User login
Product News: 07 2017
Glytone Sunscreen Lotion Broad Spectrum SPF 40
Pierre Fabre Dermo-Cosmetique USA introduces Glytone Sunscreen Lotion Broad Spectrum SPF 40, a mineral-based formula for face and body with micronized zinc oxide, octinoxate, and octisalate. The lightweight formula is water resistant for up to 40 minutes and contains hyaluronic acid to nourish the skin and help boost natural moisture levels to visibly reduce the appearance of fine lines and wrinkles. For more information, visit www.glytone-usa.com.
proactivMD
The Proactiv Company launches the proactivMD Essentials System, a 3-step acne regimen that has been reformulated to include
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
Glytone Sunscreen Lotion Broad Spectrum SPF 40
Pierre Fabre Dermo-Cosmetique USA introduces Glytone Sunscreen Lotion Broad Spectrum SPF 40, a mineral-based formula for face and body with micronized zinc oxide, octinoxate, and octisalate. The lightweight formula is water resistant for up to 40 minutes and contains hyaluronic acid to nourish the skin and help boost natural moisture levels to visibly reduce the appearance of fine lines and wrinkles. For more information, visit www.glytone-usa.com.
proactivMD
The Proactiv Company launches the proactivMD Essentials System, a 3-step acne regimen that has been reformulated to include
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
Glytone Sunscreen Lotion Broad Spectrum SPF 40
Pierre Fabre Dermo-Cosmetique USA introduces Glytone Sunscreen Lotion Broad Spectrum SPF 40, a mineral-based formula for face and body with micronized zinc oxide, octinoxate, and octisalate. The lightweight formula is water resistant for up to 40 minutes and contains hyaluronic acid to nourish the skin and help boost natural moisture levels to visibly reduce the appearance of fine lines and wrinkles. For more information, visit www.glytone-usa.com.
proactivMD
The Proactiv Company launches the proactivMD Essentials System, a 3-step acne regimen that has been reformulated to include
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
Metastatic Crohn Disease: A Review of Dermatologic Manifestations and Treatment
Almost half of Crohn disease (CD) patients experience a dermatologic manifestation of the disease. A rare entity, metastatic CD (MCD) presents a diagnostic challenge without a high index of suspicion. Its etiology is not well defined; however, it appears to be an autoimmune response to gut antigens. Herein, we review the etiology/epidemiology, diagnostic criteria, and treatment for this uncommon condition.
Epidemiology and Clinical Characteristics of MCD
Metastatic CD was first described by Parks et al1 in 1965 and refers to a diverse collection of macroscopic dermatologic manifestations in tissue not contiguous with the gastrointestinal (GI) tract. To be classified as MCD, the tissue must demonstrate characteristic histopathologic findings, which invariably include noncaseating granulomas.
Crohn disease may affect any part of the GI tract from the mouth to anus, with a multitude of associated cutaneous manifestations having been described. The terminal ileum is the most commonly affected portion of the GI tract in CD, but the large intestine also may be involved in 55% to 80% of cases.2 The incidence of non-MCD-associated anal lesions seems to correlate with intestinal involvement in that as few as 25% of patients with ileal-localized CD have anal lesions compared to nearly 80% of patients with large intestinal involvement.3
It has been estimated that 18% to 44% of patients with CD have some form of cutaneous manifestation,4 with MCD being a rare subcategory. As few as 100 cases have been described from 1965 to the present.5 The presence of MCD does not correlate well with severity of intestinal CD, and although a majority of MCD cases present after at least 6 months of GI symptoms,6 there are instances in which MCD presents without prior or existing evidence of intestinal CD.7
With regard to MCD, the term metastatic is sometimes supplanted in the literature by cutaneous to avoid any implication of cancer; however, due to a myriad of dermatologic manifestations, both terms can cause confusion. The categorization of the various types of cutaneous findings in CD is well summarized in a review by Palamaras et al8 with the following classifications: (1) granulomatous by direct extension (oral or perianal), (2) MCD lesions (genital and nongenital), (3) immune-related lesions, and (4) lesions from nutritional deficiencies. Of the cutaneous manifestations relating to CD, MCD is the least common cutaneous categorical manifestation and is further divided into subcategories of genital and nongenital lesions.8
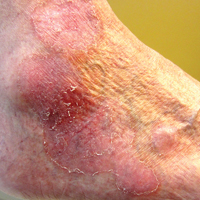
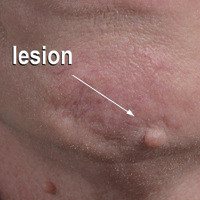
The nongenital distribution of MCD is the more common variety in adults and particularly seems to affect the legs and plantar surfaces (38%), the trunk and abdomen (24%), and the face (15%).5,9 These nongenital MCD manifestations are most commonly described as nodules, ulcerations, or erythematous to purple plaques, and less commonly described as abscesses, pustules, or papules.
The sequence of cutaneous symptoms of MCD relative to intestinal disease depends to some degree on patient age. In adults diagnosed with MCD, it has been noted that a GI flare is expected 2 months to 4 years after diagnosis; however, in children the subsequent GI flare has been noted to vary more widely from 9 months to 14 years following presentation of MCD.8 Furthermore, roughly 50% of children diagnosed with MCD present concomitantly with their first symptoms of a GI flare, whereas 70% of adults with MCD had been previously diagnosed with intestinal CD.8 In one review of 80 reported cases of MCD, 20% (16/80) had no symptoms of intestinal disease at the time of MCD diagnosis, and the majority of the asymptomatic cases were in children; interestingly, the majority of these same children were diagnosed with CD months to years later.9
Both the location and characteristics of cutaneous findings in MCD correlate with age.9 Metastatic CD has been identified in all age groups; however, lymphedema is more common in children/young adults, while nodules, ulceration, and fistulating disease are more often seen in adults.10 Affected children and adolescents with MCD range from 5 to 17 years of age, with a mean age at disease onset of 11.1 years and equal incidence in males and females.8 Adults with MCD range from 18 to 78 years of age, with a mean age at presentation of 38.4 years.8,11
Concerning anatomic location of disease, adults with MCD most commonly have nodules with or without plaques on the arms and legs and less commonly in the genital area.8 In contrast, children with MCD are more prone to genital lesions, with up to 85% of cases including some degree of genital erythematous or nonerythematous swelling with or without induration.8 Genitourinary complications of CD as a broad category, however, are estimated to occur in only 5% to 20% of intestinal CD cases in both children and adults.12
There have been conflicting reports regarding gender predilection in MCD. Based on a review by Samitz et al13 of 200 cases of CD over an 18-year period, 22% of patients with CD were found to have cutaneous manifestations--presumably not MCD but rather perianal, perineal, vulvar fistulae, fissures, or abscesses--with a male to female preponderance of almost 2 to 1. A more recent review of the literature by Palamaras et al8 in 2008 reported that contiguous non-MCD affects adult females and children more often than adult males, with 63% adult cases being female. This review seems to be more congruent with other reports in the literature implicating that females are twice as commonly affected by MCD than males.9,14
Pathophysiology
The etiology of MCD has not been well defined. One proposed mechanism of the distal tissue involvement of MCD is through passage of antigens to the skin with subsequent granulomatous response at the level of the dermis.10 Another proposed mechanism suggests antibody sensitization to gut antigens, possibly bacterial antigens, that then coincidentally cross-react with analogous skin antigens.8,14 Burgdorf11 supported this notion in a 1981 report in which it was suggested that the granulomatous reaction was related to deposition of immune complexes in the skin. Slater et al15 and Tatnall et al16 offered a variation of Burgdorf's notion, suggesting that it was sensitized T cells to circulating antigens that were the initiators of granuloma formation in the periphery.
An examination of MCD tissue in 1990 by Shum and Guenther17 under electron microscopy and immunofluorescence provided evidence against prior studies that purported to have identified immune complexes as the causative agents of MCD. In this study, the authors found no evidence of immune complexes in the dermis of MCD lesions. In addition, an attempt to react serum antibodies of a patient with MCD, which were postulated to have IgG, IgM, and IgA antibodies to specific gut antigens, yielded no response when reacted with the tongue, ileum, and colon tissue from a rat. As a culminant finding, the authors also noted MCD dermis tissue with granulomas without vasculitis, suggesting a T-cell mediated type IV hypersensitivity response with a secondary vasculitis from T-cell origin lymphokines and T-cell mediated monocyte activation.17
Research implicating other immunologic entities involved in the pathophysiology of CD such as β-2 integrin,18 CD14+ monocytes,19 and the role of the DNA repair gene MLH1 (mutL homolog 1)20 have been considered but without a clearly definitive role in the manifestations of MCD.
The utility of metronidazole in the treatment of MCD has been suggested as evidence that certain bacteria in the gut may either serve as the causative antigen or may induce its formation21; however, the causative antigen has yet to be identified, and whether it travels distally to the skin or merely resembles a similar antigen normally present in the dermis has not yet been determined. Some research has used in situ polymerase chain reaction techniques to attempt to detect similar microbial pathogens in both the vasculature of active bowel lesions and in the skin, but to date, bacterial RNA noted to be present in the gut vasculature adjacent to CD lesions has not been detected in skin lesions.22
Diagnosis
Physical Findings
Overall, it is estimated that roughly 56% of all MCD cases affect the external genitalia.23 The classic appearance of MCD includes well-demarcated ulcerations in the areas of intertriginous skin folds with or without diffuse edema and tenderness to palpation.23 Although MCD has been historically noted as having a predilection for moist skin folds, there are numerous case reports of MCD all over the body, including the face,7,24-29 retroauricular areas,30 arms and legs,16,17,31-34 lower abdomen,3,5 under the breasts,1 perineum,35 external genitalia,1,9,36-40 and even the lungs41 and bladder.42
As a dermatologic disease, MCD has been referred to as yet another great imitator, both on the macroscopic and microscopic levels.8 As such, more common causes of genital edema should be considered first and investigated based on the patient's history, physical examination, skin biopsy, lymphangiogram, ultrasound, and cystogram.43 Ultrasonography and color Doppler sonography have been shown to be helpful in patients with genital involvement. This modality can evaluate not only the presence of normal testes but also intratesticular and scrotal wall fluid, especially when the physical examination reveals swelling that makes testicle palpation more difficult.6 Clinically, the correct diagnosis of MCD often is made through suspicion of inflammatory bowel disease based on classic symptoms and/or physical findings including abdominal pain, weight loss, bloody stool, diarrhea, perianal skin tags, and anal fissures or fistulas. Any of these GI findings should prompt an intestinal biopsy to rule out any histologic evidence of CD.
Metastatic CD affecting the vulva often presents with vulvar pain and pruritus and may clinically mimic a more benign disease such as balanitis plasmacellularis, also referred to as Zoon vulvitis.23 Similar to MCD on any given body surface, there is dramatic variation in the macroscopic presentation of vulvar MCD, with physical examination findings ranging from bilateral diffuse, edematous, deeply macerated, red, ulcerated lesions over the vulva with lymphadenopathy to findings of bilateral vulvar pain with yellow drainage from the labia majora.23 There have been cases of vulvar MCD that include exquisite vulvar pain but without structural abnormalities including normal uterus, cervix, adnexa, rectovaginal septum, and rectum. In these more nebulous cases of vulvar MCD, the diagnosis often is discovered incidentally when nonspecific diagnostic imaging suggests underlying CD.23
Beyond the case-by-case variations on physical examination, the great difficulty in diagnosis, particularly in children, occurs in the absence of any GI symptoms and therefore no logical consideration of underlying CD. Consequently, there have been cases of children presenting with irritation of the vulva who were eventually diagnosed with MCD only after erroneous treatment of contact dermatitis, candidiasis, and even consideration of sexual abuse.37 Because it is so rare and obscure among practicing clinicians, the diagnosis of MCD often is considered only after irritation or swelling of the external genitalia has not responded to standard therapies. If and when the diagnosis of MCD is considered in children, it has been suggested to screen patients for anorectal stricture, as case studies have found the condition to be relatively common in this subpopulation.44
In the less common case of adults with genitourinary symptoms that suggest possible MCD, the differential diagnosis for penile or vaginal ulcers should include contact and irritant dermatitis, chronic infectious lesions (eg, hidradenitis suppurativa, actinomycosis, tuberculosis),45 sexually transmitted ulcerative diseases (eg, chancroid, lymphogranuloma venereum, herpes genitalia, granuloma inguinale),46 drug reactions, and even extramammary Paget disease.47
Histologic Findings
Because MCD has so much macroscopic variation and can present anywhere on the surface of the body, formal diagnosis relies on microscopy. As an added measure of difficulty in diagnosis, one random biopsy of a suspicious segment of tissue may not contain the expected histologic findings; therefore, clinical suspicion may warrant a second biopsy.10 There have been reported cases of an adult patient without history of CD presenting with a lesion that resembled a more common pathology, such as a genital wart, and the correct diagnosis of MCD with pseudocondylomatous morphology was made only after intestinal manifestations prompted the clinician to consider such an unusual diagnosis.48
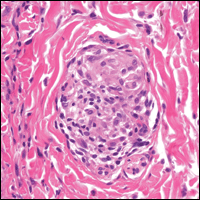
From a histopathologic perspective, MCD is characterized by discrete, noncaseating, sarcoidlike granulomas with abundant multinucleated giant cells (Langhans giant cells) in the superficial dermis (papillary), deep dermis (reticular), and adipose tissue (Figure).8,17 In the presence of concomitant intestinal disease, the granulomas of both the intestinal and dermal tissues should share the same microscopic characteristics.8 In addition, copious neutrophils and granulomas surrounding the microvasculature have been described,34 as well as general lymphocyte and plasma cell infiltrate.45 Some histologic samples have included collagen degeneration termed necrobiosis in the middle dermal layer as another variable finding in MCD.14,34
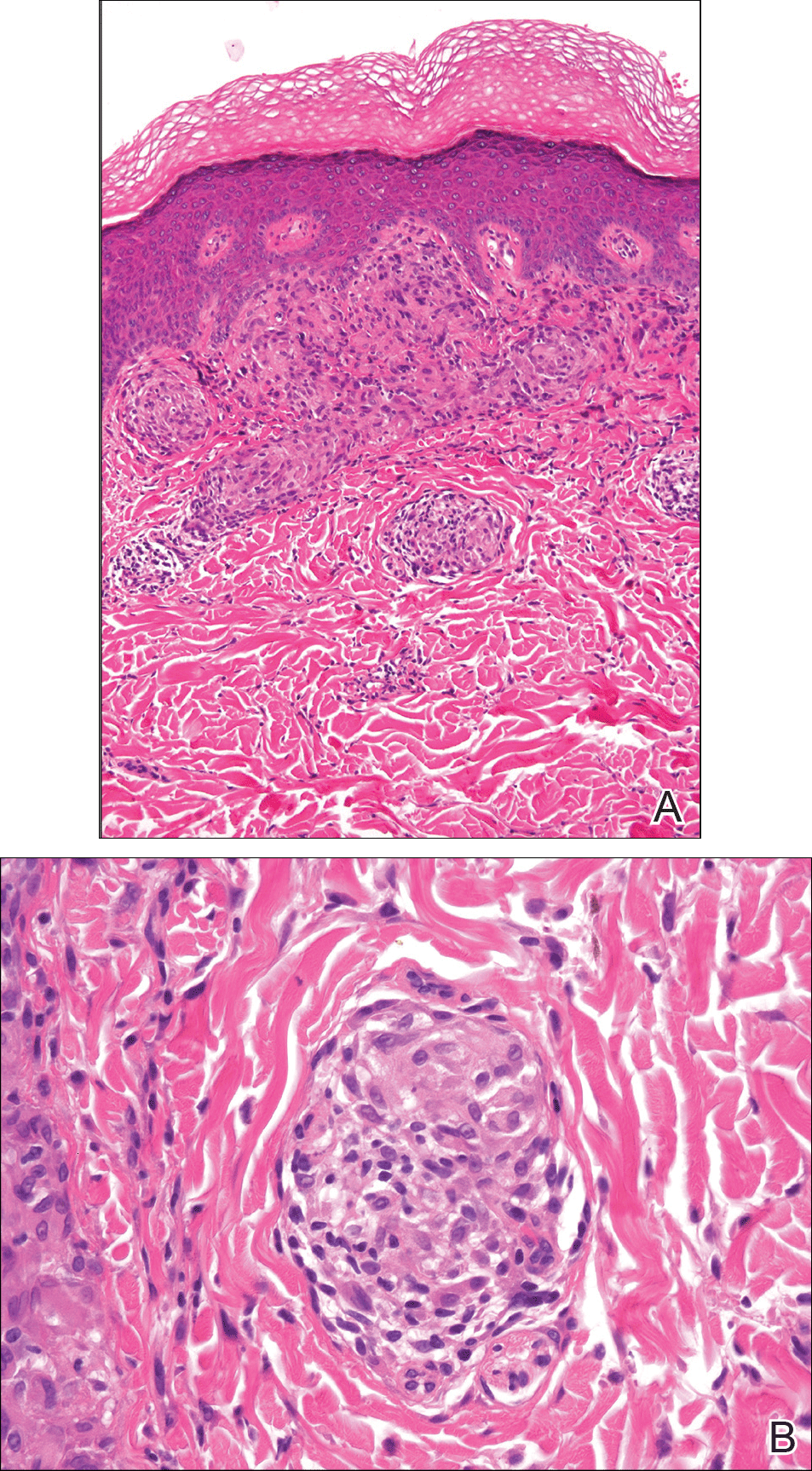
On microscopy, it has been reported that use of Verhoeff-van Gieson staining may be helpful to highlight the presence of neutrophil obstruction within the dermal vasculature, particularly the arterial lumen, as well as to aid in highlighting swelling of the endothelium with fragmentation of the internal elastic lamina.17 Although not part of the routine diagnosis, electron microscopy of MCD tissue samples have confirmed hypertrophy of the endothelial cells composing the capillaries with resulting extravasation of fibrin, red blood cells, lymphocytes, and epithelioid histiocytes.17 Observation of tissue under direct immunofluorescence has been less helpful, as it has shown only nonspecific fibrinogen deposition within the dermis and dermal vessels.17
In an article on treatment of MCD, Escher et al43 reinforced that the macroscopic findings of MCD are diverse, and the microscopic findings characteristic of MCD also can be mimicked by other etiologies such as sarcoidosis, tuberculosis, fungal infections, lymphogranuloma venereum, leishmaniasis, and connective tissue disorders.43 As such, the workup to rule out infectious, anatomic, and autoimmune etiologies should be diverse. Often, the workup for MCD will include special stains such as Ziehl-Neelsen stain to rule out Mycobacterium tuberculosis and acid-fast bacilli and Fite stain to consider atypical mycobacteria. Other tests such as tissue culture, chest radiograph, tuberculin skin test (Mantoux test), IFN-γ release assay, or polarized light microscopy may rule out infectious etiologies.9,49 Serologic testing might include VDRL test, Treponema pallidum hemagglutination assay, hepatitis B, hepatitis C, and human immunodeficiency virus.5
Crohn disease is characterized histologically by sarcoidlike noncaseating granulomas, and as such, it is important to differentiate MCD from sarcoidosis prior to histologic analysis. Sarcoidosis also can be considered much less likely with a normal chest radiograph and in the absence of increased serum calcium and angiotensin-converting enzyme levels.7 The differentiation of sarcoidosis from MCD on the microscopic scale is subtle but is sometimes facilitated in the presence of an ulcerated epidermis or lymphocytic/eosinophilic infiltrate and edema within the dermis, all suggestive of MCD.14
Metastatic CD also should be differentiated from erythema nodosum and pyoderma gangrenosum, which are among the most common cutaneous findings associated with CD.14 Pyoderma gangrenosum can be distinguished histologically by identifying copious neutrophilic infiltrate with pseudoepitheliomatous hyperplasia.50
Treatment
Because MCD is relatively rare, there are no known randomized trials suggesting a particular medical or surgical treatment. In a review of perineal MCD from 2007, the 40-year-old recommendation by Moutain3 opting for surgical debridement versus medical management still resonates, particularly for perineal disease, as an effective measure in all but the mildest of presentations.51 However, recent case reports also suggest that the tumor necrosis factor α (TNF-α) inhibitors such as infliximab and adalimumab should be considered prior to surgery even with severe perineal MCD.51 Moreover, even if medical management with TNF-α inhibitors or some combination of immunosuppressants and antibiotics does not eradicate the disease, it often helps reduce the size of the ulcers prior to surgery.52 With a limited understanding of MCD, one might think that removal of the affected bowel would eliminate cutaneous disease, but it has been shown that this strategy is not effective.53,54
The composition and location of the particular lesion affects the trajectory of treatment. For example, MCD manifesting as local ulcers and plaques has been described as responding well to topical and intralesional steroids.10,55,56 In the case of penile swelling and/or phimosis, circumcision has been helpful to improve the patient's ability to void as well as to attain and maintain erection.10 In the case of scrotal swelling secondary to MCD, early treatment (ie, within 4 to 6 months) with oral steroids and/or metronidazole is likely beneficial to prevent refractory edematous organization of the tissue.57
As a general rule, an effective treatment will include a combination of an immunosuppressant, antibiotic therapy, and sometimes surgery. The most commonly used immunosuppressant agents include topical or intralesional steroids, infliximab,43,58 cyclosporine A,59,60 dapsone, minocycline, thalidomide, methotrexate, mycophenolate mofetil, sulfasalazine, azathioprine, tacrolimus, and 6-mercaptopurine.4 Steroids have been the conventional treatment of extraintestinal manifestations of CD61; however, perineal CD has been poorly controlled with systemic steroids.62 If steroids are found not to be effective, sometimes agents such as dapsone or thalidomide are considered. One case report noted stabilization of MCD penile ulcers with oral thalidomide 300 mg once daily, oral minocycline 100 mg once daily, and topical tacrolimus 0.3% with benzocaine twice daily with continuation of prednisolone and methotrexate as parts of previously unsuccessful regimen.52
Metronidazole is perhaps the most commonly used antibiotic, having been a component of many successful regimens.4,63 For example, a 27-year-old patient with MCD presenting as a nonhealing ulcerative lesion in the subcoronal area of the penis and scrotum was treated successfully with a 6-month course of mesalamine, prednisone, and metronidazole.45 Another case report of vulvar MCD reported initial success with intravenous methylprednisolone, ciprofloxacin, and metronidazole.23 The primary limitation of metronidazole is that subsequent tapering of the dose seems to result in recurrence of disease.64 Consequently, patients must remain on the antibiotic for an indeterminate course, with dosages ranging from 5 mg/kg daily in adolescents65 to 1000 to 1500 mg daily in adults.66
Of the various immunosuppressants available, infliximab has been listed in numerous reports as a successful agent in both the induction and maintenance of extraintestinal manifestations of CD including MCD.67-71 Infliximab has been reported to be effective in the treatment of penile and scrotal edema secondary to MCD that did not respond to other immunosuppressants including oral prednisolone, azathioprine, and cyclosporine.43 Infliximab may be a good option to help heal draining fistulas, particularly in combination with an antibiotic such as metronidazole and ciprofloxacin, which helps to prevent abscess formation during healing.72 The response to infliximab has been dramatic, with resolution of cutaneous lesions after just 6 weeks in some cases.73 The dosing regimen of infliximab has been suggested at 5 mg/kg administered at 0, 2, and 6 weeks, with subsequent maintenance infusions every 10 weeks,70 or at 0, 4, and 12 weeks, with subsequent infusions every 8 weeks.43
Adalimumab may be considered as an alternative to infliximab and is potentially less allergenic as a fully humanized monoclonal antibody to TNF-α, which also has been used successfully to both induce and maintain remission of moderate to severe CD.42,74,75 Proposed dosing of adalimumab includes a loading dose of 160 mg subcutaneously on day 1, followed by an 80-mg dose 2 weeks later and a 40-mg maintenance dose every other week indefinitely.48 Of note, adalimumab has been noted in the literature to have many potential side effects, including one particular case in which severe headaches were attributed to its use.59 As a consequence of the headaches, the patient was switched from adalimumab to cyclosporine and responded well with no subsequent flare-ups on follow-up.
In summary, treatment of MCD depends on cutaneous location, severity, physician experience with certain antibiotics or immunosuppressants, availability of medication, and patient disposition. It seems reasonable to attempt medical management with one or more medical regimens before committing to surgical intervention. Furthermore, even with debridement, curettage, skin graft, or other surgical strategy, the patient is likely to require some period of immunosuppression to provide long-lasting remission.
Conclusion
Patients with inflammatory bowel disease often develop dermatologic sequelae, with MCD being a rare but serious process. Patients may present with a wide array of physical concerns and symptoms, many resembling other disease processes. As such, education and a high index of suspicion are needed for proper diagnosis and treatment.
- Parks AG, Morson BC, Pegum JS. Crohn's disease with cutaneous involvement. Proc R Soc Med. 1965;58:241-242.
- Friedman S, Blumber RS. Inflammatory bowel disease. In: Kasper DL, Braunwald E, Fauci AS, et al, eds. Harrison's Principles of Internal Medicine. 16th ed. New York, NY: McGraw-Hill; 2005:1778-1784.
- Moutain JC. Cutaneous ulceration in Crohn's disease. Gut. 1970;11:18-26.
- Lester LU, Rapini RP. Dermatologic manifestations of colonic disorders. Curr Opin Gastroenterol. 2008;25:66-73.
- Teixeira M, Machado S, Lago P, et al. Cutaneous Crohn's disease. Int J Dermatol. 2006;45:1074-1076.
- Simoneaux SF, Ball TI, Atkinson GO Jr. Scrotal swelling: unusual first presentation of Crohn's disease. Pediatr Radiol. 1995;25:375-376.
- Albuquerque A, Magro F, Rodrigues S, et al. Metastatic cutaneous Crohn's disease of the face: a case report and review of literature. Eur J Gastroenterol Hepatol. 2011;23:954-956.
- Palamaras I, El-Jabbour J, Pietropaolo N, et al. Metastatic Crohn's disease: a review. J Eur Acad Dermatol Venereol. 2008;22:1033-1043.
- Ploysangam T, Heubi JE, Eisen D, et al. Cutaneous Crohn's disease in children. J Am Acad Dermatol. 1997;36:697-704.
- Vint R, Husain E, Hassain F, et al. Metastatic Crohn's disease of the penis: two cases. Int Urol Nephrol. 2012;44:45-49.
- Burgdorf W. Cutaneous manifestations of Crohn's disease. J Am Acad Dermatol. 1981;5:689-695.
- Resnick MI, Kursh ED. Extrinsic obstruction of the ureter. In: Walsh PC, Retik AB, Stamey TA, et al, eds. Campbell's Urology. 7th ed. Philadelphia, PA: WB Saunders; 1998:400-402.
- Samitz MH, Dana AS Jr, Rosenberg P. Cutaneous vasculitis in association with Crohn's disease--review of statistics of skin complications. Cutis. 1970;6:51-56.
- Emanuel PO, Phelps RG. Metastatic Crohn's disease: a histo-pathologic study of 12 cases. J Cutan Pathol. 2008;35:457-461.
- Slater DN, Waller PC, Reilly G. Cutaneous granulomatous vasculitis: presenting features of Crohn's disease. J R Soc Med. 1985;78:589-590.
- Tatnall FM, Dodd HJ, Sarkany I. Crohn's disease with metastatic cutaneous involvement and granulomatous cheilitis. J R Soc Med. 1987;80:49-51.
- Shum DT, Guenther L. Metastatic Crohn's disease: case report and review of literature. Arch Dermatol. 1990;126:645-648.
- Bernstein CN, Sargent M, Gallatin WM. Beta2 integrin/ICAM expression in Crohn's disease. Clin Immunol Immunopathol. 1998;86:147-160.
- Grimm MC, Pavli P, Van de Pol E, et al. Evidence for a CD-14+ population of monocytes in inflammatory bowel disease mucosa--implications for pathogenesis. Clin Exp Immunol. 1995;100:291-297.
- Pokorny RM, Hofmeister A, Galandiuk S, et al. Crohn's disease and ulcerative colitis are associated with the DNA repair gene MLH1. Ann Surg. 1997;225:718-723; discussion 723-725.
- Ursing B, Kamme C. Metronidazole for Crohn's disease. Lancet. 1975;1:775-777.
- Crowson AN, Nuovo GJ, Mihm MC Jr, et al. Cutaneous manifestations of Crohn's disease, its spectrum, and pathogenesis: intracellular consensus bacterial 16S rRNA is associated with the gastrointestinal but not the cutaneous manifestations of Crohn's disease. Hum Pathol. 2003;34:1185.
- Leu S, Sun PK, Collyer J, et al. Clinical spectrum of vulva metastatic Crohn's disease. Dig Dis Sci. 2009;54:1565-1571.
- Chen W, Blume-Peytavi U, Goerdt S, et al. Metastatic Crohn's disease of the face. J Am Acad Dermatol. 1996;35:986-988.
- Ogram AE, Sobanko JF, Nigra TP. Metastatic cutaneous Crohn disease of the face: a case report and review of the literature. Cutis. 2010;85:25-27.
- Graham D, Jager D, Borum M. Metastatic Crohn's disease of the face. Dig Dis Sci. 2006;51:2062-2063.
- Biancone L, Geboes K, Spagnoli LG, et al. Metastatic Crohn's disease of the forehead. Inflamm Bowel Dis. 2002;8:101-105.
- Kolansky G, Green CK, Dubin H. Metastatic Crohn's disease of the face: an uncommon presentation. Arch Dermatol. 1993;129:1348-1349.
- Mahadevan U, Sandborn WJ. Infliximab for the treatment of orofacial Crohn's disease. Inflamm Bowel Dis. 2001;7:38-42.
- McCallum DI, Gray WM. Metastatic Crohn's disease. Br J Dermatol. 1976;95:551-554.
- Lieberman TR, Greene JF Jr. Transient subcutaneous granulomatosis of the upper extremities in Crohn's disease. Am J Gastroenterol. 1979;72:89-91.
- Kafity AA, Pellegrini AE, Fromkes JJ. Metastatic Crohn's disease: a rare cutaneous manifestation. J Clin Gastroenterol. 1993;17:300-303.
- Marotta PJ, Reynolds RP. Metastatic Crohn's disease. Am J Gastroenterol. 1996;91:373-375.
- Hackzell-Bradley M, Hedblad MA, Stephansson EA. Metastatic Crohn's disease. report of 3 cases with special reference to histopathologic findings. Arch Dermatol. 1996;132:928-932.
- van Dulleman HM, de Jong E, Slors F, et al. Treatment of therapy resistant perineal metastatic Crohn's disease after proctectomy using anti-tumor necrosis factor chimeric monoclonal antibody, cA2: report of two cases. Dis Colon Rectum. 1998;41:98-102.
- Lavery HA, Pinkerton JH, Sloan J. Crohn's disease of the vulva--two further cases. Br J Dermatol. 1985;113:359-363.
- Lally MR, Orenstein SR, Cohen BA. Crohn's disease of the vulva in an 8-year-old girl. Pediatr Dermatol. 1988;5:103-106.
- Tuffnell D, Buchan PC. Crohn's disease of the vulva in childhood. Br J Clin Pract. 1991;45:159-160.
- Schrodt BJ, Callen JP. Metastatic Crohn's disease presenting as chronic perivulvar and perirectal ulcerations in an adolescent patient. Pediatrics. 1999;103:500-502.
- Slaney G, Muller S, Clay J, et al. Crohn's disease involving the penis. Gut. 1986;27:329-333.
- Calder CJ, Lacy D, Raafat F, et al. Crohn's disease with pulmonary involvement in a 3 year old boy. Gut. 1993;34:1636-1638.
- Saha S, Fichera A, Bales G, et al. Metastatic Crohn's disease of the bladder. Inflamm Bowel Dis. 2008;14:140-142.
- Escher JC, Stoof TJ, van Deventer SJ, et al. Successful treatment of metastatic Crohn disease with infliximab. J Pediatr Gastroenterol Nutr. 2002;34:420-423.
- Saadah OI, Oliver MR, Bines JE, et al. Anorectal strictures and genital Crohn's disease: an unusual clinical association. J Pediatr Gastroenterol Nutr. 2003;36:403-406.
- Martinez-Salamanca JI, Jara J, Miralles P, et al. Metastatic Crohn's disease: penile and scrotal involvement. Scand J Urol Nephrol. 2004;38:436-437.
- Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417-429.
- Acker SM, Sahn EE, Rogers HC, et al. Genital cutaneous Crohn disease. Am J Dermatopathol. 2000;22:443-446.
- Lestre S, Ramos J, Joao A, et al. Cutaneous Crohn's disease presenting as genital warts: successful treatment with adalimumab. Eur J Dermatol. 2010;20:504-505.
- Yu JT, Chong LY, Lee KC. Metastatic Crohn's disease in a Chinese girl. Hong Kong Med J. 2006;12:467-469.
- Wilson-Jones E, Winkelmann RK. Superficial granulomatous pyoderma: a localized vegetative form of pyoderma gangrenosum. J Am Acad Dermatol. 1988;18:511-521.
- Moyes LH, Glen P, Pickford IR. Perineal metastatic Crohn's disease: a case report and review of the literature. Ann R Coll Surg Engl. 2007;89:W1-W3.
- Rajpara SM, Siddha SK, Ormerod AD, et al. Cutaneous penile and perianal Crohn's disease treated with a combination of medical and surgical interventions. Australas J Dermatol. 2008;49:21-24.
- Cockburn AG, Krolikowski J, Balogh K, et al. Crohn disease of penile and scrotal skin. Urology. 1980;15:596-598.
- Guest GD, Fink RL. Metastatic Crohn's disease: case report of an unusual variant and review of the literature. Dis Colon Rectum. 2000;43:1764-1766.
- Sangueza OP, Davis LS, Gourdin FW. Metastatic Crohn disease. South Med J. 1997;90:897-900.
- Chiba M, Iizuka M, Horie Y, et al. Metastatic Crohn's disease involving the penis. J Gastroenterol. 1997;32:817-821.
- Poon KS, Gilks CB, Masterson JS. Metastatic Crohn's disease involving the genitalia. J Urol. 2002;167:2541-2542.
- Shanahan F. Anti-TNF therapy for Crohn's disease: a perspective (infliximab is not the drug we have been waiting for). Inflamm Bowel Dis. 2000;6:137-139.
- Carranza DC, Young L. Successful treatment of metastatic Crohn's disease with cyclosporine. J Drugs Dermatol. 2008;7:789-791.
- Bardazzi F, Guidetti MS, Passarini B, et al. Cyclosporine A in metastatic Crohn's disease. Acta Derm Venereol. 1995;75:324-325.
- Faubion WA Jr, Loftus EV Jr, Harmsen WS, et al. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology. 2001;121:255-260.
- Gelbmann CM, Rogler G, Gross V, et al. Prior bowel resections, perianal disease, and a high initial Crohn's disease activity index are associated with corticosteroid resistance in active Crohn's disease. Am J Gastroenterol. 2002;97:1438-1445.
- Thukral C, Travassos WJ, Peppercorn MA. The role of antibiotics in inflammatory bowel disease. Curr Treat Options Gastroenterol. 2005;8:223-228.
- Brandt LJ, Berstein LH, Boley SJ, et al. Metronidazole therapy for perineal Crohn's disease: a follow-up study. Gastroenterology. 1982;83:383-387.
- Lehrnbecher T, Kontny HU, Jeschke R. Metastatic Crohn's disease in a 9-year-old boy. J Pediatr Gastroenterol Nutr. 1999;28:321-323.
- Abide JM. Metastatic Crohn disease: clearance with metronidazole. J Am Acad Dermatol. 2011;64:448-449.
- Rispo A, Scarpa R, Di Girolamo E, et al. Infliximab in the treatment of extra-intestinal manifestations of Crohn's disease. Scand J Rheumatol. 2005;34:387-391.
- Kaufman I, Caspi D, Yeshurun D, et al. The effect of infliximab on extraintestinal manifestations of Crohn's disease. Rheumatol Int. 2005;25:406-410.
- Konrad A, Seibold F. Response of cutaneous Crohn's disease to infliximab and methotrexate. Dig Liver Dis. 2003;35:351-356.
- Miller AM, Elliott PR, Fink R, et al. Rapid response of severe refractory metastatic Crohn's disease to infliximab. J Gastroenterol Hepatol. 2001;16:940-942.
- Chuah JH, Kim DS, Allen C, et al. Metastatic Crohn's disease of the ear. Int J Otolaryngol. 2009;2009:871567.
- Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med. 1999;340:1398-1405.
- Petrolati A, Altavilla N, Cipolla R, et al. Cutaneous metastatic Crohn's disease responsive to infliximab. Am J Gastroenterol. 2009;104:1058.
- Hanauer SB, Sandborn WJ, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn's disease: the CLASSIC-I trial. Gastroenterology. 2006;130:323-333.
- Cury DB, Moss A, Elias G, et al. Adalimumab for cutaneous metastatic Crohn's disease. Inflamm Bowel Dis. 2010;16:723-724.
Almost half of Crohn disease (CD) patients experience a dermatologic manifestation of the disease. A rare entity, metastatic CD (MCD) presents a diagnostic challenge without a high index of suspicion. Its etiology is not well defined; however, it appears to be an autoimmune response to gut antigens. Herein, we review the etiology/epidemiology, diagnostic criteria, and treatment for this uncommon condition.
Epidemiology and Clinical Characteristics of MCD
Metastatic CD was first described by Parks et al1 in 1965 and refers to a diverse collection of macroscopic dermatologic manifestations in tissue not contiguous with the gastrointestinal (GI) tract. To be classified as MCD, the tissue must demonstrate characteristic histopathologic findings, which invariably include noncaseating granulomas.
Crohn disease may affect any part of the GI tract from the mouth to anus, with a multitude of associated cutaneous manifestations having been described. The terminal ileum is the most commonly affected portion of the GI tract in CD, but the large intestine also may be involved in 55% to 80% of cases.2 The incidence of non-MCD-associated anal lesions seems to correlate with intestinal involvement in that as few as 25% of patients with ileal-localized CD have anal lesions compared to nearly 80% of patients with large intestinal involvement.3
It has been estimated that 18% to 44% of patients with CD have some form of cutaneous manifestation,4 with MCD being a rare subcategory. As few as 100 cases have been described from 1965 to the present.5 The presence of MCD does not correlate well with severity of intestinal CD, and although a majority of MCD cases present after at least 6 months of GI symptoms,6 there are instances in which MCD presents without prior or existing evidence of intestinal CD.7
With regard to MCD, the term metastatic is sometimes supplanted in the literature by cutaneous to avoid any implication of cancer; however, due to a myriad of dermatologic manifestations, both terms can cause confusion. The categorization of the various types of cutaneous findings in CD is well summarized in a review by Palamaras et al8 with the following classifications: (1) granulomatous by direct extension (oral or perianal), (2) MCD lesions (genital and nongenital), (3) immune-related lesions, and (4) lesions from nutritional deficiencies. Of the cutaneous manifestations relating to CD, MCD is the least common cutaneous categorical manifestation and is further divided into subcategories of genital and nongenital lesions.8
The nongenital distribution of MCD is the more common variety in adults and particularly seems to affect the legs and plantar surfaces (38%), the trunk and abdomen (24%), and the face (15%).5,9 These nongenital MCD manifestations are most commonly described as nodules, ulcerations, or erythematous to purple plaques, and less commonly described as abscesses, pustules, or papules.
The sequence of cutaneous symptoms of MCD relative to intestinal disease depends to some degree on patient age. In adults diagnosed with MCD, it has been noted that a GI flare is expected 2 months to 4 years after diagnosis; however, in children the subsequent GI flare has been noted to vary more widely from 9 months to 14 years following presentation of MCD.8 Furthermore, roughly 50% of children diagnosed with MCD present concomitantly with their first symptoms of a GI flare, whereas 70% of adults with MCD had been previously diagnosed with intestinal CD.8 In one review of 80 reported cases of MCD, 20% (16/80) had no symptoms of intestinal disease at the time of MCD diagnosis, and the majority of the asymptomatic cases were in children; interestingly, the majority of these same children were diagnosed with CD months to years later.9
Both the location and characteristics of cutaneous findings in MCD correlate with age.9 Metastatic CD has been identified in all age groups; however, lymphedema is more common in children/young adults, while nodules, ulceration, and fistulating disease are more often seen in adults.10 Affected children and adolescents with MCD range from 5 to 17 years of age, with a mean age at disease onset of 11.1 years and equal incidence in males and females.8 Adults with MCD range from 18 to 78 years of age, with a mean age at presentation of 38.4 years.8,11
Concerning anatomic location of disease, adults with MCD most commonly have nodules with or without plaques on the arms and legs and less commonly in the genital area.8 In contrast, children with MCD are more prone to genital lesions, with up to 85% of cases including some degree of genital erythematous or nonerythematous swelling with or without induration.8 Genitourinary complications of CD as a broad category, however, are estimated to occur in only 5% to 20% of intestinal CD cases in both children and adults.12
There have been conflicting reports regarding gender predilection in MCD. Based on a review by Samitz et al13 of 200 cases of CD over an 18-year period, 22% of patients with CD were found to have cutaneous manifestations--presumably not MCD but rather perianal, perineal, vulvar fistulae, fissures, or abscesses--with a male to female preponderance of almost 2 to 1. A more recent review of the literature by Palamaras et al8 in 2008 reported that contiguous non-MCD affects adult females and children more often than adult males, with 63% adult cases being female. This review seems to be more congruent with other reports in the literature implicating that females are twice as commonly affected by MCD than males.9,14
Pathophysiology
The etiology of MCD has not been well defined. One proposed mechanism of the distal tissue involvement of MCD is through passage of antigens to the skin with subsequent granulomatous response at the level of the dermis.10 Another proposed mechanism suggests antibody sensitization to gut antigens, possibly bacterial antigens, that then coincidentally cross-react with analogous skin antigens.8,14 Burgdorf11 supported this notion in a 1981 report in which it was suggested that the granulomatous reaction was related to deposition of immune complexes in the skin. Slater et al15 and Tatnall et al16 offered a variation of Burgdorf's notion, suggesting that it was sensitized T cells to circulating antigens that were the initiators of granuloma formation in the periphery.
An examination of MCD tissue in 1990 by Shum and Guenther17 under electron microscopy and immunofluorescence provided evidence against prior studies that purported to have identified immune complexes as the causative agents of MCD. In this study, the authors found no evidence of immune complexes in the dermis of MCD lesions. In addition, an attempt to react serum antibodies of a patient with MCD, which were postulated to have IgG, IgM, and IgA antibodies to specific gut antigens, yielded no response when reacted with the tongue, ileum, and colon tissue from a rat. As a culminant finding, the authors also noted MCD dermis tissue with granulomas without vasculitis, suggesting a T-cell mediated type IV hypersensitivity response with a secondary vasculitis from T-cell origin lymphokines and T-cell mediated monocyte activation.17
Research implicating other immunologic entities involved in the pathophysiology of CD such as β-2 integrin,18 CD14+ monocytes,19 and the role of the DNA repair gene MLH1 (mutL homolog 1)20 have been considered but without a clearly definitive role in the manifestations of MCD.
The utility of metronidazole in the treatment of MCD has been suggested as evidence that certain bacteria in the gut may either serve as the causative antigen or may induce its formation21; however, the causative antigen has yet to be identified, and whether it travels distally to the skin or merely resembles a similar antigen normally present in the dermis has not yet been determined. Some research has used in situ polymerase chain reaction techniques to attempt to detect similar microbial pathogens in both the vasculature of active bowel lesions and in the skin, but to date, bacterial RNA noted to be present in the gut vasculature adjacent to CD lesions has not been detected in skin lesions.22
Diagnosis
Physical Findings
Overall, it is estimated that roughly 56% of all MCD cases affect the external genitalia.23 The classic appearance of MCD includes well-demarcated ulcerations in the areas of intertriginous skin folds with or without diffuse edema and tenderness to palpation.23 Although MCD has been historically noted as having a predilection for moist skin folds, there are numerous case reports of MCD all over the body, including the face,7,24-29 retroauricular areas,30 arms and legs,16,17,31-34 lower abdomen,3,5 under the breasts,1 perineum,35 external genitalia,1,9,36-40 and even the lungs41 and bladder.42
As a dermatologic disease, MCD has been referred to as yet another great imitator, both on the macroscopic and microscopic levels.8 As such, more common causes of genital edema should be considered first and investigated based on the patient's history, physical examination, skin biopsy, lymphangiogram, ultrasound, and cystogram.43 Ultrasonography and color Doppler sonography have been shown to be helpful in patients with genital involvement. This modality can evaluate not only the presence of normal testes but also intratesticular and scrotal wall fluid, especially when the physical examination reveals swelling that makes testicle palpation more difficult.6 Clinically, the correct diagnosis of MCD often is made through suspicion of inflammatory bowel disease based on classic symptoms and/or physical findings including abdominal pain, weight loss, bloody stool, diarrhea, perianal skin tags, and anal fissures or fistulas. Any of these GI findings should prompt an intestinal biopsy to rule out any histologic evidence of CD.
Metastatic CD affecting the vulva often presents with vulvar pain and pruritus and may clinically mimic a more benign disease such as balanitis plasmacellularis, also referred to as Zoon vulvitis.23 Similar to MCD on any given body surface, there is dramatic variation in the macroscopic presentation of vulvar MCD, with physical examination findings ranging from bilateral diffuse, edematous, deeply macerated, red, ulcerated lesions over the vulva with lymphadenopathy to findings of bilateral vulvar pain with yellow drainage from the labia majora.23 There have been cases of vulvar MCD that include exquisite vulvar pain but without structural abnormalities including normal uterus, cervix, adnexa, rectovaginal septum, and rectum. In these more nebulous cases of vulvar MCD, the diagnosis often is discovered incidentally when nonspecific diagnostic imaging suggests underlying CD.23
Beyond the case-by-case variations on physical examination, the great difficulty in diagnosis, particularly in children, occurs in the absence of any GI symptoms and therefore no logical consideration of underlying CD. Consequently, there have been cases of children presenting with irritation of the vulva who were eventually diagnosed with MCD only after erroneous treatment of contact dermatitis, candidiasis, and even consideration of sexual abuse.37 Because it is so rare and obscure among practicing clinicians, the diagnosis of MCD often is considered only after irritation or swelling of the external genitalia has not responded to standard therapies. If and when the diagnosis of MCD is considered in children, it has been suggested to screen patients for anorectal stricture, as case studies have found the condition to be relatively common in this subpopulation.44
In the less common case of adults with genitourinary symptoms that suggest possible MCD, the differential diagnosis for penile or vaginal ulcers should include contact and irritant dermatitis, chronic infectious lesions (eg, hidradenitis suppurativa, actinomycosis, tuberculosis),45 sexually transmitted ulcerative diseases (eg, chancroid, lymphogranuloma venereum, herpes genitalia, granuloma inguinale),46 drug reactions, and even extramammary Paget disease.47
Histologic Findings
Because MCD has so much macroscopic variation and can present anywhere on the surface of the body, formal diagnosis relies on microscopy. As an added measure of difficulty in diagnosis, one random biopsy of a suspicious segment of tissue may not contain the expected histologic findings; therefore, clinical suspicion may warrant a second biopsy.10 There have been reported cases of an adult patient without history of CD presenting with a lesion that resembled a more common pathology, such as a genital wart, and the correct diagnosis of MCD with pseudocondylomatous morphology was made only after intestinal manifestations prompted the clinician to consider such an unusual diagnosis.48
From a histopathologic perspective, MCD is characterized by discrete, noncaseating, sarcoidlike granulomas with abundant multinucleated giant cells (Langhans giant cells) in the superficial dermis (papillary), deep dermis (reticular), and adipose tissue (Figure).8,17 In the presence of concomitant intestinal disease, the granulomas of both the intestinal and dermal tissues should share the same microscopic characteristics.8 In addition, copious neutrophils and granulomas surrounding the microvasculature have been described,34 as well as general lymphocyte and plasma cell infiltrate.45 Some histologic samples have included collagen degeneration termed necrobiosis in the middle dermal layer as another variable finding in MCD.14,34

On microscopy, it has been reported that use of Verhoeff-van Gieson staining may be helpful to highlight the presence of neutrophil obstruction within the dermal vasculature, particularly the arterial lumen, as well as to aid in highlighting swelling of the endothelium with fragmentation of the internal elastic lamina.17 Although not part of the routine diagnosis, electron microscopy of MCD tissue samples have confirmed hypertrophy of the endothelial cells composing the capillaries with resulting extravasation of fibrin, red blood cells, lymphocytes, and epithelioid histiocytes.17 Observation of tissue under direct immunofluorescence has been less helpful, as it has shown only nonspecific fibrinogen deposition within the dermis and dermal vessels.17
In an article on treatment of MCD, Escher et al43 reinforced that the macroscopic findings of MCD are diverse, and the microscopic findings characteristic of MCD also can be mimicked by other etiologies such as sarcoidosis, tuberculosis, fungal infections, lymphogranuloma venereum, leishmaniasis, and connective tissue disorders.43 As such, the workup to rule out infectious, anatomic, and autoimmune etiologies should be diverse. Often, the workup for MCD will include special stains such as Ziehl-Neelsen stain to rule out Mycobacterium tuberculosis and acid-fast bacilli and Fite stain to consider atypical mycobacteria. Other tests such as tissue culture, chest radiograph, tuberculin skin test (Mantoux test), IFN-γ release assay, or polarized light microscopy may rule out infectious etiologies.9,49 Serologic testing might include VDRL test, Treponema pallidum hemagglutination assay, hepatitis B, hepatitis C, and human immunodeficiency virus.5
Crohn disease is characterized histologically by sarcoidlike noncaseating granulomas, and as such, it is important to differentiate MCD from sarcoidosis prior to histologic analysis. Sarcoidosis also can be considered much less likely with a normal chest radiograph and in the absence of increased serum calcium and angiotensin-converting enzyme levels.7 The differentiation of sarcoidosis from MCD on the microscopic scale is subtle but is sometimes facilitated in the presence of an ulcerated epidermis or lymphocytic/eosinophilic infiltrate and edema within the dermis, all suggestive of MCD.14
Metastatic CD also should be differentiated from erythema nodosum and pyoderma gangrenosum, which are among the most common cutaneous findings associated with CD.14 Pyoderma gangrenosum can be distinguished histologically by identifying copious neutrophilic infiltrate with pseudoepitheliomatous hyperplasia.50
Treatment
Because MCD is relatively rare, there are no known randomized trials suggesting a particular medical or surgical treatment. In a review of perineal MCD from 2007, the 40-year-old recommendation by Moutain3 opting for surgical debridement versus medical management still resonates, particularly for perineal disease, as an effective measure in all but the mildest of presentations.51 However, recent case reports also suggest that the tumor necrosis factor α (TNF-α) inhibitors such as infliximab and adalimumab should be considered prior to surgery even with severe perineal MCD.51 Moreover, even if medical management with TNF-α inhibitors or some combination of immunosuppressants and antibiotics does not eradicate the disease, it often helps reduce the size of the ulcers prior to surgery.52 With a limited understanding of MCD, one might think that removal of the affected bowel would eliminate cutaneous disease, but it has been shown that this strategy is not effective.53,54
The composition and location of the particular lesion affects the trajectory of treatment. For example, MCD manifesting as local ulcers and plaques has been described as responding well to topical and intralesional steroids.10,55,56 In the case of penile swelling and/or phimosis, circumcision has been helpful to improve the patient's ability to void as well as to attain and maintain erection.10 In the case of scrotal swelling secondary to MCD, early treatment (ie, within 4 to 6 months) with oral steroids and/or metronidazole is likely beneficial to prevent refractory edematous organization of the tissue.57
As a general rule, an effective treatment will include a combination of an immunosuppressant, antibiotic therapy, and sometimes surgery. The most commonly used immunosuppressant agents include topical or intralesional steroids, infliximab,43,58 cyclosporine A,59,60 dapsone, minocycline, thalidomide, methotrexate, mycophenolate mofetil, sulfasalazine, azathioprine, tacrolimus, and 6-mercaptopurine.4 Steroids have been the conventional treatment of extraintestinal manifestations of CD61; however, perineal CD has been poorly controlled with systemic steroids.62 If steroids are found not to be effective, sometimes agents such as dapsone or thalidomide are considered. One case report noted stabilization of MCD penile ulcers with oral thalidomide 300 mg once daily, oral minocycline 100 mg once daily, and topical tacrolimus 0.3% with benzocaine twice daily with continuation of prednisolone and methotrexate as parts of previously unsuccessful regimen.52
Metronidazole is perhaps the most commonly used antibiotic, having been a component of many successful regimens.4,63 For example, a 27-year-old patient with MCD presenting as a nonhealing ulcerative lesion in the subcoronal area of the penis and scrotum was treated successfully with a 6-month course of mesalamine, prednisone, and metronidazole.45 Another case report of vulvar MCD reported initial success with intravenous methylprednisolone, ciprofloxacin, and metronidazole.23 The primary limitation of metronidazole is that subsequent tapering of the dose seems to result in recurrence of disease.64 Consequently, patients must remain on the antibiotic for an indeterminate course, with dosages ranging from 5 mg/kg daily in adolescents65 to 1000 to 1500 mg daily in adults.66
Of the various immunosuppressants available, infliximab has been listed in numerous reports as a successful agent in both the induction and maintenance of extraintestinal manifestations of CD including MCD.67-71 Infliximab has been reported to be effective in the treatment of penile and scrotal edema secondary to MCD that did not respond to other immunosuppressants including oral prednisolone, azathioprine, and cyclosporine.43 Infliximab may be a good option to help heal draining fistulas, particularly in combination with an antibiotic such as metronidazole and ciprofloxacin, which helps to prevent abscess formation during healing.72 The response to infliximab has been dramatic, with resolution of cutaneous lesions after just 6 weeks in some cases.73 The dosing regimen of infliximab has been suggested at 5 mg/kg administered at 0, 2, and 6 weeks, with subsequent maintenance infusions every 10 weeks,70 or at 0, 4, and 12 weeks, with subsequent infusions every 8 weeks.43
Adalimumab may be considered as an alternative to infliximab and is potentially less allergenic as a fully humanized monoclonal antibody to TNF-α, which also has been used successfully to both induce and maintain remission of moderate to severe CD.42,74,75 Proposed dosing of adalimumab includes a loading dose of 160 mg subcutaneously on day 1, followed by an 80-mg dose 2 weeks later and a 40-mg maintenance dose every other week indefinitely.48 Of note, adalimumab has been noted in the literature to have many potential side effects, including one particular case in which severe headaches were attributed to its use.59 As a consequence of the headaches, the patient was switched from adalimumab to cyclosporine and responded well with no subsequent flare-ups on follow-up.
In summary, treatment of MCD depends on cutaneous location, severity, physician experience with certain antibiotics or immunosuppressants, availability of medication, and patient disposition. It seems reasonable to attempt medical management with one or more medical regimens before committing to surgical intervention. Furthermore, even with debridement, curettage, skin graft, or other surgical strategy, the patient is likely to require some period of immunosuppression to provide long-lasting remission.
Conclusion
Patients with inflammatory bowel disease often develop dermatologic sequelae, with MCD being a rare but serious process. Patients may present with a wide array of physical concerns and symptoms, many resembling other disease processes. As such, education and a high index of suspicion are needed for proper diagnosis and treatment.
Almost half of Crohn disease (CD) patients experience a dermatologic manifestation of the disease. A rare entity, metastatic CD (MCD) presents a diagnostic challenge without a high index of suspicion. Its etiology is not well defined; however, it appears to be an autoimmune response to gut antigens. Herein, we review the etiology/epidemiology, diagnostic criteria, and treatment for this uncommon condition.
Epidemiology and Clinical Characteristics of MCD
Metastatic CD was first described by Parks et al1 in 1965 and refers to a diverse collection of macroscopic dermatologic manifestations in tissue not contiguous with the gastrointestinal (GI) tract. To be classified as MCD, the tissue must demonstrate characteristic histopathologic findings, which invariably include noncaseating granulomas.
Crohn disease may affect any part of the GI tract from the mouth to anus, with a multitude of associated cutaneous manifestations having been described. The terminal ileum is the most commonly affected portion of the GI tract in CD, but the large intestine also may be involved in 55% to 80% of cases.2 The incidence of non-MCD-associated anal lesions seems to correlate with intestinal involvement in that as few as 25% of patients with ileal-localized CD have anal lesions compared to nearly 80% of patients with large intestinal involvement.3
It has been estimated that 18% to 44% of patients with CD have some form of cutaneous manifestation,4 with MCD being a rare subcategory. As few as 100 cases have been described from 1965 to the present.5 The presence of MCD does not correlate well with severity of intestinal CD, and although a majority of MCD cases present after at least 6 months of GI symptoms,6 there are instances in which MCD presents without prior or existing evidence of intestinal CD.7
With regard to MCD, the term metastatic is sometimes supplanted in the literature by cutaneous to avoid any implication of cancer; however, due to a myriad of dermatologic manifestations, both terms can cause confusion. The categorization of the various types of cutaneous findings in CD is well summarized in a review by Palamaras et al8 with the following classifications: (1) granulomatous by direct extension (oral or perianal), (2) MCD lesions (genital and nongenital), (3) immune-related lesions, and (4) lesions from nutritional deficiencies. Of the cutaneous manifestations relating to CD, MCD is the least common cutaneous categorical manifestation and is further divided into subcategories of genital and nongenital lesions.8
The nongenital distribution of MCD is the more common variety in adults and particularly seems to affect the legs and plantar surfaces (38%), the trunk and abdomen (24%), and the face (15%).5,9 These nongenital MCD manifestations are most commonly described as nodules, ulcerations, or erythematous to purple plaques, and less commonly described as abscesses, pustules, or papules.
The sequence of cutaneous symptoms of MCD relative to intestinal disease depends to some degree on patient age. In adults diagnosed with MCD, it has been noted that a GI flare is expected 2 months to 4 years after diagnosis; however, in children the subsequent GI flare has been noted to vary more widely from 9 months to 14 years following presentation of MCD.8 Furthermore, roughly 50% of children diagnosed with MCD present concomitantly with their first symptoms of a GI flare, whereas 70% of adults with MCD had been previously diagnosed with intestinal CD.8 In one review of 80 reported cases of MCD, 20% (16/80) had no symptoms of intestinal disease at the time of MCD diagnosis, and the majority of the asymptomatic cases were in children; interestingly, the majority of these same children were diagnosed with CD months to years later.9
Both the location and characteristics of cutaneous findings in MCD correlate with age.9 Metastatic CD has been identified in all age groups; however, lymphedema is more common in children/young adults, while nodules, ulceration, and fistulating disease are more often seen in adults.10 Affected children and adolescents with MCD range from 5 to 17 years of age, with a mean age at disease onset of 11.1 years and equal incidence in males and females.8 Adults with MCD range from 18 to 78 years of age, with a mean age at presentation of 38.4 years.8,11
Concerning anatomic location of disease, adults with MCD most commonly have nodules with or without plaques on the arms and legs and less commonly in the genital area.8 In contrast, children with MCD are more prone to genital lesions, with up to 85% of cases including some degree of genital erythematous or nonerythematous swelling with or without induration.8 Genitourinary complications of CD as a broad category, however, are estimated to occur in only 5% to 20% of intestinal CD cases in both children and adults.12
There have been conflicting reports regarding gender predilection in MCD. Based on a review by Samitz et al13 of 200 cases of CD over an 18-year period, 22% of patients with CD were found to have cutaneous manifestations--presumably not MCD but rather perianal, perineal, vulvar fistulae, fissures, or abscesses--with a male to female preponderance of almost 2 to 1. A more recent review of the literature by Palamaras et al8 in 2008 reported that contiguous non-MCD affects adult females and children more often than adult males, with 63% adult cases being female. This review seems to be more congruent with other reports in the literature implicating that females are twice as commonly affected by MCD than males.9,14
Pathophysiology
The etiology of MCD has not been well defined. One proposed mechanism of the distal tissue involvement of MCD is through passage of antigens to the skin with subsequent granulomatous response at the level of the dermis.10 Another proposed mechanism suggests antibody sensitization to gut antigens, possibly bacterial antigens, that then coincidentally cross-react with analogous skin antigens.8,14 Burgdorf11 supported this notion in a 1981 report in which it was suggested that the granulomatous reaction was related to deposition of immune complexes in the skin. Slater et al15 and Tatnall et al16 offered a variation of Burgdorf's notion, suggesting that it was sensitized T cells to circulating antigens that were the initiators of granuloma formation in the periphery.
An examination of MCD tissue in 1990 by Shum and Guenther17 under electron microscopy and immunofluorescence provided evidence against prior studies that purported to have identified immune complexes as the causative agents of MCD. In this study, the authors found no evidence of immune complexes in the dermis of MCD lesions. In addition, an attempt to react serum antibodies of a patient with MCD, which were postulated to have IgG, IgM, and IgA antibodies to specific gut antigens, yielded no response when reacted with the tongue, ileum, and colon tissue from a rat. As a culminant finding, the authors also noted MCD dermis tissue with granulomas without vasculitis, suggesting a T-cell mediated type IV hypersensitivity response with a secondary vasculitis from T-cell origin lymphokines and T-cell mediated monocyte activation.17
Research implicating other immunologic entities involved in the pathophysiology of CD such as β-2 integrin,18 CD14+ monocytes,19 and the role of the DNA repair gene MLH1 (mutL homolog 1)20 have been considered but without a clearly definitive role in the manifestations of MCD.
The utility of metronidazole in the treatment of MCD has been suggested as evidence that certain bacteria in the gut may either serve as the causative antigen or may induce its formation21; however, the causative antigen has yet to be identified, and whether it travels distally to the skin or merely resembles a similar antigen normally present in the dermis has not yet been determined. Some research has used in situ polymerase chain reaction techniques to attempt to detect similar microbial pathogens in both the vasculature of active bowel lesions and in the skin, but to date, bacterial RNA noted to be present in the gut vasculature adjacent to CD lesions has not been detected in skin lesions.22
Diagnosis
Physical Findings
Overall, it is estimated that roughly 56% of all MCD cases affect the external genitalia.23 The classic appearance of MCD includes well-demarcated ulcerations in the areas of intertriginous skin folds with or without diffuse edema and tenderness to palpation.23 Although MCD has been historically noted as having a predilection for moist skin folds, there are numerous case reports of MCD all over the body, including the face,7,24-29 retroauricular areas,30 arms and legs,16,17,31-34 lower abdomen,3,5 under the breasts,1 perineum,35 external genitalia,1,9,36-40 and even the lungs41 and bladder.42
As a dermatologic disease, MCD has been referred to as yet another great imitator, both on the macroscopic and microscopic levels.8 As such, more common causes of genital edema should be considered first and investigated based on the patient's history, physical examination, skin biopsy, lymphangiogram, ultrasound, and cystogram.43 Ultrasonography and color Doppler sonography have been shown to be helpful in patients with genital involvement. This modality can evaluate not only the presence of normal testes but also intratesticular and scrotal wall fluid, especially when the physical examination reveals swelling that makes testicle palpation more difficult.6 Clinically, the correct diagnosis of MCD often is made through suspicion of inflammatory bowel disease based on classic symptoms and/or physical findings including abdominal pain, weight loss, bloody stool, diarrhea, perianal skin tags, and anal fissures or fistulas. Any of these GI findings should prompt an intestinal biopsy to rule out any histologic evidence of CD.
Metastatic CD affecting the vulva often presents with vulvar pain and pruritus and may clinically mimic a more benign disease such as balanitis plasmacellularis, also referred to as Zoon vulvitis.23 Similar to MCD on any given body surface, there is dramatic variation in the macroscopic presentation of vulvar MCD, with physical examination findings ranging from bilateral diffuse, edematous, deeply macerated, red, ulcerated lesions over the vulva with lymphadenopathy to findings of bilateral vulvar pain with yellow drainage from the labia majora.23 There have been cases of vulvar MCD that include exquisite vulvar pain but without structural abnormalities including normal uterus, cervix, adnexa, rectovaginal septum, and rectum. In these more nebulous cases of vulvar MCD, the diagnosis often is discovered incidentally when nonspecific diagnostic imaging suggests underlying CD.23
Beyond the case-by-case variations on physical examination, the great difficulty in diagnosis, particularly in children, occurs in the absence of any GI symptoms and therefore no logical consideration of underlying CD. Consequently, there have been cases of children presenting with irritation of the vulva who were eventually diagnosed with MCD only after erroneous treatment of contact dermatitis, candidiasis, and even consideration of sexual abuse.37 Because it is so rare and obscure among practicing clinicians, the diagnosis of MCD often is considered only after irritation or swelling of the external genitalia has not responded to standard therapies. If and when the diagnosis of MCD is considered in children, it has been suggested to screen patients for anorectal stricture, as case studies have found the condition to be relatively common in this subpopulation.44
In the less common case of adults with genitourinary symptoms that suggest possible MCD, the differential diagnosis for penile or vaginal ulcers should include contact and irritant dermatitis, chronic infectious lesions (eg, hidradenitis suppurativa, actinomycosis, tuberculosis),45 sexually transmitted ulcerative diseases (eg, chancroid, lymphogranuloma venereum, herpes genitalia, granuloma inguinale),46 drug reactions, and even extramammary Paget disease.47
Histologic Findings
Because MCD has so much macroscopic variation and can present anywhere on the surface of the body, formal diagnosis relies on microscopy. As an added measure of difficulty in diagnosis, one random biopsy of a suspicious segment of tissue may not contain the expected histologic findings; therefore, clinical suspicion may warrant a second biopsy.10 There have been reported cases of an adult patient without history of CD presenting with a lesion that resembled a more common pathology, such as a genital wart, and the correct diagnosis of MCD with pseudocondylomatous morphology was made only after intestinal manifestations prompted the clinician to consider such an unusual diagnosis.48
From a histopathologic perspective, MCD is characterized by discrete, noncaseating, sarcoidlike granulomas with abundant multinucleated giant cells (Langhans giant cells) in the superficial dermis (papillary), deep dermis (reticular), and adipose tissue (Figure).8,17 In the presence of concomitant intestinal disease, the granulomas of both the intestinal and dermal tissues should share the same microscopic characteristics.8 In addition, copious neutrophils and granulomas surrounding the microvasculature have been described,34 as well as general lymphocyte and plasma cell infiltrate.45 Some histologic samples have included collagen degeneration termed necrobiosis in the middle dermal layer as another variable finding in MCD.14,34

On microscopy, it has been reported that use of Verhoeff-van Gieson staining may be helpful to highlight the presence of neutrophil obstruction within the dermal vasculature, particularly the arterial lumen, as well as to aid in highlighting swelling of the endothelium with fragmentation of the internal elastic lamina.17 Although not part of the routine diagnosis, electron microscopy of MCD tissue samples have confirmed hypertrophy of the endothelial cells composing the capillaries with resulting extravasation of fibrin, red blood cells, lymphocytes, and epithelioid histiocytes.17 Observation of tissue under direct immunofluorescence has been less helpful, as it has shown only nonspecific fibrinogen deposition within the dermis and dermal vessels.17
In an article on treatment of MCD, Escher et al43 reinforced that the macroscopic findings of MCD are diverse, and the microscopic findings characteristic of MCD also can be mimicked by other etiologies such as sarcoidosis, tuberculosis, fungal infections, lymphogranuloma venereum, leishmaniasis, and connective tissue disorders.43 As such, the workup to rule out infectious, anatomic, and autoimmune etiologies should be diverse. Often, the workup for MCD will include special stains such as Ziehl-Neelsen stain to rule out Mycobacterium tuberculosis and acid-fast bacilli and Fite stain to consider atypical mycobacteria. Other tests such as tissue culture, chest radiograph, tuberculin skin test (Mantoux test), IFN-γ release assay, or polarized light microscopy may rule out infectious etiologies.9,49 Serologic testing might include VDRL test, Treponema pallidum hemagglutination assay, hepatitis B, hepatitis C, and human immunodeficiency virus.5
Crohn disease is characterized histologically by sarcoidlike noncaseating granulomas, and as such, it is important to differentiate MCD from sarcoidosis prior to histologic analysis. Sarcoidosis also can be considered much less likely with a normal chest radiograph and in the absence of increased serum calcium and angiotensin-converting enzyme levels.7 The differentiation of sarcoidosis from MCD on the microscopic scale is subtle but is sometimes facilitated in the presence of an ulcerated epidermis or lymphocytic/eosinophilic infiltrate and edema within the dermis, all suggestive of MCD.14
Metastatic CD also should be differentiated from erythema nodosum and pyoderma gangrenosum, which are among the most common cutaneous findings associated with CD.14 Pyoderma gangrenosum can be distinguished histologically by identifying copious neutrophilic infiltrate with pseudoepitheliomatous hyperplasia.50
Treatment
Because MCD is relatively rare, there are no known randomized trials suggesting a particular medical or surgical treatment. In a review of perineal MCD from 2007, the 40-year-old recommendation by Moutain3 opting for surgical debridement versus medical management still resonates, particularly for perineal disease, as an effective measure in all but the mildest of presentations.51 However, recent case reports also suggest that the tumor necrosis factor α (TNF-α) inhibitors such as infliximab and adalimumab should be considered prior to surgery even with severe perineal MCD.51 Moreover, even if medical management with TNF-α inhibitors or some combination of immunosuppressants and antibiotics does not eradicate the disease, it often helps reduce the size of the ulcers prior to surgery.52 With a limited understanding of MCD, one might think that removal of the affected bowel would eliminate cutaneous disease, but it has been shown that this strategy is not effective.53,54
The composition and location of the particular lesion affects the trajectory of treatment. For example, MCD manifesting as local ulcers and plaques has been described as responding well to topical and intralesional steroids.10,55,56 In the case of penile swelling and/or phimosis, circumcision has been helpful to improve the patient's ability to void as well as to attain and maintain erection.10 In the case of scrotal swelling secondary to MCD, early treatment (ie, within 4 to 6 months) with oral steroids and/or metronidazole is likely beneficial to prevent refractory edematous organization of the tissue.57
As a general rule, an effective treatment will include a combination of an immunosuppressant, antibiotic therapy, and sometimes surgery. The most commonly used immunosuppressant agents include topical or intralesional steroids, infliximab,43,58 cyclosporine A,59,60 dapsone, minocycline, thalidomide, methotrexate, mycophenolate mofetil, sulfasalazine, azathioprine, tacrolimus, and 6-mercaptopurine.4 Steroids have been the conventional treatment of extraintestinal manifestations of CD61; however, perineal CD has been poorly controlled with systemic steroids.62 If steroids are found not to be effective, sometimes agents such as dapsone or thalidomide are considered. One case report noted stabilization of MCD penile ulcers with oral thalidomide 300 mg once daily, oral minocycline 100 mg once daily, and topical tacrolimus 0.3% with benzocaine twice daily with continuation of prednisolone and methotrexate as parts of previously unsuccessful regimen.52
Metronidazole is perhaps the most commonly used antibiotic, having been a component of many successful regimens.4,63 For example, a 27-year-old patient with MCD presenting as a nonhealing ulcerative lesion in the subcoronal area of the penis and scrotum was treated successfully with a 6-month course of mesalamine, prednisone, and metronidazole.45 Another case report of vulvar MCD reported initial success with intravenous methylprednisolone, ciprofloxacin, and metronidazole.23 The primary limitation of metronidazole is that subsequent tapering of the dose seems to result in recurrence of disease.64 Consequently, patients must remain on the antibiotic for an indeterminate course, with dosages ranging from 5 mg/kg daily in adolescents65 to 1000 to 1500 mg daily in adults.66
Of the various immunosuppressants available, infliximab has been listed in numerous reports as a successful agent in both the induction and maintenance of extraintestinal manifestations of CD including MCD.67-71 Infliximab has been reported to be effective in the treatment of penile and scrotal edema secondary to MCD that did not respond to other immunosuppressants including oral prednisolone, azathioprine, and cyclosporine.43 Infliximab may be a good option to help heal draining fistulas, particularly in combination with an antibiotic such as metronidazole and ciprofloxacin, which helps to prevent abscess formation during healing.72 The response to infliximab has been dramatic, with resolution of cutaneous lesions after just 6 weeks in some cases.73 The dosing regimen of infliximab has been suggested at 5 mg/kg administered at 0, 2, and 6 weeks, with subsequent maintenance infusions every 10 weeks,70 or at 0, 4, and 12 weeks, with subsequent infusions every 8 weeks.43
Adalimumab may be considered as an alternative to infliximab and is potentially less allergenic as a fully humanized monoclonal antibody to TNF-α, which also has been used successfully to both induce and maintain remission of moderate to severe CD.42,74,75 Proposed dosing of adalimumab includes a loading dose of 160 mg subcutaneously on day 1, followed by an 80-mg dose 2 weeks later and a 40-mg maintenance dose every other week indefinitely.48 Of note, adalimumab has been noted in the literature to have many potential side effects, including one particular case in which severe headaches were attributed to its use.59 As a consequence of the headaches, the patient was switched from adalimumab to cyclosporine and responded well with no subsequent flare-ups on follow-up.
In summary, treatment of MCD depends on cutaneous location, severity, physician experience with certain antibiotics or immunosuppressants, availability of medication, and patient disposition. It seems reasonable to attempt medical management with one or more medical regimens before committing to surgical intervention. Furthermore, even with debridement, curettage, skin graft, or other surgical strategy, the patient is likely to require some period of immunosuppression to provide long-lasting remission.
Conclusion
Patients with inflammatory bowel disease often develop dermatologic sequelae, with MCD being a rare but serious process. Patients may present with a wide array of physical concerns and symptoms, many resembling other disease processes. As such, education and a high index of suspicion are needed for proper diagnosis and treatment.
- Parks AG, Morson BC, Pegum JS. Crohn's disease with cutaneous involvement. Proc R Soc Med. 1965;58:241-242.
- Friedman S, Blumber RS. Inflammatory bowel disease. In: Kasper DL, Braunwald E, Fauci AS, et al, eds. Harrison's Principles of Internal Medicine. 16th ed. New York, NY: McGraw-Hill; 2005:1778-1784.
- Moutain JC. Cutaneous ulceration in Crohn's disease. Gut. 1970;11:18-26.
- Lester LU, Rapini RP. Dermatologic manifestations of colonic disorders. Curr Opin Gastroenterol. 2008;25:66-73.
- Teixeira M, Machado S, Lago P, et al. Cutaneous Crohn's disease. Int J Dermatol. 2006;45:1074-1076.
- Simoneaux SF, Ball TI, Atkinson GO Jr. Scrotal swelling: unusual first presentation of Crohn's disease. Pediatr Radiol. 1995;25:375-376.
- Albuquerque A, Magro F, Rodrigues S, et al. Metastatic cutaneous Crohn's disease of the face: a case report and review of literature. Eur J Gastroenterol Hepatol. 2011;23:954-956.
- Palamaras I, El-Jabbour J, Pietropaolo N, et al. Metastatic Crohn's disease: a review. J Eur Acad Dermatol Venereol. 2008;22:1033-1043.
- Ploysangam T, Heubi JE, Eisen D, et al. Cutaneous Crohn's disease in children. J Am Acad Dermatol. 1997;36:697-704.
- Vint R, Husain E, Hassain F, et al. Metastatic Crohn's disease of the penis: two cases. Int Urol Nephrol. 2012;44:45-49.
- Burgdorf W. Cutaneous manifestations of Crohn's disease. J Am Acad Dermatol. 1981;5:689-695.
- Resnick MI, Kursh ED. Extrinsic obstruction of the ureter. In: Walsh PC, Retik AB, Stamey TA, et al, eds. Campbell's Urology. 7th ed. Philadelphia, PA: WB Saunders; 1998:400-402.
- Samitz MH, Dana AS Jr, Rosenberg P. Cutaneous vasculitis in association with Crohn's disease--review of statistics of skin complications. Cutis. 1970;6:51-56.
- Emanuel PO, Phelps RG. Metastatic Crohn's disease: a histo-pathologic study of 12 cases. J Cutan Pathol. 2008;35:457-461.
- Slater DN, Waller PC, Reilly G. Cutaneous granulomatous vasculitis: presenting features of Crohn's disease. J R Soc Med. 1985;78:589-590.
- Tatnall FM, Dodd HJ, Sarkany I. Crohn's disease with metastatic cutaneous involvement and granulomatous cheilitis. J R Soc Med. 1987;80:49-51.
- Shum DT, Guenther L. Metastatic Crohn's disease: case report and review of literature. Arch Dermatol. 1990;126:645-648.
- Bernstein CN, Sargent M, Gallatin WM. Beta2 integrin/ICAM expression in Crohn's disease. Clin Immunol Immunopathol. 1998;86:147-160.
- Grimm MC, Pavli P, Van de Pol E, et al. Evidence for a CD-14+ population of monocytes in inflammatory bowel disease mucosa--implications for pathogenesis. Clin Exp Immunol. 1995;100:291-297.
- Pokorny RM, Hofmeister A, Galandiuk S, et al. Crohn's disease and ulcerative colitis are associated with the DNA repair gene MLH1. Ann Surg. 1997;225:718-723; discussion 723-725.
- Ursing B, Kamme C. Metronidazole for Crohn's disease. Lancet. 1975;1:775-777.
- Crowson AN, Nuovo GJ, Mihm MC Jr, et al. Cutaneous manifestations of Crohn's disease, its spectrum, and pathogenesis: intracellular consensus bacterial 16S rRNA is associated with the gastrointestinal but not the cutaneous manifestations of Crohn's disease. Hum Pathol. 2003;34:1185.
- Leu S, Sun PK, Collyer J, et al. Clinical spectrum of vulva metastatic Crohn's disease. Dig Dis Sci. 2009;54:1565-1571.
- Chen W, Blume-Peytavi U, Goerdt S, et al. Metastatic Crohn's disease of the face. J Am Acad Dermatol. 1996;35:986-988.
- Ogram AE, Sobanko JF, Nigra TP. Metastatic cutaneous Crohn disease of the face: a case report and review of the literature. Cutis. 2010;85:25-27.
- Graham D, Jager D, Borum M. Metastatic Crohn's disease of the face. Dig Dis Sci. 2006;51:2062-2063.
- Biancone L, Geboes K, Spagnoli LG, et al. Metastatic Crohn's disease of the forehead. Inflamm Bowel Dis. 2002;8:101-105.
- Kolansky G, Green CK, Dubin H. Metastatic Crohn's disease of the face: an uncommon presentation. Arch Dermatol. 1993;129:1348-1349.
- Mahadevan U, Sandborn WJ. Infliximab for the treatment of orofacial Crohn's disease. Inflamm Bowel Dis. 2001;7:38-42.
- McCallum DI, Gray WM. Metastatic Crohn's disease. Br J Dermatol. 1976;95:551-554.
- Lieberman TR, Greene JF Jr. Transient subcutaneous granulomatosis of the upper extremities in Crohn's disease. Am J Gastroenterol. 1979;72:89-91.
- Kafity AA, Pellegrini AE, Fromkes JJ. Metastatic Crohn's disease: a rare cutaneous manifestation. J Clin Gastroenterol. 1993;17:300-303.
- Marotta PJ, Reynolds RP. Metastatic Crohn's disease. Am J Gastroenterol. 1996;91:373-375.
- Hackzell-Bradley M, Hedblad MA, Stephansson EA. Metastatic Crohn's disease. report of 3 cases with special reference to histopathologic findings. Arch Dermatol. 1996;132:928-932.
- van Dulleman HM, de Jong E, Slors F, et al. Treatment of therapy resistant perineal metastatic Crohn's disease after proctectomy using anti-tumor necrosis factor chimeric monoclonal antibody, cA2: report of two cases. Dis Colon Rectum. 1998;41:98-102.
- Lavery HA, Pinkerton JH, Sloan J. Crohn's disease of the vulva--two further cases. Br J Dermatol. 1985;113:359-363.
- Lally MR, Orenstein SR, Cohen BA. Crohn's disease of the vulva in an 8-year-old girl. Pediatr Dermatol. 1988;5:103-106.
- Tuffnell D, Buchan PC. Crohn's disease of the vulva in childhood. Br J Clin Pract. 1991;45:159-160.
- Schrodt BJ, Callen JP. Metastatic Crohn's disease presenting as chronic perivulvar and perirectal ulcerations in an adolescent patient. Pediatrics. 1999;103:500-502.
- Slaney G, Muller S, Clay J, et al. Crohn's disease involving the penis. Gut. 1986;27:329-333.
- Calder CJ, Lacy D, Raafat F, et al. Crohn's disease with pulmonary involvement in a 3 year old boy. Gut. 1993;34:1636-1638.
- Saha S, Fichera A, Bales G, et al. Metastatic Crohn's disease of the bladder. Inflamm Bowel Dis. 2008;14:140-142.
- Escher JC, Stoof TJ, van Deventer SJ, et al. Successful treatment of metastatic Crohn disease with infliximab. J Pediatr Gastroenterol Nutr. 2002;34:420-423.
- Saadah OI, Oliver MR, Bines JE, et al. Anorectal strictures and genital Crohn's disease: an unusual clinical association. J Pediatr Gastroenterol Nutr. 2003;36:403-406.
- Martinez-Salamanca JI, Jara J, Miralles P, et al. Metastatic Crohn's disease: penile and scrotal involvement. Scand J Urol Nephrol. 2004;38:436-437.
- Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417-429.
- Acker SM, Sahn EE, Rogers HC, et al. Genital cutaneous Crohn disease. Am J Dermatopathol. 2000;22:443-446.
- Lestre S, Ramos J, Joao A, et al. Cutaneous Crohn's disease presenting as genital warts: successful treatment with adalimumab. Eur J Dermatol. 2010;20:504-505.
- Yu JT, Chong LY, Lee KC. Metastatic Crohn's disease in a Chinese girl. Hong Kong Med J. 2006;12:467-469.
- Wilson-Jones E, Winkelmann RK. Superficial granulomatous pyoderma: a localized vegetative form of pyoderma gangrenosum. J Am Acad Dermatol. 1988;18:511-521.
- Moyes LH, Glen P, Pickford IR. Perineal metastatic Crohn's disease: a case report and review of the literature. Ann R Coll Surg Engl. 2007;89:W1-W3.
- Rajpara SM, Siddha SK, Ormerod AD, et al. Cutaneous penile and perianal Crohn's disease treated with a combination of medical and surgical interventions. Australas J Dermatol. 2008;49:21-24.
- Cockburn AG, Krolikowski J, Balogh K, et al. Crohn disease of penile and scrotal skin. Urology. 1980;15:596-598.
- Guest GD, Fink RL. Metastatic Crohn's disease: case report of an unusual variant and review of the literature. Dis Colon Rectum. 2000;43:1764-1766.
- Sangueza OP, Davis LS, Gourdin FW. Metastatic Crohn disease. South Med J. 1997;90:897-900.
- Chiba M, Iizuka M, Horie Y, et al. Metastatic Crohn's disease involving the penis. J Gastroenterol. 1997;32:817-821.
- Poon KS, Gilks CB, Masterson JS. Metastatic Crohn's disease involving the genitalia. J Urol. 2002;167:2541-2542.
- Shanahan F. Anti-TNF therapy for Crohn's disease: a perspective (infliximab is not the drug we have been waiting for). Inflamm Bowel Dis. 2000;6:137-139.
- Carranza DC, Young L. Successful treatment of metastatic Crohn's disease with cyclosporine. J Drugs Dermatol. 2008;7:789-791.
- Bardazzi F, Guidetti MS, Passarini B, et al. Cyclosporine A in metastatic Crohn's disease. Acta Derm Venereol. 1995;75:324-325.
- Faubion WA Jr, Loftus EV Jr, Harmsen WS, et al. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology. 2001;121:255-260.
- Gelbmann CM, Rogler G, Gross V, et al. Prior bowel resections, perianal disease, and a high initial Crohn's disease activity index are associated with corticosteroid resistance in active Crohn's disease. Am J Gastroenterol. 2002;97:1438-1445.
- Thukral C, Travassos WJ, Peppercorn MA. The role of antibiotics in inflammatory bowel disease. Curr Treat Options Gastroenterol. 2005;8:223-228.
- Brandt LJ, Berstein LH, Boley SJ, et al. Metronidazole therapy for perineal Crohn's disease: a follow-up study. Gastroenterology. 1982;83:383-387.
- Lehrnbecher T, Kontny HU, Jeschke R. Metastatic Crohn's disease in a 9-year-old boy. J Pediatr Gastroenterol Nutr. 1999;28:321-323.
- Abide JM. Metastatic Crohn disease: clearance with metronidazole. J Am Acad Dermatol. 2011;64:448-449.
- Rispo A, Scarpa R, Di Girolamo E, et al. Infliximab in the treatment of extra-intestinal manifestations of Crohn's disease. Scand J Rheumatol. 2005;34:387-391.
- Kaufman I, Caspi D, Yeshurun D, et al. The effect of infliximab on extraintestinal manifestations of Crohn's disease. Rheumatol Int. 2005;25:406-410.
- Konrad A, Seibold F. Response of cutaneous Crohn's disease to infliximab and methotrexate. Dig Liver Dis. 2003;35:351-356.
- Miller AM, Elliott PR, Fink R, et al. Rapid response of severe refractory metastatic Crohn's disease to infliximab. J Gastroenterol Hepatol. 2001;16:940-942.
- Chuah JH, Kim DS, Allen C, et al. Metastatic Crohn's disease of the ear. Int J Otolaryngol. 2009;2009:871567.
- Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med. 1999;340:1398-1405.
- Petrolati A, Altavilla N, Cipolla R, et al. Cutaneous metastatic Crohn's disease responsive to infliximab. Am J Gastroenterol. 2009;104:1058.
- Hanauer SB, Sandborn WJ, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn's disease: the CLASSIC-I trial. Gastroenterology. 2006;130:323-333.
- Cury DB, Moss A, Elias G, et al. Adalimumab for cutaneous metastatic Crohn's disease. Inflamm Bowel Dis. 2010;16:723-724.
- Parks AG, Morson BC, Pegum JS. Crohn's disease with cutaneous involvement. Proc R Soc Med. 1965;58:241-242.
- Friedman S, Blumber RS. Inflammatory bowel disease. In: Kasper DL, Braunwald E, Fauci AS, et al, eds. Harrison's Principles of Internal Medicine. 16th ed. New York, NY: McGraw-Hill; 2005:1778-1784.
- Moutain JC. Cutaneous ulceration in Crohn's disease. Gut. 1970;11:18-26.
- Lester LU, Rapini RP. Dermatologic manifestations of colonic disorders. Curr Opin Gastroenterol. 2008;25:66-73.
- Teixeira M, Machado S, Lago P, et al. Cutaneous Crohn's disease. Int J Dermatol. 2006;45:1074-1076.
- Simoneaux SF, Ball TI, Atkinson GO Jr. Scrotal swelling: unusual first presentation of Crohn's disease. Pediatr Radiol. 1995;25:375-376.
- Albuquerque A, Magro F, Rodrigues S, et al. Metastatic cutaneous Crohn's disease of the face: a case report and review of literature. Eur J Gastroenterol Hepatol. 2011;23:954-956.
- Palamaras I, El-Jabbour J, Pietropaolo N, et al. Metastatic Crohn's disease: a review. J Eur Acad Dermatol Venereol. 2008;22:1033-1043.
- Ploysangam T, Heubi JE, Eisen D, et al. Cutaneous Crohn's disease in children. J Am Acad Dermatol. 1997;36:697-704.
- Vint R, Husain E, Hassain F, et al. Metastatic Crohn's disease of the penis: two cases. Int Urol Nephrol. 2012;44:45-49.
- Burgdorf W. Cutaneous manifestations of Crohn's disease. J Am Acad Dermatol. 1981;5:689-695.
- Resnick MI, Kursh ED. Extrinsic obstruction of the ureter. In: Walsh PC, Retik AB, Stamey TA, et al, eds. Campbell's Urology. 7th ed. Philadelphia, PA: WB Saunders; 1998:400-402.
- Samitz MH, Dana AS Jr, Rosenberg P. Cutaneous vasculitis in association with Crohn's disease--review of statistics of skin complications. Cutis. 1970;6:51-56.
- Emanuel PO, Phelps RG. Metastatic Crohn's disease: a histo-pathologic study of 12 cases. J Cutan Pathol. 2008;35:457-461.
- Slater DN, Waller PC, Reilly G. Cutaneous granulomatous vasculitis: presenting features of Crohn's disease. J R Soc Med. 1985;78:589-590.
- Tatnall FM, Dodd HJ, Sarkany I. Crohn's disease with metastatic cutaneous involvement and granulomatous cheilitis. J R Soc Med. 1987;80:49-51.
- Shum DT, Guenther L. Metastatic Crohn's disease: case report and review of literature. Arch Dermatol. 1990;126:645-648.
- Bernstein CN, Sargent M, Gallatin WM. Beta2 integrin/ICAM expression in Crohn's disease. Clin Immunol Immunopathol. 1998;86:147-160.
- Grimm MC, Pavli P, Van de Pol E, et al. Evidence for a CD-14+ population of monocytes in inflammatory bowel disease mucosa--implications for pathogenesis. Clin Exp Immunol. 1995;100:291-297.
- Pokorny RM, Hofmeister A, Galandiuk S, et al. Crohn's disease and ulcerative colitis are associated with the DNA repair gene MLH1. Ann Surg. 1997;225:718-723; discussion 723-725.
- Ursing B, Kamme C. Metronidazole for Crohn's disease. Lancet. 1975;1:775-777.
- Crowson AN, Nuovo GJ, Mihm MC Jr, et al. Cutaneous manifestations of Crohn's disease, its spectrum, and pathogenesis: intracellular consensus bacterial 16S rRNA is associated with the gastrointestinal but not the cutaneous manifestations of Crohn's disease. Hum Pathol. 2003;34:1185.
- Leu S, Sun PK, Collyer J, et al. Clinical spectrum of vulva metastatic Crohn's disease. Dig Dis Sci. 2009;54:1565-1571.
- Chen W, Blume-Peytavi U, Goerdt S, et al. Metastatic Crohn's disease of the face. J Am Acad Dermatol. 1996;35:986-988.
- Ogram AE, Sobanko JF, Nigra TP. Metastatic cutaneous Crohn disease of the face: a case report and review of the literature. Cutis. 2010;85:25-27.
- Graham D, Jager D, Borum M. Metastatic Crohn's disease of the face. Dig Dis Sci. 2006;51:2062-2063.
- Biancone L, Geboes K, Spagnoli LG, et al. Metastatic Crohn's disease of the forehead. Inflamm Bowel Dis. 2002;8:101-105.
- Kolansky G, Green CK, Dubin H. Metastatic Crohn's disease of the face: an uncommon presentation. Arch Dermatol. 1993;129:1348-1349.
- Mahadevan U, Sandborn WJ. Infliximab for the treatment of orofacial Crohn's disease. Inflamm Bowel Dis. 2001;7:38-42.
- McCallum DI, Gray WM. Metastatic Crohn's disease. Br J Dermatol. 1976;95:551-554.
- Lieberman TR, Greene JF Jr. Transient subcutaneous granulomatosis of the upper extremities in Crohn's disease. Am J Gastroenterol. 1979;72:89-91.
- Kafity AA, Pellegrini AE, Fromkes JJ. Metastatic Crohn's disease: a rare cutaneous manifestation. J Clin Gastroenterol. 1993;17:300-303.
- Marotta PJ, Reynolds RP. Metastatic Crohn's disease. Am J Gastroenterol. 1996;91:373-375.
- Hackzell-Bradley M, Hedblad MA, Stephansson EA. Metastatic Crohn's disease. report of 3 cases with special reference to histopathologic findings. Arch Dermatol. 1996;132:928-932.
- van Dulleman HM, de Jong E, Slors F, et al. Treatment of therapy resistant perineal metastatic Crohn's disease after proctectomy using anti-tumor necrosis factor chimeric monoclonal antibody, cA2: report of two cases. Dis Colon Rectum. 1998;41:98-102.
- Lavery HA, Pinkerton JH, Sloan J. Crohn's disease of the vulva--two further cases. Br J Dermatol. 1985;113:359-363.
- Lally MR, Orenstein SR, Cohen BA. Crohn's disease of the vulva in an 8-year-old girl. Pediatr Dermatol. 1988;5:103-106.
- Tuffnell D, Buchan PC. Crohn's disease of the vulva in childhood. Br J Clin Pract. 1991;45:159-160.
- Schrodt BJ, Callen JP. Metastatic Crohn's disease presenting as chronic perivulvar and perirectal ulcerations in an adolescent patient. Pediatrics. 1999;103:500-502.
- Slaney G, Muller S, Clay J, et al. Crohn's disease involving the penis. Gut. 1986;27:329-333.
- Calder CJ, Lacy D, Raafat F, et al. Crohn's disease with pulmonary involvement in a 3 year old boy. Gut. 1993;34:1636-1638.
- Saha S, Fichera A, Bales G, et al. Metastatic Crohn's disease of the bladder. Inflamm Bowel Dis. 2008;14:140-142.
- Escher JC, Stoof TJ, van Deventer SJ, et al. Successful treatment of metastatic Crohn disease with infliximab. J Pediatr Gastroenterol Nutr. 2002;34:420-423.
- Saadah OI, Oliver MR, Bines JE, et al. Anorectal strictures and genital Crohn's disease: an unusual clinical association. J Pediatr Gastroenterol Nutr. 2003;36:403-406.
- Martinez-Salamanca JI, Jara J, Miralles P, et al. Metastatic Crohn's disease: penile and scrotal involvement. Scand J Urol Nephrol. 2004;38:436-437.
- Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417-429.
- Acker SM, Sahn EE, Rogers HC, et al. Genital cutaneous Crohn disease. Am J Dermatopathol. 2000;22:443-446.
- Lestre S, Ramos J, Joao A, et al. Cutaneous Crohn's disease presenting as genital warts: successful treatment with adalimumab. Eur J Dermatol. 2010;20:504-505.
- Yu JT, Chong LY, Lee KC. Metastatic Crohn's disease in a Chinese girl. Hong Kong Med J. 2006;12:467-469.
- Wilson-Jones E, Winkelmann RK. Superficial granulomatous pyoderma: a localized vegetative form of pyoderma gangrenosum. J Am Acad Dermatol. 1988;18:511-521.
- Moyes LH, Glen P, Pickford IR. Perineal metastatic Crohn's disease: a case report and review of the literature. Ann R Coll Surg Engl. 2007;89:W1-W3.
- Rajpara SM, Siddha SK, Ormerod AD, et al. Cutaneous penile and perianal Crohn's disease treated with a combination of medical and surgical interventions. Australas J Dermatol. 2008;49:21-24.
- Cockburn AG, Krolikowski J, Balogh K, et al. Crohn disease of penile and scrotal skin. Urology. 1980;15:596-598.
- Guest GD, Fink RL. Metastatic Crohn's disease: case report of an unusual variant and review of the literature. Dis Colon Rectum. 2000;43:1764-1766.
- Sangueza OP, Davis LS, Gourdin FW. Metastatic Crohn disease. South Med J. 1997;90:897-900.
- Chiba M, Iizuka M, Horie Y, et al. Metastatic Crohn's disease involving the penis. J Gastroenterol. 1997;32:817-821.
- Poon KS, Gilks CB, Masterson JS. Metastatic Crohn's disease involving the genitalia. J Urol. 2002;167:2541-2542.
- Shanahan F. Anti-TNF therapy for Crohn's disease: a perspective (infliximab is not the drug we have been waiting for). Inflamm Bowel Dis. 2000;6:137-139.
- Carranza DC, Young L. Successful treatment of metastatic Crohn's disease with cyclosporine. J Drugs Dermatol. 2008;7:789-791.
- Bardazzi F, Guidetti MS, Passarini B, et al. Cyclosporine A in metastatic Crohn's disease. Acta Derm Venereol. 1995;75:324-325.
- Faubion WA Jr, Loftus EV Jr, Harmsen WS, et al. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology. 2001;121:255-260.
- Gelbmann CM, Rogler G, Gross V, et al. Prior bowel resections, perianal disease, and a high initial Crohn's disease activity index are associated with corticosteroid resistance in active Crohn's disease. Am J Gastroenterol. 2002;97:1438-1445.
- Thukral C, Travassos WJ, Peppercorn MA. The role of antibiotics in inflammatory bowel disease. Curr Treat Options Gastroenterol. 2005;8:223-228.
- Brandt LJ, Berstein LH, Boley SJ, et al. Metronidazole therapy for perineal Crohn's disease: a follow-up study. Gastroenterology. 1982;83:383-387.
- Lehrnbecher T, Kontny HU, Jeschke R. Metastatic Crohn's disease in a 9-year-old boy. J Pediatr Gastroenterol Nutr. 1999;28:321-323.
- Abide JM. Metastatic Crohn disease: clearance with metronidazole. J Am Acad Dermatol. 2011;64:448-449.
- Rispo A, Scarpa R, Di Girolamo E, et al. Infliximab in the treatment of extra-intestinal manifestations of Crohn's disease. Scand J Rheumatol. 2005;34:387-391.
- Kaufman I, Caspi D, Yeshurun D, et al. The effect of infliximab on extraintestinal manifestations of Crohn's disease. Rheumatol Int. 2005;25:406-410.
- Konrad A, Seibold F. Response of cutaneous Crohn's disease to infliximab and methotrexate. Dig Liver Dis. 2003;35:351-356.
- Miller AM, Elliott PR, Fink R, et al. Rapid response of severe refractory metastatic Crohn's disease to infliximab. J Gastroenterol Hepatol. 2001;16:940-942.
- Chuah JH, Kim DS, Allen C, et al. Metastatic Crohn's disease of the ear. Int J Otolaryngol. 2009;2009:871567.
- Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med. 1999;340:1398-1405.
- Petrolati A, Altavilla N, Cipolla R, et al. Cutaneous metastatic Crohn's disease responsive to infliximab. Am J Gastroenterol. 2009;104:1058.
- Hanauer SB, Sandborn WJ, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn's disease: the CLASSIC-I trial. Gastroenterology. 2006;130:323-333.
- Cury DB, Moss A, Elias G, et al. Adalimumab for cutaneous metastatic Crohn's disease. Inflamm Bowel Dis. 2010;16:723-724.
Practice Points
- Almost half of patients with Crohn disease develop a dermatologic manifestation of the disease.
- The etiology of metastatic Crohn disease is unknown and diagnosis requires a high index of suspicion with exclusion of other processes.
Small skin abscesses: Add antibiotics to drainage
For patients who have a single, small skin abscess, the addition of oral antibiotics to standard incision and drainage of the lesion improves cure rates and decreases recurrence rates, according to a study published online June 28 in the New England Journal of Medicine.
The results of this multicenter prospective randomized double-blind placebo-controlled trial, taken together with those of another recent large study, “call into question the perception – largely based on expert opinion or smaller, underpowered, and lower-quality noninferiority trials – that cure rates do not improve with the addition of systemic antibiotic treatment after incision and drainage,” said Robert S. Daum, MD, professor of pediatrics at the University of Chicago, and his associates.
The trial involved 786 patients of all ages (64% were adults and 36% were children; mean age was 25.5 years) who had a single, uncomplicated skin abscess of 5 cm or smaller and were treated at the University of Chicago; San Francisco General Hospital; Harbor-UCLA Medical Center; Vanderbilt University Medical Center, Nashville; Washington University, St. Louis; or Emory University, Atlanta. A total of 266 patients were assigned to receive oral clindamycin, 263 to receive oral trimethoprim–sulfamethoxazole (TMP-SMX), and 257 to receive matching placebo for 10 days after the lesions were incised and drained.
At follow-up 7-10 days following the conclusion of treatment, the rates of clinical cure were 83.1% with clindamycin and 81.7% with TMP-SMX, both significantly greater than the 68.9% cure rate with placebo (P less than .001 for both comparisons). Similarly, at 1-month follow-up, 78.6% of the clindamycin group and 73.0% of the TMP-SMX group “remained cured,” compared with 62.6% of the placebo group (N Engl J Med. 2017 June 28. doi: 10.1056/NEJMoa1607033).
Among those with cultures positive for Staphylococcus aureus, cure rates 7-10 days after treatment ended were 83.5% and 83.2% in the clindamycin and TMP-SMX groups, respectively, significantly higher than in the placebo group (63.8%). Among those positive for methicillin-resistant S. aureus, cure rates were 81.7% and 84.6% in the clindamycin and TMP-SMX groups, respectively, significantly higher than in the placebo group (62.9%).
The rate of treatment-associated adverse events was higher with clindamycin (21.9%) than with TMP-SMX (11.1%) or with placebo (12.5%). The most common adverse events were diarrhea and nausea, which were mild to moderate in severity and resolved with sequelae. There were no cases of Clostridium difficile–associated diarrhea or severe allergic reactions. One patient had a hypersensitivity reaction that was considered to be related to TMP-SMX, which involved fever, rash, thrombocytopenia, and hepatitis and which resolved without sequelae.
The National Institute of Allergy and Infectious Diseases and the National Center for Research Resources supported the study. Dr. Daum reported ties to Pfizer, Dynavax, Theravance, and Merck, and his associates reported ties to numerous industry sources.
For patients who have a single, small skin abscess, the addition of oral antibiotics to standard incision and drainage of the lesion improves cure rates and decreases recurrence rates, according to a study published online June 28 in the New England Journal of Medicine.
The results of this multicenter prospective randomized double-blind placebo-controlled trial, taken together with those of another recent large study, “call into question the perception – largely based on expert opinion or smaller, underpowered, and lower-quality noninferiority trials – that cure rates do not improve with the addition of systemic antibiotic treatment after incision and drainage,” said Robert S. Daum, MD, professor of pediatrics at the University of Chicago, and his associates.
The trial involved 786 patients of all ages (64% were adults and 36% were children; mean age was 25.5 years) who had a single, uncomplicated skin abscess of 5 cm or smaller and were treated at the University of Chicago; San Francisco General Hospital; Harbor-UCLA Medical Center; Vanderbilt University Medical Center, Nashville; Washington University, St. Louis; or Emory University, Atlanta. A total of 266 patients were assigned to receive oral clindamycin, 263 to receive oral trimethoprim–sulfamethoxazole (TMP-SMX), and 257 to receive matching placebo for 10 days after the lesions were incised and drained.
At follow-up 7-10 days following the conclusion of treatment, the rates of clinical cure were 83.1% with clindamycin and 81.7% with TMP-SMX, both significantly greater than the 68.9% cure rate with placebo (P less than .001 for both comparisons). Similarly, at 1-month follow-up, 78.6% of the clindamycin group and 73.0% of the TMP-SMX group “remained cured,” compared with 62.6% of the placebo group (N Engl J Med. 2017 June 28. doi: 10.1056/NEJMoa1607033).
Among those with cultures positive for Staphylococcus aureus, cure rates 7-10 days after treatment ended were 83.5% and 83.2% in the clindamycin and TMP-SMX groups, respectively, significantly higher than in the placebo group (63.8%). Among those positive for methicillin-resistant S. aureus, cure rates were 81.7% and 84.6% in the clindamycin and TMP-SMX groups, respectively, significantly higher than in the placebo group (62.9%).
The rate of treatment-associated adverse events was higher with clindamycin (21.9%) than with TMP-SMX (11.1%) or with placebo (12.5%). The most common adverse events were diarrhea and nausea, which were mild to moderate in severity and resolved with sequelae. There were no cases of Clostridium difficile–associated diarrhea or severe allergic reactions. One patient had a hypersensitivity reaction that was considered to be related to TMP-SMX, which involved fever, rash, thrombocytopenia, and hepatitis and which resolved without sequelae.
The National Institute of Allergy and Infectious Diseases and the National Center for Research Resources supported the study. Dr. Daum reported ties to Pfizer, Dynavax, Theravance, and Merck, and his associates reported ties to numerous industry sources.
For patients who have a single, small skin abscess, the addition of oral antibiotics to standard incision and drainage of the lesion improves cure rates and decreases recurrence rates, according to a study published online June 28 in the New England Journal of Medicine.
The results of this multicenter prospective randomized double-blind placebo-controlled trial, taken together with those of another recent large study, “call into question the perception – largely based on expert opinion or smaller, underpowered, and lower-quality noninferiority trials – that cure rates do not improve with the addition of systemic antibiotic treatment after incision and drainage,” said Robert S. Daum, MD, professor of pediatrics at the University of Chicago, and his associates.
The trial involved 786 patients of all ages (64% were adults and 36% were children; mean age was 25.5 years) who had a single, uncomplicated skin abscess of 5 cm or smaller and were treated at the University of Chicago; San Francisco General Hospital; Harbor-UCLA Medical Center; Vanderbilt University Medical Center, Nashville; Washington University, St. Louis; or Emory University, Atlanta. A total of 266 patients were assigned to receive oral clindamycin, 263 to receive oral trimethoprim–sulfamethoxazole (TMP-SMX), and 257 to receive matching placebo for 10 days after the lesions were incised and drained.
At follow-up 7-10 days following the conclusion of treatment, the rates of clinical cure were 83.1% with clindamycin and 81.7% with TMP-SMX, both significantly greater than the 68.9% cure rate with placebo (P less than .001 for both comparisons). Similarly, at 1-month follow-up, 78.6% of the clindamycin group and 73.0% of the TMP-SMX group “remained cured,” compared with 62.6% of the placebo group (N Engl J Med. 2017 June 28. doi: 10.1056/NEJMoa1607033).
Among those with cultures positive for Staphylococcus aureus, cure rates 7-10 days after treatment ended were 83.5% and 83.2% in the clindamycin and TMP-SMX groups, respectively, significantly higher than in the placebo group (63.8%). Among those positive for methicillin-resistant S. aureus, cure rates were 81.7% and 84.6% in the clindamycin and TMP-SMX groups, respectively, significantly higher than in the placebo group (62.9%).
The rate of treatment-associated adverse events was higher with clindamycin (21.9%) than with TMP-SMX (11.1%) or with placebo (12.5%). The most common adverse events were diarrhea and nausea, which were mild to moderate in severity and resolved with sequelae. There were no cases of Clostridium difficile–associated diarrhea or severe allergic reactions. One patient had a hypersensitivity reaction that was considered to be related to TMP-SMX, which involved fever, rash, thrombocytopenia, and hepatitis and which resolved without sequelae.
The National Institute of Allergy and Infectious Diseases and the National Center for Research Resources supported the study. Dr. Daum reported ties to Pfizer, Dynavax, Theravance, and Merck, and his associates reported ties to numerous industry sources.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: For patients who have a single, small skin abscess, the addition of oral antibiotics to standard incision and drainage of the lesion improves cure rates and decreases recurrence rates.
Major finding: At follow-up 7-10 days following the completion of treatment, clinical cure rates were 83.1% with clindamycin and 81.7% with TMP-SMX, both significantly greater than the 68.9% cure rate with placebo.
Data source: A multicenter prospective randomized double-blind placebo-controlled trial involving 786 adults and children who had single, small skin abscesses.
Disclosures: The National Institute of Allergy and Infectious Diseases and the National Center for Research Resources supported the study. Dr. Daum reported ties to Pfizer, Dynavax, Theravance, and Merck, and his associates reported ties to numerous industry sources.
Recurring Yellowish Papules and Plaques on the Back
The Diagnosis: Nevus Lipomatosus Cutaneous Superficialis
A punch biopsy was obtained from a skin lesion, which showed orthokeratosis, irregular acanthosis, papillomatosis, intense edema in the upper dermis, and mature fat lobules that dissected collagen fibers in the reticular dermis (Figure). Classical-type nevus lipomatosus cutaneous superficialis (NLCS) was diagnosed based on these clinical and histopathological findings. The patient was referred to the plastic surgery clinic for total excision of all lesions.
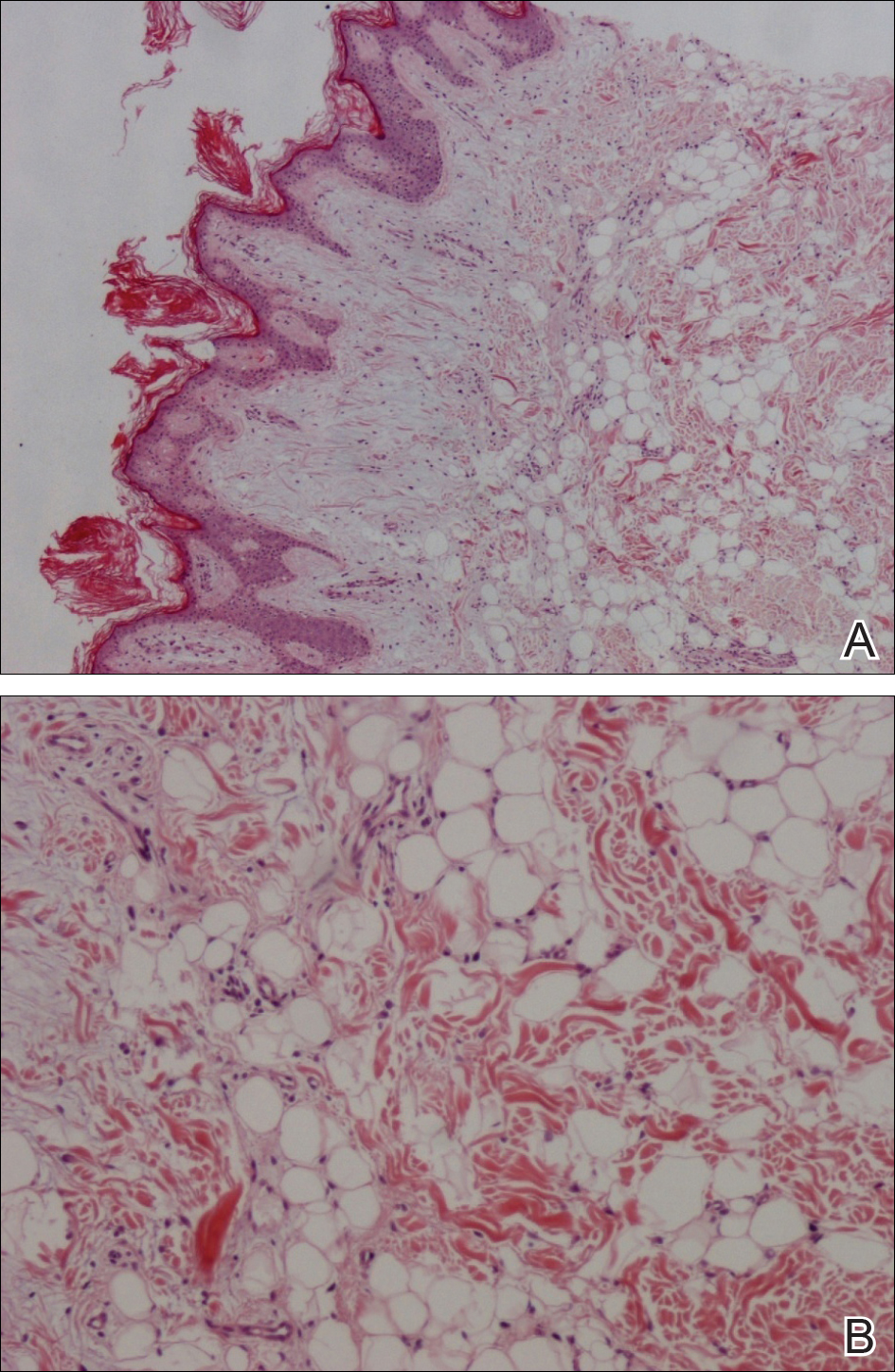
Nevus lipomatosus cutaneous superficialis is a rare hamartoma characterized by ectopic deposition of mature adipose tissue in the dermis.1 It was first described by Hoffmann and Zurhelle2 in 1921. Clinically, NLCS is classified into 2 subtypes: classical (multiple) and solitary. Classical-type NLCS is characterized by multiple pedunculated or sessile, soft, cerebriform, yellowish papules and nodules, especially in the pelvic area. Solitary-type NLCS presents as a sessile papule or nodule with no predilection for localization. Although the classical form of NLCS generally occurs in the first 2 decades of life, the solitary form usually appears in adulthood.3 Nevus lipomatosus cutaneous superficialis has no gender predilection and there is no genetic or congenital defect association.1,4
The pathogenesis of NLCS still is unknown, but some theories have been proposed, such as the development of adipose metaplasia secondary to degeneration of connective tissue, the formation of a true nevus resulting from heterotopic development of adipose tissue, and the development of mature adipocytes from pericytes in dermal vessels.1,5
Histopathology of NLCS shows clusters of ectopic mature adipose tissue in varying rates (10%-50%) between collagen bundles in the dermis. Characteristically, there is no connection between the ectopic mature adipose tissue and the subcutaneous adipose tissue.3 The differential diagnosis of NLCS includes neurofibroma, lymphangioma, sebaceous nevus, fibroepithelial polyps, leiomyoma, and lipomas.1,6
Treatment of NLCS generally involves basic surgical excision; however, patients treated with CO2 laser also have been reported in the literature.5 Because of the growth tendency and the large size of the classical form of NLCS, recurrence may occur, as in our case. In such cases, gradual surgical excision is recommended.5 We present this case to indicate that undesirable surgical results or relapse may occur in untreated patients because of lesion growth and delayed diagnosis.
- Goucha S, Khaled A, Zéglaoui F, et al. Nevus lipomatosus cutaneous superficialis: report of eight cases. Dermatol Ther (Heidelb). 2011;1:25-30.
- Hoffmann E, Zurhelle E. Ubereinen nevus lipomatodes cutaneous superficialis der linkenglutaalgegend. Arch Dermatol Syph. 1921;130:327-333.
- Patil SB, Narchal S, Paricharak M, et al. Nevus lipomatosus cutaneous superficialis: a rare case report. Iran J Med Sci. 2014;39:304-307.
- Bancalari E, Martínez-Sánchez D, Tardío JC. Nevus lipomatosus superficialis with a folliculosebaceous component: report of 2 cases. Patholog Res Int. 2011;2011:105973.
- Kim YJ, Choi JH, Kim H, et al. Recurrence of nevus lipomatosus cutaneous superficialis after CO(2) laser treatment [published online November 14, 2012]. Arch Plast Surg. 2012;39:671-673.
- Wollina U. Photoletter to the editor - nevus lipomatosus superficialis (Hoffmann-Zurhelle). three new cases including one with ulceration and one with ipsilateral gluteal hypertrophy. J Dermatol Case Rep. 2013;7:71-73.
The Diagnosis: Nevus Lipomatosus Cutaneous Superficialis
A punch biopsy was obtained from a skin lesion, which showed orthokeratosis, irregular acanthosis, papillomatosis, intense edema in the upper dermis, and mature fat lobules that dissected collagen fibers in the reticular dermis (Figure). Classical-type nevus lipomatosus cutaneous superficialis (NLCS) was diagnosed based on these clinical and histopathological findings. The patient was referred to the plastic surgery clinic for total excision of all lesions.

Nevus lipomatosus cutaneous superficialis is a rare hamartoma characterized by ectopic deposition of mature adipose tissue in the dermis.1 It was first described by Hoffmann and Zurhelle2 in 1921. Clinically, NLCS is classified into 2 subtypes: classical (multiple) and solitary. Classical-type NLCS is characterized by multiple pedunculated or sessile, soft, cerebriform, yellowish papules and nodules, especially in the pelvic area. Solitary-type NLCS presents as a sessile papule or nodule with no predilection for localization. Although the classical form of NLCS generally occurs in the first 2 decades of life, the solitary form usually appears in adulthood.3 Nevus lipomatosus cutaneous superficialis has no gender predilection and there is no genetic or congenital defect association.1,4
The pathogenesis of NLCS still is unknown, but some theories have been proposed, such as the development of adipose metaplasia secondary to degeneration of connective tissue, the formation of a true nevus resulting from heterotopic development of adipose tissue, and the development of mature adipocytes from pericytes in dermal vessels.1,5
Histopathology of NLCS shows clusters of ectopic mature adipose tissue in varying rates (10%-50%) between collagen bundles in the dermis. Characteristically, there is no connection between the ectopic mature adipose tissue and the subcutaneous adipose tissue.3 The differential diagnosis of NLCS includes neurofibroma, lymphangioma, sebaceous nevus, fibroepithelial polyps, leiomyoma, and lipomas.1,6
Treatment of NLCS generally involves basic surgical excision; however, patients treated with CO2 laser also have been reported in the literature.5 Because of the growth tendency and the large size of the classical form of NLCS, recurrence may occur, as in our case. In such cases, gradual surgical excision is recommended.5 We present this case to indicate that undesirable surgical results or relapse may occur in untreated patients because of lesion growth and delayed diagnosis.
The Diagnosis: Nevus Lipomatosus Cutaneous Superficialis
A punch biopsy was obtained from a skin lesion, which showed orthokeratosis, irregular acanthosis, papillomatosis, intense edema in the upper dermis, and mature fat lobules that dissected collagen fibers in the reticular dermis (Figure). Classical-type nevus lipomatosus cutaneous superficialis (NLCS) was diagnosed based on these clinical and histopathological findings. The patient was referred to the plastic surgery clinic for total excision of all lesions.

Nevus lipomatosus cutaneous superficialis is a rare hamartoma characterized by ectopic deposition of mature adipose tissue in the dermis.1 It was first described by Hoffmann and Zurhelle2 in 1921. Clinically, NLCS is classified into 2 subtypes: classical (multiple) and solitary. Classical-type NLCS is characterized by multiple pedunculated or sessile, soft, cerebriform, yellowish papules and nodules, especially in the pelvic area. Solitary-type NLCS presents as a sessile papule or nodule with no predilection for localization. Although the classical form of NLCS generally occurs in the first 2 decades of life, the solitary form usually appears in adulthood.3 Nevus lipomatosus cutaneous superficialis has no gender predilection and there is no genetic or congenital defect association.1,4
The pathogenesis of NLCS still is unknown, but some theories have been proposed, such as the development of adipose metaplasia secondary to degeneration of connective tissue, the formation of a true nevus resulting from heterotopic development of adipose tissue, and the development of mature adipocytes from pericytes in dermal vessels.1,5
Histopathology of NLCS shows clusters of ectopic mature adipose tissue in varying rates (10%-50%) between collagen bundles in the dermis. Characteristically, there is no connection between the ectopic mature adipose tissue and the subcutaneous adipose tissue.3 The differential diagnosis of NLCS includes neurofibroma, lymphangioma, sebaceous nevus, fibroepithelial polyps, leiomyoma, and lipomas.1,6
Treatment of NLCS generally involves basic surgical excision; however, patients treated with CO2 laser also have been reported in the literature.5 Because of the growth tendency and the large size of the classical form of NLCS, recurrence may occur, as in our case. In such cases, gradual surgical excision is recommended.5 We present this case to indicate that undesirable surgical results or relapse may occur in untreated patients because of lesion growth and delayed diagnosis.
- Goucha S, Khaled A, Zéglaoui F, et al. Nevus lipomatosus cutaneous superficialis: report of eight cases. Dermatol Ther (Heidelb). 2011;1:25-30.
- Hoffmann E, Zurhelle E. Ubereinen nevus lipomatodes cutaneous superficialis der linkenglutaalgegend. Arch Dermatol Syph. 1921;130:327-333.
- Patil SB, Narchal S, Paricharak M, et al. Nevus lipomatosus cutaneous superficialis: a rare case report. Iran J Med Sci. 2014;39:304-307.
- Bancalari E, Martínez-Sánchez D, Tardío JC. Nevus lipomatosus superficialis with a folliculosebaceous component: report of 2 cases. Patholog Res Int. 2011;2011:105973.
- Kim YJ, Choi JH, Kim H, et al. Recurrence of nevus lipomatosus cutaneous superficialis after CO(2) laser treatment [published online November 14, 2012]. Arch Plast Surg. 2012;39:671-673.
- Wollina U. Photoletter to the editor - nevus lipomatosus superficialis (Hoffmann-Zurhelle). three new cases including one with ulceration and one with ipsilateral gluteal hypertrophy. J Dermatol Case Rep. 2013;7:71-73.
- Goucha S, Khaled A, Zéglaoui F, et al. Nevus lipomatosus cutaneous superficialis: report of eight cases. Dermatol Ther (Heidelb). 2011;1:25-30.
- Hoffmann E, Zurhelle E. Ubereinen nevus lipomatodes cutaneous superficialis der linkenglutaalgegend. Arch Dermatol Syph. 1921;130:327-333.
- Patil SB, Narchal S, Paricharak M, et al. Nevus lipomatosus cutaneous superficialis: a rare case report. Iran J Med Sci. 2014;39:304-307.
- Bancalari E, Martínez-Sánchez D, Tardío JC. Nevus lipomatosus superficialis with a folliculosebaceous component: report of 2 cases. Patholog Res Int. 2011;2011:105973.
- Kim YJ, Choi JH, Kim H, et al. Recurrence of nevus lipomatosus cutaneous superficialis after CO(2) laser treatment [published online November 14, 2012]. Arch Plast Surg. 2012;39:671-673.
- Wollina U. Photoletter to the editor - nevus lipomatosus superficialis (Hoffmann-Zurhelle). three new cases including one with ulceration and one with ipsilateral gluteal hypertrophy. J Dermatol Case Rep. 2013;7:71-73.
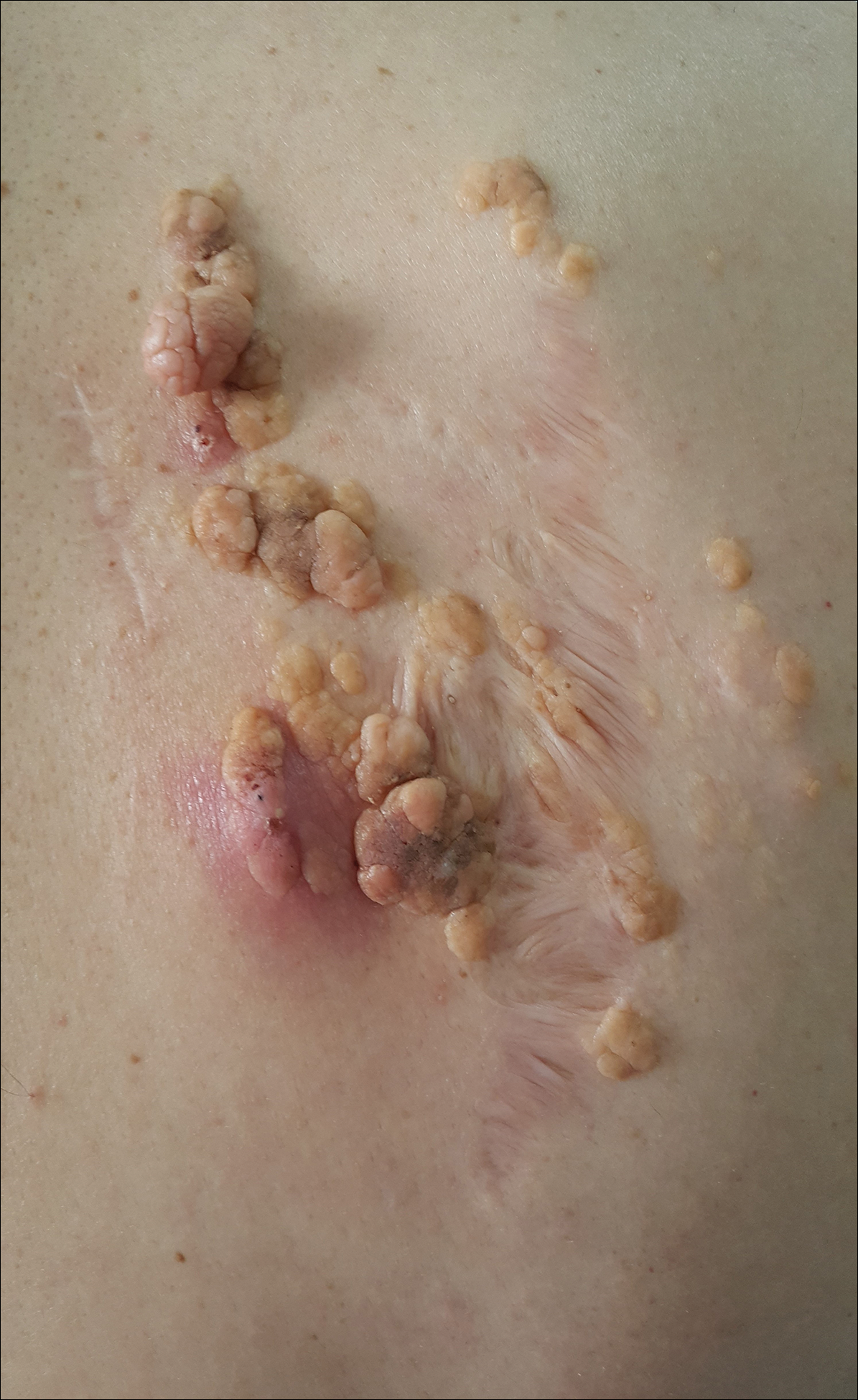
A 36-year-old man presented with a group of partially erythematous, yellowish papules and plaques ranging from 5 to 20 mm in diameter on the right side of the upper back of 20 years' duration. They were surgically excised 8 years prior but recurred and spread. The lesions occasionally were painful and tender with redness and discharge.
Desmoplastic trichoepithelioma may co-occur with BCC
SYDNEY, AUSTRALIA – Watchful waiting may not be the safest approach for managing patients with desmoplastic trichoepithelioma, according to a speaker at the annual meeting of the Australasian College of Dermatologists, who described five cases of the benign tumor combined with basal cell carcinoma.
Desmoplastic trichoepithelioma (DTE), a rare benign tumor that typically presents as a small, slow-growing, asymptomatic, skin-colored lesion on the face, with a depressed nonulcerated center and often raised edges, is managed with watchful waiting or local excision. While its key histopathologic features are narrow cords or strands of basaloid cells, numerous small keratin-filled cysts, and a surrounding desmoplastic core, DTE can be confused with morpheaform basal cell carcinoma (BCC), Tristan Blake, MD, dermatology registrar at Royal Brisbane and Womens’ Hospital, Brisbane, Australia, said at the meeting.
“At this stage, there’s no way to confidently say, looking at the slides, if those cases were desmoplastic trichoepithelioma arising in basal cell carcinoma or vice versa, or if they were a single tumor with divergent differentiation, or an occlusion of two separate tumors,” he said.
Dr. Blake added that this was the first time, to his knowledge, that such a combination had been reported, and that the finding had the potential to change the way DTE is managed.
“How can you now confidently elect to leave or watch the desmoplastic trichoepithelioma patients you have, knowing that not an insignificant portion might also harbor BCC or develop BCC in the future?” he said. This dilemma is made more acute by the fact that DTEs are typically found in younger patients and on the face, he added.
Two dermatopathologists involved in the retrospective review of cases reported that histochemistry was not particularly useful in differentiating DTE from other tumors, he noted.
Patients in the study were also interviewed about their tumors and reported no symptoms; when asked how long the lesions had been there prior to diagnosis, those who could recall said the lesions had likely been present for decades.
In an interview, Dr. Blake said that the discovery of coexisting DTE and BCC was a surprise, and cast doubt on the practice of watchful waiting.
No conflicts of interest were declared.
SYDNEY, AUSTRALIA – Watchful waiting may not be the safest approach for managing patients with desmoplastic trichoepithelioma, according to a speaker at the annual meeting of the Australasian College of Dermatologists, who described five cases of the benign tumor combined with basal cell carcinoma.
Desmoplastic trichoepithelioma (DTE), a rare benign tumor that typically presents as a small, slow-growing, asymptomatic, skin-colored lesion on the face, with a depressed nonulcerated center and often raised edges, is managed with watchful waiting or local excision. While its key histopathologic features are narrow cords or strands of basaloid cells, numerous small keratin-filled cysts, and a surrounding desmoplastic core, DTE can be confused with morpheaform basal cell carcinoma (BCC), Tristan Blake, MD, dermatology registrar at Royal Brisbane and Womens’ Hospital, Brisbane, Australia, said at the meeting.
“At this stage, there’s no way to confidently say, looking at the slides, if those cases were desmoplastic trichoepithelioma arising in basal cell carcinoma or vice versa, or if they were a single tumor with divergent differentiation, or an occlusion of two separate tumors,” he said.
Dr. Blake added that this was the first time, to his knowledge, that such a combination had been reported, and that the finding had the potential to change the way DTE is managed.
“How can you now confidently elect to leave or watch the desmoplastic trichoepithelioma patients you have, knowing that not an insignificant portion might also harbor BCC or develop BCC in the future?” he said. This dilemma is made more acute by the fact that DTEs are typically found in younger patients and on the face, he added.
Two dermatopathologists involved in the retrospective review of cases reported that histochemistry was not particularly useful in differentiating DTE from other tumors, he noted.
Patients in the study were also interviewed about their tumors and reported no symptoms; when asked how long the lesions had been there prior to diagnosis, those who could recall said the lesions had likely been present for decades.
In an interview, Dr. Blake said that the discovery of coexisting DTE and BCC was a surprise, and cast doubt on the practice of watchful waiting.
No conflicts of interest were declared.
SYDNEY, AUSTRALIA – Watchful waiting may not be the safest approach for managing patients with desmoplastic trichoepithelioma, according to a speaker at the annual meeting of the Australasian College of Dermatologists, who described five cases of the benign tumor combined with basal cell carcinoma.
Desmoplastic trichoepithelioma (DTE), a rare benign tumor that typically presents as a small, slow-growing, asymptomatic, skin-colored lesion on the face, with a depressed nonulcerated center and often raised edges, is managed with watchful waiting or local excision. While its key histopathologic features are narrow cords or strands of basaloid cells, numerous small keratin-filled cysts, and a surrounding desmoplastic core, DTE can be confused with morpheaform basal cell carcinoma (BCC), Tristan Blake, MD, dermatology registrar at Royal Brisbane and Womens’ Hospital, Brisbane, Australia, said at the meeting.
“At this stage, there’s no way to confidently say, looking at the slides, if those cases were desmoplastic trichoepithelioma arising in basal cell carcinoma or vice versa, or if they were a single tumor with divergent differentiation, or an occlusion of two separate tumors,” he said.
Dr. Blake added that this was the first time, to his knowledge, that such a combination had been reported, and that the finding had the potential to change the way DTE is managed.
“How can you now confidently elect to leave or watch the desmoplastic trichoepithelioma patients you have, knowing that not an insignificant portion might also harbor BCC or develop BCC in the future?” he said. This dilemma is made more acute by the fact that DTEs are typically found in younger patients and on the face, he added.
Two dermatopathologists involved in the retrospective review of cases reported that histochemistry was not particularly useful in differentiating DTE from other tumors, he noted.
Patients in the study were also interviewed about their tumors and reported no symptoms; when asked how long the lesions had been there prior to diagnosis, those who could recall said the lesions had likely been present for decades.
In an interview, Dr. Blake said that the discovery of coexisting DTE and BCC was a surprise, and cast doubt on the practice of watchful waiting.
No conflicts of interest were declared.
AT ACDASM 2017
Key clinical point: Watchful waiting may no longer be the obvious choice for desmoplastic trichoepithelioma, with evidence that the benign tumor may co-occur with basal cell carcinoma.
Major finding: Researchers reported five cases in which both DTE and BCC were identified in the same pathology specimen.
Data source: A retrospective review of 27 patients with DTE, which included reexamination of specimens.
Disclosures: No conflicts of interest were declared.
Perifollicular Papules on the Trunk
The Diagnosis: Disseminate and Recurrent Infundibulofolliculitis
A punch biopsy of a representative lesion on the trunk was performed. Histopathologic examination revealed a chronic lymphohistiocytic proliferation, focal spongiosis, and lymphocytic exocytosis primarily involving the isthmus of the hair follicle (Figure 1). At the follicular opening there was associated parakeratosis of the adjacent epidermis (Figure 2). Given these clinical and histopathological findings, a diagnosis of disseminate and recurrent infundibulofolliculitis (DRIF) was made.
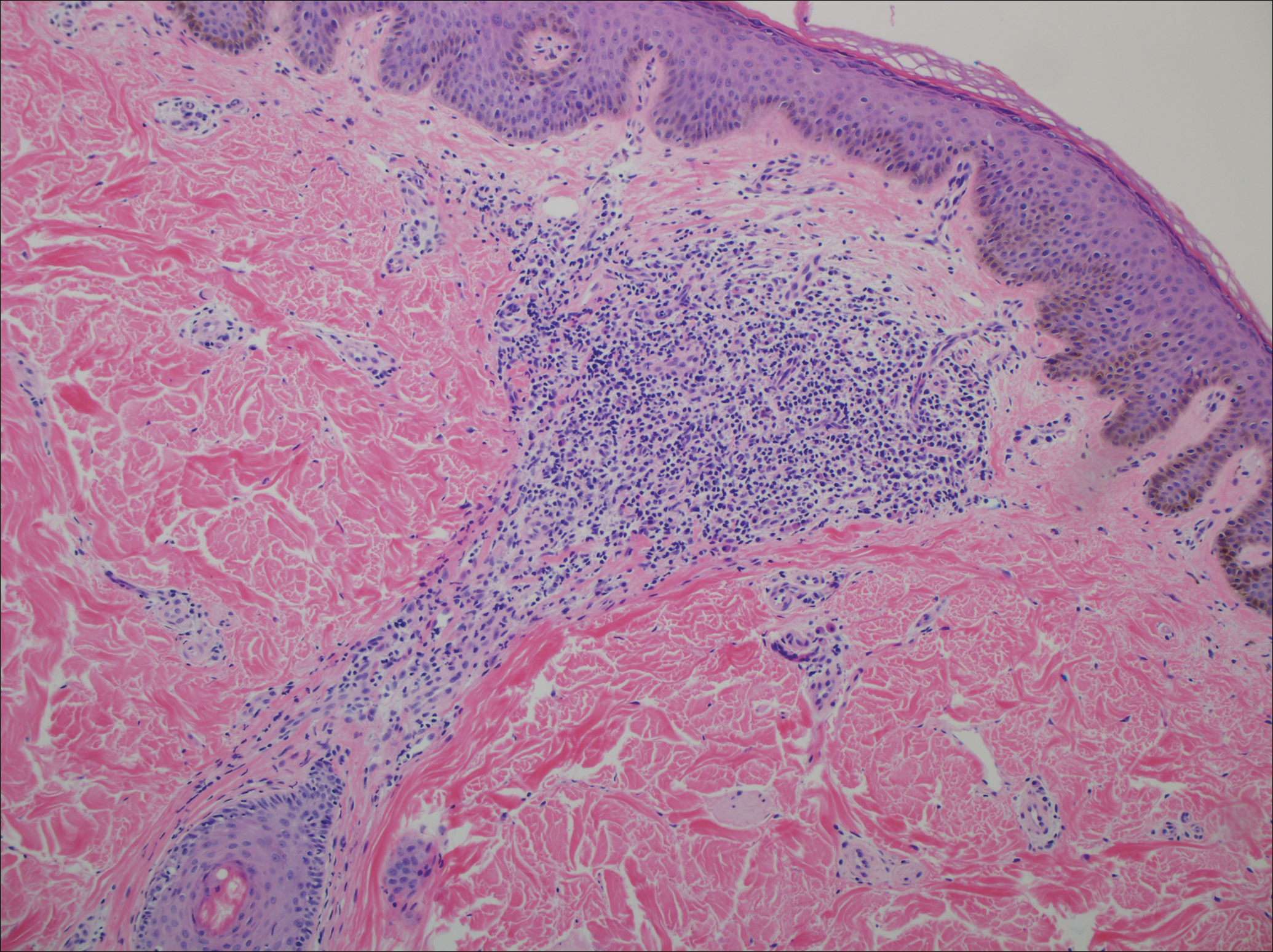
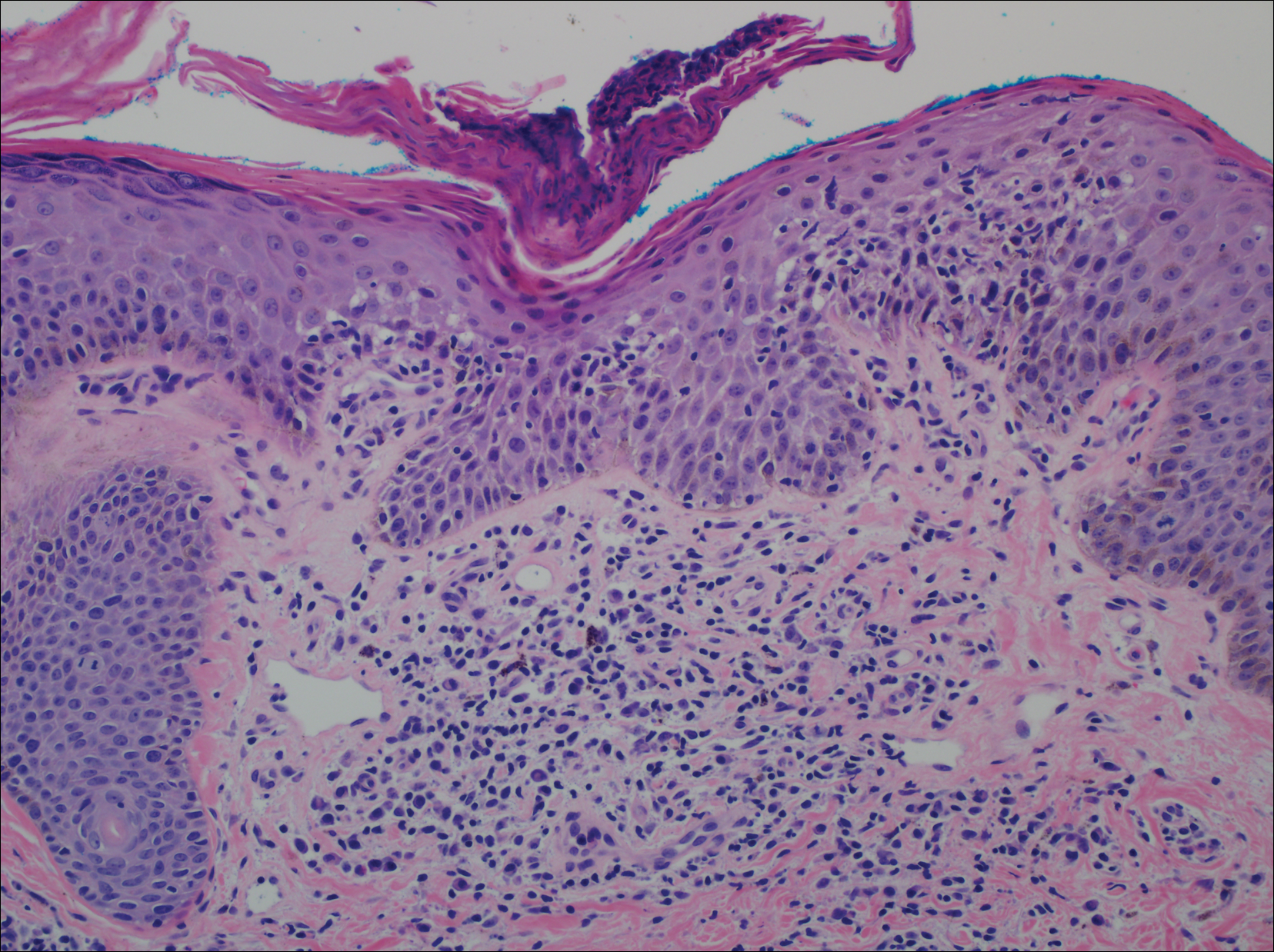
Disseminate and recurrent infundibulofolliculitis was first described by Hitch and Lund1 in 1968 in a healthy 27-year-old black man as a widespread recurrent follicular eruption. Disseminate and recurrent infundibulofolliculitis usually affects young adult males with darkly pigmented skin.2,3 It has less commonly been described in children, females, and white individuals.3,4 Associations with atopy, systemic diseases, or medications are unknown.3-6 The onset usually is sudden and the disease course may be characterized by intermittent recurrences. Pruritus usually is reported but may be mild.5
Histopathology is characterized by spongiosis centered on the infundibulum of the hair follicle and a primarily lymphocytic inflammatory infiltrate. Neutrophils also may be identified.3 Disseminate and recurrent infundibulofolliculitis can be differentiated histologically from clinically similar entities such as keratosis pilaris, which has a keratin plug filling the infundibulum; lichen nitidus, which is characterized by a clawlike downgrowth of the rete ridges surrounding a central foci of inflammation; or folliculitis, which is characterized by perifollicular suppurative inflammation.
Treatment of DRIF is anecdotal and limited to case reports. Vitamin A alone or in combination with vitamin E has been reported to lead to some improvement.5 Tetracycline-class antibiotics, keratolytics, antihistamines, and topical retinoids have not been successful, and mixed results have been seen with topical steroids.5-7 There is a reported case of improvement with a 3-week regimen of psoralen plus UVA followed by twice-weekly maintenance.8 Promising results in the treatment of DRIF have been shown with oral isotretinoin once daily.3-5 Finally, DRIF may resolve independently6; therefore, treatment of DRIF should be addressed on a case-by-case basis.
- Hitch JM, Lund HZ. Disseminate and recurrent infundibulo-folliculitis: report of a case. Arch Dermatol. 1968;97:432-435.
- Hitch JM, Lund HZ. Disseminate and recurrent infundibulo-folliculitis. Arch Dermatol. 1972;105:580-583.
- Calka O, Metin A, Ozen S. A case of disseminated and recurrent infundibulofolliculitis responsive to treatment with systemic isotretinoin. J Dermatol. 2002;29:431-434.
- Aroni K, Grapsa A, Agapitos E. Disseminate and recurrent infundibulofolliculitis: response to isotretinoin. J Drugs Dermatol. 2004;3:434-435.
- Aroni K, Aivaliotis M, Davaris P. Disseminated and recurrent infundibular folliculitis (D.R.I.F.): report of a case successfully treated with isotretinoin. J Dermatol. 1998;25:51-53.
- Owen WR, Wood C. Disseminate and recurrent infundibulofolliculitis. Arch Dermatol. 1979;115:174-175.
- Hinds GA, Heald PW. A case of disseminate and recurrent infundibulofolliculitis responsive to treatment with topical steroids. Dermatol Online J. 2008;14:11.
- Goihman-Yahr M. Disseminate and recurrent infundibulofolliculitis: response to psoralen plus UVA therapy. Int J Dermatol. 1999;38:75-78.
The Diagnosis: Disseminate and Recurrent Infundibulofolliculitis
A punch biopsy of a representative lesion on the trunk was performed. Histopathologic examination revealed a chronic lymphohistiocytic proliferation, focal spongiosis, and lymphocytic exocytosis primarily involving the isthmus of the hair follicle (Figure 1). At the follicular opening there was associated parakeratosis of the adjacent epidermis (Figure 2). Given these clinical and histopathological findings, a diagnosis of disseminate and recurrent infundibulofolliculitis (DRIF) was made.


Disseminate and recurrent infundibulofolliculitis was first described by Hitch and Lund1 in 1968 in a healthy 27-year-old black man as a widespread recurrent follicular eruption. Disseminate and recurrent infundibulofolliculitis usually affects young adult males with darkly pigmented skin.2,3 It has less commonly been described in children, females, and white individuals.3,4 Associations with atopy, systemic diseases, or medications are unknown.3-6 The onset usually is sudden and the disease course may be characterized by intermittent recurrences. Pruritus usually is reported but may be mild.5
Histopathology is characterized by spongiosis centered on the infundibulum of the hair follicle and a primarily lymphocytic inflammatory infiltrate. Neutrophils also may be identified.3 Disseminate and recurrent infundibulofolliculitis can be differentiated histologically from clinically similar entities such as keratosis pilaris, which has a keratin plug filling the infundibulum; lichen nitidus, which is characterized by a clawlike downgrowth of the rete ridges surrounding a central foci of inflammation; or folliculitis, which is characterized by perifollicular suppurative inflammation.
Treatment of DRIF is anecdotal and limited to case reports. Vitamin A alone or in combination with vitamin E has been reported to lead to some improvement.5 Tetracycline-class antibiotics, keratolytics, antihistamines, and topical retinoids have not been successful, and mixed results have been seen with topical steroids.5-7 There is a reported case of improvement with a 3-week regimen of psoralen plus UVA followed by twice-weekly maintenance.8 Promising results in the treatment of DRIF have been shown with oral isotretinoin once daily.3-5 Finally, DRIF may resolve independently6; therefore, treatment of DRIF should be addressed on a case-by-case basis.
The Diagnosis: Disseminate and Recurrent Infundibulofolliculitis
A punch biopsy of a representative lesion on the trunk was performed. Histopathologic examination revealed a chronic lymphohistiocytic proliferation, focal spongiosis, and lymphocytic exocytosis primarily involving the isthmus of the hair follicle (Figure 1). At the follicular opening there was associated parakeratosis of the adjacent epidermis (Figure 2). Given these clinical and histopathological findings, a diagnosis of disseminate and recurrent infundibulofolliculitis (DRIF) was made.


Disseminate and recurrent infundibulofolliculitis was first described by Hitch and Lund1 in 1968 in a healthy 27-year-old black man as a widespread recurrent follicular eruption. Disseminate and recurrent infundibulofolliculitis usually affects young adult males with darkly pigmented skin.2,3 It has less commonly been described in children, females, and white individuals.3,4 Associations with atopy, systemic diseases, or medications are unknown.3-6 The onset usually is sudden and the disease course may be characterized by intermittent recurrences. Pruritus usually is reported but may be mild.5
Histopathology is characterized by spongiosis centered on the infundibulum of the hair follicle and a primarily lymphocytic inflammatory infiltrate. Neutrophils also may be identified.3 Disseminate and recurrent infundibulofolliculitis can be differentiated histologically from clinically similar entities such as keratosis pilaris, which has a keratin plug filling the infundibulum; lichen nitidus, which is characterized by a clawlike downgrowth of the rete ridges surrounding a central foci of inflammation; or folliculitis, which is characterized by perifollicular suppurative inflammation.
Treatment of DRIF is anecdotal and limited to case reports. Vitamin A alone or in combination with vitamin E has been reported to lead to some improvement.5 Tetracycline-class antibiotics, keratolytics, antihistamines, and topical retinoids have not been successful, and mixed results have been seen with topical steroids.5-7 There is a reported case of improvement with a 3-week regimen of psoralen plus UVA followed by twice-weekly maintenance.8 Promising results in the treatment of DRIF have been shown with oral isotretinoin once daily.3-5 Finally, DRIF may resolve independently6; therefore, treatment of DRIF should be addressed on a case-by-case basis.
- Hitch JM, Lund HZ. Disseminate and recurrent infundibulo-folliculitis: report of a case. Arch Dermatol. 1968;97:432-435.
- Hitch JM, Lund HZ. Disseminate and recurrent infundibulo-folliculitis. Arch Dermatol. 1972;105:580-583.
- Calka O, Metin A, Ozen S. A case of disseminated and recurrent infundibulofolliculitis responsive to treatment with systemic isotretinoin. J Dermatol. 2002;29:431-434.
- Aroni K, Grapsa A, Agapitos E. Disseminate and recurrent infundibulofolliculitis: response to isotretinoin. J Drugs Dermatol. 2004;3:434-435.
- Aroni K, Aivaliotis M, Davaris P. Disseminated and recurrent infundibular folliculitis (D.R.I.F.): report of a case successfully treated with isotretinoin. J Dermatol. 1998;25:51-53.
- Owen WR, Wood C. Disseminate and recurrent infundibulofolliculitis. Arch Dermatol. 1979;115:174-175.
- Hinds GA, Heald PW. A case of disseminate and recurrent infundibulofolliculitis responsive to treatment with topical steroids. Dermatol Online J. 2008;14:11.
- Goihman-Yahr M. Disseminate and recurrent infundibulofolliculitis: response to psoralen plus UVA therapy. Int J Dermatol. 1999;38:75-78.
- Hitch JM, Lund HZ. Disseminate and recurrent infundibulo-folliculitis: report of a case. Arch Dermatol. 1968;97:432-435.
- Hitch JM, Lund HZ. Disseminate and recurrent infundibulo-folliculitis. Arch Dermatol. 1972;105:580-583.
- Calka O, Metin A, Ozen S. A case of disseminated and recurrent infundibulofolliculitis responsive to treatment with systemic isotretinoin. J Dermatol. 2002;29:431-434.
- Aroni K, Grapsa A, Agapitos E. Disseminate and recurrent infundibulofolliculitis: response to isotretinoin. J Drugs Dermatol. 2004;3:434-435.
- Aroni K, Aivaliotis M, Davaris P. Disseminated and recurrent infundibular folliculitis (D.R.I.F.): report of a case successfully treated with isotretinoin. J Dermatol. 1998;25:51-53.
- Owen WR, Wood C. Disseminate and recurrent infundibulofolliculitis. Arch Dermatol. 1979;115:174-175.
- Hinds GA, Heald PW. A case of disseminate and recurrent infundibulofolliculitis responsive to treatment with topical steroids. Dermatol Online J. 2008;14:11.
- Goihman-Yahr M. Disseminate and recurrent infundibulofolliculitis: response to psoralen plus UVA therapy. Int J Dermatol. 1999;38:75-78.
A 40-year-old black man presented with numerous perifollicular flesh-colored papules on the back, chest, abdomen, and proximal aspect of the arms of 6 years' duration. He described these lesions as persistent, nonpainful, and nonpruritic. He previously was treated with an unknown cream without any benefit. These lesions were cosmetically bothersome.
Unilateral Verrucous Porokeratosis of the Gluteal Cleft
To the Editor:
Verrucous porokeratosis of the gluteal cleft is a rare skin condition that has distinct clinical and histologic features. A review of 5 cases described a characteristic clinical presentation of a butterfly-shaped bilateral gluteal cleft lesion on most patients.1 We present an unusual case of verrucous porokeratosis presenting as a unilateral single lesion in the gluteal area that emulated seborrheic keratosis with histology consistent with verrucous porokeratosis. This case adds to the variable presentation of this unusual disease.
A 40-year-old man who presented to the dermatology clinic for a follow-up on a basal cell carcinoma of the temple region was concerned about a lesion on the left buttock of 1 year’s duration. Physical examination revealed a unilateral hyperkeratotic plaque that clinically resembled seborrheic keratosis (Figure 1). Biopsy revealed hyperkeratosis with numerous columns of parakeratosis, psoriasiform epidermal hyperplasia (Figures 2A and 2B), dyskeratotic keratinocytes (Figure 2C), pigment incontinence, and mild superficial chronic inflammation consistent with verrucous porokeratosis. The patient was treated with urea lotion but ultimately was lost to follow-up.
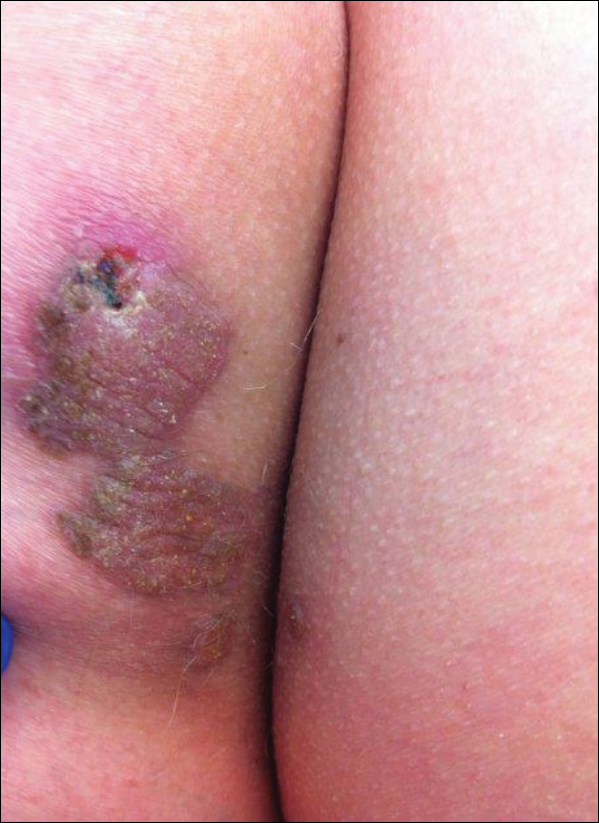
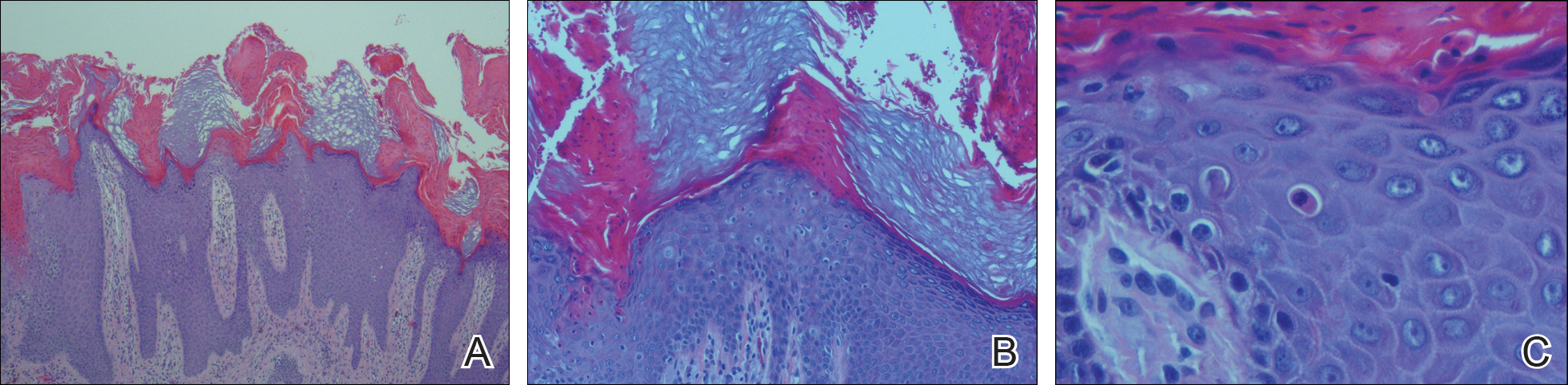
We present a unique case of unilateral verrucous porokeratosis of the gluteal cleft. The clinical differential diagnosis included seborrheic keratosis, condyloma acuminata, and inflammatory linear verrucous epidermal nevus. Histopathology was consistent with verrucous porokeratosis. Porokeratosis is a heterogeneous group of keratinization disorders containing several described variants such as classici porokeratosis of Mibelli, disseminated superficial porokeratosis, porokeratosis palmaris et plantaris disseminata, linear porokeratosis, and punctuate porokeratosis.1,2 Most patients present clinically with plaquelike bilateral (butterfly) lesions with threadlike (ridge) borders, though some patients initially have a unilateral lesion that subsequently develops into a bilateral lesion.1 The clinical course is slow growing, but it can potentially give rise to malignancies such as squamous cell carcinoma.3 Histologically, numerous columns of parakeratosis overlying epidermal cells with attenuated granular layer are observed with the concentric cornoid lamellae considered unique to the verrucous variant.1 Although our patient had only a single unilateral lesion on the gluteal cleft, the histology was consistent with verrucous porokeratosis. Our case adds to the growing clinical presentations of this unusual disease.
- Takiguchi R, White K, Clifton W, et al. Verrucous porokeratosis of the gluteal cleft (porokeratosis stychotropica): a rare disorder easily misdiagnosed. J Cutan Pathol. 2010;37:802-807.
- McGuigan K, Shurman D, Campanelli C, et al. Porokeratosis ptychotropica: a clinically distinct variant of porokeratosis. J Am Acad Dermatol. 2009;60:501-503.
- Malek J, Chedraoui A, Kibbi AG, et al. Genitogluteal porokeratosis: 10 years to make the diagnosis! Am J Dermatopathol. 2009;31:604-606.
To the Editor:
Verrucous porokeratosis of the gluteal cleft is a rare skin condition that has distinct clinical and histologic features. A review of 5 cases described a characteristic clinical presentation of a butterfly-shaped bilateral gluteal cleft lesion on most patients.1 We present an unusual case of verrucous porokeratosis presenting as a unilateral single lesion in the gluteal area that emulated seborrheic keratosis with histology consistent with verrucous porokeratosis. This case adds to the variable presentation of this unusual disease.
A 40-year-old man who presented to the dermatology clinic for a follow-up on a basal cell carcinoma of the temple region was concerned about a lesion on the left buttock of 1 year’s duration. Physical examination revealed a unilateral hyperkeratotic plaque that clinically resembled seborrheic keratosis (Figure 1). Biopsy revealed hyperkeratosis with numerous columns of parakeratosis, psoriasiform epidermal hyperplasia (Figures 2A and 2B), dyskeratotic keratinocytes (Figure 2C), pigment incontinence, and mild superficial chronic inflammation consistent with verrucous porokeratosis. The patient was treated with urea lotion but ultimately was lost to follow-up.


We present a unique case of unilateral verrucous porokeratosis of the gluteal cleft. The clinical differential diagnosis included seborrheic keratosis, condyloma acuminata, and inflammatory linear verrucous epidermal nevus. Histopathology was consistent with verrucous porokeratosis. Porokeratosis is a heterogeneous group of keratinization disorders containing several described variants such as classici porokeratosis of Mibelli, disseminated superficial porokeratosis, porokeratosis palmaris et plantaris disseminata, linear porokeratosis, and punctuate porokeratosis.1,2 Most patients present clinically with plaquelike bilateral (butterfly) lesions with threadlike (ridge) borders, though some patients initially have a unilateral lesion that subsequently develops into a bilateral lesion.1 The clinical course is slow growing, but it can potentially give rise to malignancies such as squamous cell carcinoma.3 Histologically, numerous columns of parakeratosis overlying epidermal cells with attenuated granular layer are observed with the concentric cornoid lamellae considered unique to the verrucous variant.1 Although our patient had only a single unilateral lesion on the gluteal cleft, the histology was consistent with verrucous porokeratosis. Our case adds to the growing clinical presentations of this unusual disease.
To the Editor:
Verrucous porokeratosis of the gluteal cleft is a rare skin condition that has distinct clinical and histologic features. A review of 5 cases described a characteristic clinical presentation of a butterfly-shaped bilateral gluteal cleft lesion on most patients.1 We present an unusual case of verrucous porokeratosis presenting as a unilateral single lesion in the gluteal area that emulated seborrheic keratosis with histology consistent with verrucous porokeratosis. This case adds to the variable presentation of this unusual disease.
A 40-year-old man who presented to the dermatology clinic for a follow-up on a basal cell carcinoma of the temple region was concerned about a lesion on the left buttock of 1 year’s duration. Physical examination revealed a unilateral hyperkeratotic plaque that clinically resembled seborrheic keratosis (Figure 1). Biopsy revealed hyperkeratosis with numerous columns of parakeratosis, psoriasiform epidermal hyperplasia (Figures 2A and 2B), dyskeratotic keratinocytes (Figure 2C), pigment incontinence, and mild superficial chronic inflammation consistent with verrucous porokeratosis. The patient was treated with urea lotion but ultimately was lost to follow-up.


We present a unique case of unilateral verrucous porokeratosis of the gluteal cleft. The clinical differential diagnosis included seborrheic keratosis, condyloma acuminata, and inflammatory linear verrucous epidermal nevus. Histopathology was consistent with verrucous porokeratosis. Porokeratosis is a heterogeneous group of keratinization disorders containing several described variants such as classici porokeratosis of Mibelli, disseminated superficial porokeratosis, porokeratosis palmaris et plantaris disseminata, linear porokeratosis, and punctuate porokeratosis.1,2 Most patients present clinically with plaquelike bilateral (butterfly) lesions with threadlike (ridge) borders, though some patients initially have a unilateral lesion that subsequently develops into a bilateral lesion.1 The clinical course is slow growing, but it can potentially give rise to malignancies such as squamous cell carcinoma.3 Histologically, numerous columns of parakeratosis overlying epidermal cells with attenuated granular layer are observed with the concentric cornoid lamellae considered unique to the verrucous variant.1 Although our patient had only a single unilateral lesion on the gluteal cleft, the histology was consistent with verrucous porokeratosis. Our case adds to the growing clinical presentations of this unusual disease.
- Takiguchi R, White K, Clifton W, et al. Verrucous porokeratosis of the gluteal cleft (porokeratosis stychotropica): a rare disorder easily misdiagnosed. J Cutan Pathol. 2010;37:802-807.
- McGuigan K, Shurman D, Campanelli C, et al. Porokeratosis ptychotropica: a clinically distinct variant of porokeratosis. J Am Acad Dermatol. 2009;60:501-503.
- Malek J, Chedraoui A, Kibbi AG, et al. Genitogluteal porokeratosis: 10 years to make the diagnosis! Am J Dermatopathol. 2009;31:604-606.
- Takiguchi R, White K, Clifton W, et al. Verrucous porokeratosis of the gluteal cleft (porokeratosis stychotropica): a rare disorder easily misdiagnosed. J Cutan Pathol. 2010;37:802-807.
- McGuigan K, Shurman D, Campanelli C, et al. Porokeratosis ptychotropica: a clinically distinct variant of porokeratosis. J Am Acad Dermatol. 2009;60:501-503.
- Malek J, Chedraoui A, Kibbi AG, et al. Genitogluteal porokeratosis: 10 years to make the diagnosis! Am J Dermatopathol. 2009;31:604-606.
Practice Point
- Porokeratosis of the gluteal cleft typically is bilateral but may be unilateral.
Recalcitrant Solitary Erythematous Scaly Patch on the Foot
The Diagnosis: Pagetoid Reticulosis
Histopathologic examination demonstrated a dense infiltrate and psoriasiform pattern epidermal hyperplasia (Figure, A). There was conspicuous epidermotropism of moderately enlarged, hyperchromatic lymphocytes. Intraepidermal lymphocytes were slightly larger, darker, and more convoluted than those in the subjacent dermis (Figure, B). These cells exhibited CD3+ T-cell differentiation with an abnormal CD4-CD7-CD8- phenotype (Figure, C). The histopathologic finding of atypical epidermotropic T-cell infiltrate was compatible with a rare variant of mycosis fungoides known as pagetoid reticulosis (PR). After discussing the diagnosis and treatment options, the patient elected to begin with a conservative approach to therapy. We prescribed fluocinonide ointment 0.05% twice daily under occlusion. At 1 month follow-up, the patient experienced marked improvement of the erythema and scaling of the lesion.
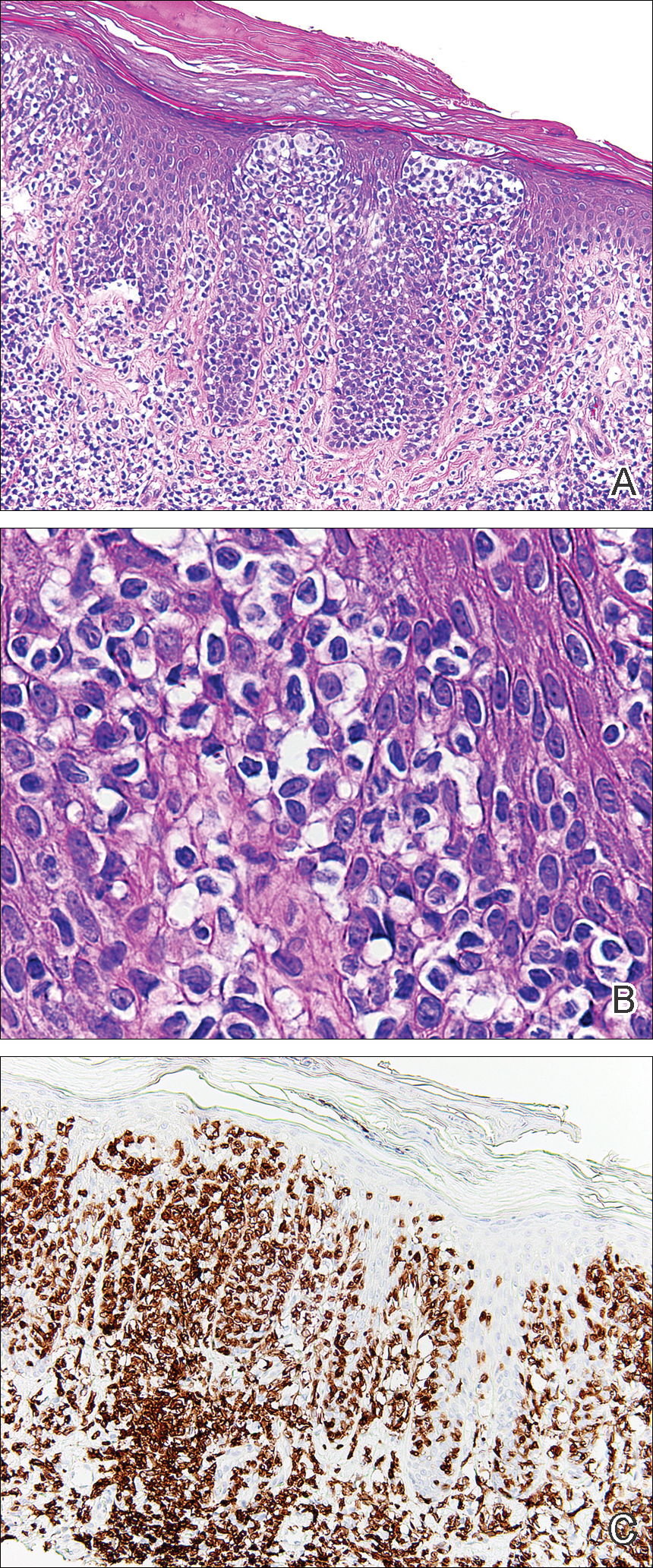
Pagetoid reticulosis is a primary cutaneous T-cell lymphoma that has been categorized as an indolent localized variant of mycosis fungoides. This rare skin disorder was originally described by Woringer and Kolopp in 19391 and was further renamed in 1973 by Braun-Falco et al.2 At that time the term pagetoid reticulosis was introduced due to similarities in histopathologic findings seen in Paget disease of the nipple. Two variants of the disease have been described since then: the localized type and the disseminated type. The localized type, also known as Woringer-Kolopp disease (WKD), typically presents as a persistent, sharply localized, scaly patch that slowly expands over several years. The lesion is classically located on the extensor surface of the hand or foot and often is asymptomatic. Due to the benign presentation, WKD can easily be confused with much more common diseases, such as psoriasis or fungal infections, resulting in a substantial delay in the diagnosis. The patient will often report a medical history notable for frequent office visits and numerous failed therapies. Even though it is exceedingly uncommon, these findings should prompt the practitioner to add WKD to their differential. The disseminated type of PR (also known as Ketron-Goodman disease) is characterized by diffuse cutaneous involvement, carries a much more progressive course, and often leads to a poor outcome.3 The histopathologic features of WKD and Ketron-Goodman disease are identical, and the 2 types are distinguished on clinical grounds alone.
Histopathologic features of PR are unique and often distinct in comparison to mycosis fungoides. Pagetoid reticulosis often is described as epidermal hyperplasia with parakeratosis, prominent acanthosis, and excessive epidermotropism of atypical lymphocytes scattered throughout the epidermis.3 The distinct pattern of epidermotropism seen in PR is the characteristic finding. Review of immunocytochemistry from reported cases has shown that CD marker expression of neoplastic T cells in PR can be variable in nature.4 Although it is known that immunophenotyping can be useful in diagnosing and distinguishing PR from other types of primary cutaneous T-cell lymphoma, the clinical significance of the observed phenotypic variation remains a mystery. As of now, it appears to be prognostically irrelevant.5
There are numerous therapeutic options available for PR. Depending on the size and extent of the disease, surgical excision and radiotherapy may be an option and are the most effective.6 For patients who are not good candidates or opt out of these options, there are various pharmacotherapies that also have proven to work. Traditional therapies include topical corticosteroids, corticosteroid injections, and phototherapy. However, more recent trials with retinoids, such as alitretinoin or bexarotene, appear to offer a promising therapeutic approach.7
Pagetoid reticulosis is a true malignant lymphoma of T-cell lineage, but it typically carries an excellent prognosis. Rare cases have been reported to progress to disseminated lymphoma.8 Therefore, long-term follow-up for a patient diagnosed with PR is recommended.
- Woringer FR, Kolopp P. Lésion érythémato-squameuse polycyclique de l'avant-bras évoluantdepuis 6 ans chez un garçonnet de 13 ans. Ann Dermatol Venereol. 1939;10:945-948.
- Braun-Falco O, Marghescu S, Wolff HH. Pagetoid reticulosis--Woringer-Kolopp's disease [in German]. Hautarzt. 1973;24:11-21.
- Haghighi B, Smoller BR, Leboit PE, et al. Pagetoid reticulosis (Woringer-Kolopp disease): an immunophenotypic, molecular, and clinicopathologic study. Mod Pathol. 2000;13:502-510.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Mourtzinos N, Puri PK, Wang G, et al. CD4/CD8 double negative pagetoid reticulosis: a case report and literature review. J Cutan Pathol. 2010;37:491-496.
- Lee J, Viakhireva N, Cesca C, et al. Clinicopathologic features and treatment outcomes in Woringer-Kolopp disease. J Am Acad Dermatol. 2008;59:706-712.
- Schmitz L, Bierhoff E, Dirschka T. Alitretinoin: an effective treatment option for pagetoid reticulosis. J Dtsch Dermatol Ges. 2013;11:1194-1195.
- Ioannides G, Engel MF, Rywlin AM. Woringer-Kolopp disease (pagetoid reticulosis). Am J Dermatopathol. 1983;5:153-158.
The Diagnosis: Pagetoid Reticulosis
Histopathologic examination demonstrated a dense infiltrate and psoriasiform pattern epidermal hyperplasia (Figure, A). There was conspicuous epidermotropism of moderately enlarged, hyperchromatic lymphocytes. Intraepidermal lymphocytes were slightly larger, darker, and more convoluted than those in the subjacent dermis (Figure, B). These cells exhibited CD3+ T-cell differentiation with an abnormal CD4-CD7-CD8- phenotype (Figure, C). The histopathologic finding of atypical epidermotropic T-cell infiltrate was compatible with a rare variant of mycosis fungoides known as pagetoid reticulosis (PR). After discussing the diagnosis and treatment options, the patient elected to begin with a conservative approach to therapy. We prescribed fluocinonide ointment 0.05% twice daily under occlusion. At 1 month follow-up, the patient experienced marked improvement of the erythema and scaling of the lesion.

Pagetoid reticulosis is a primary cutaneous T-cell lymphoma that has been categorized as an indolent localized variant of mycosis fungoides. This rare skin disorder was originally described by Woringer and Kolopp in 19391 and was further renamed in 1973 by Braun-Falco et al.2 At that time the term pagetoid reticulosis was introduced due to similarities in histopathologic findings seen in Paget disease of the nipple. Two variants of the disease have been described since then: the localized type and the disseminated type. The localized type, also known as Woringer-Kolopp disease (WKD), typically presents as a persistent, sharply localized, scaly patch that slowly expands over several years. The lesion is classically located on the extensor surface of the hand or foot and often is asymptomatic. Due to the benign presentation, WKD can easily be confused with much more common diseases, such as psoriasis or fungal infections, resulting in a substantial delay in the diagnosis. The patient will often report a medical history notable for frequent office visits and numerous failed therapies. Even though it is exceedingly uncommon, these findings should prompt the practitioner to add WKD to their differential. The disseminated type of PR (also known as Ketron-Goodman disease) is characterized by diffuse cutaneous involvement, carries a much more progressive course, and often leads to a poor outcome.3 The histopathologic features of WKD and Ketron-Goodman disease are identical, and the 2 types are distinguished on clinical grounds alone.
Histopathologic features of PR are unique and often distinct in comparison to mycosis fungoides. Pagetoid reticulosis often is described as epidermal hyperplasia with parakeratosis, prominent acanthosis, and excessive epidermotropism of atypical lymphocytes scattered throughout the epidermis.3 The distinct pattern of epidermotropism seen in PR is the characteristic finding. Review of immunocytochemistry from reported cases has shown that CD marker expression of neoplastic T cells in PR can be variable in nature.4 Although it is known that immunophenotyping can be useful in diagnosing and distinguishing PR from other types of primary cutaneous T-cell lymphoma, the clinical significance of the observed phenotypic variation remains a mystery. As of now, it appears to be prognostically irrelevant.5
There are numerous therapeutic options available for PR. Depending on the size and extent of the disease, surgical excision and radiotherapy may be an option and are the most effective.6 For patients who are not good candidates or opt out of these options, there are various pharmacotherapies that also have proven to work. Traditional therapies include topical corticosteroids, corticosteroid injections, and phototherapy. However, more recent trials with retinoids, such as alitretinoin or bexarotene, appear to offer a promising therapeutic approach.7
Pagetoid reticulosis is a true malignant lymphoma of T-cell lineage, but it typically carries an excellent prognosis. Rare cases have been reported to progress to disseminated lymphoma.8 Therefore, long-term follow-up for a patient diagnosed with PR is recommended.
The Diagnosis: Pagetoid Reticulosis
Histopathologic examination demonstrated a dense infiltrate and psoriasiform pattern epidermal hyperplasia (Figure, A). There was conspicuous epidermotropism of moderately enlarged, hyperchromatic lymphocytes. Intraepidermal lymphocytes were slightly larger, darker, and more convoluted than those in the subjacent dermis (Figure, B). These cells exhibited CD3+ T-cell differentiation with an abnormal CD4-CD7-CD8- phenotype (Figure, C). The histopathologic finding of atypical epidermotropic T-cell infiltrate was compatible with a rare variant of mycosis fungoides known as pagetoid reticulosis (PR). After discussing the diagnosis and treatment options, the patient elected to begin with a conservative approach to therapy. We prescribed fluocinonide ointment 0.05% twice daily under occlusion. At 1 month follow-up, the patient experienced marked improvement of the erythema and scaling of the lesion.

Pagetoid reticulosis is a primary cutaneous T-cell lymphoma that has been categorized as an indolent localized variant of mycosis fungoides. This rare skin disorder was originally described by Woringer and Kolopp in 19391 and was further renamed in 1973 by Braun-Falco et al.2 At that time the term pagetoid reticulosis was introduced due to similarities in histopathologic findings seen in Paget disease of the nipple. Two variants of the disease have been described since then: the localized type and the disseminated type. The localized type, also known as Woringer-Kolopp disease (WKD), typically presents as a persistent, sharply localized, scaly patch that slowly expands over several years. The lesion is classically located on the extensor surface of the hand or foot and often is asymptomatic. Due to the benign presentation, WKD can easily be confused with much more common diseases, such as psoriasis or fungal infections, resulting in a substantial delay in the diagnosis. The patient will often report a medical history notable for frequent office visits and numerous failed therapies. Even though it is exceedingly uncommon, these findings should prompt the practitioner to add WKD to their differential. The disseminated type of PR (also known as Ketron-Goodman disease) is characterized by diffuse cutaneous involvement, carries a much more progressive course, and often leads to a poor outcome.3 The histopathologic features of WKD and Ketron-Goodman disease are identical, and the 2 types are distinguished on clinical grounds alone.
Histopathologic features of PR are unique and often distinct in comparison to mycosis fungoides. Pagetoid reticulosis often is described as epidermal hyperplasia with parakeratosis, prominent acanthosis, and excessive epidermotropism of atypical lymphocytes scattered throughout the epidermis.3 The distinct pattern of epidermotropism seen in PR is the characteristic finding. Review of immunocytochemistry from reported cases has shown that CD marker expression of neoplastic T cells in PR can be variable in nature.4 Although it is known that immunophenotyping can be useful in diagnosing and distinguishing PR from other types of primary cutaneous T-cell lymphoma, the clinical significance of the observed phenotypic variation remains a mystery. As of now, it appears to be prognostically irrelevant.5
There are numerous therapeutic options available for PR. Depending on the size and extent of the disease, surgical excision and radiotherapy may be an option and are the most effective.6 For patients who are not good candidates or opt out of these options, there are various pharmacotherapies that also have proven to work. Traditional therapies include topical corticosteroids, corticosteroid injections, and phototherapy. However, more recent trials with retinoids, such as alitretinoin or bexarotene, appear to offer a promising therapeutic approach.7
Pagetoid reticulosis is a true malignant lymphoma of T-cell lineage, but it typically carries an excellent prognosis. Rare cases have been reported to progress to disseminated lymphoma.8 Therefore, long-term follow-up for a patient diagnosed with PR is recommended.
- Woringer FR, Kolopp P. Lésion érythémato-squameuse polycyclique de l'avant-bras évoluantdepuis 6 ans chez un garçonnet de 13 ans. Ann Dermatol Venereol. 1939;10:945-948.
- Braun-Falco O, Marghescu S, Wolff HH. Pagetoid reticulosis--Woringer-Kolopp's disease [in German]. Hautarzt. 1973;24:11-21.
- Haghighi B, Smoller BR, Leboit PE, et al. Pagetoid reticulosis (Woringer-Kolopp disease): an immunophenotypic, molecular, and clinicopathologic study. Mod Pathol. 2000;13:502-510.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Mourtzinos N, Puri PK, Wang G, et al. CD4/CD8 double negative pagetoid reticulosis: a case report and literature review. J Cutan Pathol. 2010;37:491-496.
- Lee J, Viakhireva N, Cesca C, et al. Clinicopathologic features and treatment outcomes in Woringer-Kolopp disease. J Am Acad Dermatol. 2008;59:706-712.
- Schmitz L, Bierhoff E, Dirschka T. Alitretinoin: an effective treatment option for pagetoid reticulosis. J Dtsch Dermatol Ges. 2013;11:1194-1195.
- Ioannides G, Engel MF, Rywlin AM. Woringer-Kolopp disease (pagetoid reticulosis). Am J Dermatopathol. 1983;5:153-158.
- Woringer FR, Kolopp P. Lésion érythémato-squameuse polycyclique de l'avant-bras évoluantdepuis 6 ans chez un garçonnet de 13 ans. Ann Dermatol Venereol. 1939;10:945-948.
- Braun-Falco O, Marghescu S, Wolff HH. Pagetoid reticulosis--Woringer-Kolopp's disease [in German]. Hautarzt. 1973;24:11-21.
- Haghighi B, Smoller BR, Leboit PE, et al. Pagetoid reticulosis (Woringer-Kolopp disease): an immunophenotypic, molecular, and clinicopathologic study. Mod Pathol. 2000;13:502-510.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Mourtzinos N, Puri PK, Wang G, et al. CD4/CD8 double negative pagetoid reticulosis: a case report and literature review. J Cutan Pathol. 2010;37:491-496.
- Lee J, Viakhireva N, Cesca C, et al. Clinicopathologic features and treatment outcomes in Woringer-Kolopp disease. J Am Acad Dermatol. 2008;59:706-712.
- Schmitz L, Bierhoff E, Dirschka T. Alitretinoin: an effective treatment option for pagetoid reticulosis. J Dtsch Dermatol Ges. 2013;11:1194-1195.
- Ioannides G, Engel MF, Rywlin AM. Woringer-Kolopp disease (pagetoid reticulosis). Am J Dermatopathol. 1983;5:153-158.
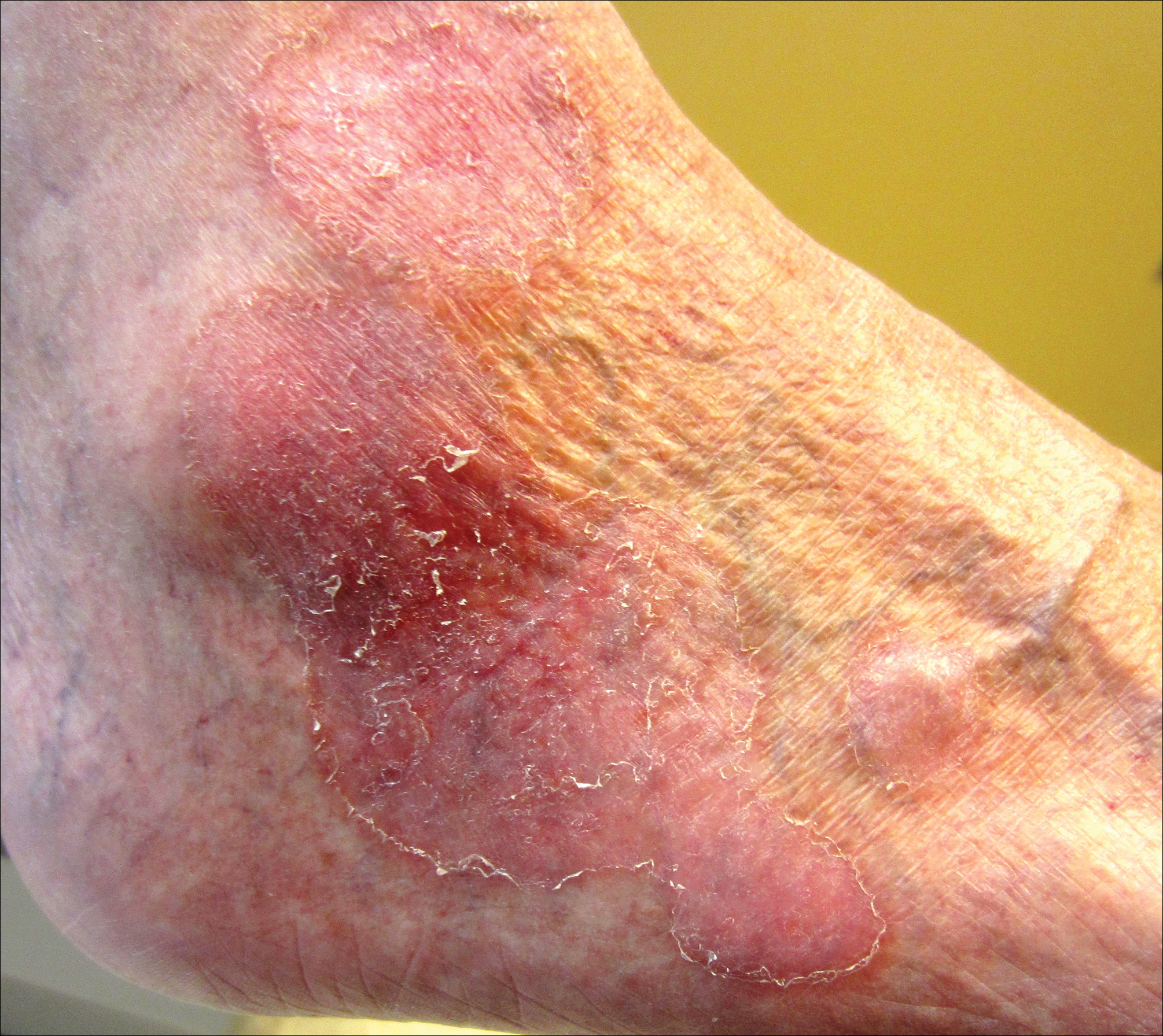
An 80-year-old man with a history of malignant melanoma and squamous cell carcinoma presented to the dermatology clinic with a chronic rash of 20 years' duration on the right ankle that extended to the instep of the right foot. His medical history was notable for hypertension and hyperlipidemia. Family history was unremarkable. The patient described the rash as red and scaly but denied associated pain or pruritus. Over the last 2 to 3 years he had tried treating the affected area with petroleum jelly, topical and oral antifungals, and mild topical steroids with minimal improvement. Complete review of systems was performed and was negative other than some mild constipation. Physical examination revealed an erythematous scaly patch on the dorsal aspect of the right ankle. Potassium hydroxide preparation and fungal culture swab yielded negative results, and a shave biopsy was performed.
Expanding Uses of Propranolol in Dermatology
Since the serendipitous discovery of expedited involution of infantile hemangiomas (IHs) with propranolol in 2008,1 current research has proliferated to discern the mechanism of action of beta-blockers in the care of IHs. Propranolol is a nonselective beta-blocker with a structure similar to catecholamines and thus competes for β-adrenergic receptors. Blocking β1-receptors is cardioselective, leading to decreased heart rate and myocardial contractility, while blocking β2-receptors leads to inhibition of smooth muscle relaxation and decreased glycogenolysis. The endothelial cells of IH express β2-adrenergic receptors; the mechanistic role of propranolol in these lesions is surmised to be due to vasoconstriction, decreased angiogenesis through inhibition of vascular endothelial growth factor, and subsequent endothelial cell apoptosis.2
After this breakthrough finding, a subsequent novel development was made when an ophthalmologist demonstrated that timolol, a topical beta-blocker, could be utilized to expedite IH involution and prevent ocular complications such as amblyopia secondary to the mass effect of the lesion. Guo and Ni3 prescribed the commercially available ophthalmologic solution of timolol maleate 0.5% for twice-daily use for 5 weeks. Remarkable reduction in the periorbital IH without rebound phenomenon was observed.3 A recent multicenter retrospective cohort of more than 700 patients with IH were treated with topical timolol with a 70% success rate, corresponding to 10% improvement from baseline; this study highlights the efficacy of timolol while confirming the safety of the medication.4
Systemic beta-blockers for IH have been used predominately for critical sites such as the nasal tip, lip, ear, perineum, and periocular area; ulcerated lesions or those that may be prone to leave a fibrofatty tissue residue after involution also have been targeted. Contraindications for use include premature infants younger than 5 weeks, infants weighing less than 2 kg, history of asthma or bronchospasm, heart rate less than 80 beats per minute, blood pressure less than 50/30 mm Hg, or hypersensitivity to the medication.5 Current guidelines for propranolol initiation vary; some dermatologists consult cardiology prior to initiation, while others perform routine vitals and an indication-driven electrocardiogram as needed based on family history of cardiac disease, maternal history of connective tissue disease, congenital heart block, or abnormal vital signs.
Given the demonstrated long-term safety of propranolol and the acceptable side-effect profile, the use of beta-blockers for IH has become increasingly mainstream. Three randomized controlled trials (RCTs) have evaluated the efficacy and minimal adverse effects of propranolol for IH. The first RCT evaluated 40 patients who received either placebo or propranolol 2 mg/kg daily (divided into 3 doses) for 6 months; IH growth stopped by week 4 in the treatment group and the largest volume difference in IH was seen at week 12.6 Léauté-Labrèze et al7 demonstrated that propranolol could be given earlier to patients and at higher doses; the treatment group included 7 patients at 3 mg/kg daily of propranolol for 15 days, followed by 15 additional days of 4 mg/kg daily of propranolol. A statistically significant (P=.004) decrease in IH volume, quantified by use of ultrasonography, was exhibited by the propranolol group.7 Lastly, the largest RCT (N=456) established the efficacy of propranolol 3 mg/kg daily for 6 months with a 60% successful treatment rate compared to 4% for patients receiving placebo.8
Given the efficacy of propranolol for IH, other investigators have experimented with nonselective beta-blockers for other dermatologic conditions. In addition to second-line use for flushing, hyperhidrosis, and adrenergic urticaria, the future of propranolol is expanding for vascular lesions in particular.9 Chow et al10 highlighted a case of progressive angiosarcoma of the scalp that responded to propranolol hydrochloride therapy at 40 mg 3 times daily with extensive regression; propranolol was given in addition to chemotherapy and radiation. The tumor was biopsied before and after propranolol therapy and exhibited a 34% decrease in the proliferative index (Ki-67).10 Interestingly, Chisholm et al11 evaluated the expression of β-adrenergic expression in 141 vascular lesions; endothelial cell expression of β2-adrenergic receptors was found positive in 100% of IHs, 67% of kaposiform hemangioendotheliomas, 41% of angiosarcomas, 50% of pyogenic granulomas, and 75% of Kaposi sarcomas, to name merely a few studied lesions.
These data have spurred physicians to further seek beta-blocker dermatologic use in specific patient populations. For example, Meseguer-Yebra et al12 employed timolol solution 0.5% twice daily for 12 weeks for 2 human immunodeficiency virus–negative patients with limited Kaposi sarcoma of the right thigh and foot; no clinical evidence of recurrence was seen at 20 months, and one of the patients had a subsequent biopsy performed with negative human herpesvirus 8 staining after therapy. In the pediatric arena, topical timolol has been used for both port-wine stains and pyogenic granulomas.13-15 Two lesions of pyogenic granulomas on the scalp of a child were treated with timolol ophthalmic solution 0.5% under occlusion for 4 weeks with resolution.15 Propranolol also has been utilized as adjunctive therapy for aggressive pediatric vascular lesions such as kaposiform hemangioendothelioma with promising results and additionally reducing the duration of therapy needed with vincristine.2
In summary, propranolol and timolol have made an indelible impression on the field of pediatric dermatology and have demonstrated a burgeoning role in the dermatologic arena. The use of nonselective beta-blockers for the management of vascular lesions can serve as adjunctive or monotherapy for certain patient populations. The relatively low adverse risk profile of propranolol makes it a versatile tool to use both systemically and topically. Although the authors of the study assessing the β2-adrenergic expression in vascular lesions admittedly stated that the positivity of the receptors does not necessarily correlate with therapeutic management, it is an interesting subject area with much potential in the future.11 This review serves to illuminate the expanding role of beta-blockers in dermatology.
- Léauté-Labrèze C, Dumas de la Roque E, Hubiche T, et al. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358:2649-2651.
- Hermans DJ, van Beynum IM, van der Vijver RJ, et al. Kaposiform hemangioendothelioma with Kasabach-Merritt syndrome: a new indication for propranolol treatment. J Pediatr Hematol Oncol. 2011;33:E171-E173.
- Guo S, Ni N. Topical treatment for capillary hemangioma of the eyelid using beta-blocker solution. Arch Ophthalmol. 2010;128:255-256.
- Püttgen K, Lucky A, Adams D, et al. Topical timolol maleate treatment of infantile hemangiomas. Pediatrics. 2016;138:3.
- Drolet BA, Frommelt PC, Chamlin SL, et al. Initiation and use of propranolol for infantile hemangioma: report of a consensus conference. Pediatrics. 2013;131:128-140.
- Hogeling M, Adams S, Wargon O. A randomized controlled trial of propranolol for infantile hemangiomas [published online July 25, 2011]. Pediatrics. 2011;128:E259-E266.
- Léauté-Labrèze C, Dumas de la Roque E, Nacka F, et al. Doubleblind randomized pilot trial evaluating the efficacy of oral propranolol on infantile haemangiomas in infants < 4 months of age. Br J Dermatol. 2013;169:181-183.
- Léauté-Labrèze C, Hoeger P, Mazereeuw-Hautier J, et al. A randomized, controlled trial of oral propranolol in infantile hemangioma. N Engl J Med. 2015;372:735-746.
- Shelley WB, Shelley ED. Adrenergic urticaria: a new form of stress induced hives. Lancet. 1985;2:1031-1033.
- Chow W, Amaya CN, Rains S, et al. Growth attenuation of cutaneous angiosarcoma with propranolol-mediated β-blockade. JAMA Dermatol. 2015;151:1226-1229.
- Chisholm KM, Chang KW, Truong MT, et al. β-adrenergic receptor expression in vascular tumors. Mod Pathol. 2012;25:1446-1451.
- Meseguer-Yebra C, Cardeñoso-Álvarez, ME, Bordel-Gómez MT, et al. Successful treatment of classic Kaposi sarcoma with topical timolol: report of two cases. Br J Dermatol. 2015;173:860-862.
- Passeron T, Maza A, Fontas E, et al. Treatment of port wine stains and pulsed dye laser and topical timolol: a multicenter randomized controlled trial. Br J Dermatol. 2014;170:1350-1353.
- Wine LL, Goff KL, Lam JM, et al. Treatment of pediatric pyogenic granulomas using β-adrenergic receptor antagonist. Pediatr Dermatol. 2014;31:203-207.
- Knöpfel N, Escudero-Góngora Mdel M, Bauzà A, et al. Timolol for the treatment of pyogenic granuloma (PG) in children. J Am Acad Dermatol. 2016;75:E105-E106.
Since the serendipitous discovery of expedited involution of infantile hemangiomas (IHs) with propranolol in 2008,1 current research has proliferated to discern the mechanism of action of beta-blockers in the care of IHs. Propranolol is a nonselective beta-blocker with a structure similar to catecholamines and thus competes for β-adrenergic receptors. Blocking β1-receptors is cardioselective, leading to decreased heart rate and myocardial contractility, while blocking β2-receptors leads to inhibition of smooth muscle relaxation and decreased glycogenolysis. The endothelial cells of IH express β2-adrenergic receptors; the mechanistic role of propranolol in these lesions is surmised to be due to vasoconstriction, decreased angiogenesis through inhibition of vascular endothelial growth factor, and subsequent endothelial cell apoptosis.2
After this breakthrough finding, a subsequent novel development was made when an ophthalmologist demonstrated that timolol, a topical beta-blocker, could be utilized to expedite IH involution and prevent ocular complications such as amblyopia secondary to the mass effect of the lesion. Guo and Ni3 prescribed the commercially available ophthalmologic solution of timolol maleate 0.5% for twice-daily use for 5 weeks. Remarkable reduction in the periorbital IH without rebound phenomenon was observed.3 A recent multicenter retrospective cohort of more than 700 patients with IH were treated with topical timolol with a 70% success rate, corresponding to 10% improvement from baseline; this study highlights the efficacy of timolol while confirming the safety of the medication.4
Systemic beta-blockers for IH have been used predominately for critical sites such as the nasal tip, lip, ear, perineum, and periocular area; ulcerated lesions or those that may be prone to leave a fibrofatty tissue residue after involution also have been targeted. Contraindications for use include premature infants younger than 5 weeks, infants weighing less than 2 kg, history of asthma or bronchospasm, heart rate less than 80 beats per minute, blood pressure less than 50/30 mm Hg, or hypersensitivity to the medication.5 Current guidelines for propranolol initiation vary; some dermatologists consult cardiology prior to initiation, while others perform routine vitals and an indication-driven electrocardiogram as needed based on family history of cardiac disease, maternal history of connective tissue disease, congenital heart block, or abnormal vital signs.
Given the demonstrated long-term safety of propranolol and the acceptable side-effect profile, the use of beta-blockers for IH has become increasingly mainstream. Three randomized controlled trials (RCTs) have evaluated the efficacy and minimal adverse effects of propranolol for IH. The first RCT evaluated 40 patients who received either placebo or propranolol 2 mg/kg daily (divided into 3 doses) for 6 months; IH growth stopped by week 4 in the treatment group and the largest volume difference in IH was seen at week 12.6 Léauté-Labrèze et al7 demonstrated that propranolol could be given earlier to patients and at higher doses; the treatment group included 7 patients at 3 mg/kg daily of propranolol for 15 days, followed by 15 additional days of 4 mg/kg daily of propranolol. A statistically significant (P=.004) decrease in IH volume, quantified by use of ultrasonography, was exhibited by the propranolol group.7 Lastly, the largest RCT (N=456) established the efficacy of propranolol 3 mg/kg daily for 6 months with a 60% successful treatment rate compared to 4% for patients receiving placebo.8
Given the efficacy of propranolol for IH, other investigators have experimented with nonselective beta-blockers for other dermatologic conditions. In addition to second-line use for flushing, hyperhidrosis, and adrenergic urticaria, the future of propranolol is expanding for vascular lesions in particular.9 Chow et al10 highlighted a case of progressive angiosarcoma of the scalp that responded to propranolol hydrochloride therapy at 40 mg 3 times daily with extensive regression; propranolol was given in addition to chemotherapy and radiation. The tumor was biopsied before and after propranolol therapy and exhibited a 34% decrease in the proliferative index (Ki-67).10 Interestingly, Chisholm et al11 evaluated the expression of β-adrenergic expression in 141 vascular lesions; endothelial cell expression of β2-adrenergic receptors was found positive in 100% of IHs, 67% of kaposiform hemangioendotheliomas, 41% of angiosarcomas, 50% of pyogenic granulomas, and 75% of Kaposi sarcomas, to name merely a few studied lesions.
These data have spurred physicians to further seek beta-blocker dermatologic use in specific patient populations. For example, Meseguer-Yebra et al12 employed timolol solution 0.5% twice daily for 12 weeks for 2 human immunodeficiency virus–negative patients with limited Kaposi sarcoma of the right thigh and foot; no clinical evidence of recurrence was seen at 20 months, and one of the patients had a subsequent biopsy performed with negative human herpesvirus 8 staining after therapy. In the pediatric arena, topical timolol has been used for both port-wine stains and pyogenic granulomas.13-15 Two lesions of pyogenic granulomas on the scalp of a child were treated with timolol ophthalmic solution 0.5% under occlusion for 4 weeks with resolution.15 Propranolol also has been utilized as adjunctive therapy for aggressive pediatric vascular lesions such as kaposiform hemangioendothelioma with promising results and additionally reducing the duration of therapy needed with vincristine.2
In summary, propranolol and timolol have made an indelible impression on the field of pediatric dermatology and have demonstrated a burgeoning role in the dermatologic arena. The use of nonselective beta-blockers for the management of vascular lesions can serve as adjunctive or monotherapy for certain patient populations. The relatively low adverse risk profile of propranolol makes it a versatile tool to use both systemically and topically. Although the authors of the study assessing the β2-adrenergic expression in vascular lesions admittedly stated that the positivity of the receptors does not necessarily correlate with therapeutic management, it is an interesting subject area with much potential in the future.11 This review serves to illuminate the expanding role of beta-blockers in dermatology.
Since the serendipitous discovery of expedited involution of infantile hemangiomas (IHs) with propranolol in 2008,1 current research has proliferated to discern the mechanism of action of beta-blockers in the care of IHs. Propranolol is a nonselective beta-blocker with a structure similar to catecholamines and thus competes for β-adrenergic receptors. Blocking β1-receptors is cardioselective, leading to decreased heart rate and myocardial contractility, while blocking β2-receptors leads to inhibition of smooth muscle relaxation and decreased glycogenolysis. The endothelial cells of IH express β2-adrenergic receptors; the mechanistic role of propranolol in these lesions is surmised to be due to vasoconstriction, decreased angiogenesis through inhibition of vascular endothelial growth factor, and subsequent endothelial cell apoptosis.2
After this breakthrough finding, a subsequent novel development was made when an ophthalmologist demonstrated that timolol, a topical beta-blocker, could be utilized to expedite IH involution and prevent ocular complications such as amblyopia secondary to the mass effect of the lesion. Guo and Ni3 prescribed the commercially available ophthalmologic solution of timolol maleate 0.5% for twice-daily use for 5 weeks. Remarkable reduction in the periorbital IH without rebound phenomenon was observed.3 A recent multicenter retrospective cohort of more than 700 patients with IH were treated with topical timolol with a 70% success rate, corresponding to 10% improvement from baseline; this study highlights the efficacy of timolol while confirming the safety of the medication.4
Systemic beta-blockers for IH have been used predominately for critical sites such as the nasal tip, lip, ear, perineum, and periocular area; ulcerated lesions or those that may be prone to leave a fibrofatty tissue residue after involution also have been targeted. Contraindications for use include premature infants younger than 5 weeks, infants weighing less than 2 kg, history of asthma or bronchospasm, heart rate less than 80 beats per minute, blood pressure less than 50/30 mm Hg, or hypersensitivity to the medication.5 Current guidelines for propranolol initiation vary; some dermatologists consult cardiology prior to initiation, while others perform routine vitals and an indication-driven electrocardiogram as needed based on family history of cardiac disease, maternal history of connective tissue disease, congenital heart block, or abnormal vital signs.
Given the demonstrated long-term safety of propranolol and the acceptable side-effect profile, the use of beta-blockers for IH has become increasingly mainstream. Three randomized controlled trials (RCTs) have evaluated the efficacy and minimal adverse effects of propranolol for IH. The first RCT evaluated 40 patients who received either placebo or propranolol 2 mg/kg daily (divided into 3 doses) for 6 months; IH growth stopped by week 4 in the treatment group and the largest volume difference in IH was seen at week 12.6 Léauté-Labrèze et al7 demonstrated that propranolol could be given earlier to patients and at higher doses; the treatment group included 7 patients at 3 mg/kg daily of propranolol for 15 days, followed by 15 additional days of 4 mg/kg daily of propranolol. A statistically significant (P=.004) decrease in IH volume, quantified by use of ultrasonography, was exhibited by the propranolol group.7 Lastly, the largest RCT (N=456) established the efficacy of propranolol 3 mg/kg daily for 6 months with a 60% successful treatment rate compared to 4% for patients receiving placebo.8
Given the efficacy of propranolol for IH, other investigators have experimented with nonselective beta-blockers for other dermatologic conditions. In addition to second-line use for flushing, hyperhidrosis, and adrenergic urticaria, the future of propranolol is expanding for vascular lesions in particular.9 Chow et al10 highlighted a case of progressive angiosarcoma of the scalp that responded to propranolol hydrochloride therapy at 40 mg 3 times daily with extensive regression; propranolol was given in addition to chemotherapy and radiation. The tumor was biopsied before and after propranolol therapy and exhibited a 34% decrease in the proliferative index (Ki-67).10 Interestingly, Chisholm et al11 evaluated the expression of β-adrenergic expression in 141 vascular lesions; endothelial cell expression of β2-adrenergic receptors was found positive in 100% of IHs, 67% of kaposiform hemangioendotheliomas, 41% of angiosarcomas, 50% of pyogenic granulomas, and 75% of Kaposi sarcomas, to name merely a few studied lesions.
These data have spurred physicians to further seek beta-blocker dermatologic use in specific patient populations. For example, Meseguer-Yebra et al12 employed timolol solution 0.5% twice daily for 12 weeks for 2 human immunodeficiency virus–negative patients with limited Kaposi sarcoma of the right thigh and foot; no clinical evidence of recurrence was seen at 20 months, and one of the patients had a subsequent biopsy performed with negative human herpesvirus 8 staining after therapy. In the pediatric arena, topical timolol has been used for both port-wine stains and pyogenic granulomas.13-15 Two lesions of pyogenic granulomas on the scalp of a child were treated with timolol ophthalmic solution 0.5% under occlusion for 4 weeks with resolution.15 Propranolol also has been utilized as adjunctive therapy for aggressive pediatric vascular lesions such as kaposiform hemangioendothelioma with promising results and additionally reducing the duration of therapy needed with vincristine.2
In summary, propranolol and timolol have made an indelible impression on the field of pediatric dermatology and have demonstrated a burgeoning role in the dermatologic arena. The use of nonselective beta-blockers for the management of vascular lesions can serve as adjunctive or monotherapy for certain patient populations. The relatively low adverse risk profile of propranolol makes it a versatile tool to use both systemically and topically. Although the authors of the study assessing the β2-adrenergic expression in vascular lesions admittedly stated that the positivity of the receptors does not necessarily correlate with therapeutic management, it is an interesting subject area with much potential in the future.11 This review serves to illuminate the expanding role of beta-blockers in dermatology.
- Léauté-Labrèze C, Dumas de la Roque E, Hubiche T, et al. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358:2649-2651.
- Hermans DJ, van Beynum IM, van der Vijver RJ, et al. Kaposiform hemangioendothelioma with Kasabach-Merritt syndrome: a new indication for propranolol treatment. J Pediatr Hematol Oncol. 2011;33:E171-E173.
- Guo S, Ni N. Topical treatment for capillary hemangioma of the eyelid using beta-blocker solution. Arch Ophthalmol. 2010;128:255-256.
- Püttgen K, Lucky A, Adams D, et al. Topical timolol maleate treatment of infantile hemangiomas. Pediatrics. 2016;138:3.
- Drolet BA, Frommelt PC, Chamlin SL, et al. Initiation and use of propranolol for infantile hemangioma: report of a consensus conference. Pediatrics. 2013;131:128-140.
- Hogeling M, Adams S, Wargon O. A randomized controlled trial of propranolol for infantile hemangiomas [published online July 25, 2011]. Pediatrics. 2011;128:E259-E266.
- Léauté-Labrèze C, Dumas de la Roque E, Nacka F, et al. Doubleblind randomized pilot trial evaluating the efficacy of oral propranolol on infantile haemangiomas in infants < 4 months of age. Br J Dermatol. 2013;169:181-183.
- Léauté-Labrèze C, Hoeger P, Mazereeuw-Hautier J, et al. A randomized, controlled trial of oral propranolol in infantile hemangioma. N Engl J Med. 2015;372:735-746.
- Shelley WB, Shelley ED. Adrenergic urticaria: a new form of stress induced hives. Lancet. 1985;2:1031-1033.
- Chow W, Amaya CN, Rains S, et al. Growth attenuation of cutaneous angiosarcoma with propranolol-mediated β-blockade. JAMA Dermatol. 2015;151:1226-1229.
- Chisholm KM, Chang KW, Truong MT, et al. β-adrenergic receptor expression in vascular tumors. Mod Pathol. 2012;25:1446-1451.
- Meseguer-Yebra C, Cardeñoso-Álvarez, ME, Bordel-Gómez MT, et al. Successful treatment of classic Kaposi sarcoma with topical timolol: report of two cases. Br J Dermatol. 2015;173:860-862.
- Passeron T, Maza A, Fontas E, et al. Treatment of port wine stains and pulsed dye laser and topical timolol: a multicenter randomized controlled trial. Br J Dermatol. 2014;170:1350-1353.
- Wine LL, Goff KL, Lam JM, et al. Treatment of pediatric pyogenic granulomas using β-adrenergic receptor antagonist. Pediatr Dermatol. 2014;31:203-207.
- Knöpfel N, Escudero-Góngora Mdel M, Bauzà A, et al. Timolol for the treatment of pyogenic granuloma (PG) in children. J Am Acad Dermatol. 2016;75:E105-E106.
- Léauté-Labrèze C, Dumas de la Roque E, Hubiche T, et al. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358:2649-2651.
- Hermans DJ, van Beynum IM, van der Vijver RJ, et al. Kaposiform hemangioendothelioma with Kasabach-Merritt syndrome: a new indication for propranolol treatment. J Pediatr Hematol Oncol. 2011;33:E171-E173.
- Guo S, Ni N. Topical treatment for capillary hemangioma of the eyelid using beta-blocker solution. Arch Ophthalmol. 2010;128:255-256.
- Püttgen K, Lucky A, Adams D, et al. Topical timolol maleate treatment of infantile hemangiomas. Pediatrics. 2016;138:3.
- Drolet BA, Frommelt PC, Chamlin SL, et al. Initiation and use of propranolol for infantile hemangioma: report of a consensus conference. Pediatrics. 2013;131:128-140.
- Hogeling M, Adams S, Wargon O. A randomized controlled trial of propranolol for infantile hemangiomas [published online July 25, 2011]. Pediatrics. 2011;128:E259-E266.
- Léauté-Labrèze C, Dumas de la Roque E, Nacka F, et al. Doubleblind randomized pilot trial evaluating the efficacy of oral propranolol on infantile haemangiomas in infants < 4 months of age. Br J Dermatol. 2013;169:181-183.
- Léauté-Labrèze C, Hoeger P, Mazereeuw-Hautier J, et al. A randomized, controlled trial of oral propranolol in infantile hemangioma. N Engl J Med. 2015;372:735-746.
- Shelley WB, Shelley ED. Adrenergic urticaria: a new form of stress induced hives. Lancet. 1985;2:1031-1033.
- Chow W, Amaya CN, Rains S, et al. Growth attenuation of cutaneous angiosarcoma with propranolol-mediated β-blockade. JAMA Dermatol. 2015;151:1226-1229.
- Chisholm KM, Chang KW, Truong MT, et al. β-adrenergic receptor expression in vascular tumors. Mod Pathol. 2012;25:1446-1451.
- Meseguer-Yebra C, Cardeñoso-Álvarez, ME, Bordel-Gómez MT, et al. Successful treatment of classic Kaposi sarcoma with topical timolol: report of two cases. Br J Dermatol. 2015;173:860-862.
- Passeron T, Maza A, Fontas E, et al. Treatment of port wine stains and pulsed dye laser and topical timolol: a multicenter randomized controlled trial. Br J Dermatol. 2014;170:1350-1353.
- Wine LL, Goff KL, Lam JM, et al. Treatment of pediatric pyogenic granulomas using β-adrenergic receptor antagonist. Pediatr Dermatol. 2014;31:203-207.
- Knöpfel N, Escudero-Góngora Mdel M, Bauzà A, et al. Timolol for the treatment of pyogenic granuloma (PG) in children. J Am Acad Dermatol. 2016;75:E105-E106.
Flesh-Colored Nodule With Underlying Sclerotic Plaque
The Diagnosis: Collision Tumor
Excisional biopsy and histopathological examination demonstrated a collision tumor composed of a benign intradermal melanocytic nevus, tumor of follicular infundibulum, and an underlying sclerosing epithelial neoplasm, with a differential diagnosis of desmoplastic trichoepithelioma, morpheaform basal cell carcinoma, and microcystic adnexal carcinoma (Figure).
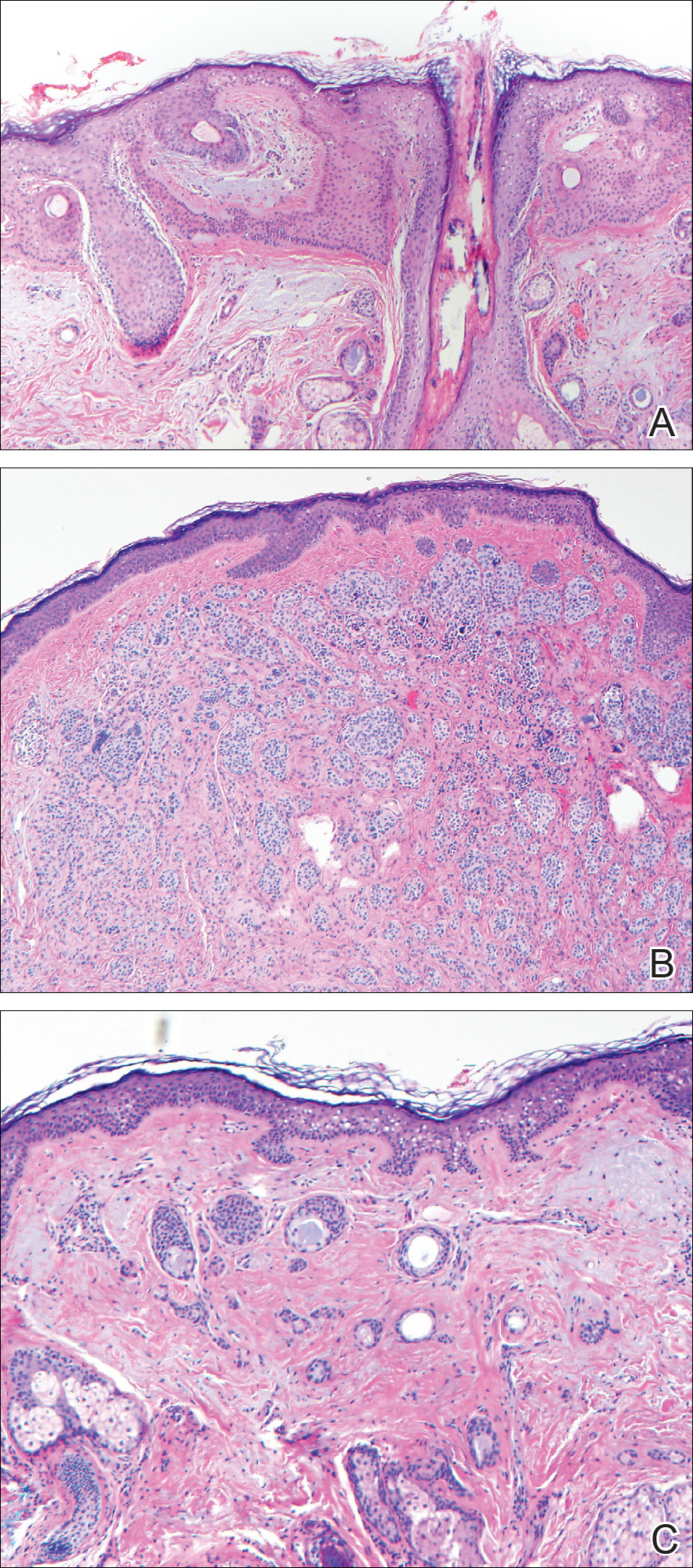
Common acquired melanocytic nevus presents clinically as a macule, papule, or nodule with smooth regular borders. The pigmented variant displays an evenly distributed pigment on the lesion. Intradermal melanocytic nevus often presents as a flesh-colored nodule, as in our case. Histopathologically, benign intradermal nevus typically is composed of a proliferation of melanocytes that exhibit dispersion as they go deeper in the dermis and maturation that manifests as melanocytes becoming smaller and more spindled in the deeper portions of the lesion.1 These 2 characteristics plus the bland cytology seen in the present case confirm the benign characteristic of this lesion (Figure, B).
In addition to the benign intradermal melanocytic nevus, an adjacent tumor of follicular infundibulum was noted. Tumor of follicular infundibulum is a rare adnexal tumor. It occurs frequently on the head and neck and shows some female predominance.2,3 Multiple lesions and eruptive lesions are rare forms that also have been reported.4 Histopathologically, the tumor demonstrates an epithelial plate that is present in the papillary dermis and is connected to the epidermis at multiple points with attachment to the follicular outer root sheath. Peripheral palisading is characteristically present above an eosinophilic basement membrane (Figure, A). Rare reports have documented sebaceous and eccrine differentiation.5,6
Tumor of follicular infundibulum has been reported to be associated with other tumors. Organoid nevus (nevus sebaceous), trichilemmal tumor, and fibroma have been reported to occur as a collision tumor with tumor of follicular infundibulum. An association with Cowden disease also has been described.7 Biopsies that represent partial samples should be interpreted cautiously, as step sections can reveal basal cell carcinoma.
The term sclerosing epithelial neoplasm describes tumors that share a paisley tielike epithelial pattern and sclerotic stroma. Small specimens often require clinicopathologic correlation (Figure, C). The differential diagnosis includes morpheaform basal cell carcinoma, desmoplastic trichoepithelioma, and microcystic adnexal carcinoma. A panel of stains using Ber-EP4, PHLDA1, cytokeratin 15, and cytokeratin 19 has been proposed to help differentiate these entities.8 CD34 and cytokeratin 20 also have been used with varying success in small specimens.9,10
- Ferringer T, Peckham S, Ko CJ, et al. Melanocytic neoplasms. In: Elston DM, Ferringer T, eds. Dermatopathology. 2nd ed. Philadelphia, PA: Elsevier Saunders; 2014:105-109.
- Headington JT. Tumors of the hair follicle. Am J Pathol. 1976;85:480-505.
- Davis DA, Cohen PR. Hair follicle nevus: case report and review of the literature. Pediatr Dermatol. 1996;13:135-138.
- Ikeda S, Kawada J, Yaguchi H, et al. A case of unilateral, systematized linear hair follicle nevi associated with epidermal nevus-like lesions. Dermatology. 2003;206:172-174.
- Mehregan AH. Hair follicle tumors of the skin. J Cutan Pathol. 1985;12:189-195.
- Mahalingam M, Bhawan J, Finn R, et al. Tumor of the follicular infundibulum with sebaceous differentiation. J Cutan Pathol. 2001;28:314-317.
- Cribier B, Grosshans E. Tumor of the follicular infundibulum: a clinicopathologic study. J Am Acad Dermatol. 1995;33:979-984.
- Sellheyer K, Nelson P, Kutzner H, et al. The immunohistochemical differential diagnosis of microcystic adnexal carcinoma, desmoplastic trichoepithelioma and morpheaform basal cell carcinoma using BerEP4 and stem cell markers. J Cutan Pathol. 2013;40:363-370.
- Abesamis-Cubillan E, El-Shabrawi-Caelen L, LeBoit PE. Merkel cells and sclerosing epithelial neoplasms. Am J Dermatopathol. 2000;22:311-315.
- Smith KJ, Williams J, Corbett D, et al. Microcystic adnexal carcinoma: an immunohistochemical study including markers of proliferation and apoptosis. Am J Surg Pathol. 2001;25:464-471.
The Diagnosis: Collision Tumor
Excisional biopsy and histopathological examination demonstrated a collision tumor composed of a benign intradermal melanocytic nevus, tumor of follicular infundibulum, and an underlying sclerosing epithelial neoplasm, with a differential diagnosis of desmoplastic trichoepithelioma, morpheaform basal cell carcinoma, and microcystic adnexal carcinoma (Figure).

Common acquired melanocytic nevus presents clinically as a macule, papule, or nodule with smooth regular borders. The pigmented variant displays an evenly distributed pigment on the lesion. Intradermal melanocytic nevus often presents as a flesh-colored nodule, as in our case. Histopathologically, benign intradermal nevus typically is composed of a proliferation of melanocytes that exhibit dispersion as they go deeper in the dermis and maturation that manifests as melanocytes becoming smaller and more spindled in the deeper portions of the lesion.1 These 2 characteristics plus the bland cytology seen in the present case confirm the benign characteristic of this lesion (Figure, B).
In addition to the benign intradermal melanocytic nevus, an adjacent tumor of follicular infundibulum was noted. Tumor of follicular infundibulum is a rare adnexal tumor. It occurs frequently on the head and neck and shows some female predominance.2,3 Multiple lesions and eruptive lesions are rare forms that also have been reported.4 Histopathologically, the tumor demonstrates an epithelial plate that is present in the papillary dermis and is connected to the epidermis at multiple points with attachment to the follicular outer root sheath. Peripheral palisading is characteristically present above an eosinophilic basement membrane (Figure, A). Rare reports have documented sebaceous and eccrine differentiation.5,6
Tumor of follicular infundibulum has been reported to be associated with other tumors. Organoid nevus (nevus sebaceous), trichilemmal tumor, and fibroma have been reported to occur as a collision tumor with tumor of follicular infundibulum. An association with Cowden disease also has been described.7 Biopsies that represent partial samples should be interpreted cautiously, as step sections can reveal basal cell carcinoma.
The term sclerosing epithelial neoplasm describes tumors that share a paisley tielike epithelial pattern and sclerotic stroma. Small specimens often require clinicopathologic correlation (Figure, C). The differential diagnosis includes morpheaform basal cell carcinoma, desmoplastic trichoepithelioma, and microcystic adnexal carcinoma. A panel of stains using Ber-EP4, PHLDA1, cytokeratin 15, and cytokeratin 19 has been proposed to help differentiate these entities.8 CD34 and cytokeratin 20 also have been used with varying success in small specimens.9,10
The Diagnosis: Collision Tumor
Excisional biopsy and histopathological examination demonstrated a collision tumor composed of a benign intradermal melanocytic nevus, tumor of follicular infundibulum, and an underlying sclerosing epithelial neoplasm, with a differential diagnosis of desmoplastic trichoepithelioma, morpheaform basal cell carcinoma, and microcystic adnexal carcinoma (Figure).

Common acquired melanocytic nevus presents clinically as a macule, papule, or nodule with smooth regular borders. The pigmented variant displays an evenly distributed pigment on the lesion. Intradermal melanocytic nevus often presents as a flesh-colored nodule, as in our case. Histopathologically, benign intradermal nevus typically is composed of a proliferation of melanocytes that exhibit dispersion as they go deeper in the dermis and maturation that manifests as melanocytes becoming smaller and more spindled in the deeper portions of the lesion.1 These 2 characteristics plus the bland cytology seen in the present case confirm the benign characteristic of this lesion (Figure, B).
In addition to the benign intradermal melanocytic nevus, an adjacent tumor of follicular infundibulum was noted. Tumor of follicular infundibulum is a rare adnexal tumor. It occurs frequently on the head and neck and shows some female predominance.2,3 Multiple lesions and eruptive lesions are rare forms that also have been reported.4 Histopathologically, the tumor demonstrates an epithelial plate that is present in the papillary dermis and is connected to the epidermis at multiple points with attachment to the follicular outer root sheath. Peripheral palisading is characteristically present above an eosinophilic basement membrane (Figure, A). Rare reports have documented sebaceous and eccrine differentiation.5,6
Tumor of follicular infundibulum has been reported to be associated with other tumors. Organoid nevus (nevus sebaceous), trichilemmal tumor, and fibroma have been reported to occur as a collision tumor with tumor of follicular infundibulum. An association with Cowden disease also has been described.7 Biopsies that represent partial samples should be interpreted cautiously, as step sections can reveal basal cell carcinoma.
The term sclerosing epithelial neoplasm describes tumors that share a paisley tielike epithelial pattern and sclerotic stroma. Small specimens often require clinicopathologic correlation (Figure, C). The differential diagnosis includes morpheaform basal cell carcinoma, desmoplastic trichoepithelioma, and microcystic adnexal carcinoma. A panel of stains using Ber-EP4, PHLDA1, cytokeratin 15, and cytokeratin 19 has been proposed to help differentiate these entities.8 CD34 and cytokeratin 20 also have been used with varying success in small specimens.9,10
- Ferringer T, Peckham S, Ko CJ, et al. Melanocytic neoplasms. In: Elston DM, Ferringer T, eds. Dermatopathology. 2nd ed. Philadelphia, PA: Elsevier Saunders; 2014:105-109.
- Headington JT. Tumors of the hair follicle. Am J Pathol. 1976;85:480-505.
- Davis DA, Cohen PR. Hair follicle nevus: case report and review of the literature. Pediatr Dermatol. 1996;13:135-138.
- Ikeda S, Kawada J, Yaguchi H, et al. A case of unilateral, systematized linear hair follicle nevi associated with epidermal nevus-like lesions. Dermatology. 2003;206:172-174.
- Mehregan AH. Hair follicle tumors of the skin. J Cutan Pathol. 1985;12:189-195.
- Mahalingam M, Bhawan J, Finn R, et al. Tumor of the follicular infundibulum with sebaceous differentiation. J Cutan Pathol. 2001;28:314-317.
- Cribier B, Grosshans E. Tumor of the follicular infundibulum: a clinicopathologic study. J Am Acad Dermatol. 1995;33:979-984.
- Sellheyer K, Nelson P, Kutzner H, et al. The immunohistochemical differential diagnosis of microcystic adnexal carcinoma, desmoplastic trichoepithelioma and morpheaform basal cell carcinoma using BerEP4 and stem cell markers. J Cutan Pathol. 2013;40:363-370.
- Abesamis-Cubillan E, El-Shabrawi-Caelen L, LeBoit PE. Merkel cells and sclerosing epithelial neoplasms. Am J Dermatopathol. 2000;22:311-315.
- Smith KJ, Williams J, Corbett D, et al. Microcystic adnexal carcinoma: an immunohistochemical study including markers of proliferation and apoptosis. Am J Surg Pathol. 2001;25:464-471.
- Ferringer T, Peckham S, Ko CJ, et al. Melanocytic neoplasms. In: Elston DM, Ferringer T, eds. Dermatopathology. 2nd ed. Philadelphia, PA: Elsevier Saunders; 2014:105-109.
- Headington JT. Tumors of the hair follicle. Am J Pathol. 1976;85:480-505.
- Davis DA, Cohen PR. Hair follicle nevus: case report and review of the literature. Pediatr Dermatol. 1996;13:135-138.
- Ikeda S, Kawada J, Yaguchi H, et al. A case of unilateral, systematized linear hair follicle nevi associated with epidermal nevus-like lesions. Dermatology. 2003;206:172-174.
- Mehregan AH. Hair follicle tumors of the skin. J Cutan Pathol. 1985;12:189-195.
- Mahalingam M, Bhawan J, Finn R, et al. Tumor of the follicular infundibulum with sebaceous differentiation. J Cutan Pathol. 2001;28:314-317.
- Cribier B, Grosshans E. Tumor of the follicular infundibulum: a clinicopathologic study. J Am Acad Dermatol. 1995;33:979-984.
- Sellheyer K, Nelson P, Kutzner H, et al. The immunohistochemical differential diagnosis of microcystic adnexal carcinoma, desmoplastic trichoepithelioma and morpheaform basal cell carcinoma using BerEP4 and stem cell markers. J Cutan Pathol. 2013;40:363-370.
- Abesamis-Cubillan E, El-Shabrawi-Caelen L, LeBoit PE. Merkel cells and sclerosing epithelial neoplasms. Am J Dermatopathol. 2000;22:311-315.
- Smith KJ, Williams J, Corbett D, et al. Microcystic adnexal carcinoma: an immunohistochemical study including markers of proliferation and apoptosis. Am J Surg Pathol. 2001;25:464-471.
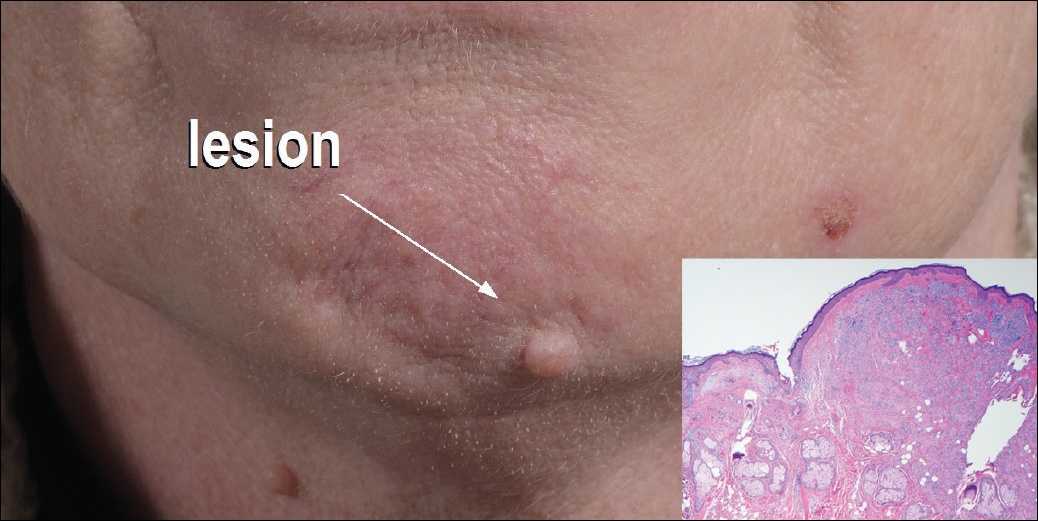
A 54-year-old man presented with a flesh-colored lesion on the chin. The nodule measured 0.6 cm in diameter. There was an underlying sclerotic plaque with indistinct borders.