User login
Are anti-TNF drugs safe for pregnant women with inflammatory bowel disease?
Yes, anti-tumor necrosis factor (anti-TNF) therapy for inflammatory bowel disease (IBD) can be continued during pregnancy.
IBD is often diagnosed and treated in women during their reproductive years. Consequently, these patients face important decisions about the management of their disease and the safety of their baby. Clinicians should be prepared to offer guidance by discussing the risks and benefits of anti-TNF agents with their pregnant patients who have IBD, as well as with those considering pregnancy.
STUDIES OF THE POTENTIAL RISKS
Anti-TNF agents are monoclonal antibodies. Infliximab, adalimumab, and golimumab are actively transported into the fetal circulation via the placenta, mainly during the second and third trimesters. Certolizumab crosses the placenta only by passive means, because it lacks the fragment crystallizable (Fc) region required for placental transfer.1
Effects on pregnancy outcomes
In a 2016 meta-analysis,2 of 1,242 pregnancies in women with IBD, 482 were in women on anti-TNF therapy. It found no statistically significant difference in rates of adverse pregnancy outcomes including congenital abnormality, preterm birth, and low birth weight.
A meta-analysis of 1,216 pregnant women with IBD found no statistically significant differences in rates of spontaneous or elective abortion, preterm birth, low birth weight, or congenital malformation in those on anti-TNF therapy vs controls.3
A systematic review of 58 studies including more than 1,500 pregnant women with IBD who were exposed to anti-TNF agents concluded that there was no association with adverse pregnancy outcomes such as spontaneous abortion, preterm delivery, stillbirth, low birth weight, congenital malformation, or infection.4
A retrospective cohort study of 66 pregnant patients with IBD from several centers in Spain found that anti-TNF or thiopurine therapy during pregnancy did not increase the risk of pregnancy complications or neonatal complications.5
Effects on newborns
Cord blood studies have shown that maternal use of infliximab and adalimumab results in a detectable serum level in newborns, while cord blood levels of certolizumab are much lower.1,6 In some studies, anti-TNF drugs were detectable in infants for up to 6 months after birth, whereas other studies found that detectable serum levels dropped soon after birth.1,7
Addressing concern about an increased risk of infection or dysfunctional immune development in newborns exposed to anti-TNF drugs in utero, a systematic review found no increased risk.4 A retrospective multicenter cohort study of 841 children also reported no association between in utero exposure to anti-TNF agents and risk of severe infection in the short term or long term (mean of 4 years).8 Additional studies are under way to determine long-term risk to the newborn.7
THE TORONTO CONSENSUS GUIDELINES
The Toronto consensus guidelines strongly recommend continuing anti-TNF therapy during pregnancy in women with IBD who began maintenance therapy before conception.6
If a patient strongly prefers to stop therapy during pregnancy to limit fetal exposure, the Toronto consensus recommends giving the last dose at 22 to 24 weeks of gestation. However, this should only be considered in patients whose IBD is in remission and at low risk of relapse.6,9
Although anti-TNF drugs may differ in terms of placental transfer, agents should not be switched in stable patients, as switching increases the risk of relapse.10
BENEFITS OF CONTINUING THERAPY
Active IBD poses a significantly greater risk to the mother and the baby than continuing anti-TNF therapy during pregnancy.1,7 The primary benefit of continuing therapy is to maintain disease remission.
Among women with active IBD at the time of conception, one-third will have improvement in disease activity during the course of their pregnancy, one-third will have no change, and one-third will have worsening of disease activity. But if IBD is in remission at the time of conception, it will remain in remission in nearly 80% of women during pregnancy.1
Women with active IBD are at increased risk of preterm delivery, low birth weight, and intrauterine growth restriction.1,2,5 Also, women with IBD have an increased risk of venous thromboembolism, particularly if they have active disease during pregnancy.1 Therefore, achieving and maintaining remission are vital in the management of the pregnant patient with IBD.
CONSIDERATIONS AFTER BIRTH: BREAST-FEEDING AND VACCINATION
Breast-feeding is considered safe. Minuscule amounts of infliximab or adalimumab are transferred in breast milk but are unlikely to result in systemic immune suppression in the infant.7
Live-attenuated vaccines should be avoided for the first 6 months in infants exposed to anti-TNF agents in utero.1,7,11 All other vaccines, including hepatitis B virus vaccine, should be given according to standard schedules.6
OUR RECOMMENDATIONS
The goal of managing IBD in women of reproductive age is to minimize the risk of adverse outcomes for both mother and baby. We recommend a team approach, working closely with a gastroenterologist and a high-risk-pregnancy obstetrician, if available.
Patients should continue anti-TNF therapy during pregnancy because evidence supports its safety. If a woman wants to stop therapy and is at low risk of relapse, we recommend giving the last dose at 22 to 24 weeks of gestation, then promptly resuming therapy postpartum.
Live-attenuated vaccines (eg, influenza, rotavirus) should be avoided for the first 6 months in babies born to mothers on anti-TNF therapy.
- Ananthakrishnan AN, Xavier RJ, Podolsky DK. Inflammatory Bowel Diseases: A Clinician’s Guide. Chichester, UK: Wiley; 2017. doi:10.1002/9781119077633
- Shihab Z, Yeomans ND, De Cruz P. Anti-tumour necrosis factor alpha therapies and inflammatory bowel disease pregnancy outcomes: a meta-analysis. J Crohns Colitis 2016; 10(8):979–988. doi:10.1093/ecco-jcc/jjv234
- Narula N, Al-Dabbagh, Dhillon A, Sands BE, Marshall JK. Anti-TNF alpha therapies are safe during pregnancy in women with inflammatory bowel disease: a systematic review and meta-analysis. Inflamm Bowel Dis 2014; 20(10):1862–1869. doi:10.1097/MIB.0000000000000092
- Nielsen OH, Loftus EV Jr, Jess T. Safety of TNF-alpha inhibitors during IBD pregnancy: a systematic review. BMC Med 2013; 11:174. doi:10.1186/1741-7015-11-174
- Casanova MJ, Chaparro M, Domenech E, et al. Safety of thiopurines and anti-TNF-alpha drugs during pregnancy in patients with inflammatory bowel disease. Am J Gastroenterol 2013; 108(3):433–440. doi:10.1038/ajg.2012.430
- Nguyen GC, Seow CH, Maxwell C, et al; IBD in Pregnancy Consensus Group; Canadian Association of Gastroenterology. The Toronto consensus statements for the management of inflammatory bowel disease in pregnancy. Gastroenterology 2016; 150(3):734–757.e1. doi:10.1053/j.gastro.2015.12.003
- Gisbert JP, Chaparro, M. Safety of anti-TNF agents during pregnancy and breastfeeding in women with inflammatory bowel disease. Am J Gastroenterol 2013; 108(9):1426–1438. doi:10.1038/ajg.2013.171
- Chaparro M, Verreth A, Lobaton T, et al. Long-term safety of in utero exposure to anti-TNF alpha drugs for the treatment of inflammatory bowel disease: results from the multicenter European TEDDY Study. Am J Gastroenterol 2018; 113(3):396–403. doi:10.1038/ajg.2017.501
- de Lima A, Zelinkova Z, van der Ent C, Steegers EA, van der Woude CJ. Tailored anti-TNF therapy during pregnancy in patients with IBD: maternal and fetal safety. Gut 2016; 65(8):1261–1268. doi:10.1136/gutjnl-2015-309321
- Van Assche G, Vermeire S, Ballet V, et al. Switch to adalimumab in patients with Crohn’s disease controlled by maintenance infliximab: prospective randomised SWITCH trial. Gut 2012; 61(2):229–234. doi:10.1136/gutjnl-2011-300755
- Saha S. Medication management in the pregnant IBD patient. Am J Gastroenterol 2017; 112(5):667–669. doi:10.1038/ajg.2017.22
Yes, anti-tumor necrosis factor (anti-TNF) therapy for inflammatory bowel disease (IBD) can be continued during pregnancy.
IBD is often diagnosed and treated in women during their reproductive years. Consequently, these patients face important decisions about the management of their disease and the safety of their baby. Clinicians should be prepared to offer guidance by discussing the risks and benefits of anti-TNF agents with their pregnant patients who have IBD, as well as with those considering pregnancy.
STUDIES OF THE POTENTIAL RISKS
Anti-TNF agents are monoclonal antibodies. Infliximab, adalimumab, and golimumab are actively transported into the fetal circulation via the placenta, mainly during the second and third trimesters. Certolizumab crosses the placenta only by passive means, because it lacks the fragment crystallizable (Fc) region required for placental transfer.1
Effects on pregnancy outcomes
In a 2016 meta-analysis,2 of 1,242 pregnancies in women with IBD, 482 were in women on anti-TNF therapy. It found no statistically significant difference in rates of adverse pregnancy outcomes including congenital abnormality, preterm birth, and low birth weight.
A meta-analysis of 1,216 pregnant women with IBD found no statistically significant differences in rates of spontaneous or elective abortion, preterm birth, low birth weight, or congenital malformation in those on anti-TNF therapy vs controls.3
A systematic review of 58 studies including more than 1,500 pregnant women with IBD who were exposed to anti-TNF agents concluded that there was no association with adverse pregnancy outcomes such as spontaneous abortion, preterm delivery, stillbirth, low birth weight, congenital malformation, or infection.4
A retrospective cohort study of 66 pregnant patients with IBD from several centers in Spain found that anti-TNF or thiopurine therapy during pregnancy did not increase the risk of pregnancy complications or neonatal complications.5
Effects on newborns
Cord blood studies have shown that maternal use of infliximab and adalimumab results in a detectable serum level in newborns, while cord blood levels of certolizumab are much lower.1,6 In some studies, anti-TNF drugs were detectable in infants for up to 6 months after birth, whereas other studies found that detectable serum levels dropped soon after birth.1,7
Addressing concern about an increased risk of infection or dysfunctional immune development in newborns exposed to anti-TNF drugs in utero, a systematic review found no increased risk.4 A retrospective multicenter cohort study of 841 children also reported no association between in utero exposure to anti-TNF agents and risk of severe infection in the short term or long term (mean of 4 years).8 Additional studies are under way to determine long-term risk to the newborn.7
THE TORONTO CONSENSUS GUIDELINES
The Toronto consensus guidelines strongly recommend continuing anti-TNF therapy during pregnancy in women with IBD who began maintenance therapy before conception.6
If a patient strongly prefers to stop therapy during pregnancy to limit fetal exposure, the Toronto consensus recommends giving the last dose at 22 to 24 weeks of gestation. However, this should only be considered in patients whose IBD is in remission and at low risk of relapse.6,9
Although anti-TNF drugs may differ in terms of placental transfer, agents should not be switched in stable patients, as switching increases the risk of relapse.10
BENEFITS OF CONTINUING THERAPY
Active IBD poses a significantly greater risk to the mother and the baby than continuing anti-TNF therapy during pregnancy.1,7 The primary benefit of continuing therapy is to maintain disease remission.
Among women with active IBD at the time of conception, one-third will have improvement in disease activity during the course of their pregnancy, one-third will have no change, and one-third will have worsening of disease activity. But if IBD is in remission at the time of conception, it will remain in remission in nearly 80% of women during pregnancy.1
Women with active IBD are at increased risk of preterm delivery, low birth weight, and intrauterine growth restriction.1,2,5 Also, women with IBD have an increased risk of venous thromboembolism, particularly if they have active disease during pregnancy.1 Therefore, achieving and maintaining remission are vital in the management of the pregnant patient with IBD.
CONSIDERATIONS AFTER BIRTH: BREAST-FEEDING AND VACCINATION
Breast-feeding is considered safe. Minuscule amounts of infliximab or adalimumab are transferred in breast milk but are unlikely to result in systemic immune suppression in the infant.7
Live-attenuated vaccines should be avoided for the first 6 months in infants exposed to anti-TNF agents in utero.1,7,11 All other vaccines, including hepatitis B virus vaccine, should be given according to standard schedules.6
OUR RECOMMENDATIONS
The goal of managing IBD in women of reproductive age is to minimize the risk of adverse outcomes for both mother and baby. We recommend a team approach, working closely with a gastroenterologist and a high-risk-pregnancy obstetrician, if available.
Patients should continue anti-TNF therapy during pregnancy because evidence supports its safety. If a woman wants to stop therapy and is at low risk of relapse, we recommend giving the last dose at 22 to 24 weeks of gestation, then promptly resuming therapy postpartum.
Live-attenuated vaccines (eg, influenza, rotavirus) should be avoided for the first 6 months in babies born to mothers on anti-TNF therapy.
Yes, anti-tumor necrosis factor (anti-TNF) therapy for inflammatory bowel disease (IBD) can be continued during pregnancy.
IBD is often diagnosed and treated in women during their reproductive years. Consequently, these patients face important decisions about the management of their disease and the safety of their baby. Clinicians should be prepared to offer guidance by discussing the risks and benefits of anti-TNF agents with their pregnant patients who have IBD, as well as with those considering pregnancy.
STUDIES OF THE POTENTIAL RISKS
Anti-TNF agents are monoclonal antibodies. Infliximab, adalimumab, and golimumab are actively transported into the fetal circulation via the placenta, mainly during the second and third trimesters. Certolizumab crosses the placenta only by passive means, because it lacks the fragment crystallizable (Fc) region required for placental transfer.1
Effects on pregnancy outcomes
In a 2016 meta-analysis,2 of 1,242 pregnancies in women with IBD, 482 were in women on anti-TNF therapy. It found no statistically significant difference in rates of adverse pregnancy outcomes including congenital abnormality, preterm birth, and low birth weight.
A meta-analysis of 1,216 pregnant women with IBD found no statistically significant differences in rates of spontaneous or elective abortion, preterm birth, low birth weight, or congenital malformation in those on anti-TNF therapy vs controls.3
A systematic review of 58 studies including more than 1,500 pregnant women with IBD who were exposed to anti-TNF agents concluded that there was no association with adverse pregnancy outcomes such as spontaneous abortion, preterm delivery, stillbirth, low birth weight, congenital malformation, or infection.4
A retrospective cohort study of 66 pregnant patients with IBD from several centers in Spain found that anti-TNF or thiopurine therapy during pregnancy did not increase the risk of pregnancy complications or neonatal complications.5
Effects on newborns
Cord blood studies have shown that maternal use of infliximab and adalimumab results in a detectable serum level in newborns, while cord blood levels of certolizumab are much lower.1,6 In some studies, anti-TNF drugs were detectable in infants for up to 6 months after birth, whereas other studies found that detectable serum levels dropped soon after birth.1,7
Addressing concern about an increased risk of infection or dysfunctional immune development in newborns exposed to anti-TNF drugs in utero, a systematic review found no increased risk.4 A retrospective multicenter cohort study of 841 children also reported no association between in utero exposure to anti-TNF agents and risk of severe infection in the short term or long term (mean of 4 years).8 Additional studies are under way to determine long-term risk to the newborn.7
THE TORONTO CONSENSUS GUIDELINES
The Toronto consensus guidelines strongly recommend continuing anti-TNF therapy during pregnancy in women with IBD who began maintenance therapy before conception.6
If a patient strongly prefers to stop therapy during pregnancy to limit fetal exposure, the Toronto consensus recommends giving the last dose at 22 to 24 weeks of gestation. However, this should only be considered in patients whose IBD is in remission and at low risk of relapse.6,9
Although anti-TNF drugs may differ in terms of placental transfer, agents should not be switched in stable patients, as switching increases the risk of relapse.10
BENEFITS OF CONTINUING THERAPY
Active IBD poses a significantly greater risk to the mother and the baby than continuing anti-TNF therapy during pregnancy.1,7 The primary benefit of continuing therapy is to maintain disease remission.
Among women with active IBD at the time of conception, one-third will have improvement in disease activity during the course of their pregnancy, one-third will have no change, and one-third will have worsening of disease activity. But if IBD is in remission at the time of conception, it will remain in remission in nearly 80% of women during pregnancy.1
Women with active IBD are at increased risk of preterm delivery, low birth weight, and intrauterine growth restriction.1,2,5 Also, women with IBD have an increased risk of venous thromboembolism, particularly if they have active disease during pregnancy.1 Therefore, achieving and maintaining remission are vital in the management of the pregnant patient with IBD.
CONSIDERATIONS AFTER BIRTH: BREAST-FEEDING AND VACCINATION
Breast-feeding is considered safe. Minuscule amounts of infliximab or adalimumab are transferred in breast milk but are unlikely to result in systemic immune suppression in the infant.7
Live-attenuated vaccines should be avoided for the first 6 months in infants exposed to anti-TNF agents in utero.1,7,11 All other vaccines, including hepatitis B virus vaccine, should be given according to standard schedules.6
OUR RECOMMENDATIONS
The goal of managing IBD in women of reproductive age is to minimize the risk of adverse outcomes for both mother and baby. We recommend a team approach, working closely with a gastroenterologist and a high-risk-pregnancy obstetrician, if available.
Patients should continue anti-TNF therapy during pregnancy because evidence supports its safety. If a woman wants to stop therapy and is at low risk of relapse, we recommend giving the last dose at 22 to 24 weeks of gestation, then promptly resuming therapy postpartum.
Live-attenuated vaccines (eg, influenza, rotavirus) should be avoided for the first 6 months in babies born to mothers on anti-TNF therapy.
- Ananthakrishnan AN, Xavier RJ, Podolsky DK. Inflammatory Bowel Diseases: A Clinician’s Guide. Chichester, UK: Wiley; 2017. doi:10.1002/9781119077633
- Shihab Z, Yeomans ND, De Cruz P. Anti-tumour necrosis factor alpha therapies and inflammatory bowel disease pregnancy outcomes: a meta-analysis. J Crohns Colitis 2016; 10(8):979–988. doi:10.1093/ecco-jcc/jjv234
- Narula N, Al-Dabbagh, Dhillon A, Sands BE, Marshall JK. Anti-TNF alpha therapies are safe during pregnancy in women with inflammatory bowel disease: a systematic review and meta-analysis. Inflamm Bowel Dis 2014; 20(10):1862–1869. doi:10.1097/MIB.0000000000000092
- Nielsen OH, Loftus EV Jr, Jess T. Safety of TNF-alpha inhibitors during IBD pregnancy: a systematic review. BMC Med 2013; 11:174. doi:10.1186/1741-7015-11-174
- Casanova MJ, Chaparro M, Domenech E, et al. Safety of thiopurines and anti-TNF-alpha drugs during pregnancy in patients with inflammatory bowel disease. Am J Gastroenterol 2013; 108(3):433–440. doi:10.1038/ajg.2012.430
- Nguyen GC, Seow CH, Maxwell C, et al; IBD in Pregnancy Consensus Group; Canadian Association of Gastroenterology. The Toronto consensus statements for the management of inflammatory bowel disease in pregnancy. Gastroenterology 2016; 150(3):734–757.e1. doi:10.1053/j.gastro.2015.12.003
- Gisbert JP, Chaparro, M. Safety of anti-TNF agents during pregnancy and breastfeeding in women with inflammatory bowel disease. Am J Gastroenterol 2013; 108(9):1426–1438. doi:10.1038/ajg.2013.171
- Chaparro M, Verreth A, Lobaton T, et al. Long-term safety of in utero exposure to anti-TNF alpha drugs for the treatment of inflammatory bowel disease: results from the multicenter European TEDDY Study. Am J Gastroenterol 2018; 113(3):396–403. doi:10.1038/ajg.2017.501
- de Lima A, Zelinkova Z, van der Ent C, Steegers EA, van der Woude CJ. Tailored anti-TNF therapy during pregnancy in patients with IBD: maternal and fetal safety. Gut 2016; 65(8):1261–1268. doi:10.1136/gutjnl-2015-309321
- Van Assche G, Vermeire S, Ballet V, et al. Switch to adalimumab in patients with Crohn’s disease controlled by maintenance infliximab: prospective randomised SWITCH trial. Gut 2012; 61(2):229–234. doi:10.1136/gutjnl-2011-300755
- Saha S. Medication management in the pregnant IBD patient. Am J Gastroenterol 2017; 112(5):667–669. doi:10.1038/ajg.2017.22
- Ananthakrishnan AN, Xavier RJ, Podolsky DK. Inflammatory Bowel Diseases: A Clinician’s Guide. Chichester, UK: Wiley; 2017. doi:10.1002/9781119077633
- Shihab Z, Yeomans ND, De Cruz P. Anti-tumour necrosis factor alpha therapies and inflammatory bowel disease pregnancy outcomes: a meta-analysis. J Crohns Colitis 2016; 10(8):979–988. doi:10.1093/ecco-jcc/jjv234
- Narula N, Al-Dabbagh, Dhillon A, Sands BE, Marshall JK. Anti-TNF alpha therapies are safe during pregnancy in women with inflammatory bowel disease: a systematic review and meta-analysis. Inflamm Bowel Dis 2014; 20(10):1862–1869. doi:10.1097/MIB.0000000000000092
- Nielsen OH, Loftus EV Jr, Jess T. Safety of TNF-alpha inhibitors during IBD pregnancy: a systematic review. BMC Med 2013; 11:174. doi:10.1186/1741-7015-11-174
- Casanova MJ, Chaparro M, Domenech E, et al. Safety of thiopurines and anti-TNF-alpha drugs during pregnancy in patients with inflammatory bowel disease. Am J Gastroenterol 2013; 108(3):433–440. doi:10.1038/ajg.2012.430
- Nguyen GC, Seow CH, Maxwell C, et al; IBD in Pregnancy Consensus Group; Canadian Association of Gastroenterology. The Toronto consensus statements for the management of inflammatory bowel disease in pregnancy. Gastroenterology 2016; 150(3):734–757.e1. doi:10.1053/j.gastro.2015.12.003
- Gisbert JP, Chaparro, M. Safety of anti-TNF agents during pregnancy and breastfeeding in women with inflammatory bowel disease. Am J Gastroenterol 2013; 108(9):1426–1438. doi:10.1038/ajg.2013.171
- Chaparro M, Verreth A, Lobaton T, et al. Long-term safety of in utero exposure to anti-TNF alpha drugs for the treatment of inflammatory bowel disease: results from the multicenter European TEDDY Study. Am J Gastroenterol 2018; 113(3):396–403. doi:10.1038/ajg.2017.501
- de Lima A, Zelinkova Z, van der Ent C, Steegers EA, van der Woude CJ. Tailored anti-TNF therapy during pregnancy in patients with IBD: maternal and fetal safety. Gut 2016; 65(8):1261–1268. doi:10.1136/gutjnl-2015-309321
- Van Assche G, Vermeire S, Ballet V, et al. Switch to adalimumab in patients with Crohn’s disease controlled by maintenance infliximab: prospective randomised SWITCH trial. Gut 2012; 61(2):229–234. doi:10.1136/gutjnl-2011-300755
- Saha S. Medication management in the pregnant IBD patient. Am J Gastroenterol 2017; 112(5):667–669. doi:10.1038/ajg.2017.22
Cannabis for chronic pain: Not a simple solution
The narrative review by Modesto-Lowe et al1 in this issue on the potential therapeutic use of cannabis for peripheral neuropathy is only the latest in a vogue string of examinations on how medical marijuana may be used to manage complex conditions. While the authors should be lauded for acknowledging that the role of cannabis in treating peripheral neuropathy is far from settled (“the unknown” in their title), the high stakes involved warrant even more stringent scrutiny than they suggest.
We are in the midst of an epidemic of chronic opioid use with massive repercussions, and it did not start overnight. Mounting calls for liberalizing narcotic use across a broad range of pain conditions accumulated gradually during the patient-advocacy era of the 1990s, with supporting “evidence” coming mostly from small uncontrolled studies, anecdotal reports, and industry pressure.2 Although cannabis and opioids are not interchangeable, we should be cautious about concluding that cannabis is effective and that it should be used to treat chronic pain.
CHRONIC PAIN IS COMPLICATED
Peripheral neuropathy, by definition, is a chronic pain condition. Unlike acute pain, chronic pain is characterized by biologic, psychologic, and social complexities that require nuance to manage and study.
Such nuance is lacking in most recent reviews of the medical use of cannabis. The conditions in question are often studied as if they were transient and acute, eg, employing short-term studies and rudimentary measures such as numeric pain-rating scales or other snapshots of pain intensity. Results of these shortsighted assessments are impossible to extrapolate to long-term outcomes.
Whether cannabis therapy for chronic pain conditions is sustainable remains to be seen. Outcomes in chronic pain should not be defined simply by pain reduction, but by other dimensions such as changes in pain-related disability and quality of life, development of pharmacologic tolerance or dependence, adverse effects, and other “collateral damage.” We are far from understanding these issues, which require highly controlled and regulated longitudinal studies.
A recent Cochrane review3 of the efficacy of cannabis-based medicines for chronic neuropathic pain found that harms might outweigh the benefits. The quality of evidence was rated as very low to moderate; the reviewers cited small sample sizes and exclusion of important subgroups of patients (eg, those with substance abuse or other psychiatric comorbidities). Such exclusions are the crux of a major problem with cannabis research: studies are not naturalistic. The gritty reality of chronic pain management is paramount, and failing to consider the high-risk biopsychosocial factors typical of patients with chronic pain is naïve and, frankly, dangerous.
COGNITIVE AND MOTIVATIONAL PROBLEMS
The true danger of cannabis lies in what we already know with certainty. As the authors discuss, cannabis undisputedly results in dose-dependent cognitive and motivational problems. If we are preaching physical therapy and home exercise to counter deconditioning, socialization to reverse depression, cognitive-behavioral therapy to increase coping, returning to work to prevent prolonged disability, and other active measures to prevent pain from becoming chronic, then why would we suggest treatments known to blunt motivation, energy, concentration, and overall mood? As a general central nervous system suppressant,4 cannabis works broadly against our best efforts to rehabilitate patients and restore their overall function.
ALL CANNABIS IS NOT THE SAME
The authors use the general term cannabis in their title, yet rightly unpack the differences between medical marijuana, tetrahydrocannabinol (THC), and cannabidiol (CBD). However, in the minds of untrained and pain-stricken patients seeking rapid relief and practical solutions, such distinctions are likely irrelevant.
The danger in the barrage of publications examining cannabis vs medical marijuana vs THC vs CBD is that they all communicate an unintentional yet problematic message: that marijuana of some sort for pain is acceptable to try. And in the face of financial pressures, changing legal landscapes, insurance coverage volatility, and access issues, are patients really going to always secure prescriptions for well-regulated CBD (lacking psychoactive THC) from thoughtful and well-informed physicians, or will they turn to convenient street suppliers?
Simplified perceptions of safety and efficacy across all cannabis products do not help. More troublesome would be to extrapolate safety to other forms of marijuana known to be dangerous, such as synthetic cannabinoids, which in some instances have been associated with catastrophic outcomes.5 The slippery slope is real: if the message becomes that some (or most) marijuana is benign or even therapeutic, what is to curb a widespread and unregulated epidemic?
YOUTH AT RISK
Some groups are more vulnerable than others to the potential negative effects of cannabis. In a study at a medical cannabis dispensary in San Francisco,6 adolescents and young adults used more marijuana than older users did and had higher rates of “use when bored” and eventual pharmacologic dependence. Sustained use of marijuana by young people places them at risk of serious psychiatric disorders, with numerous studies demonstrating the unfolding of schizophrenia, depression, bipolar disorder, and more.7
As the authors point out, cannabis may be contraindicated in those already burdened with mental health problems. If we recall that comorbid psychiatric disorders are the norm rather than the exception in chronic pain conditions,8 can we recommend cannabis therapy for most patients with chronic pain with confidence that it will not cause unintended problems? Evidence already shows that even well-established medical marijuana services attract (and perhaps unintentionally debilitate) a certain high-risk demographic: young, socioeconomically disadvantaged men with other comorbid psychiatric and substance use disorders, who ultimately rank poorly in functional health measures compared with the general population.9
NOT REEFER MADNESS, BUT REEFER CAUTION
I am not advocating the fear-mongering misinformation campaigns of the past. We should not exaggerate and warn about “reefer madness” or equate marijuana with untruths about random violence or complete bedlam. Nonetheless, concerns for widespread amotivation, worsening psychiatric states, chronic disability, and chemical dependence are very real.
Needed are tightly regulated, well-controlled, and long-term prospective studies involving isolated CBD formulations lacking THC. Over time, perhaps only formulations approved by the US Food and Drug Administration will be embraced. In the meantime, more comprehensive approaches should be recommended, such as team-based interdisciplinary rehabilitation programs that have shown efficacy in handling chronic pain complexities.10,11
If such steps are unlikely, physicians should nonetheless stand united in sending a message of cautious optimism regarding medical marijuana, educating their patients not only about recently advertised potential yet inconclusive benefits, but also about the well-known and actual certitudes of its harms for use in chronic pain management. There is plenty of bad and worse information to share with patients, and there is a slippery slope of epidemic proportions to be wary about.
- Modesto-Lowe V, Bojka R, Alvarado C. Cannabis for peripheral neuropathy: The good, the bad, and the unknown. Cleve Clin J Med 2018; 85(12):943–949. doi:10.3949/ccjm.85a.17115
- Wailoo K. Pain: A Political History. Baltimore, MD: Johns Hopkins University Press; 2014.
- Mucke M, Phillips T, Radbruch L, Petzke F, Hauser W. Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst Rev 2018; 3:CD012182. doi:10.1002/14651858.CD012182.pub2
- Lucas CJ, Galettis P, Schneider J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br J Clin Pharmacol 2018. Epub ahead of print. doi:10.1111/bcp.13710
- Patel NA, Jerry JM, Jimenez XF, Hantus ST. New-onset refractory status epilepticus associated with the use of synthetic cannabinoids. Psychosomatics 2017; 58(2):180–186. doi:10.1016/j.psym.2016.10.006
- Haug NA, Padula CB, Sottile JE, Vandrey R, Heinz AJ, Bonn-Miller MO. Cannabis use patterns and motives: a comparison of younger, middle-aged, and older medical cannabis dispensary patients. Addict Behav 2017; 72:14–20. doi:10.1016/j.addbeh.2017.03.006
- Mammen G, Rueda S, Roerecke M, Bonato S, Lev-Ran S, Rehm J. Association of cannabis with long-term clinical symptoms in anxiety and mood disorders: a systematic review of prospective studies. J Clin Psychiatry 2018; 79(4)pii:17r11839. doi:10.4088/JCP.17r11839
- Velly AM, Mohit S. Epidemiology of pain and relation to psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry 2017; pii:S0278–5846(17)30194–X. doi:10.1016/j.pnpbp.2017.05.012
- Fischer B, Ialomiteanu AR, Aeby S, Rudzinski K, Kurdyak P, Rehm J. Substance use, health, and functioning characteristics of medical marijuana program participants compared to the general adult population in Ontario (Canada). J Psychoactive Drugs 2017; 49(1):31–38. doi:10.1080/02791072.2016.1264648
- Shah A, Craner J, Cunningham JL. Medical cannabis use among patients with chronic pain in an interdisciplinary pain rehabilitation program: characterization and treatment outcomes. J Subst Abuse Treat 2017; 77:95–100. doi:10.1016/j.jsat.2017.03.012
- Stanos S. Focused review of interdisciplinary pain rehabilitation programs for chronic pain management. Curr Pain Headache Rep 2012; 16(2):147–152. doi:10.1007/s11916-012-0252-4
The narrative review by Modesto-Lowe et al1 in this issue on the potential therapeutic use of cannabis for peripheral neuropathy is only the latest in a vogue string of examinations on how medical marijuana may be used to manage complex conditions. While the authors should be lauded for acknowledging that the role of cannabis in treating peripheral neuropathy is far from settled (“the unknown” in their title), the high stakes involved warrant even more stringent scrutiny than they suggest.
We are in the midst of an epidemic of chronic opioid use with massive repercussions, and it did not start overnight. Mounting calls for liberalizing narcotic use across a broad range of pain conditions accumulated gradually during the patient-advocacy era of the 1990s, with supporting “evidence” coming mostly from small uncontrolled studies, anecdotal reports, and industry pressure.2 Although cannabis and opioids are not interchangeable, we should be cautious about concluding that cannabis is effective and that it should be used to treat chronic pain.
CHRONIC PAIN IS COMPLICATED
Peripheral neuropathy, by definition, is a chronic pain condition. Unlike acute pain, chronic pain is characterized by biologic, psychologic, and social complexities that require nuance to manage and study.
Such nuance is lacking in most recent reviews of the medical use of cannabis. The conditions in question are often studied as if they were transient and acute, eg, employing short-term studies and rudimentary measures such as numeric pain-rating scales or other snapshots of pain intensity. Results of these shortsighted assessments are impossible to extrapolate to long-term outcomes.
Whether cannabis therapy for chronic pain conditions is sustainable remains to be seen. Outcomes in chronic pain should not be defined simply by pain reduction, but by other dimensions such as changes in pain-related disability and quality of life, development of pharmacologic tolerance or dependence, adverse effects, and other “collateral damage.” We are far from understanding these issues, which require highly controlled and regulated longitudinal studies.
A recent Cochrane review3 of the efficacy of cannabis-based medicines for chronic neuropathic pain found that harms might outweigh the benefits. The quality of evidence was rated as very low to moderate; the reviewers cited small sample sizes and exclusion of important subgroups of patients (eg, those with substance abuse or other psychiatric comorbidities). Such exclusions are the crux of a major problem with cannabis research: studies are not naturalistic. The gritty reality of chronic pain management is paramount, and failing to consider the high-risk biopsychosocial factors typical of patients with chronic pain is naïve and, frankly, dangerous.
COGNITIVE AND MOTIVATIONAL PROBLEMS
The true danger of cannabis lies in what we already know with certainty. As the authors discuss, cannabis undisputedly results in dose-dependent cognitive and motivational problems. If we are preaching physical therapy and home exercise to counter deconditioning, socialization to reverse depression, cognitive-behavioral therapy to increase coping, returning to work to prevent prolonged disability, and other active measures to prevent pain from becoming chronic, then why would we suggest treatments known to blunt motivation, energy, concentration, and overall mood? As a general central nervous system suppressant,4 cannabis works broadly against our best efforts to rehabilitate patients and restore their overall function.
ALL CANNABIS IS NOT THE SAME
The authors use the general term cannabis in their title, yet rightly unpack the differences between medical marijuana, tetrahydrocannabinol (THC), and cannabidiol (CBD). However, in the minds of untrained and pain-stricken patients seeking rapid relief and practical solutions, such distinctions are likely irrelevant.
The danger in the barrage of publications examining cannabis vs medical marijuana vs THC vs CBD is that they all communicate an unintentional yet problematic message: that marijuana of some sort for pain is acceptable to try. And in the face of financial pressures, changing legal landscapes, insurance coverage volatility, and access issues, are patients really going to always secure prescriptions for well-regulated CBD (lacking psychoactive THC) from thoughtful and well-informed physicians, or will they turn to convenient street suppliers?
Simplified perceptions of safety and efficacy across all cannabis products do not help. More troublesome would be to extrapolate safety to other forms of marijuana known to be dangerous, such as synthetic cannabinoids, which in some instances have been associated with catastrophic outcomes.5 The slippery slope is real: if the message becomes that some (or most) marijuana is benign or even therapeutic, what is to curb a widespread and unregulated epidemic?
YOUTH AT RISK
Some groups are more vulnerable than others to the potential negative effects of cannabis. In a study at a medical cannabis dispensary in San Francisco,6 adolescents and young adults used more marijuana than older users did and had higher rates of “use when bored” and eventual pharmacologic dependence. Sustained use of marijuana by young people places them at risk of serious psychiatric disorders, with numerous studies demonstrating the unfolding of schizophrenia, depression, bipolar disorder, and more.7
As the authors point out, cannabis may be contraindicated in those already burdened with mental health problems. If we recall that comorbid psychiatric disorders are the norm rather than the exception in chronic pain conditions,8 can we recommend cannabis therapy for most patients with chronic pain with confidence that it will not cause unintended problems? Evidence already shows that even well-established medical marijuana services attract (and perhaps unintentionally debilitate) a certain high-risk demographic: young, socioeconomically disadvantaged men with other comorbid psychiatric and substance use disorders, who ultimately rank poorly in functional health measures compared with the general population.9
NOT REEFER MADNESS, BUT REEFER CAUTION
I am not advocating the fear-mongering misinformation campaigns of the past. We should not exaggerate and warn about “reefer madness” or equate marijuana with untruths about random violence or complete bedlam. Nonetheless, concerns for widespread amotivation, worsening psychiatric states, chronic disability, and chemical dependence are very real.
Needed are tightly regulated, well-controlled, and long-term prospective studies involving isolated CBD formulations lacking THC. Over time, perhaps only formulations approved by the US Food and Drug Administration will be embraced. In the meantime, more comprehensive approaches should be recommended, such as team-based interdisciplinary rehabilitation programs that have shown efficacy in handling chronic pain complexities.10,11
If such steps are unlikely, physicians should nonetheless stand united in sending a message of cautious optimism regarding medical marijuana, educating their patients not only about recently advertised potential yet inconclusive benefits, but also about the well-known and actual certitudes of its harms for use in chronic pain management. There is plenty of bad and worse information to share with patients, and there is a slippery slope of epidemic proportions to be wary about.
The narrative review by Modesto-Lowe et al1 in this issue on the potential therapeutic use of cannabis for peripheral neuropathy is only the latest in a vogue string of examinations on how medical marijuana may be used to manage complex conditions. While the authors should be lauded for acknowledging that the role of cannabis in treating peripheral neuropathy is far from settled (“the unknown” in their title), the high stakes involved warrant even more stringent scrutiny than they suggest.
We are in the midst of an epidemic of chronic opioid use with massive repercussions, and it did not start overnight. Mounting calls for liberalizing narcotic use across a broad range of pain conditions accumulated gradually during the patient-advocacy era of the 1990s, with supporting “evidence” coming mostly from small uncontrolled studies, anecdotal reports, and industry pressure.2 Although cannabis and opioids are not interchangeable, we should be cautious about concluding that cannabis is effective and that it should be used to treat chronic pain.
CHRONIC PAIN IS COMPLICATED
Peripheral neuropathy, by definition, is a chronic pain condition. Unlike acute pain, chronic pain is characterized by biologic, psychologic, and social complexities that require nuance to manage and study.
Such nuance is lacking in most recent reviews of the medical use of cannabis. The conditions in question are often studied as if they were transient and acute, eg, employing short-term studies and rudimentary measures such as numeric pain-rating scales or other snapshots of pain intensity. Results of these shortsighted assessments are impossible to extrapolate to long-term outcomes.
Whether cannabis therapy for chronic pain conditions is sustainable remains to be seen. Outcomes in chronic pain should not be defined simply by pain reduction, but by other dimensions such as changes in pain-related disability and quality of life, development of pharmacologic tolerance or dependence, adverse effects, and other “collateral damage.” We are far from understanding these issues, which require highly controlled and regulated longitudinal studies.
A recent Cochrane review3 of the efficacy of cannabis-based medicines for chronic neuropathic pain found that harms might outweigh the benefits. The quality of evidence was rated as very low to moderate; the reviewers cited small sample sizes and exclusion of important subgroups of patients (eg, those with substance abuse or other psychiatric comorbidities). Such exclusions are the crux of a major problem with cannabis research: studies are not naturalistic. The gritty reality of chronic pain management is paramount, and failing to consider the high-risk biopsychosocial factors typical of patients with chronic pain is naïve and, frankly, dangerous.
COGNITIVE AND MOTIVATIONAL PROBLEMS
The true danger of cannabis lies in what we already know with certainty. As the authors discuss, cannabis undisputedly results in dose-dependent cognitive and motivational problems. If we are preaching physical therapy and home exercise to counter deconditioning, socialization to reverse depression, cognitive-behavioral therapy to increase coping, returning to work to prevent prolonged disability, and other active measures to prevent pain from becoming chronic, then why would we suggest treatments known to blunt motivation, energy, concentration, and overall mood? As a general central nervous system suppressant,4 cannabis works broadly against our best efforts to rehabilitate patients and restore their overall function.
ALL CANNABIS IS NOT THE SAME
The authors use the general term cannabis in their title, yet rightly unpack the differences between medical marijuana, tetrahydrocannabinol (THC), and cannabidiol (CBD). However, in the minds of untrained and pain-stricken patients seeking rapid relief and practical solutions, such distinctions are likely irrelevant.
The danger in the barrage of publications examining cannabis vs medical marijuana vs THC vs CBD is that they all communicate an unintentional yet problematic message: that marijuana of some sort for pain is acceptable to try. And in the face of financial pressures, changing legal landscapes, insurance coverage volatility, and access issues, are patients really going to always secure prescriptions for well-regulated CBD (lacking psychoactive THC) from thoughtful and well-informed physicians, or will they turn to convenient street suppliers?
Simplified perceptions of safety and efficacy across all cannabis products do not help. More troublesome would be to extrapolate safety to other forms of marijuana known to be dangerous, such as synthetic cannabinoids, which in some instances have been associated with catastrophic outcomes.5 The slippery slope is real: if the message becomes that some (or most) marijuana is benign or even therapeutic, what is to curb a widespread and unregulated epidemic?
YOUTH AT RISK
Some groups are more vulnerable than others to the potential negative effects of cannabis. In a study at a medical cannabis dispensary in San Francisco,6 adolescents and young adults used more marijuana than older users did and had higher rates of “use when bored” and eventual pharmacologic dependence. Sustained use of marijuana by young people places them at risk of serious psychiatric disorders, with numerous studies demonstrating the unfolding of schizophrenia, depression, bipolar disorder, and more.7
As the authors point out, cannabis may be contraindicated in those already burdened with mental health problems. If we recall that comorbid psychiatric disorders are the norm rather than the exception in chronic pain conditions,8 can we recommend cannabis therapy for most patients with chronic pain with confidence that it will not cause unintended problems? Evidence already shows that even well-established medical marijuana services attract (and perhaps unintentionally debilitate) a certain high-risk demographic: young, socioeconomically disadvantaged men with other comorbid psychiatric and substance use disorders, who ultimately rank poorly in functional health measures compared with the general population.9
NOT REEFER MADNESS, BUT REEFER CAUTION
I am not advocating the fear-mongering misinformation campaigns of the past. We should not exaggerate and warn about “reefer madness” or equate marijuana with untruths about random violence or complete bedlam. Nonetheless, concerns for widespread amotivation, worsening psychiatric states, chronic disability, and chemical dependence are very real.
Needed are tightly regulated, well-controlled, and long-term prospective studies involving isolated CBD formulations lacking THC. Over time, perhaps only formulations approved by the US Food and Drug Administration will be embraced. In the meantime, more comprehensive approaches should be recommended, such as team-based interdisciplinary rehabilitation programs that have shown efficacy in handling chronic pain complexities.10,11
If such steps are unlikely, physicians should nonetheless stand united in sending a message of cautious optimism regarding medical marijuana, educating their patients not only about recently advertised potential yet inconclusive benefits, but also about the well-known and actual certitudes of its harms for use in chronic pain management. There is plenty of bad and worse information to share with patients, and there is a slippery slope of epidemic proportions to be wary about.
- Modesto-Lowe V, Bojka R, Alvarado C. Cannabis for peripheral neuropathy: The good, the bad, and the unknown. Cleve Clin J Med 2018; 85(12):943–949. doi:10.3949/ccjm.85a.17115
- Wailoo K. Pain: A Political History. Baltimore, MD: Johns Hopkins University Press; 2014.
- Mucke M, Phillips T, Radbruch L, Petzke F, Hauser W. Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst Rev 2018; 3:CD012182. doi:10.1002/14651858.CD012182.pub2
- Lucas CJ, Galettis P, Schneider J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br J Clin Pharmacol 2018. Epub ahead of print. doi:10.1111/bcp.13710
- Patel NA, Jerry JM, Jimenez XF, Hantus ST. New-onset refractory status epilepticus associated with the use of synthetic cannabinoids. Psychosomatics 2017; 58(2):180–186. doi:10.1016/j.psym.2016.10.006
- Haug NA, Padula CB, Sottile JE, Vandrey R, Heinz AJ, Bonn-Miller MO. Cannabis use patterns and motives: a comparison of younger, middle-aged, and older medical cannabis dispensary patients. Addict Behav 2017; 72:14–20. doi:10.1016/j.addbeh.2017.03.006
- Mammen G, Rueda S, Roerecke M, Bonato S, Lev-Ran S, Rehm J. Association of cannabis with long-term clinical symptoms in anxiety and mood disorders: a systematic review of prospective studies. J Clin Psychiatry 2018; 79(4)pii:17r11839. doi:10.4088/JCP.17r11839
- Velly AM, Mohit S. Epidemiology of pain and relation to psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry 2017; pii:S0278–5846(17)30194–X. doi:10.1016/j.pnpbp.2017.05.012
- Fischer B, Ialomiteanu AR, Aeby S, Rudzinski K, Kurdyak P, Rehm J. Substance use, health, and functioning characteristics of medical marijuana program participants compared to the general adult population in Ontario (Canada). J Psychoactive Drugs 2017; 49(1):31–38. doi:10.1080/02791072.2016.1264648
- Shah A, Craner J, Cunningham JL. Medical cannabis use among patients with chronic pain in an interdisciplinary pain rehabilitation program: characterization and treatment outcomes. J Subst Abuse Treat 2017; 77:95–100. doi:10.1016/j.jsat.2017.03.012
- Stanos S. Focused review of interdisciplinary pain rehabilitation programs for chronic pain management. Curr Pain Headache Rep 2012; 16(2):147–152. doi:10.1007/s11916-012-0252-4
- Modesto-Lowe V, Bojka R, Alvarado C. Cannabis for peripheral neuropathy: The good, the bad, and the unknown. Cleve Clin J Med 2018; 85(12):943–949. doi:10.3949/ccjm.85a.17115
- Wailoo K. Pain: A Political History. Baltimore, MD: Johns Hopkins University Press; 2014.
- Mucke M, Phillips T, Radbruch L, Petzke F, Hauser W. Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst Rev 2018; 3:CD012182. doi:10.1002/14651858.CD012182.pub2
- Lucas CJ, Galettis P, Schneider J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br J Clin Pharmacol 2018. Epub ahead of print. doi:10.1111/bcp.13710
- Patel NA, Jerry JM, Jimenez XF, Hantus ST. New-onset refractory status epilepticus associated with the use of synthetic cannabinoids. Psychosomatics 2017; 58(2):180–186. doi:10.1016/j.psym.2016.10.006
- Haug NA, Padula CB, Sottile JE, Vandrey R, Heinz AJ, Bonn-Miller MO. Cannabis use patterns and motives: a comparison of younger, middle-aged, and older medical cannabis dispensary patients. Addict Behav 2017; 72:14–20. doi:10.1016/j.addbeh.2017.03.006
- Mammen G, Rueda S, Roerecke M, Bonato S, Lev-Ran S, Rehm J. Association of cannabis with long-term clinical symptoms in anxiety and mood disorders: a systematic review of prospective studies. J Clin Psychiatry 2018; 79(4)pii:17r11839. doi:10.4088/JCP.17r11839
- Velly AM, Mohit S. Epidemiology of pain and relation to psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry 2017; pii:S0278–5846(17)30194–X. doi:10.1016/j.pnpbp.2017.05.012
- Fischer B, Ialomiteanu AR, Aeby S, Rudzinski K, Kurdyak P, Rehm J. Substance use, health, and functioning characteristics of medical marijuana program participants compared to the general adult population in Ontario (Canada). J Psychoactive Drugs 2017; 49(1):31–38. doi:10.1080/02791072.2016.1264648
- Shah A, Craner J, Cunningham JL. Medical cannabis use among patients with chronic pain in an interdisciplinary pain rehabilitation program: characterization and treatment outcomes. J Subst Abuse Treat 2017; 77:95–100. doi:10.1016/j.jsat.2017.03.012
- Stanos S. Focused review of interdisciplinary pain rehabilitation programs for chronic pain management. Curr Pain Headache Rep 2012; 16(2):147–152. doi:10.1007/s11916-012-0252-4
Correction: Men’s health 2018
In the article by Chaitoff et al (Men’s health 2018: BPH, prostate cancer, erectile dysfunction, supplements. Cleve Clin J Med 2018; 85(11):871–880, doi:10.3949/ccjm.85a.18011), the prostate-specific antigen level of a 60-year-old man was given as 5.1 mg/dL. The unit of measure should have been 5.1 ng/mL. This has been corrected online.
In the article by Chaitoff et al (Men’s health 2018: BPH, prostate cancer, erectile dysfunction, supplements. Cleve Clin J Med 2018; 85(11):871–880, doi:10.3949/ccjm.85a.18011), the prostate-specific antigen level of a 60-year-old man was given as 5.1 mg/dL. The unit of measure should have been 5.1 ng/mL. This has been corrected online.
In the article by Chaitoff et al (Men’s health 2018: BPH, prostate cancer, erectile dysfunction, supplements. Cleve Clin J Med 2018; 85(11):871–880, doi:10.3949/ccjm.85a.18011), the prostate-specific antigen level of a 60-year-old man was given as 5.1 mg/dL. The unit of measure should have been 5.1 ng/mL. This has been corrected online.
Dialing back opioids for chronic pain one conversation at a time
ABSTRACT
Purpose Our study examined the efficacy of a primary-care intervention in reducing opioid use among patients who have chronic non-cancer pain (CNCP). We also recorded the intervention’s effect on patients’ decisions to leave (or stay) with the primary-care practice.
Methods A family physician (FP) identified 41 patients in his practice who had CNCP of at least 6 month’s duration and were using opioids. The intervention with each patient involved an initial discussion of ethical principles, evidence-based practice, and current published guidelines. Following the discussion, patients self-selected to participate with their FP in a continuing tapering program or to accept referral to a pain center for management of their opioid medications. Tapering ranged from a 10% reduction per week to a more rapid 25% to 50% reduction every few days. Twenty-seven patients continued tapering with their FP, and 6 months later were retrospectively placed in the Taper Group. Fourteen patients chose not to pursue the tapering option and were referred to a single-modality medical pain clinic (MPC). All patients had the option of staying with the FP for other medical care.
Results At baseline and again at 6 months post-initial intervention, the MPC Group was taking significantly higher daily doses of morphine equivalents than the Taper Group. The Taper Group at 6 months was taking significantly lower average daily narcotic doses in morphine equivalents than at baseline. No significant baseline-to-6 month differences were found in the MPC Group. Contrary to many physicians’ fear of losing patients following candid discussions about opioid use, 40 of the 41 patients continued with the FP for other health needs.
Conclusions FPs can frankly discuss opioid use with their patients based on ethical principles and evidence-based recommendations and employ a tapering protocol consistent with current opioid treatment guidelines without jeopardizing the patient-physician relationship.
[polldaddy:10180698]
Opioid prescriptions for chronic noncancer pain (CNCP) have increased significantly over the past 25 years in the United States.1 Despite methodologic concerns surrounding research on opioid harms, prescription opioid misuse among CNCP patients is estimated to be 21% to 29% and prescription addiction 8% to 12%.2 Tragically, with the overall increase in opioid use for CNCP, substance-related hospital admissions and deaths due to opioid overdose have also risen.3
Increased opioid use began in 1985 when the World Health Organization expanded its ethical mandate for pain relief in dying patients to include relief from all cancer pain.3 Opioid use then accelerated following Portenoy and Foley’s 1986 article4 and the 1997 consensus statement by the American Academy of Pain Medicine (AAPM) and the American Pain Society (APS),5 with both organizations arguing that opioids have a role in the treatment of CNCP. Increased use of opioids for CNCP continued throughout the 1990s and 2000s, as many states passed legislation removing sanctions on prescribing long-term and high-dose opioid therapy, and pharmaceutical companies aggressively marketed sustained-release opioids.3
A balanced approach to opioids. While acknowledging the serious public health problems of drug abuse, addiction, and diversion of opioids from licit to illicit uses, clinical research and regulation leaders have called for a balanced approach that recognizes the legitimate medical need for opioids for CNCP. In 2009 the APS, in partnership with the AAPM, published evidence-based guidelines on chronic opioid therapy (COT) for adults with CNCP.6 In developing these guidelines, a multidisciplinary panel of experts conducted systematic reviews of available evidence and made recommendations on formulating COT for individuals, initiating and titrating therapy, regularly monitoring patients, and managing opioid-related adverse effects. Additional recommendations addressed the use of therapies focusing on psychosocial factors. The APS-AAPM guidelines received the highest rating in a systematic review critically appraising 13 guidelines that address the use of opioids for CNCP.7
Continue to: When opioid use is prolonged...
When opioid use is prolonged. Most primary care physicians are aware of the risks of prolonged opioid use, and many have successfully tapered or discontinued opioid medications for patients in acute or pre-chronic stages of pain.8 However, many physicians face the challenge of patients who have used COT for a longer time. The APS-AAPM guidelines may help primary care physicians at any stage of treating CNCP patients.
METHODS
Purpose and design. This retrospective study, which reviewed pretest-posttest findings between and within study groups, received an exempt status from Creighton University’s institutional review board. We designed the study to determine the efficacy of an intervention protocol to reduce opioid use by patients with CNCP who had been in a family physician (FP)'s panel for quite some time. Furthermore, because a common fear among primary care providers is that raising concerns with patients about their opioid use may cause those patients to leave their panel,9 our study also recorded how many patients stayed with their FP after initiation of the opioid management protocol.
Subjects. This study tracked 41 patients with CNCP in 1 FP’s panel. Inclusion criteria for participation was: 1) presence of CNCP for at least 6 months, 2) current use of opioid medication for CNCP, 3) age of at least 16 years, and 4) ability to read and write English. Two exclusion criteria were the presence of a surgically correctable condition or an organic brain syndrome or psychosis.
Clinical intervention. The FP identified eligible patients in his practice that were taking opioids for CNCP and initiated a discussion with each of them emphasizing his desire to follow the ethical principles of beneficence, nonmaleficence, respect for autonomy, and justice.10 The FP also presented his reasons for wanting the patient to stop using opioid medication. They included his beliefs that:
1) COT was not safe for the patient based on a growing body of published evidence of harm and death from COT3;
2) long-term use of opioids could lead to misuse, abuse, or addiction2;
3) prolonged opioid use paradoxically increases pain sensitivity that does not resolve
4) the patient’s current pain medications were not in line with published guidelines for use of opioids for CNCP.6
Initially, 45 patients were eligible for the study, but 4 declined participation before the intervention discussion and were immediately referred to a single-modality medical pain clinic (MPC). These patients were not included in subsequent analyses. Of the remaining 41 patients, all had a discussion with the MD about ethical principles, practice guidelines, and the importance of opioid tapering. After the discussion, patients decided whether to continue with the plan to taper their opioid therapy or to not taper their therapy and so receive a referral to an MPC.
Continue to: The 27 patients who chose to work with...
The 27 patients who chose to work with their FP started an individually tailored opioid-tapering program and were retrospectively placed in the Taper Group 6 months later. Tapering ranged from a slow 10% reduction in dosage per week to a more rapid 25% to 50% reduction every few days. Although evidence to guide specific recommendations on the rate of reduction is lacking, a slower rate may reduce unpleasant symptoms of opioid withdrawal.6 Following the patient-FP discussion, the 14 patients who chose not to pursue the tapering option were referred to an MPC for pain management, but could opt to remain with the FP for all other medical care. At 6 months post-discussion, we retrospectively assigned these 14 patients to the MPC Group.
Measures. We obtained demographic and medical information, including age, gender, race, marital status, and medication level in morphine equivalents, from the electronic health record. Medication level in morphine equivalents was recorded at the beginning of the intervention and again 6 months later. All analyses were conducted using SPSS Version 24 (IBM Corp, Armonk, NY) with P<.05 used to indicate statistical significance.
RESULTS
Between-group differences. The Taper and MPC groups did not differ significantly on demographic variables, with mean ages, respectively, at 57 and 51 years, sex 56% and 50% female, race 74% and 79% white, and marital status 48% and 50% married.
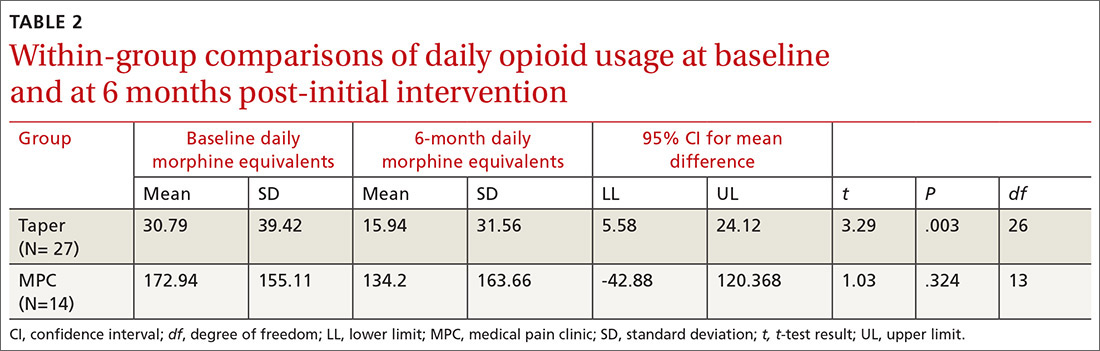
We found significant differences between the Taper and MPC groups on total daily dose in morphine equivalents at baseline and at 6 months following initial intervention. The Levene’s test for equality of variances was statistically significant, indicating unequal variances between the groups. In our SPSS analyses, we therefore used the option “equal variances not assumed.” TABLE 1 lists resultant means, standard deviations, individual sample t-test scores, and confidence intervals. The MPC Group was taking significantly higher daily doses of morphine equivalents than the Taper Group both at baseline and at 6 months following initial intervention.
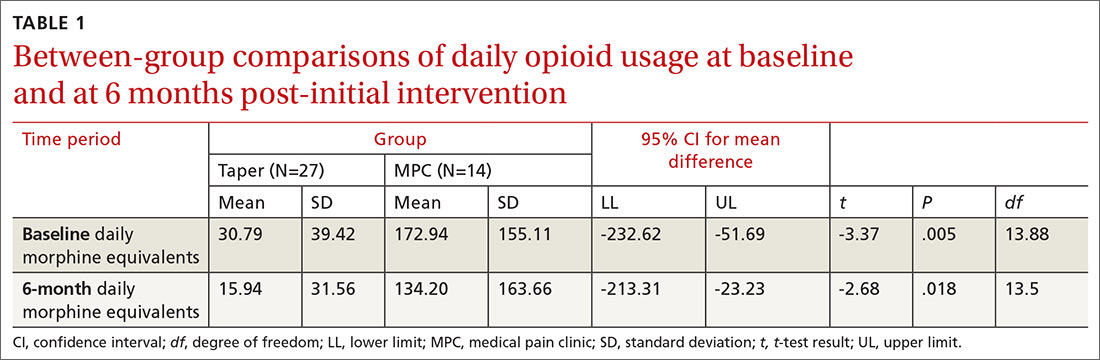
Within-group differences. Paired sample t tests indicated significant differences between baseline and 6-month average daily narcotic doses in morphine equivalents for the Taper Group. No significant difference was found between baseline and 6-month daily morphine equivalents for the MPC group. These results indicated that patients who continued opioid tapering with the FP significantly reduced their daily morphine equivalents over the 6 months of the study. Patients in the MPC Group reduced morphine equivalents over the 6 months, but the reduction was not statistically significant. Paired sample t test results are presented in TABLE 2.
Continue to: Patient retention
Patient retention. All but one of the 41 patients in the Tapering and MPC groups continued with the FP for the remainder of their health care needs. Contrary to some physicians’ fears, the patients in this study maintained continuity with their FP.
DISCUSSION
Results of this study indicate that an intervention consisting of a physician-patient discussion of ethical principles and evidence-based practice, followed by individualized opioid tapering per published guidelines, led to a significant reduction in opioid use in patients with CNCP. The Taper Group, which completed the intervention, exhibited significant morphine reductions between baseline and 6-month follow-up. This did not hold true for the MPC Group.
The MPC Group, despite participating in the discussion with the FP, chose not to complete the tapering program and was referred to a single-modality MPC where opioids were managed rather than tapered. While the MPC group reduced daily opioid dose levels, the reduction was not statistically significant. A possible reason for no difference within the MPC Group may be that they had greater dependence on opioids, as their baseline average daily dose was much higher than that in the Taper Group (173 mg vs 31 mg, respectively). Although we did not assess anxiety directly, we speculate that the MPC Group was more anxious about opioid reduction than the Taper Group, and that this anxiety potentially led 4 patients to opt out of the initial FP discussion and 14 patients to self-select out of the tapering program following the discussion.
The FP intervention was successful for the Taper Group. For MPC patients, an enhanced intervention including behavior health strategies13 might have reduced anxiety and increased motivation14 to continue tapering. Based on moderate-quality evidence, APS-AAPM guidelines strongly recommend that CNCP be viewed as a complex biopsychosocial condition. Therefore, clinicians who prescribe opioids should routinely integrate psychotherapeutic interventions, functional restoration, interdisciplinary therapy, and other adjunctive nonopioid therapies.6
Opioid tapering within multidisciplinary rehabilitation programs is possible without significant worsening of pain, mood, and function.15 Recently, an outpatient opioid-tapering support intervention showed promise for efficacy in reducing prescription opioid doses without resultant increases in pain intensity or pain interference.16
Continue to: The tapering protocol in our study...
The tapering protocol in our study and the inclusion of behavioral health co-interventions are also recommended by the 2016 guidelines published by the Center for Disease Control and Prevention.17 More information on the similarities and differences among the various guidelines is available online.18,19
Caveats with our study. Patients’ entry into the Taper or MPC groups occurred through self-selection rather than random assignment. Thus, caution is recommended in interpreting findings of the FP intervention. And, we did not measure patients’ levels of pain, so differences between groups may have been possible. In addition, the number of patients per group was relatively small, which may have accounted for the lack of significance in the MPC Group findings. Conversely, significant reductions in opioid use in the small tapering sample suggests a relatively robust intervention, despite a lack of random assignment to treatment conditions.
These findings suggest that FPs can have a frank conversation about opioid use with their patients based on ethical principles and evidence-based practice, and employ a tapering protocol consistent with current opioid treatment guidelines. Furthermore, this approach appears not to jeopardize the patient-physician relationship.
CORRESPONDENCE
Thomas P. Guck, PhD, Creighton University School of Medicine, 2412 Cuming Street, Omaha, NE 68131; [email protected].
1. Manchikanti L, Helm S, Fellows B, et al. Opioid epidemic in the United States. Pain Physician. 2012;15:ES9-ES38.
2. Vowles KE, McEntee ML, Julnes PS, et al. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. 2015;156:569-576.
3. Sullivan MD, Howe CQ. Opioid therapy for chronic pain in the United States: promises and perils. Pain. 2013;154:S94-S100.
4. Portenoy RK, Foley KM. Chronic use of opioid analgesics in non-malignant pain: report of 38 cases. Pain. 1986;25:171-186.
5. The use of opioids for the treatment of chronic pain. A consensus statement from the American Academy of Pain Medicine and the American Pain Society. Clin J Pain. 1997;13:6-8.
6. Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:113-130.
7. Nuckols TK, Anderson L, Popescu I, et al. Opioid prescribing: a systematic review and critical appraisal of guidelines for chronic pain. Ann Intern Med. 2014;160:38-47.
8. Hwang CS, Turner LW, Kruszewski SP, et al. Primary care physicians’ knowledge and attitudes regarding prescription opioid abuse and diversion. Clin J Pain. 2016;279-284.
9. Top 15 challenges facing physicians in 2015. Medical Economics. http://www.medicaleconomics.com/medical-economics/news/top-15-challenges-facing-physicians-2015?page=0,12. Accessed October 18, 2018.
10. Kotalik J. Controlling pain and reducing misuse of opioids: ethical considerations. Can Fam Physician. 2012;58:381-385.
11. Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006;104:570-587.
12. Wachholtz A, Gonzalez G. Co-morbid pain and opioid addiction: long term effect of opioid maintenance on acute pain. Drug Alcohol Depend. 2014;145:143-149.
13. Hunter CL, Goodie JL, Oordt MS, Dobmeyer AC. Integrated Behavioral Health in Primary Care. 2nd ed. Washington DC: American Psychological Association; 2017.
14. Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. 3rd ed. New York, NY: The Guilford Press; 2013.
15. Townsend CO, Kerkvliet JL, Bruce BK, et al. A longitudinal study of the efficacy of a comprehensive pain rehabilitation program with opioid withdrawal: comparison of treatment outcomes based on opioid use status at admission. Pain. 2008;140:177-189.
16. Sullivan MD, Turner JA, DiLodovico C, et al. Prescription opioid taper support for outpatients with chronic pain: a randomized controlled trial. J Pain. 2017;18:308-318.
17. Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain - United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
18. Barth KS, Guille C, McCauley J, et al. Targeting practitioners: a review of guidelines, training, and policy in pain management. Drug Alcohol Depend. 2017;173:S22-S30.
19. CDC. Common Elements in Guidelines for Prescribing Opioids for Chronic Pain. Injury Prevention & Control: Prescription Drug Overdose 2016. http://www.cdc.gov/drugoverdose/prescribing/common-elements.html. Accessed October 18, 2018.
ABSTRACT
Purpose Our study examined the efficacy of a primary-care intervention in reducing opioid use among patients who have chronic non-cancer pain (CNCP). We also recorded the intervention’s effect on patients’ decisions to leave (or stay) with the primary-care practice.
Methods A family physician (FP) identified 41 patients in his practice who had CNCP of at least 6 month’s duration and were using opioids. The intervention with each patient involved an initial discussion of ethical principles, evidence-based practice, and current published guidelines. Following the discussion, patients self-selected to participate with their FP in a continuing tapering program or to accept referral to a pain center for management of their opioid medications. Tapering ranged from a 10% reduction per week to a more rapid 25% to 50% reduction every few days. Twenty-seven patients continued tapering with their FP, and 6 months later were retrospectively placed in the Taper Group. Fourteen patients chose not to pursue the tapering option and were referred to a single-modality medical pain clinic (MPC). All patients had the option of staying with the FP for other medical care.
Results At baseline and again at 6 months post-initial intervention, the MPC Group was taking significantly higher daily doses of morphine equivalents than the Taper Group. The Taper Group at 6 months was taking significantly lower average daily narcotic doses in morphine equivalents than at baseline. No significant baseline-to-6 month differences were found in the MPC Group. Contrary to many physicians’ fear of losing patients following candid discussions about opioid use, 40 of the 41 patients continued with the FP for other health needs.
Conclusions FPs can frankly discuss opioid use with their patients based on ethical principles and evidence-based recommendations and employ a tapering protocol consistent with current opioid treatment guidelines without jeopardizing the patient-physician relationship.
[polldaddy:10180698]
Opioid prescriptions for chronic noncancer pain (CNCP) have increased significantly over the past 25 years in the United States.1 Despite methodologic concerns surrounding research on opioid harms, prescription opioid misuse among CNCP patients is estimated to be 21% to 29% and prescription addiction 8% to 12%.2 Tragically, with the overall increase in opioid use for CNCP, substance-related hospital admissions and deaths due to opioid overdose have also risen.3
Increased opioid use began in 1985 when the World Health Organization expanded its ethical mandate for pain relief in dying patients to include relief from all cancer pain.3 Opioid use then accelerated following Portenoy and Foley’s 1986 article4 and the 1997 consensus statement by the American Academy of Pain Medicine (AAPM) and the American Pain Society (APS),5 with both organizations arguing that opioids have a role in the treatment of CNCP. Increased use of opioids for CNCP continued throughout the 1990s and 2000s, as many states passed legislation removing sanctions on prescribing long-term and high-dose opioid therapy, and pharmaceutical companies aggressively marketed sustained-release opioids.3
A balanced approach to opioids. While acknowledging the serious public health problems of drug abuse, addiction, and diversion of opioids from licit to illicit uses, clinical research and regulation leaders have called for a balanced approach that recognizes the legitimate medical need for opioids for CNCP. In 2009 the APS, in partnership with the AAPM, published evidence-based guidelines on chronic opioid therapy (COT) for adults with CNCP.6 In developing these guidelines, a multidisciplinary panel of experts conducted systematic reviews of available evidence and made recommendations on formulating COT for individuals, initiating and titrating therapy, regularly monitoring patients, and managing opioid-related adverse effects. Additional recommendations addressed the use of therapies focusing on psychosocial factors. The APS-AAPM guidelines received the highest rating in a systematic review critically appraising 13 guidelines that address the use of opioids for CNCP.7
Continue to: When opioid use is prolonged...
When opioid use is prolonged. Most primary care physicians are aware of the risks of prolonged opioid use, and many have successfully tapered or discontinued opioid medications for patients in acute or pre-chronic stages of pain.8 However, many physicians face the challenge of patients who have used COT for a longer time. The APS-AAPM guidelines may help primary care physicians at any stage of treating CNCP patients.
METHODS
Purpose and design. This retrospective study, which reviewed pretest-posttest findings between and within study groups, received an exempt status from Creighton University’s institutional review board. We designed the study to determine the efficacy of an intervention protocol to reduce opioid use by patients with CNCP who had been in a family physician (FP)'s panel for quite some time. Furthermore, because a common fear among primary care providers is that raising concerns with patients about their opioid use may cause those patients to leave their panel,9 our study also recorded how many patients stayed with their FP after initiation of the opioid management protocol.
Subjects. This study tracked 41 patients with CNCP in 1 FP’s panel. Inclusion criteria for participation was: 1) presence of CNCP for at least 6 months, 2) current use of opioid medication for CNCP, 3) age of at least 16 years, and 4) ability to read and write English. Two exclusion criteria were the presence of a surgically correctable condition or an organic brain syndrome or psychosis.
Clinical intervention. The FP identified eligible patients in his practice that were taking opioids for CNCP and initiated a discussion with each of them emphasizing his desire to follow the ethical principles of beneficence, nonmaleficence, respect for autonomy, and justice.10 The FP also presented his reasons for wanting the patient to stop using opioid medication. They included his beliefs that:
1) COT was not safe for the patient based on a growing body of published evidence of harm and death from COT3;
2) long-term use of opioids could lead to misuse, abuse, or addiction2;
3) prolonged opioid use paradoxically increases pain sensitivity that does not resolve
4) the patient’s current pain medications were not in line with published guidelines for use of opioids for CNCP.6
Initially, 45 patients were eligible for the study, but 4 declined participation before the intervention discussion and were immediately referred to a single-modality medical pain clinic (MPC). These patients were not included in subsequent analyses. Of the remaining 41 patients, all had a discussion with the MD about ethical principles, practice guidelines, and the importance of opioid tapering. After the discussion, patients decided whether to continue with the plan to taper their opioid therapy or to not taper their therapy and so receive a referral to an MPC.
Continue to: The 27 patients who chose to work with...
The 27 patients who chose to work with their FP started an individually tailored opioid-tapering program and were retrospectively placed in the Taper Group 6 months later. Tapering ranged from a slow 10% reduction in dosage per week to a more rapid 25% to 50% reduction every few days. Although evidence to guide specific recommendations on the rate of reduction is lacking, a slower rate may reduce unpleasant symptoms of opioid withdrawal.6 Following the patient-FP discussion, the 14 patients who chose not to pursue the tapering option were referred to an MPC for pain management, but could opt to remain with the FP for all other medical care. At 6 months post-discussion, we retrospectively assigned these 14 patients to the MPC Group.
Measures. We obtained demographic and medical information, including age, gender, race, marital status, and medication level in morphine equivalents, from the electronic health record. Medication level in morphine equivalents was recorded at the beginning of the intervention and again 6 months later. All analyses were conducted using SPSS Version 24 (IBM Corp, Armonk, NY) with P<.05 used to indicate statistical significance.
RESULTS
Between-group differences. The Taper and MPC groups did not differ significantly on demographic variables, with mean ages, respectively, at 57 and 51 years, sex 56% and 50% female, race 74% and 79% white, and marital status 48% and 50% married.
We found significant differences between the Taper and MPC groups on total daily dose in morphine equivalents at baseline and at 6 months following initial intervention. The Levene’s test for equality of variances was statistically significant, indicating unequal variances between the groups. In our SPSS analyses, we therefore used the option “equal variances not assumed.” TABLE 1 lists resultant means, standard deviations, individual sample t-test scores, and confidence intervals. The MPC Group was taking significantly higher daily doses of morphine equivalents than the Taper Group both at baseline and at 6 months following initial intervention.

Within-group differences. Paired sample t tests indicated significant differences between baseline and 6-month average daily narcotic doses in morphine equivalents for the Taper Group. No significant difference was found between baseline and 6-month daily morphine equivalents for the MPC group. These results indicated that patients who continued opioid tapering with the FP significantly reduced their daily morphine equivalents over the 6 months of the study. Patients in the MPC Group reduced morphine equivalents over the 6 months, but the reduction was not statistically significant. Paired sample t test results are presented in TABLE 2.

Continue to: Patient retention
Patient retention. All but one of the 41 patients in the Tapering and MPC groups continued with the FP for the remainder of their health care needs. Contrary to some physicians’ fears, the patients in this study maintained continuity with their FP.
DISCUSSION
Results of this study indicate that an intervention consisting of a physician-patient discussion of ethical principles and evidence-based practice, followed by individualized opioid tapering per published guidelines, led to a significant reduction in opioid use in patients with CNCP. The Taper Group, which completed the intervention, exhibited significant morphine reductions between baseline and 6-month follow-up. This did not hold true for the MPC Group.
The MPC Group, despite participating in the discussion with the FP, chose not to complete the tapering program and was referred to a single-modality MPC where opioids were managed rather than tapered. While the MPC group reduced daily opioid dose levels, the reduction was not statistically significant. A possible reason for no difference within the MPC Group may be that they had greater dependence on opioids, as their baseline average daily dose was much higher than that in the Taper Group (173 mg vs 31 mg, respectively). Although we did not assess anxiety directly, we speculate that the MPC Group was more anxious about opioid reduction than the Taper Group, and that this anxiety potentially led 4 patients to opt out of the initial FP discussion and 14 patients to self-select out of the tapering program following the discussion.
The FP intervention was successful for the Taper Group. For MPC patients, an enhanced intervention including behavior health strategies13 might have reduced anxiety and increased motivation14 to continue tapering. Based on moderate-quality evidence, APS-AAPM guidelines strongly recommend that CNCP be viewed as a complex biopsychosocial condition. Therefore, clinicians who prescribe opioids should routinely integrate psychotherapeutic interventions, functional restoration, interdisciplinary therapy, and other adjunctive nonopioid therapies.6
Opioid tapering within multidisciplinary rehabilitation programs is possible without significant worsening of pain, mood, and function.15 Recently, an outpatient opioid-tapering support intervention showed promise for efficacy in reducing prescription opioid doses without resultant increases in pain intensity or pain interference.16
Continue to: The tapering protocol in our study...
The tapering protocol in our study and the inclusion of behavioral health co-interventions are also recommended by the 2016 guidelines published by the Center for Disease Control and Prevention.17 More information on the similarities and differences among the various guidelines is available online.18,19
Caveats with our study. Patients’ entry into the Taper or MPC groups occurred through self-selection rather than random assignment. Thus, caution is recommended in interpreting findings of the FP intervention. And, we did not measure patients’ levels of pain, so differences between groups may have been possible. In addition, the number of patients per group was relatively small, which may have accounted for the lack of significance in the MPC Group findings. Conversely, significant reductions in opioid use in the small tapering sample suggests a relatively robust intervention, despite a lack of random assignment to treatment conditions.
These findings suggest that FPs can have a frank conversation about opioid use with their patients based on ethical principles and evidence-based practice, and employ a tapering protocol consistent with current opioid treatment guidelines. Furthermore, this approach appears not to jeopardize the patient-physician relationship.
CORRESPONDENCE
Thomas P. Guck, PhD, Creighton University School of Medicine, 2412 Cuming Street, Omaha, NE 68131; [email protected].
ABSTRACT
Purpose Our study examined the efficacy of a primary-care intervention in reducing opioid use among patients who have chronic non-cancer pain (CNCP). We also recorded the intervention’s effect on patients’ decisions to leave (or stay) with the primary-care practice.
Methods A family physician (FP) identified 41 patients in his practice who had CNCP of at least 6 month’s duration and were using opioids. The intervention with each patient involved an initial discussion of ethical principles, evidence-based practice, and current published guidelines. Following the discussion, patients self-selected to participate with their FP in a continuing tapering program or to accept referral to a pain center for management of their opioid medications. Tapering ranged from a 10% reduction per week to a more rapid 25% to 50% reduction every few days. Twenty-seven patients continued tapering with their FP, and 6 months later were retrospectively placed in the Taper Group. Fourteen patients chose not to pursue the tapering option and were referred to a single-modality medical pain clinic (MPC). All patients had the option of staying with the FP for other medical care.
Results At baseline and again at 6 months post-initial intervention, the MPC Group was taking significantly higher daily doses of morphine equivalents than the Taper Group. The Taper Group at 6 months was taking significantly lower average daily narcotic doses in morphine equivalents than at baseline. No significant baseline-to-6 month differences were found in the MPC Group. Contrary to many physicians’ fear of losing patients following candid discussions about opioid use, 40 of the 41 patients continued with the FP for other health needs.
Conclusions FPs can frankly discuss opioid use with their patients based on ethical principles and evidence-based recommendations and employ a tapering protocol consistent with current opioid treatment guidelines without jeopardizing the patient-physician relationship.
[polldaddy:10180698]
Opioid prescriptions for chronic noncancer pain (CNCP) have increased significantly over the past 25 years in the United States.1 Despite methodologic concerns surrounding research on opioid harms, prescription opioid misuse among CNCP patients is estimated to be 21% to 29% and prescription addiction 8% to 12%.2 Tragically, with the overall increase in opioid use for CNCP, substance-related hospital admissions and deaths due to opioid overdose have also risen.3
Increased opioid use began in 1985 when the World Health Organization expanded its ethical mandate for pain relief in dying patients to include relief from all cancer pain.3 Opioid use then accelerated following Portenoy and Foley’s 1986 article4 and the 1997 consensus statement by the American Academy of Pain Medicine (AAPM) and the American Pain Society (APS),5 with both organizations arguing that opioids have a role in the treatment of CNCP. Increased use of opioids for CNCP continued throughout the 1990s and 2000s, as many states passed legislation removing sanctions on prescribing long-term and high-dose opioid therapy, and pharmaceutical companies aggressively marketed sustained-release opioids.3
A balanced approach to opioids. While acknowledging the serious public health problems of drug abuse, addiction, and diversion of opioids from licit to illicit uses, clinical research and regulation leaders have called for a balanced approach that recognizes the legitimate medical need for opioids for CNCP. In 2009 the APS, in partnership with the AAPM, published evidence-based guidelines on chronic opioid therapy (COT) for adults with CNCP.6 In developing these guidelines, a multidisciplinary panel of experts conducted systematic reviews of available evidence and made recommendations on formulating COT for individuals, initiating and titrating therapy, regularly monitoring patients, and managing opioid-related adverse effects. Additional recommendations addressed the use of therapies focusing on psychosocial factors. The APS-AAPM guidelines received the highest rating in a systematic review critically appraising 13 guidelines that address the use of opioids for CNCP.7
Continue to: When opioid use is prolonged...
When opioid use is prolonged. Most primary care physicians are aware of the risks of prolonged opioid use, and many have successfully tapered or discontinued opioid medications for patients in acute or pre-chronic stages of pain.8 However, many physicians face the challenge of patients who have used COT for a longer time. The APS-AAPM guidelines may help primary care physicians at any stage of treating CNCP patients.
METHODS
Purpose and design. This retrospective study, which reviewed pretest-posttest findings between and within study groups, received an exempt status from Creighton University’s institutional review board. We designed the study to determine the efficacy of an intervention protocol to reduce opioid use by patients with CNCP who had been in a family physician (FP)'s panel for quite some time. Furthermore, because a common fear among primary care providers is that raising concerns with patients about their opioid use may cause those patients to leave their panel,9 our study also recorded how many patients stayed with their FP after initiation of the opioid management protocol.
Subjects. This study tracked 41 patients with CNCP in 1 FP’s panel. Inclusion criteria for participation was: 1) presence of CNCP for at least 6 months, 2) current use of opioid medication for CNCP, 3) age of at least 16 years, and 4) ability to read and write English. Two exclusion criteria were the presence of a surgically correctable condition or an organic brain syndrome or psychosis.
Clinical intervention. The FP identified eligible patients in his practice that were taking opioids for CNCP and initiated a discussion with each of them emphasizing his desire to follow the ethical principles of beneficence, nonmaleficence, respect for autonomy, and justice.10 The FP also presented his reasons for wanting the patient to stop using opioid medication. They included his beliefs that:
1) COT was not safe for the patient based on a growing body of published evidence of harm and death from COT3;
2) long-term use of opioids could lead to misuse, abuse, or addiction2;
3) prolonged opioid use paradoxically increases pain sensitivity that does not resolve
4) the patient’s current pain medications were not in line with published guidelines for use of opioids for CNCP.6
Initially, 45 patients were eligible for the study, but 4 declined participation before the intervention discussion and were immediately referred to a single-modality medical pain clinic (MPC). These patients were not included in subsequent analyses. Of the remaining 41 patients, all had a discussion with the MD about ethical principles, practice guidelines, and the importance of opioid tapering. After the discussion, patients decided whether to continue with the plan to taper their opioid therapy or to not taper their therapy and so receive a referral to an MPC.
Continue to: The 27 patients who chose to work with...
The 27 patients who chose to work with their FP started an individually tailored opioid-tapering program and were retrospectively placed in the Taper Group 6 months later. Tapering ranged from a slow 10% reduction in dosage per week to a more rapid 25% to 50% reduction every few days. Although evidence to guide specific recommendations on the rate of reduction is lacking, a slower rate may reduce unpleasant symptoms of opioid withdrawal.6 Following the patient-FP discussion, the 14 patients who chose not to pursue the tapering option were referred to an MPC for pain management, but could opt to remain with the FP for all other medical care. At 6 months post-discussion, we retrospectively assigned these 14 patients to the MPC Group.
Measures. We obtained demographic and medical information, including age, gender, race, marital status, and medication level in morphine equivalents, from the electronic health record. Medication level in morphine equivalents was recorded at the beginning of the intervention and again 6 months later. All analyses were conducted using SPSS Version 24 (IBM Corp, Armonk, NY) with P<.05 used to indicate statistical significance.
RESULTS
Between-group differences. The Taper and MPC groups did not differ significantly on demographic variables, with mean ages, respectively, at 57 and 51 years, sex 56% and 50% female, race 74% and 79% white, and marital status 48% and 50% married.
We found significant differences between the Taper and MPC groups on total daily dose in morphine equivalents at baseline and at 6 months following initial intervention. The Levene’s test for equality of variances was statistically significant, indicating unequal variances between the groups. In our SPSS analyses, we therefore used the option “equal variances not assumed.” TABLE 1 lists resultant means, standard deviations, individual sample t-test scores, and confidence intervals. The MPC Group was taking significantly higher daily doses of morphine equivalents than the Taper Group both at baseline and at 6 months following initial intervention.

Within-group differences. Paired sample t tests indicated significant differences between baseline and 6-month average daily narcotic doses in morphine equivalents for the Taper Group. No significant difference was found between baseline and 6-month daily morphine equivalents for the MPC group. These results indicated that patients who continued opioid tapering with the FP significantly reduced their daily morphine equivalents over the 6 months of the study. Patients in the MPC Group reduced morphine equivalents over the 6 months, but the reduction was not statistically significant. Paired sample t test results are presented in TABLE 2.

Continue to: Patient retention
Patient retention. All but one of the 41 patients in the Tapering and MPC groups continued with the FP for the remainder of their health care needs. Contrary to some physicians’ fears, the patients in this study maintained continuity with their FP.
DISCUSSION
Results of this study indicate that an intervention consisting of a physician-patient discussion of ethical principles and evidence-based practice, followed by individualized opioid tapering per published guidelines, led to a significant reduction in opioid use in patients with CNCP. The Taper Group, which completed the intervention, exhibited significant morphine reductions between baseline and 6-month follow-up. This did not hold true for the MPC Group.
The MPC Group, despite participating in the discussion with the FP, chose not to complete the tapering program and was referred to a single-modality MPC where opioids were managed rather than tapered. While the MPC group reduced daily opioid dose levels, the reduction was not statistically significant. A possible reason for no difference within the MPC Group may be that they had greater dependence on opioids, as their baseline average daily dose was much higher than that in the Taper Group (173 mg vs 31 mg, respectively). Although we did not assess anxiety directly, we speculate that the MPC Group was more anxious about opioid reduction than the Taper Group, and that this anxiety potentially led 4 patients to opt out of the initial FP discussion and 14 patients to self-select out of the tapering program following the discussion.
The FP intervention was successful for the Taper Group. For MPC patients, an enhanced intervention including behavior health strategies13 might have reduced anxiety and increased motivation14 to continue tapering. Based on moderate-quality evidence, APS-AAPM guidelines strongly recommend that CNCP be viewed as a complex biopsychosocial condition. Therefore, clinicians who prescribe opioids should routinely integrate psychotherapeutic interventions, functional restoration, interdisciplinary therapy, and other adjunctive nonopioid therapies.6
Opioid tapering within multidisciplinary rehabilitation programs is possible without significant worsening of pain, mood, and function.15 Recently, an outpatient opioid-tapering support intervention showed promise for efficacy in reducing prescription opioid doses without resultant increases in pain intensity or pain interference.16
Continue to: The tapering protocol in our study...
The tapering protocol in our study and the inclusion of behavioral health co-interventions are also recommended by the 2016 guidelines published by the Center for Disease Control and Prevention.17 More information on the similarities and differences among the various guidelines is available online.18,19
Caveats with our study. Patients’ entry into the Taper or MPC groups occurred through self-selection rather than random assignment. Thus, caution is recommended in interpreting findings of the FP intervention. And, we did not measure patients’ levels of pain, so differences between groups may have been possible. In addition, the number of patients per group was relatively small, which may have accounted for the lack of significance in the MPC Group findings. Conversely, significant reductions in opioid use in the small tapering sample suggests a relatively robust intervention, despite a lack of random assignment to treatment conditions.
These findings suggest that FPs can have a frank conversation about opioid use with their patients based on ethical principles and evidence-based practice, and employ a tapering protocol consistent with current opioid treatment guidelines. Furthermore, this approach appears not to jeopardize the patient-physician relationship.
CORRESPONDENCE
Thomas P. Guck, PhD, Creighton University School of Medicine, 2412 Cuming Street, Omaha, NE 68131; [email protected].
1. Manchikanti L, Helm S, Fellows B, et al. Opioid epidemic in the United States. Pain Physician. 2012;15:ES9-ES38.
2. Vowles KE, McEntee ML, Julnes PS, et al. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. 2015;156:569-576.
3. Sullivan MD, Howe CQ. Opioid therapy for chronic pain in the United States: promises and perils. Pain. 2013;154:S94-S100.
4. Portenoy RK, Foley KM. Chronic use of opioid analgesics in non-malignant pain: report of 38 cases. Pain. 1986;25:171-186.
5. The use of opioids for the treatment of chronic pain. A consensus statement from the American Academy of Pain Medicine and the American Pain Society. Clin J Pain. 1997;13:6-8.
6. Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:113-130.
7. Nuckols TK, Anderson L, Popescu I, et al. Opioid prescribing: a systematic review and critical appraisal of guidelines for chronic pain. Ann Intern Med. 2014;160:38-47.
8. Hwang CS, Turner LW, Kruszewski SP, et al. Primary care physicians’ knowledge and attitudes regarding prescription opioid abuse and diversion. Clin J Pain. 2016;279-284.
9. Top 15 challenges facing physicians in 2015. Medical Economics. http://www.medicaleconomics.com/medical-economics/news/top-15-challenges-facing-physicians-2015?page=0,12. Accessed October 18, 2018.
10. Kotalik J. Controlling pain and reducing misuse of opioids: ethical considerations. Can Fam Physician. 2012;58:381-385.
11. Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006;104:570-587.
12. Wachholtz A, Gonzalez G. Co-morbid pain and opioid addiction: long term effect of opioid maintenance on acute pain. Drug Alcohol Depend. 2014;145:143-149.
13. Hunter CL, Goodie JL, Oordt MS, Dobmeyer AC. Integrated Behavioral Health in Primary Care. 2nd ed. Washington DC: American Psychological Association; 2017.
14. Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. 3rd ed. New York, NY: The Guilford Press; 2013.
15. Townsend CO, Kerkvliet JL, Bruce BK, et al. A longitudinal study of the efficacy of a comprehensive pain rehabilitation program with opioid withdrawal: comparison of treatment outcomes based on opioid use status at admission. Pain. 2008;140:177-189.
16. Sullivan MD, Turner JA, DiLodovico C, et al. Prescription opioid taper support for outpatients with chronic pain: a randomized controlled trial. J Pain. 2017;18:308-318.
17. Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain - United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
18. Barth KS, Guille C, McCauley J, et al. Targeting practitioners: a review of guidelines, training, and policy in pain management. Drug Alcohol Depend. 2017;173:S22-S30.
19. CDC. Common Elements in Guidelines for Prescribing Opioids for Chronic Pain. Injury Prevention & Control: Prescription Drug Overdose 2016. http://www.cdc.gov/drugoverdose/prescribing/common-elements.html. Accessed October 18, 2018.
1. Manchikanti L, Helm S, Fellows B, et al. Opioid epidemic in the United States. Pain Physician. 2012;15:ES9-ES38.
2. Vowles KE, McEntee ML, Julnes PS, et al. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. 2015;156:569-576.
3. Sullivan MD, Howe CQ. Opioid therapy for chronic pain in the United States: promises and perils. Pain. 2013;154:S94-S100.
4. Portenoy RK, Foley KM. Chronic use of opioid analgesics in non-malignant pain: report of 38 cases. Pain. 1986;25:171-186.
5. The use of opioids for the treatment of chronic pain. A consensus statement from the American Academy of Pain Medicine and the American Pain Society. Clin J Pain. 1997;13:6-8.
6. Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:113-130.
7. Nuckols TK, Anderson L, Popescu I, et al. Opioid prescribing: a systematic review and critical appraisal of guidelines for chronic pain. Ann Intern Med. 2014;160:38-47.
8. Hwang CS, Turner LW, Kruszewski SP, et al. Primary care physicians’ knowledge and attitudes regarding prescription opioid abuse and diversion. Clin J Pain. 2016;279-284.
9. Top 15 challenges facing physicians in 2015. Medical Economics. http://www.medicaleconomics.com/medical-economics/news/top-15-challenges-facing-physicians-2015?page=0,12. Accessed October 18, 2018.
10. Kotalik J. Controlling pain and reducing misuse of opioids: ethical considerations. Can Fam Physician. 2012;58:381-385.
11. Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006;104:570-587.
12. Wachholtz A, Gonzalez G. Co-morbid pain and opioid addiction: long term effect of opioid maintenance on acute pain. Drug Alcohol Depend. 2014;145:143-149.
13. Hunter CL, Goodie JL, Oordt MS, Dobmeyer AC. Integrated Behavioral Health in Primary Care. 2nd ed. Washington DC: American Psychological Association; 2017.
14. Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. 3rd ed. New York, NY: The Guilford Press; 2013.
15. Townsend CO, Kerkvliet JL, Bruce BK, et al. A longitudinal study of the efficacy of a comprehensive pain rehabilitation program with opioid withdrawal: comparison of treatment outcomes based on opioid use status at admission. Pain. 2008;140:177-189.
16. Sullivan MD, Turner JA, DiLodovico C, et al. Prescription opioid taper support for outpatients with chronic pain: a randomized controlled trial. J Pain. 2017;18:308-318.
17. Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain - United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
18. Barth KS, Guille C, McCauley J, et al. Targeting practitioners: a review of guidelines, training, and policy in pain management. Drug Alcohol Depend. 2017;173:S22-S30.
19. CDC. Common Elements in Guidelines for Prescribing Opioids for Chronic Pain. Injury Prevention & Control: Prescription Drug Overdose 2016. http://www.cdc.gov/drugoverdose/prescribing/common-elements.html. Accessed October 18, 2018.
Sexually Transmitted Infections Caused by Mycoplasma genitalium and Neisseria gonorrhoeae: Diagnosis and Treatment
From the Fargo Veterans Affairs Health Care System, Fargo, ND (Dr. Dietz, Dr. Hammer, Dr. Zegarra, and Dr. Lo), and the Queen Elizabeth Hospital, Hong Kong, China (Dr. Cho).
Abstract
- Objective: To review the management of patients with Mycoplasma genitalium and Neisseria gonorrhoeae infections.
- Methods: Review of the literature.
- Results: Mycoplasma genitalium and Neisseria gonorrhoeae are organisms that cause urethritis, cervicitis, and pelvic inflammatory disease. There is increasing antibiotic resistance to both organisms, which poses significant challenges to clinicians. Additionally, diagnostic tests for M. genitalium are not widely available, and commonly used tests for both organisms do not provide antibiotic sensitivity information. The increasing resistance of both M. genitalium and N. gonorrhoeae to currently used antimicrobial agents is alarming and warrants cautious monitoring.
- Conclusion: As the yield of new or effective antibiotic therapies has decreased over the past few years, increasing antibiotic resistance will lead to difficult treatment scenarios for sexually transmitted infections caused by these 2 organisms.
Keywords: Mycoplasma genitalium, Neisseria gonorrhoeae, antibiotic resistance, sexually transmitted infections, STIs.
The World Health Organization (WHO) estimates that more than 1 million cases of sexually transmitted Infections (STIs) are acquired every day worldwide,1 and that the majority of STIs have few or no symptoms, making diagnosis difficult. Two organisms of interest are Mycoplasma genitalium and Neisseria gonorrhoeae. In contrast to Chlamydia trachomatis, which is rarely resistant to treatment regimens, M. genitalium and N. gonorrhoeae are becoming increasingly resistant to antibiotic treatment and pose an impending threat. These bacteria can cause urethritis, cervicitis, and pelvic inflammatory disease (PID). Whereas antibiotic resistance to M. genitalium is emerging, resistance to N. gonorrhea has been a continual problem for decades. Drug resistance, especially for N. gonorrhoeae, is listed as a major threat to efforts to reduce the impact of STIs worldwide.2 In 2013, the U.S. Centers for Disease Control and Prevention (CDC) classified N. gonorrhoeae drug resistance as an urgent threat.3 As the yield of new or effective antibiotic therapies has decreased over the past few years, increasing antibiotic resistance will lead to challenging treatment scenarios for STIs caused by these 2 organisms.
Epidemiology and Pathogenesis
M. genitalium
M. genitalium is an emerging pathogen that is an etiologic agent of upper and lower genital tract STIs, such as urethritis, cervicitis, and PID.4-13 In addition, it is thought to be involved in tubal infertility and acquisition of other sexually transmitted pathogens, including HIV.7,8,13 The prevalence of M. genitalium in the general U.S. population in 2016 was reported to be approximately 17.2% for males and 16.1% for females.14 Infections are more common in patients aged 30 years and younger than in older populations.15 Also, patients self-identifying as black were found to have a higher prevalence of M. genitalium.14 This organism was first reported as being isolated from the urethras of 2 men with non-gonococcal urethritis (NGU) in London in 1980.15,16 It is a significant cause of acute and chronic NGU in males, and is estimated to account for 6% to 50% of cases of NGU.17,18M. genitalium in females has been associated with cervicitis4,9 and PID.8,10 A meta-analysis by Lis et al showed that M. genitalium infection was associated with an increased risk for preterm birth and spontaneous abortion.11 In addition, M. genitalium infections occur frequently in HIV-positive patients.19,20 M. genitalium increases susceptibility for passage of HIV across the epithelium by reducing epithelial barrier integrity.19
Beta lactams are ineffective against M. genitalium because mycoplasmas lack a cell wall and thus cell wall penicillin-binding proteins.21M. genitalium’s abilty to invade host epithelial cells is another mechanism that can protect the bacteria from antibiotic exposure.20 One of the first reports of antibiotic sensitivity testing for M. genitalium, published in 1997, noted that the organism was not susceptible to nalidixic acid, cephalosporins, penicillins, and rifampicin.22 In general, mycoplasmas are normally susceptible to antibiotics that inhibit protein synthesis,23 and initial good sensitivity to doxycycline and erythromycin was noted but this has since decreased. New antibiotics are on the horizon, but they have not been extensively tested in vivo.23
N. gonorrhoeae
Gonorrhea is the second most common STI of bacterial origin following C. trachomatis,24-26 which is rarely resistant to conventional regimens. In 2008, the World Health Organization (WHO) estimated that 106 million cases of N. gonorrhoeae infection were acquired annually and that 36.4 million adults were infected with N. gonorrhoeae.27 In the United States, the CDC estimates that gonorrhea cases are under-reported. An estimated 800,000 or more new cases are reported per year.28
The most common clinical presentations are urethritis in men and cervicitis in women.29 While urethritis is most likely to be symptomatic, only 50% of women with acute gonorrhea are symptomatic.29 In addition to lower urogenital tract infection, N. gonorrhoeae can also cause PID, ectopic pregnancy, infertility in women, and epididymitis in men.29,30 Rare complications can develop from the spread of N. gonorrhoeae to other parts of the body including the joints, eyes, cardiovascular system, and skin.29
N. gonorrhoeae can attach to the columnar epithelium and causes host innate immune-driven inflammation with neutrophil influx.29 It can avoid the immune response by varying its outer membrane protein expression. The organism is also able to acquire DNA from other Neisseria species30 and genera, which results in reduced susceptibility to therapies.
The Gonococcal Isolate Surveillance Project (GISP), established in 1986, is a collaborative project involving the CDC and STI clinics in 26 cities in the United States along with 5 regional laboratories.31 The GISP monitors susceptibilities in N. gonorrhoeae isolates obtained from roughly 6000 symptomatic men each year.31 Data collected from the GISP allows clinicians to treat infections with the correct antibiotic. Just as they observed patterns of fluoroquinolone-resistant N. gonorrhoeae, there has been a geographic progression of decreasing susceptibility to cephalosporins in recent years.31
The ease with which N. gonorrhoeae can develop resistance is particularly alarming. Sulfonamide use began in the 1930s, but resistance developed within approximately 10 years.30,32N. gonorrhoeae has acquired resistance to each therapeutic agent used for treatment over the course of its lifetime. One hypothesis is that use of single-dose therapy to rapidly treat the infection has led to treatment failure and allows for selective pressure where organisms with decreased antibiotic susceptibility are more likely to survive.30 However, there is limited evidence to support monotherapy versus combination therapy in treating N. gonorrhoeae.33,34 It is no exaggeration to say gonorrhea is now at risk of becoming an untreatable disease because of the rapid emergence of multidrug resistant N. gonorrhoeae strains worldwide.35
Diagnosis
Whether the urethritis, cervicitis, or PID is caused by N. gonorrhoeae, M. genitalium, or other non-gonococcal microorganisms (eg, C. trachomatis), no symptoms are specific to any of the microorganisms. Therefore, clinicians rely on laboratory tests to diagnose STIs caused by N. gonorrhoeae or M. genitalium.
M. genitalium
Gram Stain. Because M. genitalium lacks a cell wall, it cannot be identified by routine Gram stain.
Culture. Culturing of this fastidious bacterium might offer the advantage of assessing antibiotic susceptibility;36 however, the procedure is labor intensive and time consuming, and only a few labs in the world have the capability to perform this culture.12 Thus, this testing method is primarily undertaken for research purposes.
Serological Testing. Because of serologic cross-reactions between Mycoplasma pneumoniae and M. genitalium, there are no standardized serological tests for M. genitalium.37
Nucleic Acid Amplification Tests. M. genitalium diagnosis currently is made based exclusively on nucleic acid amplification testing (NAAT) methodology (polymerase chain reaction [PCR] or transcription-mediated amplification [TMA]), which is the only clinically useful method to detect M. genitalium. TMA for M. genitalium is commercially available in an analyte-specific reagent (ASR) format, but this has not been approved by the Food and Drug Administration (FDA).38 A study analyzing urogenital specimens from female patients via this TMA product found a 98.7% true-positive result when confirmed with repeat testing or alternative-target TMA, and only a 0.5% false-negative rate.38 There is evidence that this TMA product can be used to identify M. genitalium in urine, stool, and pharyngeal samples.39 These assays are currently available in some reference labs and large medical centers but are not widely available. Table 1 summarizes the diagnostic methods for M. genitalium.
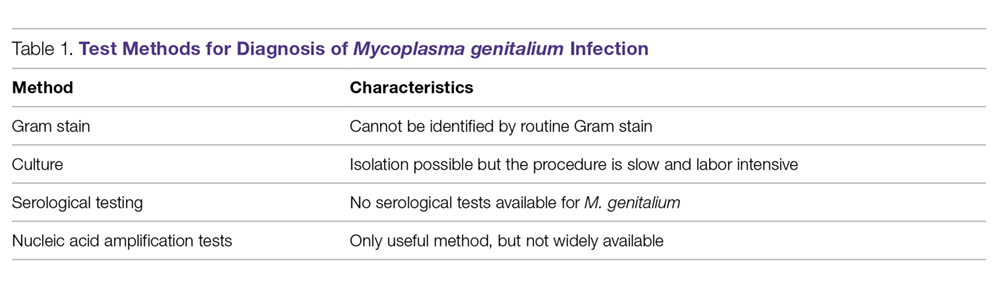
N. gonorrhoeae
Gonococcal infection can involve the urogenital tract, but can also be extra-urogenital. The method of diagnoses of urogenital infections has expanded from Gram stain of urethral or cervical discharge and the use of selective media culture (usually Thayer-Martin media)40 to molecular methods such as NAATs, which have a higher sensitivity than cultures.41,42
Gram Stain. A Gram stain that shows polymorphonuclear leukocytes with intracellular gram-negative diplococci can be considered diagnostic for N. gonorrhoeae urethritis infection in symptomatic men when samples are obtained from the urethra.43 A retrospective study of 1148 women with gonorrhea revealed that of 1049 cases of cervical gonorrhea, only 6.4% were positive by smear alone; and of 841 cases of urethral gonorrhea, only 5.1% were positive by smear alone; therefore, other diagnostic methods are generally preferred in women.44 Because Gram stain of vaginal specimens is positive in only 50% to 60% of females, its use in women and in suspected extragenital gonococcal infections is not recommended.43-45 When Gram stain was performed in asymptomatic men, the sensitivity was around 80%.39 Thus, in asymptomatic men with a high pre-test probability of having the infection, the use of other additional testing would increase the rate of detection.43
Culture. Urethral swab specimens from males with symptomatic urethritis and cervical swab samples from females with endocervical infection must be inoculated onto both a selective medium (eg, modified Thayer-Martin medium or Martin Lewis medium) and a nonselective medium (eg, chocolate agar). A selective medium is used because it can suppress the growth of contaminating organisms, and a nonselective medium is used because some strains of N. gonorrhoeae are inhibited by the vancomycin present in the selective medium.40 Specimens collected from sterile sites, such as blood, synovial fluid, and cerebrospinal fluid, should be streaked on nonselective medium such as chocolate agar. The material used for collection is critical; the preferred swabs should have plastic or wire shafts and rayon, Dacron, or calcium alginate tips. Materials such as wooden shafts or cotton tips can be toxic to N. gonorrhoeae.40 The specimen should be inoculated immediately onto the appropriate medium and transported rapidly to the laboratory, where it should be incubated at 35º to 37ºC with 5% CO2 and examined at 24 and 48 hours post collection.40 If the specimens cannot be inoculated immediately onto the appropriate medium, the specimen swab should be delivered to the lab in a special transport system that can keep the N. gonorrhoeae viable for up to 48 hours at room temperature.46
The following specimen collection techniques are recommended by the CDC:40
- In males, the cotton swab should be inserted about 2 to 3 cm into the urethral meatus and rotated 360° degrees 2 or 3 times.
- In females, collection of cervical specimens requires inserting the tip of the swab 1 to 2 centimeters into the cervical os and rotating 360° 2 or 3 times.
- Samples obtained outside of the urogenital tract: rectal specimens may be obtained by inserting the swab 3 to 4 cm into the rectal vault. Pharyngeal specimens are to be obtained from the posterior pharynx with a swab.
Culture tests allow the clinician to assess antimicrobial susceptibility and are relatively low cost when compared with nucleic acid detection tests. The sensitivity of culture ranges from 72% to 95% for symptomatic patients, but drops to 65% to 85% for asymptomatic patients.45-47 This low sensitivity is a major disadvantage of culture tests when compared to NAATs. Other disadvantages are the need for the specimens to be transported under conditions adequate to maintain the viability of organisms and the fact that 24 to 72 hours is required to report presumptive culture results.42 Antimicrobial sensitivity testing generally is not recommended; however, it is advisable to perform antimicrobial sensitivity in cases of treatment failure or disseminated gonococcal infection.12
Nucleic Acid Amplification Tests. NAATs use techniques that allow the amplification and detection of N. gonorrhoeae DNA or RNA sequences through various methods, which include assays such as PCR (eg, Amplicor; Roche, Nutley, NJ), TMA (eg, APTIMA; Gen-Probe, San Diego, CA), and strand-displacement amplification (SDA; Probe-Tec; Becton Dickinson, Franklin Lake, NJ). While PCR and SDA methods amplify bacterial DNA, TMA amplifies bacterial rRNA.41
The FDA has cleared NAATs to test endocervical, vaginal, and urethral (men) swab specimens and urine for both men and women. There are several NAATs available to test rectal, oropharyngeal, and conjunctival specimens; however, none of them are FDA-cleared. Some local and commercial laboratories have validated the reliability of these extra-urogenital NAATs.12,48 Compared to cultures, NAATs have the advantages of being more sensitive and requiring less strict collection and transport conditions. However, they are costlier than cultures, do not provide any antimicrobial susceptibility information, and have varying specificity.49,50
Rapid Tests. NAAT results are usually available in approximately 1 to 2 days, so there has been significant interest in creating technologies that would allow for a more rapid turnaround time. The GeneXpert CT/NG is a newly developed real-time PCR-based assay that can simultaneously detect C. trachomatis and N. gonorrhoeae. The advantage of this technique is the 90-minute turnaround time and its ability to process more than 90 samples at a time. The specificity of this test for N. gonorrhoeae is similar to that of other NAATs (> 99.3%), suggesting that cross-reactivity is not a significant problem.51 Table 2 summarizes the test methods used for diagnosing N. gonorrhoeae.
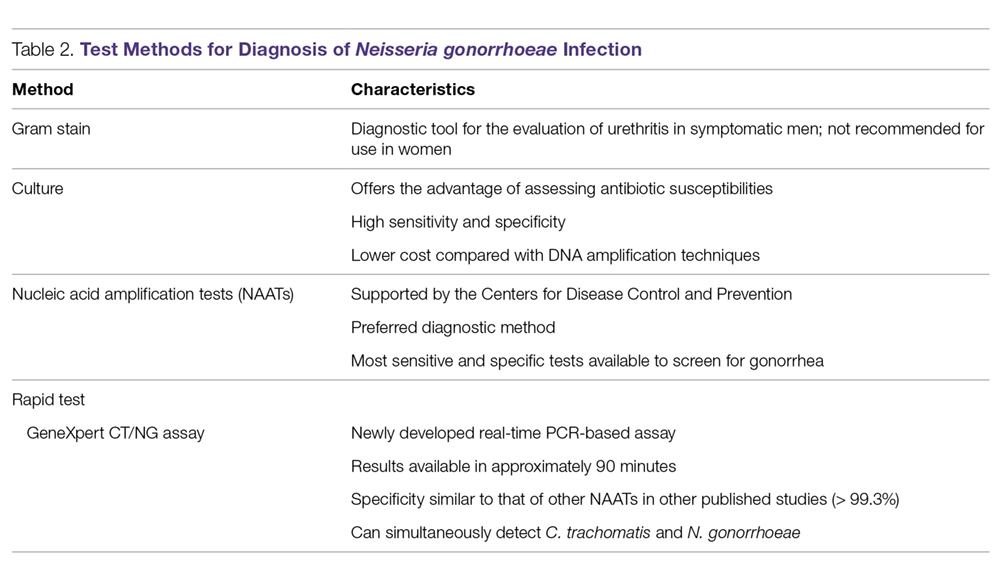
Treatment
M. genitalium
M. genitalium, Mycoplasma hominis, and the ureaplasmas (U. urealyticum and U. parvum) are generally transmitted sexually, and the natural habitat of this Mycoplasmataceae family of bacteria is the genitourinary tract. All the mycoplasmas can cause NGU, cervicitis, and PID. Presently, multiple-drug resistant M. hominis and ureaplasmas remain uncommon, but the prevalence of M. genitalium resistant to multiple antibiotics has increased significantly in recent years.23,52
In the 1990s, M. genitalium was highly sensitive to the tetracyclines in vitro,53 and doxycycline was the drug of choice for treating NGU. However, it later became apparent that doxycycline was largely ineffective in treating urethritis caused by M. genitalium.54,55
Subsequently, azithromycin, a macrolide, became popular in treating urethritis in males and cervicitis in females because it was highly active against C. trachomatis54 and M. genitalium56 and it can be given orally as a single 1-g dose, thus increasing patients’ compliance. However, azithromycin-resistant M. genitalium has rapidly emerged and rates of treatment failure with azithromycin as high as 40% have been reported in recent studies.57,58 The resistance was found to be mediated by mutations in the 23S rRNA gene upon exposure of M. genitalium to azithromycin.15,57-59 Multiple studies conducted in various countries (including the United States, Netherlands, England, and France) all found high rates of 23S rRNA gene mutations.15,57-59M. genitalium samples were analyzed using reverse transcription-PCR and Sanger sequencing of the 23S tRNA to assess rates of macrolide resistance markers. The study found that 50.8% of female participants and 42% of male participants harbored mutations indicating macrolide resistance.15
An in vitro study conducted in France showed that the respiratory fluoroquinolone moxifloxacin was highly active against mycoplasmas, including M. genitalium.60 This study and others led to the use of moxifloxacin in treating infections caused by azithromycin-resistant M. genitalium. Moxifloxacin initially was successful in treating previous treatment failure cases.61 Unfortunately, the success has been short-lived, as researchers from Japan and Australia have reported moxifloxacin treament failures.62-64 These treatment failures were related to mutations in the parC and gyrA genes.62
Because M. genitalium exhibits significantly increased resistance to the tetracyclines, macrolides, and fluoroquinolones, leading to treatment failures associated with the resistance, the recently published CDC sexually transmitted diseases guidelines (2015) do not specifically recommend or endorse one class of antibiotics over another to treat M. genitalium infections; this contrasts with their approach for other infections in which they make specific recommendations for treatment.12 The lack of clear recommendations from the CDC makes standardized treatment for this pathogen difficult. The CDC guidelines do identify M. genitalium as an emerging issue, and mention that a single 1-g dose of azithromycin should likely be recommended over doxycycline due to the low cure rate of 31% seen with doxycycline. Moxifloxacin is mentioned as a possible alternative, but it is noted that the medication has not been evaluated in clinical trials and several studies have shown failures.12
Although the existing antibiotics to treat M. genitalium infections are far from desirable, treatment approaches have been recommended:65
- Azithromycin or doxycycline should be considered for empiric treatment without documented M. genitalium infection.
- Azithromycin is suggested as the first choice in documented M. genitalium infections.
- In patients with urethritis, azithromycin is recommended over doxycycline based on multiple studies. A single 1-g dose of azithromycin is preferred to an extended regimen due to increased compliance despite the extended regimen being slightly superior in effectiveness. The single-dose regimen is associated with selection of macrolide-resistant strains.65
- Women with cervicitis and PID with documented M. genitalium infection should receive an azithromycin-containing regimen.
Although the existing antibiotics on the market could not keep up with the rapid mutations of M. genitalium, a few recent studies have provided a glimmer of hope to tackle this wily microorganism. Two recent studies from Japan demonstrated that sitafloxacin, a novel fluoroquinolone, administered 100 mg twice a day to patients with M. genitalium was superior to other older fluoroquinolones.66,67 This fluoroquinolone could turn out to be a promising first-line antibiotic for treatment of STIs caused by M. genitalium. Bissessor and colleagues conducted a prospective cohort study of M. genitalium-infected male and female patients attending a STI clinic in Melbourne, Australia, and found that oral pristinamycin is highly effective in treating the M. genitalium strains that are resistant to azithromycin and moxifloxacin.68 Jensen et al reported on the novel fluoroketolide solithromycin, which demonstrated superior in vitro activity against M. genitalium compared with doxycycline, fluoroquinolones, and other macrolides.69 Solithromycin could potentially become a new antibiotic to treat infection caused by multi-drug resistant M. genitalium.
N. gonorrhoeae
Because of increasing resistance of N. gonorrhoeae to fluoroquinolones in the United States, the CDC recommended against their routine use for all cases of gonorrhea in August 2007.70 In some countries, penicillin-, tetracycline-, and ciprofloxacin-resistance rates could be as high as 100%, and these antibacterial agents are no longer treatment options for gonorrhea. The WHO released new N. gonorrhoeae treatment guidelines in 2016 due to high-level of resistance to previously recommended fluoroquinolones and decreased susceptibility to the third-generation cephalosporins, which were a first-line recommendation in the 2003 guidelines.45 The CDC’s currently recommended regimens for the treatment of uncomplicated and disseminated gonorrheal infections are summarized in Table 3 and Table 4.12 Recommendations from the WHO guidelines are very similar to the CDC recommendations.45
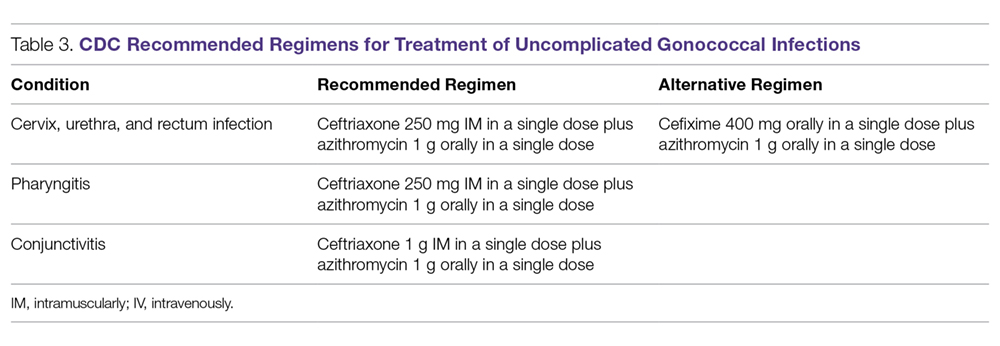
In light of the increasing resistance of N. gonorrhoeae to cephalosporins, 1 g of oral azithromycin should be added to ceftriaxone 250 mg intramuscularly in treating all cases of gonorrhea. The rationale for adding azithromycin to ceftriaxone is that azithromycin is active against N. gonorrhoeae at a different molecular target at a high dose, and it can also cover other co-pathogens.71 Unfortunately, susceptibility to cephalosporins has been decreasing rapidly.72 The greatest concern is the potential worldwide spread of the strain isolated in Kyoto, Japan, in 2009 from a patient with pharyngeal gonorrhea that was highly resistant to ceftriaxone (minimum inhibitory concentration of 2.0 to 4.0 µg/mL).73 At this time, N. gonorrhoeae isolates that are highly resistant to ceftriaxone are still rare globally.
Although cefixime is listed as an alternative treatment if ceftriaxone is not available, the 2015 CDC gonorrhea treatment guidelines note that N. gonorrhoeae is becoming more resistant to this oral third-generation cephalosporin; this increasing resistance is due in part to the genetic exchange between N. gonorrhoeae and other oral commensals actively taking place in the oral cavity, creating more resistant species. Another possible reason for cefixime resistance is that the concentration of cefixime used in treating gonococcal pharyngeal infection is subtherapeutic.74 A recent randomized multicenter trial in the United States compared 2 non-cephalosporin regimens: a single 240-mg dose of intramuscular gentamicin plus a single 2-g dose of oral azithromycin, and a single 320-mg dose of oral gemifloxacin plus a single 2-g dose of oral azithromycin. These combinations achieved 100% and 99.5% microbiological cure rates, respectively, in 401 patients with urogenital gonorrhea.75 Thus, these combination regimens can be considered as alternatives when the N. gonorrhoeae is resistant to cephalosporins or the patient is intolerant or allergic to cephalosporins.
Because N. gonorrhoeae has evolved into a “superbug,” becoming resistant to all currently available antimicrobial agents, it is important to focus on developing new agents with unique mechanisms of action to treat N. gonorrhoeae–related infections. Zoliflodacin (ETX0914), a novel topoisomerase II inhibitor, has the potential to become an effective agent to treat multi-drug resistant N. gonorrhoeae. A recent phase 2 trial demonstrated that a single oral 2000-mg dose of zoliflodacin microbiologically cleared 98% of gonorrhea patients, and some of the trial participants were infected with ciprofloxacin- or azithromycin-resistant strains.76 An additional phase 2 clinical trial compared oral zoliflodacin and intramuscular ceftriaxone. For uncomplicated urogential infections, 96% of patients in the zoliflodacin group achieved microbiologic cure versus 100% in the ceftriaxone group; however, zoliflodacin was less efficacious for pharyngeal infections.77 Gepotidacin (GSK2140944) is another new antimicrobial agent in the pipeline that looks promising. It is a novel first-in-class triazaacenaphthylene that inhibits bacterial DNA replication. A recent phase 2 clinical trial demonstrated that 1.5-g and 3-g single oral doses eradicated urogenital N. gonorrhoeae with microbiological success rates of 97% and 95%, respectively.78
Test of Cure
Because of the decreasing susceptibility of M. genitalium and N. gonorrhoeae to recommended treatment regimens, the European Guidelines consider test of cure essential in STIs caused by these 2 organisms to ensure eradication of infection and identify emerging resistance.79 However, test of cure is not routinely recommended by the CDC for these organisms in asymptomatic patients.12
Sexual Risk-Reduction Counseling
Besides aggressive treatment with appropriate antimicrobial agents, it is also essential that patients and their partners receive counseling to reduce the risk of STI. A recently published systematic review demonstrated that high-intensity counseling could decrease STI incidents in adolescents and adults.80
Conclusion
It is clear that these 2 sexually transmitted ”superbugs” are increasingly resistant to antibiotics and pose an increasing threat. Future epidemiological research and drug development studies need to be devoted to these 2 organisms, as well as to the potential development of a vaccine. This is especially important considering that antimicrobials may no longer be recommended when the prevalence of resistance to a particular antimicrobial reaches 5%, as is the case with WHO and other agencies that set the standard of ≥ 95% effectiveness for an antimicrobial to be considered as a recommended treatment.32 With current resistance rates for penicillin, ciprofloxacin, and tetracycline at close to 100% for N. gonorrhoeae in some countries,30,79 it is important to remain cognizant about current and future treatment options.
Because screening methods for M. genitalium are not available in most countries and there is not an FDA-approved screening method in the United States, M. genitalium poses a significant challenge for clinicians treating urethritis, cervicitis, and PID. Thus, the development of an effective screening method and established screening guidelines for M. genitalium is urgently needed. Better surveillance, prudent use of available antibiotics, and development of novel compounds are necessary to eliminate the impending threat caused by M. genitalium and N. gonorrhoeae.
This article is the result of work supported with resources and the use of facilities at the Fargo VA Health Care System. The contents of this manuscript do not represent the views of the Department of Veterans Affairs or the United States Government.
Corresponding author: Tze Shien Lo, MD, Veterans Affairs Medical Center, 2101 Elm Street N, Fargo, ND 58102.
Financial disclosures: None.
1. World Health Organization. Sexually transmitted infections (STIs). www.who.int/mediacentre/factsheets/fs110/en/. Fact Sheet #110. Updated August 2016. Accessed December 16, 2017.
2. World Health Organization. Growing antibiotic resistance forces updates to recommended treatment for sexually transmitted infections www.who.int/en/news-room/detail/30-08-2016-growing-antibiotic-resistance-forces-updates-to-recommended-treatment-for-sexually-transmitted-infections. Released August 30, 2016.
3. Centers for Disease Control and Prevention. Antibiotic/antimicrobial resistance biggest threats. www.cdc.gov/drugresistance/biggest_threats.html. Released February 27, 2018.
4. Taylor-Robinson D, Jensen JS. Mycoplasma genitalium: From chrysalis to multicolored butterfly. Clin Microbiol Rev. 2011;24:498-514.
5. Jensen JS. Mycoplasma genitalium: The aetiological agent of urethritis and other sexually transmitted diseases. J Eur Acad Dermatol Venereol. 2004;18:1-11.
6. Jaiyeoba O, Lazenby G, Soper DE. Recommendations and rationale for the treatment of pelvic inflammatory disease. Expert Rev Anti Infect Ther. 2011;9:61-70.
7. McGowin CL, Anderson-Smits C. Mycoplasma genitalium: An emerging cause of sexually transmitted disease in women. PLoS Pathog. 2011;7:e1001324.
8. Manhart LE, Broad JM, Golden MR. Mycoplasma genitalium: Should we treat and how? Clin Infect Dis. 2011;53 Suppl 3:S129-42.
9. Gaydos C, Maldeis NE, Hardick A, et al. Mycoplasma genitalium as a contributor to the multiple etiologies of cervicitis in women attending sexually transmitted disease clinics. Sex Transm Dis. 2009;36(1SE0):598-606.
10. Wiesenfeld HC, Hillier SL, Meyn L, et al. O04.6 Mycoplasma genitalium-Is it a pathogen in acute pelvic inflammatory disease (PID)? Sex Transm Infect. 2013 89:A34 http://sti.bmj.com/content/89/Suppl_1/A34.2. Accessed February 1, 2018.
11. Lis R, Rowhani-Rahbar A, Manhart LE. Mycoplasma genitalium infection and female reproductive tract disease: A meta-analysis. Clin Infect Dis. 2015;61:418-426.
12. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137.
13. Davies N. Mycoplasma genitalium: The need for testing and emerging diagnostic options. MLO Med Lab Obs. 2015;47:8,10-11.
14. Getman D, Jiang A, O’Donnell M, Cohen S. Mycoplasma genitalium prevalence, coinfection, and macrolide antibiotic resistance frequency in a multicenter clinical study cohort in the United States. J Clin Microbiol. 2016;54:2278-2283.
15. Tully JG, Taylor-Robinson D, Cole RM, Rose DL. A newly discovered mycoplasma in the human urogenital tract. Lancet. 1981;1(8233):1288-1291.
16. Taylor-Robinson D. The Harrison Lecture. The history and role of Mycoplasma genitalium in sexually transmitted diseases. Genitourin Med. 1995;71:1-8.
17. Horner P, Thomas B, Gilroy CB, Egger M, Taylor-Robinson D. Role of Mycoplasma genitalium and ureaplasma urealyticum in acute and chronic nongonococcal urethritis. Clin Infect Dis. 2001;32:995-1003.
18. Horner P, Blee K, O’Mahony C, et al. Clinical Effectiveness Group of the British Association of Sexual Health and HIV. 2015 UK National Guideline on the management of non-gonococcal urethritis. Int J STD AIDS. 2016;27:85-96.
19. Das K, De la Garza G, Siwak EB, et al. Mycoplasma genitalium promotes epithelial crossing and peripheral blood mononuclear cell infection by HIV-1. Int J Infect Dis. 2014;23:31-38.
20. McGowin CL, Annan RS, Quayle AJ, et al. Persistent Mycoplasma genitalium infection of human endocervical epithelial cells elicits chronic inflammatory cytokine secretion. Infect Immun. 2012;80:3842-3849.
21. Salado-Rasmussen K, Jensen JS. Mycoplasma genitalium testing pattern and macrolide resistance: A Danish nationwide retrospective survey. Clin Infect Dis. 2014;59:24-30.
22. Taylor-Robinson D, Bebear C. Antibiotic susceptibilities of mycoplasmas and treatment of mycoplasmal infections. J Antimicrob Chemother. 1997;40:622-630.
23. Taylor-Robinson D. Diagnosis and antimicrobial treatment of Mycoplasma genitalium infection: Sobering thoughts. Expert Rev Anti Infect Ther. 2014;12:715-722.
24. Ison CA. Biology of Neisseria gonorrhoeae and the clinical picture of infection. In: Gross G, Tyring SK, eds. Sexually Transmitted Infections and Sexually Transmitted Diseases.1st ed. Berlin, Heidelberg: Springer-Verlag; 2011:77-90.
25. Criss AK, Seifert HS. A bacterial siren song: Intimate interactions between neisseria and neutrophils. Nat Rev Microbiol. 2012;10:178-190.
26. Urban CF, Lourido S, Zychlinsky A. How do microbes evade neutrophil killing? Cell Microbiol. 2006;8:1687-1696.
27. World Health Organization, Dept. of Reproductive Health and Research. Global incidence and prevalence of selected curable sexually transmitted infections - 2008. www.who.int/reproductivehealth/publications/rtis/stisestimates/en/. Published 2012. Accessed February 6, 2018.
28. Centers for Disease Control and Prevention 2015 sexually transmitted diseases treatment guidelines. www.cdc.gov/std/tg2015/emerging.htm. Updated June 4, 2015.
29. Skerlev M, Culav-Koscak I. Gonorrhea: New challenges. Clin Dermatol. 2014;32:275-281.
30. Kirkcaldy RD, Ballard RC, Dowell D. Gonococcal resistance: Are cephalosporins next? Curr Infect Dis Rep. 2011;13:196-204.
31. Kidd S, Kirkcaldy R, Weinstock H, Bolan G. Tackling multidrug-resistant gonorrhea: How should we prepare for the untreatable? Expert Rev Anti Infect Ther. 2012;10:831-833.
32. Wang SA, Harvey AB, Conner SM, et al. Antimicrobial resistance for Neisseria gonorrhoeae in the United States, 1988 to 2003: The spread of fluoroquinolone resistance. Ann Intern Med. 2007;147:81-88.
33. Barbee LA, Kerani RP, Dombrowski JC, et al. A retrospective comparative study of 2-drug oral and intramuscular cephalosporin treatment regimens for pharyngeal gonorrhea. Clin Infect Dis. 2013;56:1539-434.
34. Sathia L, Ellis B, Phillip S, et al. Pharyngeal gonorrhoea - is dual therapy the way forward? Int J STD AIDS. 2007;18:647–8.
35. Tanaka M. Emergence of multidrug-resistant Neisseria gonorrhoeae strains circulating worldwide. Int J Urol. 2012;19:98-99.
36. Hamasuna R, Osada Y, Jensen JS. Isolation of Mycoplasma genitalium from first-void urine specimens by coculture with vero cells. J Clin Microbiol. 2007;45:847-850.
37. Razin S. Mycoplasma. In: Boricello SP, Murray PR, Funke G, eds. Topley & Wilson’s Microbiology and Microbial Infections. London, UK: Hodder Arnold; 2005:1957-2005.
38. Munson E, Bykowski H, Munson K, et al. Clinical laboratory assessment of Mycoplasma genitalium transcription-medicated ampliflication using primary female urogenital specimens. J Clin Microbiol. 2016;54:432-437.
39. Munson E, Wenten D, Jhansale S, et al. Expansion of comprehensive screening of male-sexually transmitted infection clinic attendees with Mycoplasma genitalium and Trichomonas vaginalis molecule assessment: a restrospective analysis. J Clin Microbiol. 2016;55:321-325.
40. Centers for Disease Control and Prevention. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae--2014. MMWR Recomm Rep. 2014;63(RR-02):1-19.
41. Boyadzhyan B, Yashina T, Yatabe JH, et al. Comparison of the APTIMA CT and GC assays with the APTIMA combo 2 assay, the Abbott LCx assay, and direct fluorescent-antibody and culture assays for detection of Chlamydia trachomatis and Neisseria gonorrhoeae. J Clin Microbiol. 2004;42:3089-3093.
42. Graseck AS, Shih SL, Peipert JF. Home versus clinic-based specimen collection for Chlamydia trachomatis and Neisseria gonorrhoeae. Expert Rev Anti Infect Ther. 2011;9:183-194.
43. Sherrard J, Barlow D. Gonorrhoea in men: Clinical and diagnostic aspects. Genitourin Med. 1996;72:422-426.
44. Goh BT, Varia KB, Ayliffe PF, Lim FK Diagnosis of gonorrhea by gram-stained smears and cultures in men and women: role of the urethral smear. Sex Transm Dis. 1985;12:135-139.
45. World Health Organization. WHO Guidelines for the Treatment of Neisseria gonorrhoeae. www.who.int/reproductivehealth/publications/rtis/gonorrhoea-treatment-guidelines/en/. Published 2016. Accessed December 16, 2017.
46. Arbique JC, Forward KR, LeBlanc J. Evaluation of four commercial transport media for the survival of Neisseria gonorrhoeae. Diagn Microbiol Infect Dis. 2000;36:163-168.
47. Schink JC, Keith LG. Problems in the culture diagnosis of gonorrhea. J Reprod Med. 1985;30(3 Suppl):244-249.
48. Marrazzo JM, Apicella MA. Neisseria gonorrhoeae (gonorrhea). In: Bennett JE, Dolin R, Blaser MJ, eds. Principles and Practice of Infectious Diseases. 8th ed. Philadelphia, PA: Elsevier; 2015:2446-2462.
49. Barry PM, Klausner JD. The use of cephalosporins for gonorrhea: The impending problem of resistance. Expert Opin Pharmacother. 2009;10:555-577.
50. Tabrizi SN, Unemo M, Limnios AE, et al. Evaluation of six commercial nucleic acid amplification tests for detection of Neisseria gonorrhoeae and other Neisseria species. J Clin Microbiol. 2011;49:3610-3615.
51. Goldenberg SD, Finn J, Sedudzi E, et al. Performance of the GeneXpert CT/NG assay compared to that of the Aptima AC2 assay for detection of rectal Chlamydia trachomatis and Neisseria gonorrhoeae by use of residual Aptima Samples. J Clin Microbiol. 2012;50:3867-3869.
52. Martin D. Mycoplasma genitalium, Mycoplasma hominis, and Ureaplasma species. In: Bennet J, Dolin R, Blaser M, eds. Principles and Practice of Infectious Diseases. 8th ed. Philadelphia, PA: Elsevier Sauders; 2015:2190-2193.
53. Hannan PC. Comparative susceptibilities of various AIDS-associated and human urogenital tract mycoplasmas and strains of Mycoplasma pneumoniae to 10 classes of antimicrobial agent in vitro. J Med Microbiol. 1998;47:1115-1122.
54. Mena LA, Mroczkowski TF, Nsuami M, Martin DH. A randomized comparison of azithromycin and doxycycline for the treatment of Mycoplasma genitalium-positive urethritis in men. Clin Infect Dis. 2009;48:1649-1654.
55. Schwebke JR, Rompalo A, Taylor S, et al. Re-evaluating the treatment of nongonococcal urethritis: Emphasizing emerging pathogens--a randomized clinical trial. Clin Infect Dis. 2011;52:163-170.
56. Bjornelius E, Anagrius C, Bojs G, et al. Antibiotic treatment of symptomatic Mycoplasma genitalium infection in Scandinavia: A controlled clinical trial. Sex Transm Infect. 2008;84:72-76.
57. Nijhuis RH, Severs TT, Van der Vegt DS, et al. High levels of macrolide resistance-associated mutations in Mycoplasma genitalium warrant antibiotic susceptibility-guided treatment. J Antimicrob Chemother. 2015;70:2515-2518.
58. Pond MJ, Nori AV, Witney AA, et al. High prevalence of antibiotic-resistant Mycoplasma genitalium in nongonococcal urethritis: The need for routine testing and the inadequacy of current treatment options. Clin Infect Dis. 2014;58:631-637.
59. Touati A, Peuchant O, Jensen JS, et al. Direct detection of macrolide resistance in Mycoplasma genitalium isolates from clinical specimens from France by use of real-time PCR and melting curve analysis. J Clin Microbiol. 2014;52:1549-1555.
60. Bebear CM, de Barbeyrac B, Pereyre S, et al. Activity of moxifloxacin against the urogenital Mycoplasmas ureaplasma spp., Mycoplasma hominis and Mycoplasma genitalium and Chlamydia trachomatis. Clin Microbiol Infect. 2008;14:801-805.
61. Jernberg E, Moghaddam A, Moi H. Azithromycin and moxifloxacin for microbiological cure of Mycoplasma genitalium infection: An open study. Int J STD AIDS. 2008;19:676-679.
62. Tagg KA, Jeoffreys NJ, Couldwell DL, et al. Fluoroquinolone and macrolide resistance-associated mutations in Mycoplasma genitalium. J Clin Microbiol. 2013;51:2245-2249.
63. Couldwell DL, Tagg KA, Jeoffreys NJ, Gilbert GL. Failure of moxifloxacin treatment in Mycoplasma genitalium infections due to macrolide and fluoroquinolone resistance. Int J STD AIDS. 2013;24:822-828.
64. Shimada Y, Deguchi T, Nakane K, et al. Emergence of clinical strains of Mycoplasma genitalium harbouring alterations in ParC associated with fluoroquinolone resistance. Int J Antimicrob Agents. 2010;36:255-258.
65. Mobley V, Seña A. Mycoplasma genitalium infection in men and women. In: UpToDate. www.uptodate.com. Last updated March 8, 2017. Accessed February 13, 2018.
66. Takahashi S, Hamasuna R, Yasuda M, et al. Clinical efficacy of sitafloxacin 100 mg twice daily for 7 days for patients with non-gonococcal urethritis. J Infect Chemother. 2013;19:941-945.
67. Ito S, Yasuda M, Seike K, et al. Clinical and microbiological outcomes in treatment of men with non-gonococcal urethritis with a 100-mg twice-daily dose regimen of sitafloxacin. J Infect Chemother. 2012;18:414-418.
68. Bissessor M, Tabrizi SN, Twin J, et al. Macrolide resistance and azithromycin failure in a Mycoplasma genitalium-infected cohort, and response of azithromycin failures to alternative antibiotic regimens. Clin Infect Dis. 2014;60:1228-1236.
69. Jensen JS, Fernandes P, Unemo M. In vitro activity of the new fluoroketolide solithromycin (CEM-101) against macrolide-resistant and -susceptible Mycoplasma genitalium strains. Antimicrob Agents Chemother. 2014;58:3151-3156.
70. Centers for Disease Control and Prevention (CDC). Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: Fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep. 2007;56:332-336.
71. Sexually transmitted diseases treatment guidelines, 2010. www.cdc.gov/std/treatment/default.htm. Published 2015. Accessed February13, 2016.
72. Centers for Disease Control and Prevention (CDC). Cephalosporin susceptibility among Neisseria gonorrhoeae isolates--United States, 2000-2010. MMWR Morb Mortal Wkly Rep. 2011;60:873-877.
73. Ohnishi M, Saika T, Hoshina S, et al. Ceftriaxone-resistant Neisseria gonorrhoeae, Japan. Emerg Infect Dis. 2011;17:148-149.
74. Centers for Disease Control and Prevention (CDC). Update to CDC’s sexually transmitted diseases treatment guidelines, 2010: Oral cephalosporins no longer a recommended treatment for gonococcal infections. MMWR Morb Mortal Wkly Rep. 2012;61:590-594.
75. Kirkcaldy RD, Weinstock HS, Moore PC, et al. The efficacy and safety of gentamicin plus azithromycin and gemifloxacin plus azithromycin as treatment of uncomplicated gonorrhea. Clin Infect Dis. 2014;59:1083-1091.
76. Seña AC, Taylor SN, Marrazzo J, et al. Microbiological cure rates and antimicrobial susceptibility of Neisseria gonorrhoeae to ETX0914 (AZD0914) in a phase II treatment trial for urogenital gonorrhea. (Poster 1308) Program and Abstract of ID Week 2016. New Orleans, LA, . October 25-30, 2016.
77. Taylor S, Marrazzo J, Batteiger B, et al. Single-dose zoliflodacin (ETX0914) for treatment of urogential gonorrhea. N Engl J Med. 2018;379:1835-1845.
78. Perry C, Dumont E, Raychaudhuri A. O05.3 A phase II, randomised, stdy in adults subjects evaluating the efficacy, safety, and tolerability of single doses of gepotidacin (GSK2140944) for treatment of uncomplicated urogenital gonorrhea. Sex Transm Infect. 2017;93(Suppl 2).
79. Bignell C, Unemo M, European STI Guidelines Editorial Board. 2012 European guideline on the diagnosis and treatment of gonorrhoea in adults. Int J STD AIDS. 2013;24:85-92.
80. O’Connor EA, Lin JS, Burda BU, et al. Behavioral sexual risk-reduction counseling in primary care to prevent sexually transmitted infections: A systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;161:874-883.
From the Fargo Veterans Affairs Health Care System, Fargo, ND (Dr. Dietz, Dr. Hammer, Dr. Zegarra, and Dr. Lo), and the Queen Elizabeth Hospital, Hong Kong, China (Dr. Cho).
Abstract
- Objective: To review the management of patients with Mycoplasma genitalium and Neisseria gonorrhoeae infections.
- Methods: Review of the literature.
- Results: Mycoplasma genitalium and Neisseria gonorrhoeae are organisms that cause urethritis, cervicitis, and pelvic inflammatory disease. There is increasing antibiotic resistance to both organisms, which poses significant challenges to clinicians. Additionally, diagnostic tests for M. genitalium are not widely available, and commonly used tests for both organisms do not provide antibiotic sensitivity information. The increasing resistance of both M. genitalium and N. gonorrhoeae to currently used antimicrobial agents is alarming and warrants cautious monitoring.
- Conclusion: As the yield of new or effective antibiotic therapies has decreased over the past few years, increasing antibiotic resistance will lead to difficult treatment scenarios for sexually transmitted infections caused by these 2 organisms.
Keywords: Mycoplasma genitalium, Neisseria gonorrhoeae, antibiotic resistance, sexually transmitted infections, STIs.
The World Health Organization (WHO) estimates that more than 1 million cases of sexually transmitted Infections (STIs) are acquired every day worldwide,1 and that the majority of STIs have few or no symptoms, making diagnosis difficult. Two organisms of interest are Mycoplasma genitalium and Neisseria gonorrhoeae. In contrast to Chlamydia trachomatis, which is rarely resistant to treatment regimens, M. genitalium and N. gonorrhoeae are becoming increasingly resistant to antibiotic treatment and pose an impending threat. These bacteria can cause urethritis, cervicitis, and pelvic inflammatory disease (PID). Whereas antibiotic resistance to M. genitalium is emerging, resistance to N. gonorrhea has been a continual problem for decades. Drug resistance, especially for N. gonorrhoeae, is listed as a major threat to efforts to reduce the impact of STIs worldwide.2 In 2013, the U.S. Centers for Disease Control and Prevention (CDC) classified N. gonorrhoeae drug resistance as an urgent threat.3 As the yield of new or effective antibiotic therapies has decreased over the past few years, increasing antibiotic resistance will lead to challenging treatment scenarios for STIs caused by these 2 organisms.
Epidemiology and Pathogenesis
M. genitalium
M. genitalium is an emerging pathogen that is an etiologic agent of upper and lower genital tract STIs, such as urethritis, cervicitis, and PID.4-13 In addition, it is thought to be involved in tubal infertility and acquisition of other sexually transmitted pathogens, including HIV.7,8,13 The prevalence of M. genitalium in the general U.S. population in 2016 was reported to be approximately 17.2% for males and 16.1% for females.14 Infections are more common in patients aged 30 years and younger than in older populations.15 Also, patients self-identifying as black were found to have a higher prevalence of M. genitalium.14 This organism was first reported as being isolated from the urethras of 2 men with non-gonococcal urethritis (NGU) in London in 1980.15,16 It is a significant cause of acute and chronic NGU in males, and is estimated to account for 6% to 50% of cases of NGU.17,18M. genitalium in females has been associated with cervicitis4,9 and PID.8,10 A meta-analysis by Lis et al showed that M. genitalium infection was associated with an increased risk for preterm birth and spontaneous abortion.11 In addition, M. genitalium infections occur frequently in HIV-positive patients.19,20 M. genitalium increases susceptibility for passage of HIV across the epithelium by reducing epithelial barrier integrity.19
Beta lactams are ineffective against M. genitalium because mycoplasmas lack a cell wall and thus cell wall penicillin-binding proteins.21M. genitalium’s abilty to invade host epithelial cells is another mechanism that can protect the bacteria from antibiotic exposure.20 One of the first reports of antibiotic sensitivity testing for M. genitalium, published in 1997, noted that the organism was not susceptible to nalidixic acid, cephalosporins, penicillins, and rifampicin.22 In general, mycoplasmas are normally susceptible to antibiotics that inhibit protein synthesis,23 and initial good sensitivity to doxycycline and erythromycin was noted but this has since decreased. New antibiotics are on the horizon, but they have not been extensively tested in vivo.23
N. gonorrhoeae
Gonorrhea is the second most common STI of bacterial origin following C. trachomatis,24-26 which is rarely resistant to conventional regimens. In 2008, the World Health Organization (WHO) estimated that 106 million cases of N. gonorrhoeae infection were acquired annually and that 36.4 million adults were infected with N. gonorrhoeae.27 In the United States, the CDC estimates that gonorrhea cases are under-reported. An estimated 800,000 or more new cases are reported per year.28
The most common clinical presentations are urethritis in men and cervicitis in women.29 While urethritis is most likely to be symptomatic, only 50% of women with acute gonorrhea are symptomatic.29 In addition to lower urogenital tract infection, N. gonorrhoeae can also cause PID, ectopic pregnancy, infertility in women, and epididymitis in men.29,30 Rare complications can develop from the spread of N. gonorrhoeae to other parts of the body including the joints, eyes, cardiovascular system, and skin.29
N. gonorrhoeae can attach to the columnar epithelium and causes host innate immune-driven inflammation with neutrophil influx.29 It can avoid the immune response by varying its outer membrane protein expression. The organism is also able to acquire DNA from other Neisseria species30 and genera, which results in reduced susceptibility to therapies.
The Gonococcal Isolate Surveillance Project (GISP), established in 1986, is a collaborative project involving the CDC and STI clinics in 26 cities in the United States along with 5 regional laboratories.31 The GISP monitors susceptibilities in N. gonorrhoeae isolates obtained from roughly 6000 symptomatic men each year.31 Data collected from the GISP allows clinicians to treat infections with the correct antibiotic. Just as they observed patterns of fluoroquinolone-resistant N. gonorrhoeae, there has been a geographic progression of decreasing susceptibility to cephalosporins in recent years.31
The ease with which N. gonorrhoeae can develop resistance is particularly alarming. Sulfonamide use began in the 1930s, but resistance developed within approximately 10 years.30,32N. gonorrhoeae has acquired resistance to each therapeutic agent used for treatment over the course of its lifetime. One hypothesis is that use of single-dose therapy to rapidly treat the infection has led to treatment failure and allows for selective pressure where organisms with decreased antibiotic susceptibility are more likely to survive.30 However, there is limited evidence to support monotherapy versus combination therapy in treating N. gonorrhoeae.33,34 It is no exaggeration to say gonorrhea is now at risk of becoming an untreatable disease because of the rapid emergence of multidrug resistant N. gonorrhoeae strains worldwide.35
Diagnosis
Whether the urethritis, cervicitis, or PID is caused by N. gonorrhoeae, M. genitalium, or other non-gonococcal microorganisms (eg, C. trachomatis), no symptoms are specific to any of the microorganisms. Therefore, clinicians rely on laboratory tests to diagnose STIs caused by N. gonorrhoeae or M. genitalium.
M. genitalium
Gram Stain. Because M. genitalium lacks a cell wall, it cannot be identified by routine Gram stain.
Culture. Culturing of this fastidious bacterium might offer the advantage of assessing antibiotic susceptibility;36 however, the procedure is labor intensive and time consuming, and only a few labs in the world have the capability to perform this culture.12 Thus, this testing method is primarily undertaken for research purposes.
Serological Testing. Because of serologic cross-reactions between Mycoplasma pneumoniae and M. genitalium, there are no standardized serological tests for M. genitalium.37
Nucleic Acid Amplification Tests. M. genitalium diagnosis currently is made based exclusively on nucleic acid amplification testing (NAAT) methodology (polymerase chain reaction [PCR] or transcription-mediated amplification [TMA]), which is the only clinically useful method to detect M. genitalium. TMA for M. genitalium is commercially available in an analyte-specific reagent (ASR) format, but this has not been approved by the Food and Drug Administration (FDA).38 A study analyzing urogenital specimens from female patients via this TMA product found a 98.7% true-positive result when confirmed with repeat testing or alternative-target TMA, and only a 0.5% false-negative rate.38 There is evidence that this TMA product can be used to identify M. genitalium in urine, stool, and pharyngeal samples.39 These assays are currently available in some reference labs and large medical centers but are not widely available. Table 1 summarizes the diagnostic methods for M. genitalium.

N. gonorrhoeae
Gonococcal infection can involve the urogenital tract, but can also be extra-urogenital. The method of diagnoses of urogenital infections has expanded from Gram stain of urethral or cervical discharge and the use of selective media culture (usually Thayer-Martin media)40 to molecular methods such as NAATs, which have a higher sensitivity than cultures.41,42
Gram Stain. A Gram stain that shows polymorphonuclear leukocytes with intracellular gram-negative diplococci can be considered diagnostic for N. gonorrhoeae urethritis infection in symptomatic men when samples are obtained from the urethra.43 A retrospective study of 1148 women with gonorrhea revealed that of 1049 cases of cervical gonorrhea, only 6.4% were positive by smear alone; and of 841 cases of urethral gonorrhea, only 5.1% were positive by smear alone; therefore, other diagnostic methods are generally preferred in women.44 Because Gram stain of vaginal specimens is positive in only 50% to 60% of females, its use in women and in suspected extragenital gonococcal infections is not recommended.43-45 When Gram stain was performed in asymptomatic men, the sensitivity was around 80%.39 Thus, in asymptomatic men with a high pre-test probability of having the infection, the use of other additional testing would increase the rate of detection.43
Culture. Urethral swab specimens from males with symptomatic urethritis and cervical swab samples from females with endocervical infection must be inoculated onto both a selective medium (eg, modified Thayer-Martin medium or Martin Lewis medium) and a nonselective medium (eg, chocolate agar). A selective medium is used because it can suppress the growth of contaminating organisms, and a nonselective medium is used because some strains of N. gonorrhoeae are inhibited by the vancomycin present in the selective medium.40 Specimens collected from sterile sites, such as blood, synovial fluid, and cerebrospinal fluid, should be streaked on nonselective medium such as chocolate agar. The material used for collection is critical; the preferred swabs should have plastic or wire shafts and rayon, Dacron, or calcium alginate tips. Materials such as wooden shafts or cotton tips can be toxic to N. gonorrhoeae.40 The specimen should be inoculated immediately onto the appropriate medium and transported rapidly to the laboratory, where it should be incubated at 35º to 37ºC with 5% CO2 and examined at 24 and 48 hours post collection.40 If the specimens cannot be inoculated immediately onto the appropriate medium, the specimen swab should be delivered to the lab in a special transport system that can keep the N. gonorrhoeae viable for up to 48 hours at room temperature.46
The following specimen collection techniques are recommended by the CDC:40
- In males, the cotton swab should be inserted about 2 to 3 cm into the urethral meatus and rotated 360° degrees 2 or 3 times.
- In females, collection of cervical specimens requires inserting the tip of the swab 1 to 2 centimeters into the cervical os and rotating 360° 2 or 3 times.
- Samples obtained outside of the urogenital tract: rectal specimens may be obtained by inserting the swab 3 to 4 cm into the rectal vault. Pharyngeal specimens are to be obtained from the posterior pharynx with a swab.
Culture tests allow the clinician to assess antimicrobial susceptibility and are relatively low cost when compared with nucleic acid detection tests. The sensitivity of culture ranges from 72% to 95% for symptomatic patients, but drops to 65% to 85% for asymptomatic patients.45-47 This low sensitivity is a major disadvantage of culture tests when compared to NAATs. Other disadvantages are the need for the specimens to be transported under conditions adequate to maintain the viability of organisms and the fact that 24 to 72 hours is required to report presumptive culture results.42 Antimicrobial sensitivity testing generally is not recommended; however, it is advisable to perform antimicrobial sensitivity in cases of treatment failure or disseminated gonococcal infection.12
Nucleic Acid Amplification Tests. NAATs use techniques that allow the amplification and detection of N. gonorrhoeae DNA or RNA sequences through various methods, which include assays such as PCR (eg, Amplicor; Roche, Nutley, NJ), TMA (eg, APTIMA; Gen-Probe, San Diego, CA), and strand-displacement amplification (SDA; Probe-Tec; Becton Dickinson, Franklin Lake, NJ). While PCR and SDA methods amplify bacterial DNA, TMA amplifies bacterial rRNA.41
The FDA has cleared NAATs to test endocervical, vaginal, and urethral (men) swab specimens and urine for both men and women. There are several NAATs available to test rectal, oropharyngeal, and conjunctival specimens; however, none of them are FDA-cleared. Some local and commercial laboratories have validated the reliability of these extra-urogenital NAATs.12,48 Compared to cultures, NAATs have the advantages of being more sensitive and requiring less strict collection and transport conditions. However, they are costlier than cultures, do not provide any antimicrobial susceptibility information, and have varying specificity.49,50
Rapid Tests. NAAT results are usually available in approximately 1 to 2 days, so there has been significant interest in creating technologies that would allow for a more rapid turnaround time. The GeneXpert CT/NG is a newly developed real-time PCR-based assay that can simultaneously detect C. trachomatis and N. gonorrhoeae. The advantage of this technique is the 90-minute turnaround time and its ability to process more than 90 samples at a time. The specificity of this test for N. gonorrhoeae is similar to that of other NAATs (> 99.3%), suggesting that cross-reactivity is not a significant problem.51 Table 2 summarizes the test methods used for diagnosing N. gonorrhoeae.

Treatment
M. genitalium
M. genitalium, Mycoplasma hominis, and the ureaplasmas (U. urealyticum and U. parvum) are generally transmitted sexually, and the natural habitat of this Mycoplasmataceae family of bacteria is the genitourinary tract. All the mycoplasmas can cause NGU, cervicitis, and PID. Presently, multiple-drug resistant M. hominis and ureaplasmas remain uncommon, but the prevalence of M. genitalium resistant to multiple antibiotics has increased significantly in recent years.23,52
In the 1990s, M. genitalium was highly sensitive to the tetracyclines in vitro,53 and doxycycline was the drug of choice for treating NGU. However, it later became apparent that doxycycline was largely ineffective in treating urethritis caused by M. genitalium.54,55
Subsequently, azithromycin, a macrolide, became popular in treating urethritis in males and cervicitis in females because it was highly active against C. trachomatis54 and M. genitalium56 and it can be given orally as a single 1-g dose, thus increasing patients’ compliance. However, azithromycin-resistant M. genitalium has rapidly emerged and rates of treatment failure with azithromycin as high as 40% have been reported in recent studies.57,58 The resistance was found to be mediated by mutations in the 23S rRNA gene upon exposure of M. genitalium to azithromycin.15,57-59 Multiple studies conducted in various countries (including the United States, Netherlands, England, and France) all found high rates of 23S rRNA gene mutations.15,57-59M. genitalium samples were analyzed using reverse transcription-PCR and Sanger sequencing of the 23S tRNA to assess rates of macrolide resistance markers. The study found that 50.8% of female participants and 42% of male participants harbored mutations indicating macrolide resistance.15
An in vitro study conducted in France showed that the respiratory fluoroquinolone moxifloxacin was highly active against mycoplasmas, including M. genitalium.60 This study and others led to the use of moxifloxacin in treating infections caused by azithromycin-resistant M. genitalium. Moxifloxacin initially was successful in treating previous treatment failure cases.61 Unfortunately, the success has been short-lived, as researchers from Japan and Australia have reported moxifloxacin treament failures.62-64 These treatment failures were related to mutations in the parC and gyrA genes.62
Because M. genitalium exhibits significantly increased resistance to the tetracyclines, macrolides, and fluoroquinolones, leading to treatment failures associated with the resistance, the recently published CDC sexually transmitted diseases guidelines (2015) do not specifically recommend or endorse one class of antibiotics over another to treat M. genitalium infections; this contrasts with their approach for other infections in which they make specific recommendations for treatment.12 The lack of clear recommendations from the CDC makes standardized treatment for this pathogen difficult. The CDC guidelines do identify M. genitalium as an emerging issue, and mention that a single 1-g dose of azithromycin should likely be recommended over doxycycline due to the low cure rate of 31% seen with doxycycline. Moxifloxacin is mentioned as a possible alternative, but it is noted that the medication has not been evaluated in clinical trials and several studies have shown failures.12
Although the existing antibiotics to treat M. genitalium infections are far from desirable, treatment approaches have been recommended:65
- Azithromycin or doxycycline should be considered for empiric treatment without documented M. genitalium infection.
- Azithromycin is suggested as the first choice in documented M. genitalium infections.
- In patients with urethritis, azithromycin is recommended over doxycycline based on multiple studies. A single 1-g dose of azithromycin is preferred to an extended regimen due to increased compliance despite the extended regimen being slightly superior in effectiveness. The single-dose regimen is associated with selection of macrolide-resistant strains.65
- Women with cervicitis and PID with documented M. genitalium infection should receive an azithromycin-containing regimen.
Although the existing antibiotics on the market could not keep up with the rapid mutations of M. genitalium, a few recent studies have provided a glimmer of hope to tackle this wily microorganism. Two recent studies from Japan demonstrated that sitafloxacin, a novel fluoroquinolone, administered 100 mg twice a day to patients with M. genitalium was superior to other older fluoroquinolones.66,67 This fluoroquinolone could turn out to be a promising first-line antibiotic for treatment of STIs caused by M. genitalium. Bissessor and colleagues conducted a prospective cohort study of M. genitalium-infected male and female patients attending a STI clinic in Melbourne, Australia, and found that oral pristinamycin is highly effective in treating the M. genitalium strains that are resistant to azithromycin and moxifloxacin.68 Jensen et al reported on the novel fluoroketolide solithromycin, which demonstrated superior in vitro activity against M. genitalium compared with doxycycline, fluoroquinolones, and other macrolides.69 Solithromycin could potentially become a new antibiotic to treat infection caused by multi-drug resistant M. genitalium.
N. gonorrhoeae
Because of increasing resistance of N. gonorrhoeae to fluoroquinolones in the United States, the CDC recommended against their routine use for all cases of gonorrhea in August 2007.70 In some countries, penicillin-, tetracycline-, and ciprofloxacin-resistance rates could be as high as 100%, and these antibacterial agents are no longer treatment options for gonorrhea. The WHO released new N. gonorrhoeae treatment guidelines in 2016 due to high-level of resistance to previously recommended fluoroquinolones and decreased susceptibility to the third-generation cephalosporins, which were a first-line recommendation in the 2003 guidelines.45 The CDC’s currently recommended regimens for the treatment of uncomplicated and disseminated gonorrheal infections are summarized in Table 3 and Table 4.12 Recommendations from the WHO guidelines are very similar to the CDC recommendations.45

In light of the increasing resistance of N. gonorrhoeae to cephalosporins, 1 g of oral azithromycin should be added to ceftriaxone 250 mg intramuscularly in treating all cases of gonorrhea. The rationale for adding azithromycin to ceftriaxone is that azithromycin is active against N. gonorrhoeae at a different molecular target at a high dose, and it can also cover other co-pathogens.71 Unfortunately, susceptibility to cephalosporins has been decreasing rapidly.72 The greatest concern is the potential worldwide spread of the strain isolated in Kyoto, Japan, in 2009 from a patient with pharyngeal gonorrhea that was highly resistant to ceftriaxone (minimum inhibitory concentration of 2.0 to 4.0 µg/mL).73 At this time, N. gonorrhoeae isolates that are highly resistant to ceftriaxone are still rare globally.

Although cefixime is listed as an alternative treatment if ceftriaxone is not available, the 2015 CDC gonorrhea treatment guidelines note that N. gonorrhoeae is becoming more resistant to this oral third-generation cephalosporin; this increasing resistance is due in part to the genetic exchange between N. gonorrhoeae and other oral commensals actively taking place in the oral cavity, creating more resistant species. Another possible reason for cefixime resistance is that the concentration of cefixime used in treating gonococcal pharyngeal infection is subtherapeutic.74 A recent randomized multicenter trial in the United States compared 2 non-cephalosporin regimens: a single 240-mg dose of intramuscular gentamicin plus a single 2-g dose of oral azithromycin, and a single 320-mg dose of oral gemifloxacin plus a single 2-g dose of oral azithromycin. These combinations achieved 100% and 99.5% microbiological cure rates, respectively, in 401 patients with urogenital gonorrhea.75 Thus, these combination regimens can be considered as alternatives when the N. gonorrhoeae is resistant to cephalosporins or the patient is intolerant or allergic to cephalosporins.
Because N. gonorrhoeae has evolved into a “superbug,” becoming resistant to all currently available antimicrobial agents, it is important to focus on developing new agents with unique mechanisms of action to treat N. gonorrhoeae–related infections. Zoliflodacin (ETX0914), a novel topoisomerase II inhibitor, has the potential to become an effective agent to treat multi-drug resistant N. gonorrhoeae. A recent phase 2 trial demonstrated that a single oral 2000-mg dose of zoliflodacin microbiologically cleared 98% of gonorrhea patients, and some of the trial participants were infected with ciprofloxacin- or azithromycin-resistant strains.76 An additional phase 2 clinical trial compared oral zoliflodacin and intramuscular ceftriaxone. For uncomplicated urogential infections, 96% of patients in the zoliflodacin group achieved microbiologic cure versus 100% in the ceftriaxone group; however, zoliflodacin was less efficacious for pharyngeal infections.77 Gepotidacin (GSK2140944) is another new antimicrobial agent in the pipeline that looks promising. It is a novel first-in-class triazaacenaphthylene that inhibits bacterial DNA replication. A recent phase 2 clinical trial demonstrated that 1.5-g and 3-g single oral doses eradicated urogenital N. gonorrhoeae with microbiological success rates of 97% and 95%, respectively.78
Test of Cure
Because of the decreasing susceptibility of M. genitalium and N. gonorrhoeae to recommended treatment regimens, the European Guidelines consider test of cure essential in STIs caused by these 2 organisms to ensure eradication of infection and identify emerging resistance.79 However, test of cure is not routinely recommended by the CDC for these organisms in asymptomatic patients.12
Sexual Risk-Reduction Counseling
Besides aggressive treatment with appropriate antimicrobial agents, it is also essential that patients and their partners receive counseling to reduce the risk of STI. A recently published systematic review demonstrated that high-intensity counseling could decrease STI incidents in adolescents and adults.80
Conclusion
It is clear that these 2 sexually transmitted ”superbugs” are increasingly resistant to antibiotics and pose an increasing threat. Future epidemiological research and drug development studies need to be devoted to these 2 organisms, as well as to the potential development of a vaccine. This is especially important considering that antimicrobials may no longer be recommended when the prevalence of resistance to a particular antimicrobial reaches 5%, as is the case with WHO and other agencies that set the standard of ≥ 95% effectiveness for an antimicrobial to be considered as a recommended treatment.32 With current resistance rates for penicillin, ciprofloxacin, and tetracycline at close to 100% for N. gonorrhoeae in some countries,30,79 it is important to remain cognizant about current and future treatment options.
Because screening methods for M. genitalium are not available in most countries and there is not an FDA-approved screening method in the United States, M. genitalium poses a significant challenge for clinicians treating urethritis, cervicitis, and PID. Thus, the development of an effective screening method and established screening guidelines for M. genitalium is urgently needed. Better surveillance, prudent use of available antibiotics, and development of novel compounds are necessary to eliminate the impending threat caused by M. genitalium and N. gonorrhoeae.
This article is the result of work supported with resources and the use of facilities at the Fargo VA Health Care System. The contents of this manuscript do not represent the views of the Department of Veterans Affairs or the United States Government.
Corresponding author: Tze Shien Lo, MD, Veterans Affairs Medical Center, 2101 Elm Street N, Fargo, ND 58102.
Financial disclosures: None.
From the Fargo Veterans Affairs Health Care System, Fargo, ND (Dr. Dietz, Dr. Hammer, Dr. Zegarra, and Dr. Lo), and the Queen Elizabeth Hospital, Hong Kong, China (Dr. Cho).
Abstract
- Objective: To review the management of patients with Mycoplasma genitalium and Neisseria gonorrhoeae infections.
- Methods: Review of the literature.
- Results: Mycoplasma genitalium and Neisseria gonorrhoeae are organisms that cause urethritis, cervicitis, and pelvic inflammatory disease. There is increasing antibiotic resistance to both organisms, which poses significant challenges to clinicians. Additionally, diagnostic tests for M. genitalium are not widely available, and commonly used tests for both organisms do not provide antibiotic sensitivity information. The increasing resistance of both M. genitalium and N. gonorrhoeae to currently used antimicrobial agents is alarming and warrants cautious monitoring.
- Conclusion: As the yield of new or effective antibiotic therapies has decreased over the past few years, increasing antibiotic resistance will lead to difficult treatment scenarios for sexually transmitted infections caused by these 2 organisms.
Keywords: Mycoplasma genitalium, Neisseria gonorrhoeae, antibiotic resistance, sexually transmitted infections, STIs.
The World Health Organization (WHO) estimates that more than 1 million cases of sexually transmitted Infections (STIs) are acquired every day worldwide,1 and that the majority of STIs have few or no symptoms, making diagnosis difficult. Two organisms of interest are Mycoplasma genitalium and Neisseria gonorrhoeae. In contrast to Chlamydia trachomatis, which is rarely resistant to treatment regimens, M. genitalium and N. gonorrhoeae are becoming increasingly resistant to antibiotic treatment and pose an impending threat. These bacteria can cause urethritis, cervicitis, and pelvic inflammatory disease (PID). Whereas antibiotic resistance to M. genitalium is emerging, resistance to N. gonorrhea has been a continual problem for decades. Drug resistance, especially for N. gonorrhoeae, is listed as a major threat to efforts to reduce the impact of STIs worldwide.2 In 2013, the U.S. Centers for Disease Control and Prevention (CDC) classified N. gonorrhoeae drug resistance as an urgent threat.3 As the yield of new or effective antibiotic therapies has decreased over the past few years, increasing antibiotic resistance will lead to challenging treatment scenarios for STIs caused by these 2 organisms.
Epidemiology and Pathogenesis
M. genitalium
M. genitalium is an emerging pathogen that is an etiologic agent of upper and lower genital tract STIs, such as urethritis, cervicitis, and PID.4-13 In addition, it is thought to be involved in tubal infertility and acquisition of other sexually transmitted pathogens, including HIV.7,8,13 The prevalence of M. genitalium in the general U.S. population in 2016 was reported to be approximately 17.2% for males and 16.1% for females.14 Infections are more common in patients aged 30 years and younger than in older populations.15 Also, patients self-identifying as black were found to have a higher prevalence of M. genitalium.14 This organism was first reported as being isolated from the urethras of 2 men with non-gonococcal urethritis (NGU) in London in 1980.15,16 It is a significant cause of acute and chronic NGU in males, and is estimated to account for 6% to 50% of cases of NGU.17,18M. genitalium in females has been associated with cervicitis4,9 and PID.8,10 A meta-analysis by Lis et al showed that M. genitalium infection was associated with an increased risk for preterm birth and spontaneous abortion.11 In addition, M. genitalium infections occur frequently in HIV-positive patients.19,20 M. genitalium increases susceptibility for passage of HIV across the epithelium by reducing epithelial barrier integrity.19
Beta lactams are ineffective against M. genitalium because mycoplasmas lack a cell wall and thus cell wall penicillin-binding proteins.21M. genitalium’s abilty to invade host epithelial cells is another mechanism that can protect the bacteria from antibiotic exposure.20 One of the first reports of antibiotic sensitivity testing for M. genitalium, published in 1997, noted that the organism was not susceptible to nalidixic acid, cephalosporins, penicillins, and rifampicin.22 In general, mycoplasmas are normally susceptible to antibiotics that inhibit protein synthesis,23 and initial good sensitivity to doxycycline and erythromycin was noted but this has since decreased. New antibiotics are on the horizon, but they have not been extensively tested in vivo.23
N. gonorrhoeae
Gonorrhea is the second most common STI of bacterial origin following C. trachomatis,24-26 which is rarely resistant to conventional regimens. In 2008, the World Health Organization (WHO) estimated that 106 million cases of N. gonorrhoeae infection were acquired annually and that 36.4 million adults were infected with N. gonorrhoeae.27 In the United States, the CDC estimates that gonorrhea cases are under-reported. An estimated 800,000 or more new cases are reported per year.28
The most common clinical presentations are urethritis in men and cervicitis in women.29 While urethritis is most likely to be symptomatic, only 50% of women with acute gonorrhea are symptomatic.29 In addition to lower urogenital tract infection, N. gonorrhoeae can also cause PID, ectopic pregnancy, infertility in women, and epididymitis in men.29,30 Rare complications can develop from the spread of N. gonorrhoeae to other parts of the body including the joints, eyes, cardiovascular system, and skin.29
N. gonorrhoeae can attach to the columnar epithelium and causes host innate immune-driven inflammation with neutrophil influx.29 It can avoid the immune response by varying its outer membrane protein expression. The organism is also able to acquire DNA from other Neisseria species30 and genera, which results in reduced susceptibility to therapies.
The Gonococcal Isolate Surveillance Project (GISP), established in 1986, is a collaborative project involving the CDC and STI clinics in 26 cities in the United States along with 5 regional laboratories.31 The GISP monitors susceptibilities in N. gonorrhoeae isolates obtained from roughly 6000 symptomatic men each year.31 Data collected from the GISP allows clinicians to treat infections with the correct antibiotic. Just as they observed patterns of fluoroquinolone-resistant N. gonorrhoeae, there has been a geographic progression of decreasing susceptibility to cephalosporins in recent years.31
The ease with which N. gonorrhoeae can develop resistance is particularly alarming. Sulfonamide use began in the 1930s, but resistance developed within approximately 10 years.30,32N. gonorrhoeae has acquired resistance to each therapeutic agent used for treatment over the course of its lifetime. One hypothesis is that use of single-dose therapy to rapidly treat the infection has led to treatment failure and allows for selective pressure where organisms with decreased antibiotic susceptibility are more likely to survive.30 However, there is limited evidence to support monotherapy versus combination therapy in treating N. gonorrhoeae.33,34 It is no exaggeration to say gonorrhea is now at risk of becoming an untreatable disease because of the rapid emergence of multidrug resistant N. gonorrhoeae strains worldwide.35
Diagnosis
Whether the urethritis, cervicitis, or PID is caused by N. gonorrhoeae, M. genitalium, or other non-gonococcal microorganisms (eg, C. trachomatis), no symptoms are specific to any of the microorganisms. Therefore, clinicians rely on laboratory tests to diagnose STIs caused by N. gonorrhoeae or M. genitalium.
M. genitalium
Gram Stain. Because M. genitalium lacks a cell wall, it cannot be identified by routine Gram stain.
Culture. Culturing of this fastidious bacterium might offer the advantage of assessing antibiotic susceptibility;36 however, the procedure is labor intensive and time consuming, and only a few labs in the world have the capability to perform this culture.12 Thus, this testing method is primarily undertaken for research purposes.
Serological Testing. Because of serologic cross-reactions between Mycoplasma pneumoniae and M. genitalium, there are no standardized serological tests for M. genitalium.37
Nucleic Acid Amplification Tests. M. genitalium diagnosis currently is made based exclusively on nucleic acid amplification testing (NAAT) methodology (polymerase chain reaction [PCR] or transcription-mediated amplification [TMA]), which is the only clinically useful method to detect M. genitalium. TMA for M. genitalium is commercially available in an analyte-specific reagent (ASR) format, but this has not been approved by the Food and Drug Administration (FDA).38 A study analyzing urogenital specimens from female patients via this TMA product found a 98.7% true-positive result when confirmed with repeat testing or alternative-target TMA, and only a 0.5% false-negative rate.38 There is evidence that this TMA product can be used to identify M. genitalium in urine, stool, and pharyngeal samples.39 These assays are currently available in some reference labs and large medical centers but are not widely available. Table 1 summarizes the diagnostic methods for M. genitalium.

N. gonorrhoeae
Gonococcal infection can involve the urogenital tract, but can also be extra-urogenital. The method of diagnoses of urogenital infections has expanded from Gram stain of urethral or cervical discharge and the use of selective media culture (usually Thayer-Martin media)40 to molecular methods such as NAATs, which have a higher sensitivity than cultures.41,42
Gram Stain. A Gram stain that shows polymorphonuclear leukocytes with intracellular gram-negative diplococci can be considered diagnostic for N. gonorrhoeae urethritis infection in symptomatic men when samples are obtained from the urethra.43 A retrospective study of 1148 women with gonorrhea revealed that of 1049 cases of cervical gonorrhea, only 6.4% were positive by smear alone; and of 841 cases of urethral gonorrhea, only 5.1% were positive by smear alone; therefore, other diagnostic methods are generally preferred in women.44 Because Gram stain of vaginal specimens is positive in only 50% to 60% of females, its use in women and in suspected extragenital gonococcal infections is not recommended.43-45 When Gram stain was performed in asymptomatic men, the sensitivity was around 80%.39 Thus, in asymptomatic men with a high pre-test probability of having the infection, the use of other additional testing would increase the rate of detection.43
Culture. Urethral swab specimens from males with symptomatic urethritis and cervical swab samples from females with endocervical infection must be inoculated onto both a selective medium (eg, modified Thayer-Martin medium or Martin Lewis medium) and a nonselective medium (eg, chocolate agar). A selective medium is used because it can suppress the growth of contaminating organisms, and a nonselective medium is used because some strains of N. gonorrhoeae are inhibited by the vancomycin present in the selective medium.40 Specimens collected from sterile sites, such as blood, synovial fluid, and cerebrospinal fluid, should be streaked on nonselective medium such as chocolate agar. The material used for collection is critical; the preferred swabs should have plastic or wire shafts and rayon, Dacron, or calcium alginate tips. Materials such as wooden shafts or cotton tips can be toxic to N. gonorrhoeae.40 The specimen should be inoculated immediately onto the appropriate medium and transported rapidly to the laboratory, where it should be incubated at 35º to 37ºC with 5% CO2 and examined at 24 and 48 hours post collection.40 If the specimens cannot be inoculated immediately onto the appropriate medium, the specimen swab should be delivered to the lab in a special transport system that can keep the N. gonorrhoeae viable for up to 48 hours at room temperature.46
The following specimen collection techniques are recommended by the CDC:40
- In males, the cotton swab should be inserted about 2 to 3 cm into the urethral meatus and rotated 360° degrees 2 or 3 times.
- In females, collection of cervical specimens requires inserting the tip of the swab 1 to 2 centimeters into the cervical os and rotating 360° 2 or 3 times.
- Samples obtained outside of the urogenital tract: rectal specimens may be obtained by inserting the swab 3 to 4 cm into the rectal vault. Pharyngeal specimens are to be obtained from the posterior pharynx with a swab.
Culture tests allow the clinician to assess antimicrobial susceptibility and are relatively low cost when compared with nucleic acid detection tests. The sensitivity of culture ranges from 72% to 95% for symptomatic patients, but drops to 65% to 85% for asymptomatic patients.45-47 This low sensitivity is a major disadvantage of culture tests when compared to NAATs. Other disadvantages are the need for the specimens to be transported under conditions adequate to maintain the viability of organisms and the fact that 24 to 72 hours is required to report presumptive culture results.42 Antimicrobial sensitivity testing generally is not recommended; however, it is advisable to perform antimicrobial sensitivity in cases of treatment failure or disseminated gonococcal infection.12
Nucleic Acid Amplification Tests. NAATs use techniques that allow the amplification and detection of N. gonorrhoeae DNA or RNA sequences through various methods, which include assays such as PCR (eg, Amplicor; Roche, Nutley, NJ), TMA (eg, APTIMA; Gen-Probe, San Diego, CA), and strand-displacement amplification (SDA; Probe-Tec; Becton Dickinson, Franklin Lake, NJ). While PCR and SDA methods amplify bacterial DNA, TMA amplifies bacterial rRNA.41
The FDA has cleared NAATs to test endocervical, vaginal, and urethral (men) swab specimens and urine for both men and women. There are several NAATs available to test rectal, oropharyngeal, and conjunctival specimens; however, none of them are FDA-cleared. Some local and commercial laboratories have validated the reliability of these extra-urogenital NAATs.12,48 Compared to cultures, NAATs have the advantages of being more sensitive and requiring less strict collection and transport conditions. However, they are costlier than cultures, do not provide any antimicrobial susceptibility information, and have varying specificity.49,50
Rapid Tests. NAAT results are usually available in approximately 1 to 2 days, so there has been significant interest in creating technologies that would allow for a more rapid turnaround time. The GeneXpert CT/NG is a newly developed real-time PCR-based assay that can simultaneously detect C. trachomatis and N. gonorrhoeae. The advantage of this technique is the 90-minute turnaround time and its ability to process more than 90 samples at a time. The specificity of this test for N. gonorrhoeae is similar to that of other NAATs (> 99.3%), suggesting that cross-reactivity is not a significant problem.51 Table 2 summarizes the test methods used for diagnosing N. gonorrhoeae.

Treatment
M. genitalium
M. genitalium, Mycoplasma hominis, and the ureaplasmas (U. urealyticum and U. parvum) are generally transmitted sexually, and the natural habitat of this Mycoplasmataceae family of bacteria is the genitourinary tract. All the mycoplasmas can cause NGU, cervicitis, and PID. Presently, multiple-drug resistant M. hominis and ureaplasmas remain uncommon, but the prevalence of M. genitalium resistant to multiple antibiotics has increased significantly in recent years.23,52
In the 1990s, M. genitalium was highly sensitive to the tetracyclines in vitro,53 and doxycycline was the drug of choice for treating NGU. However, it later became apparent that doxycycline was largely ineffective in treating urethritis caused by M. genitalium.54,55
Subsequently, azithromycin, a macrolide, became popular in treating urethritis in males and cervicitis in females because it was highly active against C. trachomatis54 and M. genitalium56 and it can be given orally as a single 1-g dose, thus increasing patients’ compliance. However, azithromycin-resistant M. genitalium has rapidly emerged and rates of treatment failure with azithromycin as high as 40% have been reported in recent studies.57,58 The resistance was found to be mediated by mutations in the 23S rRNA gene upon exposure of M. genitalium to azithromycin.15,57-59 Multiple studies conducted in various countries (including the United States, Netherlands, England, and France) all found high rates of 23S rRNA gene mutations.15,57-59M. genitalium samples were analyzed using reverse transcription-PCR and Sanger sequencing of the 23S tRNA to assess rates of macrolide resistance markers. The study found that 50.8% of female participants and 42% of male participants harbored mutations indicating macrolide resistance.15
An in vitro study conducted in France showed that the respiratory fluoroquinolone moxifloxacin was highly active against mycoplasmas, including M. genitalium.60 This study and others led to the use of moxifloxacin in treating infections caused by azithromycin-resistant M. genitalium. Moxifloxacin initially was successful in treating previous treatment failure cases.61 Unfortunately, the success has been short-lived, as researchers from Japan and Australia have reported moxifloxacin treament failures.62-64 These treatment failures were related to mutations in the parC and gyrA genes.62
Because M. genitalium exhibits significantly increased resistance to the tetracyclines, macrolides, and fluoroquinolones, leading to treatment failures associated with the resistance, the recently published CDC sexually transmitted diseases guidelines (2015) do not specifically recommend or endorse one class of antibiotics over another to treat M. genitalium infections; this contrasts with their approach for other infections in which they make specific recommendations for treatment.12 The lack of clear recommendations from the CDC makes standardized treatment for this pathogen difficult. The CDC guidelines do identify M. genitalium as an emerging issue, and mention that a single 1-g dose of azithromycin should likely be recommended over doxycycline due to the low cure rate of 31% seen with doxycycline. Moxifloxacin is mentioned as a possible alternative, but it is noted that the medication has not been evaluated in clinical trials and several studies have shown failures.12
Although the existing antibiotics to treat M. genitalium infections are far from desirable, treatment approaches have been recommended:65
- Azithromycin or doxycycline should be considered for empiric treatment without documented M. genitalium infection.
- Azithromycin is suggested as the first choice in documented M. genitalium infections.
- In patients with urethritis, azithromycin is recommended over doxycycline based on multiple studies. A single 1-g dose of azithromycin is preferred to an extended regimen due to increased compliance despite the extended regimen being slightly superior in effectiveness. The single-dose regimen is associated with selection of macrolide-resistant strains.65
- Women with cervicitis and PID with documented M. genitalium infection should receive an azithromycin-containing regimen.
Although the existing antibiotics on the market could not keep up with the rapid mutations of M. genitalium, a few recent studies have provided a glimmer of hope to tackle this wily microorganism. Two recent studies from Japan demonstrated that sitafloxacin, a novel fluoroquinolone, administered 100 mg twice a day to patients with M. genitalium was superior to other older fluoroquinolones.66,67 This fluoroquinolone could turn out to be a promising first-line antibiotic for treatment of STIs caused by M. genitalium. Bissessor and colleagues conducted a prospective cohort study of M. genitalium-infected male and female patients attending a STI clinic in Melbourne, Australia, and found that oral pristinamycin is highly effective in treating the M. genitalium strains that are resistant to azithromycin and moxifloxacin.68 Jensen et al reported on the novel fluoroketolide solithromycin, which demonstrated superior in vitro activity against M. genitalium compared with doxycycline, fluoroquinolones, and other macrolides.69 Solithromycin could potentially become a new antibiotic to treat infection caused by multi-drug resistant M. genitalium.
N. gonorrhoeae
Because of increasing resistance of N. gonorrhoeae to fluoroquinolones in the United States, the CDC recommended against their routine use for all cases of gonorrhea in August 2007.70 In some countries, penicillin-, tetracycline-, and ciprofloxacin-resistance rates could be as high as 100%, and these antibacterial agents are no longer treatment options for gonorrhea. The WHO released new N. gonorrhoeae treatment guidelines in 2016 due to high-level of resistance to previously recommended fluoroquinolones and decreased susceptibility to the third-generation cephalosporins, which were a first-line recommendation in the 2003 guidelines.45 The CDC’s currently recommended regimens for the treatment of uncomplicated and disseminated gonorrheal infections are summarized in Table 3 and Table 4.12 Recommendations from the WHO guidelines are very similar to the CDC recommendations.45

In light of the increasing resistance of N. gonorrhoeae to cephalosporins, 1 g of oral azithromycin should be added to ceftriaxone 250 mg intramuscularly in treating all cases of gonorrhea. The rationale for adding azithromycin to ceftriaxone is that azithromycin is active against N. gonorrhoeae at a different molecular target at a high dose, and it can also cover other co-pathogens.71 Unfortunately, susceptibility to cephalosporins has been decreasing rapidly.72 The greatest concern is the potential worldwide spread of the strain isolated in Kyoto, Japan, in 2009 from a patient with pharyngeal gonorrhea that was highly resistant to ceftriaxone (minimum inhibitory concentration of 2.0 to 4.0 µg/mL).73 At this time, N. gonorrhoeae isolates that are highly resistant to ceftriaxone are still rare globally.

Although cefixime is listed as an alternative treatment if ceftriaxone is not available, the 2015 CDC gonorrhea treatment guidelines note that N. gonorrhoeae is becoming more resistant to this oral third-generation cephalosporin; this increasing resistance is due in part to the genetic exchange between N. gonorrhoeae and other oral commensals actively taking place in the oral cavity, creating more resistant species. Another possible reason for cefixime resistance is that the concentration of cefixime used in treating gonococcal pharyngeal infection is subtherapeutic.74 A recent randomized multicenter trial in the United States compared 2 non-cephalosporin regimens: a single 240-mg dose of intramuscular gentamicin plus a single 2-g dose of oral azithromycin, and a single 320-mg dose of oral gemifloxacin plus a single 2-g dose of oral azithromycin. These combinations achieved 100% and 99.5% microbiological cure rates, respectively, in 401 patients with urogenital gonorrhea.75 Thus, these combination regimens can be considered as alternatives when the N. gonorrhoeae is resistant to cephalosporins or the patient is intolerant or allergic to cephalosporins.
Because N. gonorrhoeae has evolved into a “superbug,” becoming resistant to all currently available antimicrobial agents, it is important to focus on developing new agents with unique mechanisms of action to treat N. gonorrhoeae–related infections. Zoliflodacin (ETX0914), a novel topoisomerase II inhibitor, has the potential to become an effective agent to treat multi-drug resistant N. gonorrhoeae. A recent phase 2 trial demonstrated that a single oral 2000-mg dose of zoliflodacin microbiologically cleared 98% of gonorrhea patients, and some of the trial participants were infected with ciprofloxacin- or azithromycin-resistant strains.76 An additional phase 2 clinical trial compared oral zoliflodacin and intramuscular ceftriaxone. For uncomplicated urogential infections, 96% of patients in the zoliflodacin group achieved microbiologic cure versus 100% in the ceftriaxone group; however, zoliflodacin was less efficacious for pharyngeal infections.77 Gepotidacin (GSK2140944) is another new antimicrobial agent in the pipeline that looks promising. It is a novel first-in-class triazaacenaphthylene that inhibits bacterial DNA replication. A recent phase 2 clinical trial demonstrated that 1.5-g and 3-g single oral doses eradicated urogenital N. gonorrhoeae with microbiological success rates of 97% and 95%, respectively.78
Test of Cure
Because of the decreasing susceptibility of M. genitalium and N. gonorrhoeae to recommended treatment regimens, the European Guidelines consider test of cure essential in STIs caused by these 2 organisms to ensure eradication of infection and identify emerging resistance.79 However, test of cure is not routinely recommended by the CDC for these organisms in asymptomatic patients.12
Sexual Risk-Reduction Counseling
Besides aggressive treatment with appropriate antimicrobial agents, it is also essential that patients and their partners receive counseling to reduce the risk of STI. A recently published systematic review demonstrated that high-intensity counseling could decrease STI incidents in adolescents and adults.80
Conclusion
It is clear that these 2 sexually transmitted ”superbugs” are increasingly resistant to antibiotics and pose an increasing threat. Future epidemiological research and drug development studies need to be devoted to these 2 organisms, as well as to the potential development of a vaccine. This is especially important considering that antimicrobials may no longer be recommended when the prevalence of resistance to a particular antimicrobial reaches 5%, as is the case with WHO and other agencies that set the standard of ≥ 95% effectiveness for an antimicrobial to be considered as a recommended treatment.32 With current resistance rates for penicillin, ciprofloxacin, and tetracycline at close to 100% for N. gonorrhoeae in some countries,30,79 it is important to remain cognizant about current and future treatment options.
Because screening methods for M. genitalium are not available in most countries and there is not an FDA-approved screening method in the United States, M. genitalium poses a significant challenge for clinicians treating urethritis, cervicitis, and PID. Thus, the development of an effective screening method and established screening guidelines for M. genitalium is urgently needed. Better surveillance, prudent use of available antibiotics, and development of novel compounds are necessary to eliminate the impending threat caused by M. genitalium and N. gonorrhoeae.
This article is the result of work supported with resources and the use of facilities at the Fargo VA Health Care System. The contents of this manuscript do not represent the views of the Department of Veterans Affairs or the United States Government.
Corresponding author: Tze Shien Lo, MD, Veterans Affairs Medical Center, 2101 Elm Street N, Fargo, ND 58102.
Financial disclosures: None.
1. World Health Organization. Sexually transmitted infections (STIs). www.who.int/mediacentre/factsheets/fs110/en/. Fact Sheet #110. Updated August 2016. Accessed December 16, 2017.
2. World Health Organization. Growing antibiotic resistance forces updates to recommended treatment for sexually transmitted infections www.who.int/en/news-room/detail/30-08-2016-growing-antibiotic-resistance-forces-updates-to-recommended-treatment-for-sexually-transmitted-infections. Released August 30, 2016.
3. Centers for Disease Control and Prevention. Antibiotic/antimicrobial resistance biggest threats. www.cdc.gov/drugresistance/biggest_threats.html. Released February 27, 2018.
4. Taylor-Robinson D, Jensen JS. Mycoplasma genitalium: From chrysalis to multicolored butterfly. Clin Microbiol Rev. 2011;24:498-514.
5. Jensen JS. Mycoplasma genitalium: The aetiological agent of urethritis and other sexually transmitted diseases. J Eur Acad Dermatol Venereol. 2004;18:1-11.
6. Jaiyeoba O, Lazenby G, Soper DE. Recommendations and rationale for the treatment of pelvic inflammatory disease. Expert Rev Anti Infect Ther. 2011;9:61-70.
7. McGowin CL, Anderson-Smits C. Mycoplasma genitalium: An emerging cause of sexually transmitted disease in women. PLoS Pathog. 2011;7:e1001324.
8. Manhart LE, Broad JM, Golden MR. Mycoplasma genitalium: Should we treat and how? Clin Infect Dis. 2011;53 Suppl 3:S129-42.
9. Gaydos C, Maldeis NE, Hardick A, et al. Mycoplasma genitalium as a contributor to the multiple etiologies of cervicitis in women attending sexually transmitted disease clinics. Sex Transm Dis. 2009;36(1SE0):598-606.
10. Wiesenfeld HC, Hillier SL, Meyn L, et al. O04.6 Mycoplasma genitalium-Is it a pathogen in acute pelvic inflammatory disease (PID)? Sex Transm Infect. 2013 89:A34 http://sti.bmj.com/content/89/Suppl_1/A34.2. Accessed February 1, 2018.
11. Lis R, Rowhani-Rahbar A, Manhart LE. Mycoplasma genitalium infection and female reproductive tract disease: A meta-analysis. Clin Infect Dis. 2015;61:418-426.
12. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137.
13. Davies N. Mycoplasma genitalium: The need for testing and emerging diagnostic options. MLO Med Lab Obs. 2015;47:8,10-11.
14. Getman D, Jiang A, O’Donnell M, Cohen S. Mycoplasma genitalium prevalence, coinfection, and macrolide antibiotic resistance frequency in a multicenter clinical study cohort in the United States. J Clin Microbiol. 2016;54:2278-2283.
15. Tully JG, Taylor-Robinson D, Cole RM, Rose DL. A newly discovered mycoplasma in the human urogenital tract. Lancet. 1981;1(8233):1288-1291.
16. Taylor-Robinson D. The Harrison Lecture. The history and role of Mycoplasma genitalium in sexually transmitted diseases. Genitourin Med. 1995;71:1-8.
17. Horner P, Thomas B, Gilroy CB, Egger M, Taylor-Robinson D. Role of Mycoplasma genitalium and ureaplasma urealyticum in acute and chronic nongonococcal urethritis. Clin Infect Dis. 2001;32:995-1003.
18. Horner P, Blee K, O’Mahony C, et al. Clinical Effectiveness Group of the British Association of Sexual Health and HIV. 2015 UK National Guideline on the management of non-gonococcal urethritis. Int J STD AIDS. 2016;27:85-96.
19. Das K, De la Garza G, Siwak EB, et al. Mycoplasma genitalium promotes epithelial crossing and peripheral blood mononuclear cell infection by HIV-1. Int J Infect Dis. 2014;23:31-38.
20. McGowin CL, Annan RS, Quayle AJ, et al. Persistent Mycoplasma genitalium infection of human endocervical epithelial cells elicits chronic inflammatory cytokine secretion. Infect Immun. 2012;80:3842-3849.
21. Salado-Rasmussen K, Jensen JS. Mycoplasma genitalium testing pattern and macrolide resistance: A Danish nationwide retrospective survey. Clin Infect Dis. 2014;59:24-30.
22. Taylor-Robinson D, Bebear C. Antibiotic susceptibilities of mycoplasmas and treatment of mycoplasmal infections. J Antimicrob Chemother. 1997;40:622-630.
23. Taylor-Robinson D. Diagnosis and antimicrobial treatment of Mycoplasma genitalium infection: Sobering thoughts. Expert Rev Anti Infect Ther. 2014;12:715-722.
24. Ison CA. Biology of Neisseria gonorrhoeae and the clinical picture of infection. In: Gross G, Tyring SK, eds. Sexually Transmitted Infections and Sexually Transmitted Diseases.1st ed. Berlin, Heidelberg: Springer-Verlag; 2011:77-90.
25. Criss AK, Seifert HS. A bacterial siren song: Intimate interactions between neisseria and neutrophils. Nat Rev Microbiol. 2012;10:178-190.
26. Urban CF, Lourido S, Zychlinsky A. How do microbes evade neutrophil killing? Cell Microbiol. 2006;8:1687-1696.
27. World Health Organization, Dept. of Reproductive Health and Research. Global incidence and prevalence of selected curable sexually transmitted infections - 2008. www.who.int/reproductivehealth/publications/rtis/stisestimates/en/. Published 2012. Accessed February 6, 2018.
28. Centers for Disease Control and Prevention 2015 sexually transmitted diseases treatment guidelines. www.cdc.gov/std/tg2015/emerging.htm. Updated June 4, 2015.
29. Skerlev M, Culav-Koscak I. Gonorrhea: New challenges. Clin Dermatol. 2014;32:275-281.
30. Kirkcaldy RD, Ballard RC, Dowell D. Gonococcal resistance: Are cephalosporins next? Curr Infect Dis Rep. 2011;13:196-204.
31. Kidd S, Kirkcaldy R, Weinstock H, Bolan G. Tackling multidrug-resistant gonorrhea: How should we prepare for the untreatable? Expert Rev Anti Infect Ther. 2012;10:831-833.
32. Wang SA, Harvey AB, Conner SM, et al. Antimicrobial resistance for Neisseria gonorrhoeae in the United States, 1988 to 2003: The spread of fluoroquinolone resistance. Ann Intern Med. 2007;147:81-88.
33. Barbee LA, Kerani RP, Dombrowski JC, et al. A retrospective comparative study of 2-drug oral and intramuscular cephalosporin treatment regimens for pharyngeal gonorrhea. Clin Infect Dis. 2013;56:1539-434.
34. Sathia L, Ellis B, Phillip S, et al. Pharyngeal gonorrhoea - is dual therapy the way forward? Int J STD AIDS. 2007;18:647–8.
35. Tanaka M. Emergence of multidrug-resistant Neisseria gonorrhoeae strains circulating worldwide. Int J Urol. 2012;19:98-99.
36. Hamasuna R, Osada Y, Jensen JS. Isolation of Mycoplasma genitalium from first-void urine specimens by coculture with vero cells. J Clin Microbiol. 2007;45:847-850.
37. Razin S. Mycoplasma. In: Boricello SP, Murray PR, Funke G, eds. Topley & Wilson’s Microbiology and Microbial Infections. London, UK: Hodder Arnold; 2005:1957-2005.
38. Munson E, Bykowski H, Munson K, et al. Clinical laboratory assessment of Mycoplasma genitalium transcription-medicated ampliflication using primary female urogenital specimens. J Clin Microbiol. 2016;54:432-437.
39. Munson E, Wenten D, Jhansale S, et al. Expansion of comprehensive screening of male-sexually transmitted infection clinic attendees with Mycoplasma genitalium and Trichomonas vaginalis molecule assessment: a restrospective analysis. J Clin Microbiol. 2016;55:321-325.
40. Centers for Disease Control and Prevention. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae--2014. MMWR Recomm Rep. 2014;63(RR-02):1-19.
41. Boyadzhyan B, Yashina T, Yatabe JH, et al. Comparison of the APTIMA CT and GC assays with the APTIMA combo 2 assay, the Abbott LCx assay, and direct fluorescent-antibody and culture assays for detection of Chlamydia trachomatis and Neisseria gonorrhoeae. J Clin Microbiol. 2004;42:3089-3093.
42. Graseck AS, Shih SL, Peipert JF. Home versus clinic-based specimen collection for Chlamydia trachomatis and Neisseria gonorrhoeae. Expert Rev Anti Infect Ther. 2011;9:183-194.
43. Sherrard J, Barlow D. Gonorrhoea in men: Clinical and diagnostic aspects. Genitourin Med. 1996;72:422-426.
44. Goh BT, Varia KB, Ayliffe PF, Lim FK Diagnosis of gonorrhea by gram-stained smears and cultures in men and women: role of the urethral smear. Sex Transm Dis. 1985;12:135-139.
45. World Health Organization. WHO Guidelines for the Treatment of Neisseria gonorrhoeae. www.who.int/reproductivehealth/publications/rtis/gonorrhoea-treatment-guidelines/en/. Published 2016. Accessed December 16, 2017.
46. Arbique JC, Forward KR, LeBlanc J. Evaluation of four commercial transport media for the survival of Neisseria gonorrhoeae. Diagn Microbiol Infect Dis. 2000;36:163-168.
47. Schink JC, Keith LG. Problems in the culture diagnosis of gonorrhea. J Reprod Med. 1985;30(3 Suppl):244-249.
48. Marrazzo JM, Apicella MA. Neisseria gonorrhoeae (gonorrhea). In: Bennett JE, Dolin R, Blaser MJ, eds. Principles and Practice of Infectious Diseases. 8th ed. Philadelphia, PA: Elsevier; 2015:2446-2462.
49. Barry PM, Klausner JD. The use of cephalosporins for gonorrhea: The impending problem of resistance. Expert Opin Pharmacother. 2009;10:555-577.
50. Tabrizi SN, Unemo M, Limnios AE, et al. Evaluation of six commercial nucleic acid amplification tests for detection of Neisseria gonorrhoeae and other Neisseria species. J Clin Microbiol. 2011;49:3610-3615.
51. Goldenberg SD, Finn J, Sedudzi E, et al. Performance of the GeneXpert CT/NG assay compared to that of the Aptima AC2 assay for detection of rectal Chlamydia trachomatis and Neisseria gonorrhoeae by use of residual Aptima Samples. J Clin Microbiol. 2012;50:3867-3869.
52. Martin D. Mycoplasma genitalium, Mycoplasma hominis, and Ureaplasma species. In: Bennet J, Dolin R, Blaser M, eds. Principles and Practice of Infectious Diseases. 8th ed. Philadelphia, PA: Elsevier Sauders; 2015:2190-2193.
53. Hannan PC. Comparative susceptibilities of various AIDS-associated and human urogenital tract mycoplasmas and strains of Mycoplasma pneumoniae to 10 classes of antimicrobial agent in vitro. J Med Microbiol. 1998;47:1115-1122.
54. Mena LA, Mroczkowski TF, Nsuami M, Martin DH. A randomized comparison of azithromycin and doxycycline for the treatment of Mycoplasma genitalium-positive urethritis in men. Clin Infect Dis. 2009;48:1649-1654.
55. Schwebke JR, Rompalo A, Taylor S, et al. Re-evaluating the treatment of nongonococcal urethritis: Emphasizing emerging pathogens--a randomized clinical trial. Clin Infect Dis. 2011;52:163-170.
56. Bjornelius E, Anagrius C, Bojs G, et al. Antibiotic treatment of symptomatic Mycoplasma genitalium infection in Scandinavia: A controlled clinical trial. Sex Transm Infect. 2008;84:72-76.
57. Nijhuis RH, Severs TT, Van der Vegt DS, et al. High levels of macrolide resistance-associated mutations in Mycoplasma genitalium warrant antibiotic susceptibility-guided treatment. J Antimicrob Chemother. 2015;70:2515-2518.
58. Pond MJ, Nori AV, Witney AA, et al. High prevalence of antibiotic-resistant Mycoplasma genitalium in nongonococcal urethritis: The need for routine testing and the inadequacy of current treatment options. Clin Infect Dis. 2014;58:631-637.
59. Touati A, Peuchant O, Jensen JS, et al. Direct detection of macrolide resistance in Mycoplasma genitalium isolates from clinical specimens from France by use of real-time PCR and melting curve analysis. J Clin Microbiol. 2014;52:1549-1555.
60. Bebear CM, de Barbeyrac B, Pereyre S, et al. Activity of moxifloxacin against the urogenital Mycoplasmas ureaplasma spp., Mycoplasma hominis and Mycoplasma genitalium and Chlamydia trachomatis. Clin Microbiol Infect. 2008;14:801-805.
61. Jernberg E, Moghaddam A, Moi H. Azithromycin and moxifloxacin for microbiological cure of Mycoplasma genitalium infection: An open study. Int J STD AIDS. 2008;19:676-679.
62. Tagg KA, Jeoffreys NJ, Couldwell DL, et al. Fluoroquinolone and macrolide resistance-associated mutations in Mycoplasma genitalium. J Clin Microbiol. 2013;51:2245-2249.
63. Couldwell DL, Tagg KA, Jeoffreys NJ, Gilbert GL. Failure of moxifloxacin treatment in Mycoplasma genitalium infections due to macrolide and fluoroquinolone resistance. Int J STD AIDS. 2013;24:822-828.
64. Shimada Y, Deguchi T, Nakane K, et al. Emergence of clinical strains of Mycoplasma genitalium harbouring alterations in ParC associated with fluoroquinolone resistance. Int J Antimicrob Agents. 2010;36:255-258.
65. Mobley V, Seña A. Mycoplasma genitalium infection in men and women. In: UpToDate. www.uptodate.com. Last updated March 8, 2017. Accessed February 13, 2018.
66. Takahashi S, Hamasuna R, Yasuda M, et al. Clinical efficacy of sitafloxacin 100 mg twice daily for 7 days for patients with non-gonococcal urethritis. J Infect Chemother. 2013;19:941-945.
67. Ito S, Yasuda M, Seike K, et al. Clinical and microbiological outcomes in treatment of men with non-gonococcal urethritis with a 100-mg twice-daily dose regimen of sitafloxacin. J Infect Chemother. 2012;18:414-418.
68. Bissessor M, Tabrizi SN, Twin J, et al. Macrolide resistance and azithromycin failure in a Mycoplasma genitalium-infected cohort, and response of azithromycin failures to alternative antibiotic regimens. Clin Infect Dis. 2014;60:1228-1236.
69. Jensen JS, Fernandes P, Unemo M. In vitro activity of the new fluoroketolide solithromycin (CEM-101) against macrolide-resistant and -susceptible Mycoplasma genitalium strains. Antimicrob Agents Chemother. 2014;58:3151-3156.
70. Centers for Disease Control and Prevention (CDC). Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: Fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep. 2007;56:332-336.
71. Sexually transmitted diseases treatment guidelines, 2010. www.cdc.gov/std/treatment/default.htm. Published 2015. Accessed February13, 2016.
72. Centers for Disease Control and Prevention (CDC). Cephalosporin susceptibility among Neisseria gonorrhoeae isolates--United States, 2000-2010. MMWR Morb Mortal Wkly Rep. 2011;60:873-877.
73. Ohnishi M, Saika T, Hoshina S, et al. Ceftriaxone-resistant Neisseria gonorrhoeae, Japan. Emerg Infect Dis. 2011;17:148-149.
74. Centers for Disease Control and Prevention (CDC). Update to CDC’s sexually transmitted diseases treatment guidelines, 2010: Oral cephalosporins no longer a recommended treatment for gonococcal infections. MMWR Morb Mortal Wkly Rep. 2012;61:590-594.
75. Kirkcaldy RD, Weinstock HS, Moore PC, et al. The efficacy and safety of gentamicin plus azithromycin and gemifloxacin plus azithromycin as treatment of uncomplicated gonorrhea. Clin Infect Dis. 2014;59:1083-1091.
76. Seña AC, Taylor SN, Marrazzo J, et al. Microbiological cure rates and antimicrobial susceptibility of Neisseria gonorrhoeae to ETX0914 (AZD0914) in a phase II treatment trial for urogenital gonorrhea. (Poster 1308) Program and Abstract of ID Week 2016. New Orleans, LA, . October 25-30, 2016.
77. Taylor S, Marrazzo J, Batteiger B, et al. Single-dose zoliflodacin (ETX0914) for treatment of urogential gonorrhea. N Engl J Med. 2018;379:1835-1845.
78. Perry C, Dumont E, Raychaudhuri A. O05.3 A phase II, randomised, stdy in adults subjects evaluating the efficacy, safety, and tolerability of single doses of gepotidacin (GSK2140944) for treatment of uncomplicated urogenital gonorrhea. Sex Transm Infect. 2017;93(Suppl 2).
79. Bignell C, Unemo M, European STI Guidelines Editorial Board. 2012 European guideline on the diagnosis and treatment of gonorrhoea in adults. Int J STD AIDS. 2013;24:85-92.
80. O’Connor EA, Lin JS, Burda BU, et al. Behavioral sexual risk-reduction counseling in primary care to prevent sexually transmitted infections: A systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;161:874-883.
1. World Health Organization. Sexually transmitted infections (STIs). www.who.int/mediacentre/factsheets/fs110/en/. Fact Sheet #110. Updated August 2016. Accessed December 16, 2017.
2. World Health Organization. Growing antibiotic resistance forces updates to recommended treatment for sexually transmitted infections www.who.int/en/news-room/detail/30-08-2016-growing-antibiotic-resistance-forces-updates-to-recommended-treatment-for-sexually-transmitted-infections. Released August 30, 2016.
3. Centers for Disease Control and Prevention. Antibiotic/antimicrobial resistance biggest threats. www.cdc.gov/drugresistance/biggest_threats.html. Released February 27, 2018.
4. Taylor-Robinson D, Jensen JS. Mycoplasma genitalium: From chrysalis to multicolored butterfly. Clin Microbiol Rev. 2011;24:498-514.
5. Jensen JS. Mycoplasma genitalium: The aetiological agent of urethritis and other sexually transmitted diseases. J Eur Acad Dermatol Venereol. 2004;18:1-11.
6. Jaiyeoba O, Lazenby G, Soper DE. Recommendations and rationale for the treatment of pelvic inflammatory disease. Expert Rev Anti Infect Ther. 2011;9:61-70.
7. McGowin CL, Anderson-Smits C. Mycoplasma genitalium: An emerging cause of sexually transmitted disease in women. PLoS Pathog. 2011;7:e1001324.
8. Manhart LE, Broad JM, Golden MR. Mycoplasma genitalium: Should we treat and how? Clin Infect Dis. 2011;53 Suppl 3:S129-42.
9. Gaydos C, Maldeis NE, Hardick A, et al. Mycoplasma genitalium as a contributor to the multiple etiologies of cervicitis in women attending sexually transmitted disease clinics. Sex Transm Dis. 2009;36(1SE0):598-606.
10. Wiesenfeld HC, Hillier SL, Meyn L, et al. O04.6 Mycoplasma genitalium-Is it a pathogen in acute pelvic inflammatory disease (PID)? Sex Transm Infect. 2013 89:A34 http://sti.bmj.com/content/89/Suppl_1/A34.2. Accessed February 1, 2018.
11. Lis R, Rowhani-Rahbar A, Manhart LE. Mycoplasma genitalium infection and female reproductive tract disease: A meta-analysis. Clin Infect Dis. 2015;61:418-426.
12. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137.
13. Davies N. Mycoplasma genitalium: The need for testing and emerging diagnostic options. MLO Med Lab Obs. 2015;47:8,10-11.
14. Getman D, Jiang A, O’Donnell M, Cohen S. Mycoplasma genitalium prevalence, coinfection, and macrolide antibiotic resistance frequency in a multicenter clinical study cohort in the United States. J Clin Microbiol. 2016;54:2278-2283.
15. Tully JG, Taylor-Robinson D, Cole RM, Rose DL. A newly discovered mycoplasma in the human urogenital tract. Lancet. 1981;1(8233):1288-1291.
16. Taylor-Robinson D. The Harrison Lecture. The history and role of Mycoplasma genitalium in sexually transmitted diseases. Genitourin Med. 1995;71:1-8.
17. Horner P, Thomas B, Gilroy CB, Egger M, Taylor-Robinson D. Role of Mycoplasma genitalium and ureaplasma urealyticum in acute and chronic nongonococcal urethritis. Clin Infect Dis. 2001;32:995-1003.
18. Horner P, Blee K, O’Mahony C, et al. Clinical Effectiveness Group of the British Association of Sexual Health and HIV. 2015 UK National Guideline on the management of non-gonococcal urethritis. Int J STD AIDS. 2016;27:85-96.
19. Das K, De la Garza G, Siwak EB, et al. Mycoplasma genitalium promotes epithelial crossing and peripheral blood mononuclear cell infection by HIV-1. Int J Infect Dis. 2014;23:31-38.
20. McGowin CL, Annan RS, Quayle AJ, et al. Persistent Mycoplasma genitalium infection of human endocervical epithelial cells elicits chronic inflammatory cytokine secretion. Infect Immun. 2012;80:3842-3849.
21. Salado-Rasmussen K, Jensen JS. Mycoplasma genitalium testing pattern and macrolide resistance: A Danish nationwide retrospective survey. Clin Infect Dis. 2014;59:24-30.
22. Taylor-Robinson D, Bebear C. Antibiotic susceptibilities of mycoplasmas and treatment of mycoplasmal infections. J Antimicrob Chemother. 1997;40:622-630.
23. Taylor-Robinson D. Diagnosis and antimicrobial treatment of Mycoplasma genitalium infection: Sobering thoughts. Expert Rev Anti Infect Ther. 2014;12:715-722.
24. Ison CA. Biology of Neisseria gonorrhoeae and the clinical picture of infection. In: Gross G, Tyring SK, eds. Sexually Transmitted Infections and Sexually Transmitted Diseases.1st ed. Berlin, Heidelberg: Springer-Verlag; 2011:77-90.
25. Criss AK, Seifert HS. A bacterial siren song: Intimate interactions between neisseria and neutrophils. Nat Rev Microbiol. 2012;10:178-190.
26. Urban CF, Lourido S, Zychlinsky A. How do microbes evade neutrophil killing? Cell Microbiol. 2006;8:1687-1696.
27. World Health Organization, Dept. of Reproductive Health and Research. Global incidence and prevalence of selected curable sexually transmitted infections - 2008. www.who.int/reproductivehealth/publications/rtis/stisestimates/en/. Published 2012. Accessed February 6, 2018.
28. Centers for Disease Control and Prevention 2015 sexually transmitted diseases treatment guidelines. www.cdc.gov/std/tg2015/emerging.htm. Updated June 4, 2015.
29. Skerlev M, Culav-Koscak I. Gonorrhea: New challenges. Clin Dermatol. 2014;32:275-281.
30. Kirkcaldy RD, Ballard RC, Dowell D. Gonococcal resistance: Are cephalosporins next? Curr Infect Dis Rep. 2011;13:196-204.
31. Kidd S, Kirkcaldy R, Weinstock H, Bolan G. Tackling multidrug-resistant gonorrhea: How should we prepare for the untreatable? Expert Rev Anti Infect Ther. 2012;10:831-833.
32. Wang SA, Harvey AB, Conner SM, et al. Antimicrobial resistance for Neisseria gonorrhoeae in the United States, 1988 to 2003: The spread of fluoroquinolone resistance. Ann Intern Med. 2007;147:81-88.
33. Barbee LA, Kerani RP, Dombrowski JC, et al. A retrospective comparative study of 2-drug oral and intramuscular cephalosporin treatment regimens for pharyngeal gonorrhea. Clin Infect Dis. 2013;56:1539-434.
34. Sathia L, Ellis B, Phillip S, et al. Pharyngeal gonorrhoea - is dual therapy the way forward? Int J STD AIDS. 2007;18:647–8.
35. Tanaka M. Emergence of multidrug-resistant Neisseria gonorrhoeae strains circulating worldwide. Int J Urol. 2012;19:98-99.
36. Hamasuna R, Osada Y, Jensen JS. Isolation of Mycoplasma genitalium from first-void urine specimens by coculture with vero cells. J Clin Microbiol. 2007;45:847-850.
37. Razin S. Mycoplasma. In: Boricello SP, Murray PR, Funke G, eds. Topley & Wilson’s Microbiology and Microbial Infections. London, UK: Hodder Arnold; 2005:1957-2005.
38. Munson E, Bykowski H, Munson K, et al. Clinical laboratory assessment of Mycoplasma genitalium transcription-medicated ampliflication using primary female urogenital specimens. J Clin Microbiol. 2016;54:432-437.
39. Munson E, Wenten D, Jhansale S, et al. Expansion of comprehensive screening of male-sexually transmitted infection clinic attendees with Mycoplasma genitalium and Trichomonas vaginalis molecule assessment: a restrospective analysis. J Clin Microbiol. 2016;55:321-325.
40. Centers for Disease Control and Prevention. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae--2014. MMWR Recomm Rep. 2014;63(RR-02):1-19.
41. Boyadzhyan B, Yashina T, Yatabe JH, et al. Comparison of the APTIMA CT and GC assays with the APTIMA combo 2 assay, the Abbott LCx assay, and direct fluorescent-antibody and culture assays for detection of Chlamydia trachomatis and Neisseria gonorrhoeae. J Clin Microbiol. 2004;42:3089-3093.
42. Graseck AS, Shih SL, Peipert JF. Home versus clinic-based specimen collection for Chlamydia trachomatis and Neisseria gonorrhoeae. Expert Rev Anti Infect Ther. 2011;9:183-194.
43. Sherrard J, Barlow D. Gonorrhoea in men: Clinical and diagnostic aspects. Genitourin Med. 1996;72:422-426.
44. Goh BT, Varia KB, Ayliffe PF, Lim FK Diagnosis of gonorrhea by gram-stained smears and cultures in men and women: role of the urethral smear. Sex Transm Dis. 1985;12:135-139.
45. World Health Organization. WHO Guidelines for the Treatment of Neisseria gonorrhoeae. www.who.int/reproductivehealth/publications/rtis/gonorrhoea-treatment-guidelines/en/. Published 2016. Accessed December 16, 2017.
46. Arbique JC, Forward KR, LeBlanc J. Evaluation of four commercial transport media for the survival of Neisseria gonorrhoeae. Diagn Microbiol Infect Dis. 2000;36:163-168.
47. Schink JC, Keith LG. Problems in the culture diagnosis of gonorrhea. J Reprod Med. 1985;30(3 Suppl):244-249.
48. Marrazzo JM, Apicella MA. Neisseria gonorrhoeae (gonorrhea). In: Bennett JE, Dolin R, Blaser MJ, eds. Principles and Practice of Infectious Diseases. 8th ed. Philadelphia, PA: Elsevier; 2015:2446-2462.
49. Barry PM, Klausner JD. The use of cephalosporins for gonorrhea: The impending problem of resistance. Expert Opin Pharmacother. 2009;10:555-577.
50. Tabrizi SN, Unemo M, Limnios AE, et al. Evaluation of six commercial nucleic acid amplification tests for detection of Neisseria gonorrhoeae and other Neisseria species. J Clin Microbiol. 2011;49:3610-3615.
51. Goldenberg SD, Finn J, Sedudzi E, et al. Performance of the GeneXpert CT/NG assay compared to that of the Aptima AC2 assay for detection of rectal Chlamydia trachomatis and Neisseria gonorrhoeae by use of residual Aptima Samples. J Clin Microbiol. 2012;50:3867-3869.
52. Martin D. Mycoplasma genitalium, Mycoplasma hominis, and Ureaplasma species. In: Bennet J, Dolin R, Blaser M, eds. Principles and Practice of Infectious Diseases. 8th ed. Philadelphia, PA: Elsevier Sauders; 2015:2190-2193.
53. Hannan PC. Comparative susceptibilities of various AIDS-associated and human urogenital tract mycoplasmas and strains of Mycoplasma pneumoniae to 10 classes of antimicrobial agent in vitro. J Med Microbiol. 1998;47:1115-1122.
54. Mena LA, Mroczkowski TF, Nsuami M, Martin DH. A randomized comparison of azithromycin and doxycycline for the treatment of Mycoplasma genitalium-positive urethritis in men. Clin Infect Dis. 2009;48:1649-1654.
55. Schwebke JR, Rompalo A, Taylor S, et al. Re-evaluating the treatment of nongonococcal urethritis: Emphasizing emerging pathogens--a randomized clinical trial. Clin Infect Dis. 2011;52:163-170.
56. Bjornelius E, Anagrius C, Bojs G, et al. Antibiotic treatment of symptomatic Mycoplasma genitalium infection in Scandinavia: A controlled clinical trial. Sex Transm Infect. 2008;84:72-76.
57. Nijhuis RH, Severs TT, Van der Vegt DS, et al. High levels of macrolide resistance-associated mutations in Mycoplasma genitalium warrant antibiotic susceptibility-guided treatment. J Antimicrob Chemother. 2015;70:2515-2518.
58. Pond MJ, Nori AV, Witney AA, et al. High prevalence of antibiotic-resistant Mycoplasma genitalium in nongonococcal urethritis: The need for routine testing and the inadequacy of current treatment options. Clin Infect Dis. 2014;58:631-637.
59. Touati A, Peuchant O, Jensen JS, et al. Direct detection of macrolide resistance in Mycoplasma genitalium isolates from clinical specimens from France by use of real-time PCR and melting curve analysis. J Clin Microbiol. 2014;52:1549-1555.
60. Bebear CM, de Barbeyrac B, Pereyre S, et al. Activity of moxifloxacin against the urogenital Mycoplasmas ureaplasma spp., Mycoplasma hominis and Mycoplasma genitalium and Chlamydia trachomatis. Clin Microbiol Infect. 2008;14:801-805.
61. Jernberg E, Moghaddam A, Moi H. Azithromycin and moxifloxacin for microbiological cure of Mycoplasma genitalium infection: An open study. Int J STD AIDS. 2008;19:676-679.
62. Tagg KA, Jeoffreys NJ, Couldwell DL, et al. Fluoroquinolone and macrolide resistance-associated mutations in Mycoplasma genitalium. J Clin Microbiol. 2013;51:2245-2249.
63. Couldwell DL, Tagg KA, Jeoffreys NJ, Gilbert GL. Failure of moxifloxacin treatment in Mycoplasma genitalium infections due to macrolide and fluoroquinolone resistance. Int J STD AIDS. 2013;24:822-828.
64. Shimada Y, Deguchi T, Nakane K, et al. Emergence of clinical strains of Mycoplasma genitalium harbouring alterations in ParC associated with fluoroquinolone resistance. Int J Antimicrob Agents. 2010;36:255-258.
65. Mobley V, Seña A. Mycoplasma genitalium infection in men and women. In: UpToDate. www.uptodate.com. Last updated March 8, 2017. Accessed February 13, 2018.
66. Takahashi S, Hamasuna R, Yasuda M, et al. Clinical efficacy of sitafloxacin 100 mg twice daily for 7 days for patients with non-gonococcal urethritis. J Infect Chemother. 2013;19:941-945.
67. Ito S, Yasuda M, Seike K, et al. Clinical and microbiological outcomes in treatment of men with non-gonococcal urethritis with a 100-mg twice-daily dose regimen of sitafloxacin. J Infect Chemother. 2012;18:414-418.
68. Bissessor M, Tabrizi SN, Twin J, et al. Macrolide resistance and azithromycin failure in a Mycoplasma genitalium-infected cohort, and response of azithromycin failures to alternative antibiotic regimens. Clin Infect Dis. 2014;60:1228-1236.
69. Jensen JS, Fernandes P, Unemo M. In vitro activity of the new fluoroketolide solithromycin (CEM-101) against macrolide-resistant and -susceptible Mycoplasma genitalium strains. Antimicrob Agents Chemother. 2014;58:3151-3156.
70. Centers for Disease Control and Prevention (CDC). Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: Fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep. 2007;56:332-336.
71. Sexually transmitted diseases treatment guidelines, 2010. www.cdc.gov/std/treatment/default.htm. Published 2015. Accessed February13, 2016.
72. Centers for Disease Control and Prevention (CDC). Cephalosporin susceptibility among Neisseria gonorrhoeae isolates--United States, 2000-2010. MMWR Morb Mortal Wkly Rep. 2011;60:873-877.
73. Ohnishi M, Saika T, Hoshina S, et al. Ceftriaxone-resistant Neisseria gonorrhoeae, Japan. Emerg Infect Dis. 2011;17:148-149.
74. Centers for Disease Control and Prevention (CDC). Update to CDC’s sexually transmitted diseases treatment guidelines, 2010: Oral cephalosporins no longer a recommended treatment for gonococcal infections. MMWR Morb Mortal Wkly Rep. 2012;61:590-594.
75. Kirkcaldy RD, Weinstock HS, Moore PC, et al. The efficacy and safety of gentamicin plus azithromycin and gemifloxacin plus azithromycin as treatment of uncomplicated gonorrhea. Clin Infect Dis. 2014;59:1083-1091.
76. Seña AC, Taylor SN, Marrazzo J, et al. Microbiological cure rates and antimicrobial susceptibility of Neisseria gonorrhoeae to ETX0914 (AZD0914) in a phase II treatment trial for urogenital gonorrhea. (Poster 1308) Program and Abstract of ID Week 2016. New Orleans, LA, . October 25-30, 2016.
77. Taylor S, Marrazzo J, Batteiger B, et al. Single-dose zoliflodacin (ETX0914) for treatment of urogential gonorrhea. N Engl J Med. 2018;379:1835-1845.
78. Perry C, Dumont E, Raychaudhuri A. O05.3 A phase II, randomised, stdy in adults subjects evaluating the efficacy, safety, and tolerability of single doses of gepotidacin (GSK2140944) for treatment of uncomplicated urogenital gonorrhea. Sex Transm Infect. 2017;93(Suppl 2).
79. Bignell C, Unemo M, European STI Guidelines Editorial Board. 2012 European guideline on the diagnosis and treatment of gonorrhoea in adults. Int J STD AIDS. 2013;24:85-92.
80. O’Connor EA, Lin JS, Burda BU, et al. Behavioral sexual risk-reduction counseling in primary care to prevent sexually transmitted infections: A systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;161:874-883.
Influenza update 2018–2019: 100 years after the great pandemic
This centennial year update focuses primarily on immunization, but also reviews epidemiology, transmission, and treatment.
EPIDEMIOLOGY
2017–2018 was a bad season
The 2017–2018 influenza epidemic was memorable, dominated by influenza A(H3N2) viruses with morbidity and mortality rates approaching pandemic numbers. It lasted 19 weeks, killed more people than any other epidemic since 2010, particularly children, and was associated with 30,453 hospitalizations—almost twice the previous season high in some parts of the United States.2
Regrettably, 171 unvaccinated children died during 2017–2018, accounting for almost 80% of deaths.2 The mean age of the children who died was 7.1 years; 51% had at least 1 underlying medical condition placing them at risk for influenza-related complications, and 57% died after hospitalization.2
Recent estimates of the incidence of symptomatic influenza among all ages ranged from 3% to 11%, which is slightly lower than historical estimates. The rates were higher for children under age 18 than for adults.3 Interestingly, influenza A(H3N2) accounted for 50% of cases of non-mumps viral parotitis during the 2014–2015 influenza season in the United States.4
Influenza C exists but is rare
Influenza A and B account for almost all influenza-related outpatient visits and hospitalizations. Surveillance data from May 2013 through December 2016 showed that influenza C accounts for 0.5% of influenza-related outpatient visits and hospitalizations, particularly affecting children ages 6 to 24 months. Medical comorbidities and copathogens were seen in all patients requiring intensive care and in most hospitalizations.5 Diagnostic tests for influenza C are not widely available.
Dogs and cats: Factories for new flu strains?
While pigs and birds are the major reservoirs of influenza viral genetic diversity from which infection is transmitted to humans, dogs and cats have recently emerged as possible sources of novel reassortant influenza A.6 With their frequent close contact with humans, our pets may prove to pose a significant threat.
Obesity a risk factor for influenza
Obesity emerged as a risk factor for severe influenza in the 2009 pandemic. Recent data also showed that obesity increases the duration of influenza A virus shedding, thus increasing duration of contagiousness.7
Influenza a cardiovascular risk factor
Previous data showed that influenza was a risk factor for cardiovascular events. Two recent epidemiologic studies from the United Kingdom showed that laboratory-confirmed influenza was associated with higher rates of myocardial infarction and stroke for up to 4 weeks.8,9
Which strain is the biggest threat?
Predicting which emerging influenza serotype may cause the next pandemic is difficult, but influenza A(H7N9), which had not infected humans until 2013 but has since infected about 1,600 people in China and killed 37% of them, appears to have the greatest potential.10
National influenza surveillance programs and influenza-related social media applications have been developed and may get a boost from technology. A smartphone equipped with a temperature sensor can instantly detect one’s temperature with great precision. A 2018 study suggested that a smartphone-driven thermometry application correlated well with national influenza-like illness activity and improved its forecast in real time and up to 3 weeks in advance.11
TRANSMISSION
Humidity may not block transmission
Animal studies have suggested that humidity in the air interferes with transmission of airborne influenza virus, partially from biologic inactivation. But when a recent study used humidity-controlled chambers to investigate the stability of the 2009 influenza A(H1N1) virus in suspended aerosols and stationary droplets, the virus remained infectious in aerosols across a wide range of relative humidities, challenging the common belief that humidity destabilizes respiratory viruses in aerosols.12
One sick passenger may not infect the whole plane
Transmission of respiratory viruses on airplane flights has long been considered a potential avenue for spreading influenza. However, a recent study that monitored movements of individuals on 10 transcontinental US flights and simulated inflight transmission based on these data showed a low probability of direct transmission, except for passengers seated in close proximity to an infectious passenger.13
WHAT’S IN THE NEW FLU SHOT?
The 2018–2019 quadrivalent vaccine for the Northern Hemisphere14 contains the following strains:
- A/Michigan/45/2015 A(H1N1)pdm09-like virus
- A/Singapore/INFIMH-16-0019/2016 (H3N2)-like virus
- B/Colorado/06/2017-like virus (Victoria lineage)
- B/Phuket/3073/2013-like virus (Yamagata lineage).
The A(H3N2) (Singapore) and B/Victoria lineage components are new this year. The A(H3N2) strain was the main cause of the 2018 influenza epidemic in the Southern Hemisphere.
The quadrivalent live-attenuated vaccine, which was not recommended during the 2016–2017 and 2017–2018 influenza seasons, has made a comeback and is recommended for the 2018–2019 season in people for whom it is appropriate based on age and comorbidities.15 Although it was effective against influenza B and A(H3N2) viruses, it was less effective against the influenza A(H1N1)pdm09-like viruses during the 2013–2014 and 2015–2016 seasons.
A/Slovenia/2903/2015, the new A(H1N1)pdm09-like virus included in the 2018–2019 quadrivalent live-attenuated vaccine, is significantly more immunogenic than its predecessor, A/Bolivia/559/2013, but its clinical effectiveness remains to be seen.
PROMOTING VACCINATION
How effective is it?
Influenza vaccine effectiveness in the 2017–2018 influenza season was 36% overall, 67% against A(H1N1), 42% against influenza B, and 25% against A(H3N2).16 It is estimated that influenza vaccine prevents 300 to 4,000 deaths annually in the United States alone.17
A 2018 Cochrane review17 concluded that vaccination reduced the incidence of influenza by about half, with 2.3% of the population contracting the flu without vaccination compared with 0.9% with vaccination (risk ratio 0.41, 95% confidence interval 0.36–0.47). The same review found that 71 healthy adults need to be vaccinated to prevent 1 from experiencing influenza, and 29 to prevent 1 influenza-like illness.
Several recent studies showed that influenza vaccine effectiveness varied based on age and influenza serotype, with higher effectiveness in people ages 5 to 17 and ages 18 to 64 than in those age 65 and older.18–20 A mathematical model of influenza transmission and vaccination in the United States determined that even relatively low-efficacy influenza vaccines can be very useful if optimally distributed across age groups.21
Vaccination rates are low, and ‘antivaxxers’ are on the rise
Although the influenza vaccine is recommended in the United States for all people age 6 months and older regardless of the state of their health, vaccination rates remain low. In 2016, only 37% of employed adults were vaccinated. The highest rate was for government employees (45%), followed by private employees (36%), followed by the self-employed (30%).22
A national goal is to immunize 80% of all Americans and 90% of at-risk populations (which include children and the elderly).23 The number of US hospitals that require their employees to be vaccinated increased from 37.1% in 2013 to 61.4% in 2017.24 Regrettably, as of March 2018, 14 lawsuits addressing religious objections to hospital influenza vaccination mandates have been filed.25
Despite hundreds of studies demonstrating the efficacy, safety, and cost savings of influenza vaccination, the antivaccine movement has been growing in the United States and worldwide.26 All US states except West Virginia, Mississippi, and California allow nonmedical exemptions from vaccination based on religious or personal belief.27 Several US metropolitan areas represent “hot spots” for these exemptions.28 This may render such areas vulnerable to vaccine-preventable diseases, including influenza.
Herd immunity: We’re all in this together
Some argue that the potential adverse effects and the cost of vaccination outweigh the benefits, but the protective benefits of herd immunity are significant for those with comorbidities or compromised immunity.
Educating the public about herd immunity and local influenza vaccination uptake increases people’s willingness to be vaccinated.29 A key educational point is that at least 70% of a community needs to be vaccinated to prevent community outbreaks; this protects everyone, including those who do not mount a protective antibody response to influenza vaccination and those who are not vaccinated.
DOES ANNUAL VACCINATION BLUNT ITS EFFECTIVENESS?
Some studies from the 1970s and 1980s raised concern over a possible negative effect of annual influenza vaccination on vaccine effectiveness. The “antigenic distance hypothesis” holds that vaccine effectiveness is influenced by antigenic similarity between the previous season’s vaccine serotypes and the epidemic serotypes, as well as the antigenic similarity between the serotypes of the current and previous seasons.
A meta-analysis of studies from 2010 through 2015 showed significant inconsistencies in repeat vaccination effects within and between seasons and serotypes. It also showed that vaccine effectiveness may be influenced by more than 1 previous season, particularly for influenza A(H3N2), in which repeated vaccination can blunt the hemagglutinin antibody response.30
A study from Japan showed that people who needed medical attention for influenza in the previous season were at lower risk of a similar event in the current season.31 Prior-season influenza vaccination reduced current-season vaccine effectiveness only in those who did not have medically attended influenza in the prior season. This suggests that infection is more immunogenic than vaccination, but only against the serotype causing the infection and not the other serotypes included in the vaccine.
An Australian study showed that annual influenza vaccination did not decrease vaccine effectiveness against influenza-associated hospitalization. Rather, effectiveness increased by about 15% in those vaccinated in both current and previous seasons compared with those vaccinated in either season alone.32
European investigators showed that repeated seasonal influenza vaccination in the elderly prevented the need for hospitalization due to influenza A(H3N2) and B, but not A(H1N1)pdm09.33
VACCINATION IN SPECIAL POPULATIONS
High-dose vaccine for older adults
The high-dose influenza vaccine has been licensed since 2009 for use in the United States for people ages 65 and older.
Recent studies confirmed that high-dose vaccine is more effective than standard-dose vaccine in veterans34 and US Medicare beneficiaries.35
The high-dose vaccine is rapidly becoming the primary vaccine given to people ages 65 and older in retail pharmacies, where vaccination begins earlier in the season than in providers’ offices.36 Some studies have shown that the standard-dose vaccine wanes in effectiveness toward the end of the influenza season (particularly if the season is long) if it is given very early. It remains to be seen whether the same applies to the high-dose influenza vaccine.
Some advocate twice-annual influenza vaccination, particularly for older adults living in tropical and subtropical areas, where influenza seasons are more prolonged. However, a recently published study observed reductions in influenza-specific hemagglutination inhibition and cell-mediated immunity after twice-annual vaccination.37
Vaccination is beneficial during pregnancy
Many studies have shown the value of influenza vaccination during pregnancy for both mothers and their infants.
One recently published study showed that 18% of infants who developed influenza required hospitalization.38 In that study, prenatal and postpartum maternal influenza vaccination decreased the odds of influenza in infants by 61% and 53%, respectively.
Another study showed that vaccine effectiveness did not vary by gestational age at vaccination.39
Some studies have shown that influenza virus infection can increase susceptibility to certain bacterial infections. A post hoc analysis of an influenza vaccination study in pregnant women suggested that the vaccine was also associated with decreased rates of pertussis in these women.40
Factors that make vaccination less effective
Several factors including age-related frailty and iatrogenic and disease-related immunosuppression can affect vaccine effectiveness.
Frailty. A recent study showed that vaccine effectiveness was 77.6% in nonfrail older adults but only 58.7% in frail older adults.41
Immunosuppression. Temporary discontinuation of methotrexate for 2 weeks after influenza vaccination in patients with rheumatoid arthritis improves vaccine immunogenicity without precipitating disease flare.42 Solid-organ and hematopoietic stem cell transplant recipients who received influenza vaccine were less likely to develop pneumonia and require intensive care unit admission.43
The high-dose influenza vaccine is more immunogenic than the standard-dose vaccine in solid-organ transplant recipients.44
Statins are widely prescribed and have recently been associated with reduced influenza vaccine effectiveness against medically attended acute respiratory illness, but their benefits in preventing cardiovascular events outweigh this risk.45
FUTURE VACCINE CONSIDERATIONS
Moving away from eggs
During the annual egg-based production process, which takes several months, the influenza vaccine acquires antigenic changes that allow replication in eggs, particularly in the hemagglutinin protein, which mediates receptor binding. This process of egg adaptation may cause antigenic changes that decrease vaccine effectiveness against circulating viruses.
The cell-based baculovirus influenza vaccine grown in dog kidney cells has higher antigenic content and is not subject to the limitations of egg-based vaccine, although it still requires annual updates. A recombinant influenza vaccine reduces the probability of influenza-like illness by 30% compared with the egg-based influenza vaccine, but also still requires annual updates.46 The market share of these non-egg-based vaccines is small, and thus their effectiveness has yet to be demonstrated.
The US Department of Defense administered the cell-based influenza vaccine to about one-third of Armed Forces personnel, their families, and retirees in the 2017–2018 influenza seasons, and data on its effectiveness are expected in the near future.47
A universal vaccine would be ideal
The quest continues for a universal influenza vaccine, one that remains protective for several years and does not require annual updates.48 Such a vaccine would protect against seasonal epidemic influenza drift variants and pandemic strains. More people could likely be persuaded to be vaccinated once rather than every year.
An ideal universal vaccine would be suitable for all age groups, at least 75% effective against symptomatic influenza virus infection, protective against all influenza A viruses (influenza A, not B, causes pandemics and seasonal epidemics), and durable through multiple influenza seasons.51
Research and production of such a vaccine are expected to require funding of about $1 billion over the next 5 years.
Boosting effectiveness
Estimates of influenza vaccine effectiveness range from 40% to 60% in years when the vaccine viruses closely match the circulating viruses, and variably lower when they do not match. The efficacy of most other vaccines given to prevent other infections is much higher.
New technologies to improve influenza vaccine effectiveness are needed, particularly for influenza A(H3N2) viruses, which are rapidly evolving and are highly susceptible to egg-adaptive mutations in the manufacturing process.
In one study, a nanoparticle vaccine formulated with a saponin-based adjuvant induced hemagglutination inhibition responses that were even greater than those induced by the high-dose vaccine.52
Immunoglobulin A (IgA) may be a more effective vaccine target than traditional influenza vaccines that target IgG, since different parts of IgA may engage the influenza virus simultaneously.53
Vaccines can be developed more quickly than in the past. The timeline from viral sequencing to human studies with deoxyribonucleic acid plasmid vaccines decreased from 20 months in 2003 for the severe acquired respiratory syndrome coronavirus to 11 months in 2006 for influenza A/Indonesia/2006 (H5), to 4 months in 2009 for influenza A/California/2009 (H1), to 3.5 months in 2016 for Zika virus.54 This is because it is possible today to sequence a virus and insert the genetic material into a vaccine platform without ever having to grow the virus.
TREATMENT
Numerous studies have found anti-influenza medications to be effective. Nevertheless, in an analysis of the 2011–2016 influenza seasons, only 15% of high-risk patients were prescribed anti-influenza medications within 2 days of symptom onset, including 37% in those with laboratory-confirmed influenza.55 Fever was associated with an increased rate of antiviral treatment, but 25% of high-risk outpatients were afebrile. Empiric treatment of 4 high-risk outpatients with acute respiratory illness was needed to treat 1 patient with influenza.55
Treatment with a neuraminidase inhibitor within 2 days of illness has recently been shown to improve survival and shorten duration of viral shedding in patients with avian influenza A(H7N9) infection.56 Antiviral treatment within 2 days of illness is associated with improved outcomes in transplant recipients57 and with a lower risk of otitis media in children.58
Appropriate anti-influenza treatment is as important as avoiding unnecessary antibiotics. Regrettably, as many as one-third of patients with laboratory-confirmed influenza are prescribed antibiotics.59
The US Food and Drug Administration warns against fraudulent unapproved over-the-counter influenza products.60
Baloxavir marboxil
Baloxavir marboxil is a new anti-influenza medication approved in Japan in February 2018 and anticipated to be available in the United States sometime in 2019.
This prodrug is hydrolyzed in vivo to the active metabolite, which selectively inhibits cap-dependent endonuclease enzyme, a key enzyme in initiation of messenger ribonucleic acid synthesis required for influenza viral replication.61
In a double-blind phase 3 trial, the median time to alleviation of influenza symptoms is 26.5 hours shorter with baloxavir marboxil than with placebo. One tablet was as effective as 5 days of the neuraminidase inhibitor oseltamivir and was associated with greater reduction in viral load 1 day after initiation, and similar side effects.62 Of concern is the emergence of nucleic acid substitutions conferring resistance to baloxavir; this occurred in 2.2% and 9.7% of baloxavir recipients in the phase 2 and 3 trials, respectively.
CLOSING THE GAPS
Several gaps in the management of influenza persist since the 1918 pandemic.1 These include gaps in epidemiology, prevention, diagnosis, treatment, and prognosis.
- Global networks wider than current ones are needed to address this global disease and to prioritize coordination efforts.
- Establishing and strengthening clinical capacity is needed in limited resource settings. New technologies are needed to expedite vaccine development and to achieve progress toward a universal vaccine.
- Current diagnostic tests do not distinguish between seasonal and novel influenza A viruses of zoonotic origin, which are expected to cause the next pandemic.
- Current antivirals have been shown to shorten duration of illness in outpatients with uncomplicated influenza, but the benefit in hospitalized patients has been less well established.
- In 2007, resistance of seasonal influenza A(H1N1) to oseltamivir became widespread. In 2009, pandemic influenza A(H1N1), which is highly susceptible to oseltamivir, replaced the seasonal virus and remains the predominantly circulating A(H1N1) strain.
- A small-molecule fragment, N-cyclohexyaltaurine, binds to the conserved hemagglutinin receptor-binding site in a manner that mimics the binding mode of the natural receptor sialic acid. This can serve as a template to guide the development of novel broad-spectrum small-molecule anti-influenza drugs.63
- Biomarkers that can accurately predict development of severe disease in patients with influenza are needed.
- Uyeki TM, Fowler RA, Fischer WA. Gaps in the clinical management of influenza: a century since the 1918 pandemic. JAMA 2018; 320(8):755–756. doi:10.1001/jama.2018.8113
- Garten R, Blanton L, Elal AI, et al. Update: influenza activity in the United States during the 2017–18 season and composition of the 2018–19 influenza vaccine. MMWR Morb Mortal Wkly Rep 2018; 67(22):634–642. doi:10.15585/mmwr.mm6722a4
- Tokars JI, Olsen SJ, Reed C. Seasonal incidence of symptomatic influenza in the United States. Clin Infect Dis 2018; 66(10):1511–1518. doi:10.1093/cid/cix1060
- Elbadawi LI, Talley P, Rolfes MA, et al. Non-mumps viral parotitis during the 2014–2015 influenza season in the United States. Clin Infect Dis 2018. Epub ahead of print. doi:10.1093/cid/ciy137
- Thielen BK, Friedlander H, Bistodeau S, et al. Detection of influenza C viruses among outpatients and patients hospitalized for severe acute respiratory infection, Minnesota, 2013–2016. Clin Infect Dis 2018; 66(7):1092–1098. doi:10.1093/cid/cix931
- Chena Y, Trovãob NS, Wang G, et al. Emergence and evolution of novel reassortant influenza A viruses in canines in southern China. MBio 2018; 9(3):e00909–e00918. doi:10.1128/mBio.00909-18
- Maier HE, Lopez R, Sanchez N, et al. Obesity increases the duration of influenza A virus shedding in adults. J Infect Dis 2018. Epub ahead of print. doi:10.1093/infdis/jiy370
- Warren-Gash C, Blackburn R, Whitaker H, McMenamin J, Hayward AC. Laboratory-confirmed respiratory infections as triggers for acute myocardial infarction and stroke: a self-controlled case series analysis of national linked datasets from Scotland. Eur Respir J 2018; 51(3):1701794. doi:10.1183/13993003.01794-2017
- Blackburn R, Zhao H, Pebody R, Hayward A, Warren-Gash C. Laboratory-confirmed respiratory infections as predictors of hospital admission for myocardial infarction and stroke: time-series analysis of English data for 2004–2015. Clin Infect Dis 2018; 67(1):8–17. doi:10.1093/cid/cix1144
- Newsweek; Andrew S. What is disease X? Deadly bird flu virus could be next pandemic. www.newsweek.com/disease-x-bird-flu-deaths-pandemic-what-h7n9-979723. Accessed October 3, 2018.
- Miller AC, Singh I, Koehler E, Polgreen PM. A smartphone-driven thermometer application for real-time population- and individual-level influenza surveillance. Clin Infect Dis 2018; 67(3):388–397. doi:10.1093/cid/ciy073
- Kormuth KA, Lin K, Prussin AJ 2nd, et al. Influenza virus infectivity is retained in aerosols and droplets independent of relative humidity, J Infect Dis 2018; 218(5):739–747. doi:10.1093/infdis/jiy221
- Hertzberg VS, Weiss H, Elon L, et. al. Behaviors, movements, and transmission of droplet-mediated respiratory diseases during transcontinental airline flights. Proc Natl Acad Sci U S A 2018; 115(14):3623–3627. doi:10.1073/pnas.1711611115
- Grohskopf LA, Sokolow LZ, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2018–19 influenza season. MMWR Recomm Rep 2018; 67(3):1–20. doi:10.15585/mmwr.rr6703a1
- Grohskopf LA, Sokolow LZ, Fry AM, Walter EB, Jernigan DB. Update: ACIP recommendations for the use of quadrivalent live attenuated influenza vaccine (LAIV4)—United States, 2018–19 influenza season. MMWR Morb Mortal Wkly Rep 2018; 67(22):643–645. doi:10.15585/mmwr.mm6722a5
- Flannery B, Chung JR, Belongia EA, et al. Interim estimates of 2017–18 seasonal influenza vaccine effectiveness—United States, February 2018. MMWR Morb Mortal Wkly Rep 2018; 67(6):180–185. doi:10.15585/mmwr.mm6706a2
- Demicheli V, Jefferson T, Ferroni E, Rivetti A, Di Pietrantonj C. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev 2018; 2:CD001269. doi:10.1002/14651858.CD001269.pub6
- Flannery B, Smith C, Garten RJ, et al. Influence of birth cohort on effectiveness of 2015–2016 influenza vaccine against medically attended illness due to 2009 pandemic influenza A(H1N1) virus in the United States. J Infect Dis 2018; 218(2):189–196. doi:10.1093/infdis/jix634
- Rondy M, El Omeiri N, Thompson MG, Leveque A, Moren A, Sullivan SG. Effectiveness of influenza vaccines in preventing severe influenza illness among adults: a systematic review and meta-analysis of test-negative design case-control studies. J Infect 2017; 75(5):381–394. doi:10.1016/j.jinf.2017.09.010
- Stein Y, Mandelboim M, Sefty H, et al; Israeli Influenza Surveillance Network (IISN). Seasonal influenza vaccine effectiveness in preventing laboratory-confirmed influenza in primary care in Israel, 2016–2017 season: insights into novel age-specific analysis. Clin Infect Dis 2018; 66(9):1383–1391. doi:10.1093/cid/cix1013
- Sah P, Medlock J, Fitzpatrick MC, Singer BH, Galvani AP. Optimizing the impact of low-efficacy influenza vaccines. Proc Natl Acad Sci U S A 2018; 115(20):5151–5156. doi:10.1073/pnas.1802479115
- QuickStats: percentage of currently employed adults aged ≥ 18 years who received influenza vaccine in the past 12 months, by employment category—national health interview survey, United States, 2012 and 2016. MMWR Morb Mortal Wkly Rep 2018; 67(16):480. doi:10.15585/mmwr.mm6716a8
- Healthy People.gov. Immunization and infectious diseases. IID-12. Increase the percentage of children and adults who are vaccinated annually against seasonal influenza. www.healthypeople.gov/2020/topics-objectives/topic/immunization-and-infectious-diseases/objectives. Accessed October 3, 2018.
- Greene MT, Fowler KE, Ratz D, Krein SL, Bradley SF, Saint S. Changes in influenza vaccination requirements for health care personnel in US hospitals. JAMA Network Open 2018; 1(2):e180143. doi:10.1001/jamanetworkopen.2018.0143
- Opel DJ, Sonne JA, Mello MM. Vaccination without litigation—addressing religious objections to hospital influenza-vaccination mandates. N Engl J Med 2018; 378(9):785–788. doi:10.1056/NEJMp1716147
- Horowitz J. Italy loosens vaccine law just as children return to school. New York Times Sept. 20, 2018. www.nytimes.com/2018/09/20/world/europe/italy-vaccines-five-star-movement.html.
- National Conference of State Legislature. States with religious and philosophical exemptions from school immunization requirements. www.ncsl.org/research/health/school-immunization-exemption-state-laws.aspx. Accessed October 3, 2018.
- Olive JK, Hotez PJ, Damania A, Nolan MS. The state of the antivaccine movement in the United States: a focused examination of nonmedical exemptions in states and counties. PLoS Med 2018; 15(6):e1002578. doi:10.1371/journal.pmed.1002578
- Logan J, Nederhoff D, Koch B, et al. ‘What have you HEARD about the HERD?’ Does education about local influenza vaccination coverage and herd immunity affect willingness to vaccinate? Vaccine 2018; 36(28):4118–4125. doi:10.1016/j.vaccine.2018.05.037
- Belongia EA, Skowronski DM, McLean HQ, Chambers C, Sundaram ME, De Serres G. Repeated annual influenza vaccination and vaccine effectiveness: review of evidence. Expert Rev Vaccines 2017; 16(7):1–14. doi:10.1080/14760584.2017.1334554
- Saito N, Komori K, Suzuki M, et al. Negative impact of prior influenza vaccination on current influenza vaccination among people infected and not infected in prior season: a test-negative case-control study in Japan. Vaccine 2017; 35(4):687–693. doi:10.1016/j.vaccine.2016.11.024
- Cheng AC, Macartney KK, Waterer GW, Kotsimbos T, Kelly PM, Blyth CC; Influenza Complications Alert Network (FluCAN) Investigators. Repeated vaccination does not appear to impact upon influenza vaccine effectiveness against hospitalization with confirmed influenza. Clin Infect Dis 2017; 64(11):1564–1572. doi:10.1093/cid/cix209
- Rondy M, Launay O, Castilla J, et al; InNHOVE/I-MOVE+working group. Repeated seasonal influenza vaccination among elderly in Europe: effects on laboratory confirmed hospitalised influenza. Vaccine 2017; 35(34):4298–4306. doi:10.1016/j.vaccine.2017.06.088
- Young-Xu Y, van Aalst R, Mahmud SM, et al. Relative vaccine effectiveness of high-dose versus standard-dose influenza vaccines among Veterans Health Administration patients. J Infect Dis 2018; 217(11):1718–1727. doi:10.1093/infdis/jiy088
- Shay DK, Chillarige Y, Kelman J, et al. Comparative effectiveness of high-dose versus standard-dose influenza vaccines among US Medicare beneficiaries in preventing postinfluenza deaths during 2012–2013 and 2013–2014. J Infect Dis 2017; 215(4):510–517. doi:10.1093/infdis/jiw641
- Madaras-Kelly K, Remington R, Hruza H, Xu D. Comparative effectiveness of high-dose versus standard-dose influenza vaccines in preventing postinfluenza deaths. J Infect Dis 2018; 218(2):336–337. doi:10.1093/infdis/jix645
- Tam YH, Valkenburg SA, Perera RAPM, et al. Immune responses to twice-annual influenza vaccination in older adults in Hong Kong. Clin Infect Dis 2018; 66(6):904–912. doi:10.1093/cid/cix900
- Ohfuji S, Deguchi M, Tachibana D, et al; Osaka Pregnant Women Influenza Study Group. Protective effect of maternal influenza vaccination on influenza in their infants: a prospective cohort study. J Infect Dis 2018; 217(6):878–886. doi:10.1093/infdis/jix629
- Katz J, Englund JA, Steinhoff MC, et al. Impact of timing of influenza vaccination in pregnancy on transplacental antibody transfer, influenza incidence, and birth outcomes: a randomized trial in rural Nepal. Clin Infect Dis 2018; 67(3):334–340. doi:10.1093/cid/ciy090
- Nunes MC, Cutland CL, Madhi SA. Influenza vaccination during pregnancy and protection against pertussis. N Engl J Med 2018; 378(13):1257–1258. doi:10.1056/NEJMc1705208
- Andrew MK, Shinde V, Ye L, et al; Serious Outcomes Surveillance Network of the Public Health Agency of Canada/Canadian Institutes of Health Research Influenza Research Network (PCIRN) and the Toronto Invasive Bacterial Diseases Network (TIBDN). The importance of frailty in the assessment of influenza vaccine effectiveness against influenza-related hospitalization in elderly people. J Infect Dis 2017; 216(4):405–414. doi:10.1093/infdis/jix282
- Park JK, Lee YJ, Shin K, et al. Impact of temporary methotrexate discontinuation for 2 weeks on immunogenicity of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis 2018; 77(6):898–904. doi:10.1136/annrheumdis-2018-213222
- Kumar D, Ferreira VH, Blumberg E, et al. A five-year prospective multi-center evaluation of influenza infection in transplant recipients. Clin Infect Dis 2018. Epub ahead of print. doi:10.1093/cid/ciy294
- Natori Y, Shiotsuka M, Slomovic J, et al. A double-blind, randomized trial of high-dose vs standard-dose influenza vaccine in adult solid-organ transplant recipients. Clin Infect Dis 2018; 66(11):1698–1704. doi:10.1093/cid/cix1082
- Omer SB, Phadke VK, Bednarczyk BA, Chamberlain AT, Brosseau JL, Orenstein WA. Impact of statins on influenza vaccine effectiveness against medically attended acute respiratory illness. J Infect Dis 2016; 213(8):1216–1223. doi:10.1093/infdis/jiv457
- Dunkle LM, Izikson R, Patriarca P, et al. Efficacy of recombinant influenza vaccine in adults 50 years of age or older. N Engl J Med 2017; 376(25):2427–2436. doi:10.1056/NEJMoa1608862
- STAT; Branswell H. How the US military might help answer a critical question about the flu vaccine. www.statnews.com/2018/03/02/flu-vaccine-egg-production-data. Accessed October 3, 2018.
- Paules CI, Sullivan SG, Subbarao K, Fauci AS. Chasing seasonal influenza—the need for a universal influenza vaccine. N Engl J Med 2018; 378(1):7–9. doi:10.1056/NEJMp1714916
- Jin XW, Mossad SB. Avian influenza: an emerging pandemic threat. Cleve Clin J Med 2005; 72:1129-1134. pmid:16392727
- Wei WI, Brunger AT, Skehel JJ, Wiley DC. Refinement of the influenza virus hemagglutinin by simulated annealing. J Mol Biol 1990; 212(4):737–761. doi:10.1016/0022-2836(90)90234-D
- Erbelding EJ, Post DJ, Stemmy EJ, et al. A universal influenza vaccine: the strategic plan for the National Institute of Allergy and Infectious Diseases, J Infect Dis 2018; 218(3):347–354. doi:10.1093/infdis/jiy103
- Shinde V, Fries L, Wu Y, et al. Improved titers against influenza drift variants with a nanoparticle vaccine. N Engl J Med 2018; 378(24):2346–2348. doi:10.1056/NEJMc1803554
- Maurer MA, Meyer L, Bianchi M, et al. Glycosylation of human IgA directly inhibits influenza A and other sialic-acid-binding viruses. Cell Rep 2018; 23(1):90–99. doi:10.1016/j.celrep.2018.03.027
- Graham BS, Mascola JR, Fauci AS. Novel vaccine technologies: essential components of an adequate response to emerging viral diseases. JAMA 2018; 319(14):1431–1432. doi:10.1001/jama.2018.0345
- Stewart RJ, Flannery B, Chung JR, et al. Influenza antiviral prescribing for outpatients with an acute respiratory illness and at high risk for influenza-associated complications during 5 influenza seasons—United States, 2011–2016. Clin Infect Dis 2018; 66(7):1035–1041. doi:10.1093/cid/cix922
- Zheng S, Tang L, Gao H, et al. Benefit of early initiation of neuraminidase inhibitor treatment to hospitalized patients with avian influenza A(H7N9) virus. Clin Infect Dis 2018; 66(7):1054–1060. doi:10.1093/cid/cix930
- Kumar D, Ferreira VH, Blumberg E, et al. A five-year prospective multi-center evaluation of influenza infection in transplant recipients. Clin Infect Dis 2018. Epub ahead of print. doi:10.1093/cid/ciy294
- Malosh RE, Martin ET, Heikkinen T, Brooks WA, Whitley RJ, Monto AS. Efficacy and safety of oseltamivir in children: systematic review and individual patient data meta-analysis of randomized controlled trials. Clin Infect Dis 2018; 66(10):1492–1500. doi:10.1093/cid/cix1040
- Havers FP, Hicks LA, Chung JR, et al. Outpatient antibiotic prescribing for acute respiratory infections during influenza seasons. JAMA Network Open 2018; 1(2):e180243. doi:10.1001/jamanetworkopen.2018.0243
- US Food and Drug Administration. FDA warns of fraudulent and unapproved flu products. www.fda.gov/newsevents/newsroom/pressannouncements/ucm599223.htm. Accessed October 3, 2018.
- Portsmouth S, Kawaguchi K, Arai M, Tsuchiya K, Uehara T. Cap-dependent endonuclease inhibitor S-033188 for the treatment of influenza: results from a phase 3, randomized, double-blind, placebo- and active-controlled study in otherwise healthy adolescents and adults with seasonal influenza. Open Forum Infect Dis 2017; 4(suppl 1):S734. doi:10.1093/ofid/ofx180.001
- Hayden FG, Sugaya N, Hirotsu N, et al; Baloxavir Marboxil Investigators Group. Baloxavir Marboxil for uncomplicated influenza in adults and adolescents. N Engl J Med 2018; 379(10):913–923. doi:10.1056/NEJMoa1716197
- Kadam RU, Wilson IA. A small-molecule fragment that emulates binding of receptor and broadly neutralizing antibodies to influenza A hemagglutinin. Proc Natl Acad Sci U S A 2018; 115(16):4240–4245. doi:10.1073/pnas.1801999115
This centennial year update focuses primarily on immunization, but also reviews epidemiology, transmission, and treatment.
EPIDEMIOLOGY
2017–2018 was a bad season
The 2017–2018 influenza epidemic was memorable, dominated by influenza A(H3N2) viruses with morbidity and mortality rates approaching pandemic numbers. It lasted 19 weeks, killed more people than any other epidemic since 2010, particularly children, and was associated with 30,453 hospitalizations—almost twice the previous season high in some parts of the United States.2
Regrettably, 171 unvaccinated children died during 2017–2018, accounting for almost 80% of deaths.2 The mean age of the children who died was 7.1 years; 51% had at least 1 underlying medical condition placing them at risk for influenza-related complications, and 57% died after hospitalization.2
Recent estimates of the incidence of symptomatic influenza among all ages ranged from 3% to 11%, which is slightly lower than historical estimates. The rates were higher for children under age 18 than for adults.3 Interestingly, influenza A(H3N2) accounted for 50% of cases of non-mumps viral parotitis during the 2014–2015 influenza season in the United States.4
Influenza C exists but is rare
Influenza A and B account for almost all influenza-related outpatient visits and hospitalizations. Surveillance data from May 2013 through December 2016 showed that influenza C accounts for 0.5% of influenza-related outpatient visits and hospitalizations, particularly affecting children ages 6 to 24 months. Medical comorbidities and copathogens were seen in all patients requiring intensive care and in most hospitalizations.5 Diagnostic tests for influenza C are not widely available.
Dogs and cats: Factories for new flu strains?
While pigs and birds are the major reservoirs of influenza viral genetic diversity from which infection is transmitted to humans, dogs and cats have recently emerged as possible sources of novel reassortant influenza A.6 With their frequent close contact with humans, our pets may prove to pose a significant threat.
Obesity a risk factor for influenza
Obesity emerged as a risk factor for severe influenza in the 2009 pandemic. Recent data also showed that obesity increases the duration of influenza A virus shedding, thus increasing duration of contagiousness.7
Influenza a cardiovascular risk factor
Previous data showed that influenza was a risk factor for cardiovascular events. Two recent epidemiologic studies from the United Kingdom showed that laboratory-confirmed influenza was associated with higher rates of myocardial infarction and stroke for up to 4 weeks.8,9
Which strain is the biggest threat?
Predicting which emerging influenza serotype may cause the next pandemic is difficult, but influenza A(H7N9), which had not infected humans until 2013 but has since infected about 1,600 people in China and killed 37% of them, appears to have the greatest potential.10
National influenza surveillance programs and influenza-related social media applications have been developed and may get a boost from technology. A smartphone equipped with a temperature sensor can instantly detect one’s temperature with great precision. A 2018 study suggested that a smartphone-driven thermometry application correlated well with national influenza-like illness activity and improved its forecast in real time and up to 3 weeks in advance.11
TRANSMISSION
Humidity may not block transmission
Animal studies have suggested that humidity in the air interferes with transmission of airborne influenza virus, partially from biologic inactivation. But when a recent study used humidity-controlled chambers to investigate the stability of the 2009 influenza A(H1N1) virus in suspended aerosols and stationary droplets, the virus remained infectious in aerosols across a wide range of relative humidities, challenging the common belief that humidity destabilizes respiratory viruses in aerosols.12
One sick passenger may not infect the whole plane
Transmission of respiratory viruses on airplane flights has long been considered a potential avenue for spreading influenza. However, a recent study that monitored movements of individuals on 10 transcontinental US flights and simulated inflight transmission based on these data showed a low probability of direct transmission, except for passengers seated in close proximity to an infectious passenger.13
WHAT’S IN THE NEW FLU SHOT?
The 2018–2019 quadrivalent vaccine for the Northern Hemisphere14 contains the following strains:
- A/Michigan/45/2015 A(H1N1)pdm09-like virus
- A/Singapore/INFIMH-16-0019/2016 (H3N2)-like virus
- B/Colorado/06/2017-like virus (Victoria lineage)
- B/Phuket/3073/2013-like virus (Yamagata lineage).
The A(H3N2) (Singapore) and B/Victoria lineage components are new this year. The A(H3N2) strain was the main cause of the 2018 influenza epidemic in the Southern Hemisphere.
The quadrivalent live-attenuated vaccine, which was not recommended during the 2016–2017 and 2017–2018 influenza seasons, has made a comeback and is recommended for the 2018–2019 season in people for whom it is appropriate based on age and comorbidities.15 Although it was effective against influenza B and A(H3N2) viruses, it was less effective against the influenza A(H1N1)pdm09-like viruses during the 2013–2014 and 2015–2016 seasons.
A/Slovenia/2903/2015, the new A(H1N1)pdm09-like virus included in the 2018–2019 quadrivalent live-attenuated vaccine, is significantly more immunogenic than its predecessor, A/Bolivia/559/2013, but its clinical effectiveness remains to be seen.
PROMOTING VACCINATION
How effective is it?
Influenza vaccine effectiveness in the 2017–2018 influenza season was 36% overall, 67% against A(H1N1), 42% against influenza B, and 25% against A(H3N2).16 It is estimated that influenza vaccine prevents 300 to 4,000 deaths annually in the United States alone.17
A 2018 Cochrane review17 concluded that vaccination reduced the incidence of influenza by about half, with 2.3% of the population contracting the flu without vaccination compared with 0.9% with vaccination (risk ratio 0.41, 95% confidence interval 0.36–0.47). The same review found that 71 healthy adults need to be vaccinated to prevent 1 from experiencing influenza, and 29 to prevent 1 influenza-like illness.
Several recent studies showed that influenza vaccine effectiveness varied based on age and influenza serotype, with higher effectiveness in people ages 5 to 17 and ages 18 to 64 than in those age 65 and older.18–20 A mathematical model of influenza transmission and vaccination in the United States determined that even relatively low-efficacy influenza vaccines can be very useful if optimally distributed across age groups.21
Vaccination rates are low, and ‘antivaxxers’ are on the rise
Although the influenza vaccine is recommended in the United States for all people age 6 months and older regardless of the state of their health, vaccination rates remain low. In 2016, only 37% of employed adults were vaccinated. The highest rate was for government employees (45%), followed by private employees (36%), followed by the self-employed (30%).22
A national goal is to immunize 80% of all Americans and 90% of at-risk populations (which include children and the elderly).23 The number of US hospitals that require their employees to be vaccinated increased from 37.1% in 2013 to 61.4% in 2017.24 Regrettably, as of March 2018, 14 lawsuits addressing religious objections to hospital influenza vaccination mandates have been filed.25
Despite hundreds of studies demonstrating the efficacy, safety, and cost savings of influenza vaccination, the antivaccine movement has been growing in the United States and worldwide.26 All US states except West Virginia, Mississippi, and California allow nonmedical exemptions from vaccination based on religious or personal belief.27 Several US metropolitan areas represent “hot spots” for these exemptions.28 This may render such areas vulnerable to vaccine-preventable diseases, including influenza.
Herd immunity: We’re all in this together
Some argue that the potential adverse effects and the cost of vaccination outweigh the benefits, but the protective benefits of herd immunity are significant for those with comorbidities or compromised immunity.
Educating the public about herd immunity and local influenza vaccination uptake increases people’s willingness to be vaccinated.29 A key educational point is that at least 70% of a community needs to be vaccinated to prevent community outbreaks; this protects everyone, including those who do not mount a protective antibody response to influenza vaccination and those who are not vaccinated.
DOES ANNUAL VACCINATION BLUNT ITS EFFECTIVENESS?
Some studies from the 1970s and 1980s raised concern over a possible negative effect of annual influenza vaccination on vaccine effectiveness. The “antigenic distance hypothesis” holds that vaccine effectiveness is influenced by antigenic similarity between the previous season’s vaccine serotypes and the epidemic serotypes, as well as the antigenic similarity between the serotypes of the current and previous seasons.
A meta-analysis of studies from 2010 through 2015 showed significant inconsistencies in repeat vaccination effects within and between seasons and serotypes. It also showed that vaccine effectiveness may be influenced by more than 1 previous season, particularly for influenza A(H3N2), in which repeated vaccination can blunt the hemagglutinin antibody response.30
A study from Japan showed that people who needed medical attention for influenza in the previous season were at lower risk of a similar event in the current season.31 Prior-season influenza vaccination reduced current-season vaccine effectiveness only in those who did not have medically attended influenza in the prior season. This suggests that infection is more immunogenic than vaccination, but only against the serotype causing the infection and not the other serotypes included in the vaccine.
An Australian study showed that annual influenza vaccination did not decrease vaccine effectiveness against influenza-associated hospitalization. Rather, effectiveness increased by about 15% in those vaccinated in both current and previous seasons compared with those vaccinated in either season alone.32
European investigators showed that repeated seasonal influenza vaccination in the elderly prevented the need for hospitalization due to influenza A(H3N2) and B, but not A(H1N1)pdm09.33
VACCINATION IN SPECIAL POPULATIONS
High-dose vaccine for older adults
The high-dose influenza vaccine has been licensed since 2009 for use in the United States for people ages 65 and older.
Recent studies confirmed that high-dose vaccine is more effective than standard-dose vaccine in veterans34 and US Medicare beneficiaries.35
The high-dose vaccine is rapidly becoming the primary vaccine given to people ages 65 and older in retail pharmacies, where vaccination begins earlier in the season than in providers’ offices.36 Some studies have shown that the standard-dose vaccine wanes in effectiveness toward the end of the influenza season (particularly if the season is long) if it is given very early. It remains to be seen whether the same applies to the high-dose influenza vaccine.
Some advocate twice-annual influenza vaccination, particularly for older adults living in tropical and subtropical areas, where influenza seasons are more prolonged. However, a recently published study observed reductions in influenza-specific hemagglutination inhibition and cell-mediated immunity after twice-annual vaccination.37
Vaccination is beneficial during pregnancy
Many studies have shown the value of influenza vaccination during pregnancy for both mothers and their infants.
One recently published study showed that 18% of infants who developed influenza required hospitalization.38 In that study, prenatal and postpartum maternal influenza vaccination decreased the odds of influenza in infants by 61% and 53%, respectively.
Another study showed that vaccine effectiveness did not vary by gestational age at vaccination.39
Some studies have shown that influenza virus infection can increase susceptibility to certain bacterial infections. A post hoc analysis of an influenza vaccination study in pregnant women suggested that the vaccine was also associated with decreased rates of pertussis in these women.40
Factors that make vaccination less effective
Several factors including age-related frailty and iatrogenic and disease-related immunosuppression can affect vaccine effectiveness.
Frailty. A recent study showed that vaccine effectiveness was 77.6% in nonfrail older adults but only 58.7% in frail older adults.41
Immunosuppression. Temporary discontinuation of methotrexate for 2 weeks after influenza vaccination in patients with rheumatoid arthritis improves vaccine immunogenicity without precipitating disease flare.42 Solid-organ and hematopoietic stem cell transplant recipients who received influenza vaccine were less likely to develop pneumonia and require intensive care unit admission.43
The high-dose influenza vaccine is more immunogenic than the standard-dose vaccine in solid-organ transplant recipients.44
Statins are widely prescribed and have recently been associated with reduced influenza vaccine effectiveness against medically attended acute respiratory illness, but their benefits in preventing cardiovascular events outweigh this risk.45
FUTURE VACCINE CONSIDERATIONS
Moving away from eggs
During the annual egg-based production process, which takes several months, the influenza vaccine acquires antigenic changes that allow replication in eggs, particularly in the hemagglutinin protein, which mediates receptor binding. This process of egg adaptation may cause antigenic changes that decrease vaccine effectiveness against circulating viruses.
The cell-based baculovirus influenza vaccine grown in dog kidney cells has higher antigenic content and is not subject to the limitations of egg-based vaccine, although it still requires annual updates. A recombinant influenza vaccine reduces the probability of influenza-like illness by 30% compared with the egg-based influenza vaccine, but also still requires annual updates.46 The market share of these non-egg-based vaccines is small, and thus their effectiveness has yet to be demonstrated.
The US Department of Defense administered the cell-based influenza vaccine to about one-third of Armed Forces personnel, their families, and retirees in the 2017–2018 influenza seasons, and data on its effectiveness are expected in the near future.47
A universal vaccine would be ideal
The quest continues for a universal influenza vaccine, one that remains protective for several years and does not require annual updates.48 Such a vaccine would protect against seasonal epidemic influenza drift variants and pandemic strains. More people could likely be persuaded to be vaccinated once rather than every year.
An ideal universal vaccine would be suitable for all age groups, at least 75% effective against symptomatic influenza virus infection, protective against all influenza A viruses (influenza A, not B, causes pandemics and seasonal epidemics), and durable through multiple influenza seasons.51
Research and production of such a vaccine are expected to require funding of about $1 billion over the next 5 years.
Boosting effectiveness
Estimates of influenza vaccine effectiveness range from 40% to 60% in years when the vaccine viruses closely match the circulating viruses, and variably lower when they do not match. The efficacy of most other vaccines given to prevent other infections is much higher.
New technologies to improve influenza vaccine effectiveness are needed, particularly for influenza A(H3N2) viruses, which are rapidly evolving and are highly susceptible to egg-adaptive mutations in the manufacturing process.
In one study, a nanoparticle vaccine formulated with a saponin-based adjuvant induced hemagglutination inhibition responses that were even greater than those induced by the high-dose vaccine.52
Immunoglobulin A (IgA) may be a more effective vaccine target than traditional influenza vaccines that target IgG, since different parts of IgA may engage the influenza virus simultaneously.53
Vaccines can be developed more quickly than in the past. The timeline from viral sequencing to human studies with deoxyribonucleic acid plasmid vaccines decreased from 20 months in 2003 for the severe acquired respiratory syndrome coronavirus to 11 months in 2006 for influenza A/Indonesia/2006 (H5), to 4 months in 2009 for influenza A/California/2009 (H1), to 3.5 months in 2016 for Zika virus.54 This is because it is possible today to sequence a virus and insert the genetic material into a vaccine platform without ever having to grow the virus.
TREATMENT
Numerous studies have found anti-influenza medications to be effective. Nevertheless, in an analysis of the 2011–2016 influenza seasons, only 15% of high-risk patients were prescribed anti-influenza medications within 2 days of symptom onset, including 37% in those with laboratory-confirmed influenza.55 Fever was associated with an increased rate of antiviral treatment, but 25% of high-risk outpatients were afebrile. Empiric treatment of 4 high-risk outpatients with acute respiratory illness was needed to treat 1 patient with influenza.55
Treatment with a neuraminidase inhibitor within 2 days of illness has recently been shown to improve survival and shorten duration of viral shedding in patients with avian influenza A(H7N9) infection.56 Antiviral treatment within 2 days of illness is associated with improved outcomes in transplant recipients57 and with a lower risk of otitis media in children.58
Appropriate anti-influenza treatment is as important as avoiding unnecessary antibiotics. Regrettably, as many as one-third of patients with laboratory-confirmed influenza are prescribed antibiotics.59
The US Food and Drug Administration warns against fraudulent unapproved over-the-counter influenza products.60
Baloxavir marboxil
Baloxavir marboxil is a new anti-influenza medication approved in Japan in February 2018 and anticipated to be available in the United States sometime in 2019.
This prodrug is hydrolyzed in vivo to the active metabolite, which selectively inhibits cap-dependent endonuclease enzyme, a key enzyme in initiation of messenger ribonucleic acid synthesis required for influenza viral replication.61
In a double-blind phase 3 trial, the median time to alleviation of influenza symptoms is 26.5 hours shorter with baloxavir marboxil than with placebo. One tablet was as effective as 5 days of the neuraminidase inhibitor oseltamivir and was associated with greater reduction in viral load 1 day after initiation, and similar side effects.62 Of concern is the emergence of nucleic acid substitutions conferring resistance to baloxavir; this occurred in 2.2% and 9.7% of baloxavir recipients in the phase 2 and 3 trials, respectively.
CLOSING THE GAPS
Several gaps in the management of influenza persist since the 1918 pandemic.1 These include gaps in epidemiology, prevention, diagnosis, treatment, and prognosis.
- Global networks wider than current ones are needed to address this global disease and to prioritize coordination efforts.
- Establishing and strengthening clinical capacity is needed in limited resource settings. New technologies are needed to expedite vaccine development and to achieve progress toward a universal vaccine.
- Current diagnostic tests do not distinguish between seasonal and novel influenza A viruses of zoonotic origin, which are expected to cause the next pandemic.
- Current antivirals have been shown to shorten duration of illness in outpatients with uncomplicated influenza, but the benefit in hospitalized patients has been less well established.
- In 2007, resistance of seasonal influenza A(H1N1) to oseltamivir became widespread. In 2009, pandemic influenza A(H1N1), which is highly susceptible to oseltamivir, replaced the seasonal virus and remains the predominantly circulating A(H1N1) strain.
- A small-molecule fragment, N-cyclohexyaltaurine, binds to the conserved hemagglutinin receptor-binding site in a manner that mimics the binding mode of the natural receptor sialic acid. This can serve as a template to guide the development of novel broad-spectrum small-molecule anti-influenza drugs.63
- Biomarkers that can accurately predict development of severe disease in patients with influenza are needed.
This centennial year update focuses primarily on immunization, but also reviews epidemiology, transmission, and treatment.
EPIDEMIOLOGY
2017–2018 was a bad season
The 2017–2018 influenza epidemic was memorable, dominated by influenza A(H3N2) viruses with morbidity and mortality rates approaching pandemic numbers. It lasted 19 weeks, killed more people than any other epidemic since 2010, particularly children, and was associated with 30,453 hospitalizations—almost twice the previous season high in some parts of the United States.2
Regrettably, 171 unvaccinated children died during 2017–2018, accounting for almost 80% of deaths.2 The mean age of the children who died was 7.1 years; 51% had at least 1 underlying medical condition placing them at risk for influenza-related complications, and 57% died after hospitalization.2
Recent estimates of the incidence of symptomatic influenza among all ages ranged from 3% to 11%, which is slightly lower than historical estimates. The rates were higher for children under age 18 than for adults.3 Interestingly, influenza A(H3N2) accounted for 50% of cases of non-mumps viral parotitis during the 2014–2015 influenza season in the United States.4
Influenza C exists but is rare
Influenza A and B account for almost all influenza-related outpatient visits and hospitalizations. Surveillance data from May 2013 through December 2016 showed that influenza C accounts for 0.5% of influenza-related outpatient visits and hospitalizations, particularly affecting children ages 6 to 24 months. Medical comorbidities and copathogens were seen in all patients requiring intensive care and in most hospitalizations.5 Diagnostic tests for influenza C are not widely available.
Dogs and cats: Factories for new flu strains?
While pigs and birds are the major reservoirs of influenza viral genetic diversity from which infection is transmitted to humans, dogs and cats have recently emerged as possible sources of novel reassortant influenza A.6 With their frequent close contact with humans, our pets may prove to pose a significant threat.
Obesity a risk factor for influenza
Obesity emerged as a risk factor for severe influenza in the 2009 pandemic. Recent data also showed that obesity increases the duration of influenza A virus shedding, thus increasing duration of contagiousness.7
Influenza a cardiovascular risk factor
Previous data showed that influenza was a risk factor for cardiovascular events. Two recent epidemiologic studies from the United Kingdom showed that laboratory-confirmed influenza was associated with higher rates of myocardial infarction and stroke for up to 4 weeks.8,9
Which strain is the biggest threat?
Predicting which emerging influenza serotype may cause the next pandemic is difficult, but influenza A(H7N9), which had not infected humans until 2013 but has since infected about 1,600 people in China and killed 37% of them, appears to have the greatest potential.10
National influenza surveillance programs and influenza-related social media applications have been developed and may get a boost from technology. A smartphone equipped with a temperature sensor can instantly detect one’s temperature with great precision. A 2018 study suggested that a smartphone-driven thermometry application correlated well with national influenza-like illness activity and improved its forecast in real time and up to 3 weeks in advance.11
TRANSMISSION
Humidity may not block transmission
Animal studies have suggested that humidity in the air interferes with transmission of airborne influenza virus, partially from biologic inactivation. But when a recent study used humidity-controlled chambers to investigate the stability of the 2009 influenza A(H1N1) virus in suspended aerosols and stationary droplets, the virus remained infectious in aerosols across a wide range of relative humidities, challenging the common belief that humidity destabilizes respiratory viruses in aerosols.12
One sick passenger may not infect the whole plane
Transmission of respiratory viruses on airplane flights has long been considered a potential avenue for spreading influenza. However, a recent study that monitored movements of individuals on 10 transcontinental US flights and simulated inflight transmission based on these data showed a low probability of direct transmission, except for passengers seated in close proximity to an infectious passenger.13
WHAT’S IN THE NEW FLU SHOT?
The 2018–2019 quadrivalent vaccine for the Northern Hemisphere14 contains the following strains:
- A/Michigan/45/2015 A(H1N1)pdm09-like virus
- A/Singapore/INFIMH-16-0019/2016 (H3N2)-like virus
- B/Colorado/06/2017-like virus (Victoria lineage)
- B/Phuket/3073/2013-like virus (Yamagata lineage).
The A(H3N2) (Singapore) and B/Victoria lineage components are new this year. The A(H3N2) strain was the main cause of the 2018 influenza epidemic in the Southern Hemisphere.
The quadrivalent live-attenuated vaccine, which was not recommended during the 2016–2017 and 2017–2018 influenza seasons, has made a comeback and is recommended for the 2018–2019 season in people for whom it is appropriate based on age and comorbidities.15 Although it was effective against influenza B and A(H3N2) viruses, it was less effective against the influenza A(H1N1)pdm09-like viruses during the 2013–2014 and 2015–2016 seasons.
A/Slovenia/2903/2015, the new A(H1N1)pdm09-like virus included in the 2018–2019 quadrivalent live-attenuated vaccine, is significantly more immunogenic than its predecessor, A/Bolivia/559/2013, but its clinical effectiveness remains to be seen.
PROMOTING VACCINATION
How effective is it?
Influenza vaccine effectiveness in the 2017–2018 influenza season was 36% overall, 67% against A(H1N1), 42% against influenza B, and 25% against A(H3N2).16 It is estimated that influenza vaccine prevents 300 to 4,000 deaths annually in the United States alone.17
A 2018 Cochrane review17 concluded that vaccination reduced the incidence of influenza by about half, with 2.3% of the population contracting the flu without vaccination compared with 0.9% with vaccination (risk ratio 0.41, 95% confidence interval 0.36–0.47). The same review found that 71 healthy adults need to be vaccinated to prevent 1 from experiencing influenza, and 29 to prevent 1 influenza-like illness.
Several recent studies showed that influenza vaccine effectiveness varied based on age and influenza serotype, with higher effectiveness in people ages 5 to 17 and ages 18 to 64 than in those age 65 and older.18–20 A mathematical model of influenza transmission and vaccination in the United States determined that even relatively low-efficacy influenza vaccines can be very useful if optimally distributed across age groups.21
Vaccination rates are low, and ‘antivaxxers’ are on the rise
Although the influenza vaccine is recommended in the United States for all people age 6 months and older regardless of the state of their health, vaccination rates remain low. In 2016, only 37% of employed adults were vaccinated. The highest rate was for government employees (45%), followed by private employees (36%), followed by the self-employed (30%).22
A national goal is to immunize 80% of all Americans and 90% of at-risk populations (which include children and the elderly).23 The number of US hospitals that require their employees to be vaccinated increased from 37.1% in 2013 to 61.4% in 2017.24 Regrettably, as of March 2018, 14 lawsuits addressing religious objections to hospital influenza vaccination mandates have been filed.25
Despite hundreds of studies demonstrating the efficacy, safety, and cost savings of influenza vaccination, the antivaccine movement has been growing in the United States and worldwide.26 All US states except West Virginia, Mississippi, and California allow nonmedical exemptions from vaccination based on religious or personal belief.27 Several US metropolitan areas represent “hot spots” for these exemptions.28 This may render such areas vulnerable to vaccine-preventable diseases, including influenza.
Herd immunity: We’re all in this together
Some argue that the potential adverse effects and the cost of vaccination outweigh the benefits, but the protective benefits of herd immunity are significant for those with comorbidities or compromised immunity.
Educating the public about herd immunity and local influenza vaccination uptake increases people’s willingness to be vaccinated.29 A key educational point is that at least 70% of a community needs to be vaccinated to prevent community outbreaks; this protects everyone, including those who do not mount a protective antibody response to influenza vaccination and those who are not vaccinated.
DOES ANNUAL VACCINATION BLUNT ITS EFFECTIVENESS?
Some studies from the 1970s and 1980s raised concern over a possible negative effect of annual influenza vaccination on vaccine effectiveness. The “antigenic distance hypothesis” holds that vaccine effectiveness is influenced by antigenic similarity between the previous season’s vaccine serotypes and the epidemic serotypes, as well as the antigenic similarity between the serotypes of the current and previous seasons.
A meta-analysis of studies from 2010 through 2015 showed significant inconsistencies in repeat vaccination effects within and between seasons and serotypes. It also showed that vaccine effectiveness may be influenced by more than 1 previous season, particularly for influenza A(H3N2), in which repeated vaccination can blunt the hemagglutinin antibody response.30
A study from Japan showed that people who needed medical attention for influenza in the previous season were at lower risk of a similar event in the current season.31 Prior-season influenza vaccination reduced current-season vaccine effectiveness only in those who did not have medically attended influenza in the prior season. This suggests that infection is more immunogenic than vaccination, but only against the serotype causing the infection and not the other serotypes included in the vaccine.
An Australian study showed that annual influenza vaccination did not decrease vaccine effectiveness against influenza-associated hospitalization. Rather, effectiveness increased by about 15% in those vaccinated in both current and previous seasons compared with those vaccinated in either season alone.32
European investigators showed that repeated seasonal influenza vaccination in the elderly prevented the need for hospitalization due to influenza A(H3N2) and B, but not A(H1N1)pdm09.33
VACCINATION IN SPECIAL POPULATIONS
High-dose vaccine for older adults
The high-dose influenza vaccine has been licensed since 2009 for use in the United States for people ages 65 and older.
Recent studies confirmed that high-dose vaccine is more effective than standard-dose vaccine in veterans34 and US Medicare beneficiaries.35
The high-dose vaccine is rapidly becoming the primary vaccine given to people ages 65 and older in retail pharmacies, where vaccination begins earlier in the season than in providers’ offices.36 Some studies have shown that the standard-dose vaccine wanes in effectiveness toward the end of the influenza season (particularly if the season is long) if it is given very early. It remains to be seen whether the same applies to the high-dose influenza vaccine.
Some advocate twice-annual influenza vaccination, particularly for older adults living in tropical and subtropical areas, where influenza seasons are more prolonged. However, a recently published study observed reductions in influenza-specific hemagglutination inhibition and cell-mediated immunity after twice-annual vaccination.37
Vaccination is beneficial during pregnancy
Many studies have shown the value of influenza vaccination during pregnancy for both mothers and their infants.
One recently published study showed that 18% of infants who developed influenza required hospitalization.38 In that study, prenatal and postpartum maternal influenza vaccination decreased the odds of influenza in infants by 61% and 53%, respectively.
Another study showed that vaccine effectiveness did not vary by gestational age at vaccination.39
Some studies have shown that influenza virus infection can increase susceptibility to certain bacterial infections. A post hoc analysis of an influenza vaccination study in pregnant women suggested that the vaccine was also associated with decreased rates of pertussis in these women.40
Factors that make vaccination less effective
Several factors including age-related frailty and iatrogenic and disease-related immunosuppression can affect vaccine effectiveness.
Frailty. A recent study showed that vaccine effectiveness was 77.6% in nonfrail older adults but only 58.7% in frail older adults.41
Immunosuppression. Temporary discontinuation of methotrexate for 2 weeks after influenza vaccination in patients with rheumatoid arthritis improves vaccine immunogenicity without precipitating disease flare.42 Solid-organ and hematopoietic stem cell transplant recipients who received influenza vaccine were less likely to develop pneumonia and require intensive care unit admission.43
The high-dose influenza vaccine is more immunogenic than the standard-dose vaccine in solid-organ transplant recipients.44
Statins are widely prescribed and have recently been associated with reduced influenza vaccine effectiveness against medically attended acute respiratory illness, but their benefits in preventing cardiovascular events outweigh this risk.45
FUTURE VACCINE CONSIDERATIONS
Moving away from eggs
During the annual egg-based production process, which takes several months, the influenza vaccine acquires antigenic changes that allow replication in eggs, particularly in the hemagglutinin protein, which mediates receptor binding. This process of egg adaptation may cause antigenic changes that decrease vaccine effectiveness against circulating viruses.
The cell-based baculovirus influenza vaccine grown in dog kidney cells has higher antigenic content and is not subject to the limitations of egg-based vaccine, although it still requires annual updates. A recombinant influenza vaccine reduces the probability of influenza-like illness by 30% compared with the egg-based influenza vaccine, but also still requires annual updates.46 The market share of these non-egg-based vaccines is small, and thus their effectiveness has yet to be demonstrated.
The US Department of Defense administered the cell-based influenza vaccine to about one-third of Armed Forces personnel, their families, and retirees in the 2017–2018 influenza seasons, and data on its effectiveness are expected in the near future.47
A universal vaccine would be ideal
The quest continues for a universal influenza vaccine, one that remains protective for several years and does not require annual updates.48 Such a vaccine would protect against seasonal epidemic influenza drift variants and pandemic strains. More people could likely be persuaded to be vaccinated once rather than every year.
An ideal universal vaccine would be suitable for all age groups, at least 75% effective against symptomatic influenza virus infection, protective against all influenza A viruses (influenza A, not B, causes pandemics and seasonal epidemics), and durable through multiple influenza seasons.51
Research and production of such a vaccine are expected to require funding of about $1 billion over the next 5 years.
Boosting effectiveness
Estimates of influenza vaccine effectiveness range from 40% to 60% in years when the vaccine viruses closely match the circulating viruses, and variably lower when they do not match. The efficacy of most other vaccines given to prevent other infections is much higher.
New technologies to improve influenza vaccine effectiveness are needed, particularly for influenza A(H3N2) viruses, which are rapidly evolving and are highly susceptible to egg-adaptive mutations in the manufacturing process.
In one study, a nanoparticle vaccine formulated with a saponin-based adjuvant induced hemagglutination inhibition responses that were even greater than those induced by the high-dose vaccine.52
Immunoglobulin A (IgA) may be a more effective vaccine target than traditional influenza vaccines that target IgG, since different parts of IgA may engage the influenza virus simultaneously.53
Vaccines can be developed more quickly than in the past. The timeline from viral sequencing to human studies with deoxyribonucleic acid plasmid vaccines decreased from 20 months in 2003 for the severe acquired respiratory syndrome coronavirus to 11 months in 2006 for influenza A/Indonesia/2006 (H5), to 4 months in 2009 for influenza A/California/2009 (H1), to 3.5 months in 2016 for Zika virus.54 This is because it is possible today to sequence a virus and insert the genetic material into a vaccine platform without ever having to grow the virus.
TREATMENT
Numerous studies have found anti-influenza medications to be effective. Nevertheless, in an analysis of the 2011–2016 influenza seasons, only 15% of high-risk patients were prescribed anti-influenza medications within 2 days of symptom onset, including 37% in those with laboratory-confirmed influenza.55 Fever was associated with an increased rate of antiviral treatment, but 25% of high-risk outpatients were afebrile. Empiric treatment of 4 high-risk outpatients with acute respiratory illness was needed to treat 1 patient with influenza.55
Treatment with a neuraminidase inhibitor within 2 days of illness has recently been shown to improve survival and shorten duration of viral shedding in patients with avian influenza A(H7N9) infection.56 Antiviral treatment within 2 days of illness is associated with improved outcomes in transplant recipients57 and with a lower risk of otitis media in children.58
Appropriate anti-influenza treatment is as important as avoiding unnecessary antibiotics. Regrettably, as many as one-third of patients with laboratory-confirmed influenza are prescribed antibiotics.59
The US Food and Drug Administration warns against fraudulent unapproved over-the-counter influenza products.60
Baloxavir marboxil
Baloxavir marboxil is a new anti-influenza medication approved in Japan in February 2018 and anticipated to be available in the United States sometime in 2019.
This prodrug is hydrolyzed in vivo to the active metabolite, which selectively inhibits cap-dependent endonuclease enzyme, a key enzyme in initiation of messenger ribonucleic acid synthesis required for influenza viral replication.61
In a double-blind phase 3 trial, the median time to alleviation of influenza symptoms is 26.5 hours shorter with baloxavir marboxil than with placebo. One tablet was as effective as 5 days of the neuraminidase inhibitor oseltamivir and was associated with greater reduction in viral load 1 day after initiation, and similar side effects.62 Of concern is the emergence of nucleic acid substitutions conferring resistance to baloxavir; this occurred in 2.2% and 9.7% of baloxavir recipients in the phase 2 and 3 trials, respectively.
CLOSING THE GAPS
Several gaps in the management of influenza persist since the 1918 pandemic.1 These include gaps in epidemiology, prevention, diagnosis, treatment, and prognosis.
- Global networks wider than current ones are needed to address this global disease and to prioritize coordination efforts.
- Establishing and strengthening clinical capacity is needed in limited resource settings. New technologies are needed to expedite vaccine development and to achieve progress toward a universal vaccine.
- Current diagnostic tests do not distinguish between seasonal and novel influenza A viruses of zoonotic origin, which are expected to cause the next pandemic.
- Current antivirals have been shown to shorten duration of illness in outpatients with uncomplicated influenza, but the benefit in hospitalized patients has been less well established.
- In 2007, resistance of seasonal influenza A(H1N1) to oseltamivir became widespread. In 2009, pandemic influenza A(H1N1), which is highly susceptible to oseltamivir, replaced the seasonal virus and remains the predominantly circulating A(H1N1) strain.
- A small-molecule fragment, N-cyclohexyaltaurine, binds to the conserved hemagglutinin receptor-binding site in a manner that mimics the binding mode of the natural receptor sialic acid. This can serve as a template to guide the development of novel broad-spectrum small-molecule anti-influenza drugs.63
- Biomarkers that can accurately predict development of severe disease in patients with influenza are needed.
- Uyeki TM, Fowler RA, Fischer WA. Gaps in the clinical management of influenza: a century since the 1918 pandemic. JAMA 2018; 320(8):755–756. doi:10.1001/jama.2018.8113
- Garten R, Blanton L, Elal AI, et al. Update: influenza activity in the United States during the 2017–18 season and composition of the 2018–19 influenza vaccine. MMWR Morb Mortal Wkly Rep 2018; 67(22):634–642. doi:10.15585/mmwr.mm6722a4
- Tokars JI, Olsen SJ, Reed C. Seasonal incidence of symptomatic influenza in the United States. Clin Infect Dis 2018; 66(10):1511–1518. doi:10.1093/cid/cix1060
- Elbadawi LI, Talley P, Rolfes MA, et al. Non-mumps viral parotitis during the 2014–2015 influenza season in the United States. Clin Infect Dis 2018. Epub ahead of print. doi:10.1093/cid/ciy137
- Thielen BK, Friedlander H, Bistodeau S, et al. Detection of influenza C viruses among outpatients and patients hospitalized for severe acute respiratory infection, Minnesota, 2013–2016. Clin Infect Dis 2018; 66(7):1092–1098. doi:10.1093/cid/cix931
- Chena Y, Trovãob NS, Wang G, et al. Emergence and evolution of novel reassortant influenza A viruses in canines in southern China. MBio 2018; 9(3):e00909–e00918. doi:10.1128/mBio.00909-18
- Maier HE, Lopez R, Sanchez N, et al. Obesity increases the duration of influenza A virus shedding in adults. J Infect Dis 2018. Epub ahead of print. doi:10.1093/infdis/jiy370
- Warren-Gash C, Blackburn R, Whitaker H, McMenamin J, Hayward AC. Laboratory-confirmed respiratory infections as triggers for acute myocardial infarction and stroke: a self-controlled case series analysis of national linked datasets from Scotland. Eur Respir J 2018; 51(3):1701794. doi:10.1183/13993003.01794-2017
- Blackburn R, Zhao H, Pebody R, Hayward A, Warren-Gash C. Laboratory-confirmed respiratory infections as predictors of hospital admission for myocardial infarction and stroke: time-series analysis of English data for 2004–2015. Clin Infect Dis 2018; 67(1):8–17. doi:10.1093/cid/cix1144
- Newsweek; Andrew S. What is disease X? Deadly bird flu virus could be next pandemic. www.newsweek.com/disease-x-bird-flu-deaths-pandemic-what-h7n9-979723. Accessed October 3, 2018.
- Miller AC, Singh I, Koehler E, Polgreen PM. A smartphone-driven thermometer application for real-time population- and individual-level influenza surveillance. Clin Infect Dis 2018; 67(3):388–397. doi:10.1093/cid/ciy073
- Kormuth KA, Lin K, Prussin AJ 2nd, et al. Influenza virus infectivity is retained in aerosols and droplets independent of relative humidity, J Infect Dis 2018; 218(5):739–747. doi:10.1093/infdis/jiy221
- Hertzberg VS, Weiss H, Elon L, et. al. Behaviors, movements, and transmission of droplet-mediated respiratory diseases during transcontinental airline flights. Proc Natl Acad Sci U S A 2018; 115(14):3623–3627. doi:10.1073/pnas.1711611115
- Grohskopf LA, Sokolow LZ, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2018–19 influenza season. MMWR Recomm Rep 2018; 67(3):1–20. doi:10.15585/mmwr.rr6703a1
- Grohskopf LA, Sokolow LZ, Fry AM, Walter EB, Jernigan DB. Update: ACIP recommendations for the use of quadrivalent live attenuated influenza vaccine (LAIV4)—United States, 2018–19 influenza season. MMWR Morb Mortal Wkly Rep 2018; 67(22):643–645. doi:10.15585/mmwr.mm6722a5
- Flannery B, Chung JR, Belongia EA, et al. Interim estimates of 2017–18 seasonal influenza vaccine effectiveness—United States, February 2018. MMWR Morb Mortal Wkly Rep 2018; 67(6):180–185. doi:10.15585/mmwr.mm6706a2
- Demicheli V, Jefferson T, Ferroni E, Rivetti A, Di Pietrantonj C. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev 2018; 2:CD001269. doi:10.1002/14651858.CD001269.pub6
- Flannery B, Smith C, Garten RJ, et al. Influence of birth cohort on effectiveness of 2015–2016 influenza vaccine against medically attended illness due to 2009 pandemic influenza A(H1N1) virus in the United States. J Infect Dis 2018; 218(2):189–196. doi:10.1093/infdis/jix634
- Rondy M, El Omeiri N, Thompson MG, Leveque A, Moren A, Sullivan SG. Effectiveness of influenza vaccines in preventing severe influenza illness among adults: a systematic review and meta-analysis of test-negative design case-control studies. J Infect 2017; 75(5):381–394. doi:10.1016/j.jinf.2017.09.010
- Stein Y, Mandelboim M, Sefty H, et al; Israeli Influenza Surveillance Network (IISN). Seasonal influenza vaccine effectiveness in preventing laboratory-confirmed influenza in primary care in Israel, 2016–2017 season: insights into novel age-specific analysis. Clin Infect Dis 2018; 66(9):1383–1391. doi:10.1093/cid/cix1013
- Sah P, Medlock J, Fitzpatrick MC, Singer BH, Galvani AP. Optimizing the impact of low-efficacy influenza vaccines. Proc Natl Acad Sci U S A 2018; 115(20):5151–5156. doi:10.1073/pnas.1802479115
- QuickStats: percentage of currently employed adults aged ≥ 18 years who received influenza vaccine in the past 12 months, by employment category—national health interview survey, United States, 2012 and 2016. MMWR Morb Mortal Wkly Rep 2018; 67(16):480. doi:10.15585/mmwr.mm6716a8
- Healthy People.gov. Immunization and infectious diseases. IID-12. Increase the percentage of children and adults who are vaccinated annually against seasonal influenza. www.healthypeople.gov/2020/topics-objectives/topic/immunization-and-infectious-diseases/objectives. Accessed October 3, 2018.
- Greene MT, Fowler KE, Ratz D, Krein SL, Bradley SF, Saint S. Changes in influenza vaccination requirements for health care personnel in US hospitals. JAMA Network Open 2018; 1(2):e180143. doi:10.1001/jamanetworkopen.2018.0143
- Opel DJ, Sonne JA, Mello MM. Vaccination without litigation—addressing religious objections to hospital influenza-vaccination mandates. N Engl J Med 2018; 378(9):785–788. doi:10.1056/NEJMp1716147
- Horowitz J. Italy loosens vaccine law just as children return to school. New York Times Sept. 20, 2018. www.nytimes.com/2018/09/20/world/europe/italy-vaccines-five-star-movement.html.
- National Conference of State Legislature. States with religious and philosophical exemptions from school immunization requirements. www.ncsl.org/research/health/school-immunization-exemption-state-laws.aspx. Accessed October 3, 2018.
- Olive JK, Hotez PJ, Damania A, Nolan MS. The state of the antivaccine movement in the United States: a focused examination of nonmedical exemptions in states and counties. PLoS Med 2018; 15(6):e1002578. doi:10.1371/journal.pmed.1002578
- Logan J, Nederhoff D, Koch B, et al. ‘What have you HEARD about the HERD?’ Does education about local influenza vaccination coverage and herd immunity affect willingness to vaccinate? Vaccine 2018; 36(28):4118–4125. doi:10.1016/j.vaccine.2018.05.037
- Belongia EA, Skowronski DM, McLean HQ, Chambers C, Sundaram ME, De Serres G. Repeated annual influenza vaccination and vaccine effectiveness: review of evidence. Expert Rev Vaccines 2017; 16(7):1–14. doi:10.1080/14760584.2017.1334554
- Saito N, Komori K, Suzuki M, et al. Negative impact of prior influenza vaccination on current influenza vaccination among people infected and not infected in prior season: a test-negative case-control study in Japan. Vaccine 2017; 35(4):687–693. doi:10.1016/j.vaccine.2016.11.024
- Cheng AC, Macartney KK, Waterer GW, Kotsimbos T, Kelly PM, Blyth CC; Influenza Complications Alert Network (FluCAN) Investigators. Repeated vaccination does not appear to impact upon influenza vaccine effectiveness against hospitalization with confirmed influenza. Clin Infect Dis 2017; 64(11):1564–1572. doi:10.1093/cid/cix209
- Rondy M, Launay O, Castilla J, et al; InNHOVE/I-MOVE+working group. Repeated seasonal influenza vaccination among elderly in Europe: effects on laboratory confirmed hospitalised influenza. Vaccine 2017; 35(34):4298–4306. doi:10.1016/j.vaccine.2017.06.088
- Young-Xu Y, van Aalst R, Mahmud SM, et al. Relative vaccine effectiveness of high-dose versus standard-dose influenza vaccines among Veterans Health Administration patients. J Infect Dis 2018; 217(11):1718–1727. doi:10.1093/infdis/jiy088
- Shay DK, Chillarige Y, Kelman J, et al. Comparative effectiveness of high-dose versus standard-dose influenza vaccines among US Medicare beneficiaries in preventing postinfluenza deaths during 2012–2013 and 2013–2014. J Infect Dis 2017; 215(4):510–517. doi:10.1093/infdis/jiw641
- Madaras-Kelly K, Remington R, Hruza H, Xu D. Comparative effectiveness of high-dose versus standard-dose influenza vaccines in preventing postinfluenza deaths. J Infect Dis 2018; 218(2):336–337. doi:10.1093/infdis/jix645
- Tam YH, Valkenburg SA, Perera RAPM, et al. Immune responses to twice-annual influenza vaccination in older adults in Hong Kong. Clin Infect Dis 2018; 66(6):904–912. doi:10.1093/cid/cix900
- Ohfuji S, Deguchi M, Tachibana D, et al; Osaka Pregnant Women Influenza Study Group. Protective effect of maternal influenza vaccination on influenza in their infants: a prospective cohort study. J Infect Dis 2018; 217(6):878–886. doi:10.1093/infdis/jix629
- Katz J, Englund JA, Steinhoff MC, et al. Impact of timing of influenza vaccination in pregnancy on transplacental antibody transfer, influenza incidence, and birth outcomes: a randomized trial in rural Nepal. Clin Infect Dis 2018; 67(3):334–340. doi:10.1093/cid/ciy090
- Nunes MC, Cutland CL, Madhi SA. Influenza vaccination during pregnancy and protection against pertussis. N Engl J Med 2018; 378(13):1257–1258. doi:10.1056/NEJMc1705208
- Andrew MK, Shinde V, Ye L, et al; Serious Outcomes Surveillance Network of the Public Health Agency of Canada/Canadian Institutes of Health Research Influenza Research Network (PCIRN) and the Toronto Invasive Bacterial Diseases Network (TIBDN). The importance of frailty in the assessment of influenza vaccine effectiveness against influenza-related hospitalization in elderly people. J Infect Dis 2017; 216(4):405–414. doi:10.1093/infdis/jix282
- Park JK, Lee YJ, Shin K, et al. Impact of temporary methotrexate discontinuation for 2 weeks on immunogenicity of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis 2018; 77(6):898–904. doi:10.1136/annrheumdis-2018-213222
- Kumar D, Ferreira VH, Blumberg E, et al. A five-year prospective multi-center evaluation of influenza infection in transplant recipients. Clin Infect Dis 2018. Epub ahead of print. doi:10.1093/cid/ciy294
- Natori Y, Shiotsuka M, Slomovic J, et al. A double-blind, randomized trial of high-dose vs standard-dose influenza vaccine in adult solid-organ transplant recipients. Clin Infect Dis 2018; 66(11):1698–1704. doi:10.1093/cid/cix1082
- Omer SB, Phadke VK, Bednarczyk BA, Chamberlain AT, Brosseau JL, Orenstein WA. Impact of statins on influenza vaccine effectiveness against medically attended acute respiratory illness. J Infect Dis 2016; 213(8):1216–1223. doi:10.1093/infdis/jiv457
- Dunkle LM, Izikson R, Patriarca P, et al. Efficacy of recombinant influenza vaccine in adults 50 years of age or older. N Engl J Med 2017; 376(25):2427–2436. doi:10.1056/NEJMoa1608862
- STAT; Branswell H. How the US military might help answer a critical question about the flu vaccine. www.statnews.com/2018/03/02/flu-vaccine-egg-production-data. Accessed October 3, 2018.
- Paules CI, Sullivan SG, Subbarao K, Fauci AS. Chasing seasonal influenza—the need for a universal influenza vaccine. N Engl J Med 2018; 378(1):7–9. doi:10.1056/NEJMp1714916
- Jin XW, Mossad SB. Avian influenza: an emerging pandemic threat. Cleve Clin J Med 2005; 72:1129-1134. pmid:16392727
- Wei WI, Brunger AT, Skehel JJ, Wiley DC. Refinement of the influenza virus hemagglutinin by simulated annealing. J Mol Biol 1990; 212(4):737–761. doi:10.1016/0022-2836(90)90234-D
- Erbelding EJ, Post DJ, Stemmy EJ, et al. A universal influenza vaccine: the strategic plan for the National Institute of Allergy and Infectious Diseases, J Infect Dis 2018; 218(3):347–354. doi:10.1093/infdis/jiy103
- Shinde V, Fries L, Wu Y, et al. Improved titers against influenza drift variants with a nanoparticle vaccine. N Engl J Med 2018; 378(24):2346–2348. doi:10.1056/NEJMc1803554
- Maurer MA, Meyer L, Bianchi M, et al. Glycosylation of human IgA directly inhibits influenza A and other sialic-acid-binding viruses. Cell Rep 2018; 23(1):90–99. doi:10.1016/j.celrep.2018.03.027
- Graham BS, Mascola JR, Fauci AS. Novel vaccine technologies: essential components of an adequate response to emerging viral diseases. JAMA 2018; 319(14):1431–1432. doi:10.1001/jama.2018.0345
- Stewart RJ, Flannery B, Chung JR, et al. Influenza antiviral prescribing for outpatients with an acute respiratory illness and at high risk for influenza-associated complications during 5 influenza seasons—United States, 2011–2016. Clin Infect Dis 2018; 66(7):1035–1041. doi:10.1093/cid/cix922
- Zheng S, Tang L, Gao H, et al. Benefit of early initiation of neuraminidase inhibitor treatment to hospitalized patients with avian influenza A(H7N9) virus. Clin Infect Dis 2018; 66(7):1054–1060. doi:10.1093/cid/cix930
- Kumar D, Ferreira VH, Blumberg E, et al. A five-year prospective multi-center evaluation of influenza infection in transplant recipients. Clin Infect Dis 2018. Epub ahead of print. doi:10.1093/cid/ciy294
- Malosh RE, Martin ET, Heikkinen T, Brooks WA, Whitley RJ, Monto AS. Efficacy and safety of oseltamivir in children: systematic review and individual patient data meta-analysis of randomized controlled trials. Clin Infect Dis 2018; 66(10):1492–1500. doi:10.1093/cid/cix1040
- Havers FP, Hicks LA, Chung JR, et al. Outpatient antibiotic prescribing for acute respiratory infections during influenza seasons. JAMA Network Open 2018; 1(2):e180243. doi:10.1001/jamanetworkopen.2018.0243
- US Food and Drug Administration. FDA warns of fraudulent and unapproved flu products. www.fda.gov/newsevents/newsroom/pressannouncements/ucm599223.htm. Accessed October 3, 2018.
- Portsmouth S, Kawaguchi K, Arai M, Tsuchiya K, Uehara T. Cap-dependent endonuclease inhibitor S-033188 for the treatment of influenza: results from a phase 3, randomized, double-blind, placebo- and active-controlled study in otherwise healthy adolescents and adults with seasonal influenza. Open Forum Infect Dis 2017; 4(suppl 1):S734. doi:10.1093/ofid/ofx180.001
- Hayden FG, Sugaya N, Hirotsu N, et al; Baloxavir Marboxil Investigators Group. Baloxavir Marboxil for uncomplicated influenza in adults and adolescents. N Engl J Med 2018; 379(10):913–923. doi:10.1056/NEJMoa1716197
- Kadam RU, Wilson IA. A small-molecule fragment that emulates binding of receptor and broadly neutralizing antibodies to influenza A hemagglutinin. Proc Natl Acad Sci U S A 2018; 115(16):4240–4245. doi:10.1073/pnas.1801999115
- Uyeki TM, Fowler RA, Fischer WA. Gaps in the clinical management of influenza: a century since the 1918 pandemic. JAMA 2018; 320(8):755–756. doi:10.1001/jama.2018.8113
- Garten R, Blanton L, Elal AI, et al. Update: influenza activity in the United States during the 2017–18 season and composition of the 2018–19 influenza vaccine. MMWR Morb Mortal Wkly Rep 2018; 67(22):634–642. doi:10.15585/mmwr.mm6722a4
- Tokars JI, Olsen SJ, Reed C. Seasonal incidence of symptomatic influenza in the United States. Clin Infect Dis 2018; 66(10):1511–1518. doi:10.1093/cid/cix1060
- Elbadawi LI, Talley P, Rolfes MA, et al. Non-mumps viral parotitis during the 2014–2015 influenza season in the United States. Clin Infect Dis 2018. Epub ahead of print. doi:10.1093/cid/ciy137
- Thielen BK, Friedlander H, Bistodeau S, et al. Detection of influenza C viruses among outpatients and patients hospitalized for severe acute respiratory infection, Minnesota, 2013–2016. Clin Infect Dis 2018; 66(7):1092–1098. doi:10.1093/cid/cix931
- Chena Y, Trovãob NS, Wang G, et al. Emergence and evolution of novel reassortant influenza A viruses in canines in southern China. MBio 2018; 9(3):e00909–e00918. doi:10.1128/mBio.00909-18
- Maier HE, Lopez R, Sanchez N, et al. Obesity increases the duration of influenza A virus shedding in adults. J Infect Dis 2018. Epub ahead of print. doi:10.1093/infdis/jiy370
- Warren-Gash C, Blackburn R, Whitaker H, McMenamin J, Hayward AC. Laboratory-confirmed respiratory infections as triggers for acute myocardial infarction and stroke: a self-controlled case series analysis of national linked datasets from Scotland. Eur Respir J 2018; 51(3):1701794. doi:10.1183/13993003.01794-2017
- Blackburn R, Zhao H, Pebody R, Hayward A, Warren-Gash C. Laboratory-confirmed respiratory infections as predictors of hospital admission for myocardial infarction and stroke: time-series analysis of English data for 2004–2015. Clin Infect Dis 2018; 67(1):8–17. doi:10.1093/cid/cix1144
- Newsweek; Andrew S. What is disease X? Deadly bird flu virus could be next pandemic. www.newsweek.com/disease-x-bird-flu-deaths-pandemic-what-h7n9-979723. Accessed October 3, 2018.
- Miller AC, Singh I, Koehler E, Polgreen PM. A smartphone-driven thermometer application for real-time population- and individual-level influenza surveillance. Clin Infect Dis 2018; 67(3):388–397. doi:10.1093/cid/ciy073
- Kormuth KA, Lin K, Prussin AJ 2nd, et al. Influenza virus infectivity is retained in aerosols and droplets independent of relative humidity, J Infect Dis 2018; 218(5):739–747. doi:10.1093/infdis/jiy221
- Hertzberg VS, Weiss H, Elon L, et. al. Behaviors, movements, and transmission of droplet-mediated respiratory diseases during transcontinental airline flights. Proc Natl Acad Sci U S A 2018; 115(14):3623–3627. doi:10.1073/pnas.1711611115
- Grohskopf LA, Sokolow LZ, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2018–19 influenza season. MMWR Recomm Rep 2018; 67(3):1–20. doi:10.15585/mmwr.rr6703a1
- Grohskopf LA, Sokolow LZ, Fry AM, Walter EB, Jernigan DB. Update: ACIP recommendations for the use of quadrivalent live attenuated influenza vaccine (LAIV4)—United States, 2018–19 influenza season. MMWR Morb Mortal Wkly Rep 2018; 67(22):643–645. doi:10.15585/mmwr.mm6722a5
- Flannery B, Chung JR, Belongia EA, et al. Interim estimates of 2017–18 seasonal influenza vaccine effectiveness—United States, February 2018. MMWR Morb Mortal Wkly Rep 2018; 67(6):180–185. doi:10.15585/mmwr.mm6706a2
- Demicheli V, Jefferson T, Ferroni E, Rivetti A, Di Pietrantonj C. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev 2018; 2:CD001269. doi:10.1002/14651858.CD001269.pub6
- Flannery B, Smith C, Garten RJ, et al. Influence of birth cohort on effectiveness of 2015–2016 influenza vaccine against medically attended illness due to 2009 pandemic influenza A(H1N1) virus in the United States. J Infect Dis 2018; 218(2):189–196. doi:10.1093/infdis/jix634
- Rondy M, El Omeiri N, Thompson MG, Leveque A, Moren A, Sullivan SG. Effectiveness of influenza vaccines in preventing severe influenza illness among adults: a systematic review and meta-analysis of test-negative design case-control studies. J Infect 2017; 75(5):381–394. doi:10.1016/j.jinf.2017.09.010
- Stein Y, Mandelboim M, Sefty H, et al; Israeli Influenza Surveillance Network (IISN). Seasonal influenza vaccine effectiveness in preventing laboratory-confirmed influenza in primary care in Israel, 2016–2017 season: insights into novel age-specific analysis. Clin Infect Dis 2018; 66(9):1383–1391. doi:10.1093/cid/cix1013
- Sah P, Medlock J, Fitzpatrick MC, Singer BH, Galvani AP. Optimizing the impact of low-efficacy influenza vaccines. Proc Natl Acad Sci U S A 2018; 115(20):5151–5156. doi:10.1073/pnas.1802479115
- QuickStats: percentage of currently employed adults aged ≥ 18 years who received influenza vaccine in the past 12 months, by employment category—national health interview survey, United States, 2012 and 2016. MMWR Morb Mortal Wkly Rep 2018; 67(16):480. doi:10.15585/mmwr.mm6716a8
- Healthy People.gov. Immunization and infectious diseases. IID-12. Increase the percentage of children and adults who are vaccinated annually against seasonal influenza. www.healthypeople.gov/2020/topics-objectives/topic/immunization-and-infectious-diseases/objectives. Accessed October 3, 2018.
- Greene MT, Fowler KE, Ratz D, Krein SL, Bradley SF, Saint S. Changes in influenza vaccination requirements for health care personnel in US hospitals. JAMA Network Open 2018; 1(2):e180143. doi:10.1001/jamanetworkopen.2018.0143
- Opel DJ, Sonne JA, Mello MM. Vaccination without litigation—addressing religious objections to hospital influenza-vaccination mandates. N Engl J Med 2018; 378(9):785–788. doi:10.1056/NEJMp1716147
- Horowitz J. Italy loosens vaccine law just as children return to school. New York Times Sept. 20, 2018. www.nytimes.com/2018/09/20/world/europe/italy-vaccines-five-star-movement.html.
- National Conference of State Legislature. States with religious and philosophical exemptions from school immunization requirements. www.ncsl.org/research/health/school-immunization-exemption-state-laws.aspx. Accessed October 3, 2018.
- Olive JK, Hotez PJ, Damania A, Nolan MS. The state of the antivaccine movement in the United States: a focused examination of nonmedical exemptions in states and counties. PLoS Med 2018; 15(6):e1002578. doi:10.1371/journal.pmed.1002578
- Logan J, Nederhoff D, Koch B, et al. ‘What have you HEARD about the HERD?’ Does education about local influenza vaccination coverage and herd immunity affect willingness to vaccinate? Vaccine 2018; 36(28):4118–4125. doi:10.1016/j.vaccine.2018.05.037
- Belongia EA, Skowronski DM, McLean HQ, Chambers C, Sundaram ME, De Serres G. Repeated annual influenza vaccination and vaccine effectiveness: review of evidence. Expert Rev Vaccines 2017; 16(7):1–14. doi:10.1080/14760584.2017.1334554
- Saito N, Komori K, Suzuki M, et al. Negative impact of prior influenza vaccination on current influenza vaccination among people infected and not infected in prior season: a test-negative case-control study in Japan. Vaccine 2017; 35(4):687–693. doi:10.1016/j.vaccine.2016.11.024
- Cheng AC, Macartney KK, Waterer GW, Kotsimbos T, Kelly PM, Blyth CC; Influenza Complications Alert Network (FluCAN) Investigators. Repeated vaccination does not appear to impact upon influenza vaccine effectiveness against hospitalization with confirmed influenza. Clin Infect Dis 2017; 64(11):1564–1572. doi:10.1093/cid/cix209
- Rondy M, Launay O, Castilla J, et al; InNHOVE/I-MOVE+working group. Repeated seasonal influenza vaccination among elderly in Europe: effects on laboratory confirmed hospitalised influenza. Vaccine 2017; 35(34):4298–4306. doi:10.1016/j.vaccine.2017.06.088
- Young-Xu Y, van Aalst R, Mahmud SM, et al. Relative vaccine effectiveness of high-dose versus standard-dose influenza vaccines among Veterans Health Administration patients. J Infect Dis 2018; 217(11):1718–1727. doi:10.1093/infdis/jiy088
- Shay DK, Chillarige Y, Kelman J, et al. Comparative effectiveness of high-dose versus standard-dose influenza vaccines among US Medicare beneficiaries in preventing postinfluenza deaths during 2012–2013 and 2013–2014. J Infect Dis 2017; 215(4):510–517. doi:10.1093/infdis/jiw641
- Madaras-Kelly K, Remington R, Hruza H, Xu D. Comparative effectiveness of high-dose versus standard-dose influenza vaccines in preventing postinfluenza deaths. J Infect Dis 2018; 218(2):336–337. doi:10.1093/infdis/jix645
- Tam YH, Valkenburg SA, Perera RAPM, et al. Immune responses to twice-annual influenza vaccination in older adults in Hong Kong. Clin Infect Dis 2018; 66(6):904–912. doi:10.1093/cid/cix900
- Ohfuji S, Deguchi M, Tachibana D, et al; Osaka Pregnant Women Influenza Study Group. Protective effect of maternal influenza vaccination on influenza in their infants: a prospective cohort study. J Infect Dis 2018; 217(6):878–886. doi:10.1093/infdis/jix629
- Katz J, Englund JA, Steinhoff MC, et al. Impact of timing of influenza vaccination in pregnancy on transplacental antibody transfer, influenza incidence, and birth outcomes: a randomized trial in rural Nepal. Clin Infect Dis 2018; 67(3):334–340. doi:10.1093/cid/ciy090
- Nunes MC, Cutland CL, Madhi SA. Influenza vaccination during pregnancy and protection against pertussis. N Engl J Med 2018; 378(13):1257–1258. doi:10.1056/NEJMc1705208
- Andrew MK, Shinde V, Ye L, et al; Serious Outcomes Surveillance Network of the Public Health Agency of Canada/Canadian Institutes of Health Research Influenza Research Network (PCIRN) and the Toronto Invasive Bacterial Diseases Network (TIBDN). The importance of frailty in the assessment of influenza vaccine effectiveness against influenza-related hospitalization in elderly people. J Infect Dis 2017; 216(4):405–414. doi:10.1093/infdis/jix282
- Park JK, Lee YJ, Shin K, et al. Impact of temporary methotrexate discontinuation for 2 weeks on immunogenicity of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis 2018; 77(6):898–904. doi:10.1136/annrheumdis-2018-213222
- Kumar D, Ferreira VH, Blumberg E, et al. A five-year prospective multi-center evaluation of influenza infection in transplant recipients. Clin Infect Dis 2018. Epub ahead of print. doi:10.1093/cid/ciy294
- Natori Y, Shiotsuka M, Slomovic J, et al. A double-blind, randomized trial of high-dose vs standard-dose influenza vaccine in adult solid-organ transplant recipients. Clin Infect Dis 2018; 66(11):1698–1704. doi:10.1093/cid/cix1082
- Omer SB, Phadke VK, Bednarczyk BA, Chamberlain AT, Brosseau JL, Orenstein WA. Impact of statins on influenza vaccine effectiveness against medically attended acute respiratory illness. J Infect Dis 2016; 213(8):1216–1223. doi:10.1093/infdis/jiv457
- Dunkle LM, Izikson R, Patriarca P, et al. Efficacy of recombinant influenza vaccine in adults 50 years of age or older. N Engl J Med 2017; 376(25):2427–2436. doi:10.1056/NEJMoa1608862
- STAT; Branswell H. How the US military might help answer a critical question about the flu vaccine. www.statnews.com/2018/03/02/flu-vaccine-egg-production-data. Accessed October 3, 2018.
- Paules CI, Sullivan SG, Subbarao K, Fauci AS. Chasing seasonal influenza—the need for a universal influenza vaccine. N Engl J Med 2018; 378(1):7–9. doi:10.1056/NEJMp1714916
- Jin XW, Mossad SB. Avian influenza: an emerging pandemic threat. Cleve Clin J Med 2005; 72:1129-1134. pmid:16392727
- Wei WI, Brunger AT, Skehel JJ, Wiley DC. Refinement of the influenza virus hemagglutinin by simulated annealing. J Mol Biol 1990; 212(4):737–761. doi:10.1016/0022-2836(90)90234-D
- Erbelding EJ, Post DJ, Stemmy EJ, et al. A universal influenza vaccine: the strategic plan for the National Institute of Allergy and Infectious Diseases, J Infect Dis 2018; 218(3):347–354. doi:10.1093/infdis/jiy103
- Shinde V, Fries L, Wu Y, et al. Improved titers against influenza drift variants with a nanoparticle vaccine. N Engl J Med 2018; 378(24):2346–2348. doi:10.1056/NEJMc1803554
- Maurer MA, Meyer L, Bianchi M, et al. Glycosylation of human IgA directly inhibits influenza A and other sialic-acid-binding viruses. Cell Rep 2018; 23(1):90–99. doi:10.1016/j.celrep.2018.03.027
- Graham BS, Mascola JR, Fauci AS. Novel vaccine technologies: essential components of an adequate response to emerging viral diseases. JAMA 2018; 319(14):1431–1432. doi:10.1001/jama.2018.0345
- Stewart RJ, Flannery B, Chung JR, et al. Influenza antiviral prescribing for outpatients with an acute respiratory illness and at high risk for influenza-associated complications during 5 influenza seasons—United States, 2011–2016. Clin Infect Dis 2018; 66(7):1035–1041. doi:10.1093/cid/cix922
- Zheng S, Tang L, Gao H, et al. Benefit of early initiation of neuraminidase inhibitor treatment to hospitalized patients with avian influenza A(H7N9) virus. Clin Infect Dis 2018; 66(7):1054–1060. doi:10.1093/cid/cix930
- Kumar D, Ferreira VH, Blumberg E, et al. A five-year prospective multi-center evaluation of influenza infection in transplant recipients. Clin Infect Dis 2018. Epub ahead of print. doi:10.1093/cid/ciy294
- Malosh RE, Martin ET, Heikkinen T, Brooks WA, Whitley RJ, Monto AS. Efficacy and safety of oseltamivir in children: systematic review and individual patient data meta-analysis of randomized controlled trials. Clin Infect Dis 2018; 66(10):1492–1500. doi:10.1093/cid/cix1040
- Havers FP, Hicks LA, Chung JR, et al. Outpatient antibiotic prescribing for acute respiratory infections during influenza seasons. JAMA Network Open 2018; 1(2):e180243. doi:10.1001/jamanetworkopen.2018.0243
- US Food and Drug Administration. FDA warns of fraudulent and unapproved flu products. www.fda.gov/newsevents/newsroom/pressannouncements/ucm599223.htm. Accessed October 3, 2018.
- Portsmouth S, Kawaguchi K, Arai M, Tsuchiya K, Uehara T. Cap-dependent endonuclease inhibitor S-033188 for the treatment of influenza: results from a phase 3, randomized, double-blind, placebo- and active-controlled study in otherwise healthy adolescents and adults with seasonal influenza. Open Forum Infect Dis 2017; 4(suppl 1):S734. doi:10.1093/ofid/ofx180.001
- Hayden FG, Sugaya N, Hirotsu N, et al; Baloxavir Marboxil Investigators Group. Baloxavir Marboxil for uncomplicated influenza in adults and adolescents. N Engl J Med 2018; 379(10):913–923. doi:10.1056/NEJMoa1716197
- Kadam RU, Wilson IA. A small-molecule fragment that emulates binding of receptor and broadly neutralizing antibodies to influenza A hemagglutinin. Proc Natl Acad Sci U S A 2018; 115(16):4240–4245. doi:10.1073/pnas.1801999115
KEY POINTS
- Influenza A(H7N9) is a prime candidate to cause the next influenza pandemic.
- Influenza vaccine prevents 300 to 4,000 deaths in the United States every year.
- The 2018–2019 quadrivalent influenza vaccine contains updated A(H3N2) and B/Victoria lineage components different from those in the 2017–2018 Northern Hemisphere vaccine.
- The live-attenuated influenza vaccine, which was not recommended during the 2016–2017 and 2017–2018 influenza seasons, is recommended for the 2018–2019 influenza season.
- Influenza vaccine is recommended any time during pregnancy and is associated with lower infant mortality rates.
- Overall influenza vaccination rates remain below the 80% target for all Americans and 90% for at-risk populations.
Men’s health 2018: BPH, prostate cancer, erectile dysfunction, supplements
Primary care physicians are tasked with a wide variety of issues affecting men. This article reviews the latest research in 4 areas of men’s health commonly addressed in primary care:
- Medical management of benign prostatic hyperplasia (BPH)
- Prostate cancer screening and treatment
- Medical management of erectile dysfunction
- Use of supplements.
MEDICAL MANAGEMENT OF BPH
An 84-year-old man with a history of hypertension, type 2 diabetes, hyperlipidemia, BPH, mild cognitive impairment, and osteoarthritis presents for a 6-month follow-up, accompanied by his son.
Two years ago he was started on a 5-alpha reductase inhibitor and an alpha-blocker for worsening BPH symptoms. His BPH symptoms are currently under control, with an American Urological Association (AUA) symptom index score of 7 of a possible 35 (higher scores being worse).
However, both the patient and son are concerned about the number of medications he is on and wonder if some could be eliminated.
Assessment tools
BPH is a common cause of lower urinary tract symptoms in older men. Evidence-based tools to help the clinician and patient decide on when to consider treatment for symptoms are:
- The AUA symptom index1
- The International Prostate Symptom Score (IPSS).2
An AUA symptom index score or IPSS score of 8 through 19 of a possible 35 is consistent with moderate symptoms, while a score of 20 or higher indicates severe symptoms.
Combination therapy or monotherapy?
Monotherapy with an alpha-blocker or a 5-alpha reductase inhibitor is often the first-line treatment for BPH-related lower urinary tract symptoms.3 However, combination therapy with both an alpha-blocker and a 5-alpha reductase inhibitor is another evidence-based option.
The Medical Therapy of Prostatic Symptoms study,4 a randomized controlled trial, reported that long-term combination therapy reduced the risk of BPH clinical progression better than monotherapy. The same trial also found that either combination therapy or finasteride alone (a 5-alpha reductase inhibitor) reduced the risk of acute urinary retention and the future need for invasive therapy.
Monotherapy after a period of combination therapy?
There is also evidence to support switching from combination to monotherapy after an initial treatment period.
Matsukawa et al5 examined the effects of withdrawing the alpha-blocker from BPH combination therapy in a study in 140 patients. For 12 months, all patients received the alpha-blocker silodosin and the 5-alpha reductase inhibitor dutasteride. At 12 months, the remaining 132 patients (8 patients had been lost to follow-up) were randomized to continue combination therapy or to take dutasteride alone for another 12 months. They were evaluated at 0, 12, and 24 months by questionnaires (the IPSS and Overactive Bladder Symptom Score) and urodynamic testing (uroflowmetry, cystometrography, and pressure-flow studies).
There were no significant differences in subjective symptoms and bladder outlet obstruction between patients who continued combination therapy and those who switched to dutasteride monotherapy. In the monotherapy group, those whose symptoms worsened weighed more (68.8 kg vs 62.6 kg, P =.002) and had a higher body mass index (BMI) (26.2 kg/m2 vs 22.8 kg/m2, P < .001) than those whose symptoms stayed the same or got better.
These findings of successful alpha-blocker withdrawal were consistent with those of other studies.
The Symptom Management After Reducing Therapy study6 showed that 80% of men with an IPSS score less than 20 who changed to dutasteride monotherapy did not have a noticeable worsening of their symptoms.
Baldwin et al7 noted similar success after withdrawing the alpha-blocker doxazosin in patients on finasteride.
Review all medications
The National Health and Nutrition Examination Survey noted that the estimated prevalence of polypharmacy increased from 8% in 1999 to 15% in 2011.8 Many commonly used medications, such as decongestants, antihistamines, and anticholinergic agents, can worsen BPH symptoms,9 so it is reasonable to consistently review the patient’s medications to weigh the risks and benefits and determine which ones align with the patient’s personal care goals.
BPH: Take-home points
- Combination therapy with an alpha-blocker and a 5-alpha reductase inhibitor is an effective regimen for BPH.
- Polypharmacy is a significant problem in the elderly.
- Withdrawing the alpha-blocker component from BPH combination therapy can be considered after 1 year of combination therapy in patients whose symptoms have been well controlled.
PROSTATE CANCER SCREENING AND TREATMENT
A 60-year-old patient calls you after receiving his laboratory testing report from his insurance physical. His prostate-specific antigen (PSA) level is 5.1 ng/mL, and he has several questions:
- Should he have agreed to the screening?
- How effective is the screening?
- What are the next steps?
Is PSA screening useful?
Over the last few years, there has been great debate as to the utility of screening for prostate cancer.
The US Centers for Disease Control and Prevention10 reported that in 2014, an estimated 172,258 men in the United States were diagnosed with prostate cancer, but only 28,343 men died of it. These statistics support the notion that screening programs may be detecting what might otherwise be a silent disease.
The US Preventive Services Task Force (USPSTF)11 recommends against blanket PSA screening, in view of the low probability that it reduces the risk of death from prostate cancer. For men ages 55 through 69, current guidelines give a grade C recommendation to PSA screening, meaning there is moderate agreement that the benefit is likely small, and screening should be selectively offered based on professional judgment and patient preference. In men ages 70 and older who are not at high risk, the guideline gives screening a grade D recommendation, meaning there is moderate evidence that there is no benefit from the practice. This is a change from the 2012 USPSTF guidelines,12 which gave a grade D recommendation to PSA screening for all ages.
The American Urological Association13 recommends against PSA screening in men under age 40 or ages 70 and older. It does not recommend routine screening in those ages 40 to 54 at average risk, but it says the decision should be individualized in this age group in those at higher risk (eg, with a positive family history, African American). At ages 55 through 69, it recommends shared decision-making, taking into account cancer risk and life expectancy. In those who opt for screening, an interval of 2 years or more may be preferred over annual screening to reduce the risk of overdiagnosis.
The USPSTF recommendations rely heavily on data from 2 trials: the European Randomized Study of Screening for Prostate Cancer (ERSPC)14 and the Prostate, Lung, Colorectal, and Ovarian Screening (PLCO) trial.15
The ERSPC14 demonstrated that screening for prostate cancer reduced deaths from prostate cancer by 20%, with an absolute risk difference of 0.71 deaths per 1,000 men; 1,410 men would need to be screened and 48 additional cases of prostate cancer would need to be treated to prevent 1 death from prostate cancer. Screening also decreased the risk of developing metastatic disease by 30%.16 On the negative side, screening increased the risk of overdiagnosis and other harms such as bleeding, sepsis, and incontinence.
The PLCO trial,15 in contrast, found no difference in death rates between men randomly assigned to annual screening and those assigned to usual care. Differences between the trial results were thought to be due to different practice settings as well as study implementation and compliance.
Tsodikov et al17 reanalyzed data from the ERSPC and the PLCO trial using 3 different mathematical models to estimate the effects of screening in both trials compared with no screening. The analysis found no evidence that the effects of screening vs not screening differed between the 2 trials, ultimately concluding that PSA screening reduced prostate cancer deaths by 25% to 32%, which the authors inferred was primarily a result of earlier detection of cancer.
The Cluster Randomized Trial of PSA Testing for Prostate Cancer,18 published in March 2018, explored the effect of single PSA screening vs no screening on prostate cancer mortality rates in 419,582 men ages 50 through 69. Although screening detected more cases of low-risk prostate cancer, there was no significant difference in prostate cancer mortality rates after a median follow-up of 10 years. However, 10% to 15% of the control group was estimated to have also been screened, and these results do not directly speak to the efficacy of serial PSA screening.
Extended follow-up of this trial is planned to report on long-term survival benefits and whether screening lowers the risk of metastasis.
Imaging-guided prostate biopsy
Once a patient is found to have an elevated PSA level, standard practice has been to perform transrectal ultrasonography to obtain 12 core biopsy samples. The results indicate whether the prostate contains cancer, how aggressive the cancer is (Gleason score), and whether there is extracapsular extension.
In the past, magnetic resonance imaging (MRI) of the prostate before biopsy was thought to be too costly, and many insurance plans do not currently cover it.
Pahwa et al,19 however, in a cost-effectiveness study using a decision-analysis model, found that using MRI to detect lesions and then guide biopsy by triaging patients into proper treatment pathways added health benefits in a cost-effective manner in 94.05% of simulations. These benefits were found across all age groups.
This study demonstrated that doctors could use MRI to better evaluate patients for potentially harmful lesions. If a focus of cancer is found, it can be biopsied; if no cancer is seen on MRI, the patient can avoid biopsy completely. Additionally, though MRI tended to miss low-risk cancers, these cancers are thought to disproportionately lead to higher healthcare costs through unnecessary treatment. Therefore, a negative MRI study was believed to be an excellent sign that the patient does not have aggressive prostate cancer. This approach led to a net gain of 0.251 additional quality-adjusted life years compared with the standard biopsy strategy.
The Prostate MRI Imaging Study20 also found MRI to be effective in the prostate cancer workup. In this trial, 576 men who had never undergone biopsy underwent multiparametric MRI, transrectal ultrasonography-guided biopsy, and the reference standard, ie, transperineal template prostate mapping biopsy. Of those who underwent biopsy, 71% received a diagnosis of prostate cancer, and 40% had clinically significant disease. In patients with clinically significant disease, MRI was more sensitive than ultrasonography-guided biopsy (93% vs 48%, P < .0001) but less specific (41% vs 96%, P < .0001).
Based on these findings, if biopsy were performed only in those who had suspicious lesions on MRI, 27% of men with elevated PSA could avoid biopsy and its potential complications such as bleeding and sepsis, which occurred in 5.9% of the biopsy group.
The Prostate Evaluation for Clinically Important Disease: Sampling Using Image Guidance or Not? trial21 more recently studied MRI with or without targeted biopsy vs standard transrectal ultrasonography-guided biopsy in 500 men who had not undergone biopsy before, and reported similar results. MRI with or without biopsy led to fewer biopsies and less overdetection of clinically insignificant prostate cancers compared with the standard approach. Furthermore, those in the MRI-targeted biopsy group were 13% less likely to receive a diagnosis of clinically insignificant cancer than those who received the standard biopsy (adjusted difference −13 percentage points, 95% confidence interval [CI] −19 to −7, P < .001).
Together, these data provide another argument for adding multiparametric MRI to the workup of men with an elevated PSA level.
Surveillance vs treatment for prostate cancer
Once prostate cancer is diagnosed, surveillance is becoming an increasingly common management strategy.
The Prostate Cancer Intervention Versus Observation Trial (PIVOT),22 one of the largest and longest trials involving cancer patients, offered further evidence that active surveillance and less intervention for men with prostate cancer is a better approach in many cases. This trial compared prostatectomy and observation alone in a randomized fashion. Inclusion for the study required men to be medically fit for radical prostatectomy, along with having histologically confirmed localized prostate cancer (stage T1-T2NxM0 in the tumor-node-metastasis classification system) of any grade diagnosed within the last 12 months.
During 19.5 years of follow-up, 223 (61.3%) of the 364 men randomly assigned to radical prostatectomy died, compared with 245 (66.8%) of 367 men in the observation group; the difference was not statistically different (P = .06). Only 9.4% of the deaths were due to prostate cancer, 7.4% in the surgery group and 11.4% in the observation group (P = .06).
Surgery was associated with a lower all-cause mortality rate than observation in the subgroup of patients with intermediate-risk prostate cancer (defined as PSA 10–20 ng/mL and a Gleason score of 7). Surgery was also associated with less disease progression.22
This finding is in line with previous data from the Scandinavian Prostate Cancer Group Study Number 4,23 as well as the much larger Prostate Testing for Cancer and Treatment (ProtecT) trial,24 both of which reported that metastasis was 1.5 and 2.6 times as common, respectively, in participants in the active surveillance groups. However, in the PIVOT trial, those in the surgery group were significantly more likely than those in the observation group to have erectile dysfunction and urinary incontinence at 10 years.
Therefore, in men with localized disease and in those with low-risk PSA levels, both the PIVOT and ProtecT trials suggest that death from prostate cancer is uncommon and that observation may be more appropriate.
Prostate cancer: Take-home points
- A new look at 2 large trials of PSA screening strengthened evidence that testing in the right patient population can reduce deaths from prostate cancer, but a third recently published trial that found no benefit from 1-time screening may reopen debate on the topic.
- MRI offers a better method than ultrasonography-guided biopsy to triage patients thought to be at high risk of prostate cancer and tends to limit costly overtreatment of disease that likely would not cause death.
- Surgery for prostate cancer may not prolong life but could reduce disease progression, at the risk of more adverse effects.
- Shared decision-making should be practiced when deciding whether to use active surveillance or active treatment of diagnosed prostate cancer.
MANAGEMENT OF ERECTILE DYSFUNCTION
A 62-year-old man with hypertension, hyperlipidemia, peripheral artery disease, and type 2 diabetes presents for a 6-month follow-up. His medications include aspirin, metformin, lisinopril, and atorvastatin, all of which he takes without problems. Over the past several months, he has noticed that his erections are not adequate for sexual intercourse. He recently heard that a generic version of sildenafil has just become available, and he wonders if it might benefit him.
Erectile dysfunction is common, associated with chronic diseases
Erectile dysfunction, ie, persistent inability to obtain and maintain an erection sufficient to permit satisfactory sexual intercourse,25,26 is estimated to affect nearly 20% of men over the age of 20 and 75% of men over the age of 75.27
In age-adjusted models, erectile dysfunction has been shown28 to be associated with:
- History of cardiovascular disease (odds ratio [OR] 1.63, 95% CI 1.02–2.63)
- Diabetes (OR 3.90, 95% CI 2.16–7.04)
- Treated hypertension vs no hypertension (OR 2.22, 95% CI 1.30–3.80)
- Current smoking vs never smoking (OR 1.63, 95% CI 1.01–2.62)
- BMI greater than 30 kg/m2 vs less than 25 kg/m2 (OR 1.80, 95% CI 1.03–3.14).
Because of the strong association between cardiovascular disease and erectile dysfunction, the presence of one often suggests the need to screen for the other.29 While tools such as the International Index of Erectile Function (IIEF-5) have been developed to evaluate erectile dysfunction, it is most often diagnosed on the basis of clinical impression, while validated assessment methods are reserved for clinical trials.28
Multiple causes of erectile dysfunction
Erectile dysfunction arises from inadequate penile tissue response to a sexual signal. The response can be disrupted at several points. For example, damage to vascular smooth muscle cells (eg, from age or obesity) and endothelial cells (from smoking or diabetes) and narrowing of the vascular lumen (from atherosclerosis or hypertension) have all been shown to impair engorgement of the corpus cavernosum.30 In addition, denervation from prostate surgery or spinal trauma and psychogenic causes should be recognized in discussions with patients.
Drugs for erectile dysfunction
Pharmacologic management of erectile dysfunction includes oral, sublingual, intracavernosal, and intraurethral therapies.31 Treatment in primary care settings usually includes addressing underlying chronic diseases32 and prescribing phosphodiesterase-5 inhibitors (sildenafil, tadalafil, vardenafil, and avanafil). These drugs work by increasing local concentrations of cyclic guanosine monophosphate in the corpus cavernosum to induce vasodilation.33
While these 4 drugs are still patent-protected, a manufacturer has been allowed to introduce a generic version of sildenafil into US markets, and a generic version of tadalafil is expected to be available soon.
Sildenafil, tadalafil, and vardenafil have been studied and found to have some degree of effectiveness in erectile dysfunction caused by damage to the penile vasculature, denervation, and spinal cord injury.34 All drugs of this class have adverse effects including headache, facial flushing, and nasal congestion, but the drugs are generally well tolerated.35
Sildenafil and tadalafil improve IIEF-5 scores by a similar margin, raising scores on the erectile domain subsection from approximately 14 of a possible 30 to approximately 24 of 30 in a trial of both drugs.36 However, multiple crossover studies comparing the 2 drugs have shown that nearly 75% of patients prefer tadalafil to sildenafil,36,37 perhaps because of tadalafil’s longer duration of action.34
There is little evidence to suggest that vardenafil is more effective or more often preferred by patients than tadalafil or sidenafil.34,38 And though data on the newest drug on the market, avanafil, are limited, a meta-analysis concluded that it may be less effective than tadalafil and without significant differences in terms of safety.39
Other treatments
Lifestyle modifications, especially smoking cessation and exercise, have been shown to reduce the risk of erectile dysfunction with varying effect sizes across studies.40–42 Moreover, factors such as obesity, alcohol use, and smoking may cause irreversible harm, and thus a healthy lifestyle should be encouraged.41
While there is only weak evidence for the use of psychological interventions alone for treating most types of erectile dysfunction, one meta-analysis found that the combination of psychological intervention and a phosphodiesterase-5 inhibitor improved sexual satisfaction more than drug therapy alone.43
Erectile dysfunction: Take-home points
- Erectile dysfunction is common, affecting nearly 20% of men over the age of 20 and over 75% of men over the age of 75.
- Erectile dysfunction is often associated with chronic disease and may suggest the need to screen for cardiovascular disease.
- Treating underlying chronic diseases may help, and phosphodiesterase-5 inhibitors are effective; tadalafil may be most often preferred.
SUPPLEMENT USE AND MEN’S HEALTH
A 68-year-old man with a history of hypertension, BPH, and erectile dysfunction presents for a 6-month follow-up. His medication use includes lisinopril, which he takes without problems. He denies any new physical symptoms. His physical examination is unremarkable. He says he has heard about supplements that might help with his sexual performance and hopes to discuss recommendations during the visit.
A burgeoning, unregulated industry
Since the passage of the Dietary Supplement and Health Education Act in 1994, a law that decreased oversight of the supplement industry, spending on supplements has skyrocketed to over $41.1 billion each year.44 Advertisements for these products typically claim that they improve general mental and physical health, sexual and romantic performance, leanness, and muscularity.45 A national survey of men ages 57 and older reported that the most popular products were aimed at nutrition (such as multivitamins), cardiovascular health (such as omega-3 fatty acids), and chronic conditions (such as saw palmetto for BPH).46
Little evidence of efficacy
There is little evidence to support the use of most supplements to improve men’s health. For example, a study in 82,405 men found no association between mortality rates and multivitamin use (hazard ratio [HR] 1.07, 95% CI 0.96–1.19).47 Even for specific uses, such as cognitive performance, randomized trials exploring the effects of multivitamins in men have been largely negative.48
The positive trials that have been reported are often of low quality and are funded by supplement manufacturers. For example, one of the few trials that reported a positive association between multivitamin supplementation and cognition in men was underpowered (N = 51) and found improvement in only 1 of 19 cognitive domains.49 Despite the poor design and results to the contrary, this industry-funded study nevertheless concluded that multivitamins may play a role in improving elements of memory.
Evidence of possible harm from antioxidants
While not always specific to men, many meta-analyses have explored the effects of antioxidant supplements on cardiovascular and mortality risk. Most of them concluded that antioxidant supplements have no benefit and that some may actually be harmful.
For example, multiple meta-analyses of vitamin E supplementation found no cardiovascular benefit but possible increases in all-cause mortality rates in those taking high doses (risk ratio 1.04, 95% CI 1.01–1.07).50,51
Another meta-analysis of 180,938 participants in high-quality studies found an increased risk of all-cause mortality associated with independent intake of several antioxidant vitamins, including beta-carotene (risk ratio 1.07, 95% CI 1.02–1.11) and vitamin A (risk ratio 1.16, 95% CI 1.10–1.24), while intake of vitamin C and selenium had no impact on mortality.52
Similarly, although nearly 10% of US adults report taking omega-3 fatty acid supplements, a review of 24 randomized controlled trials and meta-analyses published between 2005 and 2012 concluded that only 2 supported the use of these supplements for any health benefit.53
Can supplements improve sexual function, prostate health?
To improve sexual function. A 2015 narrative review of the ingredients in General Nutrition Center’s top 30 best-selling products targeted at improving men’s sexual performance (including improving libido and erectile dysfunction) found only poor evidence for any efficacy.54 The few studies that did support the use of select supplements, including B vitamins in people with diabetes, L-arginine, and yohimbine, were deemed to be of poor quality or showed a smaller effect size compared with standard medical therapy.
To prevent prostate cancer. Studies of supplement use to improve prostate health have had mixed results. For example, multiple large case-control studies have suggested that taking vitamin D55,56 or vitamin C57 is not associated with prostate cancer risk, while increased vitamin A58,59 and E60,61 intake is associated with inconsistent increases in prostate cancer risk.
In the Selenium and Vitamin E Cancer Prevention Trial,62 a randomized controlled trial in 35,533 men, those assigned to receive vitamin E supplementation were 17% more likely to get prostate cancer than were those assigned to placebo (HR 1.17, 99% CI 1.004–1.36, P = .008).
However, there are plausible biologic links between nutraceuticals and prostate cancer. For example, studies have linked genetic polymorphisms in vitamin D receptors63 as well as intake of natural androgen receptor modulators, such as the most active polyphenol in green tea,64 to prostate cancer risk and aggressiveness in certain populations. This led a recent review to conclude that there is some biologic plausibility, but at present little epidemiologic evidence, to support any dietary supplement’s ability to broadly affect prostate cancer risk.65
Interest continues in exploring the targeted use of nutraceuticals as adjuvant therapy in specific populations at risk of prostate cancer.66,67
To treat BPH. There is a similar dearth of clinical or population-based evidence that supplements can broadly affect BPH symptoms. For example, in a 2012 Cochrane review of Serenoa repens (saw palmetto) utilizing only high-quality evidence, there was no evidence that supplement use significantly reduced lower urinary tract symptoms, nocturia, or peak urine flow in BPH patients, and this was true even when the supplement was taken at triple-strength doses.68
For other diseases. There is also limited evidence that supplements can affect other chronic diseases. For example, a meta-analysis of 3,803 patients found that glucosamine, chondroitin, and their combination had no impact on joint pain or joint space narrowing in patients with osteoarthritis of the knee or hip.69
Even when there is some evidence to suggest benefit from supplementation, study heterogeneity and varying evidence quality limit confidence in the conclusions. For example, meta-analyses suggest garlic may improve blood pressure control in those with hypertension70 and improve lipid and blood glucose control in type 2 diabetes.71 However, most of the trials included in those systematic reviews were underpowered, with samples as low as 10 patients, and many suffered from improper design, such as inadequate blinding of researchers. In addition, these meta-analyses often do not report adverse events, suggesting that higher quality studies would be needed to adequately measure event rates. As such, there is need for caution and a case-by-case review before recommending even a seemingly benign supplement like garlic to patients.
In total, there is only limited evidence to support the efficacy of supplements across many diseases and concerns common to men in primary care. This includes improving general health, cardiovascular health, sexual functioning, or other chronic diseases. While a supplement’s placebo effect may at times provide some benefit, supplements are much less strictly regulated since the passing of the 1994 act, and even vitamin supplementation has been shown to be associated with negative health outcomes. As such, a patient’s use of supplements requires careful consideration and shared decision-making.
Supplements: Take-home points
- Supplements are only loosely regulated by the federal government.
- There is some biologic but limited epidemiologic evidence for the use of multivitamins to improve cognition or mortality rates; for the use of antioxidant vitamins or omega-3 fatty acids to improve cardiovascular health; for the use of any of the top-selling sexual enhancement supplements to improve libido or erectile function; and for the use of vitamins or other supplements for improving BPH or reducing prostate cancer risk. Using supplements may in some cases be harmful.
- Given the heterogeneity of studies of supplements to manage chronic diseases and a lack of reporting of adverse events, careful consideration is needed when recommending supplements to patients.
- Barry MJ, Fowler FJ Jr, O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. J Urol 2017; 197(2S):S189–S197. doi:10.1016/j.juro.2016.10.071
- Urological Sciences Research Foundation. International Prostate Symptom Score (IPSS). http://www.usrf.org/questionnaires/AUA_SymptomScore.html. Accessed October 16, 2018.
- McVary KT, Roehrborn CG, Avins AL, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol 2011; 185(5):1793–1803. doi:10.1016/j.juro.2011.01.074
- McConnell JD, Roehrborn CG, Bautista OM, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med 2003; 349(25):2387–2398. doi:10.1056/NEJMoa030656
- Matsukawa Y, Takai S, Funahashi Y, et al. Effects of withdrawing alpha-1 blocker from the combination therapy with alpha-1 blocker and 5-alpha-reductase inhibitor in patients with lower urinary tract symptoms suggestive of benign prostatic hyperplasia: a prospective and comparative trial using urodynamics. J Urol 2017; 198(4):905–912. doi:10.1016/j.juro.2017.05.031
- Barkin J, Guimaraes M, Jacobi G, Pushkar D, Taylor S, van Vierssen Trip OB. Alpha-blocker therapy can be withdrawn in the majority of men following initial combination therapy with the dual 5a-reductase inhibitor dutasteride. Eur Urol 2003; 44(4):461–466. pmid:14499682
- Baldwin KC, Ginsberg PC, Roehrborn CG, Harkaway RC. Discontinuation of alpha-blockade after initial treatment with finasteride and doxazosin in men with lower urinary tract symptoms and clinical evidence of benign prostatic hyperplasia. Urology 2001; 58(2):203–209. pmid:11489700
- Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA 2015; 314(17):1818–1831. doi:10.1001/jama.2015.13766
- DuBeau CE, Yalla SV, Resnick NM. Improving the utility of urine flow rate to exclude outlet obstruction in men with voiding symptoms. J Am Geriatr Soc 1998; 46(9):1118–1124. pmid:9736105
- US Department of Health and Human Services Health Resources and Services Administration. United States Cancer Statistics: 1999-2014 Incidence and Mortality Web-Based Report. Atlanta; 2017. https://nccd.cdc.gov/uscs/. Accessed October 17, 2018.
- US Preventive Services Task Force. Final recommendation statement. Prostate cancer: screening. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening1. Accessed October 16, 2018.
- US Preventive Services Task Force. Archived: prostate cancer: screening. Original release date: May 2012. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/prostate-cancer-screening. Accessed October 16, 2018.
- Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA guideline. J Urol 2013; 190(2):419–426. doi:10.1016/j.juro.2013.04.119
- Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 2009; 360(13):1320–1328. doi:10.1056/NEJMoa0810084
- Andriole GL, Crawford ED, Grubb RL, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med 2009; 360(13):1310–1319. doi:10.1056/NEJMoa0810696
- Schröder FH, Hugosson J, Carlsson S, et al. Screening for prostate cancer decreases the risk of developing metastatic disease: findings from the European Randomized Study of Screening for Prostate Cancer (ERSPC). Eur Urol 2012; 62(5):745–752. doi:10.1016/j.eururo.2012.05.068
- Tsodikov A, Gulati R, Heijnsdijk EAM, et al. Reconciling the effects of screening on prostate cancer mortality in the ERSPC and PLCO trials. Ann Intern Med 2017; 167(7):449–455. doi:10.7326/M16-2586
- Martin RM, Donovan JL, Turner EL, et al. Effect of a low-intensity PSA-based screening intervention on prostate cancer mortality: the CAP randomized clinical trial. JAMA 2018; 319(9):883–895. doi:10.1001/jama.2018.0154
- Pahwa S, Schiltz NK, Ponsky LE, Lu Z, Griswold MA, Gulani V. Cost-effectiveness of MR imaging–guided strategies for detection of prostate cancer in biopsy-naive men. Radiology 2017; 285(1):157–166. doi:10.1148/radiol.2017162181
- Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017; 389(10071):815–822. doi:10.1016/S0140-6736(16)32401-1
- Kasivisvanathan V, Rannikko AS, Borghi M, et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med 2018; 378(19):1767–1777. doi:10.1056/NEJMoa1801993
- Wilt TJ, Jones KM, Barry MJ, et al. Follow-up of prostatectomy versus observation for early prostate cancer. N Engl J Med 2017; 377(2):132–142. doi:10.1056/NEJMoa1615869
- Bill-Axelson A, Holmberg L, Garmo H, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med 2014; 370(10):932–942. doi:10.1056/NEJMoa1311593
- Hamdy FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med 2016; 375(15):1415–1424. doi:10.1056/NEJMoa1606220
- Morley JE. Impotence. Am J Med 1986; 80(5):897–905. pmid:3518438
- NIH Consensus Development Panel on Impotence. NIH Consensus Conference. Impotence. JAMA 1993; 270(1):83–90. pmid:8510302
- Selvin E, Burnett AL, Platz EA. Prevalence and risk factors for erectile dysfunction in the US. Am J Med 2007; 120(2):151–157. doi:10.1016/j.amjmed.2006.06.010
- Rosen RC, Cappelleri JC, Gendrano N 3rd. The International Index of Erectile Function (IIEF): a state-of-the-science review. Int J Impot Res 2002; 14(4):226–244. doi:10.1038/sj.ijir.3900857
- Gandaglia G, Briganti A, Jackson G, et al. A systematic review of the association between erectile dysfunction and cardiovascular disease. Eur Urol 2014; 65(5):968–978. doi:10.1016/j.eururo.2013.08.023
- Heaton JPW, Adams MA. Causes of erectile dysfunction. Endocrine 2004; 23(2-3):119–123. doi:10.1385/ENDO:23:2-3:119
- Montorsi F, Salonia A, Deho F, et al. Pharmacological management of erectile dysfunction. BJU Int 2003; 91(5):446–454. pmid:12603396
- Cai X, Tian Y, Wu T, Cao CX, Bu SY, Wang KJ. The role of statins in erectile dysfunction: a systematic review and meta-analysis. Asian J Androl 2014; 16(3):461–466. doi:10.4103/1008-682X.123678
- Webb DJ, Freestone S, Allen MJ, Muirhead GJ. Sildenafil citrate and blood-pressure–lowering drugs: results of drug interaction studies with an organic nitrate and a calcium antagonist. Am J Cardiol 1999; 83(5):21C–28C. pmid:10078539
- Doggrell SA. Comparison of clinical trials with sildenafil, vardenafil and tadalafil in erectile dysfunction. Expert Opin Pharmacother 2005; 6(1):75–84. doi:10.1517/14656566.6.1.75
- Gresser U, Gleiter CH. Erectile dysfunction: comparison of efficacy and side effects of the PDE-5 inhibitors sildenafil, vardenafil and tadalafil—review of the literature. Eur J Med Res 2002; 7(10):435–446. pmid:12435622
- Eardley I, Mirone V, Montorsi F, et al. An open-label, multicentre, randomized, crossover study comparing sildenafil citrate and tadalafil for treating erectile dysfunction in men naive to phosphodiesterase 5 inhibitor therapy. BJU Int 2005; 96(9):1323–1332. doi:10.1111/j.1464-410X.2005.05892.x
- von Keitz A, Rajfer J, Segal S, et al. A multicenter, randomized, double-blind, crossover study to evaluate patient preference between tadalafil and sildenafil. Eur Urol 2004; 45(4):499–509. doi:10.1016/j.eururo.2003.11.030
- Martin-Morales A, Haro JM, Beardsworth A, Bertsch J, Kontodimas S; EDOS Group. Therapeutic effectiveness and patient satisfaction after 6 months of treatment with tadalafil, sildenafil, and vardenafil: results from the erectile dysfunction observational study (EDOS). Eur Urol 2007; 51(2):541–550. doi:10.1016/j.eururo.2006.09.027
- Yuan J, Zhang R, Yang Z, et al. Comparative effectiveness and safety of oral phosphodiesterase type 5 inhibitors for erectile dysfunction: a systematic review and network meta-analysis. Eur Urol 2013; 63(5):902–912. doi:10.1016/j.eururo.2013.01.012
- Cao S, Yin X, Wang Y, Zhou H, Song F, Lu Z. Smoking and risk of erectile dysfunction: systematic review of observational studies with meta-analysis. PLoS One 2013; 8(4):e60443. doi:10.1371/journal.pone.0060443
- Derby CA, Mohr BA, Goldstein I, Feldman HA, Johannes CB, McKinlay JB. Modifiable risk factors and erectile dysfunction: can lifestyle changes modify risk? Urology 2000; 56(2):302–306. pmid:10925098
- Esposito K, Giugliano F, Di Palo C, et al. Effect of lifestyle changes on erectile dysfunction in obese men: a randomized controlled trial. JAMA 2004; 291(24):2978–2984. doi:10.1001/jama.291.24.2978
- Schmidt HM, Munder T, Gerger H, Frühauf S, Barth J. Combination of psychological intervention and phosphodiesterase-5 inhibitors for erectile dysfunction: a narrative review and meta-analysis. J Sex Med 2014; 11(6):1376–1391. doi:10.1111/jsm.12520
- New Hope Network. Supplement Business Report 2017. Boulder; 2017. http://images.info.newhope.com/Web/NewHopeNaturalMedia/%7B3a3f3b03-6130-41d4-9e66-84f29eeebe44%7D_2017_Supplement_Business_Report_-_Extended_TOC.pdf. Accessed October 16, 2018.
- Labre MP. Burn fat, build muscle: a content analysis of men’s health and men’s fitness. Int J Mens Health 2005; 4(2):187–200.
- Qato DM, Alexander GC, Conti RM, Johnson M, Schumm P, Lindau ST. Use of prescription and over-the-counter medications and dietary supplements among older adults in the United States. JAMA 2008; 300(24):2867–2878. doi:10.1001/jama.2008.892
- Park SY, Murphy SP, Wilkens LR, Henderson BE, Kolonel LN. Multivitamin use and the risk of mortality and cancer incidence: the multiethnic cohort study. Am J Epidemiol 2011; 173(8):906–914. doi:10.1093/aje/kwq447
- McNeill G, Avenell A, Campbell MK, et al. Effect of multivitamin and multimineral supplementation on cognitive function in men and women aged 65 years and over: a randomised controlled trial. Nutr J 2007; 6(1):10. doi:10.1186/1475-2891-6-10
- Harris E, Macpherson H, Vitetta L, Kirk J, Sali A, Pipingas A. Effects of a multivitamin, mineral and herbal supplement on cognition and blood biomarkers in older men: a randomised, placebo-controlled trial. Hum Psychopharmacol Clin Exp 2012; 27(4):370–377. doi:10.1002/hup.2236
- Vivekananthan DP, Penn MS, Sapp SK, Hsu A, Topol EJ. Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Lancet 2003; 361(9374):2017–2023. doi:10.1016/S0140-6736(03)13637-9
- Miller ER, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med 2005; 142(1):37–46. pmid:15537682
- Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA 2007; 297(8):842–857. doi:10.1001/jama.297.8.842
- Grey A, Bolland M. Clinical trial evidence and use of fish oil supplements. JAMA Intern Med 2014; 174(3):460–462. doi:10.1001/jamainternmed.2013.12765
- Cui T, Kovell RC, Brooks DC, Terlecki RP. A urologist’s guide to ingredients found in top-selling nutraceuticals for men’s sexual health. J Sex Med 2015; 12(11):2105–2117. doi:10.1111/jsm.13013
- Schenk JM, Till CA, Tangen CM, et al. Serum 25-hydroxyvitamin D concentrations and risk of prostate cancer: results from the Prostate Cancer Prevention Trial. Cancer Epidemiol Prev Biomarkers 2014; 23(8):1484–1493. doi:10.1158/1055-9965.EPI-13-1340
- Albanes D, Mondul AM, Yu K, et al. Serum 25-hydroxy vitamin D and prostate cancer risk in a large nested case-control study. Cancer Epidemiol Prev Biomarkers 2011; 20(9):1850–1860. doi:10.1158/1055-9965.EPI-11-0403
- Roswall N, Larsen SB, Friis S, et al. Micronutrient intake and risk of prostate cancer in a cohort of middle-aged, Danish men. Cancer Causes Control 2013; 24(6):1129–1135. doi:10.1007/s10552-013-0190-4
- Mondul AM, Watters JL, Männistö S, et al. Serum retinol and risk of prostate cancer. Am J Epidemiol 2011; 173(7):813-821. doi:10.1093/aje/kwq429
- Schenk JM, Riboli E, Chatterjee N, et al. Serum retinol and prostate cancer risk: a nested case-control study in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol Prev Biomarkers 2009; 18(4):1227–1231. doi:10.1158/1055-9965.EPI-08-0984
- Bidoli E, Talamini R, Zucchetto A, et al. Dietary vitamins E and C and prostate cancer risk. Acta Oncol 2009; 48(6):890–894. doi:10.1080/02841860902946546
- Wright ME, Weinstein SJ, Lawson KA, et al. Supplemental and dietary vitamin E intakes and risk of prostate cancer in a large prospective study. Cancer Epidemiol Prev Biomarkers 2007; 16(6):1128–1135. doi:10.1158/1055-9965.EPI-06-1071
- Klein EA, Thompson IM, Tangen CM, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011; 306(14):1549–1556. doi:10.1001/jama.2011.1437
- Jingwi EY, Abbas M, Ricks-Santi L, et al. Vitamin D receptor genetic polymorphisms are associated with PSA level, Gleason score and prostate cancer risk in African-American men. Anticancer Res 2015; 35(3):1549–1558. pmid:25750310
- Siddiqui IA, Asim M, Hafeez BB, Adhami VM, Tarapore RS, Mukhtar H. Green tea polyphenol EGCG blunts androgen receptor function in prostate cancer. FASEB J 2011; 25(4):1198–1207. doi:10.1096/fj.10-167924
- Yacoubian A, Dargham RA, Khauli RB, Bachir BG. Overview of dietary supplements in prostate cancer. Curr Urol Rep 2016; 17(11):78. doi:10.1007/s11934-016-0637-8
- Kallifatidis G, Hoy JJ, Lokeshwar BL. Bioactive natural products for chemoprevention and treatment of castration-resistant prostate cancer. Semin Cancer Biol 2016; 40:160–169. doi:10.1016/j.semcancer.2016.06.003
- Shui IM, Mondul AM, Lindström S, et al. Circulating vitamin D, vitamin D–related genetic variation, and risk of fatal prostate cancer in the National Cancer Institute Breast and Prostate Cancer Cohort Consortium. Cancer 2015; 121(12):1949–1956. doi:10.1002/cncr.29320
- Tacklind J, MacDonald R, Rutks I, Stanke JU, Wilt TJ. Serenoa repens for benign prostatic hyperplasia. Cochrane Database Syst Rev 2012; 12:CD001423. doi:10.1002/14651858.CD001423.pub3
- Wandel S, Jüni P, Tendal B, et al. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis. BMJ 2010; 341:c4675. doi:10.1136/bmj.c4675
- Reinhart KM, Coleman CI, Teevan C, Vachhani P, White CM. Effects of garlic on blood pressure in patients with and without systolic hypertension: a meta-analysis. Ann Pharmacother 2008; 42(12):1766–1771. doi:10.1345/aph.1L319
- Wang J, Zhang X, Lan H, Wang W. Effect of garlic supplement in the management of type 2 diabetes mellitus (T2DM): a meta-analysis of randomized controlled trials. Food Nutr Res 2017; 61(1):1377571. doi:10.1080/16546628.2017.1377571
Primary care physicians are tasked with a wide variety of issues affecting men. This article reviews the latest research in 4 areas of men’s health commonly addressed in primary care:
- Medical management of benign prostatic hyperplasia (BPH)
- Prostate cancer screening and treatment
- Medical management of erectile dysfunction
- Use of supplements.
MEDICAL MANAGEMENT OF BPH
An 84-year-old man with a history of hypertension, type 2 diabetes, hyperlipidemia, BPH, mild cognitive impairment, and osteoarthritis presents for a 6-month follow-up, accompanied by his son.
Two years ago he was started on a 5-alpha reductase inhibitor and an alpha-blocker for worsening BPH symptoms. His BPH symptoms are currently under control, with an American Urological Association (AUA) symptom index score of 7 of a possible 35 (higher scores being worse).
However, both the patient and son are concerned about the number of medications he is on and wonder if some could be eliminated.
Assessment tools
BPH is a common cause of lower urinary tract symptoms in older men. Evidence-based tools to help the clinician and patient decide on when to consider treatment for symptoms are:
- The AUA symptom index1
- The International Prostate Symptom Score (IPSS).2
An AUA symptom index score or IPSS score of 8 through 19 of a possible 35 is consistent with moderate symptoms, while a score of 20 or higher indicates severe symptoms.
Combination therapy or monotherapy?
Monotherapy with an alpha-blocker or a 5-alpha reductase inhibitor is often the first-line treatment for BPH-related lower urinary tract symptoms.3 However, combination therapy with both an alpha-blocker and a 5-alpha reductase inhibitor is another evidence-based option.
The Medical Therapy of Prostatic Symptoms study,4 a randomized controlled trial, reported that long-term combination therapy reduced the risk of BPH clinical progression better than monotherapy. The same trial also found that either combination therapy or finasteride alone (a 5-alpha reductase inhibitor) reduced the risk of acute urinary retention and the future need for invasive therapy.
Monotherapy after a period of combination therapy?
There is also evidence to support switching from combination to monotherapy after an initial treatment period.
Matsukawa et al5 examined the effects of withdrawing the alpha-blocker from BPH combination therapy in a study in 140 patients. For 12 months, all patients received the alpha-blocker silodosin and the 5-alpha reductase inhibitor dutasteride. At 12 months, the remaining 132 patients (8 patients had been lost to follow-up) were randomized to continue combination therapy or to take dutasteride alone for another 12 months. They were evaluated at 0, 12, and 24 months by questionnaires (the IPSS and Overactive Bladder Symptom Score) and urodynamic testing (uroflowmetry, cystometrography, and pressure-flow studies).
There were no significant differences in subjective symptoms and bladder outlet obstruction between patients who continued combination therapy and those who switched to dutasteride monotherapy. In the monotherapy group, those whose symptoms worsened weighed more (68.8 kg vs 62.6 kg, P =.002) and had a higher body mass index (BMI) (26.2 kg/m2 vs 22.8 kg/m2, P < .001) than those whose symptoms stayed the same or got better.
These findings of successful alpha-blocker withdrawal were consistent with those of other studies.
The Symptom Management After Reducing Therapy study6 showed that 80% of men with an IPSS score less than 20 who changed to dutasteride monotherapy did not have a noticeable worsening of their symptoms.
Baldwin et al7 noted similar success after withdrawing the alpha-blocker doxazosin in patients on finasteride.
Review all medications
The National Health and Nutrition Examination Survey noted that the estimated prevalence of polypharmacy increased from 8% in 1999 to 15% in 2011.8 Many commonly used medications, such as decongestants, antihistamines, and anticholinergic agents, can worsen BPH symptoms,9 so it is reasonable to consistently review the patient’s medications to weigh the risks and benefits and determine which ones align with the patient’s personal care goals.
BPH: Take-home points
- Combination therapy with an alpha-blocker and a 5-alpha reductase inhibitor is an effective regimen for BPH.
- Polypharmacy is a significant problem in the elderly.
- Withdrawing the alpha-blocker component from BPH combination therapy can be considered after 1 year of combination therapy in patients whose symptoms have been well controlled.
PROSTATE CANCER SCREENING AND TREATMENT
A 60-year-old patient calls you after receiving his laboratory testing report from his insurance physical. His prostate-specific antigen (PSA) level is 5.1 ng/mL, and he has several questions:
- Should he have agreed to the screening?
- How effective is the screening?
- What are the next steps?
Is PSA screening useful?
Over the last few years, there has been great debate as to the utility of screening for prostate cancer.
The US Centers for Disease Control and Prevention10 reported that in 2014, an estimated 172,258 men in the United States were diagnosed with prostate cancer, but only 28,343 men died of it. These statistics support the notion that screening programs may be detecting what might otherwise be a silent disease.
The US Preventive Services Task Force (USPSTF)11 recommends against blanket PSA screening, in view of the low probability that it reduces the risk of death from prostate cancer. For men ages 55 through 69, current guidelines give a grade C recommendation to PSA screening, meaning there is moderate agreement that the benefit is likely small, and screening should be selectively offered based on professional judgment and patient preference. In men ages 70 and older who are not at high risk, the guideline gives screening a grade D recommendation, meaning there is moderate evidence that there is no benefit from the practice. This is a change from the 2012 USPSTF guidelines,12 which gave a grade D recommendation to PSA screening for all ages.
The American Urological Association13 recommends against PSA screening in men under age 40 or ages 70 and older. It does not recommend routine screening in those ages 40 to 54 at average risk, but it says the decision should be individualized in this age group in those at higher risk (eg, with a positive family history, African American). At ages 55 through 69, it recommends shared decision-making, taking into account cancer risk and life expectancy. In those who opt for screening, an interval of 2 years or more may be preferred over annual screening to reduce the risk of overdiagnosis.
The USPSTF recommendations rely heavily on data from 2 trials: the European Randomized Study of Screening for Prostate Cancer (ERSPC)14 and the Prostate, Lung, Colorectal, and Ovarian Screening (PLCO) trial.15
The ERSPC14 demonstrated that screening for prostate cancer reduced deaths from prostate cancer by 20%, with an absolute risk difference of 0.71 deaths per 1,000 men; 1,410 men would need to be screened and 48 additional cases of prostate cancer would need to be treated to prevent 1 death from prostate cancer. Screening also decreased the risk of developing metastatic disease by 30%.16 On the negative side, screening increased the risk of overdiagnosis and other harms such as bleeding, sepsis, and incontinence.
The PLCO trial,15 in contrast, found no difference in death rates between men randomly assigned to annual screening and those assigned to usual care. Differences between the trial results were thought to be due to different practice settings as well as study implementation and compliance.
Tsodikov et al17 reanalyzed data from the ERSPC and the PLCO trial using 3 different mathematical models to estimate the effects of screening in both trials compared with no screening. The analysis found no evidence that the effects of screening vs not screening differed between the 2 trials, ultimately concluding that PSA screening reduced prostate cancer deaths by 25% to 32%, which the authors inferred was primarily a result of earlier detection of cancer.
The Cluster Randomized Trial of PSA Testing for Prostate Cancer,18 published in March 2018, explored the effect of single PSA screening vs no screening on prostate cancer mortality rates in 419,582 men ages 50 through 69. Although screening detected more cases of low-risk prostate cancer, there was no significant difference in prostate cancer mortality rates after a median follow-up of 10 years. However, 10% to 15% of the control group was estimated to have also been screened, and these results do not directly speak to the efficacy of serial PSA screening.
Extended follow-up of this trial is planned to report on long-term survival benefits and whether screening lowers the risk of metastasis.
Imaging-guided prostate biopsy
Once a patient is found to have an elevated PSA level, standard practice has been to perform transrectal ultrasonography to obtain 12 core biopsy samples. The results indicate whether the prostate contains cancer, how aggressive the cancer is (Gleason score), and whether there is extracapsular extension.
In the past, magnetic resonance imaging (MRI) of the prostate before biopsy was thought to be too costly, and many insurance plans do not currently cover it.
Pahwa et al,19 however, in a cost-effectiveness study using a decision-analysis model, found that using MRI to detect lesions and then guide biopsy by triaging patients into proper treatment pathways added health benefits in a cost-effective manner in 94.05% of simulations. These benefits were found across all age groups.
This study demonstrated that doctors could use MRI to better evaluate patients for potentially harmful lesions. If a focus of cancer is found, it can be biopsied; if no cancer is seen on MRI, the patient can avoid biopsy completely. Additionally, though MRI tended to miss low-risk cancers, these cancers are thought to disproportionately lead to higher healthcare costs through unnecessary treatment. Therefore, a negative MRI study was believed to be an excellent sign that the patient does not have aggressive prostate cancer. This approach led to a net gain of 0.251 additional quality-adjusted life years compared with the standard biopsy strategy.
The Prostate MRI Imaging Study20 also found MRI to be effective in the prostate cancer workup. In this trial, 576 men who had never undergone biopsy underwent multiparametric MRI, transrectal ultrasonography-guided biopsy, and the reference standard, ie, transperineal template prostate mapping biopsy. Of those who underwent biopsy, 71% received a diagnosis of prostate cancer, and 40% had clinically significant disease. In patients with clinically significant disease, MRI was more sensitive than ultrasonography-guided biopsy (93% vs 48%, P < .0001) but less specific (41% vs 96%, P < .0001).
Based on these findings, if biopsy were performed only in those who had suspicious lesions on MRI, 27% of men with elevated PSA could avoid biopsy and its potential complications such as bleeding and sepsis, which occurred in 5.9% of the biopsy group.
The Prostate Evaluation for Clinically Important Disease: Sampling Using Image Guidance or Not? trial21 more recently studied MRI with or without targeted biopsy vs standard transrectal ultrasonography-guided biopsy in 500 men who had not undergone biopsy before, and reported similar results. MRI with or without biopsy led to fewer biopsies and less overdetection of clinically insignificant prostate cancers compared with the standard approach. Furthermore, those in the MRI-targeted biopsy group were 13% less likely to receive a diagnosis of clinically insignificant cancer than those who received the standard biopsy (adjusted difference −13 percentage points, 95% confidence interval [CI] −19 to −7, P < .001).
Together, these data provide another argument for adding multiparametric MRI to the workup of men with an elevated PSA level.
Surveillance vs treatment for prostate cancer
Once prostate cancer is diagnosed, surveillance is becoming an increasingly common management strategy.
The Prostate Cancer Intervention Versus Observation Trial (PIVOT),22 one of the largest and longest trials involving cancer patients, offered further evidence that active surveillance and less intervention for men with prostate cancer is a better approach in many cases. This trial compared prostatectomy and observation alone in a randomized fashion. Inclusion for the study required men to be medically fit for radical prostatectomy, along with having histologically confirmed localized prostate cancer (stage T1-T2NxM0 in the tumor-node-metastasis classification system) of any grade diagnosed within the last 12 months.
During 19.5 years of follow-up, 223 (61.3%) of the 364 men randomly assigned to radical prostatectomy died, compared with 245 (66.8%) of 367 men in the observation group; the difference was not statistically different (P = .06). Only 9.4% of the deaths were due to prostate cancer, 7.4% in the surgery group and 11.4% in the observation group (P = .06).
Surgery was associated with a lower all-cause mortality rate than observation in the subgroup of patients with intermediate-risk prostate cancer (defined as PSA 10–20 ng/mL and a Gleason score of 7). Surgery was also associated with less disease progression.22
This finding is in line with previous data from the Scandinavian Prostate Cancer Group Study Number 4,23 as well as the much larger Prostate Testing for Cancer and Treatment (ProtecT) trial,24 both of which reported that metastasis was 1.5 and 2.6 times as common, respectively, in participants in the active surveillance groups. However, in the PIVOT trial, those in the surgery group were significantly more likely than those in the observation group to have erectile dysfunction and urinary incontinence at 10 years.
Therefore, in men with localized disease and in those with low-risk PSA levels, both the PIVOT and ProtecT trials suggest that death from prostate cancer is uncommon and that observation may be more appropriate.
Prostate cancer: Take-home points
- A new look at 2 large trials of PSA screening strengthened evidence that testing in the right patient population can reduce deaths from prostate cancer, but a third recently published trial that found no benefit from 1-time screening may reopen debate on the topic.
- MRI offers a better method than ultrasonography-guided biopsy to triage patients thought to be at high risk of prostate cancer and tends to limit costly overtreatment of disease that likely would not cause death.
- Surgery for prostate cancer may not prolong life but could reduce disease progression, at the risk of more adverse effects.
- Shared decision-making should be practiced when deciding whether to use active surveillance or active treatment of diagnosed prostate cancer.
MANAGEMENT OF ERECTILE DYSFUNCTION
A 62-year-old man with hypertension, hyperlipidemia, peripheral artery disease, and type 2 diabetes presents for a 6-month follow-up. His medications include aspirin, metformin, lisinopril, and atorvastatin, all of which he takes without problems. Over the past several months, he has noticed that his erections are not adequate for sexual intercourse. He recently heard that a generic version of sildenafil has just become available, and he wonders if it might benefit him.
Erectile dysfunction is common, associated with chronic diseases
Erectile dysfunction, ie, persistent inability to obtain and maintain an erection sufficient to permit satisfactory sexual intercourse,25,26 is estimated to affect nearly 20% of men over the age of 20 and 75% of men over the age of 75.27
In age-adjusted models, erectile dysfunction has been shown28 to be associated with:
- History of cardiovascular disease (odds ratio [OR] 1.63, 95% CI 1.02–2.63)
- Diabetes (OR 3.90, 95% CI 2.16–7.04)
- Treated hypertension vs no hypertension (OR 2.22, 95% CI 1.30–3.80)
- Current smoking vs never smoking (OR 1.63, 95% CI 1.01–2.62)
- BMI greater than 30 kg/m2 vs less than 25 kg/m2 (OR 1.80, 95% CI 1.03–3.14).
Because of the strong association between cardiovascular disease and erectile dysfunction, the presence of one often suggests the need to screen for the other.29 While tools such as the International Index of Erectile Function (IIEF-5) have been developed to evaluate erectile dysfunction, it is most often diagnosed on the basis of clinical impression, while validated assessment methods are reserved for clinical trials.28
Multiple causes of erectile dysfunction
Erectile dysfunction arises from inadequate penile tissue response to a sexual signal. The response can be disrupted at several points. For example, damage to vascular smooth muscle cells (eg, from age or obesity) and endothelial cells (from smoking or diabetes) and narrowing of the vascular lumen (from atherosclerosis or hypertension) have all been shown to impair engorgement of the corpus cavernosum.30 In addition, denervation from prostate surgery or spinal trauma and psychogenic causes should be recognized in discussions with patients.
Drugs for erectile dysfunction
Pharmacologic management of erectile dysfunction includes oral, sublingual, intracavernosal, and intraurethral therapies.31 Treatment in primary care settings usually includes addressing underlying chronic diseases32 and prescribing phosphodiesterase-5 inhibitors (sildenafil, tadalafil, vardenafil, and avanafil). These drugs work by increasing local concentrations of cyclic guanosine monophosphate in the corpus cavernosum to induce vasodilation.33
While these 4 drugs are still patent-protected, a manufacturer has been allowed to introduce a generic version of sildenafil into US markets, and a generic version of tadalafil is expected to be available soon.
Sildenafil, tadalafil, and vardenafil have been studied and found to have some degree of effectiveness in erectile dysfunction caused by damage to the penile vasculature, denervation, and spinal cord injury.34 All drugs of this class have adverse effects including headache, facial flushing, and nasal congestion, but the drugs are generally well tolerated.35
Sildenafil and tadalafil improve IIEF-5 scores by a similar margin, raising scores on the erectile domain subsection from approximately 14 of a possible 30 to approximately 24 of 30 in a trial of both drugs.36 However, multiple crossover studies comparing the 2 drugs have shown that nearly 75% of patients prefer tadalafil to sildenafil,36,37 perhaps because of tadalafil’s longer duration of action.34
There is little evidence to suggest that vardenafil is more effective or more often preferred by patients than tadalafil or sidenafil.34,38 And though data on the newest drug on the market, avanafil, are limited, a meta-analysis concluded that it may be less effective than tadalafil and without significant differences in terms of safety.39
Other treatments
Lifestyle modifications, especially smoking cessation and exercise, have been shown to reduce the risk of erectile dysfunction with varying effect sizes across studies.40–42 Moreover, factors such as obesity, alcohol use, and smoking may cause irreversible harm, and thus a healthy lifestyle should be encouraged.41
While there is only weak evidence for the use of psychological interventions alone for treating most types of erectile dysfunction, one meta-analysis found that the combination of psychological intervention and a phosphodiesterase-5 inhibitor improved sexual satisfaction more than drug therapy alone.43
Erectile dysfunction: Take-home points
- Erectile dysfunction is common, affecting nearly 20% of men over the age of 20 and over 75% of men over the age of 75.
- Erectile dysfunction is often associated with chronic disease and may suggest the need to screen for cardiovascular disease.
- Treating underlying chronic diseases may help, and phosphodiesterase-5 inhibitors are effective; tadalafil may be most often preferred.
SUPPLEMENT USE AND MEN’S HEALTH
A 68-year-old man with a history of hypertension, BPH, and erectile dysfunction presents for a 6-month follow-up. His medication use includes lisinopril, which he takes without problems. He denies any new physical symptoms. His physical examination is unremarkable. He says he has heard about supplements that might help with his sexual performance and hopes to discuss recommendations during the visit.
A burgeoning, unregulated industry
Since the passage of the Dietary Supplement and Health Education Act in 1994, a law that decreased oversight of the supplement industry, spending on supplements has skyrocketed to over $41.1 billion each year.44 Advertisements for these products typically claim that they improve general mental and physical health, sexual and romantic performance, leanness, and muscularity.45 A national survey of men ages 57 and older reported that the most popular products were aimed at nutrition (such as multivitamins), cardiovascular health (such as omega-3 fatty acids), and chronic conditions (such as saw palmetto for BPH).46
Little evidence of efficacy
There is little evidence to support the use of most supplements to improve men’s health. For example, a study in 82,405 men found no association between mortality rates and multivitamin use (hazard ratio [HR] 1.07, 95% CI 0.96–1.19).47 Even for specific uses, such as cognitive performance, randomized trials exploring the effects of multivitamins in men have been largely negative.48
The positive trials that have been reported are often of low quality and are funded by supplement manufacturers. For example, one of the few trials that reported a positive association between multivitamin supplementation and cognition in men was underpowered (N = 51) and found improvement in only 1 of 19 cognitive domains.49 Despite the poor design and results to the contrary, this industry-funded study nevertheless concluded that multivitamins may play a role in improving elements of memory.
Evidence of possible harm from antioxidants
While not always specific to men, many meta-analyses have explored the effects of antioxidant supplements on cardiovascular and mortality risk. Most of them concluded that antioxidant supplements have no benefit and that some may actually be harmful.
For example, multiple meta-analyses of vitamin E supplementation found no cardiovascular benefit but possible increases in all-cause mortality rates in those taking high doses (risk ratio 1.04, 95% CI 1.01–1.07).50,51
Another meta-analysis of 180,938 participants in high-quality studies found an increased risk of all-cause mortality associated with independent intake of several antioxidant vitamins, including beta-carotene (risk ratio 1.07, 95% CI 1.02–1.11) and vitamin A (risk ratio 1.16, 95% CI 1.10–1.24), while intake of vitamin C and selenium had no impact on mortality.52
Similarly, although nearly 10% of US adults report taking omega-3 fatty acid supplements, a review of 24 randomized controlled trials and meta-analyses published between 2005 and 2012 concluded that only 2 supported the use of these supplements for any health benefit.53
Can supplements improve sexual function, prostate health?
To improve sexual function. A 2015 narrative review of the ingredients in General Nutrition Center’s top 30 best-selling products targeted at improving men’s sexual performance (including improving libido and erectile dysfunction) found only poor evidence for any efficacy.54 The few studies that did support the use of select supplements, including B vitamins in people with diabetes, L-arginine, and yohimbine, were deemed to be of poor quality or showed a smaller effect size compared with standard medical therapy.
To prevent prostate cancer. Studies of supplement use to improve prostate health have had mixed results. For example, multiple large case-control studies have suggested that taking vitamin D55,56 or vitamin C57 is not associated with prostate cancer risk, while increased vitamin A58,59 and E60,61 intake is associated with inconsistent increases in prostate cancer risk.
In the Selenium and Vitamin E Cancer Prevention Trial,62 a randomized controlled trial in 35,533 men, those assigned to receive vitamin E supplementation were 17% more likely to get prostate cancer than were those assigned to placebo (HR 1.17, 99% CI 1.004–1.36, P = .008).
However, there are plausible biologic links between nutraceuticals and prostate cancer. For example, studies have linked genetic polymorphisms in vitamin D receptors63 as well as intake of natural androgen receptor modulators, such as the most active polyphenol in green tea,64 to prostate cancer risk and aggressiveness in certain populations. This led a recent review to conclude that there is some biologic plausibility, but at present little epidemiologic evidence, to support any dietary supplement’s ability to broadly affect prostate cancer risk.65
Interest continues in exploring the targeted use of nutraceuticals as adjuvant therapy in specific populations at risk of prostate cancer.66,67
To treat BPH. There is a similar dearth of clinical or population-based evidence that supplements can broadly affect BPH symptoms. For example, in a 2012 Cochrane review of Serenoa repens (saw palmetto) utilizing only high-quality evidence, there was no evidence that supplement use significantly reduced lower urinary tract symptoms, nocturia, or peak urine flow in BPH patients, and this was true even when the supplement was taken at triple-strength doses.68
For other diseases. There is also limited evidence that supplements can affect other chronic diseases. For example, a meta-analysis of 3,803 patients found that glucosamine, chondroitin, and their combination had no impact on joint pain or joint space narrowing in patients with osteoarthritis of the knee or hip.69
Even when there is some evidence to suggest benefit from supplementation, study heterogeneity and varying evidence quality limit confidence in the conclusions. For example, meta-analyses suggest garlic may improve blood pressure control in those with hypertension70 and improve lipid and blood glucose control in type 2 diabetes.71 However, most of the trials included in those systematic reviews were underpowered, with samples as low as 10 patients, and many suffered from improper design, such as inadequate blinding of researchers. In addition, these meta-analyses often do not report adverse events, suggesting that higher quality studies would be needed to adequately measure event rates. As such, there is need for caution and a case-by-case review before recommending even a seemingly benign supplement like garlic to patients.
In total, there is only limited evidence to support the efficacy of supplements across many diseases and concerns common to men in primary care. This includes improving general health, cardiovascular health, sexual functioning, or other chronic diseases. While a supplement’s placebo effect may at times provide some benefit, supplements are much less strictly regulated since the passing of the 1994 act, and even vitamin supplementation has been shown to be associated with negative health outcomes. As such, a patient’s use of supplements requires careful consideration and shared decision-making.
Supplements: Take-home points
- Supplements are only loosely regulated by the federal government.
- There is some biologic but limited epidemiologic evidence for the use of multivitamins to improve cognition or mortality rates; for the use of antioxidant vitamins or omega-3 fatty acids to improve cardiovascular health; for the use of any of the top-selling sexual enhancement supplements to improve libido or erectile function; and for the use of vitamins or other supplements for improving BPH or reducing prostate cancer risk. Using supplements may in some cases be harmful.
- Given the heterogeneity of studies of supplements to manage chronic diseases and a lack of reporting of adverse events, careful consideration is needed when recommending supplements to patients.
Primary care physicians are tasked with a wide variety of issues affecting men. This article reviews the latest research in 4 areas of men’s health commonly addressed in primary care:
- Medical management of benign prostatic hyperplasia (BPH)
- Prostate cancer screening and treatment
- Medical management of erectile dysfunction
- Use of supplements.
MEDICAL MANAGEMENT OF BPH
An 84-year-old man with a history of hypertension, type 2 diabetes, hyperlipidemia, BPH, mild cognitive impairment, and osteoarthritis presents for a 6-month follow-up, accompanied by his son.
Two years ago he was started on a 5-alpha reductase inhibitor and an alpha-blocker for worsening BPH symptoms. His BPH symptoms are currently under control, with an American Urological Association (AUA) symptom index score of 7 of a possible 35 (higher scores being worse).
However, both the patient and son are concerned about the number of medications he is on and wonder if some could be eliminated.
Assessment tools
BPH is a common cause of lower urinary tract symptoms in older men. Evidence-based tools to help the clinician and patient decide on when to consider treatment for symptoms are:
- The AUA symptom index1
- The International Prostate Symptom Score (IPSS).2
An AUA symptom index score or IPSS score of 8 through 19 of a possible 35 is consistent with moderate symptoms, while a score of 20 or higher indicates severe symptoms.
Combination therapy or monotherapy?
Monotherapy with an alpha-blocker or a 5-alpha reductase inhibitor is often the first-line treatment for BPH-related lower urinary tract symptoms.3 However, combination therapy with both an alpha-blocker and a 5-alpha reductase inhibitor is another evidence-based option.
The Medical Therapy of Prostatic Symptoms study,4 a randomized controlled trial, reported that long-term combination therapy reduced the risk of BPH clinical progression better than monotherapy. The same trial also found that either combination therapy or finasteride alone (a 5-alpha reductase inhibitor) reduced the risk of acute urinary retention and the future need for invasive therapy.
Monotherapy after a period of combination therapy?
There is also evidence to support switching from combination to monotherapy after an initial treatment period.
Matsukawa et al5 examined the effects of withdrawing the alpha-blocker from BPH combination therapy in a study in 140 patients. For 12 months, all patients received the alpha-blocker silodosin and the 5-alpha reductase inhibitor dutasteride. At 12 months, the remaining 132 patients (8 patients had been lost to follow-up) were randomized to continue combination therapy or to take dutasteride alone for another 12 months. They were evaluated at 0, 12, and 24 months by questionnaires (the IPSS and Overactive Bladder Symptom Score) and urodynamic testing (uroflowmetry, cystometrography, and pressure-flow studies).
There were no significant differences in subjective symptoms and bladder outlet obstruction between patients who continued combination therapy and those who switched to dutasteride monotherapy. In the monotherapy group, those whose symptoms worsened weighed more (68.8 kg vs 62.6 kg, P =.002) and had a higher body mass index (BMI) (26.2 kg/m2 vs 22.8 kg/m2, P < .001) than those whose symptoms stayed the same or got better.
These findings of successful alpha-blocker withdrawal were consistent with those of other studies.
The Symptom Management After Reducing Therapy study6 showed that 80% of men with an IPSS score less than 20 who changed to dutasteride monotherapy did not have a noticeable worsening of their symptoms.
Baldwin et al7 noted similar success after withdrawing the alpha-blocker doxazosin in patients on finasteride.
Review all medications
The National Health and Nutrition Examination Survey noted that the estimated prevalence of polypharmacy increased from 8% in 1999 to 15% in 2011.8 Many commonly used medications, such as decongestants, antihistamines, and anticholinergic agents, can worsen BPH symptoms,9 so it is reasonable to consistently review the patient’s medications to weigh the risks and benefits and determine which ones align with the patient’s personal care goals.
BPH: Take-home points
- Combination therapy with an alpha-blocker and a 5-alpha reductase inhibitor is an effective regimen for BPH.
- Polypharmacy is a significant problem in the elderly.
- Withdrawing the alpha-blocker component from BPH combination therapy can be considered after 1 year of combination therapy in patients whose symptoms have been well controlled.
PROSTATE CANCER SCREENING AND TREATMENT
A 60-year-old patient calls you after receiving his laboratory testing report from his insurance physical. His prostate-specific antigen (PSA) level is 5.1 ng/mL, and he has several questions:
- Should he have agreed to the screening?
- How effective is the screening?
- What are the next steps?
Is PSA screening useful?
Over the last few years, there has been great debate as to the utility of screening for prostate cancer.
The US Centers for Disease Control and Prevention10 reported that in 2014, an estimated 172,258 men in the United States were diagnosed with prostate cancer, but only 28,343 men died of it. These statistics support the notion that screening programs may be detecting what might otherwise be a silent disease.
The US Preventive Services Task Force (USPSTF)11 recommends against blanket PSA screening, in view of the low probability that it reduces the risk of death from prostate cancer. For men ages 55 through 69, current guidelines give a grade C recommendation to PSA screening, meaning there is moderate agreement that the benefit is likely small, and screening should be selectively offered based on professional judgment and patient preference. In men ages 70 and older who are not at high risk, the guideline gives screening a grade D recommendation, meaning there is moderate evidence that there is no benefit from the practice. This is a change from the 2012 USPSTF guidelines,12 which gave a grade D recommendation to PSA screening for all ages.
The American Urological Association13 recommends against PSA screening in men under age 40 or ages 70 and older. It does not recommend routine screening in those ages 40 to 54 at average risk, but it says the decision should be individualized in this age group in those at higher risk (eg, with a positive family history, African American). At ages 55 through 69, it recommends shared decision-making, taking into account cancer risk and life expectancy. In those who opt for screening, an interval of 2 years or more may be preferred over annual screening to reduce the risk of overdiagnosis.
The USPSTF recommendations rely heavily on data from 2 trials: the European Randomized Study of Screening for Prostate Cancer (ERSPC)14 and the Prostate, Lung, Colorectal, and Ovarian Screening (PLCO) trial.15
The ERSPC14 demonstrated that screening for prostate cancer reduced deaths from prostate cancer by 20%, with an absolute risk difference of 0.71 deaths per 1,000 men; 1,410 men would need to be screened and 48 additional cases of prostate cancer would need to be treated to prevent 1 death from prostate cancer. Screening also decreased the risk of developing metastatic disease by 30%.16 On the negative side, screening increased the risk of overdiagnosis and other harms such as bleeding, sepsis, and incontinence.
The PLCO trial,15 in contrast, found no difference in death rates between men randomly assigned to annual screening and those assigned to usual care. Differences between the trial results were thought to be due to different practice settings as well as study implementation and compliance.
Tsodikov et al17 reanalyzed data from the ERSPC and the PLCO trial using 3 different mathematical models to estimate the effects of screening in both trials compared with no screening. The analysis found no evidence that the effects of screening vs not screening differed between the 2 trials, ultimately concluding that PSA screening reduced prostate cancer deaths by 25% to 32%, which the authors inferred was primarily a result of earlier detection of cancer.
The Cluster Randomized Trial of PSA Testing for Prostate Cancer,18 published in March 2018, explored the effect of single PSA screening vs no screening on prostate cancer mortality rates in 419,582 men ages 50 through 69. Although screening detected more cases of low-risk prostate cancer, there was no significant difference in prostate cancer mortality rates after a median follow-up of 10 years. However, 10% to 15% of the control group was estimated to have also been screened, and these results do not directly speak to the efficacy of serial PSA screening.
Extended follow-up of this trial is planned to report on long-term survival benefits and whether screening lowers the risk of metastasis.
Imaging-guided prostate biopsy
Once a patient is found to have an elevated PSA level, standard practice has been to perform transrectal ultrasonography to obtain 12 core biopsy samples. The results indicate whether the prostate contains cancer, how aggressive the cancer is (Gleason score), and whether there is extracapsular extension.
In the past, magnetic resonance imaging (MRI) of the prostate before biopsy was thought to be too costly, and many insurance plans do not currently cover it.
Pahwa et al,19 however, in a cost-effectiveness study using a decision-analysis model, found that using MRI to detect lesions and then guide biopsy by triaging patients into proper treatment pathways added health benefits in a cost-effective manner in 94.05% of simulations. These benefits were found across all age groups.
This study demonstrated that doctors could use MRI to better evaluate patients for potentially harmful lesions. If a focus of cancer is found, it can be biopsied; if no cancer is seen on MRI, the patient can avoid biopsy completely. Additionally, though MRI tended to miss low-risk cancers, these cancers are thought to disproportionately lead to higher healthcare costs through unnecessary treatment. Therefore, a negative MRI study was believed to be an excellent sign that the patient does not have aggressive prostate cancer. This approach led to a net gain of 0.251 additional quality-adjusted life years compared with the standard biopsy strategy.
The Prostate MRI Imaging Study20 also found MRI to be effective in the prostate cancer workup. In this trial, 576 men who had never undergone biopsy underwent multiparametric MRI, transrectal ultrasonography-guided biopsy, and the reference standard, ie, transperineal template prostate mapping biopsy. Of those who underwent biopsy, 71% received a diagnosis of prostate cancer, and 40% had clinically significant disease. In patients with clinically significant disease, MRI was more sensitive than ultrasonography-guided biopsy (93% vs 48%, P < .0001) but less specific (41% vs 96%, P < .0001).
Based on these findings, if biopsy were performed only in those who had suspicious lesions on MRI, 27% of men with elevated PSA could avoid biopsy and its potential complications such as bleeding and sepsis, which occurred in 5.9% of the biopsy group.
The Prostate Evaluation for Clinically Important Disease: Sampling Using Image Guidance or Not? trial21 more recently studied MRI with or without targeted biopsy vs standard transrectal ultrasonography-guided biopsy in 500 men who had not undergone biopsy before, and reported similar results. MRI with or without biopsy led to fewer biopsies and less overdetection of clinically insignificant prostate cancers compared with the standard approach. Furthermore, those in the MRI-targeted biopsy group were 13% less likely to receive a diagnosis of clinically insignificant cancer than those who received the standard biopsy (adjusted difference −13 percentage points, 95% confidence interval [CI] −19 to −7, P < .001).
Together, these data provide another argument for adding multiparametric MRI to the workup of men with an elevated PSA level.
Surveillance vs treatment for prostate cancer
Once prostate cancer is diagnosed, surveillance is becoming an increasingly common management strategy.
The Prostate Cancer Intervention Versus Observation Trial (PIVOT),22 one of the largest and longest trials involving cancer patients, offered further evidence that active surveillance and less intervention for men with prostate cancer is a better approach in many cases. This trial compared prostatectomy and observation alone in a randomized fashion. Inclusion for the study required men to be medically fit for radical prostatectomy, along with having histologically confirmed localized prostate cancer (stage T1-T2NxM0 in the tumor-node-metastasis classification system) of any grade diagnosed within the last 12 months.
During 19.5 years of follow-up, 223 (61.3%) of the 364 men randomly assigned to radical prostatectomy died, compared with 245 (66.8%) of 367 men in the observation group; the difference was not statistically different (P = .06). Only 9.4% of the deaths were due to prostate cancer, 7.4% in the surgery group and 11.4% in the observation group (P = .06).
Surgery was associated with a lower all-cause mortality rate than observation in the subgroup of patients with intermediate-risk prostate cancer (defined as PSA 10–20 ng/mL and a Gleason score of 7). Surgery was also associated with less disease progression.22
This finding is in line with previous data from the Scandinavian Prostate Cancer Group Study Number 4,23 as well as the much larger Prostate Testing for Cancer and Treatment (ProtecT) trial,24 both of which reported that metastasis was 1.5 and 2.6 times as common, respectively, in participants in the active surveillance groups. However, in the PIVOT trial, those in the surgery group were significantly more likely than those in the observation group to have erectile dysfunction and urinary incontinence at 10 years.
Therefore, in men with localized disease and in those with low-risk PSA levels, both the PIVOT and ProtecT trials suggest that death from prostate cancer is uncommon and that observation may be more appropriate.
Prostate cancer: Take-home points
- A new look at 2 large trials of PSA screening strengthened evidence that testing in the right patient population can reduce deaths from prostate cancer, but a third recently published trial that found no benefit from 1-time screening may reopen debate on the topic.
- MRI offers a better method than ultrasonography-guided biopsy to triage patients thought to be at high risk of prostate cancer and tends to limit costly overtreatment of disease that likely would not cause death.
- Surgery for prostate cancer may not prolong life but could reduce disease progression, at the risk of more adverse effects.
- Shared decision-making should be practiced when deciding whether to use active surveillance or active treatment of diagnosed prostate cancer.
MANAGEMENT OF ERECTILE DYSFUNCTION
A 62-year-old man with hypertension, hyperlipidemia, peripheral artery disease, and type 2 diabetes presents for a 6-month follow-up. His medications include aspirin, metformin, lisinopril, and atorvastatin, all of which he takes without problems. Over the past several months, he has noticed that his erections are not adequate for sexual intercourse. He recently heard that a generic version of sildenafil has just become available, and he wonders if it might benefit him.
Erectile dysfunction is common, associated with chronic diseases
Erectile dysfunction, ie, persistent inability to obtain and maintain an erection sufficient to permit satisfactory sexual intercourse,25,26 is estimated to affect nearly 20% of men over the age of 20 and 75% of men over the age of 75.27
In age-adjusted models, erectile dysfunction has been shown28 to be associated with:
- History of cardiovascular disease (odds ratio [OR] 1.63, 95% CI 1.02–2.63)
- Diabetes (OR 3.90, 95% CI 2.16–7.04)
- Treated hypertension vs no hypertension (OR 2.22, 95% CI 1.30–3.80)
- Current smoking vs never smoking (OR 1.63, 95% CI 1.01–2.62)
- BMI greater than 30 kg/m2 vs less than 25 kg/m2 (OR 1.80, 95% CI 1.03–3.14).
Because of the strong association between cardiovascular disease and erectile dysfunction, the presence of one often suggests the need to screen for the other.29 While tools such as the International Index of Erectile Function (IIEF-5) have been developed to evaluate erectile dysfunction, it is most often diagnosed on the basis of clinical impression, while validated assessment methods are reserved for clinical trials.28
Multiple causes of erectile dysfunction
Erectile dysfunction arises from inadequate penile tissue response to a sexual signal. The response can be disrupted at several points. For example, damage to vascular smooth muscle cells (eg, from age or obesity) and endothelial cells (from smoking or diabetes) and narrowing of the vascular lumen (from atherosclerosis or hypertension) have all been shown to impair engorgement of the corpus cavernosum.30 In addition, denervation from prostate surgery or spinal trauma and psychogenic causes should be recognized in discussions with patients.
Drugs for erectile dysfunction
Pharmacologic management of erectile dysfunction includes oral, sublingual, intracavernosal, and intraurethral therapies.31 Treatment in primary care settings usually includes addressing underlying chronic diseases32 and prescribing phosphodiesterase-5 inhibitors (sildenafil, tadalafil, vardenafil, and avanafil). These drugs work by increasing local concentrations of cyclic guanosine monophosphate in the corpus cavernosum to induce vasodilation.33
While these 4 drugs are still patent-protected, a manufacturer has been allowed to introduce a generic version of sildenafil into US markets, and a generic version of tadalafil is expected to be available soon.
Sildenafil, tadalafil, and vardenafil have been studied and found to have some degree of effectiveness in erectile dysfunction caused by damage to the penile vasculature, denervation, and spinal cord injury.34 All drugs of this class have adverse effects including headache, facial flushing, and nasal congestion, but the drugs are generally well tolerated.35
Sildenafil and tadalafil improve IIEF-5 scores by a similar margin, raising scores on the erectile domain subsection from approximately 14 of a possible 30 to approximately 24 of 30 in a trial of both drugs.36 However, multiple crossover studies comparing the 2 drugs have shown that nearly 75% of patients prefer tadalafil to sildenafil,36,37 perhaps because of tadalafil’s longer duration of action.34
There is little evidence to suggest that vardenafil is more effective or more often preferred by patients than tadalafil or sidenafil.34,38 And though data on the newest drug on the market, avanafil, are limited, a meta-analysis concluded that it may be less effective than tadalafil and without significant differences in terms of safety.39
Other treatments
Lifestyle modifications, especially smoking cessation and exercise, have been shown to reduce the risk of erectile dysfunction with varying effect sizes across studies.40–42 Moreover, factors such as obesity, alcohol use, and smoking may cause irreversible harm, and thus a healthy lifestyle should be encouraged.41
While there is only weak evidence for the use of psychological interventions alone for treating most types of erectile dysfunction, one meta-analysis found that the combination of psychological intervention and a phosphodiesterase-5 inhibitor improved sexual satisfaction more than drug therapy alone.43
Erectile dysfunction: Take-home points
- Erectile dysfunction is common, affecting nearly 20% of men over the age of 20 and over 75% of men over the age of 75.
- Erectile dysfunction is often associated with chronic disease and may suggest the need to screen for cardiovascular disease.
- Treating underlying chronic diseases may help, and phosphodiesterase-5 inhibitors are effective; tadalafil may be most often preferred.
SUPPLEMENT USE AND MEN’S HEALTH
A 68-year-old man with a history of hypertension, BPH, and erectile dysfunction presents for a 6-month follow-up. His medication use includes lisinopril, which he takes without problems. He denies any new physical symptoms. His physical examination is unremarkable. He says he has heard about supplements that might help with his sexual performance and hopes to discuss recommendations during the visit.
A burgeoning, unregulated industry
Since the passage of the Dietary Supplement and Health Education Act in 1994, a law that decreased oversight of the supplement industry, spending on supplements has skyrocketed to over $41.1 billion each year.44 Advertisements for these products typically claim that they improve general mental and physical health, sexual and romantic performance, leanness, and muscularity.45 A national survey of men ages 57 and older reported that the most popular products were aimed at nutrition (such as multivitamins), cardiovascular health (such as omega-3 fatty acids), and chronic conditions (such as saw palmetto for BPH).46
Little evidence of efficacy
There is little evidence to support the use of most supplements to improve men’s health. For example, a study in 82,405 men found no association between mortality rates and multivitamin use (hazard ratio [HR] 1.07, 95% CI 0.96–1.19).47 Even for specific uses, such as cognitive performance, randomized trials exploring the effects of multivitamins in men have been largely negative.48
The positive trials that have been reported are often of low quality and are funded by supplement manufacturers. For example, one of the few trials that reported a positive association between multivitamin supplementation and cognition in men was underpowered (N = 51) and found improvement in only 1 of 19 cognitive domains.49 Despite the poor design and results to the contrary, this industry-funded study nevertheless concluded that multivitamins may play a role in improving elements of memory.
Evidence of possible harm from antioxidants
While not always specific to men, many meta-analyses have explored the effects of antioxidant supplements on cardiovascular and mortality risk. Most of them concluded that antioxidant supplements have no benefit and that some may actually be harmful.
For example, multiple meta-analyses of vitamin E supplementation found no cardiovascular benefit but possible increases in all-cause mortality rates in those taking high doses (risk ratio 1.04, 95% CI 1.01–1.07).50,51
Another meta-analysis of 180,938 participants in high-quality studies found an increased risk of all-cause mortality associated with independent intake of several antioxidant vitamins, including beta-carotene (risk ratio 1.07, 95% CI 1.02–1.11) and vitamin A (risk ratio 1.16, 95% CI 1.10–1.24), while intake of vitamin C and selenium had no impact on mortality.52
Similarly, although nearly 10% of US adults report taking omega-3 fatty acid supplements, a review of 24 randomized controlled trials and meta-analyses published between 2005 and 2012 concluded that only 2 supported the use of these supplements for any health benefit.53
Can supplements improve sexual function, prostate health?
To improve sexual function. A 2015 narrative review of the ingredients in General Nutrition Center’s top 30 best-selling products targeted at improving men’s sexual performance (including improving libido and erectile dysfunction) found only poor evidence for any efficacy.54 The few studies that did support the use of select supplements, including B vitamins in people with diabetes, L-arginine, and yohimbine, were deemed to be of poor quality or showed a smaller effect size compared with standard medical therapy.
To prevent prostate cancer. Studies of supplement use to improve prostate health have had mixed results. For example, multiple large case-control studies have suggested that taking vitamin D55,56 or vitamin C57 is not associated with prostate cancer risk, while increased vitamin A58,59 and E60,61 intake is associated with inconsistent increases in prostate cancer risk.
In the Selenium and Vitamin E Cancer Prevention Trial,62 a randomized controlled trial in 35,533 men, those assigned to receive vitamin E supplementation were 17% more likely to get prostate cancer than were those assigned to placebo (HR 1.17, 99% CI 1.004–1.36, P = .008).
However, there are plausible biologic links between nutraceuticals and prostate cancer. For example, studies have linked genetic polymorphisms in vitamin D receptors63 as well as intake of natural androgen receptor modulators, such as the most active polyphenol in green tea,64 to prostate cancer risk and aggressiveness in certain populations. This led a recent review to conclude that there is some biologic plausibility, but at present little epidemiologic evidence, to support any dietary supplement’s ability to broadly affect prostate cancer risk.65
Interest continues in exploring the targeted use of nutraceuticals as adjuvant therapy in specific populations at risk of prostate cancer.66,67
To treat BPH. There is a similar dearth of clinical or population-based evidence that supplements can broadly affect BPH symptoms. For example, in a 2012 Cochrane review of Serenoa repens (saw palmetto) utilizing only high-quality evidence, there was no evidence that supplement use significantly reduced lower urinary tract symptoms, nocturia, or peak urine flow in BPH patients, and this was true even when the supplement was taken at triple-strength doses.68
For other diseases. There is also limited evidence that supplements can affect other chronic diseases. For example, a meta-analysis of 3,803 patients found that glucosamine, chondroitin, and their combination had no impact on joint pain or joint space narrowing in patients with osteoarthritis of the knee or hip.69
Even when there is some evidence to suggest benefit from supplementation, study heterogeneity and varying evidence quality limit confidence in the conclusions. For example, meta-analyses suggest garlic may improve blood pressure control in those with hypertension70 and improve lipid and blood glucose control in type 2 diabetes.71 However, most of the trials included in those systematic reviews were underpowered, with samples as low as 10 patients, and many suffered from improper design, such as inadequate blinding of researchers. In addition, these meta-analyses often do not report adverse events, suggesting that higher quality studies would be needed to adequately measure event rates. As such, there is need for caution and a case-by-case review before recommending even a seemingly benign supplement like garlic to patients.
In total, there is only limited evidence to support the efficacy of supplements across many diseases and concerns common to men in primary care. This includes improving general health, cardiovascular health, sexual functioning, or other chronic diseases. While a supplement’s placebo effect may at times provide some benefit, supplements are much less strictly regulated since the passing of the 1994 act, and even vitamin supplementation has been shown to be associated with negative health outcomes. As such, a patient’s use of supplements requires careful consideration and shared decision-making.
Supplements: Take-home points
- Supplements are only loosely regulated by the federal government.
- There is some biologic but limited epidemiologic evidence for the use of multivitamins to improve cognition or mortality rates; for the use of antioxidant vitamins or omega-3 fatty acids to improve cardiovascular health; for the use of any of the top-selling sexual enhancement supplements to improve libido or erectile function; and for the use of vitamins or other supplements for improving BPH or reducing prostate cancer risk. Using supplements may in some cases be harmful.
- Given the heterogeneity of studies of supplements to manage chronic diseases and a lack of reporting of adverse events, careful consideration is needed when recommending supplements to patients.
- Barry MJ, Fowler FJ Jr, O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. J Urol 2017; 197(2S):S189–S197. doi:10.1016/j.juro.2016.10.071
- Urological Sciences Research Foundation. International Prostate Symptom Score (IPSS). http://www.usrf.org/questionnaires/AUA_SymptomScore.html. Accessed October 16, 2018.
- McVary KT, Roehrborn CG, Avins AL, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol 2011; 185(5):1793–1803. doi:10.1016/j.juro.2011.01.074
- McConnell JD, Roehrborn CG, Bautista OM, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med 2003; 349(25):2387–2398. doi:10.1056/NEJMoa030656
- Matsukawa Y, Takai S, Funahashi Y, et al. Effects of withdrawing alpha-1 blocker from the combination therapy with alpha-1 blocker and 5-alpha-reductase inhibitor in patients with lower urinary tract symptoms suggestive of benign prostatic hyperplasia: a prospective and comparative trial using urodynamics. J Urol 2017; 198(4):905–912. doi:10.1016/j.juro.2017.05.031
- Barkin J, Guimaraes M, Jacobi G, Pushkar D, Taylor S, van Vierssen Trip OB. Alpha-blocker therapy can be withdrawn in the majority of men following initial combination therapy with the dual 5a-reductase inhibitor dutasteride. Eur Urol 2003; 44(4):461–466. pmid:14499682
- Baldwin KC, Ginsberg PC, Roehrborn CG, Harkaway RC. Discontinuation of alpha-blockade after initial treatment with finasteride and doxazosin in men with lower urinary tract symptoms and clinical evidence of benign prostatic hyperplasia. Urology 2001; 58(2):203–209. pmid:11489700
- Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA 2015; 314(17):1818–1831. doi:10.1001/jama.2015.13766
- DuBeau CE, Yalla SV, Resnick NM. Improving the utility of urine flow rate to exclude outlet obstruction in men with voiding symptoms. J Am Geriatr Soc 1998; 46(9):1118–1124. pmid:9736105
- US Department of Health and Human Services Health Resources and Services Administration. United States Cancer Statistics: 1999-2014 Incidence and Mortality Web-Based Report. Atlanta; 2017. https://nccd.cdc.gov/uscs/. Accessed October 17, 2018.
- US Preventive Services Task Force. Final recommendation statement. Prostate cancer: screening. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening1. Accessed October 16, 2018.
- US Preventive Services Task Force. Archived: prostate cancer: screening. Original release date: May 2012. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/prostate-cancer-screening. Accessed October 16, 2018.
- Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA guideline. J Urol 2013; 190(2):419–426. doi:10.1016/j.juro.2013.04.119
- Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 2009; 360(13):1320–1328. doi:10.1056/NEJMoa0810084
- Andriole GL, Crawford ED, Grubb RL, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med 2009; 360(13):1310–1319. doi:10.1056/NEJMoa0810696
- Schröder FH, Hugosson J, Carlsson S, et al. Screening for prostate cancer decreases the risk of developing metastatic disease: findings from the European Randomized Study of Screening for Prostate Cancer (ERSPC). Eur Urol 2012; 62(5):745–752. doi:10.1016/j.eururo.2012.05.068
- Tsodikov A, Gulati R, Heijnsdijk EAM, et al. Reconciling the effects of screening on prostate cancer mortality in the ERSPC and PLCO trials. Ann Intern Med 2017; 167(7):449–455. doi:10.7326/M16-2586
- Martin RM, Donovan JL, Turner EL, et al. Effect of a low-intensity PSA-based screening intervention on prostate cancer mortality: the CAP randomized clinical trial. JAMA 2018; 319(9):883–895. doi:10.1001/jama.2018.0154
- Pahwa S, Schiltz NK, Ponsky LE, Lu Z, Griswold MA, Gulani V. Cost-effectiveness of MR imaging–guided strategies for detection of prostate cancer in biopsy-naive men. Radiology 2017; 285(1):157–166. doi:10.1148/radiol.2017162181
- Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017; 389(10071):815–822. doi:10.1016/S0140-6736(16)32401-1
- Kasivisvanathan V, Rannikko AS, Borghi M, et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med 2018; 378(19):1767–1777. doi:10.1056/NEJMoa1801993
- Wilt TJ, Jones KM, Barry MJ, et al. Follow-up of prostatectomy versus observation for early prostate cancer. N Engl J Med 2017; 377(2):132–142. doi:10.1056/NEJMoa1615869
- Bill-Axelson A, Holmberg L, Garmo H, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med 2014; 370(10):932–942. doi:10.1056/NEJMoa1311593
- Hamdy FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med 2016; 375(15):1415–1424. doi:10.1056/NEJMoa1606220
- Morley JE. Impotence. Am J Med 1986; 80(5):897–905. pmid:3518438
- NIH Consensus Development Panel on Impotence. NIH Consensus Conference. Impotence. JAMA 1993; 270(1):83–90. pmid:8510302
- Selvin E, Burnett AL, Platz EA. Prevalence and risk factors for erectile dysfunction in the US. Am J Med 2007; 120(2):151–157. doi:10.1016/j.amjmed.2006.06.010
- Rosen RC, Cappelleri JC, Gendrano N 3rd. The International Index of Erectile Function (IIEF): a state-of-the-science review. Int J Impot Res 2002; 14(4):226–244. doi:10.1038/sj.ijir.3900857
- Gandaglia G, Briganti A, Jackson G, et al. A systematic review of the association between erectile dysfunction and cardiovascular disease. Eur Urol 2014; 65(5):968–978. doi:10.1016/j.eururo.2013.08.023
- Heaton JPW, Adams MA. Causes of erectile dysfunction. Endocrine 2004; 23(2-3):119–123. doi:10.1385/ENDO:23:2-3:119
- Montorsi F, Salonia A, Deho F, et al. Pharmacological management of erectile dysfunction. BJU Int 2003; 91(5):446–454. pmid:12603396
- Cai X, Tian Y, Wu T, Cao CX, Bu SY, Wang KJ. The role of statins in erectile dysfunction: a systematic review and meta-analysis. Asian J Androl 2014; 16(3):461–466. doi:10.4103/1008-682X.123678
- Webb DJ, Freestone S, Allen MJ, Muirhead GJ. Sildenafil citrate and blood-pressure–lowering drugs: results of drug interaction studies with an organic nitrate and a calcium antagonist. Am J Cardiol 1999; 83(5):21C–28C. pmid:10078539
- Doggrell SA. Comparison of clinical trials with sildenafil, vardenafil and tadalafil in erectile dysfunction. Expert Opin Pharmacother 2005; 6(1):75–84. doi:10.1517/14656566.6.1.75
- Gresser U, Gleiter CH. Erectile dysfunction: comparison of efficacy and side effects of the PDE-5 inhibitors sildenafil, vardenafil and tadalafil—review of the literature. Eur J Med Res 2002; 7(10):435–446. pmid:12435622
- Eardley I, Mirone V, Montorsi F, et al. An open-label, multicentre, randomized, crossover study comparing sildenafil citrate and tadalafil for treating erectile dysfunction in men naive to phosphodiesterase 5 inhibitor therapy. BJU Int 2005; 96(9):1323–1332. doi:10.1111/j.1464-410X.2005.05892.x
- von Keitz A, Rajfer J, Segal S, et al. A multicenter, randomized, double-blind, crossover study to evaluate patient preference between tadalafil and sildenafil. Eur Urol 2004; 45(4):499–509. doi:10.1016/j.eururo.2003.11.030
- Martin-Morales A, Haro JM, Beardsworth A, Bertsch J, Kontodimas S; EDOS Group. Therapeutic effectiveness and patient satisfaction after 6 months of treatment with tadalafil, sildenafil, and vardenafil: results from the erectile dysfunction observational study (EDOS). Eur Urol 2007; 51(2):541–550. doi:10.1016/j.eururo.2006.09.027
- Yuan J, Zhang R, Yang Z, et al. Comparative effectiveness and safety of oral phosphodiesterase type 5 inhibitors for erectile dysfunction: a systematic review and network meta-analysis. Eur Urol 2013; 63(5):902–912. doi:10.1016/j.eururo.2013.01.012
- Cao S, Yin X, Wang Y, Zhou H, Song F, Lu Z. Smoking and risk of erectile dysfunction: systematic review of observational studies with meta-analysis. PLoS One 2013; 8(4):e60443. doi:10.1371/journal.pone.0060443
- Derby CA, Mohr BA, Goldstein I, Feldman HA, Johannes CB, McKinlay JB. Modifiable risk factors and erectile dysfunction: can lifestyle changes modify risk? Urology 2000; 56(2):302–306. pmid:10925098
- Esposito K, Giugliano F, Di Palo C, et al. Effect of lifestyle changes on erectile dysfunction in obese men: a randomized controlled trial. JAMA 2004; 291(24):2978–2984. doi:10.1001/jama.291.24.2978
- Schmidt HM, Munder T, Gerger H, Frühauf S, Barth J. Combination of psychological intervention and phosphodiesterase-5 inhibitors for erectile dysfunction: a narrative review and meta-analysis. J Sex Med 2014; 11(6):1376–1391. doi:10.1111/jsm.12520
- New Hope Network. Supplement Business Report 2017. Boulder; 2017. http://images.info.newhope.com/Web/NewHopeNaturalMedia/%7B3a3f3b03-6130-41d4-9e66-84f29eeebe44%7D_2017_Supplement_Business_Report_-_Extended_TOC.pdf. Accessed October 16, 2018.
- Labre MP. Burn fat, build muscle: a content analysis of men’s health and men’s fitness. Int J Mens Health 2005; 4(2):187–200.
- Qato DM, Alexander GC, Conti RM, Johnson M, Schumm P, Lindau ST. Use of prescription and over-the-counter medications and dietary supplements among older adults in the United States. JAMA 2008; 300(24):2867–2878. doi:10.1001/jama.2008.892
- Park SY, Murphy SP, Wilkens LR, Henderson BE, Kolonel LN. Multivitamin use and the risk of mortality and cancer incidence: the multiethnic cohort study. Am J Epidemiol 2011; 173(8):906–914. doi:10.1093/aje/kwq447
- McNeill G, Avenell A, Campbell MK, et al. Effect of multivitamin and multimineral supplementation on cognitive function in men and women aged 65 years and over: a randomised controlled trial. Nutr J 2007; 6(1):10. doi:10.1186/1475-2891-6-10
- Harris E, Macpherson H, Vitetta L, Kirk J, Sali A, Pipingas A. Effects of a multivitamin, mineral and herbal supplement on cognition and blood biomarkers in older men: a randomised, placebo-controlled trial. Hum Psychopharmacol Clin Exp 2012; 27(4):370–377. doi:10.1002/hup.2236
- Vivekananthan DP, Penn MS, Sapp SK, Hsu A, Topol EJ. Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Lancet 2003; 361(9374):2017–2023. doi:10.1016/S0140-6736(03)13637-9
- Miller ER, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med 2005; 142(1):37–46. pmid:15537682
- Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA 2007; 297(8):842–857. doi:10.1001/jama.297.8.842
- Grey A, Bolland M. Clinical trial evidence and use of fish oil supplements. JAMA Intern Med 2014; 174(3):460–462. doi:10.1001/jamainternmed.2013.12765
- Cui T, Kovell RC, Brooks DC, Terlecki RP. A urologist’s guide to ingredients found in top-selling nutraceuticals for men’s sexual health. J Sex Med 2015; 12(11):2105–2117. doi:10.1111/jsm.13013
- Schenk JM, Till CA, Tangen CM, et al. Serum 25-hydroxyvitamin D concentrations and risk of prostate cancer: results from the Prostate Cancer Prevention Trial. Cancer Epidemiol Prev Biomarkers 2014; 23(8):1484–1493. doi:10.1158/1055-9965.EPI-13-1340
- Albanes D, Mondul AM, Yu K, et al. Serum 25-hydroxy vitamin D and prostate cancer risk in a large nested case-control study. Cancer Epidemiol Prev Biomarkers 2011; 20(9):1850–1860. doi:10.1158/1055-9965.EPI-11-0403
- Roswall N, Larsen SB, Friis S, et al. Micronutrient intake and risk of prostate cancer in a cohort of middle-aged, Danish men. Cancer Causes Control 2013; 24(6):1129–1135. doi:10.1007/s10552-013-0190-4
- Mondul AM, Watters JL, Männistö S, et al. Serum retinol and risk of prostate cancer. Am J Epidemiol 2011; 173(7):813-821. doi:10.1093/aje/kwq429
- Schenk JM, Riboli E, Chatterjee N, et al. Serum retinol and prostate cancer risk: a nested case-control study in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol Prev Biomarkers 2009; 18(4):1227–1231. doi:10.1158/1055-9965.EPI-08-0984
- Bidoli E, Talamini R, Zucchetto A, et al. Dietary vitamins E and C and prostate cancer risk. Acta Oncol 2009; 48(6):890–894. doi:10.1080/02841860902946546
- Wright ME, Weinstein SJ, Lawson KA, et al. Supplemental and dietary vitamin E intakes and risk of prostate cancer in a large prospective study. Cancer Epidemiol Prev Biomarkers 2007; 16(6):1128–1135. doi:10.1158/1055-9965.EPI-06-1071
- Klein EA, Thompson IM, Tangen CM, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011; 306(14):1549–1556. doi:10.1001/jama.2011.1437
- Jingwi EY, Abbas M, Ricks-Santi L, et al. Vitamin D receptor genetic polymorphisms are associated with PSA level, Gleason score and prostate cancer risk in African-American men. Anticancer Res 2015; 35(3):1549–1558. pmid:25750310
- Siddiqui IA, Asim M, Hafeez BB, Adhami VM, Tarapore RS, Mukhtar H. Green tea polyphenol EGCG blunts androgen receptor function in prostate cancer. FASEB J 2011; 25(4):1198–1207. doi:10.1096/fj.10-167924
- Yacoubian A, Dargham RA, Khauli RB, Bachir BG. Overview of dietary supplements in prostate cancer. Curr Urol Rep 2016; 17(11):78. doi:10.1007/s11934-016-0637-8
- Kallifatidis G, Hoy JJ, Lokeshwar BL. Bioactive natural products for chemoprevention and treatment of castration-resistant prostate cancer. Semin Cancer Biol 2016; 40:160–169. doi:10.1016/j.semcancer.2016.06.003
- Shui IM, Mondul AM, Lindström S, et al. Circulating vitamin D, vitamin D–related genetic variation, and risk of fatal prostate cancer in the National Cancer Institute Breast and Prostate Cancer Cohort Consortium. Cancer 2015; 121(12):1949–1956. doi:10.1002/cncr.29320
- Tacklind J, MacDonald R, Rutks I, Stanke JU, Wilt TJ. Serenoa repens for benign prostatic hyperplasia. Cochrane Database Syst Rev 2012; 12:CD001423. doi:10.1002/14651858.CD001423.pub3
- Wandel S, Jüni P, Tendal B, et al. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis. BMJ 2010; 341:c4675. doi:10.1136/bmj.c4675
- Reinhart KM, Coleman CI, Teevan C, Vachhani P, White CM. Effects of garlic on blood pressure in patients with and without systolic hypertension: a meta-analysis. Ann Pharmacother 2008; 42(12):1766–1771. doi:10.1345/aph.1L319
- Wang J, Zhang X, Lan H, Wang W. Effect of garlic supplement in the management of type 2 diabetes mellitus (T2DM): a meta-analysis of randomized controlled trials. Food Nutr Res 2017; 61(1):1377571. doi:10.1080/16546628.2017.1377571
- Barry MJ, Fowler FJ Jr, O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. J Urol 2017; 197(2S):S189–S197. doi:10.1016/j.juro.2016.10.071
- Urological Sciences Research Foundation. International Prostate Symptom Score (IPSS). http://www.usrf.org/questionnaires/AUA_SymptomScore.html. Accessed October 16, 2018.
- McVary KT, Roehrborn CG, Avins AL, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol 2011; 185(5):1793–1803. doi:10.1016/j.juro.2011.01.074
- McConnell JD, Roehrborn CG, Bautista OM, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med 2003; 349(25):2387–2398. doi:10.1056/NEJMoa030656
- Matsukawa Y, Takai S, Funahashi Y, et al. Effects of withdrawing alpha-1 blocker from the combination therapy with alpha-1 blocker and 5-alpha-reductase inhibitor in patients with lower urinary tract symptoms suggestive of benign prostatic hyperplasia: a prospective and comparative trial using urodynamics. J Urol 2017; 198(4):905–912. doi:10.1016/j.juro.2017.05.031
- Barkin J, Guimaraes M, Jacobi G, Pushkar D, Taylor S, van Vierssen Trip OB. Alpha-blocker therapy can be withdrawn in the majority of men following initial combination therapy with the dual 5a-reductase inhibitor dutasteride. Eur Urol 2003; 44(4):461–466. pmid:14499682
- Baldwin KC, Ginsberg PC, Roehrborn CG, Harkaway RC. Discontinuation of alpha-blockade after initial treatment with finasteride and doxazosin in men with lower urinary tract symptoms and clinical evidence of benign prostatic hyperplasia. Urology 2001; 58(2):203–209. pmid:11489700
- Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA 2015; 314(17):1818–1831. doi:10.1001/jama.2015.13766
- DuBeau CE, Yalla SV, Resnick NM. Improving the utility of urine flow rate to exclude outlet obstruction in men with voiding symptoms. J Am Geriatr Soc 1998; 46(9):1118–1124. pmid:9736105
- US Department of Health and Human Services Health Resources and Services Administration. United States Cancer Statistics: 1999-2014 Incidence and Mortality Web-Based Report. Atlanta; 2017. https://nccd.cdc.gov/uscs/. Accessed October 17, 2018.
- US Preventive Services Task Force. Final recommendation statement. Prostate cancer: screening. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening1. Accessed October 16, 2018.
- US Preventive Services Task Force. Archived: prostate cancer: screening. Original release date: May 2012. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/prostate-cancer-screening. Accessed October 16, 2018.
- Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA guideline. J Urol 2013; 190(2):419–426. doi:10.1016/j.juro.2013.04.119
- Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 2009; 360(13):1320–1328. doi:10.1056/NEJMoa0810084
- Andriole GL, Crawford ED, Grubb RL, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med 2009; 360(13):1310–1319. doi:10.1056/NEJMoa0810696
- Schröder FH, Hugosson J, Carlsson S, et al. Screening for prostate cancer decreases the risk of developing metastatic disease: findings from the European Randomized Study of Screening for Prostate Cancer (ERSPC). Eur Urol 2012; 62(5):745–752. doi:10.1016/j.eururo.2012.05.068
- Tsodikov A, Gulati R, Heijnsdijk EAM, et al. Reconciling the effects of screening on prostate cancer mortality in the ERSPC and PLCO trials. Ann Intern Med 2017; 167(7):449–455. doi:10.7326/M16-2586
- Martin RM, Donovan JL, Turner EL, et al. Effect of a low-intensity PSA-based screening intervention on prostate cancer mortality: the CAP randomized clinical trial. JAMA 2018; 319(9):883–895. doi:10.1001/jama.2018.0154
- Pahwa S, Schiltz NK, Ponsky LE, Lu Z, Griswold MA, Gulani V. Cost-effectiveness of MR imaging–guided strategies for detection of prostate cancer in biopsy-naive men. Radiology 2017; 285(1):157–166. doi:10.1148/radiol.2017162181
- Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017; 389(10071):815–822. doi:10.1016/S0140-6736(16)32401-1
- Kasivisvanathan V, Rannikko AS, Borghi M, et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med 2018; 378(19):1767–1777. doi:10.1056/NEJMoa1801993
- Wilt TJ, Jones KM, Barry MJ, et al. Follow-up of prostatectomy versus observation for early prostate cancer. N Engl J Med 2017; 377(2):132–142. doi:10.1056/NEJMoa1615869
- Bill-Axelson A, Holmberg L, Garmo H, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med 2014; 370(10):932–942. doi:10.1056/NEJMoa1311593
- Hamdy FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med 2016; 375(15):1415–1424. doi:10.1056/NEJMoa1606220
- Morley JE. Impotence. Am J Med 1986; 80(5):897–905. pmid:3518438
- NIH Consensus Development Panel on Impotence. NIH Consensus Conference. Impotence. JAMA 1993; 270(1):83–90. pmid:8510302
- Selvin E, Burnett AL, Platz EA. Prevalence and risk factors for erectile dysfunction in the US. Am J Med 2007; 120(2):151–157. doi:10.1016/j.amjmed.2006.06.010
- Rosen RC, Cappelleri JC, Gendrano N 3rd. The International Index of Erectile Function (IIEF): a state-of-the-science review. Int J Impot Res 2002; 14(4):226–244. doi:10.1038/sj.ijir.3900857
- Gandaglia G, Briganti A, Jackson G, et al. A systematic review of the association between erectile dysfunction and cardiovascular disease. Eur Urol 2014; 65(5):968–978. doi:10.1016/j.eururo.2013.08.023
- Heaton JPW, Adams MA. Causes of erectile dysfunction. Endocrine 2004; 23(2-3):119–123. doi:10.1385/ENDO:23:2-3:119
- Montorsi F, Salonia A, Deho F, et al. Pharmacological management of erectile dysfunction. BJU Int 2003; 91(5):446–454. pmid:12603396
- Cai X, Tian Y, Wu T, Cao CX, Bu SY, Wang KJ. The role of statins in erectile dysfunction: a systematic review and meta-analysis. Asian J Androl 2014; 16(3):461–466. doi:10.4103/1008-682X.123678
- Webb DJ, Freestone S, Allen MJ, Muirhead GJ. Sildenafil citrate and blood-pressure–lowering drugs: results of drug interaction studies with an organic nitrate and a calcium antagonist. Am J Cardiol 1999; 83(5):21C–28C. pmid:10078539
- Doggrell SA. Comparison of clinical trials with sildenafil, vardenafil and tadalafil in erectile dysfunction. Expert Opin Pharmacother 2005; 6(1):75–84. doi:10.1517/14656566.6.1.75
- Gresser U, Gleiter CH. Erectile dysfunction: comparison of efficacy and side effects of the PDE-5 inhibitors sildenafil, vardenafil and tadalafil—review of the literature. Eur J Med Res 2002; 7(10):435–446. pmid:12435622
- Eardley I, Mirone V, Montorsi F, et al. An open-label, multicentre, randomized, crossover study comparing sildenafil citrate and tadalafil for treating erectile dysfunction in men naive to phosphodiesterase 5 inhibitor therapy. BJU Int 2005; 96(9):1323–1332. doi:10.1111/j.1464-410X.2005.05892.x
- von Keitz A, Rajfer J, Segal S, et al. A multicenter, randomized, double-blind, crossover study to evaluate patient preference between tadalafil and sildenafil. Eur Urol 2004; 45(4):499–509. doi:10.1016/j.eururo.2003.11.030
- Martin-Morales A, Haro JM, Beardsworth A, Bertsch J, Kontodimas S; EDOS Group. Therapeutic effectiveness and patient satisfaction after 6 months of treatment with tadalafil, sildenafil, and vardenafil: results from the erectile dysfunction observational study (EDOS). Eur Urol 2007; 51(2):541–550. doi:10.1016/j.eururo.2006.09.027
- Yuan J, Zhang R, Yang Z, et al. Comparative effectiveness and safety of oral phosphodiesterase type 5 inhibitors for erectile dysfunction: a systematic review and network meta-analysis. Eur Urol 2013; 63(5):902–912. doi:10.1016/j.eururo.2013.01.012
- Cao S, Yin X, Wang Y, Zhou H, Song F, Lu Z. Smoking and risk of erectile dysfunction: systematic review of observational studies with meta-analysis. PLoS One 2013; 8(4):e60443. doi:10.1371/journal.pone.0060443
- Derby CA, Mohr BA, Goldstein I, Feldman HA, Johannes CB, McKinlay JB. Modifiable risk factors and erectile dysfunction: can lifestyle changes modify risk? Urology 2000; 56(2):302–306. pmid:10925098
- Esposito K, Giugliano F, Di Palo C, et al. Effect of lifestyle changes on erectile dysfunction in obese men: a randomized controlled trial. JAMA 2004; 291(24):2978–2984. doi:10.1001/jama.291.24.2978
- Schmidt HM, Munder T, Gerger H, Frühauf S, Barth J. Combination of psychological intervention and phosphodiesterase-5 inhibitors for erectile dysfunction: a narrative review and meta-analysis. J Sex Med 2014; 11(6):1376–1391. doi:10.1111/jsm.12520
- New Hope Network. Supplement Business Report 2017. Boulder; 2017. http://images.info.newhope.com/Web/NewHopeNaturalMedia/%7B3a3f3b03-6130-41d4-9e66-84f29eeebe44%7D_2017_Supplement_Business_Report_-_Extended_TOC.pdf. Accessed October 16, 2018.
- Labre MP. Burn fat, build muscle: a content analysis of men’s health and men’s fitness. Int J Mens Health 2005; 4(2):187–200.
- Qato DM, Alexander GC, Conti RM, Johnson M, Schumm P, Lindau ST. Use of prescription and over-the-counter medications and dietary supplements among older adults in the United States. JAMA 2008; 300(24):2867–2878. doi:10.1001/jama.2008.892
- Park SY, Murphy SP, Wilkens LR, Henderson BE, Kolonel LN. Multivitamin use and the risk of mortality and cancer incidence: the multiethnic cohort study. Am J Epidemiol 2011; 173(8):906–914. doi:10.1093/aje/kwq447
- McNeill G, Avenell A, Campbell MK, et al. Effect of multivitamin and multimineral supplementation on cognitive function in men and women aged 65 years and over: a randomised controlled trial. Nutr J 2007; 6(1):10. doi:10.1186/1475-2891-6-10
- Harris E, Macpherson H, Vitetta L, Kirk J, Sali A, Pipingas A. Effects of a multivitamin, mineral and herbal supplement on cognition and blood biomarkers in older men: a randomised, placebo-controlled trial. Hum Psychopharmacol Clin Exp 2012; 27(4):370–377. doi:10.1002/hup.2236
- Vivekananthan DP, Penn MS, Sapp SK, Hsu A, Topol EJ. Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Lancet 2003; 361(9374):2017–2023. doi:10.1016/S0140-6736(03)13637-9
- Miller ER, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med 2005; 142(1):37–46. pmid:15537682
- Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA 2007; 297(8):842–857. doi:10.1001/jama.297.8.842
- Grey A, Bolland M. Clinical trial evidence and use of fish oil supplements. JAMA Intern Med 2014; 174(3):460–462. doi:10.1001/jamainternmed.2013.12765
- Cui T, Kovell RC, Brooks DC, Terlecki RP. A urologist’s guide to ingredients found in top-selling nutraceuticals for men’s sexual health. J Sex Med 2015; 12(11):2105–2117. doi:10.1111/jsm.13013
- Schenk JM, Till CA, Tangen CM, et al. Serum 25-hydroxyvitamin D concentrations and risk of prostate cancer: results from the Prostate Cancer Prevention Trial. Cancer Epidemiol Prev Biomarkers 2014; 23(8):1484–1493. doi:10.1158/1055-9965.EPI-13-1340
- Albanes D, Mondul AM, Yu K, et al. Serum 25-hydroxy vitamin D and prostate cancer risk in a large nested case-control study. Cancer Epidemiol Prev Biomarkers 2011; 20(9):1850–1860. doi:10.1158/1055-9965.EPI-11-0403
- Roswall N, Larsen SB, Friis S, et al. Micronutrient intake and risk of prostate cancer in a cohort of middle-aged, Danish men. Cancer Causes Control 2013; 24(6):1129–1135. doi:10.1007/s10552-013-0190-4
- Mondul AM, Watters JL, Männistö S, et al. Serum retinol and risk of prostate cancer. Am J Epidemiol 2011; 173(7):813-821. doi:10.1093/aje/kwq429
- Schenk JM, Riboli E, Chatterjee N, et al. Serum retinol and prostate cancer risk: a nested case-control study in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol Prev Biomarkers 2009; 18(4):1227–1231. doi:10.1158/1055-9965.EPI-08-0984
- Bidoli E, Talamini R, Zucchetto A, et al. Dietary vitamins E and C and prostate cancer risk. Acta Oncol 2009; 48(6):890–894. doi:10.1080/02841860902946546
- Wright ME, Weinstein SJ, Lawson KA, et al. Supplemental and dietary vitamin E intakes and risk of prostate cancer in a large prospective study. Cancer Epidemiol Prev Biomarkers 2007; 16(6):1128–1135. doi:10.1158/1055-9965.EPI-06-1071
- Klein EA, Thompson IM, Tangen CM, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011; 306(14):1549–1556. doi:10.1001/jama.2011.1437
- Jingwi EY, Abbas M, Ricks-Santi L, et al. Vitamin D receptor genetic polymorphisms are associated with PSA level, Gleason score and prostate cancer risk in African-American men. Anticancer Res 2015; 35(3):1549–1558. pmid:25750310
- Siddiqui IA, Asim M, Hafeez BB, Adhami VM, Tarapore RS, Mukhtar H. Green tea polyphenol EGCG blunts androgen receptor function in prostate cancer. FASEB J 2011; 25(4):1198–1207. doi:10.1096/fj.10-167924
- Yacoubian A, Dargham RA, Khauli RB, Bachir BG. Overview of dietary supplements in prostate cancer. Curr Urol Rep 2016; 17(11):78. doi:10.1007/s11934-016-0637-8
- Kallifatidis G, Hoy JJ, Lokeshwar BL. Bioactive natural products for chemoprevention and treatment of castration-resistant prostate cancer. Semin Cancer Biol 2016; 40:160–169. doi:10.1016/j.semcancer.2016.06.003
- Shui IM, Mondul AM, Lindström S, et al. Circulating vitamin D, vitamin D–related genetic variation, and risk of fatal prostate cancer in the National Cancer Institute Breast and Prostate Cancer Cohort Consortium. Cancer 2015; 121(12):1949–1956. doi:10.1002/cncr.29320
- Tacklind J, MacDonald R, Rutks I, Stanke JU, Wilt TJ. Serenoa repens for benign prostatic hyperplasia. Cochrane Database Syst Rev 2012; 12:CD001423. doi:10.1002/14651858.CD001423.pub3
- Wandel S, Jüni P, Tendal B, et al. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis. BMJ 2010; 341:c4675. doi:10.1136/bmj.c4675
- Reinhart KM, Coleman CI, Teevan C, Vachhani P, White CM. Effects of garlic on blood pressure in patients with and without systolic hypertension: a meta-analysis. Ann Pharmacother 2008; 42(12):1766–1771. doi:10.1345/aph.1L319
- Wang J, Zhang X, Lan H, Wang W. Effect of garlic supplement in the management of type 2 diabetes mellitus (T2DM): a meta-analysis of randomized controlled trials. Food Nutr Res 2017; 61(1):1377571. doi:10.1080/16546628.2017.1377571
KEY POINTS
- The combination of an alpha-blocker and a 5-alpha reductase inhibitor is an effective regimen for BPH. Withdrawing the alpha-blocker from the combination can be considered if symptoms have been well controlled after 1 year of combination therapy.
- A new look at 2 large trials of prostate-specific antigen screening strengthened evidence that testing in the right patient population can reduce deaths from prostate cancer, but a third recently published trial found no benefit to 1-time screening.
- Magnetic resonance imaging offers a better method than ultrasonography-guided biopsy to triage patients thought to be at high risk of prostate cancer and tends to limit costly overtreatment of disease that likely would not cause death.
- Erectile dysfunction is often associated with chronic disease and may suggest the need to screen for cardiovascular disease.
Bisphosphonate-related atypical femoral fracture: Managing a rare but serious complication
Bisphosphonate therapy minimizes bone loss and reduces fracture risk by up to 50% in patients with osteoporosis,1 but it is also associated with increased risks of osteonecrosis of the jaw and atypical femoral fracture. Although atypical femoral fractures are rare, they can have a devastating effect. Patient concern about this complication has contributed to a decrease in bisphosphonate use by about half in the last decade or so,2,3 and we fear this could result in an increase in hip fracture rates.
In this article, we examine the evidence on bisphosphonate-associated atypical femoral fractures, including risks, pathogenesis, treatment, and prevention.
ATYPICAL FRACTURES INVOLVE THE FEMORAL SHAFT, NOT THE HEAD
An atypical femoral fracture is a transverse fracture of the femoral shaft (diaphysis), defined by both clinical criteria and radiographic appearance.
To be defined as atypical, a femoral fracture must meet 4 of the following 5 criteria4:
- Occurs with minimal or no trauma
- Has a predominantly transverse fracture line, originating at the lateral cortex and sometimes becoming oblique as it progresses medially across the femur
- Extends through both cortices and may be associated with a medial spike (complete fractures); or involves only the lateral cortex (incomplete fractures)
- Is noncomminuted or minimally comminuted
- Shows localized periosteal or endosteal thickening (termed “beaking” or “flaring”) of the lateral cortex at the fracture site.
Several minor features are also important but are not required, eg:
- Cortical thickening of the femoral shaft
- Unilateral or bilateral prodromal pain preceding the fracture
- Bilateral incomplete or complete femoral diaphysis fractures
- Delayed fracture healing.
Atypical femoral fracture can occur anywhere along the shaft, from just distal to the lesser trochanter to just proximal to the supracondylar flare. However, most occur in 2 areas, with 1 cluster centered at about 41 mm from the lesser trochanter (more common in relatively younger patients) and the other at 187 mm.5
ABSOLUTE RISK IS LOW BUT INCREASES WITH LONGER USE
Atypical femoral fractures are rare. Schilcher et al6 reviewed radiographs of 1,234 women who had a subtrochanteric or shaft fracture and found 59 (4.6%) of fractures were atypical. In a systematic review of 14 studies,7 the incidence ranged from 3.0 to 9.8 cases per 100,000 patient-years.
Furthermore, not all atypical femoral fractures are in bisphosphonate users: 7.4% were in nonusers in 1 series8 and 22% in another.9
Nevertheless, most studies show that bisphosphonate use increases the incidence of atypical femoral fracture, and the incidence increases with duration of use, especially after 3 years.7
An international task force of the American Society for Bone and Mineral Research listed the absolute risk as between 3.2 and 50 cases per 100,000 patient-years, with longer use (> 5 years) increasing the risk to about 100 per 100,000 patient-years.4 After stopping bisphosphonate therapy, the risk diminished by 70% per year.9
In another study, for 0.1 to 1.9 years of therapy, the age-adjusted atypical fracture rates were 1.78 per 100,000 per year (95% confidence interval [CI] 1.5–2.0), increasing to 113.1 per 100,000 per year (95% CI 69.3–156.8) with exposure from 8 to 9.9 years.10
A case-control study found that more than 5 years of bisphosphonate use increased the fracture risk by an odds ratio of 2.74 (95% CI 1.25–6.02).11
The incidence of typical femoral fracture was higher in those who adhered better to their oral bisphosphonate regimen in some studies,12 but the opposite was true in others.13
The benefits of bisphosphonate therapy in reducing fracture risk, however, outweigh the risk of atypical fracture.4
We do not know whether the rate of atypical femoral fracture is increasing. A review of Kaiser Permanente Northwest records found that the rates of atypical femoral shaft fracture had remained stable from 1996 to 2009. However, 61.9% of patients who met the strict radiographic criteria had taken oral bisphosphonates.14 These data suggest that bisphosphonate use has not increased the overall population-based risk for subtrochanteric and femoral shaft fractures, but that bisphosphonates and other risk factors may have increased the likelihood that such fractures will exhibit atypical radiographic features.
A population-based study in Denmark13 found that alendronate use longer than 10 years was associated with an adjusted 30% lower risk of hip fracture and no increase in the risk of subtrochanteric and femoral shaft fracture. In addition, the risk of subtrochanteric and femoral shaft fracture was lower with high adherence to alendronate treatment (based on medication possession ratio > 80%) compared with low adherence (ratio < 50%) (odds ratio 0.88, 95% CI 0.77–0.99). The risk was not increased in current vs past users.
The Danish study13 used the coding of the 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10) to identify subtrochanteric and femoral shaft fractures without radiologic review for atypical radiographic features. The lack of specific ICD-10 coding for subtrochanteric and femoral shaft fractures with atypical radiographic features has limited our knowledge of their incidence.
Contralateral fracture in more than one-fourth of cases
After an atypical femoral fracture, patients have a significant risk of fracture on the contralateral side. In a case-control study, 28% of patients with atypical femoral fracture suffered a contralateral fracture, compared with 0.9% of patients presenting with a typical fracture pattern (odds ratio 42.6, 95% CI 12.8–142.4).15
Contralateral fracture occurs from 1 month to 4 years after the index atypical femoral fracture.16
There are reports of bisphosphonate-related low-impact fractures in other sites such as the tibia17 and forearm.18 However, they may be too rare to warrant screening.
Mortality rates
A Swedish database study found that patients with atypical femoral fractures, whether bisphosphonate users or nonusers, do not have higher mortality rates than patients with ordinary subtrochanteric or femoral shaft fractures.19 Furthermore, the mortality rates for those with atypical femoral fracture were similar to rates in the general population. In contrast, patients with an ordinary femoral fracture had a higher mortality risk than the general population.19
Other studies suggest that atypical femoral fracture may be associated with a less favorable prognosis in older patients,20 but this could be due to differences in demographics, treatment adherence, or postfracture care.21
In addition, functional outcomes as measured by independent mobility at discharge and at 3 months were comparable between patients with atypical fracture and those with typical fracture.22
IMAGING STUDIES
If a long-term bisphosphonate user presents with hip, thigh, or groin pain, imaging studies are recommended.
Plain radiography
Radiography is usually the first step and should include a frontal view of the pelvis (Figure 1) and 2 views of the full length of each femur. If radiography is not conclusive, bone scan or magnetic resonance imaging (MRI) should be considered.
A linear cortex transverse fracture pattern and focal lateral cortical thickening are the most sensitive and specific radiographic features.23,24 Because of the risk of fracture on the contralateral side, radiographic study of that side is recommended as well.
Computed tomography
Computed tomography (CT) is not sensitive for early stress fractures and, given the radiation burden, is not recommended in the workup of atypical fracture.
Bone scanning
Bone scanning using technetium 99m-labeled methylene diphosphonate with a gamma camera shows active bone turnover. Stress fractures and atypical femoral fractures are most easily identified in the third (delayed) phase of the bone scan. Although bone scanning is highly sensitive, the specificity is limited by lack of spatial resolution. Atypical femoral fracture appears as increased activity in the subtrochanteric region with a predilection for the lateral cortex.
Dual-energy x-ray absorptiometry
Conventional dual-energy x-ray absorptiometry (DXA) extends only to 1 to 2 cm below the lesser trochanter and can therefore miss atypical fractures, which usually occur farther down. The overall detection rate for DXA was 61% in a sample of 33 patients.25
Newer scanners can look at the entire femoral shaft.26 In addition, newer software can quantify focal thickening (beaking) of the lateral cortex and screen patients who have no symptoms. The results of serial measurements can be graphed so that the practitioner can view trends to help assess or rule out potential asymptomatic atypical femoral fracture.
A localized reaction (periosteal thickening of the lateral cortex or beaking) often precedes atypical femoral fracture. A 2017 study reported that patients with high localized reaction (mean height 3.3 mm) that was of the pointed type and was accompanied by prodromal pain had an increased risk of complete or incomplete atypical femoral fracture at that site.27 This finding is used by the newer DXA software. The predictive value of beaking on extended femoral DXA may be as high as 83%.26
Magnetic resonance imaging
The MRI characteristics of atypical femoral fracture are similar to those of other stress fractures except that there is a lateral-to-medial pattern rather than a medial pattern. The earliest findings include periosteal reaction about the lateral cortex with a normal marrow signal.
MRI may be of particular benefit in patients with known atypical femoral fracture to screen the contralateral leg. It should image the entire length of both femurs. Contrast enhancement is not needed.
Regardless of whether initial findings were discovered on conventional radiographs or DXA, MRI confirmation is needed. Radionuclide bone scanning is currently not recommended because it lacks specificity. Combination imaging is recommended, with either radiography plus MRI or DXA plus MRI.
DIFFERENTIAL DIAGNOSIS
The differential diagnosis of atypical femoral fracture includes stress fracture, pathologic fracture, hypophosphatasia, and osteogenesis imperfecta.28 Hypophosphatemic osteomalacia can cause Looser zones, which can be confused with atypical femoral fractures but usually occur on the medial side.4 Stress fracture of the femur can occur below the lesser trochanter but usually begins in the medial, not the lateral, cortex.
Pathologic fractures from underlying osseous lesions can mimic the cortical beaking of bisphosphonate-related fracture, but they usually show the associated underlying lucent lesion and poorly defined margins. A sinus tract along the region of a chronic osteomyelitis may also appear similar.
Hypophosphatasia is an inborn error of metabolism caused by a loss-of-function mutation in the gene encoding alkaline phosphatase, resulting in pyrophosphate accumulation and causing osteomalacia from impaired mineralization. This can result in femoral pseudofracture that is often bilateral and occurs in the subtrochanteric region.29
ADDITIONAL RISK FACTORS
Patients with atypical femoral fracture are generally a heterogeneous group, but there are risk factors to note other than bisphosphonate exposure.
Asian women had a risk 8 times higher than white women in 1 study.30
Bone geometry. Mahjoub et al8 reported that compared with controls, patients with atypical femoral fracture had greater offset of the femoral shaft from the center of rotation of the femoral head, a more acute angle between the femoral neck and shaft, and greater proximal cortical thickness.
Medications. In addition to bisphosphonates, other drugs associated with atypical femoral fracture include RANK-ligand inhibitors such as denosumab (another drug for osteoporosis),31 glucocorticoids,32,33 and proton pump inhibitors.32,33
Genetics. Three sisters with atypical femoral fracture were found to have 37 rare mutations in 34 genes, including one in the GGPS1 gene, which codes for geranylgeranyl pyrophosphate synthase—an enzyme that bisphosphonates inhibit.34
Medical conditions other than osteoporosis include collagen diseases, chronic pulmonary disease, asthma, rheumatoid arthritis, and diabetes.35
Clinical recommendations
Current recommendations are to reevaluate bisphosphonate use in patients with osteoporosis after 5 or more years of therapy.36
Given that patients with osteoporosis are at increased risk of typical fracture, those at higher risk should be considered for continued bisphosphonate therapy. Factors for high risk include the following:
- History of fracture on therapy
- Hip T score –2.5 or lower
- Older age (≥ 70)
- Other strong risk factors for fracture such as smoking, alcohol use, corticosteroid use, rheumatoid arthritis, and family history
- World Health Organization FRAX fracture risk score above the country-specific threshold.
Those at lower risk should be considered for a 2- to 3-year bisphosphonate holiday with periodic reevaluation of bone density and, possibly, bone markers.36
WHAT IS THE UNDERLYING PATHOPHYSIOLOGY?
The mechanism by which bisphosphonates increase the risk of atypical femoral fracture is not clear. These drugs work by suppressing bone turnover; however, in theory, prolonged use could suppress it too much and increase bone fragility.
One hypothesis is that bisphosphonates impair the toughening of cortical bone, an important barrier to clinical fracture. This is supported by a study that found bisphosphonate users with atypical femoral fracture had deficits in intrinsic and extrinsic bone toughness, perhaps due to treatment-related increases in matrix mineralization.37 Although this study and others showed an increase in matrix mineralization and reduced mineralization heterogeneity with bisphosphonate use,38,39 it is unclear whether such changes contributed to reduced toughness or to atypical femoral fracture.
Changes in the skeletal geometry of the lower limb such as femoral neck-shaft angle and femoral curvature alter the stresses and strains experienced by the femoral diaphysis with loading. Because the incidence of incomplete atypical femoral fracture is much greater than that of complete fracture, most incomplete atypical femoral fractures heal before the fracture progresses.
Ultimately, all fractures, including atypical femoral fractures, occur when mechanical stress and strain exceed bone strength.
Antiresorptive drugs such as bisphosphonates, estrogen, calcitonin, and RANK ligand inhibitors prevent hip fracture by increasing the strength of the proximal femur—perhaps at the expense of the strength (or toughness) of the subtrochanteric shaft. It is also possible that treatment-related increases in hip strength (and reduced hip fracture rates) promote or sustain the transfer of stress and strain to femoral regions that experience lesser or no increases in strength from treatment, which likely includes the shaft.40,41
CT studies in Japanese women with osteoporosis have shown that 2 years of zoledronate therapy had greater effects in the hip than in the femoral shaft, with significant increases in cortical thickness and volumetric bone mineral density at the femoral neck and intertrochanteric region compared with baseline.42 But zoledronate did not increase femoral shaft cortical thickness and caused only a minor increase in femoral shaft volumetric bone mineral density. Fracture patterns may have depended on damage and effects of bone turnover on mass and structure.
This hypothetical scenario portrays a possible “hip survival bias” mechanism for atypical femoral fracture, with the association with antiresorptive drugs arising from greater stress and strain in cortical regions where these fractures occur rather than from treatment-related reductions in cortical bone strength or toughness.
PRODROMAL PAIN IS COMMON
From 32% to 76% of patients who have incomplete or developing atypical femoral fracture present with a prodrome of groin or hip pain.4,43 Prodromal pain occurs any time from 2 weeks to several years before the fracture, presenting as pain in the anterior or lateral thigh or in the groin.
Prodromal pain in a patient on antiresorptive therapy should be a signal for the clinician to obtain a radiograph of the hip and to look for contralateral symptoms and fractures. The most common mechanism of injury appears to be a ground-level fall or even a nontraumatic activity such as walking or stepping off a curb.
MEDICAL MANAGEMENT
In bisphosphonate users with radiographic evidence of atypical femoral fracture, the bisphosphonate should be discontinued and the patient assessed for calcium and vitamin D deficiency, with supplements prescribed if needed.4
For patients with incomplete fracture and persistent pain after 3 months of medical management, prophylactic surgical nail fixation is recommended to prevent complete fracture.
Teriparatide, which has been associated with enhanced bone fracture healing, is a possible treatment to promote healing of atypical femoral fracture, either alone or as an adjunct to surgical fixation. A systematic review published in 2015 supported the use of teriparatide for enhancing fracture healing in atypical femoral fracture.44 In addition, a 10-patient series45 showed that incomplete fractures without radiolucent lines responded to teriparatide alone, whereas those with radiolucent lines needed intramedullary nailing.
These results suggest that teriparatide works best when the fracture site is stable, either inherently or with surgical fixation.
ORTHOPEDIC CARE
Orthopedic care for atypical femoral fracture differs depending on whether the patient experiences pain and whether the fracture is incomplete or complete. Figure 2 shows a treatment algorithm for atypical femoral fracture.
These are difficult fractures to manage, complicated by delayed healing in the elderly, complex displacement patterns, altered bone geometry, and risk of fracture in the opposite limb, all of which raise questions about recommending protected weight-bearing exercise.
Furthermore, atypical femoral fracture is often associated with increased anterolateral bowing of the femur, making it difficult to insert an intramedullary nail: the radius of curvature of the bone is shorter than that of a standard femoral nail. This mismatch can lead to intraoperative complications such as iatrogenic fracture during prophylactic nailing, malunion from excess straightening of the femur (which can itself lead to leg length discrepancy), and gapping of the fracture site, particularly on the medial side.
Intramedullary nailing for complete fracture
Intramedullary nailing is the first-line treatment for complete atypical femoral fracture, although the risk of delayed healing and revision surgery may be somewhat higher than with typical femoral fracture.46 Prophylactic intramedullary nailing should be considered for a patient with intractable pain.2
A radiograph of the opposite leg should be obtained routinely, looking for an asymptomatic fracture. Bisphosphonates should be discontinued and calcium and vitamin D continued. Teriparatide therapy can be considered as an alternative treatment.
Conservative management for incomplete fracture without pain
Incomplete atypical femoral fracture unaccompanied by pain can be followed conservatively.47 In addition to stopping antiresorptive therapy, patients need to avoid high-impact and repetitive-impact activities such as jumping or running. If pain occurs, patients should begin protected weight-bearing exercise.
Treatment is uncertain for incomplete fracture with pain
For patients with incomplete atypical femoral fracture and pain, treatment is controversial. Regimens that include 2 to 3 months of protected weight-bearing exercise, a full metabolic bone workup, calcium and vitamin D supplementation, and anabolic bone agents have produced some success. Some authors have reported poor results from conservative care, with few patients achieving pain relief or signs of complete healing.48,49 Additionally, if an incomplete fracture is found in the opposite femur, protected weight-bearing of both legs may not be possible.
Patients with incomplete fracture should be monitored regularly with radiography and physical examination. If there is progression of the fracture, escalation of pain, or failure to heal within 2 to 3 months, then surgical treatment is necessary.
Prophylactic placement of an intramedullary nail to prevent completion of the fracture and allow a return to full weight-bearing is generally advised.50 A long locking plate can be used if bone deformities make it difficult to place an intramedullary nail; however, nails are preferred because they allow formation of endochondral callus, which can be helpful in these difficult-to-heal fractures.
Results from retrospective reviews have shown that surgically treated patients with bisphosphonate-associated incomplete atypical femoral fracture were more likely than those treated nonsurgically to be pain-free (81% vs 64%) and have radiographic healing (100% vs 18% at final follow-up).46 Results have also been positive for those with complete atypical femoral fracture. At 6 months, 64% of surgically treated patients were pain-free and 98% were radiographically healed.51
The unusual geometry of the femur in patients with atypical femoral fracture and the presence of intramedullary cortical callus makes the placement of an intramedullary femoral rod more complex than in typical femoral fracture.8
Intramedullary nailing of atypical femoral fracture is a challenge for even the most experienced surgeon, and vigilance is imperative to avoid iatrogenic fracture and malunion.
MANY QUESTIONS REMAIN
We need more studies on the pathophysiology of bisphosphonate-associated atypical femoral fracture, the value of periodic screening with DXA, and which factors predict high risk (eg, Asian ethnicity, use of certain medications, femoral geometry). In addition, we need more data on the success of conservative management of incomplete fracture, including use of teriparatide.
- Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 1996; 348(9041):1535–1541. pmid:8950879
- Jha S, Wang Z, Laucis N, Bhattacharyya T. Trends in media reports, oral bisphosphonate prescriptions, and hip fractures 1996–2012: an ecological analysis. J Bone Miner Res 2015; 30(12):2179–2187. doi:10.1002/jbmr.2565
- Solomon DH, Johnston SS, Boytsov NN, McMorrow D, Lane JM, Krohn KD. Osteoporosis medication use after hip fracture in US patients between 2002 and 2011. J Bone Miner Res 2014; 29(9):1929–1937. doi:10.1002/jbmr.2202
- Shane E, Burr D, Abrahamsen B, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2014; 29(1):1–23. doi:10.1002/jbmr.1998
- Koeppen VA, Schilcher J, Aspenberg P. Dichotomous location of 160 atypical femoral fractures. Acta Orthop 2013; 84(6):561–564. doi:10.3109/17453674.2013.866193
- Schilcher J, Koeppen V, Aspenberg P, Michäelsson K. Risk of atypical femoral fracture during and after bisphosphonate use. Acta Orthop 2015; 86(1):100–107. doi:10.3109/17453674.2015.1004149
- Khow KS, Shibu P, Yu SC, Chehade MJ, Visvanathan R. Epidemiology and postoperative outcomes of atypical femoral fractures in older adults: a systematic review. J Nutr Health Aging 2017; 21(1):83–91. doi:10.1007/s12603-015-0652-3
- Mahjoub Z, Jean S, Leclerc JT, et al. Incidence and characteristics of atypical femoral fractures: clinical and geometrical data. J Bone Miner Res 2016; 31(4):767–776. doi:10.1002/jbmr.2748
- Schilcher J, Michaelsson K, Aspenberg P. Bisphosphonate use and atypical fractures of the femoral shaft. N Engl J Med 2011; 364(18):1728–1737. doi:10.1056/NEJMoa1010650
- Dell RM, Adams AL, Greene DF, et al. Incidence of atypical nontraumatic diaphyseal fractures of the femur. J Bone Miner Res 2012; 27(12):2544–2550. doi:10.1002/jbmr.1719
- Park-Wyllie LY, Mamdani MM, Juurlink DN, et al. Bisphosphonate use and the risk of subtrochanteric or femoral shaft fractures in older women. JAMA 2011; 305(8):783–789. doi:10.1001/jama.2011.190
- Wang Z, Ward MM, Chan L, Bhattacharyya T. Adherence to oral bisphosphonates and the risk of subtrochanteric and femoral shaft fractures among female Medicare beneficiaries. Osteoporos Int 2014; 25(8):2109–2116. doi:10.1007/s00198-014-2738-x
- Abrahamsen B, Eiken P, Prieto-Alhambra D, Eastell R. Risk of hip, subtrochanteric, and femoral shaft fractures among mid and long term users of alendronate: nationwide cohort and nested case-control study. BMJ 2016; 353:i3365. doi:10.1136/bmj.i3365
- Feldstein AC, Black D, Perrin N, et al. Incidence and demography of femur fractures with and without atypical features. J Bone Miner Res 2012; 27(5):977–986. doi:10.1002/jbmr.1550
- Meier RP, Perneger TV, Stern R, Rizzoli R, Peter RE. Increasing occurrence of atypical femoral fractures associated with bisphosphonate use. Arch Intern Med 2012; 172(12):930–936. doi:10.1001/archinternmed.2012.1796
- La Rocca Vieira R, Rosenberg ZS, Allison MB, Im SA, Babb J, Peck V. Frequency of incomplete atypical femoral fractures in asymptomatic patients on long term bisphosphonate therapy. AJR Am J Roentgenol 2012; 198(5):1144–1151. doi:10.2214/AJR.11.7442
- Bissonnette L, April PM, Dumais R, Boire G, Roux S. Atypical fracture of the tibial diaphysis associated with bisphosphonate therapy: a case report. Bone 2013; 56(2):406–409. doi:10.1016/j.bone.2013.07.012
- Moon J, Bither N, Lee T. Atypical forearm fractures associated with long-term use of bisphosphonate. Arch Orthop Trauma Surg 2013; 133(7):889–892. doi:10.1007/s00402-013-1760-3
- Kharazmi M, Hallberg P, Schilcher J, Aspenberg P, Michaëlsson K. Mortality after atypical femoral fractures: a cohort study. J Bone Miner Res 2016; 31(3):491–497. doi:10.1002/jbmr.2767
- Medin E, Goude F, Melberg HO, Tediosi F, Belicza E, Peltola M; EuroHOPE Study Group. European regional differences in all-cause mortality and length of stay for patients with hip fracture. Health Econ 2015; 24(suppl 2):53–64. doi:10.1002/hec.3278
- Abrahamsen B, Prieto-Alhambra D. Patients with atypical femur fractures have the same mortality as the background population-drug channeling bias, bisphosphonate effects and public health implications. J Bone Miner Res 2016; 31(3):488–490. doi:10.1002/jbmr.2801
- Khow KS, Paterson F, Shibu P, Yu SC, Chehade MJ, Visvanathan R. Outcomes between older adults with atypical and typical femoral fractures are comparable. Injury 2017; 48(2):394–398. doi:10.1016/j.injury.2016.10.035
- Adams AL, Xue F, Chantra JQ, et al. Sensitivity and specificity of radiographic characteristics in atypical femoral fractures. Osteoporos Int 2017; 28(1):413–417. doi:10.1007/s00198-016-3809-y
- Rosenberg ZS, La Rocca Vieira R, Chan SS, et al. Bisphosphonate-related complete atypical subtrochanteric femoral fractures: diagnostic utility of radiography. AJR Am J Roentgenol 2011; 197(4):954–960. doi:10.2214/AJR.10.6262
- Kim S, Yang KH, Lim H, et al. Detection of prefracture hip lesions in atypical subtrochanteric fracture with dual-energy x-ray absorptiometry images. Radiology 2014; 270(2):487–495. doi:10.1148/radiol.13122691
- van de Laarschot DM, Smits AA, Buitendijk SK, Stegenga MT, Zillikens MC. Screening for atypical femur fractures using extended femur scans by DXA. J Bone Miner Res 2017; 32(8):1632–1639. doi:10.1002/jbmr.3164
- Sato H, Kondo N, Nakatsue T, et al. High and pointed type of femoral localized reaction frequently extends to complete an incomplete atypical femoral fracture in patients with autoimmune diseases on long-term glucocorticoids and bisphosphonates. Osteoporos Int 2017; 28(8):2367–2376. doi:10.1007/s00198-017-4038-8
- Giaconi JC, Watterson CT. Bisphosphonate-related atypical femur fractures and the radiographic features. In: Silverman SL, Abrahamsen B, eds. The Duration and Safety of Osteoporosis Treatment. Switzerland: Springer International Publishing; 2016:107–124. doi:10.1007/978-3-319-23639-1
- Whyte MP. Atypical femoral fractures, bisphosphonates, and adult hypophosphatasia. J Bone Miner Res 2009; 24(6):1132–1134. doi:10.1359/jbmr.081253
- Lo JC, Hui RL, Grimsrud CD, et al. The association of race/ethnicity and risk of atypical femoral fracture among older women receiving oral bisphosphonate therapy. Bone 2016; 85:142–147. doi:10.1016/j.bone.2016.01.002
- Bone HG, Wagman RB, Brandi ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol 2017; 5(7):513–523. doi:10.1016/S2213-8587(17)30138-9
- Koh JH, Myong JP, Yoo J, et al. Predisposing factors associated with atypical femur fracture among postmenopausal Korean women receiving bisphosphonate therapy: 8 years' experience in a single center. Osteoporos Int 2017; 28(11):3251–3259. doi:10.1007/s00198-017-4169-y
- Kim D, Sung YK, Cho SK, Han M, Kim YS. Factors associated with atypical femoral fracture. Rheumatol Int 2016; 36(1):65–71. doi:10.1007/s00296-015-3323-0
- Roca-Ayats N, Balcells S, Garcia-Giralt N, et al. GGPS1 mutation and atypical femoral fractures with bisphosphonates. N Engl J Med 2017; 376(18):1794–1795. doi:10.1056/NEJMc1612804
- Giusti A, Hamdy NA, Dekkers OM, Ramautar SR, Dijkstra S, Papapoulos SE. Atypical fractures and bisphosphonate therapy: a cohort study of patients with femoral fracture with radiographic adjudication of fracture site and features. Bone 2011; 48(5):966–971. doi:10.1016/j.bone.2010.12.033
- Adler RA, El-Hajj Fuleihan G, Bauer DC, et al. Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2016; 31(1):16–35. doi:10.1002/jbmr.2708
- Lloyd AA, Gludovatz B, Riedel C, et al. Atypical fracture with long-term bisphosphonate therapy is associated with altered cortical composition and reduced fracture resistance. Proc Natl Acad Sci USA 2017; 114(33):8722–8727. doi:10.1073/pnas.1704460114
- Ettinger B, Burr DB, Ritchie RO. Proposed pathogenesis for atypical femoral fractures; lessons from materials research. Bone 2013; 55(2):495–500. doi:10.1016/j.bone.2013.02.004
- Burr DB, Liu Z, Allen MR. Duration-dependent effects of clinically relevant oral alendronate doses on cortical bone toughness in beagle dogs. Bone 2015; 71:58–62. doi:10.1016/j.bone.2014.10.010
- Sasaki S, Miyakoshi N, Hongo M, Kasukawa Y, Shimada Y. Low-energy diaphyseal femoral fractures associated with bisphosphonate use and severe curved femur: a case series. J Bone Miner Metab 2012; 30(5):561–567. doi:10.1007/s00774-012-0358-0
- Pulkkinen P, Gluer C, Jamsa T. Investigation of differences between hip fracture types: a worthy strategy of improved risk assessment and fracture prevention. Bone 2011; 49(4):600–604. doi:10.1016/j.bone.2011.07.022
- Ito M, Sone T, Shiraki M, et al. The effect of once-yearly zoledronic acid on hip structural and biomechanical properties derived using computed tomography (CT) in Japanese women with osteoporosis. Bone 2018; 106:179–186. doi:10.1016/j.bone.2017.10.013
- Bogdan Y, Einhorn TA. Clinical presentation of atypical femur fractures. In: Silverman SL, Abrahamsen B, eds. The Duration and Safety of Osteoporosis Treatment. Switzerland: Springer International Publishing; 2016:137–140. doi:10.1007/978-3-319-23639-1
- Im GI, Lee SH. Effect of teriparatide on healing of atypical femoral fractures: a systemic review. J Bone Metab 2015; 22(4):183–189. doi:10.11005/jbm.2015.22.4.183
- Saleh A, Hegde VV, Potty AG, Schneider R, Cornell CN, Lane JM. Management strategy for symptomatic bisphosphonate-associated incomplete atypical femoral fractures. HSS J 2012; 8(2):103–110. doi:10.1007/s11420-012-9275-y
- Egol KA, Park JH, Prensky C, Rosenberg ZS, Peck V, Tejwani NC. Surgical treatment improves clinical and functional outcomes for patients who sustain incomplete bisphosphonate-related femur fractures. J Orthop Trauma 2013; 27(6):331–335. doi:10.1097/BOT.0b013e31827240ae
- Koh A, Guerado E, Giannoudis PV. Atypical femoral fractures related to bisphosphonate treatment: issues and controversies related to their surgical management. Bone Joint J 2017; 99-B(3):295–302. doi:10.1302/0301-620X.99B3.BJJ-2016-0276.R2
- Oh CW, Oh JK, Park KC, Kim JW, Yoon YC. Prophylactic nailing of incomplete atypical femoral fractures. ScientificWorldJournal 2013; 2013:450148. doi:10.1155/2013/450148
- Ha YC, Cho MR, Park KH, Kim SY, Koo KH. Is surgery necessary for femoral insufficiency fractures after long-term bisphosphonate therapy? Clin Orthop Relat Res 2010; 468(12):3393–3398. doi:10.1007/s11999-010-1583-2
- Tosounidis TH, Lampropoulou-Adamidou, Kanakaris NK. Intramedullary nailing of sequential bilateral atypical subtrochanteric fractures and the management of distal femoral intraoperative fracture. J Orthop Trauma 2015 Jun 11. Epub ahead of print. doi:10.1097/BOT.0000000000000370
- Egol KA, Park JH, Rosenberg ZS, Peck V, Tejwani NC. Healing delayed but generally reliable after bisphosphonate-associated complete femur fractures treated with IM nails. Clin Orthop Relat Res 2014; 472(9):2728–2734. doi:10.1007/s11999-013-2963-1
Bisphosphonate therapy minimizes bone loss and reduces fracture risk by up to 50% in patients with osteoporosis,1 but it is also associated with increased risks of osteonecrosis of the jaw and atypical femoral fracture. Although atypical femoral fractures are rare, they can have a devastating effect. Patient concern about this complication has contributed to a decrease in bisphosphonate use by about half in the last decade or so,2,3 and we fear this could result in an increase in hip fracture rates.
In this article, we examine the evidence on bisphosphonate-associated atypical femoral fractures, including risks, pathogenesis, treatment, and prevention.
ATYPICAL FRACTURES INVOLVE THE FEMORAL SHAFT, NOT THE HEAD
An atypical femoral fracture is a transverse fracture of the femoral shaft (diaphysis), defined by both clinical criteria and radiographic appearance.
To be defined as atypical, a femoral fracture must meet 4 of the following 5 criteria4:
- Occurs with minimal or no trauma
- Has a predominantly transverse fracture line, originating at the lateral cortex and sometimes becoming oblique as it progresses medially across the femur
- Extends through both cortices and may be associated with a medial spike (complete fractures); or involves only the lateral cortex (incomplete fractures)
- Is noncomminuted or minimally comminuted
- Shows localized periosteal or endosteal thickening (termed “beaking” or “flaring”) of the lateral cortex at the fracture site.
Several minor features are also important but are not required, eg:
- Cortical thickening of the femoral shaft
- Unilateral or bilateral prodromal pain preceding the fracture
- Bilateral incomplete or complete femoral diaphysis fractures
- Delayed fracture healing.
Atypical femoral fracture can occur anywhere along the shaft, from just distal to the lesser trochanter to just proximal to the supracondylar flare. However, most occur in 2 areas, with 1 cluster centered at about 41 mm from the lesser trochanter (more common in relatively younger patients) and the other at 187 mm.5
ABSOLUTE RISK IS LOW BUT INCREASES WITH LONGER USE
Atypical femoral fractures are rare. Schilcher et al6 reviewed radiographs of 1,234 women who had a subtrochanteric or shaft fracture and found 59 (4.6%) of fractures were atypical. In a systematic review of 14 studies,7 the incidence ranged from 3.0 to 9.8 cases per 100,000 patient-years.
Furthermore, not all atypical femoral fractures are in bisphosphonate users: 7.4% were in nonusers in 1 series8 and 22% in another.9
Nevertheless, most studies show that bisphosphonate use increases the incidence of atypical femoral fracture, and the incidence increases with duration of use, especially after 3 years.7
An international task force of the American Society for Bone and Mineral Research listed the absolute risk as between 3.2 and 50 cases per 100,000 patient-years, with longer use (> 5 years) increasing the risk to about 100 per 100,000 patient-years.4 After stopping bisphosphonate therapy, the risk diminished by 70% per year.9
In another study, for 0.1 to 1.9 years of therapy, the age-adjusted atypical fracture rates were 1.78 per 100,000 per year (95% confidence interval [CI] 1.5–2.0), increasing to 113.1 per 100,000 per year (95% CI 69.3–156.8) with exposure from 8 to 9.9 years.10
A case-control study found that more than 5 years of bisphosphonate use increased the fracture risk by an odds ratio of 2.74 (95% CI 1.25–6.02).11
The incidence of typical femoral fracture was higher in those who adhered better to their oral bisphosphonate regimen in some studies,12 but the opposite was true in others.13
The benefits of bisphosphonate therapy in reducing fracture risk, however, outweigh the risk of atypical fracture.4
We do not know whether the rate of atypical femoral fracture is increasing. A review of Kaiser Permanente Northwest records found that the rates of atypical femoral shaft fracture had remained stable from 1996 to 2009. However, 61.9% of patients who met the strict radiographic criteria had taken oral bisphosphonates.14 These data suggest that bisphosphonate use has not increased the overall population-based risk for subtrochanteric and femoral shaft fractures, but that bisphosphonates and other risk factors may have increased the likelihood that such fractures will exhibit atypical radiographic features.
A population-based study in Denmark13 found that alendronate use longer than 10 years was associated with an adjusted 30% lower risk of hip fracture and no increase in the risk of subtrochanteric and femoral shaft fracture. In addition, the risk of subtrochanteric and femoral shaft fracture was lower with high adherence to alendronate treatment (based on medication possession ratio > 80%) compared with low adherence (ratio < 50%) (odds ratio 0.88, 95% CI 0.77–0.99). The risk was not increased in current vs past users.
The Danish study13 used the coding of the 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10) to identify subtrochanteric and femoral shaft fractures without radiologic review for atypical radiographic features. The lack of specific ICD-10 coding for subtrochanteric and femoral shaft fractures with atypical radiographic features has limited our knowledge of their incidence.
Contralateral fracture in more than one-fourth of cases
After an atypical femoral fracture, patients have a significant risk of fracture on the contralateral side. In a case-control study, 28% of patients with atypical femoral fracture suffered a contralateral fracture, compared with 0.9% of patients presenting with a typical fracture pattern (odds ratio 42.6, 95% CI 12.8–142.4).15
Contralateral fracture occurs from 1 month to 4 years after the index atypical femoral fracture.16
There are reports of bisphosphonate-related low-impact fractures in other sites such as the tibia17 and forearm.18 However, they may be too rare to warrant screening.
Mortality rates
A Swedish database study found that patients with atypical femoral fractures, whether bisphosphonate users or nonusers, do not have higher mortality rates than patients with ordinary subtrochanteric or femoral shaft fractures.19 Furthermore, the mortality rates for those with atypical femoral fracture were similar to rates in the general population. In contrast, patients with an ordinary femoral fracture had a higher mortality risk than the general population.19
Other studies suggest that atypical femoral fracture may be associated with a less favorable prognosis in older patients,20 but this could be due to differences in demographics, treatment adherence, or postfracture care.21
In addition, functional outcomes as measured by independent mobility at discharge and at 3 months were comparable between patients with atypical fracture and those with typical fracture.22
IMAGING STUDIES
If a long-term bisphosphonate user presents with hip, thigh, or groin pain, imaging studies are recommended.
Plain radiography
Radiography is usually the first step and should include a frontal view of the pelvis (Figure 1) and 2 views of the full length of each femur. If radiography is not conclusive, bone scan or magnetic resonance imaging (MRI) should be considered.
A linear cortex transverse fracture pattern and focal lateral cortical thickening are the most sensitive and specific radiographic features.23,24 Because of the risk of fracture on the contralateral side, radiographic study of that side is recommended as well.
Computed tomography
Computed tomography (CT) is not sensitive for early stress fractures and, given the radiation burden, is not recommended in the workup of atypical fracture.
Bone scanning
Bone scanning using technetium 99m-labeled methylene diphosphonate with a gamma camera shows active bone turnover. Stress fractures and atypical femoral fractures are most easily identified in the third (delayed) phase of the bone scan. Although bone scanning is highly sensitive, the specificity is limited by lack of spatial resolution. Atypical femoral fracture appears as increased activity in the subtrochanteric region with a predilection for the lateral cortex.
Dual-energy x-ray absorptiometry
Conventional dual-energy x-ray absorptiometry (DXA) extends only to 1 to 2 cm below the lesser trochanter and can therefore miss atypical fractures, which usually occur farther down. The overall detection rate for DXA was 61% in a sample of 33 patients.25
Newer scanners can look at the entire femoral shaft.26 In addition, newer software can quantify focal thickening (beaking) of the lateral cortex and screen patients who have no symptoms. The results of serial measurements can be graphed so that the practitioner can view trends to help assess or rule out potential asymptomatic atypical femoral fracture.
A localized reaction (periosteal thickening of the lateral cortex or beaking) often precedes atypical femoral fracture. A 2017 study reported that patients with high localized reaction (mean height 3.3 mm) that was of the pointed type and was accompanied by prodromal pain had an increased risk of complete or incomplete atypical femoral fracture at that site.27 This finding is used by the newer DXA software. The predictive value of beaking on extended femoral DXA may be as high as 83%.26
Magnetic resonance imaging
The MRI characteristics of atypical femoral fracture are similar to those of other stress fractures except that there is a lateral-to-medial pattern rather than a medial pattern. The earliest findings include periosteal reaction about the lateral cortex with a normal marrow signal.
MRI may be of particular benefit in patients with known atypical femoral fracture to screen the contralateral leg. It should image the entire length of both femurs. Contrast enhancement is not needed.
Regardless of whether initial findings were discovered on conventional radiographs or DXA, MRI confirmation is needed. Radionuclide bone scanning is currently not recommended because it lacks specificity. Combination imaging is recommended, with either radiography plus MRI or DXA plus MRI.
DIFFERENTIAL DIAGNOSIS
The differential diagnosis of atypical femoral fracture includes stress fracture, pathologic fracture, hypophosphatasia, and osteogenesis imperfecta.28 Hypophosphatemic osteomalacia can cause Looser zones, which can be confused with atypical femoral fractures but usually occur on the medial side.4 Stress fracture of the femur can occur below the lesser trochanter but usually begins in the medial, not the lateral, cortex.
Pathologic fractures from underlying osseous lesions can mimic the cortical beaking of bisphosphonate-related fracture, but they usually show the associated underlying lucent lesion and poorly defined margins. A sinus tract along the region of a chronic osteomyelitis may also appear similar.
Hypophosphatasia is an inborn error of metabolism caused by a loss-of-function mutation in the gene encoding alkaline phosphatase, resulting in pyrophosphate accumulation and causing osteomalacia from impaired mineralization. This can result in femoral pseudofracture that is often bilateral and occurs in the subtrochanteric region.29
ADDITIONAL RISK FACTORS
Patients with atypical femoral fracture are generally a heterogeneous group, but there are risk factors to note other than bisphosphonate exposure.
Asian women had a risk 8 times higher than white women in 1 study.30
Bone geometry. Mahjoub et al8 reported that compared with controls, patients with atypical femoral fracture had greater offset of the femoral shaft from the center of rotation of the femoral head, a more acute angle between the femoral neck and shaft, and greater proximal cortical thickness.
Medications. In addition to bisphosphonates, other drugs associated with atypical femoral fracture include RANK-ligand inhibitors such as denosumab (another drug for osteoporosis),31 glucocorticoids,32,33 and proton pump inhibitors.32,33
Genetics. Three sisters with atypical femoral fracture were found to have 37 rare mutations in 34 genes, including one in the GGPS1 gene, which codes for geranylgeranyl pyrophosphate synthase—an enzyme that bisphosphonates inhibit.34
Medical conditions other than osteoporosis include collagen diseases, chronic pulmonary disease, asthma, rheumatoid arthritis, and diabetes.35
Clinical recommendations
Current recommendations are to reevaluate bisphosphonate use in patients with osteoporosis after 5 or more years of therapy.36
Given that patients with osteoporosis are at increased risk of typical fracture, those at higher risk should be considered for continued bisphosphonate therapy. Factors for high risk include the following:
- History of fracture on therapy
- Hip T score –2.5 or lower
- Older age (≥ 70)
- Other strong risk factors for fracture such as smoking, alcohol use, corticosteroid use, rheumatoid arthritis, and family history
- World Health Organization FRAX fracture risk score above the country-specific threshold.
Those at lower risk should be considered for a 2- to 3-year bisphosphonate holiday with periodic reevaluation of bone density and, possibly, bone markers.36
WHAT IS THE UNDERLYING PATHOPHYSIOLOGY?
The mechanism by which bisphosphonates increase the risk of atypical femoral fracture is not clear. These drugs work by suppressing bone turnover; however, in theory, prolonged use could suppress it too much and increase bone fragility.
One hypothesis is that bisphosphonates impair the toughening of cortical bone, an important barrier to clinical fracture. This is supported by a study that found bisphosphonate users with atypical femoral fracture had deficits in intrinsic and extrinsic bone toughness, perhaps due to treatment-related increases in matrix mineralization.37 Although this study and others showed an increase in matrix mineralization and reduced mineralization heterogeneity with bisphosphonate use,38,39 it is unclear whether such changes contributed to reduced toughness or to atypical femoral fracture.
Changes in the skeletal geometry of the lower limb such as femoral neck-shaft angle and femoral curvature alter the stresses and strains experienced by the femoral diaphysis with loading. Because the incidence of incomplete atypical femoral fracture is much greater than that of complete fracture, most incomplete atypical femoral fractures heal before the fracture progresses.
Ultimately, all fractures, including atypical femoral fractures, occur when mechanical stress and strain exceed bone strength.
Antiresorptive drugs such as bisphosphonates, estrogen, calcitonin, and RANK ligand inhibitors prevent hip fracture by increasing the strength of the proximal femur—perhaps at the expense of the strength (or toughness) of the subtrochanteric shaft. It is also possible that treatment-related increases in hip strength (and reduced hip fracture rates) promote or sustain the transfer of stress and strain to femoral regions that experience lesser or no increases in strength from treatment, which likely includes the shaft.40,41
CT studies in Japanese women with osteoporosis have shown that 2 years of zoledronate therapy had greater effects in the hip than in the femoral shaft, with significant increases in cortical thickness and volumetric bone mineral density at the femoral neck and intertrochanteric region compared with baseline.42 But zoledronate did not increase femoral shaft cortical thickness and caused only a minor increase in femoral shaft volumetric bone mineral density. Fracture patterns may have depended on damage and effects of bone turnover on mass and structure.
This hypothetical scenario portrays a possible “hip survival bias” mechanism for atypical femoral fracture, with the association with antiresorptive drugs arising from greater stress and strain in cortical regions where these fractures occur rather than from treatment-related reductions in cortical bone strength or toughness.
PRODROMAL PAIN IS COMMON
From 32% to 76% of patients who have incomplete or developing atypical femoral fracture present with a prodrome of groin or hip pain.4,43 Prodromal pain occurs any time from 2 weeks to several years before the fracture, presenting as pain in the anterior or lateral thigh or in the groin.
Prodromal pain in a patient on antiresorptive therapy should be a signal for the clinician to obtain a radiograph of the hip and to look for contralateral symptoms and fractures. The most common mechanism of injury appears to be a ground-level fall or even a nontraumatic activity such as walking or stepping off a curb.
MEDICAL MANAGEMENT
In bisphosphonate users with radiographic evidence of atypical femoral fracture, the bisphosphonate should be discontinued and the patient assessed for calcium and vitamin D deficiency, with supplements prescribed if needed.4
For patients with incomplete fracture and persistent pain after 3 months of medical management, prophylactic surgical nail fixation is recommended to prevent complete fracture.
Teriparatide, which has been associated with enhanced bone fracture healing, is a possible treatment to promote healing of atypical femoral fracture, either alone or as an adjunct to surgical fixation. A systematic review published in 2015 supported the use of teriparatide for enhancing fracture healing in atypical femoral fracture.44 In addition, a 10-patient series45 showed that incomplete fractures without radiolucent lines responded to teriparatide alone, whereas those with radiolucent lines needed intramedullary nailing.
These results suggest that teriparatide works best when the fracture site is stable, either inherently or with surgical fixation.
ORTHOPEDIC CARE
Orthopedic care for atypical femoral fracture differs depending on whether the patient experiences pain and whether the fracture is incomplete or complete. Figure 2 shows a treatment algorithm for atypical femoral fracture.
These are difficult fractures to manage, complicated by delayed healing in the elderly, complex displacement patterns, altered bone geometry, and risk of fracture in the opposite limb, all of which raise questions about recommending protected weight-bearing exercise.
Furthermore, atypical femoral fracture is often associated with increased anterolateral bowing of the femur, making it difficult to insert an intramedullary nail: the radius of curvature of the bone is shorter than that of a standard femoral nail. This mismatch can lead to intraoperative complications such as iatrogenic fracture during prophylactic nailing, malunion from excess straightening of the femur (which can itself lead to leg length discrepancy), and gapping of the fracture site, particularly on the medial side.
Intramedullary nailing for complete fracture
Intramedullary nailing is the first-line treatment for complete atypical femoral fracture, although the risk of delayed healing and revision surgery may be somewhat higher than with typical femoral fracture.46 Prophylactic intramedullary nailing should be considered for a patient with intractable pain.2
A radiograph of the opposite leg should be obtained routinely, looking for an asymptomatic fracture. Bisphosphonates should be discontinued and calcium and vitamin D continued. Teriparatide therapy can be considered as an alternative treatment.
Conservative management for incomplete fracture without pain
Incomplete atypical femoral fracture unaccompanied by pain can be followed conservatively.47 In addition to stopping antiresorptive therapy, patients need to avoid high-impact and repetitive-impact activities such as jumping or running. If pain occurs, patients should begin protected weight-bearing exercise.
Treatment is uncertain for incomplete fracture with pain
For patients with incomplete atypical femoral fracture and pain, treatment is controversial. Regimens that include 2 to 3 months of protected weight-bearing exercise, a full metabolic bone workup, calcium and vitamin D supplementation, and anabolic bone agents have produced some success. Some authors have reported poor results from conservative care, with few patients achieving pain relief or signs of complete healing.48,49 Additionally, if an incomplete fracture is found in the opposite femur, protected weight-bearing of both legs may not be possible.
Patients with incomplete fracture should be monitored regularly with radiography and physical examination. If there is progression of the fracture, escalation of pain, or failure to heal within 2 to 3 months, then surgical treatment is necessary.
Prophylactic placement of an intramedullary nail to prevent completion of the fracture and allow a return to full weight-bearing is generally advised.50 A long locking plate can be used if bone deformities make it difficult to place an intramedullary nail; however, nails are preferred because they allow formation of endochondral callus, which can be helpful in these difficult-to-heal fractures.
Results from retrospective reviews have shown that surgically treated patients with bisphosphonate-associated incomplete atypical femoral fracture were more likely than those treated nonsurgically to be pain-free (81% vs 64%) and have radiographic healing (100% vs 18% at final follow-up).46 Results have also been positive for those with complete atypical femoral fracture. At 6 months, 64% of surgically treated patients were pain-free and 98% were radiographically healed.51
The unusual geometry of the femur in patients with atypical femoral fracture and the presence of intramedullary cortical callus makes the placement of an intramedullary femoral rod more complex than in typical femoral fracture.8
Intramedullary nailing of atypical femoral fracture is a challenge for even the most experienced surgeon, and vigilance is imperative to avoid iatrogenic fracture and malunion.
MANY QUESTIONS REMAIN
We need more studies on the pathophysiology of bisphosphonate-associated atypical femoral fracture, the value of periodic screening with DXA, and which factors predict high risk (eg, Asian ethnicity, use of certain medications, femoral geometry). In addition, we need more data on the success of conservative management of incomplete fracture, including use of teriparatide.
Bisphosphonate therapy minimizes bone loss and reduces fracture risk by up to 50% in patients with osteoporosis,1 but it is also associated with increased risks of osteonecrosis of the jaw and atypical femoral fracture. Although atypical femoral fractures are rare, they can have a devastating effect. Patient concern about this complication has contributed to a decrease in bisphosphonate use by about half in the last decade or so,2,3 and we fear this could result in an increase in hip fracture rates.
In this article, we examine the evidence on bisphosphonate-associated atypical femoral fractures, including risks, pathogenesis, treatment, and prevention.
ATYPICAL FRACTURES INVOLVE THE FEMORAL SHAFT, NOT THE HEAD
An atypical femoral fracture is a transverse fracture of the femoral shaft (diaphysis), defined by both clinical criteria and radiographic appearance.
To be defined as atypical, a femoral fracture must meet 4 of the following 5 criteria4:
- Occurs with minimal or no trauma
- Has a predominantly transverse fracture line, originating at the lateral cortex and sometimes becoming oblique as it progresses medially across the femur
- Extends through both cortices and may be associated with a medial spike (complete fractures); or involves only the lateral cortex (incomplete fractures)
- Is noncomminuted or minimally comminuted
- Shows localized periosteal or endosteal thickening (termed “beaking” or “flaring”) of the lateral cortex at the fracture site.
Several minor features are also important but are not required, eg:
- Cortical thickening of the femoral shaft
- Unilateral or bilateral prodromal pain preceding the fracture
- Bilateral incomplete or complete femoral diaphysis fractures
- Delayed fracture healing.
Atypical femoral fracture can occur anywhere along the shaft, from just distal to the lesser trochanter to just proximal to the supracondylar flare. However, most occur in 2 areas, with 1 cluster centered at about 41 mm from the lesser trochanter (more common in relatively younger patients) and the other at 187 mm.5
ABSOLUTE RISK IS LOW BUT INCREASES WITH LONGER USE
Atypical femoral fractures are rare. Schilcher et al6 reviewed radiographs of 1,234 women who had a subtrochanteric or shaft fracture and found 59 (4.6%) of fractures were atypical. In a systematic review of 14 studies,7 the incidence ranged from 3.0 to 9.8 cases per 100,000 patient-years.
Furthermore, not all atypical femoral fractures are in bisphosphonate users: 7.4% were in nonusers in 1 series8 and 22% in another.9
Nevertheless, most studies show that bisphosphonate use increases the incidence of atypical femoral fracture, and the incidence increases with duration of use, especially after 3 years.7
An international task force of the American Society for Bone and Mineral Research listed the absolute risk as between 3.2 and 50 cases per 100,000 patient-years, with longer use (> 5 years) increasing the risk to about 100 per 100,000 patient-years.4 After stopping bisphosphonate therapy, the risk diminished by 70% per year.9
In another study, for 0.1 to 1.9 years of therapy, the age-adjusted atypical fracture rates were 1.78 per 100,000 per year (95% confidence interval [CI] 1.5–2.0), increasing to 113.1 per 100,000 per year (95% CI 69.3–156.8) with exposure from 8 to 9.9 years.10
A case-control study found that more than 5 years of bisphosphonate use increased the fracture risk by an odds ratio of 2.74 (95% CI 1.25–6.02).11
The incidence of typical femoral fracture was higher in those who adhered better to their oral bisphosphonate regimen in some studies,12 but the opposite was true in others.13
The benefits of bisphosphonate therapy in reducing fracture risk, however, outweigh the risk of atypical fracture.4
We do not know whether the rate of atypical femoral fracture is increasing. A review of Kaiser Permanente Northwest records found that the rates of atypical femoral shaft fracture had remained stable from 1996 to 2009. However, 61.9% of patients who met the strict radiographic criteria had taken oral bisphosphonates.14 These data suggest that bisphosphonate use has not increased the overall population-based risk for subtrochanteric and femoral shaft fractures, but that bisphosphonates and other risk factors may have increased the likelihood that such fractures will exhibit atypical radiographic features.
A population-based study in Denmark13 found that alendronate use longer than 10 years was associated with an adjusted 30% lower risk of hip fracture and no increase in the risk of subtrochanteric and femoral shaft fracture. In addition, the risk of subtrochanteric and femoral shaft fracture was lower with high adherence to alendronate treatment (based on medication possession ratio > 80%) compared with low adherence (ratio < 50%) (odds ratio 0.88, 95% CI 0.77–0.99). The risk was not increased in current vs past users.
The Danish study13 used the coding of the 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10) to identify subtrochanteric and femoral shaft fractures without radiologic review for atypical radiographic features. The lack of specific ICD-10 coding for subtrochanteric and femoral shaft fractures with atypical radiographic features has limited our knowledge of their incidence.
Contralateral fracture in more than one-fourth of cases
After an atypical femoral fracture, patients have a significant risk of fracture on the contralateral side. In a case-control study, 28% of patients with atypical femoral fracture suffered a contralateral fracture, compared with 0.9% of patients presenting with a typical fracture pattern (odds ratio 42.6, 95% CI 12.8–142.4).15
Contralateral fracture occurs from 1 month to 4 years after the index atypical femoral fracture.16
There are reports of bisphosphonate-related low-impact fractures in other sites such as the tibia17 and forearm.18 However, they may be too rare to warrant screening.
Mortality rates
A Swedish database study found that patients with atypical femoral fractures, whether bisphosphonate users or nonusers, do not have higher mortality rates than patients with ordinary subtrochanteric or femoral shaft fractures.19 Furthermore, the mortality rates for those with atypical femoral fracture were similar to rates in the general population. In contrast, patients with an ordinary femoral fracture had a higher mortality risk than the general population.19
Other studies suggest that atypical femoral fracture may be associated with a less favorable prognosis in older patients,20 but this could be due to differences in demographics, treatment adherence, or postfracture care.21
In addition, functional outcomes as measured by independent mobility at discharge and at 3 months were comparable between patients with atypical fracture and those with typical fracture.22
IMAGING STUDIES
If a long-term bisphosphonate user presents with hip, thigh, or groin pain, imaging studies are recommended.
Plain radiography
Radiography is usually the first step and should include a frontal view of the pelvis (Figure 1) and 2 views of the full length of each femur. If radiography is not conclusive, bone scan or magnetic resonance imaging (MRI) should be considered.
A linear cortex transverse fracture pattern and focal lateral cortical thickening are the most sensitive and specific radiographic features.23,24 Because of the risk of fracture on the contralateral side, radiographic study of that side is recommended as well.
Computed tomography
Computed tomography (CT) is not sensitive for early stress fractures and, given the radiation burden, is not recommended in the workup of atypical fracture.
Bone scanning
Bone scanning using technetium 99m-labeled methylene diphosphonate with a gamma camera shows active bone turnover. Stress fractures and atypical femoral fractures are most easily identified in the third (delayed) phase of the bone scan. Although bone scanning is highly sensitive, the specificity is limited by lack of spatial resolution. Atypical femoral fracture appears as increased activity in the subtrochanteric region with a predilection for the lateral cortex.
Dual-energy x-ray absorptiometry
Conventional dual-energy x-ray absorptiometry (DXA) extends only to 1 to 2 cm below the lesser trochanter and can therefore miss atypical fractures, which usually occur farther down. The overall detection rate for DXA was 61% in a sample of 33 patients.25
Newer scanners can look at the entire femoral shaft.26 In addition, newer software can quantify focal thickening (beaking) of the lateral cortex and screen patients who have no symptoms. The results of serial measurements can be graphed so that the practitioner can view trends to help assess or rule out potential asymptomatic atypical femoral fracture.
A localized reaction (periosteal thickening of the lateral cortex or beaking) often precedes atypical femoral fracture. A 2017 study reported that patients with high localized reaction (mean height 3.3 mm) that was of the pointed type and was accompanied by prodromal pain had an increased risk of complete or incomplete atypical femoral fracture at that site.27 This finding is used by the newer DXA software. The predictive value of beaking on extended femoral DXA may be as high as 83%.26
Magnetic resonance imaging
The MRI characteristics of atypical femoral fracture are similar to those of other stress fractures except that there is a lateral-to-medial pattern rather than a medial pattern. The earliest findings include periosteal reaction about the lateral cortex with a normal marrow signal.
MRI may be of particular benefit in patients with known atypical femoral fracture to screen the contralateral leg. It should image the entire length of both femurs. Contrast enhancement is not needed.
Regardless of whether initial findings were discovered on conventional radiographs or DXA, MRI confirmation is needed. Radionuclide bone scanning is currently not recommended because it lacks specificity. Combination imaging is recommended, with either radiography plus MRI or DXA plus MRI.
DIFFERENTIAL DIAGNOSIS
The differential diagnosis of atypical femoral fracture includes stress fracture, pathologic fracture, hypophosphatasia, and osteogenesis imperfecta.28 Hypophosphatemic osteomalacia can cause Looser zones, which can be confused with atypical femoral fractures but usually occur on the medial side.4 Stress fracture of the femur can occur below the lesser trochanter but usually begins in the medial, not the lateral, cortex.
Pathologic fractures from underlying osseous lesions can mimic the cortical beaking of bisphosphonate-related fracture, but they usually show the associated underlying lucent lesion and poorly defined margins. A sinus tract along the region of a chronic osteomyelitis may also appear similar.
Hypophosphatasia is an inborn error of metabolism caused by a loss-of-function mutation in the gene encoding alkaline phosphatase, resulting in pyrophosphate accumulation and causing osteomalacia from impaired mineralization. This can result in femoral pseudofracture that is often bilateral and occurs in the subtrochanteric region.29
ADDITIONAL RISK FACTORS
Patients with atypical femoral fracture are generally a heterogeneous group, but there are risk factors to note other than bisphosphonate exposure.
Asian women had a risk 8 times higher than white women in 1 study.30
Bone geometry. Mahjoub et al8 reported that compared with controls, patients with atypical femoral fracture had greater offset of the femoral shaft from the center of rotation of the femoral head, a more acute angle between the femoral neck and shaft, and greater proximal cortical thickness.
Medications. In addition to bisphosphonates, other drugs associated with atypical femoral fracture include RANK-ligand inhibitors such as denosumab (another drug for osteoporosis),31 glucocorticoids,32,33 and proton pump inhibitors.32,33
Genetics. Three sisters with atypical femoral fracture were found to have 37 rare mutations in 34 genes, including one in the GGPS1 gene, which codes for geranylgeranyl pyrophosphate synthase—an enzyme that bisphosphonates inhibit.34
Medical conditions other than osteoporosis include collagen diseases, chronic pulmonary disease, asthma, rheumatoid arthritis, and diabetes.35
Clinical recommendations
Current recommendations are to reevaluate bisphosphonate use in patients with osteoporosis after 5 or more years of therapy.36
Given that patients with osteoporosis are at increased risk of typical fracture, those at higher risk should be considered for continued bisphosphonate therapy. Factors for high risk include the following:
- History of fracture on therapy
- Hip T score –2.5 or lower
- Older age (≥ 70)
- Other strong risk factors for fracture such as smoking, alcohol use, corticosteroid use, rheumatoid arthritis, and family history
- World Health Organization FRAX fracture risk score above the country-specific threshold.
Those at lower risk should be considered for a 2- to 3-year bisphosphonate holiday with periodic reevaluation of bone density and, possibly, bone markers.36
WHAT IS THE UNDERLYING PATHOPHYSIOLOGY?
The mechanism by which bisphosphonates increase the risk of atypical femoral fracture is not clear. These drugs work by suppressing bone turnover; however, in theory, prolonged use could suppress it too much and increase bone fragility.
One hypothesis is that bisphosphonates impair the toughening of cortical bone, an important barrier to clinical fracture. This is supported by a study that found bisphosphonate users with atypical femoral fracture had deficits in intrinsic and extrinsic bone toughness, perhaps due to treatment-related increases in matrix mineralization.37 Although this study and others showed an increase in matrix mineralization and reduced mineralization heterogeneity with bisphosphonate use,38,39 it is unclear whether such changes contributed to reduced toughness or to atypical femoral fracture.
Changes in the skeletal geometry of the lower limb such as femoral neck-shaft angle and femoral curvature alter the stresses and strains experienced by the femoral diaphysis with loading. Because the incidence of incomplete atypical femoral fracture is much greater than that of complete fracture, most incomplete atypical femoral fractures heal before the fracture progresses.
Ultimately, all fractures, including atypical femoral fractures, occur when mechanical stress and strain exceed bone strength.
Antiresorptive drugs such as bisphosphonates, estrogen, calcitonin, and RANK ligand inhibitors prevent hip fracture by increasing the strength of the proximal femur—perhaps at the expense of the strength (or toughness) of the subtrochanteric shaft. It is also possible that treatment-related increases in hip strength (and reduced hip fracture rates) promote or sustain the transfer of stress and strain to femoral regions that experience lesser or no increases in strength from treatment, which likely includes the shaft.40,41
CT studies in Japanese women with osteoporosis have shown that 2 years of zoledronate therapy had greater effects in the hip than in the femoral shaft, with significant increases in cortical thickness and volumetric bone mineral density at the femoral neck and intertrochanteric region compared with baseline.42 But zoledronate did not increase femoral shaft cortical thickness and caused only a minor increase in femoral shaft volumetric bone mineral density. Fracture patterns may have depended on damage and effects of bone turnover on mass and structure.
This hypothetical scenario portrays a possible “hip survival bias” mechanism for atypical femoral fracture, with the association with antiresorptive drugs arising from greater stress and strain in cortical regions where these fractures occur rather than from treatment-related reductions in cortical bone strength or toughness.
PRODROMAL PAIN IS COMMON
From 32% to 76% of patients who have incomplete or developing atypical femoral fracture present with a prodrome of groin or hip pain.4,43 Prodromal pain occurs any time from 2 weeks to several years before the fracture, presenting as pain in the anterior or lateral thigh or in the groin.
Prodromal pain in a patient on antiresorptive therapy should be a signal for the clinician to obtain a radiograph of the hip and to look for contralateral symptoms and fractures. The most common mechanism of injury appears to be a ground-level fall or even a nontraumatic activity such as walking or stepping off a curb.
MEDICAL MANAGEMENT
In bisphosphonate users with radiographic evidence of atypical femoral fracture, the bisphosphonate should be discontinued and the patient assessed for calcium and vitamin D deficiency, with supplements prescribed if needed.4
For patients with incomplete fracture and persistent pain after 3 months of medical management, prophylactic surgical nail fixation is recommended to prevent complete fracture.
Teriparatide, which has been associated with enhanced bone fracture healing, is a possible treatment to promote healing of atypical femoral fracture, either alone or as an adjunct to surgical fixation. A systematic review published in 2015 supported the use of teriparatide for enhancing fracture healing in atypical femoral fracture.44 In addition, a 10-patient series45 showed that incomplete fractures without radiolucent lines responded to teriparatide alone, whereas those with radiolucent lines needed intramedullary nailing.
These results suggest that teriparatide works best when the fracture site is stable, either inherently or with surgical fixation.
ORTHOPEDIC CARE
Orthopedic care for atypical femoral fracture differs depending on whether the patient experiences pain and whether the fracture is incomplete or complete. Figure 2 shows a treatment algorithm for atypical femoral fracture.
These are difficult fractures to manage, complicated by delayed healing in the elderly, complex displacement patterns, altered bone geometry, and risk of fracture in the opposite limb, all of which raise questions about recommending protected weight-bearing exercise.
Furthermore, atypical femoral fracture is often associated with increased anterolateral bowing of the femur, making it difficult to insert an intramedullary nail: the radius of curvature of the bone is shorter than that of a standard femoral nail. This mismatch can lead to intraoperative complications such as iatrogenic fracture during prophylactic nailing, malunion from excess straightening of the femur (which can itself lead to leg length discrepancy), and gapping of the fracture site, particularly on the medial side.
Intramedullary nailing for complete fracture
Intramedullary nailing is the first-line treatment for complete atypical femoral fracture, although the risk of delayed healing and revision surgery may be somewhat higher than with typical femoral fracture.46 Prophylactic intramedullary nailing should be considered for a patient with intractable pain.2
A radiograph of the opposite leg should be obtained routinely, looking for an asymptomatic fracture. Bisphosphonates should be discontinued and calcium and vitamin D continued. Teriparatide therapy can be considered as an alternative treatment.
Conservative management for incomplete fracture without pain
Incomplete atypical femoral fracture unaccompanied by pain can be followed conservatively.47 In addition to stopping antiresorptive therapy, patients need to avoid high-impact and repetitive-impact activities such as jumping or running. If pain occurs, patients should begin protected weight-bearing exercise.
Treatment is uncertain for incomplete fracture with pain
For patients with incomplete atypical femoral fracture and pain, treatment is controversial. Regimens that include 2 to 3 months of protected weight-bearing exercise, a full metabolic bone workup, calcium and vitamin D supplementation, and anabolic bone agents have produced some success. Some authors have reported poor results from conservative care, with few patients achieving pain relief or signs of complete healing.48,49 Additionally, if an incomplete fracture is found in the opposite femur, protected weight-bearing of both legs may not be possible.
Patients with incomplete fracture should be monitored regularly with radiography and physical examination. If there is progression of the fracture, escalation of pain, or failure to heal within 2 to 3 months, then surgical treatment is necessary.
Prophylactic placement of an intramedullary nail to prevent completion of the fracture and allow a return to full weight-bearing is generally advised.50 A long locking plate can be used if bone deformities make it difficult to place an intramedullary nail; however, nails are preferred because they allow formation of endochondral callus, which can be helpful in these difficult-to-heal fractures.
Results from retrospective reviews have shown that surgically treated patients with bisphosphonate-associated incomplete atypical femoral fracture were more likely than those treated nonsurgically to be pain-free (81% vs 64%) and have radiographic healing (100% vs 18% at final follow-up).46 Results have also been positive for those with complete atypical femoral fracture. At 6 months, 64% of surgically treated patients were pain-free and 98% were radiographically healed.51
The unusual geometry of the femur in patients with atypical femoral fracture and the presence of intramedullary cortical callus makes the placement of an intramedullary femoral rod more complex than in typical femoral fracture.8
Intramedullary nailing of atypical femoral fracture is a challenge for even the most experienced surgeon, and vigilance is imperative to avoid iatrogenic fracture and malunion.
MANY QUESTIONS REMAIN
We need more studies on the pathophysiology of bisphosphonate-associated atypical femoral fracture, the value of periodic screening with DXA, and which factors predict high risk (eg, Asian ethnicity, use of certain medications, femoral geometry). In addition, we need more data on the success of conservative management of incomplete fracture, including use of teriparatide.
- Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 1996; 348(9041):1535–1541. pmid:8950879
- Jha S, Wang Z, Laucis N, Bhattacharyya T. Trends in media reports, oral bisphosphonate prescriptions, and hip fractures 1996–2012: an ecological analysis. J Bone Miner Res 2015; 30(12):2179–2187. doi:10.1002/jbmr.2565
- Solomon DH, Johnston SS, Boytsov NN, McMorrow D, Lane JM, Krohn KD. Osteoporosis medication use after hip fracture in US patients between 2002 and 2011. J Bone Miner Res 2014; 29(9):1929–1937. doi:10.1002/jbmr.2202
- Shane E, Burr D, Abrahamsen B, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2014; 29(1):1–23. doi:10.1002/jbmr.1998
- Koeppen VA, Schilcher J, Aspenberg P. Dichotomous location of 160 atypical femoral fractures. Acta Orthop 2013; 84(6):561–564. doi:10.3109/17453674.2013.866193
- Schilcher J, Koeppen V, Aspenberg P, Michäelsson K. Risk of atypical femoral fracture during and after bisphosphonate use. Acta Orthop 2015; 86(1):100–107. doi:10.3109/17453674.2015.1004149
- Khow KS, Shibu P, Yu SC, Chehade MJ, Visvanathan R. Epidemiology and postoperative outcomes of atypical femoral fractures in older adults: a systematic review. J Nutr Health Aging 2017; 21(1):83–91. doi:10.1007/s12603-015-0652-3
- Mahjoub Z, Jean S, Leclerc JT, et al. Incidence and characteristics of atypical femoral fractures: clinical and geometrical data. J Bone Miner Res 2016; 31(4):767–776. doi:10.1002/jbmr.2748
- Schilcher J, Michaelsson K, Aspenberg P. Bisphosphonate use and atypical fractures of the femoral shaft. N Engl J Med 2011; 364(18):1728–1737. doi:10.1056/NEJMoa1010650
- Dell RM, Adams AL, Greene DF, et al. Incidence of atypical nontraumatic diaphyseal fractures of the femur. J Bone Miner Res 2012; 27(12):2544–2550. doi:10.1002/jbmr.1719
- Park-Wyllie LY, Mamdani MM, Juurlink DN, et al. Bisphosphonate use and the risk of subtrochanteric or femoral shaft fractures in older women. JAMA 2011; 305(8):783–789. doi:10.1001/jama.2011.190
- Wang Z, Ward MM, Chan L, Bhattacharyya T. Adherence to oral bisphosphonates and the risk of subtrochanteric and femoral shaft fractures among female Medicare beneficiaries. Osteoporos Int 2014; 25(8):2109–2116. doi:10.1007/s00198-014-2738-x
- Abrahamsen B, Eiken P, Prieto-Alhambra D, Eastell R. Risk of hip, subtrochanteric, and femoral shaft fractures among mid and long term users of alendronate: nationwide cohort and nested case-control study. BMJ 2016; 353:i3365. doi:10.1136/bmj.i3365
- Feldstein AC, Black D, Perrin N, et al. Incidence and demography of femur fractures with and without atypical features. J Bone Miner Res 2012; 27(5):977–986. doi:10.1002/jbmr.1550
- Meier RP, Perneger TV, Stern R, Rizzoli R, Peter RE. Increasing occurrence of atypical femoral fractures associated with bisphosphonate use. Arch Intern Med 2012; 172(12):930–936. doi:10.1001/archinternmed.2012.1796
- La Rocca Vieira R, Rosenberg ZS, Allison MB, Im SA, Babb J, Peck V. Frequency of incomplete atypical femoral fractures in asymptomatic patients on long term bisphosphonate therapy. AJR Am J Roentgenol 2012; 198(5):1144–1151. doi:10.2214/AJR.11.7442
- Bissonnette L, April PM, Dumais R, Boire G, Roux S. Atypical fracture of the tibial diaphysis associated with bisphosphonate therapy: a case report. Bone 2013; 56(2):406–409. doi:10.1016/j.bone.2013.07.012
- Moon J, Bither N, Lee T. Atypical forearm fractures associated with long-term use of bisphosphonate. Arch Orthop Trauma Surg 2013; 133(7):889–892. doi:10.1007/s00402-013-1760-3
- Kharazmi M, Hallberg P, Schilcher J, Aspenberg P, Michaëlsson K. Mortality after atypical femoral fractures: a cohort study. J Bone Miner Res 2016; 31(3):491–497. doi:10.1002/jbmr.2767
- Medin E, Goude F, Melberg HO, Tediosi F, Belicza E, Peltola M; EuroHOPE Study Group. European regional differences in all-cause mortality and length of stay for patients with hip fracture. Health Econ 2015; 24(suppl 2):53–64. doi:10.1002/hec.3278
- Abrahamsen B, Prieto-Alhambra D. Patients with atypical femur fractures have the same mortality as the background population-drug channeling bias, bisphosphonate effects and public health implications. J Bone Miner Res 2016; 31(3):488–490. doi:10.1002/jbmr.2801
- Khow KS, Paterson F, Shibu P, Yu SC, Chehade MJ, Visvanathan R. Outcomes between older adults with atypical and typical femoral fractures are comparable. Injury 2017; 48(2):394–398. doi:10.1016/j.injury.2016.10.035
- Adams AL, Xue F, Chantra JQ, et al. Sensitivity and specificity of radiographic characteristics in atypical femoral fractures. Osteoporos Int 2017; 28(1):413–417. doi:10.1007/s00198-016-3809-y
- Rosenberg ZS, La Rocca Vieira R, Chan SS, et al. Bisphosphonate-related complete atypical subtrochanteric femoral fractures: diagnostic utility of radiography. AJR Am J Roentgenol 2011; 197(4):954–960. doi:10.2214/AJR.10.6262
- Kim S, Yang KH, Lim H, et al. Detection of prefracture hip lesions in atypical subtrochanteric fracture with dual-energy x-ray absorptiometry images. Radiology 2014; 270(2):487–495. doi:10.1148/radiol.13122691
- van de Laarschot DM, Smits AA, Buitendijk SK, Stegenga MT, Zillikens MC. Screening for atypical femur fractures using extended femur scans by DXA. J Bone Miner Res 2017; 32(8):1632–1639. doi:10.1002/jbmr.3164
- Sato H, Kondo N, Nakatsue T, et al. High and pointed type of femoral localized reaction frequently extends to complete an incomplete atypical femoral fracture in patients with autoimmune diseases on long-term glucocorticoids and bisphosphonates. Osteoporos Int 2017; 28(8):2367–2376. doi:10.1007/s00198-017-4038-8
- Giaconi JC, Watterson CT. Bisphosphonate-related atypical femur fractures and the radiographic features. In: Silverman SL, Abrahamsen B, eds. The Duration and Safety of Osteoporosis Treatment. Switzerland: Springer International Publishing; 2016:107–124. doi:10.1007/978-3-319-23639-1
- Whyte MP. Atypical femoral fractures, bisphosphonates, and adult hypophosphatasia. J Bone Miner Res 2009; 24(6):1132–1134. doi:10.1359/jbmr.081253
- Lo JC, Hui RL, Grimsrud CD, et al. The association of race/ethnicity and risk of atypical femoral fracture among older women receiving oral bisphosphonate therapy. Bone 2016; 85:142–147. doi:10.1016/j.bone.2016.01.002
- Bone HG, Wagman RB, Brandi ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol 2017; 5(7):513–523. doi:10.1016/S2213-8587(17)30138-9
- Koh JH, Myong JP, Yoo J, et al. Predisposing factors associated with atypical femur fracture among postmenopausal Korean women receiving bisphosphonate therapy: 8 years' experience in a single center. Osteoporos Int 2017; 28(11):3251–3259. doi:10.1007/s00198-017-4169-y
- Kim D, Sung YK, Cho SK, Han M, Kim YS. Factors associated with atypical femoral fracture. Rheumatol Int 2016; 36(1):65–71. doi:10.1007/s00296-015-3323-0
- Roca-Ayats N, Balcells S, Garcia-Giralt N, et al. GGPS1 mutation and atypical femoral fractures with bisphosphonates. N Engl J Med 2017; 376(18):1794–1795. doi:10.1056/NEJMc1612804
- Giusti A, Hamdy NA, Dekkers OM, Ramautar SR, Dijkstra S, Papapoulos SE. Atypical fractures and bisphosphonate therapy: a cohort study of patients with femoral fracture with radiographic adjudication of fracture site and features. Bone 2011; 48(5):966–971. doi:10.1016/j.bone.2010.12.033
- Adler RA, El-Hajj Fuleihan G, Bauer DC, et al. Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2016; 31(1):16–35. doi:10.1002/jbmr.2708
- Lloyd AA, Gludovatz B, Riedel C, et al. Atypical fracture with long-term bisphosphonate therapy is associated with altered cortical composition and reduced fracture resistance. Proc Natl Acad Sci USA 2017; 114(33):8722–8727. doi:10.1073/pnas.1704460114
- Ettinger B, Burr DB, Ritchie RO. Proposed pathogenesis for atypical femoral fractures; lessons from materials research. Bone 2013; 55(2):495–500. doi:10.1016/j.bone.2013.02.004
- Burr DB, Liu Z, Allen MR. Duration-dependent effects of clinically relevant oral alendronate doses on cortical bone toughness in beagle dogs. Bone 2015; 71:58–62. doi:10.1016/j.bone.2014.10.010
- Sasaki S, Miyakoshi N, Hongo M, Kasukawa Y, Shimada Y. Low-energy diaphyseal femoral fractures associated with bisphosphonate use and severe curved femur: a case series. J Bone Miner Metab 2012; 30(5):561–567. doi:10.1007/s00774-012-0358-0
- Pulkkinen P, Gluer C, Jamsa T. Investigation of differences between hip fracture types: a worthy strategy of improved risk assessment and fracture prevention. Bone 2011; 49(4):600–604. doi:10.1016/j.bone.2011.07.022
- Ito M, Sone T, Shiraki M, et al. The effect of once-yearly zoledronic acid on hip structural and biomechanical properties derived using computed tomography (CT) in Japanese women with osteoporosis. Bone 2018; 106:179–186. doi:10.1016/j.bone.2017.10.013
- Bogdan Y, Einhorn TA. Clinical presentation of atypical femur fractures. In: Silverman SL, Abrahamsen B, eds. The Duration and Safety of Osteoporosis Treatment. Switzerland: Springer International Publishing; 2016:137–140. doi:10.1007/978-3-319-23639-1
- Im GI, Lee SH. Effect of teriparatide on healing of atypical femoral fractures: a systemic review. J Bone Metab 2015; 22(4):183–189. doi:10.11005/jbm.2015.22.4.183
- Saleh A, Hegde VV, Potty AG, Schneider R, Cornell CN, Lane JM. Management strategy for symptomatic bisphosphonate-associated incomplete atypical femoral fractures. HSS J 2012; 8(2):103–110. doi:10.1007/s11420-012-9275-y
- Egol KA, Park JH, Prensky C, Rosenberg ZS, Peck V, Tejwani NC. Surgical treatment improves clinical and functional outcomes for patients who sustain incomplete bisphosphonate-related femur fractures. J Orthop Trauma 2013; 27(6):331–335. doi:10.1097/BOT.0b013e31827240ae
- Koh A, Guerado E, Giannoudis PV. Atypical femoral fractures related to bisphosphonate treatment: issues and controversies related to their surgical management. Bone Joint J 2017; 99-B(3):295–302. doi:10.1302/0301-620X.99B3.BJJ-2016-0276.R2
- Oh CW, Oh JK, Park KC, Kim JW, Yoon YC. Prophylactic nailing of incomplete atypical femoral fractures. ScientificWorldJournal 2013; 2013:450148. doi:10.1155/2013/450148
- Ha YC, Cho MR, Park KH, Kim SY, Koo KH. Is surgery necessary for femoral insufficiency fractures after long-term bisphosphonate therapy? Clin Orthop Relat Res 2010; 468(12):3393–3398. doi:10.1007/s11999-010-1583-2
- Tosounidis TH, Lampropoulou-Adamidou, Kanakaris NK. Intramedullary nailing of sequential bilateral atypical subtrochanteric fractures and the management of distal femoral intraoperative fracture. J Orthop Trauma 2015 Jun 11. Epub ahead of print. doi:10.1097/BOT.0000000000000370
- Egol KA, Park JH, Rosenberg ZS, Peck V, Tejwani NC. Healing delayed but generally reliable after bisphosphonate-associated complete femur fractures treated with IM nails. Clin Orthop Relat Res 2014; 472(9):2728–2734. doi:10.1007/s11999-013-2963-1
- Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 1996; 348(9041):1535–1541. pmid:8950879
- Jha S, Wang Z, Laucis N, Bhattacharyya T. Trends in media reports, oral bisphosphonate prescriptions, and hip fractures 1996–2012: an ecological analysis. J Bone Miner Res 2015; 30(12):2179–2187. doi:10.1002/jbmr.2565
- Solomon DH, Johnston SS, Boytsov NN, McMorrow D, Lane JM, Krohn KD. Osteoporosis medication use after hip fracture in US patients between 2002 and 2011. J Bone Miner Res 2014; 29(9):1929–1937. doi:10.1002/jbmr.2202
- Shane E, Burr D, Abrahamsen B, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2014; 29(1):1–23. doi:10.1002/jbmr.1998
- Koeppen VA, Schilcher J, Aspenberg P. Dichotomous location of 160 atypical femoral fractures. Acta Orthop 2013; 84(6):561–564. doi:10.3109/17453674.2013.866193
- Schilcher J, Koeppen V, Aspenberg P, Michäelsson K. Risk of atypical femoral fracture during and after bisphosphonate use. Acta Orthop 2015; 86(1):100–107. doi:10.3109/17453674.2015.1004149
- Khow KS, Shibu P, Yu SC, Chehade MJ, Visvanathan R. Epidemiology and postoperative outcomes of atypical femoral fractures in older adults: a systematic review. J Nutr Health Aging 2017; 21(1):83–91. doi:10.1007/s12603-015-0652-3
- Mahjoub Z, Jean S, Leclerc JT, et al. Incidence and characteristics of atypical femoral fractures: clinical and geometrical data. J Bone Miner Res 2016; 31(4):767–776. doi:10.1002/jbmr.2748
- Schilcher J, Michaelsson K, Aspenberg P. Bisphosphonate use and atypical fractures of the femoral shaft. N Engl J Med 2011; 364(18):1728–1737. doi:10.1056/NEJMoa1010650
- Dell RM, Adams AL, Greene DF, et al. Incidence of atypical nontraumatic diaphyseal fractures of the femur. J Bone Miner Res 2012; 27(12):2544–2550. doi:10.1002/jbmr.1719
- Park-Wyllie LY, Mamdani MM, Juurlink DN, et al. Bisphosphonate use and the risk of subtrochanteric or femoral shaft fractures in older women. JAMA 2011; 305(8):783–789. doi:10.1001/jama.2011.190
- Wang Z, Ward MM, Chan L, Bhattacharyya T. Adherence to oral bisphosphonates and the risk of subtrochanteric and femoral shaft fractures among female Medicare beneficiaries. Osteoporos Int 2014; 25(8):2109–2116. doi:10.1007/s00198-014-2738-x
- Abrahamsen B, Eiken P, Prieto-Alhambra D, Eastell R. Risk of hip, subtrochanteric, and femoral shaft fractures among mid and long term users of alendronate: nationwide cohort and nested case-control study. BMJ 2016; 353:i3365. doi:10.1136/bmj.i3365
- Feldstein AC, Black D, Perrin N, et al. Incidence and demography of femur fractures with and without atypical features. J Bone Miner Res 2012; 27(5):977–986. doi:10.1002/jbmr.1550
- Meier RP, Perneger TV, Stern R, Rizzoli R, Peter RE. Increasing occurrence of atypical femoral fractures associated with bisphosphonate use. Arch Intern Med 2012; 172(12):930–936. doi:10.1001/archinternmed.2012.1796
- La Rocca Vieira R, Rosenberg ZS, Allison MB, Im SA, Babb J, Peck V. Frequency of incomplete atypical femoral fractures in asymptomatic patients on long term bisphosphonate therapy. AJR Am J Roentgenol 2012; 198(5):1144–1151. doi:10.2214/AJR.11.7442
- Bissonnette L, April PM, Dumais R, Boire G, Roux S. Atypical fracture of the tibial diaphysis associated with bisphosphonate therapy: a case report. Bone 2013; 56(2):406–409. doi:10.1016/j.bone.2013.07.012
- Moon J, Bither N, Lee T. Atypical forearm fractures associated with long-term use of bisphosphonate. Arch Orthop Trauma Surg 2013; 133(7):889–892. doi:10.1007/s00402-013-1760-3
- Kharazmi M, Hallberg P, Schilcher J, Aspenberg P, Michaëlsson K. Mortality after atypical femoral fractures: a cohort study. J Bone Miner Res 2016; 31(3):491–497. doi:10.1002/jbmr.2767
- Medin E, Goude F, Melberg HO, Tediosi F, Belicza E, Peltola M; EuroHOPE Study Group. European regional differences in all-cause mortality and length of stay for patients with hip fracture. Health Econ 2015; 24(suppl 2):53–64. doi:10.1002/hec.3278
- Abrahamsen B, Prieto-Alhambra D. Patients with atypical femur fractures have the same mortality as the background population-drug channeling bias, bisphosphonate effects and public health implications. J Bone Miner Res 2016; 31(3):488–490. doi:10.1002/jbmr.2801
- Khow KS, Paterson F, Shibu P, Yu SC, Chehade MJ, Visvanathan R. Outcomes between older adults with atypical and typical femoral fractures are comparable. Injury 2017; 48(2):394–398. doi:10.1016/j.injury.2016.10.035
- Adams AL, Xue F, Chantra JQ, et al. Sensitivity and specificity of radiographic characteristics in atypical femoral fractures. Osteoporos Int 2017; 28(1):413–417. doi:10.1007/s00198-016-3809-y
- Rosenberg ZS, La Rocca Vieira R, Chan SS, et al. Bisphosphonate-related complete atypical subtrochanteric femoral fractures: diagnostic utility of radiography. AJR Am J Roentgenol 2011; 197(4):954–960. doi:10.2214/AJR.10.6262
- Kim S, Yang KH, Lim H, et al. Detection of prefracture hip lesions in atypical subtrochanteric fracture with dual-energy x-ray absorptiometry images. Radiology 2014; 270(2):487–495. doi:10.1148/radiol.13122691
- van de Laarschot DM, Smits AA, Buitendijk SK, Stegenga MT, Zillikens MC. Screening for atypical femur fractures using extended femur scans by DXA. J Bone Miner Res 2017; 32(8):1632–1639. doi:10.1002/jbmr.3164
- Sato H, Kondo N, Nakatsue T, et al. High and pointed type of femoral localized reaction frequently extends to complete an incomplete atypical femoral fracture in patients with autoimmune diseases on long-term glucocorticoids and bisphosphonates. Osteoporos Int 2017; 28(8):2367–2376. doi:10.1007/s00198-017-4038-8
- Giaconi JC, Watterson CT. Bisphosphonate-related atypical femur fractures and the radiographic features. In: Silverman SL, Abrahamsen B, eds. The Duration and Safety of Osteoporosis Treatment. Switzerland: Springer International Publishing; 2016:107–124. doi:10.1007/978-3-319-23639-1
- Whyte MP. Atypical femoral fractures, bisphosphonates, and adult hypophosphatasia. J Bone Miner Res 2009; 24(6):1132–1134. doi:10.1359/jbmr.081253
- Lo JC, Hui RL, Grimsrud CD, et al. The association of race/ethnicity and risk of atypical femoral fracture among older women receiving oral bisphosphonate therapy. Bone 2016; 85:142–147. doi:10.1016/j.bone.2016.01.002
- Bone HG, Wagman RB, Brandi ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol 2017; 5(7):513–523. doi:10.1016/S2213-8587(17)30138-9
- Koh JH, Myong JP, Yoo J, et al. Predisposing factors associated with atypical femur fracture among postmenopausal Korean women receiving bisphosphonate therapy: 8 years' experience in a single center. Osteoporos Int 2017; 28(11):3251–3259. doi:10.1007/s00198-017-4169-y
- Kim D, Sung YK, Cho SK, Han M, Kim YS. Factors associated with atypical femoral fracture. Rheumatol Int 2016; 36(1):65–71. doi:10.1007/s00296-015-3323-0
- Roca-Ayats N, Balcells S, Garcia-Giralt N, et al. GGPS1 mutation and atypical femoral fractures with bisphosphonates. N Engl J Med 2017; 376(18):1794–1795. doi:10.1056/NEJMc1612804
- Giusti A, Hamdy NA, Dekkers OM, Ramautar SR, Dijkstra S, Papapoulos SE. Atypical fractures and bisphosphonate therapy: a cohort study of patients with femoral fracture with radiographic adjudication of fracture site and features. Bone 2011; 48(5):966–971. doi:10.1016/j.bone.2010.12.033
- Adler RA, El-Hajj Fuleihan G, Bauer DC, et al. Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2016; 31(1):16–35. doi:10.1002/jbmr.2708
- Lloyd AA, Gludovatz B, Riedel C, et al. Atypical fracture with long-term bisphosphonate therapy is associated with altered cortical composition and reduced fracture resistance. Proc Natl Acad Sci USA 2017; 114(33):8722–8727. doi:10.1073/pnas.1704460114
- Ettinger B, Burr DB, Ritchie RO. Proposed pathogenesis for atypical femoral fractures; lessons from materials research. Bone 2013; 55(2):495–500. doi:10.1016/j.bone.2013.02.004
- Burr DB, Liu Z, Allen MR. Duration-dependent effects of clinically relevant oral alendronate doses on cortical bone toughness in beagle dogs. Bone 2015; 71:58–62. doi:10.1016/j.bone.2014.10.010
- Sasaki S, Miyakoshi N, Hongo M, Kasukawa Y, Shimada Y. Low-energy diaphyseal femoral fractures associated with bisphosphonate use and severe curved femur: a case series. J Bone Miner Metab 2012; 30(5):561–567. doi:10.1007/s00774-012-0358-0
- Pulkkinen P, Gluer C, Jamsa T. Investigation of differences between hip fracture types: a worthy strategy of improved risk assessment and fracture prevention. Bone 2011; 49(4):600–604. doi:10.1016/j.bone.2011.07.022
- Ito M, Sone T, Shiraki M, et al. The effect of once-yearly zoledronic acid on hip structural and biomechanical properties derived using computed tomography (CT) in Japanese women with osteoporosis. Bone 2018; 106:179–186. doi:10.1016/j.bone.2017.10.013
- Bogdan Y, Einhorn TA. Clinical presentation of atypical femur fractures. In: Silverman SL, Abrahamsen B, eds. The Duration and Safety of Osteoporosis Treatment. Switzerland: Springer International Publishing; 2016:137–140. doi:10.1007/978-3-319-23639-1
- Im GI, Lee SH. Effect of teriparatide on healing of atypical femoral fractures: a systemic review. J Bone Metab 2015; 22(4):183–189. doi:10.11005/jbm.2015.22.4.183
- Saleh A, Hegde VV, Potty AG, Schneider R, Cornell CN, Lane JM. Management strategy for symptomatic bisphosphonate-associated incomplete atypical femoral fractures. HSS J 2012; 8(2):103–110. doi:10.1007/s11420-012-9275-y
- Egol KA, Park JH, Prensky C, Rosenberg ZS, Peck V, Tejwani NC. Surgical treatment improves clinical and functional outcomes for patients who sustain incomplete bisphosphonate-related femur fractures. J Orthop Trauma 2013; 27(6):331–335. doi:10.1097/BOT.0b013e31827240ae
- Koh A, Guerado E, Giannoudis PV. Atypical femoral fractures related to bisphosphonate treatment: issues and controversies related to their surgical management. Bone Joint J 2017; 99-B(3):295–302. doi:10.1302/0301-620X.99B3.BJJ-2016-0276.R2
- Oh CW, Oh JK, Park KC, Kim JW, Yoon YC. Prophylactic nailing of incomplete atypical femoral fractures. ScientificWorldJournal 2013; 2013:450148. doi:10.1155/2013/450148
- Ha YC, Cho MR, Park KH, Kim SY, Koo KH. Is surgery necessary for femoral insufficiency fractures after long-term bisphosphonate therapy? Clin Orthop Relat Res 2010; 468(12):3393–3398. doi:10.1007/s11999-010-1583-2
- Tosounidis TH, Lampropoulou-Adamidou, Kanakaris NK. Intramedullary nailing of sequential bilateral atypical subtrochanteric fractures and the management of distal femoral intraoperative fracture. J Orthop Trauma 2015 Jun 11. Epub ahead of print. doi:10.1097/BOT.0000000000000370
- Egol KA, Park JH, Rosenberg ZS, Peck V, Tejwani NC. Healing delayed but generally reliable after bisphosphonate-associated complete femur fractures treated with IM nails. Clin Orthop Relat Res 2014; 472(9):2728–2734. doi:10.1007/s11999-013-2963-1
KEY POINTS
- The benefits of bisphosphonate therapy in reducing fracture risk outweigh the risk of atypical fracture.
- Bisphosphonate use for longer than 5 years greatly increases the risk of atypical femoral fracture.
- Treatment of atypical femoral fracture varies depending on whether the patient has pain and whether the fracture is complete or incomplete.
A physician’s response to observational studies of opioid prescribing
Several months ago, we invited readers to submit short personalized commentaries on articles that changed the way they approach a specific clinical problem and the way they take care of patients. In this issue of the Journal, addiction specialist Charles Reznikoff, MD, discusses 3 observational studies that focused on how prescribing opioids for acute pain can lead to chronic opioid use and addiction, and how these studies have influenced his practice.
Although observational studies rank lower on the level-of-evidence scale than randomized controlled trials, they can intellectually stimulate and inform us in ways that lead us to modify how we deliver clinical care.
The initial prescribing of pain medications and the management of patients with chronic pain are currently under intense scrutiny, and are the topic of much discussion in the United States. The opioid epidemic has spilled over into all aspects of daily life, far beyond the medical community. But since we physicians are the only legal and regulated source of narcotics and other pain medications, we are under the microscope—and rightly so.
We, our patients, the pharmaceutical industry, legislators, and the law enforcement community struggle to navigate a complex maze, one with moving walls. Not long ago, physicians were told that we were not attentive enough to our patients’ suffering and needed to do better at relieving it. “Pain” became a vital sign and a recorded metric of quality care. Some excellent changes evolved from this focus, such as increased emphasis on postoperative regional and local pain control. But pain measurements continue to be recorded at every outpatient visit, an almost mindless requirement.
Recently, a patient with lupus nephritis whom I was seeing for blood pressure management reported a pain level of 8 on a scale of 10. I confess that I usually don’t even look at these metrics, but for whatever reason I saw her answer. I asked her about it. She had burned her finger while cooking and said, “I had no idea what number to pick. I picked 8. It’s no big deal.”
But the ongoing emphasis on this metric may lead some patients to expect total pain relief, a problematic expectation in those with chronic pain syndromes such as fibromyalgia. As Dr. Reznikoff points out, a large proportion of patients report they have chronic pain, and many (but clearly not all) suffer from recognized or masked chronic anxiety and depression disorders1 that may well influence how they use pain medications.
Thus, while physicians indeed are on the front lines of offering initial prescriptions for pain medications, we remain betwixt and between in the challenges of responding to the immediate needs of our patients while trying to predict the long-term effects of our prescription on the individual patient and of our prescribing patterns on society in general.
I again welcome your submissions describing how individual publications have affected your personal approach to managing patients and specific diseases. We will publish selected contributions in print and online.
- Tsang A, Von Korff M, Lee S, et al. Common chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety disorders. J Pain 2008; 9(10):883–891. doi:10.1016/j.jpain.2008.05.005
Several months ago, we invited readers to submit short personalized commentaries on articles that changed the way they approach a specific clinical problem and the way they take care of patients. In this issue of the Journal, addiction specialist Charles Reznikoff, MD, discusses 3 observational studies that focused on how prescribing opioids for acute pain can lead to chronic opioid use and addiction, and how these studies have influenced his practice.
Although observational studies rank lower on the level-of-evidence scale than randomized controlled trials, they can intellectually stimulate and inform us in ways that lead us to modify how we deliver clinical care.
The initial prescribing of pain medications and the management of patients with chronic pain are currently under intense scrutiny, and are the topic of much discussion in the United States. The opioid epidemic has spilled over into all aspects of daily life, far beyond the medical community. But since we physicians are the only legal and regulated source of narcotics and other pain medications, we are under the microscope—and rightly so.
We, our patients, the pharmaceutical industry, legislators, and the law enforcement community struggle to navigate a complex maze, one with moving walls. Not long ago, physicians were told that we were not attentive enough to our patients’ suffering and needed to do better at relieving it. “Pain” became a vital sign and a recorded metric of quality care. Some excellent changes evolved from this focus, such as increased emphasis on postoperative regional and local pain control. But pain measurements continue to be recorded at every outpatient visit, an almost mindless requirement.
Recently, a patient with lupus nephritis whom I was seeing for blood pressure management reported a pain level of 8 on a scale of 10. I confess that I usually don’t even look at these metrics, but for whatever reason I saw her answer. I asked her about it. She had burned her finger while cooking and said, “I had no idea what number to pick. I picked 8. It’s no big deal.”
But the ongoing emphasis on this metric may lead some patients to expect total pain relief, a problematic expectation in those with chronic pain syndromes such as fibromyalgia. As Dr. Reznikoff points out, a large proportion of patients report they have chronic pain, and many (but clearly not all) suffer from recognized or masked chronic anxiety and depression disorders1 that may well influence how they use pain medications.
Thus, while physicians indeed are on the front lines of offering initial prescriptions for pain medications, we remain betwixt and between in the challenges of responding to the immediate needs of our patients while trying to predict the long-term effects of our prescription on the individual patient and of our prescribing patterns on society in general.
I again welcome your submissions describing how individual publications have affected your personal approach to managing patients and specific diseases. We will publish selected contributions in print and online.
Several months ago, we invited readers to submit short personalized commentaries on articles that changed the way they approach a specific clinical problem and the way they take care of patients. In this issue of the Journal, addiction specialist Charles Reznikoff, MD, discusses 3 observational studies that focused on how prescribing opioids for acute pain can lead to chronic opioid use and addiction, and how these studies have influenced his practice.
Although observational studies rank lower on the level-of-evidence scale than randomized controlled trials, they can intellectually stimulate and inform us in ways that lead us to modify how we deliver clinical care.
The initial prescribing of pain medications and the management of patients with chronic pain are currently under intense scrutiny, and are the topic of much discussion in the United States. The opioid epidemic has spilled over into all aspects of daily life, far beyond the medical community. But since we physicians are the only legal and regulated source of narcotics and other pain medications, we are under the microscope—and rightly so.
We, our patients, the pharmaceutical industry, legislators, and the law enforcement community struggle to navigate a complex maze, one with moving walls. Not long ago, physicians were told that we were not attentive enough to our patients’ suffering and needed to do better at relieving it. “Pain” became a vital sign and a recorded metric of quality care. Some excellent changes evolved from this focus, such as increased emphasis on postoperative regional and local pain control. But pain measurements continue to be recorded at every outpatient visit, an almost mindless requirement.
Recently, a patient with lupus nephritis whom I was seeing for blood pressure management reported a pain level of 8 on a scale of 10. I confess that I usually don’t even look at these metrics, but for whatever reason I saw her answer. I asked her about it. She had burned her finger while cooking and said, “I had no idea what number to pick. I picked 8. It’s no big deal.”
But the ongoing emphasis on this metric may lead some patients to expect total pain relief, a problematic expectation in those with chronic pain syndromes such as fibromyalgia. As Dr. Reznikoff points out, a large proportion of patients report they have chronic pain, and many (but clearly not all) suffer from recognized or masked chronic anxiety and depression disorders1 that may well influence how they use pain medications.
Thus, while physicians indeed are on the front lines of offering initial prescriptions for pain medications, we remain betwixt and between in the challenges of responding to the immediate needs of our patients while trying to predict the long-term effects of our prescription on the individual patient and of our prescribing patterns on society in general.
I again welcome your submissions describing how individual publications have affected your personal approach to managing patients and specific diseases. We will publish selected contributions in print and online.
- Tsang A, Von Korff M, Lee S, et al. Common chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety disorders. J Pain 2008; 9(10):883–891. doi:10.1016/j.jpain.2008.05.005
- Tsang A, Von Korff M, Lee S, et al. Common chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety disorders. J Pain 2008; 9(10):883–891. doi:10.1016/j.jpain.2008.05.005
Correction: Genitourinary syndrome of menopause