these patients are established with an ob.gyn. and are closely monitored to make sure that we optimize their care and give them the best outcome possible for mom and baby.”
Dr. Hsiao disclosed that she is on the board of directors for the Hidradenitis Suppurativa Foundation. She has also served as an advisor for Novartis, UCB, and Boehringer Ingelheim and as a speaker and advisor for AbbVie.
PORTLAND, ORE. – The recurrent boils, abscesses, and nodules of the chronic inflammatory skin condition hidradenitis suppurativa (HS) may improve during pregnancy for a subset of women, but for many, pregnancy does not change the disease course and may worsen symptoms.
In addition, HS appears to be a risk factor for adverse pregnancy and maternal outcomes.
“This is relevant, because in the United States, HS disproportionately impacts women compared with men by a ratio of about 3:1,” Jennifer Hsiao, MD, said at the annual meeting of the Pacific Dermatologic Association.
“Also, the highest prevalence of HS is among people in their 20s and 30s, so in their practice, clinicians will encounter female patients with HS who are either pregnant or actively thinking about getting pregnant,” she said.
During a wide-ranging presentation, Dr. Hsiao of the department of dermatology at the University of Southern California, Los Angeles, described the impact of pregnancy on HS, identified appropriate treatment options for this population of patients, and discussed HS comorbidities that may be exacerbated during pregnancy.
She began by noting that levels of progesterone and estrogen both rise during pregnancy. Progesterone is known to suppress development and function of Th1 and Th17 T cells, but the effect of estrogen on inflammation is less well known. At the same time, serum levels of interleukin (IL)-1 receptor antagonist and soluble TNF-alpha receptor both increase during pregnancy.
“This would lead to serum IL-1 and TNF-alpha falling, sort of like the way that we give anti–IL-1 and TNF blockers as HS treatments,” she explained. “So, presumably that might be helpful during HS in pregnancy. On the flip side, pregnancy weight gain can exacerbate HS, with increased friction between skin folds. In addition, just having more adipocytes can promote secretion of proinflammatory cytokines like TNF-alpha.”
To better understand the effect of pregnancy on patients with HS, Dr. Hsiao and colleagues conducted a systematic review and meta-analysis on the topic published in Dermatology. They included eight studies in which a total of 672 patients self-reported their HS disease course during pregnancy and 164 self-reported whether they had a postpartum HS flare or not. On pooled analyses, HS improved in 24% of patients but worsened in 20%. In addition, 60% of patients experienced a postpartum flare.
“So, at this point in time, based on the literature, it would be fair to tell your patient that during pregnancy, HS has a mixed response,” Dr. Hsiao said. “About 25% may have improvement, but for the rest, HS symptoms may be unchanged or even worsen. That’s why it’s so important to be in contact with your pregnant patients, because not only may they have to stay on treatment, but they might also have to escalate [their treatment] during pregnancy.”
Lifestyle modifications to discuss with pregnant HS patients include appropriate weight gain during pregnancy, smoking cessation, and avoidance of tight-fitting clothing, “since friction can make things worse,” she said. Topical antibiotics safe to use during pregnancy for patients with mild HS include clindamycin 1%, erythromycin 2%, and metronidazole 0.75% applied twice per day to active lesions, she continued.
As for systemic therapies, some data exist to support the use of metformin 500 mg once daily, titrating up to twice or – if needed and tolerated – three times daily for patients with mild to moderate HS, she said, referencing a paper published in the Journal of the European Academy of Dermatology and Venereology.
Zinc gluconate is another potential option. Of 22 nonpregnant HS patients with Hurley stage I-II disease who were treated with zinc gluconate 90 mg daily, 8 had a complete remission of HS and 14 had partial remission, according to a report in Dermatology.
“Zinc supplementation of up to 50 mg daily has shown no effect on neonatal or maternal outcomes at birth based on existing medical literature,” Dr. Hsiao added.
Among antibiotics, injections of intralesional Kenalog 5-10 mg/mL have been shown to decrease pain and inflammation in acute HS lesions and are unlikely to pose significant risks during pregnancy, but a course of systemic antibiotics may be warranted in moderate to severe disease, she said. These include, but are not limited to, clindamycin, erythromycin base, cephalexin, or metronidazole.
“In addition, some of my HS colleagues and I will also use other antibiotics such as Augmentin [amoxicillin/clavulanate] or cefdinir for HS and these are also generally considered safe to use in pregnancy,” she said. “Caution is advised with using rifampin, dapsone, and moxifloxacin during pregnancy.”
As for biologic agents, the first-line option is adalimumab, which is currently the only Food and Drug Administration–approved treatment for HS.
“There is also good efficacy data for infliximab,” she said. “Etanercept has less placental transfer than adalimumab or infliximab so it’s safer to use in pregnancy, but it has inconsistent data for efficacy in HS, so I would generally avoid using it to treat HS and reach for adalimumab or infliximab instead.”
Data on TNF-alpha inhibitors from the GI and rheumatology literature have demonstrated that there is minimal placental transport of maternal antibodies during the first two trimesters of pregnancy.
“It’s at the beginning of the third trimester that the placental transfer of antibodies picks up,” she said. “At that point in time, you can have a discussion with the patient: do you want to stay on treatment and treat through, or do you want to consider being taken off the medication? I think this is a discussion that needs to be had, because let’s say you peel off adalimumab or infliximab and they have severe HS flares. I’m not sure that leads to a better outcome. I usually treat through for my pregnant patients.”
To better understand clinician practice patterns on the management of HS in pregnancy, Dr. Hsiao and Erin Collier, MD, MPH, of University of California, Los Angeles, and colleagues distributed an online survey to HS specialists in North America. They reported the findings in the International Journal of Women’s Dermatology.
Of the 49 respondents, 36 (73%) directed an HS specialty clinic and 29 (59%) reported having prescribed or continued a biologic agent in a pregnant HS patient. The top three biologics prescribed were adalimumab (90%), infliximab (41%), and certolizumab pegol (34%). Dr. Hsiao noted that certolizumab pegol is a pegylated anti-TNF, so it lacks an Fc region on the medication.
“This means that it cannot be actively transported by the neonatal Fc receptor on the placenta, thus resulting in minimal placental transmission,” she said. “The main issue is that there is little data on its efficacy in HS, but it’s a reasonable option to consider in a pregnant patient, especially in a patient with severe HS who asks, ‘what’s the safest biologic that I can go on?’ But you’d have to discuss with the patient that in terms of efficacy data, there is much less in the literature compared to adalimumab or infliximab.”
Breastfeeding while on anti–TNF-alpha biologics is considered safe. “There are minimal amounts of medication in breast milk,” she said. “If any gets through, infant gastric digestion is thought to take care of the rest. Of note, babies born to mothers who are continually treated with biologic agents should not be given live vaccinations for 6 months after birth.”
In a single-center study, Dr. Hsiao and colleagues retrospectively examined pregnancy complications, pregnancy outcomes, and neonatal outcomes in patients with HS. The study population included 202 pregnancies in 127 HS patients. Of 134 babies born to mothers with HS, 74% were breastfed and 24% were bottle-fed, and presence of HS lesions on the breast was significantly associated with not breastfeeding.
“So, when we see these patients, if moms decide to breastfeed and they have lesions on the breast, it would be helpful to discuss expectations and perhaps treat HS breast lesions early, so the breastfeeding process may go more smoothly for them after they deliver,” said Dr. Hsiao, who is one of the editors of the textbook “A Comprehensive Guide to Hidradenitis Suppurativa” (Elsevier, 2021). Safety-related resources that she recommends for clinicians include Mother to Baby and the Drugs and Lactation Database (LactMed).
Dr. Hsiao concluded her presentation by spotlighting the influence of pregnancy on HS comorbidities. Patients with HS already have a higher prevalence of depression and anxiety compared to controls. “Pregnancy can exacerbate underlying mood disorders in patients,” she said. “That’s why monitoring the patient’s mood and coordinating mental health care with the patient’s primary care physician and ob.gyn. is important.”
In addition, pregnancy-related changes in body mass index, blood pressure, lipid metabolism, and glucose tolerance trend toward changes seen in metabolic syndrome, she said, and HS patients are already at higher risk of metabolic syndrome compared with the general population.
HS may also compromise a patient’s ability to have a healthy pregnancy. Dr. Hsiao worked with Amit Garg, MD, and colleagues on a study that drew from the IBM MarketScan Commercial Claims Database to evaluate adverse pregnancy and maternal outcomes in women with HS between Jan. 1, 2011, and Sept. 30, 2015.
After the researchers adjusted for age, race, smoking status, and other comorbidities, they found that HS pregnancies were independently associated with spontaneous abortion (odds ratio, 1.20), gestational diabetes (OR, 1.26), and cesarean section (OR, 1.09). The findings were published in the Journal of the American Academy of Dermatology.
A separate study that used the same database found comparable results, also published in the Journal of the American Academy of Dermatology. “What I say to patients right now is, ‘there are many women with HS who have healthy pregnancies and deliver healthy babies, but HS could be a risk factor for a higher-risk pregnancy.’ It’s important that these patients are established with an ob.gyn. and are closely monitored to make sure that we optimize their care and give them the best outcome possible for mom and baby.”
Dr. Hsiao disclosed that she is on the board of directors for the Hidradenitis Suppurativa Foundation. She has also served as an advisor for Novartis, UCB, and Boehringer Ingelheim and as a speaker and advisor for AbbVie.
AT PDA 2022
Popliteal plaques
Both a Wood lamp examination and a potassium hydroxide (KOH) prep returned negative results. Those findings, combined with the patient’s month-long antifungal medication adherence, helped to rule out other diagnoses. Based on history and examination, the patient was diagnosed with erythrasma.
Erythrasma is a skin infection caused by the gram-positive bacteria Corynebacterium minutissimum1 that usually manifests in moist intertriginous areas. Sometimes it is secondary to fungal or yeast infections, local skin irritation due to friction, or due to maceration of the skin from persistent moisture. The Wood lamp examination can show coral-red fluorescence in erythrasma, but recent bathing (as in this case) may limit this finding.1
The differential diagnosis of erythematous plaques in an intertriginous area includes inverse psoriasis. However, this patient had no nail changes, joint difficulties, or other rashes consistent with psoriasis. Macerated, erythematous inflammatory changes in intertriginous areas are always concerning for fungal infections (eg, yeast infection, tinea corporis), especially with the presence of any scale. In this case, the patient’s medication regimen helped to rule out these types of conditions.
First-line therapy for erythrasma includes topical antibiotics: clindamycin, erythromycin, mupirocin, and fusidic acid. Systemic antibiotics in the tetracycline family and macrolides may also be used but have a higher risk of adverse effects. Keeping the affected area dry is a useful adjunct to pharmacologic therapy.
The patient was treated with topical clindamycin bid for 7 days. By her 2-month follow-up appointment, there were no residual skin changes. Had the plaques persisted, the possibility of inverse psoriasis would have been revisited, with either presumptive treatment prescribed or biopsy performed to establish the diagnosis.
Photo courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Forouzan P, Cohen PR. Erythrasma revisited: diagnosis, differential diagnoses, and comprehensive review of treatment. Cureus. 2020;12:e10733. doi: 10.7759/cureus.10733

Both a Wood lamp examination and a potassium hydroxide (KOH) prep returned negative results. Those findings, combined with the patient’s month-long antifungal medication adherence, helped to rule out other diagnoses. Based on history and examination, the patient was diagnosed with erythrasma.
Erythrasma is a skin infection caused by the gram-positive bacteria Corynebacterium minutissimum1 that usually manifests in moist intertriginous areas. Sometimes it is secondary to fungal or yeast infections, local skin irritation due to friction, or due to maceration of the skin from persistent moisture. The Wood lamp examination can show coral-red fluorescence in erythrasma, but recent bathing (as in this case) may limit this finding.1
The differential diagnosis of erythematous plaques in an intertriginous area includes inverse psoriasis. However, this patient had no nail changes, joint difficulties, or other rashes consistent with psoriasis. Macerated, erythematous inflammatory changes in intertriginous areas are always concerning for fungal infections (eg, yeast infection, tinea corporis), especially with the presence of any scale. In this case, the patient’s medication regimen helped to rule out these types of conditions.
First-line therapy for erythrasma includes topical antibiotics: clindamycin, erythromycin, mupirocin, and fusidic acid. Systemic antibiotics in the tetracycline family and macrolides may also be used but have a higher risk of adverse effects. Keeping the affected area dry is a useful adjunct to pharmacologic therapy.
The patient was treated with topical clindamycin bid for 7 days. By her 2-month follow-up appointment, there were no residual skin changes. Had the plaques persisted, the possibility of inverse psoriasis would have been revisited, with either presumptive treatment prescribed or biopsy performed to establish the diagnosis.
Photo courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

Both a Wood lamp examination and a potassium hydroxide (KOH) prep returned negative results. Those findings, combined with the patient’s month-long antifungal medication adherence, helped to rule out other diagnoses. Based on history and examination, the patient was diagnosed with erythrasma.
Erythrasma is a skin infection caused by the gram-positive bacteria Corynebacterium minutissimum1 that usually manifests in moist intertriginous areas. Sometimes it is secondary to fungal or yeast infections, local skin irritation due to friction, or due to maceration of the skin from persistent moisture. The Wood lamp examination can show coral-red fluorescence in erythrasma, but recent bathing (as in this case) may limit this finding.1
The differential diagnosis of erythematous plaques in an intertriginous area includes inverse psoriasis. However, this patient had no nail changes, joint difficulties, or other rashes consistent with psoriasis. Macerated, erythematous inflammatory changes in intertriginous areas are always concerning for fungal infections (eg, yeast infection, tinea corporis), especially with the presence of any scale. In this case, the patient’s medication regimen helped to rule out these types of conditions.
First-line therapy for erythrasma includes topical antibiotics: clindamycin, erythromycin, mupirocin, and fusidic acid. Systemic antibiotics in the tetracycline family and macrolides may also be used but have a higher risk of adverse effects. Keeping the affected area dry is a useful adjunct to pharmacologic therapy.
The patient was treated with topical clindamycin bid for 7 days. By her 2-month follow-up appointment, there were no residual skin changes. Had the plaques persisted, the possibility of inverse psoriasis would have been revisited, with either presumptive treatment prescribed or biopsy performed to establish the diagnosis.
Photo courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Forouzan P, Cohen PR. Erythrasma revisited: diagnosis, differential diagnoses, and comprehensive review of treatment. Cureus. 2020;12:e10733. doi: 10.7759/cureus.10733
1. Forouzan P, Cohen PR. Erythrasma revisited: diagnosis, differential diagnoses, and comprehensive review of treatment. Cureus. 2020;12:e10733. doi: 10.7759/cureus.10733
In one state, pandemic tamped down lice and scabies cases
.
When COVID-19 was declared a public health emergency by the World Health Organization in March 2020, many countries including the United States enacted lockdown and isolation measures to help contain the spread of the disease. Since scabies and lice are both spread by direct contact, “we hypothesized that the nationwide lockdown would influence the transmission of these two conditions among individuals,” wrote Marianne Bonanno, MD, of the University of North Carolina, Chapel Hill, and colleagues.
“The pandemic created a unique opportunity for real-life observations following physical distancing measures being put in place,” coauthor Christopher Sayed, MD, associate professor of dermatology at UNC, said in an interview. “It makes intuitive sense that since lice and scabies spread by cost physical contact that rates would decrease with school closures and other physical distancing measures. Reports from other countries in which extended families more often live together and were forced to spend more time in close quarters saw increased rates so it was interesting to see this contrast,” he noted.
In the study, the researchers reviewed data from 1,858 cases of adult scabies, 893 cases of pediatric scabies, and 804 cases of pediatric lice reported in North Carolina between March 2017 and February 2021. They compared monthly cases of scabies and lice, and prescriptions during the period before the pandemic (March 2017 to February 2020), and during the pandemic (March 2020 to February 2021).
Pediatric lice cases decreased by 60.6% over the study period (P < .001). Significant decreases also occurred in adult scabies (31.1%, P < .001) and pediatric scabies (39%, P < .01).
The number of prescriptions for lice and scabies also decreased significantly (P < .01) during the study period, although these numbers differed from the actual cases. Prescriptions decreased by 41.4%, 29.9%, and 69.3% for pediatric scabies, adult scabies, and pediatric lice, respectively.
Both pediatric scabies and pediatric lice showed a greater drop in prescriptions than in cases, while the drop in prescriptions for adult scabies was slightly less than the drop in cases.
The difference in the decreased numbers between cases and prescriptions may stem from the decrease in close contacts during the pandemic, which decreased the need for multiple prescriptions, but other potential explanations could be examined in future studies, the researchers wrote in their discussion.
The study findings were limited by several factors including the cross-sectional design and potential underdiagnosis and underreporting, as well as the focus only on a population in a single state, which may limit generalizability, the researchers noted.
However, the results offer preliminary insights on the impact of COVID-19 restrictions on scabies and lice, and suggest the potential value of physical distancing to reduce transmission of both conditions, especially in settings such as schools and prisons, to help contain future outbreaks, they concluded.
The study findings reinforce physical contact as the likely route of disease transmission, for lice and scabies, Dr. Sayed said in the interview. “It’s possible distancing measures on a small scale could be considered for outbreaks in institutional settings, though the risks of these infestations are much lower than with COVID-19,” he said. “It will be interesting to observe trends as physical distancing measures end to see if cases rebound in the next few years,” he added.
Drop in cases likely temporary
“Examining the epidemiology of different infectious diseases over time is an interesting and important area of study,” said Sheilagh Maguiness, MD, associate professor of dermatology and pediatrics at the University of Minnesota, Minneapolis, who was asked to comment on the results.
“The pandemic dramatically altered the daily lives of adults and children across the globe, and we can learn a lot from studying how social distancing and prolonged masking has made an impact on the incidence and prevalence of different infectious illnesses in the country and across the world,” she said in an interview.
Dr. Maguiness said she was not surprised by the study findings. “In fact, other countries have published similar studies documenting a reduction in both head lice and scabies infestations during the time of the pandemic,” she said. “In France, it was noted that during March to December 2020, there was a reduction in sales for topical head lice and scabies treatments of 44% and 14%, respectively. Similarly, a study from Argentina documented a decline in head lice infestations by about 25% among children,” she said.
“I personally noted a marked decrease in both of these diagnoses among children in my own clinic,” she added.
“Since both of these conditions are spread through close physical contact with others, it makes sense that there would be a steep decline in ectoparasitic infections during times of social distancing. However, anecdotally we are now diagnosing and treating these infestations again more regularly in our clinic,” said Dr. Maguiness. “As social distancing relaxes, I would expect that the incidence of both head lice and scabies will again increase.”
The study received no outside funding. The researchers and Dr. Maguiness had no financial conflicts to disclose.
.
When COVID-19 was declared a public health emergency by the World Health Organization in March 2020, many countries including the United States enacted lockdown and isolation measures to help contain the spread of the disease. Since scabies and lice are both spread by direct contact, “we hypothesized that the nationwide lockdown would influence the transmission of these two conditions among individuals,” wrote Marianne Bonanno, MD, of the University of North Carolina, Chapel Hill, and colleagues.
“The pandemic created a unique opportunity for real-life observations following physical distancing measures being put in place,” coauthor Christopher Sayed, MD, associate professor of dermatology at UNC, said in an interview. “It makes intuitive sense that since lice and scabies spread by cost physical contact that rates would decrease with school closures and other physical distancing measures. Reports from other countries in which extended families more often live together and were forced to spend more time in close quarters saw increased rates so it was interesting to see this contrast,” he noted.
In the study, the researchers reviewed data from 1,858 cases of adult scabies, 893 cases of pediatric scabies, and 804 cases of pediatric lice reported in North Carolina between March 2017 and February 2021. They compared monthly cases of scabies and lice, and prescriptions during the period before the pandemic (March 2017 to February 2020), and during the pandemic (March 2020 to February 2021).
Pediatric lice cases decreased by 60.6% over the study period (P < .001). Significant decreases also occurred in adult scabies (31.1%, P < .001) and pediatric scabies (39%, P < .01).
The number of prescriptions for lice and scabies also decreased significantly (P < .01) during the study period, although these numbers differed from the actual cases. Prescriptions decreased by 41.4%, 29.9%, and 69.3% for pediatric scabies, adult scabies, and pediatric lice, respectively.
Both pediatric scabies and pediatric lice showed a greater drop in prescriptions than in cases, while the drop in prescriptions for adult scabies was slightly less than the drop in cases.
The difference in the decreased numbers between cases and prescriptions may stem from the decrease in close contacts during the pandemic, which decreased the need for multiple prescriptions, but other potential explanations could be examined in future studies, the researchers wrote in their discussion.
The study findings were limited by several factors including the cross-sectional design and potential underdiagnosis and underreporting, as well as the focus only on a population in a single state, which may limit generalizability, the researchers noted.
However, the results offer preliminary insights on the impact of COVID-19 restrictions on scabies and lice, and suggest the potential value of physical distancing to reduce transmission of both conditions, especially in settings such as schools and prisons, to help contain future outbreaks, they concluded.
The study findings reinforce physical contact as the likely route of disease transmission, for lice and scabies, Dr. Sayed said in the interview. “It’s possible distancing measures on a small scale could be considered for outbreaks in institutional settings, though the risks of these infestations are much lower than with COVID-19,” he said. “It will be interesting to observe trends as physical distancing measures end to see if cases rebound in the next few years,” he added.
Drop in cases likely temporary
“Examining the epidemiology of different infectious diseases over time is an interesting and important area of study,” said Sheilagh Maguiness, MD, associate professor of dermatology and pediatrics at the University of Minnesota, Minneapolis, who was asked to comment on the results.
“The pandemic dramatically altered the daily lives of adults and children across the globe, and we can learn a lot from studying how social distancing and prolonged masking has made an impact on the incidence and prevalence of different infectious illnesses in the country and across the world,” she said in an interview.
Dr. Maguiness said she was not surprised by the study findings. “In fact, other countries have published similar studies documenting a reduction in both head lice and scabies infestations during the time of the pandemic,” she said. “In France, it was noted that during March to December 2020, there was a reduction in sales for topical head lice and scabies treatments of 44% and 14%, respectively. Similarly, a study from Argentina documented a decline in head lice infestations by about 25% among children,” she said.
“I personally noted a marked decrease in both of these diagnoses among children in my own clinic,” she added.
“Since both of these conditions are spread through close physical contact with others, it makes sense that there would be a steep decline in ectoparasitic infections during times of social distancing. However, anecdotally we are now diagnosing and treating these infestations again more regularly in our clinic,” said Dr. Maguiness. “As social distancing relaxes, I would expect that the incidence of both head lice and scabies will again increase.”
The study received no outside funding. The researchers and Dr. Maguiness had no financial conflicts to disclose.
.
When COVID-19 was declared a public health emergency by the World Health Organization in March 2020, many countries including the United States enacted lockdown and isolation measures to help contain the spread of the disease. Since scabies and lice are both spread by direct contact, “we hypothesized that the nationwide lockdown would influence the transmission of these two conditions among individuals,” wrote Marianne Bonanno, MD, of the University of North Carolina, Chapel Hill, and colleagues.
“The pandemic created a unique opportunity for real-life observations following physical distancing measures being put in place,” coauthor Christopher Sayed, MD, associate professor of dermatology at UNC, said in an interview. “It makes intuitive sense that since lice and scabies spread by cost physical contact that rates would decrease with school closures and other physical distancing measures. Reports from other countries in which extended families more often live together and were forced to spend more time in close quarters saw increased rates so it was interesting to see this contrast,” he noted.
In the study, the researchers reviewed data from 1,858 cases of adult scabies, 893 cases of pediatric scabies, and 804 cases of pediatric lice reported in North Carolina between March 2017 and February 2021. They compared monthly cases of scabies and lice, and prescriptions during the period before the pandemic (March 2017 to February 2020), and during the pandemic (March 2020 to February 2021).
Pediatric lice cases decreased by 60.6% over the study period (P < .001). Significant decreases also occurred in adult scabies (31.1%, P < .001) and pediatric scabies (39%, P < .01).
The number of prescriptions for lice and scabies also decreased significantly (P < .01) during the study period, although these numbers differed from the actual cases. Prescriptions decreased by 41.4%, 29.9%, and 69.3% for pediatric scabies, adult scabies, and pediatric lice, respectively.
Both pediatric scabies and pediatric lice showed a greater drop in prescriptions than in cases, while the drop in prescriptions for adult scabies was slightly less than the drop in cases.
The difference in the decreased numbers between cases and prescriptions may stem from the decrease in close contacts during the pandemic, which decreased the need for multiple prescriptions, but other potential explanations could be examined in future studies, the researchers wrote in their discussion.
The study findings were limited by several factors including the cross-sectional design and potential underdiagnosis and underreporting, as well as the focus only on a population in a single state, which may limit generalizability, the researchers noted.
However, the results offer preliminary insights on the impact of COVID-19 restrictions on scabies and lice, and suggest the potential value of physical distancing to reduce transmission of both conditions, especially in settings such as schools and prisons, to help contain future outbreaks, they concluded.
The study findings reinforce physical contact as the likely route of disease transmission, for lice and scabies, Dr. Sayed said in the interview. “It’s possible distancing measures on a small scale could be considered for outbreaks in institutional settings, though the risks of these infestations are much lower than with COVID-19,” he said. “It will be interesting to observe trends as physical distancing measures end to see if cases rebound in the next few years,” he added.
Drop in cases likely temporary
“Examining the epidemiology of different infectious diseases over time is an interesting and important area of study,” said Sheilagh Maguiness, MD, associate professor of dermatology and pediatrics at the University of Minnesota, Minneapolis, who was asked to comment on the results.
“The pandemic dramatically altered the daily lives of adults and children across the globe, and we can learn a lot from studying how social distancing and prolonged masking has made an impact on the incidence and prevalence of different infectious illnesses in the country and across the world,” she said in an interview.
Dr. Maguiness said she was not surprised by the study findings. “In fact, other countries have published similar studies documenting a reduction in both head lice and scabies infestations during the time of the pandemic,” she said. “In France, it was noted that during March to December 2020, there was a reduction in sales for topical head lice and scabies treatments of 44% and 14%, respectively. Similarly, a study from Argentina documented a decline in head lice infestations by about 25% among children,” she said.
“I personally noted a marked decrease in both of these diagnoses among children in my own clinic,” she added.
“Since both of these conditions are spread through close physical contact with others, it makes sense that there would be a steep decline in ectoparasitic infections during times of social distancing. However, anecdotally we are now diagnosing and treating these infestations again more regularly in our clinic,” said Dr. Maguiness. “As social distancing relaxes, I would expect that the incidence of both head lice and scabies will again increase.”
The study received no outside funding. The researchers and Dr. Maguiness had no financial conflicts to disclose.
FROM PEDIATRIC DERMATOLOGY
FDA acts against sales of unapproved mole and skin tag products on Amazon, other sites
according to a press release issued on Aug. 9.
In addition to Amazon.com, the other two companies are Ariella Naturals, and Justified Laboratories.
Currently, no over-the-counter products are FDA-approved for the at-home removal of moles and skin tags, and use of unapproved products could be dangerous to consumers, according to the statement. These products may be sold as ointments, gels, sticks, or liquids, and may contain high concentrations of salicylic acid or other harmful ingredients. Introducing unapproved products in to interstate commerce violates the Federal Food, Drug, and Cosmetic Act.
Two products sold on Amazon are the “Deisana Skin Tag Remover, Mole Remover and Repair Gel Set” and “Skincell Mole Skin Tag Corrector Serum,” according to the letter sent to Amazon.
The warning letters alert the three companies that they have 15 days from receipt to address any violations. However, warning letters are not a final FDA action, according to the statement.
“The agency’s rigorous surveillance works to identify threats to public health and stop these products from reaching our communities,” Donald D. Ashley, JD, director of the Office of Compliance in the FDA’s Center for Drug Evaluation and Research, said in the press release. “This includes where online retailers like Amazon are involved in the interstate sale of unapproved drug products. We will continue to work diligently to ensure that online retailers do not sell products that violate federal law,” he added.
The statement emphasized that moles should be evaluated by a health care professional, as attempts at self-diagnosis and at-home treatment could lead to a delayed cancer diagnosis, and potentially to cancer progression.
Products marketed to consumers for at-home removal of moles, skin tags, and other skin lesions could cause injuries, infections, and scarring, according to a related consumer update first posted by the FDA in June, which was updated after the warning letters were sent out.
Consumers and health care professionals are encouraged to report any adverse events related to mole removal or skin tag removal products to the agency’s MedWatch Adverse Event Reporting program.
The FDA also offers an online guide, BeSafeRx, with advice for consumers about potential risks of using online pharmacies and how to do so safely.
according to a press release issued on Aug. 9.
In addition to Amazon.com, the other two companies are Ariella Naturals, and Justified Laboratories.
Currently, no over-the-counter products are FDA-approved for the at-home removal of moles and skin tags, and use of unapproved products could be dangerous to consumers, according to the statement. These products may be sold as ointments, gels, sticks, or liquids, and may contain high concentrations of salicylic acid or other harmful ingredients. Introducing unapproved products in to interstate commerce violates the Federal Food, Drug, and Cosmetic Act.
Two products sold on Amazon are the “Deisana Skin Tag Remover, Mole Remover and Repair Gel Set” and “Skincell Mole Skin Tag Corrector Serum,” according to the letter sent to Amazon.
The warning letters alert the three companies that they have 15 days from receipt to address any violations. However, warning letters are not a final FDA action, according to the statement.
“The agency’s rigorous surveillance works to identify threats to public health and stop these products from reaching our communities,” Donald D. Ashley, JD, director of the Office of Compliance in the FDA’s Center for Drug Evaluation and Research, said in the press release. “This includes where online retailers like Amazon are involved in the interstate sale of unapproved drug products. We will continue to work diligently to ensure that online retailers do not sell products that violate federal law,” he added.
The statement emphasized that moles should be evaluated by a health care professional, as attempts at self-diagnosis and at-home treatment could lead to a delayed cancer diagnosis, and potentially to cancer progression.
Products marketed to consumers for at-home removal of moles, skin tags, and other skin lesions could cause injuries, infections, and scarring, according to a related consumer update first posted by the FDA in June, which was updated after the warning letters were sent out.
Consumers and health care professionals are encouraged to report any adverse events related to mole removal or skin tag removal products to the agency’s MedWatch Adverse Event Reporting program.
The FDA also offers an online guide, BeSafeRx, with advice for consumers about potential risks of using online pharmacies and how to do so safely.
according to a press release issued on Aug. 9.
In addition to Amazon.com, the other two companies are Ariella Naturals, and Justified Laboratories.
Currently, no over-the-counter products are FDA-approved for the at-home removal of moles and skin tags, and use of unapproved products could be dangerous to consumers, according to the statement. These products may be sold as ointments, gels, sticks, or liquids, and may contain high concentrations of salicylic acid or other harmful ingredients. Introducing unapproved products in to interstate commerce violates the Federal Food, Drug, and Cosmetic Act.
Two products sold on Amazon are the “Deisana Skin Tag Remover, Mole Remover and Repair Gel Set” and “Skincell Mole Skin Tag Corrector Serum,” according to the letter sent to Amazon.
The warning letters alert the three companies that they have 15 days from receipt to address any violations. However, warning letters are not a final FDA action, according to the statement.
“The agency’s rigorous surveillance works to identify threats to public health and stop these products from reaching our communities,” Donald D. Ashley, JD, director of the Office of Compliance in the FDA’s Center for Drug Evaluation and Research, said in the press release. “This includes where online retailers like Amazon are involved in the interstate sale of unapproved drug products. We will continue to work diligently to ensure that online retailers do not sell products that violate federal law,” he added.
The statement emphasized that moles should be evaluated by a health care professional, as attempts at self-diagnosis and at-home treatment could lead to a delayed cancer diagnosis, and potentially to cancer progression.
Products marketed to consumers for at-home removal of moles, skin tags, and other skin lesions could cause injuries, infections, and scarring, according to a related consumer update first posted by the FDA in June, which was updated after the warning letters were sent out.
Consumers and health care professionals are encouraged to report any adverse events related to mole removal or skin tag removal products to the agency’s MedWatch Adverse Event Reporting program.
The FDA also offers an online guide, BeSafeRx, with advice for consumers about potential risks of using online pharmacies and how to do so safely.