User login
Low copays drive better adherence to new diabetes drugs
TOPLINE:
The less U.S. patients pay out of pocket for drugs that often have high copays, such as sodium-glucose cotransporter 2 (SGLT2) inhibitors or glucagon-like peptide-1 (GLP-1) agonists, the more adherent they are.
METHODOLOGY:
- Review of 90,041 U.S. adults who started a GLP-1 agonist (n = 39,149) or SGLT2 inhibitor (n = 50,892) in 2014-2020.
- Participants had type 2 diabetes, heart failure, or both.
- Data are from Clinformatics Data Mart, including both commercial and Medicare health insurance plans.
- Primary outcome: 12-month adherence to prescribed GLP-1 agonist or SGLT2 inhibitor.
TAKEAWAYS:
- U.S. adults with a lower drug copay had significantly higher odds of 12-month adherence to GLP-1 agonists and SGLT2 inhibitors, compared with those with a higher copay.
- These differences persisted after controlling for patient demographic, clinical, and socioeconomic covariates.
- After full adjustment, patients with a high copay (≥ $50/month) were, after 12 months, 53% less likely to adhere to an SGLT2 inhibitor and 32% less likely to adhere to a GLP-1 agonist, compared with patients with a low copay (< $10/month) for these agents.
IN PRACTICE:
“Lowering high out-of-pocket prescription costs may be key to improving adherence to guideline-recommended therapies and advancing overall quality of care in patients with type 2 diabetes and heart failure,” say the authors.
STUDY DETAILS:
The study was led by Utibe R. Essien, MD, from the University of California, Los Angeles, and Balvindar Singh, MD, PhD, from the University of Pittsburgh, and included several authors from other U.S. centers.
LIMITATIONS:
Study could not exclude residual confounding.
Generalizability uncertain for those without health insurance or with public insurance.
Study did not have information on patient preferences associated with medication use, including specific reasons for poor adherence.
Possible misclassifications of type 2 diabetes and heart failure diagnoses or medical comorbidities.
Study could not assess how copayments influenced initial prescription receipt or abandonment at the pharmacy, nor other factors including possible price inflation.
DISCLOSURES:
The study received no commercial funding. One author (not a lead author) is an advisor to several drug companies, including ones that market SGLT2 inhibitors or GLP-1 agonists.
A version of this article first appeared on Medscape.com.
TOPLINE:
The less U.S. patients pay out of pocket for drugs that often have high copays, such as sodium-glucose cotransporter 2 (SGLT2) inhibitors or glucagon-like peptide-1 (GLP-1) agonists, the more adherent they are.
METHODOLOGY:
- Review of 90,041 U.S. adults who started a GLP-1 agonist (n = 39,149) or SGLT2 inhibitor (n = 50,892) in 2014-2020.
- Participants had type 2 diabetes, heart failure, or both.
- Data are from Clinformatics Data Mart, including both commercial and Medicare health insurance plans.
- Primary outcome: 12-month adherence to prescribed GLP-1 agonist or SGLT2 inhibitor.
TAKEAWAYS:
- U.S. adults with a lower drug copay had significantly higher odds of 12-month adherence to GLP-1 agonists and SGLT2 inhibitors, compared with those with a higher copay.
- These differences persisted after controlling for patient demographic, clinical, and socioeconomic covariates.
- After full adjustment, patients with a high copay (≥ $50/month) were, after 12 months, 53% less likely to adhere to an SGLT2 inhibitor and 32% less likely to adhere to a GLP-1 agonist, compared with patients with a low copay (< $10/month) for these agents.
IN PRACTICE:
“Lowering high out-of-pocket prescription costs may be key to improving adherence to guideline-recommended therapies and advancing overall quality of care in patients with type 2 diabetes and heart failure,” say the authors.
STUDY DETAILS:
The study was led by Utibe R. Essien, MD, from the University of California, Los Angeles, and Balvindar Singh, MD, PhD, from the University of Pittsburgh, and included several authors from other U.S. centers.
LIMITATIONS:
Study could not exclude residual confounding.
Generalizability uncertain for those without health insurance or with public insurance.
Study did not have information on patient preferences associated with medication use, including specific reasons for poor adherence.
Possible misclassifications of type 2 diabetes and heart failure diagnoses or medical comorbidities.
Study could not assess how copayments influenced initial prescription receipt or abandonment at the pharmacy, nor other factors including possible price inflation.
DISCLOSURES:
The study received no commercial funding. One author (not a lead author) is an advisor to several drug companies, including ones that market SGLT2 inhibitors or GLP-1 agonists.
A version of this article first appeared on Medscape.com.
TOPLINE:
The less U.S. patients pay out of pocket for drugs that often have high copays, such as sodium-glucose cotransporter 2 (SGLT2) inhibitors or glucagon-like peptide-1 (GLP-1) agonists, the more adherent they are.
METHODOLOGY:
- Review of 90,041 U.S. adults who started a GLP-1 agonist (n = 39,149) or SGLT2 inhibitor (n = 50,892) in 2014-2020.
- Participants had type 2 diabetes, heart failure, or both.
- Data are from Clinformatics Data Mart, including both commercial and Medicare health insurance plans.
- Primary outcome: 12-month adherence to prescribed GLP-1 agonist or SGLT2 inhibitor.
TAKEAWAYS:
- U.S. adults with a lower drug copay had significantly higher odds of 12-month adherence to GLP-1 agonists and SGLT2 inhibitors, compared with those with a higher copay.
- These differences persisted after controlling for patient demographic, clinical, and socioeconomic covariates.
- After full adjustment, patients with a high copay (≥ $50/month) were, after 12 months, 53% less likely to adhere to an SGLT2 inhibitor and 32% less likely to adhere to a GLP-1 agonist, compared with patients with a low copay (< $10/month) for these agents.
IN PRACTICE:
“Lowering high out-of-pocket prescription costs may be key to improving adherence to guideline-recommended therapies and advancing overall quality of care in patients with type 2 diabetes and heart failure,” say the authors.
STUDY DETAILS:
The study was led by Utibe R. Essien, MD, from the University of California, Los Angeles, and Balvindar Singh, MD, PhD, from the University of Pittsburgh, and included several authors from other U.S. centers.
LIMITATIONS:
Study could not exclude residual confounding.
Generalizability uncertain for those without health insurance or with public insurance.
Study did not have information on patient preferences associated with medication use, including specific reasons for poor adherence.
Possible misclassifications of type 2 diabetes and heart failure diagnoses or medical comorbidities.
Study could not assess how copayments influenced initial prescription receipt or abandonment at the pharmacy, nor other factors including possible price inflation.
DISCLOSURES:
The study received no commercial funding. One author (not a lead author) is an advisor to several drug companies, including ones that market SGLT2 inhibitors or GLP-1 agonists.
A version of this article first appeared on Medscape.com.
Is education or screening better for type 1 diabetes?
After 100 years of insulin therapy, teplizumab, an immunotherapy for early-stage type 1 diabetes, has been approved for the first time in the United States and has been shown to delay the manifestation of clinical diabetes by 3 years on average. at the Diabetes Congress in Berlin.
Anette-Gabriele Ziegler, MD, PhD, director of the Institute for Diabetes Research in Helmholtz Munich, argued that voluntary screening for type 1 diabetes should be included in standard care. “The first immunotherapy that delays type 1 diabetes has been approved in the U.S. for early stage 2. And this early stage can only be identified through prior screening, since no symptoms have manifested by this stage,” she said. This is the only way in which as many people as possible, particularly children, will benefit from the disease-delaying therapy, she added.
Two autoantibodies
One biomarker for the early diagnosis of type 1 diabetes is evidence of at least two positive islet cell antibodies. In one study of more than 13,000 children who were observed for 20 years, the specificity of these antibodies was 100%. “Every single child with a positive autoantibody test developed type 1 diabetes later on in their life,” Dr. Ziegler said. “Based on the results of this study, the early stages of type 1 diabetes were added to multiple guidelines.”
The early stage of type 1 diabetes is divided into the following three phases, depending on autoantibody detection and the level of glucose metabolism:
- Early stage 1: Two or more islet autoantibodies and normoglycemia.
- Early stage 2: Two or more islet autoantibodies and dysglycemia.
- Early stage 3: Symptoms, hyperglycemia, insulin therapy.
The aim of the ongoing FR1DA study is to ascertain whether the general population could also be screened for type 1 diabetes using this autoantibody. “Since 2015, children of kindergarten and school age have undergone screening, and to date, more than 170,000 have been tested,” said Dr. Ziegler. “At least two autoantibodies were detected in 0.3% of those screened.”
Education and care
The families of the children in whom early-stage type 1 diabetes was diagnosed were invited to take an oral glucose tolerance test (OGTT), to undergo measurement of hemoglobin A1c, and to take part in training and monitoring. “Education and competent, ongoing care are crucial for the efficacy of the screening,” Dr. Ziegler emphasized.
The OGTT revealed that 85% of the FR1DA children were still in early stage 1, another 11% were in early stage 2, and the remaining 4% were in early stage 3.
“Unfortunately, the 4% could no longer benefit from teplizumab, since the medication is not approved for manifest diabetes,” said Dr. Ziegler. “However, the 11% could receive teplizumab immediately, and then later on, the 85%, when they developed stage 2. Therefore, further observation of the children is also important.”
The speed at which the disease progresses from early stage 1 to early stage 2 can be stratified using IA2 antibodies, the 90-minute OGTT glucose value, and the HbA1c value. With regard to progression to clinical type 1 diabetes (stage 3), it was observed that the progression risk for the FR1DA children was similar to that of international birth cohorts with increased genetic risk. “Of course, there is still no 20-year follow-up like for BABYDIAB, DIPP, and DAISY, but as of yet, the progression rate is practically identical,” said Dr. Ziegler.
Dubious benefits?
The advantages of screening for type 1 diabetes would not be limited to potential access to preventive therapies and a smooth transition to insulin therapy at the correct point in time, according to Dr. Ziegler. Participation in the FR1DA study dramatically reduced the risk of diabetic ketoacidosis (DKA). Between 2015 and 2023, the overall rate of ketoacidosis associated with the manifestation of clinical type 1 diabetes was 4.3%. In contrast, the general DKA rate in Germany has remained largely unchanged for the last 2 decades at between 20% and 25%.
In addition, the FR1DA children exhibited better beta cell function and better metabolic function at clinical diagnosis of type 1 diabetes. This finding was observed in a comparison with children with a spontaneous diabetes diagnosis from the DiMelli study. “It is important that there is a lot of data that shows how, in the long term, this is associated with a better morbidity and mortality,” said Dr. Ziegler.
Despite the impressive data from the FR1DA study, not all diabetes experts are convinced that a general screening for type 1 diabetes would be beneficial. Beate Karges, MD, PhD, of the Clinic for Pediatric and Adolescent Medicine of the Bethlehem Hospital Stolberg (Germany) and the endocrinology and diabetology department at the University Hospital Aachen (Germany), stressed, “Screening makes sense if the disease is curable in the preclinical phase or if there is a significantly better prognosis in the event of early diagnosis and treatment.”
Severe side effects
Even with an early-stage diagnosis, curing type 1 diabetes is impossible. The new anti-CD3 antibody teplizumab merely delays the manifestation of symptoms for 3 years. However, this delay has its price. The summary of product characteristics for teplizumab contains warnings of severe lymphopenia lasting many weeks, cytokine release syndrome, severe infections, and hypersensitivity reactions. Furthermore, vaccinations may not be administered during teplizumab treatment and therefore must be completed in advance.
“Preventing type 1 diabetes is still not possible, we can only delay it, and the long-term efficacy and safety of this immunotherapy are not clear,” said Dr. Karges. She added that a significant reduction in the DKA rate – as observed in the FR1DA study – may be possible even without screening. This possibility was demonstrated by a model project in Stuttgart, Germany, in which the DKA rate was significantly reduced through education alone.
Education reduces ketoacidosis
“The families were given information about the early signs of type 1 diabetes during the education investigation. Through this [education], a reduction in the ketoacidosis rate from 28% to 16% was achieved,” said Dr. Karges. It is also known from studies of familial type 1 diabetes that secondary sufferers in the family only exhibit a DKA rate of 7%. “Through education within the family and awareness campaigns, the DKA rate can be reduced by 40%-65%,” said Dr. Karges.
Dr. Karges also doubts whether starting insulin therapy earlier “at the correct point in time” elicits long-term advantages. Secondary sufferers with familial type 1 diabetes have better HbA1c values in the first few years after diagnosis. “But as they progress beyond 2, towards 10 years, the difference in HbA1c values diminishes,” said Dr. Karges.
Whether the patient has DKA at type 1 diabetes diagnosis also seems to make little difference in the long term. “There is also no difference in the HbA1c value in the 2-10 years after diagnosis,” said Dr. Karges. “Glycemic control is not permanently improved in the event that treatment is started early,” she concluded.
“Type 1 diabetes can be delayed with an immune intervention, but to do so, we must also accept possible severe side effects in an otherwise healthy child,” she said. On the other hand, type 1 diabetes can be treated well. “With pumps and continuous glucose monitoring, insulin therapy in children and adolescents has become significantly safer and more effective,” she said.
New therapeutic options
Whether voluntary screening for type 1 diabetes eventually finds its way into standard care depends on the further development of preventive medications. Dr. Ziegler stressed that future preventive therapy does not need to be limited to the anti-CD3 antibody teplizumab.
For example, strategies such as high-dose oral insulin therapy are being investigated. Verapamil, which is used to treat hypertension, is also promising, since with it, beta cells were retained in early stage 3, and it improved their function. The fusion protein abatacept fell short of statistical significance in a recently published study. For Dr. Ziegler, one thing remains true. “The therapy of type 1 diabetes is about to undergo a renaissance.”
This article was translated from the Medscape German Edition. A version of this article appeared on Medscape.com.
After 100 years of insulin therapy, teplizumab, an immunotherapy for early-stage type 1 diabetes, has been approved for the first time in the United States and has been shown to delay the manifestation of clinical diabetes by 3 years on average. at the Diabetes Congress in Berlin.
Anette-Gabriele Ziegler, MD, PhD, director of the Institute for Diabetes Research in Helmholtz Munich, argued that voluntary screening for type 1 diabetes should be included in standard care. “The first immunotherapy that delays type 1 diabetes has been approved in the U.S. for early stage 2. And this early stage can only be identified through prior screening, since no symptoms have manifested by this stage,” she said. This is the only way in which as many people as possible, particularly children, will benefit from the disease-delaying therapy, she added.
Two autoantibodies
One biomarker for the early diagnosis of type 1 diabetes is evidence of at least two positive islet cell antibodies. In one study of more than 13,000 children who were observed for 20 years, the specificity of these antibodies was 100%. “Every single child with a positive autoantibody test developed type 1 diabetes later on in their life,” Dr. Ziegler said. “Based on the results of this study, the early stages of type 1 diabetes were added to multiple guidelines.”
The early stage of type 1 diabetes is divided into the following three phases, depending on autoantibody detection and the level of glucose metabolism:
- Early stage 1: Two or more islet autoantibodies and normoglycemia.
- Early stage 2: Two or more islet autoantibodies and dysglycemia.
- Early stage 3: Symptoms, hyperglycemia, insulin therapy.
The aim of the ongoing FR1DA study is to ascertain whether the general population could also be screened for type 1 diabetes using this autoantibody. “Since 2015, children of kindergarten and school age have undergone screening, and to date, more than 170,000 have been tested,” said Dr. Ziegler. “At least two autoantibodies were detected in 0.3% of those screened.”
Education and care
The families of the children in whom early-stage type 1 diabetes was diagnosed were invited to take an oral glucose tolerance test (OGTT), to undergo measurement of hemoglobin A1c, and to take part in training and monitoring. “Education and competent, ongoing care are crucial for the efficacy of the screening,” Dr. Ziegler emphasized.
The OGTT revealed that 85% of the FR1DA children were still in early stage 1, another 11% were in early stage 2, and the remaining 4% were in early stage 3.
“Unfortunately, the 4% could no longer benefit from teplizumab, since the medication is not approved for manifest diabetes,” said Dr. Ziegler. “However, the 11% could receive teplizumab immediately, and then later on, the 85%, when they developed stage 2. Therefore, further observation of the children is also important.”
The speed at which the disease progresses from early stage 1 to early stage 2 can be stratified using IA2 antibodies, the 90-minute OGTT glucose value, and the HbA1c value. With regard to progression to clinical type 1 diabetes (stage 3), it was observed that the progression risk for the FR1DA children was similar to that of international birth cohorts with increased genetic risk. “Of course, there is still no 20-year follow-up like for BABYDIAB, DIPP, and DAISY, but as of yet, the progression rate is practically identical,” said Dr. Ziegler.
Dubious benefits?
The advantages of screening for type 1 diabetes would not be limited to potential access to preventive therapies and a smooth transition to insulin therapy at the correct point in time, according to Dr. Ziegler. Participation in the FR1DA study dramatically reduced the risk of diabetic ketoacidosis (DKA). Between 2015 and 2023, the overall rate of ketoacidosis associated with the manifestation of clinical type 1 diabetes was 4.3%. In contrast, the general DKA rate in Germany has remained largely unchanged for the last 2 decades at between 20% and 25%.
In addition, the FR1DA children exhibited better beta cell function and better metabolic function at clinical diagnosis of type 1 diabetes. This finding was observed in a comparison with children with a spontaneous diabetes diagnosis from the DiMelli study. “It is important that there is a lot of data that shows how, in the long term, this is associated with a better morbidity and mortality,” said Dr. Ziegler.
Despite the impressive data from the FR1DA study, not all diabetes experts are convinced that a general screening for type 1 diabetes would be beneficial. Beate Karges, MD, PhD, of the Clinic for Pediatric and Adolescent Medicine of the Bethlehem Hospital Stolberg (Germany) and the endocrinology and diabetology department at the University Hospital Aachen (Germany), stressed, “Screening makes sense if the disease is curable in the preclinical phase or if there is a significantly better prognosis in the event of early diagnosis and treatment.”
Severe side effects
Even with an early-stage diagnosis, curing type 1 diabetes is impossible. The new anti-CD3 antibody teplizumab merely delays the manifestation of symptoms for 3 years. However, this delay has its price. The summary of product characteristics for teplizumab contains warnings of severe lymphopenia lasting many weeks, cytokine release syndrome, severe infections, and hypersensitivity reactions. Furthermore, vaccinations may not be administered during teplizumab treatment and therefore must be completed in advance.
“Preventing type 1 diabetes is still not possible, we can only delay it, and the long-term efficacy and safety of this immunotherapy are not clear,” said Dr. Karges. She added that a significant reduction in the DKA rate – as observed in the FR1DA study – may be possible even without screening. This possibility was demonstrated by a model project in Stuttgart, Germany, in which the DKA rate was significantly reduced through education alone.
Education reduces ketoacidosis
“The families were given information about the early signs of type 1 diabetes during the education investigation. Through this [education], a reduction in the ketoacidosis rate from 28% to 16% was achieved,” said Dr. Karges. It is also known from studies of familial type 1 diabetes that secondary sufferers in the family only exhibit a DKA rate of 7%. “Through education within the family and awareness campaigns, the DKA rate can be reduced by 40%-65%,” said Dr. Karges.
Dr. Karges also doubts whether starting insulin therapy earlier “at the correct point in time” elicits long-term advantages. Secondary sufferers with familial type 1 diabetes have better HbA1c values in the first few years after diagnosis. “But as they progress beyond 2, towards 10 years, the difference in HbA1c values diminishes,” said Dr. Karges.
Whether the patient has DKA at type 1 diabetes diagnosis also seems to make little difference in the long term. “There is also no difference in the HbA1c value in the 2-10 years after diagnosis,” said Dr. Karges. “Glycemic control is not permanently improved in the event that treatment is started early,” she concluded.
“Type 1 diabetes can be delayed with an immune intervention, but to do so, we must also accept possible severe side effects in an otherwise healthy child,” she said. On the other hand, type 1 diabetes can be treated well. “With pumps and continuous glucose monitoring, insulin therapy in children and adolescents has become significantly safer and more effective,” she said.
New therapeutic options
Whether voluntary screening for type 1 diabetes eventually finds its way into standard care depends on the further development of preventive medications. Dr. Ziegler stressed that future preventive therapy does not need to be limited to the anti-CD3 antibody teplizumab.
For example, strategies such as high-dose oral insulin therapy are being investigated. Verapamil, which is used to treat hypertension, is also promising, since with it, beta cells were retained in early stage 3, and it improved their function. The fusion protein abatacept fell short of statistical significance in a recently published study. For Dr. Ziegler, one thing remains true. “The therapy of type 1 diabetes is about to undergo a renaissance.”
This article was translated from the Medscape German Edition. A version of this article appeared on Medscape.com.
After 100 years of insulin therapy, teplizumab, an immunotherapy for early-stage type 1 diabetes, has been approved for the first time in the United States and has been shown to delay the manifestation of clinical diabetes by 3 years on average. at the Diabetes Congress in Berlin.
Anette-Gabriele Ziegler, MD, PhD, director of the Institute for Diabetes Research in Helmholtz Munich, argued that voluntary screening for type 1 diabetes should be included in standard care. “The first immunotherapy that delays type 1 diabetes has been approved in the U.S. for early stage 2. And this early stage can only be identified through prior screening, since no symptoms have manifested by this stage,” she said. This is the only way in which as many people as possible, particularly children, will benefit from the disease-delaying therapy, she added.
Two autoantibodies
One biomarker for the early diagnosis of type 1 diabetes is evidence of at least two positive islet cell antibodies. In one study of more than 13,000 children who were observed for 20 years, the specificity of these antibodies was 100%. “Every single child with a positive autoantibody test developed type 1 diabetes later on in their life,” Dr. Ziegler said. “Based on the results of this study, the early stages of type 1 diabetes were added to multiple guidelines.”
The early stage of type 1 diabetes is divided into the following three phases, depending on autoantibody detection and the level of glucose metabolism:
- Early stage 1: Two or more islet autoantibodies and normoglycemia.
- Early stage 2: Two or more islet autoantibodies and dysglycemia.
- Early stage 3: Symptoms, hyperglycemia, insulin therapy.
The aim of the ongoing FR1DA study is to ascertain whether the general population could also be screened for type 1 diabetes using this autoantibody. “Since 2015, children of kindergarten and school age have undergone screening, and to date, more than 170,000 have been tested,” said Dr. Ziegler. “At least two autoantibodies were detected in 0.3% of those screened.”
Education and care
The families of the children in whom early-stage type 1 diabetes was diagnosed were invited to take an oral glucose tolerance test (OGTT), to undergo measurement of hemoglobin A1c, and to take part in training and monitoring. “Education and competent, ongoing care are crucial for the efficacy of the screening,” Dr. Ziegler emphasized.
The OGTT revealed that 85% of the FR1DA children were still in early stage 1, another 11% were in early stage 2, and the remaining 4% were in early stage 3.
“Unfortunately, the 4% could no longer benefit from teplizumab, since the medication is not approved for manifest diabetes,” said Dr. Ziegler. “However, the 11% could receive teplizumab immediately, and then later on, the 85%, when they developed stage 2. Therefore, further observation of the children is also important.”
The speed at which the disease progresses from early stage 1 to early stage 2 can be stratified using IA2 antibodies, the 90-minute OGTT glucose value, and the HbA1c value. With regard to progression to clinical type 1 diabetes (stage 3), it was observed that the progression risk for the FR1DA children was similar to that of international birth cohorts with increased genetic risk. “Of course, there is still no 20-year follow-up like for BABYDIAB, DIPP, and DAISY, but as of yet, the progression rate is practically identical,” said Dr. Ziegler.
Dubious benefits?
The advantages of screening for type 1 diabetes would not be limited to potential access to preventive therapies and a smooth transition to insulin therapy at the correct point in time, according to Dr. Ziegler. Participation in the FR1DA study dramatically reduced the risk of diabetic ketoacidosis (DKA). Between 2015 and 2023, the overall rate of ketoacidosis associated with the manifestation of clinical type 1 diabetes was 4.3%. In contrast, the general DKA rate in Germany has remained largely unchanged for the last 2 decades at between 20% and 25%.
In addition, the FR1DA children exhibited better beta cell function and better metabolic function at clinical diagnosis of type 1 diabetes. This finding was observed in a comparison with children with a spontaneous diabetes diagnosis from the DiMelli study. “It is important that there is a lot of data that shows how, in the long term, this is associated with a better morbidity and mortality,” said Dr. Ziegler.
Despite the impressive data from the FR1DA study, not all diabetes experts are convinced that a general screening for type 1 diabetes would be beneficial. Beate Karges, MD, PhD, of the Clinic for Pediatric and Adolescent Medicine of the Bethlehem Hospital Stolberg (Germany) and the endocrinology and diabetology department at the University Hospital Aachen (Germany), stressed, “Screening makes sense if the disease is curable in the preclinical phase or if there is a significantly better prognosis in the event of early diagnosis and treatment.”
Severe side effects
Even with an early-stage diagnosis, curing type 1 diabetes is impossible. The new anti-CD3 antibody teplizumab merely delays the manifestation of symptoms for 3 years. However, this delay has its price. The summary of product characteristics for teplizumab contains warnings of severe lymphopenia lasting many weeks, cytokine release syndrome, severe infections, and hypersensitivity reactions. Furthermore, vaccinations may not be administered during teplizumab treatment and therefore must be completed in advance.
“Preventing type 1 diabetes is still not possible, we can only delay it, and the long-term efficacy and safety of this immunotherapy are not clear,” said Dr. Karges. She added that a significant reduction in the DKA rate – as observed in the FR1DA study – may be possible even without screening. This possibility was demonstrated by a model project in Stuttgart, Germany, in which the DKA rate was significantly reduced through education alone.
Education reduces ketoacidosis
“The families were given information about the early signs of type 1 diabetes during the education investigation. Through this [education], a reduction in the ketoacidosis rate from 28% to 16% was achieved,” said Dr. Karges. It is also known from studies of familial type 1 diabetes that secondary sufferers in the family only exhibit a DKA rate of 7%. “Through education within the family and awareness campaigns, the DKA rate can be reduced by 40%-65%,” said Dr. Karges.
Dr. Karges also doubts whether starting insulin therapy earlier “at the correct point in time” elicits long-term advantages. Secondary sufferers with familial type 1 diabetes have better HbA1c values in the first few years after diagnosis. “But as they progress beyond 2, towards 10 years, the difference in HbA1c values diminishes,” said Dr. Karges.
Whether the patient has DKA at type 1 diabetes diagnosis also seems to make little difference in the long term. “There is also no difference in the HbA1c value in the 2-10 years after diagnosis,” said Dr. Karges. “Glycemic control is not permanently improved in the event that treatment is started early,” she concluded.
“Type 1 diabetes can be delayed with an immune intervention, but to do so, we must also accept possible severe side effects in an otherwise healthy child,” she said. On the other hand, type 1 diabetes can be treated well. “With pumps and continuous glucose monitoring, insulin therapy in children and adolescents has become significantly safer and more effective,” she said.
New therapeutic options
Whether voluntary screening for type 1 diabetes eventually finds its way into standard care depends on the further development of preventive medications. Dr. Ziegler stressed that future preventive therapy does not need to be limited to the anti-CD3 antibody teplizumab.
For example, strategies such as high-dose oral insulin therapy are being investigated. Verapamil, which is used to treat hypertension, is also promising, since with it, beta cells were retained in early stage 3, and it improved their function. The fusion protein abatacept fell short of statistical significance in a recently published study. For Dr. Ziegler, one thing remains true. “The therapy of type 1 diabetes is about to undergo a renaissance.”
This article was translated from the Medscape German Edition. A version of this article appeared on Medscape.com.
FDA OKs empagliflozin for children with type 2 diabetes
aged 10 years and older.
This approval represents only the second oral treatment option for children and adolescents with type 2 diabetes after metformin; the latter appears to be less effective for pediatric patients than for adults.
Injectable glucagonlike peptide–1 (GLP-1) agonists are also available for youth with type 2 diabetes. These include daily liraglutide (Victoza) and once-weekly extended-release exenatide (Bydureon/Bydureon BCise).
Jardiance has been approved for adults with type 2 diabetes since 2014, and Synjardy has been approved since 2015.
“Compared to adults, children with type 2 diabetes have limited treatment options, even though the disease and symptom onset generally progress more rapidly in children,” said Michelle Carey, MD, MPH.
“Today’s approvals provide much-needed additional treatment options for children with type 2 diabetes,” added Dr. Carey, associate director for therapeutic review for the division of diabetes, lipid disorders, and obesity in the FDA’s Center for Drug Evaluation and Research.
Type 2 diabetes rising exponentially in children, mainly non-Whites
Type 2 diabetes is rising exponentially in children and adolescents in the United States.
Data from the SEARCH for Diabetes in Youth study show that the incidence of type 2 diabetes among youth rose by about 5% per year between 2002 and 2015, and it continues to rise.
A more recent study found that a doubling of cases occurred during the pandemic, with youth often presenting with more severe disease. The majority of cases are among non-White racial groups.
Safety and efficacy data for empagliflozin for children came from the Diabetes Study of Linagliptin and Empagliflozin in Children and Adolescents (DINAMO) trial. That trial included 157 patients aged 10-17 years with A1c of 7% or above. Patients were randomly assigned to receive empagliflozin 10 mg or 25 mg daily, linagliptin (a DPP-4 inhibitor) 5 mg, or placebo for 26 weeks. Over 90% were also taking metformin, 40% in combination with insulin. All patients were given diet and exercise advice.
At week 26, the children treated with empagliflozin showed an average 0.2 percentage point decrease in A1c, compared with a 0.7-point increase among those taking placebo. Use of empagliflozin was also associated with lower fasting plasma glucose levels compared with placebo.
Side effects were similar to those seen in adults except for a higher risk of hypoglycemia, regardless of other glucose-lowering therapies that were being taken.
Reduction in A1c for participants treated with linagliptin was not statistically significant in comparison with placebo. There was a numerical reduction of 0.34% (P = .2935).
“Across the lifespan, we know that people living with type 2 diabetes have a high risk for many diabetes complications, so it’s important to recognize and treat diabetes early in its course,” Lori Laffel, MD, lead investigator of the DINAMO study, said in a press release from BI.
“These findings are particularly important given the need for more therapeutic options, especially oral agents, to manage type 2 diabetes in young people as, to date, metformin [has been] the only globally available oral treatment for youth,” added Dr. Laffel, chief of the pediatric, adolescent, and young adult section at the Joslin Diabetes Center and professor of pediatrics at Harvard Medical School, Boston.
A version of this article first appeared on Medscape.com.
aged 10 years and older.
This approval represents only the second oral treatment option for children and adolescents with type 2 diabetes after metformin; the latter appears to be less effective for pediatric patients than for adults.
Injectable glucagonlike peptide–1 (GLP-1) agonists are also available for youth with type 2 diabetes. These include daily liraglutide (Victoza) and once-weekly extended-release exenatide (Bydureon/Bydureon BCise).
Jardiance has been approved for adults with type 2 diabetes since 2014, and Synjardy has been approved since 2015.
“Compared to adults, children with type 2 diabetes have limited treatment options, even though the disease and symptom onset generally progress more rapidly in children,” said Michelle Carey, MD, MPH.
“Today’s approvals provide much-needed additional treatment options for children with type 2 diabetes,” added Dr. Carey, associate director for therapeutic review for the division of diabetes, lipid disorders, and obesity in the FDA’s Center for Drug Evaluation and Research.
Type 2 diabetes rising exponentially in children, mainly non-Whites
Type 2 diabetes is rising exponentially in children and adolescents in the United States.
Data from the SEARCH for Diabetes in Youth study show that the incidence of type 2 diabetes among youth rose by about 5% per year between 2002 and 2015, and it continues to rise.
A more recent study found that a doubling of cases occurred during the pandemic, with youth often presenting with more severe disease. The majority of cases are among non-White racial groups.
Safety and efficacy data for empagliflozin for children came from the Diabetes Study of Linagliptin and Empagliflozin in Children and Adolescents (DINAMO) trial. That trial included 157 patients aged 10-17 years with A1c of 7% or above. Patients were randomly assigned to receive empagliflozin 10 mg or 25 mg daily, linagliptin (a DPP-4 inhibitor) 5 mg, or placebo for 26 weeks. Over 90% were also taking metformin, 40% in combination with insulin. All patients were given diet and exercise advice.
At week 26, the children treated with empagliflozin showed an average 0.2 percentage point decrease in A1c, compared with a 0.7-point increase among those taking placebo. Use of empagliflozin was also associated with lower fasting plasma glucose levels compared with placebo.
Side effects were similar to those seen in adults except for a higher risk of hypoglycemia, regardless of other glucose-lowering therapies that were being taken.
Reduction in A1c for participants treated with linagliptin was not statistically significant in comparison with placebo. There was a numerical reduction of 0.34% (P = .2935).
“Across the lifespan, we know that people living with type 2 diabetes have a high risk for many diabetes complications, so it’s important to recognize and treat diabetes early in its course,” Lori Laffel, MD, lead investigator of the DINAMO study, said in a press release from BI.
“These findings are particularly important given the need for more therapeutic options, especially oral agents, to manage type 2 diabetes in young people as, to date, metformin [has been] the only globally available oral treatment for youth,” added Dr. Laffel, chief of the pediatric, adolescent, and young adult section at the Joslin Diabetes Center and professor of pediatrics at Harvard Medical School, Boston.
A version of this article first appeared on Medscape.com.
aged 10 years and older.
This approval represents only the second oral treatment option for children and adolescents with type 2 diabetes after metformin; the latter appears to be less effective for pediatric patients than for adults.
Injectable glucagonlike peptide–1 (GLP-1) agonists are also available for youth with type 2 diabetes. These include daily liraglutide (Victoza) and once-weekly extended-release exenatide (Bydureon/Bydureon BCise).
Jardiance has been approved for adults with type 2 diabetes since 2014, and Synjardy has been approved since 2015.
“Compared to adults, children with type 2 diabetes have limited treatment options, even though the disease and symptom onset generally progress more rapidly in children,” said Michelle Carey, MD, MPH.
“Today’s approvals provide much-needed additional treatment options for children with type 2 diabetes,” added Dr. Carey, associate director for therapeutic review for the division of diabetes, lipid disorders, and obesity in the FDA’s Center for Drug Evaluation and Research.
Type 2 diabetes rising exponentially in children, mainly non-Whites
Type 2 diabetes is rising exponentially in children and adolescents in the United States.
Data from the SEARCH for Diabetes in Youth study show that the incidence of type 2 diabetes among youth rose by about 5% per year between 2002 and 2015, and it continues to rise.
A more recent study found that a doubling of cases occurred during the pandemic, with youth often presenting with more severe disease. The majority of cases are among non-White racial groups.
Safety and efficacy data for empagliflozin for children came from the Diabetes Study of Linagliptin and Empagliflozin in Children and Adolescents (DINAMO) trial. That trial included 157 patients aged 10-17 years with A1c of 7% or above. Patients were randomly assigned to receive empagliflozin 10 mg or 25 mg daily, linagliptin (a DPP-4 inhibitor) 5 mg, or placebo for 26 weeks. Over 90% were also taking metformin, 40% in combination with insulin. All patients were given diet and exercise advice.
At week 26, the children treated with empagliflozin showed an average 0.2 percentage point decrease in A1c, compared with a 0.7-point increase among those taking placebo. Use of empagliflozin was also associated with lower fasting plasma glucose levels compared with placebo.
Side effects were similar to those seen in adults except for a higher risk of hypoglycemia, regardless of other glucose-lowering therapies that were being taken.
Reduction in A1c for participants treated with linagliptin was not statistically significant in comparison with placebo. There was a numerical reduction of 0.34% (P = .2935).
“Across the lifespan, we know that people living with type 2 diabetes have a high risk for many diabetes complications, so it’s important to recognize and treat diabetes early in its course,” Lori Laffel, MD, lead investigator of the DINAMO study, said in a press release from BI.
“These findings are particularly important given the need for more therapeutic options, especially oral agents, to manage type 2 diabetes in young people as, to date, metformin [has been] the only globally available oral treatment for youth,” added Dr. Laffel, chief of the pediatric, adolescent, and young adult section at the Joslin Diabetes Center and professor of pediatrics at Harvard Medical School, Boston.
A version of this article first appeared on Medscape.com.
NAFLD increases risk for severe infections
People with nonalcoholic fatty liver disease (NAFLD) are more likely to develop severe infections requiring hospitalization, according to findings from a large Swedish cohort study.
The increased risk was equal to one extra severe infection in every six patients with NAFLD by 20 years after diagnosis, wrote Fahim Ebrahimi, MD, of the Karolinska Institute in Stockholm, and coauthors.
“Accumulating evidence suggests that NAFLD can affect multiple organ systems, which is not surprising, as the liver has multiple functions – regulating metabolism and being a central organ of the immune system,” Dr. Ebrahimi said in an interview.
The study was published online in Clinical Gastroenterology and Hepatology.
“Up to a fifth of cells in the liver are immune cells that process numerous antigens and pathogens from the gastrointestinal tract,” Dr. Ebrahimi noted. “We were intrigued by experimental studies showing that, in NAFLD, many of these key immune cells become dysfunctional at various levels, which may affect disease progression, but at the same time also increase the susceptibility to viral, bacterial, and fungal infections.”
Patients with NAFLD have metabolic risk factors known to increase infection risk, but a smaller study by a different research group had found that NAFLD could independently predispose patients to bacterial infections.
To further explore a connection between NAFLD and infection risk, the researchers looked at data for 12,133 Swedish adults with simple steatosis, nonfibrotic steatohepatitis, noncirrhotic fibrosis, or cirrhosis caused by NAFLD confirmed by liver biopsies performed between 1969 and 2017.
Each patient was matched to five or more contemporary controls from the general population by age, sex, and region of residence. The authors conducted an additional analysis that also adjusted for education, country of birth, and baseline clinical comorbidities, including diabetes, obesity, dyslipidemia, and hypertension, as well as hospitalization preceding the biopsy and chronic obstructive pulmonary disease.
The primary endpoint was severe infections requiring hospital admission. Secondary endpoints included seven prespecified infection subgroups: sepsis; respiratory tract; most gastrointestinal infections; bacterial peritonitis; urogenital; muscle, skin, and soft tissue; and other infections.
Elevated risk at all NAFLD stages
Dr. Ebrahimi and colleagues found that over a median follow-up of 14 years, patients with NAFLD had a higher incidence of severe infections – most often respiratory or urinary tract infections – compared with those without NAFLD (32% vs. 17%, respectively).
Biopsy-confirmed NAFLD was also associated with a 71% higher hazard and a 20-year absolute excess risk of 17.3% for severe infections requiring hospital admission versus comparators. The elevated risk showed up in patients with steatosis and increased with the severity of NAFLD. Simple steatosis saw a 64% higher risk (adjusted hazard ratio, 1.64; 95% confidence interval, 1.55-1.73), whereas patients with cirrhosis saw a more than twofold higher risk, compared with controls (aHR, 2.32; 95% CI, 1.92-2.82).
When Dr. Ebrahimi and colleagues adjusted for parameters of the metabolic syndrome, they found an independent increased risk for severe infection. For patients with NAFLD, the increased risk may come from greater susceptibility to infections in general or to a more severe course of infections.
“Our study clearly demonstrates the complexity and high disease burden associated with NAFLD,” Dr. Ebrahimi said. “We are beginning to understand the different layers involved and will eventually move away from a liver-centric view to a more holistic view of the disease.”
Clinicians caring for patients with NAFLD need to be aware of the increased risk for infection, Dr. Ebrahimi said. They also should assess their patients’ vaccination status, and seek to control modifiable risk factors, such as diabetes.
Nancy Reau, MD, of Rush University, Chicago, described the study’s message as important.
“Patients with NAFLD and advancing liver disease are at risk for severe infections,” Dr. Reau said. “When we consider the fact that patients with advanced liver disease tend to die from infectious complications, awareness leading to early recognition and efficient treatment is imperative.”
The authors acknowledged the following limitations: only severe infections requiring hospitalization could be captured; whether infection led to decompensation or vice versa among patients with cirrhosis could not be determined; and detailed data on smoking, alcohol, vaccinations, body mass, and other potentially relevant measures were not available.
The Swiss National Science Foundation, Syskonen Svensson Foundation, and Bengt Ihre Foundation provided grants to Dr. Ebrahimi or coauthors. One coauthor disclosed previous research funding from Janssen and MSD. Dr. Reau disclosed receiving research support and consulting fees from AbbVie and Gilead, as well as consulting fees from Arbutus, Intercept, and Salix.
A version of this article first appeared on Medscape.com.
People with nonalcoholic fatty liver disease (NAFLD) are more likely to develop severe infections requiring hospitalization, according to findings from a large Swedish cohort study.
The increased risk was equal to one extra severe infection in every six patients with NAFLD by 20 years after diagnosis, wrote Fahim Ebrahimi, MD, of the Karolinska Institute in Stockholm, and coauthors.
“Accumulating evidence suggests that NAFLD can affect multiple organ systems, which is not surprising, as the liver has multiple functions – regulating metabolism and being a central organ of the immune system,” Dr. Ebrahimi said in an interview.
The study was published online in Clinical Gastroenterology and Hepatology.
“Up to a fifth of cells in the liver are immune cells that process numerous antigens and pathogens from the gastrointestinal tract,” Dr. Ebrahimi noted. “We were intrigued by experimental studies showing that, in NAFLD, many of these key immune cells become dysfunctional at various levels, which may affect disease progression, but at the same time also increase the susceptibility to viral, bacterial, and fungal infections.”
Patients with NAFLD have metabolic risk factors known to increase infection risk, but a smaller study by a different research group had found that NAFLD could independently predispose patients to bacterial infections.
To further explore a connection between NAFLD and infection risk, the researchers looked at data for 12,133 Swedish adults with simple steatosis, nonfibrotic steatohepatitis, noncirrhotic fibrosis, or cirrhosis caused by NAFLD confirmed by liver biopsies performed between 1969 and 2017.
Each patient was matched to five or more contemporary controls from the general population by age, sex, and region of residence. The authors conducted an additional analysis that also adjusted for education, country of birth, and baseline clinical comorbidities, including diabetes, obesity, dyslipidemia, and hypertension, as well as hospitalization preceding the biopsy and chronic obstructive pulmonary disease.
The primary endpoint was severe infections requiring hospital admission. Secondary endpoints included seven prespecified infection subgroups: sepsis; respiratory tract; most gastrointestinal infections; bacterial peritonitis; urogenital; muscle, skin, and soft tissue; and other infections.
Elevated risk at all NAFLD stages
Dr. Ebrahimi and colleagues found that over a median follow-up of 14 years, patients with NAFLD had a higher incidence of severe infections – most often respiratory or urinary tract infections – compared with those without NAFLD (32% vs. 17%, respectively).
Biopsy-confirmed NAFLD was also associated with a 71% higher hazard and a 20-year absolute excess risk of 17.3% for severe infections requiring hospital admission versus comparators. The elevated risk showed up in patients with steatosis and increased with the severity of NAFLD. Simple steatosis saw a 64% higher risk (adjusted hazard ratio, 1.64; 95% confidence interval, 1.55-1.73), whereas patients with cirrhosis saw a more than twofold higher risk, compared with controls (aHR, 2.32; 95% CI, 1.92-2.82).
When Dr. Ebrahimi and colleagues adjusted for parameters of the metabolic syndrome, they found an independent increased risk for severe infection. For patients with NAFLD, the increased risk may come from greater susceptibility to infections in general or to a more severe course of infections.
“Our study clearly demonstrates the complexity and high disease burden associated with NAFLD,” Dr. Ebrahimi said. “We are beginning to understand the different layers involved and will eventually move away from a liver-centric view to a more holistic view of the disease.”
Clinicians caring for patients with NAFLD need to be aware of the increased risk for infection, Dr. Ebrahimi said. They also should assess their patients’ vaccination status, and seek to control modifiable risk factors, such as diabetes.
Nancy Reau, MD, of Rush University, Chicago, described the study’s message as important.
“Patients with NAFLD and advancing liver disease are at risk for severe infections,” Dr. Reau said. “When we consider the fact that patients with advanced liver disease tend to die from infectious complications, awareness leading to early recognition and efficient treatment is imperative.”
The authors acknowledged the following limitations: only severe infections requiring hospitalization could be captured; whether infection led to decompensation or vice versa among patients with cirrhosis could not be determined; and detailed data on smoking, alcohol, vaccinations, body mass, and other potentially relevant measures were not available.
The Swiss National Science Foundation, Syskonen Svensson Foundation, and Bengt Ihre Foundation provided grants to Dr. Ebrahimi or coauthors. One coauthor disclosed previous research funding from Janssen and MSD. Dr. Reau disclosed receiving research support and consulting fees from AbbVie and Gilead, as well as consulting fees from Arbutus, Intercept, and Salix.
A version of this article first appeared on Medscape.com.
People with nonalcoholic fatty liver disease (NAFLD) are more likely to develop severe infections requiring hospitalization, according to findings from a large Swedish cohort study.
The increased risk was equal to one extra severe infection in every six patients with NAFLD by 20 years after diagnosis, wrote Fahim Ebrahimi, MD, of the Karolinska Institute in Stockholm, and coauthors.
“Accumulating evidence suggests that NAFLD can affect multiple organ systems, which is not surprising, as the liver has multiple functions – regulating metabolism and being a central organ of the immune system,” Dr. Ebrahimi said in an interview.
The study was published online in Clinical Gastroenterology and Hepatology.
“Up to a fifth of cells in the liver are immune cells that process numerous antigens and pathogens from the gastrointestinal tract,” Dr. Ebrahimi noted. “We were intrigued by experimental studies showing that, in NAFLD, many of these key immune cells become dysfunctional at various levels, which may affect disease progression, but at the same time also increase the susceptibility to viral, bacterial, and fungal infections.”
Patients with NAFLD have metabolic risk factors known to increase infection risk, but a smaller study by a different research group had found that NAFLD could independently predispose patients to bacterial infections.
To further explore a connection between NAFLD and infection risk, the researchers looked at data for 12,133 Swedish adults with simple steatosis, nonfibrotic steatohepatitis, noncirrhotic fibrosis, or cirrhosis caused by NAFLD confirmed by liver biopsies performed between 1969 and 2017.
Each patient was matched to five or more contemporary controls from the general population by age, sex, and region of residence. The authors conducted an additional analysis that also adjusted for education, country of birth, and baseline clinical comorbidities, including diabetes, obesity, dyslipidemia, and hypertension, as well as hospitalization preceding the biopsy and chronic obstructive pulmonary disease.
The primary endpoint was severe infections requiring hospital admission. Secondary endpoints included seven prespecified infection subgroups: sepsis; respiratory tract; most gastrointestinal infections; bacterial peritonitis; urogenital; muscle, skin, and soft tissue; and other infections.
Elevated risk at all NAFLD stages
Dr. Ebrahimi and colleagues found that over a median follow-up of 14 years, patients with NAFLD had a higher incidence of severe infections – most often respiratory or urinary tract infections – compared with those without NAFLD (32% vs. 17%, respectively).
Biopsy-confirmed NAFLD was also associated with a 71% higher hazard and a 20-year absolute excess risk of 17.3% for severe infections requiring hospital admission versus comparators. The elevated risk showed up in patients with steatosis and increased with the severity of NAFLD. Simple steatosis saw a 64% higher risk (adjusted hazard ratio, 1.64; 95% confidence interval, 1.55-1.73), whereas patients with cirrhosis saw a more than twofold higher risk, compared with controls (aHR, 2.32; 95% CI, 1.92-2.82).
When Dr. Ebrahimi and colleagues adjusted for parameters of the metabolic syndrome, they found an independent increased risk for severe infection. For patients with NAFLD, the increased risk may come from greater susceptibility to infections in general or to a more severe course of infections.
“Our study clearly demonstrates the complexity and high disease burden associated with NAFLD,” Dr. Ebrahimi said. “We are beginning to understand the different layers involved and will eventually move away from a liver-centric view to a more holistic view of the disease.”
Clinicians caring for patients with NAFLD need to be aware of the increased risk for infection, Dr. Ebrahimi said. They also should assess their patients’ vaccination status, and seek to control modifiable risk factors, such as diabetes.
Nancy Reau, MD, of Rush University, Chicago, described the study’s message as important.
“Patients with NAFLD and advancing liver disease are at risk for severe infections,” Dr. Reau said. “When we consider the fact that patients with advanced liver disease tend to die from infectious complications, awareness leading to early recognition and efficient treatment is imperative.”
The authors acknowledged the following limitations: only severe infections requiring hospitalization could be captured; whether infection led to decompensation or vice versa among patients with cirrhosis could not be determined; and detailed data on smoking, alcohol, vaccinations, body mass, and other potentially relevant measures were not available.
The Swiss National Science Foundation, Syskonen Svensson Foundation, and Bengt Ihre Foundation provided grants to Dr. Ebrahimi or coauthors. One coauthor disclosed previous research funding from Janssen and MSD. Dr. Reau disclosed receiving research support and consulting fees from AbbVie and Gilead, as well as consulting fees from Arbutus, Intercept, and Salix.
A version of this article first appeared on Medscape.com.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Hold Ozempic before surgery to optimize patient safety?
Semaglutide and related drugs for weight loss have co-opted bariatric medicine in recent months. They have also raised serious questions for hospital-based clinicians who wonder whether the drugs may pose risks to surgery patients undergoing anesthesia.
at this point. Official guidance on best practices has not yet caught up to surging popularity of this and other glucagon-like peptide-1 (GLP-1) agonists for weight loss.
Ozempic is indicated for treating type 2 diabetes but also is prescribed off-label for weight loss. Other GLP-1 agents from Novo Nordisk, Wegovy (semaglutide) and Saxenda (liraglutide) injections, are Food and Drug Administration–approved for weight loss. These medications work by decreasing hunger and lowering how much people eat. Semaglutide also is available as a once-daily tablet for type 2 diabetes (Rybelsus).
The American Society of Anesthesiologists (ASA) has been working on guidance on the drugs. “It’s a really hot issue now. We are getting emails from our members looking for guidance,” ASA president Michael Champeau, MD, said in an interview.
But despite the interest in how the medications might affect surgery patients and interact with anesthesia, relatively little evidence exists in the literature beyond case studies. So the society is not issuing official recommendations at this point.
“We’re going to just be calling it ‘guidance’ for right now because of the paucity of the scientific literature,” said Dr. Champeau, adjunct clinical professor of anesthesiology, perioperative, and pain medicine at Stanford (Calif.) University. “It’s probably not going to have words like ‘must; it will probably have words like ‘should’ or ‘should consider.’ “
The ASA guidance could be out in written form soon, Dr. Champeau added.
Meanwhile, whether physicians should advise stopping these medications 24 hours, 48 hours, or up to 2 weeks before surgery remains unknown.
In search of some consensus, John Shields, MD, an orthopedic surgeon at Atrium Health Wake Forest Baptist Davie Medical Center in Bermuda Run, N.C., asked colleagues on #MedTwitter: “Anyone have guidelines for ozempic around time of surgery? – holding med? – how long NPO?”
Because a full stomach can interfere with anesthesia, clinicians often advise people to stop eating and drinking 12-24 hours before elective procedures (NPO). In the case of once-weekly GLP-1 injections, which can slow gastric emptying, the optimal timeframe remains an open question. The main concern is aspiration, where a patient actively vomits while under anesthesia or their stomach contents passively come back up.
Dr. Shields’ Twitter post garnered significant reaction and comments. Within 4 days, the post was retweeted 30 times and received 72 replies and comments. Dr. Shields noted the general consensus was to hold semaglutide for 1-2 weeks before a procedure. Other suggestions included recommending a liquid diet only for 24-48 hours before surgery, recommending an NPO protocol 24-36 hours in advance, or adjusting the weekly injection so the last dose is taken 5-6 days before surgery.
Anesthesiologist Cliff Gevirtz, MD, has encountered only a few surgical patients so far taking a GLP-1 for weight loss. “And thankfully no aspiration,” added Dr. Gevirtz, clinical director of office-based ambulatory anesthesia services at Somnia Anesthesia in Harrison, N.Y.
To minimize risk, some physicians will perform an ultrasound scan to assess the contents of the stomach. If surgery is elective in a patient with a full stomach, the procedure can get postponed. Another option is to proceed with the case but treat the patient as anesthesiologists approach an emergency procedure. To be safe, many will treat the case as if the patient has a full stomach.
Dr. Gevirtz said he would treat the patient as a ‘full stomach’ and perform a rapid sequence induction with cricoid pressure. He would then extubate the patient once laryngeal reflexes return.
A rapid-sequence induction involves giving the medicine that makes a patient go to sleep, giving another medicine that paralyzes them quickly, then inserting a breathing tube – all within about 30 seconds. Cricoid pressure involves pushing on the neck during intubation to try to seal off the top of the esophagus and again minimize the chances of food coming back up.
Giving metoclopramide 30 minutes before surgery is another option, Dr. Gevirtz said. Metoclopramide can hasten the emptying of stomach contents. Administration in advance is important because waiting for the drug to work can prolong time in the operating room.
Is holding semaglutide before surgery a relevant clinical question? “Yes, very much so,” said Ronnie Fass, MD, division director of gastroenterology and hepatology and the medical director of the Digestive Health Center at The MetroHealth System in Cleveland.
Dr. Fass recommended different strategies based on the semaglutide indication. Currently, clinicians at MetroHealth instruct patients to discontinue diabetic medications the day of surgery. For those who take semaglutide for diabetes, and because the medication is taken once a week, “there is growing discussion among surgeons that the medication should not be stopped prior to surgery. This is to ensure that patients’ diabetes is well controlled before and during surgery,” Dr. Fass said.
In patients taking semaglutide for weight loss only, “there is no clear answer at this point,” he said.
Dr. Fass said the question is complicated by the fact that the medication is taken once a week. “It brings up important questions about the use of the medication during surgery, which may increase the likelihood of side effects in general and for certain types of surgery. Personally, if a patient is taking [semaglutide] for weight loss only, I would consider stopping the medication before surgery.”
The ASA was able to act quickly because it already had an expert task force review how long people should fast before surgery last year – before the explosion in popularity of the GLP-1 agonists.
Although it is still a work in progress, Dr. Champeau offered “a peek” at the recommendations. “The guidance is going to look at how far in advance the drugs should be stopped, rather than looking at making people fast even longer” before surgery, he said. “There’s just no data on that latter question.”
A version of this article originally appeared on Medscape.com.
Semaglutide and related drugs for weight loss have co-opted bariatric medicine in recent months. They have also raised serious questions for hospital-based clinicians who wonder whether the drugs may pose risks to surgery patients undergoing anesthesia.
at this point. Official guidance on best practices has not yet caught up to surging popularity of this and other glucagon-like peptide-1 (GLP-1) agonists for weight loss.
Ozempic is indicated for treating type 2 diabetes but also is prescribed off-label for weight loss. Other GLP-1 agents from Novo Nordisk, Wegovy (semaglutide) and Saxenda (liraglutide) injections, are Food and Drug Administration–approved for weight loss. These medications work by decreasing hunger and lowering how much people eat. Semaglutide also is available as a once-daily tablet for type 2 diabetes (Rybelsus).
The American Society of Anesthesiologists (ASA) has been working on guidance on the drugs. “It’s a really hot issue now. We are getting emails from our members looking for guidance,” ASA president Michael Champeau, MD, said in an interview.
But despite the interest in how the medications might affect surgery patients and interact with anesthesia, relatively little evidence exists in the literature beyond case studies. So the society is not issuing official recommendations at this point.
“We’re going to just be calling it ‘guidance’ for right now because of the paucity of the scientific literature,” said Dr. Champeau, adjunct clinical professor of anesthesiology, perioperative, and pain medicine at Stanford (Calif.) University. “It’s probably not going to have words like ‘must; it will probably have words like ‘should’ or ‘should consider.’ “
The ASA guidance could be out in written form soon, Dr. Champeau added.
Meanwhile, whether physicians should advise stopping these medications 24 hours, 48 hours, or up to 2 weeks before surgery remains unknown.
In search of some consensus, John Shields, MD, an orthopedic surgeon at Atrium Health Wake Forest Baptist Davie Medical Center in Bermuda Run, N.C., asked colleagues on #MedTwitter: “Anyone have guidelines for ozempic around time of surgery? – holding med? – how long NPO?”
Because a full stomach can interfere with anesthesia, clinicians often advise people to stop eating and drinking 12-24 hours before elective procedures (NPO). In the case of once-weekly GLP-1 injections, which can slow gastric emptying, the optimal timeframe remains an open question. The main concern is aspiration, where a patient actively vomits while under anesthesia or their stomach contents passively come back up.
Dr. Shields’ Twitter post garnered significant reaction and comments. Within 4 days, the post was retweeted 30 times and received 72 replies and comments. Dr. Shields noted the general consensus was to hold semaglutide for 1-2 weeks before a procedure. Other suggestions included recommending a liquid diet only for 24-48 hours before surgery, recommending an NPO protocol 24-36 hours in advance, or adjusting the weekly injection so the last dose is taken 5-6 days before surgery.
Anesthesiologist Cliff Gevirtz, MD, has encountered only a few surgical patients so far taking a GLP-1 for weight loss. “And thankfully no aspiration,” added Dr. Gevirtz, clinical director of office-based ambulatory anesthesia services at Somnia Anesthesia in Harrison, N.Y.
To minimize risk, some physicians will perform an ultrasound scan to assess the contents of the stomach. If surgery is elective in a patient with a full stomach, the procedure can get postponed. Another option is to proceed with the case but treat the patient as anesthesiologists approach an emergency procedure. To be safe, many will treat the case as if the patient has a full stomach.
Dr. Gevirtz said he would treat the patient as a ‘full stomach’ and perform a rapid sequence induction with cricoid pressure. He would then extubate the patient once laryngeal reflexes return.
A rapid-sequence induction involves giving the medicine that makes a patient go to sleep, giving another medicine that paralyzes them quickly, then inserting a breathing tube – all within about 30 seconds. Cricoid pressure involves pushing on the neck during intubation to try to seal off the top of the esophagus and again minimize the chances of food coming back up.
Giving metoclopramide 30 minutes before surgery is another option, Dr. Gevirtz said. Metoclopramide can hasten the emptying of stomach contents. Administration in advance is important because waiting for the drug to work can prolong time in the operating room.
Is holding semaglutide before surgery a relevant clinical question? “Yes, very much so,” said Ronnie Fass, MD, division director of gastroenterology and hepatology and the medical director of the Digestive Health Center at The MetroHealth System in Cleveland.
Dr. Fass recommended different strategies based on the semaglutide indication. Currently, clinicians at MetroHealth instruct patients to discontinue diabetic medications the day of surgery. For those who take semaglutide for diabetes, and because the medication is taken once a week, “there is growing discussion among surgeons that the medication should not be stopped prior to surgery. This is to ensure that patients’ diabetes is well controlled before and during surgery,” Dr. Fass said.
In patients taking semaglutide for weight loss only, “there is no clear answer at this point,” he said.
Dr. Fass said the question is complicated by the fact that the medication is taken once a week. “It brings up important questions about the use of the medication during surgery, which may increase the likelihood of side effects in general and for certain types of surgery. Personally, if a patient is taking [semaglutide] for weight loss only, I would consider stopping the medication before surgery.”
The ASA was able to act quickly because it already had an expert task force review how long people should fast before surgery last year – before the explosion in popularity of the GLP-1 agonists.
Although it is still a work in progress, Dr. Champeau offered “a peek” at the recommendations. “The guidance is going to look at how far in advance the drugs should be stopped, rather than looking at making people fast even longer” before surgery, he said. “There’s just no data on that latter question.”
A version of this article originally appeared on Medscape.com.
Semaglutide and related drugs for weight loss have co-opted bariatric medicine in recent months. They have also raised serious questions for hospital-based clinicians who wonder whether the drugs may pose risks to surgery patients undergoing anesthesia.
at this point. Official guidance on best practices has not yet caught up to surging popularity of this and other glucagon-like peptide-1 (GLP-1) agonists for weight loss.
Ozempic is indicated for treating type 2 diabetes but also is prescribed off-label for weight loss. Other GLP-1 agents from Novo Nordisk, Wegovy (semaglutide) and Saxenda (liraglutide) injections, are Food and Drug Administration–approved for weight loss. These medications work by decreasing hunger and lowering how much people eat. Semaglutide also is available as a once-daily tablet for type 2 diabetes (Rybelsus).
The American Society of Anesthesiologists (ASA) has been working on guidance on the drugs. “It’s a really hot issue now. We are getting emails from our members looking for guidance,” ASA president Michael Champeau, MD, said in an interview.
But despite the interest in how the medications might affect surgery patients and interact with anesthesia, relatively little evidence exists in the literature beyond case studies. So the society is not issuing official recommendations at this point.
“We’re going to just be calling it ‘guidance’ for right now because of the paucity of the scientific literature,” said Dr. Champeau, adjunct clinical professor of anesthesiology, perioperative, and pain medicine at Stanford (Calif.) University. “It’s probably not going to have words like ‘must; it will probably have words like ‘should’ or ‘should consider.’ “
The ASA guidance could be out in written form soon, Dr. Champeau added.
Meanwhile, whether physicians should advise stopping these medications 24 hours, 48 hours, or up to 2 weeks before surgery remains unknown.
In search of some consensus, John Shields, MD, an orthopedic surgeon at Atrium Health Wake Forest Baptist Davie Medical Center in Bermuda Run, N.C., asked colleagues on #MedTwitter: “Anyone have guidelines for ozempic around time of surgery? – holding med? – how long NPO?”
Because a full stomach can interfere with anesthesia, clinicians often advise people to stop eating and drinking 12-24 hours before elective procedures (NPO). In the case of once-weekly GLP-1 injections, which can slow gastric emptying, the optimal timeframe remains an open question. The main concern is aspiration, where a patient actively vomits while under anesthesia or their stomach contents passively come back up.
Dr. Shields’ Twitter post garnered significant reaction and comments. Within 4 days, the post was retweeted 30 times and received 72 replies and comments. Dr. Shields noted the general consensus was to hold semaglutide for 1-2 weeks before a procedure. Other suggestions included recommending a liquid diet only for 24-48 hours before surgery, recommending an NPO protocol 24-36 hours in advance, or adjusting the weekly injection so the last dose is taken 5-6 days before surgery.
Anesthesiologist Cliff Gevirtz, MD, has encountered only a few surgical patients so far taking a GLP-1 for weight loss. “And thankfully no aspiration,” added Dr. Gevirtz, clinical director of office-based ambulatory anesthesia services at Somnia Anesthesia in Harrison, N.Y.
To minimize risk, some physicians will perform an ultrasound scan to assess the contents of the stomach. If surgery is elective in a patient with a full stomach, the procedure can get postponed. Another option is to proceed with the case but treat the patient as anesthesiologists approach an emergency procedure. To be safe, many will treat the case as if the patient has a full stomach.
Dr. Gevirtz said he would treat the patient as a ‘full stomach’ and perform a rapid sequence induction with cricoid pressure. He would then extubate the patient once laryngeal reflexes return.
A rapid-sequence induction involves giving the medicine that makes a patient go to sleep, giving another medicine that paralyzes them quickly, then inserting a breathing tube – all within about 30 seconds. Cricoid pressure involves pushing on the neck during intubation to try to seal off the top of the esophagus and again minimize the chances of food coming back up.
Giving metoclopramide 30 minutes before surgery is another option, Dr. Gevirtz said. Metoclopramide can hasten the emptying of stomach contents. Administration in advance is important because waiting for the drug to work can prolong time in the operating room.
Is holding semaglutide before surgery a relevant clinical question? “Yes, very much so,” said Ronnie Fass, MD, division director of gastroenterology and hepatology and the medical director of the Digestive Health Center at The MetroHealth System in Cleveland.
Dr. Fass recommended different strategies based on the semaglutide indication. Currently, clinicians at MetroHealth instruct patients to discontinue diabetic medications the day of surgery. For those who take semaglutide for diabetes, and because the medication is taken once a week, “there is growing discussion among surgeons that the medication should not be stopped prior to surgery. This is to ensure that patients’ diabetes is well controlled before and during surgery,” Dr. Fass said.
In patients taking semaglutide for weight loss only, “there is no clear answer at this point,” he said.
Dr. Fass said the question is complicated by the fact that the medication is taken once a week. “It brings up important questions about the use of the medication during surgery, which may increase the likelihood of side effects in general and for certain types of surgery. Personally, if a patient is taking [semaglutide] for weight loss only, I would consider stopping the medication before surgery.”
The ASA was able to act quickly because it already had an expert task force review how long people should fast before surgery last year – before the explosion in popularity of the GLP-1 agonists.
Although it is still a work in progress, Dr. Champeau offered “a peek” at the recommendations. “The guidance is going to look at how far in advance the drugs should be stopped, rather than looking at making people fast even longer” before surgery, he said. “There’s just no data on that latter question.”
A version of this article originally appeared on Medscape.com.
A ‘one-stop shop’: New guidance on hormones and aging
The idea of the statement “is to be complete, but also to clarify some misunderstandings. ...We tried to be very clear in the language about what we know, where we can go, where we shouldn’t go, and what we still need to learn,” statement coauthor Cynthia A. Stuenkel, MD, of the University of California, San Diego, said in an interview.
The document is divided into nine parts or axes: growth hormone, adrenal, ovarian, testicular, thyroid, osteoporosis, vitamin D deficiency, type 2 diabetes, and water metabolism. Each section covers natural history and observational data in older individuals, available therapies, clinical trial data on efficacy and safety in older individuals, bulleted “key points,” and research gaps.
“Hormones and Aging: An Endocrine Society Scientific Statement” was presented at the annual meeting of the Endocrine Society and published online in the Journal of Clinical Endocrinology & Metabolism.
During a press briefing, writing group chair Anne R. Cappola, MD, of the University of Pennsylvania, Philadelphia, said the goal is to “provide a really concise summary across each of these areas. ... There are multiple hormonal changes that occur with age, so we really couldn’t limit ourselves to just one gland or the few that we commonly think about. We wanted to cover all the axes.”
The statement tackles several controversial areas, including hormone therapy for menopausal symptoms in women and hypogonadal symptoms in men, diabetes treatment goals in older adults, distinguishing between age-associated changes in thyroid function and early hypothyroidism, and vitamin D supplementation in older adults.
“Hormones have these almost mythical qualities to some people. ... ‘If I just had my hormones back the way they were, it would all work out.’ What we want to do is make sure that patients are being treated appropriately and that their symptoms are being heard and managed and ascribed to the appropriate problems and not necessarily to hormonal problems when they are not. ... Part of what we need to do is [provide] the evidence that we have, which includes evidence of when not to prescribe as well as [when] to prescribe,” Dr. Cappola said.
Not designed to be read all at once
In the menopause section, for example, one “key point” is that menopausal symptoms are common, vary in degree and bother, and can be effectively treated with a variety of therapies proven effective in randomized clinical trials. Another key point is that menopausal hormone therapy is safest for women who are younger than 60 years and less than 10 years since starting menopause.
“It’s almost 20 years since the original Women’s Health Initiative, and that led to an incredible falloff of prescribing hormone therapy and a falloff in teaching of our students, residents, fellows, and practitioners about [menopausal] hormone therapy. ... Hopefully, by issuing this kind of aging statement it gets people to read, think, and learn more. And, hopefully, we can improve the education of physicians. ... Menopause is a universal experience. Clinicians should know about it,” noted Dr. Stuenkel, who chaired the menopause section writing panel.
In the type 2 diabetes section, in the bullet points it is noted that oral glucose tolerance testing may reveal abnormal glucose status in older adults that are not picked up with hemoglobin A1c or fasting glucose levels and that glycemic targets should be individualized.
Asked to comment on the statement, Michele Bellantoni, MD, said: “This was a huge undertaking because there are so many areas of expertise here. I thought they did a very good job of reviewing the literature and showing each of the different hormonal axes. ... It’s a good go-to review.”
“I thought it was a very good attempt to catalog and provide opportunities for policy, and particularly at [the National Institutes of Health], as they look at funding to show where are these gaps and to support appropriate research. I think the most important aspect to come of this is identifying research gaps for funding opportunities. I very much support that,” noted Dr. Bellantoni, who is clinical director of the division of geriatric medicine at Johns Hopkins University, Baltimore.
However, she also said that the 40-page document might be a bit much for busy clinicians, despite the bullet points at the end of each section.
“I would love to see an editorial that puts into perspective the take-home messages or a subsequent article that distills this into every day practice of care of older adults, both preventative and treatment care. ... I think that would be so useful.”
During the briefing, Dr. Cappola noted that the document need not be read all at once.
“It ended up being a large document, but you should not be intimidated by it because each section is only about 2,000 words. So, it’s really a kind of one-stop shop to be able to look across all these axes at once. We also wanted people to think about the common themes that occur across all these axes when considering what’s going on right now and for future research,” she said.
Dr. Stuenkel, Dr. Cappola, and Dr. Bellantoni reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The idea of the statement “is to be complete, but also to clarify some misunderstandings. ...We tried to be very clear in the language about what we know, where we can go, where we shouldn’t go, and what we still need to learn,” statement coauthor Cynthia A. Stuenkel, MD, of the University of California, San Diego, said in an interview.
The document is divided into nine parts or axes: growth hormone, adrenal, ovarian, testicular, thyroid, osteoporosis, vitamin D deficiency, type 2 diabetes, and water metabolism. Each section covers natural history and observational data in older individuals, available therapies, clinical trial data on efficacy and safety in older individuals, bulleted “key points,” and research gaps.
“Hormones and Aging: An Endocrine Society Scientific Statement” was presented at the annual meeting of the Endocrine Society and published online in the Journal of Clinical Endocrinology & Metabolism.
During a press briefing, writing group chair Anne R. Cappola, MD, of the University of Pennsylvania, Philadelphia, said the goal is to “provide a really concise summary across each of these areas. ... There are multiple hormonal changes that occur with age, so we really couldn’t limit ourselves to just one gland or the few that we commonly think about. We wanted to cover all the axes.”
The statement tackles several controversial areas, including hormone therapy for menopausal symptoms in women and hypogonadal symptoms in men, diabetes treatment goals in older adults, distinguishing between age-associated changes in thyroid function and early hypothyroidism, and vitamin D supplementation in older adults.
“Hormones have these almost mythical qualities to some people. ... ‘If I just had my hormones back the way they were, it would all work out.’ What we want to do is make sure that patients are being treated appropriately and that their symptoms are being heard and managed and ascribed to the appropriate problems and not necessarily to hormonal problems when they are not. ... Part of what we need to do is [provide] the evidence that we have, which includes evidence of when not to prescribe as well as [when] to prescribe,” Dr. Cappola said.
Not designed to be read all at once
In the menopause section, for example, one “key point” is that menopausal symptoms are common, vary in degree and bother, and can be effectively treated with a variety of therapies proven effective in randomized clinical trials. Another key point is that menopausal hormone therapy is safest for women who are younger than 60 years and less than 10 years since starting menopause.
“It’s almost 20 years since the original Women’s Health Initiative, and that led to an incredible falloff of prescribing hormone therapy and a falloff in teaching of our students, residents, fellows, and practitioners about [menopausal] hormone therapy. ... Hopefully, by issuing this kind of aging statement it gets people to read, think, and learn more. And, hopefully, we can improve the education of physicians. ... Menopause is a universal experience. Clinicians should know about it,” noted Dr. Stuenkel, who chaired the menopause section writing panel.
In the type 2 diabetes section, in the bullet points it is noted that oral glucose tolerance testing may reveal abnormal glucose status in older adults that are not picked up with hemoglobin A1c or fasting glucose levels and that glycemic targets should be individualized.
Asked to comment on the statement, Michele Bellantoni, MD, said: “This was a huge undertaking because there are so many areas of expertise here. I thought they did a very good job of reviewing the literature and showing each of the different hormonal axes. ... It’s a good go-to review.”
“I thought it was a very good attempt to catalog and provide opportunities for policy, and particularly at [the National Institutes of Health], as they look at funding to show where are these gaps and to support appropriate research. I think the most important aspect to come of this is identifying research gaps for funding opportunities. I very much support that,” noted Dr. Bellantoni, who is clinical director of the division of geriatric medicine at Johns Hopkins University, Baltimore.
However, she also said that the 40-page document might be a bit much for busy clinicians, despite the bullet points at the end of each section.
“I would love to see an editorial that puts into perspective the take-home messages or a subsequent article that distills this into every day practice of care of older adults, both preventative and treatment care. ... I think that would be so useful.”
During the briefing, Dr. Cappola noted that the document need not be read all at once.
“It ended up being a large document, but you should not be intimidated by it because each section is only about 2,000 words. So, it’s really a kind of one-stop shop to be able to look across all these axes at once. We also wanted people to think about the common themes that occur across all these axes when considering what’s going on right now and for future research,” she said.
Dr. Stuenkel, Dr. Cappola, and Dr. Bellantoni reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The idea of the statement “is to be complete, but also to clarify some misunderstandings. ...We tried to be very clear in the language about what we know, where we can go, where we shouldn’t go, and what we still need to learn,” statement coauthor Cynthia A. Stuenkel, MD, of the University of California, San Diego, said in an interview.
The document is divided into nine parts or axes: growth hormone, adrenal, ovarian, testicular, thyroid, osteoporosis, vitamin D deficiency, type 2 diabetes, and water metabolism. Each section covers natural history and observational data in older individuals, available therapies, clinical trial data on efficacy and safety in older individuals, bulleted “key points,” and research gaps.
“Hormones and Aging: An Endocrine Society Scientific Statement” was presented at the annual meeting of the Endocrine Society and published online in the Journal of Clinical Endocrinology & Metabolism.
During a press briefing, writing group chair Anne R. Cappola, MD, of the University of Pennsylvania, Philadelphia, said the goal is to “provide a really concise summary across each of these areas. ... There are multiple hormonal changes that occur with age, so we really couldn’t limit ourselves to just one gland or the few that we commonly think about. We wanted to cover all the axes.”
The statement tackles several controversial areas, including hormone therapy for menopausal symptoms in women and hypogonadal symptoms in men, diabetes treatment goals in older adults, distinguishing between age-associated changes in thyroid function and early hypothyroidism, and vitamin D supplementation in older adults.
“Hormones have these almost mythical qualities to some people. ... ‘If I just had my hormones back the way they were, it would all work out.’ What we want to do is make sure that patients are being treated appropriately and that their symptoms are being heard and managed and ascribed to the appropriate problems and not necessarily to hormonal problems when they are not. ... Part of what we need to do is [provide] the evidence that we have, which includes evidence of when not to prescribe as well as [when] to prescribe,” Dr. Cappola said.
Not designed to be read all at once
In the menopause section, for example, one “key point” is that menopausal symptoms are common, vary in degree and bother, and can be effectively treated with a variety of therapies proven effective in randomized clinical trials. Another key point is that menopausal hormone therapy is safest for women who are younger than 60 years and less than 10 years since starting menopause.
“It’s almost 20 years since the original Women’s Health Initiative, and that led to an incredible falloff of prescribing hormone therapy and a falloff in teaching of our students, residents, fellows, and practitioners about [menopausal] hormone therapy. ... Hopefully, by issuing this kind of aging statement it gets people to read, think, and learn more. And, hopefully, we can improve the education of physicians. ... Menopause is a universal experience. Clinicians should know about it,” noted Dr. Stuenkel, who chaired the menopause section writing panel.
In the type 2 diabetes section, in the bullet points it is noted that oral glucose tolerance testing may reveal abnormal glucose status in older adults that are not picked up with hemoglobin A1c or fasting glucose levels and that glycemic targets should be individualized.
Asked to comment on the statement, Michele Bellantoni, MD, said: “This was a huge undertaking because there are so many areas of expertise here. I thought they did a very good job of reviewing the literature and showing each of the different hormonal axes. ... It’s a good go-to review.”
“I thought it was a very good attempt to catalog and provide opportunities for policy, and particularly at [the National Institutes of Health], as they look at funding to show where are these gaps and to support appropriate research. I think the most important aspect to come of this is identifying research gaps for funding opportunities. I very much support that,” noted Dr. Bellantoni, who is clinical director of the division of geriatric medicine at Johns Hopkins University, Baltimore.
However, she also said that the 40-page document might be a bit much for busy clinicians, despite the bullet points at the end of each section.
“I would love to see an editorial that puts into perspective the take-home messages or a subsequent article that distills this into every day practice of care of older adults, both preventative and treatment care. ... I think that would be so useful.”
During the briefing, Dr. Cappola noted that the document need not be read all at once.
“It ended up being a large document, but you should not be intimidated by it because each section is only about 2,000 words. So, it’s really a kind of one-stop shop to be able to look across all these axes at once. We also wanted people to think about the common themes that occur across all these axes when considering what’s going on right now and for future research,” she said.
Dr. Stuenkel, Dr. Cappola, and Dr. Bellantoni reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ENDO 2023
Depression drives metabolic syndrome
Previous research has established a connection between metabolic syndrome and depression, but data on the increased risk for depressed individuals to develop metabolic syndrome (MetS) are lacking, wrote Lara Onofre Ferriani, PhD, of Federal University of Espírito Santo, Vitoria, Brazil, and colleagues.
“Individuals with MetS and depression have increased levels of inflammatory markers, and it is speculated that inflammation could mediate this comorbidity,” they said.
In a study published in the Journal of Psychiatric Research, the investigators reviewed data from 13,883 participants in the Brazilian Longitudinal Study of Adult Health; all were civil servants at universities in Brazil. The participants ranged from 35 to 74 years of age, with a mean age of 51.9 years; 54.3% were women; and 52.4% were white; the mean follow-up period was 3.8 years.
The primary outcome was the association between depression diagnosis and severity on components of MetS at baseline and over a 4-year period. Participants were classified by MetS trajectory as recovered, incident, or persistent, and classified by depression status as without depression or with a mild, moderate, or severe current depressive episode. Depression status was based on the Clinical Interview Schedule Revised. MetS components and diagnosis were based on the National Cholesterol Education Program Adult Treatment Panel III.
In a logistic regression analysis, baseline depression was positively associated with recovered, incident, and persistent MetS (odds ratios, 1.59, 1.45, and 1.70, respectively).
Depression at baseline also was significantly associated with separate components of MetS: large waist circumference, high triglycerides, low high-density lipoprotein cholesterol, and hyperglycemia, with odds ratios of 1.47, 1.23, 1.30, and 1.38, respectively.
Although not seen at baseline, a significant positive association between baseline depression and the presence of three or more MetS components was noted at follow-up, with a positive dose-response effect, the researchers wrote in their discussion.
Not all associations were statistically significant, but this was mainly because of the small number of cases of moderate and severe depression, they said. However, the magnitude of associations was greater in severe depression, when compared with moderate and mild, which suggests that the risk of MetS may be higher in this population, they added.
The study findings were limited by several factors including the possible misclassification of depression, inability to differentiate among depressive subtypes, and the potential lack of generalizability to other populations beyond Brazilian civil servants, the researchers noted.
However, the results were strengthened by the large sample size and support the role of depression as a risk factor for MetS, they said. More research is needed to determine a bidirectional relationship and to assess the trajectory of depression after MetS develops, but the findings “highlight the need to investigate and manage metabolic and cardiovascular alterations in the presence of depression in clinical settings,” they concluded.
The study was supported by the Brazilian Ministry of Health (Science and Technology Department) and the Brazilian Ministry of Science, Technology and Innovation FINEP and CNPq, and by the Coordenaçaõ de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES). The researchers had no financial conflicts to disclose.
Previous research has established a connection between metabolic syndrome and depression, but data on the increased risk for depressed individuals to develop metabolic syndrome (MetS) are lacking, wrote Lara Onofre Ferriani, PhD, of Federal University of Espírito Santo, Vitoria, Brazil, and colleagues.
“Individuals with MetS and depression have increased levels of inflammatory markers, and it is speculated that inflammation could mediate this comorbidity,” they said.
In a study published in the Journal of Psychiatric Research, the investigators reviewed data from 13,883 participants in the Brazilian Longitudinal Study of Adult Health; all were civil servants at universities in Brazil. The participants ranged from 35 to 74 years of age, with a mean age of 51.9 years; 54.3% were women; and 52.4% were white; the mean follow-up period was 3.8 years.
The primary outcome was the association between depression diagnosis and severity on components of MetS at baseline and over a 4-year period. Participants were classified by MetS trajectory as recovered, incident, or persistent, and classified by depression status as without depression or with a mild, moderate, or severe current depressive episode. Depression status was based on the Clinical Interview Schedule Revised. MetS components and diagnosis were based on the National Cholesterol Education Program Adult Treatment Panel III.
In a logistic regression analysis, baseline depression was positively associated with recovered, incident, and persistent MetS (odds ratios, 1.59, 1.45, and 1.70, respectively).
Depression at baseline also was significantly associated with separate components of MetS: large waist circumference, high triglycerides, low high-density lipoprotein cholesterol, and hyperglycemia, with odds ratios of 1.47, 1.23, 1.30, and 1.38, respectively.
Although not seen at baseline, a significant positive association between baseline depression and the presence of three or more MetS components was noted at follow-up, with a positive dose-response effect, the researchers wrote in their discussion.
Not all associations were statistically significant, but this was mainly because of the small number of cases of moderate and severe depression, they said. However, the magnitude of associations was greater in severe depression, when compared with moderate and mild, which suggests that the risk of MetS may be higher in this population, they added.
The study findings were limited by several factors including the possible misclassification of depression, inability to differentiate among depressive subtypes, and the potential lack of generalizability to other populations beyond Brazilian civil servants, the researchers noted.
However, the results were strengthened by the large sample size and support the role of depression as a risk factor for MetS, they said. More research is needed to determine a bidirectional relationship and to assess the trajectory of depression after MetS develops, but the findings “highlight the need to investigate and manage metabolic and cardiovascular alterations in the presence of depression in clinical settings,” they concluded.
The study was supported by the Brazilian Ministry of Health (Science and Technology Department) and the Brazilian Ministry of Science, Technology and Innovation FINEP and CNPq, and by the Coordenaçaõ de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES). The researchers had no financial conflicts to disclose.
Previous research has established a connection between metabolic syndrome and depression, but data on the increased risk for depressed individuals to develop metabolic syndrome (MetS) are lacking, wrote Lara Onofre Ferriani, PhD, of Federal University of Espírito Santo, Vitoria, Brazil, and colleagues.
“Individuals with MetS and depression have increased levels of inflammatory markers, and it is speculated that inflammation could mediate this comorbidity,” they said.
In a study published in the Journal of Psychiatric Research, the investigators reviewed data from 13,883 participants in the Brazilian Longitudinal Study of Adult Health; all were civil servants at universities in Brazil. The participants ranged from 35 to 74 years of age, with a mean age of 51.9 years; 54.3% were women; and 52.4% were white; the mean follow-up period was 3.8 years.
The primary outcome was the association between depression diagnosis and severity on components of MetS at baseline and over a 4-year period. Participants were classified by MetS trajectory as recovered, incident, or persistent, and classified by depression status as without depression or with a mild, moderate, or severe current depressive episode. Depression status was based on the Clinical Interview Schedule Revised. MetS components and diagnosis were based on the National Cholesterol Education Program Adult Treatment Panel III.
In a logistic regression analysis, baseline depression was positively associated with recovered, incident, and persistent MetS (odds ratios, 1.59, 1.45, and 1.70, respectively).
Depression at baseline also was significantly associated with separate components of MetS: large waist circumference, high triglycerides, low high-density lipoprotein cholesterol, and hyperglycemia, with odds ratios of 1.47, 1.23, 1.30, and 1.38, respectively.
Although not seen at baseline, a significant positive association between baseline depression and the presence of three or more MetS components was noted at follow-up, with a positive dose-response effect, the researchers wrote in their discussion.
Not all associations were statistically significant, but this was mainly because of the small number of cases of moderate and severe depression, they said. However, the magnitude of associations was greater in severe depression, when compared with moderate and mild, which suggests that the risk of MetS may be higher in this population, they added.
The study findings were limited by several factors including the possible misclassification of depression, inability to differentiate among depressive subtypes, and the potential lack of generalizability to other populations beyond Brazilian civil servants, the researchers noted.
However, the results were strengthened by the large sample size and support the role of depression as a risk factor for MetS, they said. More research is needed to determine a bidirectional relationship and to assess the trajectory of depression after MetS develops, but the findings “highlight the need to investigate and manage metabolic and cardiovascular alterations in the presence of depression in clinical settings,” they concluded.
The study was supported by the Brazilian Ministry of Health (Science and Technology Department) and the Brazilian Ministry of Science, Technology and Innovation FINEP and CNPq, and by the Coordenaçaõ de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES). The researchers had no financial conflicts to disclose.
FROM THE JOURNAL OF PSYCHIATRIC RESEARCH
Could semaglutide treat addiction as well as obesity?
As demand for semaglutide for weight loss grew following approval of Wegovy by the U.S. Food and Drug Administration in 2021, anecdotal reports of unexpected potential added benefits also began to surface.
Some patients taking these drugs for type 2 diabetes or weight loss also lost interest in addictive and compulsive behaviors such as drinking alcohol, smoking, shopping, nail biting, and skin picking, as reported in articles in the New York Times and The Atlantic, among others.
There is also some preliminary research to support these observations.
This news organization invited three experts to weigh in.
Recent and upcoming studies
The senior author of a recent randomized controlled trial of 127 patients with alcohol use disorder (AUD), Anders Fink-Jensen, MD, said: “I hope that GLP-1 analogs in the future can be used against AUD, but before that can happen, several GLP-1 trials [are needed to] prove an effect on alcohol intake.”
His study involved patients who received exenatide (Byetta, Bydureon, AstraZeneca), the first-generation GLP-1 agonist approved for type 2 diabetes, over 26 weeks, but treatment did not reduce the number of heavy drinking days (the primary outcome), compared with placebo.
However, in post hoc, exploratory analyses, heavy drinking days and total alcohol intake were significantly reduced in the subgroup of patients with AUD and obesity (body mass index > 30 kg/m2).
The participants were also shown pictures of alcohol or neutral subjects while they underwent functional magnetic resonance imaging. Those who had received exenatide, compared with placebo, had significantly less activation of brain reward centers when shown the pictures of alcohol.
“Something is happening in the brain and activation of the reward center is hampered by the GLP-1 compound,” Dr. Fink-Jensen, a clinical psychologist at the Psychiatric Centre Copenhagen, remarked in an email.
“If patients with AUD already fulfill the criteria for semaglutide (or other GLP-1 analogs) by having type 2 diabetes and/or a BMI over 30 kg/m2, they can of course use the compound right now,” he noted.
His team is also beginning a study in patients with AUD and a BMI ≥ 30 kg/m2 to investigate the effects on alcohol intake of semaglutide up to 2.4 mg weekly, the maximum dose currently approved for obesity in the United States.
“Based on the potency of exenatide and semaglutide,” Dr. Fink-Jensen said, “we expect that semaglutide will cause a stronger reduction in alcohol intake” than exenatide.
Animal studies have also shown that GLP-1 agonists suppress alcohol-induced reward, alcohol intake, motivation to consume alcohol, alcohol seeking, and relapse drinking of alcohol, Elisabet Jerlhag Holm, PhD, noted.
Interestingly, these agents also suppress the reward, intake, and motivation to consume other addictive drugs like cocaine, amphetamine, nicotine, and some opioids, Jerlhag Holm, professor, department of pharmacology, University of Gothenburg, Sweden, noted in an email.
In a recently published preclinical study, her group provides evidence to help explain anecdotal reports from patients with obesity treated with semaglutide who claim they also reduced their alcohol intake. In the study, semaglutide both reduced alcohol intake (and relapse-like drinking) and decreased body weight of rats of both sexes.
“Future research should explore the possibility of semaglutide decreasing alcohol intake in patients with AUD, particularly those who are overweight,” said Prof. Holm.
“AUD is a heterogenous disorder, and one medication is most likely not helpful for all AUD patients,” she added. “Therefore, an arsenal of different medications is beneficial when treating AUD.”
Janice J. Hwang, MD, MHS, echoed these thoughts: “Anecdotally, there are a lot of reports from patients (and in the news) that this class of medication [GLP-1 agonists] impacts cravings and could impact addictive behaviors.”
“I would say, overall, the jury is still out,” as to whether anecdotal reports of GLP-1 agonists curbing addictions will be borne out in randomized controlled trials.
“I think it is much too early to tell” whether these drugs might be approved for treating addictions without more solid clinical trial data, noted Dr. Hwang, who is an associate professor of medicine and chief, division of endocrinology and metabolism, at the University of North Carolina at Chapel Hill.
Meanwhile, another research group at the University of North Carolina at Chapel Hill, led by psychiatrist Christian Hendershot, PhD, is conducting a clinical trial in 48 participants with AUD who are also smokers.
They aim to determine if patients who receive semaglutide at escalating doses (0.25 mg to 1.0 mg per week via subcutaneous injection) over 9 weeks will consume less alcohol (the primary outcome) and smoke less (a secondary outcome) than those who receive a sham placebo injection. Results are expected in October 2023.
Dr. Fink-Jensen has received an unrestricted research grant from Novo Nordisk to investigate the effects of GLP-1 receptor stimulation on weight gain and metabolic disturbances in patients with schizophrenia treated with an antipsychotic.
A version of this article first appeared on Medscape.com.
As demand for semaglutide for weight loss grew following approval of Wegovy by the U.S. Food and Drug Administration in 2021, anecdotal reports of unexpected potential added benefits also began to surface.
Some patients taking these drugs for type 2 diabetes or weight loss also lost interest in addictive and compulsive behaviors such as drinking alcohol, smoking, shopping, nail biting, and skin picking, as reported in articles in the New York Times and The Atlantic, among others.
There is also some preliminary research to support these observations.
This news organization invited three experts to weigh in.
Recent and upcoming studies
The senior author of a recent randomized controlled trial of 127 patients with alcohol use disorder (AUD), Anders Fink-Jensen, MD, said: “I hope that GLP-1 analogs in the future can be used against AUD, but before that can happen, several GLP-1 trials [are needed to] prove an effect on alcohol intake.”
His study involved patients who received exenatide (Byetta, Bydureon, AstraZeneca), the first-generation GLP-1 agonist approved for type 2 diabetes, over 26 weeks, but treatment did not reduce the number of heavy drinking days (the primary outcome), compared with placebo.
However, in post hoc, exploratory analyses, heavy drinking days and total alcohol intake were significantly reduced in the subgroup of patients with AUD and obesity (body mass index > 30 kg/m2).
The participants were also shown pictures of alcohol or neutral subjects while they underwent functional magnetic resonance imaging. Those who had received exenatide, compared with placebo, had significantly less activation of brain reward centers when shown the pictures of alcohol.
“Something is happening in the brain and activation of the reward center is hampered by the GLP-1 compound,” Dr. Fink-Jensen, a clinical psychologist at the Psychiatric Centre Copenhagen, remarked in an email.
“If patients with AUD already fulfill the criteria for semaglutide (or other GLP-1 analogs) by having type 2 diabetes and/or a BMI over 30 kg/m2, they can of course use the compound right now,” he noted.
His team is also beginning a study in patients with AUD and a BMI ≥ 30 kg/m2 to investigate the effects on alcohol intake of semaglutide up to 2.4 mg weekly, the maximum dose currently approved for obesity in the United States.
“Based on the potency of exenatide and semaglutide,” Dr. Fink-Jensen said, “we expect that semaglutide will cause a stronger reduction in alcohol intake” than exenatide.
Animal studies have also shown that GLP-1 agonists suppress alcohol-induced reward, alcohol intake, motivation to consume alcohol, alcohol seeking, and relapse drinking of alcohol, Elisabet Jerlhag Holm, PhD, noted.
Interestingly, these agents also suppress the reward, intake, and motivation to consume other addictive drugs like cocaine, amphetamine, nicotine, and some opioids, Jerlhag Holm, professor, department of pharmacology, University of Gothenburg, Sweden, noted in an email.
In a recently published preclinical study, her group provides evidence to help explain anecdotal reports from patients with obesity treated with semaglutide who claim they also reduced their alcohol intake. In the study, semaglutide both reduced alcohol intake (and relapse-like drinking) and decreased body weight of rats of both sexes.
“Future research should explore the possibility of semaglutide decreasing alcohol intake in patients with AUD, particularly those who are overweight,” said Prof. Holm.
“AUD is a heterogenous disorder, and one medication is most likely not helpful for all AUD patients,” she added. “Therefore, an arsenal of different medications is beneficial when treating AUD.”
Janice J. Hwang, MD, MHS, echoed these thoughts: “Anecdotally, there are a lot of reports from patients (and in the news) that this class of medication [GLP-1 agonists] impacts cravings and could impact addictive behaviors.”
“I would say, overall, the jury is still out,” as to whether anecdotal reports of GLP-1 agonists curbing addictions will be borne out in randomized controlled trials.
“I think it is much too early to tell” whether these drugs might be approved for treating addictions without more solid clinical trial data, noted Dr. Hwang, who is an associate professor of medicine and chief, division of endocrinology and metabolism, at the University of North Carolina at Chapel Hill.
Meanwhile, another research group at the University of North Carolina at Chapel Hill, led by psychiatrist Christian Hendershot, PhD, is conducting a clinical trial in 48 participants with AUD who are also smokers.
They aim to determine if patients who receive semaglutide at escalating doses (0.25 mg to 1.0 mg per week via subcutaneous injection) over 9 weeks will consume less alcohol (the primary outcome) and smoke less (a secondary outcome) than those who receive a sham placebo injection. Results are expected in October 2023.
Dr. Fink-Jensen has received an unrestricted research grant from Novo Nordisk to investigate the effects of GLP-1 receptor stimulation on weight gain and metabolic disturbances in patients with schizophrenia treated with an antipsychotic.
A version of this article first appeared on Medscape.com.
As demand for semaglutide for weight loss grew following approval of Wegovy by the U.S. Food and Drug Administration in 2021, anecdotal reports of unexpected potential added benefits also began to surface.
Some patients taking these drugs for type 2 diabetes or weight loss also lost interest in addictive and compulsive behaviors such as drinking alcohol, smoking, shopping, nail biting, and skin picking, as reported in articles in the New York Times and The Atlantic, among others.
There is also some preliminary research to support these observations.
This news organization invited three experts to weigh in.
Recent and upcoming studies
The senior author of a recent randomized controlled trial of 127 patients with alcohol use disorder (AUD), Anders Fink-Jensen, MD, said: “I hope that GLP-1 analogs in the future can be used against AUD, but before that can happen, several GLP-1 trials [are needed to] prove an effect on alcohol intake.”
His study involved patients who received exenatide (Byetta, Bydureon, AstraZeneca), the first-generation GLP-1 agonist approved for type 2 diabetes, over 26 weeks, but treatment did not reduce the number of heavy drinking days (the primary outcome), compared with placebo.
However, in post hoc, exploratory analyses, heavy drinking days and total alcohol intake were significantly reduced in the subgroup of patients with AUD and obesity (body mass index > 30 kg/m2).
The participants were also shown pictures of alcohol or neutral subjects while they underwent functional magnetic resonance imaging. Those who had received exenatide, compared with placebo, had significantly less activation of brain reward centers when shown the pictures of alcohol.
“Something is happening in the brain and activation of the reward center is hampered by the GLP-1 compound,” Dr. Fink-Jensen, a clinical psychologist at the Psychiatric Centre Copenhagen, remarked in an email.
“If patients with AUD already fulfill the criteria for semaglutide (or other GLP-1 analogs) by having type 2 diabetes and/or a BMI over 30 kg/m2, they can of course use the compound right now,” he noted.
His team is also beginning a study in patients with AUD and a BMI ≥ 30 kg/m2 to investigate the effects on alcohol intake of semaglutide up to 2.4 mg weekly, the maximum dose currently approved for obesity in the United States.
“Based on the potency of exenatide and semaglutide,” Dr. Fink-Jensen said, “we expect that semaglutide will cause a stronger reduction in alcohol intake” than exenatide.
Animal studies have also shown that GLP-1 agonists suppress alcohol-induced reward, alcohol intake, motivation to consume alcohol, alcohol seeking, and relapse drinking of alcohol, Elisabet Jerlhag Holm, PhD, noted.
Interestingly, these agents also suppress the reward, intake, and motivation to consume other addictive drugs like cocaine, amphetamine, nicotine, and some opioids, Jerlhag Holm, professor, department of pharmacology, University of Gothenburg, Sweden, noted in an email.
In a recently published preclinical study, her group provides evidence to help explain anecdotal reports from patients with obesity treated with semaglutide who claim they also reduced their alcohol intake. In the study, semaglutide both reduced alcohol intake (and relapse-like drinking) and decreased body weight of rats of both sexes.
“Future research should explore the possibility of semaglutide decreasing alcohol intake in patients with AUD, particularly those who are overweight,” said Prof. Holm.
“AUD is a heterogenous disorder, and one medication is most likely not helpful for all AUD patients,” she added. “Therefore, an arsenal of different medications is beneficial when treating AUD.”
Janice J. Hwang, MD, MHS, echoed these thoughts: “Anecdotally, there are a lot of reports from patients (and in the news) that this class of medication [GLP-1 agonists] impacts cravings and could impact addictive behaviors.”
“I would say, overall, the jury is still out,” as to whether anecdotal reports of GLP-1 agonists curbing addictions will be borne out in randomized controlled trials.
“I think it is much too early to tell” whether these drugs might be approved for treating addictions without more solid clinical trial data, noted Dr. Hwang, who is an associate professor of medicine and chief, division of endocrinology and metabolism, at the University of North Carolina at Chapel Hill.
Meanwhile, another research group at the University of North Carolina at Chapel Hill, led by psychiatrist Christian Hendershot, PhD, is conducting a clinical trial in 48 participants with AUD who are also smokers.
They aim to determine if patients who receive semaglutide at escalating doses (0.25 mg to 1.0 mg per week via subcutaneous injection) over 9 weeks will consume less alcohol (the primary outcome) and smoke less (a secondary outcome) than those who receive a sham placebo injection. Results are expected in October 2023.
Dr. Fink-Jensen has received an unrestricted research grant from Novo Nordisk to investigate the effects of GLP-1 receptor stimulation on weight gain and metabolic disturbances in patients with schizophrenia treated with an antipsychotic.
A version of this article first appeared on Medscape.com.
Patient with newly diagnosed type 2 diabetes? Remember these steps
Nearly 40 antihyperglycemic agents have been approved by the US Food and Drug Administration (FDA) since the approval of human insulin in 1982.1 In addition, existing antihyperglycemic medications are constantly gaining FDA approval for new indications for common type 2 diabetes (T2D) comorbidities. For example, in addition to their glycemic benefits, the sodium-glucose cotransporter-2 (SGLT2) inhibitors have been approved for use in patients with T2D and established atherosclerotic cardiovascular disease (ASCVD) to reduce the risk for major adverse cardiovascular events (MACE; canagliflozin), risk for hospitalization for heart failure (dapagliflozin), and cardiovascular death (empagliflozin).2-4
The plethora of new agents and new data for existing agents, coupled with the annual release of guidelines from the American Diabetes Association (ADA) and practice recommendations from several other professional organizations,5-7 make it challenging for family physicians to stay current and provide the most up-to-date, evidence-based care. In this article, we provide advice on how to approach the screening, diagnosis, and evaluation of T2D, and on how to manage newly diagnosed T2D.
Screening, Dx, and evaluation: A quick review
Screening
Screening recommendations vary among professional organizations (TABLE 15,6,8). The US Preventive Services Task Force (USPSTF) recommends screening adults ages 35 to 70 years who are overweight or obese. Clinicians also can consider screening patients with a higher risk for diabetes.5 The ADA suggests screening all adults starting at 35 years, regardless of risk factors.8 Asymptomatic adults of any age with overweight or obesity and 1 or more risk factors should be screened.8
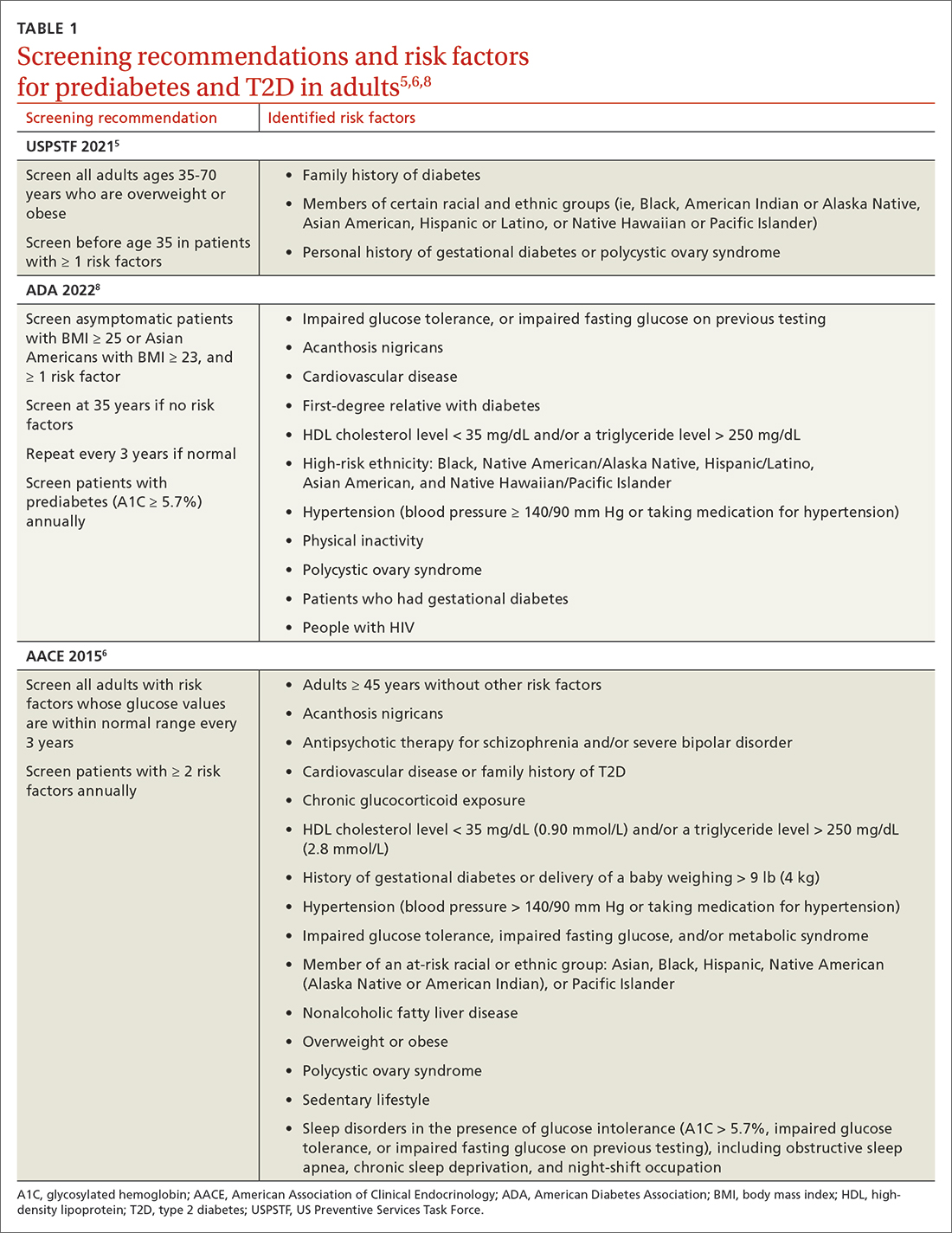
Making the diagnosis
The initial diagnosis of diabetes can be made by a fasting plasma glucose level ≥ 126 mg/dL (7.0 mmol/L); a 2-hour plasma glucose level ≥ 200 mg/dL (11.0 mmol/L) following an oral glucose tolerance test; or an A1C level ≥ 6.5%. Prioritize lab-drawn A1C measurements over point-of-care tests to diagnose T2D. In patients with classic symptoms of hyperglycemia, a random plasma glucose level ≥ 200 mg/dL (11.0 mmol/L) is also diagnostic. Generally, these tests are considered equally appropriate in screening for diabetes and may be used to detect prediabetes. In the absence of clear symptoms of hyperglycemia, the diagnosis of diabetes requires 2 abnormal screening test results, either via 1 blood sample (such as an abnormal A1C and glucose) or 2 separate blood samples of the same test. Further evaluation is advised if there is discordance between the 2 samples.8
Extended evaluations
Patients with newly diagnosed T2D require a thorough evaluation for comorbidities and complications of diabetes. Refer patients to an ophthalmologist for a dilated eye examination, with subsequent exams occurring every 1 to 2 years.6,9 Additional referrals for diabetes education, family planning for women of reproductive age, and dental, social, or mental health services may be clinically appropriate.9
Setting goals for glycemic control
Glycemic control is commonly monitored by the A1C level and by blood glucose monitoring either through traditional point-of-care glucometers or continuous glucose monitors (CGMs).10 Generally, CGMs provide more glycemic data than traditional glucometers and may cue patients to choose healthier dietary options and engage in physical exercise.11 Patients with T2D who use CGMs exhibit lower A1Cs, greater time in glycemic range, and reduced hypoglycemic episodes.11 Generally, CGMs are reserved for patients with type 1 diabetes and patients with T2D who use multiple daily injections, subcutaneous insulin infusions, or basal insulin only.12 Most professional organizations recommend that clinicians consider patient-specific factors to set individualized glycemic goals.6,10,13,14 For example, more stringent glycemic goals could be pursued for patients with longer life expectancy, shorter disease duration, absence of complications (eg, nephropathy, neuropathy, or cardiovascular disease), fewer comorbid conditions, lower hypoglycemia risk, or higher cognitive function.6
More specific A1C goals vary by professional organization. For nonpregnant adults, the ADA recommends an A1C goal of < 7% and a preprandial blood glucose level of 80 to 130 mg/dL (4.4-7.2 mmol/L).10 However, a lower A1C goal may be appropriate if it can be attained safely without causing hypoglycemia or other adverse effects.10 The AACE suggests an A1C goal of ≤ 6.5% and a fasting blood glucose level of < 110 mg/dL when it can be achieved safely.6 More stringent A1C goals may reduce long-term micro- and macrovascular complications—especially in patients with newly diagnosed T2D.10 While older studies such as the ACCORD trial found increased mortality in groups with more stringent glycemic targets, they did not include newer agents (SGLT2 inhibitors or glucagon-like peptide-1 [GLP-1] receptor agonists) that reduce cardiovascular events by mechanisms outside their glycemic-lowering effect. With these newer agents, more aggressive A1C goals can be targeted safely in select patients, particularly those with long life expectancy.10 Both the ADA and AACE recommend a less stringent A1C goal of 7% to 8% for patients with limited life expectancy or risks (eg, a history of hypoglycemia) that outweigh expected benefits.6,10
Continue to: Lifestyle modifications
Lifestyle modifications: As important as medication
Nutrition
The energy-dense Western diet, combined with sedentary behavior, are thought to be a primary cause of T2D.15 Therefore, include lifestyle modifications in the initial management of newly diagnosed T2D. Diets that replace carbohydrates with saturated and trans fats are related to increased mortality in patients with T2D.16 Increased consumption of vegetables, fruits, legumes, nuts, fish, cereal, and oils reduces concentrations of saturated and trans fats and increases dietary intake of monounsaturated fatty acids, fiber, antioxidants, and polyphenols.17
Increasing the intake of fiber, an undigestible carbohydrate, offers numerous benefits in T2D management. High-fiber diets can help regulate blood sugar and lipid levels, increase satiety, reduce inflammation, aid in weight management, and reduce premature mortality.18 Insoluble fiber, found in foods such as whole wheat flour, nuts, and cauliflower, helps food pass more quickly through the stomach and intestines and adds bulk to stool. Soluble fiber, found in foods such as chickpeas, lentils, and Brussels sprouts, absorbs water and forms a gel-like substance that protects nutrients from digestive enzymes and slows down digestion. The result is a more gradual rise in postprandial glucose levels and improved insulin sensitivity.19 Dietary fiber may produce short-chain fatty acids which in turn activate incretin secretion and stimulate a glucose-dependent release of insulin from the pancreas.20
Simple dietary substitutions, such as whole grains and legumes for white rice, can reduce fasting blood glucose and A1C levels.21 In a randomized controlled trial (RCT), increasing whole grain oat intake improved measures of glycemic control, reducing A1C by 1% at 1-year follow-up.19 Encourage patients with T2D to increase consumption of high-fiber foods and replace animal fats and refined grains with vegetable fats (eg, nuts, avocados, olives). Nutritional therapies should be individualized, taking into account personal preferences and cultural customs.22 Nutritional habits may be based on race/ethnicity, religion/spirituality, or even the city in which an individual resides. Nutrition recommendations should account for these differences as well as access to healthy foods. For instance, ethnic groups whose dietary patterns include tortillas could be counseled to choose high-fiber options such as corn instead of flour tortillas and to incorporate vegetables in place of high-fat foods. Additionally, ethnic groups who favor using animal fats in foods such as greens could be advised on ways to add flavor to vegetables without adding saturated fats. Taking this approach may lessen barriers to change and increase ability to make dietary modifications.23
Exercise
Encourage all patients with T2D to exercise regularly. The atherosclerotic plaques found in patients with T2D have increased inflammatory properties and result in worse cardiovascular outcomes compared with plaques in individuals without T2D.24 Regular exercise reduces levels of pro-inflammatory markers—C-reactive protein, interleukin (IL)-6, and tumor necrosis factor alpha—and increases levels of anti-inflammatory markers (IL-4 and IL-10).24 Regular exercise can improve body composition, physical fitness, lipid and glucose metabolism, and insulin sensitivity.25,26
A meta-analysis of RCTs demonstrated that structured exercise > 150 minutes per week resulted in A1C reductions of 0.89%,27 which is comparable to the effect of many oral antihyperglycemic medications.26 The Health Benefits of Aerobic and Resistance Training in individuals with T2D (HART-D) and Diabetes Aerobic and Resistance Exercise (DARE) studies demonstrated that combining endurance and resistance training was superior for improving glycemic control, cardiorespiratory fitness, and body composition, than using either type of training alone.25 Both the American College of Sports Medicine (ACSM) and the ADA recommend that adults engage in at least 150 total minutes of moderate-intensity aerobic activity per week and resistance training 2 to 3 times weekly.26 ACSM defines moderate-intensity exercise as 65% to 75% of maximal heart rate, a rating of perceived exertion of 3 to 4, or a step rate of 100 steps per minute.28
Continue to: Because of their longitudinal relationships...
Because of their longitudinal relationships with patients, family physicians are in an optimal position to assess a patient’s physical capacity level and provide individualized counseling. Several systematic reviews have demonstrated that counseling on exercise increases patients’ participation in physical activity.29 Encourage your patients with T2D to exercise regularly, considering each individual’s ability to engage in physical activity.
Weight loss
Include weight management in the initial treatment of patients with newly diagnosed T2D. Weight loss decreases hepatic glucose production and increases peripheral insulin sensitivity and insulin secretion.30 Moderate decreases in weight (5%-10%) can reduce complications related to diabetes, and sustained significant weight loss (> 10%) can potentially cause T2D remission (A1C < 6.5% after stopping diabetes medications).31,32
Diabetes self-management education supports patients by giving them tools for making and maintaining lifestyle changes. Understanding individual barriers to change and addressing these during motivational interviews is important. Through a qualitative interview study, participants in a diabetes self-management program revealed 4 factors that motivated them to maintain lifestyle changes: support from others, experiencing the impact of the changes they made, fear of T2D complications, and forming new habits.33 Family physicians are key in helping patients acquire knowledge and support to make the lifestyle modifications needed to manage newly diagnosed T2D.
Individualized pharmacotherapy considerations
For decades, the initial pharmacotherapeutic regimen for patients with newly diagnosed T2D considered the patient’s baseline A1C as a major driver for therapy. Metformin has been the mainstay in T2D treatment due to its clinical efficacy, minimal risk for hypoglycemia, and low cost. Regardless of the regimen, pharmacotherapy should be initiated at the time of T2D diagnosis in conjunction with the aforementioned lifestyle modifications.34
When selecting pharmacotherapy, practice guidelines recommend considering the efficacy and adverse effects of medications, patient-specific comorbidities, adherence, cost, and a patient’s lifestyle factors.34 Drug classes with pertinent information are listed in TABLE 2.34-54 After starting medication, monitor the A1C level every 3 months to determine whether therapy should be intensified. Patients should have their labs drawn ahead of the quarterly visit, or point-of-care measurements may be used to facilitate in-person patient–provider discussions.
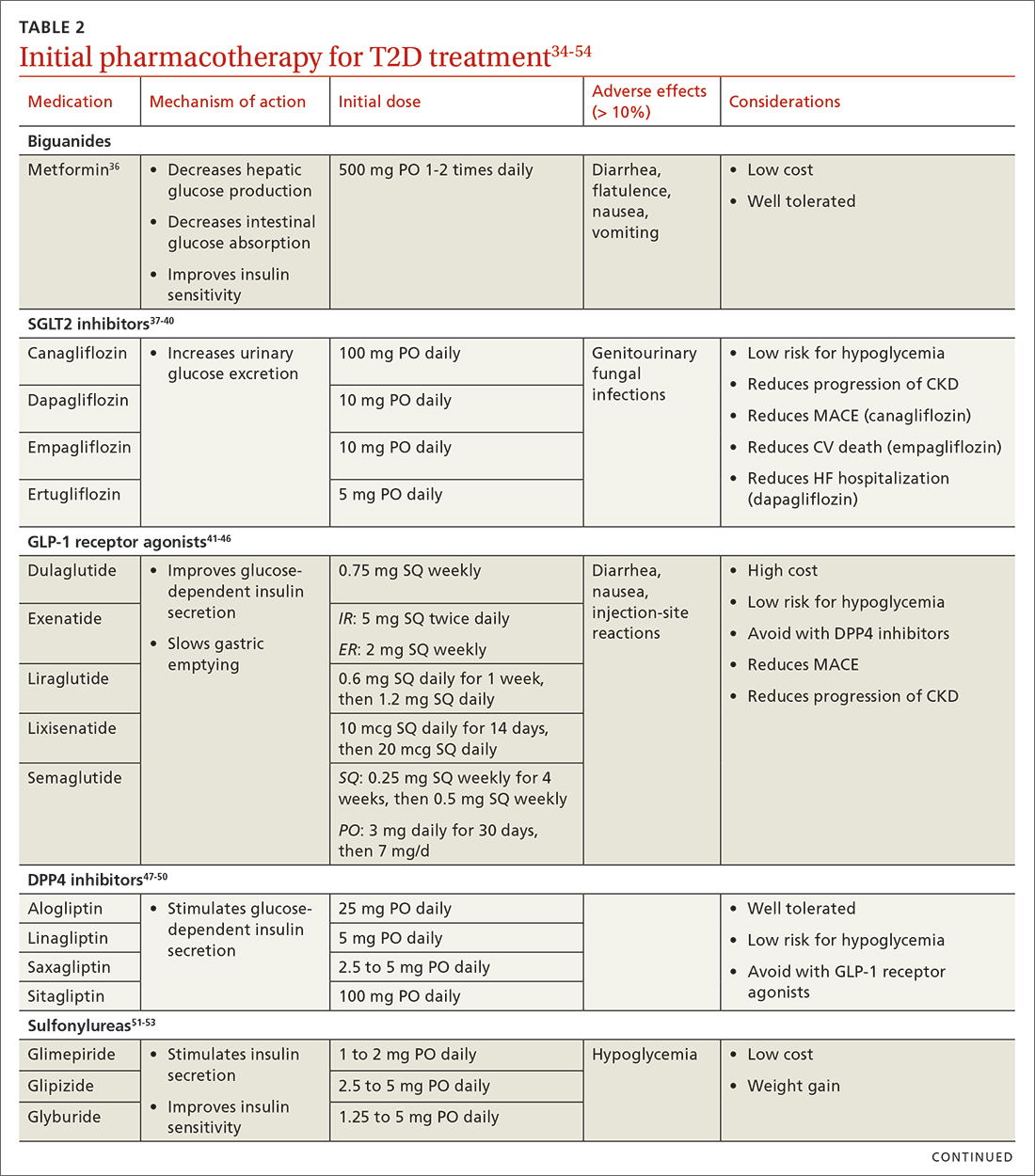
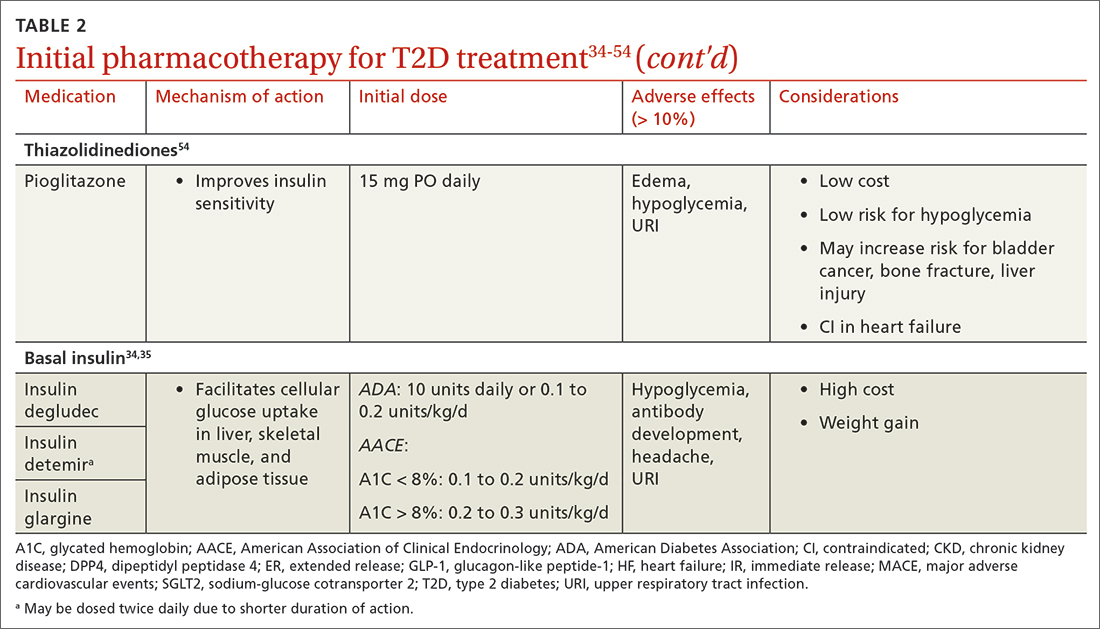
Continue to: Consider patient-specific factors when starting pharmacotherapy
Consider patient-specific factors when starting pharmacotherapy
ASCVD. Regardless of baseline glycemic control, offer patients who have ASCVD, or who are at high risk for it, an SGLT2 inhibitor (canagliflozin, dapagliflozin, or empagliflozin) or a long-acting GLP-1 receptor agonist (dulaglutide, liraglutide, or semaglutide).34,35 SGLT2 inhibitors reduced the risk for MACE by 11% in patients with established ASCVD.55 They also reduced a composite outcome of cardiovascular death or hospitalization for heart failure by 23% in patients with or without ASCVD or heart failure at baseline.55 GLP-1 receptor agonists offer a similar reduction in MACE to SGLT2 inhibitors, but they do not have significant effects in heart failure.56 Thiazolidinediones (TZDs), saxagliptin, and alogliptin should be avoided in patients with heart failure.57 TZDs may reduce the risk for recurrent stroke in patients with T2D.58
Chronic kidney disease (CKD). As with ASCVD, prioritize SGLT2 inhibitors and GLP-1 receptor agonists in patients with CKD. While both classes reduced the risk for progression of kidney disease such as macroalbuminuria, SGLT2 inhibitors offer additional benefits in their reduction of the worsening of estimated glomerular filtration rate, end-stage kidney disease, and renal death.56
Obesity. Consider the effect of each drug class on weight when making initial treatment choices, taking special care to minimize weight gain and potentially promote weight loss.34 The ADA prefers GLP-1 receptor agonists, but also suggests SGLT2 inhibitors in these patients. While all GLP-1 receptor agonists have an impact on weight, weekly subcutaneous semaglutide offers the most pronounced weight loss of 2 to 7 kg over 56 weeks.59 SGLT2 inhibitors promote sustainable weight loss to a lesser degree, contributing to an average loss of 3 kg at 2 years.60 Weight gain is common in patients taking sulfonylureas (2.01-2.3 kg)31 and insulin (3-9 kg weight gain in the first year)61 and should be avoided in patients with T2D and obesity.34
Hypoglycemia risk. In addition to counseling patients on hypoglycemia management and prescribing glucagon rescue kits, offer medications with no or very low risk for hypoglycemia (eg, GLP-1 receptor agonists, SGLT2 inhibitors, dipeptidyl peptidase-4 inhibitors, and TZDs). Generally, avoid insulin and sulfonylureas in patients in whom hypoglycemia is a major concern (eg, older adults, individuals with labile blood glucose levels).34 Patients with reduced renal function are at higher risk for hypoglycemia with insulin or sulfonylureas due to reduced drug clearance. However, insulin is often the only treatment for patients with advanced renal disease. Pay close attention to insulin dosing in patients with advanced renal disease, which may necessitate lower doses and smaller dose adjustments due to this risk.
Social determinants of health. Medication access and cost is a major burden in T2D management and should be considered for every patient. Compared with the period of 2005 to 2007, the annual cost of diabetes medications for an individual in 2015 to 2017 increased by 147%, rising from $1106 to $2727 per year.62 This increase is driven by the cost of insulin and newer medications without generic options.62 Identify local resources in your community, such as patient assistance programs and pharmacies with reduced-price generic prescription programs, which may be useful for patients who are underinsured or uninsured.
Continue to: Even if cost weren't an issue...
Even if cost weren’t an issue, many medications such as insulin and GLP-1 receptor agonists should be kept refrigerated and are only stable at room temperature for a limited time. Medications that are stable at room temperature should be prioritized in patients with limited or inconsistent access to refrigeration or unstable housing who may find it difficult to store their medications appropriately.
Do not delay insulin initiation in patients with high baseline A1C
Whenever possible, a GLP-1 receptor agonist is the preferred injectable medication to insulin. Starting insulin introduces numerous risks, including hypoglycemia, weight gain, and stigma. However, in the patient with newly diagnosed T2D, choose basal insulin when the baseline hyperglycemia is severe,34 as indicated by:
- blood glucose > 300 mg/dL (16.7 mmol/L),
- A1C > 10% (86 mmol/mol),
- symptoms of hyperglycemia (polyuria or polydipsia), or
- evidence of catabolism (weight loss, hypertriglyceridemia, ketosis).
Basal insulin analogs are preferred over NPH given their reduced variability, dosing, and hypoglycemic risk.35 Mixed insulins may be used if a patient is unable to afford an insulin analog, which can be quite costly. However, extensive counseling on dosing and management of hypoglycemia is crucial to patient safety with these agents. The ADA recommends initiating 0.1 to 0.2 units/kg of basal insulin daily or 10 units daily.34 The AACE follows this recommendation for patients with baseline A1C < 8%, but it proposes a more aggressive initiation of 0.2 to 0.3 units/kg/d for patients with baseline A1C > 8%.35 Titrate the dose by 2 units every 3 days to reach the target fasting blood glucose level. As hyperglycemia resolves, simplify the regimen and transition to noninsulin options per the previously discussed considerations.
It’s not just about glycemic control
In addition to the direct effects of hyperglycemia, a T2D diagnosis introduces an increased risk for ASCVD, a reduced ability to fight infection, and heightened risk for depression. Order a lipid panel at the time of T2D diagnosis and initiate lipid management as needed (TABLE 335,63,64). Both the ADA and the American Heart Association recommend starting a moderate-intensity statin as primary prevention for all patients with T2D between 40 and 75 years of age regardless of the 10-year ASCVD risk.63 The AACE uses specific lipid targets and recommends moderate- to high-intensity statin therapy for patients with T2D.35 All recommendations by professional organizations list high-intensity statins for patients with established ASCVD.
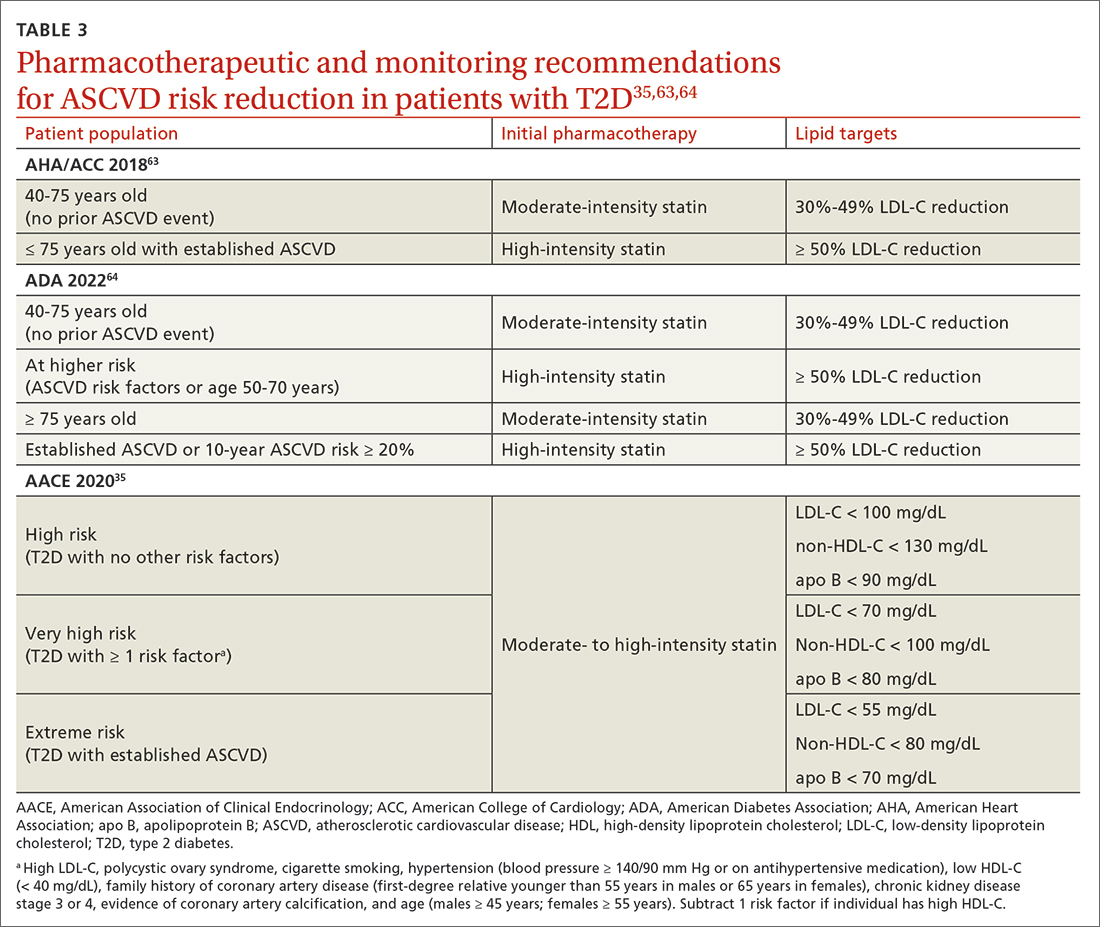
It is also vital to recommend that patients with newly diagnosed T2D remain up to date on all indicated vaccinations. They should promptly receive the hepatitis B and pneumococcal vaccines if they have not already done so for a previous indication. COVID-19 and annual influenza vaccines also should be prioritized for these patients.65
Finally, patients with diabetes are twice as likely to develop depression than patients without diabetes.66 Individuals with T2D and depression exhibit poorer medication adherence, lifestyle choices, and glycemic control.66 Screen for and treat these issues in all patients with T2D across the course of the disease.
Overall, work closely with patients to support them in managing their new diagnosis with evidence-based pharmacologic and nonpharmacologic approaches. The importance of lifestyle changes including high-fiber diets, regular exercise, and weight loss should not be overlooked. Do not delay starting pharmacotherapy after diagnosing T2D and consider medication-specific and patient-specific factors to individualize therapy, improve adherence, and prevent complications.
CORRESPONDENCE
Jennie B. Jarrett, PharmD, MMedEd, 833 South Wood Street (MC 886), Chicago, IL 60612; [email protected]
1. Dahlén AD, Dashi G, Maslov I, et al. Trends in antidiabetic drug discovery: FDA approved drugs, new drugs in clinical trials and global sales. Front Pharmacol. 2022;12. Accessed April 19, 2023. www.frontiersin.org/article/10.3389/fphar.2021.807548
2. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128. doi: 10.1056/NEJMoa1504720
3. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644-657. doi: 10.1056/NEJMoa1611925
4. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357. doi: 10.1056/NEJMoa1812389
5. Davidson KW, Barry MJ, et al. Screening for prediabetes and type 2 diabetes: US Preventive Services Task Force recommendation statement. JAMA. 2021;326:736-743. doi: 10.1001/jama. 2021.12531
6. Handelsman Y, Bloomgarden ZT, Grunberger G, et al. American Association of Clinical Endocrinologists and American College of Endocrinology - clinical practice guidelines for developing a diabetes mellitus comprehensive care plan - 2015. Endocr Pract. 2015;21(suppl 1):1-87. doi: 10.4158/EP15672.GL
7. ADA. Introduction: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S1-S2. doi: 10.2337/dc22-Sint
8. ADA Professional Practice Committee. Classification and diagnosis of diabetes: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S17-S38. doi: 10.2337/dc22-S002
9. ADA Professional Practice Committee. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S46-S59. doi: 10.2337/dc22-S004
10. ADA Professional Practice Committee. Glycemic targets: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S83-S96. doi: 10.2337/dc22-S006
11. Janapala RN, Jayaraj JS, Fathima N, et al. Continuous glucose monitoring versus self-monitoring of blood glucose in type 2 diabetes mellitus: a systematic review with meta-analysis. Cureus. 2019;11:e5634. doi: 10.7759/cureus.5634
12. ADA Professional Practice Committee. Diabetes technology: standards of medical care in diabetes - 2022. Diabetes Care. 2021;45(suppl 1):S97-S112. doi: 10.2337/dc22-S007
13. Qaseem A, Wilt TJ, Kansagara D, et al. Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: a guidance statement update from the American College of Physicians. Ann Intern Med. 2018;168:569-576. doi: 10.7326/M17-0939
14. Moran GM, Bakhai C, Song SH, et al, Guideline Committee. Type 2 diabetes: summary of updated NICE guidance. BMJ. 2022;377:o775. doi: 10.1136/bmj.o775
15. Kolb H, Martin S. Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med. 2017;15:131. doi: 10.1186/s12916-017-0901-x
16. McMacken M, Shah S. A plant-based diet for the prevention and treatment of type 2 diabetes. J Geriatr Cardiol. 2017;14:342-354. doi: 10.11909/j.issn.1671-5411.2017.05.009
17. Asif M. The prevention and control the type-2 diabetes by changing lifestyle and dietary pattern. J Educ Health Promot. 2014;3:1. doi: 10.4103/2277-9531.127541
18. Reynolds AN, Akerman AP, Mann J. Dietary fibre and whole grains in diabetes management: systematic review and meta-analyses. PLoS Med. 2020;17(3):e1003053. doi: 10.1371/journal.pmed.1003053
19. Li X, Cai X, Ma X, et al. Short- and long-term effects of wholegrain oat intake on weight management and glucolipid metabolism in overweight type-2 diabetics: a randomized control trial. Nutrients. 2016;8:549. doi: 10.3390/nu8090549
20. Fujii H, Iwase M, Ohkuma T, et al. Impact of dietary fiber intake on glycemic control, cardiovascular risk factors and chronic kidney disease in Japanese patients with type 2 diabetes mellitus: the Fukuoka Diabetes Registry. Nutr J. 2013;12:159. doi: 10.1186/1475-2891-12-159
21. Kim M, Jeung SR, Jeong TS, et al. Replacing with whole grains and legumes reduces Lp-PLA2 activities in plasma and PBMCs in patients with prediabetes or T2D. J Lipid Res. 2014;55:1762-1771. doi: 10.1194/jlr.M044834
22. Evert AB, Dennison M, Gardner CD, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care. 2019;42:731-754. doi: 10.2337/dci19-0014
23. Caballero AE. The “a to z” of managing type 2 diabetes in culturally diverse populations. Front Endocrinol. 2018;9:479. doi: 10.3389/fendo.2018.00479
24. Golbidi S, Badran M, Laher I. Antioxidant and anti-inflammatory effects of exercise in diabetic patients. Exp Diabetes Res. 2012; 2012:941868. doi: 10.1155/2012/941868
25. Karstoft K, Pedersen BK. Exercise and type 2 diabetes: focus on metabolism and inflammation. Immunol Cell Biol. 2016;94:146-150. doi: 10.1038/icb.2015.101
26. Dugan JA. Exercise recommendations for patients with type 2 diabetes. JAAPA. 2016;29:13-18. doi: 10.1097/01.JAA. 0000475460.77476.f6
27. Umpierre D, Ribeiro PA, Kramer CK, et al. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2011;305:1790–1799. doi: 10.1001/jama.2011.576
28. Zuhl M. Tips for monitoring aerobic exercise intensity. 2020. Accessed April 19, 2023. www.acsm.org/docs/default-source/files-for-resource-library/exercise-intensity-infographic.pdf? sfvrsn=f467c793_2
29. Williams A, Radford J, O’Brien J, Davison K. Type 2 diabetes and the medicine of exercise: the role of general practice in ensuring exercise is part of every patient’s plan. Aust J Gen Pract. 2020;49:189-193. doi: 10.31128/AJGP-09-19-5091
30. Grams J, Garvey WT. Weight loss and the prevention and treatment of type 2 diabetes using lifestyle therapy, pharmacotherapy, and bariatric surgery: mechanisms of action. Curr Obes Rep. 2015;4:287-302. doi: 10.1007/s13679-015-0155-x
31. Apovian CM, Okemah J, O’Neil PM. Body weight considerations in the management of type 2 diabetes. Adv Ther. 2019;36:44-58. doi: 10.1007/s12325-018-0824-8
32. Lean MEJ, Leslie WS, Barnes AC, et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol. 2019;7:344-355. doi: 10.1016/S2213-8587(19)30068-3
33. Rise MB, Pellerud A, Rygg LØ, et al. Making and maintaining lifestyle changes after participating in group based type 2 diabetes self-management educations: a qualitative study. PLoS One. 2013;8:e64009. doi: 10.1371/journal.pone.0064009
34. ADA Professional Practice Committee. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S125-S143. doi: 10.2337/dc22-S009
35. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract. 2020;26:107-139. doi: 10.4158/CS-2019-0472
36. Metformin. Package insert. Bristol-Myers Squibb Company; 2017.
37. Invokana (canagliflozin). Package insert. Janssen Pharmaceuticals, Inc; 2020.
38. Farxiga (dapagliflozin). Package insert. AstraZeneca Pharmaceuticals LP; 2021.
39. Jardiance (empagliflozin). Package insert. Boehringer Ingelheim Pharmaceuticals, Inc; 2022.
40. Steglatro (ertugliflozin). Package insert. Merck & Co, Inc; 2021.
41. Trulicity (dulaglutide). Package insert. Lilly USA, LLC; 2022.
42. Byetta (exenatide). Package insert. AstraZeneca Canada Inc; 2022.
43. Bydureon (exenatide ER). Package insert. AstraZeneca Pharmaceuticals LP; 2022.
44. Victoza (liraglutide). Package insert. Novo Nordisk; 2022.
45. Adlyxin (lixisenatide). Package insert. Sanofi-Aventis US LLC; 2022.
46. Ozempic (semaglutide). Package insert. Novo Nordisk; 2022.
47. Alogliptin. Package insert. Takeda Pharmaceuticals USA, Inc; 2022.
48. Linagliptin. Package insert. Boehringer Ingelheim Pharmaceuticals, Inc; 2022.
49. Saxagliptin. Package insert. AstraZeneca Pharmaceuticals LP; 2019.
50. Januvia (sitagliptin). Package insert. Merck Sharp & Dohme LLC; 2022.
51. Glimepiride. Package insert. Sanofi-Aventis US LLC; 2009.
52. Glipizide. Package insert. Roerig; 2023.
53. Glyburide. Package insert. Sanofi-Aventis US LLC; 2009.
54. Pioglitazone. Package insert. Northstar Rx LLC; 2022.
55. Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31-39. doi: 10.1016/S0140-6736(18)32590-X
56. Zelniker TA, Wiviott SD, Raz I, et al. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation. 2019;139:2022-2031. doi: 10.1161/CIRCULATIONAHA.118.038868
57. FDA. FDA Drug Safety Communication: FDA adds warnings about heart failure risk to labels of type 2 diabetes medicines containing saxagliptin and alogliptin. Accessed April 19, 2023. www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-adds-warnings-about-heart-failure-risk-labels-type-2-diabetes
58. Wilcox R, Bousser MG, Betteridge DJ, et al. Effects of pioglitazone in patients with type 2 diabetes with or without previous stroke: results from PROactive (PROspective pioglitAzone Clinical Trial In macroVascular Events 04). Stroke. 2007;38:865-873. doi: 10.1161/01.STR.0000257974.06317.49
59. Lingvay I, Hansen T, Macura S, et al. Superior weight loss with once-weekly semaglutide versus other glucagon-like peptide-1 receptor agonists is independent of gastrointestinal adverse events. BMJ Open Diabetes Res Care. 2020;8:e001706. doi: 10.1136/bmjdrc-2020-001706
60. Liu XY, Zhang N, Chen R, et al. Efficacy and safety of sodium-glucose cotransporter 2 inhibitors in type 2 diabetes: a meta-analysis of randomized controlled trials for 1 to 2 years. J Diabetes Complications. 2015;29:1295-1303. doi: 10.1016/j.jdiacomp.2015.07.011
61. Brown A, Guess N, Dornhorst A, et al. Insulin-associated weight gain in obese type 2 diabetes mellitus patients: what can be done? Diabetes Obes Metab. 2017;19:1655-1668. doi: 10.1111/dom.13009
62. Zhou X, Shrestha SS, Shao H, et al. Factors contributing to the rising national cost of glucose-lowering medicines for diabetes during 2005-2007 and 2015-2017. Diabetes Care. 2020;43:2396-2402. doi: 10.2337/dc19-2273
63. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082-e1143. doi: 10.1161/CIR.0000000000000625
64. ADA Professional Practice Committee. Cardiovascular disease and risk management: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S144-S174. doi: 10.2337/dc22-S010
65. CDC. Adult immunization schedule by medical condition and other indication. 2022. Accessed April 19, 2023. www.cdc.gov/vaccines/schedules/hcp/imz/adult-conditions.htm
66. Semenkovich K, Brown ME, Svrakic DM, et al. Depression in type 2 diabetes mellitus: prevalence, impact, and treatment. Drugs. 2015;75:577-587. doi: 10.1007/s40265-015-0347-4
Nearly 40 antihyperglycemic agents have been approved by the US Food and Drug Administration (FDA) since the approval of human insulin in 1982.1 In addition, existing antihyperglycemic medications are constantly gaining FDA approval for new indications for common type 2 diabetes (T2D) comorbidities. For example, in addition to their glycemic benefits, the sodium-glucose cotransporter-2 (SGLT2) inhibitors have been approved for use in patients with T2D and established atherosclerotic cardiovascular disease (ASCVD) to reduce the risk for major adverse cardiovascular events (MACE; canagliflozin), risk for hospitalization for heart failure (dapagliflozin), and cardiovascular death (empagliflozin).2-4
The plethora of new agents and new data for existing agents, coupled with the annual release of guidelines from the American Diabetes Association (ADA) and practice recommendations from several other professional organizations,5-7 make it challenging for family physicians to stay current and provide the most up-to-date, evidence-based care. In this article, we provide advice on how to approach the screening, diagnosis, and evaluation of T2D, and on how to manage newly diagnosed T2D.
Screening, Dx, and evaluation: A quick review
Screening
Screening recommendations vary among professional organizations (TABLE 15,6,8). The US Preventive Services Task Force (USPSTF) recommends screening adults ages 35 to 70 years who are overweight or obese. Clinicians also can consider screening patients with a higher risk for diabetes.5 The ADA suggests screening all adults starting at 35 years, regardless of risk factors.8 Asymptomatic adults of any age with overweight or obesity and 1 or more risk factors should be screened.8

Making the diagnosis
The initial diagnosis of diabetes can be made by a fasting plasma glucose level ≥ 126 mg/dL (7.0 mmol/L); a 2-hour plasma glucose level ≥ 200 mg/dL (11.0 mmol/L) following an oral glucose tolerance test; or an A1C level ≥ 6.5%. Prioritize lab-drawn A1C measurements over point-of-care tests to diagnose T2D. In patients with classic symptoms of hyperglycemia, a random plasma glucose level ≥ 200 mg/dL (11.0 mmol/L) is also diagnostic. Generally, these tests are considered equally appropriate in screening for diabetes and may be used to detect prediabetes. In the absence of clear symptoms of hyperglycemia, the diagnosis of diabetes requires 2 abnormal screening test results, either via 1 blood sample (such as an abnormal A1C and glucose) or 2 separate blood samples of the same test. Further evaluation is advised if there is discordance between the 2 samples.8
Extended evaluations
Patients with newly diagnosed T2D require a thorough evaluation for comorbidities and complications of diabetes. Refer patients to an ophthalmologist for a dilated eye examination, with subsequent exams occurring every 1 to 2 years.6,9 Additional referrals for diabetes education, family planning for women of reproductive age, and dental, social, or mental health services may be clinically appropriate.9
Setting goals for glycemic control
Glycemic control is commonly monitored by the A1C level and by blood glucose monitoring either through traditional point-of-care glucometers or continuous glucose monitors (CGMs).10 Generally, CGMs provide more glycemic data than traditional glucometers and may cue patients to choose healthier dietary options and engage in physical exercise.11 Patients with T2D who use CGMs exhibit lower A1Cs, greater time in glycemic range, and reduced hypoglycemic episodes.11 Generally, CGMs are reserved for patients with type 1 diabetes and patients with T2D who use multiple daily injections, subcutaneous insulin infusions, or basal insulin only.12 Most professional organizations recommend that clinicians consider patient-specific factors to set individualized glycemic goals.6,10,13,14 For example, more stringent glycemic goals could be pursued for patients with longer life expectancy, shorter disease duration, absence of complications (eg, nephropathy, neuropathy, or cardiovascular disease), fewer comorbid conditions, lower hypoglycemia risk, or higher cognitive function.6
More specific A1C goals vary by professional organization. For nonpregnant adults, the ADA recommends an A1C goal of < 7% and a preprandial blood glucose level of 80 to 130 mg/dL (4.4-7.2 mmol/L).10 However, a lower A1C goal may be appropriate if it can be attained safely without causing hypoglycemia or other adverse effects.10 The AACE suggests an A1C goal of ≤ 6.5% and a fasting blood glucose level of < 110 mg/dL when it can be achieved safely.6 More stringent A1C goals may reduce long-term micro- and macrovascular complications—especially in patients with newly diagnosed T2D.10 While older studies such as the ACCORD trial found increased mortality in groups with more stringent glycemic targets, they did not include newer agents (SGLT2 inhibitors or glucagon-like peptide-1 [GLP-1] receptor agonists) that reduce cardiovascular events by mechanisms outside their glycemic-lowering effect. With these newer agents, more aggressive A1C goals can be targeted safely in select patients, particularly those with long life expectancy.10 Both the ADA and AACE recommend a less stringent A1C goal of 7% to 8% for patients with limited life expectancy or risks (eg, a history of hypoglycemia) that outweigh expected benefits.6,10
Continue to: Lifestyle modifications
Lifestyle modifications: As important as medication
Nutrition
The energy-dense Western diet, combined with sedentary behavior, are thought to be a primary cause of T2D.15 Therefore, include lifestyle modifications in the initial management of newly diagnosed T2D. Diets that replace carbohydrates with saturated and trans fats are related to increased mortality in patients with T2D.16 Increased consumption of vegetables, fruits, legumes, nuts, fish, cereal, and oils reduces concentrations of saturated and trans fats and increases dietary intake of monounsaturated fatty acids, fiber, antioxidants, and polyphenols.17
Increasing the intake of fiber, an undigestible carbohydrate, offers numerous benefits in T2D management. High-fiber diets can help regulate blood sugar and lipid levels, increase satiety, reduce inflammation, aid in weight management, and reduce premature mortality.18 Insoluble fiber, found in foods such as whole wheat flour, nuts, and cauliflower, helps food pass more quickly through the stomach and intestines and adds bulk to stool. Soluble fiber, found in foods such as chickpeas, lentils, and Brussels sprouts, absorbs water and forms a gel-like substance that protects nutrients from digestive enzymes and slows down digestion. The result is a more gradual rise in postprandial glucose levels and improved insulin sensitivity.19 Dietary fiber may produce short-chain fatty acids which in turn activate incretin secretion and stimulate a glucose-dependent release of insulin from the pancreas.20
Simple dietary substitutions, such as whole grains and legumes for white rice, can reduce fasting blood glucose and A1C levels.21 In a randomized controlled trial (RCT), increasing whole grain oat intake improved measures of glycemic control, reducing A1C by 1% at 1-year follow-up.19 Encourage patients with T2D to increase consumption of high-fiber foods and replace animal fats and refined grains with vegetable fats (eg, nuts, avocados, olives). Nutritional therapies should be individualized, taking into account personal preferences and cultural customs.22 Nutritional habits may be based on race/ethnicity, religion/spirituality, or even the city in which an individual resides. Nutrition recommendations should account for these differences as well as access to healthy foods. For instance, ethnic groups whose dietary patterns include tortillas could be counseled to choose high-fiber options such as corn instead of flour tortillas and to incorporate vegetables in place of high-fat foods. Additionally, ethnic groups who favor using animal fats in foods such as greens could be advised on ways to add flavor to vegetables without adding saturated fats. Taking this approach may lessen barriers to change and increase ability to make dietary modifications.23
Exercise
Encourage all patients with T2D to exercise regularly. The atherosclerotic plaques found in patients with T2D have increased inflammatory properties and result in worse cardiovascular outcomes compared with plaques in individuals without T2D.24 Regular exercise reduces levels of pro-inflammatory markers—C-reactive protein, interleukin (IL)-6, and tumor necrosis factor alpha—and increases levels of anti-inflammatory markers (IL-4 and IL-10).24 Regular exercise can improve body composition, physical fitness, lipid and glucose metabolism, and insulin sensitivity.25,26
A meta-analysis of RCTs demonstrated that structured exercise > 150 minutes per week resulted in A1C reductions of 0.89%,27 which is comparable to the effect of many oral antihyperglycemic medications.26 The Health Benefits of Aerobic and Resistance Training in individuals with T2D (HART-D) and Diabetes Aerobic and Resistance Exercise (DARE) studies demonstrated that combining endurance and resistance training was superior for improving glycemic control, cardiorespiratory fitness, and body composition, than using either type of training alone.25 Both the American College of Sports Medicine (ACSM) and the ADA recommend that adults engage in at least 150 total minutes of moderate-intensity aerobic activity per week and resistance training 2 to 3 times weekly.26 ACSM defines moderate-intensity exercise as 65% to 75% of maximal heart rate, a rating of perceived exertion of 3 to 4, or a step rate of 100 steps per minute.28
Continue to: Because of their longitudinal relationships...
Because of their longitudinal relationships with patients, family physicians are in an optimal position to assess a patient’s physical capacity level and provide individualized counseling. Several systematic reviews have demonstrated that counseling on exercise increases patients’ participation in physical activity.29 Encourage your patients with T2D to exercise regularly, considering each individual’s ability to engage in physical activity.
Weight loss
Include weight management in the initial treatment of patients with newly diagnosed T2D. Weight loss decreases hepatic glucose production and increases peripheral insulin sensitivity and insulin secretion.30 Moderate decreases in weight (5%-10%) can reduce complications related to diabetes, and sustained significant weight loss (> 10%) can potentially cause T2D remission (A1C < 6.5% after stopping diabetes medications).31,32
Diabetes self-management education supports patients by giving them tools for making and maintaining lifestyle changes. Understanding individual barriers to change and addressing these during motivational interviews is important. Through a qualitative interview study, participants in a diabetes self-management program revealed 4 factors that motivated them to maintain lifestyle changes: support from others, experiencing the impact of the changes they made, fear of T2D complications, and forming new habits.33 Family physicians are key in helping patients acquire knowledge and support to make the lifestyle modifications needed to manage newly diagnosed T2D.
Individualized pharmacotherapy considerations
For decades, the initial pharmacotherapeutic regimen for patients with newly diagnosed T2D considered the patient’s baseline A1C as a major driver for therapy. Metformin has been the mainstay in T2D treatment due to its clinical efficacy, minimal risk for hypoglycemia, and low cost. Regardless of the regimen, pharmacotherapy should be initiated at the time of T2D diagnosis in conjunction with the aforementioned lifestyle modifications.34
When selecting pharmacotherapy, practice guidelines recommend considering the efficacy and adverse effects of medications, patient-specific comorbidities, adherence, cost, and a patient’s lifestyle factors.34 Drug classes with pertinent information are listed in TABLE 2.34-54 After starting medication, monitor the A1C level every 3 months to determine whether therapy should be intensified. Patients should have their labs drawn ahead of the quarterly visit, or point-of-care measurements may be used to facilitate in-person patient–provider discussions.


Continue to: Consider patient-specific factors when starting pharmacotherapy
Consider patient-specific factors when starting pharmacotherapy
ASCVD. Regardless of baseline glycemic control, offer patients who have ASCVD, or who are at high risk for it, an SGLT2 inhibitor (canagliflozin, dapagliflozin, or empagliflozin) or a long-acting GLP-1 receptor agonist (dulaglutide, liraglutide, or semaglutide).34,35 SGLT2 inhibitors reduced the risk for MACE by 11% in patients with established ASCVD.55 They also reduced a composite outcome of cardiovascular death or hospitalization for heart failure by 23% in patients with or without ASCVD or heart failure at baseline.55 GLP-1 receptor agonists offer a similar reduction in MACE to SGLT2 inhibitors, but they do not have significant effects in heart failure.56 Thiazolidinediones (TZDs), saxagliptin, and alogliptin should be avoided in patients with heart failure.57 TZDs may reduce the risk for recurrent stroke in patients with T2D.58
Chronic kidney disease (CKD). As with ASCVD, prioritize SGLT2 inhibitors and GLP-1 receptor agonists in patients with CKD. While both classes reduced the risk for progression of kidney disease such as macroalbuminuria, SGLT2 inhibitors offer additional benefits in their reduction of the worsening of estimated glomerular filtration rate, end-stage kidney disease, and renal death.56
Obesity. Consider the effect of each drug class on weight when making initial treatment choices, taking special care to minimize weight gain and potentially promote weight loss.34 The ADA prefers GLP-1 receptor agonists, but also suggests SGLT2 inhibitors in these patients. While all GLP-1 receptor agonists have an impact on weight, weekly subcutaneous semaglutide offers the most pronounced weight loss of 2 to 7 kg over 56 weeks.59 SGLT2 inhibitors promote sustainable weight loss to a lesser degree, contributing to an average loss of 3 kg at 2 years.60 Weight gain is common in patients taking sulfonylureas (2.01-2.3 kg)31 and insulin (3-9 kg weight gain in the first year)61 and should be avoided in patients with T2D and obesity.34
Hypoglycemia risk. In addition to counseling patients on hypoglycemia management and prescribing glucagon rescue kits, offer medications with no or very low risk for hypoglycemia (eg, GLP-1 receptor agonists, SGLT2 inhibitors, dipeptidyl peptidase-4 inhibitors, and TZDs). Generally, avoid insulin and sulfonylureas in patients in whom hypoglycemia is a major concern (eg, older adults, individuals with labile blood glucose levels).34 Patients with reduced renal function are at higher risk for hypoglycemia with insulin or sulfonylureas due to reduced drug clearance. However, insulin is often the only treatment for patients with advanced renal disease. Pay close attention to insulin dosing in patients with advanced renal disease, which may necessitate lower doses and smaller dose adjustments due to this risk.
Social determinants of health. Medication access and cost is a major burden in T2D management and should be considered for every patient. Compared with the period of 2005 to 2007, the annual cost of diabetes medications for an individual in 2015 to 2017 increased by 147%, rising from $1106 to $2727 per year.62 This increase is driven by the cost of insulin and newer medications without generic options.62 Identify local resources in your community, such as patient assistance programs and pharmacies with reduced-price generic prescription programs, which may be useful for patients who are underinsured or uninsured.
Continue to: Even if cost weren't an issue...
Even if cost weren’t an issue, many medications such as insulin and GLP-1 receptor agonists should be kept refrigerated and are only stable at room temperature for a limited time. Medications that are stable at room temperature should be prioritized in patients with limited or inconsistent access to refrigeration or unstable housing who may find it difficult to store their medications appropriately.
Do not delay insulin initiation in patients with high baseline A1C
Whenever possible, a GLP-1 receptor agonist is the preferred injectable medication to insulin. Starting insulin introduces numerous risks, including hypoglycemia, weight gain, and stigma. However, in the patient with newly diagnosed T2D, choose basal insulin when the baseline hyperglycemia is severe,34 as indicated by:
- blood glucose > 300 mg/dL (16.7 mmol/L),
- A1C > 10% (86 mmol/mol),
- symptoms of hyperglycemia (polyuria or polydipsia), or
- evidence of catabolism (weight loss, hypertriglyceridemia, ketosis).
Basal insulin analogs are preferred over NPH given their reduced variability, dosing, and hypoglycemic risk.35 Mixed insulins may be used if a patient is unable to afford an insulin analog, which can be quite costly. However, extensive counseling on dosing and management of hypoglycemia is crucial to patient safety with these agents. The ADA recommends initiating 0.1 to 0.2 units/kg of basal insulin daily or 10 units daily.34 The AACE follows this recommendation for patients with baseline A1C < 8%, but it proposes a more aggressive initiation of 0.2 to 0.3 units/kg/d for patients with baseline A1C > 8%.35 Titrate the dose by 2 units every 3 days to reach the target fasting blood glucose level. As hyperglycemia resolves, simplify the regimen and transition to noninsulin options per the previously discussed considerations.
It’s not just about glycemic control
In addition to the direct effects of hyperglycemia, a T2D diagnosis introduces an increased risk for ASCVD, a reduced ability to fight infection, and heightened risk for depression. Order a lipid panel at the time of T2D diagnosis and initiate lipid management as needed (TABLE 335,63,64). Both the ADA and the American Heart Association recommend starting a moderate-intensity statin as primary prevention for all patients with T2D between 40 and 75 years of age regardless of the 10-year ASCVD risk.63 The AACE uses specific lipid targets and recommends moderate- to high-intensity statin therapy for patients with T2D.35 All recommendations by professional organizations list high-intensity statins for patients with established ASCVD.

It is also vital to recommend that patients with newly diagnosed T2D remain up to date on all indicated vaccinations. They should promptly receive the hepatitis B and pneumococcal vaccines if they have not already done so for a previous indication. COVID-19 and annual influenza vaccines also should be prioritized for these patients.65
Finally, patients with diabetes are twice as likely to develop depression than patients without diabetes.66 Individuals with T2D and depression exhibit poorer medication adherence, lifestyle choices, and glycemic control.66 Screen for and treat these issues in all patients with T2D across the course of the disease.
Overall, work closely with patients to support them in managing their new diagnosis with evidence-based pharmacologic and nonpharmacologic approaches. The importance of lifestyle changes including high-fiber diets, regular exercise, and weight loss should not be overlooked. Do not delay starting pharmacotherapy after diagnosing T2D and consider medication-specific and patient-specific factors to individualize therapy, improve adherence, and prevent complications.
CORRESPONDENCE
Jennie B. Jarrett, PharmD, MMedEd, 833 South Wood Street (MC 886), Chicago, IL 60612; [email protected]
Nearly 40 antihyperglycemic agents have been approved by the US Food and Drug Administration (FDA) since the approval of human insulin in 1982.1 In addition, existing antihyperglycemic medications are constantly gaining FDA approval for new indications for common type 2 diabetes (T2D) comorbidities. For example, in addition to their glycemic benefits, the sodium-glucose cotransporter-2 (SGLT2) inhibitors have been approved for use in patients with T2D and established atherosclerotic cardiovascular disease (ASCVD) to reduce the risk for major adverse cardiovascular events (MACE; canagliflozin), risk for hospitalization for heart failure (dapagliflozin), and cardiovascular death (empagliflozin).2-4
The plethora of new agents and new data for existing agents, coupled with the annual release of guidelines from the American Diabetes Association (ADA) and practice recommendations from several other professional organizations,5-7 make it challenging for family physicians to stay current and provide the most up-to-date, evidence-based care. In this article, we provide advice on how to approach the screening, diagnosis, and evaluation of T2D, and on how to manage newly diagnosed T2D.
Screening, Dx, and evaluation: A quick review
Screening
Screening recommendations vary among professional organizations (TABLE 15,6,8). The US Preventive Services Task Force (USPSTF) recommends screening adults ages 35 to 70 years who are overweight or obese. Clinicians also can consider screening patients with a higher risk for diabetes.5 The ADA suggests screening all adults starting at 35 years, regardless of risk factors.8 Asymptomatic adults of any age with overweight or obesity and 1 or more risk factors should be screened.8

Making the diagnosis
The initial diagnosis of diabetes can be made by a fasting plasma glucose level ≥ 126 mg/dL (7.0 mmol/L); a 2-hour plasma glucose level ≥ 200 mg/dL (11.0 mmol/L) following an oral glucose tolerance test; or an A1C level ≥ 6.5%. Prioritize lab-drawn A1C measurements over point-of-care tests to diagnose T2D. In patients with classic symptoms of hyperglycemia, a random plasma glucose level ≥ 200 mg/dL (11.0 mmol/L) is also diagnostic. Generally, these tests are considered equally appropriate in screening for diabetes and may be used to detect prediabetes. In the absence of clear symptoms of hyperglycemia, the diagnosis of diabetes requires 2 abnormal screening test results, either via 1 blood sample (such as an abnormal A1C and glucose) or 2 separate blood samples of the same test. Further evaluation is advised if there is discordance between the 2 samples.8
Extended evaluations
Patients with newly diagnosed T2D require a thorough evaluation for comorbidities and complications of diabetes. Refer patients to an ophthalmologist for a dilated eye examination, with subsequent exams occurring every 1 to 2 years.6,9 Additional referrals for diabetes education, family planning for women of reproductive age, and dental, social, or mental health services may be clinically appropriate.9
Setting goals for glycemic control
Glycemic control is commonly monitored by the A1C level and by blood glucose monitoring either through traditional point-of-care glucometers or continuous glucose monitors (CGMs).10 Generally, CGMs provide more glycemic data than traditional glucometers and may cue patients to choose healthier dietary options and engage in physical exercise.11 Patients with T2D who use CGMs exhibit lower A1Cs, greater time in glycemic range, and reduced hypoglycemic episodes.11 Generally, CGMs are reserved for patients with type 1 diabetes and patients with T2D who use multiple daily injections, subcutaneous insulin infusions, or basal insulin only.12 Most professional organizations recommend that clinicians consider patient-specific factors to set individualized glycemic goals.6,10,13,14 For example, more stringent glycemic goals could be pursued for patients with longer life expectancy, shorter disease duration, absence of complications (eg, nephropathy, neuropathy, or cardiovascular disease), fewer comorbid conditions, lower hypoglycemia risk, or higher cognitive function.6
More specific A1C goals vary by professional organization. For nonpregnant adults, the ADA recommends an A1C goal of < 7% and a preprandial blood glucose level of 80 to 130 mg/dL (4.4-7.2 mmol/L).10 However, a lower A1C goal may be appropriate if it can be attained safely without causing hypoglycemia or other adverse effects.10 The AACE suggests an A1C goal of ≤ 6.5% and a fasting blood glucose level of < 110 mg/dL when it can be achieved safely.6 More stringent A1C goals may reduce long-term micro- and macrovascular complications—especially in patients with newly diagnosed T2D.10 While older studies such as the ACCORD trial found increased mortality in groups with more stringent glycemic targets, they did not include newer agents (SGLT2 inhibitors or glucagon-like peptide-1 [GLP-1] receptor agonists) that reduce cardiovascular events by mechanisms outside their glycemic-lowering effect. With these newer agents, more aggressive A1C goals can be targeted safely in select patients, particularly those with long life expectancy.10 Both the ADA and AACE recommend a less stringent A1C goal of 7% to 8% for patients with limited life expectancy or risks (eg, a history of hypoglycemia) that outweigh expected benefits.6,10
Continue to: Lifestyle modifications
Lifestyle modifications: As important as medication
Nutrition
The energy-dense Western diet, combined with sedentary behavior, are thought to be a primary cause of T2D.15 Therefore, include lifestyle modifications in the initial management of newly diagnosed T2D. Diets that replace carbohydrates with saturated and trans fats are related to increased mortality in patients with T2D.16 Increased consumption of vegetables, fruits, legumes, nuts, fish, cereal, and oils reduces concentrations of saturated and trans fats and increases dietary intake of monounsaturated fatty acids, fiber, antioxidants, and polyphenols.17
Increasing the intake of fiber, an undigestible carbohydrate, offers numerous benefits in T2D management. High-fiber diets can help regulate blood sugar and lipid levels, increase satiety, reduce inflammation, aid in weight management, and reduce premature mortality.18 Insoluble fiber, found in foods such as whole wheat flour, nuts, and cauliflower, helps food pass more quickly through the stomach and intestines and adds bulk to stool. Soluble fiber, found in foods such as chickpeas, lentils, and Brussels sprouts, absorbs water and forms a gel-like substance that protects nutrients from digestive enzymes and slows down digestion. The result is a more gradual rise in postprandial glucose levels and improved insulin sensitivity.19 Dietary fiber may produce short-chain fatty acids which in turn activate incretin secretion and stimulate a glucose-dependent release of insulin from the pancreas.20
Simple dietary substitutions, such as whole grains and legumes for white rice, can reduce fasting blood glucose and A1C levels.21 In a randomized controlled trial (RCT), increasing whole grain oat intake improved measures of glycemic control, reducing A1C by 1% at 1-year follow-up.19 Encourage patients with T2D to increase consumption of high-fiber foods and replace animal fats and refined grains with vegetable fats (eg, nuts, avocados, olives). Nutritional therapies should be individualized, taking into account personal preferences and cultural customs.22 Nutritional habits may be based on race/ethnicity, religion/spirituality, or even the city in which an individual resides. Nutrition recommendations should account for these differences as well as access to healthy foods. For instance, ethnic groups whose dietary patterns include tortillas could be counseled to choose high-fiber options such as corn instead of flour tortillas and to incorporate vegetables in place of high-fat foods. Additionally, ethnic groups who favor using animal fats in foods such as greens could be advised on ways to add flavor to vegetables without adding saturated fats. Taking this approach may lessen barriers to change and increase ability to make dietary modifications.23
Exercise
Encourage all patients with T2D to exercise regularly. The atherosclerotic plaques found in patients with T2D have increased inflammatory properties and result in worse cardiovascular outcomes compared with plaques in individuals without T2D.24 Regular exercise reduces levels of pro-inflammatory markers—C-reactive protein, interleukin (IL)-6, and tumor necrosis factor alpha—and increases levels of anti-inflammatory markers (IL-4 and IL-10).24 Regular exercise can improve body composition, physical fitness, lipid and glucose metabolism, and insulin sensitivity.25,26
A meta-analysis of RCTs demonstrated that structured exercise > 150 minutes per week resulted in A1C reductions of 0.89%,27 which is comparable to the effect of many oral antihyperglycemic medications.26 The Health Benefits of Aerobic and Resistance Training in individuals with T2D (HART-D) and Diabetes Aerobic and Resistance Exercise (DARE) studies demonstrated that combining endurance and resistance training was superior for improving glycemic control, cardiorespiratory fitness, and body composition, than using either type of training alone.25 Both the American College of Sports Medicine (ACSM) and the ADA recommend that adults engage in at least 150 total minutes of moderate-intensity aerobic activity per week and resistance training 2 to 3 times weekly.26 ACSM defines moderate-intensity exercise as 65% to 75% of maximal heart rate, a rating of perceived exertion of 3 to 4, or a step rate of 100 steps per minute.28
Continue to: Because of their longitudinal relationships...
Because of their longitudinal relationships with patients, family physicians are in an optimal position to assess a patient’s physical capacity level and provide individualized counseling. Several systematic reviews have demonstrated that counseling on exercise increases patients’ participation in physical activity.29 Encourage your patients with T2D to exercise regularly, considering each individual’s ability to engage in physical activity.
Weight loss
Include weight management in the initial treatment of patients with newly diagnosed T2D. Weight loss decreases hepatic glucose production and increases peripheral insulin sensitivity and insulin secretion.30 Moderate decreases in weight (5%-10%) can reduce complications related to diabetes, and sustained significant weight loss (> 10%) can potentially cause T2D remission (A1C < 6.5% after stopping diabetes medications).31,32
Diabetes self-management education supports patients by giving them tools for making and maintaining lifestyle changes. Understanding individual barriers to change and addressing these during motivational interviews is important. Through a qualitative interview study, participants in a diabetes self-management program revealed 4 factors that motivated them to maintain lifestyle changes: support from others, experiencing the impact of the changes they made, fear of T2D complications, and forming new habits.33 Family physicians are key in helping patients acquire knowledge and support to make the lifestyle modifications needed to manage newly diagnosed T2D.
Individualized pharmacotherapy considerations
For decades, the initial pharmacotherapeutic regimen for patients with newly diagnosed T2D considered the patient’s baseline A1C as a major driver for therapy. Metformin has been the mainstay in T2D treatment due to its clinical efficacy, minimal risk for hypoglycemia, and low cost. Regardless of the regimen, pharmacotherapy should be initiated at the time of T2D diagnosis in conjunction with the aforementioned lifestyle modifications.34
When selecting pharmacotherapy, practice guidelines recommend considering the efficacy and adverse effects of medications, patient-specific comorbidities, adherence, cost, and a patient’s lifestyle factors.34 Drug classes with pertinent information are listed in TABLE 2.34-54 After starting medication, monitor the A1C level every 3 months to determine whether therapy should be intensified. Patients should have their labs drawn ahead of the quarterly visit, or point-of-care measurements may be used to facilitate in-person patient–provider discussions.


Continue to: Consider patient-specific factors when starting pharmacotherapy
Consider patient-specific factors when starting pharmacotherapy
ASCVD. Regardless of baseline glycemic control, offer patients who have ASCVD, or who are at high risk for it, an SGLT2 inhibitor (canagliflozin, dapagliflozin, or empagliflozin) or a long-acting GLP-1 receptor agonist (dulaglutide, liraglutide, or semaglutide).34,35 SGLT2 inhibitors reduced the risk for MACE by 11% in patients with established ASCVD.55 They also reduced a composite outcome of cardiovascular death or hospitalization for heart failure by 23% in patients with or without ASCVD or heart failure at baseline.55 GLP-1 receptor agonists offer a similar reduction in MACE to SGLT2 inhibitors, but they do not have significant effects in heart failure.56 Thiazolidinediones (TZDs), saxagliptin, and alogliptin should be avoided in patients with heart failure.57 TZDs may reduce the risk for recurrent stroke in patients with T2D.58
Chronic kidney disease (CKD). As with ASCVD, prioritize SGLT2 inhibitors and GLP-1 receptor agonists in patients with CKD. While both classes reduced the risk for progression of kidney disease such as macroalbuminuria, SGLT2 inhibitors offer additional benefits in their reduction of the worsening of estimated glomerular filtration rate, end-stage kidney disease, and renal death.56
Obesity. Consider the effect of each drug class on weight when making initial treatment choices, taking special care to minimize weight gain and potentially promote weight loss.34 The ADA prefers GLP-1 receptor agonists, but also suggests SGLT2 inhibitors in these patients. While all GLP-1 receptor agonists have an impact on weight, weekly subcutaneous semaglutide offers the most pronounced weight loss of 2 to 7 kg over 56 weeks.59 SGLT2 inhibitors promote sustainable weight loss to a lesser degree, contributing to an average loss of 3 kg at 2 years.60 Weight gain is common in patients taking sulfonylureas (2.01-2.3 kg)31 and insulin (3-9 kg weight gain in the first year)61 and should be avoided in patients with T2D and obesity.34
Hypoglycemia risk. In addition to counseling patients on hypoglycemia management and prescribing glucagon rescue kits, offer medications with no or very low risk for hypoglycemia (eg, GLP-1 receptor agonists, SGLT2 inhibitors, dipeptidyl peptidase-4 inhibitors, and TZDs). Generally, avoid insulin and sulfonylureas in patients in whom hypoglycemia is a major concern (eg, older adults, individuals with labile blood glucose levels).34 Patients with reduced renal function are at higher risk for hypoglycemia with insulin or sulfonylureas due to reduced drug clearance. However, insulin is often the only treatment for patients with advanced renal disease. Pay close attention to insulin dosing in patients with advanced renal disease, which may necessitate lower doses and smaller dose adjustments due to this risk.
Social determinants of health. Medication access and cost is a major burden in T2D management and should be considered for every patient. Compared with the period of 2005 to 2007, the annual cost of diabetes medications for an individual in 2015 to 2017 increased by 147%, rising from $1106 to $2727 per year.62 This increase is driven by the cost of insulin and newer medications without generic options.62 Identify local resources in your community, such as patient assistance programs and pharmacies with reduced-price generic prescription programs, which may be useful for patients who are underinsured or uninsured.
Continue to: Even if cost weren't an issue...
Even if cost weren’t an issue, many medications such as insulin and GLP-1 receptor agonists should be kept refrigerated and are only stable at room temperature for a limited time. Medications that are stable at room temperature should be prioritized in patients with limited or inconsistent access to refrigeration or unstable housing who may find it difficult to store their medications appropriately.
Do not delay insulin initiation in patients with high baseline A1C
Whenever possible, a GLP-1 receptor agonist is the preferred injectable medication to insulin. Starting insulin introduces numerous risks, including hypoglycemia, weight gain, and stigma. However, in the patient with newly diagnosed T2D, choose basal insulin when the baseline hyperglycemia is severe,34 as indicated by:
- blood glucose > 300 mg/dL (16.7 mmol/L),
- A1C > 10% (86 mmol/mol),
- symptoms of hyperglycemia (polyuria or polydipsia), or
- evidence of catabolism (weight loss, hypertriglyceridemia, ketosis).
Basal insulin analogs are preferred over NPH given their reduced variability, dosing, and hypoglycemic risk.35 Mixed insulins may be used if a patient is unable to afford an insulin analog, which can be quite costly. However, extensive counseling on dosing and management of hypoglycemia is crucial to patient safety with these agents. The ADA recommends initiating 0.1 to 0.2 units/kg of basal insulin daily or 10 units daily.34 The AACE follows this recommendation for patients with baseline A1C < 8%, but it proposes a more aggressive initiation of 0.2 to 0.3 units/kg/d for patients with baseline A1C > 8%.35 Titrate the dose by 2 units every 3 days to reach the target fasting blood glucose level. As hyperglycemia resolves, simplify the regimen and transition to noninsulin options per the previously discussed considerations.
It’s not just about glycemic control
In addition to the direct effects of hyperglycemia, a T2D diagnosis introduces an increased risk for ASCVD, a reduced ability to fight infection, and heightened risk for depression. Order a lipid panel at the time of T2D diagnosis and initiate lipid management as needed (TABLE 335,63,64). Both the ADA and the American Heart Association recommend starting a moderate-intensity statin as primary prevention for all patients with T2D between 40 and 75 years of age regardless of the 10-year ASCVD risk.63 The AACE uses specific lipid targets and recommends moderate- to high-intensity statin therapy for patients with T2D.35 All recommendations by professional organizations list high-intensity statins for patients with established ASCVD.

It is also vital to recommend that patients with newly diagnosed T2D remain up to date on all indicated vaccinations. They should promptly receive the hepatitis B and pneumococcal vaccines if they have not already done so for a previous indication. COVID-19 and annual influenza vaccines also should be prioritized for these patients.65
Finally, patients with diabetes are twice as likely to develop depression than patients without diabetes.66 Individuals with T2D and depression exhibit poorer medication adherence, lifestyle choices, and glycemic control.66 Screen for and treat these issues in all patients with T2D across the course of the disease.
Overall, work closely with patients to support them in managing their new diagnosis with evidence-based pharmacologic and nonpharmacologic approaches. The importance of lifestyle changes including high-fiber diets, regular exercise, and weight loss should not be overlooked. Do not delay starting pharmacotherapy after diagnosing T2D and consider medication-specific and patient-specific factors to individualize therapy, improve adherence, and prevent complications.
CORRESPONDENCE
Jennie B. Jarrett, PharmD, MMedEd, 833 South Wood Street (MC 886), Chicago, IL 60612; [email protected]
1. Dahlén AD, Dashi G, Maslov I, et al. Trends in antidiabetic drug discovery: FDA approved drugs, new drugs in clinical trials and global sales. Front Pharmacol. 2022;12. Accessed April 19, 2023. www.frontiersin.org/article/10.3389/fphar.2021.807548
2. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128. doi: 10.1056/NEJMoa1504720
3. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644-657. doi: 10.1056/NEJMoa1611925
4. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357. doi: 10.1056/NEJMoa1812389
5. Davidson KW, Barry MJ, et al. Screening for prediabetes and type 2 diabetes: US Preventive Services Task Force recommendation statement. JAMA. 2021;326:736-743. doi: 10.1001/jama. 2021.12531
6. Handelsman Y, Bloomgarden ZT, Grunberger G, et al. American Association of Clinical Endocrinologists and American College of Endocrinology - clinical practice guidelines for developing a diabetes mellitus comprehensive care plan - 2015. Endocr Pract. 2015;21(suppl 1):1-87. doi: 10.4158/EP15672.GL
7. ADA. Introduction: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S1-S2. doi: 10.2337/dc22-Sint
8. ADA Professional Practice Committee. Classification and diagnosis of diabetes: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S17-S38. doi: 10.2337/dc22-S002
9. ADA Professional Practice Committee. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S46-S59. doi: 10.2337/dc22-S004
10. ADA Professional Practice Committee. Glycemic targets: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S83-S96. doi: 10.2337/dc22-S006
11. Janapala RN, Jayaraj JS, Fathima N, et al. Continuous glucose monitoring versus self-monitoring of blood glucose in type 2 diabetes mellitus: a systematic review with meta-analysis. Cureus. 2019;11:e5634. doi: 10.7759/cureus.5634
12. ADA Professional Practice Committee. Diabetes technology: standards of medical care in diabetes - 2022. Diabetes Care. 2021;45(suppl 1):S97-S112. doi: 10.2337/dc22-S007
13. Qaseem A, Wilt TJ, Kansagara D, et al. Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: a guidance statement update from the American College of Physicians. Ann Intern Med. 2018;168:569-576. doi: 10.7326/M17-0939
14. Moran GM, Bakhai C, Song SH, et al, Guideline Committee. Type 2 diabetes: summary of updated NICE guidance. BMJ. 2022;377:o775. doi: 10.1136/bmj.o775
15. Kolb H, Martin S. Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med. 2017;15:131. doi: 10.1186/s12916-017-0901-x
16. McMacken M, Shah S. A plant-based diet for the prevention and treatment of type 2 diabetes. J Geriatr Cardiol. 2017;14:342-354. doi: 10.11909/j.issn.1671-5411.2017.05.009
17. Asif M. The prevention and control the type-2 diabetes by changing lifestyle and dietary pattern. J Educ Health Promot. 2014;3:1. doi: 10.4103/2277-9531.127541
18. Reynolds AN, Akerman AP, Mann J. Dietary fibre and whole grains in diabetes management: systematic review and meta-analyses. PLoS Med. 2020;17(3):e1003053. doi: 10.1371/journal.pmed.1003053
19. Li X, Cai X, Ma X, et al. Short- and long-term effects of wholegrain oat intake on weight management and glucolipid metabolism in overweight type-2 diabetics: a randomized control trial. Nutrients. 2016;8:549. doi: 10.3390/nu8090549
20. Fujii H, Iwase M, Ohkuma T, et al. Impact of dietary fiber intake on glycemic control, cardiovascular risk factors and chronic kidney disease in Japanese patients with type 2 diabetes mellitus: the Fukuoka Diabetes Registry. Nutr J. 2013;12:159. doi: 10.1186/1475-2891-12-159
21. Kim M, Jeung SR, Jeong TS, et al. Replacing with whole grains and legumes reduces Lp-PLA2 activities in plasma and PBMCs in patients with prediabetes or T2D. J Lipid Res. 2014;55:1762-1771. doi: 10.1194/jlr.M044834
22. Evert AB, Dennison M, Gardner CD, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care. 2019;42:731-754. doi: 10.2337/dci19-0014
23. Caballero AE. The “a to z” of managing type 2 diabetes in culturally diverse populations. Front Endocrinol. 2018;9:479. doi: 10.3389/fendo.2018.00479
24. Golbidi S, Badran M, Laher I. Antioxidant and anti-inflammatory effects of exercise in diabetic patients. Exp Diabetes Res. 2012; 2012:941868. doi: 10.1155/2012/941868
25. Karstoft K, Pedersen BK. Exercise and type 2 diabetes: focus on metabolism and inflammation. Immunol Cell Biol. 2016;94:146-150. doi: 10.1038/icb.2015.101
26. Dugan JA. Exercise recommendations for patients with type 2 diabetes. JAAPA. 2016;29:13-18. doi: 10.1097/01.JAA. 0000475460.77476.f6
27. Umpierre D, Ribeiro PA, Kramer CK, et al. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2011;305:1790–1799. doi: 10.1001/jama.2011.576
28. Zuhl M. Tips for monitoring aerobic exercise intensity. 2020. Accessed April 19, 2023. www.acsm.org/docs/default-source/files-for-resource-library/exercise-intensity-infographic.pdf? sfvrsn=f467c793_2
29. Williams A, Radford J, O’Brien J, Davison K. Type 2 diabetes and the medicine of exercise: the role of general practice in ensuring exercise is part of every patient’s plan. Aust J Gen Pract. 2020;49:189-193. doi: 10.31128/AJGP-09-19-5091
30. Grams J, Garvey WT. Weight loss and the prevention and treatment of type 2 diabetes using lifestyle therapy, pharmacotherapy, and bariatric surgery: mechanisms of action. Curr Obes Rep. 2015;4:287-302. doi: 10.1007/s13679-015-0155-x
31. Apovian CM, Okemah J, O’Neil PM. Body weight considerations in the management of type 2 diabetes. Adv Ther. 2019;36:44-58. doi: 10.1007/s12325-018-0824-8
32. Lean MEJ, Leslie WS, Barnes AC, et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol. 2019;7:344-355. doi: 10.1016/S2213-8587(19)30068-3
33. Rise MB, Pellerud A, Rygg LØ, et al. Making and maintaining lifestyle changes after participating in group based type 2 diabetes self-management educations: a qualitative study. PLoS One. 2013;8:e64009. doi: 10.1371/journal.pone.0064009
34. ADA Professional Practice Committee. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S125-S143. doi: 10.2337/dc22-S009
35. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract. 2020;26:107-139. doi: 10.4158/CS-2019-0472
36. Metformin. Package insert. Bristol-Myers Squibb Company; 2017.
37. Invokana (canagliflozin). Package insert. Janssen Pharmaceuticals, Inc; 2020.
38. Farxiga (dapagliflozin). Package insert. AstraZeneca Pharmaceuticals LP; 2021.
39. Jardiance (empagliflozin). Package insert. Boehringer Ingelheim Pharmaceuticals, Inc; 2022.
40. Steglatro (ertugliflozin). Package insert. Merck & Co, Inc; 2021.
41. Trulicity (dulaglutide). Package insert. Lilly USA, LLC; 2022.
42. Byetta (exenatide). Package insert. AstraZeneca Canada Inc; 2022.
43. Bydureon (exenatide ER). Package insert. AstraZeneca Pharmaceuticals LP; 2022.
44. Victoza (liraglutide). Package insert. Novo Nordisk; 2022.
45. Adlyxin (lixisenatide). Package insert. Sanofi-Aventis US LLC; 2022.
46. Ozempic (semaglutide). Package insert. Novo Nordisk; 2022.
47. Alogliptin. Package insert. Takeda Pharmaceuticals USA, Inc; 2022.
48. Linagliptin. Package insert. Boehringer Ingelheim Pharmaceuticals, Inc; 2022.
49. Saxagliptin. Package insert. AstraZeneca Pharmaceuticals LP; 2019.
50. Januvia (sitagliptin). Package insert. Merck Sharp & Dohme LLC; 2022.
51. Glimepiride. Package insert. Sanofi-Aventis US LLC; 2009.
52. Glipizide. Package insert. Roerig; 2023.
53. Glyburide. Package insert. Sanofi-Aventis US LLC; 2009.
54. Pioglitazone. Package insert. Northstar Rx LLC; 2022.
55. Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31-39. doi: 10.1016/S0140-6736(18)32590-X
56. Zelniker TA, Wiviott SD, Raz I, et al. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation. 2019;139:2022-2031. doi: 10.1161/CIRCULATIONAHA.118.038868
57. FDA. FDA Drug Safety Communication: FDA adds warnings about heart failure risk to labels of type 2 diabetes medicines containing saxagliptin and alogliptin. Accessed April 19, 2023. www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-adds-warnings-about-heart-failure-risk-labels-type-2-diabetes
58. Wilcox R, Bousser MG, Betteridge DJ, et al. Effects of pioglitazone in patients with type 2 diabetes with or without previous stroke: results from PROactive (PROspective pioglitAzone Clinical Trial In macroVascular Events 04). Stroke. 2007;38:865-873. doi: 10.1161/01.STR.0000257974.06317.49
59. Lingvay I, Hansen T, Macura S, et al. Superior weight loss with once-weekly semaglutide versus other glucagon-like peptide-1 receptor agonists is independent of gastrointestinal adverse events. BMJ Open Diabetes Res Care. 2020;8:e001706. doi: 10.1136/bmjdrc-2020-001706
60. Liu XY, Zhang N, Chen R, et al. Efficacy and safety of sodium-glucose cotransporter 2 inhibitors in type 2 diabetes: a meta-analysis of randomized controlled trials for 1 to 2 years. J Diabetes Complications. 2015;29:1295-1303. doi: 10.1016/j.jdiacomp.2015.07.011
61. Brown A, Guess N, Dornhorst A, et al. Insulin-associated weight gain in obese type 2 diabetes mellitus patients: what can be done? Diabetes Obes Metab. 2017;19:1655-1668. doi: 10.1111/dom.13009
62. Zhou X, Shrestha SS, Shao H, et al. Factors contributing to the rising national cost of glucose-lowering medicines for diabetes during 2005-2007 and 2015-2017. Diabetes Care. 2020;43:2396-2402. doi: 10.2337/dc19-2273
63. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082-e1143. doi: 10.1161/CIR.0000000000000625
64. ADA Professional Practice Committee. Cardiovascular disease and risk management: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S144-S174. doi: 10.2337/dc22-S010
65. CDC. Adult immunization schedule by medical condition and other indication. 2022. Accessed April 19, 2023. www.cdc.gov/vaccines/schedules/hcp/imz/adult-conditions.htm
66. Semenkovich K, Brown ME, Svrakic DM, et al. Depression in type 2 diabetes mellitus: prevalence, impact, and treatment. Drugs. 2015;75:577-587. doi: 10.1007/s40265-015-0347-4
1. Dahlén AD, Dashi G, Maslov I, et al. Trends in antidiabetic drug discovery: FDA approved drugs, new drugs in clinical trials and global sales. Front Pharmacol. 2022;12. Accessed April 19, 2023. www.frontiersin.org/article/10.3389/fphar.2021.807548
2. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128. doi: 10.1056/NEJMoa1504720
3. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644-657. doi: 10.1056/NEJMoa1611925
4. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357. doi: 10.1056/NEJMoa1812389
5. Davidson KW, Barry MJ, et al. Screening for prediabetes and type 2 diabetes: US Preventive Services Task Force recommendation statement. JAMA. 2021;326:736-743. doi: 10.1001/jama. 2021.12531
6. Handelsman Y, Bloomgarden ZT, Grunberger G, et al. American Association of Clinical Endocrinologists and American College of Endocrinology - clinical practice guidelines for developing a diabetes mellitus comprehensive care plan - 2015. Endocr Pract. 2015;21(suppl 1):1-87. doi: 10.4158/EP15672.GL
7. ADA. Introduction: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S1-S2. doi: 10.2337/dc22-Sint
8. ADA Professional Practice Committee. Classification and diagnosis of diabetes: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S17-S38. doi: 10.2337/dc22-S002
9. ADA Professional Practice Committee. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S46-S59. doi: 10.2337/dc22-S004
10. ADA Professional Practice Committee. Glycemic targets: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S83-S96. doi: 10.2337/dc22-S006
11. Janapala RN, Jayaraj JS, Fathima N, et al. Continuous glucose monitoring versus self-monitoring of blood glucose in type 2 diabetes mellitus: a systematic review with meta-analysis. Cureus. 2019;11:e5634. doi: 10.7759/cureus.5634
12. ADA Professional Practice Committee. Diabetes technology: standards of medical care in diabetes - 2022. Diabetes Care. 2021;45(suppl 1):S97-S112. doi: 10.2337/dc22-S007
13. Qaseem A, Wilt TJ, Kansagara D, et al. Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: a guidance statement update from the American College of Physicians. Ann Intern Med. 2018;168:569-576. doi: 10.7326/M17-0939
14. Moran GM, Bakhai C, Song SH, et al, Guideline Committee. Type 2 diabetes: summary of updated NICE guidance. BMJ. 2022;377:o775. doi: 10.1136/bmj.o775
15. Kolb H, Martin S. Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med. 2017;15:131. doi: 10.1186/s12916-017-0901-x
16. McMacken M, Shah S. A plant-based diet for the prevention and treatment of type 2 diabetes. J Geriatr Cardiol. 2017;14:342-354. doi: 10.11909/j.issn.1671-5411.2017.05.009
17. Asif M. The prevention and control the type-2 diabetes by changing lifestyle and dietary pattern. J Educ Health Promot. 2014;3:1. doi: 10.4103/2277-9531.127541
18. Reynolds AN, Akerman AP, Mann J. Dietary fibre and whole grains in diabetes management: systematic review and meta-analyses. PLoS Med. 2020;17(3):e1003053. doi: 10.1371/journal.pmed.1003053
19. Li X, Cai X, Ma X, et al. Short- and long-term effects of wholegrain oat intake on weight management and glucolipid metabolism in overweight type-2 diabetics: a randomized control trial. Nutrients. 2016;8:549. doi: 10.3390/nu8090549
20. Fujii H, Iwase M, Ohkuma T, et al. Impact of dietary fiber intake on glycemic control, cardiovascular risk factors and chronic kidney disease in Japanese patients with type 2 diabetes mellitus: the Fukuoka Diabetes Registry. Nutr J. 2013;12:159. doi: 10.1186/1475-2891-12-159
21. Kim M, Jeung SR, Jeong TS, et al. Replacing with whole grains and legumes reduces Lp-PLA2 activities in plasma and PBMCs in patients with prediabetes or T2D. J Lipid Res. 2014;55:1762-1771. doi: 10.1194/jlr.M044834
22. Evert AB, Dennison M, Gardner CD, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care. 2019;42:731-754. doi: 10.2337/dci19-0014
23. Caballero AE. The “a to z” of managing type 2 diabetes in culturally diverse populations. Front Endocrinol. 2018;9:479. doi: 10.3389/fendo.2018.00479
24. Golbidi S, Badran M, Laher I. Antioxidant and anti-inflammatory effects of exercise in diabetic patients. Exp Diabetes Res. 2012; 2012:941868. doi: 10.1155/2012/941868
25. Karstoft K, Pedersen BK. Exercise and type 2 diabetes: focus on metabolism and inflammation. Immunol Cell Biol. 2016;94:146-150. doi: 10.1038/icb.2015.101
26. Dugan JA. Exercise recommendations for patients with type 2 diabetes. JAAPA. 2016;29:13-18. doi: 10.1097/01.JAA. 0000475460.77476.f6
27. Umpierre D, Ribeiro PA, Kramer CK, et al. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2011;305:1790–1799. doi: 10.1001/jama.2011.576
28. Zuhl M. Tips for monitoring aerobic exercise intensity. 2020. Accessed April 19, 2023. www.acsm.org/docs/default-source/files-for-resource-library/exercise-intensity-infographic.pdf? sfvrsn=f467c793_2
29. Williams A, Radford J, O’Brien J, Davison K. Type 2 diabetes and the medicine of exercise: the role of general practice in ensuring exercise is part of every patient’s plan. Aust J Gen Pract. 2020;49:189-193. doi: 10.31128/AJGP-09-19-5091
30. Grams J, Garvey WT. Weight loss and the prevention and treatment of type 2 diabetes using lifestyle therapy, pharmacotherapy, and bariatric surgery: mechanisms of action. Curr Obes Rep. 2015;4:287-302. doi: 10.1007/s13679-015-0155-x
31. Apovian CM, Okemah J, O’Neil PM. Body weight considerations in the management of type 2 diabetes. Adv Ther. 2019;36:44-58. doi: 10.1007/s12325-018-0824-8
32. Lean MEJ, Leslie WS, Barnes AC, et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol. 2019;7:344-355. doi: 10.1016/S2213-8587(19)30068-3
33. Rise MB, Pellerud A, Rygg LØ, et al. Making and maintaining lifestyle changes after participating in group based type 2 diabetes self-management educations: a qualitative study. PLoS One. 2013;8:e64009. doi: 10.1371/journal.pone.0064009
34. ADA Professional Practice Committee. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S125-S143. doi: 10.2337/dc22-S009
35. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract. 2020;26:107-139. doi: 10.4158/CS-2019-0472
36. Metformin. Package insert. Bristol-Myers Squibb Company; 2017.
37. Invokana (canagliflozin). Package insert. Janssen Pharmaceuticals, Inc; 2020.
38. Farxiga (dapagliflozin). Package insert. AstraZeneca Pharmaceuticals LP; 2021.
39. Jardiance (empagliflozin). Package insert. Boehringer Ingelheim Pharmaceuticals, Inc; 2022.
40. Steglatro (ertugliflozin). Package insert. Merck & Co, Inc; 2021.
41. Trulicity (dulaglutide). Package insert. Lilly USA, LLC; 2022.
42. Byetta (exenatide). Package insert. AstraZeneca Canada Inc; 2022.
43. Bydureon (exenatide ER). Package insert. AstraZeneca Pharmaceuticals LP; 2022.
44. Victoza (liraglutide). Package insert. Novo Nordisk; 2022.
45. Adlyxin (lixisenatide). Package insert. Sanofi-Aventis US LLC; 2022.
46. Ozempic (semaglutide). Package insert. Novo Nordisk; 2022.
47. Alogliptin. Package insert. Takeda Pharmaceuticals USA, Inc; 2022.
48. Linagliptin. Package insert. Boehringer Ingelheim Pharmaceuticals, Inc; 2022.
49. Saxagliptin. Package insert. AstraZeneca Pharmaceuticals LP; 2019.
50. Januvia (sitagliptin). Package insert. Merck Sharp & Dohme LLC; 2022.
51. Glimepiride. Package insert. Sanofi-Aventis US LLC; 2009.
52. Glipizide. Package insert. Roerig; 2023.
53. Glyburide. Package insert. Sanofi-Aventis US LLC; 2009.
54. Pioglitazone. Package insert. Northstar Rx LLC; 2022.
55. Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31-39. doi: 10.1016/S0140-6736(18)32590-X
56. Zelniker TA, Wiviott SD, Raz I, et al. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation. 2019;139:2022-2031. doi: 10.1161/CIRCULATIONAHA.118.038868
57. FDA. FDA Drug Safety Communication: FDA adds warnings about heart failure risk to labels of type 2 diabetes medicines containing saxagliptin and alogliptin. Accessed April 19, 2023. www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-adds-warnings-about-heart-failure-risk-labels-type-2-diabetes
58. Wilcox R, Bousser MG, Betteridge DJ, et al. Effects of pioglitazone in patients with type 2 diabetes with or without previous stroke: results from PROactive (PROspective pioglitAzone Clinical Trial In macroVascular Events 04). Stroke. 2007;38:865-873. doi: 10.1161/01.STR.0000257974.06317.49
59. Lingvay I, Hansen T, Macura S, et al. Superior weight loss with once-weekly semaglutide versus other glucagon-like peptide-1 receptor agonists is independent of gastrointestinal adverse events. BMJ Open Diabetes Res Care. 2020;8:e001706. doi: 10.1136/bmjdrc-2020-001706
60. Liu XY, Zhang N, Chen R, et al. Efficacy and safety of sodium-glucose cotransporter 2 inhibitors in type 2 diabetes: a meta-analysis of randomized controlled trials for 1 to 2 years. J Diabetes Complications. 2015;29:1295-1303. doi: 10.1016/j.jdiacomp.2015.07.011
61. Brown A, Guess N, Dornhorst A, et al. Insulin-associated weight gain in obese type 2 diabetes mellitus patients: what can be done? Diabetes Obes Metab. 2017;19:1655-1668. doi: 10.1111/dom.13009
62. Zhou X, Shrestha SS, Shao H, et al. Factors contributing to the rising national cost of glucose-lowering medicines for diabetes during 2005-2007 and 2015-2017. Diabetes Care. 2020;43:2396-2402. doi: 10.2337/dc19-2273
63. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082-e1143. doi: 10.1161/CIR.0000000000000625
64. ADA Professional Practice Committee. Cardiovascular disease and risk management: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S144-S174. doi: 10.2337/dc22-S010
65. CDC. Adult immunization schedule by medical condition and other indication. 2022. Accessed April 19, 2023. www.cdc.gov/vaccines/schedules/hcp/imz/adult-conditions.htm
66. Semenkovich K, Brown ME, Svrakic DM, et al. Depression in type 2 diabetes mellitus: prevalence, impact, and treatment. Drugs. 2015;75:577-587. doi: 10.1007/s40265-015-0347-4
PRACTICE RECOMMENDATIONS
› Individualize lifestyle modifications, considering personal and cultural experiences, health literacy, access to healthy foods, willingness and ability to make behavior changes, and barriers to change. C
› Initiate medication therapy at diagnosis, considering medication efficacy and cost, hypoglycemia risk, weight effects, benefits in cardiovascular and kidney disease, and patient-specific comorbidities. C
› Start basal insulin as first-line therapy in patients with severe baseline hyperglycemia, symptoms of hyperglycemia, or evidence of catabolism. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Low-carb breakfast key to lower glucose variability in T2D?
These findings from a 3-month randomized study in 121 patients in Canada and Australia were published online recently in the American Journal of Clinical Nutrition.
The researchers aimed to determine whether a low-carbohydrate, high-fat breakfast (focused around eggs), compared with a standard, low-fat control breakfast (designed to have no/minimal eggs), would improve blood glucose control in individuals with type 2 diabetes.
“We’ve determined that if the first meal of the day is low-carb and higher in protein and fat we can limit hyperglycemic swings,” lead author Barbara Oliveira, PhD, School of Health and Exercise Sciences, University of British Columbia, Kelowna, said in a press release from the university.
“Having fewer carbs for breakfast not only aligns better with how people with [type 2 diabetes] handle glucose throughout the day,” she noted, “but it also has incredible potential for people with [type 2 diabetes] who struggle with their glucose levels in the morning.”
“By making a small adjustment to the carb content of a single meal rather than the entire diet,” Dr. Oliveira added, “we have the potential to increase adherence significantly while still obtaining significant benefits.”
The researchers conclude that “this trial provides evidence that advice to consume a low-carbohydrate breakfast could be a simple, feasible, and effective approach to manage postprandial hyperglycemia and lower glycemic variability in people living with type 2 diabetes.”
Could breakfast tweak improve glucose control?
People with type 2 diabetes have higher levels of insulin resistance and greater glucose intolerance in the morning, the researchers write.
And consuming a low-fat, high-carbohydrate meal in line with most dietary guidelines appears to incur the highest hyperglycemia spike and leads to higher glycemic variability.
They speculated that eating a low-carb breakfast, compared with a low-fat breakfast, might be an easy way to mitigate this.
They recruited participants from online ads in three provinces in Canada and four states in Australia, and they conducted the study from a site in British Columbia and one in Wollongong, Australia.
The participants were aged 20-79 years and diagnosed with type 2 diabetes. They also had a current hemoglobin A1c < 8.5% and no allergies to eggs, and they were able to follow remote, online guidance.
After screening, the participants had a phone or video conference call with a member of the research team who explained the study.
The researchers randomly assigned 75 participants in Canada and 46 participants in Australia 1:1 to the low-carbohydrate intervention or the control intervention.
The participants had a mean age of 64 and 53% were women. They had a mean weight of 93 kg (204 lb), body mass index of 32 kg/m2, and A1c of 7.0%.
Registered dietitians in Canada and Australia each designed 8-10 recipes/menus for low-carb breakfasts and an equal number of recipes/menus for control (low-fat) breakfasts that were specific for those countries.
Each recipe contains about 450 kcal, and they are available in Supplemental Appendix 1A and 1B, with the article.
Each low-carbohydrate breakfast contains about 25 g protein, 8 g carbohydrates, and 37 g fat. For example, one breakfast is a three-egg omelet with spinach.
Each control (low-fat) recipe contains about 20 g protein, 56 g carbohydrates, and 15 g fat. For example, one breakfast is a small blueberry muffin and a small plain Greek yogurt.
The participants were advised to select one of these breakfasts every day and follow it exactly (they were also required to upload a photograph of their breakfast every morning). They were not given any guidance or calorie restriction for the other meals of the day.
The participants also filled in 3-day food records and answered a questionnaire about exercise, hunger, and satiety, at the beginning, middle, and end of the intervention.
They provided self-reported height, weight, and waist circumference, and they were given requisitions for blood tests for A1c to be done at a local laboratory, at the beginning and end of the intervention.
The participants also wore a continuous glucose monitor (CGM) during the first and last 14 days of the intervention.
Intervention improved CGM measures
There was no significant difference in the primary outcome, change in A1c, at the end of 12 weeks, in the two groups. The mean A1c decreased by 0.3% in the intervention group vs 0.1% in the control group (P = .06).
Similarly, in secondary outcomes, weight and BMI each decreased about 1% and waist circumference decreased by about 2.5 cm in each group at 12 weeks (no significant difference). There were also no significant differences in hunger, satiety, or physical activity between the two groups.
However, the 24-hour CGM data showed that mean and maximum glucose, glycemic variability, and time above range were all significantly lower in participants in the low-carbohydrate breakfast intervention group vs. those in the control group (all P < .05).
Time in range was significantly higher among participants in the intervention group (P < .05).
In addition, the 2-hour postprandial CGM data showed that mean glucose and maximum glucose after breakfast were lower in participants in the low-carbohydrate breakfast group than in the control group.
This work was supported by investigator-initiated operating grants to senior author Jonathan P. Little, PhD, School of Health and Exercise Sciences, University of British Columbia, from the Egg Nutrition Center, United States, and Egg Farmers of Canada. The authors declare that they have no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
These findings from a 3-month randomized study in 121 patients in Canada and Australia were published online recently in the American Journal of Clinical Nutrition.
The researchers aimed to determine whether a low-carbohydrate, high-fat breakfast (focused around eggs), compared with a standard, low-fat control breakfast (designed to have no/minimal eggs), would improve blood glucose control in individuals with type 2 diabetes.
“We’ve determined that if the first meal of the day is low-carb and higher in protein and fat we can limit hyperglycemic swings,” lead author Barbara Oliveira, PhD, School of Health and Exercise Sciences, University of British Columbia, Kelowna, said in a press release from the university.
“Having fewer carbs for breakfast not only aligns better with how people with [type 2 diabetes] handle glucose throughout the day,” she noted, “but it also has incredible potential for people with [type 2 diabetes] who struggle with their glucose levels in the morning.”
“By making a small adjustment to the carb content of a single meal rather than the entire diet,” Dr. Oliveira added, “we have the potential to increase adherence significantly while still obtaining significant benefits.”
The researchers conclude that “this trial provides evidence that advice to consume a low-carbohydrate breakfast could be a simple, feasible, and effective approach to manage postprandial hyperglycemia and lower glycemic variability in people living with type 2 diabetes.”
Could breakfast tweak improve glucose control?
People with type 2 diabetes have higher levels of insulin resistance and greater glucose intolerance in the morning, the researchers write.
And consuming a low-fat, high-carbohydrate meal in line with most dietary guidelines appears to incur the highest hyperglycemia spike and leads to higher glycemic variability.
They speculated that eating a low-carb breakfast, compared with a low-fat breakfast, might be an easy way to mitigate this.
They recruited participants from online ads in three provinces in Canada and four states in Australia, and they conducted the study from a site in British Columbia and one in Wollongong, Australia.
The participants were aged 20-79 years and diagnosed with type 2 diabetes. They also had a current hemoglobin A1c < 8.5% and no allergies to eggs, and they were able to follow remote, online guidance.
After screening, the participants had a phone or video conference call with a member of the research team who explained the study.
The researchers randomly assigned 75 participants in Canada and 46 participants in Australia 1:1 to the low-carbohydrate intervention or the control intervention.
The participants had a mean age of 64 and 53% were women. They had a mean weight of 93 kg (204 lb), body mass index of 32 kg/m2, and A1c of 7.0%.
Registered dietitians in Canada and Australia each designed 8-10 recipes/menus for low-carb breakfasts and an equal number of recipes/menus for control (low-fat) breakfasts that were specific for those countries.
Each recipe contains about 450 kcal, and they are available in Supplemental Appendix 1A and 1B, with the article.
Each low-carbohydrate breakfast contains about 25 g protein, 8 g carbohydrates, and 37 g fat. For example, one breakfast is a three-egg omelet with spinach.
Each control (low-fat) recipe contains about 20 g protein, 56 g carbohydrates, and 15 g fat. For example, one breakfast is a small blueberry muffin and a small plain Greek yogurt.
The participants were advised to select one of these breakfasts every day and follow it exactly (they were also required to upload a photograph of their breakfast every morning). They were not given any guidance or calorie restriction for the other meals of the day.
The participants also filled in 3-day food records and answered a questionnaire about exercise, hunger, and satiety, at the beginning, middle, and end of the intervention.
They provided self-reported height, weight, and waist circumference, and they were given requisitions for blood tests for A1c to be done at a local laboratory, at the beginning and end of the intervention.
The participants also wore a continuous glucose monitor (CGM) during the first and last 14 days of the intervention.
Intervention improved CGM measures
There was no significant difference in the primary outcome, change in A1c, at the end of 12 weeks, in the two groups. The mean A1c decreased by 0.3% in the intervention group vs 0.1% in the control group (P = .06).
Similarly, in secondary outcomes, weight and BMI each decreased about 1% and waist circumference decreased by about 2.5 cm in each group at 12 weeks (no significant difference). There were also no significant differences in hunger, satiety, or physical activity between the two groups.
However, the 24-hour CGM data showed that mean and maximum glucose, glycemic variability, and time above range were all significantly lower in participants in the low-carbohydrate breakfast intervention group vs. those in the control group (all P < .05).
Time in range was significantly higher among participants in the intervention group (P < .05).
In addition, the 2-hour postprandial CGM data showed that mean glucose and maximum glucose after breakfast were lower in participants in the low-carbohydrate breakfast group than in the control group.
This work was supported by investigator-initiated operating grants to senior author Jonathan P. Little, PhD, School of Health and Exercise Sciences, University of British Columbia, from the Egg Nutrition Center, United States, and Egg Farmers of Canada. The authors declare that they have no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
These findings from a 3-month randomized study in 121 patients in Canada and Australia were published online recently in the American Journal of Clinical Nutrition.
The researchers aimed to determine whether a low-carbohydrate, high-fat breakfast (focused around eggs), compared with a standard, low-fat control breakfast (designed to have no/minimal eggs), would improve blood glucose control in individuals with type 2 diabetes.
“We’ve determined that if the first meal of the day is low-carb and higher in protein and fat we can limit hyperglycemic swings,” lead author Barbara Oliveira, PhD, School of Health and Exercise Sciences, University of British Columbia, Kelowna, said in a press release from the university.
“Having fewer carbs for breakfast not only aligns better with how people with [type 2 diabetes] handle glucose throughout the day,” she noted, “but it also has incredible potential for people with [type 2 diabetes] who struggle with their glucose levels in the morning.”
“By making a small adjustment to the carb content of a single meal rather than the entire diet,” Dr. Oliveira added, “we have the potential to increase adherence significantly while still obtaining significant benefits.”
The researchers conclude that “this trial provides evidence that advice to consume a low-carbohydrate breakfast could be a simple, feasible, and effective approach to manage postprandial hyperglycemia and lower glycemic variability in people living with type 2 diabetes.”
Could breakfast tweak improve glucose control?
People with type 2 diabetes have higher levels of insulin resistance and greater glucose intolerance in the morning, the researchers write.
And consuming a low-fat, high-carbohydrate meal in line with most dietary guidelines appears to incur the highest hyperglycemia spike and leads to higher glycemic variability.
They speculated that eating a low-carb breakfast, compared with a low-fat breakfast, might be an easy way to mitigate this.
They recruited participants from online ads in three provinces in Canada and four states in Australia, and they conducted the study from a site in British Columbia and one in Wollongong, Australia.
The participants were aged 20-79 years and diagnosed with type 2 diabetes. They also had a current hemoglobin A1c < 8.5% and no allergies to eggs, and they were able to follow remote, online guidance.
After screening, the participants had a phone or video conference call with a member of the research team who explained the study.
The researchers randomly assigned 75 participants in Canada and 46 participants in Australia 1:1 to the low-carbohydrate intervention or the control intervention.
The participants had a mean age of 64 and 53% were women. They had a mean weight of 93 kg (204 lb), body mass index of 32 kg/m2, and A1c of 7.0%.
Registered dietitians in Canada and Australia each designed 8-10 recipes/menus for low-carb breakfasts and an equal number of recipes/menus for control (low-fat) breakfasts that were specific for those countries.
Each recipe contains about 450 kcal, and they are available in Supplemental Appendix 1A and 1B, with the article.
Each low-carbohydrate breakfast contains about 25 g protein, 8 g carbohydrates, and 37 g fat. For example, one breakfast is a three-egg omelet with spinach.
Each control (low-fat) recipe contains about 20 g protein, 56 g carbohydrates, and 15 g fat. For example, one breakfast is a small blueberry muffin and a small plain Greek yogurt.
The participants were advised to select one of these breakfasts every day and follow it exactly (they were also required to upload a photograph of their breakfast every morning). They were not given any guidance or calorie restriction for the other meals of the day.
The participants also filled in 3-day food records and answered a questionnaire about exercise, hunger, and satiety, at the beginning, middle, and end of the intervention.
They provided self-reported height, weight, and waist circumference, and they were given requisitions for blood tests for A1c to be done at a local laboratory, at the beginning and end of the intervention.
The participants also wore a continuous glucose monitor (CGM) during the first and last 14 days of the intervention.
Intervention improved CGM measures
There was no significant difference in the primary outcome, change in A1c, at the end of 12 weeks, in the two groups. The mean A1c decreased by 0.3% in the intervention group vs 0.1% in the control group (P = .06).
Similarly, in secondary outcomes, weight and BMI each decreased about 1% and waist circumference decreased by about 2.5 cm in each group at 12 weeks (no significant difference). There were also no significant differences in hunger, satiety, or physical activity between the two groups.
However, the 24-hour CGM data showed that mean and maximum glucose, glycemic variability, and time above range were all significantly lower in participants in the low-carbohydrate breakfast intervention group vs. those in the control group (all P < .05).
Time in range was significantly higher among participants in the intervention group (P < .05).
In addition, the 2-hour postprandial CGM data showed that mean glucose and maximum glucose after breakfast were lower in participants in the low-carbohydrate breakfast group than in the control group.
This work was supported by investigator-initiated operating grants to senior author Jonathan P. Little, PhD, School of Health and Exercise Sciences, University of British Columbia, from the Egg Nutrition Center, United States, and Egg Farmers of Canada. The authors declare that they have no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF CLINICAL NUTRITION






