User login
Atypical Features of COVID-19: A Literature Review
From the University of Florida College of Medicine, Division of Infectious Diseases and Global Medicine, Gainesville, FL.
Abstract
- Objective: To review current reports on atypical manifestations of coronavirus disease 2019 (COVID-19).
- Methods: Review of the literature.
- Results: Evidence regarding atypical features of COVID-19 is accumulating. SARS-CoV-2 can infect human cells that express the angiotensin-converting enzyme 2 receptor, which would allow for a broad spectrum of illnesses affecting the renal, cardiac, and gastrointestinal organ systems. Neurologic, cutaneous, and musculoskeletal manifestations have also been reported. The potential for SARS-CoV-2 to induce a hypercoagulable state provides another avenue for the virus to indirectly damage various organ systems, as evidenced by reports of cerebrovascular disease, myocardial injury, and a chilblain-like rash in patients with COVID-19.
- Conclusion: Because the signs and symptoms of COVID-19 may occur with varying frequency across populations, it is important to keep differentials broad when assessing patients with a clinical illness that may indeed be COVID-19.
Keywords: coronavirus; severe acute respiratory syndrome coronavirus-2; SARS-CoV-2; pandemic.
Coronavirus disease 2019 (COVID-19), the syndrome caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), was first reported in Wuhan, China, in early December 2019.1 Since then, the virus has spread quickly around the world, with the World Health Organization (WHO) declaring the coronavirus outbreak a global pandemic on March 11, 2020. As of May 21, 2020, more than 5,000,000 cases of COVID-19 have been confirmed, and more than 328,000 deaths related to COVID-19 have been reported globally.2 These numbers are expected to increase, due to the reproduction number (R0) of SARS-CoV-2. R0 represents the number of new infections generated by an infectious person in a totally naïve population.3 The WHO estimates that the R0 of SARS-CoV-2 is 1.95, with other estimates ranging from 1.4 to 6.49.3 To control the pathogen, the R0 needs to be brought under a value of 1.
A fundamental tool in lowering the R0 is prompt testing and isolation of those who display signs and symptoms of infection. SARS-CoV-2 is still a novel pathogen about which we know relatively little. The common symptoms of COVID-19 are now well known—including fever, fatigue, anorexia, cough, and shortness of breath—but atypical manifestations of this viral continue to be reported and described. To help clinicians across specialties and settings identify patients with possible infection, we have summarized findings from current reports on COVID-19 manifestations involving the renal, cardiac, gastrointestinal (GI), and other organ systems.
Renal
During the 2003 SARS-CoV-1 outbreak, acute kidney injury (AKI) was an uncommon complication of the infection, but early reports suggest that AKI may occur more commonly with COVID-19.4 In a study of 193 patients with laboratory-confirmed COVID-19 treated in 3 Chinese hospitals, 59% presented with proteinuria, 44% with hematuria, 14% with increased blood urea nitrogen, and 10% with increased levels of serum creatinine.4 These markers, indicative of AKI, may be associated with increased mortality. Among this cohort, those with AKI had a mortality risk 5.3 times higher than those who did not have AKI.4 The pathophysiology of renal disease in COVID-19 may be related to dehydration or inflammatory mediators, causing decreased renal perfusion and cytokine storm, but evidence also suggests that SARS-CoV-2 is able to directly infect kidney cells.5 The virus infects cells by using angiotensin-converting enzyme 2 (ACE2) on the cell membrane as a cell entry receptor; ACE2 is expressed on the kidney, heart, and GI cells, and this may allow SARS-CoV-2 to directly infect and damage these organs. Other potential mechanisms of renal injury include overproduction of proinflammatory cytokines and administration of nephrotoxic drugs. No matter the mechanism, however, increased serum creatinine and blood urea nitrogen correlate with an increased likelihood of requiring intensive care unit (ICU) admission.6 Therefore, clinicians should carefully monitor renal function in patients with COVID-19.
Cardiac
In a report of 138 Chinese patients hospitalized for COVID-19, 36 required ICU admission: 44.4% of these had arrhythmias and 22.2% had developed acute cardiac injury.6 In addition, the cardiac cell injury biomarker troponin I was more likely to be elevated in ICU patients.6 A study of 21 patients admitted to the ICU in Washington State found elevated levels of brain natriuretic peptide.7 These biomarkers reflect the presence of myocardial stress, but do not necessarily indicate direct myocardial infection. Case reports of fulminant myocarditis in those with COVID-19 have begun to surface, however.8,9 An examination of 68 deaths in persons with COVID-19 concluded that 7% were caused by myocarditis with circulatory failure.10
The pathophysiology of myocardial injury in COVID-19 is likely multifactorial. This includes increased inflammatory mediators, hypoxemia, and metabolic changes that can directly damage myocardial tissue. These factors can also exacerbate comorbid conditions, such as coronary artery disease, leading to ischemia and dysfunction of preexisting electrical conduction abnormalities. However, pathologic evidence of myocarditis and the presence of the ACE2 receptor, which may be a mediator of cardiac function, on cardiac muscle cells suggest that SARS-CoV-2 is capable of directly infecting and damaging myocardial cells. Other proposed mechanisms include infection-mediated downregulation of ACE2, causing cardiac dysfunction, or thrombus formation.11 Although respiratory failure is the most common source of advanced illness in COVID-19 patients, myocarditis and arrhythmias can be life-threatening manifestations of the disease.
Gastrointestinal
As noted, ACE2 is expressed in the GI tract. In 73 patients hospitalized for COVID-19, 53.4% tested positive for SARS-CoV-2 RNA in stool, and 23.4% continued to have RNA-positive stool samples even after their respiratory samples tested negative.12 These findings suggest the potential for SARS-CoV-2 to spread through fecal-oral transmission in those who are asymptomatic, pre-symptomatic, or symptomatic. This mode of transmission has yet to be determined conclusively, and more research is needed. However, GI symptoms have been reported in persons with COVID-19. Among 138 hospitalized patients, 10.1% had complaints of diarrhea and nausea and 3.6% reported vomiting.6 Those who reported nausea and diarrhea noted that they developed these symptoms 1 to 2 days before they developed fever.6 Also, among a cohort of 1099 Chinese patients with COVID-19, 3.8% complained of diarrhea.13 Although diarrhea does not occur in a majority of patients, GI complaints, such as nausea, vomiting, or diarrhea, should raise clinical suspicion for COVID-19, and in known areas of active transmission, testing of patients with GI symptoms is likely warranted.
Ocular
Ocular manifestations of COVID-19 are now being described, and should be taken into consideration when examining a patient. In a study of 38 patients with COVID-19 from Hubei province, China, 31.6% had ocular findings consistent with conjunctivitis, including conjunctival hyperemia, chemosis, epiphora, and increased ocular secretions.14 SARS-CoV-2 was detected in conjunctival and nasopharyngeal samples in 2 patients from this cohort. Conjunctival congestion was reported in a cohort of 1099 patients with COVID-19 treated at multiple centers throughout China, but at a much lower incidence, approximately 0.8%.13 Because SARS-CoV-2 can cause conjunctival disease and has been detected in samples from the external surface of the eye, it appears the virus is transmissible from tears or contact with the eye itself.
Neurologic
Common reported neurologic symptoms include dizziness, headache, impaired consciousness, ataxia, and cerebrovascular events. In a cohort of 214 patients from Wuhan, China, 36.4% had some form of neurological insult.15 These symptoms were more common in those with severe illness (P = 0.02).15 Two interesting neurologic symptoms that have been described are anosmia (loss of smell) and ageusia (loss of taste), which are being found primarily in tandem. It is still unclear how many people with COVID-19 are experiencing these symptoms, but a report from Italy estimates 19.4% of 320 patients examined had chemosensory dysfunction.16 The aforementioned report from Wuhan, China, found that 5.1% had anosmia and 5.6% had ageusia.15 The presence of anosmia/ageusia in some patients suggests that SARS-CoV-2 may enter the central nervous system (CNS) through a retrograde neuronal route.15 In addition, a case report from Japan described a 24-year-old man who presented with meningitis/encephalitis and had SARS-CoV-2 RNA present in his cerebrospinal fluid, showing that SARS-CoV-2 can penetrate into the CNS.17
SARS-CoV-2 may also have an association with Guillain–Barré syndrome, as this condition was reported in 5 patients from 3 hospitals in Northern Italy.18 The symptoms of Guillain–Barré syndrome presented 5 to 10 days after the typical COVID-19 symptoms, and evolved over 36 hours to 4 days afterwards. Four of the 5 patients experienced flaccid tetraparesis or tetraplegia, and 3 required mechanical ventilation.18
Another possible cause of neurologic injury in COVID-19 is damage to endothelial cells in cerebral blood vessels, causing thrombus formation and possibly increasing the risk of acute ischemic stroke.15,19 Supporting this mechanism of injury, significantly lower platelet counts were noted in patients with CNS symptoms (P = 0.005).15 Other hematological impacts of COVID-19 have been reported, particularly hypercoagulability, as evidenced by elevated D-dimer levels.13,20 This hypercoagulable state is linked to overproduction of proinflammatory cytokines (cytokine storm), leading to dysregulation of coagulation pathways and reduced concentrations of anticoagulants, such as protein C, antithrombin III, and tissue factor pathway inhibitor.21
Cutaneous
Cutaneous findings emerging in persons with COVID-19 demonstrate features of small-vessel and capillary occlusion, including erythematous skin eruptions and petechial rash. One report from Italy noted that 20.4% of patients with COVID-19 (n = 88) had a cutaneous finding, with a cutaneous manifestation developing in 8 at the onset of illness and in 10 following hospital admission.22 Fourteen patients had an erythematous rash, primarily on the trunk, with 3 patients having a diffuse urticarial appearing rash, and 1 patient developing vesicles.22 The severity of illness did not appear to correlate with the cutaneous manifestation, and the lesions healed within a few days.
One case report described a patient from Bangkok who was thought to be suffering from dengue fever, but was found to have SARS-CoV-2 infection. He initially presented with skin rash and petechiae, and later developed respiratory disease.23
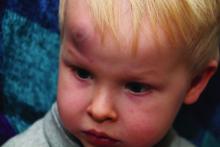
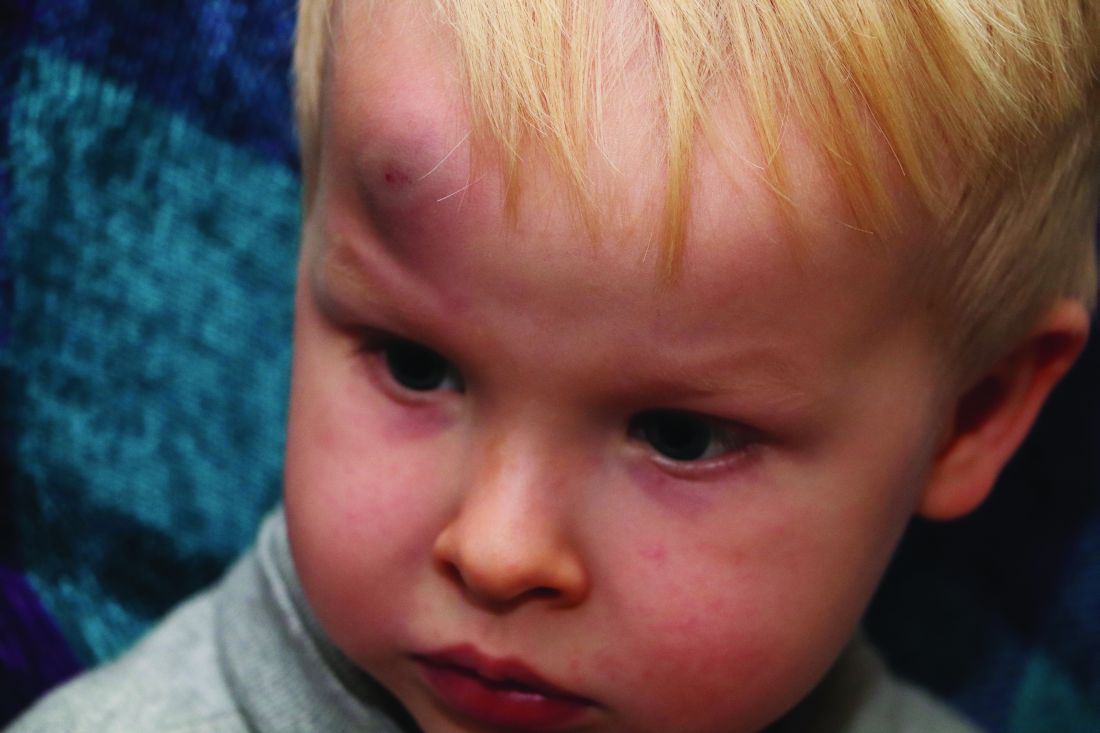
Other dermatologic findings of COVID-19 resemble chilblains disease, colloquially referred to as “COVID toes.” Two women, 27 and 35 years old, presented to a dermatology clinic in Qatar with a chief complaint of skin rash, described as red-purple papules on the dorsal aspects of the fingers bilaterally.22 Both patients had an unremarkable medical and drug history, but recent travel to the United Kingdom dictated SARS-CoV-2 screening, which was positive.24 An Italian case report describes a 23-year-old man who tested positive for SARS-CoV-2 and had violaceous plaques on an erythematous background on his feet, without any lesions on his hands.25 Since chilblains is less common in the warmer months and these events correspond with the COVID-19 pandemic, SARS-CoV-2 infection is the suspected etiology. The pathophysiology of these lesions is unclear, and more research is needed. As more data become available, we may see cutaneous manifestations in patients with COVID-19 similar to those commonly reported with other viral infectious processes.
Musculoskeletal
Of 138 patients hospitalized in Wuhan, China, for COVID-19, 34.8% presented with myalgia; the presence of myalgia does not appear to be correlated with an increased likelihood of ICU admission.6 Myalgia or arthralgia was also reported in 14.9% among the cohort of 1099 COVID-19 patients in China.13 These musculoskeletal symptoms are described among large muscle groups found in the extremities, trunk, and back, and should raise suspicion in patients who present with other signs and symptoms concerning for COVID-19.
Conclusion
Evidence regarding atypical features of COVID-19 is accumulating. SARS-CoV-2 can infect a human cells that express the ACE2 receptor, which would allow for a broad spectrum of illnesses. The potential for SARS-CoV-2 to induce a hypercoagulable state allows it to indirectly damage various organ systems,20 leading to cerebrovascular disease, myocardial injury, and a chilblain-like rash. Clinicians must be aware of these unique features, as early recognition of persons who present with COVID-19 will allow for prompt testing, institution of infection control and isolation practices, and treatment, as needed, among those infected. Also, this is a pandemic involving a novel virus affecting different populations throughout the world, and these signs and symptoms may occur with varying frequency across populations. Therefore, it is important to keep differentials broad when assessing patients with a clinical illness that may indeed be COVID-19.
Corresponding author: Norman L. Beatty, MD, [email protected].
Financial disclosures: None.
1. WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020 [press release]. World Health Organization; March 11, 2020.
2. Coronavirus COVID-19 Global Cases by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. Johns Hopkins CSSE. https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6 Accessed May 15, 2020.
3. Liu Y, Gayle AA, Wilder-Smith A, Rocklöv J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Travel Med. 2020;27(2):taaa021. doi:10.1093/jtm/taaa021
4. Li Z, Wu M, Guo J, et al. Caution on kidney dysfunctions of 2019-nCoV patients. medRxiv preprint. doi: 10.1101/2020.02.08.20021212
5. Li W, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450-454. doi: 10.1038/nature02145.
6. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. doi:10.1001/jama.2020.1585
7. Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323:1612‐1614. doi:10.1001/jama.2020.4326
8. Chen C, Zhou Y, Wang DW. SARS-CoV-2: a potential novel etiology of fulminant myocarditis. Herz. 2020;45:230-232. doi: 10.1007/s00059-020-04909-z
9. Hu H, Ma F, Wei X, Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. 2020 Mar 16;ehaa190. doi: 10.1093/eurheartj/ehaa190
10. Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846-848. doi:10.1007/s00134-020-05991-x
11. Akhmerov A, Marban E. COVID-19 and the heart. Circ Res. 2020;126:1443-1455. doi:10.1161/CIRCRESAHA.120.317055
12. Xiao F, Tang M, Zheng X, et al. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158:1831-1833. doi: 10.1053/j.gastro.2020.02.055
13. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1078-1720. doi: 10.1056/NEJMoa2002032
14. Wu P, Duan F, Luo C, et al. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020 Mar 31;e201291. doi: 10.1001/jamaophthalmol.2020.1291
15. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020 Apr 10. doi: 10.1001/jamaneurol.2020.1127
16. Vaira LA, Salzano G, Deiana G, De Riu G. Anosmia and ageusia: common findings in COVID-19 patients. Laryngoscope. 2020 Apr 1. doi: 10.1002/lary.28692
17. Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS-coronavirus-2. Int J Infect Dis. 2020;94:55-58. doi: 10.1016/j.ijid.2020.03.062
18. Toscano G, Palmerini F, Ravaglia S, et al. Guillain–Barré syndrome associated with SARS-CoV-2. N Engl J Med. 2020 Apr 17;NEJMc2009191. doi:10.1056/nejmc2009191
19. Dafer RM, Osteraas ND, Biller J. Acute stroke care in the coronavirus disease 2019 pandemic. J Stroke Cerebrovascular Dis. 2020 Apr 17:104881. doi: 10.1016/j.jstrokecerebrovasdis.2020.104881
20. Terpos E, Ntanasis-Stathopoulos I, Elalamy I, et al. Hematological findings and complications of COVID-19. Am J Hematol. 2020;10.1002/ajh.25829. doi:10.1002/ajh.25829
21. Jose RJ, Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. 2020;S2213-2600(20)30216-2. doi:10.1016/S2213-2600(20)30216-2
22. Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020 Mar 26. doi: 10.1111/jdv.16387
23. Joob B, Wiwanitkit V. COVID-19 can present with a rash and be mistaken for dengue. J Am Acad Dermatol. 2020;82(5):e177. doi: 10.1016/j.jaad.2020.03.036
24. Alramthan A, Aldaraji W. A Case of COVID‐19 presenting in clinical picture resembling chilblains disease. First report from the Middle East. Clin Exp Dermatol. 2020 Apr 17. doi: 10.1111/ced.14243
25. Kolivras A, Dehavay F, Delplace D, et al. Coronavirus (COVID-19) infection–induced chilblains: a case report with histopathologic findings. JAAD Case Rep. 2020 Apr 18. doi: 10.1016/j.jdcr.2020.04.011
From the University of Florida College of Medicine, Division of Infectious Diseases and Global Medicine, Gainesville, FL.
Abstract
- Objective: To review current reports on atypical manifestations of coronavirus disease 2019 (COVID-19).
- Methods: Review of the literature.
- Results: Evidence regarding atypical features of COVID-19 is accumulating. SARS-CoV-2 can infect human cells that express the angiotensin-converting enzyme 2 receptor, which would allow for a broad spectrum of illnesses affecting the renal, cardiac, and gastrointestinal organ systems. Neurologic, cutaneous, and musculoskeletal manifestations have also been reported. The potential for SARS-CoV-2 to induce a hypercoagulable state provides another avenue for the virus to indirectly damage various organ systems, as evidenced by reports of cerebrovascular disease, myocardial injury, and a chilblain-like rash in patients with COVID-19.
- Conclusion: Because the signs and symptoms of COVID-19 may occur with varying frequency across populations, it is important to keep differentials broad when assessing patients with a clinical illness that may indeed be COVID-19.
Keywords: coronavirus; severe acute respiratory syndrome coronavirus-2; SARS-CoV-2; pandemic.
Coronavirus disease 2019 (COVID-19), the syndrome caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), was first reported in Wuhan, China, in early December 2019.1 Since then, the virus has spread quickly around the world, with the World Health Organization (WHO) declaring the coronavirus outbreak a global pandemic on March 11, 2020. As of May 21, 2020, more than 5,000,000 cases of COVID-19 have been confirmed, and more than 328,000 deaths related to COVID-19 have been reported globally.2 These numbers are expected to increase, due to the reproduction number (R0) of SARS-CoV-2. R0 represents the number of new infections generated by an infectious person in a totally naïve population.3 The WHO estimates that the R0 of SARS-CoV-2 is 1.95, with other estimates ranging from 1.4 to 6.49.3 To control the pathogen, the R0 needs to be brought under a value of 1.
A fundamental tool in lowering the R0 is prompt testing and isolation of those who display signs and symptoms of infection. SARS-CoV-2 is still a novel pathogen about which we know relatively little. The common symptoms of COVID-19 are now well known—including fever, fatigue, anorexia, cough, and shortness of breath—but atypical manifestations of this viral continue to be reported and described. To help clinicians across specialties and settings identify patients with possible infection, we have summarized findings from current reports on COVID-19 manifestations involving the renal, cardiac, gastrointestinal (GI), and other organ systems.
Renal
During the 2003 SARS-CoV-1 outbreak, acute kidney injury (AKI) was an uncommon complication of the infection, but early reports suggest that AKI may occur more commonly with COVID-19.4 In a study of 193 patients with laboratory-confirmed COVID-19 treated in 3 Chinese hospitals, 59% presented with proteinuria, 44% with hematuria, 14% with increased blood urea nitrogen, and 10% with increased levels of serum creatinine.4 These markers, indicative of AKI, may be associated with increased mortality. Among this cohort, those with AKI had a mortality risk 5.3 times higher than those who did not have AKI.4 The pathophysiology of renal disease in COVID-19 may be related to dehydration or inflammatory mediators, causing decreased renal perfusion and cytokine storm, but evidence also suggests that SARS-CoV-2 is able to directly infect kidney cells.5 The virus infects cells by using angiotensin-converting enzyme 2 (ACE2) on the cell membrane as a cell entry receptor; ACE2 is expressed on the kidney, heart, and GI cells, and this may allow SARS-CoV-2 to directly infect and damage these organs. Other potential mechanisms of renal injury include overproduction of proinflammatory cytokines and administration of nephrotoxic drugs. No matter the mechanism, however, increased serum creatinine and blood urea nitrogen correlate with an increased likelihood of requiring intensive care unit (ICU) admission.6 Therefore, clinicians should carefully monitor renal function in patients with COVID-19.
Cardiac
In a report of 138 Chinese patients hospitalized for COVID-19, 36 required ICU admission: 44.4% of these had arrhythmias and 22.2% had developed acute cardiac injury.6 In addition, the cardiac cell injury biomarker troponin I was more likely to be elevated in ICU patients.6 A study of 21 patients admitted to the ICU in Washington State found elevated levels of brain natriuretic peptide.7 These biomarkers reflect the presence of myocardial stress, but do not necessarily indicate direct myocardial infection. Case reports of fulminant myocarditis in those with COVID-19 have begun to surface, however.8,9 An examination of 68 deaths in persons with COVID-19 concluded that 7% were caused by myocarditis with circulatory failure.10
The pathophysiology of myocardial injury in COVID-19 is likely multifactorial. This includes increased inflammatory mediators, hypoxemia, and metabolic changes that can directly damage myocardial tissue. These factors can also exacerbate comorbid conditions, such as coronary artery disease, leading to ischemia and dysfunction of preexisting electrical conduction abnormalities. However, pathologic evidence of myocarditis and the presence of the ACE2 receptor, which may be a mediator of cardiac function, on cardiac muscle cells suggest that SARS-CoV-2 is capable of directly infecting and damaging myocardial cells. Other proposed mechanisms include infection-mediated downregulation of ACE2, causing cardiac dysfunction, or thrombus formation.11 Although respiratory failure is the most common source of advanced illness in COVID-19 patients, myocarditis and arrhythmias can be life-threatening manifestations of the disease.
Gastrointestinal
As noted, ACE2 is expressed in the GI tract. In 73 patients hospitalized for COVID-19, 53.4% tested positive for SARS-CoV-2 RNA in stool, and 23.4% continued to have RNA-positive stool samples even after their respiratory samples tested negative.12 These findings suggest the potential for SARS-CoV-2 to spread through fecal-oral transmission in those who are asymptomatic, pre-symptomatic, or symptomatic. This mode of transmission has yet to be determined conclusively, and more research is needed. However, GI symptoms have been reported in persons with COVID-19. Among 138 hospitalized patients, 10.1% had complaints of diarrhea and nausea and 3.6% reported vomiting.6 Those who reported nausea and diarrhea noted that they developed these symptoms 1 to 2 days before they developed fever.6 Also, among a cohort of 1099 Chinese patients with COVID-19, 3.8% complained of diarrhea.13 Although diarrhea does not occur in a majority of patients, GI complaints, such as nausea, vomiting, or diarrhea, should raise clinical suspicion for COVID-19, and in known areas of active transmission, testing of patients with GI symptoms is likely warranted.
Ocular
Ocular manifestations of COVID-19 are now being described, and should be taken into consideration when examining a patient. In a study of 38 patients with COVID-19 from Hubei province, China, 31.6% had ocular findings consistent with conjunctivitis, including conjunctival hyperemia, chemosis, epiphora, and increased ocular secretions.14 SARS-CoV-2 was detected in conjunctival and nasopharyngeal samples in 2 patients from this cohort. Conjunctival congestion was reported in a cohort of 1099 patients with COVID-19 treated at multiple centers throughout China, but at a much lower incidence, approximately 0.8%.13 Because SARS-CoV-2 can cause conjunctival disease and has been detected in samples from the external surface of the eye, it appears the virus is transmissible from tears or contact with the eye itself.
Neurologic
Common reported neurologic symptoms include dizziness, headache, impaired consciousness, ataxia, and cerebrovascular events. In a cohort of 214 patients from Wuhan, China, 36.4% had some form of neurological insult.15 These symptoms were more common in those with severe illness (P = 0.02).15 Two interesting neurologic symptoms that have been described are anosmia (loss of smell) and ageusia (loss of taste), which are being found primarily in tandem. It is still unclear how many people with COVID-19 are experiencing these symptoms, but a report from Italy estimates 19.4% of 320 patients examined had chemosensory dysfunction.16 The aforementioned report from Wuhan, China, found that 5.1% had anosmia and 5.6% had ageusia.15 The presence of anosmia/ageusia in some patients suggests that SARS-CoV-2 may enter the central nervous system (CNS) through a retrograde neuronal route.15 In addition, a case report from Japan described a 24-year-old man who presented with meningitis/encephalitis and had SARS-CoV-2 RNA present in his cerebrospinal fluid, showing that SARS-CoV-2 can penetrate into the CNS.17
SARS-CoV-2 may also have an association with Guillain–Barré syndrome, as this condition was reported in 5 patients from 3 hospitals in Northern Italy.18 The symptoms of Guillain–Barré syndrome presented 5 to 10 days after the typical COVID-19 symptoms, and evolved over 36 hours to 4 days afterwards. Four of the 5 patients experienced flaccid tetraparesis or tetraplegia, and 3 required mechanical ventilation.18
Another possible cause of neurologic injury in COVID-19 is damage to endothelial cells in cerebral blood vessels, causing thrombus formation and possibly increasing the risk of acute ischemic stroke.15,19 Supporting this mechanism of injury, significantly lower platelet counts were noted in patients with CNS symptoms (P = 0.005).15 Other hematological impacts of COVID-19 have been reported, particularly hypercoagulability, as evidenced by elevated D-dimer levels.13,20 This hypercoagulable state is linked to overproduction of proinflammatory cytokines (cytokine storm), leading to dysregulation of coagulation pathways and reduced concentrations of anticoagulants, such as protein C, antithrombin III, and tissue factor pathway inhibitor.21
Cutaneous
Cutaneous findings emerging in persons with COVID-19 demonstrate features of small-vessel and capillary occlusion, including erythematous skin eruptions and petechial rash. One report from Italy noted that 20.4% of patients with COVID-19 (n = 88) had a cutaneous finding, with a cutaneous manifestation developing in 8 at the onset of illness and in 10 following hospital admission.22 Fourteen patients had an erythematous rash, primarily on the trunk, with 3 patients having a diffuse urticarial appearing rash, and 1 patient developing vesicles.22 The severity of illness did not appear to correlate with the cutaneous manifestation, and the lesions healed within a few days.
One case report described a patient from Bangkok who was thought to be suffering from dengue fever, but was found to have SARS-CoV-2 infection. He initially presented with skin rash and petechiae, and later developed respiratory disease.23
Other dermatologic findings of COVID-19 resemble chilblains disease, colloquially referred to as “COVID toes.” Two women, 27 and 35 years old, presented to a dermatology clinic in Qatar with a chief complaint of skin rash, described as red-purple papules on the dorsal aspects of the fingers bilaterally.22 Both patients had an unremarkable medical and drug history, but recent travel to the United Kingdom dictated SARS-CoV-2 screening, which was positive.24 An Italian case report describes a 23-year-old man who tested positive for SARS-CoV-2 and had violaceous plaques on an erythematous background on his feet, without any lesions on his hands.25 Since chilblains is less common in the warmer months and these events correspond with the COVID-19 pandemic, SARS-CoV-2 infection is the suspected etiology. The pathophysiology of these lesions is unclear, and more research is needed. As more data become available, we may see cutaneous manifestations in patients with COVID-19 similar to those commonly reported with other viral infectious processes.
Musculoskeletal
Of 138 patients hospitalized in Wuhan, China, for COVID-19, 34.8% presented with myalgia; the presence of myalgia does not appear to be correlated with an increased likelihood of ICU admission.6 Myalgia or arthralgia was also reported in 14.9% among the cohort of 1099 COVID-19 patients in China.13 These musculoskeletal symptoms are described among large muscle groups found in the extremities, trunk, and back, and should raise suspicion in patients who present with other signs and symptoms concerning for COVID-19.
Conclusion
Evidence regarding atypical features of COVID-19 is accumulating. SARS-CoV-2 can infect a human cells that express the ACE2 receptor, which would allow for a broad spectrum of illnesses. The potential for SARS-CoV-2 to induce a hypercoagulable state allows it to indirectly damage various organ systems,20 leading to cerebrovascular disease, myocardial injury, and a chilblain-like rash. Clinicians must be aware of these unique features, as early recognition of persons who present with COVID-19 will allow for prompt testing, institution of infection control and isolation practices, and treatment, as needed, among those infected. Also, this is a pandemic involving a novel virus affecting different populations throughout the world, and these signs and symptoms may occur with varying frequency across populations. Therefore, it is important to keep differentials broad when assessing patients with a clinical illness that may indeed be COVID-19.
Corresponding author: Norman L. Beatty, MD, [email protected].
Financial disclosures: None.
From the University of Florida College of Medicine, Division of Infectious Diseases and Global Medicine, Gainesville, FL.
Abstract
- Objective: To review current reports on atypical manifestations of coronavirus disease 2019 (COVID-19).
- Methods: Review of the literature.
- Results: Evidence regarding atypical features of COVID-19 is accumulating. SARS-CoV-2 can infect human cells that express the angiotensin-converting enzyme 2 receptor, which would allow for a broad spectrum of illnesses affecting the renal, cardiac, and gastrointestinal organ systems. Neurologic, cutaneous, and musculoskeletal manifestations have also been reported. The potential for SARS-CoV-2 to induce a hypercoagulable state provides another avenue for the virus to indirectly damage various organ systems, as evidenced by reports of cerebrovascular disease, myocardial injury, and a chilblain-like rash in patients with COVID-19.
- Conclusion: Because the signs and symptoms of COVID-19 may occur with varying frequency across populations, it is important to keep differentials broad when assessing patients with a clinical illness that may indeed be COVID-19.
Keywords: coronavirus; severe acute respiratory syndrome coronavirus-2; SARS-CoV-2; pandemic.
Coronavirus disease 2019 (COVID-19), the syndrome caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), was first reported in Wuhan, China, in early December 2019.1 Since then, the virus has spread quickly around the world, with the World Health Organization (WHO) declaring the coronavirus outbreak a global pandemic on March 11, 2020. As of May 21, 2020, more than 5,000,000 cases of COVID-19 have been confirmed, and more than 328,000 deaths related to COVID-19 have been reported globally.2 These numbers are expected to increase, due to the reproduction number (R0) of SARS-CoV-2. R0 represents the number of new infections generated by an infectious person in a totally naïve population.3 The WHO estimates that the R0 of SARS-CoV-2 is 1.95, with other estimates ranging from 1.4 to 6.49.3 To control the pathogen, the R0 needs to be brought under a value of 1.
A fundamental tool in lowering the R0 is prompt testing and isolation of those who display signs and symptoms of infection. SARS-CoV-2 is still a novel pathogen about which we know relatively little. The common symptoms of COVID-19 are now well known—including fever, fatigue, anorexia, cough, and shortness of breath—but atypical manifestations of this viral continue to be reported and described. To help clinicians across specialties and settings identify patients with possible infection, we have summarized findings from current reports on COVID-19 manifestations involving the renal, cardiac, gastrointestinal (GI), and other organ systems.
Renal
During the 2003 SARS-CoV-1 outbreak, acute kidney injury (AKI) was an uncommon complication of the infection, but early reports suggest that AKI may occur more commonly with COVID-19.4 In a study of 193 patients with laboratory-confirmed COVID-19 treated in 3 Chinese hospitals, 59% presented with proteinuria, 44% with hematuria, 14% with increased blood urea nitrogen, and 10% with increased levels of serum creatinine.4 These markers, indicative of AKI, may be associated with increased mortality. Among this cohort, those with AKI had a mortality risk 5.3 times higher than those who did not have AKI.4 The pathophysiology of renal disease in COVID-19 may be related to dehydration or inflammatory mediators, causing decreased renal perfusion and cytokine storm, but evidence also suggests that SARS-CoV-2 is able to directly infect kidney cells.5 The virus infects cells by using angiotensin-converting enzyme 2 (ACE2) on the cell membrane as a cell entry receptor; ACE2 is expressed on the kidney, heart, and GI cells, and this may allow SARS-CoV-2 to directly infect and damage these organs. Other potential mechanisms of renal injury include overproduction of proinflammatory cytokines and administration of nephrotoxic drugs. No matter the mechanism, however, increased serum creatinine and blood urea nitrogen correlate with an increased likelihood of requiring intensive care unit (ICU) admission.6 Therefore, clinicians should carefully monitor renal function in patients with COVID-19.
Cardiac
In a report of 138 Chinese patients hospitalized for COVID-19, 36 required ICU admission: 44.4% of these had arrhythmias and 22.2% had developed acute cardiac injury.6 In addition, the cardiac cell injury biomarker troponin I was more likely to be elevated in ICU patients.6 A study of 21 patients admitted to the ICU in Washington State found elevated levels of brain natriuretic peptide.7 These biomarkers reflect the presence of myocardial stress, but do not necessarily indicate direct myocardial infection. Case reports of fulminant myocarditis in those with COVID-19 have begun to surface, however.8,9 An examination of 68 deaths in persons with COVID-19 concluded that 7% were caused by myocarditis with circulatory failure.10
The pathophysiology of myocardial injury in COVID-19 is likely multifactorial. This includes increased inflammatory mediators, hypoxemia, and metabolic changes that can directly damage myocardial tissue. These factors can also exacerbate comorbid conditions, such as coronary artery disease, leading to ischemia and dysfunction of preexisting electrical conduction abnormalities. However, pathologic evidence of myocarditis and the presence of the ACE2 receptor, which may be a mediator of cardiac function, on cardiac muscle cells suggest that SARS-CoV-2 is capable of directly infecting and damaging myocardial cells. Other proposed mechanisms include infection-mediated downregulation of ACE2, causing cardiac dysfunction, or thrombus formation.11 Although respiratory failure is the most common source of advanced illness in COVID-19 patients, myocarditis and arrhythmias can be life-threatening manifestations of the disease.
Gastrointestinal
As noted, ACE2 is expressed in the GI tract. In 73 patients hospitalized for COVID-19, 53.4% tested positive for SARS-CoV-2 RNA in stool, and 23.4% continued to have RNA-positive stool samples even after their respiratory samples tested negative.12 These findings suggest the potential for SARS-CoV-2 to spread through fecal-oral transmission in those who are asymptomatic, pre-symptomatic, or symptomatic. This mode of transmission has yet to be determined conclusively, and more research is needed. However, GI symptoms have been reported in persons with COVID-19. Among 138 hospitalized patients, 10.1% had complaints of diarrhea and nausea and 3.6% reported vomiting.6 Those who reported nausea and diarrhea noted that they developed these symptoms 1 to 2 days before they developed fever.6 Also, among a cohort of 1099 Chinese patients with COVID-19, 3.8% complained of diarrhea.13 Although diarrhea does not occur in a majority of patients, GI complaints, such as nausea, vomiting, or diarrhea, should raise clinical suspicion for COVID-19, and in known areas of active transmission, testing of patients with GI symptoms is likely warranted.
Ocular
Ocular manifestations of COVID-19 are now being described, and should be taken into consideration when examining a patient. In a study of 38 patients with COVID-19 from Hubei province, China, 31.6% had ocular findings consistent with conjunctivitis, including conjunctival hyperemia, chemosis, epiphora, and increased ocular secretions.14 SARS-CoV-2 was detected in conjunctival and nasopharyngeal samples in 2 patients from this cohort. Conjunctival congestion was reported in a cohort of 1099 patients with COVID-19 treated at multiple centers throughout China, but at a much lower incidence, approximately 0.8%.13 Because SARS-CoV-2 can cause conjunctival disease and has been detected in samples from the external surface of the eye, it appears the virus is transmissible from tears or contact with the eye itself.
Neurologic
Common reported neurologic symptoms include dizziness, headache, impaired consciousness, ataxia, and cerebrovascular events. In a cohort of 214 patients from Wuhan, China, 36.4% had some form of neurological insult.15 These symptoms were more common in those with severe illness (P = 0.02).15 Two interesting neurologic symptoms that have been described are anosmia (loss of smell) and ageusia (loss of taste), which are being found primarily in tandem. It is still unclear how many people with COVID-19 are experiencing these symptoms, but a report from Italy estimates 19.4% of 320 patients examined had chemosensory dysfunction.16 The aforementioned report from Wuhan, China, found that 5.1% had anosmia and 5.6% had ageusia.15 The presence of anosmia/ageusia in some patients suggests that SARS-CoV-2 may enter the central nervous system (CNS) through a retrograde neuronal route.15 In addition, a case report from Japan described a 24-year-old man who presented with meningitis/encephalitis and had SARS-CoV-2 RNA present in his cerebrospinal fluid, showing that SARS-CoV-2 can penetrate into the CNS.17
SARS-CoV-2 may also have an association with Guillain–Barré syndrome, as this condition was reported in 5 patients from 3 hospitals in Northern Italy.18 The symptoms of Guillain–Barré syndrome presented 5 to 10 days after the typical COVID-19 symptoms, and evolved over 36 hours to 4 days afterwards. Four of the 5 patients experienced flaccid tetraparesis or tetraplegia, and 3 required mechanical ventilation.18
Another possible cause of neurologic injury in COVID-19 is damage to endothelial cells in cerebral blood vessels, causing thrombus formation and possibly increasing the risk of acute ischemic stroke.15,19 Supporting this mechanism of injury, significantly lower platelet counts were noted in patients with CNS symptoms (P = 0.005).15 Other hematological impacts of COVID-19 have been reported, particularly hypercoagulability, as evidenced by elevated D-dimer levels.13,20 This hypercoagulable state is linked to overproduction of proinflammatory cytokines (cytokine storm), leading to dysregulation of coagulation pathways and reduced concentrations of anticoagulants, such as protein C, antithrombin III, and tissue factor pathway inhibitor.21
Cutaneous
Cutaneous findings emerging in persons with COVID-19 demonstrate features of small-vessel and capillary occlusion, including erythematous skin eruptions and petechial rash. One report from Italy noted that 20.4% of patients with COVID-19 (n = 88) had a cutaneous finding, with a cutaneous manifestation developing in 8 at the onset of illness and in 10 following hospital admission.22 Fourteen patients had an erythematous rash, primarily on the trunk, with 3 patients having a diffuse urticarial appearing rash, and 1 patient developing vesicles.22 The severity of illness did not appear to correlate with the cutaneous manifestation, and the lesions healed within a few days.
One case report described a patient from Bangkok who was thought to be suffering from dengue fever, but was found to have SARS-CoV-2 infection. He initially presented with skin rash and petechiae, and later developed respiratory disease.23
Other dermatologic findings of COVID-19 resemble chilblains disease, colloquially referred to as “COVID toes.” Two women, 27 and 35 years old, presented to a dermatology clinic in Qatar with a chief complaint of skin rash, described as red-purple papules on the dorsal aspects of the fingers bilaterally.22 Both patients had an unremarkable medical and drug history, but recent travel to the United Kingdom dictated SARS-CoV-2 screening, which was positive.24 An Italian case report describes a 23-year-old man who tested positive for SARS-CoV-2 and had violaceous plaques on an erythematous background on his feet, without any lesions on his hands.25 Since chilblains is less common in the warmer months and these events correspond with the COVID-19 pandemic, SARS-CoV-2 infection is the suspected etiology. The pathophysiology of these lesions is unclear, and more research is needed. As more data become available, we may see cutaneous manifestations in patients with COVID-19 similar to those commonly reported with other viral infectious processes.
Musculoskeletal
Of 138 patients hospitalized in Wuhan, China, for COVID-19, 34.8% presented with myalgia; the presence of myalgia does not appear to be correlated with an increased likelihood of ICU admission.6 Myalgia or arthralgia was also reported in 14.9% among the cohort of 1099 COVID-19 patients in China.13 These musculoskeletal symptoms are described among large muscle groups found in the extremities, trunk, and back, and should raise suspicion in patients who present with other signs and symptoms concerning for COVID-19.
Conclusion
Evidence regarding atypical features of COVID-19 is accumulating. SARS-CoV-2 can infect a human cells that express the ACE2 receptor, which would allow for a broad spectrum of illnesses. The potential for SARS-CoV-2 to induce a hypercoagulable state allows it to indirectly damage various organ systems,20 leading to cerebrovascular disease, myocardial injury, and a chilblain-like rash. Clinicians must be aware of these unique features, as early recognition of persons who present with COVID-19 will allow for prompt testing, institution of infection control and isolation practices, and treatment, as needed, among those infected. Also, this is a pandemic involving a novel virus affecting different populations throughout the world, and these signs and symptoms may occur with varying frequency across populations. Therefore, it is important to keep differentials broad when assessing patients with a clinical illness that may indeed be COVID-19.
Corresponding author: Norman L. Beatty, MD, [email protected].
Financial disclosures: None.
1. WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020 [press release]. World Health Organization; March 11, 2020.
2. Coronavirus COVID-19 Global Cases by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. Johns Hopkins CSSE. https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6 Accessed May 15, 2020.
3. Liu Y, Gayle AA, Wilder-Smith A, Rocklöv J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Travel Med. 2020;27(2):taaa021. doi:10.1093/jtm/taaa021
4. Li Z, Wu M, Guo J, et al. Caution on kidney dysfunctions of 2019-nCoV patients. medRxiv preprint. doi: 10.1101/2020.02.08.20021212
5. Li W, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450-454. doi: 10.1038/nature02145.
6. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. doi:10.1001/jama.2020.1585
7. Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323:1612‐1614. doi:10.1001/jama.2020.4326
8. Chen C, Zhou Y, Wang DW. SARS-CoV-2: a potential novel etiology of fulminant myocarditis. Herz. 2020;45:230-232. doi: 10.1007/s00059-020-04909-z
9. Hu H, Ma F, Wei X, Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. 2020 Mar 16;ehaa190. doi: 10.1093/eurheartj/ehaa190
10. Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846-848. doi:10.1007/s00134-020-05991-x
11. Akhmerov A, Marban E. COVID-19 and the heart. Circ Res. 2020;126:1443-1455. doi:10.1161/CIRCRESAHA.120.317055
12. Xiao F, Tang M, Zheng X, et al. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158:1831-1833. doi: 10.1053/j.gastro.2020.02.055
13. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1078-1720. doi: 10.1056/NEJMoa2002032
14. Wu P, Duan F, Luo C, et al. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020 Mar 31;e201291. doi: 10.1001/jamaophthalmol.2020.1291
15. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020 Apr 10. doi: 10.1001/jamaneurol.2020.1127
16. Vaira LA, Salzano G, Deiana G, De Riu G. Anosmia and ageusia: common findings in COVID-19 patients. Laryngoscope. 2020 Apr 1. doi: 10.1002/lary.28692
17. Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS-coronavirus-2. Int J Infect Dis. 2020;94:55-58. doi: 10.1016/j.ijid.2020.03.062
18. Toscano G, Palmerini F, Ravaglia S, et al. Guillain–Barré syndrome associated with SARS-CoV-2. N Engl J Med. 2020 Apr 17;NEJMc2009191. doi:10.1056/nejmc2009191
19. Dafer RM, Osteraas ND, Biller J. Acute stroke care in the coronavirus disease 2019 pandemic. J Stroke Cerebrovascular Dis. 2020 Apr 17:104881. doi: 10.1016/j.jstrokecerebrovasdis.2020.104881
20. Terpos E, Ntanasis-Stathopoulos I, Elalamy I, et al. Hematological findings and complications of COVID-19. Am J Hematol. 2020;10.1002/ajh.25829. doi:10.1002/ajh.25829
21. Jose RJ, Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. 2020;S2213-2600(20)30216-2. doi:10.1016/S2213-2600(20)30216-2
22. Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020 Mar 26. doi: 10.1111/jdv.16387
23. Joob B, Wiwanitkit V. COVID-19 can present with a rash and be mistaken for dengue. J Am Acad Dermatol. 2020;82(5):e177. doi: 10.1016/j.jaad.2020.03.036
24. Alramthan A, Aldaraji W. A Case of COVID‐19 presenting in clinical picture resembling chilblains disease. First report from the Middle East. Clin Exp Dermatol. 2020 Apr 17. doi: 10.1111/ced.14243
25. Kolivras A, Dehavay F, Delplace D, et al. Coronavirus (COVID-19) infection–induced chilblains: a case report with histopathologic findings. JAAD Case Rep. 2020 Apr 18. doi: 10.1016/j.jdcr.2020.04.011
1. WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020 [press release]. World Health Organization; March 11, 2020.
2. Coronavirus COVID-19 Global Cases by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. Johns Hopkins CSSE. https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6 Accessed May 15, 2020.
3. Liu Y, Gayle AA, Wilder-Smith A, Rocklöv J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Travel Med. 2020;27(2):taaa021. doi:10.1093/jtm/taaa021
4. Li Z, Wu M, Guo J, et al. Caution on kidney dysfunctions of 2019-nCoV patients. medRxiv preprint. doi: 10.1101/2020.02.08.20021212
5. Li W, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450-454. doi: 10.1038/nature02145.
6. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. doi:10.1001/jama.2020.1585
7. Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323:1612‐1614. doi:10.1001/jama.2020.4326
8. Chen C, Zhou Y, Wang DW. SARS-CoV-2: a potential novel etiology of fulminant myocarditis. Herz. 2020;45:230-232. doi: 10.1007/s00059-020-04909-z
9. Hu H, Ma F, Wei X, Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. 2020 Mar 16;ehaa190. doi: 10.1093/eurheartj/ehaa190
10. Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846-848. doi:10.1007/s00134-020-05991-x
11. Akhmerov A, Marban E. COVID-19 and the heart. Circ Res. 2020;126:1443-1455. doi:10.1161/CIRCRESAHA.120.317055
12. Xiao F, Tang M, Zheng X, et al. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158:1831-1833. doi: 10.1053/j.gastro.2020.02.055
13. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1078-1720. doi: 10.1056/NEJMoa2002032
14. Wu P, Duan F, Luo C, et al. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020 Mar 31;e201291. doi: 10.1001/jamaophthalmol.2020.1291
15. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020 Apr 10. doi: 10.1001/jamaneurol.2020.1127
16. Vaira LA, Salzano G, Deiana G, De Riu G. Anosmia and ageusia: common findings in COVID-19 patients. Laryngoscope. 2020 Apr 1. doi: 10.1002/lary.28692
17. Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS-coronavirus-2. Int J Infect Dis. 2020;94:55-58. doi: 10.1016/j.ijid.2020.03.062
18. Toscano G, Palmerini F, Ravaglia S, et al. Guillain–Barré syndrome associated with SARS-CoV-2. N Engl J Med. 2020 Apr 17;NEJMc2009191. doi:10.1056/nejmc2009191
19. Dafer RM, Osteraas ND, Biller J. Acute stroke care in the coronavirus disease 2019 pandemic. J Stroke Cerebrovascular Dis. 2020 Apr 17:104881. doi: 10.1016/j.jstrokecerebrovasdis.2020.104881
20. Terpos E, Ntanasis-Stathopoulos I, Elalamy I, et al. Hematological findings and complications of COVID-19. Am J Hematol. 2020;10.1002/ajh.25829. doi:10.1002/ajh.25829
21. Jose RJ, Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. 2020;S2213-2600(20)30216-2. doi:10.1016/S2213-2600(20)30216-2
22. Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020 Mar 26. doi: 10.1111/jdv.16387
23. Joob B, Wiwanitkit V. COVID-19 can present with a rash and be mistaken for dengue. J Am Acad Dermatol. 2020;82(5):e177. doi: 10.1016/j.jaad.2020.03.036
24. Alramthan A, Aldaraji W. A Case of COVID‐19 presenting in clinical picture resembling chilblains disease. First report from the Middle East. Clin Exp Dermatol. 2020 Apr 17. doi: 10.1111/ced.14243
25. Kolivras A, Dehavay F, Delplace D, et al. Coronavirus (COVID-19) infection–induced chilblains: a case report with histopathologic findings. JAAD Case Rep. 2020 Apr 18. doi: 10.1016/j.jdcr.2020.04.011
Use of an Electronic Alert Tool to Prevent Readmissions Following Coronary Artery Bypass Graft Surgery
From the University of North Carolina at Wilmington School of Nursing (Dr. Smith and Dr. Turrise), the New Hanover Regional Medical Center Heart Center (Mr. Jordan), the Coastal Carolinas Health Alliance and Coastal Connect Health Information Exchange (Ms. Robertson), and Coastal Thoracic Surgical Associates (Dr. Kane), Wilmington, NC.
Abstract
Objective: Cardiothoracic (CT) surgeons at our medical center were not receiving timely notification when their coronary artery bypass graft (CABG) surgery patients were admitted to the medical center or to other hospitals. The CT surgical team worked with a health alliance in southeastern North Carolina to implement health information exchange (HIE) real-time electronic notifications for their CABG patients who presented to the hospital’s emergency department (ED) or any ED affiliated with the medical center. The alert tool notifies team members about patient encounters, driving timely clinical engagement.
Methods: The CT team provided the HIE team with the names of CABG surgery patients, which were loaded into the alert tool. When a patient on the list presented to the hospital ED or its affiliates, the alert tool sent a real-time electronic notification to the Cardiac Surgical Services nurse coordinator. This intervention prompted the assessment and disposition of CABG patients, while in the ED, by the CT surgical team.
Results: Over a 16-month period (September 2017-December 2018), the names of 614 post-CABG patients were input into the HIE for tracking. Of these patients, 47 were treated and discharged from the ED; 31 were admitted for observation; 44 were readmitted for inpatient care; and 492 did not have a qualifying event requiring a notification alert. Following implementation of this practice change, the 30-day readmission rate for patients who underwent CABG at our institution decreased from 10% to 7.2%.
Conclusion: Utilizing a real-time alert tool resulted in immediate notification of the CT team when 1 of their patients presented to the ED. This afforded the CT surgical team an opportunity to intervene in the care of their patients, which in turn led to improved quality of care, physician communication and collaboration, and patient outcomes, such as preventable 30-day readmissions.
Keywords: electronic health record; real-time electronic notification; CABG; process improvement.
Unplanned 30-day hospital readmissions of patients who have undergone coronary artery bypass graft (CABG) surgery contribute to higher overall health care costs. CABG is 1 of the conditions/procedures that the Centers for Medicare and Medicaid Services (CMS) monitors for excess readmissions.1 Readmission rates for CABG-related conditions at 30 days post-surgery are reported to be between 16% and 20% for US hospitals.2 Readmissions are not only financially costly, but also have been associated with worse patient outcomes and decreased patient satisfaction.3 Common diagnoses for post-CABG admission include atrial fibrillation, pleural effusion, and wound infection.
The facility where this project was implemented had a 10% post-CABG admission rate for patients across all payers. While this rate is below the national average of 13.2%, the cardiothoracic (CT) surgical team was not being notified in a timely manner when their post-CABG patients were readmitted. The Lean team used the A3 problem-solving process to develop strategies that would reduce these readmissions and improve the care of their patients.
We explored the use of electronic alerts in managing post-CABG patients by conducting a literature search using the terms electronic alerts in patient care, patient engagement in the emergency department, electronic alerts in CABG, real-time notifications to prevent readmission, and CABG readmission. Databases searched were PubMed, Google Scholar, Cumulative Index of Nursing and Allied Health Literature, ProQuest, and ScienceDirect. This search resulted in studies focused on the use of electronic health record (EHR) alerts as a clinical decision-support tool; for example, patient demographic and assessment data are entered into the EHR, and the clinician is prompted with “performance” recommendations (eg, consider electrocardiogram and aspirin).4 In a paper by Engelman and Benjamin,5 the authors discuss the importance of the engaged physician and note that, in their emergency department (ED), an electronic notification is sent when a postoperative patient presents; however, the notification goes to the inpatient service for timely review and disposition. There was no literature that discussed the use of an electronic alert tool as a real-time patient engagement strategy that resulted in a practice change specific to the CT surgical team.
Our process improvement project focused on alerting the CT surgical team when a post-CABG patient presented to the ED, allowing them to evaluate the patient in real time and determine whether the chief complaint was related to the CABG and whether further evaluation by the CT surgeon was required. Specifically, we wanted to determine whether a real-time electronic alert that notified the CT surgical team about post-op CABG patients presenting to the ED would result in timely patient engagement, avoidance of readmissions, and an enhanced patient experience. During this project, alerts were sent to the CT surgical team notifying them of a post-CABG patient presenting to the ED or being directly admitted from home on physician orders, a provider’s office, or inpatient rehabilitation; however, the focus of this article is specifically on the notification regarding post-CABG patients presenting to the ED.
Prior to implementing the electronic notification project, the team developed and implemented several internal and external readmission reduction and prevention strategies for CABG patients. An in-house strategy involved a process whereby patients would receive their discharge medications prior to being discharged from the hospital post-CABG, thereby avoiding potential delays in the patient obtaining medications. When examining post-CABG patient readmissions, the primary conditions that led to readmission were fluid overload, pleural effusion, and atrial fibrillation. As such, a second in-house strategy was developed for post-CABG patients presenting to the ED with atrial fibrillation. The newly established protocol allowed patients to be monitored and treated in the cardiac observation unit. In addition, external strategies, including an outpatient furosemide protocol for home health nurses and an outpatient thoracentesis program and order set, were established (eg, for patients with congestive heart failure, shortness of breath).
Methods
Setting
The regional medical center where this project was implemented is the ninth largest hospital in North Carolina and the largest county-owned public hospital in the state. It is a tertiary care center and teaching hospital with 3 hospital campuses and 855 licensed beds. The medical center was included in the 100 Safecare Hospitals list by the Safecare Group; received a grade “A” Hospital Safety Score from the Leapfrog Group; and is 1 of America’s Top 100 Hospitals for Patient Experience.
Real-Time Notification Project
A regional hospital alliance in southeastern North Carolina established a health information exchange (HIE) with its member hospitals and office-based physicians to enable electronic exchange of patient information to improve quality, safety, and efficiency in health care delivery. Our medical center is part of this alliance. The HIE is a digital platform that facilitates the sharing of information between disparate connected EHR systems, and offers a portal for practices and hospitals to access patient information across North Carolina, South Carolina (via SC HIE), and nationwide (select dialysis centers). More specifically, approved providers and team members are able to access, in real time, patient-care encounter documents from other care settings (eg, acute, post-acute, ambulatory) via the HIE. Additionally, approved care entities can query-retrieve web portal information to support patient outcome improvement strategies. A partnership discussion highlighted the opportunity to utilize the HIE’s capabilities, such as real-time notification, to facilitate workflow (eg, when a patient presents to the ED, the HIE can provide access to health information at the point of care). In this capacity, the alert tool notifies care team members about patient encounters to drive timely clinical engagement for care transitions.
In January 2017, we began discussions on using the HIE to facilitate real-time electronic tracking in the Cardiac Surgical Services department at our medical center. Persons involved in these discussions included the cardiovascular (CV) team (comprised of case managers, department managers and coordinators, program coordinators, administrators, and support services [eg, pre-admission testing and home health staff]) and CT surgeons. At that time, CABG readmissions were manually tracked, and the real-time notification tool was being used in other departments (eg, in case management for tracking readmissions). The entire team was part of the initial decision meeting to pursue this possibility. The CV team reached consensus in June 2017 and proposed extending the use of the alert tool to the post-CABG population presenting to the ED (or any ED affiliated with the medical center) or admitted directly to the medical center.
The HIE staff met with the Cardiac Surgical Services team to tailor and develop the logistics of the project, such as who would be notified and how. The goals of the project were to support appropriate care intervention, reduce preventable hospital readmissions, and improve quality of care through enhanced provider communication and engagement. To achieve these goals, on the day of discharge the Cardiac Surgical Services coordinator provided the HIE team with the names of patients who had undergone CABG surgery. This patient list was loaded into the alert tool and continually updated. At 31 days, patient names were removed from the list. When a patient on the list presented to the hospital ED, the alert tool sent 2 real-time electronic notifications, an email and a text message, to the Cardiac Surgical Services coordinator, noting that a patient event occurred. Personal information was not included in the alert in order to protect patient information and comply with Health Insurance Portability and Accountability Act regulations.
The alert prompted the Cardiac Surgical Services coordinator to securely access patient information to identify and, if necessary, visit the patient. Then, based on the information gathered by the Cardiac Surgical Services coordinator, a Situation-Background-Assessment-Recommendation report was relayed to the CT surgeon, who then determined whether intervention by the CT surgical team was warranted. This process, on average, took approximately 30 minutes to complete. This was a key change in processes, one that allowed post-CABG patients to be seen by the CT surgical team while in the ED. If the issue was related to the CABG surgery, the CT surgeons could then determine an appropriate course of action, including admission or implementation of another protocol, such as the home furosemide protocol. For patients directly admitted, the surgeon contacted the admitting provider to discuss the level of care required (ie, observation or inpatient admission and treatment).
Biweekly CV team meetings were conducted during the implementation of the real-time notification alert tool. At each meeting, updates were provided on notifications received, patients who were missed by the notification process, and how well the real-time alerts were working to enhance care and appropriate disposition.
Measurements
Clinical performance data included total notifications, total number of ED visits, ED disposition (inpatient admission, observation, discharge), total number of direct admissions, direct admissions to observation, direct inpatient admissions, and patients missed by the notification process (eg, due to data entry errors, omissions of information [suffix of junior or senior], as well as programming bugs). Finally, the number of observation admissions converted to inpatient admissions was collected and further analyzed to inform needed process changes.
The Cardiac Surgical Services coordinator collected, entered, and maintained data using Excel. Data were obtained from the EHR, recorded in Excel, and analyzed using basic descriptive statistics in an ongoing fashion. Particular attention was focused on problems with the notification process (eg, patients being missed due to errors in data entry) and summarizing information to keep the Cardiac Surgical Services team updated on the progress of the process improvement. This project did not require staff protections or considerations, and because this was not a research study Institutional Review Board approval was not required.
Results
This practice change was implemented in September 2017 and led to improvements in care quality, as evidenced by improved physician communication and collaboration. In the 16-month period from implementation through December 2018, the names of 614 post-CABG patients were input into the HIE for tracking. Of these patients, 47 were treated and discharged from the ED; 31 were admitted for observation; and 44 were readmitted for inpatient care. The remaining 492 patients did not have a qualifying event requiring a notification alert. Clinical performance data from this period included 70 ED visits, 21 direct admissions, 19 direct admissions to observation, 5 patients missed by the notification process, and 4 observation admissions converted to inpatient admissions. A reduction in the CABG readmission rate from 10% in September 2017 to 7.2% in December 2018 was also noted.
Discussion
The aim of this process improvement project was to determine whether a real-time electronic alert that notified the CT surgical team about post-op CABG patients presenting to the ED would result in timely patient engagement, avoidance of readmissions, and an enhanced patient experience. This practice change has been successful, following 16 months of implementation and process refinement. Integrating a real-time electronic alert with a supporting action plan and care protocols resulted in timely patient engagement and avoidance of readmission of post-CABG patients.
Early notification of possible post-CABG readmissions became a standard-of-care process within the Cardiac Surgical Services department, with expansion to all CT post-op patients. Leveraging HIE technology to support quality improvement processes was also viewed by other departments as relevant and beneficial. For example, the hospital stroke and orthopedic-spine teams established their own processes for receiving real-time alerts.
There were several lessons learned during this project. First, gaining 100% physician buy-in to collaborative communication proved to be critical to the project’s success. The CV team was surprised by the length of time (approximately 8-10 months) it took for the practice change to be adopted by the physicians. In part, some of this delay in adoption resulted from medical staff turnover, primarily in the medical resident training rotations. Collaborative communication was key. The CT surgeons spoke with ED leadership and hospitalist services to explain the readmission reduction project and the use of an electronic alert tool. The CT surgeons also communicated to the ED physicians, hospitalists, and cardiologists that the Cardiac Surgical Services coordinator would be involved in the process and discussions regarding patientss care. Additionally, the CT surgeons authored the furosemide protocol and then committed to its use in the home health setting, further highlighting the role of collaborative communication in avoiding readmissions.
Another key step in this quality improvement project was determining who should receive the alert notifications. At the onset of the project, all notifications were sent to 1 person, the Cardiac Surgical Services coordinator. While this seemed logical in the initial stage of the project, it was unsustainable, as the receipt of the alert and the subsequent notification of the CT surgeon depended on 1 person and their availability. Approximately 10 months into the project, the notification process was further refined, with the cardiovascular intensive care unit charge nurse becoming the point of contact for the alerts. The Cardiac Surgical Services coordinator, in collaboration with nursing leaders and CT surgeons, completed a Lean Standard Work template outlining the major steps and the associated responsibilities (for the cardiovascular intensive care unit charge nurse, CT surgeon and on-call surgeon, Cardiac Surgical Services coordinator) in the process of receiving notifications, collecting patient assessment data, and reporting notifications to the CT surgeons.
Establishing adequate support mechanisms during a practice change is also important. For instance, we had to dedicate personnel time for data collection and analysis and involve additional nursing or other qualified personnel in the new process to avoid depending on a single person for the project’s success. Additional considerations were establishing criteria for surgeon notification and defining an appropriate time frame for notification (eg, urgent versus next-day notifications). We accomplished these activities approximately 10 months into the project, after it became apparent at CV team meeting discussions that further clarification of criteria and timelines was needed.
Some aspects of the project unfolded as planned, while others presented opportunities for improvement. For example, the alert notification process worked as envisioned; however, as previously mentioned, the process needed to be more inclusive to ensure there is always a charge nurse on duty to receive the alert notification, rather than just the Cardiac Surgical Services coordinator, who may not always be at the hospital. The outpatient thoracentesis program was well planned and effectively implemented. This program provided an avenue for patients who had symptoms of pleural effusion to be treated in an outpatient setting, rather than requiring an inpatient stay. Opportunities for improvement included addressing the inconsistent use of the home health furosemide protocol (developed in 2016), and the need for continued interprofessional and interdepartmental communication and coordination. For example, we had to inform the ED physicians and staff who rotate or are new to the ED about established processes and protocols in place for managing post-CABG patients who present to the ED.
The primary limitation of this project was the inability to measure the enhanced patient experience, which was 1 of the stated project goals. This goal became secondary because of more pressing issues, specifically, interorganizational collaboration (eg, hospital EHR, HIE, and CT surgical team) and tailoring the functionality of the electronic alert tool to the project. Developing and implementing measures of enhanced patient experience were not feasible during this implementation. Additionally, because this was not a research study, it was not possible to determine cause and effect or to control for confounders, such as a sicker, older cohort with more comorbid conditions, during the comparison period. Finally, although this process improvement project was conducted at a regional medical center that is the only facility performing CABG within the region, patients may have presented to another facility for an event that led to a readmission. Because readmissions to other facilities could not be captured, it is possible that the actual readmission rate was higher than the rate reported here.
Conclusions and Implications
Utilizing a real-time alert from the HIE to the CT surgical team resulted in CT surgeons being immediately made aware when their patients presented to the ED, allowing the CT surgical team the opportunity to intervene, as appropriate, in the care of their patients. Furthermore, this real-time notification and intervention resulted in timely patient engagement and, in some cases, avoidance of readmissions. Currently, patients are monitored for readmission within 30 days of discharge. In the future, the time will expand to 91 days, in preparation for participation in the CMS bundle payment program for CABG surgery.
This practice change can be used in organizations that do not have or participate in a HIE. In fact, these real-time alert applications may be available through an EHR already in use within the organization. The use of the alert requires collaborative communication and having supporting protocols in place to guide decision-making and care of post-CABG patients presenting to the ED.
There appears to be a gap in the literature discussing the use of an electronic alert tool as a real-time patient engagement strategy for post-CABG patients presenting to the ED. As such, this project contributes important results and lessons learned for other hospital service lines/departments that might consider implementing a similar process. Next steps include designing and conducting methodologically rigorous research studies based on this process improvement project to examine mortality rates as an outcome, and designing a more specific measure of patient experience, as the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey only provides hospital-level data.
Corresponding author: Stephanie D. Smith, PhD, RN, UNCW School of Nursing, 601 South College Road, Wilmington, NC 28403; [email protected].
Funding disclosures: None.
1. Hannan EL, Zhong Y, Lahey SJ, et al. 30-day readmissions after coronary artery bypass graft surgery in New York State. JACC Cardiovasc Interv. 2011;4:569-576.
2. Feng TR, White R, Gaber-Baylis L, et al. Coronary artery bypass graft readmission rates and risk factors- A retrospective cohort study. Int J Surg. 2018;54 (Part A):7-17.
3. Donndorf P, Kaminski A. “Return to sender” or “consider it done”?! The importance of reducing hospital readmission after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2017;154:1298-1299.
4. Sequist TD, Morong SM, Marston A, et al. Electronic risk alerts to improve primary care management of chest pain: A randomized, controlled trial. J Gen Intern Med. 2012;27:438-444.
5. Engelman D, Benjamin EM. Physician engagement: The “secret sauce” to success in bundled health care. Am J Med Qual. 2018;33:100-102.
From the University of North Carolina at Wilmington School of Nursing (Dr. Smith and Dr. Turrise), the New Hanover Regional Medical Center Heart Center (Mr. Jordan), the Coastal Carolinas Health Alliance and Coastal Connect Health Information Exchange (Ms. Robertson), and Coastal Thoracic Surgical Associates (Dr. Kane), Wilmington, NC.
Abstract
Objective: Cardiothoracic (CT) surgeons at our medical center were not receiving timely notification when their coronary artery bypass graft (CABG) surgery patients were admitted to the medical center or to other hospitals. The CT surgical team worked with a health alliance in southeastern North Carolina to implement health information exchange (HIE) real-time electronic notifications for their CABG patients who presented to the hospital’s emergency department (ED) or any ED affiliated with the medical center. The alert tool notifies team members about patient encounters, driving timely clinical engagement.
Methods: The CT team provided the HIE team with the names of CABG surgery patients, which were loaded into the alert tool. When a patient on the list presented to the hospital ED or its affiliates, the alert tool sent a real-time electronic notification to the Cardiac Surgical Services nurse coordinator. This intervention prompted the assessment and disposition of CABG patients, while in the ED, by the CT surgical team.
Results: Over a 16-month period (September 2017-December 2018), the names of 614 post-CABG patients were input into the HIE for tracking. Of these patients, 47 were treated and discharged from the ED; 31 were admitted for observation; 44 were readmitted for inpatient care; and 492 did not have a qualifying event requiring a notification alert. Following implementation of this practice change, the 30-day readmission rate for patients who underwent CABG at our institution decreased from 10% to 7.2%.
Conclusion: Utilizing a real-time alert tool resulted in immediate notification of the CT team when 1 of their patients presented to the ED. This afforded the CT surgical team an opportunity to intervene in the care of their patients, which in turn led to improved quality of care, physician communication and collaboration, and patient outcomes, such as preventable 30-day readmissions.
Keywords: electronic health record; real-time electronic notification; CABG; process improvement.
Unplanned 30-day hospital readmissions of patients who have undergone coronary artery bypass graft (CABG) surgery contribute to higher overall health care costs. CABG is 1 of the conditions/procedures that the Centers for Medicare and Medicaid Services (CMS) monitors for excess readmissions.1 Readmission rates for CABG-related conditions at 30 days post-surgery are reported to be between 16% and 20% for US hospitals.2 Readmissions are not only financially costly, but also have been associated with worse patient outcomes and decreased patient satisfaction.3 Common diagnoses for post-CABG admission include atrial fibrillation, pleural effusion, and wound infection.
The facility where this project was implemented had a 10% post-CABG admission rate for patients across all payers. While this rate is below the national average of 13.2%, the cardiothoracic (CT) surgical team was not being notified in a timely manner when their post-CABG patients were readmitted. The Lean team used the A3 problem-solving process to develop strategies that would reduce these readmissions and improve the care of their patients.
We explored the use of electronic alerts in managing post-CABG patients by conducting a literature search using the terms electronic alerts in patient care, patient engagement in the emergency department, electronic alerts in CABG, real-time notifications to prevent readmission, and CABG readmission. Databases searched were PubMed, Google Scholar, Cumulative Index of Nursing and Allied Health Literature, ProQuest, and ScienceDirect. This search resulted in studies focused on the use of electronic health record (EHR) alerts as a clinical decision-support tool; for example, patient demographic and assessment data are entered into the EHR, and the clinician is prompted with “performance” recommendations (eg, consider electrocardiogram and aspirin).4 In a paper by Engelman and Benjamin,5 the authors discuss the importance of the engaged physician and note that, in their emergency department (ED), an electronic notification is sent when a postoperative patient presents; however, the notification goes to the inpatient service for timely review and disposition. There was no literature that discussed the use of an electronic alert tool as a real-time patient engagement strategy that resulted in a practice change specific to the CT surgical team.
Our process improvement project focused on alerting the CT surgical team when a post-CABG patient presented to the ED, allowing them to evaluate the patient in real time and determine whether the chief complaint was related to the CABG and whether further evaluation by the CT surgeon was required. Specifically, we wanted to determine whether a real-time electronic alert that notified the CT surgical team about post-op CABG patients presenting to the ED would result in timely patient engagement, avoidance of readmissions, and an enhanced patient experience. During this project, alerts were sent to the CT surgical team notifying them of a post-CABG patient presenting to the ED or being directly admitted from home on physician orders, a provider’s office, or inpatient rehabilitation; however, the focus of this article is specifically on the notification regarding post-CABG patients presenting to the ED.
Prior to implementing the electronic notification project, the team developed and implemented several internal and external readmission reduction and prevention strategies for CABG patients. An in-house strategy involved a process whereby patients would receive their discharge medications prior to being discharged from the hospital post-CABG, thereby avoiding potential delays in the patient obtaining medications. When examining post-CABG patient readmissions, the primary conditions that led to readmission were fluid overload, pleural effusion, and atrial fibrillation. As such, a second in-house strategy was developed for post-CABG patients presenting to the ED with atrial fibrillation. The newly established protocol allowed patients to be monitored and treated in the cardiac observation unit. In addition, external strategies, including an outpatient furosemide protocol for home health nurses and an outpatient thoracentesis program and order set, were established (eg, for patients with congestive heart failure, shortness of breath).
Methods
Setting
The regional medical center where this project was implemented is the ninth largest hospital in North Carolina and the largest county-owned public hospital in the state. It is a tertiary care center and teaching hospital with 3 hospital campuses and 855 licensed beds. The medical center was included in the 100 Safecare Hospitals list by the Safecare Group; received a grade “A” Hospital Safety Score from the Leapfrog Group; and is 1 of America’s Top 100 Hospitals for Patient Experience.
Real-Time Notification Project
A regional hospital alliance in southeastern North Carolina established a health information exchange (HIE) with its member hospitals and office-based physicians to enable electronic exchange of patient information to improve quality, safety, and efficiency in health care delivery. Our medical center is part of this alliance. The HIE is a digital platform that facilitates the sharing of information between disparate connected EHR systems, and offers a portal for practices and hospitals to access patient information across North Carolina, South Carolina (via SC HIE), and nationwide (select dialysis centers). More specifically, approved providers and team members are able to access, in real time, patient-care encounter documents from other care settings (eg, acute, post-acute, ambulatory) via the HIE. Additionally, approved care entities can query-retrieve web portal information to support patient outcome improvement strategies. A partnership discussion highlighted the opportunity to utilize the HIE’s capabilities, such as real-time notification, to facilitate workflow (eg, when a patient presents to the ED, the HIE can provide access to health information at the point of care). In this capacity, the alert tool notifies care team members about patient encounters to drive timely clinical engagement for care transitions.
In January 2017, we began discussions on using the HIE to facilitate real-time electronic tracking in the Cardiac Surgical Services department at our medical center. Persons involved in these discussions included the cardiovascular (CV) team (comprised of case managers, department managers and coordinators, program coordinators, administrators, and support services [eg, pre-admission testing and home health staff]) and CT surgeons. At that time, CABG readmissions were manually tracked, and the real-time notification tool was being used in other departments (eg, in case management for tracking readmissions). The entire team was part of the initial decision meeting to pursue this possibility. The CV team reached consensus in June 2017 and proposed extending the use of the alert tool to the post-CABG population presenting to the ED (or any ED affiliated with the medical center) or admitted directly to the medical center.
The HIE staff met with the Cardiac Surgical Services team to tailor and develop the logistics of the project, such as who would be notified and how. The goals of the project were to support appropriate care intervention, reduce preventable hospital readmissions, and improve quality of care through enhanced provider communication and engagement. To achieve these goals, on the day of discharge the Cardiac Surgical Services coordinator provided the HIE team with the names of patients who had undergone CABG surgery. This patient list was loaded into the alert tool and continually updated. At 31 days, patient names were removed from the list. When a patient on the list presented to the hospital ED, the alert tool sent 2 real-time electronic notifications, an email and a text message, to the Cardiac Surgical Services coordinator, noting that a patient event occurred. Personal information was not included in the alert in order to protect patient information and comply with Health Insurance Portability and Accountability Act regulations.
The alert prompted the Cardiac Surgical Services coordinator to securely access patient information to identify and, if necessary, visit the patient. Then, based on the information gathered by the Cardiac Surgical Services coordinator, a Situation-Background-Assessment-Recommendation report was relayed to the CT surgeon, who then determined whether intervention by the CT surgical team was warranted. This process, on average, took approximately 30 minutes to complete. This was a key change in processes, one that allowed post-CABG patients to be seen by the CT surgical team while in the ED. If the issue was related to the CABG surgery, the CT surgeons could then determine an appropriate course of action, including admission or implementation of another protocol, such as the home furosemide protocol. For patients directly admitted, the surgeon contacted the admitting provider to discuss the level of care required (ie, observation or inpatient admission and treatment).
Biweekly CV team meetings were conducted during the implementation of the real-time notification alert tool. At each meeting, updates were provided on notifications received, patients who were missed by the notification process, and how well the real-time alerts were working to enhance care and appropriate disposition.
Measurements
Clinical performance data included total notifications, total number of ED visits, ED disposition (inpatient admission, observation, discharge), total number of direct admissions, direct admissions to observation, direct inpatient admissions, and patients missed by the notification process (eg, due to data entry errors, omissions of information [suffix of junior or senior], as well as programming bugs). Finally, the number of observation admissions converted to inpatient admissions was collected and further analyzed to inform needed process changes.
The Cardiac Surgical Services coordinator collected, entered, and maintained data using Excel. Data were obtained from the EHR, recorded in Excel, and analyzed using basic descriptive statistics in an ongoing fashion. Particular attention was focused on problems with the notification process (eg, patients being missed due to errors in data entry) and summarizing information to keep the Cardiac Surgical Services team updated on the progress of the process improvement. This project did not require staff protections or considerations, and because this was not a research study Institutional Review Board approval was not required.
Results
This practice change was implemented in September 2017 and led to improvements in care quality, as evidenced by improved physician communication and collaboration. In the 16-month period from implementation through December 2018, the names of 614 post-CABG patients were input into the HIE for tracking. Of these patients, 47 were treated and discharged from the ED; 31 were admitted for observation; and 44 were readmitted for inpatient care. The remaining 492 patients did not have a qualifying event requiring a notification alert. Clinical performance data from this period included 70 ED visits, 21 direct admissions, 19 direct admissions to observation, 5 patients missed by the notification process, and 4 observation admissions converted to inpatient admissions. A reduction in the CABG readmission rate from 10% in September 2017 to 7.2% in December 2018 was also noted.
Discussion
The aim of this process improvement project was to determine whether a real-time electronic alert that notified the CT surgical team about post-op CABG patients presenting to the ED would result in timely patient engagement, avoidance of readmissions, and an enhanced patient experience. This practice change has been successful, following 16 months of implementation and process refinement. Integrating a real-time electronic alert with a supporting action plan and care protocols resulted in timely patient engagement and avoidance of readmission of post-CABG patients.
Early notification of possible post-CABG readmissions became a standard-of-care process within the Cardiac Surgical Services department, with expansion to all CT post-op patients. Leveraging HIE technology to support quality improvement processes was also viewed by other departments as relevant and beneficial. For example, the hospital stroke and orthopedic-spine teams established their own processes for receiving real-time alerts.
There were several lessons learned during this project. First, gaining 100% physician buy-in to collaborative communication proved to be critical to the project’s success. The CV team was surprised by the length of time (approximately 8-10 months) it took for the practice change to be adopted by the physicians. In part, some of this delay in adoption resulted from medical staff turnover, primarily in the medical resident training rotations. Collaborative communication was key. The CT surgeons spoke with ED leadership and hospitalist services to explain the readmission reduction project and the use of an electronic alert tool. The CT surgeons also communicated to the ED physicians, hospitalists, and cardiologists that the Cardiac Surgical Services coordinator would be involved in the process and discussions regarding patientss care. Additionally, the CT surgeons authored the furosemide protocol and then committed to its use in the home health setting, further highlighting the role of collaborative communication in avoiding readmissions.
Another key step in this quality improvement project was determining who should receive the alert notifications. At the onset of the project, all notifications were sent to 1 person, the Cardiac Surgical Services coordinator. While this seemed logical in the initial stage of the project, it was unsustainable, as the receipt of the alert and the subsequent notification of the CT surgeon depended on 1 person and their availability. Approximately 10 months into the project, the notification process was further refined, with the cardiovascular intensive care unit charge nurse becoming the point of contact for the alerts. The Cardiac Surgical Services coordinator, in collaboration with nursing leaders and CT surgeons, completed a Lean Standard Work template outlining the major steps and the associated responsibilities (for the cardiovascular intensive care unit charge nurse, CT surgeon and on-call surgeon, Cardiac Surgical Services coordinator) in the process of receiving notifications, collecting patient assessment data, and reporting notifications to the CT surgeons.
Establishing adequate support mechanisms during a practice change is also important. For instance, we had to dedicate personnel time for data collection and analysis and involve additional nursing or other qualified personnel in the new process to avoid depending on a single person for the project’s success. Additional considerations were establishing criteria for surgeon notification and defining an appropriate time frame for notification (eg, urgent versus next-day notifications). We accomplished these activities approximately 10 months into the project, after it became apparent at CV team meeting discussions that further clarification of criteria and timelines was needed.
Some aspects of the project unfolded as planned, while others presented opportunities for improvement. For example, the alert notification process worked as envisioned; however, as previously mentioned, the process needed to be more inclusive to ensure there is always a charge nurse on duty to receive the alert notification, rather than just the Cardiac Surgical Services coordinator, who may not always be at the hospital. The outpatient thoracentesis program was well planned and effectively implemented. This program provided an avenue for patients who had symptoms of pleural effusion to be treated in an outpatient setting, rather than requiring an inpatient stay. Opportunities for improvement included addressing the inconsistent use of the home health furosemide protocol (developed in 2016), and the need for continued interprofessional and interdepartmental communication and coordination. For example, we had to inform the ED physicians and staff who rotate or are new to the ED about established processes and protocols in place for managing post-CABG patients who present to the ED.
The primary limitation of this project was the inability to measure the enhanced patient experience, which was 1 of the stated project goals. This goal became secondary because of more pressing issues, specifically, interorganizational collaboration (eg, hospital EHR, HIE, and CT surgical team) and tailoring the functionality of the electronic alert tool to the project. Developing and implementing measures of enhanced patient experience were not feasible during this implementation. Additionally, because this was not a research study, it was not possible to determine cause and effect or to control for confounders, such as a sicker, older cohort with more comorbid conditions, during the comparison period. Finally, although this process improvement project was conducted at a regional medical center that is the only facility performing CABG within the region, patients may have presented to another facility for an event that led to a readmission. Because readmissions to other facilities could not be captured, it is possible that the actual readmission rate was higher than the rate reported here.
Conclusions and Implications
Utilizing a real-time alert from the HIE to the CT surgical team resulted in CT surgeons being immediately made aware when their patients presented to the ED, allowing the CT surgical team the opportunity to intervene, as appropriate, in the care of their patients. Furthermore, this real-time notification and intervention resulted in timely patient engagement and, in some cases, avoidance of readmissions. Currently, patients are monitored for readmission within 30 days of discharge. In the future, the time will expand to 91 days, in preparation for participation in the CMS bundle payment program for CABG surgery.
This practice change can be used in organizations that do not have or participate in a HIE. In fact, these real-time alert applications may be available through an EHR already in use within the organization. The use of the alert requires collaborative communication and having supporting protocols in place to guide decision-making and care of post-CABG patients presenting to the ED.
There appears to be a gap in the literature discussing the use of an electronic alert tool as a real-time patient engagement strategy for post-CABG patients presenting to the ED. As such, this project contributes important results and lessons learned for other hospital service lines/departments that might consider implementing a similar process. Next steps include designing and conducting methodologically rigorous research studies based on this process improvement project to examine mortality rates as an outcome, and designing a more specific measure of patient experience, as the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey only provides hospital-level data.
Corresponding author: Stephanie D. Smith, PhD, RN, UNCW School of Nursing, 601 South College Road, Wilmington, NC 28403; [email protected].
Funding disclosures: None.
From the University of North Carolina at Wilmington School of Nursing (Dr. Smith and Dr. Turrise), the New Hanover Regional Medical Center Heart Center (Mr. Jordan), the Coastal Carolinas Health Alliance and Coastal Connect Health Information Exchange (Ms. Robertson), and Coastal Thoracic Surgical Associates (Dr. Kane), Wilmington, NC.
Abstract
Objective: Cardiothoracic (CT) surgeons at our medical center were not receiving timely notification when their coronary artery bypass graft (CABG) surgery patients were admitted to the medical center or to other hospitals. The CT surgical team worked with a health alliance in southeastern North Carolina to implement health information exchange (HIE) real-time electronic notifications for their CABG patients who presented to the hospital’s emergency department (ED) or any ED affiliated with the medical center. The alert tool notifies team members about patient encounters, driving timely clinical engagement.
Methods: The CT team provided the HIE team with the names of CABG surgery patients, which were loaded into the alert tool. When a patient on the list presented to the hospital ED or its affiliates, the alert tool sent a real-time electronic notification to the Cardiac Surgical Services nurse coordinator. This intervention prompted the assessment and disposition of CABG patients, while in the ED, by the CT surgical team.
Results: Over a 16-month period (September 2017-December 2018), the names of 614 post-CABG patients were input into the HIE for tracking. Of these patients, 47 were treated and discharged from the ED; 31 were admitted for observation; 44 were readmitted for inpatient care; and 492 did not have a qualifying event requiring a notification alert. Following implementation of this practice change, the 30-day readmission rate for patients who underwent CABG at our institution decreased from 10% to 7.2%.
Conclusion: Utilizing a real-time alert tool resulted in immediate notification of the CT team when 1 of their patients presented to the ED. This afforded the CT surgical team an opportunity to intervene in the care of their patients, which in turn led to improved quality of care, physician communication and collaboration, and patient outcomes, such as preventable 30-day readmissions.
Keywords: electronic health record; real-time electronic notification; CABG; process improvement.
Unplanned 30-day hospital readmissions of patients who have undergone coronary artery bypass graft (CABG) surgery contribute to higher overall health care costs. CABG is 1 of the conditions/procedures that the Centers for Medicare and Medicaid Services (CMS) monitors for excess readmissions.1 Readmission rates for CABG-related conditions at 30 days post-surgery are reported to be between 16% and 20% for US hospitals.2 Readmissions are not only financially costly, but also have been associated with worse patient outcomes and decreased patient satisfaction.3 Common diagnoses for post-CABG admission include atrial fibrillation, pleural effusion, and wound infection.
The facility where this project was implemented had a 10% post-CABG admission rate for patients across all payers. While this rate is below the national average of 13.2%, the cardiothoracic (CT) surgical team was not being notified in a timely manner when their post-CABG patients were readmitted. The Lean team used the A3 problem-solving process to develop strategies that would reduce these readmissions and improve the care of their patients.
We explored the use of electronic alerts in managing post-CABG patients by conducting a literature search using the terms electronic alerts in patient care, patient engagement in the emergency department, electronic alerts in CABG, real-time notifications to prevent readmission, and CABG readmission. Databases searched were PubMed, Google Scholar, Cumulative Index of Nursing and Allied Health Literature, ProQuest, and ScienceDirect. This search resulted in studies focused on the use of electronic health record (EHR) alerts as a clinical decision-support tool; for example, patient demographic and assessment data are entered into the EHR, and the clinician is prompted with “performance” recommendations (eg, consider electrocardiogram and aspirin).4 In a paper by Engelman and Benjamin,5 the authors discuss the importance of the engaged physician and note that, in their emergency department (ED), an electronic notification is sent when a postoperative patient presents; however, the notification goes to the inpatient service for timely review and disposition. There was no literature that discussed the use of an electronic alert tool as a real-time patient engagement strategy that resulted in a practice change specific to the CT surgical team.
Our process improvement project focused on alerting the CT surgical team when a post-CABG patient presented to the ED, allowing them to evaluate the patient in real time and determine whether the chief complaint was related to the CABG and whether further evaluation by the CT surgeon was required. Specifically, we wanted to determine whether a real-time electronic alert that notified the CT surgical team about post-op CABG patients presenting to the ED would result in timely patient engagement, avoidance of readmissions, and an enhanced patient experience. During this project, alerts were sent to the CT surgical team notifying them of a post-CABG patient presenting to the ED or being directly admitted from home on physician orders, a provider’s office, or inpatient rehabilitation; however, the focus of this article is specifically on the notification regarding post-CABG patients presenting to the ED.
Prior to implementing the electronic notification project, the team developed and implemented several internal and external readmission reduction and prevention strategies for CABG patients. An in-house strategy involved a process whereby patients would receive their discharge medications prior to being discharged from the hospital post-CABG, thereby avoiding potential delays in the patient obtaining medications. When examining post-CABG patient readmissions, the primary conditions that led to readmission were fluid overload, pleural effusion, and atrial fibrillation. As such, a second in-house strategy was developed for post-CABG patients presenting to the ED with atrial fibrillation. The newly established protocol allowed patients to be monitored and treated in the cardiac observation unit. In addition, external strategies, including an outpatient furosemide protocol for home health nurses and an outpatient thoracentesis program and order set, were established (eg, for patients with congestive heart failure, shortness of breath).
Methods
Setting
The regional medical center where this project was implemented is the ninth largest hospital in North Carolina and the largest county-owned public hospital in the state. It is a tertiary care center and teaching hospital with 3 hospital campuses and 855 licensed beds. The medical center was included in the 100 Safecare Hospitals list by the Safecare Group; received a grade “A” Hospital Safety Score from the Leapfrog Group; and is 1 of America’s Top 100 Hospitals for Patient Experience.
Real-Time Notification Project
A regional hospital alliance in southeastern North Carolina established a health information exchange (HIE) with its member hospitals and office-based physicians to enable electronic exchange of patient information to improve quality, safety, and efficiency in health care delivery. Our medical center is part of this alliance. The HIE is a digital platform that facilitates the sharing of information between disparate connected EHR systems, and offers a portal for practices and hospitals to access patient information across North Carolina, South Carolina (via SC HIE), and nationwide (select dialysis centers). More specifically, approved providers and team members are able to access, in real time, patient-care encounter documents from other care settings (eg, acute, post-acute, ambulatory) via the HIE. Additionally, approved care entities can query-retrieve web portal information to support patient outcome improvement strategies. A partnership discussion highlighted the opportunity to utilize the HIE’s capabilities, such as real-time notification, to facilitate workflow (eg, when a patient presents to the ED, the HIE can provide access to health information at the point of care). In this capacity, the alert tool notifies care team members about patient encounters to drive timely clinical engagement for care transitions.
In January 2017, we began discussions on using the HIE to facilitate real-time electronic tracking in the Cardiac Surgical Services department at our medical center. Persons involved in these discussions included the cardiovascular (CV) team (comprised of case managers, department managers and coordinators, program coordinators, administrators, and support services [eg, pre-admission testing and home health staff]) and CT surgeons. At that time, CABG readmissions were manually tracked, and the real-time notification tool was being used in other departments (eg, in case management for tracking readmissions). The entire team was part of the initial decision meeting to pursue this possibility. The CV team reached consensus in June 2017 and proposed extending the use of the alert tool to the post-CABG population presenting to the ED (or any ED affiliated with the medical center) or admitted directly to the medical center.
The HIE staff met with the Cardiac Surgical Services team to tailor and develop the logistics of the project, such as who would be notified and how. The goals of the project were to support appropriate care intervention, reduce preventable hospital readmissions, and improve quality of care through enhanced provider communication and engagement. To achieve these goals, on the day of discharge the Cardiac Surgical Services coordinator provided the HIE team with the names of patients who had undergone CABG surgery. This patient list was loaded into the alert tool and continually updated. At 31 days, patient names were removed from the list. When a patient on the list presented to the hospital ED, the alert tool sent 2 real-time electronic notifications, an email and a text message, to the Cardiac Surgical Services coordinator, noting that a patient event occurred. Personal information was not included in the alert in order to protect patient information and comply with Health Insurance Portability and Accountability Act regulations.
The alert prompted the Cardiac Surgical Services coordinator to securely access patient information to identify and, if necessary, visit the patient. Then, based on the information gathered by the Cardiac Surgical Services coordinator, a Situation-Background-Assessment-Recommendation report was relayed to the CT surgeon, who then determined whether intervention by the CT surgical team was warranted. This process, on average, took approximately 30 minutes to complete. This was a key change in processes, one that allowed post-CABG patients to be seen by the CT surgical team while in the ED. If the issue was related to the CABG surgery, the CT surgeons could then determine an appropriate course of action, including admission or implementation of another protocol, such as the home furosemide protocol. For patients directly admitted, the surgeon contacted the admitting provider to discuss the level of care required (ie, observation or inpatient admission and treatment).
Biweekly CV team meetings were conducted during the implementation of the real-time notification alert tool. At each meeting, updates were provided on notifications received, patients who were missed by the notification process, and how well the real-time alerts were working to enhance care and appropriate disposition.
Measurements
Clinical performance data included total notifications, total number of ED visits, ED disposition (inpatient admission, observation, discharge), total number of direct admissions, direct admissions to observation, direct inpatient admissions, and patients missed by the notification process (eg, due to data entry errors, omissions of information [suffix of junior or senior], as well as programming bugs). Finally, the number of observation admissions converted to inpatient admissions was collected and further analyzed to inform needed process changes.
The Cardiac Surgical Services coordinator collected, entered, and maintained data using Excel. Data were obtained from the EHR, recorded in Excel, and analyzed using basic descriptive statistics in an ongoing fashion. Particular attention was focused on problems with the notification process (eg, patients being missed due to errors in data entry) and summarizing information to keep the Cardiac Surgical Services team updated on the progress of the process improvement. This project did not require staff protections or considerations, and because this was not a research study Institutional Review Board approval was not required.
Results
This practice change was implemented in September 2017 and led to improvements in care quality, as evidenced by improved physician communication and collaboration. In the 16-month period from implementation through December 2018, the names of 614 post-CABG patients were input into the HIE for tracking. Of these patients, 47 were treated and discharged from the ED; 31 were admitted for observation; and 44 were readmitted for inpatient care. The remaining 492 patients did not have a qualifying event requiring a notification alert. Clinical performance data from this period included 70 ED visits, 21 direct admissions, 19 direct admissions to observation, 5 patients missed by the notification process, and 4 observation admissions converted to inpatient admissions. A reduction in the CABG readmission rate from 10% in September 2017 to 7.2% in December 2018 was also noted.
Discussion
The aim of this process improvement project was to determine whether a real-time electronic alert that notified the CT surgical team about post-op CABG patients presenting to the ED would result in timely patient engagement, avoidance of readmissions, and an enhanced patient experience. This practice change has been successful, following 16 months of implementation and process refinement. Integrating a real-time electronic alert with a supporting action plan and care protocols resulted in timely patient engagement and avoidance of readmission of post-CABG patients.
Early notification of possible post-CABG readmissions became a standard-of-care process within the Cardiac Surgical Services department, with expansion to all CT post-op patients. Leveraging HIE technology to support quality improvement processes was also viewed by other departments as relevant and beneficial. For example, the hospital stroke and orthopedic-spine teams established their own processes for receiving real-time alerts.
There were several lessons learned during this project. First, gaining 100% physician buy-in to collaborative communication proved to be critical to the project’s success. The CV team was surprised by the length of time (approximately 8-10 months) it took for the practice change to be adopted by the physicians. In part, some of this delay in adoption resulted from medical staff turnover, primarily in the medical resident training rotations. Collaborative communication was key. The CT surgeons spoke with ED leadership and hospitalist services to explain the readmission reduction project and the use of an electronic alert tool. The CT surgeons also communicated to the ED physicians, hospitalists, and cardiologists that the Cardiac Surgical Services coordinator would be involved in the process and discussions regarding patientss care. Additionally, the CT surgeons authored the furosemide protocol and then committed to its use in the home health setting, further highlighting the role of collaborative communication in avoiding readmissions.
Another key step in this quality improvement project was determining who should receive the alert notifications. At the onset of the project, all notifications were sent to 1 person, the Cardiac Surgical Services coordinator. While this seemed logical in the initial stage of the project, it was unsustainable, as the receipt of the alert and the subsequent notification of the CT surgeon depended on 1 person and their availability. Approximately 10 months into the project, the notification process was further refined, with the cardiovascular intensive care unit charge nurse becoming the point of contact for the alerts. The Cardiac Surgical Services coordinator, in collaboration with nursing leaders and CT surgeons, completed a Lean Standard Work template outlining the major steps and the associated responsibilities (for the cardiovascular intensive care unit charge nurse, CT surgeon and on-call surgeon, Cardiac Surgical Services coordinator) in the process of receiving notifications, collecting patient assessment data, and reporting notifications to the CT surgeons.
Establishing adequate support mechanisms during a practice change is also important. For instance, we had to dedicate personnel time for data collection and analysis and involve additional nursing or other qualified personnel in the new process to avoid depending on a single person for the project’s success. Additional considerations were establishing criteria for surgeon notification and defining an appropriate time frame for notification (eg, urgent versus next-day notifications). We accomplished these activities approximately 10 months into the project, after it became apparent at CV team meeting discussions that further clarification of criteria and timelines was needed.
Some aspects of the project unfolded as planned, while others presented opportunities for improvement. For example, the alert notification process worked as envisioned; however, as previously mentioned, the process needed to be more inclusive to ensure there is always a charge nurse on duty to receive the alert notification, rather than just the Cardiac Surgical Services coordinator, who may not always be at the hospital. The outpatient thoracentesis program was well planned and effectively implemented. This program provided an avenue for patients who had symptoms of pleural effusion to be treated in an outpatient setting, rather than requiring an inpatient stay. Opportunities for improvement included addressing the inconsistent use of the home health furosemide protocol (developed in 2016), and the need for continued interprofessional and interdepartmental communication and coordination. For example, we had to inform the ED physicians and staff who rotate or are new to the ED about established processes and protocols in place for managing post-CABG patients who present to the ED.
The primary limitation of this project was the inability to measure the enhanced patient experience, which was 1 of the stated project goals. This goal became secondary because of more pressing issues, specifically, interorganizational collaboration (eg, hospital EHR, HIE, and CT surgical team) and tailoring the functionality of the electronic alert tool to the project. Developing and implementing measures of enhanced patient experience were not feasible during this implementation. Additionally, because this was not a research study, it was not possible to determine cause and effect or to control for confounders, such as a sicker, older cohort with more comorbid conditions, during the comparison period. Finally, although this process improvement project was conducted at a regional medical center that is the only facility performing CABG within the region, patients may have presented to another facility for an event that led to a readmission. Because readmissions to other facilities could not be captured, it is possible that the actual readmission rate was higher than the rate reported here.
Conclusions and Implications
Utilizing a real-time alert from the HIE to the CT surgical team resulted in CT surgeons being immediately made aware when their patients presented to the ED, allowing the CT surgical team the opportunity to intervene, as appropriate, in the care of their patients. Furthermore, this real-time notification and intervention resulted in timely patient engagement and, in some cases, avoidance of readmissions. Currently, patients are monitored for readmission within 30 days of discharge. In the future, the time will expand to 91 days, in preparation for participation in the CMS bundle payment program for CABG surgery.
This practice change can be used in organizations that do not have or participate in a HIE. In fact, these real-time alert applications may be available through an EHR already in use within the organization. The use of the alert requires collaborative communication and having supporting protocols in place to guide decision-making and care of post-CABG patients presenting to the ED.
There appears to be a gap in the literature discussing the use of an electronic alert tool as a real-time patient engagement strategy for post-CABG patients presenting to the ED. As such, this project contributes important results and lessons learned for other hospital service lines/departments that might consider implementing a similar process. Next steps include designing and conducting methodologically rigorous research studies based on this process improvement project to examine mortality rates as an outcome, and designing a more specific measure of patient experience, as the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey only provides hospital-level data.
Corresponding author: Stephanie D. Smith, PhD, RN, UNCW School of Nursing, 601 South College Road, Wilmington, NC 28403; [email protected].
Funding disclosures: None.
1. Hannan EL, Zhong Y, Lahey SJ, et al. 30-day readmissions after coronary artery bypass graft surgery in New York State. JACC Cardiovasc Interv. 2011;4:569-576.
2. Feng TR, White R, Gaber-Baylis L, et al. Coronary artery bypass graft readmission rates and risk factors- A retrospective cohort study. Int J Surg. 2018;54 (Part A):7-17.
3. Donndorf P, Kaminski A. “Return to sender” or “consider it done”?! The importance of reducing hospital readmission after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2017;154:1298-1299.
4. Sequist TD, Morong SM, Marston A, et al. Electronic risk alerts to improve primary care management of chest pain: A randomized, controlled trial. J Gen Intern Med. 2012;27:438-444.
5. Engelman D, Benjamin EM. Physician engagement: The “secret sauce” to success in bundled health care. Am J Med Qual. 2018;33:100-102.
1. Hannan EL, Zhong Y, Lahey SJ, et al. 30-day readmissions after coronary artery bypass graft surgery in New York State. JACC Cardiovasc Interv. 2011;4:569-576.
2. Feng TR, White R, Gaber-Baylis L, et al. Coronary artery bypass graft readmission rates and risk factors- A retrospective cohort study. Int J Surg. 2018;54 (Part A):7-17.
3. Donndorf P, Kaminski A. “Return to sender” or “consider it done”?! The importance of reducing hospital readmission after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2017;154:1298-1299.
4. Sequist TD, Morong SM, Marston A, et al. Electronic risk alerts to improve primary care management of chest pain: A randomized, controlled trial. J Gen Intern Med. 2012;27:438-444.
5. Engelman D, Benjamin EM. Physician engagement: The “secret sauce” to success in bundled health care. Am J Med Qual. 2018;33:100-102.
AAP adds specifics to policy on abusive head trauma
the American Academy of Pediatrics said in an updated policy statement.
Abusive head trauma (AHT) is fatal in approximately one-quarter of cases in infants during the first year of life, and less-obvious clinical signs such as vomiting and fussiness often are missed, wrote Sandeep K. Narang, MD, JD, of Northwestern University, Chicago, and colleagues on the AAP Council on Child Abuse and Neglect.
In a policy statement published in Pediatrics, the AAP cautioned physicians to remain vigilant for signs that are common in AHT cases. In particular, bruising on the torso, ears, and neck in children aged younger than 4 years, or any bruising in infants younger than 4 months should be a red flag. In addition, the most recent data indicate that apnea and retinal hemorrhages are more common in cases of abuse than in accidental injuries. The AAP also recommends a skeletal survey in suspected AHT for children younger than 2 years to identify occult fractures.
“Oral injuries in infants, such as frenulum tears, may also accompany or precede AHT,” Dr. Narang and associates said.
In addition, secondary brain injury as a result of AHT can lead to poor outcomes that may be observed. “Almost 70% of survivors of AHT have some degree of lasting neurologic impairment, including static encephalopathy, intellectual disability, cerebral palsy, cortical blindness, seizure disorders, behavior problems, and learning disabilities,” according to the statement.
Endocrine dysfunction also is common in children with a history of AHT, but might not present until years later, the authors noted.
When AHT is suspected in a patient, the policy statement recommends that a subspecialist in child abuse pediatrics or in related areas including radiology, ophthalmology, neurosurgery, neurology, and general pediatric surgery “should also be consulted when necessary to ensure a complete and accurate evaluation.”
Although falls from a height of 1.5 m or 5 feet often are used as an explanation for AHT injuries, “numerous lines of clinical research have clarified the extreme rarity of short falls as a cause of severe neurologic injury or death in young infants,” Dr. Narang and associates wrote.
Other recommendations in the updated policy encourage use of the term “abusive head trauma” in medical communications, as well as encourage caregivers to serve as a medical home for survivors of AHT or refer them to medical homes for rehabilitation and monitoring. Parents and caregivers may need to be educated about the dangers of shaking or striking an infant, shown safe ways to manage a crying baby, and given tools to manage their own stress and frustration.
Physicians are legally required to report suspected cases of child abuse or neglect, and should be prepared to educate stakeholders if you are called on to work with legal and child protective services about the science behind AHT.
“The role of the pediatric practitioner is not to apportion blame or investigate potential criminal activity but to identify the medical problem, evaluate and treat the child’s injuries, and offer honest medical information to parents, families, investigators, and attorneys and/or judges,” Dr. Narang and associates wrote.
This policy statement updates the previous policy statement issued in 2009 and affirmed in 2013. The policy had no external funding, and the authors had no financial conflicts to disclose. Dr. Narang, Amanda Fingarson, DO, and James Lukefahr, MD, have served as paid expert witnesses/consultants in cases of abusive head trauma in infants and children.
SOURCE: Narang SK et al. Pediatrics. 2020 Mar 23. doi: 10.1542/peds.2020-0203.
the American Academy of Pediatrics said in an updated policy statement.
Abusive head trauma (AHT) is fatal in approximately one-quarter of cases in infants during the first year of life, and less-obvious clinical signs such as vomiting and fussiness often are missed, wrote Sandeep K. Narang, MD, JD, of Northwestern University, Chicago, and colleagues on the AAP Council on Child Abuse and Neglect.
In a policy statement published in Pediatrics, the AAP cautioned physicians to remain vigilant for signs that are common in AHT cases. In particular, bruising on the torso, ears, and neck in children aged younger than 4 years, or any bruising in infants younger than 4 months should be a red flag. In addition, the most recent data indicate that apnea and retinal hemorrhages are more common in cases of abuse than in accidental injuries. The AAP also recommends a skeletal survey in suspected AHT for children younger than 2 years to identify occult fractures.
“Oral injuries in infants, such as frenulum tears, may also accompany or precede AHT,” Dr. Narang and associates said.
In addition, secondary brain injury as a result of AHT can lead to poor outcomes that may be observed. “Almost 70% of survivors of AHT have some degree of lasting neurologic impairment, including static encephalopathy, intellectual disability, cerebral palsy, cortical blindness, seizure disorders, behavior problems, and learning disabilities,” according to the statement.
Endocrine dysfunction also is common in children with a history of AHT, but might not present until years later, the authors noted.
When AHT is suspected in a patient, the policy statement recommends that a subspecialist in child abuse pediatrics or in related areas including radiology, ophthalmology, neurosurgery, neurology, and general pediatric surgery “should also be consulted when necessary to ensure a complete and accurate evaluation.”
Although falls from a height of 1.5 m or 5 feet often are used as an explanation for AHT injuries, “numerous lines of clinical research have clarified the extreme rarity of short falls as a cause of severe neurologic injury or death in young infants,” Dr. Narang and associates wrote.
Other recommendations in the updated policy encourage use of the term “abusive head trauma” in medical communications, as well as encourage caregivers to serve as a medical home for survivors of AHT or refer them to medical homes for rehabilitation and monitoring. Parents and caregivers may need to be educated about the dangers of shaking or striking an infant, shown safe ways to manage a crying baby, and given tools to manage their own stress and frustration.
Physicians are legally required to report suspected cases of child abuse or neglect, and should be prepared to educate stakeholders if you are called on to work with legal and child protective services about the science behind AHT.
“The role of the pediatric practitioner is not to apportion blame or investigate potential criminal activity but to identify the medical problem, evaluate and treat the child’s injuries, and offer honest medical information to parents, families, investigators, and attorneys and/or judges,” Dr. Narang and associates wrote.
This policy statement updates the previous policy statement issued in 2009 and affirmed in 2013. The policy had no external funding, and the authors had no financial conflicts to disclose. Dr. Narang, Amanda Fingarson, DO, and James Lukefahr, MD, have served as paid expert witnesses/consultants in cases of abusive head trauma in infants and children.
SOURCE: Narang SK et al. Pediatrics. 2020 Mar 23. doi: 10.1542/peds.2020-0203.
the American Academy of Pediatrics said in an updated policy statement.
Abusive head trauma (AHT) is fatal in approximately one-quarter of cases in infants during the first year of life, and less-obvious clinical signs such as vomiting and fussiness often are missed, wrote Sandeep K. Narang, MD, JD, of Northwestern University, Chicago, and colleagues on the AAP Council on Child Abuse and Neglect.
In a policy statement published in Pediatrics, the AAP cautioned physicians to remain vigilant for signs that are common in AHT cases. In particular, bruising on the torso, ears, and neck in children aged younger than 4 years, or any bruising in infants younger than 4 months should be a red flag. In addition, the most recent data indicate that apnea and retinal hemorrhages are more common in cases of abuse than in accidental injuries. The AAP also recommends a skeletal survey in suspected AHT for children younger than 2 years to identify occult fractures.
“Oral injuries in infants, such as frenulum tears, may also accompany or precede AHT,” Dr. Narang and associates said.
In addition, secondary brain injury as a result of AHT can lead to poor outcomes that may be observed. “Almost 70% of survivors of AHT have some degree of lasting neurologic impairment, including static encephalopathy, intellectual disability, cerebral palsy, cortical blindness, seizure disorders, behavior problems, and learning disabilities,” according to the statement.
Endocrine dysfunction also is common in children with a history of AHT, but might not present until years later, the authors noted.
When AHT is suspected in a patient, the policy statement recommends that a subspecialist in child abuse pediatrics or in related areas including radiology, ophthalmology, neurosurgery, neurology, and general pediatric surgery “should also be consulted when necessary to ensure a complete and accurate evaluation.”
Although falls from a height of 1.5 m or 5 feet often are used as an explanation for AHT injuries, “numerous lines of clinical research have clarified the extreme rarity of short falls as a cause of severe neurologic injury or death in young infants,” Dr. Narang and associates wrote.
Other recommendations in the updated policy encourage use of the term “abusive head trauma” in medical communications, as well as encourage caregivers to serve as a medical home for survivors of AHT or refer them to medical homes for rehabilitation and monitoring. Parents and caregivers may need to be educated about the dangers of shaking or striking an infant, shown safe ways to manage a crying baby, and given tools to manage their own stress and frustration.
Physicians are legally required to report suspected cases of child abuse or neglect, and should be prepared to educate stakeholders if you are called on to work with legal and child protective services about the science behind AHT.
“The role of the pediatric practitioner is not to apportion blame or investigate potential criminal activity but to identify the medical problem, evaluate and treat the child’s injuries, and offer honest medical information to parents, families, investigators, and attorneys and/or judges,” Dr. Narang and associates wrote.
This policy statement updates the previous policy statement issued in 2009 and affirmed in 2013. The policy had no external funding, and the authors had no financial conflicts to disclose. Dr. Narang, Amanda Fingarson, DO, and James Lukefahr, MD, have served as paid expert witnesses/consultants in cases of abusive head trauma in infants and children.
SOURCE: Narang SK et al. Pediatrics. 2020 Mar 23. doi: 10.1542/peds.2020-0203.
FROM PEDIATRICS
Guidance defines vaping-related respiratory syndrome
ORLANDO – Knowledge of vaping devices, familiarity with terminology, and the ability to quickly pinpoint individuals at risk of lung injury are just a few skills that can help critical care professionals confronted with patients who may have vaping-associated lung disease, according to a new guidance document.
The guidance offers a risk-stratification system that classifies patients into groups based on exposure, symptoms, and imaging results, and provides specific evaluation needs and management strategies for each. The guidance is designed to help critical care professionals efficiently identify those at high risk of respiratory failure.
Physicians also need to communicate with patients to identify what substances are being vaped and develop effective methods to encourage abstinence, according to the authors, led by Craig M. Lilly, MD, FCCP, professor of medicine, anesthesiology, and surgery at the University of Massachusetts, Worcester.
“I would encourage every intensivist, when they leave their intensive care unit at night, [to ask], ‘have I advised against vaping today?’ ” Dr. Lilly said at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
The guidelines, concurrently published as a review article in Critical Care Explorations, propose the term vaping-associated respiratory distress syndrome (VARDS), which the authors say constitutes an acute and progressive respiratory syndrome marked by pathologic changes of lung injury and potentially life-threatening hypoxemic respiratory failure.
They also introduce the three-group Worcester classification system, which is intended to triage vaping-exposed individuals for risk of VARDS based on the presence or absence of vaping-related symptoms and infiltrates, and normal or abnormal oxygen saturation.
“It’s very simple,” said Dr. Lilly, who added that the risk stratification model was developed at the request of Massachusetts public health officials.
Patients with vaping exposure but no symptoms attributable to vaping, such as cough, chest pain, or weight loss, are classified as Worcester Low Risk and testing is not recommended, he said.
By contrast, individuals are considered Worcester Medium Risk if they have vaping exposure, symptoms, and a vaping-associated abnormal pattern on imaging, but no hypoxemia; the presence of hypoxemia would tip the scale toward Worcester High Risk.
“Most patients that have died from vaping have been sent out of emergency rooms when they were noted to be hypoxic,” Dr. Lilly told meeting attendees.
Louella B. Amos, MD, a pediatric pulmonologist at Children’s Hospital of Wisconsin in Milwaukee, said she expects the guidance and risk stratification system will be useful not only for critical care specialists, but for other health care providers as well.
“It’s important to make decisions relatively quickly, depending on the severity of symptoms, and I think this is nice and simple,” Dr. Amos said in an interview.
“We always triage when we see patients, either at the door or in our clinic, or behind that, even in the hospital,” she said. “So I think this can be a great tool for everybody, not only the intensivist, but people who are triaging at the front.”
Management of individuals at low risk of VARDS begins with encouragement of abstinence. “We think that every vaping patient should be advised to quit vaping,” Dr. Lilly said. Patients who are interested in quitting who have not yet worked with someone in their health care team whom they trust can be referred to their primary care physicians for counseling, he added, while those struggling with addiction, unable to quit, and unable to partner with a primary care physician can be referred to an addiction medicine specialist.
For moderate-risk patients, vaping cessation is “absolutely mandatory,” said Dr. Lilly, who recommended monitoring of vaping abstinence, outpatient evaluation based on imaging studies, and adequate follow-up to ensure symptoms resolve, tests normalize, and daily activities bounce back to baseline levels.
The guidance offers more extensive recommendations for the VARDS high-risk group, including supervised vaping abstinence, continuous pulse oximetry, and early intervention with noninvasive ventilation, and mechanical ventilation if required, Dr. Lilly said.
Judging vaping exposure is challenging, requiring clinicians to have a familiarity with the many different devices that are available.
Beyond device type, he added, it’s important to know the various terms for devices and lingo that patients may use to describe them, what solutions are vaped, whether those solutions are commercially prepared or off the street, the dose the device delivers, and a number of other factors, he said.
Clinical evaluation typically comes down to unexplained cough, chest pain, weight loss, fatigue, or dyspnea, though one other clue is whether there are gastrointestinal symptoms: “The same way that aerosols can go down to the lungs, they also go into the GI tract, and when nausea, vomiting, or cramping abdominal pain is tightly associated with vaping exposure, one should assume that the patient has been toxin exposed,” he explained.
Dr. Lilly said he had no financial relationships to disclose.
ORLANDO – Knowledge of vaping devices, familiarity with terminology, and the ability to quickly pinpoint individuals at risk of lung injury are just a few skills that can help critical care professionals confronted with patients who may have vaping-associated lung disease, according to a new guidance document.
The guidance offers a risk-stratification system that classifies patients into groups based on exposure, symptoms, and imaging results, and provides specific evaluation needs and management strategies for each. The guidance is designed to help critical care professionals efficiently identify those at high risk of respiratory failure.
Physicians also need to communicate with patients to identify what substances are being vaped and develop effective methods to encourage abstinence, according to the authors, led by Craig M. Lilly, MD, FCCP, professor of medicine, anesthesiology, and surgery at the University of Massachusetts, Worcester.
“I would encourage every intensivist, when they leave their intensive care unit at night, [to ask], ‘have I advised against vaping today?’ ” Dr. Lilly said at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
The guidelines, concurrently published as a review article in Critical Care Explorations, propose the term vaping-associated respiratory distress syndrome (VARDS), which the authors say constitutes an acute and progressive respiratory syndrome marked by pathologic changes of lung injury and potentially life-threatening hypoxemic respiratory failure.
They also introduce the three-group Worcester classification system, which is intended to triage vaping-exposed individuals for risk of VARDS based on the presence or absence of vaping-related symptoms and infiltrates, and normal or abnormal oxygen saturation.
“It’s very simple,” said Dr. Lilly, who added that the risk stratification model was developed at the request of Massachusetts public health officials.
Patients with vaping exposure but no symptoms attributable to vaping, such as cough, chest pain, or weight loss, are classified as Worcester Low Risk and testing is not recommended, he said.
By contrast, individuals are considered Worcester Medium Risk if they have vaping exposure, symptoms, and a vaping-associated abnormal pattern on imaging, but no hypoxemia; the presence of hypoxemia would tip the scale toward Worcester High Risk.
“Most patients that have died from vaping have been sent out of emergency rooms when they were noted to be hypoxic,” Dr. Lilly told meeting attendees.
Louella B. Amos, MD, a pediatric pulmonologist at Children’s Hospital of Wisconsin in Milwaukee, said she expects the guidance and risk stratification system will be useful not only for critical care specialists, but for other health care providers as well.
“It’s important to make decisions relatively quickly, depending on the severity of symptoms, and I think this is nice and simple,” Dr. Amos said in an interview.
“We always triage when we see patients, either at the door or in our clinic, or behind that, even in the hospital,” she said. “So I think this can be a great tool for everybody, not only the intensivist, but people who are triaging at the front.”
Management of individuals at low risk of VARDS begins with encouragement of abstinence. “We think that every vaping patient should be advised to quit vaping,” Dr. Lilly said. Patients who are interested in quitting who have not yet worked with someone in their health care team whom they trust can be referred to their primary care physicians for counseling, he added, while those struggling with addiction, unable to quit, and unable to partner with a primary care physician can be referred to an addiction medicine specialist.
For moderate-risk patients, vaping cessation is “absolutely mandatory,” said Dr. Lilly, who recommended monitoring of vaping abstinence, outpatient evaluation based on imaging studies, and adequate follow-up to ensure symptoms resolve, tests normalize, and daily activities bounce back to baseline levels.
The guidance offers more extensive recommendations for the VARDS high-risk group, including supervised vaping abstinence, continuous pulse oximetry, and early intervention with noninvasive ventilation, and mechanical ventilation if required, Dr. Lilly said.
Judging vaping exposure is challenging, requiring clinicians to have a familiarity with the many different devices that are available.
Beyond device type, he added, it’s important to know the various terms for devices and lingo that patients may use to describe them, what solutions are vaped, whether those solutions are commercially prepared or off the street, the dose the device delivers, and a number of other factors, he said.
Clinical evaluation typically comes down to unexplained cough, chest pain, weight loss, fatigue, or dyspnea, though one other clue is whether there are gastrointestinal symptoms: “The same way that aerosols can go down to the lungs, they also go into the GI tract, and when nausea, vomiting, or cramping abdominal pain is tightly associated with vaping exposure, one should assume that the patient has been toxin exposed,” he explained.
Dr. Lilly said he had no financial relationships to disclose.
ORLANDO – Knowledge of vaping devices, familiarity with terminology, and the ability to quickly pinpoint individuals at risk of lung injury are just a few skills that can help critical care professionals confronted with patients who may have vaping-associated lung disease, according to a new guidance document.
The guidance offers a risk-stratification system that classifies patients into groups based on exposure, symptoms, and imaging results, and provides specific evaluation needs and management strategies for each. The guidance is designed to help critical care professionals efficiently identify those at high risk of respiratory failure.
Physicians also need to communicate with patients to identify what substances are being vaped and develop effective methods to encourage abstinence, according to the authors, led by Craig M. Lilly, MD, FCCP, professor of medicine, anesthesiology, and surgery at the University of Massachusetts, Worcester.
“I would encourage every intensivist, when they leave their intensive care unit at night, [to ask], ‘have I advised against vaping today?’ ” Dr. Lilly said at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
The guidelines, concurrently published as a review article in Critical Care Explorations, propose the term vaping-associated respiratory distress syndrome (VARDS), which the authors say constitutes an acute and progressive respiratory syndrome marked by pathologic changes of lung injury and potentially life-threatening hypoxemic respiratory failure.
They also introduce the three-group Worcester classification system, which is intended to triage vaping-exposed individuals for risk of VARDS based on the presence or absence of vaping-related symptoms and infiltrates, and normal or abnormal oxygen saturation.
“It’s very simple,” said Dr. Lilly, who added that the risk stratification model was developed at the request of Massachusetts public health officials.
Patients with vaping exposure but no symptoms attributable to vaping, such as cough, chest pain, or weight loss, are classified as Worcester Low Risk and testing is not recommended, he said.
By contrast, individuals are considered Worcester Medium Risk if they have vaping exposure, symptoms, and a vaping-associated abnormal pattern on imaging, but no hypoxemia; the presence of hypoxemia would tip the scale toward Worcester High Risk.
“Most patients that have died from vaping have been sent out of emergency rooms when they were noted to be hypoxic,” Dr. Lilly told meeting attendees.
Louella B. Amos, MD, a pediatric pulmonologist at Children’s Hospital of Wisconsin in Milwaukee, said she expects the guidance and risk stratification system will be useful not only for critical care specialists, but for other health care providers as well.
“It’s important to make decisions relatively quickly, depending on the severity of symptoms, and I think this is nice and simple,” Dr. Amos said in an interview.
“We always triage when we see patients, either at the door or in our clinic, or behind that, even in the hospital,” she said. “So I think this can be a great tool for everybody, not only the intensivist, but people who are triaging at the front.”
Management of individuals at low risk of VARDS begins with encouragement of abstinence. “We think that every vaping patient should be advised to quit vaping,” Dr. Lilly said. Patients who are interested in quitting who have not yet worked with someone in their health care team whom they trust can be referred to their primary care physicians for counseling, he added, while those struggling with addiction, unable to quit, and unable to partner with a primary care physician can be referred to an addiction medicine specialist.
For moderate-risk patients, vaping cessation is “absolutely mandatory,” said Dr. Lilly, who recommended monitoring of vaping abstinence, outpatient evaluation based on imaging studies, and adequate follow-up to ensure symptoms resolve, tests normalize, and daily activities bounce back to baseline levels.
The guidance offers more extensive recommendations for the VARDS high-risk group, including supervised vaping abstinence, continuous pulse oximetry, and early intervention with noninvasive ventilation, and mechanical ventilation if required, Dr. Lilly said.
Judging vaping exposure is challenging, requiring clinicians to have a familiarity with the many different devices that are available.
Beyond device type, he added, it’s important to know the various terms for devices and lingo that patients may use to describe them, what solutions are vaped, whether those solutions are commercially prepared or off the street, the dose the device delivers, and a number of other factors, he said.
Clinical evaluation typically comes down to unexplained cough, chest pain, weight loss, fatigue, or dyspnea, though one other clue is whether there are gastrointestinal symptoms: “The same way that aerosols can go down to the lungs, they also go into the GI tract, and when nausea, vomiting, or cramping abdominal pain is tightly associated with vaping exposure, one should assume that the patient has been toxin exposed,” he explained.
Dr. Lilly said he had no financial relationships to disclose.
REPORTING FROM CCC49
Opioid use disorder up in sepsis hospitalizations
ORLANDO –
The prevalence of opioid use disorder (OUD) has significantly increased over the past 15 years, the analysis further shows.
Results of the study, presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine, further suggested that OUD disproportionately contributes to sepsis deaths in younger, healthier patients.
Together, these findings underscore the importance of ongoing efforts to address the opioid epidemic in the United States, according to researcher Mohammad Alrawashdeh, PhD, MSN, a postdoctoral research fellow with Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston.
“In addition to ongoing efforts to combat the opioid crisis, future public health interventions should focus on increasing awareness, recognition, and aggressive treatment of sepsis in this population,” Dr. Alrawashdeh said in an oral presentation of the study.
This study fills an important knowledge gap regarding the connection between OUD and sepsis, according to Greg S. Martin, MD, MS, FCCM, professor of medicine in pulmonary critical care at Emory University, Atlanta, and secretary for the Society of Critical Care Medicine.
“We’ve not really ever been able to piece together the relationship between opioid use disorders and sepsis,” Dr. Martin said in an interview. “It’s not that people wouldn’t suspect that there’s a connection – it’s more that we have simply not been able to get the kind of data that you can use, like they’ve done here, that really helps you to answer that question.”
The study suggests not only that OUD and sepsis are linked, Dr. Martin added, but that health care providers need to be prepared to potentially see further increases in the number of patients with OUD seen in the intensive care unit.
“Both of those are things that we certainly need to be aware of, both from the individual practitioner perspective and also the public health planning perspective,” he said.
The retrospective study by Dr. Alrawashdeh and coinvestigators focused on electronic health record data for adults admitted to 373 hospitals in the United States between 2009 and 2015, including 375,479 who had sepsis.
Over time, there was a significant increase in the prevalence of OUD among those hospitalized for sepsis, from less than 2.0% in 2009 to more than 3% in 2015, representing a significant 77.3% increase. In general, the prevalence of sepsis was significantly higher among hospitalized patients with OUD compared with patients without the disorder, at 7.2% and 5.6%, respectively.
The sepsis patients with OUD tended to be younger, healthier, and more likely to be white compared with patients without OUD, according to the report. Moreover, the sepsis patients with OUD more often had endocarditis and gram-positive and fungal bloodstream infections. They also required more mechanical ventilation and had more ICU admissions, with longer stays in both the ICU and hospital.
The OUD patients accounted for 2.1% of sepsis-associated deaths overall, but 3.3% of those deaths in healthy patients, and 7.1% of deaths among younger patients, according to the report.
Those findings provide some clues that could help guide clinical practice, according to Dr. Martin. For example, the data show a nearly fivefold increased risk of endocarditis with OUD (3.9% versus 0.7%), which may inform screening practices.
“While we don’t necessarily screen every sepsis patient for endocarditis, if it’s an opioid use disorder patient – particularly one with a bloodstream infection – then that’s almost certainly something you should be doing,” Dr. Martin said.
The data suggest gram-positive bacterial and fungal infections will more likely be encountered among these patients, which could guide empiric treatment, he said.
Providers specializing in OUD should have a heightened awareness of the potential for infection and sepsis among those patients, and perhaps be more attuned to fever and other signs of infection that might warrant a referral or additional care, Dr. Martin added.
Dr. Alrawashdeh reported no disclosures related to the study.
SOURCE: Alrawashdeh M et al. Crit Care Med. 2020 Jan;48(1):28. Abstract 56.
ORLANDO –
The prevalence of opioid use disorder (OUD) has significantly increased over the past 15 years, the analysis further shows.
Results of the study, presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine, further suggested that OUD disproportionately contributes to sepsis deaths in younger, healthier patients.
Together, these findings underscore the importance of ongoing efforts to address the opioid epidemic in the United States, according to researcher Mohammad Alrawashdeh, PhD, MSN, a postdoctoral research fellow with Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston.
“In addition to ongoing efforts to combat the opioid crisis, future public health interventions should focus on increasing awareness, recognition, and aggressive treatment of sepsis in this population,” Dr. Alrawashdeh said in an oral presentation of the study.
This study fills an important knowledge gap regarding the connection between OUD and sepsis, according to Greg S. Martin, MD, MS, FCCM, professor of medicine in pulmonary critical care at Emory University, Atlanta, and secretary for the Society of Critical Care Medicine.
“We’ve not really ever been able to piece together the relationship between opioid use disorders and sepsis,” Dr. Martin said in an interview. “It’s not that people wouldn’t suspect that there’s a connection – it’s more that we have simply not been able to get the kind of data that you can use, like they’ve done here, that really helps you to answer that question.”
The study suggests not only that OUD and sepsis are linked, Dr. Martin added, but that health care providers need to be prepared to potentially see further increases in the number of patients with OUD seen in the intensive care unit.
“Both of those are things that we certainly need to be aware of, both from the individual practitioner perspective and also the public health planning perspective,” he said.
The retrospective study by Dr. Alrawashdeh and coinvestigators focused on electronic health record data for adults admitted to 373 hospitals in the United States between 2009 and 2015, including 375,479 who had sepsis.
Over time, there was a significant increase in the prevalence of OUD among those hospitalized for sepsis, from less than 2.0% in 2009 to more than 3% in 2015, representing a significant 77.3% increase. In general, the prevalence of sepsis was significantly higher among hospitalized patients with OUD compared with patients without the disorder, at 7.2% and 5.6%, respectively.
The sepsis patients with OUD tended to be younger, healthier, and more likely to be white compared with patients without OUD, according to the report. Moreover, the sepsis patients with OUD more often had endocarditis and gram-positive and fungal bloodstream infections. They also required more mechanical ventilation and had more ICU admissions, with longer stays in both the ICU and hospital.
The OUD patients accounted for 2.1% of sepsis-associated deaths overall, but 3.3% of those deaths in healthy patients, and 7.1% of deaths among younger patients, according to the report.
Those findings provide some clues that could help guide clinical practice, according to Dr. Martin. For example, the data show a nearly fivefold increased risk of endocarditis with OUD (3.9% versus 0.7%), which may inform screening practices.
“While we don’t necessarily screen every sepsis patient for endocarditis, if it’s an opioid use disorder patient – particularly one with a bloodstream infection – then that’s almost certainly something you should be doing,” Dr. Martin said.
The data suggest gram-positive bacterial and fungal infections will more likely be encountered among these patients, which could guide empiric treatment, he said.
Providers specializing in OUD should have a heightened awareness of the potential for infection and sepsis among those patients, and perhaps be more attuned to fever and other signs of infection that might warrant a referral or additional care, Dr. Martin added.
Dr. Alrawashdeh reported no disclosures related to the study.
SOURCE: Alrawashdeh M et al. Crit Care Med. 2020 Jan;48(1):28. Abstract 56.
ORLANDO –
The prevalence of opioid use disorder (OUD) has significantly increased over the past 15 years, the analysis further shows.
Results of the study, presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine, further suggested that OUD disproportionately contributes to sepsis deaths in younger, healthier patients.
Together, these findings underscore the importance of ongoing efforts to address the opioid epidemic in the United States, according to researcher Mohammad Alrawashdeh, PhD, MSN, a postdoctoral research fellow with Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston.
“In addition to ongoing efforts to combat the opioid crisis, future public health interventions should focus on increasing awareness, recognition, and aggressive treatment of sepsis in this population,” Dr. Alrawashdeh said in an oral presentation of the study.
This study fills an important knowledge gap regarding the connection between OUD and sepsis, according to Greg S. Martin, MD, MS, FCCM, professor of medicine in pulmonary critical care at Emory University, Atlanta, and secretary for the Society of Critical Care Medicine.
“We’ve not really ever been able to piece together the relationship between opioid use disorders and sepsis,” Dr. Martin said in an interview. “It’s not that people wouldn’t suspect that there’s a connection – it’s more that we have simply not been able to get the kind of data that you can use, like they’ve done here, that really helps you to answer that question.”
The study suggests not only that OUD and sepsis are linked, Dr. Martin added, but that health care providers need to be prepared to potentially see further increases in the number of patients with OUD seen in the intensive care unit.
“Both of those are things that we certainly need to be aware of, both from the individual practitioner perspective and also the public health planning perspective,” he said.
The retrospective study by Dr. Alrawashdeh and coinvestigators focused on electronic health record data for adults admitted to 373 hospitals in the United States between 2009 and 2015, including 375,479 who had sepsis.
Over time, there was a significant increase in the prevalence of OUD among those hospitalized for sepsis, from less than 2.0% in 2009 to more than 3% in 2015, representing a significant 77.3% increase. In general, the prevalence of sepsis was significantly higher among hospitalized patients with OUD compared with patients without the disorder, at 7.2% and 5.6%, respectively.
The sepsis patients with OUD tended to be younger, healthier, and more likely to be white compared with patients without OUD, according to the report. Moreover, the sepsis patients with OUD more often had endocarditis and gram-positive and fungal bloodstream infections. They also required more mechanical ventilation and had more ICU admissions, with longer stays in both the ICU and hospital.
The OUD patients accounted for 2.1% of sepsis-associated deaths overall, but 3.3% of those deaths in healthy patients, and 7.1% of deaths among younger patients, according to the report.
Those findings provide some clues that could help guide clinical practice, according to Dr. Martin. For example, the data show a nearly fivefold increased risk of endocarditis with OUD (3.9% versus 0.7%), which may inform screening practices.
“While we don’t necessarily screen every sepsis patient for endocarditis, if it’s an opioid use disorder patient – particularly one with a bloodstream infection – then that’s almost certainly something you should be doing,” Dr. Martin said.
The data suggest gram-positive bacterial and fungal infections will more likely be encountered among these patients, which could guide empiric treatment, he said.
Providers specializing in OUD should have a heightened awareness of the potential for infection and sepsis among those patients, and perhaps be more attuned to fever and other signs of infection that might warrant a referral or additional care, Dr. Martin added.
Dr. Alrawashdeh reported no disclosures related to the study.
SOURCE: Alrawashdeh M et al. Crit Care Med. 2020 Jan;48(1):28. Abstract 56.
REPORTING FROM CCC49
2019-nCoV: Structure, characteristics of key potential therapy target determined
Researchers have identified the structure of a protein that could turn out to be a potential vaccine target for the 2019-nCoV.
As is typical of other coronaviruses, 2019-nCoV makes use of a densely glycosylated spike protein to gain entry into host cells. The spike protein is a trimeric class I fusion protein that exists in a metastable prefusion conformation that undergoes a dramatic structural rearrangement to fuse the viral membrane with the host-cell membrane, according to Daniel Wrapp of the University of Texas at Austin and colleagues.
The researchers performed a study to synthesize and determine the 3-D structure of the spike protein because it is a logical target for vaccine development and for the development of targeted therapeutics for COVID-19, the disease caused by the virus.
“As soon as we knew this was a coronavirus, we felt we had to jump at it,” senior author Jason S. McLellan, PhD, associate professor of molecular science, said in a press release from the University, “because we could be one of the first ones to get this structure. We knew exactly what mutations to put into this because we’ve already shown these mutations work for a bunch of other coronaviruses.”
Because recent reports by other researchers demonstrated that 2019-nCoV and SARS-CoV spike proteins share the same functional host-cell receptor–angiotensin-converting enzyme 2 (ACE2), Dr. McLellan and his colleagues examined the relation between the two viruses. They found biophysical and structural evidence that the 2019-nCoV spike protein binds ACE2 with higher affinity than the closely related SARS-CoV spike protein. “The high affinity of 2019-nCoV S for human ACE2 may contribute to the apparent ease with which 2019-nCoV can spread from human-to-human; however, additional studies are needed to investigate this possibility,” the researchers wrote.
Focusing their attention on the receptor-binding domain (RBD) of the 2019-nCoV spike protein, they tested several published SARS-CoV RBD-specific monoclonal antibodies against it and found that these antibodies showed no appreciable binding to 2019-nCoV spike protein, which suggests limited antibody cross-reactivity. For this reason, they suggested that future antibody isolation and therapeutic design efforts will benefit from specifically using 2019-nCoV spike proteins as probes.
“This information will support precision vaccine design and discovery of anti-viral therapeutics, accelerating medical countermeasure development,” they concluded.
The research was supported in part by an National Institutes of Health/National Institute of Allergy and Infectious Diseases grant and by intramural funding from the National Institute of Allergy and Infectious Diseases. Four authors are inventors on US patent application No. 62/412,703 (Prefusion Coronavirus Spike Proteins and Their Use) and all are inventors on US patent application No. 62/972,886 (2019-nCoV Vaccine).
SOURCE: Wrapp D et al. Science. 2020 Feb 19. doi: 10.1126/science.abb2507.
Researchers have identified the structure of a protein that could turn out to be a potential vaccine target for the 2019-nCoV.
As is typical of other coronaviruses, 2019-nCoV makes use of a densely glycosylated spike protein to gain entry into host cells. The spike protein is a trimeric class I fusion protein that exists in a metastable prefusion conformation that undergoes a dramatic structural rearrangement to fuse the viral membrane with the host-cell membrane, according to Daniel Wrapp of the University of Texas at Austin and colleagues.
The researchers performed a study to synthesize and determine the 3-D structure of the spike protein because it is a logical target for vaccine development and for the development of targeted therapeutics for COVID-19, the disease caused by the virus.
“As soon as we knew this was a coronavirus, we felt we had to jump at it,” senior author Jason S. McLellan, PhD, associate professor of molecular science, said in a press release from the University, “because we could be one of the first ones to get this structure. We knew exactly what mutations to put into this because we’ve already shown these mutations work for a bunch of other coronaviruses.”
Because recent reports by other researchers demonstrated that 2019-nCoV and SARS-CoV spike proteins share the same functional host-cell receptor–angiotensin-converting enzyme 2 (ACE2), Dr. McLellan and his colleagues examined the relation between the two viruses. They found biophysical and structural evidence that the 2019-nCoV spike protein binds ACE2 with higher affinity than the closely related SARS-CoV spike protein. “The high affinity of 2019-nCoV S for human ACE2 may contribute to the apparent ease with which 2019-nCoV can spread from human-to-human; however, additional studies are needed to investigate this possibility,” the researchers wrote.
Focusing their attention on the receptor-binding domain (RBD) of the 2019-nCoV spike protein, they tested several published SARS-CoV RBD-specific monoclonal antibodies against it and found that these antibodies showed no appreciable binding to 2019-nCoV spike protein, which suggests limited antibody cross-reactivity. For this reason, they suggested that future antibody isolation and therapeutic design efforts will benefit from specifically using 2019-nCoV spike proteins as probes.
“This information will support precision vaccine design and discovery of anti-viral therapeutics, accelerating medical countermeasure development,” they concluded.
The research was supported in part by an National Institutes of Health/National Institute of Allergy and Infectious Diseases grant and by intramural funding from the National Institute of Allergy and Infectious Diseases. Four authors are inventors on US patent application No. 62/412,703 (Prefusion Coronavirus Spike Proteins and Their Use) and all are inventors on US patent application No. 62/972,886 (2019-nCoV Vaccine).
SOURCE: Wrapp D et al. Science. 2020 Feb 19. doi: 10.1126/science.abb2507.
Researchers have identified the structure of a protein that could turn out to be a potential vaccine target for the 2019-nCoV.
As is typical of other coronaviruses, 2019-nCoV makes use of a densely glycosylated spike protein to gain entry into host cells. The spike protein is a trimeric class I fusion protein that exists in a metastable prefusion conformation that undergoes a dramatic structural rearrangement to fuse the viral membrane with the host-cell membrane, according to Daniel Wrapp of the University of Texas at Austin and colleagues.
The researchers performed a study to synthesize and determine the 3-D structure of the spike protein because it is a logical target for vaccine development and for the development of targeted therapeutics for COVID-19, the disease caused by the virus.
“As soon as we knew this was a coronavirus, we felt we had to jump at it,” senior author Jason S. McLellan, PhD, associate professor of molecular science, said in a press release from the University, “because we could be one of the first ones to get this structure. We knew exactly what mutations to put into this because we’ve already shown these mutations work for a bunch of other coronaviruses.”
Because recent reports by other researchers demonstrated that 2019-nCoV and SARS-CoV spike proteins share the same functional host-cell receptor–angiotensin-converting enzyme 2 (ACE2), Dr. McLellan and his colleagues examined the relation between the two viruses. They found biophysical and structural evidence that the 2019-nCoV spike protein binds ACE2 with higher affinity than the closely related SARS-CoV spike protein. “The high affinity of 2019-nCoV S for human ACE2 may contribute to the apparent ease with which 2019-nCoV can spread from human-to-human; however, additional studies are needed to investigate this possibility,” the researchers wrote.
Focusing their attention on the receptor-binding domain (RBD) of the 2019-nCoV spike protein, they tested several published SARS-CoV RBD-specific monoclonal antibodies against it and found that these antibodies showed no appreciable binding to 2019-nCoV spike protein, which suggests limited antibody cross-reactivity. For this reason, they suggested that future antibody isolation and therapeutic design efforts will benefit from specifically using 2019-nCoV spike proteins as probes.
“This information will support precision vaccine design and discovery of anti-viral therapeutics, accelerating medical countermeasure development,” they concluded.
The research was supported in part by an National Institutes of Health/National Institute of Allergy and Infectious Diseases grant and by intramural funding from the National Institute of Allergy and Infectious Diseases. Four authors are inventors on US patent application No. 62/412,703 (Prefusion Coronavirus Spike Proteins and Their Use) and all are inventors on US patent application No. 62/972,886 (2019-nCoV Vaccine).
SOURCE: Wrapp D et al. Science. 2020 Feb 19. doi: 10.1126/science.abb2507.
FROM SCIENCE
‘A glimmer of hope’ for stroke/mortality benefit with AFib catheter ablation
SNOWMASS, COLO. – stroke, major bleeding, or cardiac arrest, compared with rhythm and/or rate control drugs in a propensity score–weighted, retrospective, observational study.
Findings of the investigation, which included more than 183,000 real-world patients in routine clinical practice, were reported by Peter S. Noseworthy, MD, during the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
The results breathe new life into the controversy created by the previously reported CABANA trial (Catheter Ablation vs. Antiarrhythmic Drug Therapy for Atrial Fibrillation), a 10-country study in which 2,204 patients with atrial fibrillation (AFib) were randomized to catheter ablation or antiarrhythmic and/or rhythm control medications and followed for a mean of about 4 years. CABANA yielded a negative result (JAMA. 2019 Apr 2;321[13]:1261-74), with the prespecified intent-to-treat analysis indicating no significant between-group difference in the primary composite endpoint – the very same one that was positive in the large observational study.
However, CABANA was marred by major problems arising from protocol deviations: Nearly 28% of patients assigned to medical therapy crossed over to catheter ablation, typically because their antiarrhythmic drugs failed, and 10% of patients randomized to catheter ablation never got it. This muddies the waters when trying to identify a true stroke/mortality benefit for catheter ablation, if indeed any such benefit was actually present.
Here’s where the controversy arose: While CABANA must be called a negative trial based upon the disappointing results of the intent-to-treat analysis, a prespecified post hoc analysis of patients as actually treated showed a statistically significant 27% relative risk reduction for the primary composite endpoint in the catheter ablation group. That’s strikingly similar to the 30% relative risk reduction for catheter ablation seen in the huge observational study, where the CABANA-type primary outcome occurred in 22.5% of the medically managed patients and 16.8% of those who underwent catheter ablation, noted Dr. Noseworthy, professor of medicine and director of heart rhythm and physiology at the Mayo Clinic in Rochester, Minn.
He ought to know: He was both an investigator in CABANA and first author of the published observational study (Eur Heart J. 2019 Apr 21;40[16]:1257-64).
In the observational study, Dr. Noseworthy and coinvestigators utilized a huge U.S. administrative health claims database in order to identify a nationally representative group of 183,760 AFib patients, 12,032 of whom were treated with catheter ablation and the rest with antiarrhythmic and/or rhythm control drugs during the same years the CABANA trial was enrolling patients. The two groups were balanced using propensity score weighting to adjust for baseline differences in 90 variables.
The investigators sought to learn if the CABANA study population was representative of real-world AFib patients, and whether the observational experience could help resolve the CABANA controversy. It turned out that most AFib patients seen in daily clinical practice were CABANA like; that is, 74% of them would have been eligible for the clinical trial because they were symptomatic, over age 65, or younger than 65 with at least one CHADS2 stroke risk factor. About 22% of the large real-world sample would have been excluded from CABANA because they’d failed on amiodarone and other antiarrhythmic agents or had previously undergone ablation. About 4% of patients failed to meet the CABANA inclusion criteria.
The risk reduction for the composite endpoint associated with catheter ablation in the large retrospective study was greatest in the CABANA-like patients, at 30%. It was less robust but still statistically significant at 15% in patients who met at least one of the exclusion criteria for the trial.
The sheer size of this study provides greater statistical power than in CABANA. Of course, a nonrandomized, propensity score–based comparison such as this is always susceptible to confounding, even after adjustment for 90 variables. But the observational study does offer “a glimmer of hope” that catheter ablation, done in the right patients, might confer a stroke risk reduction and mortality benefit, he said.
The 33% relative risk reduction in the small group of real-world patients who failed to meet the CABANA inclusion criteria, while numerically impressive, wasn’t close to statistical significance, probably because event rates in that population were so low.
“Even if you could reduce stroke risk with ablation in that low-risk group, it would be a very inefficient way to reduce the population burden of stroke,” Dr. Noseworthy observed.
Putting together the results of CABANA and the large observational study to sum up his view of where catheter ablation for AF[ib] stands today, Dr. Noseworthy commented, “Ablation is reasonable for symptom control in many patients, basically anyone who is either breaking through on drugs or doesn’t want to take the drugs and is highly symptomatic. And there may be a small stroke and/or mortality benefit for people who are in the sweet spot – and those are people who look a lot like the patients enrolled in CABANA.”
Patients who met the exclusion criteria for CABANA are too advanced in their AFib to be likely to derive a stroke or mortality benefit from catheter ablation. “It’s very hard to move the needle in these patients with either a drug or catheter ablation approach. I wouldn’t try to reduce the risk of stroke here with an expensive and invasive procedure,” the electrophysiologist concluded.
He reported having no financial conflicts regarding his presentation.
SNOWMASS, COLO. – stroke, major bleeding, or cardiac arrest, compared with rhythm and/or rate control drugs in a propensity score–weighted, retrospective, observational study.
Findings of the investigation, which included more than 183,000 real-world patients in routine clinical practice, were reported by Peter S. Noseworthy, MD, during the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
The results breathe new life into the controversy created by the previously reported CABANA trial (Catheter Ablation vs. Antiarrhythmic Drug Therapy for Atrial Fibrillation), a 10-country study in which 2,204 patients with atrial fibrillation (AFib) were randomized to catheter ablation or antiarrhythmic and/or rhythm control medications and followed for a mean of about 4 years. CABANA yielded a negative result (JAMA. 2019 Apr 2;321[13]:1261-74), with the prespecified intent-to-treat analysis indicating no significant between-group difference in the primary composite endpoint – the very same one that was positive in the large observational study.
However, CABANA was marred by major problems arising from protocol deviations: Nearly 28% of patients assigned to medical therapy crossed over to catheter ablation, typically because their antiarrhythmic drugs failed, and 10% of patients randomized to catheter ablation never got it. This muddies the waters when trying to identify a true stroke/mortality benefit for catheter ablation, if indeed any such benefit was actually present.
Here’s where the controversy arose: While CABANA must be called a negative trial based upon the disappointing results of the intent-to-treat analysis, a prespecified post hoc analysis of patients as actually treated showed a statistically significant 27% relative risk reduction for the primary composite endpoint in the catheter ablation group. That’s strikingly similar to the 30% relative risk reduction for catheter ablation seen in the huge observational study, where the CABANA-type primary outcome occurred in 22.5% of the medically managed patients and 16.8% of those who underwent catheter ablation, noted Dr. Noseworthy, professor of medicine and director of heart rhythm and physiology at the Mayo Clinic in Rochester, Minn.
He ought to know: He was both an investigator in CABANA and first author of the published observational study (Eur Heart J. 2019 Apr 21;40[16]:1257-64).
In the observational study, Dr. Noseworthy and coinvestigators utilized a huge U.S. administrative health claims database in order to identify a nationally representative group of 183,760 AFib patients, 12,032 of whom were treated with catheter ablation and the rest with antiarrhythmic and/or rhythm control drugs during the same years the CABANA trial was enrolling patients. The two groups were balanced using propensity score weighting to adjust for baseline differences in 90 variables.
The investigators sought to learn if the CABANA study population was representative of real-world AFib patients, and whether the observational experience could help resolve the CABANA controversy. It turned out that most AFib patients seen in daily clinical practice were CABANA like; that is, 74% of them would have been eligible for the clinical trial because they were symptomatic, over age 65, or younger than 65 with at least one CHADS2 stroke risk factor. About 22% of the large real-world sample would have been excluded from CABANA because they’d failed on amiodarone and other antiarrhythmic agents or had previously undergone ablation. About 4% of patients failed to meet the CABANA inclusion criteria.
The risk reduction for the composite endpoint associated with catheter ablation in the large retrospective study was greatest in the CABANA-like patients, at 30%. It was less robust but still statistically significant at 15% in patients who met at least one of the exclusion criteria for the trial.
The sheer size of this study provides greater statistical power than in CABANA. Of course, a nonrandomized, propensity score–based comparison such as this is always susceptible to confounding, even after adjustment for 90 variables. But the observational study does offer “a glimmer of hope” that catheter ablation, done in the right patients, might confer a stroke risk reduction and mortality benefit, he said.
The 33% relative risk reduction in the small group of real-world patients who failed to meet the CABANA inclusion criteria, while numerically impressive, wasn’t close to statistical significance, probably because event rates in that population were so low.
“Even if you could reduce stroke risk with ablation in that low-risk group, it would be a very inefficient way to reduce the population burden of stroke,” Dr. Noseworthy observed.
Putting together the results of CABANA and the large observational study to sum up his view of where catheter ablation for AF[ib] stands today, Dr. Noseworthy commented, “Ablation is reasonable for symptom control in many patients, basically anyone who is either breaking through on drugs or doesn’t want to take the drugs and is highly symptomatic. And there may be a small stroke and/or mortality benefit for people who are in the sweet spot – and those are people who look a lot like the patients enrolled in CABANA.”
Patients who met the exclusion criteria for CABANA are too advanced in their AFib to be likely to derive a stroke or mortality benefit from catheter ablation. “It’s very hard to move the needle in these patients with either a drug or catheter ablation approach. I wouldn’t try to reduce the risk of stroke here with an expensive and invasive procedure,” the electrophysiologist concluded.
He reported having no financial conflicts regarding his presentation.
SNOWMASS, COLO. – stroke, major bleeding, or cardiac arrest, compared with rhythm and/or rate control drugs in a propensity score–weighted, retrospective, observational study.
Findings of the investigation, which included more than 183,000 real-world patients in routine clinical practice, were reported by Peter S. Noseworthy, MD, during the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
The results breathe new life into the controversy created by the previously reported CABANA trial (Catheter Ablation vs. Antiarrhythmic Drug Therapy for Atrial Fibrillation), a 10-country study in which 2,204 patients with atrial fibrillation (AFib) were randomized to catheter ablation or antiarrhythmic and/or rhythm control medications and followed for a mean of about 4 years. CABANA yielded a negative result (JAMA. 2019 Apr 2;321[13]:1261-74), with the prespecified intent-to-treat analysis indicating no significant between-group difference in the primary composite endpoint – the very same one that was positive in the large observational study.
However, CABANA was marred by major problems arising from protocol deviations: Nearly 28% of patients assigned to medical therapy crossed over to catheter ablation, typically because their antiarrhythmic drugs failed, and 10% of patients randomized to catheter ablation never got it. This muddies the waters when trying to identify a true stroke/mortality benefit for catheter ablation, if indeed any such benefit was actually present.
Here’s where the controversy arose: While CABANA must be called a negative trial based upon the disappointing results of the intent-to-treat analysis, a prespecified post hoc analysis of patients as actually treated showed a statistically significant 27% relative risk reduction for the primary composite endpoint in the catheter ablation group. That’s strikingly similar to the 30% relative risk reduction for catheter ablation seen in the huge observational study, where the CABANA-type primary outcome occurred in 22.5% of the medically managed patients and 16.8% of those who underwent catheter ablation, noted Dr. Noseworthy, professor of medicine and director of heart rhythm and physiology at the Mayo Clinic in Rochester, Minn.
He ought to know: He was both an investigator in CABANA and first author of the published observational study (Eur Heart J. 2019 Apr 21;40[16]:1257-64).
In the observational study, Dr. Noseworthy and coinvestigators utilized a huge U.S. administrative health claims database in order to identify a nationally representative group of 183,760 AFib patients, 12,032 of whom were treated with catheter ablation and the rest with antiarrhythmic and/or rhythm control drugs during the same years the CABANA trial was enrolling patients. The two groups were balanced using propensity score weighting to adjust for baseline differences in 90 variables.
The investigators sought to learn if the CABANA study population was representative of real-world AFib patients, and whether the observational experience could help resolve the CABANA controversy. It turned out that most AFib patients seen in daily clinical practice were CABANA like; that is, 74% of them would have been eligible for the clinical trial because they were symptomatic, over age 65, or younger than 65 with at least one CHADS2 stroke risk factor. About 22% of the large real-world sample would have been excluded from CABANA because they’d failed on amiodarone and other antiarrhythmic agents or had previously undergone ablation. About 4% of patients failed to meet the CABANA inclusion criteria.
The risk reduction for the composite endpoint associated with catheter ablation in the large retrospective study was greatest in the CABANA-like patients, at 30%. It was less robust but still statistically significant at 15% in patients who met at least one of the exclusion criteria for the trial.
The sheer size of this study provides greater statistical power than in CABANA. Of course, a nonrandomized, propensity score–based comparison such as this is always susceptible to confounding, even after adjustment for 90 variables. But the observational study does offer “a glimmer of hope” that catheter ablation, done in the right patients, might confer a stroke risk reduction and mortality benefit, he said.
The 33% relative risk reduction in the small group of real-world patients who failed to meet the CABANA inclusion criteria, while numerically impressive, wasn’t close to statistical significance, probably because event rates in that population were so low.
“Even if you could reduce stroke risk with ablation in that low-risk group, it would be a very inefficient way to reduce the population burden of stroke,” Dr. Noseworthy observed.
Putting together the results of CABANA and the large observational study to sum up his view of where catheter ablation for AF[ib] stands today, Dr. Noseworthy commented, “Ablation is reasonable for symptom control in many patients, basically anyone who is either breaking through on drugs or doesn’t want to take the drugs and is highly symptomatic. And there may be a small stroke and/or mortality benefit for people who are in the sweet spot – and those are people who look a lot like the patients enrolled in CABANA.”
Patients who met the exclusion criteria for CABANA are too advanced in their AFib to be likely to derive a stroke or mortality benefit from catheter ablation. “It’s very hard to move the needle in these patients with either a drug or catheter ablation approach. I wouldn’t try to reduce the risk of stroke here with an expensive and invasive procedure,” the electrophysiologist concluded.
He reported having no financial conflicts regarding his presentation.
REPORTING FROM ACC SNOWMASS 2020
IBD quality initiative slashes ED utilization
AUSTIN, TEX. – A quality improvement initiative aimed at patients with inflammatory bowel disease (IBD) has reduced emergency department visits and hospitalizations by 20% or more and slashed opioid use by half, according to study results presented at the Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
After 15 months, the quality improvement program saw emergency department visit rates decline from 18% to 14%, a 22% relative decrease, Gil Y. Melmed, MD, of Cedars-Sinai Medical Center, Los Angeles, said. Additionally, the study documented a similar decrease in the rate of hospitalization, declining from 14% to 11%, while opioid utilization rates declined from 8% to 4%. “We also found decreases in special-cause variation in other measures of interest, including CT scan utilization as well as corticosteroid use, which was reduced 29% during the course of the program,” he said.
The quality initiative was conducted through the Crohn’s & Colitis Foundation as an outgrowth of its IBD Qorus quality improvement program. The 15-month study involved 20,392 patient visits at 15 academic and 11 private/community practices from January 2018 to April 2019. “This specific project within Qorus is focused specifically around the concept of improving access during times of urgent care need,” Dr. Melmed told this news organization. The goal was to identify practice changes that can drive improvement.
The intervention consisted of 19 different strategies, called a “Change Package,” and participating sites could choose to test and implement one or more of them, Dr. Melmed said. Some examples included designating urgent care slots in the clinic schedule, installing a nurse hotline, a weekly “huddle” to review high-risk patients, and patient education on using urgent care.
One of the drivers of the program was to provide immediate care improvement to patients, Dr. Melmed said in the interview. “As opposed to investments into the cure of IBD that we need, but which can take years to develop, this research has immediate, practical applicability for patients today,” he said.
“The fact that we were able to demonstrate reduction in emergency room utilization and hospitalization, steroid use, and narcotic use has really energized the work that we were doing. We can now show that very-low-cost process changes at a site level lead to robust improvement in patient outcomes. These changes are potentially implementable in any practice setting,” Dr. Melmed said in the interview.
After Dr. Melmed’s presentation, Maria T. Abreu, MD, director of the Crohn’s and Colitis Center at the University of Miami, asked about the cost of the interventions. Dr. Melmed said the costs were nominal, such as paying for a new phone line for a patient hotline. “But overall the cost really involved in the program was the time that it took to review the high-risk list on a weekly basis with the team, and that is essentially a 15-minute huddle,” he said.
Later, Dr. Abreu said in an interview that the program was “a terrific example of how measuring outcomes and sharing ideas can make huge impacts in the lives of patients.” She added, “An enormous amount of money is spent on clinical trials of expensive biologics which have revolutionized treatment, yet the humanistic aspects of our care have just as great of an impact. In this study, each center focused on ways they could lower ER visits and hospitalizations. One size did not fit all, yet they could learn from each other. The very platform they used to conduct the study is a model for all of us.”
Corey A. Siegel, MD, of the Dartmouth-Hitchcock Medical Center, Lebanon, N.H., and Dr. Melmed's coprincipal investigator on Qorus, said the quality initiative now includes 49 GI practices across the country with plans to grow to 60 by the end of the year. "We have created this 'collaboratory' for providers from actross the country to work togetherr to learn how to best deliver high-qulaity care for patients with IBD," he said.
Another feature of the quality initiative allowed participating sites to see how they compared with others anonymously, Dr. Melmed said. “Using the data, we called out high-performing sites to teach the rest of us what they were doing that enabled them to improve, so that all of us could learn from their successes,” he said.
The researchers are aiming to evaluate costs and identify the most successful interventions, with the plan to present the latter at Digestive Disease Week® 2020 and use them to develop a toolkit practices can use. “Ultimately,” said Dr. Melmed, “this is scalable.”
Dr. Melmed disclosed financial relationships with AbbVie, Boehringer-Ingelheim, Celgene, Jannsen, GSK, Medtronic, Pfizer, Samsung Bioepis, Takeda, and Techlab; IBD Qorus receives support from Abbvie, AMAG, Helmsley Charitable Trust, Janssen, Nephoroceuticals, Pfizer, Takeda, and UCB.
SOURCE: Melmed GT et al. Crohn’s & Colitis Congress 2020, Session 28.
AUSTIN, TEX. – A quality improvement initiative aimed at patients with inflammatory bowel disease (IBD) has reduced emergency department visits and hospitalizations by 20% or more and slashed opioid use by half, according to study results presented at the Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
After 15 months, the quality improvement program saw emergency department visit rates decline from 18% to 14%, a 22% relative decrease, Gil Y. Melmed, MD, of Cedars-Sinai Medical Center, Los Angeles, said. Additionally, the study documented a similar decrease in the rate of hospitalization, declining from 14% to 11%, while opioid utilization rates declined from 8% to 4%. “We also found decreases in special-cause variation in other measures of interest, including CT scan utilization as well as corticosteroid use, which was reduced 29% during the course of the program,” he said.
The quality initiative was conducted through the Crohn’s & Colitis Foundation as an outgrowth of its IBD Qorus quality improvement program. The 15-month study involved 20,392 patient visits at 15 academic and 11 private/community practices from January 2018 to April 2019. “This specific project within Qorus is focused specifically around the concept of improving access during times of urgent care need,” Dr. Melmed told this news organization. The goal was to identify practice changes that can drive improvement.
The intervention consisted of 19 different strategies, called a “Change Package,” and participating sites could choose to test and implement one or more of them, Dr. Melmed said. Some examples included designating urgent care slots in the clinic schedule, installing a nurse hotline, a weekly “huddle” to review high-risk patients, and patient education on using urgent care.
One of the drivers of the program was to provide immediate care improvement to patients, Dr. Melmed said in the interview. “As opposed to investments into the cure of IBD that we need, but which can take years to develop, this research has immediate, practical applicability for patients today,” he said.
“The fact that we were able to demonstrate reduction in emergency room utilization and hospitalization, steroid use, and narcotic use has really energized the work that we were doing. We can now show that very-low-cost process changes at a site level lead to robust improvement in patient outcomes. These changes are potentially implementable in any practice setting,” Dr. Melmed said in the interview.
After Dr. Melmed’s presentation, Maria T. Abreu, MD, director of the Crohn’s and Colitis Center at the University of Miami, asked about the cost of the interventions. Dr. Melmed said the costs were nominal, such as paying for a new phone line for a patient hotline. “But overall the cost really involved in the program was the time that it took to review the high-risk list on a weekly basis with the team, and that is essentially a 15-minute huddle,” he said.
Later, Dr. Abreu said in an interview that the program was “a terrific example of how measuring outcomes and sharing ideas can make huge impacts in the lives of patients.” She added, “An enormous amount of money is spent on clinical trials of expensive biologics which have revolutionized treatment, yet the humanistic aspects of our care have just as great of an impact. In this study, each center focused on ways they could lower ER visits and hospitalizations. One size did not fit all, yet they could learn from each other. The very platform they used to conduct the study is a model for all of us.”
Corey A. Siegel, MD, of the Dartmouth-Hitchcock Medical Center, Lebanon, N.H., and Dr. Melmed's coprincipal investigator on Qorus, said the quality initiative now includes 49 GI practices across the country with plans to grow to 60 by the end of the year. "We have created this 'collaboratory' for providers from actross the country to work togetherr to learn how to best deliver high-qulaity care for patients with IBD," he said.
Another feature of the quality initiative allowed participating sites to see how they compared with others anonymously, Dr. Melmed said. “Using the data, we called out high-performing sites to teach the rest of us what they were doing that enabled them to improve, so that all of us could learn from their successes,” he said.
The researchers are aiming to evaluate costs and identify the most successful interventions, with the plan to present the latter at Digestive Disease Week® 2020 and use them to develop a toolkit practices can use. “Ultimately,” said Dr. Melmed, “this is scalable.”
Dr. Melmed disclosed financial relationships with AbbVie, Boehringer-Ingelheim, Celgene, Jannsen, GSK, Medtronic, Pfizer, Samsung Bioepis, Takeda, and Techlab; IBD Qorus receives support from Abbvie, AMAG, Helmsley Charitable Trust, Janssen, Nephoroceuticals, Pfizer, Takeda, and UCB.
SOURCE: Melmed GT et al. Crohn’s & Colitis Congress 2020, Session 28.
AUSTIN, TEX. – A quality improvement initiative aimed at patients with inflammatory bowel disease (IBD) has reduced emergency department visits and hospitalizations by 20% or more and slashed opioid use by half, according to study results presented at the Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
After 15 months, the quality improvement program saw emergency department visit rates decline from 18% to 14%, a 22% relative decrease, Gil Y. Melmed, MD, of Cedars-Sinai Medical Center, Los Angeles, said. Additionally, the study documented a similar decrease in the rate of hospitalization, declining from 14% to 11%, while opioid utilization rates declined from 8% to 4%. “We also found decreases in special-cause variation in other measures of interest, including CT scan utilization as well as corticosteroid use, which was reduced 29% during the course of the program,” he said.
The quality initiative was conducted through the Crohn’s & Colitis Foundation as an outgrowth of its IBD Qorus quality improvement program. The 15-month study involved 20,392 patient visits at 15 academic and 11 private/community practices from January 2018 to April 2019. “This specific project within Qorus is focused specifically around the concept of improving access during times of urgent care need,” Dr. Melmed told this news organization. The goal was to identify practice changes that can drive improvement.
The intervention consisted of 19 different strategies, called a “Change Package,” and participating sites could choose to test and implement one or more of them, Dr. Melmed said. Some examples included designating urgent care slots in the clinic schedule, installing a nurse hotline, a weekly “huddle” to review high-risk patients, and patient education on using urgent care.
One of the drivers of the program was to provide immediate care improvement to patients, Dr. Melmed said in the interview. “As opposed to investments into the cure of IBD that we need, but which can take years to develop, this research has immediate, practical applicability for patients today,” he said.
“The fact that we were able to demonstrate reduction in emergency room utilization and hospitalization, steroid use, and narcotic use has really energized the work that we were doing. We can now show that very-low-cost process changes at a site level lead to robust improvement in patient outcomes. These changes are potentially implementable in any practice setting,” Dr. Melmed said in the interview.
After Dr. Melmed’s presentation, Maria T. Abreu, MD, director of the Crohn’s and Colitis Center at the University of Miami, asked about the cost of the interventions. Dr. Melmed said the costs were nominal, such as paying for a new phone line for a patient hotline. “But overall the cost really involved in the program was the time that it took to review the high-risk list on a weekly basis with the team, and that is essentially a 15-minute huddle,” he said.
Later, Dr. Abreu said in an interview that the program was “a terrific example of how measuring outcomes and sharing ideas can make huge impacts in the lives of patients.” She added, “An enormous amount of money is spent on clinical trials of expensive biologics which have revolutionized treatment, yet the humanistic aspects of our care have just as great of an impact. In this study, each center focused on ways they could lower ER visits and hospitalizations. One size did not fit all, yet they could learn from each other. The very platform they used to conduct the study is a model for all of us.”
Corey A. Siegel, MD, of the Dartmouth-Hitchcock Medical Center, Lebanon, N.H., and Dr. Melmed's coprincipal investigator on Qorus, said the quality initiative now includes 49 GI practices across the country with plans to grow to 60 by the end of the year. "We have created this 'collaboratory' for providers from actross the country to work togetherr to learn how to best deliver high-qulaity care for patients with IBD," he said.
Another feature of the quality initiative allowed participating sites to see how they compared with others anonymously, Dr. Melmed said. “Using the data, we called out high-performing sites to teach the rest of us what they were doing that enabled them to improve, so that all of us could learn from their successes,” he said.
The researchers are aiming to evaluate costs and identify the most successful interventions, with the plan to present the latter at Digestive Disease Week® 2020 and use them to develop a toolkit practices can use. “Ultimately,” said Dr. Melmed, “this is scalable.”
Dr. Melmed disclosed financial relationships with AbbVie, Boehringer-Ingelheim, Celgene, Jannsen, GSK, Medtronic, Pfizer, Samsung Bioepis, Takeda, and Techlab; IBD Qorus receives support from Abbvie, AMAG, Helmsley Charitable Trust, Janssen, Nephoroceuticals, Pfizer, Takeda, and UCB.
SOURCE: Melmed GT et al. Crohn’s & Colitis Congress 2020, Session 28.
REPORTING FROM CROHN’S & COLITIS CONGRESS
Cardiac arrest: Targeted temperature management a game changer
SNOWMASS, COLO. – Targeted temperature management maintained at 32-36 degrees Celsius is now a strong class I recommendation for all comatose patients who experience return of spontaneous circulation after out-of-hospital cardiac arrest, including those with nonshockable rhythms, Erin A. Bohula, MD, PhD, said at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
“Our practice is that ,” said Dr. Bohula, a cardiologist and critical care specialist at Brigham and Women’s Hospital and Harvard Medical School, Boston.
The current ACC/AHA guidelines declare: “There are essentially no patients for whom temperature control somewhere in the range between 32 degrees C [89.6 F) and 36 degrees C [96.8 F] is contraindicated.” The writing committee cited “recent clinical trial data enrolling patients with all rhythms, the rarity of adverse effects in trials, the high neurologic morbidity and mortality without any specific interventions, and the preponderance of data suggesting that temperature is an important variable for neurologic recovery” (Circulation. 2015 Nov 3;132[18 Suppl 2]:S465-82).
“That’s a pretty strong statement,” Dr. Bohula observed.
The current guidelines, which date back to 2015, give a class I, level of evidence B recommendation for targeted temperature management (TTM) in patients who are comatose with return of spontaneous circulation (ROSC) after out-of-hospital cardiac arrest involving ventricular fibrillation or pulseless ventricular fibrillation. The bedside definition of comatose is lack of meaningful response to verbal commands to squeeze hands, blink, or move toes.
The current recommendation for TTM in patients resuscitated from out-of-hospital cardiac arrest with a nonshockable rhythm is class I, level of evidence C, meaning it’s based on expert consensus. However, that recommendation is now out of date and due for a level-of-evidence upgrade in light of the recent results of the French HYPERION trial, an open-label randomized trial of 584 patients resuscitated from cardiac arrest with a nonshockable rhythm. Although 90-day mortality was similarly high in the TTM and targeted normothermia groups, the rate of favorable neurologic outcome as assessed by a Cerebral Performance Category scale score of 1 or 2 was 10.2% in the TTM group, significantly better than the 5.7% rate in controls (N Engl J Med. 2019 Dec 12;381[24]:2327-37).
The 2010, ACC/AHA guidelines recommended a TTM range of 32-34 degrees C, but on the basis of subsequent persuasive randomized trial data, that range was broadened to 32-36 degrees C in the 2015 guidelines, with a class IB recommendation. Maintenance of TTM for at least 24 hours has a IIa, level of evidence C recommendation in the current guidelines.
The guidelines emphasize that specific features may favor selection of one temperature for TTM over another. For example, patients with seizures or cerebral edema might be better off with TTM at a lower temperature, while a higher temperature may be best for those with bleeding or severe bradycardia. At Brigham and Women’s Hospital, the default temperature is 33 degrees C. However, TTM with a goal of 36 degrees C is seriously considered in patients with recent head trauma, major surgery within the past 2 weeks, refractory hypotension, severe sepsis, pregnancy, or high bleeding risk. Rewarming is done at a rate of 0.25 degrees C per hour, with sedation maintained until the patient has been returned to 98.6 degrees F, according to Dr. Bohula.
Based on several negative studies of TTM using rapid infusion of chilled fluids in the ambulance en route to the hospital, the guidelines rate that practice class IIIA, meaning don’t do it. Avoidance of a systolic blood pressure below 90 mm Hg and a mean arterial pressure of less than 65 mm Hg gets a class IIb level of evidence C recommendation to lessen the risk of cerebral hypoxia.
TTM a major breakthrough
Prior to the introduction of TTM, comatose patients with ROSC after out-of-hospital cardiac arrest had a dreadful prognosis, with survival rates of 1%-10% in registry studies. In contrast, the survival rate in the landmark TTM clinical trials was 50%-60%. And while that’s a dramatic improvement, ROSC after cardiac arrest remains a high-mortality condition. Dr. Bohula was first author of a report by the Critical Care Cardiology Trials Network, composed of 16 tertiary cardiac intensive care units in the United States and Canada. Cardiac arrest was the primary indication for 8.7% of 3,049 consecutive admissions, and its 38% mortality rate was the highest of all cardiac critical care indications (JAMA Cardiol. 2019 Jul 24;4[9]:928-35).
TTM was developed in response to a recognition that two-thirds of deaths in patients who make it to the hospital after out-of-hospital cardiac arrest are neurologic – the result of brain anoxia – rather than being due to the myocardial ischemia that may have initially brought them to medical attention.
“Time is brain cells, the same way we think of time as cardiac muscle,” Dr. Bohula observed.
The main idea behind therapeutic hypothermia is that it lowers the cerebral metabolic rate of oxygen to reduce the consequences of ongoing anoxia. The brain doesn’t require as much perfusion when cooled.
TTM has other beneficial neurologic effects as well: It reduces cerebral blood volume via autoregulation, decreases intracranial pressure, and blunts the inflammatory response involved in the postcardiac arrest syndrome. In addition, TTM has anticonvulsant properties, an important effect because seizures and/or myoclonus occur in up to 15% of adults who achieve ROSC after cardiac arrest – and in even more of those who are comatose after doing so. And seizures increase the brain’s metabolic rate threefold, resulting in more cerebral ischemic injury, she explained.
Seizure activity can be difficult to distinguish from shivering in a patient on TTM. For this reason Dr. Bohula recommends putting patients on continuous EEG monitoring from the time of admission, as is the routine practice at the Brigham.
She reported serving as a consultant to Daiichi Sankyo, Servier, Lexicon, Kowa, Merck, Novartis, Novo Nordisk, and the National Institutes of Health. In addition, she generates institutional research grants provided by a half-dozen pharmaceutical companies.
SNOWMASS, COLO. – Targeted temperature management maintained at 32-36 degrees Celsius is now a strong class I recommendation for all comatose patients who experience return of spontaneous circulation after out-of-hospital cardiac arrest, including those with nonshockable rhythms, Erin A. Bohula, MD, PhD, said at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
“Our practice is that ,” said Dr. Bohula, a cardiologist and critical care specialist at Brigham and Women’s Hospital and Harvard Medical School, Boston.
The current ACC/AHA guidelines declare: “There are essentially no patients for whom temperature control somewhere in the range between 32 degrees C [89.6 F) and 36 degrees C [96.8 F] is contraindicated.” The writing committee cited “recent clinical trial data enrolling patients with all rhythms, the rarity of adverse effects in trials, the high neurologic morbidity and mortality without any specific interventions, and the preponderance of data suggesting that temperature is an important variable for neurologic recovery” (Circulation. 2015 Nov 3;132[18 Suppl 2]:S465-82).
“That’s a pretty strong statement,” Dr. Bohula observed.
The current guidelines, which date back to 2015, give a class I, level of evidence B recommendation for targeted temperature management (TTM) in patients who are comatose with return of spontaneous circulation (ROSC) after out-of-hospital cardiac arrest involving ventricular fibrillation or pulseless ventricular fibrillation. The bedside definition of comatose is lack of meaningful response to verbal commands to squeeze hands, blink, or move toes.
The current recommendation for TTM in patients resuscitated from out-of-hospital cardiac arrest with a nonshockable rhythm is class I, level of evidence C, meaning it’s based on expert consensus. However, that recommendation is now out of date and due for a level-of-evidence upgrade in light of the recent results of the French HYPERION trial, an open-label randomized trial of 584 patients resuscitated from cardiac arrest with a nonshockable rhythm. Although 90-day mortality was similarly high in the TTM and targeted normothermia groups, the rate of favorable neurologic outcome as assessed by a Cerebral Performance Category scale score of 1 or 2 was 10.2% in the TTM group, significantly better than the 5.7% rate in controls (N Engl J Med. 2019 Dec 12;381[24]:2327-37).
The 2010, ACC/AHA guidelines recommended a TTM range of 32-34 degrees C, but on the basis of subsequent persuasive randomized trial data, that range was broadened to 32-36 degrees C in the 2015 guidelines, with a class IB recommendation. Maintenance of TTM for at least 24 hours has a IIa, level of evidence C recommendation in the current guidelines.
The guidelines emphasize that specific features may favor selection of one temperature for TTM over another. For example, patients with seizures or cerebral edema might be better off with TTM at a lower temperature, while a higher temperature may be best for those with bleeding or severe bradycardia. At Brigham and Women’s Hospital, the default temperature is 33 degrees C. However, TTM with a goal of 36 degrees C is seriously considered in patients with recent head trauma, major surgery within the past 2 weeks, refractory hypotension, severe sepsis, pregnancy, or high bleeding risk. Rewarming is done at a rate of 0.25 degrees C per hour, with sedation maintained until the patient has been returned to 98.6 degrees F, according to Dr. Bohula.
Based on several negative studies of TTM using rapid infusion of chilled fluids in the ambulance en route to the hospital, the guidelines rate that practice class IIIA, meaning don’t do it. Avoidance of a systolic blood pressure below 90 mm Hg and a mean arterial pressure of less than 65 mm Hg gets a class IIb level of evidence C recommendation to lessen the risk of cerebral hypoxia.
TTM a major breakthrough
Prior to the introduction of TTM, comatose patients with ROSC after out-of-hospital cardiac arrest had a dreadful prognosis, with survival rates of 1%-10% in registry studies. In contrast, the survival rate in the landmark TTM clinical trials was 50%-60%. And while that’s a dramatic improvement, ROSC after cardiac arrest remains a high-mortality condition. Dr. Bohula was first author of a report by the Critical Care Cardiology Trials Network, composed of 16 tertiary cardiac intensive care units in the United States and Canada. Cardiac arrest was the primary indication for 8.7% of 3,049 consecutive admissions, and its 38% mortality rate was the highest of all cardiac critical care indications (JAMA Cardiol. 2019 Jul 24;4[9]:928-35).
TTM was developed in response to a recognition that two-thirds of deaths in patients who make it to the hospital after out-of-hospital cardiac arrest are neurologic – the result of brain anoxia – rather than being due to the myocardial ischemia that may have initially brought them to medical attention.
“Time is brain cells, the same way we think of time as cardiac muscle,” Dr. Bohula observed.
The main idea behind therapeutic hypothermia is that it lowers the cerebral metabolic rate of oxygen to reduce the consequences of ongoing anoxia. The brain doesn’t require as much perfusion when cooled.
TTM has other beneficial neurologic effects as well: It reduces cerebral blood volume via autoregulation, decreases intracranial pressure, and blunts the inflammatory response involved in the postcardiac arrest syndrome. In addition, TTM has anticonvulsant properties, an important effect because seizures and/or myoclonus occur in up to 15% of adults who achieve ROSC after cardiac arrest – and in even more of those who are comatose after doing so. And seizures increase the brain’s metabolic rate threefold, resulting in more cerebral ischemic injury, she explained.
Seizure activity can be difficult to distinguish from shivering in a patient on TTM. For this reason Dr. Bohula recommends putting patients on continuous EEG monitoring from the time of admission, as is the routine practice at the Brigham.
She reported serving as a consultant to Daiichi Sankyo, Servier, Lexicon, Kowa, Merck, Novartis, Novo Nordisk, and the National Institutes of Health. In addition, she generates institutional research grants provided by a half-dozen pharmaceutical companies.
SNOWMASS, COLO. – Targeted temperature management maintained at 32-36 degrees Celsius is now a strong class I recommendation for all comatose patients who experience return of spontaneous circulation after out-of-hospital cardiac arrest, including those with nonshockable rhythms, Erin A. Bohula, MD, PhD, said at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
“Our practice is that ,” said Dr. Bohula, a cardiologist and critical care specialist at Brigham and Women’s Hospital and Harvard Medical School, Boston.
The current ACC/AHA guidelines declare: “There are essentially no patients for whom temperature control somewhere in the range between 32 degrees C [89.6 F) and 36 degrees C [96.8 F] is contraindicated.” The writing committee cited “recent clinical trial data enrolling patients with all rhythms, the rarity of adverse effects in trials, the high neurologic morbidity and mortality without any specific interventions, and the preponderance of data suggesting that temperature is an important variable for neurologic recovery” (Circulation. 2015 Nov 3;132[18 Suppl 2]:S465-82).
“That’s a pretty strong statement,” Dr. Bohula observed.
The current guidelines, which date back to 2015, give a class I, level of evidence B recommendation for targeted temperature management (TTM) in patients who are comatose with return of spontaneous circulation (ROSC) after out-of-hospital cardiac arrest involving ventricular fibrillation or pulseless ventricular fibrillation. The bedside definition of comatose is lack of meaningful response to verbal commands to squeeze hands, blink, or move toes.
The current recommendation for TTM in patients resuscitated from out-of-hospital cardiac arrest with a nonshockable rhythm is class I, level of evidence C, meaning it’s based on expert consensus. However, that recommendation is now out of date and due for a level-of-evidence upgrade in light of the recent results of the French HYPERION trial, an open-label randomized trial of 584 patients resuscitated from cardiac arrest with a nonshockable rhythm. Although 90-day mortality was similarly high in the TTM and targeted normothermia groups, the rate of favorable neurologic outcome as assessed by a Cerebral Performance Category scale score of 1 or 2 was 10.2% in the TTM group, significantly better than the 5.7% rate in controls (N Engl J Med. 2019 Dec 12;381[24]:2327-37).
The 2010, ACC/AHA guidelines recommended a TTM range of 32-34 degrees C, but on the basis of subsequent persuasive randomized trial data, that range was broadened to 32-36 degrees C in the 2015 guidelines, with a class IB recommendation. Maintenance of TTM for at least 24 hours has a IIa, level of evidence C recommendation in the current guidelines.
The guidelines emphasize that specific features may favor selection of one temperature for TTM over another. For example, patients with seizures or cerebral edema might be better off with TTM at a lower temperature, while a higher temperature may be best for those with bleeding or severe bradycardia. At Brigham and Women’s Hospital, the default temperature is 33 degrees C. However, TTM with a goal of 36 degrees C is seriously considered in patients with recent head trauma, major surgery within the past 2 weeks, refractory hypotension, severe sepsis, pregnancy, or high bleeding risk. Rewarming is done at a rate of 0.25 degrees C per hour, with sedation maintained until the patient has been returned to 98.6 degrees F, according to Dr. Bohula.
Based on several negative studies of TTM using rapid infusion of chilled fluids in the ambulance en route to the hospital, the guidelines rate that practice class IIIA, meaning don’t do it. Avoidance of a systolic blood pressure below 90 mm Hg and a mean arterial pressure of less than 65 mm Hg gets a class IIb level of evidence C recommendation to lessen the risk of cerebral hypoxia.
TTM a major breakthrough
Prior to the introduction of TTM, comatose patients with ROSC after out-of-hospital cardiac arrest had a dreadful prognosis, with survival rates of 1%-10% in registry studies. In contrast, the survival rate in the landmark TTM clinical trials was 50%-60%. And while that’s a dramatic improvement, ROSC after cardiac arrest remains a high-mortality condition. Dr. Bohula was first author of a report by the Critical Care Cardiology Trials Network, composed of 16 tertiary cardiac intensive care units in the United States and Canada. Cardiac arrest was the primary indication for 8.7% of 3,049 consecutive admissions, and its 38% mortality rate was the highest of all cardiac critical care indications (JAMA Cardiol. 2019 Jul 24;4[9]:928-35).
TTM was developed in response to a recognition that two-thirds of deaths in patients who make it to the hospital after out-of-hospital cardiac arrest are neurologic – the result of brain anoxia – rather than being due to the myocardial ischemia that may have initially brought them to medical attention.
“Time is brain cells, the same way we think of time as cardiac muscle,” Dr. Bohula observed.
The main idea behind therapeutic hypothermia is that it lowers the cerebral metabolic rate of oxygen to reduce the consequences of ongoing anoxia. The brain doesn’t require as much perfusion when cooled.
TTM has other beneficial neurologic effects as well: It reduces cerebral blood volume via autoregulation, decreases intracranial pressure, and blunts the inflammatory response involved in the postcardiac arrest syndrome. In addition, TTM has anticonvulsant properties, an important effect because seizures and/or myoclonus occur in up to 15% of adults who achieve ROSC after cardiac arrest – and in even more of those who are comatose after doing so. And seizures increase the brain’s metabolic rate threefold, resulting in more cerebral ischemic injury, she explained.
Seizure activity can be difficult to distinguish from shivering in a patient on TTM. For this reason Dr. Bohula recommends putting patients on continuous EEG monitoring from the time of admission, as is the routine practice at the Brigham.
She reported serving as a consultant to Daiichi Sankyo, Servier, Lexicon, Kowa, Merck, Novartis, Novo Nordisk, and the National Institutes of Health. In addition, she generates institutional research grants provided by a half-dozen pharmaceutical companies.
EXPERT ANALYSIS FROM ACC SNOWMASS 2020
HHS declares coronavirus emergency, orders quarantine
The federal government declared a formal public health emergency on Jan. 31 to aid in the response to the 2019 Novel Coronavirus (2019-nCoV). The declaration, issued by Health and Human Services Secretary Alex. M. Azar II gives state, tribal, and local health departments additional flexibility to request assistance from the federal government in responding to the coronavirus.
"While this virus poses a serious public health threat, the risk to the American public remains low at this time, and we are working to keep this risk low."*
2019-nCoV—the first such action taken by the Centers for Disease Control and Prevention in more than 50 years.
“This decision is based on the current scientific facts,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during a press briefing Jan. 31. “While we understand the action seems drastic, our goal today, tomorrow, and always continues to be the safety of the American public. We would rather be remembered for over-reacting than under-reacting.”
These actions come on the heels of the World Health Organization’s Jan. 30 declaration of 2019-nCoV as a public health emergency of international concern, and from a recent spike in cases reported by Chinese health officials. “Every day this week China has reported additional cases,” Dr. Messonnier said. “Today’s numbers are a 26% increase since yesterday. Over the course of the last week, there have been nearly 7,000 new cases reported. This tells us the virus is continuing to spread rapidly in China. The reported deaths have continued to rise as well. In addition, locations outside China have continued to report cases. There have been an increasing number of reports of person-to-person spread, and now, most recently, a report in the New England Journal of Medicine of asymptomatic spread.”

The quarantine of passengers will last 14 days from when the plane left Wuhan, China. Martin Cetron, MD, who directs the CDC’s Division of Global Migration and Quarantine, said that the quarantine order “offers the greatest level of protection for the American public in preventing introduction and spread. That is our primary concern. Prior epidemics suggest that when people are properly informed, they’re usually very compliant with this request to restrict their movement. This allows someone who would become symptomatic to be rapidly identified. Offering early, rapid diagnosis of their illness could alleviate a lot of anxiety and uncertainty. In addition, this is a protective effect on family members. No individual wants to be the source of introducing or exposing a family member or a loved one to their virus. Additionally, this is part of their civic responsibility to protect their communities.”
* This story was updated on 01/31/2020.
The federal government declared a formal public health emergency on Jan. 31 to aid in the response to the 2019 Novel Coronavirus (2019-nCoV). The declaration, issued by Health and Human Services Secretary Alex. M. Azar II gives state, tribal, and local health departments additional flexibility to request assistance from the federal government in responding to the coronavirus.
"While this virus poses a serious public health threat, the risk to the American public remains low at this time, and we are working to keep this risk low."*
2019-nCoV—the first such action taken by the Centers for Disease Control and Prevention in more than 50 years.
“This decision is based on the current scientific facts,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during a press briefing Jan. 31. “While we understand the action seems drastic, our goal today, tomorrow, and always continues to be the safety of the American public. We would rather be remembered for over-reacting than under-reacting.”
These actions come on the heels of the World Health Organization’s Jan. 30 declaration of 2019-nCoV as a public health emergency of international concern, and from a recent spike in cases reported by Chinese health officials. “Every day this week China has reported additional cases,” Dr. Messonnier said. “Today’s numbers are a 26% increase since yesterday. Over the course of the last week, there have been nearly 7,000 new cases reported. This tells us the virus is continuing to spread rapidly in China. The reported deaths have continued to rise as well. In addition, locations outside China have continued to report cases. There have been an increasing number of reports of person-to-person spread, and now, most recently, a report in the New England Journal of Medicine of asymptomatic spread.”

The quarantine of passengers will last 14 days from when the plane left Wuhan, China. Martin Cetron, MD, who directs the CDC’s Division of Global Migration and Quarantine, said that the quarantine order “offers the greatest level of protection for the American public in preventing introduction and spread. That is our primary concern. Prior epidemics suggest that when people are properly informed, they’re usually very compliant with this request to restrict their movement. This allows someone who would become symptomatic to be rapidly identified. Offering early, rapid diagnosis of their illness could alleviate a lot of anxiety and uncertainty. In addition, this is a protective effect on family members. No individual wants to be the source of introducing or exposing a family member or a loved one to their virus. Additionally, this is part of their civic responsibility to protect their communities.”
* This story was updated on 01/31/2020.
The federal government declared a formal public health emergency on Jan. 31 to aid in the response to the 2019 Novel Coronavirus (2019-nCoV). The declaration, issued by Health and Human Services Secretary Alex. M. Azar II gives state, tribal, and local health departments additional flexibility to request assistance from the federal government in responding to the coronavirus.
"While this virus poses a serious public health threat, the risk to the American public remains low at this time, and we are working to keep this risk low."*
2019-nCoV—the first such action taken by the Centers for Disease Control and Prevention in more than 50 years.
“This decision is based on the current scientific facts,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during a press briefing Jan. 31. “While we understand the action seems drastic, our goal today, tomorrow, and always continues to be the safety of the American public. We would rather be remembered for over-reacting than under-reacting.”
These actions come on the heels of the World Health Organization’s Jan. 30 declaration of 2019-nCoV as a public health emergency of international concern, and from a recent spike in cases reported by Chinese health officials. “Every day this week China has reported additional cases,” Dr. Messonnier said. “Today’s numbers are a 26% increase since yesterday. Over the course of the last week, there have been nearly 7,000 new cases reported. This tells us the virus is continuing to spread rapidly in China. The reported deaths have continued to rise as well. In addition, locations outside China have continued to report cases. There have been an increasing number of reports of person-to-person spread, and now, most recently, a report in the New England Journal of Medicine of asymptomatic spread.”

The quarantine of passengers will last 14 days from when the plane left Wuhan, China. Martin Cetron, MD, who directs the CDC’s Division of Global Migration and Quarantine, said that the quarantine order “offers the greatest level of protection for the American public in preventing introduction and spread. That is our primary concern. Prior epidemics suggest that when people are properly informed, they’re usually very compliant with this request to restrict their movement. This allows someone who would become symptomatic to be rapidly identified. Offering early, rapid diagnosis of their illness could alleviate a lot of anxiety and uncertainty. In addition, this is a protective effect on family members. No individual wants to be the source of introducing or exposing a family member or a loved one to their virus. Additionally, this is part of their civic responsibility to protect their communities.”
* This story was updated on 01/31/2020.