User login
VIDEO: Triple therapy study and new recommendations provide guidance on CAPS
MADRID – Catastrophic antiphospholipid syndrome (CAPS) is associated with a high mortality rate, but new research presented at the European Congress of Rheumatology shows that patient survival can be significantly improved by a triple therapy treatment approach.
Researchers at the Congress also presented clinical practice guidelines for the diagnosis and management of the rare disease, which accounts for just 1% of patients with antiphospholipid syndrome (APS).
CAPS is characterized by a fast onset of widespread thrombosis, mainly in the small vessels, and, often, microangiopathic hemolytic anemia is seen in the laboratory. If undiagnosed or left untreated, patients may present with multiorgan failure needing intensive care treatment, which can be fatal in up to 50% of cases.
At the Congress, Ignasi Rodríguez-Pintó, MD, presented new data from the CAPS Registry that looks at the combined effect of anticoagulation, corticosteroids, and plasma exchange or intravenous immunoglobulins on the survival of patients with CAPS as well as the new clinical practice guidelines.
CAPS Registry study
The aim of the study Dr. Rodríguez-Pintó presented on behalf of the CAPS Registry Project Group was to determine what, if any, survival benefit would be incurred from a triple therapy approach when compared with other different combinations of anticoagulation, corticosteroids, and plasma exchange or intravenous immunoglobulins, or none of these treatments.
Although the triple therapy treatment approach is already being used in practice, its use is largely empirical, Dr. Rodríguez-Pintó of the department of autoimmune disease at the Hospital Clinic, Barcelona, explained in a video interview.
The investigators derived their data from episodes of CAPS occurring in patients in the CAPS Registry from the European Forum on Antiphospholipid Antibodies. This international registry was set up in 2000 and has been assembling the clinical, laboratory, and therapeutic findings of patients with CAPS for almost 20 years.
“We observed 525 episodes of CAPS in 502 patients. That means that some patients had two to three episodes of CAPS,” Dr. Rodríguez-Pintó said. Data on 38 episodes of CAPS had to be excluded from the analysis because of missing information, which left 487 episodes occurring in 471 patients.
The mean age of the 471 patients included in the analysis was 38 years. The majority (67.9%) were female and had primary (68.8%) APS. Triple therapy was given to about 40% of patients who experienced CAPS, with about 57% receiving other combinations of drugs and 2.5% receiving no treatment for CAPS.
Overall, 177 of the 487 (36.3%) episodes of CAPS were fatal.
“Triple therapy was associated with a higher chance of survival when compared to other combinations or to none of these treatments,” Dr. Rodríguez-Pintó said.
While 28% of patients with CAPS died in the triple therapy group, mortality was 41% with other combinations of treatments and 75% with none of these treatments.
All-cause mortality was reduced by 47% with triple therapy, compared with none of these treatments. The adjusted odds ratio (aOR) when comparing survival between triple therapy and no treatment was 7.7, with a 95% confidence interval of 2.0 to 29.7. The aOR comparing other drug combinations versus none of these treatments was 6.8 (95% CI, 1.7-29.6).
“For a long time, we have been saying that triple therapy would probably be the best approach, but we had no firm evidence,” Dr. Rodríguez-Pintó said.
“So, this is the first time that we have clear clinical evidence of the benefit of these approaches, and I think that these results are important because they will give us more confidence in how we treat patients and help develop guidance on [the treatment’s] use in the future.”
Guidelines
A steering committee composed of representatives from the European Commission–funded RARE-BestPractices project and McMaster University in Hamilton, Ont., used GRADE methodology to develop the guidelines for CAPS diagnosis and management. The committee answered three diagnostic and seven treatment questions that originated from a panel of 19 international stakeholders, including Dr. Rodríguez-Pintó, through systematic reviews of the literature that used Cochrane criteria.
Although the review of studies did not include the study of CAPS Registry data that Dr. Rodríguez-Pintó and his colleagues conducted, he said that the recommendations still confirm the value of using a triple therapy approach to treatment.
The panel created three diagnostic recommendations for patients suspected of having CAPS, all of which were conditional and based on very low certainty of evidence: use preliminary CAPS classification criteria to diagnose CAPS; use or nonuse of biopsy, depending on the circumstances, because of its high specificity but possibly low sensitivity for thrombotic microangiopathy; and test for antiphospholipid antibodies, which should not delay initiation of treatment.
All seven first-line treatment recommendations that the panel developed relied on a very low certainty of evidence, and most were conditional:
- Triple therapy combination treatment with corticosteroids, heparin, and plasma exchange or intravenous immunoglobulins instead of a single agent or other combination treatments.
- Therapeutic dose anticoagulation was one of only two treatment recommendations to be considered “strong,” but use of direct oral anticoagulants is not advised.
- Therapeutic plasma exchange is recommended for use with other therapies and should be strongly considered for patients with microangiopathic hemolytic anemia.
- Intravenous immunoglobulin is advised for use in conjunction with other therapies and should be given special consideration for patients with immune thrombocytopenia or renal insufficiency.
- Antiplatelet agents are conditionally recommended as an add-on therapy, but their potential mortality benefit is tempered by increased risk of bleeding when used with anticoagulants. Strong consideration should be given to their use as an alternative therapy to anticoagulation when anticoagulation is contraindicated for a reason other than bleeding.
- Rituximab (Rituxan) should not be used because of little available data on its use, uncertainty regarding long-term consequences, and its expense – except for refractory cases where other therapies have been insufficient.
- Corticosteroids should not be used because of their lack of efficacy in CAPS when used alone and potential for adverse effects, except for certain circumstances where they may be indicated.
The authors of the guidelines emphasized that these recommendations are not meant to apply to every CAPS patient. They also noted that the available evidence did not allow for temporal analysis of treatments and that conclusions could not be drawn regarding “first-line” versus “second-line” therapies.
None of the authors of the registry study or the guidelines had relevant conflicts of interest to declare.
MADRID – Catastrophic antiphospholipid syndrome (CAPS) is associated with a high mortality rate, but new research presented at the European Congress of Rheumatology shows that patient survival can be significantly improved by a triple therapy treatment approach.
Researchers at the Congress also presented clinical practice guidelines for the diagnosis and management of the rare disease, which accounts for just 1% of patients with antiphospholipid syndrome (APS).
CAPS is characterized by a fast onset of widespread thrombosis, mainly in the small vessels, and, often, microangiopathic hemolytic anemia is seen in the laboratory. If undiagnosed or left untreated, patients may present with multiorgan failure needing intensive care treatment, which can be fatal in up to 50% of cases.
At the Congress, Ignasi Rodríguez-Pintó, MD, presented new data from the CAPS Registry that looks at the combined effect of anticoagulation, corticosteroids, and plasma exchange or intravenous immunoglobulins on the survival of patients with CAPS as well as the new clinical practice guidelines.
CAPS Registry study
The aim of the study Dr. Rodríguez-Pintó presented on behalf of the CAPS Registry Project Group was to determine what, if any, survival benefit would be incurred from a triple therapy approach when compared with other different combinations of anticoagulation, corticosteroids, and plasma exchange or intravenous immunoglobulins, or none of these treatments.
Although the triple therapy treatment approach is already being used in practice, its use is largely empirical, Dr. Rodríguez-Pintó of the department of autoimmune disease at the Hospital Clinic, Barcelona, explained in a video interview.
The investigators derived their data from episodes of CAPS occurring in patients in the CAPS Registry from the European Forum on Antiphospholipid Antibodies. This international registry was set up in 2000 and has been assembling the clinical, laboratory, and therapeutic findings of patients with CAPS for almost 20 years.
“We observed 525 episodes of CAPS in 502 patients. That means that some patients had two to three episodes of CAPS,” Dr. Rodríguez-Pintó said. Data on 38 episodes of CAPS had to be excluded from the analysis because of missing information, which left 487 episodes occurring in 471 patients.
The mean age of the 471 patients included in the analysis was 38 years. The majority (67.9%) were female and had primary (68.8%) APS. Triple therapy was given to about 40% of patients who experienced CAPS, with about 57% receiving other combinations of drugs and 2.5% receiving no treatment for CAPS.
Overall, 177 of the 487 (36.3%) episodes of CAPS were fatal.
“Triple therapy was associated with a higher chance of survival when compared to other combinations or to none of these treatments,” Dr. Rodríguez-Pintó said.
While 28% of patients with CAPS died in the triple therapy group, mortality was 41% with other combinations of treatments and 75% with none of these treatments.
All-cause mortality was reduced by 47% with triple therapy, compared with none of these treatments. The adjusted odds ratio (aOR) when comparing survival between triple therapy and no treatment was 7.7, with a 95% confidence interval of 2.0 to 29.7. The aOR comparing other drug combinations versus none of these treatments was 6.8 (95% CI, 1.7-29.6).
“For a long time, we have been saying that triple therapy would probably be the best approach, but we had no firm evidence,” Dr. Rodríguez-Pintó said.
“So, this is the first time that we have clear clinical evidence of the benefit of these approaches, and I think that these results are important because they will give us more confidence in how we treat patients and help develop guidance on [the treatment’s] use in the future.”
Guidelines
A steering committee composed of representatives from the European Commission–funded RARE-BestPractices project and McMaster University in Hamilton, Ont., used GRADE methodology to develop the guidelines for CAPS diagnosis and management. The committee answered three diagnostic and seven treatment questions that originated from a panel of 19 international stakeholders, including Dr. Rodríguez-Pintó, through systematic reviews of the literature that used Cochrane criteria.
Although the review of studies did not include the study of CAPS Registry data that Dr. Rodríguez-Pintó and his colleagues conducted, he said that the recommendations still confirm the value of using a triple therapy approach to treatment.
The panel created three diagnostic recommendations for patients suspected of having CAPS, all of which were conditional and based on very low certainty of evidence: use preliminary CAPS classification criteria to diagnose CAPS; use or nonuse of biopsy, depending on the circumstances, because of its high specificity but possibly low sensitivity for thrombotic microangiopathy; and test for antiphospholipid antibodies, which should not delay initiation of treatment.
All seven first-line treatment recommendations that the panel developed relied on a very low certainty of evidence, and most were conditional:
- Triple therapy combination treatment with corticosteroids, heparin, and plasma exchange or intravenous immunoglobulins instead of a single agent or other combination treatments.
- Therapeutic dose anticoagulation was one of only two treatment recommendations to be considered “strong,” but use of direct oral anticoagulants is not advised.
- Therapeutic plasma exchange is recommended for use with other therapies and should be strongly considered for patients with microangiopathic hemolytic anemia.
- Intravenous immunoglobulin is advised for use in conjunction with other therapies and should be given special consideration for patients with immune thrombocytopenia or renal insufficiency.
- Antiplatelet agents are conditionally recommended as an add-on therapy, but their potential mortality benefit is tempered by increased risk of bleeding when used with anticoagulants. Strong consideration should be given to their use as an alternative therapy to anticoagulation when anticoagulation is contraindicated for a reason other than bleeding.
- Rituximab (Rituxan) should not be used because of little available data on its use, uncertainty regarding long-term consequences, and its expense – except for refractory cases where other therapies have been insufficient.
- Corticosteroids should not be used because of their lack of efficacy in CAPS when used alone and potential for adverse effects, except for certain circumstances where they may be indicated.
The authors of the guidelines emphasized that these recommendations are not meant to apply to every CAPS patient. They also noted that the available evidence did not allow for temporal analysis of treatments and that conclusions could not be drawn regarding “first-line” versus “second-line” therapies.
None of the authors of the registry study or the guidelines had relevant conflicts of interest to declare.
MADRID – Catastrophic antiphospholipid syndrome (CAPS) is associated with a high mortality rate, but new research presented at the European Congress of Rheumatology shows that patient survival can be significantly improved by a triple therapy treatment approach.
Researchers at the Congress also presented clinical practice guidelines for the diagnosis and management of the rare disease, which accounts for just 1% of patients with antiphospholipid syndrome (APS).
CAPS is characterized by a fast onset of widespread thrombosis, mainly in the small vessels, and, often, microangiopathic hemolytic anemia is seen in the laboratory. If undiagnosed or left untreated, patients may present with multiorgan failure needing intensive care treatment, which can be fatal in up to 50% of cases.
At the Congress, Ignasi Rodríguez-Pintó, MD, presented new data from the CAPS Registry that looks at the combined effect of anticoagulation, corticosteroids, and plasma exchange or intravenous immunoglobulins on the survival of patients with CAPS as well as the new clinical practice guidelines.
CAPS Registry study
The aim of the study Dr. Rodríguez-Pintó presented on behalf of the CAPS Registry Project Group was to determine what, if any, survival benefit would be incurred from a triple therapy approach when compared with other different combinations of anticoagulation, corticosteroids, and plasma exchange or intravenous immunoglobulins, or none of these treatments.
Although the triple therapy treatment approach is already being used in practice, its use is largely empirical, Dr. Rodríguez-Pintó of the department of autoimmune disease at the Hospital Clinic, Barcelona, explained in a video interview.
The investigators derived their data from episodes of CAPS occurring in patients in the CAPS Registry from the European Forum on Antiphospholipid Antibodies. This international registry was set up in 2000 and has been assembling the clinical, laboratory, and therapeutic findings of patients with CAPS for almost 20 years.
“We observed 525 episodes of CAPS in 502 patients. That means that some patients had two to three episodes of CAPS,” Dr. Rodríguez-Pintó said. Data on 38 episodes of CAPS had to be excluded from the analysis because of missing information, which left 487 episodes occurring in 471 patients.
The mean age of the 471 patients included in the analysis was 38 years. The majority (67.9%) were female and had primary (68.8%) APS. Triple therapy was given to about 40% of patients who experienced CAPS, with about 57% receiving other combinations of drugs and 2.5% receiving no treatment for CAPS.
Overall, 177 of the 487 (36.3%) episodes of CAPS were fatal.
“Triple therapy was associated with a higher chance of survival when compared to other combinations or to none of these treatments,” Dr. Rodríguez-Pintó said.
While 28% of patients with CAPS died in the triple therapy group, mortality was 41% with other combinations of treatments and 75% with none of these treatments.
All-cause mortality was reduced by 47% with triple therapy, compared with none of these treatments. The adjusted odds ratio (aOR) when comparing survival between triple therapy and no treatment was 7.7, with a 95% confidence interval of 2.0 to 29.7. The aOR comparing other drug combinations versus none of these treatments was 6.8 (95% CI, 1.7-29.6).
“For a long time, we have been saying that triple therapy would probably be the best approach, but we had no firm evidence,” Dr. Rodríguez-Pintó said.
“So, this is the first time that we have clear clinical evidence of the benefit of these approaches, and I think that these results are important because they will give us more confidence in how we treat patients and help develop guidance on [the treatment’s] use in the future.”
Guidelines
A steering committee composed of representatives from the European Commission–funded RARE-BestPractices project and McMaster University in Hamilton, Ont., used GRADE methodology to develop the guidelines for CAPS diagnosis and management. The committee answered three diagnostic and seven treatment questions that originated from a panel of 19 international stakeholders, including Dr. Rodríguez-Pintó, through systematic reviews of the literature that used Cochrane criteria.
Although the review of studies did not include the study of CAPS Registry data that Dr. Rodríguez-Pintó and his colleagues conducted, he said that the recommendations still confirm the value of using a triple therapy approach to treatment.
The panel created three diagnostic recommendations for patients suspected of having CAPS, all of which were conditional and based on very low certainty of evidence: use preliminary CAPS classification criteria to diagnose CAPS; use or nonuse of biopsy, depending on the circumstances, because of its high specificity but possibly low sensitivity for thrombotic microangiopathy; and test for antiphospholipid antibodies, which should not delay initiation of treatment.
All seven first-line treatment recommendations that the panel developed relied on a very low certainty of evidence, and most were conditional:
- Triple therapy combination treatment with corticosteroids, heparin, and plasma exchange or intravenous immunoglobulins instead of a single agent or other combination treatments.
- Therapeutic dose anticoagulation was one of only two treatment recommendations to be considered “strong,” but use of direct oral anticoagulants is not advised.
- Therapeutic plasma exchange is recommended for use with other therapies and should be strongly considered for patients with microangiopathic hemolytic anemia.
- Intravenous immunoglobulin is advised for use in conjunction with other therapies and should be given special consideration for patients with immune thrombocytopenia or renal insufficiency.
- Antiplatelet agents are conditionally recommended as an add-on therapy, but their potential mortality benefit is tempered by increased risk of bleeding when used with anticoagulants. Strong consideration should be given to their use as an alternative therapy to anticoagulation when anticoagulation is contraindicated for a reason other than bleeding.
- Rituximab (Rituxan) should not be used because of little available data on its use, uncertainty regarding long-term consequences, and its expense – except for refractory cases where other therapies have been insufficient.
- Corticosteroids should not be used because of their lack of efficacy in CAPS when used alone and potential for adverse effects, except for certain circumstances where they may be indicated.
The authors of the guidelines emphasized that these recommendations are not meant to apply to every CAPS patient. They also noted that the available evidence did not allow for temporal analysis of treatments and that conclusions could not be drawn regarding “first-line” versus “second-line” therapies.
None of the authors of the registry study or the guidelines had relevant conflicts of interest to declare.
AT THE EULAR 2017 CONGRESS
Key clinical point:
Major finding: Mortality was 28% with triple therapy, 41% with other combinations of treatments, and 75% with none of these treatments.
Data source: A registry study of 471 CAPS patients and clinical practice guidelines for CAPS.
Disclosures: None of the authors of the registry study or the guidelines had relevant conflicts of interest to declare.
Cerebral Venous Thrombosis
Cerebral venous thrombosis (CVT) is a rare cerebrovascular disease that affects about 5 in 1 million people each year and accounts for 0.5% of all strokes.1 Previously it was thought to be caused most commonly by infections (eg, mastoiditis, otitis, meningitis) affecting the superior sagittal sinus and often resulting in focal neurologic deficits, seizures, coma, and death. Although local and systemic infections are still prominent risk factors in its development, CVT is now primarily recognized as a nonseptic disease with a wide spectrum of clinical presentations.
Cerebral venous thrombosis causes reduced outflow of blood and cerebrospinal fluid, which in half of affected patients leads to significant venous infarct. As opposed to arterial infarctions, CVT mainly affects children and young adults; it is an important cause of stroke in the younger population.2 There is significant overlap of the many risk factors for CVT and those for venous thromboembolism (VTE): cancer, obesity, genetic thrombophilia, trauma, infection, and prior neurosurgery. However, the most common acquired risk factors for CVT are oral contraceptive use and pregnancy, which explains why CVT is 3 times more likely to occur in young and middle-aged women.3
Cerebral venous thrombosis was first recognized by a French physician in the 19th century, a time when the condition was diagnosed at autopsy, which typically showed hemorrhagic lesions at the thrombus site.4 For many years heparin was contraindicated in the treatment of CVT, and only within the past 25 years did advances in neuroimaging allow for earlier diagnosis and change perspectives on the management of this disease.
Cerebral venous thrombosis occurs from formation of a thrombus within the cerebral venous sinuses, leading to elevated intracranial pressure and eventually ischemia or intracranial hemorrhage. Improved imaging techniques, notably magnetic resonance imaging (MRI) and computed tomography (CT) venography, allow physicians to identify thrombus formation earlier and begin anticoagulation therapy with heparin before infarction. A meta-analysis of studies found that heparin was safer and more efficacious in treating CVT compared with placebo.5 Furthermore, several small randomized trials found treatment with unfractionated heparin (UFH) or low-molecularweight heparin (LMWH) was not associated with higher risk of hemorrhagic stroke in these patients.6-8
Despite improvements in imaging modalities, in many cases diagnosis is delayed, as most patients with CVT have a wide spectrum of presentations with nonspecific symptoms, such as headache, seizure, focal sensorimotor deficits, and papilledema.9 Clinical presentations of CVT depend on a variety of factors, including age, time of onset, CVT location, and presence of parenchymal lesions. Isolated headache is the most common initial symptom, and in many cases is the only presenting manifestation of CVT.1 Encephalopathy with multifocal signs, delirium, or dysfunction in executive functions most commonly occurs in elderly populations.
Cavernous sinus thrombosis most commonly produces generalized headaches, orbital pain, proptosis, and diplopia, whereas cortical vein thrombosis often produces seizures and focal sensorimotor deficits. Aphasia may be present in patients with isolated left transverse sinus thrombosis. In the presence of deep cerebral venous occlusion, patients can present in coma or with severe cognitive deficits and widespread paresis.10 Thrombosis of different veins and sinuses results in a wide spectrum of diverse clinical pictures, posing a diagnostic challenge and affecting clinical outcomes.
Given the variable and often nonspecific clinical presentations of these cases, unenhanced CT typically is the first imaging study ordered. According to the literature, noncontrast CT is not sensitive (25%-56%) in detecting CVT, and findings are normal in up to 26% of patients, rarely providing a specific diagnosis.11 Furthermore, visualization on MRI can be difficult during the acute phase of CVT, as the thrombus initially appears isointense on T1-weighted images and gradually becomes hyperintense over the course of the disease process.12 These difficulties with the usual first-choice imaging examinations often result in a delay in diagnosing CVT. However, several points on close examination of these imaging studies may help physicians establish a high index of clinical suspicion and order the appropriate follow-up studies for CVT.
The authors report the case of a patient who presented with a 1-week history of confusion, headaches, and dizziness. His nonspecific presentation along with the absence of obvious signs of CVT on imaging prolonged his diagnosis and the initiation of an appropriate treatment plan.
Case Report
A 57-year-old white air-conditioning mechanic presented to the emergency department (ED) with a 1-week history of gradual-onset confusion, severe headaches, dizziness, light-headedness, poor memory, and increased sleep. Two days earlier, he presented with similar symptoms to an outside facility, where he was admitted and underwent a workup for stroke, hemorrhage, and cardiac abnormalities—including noncontrast CT of the head. With nothing of clinical significance found, the patient was discharged and was advised to follow up on an outpatient basis.
Persisting symptoms brought the patient to the ED 1 day later, and he was admitted. He described severe, progressive, generalized headaches that were more severe when he was lying down at night and waking in the morning. He did not report that the headaches worsened with coughing or straining, and he reported no history of trauma, neck stiffness, vision change, seizures, and migraines. His medical history was significant for hypertension, dyslipidemia, and in 2011, an unprovoked deep vein thrombosis (DVT) in the right leg. He reported no history of tobacco, alcohol, or illicit drug use. He had served in the U.S. Navy, working in electronics, intelligence, data systems, and satellite communications.
On initial physical examination, the patient was afebrile and lethargic, and his blood pressure was mildly elevated (144/83 mm Hg). Cardiopulmonary examination was normal. Neurologic examination revealed no severe focal deficits, and cranial nerves II to XII were intact. Funduscopic examination was normal, with no papilledema noted. Motor strength was 5/5 bilaterally in the deltoids, biceps, triceps, radial and ulnar wrist extensors, iliopsoas, quadriceps, hamstrings, tibialis anterior and posterior, fibulares, and gastrocnemius. Pinprick sensation and light-touch sensation were decreased within the lateral bicipital region of the left upper extremity. Pinprick sensation was intact bilaterally in 1-inch increments at all other distributions along the medial and lateral aspects of the upper and lower extremities. Muscle tone and bulk were normal in all extremities. Reflexes were 2+ bilaterally in the biceps, brachioradialis, triceps, quadriceps, and Achilles. The Babinski sign was absent bilaterally, the finger-tonose and heel-to-shin tests were normal, the Romberg sign was absent, and there was no evidence of pronator drift. Laboratory test results were normal except for slightly elevated hemoglobin (17.5 g/dL) and D-dimer (588 ng/mL) levels.
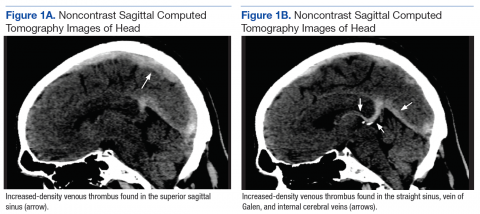
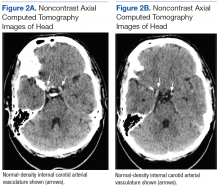
Although noncontrast CT of the head initially showed no acute intracranial abnormalities, retrospective close comparison with the arterial system revealed slightly increased attenuation in the superior sagittal sinus, straight sinus, vein of Galen, and internal cerebral veins (Figures 1A and 1B) relative to the arterial carotid anterior circulation (Figures 2A and 2B).
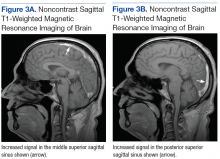
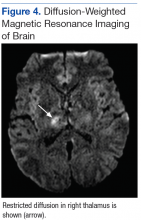
Subsequent brain MRI without contrast showed a hyperintense T1 signal involving the superior sagittal sinus (Figures 3A and 3B), extending into the straight sinus and the vein of Galen. Magnetic resonance imaging with contrast demonstrated a prominent filling defect primarily in the superior sagittal sinus, in the right transverse sinus, and in the vein of Galen. Diffusion-weighted brain MRI sequence showed restricted diffusion localized to the right thalamic area (Figure 4) and no evidence of hemorrhage.
Treatment
International guidelines recommend using heparin to achieve rapid anticoagulation, stop the thrombotic process, and prevent extension of the thrombus.13 Theoretically, more rapid recanalization may have been achieved by performing endovascular thrombectomy in the present case. However, severe bleeding complications, combined with higher cost and the limited availability of clinicians experienced in treating this rare disease, convince physicians to rely on heparin as first-line treatment for CVT.14 A small randomized clinical trial found LMWH safer and more efficacious than UFH in treating CVT.15 After stabilization, oral anticoagulation therapy is used to maintain an international normalized ratio (INR) between 2.0 and 3.0 for at least 3 months.14
Given these findings, the patient was initially treated with LMWH. Eventually he was switched to oral warfarin and showed signs of clinical improvement. A hypercoagulability state workup revealed that the patient was heterozygous for the prothrombin G20210A mutation, and he was discharged and instructed to continue the oral warfarin therapy.
On follow-up, the hematology and neurology team initiated indefinite treatment with warfarin for his genetic hypercoagulability state. Monitoring of the dose of anticoagulation therapy was started to maintain INR between 2.0 and 3.0. The patient began coming to the office for INR monitoring every 2 to 3 weeks, and his most recent INR, in May 2017, was 2.66. He is taking 7.5 mg of warfarin on Wednesdays and Sundays and 5 mg on all other days and currently does not report any progressive neurologic deficits.
Discussion
The clinical findings of CVT and the hypercoagulability state workup revealed that the patient was heterozygous for the prothrombin G20210A mutation. Prothrombin is the precursor to thrombin, which is a key regulator of the clotting cascade and a promoter of coagulation. Carriers of the mutation have elevated levels of blood plasma prothrombin and have been associated with a 4 times higher risk for VTE.16
Several large studies and systematic reviews have confirmed that the prothrombin G20210A mutation is associated with higher rates of VTE, leading to an increased risk for DVT of the leg or pulmonary embolism.17-19 More specifically, a metaanalysis of 15 case–control studies found strong associations between the mutation and CVT.20 Despite this significant association, studies are inconclusive about whether heterozygosity for the mutation is associated with increased rates of recurrent CVT or other VTE in the absence of other risk factors, such as oral contraceptive use, trauma, malignancy, and infection.21-23 Therefore, the optimal duration of anticoagulation therapy for CVT is not well established in patients with the mutation. However, the present patient was started on indefinite anticoagulation therapy because the underlying etiology of the CVT was not reversible or transient, and this CVT was his second episode of VTE, following a 2011 DVT in the right leg.
The case discussed here illustrates the clinical presentation and diagnostic complexities of CVT. Two days before coming to the ED, the patient presented to an outside facility and underwent a workup for nonspecific symptoms (eg, confusion, headaches). Due to the nonspecific presentation associated with CVT, a detailed history is imperative to distinguish symptoms suggesting increased intracranial pressure, such as headaches worse when lying down or present in the morning, with a high clinical suspicion of CVT. The ability to attain these specific details leads clinicians toward obtaining the necessary imaging studies for potential CVT patients, and may prevent delay in diagnosis and treatment. The thrombus in CVT initially consists of deoxyhemoglobin and appears on MRI as an isointense signal on T1-weighted images and a hypointense signal on T2-weighted images; over subsequent days, the thrombus changes to methemoglobin and appears as a slightly hyperintense signal on both T1- and T2-weighted images.24
During this phase, there are some false negatives, as the thrombus can be mistaken for imaging artifacts, hematocrit elevations, or low flow of normal venous blood. Given the clinical findings and imaging studies, it is essential to distinguish CVT from other benign etiologies. Earlier diagnosis and initiation of anticoagulation therapy may have precluded the small amount of localized ischemic changes in this patient’s right thalamus, thus preventing the mild sensory loss in the left upper extremity. With the variable and nonspecific clinical presentations and the difficulties in identifying CVT with first-line imaging, progression of thrombus formation may lead to severe focal neurologic deficits, coma, or death.
Using CT imaging studies to compare the blood in the draining cerebral sinuses with the blood in the arterial system can help distinguish CVT from other etiologies of hyperdense abnormalities, such as increased hematocrit or decreased flow. Retrospective close examination of the present patient’s noncontrast CT images of the head and brain revealed slight hyperdensity in the cerebral sinuses compared with the arterial blood, suggesting the presence of thrombus formation in the cerebral veins. As CT is often the first study used to evaluate the nonspecific clinical presentations of these patients, identifying subtle signaldensity differences between the arterial and venous systems could guide physicians in identifying CVT earlier.
The authors reiterate the importance of meticulous imaging interpretation in light of the entire clinical picture: In these patients, it is imperative to have a high index of clinical suspicion for CVT in order to prevent more serious complications, such as ischemic or hemorrhagic stroke.
1. Bousser MG, Ferro JM. Cerebral venous thrombosis: an update. Lancet Neurol. 2007;6(2):162-170.
2. Coutinho JM. Cerebral venous thrombosis. J Thromb Haemost. 2015;13(suppl 1):S238-S244.
3. Coutinho JM, Ferro JM, Canhão P, et al. Cerebral venous and sinus thrombosis in women. Stroke. 2009;40(7):2356-2361.
4. Zuurbier SM, Coutinho JM. Cerebral venous thrombosis. Adv Exp Med Biol. 2017;906:183-193.
5. Einhäupl KM, Villringer A, Meister W, et al. Heparin treatment in sinus venous thrombosis. Lancet. 1991;338(8767):597-600.
6. de Bruijn SF, Stam J. Randomized, placebocontrolled trial of anticoagulant treatment with lowmolecular-weight heparin for cerebral sinus thrombosis. Stroke. 1999;30(3):484-488.
7. Nagaraja D, Haridas T, Taly AB, Veerendrakumar M, SubbuKrishna DK. Puerperal cerebral venous thrombosis: therapeutic benefit of low dose heparin. Neurol India. 1999;47(1):43-46.
8. Coutinho JM, de Bruijn SF, deVeber G, Stam J. Anticoagulation for cerebral venous sinus thrombosis. Stroke. 2012;43(4):e41-e42.
9. Sassi SB, Touati N, Baccouche H, Drissi C, Romdhane NB, Hentati F. Cerebral venous thrombosis. Clin Appl Thromb Hemost. 2016:1076029616665168. [Epub ahead of print.]
10. Ferro JM, Canhão P. Cerebral venous sinus thrombosis: update on diagnosis and management. Curr Cardiol Rep. 2014;16(9):523.
11. Albright KC, Freeman WD, Kruse BT. Cerebral venous thrombosis. J Emerg Med. 2010;38(2):238-239.
12. Lafitte F, Boukobza M, Guichard JP, et al. MRI and MRA for diagnosis and follow-up of cerebral venous thrombosis (CVT). Clin Radiol. 1997;52(9):672-679.
13. Saposnik G, Barinagarrementeria F, Brown RD Jr, et al; American Heart Association Stroke Council and Council on Epidemiology and Prevention. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(4):1158-1192.
14. Coutinho JM, Middeldorp S, Stam J. Advances in the treatment of cerebral venous thrombosis. Curr Treat Options Neurol. 2014;16(7):299.
15. Coutinho JM, Ferro JM, Canhão P, Barinagarrementeria F, Bousser MG, Stam J; ISCVT Investigators. Unfractionated or low-molecular weight heparin for the treatment of cerebral venous thrombosis. Stroke. 2010;41(11):2575-2580.
16. Rosendaal FR. Venous thrombosis: the role of genes, environment, and behavior. Hematology Am Soc Hematol Educ Program. 2005:1-12.
17. Dentali F, Crowther M, Ageno W. Thrombophilic abnormalities, oral contraceptives, and risk of cerebral vein thrombosis: a meta-analysis. Blood.2006;107(7):2766-2773.
18. Salomon O, Steinberg DM, Zivelin A, et al. Single and combined prothrombotic factors in patients with idiopathic venous thromboembolism: prevalence and risk assessment. Arterioscler Thromb Vasc Biol. 1999;19(3):511-518.
19. Emmerich J, Rosendaal FR, Cattaneo M, et al. Combined effect of factor V Leiden and prothrombin 20210A on the risk of venous thromboembolism— pooled analysis of 8 case–control studies including 2310 cases and 3204 controls. Study Group for Pooled-Analysis in Venous Thromboembolism. Thromb Haemost. 2001;86(3):809-816.
20. Lauw MN, Barco S, Coutinho JM, Middeldorp S. Cerebral venous thrombosis and thrombophilia: a systematic review and meta-analysis. Semin Thromb Hemost. 2013;39(8):913-927.
21. Dentali F, Poli D, Scoditti U, et al. Long-term outcomes of patients with cerebral vein thrombosis: a multicenter study. J Thromb Haemost. 2012;10(7):1297-1302.
22. Martinelli I, Bucciarelli P, Passamonti SM, Battaglioli T, Previtali E, Mannucci PM. Long-term evaluation of the risk of recurrence after cerebral sinus-venous thrombosis. Circulation. 2010;121(25):2740-2746.
23. Gosk-Bierska I, Wysokinski W, Brown RD Jr, et al. Cerebral venous sinus thrombosis: incidence of venous thrombosis recurrence and survival. Neurology. 2006;67(5):814-819.
24. Galidie G, Le Gall R, Cordoliani YS, Pharaboz C, Le Marec E, Cosnard G. Thrombosis of the cerebral veins. X-ray computed tomography and MRI imaging. 11 cases [in French]. J Radiol. 1992;73(3):175-190
Cerebral venous thrombosis (CVT) is a rare cerebrovascular disease that affects about 5 in 1 million people each year and accounts for 0.5% of all strokes.1 Previously it was thought to be caused most commonly by infections (eg, mastoiditis, otitis, meningitis) affecting the superior sagittal sinus and often resulting in focal neurologic deficits, seizures, coma, and death. Although local and systemic infections are still prominent risk factors in its development, CVT is now primarily recognized as a nonseptic disease with a wide spectrum of clinical presentations.
Cerebral venous thrombosis causes reduced outflow of blood and cerebrospinal fluid, which in half of affected patients leads to significant venous infarct. As opposed to arterial infarctions, CVT mainly affects children and young adults; it is an important cause of stroke in the younger population.2 There is significant overlap of the many risk factors for CVT and those for venous thromboembolism (VTE): cancer, obesity, genetic thrombophilia, trauma, infection, and prior neurosurgery. However, the most common acquired risk factors for CVT are oral contraceptive use and pregnancy, which explains why CVT is 3 times more likely to occur in young and middle-aged women.3
Cerebral venous thrombosis was first recognized by a French physician in the 19th century, a time when the condition was diagnosed at autopsy, which typically showed hemorrhagic lesions at the thrombus site.4 For many years heparin was contraindicated in the treatment of CVT, and only within the past 25 years did advances in neuroimaging allow for earlier diagnosis and change perspectives on the management of this disease.
Cerebral venous thrombosis occurs from formation of a thrombus within the cerebral venous sinuses, leading to elevated intracranial pressure and eventually ischemia or intracranial hemorrhage. Improved imaging techniques, notably magnetic resonance imaging (MRI) and computed tomography (CT) venography, allow physicians to identify thrombus formation earlier and begin anticoagulation therapy with heparin before infarction. A meta-analysis of studies found that heparin was safer and more efficacious in treating CVT compared with placebo.5 Furthermore, several small randomized trials found treatment with unfractionated heparin (UFH) or low-molecularweight heparin (LMWH) was not associated with higher risk of hemorrhagic stroke in these patients.6-8
Despite improvements in imaging modalities, in many cases diagnosis is delayed, as most patients with CVT have a wide spectrum of presentations with nonspecific symptoms, such as headache, seizure, focal sensorimotor deficits, and papilledema.9 Clinical presentations of CVT depend on a variety of factors, including age, time of onset, CVT location, and presence of parenchymal lesions. Isolated headache is the most common initial symptom, and in many cases is the only presenting manifestation of CVT.1 Encephalopathy with multifocal signs, delirium, or dysfunction in executive functions most commonly occurs in elderly populations.
Cavernous sinus thrombosis most commonly produces generalized headaches, orbital pain, proptosis, and diplopia, whereas cortical vein thrombosis often produces seizures and focal sensorimotor deficits. Aphasia may be present in patients with isolated left transverse sinus thrombosis. In the presence of deep cerebral venous occlusion, patients can present in coma or with severe cognitive deficits and widespread paresis.10 Thrombosis of different veins and sinuses results in a wide spectrum of diverse clinical pictures, posing a diagnostic challenge and affecting clinical outcomes.
Given the variable and often nonspecific clinical presentations of these cases, unenhanced CT typically is the first imaging study ordered. According to the literature, noncontrast CT is not sensitive (25%-56%) in detecting CVT, and findings are normal in up to 26% of patients, rarely providing a specific diagnosis.11 Furthermore, visualization on MRI can be difficult during the acute phase of CVT, as the thrombus initially appears isointense on T1-weighted images and gradually becomes hyperintense over the course of the disease process.12 These difficulties with the usual first-choice imaging examinations often result in a delay in diagnosing CVT. However, several points on close examination of these imaging studies may help physicians establish a high index of clinical suspicion and order the appropriate follow-up studies for CVT.
The authors report the case of a patient who presented with a 1-week history of confusion, headaches, and dizziness. His nonspecific presentation along with the absence of obvious signs of CVT on imaging prolonged his diagnosis and the initiation of an appropriate treatment plan.
Case Report
A 57-year-old white air-conditioning mechanic presented to the emergency department (ED) with a 1-week history of gradual-onset confusion, severe headaches, dizziness, light-headedness, poor memory, and increased sleep. Two days earlier, he presented with similar symptoms to an outside facility, where he was admitted and underwent a workup for stroke, hemorrhage, and cardiac abnormalities—including noncontrast CT of the head. With nothing of clinical significance found, the patient was discharged and was advised to follow up on an outpatient basis.
Persisting symptoms brought the patient to the ED 1 day later, and he was admitted. He described severe, progressive, generalized headaches that were more severe when he was lying down at night and waking in the morning. He did not report that the headaches worsened with coughing or straining, and he reported no history of trauma, neck stiffness, vision change, seizures, and migraines. His medical history was significant for hypertension, dyslipidemia, and in 2011, an unprovoked deep vein thrombosis (DVT) in the right leg. He reported no history of tobacco, alcohol, or illicit drug use. He had served in the U.S. Navy, working in electronics, intelligence, data systems, and satellite communications.
On initial physical examination, the patient was afebrile and lethargic, and his blood pressure was mildly elevated (144/83 mm Hg). Cardiopulmonary examination was normal. Neurologic examination revealed no severe focal deficits, and cranial nerves II to XII were intact. Funduscopic examination was normal, with no papilledema noted. Motor strength was 5/5 bilaterally in the deltoids, biceps, triceps, radial and ulnar wrist extensors, iliopsoas, quadriceps, hamstrings, tibialis anterior and posterior, fibulares, and gastrocnemius. Pinprick sensation and light-touch sensation were decreased within the lateral bicipital region of the left upper extremity. Pinprick sensation was intact bilaterally in 1-inch increments at all other distributions along the medial and lateral aspects of the upper and lower extremities. Muscle tone and bulk were normal in all extremities. Reflexes were 2+ bilaterally in the biceps, brachioradialis, triceps, quadriceps, and Achilles. The Babinski sign was absent bilaterally, the finger-tonose and heel-to-shin tests were normal, the Romberg sign was absent, and there was no evidence of pronator drift. Laboratory test results were normal except for slightly elevated hemoglobin (17.5 g/dL) and D-dimer (588 ng/mL) levels.
Although noncontrast CT of the head initially showed no acute intracranial abnormalities, retrospective close comparison with the arterial system revealed slightly increased attenuation in the superior sagittal sinus, straight sinus, vein of Galen, and internal cerebral veins (Figures 1A and 1B) relative to the arterial carotid anterior circulation (Figures 2A and 2B).
Subsequent brain MRI without contrast showed a hyperintense T1 signal involving the superior sagittal sinus (Figures 3A and 3B), extending into the straight sinus and the vein of Galen. Magnetic resonance imaging with contrast demonstrated a prominent filling defect primarily in the superior sagittal sinus, in the right transverse sinus, and in the vein of Galen. Diffusion-weighted brain MRI sequence showed restricted diffusion localized to the right thalamic area (Figure 4) and no evidence of hemorrhage.
Treatment
International guidelines recommend using heparin to achieve rapid anticoagulation, stop the thrombotic process, and prevent extension of the thrombus.13 Theoretically, more rapid recanalization may have been achieved by performing endovascular thrombectomy in the present case. However, severe bleeding complications, combined with higher cost and the limited availability of clinicians experienced in treating this rare disease, convince physicians to rely on heparin as first-line treatment for CVT.14 A small randomized clinical trial found LMWH safer and more efficacious than UFH in treating CVT.15 After stabilization, oral anticoagulation therapy is used to maintain an international normalized ratio (INR) between 2.0 and 3.0 for at least 3 months.14
Given these findings, the patient was initially treated with LMWH. Eventually he was switched to oral warfarin and showed signs of clinical improvement. A hypercoagulability state workup revealed that the patient was heterozygous for the prothrombin G20210A mutation, and he was discharged and instructed to continue the oral warfarin therapy.
On follow-up, the hematology and neurology team initiated indefinite treatment with warfarin for his genetic hypercoagulability state. Monitoring of the dose of anticoagulation therapy was started to maintain INR between 2.0 and 3.0. The patient began coming to the office for INR monitoring every 2 to 3 weeks, and his most recent INR, in May 2017, was 2.66. He is taking 7.5 mg of warfarin on Wednesdays and Sundays and 5 mg on all other days and currently does not report any progressive neurologic deficits.
Discussion
The clinical findings of CVT and the hypercoagulability state workup revealed that the patient was heterozygous for the prothrombin G20210A mutation. Prothrombin is the precursor to thrombin, which is a key regulator of the clotting cascade and a promoter of coagulation. Carriers of the mutation have elevated levels of blood plasma prothrombin and have been associated with a 4 times higher risk for VTE.16
Several large studies and systematic reviews have confirmed that the prothrombin G20210A mutation is associated with higher rates of VTE, leading to an increased risk for DVT of the leg or pulmonary embolism.17-19 More specifically, a metaanalysis of 15 case–control studies found strong associations between the mutation and CVT.20 Despite this significant association, studies are inconclusive about whether heterozygosity for the mutation is associated with increased rates of recurrent CVT or other VTE in the absence of other risk factors, such as oral contraceptive use, trauma, malignancy, and infection.21-23 Therefore, the optimal duration of anticoagulation therapy for CVT is not well established in patients with the mutation. However, the present patient was started on indefinite anticoagulation therapy because the underlying etiology of the CVT was not reversible or transient, and this CVT was his second episode of VTE, following a 2011 DVT in the right leg.
The case discussed here illustrates the clinical presentation and diagnostic complexities of CVT. Two days before coming to the ED, the patient presented to an outside facility and underwent a workup for nonspecific symptoms (eg, confusion, headaches). Due to the nonspecific presentation associated with CVT, a detailed history is imperative to distinguish symptoms suggesting increased intracranial pressure, such as headaches worse when lying down or present in the morning, with a high clinical suspicion of CVT. The ability to attain these specific details leads clinicians toward obtaining the necessary imaging studies for potential CVT patients, and may prevent delay in diagnosis and treatment. The thrombus in CVT initially consists of deoxyhemoglobin and appears on MRI as an isointense signal on T1-weighted images and a hypointense signal on T2-weighted images; over subsequent days, the thrombus changes to methemoglobin and appears as a slightly hyperintense signal on both T1- and T2-weighted images.24
During this phase, there are some false negatives, as the thrombus can be mistaken for imaging artifacts, hematocrit elevations, or low flow of normal venous blood. Given the clinical findings and imaging studies, it is essential to distinguish CVT from other benign etiologies. Earlier diagnosis and initiation of anticoagulation therapy may have precluded the small amount of localized ischemic changes in this patient’s right thalamus, thus preventing the mild sensory loss in the left upper extremity. With the variable and nonspecific clinical presentations and the difficulties in identifying CVT with first-line imaging, progression of thrombus formation may lead to severe focal neurologic deficits, coma, or death.
Using CT imaging studies to compare the blood in the draining cerebral sinuses with the blood in the arterial system can help distinguish CVT from other etiologies of hyperdense abnormalities, such as increased hematocrit or decreased flow. Retrospective close examination of the present patient’s noncontrast CT images of the head and brain revealed slight hyperdensity in the cerebral sinuses compared with the arterial blood, suggesting the presence of thrombus formation in the cerebral veins. As CT is often the first study used to evaluate the nonspecific clinical presentations of these patients, identifying subtle signaldensity differences between the arterial and venous systems could guide physicians in identifying CVT earlier.
The authors reiterate the importance of meticulous imaging interpretation in light of the entire clinical picture: In these patients, it is imperative to have a high index of clinical suspicion for CVT in order to prevent more serious complications, such as ischemic or hemorrhagic stroke.
Cerebral venous thrombosis (CVT) is a rare cerebrovascular disease that affects about 5 in 1 million people each year and accounts for 0.5% of all strokes.1 Previously it was thought to be caused most commonly by infections (eg, mastoiditis, otitis, meningitis) affecting the superior sagittal sinus and often resulting in focal neurologic deficits, seizures, coma, and death. Although local and systemic infections are still prominent risk factors in its development, CVT is now primarily recognized as a nonseptic disease with a wide spectrum of clinical presentations.
Cerebral venous thrombosis causes reduced outflow of blood and cerebrospinal fluid, which in half of affected patients leads to significant venous infarct. As opposed to arterial infarctions, CVT mainly affects children and young adults; it is an important cause of stroke in the younger population.2 There is significant overlap of the many risk factors for CVT and those for venous thromboembolism (VTE): cancer, obesity, genetic thrombophilia, trauma, infection, and prior neurosurgery. However, the most common acquired risk factors for CVT are oral contraceptive use and pregnancy, which explains why CVT is 3 times more likely to occur in young and middle-aged women.3
Cerebral venous thrombosis was first recognized by a French physician in the 19th century, a time when the condition was diagnosed at autopsy, which typically showed hemorrhagic lesions at the thrombus site.4 For many years heparin was contraindicated in the treatment of CVT, and only within the past 25 years did advances in neuroimaging allow for earlier diagnosis and change perspectives on the management of this disease.
Cerebral venous thrombosis occurs from formation of a thrombus within the cerebral venous sinuses, leading to elevated intracranial pressure and eventually ischemia or intracranial hemorrhage. Improved imaging techniques, notably magnetic resonance imaging (MRI) and computed tomography (CT) venography, allow physicians to identify thrombus formation earlier and begin anticoagulation therapy with heparin before infarction. A meta-analysis of studies found that heparin was safer and more efficacious in treating CVT compared with placebo.5 Furthermore, several small randomized trials found treatment with unfractionated heparin (UFH) or low-molecularweight heparin (LMWH) was not associated with higher risk of hemorrhagic stroke in these patients.6-8
Despite improvements in imaging modalities, in many cases diagnosis is delayed, as most patients with CVT have a wide spectrum of presentations with nonspecific symptoms, such as headache, seizure, focal sensorimotor deficits, and papilledema.9 Clinical presentations of CVT depend on a variety of factors, including age, time of onset, CVT location, and presence of parenchymal lesions. Isolated headache is the most common initial symptom, and in many cases is the only presenting manifestation of CVT.1 Encephalopathy with multifocal signs, delirium, or dysfunction in executive functions most commonly occurs in elderly populations.
Cavernous sinus thrombosis most commonly produces generalized headaches, orbital pain, proptosis, and diplopia, whereas cortical vein thrombosis often produces seizures and focal sensorimotor deficits. Aphasia may be present in patients with isolated left transverse sinus thrombosis. In the presence of deep cerebral venous occlusion, patients can present in coma or with severe cognitive deficits and widespread paresis.10 Thrombosis of different veins and sinuses results in a wide spectrum of diverse clinical pictures, posing a diagnostic challenge and affecting clinical outcomes.
Given the variable and often nonspecific clinical presentations of these cases, unenhanced CT typically is the first imaging study ordered. According to the literature, noncontrast CT is not sensitive (25%-56%) in detecting CVT, and findings are normal in up to 26% of patients, rarely providing a specific diagnosis.11 Furthermore, visualization on MRI can be difficult during the acute phase of CVT, as the thrombus initially appears isointense on T1-weighted images and gradually becomes hyperintense over the course of the disease process.12 These difficulties with the usual first-choice imaging examinations often result in a delay in diagnosing CVT. However, several points on close examination of these imaging studies may help physicians establish a high index of clinical suspicion and order the appropriate follow-up studies for CVT.
The authors report the case of a patient who presented with a 1-week history of confusion, headaches, and dizziness. His nonspecific presentation along with the absence of obvious signs of CVT on imaging prolonged his diagnosis and the initiation of an appropriate treatment plan.
Case Report
A 57-year-old white air-conditioning mechanic presented to the emergency department (ED) with a 1-week history of gradual-onset confusion, severe headaches, dizziness, light-headedness, poor memory, and increased sleep. Two days earlier, he presented with similar symptoms to an outside facility, where he was admitted and underwent a workup for stroke, hemorrhage, and cardiac abnormalities—including noncontrast CT of the head. With nothing of clinical significance found, the patient was discharged and was advised to follow up on an outpatient basis.
Persisting symptoms brought the patient to the ED 1 day later, and he was admitted. He described severe, progressive, generalized headaches that were more severe when he was lying down at night and waking in the morning. He did not report that the headaches worsened with coughing or straining, and he reported no history of trauma, neck stiffness, vision change, seizures, and migraines. His medical history was significant for hypertension, dyslipidemia, and in 2011, an unprovoked deep vein thrombosis (DVT) in the right leg. He reported no history of tobacco, alcohol, or illicit drug use. He had served in the U.S. Navy, working in electronics, intelligence, data systems, and satellite communications.
On initial physical examination, the patient was afebrile and lethargic, and his blood pressure was mildly elevated (144/83 mm Hg). Cardiopulmonary examination was normal. Neurologic examination revealed no severe focal deficits, and cranial nerves II to XII were intact. Funduscopic examination was normal, with no papilledema noted. Motor strength was 5/5 bilaterally in the deltoids, biceps, triceps, radial and ulnar wrist extensors, iliopsoas, quadriceps, hamstrings, tibialis anterior and posterior, fibulares, and gastrocnemius. Pinprick sensation and light-touch sensation were decreased within the lateral bicipital region of the left upper extremity. Pinprick sensation was intact bilaterally in 1-inch increments at all other distributions along the medial and lateral aspects of the upper and lower extremities. Muscle tone and bulk were normal in all extremities. Reflexes were 2+ bilaterally in the biceps, brachioradialis, triceps, quadriceps, and Achilles. The Babinski sign was absent bilaterally, the finger-tonose and heel-to-shin tests were normal, the Romberg sign was absent, and there was no evidence of pronator drift. Laboratory test results were normal except for slightly elevated hemoglobin (17.5 g/dL) and D-dimer (588 ng/mL) levels.
Although noncontrast CT of the head initially showed no acute intracranial abnormalities, retrospective close comparison with the arterial system revealed slightly increased attenuation in the superior sagittal sinus, straight sinus, vein of Galen, and internal cerebral veins (Figures 1A and 1B) relative to the arterial carotid anterior circulation (Figures 2A and 2B).
Subsequent brain MRI without contrast showed a hyperintense T1 signal involving the superior sagittal sinus (Figures 3A and 3B), extending into the straight sinus and the vein of Galen. Magnetic resonance imaging with contrast demonstrated a prominent filling defect primarily in the superior sagittal sinus, in the right transverse sinus, and in the vein of Galen. Diffusion-weighted brain MRI sequence showed restricted diffusion localized to the right thalamic area (Figure 4) and no evidence of hemorrhage.
Treatment
International guidelines recommend using heparin to achieve rapid anticoagulation, stop the thrombotic process, and prevent extension of the thrombus.13 Theoretically, more rapid recanalization may have been achieved by performing endovascular thrombectomy in the present case. However, severe bleeding complications, combined with higher cost and the limited availability of clinicians experienced in treating this rare disease, convince physicians to rely on heparin as first-line treatment for CVT.14 A small randomized clinical trial found LMWH safer and more efficacious than UFH in treating CVT.15 After stabilization, oral anticoagulation therapy is used to maintain an international normalized ratio (INR) between 2.0 and 3.0 for at least 3 months.14
Given these findings, the patient was initially treated with LMWH. Eventually he was switched to oral warfarin and showed signs of clinical improvement. A hypercoagulability state workup revealed that the patient was heterozygous for the prothrombin G20210A mutation, and he was discharged and instructed to continue the oral warfarin therapy.
On follow-up, the hematology and neurology team initiated indefinite treatment with warfarin for his genetic hypercoagulability state. Monitoring of the dose of anticoagulation therapy was started to maintain INR between 2.0 and 3.0. The patient began coming to the office for INR monitoring every 2 to 3 weeks, and his most recent INR, in May 2017, was 2.66. He is taking 7.5 mg of warfarin on Wednesdays and Sundays and 5 mg on all other days and currently does not report any progressive neurologic deficits.
Discussion
The clinical findings of CVT and the hypercoagulability state workup revealed that the patient was heterozygous for the prothrombin G20210A mutation. Prothrombin is the precursor to thrombin, which is a key regulator of the clotting cascade and a promoter of coagulation. Carriers of the mutation have elevated levels of blood plasma prothrombin and have been associated with a 4 times higher risk for VTE.16
Several large studies and systematic reviews have confirmed that the prothrombin G20210A mutation is associated with higher rates of VTE, leading to an increased risk for DVT of the leg or pulmonary embolism.17-19 More specifically, a metaanalysis of 15 case–control studies found strong associations between the mutation and CVT.20 Despite this significant association, studies are inconclusive about whether heterozygosity for the mutation is associated with increased rates of recurrent CVT or other VTE in the absence of other risk factors, such as oral contraceptive use, trauma, malignancy, and infection.21-23 Therefore, the optimal duration of anticoagulation therapy for CVT is not well established in patients with the mutation. However, the present patient was started on indefinite anticoagulation therapy because the underlying etiology of the CVT was not reversible or transient, and this CVT was his second episode of VTE, following a 2011 DVT in the right leg.
The case discussed here illustrates the clinical presentation and diagnostic complexities of CVT. Two days before coming to the ED, the patient presented to an outside facility and underwent a workup for nonspecific symptoms (eg, confusion, headaches). Due to the nonspecific presentation associated with CVT, a detailed history is imperative to distinguish symptoms suggesting increased intracranial pressure, such as headaches worse when lying down or present in the morning, with a high clinical suspicion of CVT. The ability to attain these specific details leads clinicians toward obtaining the necessary imaging studies for potential CVT patients, and may prevent delay in diagnosis and treatment. The thrombus in CVT initially consists of deoxyhemoglobin and appears on MRI as an isointense signal on T1-weighted images and a hypointense signal on T2-weighted images; over subsequent days, the thrombus changes to methemoglobin and appears as a slightly hyperintense signal on both T1- and T2-weighted images.24
During this phase, there are some false negatives, as the thrombus can be mistaken for imaging artifacts, hematocrit elevations, or low flow of normal venous blood. Given the clinical findings and imaging studies, it is essential to distinguish CVT from other benign etiologies. Earlier diagnosis and initiation of anticoagulation therapy may have precluded the small amount of localized ischemic changes in this patient’s right thalamus, thus preventing the mild sensory loss in the left upper extremity. With the variable and nonspecific clinical presentations and the difficulties in identifying CVT with first-line imaging, progression of thrombus formation may lead to severe focal neurologic deficits, coma, or death.
Using CT imaging studies to compare the blood in the draining cerebral sinuses with the blood in the arterial system can help distinguish CVT from other etiologies of hyperdense abnormalities, such as increased hematocrit or decreased flow. Retrospective close examination of the present patient’s noncontrast CT images of the head and brain revealed slight hyperdensity in the cerebral sinuses compared with the arterial blood, suggesting the presence of thrombus formation in the cerebral veins. As CT is often the first study used to evaluate the nonspecific clinical presentations of these patients, identifying subtle signaldensity differences between the arterial and venous systems could guide physicians in identifying CVT earlier.
The authors reiterate the importance of meticulous imaging interpretation in light of the entire clinical picture: In these patients, it is imperative to have a high index of clinical suspicion for CVT in order to prevent more serious complications, such as ischemic or hemorrhagic stroke.
1. Bousser MG, Ferro JM. Cerebral venous thrombosis: an update. Lancet Neurol. 2007;6(2):162-170.
2. Coutinho JM. Cerebral venous thrombosis. J Thromb Haemost. 2015;13(suppl 1):S238-S244.
3. Coutinho JM, Ferro JM, Canhão P, et al. Cerebral venous and sinus thrombosis in women. Stroke. 2009;40(7):2356-2361.
4. Zuurbier SM, Coutinho JM. Cerebral venous thrombosis. Adv Exp Med Biol. 2017;906:183-193.
5. Einhäupl KM, Villringer A, Meister W, et al. Heparin treatment in sinus venous thrombosis. Lancet. 1991;338(8767):597-600.
6. de Bruijn SF, Stam J. Randomized, placebocontrolled trial of anticoagulant treatment with lowmolecular-weight heparin for cerebral sinus thrombosis. Stroke. 1999;30(3):484-488.
7. Nagaraja D, Haridas T, Taly AB, Veerendrakumar M, SubbuKrishna DK. Puerperal cerebral venous thrombosis: therapeutic benefit of low dose heparin. Neurol India. 1999;47(1):43-46.
8. Coutinho JM, de Bruijn SF, deVeber G, Stam J. Anticoagulation for cerebral venous sinus thrombosis. Stroke. 2012;43(4):e41-e42.
9. Sassi SB, Touati N, Baccouche H, Drissi C, Romdhane NB, Hentati F. Cerebral venous thrombosis. Clin Appl Thromb Hemost. 2016:1076029616665168. [Epub ahead of print.]
10. Ferro JM, Canhão P. Cerebral venous sinus thrombosis: update on diagnosis and management. Curr Cardiol Rep. 2014;16(9):523.
11. Albright KC, Freeman WD, Kruse BT. Cerebral venous thrombosis. J Emerg Med. 2010;38(2):238-239.
12. Lafitte F, Boukobza M, Guichard JP, et al. MRI and MRA for diagnosis and follow-up of cerebral venous thrombosis (CVT). Clin Radiol. 1997;52(9):672-679.
13. Saposnik G, Barinagarrementeria F, Brown RD Jr, et al; American Heart Association Stroke Council and Council on Epidemiology and Prevention. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(4):1158-1192.
14. Coutinho JM, Middeldorp S, Stam J. Advances in the treatment of cerebral venous thrombosis. Curr Treat Options Neurol. 2014;16(7):299.
15. Coutinho JM, Ferro JM, Canhão P, Barinagarrementeria F, Bousser MG, Stam J; ISCVT Investigators. Unfractionated or low-molecular weight heparin for the treatment of cerebral venous thrombosis. Stroke. 2010;41(11):2575-2580.
16. Rosendaal FR. Venous thrombosis: the role of genes, environment, and behavior. Hematology Am Soc Hematol Educ Program. 2005:1-12.
17. Dentali F, Crowther M, Ageno W. Thrombophilic abnormalities, oral contraceptives, and risk of cerebral vein thrombosis: a meta-analysis. Blood.2006;107(7):2766-2773.
18. Salomon O, Steinberg DM, Zivelin A, et al. Single and combined prothrombotic factors in patients with idiopathic venous thromboembolism: prevalence and risk assessment. Arterioscler Thromb Vasc Biol. 1999;19(3):511-518.
19. Emmerich J, Rosendaal FR, Cattaneo M, et al. Combined effect of factor V Leiden and prothrombin 20210A on the risk of venous thromboembolism— pooled analysis of 8 case–control studies including 2310 cases and 3204 controls. Study Group for Pooled-Analysis in Venous Thromboembolism. Thromb Haemost. 2001;86(3):809-816.
20. Lauw MN, Barco S, Coutinho JM, Middeldorp S. Cerebral venous thrombosis and thrombophilia: a systematic review and meta-analysis. Semin Thromb Hemost. 2013;39(8):913-927.
21. Dentali F, Poli D, Scoditti U, et al. Long-term outcomes of patients with cerebral vein thrombosis: a multicenter study. J Thromb Haemost. 2012;10(7):1297-1302.
22. Martinelli I, Bucciarelli P, Passamonti SM, Battaglioli T, Previtali E, Mannucci PM. Long-term evaluation of the risk of recurrence after cerebral sinus-venous thrombosis. Circulation. 2010;121(25):2740-2746.
23. Gosk-Bierska I, Wysokinski W, Brown RD Jr, et al. Cerebral venous sinus thrombosis: incidence of venous thrombosis recurrence and survival. Neurology. 2006;67(5):814-819.
24. Galidie G, Le Gall R, Cordoliani YS, Pharaboz C, Le Marec E, Cosnard G. Thrombosis of the cerebral veins. X-ray computed tomography and MRI imaging. 11 cases [in French]. J Radiol. 1992;73(3):175-190
1. Bousser MG, Ferro JM. Cerebral venous thrombosis: an update. Lancet Neurol. 2007;6(2):162-170.
2. Coutinho JM. Cerebral venous thrombosis. J Thromb Haemost. 2015;13(suppl 1):S238-S244.
3. Coutinho JM, Ferro JM, Canhão P, et al. Cerebral venous and sinus thrombosis in women. Stroke. 2009;40(7):2356-2361.
4. Zuurbier SM, Coutinho JM. Cerebral venous thrombosis. Adv Exp Med Biol. 2017;906:183-193.
5. Einhäupl KM, Villringer A, Meister W, et al. Heparin treatment in sinus venous thrombosis. Lancet. 1991;338(8767):597-600.
6. de Bruijn SF, Stam J. Randomized, placebocontrolled trial of anticoagulant treatment with lowmolecular-weight heparin for cerebral sinus thrombosis. Stroke. 1999;30(3):484-488.
7. Nagaraja D, Haridas T, Taly AB, Veerendrakumar M, SubbuKrishna DK. Puerperal cerebral venous thrombosis: therapeutic benefit of low dose heparin. Neurol India. 1999;47(1):43-46.
8. Coutinho JM, de Bruijn SF, deVeber G, Stam J. Anticoagulation for cerebral venous sinus thrombosis. Stroke. 2012;43(4):e41-e42.
9. Sassi SB, Touati N, Baccouche H, Drissi C, Romdhane NB, Hentati F. Cerebral venous thrombosis. Clin Appl Thromb Hemost. 2016:1076029616665168. [Epub ahead of print.]
10. Ferro JM, Canhão P. Cerebral venous sinus thrombosis: update on diagnosis and management. Curr Cardiol Rep. 2014;16(9):523.
11. Albright KC, Freeman WD, Kruse BT. Cerebral venous thrombosis. J Emerg Med. 2010;38(2):238-239.
12. Lafitte F, Boukobza M, Guichard JP, et al. MRI and MRA for diagnosis and follow-up of cerebral venous thrombosis (CVT). Clin Radiol. 1997;52(9):672-679.
13. Saposnik G, Barinagarrementeria F, Brown RD Jr, et al; American Heart Association Stroke Council and Council on Epidemiology and Prevention. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(4):1158-1192.
14. Coutinho JM, Middeldorp S, Stam J. Advances in the treatment of cerebral venous thrombosis. Curr Treat Options Neurol. 2014;16(7):299.
15. Coutinho JM, Ferro JM, Canhão P, Barinagarrementeria F, Bousser MG, Stam J; ISCVT Investigators. Unfractionated or low-molecular weight heparin for the treatment of cerebral venous thrombosis. Stroke. 2010;41(11):2575-2580.
16. Rosendaal FR. Venous thrombosis: the role of genes, environment, and behavior. Hematology Am Soc Hematol Educ Program. 2005:1-12.
17. Dentali F, Crowther M, Ageno W. Thrombophilic abnormalities, oral contraceptives, and risk of cerebral vein thrombosis: a meta-analysis. Blood.2006;107(7):2766-2773.
18. Salomon O, Steinberg DM, Zivelin A, et al. Single and combined prothrombotic factors in patients with idiopathic venous thromboembolism: prevalence and risk assessment. Arterioscler Thromb Vasc Biol. 1999;19(3):511-518.
19. Emmerich J, Rosendaal FR, Cattaneo M, et al. Combined effect of factor V Leiden and prothrombin 20210A on the risk of venous thromboembolism— pooled analysis of 8 case–control studies including 2310 cases and 3204 controls. Study Group for Pooled-Analysis in Venous Thromboembolism. Thromb Haemost. 2001;86(3):809-816.
20. Lauw MN, Barco S, Coutinho JM, Middeldorp S. Cerebral venous thrombosis and thrombophilia: a systematic review and meta-analysis. Semin Thromb Hemost. 2013;39(8):913-927.
21. Dentali F, Poli D, Scoditti U, et al. Long-term outcomes of patients with cerebral vein thrombosis: a multicenter study. J Thromb Haemost. 2012;10(7):1297-1302.
22. Martinelli I, Bucciarelli P, Passamonti SM, Battaglioli T, Previtali E, Mannucci PM. Long-term evaluation of the risk of recurrence after cerebral sinus-venous thrombosis. Circulation. 2010;121(25):2740-2746.
23. Gosk-Bierska I, Wysokinski W, Brown RD Jr, et al. Cerebral venous sinus thrombosis: incidence of venous thrombosis recurrence and survival. Neurology. 2006;67(5):814-819.
24. Galidie G, Le Gall R, Cordoliani YS, Pharaboz C, Le Marec E, Cosnard G. Thrombosis of the cerebral veins. X-ray computed tomography and MRI imaging. 11 cases [in French]. J Radiol. 1992;73(3):175-190
Idarucizumab reversed dabigatran completely and rapidly in study
One IV 5-g dose of idarucizumab completely, rapidly, and safely reversed the anticoagulant effect of dabigatran, according to final results for 503 patients in the multicenter, prospective, open-label, uncontrolled RE-VERSE AD study.
Uncontrolled bleeding stopped a median of 2.5 hours after 134 patients received idarucizumab. In a separate group of 202 patients, 197 were able to undergo urgent procedures after a median of 1.6 hours, Charles V. Pollack Jr., MD, and his associates reported at the International Society on Thrombosis and Haemostasis congress. The report was simultaneously published in the New England Journal of Medicine.
Idarucizumab was specifically developed to reverse the anticoagulant effect of dabigatran. Many countries have already licensed the humanized monoclonal antibody fragment based on interim results for the first 90 patients enrolled in the Reversal Effects of Idarucizumab on Active Dabigatran (RE-VERSE AD) study (NCT02104947), noted Dr. Pollack, of Thomas Jefferson University, Philadelphia.
The final RE-VERSE AD cohort included 301 patients with uncontrolled gastrointestinal, intracranial, or trauma-related bleeding and 202 patients who needed urgent procedures. Participants from both groups typically were white, in their late 70s (age range, 21-96 years), and receiving 110 mg (75-150 mg) dabigatran twice daily. The primary endpoint was maximum percentage reversal within 4 hours after patients received idarucizumab, based on diluted thrombin time and ecarin clotting time.
The median maximum percentage reversal of dabigatran was 100% (95% confidence interval, 100% to 100%) in more than 98% of patients, and the effect usually lasted 24 hours. Among patients who underwent procedures, intraprocedural hemostasis was considered normal in 93% of cases, mildly abnormal in 5% of cases, and moderately abnormal in 2% of cases, the researchers noted. Seven patients received another dose of idarucizumab after developing recurrent or postoperative bleeding.
A total of 24 patients had an adjudicated thrombotic event within 30 days after receiving idarucizumab. These events included pulmonary embolism, systemic embolism, ischemic stroke, deep vein thrombosis, and myocardial infarction. The fact that many patients did not restart anticoagulation could have contributed to these thrombotic events, the researchers asserted. They noted that idarucizumab had no procoagulant activity in studies of animals and healthy human volunteers.
About 19% of patients in both groups died within 90 days. “Patients enrolled in this study were elderly, had numerous coexisting conditions, and presented with serious index events, such as intracranial hemorrhage, multiple trauma, sepsis, acute abdomen, or open fracture,” the investigators wrote. “Most of the deaths that occurred within 5 days after enrollment appeared to be related to the severity of the index event or to coexisting conditions, such as respiratory failure or multiple organ failure, whereas deaths that occurred after 30 days were more likely to be independent events or related to coexisting conditions.”
Boehringer Ingelheim Pharmaceuticals provided funding. Dr. Pollack disclosed grant support from Boehringer Ingelheim during the course of the study and ties to Daiichi Sankyo, Portola, CSL Behring, Bristol-Myers Squibb/Pfizer, Janssen Pharma, and AstraZeneca. Eighteen coinvestigators also disclosed ties to Boehringer Ingelheim and a number of other pharmaceutical companies. Two coinvestigators had no relevant financial disclosures.
One IV 5-g dose of idarucizumab completely, rapidly, and safely reversed the anticoagulant effect of dabigatran, according to final results for 503 patients in the multicenter, prospective, open-label, uncontrolled RE-VERSE AD study.
Uncontrolled bleeding stopped a median of 2.5 hours after 134 patients received idarucizumab. In a separate group of 202 patients, 197 were able to undergo urgent procedures after a median of 1.6 hours, Charles V. Pollack Jr., MD, and his associates reported at the International Society on Thrombosis and Haemostasis congress. The report was simultaneously published in the New England Journal of Medicine.
Idarucizumab was specifically developed to reverse the anticoagulant effect of dabigatran. Many countries have already licensed the humanized monoclonal antibody fragment based on interim results for the first 90 patients enrolled in the Reversal Effects of Idarucizumab on Active Dabigatran (RE-VERSE AD) study (NCT02104947), noted Dr. Pollack, of Thomas Jefferson University, Philadelphia.
The final RE-VERSE AD cohort included 301 patients with uncontrolled gastrointestinal, intracranial, or trauma-related bleeding and 202 patients who needed urgent procedures. Participants from both groups typically were white, in their late 70s (age range, 21-96 years), and receiving 110 mg (75-150 mg) dabigatran twice daily. The primary endpoint was maximum percentage reversal within 4 hours after patients received idarucizumab, based on diluted thrombin time and ecarin clotting time.
The median maximum percentage reversal of dabigatran was 100% (95% confidence interval, 100% to 100%) in more than 98% of patients, and the effect usually lasted 24 hours. Among patients who underwent procedures, intraprocedural hemostasis was considered normal in 93% of cases, mildly abnormal in 5% of cases, and moderately abnormal in 2% of cases, the researchers noted. Seven patients received another dose of idarucizumab after developing recurrent or postoperative bleeding.
A total of 24 patients had an adjudicated thrombotic event within 30 days after receiving idarucizumab. These events included pulmonary embolism, systemic embolism, ischemic stroke, deep vein thrombosis, and myocardial infarction. The fact that many patients did not restart anticoagulation could have contributed to these thrombotic events, the researchers asserted. They noted that idarucizumab had no procoagulant activity in studies of animals and healthy human volunteers.
About 19% of patients in both groups died within 90 days. “Patients enrolled in this study were elderly, had numerous coexisting conditions, and presented with serious index events, such as intracranial hemorrhage, multiple trauma, sepsis, acute abdomen, or open fracture,” the investigators wrote. “Most of the deaths that occurred within 5 days after enrollment appeared to be related to the severity of the index event or to coexisting conditions, such as respiratory failure or multiple organ failure, whereas deaths that occurred after 30 days were more likely to be independent events or related to coexisting conditions.”
Boehringer Ingelheim Pharmaceuticals provided funding. Dr. Pollack disclosed grant support from Boehringer Ingelheim during the course of the study and ties to Daiichi Sankyo, Portola, CSL Behring, Bristol-Myers Squibb/Pfizer, Janssen Pharma, and AstraZeneca. Eighteen coinvestigators also disclosed ties to Boehringer Ingelheim and a number of other pharmaceutical companies. Two coinvestigators had no relevant financial disclosures.
One IV 5-g dose of idarucizumab completely, rapidly, and safely reversed the anticoagulant effect of dabigatran, according to final results for 503 patients in the multicenter, prospective, open-label, uncontrolled RE-VERSE AD study.
Uncontrolled bleeding stopped a median of 2.5 hours after 134 patients received idarucizumab. In a separate group of 202 patients, 197 were able to undergo urgent procedures after a median of 1.6 hours, Charles V. Pollack Jr., MD, and his associates reported at the International Society on Thrombosis and Haemostasis congress. The report was simultaneously published in the New England Journal of Medicine.
Idarucizumab was specifically developed to reverse the anticoagulant effect of dabigatran. Many countries have already licensed the humanized monoclonal antibody fragment based on interim results for the first 90 patients enrolled in the Reversal Effects of Idarucizumab on Active Dabigatran (RE-VERSE AD) study (NCT02104947), noted Dr. Pollack, of Thomas Jefferson University, Philadelphia.
The final RE-VERSE AD cohort included 301 patients with uncontrolled gastrointestinal, intracranial, or trauma-related bleeding and 202 patients who needed urgent procedures. Participants from both groups typically were white, in their late 70s (age range, 21-96 years), and receiving 110 mg (75-150 mg) dabigatran twice daily. The primary endpoint was maximum percentage reversal within 4 hours after patients received idarucizumab, based on diluted thrombin time and ecarin clotting time.
The median maximum percentage reversal of dabigatran was 100% (95% confidence interval, 100% to 100%) in more than 98% of patients, and the effect usually lasted 24 hours. Among patients who underwent procedures, intraprocedural hemostasis was considered normal in 93% of cases, mildly abnormal in 5% of cases, and moderately abnormal in 2% of cases, the researchers noted. Seven patients received another dose of idarucizumab after developing recurrent or postoperative bleeding.
A total of 24 patients had an adjudicated thrombotic event within 30 days after receiving idarucizumab. These events included pulmonary embolism, systemic embolism, ischemic stroke, deep vein thrombosis, and myocardial infarction. The fact that many patients did not restart anticoagulation could have contributed to these thrombotic events, the researchers asserted. They noted that idarucizumab had no procoagulant activity in studies of animals and healthy human volunteers.
About 19% of patients in both groups died within 90 days. “Patients enrolled in this study were elderly, had numerous coexisting conditions, and presented with serious index events, such as intracranial hemorrhage, multiple trauma, sepsis, acute abdomen, or open fracture,” the investigators wrote. “Most of the deaths that occurred within 5 days after enrollment appeared to be related to the severity of the index event or to coexisting conditions, such as respiratory failure or multiple organ failure, whereas deaths that occurred after 30 days were more likely to be independent events or related to coexisting conditions.”
Boehringer Ingelheim Pharmaceuticals provided funding. Dr. Pollack disclosed grant support from Boehringer Ingelheim during the course of the study and ties to Daiichi Sankyo, Portola, CSL Behring, Bristol-Myers Squibb/Pfizer, Janssen Pharma, and AstraZeneca. Eighteen coinvestigators also disclosed ties to Boehringer Ingelheim and a number of other pharmaceutical companies. Two coinvestigators had no relevant financial disclosures.
FROM 2017 ISTH CONGRESS
Key clinical point:
Major finding: Uncontrolled bleeding stopped a median of 2.5 hours after 134 patients received idarucizumab. In a separate group, 197 patients were able to undergo urgent procedures after a median of 1.6 hours.
Data source: A multicenter, prospective, open-label study of 503 patients (RE-VERSE AD).
Disclosures: Boehringer Ingelheim Pharmaceuticals provided funding. Dr. Pollack disclosed grant support from Boehringer Ingelheim during the course of the study and ties to Daiichi Sankyo, Portola, CSL Behring, BMS/Pfizer, Janssen Pharma, and AstraZeneca. Eighteen coinvestigators disclosed ties to Boehringer Ingelheim and a number of other pharmaceutical companies. Two coinvestigators had no relevant financial disclosures.
VIDEO: Meta-analysis favors anticoagulation for patients with cirrhosis and portal vein thrombosis
Patients with cirrhosis and portal vein thrombosis (PVT) who received anticoagulation therapy had nearly fivefold greater odds of recanalization compared with untreated patients, and were no more likely to experience major or minor bleeding, in a pooled analysis of eight studies published in the August issue of Gastroenterology (doi: 10.1053/j.gastro.2017.04.042).
Rates of any recanalization were 71% in treated patients and 42% in untreated patients (P less than .0001), wrote Lorenzo Loffredo, MD, of Sapienza University, Rome, and his coinvestigators. Rates of complete recanalization were 53% and 33%, respectively (P = .002), rates of spontaneous variceal bleeding were 2% and 12% (P = .04), and bleeding affected 11% of patients in each group. Together, the findings “show that anticoagulants are efficacious and safe for treatment of portal vein thrombosis in cirrhotic patients,” although larger, interventional clinical trials are needed to pinpoint the clinical role of anticoagulation in cirrhotic patients with PVT, the reviewers reported.
Source: American Gastroenterological Association
Bleeding from portal hypertension is a major complication in cirrhosis, but PVT affects about 20% of patients and predicts poor outcomes, they noted. Anticoagulation in this setting can be difficult because patients often have concurrent coagulopathies that are hard to assess with standard techniques, such as PT-INR (international normalized ratio). Although some studies support anticoagulating these patients, data are limited. Therefore, the reviewers searched PubMed, the ISI Web of Science, SCOPUS, and the Cochrane database through Feb. 14, 2017, for trials comparing anticoagulation with no treatment in patients with cirrhosis and PVT.
This search yielded eight trials of 353 patients who received low-molecular-weight heparin, warfarin, or no treatment for about 6 months, with a typical follow-up period of 2 years. The reviewers found no evidence of publication bias or significant heterogeneity among the trials. Six studies evaluated complete recanalization, another set of six studies tracked progression of PVT, a third set of six studies evaluated major or minor bleeding events, and four studies evaluated spontaneous variceal bleeding. Compared with no treatment, anticoagulation was tied to a significantly greater likelihood of complete recanalization (pooled odds ratio, 3.4; 95% confidence interval, 1.5-7.4; P = .002), a significantly lower chance of PVT progressing (9% vs. 33%; pooled odds ratio, 0.14; 95% CI, 0.06-0.31; P less than .0001), no difference in bleeding rates (11% in each pooled group), and a significantly lower risk of spontaneous variceal bleeding (OR, 0.23; 95% CI, 0.06-0.94; P = .04).
“Metaregression analysis showed that duration of anticoagulation did not influence outcomes,” the reviewers wrote. “Low-molecular-weight heparin, but not warfarin, was significantly associated with a complete PVT resolution as compared to untreated patients, while both low-molecular-weight heparin and warfarin were effective in reducing PVT progression.” That finding merits careful interpretation, however, because most studies on warfarin were retrospective and lacked data on the quality of anticoagulation, they added.
“It is a challenge to treat patients with cirrhosis using anticoagulants because of the perception that the coexistent coagulopathy could promote bleeding,” the researchers wrote. Nonetheless, their analysis suggests that anticoagulation has significant benefits and does not increase bleeding risk, regardless of the severity of liver failure, they concluded.
The reviewers reported having no funding sources or conflicts of interest.
Patients with cirrhosis and portal vein thrombosis (PVT) who received anticoagulation therapy had nearly fivefold greater odds of recanalization compared with untreated patients, and were no more likely to experience major or minor bleeding, in a pooled analysis of eight studies published in the August issue of Gastroenterology (doi: 10.1053/j.gastro.2017.04.042).
Rates of any recanalization were 71% in treated patients and 42% in untreated patients (P less than .0001), wrote Lorenzo Loffredo, MD, of Sapienza University, Rome, and his coinvestigators. Rates of complete recanalization were 53% and 33%, respectively (P = .002), rates of spontaneous variceal bleeding were 2% and 12% (P = .04), and bleeding affected 11% of patients in each group. Together, the findings “show that anticoagulants are efficacious and safe for treatment of portal vein thrombosis in cirrhotic patients,” although larger, interventional clinical trials are needed to pinpoint the clinical role of anticoagulation in cirrhotic patients with PVT, the reviewers reported.
Source: American Gastroenterological Association
Bleeding from portal hypertension is a major complication in cirrhosis, but PVT affects about 20% of patients and predicts poor outcomes, they noted. Anticoagulation in this setting can be difficult because patients often have concurrent coagulopathies that are hard to assess with standard techniques, such as PT-INR (international normalized ratio). Although some studies support anticoagulating these patients, data are limited. Therefore, the reviewers searched PubMed, the ISI Web of Science, SCOPUS, and the Cochrane database through Feb. 14, 2017, for trials comparing anticoagulation with no treatment in patients with cirrhosis and PVT.
This search yielded eight trials of 353 patients who received low-molecular-weight heparin, warfarin, or no treatment for about 6 months, with a typical follow-up period of 2 years. The reviewers found no evidence of publication bias or significant heterogeneity among the trials. Six studies evaluated complete recanalization, another set of six studies tracked progression of PVT, a third set of six studies evaluated major or minor bleeding events, and four studies evaluated spontaneous variceal bleeding. Compared with no treatment, anticoagulation was tied to a significantly greater likelihood of complete recanalization (pooled odds ratio, 3.4; 95% confidence interval, 1.5-7.4; P = .002), a significantly lower chance of PVT progressing (9% vs. 33%; pooled odds ratio, 0.14; 95% CI, 0.06-0.31; P less than .0001), no difference in bleeding rates (11% in each pooled group), and a significantly lower risk of spontaneous variceal bleeding (OR, 0.23; 95% CI, 0.06-0.94; P = .04).
“Metaregression analysis showed that duration of anticoagulation did not influence outcomes,” the reviewers wrote. “Low-molecular-weight heparin, but not warfarin, was significantly associated with a complete PVT resolution as compared to untreated patients, while both low-molecular-weight heparin and warfarin were effective in reducing PVT progression.” That finding merits careful interpretation, however, because most studies on warfarin were retrospective and lacked data on the quality of anticoagulation, they added.
“It is a challenge to treat patients with cirrhosis using anticoagulants because of the perception that the coexistent coagulopathy could promote bleeding,” the researchers wrote. Nonetheless, their analysis suggests that anticoagulation has significant benefits and does not increase bleeding risk, regardless of the severity of liver failure, they concluded.
The reviewers reported having no funding sources or conflicts of interest.
Patients with cirrhosis and portal vein thrombosis (PVT) who received anticoagulation therapy had nearly fivefold greater odds of recanalization compared with untreated patients, and were no more likely to experience major or minor bleeding, in a pooled analysis of eight studies published in the August issue of Gastroenterology (doi: 10.1053/j.gastro.2017.04.042).
Rates of any recanalization were 71% in treated patients and 42% in untreated patients (P less than .0001), wrote Lorenzo Loffredo, MD, of Sapienza University, Rome, and his coinvestigators. Rates of complete recanalization were 53% and 33%, respectively (P = .002), rates of spontaneous variceal bleeding were 2% and 12% (P = .04), and bleeding affected 11% of patients in each group. Together, the findings “show that anticoagulants are efficacious and safe for treatment of portal vein thrombosis in cirrhotic patients,” although larger, interventional clinical trials are needed to pinpoint the clinical role of anticoagulation in cirrhotic patients with PVT, the reviewers reported.
Source: American Gastroenterological Association
Bleeding from portal hypertension is a major complication in cirrhosis, but PVT affects about 20% of patients and predicts poor outcomes, they noted. Anticoagulation in this setting can be difficult because patients often have concurrent coagulopathies that are hard to assess with standard techniques, such as PT-INR (international normalized ratio). Although some studies support anticoagulating these patients, data are limited. Therefore, the reviewers searched PubMed, the ISI Web of Science, SCOPUS, and the Cochrane database through Feb. 14, 2017, for trials comparing anticoagulation with no treatment in patients with cirrhosis and PVT.
This search yielded eight trials of 353 patients who received low-molecular-weight heparin, warfarin, or no treatment for about 6 months, with a typical follow-up period of 2 years. The reviewers found no evidence of publication bias or significant heterogeneity among the trials. Six studies evaluated complete recanalization, another set of six studies tracked progression of PVT, a third set of six studies evaluated major or minor bleeding events, and four studies evaluated spontaneous variceal bleeding. Compared with no treatment, anticoagulation was tied to a significantly greater likelihood of complete recanalization (pooled odds ratio, 3.4; 95% confidence interval, 1.5-7.4; P = .002), a significantly lower chance of PVT progressing (9% vs. 33%; pooled odds ratio, 0.14; 95% CI, 0.06-0.31; P less than .0001), no difference in bleeding rates (11% in each pooled group), and a significantly lower risk of spontaneous variceal bleeding (OR, 0.23; 95% CI, 0.06-0.94; P = .04).
“Metaregression analysis showed that duration of anticoagulation did not influence outcomes,” the reviewers wrote. “Low-molecular-weight heparin, but not warfarin, was significantly associated with a complete PVT resolution as compared to untreated patients, while both low-molecular-weight heparin and warfarin were effective in reducing PVT progression.” That finding merits careful interpretation, however, because most studies on warfarin were retrospective and lacked data on the quality of anticoagulation, they added.
“It is a challenge to treat patients with cirrhosis using anticoagulants because of the perception that the coexistent coagulopathy could promote bleeding,” the researchers wrote. Nonetheless, their analysis suggests that anticoagulation has significant benefits and does not increase bleeding risk, regardless of the severity of liver failure, they concluded.
The reviewers reported having no funding sources or conflicts of interest.
FROM GASTROENTEROLOGY
Key clinical point: Anticoagulation produced favorable outcomes with no increase in bleeding risk in patients with cirrhosis and portal vein thrombosis.
Major finding: Rates of any recanalization were 71% in treated patients and 42% in untreated patients (P less than .0001); rates of complete recanalization were 53% and 33%, respectively (P = .002), rates of spontaneous variceal bleeding were 2% and 12% (P = .04), and bleeding affected 11% of patients in each group.
Data source: A systematic review and meta-analysis of eight studies of 353 patients with cirrhosis and portal vein thrombosis.
Disclosures: The reviewers reported having no funding sources or conflicts of interest.
FDA approves betrixaban for VTE prophylaxis
Betrixaban, a factor Xa inhibitor, has been approved for the prophylaxis of venous thromboembolism (VTE) in at-risk adult patients hospitalized with an acute illness, according to an announcement from the Food and Drug Administration.
Approval was based on results from a randomized, double-blind clinical trial in which over 7,000 hospitalized patients at risk for VTE received either extended-duration betrixaban (35-42 days) or short duration enoxaparin (6-14 days), a low molecular weight heparin administered subcutaneously. The rate of deep vein thrombosis, nonfatal pulmonary embolism, or VTE-related death was 4.4% among patients receiving betrixaban and 6% among patients receiving enoxaparin (relative risk, 0.75; 95% confidence interval: 0.61, 0.91).
The recommended dosage for betrixaban is 80 mg per day for 35-42 days at the same time every day with food, after a dose of 160 mg on the first day of treatment.
Betrixaban will be marketed as Bevyxxa by Portola.
Find the full FDA announcement and prescribing information on the FDA website.
Betrixaban, a factor Xa inhibitor, has been approved for the prophylaxis of venous thromboembolism (VTE) in at-risk adult patients hospitalized with an acute illness, according to an announcement from the Food and Drug Administration.
Approval was based on results from a randomized, double-blind clinical trial in which over 7,000 hospitalized patients at risk for VTE received either extended-duration betrixaban (35-42 days) or short duration enoxaparin (6-14 days), a low molecular weight heparin administered subcutaneously. The rate of deep vein thrombosis, nonfatal pulmonary embolism, or VTE-related death was 4.4% among patients receiving betrixaban and 6% among patients receiving enoxaparin (relative risk, 0.75; 95% confidence interval: 0.61, 0.91).
The recommended dosage for betrixaban is 80 mg per day for 35-42 days at the same time every day with food, after a dose of 160 mg on the first day of treatment.
Betrixaban will be marketed as Bevyxxa by Portola.
Find the full FDA announcement and prescribing information on the FDA website.
Betrixaban, a factor Xa inhibitor, has been approved for the prophylaxis of venous thromboembolism (VTE) in at-risk adult patients hospitalized with an acute illness, according to an announcement from the Food and Drug Administration.
Approval was based on results from a randomized, double-blind clinical trial in which over 7,000 hospitalized patients at risk for VTE received either extended-duration betrixaban (35-42 days) or short duration enoxaparin (6-14 days), a low molecular weight heparin administered subcutaneously. The rate of deep vein thrombosis, nonfatal pulmonary embolism, or VTE-related death was 4.4% among patients receiving betrixaban and 6% among patients receiving enoxaparin (relative risk, 0.75; 95% confidence interval: 0.61, 0.91).
The recommended dosage for betrixaban is 80 mg per day for 35-42 days at the same time every day with food, after a dose of 160 mg on the first day of treatment.
Betrixaban will be marketed as Bevyxxa by Portola.
Find the full FDA announcement and prescribing information on the FDA website.
Aspirin triples major bleeding risk after age 75 years
Older adults who take aspirin daily are at greater risk for serious bleeding than previously thought, based on data from roughly 3,000 patients.
“The risk of upper gastrointestinal bleeding on antiplatelet treatment increases with age, but it is uncertain whether older age alone is a sufficient indicator of high risk to justify routine coprescription of PPIs [proton pump inhibitors],” wrote Linxin Li, DPhil, of the University of Oxford (England) and her colleagues.
To assess the rate of bleeding among older adults on long-term aspirin therapy, Dr. Li and her colleagues reviewed data from the Oxford Vascular Study, a prospective population-based study of 3,166 patients. Of those, 1,584 were younger than 75 years, with an average age of 61 years, and 1,582 were at least 75 years old, with average age of 83 years. Patients were followed at 30 days, 6 months, and 1, 5, and 10 years to determine bleeding, recurrent ischemic events, and disability (Lancet. 2017. doi: 10.1016/S0140-6736[17]30770-5).
In addition, more than twice the major upper GI bleeds were disabling or fatal in adults aged 75 years and older than in the younger patients (62% vs. 25%).
Only a third of the patients in the study were taking proton pump inhibitors (PPIs), partly because current clinical guidelines don’t specifically recommend their use and partly in the absence of an accepted definition of which patients are at high risk for upper GI bleeding, the researchers said. They estimated that the number needed to treat with PPIs to prevent a major GI bleed after 5 years decreased with age: “80 for patients younger than 65 years, 75 for patients aged 65-74 years, 23 for patients aged 75-84 years, and 21 for patients aged 85 years or older.” In addition, the number needed to treat with PPIs to prevent a disabling or fatal upper GI bleed after 5 years was 338 for patients younger than 65 years but dropped to 25 for patients aged 85 years and older.
The findings were limited by the observational nature of the study and inability to show that increased risk of bleeding was caused by aspirin alone, the researchers said. However, based on the data, “age 75 years would be an appropriate threshold to start a PPI both in patients newly initiated on antiplatelet drugs and in patients on established treatment,” they wrote.
The study data were taken from the Oxford Vascular Study, which was funded by the National Institute of Health Research and several other research institutions. Corresponding author Peter Rothwell, MD, disclosed financial relationships with Bayer.
In patients with stroke with a cardiac source of embolism who qualify for oral anticoagulation, we obsess about the association between benefit and bleeding risk. Specific risk scores were developed to assess the bleeding risk for patients with atrial fibrillation who qualified for anticoagulation. However, similar risk scores are not applied for patients who undergo long-term prevention with antiplatelet therapy.
On the basis of this study’s results, the benefit-risk association in long-term antiplatelet therapy should be evaluated every 3-5 years in patients older than 75 years, and PPIs should be used in patients on antiplatelet therapy who are at least 75 years old or in patients with a history of gastrointestinal bleeds.
Hans-Christoph Diener, MD, of the department of neurology at the University Duisburg-Essen in Essen, Germany, made these remarks in an accompanying editorial (Lancet. 2017 Jun 13. doi: 10.1016/S0140-6736[17]31507-6). He disclosed relationships with multiple companies, including AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Merck, and Novartis.
In patients with stroke with a cardiac source of embolism who qualify for oral anticoagulation, we obsess about the association between benefit and bleeding risk. Specific risk scores were developed to assess the bleeding risk for patients with atrial fibrillation who qualified for anticoagulation. However, similar risk scores are not applied for patients who undergo long-term prevention with antiplatelet therapy.
On the basis of this study’s results, the benefit-risk association in long-term antiplatelet therapy should be evaluated every 3-5 years in patients older than 75 years, and PPIs should be used in patients on antiplatelet therapy who are at least 75 years old or in patients with a history of gastrointestinal bleeds.
Hans-Christoph Diener, MD, of the department of neurology at the University Duisburg-Essen in Essen, Germany, made these remarks in an accompanying editorial (Lancet. 2017 Jun 13. doi: 10.1016/S0140-6736[17]31507-6). He disclosed relationships with multiple companies, including AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Merck, and Novartis.
In patients with stroke with a cardiac source of embolism who qualify for oral anticoagulation, we obsess about the association between benefit and bleeding risk. Specific risk scores were developed to assess the bleeding risk for patients with atrial fibrillation who qualified for anticoagulation. However, similar risk scores are not applied for patients who undergo long-term prevention with antiplatelet therapy.
On the basis of this study’s results, the benefit-risk association in long-term antiplatelet therapy should be evaluated every 3-5 years in patients older than 75 years, and PPIs should be used in patients on antiplatelet therapy who are at least 75 years old or in patients with a history of gastrointestinal bleeds.
Hans-Christoph Diener, MD, of the department of neurology at the University Duisburg-Essen in Essen, Germany, made these remarks in an accompanying editorial (Lancet. 2017 Jun 13. doi: 10.1016/S0140-6736[17]31507-6). He disclosed relationships with multiple companies, including AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Merck, and Novartis.
Older adults who take aspirin daily are at greater risk for serious bleeding than previously thought, based on data from roughly 3,000 patients.
“The risk of upper gastrointestinal bleeding on antiplatelet treatment increases with age, but it is uncertain whether older age alone is a sufficient indicator of high risk to justify routine coprescription of PPIs [proton pump inhibitors],” wrote Linxin Li, DPhil, of the University of Oxford (England) and her colleagues.
To assess the rate of bleeding among older adults on long-term aspirin therapy, Dr. Li and her colleagues reviewed data from the Oxford Vascular Study, a prospective population-based study of 3,166 patients. Of those, 1,584 were younger than 75 years, with an average age of 61 years, and 1,582 were at least 75 years old, with average age of 83 years. Patients were followed at 30 days, 6 months, and 1, 5, and 10 years to determine bleeding, recurrent ischemic events, and disability (Lancet. 2017. doi: 10.1016/S0140-6736[17]30770-5).
In addition, more than twice the major upper GI bleeds were disabling or fatal in adults aged 75 years and older than in the younger patients (62% vs. 25%).
Only a third of the patients in the study were taking proton pump inhibitors (PPIs), partly because current clinical guidelines don’t specifically recommend their use and partly in the absence of an accepted definition of which patients are at high risk for upper GI bleeding, the researchers said. They estimated that the number needed to treat with PPIs to prevent a major GI bleed after 5 years decreased with age: “80 for patients younger than 65 years, 75 for patients aged 65-74 years, 23 for patients aged 75-84 years, and 21 for patients aged 85 years or older.” In addition, the number needed to treat with PPIs to prevent a disabling or fatal upper GI bleed after 5 years was 338 for patients younger than 65 years but dropped to 25 for patients aged 85 years and older.
The findings were limited by the observational nature of the study and inability to show that increased risk of bleeding was caused by aspirin alone, the researchers said. However, based on the data, “age 75 years would be an appropriate threshold to start a PPI both in patients newly initiated on antiplatelet drugs and in patients on established treatment,” they wrote.
The study data were taken from the Oxford Vascular Study, which was funded by the National Institute of Health Research and several other research institutions. Corresponding author Peter Rothwell, MD, disclosed financial relationships with Bayer.
Older adults who take aspirin daily are at greater risk for serious bleeding than previously thought, based on data from roughly 3,000 patients.
“The risk of upper gastrointestinal bleeding on antiplatelet treatment increases with age, but it is uncertain whether older age alone is a sufficient indicator of high risk to justify routine coprescription of PPIs [proton pump inhibitors],” wrote Linxin Li, DPhil, of the University of Oxford (England) and her colleagues.
To assess the rate of bleeding among older adults on long-term aspirin therapy, Dr. Li and her colleagues reviewed data from the Oxford Vascular Study, a prospective population-based study of 3,166 patients. Of those, 1,584 were younger than 75 years, with an average age of 61 years, and 1,582 were at least 75 years old, with average age of 83 years. Patients were followed at 30 days, 6 months, and 1, 5, and 10 years to determine bleeding, recurrent ischemic events, and disability (Lancet. 2017. doi: 10.1016/S0140-6736[17]30770-5).
In addition, more than twice the major upper GI bleeds were disabling or fatal in adults aged 75 years and older than in the younger patients (62% vs. 25%).
Only a third of the patients in the study were taking proton pump inhibitors (PPIs), partly because current clinical guidelines don’t specifically recommend their use and partly in the absence of an accepted definition of which patients are at high risk for upper GI bleeding, the researchers said. They estimated that the number needed to treat with PPIs to prevent a major GI bleed after 5 years decreased with age: “80 for patients younger than 65 years, 75 for patients aged 65-74 years, 23 for patients aged 75-84 years, and 21 for patients aged 85 years or older.” In addition, the number needed to treat with PPIs to prevent a disabling or fatal upper GI bleed after 5 years was 338 for patients younger than 65 years but dropped to 25 for patients aged 85 years and older.
The findings were limited by the observational nature of the study and inability to show that increased risk of bleeding was caused by aspirin alone, the researchers said. However, based on the data, “age 75 years would be an appropriate threshold to start a PPI both in patients newly initiated on antiplatelet drugs and in patients on established treatment,” they wrote.
The study data were taken from the Oxford Vascular Study, which was funded by the National Institute of Health Research and several other research institutions. Corresponding author Peter Rothwell, MD, disclosed financial relationships with Bayer.
FROM THE LANCET
Key clinical point:
Major finding: The annual rate of life-threatening or fatal bleeding episodes was less than 0.5% for patients younger than 65 years but rose to 1.5% in those aged 75-84 years and 2.5% in those aged 85 years and older.
Data source: A prospective, population-based cohort study of 3,166 adults who had one transient ischemic attack, ischemic stroke, or MI and who were treated with antiplatelet drugs.
Disclosures: The study data were taken from the Oxford Vascular Study, which was funded by the Wellcome Trust, Wolfson Foundation, British Heart Foundation, Dunhill Medical Trust, the National Institute of Health Research (NIHR), and the NIHR Oxford Biomedical Research Centre. Corresponding author Peter Rothwell, MD, disclosed financial relationships with Bayer.
Low-dose aspirin bests dual-antiplatelet therapy in TAVR
PARIS – Single-antiplatelet therapy with low-dose aspirin following transcatheter aortic valve replacement (TAVR) reduced the occurrence of major adverse events, compared with guideline-recommended dual-antiplatelet therapy (DAPT), in the randomized ARTE trial.
The TAVR guideline recommendation for DAPT with low-dose aspirin plus clopidogrel is not based on evidence. It relies on expert opinion. ARTE (Aspirin Versus Aspirin + Clopidogrel Following TAVR) is the first sizable randomized trial to address the safety and efficacy of aspirin alone versus DAPT in the setting of TAVR, Josep Rodés-Cabau, MD, noted in presenting the ARTE results at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
ARTE was a multicenter, prospective, international open-label study of 222 TAVR patients who were randomized to 3 months of single-antiplatelet therapy (SAPT) with aspirin at 80-100 mg/day or to DAPT with aspirin at 80-100 mg/day plus clopidogrel at 75 mg/day after a single 300-mg loading dose. Participants had a mean Society of Thoracic Surgery Predicted Risk of Mortality score of 6.3%. The vast majority of participants received the balloon-expandable Edwards Lifesciences Sapien XT valve. The remainder got the Sapien 3 valve.
The primary outcome was the 3-month composite of death, MI, major or life-threatening bleeding, or stroke or transient ischemic attack. It occurred in 15.3% of the DAPT group and 7.2% on SAPT, a difference that didn’t reach statistical significance (P = .065) because of small patient numbers.

All subjects were on a proton pump inhibitor. The type, timing, and severity of bleeding events differed between the two study arms. All 4 bleeding events in the SAPT group were vascular in nature, while 5 of the 12 in the DAPT group were gastrointestinal. All the bleeding events in the SAPT group occurred within 72 hours after TAVR, whereas 5 of 12 in the DAPT recipients occurred later. Only one patient on SAPT experienced life-threatening bleeding, compared with seven DAPT patients who did.
“There were two prior smaller studies before ours,” according to Dr. Rodés-Cabau of Laval University in Quebec City. “One showed no differences, and an Italian one showed a tendency toward more bleeding with DAPT. So, I think there has been no sign to date that adding clopidogrel protects this group of patients from anything.”
Discussant Luis Nombela-Franco, MD, an interventional cardiologist at San Carlos Hospital in Madrid, pronounced the ARTE trial guideline-changing despite its limitations.
ARTE was supported by grants from Edwards Lifesciences and the Quebec Heart and Lung Institute.
Simultaneous with Dr. Rodés-Cabau’s presentation in Paris, the ARTE trial was published online (JACC Cardiovasc Interv. 2017 May 11. pii: S1936-8798[17]30812-9).
PARIS – Single-antiplatelet therapy with low-dose aspirin following transcatheter aortic valve replacement (TAVR) reduced the occurrence of major adverse events, compared with guideline-recommended dual-antiplatelet therapy (DAPT), in the randomized ARTE trial.
The TAVR guideline recommendation for DAPT with low-dose aspirin plus clopidogrel is not based on evidence. It relies on expert opinion. ARTE (Aspirin Versus Aspirin + Clopidogrel Following TAVR) is the first sizable randomized trial to address the safety and efficacy of aspirin alone versus DAPT in the setting of TAVR, Josep Rodés-Cabau, MD, noted in presenting the ARTE results at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
ARTE was a multicenter, prospective, international open-label study of 222 TAVR patients who were randomized to 3 months of single-antiplatelet therapy (SAPT) with aspirin at 80-100 mg/day or to DAPT with aspirin at 80-100 mg/day plus clopidogrel at 75 mg/day after a single 300-mg loading dose. Participants had a mean Society of Thoracic Surgery Predicted Risk of Mortality score of 6.3%. The vast majority of participants received the balloon-expandable Edwards Lifesciences Sapien XT valve. The remainder got the Sapien 3 valve.
The primary outcome was the 3-month composite of death, MI, major or life-threatening bleeding, or stroke or transient ischemic attack. It occurred in 15.3% of the DAPT group and 7.2% on SAPT, a difference that didn’t reach statistical significance (P = .065) because of small patient numbers.

All subjects were on a proton pump inhibitor. The type, timing, and severity of bleeding events differed between the two study arms. All 4 bleeding events in the SAPT group were vascular in nature, while 5 of the 12 in the DAPT group were gastrointestinal. All the bleeding events in the SAPT group occurred within 72 hours after TAVR, whereas 5 of 12 in the DAPT recipients occurred later. Only one patient on SAPT experienced life-threatening bleeding, compared with seven DAPT patients who did.
“There were two prior smaller studies before ours,” according to Dr. Rodés-Cabau of Laval University in Quebec City. “One showed no differences, and an Italian one showed a tendency toward more bleeding with DAPT. So, I think there has been no sign to date that adding clopidogrel protects this group of patients from anything.”
Discussant Luis Nombela-Franco, MD, an interventional cardiologist at San Carlos Hospital in Madrid, pronounced the ARTE trial guideline-changing despite its limitations.
ARTE was supported by grants from Edwards Lifesciences and the Quebec Heart and Lung Institute.
Simultaneous with Dr. Rodés-Cabau’s presentation in Paris, the ARTE trial was published online (JACC Cardiovasc Interv. 2017 May 11. pii: S1936-8798[17]30812-9).
PARIS – Single-antiplatelet therapy with low-dose aspirin following transcatheter aortic valve replacement (TAVR) reduced the occurrence of major adverse events, compared with guideline-recommended dual-antiplatelet therapy (DAPT), in the randomized ARTE trial.
The TAVR guideline recommendation for DAPT with low-dose aspirin plus clopidogrel is not based on evidence. It relies on expert opinion. ARTE (Aspirin Versus Aspirin + Clopidogrel Following TAVR) is the first sizable randomized trial to address the safety and efficacy of aspirin alone versus DAPT in the setting of TAVR, Josep Rodés-Cabau, MD, noted in presenting the ARTE results at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
ARTE was a multicenter, prospective, international open-label study of 222 TAVR patients who were randomized to 3 months of single-antiplatelet therapy (SAPT) with aspirin at 80-100 mg/day or to DAPT with aspirin at 80-100 mg/day plus clopidogrel at 75 mg/day after a single 300-mg loading dose. Participants had a mean Society of Thoracic Surgery Predicted Risk of Mortality score of 6.3%. The vast majority of participants received the balloon-expandable Edwards Lifesciences Sapien XT valve. The remainder got the Sapien 3 valve.
The primary outcome was the 3-month composite of death, MI, major or life-threatening bleeding, or stroke or transient ischemic attack. It occurred in 15.3% of the DAPT group and 7.2% on SAPT, a difference that didn’t reach statistical significance (P = .065) because of small patient numbers.

All subjects were on a proton pump inhibitor. The type, timing, and severity of bleeding events differed between the two study arms. All 4 bleeding events in the SAPT group were vascular in nature, while 5 of the 12 in the DAPT group were gastrointestinal. All the bleeding events in the SAPT group occurred within 72 hours after TAVR, whereas 5 of 12 in the DAPT recipients occurred later. Only one patient on SAPT experienced life-threatening bleeding, compared with seven DAPT patients who did.
“There were two prior smaller studies before ours,” according to Dr. Rodés-Cabau of Laval University in Quebec City. “One showed no differences, and an Italian one showed a tendency toward more bleeding with DAPT. So, I think there has been no sign to date that adding clopidogrel protects this group of patients from anything.”
Discussant Luis Nombela-Franco, MD, an interventional cardiologist at San Carlos Hospital in Madrid, pronounced the ARTE trial guideline-changing despite its limitations.
ARTE was supported by grants from Edwards Lifesciences and the Quebec Heart and Lung Institute.
Simultaneous with Dr. Rodés-Cabau’s presentation in Paris, the ARTE trial was published online (JACC Cardiovasc Interv. 2017 May 11. pii: S1936-8798[17]30812-9).
AT EUROPCR
Key clinical point:
Major finding: The 3-month composite of death, MI, major or life-threatening bleeding, or stroke or transient ischemic attack occurred in 15.3% of TAVR patients randomized to DAPT with low-dose aspirin plus clopidogrel, compared with 7.2% on aspirin only.
Data source: A randomized, multicenter, international, prospective open-label trial in 222 TAVR patients.
Disclosures: The presenter reported receiving research grants from Edwards Lifesciences and the Quebec Heart and Lung Institute, which supported the ARTE trial.
Game over: VTE is a risk in obese, sedentary teens
MONTREAL – It’s well known that airplane passengers, condemned to sit for endless hours in the claustrophobic cabins of the unfriendly skies, are at increased risk for venous thromboembolic events (VTEs). Less well documented, however, is the VTE risk encountered by overweight or obese teens who while their hours away playing video games.
“This is becoming a sedentary-type risk factor,” said Mira A. Kohorst, MD, from the division of pediatric hematology-oncology at the Mayo Clinic in Rochester, Minn.
Dr. Kohorst and her colleagues reported on a small but troubling trend of VTE episodes that they observed in teen boys over the last few years. They refer to obesity, sedentary lifestyle, and gaming as “the new thrombophilia cocktail in adolescent males.”
The reported incidence of pediatric VTE ranges from 0.7 to 4.9 per 100,000 person years, considerably lower than the 1 in 1000 estimated incidences reported in adults. But, thanks to the growing incidence of obesity in children, which more than doubled from 1980 to 2012 and quadrupled in teens age 12-19 years from 5% to 21%, youngsters appear to be catching up in the VTE department, the investigators reported.
“Given the direct mortality rate of 2% [that is] associated with VTE and risk for postthrombotic syndrome of 26%, it is important to understand underlying modifiable risk factors,” they wrote.
To do this, they retrospectively reviewed records of children who presented with VTE in their center.
All play, no exercise
The authors described three cases, including that of an 18-year old boy with a body mass index (BMI) of 37 kg/m2, putting him squarely in the obese category. This lad, who spent 12 or more hours a day playing video games and was sedentary at other times as well, presented with bilateral pulmonary emboli and an associated right lower lobe infarction. Testing for thrombophilia showed that he was heterozygous for factor V Leiden but did not have other coagulation abnormalities. He was started on enoxaparin (Lovenox) and then transitioned to apixaban (Eliquis) for a total of 6 months of thromboprophylaxis. He was counseled about modifying his lifestyle and did not have a recurrence after 14 months of follow-up.
A similarly sedentary 17-year old male with an even higher BMI (39 kg/m2) presented with bilateral basilar pulmonary emboli and infarctions in association with a left femoral deep vein thrombosis. This patients also had factor V Leiden heterozygosity and the May-Thurner (iliac vein compression) syndrome. He was treated for a total of 6 months with warfarin followed by rivaroxaban (Xarelto) and was counseled about lifestyle changes but was unable to lose weight. Eight months after completing therapy, he had a second extensive deep vein thrombosis, this time in his right leg, and was restarted on rivaroxaban.
The third patient, a morbidly obese (BMI 56 kg/m2) 13-year-old boy, presented with left lower lobe pulmonary embolism following 3 weeks of immobility caused by the Guillain-Barré syndrome. As in the other cases, he confessed to a sedentary lifestyle and a predilection for gaming. His father had previously developed a line-associated thrombus. The family declined thrombophilia testing. The patient received 3 months of enoxaparin. He has not been followed since discontinuing therapy.
Move it, kid!
The risk of VTE in adolescent boys, especially obese and extreme gamers who spend most of their waking hours in a chair staring at a screen, is similar to that for adolescent girls who use oral contraceptives, Dr. Kohorst and her colleagues said.
“Many case reports link prolonged ‘gaming’ to thrombosis and fatal pulmonary emboli. Additionally, prolonged television viewing has become a documented risk factor for mortality from pulmonary emboli,” the investigators wrote.
They recommend that clinicians ask adolescents about their gaming and TV-watching habits and encourage them to become more active to lower their risk for VTE.
The study was internally supported. Dr. Kohorst and colleagues reported no relevant disclosures.
MONTREAL – It’s well known that airplane passengers, condemned to sit for endless hours in the claustrophobic cabins of the unfriendly skies, are at increased risk for venous thromboembolic events (VTEs). Less well documented, however, is the VTE risk encountered by overweight or obese teens who while their hours away playing video games.
“This is becoming a sedentary-type risk factor,” said Mira A. Kohorst, MD, from the division of pediatric hematology-oncology at the Mayo Clinic in Rochester, Minn.
Dr. Kohorst and her colleagues reported on a small but troubling trend of VTE episodes that they observed in teen boys over the last few years. They refer to obesity, sedentary lifestyle, and gaming as “the new thrombophilia cocktail in adolescent males.”
The reported incidence of pediatric VTE ranges from 0.7 to 4.9 per 100,000 person years, considerably lower than the 1 in 1000 estimated incidences reported in adults. But, thanks to the growing incidence of obesity in children, which more than doubled from 1980 to 2012 and quadrupled in teens age 12-19 years from 5% to 21%, youngsters appear to be catching up in the VTE department, the investigators reported.
“Given the direct mortality rate of 2% [that is] associated with VTE and risk for postthrombotic syndrome of 26%, it is important to understand underlying modifiable risk factors,” they wrote.
To do this, they retrospectively reviewed records of children who presented with VTE in their center.
All play, no exercise
The authors described three cases, including that of an 18-year old boy with a body mass index (BMI) of 37 kg/m2, putting him squarely in the obese category. This lad, who spent 12 or more hours a day playing video games and was sedentary at other times as well, presented with bilateral pulmonary emboli and an associated right lower lobe infarction. Testing for thrombophilia showed that he was heterozygous for factor V Leiden but did not have other coagulation abnormalities. He was started on enoxaparin (Lovenox) and then transitioned to apixaban (Eliquis) for a total of 6 months of thromboprophylaxis. He was counseled about modifying his lifestyle and did not have a recurrence after 14 months of follow-up.
A similarly sedentary 17-year old male with an even higher BMI (39 kg/m2) presented with bilateral basilar pulmonary emboli and infarctions in association with a left femoral deep vein thrombosis. This patients also had factor V Leiden heterozygosity and the May-Thurner (iliac vein compression) syndrome. He was treated for a total of 6 months with warfarin followed by rivaroxaban (Xarelto) and was counseled about lifestyle changes but was unable to lose weight. Eight months after completing therapy, he had a second extensive deep vein thrombosis, this time in his right leg, and was restarted on rivaroxaban.
The third patient, a morbidly obese (BMI 56 kg/m2) 13-year-old boy, presented with left lower lobe pulmonary embolism following 3 weeks of immobility caused by the Guillain-Barré syndrome. As in the other cases, he confessed to a sedentary lifestyle and a predilection for gaming. His father had previously developed a line-associated thrombus. The family declined thrombophilia testing. The patient received 3 months of enoxaparin. He has not been followed since discontinuing therapy.
Move it, kid!
The risk of VTE in adolescent boys, especially obese and extreme gamers who spend most of their waking hours in a chair staring at a screen, is similar to that for adolescent girls who use oral contraceptives, Dr. Kohorst and her colleagues said.
“Many case reports link prolonged ‘gaming’ to thrombosis and fatal pulmonary emboli. Additionally, prolonged television viewing has become a documented risk factor for mortality from pulmonary emboli,” the investigators wrote.
They recommend that clinicians ask adolescents about their gaming and TV-watching habits and encourage them to become more active to lower their risk for VTE.
The study was internally supported. Dr. Kohorst and colleagues reported no relevant disclosures.
MONTREAL – It’s well known that airplane passengers, condemned to sit for endless hours in the claustrophobic cabins of the unfriendly skies, are at increased risk for venous thromboembolic events (VTEs). Less well documented, however, is the VTE risk encountered by overweight or obese teens who while their hours away playing video games.
“This is becoming a sedentary-type risk factor,” said Mira A. Kohorst, MD, from the division of pediatric hematology-oncology at the Mayo Clinic in Rochester, Minn.
Dr. Kohorst and her colleagues reported on a small but troubling trend of VTE episodes that they observed in teen boys over the last few years. They refer to obesity, sedentary lifestyle, and gaming as “the new thrombophilia cocktail in adolescent males.”
The reported incidence of pediatric VTE ranges from 0.7 to 4.9 per 100,000 person years, considerably lower than the 1 in 1000 estimated incidences reported in adults. But, thanks to the growing incidence of obesity in children, which more than doubled from 1980 to 2012 and quadrupled in teens age 12-19 years from 5% to 21%, youngsters appear to be catching up in the VTE department, the investigators reported.
“Given the direct mortality rate of 2% [that is] associated with VTE and risk for postthrombotic syndrome of 26%, it is important to understand underlying modifiable risk factors,” they wrote.
To do this, they retrospectively reviewed records of children who presented with VTE in their center.
All play, no exercise
The authors described three cases, including that of an 18-year old boy with a body mass index (BMI) of 37 kg/m2, putting him squarely in the obese category. This lad, who spent 12 or more hours a day playing video games and was sedentary at other times as well, presented with bilateral pulmonary emboli and an associated right lower lobe infarction. Testing for thrombophilia showed that he was heterozygous for factor V Leiden but did not have other coagulation abnormalities. He was started on enoxaparin (Lovenox) and then transitioned to apixaban (Eliquis) for a total of 6 months of thromboprophylaxis. He was counseled about modifying his lifestyle and did not have a recurrence after 14 months of follow-up.
A similarly sedentary 17-year old male with an even higher BMI (39 kg/m2) presented with bilateral basilar pulmonary emboli and infarctions in association with a left femoral deep vein thrombosis. This patients also had factor V Leiden heterozygosity and the May-Thurner (iliac vein compression) syndrome. He was treated for a total of 6 months with warfarin followed by rivaroxaban (Xarelto) and was counseled about lifestyle changes but was unable to lose weight. Eight months after completing therapy, he had a second extensive deep vein thrombosis, this time in his right leg, and was restarted on rivaroxaban.
The third patient, a morbidly obese (BMI 56 kg/m2) 13-year-old boy, presented with left lower lobe pulmonary embolism following 3 weeks of immobility caused by the Guillain-Barré syndrome. As in the other cases, he confessed to a sedentary lifestyle and a predilection for gaming. His father had previously developed a line-associated thrombus. The family declined thrombophilia testing. The patient received 3 months of enoxaparin. He has not been followed since discontinuing therapy.
Move it, kid!
The risk of VTE in adolescent boys, especially obese and extreme gamers who spend most of their waking hours in a chair staring at a screen, is similar to that for adolescent girls who use oral contraceptives, Dr. Kohorst and her colleagues said.
“Many case reports link prolonged ‘gaming’ to thrombosis and fatal pulmonary emboli. Additionally, prolonged television viewing has become a documented risk factor for mortality from pulmonary emboli,” the investigators wrote.
They recommend that clinicians ask adolescents about their gaming and TV-watching habits and encourage them to become more active to lower their risk for VTE.
The study was internally supported. Dr. Kohorst and colleagues reported no relevant disclosures.
FROM ASPHO 2017
Key clinical point: Obesity and a sedentary lifestyle are risk factors for venous thromboembolic events in teens, as well as adults.
Major finding: Teen boys who were obese and spent much of their day playing video games presented with VTE.
Data source: Retrospective review and case series.
Disclosures: The study was internally supported. Dr. Kohorst and colleagues reported no relevant disclosures.
Antithrombotics no deterrent for emergent lap appendectomy
HOUSTON – Few studies have looked at the risk of irreversible antithrombotic therapy in patients who need emergent or urgent laparoscopic appendectomy, but a new study showed that the operation poses no significantly greater risk for such patients, compared with people who are not on antithrombotics.
The study’s findings do not apply to all patients on anticoagulation, specifically those on new novel oral anticoagulants (NOACs), said Christopher Pearcy, MD, of Methodist Dallas Medical Center.
NOAC agents include dabigatran, rivaroxaban, and apixaban.
Appendicitis is the third most common indication for abdominal surgery in the elderly, Dr. Pearcy noted, and their mortality rates are eight times greater than those of younger patients. However, these patients often proceed to operation with minimal workup, “given that laparoscopic appendectomy is a relatively benign procedure,” he said at the annual meeting of the Society of American Gastrointestinal and Endoscopic Surgeons.
The retrospective study evaluated two groups of 195 patients who had urgent or emergent laparoscopic appendectomy at three centers from 2010 to 2014. One group was on irreversible antithrombotic therapy, and the other served as controls.
The primary outcomes were blood loss, transfusion requirement, and mortality. Secondary outcomes were duration of operation, length of hospital stay, rates of infections, complications, and 30-day readmissions.
“Compared with controls, we didn’t find any significant difference in any outcome whatsoever after laparoscopic appendectomy in patients on prehospital antithrombotic therapy,” Dr. Pearcy said.
Specifically, average estimated blood loss was 18 cc in controls vs. 22 cc in patients on antithrombotics, and mortality was 0% in the former vs. 1% in the latter. Patients on antithrombotics had a lower rate of complications: 3% vs. 11%.
Dr. Pearcy discussed a case of a 70-year-old man with acute appendicitis. He had a history of coronary artery disease, hypertension, hyperlipidemia, type 2 diabetes, and stroke, and was taking clopidogrel and aspirin daily.
“Is it safe to proceed with surgery given this patient’s irreversible antithrombotic therapy? We would say yes,” he said.
Dr. Pearcy reported having no financial disclosures.
HOUSTON – Few studies have looked at the risk of irreversible antithrombotic therapy in patients who need emergent or urgent laparoscopic appendectomy, but a new study showed that the operation poses no significantly greater risk for such patients, compared with people who are not on antithrombotics.
The study’s findings do not apply to all patients on anticoagulation, specifically those on new novel oral anticoagulants (NOACs), said Christopher Pearcy, MD, of Methodist Dallas Medical Center.
NOAC agents include dabigatran, rivaroxaban, and apixaban.
Appendicitis is the third most common indication for abdominal surgery in the elderly, Dr. Pearcy noted, and their mortality rates are eight times greater than those of younger patients. However, these patients often proceed to operation with minimal workup, “given that laparoscopic appendectomy is a relatively benign procedure,” he said at the annual meeting of the Society of American Gastrointestinal and Endoscopic Surgeons.
The retrospective study evaluated two groups of 195 patients who had urgent or emergent laparoscopic appendectomy at three centers from 2010 to 2014. One group was on irreversible antithrombotic therapy, and the other served as controls.
The primary outcomes were blood loss, transfusion requirement, and mortality. Secondary outcomes were duration of operation, length of hospital stay, rates of infections, complications, and 30-day readmissions.
“Compared with controls, we didn’t find any significant difference in any outcome whatsoever after laparoscopic appendectomy in patients on prehospital antithrombotic therapy,” Dr. Pearcy said.
Specifically, average estimated blood loss was 18 cc in controls vs. 22 cc in patients on antithrombotics, and mortality was 0% in the former vs. 1% in the latter. Patients on antithrombotics had a lower rate of complications: 3% vs. 11%.
Dr. Pearcy discussed a case of a 70-year-old man with acute appendicitis. He had a history of coronary artery disease, hypertension, hyperlipidemia, type 2 diabetes, and stroke, and was taking clopidogrel and aspirin daily.
“Is it safe to proceed with surgery given this patient’s irreversible antithrombotic therapy? We would say yes,” he said.
Dr. Pearcy reported having no financial disclosures.
HOUSTON – Few studies have looked at the risk of irreversible antithrombotic therapy in patients who need emergent or urgent laparoscopic appendectomy, but a new study showed that the operation poses no significantly greater risk for such patients, compared with people who are not on antithrombotics.
The study’s findings do not apply to all patients on anticoagulation, specifically those on new novel oral anticoagulants (NOACs), said Christopher Pearcy, MD, of Methodist Dallas Medical Center.
NOAC agents include dabigatran, rivaroxaban, and apixaban.
Appendicitis is the third most common indication for abdominal surgery in the elderly, Dr. Pearcy noted, and their mortality rates are eight times greater than those of younger patients. However, these patients often proceed to operation with minimal workup, “given that laparoscopic appendectomy is a relatively benign procedure,” he said at the annual meeting of the Society of American Gastrointestinal and Endoscopic Surgeons.
The retrospective study evaluated two groups of 195 patients who had urgent or emergent laparoscopic appendectomy at three centers from 2010 to 2014. One group was on irreversible antithrombotic therapy, and the other served as controls.
The primary outcomes were blood loss, transfusion requirement, and mortality. Secondary outcomes were duration of operation, length of hospital stay, rates of infections, complications, and 30-day readmissions.
“Compared with controls, we didn’t find any significant difference in any outcome whatsoever after laparoscopic appendectomy in patients on prehospital antithrombotic therapy,” Dr. Pearcy said.
Specifically, average estimated blood loss was 18 cc in controls vs. 22 cc in patients on antithrombotics, and mortality was 0% in the former vs. 1% in the latter. Patients on antithrombotics had a lower rate of complications: 3% vs. 11%.
Dr. Pearcy discussed a case of a 70-year-old man with acute appendicitis. He had a history of coronary artery disease, hypertension, hyperlipidemia, type 2 diabetes, and stroke, and was taking clopidogrel and aspirin daily.
“Is it safe to proceed with surgery given this patient’s irreversible antithrombotic therapy? We would say yes,” he said.
Dr. Pearcy reported having no financial disclosures.
AT SAGES 2017
Key clinical point: Emergent laparoscopic appendectomy poses no significant risk for patients on irreversible antithrombotic therapy.
Major finding: Average estimated blood loss was 18 cc in controls vs. 22 cc in patients on antithrombotics, and mortality was 0% vs. 1%, respectively.
Data source: A retrospective study of 390 patients who had urgent or emergent laparoscopic appendectomy at three centers from 2010 to 2014.
Disclosures: Dr. Pearcy reported having no financial disclosures.
Anticoagulant resumption after ICH aids patients
HOUSTON – Even when patients on an oral anticoagulant have the dreaded complication of an intracerebral hemorrhage, resumption of their oral anticoagulation regimen appears to produce the best midterm outcomes, based on a meta-analysis of data from more than 1,000 patients collected in three observational studies.
Resumption of oral anticoagulation therapy (OAT) is a “major dilemma” when managing patients who developed an intracerebral hemorrhage (ICH) while on OAT, said Alessandro Biffi, MD, explaining why he performed this meta-analysis that he presented at the International Stroke Conference sponsored by the American Heart Association.
He used individual patient data collected from a total of 1,027 patients enrolled in any of three different observational studies: the German-wide Multicenter Analysis of Oral Anticoagulation Associated Intracerebral Hemorrhage (RETRACE) study, the MGH longitudinal ICH study, or the Ethnic/Racial Variations of Intracerebral Hemorrhage (ERICH) study. Overall 26% of the patients resumed OAT following their ICH, although the rate ranged from a low of 20% in one study to a high of 42% in another. The vast majority of patients received a vitamin K antagonist as their anticoagulant; very few received a new oral anticoagulant.
Using propensity score matching to compare similar patients who resumed or stayed off OAT, Dr. Biffi found that, during the year following the index ICH, mortality was 71%-74% lower among patients who resumed OAT. Recurrent all-cause stroke was 49%-55% lower with resumed OAT, and favorable functional outcomes (a score of 0-3 on the modified Rankin scale) were more than fourfold higher with OAT resumption, he reported.
Dr. Biffi calculated these relative rates, both for patients with a lumbar location of their ICH and for those with a nonlumbar location, and found that location had no influence on responsiveness to OAT. Patients with an index ICH in a lumbar location had a trend toward more recurrent ICH on OAT, a 26% higher rate relative to patients not resumed on OAT, but this difference fell short of statistical significance.
The only factor he found that linked with whether or not patients resumed OAT was the severity of their index ICH. The more severe their bleed, the less likely were patients to resume. Aside from that, “there is a lot of variation in practice,” he said. “We are gathering additional data” to try to further address this question.
Dr. Biffi had no disclosures.
[email protected]
On Twitter @mitchelzoler
Resuming oral anticoagulation following an intracerebral hemorrhage is one of the most vexing problems today in vascular neurology. It’s a situation that often happens, and it will grow increasingly more common as the number of patients with atrial fibrillation escalates and even more people start oral anticoagulation.
It’s also very important to remember that patients like these who need oral anticoagulation but now have a history of ICH must have all their other cardiovascular disease risk factors very well controlled: their blood pressure, their diabetes, their smoking, etc. Oral anticoagulation may be important for these patients, but tight risk factor control is even more important.
I agree with Dr. Biffi that a prospective, randomized trial is the best way to get more information to help guide resuming oral anticoagulation. Observational studies are significantly limited by ascertainment bias, and for these patients there are also many variables – at least a dozen – that can influence whether or not a patient resumes oral anticoagulation. Dr. Biffi’s findings are interesting, but the limitations of his data prevent the results from being truly compelling.
It would be very helpful to have data from a trial that randomized ICH patients who required anticoagulation to a full-dose NOAC, a reduced-dose NOAC, or aspirin and see which group had the best long-term outcome. Whatever the results, it would change practice. It’s intriguing to speculate that a reduced-dose NOAC might provide adequate ischemic protection with a reduced risk for more bleeding.
Mark J. Alberts, MD , is chief of neurology at Hartford (Conn.) Hospital. He had no disclosures. He made these comments in an interview and during a press conference.
Resuming oral anticoagulation following an intracerebral hemorrhage is one of the most vexing problems today in vascular neurology. It’s a situation that often happens, and it will grow increasingly more common as the number of patients with atrial fibrillation escalates and even more people start oral anticoagulation.
It’s also very important to remember that patients like these who need oral anticoagulation but now have a history of ICH must have all their other cardiovascular disease risk factors very well controlled: their blood pressure, their diabetes, their smoking, etc. Oral anticoagulation may be important for these patients, but tight risk factor control is even more important.
I agree with Dr. Biffi that a prospective, randomized trial is the best way to get more information to help guide resuming oral anticoagulation. Observational studies are significantly limited by ascertainment bias, and for these patients there are also many variables – at least a dozen – that can influence whether or not a patient resumes oral anticoagulation. Dr. Biffi’s findings are interesting, but the limitations of his data prevent the results from being truly compelling.
It would be very helpful to have data from a trial that randomized ICH patients who required anticoagulation to a full-dose NOAC, a reduced-dose NOAC, or aspirin and see which group had the best long-term outcome. Whatever the results, it would change practice. It’s intriguing to speculate that a reduced-dose NOAC might provide adequate ischemic protection with a reduced risk for more bleeding.
Mark J. Alberts, MD , is chief of neurology at Hartford (Conn.) Hospital. He had no disclosures. He made these comments in an interview and during a press conference.
Resuming oral anticoagulation following an intracerebral hemorrhage is one of the most vexing problems today in vascular neurology. It’s a situation that often happens, and it will grow increasingly more common as the number of patients with atrial fibrillation escalates and even more people start oral anticoagulation.
It’s also very important to remember that patients like these who need oral anticoagulation but now have a history of ICH must have all their other cardiovascular disease risk factors very well controlled: their blood pressure, their diabetes, their smoking, etc. Oral anticoagulation may be important for these patients, but tight risk factor control is even more important.
I agree with Dr. Biffi that a prospective, randomized trial is the best way to get more information to help guide resuming oral anticoagulation. Observational studies are significantly limited by ascertainment bias, and for these patients there are also many variables – at least a dozen – that can influence whether or not a patient resumes oral anticoagulation. Dr. Biffi’s findings are interesting, but the limitations of his data prevent the results from being truly compelling.
It would be very helpful to have data from a trial that randomized ICH patients who required anticoagulation to a full-dose NOAC, a reduced-dose NOAC, or aspirin and see which group had the best long-term outcome. Whatever the results, it would change practice. It’s intriguing to speculate that a reduced-dose NOAC might provide adequate ischemic protection with a reduced risk for more bleeding.
Mark J. Alberts, MD , is chief of neurology at Hartford (Conn.) Hospital. He had no disclosures. He made these comments in an interview and during a press conference.
HOUSTON – Even when patients on an oral anticoagulant have the dreaded complication of an intracerebral hemorrhage, resumption of their oral anticoagulation regimen appears to produce the best midterm outcomes, based on a meta-analysis of data from more than 1,000 patients collected in three observational studies.
Resumption of oral anticoagulation therapy (OAT) is a “major dilemma” when managing patients who developed an intracerebral hemorrhage (ICH) while on OAT, said Alessandro Biffi, MD, explaining why he performed this meta-analysis that he presented at the International Stroke Conference sponsored by the American Heart Association.
He used individual patient data collected from a total of 1,027 patients enrolled in any of three different observational studies: the German-wide Multicenter Analysis of Oral Anticoagulation Associated Intracerebral Hemorrhage (RETRACE) study, the MGH longitudinal ICH study, or the Ethnic/Racial Variations of Intracerebral Hemorrhage (ERICH) study. Overall 26% of the patients resumed OAT following their ICH, although the rate ranged from a low of 20% in one study to a high of 42% in another. The vast majority of patients received a vitamin K antagonist as their anticoagulant; very few received a new oral anticoagulant.
Using propensity score matching to compare similar patients who resumed or stayed off OAT, Dr. Biffi found that, during the year following the index ICH, mortality was 71%-74% lower among patients who resumed OAT. Recurrent all-cause stroke was 49%-55% lower with resumed OAT, and favorable functional outcomes (a score of 0-3 on the modified Rankin scale) were more than fourfold higher with OAT resumption, he reported.
Dr. Biffi calculated these relative rates, both for patients with a lumbar location of their ICH and for those with a nonlumbar location, and found that location had no influence on responsiveness to OAT. Patients with an index ICH in a lumbar location had a trend toward more recurrent ICH on OAT, a 26% higher rate relative to patients not resumed on OAT, but this difference fell short of statistical significance.
The only factor he found that linked with whether or not patients resumed OAT was the severity of their index ICH. The more severe their bleed, the less likely were patients to resume. Aside from that, “there is a lot of variation in practice,” he said. “We are gathering additional data” to try to further address this question.
Dr. Biffi had no disclosures.
[email protected]
On Twitter @mitchelzoler
HOUSTON – Even when patients on an oral anticoagulant have the dreaded complication of an intracerebral hemorrhage, resumption of their oral anticoagulation regimen appears to produce the best midterm outcomes, based on a meta-analysis of data from more than 1,000 patients collected in three observational studies.
Resumption of oral anticoagulation therapy (OAT) is a “major dilemma” when managing patients who developed an intracerebral hemorrhage (ICH) while on OAT, said Alessandro Biffi, MD, explaining why he performed this meta-analysis that he presented at the International Stroke Conference sponsored by the American Heart Association.
He used individual patient data collected from a total of 1,027 patients enrolled in any of three different observational studies: the German-wide Multicenter Analysis of Oral Anticoagulation Associated Intracerebral Hemorrhage (RETRACE) study, the MGH longitudinal ICH study, or the Ethnic/Racial Variations of Intracerebral Hemorrhage (ERICH) study. Overall 26% of the patients resumed OAT following their ICH, although the rate ranged from a low of 20% in one study to a high of 42% in another. The vast majority of patients received a vitamin K antagonist as their anticoagulant; very few received a new oral anticoagulant.
Using propensity score matching to compare similar patients who resumed or stayed off OAT, Dr. Biffi found that, during the year following the index ICH, mortality was 71%-74% lower among patients who resumed OAT. Recurrent all-cause stroke was 49%-55% lower with resumed OAT, and favorable functional outcomes (a score of 0-3 on the modified Rankin scale) were more than fourfold higher with OAT resumption, he reported.
Dr. Biffi calculated these relative rates, both for patients with a lumbar location of their ICH and for those with a nonlumbar location, and found that location had no influence on responsiveness to OAT. Patients with an index ICH in a lumbar location had a trend toward more recurrent ICH on OAT, a 26% higher rate relative to patients not resumed on OAT, but this difference fell short of statistical significance.
The only factor he found that linked with whether or not patients resumed OAT was the severity of their index ICH. The more severe their bleed, the less likely were patients to resume. Aside from that, “there is a lot of variation in practice,” he said. “We are gathering additional data” to try to further address this question.
Dr. Biffi had no disclosures.
[email protected]
On Twitter @mitchelzoler
AT THE INTERNATIONAL STROKE CONFERENCE
Key clinical point:
Major finding: One-year mortality was 71%-74% lower among patients who resumed oral anticoagulation relative to those who did not.
Data source: Meta-analysis of data from 1,027 patients collected in three observational studies.
Disclosures: Dr. Biffi had no disclosures.