User login
Social media makes kids with type 1 diabetes feel less alone
After being diagnosed with type 1 diabetes in 2021, British teenager Johnny Bailey felt isolated. That’s when he turned to social media, where he found others living with type 1 diabetes. He began to share his experience and now has more than 329,000 followers on his TikTok account, where he regularly posts videos.
These include short clips of him demonstrating how he changes his FreeStyle Libre sensor for his flash glucose monitor. In the videos, Johnny appropriately places his sensor on the back of his arm with background music, makes facial expressions, and transforms a dreaded diabetes-related task into an experience that appears fun and entertaining. In the limited videos I was able to review, he follows all the appropriate steps for sensor placement.
Many youths living with type 1 diabetes struggle with living with a chronic medical condition. Because type 1 diabetes is a rare condition, affecting about 1 in 500 children in the United States, many youth may not meet anyone else their age with type 1 diabetes through school, social events, or extracurricular activities.
For adolescents with intensively managed conditions like type 1 diabetes, this can present numerous psychosocial challenges – specifically, many youth experience shame or stigma associated with managing type 1 diabetes.
Diabetes-specific tasks may include wearing an insulin pump, monitoring blood glucose with finger pricks or a continuous glucose monitor (CGM), giving injections of insulin before meals and snacks, adjusting times for meals and snacks based on metabolic needs, waking up in the middle of the night to treat high or low blood glucose – the list goes on and on.
One study estimated that the average time it takes a child with type 1 diabetes to perform diabetes-specific tasks is over 5 hours per day.
Although much of this diabetes management time is spent by parents, as children get older and become teenagers, they are gradually transitioning to taking on more of this responsibility themselves. Wearing diabetes technology (insulin pumps and CGMs) can draw unwanted attention, leading to diabetes-specific body image concerns. Kids may also have to excuse themselves from an activity to treat a low or high blood glucose, creating uncomfortable situations when others inquire about why the activity was interrupted. As a result, many youths will avoid managing their diabetes properly to avoid drawing unwanted attention, consequently put their health at risk.
Those who are afraid of placing their glucose sensor owing to fear of pain may be reassured by seeing Johnny placing his sensor with a smile on his face. Some of his content also highlights other stigmatizing situations that teens may face, for example someone with a judgmental look questioning why he needed to give an insulin injection here.
This highlights an important concept – that people with type 1 diabetes may face criticism when dosing insulin in public, but it doesn’t mean they should feel forced to manage diabetes in private unless they choose to. Johnny is an inspirational individual who has bravely taken his type 1 diabetes experiences and used his creative skills to make these seemingly boring health-related tasks fun, interesting, and accessible.
Social media has become an outlet for people with type 1 diabetes to connect with others who can relate to their experiences.
However, there’s another side to consider. Although social media may provide a great source of support for youth, it may also adversely affect mental health. Just as quickly as social media outlets have grown, so has concern over excessive social media use and its impact on adolescents’ mental health. There’s a growing body of literature that describes the negative mental health aspects related to social media use.
Some adolescents struggling to manage type 1 diabetes may feel worse when seeing others thrive on social media, which has the potential to worsen stigma and shame. Youth may wonder how someone else is able to manage their type 1 diabetes so well when they are facing so many challenges.
Short videos on social media provide an incomplete picture of living with type 1 diabetes – just a glimpse into others’ lives, and only the parts that they want others to see. Managing a chronic condition can’t be fully represented in 10-second videos. And if youths choose to post their type 1 diabetes experiences on social media, they also risk receiving backlash or criticism, which can negatively their impact mental health in return.
Furthermore, the content being posted may not always be accurate or educational, leading to the potential for some youth to misunderstand type 1 diabetes.
Although I wouldn’t discourage youth with type 1 diabetes from engaging on social media and viewing diabetes-related content, they need to know that social media is flooded with misinformation. Creating an open space for youth to ask their clinicians questions about type 1 diabetes–related topics they view on social media is vital to ensuring they are viewing accurate information, so they are able to continue to manage their diabetes safely.
As a pediatric endocrinologist, I sometimes share resources on social media with patients if I believe it will help them cope with their type 1 diabetes diagnosis and management. I have had numerous patients – many of whom have struggled to accept their diagnosis – mention with joy and excitement that they were following an organization addressing type 1 diabetes on social media.
When making suggestions, I may refer them to The Diabetes Link, an organization with resources for young adults with type 1 diabetes that creates a space to connect with other young adults with type 1 diabetes. diaTribe is another organization created and led by people with diabetes that has a plethora of resources and provides evidence-based education for patients. I have also shared Project 50-in-50, which highlights two individuals with type 1 diabetes hiking the highest peak in each state in less than 50 days. Being able to see type 1 diabetes in a positive light is a huge step toward a more positive outlook on diabetes management.
Dr. Nally is an assistant professor, department of pediatrics, and a pediatric endocrinologist, division of pediatric endocrinology, at Yale University, New Haven, Conn. She reported conflicts of interest with Medtronic and the National Institutes of Health.
A version of this article first appeared on Medscape.com.
After being diagnosed with type 1 diabetes in 2021, British teenager Johnny Bailey felt isolated. That’s when he turned to social media, where he found others living with type 1 diabetes. He began to share his experience and now has more than 329,000 followers on his TikTok account, where he regularly posts videos.
These include short clips of him demonstrating how he changes his FreeStyle Libre sensor for his flash glucose monitor. In the videos, Johnny appropriately places his sensor on the back of his arm with background music, makes facial expressions, and transforms a dreaded diabetes-related task into an experience that appears fun and entertaining. In the limited videos I was able to review, he follows all the appropriate steps for sensor placement.
Many youths living with type 1 diabetes struggle with living with a chronic medical condition. Because type 1 diabetes is a rare condition, affecting about 1 in 500 children in the United States, many youth may not meet anyone else their age with type 1 diabetes through school, social events, or extracurricular activities.
For adolescents with intensively managed conditions like type 1 diabetes, this can present numerous psychosocial challenges – specifically, many youth experience shame or stigma associated with managing type 1 diabetes.
Diabetes-specific tasks may include wearing an insulin pump, monitoring blood glucose with finger pricks or a continuous glucose monitor (CGM), giving injections of insulin before meals and snacks, adjusting times for meals and snacks based on metabolic needs, waking up in the middle of the night to treat high or low blood glucose – the list goes on and on.
One study estimated that the average time it takes a child with type 1 diabetes to perform diabetes-specific tasks is over 5 hours per day.
Although much of this diabetes management time is spent by parents, as children get older and become teenagers, they are gradually transitioning to taking on more of this responsibility themselves. Wearing diabetes technology (insulin pumps and CGMs) can draw unwanted attention, leading to diabetes-specific body image concerns. Kids may also have to excuse themselves from an activity to treat a low or high blood glucose, creating uncomfortable situations when others inquire about why the activity was interrupted. As a result, many youths will avoid managing their diabetes properly to avoid drawing unwanted attention, consequently put their health at risk.
Those who are afraid of placing their glucose sensor owing to fear of pain may be reassured by seeing Johnny placing his sensor with a smile on his face. Some of his content also highlights other stigmatizing situations that teens may face, for example someone with a judgmental look questioning why he needed to give an insulin injection here.
This highlights an important concept – that people with type 1 diabetes may face criticism when dosing insulin in public, but it doesn’t mean they should feel forced to manage diabetes in private unless they choose to. Johnny is an inspirational individual who has bravely taken his type 1 diabetes experiences and used his creative skills to make these seemingly boring health-related tasks fun, interesting, and accessible.
Social media has become an outlet for people with type 1 diabetes to connect with others who can relate to their experiences.
However, there’s another side to consider. Although social media may provide a great source of support for youth, it may also adversely affect mental health. Just as quickly as social media outlets have grown, so has concern over excessive social media use and its impact on adolescents’ mental health. There’s a growing body of literature that describes the negative mental health aspects related to social media use.
Some adolescents struggling to manage type 1 diabetes may feel worse when seeing others thrive on social media, which has the potential to worsen stigma and shame. Youth may wonder how someone else is able to manage their type 1 diabetes so well when they are facing so many challenges.
Short videos on social media provide an incomplete picture of living with type 1 diabetes – just a glimpse into others’ lives, and only the parts that they want others to see. Managing a chronic condition can’t be fully represented in 10-second videos. And if youths choose to post their type 1 diabetes experiences on social media, they also risk receiving backlash or criticism, which can negatively their impact mental health in return.
Furthermore, the content being posted may not always be accurate or educational, leading to the potential for some youth to misunderstand type 1 diabetes.
Although I wouldn’t discourage youth with type 1 diabetes from engaging on social media and viewing diabetes-related content, they need to know that social media is flooded with misinformation. Creating an open space for youth to ask their clinicians questions about type 1 diabetes–related topics they view on social media is vital to ensuring they are viewing accurate information, so they are able to continue to manage their diabetes safely.
As a pediatric endocrinologist, I sometimes share resources on social media with patients if I believe it will help them cope with their type 1 diabetes diagnosis and management. I have had numerous patients – many of whom have struggled to accept their diagnosis – mention with joy and excitement that they were following an organization addressing type 1 diabetes on social media.
When making suggestions, I may refer them to The Diabetes Link, an organization with resources for young adults with type 1 diabetes that creates a space to connect with other young adults with type 1 diabetes. diaTribe is another organization created and led by people with diabetes that has a plethora of resources and provides evidence-based education for patients. I have also shared Project 50-in-50, which highlights two individuals with type 1 diabetes hiking the highest peak in each state in less than 50 days. Being able to see type 1 diabetes in a positive light is a huge step toward a more positive outlook on diabetes management.
Dr. Nally is an assistant professor, department of pediatrics, and a pediatric endocrinologist, division of pediatric endocrinology, at Yale University, New Haven, Conn. She reported conflicts of interest with Medtronic and the National Institutes of Health.
A version of this article first appeared on Medscape.com.
After being diagnosed with type 1 diabetes in 2021, British teenager Johnny Bailey felt isolated. That’s when he turned to social media, where he found others living with type 1 diabetes. He began to share his experience and now has more than 329,000 followers on his TikTok account, where he regularly posts videos.
These include short clips of him demonstrating how he changes his FreeStyle Libre sensor for his flash glucose monitor. In the videos, Johnny appropriately places his sensor on the back of his arm with background music, makes facial expressions, and transforms a dreaded diabetes-related task into an experience that appears fun and entertaining. In the limited videos I was able to review, he follows all the appropriate steps for sensor placement.
Many youths living with type 1 diabetes struggle with living with a chronic medical condition. Because type 1 diabetes is a rare condition, affecting about 1 in 500 children in the United States, many youth may not meet anyone else their age with type 1 diabetes through school, social events, or extracurricular activities.
For adolescents with intensively managed conditions like type 1 diabetes, this can present numerous psychosocial challenges – specifically, many youth experience shame or stigma associated with managing type 1 diabetes.
Diabetes-specific tasks may include wearing an insulin pump, monitoring blood glucose with finger pricks or a continuous glucose monitor (CGM), giving injections of insulin before meals and snacks, adjusting times for meals and snacks based on metabolic needs, waking up in the middle of the night to treat high or low blood glucose – the list goes on and on.
One study estimated that the average time it takes a child with type 1 diabetes to perform diabetes-specific tasks is over 5 hours per day.
Although much of this diabetes management time is spent by parents, as children get older and become teenagers, they are gradually transitioning to taking on more of this responsibility themselves. Wearing diabetes technology (insulin pumps and CGMs) can draw unwanted attention, leading to diabetes-specific body image concerns. Kids may also have to excuse themselves from an activity to treat a low or high blood glucose, creating uncomfortable situations when others inquire about why the activity was interrupted. As a result, many youths will avoid managing their diabetes properly to avoid drawing unwanted attention, consequently put their health at risk.
Those who are afraid of placing their glucose sensor owing to fear of pain may be reassured by seeing Johnny placing his sensor with a smile on his face. Some of his content also highlights other stigmatizing situations that teens may face, for example someone with a judgmental look questioning why he needed to give an insulin injection here.
This highlights an important concept – that people with type 1 diabetes may face criticism when dosing insulin in public, but it doesn’t mean they should feel forced to manage diabetes in private unless they choose to. Johnny is an inspirational individual who has bravely taken his type 1 diabetes experiences and used his creative skills to make these seemingly boring health-related tasks fun, interesting, and accessible.
Social media has become an outlet for people with type 1 diabetes to connect with others who can relate to their experiences.
However, there’s another side to consider. Although social media may provide a great source of support for youth, it may also adversely affect mental health. Just as quickly as social media outlets have grown, so has concern over excessive social media use and its impact on adolescents’ mental health. There’s a growing body of literature that describes the negative mental health aspects related to social media use.
Some adolescents struggling to manage type 1 diabetes may feel worse when seeing others thrive on social media, which has the potential to worsen stigma and shame. Youth may wonder how someone else is able to manage their type 1 diabetes so well when they are facing so many challenges.
Short videos on social media provide an incomplete picture of living with type 1 diabetes – just a glimpse into others’ lives, and only the parts that they want others to see. Managing a chronic condition can’t be fully represented in 10-second videos. And if youths choose to post their type 1 diabetes experiences on social media, they also risk receiving backlash or criticism, which can negatively their impact mental health in return.
Furthermore, the content being posted may not always be accurate or educational, leading to the potential for some youth to misunderstand type 1 diabetes.
Although I wouldn’t discourage youth with type 1 diabetes from engaging on social media and viewing diabetes-related content, they need to know that social media is flooded with misinformation. Creating an open space for youth to ask their clinicians questions about type 1 diabetes–related topics they view on social media is vital to ensuring they are viewing accurate information, so they are able to continue to manage their diabetes safely.
As a pediatric endocrinologist, I sometimes share resources on social media with patients if I believe it will help them cope with their type 1 diabetes diagnosis and management. I have had numerous patients – many of whom have struggled to accept their diagnosis – mention with joy and excitement that they were following an organization addressing type 1 diabetes on social media.
When making suggestions, I may refer them to The Diabetes Link, an organization with resources for young adults with type 1 diabetes that creates a space to connect with other young adults with type 1 diabetes. diaTribe is another organization created and led by people with diabetes that has a plethora of resources and provides evidence-based education for patients. I have also shared Project 50-in-50, which highlights two individuals with type 1 diabetes hiking the highest peak in each state in less than 50 days. Being able to see type 1 diabetes in a positive light is a huge step toward a more positive outlook on diabetes management.
Dr. Nally is an assistant professor, department of pediatrics, and a pediatric endocrinologist, division of pediatric endocrinology, at Yale University, New Haven, Conn. She reported conflicts of interest with Medtronic and the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Weight loss with semaglutide maintained for up to 3 years
Once weekly glucagon-like peptide 1 receptor agonist (GLP-1 RA) semaglutide (Ozempic, Novo Nordisk) significantly improved hemoglobin A1c level and body weight for up to 3 years in a large cohort of adults with type 2 diabetes, show real-world data from Israel.
and, in particular, in those patients with higher adherence to the therapy.
Avraham Karasik, MD, from the Institute of Research and Innovation at Maccabi Health Services, Tel Aviv, led the study and presented the work as a poster at this year’s annual meeting of the European Association for the Study of Diabetes.
“We found a clinically relevant improvement in blood sugar control and weight loss after 6 months of treatment, comparable with that seen in randomized trials,” said Dr. Karasik during an interview. “Importantly, these effects were sustained for up to 3 years, supporting the use of once weekly semaglutide for the long-term management of type 2 diabetes.”
Esther Walden, RN, deputy head of care at Diabetes UK, appreciated that the real-world findings reflected those seen in the randomized controlled trials. “This study suggests that improvements in blood sugars and weight loss can potentially be sustained in the longer term for adults with type 2 diabetes taking semaglutide as prescribed.”
Large scale, long term, and real world
Dr. Karasik explained that in Israel, there are many early adopters of once weekly semaglutide, and as such, it made for a large sample size, with a significant use duration for the retrospective study. “It’s a popular drug and there are lots of questions about durability of effect,” he pointed out.
Though evidence from randomized controlled trials support the effectiveness of once weekly semaglutide to treat type 2 diabetes, these studies are mostly of relatively short follow-up, explained Dr. Karasik, pointing out that long-term, large-scale, real-world data are needed. “In real life, people are acting differently to the trial setting and some adhere while others don’t, so it was interesting to see the durability as well as what happens when people discontinue treatment or adhere less.”
“Unsurprisingly, people who had a higher proportion of days covered ([PDC]; the total days of semaglutide use as a proportion of the total number of days followed up) had a higher effect,” explained Dr. Karasik, adding that, “if you don’t take it, it doesn’t work.”
A total of 23,442 patients were included in the study, with 6,049 followed up for 2 years or more. Mean baseline A1c was 7.6%-7.9%; body mass index (BMI) was 33.7-33.8 kg/m2; metformin was taken by 84%-88% of participants; insulin was taken by 30%; and 31% were treated with another GLP-1 RA prior to receiving semaglutide.
For study inclusion, participants were required to have had redeemed at least one prescription for subcutaneous semaglutide (0.25, 0.5, or 1 mg), and had at least one A1c measurement 12 months before and around 6 months after the start of semaglutide.
The primary outcome was change in A1c from baseline to the end of the follow-up at 6, 12, 18, 24, 30, and 36 months. Key secondary outcomes included change in body weight from baseline to the end of the follow-up (36 months); change in A1c and body weight in subgroups of patients who were persistently on therapy (at 12, 24, 36 months); and change in A1c and body weight in subgroups stratified by baseline characteristics. There was also an exploratory outcome, which was change in A1c and weight after treatment discontinuation. Dr. Karasik presented some of these results in his poster.
Median follow-up was 17.6 months in the total population and was 29.9 months in those who persisted with therapy for 2 years or more. “We have over 23,000 participants so it’s a large group, and these are not selected patients so the generalizability is better.”
Three-year sustained effect
Results from the total population showed that A1c lowered by a mean of 0.77% (from 7.6% to 6.8%) and body weight reduced by 4.7 kg (from 94.1 kg to 89.7 kg) after 6 months of treatment. These reductions were maintained during 3 years of follow-up in around 1,000 patients.
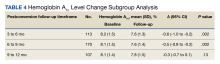
A significant 75% of participants adhered to once weekly semaglutide (PDC of more than 60%) within the first 6 months. In patients who used semaglutide for at least 2 years, those with high adherence (PDC of at least 80%) showed an A1c reduction of 0.76% after 24 months and of 0.43% after 36 months. Body weight was reduced by 6.0 kg after 24 months and 5.8 kg after 36 months.
Reductions in both A1c and weight were lower in patients with PDC of below 60%, compared with those with PDC of 60%-79% or 80% or over (statistically significant difference of P < .05 for between-groups differences for both outcomes across maximum follow-up time).
As expected, among patients who were GLP-1 RA–naive, reductions in A1c level and body weight were more pronounced, compared with GLP-1 RA–experienced patients (A1c reduction, –0.87% vs. –0.54%; weight loss, –5.5 kg vs. –3.0 kg, respectively; P < .001 for between-groups difference for both outcomes).
Dr. Karasik reported that some patients who stopped taking semaglutide did not regain weight immediately and that this potential residual effect after treatment discontinuation merits additional investigation. “This is not like in the randomized controlled trials. I don’t know how to interpret it, but that’s the observation. A1c did increase a little when they stopped therapy, compared to those with PDC [of 60%-79% or 80% or over] (P < .05 for between-groups difference for both outcomes in most follow-up time).”
He also highlighted that in regard to the long-term outcomes, “unlike many drugs where the effect fades out with time, here we don’t see that happening. This is another encouraging point.”
Dr. Karasik declares speaker fees and grants from Novo Nordisk, Boehringer Ingelheim, and AstraZeneca. The study was supported by Novo Nordisk.
A version of this article appeared on Medscape.com.
Once weekly glucagon-like peptide 1 receptor agonist (GLP-1 RA) semaglutide (Ozempic, Novo Nordisk) significantly improved hemoglobin A1c level and body weight for up to 3 years in a large cohort of adults with type 2 diabetes, show real-world data from Israel.
and, in particular, in those patients with higher adherence to the therapy.
Avraham Karasik, MD, from the Institute of Research and Innovation at Maccabi Health Services, Tel Aviv, led the study and presented the work as a poster at this year’s annual meeting of the European Association for the Study of Diabetes.
“We found a clinically relevant improvement in blood sugar control and weight loss after 6 months of treatment, comparable with that seen in randomized trials,” said Dr. Karasik during an interview. “Importantly, these effects were sustained for up to 3 years, supporting the use of once weekly semaglutide for the long-term management of type 2 diabetes.”
Esther Walden, RN, deputy head of care at Diabetes UK, appreciated that the real-world findings reflected those seen in the randomized controlled trials. “This study suggests that improvements in blood sugars and weight loss can potentially be sustained in the longer term for adults with type 2 diabetes taking semaglutide as prescribed.”
Large scale, long term, and real world
Dr. Karasik explained that in Israel, there are many early adopters of once weekly semaglutide, and as such, it made for a large sample size, with a significant use duration for the retrospective study. “It’s a popular drug and there are lots of questions about durability of effect,” he pointed out.
Though evidence from randomized controlled trials support the effectiveness of once weekly semaglutide to treat type 2 diabetes, these studies are mostly of relatively short follow-up, explained Dr. Karasik, pointing out that long-term, large-scale, real-world data are needed. “In real life, people are acting differently to the trial setting and some adhere while others don’t, so it was interesting to see the durability as well as what happens when people discontinue treatment or adhere less.”
“Unsurprisingly, people who had a higher proportion of days covered ([PDC]; the total days of semaglutide use as a proportion of the total number of days followed up) had a higher effect,” explained Dr. Karasik, adding that, “if you don’t take it, it doesn’t work.”
A total of 23,442 patients were included in the study, with 6,049 followed up for 2 years or more. Mean baseline A1c was 7.6%-7.9%; body mass index (BMI) was 33.7-33.8 kg/m2; metformin was taken by 84%-88% of participants; insulin was taken by 30%; and 31% were treated with another GLP-1 RA prior to receiving semaglutide.
For study inclusion, participants were required to have had redeemed at least one prescription for subcutaneous semaglutide (0.25, 0.5, or 1 mg), and had at least one A1c measurement 12 months before and around 6 months after the start of semaglutide.
The primary outcome was change in A1c from baseline to the end of the follow-up at 6, 12, 18, 24, 30, and 36 months. Key secondary outcomes included change in body weight from baseline to the end of the follow-up (36 months); change in A1c and body weight in subgroups of patients who were persistently on therapy (at 12, 24, 36 months); and change in A1c and body weight in subgroups stratified by baseline characteristics. There was also an exploratory outcome, which was change in A1c and weight after treatment discontinuation. Dr. Karasik presented some of these results in his poster.
Median follow-up was 17.6 months in the total population and was 29.9 months in those who persisted with therapy for 2 years or more. “We have over 23,000 participants so it’s a large group, and these are not selected patients so the generalizability is better.”
Three-year sustained effect
Results from the total population showed that A1c lowered by a mean of 0.77% (from 7.6% to 6.8%) and body weight reduced by 4.7 kg (from 94.1 kg to 89.7 kg) after 6 months of treatment. These reductions were maintained during 3 years of follow-up in around 1,000 patients.
A significant 75% of participants adhered to once weekly semaglutide (PDC of more than 60%) within the first 6 months. In patients who used semaglutide for at least 2 years, those with high adherence (PDC of at least 80%) showed an A1c reduction of 0.76% after 24 months and of 0.43% after 36 months. Body weight was reduced by 6.0 kg after 24 months and 5.8 kg after 36 months.
Reductions in both A1c and weight were lower in patients with PDC of below 60%, compared with those with PDC of 60%-79% or 80% or over (statistically significant difference of P < .05 for between-groups differences for both outcomes across maximum follow-up time).
As expected, among patients who were GLP-1 RA–naive, reductions in A1c level and body weight were more pronounced, compared with GLP-1 RA–experienced patients (A1c reduction, –0.87% vs. –0.54%; weight loss, –5.5 kg vs. –3.0 kg, respectively; P < .001 for between-groups difference for both outcomes).
Dr. Karasik reported that some patients who stopped taking semaglutide did not regain weight immediately and that this potential residual effect after treatment discontinuation merits additional investigation. “This is not like in the randomized controlled trials. I don’t know how to interpret it, but that’s the observation. A1c did increase a little when they stopped therapy, compared to those with PDC [of 60%-79% or 80% or over] (P < .05 for between-groups difference for both outcomes in most follow-up time).”
He also highlighted that in regard to the long-term outcomes, “unlike many drugs where the effect fades out with time, here we don’t see that happening. This is another encouraging point.”
Dr. Karasik declares speaker fees and grants from Novo Nordisk, Boehringer Ingelheim, and AstraZeneca. The study was supported by Novo Nordisk.
A version of this article appeared on Medscape.com.
Once weekly glucagon-like peptide 1 receptor agonist (GLP-1 RA) semaglutide (Ozempic, Novo Nordisk) significantly improved hemoglobin A1c level and body weight for up to 3 years in a large cohort of adults with type 2 diabetes, show real-world data from Israel.
and, in particular, in those patients with higher adherence to the therapy.
Avraham Karasik, MD, from the Institute of Research and Innovation at Maccabi Health Services, Tel Aviv, led the study and presented the work as a poster at this year’s annual meeting of the European Association for the Study of Diabetes.
“We found a clinically relevant improvement in blood sugar control and weight loss after 6 months of treatment, comparable with that seen in randomized trials,” said Dr. Karasik during an interview. “Importantly, these effects were sustained for up to 3 years, supporting the use of once weekly semaglutide for the long-term management of type 2 diabetes.”
Esther Walden, RN, deputy head of care at Diabetes UK, appreciated that the real-world findings reflected those seen in the randomized controlled trials. “This study suggests that improvements in blood sugars and weight loss can potentially be sustained in the longer term for adults with type 2 diabetes taking semaglutide as prescribed.”
Large scale, long term, and real world
Dr. Karasik explained that in Israel, there are many early adopters of once weekly semaglutide, and as such, it made for a large sample size, with a significant use duration for the retrospective study. “It’s a popular drug and there are lots of questions about durability of effect,” he pointed out.
Though evidence from randomized controlled trials support the effectiveness of once weekly semaglutide to treat type 2 diabetes, these studies are mostly of relatively short follow-up, explained Dr. Karasik, pointing out that long-term, large-scale, real-world data are needed. “In real life, people are acting differently to the trial setting and some adhere while others don’t, so it was interesting to see the durability as well as what happens when people discontinue treatment or adhere less.”
“Unsurprisingly, people who had a higher proportion of days covered ([PDC]; the total days of semaglutide use as a proportion of the total number of days followed up) had a higher effect,” explained Dr. Karasik, adding that, “if you don’t take it, it doesn’t work.”
A total of 23,442 patients were included in the study, with 6,049 followed up for 2 years or more. Mean baseline A1c was 7.6%-7.9%; body mass index (BMI) was 33.7-33.8 kg/m2; metformin was taken by 84%-88% of participants; insulin was taken by 30%; and 31% were treated with another GLP-1 RA prior to receiving semaglutide.
For study inclusion, participants were required to have had redeemed at least one prescription for subcutaneous semaglutide (0.25, 0.5, or 1 mg), and had at least one A1c measurement 12 months before and around 6 months after the start of semaglutide.
The primary outcome was change in A1c from baseline to the end of the follow-up at 6, 12, 18, 24, 30, and 36 months. Key secondary outcomes included change in body weight from baseline to the end of the follow-up (36 months); change in A1c and body weight in subgroups of patients who were persistently on therapy (at 12, 24, 36 months); and change in A1c and body weight in subgroups stratified by baseline characteristics. There was also an exploratory outcome, which was change in A1c and weight after treatment discontinuation. Dr. Karasik presented some of these results in his poster.
Median follow-up was 17.6 months in the total population and was 29.9 months in those who persisted with therapy for 2 years or more. “We have over 23,000 participants so it’s a large group, and these are not selected patients so the generalizability is better.”
Three-year sustained effect
Results from the total population showed that A1c lowered by a mean of 0.77% (from 7.6% to 6.8%) and body weight reduced by 4.7 kg (from 94.1 kg to 89.7 kg) after 6 months of treatment. These reductions were maintained during 3 years of follow-up in around 1,000 patients.
A significant 75% of participants adhered to once weekly semaglutide (PDC of more than 60%) within the first 6 months. In patients who used semaglutide for at least 2 years, those with high adherence (PDC of at least 80%) showed an A1c reduction of 0.76% after 24 months and of 0.43% after 36 months. Body weight was reduced by 6.0 kg after 24 months and 5.8 kg after 36 months.
Reductions in both A1c and weight were lower in patients with PDC of below 60%, compared with those with PDC of 60%-79% or 80% or over (statistically significant difference of P < .05 for between-groups differences for both outcomes across maximum follow-up time).
As expected, among patients who were GLP-1 RA–naive, reductions in A1c level and body weight were more pronounced, compared with GLP-1 RA–experienced patients (A1c reduction, –0.87% vs. –0.54%; weight loss, –5.5 kg vs. –3.0 kg, respectively; P < .001 for between-groups difference for both outcomes).
Dr. Karasik reported that some patients who stopped taking semaglutide did not regain weight immediately and that this potential residual effect after treatment discontinuation merits additional investigation. “This is not like in the randomized controlled trials. I don’t know how to interpret it, but that’s the observation. A1c did increase a little when they stopped therapy, compared to those with PDC [of 60%-79% or 80% or over] (P < .05 for between-groups difference for both outcomes in most follow-up time).”
He also highlighted that in regard to the long-term outcomes, “unlike many drugs where the effect fades out with time, here we don’t see that happening. This is another encouraging point.”
Dr. Karasik declares speaker fees and grants from Novo Nordisk, Boehringer Ingelheim, and AstraZeneca. The study was supported by Novo Nordisk.
A version of this article appeared on Medscape.com.
FROM EASD 2023
Tirzepatide with insulin glargine improves type 2 diabetes
HAMBURG, GERMANY – Once-weekly tirzepatide (Mounjaro, Lilly) added to insulin glargine resulted in greater reductions in hemoglobin A1c along with more weight loss and less hypoglycemia, compared with prandial insulin lispro (Humalog, Sanofi), for patients with inadequately controlled type 2 diabetes, show data from the SURPASS-6 randomized clinical trial.
It also resulted in a higher percentage of participants meeting an A1c target of less than 7.0%, wrote the researchers, whose study was presented at the annual meeting of the European Association for the Study of Diabetes and was published simultaneously in JAMA.
Also, daily insulin glargine use was substantially lower among participants who received tirzepatide, compared with insulin lispro. Insulin glargine was administered at a dosage 13 IU/day; insulin lispro was administered at a dosage of 62 IU/day. “At the highest dose, some patients stopped their insulin [glargine] in the tirzepatide arm,” said Juan Pablo Frias, MD, medical director and principal investigator of Velocity Clinical Research, Los Angeles, who presented the findings. “We demonstrated clinically meaningful and superior glycemic and body weight control with tirzepatide compared with insulin lispro, while tirzepatide was also associated with less clinically significant hypoglycemia.”
Weight improved for participants who received tirzepatide compared with those who received insulin lispro, at –10 kg and +4 kg respectively. The rate of clinically significant hypoglycemia (blood glucose < 54 mg/dL) or severe hypoglycemia was tenfold lower with tirzepatide, compared with insulin lispro.
The session dedicated to tirzepatide was comoderated by Apostolos Tsapas, MD, professor of medicine and diabetes, Aristotle University, Thessaloniki, Greece, and Konstantinos Toulis, MD, consultant in endocrinology and diabetes, General Military Hospital, Thessaloniki, Greece. Dr. Toulis remarked that, in the chronic disease setting, management and treatment intensification are challenging to integrate, and there are barriers to adoption in routine practice. “This is particularly true when it adds complexity, as in the case of multiple prandial insulin injections on top of basal insulin in suboptimally treated individuals with type 2 diabetes.
“Demonstrating superiority over insulin lispro in terms of the so-called trio of A1c, weight loss, and hypoglycemic events, tirzepatide offers both a simpler to adhere to and a more efficacious treatment intensification option.” He noted that, while long-term safety data are awaited, “this seems to be a definite step forward from any viewpoint, with the possible exception of the taxpayer’s perspective.”
Dr. Tsapas added: “These data further support the very high dual glucose and weight efficacy of tirzepatide and the primary role of incretin-related therapies amongst the injectables for the treatment of type 2 diabetes.”
Tirzepatide 5, 10, 15 mg vs. insulin lispro in addition to insulin glargine
The researchers aimed to assess the efficacy and safety of adding once-weekly tirzepatide, compared with thrice-daily prandial insulin lispro, as an adjunctive therapy to insulin glargine for patients with type 2 diabetes that was inadequately controlled with basal insulin.
Tirzepatide activates the body’s receptors for glucose-dependent insulinotropic polypeptide and glucagonlike peptide–1 (GLP-1). The study authors noted that “recent guidelines support adding an injectable incretin-related therapy such as GLP-1 receptor agonist for glycemic control, rather than basal insulin, when oral medications are inadequate.”
The open-label, phase 3b clinical trial drew data from 135 sites across 15 countries and included 1,428 adults with type 2 diabetes who were taking basal insulin. Participants were randomly assigned in a 1:1:1:3 ratio to receive once-weekly subcutaneous injections of tirzepatide (5 mg [n = 243], 10 mg [n = 238], or 15 mg [n = 236]) or prandial thrice-daily insulin lispro (n = 708).
Both arms were well matched. The average age was 60 years, and 60% of participants were women. The average amount of time patients had type 2 diabetes was 14 years; 85% of participants continued taking metformin. The average A1c level was 8.8% at baseline. Patients were categorized as having obesity (average body mass index, 33 kg/m2). The average insulin glargine dose was 46 units, or 0.5 units/kg.
Outcomes included noninferiority of tirzepatide (pooled cohort) compared with insulin lispro, both in addition to insulin glargine; and A1c change from baseline to week 52 (noninferiority margin, 0.3%). Key secondary endpoints included change in body weight and percentage of participants who achieved an A1c target of less than 7.0%.
About 90% of participants who received the study drug completed the study, said Dr. Frias. “Only 0.5% of tirzepatide patients needed rescue therapy, while only 2% of the insulin lispro did.”
Prior to optimization, the average insulin glargine dose was 42 IU/kg; during optimization, it rose to an average of 46 IU/kg. “At 52 weeks, those on basal-bolus insulin found their insulin glargine dose stayed flat while insulin lispro was 62 units,” reported Dr. Frias. “The three tirzepatide doses show a reduction in insulin glargine, such that the pooled dose reached an average of 11 units, while 20% actually came off their basal insulin altogether [pooled tirzepatide].”
Tirzepatide (pooled) led to the recommended A1c target of less than 7.0% for 68% of patients versus 36% of patients in the insulin lispro group.
About 68% of the patients who received tirzepatide (pooled) achieved the recommended A1c target of less than 7.0% versus 36% of patients in the insulin lispro group.
“Individual tirzepatide doses and pooled doses showed significant reduction in A1c and up to a 2.5% reduction,” Dr. Frias added. “Normoglycemia was obtained by a greater proportion of patients on tirzepatide doses versus basal-bolus insulin – one-third in the 15-mg tirzepatide dose.”
Body weight reduction of 10% or more with tirzepatide
Further, at week 52, weight loss of 5% or more was achieved by 75.4% of participants in the pooled tirzepatide group, compared with 6.3% in the prandial lispro group. The weight loss was accompanied by clinically relevant improvements in cardiometabolic parameters.
In an exploratory analysis, weight loss of 10% or more was achieved by a mean of 48.9% of pooled tirzepatide-treated participants at week 52, compared with 2% of those taking insulin lispro, said Dr. Frias.
“It is possible that the body weight loss induced by tirzepatide therapy and its reported effect in reducing liver fat content may have led to an improvement in insulin sensitivity and decreased insulin requirements,” wrote the researchers in their article.
Hypoglycemia risk and the weight gain observed with complex insulin regimens that include prandial insulin have been main limitations to optimally up-titrate insulin therapy in clinical practice, wrote the authors.
Dr. Frias noted that, in this study, 48% of patients who received insulin lispro experienced clinically significant hypoglycemia, while only 10% of patients in the tirzepatide arms did. “This was 0.4 episodes per patient-year versus 4.4 in tirzepatide and insulin lispro respectively.”
There were more reports of adverse events among the tirzepatide groups than the insulin lispro group. “Typically, with tirzepatide, the commonest adverse events were GI in origin and were mild to moderate.” Rates were 14%-26% for nausea, 11%-15% for diarrhea, and 5%-13% for vomiting.
The study was sponsored by Eli Lilly. Dr. Frias has received grants from Eli Lilly paid to his institution during the conduct of the study and grants, personal fees, or nonfinancial support from Boehringer Ingelheim, Pfizer, Merck, Altimmune, 89BIO, Akero, Carmot Therapeutics, Intercept, Janssen, Madrigal, Novartis, Eli Lilly, Sanofi, and Novo Nordisk outside the submitted work. Dr. Toulis and Dr. Tsapas declared no relevant disclosures.
A version of this article first appeared on Medscape.com.
HAMBURG, GERMANY – Once-weekly tirzepatide (Mounjaro, Lilly) added to insulin glargine resulted in greater reductions in hemoglobin A1c along with more weight loss and less hypoglycemia, compared with prandial insulin lispro (Humalog, Sanofi), for patients with inadequately controlled type 2 diabetes, show data from the SURPASS-6 randomized clinical trial.
It also resulted in a higher percentage of participants meeting an A1c target of less than 7.0%, wrote the researchers, whose study was presented at the annual meeting of the European Association for the Study of Diabetes and was published simultaneously in JAMA.
Also, daily insulin glargine use was substantially lower among participants who received tirzepatide, compared with insulin lispro. Insulin glargine was administered at a dosage 13 IU/day; insulin lispro was administered at a dosage of 62 IU/day. “At the highest dose, some patients stopped their insulin [glargine] in the tirzepatide arm,” said Juan Pablo Frias, MD, medical director and principal investigator of Velocity Clinical Research, Los Angeles, who presented the findings. “We demonstrated clinically meaningful and superior glycemic and body weight control with tirzepatide compared with insulin lispro, while tirzepatide was also associated with less clinically significant hypoglycemia.”
Weight improved for participants who received tirzepatide compared with those who received insulin lispro, at –10 kg and +4 kg respectively. The rate of clinically significant hypoglycemia (blood glucose < 54 mg/dL) or severe hypoglycemia was tenfold lower with tirzepatide, compared with insulin lispro.
The session dedicated to tirzepatide was comoderated by Apostolos Tsapas, MD, professor of medicine and diabetes, Aristotle University, Thessaloniki, Greece, and Konstantinos Toulis, MD, consultant in endocrinology and diabetes, General Military Hospital, Thessaloniki, Greece. Dr. Toulis remarked that, in the chronic disease setting, management and treatment intensification are challenging to integrate, and there are barriers to adoption in routine practice. “This is particularly true when it adds complexity, as in the case of multiple prandial insulin injections on top of basal insulin in suboptimally treated individuals with type 2 diabetes.
“Demonstrating superiority over insulin lispro in terms of the so-called trio of A1c, weight loss, and hypoglycemic events, tirzepatide offers both a simpler to adhere to and a more efficacious treatment intensification option.” He noted that, while long-term safety data are awaited, “this seems to be a definite step forward from any viewpoint, with the possible exception of the taxpayer’s perspective.”
Dr. Tsapas added: “These data further support the very high dual glucose and weight efficacy of tirzepatide and the primary role of incretin-related therapies amongst the injectables for the treatment of type 2 diabetes.”
Tirzepatide 5, 10, 15 mg vs. insulin lispro in addition to insulin glargine
The researchers aimed to assess the efficacy and safety of adding once-weekly tirzepatide, compared with thrice-daily prandial insulin lispro, as an adjunctive therapy to insulin glargine for patients with type 2 diabetes that was inadequately controlled with basal insulin.
Tirzepatide activates the body’s receptors for glucose-dependent insulinotropic polypeptide and glucagonlike peptide–1 (GLP-1). The study authors noted that “recent guidelines support adding an injectable incretin-related therapy such as GLP-1 receptor agonist for glycemic control, rather than basal insulin, when oral medications are inadequate.”
The open-label, phase 3b clinical trial drew data from 135 sites across 15 countries and included 1,428 adults with type 2 diabetes who were taking basal insulin. Participants were randomly assigned in a 1:1:1:3 ratio to receive once-weekly subcutaneous injections of tirzepatide (5 mg [n = 243], 10 mg [n = 238], or 15 mg [n = 236]) or prandial thrice-daily insulin lispro (n = 708).
Both arms were well matched. The average age was 60 years, and 60% of participants were women. The average amount of time patients had type 2 diabetes was 14 years; 85% of participants continued taking metformin. The average A1c level was 8.8% at baseline. Patients were categorized as having obesity (average body mass index, 33 kg/m2). The average insulin glargine dose was 46 units, or 0.5 units/kg.
Outcomes included noninferiority of tirzepatide (pooled cohort) compared with insulin lispro, both in addition to insulin glargine; and A1c change from baseline to week 52 (noninferiority margin, 0.3%). Key secondary endpoints included change in body weight and percentage of participants who achieved an A1c target of less than 7.0%.
About 90% of participants who received the study drug completed the study, said Dr. Frias. “Only 0.5% of tirzepatide patients needed rescue therapy, while only 2% of the insulin lispro did.”
Prior to optimization, the average insulin glargine dose was 42 IU/kg; during optimization, it rose to an average of 46 IU/kg. “At 52 weeks, those on basal-bolus insulin found their insulin glargine dose stayed flat while insulin lispro was 62 units,” reported Dr. Frias. “The three tirzepatide doses show a reduction in insulin glargine, such that the pooled dose reached an average of 11 units, while 20% actually came off their basal insulin altogether [pooled tirzepatide].”
Tirzepatide (pooled) led to the recommended A1c target of less than 7.0% for 68% of patients versus 36% of patients in the insulin lispro group.
About 68% of the patients who received tirzepatide (pooled) achieved the recommended A1c target of less than 7.0% versus 36% of patients in the insulin lispro group.
“Individual tirzepatide doses and pooled doses showed significant reduction in A1c and up to a 2.5% reduction,” Dr. Frias added. “Normoglycemia was obtained by a greater proportion of patients on tirzepatide doses versus basal-bolus insulin – one-third in the 15-mg tirzepatide dose.”
Body weight reduction of 10% or more with tirzepatide
Further, at week 52, weight loss of 5% or more was achieved by 75.4% of participants in the pooled tirzepatide group, compared with 6.3% in the prandial lispro group. The weight loss was accompanied by clinically relevant improvements in cardiometabolic parameters.
In an exploratory analysis, weight loss of 10% or more was achieved by a mean of 48.9% of pooled tirzepatide-treated participants at week 52, compared with 2% of those taking insulin lispro, said Dr. Frias.
“It is possible that the body weight loss induced by tirzepatide therapy and its reported effect in reducing liver fat content may have led to an improvement in insulin sensitivity and decreased insulin requirements,” wrote the researchers in their article.
Hypoglycemia risk and the weight gain observed with complex insulin regimens that include prandial insulin have been main limitations to optimally up-titrate insulin therapy in clinical practice, wrote the authors.
Dr. Frias noted that, in this study, 48% of patients who received insulin lispro experienced clinically significant hypoglycemia, while only 10% of patients in the tirzepatide arms did. “This was 0.4 episodes per patient-year versus 4.4 in tirzepatide and insulin lispro respectively.”
There were more reports of adverse events among the tirzepatide groups than the insulin lispro group. “Typically, with tirzepatide, the commonest adverse events were GI in origin and were mild to moderate.” Rates were 14%-26% for nausea, 11%-15% for diarrhea, and 5%-13% for vomiting.
The study was sponsored by Eli Lilly. Dr. Frias has received grants from Eli Lilly paid to his institution during the conduct of the study and grants, personal fees, or nonfinancial support from Boehringer Ingelheim, Pfizer, Merck, Altimmune, 89BIO, Akero, Carmot Therapeutics, Intercept, Janssen, Madrigal, Novartis, Eli Lilly, Sanofi, and Novo Nordisk outside the submitted work. Dr. Toulis and Dr. Tsapas declared no relevant disclosures.
A version of this article first appeared on Medscape.com.
HAMBURG, GERMANY – Once-weekly tirzepatide (Mounjaro, Lilly) added to insulin glargine resulted in greater reductions in hemoglobin A1c along with more weight loss and less hypoglycemia, compared with prandial insulin lispro (Humalog, Sanofi), for patients with inadequately controlled type 2 diabetes, show data from the SURPASS-6 randomized clinical trial.
It also resulted in a higher percentage of participants meeting an A1c target of less than 7.0%, wrote the researchers, whose study was presented at the annual meeting of the European Association for the Study of Diabetes and was published simultaneously in JAMA.
Also, daily insulin glargine use was substantially lower among participants who received tirzepatide, compared with insulin lispro. Insulin glargine was administered at a dosage 13 IU/day; insulin lispro was administered at a dosage of 62 IU/day. “At the highest dose, some patients stopped their insulin [glargine] in the tirzepatide arm,” said Juan Pablo Frias, MD, medical director and principal investigator of Velocity Clinical Research, Los Angeles, who presented the findings. “We demonstrated clinically meaningful and superior glycemic and body weight control with tirzepatide compared with insulin lispro, while tirzepatide was also associated with less clinically significant hypoglycemia.”
Weight improved for participants who received tirzepatide compared with those who received insulin lispro, at –10 kg and +4 kg respectively. The rate of clinically significant hypoglycemia (blood glucose < 54 mg/dL) or severe hypoglycemia was tenfold lower with tirzepatide, compared with insulin lispro.
The session dedicated to tirzepatide was comoderated by Apostolos Tsapas, MD, professor of medicine and diabetes, Aristotle University, Thessaloniki, Greece, and Konstantinos Toulis, MD, consultant in endocrinology and diabetes, General Military Hospital, Thessaloniki, Greece. Dr. Toulis remarked that, in the chronic disease setting, management and treatment intensification are challenging to integrate, and there are barriers to adoption in routine practice. “This is particularly true when it adds complexity, as in the case of multiple prandial insulin injections on top of basal insulin in suboptimally treated individuals with type 2 diabetes.
“Demonstrating superiority over insulin lispro in terms of the so-called trio of A1c, weight loss, and hypoglycemic events, tirzepatide offers both a simpler to adhere to and a more efficacious treatment intensification option.” He noted that, while long-term safety data are awaited, “this seems to be a definite step forward from any viewpoint, with the possible exception of the taxpayer’s perspective.”
Dr. Tsapas added: “These data further support the very high dual glucose and weight efficacy of tirzepatide and the primary role of incretin-related therapies amongst the injectables for the treatment of type 2 diabetes.”
Tirzepatide 5, 10, 15 mg vs. insulin lispro in addition to insulin glargine
The researchers aimed to assess the efficacy and safety of adding once-weekly tirzepatide, compared with thrice-daily prandial insulin lispro, as an adjunctive therapy to insulin glargine for patients with type 2 diabetes that was inadequately controlled with basal insulin.
Tirzepatide activates the body’s receptors for glucose-dependent insulinotropic polypeptide and glucagonlike peptide–1 (GLP-1). The study authors noted that “recent guidelines support adding an injectable incretin-related therapy such as GLP-1 receptor agonist for glycemic control, rather than basal insulin, when oral medications are inadequate.”
The open-label, phase 3b clinical trial drew data from 135 sites across 15 countries and included 1,428 adults with type 2 diabetes who were taking basal insulin. Participants were randomly assigned in a 1:1:1:3 ratio to receive once-weekly subcutaneous injections of tirzepatide (5 mg [n = 243], 10 mg [n = 238], or 15 mg [n = 236]) or prandial thrice-daily insulin lispro (n = 708).
Both arms were well matched. The average age was 60 years, and 60% of participants were women. The average amount of time patients had type 2 diabetes was 14 years; 85% of participants continued taking metformin. The average A1c level was 8.8% at baseline. Patients were categorized as having obesity (average body mass index, 33 kg/m2). The average insulin glargine dose was 46 units, or 0.5 units/kg.
Outcomes included noninferiority of tirzepatide (pooled cohort) compared with insulin lispro, both in addition to insulin glargine; and A1c change from baseline to week 52 (noninferiority margin, 0.3%). Key secondary endpoints included change in body weight and percentage of participants who achieved an A1c target of less than 7.0%.
About 90% of participants who received the study drug completed the study, said Dr. Frias. “Only 0.5% of tirzepatide patients needed rescue therapy, while only 2% of the insulin lispro did.”
Prior to optimization, the average insulin glargine dose was 42 IU/kg; during optimization, it rose to an average of 46 IU/kg. “At 52 weeks, those on basal-bolus insulin found their insulin glargine dose stayed flat while insulin lispro was 62 units,” reported Dr. Frias. “The three tirzepatide doses show a reduction in insulin glargine, such that the pooled dose reached an average of 11 units, while 20% actually came off their basal insulin altogether [pooled tirzepatide].”
Tirzepatide (pooled) led to the recommended A1c target of less than 7.0% for 68% of patients versus 36% of patients in the insulin lispro group.
About 68% of the patients who received tirzepatide (pooled) achieved the recommended A1c target of less than 7.0% versus 36% of patients in the insulin lispro group.
“Individual tirzepatide doses and pooled doses showed significant reduction in A1c and up to a 2.5% reduction,” Dr. Frias added. “Normoglycemia was obtained by a greater proportion of patients on tirzepatide doses versus basal-bolus insulin – one-third in the 15-mg tirzepatide dose.”
Body weight reduction of 10% or more with tirzepatide
Further, at week 52, weight loss of 5% or more was achieved by 75.4% of participants in the pooled tirzepatide group, compared with 6.3% in the prandial lispro group. The weight loss was accompanied by clinically relevant improvements in cardiometabolic parameters.
In an exploratory analysis, weight loss of 10% or more was achieved by a mean of 48.9% of pooled tirzepatide-treated participants at week 52, compared with 2% of those taking insulin lispro, said Dr. Frias.
“It is possible that the body weight loss induced by tirzepatide therapy and its reported effect in reducing liver fat content may have led to an improvement in insulin sensitivity and decreased insulin requirements,” wrote the researchers in their article.
Hypoglycemia risk and the weight gain observed with complex insulin regimens that include prandial insulin have been main limitations to optimally up-titrate insulin therapy in clinical practice, wrote the authors.
Dr. Frias noted that, in this study, 48% of patients who received insulin lispro experienced clinically significant hypoglycemia, while only 10% of patients in the tirzepatide arms did. “This was 0.4 episodes per patient-year versus 4.4 in tirzepatide and insulin lispro respectively.”
There were more reports of adverse events among the tirzepatide groups than the insulin lispro group. “Typically, with tirzepatide, the commonest adverse events were GI in origin and were mild to moderate.” Rates were 14%-26% for nausea, 11%-15% for diarrhea, and 5%-13% for vomiting.
The study was sponsored by Eli Lilly. Dr. Frias has received grants from Eli Lilly paid to his institution during the conduct of the study and grants, personal fees, or nonfinancial support from Boehringer Ingelheim, Pfizer, Merck, Altimmune, 89BIO, Akero, Carmot Therapeutics, Intercept, Janssen, Madrigal, Novartis, Eli Lilly, Sanofi, and Novo Nordisk outside the submitted work. Dr. Toulis and Dr. Tsapas declared no relevant disclosures.
A version of this article first appeared on Medscape.com.
AT EASD 2023
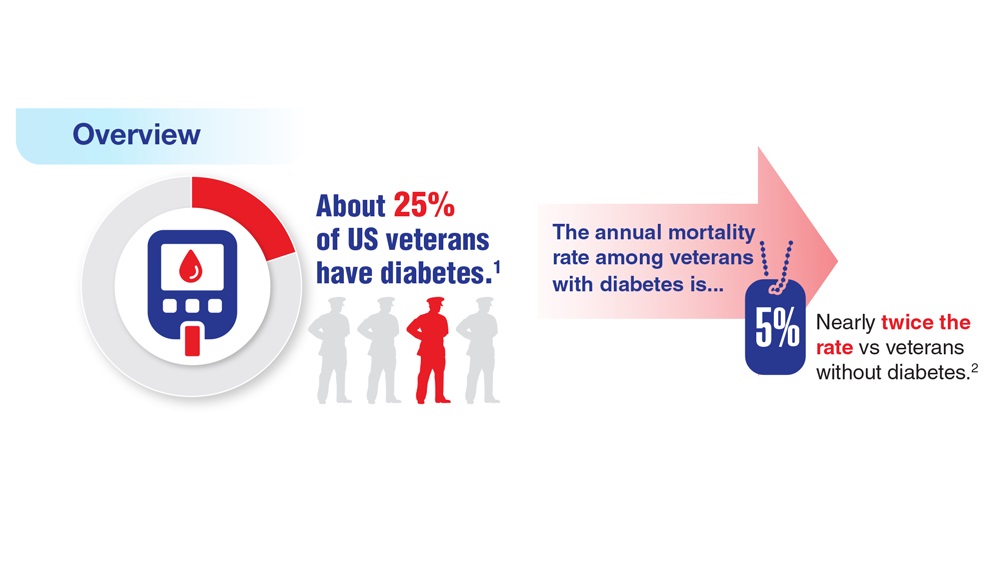
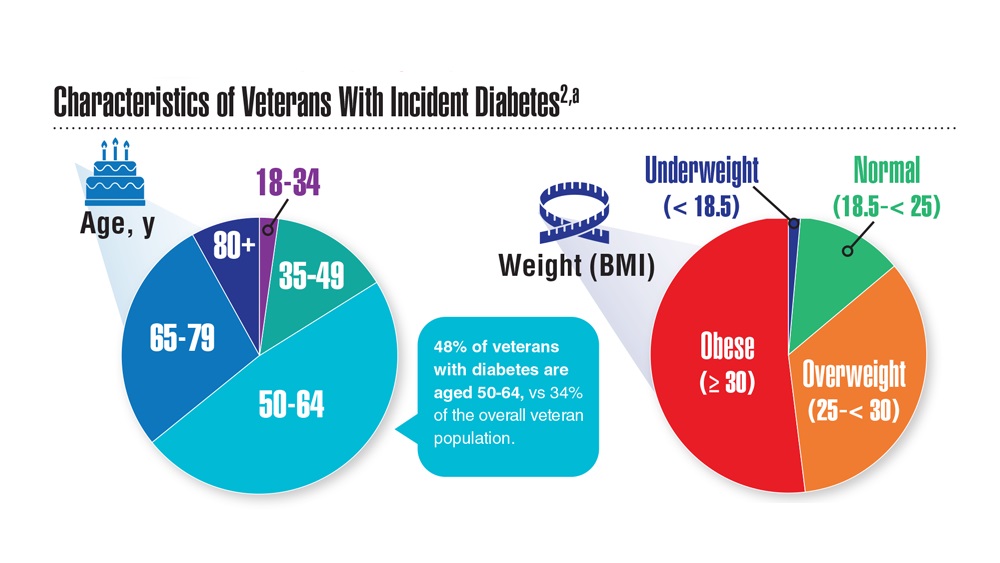
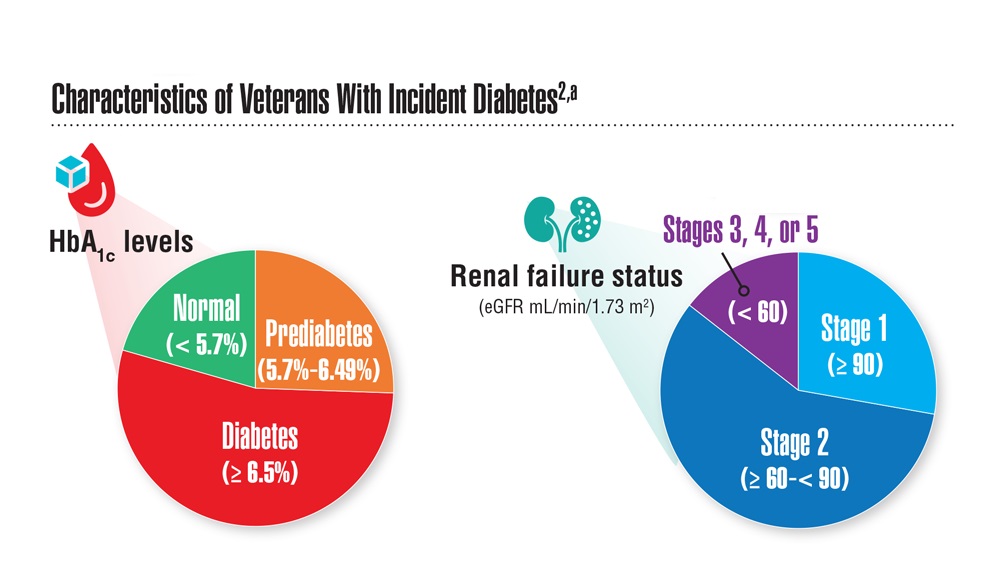
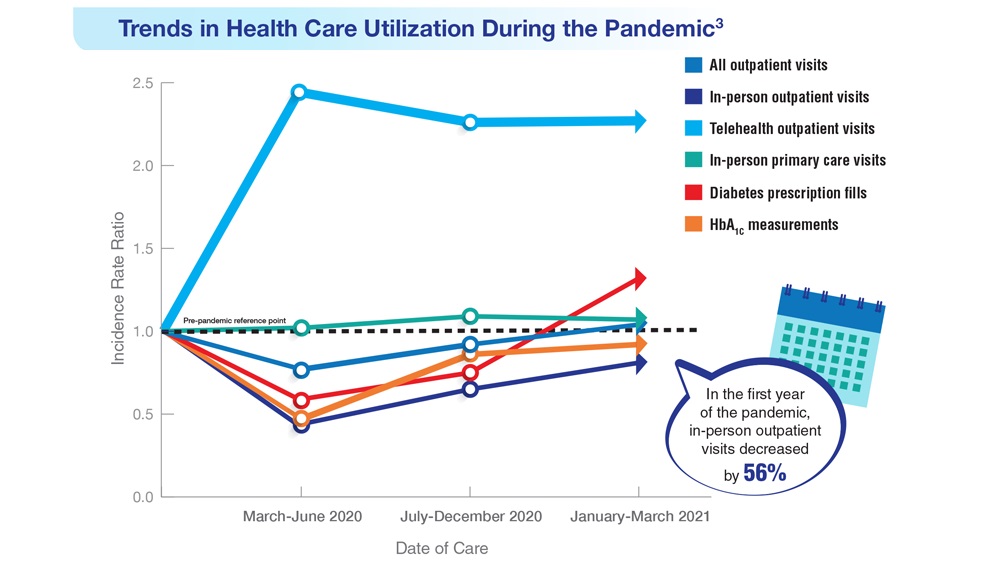
Federal Health Care Data Trends 2023
Federal Health Care Data Trends (click to view the digital edition) is a special supplement to Federal Practitioner, highlighting the latest research and study outcomes related to the health of veteran and active-duty populations.
In this issue:
- Limb Loss and Prostheses
- Neurology
- Cardiology
- Mental Health
- Diabetes
- Rheumatoid Arthritis
- Respiratory illnesses
- Women's Health
- HPV and Related Cancers
Federal Health Care Data Trends (click to view the digital edition) is a special supplement to Federal Practitioner, highlighting the latest research and study outcomes related to the health of veteran and active-duty populations.
In this issue:
- Limb Loss and Prostheses
- Neurology
- Cardiology
- Mental Health
- Diabetes
- Rheumatoid Arthritis
- Respiratory illnesses
- Women's Health
- HPV and Related Cancers
Federal Health Care Data Trends (click to view the digital edition) is a special supplement to Federal Practitioner, highlighting the latest research and study outcomes related to the health of veteran and active-duty populations.
In this issue:
- Limb Loss and Prostheses
- Neurology
- Cardiology
- Mental Health
- Diabetes
- Rheumatoid Arthritis
- Respiratory illnesses
- Women's Health
- HPV and Related Cancers
Metformin treatment shows benefit in gestational diabetes
HAMBURG –
Overall, the trial’s primary outcome, a composite of insulin initiation or a fasting glucose level ≥ 5.1 mmol/L (92 mg/dL) at gestation weeks 32 or 38, did not differ between women with gestational diabetes randomly assigned to either placebo or metformin. However, women taking metformin were significantly less likely to require insulin and had significantly lower fasting blood glucose levels at weeks 32 and 38.
“With a composite outcome it’s more difficult to find a positive result ... So, although the primary composite outcome was not positive, the components of the primary outcome that are clinically meaningful were positive,” lead study author Fidelma Dunne, PhD, professor and endocrine consultant at the University of Galway, Ireland, said in an interview.
There were no differences in maternal or neonatal morbidities, but there was a nonsignificant increase in small for gestational age (SGA), a finding that has been seen in some but not all previous studies of metformin use in gestational diabetes.
Dr. Dunne presented the findings on Oct. 3 at the annual meeting of the European Association for the Study of Diabetes. The results were simultaneously published in JAMA.
Current recommendations from the United Kingdom’s National Institute for Health and Care Excellence say metformin is a suitable first-line therapy for gestational diabetes. However, both the American Diabetes Association and the Society of Maternal-Fetal Medicine do not, particularly for pregnancies with hypertension or preeclampsia or in those who are at risk for intrauterine growth restriction.
“Gestational diabetes is now reaching epidemic proportions. And of course, the vast majority of these women are in low- and middle-income countries where insulin might not be available, or the storage may not allow it to be used effectively. If you have a medication that in the majority of women is safe and effective it may actually help a lot of women in [those regions],” Dr. Dunne said.
Moreover, she noted, “women with gestational diabetes are testing their sugar with finger pricks four to seven times per day and we ask them to take insulin one to four times a day. So if you can relieve any of that pain related to treatment of their condition than that is a benefit for the women as well.”
Asked to comment, Katrien Benhalima, MD, PhD, of University Hospital Gasthuisberg, KU Leuven, Belgium, said, “I think it’s an interesting study because they investigated something novel, to initiate immediately metformin or placebo. Normally what we do with gestational diabetes is once we get the diagnosis, we treat them with lifestyle, and if that’s insufficient then we start with medical therapy. So this is a novel approach.”
She also agreed with Dr. Dunne that the lack of significance for the primary outcome “isn’t an issue of power but it is a composite outcome. If you look at the individual outcomes, as can be expected, the women taking metformin had less need for insulin treatment.”
But, Dr. Benhalima said, the study still leaves open the SGA issue. “It wasn’t significant, but it’s still something we are worried about in the sense that we feel we need more data, especially in the long-term for the offspring health ... You really need to follow them for 10 years or longer to see an effect.”
So for now, Dr. Benhalima said that she wouldn’t use metformin as a first-line treatment for gestational diabetes. “Normally if lifestyle isn’t enough we will still start insulin ... Another issue is why would you offer everybody medical treatment when pregnancy outcomes can be met with lifestyle alone?”
Then again, she added, “of course metformin is easier than an injection. Treatment satisfaction is improved, and the cost is less.”
Primary outcome didn’t differ, but study findings point toward metformin benefit
The double-blind, placebo-controlled trial was conducted at two sites in Ireland, with 510 individuals (535 gestational diabetes pregnancies) enrolled between June 2017 and September 2022. In addition to usual care, they were randomly assigned 1:1 to either placebo or metformin (maximum 2,500 mg) at the time of gestational diabetes diagnosis and continued until delivery.
The primary outcome, a composite of insulin initiation or a fasting glucose ≥ 5.1 mmol/L at gestation weeks 32 or 38, did not differ significantly between the two groups, with risk ratio 0.89 (P = 0.13).
Insulin initiation occurred in 38.4% of the metformin and 51.1% of the placebo groups (relative risk, 0.75, P = .004). The amount of insulin required at the last assessment prior to delivery did not differ between the two groups (P = .17).
Mean fasting glucose was significantly lower with metformin vs. placebo at gestational week 32 (4.9 vs. 5.0 mmol/L; P = .03) and at gestational week 38 (4.5 vs 4.7 mmol/L; P < .001).
On average, those in the metformin group gained less weight between randomization and delivery (0.8 kg vs. 2.0 kg; P = .003).
Gestational week at delivery didn’t differ between the groups, both 39.1 weeks, nor did preterm births prior to 37 weeks’ gestation (9.2% metformin vs. 6.5% placebo; P = .33) or any other pregnancy-related complications.
More participants in the metformin group said that they would choose the drug compared with placebo (76.2% vs. 67.1%, P = .04).
Mean birth weight was lower in the metformin group compared with placebo, 3,393 g vs. 3,506 g (P = .005), with fewer weighing > 4,000 g (7.6% vs. 14.8%; P = .02) or being large for gestational age, i.e., above the 90th percentile (6.5% vs. 14.9%; P = .003).
Proportions of offspring that were SGA (less than 10th percentile) were 5.7% in the metformin group vs. 2.7% with placebo (P = .13).
There were no other significant differences in neonatal variables.
Dr. Dunne told this news organization that her group has recently received funding for long-term follow-up of the SGA offspring. “As other papers have pointed out, if there’s any hint of SGA that’s really important to follow up. So we’re now beginning our longitudinal follow up of the mother and infants to see if the small number that were SGA will in fact turn out to have an increase in body mass index and weight in their childhood and adolescent years.”
The trial was funded by the Health Review Board (HRB) of Ireland, coordinated by the HRB-Clinical Research Facility Galway, and sponsored by the University of Galway, Ireland. Metformin and matched placebo were provided by Merck Healthcare KGaA, Darmstadt, Germany (operating as EMD Serono in the United States), and blood glucose monitoring strips were provided by Ascensia.
Dr. Dunne reported nonfinancial support from Merck and matched placebo and nonfinancial support from Ascensia during the conduct of the study. Dr. Benhalima receives research funds from Flemish Research Fund, study medication from Novo Nordisk, and devices and unrestricted grants from Medtronic and Dexcom.
A version of this article appeared on Medscape.com.
HAMBURG –
Overall, the trial’s primary outcome, a composite of insulin initiation or a fasting glucose level ≥ 5.1 mmol/L (92 mg/dL) at gestation weeks 32 or 38, did not differ between women with gestational diabetes randomly assigned to either placebo or metformin. However, women taking metformin were significantly less likely to require insulin and had significantly lower fasting blood glucose levels at weeks 32 and 38.
“With a composite outcome it’s more difficult to find a positive result ... So, although the primary composite outcome was not positive, the components of the primary outcome that are clinically meaningful were positive,” lead study author Fidelma Dunne, PhD, professor and endocrine consultant at the University of Galway, Ireland, said in an interview.
There were no differences in maternal or neonatal morbidities, but there was a nonsignificant increase in small for gestational age (SGA), a finding that has been seen in some but not all previous studies of metformin use in gestational diabetes.
Dr. Dunne presented the findings on Oct. 3 at the annual meeting of the European Association for the Study of Diabetes. The results were simultaneously published in JAMA.
Current recommendations from the United Kingdom’s National Institute for Health and Care Excellence say metformin is a suitable first-line therapy for gestational diabetes. However, both the American Diabetes Association and the Society of Maternal-Fetal Medicine do not, particularly for pregnancies with hypertension or preeclampsia or in those who are at risk for intrauterine growth restriction.
“Gestational diabetes is now reaching epidemic proportions. And of course, the vast majority of these women are in low- and middle-income countries where insulin might not be available, or the storage may not allow it to be used effectively. If you have a medication that in the majority of women is safe and effective it may actually help a lot of women in [those regions],” Dr. Dunne said.
Moreover, she noted, “women with gestational diabetes are testing their sugar with finger pricks four to seven times per day and we ask them to take insulin one to four times a day. So if you can relieve any of that pain related to treatment of their condition than that is a benefit for the women as well.”
Asked to comment, Katrien Benhalima, MD, PhD, of University Hospital Gasthuisberg, KU Leuven, Belgium, said, “I think it’s an interesting study because they investigated something novel, to initiate immediately metformin or placebo. Normally what we do with gestational diabetes is once we get the diagnosis, we treat them with lifestyle, and if that’s insufficient then we start with medical therapy. So this is a novel approach.”
She also agreed with Dr. Dunne that the lack of significance for the primary outcome “isn’t an issue of power but it is a composite outcome. If you look at the individual outcomes, as can be expected, the women taking metformin had less need for insulin treatment.”
But, Dr. Benhalima said, the study still leaves open the SGA issue. “It wasn’t significant, but it’s still something we are worried about in the sense that we feel we need more data, especially in the long-term for the offspring health ... You really need to follow them for 10 years or longer to see an effect.”
So for now, Dr. Benhalima said that she wouldn’t use metformin as a first-line treatment for gestational diabetes. “Normally if lifestyle isn’t enough we will still start insulin ... Another issue is why would you offer everybody medical treatment when pregnancy outcomes can be met with lifestyle alone?”
Then again, she added, “of course metformin is easier than an injection. Treatment satisfaction is improved, and the cost is less.”
Primary outcome didn’t differ, but study findings point toward metformin benefit
The double-blind, placebo-controlled trial was conducted at two sites in Ireland, with 510 individuals (535 gestational diabetes pregnancies) enrolled between June 2017 and September 2022. In addition to usual care, they were randomly assigned 1:1 to either placebo or metformin (maximum 2,500 mg) at the time of gestational diabetes diagnosis and continued until delivery.
The primary outcome, a composite of insulin initiation or a fasting glucose ≥ 5.1 mmol/L at gestation weeks 32 or 38, did not differ significantly between the two groups, with risk ratio 0.89 (P = 0.13).
Insulin initiation occurred in 38.4% of the metformin and 51.1% of the placebo groups (relative risk, 0.75, P = .004). The amount of insulin required at the last assessment prior to delivery did not differ between the two groups (P = .17).
Mean fasting glucose was significantly lower with metformin vs. placebo at gestational week 32 (4.9 vs. 5.0 mmol/L; P = .03) and at gestational week 38 (4.5 vs 4.7 mmol/L; P < .001).
On average, those in the metformin group gained less weight between randomization and delivery (0.8 kg vs. 2.0 kg; P = .003).
Gestational week at delivery didn’t differ between the groups, both 39.1 weeks, nor did preterm births prior to 37 weeks’ gestation (9.2% metformin vs. 6.5% placebo; P = .33) or any other pregnancy-related complications.
More participants in the metformin group said that they would choose the drug compared with placebo (76.2% vs. 67.1%, P = .04).
Mean birth weight was lower in the metformin group compared with placebo, 3,393 g vs. 3,506 g (P = .005), with fewer weighing > 4,000 g (7.6% vs. 14.8%; P = .02) or being large for gestational age, i.e., above the 90th percentile (6.5% vs. 14.9%; P = .003).
Proportions of offspring that were SGA (less than 10th percentile) were 5.7% in the metformin group vs. 2.7% with placebo (P = .13).
There were no other significant differences in neonatal variables.
Dr. Dunne told this news organization that her group has recently received funding for long-term follow-up of the SGA offspring. “As other papers have pointed out, if there’s any hint of SGA that’s really important to follow up. So we’re now beginning our longitudinal follow up of the mother and infants to see if the small number that were SGA will in fact turn out to have an increase in body mass index and weight in their childhood and adolescent years.”
The trial was funded by the Health Review Board (HRB) of Ireland, coordinated by the HRB-Clinical Research Facility Galway, and sponsored by the University of Galway, Ireland. Metformin and matched placebo were provided by Merck Healthcare KGaA, Darmstadt, Germany (operating as EMD Serono in the United States), and blood glucose monitoring strips were provided by Ascensia.
Dr. Dunne reported nonfinancial support from Merck and matched placebo and nonfinancial support from Ascensia during the conduct of the study. Dr. Benhalima receives research funds from Flemish Research Fund, study medication from Novo Nordisk, and devices and unrestricted grants from Medtronic and Dexcom.
A version of this article appeared on Medscape.com.
HAMBURG –
Overall, the trial’s primary outcome, a composite of insulin initiation or a fasting glucose level ≥ 5.1 mmol/L (92 mg/dL) at gestation weeks 32 or 38, did not differ between women with gestational diabetes randomly assigned to either placebo or metformin. However, women taking metformin were significantly less likely to require insulin and had significantly lower fasting blood glucose levels at weeks 32 and 38.
“With a composite outcome it’s more difficult to find a positive result ... So, although the primary composite outcome was not positive, the components of the primary outcome that are clinically meaningful were positive,” lead study author Fidelma Dunne, PhD, professor and endocrine consultant at the University of Galway, Ireland, said in an interview.
There were no differences in maternal or neonatal morbidities, but there was a nonsignificant increase in small for gestational age (SGA), a finding that has been seen in some but not all previous studies of metformin use in gestational diabetes.
Dr. Dunne presented the findings on Oct. 3 at the annual meeting of the European Association for the Study of Diabetes. The results were simultaneously published in JAMA.
Current recommendations from the United Kingdom’s National Institute for Health and Care Excellence say metformin is a suitable first-line therapy for gestational diabetes. However, both the American Diabetes Association and the Society of Maternal-Fetal Medicine do not, particularly for pregnancies with hypertension or preeclampsia or in those who are at risk for intrauterine growth restriction.
“Gestational diabetes is now reaching epidemic proportions. And of course, the vast majority of these women are in low- and middle-income countries where insulin might not be available, or the storage may not allow it to be used effectively. If you have a medication that in the majority of women is safe and effective it may actually help a lot of women in [those regions],” Dr. Dunne said.
Moreover, she noted, “women with gestational diabetes are testing their sugar with finger pricks four to seven times per day and we ask them to take insulin one to four times a day. So if you can relieve any of that pain related to treatment of their condition than that is a benefit for the women as well.”
Asked to comment, Katrien Benhalima, MD, PhD, of University Hospital Gasthuisberg, KU Leuven, Belgium, said, “I think it’s an interesting study because they investigated something novel, to initiate immediately metformin or placebo. Normally what we do with gestational diabetes is once we get the diagnosis, we treat them with lifestyle, and if that’s insufficient then we start with medical therapy. So this is a novel approach.”
She also agreed with Dr. Dunne that the lack of significance for the primary outcome “isn’t an issue of power but it is a composite outcome. If you look at the individual outcomes, as can be expected, the women taking metformin had less need for insulin treatment.”
But, Dr. Benhalima said, the study still leaves open the SGA issue. “It wasn’t significant, but it’s still something we are worried about in the sense that we feel we need more data, especially in the long-term for the offspring health ... You really need to follow them for 10 years or longer to see an effect.”
So for now, Dr. Benhalima said that she wouldn’t use metformin as a first-line treatment for gestational diabetes. “Normally if lifestyle isn’t enough we will still start insulin ... Another issue is why would you offer everybody medical treatment when pregnancy outcomes can be met with lifestyle alone?”
Then again, she added, “of course metformin is easier than an injection. Treatment satisfaction is improved, and the cost is less.”
Primary outcome didn’t differ, but study findings point toward metformin benefit
The double-blind, placebo-controlled trial was conducted at two sites in Ireland, with 510 individuals (535 gestational diabetes pregnancies) enrolled between June 2017 and September 2022. In addition to usual care, they were randomly assigned 1:1 to either placebo or metformin (maximum 2,500 mg) at the time of gestational diabetes diagnosis and continued until delivery.
The primary outcome, a composite of insulin initiation or a fasting glucose ≥ 5.1 mmol/L at gestation weeks 32 or 38, did not differ significantly between the two groups, with risk ratio 0.89 (P = 0.13).
Insulin initiation occurred in 38.4% of the metformin and 51.1% of the placebo groups (relative risk, 0.75, P = .004). The amount of insulin required at the last assessment prior to delivery did not differ between the two groups (P = .17).
Mean fasting glucose was significantly lower with metformin vs. placebo at gestational week 32 (4.9 vs. 5.0 mmol/L; P = .03) and at gestational week 38 (4.5 vs 4.7 mmol/L; P < .001).
On average, those in the metformin group gained less weight between randomization and delivery (0.8 kg vs. 2.0 kg; P = .003).
Gestational week at delivery didn’t differ between the groups, both 39.1 weeks, nor did preterm births prior to 37 weeks’ gestation (9.2% metformin vs. 6.5% placebo; P = .33) or any other pregnancy-related complications.
More participants in the metformin group said that they would choose the drug compared with placebo (76.2% vs. 67.1%, P = .04).
Mean birth weight was lower in the metformin group compared with placebo, 3,393 g vs. 3,506 g (P = .005), with fewer weighing > 4,000 g (7.6% vs. 14.8%; P = .02) or being large for gestational age, i.e., above the 90th percentile (6.5% vs. 14.9%; P = .003).
Proportions of offspring that were SGA (less than 10th percentile) were 5.7% in the metformin group vs. 2.7% with placebo (P = .13).
There were no other significant differences in neonatal variables.
Dr. Dunne told this news organization that her group has recently received funding for long-term follow-up of the SGA offspring. “As other papers have pointed out, if there’s any hint of SGA that’s really important to follow up. So we’re now beginning our longitudinal follow up of the mother and infants to see if the small number that were SGA will in fact turn out to have an increase in body mass index and weight in their childhood and adolescent years.”
The trial was funded by the Health Review Board (HRB) of Ireland, coordinated by the HRB-Clinical Research Facility Galway, and sponsored by the University of Galway, Ireland. Metformin and matched placebo were provided by Merck Healthcare KGaA, Darmstadt, Germany (operating as EMD Serono in the United States), and blood glucose monitoring strips were provided by Ascensia.
Dr. Dunne reported nonfinancial support from Merck and matched placebo and nonfinancial support from Ascensia during the conduct of the study. Dr. Benhalima receives research funds from Flemish Research Fund, study medication from Novo Nordisk, and devices and unrestricted grants from Medtronic and Dexcom.
A version of this article appeared on Medscape.com.
AT EASD 2023
Data Trends 2023: Diabetes
- US Department of Veterans Affairs. Nutrition and food services. Diabetes information. Updated December 1, 2022. Accessed April 14, 2023. https://www.nutrition.va.gov/Diabetes.asp
- Avramovic S et al. BMJ Open. 2020;10(12):e039489. doi:10.1136/bmjopen-2020-039489
- Adhikari S et al. BMC Health Serv Res. 2023;23(1):41. doi:10.1186/s12913-023-09057-8
- Zhou P et al. J Diabetes Metab Disord. 2022;21(1):759-768. doi:10.1007/s40200-022-01049-5
- Lamprea-Montealegre JA et al. JAMA. 2022;328(9):861-871. doi:10.1001/jama.2022.13885
- Fairman KA, Buckley K. Health Psychol. 2021;40(1):1-10. doi:10.1037/hea0000889
- US Department of Veterans Affairs. Nutrition and food services. Diabetes information. Updated December 1, 2022. Accessed April 14, 2023. https://www.nutrition.va.gov/Diabetes.asp
- Avramovic S et al. BMJ Open. 2020;10(12):e039489. doi:10.1136/bmjopen-2020-039489
- Adhikari S et al. BMC Health Serv Res. 2023;23(1):41. doi:10.1186/s12913-023-09057-8
- Zhou P et al. J Diabetes Metab Disord. 2022;21(1):759-768. doi:10.1007/s40200-022-01049-5
- Lamprea-Montealegre JA et al. JAMA. 2022;328(9):861-871. doi:10.1001/jama.2022.13885
- Fairman KA, Buckley K. Health Psychol. 2021;40(1):1-10. doi:10.1037/hea0000889
- US Department of Veterans Affairs. Nutrition and food services. Diabetes information. Updated December 1, 2022. Accessed April 14, 2023. https://www.nutrition.va.gov/Diabetes.asp
- Avramovic S et al. BMJ Open. 2020;10(12):e039489. doi:10.1136/bmjopen-2020-039489
- Adhikari S et al. BMC Health Serv Res. 2023;23(1):41. doi:10.1186/s12913-023-09057-8
- Zhou P et al. J Diabetes Metab Disord. 2022;21(1):759-768. doi:10.1007/s40200-022-01049-5
- Lamprea-Montealegre JA et al. JAMA. 2022;328(9):861-871. doi:10.1001/jama.2022.13885
- Fairman KA, Buckley K. Health Psychol. 2021;40(1):1-10. doi:10.1037/hea0000889
Impact of Liraglutide to Semaglutide Conversion on Glycemic Control and Cost Savings at a Veterans Affairs Medical Center
Semaglutide and liraglutide are glucagon-like peptide 1 receptor agonists (GLP-1 RAs) that are approved by the US Food and Drug Administration as subcutaneous injections for patients with type 2 diabetes mellitus (T2DM). Both are recommended by the American Diabetes Association (ADA) as first-line options for patients with concomitant atherosclerotic cardiovascular (CV) disease and exert therapeutic effect via incretin-like mechanisms.1 These agents lower blood glucose levels by stimulating insulin release, increasing the body’s sensitivity to insulin, and inhibiting inappropriate glucagon secretion.2,3 They also slow gastric emptying, resulting in decreased appetite and potential weight loss.4
The SUSTAIN (1-7) trials concluded that semaglutide presented an equivalent safety profile and greater efficacy compared with other GLP-1 RAs, including exenatide and dulaglutide.2 The SUSTAIN-10 open-label, head-to-head trial evaluating 1 mg semaglutide once weekly vs 1.2 mg liraglutide daily concluded that semaglutide was superior in hemoglobin A1c (HbA1c) and body weight reduction compared with liraglutide, with slightly increased gastrointestinal (GI) adverse effects (AEs).5 Similar to the LEADER trial assessing liraglutide, SUSTAIN-6 evaluated semaglutide in patients at increased CV risk and found that compared with placebo, semaglutide decreased rates of serious CV events, such as CV death, myocardial infarction, and stroke and were similar to the CV outcomes in the LEADER trial.2,6 Although initial results of the SUSTAIN-6 trial were thought to be nearly equivalent to the LEADER trial, analyses later published comparing both trials noted that semaglutide had more potent HbA1c lowering and weight loss benefit when compared with liraglutide.2,6 The cardioprotective outcomes of SUSTAIN-6 qualified semaglutide for inclusion in the current ADA Standards of Medical Care recommendations for CV risk reduction.6,7 However, despite the CV safety profile and efficacy associated with semaglutide, the SUSTAIN-6 trial noted an increased risk of diabetic retinopathy (DR) complications in 50 of 1648 patients (3%) treated with semaglutide compared with 29 of 1649 (1.8%) who received placebo (P = .02; hazard ratio, 1.76; 95% CI, 1.11-2.78).6 Of the 79 total patients who experienced retinopathy complications, 66 had retinopathy at baseline (42 of 50 [84%]) in the semaglutide group; 24 of 29 [83%] in the placebo group).6 Worsening of DR became one of the most notable AEs of semaglutide evaluated in clinical trials. This further deemed the effect as a warning in the semaglutide package insert to assist clinicians with treatment decisions.
As part of a US Department of Veterans Affairs (VA) National Lost Opportunity Cost Savings Initiative, which encompasses administrative efforts to promote more cost-effective yet safe and efficacious therapy options for veterans, the Michael E. DeBakey VA Medical Center (MEDVAMC) in Houston, Texas, converted a portion of patients with T2DM established on liraglutide to semaglutide. The 30-day supply cost of the 2-pack liraglutide 6 mg/mL (3 mL) injection pens for the MEDVAMC was $197.64. The 30-day supply cost for the singular multidose semaglutide 0.5 mg/0.375 mL (1.5 mL) injection pen was $115.15. Cost savings for the MEDVAMC facility were initially estimated to reach $642,522.
The subset of patients converted had to have undergone teleretinal imaging and not have a diagnosis of nonproliferative DR (NPDR), proliferative DR (PDR), or PDR with or without
In the fall of 2021, there was also a standing list of patients on liraglutide who were not converted due to a lack of teleretinal imaging. As a result, there was potential for a quality improvement (QI) intervention to target this patient population, which could result in further cost savings for MEDVAMC and improved glycemic control because of increased conversion from liraglutide to semaglutide. The purpose of this project was to perform a QI assessment on this subset of patients both initially converted from liraglutide to semaglutide, and those who were yet to be converted due to a lack of teleretinal imaging to determine the impact on glycemic control and cost savings.
Methods
This QI project was a single-center, prospective cohort study with a retrospective chart review of veterans with T2DM converted from liraglutide to semaglutide at the MEDVAMC. Patient data were collected from the Computerized Patient Record System (CPRS) between March 1, 2021, and November 30, 2021. An initial subset of patients was converted to semaglutide in March and April 2021. Patients initially excluded underwent a second chart review to determine whether they truly met exclusion criteria. Patients who did not have a definitive diagnosis of NPDR or PDR, those due for updated teleretinal imaging, as well as those with updated teleretinal imaging that excluded NPDR or PDR were targeted for clinician education interventions.
Following this intervention, a subset of patients with negative DR findings were converted from liraglutide to semaglutide. Primary care and endocrinology clinicians were notified that patients who met the criteria should be referred for teleretinal imaging if no updated results were present or that patients were eligible for semaglutide conversion based on negative findings. Both patients who were initially converted as well as those converted following education were included for data collection/analysis of glycemic control via HbA1c and blood glucose levels.
Cost savings were evaluated using outpatient pharmacy procurement pricing data. This project was approved by the MEDVAMC Quality Assurance and Regulatory Affairs Office.
Participants
Patients included in the study were adults aged ≥ 18 years with T2DM, converted from liraglutide 0.6 and 1.2 mg daily to semaglutide 0.25 mg weekly (titrated to 0.5 mg weekly after 4 weeks), and had an active prescription for semaglutide, with or without insulin or other oral antihyperglycemics. Patients with NPDR or PDR, type 1 DM, no HbA1c data, no filled semaglutide prescriptions, insulin pumps, and those without teleretinal imaging within the postintervention period or who died during the study period were excluded.
Patient baseline characteristics collected included demographic data, CV comorbidities, antihyperglycemic medications, and changes in insulin doses. Parameters analyzed at baseline and 3 to 12 months postconversion included body weight, HbA1c, and blood glucose levels.
Outcomes
The primary objectives of this QI project were to assess glycemic control (via changes in HbA1c levels) and cost savings following patient conversion from liraglutide to semaglutide. A second objective was to educate clinicians for referral of T2DM patients without teleretinal imaging in the past 2 years.
The purpose of the latter objective was to encourage conversion from liraglutide to semaglutide in the absence of DR. We predicted that 50% of patients with clinician education would be converted. Secondary objectives included assessing body weight differences, evaluating modifications in diabetes regimen, and documenting AEs. We predicted that glycemic control would either remain stable or improve with conversion to semaglutide.
Statistical Analysis
Patient demographic data were analyzed using descriptive statistics. Quantitative data (HbA1c, blood glucose, and body weight differences as continuous variables) were analyzed using a paired Student t test, and categorical variables were analyzed using the χ2 test.
Results
During the study period, 692 patients were identified with active liraglutide prescriptions (Figure). Of these, 49 patients who were initially excluded due to outdated teleretinal imaging or negative findings met the criteria for clinician education, and 14 of those 49 patients (28.6%) were converted from liraglutide to semaglutide. Thirty-three patients (67.3%) did not schedule teleretinal imaging or did not convert to semaglutide following negative teleretinal findings. Two patients (4.1%) either scheduled or proceeded with teleretinal imaging, without any further action from the clinician.
Including the 14 patients converted posteducational intervention, 425 patients were converted to semaglutide. Excluded from analysis were 121 patients: 57 for incomplete HbA1c data or no filled semaglutide prescription; 30 for HbA1c and weight data outside of the study timeframe; 25 died of causes unrelated to the project; 8 had insulin pumps; and 1 was diagnosed with late-onset type 1 DM. The final sample was 304 patients who underwent analysis.
Two hundred seventy-three patients (89.8%) were male, and 180 (59.2%) were White (Table 1). The mean (SD) age of patients was 65.9 (9.6) years, and 236 (77.6%) were established on insulin therapy (either basal, bolus, or a combination). The 3 most common antihyperglycemic agents (other than insulin) that patients used included 185 metformin (60.9%), 104 empagliflozin (34.2%), and 50 glipizide (16.4%) prescriptions.
Most patients had CV disease. Three hundred patients (98.7%) had comorbid hypertension, 298 (98.0%) had hyperlipidemia, and 114 (37.5%) had coronary artery disease (Table 2). Other diseases that patients were concomitantly diagnosed with included peripheral vascular disease, heart failure, history of stroke or transient ischemic attack, and history of myocardial infarction.
Documented AEs included 83 patients (27.3%) with hypoglycemia at any point within 3 to 12 months of conversion and 25 patients (8.2%) with mainly GI-related events, including nausea, vomiting, diarrhea, decreased appetite, and abdominal pain. Six patients (2.0%) had a new diagnosis of DR 3 to 12 months postconversion.
Glycemic Control and Weight Changes
At baseline, mean (SD) HbA1c was 8.1% (1.5), blood glucose was 187.4 (44.2) mg/dL, and body weight was 112.9 (23.0) kg (Table 3). In the timeframe evaluated (3 to 12 months postconversion), patients’ mean (SD) HbA1c was found to have significantly decreased to 7.6% (1.4) (P < .001; 95% CI, -0.7 to -0.3), blood glucose decreased to 172.6 (39.0) mg/dL (P < .001; 95% CI, -19.3 to -10.2), and body weight decreased to 105.2 (32.3) kg (P < .001; 95% CI, -10.6 to -4.8). All parameters evaluated were deemed statistically significant.
Further analyses evaluating specific changes in HbA1c observed postconversion are as follows: 199 patients (65.5%) experienced a decrease, 92 (30.3%) experienced an increase, and 13 (4.3%) experienced no change in their HbA1c.
As the timeframe was fairly broad to assess HbA1c changes, a prespecified subgroup analysis was conducted to determine specific changes in HbA1c within 3 to 6, 6 to 9, and 9 to 12 months postconversion (Table 4). At 3 to 6 months postconversion, patient mean (SD) HbA1c levels significantly decreased from 8.2% (1.5) at baseline to 7.6% (1.3) postconversion (P = .002; 95% CI, -1.0 to -0.2). At 6 to 9 months postconversion, the mean (SD) HbA1c significantly decreased from 8.1% (1.5) at baseline to 7.6% (1.4) postconversion (P = .002; 95% CI, -0.8 to -0.2).
Glucose-Lowering Agent Adjustments
One hundred thirteen patients (37.2%) required no changes to their antihyperglycemic regimen with the conversion, 85 (28.0%) required increased insulin doses, and 77 (25.3%) required decreased insulin doses (Table 5). Forty-five (14.8%) patients underwent discontinuation of either insulin or other antihyperglycemic agents; 44 (14.5%) had other antihyperglycemic agents dose increased, 39 (12.8%) required adding other glucose-lowering agents, 28 (9.2%) discontinued semaglutide, and 10 (3.3%) had other glucose-lowering medication doses decreased.
Cost Savings
Cost savings were evaluated using the MEDVAMC outpatient pharmacy procurement service. The total cost savings per patient per month was $82.49. For the 411 preclinician education patients converted to semaglutide, this resulted in a prospective annual cost savings of $406,840.68. An additional $13,858.32 was saved due to the intervention/clinician education for 14 patients converted to semaglutide. The total annual cost savings was $420,699.00.
Discussion
Overall, glycemic control significantly improved with veterans’ conversion from liraglutide to semaglutide. Not only were significant changes noted with HbA1c levels and weight, but consistencies were noted with mean HbA1c decrease and weight loss expected of GLP-1 RAs noted in clinical trials. The typical range for HbA1c changes expected is -1% to -2% and weight loss of 1 to 6 kg.4,7 Data from the LEAD-5 and SUSTAIN-4 trials, evaluating glycemic control in liraglutide and semaglutide, respectively, have noted comparable yet slightly more potent HbA1c decreases (-1.33% for liraglutide 1.8 mg daily vs -1.2% and -1.6% for semaglutide 0.5 mg and 1 mg weekly, respectively).8,9 However, more robust weight loss has been noted with semaglutide vs liraglutide (-4.62 kg for semaglutide 0.5 mg weekly and -6.33 kg for semaglutide 1 mg weekly vs -3.43 kg for liraglutide 1.8 mg daily).8,9 Results from the SUSTAIN-10 trial also noted mean changes in HbA1c of -1.7% for semaglutide 1 mg weekly vs -1.0% for liraglutide 1.2 mg daily; mean body weight differences were -5.8 kg for semaglutide and -1.9 kg for liraglutide at their respective doses.5 The mean weight loss noted with this QI project is consistent with prior trials of semaglutide.
Of note, 44 patients (14.5%) required the dosage increase of either one or multiple additional glucose-lowering agents at any time point within the 3- to 12-month period. Of those patients, 38 (86.4%) underwent further semaglutide dose titration to 1 mg weekly. Common reasons for a further dose increase to 1 mg weekly were an indication for more robust HbA1c lowering, a desire to decrease patients’ either basal or bolus insulin requirements, or a treatment goal of completely titrating patients off insulin.
It is uncertain why 30.3% of patients experienced an increase in HbA1c and 4.3% experienced no change. However, possibilities for the divergence in HbA1c outcomes in these subsets of patients may include suboptimal adherence to semaglutide or other antihyperglycemic agents as indicated by clinicians or nonadherence to dietary and lifestyle modifications.
Most patients (65.5%) experienced a decrease in HbA1c because of conversion to semaglutide, and
At the MEDVAMC, liraglutide is a nonformulary agent and semaglutide is now the formulary-preferred option. For patients with uncontrolled T2DM, if a GLP-1 RA is desired for therapy, clinicians are to place a prior authorization drug request (PADR) consultation for semaglutide for further evaluation and review of VA Criteria for Use (CFU) by clinical pharmacist practitioners. Liraglutide is the alternative option if patients do not meet the CFU for semaglutide (ie, have a diagnosis of DR among other exclusions). However, the semaglutide CFU was updated in April 2022 to exclude those specifically diagnosed with PDR, severe NPDR, and macular edema unless an ophthalmologist deems semaglutide acceptable. This indicates that patients with mild-to-moderate NPDR (who were originally excluded from this QI project) are now eligible to receive semaglutide. The incidence of new DR diagnoses (2%) observed in this study could indicate an unclear relationship between semaglutide and increased rates of DR; however, no definitive correlation can be established due to the retrospective nature of this project. The implications of the results of this QI project in relation to the updated CFU remain undetermined.
Due to the comparable improvements in HbA1c and more robust weight loss noted with semaglutide vs liraglutide, we deem it appropriate to select semaglutide as the more cost-efficient GLP-1 RA and formulary preferred option. The data of this QI project supports the overall safety and treatment utility of this option. Although significant cost savings were achieved (> $400,000), the long-term benefit of the liraglutide to semaglutide conversion remains unknown.
Strengths and Limitations
Strengths of this project include the large sample size, its setting in a large VA medical center, and the evaluation of multiple outcomes beyond HbA1c for assessment of glycemic control (ie, mean blood glucose, insulin titration, and dose adjustment of other glucose-lowering agents).
Limitations of this study include the retrospective chart review used for data collection, limited accuracy of objective data due to the COVID-19 pandemic, and inconsistencies with documentation in patients’ electronic health records. As a protective measure in the height of the pandemic between March 2021 and November 2021, the VA promoted using telephone and virtual-visit clinics to minimize exposure for patients with nonurgent follow-up needs. Patient hesitance to present to the clinic in person due to COVID-19 was also a significant factor in obtaining objective follow-up data. As a result, less accurate and timely baseline and postconversion weight and HbA1c data resulted, leading to our decision to extend the timeframe evaluated postconversion to 3 to 12 months. We also noted inconsistencies with documentation in CPRS. Unless veterans were closely followed by clinical pharmacist practitioners or endocrine consultation service clinicians, it was more difficult to follow and document trends of insulin titration to assess the impact of semaglutide conversion. The number of AEs, including hypoglycemia and GI intolerance, were also not consistently documented within the CPRS, and the frequency of AEs may be underestimated.
Another possible limitation regarding the interpretation of the results includes the portion of patients titrated up to semaglutide 1 mg weekly. As the focal point of this project was to review changes in glycemic control in the conversion to semaglutide 0.5 mg, this population of patients converted to 1 mg could potentially overestimate the HbA1c and weight changes described, as it is consistent with the SUSTAIN trials that show more robust decreases in those parameters described earlier.
Conclusions
A subset of patients with T2DM converted from liraglutide to semaglutide experienced significant changes in glycemic control and body weight. Significant differences were noted for a decreased HbA1c, decreased mean blood glucose, and weight loss. A fair portion of patients’ antihyperglycemic regimens required no changes on conversion to semaglutide. Although the semaglutide discontinuation rate neared 10%, AEs that may have contributed to this discontinuation rate included hypoglycemia and GI intolerance. Clinician education resulted in a substantial number of patients undergoing teleretinal imaging and further conversion to semaglutide; however, due to the low conversion response rate, a more effective method of educating clinicians is warranted. Although the semaglutide cost savings initiative at MEDVAMC resulted in significant savings, a full cost-effective analysis is needed to assess more comprehensive institution savings.
1. ElSayed NA, Aleppo G, Aroda VR, et al. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46(suppl 1):S140-S157. doi:10.2337/dc23-S009
2. Aroda VR, Ahmann A, Cariou B, et al. Comparative efficacy, safety, and cardiovascular outcome with once-weekly subcutaneous semaglutide in the treatment of type 2 diabetes: insights from the SUSTAIN 1-7 trials. Diabetes Metab. 2019;45(5):409-418. doi:10.1016/j.diabet.2018.12.001
3. Trujillo JM, Nuffer W, Smith BA. GLP-1 receptor agonists: an updated review of head-to-head clinical studies. Ther Adv Endocrinol Metab. 2021;12:2042018821997320. Published 2021 Mar 9. doi:10.1177/2042018821997320
4. Drucker DJ. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. 2018;27(4):740-756. doi:10.1016/j.cmet.2018.03.001
5. Capehorn MS, Catarig AM, Furberg JK, et al. Efficacy and safety of once-weekly semaglutide 1.0mg vs once-daily liraglutide 1.2mg as add-on to 1-3 oral antidiabetic drugs in subjects with type 2 diabetes (SUSTAIN 10). Diabetes Metab. 2020;46(2):100-109. doi:10.1016/j.diabet.2019.101117
6. Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834-1844. doi:10.1056/NEJMoa1607141
7. ElSayed NA, Aleppo G, Aroda VR, et al. 10. Cardiovascular Disease and Risk Management: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46(suppl 1):S158-S190. doi:10.2337/dc23-S010
8. Russell-Jones D, Vaag A, Schmitz O, et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia. 2009;52(10):2046-2055. doi:10.1007/s00125-009-1472-y
9. Aroda VR, Bain SC, Cariou B, et al. Efficacy and safety of once-weekly semaglutide versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulin-naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open-label, parallel-group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol. 2017;5(5):355-366. doi:10.1016/S2213-8587(17)30085-2
Semaglutide and liraglutide are glucagon-like peptide 1 receptor agonists (GLP-1 RAs) that are approved by the US Food and Drug Administration as subcutaneous injections for patients with type 2 diabetes mellitus (T2DM). Both are recommended by the American Diabetes Association (ADA) as first-line options for patients with concomitant atherosclerotic cardiovascular (CV) disease and exert therapeutic effect via incretin-like mechanisms.1 These agents lower blood glucose levels by stimulating insulin release, increasing the body’s sensitivity to insulin, and inhibiting inappropriate glucagon secretion.2,3 They also slow gastric emptying, resulting in decreased appetite and potential weight loss.4
The SUSTAIN (1-7) trials concluded that semaglutide presented an equivalent safety profile and greater efficacy compared with other GLP-1 RAs, including exenatide and dulaglutide.2 The SUSTAIN-10 open-label, head-to-head trial evaluating 1 mg semaglutide once weekly vs 1.2 mg liraglutide daily concluded that semaglutide was superior in hemoglobin A1c (HbA1c) and body weight reduction compared with liraglutide, with slightly increased gastrointestinal (GI) adverse effects (AEs).5 Similar to the LEADER trial assessing liraglutide, SUSTAIN-6 evaluated semaglutide in patients at increased CV risk and found that compared with placebo, semaglutide decreased rates of serious CV events, such as CV death, myocardial infarction, and stroke and were similar to the CV outcomes in the LEADER trial.2,6 Although initial results of the SUSTAIN-6 trial were thought to be nearly equivalent to the LEADER trial, analyses later published comparing both trials noted that semaglutide had more potent HbA1c lowering and weight loss benefit when compared with liraglutide.2,6 The cardioprotective outcomes of SUSTAIN-6 qualified semaglutide for inclusion in the current ADA Standards of Medical Care recommendations for CV risk reduction.6,7 However, despite the CV safety profile and efficacy associated with semaglutide, the SUSTAIN-6 trial noted an increased risk of diabetic retinopathy (DR) complications in 50 of 1648 patients (3%) treated with semaglutide compared with 29 of 1649 (1.8%) who received placebo (P = .02; hazard ratio, 1.76; 95% CI, 1.11-2.78).6 Of the 79 total patients who experienced retinopathy complications, 66 had retinopathy at baseline (42 of 50 [84%]) in the semaglutide group; 24 of 29 [83%] in the placebo group).6 Worsening of DR became one of the most notable AEs of semaglutide evaluated in clinical trials. This further deemed the effect as a warning in the semaglutide package insert to assist clinicians with treatment decisions.
As part of a US Department of Veterans Affairs (VA) National Lost Opportunity Cost Savings Initiative, which encompasses administrative efforts to promote more cost-effective yet safe and efficacious therapy options for veterans, the Michael E. DeBakey VA Medical Center (MEDVAMC) in Houston, Texas, converted a portion of patients with T2DM established on liraglutide to semaglutide. The 30-day supply cost of the 2-pack liraglutide 6 mg/mL (3 mL) injection pens for the MEDVAMC was $197.64. The 30-day supply cost for the singular multidose semaglutide 0.5 mg/0.375 mL (1.5 mL) injection pen was $115.15. Cost savings for the MEDVAMC facility were initially estimated to reach $642,522.
The subset of patients converted had to have undergone teleretinal imaging and not have a diagnosis of nonproliferative DR (NPDR), proliferative DR (PDR), or PDR with or without
In the fall of 2021, there was also a standing list of patients on liraglutide who were not converted due to a lack of teleretinal imaging. As a result, there was potential for a quality improvement (QI) intervention to target this patient population, which could result in further cost savings for MEDVAMC and improved glycemic control because of increased conversion from liraglutide to semaglutide. The purpose of this project was to perform a QI assessment on this subset of patients both initially converted from liraglutide to semaglutide, and those who were yet to be converted due to a lack of teleretinal imaging to determine the impact on glycemic control and cost savings.
Methods
This QI project was a single-center, prospective cohort study with a retrospective chart review of veterans with T2DM converted from liraglutide to semaglutide at the MEDVAMC. Patient data were collected from the Computerized Patient Record System (CPRS) between March 1, 2021, and November 30, 2021. An initial subset of patients was converted to semaglutide in March and April 2021. Patients initially excluded underwent a second chart review to determine whether they truly met exclusion criteria. Patients who did not have a definitive diagnosis of NPDR or PDR, those due for updated teleretinal imaging, as well as those with updated teleretinal imaging that excluded NPDR or PDR were targeted for clinician education interventions.
Following this intervention, a subset of patients with negative DR findings were converted from liraglutide to semaglutide. Primary care and endocrinology clinicians were notified that patients who met the criteria should be referred for teleretinal imaging if no updated results were present or that patients were eligible for semaglutide conversion based on negative findings. Both patients who were initially converted as well as those converted following education were included for data collection/analysis of glycemic control via HbA1c and blood glucose levels.
Cost savings were evaluated using outpatient pharmacy procurement pricing data. This project was approved by the MEDVAMC Quality Assurance and Regulatory Affairs Office.
Participants
Patients included in the study were adults aged ≥ 18 years with T2DM, converted from liraglutide 0.6 and 1.2 mg daily to semaglutide 0.25 mg weekly (titrated to 0.5 mg weekly after 4 weeks), and had an active prescription for semaglutide, with or without insulin or other oral antihyperglycemics. Patients with NPDR or PDR, type 1 DM, no HbA1c data, no filled semaglutide prescriptions, insulin pumps, and those without teleretinal imaging within the postintervention period or who died during the study period were excluded.
Patient baseline characteristics collected included demographic data, CV comorbidities, antihyperglycemic medications, and changes in insulin doses. Parameters analyzed at baseline and 3 to 12 months postconversion included body weight, HbA1c, and blood glucose levels.
Outcomes
The primary objectives of this QI project were to assess glycemic control (via changes in HbA1c levels) and cost savings following patient conversion from liraglutide to semaglutide. A second objective was to educate clinicians for referral of T2DM patients without teleretinal imaging in the past 2 years.
The purpose of the latter objective was to encourage conversion from liraglutide to semaglutide in the absence of DR. We predicted that 50% of patients with clinician education would be converted. Secondary objectives included assessing body weight differences, evaluating modifications in diabetes regimen, and documenting AEs. We predicted that glycemic control would either remain stable or improve with conversion to semaglutide.
Statistical Analysis
Patient demographic data were analyzed using descriptive statistics. Quantitative data (HbA1c, blood glucose, and body weight differences as continuous variables) were analyzed using a paired Student t test, and categorical variables were analyzed using the χ2 test.
Results
During the study period, 692 patients were identified with active liraglutide prescriptions (Figure). Of these, 49 patients who were initially excluded due to outdated teleretinal imaging or negative findings met the criteria for clinician education, and 14 of those 49 patients (28.6%) were converted from liraglutide to semaglutide. Thirty-three patients (67.3%) did not schedule teleretinal imaging or did not convert to semaglutide following negative teleretinal findings. Two patients (4.1%) either scheduled or proceeded with teleretinal imaging, without any further action from the clinician.
Including the 14 patients converted posteducational intervention, 425 patients were converted to semaglutide. Excluded from analysis were 121 patients: 57 for incomplete HbA1c data or no filled semaglutide prescription; 30 for HbA1c and weight data outside of the study timeframe; 25 died of causes unrelated to the project; 8 had insulin pumps; and 1 was diagnosed with late-onset type 1 DM. The final sample was 304 patients who underwent analysis.
Two hundred seventy-three patients (89.8%) were male, and 180 (59.2%) were White (Table 1). The mean (SD) age of patients was 65.9 (9.6) years, and 236 (77.6%) were established on insulin therapy (either basal, bolus, or a combination). The 3 most common antihyperglycemic agents (other than insulin) that patients used included 185 metformin (60.9%), 104 empagliflozin (34.2%), and 50 glipizide (16.4%) prescriptions.
Most patients had CV disease. Three hundred patients (98.7%) had comorbid hypertension, 298 (98.0%) had hyperlipidemia, and 114 (37.5%) had coronary artery disease (Table 2). Other diseases that patients were concomitantly diagnosed with included peripheral vascular disease, heart failure, history of stroke or transient ischemic attack, and history of myocardial infarction.
Documented AEs included 83 patients (27.3%) with hypoglycemia at any point within 3 to 12 months of conversion and 25 patients (8.2%) with mainly GI-related events, including nausea, vomiting, diarrhea, decreased appetite, and abdominal pain. Six patients (2.0%) had a new diagnosis of DR 3 to 12 months postconversion.
Glycemic Control and Weight Changes
At baseline, mean (SD) HbA1c was 8.1% (1.5), blood glucose was 187.4 (44.2) mg/dL, and body weight was 112.9 (23.0) kg (Table 3). In the timeframe evaluated (3 to 12 months postconversion), patients’ mean (SD) HbA1c was found to have significantly decreased to 7.6% (1.4) (P < .001; 95% CI, -0.7 to -0.3), blood glucose decreased to 172.6 (39.0) mg/dL (P < .001; 95% CI, -19.3 to -10.2), and body weight decreased to 105.2 (32.3) kg (P < .001; 95% CI, -10.6 to -4.8). All parameters evaluated were deemed statistically significant.
Further analyses evaluating specific changes in HbA1c observed postconversion are as follows: 199 patients (65.5%) experienced a decrease, 92 (30.3%) experienced an increase, and 13 (4.3%) experienced no change in their HbA1c.
As the timeframe was fairly broad to assess HbA1c changes, a prespecified subgroup analysis was conducted to determine specific changes in HbA1c within 3 to 6, 6 to 9, and 9 to 12 months postconversion (Table 4). At 3 to 6 months postconversion, patient mean (SD) HbA1c levels significantly decreased from 8.2% (1.5) at baseline to 7.6% (1.3) postconversion (P = .002; 95% CI, -1.0 to -0.2). At 6 to 9 months postconversion, the mean (SD) HbA1c significantly decreased from 8.1% (1.5) at baseline to 7.6% (1.4) postconversion (P = .002; 95% CI, -0.8 to -0.2).
Glucose-Lowering Agent Adjustments
One hundred thirteen patients (37.2%) required no changes to their antihyperglycemic regimen with the conversion, 85 (28.0%) required increased insulin doses, and 77 (25.3%) required decreased insulin doses (Table 5). Forty-five (14.8%) patients underwent discontinuation of either insulin or other antihyperglycemic agents; 44 (14.5%) had other antihyperglycemic agents dose increased, 39 (12.8%) required adding other glucose-lowering agents, 28 (9.2%) discontinued semaglutide, and 10 (3.3%) had other glucose-lowering medication doses decreased.
Cost Savings
Cost savings were evaluated using the MEDVAMC outpatient pharmacy procurement service. The total cost savings per patient per month was $82.49. For the 411 preclinician education patients converted to semaglutide, this resulted in a prospective annual cost savings of $406,840.68. An additional $13,858.32 was saved due to the intervention/clinician education for 14 patients converted to semaglutide. The total annual cost savings was $420,699.00.
Discussion
Overall, glycemic control significantly improved with veterans’ conversion from liraglutide to semaglutide. Not only were significant changes noted with HbA1c levels and weight, but consistencies were noted with mean HbA1c decrease and weight loss expected of GLP-1 RAs noted in clinical trials. The typical range for HbA1c changes expected is -1% to -2% and weight loss of 1 to 6 kg.4,7 Data from the LEAD-5 and SUSTAIN-4 trials, evaluating glycemic control in liraglutide and semaglutide, respectively, have noted comparable yet slightly more potent HbA1c decreases (-1.33% for liraglutide 1.8 mg daily vs -1.2% and -1.6% for semaglutide 0.5 mg and 1 mg weekly, respectively).8,9 However, more robust weight loss has been noted with semaglutide vs liraglutide (-4.62 kg for semaglutide 0.5 mg weekly and -6.33 kg for semaglutide 1 mg weekly vs -3.43 kg for liraglutide 1.8 mg daily).8,9 Results from the SUSTAIN-10 trial also noted mean changes in HbA1c of -1.7% for semaglutide 1 mg weekly vs -1.0% for liraglutide 1.2 mg daily; mean body weight differences were -5.8 kg for semaglutide and -1.9 kg for liraglutide at their respective doses.5 The mean weight loss noted with this QI project is consistent with prior trials of semaglutide.
Of note, 44 patients (14.5%) required the dosage increase of either one or multiple additional glucose-lowering agents at any time point within the 3- to 12-month period. Of those patients, 38 (86.4%) underwent further semaglutide dose titration to 1 mg weekly. Common reasons for a further dose increase to 1 mg weekly were an indication for more robust HbA1c lowering, a desire to decrease patients’ either basal or bolus insulin requirements, or a treatment goal of completely titrating patients off insulin.
It is uncertain why 30.3% of patients experienced an increase in HbA1c and 4.3% experienced no change. However, possibilities for the divergence in HbA1c outcomes in these subsets of patients may include suboptimal adherence to semaglutide or other antihyperglycemic agents as indicated by clinicians or nonadherence to dietary and lifestyle modifications.
Most patients (65.5%) experienced a decrease in HbA1c because of conversion to semaglutide, and
At the MEDVAMC, liraglutide is a nonformulary agent and semaglutide is now the formulary-preferred option. For patients with uncontrolled T2DM, if a GLP-1 RA is desired for therapy, clinicians are to place a prior authorization drug request (PADR) consultation for semaglutide for further evaluation and review of VA Criteria for Use (CFU) by clinical pharmacist practitioners. Liraglutide is the alternative option if patients do not meet the CFU for semaglutide (ie, have a diagnosis of DR among other exclusions). However, the semaglutide CFU was updated in April 2022 to exclude those specifically diagnosed with PDR, severe NPDR, and macular edema unless an ophthalmologist deems semaglutide acceptable. This indicates that patients with mild-to-moderate NPDR (who were originally excluded from this QI project) are now eligible to receive semaglutide. The incidence of new DR diagnoses (2%) observed in this study could indicate an unclear relationship between semaglutide and increased rates of DR; however, no definitive correlation can be established due to the retrospective nature of this project. The implications of the results of this QI project in relation to the updated CFU remain undetermined.
Due to the comparable improvements in HbA1c and more robust weight loss noted with semaglutide vs liraglutide, we deem it appropriate to select semaglutide as the more cost-efficient GLP-1 RA and formulary preferred option. The data of this QI project supports the overall safety and treatment utility of this option. Although significant cost savings were achieved (> $400,000), the long-term benefit of the liraglutide to semaglutide conversion remains unknown.
Strengths and Limitations
Strengths of this project include the large sample size, its setting in a large VA medical center, and the evaluation of multiple outcomes beyond HbA1c for assessment of glycemic control (ie, mean blood glucose, insulin titration, and dose adjustment of other glucose-lowering agents).
Limitations of this study include the retrospective chart review used for data collection, limited accuracy of objective data due to the COVID-19 pandemic, and inconsistencies with documentation in patients’ electronic health records. As a protective measure in the height of the pandemic between March 2021 and November 2021, the VA promoted using telephone and virtual-visit clinics to minimize exposure for patients with nonurgent follow-up needs. Patient hesitance to present to the clinic in person due to COVID-19 was also a significant factor in obtaining objective follow-up data. As a result, less accurate and timely baseline and postconversion weight and HbA1c data resulted, leading to our decision to extend the timeframe evaluated postconversion to 3 to 12 months. We also noted inconsistencies with documentation in CPRS. Unless veterans were closely followed by clinical pharmacist practitioners or endocrine consultation service clinicians, it was more difficult to follow and document trends of insulin titration to assess the impact of semaglutide conversion. The number of AEs, including hypoglycemia and GI intolerance, were also not consistently documented within the CPRS, and the frequency of AEs may be underestimated.
Another possible limitation regarding the interpretation of the results includes the portion of patients titrated up to semaglutide 1 mg weekly. As the focal point of this project was to review changes in glycemic control in the conversion to semaglutide 0.5 mg, this population of patients converted to 1 mg could potentially overestimate the HbA1c and weight changes described, as it is consistent with the SUSTAIN trials that show more robust decreases in those parameters described earlier.
Conclusions
A subset of patients with T2DM converted from liraglutide to semaglutide experienced significant changes in glycemic control and body weight. Significant differences were noted for a decreased HbA1c, decreased mean blood glucose, and weight loss. A fair portion of patients’ antihyperglycemic regimens required no changes on conversion to semaglutide. Although the semaglutide discontinuation rate neared 10%, AEs that may have contributed to this discontinuation rate included hypoglycemia and GI intolerance. Clinician education resulted in a substantial number of patients undergoing teleretinal imaging and further conversion to semaglutide; however, due to the low conversion response rate, a more effective method of educating clinicians is warranted. Although the semaglutide cost savings initiative at MEDVAMC resulted in significant savings, a full cost-effective analysis is needed to assess more comprehensive institution savings.
Semaglutide and liraglutide are glucagon-like peptide 1 receptor agonists (GLP-1 RAs) that are approved by the US Food and Drug Administration as subcutaneous injections for patients with type 2 diabetes mellitus (T2DM). Both are recommended by the American Diabetes Association (ADA) as first-line options for patients with concomitant atherosclerotic cardiovascular (CV) disease and exert therapeutic effect via incretin-like mechanisms.1 These agents lower blood glucose levels by stimulating insulin release, increasing the body’s sensitivity to insulin, and inhibiting inappropriate glucagon secretion.2,3 They also slow gastric emptying, resulting in decreased appetite and potential weight loss.4
The SUSTAIN (1-7) trials concluded that semaglutide presented an equivalent safety profile and greater efficacy compared with other GLP-1 RAs, including exenatide and dulaglutide.2 The SUSTAIN-10 open-label, head-to-head trial evaluating 1 mg semaglutide once weekly vs 1.2 mg liraglutide daily concluded that semaglutide was superior in hemoglobin A1c (HbA1c) and body weight reduction compared with liraglutide, with slightly increased gastrointestinal (GI) adverse effects (AEs).5 Similar to the LEADER trial assessing liraglutide, SUSTAIN-6 evaluated semaglutide in patients at increased CV risk and found that compared with placebo, semaglutide decreased rates of serious CV events, such as CV death, myocardial infarction, and stroke and were similar to the CV outcomes in the LEADER trial.2,6 Although initial results of the SUSTAIN-6 trial were thought to be nearly equivalent to the LEADER trial, analyses later published comparing both trials noted that semaglutide had more potent HbA1c lowering and weight loss benefit when compared with liraglutide.2,6 The cardioprotective outcomes of SUSTAIN-6 qualified semaglutide for inclusion in the current ADA Standards of Medical Care recommendations for CV risk reduction.6,7 However, despite the CV safety profile and efficacy associated with semaglutide, the SUSTAIN-6 trial noted an increased risk of diabetic retinopathy (DR) complications in 50 of 1648 patients (3%) treated with semaglutide compared with 29 of 1649 (1.8%) who received placebo (P = .02; hazard ratio, 1.76; 95% CI, 1.11-2.78).6 Of the 79 total patients who experienced retinopathy complications, 66 had retinopathy at baseline (42 of 50 [84%]) in the semaglutide group; 24 of 29 [83%] in the placebo group).6 Worsening of DR became one of the most notable AEs of semaglutide evaluated in clinical trials. This further deemed the effect as a warning in the semaglutide package insert to assist clinicians with treatment decisions.
As part of a US Department of Veterans Affairs (VA) National Lost Opportunity Cost Savings Initiative, which encompasses administrative efforts to promote more cost-effective yet safe and efficacious therapy options for veterans, the Michael E. DeBakey VA Medical Center (MEDVAMC) in Houston, Texas, converted a portion of patients with T2DM established on liraglutide to semaglutide. The 30-day supply cost of the 2-pack liraglutide 6 mg/mL (3 mL) injection pens for the MEDVAMC was $197.64. The 30-day supply cost for the singular multidose semaglutide 0.5 mg/0.375 mL (1.5 mL) injection pen was $115.15. Cost savings for the MEDVAMC facility were initially estimated to reach $642,522.
The subset of patients converted had to have undergone teleretinal imaging and not have a diagnosis of nonproliferative DR (NPDR), proliferative DR (PDR), or PDR with or without
In the fall of 2021, there was also a standing list of patients on liraglutide who were not converted due to a lack of teleretinal imaging. As a result, there was potential for a quality improvement (QI) intervention to target this patient population, which could result in further cost savings for MEDVAMC and improved glycemic control because of increased conversion from liraglutide to semaglutide. The purpose of this project was to perform a QI assessment on this subset of patients both initially converted from liraglutide to semaglutide, and those who were yet to be converted due to a lack of teleretinal imaging to determine the impact on glycemic control and cost savings.
Methods
This QI project was a single-center, prospective cohort study with a retrospective chart review of veterans with T2DM converted from liraglutide to semaglutide at the MEDVAMC. Patient data were collected from the Computerized Patient Record System (CPRS) between March 1, 2021, and November 30, 2021. An initial subset of patients was converted to semaglutide in March and April 2021. Patients initially excluded underwent a second chart review to determine whether they truly met exclusion criteria. Patients who did not have a definitive diagnosis of NPDR or PDR, those due for updated teleretinal imaging, as well as those with updated teleretinal imaging that excluded NPDR or PDR were targeted for clinician education interventions.
Following this intervention, a subset of patients with negative DR findings were converted from liraglutide to semaglutide. Primary care and endocrinology clinicians were notified that patients who met the criteria should be referred for teleretinal imaging if no updated results were present or that patients were eligible for semaglutide conversion based on negative findings. Both patients who were initially converted as well as those converted following education were included for data collection/analysis of glycemic control via HbA1c and blood glucose levels.
Cost savings were evaluated using outpatient pharmacy procurement pricing data. This project was approved by the MEDVAMC Quality Assurance and Regulatory Affairs Office.
Participants
Patients included in the study were adults aged ≥ 18 years with T2DM, converted from liraglutide 0.6 and 1.2 mg daily to semaglutide 0.25 mg weekly (titrated to 0.5 mg weekly after 4 weeks), and had an active prescription for semaglutide, with or without insulin or other oral antihyperglycemics. Patients with NPDR or PDR, type 1 DM, no HbA1c data, no filled semaglutide prescriptions, insulin pumps, and those without teleretinal imaging within the postintervention period or who died during the study period were excluded.
Patient baseline characteristics collected included demographic data, CV comorbidities, antihyperglycemic medications, and changes in insulin doses. Parameters analyzed at baseline and 3 to 12 months postconversion included body weight, HbA1c, and blood glucose levels.
Outcomes
The primary objectives of this QI project were to assess glycemic control (via changes in HbA1c levels) and cost savings following patient conversion from liraglutide to semaglutide. A second objective was to educate clinicians for referral of T2DM patients without teleretinal imaging in the past 2 years.
The purpose of the latter objective was to encourage conversion from liraglutide to semaglutide in the absence of DR. We predicted that 50% of patients with clinician education would be converted. Secondary objectives included assessing body weight differences, evaluating modifications in diabetes regimen, and documenting AEs. We predicted that glycemic control would either remain stable or improve with conversion to semaglutide.
Statistical Analysis
Patient demographic data were analyzed using descriptive statistics. Quantitative data (HbA1c, blood glucose, and body weight differences as continuous variables) were analyzed using a paired Student t test, and categorical variables were analyzed using the χ2 test.
Results
During the study period, 692 patients were identified with active liraglutide prescriptions (Figure). Of these, 49 patients who were initially excluded due to outdated teleretinal imaging or negative findings met the criteria for clinician education, and 14 of those 49 patients (28.6%) were converted from liraglutide to semaglutide. Thirty-three patients (67.3%) did not schedule teleretinal imaging or did not convert to semaglutide following negative teleretinal findings. Two patients (4.1%) either scheduled or proceeded with teleretinal imaging, without any further action from the clinician.
Including the 14 patients converted posteducational intervention, 425 patients were converted to semaglutide. Excluded from analysis were 121 patients: 57 for incomplete HbA1c data or no filled semaglutide prescription; 30 for HbA1c and weight data outside of the study timeframe; 25 died of causes unrelated to the project; 8 had insulin pumps; and 1 was diagnosed with late-onset type 1 DM. The final sample was 304 patients who underwent analysis.
Two hundred seventy-three patients (89.8%) were male, and 180 (59.2%) were White (Table 1). The mean (SD) age of patients was 65.9 (9.6) years, and 236 (77.6%) were established on insulin therapy (either basal, bolus, or a combination). The 3 most common antihyperglycemic agents (other than insulin) that patients used included 185 metformin (60.9%), 104 empagliflozin (34.2%), and 50 glipizide (16.4%) prescriptions.
Most patients had CV disease. Three hundred patients (98.7%) had comorbid hypertension, 298 (98.0%) had hyperlipidemia, and 114 (37.5%) had coronary artery disease (Table 2). Other diseases that patients were concomitantly diagnosed with included peripheral vascular disease, heart failure, history of stroke or transient ischemic attack, and history of myocardial infarction.
Documented AEs included 83 patients (27.3%) with hypoglycemia at any point within 3 to 12 months of conversion and 25 patients (8.2%) with mainly GI-related events, including nausea, vomiting, diarrhea, decreased appetite, and abdominal pain. Six patients (2.0%) had a new diagnosis of DR 3 to 12 months postconversion.
Glycemic Control and Weight Changes
At baseline, mean (SD) HbA1c was 8.1% (1.5), blood glucose was 187.4 (44.2) mg/dL, and body weight was 112.9 (23.0) kg (Table 3). In the timeframe evaluated (3 to 12 months postconversion), patients’ mean (SD) HbA1c was found to have significantly decreased to 7.6% (1.4) (P < .001; 95% CI, -0.7 to -0.3), blood glucose decreased to 172.6 (39.0) mg/dL (P < .001; 95% CI, -19.3 to -10.2), and body weight decreased to 105.2 (32.3) kg (P < .001; 95% CI, -10.6 to -4.8). All parameters evaluated were deemed statistically significant.
Further analyses evaluating specific changes in HbA1c observed postconversion are as follows: 199 patients (65.5%) experienced a decrease, 92 (30.3%) experienced an increase, and 13 (4.3%) experienced no change in their HbA1c.
As the timeframe was fairly broad to assess HbA1c changes, a prespecified subgroup analysis was conducted to determine specific changes in HbA1c within 3 to 6, 6 to 9, and 9 to 12 months postconversion (Table 4). At 3 to 6 months postconversion, patient mean (SD) HbA1c levels significantly decreased from 8.2% (1.5) at baseline to 7.6% (1.3) postconversion (P = .002; 95% CI, -1.0 to -0.2). At 6 to 9 months postconversion, the mean (SD) HbA1c significantly decreased from 8.1% (1.5) at baseline to 7.6% (1.4) postconversion (P = .002; 95% CI, -0.8 to -0.2).
Glucose-Lowering Agent Adjustments
One hundred thirteen patients (37.2%) required no changes to their antihyperglycemic regimen with the conversion, 85 (28.0%) required increased insulin doses, and 77 (25.3%) required decreased insulin doses (Table 5). Forty-five (14.8%) patients underwent discontinuation of either insulin or other antihyperglycemic agents; 44 (14.5%) had other antihyperglycemic agents dose increased, 39 (12.8%) required adding other glucose-lowering agents, 28 (9.2%) discontinued semaglutide, and 10 (3.3%) had other glucose-lowering medication doses decreased.
Cost Savings
Cost savings were evaluated using the MEDVAMC outpatient pharmacy procurement service. The total cost savings per patient per month was $82.49. For the 411 preclinician education patients converted to semaglutide, this resulted in a prospective annual cost savings of $406,840.68. An additional $13,858.32 was saved due to the intervention/clinician education for 14 patients converted to semaglutide. The total annual cost savings was $420,699.00.
Discussion
Overall, glycemic control significantly improved with veterans’ conversion from liraglutide to semaglutide. Not only were significant changes noted with HbA1c levels and weight, but consistencies were noted with mean HbA1c decrease and weight loss expected of GLP-1 RAs noted in clinical trials. The typical range for HbA1c changes expected is -1% to -2% and weight loss of 1 to 6 kg.4,7 Data from the LEAD-5 and SUSTAIN-4 trials, evaluating glycemic control in liraglutide and semaglutide, respectively, have noted comparable yet slightly more potent HbA1c decreases (-1.33% for liraglutide 1.8 mg daily vs -1.2% and -1.6% for semaglutide 0.5 mg and 1 mg weekly, respectively).8,9 However, more robust weight loss has been noted with semaglutide vs liraglutide (-4.62 kg for semaglutide 0.5 mg weekly and -6.33 kg for semaglutide 1 mg weekly vs -3.43 kg for liraglutide 1.8 mg daily).8,9 Results from the SUSTAIN-10 trial also noted mean changes in HbA1c of -1.7% for semaglutide 1 mg weekly vs -1.0% for liraglutide 1.2 mg daily; mean body weight differences were -5.8 kg for semaglutide and -1.9 kg for liraglutide at their respective doses.5 The mean weight loss noted with this QI project is consistent with prior trials of semaglutide.
Of note, 44 patients (14.5%) required the dosage increase of either one or multiple additional glucose-lowering agents at any time point within the 3- to 12-month period. Of those patients, 38 (86.4%) underwent further semaglutide dose titration to 1 mg weekly. Common reasons for a further dose increase to 1 mg weekly were an indication for more robust HbA1c lowering, a desire to decrease patients’ either basal or bolus insulin requirements, or a treatment goal of completely titrating patients off insulin.
It is uncertain why 30.3% of patients experienced an increase in HbA1c and 4.3% experienced no change. However, possibilities for the divergence in HbA1c outcomes in these subsets of patients may include suboptimal adherence to semaglutide or other antihyperglycemic agents as indicated by clinicians or nonadherence to dietary and lifestyle modifications.
Most patients (65.5%) experienced a decrease in HbA1c because of conversion to semaglutide, and
At the MEDVAMC, liraglutide is a nonformulary agent and semaglutide is now the formulary-preferred option. For patients with uncontrolled T2DM, if a GLP-1 RA is desired for therapy, clinicians are to place a prior authorization drug request (PADR) consultation for semaglutide for further evaluation and review of VA Criteria for Use (CFU) by clinical pharmacist practitioners. Liraglutide is the alternative option if patients do not meet the CFU for semaglutide (ie, have a diagnosis of DR among other exclusions). However, the semaglutide CFU was updated in April 2022 to exclude those specifically diagnosed with PDR, severe NPDR, and macular edema unless an ophthalmologist deems semaglutide acceptable. This indicates that patients with mild-to-moderate NPDR (who were originally excluded from this QI project) are now eligible to receive semaglutide. The incidence of new DR diagnoses (2%) observed in this study could indicate an unclear relationship between semaglutide and increased rates of DR; however, no definitive correlation can be established due to the retrospective nature of this project. The implications of the results of this QI project in relation to the updated CFU remain undetermined.
Due to the comparable improvements in HbA1c and more robust weight loss noted with semaglutide vs liraglutide, we deem it appropriate to select semaglutide as the more cost-efficient GLP-1 RA and formulary preferred option. The data of this QI project supports the overall safety and treatment utility of this option. Although significant cost savings were achieved (> $400,000), the long-term benefit of the liraglutide to semaglutide conversion remains unknown.
Strengths and Limitations
Strengths of this project include the large sample size, its setting in a large VA medical center, and the evaluation of multiple outcomes beyond HbA1c for assessment of glycemic control (ie, mean blood glucose, insulin titration, and dose adjustment of other glucose-lowering agents).
Limitations of this study include the retrospective chart review used for data collection, limited accuracy of objective data due to the COVID-19 pandemic, and inconsistencies with documentation in patients’ electronic health records. As a protective measure in the height of the pandemic between March 2021 and November 2021, the VA promoted using telephone and virtual-visit clinics to minimize exposure for patients with nonurgent follow-up needs. Patient hesitance to present to the clinic in person due to COVID-19 was also a significant factor in obtaining objective follow-up data. As a result, less accurate and timely baseline and postconversion weight and HbA1c data resulted, leading to our decision to extend the timeframe evaluated postconversion to 3 to 12 months. We also noted inconsistencies with documentation in CPRS. Unless veterans were closely followed by clinical pharmacist practitioners or endocrine consultation service clinicians, it was more difficult to follow and document trends of insulin titration to assess the impact of semaglutide conversion. The number of AEs, including hypoglycemia and GI intolerance, were also not consistently documented within the CPRS, and the frequency of AEs may be underestimated.
Another possible limitation regarding the interpretation of the results includes the portion of patients titrated up to semaglutide 1 mg weekly. As the focal point of this project was to review changes in glycemic control in the conversion to semaglutide 0.5 mg, this population of patients converted to 1 mg could potentially overestimate the HbA1c and weight changes described, as it is consistent with the SUSTAIN trials that show more robust decreases in those parameters described earlier.
Conclusions
A subset of patients with T2DM converted from liraglutide to semaglutide experienced significant changes in glycemic control and body weight. Significant differences were noted for a decreased HbA1c, decreased mean blood glucose, and weight loss. A fair portion of patients’ antihyperglycemic regimens required no changes on conversion to semaglutide. Although the semaglutide discontinuation rate neared 10%, AEs that may have contributed to this discontinuation rate included hypoglycemia and GI intolerance. Clinician education resulted in a substantial number of patients undergoing teleretinal imaging and further conversion to semaglutide; however, due to the low conversion response rate, a more effective method of educating clinicians is warranted. Although the semaglutide cost savings initiative at MEDVAMC resulted in significant savings, a full cost-effective analysis is needed to assess more comprehensive institution savings.
1. ElSayed NA, Aleppo G, Aroda VR, et al. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46(suppl 1):S140-S157. doi:10.2337/dc23-S009
2. Aroda VR, Ahmann A, Cariou B, et al. Comparative efficacy, safety, and cardiovascular outcome with once-weekly subcutaneous semaglutide in the treatment of type 2 diabetes: insights from the SUSTAIN 1-7 trials. Diabetes Metab. 2019;45(5):409-418. doi:10.1016/j.diabet.2018.12.001
3. Trujillo JM, Nuffer W, Smith BA. GLP-1 receptor agonists: an updated review of head-to-head clinical studies. Ther Adv Endocrinol Metab. 2021;12:2042018821997320. Published 2021 Mar 9. doi:10.1177/2042018821997320
4. Drucker DJ. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. 2018;27(4):740-756. doi:10.1016/j.cmet.2018.03.001
5. Capehorn MS, Catarig AM, Furberg JK, et al. Efficacy and safety of once-weekly semaglutide 1.0mg vs once-daily liraglutide 1.2mg as add-on to 1-3 oral antidiabetic drugs in subjects with type 2 diabetes (SUSTAIN 10). Diabetes Metab. 2020;46(2):100-109. doi:10.1016/j.diabet.2019.101117
6. Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834-1844. doi:10.1056/NEJMoa1607141
7. ElSayed NA, Aleppo G, Aroda VR, et al. 10. Cardiovascular Disease and Risk Management: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46(suppl 1):S158-S190. doi:10.2337/dc23-S010
8. Russell-Jones D, Vaag A, Schmitz O, et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia. 2009;52(10):2046-2055. doi:10.1007/s00125-009-1472-y
9. Aroda VR, Bain SC, Cariou B, et al. Efficacy and safety of once-weekly semaglutide versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulin-naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open-label, parallel-group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol. 2017;5(5):355-366. doi:10.1016/S2213-8587(17)30085-2
1. ElSayed NA, Aleppo G, Aroda VR, et al. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46(suppl 1):S140-S157. doi:10.2337/dc23-S009
2. Aroda VR, Ahmann A, Cariou B, et al. Comparative efficacy, safety, and cardiovascular outcome with once-weekly subcutaneous semaglutide in the treatment of type 2 diabetes: insights from the SUSTAIN 1-7 trials. Diabetes Metab. 2019;45(5):409-418. doi:10.1016/j.diabet.2018.12.001
3. Trujillo JM, Nuffer W, Smith BA. GLP-1 receptor agonists: an updated review of head-to-head clinical studies. Ther Adv Endocrinol Metab. 2021;12:2042018821997320. Published 2021 Mar 9. doi:10.1177/2042018821997320
4. Drucker DJ. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. 2018;27(4):740-756. doi:10.1016/j.cmet.2018.03.001
5. Capehorn MS, Catarig AM, Furberg JK, et al. Efficacy and safety of once-weekly semaglutide 1.0mg vs once-daily liraglutide 1.2mg as add-on to 1-3 oral antidiabetic drugs in subjects with type 2 diabetes (SUSTAIN 10). Diabetes Metab. 2020;46(2):100-109. doi:10.1016/j.diabet.2019.101117
6. Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834-1844. doi:10.1056/NEJMoa1607141
7. ElSayed NA, Aleppo G, Aroda VR, et al. 10. Cardiovascular Disease and Risk Management: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46(suppl 1):S158-S190. doi:10.2337/dc23-S010
8. Russell-Jones D, Vaag A, Schmitz O, et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia. 2009;52(10):2046-2055. doi:10.1007/s00125-009-1472-y
9. Aroda VR, Bain SC, Cariou B, et al. Efficacy and safety of once-weekly semaglutide versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulin-naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open-label, parallel-group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol. 2017;5(5):355-366. doi:10.1016/S2213-8587(17)30085-2
Menstruation linked to underdiagnosis of type 2 diabetes?
The analysis estimates that an additional 17% of undiagnosed women younger than 50 years could be reclassified as having T2D, and that women under 50 had an A1c distribution that was markedly lower than that of men under 50, by a mean of 1.6 mmol/mol.
In a study that will be presented at this year’s annual meeting of the European Association for the Study of Diabetes (EASD), the researchers wanted to investigate whether a contributing factor to late diagnosis of T2D in women under 50 may be the difference in A1c levels due to hemoglobin replacement linked to menstrual blood loss.
The study was published online in Diabetes Therapy. “If the threshold for diagnosis of diabetes ... was lowered by 2 mmol/mol in women under the age of 50, an additional 17% of these women (approximately equivalent to 35,000 women in England and Wales) would be diagnosed with diabetes ... which may contribute to up to 64% of the difference in mortality rates between men/women with diabetes mellitus aged 16-50 years,” the researchers noted.
They added that A1c levels in women under 50 years were found to be consistently lower than those in men, and with A1c levels in women reaching the equivalent of those in men up to 10 years later, this “may result in delayed diagnosis of diabetes mellitus in premenopausal women.”
Noting that the study was observational, senior author Adrian Heald, MD, consultant endocrinologist, Salford (England) Royal NHS Foundation Trust, said that it “may be the case that prediabetes and type 2 diabetes in women are not being spotted because the set point needs to be slightly lower, but a systematic study sampling from the population of at-risk individuals is needed further to our findings.
“We also need to refer back to use of the glucose tolerance test, because A1c has been used for the past 15 years but it is not the gold standard,” added Dr. Heald. “Clinicians have often wondered if patients might be missed with A1c measurement, or even overdiagnosed.”
Lucy Chambers, PhD, from Diabetes UK, acknowledged that the research was valuable but added: “More research on sex differences in thresholds for a type 2 diagnosis is needed to inform any changes to clinical practice. In the meantime, we encourage clinicians to follow the current guidance of not ruling out type 2 diabetes based on a one-off A1c below the diagnostic threshold.”
But in support of greater understanding around the sex differences in A1c diagnostic thresholds, Dr. Chambers added: “Receiving an accurate and timely diagnosis ensures that women get the treatment and support needed to manage their type 2 diabetes and avoid long-term complications, including heart disease, where sex-based inequalities in care already contribute to poorer outcomes for women.”
Effect of A1c reference range on T2D diagnosis and associated CVD
Compared with men, women with T2D have poorer glycemic control; a higher risk for cardiovascular (CV) complications; reduced life expectancy (5.3 years shorter vs. 4.5 years shorter); and a higher risk factor burden, such as obesity and hypertension at diagnosis.
In addition, T2D is a stronger risk factor for CV disease (CVD) in women than in men, and those aged 35-59 years who receive a diagnosis have the highest relative CV death risk across all age and sex groups.
The researchers pointed out that previous studies have observed differences in A1c relative to menopause, and they too found that “A1c levels rose after the age of 50 in women.”
However, they noted that the implication of differing A1c reference ranges on delayed diabetes diagnosis with worsening CV risk profile had not been previously recognized and that their study “[h]ighlights for the first time that, while 1.6 mmol/mol may appear only a small difference in terms of laboratory measurement, at population level this has implications for significant number of premenopausal women.”
The researchers initially observed the trend in local data in Salford, in the northwest of England. “These ... data highlighted that women seemed to be diagnosed with type 2 diabetes at an older age, so we wanted to examine what the source of that might be,” study author Mike Stedman, BSc, director, Res Consortium, Andover, England, said in an interview.
Dr. Stedman and his colleagues assessed the sex and age differences of A1c in individuals who had not been diagnosed with diabetes (A1c ≤ 48 mmol/mol [≤ 6.5%]). “We looked at data from other labs [in addition to those in Salford, totaling 938,678 people] to see if this was a local phenomenon. They could only provide more recent data, but these also showed a similar pattern,” he added.
Finally, Dr. Stedman, Dr. Heald, and their colleagues estimated the possible national impact by extrapolating findings based on population data from the UK Office of National Statistics and on National Diabetes Audit data for type 2 diabetes prevalence and related excess mortality. This brought them to the conclusion that T2D would be diagnosed in an additional 17% of women if the threshold were lowered by 2 mmol/mol, to 46 mmol/mol, in women under 50 years.
Lower A1c in women under 50 may delay T2D diagnosis by up to 10 years
The analysis found that the median A1c increased with age, with values in women younger than 50 years consistently being 1 mmol/mol lower than values in men. In contrast, A1c values in women over 50 years were equivalent to those in men.
However, at age 50 years, compared with men, A1c in women was found to lag by approximately 5 years. Women under 50 had an A1c distribution that was lower than that of men by an average of 1.6 mmol/mol (4.7% of mean; P < .0001), whereas this difference in individuals aged 50 years or older was less pronounced (P < .0001).
The authors wrote that “an undermeasurement of approximately 1.6 mmol/mol A1c in women may delay their diabetes ... diagnosis by up to 10 years.”
Further analysis showed that, at an A1c of 48 mmol/mol, 50% fewer women than men under the age of 50 could be diagnosed with T2D, whereas only 20% fewer women than men aged 50 years or older could be diagnosed with T2D.
Lowering the A1c threshold for diagnosis of T2D from 48 mmol/mol to 46 mmol/mol in women under 50 led to an estimate that an additional 35,345 undiagnosed women in England could be reclassified as having a T2D diagnosis.
The authors pointed out that “gender difference in adverse cardiovascular risk factors are known to be present prior to the development of [type 2] diabetes” and that “once diagnosed, atherosclerotic CVD prevalence is twice as high in patients with diabetes ... compared to those without a diagnosis.”
Dr. Heald added that there is always the possibility that other factors might be at play and that the work posed questions rather than presented answers.
Taking a pragmatic view, the researchers suggested that “one alternative approach may be to offer further assessment using fasting plasma glucose or oral glucose tolerance testing in those with A1c values of 46 or 47 mmol/mol.”
“In anyone with an early diagnosis of type 2 diabetes, in addition to dietary modification and especially if there is cardiovascular risk, then one might start them on metformin due to the cardiovascular benefits as well as the sugar-lowering effects,” said Dr. Heald, adding that “we certainly don’t want women missing out on metformin that could have huge benefits in the longer term.”
Dr. Stedman and Dr. Heald declared no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; and no other relationships or activities that could appear to have influenced the submitted work. Dr. Chambers has declared no conflicts.
A version of this article appeared on Medscape.com.
The analysis estimates that an additional 17% of undiagnosed women younger than 50 years could be reclassified as having T2D, and that women under 50 had an A1c distribution that was markedly lower than that of men under 50, by a mean of 1.6 mmol/mol.
In a study that will be presented at this year’s annual meeting of the European Association for the Study of Diabetes (EASD), the researchers wanted to investigate whether a contributing factor to late diagnosis of T2D in women under 50 may be the difference in A1c levels due to hemoglobin replacement linked to menstrual blood loss.
The study was published online in Diabetes Therapy. “If the threshold for diagnosis of diabetes ... was lowered by 2 mmol/mol in women under the age of 50, an additional 17% of these women (approximately equivalent to 35,000 women in England and Wales) would be diagnosed with diabetes ... which may contribute to up to 64% of the difference in mortality rates between men/women with diabetes mellitus aged 16-50 years,” the researchers noted.
They added that A1c levels in women under 50 years were found to be consistently lower than those in men, and with A1c levels in women reaching the equivalent of those in men up to 10 years later, this “may result in delayed diagnosis of diabetes mellitus in premenopausal women.”
Noting that the study was observational, senior author Adrian Heald, MD, consultant endocrinologist, Salford (England) Royal NHS Foundation Trust, said that it “may be the case that prediabetes and type 2 diabetes in women are not being spotted because the set point needs to be slightly lower, but a systematic study sampling from the population of at-risk individuals is needed further to our findings.
“We also need to refer back to use of the glucose tolerance test, because A1c has been used for the past 15 years but it is not the gold standard,” added Dr. Heald. “Clinicians have often wondered if patients might be missed with A1c measurement, or even overdiagnosed.”
Lucy Chambers, PhD, from Diabetes UK, acknowledged that the research was valuable but added: “More research on sex differences in thresholds for a type 2 diagnosis is needed to inform any changes to clinical practice. In the meantime, we encourage clinicians to follow the current guidance of not ruling out type 2 diabetes based on a one-off A1c below the diagnostic threshold.”
But in support of greater understanding around the sex differences in A1c diagnostic thresholds, Dr. Chambers added: “Receiving an accurate and timely diagnosis ensures that women get the treatment and support needed to manage their type 2 diabetes and avoid long-term complications, including heart disease, where sex-based inequalities in care already contribute to poorer outcomes for women.”
Effect of A1c reference range on T2D diagnosis and associated CVD
Compared with men, women with T2D have poorer glycemic control; a higher risk for cardiovascular (CV) complications; reduced life expectancy (5.3 years shorter vs. 4.5 years shorter); and a higher risk factor burden, such as obesity and hypertension at diagnosis.
In addition, T2D is a stronger risk factor for CV disease (CVD) in women than in men, and those aged 35-59 years who receive a diagnosis have the highest relative CV death risk across all age and sex groups.
The researchers pointed out that previous studies have observed differences in A1c relative to menopause, and they too found that “A1c levels rose after the age of 50 in women.”
However, they noted that the implication of differing A1c reference ranges on delayed diabetes diagnosis with worsening CV risk profile had not been previously recognized and that their study “[h]ighlights for the first time that, while 1.6 mmol/mol may appear only a small difference in terms of laboratory measurement, at population level this has implications for significant number of premenopausal women.”
The researchers initially observed the trend in local data in Salford, in the northwest of England. “These ... data highlighted that women seemed to be diagnosed with type 2 diabetes at an older age, so we wanted to examine what the source of that might be,” study author Mike Stedman, BSc, director, Res Consortium, Andover, England, said in an interview.
Dr. Stedman and his colleagues assessed the sex and age differences of A1c in individuals who had not been diagnosed with diabetes (A1c ≤ 48 mmol/mol [≤ 6.5%]). “We looked at data from other labs [in addition to those in Salford, totaling 938,678 people] to see if this was a local phenomenon. They could only provide more recent data, but these also showed a similar pattern,” he added.
Finally, Dr. Stedman, Dr. Heald, and their colleagues estimated the possible national impact by extrapolating findings based on population data from the UK Office of National Statistics and on National Diabetes Audit data for type 2 diabetes prevalence and related excess mortality. This brought them to the conclusion that T2D would be diagnosed in an additional 17% of women if the threshold were lowered by 2 mmol/mol, to 46 mmol/mol, in women under 50 years.
Lower A1c in women under 50 may delay T2D diagnosis by up to 10 years
The analysis found that the median A1c increased with age, with values in women younger than 50 years consistently being 1 mmol/mol lower than values in men. In contrast, A1c values in women over 50 years were equivalent to those in men.
However, at age 50 years, compared with men, A1c in women was found to lag by approximately 5 years. Women under 50 had an A1c distribution that was lower than that of men by an average of 1.6 mmol/mol (4.7% of mean; P < .0001), whereas this difference in individuals aged 50 years or older was less pronounced (P < .0001).
The authors wrote that “an undermeasurement of approximately 1.6 mmol/mol A1c in women may delay their diabetes ... diagnosis by up to 10 years.”
Further analysis showed that, at an A1c of 48 mmol/mol, 50% fewer women than men under the age of 50 could be diagnosed with T2D, whereas only 20% fewer women than men aged 50 years or older could be diagnosed with T2D.
Lowering the A1c threshold for diagnosis of T2D from 48 mmol/mol to 46 mmol/mol in women under 50 led to an estimate that an additional 35,345 undiagnosed women in England could be reclassified as having a T2D diagnosis.
The authors pointed out that “gender difference in adverse cardiovascular risk factors are known to be present prior to the development of [type 2] diabetes” and that “once diagnosed, atherosclerotic CVD prevalence is twice as high in patients with diabetes ... compared to those without a diagnosis.”
Dr. Heald added that there is always the possibility that other factors might be at play and that the work posed questions rather than presented answers.
Taking a pragmatic view, the researchers suggested that “one alternative approach may be to offer further assessment using fasting plasma glucose or oral glucose tolerance testing in those with A1c values of 46 or 47 mmol/mol.”
“In anyone with an early diagnosis of type 2 diabetes, in addition to dietary modification and especially if there is cardiovascular risk, then one might start them on metformin due to the cardiovascular benefits as well as the sugar-lowering effects,” said Dr. Heald, adding that “we certainly don’t want women missing out on metformin that could have huge benefits in the longer term.”
Dr. Stedman and Dr. Heald declared no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; and no other relationships or activities that could appear to have influenced the submitted work. Dr. Chambers has declared no conflicts.
A version of this article appeared on Medscape.com.
The analysis estimates that an additional 17% of undiagnosed women younger than 50 years could be reclassified as having T2D, and that women under 50 had an A1c distribution that was markedly lower than that of men under 50, by a mean of 1.6 mmol/mol.
In a study that will be presented at this year’s annual meeting of the European Association for the Study of Diabetes (EASD), the researchers wanted to investigate whether a contributing factor to late diagnosis of T2D in women under 50 may be the difference in A1c levels due to hemoglobin replacement linked to menstrual blood loss.
The study was published online in Diabetes Therapy. “If the threshold for diagnosis of diabetes ... was lowered by 2 mmol/mol in women under the age of 50, an additional 17% of these women (approximately equivalent to 35,000 women in England and Wales) would be diagnosed with diabetes ... which may contribute to up to 64% of the difference in mortality rates between men/women with diabetes mellitus aged 16-50 years,” the researchers noted.
They added that A1c levels in women under 50 years were found to be consistently lower than those in men, and with A1c levels in women reaching the equivalent of those in men up to 10 years later, this “may result in delayed diagnosis of diabetes mellitus in premenopausal women.”
Noting that the study was observational, senior author Adrian Heald, MD, consultant endocrinologist, Salford (England) Royal NHS Foundation Trust, said that it “may be the case that prediabetes and type 2 diabetes in women are not being spotted because the set point needs to be slightly lower, but a systematic study sampling from the population of at-risk individuals is needed further to our findings.
“We also need to refer back to use of the glucose tolerance test, because A1c has been used for the past 15 years but it is not the gold standard,” added Dr. Heald. “Clinicians have often wondered if patients might be missed with A1c measurement, or even overdiagnosed.”
Lucy Chambers, PhD, from Diabetes UK, acknowledged that the research was valuable but added: “More research on sex differences in thresholds for a type 2 diagnosis is needed to inform any changes to clinical practice. In the meantime, we encourage clinicians to follow the current guidance of not ruling out type 2 diabetes based on a one-off A1c below the diagnostic threshold.”
But in support of greater understanding around the sex differences in A1c diagnostic thresholds, Dr. Chambers added: “Receiving an accurate and timely diagnosis ensures that women get the treatment and support needed to manage their type 2 diabetes and avoid long-term complications, including heart disease, where sex-based inequalities in care already contribute to poorer outcomes for women.”
Effect of A1c reference range on T2D diagnosis and associated CVD
Compared with men, women with T2D have poorer glycemic control; a higher risk for cardiovascular (CV) complications; reduced life expectancy (5.3 years shorter vs. 4.5 years shorter); and a higher risk factor burden, such as obesity and hypertension at diagnosis.
In addition, T2D is a stronger risk factor for CV disease (CVD) in women than in men, and those aged 35-59 years who receive a diagnosis have the highest relative CV death risk across all age and sex groups.
The researchers pointed out that previous studies have observed differences in A1c relative to menopause, and they too found that “A1c levels rose after the age of 50 in women.”
However, they noted that the implication of differing A1c reference ranges on delayed diabetes diagnosis with worsening CV risk profile had not been previously recognized and that their study “[h]ighlights for the first time that, while 1.6 mmol/mol may appear only a small difference in terms of laboratory measurement, at population level this has implications for significant number of premenopausal women.”
The researchers initially observed the trend in local data in Salford, in the northwest of England. “These ... data highlighted that women seemed to be diagnosed with type 2 diabetes at an older age, so we wanted to examine what the source of that might be,” study author Mike Stedman, BSc, director, Res Consortium, Andover, England, said in an interview.
Dr. Stedman and his colleagues assessed the sex and age differences of A1c in individuals who had not been diagnosed with diabetes (A1c ≤ 48 mmol/mol [≤ 6.5%]). “We looked at data from other labs [in addition to those in Salford, totaling 938,678 people] to see if this was a local phenomenon. They could only provide more recent data, but these also showed a similar pattern,” he added.
Finally, Dr. Stedman, Dr. Heald, and their colleagues estimated the possible national impact by extrapolating findings based on population data from the UK Office of National Statistics and on National Diabetes Audit data for type 2 diabetes prevalence and related excess mortality. This brought them to the conclusion that T2D would be diagnosed in an additional 17% of women if the threshold were lowered by 2 mmol/mol, to 46 mmol/mol, in women under 50 years.
Lower A1c in women under 50 may delay T2D diagnosis by up to 10 years
The analysis found that the median A1c increased with age, with values in women younger than 50 years consistently being 1 mmol/mol lower than values in men. In contrast, A1c values in women over 50 years were equivalent to those in men.
However, at age 50 years, compared with men, A1c in women was found to lag by approximately 5 years. Women under 50 had an A1c distribution that was lower than that of men by an average of 1.6 mmol/mol (4.7% of mean; P < .0001), whereas this difference in individuals aged 50 years or older was less pronounced (P < .0001).
The authors wrote that “an undermeasurement of approximately 1.6 mmol/mol A1c in women may delay their diabetes ... diagnosis by up to 10 years.”
Further analysis showed that, at an A1c of 48 mmol/mol, 50% fewer women than men under the age of 50 could be diagnosed with T2D, whereas only 20% fewer women than men aged 50 years or older could be diagnosed with T2D.
Lowering the A1c threshold for diagnosis of T2D from 48 mmol/mol to 46 mmol/mol in women under 50 led to an estimate that an additional 35,345 undiagnosed women in England could be reclassified as having a T2D diagnosis.
The authors pointed out that “gender difference in adverse cardiovascular risk factors are known to be present prior to the development of [type 2] diabetes” and that “once diagnosed, atherosclerotic CVD prevalence is twice as high in patients with diabetes ... compared to those without a diagnosis.”
Dr. Heald added that there is always the possibility that other factors might be at play and that the work posed questions rather than presented answers.
Taking a pragmatic view, the researchers suggested that “one alternative approach may be to offer further assessment using fasting plasma glucose or oral glucose tolerance testing in those with A1c values of 46 or 47 mmol/mol.”
“In anyone with an early diagnosis of type 2 diabetes, in addition to dietary modification and especially if there is cardiovascular risk, then one might start them on metformin due to the cardiovascular benefits as well as the sugar-lowering effects,” said Dr. Heald, adding that “we certainly don’t want women missing out on metformin that could have huge benefits in the longer term.”
Dr. Stedman and Dr. Heald declared no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; and no other relationships or activities that could appear to have influenced the submitted work. Dr. Chambers has declared no conflicts.
A version of this article appeared on Medscape.com.
FROM EASD 2023
Can zoo poo help manage diabetic foot ulcers?
In a striking convergence of veterinary biology and medical science, researchers from the University of Sheffield (England) have unveiled findings that could potentially advance the treatment of diabetic foot ulcers, a condition affecting an estimated 18.6 million people worldwide. The unexpected ingredient in this potentially transformative therapy? Feces from endangered species, sourced from Yorkshire Wildlife Park, Doncaster, England.
The scourge of antibiotic resistance
Diabetic foot ulcers are a significant challenge in health care, not only because of their prevalence but also because of the alarming rise of antibiotic-resistant bacterial infections. Current antibiotic treatments frequently fail, leading to life-altering consequences like amputations and significant health care costs – estimated at one-third of the total direct costs of diabetes care. The critical need for alternative therapies has propelled scientists into a pressing search for novel antimicrobial agents.
A pioneering approach: zoo poo as bioactive goldmine
Led by Professor Graham Stafford, chair of molecular microbiology at the University of Sheffield, the research team began to explore a rather unorthodox resource: the fecal matter of endangered animals like Guinea baboons, lemurs, and Visayan pigs. While such a source might seem surprising at first glance, the rationale becomes clear when considering the nature of bacteriophages.
What are bacteriophages?
Bacteriophages, commonly known as phages, are viruses that selectively target and kill bacteria. Despite being the most prevalent biological entities on Earth, their therapeutic potential has remained largely untapped. What makes bacteriophages particularly interesting is their ability to kill antibiotic-resistant bacteria – a feature making them prime candidates for treating otherwise unmanageable diabetic foot ulcers. (Armstrong DG, et al; Fish R, et al).
Findings and future directions
Professor Stafford and his team discovered that the feces of several endangered animals harbored bacteriophages capable of killing bacterial strains resistant to antibiotics. The findings not only hold promise for a groundbreaking treatment but also provide another compelling reason to conserve endangered species: Their inherent biodiversity might contain cures for a range of infectious diseases.
While research is ongoing and clinical trials have not yet begun, the preliminary results are overwhelmingly promising.
We often look to complex technologies and synthetic materials for medical science breakthroughs, yet sometimes the most innovative solutions can be found in the most overlooked places. In this case, the feces of endangered species could turn out to be a vital asset in battling antibiotic resistance, thus affecting diabetic foot care in ways we never imagined possible.
The research conducted at the University of Sheffield also serves as a powerful argument for a One Health approach – a multidisciplinary field focusing on the interconnectedness of human, animal, and environmental health.
This intriguing work reaffirms the need for an interdisciplinary approach in tackling the world’s pressing health care challenges. The collaborative efforts between the University of Sheffield and Yorkshire Wildlife Park exemplify how academic research and conservation can come together to yield solutions for some of the most devastating and costly health conditions, while also underscoring the invaluable role that biodiversity plays in our collective well-being. Here’s to teaming up to act against amputation worldwide.
Dr. Armstrong is professor of surgery and director of limb preservation at University of Southern California, Los Angeles. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
In a striking convergence of veterinary biology and medical science, researchers from the University of Sheffield (England) have unveiled findings that could potentially advance the treatment of diabetic foot ulcers, a condition affecting an estimated 18.6 million people worldwide. The unexpected ingredient in this potentially transformative therapy? Feces from endangered species, sourced from Yorkshire Wildlife Park, Doncaster, England.
The scourge of antibiotic resistance
Diabetic foot ulcers are a significant challenge in health care, not only because of their prevalence but also because of the alarming rise of antibiotic-resistant bacterial infections. Current antibiotic treatments frequently fail, leading to life-altering consequences like amputations and significant health care costs – estimated at one-third of the total direct costs of diabetes care. The critical need for alternative therapies has propelled scientists into a pressing search for novel antimicrobial agents.
A pioneering approach: zoo poo as bioactive goldmine
Led by Professor Graham Stafford, chair of molecular microbiology at the University of Sheffield, the research team began to explore a rather unorthodox resource: the fecal matter of endangered animals like Guinea baboons, lemurs, and Visayan pigs. While such a source might seem surprising at first glance, the rationale becomes clear when considering the nature of bacteriophages.
What are bacteriophages?
Bacteriophages, commonly known as phages, are viruses that selectively target and kill bacteria. Despite being the most prevalent biological entities on Earth, their therapeutic potential has remained largely untapped. What makes bacteriophages particularly interesting is their ability to kill antibiotic-resistant bacteria – a feature making them prime candidates for treating otherwise unmanageable diabetic foot ulcers. (Armstrong DG, et al; Fish R, et al).
Findings and future directions
Professor Stafford and his team discovered that the feces of several endangered animals harbored bacteriophages capable of killing bacterial strains resistant to antibiotics. The findings not only hold promise for a groundbreaking treatment but also provide another compelling reason to conserve endangered species: Their inherent biodiversity might contain cures for a range of infectious diseases.
While research is ongoing and clinical trials have not yet begun, the preliminary results are overwhelmingly promising.
We often look to complex technologies and synthetic materials for medical science breakthroughs, yet sometimes the most innovative solutions can be found in the most overlooked places. In this case, the feces of endangered species could turn out to be a vital asset in battling antibiotic resistance, thus affecting diabetic foot care in ways we never imagined possible.
The research conducted at the University of Sheffield also serves as a powerful argument for a One Health approach – a multidisciplinary field focusing on the interconnectedness of human, animal, and environmental health.
This intriguing work reaffirms the need for an interdisciplinary approach in tackling the world’s pressing health care challenges. The collaborative efforts between the University of Sheffield and Yorkshire Wildlife Park exemplify how academic research and conservation can come together to yield solutions for some of the most devastating and costly health conditions, while also underscoring the invaluable role that biodiversity plays in our collective well-being. Here’s to teaming up to act against amputation worldwide.
Dr. Armstrong is professor of surgery and director of limb preservation at University of Southern California, Los Angeles. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
In a striking convergence of veterinary biology and medical science, researchers from the University of Sheffield (England) have unveiled findings that could potentially advance the treatment of diabetic foot ulcers, a condition affecting an estimated 18.6 million people worldwide. The unexpected ingredient in this potentially transformative therapy? Feces from endangered species, sourced from Yorkshire Wildlife Park, Doncaster, England.
The scourge of antibiotic resistance
Diabetic foot ulcers are a significant challenge in health care, not only because of their prevalence but also because of the alarming rise of antibiotic-resistant bacterial infections. Current antibiotic treatments frequently fail, leading to life-altering consequences like amputations and significant health care costs – estimated at one-third of the total direct costs of diabetes care. The critical need for alternative therapies has propelled scientists into a pressing search for novel antimicrobial agents.
A pioneering approach: zoo poo as bioactive goldmine
Led by Professor Graham Stafford, chair of molecular microbiology at the University of Sheffield, the research team began to explore a rather unorthodox resource: the fecal matter of endangered animals like Guinea baboons, lemurs, and Visayan pigs. While such a source might seem surprising at first glance, the rationale becomes clear when considering the nature of bacteriophages.
What are bacteriophages?
Bacteriophages, commonly known as phages, are viruses that selectively target and kill bacteria. Despite being the most prevalent biological entities on Earth, their therapeutic potential has remained largely untapped. What makes bacteriophages particularly interesting is their ability to kill antibiotic-resistant bacteria – a feature making them prime candidates for treating otherwise unmanageable diabetic foot ulcers. (Armstrong DG, et al; Fish R, et al).
Findings and future directions
Professor Stafford and his team discovered that the feces of several endangered animals harbored bacteriophages capable of killing bacterial strains resistant to antibiotics. The findings not only hold promise for a groundbreaking treatment but also provide another compelling reason to conserve endangered species: Their inherent biodiversity might contain cures for a range of infectious diseases.
While research is ongoing and clinical trials have not yet begun, the preliminary results are overwhelmingly promising.
We often look to complex technologies and synthetic materials for medical science breakthroughs, yet sometimes the most innovative solutions can be found in the most overlooked places. In this case, the feces of endangered species could turn out to be a vital asset in battling antibiotic resistance, thus affecting diabetic foot care in ways we never imagined possible.
The research conducted at the University of Sheffield also serves as a powerful argument for a One Health approach – a multidisciplinary field focusing on the interconnectedness of human, animal, and environmental health.
This intriguing work reaffirms the need for an interdisciplinary approach in tackling the world’s pressing health care challenges. The collaborative efforts between the University of Sheffield and Yorkshire Wildlife Park exemplify how academic research and conservation can come together to yield solutions for some of the most devastating and costly health conditions, while also underscoring the invaluable role that biodiversity plays in our collective well-being. Here’s to teaming up to act against amputation worldwide.
Dr. Armstrong is professor of surgery and director of limb preservation at University of Southern California, Los Angeles. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
When to prescribe semaglutide?
A 36-year-old woman presents to your office for assistance with weight loss. She usually weighs around 150 lb, but she had two pregnancies in the past 4 years and has gained 70 lb. Her current weight is 220 lb with a body mass index (BMI) of 36.6 kg/m2, and she has been unable to lose any weight despite diet and exercise. She reports back pain and generalized fatigue but is primarily worried about developing type 2 diabetes, which runs in her family. Her insurance covers weight loss medications, but
More and more people are turning to “medical weight management” to drop pounds and improve their health. This is a strategy that adds pharmacotherapy to lifestyle modifications to treat the chronic disease of obesity, and it is analogous to the treatment of high blood pressure or high cholesterol with medications.
This patient meets the criteria set forth by the American Heart Association, American College of Cardiology, and The Obesity Society for the management of obesity with antiobesity medications:
- BMI ≥ 30 or BMI ≥ 27 with weight-related comorbidities and
- Has been unable to achieve ≥ 5% weight loss with lifestyle changes alone.
Several U.S. Food and Drug Administration–approved antiobesity medications have been proven to cause clinically significant weight loss:
- orlistat (Alli or Xenical).
- phentermine/topiramate (Qsymia).
- naltrexone/bupropion (Contrave).
- liraglutide 3.0 mg subcutaneously daily (Saxenda).
- semaglutide 2.4 mg subcutaneously weekly (Wegovy).
When considering an antiobesity medication for a patient, it’s important to discuss efficacy, side-effect profile, contraindications, cost and coverage, and long-term use.
In this commentary, we’ll specifically focus on semaglutide (Wegovy) as it is currently the most effective FDA-approved medication for weight loss.
Efficacy
In a phase 3 clinical trial, patients on semaglutide 2.4 mg weekly lost an average of 15% of their body weight at 68 weeks, or approximately 33 lb. It is important to note that there is variability in treatment response to semaglutide 2.4 mg, just like with any other medication. About 1 in 3 individuals lost ≥ 20% of their weight, but about 1 in every 10 patients did not lose any weight.
In this patient, who has a family history of type 2 diabetes, weight loss with semaglutide 2.4 mg will probably reduce her risk of developing diabetes. With just 5%-10% weight loss, she will see improvements in her blood glucose, blood pressure, and cholesterol. Even greater weight loss (≥ 10%) has been associated with resolution of fatty liver and sleep apnea.
Side effects
Before starting semaglutide, patients should be counseled about potential gastrointestinal side effects, including nausea, upset stomach, diarrhea, constipation, and reflux.
Side effects can be managed with dietary modifications, over-the-counter treatments, and slow dose escalation. Some common tips include:
- Eat slowly.
- Eat a bland diet.
- Avoid fatty or fried foods.
- Avoid lying down immediately after eating.
- Prioritize water and fiber intake to mitigate constipation.
- Use over-the-counter treatments as needed (for example, laxative for constipation).
Most of these side effects are present only during dose escalation and resolve once the patient is on a stable dose.
Patients should be counseled about the less than 1% risk for gallbladder issues or pancreatitis. They should be instructed to go to an urgent care or emergency room if they develop severe abdominal pain, recurrent vomiting, or the inability to eat or drink.
Contraindications
We don’t prescribe GLP-1 receptor agonists, including semaglutide 2.4 mg, in patients with a personal or family history of medullary thyroid cancer. GLP-1 agonists are contraindicated in patients with a history of pancreatitis or gastroparesis. All FDA-approved antiobesity medications are contraindicated in women who are breastfeeding or trying for pregnancy. If this patient would like to pursue pregnancy again, semaglutide 2.4 mg should be stopped 2 months prior to conception.
Access
In this case, the patient’s insurance covered semaglutide 2.4 mg with a copay of $25 per month. Without insurance, semaglutide 2.4 mg (Wegovy) costs about $1,400 per month, and semaglutide 2.0 mg (Ozempic), the formulation approved for type 2 diabetes, costs up to $1,000 per month. These price ranges are often cost-prohibitive and unsustainable, especially because these medications are intended for long-term use.
Currently, Medicare does not cover antiobesity medications nor do most state Medicaid plans. Therefore, these medications are usually not considered by patients who have Medicare or Medicaid insurance.
Because insurance coverage varies and out-of-pocket costs can be prohibitive, many individuals seek other ways of acquiring semaglutide. The off-label use of semaglutide 2.0 mg (Ozempic) for obesity is scientifically supported and safe, whereas the use of compounded semaglutide is risky due to lack of regulation.
Compounded semaglutide should be avoided, given that these products are not controlled by the FDA, and adverse events have been reported in connection with compounded semaglutide.
In our clinical practice, patients have reported advertisements for “generic semaglutide” compounded with vitamins like vitamin B12 or B6. This is a significant area of concern because some vitamins (for instance, vitamin B6) are toxic at high doses.
We discussed the dangers of compounded semaglutide with our patient and told her that this isn’t something we recommend prescribing. If the patient didn’t want to wait for semaglutide 2.4 mg to be available at her pharmacy, we discussed alternative medications used for the management of obesity, such as other FDA-approved GLP-1 agonists (that is, liraglutide 3.0 mg) and off-label medications. In this case, the patient opted to wait for semaglutide 2.4 mg because she preferred a weekly injectable medication, given her busy lifestyle as a new mom.
Dr. Schmitz, of Weill Cornell Medicine, New York, disclosed no relevant financial relationships. Dr. Tchang, of Weill Cornell Medicine and the Iris Cantor Women's Health Center, both in New York, serves or has served as a director, officer, partner, employee, advisor, consultant, or trustee for Gelesis and Novo Nordisk, and has received income from Gelesis.
A version of this article first appeared on Medscape.com.
A 36-year-old woman presents to your office for assistance with weight loss. She usually weighs around 150 lb, but she had two pregnancies in the past 4 years and has gained 70 lb. Her current weight is 220 lb with a body mass index (BMI) of 36.6 kg/m2, and she has been unable to lose any weight despite diet and exercise. She reports back pain and generalized fatigue but is primarily worried about developing type 2 diabetes, which runs in her family. Her insurance covers weight loss medications, but
More and more people are turning to “medical weight management” to drop pounds and improve their health. This is a strategy that adds pharmacotherapy to lifestyle modifications to treat the chronic disease of obesity, and it is analogous to the treatment of high blood pressure or high cholesterol with medications.
This patient meets the criteria set forth by the American Heart Association, American College of Cardiology, and The Obesity Society for the management of obesity with antiobesity medications:
- BMI ≥ 30 or BMI ≥ 27 with weight-related comorbidities and
- Has been unable to achieve ≥ 5% weight loss with lifestyle changes alone.
Several U.S. Food and Drug Administration–approved antiobesity medications have been proven to cause clinically significant weight loss:
- orlistat (Alli or Xenical).
- phentermine/topiramate (Qsymia).
- naltrexone/bupropion (Contrave).
- liraglutide 3.0 mg subcutaneously daily (Saxenda).
- semaglutide 2.4 mg subcutaneously weekly (Wegovy).
When considering an antiobesity medication for a patient, it’s important to discuss efficacy, side-effect profile, contraindications, cost and coverage, and long-term use.
In this commentary, we’ll specifically focus on semaglutide (Wegovy) as it is currently the most effective FDA-approved medication for weight loss.
Efficacy
In a phase 3 clinical trial, patients on semaglutide 2.4 mg weekly lost an average of 15% of their body weight at 68 weeks, or approximately 33 lb. It is important to note that there is variability in treatment response to semaglutide 2.4 mg, just like with any other medication. About 1 in 3 individuals lost ≥ 20% of their weight, but about 1 in every 10 patients did not lose any weight.
In this patient, who has a family history of type 2 diabetes, weight loss with semaglutide 2.4 mg will probably reduce her risk of developing diabetes. With just 5%-10% weight loss, she will see improvements in her blood glucose, blood pressure, and cholesterol. Even greater weight loss (≥ 10%) has been associated with resolution of fatty liver and sleep apnea.
Side effects
Before starting semaglutide, patients should be counseled about potential gastrointestinal side effects, including nausea, upset stomach, diarrhea, constipation, and reflux.
Side effects can be managed with dietary modifications, over-the-counter treatments, and slow dose escalation. Some common tips include:
- Eat slowly.
- Eat a bland diet.
- Avoid fatty or fried foods.
- Avoid lying down immediately after eating.
- Prioritize water and fiber intake to mitigate constipation.
- Use over-the-counter treatments as needed (for example, laxative for constipation).
Most of these side effects are present only during dose escalation and resolve once the patient is on a stable dose.
Patients should be counseled about the less than 1% risk for gallbladder issues or pancreatitis. They should be instructed to go to an urgent care or emergency room if they develop severe abdominal pain, recurrent vomiting, or the inability to eat or drink.
Contraindications
We don’t prescribe GLP-1 receptor agonists, including semaglutide 2.4 mg, in patients with a personal or family history of medullary thyroid cancer. GLP-1 agonists are contraindicated in patients with a history of pancreatitis or gastroparesis. All FDA-approved antiobesity medications are contraindicated in women who are breastfeeding or trying for pregnancy. If this patient would like to pursue pregnancy again, semaglutide 2.4 mg should be stopped 2 months prior to conception.
Access
In this case, the patient’s insurance covered semaglutide 2.4 mg with a copay of $25 per month. Without insurance, semaglutide 2.4 mg (Wegovy) costs about $1,400 per month, and semaglutide 2.0 mg (Ozempic), the formulation approved for type 2 diabetes, costs up to $1,000 per month. These price ranges are often cost-prohibitive and unsustainable, especially because these medications are intended for long-term use.
Currently, Medicare does not cover antiobesity medications nor do most state Medicaid plans. Therefore, these medications are usually not considered by patients who have Medicare or Medicaid insurance.
Because insurance coverage varies and out-of-pocket costs can be prohibitive, many individuals seek other ways of acquiring semaglutide. The off-label use of semaglutide 2.0 mg (Ozempic) for obesity is scientifically supported and safe, whereas the use of compounded semaglutide is risky due to lack of regulation.
Compounded semaglutide should be avoided, given that these products are not controlled by the FDA, and adverse events have been reported in connection with compounded semaglutide.
In our clinical practice, patients have reported advertisements for “generic semaglutide” compounded with vitamins like vitamin B12 or B6. This is a significant area of concern because some vitamins (for instance, vitamin B6) are toxic at high doses.
We discussed the dangers of compounded semaglutide with our patient and told her that this isn’t something we recommend prescribing. If the patient didn’t want to wait for semaglutide 2.4 mg to be available at her pharmacy, we discussed alternative medications used for the management of obesity, such as other FDA-approved GLP-1 agonists (that is, liraglutide 3.0 mg) and off-label medications. In this case, the patient opted to wait for semaglutide 2.4 mg because she preferred a weekly injectable medication, given her busy lifestyle as a new mom.
Dr. Schmitz, of Weill Cornell Medicine, New York, disclosed no relevant financial relationships. Dr. Tchang, of Weill Cornell Medicine and the Iris Cantor Women's Health Center, both in New York, serves or has served as a director, officer, partner, employee, advisor, consultant, or trustee for Gelesis and Novo Nordisk, and has received income from Gelesis.
A version of this article first appeared on Medscape.com.
A 36-year-old woman presents to your office for assistance with weight loss. She usually weighs around 150 lb, but she had two pregnancies in the past 4 years and has gained 70 lb. Her current weight is 220 lb with a body mass index (BMI) of 36.6 kg/m2, and she has been unable to lose any weight despite diet and exercise. She reports back pain and generalized fatigue but is primarily worried about developing type 2 diabetes, which runs in her family. Her insurance covers weight loss medications, but
More and more people are turning to “medical weight management” to drop pounds and improve their health. This is a strategy that adds pharmacotherapy to lifestyle modifications to treat the chronic disease of obesity, and it is analogous to the treatment of high blood pressure or high cholesterol with medications.
This patient meets the criteria set forth by the American Heart Association, American College of Cardiology, and The Obesity Society for the management of obesity with antiobesity medications:
- BMI ≥ 30 or BMI ≥ 27 with weight-related comorbidities and
- Has been unable to achieve ≥ 5% weight loss with lifestyle changes alone.
Several U.S. Food and Drug Administration–approved antiobesity medications have been proven to cause clinically significant weight loss:
- orlistat (Alli or Xenical).
- phentermine/topiramate (Qsymia).
- naltrexone/bupropion (Contrave).
- liraglutide 3.0 mg subcutaneously daily (Saxenda).
- semaglutide 2.4 mg subcutaneously weekly (Wegovy).
When considering an antiobesity medication for a patient, it’s important to discuss efficacy, side-effect profile, contraindications, cost and coverage, and long-term use.
In this commentary, we’ll specifically focus on semaglutide (Wegovy) as it is currently the most effective FDA-approved medication for weight loss.
Efficacy
In a phase 3 clinical trial, patients on semaglutide 2.4 mg weekly lost an average of 15% of their body weight at 68 weeks, or approximately 33 lb. It is important to note that there is variability in treatment response to semaglutide 2.4 mg, just like with any other medication. About 1 in 3 individuals lost ≥ 20% of their weight, but about 1 in every 10 patients did not lose any weight.
In this patient, who has a family history of type 2 diabetes, weight loss with semaglutide 2.4 mg will probably reduce her risk of developing diabetes. With just 5%-10% weight loss, she will see improvements in her blood glucose, blood pressure, and cholesterol. Even greater weight loss (≥ 10%) has been associated with resolution of fatty liver and sleep apnea.
Side effects
Before starting semaglutide, patients should be counseled about potential gastrointestinal side effects, including nausea, upset stomach, diarrhea, constipation, and reflux.
Side effects can be managed with dietary modifications, over-the-counter treatments, and slow dose escalation. Some common tips include:
- Eat slowly.
- Eat a bland diet.
- Avoid fatty or fried foods.
- Avoid lying down immediately after eating.
- Prioritize water and fiber intake to mitigate constipation.
- Use over-the-counter treatments as needed (for example, laxative for constipation).
Most of these side effects are present only during dose escalation and resolve once the patient is on a stable dose.
Patients should be counseled about the less than 1% risk for gallbladder issues or pancreatitis. They should be instructed to go to an urgent care or emergency room if they develop severe abdominal pain, recurrent vomiting, or the inability to eat or drink.
Contraindications
We don’t prescribe GLP-1 receptor agonists, including semaglutide 2.4 mg, in patients with a personal or family history of medullary thyroid cancer. GLP-1 agonists are contraindicated in patients with a history of pancreatitis or gastroparesis. All FDA-approved antiobesity medications are contraindicated in women who are breastfeeding or trying for pregnancy. If this patient would like to pursue pregnancy again, semaglutide 2.4 mg should be stopped 2 months prior to conception.
Access
In this case, the patient’s insurance covered semaglutide 2.4 mg with a copay of $25 per month. Without insurance, semaglutide 2.4 mg (Wegovy) costs about $1,400 per month, and semaglutide 2.0 mg (Ozempic), the formulation approved for type 2 diabetes, costs up to $1,000 per month. These price ranges are often cost-prohibitive and unsustainable, especially because these medications are intended for long-term use.
Currently, Medicare does not cover antiobesity medications nor do most state Medicaid plans. Therefore, these medications are usually not considered by patients who have Medicare or Medicaid insurance.
Because insurance coverage varies and out-of-pocket costs can be prohibitive, many individuals seek other ways of acquiring semaglutide. The off-label use of semaglutide 2.0 mg (Ozempic) for obesity is scientifically supported and safe, whereas the use of compounded semaglutide is risky due to lack of regulation.
Compounded semaglutide should be avoided, given that these products are not controlled by the FDA, and adverse events have been reported in connection with compounded semaglutide.
In our clinical practice, patients have reported advertisements for “generic semaglutide” compounded with vitamins like vitamin B12 or B6. This is a significant area of concern because some vitamins (for instance, vitamin B6) are toxic at high doses.
We discussed the dangers of compounded semaglutide with our patient and told her that this isn’t something we recommend prescribing. If the patient didn’t want to wait for semaglutide 2.4 mg to be available at her pharmacy, we discussed alternative medications used for the management of obesity, such as other FDA-approved GLP-1 agonists (that is, liraglutide 3.0 mg) and off-label medications. In this case, the patient opted to wait for semaglutide 2.4 mg because she preferred a weekly injectable medication, given her busy lifestyle as a new mom.
Dr. Schmitz, of Weill Cornell Medicine, New York, disclosed no relevant financial relationships. Dr. Tchang, of Weill Cornell Medicine and the Iris Cantor Women's Health Center, both in New York, serves or has served as a director, officer, partner, employee, advisor, consultant, or trustee for Gelesis and Novo Nordisk, and has received income from Gelesis.
A version of this article first appeared on Medscape.com.