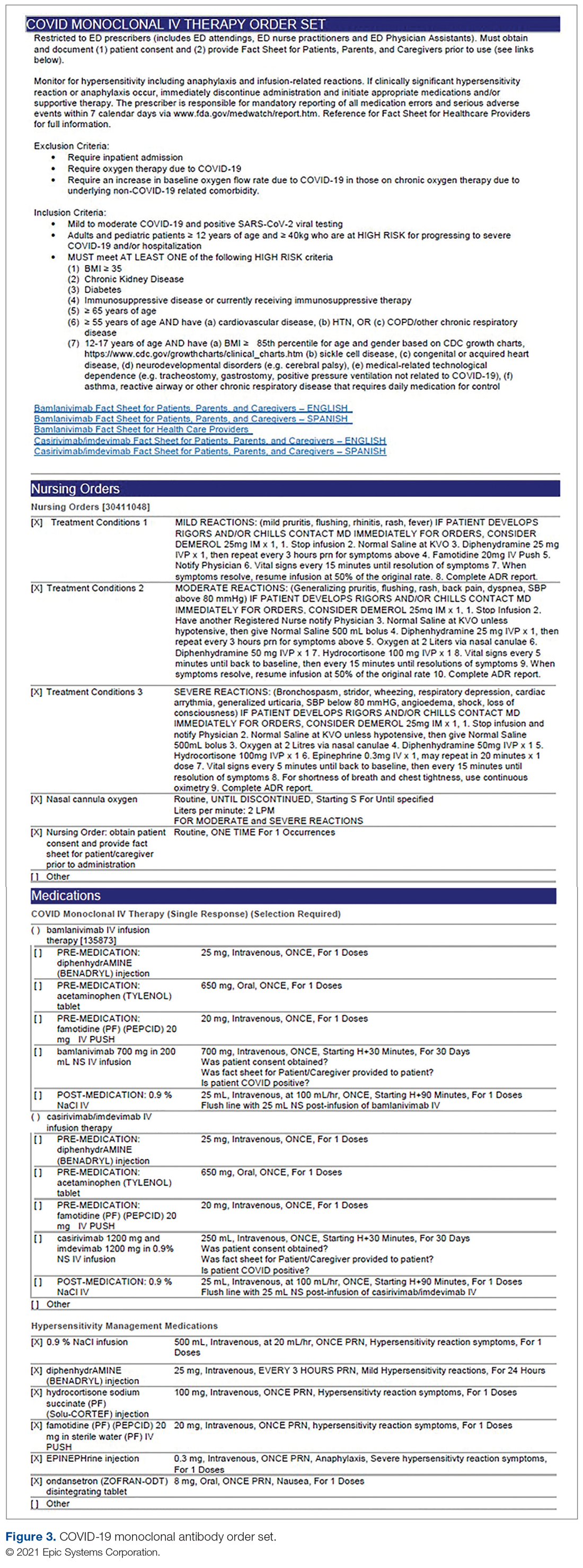
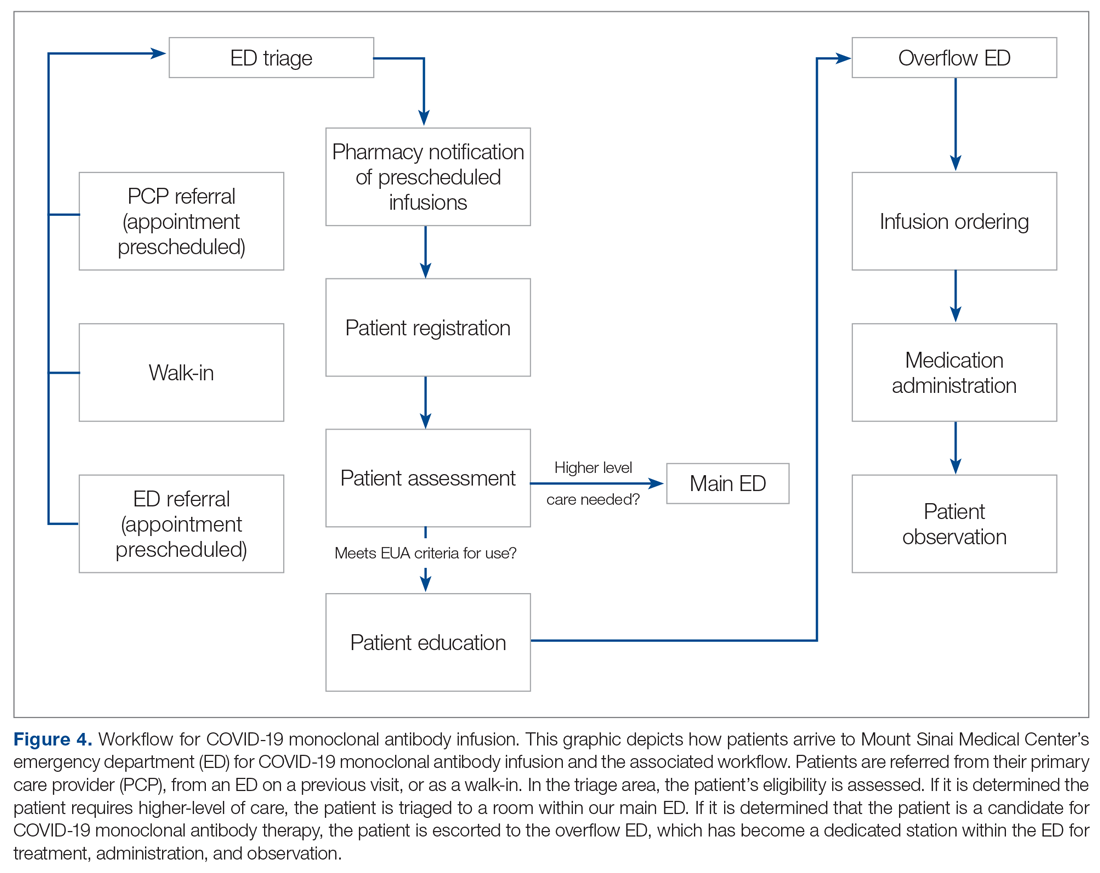
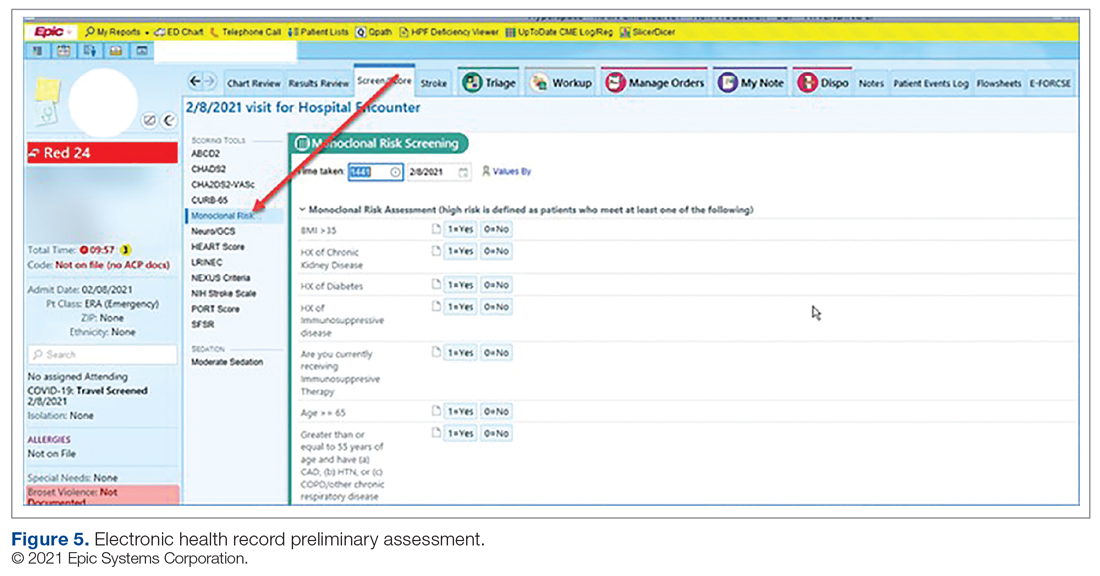
User login
COVID-19: One Patient at a Time
I will never forget the first time I cared for a patient who tested positive for COVID-19. It was March 2020, and I was evaluating a patient in the emergency department (ED). At the time we knew very little about this virus and how it is transmitted. We had all seen the images from Wuhan, China, and had appropriate fear of the lethality of the virus, but there was not yet a clear understanding as to how best to keep health care practitioners safe as they cared for patients with COVID-19.
That evening I received a page that a middle-aged man who had tested positive for COVID-19 was in the ED with fever, cough, and hypoxia. As a hospitalist, my role is to care for these patients, those admitted to stay overnight in the hospital. Before going to see the patient, I watched a video on how to properly don personal protective equipment (PPE). I walked to the ED and suited up with a surgical mask, goggles, disposable gown, and gloves. I was very conscious of the amount of time I spent in that patient’s room, and tried to stand at the foot of the bed as much as possible so as to maximize the distance between our faces when we talked.
Upon finishing my assessment, I took off my PPE and exited the room but kept wondering if I had done so correctly. That night when I came home, I slept in the guest bedroom to minimize the risk of transmission of the virus to my wife. For the next 7 days I was terrified that I had been exposed to the virus, worried that I hadn’t worn my mask properly, or that I exposed myself to contamination when taking off my goggles and gown. I was hyperaware of my breathing and temperature, wondering if that scratch in my throat was the first sign of something worse. I never did develop any symptoms of illness but the amount of stress I felt that week was enormous.
Over the subsequent weeks I became much more comfortable with putting on and taking off PPE since the volume of COVID patients kept increasing to the point that more than 80% of the hospital patient census consisted of COVID-19 infections. Those patient interactions became less awkward once I could stop worrying about the PPE and focus on providing patient care.
Unfortunately, patient after patient entered the hospital, all with the same symptoms: cough, fever, and hypoxia. Medically there was little decision-making necessary as care was mostly supportive with supplemental oxygen to give these patients time to recover. Instead, I focused on understanding each patient’s symptoms and thinking about what could be offered to relieve bothersome symptoms. These patients were isolated in their hospital rooms – denied visitors and their interactions with hospital staff involved layers and layers of protective barrier. I sought to overcome those physical barriers through personal connection – learning about a patient’s hobbies, asking about their families, or reminiscing about one of their favorite trips.
Despite this supportive care, many patients ended up intubated in the intensive care unit. Many eventually improved, and we celebrated those individuals – a victory at a time. We even counted the COVID discharges with a running tally; first 10, then a few dozen, and eventually the number climbed into the triple digits. But not every patient was so fortunate. Hearing about a 40-something who passed away hit too close to home – what if that were me?
The hospitalists I work with rose to the occasion. We feared the virus but still showed up for work because the patients needed us and we had job obligations to honor. Everyone else was stuck at home during lockdown but we still got in our cars and drove to the hospital, suited up in our PPE, and cared for terrified patients that were struggling to breathe.
There was a satisfaction in having a job to do and being able to contribute during this time of global crisis. Staying busy gave our minds something to focus on and helped us feel a sense of purpose. Some of us stayed late to coordinate staffing. Others helped to disseminate practice guidelines and clinical knowledge. While others lent a hand wherever they could to pitch in. That sense of camaraderie served as plenty of motivation.
During the early stages of the pandemic, there was a sense that this crisis that would end after a few months and life would return to normal. By May, we experienced a dramatic decline in the number of hospitalized patients with COVID-19, which resulted in a real sense of optimism. But soon it became apparent that this pandemic was not going away anytime soon.
Cases nationwide began rising again over the summer. We saw a steady trickle of new admissions at our hospital month after month until the fall when the rate of admissions accelerated again. The hospital reactivated our surge plan, increased staffing, and confronted the new surge with growing dread. That first surge was all endorphins – but fatigue set in by the time the second wave hit. The volunteerism and sense of “we are in this together” just did not exist anymore. The stories about health care heroes in the broader community waned and the outside world seemingly had moved on from thinking about the pandemic.
Yet we remained, caring for patients with cough, fever, and low oxygen saturation. It was like living through a movie we had already seen before. We knew what we were supposed to do and we followed the script. But now it felt too much like a routine.
It has been a very long 14 months since I first cared for a patient with COVID-19. For much of this time it felt like we were just stuck on a treadmill, passing the time but not making any significant progress towards a post-COVID future state. How many times over this year did we push that date forward in our minds when “life would go back to normal”?
Now, we have reason for hope. More than 100 million Americans have been vaccinated and that number rises daily. The vaccines are remarkably effective, they are making a real difference in reducing the number of patients with COVID-19 at the hospital, and our level of daily anxiety is lower. There is still much uncertainty about the future, but at least we can feel proud of our service over the last year — proud of showing up and donning that PPE. And so, we continue one patient at a time.
Corresponding author: James A. Colbert, MD, Attending Hospitalist, Newton-Wellesley Hospital, 2014 Washington St, Newton, MA, 02462, Senior Medical Director, Blue Cross Blue Shield of Massachusetts; [email protected].
Financial disclosures: None.
I will never forget the first time I cared for a patient who tested positive for COVID-19. It was March 2020, and I was evaluating a patient in the emergency department (ED). At the time we knew very little about this virus and how it is transmitted. We had all seen the images from Wuhan, China, and had appropriate fear of the lethality of the virus, but there was not yet a clear understanding as to how best to keep health care practitioners safe as they cared for patients with COVID-19.
That evening I received a page that a middle-aged man who had tested positive for COVID-19 was in the ED with fever, cough, and hypoxia. As a hospitalist, my role is to care for these patients, those admitted to stay overnight in the hospital. Before going to see the patient, I watched a video on how to properly don personal protective equipment (PPE). I walked to the ED and suited up with a surgical mask, goggles, disposable gown, and gloves. I was very conscious of the amount of time I spent in that patient’s room, and tried to stand at the foot of the bed as much as possible so as to maximize the distance between our faces when we talked.
Upon finishing my assessment, I took off my PPE and exited the room but kept wondering if I had done so correctly. That night when I came home, I slept in the guest bedroom to minimize the risk of transmission of the virus to my wife. For the next 7 days I was terrified that I had been exposed to the virus, worried that I hadn’t worn my mask properly, or that I exposed myself to contamination when taking off my goggles and gown. I was hyperaware of my breathing and temperature, wondering if that scratch in my throat was the first sign of something worse. I never did develop any symptoms of illness but the amount of stress I felt that week was enormous.
Over the subsequent weeks I became much more comfortable with putting on and taking off PPE since the volume of COVID patients kept increasing to the point that more than 80% of the hospital patient census consisted of COVID-19 infections. Those patient interactions became less awkward once I could stop worrying about the PPE and focus on providing patient care.
Unfortunately, patient after patient entered the hospital, all with the same symptoms: cough, fever, and hypoxia. Medically there was little decision-making necessary as care was mostly supportive with supplemental oxygen to give these patients time to recover. Instead, I focused on understanding each patient’s symptoms and thinking about what could be offered to relieve bothersome symptoms. These patients were isolated in their hospital rooms – denied visitors and their interactions with hospital staff involved layers and layers of protective barrier. I sought to overcome those physical barriers through personal connection – learning about a patient’s hobbies, asking about their families, or reminiscing about one of their favorite trips.
Despite this supportive care, many patients ended up intubated in the intensive care unit. Many eventually improved, and we celebrated those individuals – a victory at a time. We even counted the COVID discharges with a running tally; first 10, then a few dozen, and eventually the number climbed into the triple digits. But not every patient was so fortunate. Hearing about a 40-something who passed away hit too close to home – what if that were me?
The hospitalists I work with rose to the occasion. We feared the virus but still showed up for work because the patients needed us and we had job obligations to honor. Everyone else was stuck at home during lockdown but we still got in our cars and drove to the hospital, suited up in our PPE, and cared for terrified patients that were struggling to breathe.
There was a satisfaction in having a job to do and being able to contribute during this time of global crisis. Staying busy gave our minds something to focus on and helped us feel a sense of purpose. Some of us stayed late to coordinate staffing. Others helped to disseminate practice guidelines and clinical knowledge. While others lent a hand wherever they could to pitch in. That sense of camaraderie served as plenty of motivation.
During the early stages of the pandemic, there was a sense that this crisis that would end after a few months and life would return to normal. By May, we experienced a dramatic decline in the number of hospitalized patients with COVID-19, which resulted in a real sense of optimism. But soon it became apparent that this pandemic was not going away anytime soon.
Cases nationwide began rising again over the summer. We saw a steady trickle of new admissions at our hospital month after month until the fall when the rate of admissions accelerated again. The hospital reactivated our surge plan, increased staffing, and confronted the new surge with growing dread. That first surge was all endorphins – but fatigue set in by the time the second wave hit. The volunteerism and sense of “we are in this together” just did not exist anymore. The stories about health care heroes in the broader community waned and the outside world seemingly had moved on from thinking about the pandemic.
Yet we remained, caring for patients with cough, fever, and low oxygen saturation. It was like living through a movie we had already seen before. We knew what we were supposed to do and we followed the script. But now it felt too much like a routine.
It has been a very long 14 months since I first cared for a patient with COVID-19. For much of this time it felt like we were just stuck on a treadmill, passing the time but not making any significant progress towards a post-COVID future state. How many times over this year did we push that date forward in our minds when “life would go back to normal”?
Now, we have reason for hope. More than 100 million Americans have been vaccinated and that number rises daily. The vaccines are remarkably effective, they are making a real difference in reducing the number of patients with COVID-19 at the hospital, and our level of daily anxiety is lower. There is still much uncertainty about the future, but at least we can feel proud of our service over the last year — proud of showing up and donning that PPE. And so, we continue one patient at a time.
Corresponding author: James A. Colbert, MD, Attending Hospitalist, Newton-Wellesley Hospital, 2014 Washington St, Newton, MA, 02462, Senior Medical Director, Blue Cross Blue Shield of Massachusetts; [email protected].
Financial disclosures: None.
I will never forget the first time I cared for a patient who tested positive for COVID-19. It was March 2020, and I was evaluating a patient in the emergency department (ED). At the time we knew very little about this virus and how it is transmitted. We had all seen the images from Wuhan, China, and had appropriate fear of the lethality of the virus, but there was not yet a clear understanding as to how best to keep health care practitioners safe as they cared for patients with COVID-19.
That evening I received a page that a middle-aged man who had tested positive for COVID-19 was in the ED with fever, cough, and hypoxia. As a hospitalist, my role is to care for these patients, those admitted to stay overnight in the hospital. Before going to see the patient, I watched a video on how to properly don personal protective equipment (PPE). I walked to the ED and suited up with a surgical mask, goggles, disposable gown, and gloves. I was very conscious of the amount of time I spent in that patient’s room, and tried to stand at the foot of the bed as much as possible so as to maximize the distance between our faces when we talked.
Upon finishing my assessment, I took off my PPE and exited the room but kept wondering if I had done so correctly. That night when I came home, I slept in the guest bedroom to minimize the risk of transmission of the virus to my wife. For the next 7 days I was terrified that I had been exposed to the virus, worried that I hadn’t worn my mask properly, or that I exposed myself to contamination when taking off my goggles and gown. I was hyperaware of my breathing and temperature, wondering if that scratch in my throat was the first sign of something worse. I never did develop any symptoms of illness but the amount of stress I felt that week was enormous.
Over the subsequent weeks I became much more comfortable with putting on and taking off PPE since the volume of COVID patients kept increasing to the point that more than 80% of the hospital patient census consisted of COVID-19 infections. Those patient interactions became less awkward once I could stop worrying about the PPE and focus on providing patient care.
Unfortunately, patient after patient entered the hospital, all with the same symptoms: cough, fever, and hypoxia. Medically there was little decision-making necessary as care was mostly supportive with supplemental oxygen to give these patients time to recover. Instead, I focused on understanding each patient’s symptoms and thinking about what could be offered to relieve bothersome symptoms. These patients were isolated in their hospital rooms – denied visitors and their interactions with hospital staff involved layers and layers of protective barrier. I sought to overcome those physical barriers through personal connection – learning about a patient’s hobbies, asking about their families, or reminiscing about one of their favorite trips.
Despite this supportive care, many patients ended up intubated in the intensive care unit. Many eventually improved, and we celebrated those individuals – a victory at a time. We even counted the COVID discharges with a running tally; first 10, then a few dozen, and eventually the number climbed into the triple digits. But not every patient was so fortunate. Hearing about a 40-something who passed away hit too close to home – what if that were me?
The hospitalists I work with rose to the occasion. We feared the virus but still showed up for work because the patients needed us and we had job obligations to honor. Everyone else was stuck at home during lockdown but we still got in our cars and drove to the hospital, suited up in our PPE, and cared for terrified patients that were struggling to breathe.
There was a satisfaction in having a job to do and being able to contribute during this time of global crisis. Staying busy gave our minds something to focus on and helped us feel a sense of purpose. Some of us stayed late to coordinate staffing. Others helped to disseminate practice guidelines and clinical knowledge. While others lent a hand wherever they could to pitch in. That sense of camaraderie served as plenty of motivation.
During the early stages of the pandemic, there was a sense that this crisis that would end after a few months and life would return to normal. By May, we experienced a dramatic decline in the number of hospitalized patients with COVID-19, which resulted in a real sense of optimism. But soon it became apparent that this pandemic was not going away anytime soon.
Cases nationwide began rising again over the summer. We saw a steady trickle of new admissions at our hospital month after month until the fall when the rate of admissions accelerated again. The hospital reactivated our surge plan, increased staffing, and confronted the new surge with growing dread. That first surge was all endorphins – but fatigue set in by the time the second wave hit. The volunteerism and sense of “we are in this together” just did not exist anymore. The stories about health care heroes in the broader community waned and the outside world seemingly had moved on from thinking about the pandemic.
Yet we remained, caring for patients with cough, fever, and low oxygen saturation. It was like living through a movie we had already seen before. We knew what we were supposed to do and we followed the script. But now it felt too much like a routine.
It has been a very long 14 months since I first cared for a patient with COVID-19. For much of this time it felt like we were just stuck on a treadmill, passing the time but not making any significant progress towards a post-COVID future state. How many times over this year did we push that date forward in our minds when “life would go back to normal”?
Now, we have reason for hope. More than 100 million Americans have been vaccinated and that number rises daily. The vaccines are remarkably effective, they are making a real difference in reducing the number of patients with COVID-19 at the hospital, and our level of daily anxiety is lower. There is still much uncertainty about the future, but at least we can feel proud of our service over the last year — proud of showing up and donning that PPE. And so, we continue one patient at a time.
Corresponding author: James A. Colbert, MD, Attending Hospitalist, Newton-Wellesley Hospital, 2014 Washington St, Newton, MA, 02462, Senior Medical Director, Blue Cross Blue Shield of Massachusetts; [email protected].
Financial disclosures: None.
Impact of Hospitalist Programs on Perceived Care Quality, Interprofessional Collaboration, and Communication: Lessons from Implementation of 3 Hospital Medicine Programs in Canada
From the Fraser Health Authority, Surrey, BC, Canada (Drs. Yousefi and Paletta), and Catalyst Consulting Inc., Vancouver, BC, Canada (Elayne McIvor).
Objective: Despite the ongoing growth in the number of hospitalist programs in Canada, their impact on the quality of interprofessional communication, teamwork, and staff satisfaction is not well known. This study aimed to evaluate perceptions of frontline care providers and hospital managers about the impact of the implementation of 3 new hospitalist services on care quality, teamwork, and interprofessional communication.
Design: We used an online survey and semistructured interviews to evaluate respondents’ views on quality of interprofessional communication and collaboration, impact of the new services on quality of care, and overall staff satisfaction with the new inpatient care model.
Setting: Integrated Regional Health Authority in British Columbia, Canada.
Participants: Participants included hospital administrators, frontline care providers (across a range of professions), and hospital and community-based physicians.
Results: The majority of respondents reported high levels of satisfaction with their new hospital medicine services. They identified improvements in interprofessional collaboration and communication between hospitalists and other professionals, which were attributed to enhanced onsite presence of physicians. They also perceived improvements in quality of care and efficiency. On the other hand, they identified a number of challenges with the change process, and raised concerns about the impact of patient handoffs on care quality and efficiency.
Conclusion: Across 3 very different acute care settings, the implementation of a hospitalist service was widely perceived to have resulted in improved teamwork, quality of care, and interprofessional communication.
Keywords: hospital medicine; hospitalist; teamwork; interprofessional collaboration.
Over the past 2 decades, the hospitalist model has become prevalent in Canada and internationally.1 Hospitalist care has been associated with improvements in efficiency and quality of care.2-6 However, less is known about its impact on the quality of interprofessional communication, teamwork, and staff satisfaction. In a 2012 study of a specialized orthopedic facility in the Greater Toronto Area (GTA), Ontario, Webster et al found a pervasive perception among interviewees that the addition of a hospitalist resulted in improved patient safety, expedited transfers, enhanced communication with Primary Care Providers (PCPs), and better continuity of care.7 They also identified enhanced collaboration among providers since the addition of the hospitalist to the care team. In another study of 5 community hospitals in the GTA, Conn et al8 found that staff on General Internal Medicine wards where hospitalists worked described superior interprofessional collaboration, deeper interpersonal relationships between physicians and other care team members, and a higher sense of “team-based care.”
Fraser Health Authority (FH) is an integrated regional health system with one of the largest regional Hospital Medicine (HM) networks in Canada.9 Over the past 2 decades, FH has implemented a number of HM services in its acute care facilities across a range of small and large community and academic hospitals. More recently, 3 hospitalist services were implemented over a 2-year period: new HM services in a tertiary referral center (Site A, July 2016) and a small community hospital (Site B, December 2016), and reintroduction of a hospitalist service in a medium-sized community hospital (Site C, January 2017). This provided a unique opportunity to assess the impact of the implementation of the hospitalist model across a range of facilities. The main objectives of this evaluation were to understand the level of physician, nursing, allied staff, and hospital administration satisfaction with the new hospitalist model, as well as the perceived impact of the service on efficiency and quality of care. As such, FH engaged an external consultant (EM) to conduct a comprehensive evaluation of the introduction of its latest HM services.
Methods
Setting
Hospital medicine services are currently available in 10 of 12 acute care facilities within the FH system. The 3 sites described in this evaluation constitute the most recent sites where a hospitalist service was implemented.
Site A is a 272-bed tertiary referral center situated in a rapidly growing community. At the time of our evaluation, 21 Full Time Equivalent (FTE) hospitalists cared for an average of 126 patients, which constituted the majority of adult medical patients. Each day, 8 individuals rounded on admitted patients (average individual census: 16) with another person providing in-house, evening, and overnight coverage. An additional flexible shift during the early afternoon helped with Emergency Department (ED) admissions.
Site B is small, 45-bed community hospital in a semi-rural community. The hospitalist service began in December 2016, with 4 FTE hospitalists caring for an average of 28 patients daily. This constituted 2 hospitalists rounding daily on admitted patients, with on-call coverage provided from home.
Site C is a 188-bed community hospital with a hospitalist service initially introduced in 2005. In 2016, the program was disbanded and the site moved back to a primarily community-based model, in which family physicians in the community were invited to assume the care of hospitalized patients. However, the hospitalist program had to be reintroduced in January 2017 due to poor uptake among PCPs in the community. At the time of evaluation, 19 FTE hospitalists (with 7 hospitalists working daily) provided most responsible physician care to a daily census of 116 patients (average individual census: 16). The program also covered ED admissions in-house until midnight, with overnight call provided from home.
Approach
We adopted a utilization-focused evaluation approach to guide our investigation. In this approach, the assessment is deliberately planned and conducted in a way that it maximizes the likelihood that findings would be used by the organization to inform learning, adaptations, and decision-making.11 To enable this, the evaluator identified the primary intended recipients and engaged them at the start of the evaluation process to understand the main intended uses of the project. Moreover, the evaluator ensured that these intended uses of the evaluation guided all other decisions made throughout the process.
We collected data using an online survey of the staff at the 3 facilities, complemented by a series of semistructured qualitative interviews with FH administrators and frontline providers.
Online survey
We conducted an open online survey of a broad range of stakeholders who worked in the 3 facilities. To develop the questionnaire, we searched our department’s archives for previous surveys conducted from 2001 to 2005. We also interviewed the regional HM program management team to identify priority areas and reached out to the local leadership of the 3 acute care facilities for their input and support of the project. We refined the survey through several iterations, seeking input from experts in the FH Department of Evaluation and Research. The final questionnaire contained 10 items, including a mix of closed- and open-ended questions (Appendix A).
To reach the target audience, we collaborated with each hospital’s local leadership as well as the Divisions of Family Practice (DFP) that support local community PCPs in each hospital community.10 Existing email lists were compiled to create a master electronic survey distribution list. The initial invitation and 3 subsequent reminders were disseminated to the following target groups: hospital physicians (both hospitalists and nonhospitalists), PCPs, nursing and other allied professionals, administrators, and DFP leadership.
The survey consent form, background information, questions, and online platform (SimpleSurvey, Montreal, QC) were approved by FH’s Privacy Department. All respondents were required to provide their consent and able to withdraw at any time. Survey responses were kept anonymous and confidential, with results captured automatically into a spreadsheet by the survey platform. As an incentive for participation, respondents had the opportunity to win 1 of 3 $100 Visa gift cards. Personal contact information provided for the prize draw was collected in a separate survey that could not link back to respondents’ answers. The survey was trialed several times by the evaluation team to address any technical challenges before dissemination to the targeted participants.
Qualitative interviews
We conducted semistructured interviews with a purposive sample of FH administrators and frontline providers (Appendix B). The interview questions broadly mirrored the survey but allowed for more in-depth exploration of constructs. Interviewees were recruited through email invitations to selected senior and mid-level local and regional administrators, asking interviewees to refer our team to other contacts, and inviting survey respondents to voluntarily participate in a follow-up interview. One of the authors (EM), a Credentialed Evaluator, conducted all the one-time interviews either in-person at the individual participant’s workplace or by telephone. She did not have pre-existing relationships with any of the interviewees. Interviews were recorded and transcribed for analysis. Interviewees were required to consent to participate and understood that they could withdraw at any point. They were not offered incentives to participate. Interviews were carried out until thematic saturation was reached.
Analysis
A content analysis approach was employed for all qualitative data, which included open-ended responses from the online survey and interview transcripts. One of the authors (EM) conducted the analysis. The following steps were followed in the inductive content analysis process: repeated reading of the raw data, generation of initial thematic codes, organizing and sorting codes into categories (ie, main vs subcategories), coding of all data, quantifying codes, and interpreting themes. When responding to open-ended questions, respondents often provided multiple answers per question. Each of the respondents’ answers were coded. In alignment with the inductive nature of the analysis process, themes emerged organically from the data rather than the researchers using preconceived theories and categories to code the text. This was achieved by postponing the review of relevant literature on the topic until after the analysis was complete and using an external evaluation consultant (with no prior relationship to FH and limited theoretical knowledge of the topic matter) to analyze the data. Descriptive statistics were run on quantitative data in SPSS (v.24, IBM, Armonk, NY). For survey responses to be included in the analysis, the respondents needed to indicate which site they worked at and were required to answer at least 1 other survey question. One interviewee was excluded from the analysis since they were not familiar with the hospitalist model at their site.
Ethics approval
The evaluation protocol was reviewed by FH Department of Evaluation and Research and was deemed exempt from formal research ethics review.
Results
A total of 377 individuals responded to the online survey between January 8 and February 28, 2018 (response rate 14%). The distribution of respondents generally reflected the size of the respective acute care facilities. Compared to the overall sampled population, fewer nurses participated in the survey (45% vs 64%) while the rate of participation for Unit Clerks (14% vs 16%) and allied professionals (12% vs 16%) were similar.
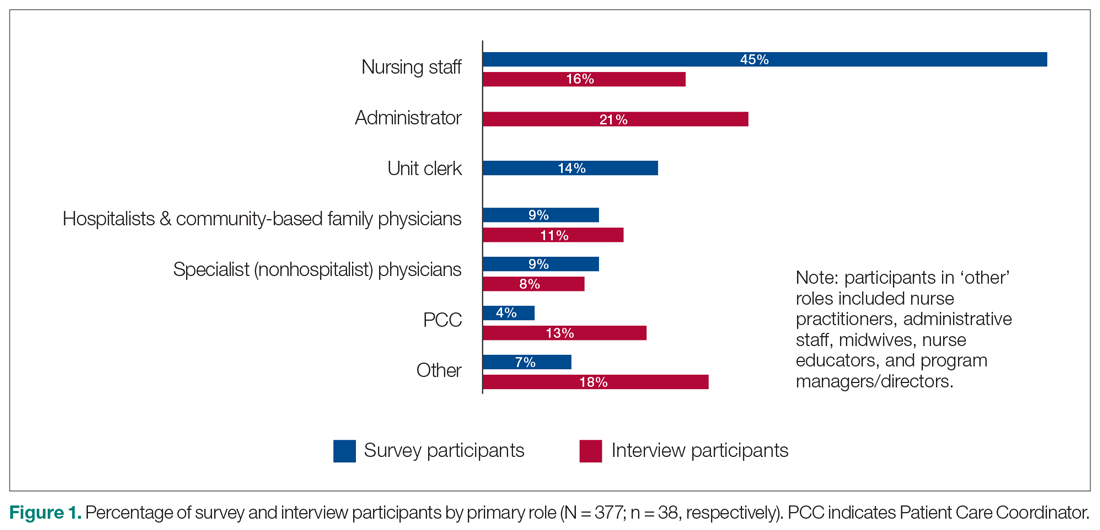
Out of the 45 people approached for an interview, a total of 38 were conducted from January 3 to March 5, 2018 (response rate 84%). The interviews lasted an average of 42 minutes. Interviewees represented a range of administrative and health professional roles (Figure 1). Some interviewees held multiple positions.
Satisfaction with HM service
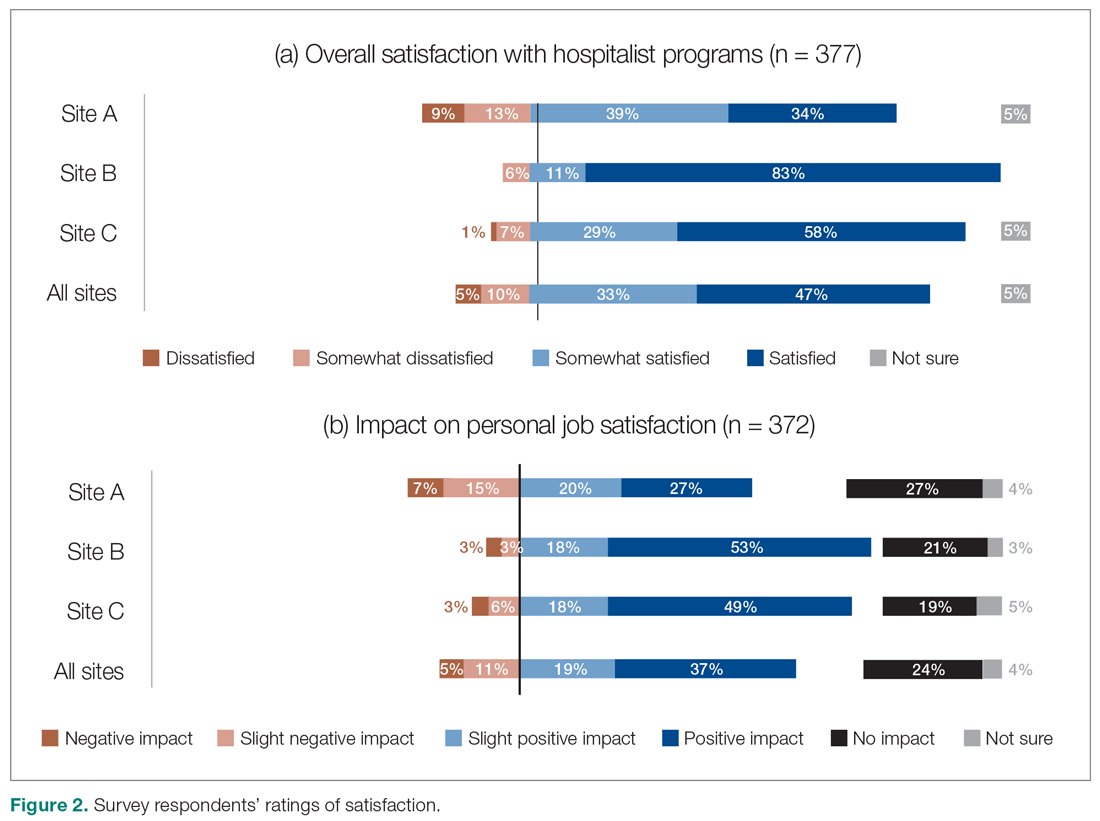
Across all sites, survey respondents reported high levels of satisfaction with their respective HM services and identified positive impacts on their job satisfaction (Figure 2). Almost all interviewees similarly expressed high satisfaction levels with their HM services (95%; n = 36).
Perceptions of HM service performance
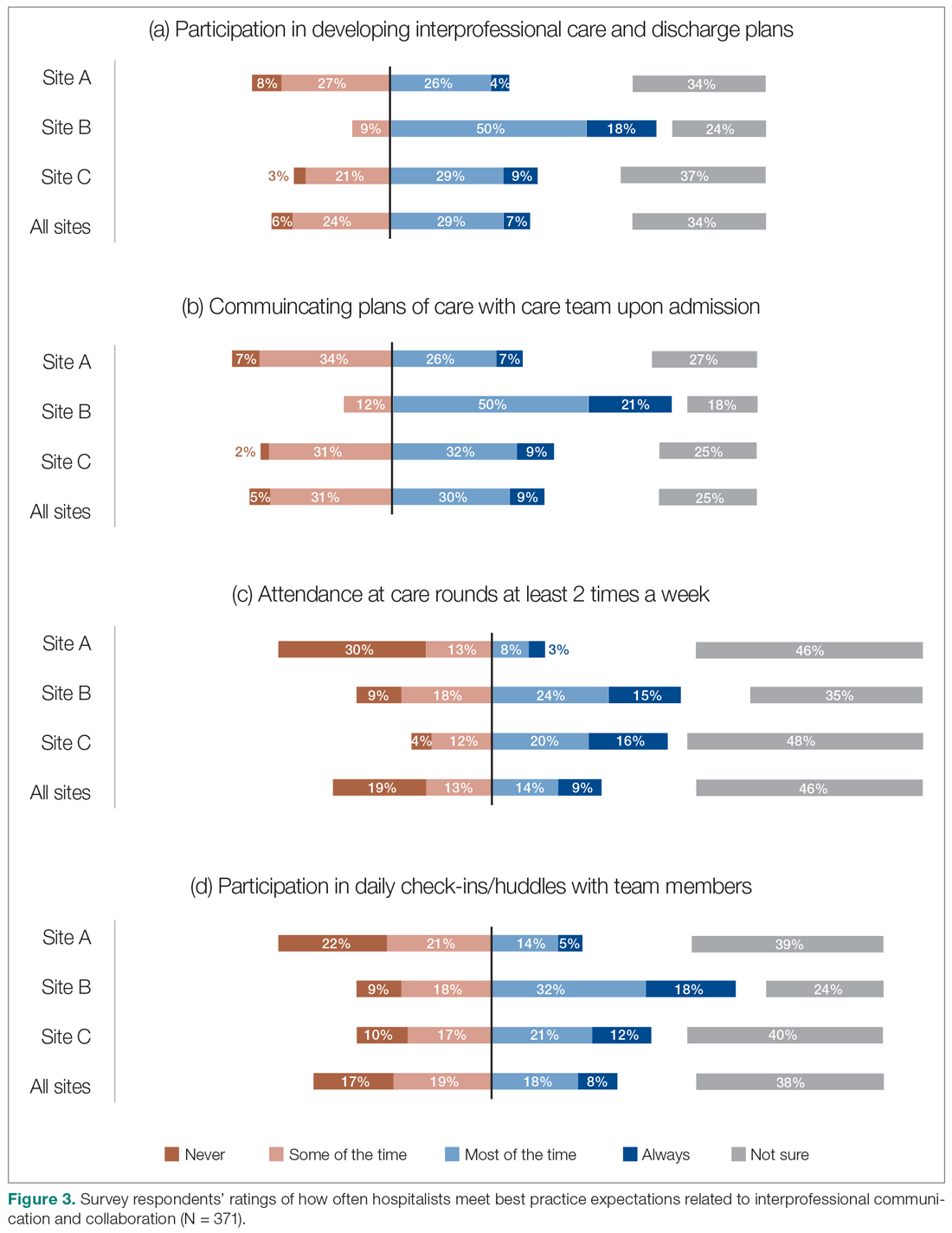
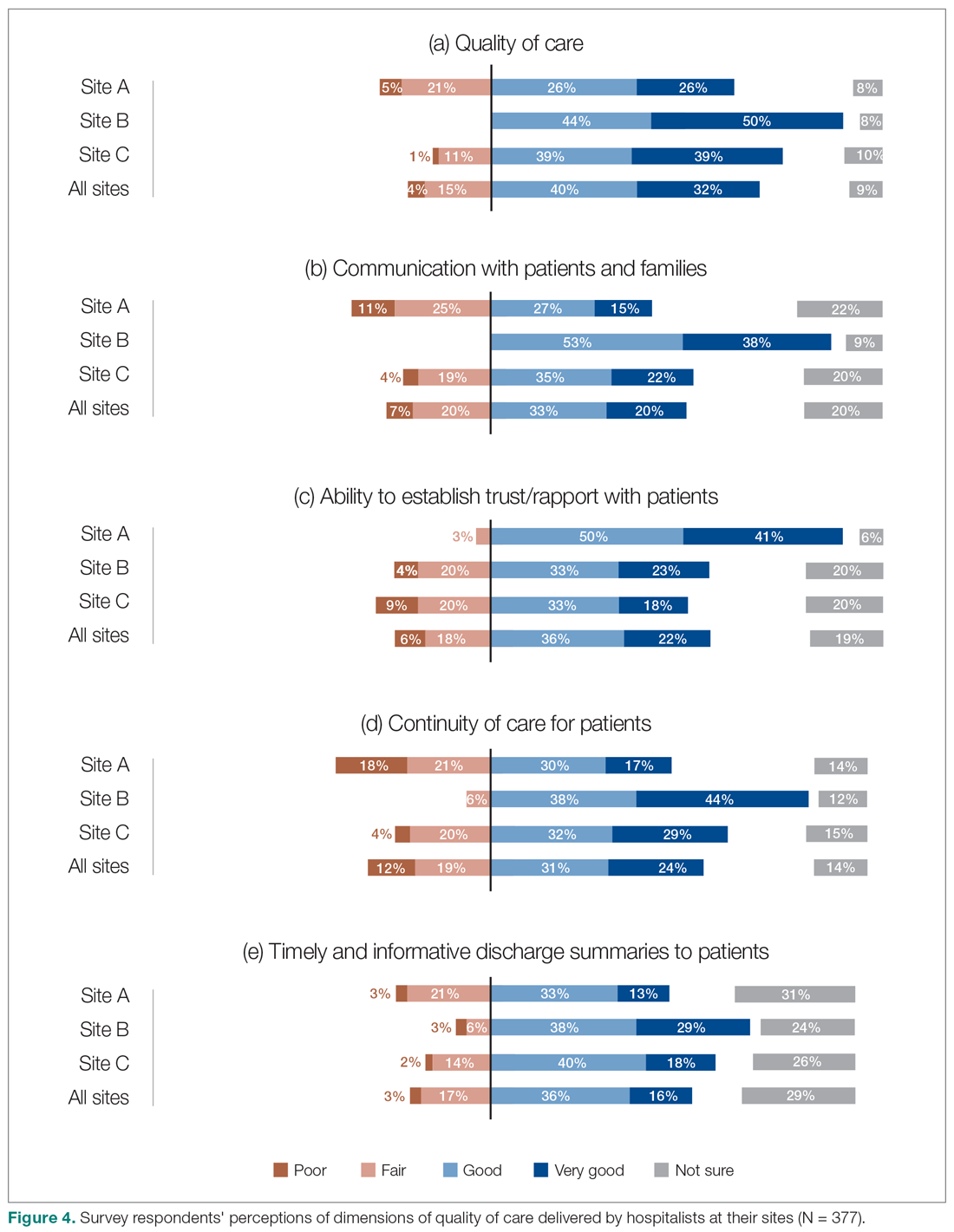
Survey respondents rated the strength of hospitalists’ interprofessional communication and collaboration with other physicians and with care teams. Roughly two-thirds reported that overall hospitalist communication was “good” or “very good.” We also asked participants to rate the frequency at which hospitalists met best practice expectations related to interprofessional teamwork. Across all sites, similar proportions of respondents (23% to 39%) reported that these best practices were met “most of the time” or “always” (Figure 3). Survey questions also assessed perceptions of respondents about the quality and safety of care provided by hospitalists (Figure 4).
Perceptions of the impact of the HM service postimplementation
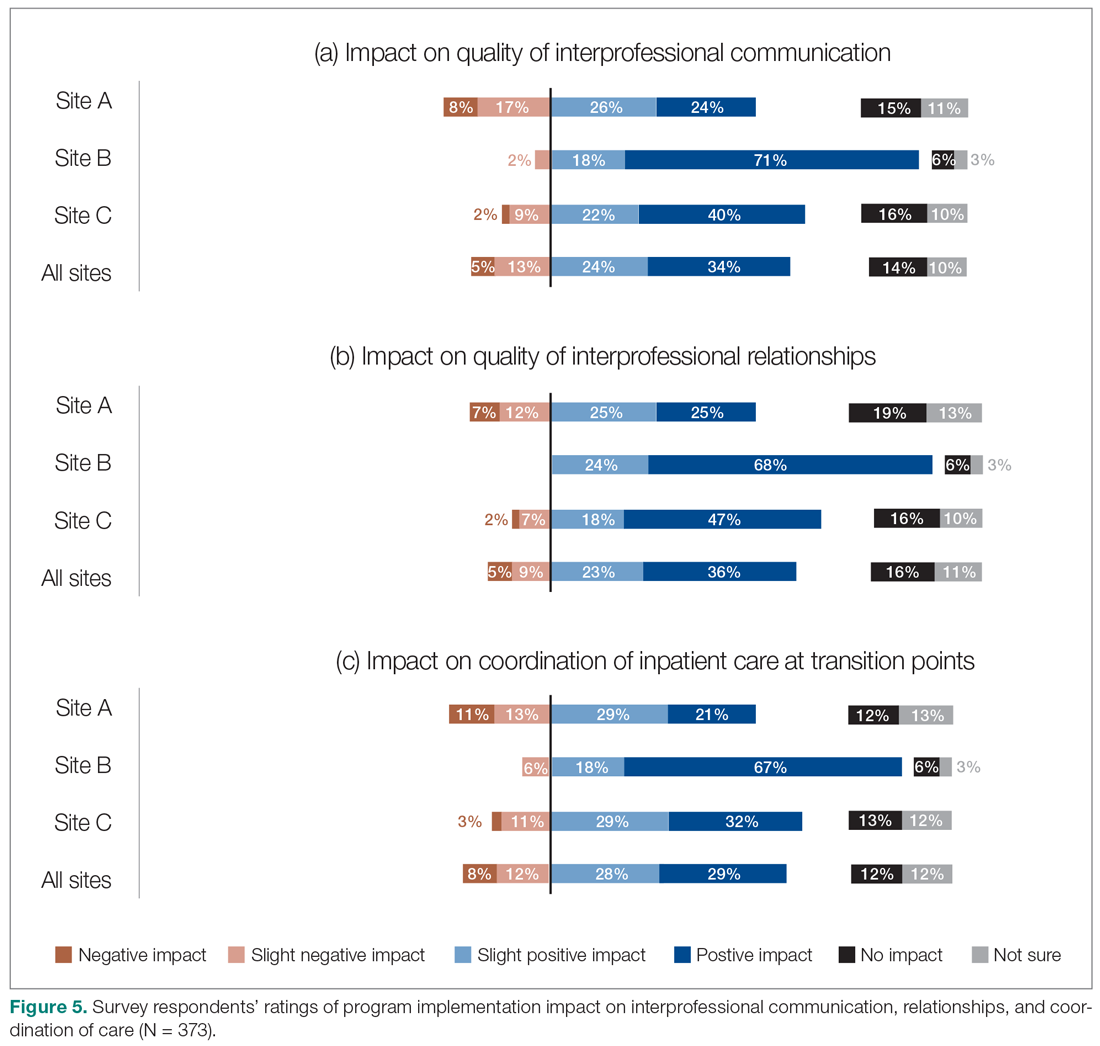
The majority of survey respondents reported improvements in the quality of communication, professional relationships, and coordination of inpatient care at transition points after the implementation of the HM service (Figure 5). This was also reflected in interviews, where some indicated that it was easier to communicate with hospitalists due to their on-site presence, accessibility, and 24/7 availability (n = 21). They also described improved collaboration within the care teams (n = 7), and easier communication with hospitalists because they were approachable, willing, and receptive (n = 4).
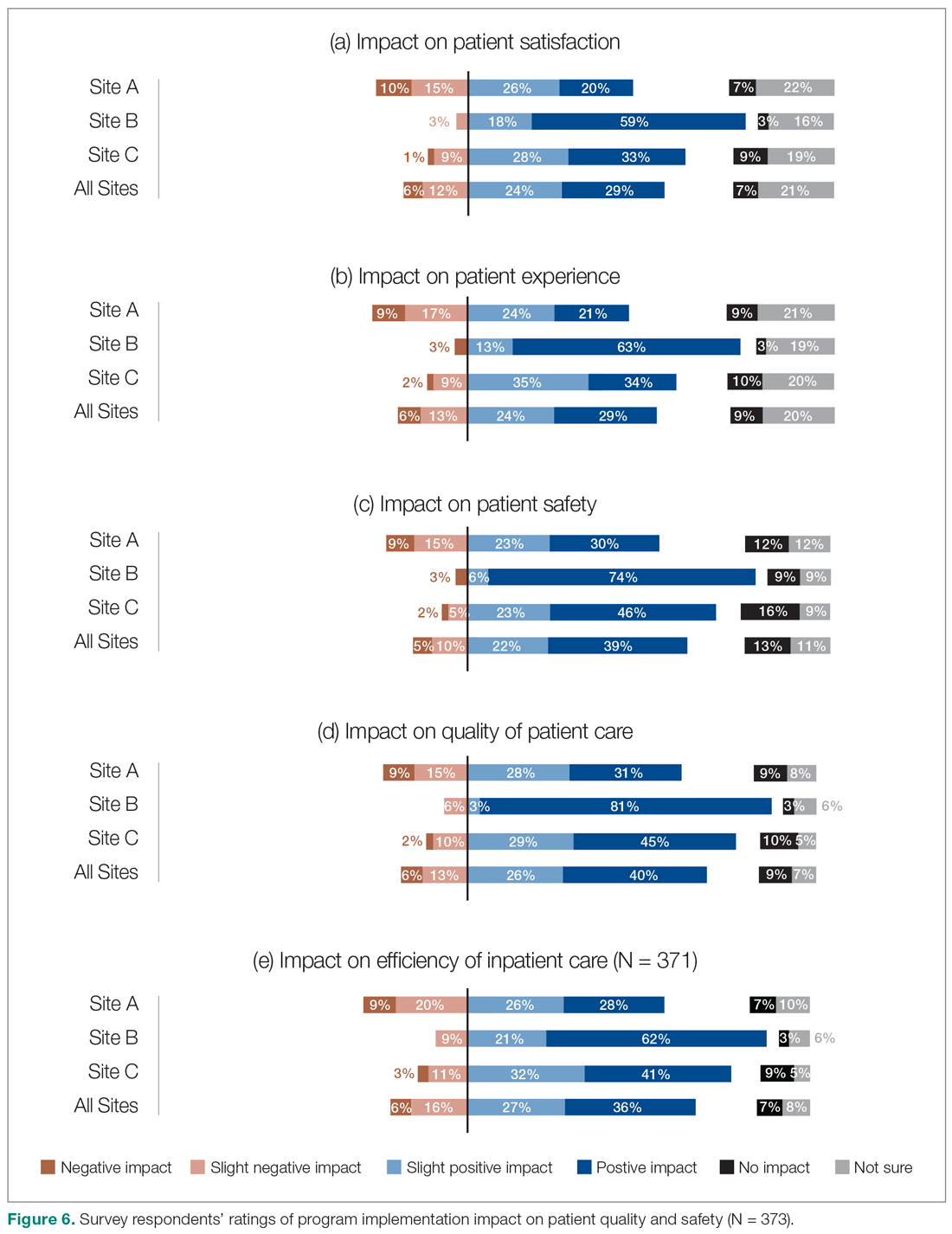
We also asked the survey respondents to assess the impact of the new hospitalist model on different dimensions of care quality, including patient satisfaction, patient experience, efficiency, and overall quality of care (Figure 6). Findings were comparable across these dimensions, with roughly 50-60% of respondents noting positive changes compared to before the implementation of the programs. However, most interviewees identified both positive and negative effects in these areas. Positive impacts included hospitalist on-site presence leading to better accessibility and timeliness of care (n = 5), hospitalists providing continuity to patients/families by working for weeklong rotations (n = 6), hospitalists being particularly skilled at managing complex clinical presentations (n = 2), and hospitalists being able to spend more time with patients (n = 2). On the other hand, some interviewees noted that patients and families did not like seeing multiple doctors due to frequent handoffs between hospitalists (n = 12). They also raised concerns that hospitalists did not know patients’ histories or had relationships with them, potentially leading to longer length of stay and unnecessary investigations (n = 8).
Site-to-site ratings of satisfaction and performance
Survey respondents’ satisfaction and performance ratings varied substantially site-to-site. Across all areas assessed, ratings were consistently highest at Site B (the smallest institution in our evaluation and the most recent addition to the HM network in the health authority). These differences were statistically significant across all survey questions asked.
Discussion
Findings from this study provide insight into the experiences of frontline health care professionals and administrators with the implementation of new HM services across a range of small to large acute care facilities. They indicate that the majority of respondents reported high levels of satisfaction with their hospitalist services. Most also indicated that the service had resulted in improvements compared to prior inpatient care models.
Over half of the survey respondents, and the majority of interviewees, reported a positive impact on interprofessional communication and collaboration. This was largely attributed to enhanced accessibility and availability of hospitalists:
- "Being on-site lends itself to better communication because they’re accessible. Hospitalists always answer the phone, but the general practitioners (GP) don’t always since they may be with other patients." (Dietician, Site A)
- "A big strength is that we have physician presence on the unit all day during scheduled hours, which makes us more accessible to nurses and more able to follow up on patients that we have concerns about." (Physician Leader, Site B)
However, the ratings dropped substantially when they were asked to assess adherence to specific best practices of such communication and collaboration, such as participation in daily check-ins or attendance at team care rounds (Figure 3). Interdisciplinary clinical rounds have been identified as a tool to improve the effectiveness of care teams.12 A number of elements have been identified as key components of effective rounds.13 Bedside rounds have also been found to enhance communication and teamwork.14,15 In our study, the discrepancy between overall high levels of satisfaction with hospitalists’ communication/collaboration despite low scores on participation in more concrete activities may illustrate the importance of informal and ad hoc opportunities for interactions between hospitalists and other care providers that result from the enhanced presence of hospitalists on care units.8 Outside of formal rounds, hospitalists have the ability to interact with other care providers throughout their shifts. Prior studies have shown that hospitalists spend a significant portion of their time communicating with other care team members throughout their workdays.16 At the same time, the amount of time spent on communication should be balanced against the need for provision of direct care at the bedside. Future research should aim to identify the right balance between these competing priorities, and to understand the nature and quality of the communication between various care providers.
We also aimed to understand the perceptions of study participants about the impact of the HM service on quality of care. Survey participants not only expressed reasonable satisfaction with various aspects of hospitalists’ performance, but also described a positive impact on care quality after the implementation of their new services. This was also reflected in the interviews:
- "The clinical knowledge of the new hospitalists is far better. Some are internal medicine trained, so they bring better knowledge and skills. I feel comfortable that they can take patients and manage them. I wasn’t always comfortable with doing that in the past." (Emergency Physician, Site C)
- "Hospitalists are really familiar with acute care and how it works. They’ve become more familiar with the discharge planning system and thus know more about the resources available. And even something as simple as knowing which forms to use." (Dietician, Site A)
It must be noted that these observations should ideally be corroborated through a robust before-after analysis of various quality measures. While such an analysis was beyond the scope of our current project, we have previously demonstrated that across our network (including the 3 sites included in our evaluation) hospitalist care is associated with lower mortality and readmission rates.4 Our findings appear to confirm previous suggestions that hospitalists’ dedicated focus on inpatient care may allow them to develop enhanced skills in the management of common conditions in the acute care setting17 which can be perceived to be of value to other hospital-based care providers.
The issue of frequent handover among hospitalists was the most commonly identified challenge by both survey respondents and interviewees:
- "They’re very reluctant to discharge patients if it’s their first day with the patient. Even if the previous hospitalist said they were ready for discharge, the new doc wants to run all of their own tests before they feel comfortable. Maybe it’s a trust issue between hospitalists when they hand patients over. It’s also being personally liable for patients if you discharge them." (Patient Care Coordinator, Site A)
- "Communication is an issue. There’s lots of turnover in hospitalists. Relationships were closer with GPs because we had so much more interaction with particular individuals." (Hospitalist Physician Leader, Site A)
It must be noted that we conducted our evaluation in a relatively short time span (within 2 years) after the 3 services were implemented. Developing trust among a large number of hospitalists newly recruited to these programs can take time and may be a factor that can explain the reluctance of some to discharge patients after handoffs. However, concerns about discontinuity of care inherent in the hospitalist model are not new.18,19 Better continuity has been associated with higher probability of patient discharges20 and improved outcomes.21 To address this challenge, the hospitalist community has focused on defining the core competencies associated with high quality handovers,22 and deliberate efforts to improve the quality of handoffs through quality improvement methodologies.23 Our study participants similarly identified these measures as potential solutions. Despite this, addressing hospitalist continuity of care remains a pressing challenge for the broader hospitalist community.24
Our evaluation has a number of methodological limitations. First, the survey response rate was only 14%, which raises questions about nonresponse bias and the representativeness of the findings to the larger population of interest. While the distribution of respondents was largely similar to the overall sampled population, a number of factors may have impacted our response rate. For example, we were only able to distribute our survey to health care providers’ institutional email addresses. Moreover, while we provided incentives for participation and sent out a number of reminders, we solely relied on one communication modality (ie, electronic communication) and did not utilize other methods (such as posters, reminder at meetings, in-person invitations). Second, while the survey included a number of open-ended questions, many of these responses were at times brief and difficult to interpret and were not included in the analysis. Third, all data collected were self-reported. For example, we could not corroborate comments about participation in interdisciplinary rounds by objective measures such as attendance records or direct observation. Self-report data is subjective in nature and is vulnerable to a range of biases, such as social desirability bias.25 Finally, patient satisfaction and experience with hospitalist care were not assessed by patients themselves. Ideally, standardized cross-site indicators should validate our patient-related results.
As mentioned above, hospitalist performance ratings varied substantially from site-to-site and were consistently higher at Site B (a small community hospital in a semi-rural area), followed by Site C (a medium-sized community hospital) and Site A (a tertiary referral center). The variability in program ratings and perceived hospitalist impacts between sites could be due to a variety of factors, such as the degree of change between the past and current models at each site, differences in hospitalist hiring processes, hospital size and culture, and differences in service design and operations. It may also be related to the timing of the introduction of the HM service, as Site B was the most recent site where the service was established. As such, there may be an element of recall bias behind the observed discrepancies. This highlights the importance of local context on respondent perceptions and suggests that our results may not be generalizable to other institutions with different attributes and characteristics.
Conclusion
Findings from this study have demonstrated that the recent hospitalist services in our health system have improved overall levels of interprofessional communication and teamwork, as well as perceptions of care quality among the majority of participants who reported high levels of satisfaction with their programs. Our findings further highlight the issue of frequent handovers among hospitalists as a pressing and ongoing challenge.
Corresponding Author: Vandad Yousefi, MD, CCFP, Past Regional Department Head – Hospital Medicine, Fraser Health Authority, Central City Tower, Suite 400, 13450 – 102nd Ave, Surrey, BC V3T 0H1; [email protected].
Financial disclosures: This project was funded by the Fraser Health Authority, which provided the funding for hiring of the external consultant to design, implement, and analyze the results of the evaluation program in collaboration with the Regional Hospitalist Program at Fraser Health.
1. Yousefi V, Wilton D. Re-designing Hospital Care: Learning from the Experience of Hospital Medicine in Canada. Journal of Global Health Care Systems. 2011;1(3).
2. White HL. Assessing the Prevalence, Penetration and Performance of Hospital Physicians in Ontario: Implications for the Quality and Efficiency of Inpatient Care. Doctoral Thesis; 2016.
3. Yousefi V, Chong CA. Does implementation of a hospitalist program in a Canadian community hospital improve measures of quality of care and utilization? An observational comparative analysis of hospitalists vs. traditional care providers. BMC Health Serv Res. 2013;13:204.
4. Yousefi V, Hejazi S, Lam A. Impact of Hospitalists on Care Outcomes in a Large Integrated Health System in British Columbia. Journal of Clinical Outcomes Management. 2020;27(2):59-72.
5. Salim SA, Elmaraezy A, Pamarthy A, et al. Impact of hospitalists on the efficiency of inpatient care and patient satisfaction: a systematic review and meta-analysis. J Community Hosp Intern Med Perspect. 2019;9(2):121-134.
6. Peterson MC. A systematic review of outcomes and quality measures in adult patients cared for by hospitalists vs nonhospitalists. Mayo Clinic Proc. 2009;84(3):248-254.
7. Webster F, Bremner S, Jackson M, et al. The impact of a hospitalist on role boundaries in an orthopedic environment. J Multidiscip Healthc. 2012;5:249-256.
8. Gotlib Conn L, Reeves S, Dainty K, et al. Interprofessional communication with hospitalist and consultant physicians in general internal medicine: a qualitative study. BMC Health Serv Res. 2012; 12:437.
9. About Fraser Health. Fraser Health Authority. Updated 2018. Accessed January 30, 2019. https://www.fraserhealth.ca/about-us/about-fraser-health#.XFJrl9JKiUk
10. Divisions of Family Practice. Accessed May 2, 2020. https://www.divisionsbc.ca/provincial/about-us
11. Patton MQ. Essentials of Utilization-Focused Evaluation. 2012. Sage Publications, Inc; 2011.
12. Buljac-Samardzic M, Doekhie KD, van Wijngaarden JDH. Interventions to improve team effectiveness within health care: a systematic review of the past decade. Hum Resour Health. 2020;18(1):2.
13. Verhaegh KJ, Seller-Boersma A, Simons R, et al. An exploratory study of healthcare professionals’ perceptions of interprofessional communication and collaboration. J Interprof Care. 2017;31(3):397-400.
14. O’Leary KJ, Johnson JK, Manojlovich M, et al. Redesigning systems to improve teamwork and quality for hospitalized patients (RESET): study protocol evaluating the effect of mentored implementation to redesign clinical microsystems. BMC Health Serv Res. 2019;19(1):293.
15. Stein J, Payne C, Methvin A, et al. Reorganizing a hospital ward as an accountable care unit. J Hosp Med. 2015;10(1):36-40.
16. Yousefi V. How Canadian hospitalists spend their time - A work-sampling study within a hospital medicine program in Ontario. Journal of Clinical Outcomes Management. 2011;18(4):159.
17. Marinella MA: Hospitalists-Where They Came from, Who They Are, and What They Do. Hosp Physician. 2002;38(5):32-36.
18. Wachter RM. An introduction to the hospitalist model. Ann Intern Med. 1999;130(4 Pt 2):338-342.
19. Wachter RM, Goldman L. The hospitalist movement 5 years later. JAMA. 2002;287(4):487-494.
20. van Walraven C. The Influence of Inpatient Physician Continuity on Hospital Discharge. J Gen Intern Med. 2019;34(9):1709-1714.
21. Goodwin JS, Li S, Kuo YF. Association of the Work Schedules of Hospitalists With Patient Outcomes of Hospitalization. JAMA Intern Med. 2020;180(2):215-222.
22. Nichani S, Fitterman N, Lukela M, Crocker J, the Society of Hospital Medicine, Patient Handoff. 2017 Hospital Medicine Revised Core Competencies. J Hosp Med. 2017;4:S74.
23. Lo HY, Mullan PC, Lye C, et al. A QI initiative: implementing a patient handoff checklist for pediatric hospitalist attendings. BMJ Qual Improv Rep. 2016;5(1):u212920.w5661.
24. Wachter RM, Goldman L. Zero to 50,000 - The 20th Anniversary of the Hospitalist. N Engl J Med. 2016;375(11):1009-1011.
25. Grimm, P. Social Desirability Bias. In: Sheth J, Malhotra N, eds. Wiley International Encyclopedia of Marketing. John Wiley & Sons, Ltd; 2010.
From the Fraser Health Authority, Surrey, BC, Canada (Drs. Yousefi and Paletta), and Catalyst Consulting Inc., Vancouver, BC, Canada (Elayne McIvor).
Objective: Despite the ongoing growth in the number of hospitalist programs in Canada, their impact on the quality of interprofessional communication, teamwork, and staff satisfaction is not well known. This study aimed to evaluate perceptions of frontline care providers and hospital managers about the impact of the implementation of 3 new hospitalist services on care quality, teamwork, and interprofessional communication.
Design: We used an online survey and semistructured interviews to evaluate respondents’ views on quality of interprofessional communication and collaboration, impact of the new services on quality of care, and overall staff satisfaction with the new inpatient care model.
Setting: Integrated Regional Health Authority in British Columbia, Canada.
Participants: Participants included hospital administrators, frontline care providers (across a range of professions), and hospital and community-based physicians.
Results: The majority of respondents reported high levels of satisfaction with their new hospital medicine services. They identified improvements in interprofessional collaboration and communication between hospitalists and other professionals, which were attributed to enhanced onsite presence of physicians. They also perceived improvements in quality of care and efficiency. On the other hand, they identified a number of challenges with the change process, and raised concerns about the impact of patient handoffs on care quality and efficiency.
Conclusion: Across 3 very different acute care settings, the implementation of a hospitalist service was widely perceived to have resulted in improved teamwork, quality of care, and interprofessional communication.
Keywords: hospital medicine; hospitalist; teamwork; interprofessional collaboration.
Over the past 2 decades, the hospitalist model has become prevalent in Canada and internationally.1 Hospitalist care has been associated with improvements in efficiency and quality of care.2-6 However, less is known about its impact on the quality of interprofessional communication, teamwork, and staff satisfaction. In a 2012 study of a specialized orthopedic facility in the Greater Toronto Area (GTA), Ontario, Webster et al found a pervasive perception among interviewees that the addition of a hospitalist resulted in improved patient safety, expedited transfers, enhanced communication with Primary Care Providers (PCPs), and better continuity of care.7 They also identified enhanced collaboration among providers since the addition of the hospitalist to the care team. In another study of 5 community hospitals in the GTA, Conn et al8 found that staff on General Internal Medicine wards where hospitalists worked described superior interprofessional collaboration, deeper interpersonal relationships between physicians and other care team members, and a higher sense of “team-based care.”
Fraser Health Authority (FH) is an integrated regional health system with one of the largest regional Hospital Medicine (HM) networks in Canada.9 Over the past 2 decades, FH has implemented a number of HM services in its acute care facilities across a range of small and large community and academic hospitals. More recently, 3 hospitalist services were implemented over a 2-year period: new HM services in a tertiary referral center (Site A, July 2016) and a small community hospital (Site B, December 2016), and reintroduction of a hospitalist service in a medium-sized community hospital (Site C, January 2017). This provided a unique opportunity to assess the impact of the implementation of the hospitalist model across a range of facilities. The main objectives of this evaluation were to understand the level of physician, nursing, allied staff, and hospital administration satisfaction with the new hospitalist model, as well as the perceived impact of the service on efficiency and quality of care. As such, FH engaged an external consultant (EM) to conduct a comprehensive evaluation of the introduction of its latest HM services.
Methods
Setting
Hospital medicine services are currently available in 10 of 12 acute care facilities within the FH system. The 3 sites described in this evaluation constitute the most recent sites where a hospitalist service was implemented.
Site A is a 272-bed tertiary referral center situated in a rapidly growing community. At the time of our evaluation, 21 Full Time Equivalent (FTE) hospitalists cared for an average of 126 patients, which constituted the majority of adult medical patients. Each day, 8 individuals rounded on admitted patients (average individual census: 16) with another person providing in-house, evening, and overnight coverage. An additional flexible shift during the early afternoon helped with Emergency Department (ED) admissions.
Site B is small, 45-bed community hospital in a semi-rural community. The hospitalist service began in December 2016, with 4 FTE hospitalists caring for an average of 28 patients daily. This constituted 2 hospitalists rounding daily on admitted patients, with on-call coverage provided from home.
Site C is a 188-bed community hospital with a hospitalist service initially introduced in 2005. In 2016, the program was disbanded and the site moved back to a primarily community-based model, in which family physicians in the community were invited to assume the care of hospitalized patients. However, the hospitalist program had to be reintroduced in January 2017 due to poor uptake among PCPs in the community. At the time of evaluation, 19 FTE hospitalists (with 7 hospitalists working daily) provided most responsible physician care to a daily census of 116 patients (average individual census: 16). The program also covered ED admissions in-house until midnight, with overnight call provided from home.
Approach
We adopted a utilization-focused evaluation approach to guide our investigation. In this approach, the assessment is deliberately planned and conducted in a way that it maximizes the likelihood that findings would be used by the organization to inform learning, adaptations, and decision-making.11 To enable this, the evaluator identified the primary intended recipients and engaged them at the start of the evaluation process to understand the main intended uses of the project. Moreover, the evaluator ensured that these intended uses of the evaluation guided all other decisions made throughout the process.
We collected data using an online survey of the staff at the 3 facilities, complemented by a series of semistructured qualitative interviews with FH administrators and frontline providers.
Online survey
We conducted an open online survey of a broad range of stakeholders who worked in the 3 facilities. To develop the questionnaire, we searched our department’s archives for previous surveys conducted from 2001 to 2005. We also interviewed the regional HM program management team to identify priority areas and reached out to the local leadership of the 3 acute care facilities for their input and support of the project. We refined the survey through several iterations, seeking input from experts in the FH Department of Evaluation and Research. The final questionnaire contained 10 items, including a mix of closed- and open-ended questions (Appendix A).
To reach the target audience, we collaborated with each hospital’s local leadership as well as the Divisions of Family Practice (DFP) that support local community PCPs in each hospital community.10 Existing email lists were compiled to create a master electronic survey distribution list. The initial invitation and 3 subsequent reminders were disseminated to the following target groups: hospital physicians (both hospitalists and nonhospitalists), PCPs, nursing and other allied professionals, administrators, and DFP leadership.
The survey consent form, background information, questions, and online platform (SimpleSurvey, Montreal, QC) were approved by FH’s Privacy Department. All respondents were required to provide their consent and able to withdraw at any time. Survey responses were kept anonymous and confidential, with results captured automatically into a spreadsheet by the survey platform. As an incentive for participation, respondents had the opportunity to win 1 of 3 $100 Visa gift cards. Personal contact information provided for the prize draw was collected in a separate survey that could not link back to respondents’ answers. The survey was trialed several times by the evaluation team to address any technical challenges before dissemination to the targeted participants.
Qualitative interviews
We conducted semistructured interviews with a purposive sample of FH administrators and frontline providers (Appendix B). The interview questions broadly mirrored the survey but allowed for more in-depth exploration of constructs. Interviewees were recruited through email invitations to selected senior and mid-level local and regional administrators, asking interviewees to refer our team to other contacts, and inviting survey respondents to voluntarily participate in a follow-up interview. One of the authors (EM), a Credentialed Evaluator, conducted all the one-time interviews either in-person at the individual participant’s workplace or by telephone. She did not have pre-existing relationships with any of the interviewees. Interviews were recorded and transcribed for analysis. Interviewees were required to consent to participate and understood that they could withdraw at any point. They were not offered incentives to participate. Interviews were carried out until thematic saturation was reached.
Analysis
A content analysis approach was employed for all qualitative data, which included open-ended responses from the online survey and interview transcripts. One of the authors (EM) conducted the analysis. The following steps were followed in the inductive content analysis process: repeated reading of the raw data, generation of initial thematic codes, organizing and sorting codes into categories (ie, main vs subcategories), coding of all data, quantifying codes, and interpreting themes. When responding to open-ended questions, respondents often provided multiple answers per question. Each of the respondents’ answers were coded. In alignment with the inductive nature of the analysis process, themes emerged organically from the data rather than the researchers using preconceived theories and categories to code the text. This was achieved by postponing the review of relevant literature on the topic until after the analysis was complete and using an external evaluation consultant (with no prior relationship to FH and limited theoretical knowledge of the topic matter) to analyze the data. Descriptive statistics were run on quantitative data in SPSS (v.24, IBM, Armonk, NY). For survey responses to be included in the analysis, the respondents needed to indicate which site they worked at and were required to answer at least 1 other survey question. One interviewee was excluded from the analysis since they were not familiar with the hospitalist model at their site.
Ethics approval
The evaluation protocol was reviewed by FH Department of Evaluation and Research and was deemed exempt from formal research ethics review.
Results
A total of 377 individuals responded to the online survey between January 8 and February 28, 2018 (response rate 14%). The distribution of respondents generally reflected the size of the respective acute care facilities. Compared to the overall sampled population, fewer nurses participated in the survey (45% vs 64%) while the rate of participation for Unit Clerks (14% vs 16%) and allied professionals (12% vs 16%) were similar.
Out of the 45 people approached for an interview, a total of 38 were conducted from January 3 to March 5, 2018 (response rate 84%). The interviews lasted an average of 42 minutes. Interviewees represented a range of administrative and health professional roles (Figure 1). Some interviewees held multiple positions.
Satisfaction with HM service
Across all sites, survey respondents reported high levels of satisfaction with their respective HM services and identified positive impacts on their job satisfaction (Figure 2). Almost all interviewees similarly expressed high satisfaction levels with their HM services (95%; n = 36).
Perceptions of HM service performance
Survey respondents rated the strength of hospitalists’ interprofessional communication and collaboration with other physicians and with care teams. Roughly two-thirds reported that overall hospitalist communication was “good” or “very good.” We also asked participants to rate the frequency at which hospitalists met best practice expectations related to interprofessional teamwork. Across all sites, similar proportions of respondents (23% to 39%) reported that these best practices were met “most of the time” or “always” (Figure 3). Survey questions also assessed perceptions of respondents about the quality and safety of care provided by hospitalists (Figure 4).
Perceptions of the impact of the HM service postimplementation
The majority of survey respondents reported improvements in the quality of communication, professional relationships, and coordination of inpatient care at transition points after the implementation of the HM service (Figure 5). This was also reflected in interviews, where some indicated that it was easier to communicate with hospitalists due to their on-site presence, accessibility, and 24/7 availability (n = 21). They also described improved collaboration within the care teams (n = 7), and easier communication with hospitalists because they were approachable, willing, and receptive (n = 4).
We also asked the survey respondents to assess the impact of the new hospitalist model on different dimensions of care quality, including patient satisfaction, patient experience, efficiency, and overall quality of care (Figure 6). Findings were comparable across these dimensions, with roughly 50-60% of respondents noting positive changes compared to before the implementation of the programs. However, most interviewees identified both positive and negative effects in these areas. Positive impacts included hospitalist on-site presence leading to better accessibility and timeliness of care (n = 5), hospitalists providing continuity to patients/families by working for weeklong rotations (n = 6), hospitalists being particularly skilled at managing complex clinical presentations (n = 2), and hospitalists being able to spend more time with patients (n = 2). On the other hand, some interviewees noted that patients and families did not like seeing multiple doctors due to frequent handoffs between hospitalists (n = 12). They also raised concerns that hospitalists did not know patients’ histories or had relationships with them, potentially leading to longer length of stay and unnecessary investigations (n = 8).
Site-to-site ratings of satisfaction and performance
Survey respondents’ satisfaction and performance ratings varied substantially site-to-site. Across all areas assessed, ratings were consistently highest at Site B (the smallest institution in our evaluation and the most recent addition to the HM network in the health authority). These differences were statistically significant across all survey questions asked.
Discussion
Findings from this study provide insight into the experiences of frontline health care professionals and administrators with the implementation of new HM services across a range of small to large acute care facilities. They indicate that the majority of respondents reported high levels of satisfaction with their hospitalist services. Most also indicated that the service had resulted in improvements compared to prior inpatient care models.
Over half of the survey respondents, and the majority of interviewees, reported a positive impact on interprofessional communication and collaboration. This was largely attributed to enhanced accessibility and availability of hospitalists:
- "Being on-site lends itself to better communication because they’re accessible. Hospitalists always answer the phone, but the general practitioners (GP) don’t always since they may be with other patients." (Dietician, Site A)
- "A big strength is that we have physician presence on the unit all day during scheduled hours, which makes us more accessible to nurses and more able to follow up on patients that we have concerns about." (Physician Leader, Site B)
However, the ratings dropped substantially when they were asked to assess adherence to specific best practices of such communication and collaboration, such as participation in daily check-ins or attendance at team care rounds (Figure 3). Interdisciplinary clinical rounds have been identified as a tool to improve the effectiveness of care teams.12 A number of elements have been identified as key components of effective rounds.13 Bedside rounds have also been found to enhance communication and teamwork.14,15 In our study, the discrepancy between overall high levels of satisfaction with hospitalists’ communication/collaboration despite low scores on participation in more concrete activities may illustrate the importance of informal and ad hoc opportunities for interactions between hospitalists and other care providers that result from the enhanced presence of hospitalists on care units.8 Outside of formal rounds, hospitalists have the ability to interact with other care providers throughout their shifts. Prior studies have shown that hospitalists spend a significant portion of their time communicating with other care team members throughout their workdays.16 At the same time, the amount of time spent on communication should be balanced against the need for provision of direct care at the bedside. Future research should aim to identify the right balance between these competing priorities, and to understand the nature and quality of the communication between various care providers.
We also aimed to understand the perceptions of study participants about the impact of the HM service on quality of care. Survey participants not only expressed reasonable satisfaction with various aspects of hospitalists’ performance, but also described a positive impact on care quality after the implementation of their new services. This was also reflected in the interviews:
- "The clinical knowledge of the new hospitalists is far better. Some are internal medicine trained, so they bring better knowledge and skills. I feel comfortable that they can take patients and manage them. I wasn’t always comfortable with doing that in the past." (Emergency Physician, Site C)
- "Hospitalists are really familiar with acute care and how it works. They’ve become more familiar with the discharge planning system and thus know more about the resources available. And even something as simple as knowing which forms to use." (Dietician, Site A)
It must be noted that these observations should ideally be corroborated through a robust before-after analysis of various quality measures. While such an analysis was beyond the scope of our current project, we have previously demonstrated that across our network (including the 3 sites included in our evaluation) hospitalist care is associated with lower mortality and readmission rates.4 Our findings appear to confirm previous suggestions that hospitalists’ dedicated focus on inpatient care may allow them to develop enhanced skills in the management of common conditions in the acute care setting17 which can be perceived to be of value to other hospital-based care providers.
The issue of frequent handover among hospitalists was the most commonly identified challenge by both survey respondents and interviewees:
- "They’re very reluctant to discharge patients if it’s their first day with the patient. Even if the previous hospitalist said they were ready for discharge, the new doc wants to run all of their own tests before they feel comfortable. Maybe it’s a trust issue between hospitalists when they hand patients over. It’s also being personally liable for patients if you discharge them." (Patient Care Coordinator, Site A)
- "Communication is an issue. There’s lots of turnover in hospitalists. Relationships were closer with GPs because we had so much more interaction with particular individuals." (Hospitalist Physician Leader, Site A)
It must be noted that we conducted our evaluation in a relatively short time span (within 2 years) after the 3 services were implemented. Developing trust among a large number of hospitalists newly recruited to these programs can take time and may be a factor that can explain the reluctance of some to discharge patients after handoffs. However, concerns about discontinuity of care inherent in the hospitalist model are not new.18,19 Better continuity has been associated with higher probability of patient discharges20 and improved outcomes.21 To address this challenge, the hospitalist community has focused on defining the core competencies associated with high quality handovers,22 and deliberate efforts to improve the quality of handoffs through quality improvement methodologies.23 Our study participants similarly identified these measures as potential solutions. Despite this, addressing hospitalist continuity of care remains a pressing challenge for the broader hospitalist community.24
Our evaluation has a number of methodological limitations. First, the survey response rate was only 14%, which raises questions about nonresponse bias and the representativeness of the findings to the larger population of interest. While the distribution of respondents was largely similar to the overall sampled population, a number of factors may have impacted our response rate. For example, we were only able to distribute our survey to health care providers’ institutional email addresses. Moreover, while we provided incentives for participation and sent out a number of reminders, we solely relied on one communication modality (ie, electronic communication) and did not utilize other methods (such as posters, reminder at meetings, in-person invitations). Second, while the survey included a number of open-ended questions, many of these responses were at times brief and difficult to interpret and were not included in the analysis. Third, all data collected were self-reported. For example, we could not corroborate comments about participation in interdisciplinary rounds by objective measures such as attendance records or direct observation. Self-report data is subjective in nature and is vulnerable to a range of biases, such as social desirability bias.25 Finally, patient satisfaction and experience with hospitalist care were not assessed by patients themselves. Ideally, standardized cross-site indicators should validate our patient-related results.
As mentioned above, hospitalist performance ratings varied substantially from site-to-site and were consistently higher at Site B (a small community hospital in a semi-rural area), followed by Site C (a medium-sized community hospital) and Site A (a tertiary referral center). The variability in program ratings and perceived hospitalist impacts between sites could be due to a variety of factors, such as the degree of change between the past and current models at each site, differences in hospitalist hiring processes, hospital size and culture, and differences in service design and operations. It may also be related to the timing of the introduction of the HM service, as Site B was the most recent site where the service was established. As such, there may be an element of recall bias behind the observed discrepancies. This highlights the importance of local context on respondent perceptions and suggests that our results may not be generalizable to other institutions with different attributes and characteristics.
Conclusion
Findings from this study have demonstrated that the recent hospitalist services in our health system have improved overall levels of interprofessional communication and teamwork, as well as perceptions of care quality among the majority of participants who reported high levels of satisfaction with their programs. Our findings further highlight the issue of frequent handovers among hospitalists as a pressing and ongoing challenge.
Corresponding Author: Vandad Yousefi, MD, CCFP, Past Regional Department Head – Hospital Medicine, Fraser Health Authority, Central City Tower, Suite 400, 13450 – 102nd Ave, Surrey, BC V3T 0H1; [email protected].
Financial disclosures: This project was funded by the Fraser Health Authority, which provided the funding for hiring of the external consultant to design, implement, and analyze the results of the evaluation program in collaboration with the Regional Hospitalist Program at Fraser Health.
From the Fraser Health Authority, Surrey, BC, Canada (Drs. Yousefi and Paletta), and Catalyst Consulting Inc., Vancouver, BC, Canada (Elayne McIvor).
Objective: Despite the ongoing growth in the number of hospitalist programs in Canada, their impact on the quality of interprofessional communication, teamwork, and staff satisfaction is not well known. This study aimed to evaluate perceptions of frontline care providers and hospital managers about the impact of the implementation of 3 new hospitalist services on care quality, teamwork, and interprofessional communication.
Design: We used an online survey and semistructured interviews to evaluate respondents’ views on quality of interprofessional communication and collaboration, impact of the new services on quality of care, and overall staff satisfaction with the new inpatient care model.
Setting: Integrated Regional Health Authority in British Columbia, Canada.
Participants: Participants included hospital administrators, frontline care providers (across a range of professions), and hospital and community-based physicians.
Results: The majority of respondents reported high levels of satisfaction with their new hospital medicine services. They identified improvements in interprofessional collaboration and communication between hospitalists and other professionals, which were attributed to enhanced onsite presence of physicians. They also perceived improvements in quality of care and efficiency. On the other hand, they identified a number of challenges with the change process, and raised concerns about the impact of patient handoffs on care quality and efficiency.
Conclusion: Across 3 very different acute care settings, the implementation of a hospitalist service was widely perceived to have resulted in improved teamwork, quality of care, and interprofessional communication.
Keywords: hospital medicine; hospitalist; teamwork; interprofessional collaboration.
Over the past 2 decades, the hospitalist model has become prevalent in Canada and internationally.1 Hospitalist care has been associated with improvements in efficiency and quality of care.2-6 However, less is known about its impact on the quality of interprofessional communication, teamwork, and staff satisfaction. In a 2012 study of a specialized orthopedic facility in the Greater Toronto Area (GTA), Ontario, Webster et al found a pervasive perception among interviewees that the addition of a hospitalist resulted in improved patient safety, expedited transfers, enhanced communication with Primary Care Providers (PCPs), and better continuity of care.7 They also identified enhanced collaboration among providers since the addition of the hospitalist to the care team. In another study of 5 community hospitals in the GTA, Conn et al8 found that staff on General Internal Medicine wards where hospitalists worked described superior interprofessional collaboration, deeper interpersonal relationships between physicians and other care team members, and a higher sense of “team-based care.”
Fraser Health Authority (FH) is an integrated regional health system with one of the largest regional Hospital Medicine (HM) networks in Canada.9 Over the past 2 decades, FH has implemented a number of HM services in its acute care facilities across a range of small and large community and academic hospitals. More recently, 3 hospitalist services were implemented over a 2-year period: new HM services in a tertiary referral center (Site A, July 2016) and a small community hospital (Site B, December 2016), and reintroduction of a hospitalist service in a medium-sized community hospital (Site C, January 2017). This provided a unique opportunity to assess the impact of the implementation of the hospitalist model across a range of facilities. The main objectives of this evaluation were to understand the level of physician, nursing, allied staff, and hospital administration satisfaction with the new hospitalist model, as well as the perceived impact of the service on efficiency and quality of care. As such, FH engaged an external consultant (EM) to conduct a comprehensive evaluation of the introduction of its latest HM services.
Methods
Setting
Hospital medicine services are currently available in 10 of 12 acute care facilities within the FH system. The 3 sites described in this evaluation constitute the most recent sites where a hospitalist service was implemented.
Site A is a 272-bed tertiary referral center situated in a rapidly growing community. At the time of our evaluation, 21 Full Time Equivalent (FTE) hospitalists cared for an average of 126 patients, which constituted the majority of adult medical patients. Each day, 8 individuals rounded on admitted patients (average individual census: 16) with another person providing in-house, evening, and overnight coverage. An additional flexible shift during the early afternoon helped with Emergency Department (ED) admissions.
Site B is small, 45-bed community hospital in a semi-rural community. The hospitalist service began in December 2016, with 4 FTE hospitalists caring for an average of 28 patients daily. This constituted 2 hospitalists rounding daily on admitted patients, with on-call coverage provided from home.
Site C is a 188-bed community hospital with a hospitalist service initially introduced in 2005. In 2016, the program was disbanded and the site moved back to a primarily community-based model, in which family physicians in the community were invited to assume the care of hospitalized patients. However, the hospitalist program had to be reintroduced in January 2017 due to poor uptake among PCPs in the community. At the time of evaluation, 19 FTE hospitalists (with 7 hospitalists working daily) provided most responsible physician care to a daily census of 116 patients (average individual census: 16). The program also covered ED admissions in-house until midnight, with overnight call provided from home.
Approach
We adopted a utilization-focused evaluation approach to guide our investigation. In this approach, the assessment is deliberately planned and conducted in a way that it maximizes the likelihood that findings would be used by the organization to inform learning, adaptations, and decision-making.11 To enable this, the evaluator identified the primary intended recipients and engaged them at the start of the evaluation process to understand the main intended uses of the project. Moreover, the evaluator ensured that these intended uses of the evaluation guided all other decisions made throughout the process.
We collected data using an online survey of the staff at the 3 facilities, complemented by a series of semistructured qualitative interviews with FH administrators and frontline providers.
Online survey
We conducted an open online survey of a broad range of stakeholders who worked in the 3 facilities. To develop the questionnaire, we searched our department’s archives for previous surveys conducted from 2001 to 2005. We also interviewed the regional HM program management team to identify priority areas and reached out to the local leadership of the 3 acute care facilities for their input and support of the project. We refined the survey through several iterations, seeking input from experts in the FH Department of Evaluation and Research. The final questionnaire contained 10 items, including a mix of closed- and open-ended questions (Appendix A).
To reach the target audience, we collaborated with each hospital’s local leadership as well as the Divisions of Family Practice (DFP) that support local community PCPs in each hospital community.10 Existing email lists were compiled to create a master electronic survey distribution list. The initial invitation and 3 subsequent reminders were disseminated to the following target groups: hospital physicians (both hospitalists and nonhospitalists), PCPs, nursing and other allied professionals, administrators, and DFP leadership.
The survey consent form, background information, questions, and online platform (SimpleSurvey, Montreal, QC) were approved by FH’s Privacy Department. All respondents were required to provide their consent and able to withdraw at any time. Survey responses were kept anonymous and confidential, with results captured automatically into a spreadsheet by the survey platform. As an incentive for participation, respondents had the opportunity to win 1 of 3 $100 Visa gift cards. Personal contact information provided for the prize draw was collected in a separate survey that could not link back to respondents’ answers. The survey was trialed several times by the evaluation team to address any technical challenges before dissemination to the targeted participants.
Qualitative interviews
We conducted semistructured interviews with a purposive sample of FH administrators and frontline providers (Appendix B). The interview questions broadly mirrored the survey but allowed for more in-depth exploration of constructs. Interviewees were recruited through email invitations to selected senior and mid-level local and regional administrators, asking interviewees to refer our team to other contacts, and inviting survey respondents to voluntarily participate in a follow-up interview. One of the authors (EM), a Credentialed Evaluator, conducted all the one-time interviews either in-person at the individual participant’s workplace or by telephone. She did not have pre-existing relationships with any of the interviewees. Interviews were recorded and transcribed for analysis. Interviewees were required to consent to participate and understood that they could withdraw at any point. They were not offered incentives to participate. Interviews were carried out until thematic saturation was reached.
Analysis
A content analysis approach was employed for all qualitative data, which included open-ended responses from the online survey and interview transcripts. One of the authors (EM) conducted the analysis. The following steps were followed in the inductive content analysis process: repeated reading of the raw data, generation of initial thematic codes, organizing and sorting codes into categories (ie, main vs subcategories), coding of all data, quantifying codes, and interpreting themes. When responding to open-ended questions, respondents often provided multiple answers per question. Each of the respondents’ answers were coded. In alignment with the inductive nature of the analysis process, themes emerged organically from the data rather than the researchers using preconceived theories and categories to code the text. This was achieved by postponing the review of relevant literature on the topic until after the analysis was complete and using an external evaluation consultant (with no prior relationship to FH and limited theoretical knowledge of the topic matter) to analyze the data. Descriptive statistics were run on quantitative data in SPSS (v.24, IBM, Armonk, NY). For survey responses to be included in the analysis, the respondents needed to indicate which site they worked at and were required to answer at least 1 other survey question. One interviewee was excluded from the analysis since they were not familiar with the hospitalist model at their site.
Ethics approval
The evaluation protocol was reviewed by FH Department of Evaluation and Research and was deemed exempt from formal research ethics review.
Results
A total of 377 individuals responded to the online survey between January 8 and February 28, 2018 (response rate 14%). The distribution of respondents generally reflected the size of the respective acute care facilities. Compared to the overall sampled population, fewer nurses participated in the survey (45% vs 64%) while the rate of participation for Unit Clerks (14% vs 16%) and allied professionals (12% vs 16%) were similar.
Out of the 45 people approached for an interview, a total of 38 were conducted from January 3 to March 5, 2018 (response rate 84%). The interviews lasted an average of 42 minutes. Interviewees represented a range of administrative and health professional roles (Figure 1). Some interviewees held multiple positions.
Satisfaction with HM service
Across all sites, survey respondents reported high levels of satisfaction with their respective HM services and identified positive impacts on their job satisfaction (Figure 2). Almost all interviewees similarly expressed high satisfaction levels with their HM services (95%; n = 36).
Perceptions of HM service performance
Survey respondents rated the strength of hospitalists’ interprofessional communication and collaboration with other physicians and with care teams. Roughly two-thirds reported that overall hospitalist communication was “good” or “very good.” We also asked participants to rate the frequency at which hospitalists met best practice expectations related to interprofessional teamwork. Across all sites, similar proportions of respondents (23% to 39%) reported that these best practices were met “most of the time” or “always” (Figure 3). Survey questions also assessed perceptions of respondents about the quality and safety of care provided by hospitalists (Figure 4).
Perceptions of the impact of the HM service postimplementation
The majority of survey respondents reported improvements in the quality of communication, professional relationships, and coordination of inpatient care at transition points after the implementation of the HM service (Figure 5). This was also reflected in interviews, where some indicated that it was easier to communicate with hospitalists due to their on-site presence, accessibility, and 24/7 availability (n = 21). They also described improved collaboration within the care teams (n = 7), and easier communication with hospitalists because they were approachable, willing, and receptive (n = 4).
We also asked the survey respondents to assess the impact of the new hospitalist model on different dimensions of care quality, including patient satisfaction, patient experience, efficiency, and overall quality of care (Figure 6). Findings were comparable across these dimensions, with roughly 50-60% of respondents noting positive changes compared to before the implementation of the programs. However, most interviewees identified both positive and negative effects in these areas. Positive impacts included hospitalist on-site presence leading to better accessibility and timeliness of care (n = 5), hospitalists providing continuity to patients/families by working for weeklong rotations (n = 6), hospitalists being particularly skilled at managing complex clinical presentations (n = 2), and hospitalists being able to spend more time with patients (n = 2). On the other hand, some interviewees noted that patients and families did not like seeing multiple doctors due to frequent handoffs between hospitalists (n = 12). They also raised concerns that hospitalists did not know patients’ histories or had relationships with them, potentially leading to longer length of stay and unnecessary investigations (n = 8).
Site-to-site ratings of satisfaction and performance
Survey respondents’ satisfaction and performance ratings varied substantially site-to-site. Across all areas assessed, ratings were consistently highest at Site B (the smallest institution in our evaluation and the most recent addition to the HM network in the health authority). These differences were statistically significant across all survey questions asked.
Discussion
Findings from this study provide insight into the experiences of frontline health care professionals and administrators with the implementation of new HM services across a range of small to large acute care facilities. They indicate that the majority of respondents reported high levels of satisfaction with their hospitalist services. Most also indicated that the service had resulted in improvements compared to prior inpatient care models.
Over half of the survey respondents, and the majority of interviewees, reported a positive impact on interprofessional communication and collaboration. This was largely attributed to enhanced accessibility and availability of hospitalists:
- "Being on-site lends itself to better communication because they’re accessible. Hospitalists always answer the phone, but the general practitioners (GP) don’t always since they may be with other patients." (Dietician, Site A)
- "A big strength is that we have physician presence on the unit all day during scheduled hours, which makes us more accessible to nurses and more able to follow up on patients that we have concerns about." (Physician Leader, Site B)
However, the ratings dropped substantially when they were asked to assess adherence to specific best practices of such communication and collaboration, such as participation in daily check-ins or attendance at team care rounds (Figure 3). Interdisciplinary clinical rounds have been identified as a tool to improve the effectiveness of care teams.12 A number of elements have been identified as key components of effective rounds.13 Bedside rounds have also been found to enhance communication and teamwork.14,15 In our study, the discrepancy between overall high levels of satisfaction with hospitalists’ communication/collaboration despite low scores on participation in more concrete activities may illustrate the importance of informal and ad hoc opportunities for interactions between hospitalists and other care providers that result from the enhanced presence of hospitalists on care units.8 Outside of formal rounds, hospitalists have the ability to interact with other care providers throughout their shifts. Prior studies have shown that hospitalists spend a significant portion of their time communicating with other care team members throughout their workdays.16 At the same time, the amount of time spent on communication should be balanced against the need for provision of direct care at the bedside. Future research should aim to identify the right balance between these competing priorities, and to understand the nature and quality of the communication between various care providers.
We also aimed to understand the perceptions of study participants about the impact of the HM service on quality of care. Survey participants not only expressed reasonable satisfaction with various aspects of hospitalists’ performance, but also described a positive impact on care quality after the implementation of their new services. This was also reflected in the interviews:
- "The clinical knowledge of the new hospitalists is far better. Some are internal medicine trained, so they bring better knowledge and skills. I feel comfortable that they can take patients and manage them. I wasn’t always comfortable with doing that in the past." (Emergency Physician, Site C)
- "Hospitalists are really familiar with acute care and how it works. They’ve become more familiar with the discharge planning system and thus know more about the resources available. And even something as simple as knowing which forms to use." (Dietician, Site A)
It must be noted that these observations should ideally be corroborated through a robust before-after analysis of various quality measures. While such an analysis was beyond the scope of our current project, we have previously demonstrated that across our network (including the 3 sites included in our evaluation) hospitalist care is associated with lower mortality and readmission rates.4 Our findings appear to confirm previous suggestions that hospitalists’ dedicated focus on inpatient care may allow them to develop enhanced skills in the management of common conditions in the acute care setting17 which can be perceived to be of value to other hospital-based care providers.
The issue of frequent handover among hospitalists was the most commonly identified challenge by both survey respondents and interviewees:
- "They’re very reluctant to discharge patients if it’s their first day with the patient. Even if the previous hospitalist said they were ready for discharge, the new doc wants to run all of their own tests before they feel comfortable. Maybe it’s a trust issue between hospitalists when they hand patients over. It’s also being personally liable for patients if you discharge them." (Patient Care Coordinator, Site A)
- "Communication is an issue. There’s lots of turnover in hospitalists. Relationships were closer with GPs because we had so much more interaction with particular individuals." (Hospitalist Physician Leader, Site A)
It must be noted that we conducted our evaluation in a relatively short time span (within 2 years) after the 3 services were implemented. Developing trust among a large number of hospitalists newly recruited to these programs can take time and may be a factor that can explain the reluctance of some to discharge patients after handoffs. However, concerns about discontinuity of care inherent in the hospitalist model are not new.18,19 Better continuity has been associated with higher probability of patient discharges20 and improved outcomes.21 To address this challenge, the hospitalist community has focused on defining the core competencies associated with high quality handovers,22 and deliberate efforts to improve the quality of handoffs through quality improvement methodologies.23 Our study participants similarly identified these measures as potential solutions. Despite this, addressing hospitalist continuity of care remains a pressing challenge for the broader hospitalist community.24
Our evaluation has a number of methodological limitations. First, the survey response rate was only 14%, which raises questions about nonresponse bias and the representativeness of the findings to the larger population of interest. While the distribution of respondents was largely similar to the overall sampled population, a number of factors may have impacted our response rate. For example, we were only able to distribute our survey to health care providers’ institutional email addresses. Moreover, while we provided incentives for participation and sent out a number of reminders, we solely relied on one communication modality (ie, electronic communication) and did not utilize other methods (such as posters, reminder at meetings, in-person invitations). Second, while the survey included a number of open-ended questions, many of these responses were at times brief and difficult to interpret and were not included in the analysis. Third, all data collected were self-reported. For example, we could not corroborate comments about participation in interdisciplinary rounds by objective measures such as attendance records or direct observation. Self-report data is subjective in nature and is vulnerable to a range of biases, such as social desirability bias.25 Finally, patient satisfaction and experience with hospitalist care were not assessed by patients themselves. Ideally, standardized cross-site indicators should validate our patient-related results.
As mentioned above, hospitalist performance ratings varied substantially from site-to-site and were consistently higher at Site B (a small community hospital in a semi-rural area), followed by Site C (a medium-sized community hospital) and Site A (a tertiary referral center). The variability in program ratings and perceived hospitalist impacts between sites could be due to a variety of factors, such as the degree of change between the past and current models at each site, differences in hospitalist hiring processes, hospital size and culture, and differences in service design and operations. It may also be related to the timing of the introduction of the HM service, as Site B was the most recent site where the service was established. As such, there may be an element of recall bias behind the observed discrepancies. This highlights the importance of local context on respondent perceptions and suggests that our results may not be generalizable to other institutions with different attributes and characteristics.
Conclusion
Findings from this study have demonstrated that the recent hospitalist services in our health system have improved overall levels of interprofessional communication and teamwork, as well as perceptions of care quality among the majority of participants who reported high levels of satisfaction with their programs. Our findings further highlight the issue of frequent handovers among hospitalists as a pressing and ongoing challenge.
Corresponding Author: Vandad Yousefi, MD, CCFP, Past Regional Department Head – Hospital Medicine, Fraser Health Authority, Central City Tower, Suite 400, 13450 – 102nd Ave, Surrey, BC V3T 0H1; [email protected].
Financial disclosures: This project was funded by the Fraser Health Authority, which provided the funding for hiring of the external consultant to design, implement, and analyze the results of the evaluation program in collaboration with the Regional Hospitalist Program at Fraser Health.
1. Yousefi V, Wilton D. Re-designing Hospital Care: Learning from the Experience of Hospital Medicine in Canada. Journal of Global Health Care Systems. 2011;1(3).
2. White HL. Assessing the Prevalence, Penetration and Performance of Hospital Physicians in Ontario: Implications for the Quality and Efficiency of Inpatient Care. Doctoral Thesis; 2016.
3. Yousefi V, Chong CA. Does implementation of a hospitalist program in a Canadian community hospital improve measures of quality of care and utilization? An observational comparative analysis of hospitalists vs. traditional care providers. BMC Health Serv Res. 2013;13:204.
4. Yousefi V, Hejazi S, Lam A. Impact of Hospitalists on Care Outcomes in a Large Integrated Health System in British Columbia. Journal of Clinical Outcomes Management. 2020;27(2):59-72.
5. Salim SA, Elmaraezy A, Pamarthy A, et al. Impact of hospitalists on the efficiency of inpatient care and patient satisfaction: a systematic review and meta-analysis. J Community Hosp Intern Med Perspect. 2019;9(2):121-134.
6. Peterson MC. A systematic review of outcomes and quality measures in adult patients cared for by hospitalists vs nonhospitalists. Mayo Clinic Proc. 2009;84(3):248-254.
7. Webster F, Bremner S, Jackson M, et al. The impact of a hospitalist on role boundaries in an orthopedic environment. J Multidiscip Healthc. 2012;5:249-256.
8. Gotlib Conn L, Reeves S, Dainty K, et al. Interprofessional communication with hospitalist and consultant physicians in general internal medicine: a qualitative study. BMC Health Serv Res. 2012; 12:437.
9. About Fraser Health. Fraser Health Authority. Updated 2018. Accessed January 30, 2019. https://www.fraserhealth.ca/about-us/about-fraser-health#.XFJrl9JKiUk
10. Divisions of Family Practice. Accessed May 2, 2020. https://www.divisionsbc.ca/provincial/about-us
11. Patton MQ. Essentials of Utilization-Focused Evaluation. 2012. Sage Publications, Inc; 2011.
12. Buljac-Samardzic M, Doekhie KD, van Wijngaarden JDH. Interventions to improve team effectiveness within health care: a systematic review of the past decade. Hum Resour Health. 2020;18(1):2.
13. Verhaegh KJ, Seller-Boersma A, Simons R, et al. An exploratory study of healthcare professionals’ perceptions of interprofessional communication and collaboration. J Interprof Care. 2017;31(3):397-400.
14. O’Leary KJ, Johnson JK, Manojlovich M, et al. Redesigning systems to improve teamwork and quality for hospitalized patients (RESET): study protocol evaluating the effect of mentored implementation to redesign clinical microsystems. BMC Health Serv Res. 2019;19(1):293.
15. Stein J, Payne C, Methvin A, et al. Reorganizing a hospital ward as an accountable care unit. J Hosp Med. 2015;10(1):36-40.
16. Yousefi V. How Canadian hospitalists spend their time - A work-sampling study within a hospital medicine program in Ontario. Journal of Clinical Outcomes Management. 2011;18(4):159.
17. Marinella MA: Hospitalists-Where They Came from, Who They Are, and What They Do. Hosp Physician. 2002;38(5):32-36.
18. Wachter RM. An introduction to the hospitalist model. Ann Intern Med. 1999;130(4 Pt 2):338-342.
19. Wachter RM, Goldman L. The hospitalist movement 5 years later. JAMA. 2002;287(4):487-494.
20. van Walraven C. The Influence of Inpatient Physician Continuity on Hospital Discharge. J Gen Intern Med. 2019;34(9):1709-1714.
21. Goodwin JS, Li S, Kuo YF. Association of the Work Schedules of Hospitalists With Patient Outcomes of Hospitalization. JAMA Intern Med. 2020;180(2):215-222.
22. Nichani S, Fitterman N, Lukela M, Crocker J, the Society of Hospital Medicine, Patient Handoff. 2017 Hospital Medicine Revised Core Competencies. J Hosp Med. 2017;4:S74.
23. Lo HY, Mullan PC, Lye C, et al. A QI initiative: implementing a patient handoff checklist for pediatric hospitalist attendings. BMJ Qual Improv Rep. 2016;5(1):u212920.w5661.
24. Wachter RM, Goldman L. Zero to 50,000 - The 20th Anniversary of the Hospitalist. N Engl J Med. 2016;375(11):1009-1011.
25. Grimm, P. Social Desirability Bias. In: Sheth J, Malhotra N, eds. Wiley International Encyclopedia of Marketing. John Wiley & Sons, Ltd; 2010.
1. Yousefi V, Wilton D. Re-designing Hospital Care: Learning from the Experience of Hospital Medicine in Canada. Journal of Global Health Care Systems. 2011;1(3).
2. White HL. Assessing the Prevalence, Penetration and Performance of Hospital Physicians in Ontario: Implications for the Quality and Efficiency of Inpatient Care. Doctoral Thesis; 2016.
3. Yousefi V, Chong CA. Does implementation of a hospitalist program in a Canadian community hospital improve measures of quality of care and utilization? An observational comparative analysis of hospitalists vs. traditional care providers. BMC Health Serv Res. 2013;13:204.
4. Yousefi V, Hejazi S, Lam A. Impact of Hospitalists on Care Outcomes in a Large Integrated Health System in British Columbia. Journal of Clinical Outcomes Management. 2020;27(2):59-72.
5. Salim SA, Elmaraezy A, Pamarthy A, et al. Impact of hospitalists on the efficiency of inpatient care and patient satisfaction: a systematic review and meta-analysis. J Community Hosp Intern Med Perspect. 2019;9(2):121-134.
6. Peterson MC. A systematic review of outcomes and quality measures in adult patients cared for by hospitalists vs nonhospitalists. Mayo Clinic Proc. 2009;84(3):248-254.
7. Webster F, Bremner S, Jackson M, et al. The impact of a hospitalist on role boundaries in an orthopedic environment. J Multidiscip Healthc. 2012;5:249-256.
8. Gotlib Conn L, Reeves S, Dainty K, et al. Interprofessional communication with hospitalist and consultant physicians in general internal medicine: a qualitative study. BMC Health Serv Res. 2012; 12:437.
9. About Fraser Health. Fraser Health Authority. Updated 2018. Accessed January 30, 2019. https://www.fraserhealth.ca/about-us/about-fraser-health#.XFJrl9JKiUk
10. Divisions of Family Practice. Accessed May 2, 2020. https://www.divisionsbc.ca/provincial/about-us
11. Patton MQ. Essentials of Utilization-Focused Evaluation. 2012. Sage Publications, Inc; 2011.
12. Buljac-Samardzic M, Doekhie KD, van Wijngaarden JDH. Interventions to improve team effectiveness within health care: a systematic review of the past decade. Hum Resour Health. 2020;18(1):2.
13. Verhaegh KJ, Seller-Boersma A, Simons R, et al. An exploratory study of healthcare professionals’ perceptions of interprofessional communication and collaboration. J Interprof Care. 2017;31(3):397-400.
14. O’Leary KJ, Johnson JK, Manojlovich M, et al. Redesigning systems to improve teamwork and quality for hospitalized patients (RESET): study protocol evaluating the effect of mentored implementation to redesign clinical microsystems. BMC Health Serv Res. 2019;19(1):293.
15. Stein J, Payne C, Methvin A, et al. Reorganizing a hospital ward as an accountable care unit. J Hosp Med. 2015;10(1):36-40.
16. Yousefi V. How Canadian hospitalists spend their time - A work-sampling study within a hospital medicine program in Ontario. Journal of Clinical Outcomes Management. 2011;18(4):159.
17. Marinella MA: Hospitalists-Where They Came from, Who They Are, and What They Do. Hosp Physician. 2002;38(5):32-36.
18. Wachter RM. An introduction to the hospitalist model. Ann Intern Med. 1999;130(4 Pt 2):338-342.
19. Wachter RM, Goldman L. The hospitalist movement 5 years later. JAMA. 2002;287(4):487-494.
20. van Walraven C. The Influence of Inpatient Physician Continuity on Hospital Discharge. J Gen Intern Med. 2019;34(9):1709-1714.
21. Goodwin JS, Li S, Kuo YF. Association of the Work Schedules of Hospitalists With Patient Outcomes of Hospitalization. JAMA Intern Med. 2020;180(2):215-222.
22. Nichani S, Fitterman N, Lukela M, Crocker J, the Society of Hospital Medicine, Patient Handoff. 2017 Hospital Medicine Revised Core Competencies. J Hosp Med. 2017;4:S74.
23. Lo HY, Mullan PC, Lye C, et al. A QI initiative: implementing a patient handoff checklist for pediatric hospitalist attendings. BMJ Qual Improv Rep. 2016;5(1):u212920.w5661.
24. Wachter RM, Goldman L. Zero to 50,000 - The 20th Anniversary of the Hospitalist. N Engl J Med. 2016;375(11):1009-1011.
25. Grimm, P. Social Desirability Bias. In: Sheth J, Malhotra N, eds. Wiley International Encyclopedia of Marketing. John Wiley & Sons, Ltd; 2010.
COVID-19 Monoclonal Antibody Infusions: A Multidisciplinary Initiative to Operationalize EUA Novel Treatment Options
From Mount Sinai Medical Center, Miami Beach, FL.
Abstract
Objective: To develop and implement a process for administering COVID-19 monoclonal antibody infusions for outpatients with mild or moderate COVID-19 at high risk for hospitalization, using multidisciplinary collaboration, US Food and Drug Administration (FDA) guidance, and infection prevention standards.
Methods: When monoclonal antibody therapy became available for mild or moderate COVID-19 outpatients via Emergency Use Authorization (EUA), our institution sought to provide this therapy option to our patients. We describe the process for planning, implementing, and maintaining a successful program for administering novel therapies based on FDA guidance and infection prevention standards. Key components of our implementation process were multidisciplinary planning involving decision makers and stakeholders; setting realistic goals in the process; team communication; and measuring and reporting quality improvement on a regular basis.
Results: A total of 790 COVID-19 monoclonal antibody infusions were administered from November 20, 2020 to March 5, 2021. Steps to minimize the likelihood of adverse drug reactions were implemented and a low incidence (< 1%) has occurred. There has been no concern from staff regarding infection during the process. Rarely, patients have raised cost-related concerns, typically due to incomplete communication regarding billing prior to the infusion. Patients, families, nursing staff, physicians, pharmacy, and hospital administration have expressed satisfaction with the program.
Conclusion: This process can provide a template for other hospitals or health care delivery facilities to provide novel therapies to patients with mild or moderate COVID-19 in a safe and effective manner.
Keywords: COVID-19; monoclonal antibody; infusion; emergency use authorization.
SARS-CoV-2 and the disease it causes, COVID-19, have transformed from scientific vernacular to common household terms. It began with a cluster of pneumonia cases of unknown etiology in December 2019 in Wuhan, China, with physicians there reporting a novel coronavirus strain (2019-nCoV), now referred to as SARS-CoV-2. Rapid spread of this virus resulted in the World Health Organization (WHO) declaring an international public health emergency. Since this time, the virus has evolved into a worldwide pandemic. COVID-19 has dramatically impacted our society, resulting in more than 2.63 million global deaths as of this writing, of which more than 527,000 deaths have occurred in the United States.1 This novel virus has resulted in a flurry of literature, research, therapies, and collaboration across multiple disciplines in an effort to prevent, treat, and mitigate cases and complications of this disease.
On November 9, 2020, and November 21, 2020, the US Food and Drug Administration (FDA) issued Emergency Use Authorizations (EUA) for 2 novel COVID-19 monoclonal therapies, bamlanivimab2-3 and casirivimab/imdevimab,3-4 respectively. The EUAs granted permission for these therapies to be administered for the treatment of mild to moderate COVID-19 in adult and pediatric patients (≥ 12 years and weighing at least 40 kg) with positive results of direct SARS-CoV-2 viral testing and who are at high risk for progressing to severe COVID-19 and/or hospitalization. The therapies work by targeting the SARS-CoV-2 spike protein and subsequent attachment to human angiotensin-converting enzyme 2 receptors. Clinical trial data leading to the EUA demonstrated a reduction in viral load, safe outcome, and most importantly, fewer hospitalization and emergency room visits, as compared to the placebo group.5-7 The use of monoclonal antibodies is not new and gained recognition during the Ebola crisis, when the monoclonal antibody to the Ebola virus showed a significant survival benefit.8 Providing monoclonal antibody therapy soon after symptom onset aligns with a shift from the onset of the pandemic to the current focus on the administration of pharmaceutical therapy early in the disease course. This shift prevents progression to severe COVID-19, with the goal of reducing patient mortality, hospitalizations, and strain on health care systems.
The availability of novel neutralizing monoclonal antibodies for COVID-19 led to discussions of how to incorporate these therapies as new options for patients. Our institution networked with colleagues from multiple disciplines to discuss processes and policies for the safe administration of the monoclonal antibody infusion therapies. Federal health leaders urge more use of monoclonal antibodies, but many hospitals have been unable to successfully implement infusions due to staff and logistical challenges.9 This article presents a viable process that hospitals can use to provide these novel therapies to outpatients with mild to moderate COVID-19.
The Mount Sinai Medical Center, Florida Experience
Mount Sinai Medical Center in Miami Beach, Florida, is the largest private, independent, not-for-profit teaching hospital in South Florida, comprising 672 licensed beds and supporting 150,000 emergency department (ED) visits annually. Per the EUA criteria for use, COVID-19 monoclonal antibody therapies are not authorized for patients who are hospitalized or who require oxygen therapy due to COVID-19. Therefore, options for outpatient administration needed to be evaluated. Directly following the first EUA press release, a task force of key stakeholders was assembled to brainstorm and develop a process to offer this therapy to the community. A multidisciplinary task force with representation from the ED, nursing, primary care, hospital medicine, pharmacy, risk management, billing, information technology, infection prevention, and senior level leadership participated (Table).
The task force reviewed institutional outpatient locations to determine whether offering this service would be feasible (eg, ED, ambulatory care facilities, cancer center). The ED was selected because it would offer the largest array of appointment times to meet the community needs with around-the-clock availability. While Mount Sinai Medical Center offers care in 3 emergency center locations in Aventura, Hialeah, and Miami Beach, it was determined to initiate the infusions at the main campus center in Miami Beach only. The main campus affords an onsite pharmacy with suitable staffing to prepare the anticipated volume of infusions in a timely manner, as both therapies have short stabilities following preparation. Thus, it was decided that patients from freestanding emergency centers in Aventura and Hialeah would be moved to the Miami Beach ED location to receive therapy. Operating at a single site also allowed for more rapid implementation, monitoring, and ability to make modifications more easily. Discussions for the possible expansion of COVID-19 monoclonal antibody infusions at satellite locations are underway.
On November 20, 2020, 11 days after the formation of the multidisciplinary task force, the first COVID-19 monoclonal infusion was successfully administered. Figure 1 depicts the timeline from assessment to program implementation. Critical to implementation was the involvement of decision makers from all necessary departments early in the planning process to ensure that standard operating procedures were followed and that the patients, community, and organization had a positive experience. This allowed for simultaneous planning of electronic health record (Epic; EHR) builds, departmental workflows, and staff education, as described in the following section. Figure 2 shows the patient safety activities included in the implementation process.
Key Stakeholder Involvement and Workflow
On the day of bamlanivimab EUA release, email communication was shared among hospital leadership with details of the press release. Departments were quickly involved to initiate a task force to assess if and how this therapy could be offered at Mount Sinai Medical Center. The following sections explain the role of each stakeholder and their essential role to operationalize these novel EUA treatment options. The task force was organized and led by our chief medical officer and chief nursing officer.
Information Technology
Medication Ordering and Documentation EHR and Smart Pumps. Early in the pandemic, the antimicrobial stewardship (ASP) clinical coordinator became the designated point person for pharmacy assessment of novel COVID-19 therapies. As such, this pharmacist began reviewing the bamlanivimab and, later, the casirivimab/imdevimab EUA Fact Sheet for Health Care Providers. All necessary elements for the complete and safe ordering and dispensing of the medication were developed and reviewed by pharmacy administration and ED nursing leadership for input, prior to submitting to the information technology team for implementation. Building the COVID-19 monoclonal medication records into the EHR allowed for detailed direction (ie, administration and preparation instructions) to be consistently applied. The medication records were also built into hospital smart pumps so that nurses could access prepopulated, accurate volumes and infusion rates to minimize errors.
Order Set Development. The pharmacy medication build was added to a comprehensive order set (Figure 3), which was then developed to guide prescribers and standardize the process around ordering of COVID-19 monoclonal therapies. While these therapies are new, oncology monoclonal therapies are regularly administered to outpatients at Mount Sinai Cancer Center. The cancer center was therefore consulted on their process surrounding best practices in administration of monoclonal antibody therapies. This included protocols for medications used in pretreatment and management of hypersensitivity reactions and potential adverse drug reactions of both COVID-19 monoclonal therapies. These medication orders were selected by default in the order set to ensure that all patients received premedications aimed at minimizing the risk of hypersensitivity reaction, and had as-needed medication orders, in the event a hypersensitivity reaction occurred. Reducing hypersensitivity reaction risk is important as well to increase the likelihood that the patient would receive full therapy, as management of this adverse drug reactions involves possible cessation of therapy depending on the level of severity. The pharmacy department also ensured these medications were stocked in ED automated dispensing cabinets to promote quick access. In addition to the aforementioned nursing orders, we added EUA criteria for use and hyperlinks to the Fact Sheets for Patients and Caregivers and Health Care Providers for each monoclonal therapy, and restricted ordering to ED physicians, nurse practitioners, and physician assistants.
The order set underwent multidisciplinary review by pharmacy administration, the chair of emergency medicine, physicians, and ED nursing leadership prior to presentation and approval by the Pharmacy and Therapeutics Committee. Lastly, at time of implementation, the order set was added to the ED preference list, preventing inpatient access. Additionally, as a patient safety action, free- standing orders of COVID-19 monoclonal therapies were disabled, so providers could only order therapies via the approved, comprehensive order set.
Preliminary Assessment Tool. A provider assessment tool was developed to document patient-specific EUA criteria for use during initial assessment (Figure 4). This tool serves as a checklist and is visible to the full multidisciplinary team in the patient’s EHR. It is used as a resource at the time of pharmacist verification and ED physician assessment to ensure criteria for use are met.
Outpatient Offices
Patient Referral. Patients with symptoms or concerns of COVID-19 exposure can make physician appointments via telemedicine or in person at Mount Sinai Medical Center’s primary care and specialty offices. At the time of patient encounter, physicians suspecting a COVID-19 diagnosis will refer patients for outpatient COVID-19 polymerase chain reaction (PCR) laboratory testing, which has an approximate 24-hour turnaround to results. Physicians also assess whether the patient meets EUA criteria for use, pending results of testing. In the event a patient meets EUA criteria for use, the physician provides patient counseling and requests verbal consent. Following this, the physician enters a note in the EHR describing the patient’s condition, criteria for use evaluation, and the patient’s verbal agreement to therapy. This preliminary screening is beneficial to begin planning with both the patient and ED to minimize delays. Patients are notified of the results of their test once available. If the COVID-19 PCR test returns positive, the physician will call the ED at the main campus and schedule the patient for COVID-19 monoclonal therapy. As the desired timeframe for administering COVID-19 monoclonal therapies is within less than 10 days of symptom onset, timely scheduling of appointments is crucial. Infusion appointments are typically provided the same or next day. The patients are informed that they must bring documentation of their positive COVID-19 PCR test to their ED visit. Lastly, because patients are pretreated with medication that may potentially impair driving, they are instructed that they cannot drive themselves home; ride shares also are not allowed in order to limit the spread of infection.
Emergency Department
Patient Arrival and Screening. A COVID-19 patient can be evaluated in the ED 1 of 2 ways. The first option is via outpatient office referral, as described previously. Upon arrival to the ED, a second screening is performed to ensure the patient still meets EUA criteria for use and the positive COVID-19 PCR test result is confirmed. If the patient no longer meets criteria, the patient is triaged accordingly, including evaluation for higher-level care (eg, supplemental oxygen, hospital admission). The second optoion is via new patient walk-ins without outpatient physician referral (Figure 4). In these cases, an initial screening is performed, documenting EUA criteria for use in the preliminary assessment (Figure 5). Physicians will consider an outside COVID-19 test as valid, so long as documentation is readily available confirming a positive PCR result. Otherwise, an in-house COVID-19 PCR test will be performed, which has a 2-hour turnaround time.
Infusion Schedule. The ED offers a total of 16 COVID-19 monoclonal infusions slots daily. These are broken up into 4 infusion time blocks (eg, 8
Patient Education. Prior to administration of the monoclonal therapy, physician and nursing staff obtain a formal, written patient consent for therapy and provide patients with the option of participating in the institutional review board (IRB) approved study. Details of this are discussed in the risk management and IRB sections of the article. Nursing staff also provides the medication-specific Fact Sheet for Patients and Caregivers in either Spanish or English, which is also included as a hyperlink on the COVID-19 Monoclonal Antibody Order Set for ease of access. Interpreter services are available for patients who speak other languages. An ED decentralized pharmacist is also available onsite Monday through Friday from 12
Infusion Ordering. Once the patient is ready to begin therapy, the he/she is brought to a dedicated overflow area of the ED. There are few, if any, patients in this location, and it is adjacent to the main emergency center for easy access by the patients, nurses, pharmacists, and physicians. The physician then enters orders in the EHR using the COVID-19 Monoclonal Antibody Order Set (Figure 3). Three discrete questions were built into the medication order: (1) Was patient consent obtained? (2) Was the Fact Sheet for Patient/Caregiver provided to the patient? (3) Is the patient COVID-19 PCR-positive? These questions were built as hard stops so that the medication orders cannot be placed without a response. This serves as another double-check to ensure processes are followed and helps facilitate timely verification by the pharmacist.
Medication Administration. One nurse is dedicated to administering the monoclonal therapies scheduled at 8
Pharmacy Department
Medication Receipt Process. Inventory is currently allocated biweekly from the state department of health and will soon be transitioning to a direct order system. The pharmacy technician in charge of deliveries notifies the pharmacy Antimicrobial Stewardship Program (ASP) clinical coordinator upon receipt of the monoclonal therapies. Bamlanivimab is supplied as 1 vial per dose, whereas casirivimab/imdevimab is supplied as 4 vials or 8 vials per dose, depending how it is shipped. To reduce the likelihood of medication errors, the ASP clinical coordinator assembles each of the casirivimab/imdevimab vials into kits, where 1 kit equals 1 dose. Labels are then affixed to each kit indicating the medication name, number of vials which equal a full dose, and pharmacist signature. The kits are stored in a dedicated refrigerator, and inventory logs are affixed to the outside of the refrigerator and updated daily. This inventory is also communicated daily to ED physician, nursing, and pharmacy leadership, as well as the director of patient safety, who reports weekly usage to the state Department of Health and Human Services. These weekly reports are used to determine allocation amounts.
Medication Verification and Delivery. The Mount Sinai Medical Center pharmacist staffing model consists of centralized order entry and specialized, decentralized positions. All orders are verified by the ED pharmacist when scheduled (not a 24/7 service) and by the designated pharmacist for all other times. At the time of medication verification, the pharmacist documents patient-specific EUA criteria for use and confirms that consent was obtained and the Fact Sheet for Patients/Caregivers was provided. A pharmacist intervention was developed to assist with this documentation. Pharmacists input smart text “.COVIDmonoclonal” and a drop-down menu of EUA criteria for use appears. The pharmacist reviews the patient care notes and medication order question responses to ascertain this information, contacting the ED prescriber if further clarification is required. This verification serves as another check to ensure processes put in place are followed. Lastly, intravenous preparation and delivery are electronically recorded in the EHR, and the medications require nursing signature at the time of delivery to ensure a formal chain of custody.
Risk Management
At Mount Sinai Medical Center, all EUA and investigational therapies require patient consent. Consistent with this requirement, a COVID-19 monoclonal specific consent was developed by risk management. This is provided to every patient receiving a COVID-19 monoclonal infusion, in addition to the FDA EUA Fact Sheet for Patients and Caregivers, and documented as part of their EHR. The questions providers must answer are built into the order set to ensure this process is followed and these patient safety checks are incorporated into the workflow.
Billing and Finance Department
In alignment with Mount Sinai Medical Center’s mission to provide high-quality health care to its diverse community through teaching, research, charity care, and financial responsibility, it was determined that this therapy would be provided to all patients regardless of insurance type, including those who are uninsured. The billing and finance department was consulted prior to this service being offered, to provide patients with accurate and pertinent information. The billing and finance department provided guidance on how to document patient encounters at time of registration to facilitate appropriate billing. At this time, the medication is free of charge, but nonmedication-related ED fees apply. This is explained to patients so there is a clear understanding prior to booking their appointment.
Infection Prevention
As patients receiving COVID-19 monoclonal therapies can transmit the virus to others, measures to ensure protection for other patients and staff are vital. To minimize exposure, specific nursing and physician staff from the ED are assigned to the treatment of these patients, and patients receive infusions and postobservation monitoring in a designated wing of the ED. Additionally, all staff who interact with these patients are required to don full personal protective equipment. This includes not only physicians and nurses but all specialties such as physician assistants, nurse practitioners, pharmacists, and laboratory technicians. Moreover, patients are not permitted to go home in a ride share and are counseled on Centers for Disease Control and Prevention quarantining following infusion.
Measurement of Process and Outcomes and Reporting
IRB approval was sought and obtained early during initiation of this service, allowing study consent to be offered to patients at the time general consent was obtained, which maximized patient recruitment and streamlined workflow. The study is a prospective observational research study to determine the impact of administration of COVID-19 monoclonal antibody therapy on length of symptoms, chronic illness, and rate of hospitalization. Most patients were eager to participate and offer their assistance to the scientific community during this pandemic.
Staff Education
In order to successfully implement this multidisciplinary EUA treatment option, comprehensive staff education was paramount after the workflow was developed. Prior to the first day of infusions, nurses and pharmacists were provided education during multiple huddle announcements. The pharmacy team also provided screen captures via email to the pharmacists so they could become familiar with the order set, intervention documentation, and location of the preliminary assessment of EUA criteria for use at the time of order verification. The emergency medicine department chair and chief medical officer also provided education via several virtual meetings and email to referring physicians (specialists and primary care) and residents in the emergency centers involved in COVID-19 monoclonal therapy-related patient care.
Factors Contributing to Success
We believe the reasons for continued success of this process are multifactorial and include the following key elements. Multidisciplinary planning, which included decision makers and all stakeholders, began at the time the idea was conceived. This allowed quick implementation of this service by efficiently navigating barriers to engaging impacted staff early on. Throughout this process, the authors set realistic step-wise goals. While navigating through the many details to implementation described, we also kept in mind the big picture, which was to provide this potentially lifesaving therapy to as many qualifying members of our community as possible. This included being flexible with the process and adapting when needed to achieve this ultimate goal. A focus on safety remained a priority to minimize possible errors and enhance patient and staff satisfaction. The optimization of the EHR streamlined workflow, provided point-of-care resources, and enhanced patient safety. Additionally, the target date set for implementation allowed staff and department leads adequate time to plan for and anticipate the changes. Serving only 1 patient on the first day allowed time for staff to experience this new process hands-on and provided opportunity for focused education. This team communication was essential to implementing this project, including staff training of processes and procedures prior to go-live. Early incorporation of IRB approval allowed the experience to be assessed and considered for contribution to the scientific literature to tackle this novel virus that has impacted our communities locally, nationally, and abroad. Moreover, continued measurement and reporting on a regular basis leads to performance improvement. The process outlined here can be adapted to incorporate other new therapies in the future, such as the recent February 9, 2021, EUA of the COVID-19 monoclonal antibody combination bamlanivimab and etesevimab.10
Conclusion
We administered 790 COVID-19 monoclonal antibody infusions between November 20, 2020 and March 5, 2021. Steps to minimize the likelihood of hypersensitivity reactions were implemented, and a low incidence (< 1%) has been observed. There has been no incidence of infection, concern from staff about infection prevention, or risk of infection during the processes. There have been very infrequent cost-related concerns raised by patients, typically due to incomplete communication regarding billing prior to the infusion. To address these issues, staff education has been provided to enhance patient instruction on this topic. The program has provided patient and family satisfaction, as well nursing, physician, pharmacist, clinical staff, and hospital administration pride and gratification. Setting up a new program to provide a 4-hour patient encounter to infuse therapy to high-risk patients with COVID-19 requires commitment and effort. This article describes the experience, ideas, and formula others may consider using to set up such a program. Through networking and formal phone calls and meetings about monoclonal antibody therapy, we have heard about other institutions who have not been able to institute this program due to various barriers to implementation. We hope our experience serves as a resource for others to provide this therapy to their patients and expand access in an effort to mitigate COVID-19 consequences and cases affecting our communities.
Corresponding author: Kathleen Jodoin, PharmD, BCPS, Mount Sinai Medical Center, 4300 Alton Rd, Miami Beach, FL 33140; [email protected].
Financial disclosures: None.
1. COVID Data Tracker. Center for Disease Control and Prevention. https://covid.cdc.gov/covid-data-tracker/#global-counts-rates. Accessed March 12, 2021.
2. Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of Bamlanivimab. US Food and Drug Administration. Updated February 2021. Accessed March 9, 2021. https://www.fda.gov/media/143603/download
3. Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibodies for Treatment of COVID-19 | FDA. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibodies-treatment-covid-19. Accessed February 14, 2021.
4. Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of Casirivimab and Imdevimab. US Food and Drug Administration. Updated December 2020. Accessed March 9, 2021. https://www.fda.gov/media/143892/download
5. Chen P, Nirula A, Heller B, et al. SARS-CoV-2 Neutralizing antibody LY-CoV555 in outpatients with COVID-19. N Engl J Med. 2021;384(3):229-237. doi:10.1056/NEJMoa2029849
6. Gottlieb RL, Nirula A, Chen P, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. 10.1JAMA. 2021;325(7):632-644. doi:10.1001/jama.2021.0202
7. Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. 10.1N Engl J Med. 2021;384:238-251. doi:10.1056/nejmoa2035002
8. Mulangu S, Dodd LE, Davey RT Jr, et al. A randomized, controlled trial of Ebola virus disease therapeutics. 10.1N Engl J Med. 2019;381:2293-2303. doi:10.1056/NEJMoa1910993
9. Boyle, P. Can an experimental treatment keep COVID-19 patients out of hospitals? Association of American Medical Colleges. January 29, 2021. Accessed March 9, 2021. https://www.aamc.org/news-insights/can-experimental-treatment-keep-covid-19-patients-out-hospitals
10. Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of Bamlanivimab and Etesevimab. US Food and Drug Administration. Updated February 2021. Accessed March 9, 2021. https://www.fda.gov/media/145802/download
From Mount Sinai Medical Center, Miami Beach, FL.
Abstract
Objective: To develop and implement a process for administering COVID-19 monoclonal antibody infusions for outpatients with mild or moderate COVID-19 at high risk for hospitalization, using multidisciplinary collaboration, US Food and Drug Administration (FDA) guidance, and infection prevention standards.
Methods: When monoclonal antibody therapy became available for mild or moderate COVID-19 outpatients via Emergency Use Authorization (EUA), our institution sought to provide this therapy option to our patients. We describe the process for planning, implementing, and maintaining a successful program for administering novel therapies based on FDA guidance and infection prevention standards. Key components of our implementation process were multidisciplinary planning involving decision makers and stakeholders; setting realistic goals in the process; team communication; and measuring and reporting quality improvement on a regular basis.
Results: A total of 790 COVID-19 monoclonal antibody infusions were administered from November 20, 2020 to March 5, 2021. Steps to minimize the likelihood of adverse drug reactions were implemented and a low incidence (< 1%) has occurred. There has been no concern from staff regarding infection during the process. Rarely, patients have raised cost-related concerns, typically due to incomplete communication regarding billing prior to the infusion. Patients, families, nursing staff, physicians, pharmacy, and hospital administration have expressed satisfaction with the program.
Conclusion: This process can provide a template for other hospitals or health care delivery facilities to provide novel therapies to patients with mild or moderate COVID-19 in a safe and effective manner.
Keywords: COVID-19; monoclonal antibody; infusion; emergency use authorization.
SARS-CoV-2 and the disease it causes, COVID-19, have transformed from scientific vernacular to common household terms. It began with a cluster of pneumonia cases of unknown etiology in December 2019 in Wuhan, China, with physicians there reporting a novel coronavirus strain (2019-nCoV), now referred to as SARS-CoV-2. Rapid spread of this virus resulted in the World Health Organization (WHO) declaring an international public health emergency. Since this time, the virus has evolved into a worldwide pandemic. COVID-19 has dramatically impacted our society, resulting in more than 2.63 million global deaths as of this writing, of which more than 527,000 deaths have occurred in the United States.1 This novel virus has resulted in a flurry of literature, research, therapies, and collaboration across multiple disciplines in an effort to prevent, treat, and mitigate cases and complications of this disease.
On November 9, 2020, and November 21, 2020, the US Food and Drug Administration (FDA) issued Emergency Use Authorizations (EUA) for 2 novel COVID-19 monoclonal therapies, bamlanivimab2-3 and casirivimab/imdevimab,3-4 respectively. The EUAs granted permission for these therapies to be administered for the treatment of mild to moderate COVID-19 in adult and pediatric patients (≥ 12 years and weighing at least 40 kg) with positive results of direct SARS-CoV-2 viral testing and who are at high risk for progressing to severe COVID-19 and/or hospitalization. The therapies work by targeting the SARS-CoV-2 spike protein and subsequent attachment to human angiotensin-converting enzyme 2 receptors. Clinical trial data leading to the EUA demonstrated a reduction in viral load, safe outcome, and most importantly, fewer hospitalization and emergency room visits, as compared to the placebo group.5-7 The use of monoclonal antibodies is not new and gained recognition during the Ebola crisis, when the monoclonal antibody to the Ebola virus showed a significant survival benefit.8 Providing monoclonal antibody therapy soon after symptom onset aligns with a shift from the onset of the pandemic to the current focus on the administration of pharmaceutical therapy early in the disease course. This shift prevents progression to severe COVID-19, with the goal of reducing patient mortality, hospitalizations, and strain on health care systems.
The availability of novel neutralizing monoclonal antibodies for COVID-19 led to discussions of how to incorporate these therapies as new options for patients. Our institution networked with colleagues from multiple disciplines to discuss processes and policies for the safe administration of the monoclonal antibody infusion therapies. Federal health leaders urge more use of monoclonal antibodies, but many hospitals have been unable to successfully implement infusions due to staff and logistical challenges.9 This article presents a viable process that hospitals can use to provide these novel therapies to outpatients with mild to moderate COVID-19.
The Mount Sinai Medical Center, Florida Experience
Mount Sinai Medical Center in Miami Beach, Florida, is the largest private, independent, not-for-profit teaching hospital in South Florida, comprising 672 licensed beds and supporting 150,000 emergency department (ED) visits annually. Per the EUA criteria for use, COVID-19 monoclonal antibody therapies are not authorized for patients who are hospitalized or who require oxygen therapy due to COVID-19. Therefore, options for outpatient administration needed to be evaluated. Directly following the first EUA press release, a task force of key stakeholders was assembled to brainstorm and develop a process to offer this therapy to the community. A multidisciplinary task force with representation from the ED, nursing, primary care, hospital medicine, pharmacy, risk management, billing, information technology, infection prevention, and senior level leadership participated (Table).
The task force reviewed institutional outpatient locations to determine whether offering this service would be feasible (eg, ED, ambulatory care facilities, cancer center). The ED was selected because it would offer the largest array of appointment times to meet the community needs with around-the-clock availability. While Mount Sinai Medical Center offers care in 3 emergency center locations in Aventura, Hialeah, and Miami Beach, it was determined to initiate the infusions at the main campus center in Miami Beach only. The main campus affords an onsite pharmacy with suitable staffing to prepare the anticipated volume of infusions in a timely manner, as both therapies have short stabilities following preparation. Thus, it was decided that patients from freestanding emergency centers in Aventura and Hialeah would be moved to the Miami Beach ED location to receive therapy. Operating at a single site also allowed for more rapid implementation, monitoring, and ability to make modifications more easily. Discussions for the possible expansion of COVID-19 monoclonal antibody infusions at satellite locations are underway.
On November 20, 2020, 11 days after the formation of the multidisciplinary task force, the first COVID-19 monoclonal infusion was successfully administered. Figure 1 depicts the timeline from assessment to program implementation. Critical to implementation was the involvement of decision makers from all necessary departments early in the planning process to ensure that standard operating procedures were followed and that the patients, community, and organization had a positive experience. This allowed for simultaneous planning of electronic health record (Epic; EHR) builds, departmental workflows, and staff education, as described in the following section. Figure 2 shows the patient safety activities included in the implementation process.
Key Stakeholder Involvement and Workflow
On the day of bamlanivimab EUA release, email communication was shared among hospital leadership with details of the press release. Departments were quickly involved to initiate a task force to assess if and how this therapy could be offered at Mount Sinai Medical Center. The following sections explain the role of each stakeholder and their essential role to operationalize these novel EUA treatment options. The task force was organized and led by our chief medical officer and chief nursing officer.
Information Technology
Medication Ordering and Documentation EHR and Smart Pumps. Early in the pandemic, the antimicrobial stewardship (ASP) clinical coordinator became the designated point person for pharmacy assessment of novel COVID-19 therapies. As such, this pharmacist began reviewing the bamlanivimab and, later, the casirivimab/imdevimab EUA Fact Sheet for Health Care Providers. All necessary elements for the complete and safe ordering and dispensing of the medication were developed and reviewed by pharmacy administration and ED nursing leadership for input, prior to submitting to the information technology team for implementation. Building the COVID-19 monoclonal medication records into the EHR allowed for detailed direction (ie, administration and preparation instructions) to be consistently applied. The medication records were also built into hospital smart pumps so that nurses could access prepopulated, accurate volumes and infusion rates to minimize errors.
Order Set Development. The pharmacy medication build was added to a comprehensive order set (Figure 3), which was then developed to guide prescribers and standardize the process around ordering of COVID-19 monoclonal therapies. While these therapies are new, oncology monoclonal therapies are regularly administered to outpatients at Mount Sinai Cancer Center. The cancer center was therefore consulted on their process surrounding best practices in administration of monoclonal antibody therapies. This included protocols for medications used in pretreatment and management of hypersensitivity reactions and potential adverse drug reactions of both COVID-19 monoclonal therapies. These medication orders were selected by default in the order set to ensure that all patients received premedications aimed at minimizing the risk of hypersensitivity reaction, and had as-needed medication orders, in the event a hypersensitivity reaction occurred. Reducing hypersensitivity reaction risk is important as well to increase the likelihood that the patient would receive full therapy, as management of this adverse drug reactions involves possible cessation of therapy depending on the level of severity. The pharmacy department also ensured these medications were stocked in ED automated dispensing cabinets to promote quick access. In addition to the aforementioned nursing orders, we added EUA criteria for use and hyperlinks to the Fact Sheets for Patients and Caregivers and Health Care Providers for each monoclonal therapy, and restricted ordering to ED physicians, nurse practitioners, and physician assistants.
The order set underwent multidisciplinary review by pharmacy administration, the chair of emergency medicine, physicians, and ED nursing leadership prior to presentation and approval by the Pharmacy and Therapeutics Committee. Lastly, at time of implementation, the order set was added to the ED preference list, preventing inpatient access. Additionally, as a patient safety action, free- standing orders of COVID-19 monoclonal therapies were disabled, so providers could only order therapies via the approved, comprehensive order set.
Preliminary Assessment Tool. A provider assessment tool was developed to document patient-specific EUA criteria for use during initial assessment (Figure 4). This tool serves as a checklist and is visible to the full multidisciplinary team in the patient’s EHR. It is used as a resource at the time of pharmacist verification and ED physician assessment to ensure criteria for use are met.
Outpatient Offices
Patient Referral. Patients with symptoms or concerns of COVID-19 exposure can make physician appointments via telemedicine or in person at Mount Sinai Medical Center’s primary care and specialty offices. At the time of patient encounter, physicians suspecting a COVID-19 diagnosis will refer patients for outpatient COVID-19 polymerase chain reaction (PCR) laboratory testing, which has an approximate 24-hour turnaround to results. Physicians also assess whether the patient meets EUA criteria for use, pending results of testing. In the event a patient meets EUA criteria for use, the physician provides patient counseling and requests verbal consent. Following this, the physician enters a note in the EHR describing the patient’s condition, criteria for use evaluation, and the patient’s verbal agreement to therapy. This preliminary screening is beneficial to begin planning with both the patient and ED to minimize delays. Patients are notified of the results of their test once available. If the COVID-19 PCR test returns positive, the physician will call the ED at the main campus and schedule the patient for COVID-19 monoclonal therapy. As the desired timeframe for administering COVID-19 monoclonal therapies is within less than 10 days of symptom onset, timely scheduling of appointments is crucial. Infusion appointments are typically provided the same or next day. The patients are informed that they must bring documentation of their positive COVID-19 PCR test to their ED visit. Lastly, because patients are pretreated with medication that may potentially impair driving, they are instructed that they cannot drive themselves home; ride shares also are not allowed in order to limit the spread of infection.
Emergency Department
Patient Arrival and Screening. A COVID-19 patient can be evaluated in the ED 1 of 2 ways. The first option is via outpatient office referral, as described previously. Upon arrival to the ED, a second screening is performed to ensure the patient still meets EUA criteria for use and the positive COVID-19 PCR test result is confirmed. If the patient no longer meets criteria, the patient is triaged accordingly, including evaluation for higher-level care (eg, supplemental oxygen, hospital admission). The second optoion is via new patient walk-ins without outpatient physician referral (Figure 4). In these cases, an initial screening is performed, documenting EUA criteria for use in the preliminary assessment (Figure 5). Physicians will consider an outside COVID-19 test as valid, so long as documentation is readily available confirming a positive PCR result. Otherwise, an in-house COVID-19 PCR test will be performed, which has a 2-hour turnaround time.
Infusion Schedule. The ED offers a total of 16 COVID-19 monoclonal infusions slots daily. These are broken up into 4 infusion time blocks (eg, 8
Patient Education. Prior to administration of the monoclonal therapy, physician and nursing staff obtain a formal, written patient consent for therapy and provide patients with the option of participating in the institutional review board (IRB) approved study. Details of this are discussed in the risk management and IRB sections of the article. Nursing staff also provides the medication-specific Fact Sheet for Patients and Caregivers in either Spanish or English, which is also included as a hyperlink on the COVID-19 Monoclonal Antibody Order Set for ease of access. Interpreter services are available for patients who speak other languages. An ED decentralized pharmacist is also available onsite Monday through Friday from 12
Infusion Ordering. Once the patient is ready to begin therapy, the he/she is brought to a dedicated overflow area of the ED. There are few, if any, patients in this location, and it is adjacent to the main emergency center for easy access by the patients, nurses, pharmacists, and physicians. The physician then enters orders in the EHR using the COVID-19 Monoclonal Antibody Order Set (Figure 3). Three discrete questions were built into the medication order: (1) Was patient consent obtained? (2) Was the Fact Sheet for Patient/Caregiver provided to the patient? (3) Is the patient COVID-19 PCR-positive? These questions were built as hard stops so that the medication orders cannot be placed without a response. This serves as another double-check to ensure processes are followed and helps facilitate timely verification by the pharmacist.
Medication Administration. One nurse is dedicated to administering the monoclonal therapies scheduled at 8
Pharmacy Department
Medication Receipt Process. Inventory is currently allocated biweekly from the state department of health and will soon be transitioning to a direct order system. The pharmacy technician in charge of deliveries notifies the pharmacy Antimicrobial Stewardship Program (ASP) clinical coordinator upon receipt of the monoclonal therapies. Bamlanivimab is supplied as 1 vial per dose, whereas casirivimab/imdevimab is supplied as 4 vials or 8 vials per dose, depending how it is shipped. To reduce the likelihood of medication errors, the ASP clinical coordinator assembles each of the casirivimab/imdevimab vials into kits, where 1 kit equals 1 dose. Labels are then affixed to each kit indicating the medication name, number of vials which equal a full dose, and pharmacist signature. The kits are stored in a dedicated refrigerator, and inventory logs are affixed to the outside of the refrigerator and updated daily. This inventory is also communicated daily to ED physician, nursing, and pharmacy leadership, as well as the director of patient safety, who reports weekly usage to the state Department of Health and Human Services. These weekly reports are used to determine allocation amounts.
Medication Verification and Delivery. The Mount Sinai Medical Center pharmacist staffing model consists of centralized order entry and specialized, decentralized positions. All orders are verified by the ED pharmacist when scheduled (not a 24/7 service) and by the designated pharmacist for all other times. At the time of medication verification, the pharmacist documents patient-specific EUA criteria for use and confirms that consent was obtained and the Fact Sheet for Patients/Caregivers was provided. A pharmacist intervention was developed to assist with this documentation. Pharmacists input smart text “.COVIDmonoclonal” and a drop-down menu of EUA criteria for use appears. The pharmacist reviews the patient care notes and medication order question responses to ascertain this information, contacting the ED prescriber if further clarification is required. This verification serves as another check to ensure processes put in place are followed. Lastly, intravenous preparation and delivery are electronically recorded in the EHR, and the medications require nursing signature at the time of delivery to ensure a formal chain of custody.
Risk Management
At Mount Sinai Medical Center, all EUA and investigational therapies require patient consent. Consistent with this requirement, a COVID-19 monoclonal specific consent was developed by risk management. This is provided to every patient receiving a COVID-19 monoclonal infusion, in addition to the FDA EUA Fact Sheet for Patients and Caregivers, and documented as part of their EHR. The questions providers must answer are built into the order set to ensure this process is followed and these patient safety checks are incorporated into the workflow.
Billing and Finance Department
In alignment with Mount Sinai Medical Center’s mission to provide high-quality health care to its diverse community through teaching, research, charity care, and financial responsibility, it was determined that this therapy would be provided to all patients regardless of insurance type, including those who are uninsured. The billing and finance department was consulted prior to this service being offered, to provide patients with accurate and pertinent information. The billing and finance department provided guidance on how to document patient encounters at time of registration to facilitate appropriate billing. At this time, the medication is free of charge, but nonmedication-related ED fees apply. This is explained to patients so there is a clear understanding prior to booking their appointment.
Infection Prevention
As patients receiving COVID-19 monoclonal therapies can transmit the virus to others, measures to ensure protection for other patients and staff are vital. To minimize exposure, specific nursing and physician staff from the ED are assigned to the treatment of these patients, and patients receive infusions and postobservation monitoring in a designated wing of the ED. Additionally, all staff who interact with these patients are required to don full personal protective equipment. This includes not only physicians and nurses but all specialties such as physician assistants, nurse practitioners, pharmacists, and laboratory technicians. Moreover, patients are not permitted to go home in a ride share and are counseled on Centers for Disease Control and Prevention quarantining following infusion.
Measurement of Process and Outcomes and Reporting
IRB approval was sought and obtained early during initiation of this service, allowing study consent to be offered to patients at the time general consent was obtained, which maximized patient recruitment and streamlined workflow. The study is a prospective observational research study to determine the impact of administration of COVID-19 monoclonal antibody therapy on length of symptoms, chronic illness, and rate of hospitalization. Most patients were eager to participate and offer their assistance to the scientific community during this pandemic.
Staff Education
In order to successfully implement this multidisciplinary EUA treatment option, comprehensive staff education was paramount after the workflow was developed. Prior to the first day of infusions, nurses and pharmacists were provided education during multiple huddle announcements. The pharmacy team also provided screen captures via email to the pharmacists so they could become familiar with the order set, intervention documentation, and location of the preliminary assessment of EUA criteria for use at the time of order verification. The emergency medicine department chair and chief medical officer also provided education via several virtual meetings and email to referring physicians (specialists and primary care) and residents in the emergency centers involved in COVID-19 monoclonal therapy-related patient care.
Factors Contributing to Success
We believe the reasons for continued success of this process are multifactorial and include the following key elements. Multidisciplinary planning, which included decision makers and all stakeholders, began at the time the idea was conceived. This allowed quick implementation of this service by efficiently navigating barriers to engaging impacted staff early on. Throughout this process, the authors set realistic step-wise goals. While navigating through the many details to implementation described, we also kept in mind the big picture, which was to provide this potentially lifesaving therapy to as many qualifying members of our community as possible. This included being flexible with the process and adapting when needed to achieve this ultimate goal. A focus on safety remained a priority to minimize possible errors and enhance patient and staff satisfaction. The optimization of the EHR streamlined workflow, provided point-of-care resources, and enhanced patient safety. Additionally, the target date set for implementation allowed staff and department leads adequate time to plan for and anticipate the changes. Serving only 1 patient on the first day allowed time for staff to experience this new process hands-on and provided opportunity for focused education. This team communication was essential to implementing this project, including staff training of processes and procedures prior to go-live. Early incorporation of IRB approval allowed the experience to be assessed and considered for contribution to the scientific literature to tackle this novel virus that has impacted our communities locally, nationally, and abroad. Moreover, continued measurement and reporting on a regular basis leads to performance improvement. The process outlined here can be adapted to incorporate other new therapies in the future, such as the recent February 9, 2021, EUA of the COVID-19 monoclonal antibody combination bamlanivimab and etesevimab.10
Conclusion
We administered 790 COVID-19 monoclonal antibody infusions between November 20, 2020 and March 5, 2021. Steps to minimize the likelihood of hypersensitivity reactions were implemented, and a low incidence (< 1%) has been observed. There has been no incidence of infection, concern from staff about infection prevention, or risk of infection during the processes. There have been very infrequent cost-related concerns raised by patients, typically due to incomplete communication regarding billing prior to the infusion. To address these issues, staff education has been provided to enhance patient instruction on this topic. The program has provided patient and family satisfaction, as well nursing, physician, pharmacist, clinical staff, and hospital administration pride and gratification. Setting up a new program to provide a 4-hour patient encounter to infuse therapy to high-risk patients with COVID-19 requires commitment and effort. This article describes the experience, ideas, and formula others may consider using to set up such a program. Through networking and formal phone calls and meetings about monoclonal antibody therapy, we have heard about other institutions who have not been able to institute this program due to various barriers to implementation. We hope our experience serves as a resource for others to provide this therapy to their patients and expand access in an effort to mitigate COVID-19 consequences and cases affecting our communities.
Corresponding author: Kathleen Jodoin, PharmD, BCPS, Mount Sinai Medical Center, 4300 Alton Rd, Miami Beach, FL 33140; [email protected].
Financial disclosures: None.
From Mount Sinai Medical Center, Miami Beach, FL.
Abstract
Objective: To develop and implement a process for administering COVID-19 monoclonal antibody infusions for outpatients with mild or moderate COVID-19 at high risk for hospitalization, using multidisciplinary collaboration, US Food and Drug Administration (FDA) guidance, and infection prevention standards.
Methods: When monoclonal antibody therapy became available for mild or moderate COVID-19 outpatients via Emergency Use Authorization (EUA), our institution sought to provide this therapy option to our patients. We describe the process for planning, implementing, and maintaining a successful program for administering novel therapies based on FDA guidance and infection prevention standards. Key components of our implementation process were multidisciplinary planning involving decision makers and stakeholders; setting realistic goals in the process; team communication; and measuring and reporting quality improvement on a regular basis.
Results: A total of 790 COVID-19 monoclonal antibody infusions were administered from November 20, 2020 to March 5, 2021. Steps to minimize the likelihood of adverse drug reactions were implemented and a low incidence (< 1%) has occurred. There has been no concern from staff regarding infection during the process. Rarely, patients have raised cost-related concerns, typically due to incomplete communication regarding billing prior to the infusion. Patients, families, nursing staff, physicians, pharmacy, and hospital administration have expressed satisfaction with the program.
Conclusion: This process can provide a template for other hospitals or health care delivery facilities to provide novel therapies to patients with mild or moderate COVID-19 in a safe and effective manner.
Keywords: COVID-19; monoclonal antibody; infusion; emergency use authorization.
SARS-CoV-2 and the disease it causes, COVID-19, have transformed from scientific vernacular to common household terms. It began with a cluster of pneumonia cases of unknown etiology in December 2019 in Wuhan, China, with physicians there reporting a novel coronavirus strain (2019-nCoV), now referred to as SARS-CoV-2. Rapid spread of this virus resulted in the World Health Organization (WHO) declaring an international public health emergency. Since this time, the virus has evolved into a worldwide pandemic. COVID-19 has dramatically impacted our society, resulting in more than 2.63 million global deaths as of this writing, of which more than 527,000 deaths have occurred in the United States.1 This novel virus has resulted in a flurry of literature, research, therapies, and collaboration across multiple disciplines in an effort to prevent, treat, and mitigate cases and complications of this disease.
On November 9, 2020, and November 21, 2020, the US Food and Drug Administration (FDA) issued Emergency Use Authorizations (EUA) for 2 novel COVID-19 monoclonal therapies, bamlanivimab2-3 and casirivimab/imdevimab,3-4 respectively. The EUAs granted permission for these therapies to be administered for the treatment of mild to moderate COVID-19 in adult and pediatric patients (≥ 12 years and weighing at least 40 kg) with positive results of direct SARS-CoV-2 viral testing and who are at high risk for progressing to severe COVID-19 and/or hospitalization. The therapies work by targeting the SARS-CoV-2 spike protein and subsequent attachment to human angiotensin-converting enzyme 2 receptors. Clinical trial data leading to the EUA demonstrated a reduction in viral load, safe outcome, and most importantly, fewer hospitalization and emergency room visits, as compared to the placebo group.5-7 The use of monoclonal antibodies is not new and gained recognition during the Ebola crisis, when the monoclonal antibody to the Ebola virus showed a significant survival benefit.8 Providing monoclonal antibody therapy soon after symptom onset aligns with a shift from the onset of the pandemic to the current focus on the administration of pharmaceutical therapy early in the disease course. This shift prevents progression to severe COVID-19, with the goal of reducing patient mortality, hospitalizations, and strain on health care systems.
The availability of novel neutralizing monoclonal antibodies for COVID-19 led to discussions of how to incorporate these therapies as new options for patients. Our institution networked with colleagues from multiple disciplines to discuss processes and policies for the safe administration of the monoclonal antibody infusion therapies. Federal health leaders urge more use of monoclonal antibodies, but many hospitals have been unable to successfully implement infusions due to staff and logistical challenges.9 This article presents a viable process that hospitals can use to provide these novel therapies to outpatients with mild to moderate COVID-19.
The Mount Sinai Medical Center, Florida Experience
Mount Sinai Medical Center in Miami Beach, Florida, is the largest private, independent, not-for-profit teaching hospital in South Florida, comprising 672 licensed beds and supporting 150,000 emergency department (ED) visits annually. Per the EUA criteria for use, COVID-19 monoclonal antibody therapies are not authorized for patients who are hospitalized or who require oxygen therapy due to COVID-19. Therefore, options for outpatient administration needed to be evaluated. Directly following the first EUA press release, a task force of key stakeholders was assembled to brainstorm and develop a process to offer this therapy to the community. A multidisciplinary task force with representation from the ED, nursing, primary care, hospital medicine, pharmacy, risk management, billing, information technology, infection prevention, and senior level leadership participated (Table).
The task force reviewed institutional outpatient locations to determine whether offering this service would be feasible (eg, ED, ambulatory care facilities, cancer center). The ED was selected because it would offer the largest array of appointment times to meet the community needs with around-the-clock availability. While Mount Sinai Medical Center offers care in 3 emergency center locations in Aventura, Hialeah, and Miami Beach, it was determined to initiate the infusions at the main campus center in Miami Beach only. The main campus affords an onsite pharmacy with suitable staffing to prepare the anticipated volume of infusions in a timely manner, as both therapies have short stabilities following preparation. Thus, it was decided that patients from freestanding emergency centers in Aventura and Hialeah would be moved to the Miami Beach ED location to receive therapy. Operating at a single site also allowed for more rapid implementation, monitoring, and ability to make modifications more easily. Discussions for the possible expansion of COVID-19 monoclonal antibody infusions at satellite locations are underway.
On November 20, 2020, 11 days after the formation of the multidisciplinary task force, the first COVID-19 monoclonal infusion was successfully administered. Figure 1 depicts the timeline from assessment to program implementation. Critical to implementation was the involvement of decision makers from all necessary departments early in the planning process to ensure that standard operating procedures were followed and that the patients, community, and organization had a positive experience. This allowed for simultaneous planning of electronic health record (Epic; EHR) builds, departmental workflows, and staff education, as described in the following section. Figure 2 shows the patient safety activities included in the implementation process.
Key Stakeholder Involvement and Workflow
On the day of bamlanivimab EUA release, email communication was shared among hospital leadership with details of the press release. Departments were quickly involved to initiate a task force to assess if and how this therapy could be offered at Mount Sinai Medical Center. The following sections explain the role of each stakeholder and their essential role to operationalize these novel EUA treatment options. The task force was organized and led by our chief medical officer and chief nursing officer.
Information Technology
Medication Ordering and Documentation EHR and Smart Pumps. Early in the pandemic, the antimicrobial stewardship (ASP) clinical coordinator became the designated point person for pharmacy assessment of novel COVID-19 therapies. As such, this pharmacist began reviewing the bamlanivimab and, later, the casirivimab/imdevimab EUA Fact Sheet for Health Care Providers. All necessary elements for the complete and safe ordering and dispensing of the medication were developed and reviewed by pharmacy administration and ED nursing leadership for input, prior to submitting to the information technology team for implementation. Building the COVID-19 monoclonal medication records into the EHR allowed for detailed direction (ie, administration and preparation instructions) to be consistently applied. The medication records were also built into hospital smart pumps so that nurses could access prepopulated, accurate volumes and infusion rates to minimize errors.
Order Set Development. The pharmacy medication build was added to a comprehensive order set (Figure 3), which was then developed to guide prescribers and standardize the process around ordering of COVID-19 monoclonal therapies. While these therapies are new, oncology monoclonal therapies are regularly administered to outpatients at Mount Sinai Cancer Center. The cancer center was therefore consulted on their process surrounding best practices in administration of monoclonal antibody therapies. This included protocols for medications used in pretreatment and management of hypersensitivity reactions and potential adverse drug reactions of both COVID-19 monoclonal therapies. These medication orders were selected by default in the order set to ensure that all patients received premedications aimed at minimizing the risk of hypersensitivity reaction, and had as-needed medication orders, in the event a hypersensitivity reaction occurred. Reducing hypersensitivity reaction risk is important as well to increase the likelihood that the patient would receive full therapy, as management of this adverse drug reactions involves possible cessation of therapy depending on the level of severity. The pharmacy department also ensured these medications were stocked in ED automated dispensing cabinets to promote quick access. In addition to the aforementioned nursing orders, we added EUA criteria for use and hyperlinks to the Fact Sheets for Patients and Caregivers and Health Care Providers for each monoclonal therapy, and restricted ordering to ED physicians, nurse practitioners, and physician assistants.
The order set underwent multidisciplinary review by pharmacy administration, the chair of emergency medicine, physicians, and ED nursing leadership prior to presentation and approval by the Pharmacy and Therapeutics Committee. Lastly, at time of implementation, the order set was added to the ED preference list, preventing inpatient access. Additionally, as a patient safety action, free- standing orders of COVID-19 monoclonal therapies were disabled, so providers could only order therapies via the approved, comprehensive order set.
Preliminary Assessment Tool. A provider assessment tool was developed to document patient-specific EUA criteria for use during initial assessment (Figure 4). This tool serves as a checklist and is visible to the full multidisciplinary team in the patient’s EHR. It is used as a resource at the time of pharmacist verification and ED physician assessment to ensure criteria for use are met.
Outpatient Offices
Patient Referral. Patients with symptoms or concerns of COVID-19 exposure can make physician appointments via telemedicine or in person at Mount Sinai Medical Center’s primary care and specialty offices. At the time of patient encounter, physicians suspecting a COVID-19 diagnosis will refer patients for outpatient COVID-19 polymerase chain reaction (PCR) laboratory testing, which has an approximate 24-hour turnaround to results. Physicians also assess whether the patient meets EUA criteria for use, pending results of testing. In the event a patient meets EUA criteria for use, the physician provides patient counseling and requests verbal consent. Following this, the physician enters a note in the EHR describing the patient’s condition, criteria for use evaluation, and the patient’s verbal agreement to therapy. This preliminary screening is beneficial to begin planning with both the patient and ED to minimize delays. Patients are notified of the results of their test once available. If the COVID-19 PCR test returns positive, the physician will call the ED at the main campus and schedule the patient for COVID-19 monoclonal therapy. As the desired timeframe for administering COVID-19 monoclonal therapies is within less than 10 days of symptom onset, timely scheduling of appointments is crucial. Infusion appointments are typically provided the same or next day. The patients are informed that they must bring documentation of their positive COVID-19 PCR test to their ED visit. Lastly, because patients are pretreated with medication that may potentially impair driving, they are instructed that they cannot drive themselves home; ride shares also are not allowed in order to limit the spread of infection.
Emergency Department
Patient Arrival and Screening. A COVID-19 patient can be evaluated in the ED 1 of 2 ways. The first option is via outpatient office referral, as described previously. Upon arrival to the ED, a second screening is performed to ensure the patient still meets EUA criteria for use and the positive COVID-19 PCR test result is confirmed. If the patient no longer meets criteria, the patient is triaged accordingly, including evaluation for higher-level care (eg, supplemental oxygen, hospital admission). The second optoion is via new patient walk-ins without outpatient physician referral (Figure 4). In these cases, an initial screening is performed, documenting EUA criteria for use in the preliminary assessment (Figure 5). Physicians will consider an outside COVID-19 test as valid, so long as documentation is readily available confirming a positive PCR result. Otherwise, an in-house COVID-19 PCR test will be performed, which has a 2-hour turnaround time.
Infusion Schedule. The ED offers a total of 16 COVID-19 monoclonal infusions slots daily. These are broken up into 4 infusion time blocks (eg, 8
Patient Education. Prior to administration of the monoclonal therapy, physician and nursing staff obtain a formal, written patient consent for therapy and provide patients with the option of participating in the institutional review board (IRB) approved study. Details of this are discussed in the risk management and IRB sections of the article. Nursing staff also provides the medication-specific Fact Sheet for Patients and Caregivers in either Spanish or English, which is also included as a hyperlink on the COVID-19 Monoclonal Antibody Order Set for ease of access. Interpreter services are available for patients who speak other languages. An ED decentralized pharmacist is also available onsite Monday through Friday from 12
Infusion Ordering. Once the patient is ready to begin therapy, the he/she is brought to a dedicated overflow area of the ED. There are few, if any, patients in this location, and it is adjacent to the main emergency center for easy access by the patients, nurses, pharmacists, and physicians. The physician then enters orders in the EHR using the COVID-19 Monoclonal Antibody Order Set (Figure 3). Three discrete questions were built into the medication order: (1) Was patient consent obtained? (2) Was the Fact Sheet for Patient/Caregiver provided to the patient? (3) Is the patient COVID-19 PCR-positive? These questions were built as hard stops so that the medication orders cannot be placed without a response. This serves as another double-check to ensure processes are followed and helps facilitate timely verification by the pharmacist.
Medication Administration. One nurse is dedicated to administering the monoclonal therapies scheduled at 8
Pharmacy Department
Medication Receipt Process. Inventory is currently allocated biweekly from the state department of health and will soon be transitioning to a direct order system. The pharmacy technician in charge of deliveries notifies the pharmacy Antimicrobial Stewardship Program (ASP) clinical coordinator upon receipt of the monoclonal therapies. Bamlanivimab is supplied as 1 vial per dose, whereas casirivimab/imdevimab is supplied as 4 vials or 8 vials per dose, depending how it is shipped. To reduce the likelihood of medication errors, the ASP clinical coordinator assembles each of the casirivimab/imdevimab vials into kits, where 1 kit equals 1 dose. Labels are then affixed to each kit indicating the medication name, number of vials which equal a full dose, and pharmacist signature. The kits are stored in a dedicated refrigerator, and inventory logs are affixed to the outside of the refrigerator and updated daily. This inventory is also communicated daily to ED physician, nursing, and pharmacy leadership, as well as the director of patient safety, who reports weekly usage to the state Department of Health and Human Services. These weekly reports are used to determine allocation amounts.
Medication Verification and Delivery. The Mount Sinai Medical Center pharmacist staffing model consists of centralized order entry and specialized, decentralized positions. All orders are verified by the ED pharmacist when scheduled (not a 24/7 service) and by the designated pharmacist for all other times. At the time of medication verification, the pharmacist documents patient-specific EUA criteria for use and confirms that consent was obtained and the Fact Sheet for Patients/Caregivers was provided. A pharmacist intervention was developed to assist with this documentation. Pharmacists input smart text “.COVIDmonoclonal” and a drop-down menu of EUA criteria for use appears. The pharmacist reviews the patient care notes and medication order question responses to ascertain this information, contacting the ED prescriber if further clarification is required. This verification serves as another check to ensure processes put in place are followed. Lastly, intravenous preparation and delivery are electronically recorded in the EHR, and the medications require nursing signature at the time of delivery to ensure a formal chain of custody.
Risk Management
At Mount Sinai Medical Center, all EUA and investigational therapies require patient consent. Consistent with this requirement, a COVID-19 monoclonal specific consent was developed by risk management. This is provided to every patient receiving a COVID-19 monoclonal infusion, in addition to the FDA EUA Fact Sheet for Patients and Caregivers, and documented as part of their EHR. The questions providers must answer are built into the order set to ensure this process is followed and these patient safety checks are incorporated into the workflow.
Billing and Finance Department
In alignment with Mount Sinai Medical Center’s mission to provide high-quality health care to its diverse community through teaching, research, charity care, and financial responsibility, it was determined that this therapy would be provided to all patients regardless of insurance type, including those who are uninsured. The billing and finance department was consulted prior to this service being offered, to provide patients with accurate and pertinent information. The billing and finance department provided guidance on how to document patient encounters at time of registration to facilitate appropriate billing. At this time, the medication is free of charge, but nonmedication-related ED fees apply. This is explained to patients so there is a clear understanding prior to booking their appointment.
Infection Prevention
As patients receiving COVID-19 monoclonal therapies can transmit the virus to others, measures to ensure protection for other patients and staff are vital. To minimize exposure, specific nursing and physician staff from the ED are assigned to the treatment of these patients, and patients receive infusions and postobservation monitoring in a designated wing of the ED. Additionally, all staff who interact with these patients are required to don full personal protective equipment. This includes not only physicians and nurses but all specialties such as physician assistants, nurse practitioners, pharmacists, and laboratory technicians. Moreover, patients are not permitted to go home in a ride share and are counseled on Centers for Disease Control and Prevention quarantining following infusion.
Measurement of Process and Outcomes and Reporting
IRB approval was sought and obtained early during initiation of this service, allowing study consent to be offered to patients at the time general consent was obtained, which maximized patient recruitment and streamlined workflow. The study is a prospective observational research study to determine the impact of administration of COVID-19 monoclonal antibody therapy on length of symptoms, chronic illness, and rate of hospitalization. Most patients were eager to participate and offer their assistance to the scientific community during this pandemic.
Staff Education
In order to successfully implement this multidisciplinary EUA treatment option, comprehensive staff education was paramount after the workflow was developed. Prior to the first day of infusions, nurses and pharmacists were provided education during multiple huddle announcements. The pharmacy team also provided screen captures via email to the pharmacists so they could become familiar with the order set, intervention documentation, and location of the preliminary assessment of EUA criteria for use at the time of order verification. The emergency medicine department chair and chief medical officer also provided education via several virtual meetings and email to referring physicians (specialists and primary care) and residents in the emergency centers involved in COVID-19 monoclonal therapy-related patient care.
Factors Contributing to Success
We believe the reasons for continued success of this process are multifactorial and include the following key elements. Multidisciplinary planning, which included decision makers and all stakeholders, began at the time the idea was conceived. This allowed quick implementation of this service by efficiently navigating barriers to engaging impacted staff early on. Throughout this process, the authors set realistic step-wise goals. While navigating through the many details to implementation described, we also kept in mind the big picture, which was to provide this potentially lifesaving therapy to as many qualifying members of our community as possible. This included being flexible with the process and adapting when needed to achieve this ultimate goal. A focus on safety remained a priority to minimize possible errors and enhance patient and staff satisfaction. The optimization of the EHR streamlined workflow, provided point-of-care resources, and enhanced patient safety. Additionally, the target date set for implementation allowed staff and department leads adequate time to plan for and anticipate the changes. Serving only 1 patient on the first day allowed time for staff to experience this new process hands-on and provided opportunity for focused education. This team communication was essential to implementing this project, including staff training of processes and procedures prior to go-live. Early incorporation of IRB approval allowed the experience to be assessed and considered for contribution to the scientific literature to tackle this novel virus that has impacted our communities locally, nationally, and abroad. Moreover, continued measurement and reporting on a regular basis leads to performance improvement. The process outlined here can be adapted to incorporate other new therapies in the future, such as the recent February 9, 2021, EUA of the COVID-19 monoclonal antibody combination bamlanivimab and etesevimab.10
Conclusion
We administered 790 COVID-19 monoclonal antibody infusions between November 20, 2020 and March 5, 2021. Steps to minimize the likelihood of hypersensitivity reactions were implemented, and a low incidence (< 1%) has been observed. There has been no incidence of infection, concern from staff about infection prevention, or risk of infection during the processes. There have been very infrequent cost-related concerns raised by patients, typically due to incomplete communication regarding billing prior to the infusion. To address these issues, staff education has been provided to enhance patient instruction on this topic. The program has provided patient and family satisfaction, as well nursing, physician, pharmacist, clinical staff, and hospital administration pride and gratification. Setting up a new program to provide a 4-hour patient encounter to infuse therapy to high-risk patients with COVID-19 requires commitment and effort. This article describes the experience, ideas, and formula others may consider using to set up such a program. Through networking and formal phone calls and meetings about monoclonal antibody therapy, we have heard about other institutions who have not been able to institute this program due to various barriers to implementation. We hope our experience serves as a resource for others to provide this therapy to their patients and expand access in an effort to mitigate COVID-19 consequences and cases affecting our communities.
Corresponding author: Kathleen Jodoin, PharmD, BCPS, Mount Sinai Medical Center, 4300 Alton Rd, Miami Beach, FL 33140; [email protected].
Financial disclosures: None.
1. COVID Data Tracker. Center for Disease Control and Prevention. https://covid.cdc.gov/covid-data-tracker/#global-counts-rates. Accessed March 12, 2021.
2. Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of Bamlanivimab. US Food and Drug Administration. Updated February 2021. Accessed March 9, 2021. https://www.fda.gov/media/143603/download
3. Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibodies for Treatment of COVID-19 | FDA. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibodies-treatment-covid-19. Accessed February 14, 2021.
4. Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of Casirivimab and Imdevimab. US Food and Drug Administration. Updated December 2020. Accessed March 9, 2021. https://www.fda.gov/media/143892/download
5. Chen P, Nirula A, Heller B, et al. SARS-CoV-2 Neutralizing antibody LY-CoV555 in outpatients with COVID-19. N Engl J Med. 2021;384(3):229-237. doi:10.1056/NEJMoa2029849
6. Gottlieb RL, Nirula A, Chen P, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. 10.1JAMA. 2021;325(7):632-644. doi:10.1001/jama.2021.0202
7. Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. 10.1N Engl J Med. 2021;384:238-251. doi:10.1056/nejmoa2035002
8. Mulangu S, Dodd LE, Davey RT Jr, et al. A randomized, controlled trial of Ebola virus disease therapeutics. 10.1N Engl J Med. 2019;381:2293-2303. doi:10.1056/NEJMoa1910993
9. Boyle, P. Can an experimental treatment keep COVID-19 patients out of hospitals? Association of American Medical Colleges. January 29, 2021. Accessed March 9, 2021. https://www.aamc.org/news-insights/can-experimental-treatment-keep-covid-19-patients-out-hospitals
10. Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of Bamlanivimab and Etesevimab. US Food and Drug Administration. Updated February 2021. Accessed March 9, 2021. https://www.fda.gov/media/145802/download
1. COVID Data Tracker. Center for Disease Control and Prevention. https://covid.cdc.gov/covid-data-tracker/#global-counts-rates. Accessed March 12, 2021.
2. Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of Bamlanivimab. US Food and Drug Administration. Updated February 2021. Accessed March 9, 2021. https://www.fda.gov/media/143603/download
3. Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibodies for Treatment of COVID-19 | FDA. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibodies-treatment-covid-19. Accessed February 14, 2021.
4. Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of Casirivimab and Imdevimab. US Food and Drug Administration. Updated December 2020. Accessed March 9, 2021. https://www.fda.gov/media/143892/download
5. Chen P, Nirula A, Heller B, et al. SARS-CoV-2 Neutralizing antibody LY-CoV555 in outpatients with COVID-19. N Engl J Med. 2021;384(3):229-237. doi:10.1056/NEJMoa2029849
6. Gottlieb RL, Nirula A, Chen P, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. 10.1JAMA. 2021;325(7):632-644. doi:10.1001/jama.2021.0202
7. Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. 10.1N Engl J Med. 2021;384:238-251. doi:10.1056/nejmoa2035002
8. Mulangu S, Dodd LE, Davey RT Jr, et al. A randomized, controlled trial of Ebola virus disease therapeutics. 10.1N Engl J Med. 2019;381:2293-2303. doi:10.1056/NEJMoa1910993
9. Boyle, P. Can an experimental treatment keep COVID-19 patients out of hospitals? Association of American Medical Colleges. January 29, 2021. Accessed March 9, 2021. https://www.aamc.org/news-insights/can-experimental-treatment-keep-covid-19-patients-out-hospitals
10. Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of Bamlanivimab and Etesevimab. US Food and Drug Administration. Updated February 2021. Accessed March 9, 2021. https://www.fda.gov/media/145802/download
Use of Fecal Immunochemical Testing in Acute Patient Care in a Safety Net Hospital System
From Baylor College of Medicine, Houston, TX (Drs. Spezia-Lindner, Montealegre, Muldrew, and Suarez) and Harris Health System, Houston, TX (Shanna L. Harris, Maria Daheri, and Drs. Muldrew and Suarez).
Abstract
Objective: To characterize and analyze the prevalence, indications for, and outcomes of fecal immunochemical testing (FIT) in acute patient care within a safety net health care system’s emergency departments (EDs) and inpatient settings.
Design: Retrospective cohort study derived from administrative data.
Setting: A large, urban, safety net health care delivery system in Texas. The data gathered were from the health care system’s 2 primary hospitals and their associated EDs. This health care system utilizes FIT exclusively for fecal occult blood testing.
Participants: Adults ≥18 years who underwent FIT in the ED or inpatient setting between August 2016 and March 2017. Chart review abstractions were performed on a sample (n = 382) from the larger subset.
Measurements: Primary data points included total FITs performed in acute patient care during the study period, basic demographic data, FIT indications, FIT result, receipt of invasive diagnostic follow-up, and result of invasive diagnostic follow-up. Multivariable log-binomial regression was used to calculate risk ratios (RRs) to assess the association between FIT result and receipt of diagnostic follow-up. Chi-square analysis was used to compare the proportion of abnormal findings on diagnostic follow-up by FIT result.
Results: During the 8-month study period, 2718 FITs were performed in the ED and inpatient setting, comprising 5.7% of system-wide FITs. Of the 382 patients included in the chart review who underwent acute care FIT, a majority had their test performed in the ED (304, 79.6%), 133 of which were positive (34.8%). The most common indication for FIT was evidence of overt gastrointestinal (GI) bleed (207, 54.2%), followed by anemia (84, 22.0%). While a positive FIT result was significantly associated with obtaining a diagnostic exam in multivariate analysis (RR, 1.72; P < 0.001), having signs of overt GI bleeding was a stronger predictor of diagnostic follow-up (RR, 2.00; P = 0.003). Of patients who underwent FIT and received diagnostic follow-up (n = 110), 48.2% were FIT negative. These patients were just as likely to have an abnormal finding as FIT-positive patients (90.6% vs 91.2%; P = 0.86). Of the 382 patients in the study, 4 (1.0%) were subsequently diagnosed with colorectal cancer (CRC). Of those 4 patients, 1 (25%) was FIT positive.
Conclusion: FIT is being utilized in acute patient care outside of its established indication for CRC screening in asymptomatic, average-risk adults. Our study demonstrates that FIT is not useful in acute patient care.
Keywords: FOBT; FIT; fecal immunochemical testing; inpatient.
Colorectal cancer (CRC) is the second leading cause of cancer-related mortality in the United States. It is estimated that in 2020, 147,950 individuals will be diagnosed with invasive CRC and 53,200 will die from it.1 While the overall incidence has been declining for decades, it is rising in young adults.2–4 Screening using direct visualization procedures (colonoscopy and sigmoidoscopy) and stool-based tests has been demonstrated to improve detection of precancerous and early cancerous lesions, thereby reducing CRC mortality.5 However, screening rates in the United States are suboptimal, with only 68.8% of adults aged 50 to 75 years screened according to guidelines in 2018.6Stool-based testing is a well-established and validated screening measure for CRC in asymptomatic individuals at average risk. Its widespread use in this population has been shown to cost-effectively screen for CRC among adults 50 years of age and older.5,7 Presently, the 2 most commonly used stool-based assays in the US health care system are guaiac-based tests (guaiac fecal occult blood test [gFOBT], Hemoccult) and
Despite the exclusive validation of FOBTs for use in CRC screening, studies have demonstrated that they are commonly used for a multitude of additional indications in emergency department (ED) and inpatient settings, most aimed at detecting or confirming GI blood loss. This may lead to inappropriate patient management, including the receipt of unnecessary follow-up procedures, which can incur significant costs to the patient and the health system.13-19 These costs may be particularly burdensome in safety net health systems (ie, those that offer access to care regardless of the patient’s ability to pay), which serve a large proportion of socioeconomically disadvantaged individuals in the United States.20,21 To our knowledge, no published study to date has specifically investigated the role of FIT in acute patient management.
This study characterizes the use of FIT in acute patient care within a large, urban, safety net health care system. Through a retrospective review of administrative data and patient charts, we evaluated FIT use prevalence, indications, and patient outcomes in the ED and inpatient settings.
Methods
Setting
This study was conducted in a large, urban, county-based integrated delivery system in Houston, Texas, that provides health care services to one of the largest uninsured and underinsured populations in the country.22 The health system includes 2 main hospitals and more than 20 ambulatory care clinics. Within its ambulatory care clinics, the health system implements a population-based screening strategy using stool-based testing. All adults aged 50 years or older who are due for FIT are identified through the health-maintenance module of the electronic medical record (EMR) and offered a take-home FIT. The health system utilizes FIT exclusively (OC-Light S FIT, Polymedco, Cortlandt Manor, NY); no guaiac-based assays are available.
Design and Data Collection
We began by using administrative records to determine the proportion of FITs conducted health system-wide that were ordered and completed in the acute care setting over the study period (August 2016-March 2017). Specifically, we used aggregate quality metric reports, which quantify the number of FITs conducted at each health system clinic and hospital each month, to calculate the proportion of FITs done in the ED and inpatient hospital setting.
We then conducted a retrospective cohort study of 382 adult patients who received FIT in the EDs and inpatient wards in both of the health system’s hospitals over the study period. All data were collected by retrospective chart review in Epic (Madison, WI) EMRs. Sampling was performed by selecting the medical record numbers corresponding to the first 50 completed FITs chronologically each month over the 8-month period, with a total of 400 charts reviewed.
Data collected included basic patient demographics, location of FIT ordering (ED vs inpatient), primary service ordering FIT, FIT indication, FIT result, and receipt and results of invasive diagnostic follow-up. Demographics collected included age, biological sex, race (self-selected), and insurance coverage.
FIT indication was determined based on resident or attending physician notes. The history of present illness, physical exam, and assessment and plan section of notes were reviewed by the lead author for a specific statement of indication for FIT or for evidence of clinical presentation for which FIT could reasonably be ordered. Indications were iteratively reviewed and collapsed into 6 different categories: anemia, iron deficiency with or without anemia, overt GIB, suspected GIB/miscellaneous, non-bloody diarrhea, and no indication identified. Overt GIB was defined as reported or witnessed hematemesis, coffee-ground emesis, hematochezia, bright red blood per rectum, or melena irrespective of time frame (current or remote) or chronicity (acute, subacute, or chronic). In cases where signs of overt bleed were not witnessed by medical professionals, determination of conditions such as melena or coffee-ground emesis were made based on health care providers’ assessment of patient history as documented in his or her notes. Suspected GIB/miscellaneous was defined with the following parameters: any new drop in hemoglobin, abdominal pain, anorectal pain, non-bloody vomiting, hemoptysis, isolated rising blood urea nitrogen, or patient noticing blood on self, clothing, or in the commode without an identified source. Patients who were anemic and found to have iron deficiency on recent lab studies (within 6 months) were reflexively categorized into iron deficiency with or without anemia as opposed to the “anemia” category, which was comprised of any anemia without recent iron studies or non-iron deficient anemia. FIT result was determined by test result entry in Epic, with results either reading positive or negative.
Diagnostic follow-up, for our purposes, was defined as receipt of an invasive procedure or surgery, including esophagogastroduodenoscopy (EGD), colonoscopy, flexible sigmoidoscopy, diagnostic and/or therapeutic abdominal surgical intervention, or any combination of these. Results of diagnostic follow-up were coded as normal or abnormal. A normal result was determined if all procedures performed were listed as normal or as “no pathological findings” on the operative or endoscopic report. Any reported pathologic findings on the operative/endoscopic report were coded as abnormal.
Statistical Analysis
Proportions were used to describe demographic characteristics of patients who received a FIT in acute hospital settings. Bivariable tables and Chi-square tests were used to compare indications and outcomes for FIT-positive and FIT-negative patients. The association between receipt of an invasive diagnostic follow-up (outcome) and the results of an inpatient FIT (predictor) was assessed using multivariable log-binomial regression to calculate risk ratios (RRs) and corresponding 95% confidence intervals. Log-binomial regression was used over logistic regression given that adjusted odds ratios generated by logistic regression often overestimate the association between the risk factor and the outcome when the outcome is common,23 as in the case of diagnostic follow-up. The model was adjusted for variables selected a priori, specifically, age, gender, and FIT indication. Chi-square analysis was used to compare the proportion of abnormal findings on diagnostic follow-up by FIT result (negative vs positive).
Results
During the 8-month study period, there were 2718 FITs ordered and completed in the acute care setting, compared to 44,662 FITs ordered and completed in the outpatient setting (5.7% performed during acute care).
Among the 400 charts reviewed, 7 were excluded from the analysis because they were duplicates from the same patient, and 11 were excluded due to insufficient information in the patient’s medical record, resulting in 382 patients included in the analysis. Patient demographic characteristics are described in Table 1. Patients were predominantly Hispanic/Latino or Black/African American (51.0% and 32.5%, respectively), a majority had insurance through the county health system (50.5%), and most were male (58.1%). The average age of those receiving FIT was 52 years (standard deviation, 14.8 years), with 40.8% being under the age of 50. For a majority of patients, FIT was ordered in the ED by emergency medicine providers (79.8%). The remaining FITs were ordered by providers in 12 different inpatient departments. Of the FITs ordered, 35.1% were positive.
Indications for ordering FIT are listed in Table 2. The largest proportion of FITs were ordered for overt signs of GIB (54.2%), followed by anemia (22.0%), suspected GIB/miscellaneous reasons (12.3%), iron deficiency with or without anemia (7.6%), and non-bloody diarrhea (2.1%). In 1.8% of cases, no indication for FIT was found in the EMR. No FITs were ordered for the indication of CRC detection. Of these indication categories, overt GIB yielded the highest percentage of FIT positive results (44.0%), and non-bloody diarrhea yielded the lowest (0%).
A total of 110 patients (28.7%) underwent FIT and received invasive diagnostic follow-up. Of these 110 patients, 57 (51.8%) underwent EGD (2 of whom had further surgical intervention), 21 (19.1%) underwent colonoscopy (1 of whom had further surgical intervention), 25 (22.7%) underwent dual EGD and colonoscopy, 1 (0.9%) underwent flexible sigmoidoscopy, and 6 (5.5%) directly underwent abdominal surgical intervention. There was a significantly higher rate of diagnostic follow-up for FIT-positive vs FIT-negative patients (42.9% vs 21.3%; P < 0.001). However, of the 110 patients who underwent subsequent diagnostic follow-up, 48.2% were FIT negative. FIT-negative patients who received diagnostic follow-up were just as likely to have an abnormal finding as FIT-positive patients (90.6% vs 91.2%; P = 0.86).
Of the 382 patients in the study, 4 were diagnosed with CRC through diagnostic follow-up (1.0%). Of those 4 patients, 1 was FIT positive.
The results of the multivariable analyses to evaluate predictors of diagnostic colonoscopy are described in Table 3. Variables in the final model were FITresult, age, and FIT indication. After adjusting for other variables in the model, receipt of diagnostic follow-up was significantly associated with having a positive FIT (adjusted RR, 1.72; P < 0.001) and an overt GIB as an indication (adjusted RR, 2.00; P < 0.01).
Discussion
During the time frame of our study, 5.7% of all FITs ordered within our health system were ordered in the acute patient care setting at our hospitals. The most common indication was overt GIB, which was the indication for 54.2% of patients. Of note, none of the FITs ordered in the acute patient care setting were ordered for CRC screening. These findings support the evidence in the literature that stool-based screening tests, including FIT, are commonly used in US health care systems for diagnostic purposes and risk stratification in acute patient care to detect GIBs.13-18
Our data suggest that FIT was not a clinically useful test in determining a patient’s need for diagnostic follow-up. While having a positive FIT was significantly associated with obtaining a diagnostic exam in multivariate analysis (RR, 1.72), having signs of overt GI bleeding was a stronger predictor of diagnostic follow-up (RR, 2.00). This salient finding is evidence that a thorough clinical history and physical exam may more strongly predict whether a patient will undergo endoscopy or other follow-up than a FIT result. These findings support other studies in the literature that have called into question the utility of FOBTs in these acute settings.13-19 Under such circumstances, FOBTs have been shown to rarely influence patient management and thus represent an unnecessary expense.13–17 Additionally, in some cases, FOBT use in these settings may negatively affect patient outcomes. Such adverse effects include delaying treatment until results are returned or obfuscating indicated management with the results (eg, a patient with indications for colonoscopy not being referred due to a negative FOBT).13,14,17
We found that, for patients who subsequently went on to have diagnostic follow-up (most commonly endoscopy), there was no difference in the likelihood of FIT-positive and FIT-negative patients to have an abnormality discovered (91.2% vs 90.6%; P = 0.86). This analysis demonstrates no post-hoc support for FIT positivity as a predictor of presence of pathology in patients who were discriminately selected for diagnostic follow-up on clinical grounds by gastroenterologists and surgeons. It does, however, further support that clinical judgment about the need for diagnostic follow-up—irrespective of FIT result—has a very high yield for discovery of pathology in the acute setting.
There are multiple reasons why FOBTs, and specifically FIT, contribute little in management decisions for patients with suspected GI blood loss. Use of FIT raises concern for both false-negatives and false-positives when used outside of its indication. Regarding false- negatives, FIT is an unreliable test for detection of blood loss from the upper GI tract. As FITs utilize antibodies to detect the presence of globin, a byproduct of red blood cell breakdown, it is expected that FIT would fail to detect many cases of upper GI bleeding, as globin is broken down in the upper GI tract.24 This fact is part of what has made FIT a more effective CRC screening test than its guaiac-based counterparts—it has greater specificity for lower GI tract blood loss compared to tests relying on detection of heme.8 While guaiac-based assays like Hemoccult have also been shown to be poor tests in acute patient care, they may more frequently, though still unreliably, detect blood of upper GI origin. We believe that part of the ongoing use of FIT in patients with a suspected upper GIB may be from lack of understanding among providers on the mechanistic difference between gFOBTs and FITs, even though gFOBTs also yield highly unreliable results.
FIT does not have the same risk of false-positive results that guaiac-based tests have, which can yield positive results with extra-intestinal blood ingestion, aspirin, or alcohol use; insignificant GI bleeding; and consumption of peroxidase-containing foods.13,17,25 However, from a clinical standpoint, there are several scenarios of insignificant bleeding that would yield a positive FIT result, such as hemorrhoids, which are common in the US population.26,27 Additionally, in the ED, where most FITs were performed in our study, it is possible that samples for FITs are being obtained via digital rectal exam (DRE) given patients’ acuity of medical conditions and time constraints. However, FIT has been validated when using a formed stool sample. Obtaining FIT via DRE may lead to microtrauma to the rectum, which could hypothetically yield a positive FIT.
Strengths of this study include its use of in-depth chart data on a large number of FIT-positive patients, which allowed us to discern indications, outcomes, and other clinical data that may have influenced clinical decision-making. Additionally, whereas other studies that address FOBT use in acute patient care have focused on guaiac-based assays, our findings regarding the lack of utility of FIT are novel and have particular relevance as FITs continue to grow in popularity. Nonetheless, there are certain limitations future research should seek to address. In this study, the diagnostic follow-up result was coded by presence or absence of pathologic findings but did not qualify findings by severity or attempt to determine whether the pathology noted on diagnostic follow-up was the definitive source of the suspected GI bleed. These variables could help determine whether there was a difference in severity of bleeding between FIT-positive and FIT-negative patients and could potentially be studied with a prospective research design. Our own study was not designed to address the question of whether FIT result informs patient management decisions. To answer this directly, interviews would have to be conducted with those making the follow-up decision (ie, endoscopists and surgeons). Additionally, this study was not adequately powered to make determinations on the efficacy of FIT in the acute care setting for detection of CRC. As mentioned, only 1 of the 4 patients (25%) who went on to be diagnosed with CRC on follow-up was initially FIT-positive. This would require further investigation.
Conclusion
FIT is being utilized for diagnostic purposes in the acute care of symptomatic patients, which is a misuse of an established screening test for CRC. While our study was not designed to answer whether and how often a FIT result informs subsequent patient management, our results indicate that FIT is an ineffective diagnostic and risk-stratification tool when used in the acute care setting. Our findings add to existing evidence that indicates FOBTs should not be used in acute patient care.
Taken as a whole, the results of our study add to a growing body of evidence demonstrating no role for FOBTs, and specifically FIT, in acute patient care. In light of this evidence, some health care systems have already demonstrated success with system-wide disinvestment from the test in acute patient care settings, with one group publishing about their disinvestment process.28 After completion of our study, our preliminary data were presented to leadership from the internal medicine, emergency medicine, and laboratory divisions within our health care delivery system to galvanize complete disinvestment of FIT from acute care at our hospitals, a policy that was put into effect in July 2019.
Corresponding author: Nathaniel J. Spezia-Lindner, MD, Baylor College of Medicine, 7200 Cambridge St, BCM 903, Ste A10.197, Houston, TX 77030; [email protected].
Financial disclosures: None.
Funding: Cancer Prevention and Research Institute of Texas, CPRIT (PP170094, PDs: ML Jibaja-Weiss and JR Montealegre).
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. 10.1CA Cancer 10.1J Clin. 2020;70(1):7-30.
2. Howlader NN, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975-2014. National Cancer Institute; 2017:1-2.
3. Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States, 1974–2013. 10.1J Natl Cancer Inst. 2017;109(8):djw322.
4. Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. 10.25JAMA Surg. 2015;150(1):17-22.
5. Lin JS, Piper MA, Perdue LA, et al. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. 10.25JAMA. 2016;315(23):2576-2594.
6. Centers for Disease Control and Prevention (CDC). Use of colorectal cancer screening tests. Behavioral Risk Factor Surveillance System. October 22, 2019. Accessed February 10, 2021. https://www.cdc.gov/cancer/colorectal/statistics/use-screening-tests-BRFSS.htm
7. Hewitson P, Glasziou PP, Irwig L, et al. Screening for colorectal cancer using the fecal occult blood test, Hemoccult. 10.25Cochrane Database Syst Rev. 2007;2007(1):CD001216.
8. Bujanda L, Lanas Á, Quintero E, et al. Effect of aspirin and antiplatelet drugs on the outcome of the fecal immunochemical test. 10.25Mayo Clin Proc. 2013;88(7):683-689.
9. Allison JE, Sakoda LC, Levin TR, et al. Screening for colorectal neoplasms with new fecal occult blood tests: update on performance characteristics. 10.25J Natl Cancer Inst. 2007;99(19):1462-1470.
10. Dancourt V, Lejeune C, Lepage C, et al. Immunochemical faecal occult blood tests are superior to guaiac-based tests for the detection of colorectal neoplasms. 10.25Eur J Cancer. 2008;44(15):2254-2258.
11. Hol L, Wilschut JA, van Ballegooijen M, et al. Screening for colorectal cancer: random comparison of guaiac and immunochemical faecal occult blood testing at different cut-off levels. 10.25Br J Cancer. 2009;100(7):1103-1110.
12. Levi Z, Birkenfeld S, Vilkin A, et al. A higher detection rate for colorectal cancer and advanced adenomatous polyp for screening with immunochemical fecal occult blood test than guaiac fecal occult blood test, despite lower compliance rate. A prospective, controlled, feasibility study. Int J Cancer. 2011;128(10):2415-2424.
13. Friedman A, Chan A, Chin LC, et al. Use and abuse of faecal occult blood tests in an acute hospital inpatient setting. Intern Med J. 2010;40(2):107-111.
14. Narula N, Ulic D, Al-Dabbagh R, et al. Fecal occult blood testing as a diagnostic test in symptomatic patients is not useful: a retrospective chart review. Can J Gastroenterol Hepatol. 2014;28(8):421-426.
15. Ip S, Sokoro AA, Kaita L, et al. Use of fecal occult blood testing in hospitalized patients: results of an audit. Can J Gastroenterol Hepatol. 2014;28(9):489-494.
16. Mosadeghi S, Ren H, Catungal J, et al. Utilization of fecal occult blood test in the acute hospital setting and its impact on clinical management and outcomes. J Postgrad Med. 2016;62(2):91-95.
17. van Rijn AF, Stroobants AK, Deutekom M, et al. Inappropriate use of the faecal occult blood test in a university hospital in the Netherlands. Eur J Gastroenterol Hepatol. 2012;24(11):1266-1269.
18. Sharma VK, Komanduri S, Nayyar S, et al. An audit of the utility of in-patient fecal occult blood testing. Am J Gastroenterol. 2001;96(4):1256-1260.
19. Chiang TH, Lee YC, Tu CH, et al. Performance of the immunochemical fecal occult blood test in predicting lesions in the lower gastrointestinal tract. CMAJ. 2011;183(13):1474-1481.
20. Chokshi DA, Chang JE, Wilson RM. Health reform and the changing safety net in the United States. N Engl J Med. 2016;375(18):1790-1796.
21. Nguyen OK, Makam AN, Halm EA. National use of safety net clinics for primary care among adults with non-Medicaid insurance in the United States. PLoS One. 2016;11(3):e0151610.
22. United States Census Bureau. American Community Survey. Selected Economic Characteristics. 2019. Accessed February 20, 2021. https://data.census.gov/cedsci/table?q=ACSDP1Y2019.DP03%20Texas&g=0400000US48&tid=ACSDP1Y2019.DP03&hidePreview=true
23. McNutt LA, Wu C, Xue X, et al. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157(10):940-943.
24. Rockey DC. Occult gastrointestinal bleeding. Gastroenterol Clin North Am. 2005;34(4):699-718.
25. Macrae FA, St John DJ. Relationship between patterns of bleeding and Hemoccult sensitivity in patients with colorectal cancers or adenomas. Gastroenterology. 1982;82(5 pt 1):891-898.
26. Johanson JF, Sonnenberg A. The prevalence of hemorrhoids and chronic constipation: an epidemiologic study. Gastroenterology. 1990;98(2):380-386.
27. Fleming JL, Ahlquist DA, McGill DB, et al. Influence of aspirin and ethanol on fecal blood levels as determined by using the HemoQuant assay. Mayo Clin Proc. 1987;62(3):159-163.
28. Gupta A, Tang Z, Agrawal D. Eliminating in-hospital fecal occult blood testing: our experience with disinvestment. Am J Med. 2018;131(7):760-763.
From Baylor College of Medicine, Houston, TX (Drs. Spezia-Lindner, Montealegre, Muldrew, and Suarez) and Harris Health System, Houston, TX (Shanna L. Harris, Maria Daheri, and Drs. Muldrew and Suarez).
Abstract
Objective: To characterize and analyze the prevalence, indications for, and outcomes of fecal immunochemical testing (FIT) in acute patient care within a safety net health care system’s emergency departments (EDs) and inpatient settings.
Design: Retrospective cohort study derived from administrative data.
Setting: A large, urban, safety net health care delivery system in Texas. The data gathered were from the health care system’s 2 primary hospitals and their associated EDs. This health care system utilizes FIT exclusively for fecal occult blood testing.
Participants: Adults ≥18 years who underwent FIT in the ED or inpatient setting between August 2016 and March 2017. Chart review abstractions were performed on a sample (n = 382) from the larger subset.
Measurements: Primary data points included total FITs performed in acute patient care during the study period, basic demographic data, FIT indications, FIT result, receipt of invasive diagnostic follow-up, and result of invasive diagnostic follow-up. Multivariable log-binomial regression was used to calculate risk ratios (RRs) to assess the association between FIT result and receipt of diagnostic follow-up. Chi-square analysis was used to compare the proportion of abnormal findings on diagnostic follow-up by FIT result.
Results: During the 8-month study period, 2718 FITs were performed in the ED and inpatient setting, comprising 5.7% of system-wide FITs. Of the 382 patients included in the chart review who underwent acute care FIT, a majority had their test performed in the ED (304, 79.6%), 133 of which were positive (34.8%). The most common indication for FIT was evidence of overt gastrointestinal (GI) bleed (207, 54.2%), followed by anemia (84, 22.0%). While a positive FIT result was significantly associated with obtaining a diagnostic exam in multivariate analysis (RR, 1.72; P < 0.001), having signs of overt GI bleeding was a stronger predictor of diagnostic follow-up (RR, 2.00; P = 0.003). Of patients who underwent FIT and received diagnostic follow-up (n = 110), 48.2% were FIT negative. These patients were just as likely to have an abnormal finding as FIT-positive patients (90.6% vs 91.2%; P = 0.86). Of the 382 patients in the study, 4 (1.0%) were subsequently diagnosed with colorectal cancer (CRC). Of those 4 patients, 1 (25%) was FIT positive.
Conclusion: FIT is being utilized in acute patient care outside of its established indication for CRC screening in asymptomatic, average-risk adults. Our study demonstrates that FIT is not useful in acute patient care.
Keywords: FOBT; FIT; fecal immunochemical testing; inpatient.
Colorectal cancer (CRC) is the second leading cause of cancer-related mortality in the United States. It is estimated that in 2020, 147,950 individuals will be diagnosed with invasive CRC and 53,200 will die from it.1 While the overall incidence has been declining for decades, it is rising in young adults.2–4 Screening using direct visualization procedures (colonoscopy and sigmoidoscopy) and stool-based tests has been demonstrated to improve detection of precancerous and early cancerous lesions, thereby reducing CRC mortality.5 However, screening rates in the United States are suboptimal, with only 68.8% of adults aged 50 to 75 years screened according to guidelines in 2018.6Stool-based testing is a well-established and validated screening measure for CRC in asymptomatic individuals at average risk. Its widespread use in this population has been shown to cost-effectively screen for CRC among adults 50 years of age and older.5,7 Presently, the 2 most commonly used stool-based assays in the US health care system are guaiac-based tests (guaiac fecal occult blood test [gFOBT], Hemoccult) and
Despite the exclusive validation of FOBTs for use in CRC screening, studies have demonstrated that they are commonly used for a multitude of additional indications in emergency department (ED) and inpatient settings, most aimed at detecting or confirming GI blood loss. This may lead to inappropriate patient management, including the receipt of unnecessary follow-up procedures, which can incur significant costs to the patient and the health system.13-19 These costs may be particularly burdensome in safety net health systems (ie, those that offer access to care regardless of the patient’s ability to pay), which serve a large proportion of socioeconomically disadvantaged individuals in the United States.20,21 To our knowledge, no published study to date has specifically investigated the role of FIT in acute patient management.
This study characterizes the use of FIT in acute patient care within a large, urban, safety net health care system. Through a retrospective review of administrative data and patient charts, we evaluated FIT use prevalence, indications, and patient outcomes in the ED and inpatient settings.
Methods
Setting
This study was conducted in a large, urban, county-based integrated delivery system in Houston, Texas, that provides health care services to one of the largest uninsured and underinsured populations in the country.22 The health system includes 2 main hospitals and more than 20 ambulatory care clinics. Within its ambulatory care clinics, the health system implements a population-based screening strategy using stool-based testing. All adults aged 50 years or older who are due for FIT are identified through the health-maintenance module of the electronic medical record (EMR) and offered a take-home FIT. The health system utilizes FIT exclusively (OC-Light S FIT, Polymedco, Cortlandt Manor, NY); no guaiac-based assays are available.
Design and Data Collection
We began by using administrative records to determine the proportion of FITs conducted health system-wide that were ordered and completed in the acute care setting over the study period (August 2016-March 2017). Specifically, we used aggregate quality metric reports, which quantify the number of FITs conducted at each health system clinic and hospital each month, to calculate the proportion of FITs done in the ED and inpatient hospital setting.
We then conducted a retrospective cohort study of 382 adult patients who received FIT in the EDs and inpatient wards in both of the health system’s hospitals over the study period. All data were collected by retrospective chart review in Epic (Madison, WI) EMRs. Sampling was performed by selecting the medical record numbers corresponding to the first 50 completed FITs chronologically each month over the 8-month period, with a total of 400 charts reviewed.
Data collected included basic patient demographics, location of FIT ordering (ED vs inpatient), primary service ordering FIT, FIT indication, FIT result, and receipt and results of invasive diagnostic follow-up. Demographics collected included age, biological sex, race (self-selected), and insurance coverage.
FIT indication was determined based on resident or attending physician notes. The history of present illness, physical exam, and assessment and plan section of notes were reviewed by the lead author for a specific statement of indication for FIT or for evidence of clinical presentation for which FIT could reasonably be ordered. Indications were iteratively reviewed and collapsed into 6 different categories: anemia, iron deficiency with or without anemia, overt GIB, suspected GIB/miscellaneous, non-bloody diarrhea, and no indication identified. Overt GIB was defined as reported or witnessed hematemesis, coffee-ground emesis, hematochezia, bright red blood per rectum, or melena irrespective of time frame (current or remote) or chronicity (acute, subacute, or chronic). In cases where signs of overt bleed were not witnessed by medical professionals, determination of conditions such as melena or coffee-ground emesis were made based on health care providers’ assessment of patient history as documented in his or her notes. Suspected GIB/miscellaneous was defined with the following parameters: any new drop in hemoglobin, abdominal pain, anorectal pain, non-bloody vomiting, hemoptysis, isolated rising blood urea nitrogen, or patient noticing blood on self, clothing, or in the commode without an identified source. Patients who were anemic and found to have iron deficiency on recent lab studies (within 6 months) were reflexively categorized into iron deficiency with or without anemia as opposed to the “anemia” category, which was comprised of any anemia without recent iron studies or non-iron deficient anemia. FIT result was determined by test result entry in Epic, with results either reading positive or negative.
Diagnostic follow-up, for our purposes, was defined as receipt of an invasive procedure or surgery, including esophagogastroduodenoscopy (EGD), colonoscopy, flexible sigmoidoscopy, diagnostic and/or therapeutic abdominal surgical intervention, or any combination of these. Results of diagnostic follow-up were coded as normal or abnormal. A normal result was determined if all procedures performed were listed as normal or as “no pathological findings” on the operative or endoscopic report. Any reported pathologic findings on the operative/endoscopic report were coded as abnormal.
Statistical Analysis
Proportions were used to describe demographic characteristics of patients who received a FIT in acute hospital settings. Bivariable tables and Chi-square tests were used to compare indications and outcomes for FIT-positive and FIT-negative patients. The association between receipt of an invasive diagnostic follow-up (outcome) and the results of an inpatient FIT (predictor) was assessed using multivariable log-binomial regression to calculate risk ratios (RRs) and corresponding 95% confidence intervals. Log-binomial regression was used over logistic regression given that adjusted odds ratios generated by logistic regression often overestimate the association between the risk factor and the outcome when the outcome is common,23 as in the case of diagnostic follow-up. The model was adjusted for variables selected a priori, specifically, age, gender, and FIT indication. Chi-square analysis was used to compare the proportion of abnormal findings on diagnostic follow-up by FIT result (negative vs positive).
Results
During the 8-month study period, there were 2718 FITs ordered and completed in the acute care setting, compared to 44,662 FITs ordered and completed in the outpatient setting (5.7% performed during acute care).
Among the 400 charts reviewed, 7 were excluded from the analysis because they were duplicates from the same patient, and 11 were excluded due to insufficient information in the patient’s medical record, resulting in 382 patients included in the analysis. Patient demographic characteristics are described in Table 1. Patients were predominantly Hispanic/Latino or Black/African American (51.0% and 32.5%, respectively), a majority had insurance through the county health system (50.5%), and most were male (58.1%). The average age of those receiving FIT was 52 years (standard deviation, 14.8 years), with 40.8% being under the age of 50. For a majority of patients, FIT was ordered in the ED by emergency medicine providers (79.8%). The remaining FITs were ordered by providers in 12 different inpatient departments. Of the FITs ordered, 35.1% were positive.
Indications for ordering FIT are listed in Table 2. The largest proportion of FITs were ordered for overt signs of GIB (54.2%), followed by anemia (22.0%), suspected GIB/miscellaneous reasons (12.3%), iron deficiency with or without anemia (7.6%), and non-bloody diarrhea (2.1%). In 1.8% of cases, no indication for FIT was found in the EMR. No FITs were ordered for the indication of CRC detection. Of these indication categories, overt GIB yielded the highest percentage of FIT positive results (44.0%), and non-bloody diarrhea yielded the lowest (0%).
A total of 110 patients (28.7%) underwent FIT and received invasive diagnostic follow-up. Of these 110 patients, 57 (51.8%) underwent EGD (2 of whom had further surgical intervention), 21 (19.1%) underwent colonoscopy (1 of whom had further surgical intervention), 25 (22.7%) underwent dual EGD and colonoscopy, 1 (0.9%) underwent flexible sigmoidoscopy, and 6 (5.5%) directly underwent abdominal surgical intervention. There was a significantly higher rate of diagnostic follow-up for FIT-positive vs FIT-negative patients (42.9% vs 21.3%; P < 0.001). However, of the 110 patients who underwent subsequent diagnostic follow-up, 48.2% were FIT negative. FIT-negative patients who received diagnostic follow-up were just as likely to have an abnormal finding as FIT-positive patients (90.6% vs 91.2%; P = 0.86).
Of the 382 patients in the study, 4 were diagnosed with CRC through diagnostic follow-up (1.0%). Of those 4 patients, 1 was FIT positive.
The results of the multivariable analyses to evaluate predictors of diagnostic colonoscopy are described in Table 3. Variables in the final model were FITresult, age, and FIT indication. After adjusting for other variables in the model, receipt of diagnostic follow-up was significantly associated with having a positive FIT (adjusted RR, 1.72; P < 0.001) and an overt GIB as an indication (adjusted RR, 2.00; P < 0.01).
Discussion
During the time frame of our study, 5.7% of all FITs ordered within our health system were ordered in the acute patient care setting at our hospitals. The most common indication was overt GIB, which was the indication for 54.2% of patients. Of note, none of the FITs ordered in the acute patient care setting were ordered for CRC screening. These findings support the evidence in the literature that stool-based screening tests, including FIT, are commonly used in US health care systems for diagnostic purposes and risk stratification in acute patient care to detect GIBs.13-18
Our data suggest that FIT was not a clinically useful test in determining a patient’s need for diagnostic follow-up. While having a positive FIT was significantly associated with obtaining a diagnostic exam in multivariate analysis (RR, 1.72), having signs of overt GI bleeding was a stronger predictor of diagnostic follow-up (RR, 2.00). This salient finding is evidence that a thorough clinical history and physical exam may more strongly predict whether a patient will undergo endoscopy or other follow-up than a FIT result. These findings support other studies in the literature that have called into question the utility of FOBTs in these acute settings.13-19 Under such circumstances, FOBTs have been shown to rarely influence patient management and thus represent an unnecessary expense.13–17 Additionally, in some cases, FOBT use in these settings may negatively affect patient outcomes. Such adverse effects include delaying treatment until results are returned or obfuscating indicated management with the results (eg, a patient with indications for colonoscopy not being referred due to a negative FOBT).13,14,17
We found that, for patients who subsequently went on to have diagnostic follow-up (most commonly endoscopy), there was no difference in the likelihood of FIT-positive and FIT-negative patients to have an abnormality discovered (91.2% vs 90.6%; P = 0.86). This analysis demonstrates no post-hoc support for FIT positivity as a predictor of presence of pathology in patients who were discriminately selected for diagnostic follow-up on clinical grounds by gastroenterologists and surgeons. It does, however, further support that clinical judgment about the need for diagnostic follow-up—irrespective of FIT result—has a very high yield for discovery of pathology in the acute setting.
There are multiple reasons why FOBTs, and specifically FIT, contribute little in management decisions for patients with suspected GI blood loss. Use of FIT raises concern for both false-negatives and false-positives when used outside of its indication. Regarding false- negatives, FIT is an unreliable test for detection of blood loss from the upper GI tract. As FITs utilize antibodies to detect the presence of globin, a byproduct of red blood cell breakdown, it is expected that FIT would fail to detect many cases of upper GI bleeding, as globin is broken down in the upper GI tract.24 This fact is part of what has made FIT a more effective CRC screening test than its guaiac-based counterparts—it has greater specificity for lower GI tract blood loss compared to tests relying on detection of heme.8 While guaiac-based assays like Hemoccult have also been shown to be poor tests in acute patient care, they may more frequently, though still unreliably, detect blood of upper GI origin. We believe that part of the ongoing use of FIT in patients with a suspected upper GIB may be from lack of understanding among providers on the mechanistic difference between gFOBTs and FITs, even though gFOBTs also yield highly unreliable results.
FIT does not have the same risk of false-positive results that guaiac-based tests have, which can yield positive results with extra-intestinal blood ingestion, aspirin, or alcohol use; insignificant GI bleeding; and consumption of peroxidase-containing foods.13,17,25 However, from a clinical standpoint, there are several scenarios of insignificant bleeding that would yield a positive FIT result, such as hemorrhoids, which are common in the US population.26,27 Additionally, in the ED, where most FITs were performed in our study, it is possible that samples for FITs are being obtained via digital rectal exam (DRE) given patients’ acuity of medical conditions and time constraints. However, FIT has been validated when using a formed stool sample. Obtaining FIT via DRE may lead to microtrauma to the rectum, which could hypothetically yield a positive FIT.
Strengths of this study include its use of in-depth chart data on a large number of FIT-positive patients, which allowed us to discern indications, outcomes, and other clinical data that may have influenced clinical decision-making. Additionally, whereas other studies that address FOBT use in acute patient care have focused on guaiac-based assays, our findings regarding the lack of utility of FIT are novel and have particular relevance as FITs continue to grow in popularity. Nonetheless, there are certain limitations future research should seek to address. In this study, the diagnostic follow-up result was coded by presence or absence of pathologic findings but did not qualify findings by severity or attempt to determine whether the pathology noted on diagnostic follow-up was the definitive source of the suspected GI bleed. These variables could help determine whether there was a difference in severity of bleeding between FIT-positive and FIT-negative patients and could potentially be studied with a prospective research design. Our own study was not designed to address the question of whether FIT result informs patient management decisions. To answer this directly, interviews would have to be conducted with those making the follow-up decision (ie, endoscopists and surgeons). Additionally, this study was not adequately powered to make determinations on the efficacy of FIT in the acute care setting for detection of CRC. As mentioned, only 1 of the 4 patients (25%) who went on to be diagnosed with CRC on follow-up was initially FIT-positive. This would require further investigation.
Conclusion
FIT is being utilized for diagnostic purposes in the acute care of symptomatic patients, which is a misuse of an established screening test for CRC. While our study was not designed to answer whether and how often a FIT result informs subsequent patient management, our results indicate that FIT is an ineffective diagnostic and risk-stratification tool when used in the acute care setting. Our findings add to existing evidence that indicates FOBTs should not be used in acute patient care.
Taken as a whole, the results of our study add to a growing body of evidence demonstrating no role for FOBTs, and specifically FIT, in acute patient care. In light of this evidence, some health care systems have already demonstrated success with system-wide disinvestment from the test in acute patient care settings, with one group publishing about their disinvestment process.28 After completion of our study, our preliminary data were presented to leadership from the internal medicine, emergency medicine, and laboratory divisions within our health care delivery system to galvanize complete disinvestment of FIT from acute care at our hospitals, a policy that was put into effect in July 2019.
Corresponding author: Nathaniel J. Spezia-Lindner, MD, Baylor College of Medicine, 7200 Cambridge St, BCM 903, Ste A10.197, Houston, TX 77030; [email protected].
Financial disclosures: None.
Funding: Cancer Prevention and Research Institute of Texas, CPRIT (PP170094, PDs: ML Jibaja-Weiss and JR Montealegre).
From Baylor College of Medicine, Houston, TX (Drs. Spezia-Lindner, Montealegre, Muldrew, and Suarez) and Harris Health System, Houston, TX (Shanna L. Harris, Maria Daheri, and Drs. Muldrew and Suarez).
Abstract
Objective: To characterize and analyze the prevalence, indications for, and outcomes of fecal immunochemical testing (FIT) in acute patient care within a safety net health care system’s emergency departments (EDs) and inpatient settings.
Design: Retrospective cohort study derived from administrative data.
Setting: A large, urban, safety net health care delivery system in Texas. The data gathered were from the health care system’s 2 primary hospitals and their associated EDs. This health care system utilizes FIT exclusively for fecal occult blood testing.
Participants: Adults ≥18 years who underwent FIT in the ED or inpatient setting between August 2016 and March 2017. Chart review abstractions were performed on a sample (n = 382) from the larger subset.
Measurements: Primary data points included total FITs performed in acute patient care during the study period, basic demographic data, FIT indications, FIT result, receipt of invasive diagnostic follow-up, and result of invasive diagnostic follow-up. Multivariable log-binomial regression was used to calculate risk ratios (RRs) to assess the association between FIT result and receipt of diagnostic follow-up. Chi-square analysis was used to compare the proportion of abnormal findings on diagnostic follow-up by FIT result.
Results: During the 8-month study period, 2718 FITs were performed in the ED and inpatient setting, comprising 5.7% of system-wide FITs. Of the 382 patients included in the chart review who underwent acute care FIT, a majority had their test performed in the ED (304, 79.6%), 133 of which were positive (34.8%). The most common indication for FIT was evidence of overt gastrointestinal (GI) bleed (207, 54.2%), followed by anemia (84, 22.0%). While a positive FIT result was significantly associated with obtaining a diagnostic exam in multivariate analysis (RR, 1.72; P < 0.001), having signs of overt GI bleeding was a stronger predictor of diagnostic follow-up (RR, 2.00; P = 0.003). Of patients who underwent FIT and received diagnostic follow-up (n = 110), 48.2% were FIT negative. These patients were just as likely to have an abnormal finding as FIT-positive patients (90.6% vs 91.2%; P = 0.86). Of the 382 patients in the study, 4 (1.0%) were subsequently diagnosed with colorectal cancer (CRC). Of those 4 patients, 1 (25%) was FIT positive.
Conclusion: FIT is being utilized in acute patient care outside of its established indication for CRC screening in asymptomatic, average-risk adults. Our study demonstrates that FIT is not useful in acute patient care.
Keywords: FOBT; FIT; fecal immunochemical testing; inpatient.
Colorectal cancer (CRC) is the second leading cause of cancer-related mortality in the United States. It is estimated that in 2020, 147,950 individuals will be diagnosed with invasive CRC and 53,200 will die from it.1 While the overall incidence has been declining for decades, it is rising in young adults.2–4 Screening using direct visualization procedures (colonoscopy and sigmoidoscopy) and stool-based tests has been demonstrated to improve detection of precancerous and early cancerous lesions, thereby reducing CRC mortality.5 However, screening rates in the United States are suboptimal, with only 68.8% of adults aged 50 to 75 years screened according to guidelines in 2018.6Stool-based testing is a well-established and validated screening measure for CRC in asymptomatic individuals at average risk. Its widespread use in this population has been shown to cost-effectively screen for CRC among adults 50 years of age and older.5,7 Presently, the 2 most commonly used stool-based assays in the US health care system are guaiac-based tests (guaiac fecal occult blood test [gFOBT], Hemoccult) and
Despite the exclusive validation of FOBTs for use in CRC screening, studies have demonstrated that they are commonly used for a multitude of additional indications in emergency department (ED) and inpatient settings, most aimed at detecting or confirming GI blood loss. This may lead to inappropriate patient management, including the receipt of unnecessary follow-up procedures, which can incur significant costs to the patient and the health system.13-19 These costs may be particularly burdensome in safety net health systems (ie, those that offer access to care regardless of the patient’s ability to pay), which serve a large proportion of socioeconomically disadvantaged individuals in the United States.20,21 To our knowledge, no published study to date has specifically investigated the role of FIT in acute patient management.
This study characterizes the use of FIT in acute patient care within a large, urban, safety net health care system. Through a retrospective review of administrative data and patient charts, we evaluated FIT use prevalence, indications, and patient outcomes in the ED and inpatient settings.
Methods
Setting
This study was conducted in a large, urban, county-based integrated delivery system in Houston, Texas, that provides health care services to one of the largest uninsured and underinsured populations in the country.22 The health system includes 2 main hospitals and more than 20 ambulatory care clinics. Within its ambulatory care clinics, the health system implements a population-based screening strategy using stool-based testing. All adults aged 50 years or older who are due for FIT are identified through the health-maintenance module of the electronic medical record (EMR) and offered a take-home FIT. The health system utilizes FIT exclusively (OC-Light S FIT, Polymedco, Cortlandt Manor, NY); no guaiac-based assays are available.
Design and Data Collection
We began by using administrative records to determine the proportion of FITs conducted health system-wide that were ordered and completed in the acute care setting over the study period (August 2016-March 2017). Specifically, we used aggregate quality metric reports, which quantify the number of FITs conducted at each health system clinic and hospital each month, to calculate the proportion of FITs done in the ED and inpatient hospital setting.
We then conducted a retrospective cohort study of 382 adult patients who received FIT in the EDs and inpatient wards in both of the health system’s hospitals over the study period. All data were collected by retrospective chart review in Epic (Madison, WI) EMRs. Sampling was performed by selecting the medical record numbers corresponding to the first 50 completed FITs chronologically each month over the 8-month period, with a total of 400 charts reviewed.
Data collected included basic patient demographics, location of FIT ordering (ED vs inpatient), primary service ordering FIT, FIT indication, FIT result, and receipt and results of invasive diagnostic follow-up. Demographics collected included age, biological sex, race (self-selected), and insurance coverage.
FIT indication was determined based on resident or attending physician notes. The history of present illness, physical exam, and assessment and plan section of notes were reviewed by the lead author for a specific statement of indication for FIT or for evidence of clinical presentation for which FIT could reasonably be ordered. Indications were iteratively reviewed and collapsed into 6 different categories: anemia, iron deficiency with or without anemia, overt GIB, suspected GIB/miscellaneous, non-bloody diarrhea, and no indication identified. Overt GIB was defined as reported or witnessed hematemesis, coffee-ground emesis, hematochezia, bright red blood per rectum, or melena irrespective of time frame (current or remote) or chronicity (acute, subacute, or chronic). In cases where signs of overt bleed were not witnessed by medical professionals, determination of conditions such as melena or coffee-ground emesis were made based on health care providers’ assessment of patient history as documented in his or her notes. Suspected GIB/miscellaneous was defined with the following parameters: any new drop in hemoglobin, abdominal pain, anorectal pain, non-bloody vomiting, hemoptysis, isolated rising blood urea nitrogen, or patient noticing blood on self, clothing, or in the commode without an identified source. Patients who were anemic and found to have iron deficiency on recent lab studies (within 6 months) were reflexively categorized into iron deficiency with or without anemia as opposed to the “anemia” category, which was comprised of any anemia without recent iron studies or non-iron deficient anemia. FIT result was determined by test result entry in Epic, with results either reading positive or negative.
Diagnostic follow-up, for our purposes, was defined as receipt of an invasive procedure or surgery, including esophagogastroduodenoscopy (EGD), colonoscopy, flexible sigmoidoscopy, diagnostic and/or therapeutic abdominal surgical intervention, or any combination of these. Results of diagnostic follow-up were coded as normal or abnormal. A normal result was determined if all procedures performed were listed as normal or as “no pathological findings” on the operative or endoscopic report. Any reported pathologic findings on the operative/endoscopic report were coded as abnormal.
Statistical Analysis
Proportions were used to describe demographic characteristics of patients who received a FIT in acute hospital settings. Bivariable tables and Chi-square tests were used to compare indications and outcomes for FIT-positive and FIT-negative patients. The association between receipt of an invasive diagnostic follow-up (outcome) and the results of an inpatient FIT (predictor) was assessed using multivariable log-binomial regression to calculate risk ratios (RRs) and corresponding 95% confidence intervals. Log-binomial regression was used over logistic regression given that adjusted odds ratios generated by logistic regression often overestimate the association between the risk factor and the outcome when the outcome is common,23 as in the case of diagnostic follow-up. The model was adjusted for variables selected a priori, specifically, age, gender, and FIT indication. Chi-square analysis was used to compare the proportion of abnormal findings on diagnostic follow-up by FIT result (negative vs positive).
Results
During the 8-month study period, there were 2718 FITs ordered and completed in the acute care setting, compared to 44,662 FITs ordered and completed in the outpatient setting (5.7% performed during acute care).
Among the 400 charts reviewed, 7 were excluded from the analysis because they were duplicates from the same patient, and 11 were excluded due to insufficient information in the patient’s medical record, resulting in 382 patients included in the analysis. Patient demographic characteristics are described in Table 1. Patients were predominantly Hispanic/Latino or Black/African American (51.0% and 32.5%, respectively), a majority had insurance through the county health system (50.5%), and most were male (58.1%). The average age of those receiving FIT was 52 years (standard deviation, 14.8 years), with 40.8% being under the age of 50. For a majority of patients, FIT was ordered in the ED by emergency medicine providers (79.8%). The remaining FITs were ordered by providers in 12 different inpatient departments. Of the FITs ordered, 35.1% were positive.
Indications for ordering FIT are listed in Table 2. The largest proportion of FITs were ordered for overt signs of GIB (54.2%), followed by anemia (22.0%), suspected GIB/miscellaneous reasons (12.3%), iron deficiency with or without anemia (7.6%), and non-bloody diarrhea (2.1%). In 1.8% of cases, no indication for FIT was found in the EMR. No FITs were ordered for the indication of CRC detection. Of these indication categories, overt GIB yielded the highest percentage of FIT positive results (44.0%), and non-bloody diarrhea yielded the lowest (0%).
A total of 110 patients (28.7%) underwent FIT and received invasive diagnostic follow-up. Of these 110 patients, 57 (51.8%) underwent EGD (2 of whom had further surgical intervention), 21 (19.1%) underwent colonoscopy (1 of whom had further surgical intervention), 25 (22.7%) underwent dual EGD and colonoscopy, 1 (0.9%) underwent flexible sigmoidoscopy, and 6 (5.5%) directly underwent abdominal surgical intervention. There was a significantly higher rate of diagnostic follow-up for FIT-positive vs FIT-negative patients (42.9% vs 21.3%; P < 0.001). However, of the 110 patients who underwent subsequent diagnostic follow-up, 48.2% were FIT negative. FIT-negative patients who received diagnostic follow-up were just as likely to have an abnormal finding as FIT-positive patients (90.6% vs 91.2%; P = 0.86).
Of the 382 patients in the study, 4 were diagnosed with CRC through diagnostic follow-up (1.0%). Of those 4 patients, 1 was FIT positive.
The results of the multivariable analyses to evaluate predictors of diagnostic colonoscopy are described in Table 3. Variables in the final model were FITresult, age, and FIT indication. After adjusting for other variables in the model, receipt of diagnostic follow-up was significantly associated with having a positive FIT (adjusted RR, 1.72; P < 0.001) and an overt GIB as an indication (adjusted RR, 2.00; P < 0.01).
Discussion
During the time frame of our study, 5.7% of all FITs ordered within our health system were ordered in the acute patient care setting at our hospitals. The most common indication was overt GIB, which was the indication for 54.2% of patients. Of note, none of the FITs ordered in the acute patient care setting were ordered for CRC screening. These findings support the evidence in the literature that stool-based screening tests, including FIT, are commonly used in US health care systems for diagnostic purposes and risk stratification in acute patient care to detect GIBs.13-18
Our data suggest that FIT was not a clinically useful test in determining a patient’s need for diagnostic follow-up. While having a positive FIT was significantly associated with obtaining a diagnostic exam in multivariate analysis (RR, 1.72), having signs of overt GI bleeding was a stronger predictor of diagnostic follow-up (RR, 2.00). This salient finding is evidence that a thorough clinical history and physical exam may more strongly predict whether a patient will undergo endoscopy or other follow-up than a FIT result. These findings support other studies in the literature that have called into question the utility of FOBTs in these acute settings.13-19 Under such circumstances, FOBTs have been shown to rarely influence patient management and thus represent an unnecessary expense.13–17 Additionally, in some cases, FOBT use in these settings may negatively affect patient outcomes. Such adverse effects include delaying treatment until results are returned or obfuscating indicated management with the results (eg, a patient with indications for colonoscopy not being referred due to a negative FOBT).13,14,17
We found that, for patients who subsequently went on to have diagnostic follow-up (most commonly endoscopy), there was no difference in the likelihood of FIT-positive and FIT-negative patients to have an abnormality discovered (91.2% vs 90.6%; P = 0.86). This analysis demonstrates no post-hoc support for FIT positivity as a predictor of presence of pathology in patients who were discriminately selected for diagnostic follow-up on clinical grounds by gastroenterologists and surgeons. It does, however, further support that clinical judgment about the need for diagnostic follow-up—irrespective of FIT result—has a very high yield for discovery of pathology in the acute setting.
There are multiple reasons why FOBTs, and specifically FIT, contribute little in management decisions for patients with suspected GI blood loss. Use of FIT raises concern for both false-negatives and false-positives when used outside of its indication. Regarding false- negatives, FIT is an unreliable test for detection of blood loss from the upper GI tract. As FITs utilize antibodies to detect the presence of globin, a byproduct of red blood cell breakdown, it is expected that FIT would fail to detect many cases of upper GI bleeding, as globin is broken down in the upper GI tract.24 This fact is part of what has made FIT a more effective CRC screening test than its guaiac-based counterparts—it has greater specificity for lower GI tract blood loss compared to tests relying on detection of heme.8 While guaiac-based assays like Hemoccult have also been shown to be poor tests in acute patient care, they may more frequently, though still unreliably, detect blood of upper GI origin. We believe that part of the ongoing use of FIT in patients with a suspected upper GIB may be from lack of understanding among providers on the mechanistic difference between gFOBTs and FITs, even though gFOBTs also yield highly unreliable results.
FIT does not have the same risk of false-positive results that guaiac-based tests have, which can yield positive results with extra-intestinal blood ingestion, aspirin, or alcohol use; insignificant GI bleeding; and consumption of peroxidase-containing foods.13,17,25 However, from a clinical standpoint, there are several scenarios of insignificant bleeding that would yield a positive FIT result, such as hemorrhoids, which are common in the US population.26,27 Additionally, in the ED, where most FITs were performed in our study, it is possible that samples for FITs are being obtained via digital rectal exam (DRE) given patients’ acuity of medical conditions and time constraints. However, FIT has been validated when using a formed stool sample. Obtaining FIT via DRE may lead to microtrauma to the rectum, which could hypothetically yield a positive FIT.
Strengths of this study include its use of in-depth chart data on a large number of FIT-positive patients, which allowed us to discern indications, outcomes, and other clinical data that may have influenced clinical decision-making. Additionally, whereas other studies that address FOBT use in acute patient care have focused on guaiac-based assays, our findings regarding the lack of utility of FIT are novel and have particular relevance as FITs continue to grow in popularity. Nonetheless, there are certain limitations future research should seek to address. In this study, the diagnostic follow-up result was coded by presence or absence of pathologic findings but did not qualify findings by severity or attempt to determine whether the pathology noted on diagnostic follow-up was the definitive source of the suspected GI bleed. These variables could help determine whether there was a difference in severity of bleeding between FIT-positive and FIT-negative patients and could potentially be studied with a prospective research design. Our own study was not designed to address the question of whether FIT result informs patient management decisions. To answer this directly, interviews would have to be conducted with those making the follow-up decision (ie, endoscopists and surgeons). Additionally, this study was not adequately powered to make determinations on the efficacy of FIT in the acute care setting for detection of CRC. As mentioned, only 1 of the 4 patients (25%) who went on to be diagnosed with CRC on follow-up was initially FIT-positive. This would require further investigation.
Conclusion
FIT is being utilized for diagnostic purposes in the acute care of symptomatic patients, which is a misuse of an established screening test for CRC. While our study was not designed to answer whether and how often a FIT result informs subsequent patient management, our results indicate that FIT is an ineffective diagnostic and risk-stratification tool when used in the acute care setting. Our findings add to existing evidence that indicates FOBTs should not be used in acute patient care.
Taken as a whole, the results of our study add to a growing body of evidence demonstrating no role for FOBTs, and specifically FIT, in acute patient care. In light of this evidence, some health care systems have already demonstrated success with system-wide disinvestment from the test in acute patient care settings, with one group publishing about their disinvestment process.28 After completion of our study, our preliminary data were presented to leadership from the internal medicine, emergency medicine, and laboratory divisions within our health care delivery system to galvanize complete disinvestment of FIT from acute care at our hospitals, a policy that was put into effect in July 2019.
Corresponding author: Nathaniel J. Spezia-Lindner, MD, Baylor College of Medicine, 7200 Cambridge St, BCM 903, Ste A10.197, Houston, TX 77030; [email protected].
Financial disclosures: None.
Funding: Cancer Prevention and Research Institute of Texas, CPRIT (PP170094, PDs: ML Jibaja-Weiss and JR Montealegre).
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. 10.1CA Cancer 10.1J Clin. 2020;70(1):7-30.
2. Howlader NN, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975-2014. National Cancer Institute; 2017:1-2.
3. Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States, 1974–2013. 10.1J Natl Cancer Inst. 2017;109(8):djw322.
4. Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. 10.25JAMA Surg. 2015;150(1):17-22.
5. Lin JS, Piper MA, Perdue LA, et al. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. 10.25JAMA. 2016;315(23):2576-2594.
6. Centers for Disease Control and Prevention (CDC). Use of colorectal cancer screening tests. Behavioral Risk Factor Surveillance System. October 22, 2019. Accessed February 10, 2021. https://www.cdc.gov/cancer/colorectal/statistics/use-screening-tests-BRFSS.htm
7. Hewitson P, Glasziou PP, Irwig L, et al. Screening for colorectal cancer using the fecal occult blood test, Hemoccult. 10.25Cochrane Database Syst Rev. 2007;2007(1):CD001216.
8. Bujanda L, Lanas Á, Quintero E, et al. Effect of aspirin and antiplatelet drugs on the outcome of the fecal immunochemical test. 10.25Mayo Clin Proc. 2013;88(7):683-689.
9. Allison JE, Sakoda LC, Levin TR, et al. Screening for colorectal neoplasms with new fecal occult blood tests: update on performance characteristics. 10.25J Natl Cancer Inst. 2007;99(19):1462-1470.
10. Dancourt V, Lejeune C, Lepage C, et al. Immunochemical faecal occult blood tests are superior to guaiac-based tests for the detection of colorectal neoplasms. 10.25Eur J Cancer. 2008;44(15):2254-2258.
11. Hol L, Wilschut JA, van Ballegooijen M, et al. Screening for colorectal cancer: random comparison of guaiac and immunochemical faecal occult blood testing at different cut-off levels. 10.25Br J Cancer. 2009;100(7):1103-1110.
12. Levi Z, Birkenfeld S, Vilkin A, et al. A higher detection rate for colorectal cancer and advanced adenomatous polyp for screening with immunochemical fecal occult blood test than guaiac fecal occult blood test, despite lower compliance rate. A prospective, controlled, feasibility study. Int J Cancer. 2011;128(10):2415-2424.
13. Friedman A, Chan A, Chin LC, et al. Use and abuse of faecal occult blood tests in an acute hospital inpatient setting. Intern Med J. 2010;40(2):107-111.
14. Narula N, Ulic D, Al-Dabbagh R, et al. Fecal occult blood testing as a diagnostic test in symptomatic patients is not useful: a retrospective chart review. Can J Gastroenterol Hepatol. 2014;28(8):421-426.
15. Ip S, Sokoro AA, Kaita L, et al. Use of fecal occult blood testing in hospitalized patients: results of an audit. Can J Gastroenterol Hepatol. 2014;28(9):489-494.
16. Mosadeghi S, Ren H, Catungal J, et al. Utilization of fecal occult blood test in the acute hospital setting and its impact on clinical management and outcomes. J Postgrad Med. 2016;62(2):91-95.
17. van Rijn AF, Stroobants AK, Deutekom M, et al. Inappropriate use of the faecal occult blood test in a university hospital in the Netherlands. Eur J Gastroenterol Hepatol. 2012;24(11):1266-1269.
18. Sharma VK, Komanduri S, Nayyar S, et al. An audit of the utility of in-patient fecal occult blood testing. Am J Gastroenterol. 2001;96(4):1256-1260.
19. Chiang TH, Lee YC, Tu CH, et al. Performance of the immunochemical fecal occult blood test in predicting lesions in the lower gastrointestinal tract. CMAJ. 2011;183(13):1474-1481.
20. Chokshi DA, Chang JE, Wilson RM. Health reform and the changing safety net in the United States. N Engl J Med. 2016;375(18):1790-1796.
21. Nguyen OK, Makam AN, Halm EA. National use of safety net clinics for primary care among adults with non-Medicaid insurance in the United States. PLoS One. 2016;11(3):e0151610.
22. United States Census Bureau. American Community Survey. Selected Economic Characteristics. 2019. Accessed February 20, 2021. https://data.census.gov/cedsci/table?q=ACSDP1Y2019.DP03%20Texas&g=0400000US48&tid=ACSDP1Y2019.DP03&hidePreview=true
23. McNutt LA, Wu C, Xue X, et al. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157(10):940-943.
24. Rockey DC. Occult gastrointestinal bleeding. Gastroenterol Clin North Am. 2005;34(4):699-718.
25. Macrae FA, St John DJ. Relationship between patterns of bleeding and Hemoccult sensitivity in patients with colorectal cancers or adenomas. Gastroenterology. 1982;82(5 pt 1):891-898.
26. Johanson JF, Sonnenberg A. The prevalence of hemorrhoids and chronic constipation: an epidemiologic study. Gastroenterology. 1990;98(2):380-386.
27. Fleming JL, Ahlquist DA, McGill DB, et al. Influence of aspirin and ethanol on fecal blood levels as determined by using the HemoQuant assay. Mayo Clin Proc. 1987;62(3):159-163.
28. Gupta A, Tang Z, Agrawal D. Eliminating in-hospital fecal occult blood testing: our experience with disinvestment. Am J Med. 2018;131(7):760-763.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. 10.1CA Cancer 10.1J Clin. 2020;70(1):7-30.
2. Howlader NN, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975-2014. National Cancer Institute; 2017:1-2.
3. Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States, 1974–2013. 10.1J Natl Cancer Inst. 2017;109(8):djw322.
4. Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. 10.25JAMA Surg. 2015;150(1):17-22.
5. Lin JS, Piper MA, Perdue LA, et al. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. 10.25JAMA. 2016;315(23):2576-2594.
6. Centers for Disease Control and Prevention (CDC). Use of colorectal cancer screening tests. Behavioral Risk Factor Surveillance System. October 22, 2019. Accessed February 10, 2021. https://www.cdc.gov/cancer/colorectal/statistics/use-screening-tests-BRFSS.htm
7. Hewitson P, Glasziou PP, Irwig L, et al. Screening for colorectal cancer using the fecal occult blood test, Hemoccult. 10.25Cochrane Database Syst Rev. 2007;2007(1):CD001216.
8. Bujanda L, Lanas Á, Quintero E, et al. Effect of aspirin and antiplatelet drugs on the outcome of the fecal immunochemical test. 10.25Mayo Clin Proc. 2013;88(7):683-689.
9. Allison JE, Sakoda LC, Levin TR, et al. Screening for colorectal neoplasms with new fecal occult blood tests: update on performance characteristics. 10.25J Natl Cancer Inst. 2007;99(19):1462-1470.
10. Dancourt V, Lejeune C, Lepage C, et al. Immunochemical faecal occult blood tests are superior to guaiac-based tests for the detection of colorectal neoplasms. 10.25Eur J Cancer. 2008;44(15):2254-2258.
11. Hol L, Wilschut JA, van Ballegooijen M, et al. Screening for colorectal cancer: random comparison of guaiac and immunochemical faecal occult blood testing at different cut-off levels. 10.25Br J Cancer. 2009;100(7):1103-1110.
12. Levi Z, Birkenfeld S, Vilkin A, et al. A higher detection rate for colorectal cancer and advanced adenomatous polyp for screening with immunochemical fecal occult blood test than guaiac fecal occult blood test, despite lower compliance rate. A prospective, controlled, feasibility study. Int J Cancer. 2011;128(10):2415-2424.
13. Friedman A, Chan A, Chin LC, et al. Use and abuse of faecal occult blood tests in an acute hospital inpatient setting. Intern Med J. 2010;40(2):107-111.
14. Narula N, Ulic D, Al-Dabbagh R, et al. Fecal occult blood testing as a diagnostic test in symptomatic patients is not useful: a retrospective chart review. Can J Gastroenterol Hepatol. 2014;28(8):421-426.
15. Ip S, Sokoro AA, Kaita L, et al. Use of fecal occult blood testing in hospitalized patients: results of an audit. Can J Gastroenterol Hepatol. 2014;28(9):489-494.
16. Mosadeghi S, Ren H, Catungal J, et al. Utilization of fecal occult blood test in the acute hospital setting and its impact on clinical management and outcomes. J Postgrad Med. 2016;62(2):91-95.
17. van Rijn AF, Stroobants AK, Deutekom M, et al. Inappropriate use of the faecal occult blood test in a university hospital in the Netherlands. Eur J Gastroenterol Hepatol. 2012;24(11):1266-1269.
18. Sharma VK, Komanduri S, Nayyar S, et al. An audit of the utility of in-patient fecal occult blood testing. Am J Gastroenterol. 2001;96(4):1256-1260.
19. Chiang TH, Lee YC, Tu CH, et al. Performance of the immunochemical fecal occult blood test in predicting lesions in the lower gastrointestinal tract. CMAJ. 2011;183(13):1474-1481.
20. Chokshi DA, Chang JE, Wilson RM. Health reform and the changing safety net in the United States. N Engl J Med. 2016;375(18):1790-1796.
21. Nguyen OK, Makam AN, Halm EA. National use of safety net clinics for primary care among adults with non-Medicaid insurance in the United States. PLoS One. 2016;11(3):e0151610.
22. United States Census Bureau. American Community Survey. Selected Economic Characteristics. 2019. Accessed February 20, 2021. https://data.census.gov/cedsci/table?q=ACSDP1Y2019.DP03%20Texas&g=0400000US48&tid=ACSDP1Y2019.DP03&hidePreview=true
23. McNutt LA, Wu C, Xue X, et al. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157(10):940-943.
24. Rockey DC. Occult gastrointestinal bleeding. Gastroenterol Clin North Am. 2005;34(4):699-718.
25. Macrae FA, St John DJ. Relationship between patterns of bleeding and Hemoccult sensitivity in patients with colorectal cancers or adenomas. Gastroenterology. 1982;82(5 pt 1):891-898.
26. Johanson JF, Sonnenberg A. The prevalence of hemorrhoids and chronic constipation: an epidemiologic study. Gastroenterology. 1990;98(2):380-386.
27. Fleming JL, Ahlquist DA, McGill DB, et al. Influence of aspirin and ethanol on fecal blood levels as determined by using the HemoQuant assay. Mayo Clin Proc. 1987;62(3):159-163.
28. Gupta A, Tang Z, Agrawal D. Eliminating in-hospital fecal occult blood testing: our experience with disinvestment. Am J Med. 2018;131(7):760-763.
COVID-19 Screening and Testing Among Patients With Neurologic Dysfunction: The Neuro-COVID-19 Time-out Process and Checklist
From the University of Mississippi Medical Center, Department of Neurology, Division of Neuroscience Intensive Care, Jackson, MS.
Abstract
Objective: To test a coronavirus disease 2019 (COVID-19) screening tool to identify patients who qualify for testing among patients with neurologic dysfunction who are unable to answer the usual screening questions, which could help to prevent unprotected exposure of patients and health care workers to COVID-19.
Methods: The Neuro-COVID-19 Time-out Process and Checklist (NCOT-PC) was implemented at our institution for 1 week as a quality improvement project to improve the pathway for COVID-19 screening and testing among patients with neurologic dysfunction.
Results: A total of 14 new patients were admitted into the neuroscience intensive care unit (NSICU) service during the pilot period. The NCOT-PC was utilized on 9 (64%) patients with neurologic dysfunction; 7 of these patients were found to have a likelihood of requiring testing based on the NCOT-PC and were subsequently screened for COVID-19 testing by contacting the institution’s COVID-19 testing hotline (Med-Com). All these patients were subsequently transitioned into person-under-investigation status based on the determination from Med-Com. The NSICU staff involved were able to utilize NCOT-PC without issues. The NCOT-PC was immediately adopted into the NSICU process.
Conclusion: Use of the NCOT-PC tool was found to be feasible and improved the screening methodology of patients with neurologic dysfunction.
Keywords: coronavirus; health care planning; quality improvement; patient safety; medical decision-making; neuroscience intensive care unit.
The coronavirus disease 2019 (COVID-19) pandemic has altered various standard emergent care pathways. Current recommendations regarding COVID-19 screening for testing involve asking patients about their symptoms, including fever, cough, chest pain, and dyspnea.1 This standard screening method poses a problem when caring for patients with neurologic dysfunction. COVID-19 patients may pre-sent with conditions that affect their ability to answer questions, such as stroke, encephalitis, neuromuscular disorders, or headache, and that may preclude the use of standard screening for testing.2 Patients with acute neurologic dysfunction who cannot undergo standard screening may leave the emergency department (ED) and transition into the neuroscience intensive care unit (NSICU) or any intensive care unit (ICU) without a reliable COVID-19 screening test.
The Protected Code Stroke pathway offers protection in the emergent setting for patients with stroke when their COVID-19 status is unknown.3 A similar process has been applied at our institution for emergent management of patients with cerebrovascular disease (stroke, intracerebral hemorrhage, and subarachnoid hemorrhage). However, the process from the ED after designating “difficult to screen” patients as persons under investigation (PUI) is unclear. The Centers for Disease Control and Prevention (CDC) has delineated the priorities for testing, with not all declared PUIs requiring testing.4 This poses a great challenge, because patients designated as PUIs require the same management as a COVID-19-positive patient, with negative-pressure isolation rooms as well as use of protective personal equipment (PPE), which may not be readily available. It was also recognized that, because the ED staff can be overwhelmed by COVID-19 patients, there may not be enough time to perform detailed screening of patients with neurologic dysfunction and that “reverse masking” may not be done consistently for nonintubated patients. This may place patients and health care workers at risk of unprotected exposure.
Recognizing these challenges, we created a Neuro-COVID-19 Time-out Process and Checklist (NCOT-PC) as a quality improvement project. The aim of this project was to improve and standardize the current process of identifying patients with neurologic dysfunction who require COVID-19 testing to decrease the risk of unprotected exposure of patients and health care workers.
Methods
Patients and Definitions
This quality improvement project was undertaken at the University of Mississippi Medical Center NSICU. Because this was a quality improvement project, an Institutional Review Board exemption was granted.
The NCOT-PC was utilized in consecutive patients with neurologic dysfunction admitted to the NSICU during a period of 1 week. “Neurologic dysfunction” encompasses any neurologic illness affecting the mental status and/or level of alertness, subsequently precluding the ability to reliably screen the patient utilizing standard COVID-19 screening. “Med-Com” at our institution is the equivalent of the national COVID-19 testing hotline, where our institution’s infectious diseases experts screen calls for testing and determine whether testing is warranted. “Unprotected exposure” means exposure to COVID-19 without adequate and appropriate PPE.
Quality Improvement Process
As more PUIs were being admitted to the institution, we used the Plan-Do-Study-Act method for process improvements in the NSICU.5 NSICU stakeholders, including attendings, the nurse manager, and nurse practitioners (NPs), developed an algorithm to facilitate the coordination of the NSICU staff in screening patients to identify those with a high likelihood of needing COVID-19 testing upon arrival in the NSICU (Figure 1). Once the NCOT-PC was finalized, NSICU stakeholders were educated regarding the use of this screening tool.
The checklist clinicians review when screening patients is shown in Figure 2. The risk factors comprising the checklist include patient history and clinical and radiographic characteristics that have been shown to be relevant for identifying patients with COVID-19.6,7 The imaging criteria utilize imaging that is part of the standard of care for NSICU patients. For example, computed tomography angiogram of the head and neck performed as part of the acute stroke protocol captures the upper part of the chest. These images are utilized for their incidental findings, such as apical ground-glass opacities and tree-in-bud formation. The risk factors applicable to the patient determine whether the clinician will call Med-Com for testing approval. Institutional COVID-19 processes were then followed accordingly.8 The decision from Med-Com was considered final, and no deviation from institutional policies was allowed.
NCOT-PC was utilized for consecutive days for 1 week before re-evaluation of its feasibility and adaptability.
Data Collection and Analysis
Consecutive patients with neurologic dysfunction admitted into the NSICU were assigned nonlinkable patient numbers. No identifiers were collected for the purpose of this project. The primary diagnosis for admission, the neurologic dysfunction that precluded standard screening, and checklist components that the patient fulfilled were collected.
To assess the tool’s feasibility, feedback regarding the ease of use of the NCOT-PC was gathered from the nurses, NPs, charge nurses, fellows, and other attendings. To assess the utility of the NCOT-PC in identifying patients who will be approved for COVID-19 testing, we calculated the proportion of patients who were deemed to have a high likelihood of testing and the proportion of patients who were approved for testing. Descriptive statistics were used, as applicable for the project, to summarize the utility of the NCOT-PC.
Results
We found that the NCOT-PC can be easily used by clinicians. The NSICU staff did not communicate any implementation issues, and since the NCOT-PC was implemented, no problems have been identified.
During the pilot period of the NCOT-PC, 14 new patients were admitted to the NSICU service. Nine (64%) of these had neurologic dysfunction, and the NCOT-PC was used to determine whether Med-Com should be called based on the patients’ likelihood (high vs low) of needing a COVID-19 test. Of those patients with neurologic dysfunction, 7 (78%) were deemed to have a high likelihood of needing a COVID-19 test based on the NCOT-PC. Med-Com was contacted regarding these patients, and all were deemed to require the COVID-19 test by Med-Com and were transitioned into PUI status per institutional policy (Table).
Discussion
The NCOT-PC project improved and standardized the process of identifying and screening patients with neurologic dysfunction for COVID-19 testing. The screening tool is feasible to use, and it decreased inadvertent unprotected exposure of patients and health care workers.
The NCOT-PC was easy to administer. Educating the staff regarding the new process took only a few minutes and involved a meeting with the nurse manager, NPs, fellows, residents, and attendings. We found that this process works well in tandem with the standard institutional processes in place in terms of Protected Code Stroke pathway, PUI isolation, PPE use, and Med-Com screening for COVID-19 testing. Med-Com was called only if the patient fulfilled the checklist criteria. In addition, no extra cost was attributed to implementing the NCOT-PC, since we utilized imaging that was already done as part of the standard of care for patients with neurologic dysfunction.
The standardization of the process of screening for COVID-19 testing among patients with neurologic dysfunction improved patient selection. Before the NCOT-PC, there was no consistency in terms of who should get tested and the reason for testing patients with neurologic dysfunction. Patients can pass through the ED and arrive in the NSICU with an unclear screening status, which may cause inadvertent patient and health care worker exposure to COVID-19. With the NCOT-PC, we have avoided instances of inadvertent staff or patient exposure in the NSICU.
The NCOT-PC was adopted into the NSICU process after the first week it was piloted. Beyond the NSICU, the application of the NCOT-PC can be extended to any patient presentation that precludes standard screening, such as ED and interhospital transfers for stroke codes, trauma codes, code blue, or myocardial infarction codes. In our department, as we started the process of PCS for stroke codes, we included NCOT-PC for stroke patients with neurologic dysfunction.
The results of our initiative are largely limited by the decision-making process of Med-Com when patients are called in for testing. At the time of our project, there were no specific criteria used for patients with altered mental status, except for the standard screening methods, and it was through clinician-to-clinician discussion that testing decisions were made. Another limitation is the short period of time that the NCOT-PC was applied before adoption.
In summary, the NCOT-PC tool improved the screening process for COVID-19 testing in patients with neurologic dysfunction admitted to the NSICU. It was feasible and prevented unprotected staff and patient exposure to COVID-19. The NCOT-PC functionality was compatible with institutional COVID-19 policies in place, which contributed to its overall sustainability.
The Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) were utilized in preparing this manuscript.9
Acknowledgment: The authors thank the University of Mississippi Medical Center NSICU staff for their input with implementation of the NCOT-PC.
Corresponding author: Prashant A. Natteru, MD, University of Mississippi Medical Center, Department of Neurology, 2500 North State St., Jackson, MS 39216; [email protected].
Financial disclosures: None.
1. Coronavirus disease 2019 (COVID-19) Symptoms. www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. Accessed April 9, 2020.
2. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:1-9.
3. Khosravani H, Rajendram P, Notario L, et al. Protected code stroke: hyperacute stroke management during the coronavirus disease 2019. (COVID-19) pandemic. Stroke. 2020;51:1891-1895.
4. Coronavirus disease 2019 (COVID-19) evaluation and testing. www.cdc.gov/coronavirus/2019-nCoV/hcp/clinical-criteria.html. Accessed April 9, 2020.
5. Plan-Do-Study-Act Worksheet. Institute for Healthcare Improvement website. www.ihi.org/resources/Pages/Tools/PlanDoStudyActWorksheet.aspx. Accessed March 31,2020.
6. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;10.1002/jmv.25728.
7. Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis. 2020;101623.
8. UMMC’s COVID-19 Clinical Processes. www.umc.edu/CoronaVirus/Mississippi-Health-Care-Professionals/Clinical-Resources/Clinical-Resources.html. Accessed April 9, 2020.
9. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): Revised Publication Guidelines from a Detailed Consensus Process. The EQUATOR Network. www.equator-network.org/reporting-guidelines/squire/. Accessed May 12, 2020.
From the University of Mississippi Medical Center, Department of Neurology, Division of Neuroscience Intensive Care, Jackson, MS.
Abstract
Objective: To test a coronavirus disease 2019 (COVID-19) screening tool to identify patients who qualify for testing among patients with neurologic dysfunction who are unable to answer the usual screening questions, which could help to prevent unprotected exposure of patients and health care workers to COVID-19.
Methods: The Neuro-COVID-19 Time-out Process and Checklist (NCOT-PC) was implemented at our institution for 1 week as a quality improvement project to improve the pathway for COVID-19 screening and testing among patients with neurologic dysfunction.
Results: A total of 14 new patients were admitted into the neuroscience intensive care unit (NSICU) service during the pilot period. The NCOT-PC was utilized on 9 (64%) patients with neurologic dysfunction; 7 of these patients were found to have a likelihood of requiring testing based on the NCOT-PC and were subsequently screened for COVID-19 testing by contacting the institution’s COVID-19 testing hotline (Med-Com). All these patients were subsequently transitioned into person-under-investigation status based on the determination from Med-Com. The NSICU staff involved were able to utilize NCOT-PC without issues. The NCOT-PC was immediately adopted into the NSICU process.
Conclusion: Use of the NCOT-PC tool was found to be feasible and improved the screening methodology of patients with neurologic dysfunction.
Keywords: coronavirus; health care planning; quality improvement; patient safety; medical decision-making; neuroscience intensive care unit.
The coronavirus disease 2019 (COVID-19) pandemic has altered various standard emergent care pathways. Current recommendations regarding COVID-19 screening for testing involve asking patients about their symptoms, including fever, cough, chest pain, and dyspnea.1 This standard screening method poses a problem when caring for patients with neurologic dysfunction. COVID-19 patients may pre-sent with conditions that affect their ability to answer questions, such as stroke, encephalitis, neuromuscular disorders, or headache, and that may preclude the use of standard screening for testing.2 Patients with acute neurologic dysfunction who cannot undergo standard screening may leave the emergency department (ED) and transition into the neuroscience intensive care unit (NSICU) or any intensive care unit (ICU) without a reliable COVID-19 screening test.
The Protected Code Stroke pathway offers protection in the emergent setting for patients with stroke when their COVID-19 status is unknown.3 A similar process has been applied at our institution for emergent management of patients with cerebrovascular disease (stroke, intracerebral hemorrhage, and subarachnoid hemorrhage). However, the process from the ED after designating “difficult to screen” patients as persons under investigation (PUI) is unclear. The Centers for Disease Control and Prevention (CDC) has delineated the priorities for testing, with not all declared PUIs requiring testing.4 This poses a great challenge, because patients designated as PUIs require the same management as a COVID-19-positive patient, with negative-pressure isolation rooms as well as use of protective personal equipment (PPE), which may not be readily available. It was also recognized that, because the ED staff can be overwhelmed by COVID-19 patients, there may not be enough time to perform detailed screening of patients with neurologic dysfunction and that “reverse masking” may not be done consistently for nonintubated patients. This may place patients and health care workers at risk of unprotected exposure.
Recognizing these challenges, we created a Neuro-COVID-19 Time-out Process and Checklist (NCOT-PC) as a quality improvement project. The aim of this project was to improve and standardize the current process of identifying patients with neurologic dysfunction who require COVID-19 testing to decrease the risk of unprotected exposure of patients and health care workers.
Methods
Patients and Definitions
This quality improvement project was undertaken at the University of Mississippi Medical Center NSICU. Because this was a quality improvement project, an Institutional Review Board exemption was granted.
The NCOT-PC was utilized in consecutive patients with neurologic dysfunction admitted to the NSICU during a period of 1 week. “Neurologic dysfunction” encompasses any neurologic illness affecting the mental status and/or level of alertness, subsequently precluding the ability to reliably screen the patient utilizing standard COVID-19 screening. “Med-Com” at our institution is the equivalent of the national COVID-19 testing hotline, where our institution’s infectious diseases experts screen calls for testing and determine whether testing is warranted. “Unprotected exposure” means exposure to COVID-19 without adequate and appropriate PPE.
Quality Improvement Process
As more PUIs were being admitted to the institution, we used the Plan-Do-Study-Act method for process improvements in the NSICU.5 NSICU stakeholders, including attendings, the nurse manager, and nurse practitioners (NPs), developed an algorithm to facilitate the coordination of the NSICU staff in screening patients to identify those with a high likelihood of needing COVID-19 testing upon arrival in the NSICU (Figure 1). Once the NCOT-PC was finalized, NSICU stakeholders were educated regarding the use of this screening tool.
The checklist clinicians review when screening patients is shown in Figure 2. The risk factors comprising the checklist include patient history and clinical and radiographic characteristics that have been shown to be relevant for identifying patients with COVID-19.6,7 The imaging criteria utilize imaging that is part of the standard of care for NSICU patients. For example, computed tomography angiogram of the head and neck performed as part of the acute stroke protocol captures the upper part of the chest. These images are utilized for their incidental findings, such as apical ground-glass opacities and tree-in-bud formation. The risk factors applicable to the patient determine whether the clinician will call Med-Com for testing approval. Institutional COVID-19 processes were then followed accordingly.8 The decision from Med-Com was considered final, and no deviation from institutional policies was allowed.
NCOT-PC was utilized for consecutive days for 1 week before re-evaluation of its feasibility and adaptability.
Data Collection and Analysis
Consecutive patients with neurologic dysfunction admitted into the NSICU were assigned nonlinkable patient numbers. No identifiers were collected for the purpose of this project. The primary diagnosis for admission, the neurologic dysfunction that precluded standard screening, and checklist components that the patient fulfilled were collected.
To assess the tool’s feasibility, feedback regarding the ease of use of the NCOT-PC was gathered from the nurses, NPs, charge nurses, fellows, and other attendings. To assess the utility of the NCOT-PC in identifying patients who will be approved for COVID-19 testing, we calculated the proportion of patients who were deemed to have a high likelihood of testing and the proportion of patients who were approved for testing. Descriptive statistics were used, as applicable for the project, to summarize the utility of the NCOT-PC.
Results
We found that the NCOT-PC can be easily used by clinicians. The NSICU staff did not communicate any implementation issues, and since the NCOT-PC was implemented, no problems have been identified.
During the pilot period of the NCOT-PC, 14 new patients were admitted to the NSICU service. Nine (64%) of these had neurologic dysfunction, and the NCOT-PC was used to determine whether Med-Com should be called based on the patients’ likelihood (high vs low) of needing a COVID-19 test. Of those patients with neurologic dysfunction, 7 (78%) were deemed to have a high likelihood of needing a COVID-19 test based on the NCOT-PC. Med-Com was contacted regarding these patients, and all were deemed to require the COVID-19 test by Med-Com and were transitioned into PUI status per institutional policy (Table).
Discussion
The NCOT-PC project improved and standardized the process of identifying and screening patients with neurologic dysfunction for COVID-19 testing. The screening tool is feasible to use, and it decreased inadvertent unprotected exposure of patients and health care workers.
The NCOT-PC was easy to administer. Educating the staff regarding the new process took only a few minutes and involved a meeting with the nurse manager, NPs, fellows, residents, and attendings. We found that this process works well in tandem with the standard institutional processes in place in terms of Protected Code Stroke pathway, PUI isolation, PPE use, and Med-Com screening for COVID-19 testing. Med-Com was called only if the patient fulfilled the checklist criteria. In addition, no extra cost was attributed to implementing the NCOT-PC, since we utilized imaging that was already done as part of the standard of care for patients with neurologic dysfunction.
The standardization of the process of screening for COVID-19 testing among patients with neurologic dysfunction improved patient selection. Before the NCOT-PC, there was no consistency in terms of who should get tested and the reason for testing patients with neurologic dysfunction. Patients can pass through the ED and arrive in the NSICU with an unclear screening status, which may cause inadvertent patient and health care worker exposure to COVID-19. With the NCOT-PC, we have avoided instances of inadvertent staff or patient exposure in the NSICU.
The NCOT-PC was adopted into the NSICU process after the first week it was piloted. Beyond the NSICU, the application of the NCOT-PC can be extended to any patient presentation that precludes standard screening, such as ED and interhospital transfers for stroke codes, trauma codes, code blue, or myocardial infarction codes. In our department, as we started the process of PCS for stroke codes, we included NCOT-PC for stroke patients with neurologic dysfunction.
The results of our initiative are largely limited by the decision-making process of Med-Com when patients are called in for testing. At the time of our project, there were no specific criteria used for patients with altered mental status, except for the standard screening methods, and it was through clinician-to-clinician discussion that testing decisions were made. Another limitation is the short period of time that the NCOT-PC was applied before adoption.
In summary, the NCOT-PC tool improved the screening process for COVID-19 testing in patients with neurologic dysfunction admitted to the NSICU. It was feasible and prevented unprotected staff and patient exposure to COVID-19. The NCOT-PC functionality was compatible with institutional COVID-19 policies in place, which contributed to its overall sustainability.
The Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) were utilized in preparing this manuscript.9
Acknowledgment: The authors thank the University of Mississippi Medical Center NSICU staff for their input with implementation of the NCOT-PC.
Corresponding author: Prashant A. Natteru, MD, University of Mississippi Medical Center, Department of Neurology, 2500 North State St., Jackson, MS 39216; [email protected].
Financial disclosures: None.
From the University of Mississippi Medical Center, Department of Neurology, Division of Neuroscience Intensive Care, Jackson, MS.
Abstract
Objective: To test a coronavirus disease 2019 (COVID-19) screening tool to identify patients who qualify for testing among patients with neurologic dysfunction who are unable to answer the usual screening questions, which could help to prevent unprotected exposure of patients and health care workers to COVID-19.
Methods: The Neuro-COVID-19 Time-out Process and Checklist (NCOT-PC) was implemented at our institution for 1 week as a quality improvement project to improve the pathway for COVID-19 screening and testing among patients with neurologic dysfunction.
Results: A total of 14 new patients were admitted into the neuroscience intensive care unit (NSICU) service during the pilot period. The NCOT-PC was utilized on 9 (64%) patients with neurologic dysfunction; 7 of these patients were found to have a likelihood of requiring testing based on the NCOT-PC and were subsequently screened for COVID-19 testing by contacting the institution’s COVID-19 testing hotline (Med-Com). All these patients were subsequently transitioned into person-under-investigation status based on the determination from Med-Com. The NSICU staff involved were able to utilize NCOT-PC without issues. The NCOT-PC was immediately adopted into the NSICU process.
Conclusion: Use of the NCOT-PC tool was found to be feasible and improved the screening methodology of patients with neurologic dysfunction.
Keywords: coronavirus; health care planning; quality improvement; patient safety; medical decision-making; neuroscience intensive care unit.
The coronavirus disease 2019 (COVID-19) pandemic has altered various standard emergent care pathways. Current recommendations regarding COVID-19 screening for testing involve asking patients about their symptoms, including fever, cough, chest pain, and dyspnea.1 This standard screening method poses a problem when caring for patients with neurologic dysfunction. COVID-19 patients may pre-sent with conditions that affect their ability to answer questions, such as stroke, encephalitis, neuromuscular disorders, or headache, and that may preclude the use of standard screening for testing.2 Patients with acute neurologic dysfunction who cannot undergo standard screening may leave the emergency department (ED) and transition into the neuroscience intensive care unit (NSICU) or any intensive care unit (ICU) without a reliable COVID-19 screening test.
The Protected Code Stroke pathway offers protection in the emergent setting for patients with stroke when their COVID-19 status is unknown.3 A similar process has been applied at our institution for emergent management of patients with cerebrovascular disease (stroke, intracerebral hemorrhage, and subarachnoid hemorrhage). However, the process from the ED after designating “difficult to screen” patients as persons under investigation (PUI) is unclear. The Centers for Disease Control and Prevention (CDC) has delineated the priorities for testing, with not all declared PUIs requiring testing.4 This poses a great challenge, because patients designated as PUIs require the same management as a COVID-19-positive patient, with negative-pressure isolation rooms as well as use of protective personal equipment (PPE), which may not be readily available. It was also recognized that, because the ED staff can be overwhelmed by COVID-19 patients, there may not be enough time to perform detailed screening of patients with neurologic dysfunction and that “reverse masking” may not be done consistently for nonintubated patients. This may place patients and health care workers at risk of unprotected exposure.
Recognizing these challenges, we created a Neuro-COVID-19 Time-out Process and Checklist (NCOT-PC) as a quality improvement project. The aim of this project was to improve and standardize the current process of identifying patients with neurologic dysfunction who require COVID-19 testing to decrease the risk of unprotected exposure of patients and health care workers.
Methods
Patients and Definitions
This quality improvement project was undertaken at the University of Mississippi Medical Center NSICU. Because this was a quality improvement project, an Institutional Review Board exemption was granted.
The NCOT-PC was utilized in consecutive patients with neurologic dysfunction admitted to the NSICU during a period of 1 week. “Neurologic dysfunction” encompasses any neurologic illness affecting the mental status and/or level of alertness, subsequently precluding the ability to reliably screen the patient utilizing standard COVID-19 screening. “Med-Com” at our institution is the equivalent of the national COVID-19 testing hotline, where our institution’s infectious diseases experts screen calls for testing and determine whether testing is warranted. “Unprotected exposure” means exposure to COVID-19 without adequate and appropriate PPE.
Quality Improvement Process
As more PUIs were being admitted to the institution, we used the Plan-Do-Study-Act method for process improvements in the NSICU.5 NSICU stakeholders, including attendings, the nurse manager, and nurse practitioners (NPs), developed an algorithm to facilitate the coordination of the NSICU staff in screening patients to identify those with a high likelihood of needing COVID-19 testing upon arrival in the NSICU (Figure 1). Once the NCOT-PC was finalized, NSICU stakeholders were educated regarding the use of this screening tool.
The checklist clinicians review when screening patients is shown in Figure 2. The risk factors comprising the checklist include patient history and clinical and radiographic characteristics that have been shown to be relevant for identifying patients with COVID-19.6,7 The imaging criteria utilize imaging that is part of the standard of care for NSICU patients. For example, computed tomography angiogram of the head and neck performed as part of the acute stroke protocol captures the upper part of the chest. These images are utilized for their incidental findings, such as apical ground-glass opacities and tree-in-bud formation. The risk factors applicable to the patient determine whether the clinician will call Med-Com for testing approval. Institutional COVID-19 processes were then followed accordingly.8 The decision from Med-Com was considered final, and no deviation from institutional policies was allowed.
NCOT-PC was utilized for consecutive days for 1 week before re-evaluation of its feasibility and adaptability.
Data Collection and Analysis
Consecutive patients with neurologic dysfunction admitted into the NSICU were assigned nonlinkable patient numbers. No identifiers were collected for the purpose of this project. The primary diagnosis for admission, the neurologic dysfunction that precluded standard screening, and checklist components that the patient fulfilled were collected.
To assess the tool’s feasibility, feedback regarding the ease of use of the NCOT-PC was gathered from the nurses, NPs, charge nurses, fellows, and other attendings. To assess the utility of the NCOT-PC in identifying patients who will be approved for COVID-19 testing, we calculated the proportion of patients who were deemed to have a high likelihood of testing and the proportion of patients who were approved for testing. Descriptive statistics were used, as applicable for the project, to summarize the utility of the NCOT-PC.
Results
We found that the NCOT-PC can be easily used by clinicians. The NSICU staff did not communicate any implementation issues, and since the NCOT-PC was implemented, no problems have been identified.
During the pilot period of the NCOT-PC, 14 new patients were admitted to the NSICU service. Nine (64%) of these had neurologic dysfunction, and the NCOT-PC was used to determine whether Med-Com should be called based on the patients’ likelihood (high vs low) of needing a COVID-19 test. Of those patients with neurologic dysfunction, 7 (78%) were deemed to have a high likelihood of needing a COVID-19 test based on the NCOT-PC. Med-Com was contacted regarding these patients, and all were deemed to require the COVID-19 test by Med-Com and were transitioned into PUI status per institutional policy (Table).
Discussion
The NCOT-PC project improved and standardized the process of identifying and screening patients with neurologic dysfunction for COVID-19 testing. The screening tool is feasible to use, and it decreased inadvertent unprotected exposure of patients and health care workers.
The NCOT-PC was easy to administer. Educating the staff regarding the new process took only a few minutes and involved a meeting with the nurse manager, NPs, fellows, residents, and attendings. We found that this process works well in tandem with the standard institutional processes in place in terms of Protected Code Stroke pathway, PUI isolation, PPE use, and Med-Com screening for COVID-19 testing. Med-Com was called only if the patient fulfilled the checklist criteria. In addition, no extra cost was attributed to implementing the NCOT-PC, since we utilized imaging that was already done as part of the standard of care for patients with neurologic dysfunction.
The standardization of the process of screening for COVID-19 testing among patients with neurologic dysfunction improved patient selection. Before the NCOT-PC, there was no consistency in terms of who should get tested and the reason for testing patients with neurologic dysfunction. Patients can pass through the ED and arrive in the NSICU with an unclear screening status, which may cause inadvertent patient and health care worker exposure to COVID-19. With the NCOT-PC, we have avoided instances of inadvertent staff or patient exposure in the NSICU.
The NCOT-PC was adopted into the NSICU process after the first week it was piloted. Beyond the NSICU, the application of the NCOT-PC can be extended to any patient presentation that precludes standard screening, such as ED and interhospital transfers for stroke codes, trauma codes, code blue, or myocardial infarction codes. In our department, as we started the process of PCS for stroke codes, we included NCOT-PC for stroke patients with neurologic dysfunction.
The results of our initiative are largely limited by the decision-making process of Med-Com when patients are called in for testing. At the time of our project, there were no specific criteria used for patients with altered mental status, except for the standard screening methods, and it was through clinician-to-clinician discussion that testing decisions were made. Another limitation is the short period of time that the NCOT-PC was applied before adoption.
In summary, the NCOT-PC tool improved the screening process for COVID-19 testing in patients with neurologic dysfunction admitted to the NSICU. It was feasible and prevented unprotected staff and patient exposure to COVID-19. The NCOT-PC functionality was compatible with institutional COVID-19 policies in place, which contributed to its overall sustainability.
The Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) were utilized in preparing this manuscript.9
Acknowledgment: The authors thank the University of Mississippi Medical Center NSICU staff for their input with implementation of the NCOT-PC.
Corresponding author: Prashant A. Natteru, MD, University of Mississippi Medical Center, Department of Neurology, 2500 North State St., Jackson, MS 39216; [email protected].
Financial disclosures: None.
1. Coronavirus disease 2019 (COVID-19) Symptoms. www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. Accessed April 9, 2020.
2. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:1-9.
3. Khosravani H, Rajendram P, Notario L, et al. Protected code stroke: hyperacute stroke management during the coronavirus disease 2019. (COVID-19) pandemic. Stroke. 2020;51:1891-1895.
4. Coronavirus disease 2019 (COVID-19) evaluation and testing. www.cdc.gov/coronavirus/2019-nCoV/hcp/clinical-criteria.html. Accessed April 9, 2020.
5. Plan-Do-Study-Act Worksheet. Institute for Healthcare Improvement website. www.ihi.org/resources/Pages/Tools/PlanDoStudyActWorksheet.aspx. Accessed March 31,2020.
6. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;10.1002/jmv.25728.
7. Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis. 2020;101623.
8. UMMC’s COVID-19 Clinical Processes. www.umc.edu/CoronaVirus/Mississippi-Health-Care-Professionals/Clinical-Resources/Clinical-Resources.html. Accessed April 9, 2020.
9. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): Revised Publication Guidelines from a Detailed Consensus Process. The EQUATOR Network. www.equator-network.org/reporting-guidelines/squire/. Accessed May 12, 2020.
1. Coronavirus disease 2019 (COVID-19) Symptoms. www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. Accessed April 9, 2020.
2. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:1-9.
3. Khosravani H, Rajendram P, Notario L, et al. Protected code stroke: hyperacute stroke management during the coronavirus disease 2019. (COVID-19) pandemic. Stroke. 2020;51:1891-1895.
4. Coronavirus disease 2019 (COVID-19) evaluation and testing. www.cdc.gov/coronavirus/2019-nCoV/hcp/clinical-criteria.html. Accessed April 9, 2020.
5. Plan-Do-Study-Act Worksheet. Institute for Healthcare Improvement website. www.ihi.org/resources/Pages/Tools/PlanDoStudyActWorksheet.aspx. Accessed March 31,2020.
6. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;10.1002/jmv.25728.
7. Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis. 2020;101623.
8. UMMC’s COVID-19 Clinical Processes. www.umc.edu/CoronaVirus/Mississippi-Health-Care-Professionals/Clinical-Resources/Clinical-Resources.html. Accessed April 9, 2020.
9. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): Revised Publication Guidelines from a Detailed Consensus Process. The EQUATOR Network. www.equator-network.org/reporting-guidelines/squire/. Accessed May 12, 2020.
“I Really Didn’t Want To Come In”: The Unseen Effects of COVID-19 on Children
The Children’s Hospital of Philadelphia, Philadelphia, PA.
The effects of COVID-19 on children’s health are multifaceted. In comparison to adults, children typically experience far milder physical consequences when infected with the virus. A notable exception is the newly described multisystem inflammatory syndrome associated with COVID-19 (MIS-C), which has proven to be a source of significant morbidity among the children it affects.1 Nevertheless, even those children not infected with COVID-19 have suffered due to the disease. School closures have deprived children of opportunities for social and academic growth and, in some cases, the provision of food, social services, medication administration, and many different therapies. Social distancing rules have limited play among children, which is crucial to their development and mental health. The impact on children who have lost family members, including parents, is monumental. Amidst all of this observable suffering, however, the pandemic poses a less visible threat to the health of children.
It is well documented that concern about exposure to COVID-19 has led many adults to avoid emergency departments (EDs) around the world. We believe parents may be avoiding ED visits for their children for the same reason. In the United States, ED volumes dropped approximately 50% during spring 2020.2 While EDs saw increasing, and at times overwhelming, numbers of patients with COVID-19, the number of patients presenting with other life-threatening medical issues, including heart attacks and strokes, declined.3,4 Data from the National Center for Health Statistics this past spring revealed nationwide increases in deaths due to nonrespiratory causes such as diabetes, heart disease, and stroke.5 ED avoidance and unprecedented lack of access to outpatient care, though with the intent to reduce overall risk, are likely significant contributors to these deaths.
Pediatric patients, especially the most vulnerable, are similarly at risk for deleterious health-related consequences from ED avoidance and from limited access to primary and outpatient specialty care. Data from Europe indicate dramatic drops in pediatric ED (PED) volumes, as well as an increase in the proportion of ED visits leading to hospitalization.6,7 These studies suggest that when patients do ultimately present to the PED, they may be more seriously ill.
At our institution, we have seen many COVID-19-negative patients whose medical care has been negatively influenced by the pandemic. A few months ago, a 1-month-old infant with an underlying health condition presented to the PED in extremis after weeks of progressively worsening feeding issues. The infant had been closely followed by the primary care provider (PCP) and subspecialty team via phone calls, televisits, and some office visits. Both physicians and parents had tried to resolve the feeding issues within the outpatient context, explicitly hoping to avoid potential exposure of this fragile patient to COVID-19 in the hospital. On eventual presentation to the PED, the infant was profoundly dehydrated, with significant electrolyte derangement and an acute abdomen, requiring admission to the intensive care unit. Ultimately, a new diagnosis of Hirschsprung disease was made, and the infant was hospitalized for several weeks for weight gain.
Later this summer, a school-aged child with a history of poorly controlled type 1 diabetes presented to an affiliated community hospital comatose and with Kussmaul respirations. Prior to the pandemic, a school nurse administered the child’s morning insulin. Since school closed, the patient had been responsible for administering this dose of insulin while the parents worked outside the home. Despite close and frequent communication between the patient’s endocrinology team and the family, the patient’s glucose and ketone levels began to rise. The parent administered repeated boluses of insulin at home in an attempt to avoid the perceived exposure risk associated with an ED visit. On presentation to the PED, the patient was profoundly altered, with a pH of 7.0. When transfer to a tertiary care center was recommended, the patient’s parent expressed persistent concerns about COVID-19 exposure in the larger hospital, although ultimately consent to transfer was given.
A third case from this summer provides an example of a different type of patient affected by COVID-19: the neonate whose birth circumstances were altered due to the virus. A 3-day-old, full-term infant presented to the ED with hypothermia after PCP referral. The parents had considered both home birth and hospital delivery earlier in the pregnancy, ultimately opting for home birth due to concerns about COVID-19 exposure in the hospital. The pregnancy and delivery were uncomplicated. The neonate did not receive the first hepatitis B vaccine, erythromycin eye ointment, or vitamin K after delivery. In the first 3 days of life, the patient had voided once and stooled once per day. The patient’s mother, inexperienced with breastfeeding and without access to a lactation consultant, was unsure about latch or emptying of her breasts. At the first pediatrician visit, the infant was noted to be hypothermic to 35°C, intermittently bradycardic to the 80s, and with diminished arousal. In the PED, a full sepsis work-up was initiated. Though multiple attempts were made by different providers, only a minimal amount of blood could be drawn, presumably due to dehydration. Of note, the neonate received vitamin K subcutaneously prior to lumbar puncture.
Pediatricians across the country have gone to great lengths to protect their patients and to provide high-quality care both inside and outside the office during this unprecedented time. Nevertheless, these 3 cases illustrate the detrimental effects of COVID-19 on the delivery of pediatric health care. The first 2 cases in particular demonstrate the limitations of even close and consistent phone and televisit follow-up. Telehealth has provided a lifeline for patients and families during the pandemic, and, in most cases, has provided an excellent temporary substitution for office visits. There are, however, limitations to care without physical evaluation. Had the children in the first 2 cases been evaluated in person sooner, they may have been referred to a higher level of care more expediently. Likewise, in all 3 cases, parental reservations about exposing their children to COVID-19 through a trip to the hospital, however well-intentioned, likely played a role in the eventual severity of illness with which each child presented to the hospital.
If we are encountering children in the PED with severe illness due to delayed presentation to care, what about the children we aren’t seeing? As COVID-19 cases rise daily in the United States, we must be aware of the possibility of ED avoidance. We propose a multimodal approach to combat this dangerous phenomenon. Inpatient and ED-based pediatricians must maintain clear and open lines of communication with outpatient colleagues so that we can partner in considering which cases warrant prompt ED evaluation, even in the midst of a pandemic. All pediatricians must remind families that our hospitals remain open and ready to treat children safely. We must promote community awareness of the numerous safety precautions we take every day so that patients and families can feel comfortable seeking care at the hospital; the message of ED and hospital safety must be even more robust for caregivers of our particularly vulnerable children. As always, how we communicate with patients and their families matters. Validating and addressing concerns about COVID-19 exposure, while providing reassurance about the safety of our hospitals, could save children’s lives.
Acknowledgment: Thank you to Dr. Cynthia Mollen and Dr. Kathy Shaw for their reviews of the manuscript.
Corresponding author: Regina L. Toto, MD, Department of Pediatrics, The Children’s Hospital of Philadelphia, 3401 Civic Center Blvd., Philadelphia, PA 19104; [email protected].
Financial disclosures: None.
Keywords: coronavirus; pediatric; children; access to care; emergency department.
1. Riphagen S, Gomez X, Gonzalez-Martinez C, et al. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607-1608.
2. Wong LE, Hawkins JE, Langness S, et al. Where are all the patients? addressing COVID-19 fear to encourage sick patients to seek emergency care. NEJM Catalyst. 2020. doi:10.1056/CAT.20.0193
3. Moroni F, Gramegna M, Ajello S, et al. Collateral damage: medical care avoidance behavior among patients with acute coronary syndrome during the COVID-19 pandemic. JACC. 2020. doi:10.1016/j.jaccas.2020.04.010
4. Deerberg-Wittram J, Knothe C. Do not stay home: we are ready for you. NEJM Catalyst. 2020. doi:10.1056/CAT.20.0146
5. Woolf SH, Chapman DA, Sabo RT, et al. Excess deaths From COVID-19 and other causes, March-April 2020. JAMA. 2020. doi:10.1001.jama.2020.11787
6. Lazzerini M, Barbi E, Apicella A, et al. Delayed access or provision of care in Italy resulting from fear of COVID-19. Lancet Child Adolesc Health. 2020;4:E10-1.
7. Happle C, Dopfer C, Wetzke M, et al. Covid-19 related reduction in paediatric emergency healthcare utilization--a concerning trend. BMC Pediatrics. [under review]. 2020. doi:10.21203/rs.3.rs-2
The Children’s Hospital of Philadelphia, Philadelphia, PA.
The effects of COVID-19 on children’s health are multifaceted. In comparison to adults, children typically experience far milder physical consequences when infected with the virus. A notable exception is the newly described multisystem inflammatory syndrome associated with COVID-19 (MIS-C), which has proven to be a source of significant morbidity among the children it affects.1 Nevertheless, even those children not infected with COVID-19 have suffered due to the disease. School closures have deprived children of opportunities for social and academic growth and, in some cases, the provision of food, social services, medication administration, and many different therapies. Social distancing rules have limited play among children, which is crucial to their development and mental health. The impact on children who have lost family members, including parents, is monumental. Amidst all of this observable suffering, however, the pandemic poses a less visible threat to the health of children.
It is well documented that concern about exposure to COVID-19 has led many adults to avoid emergency departments (EDs) around the world. We believe parents may be avoiding ED visits for their children for the same reason. In the United States, ED volumes dropped approximately 50% during spring 2020.2 While EDs saw increasing, and at times overwhelming, numbers of patients with COVID-19, the number of patients presenting with other life-threatening medical issues, including heart attacks and strokes, declined.3,4 Data from the National Center for Health Statistics this past spring revealed nationwide increases in deaths due to nonrespiratory causes such as diabetes, heart disease, and stroke.5 ED avoidance and unprecedented lack of access to outpatient care, though with the intent to reduce overall risk, are likely significant contributors to these deaths.
Pediatric patients, especially the most vulnerable, are similarly at risk for deleterious health-related consequences from ED avoidance and from limited access to primary and outpatient specialty care. Data from Europe indicate dramatic drops in pediatric ED (PED) volumes, as well as an increase in the proportion of ED visits leading to hospitalization.6,7 These studies suggest that when patients do ultimately present to the PED, they may be more seriously ill.
At our institution, we have seen many COVID-19-negative patients whose medical care has been negatively influenced by the pandemic. A few months ago, a 1-month-old infant with an underlying health condition presented to the PED in extremis after weeks of progressively worsening feeding issues. The infant had been closely followed by the primary care provider (PCP) and subspecialty team via phone calls, televisits, and some office visits. Both physicians and parents had tried to resolve the feeding issues within the outpatient context, explicitly hoping to avoid potential exposure of this fragile patient to COVID-19 in the hospital. On eventual presentation to the PED, the infant was profoundly dehydrated, with significant electrolyte derangement and an acute abdomen, requiring admission to the intensive care unit. Ultimately, a new diagnosis of Hirschsprung disease was made, and the infant was hospitalized for several weeks for weight gain.
Later this summer, a school-aged child with a history of poorly controlled type 1 diabetes presented to an affiliated community hospital comatose and with Kussmaul respirations. Prior to the pandemic, a school nurse administered the child’s morning insulin. Since school closed, the patient had been responsible for administering this dose of insulin while the parents worked outside the home. Despite close and frequent communication between the patient’s endocrinology team and the family, the patient’s glucose and ketone levels began to rise. The parent administered repeated boluses of insulin at home in an attempt to avoid the perceived exposure risk associated with an ED visit. On presentation to the PED, the patient was profoundly altered, with a pH of 7.0. When transfer to a tertiary care center was recommended, the patient’s parent expressed persistent concerns about COVID-19 exposure in the larger hospital, although ultimately consent to transfer was given.
A third case from this summer provides an example of a different type of patient affected by COVID-19: the neonate whose birth circumstances were altered due to the virus. A 3-day-old, full-term infant presented to the ED with hypothermia after PCP referral. The parents had considered both home birth and hospital delivery earlier in the pregnancy, ultimately opting for home birth due to concerns about COVID-19 exposure in the hospital. The pregnancy and delivery were uncomplicated. The neonate did not receive the first hepatitis B vaccine, erythromycin eye ointment, or vitamin K after delivery. In the first 3 days of life, the patient had voided once and stooled once per day. The patient’s mother, inexperienced with breastfeeding and without access to a lactation consultant, was unsure about latch or emptying of her breasts. At the first pediatrician visit, the infant was noted to be hypothermic to 35°C, intermittently bradycardic to the 80s, and with diminished arousal. In the PED, a full sepsis work-up was initiated. Though multiple attempts were made by different providers, only a minimal amount of blood could be drawn, presumably due to dehydration. Of note, the neonate received vitamin K subcutaneously prior to lumbar puncture.
Pediatricians across the country have gone to great lengths to protect their patients and to provide high-quality care both inside and outside the office during this unprecedented time. Nevertheless, these 3 cases illustrate the detrimental effects of COVID-19 on the delivery of pediatric health care. The first 2 cases in particular demonstrate the limitations of even close and consistent phone and televisit follow-up. Telehealth has provided a lifeline for patients and families during the pandemic, and, in most cases, has provided an excellent temporary substitution for office visits. There are, however, limitations to care without physical evaluation. Had the children in the first 2 cases been evaluated in person sooner, they may have been referred to a higher level of care more expediently. Likewise, in all 3 cases, parental reservations about exposing their children to COVID-19 through a trip to the hospital, however well-intentioned, likely played a role in the eventual severity of illness with which each child presented to the hospital.
If we are encountering children in the PED with severe illness due to delayed presentation to care, what about the children we aren’t seeing? As COVID-19 cases rise daily in the United States, we must be aware of the possibility of ED avoidance. We propose a multimodal approach to combat this dangerous phenomenon. Inpatient and ED-based pediatricians must maintain clear and open lines of communication with outpatient colleagues so that we can partner in considering which cases warrant prompt ED evaluation, even in the midst of a pandemic. All pediatricians must remind families that our hospitals remain open and ready to treat children safely. We must promote community awareness of the numerous safety precautions we take every day so that patients and families can feel comfortable seeking care at the hospital; the message of ED and hospital safety must be even more robust for caregivers of our particularly vulnerable children. As always, how we communicate with patients and their families matters. Validating and addressing concerns about COVID-19 exposure, while providing reassurance about the safety of our hospitals, could save children’s lives.
Acknowledgment: Thank you to Dr. Cynthia Mollen and Dr. Kathy Shaw for their reviews of the manuscript.
Corresponding author: Regina L. Toto, MD, Department of Pediatrics, The Children’s Hospital of Philadelphia, 3401 Civic Center Blvd., Philadelphia, PA 19104; [email protected].
Financial disclosures: None.
Keywords: coronavirus; pediatric; children; access to care; emergency department.
The Children’s Hospital of Philadelphia, Philadelphia, PA.
The effects of COVID-19 on children’s health are multifaceted. In comparison to adults, children typically experience far milder physical consequences when infected with the virus. A notable exception is the newly described multisystem inflammatory syndrome associated with COVID-19 (MIS-C), which has proven to be a source of significant morbidity among the children it affects.1 Nevertheless, even those children not infected with COVID-19 have suffered due to the disease. School closures have deprived children of opportunities for social and academic growth and, in some cases, the provision of food, social services, medication administration, and many different therapies. Social distancing rules have limited play among children, which is crucial to their development and mental health. The impact on children who have lost family members, including parents, is monumental. Amidst all of this observable suffering, however, the pandemic poses a less visible threat to the health of children.
It is well documented that concern about exposure to COVID-19 has led many adults to avoid emergency departments (EDs) around the world. We believe parents may be avoiding ED visits for their children for the same reason. In the United States, ED volumes dropped approximately 50% during spring 2020.2 While EDs saw increasing, and at times overwhelming, numbers of patients with COVID-19, the number of patients presenting with other life-threatening medical issues, including heart attacks and strokes, declined.3,4 Data from the National Center for Health Statistics this past spring revealed nationwide increases in deaths due to nonrespiratory causes such as diabetes, heart disease, and stroke.5 ED avoidance and unprecedented lack of access to outpatient care, though with the intent to reduce overall risk, are likely significant contributors to these deaths.
Pediatric patients, especially the most vulnerable, are similarly at risk for deleterious health-related consequences from ED avoidance and from limited access to primary and outpatient specialty care. Data from Europe indicate dramatic drops in pediatric ED (PED) volumes, as well as an increase in the proportion of ED visits leading to hospitalization.6,7 These studies suggest that when patients do ultimately present to the PED, they may be more seriously ill.
At our institution, we have seen many COVID-19-negative patients whose medical care has been negatively influenced by the pandemic. A few months ago, a 1-month-old infant with an underlying health condition presented to the PED in extremis after weeks of progressively worsening feeding issues. The infant had been closely followed by the primary care provider (PCP) and subspecialty team via phone calls, televisits, and some office visits. Both physicians and parents had tried to resolve the feeding issues within the outpatient context, explicitly hoping to avoid potential exposure of this fragile patient to COVID-19 in the hospital. On eventual presentation to the PED, the infant was profoundly dehydrated, with significant electrolyte derangement and an acute abdomen, requiring admission to the intensive care unit. Ultimately, a new diagnosis of Hirschsprung disease was made, and the infant was hospitalized for several weeks for weight gain.
Later this summer, a school-aged child with a history of poorly controlled type 1 diabetes presented to an affiliated community hospital comatose and with Kussmaul respirations. Prior to the pandemic, a school nurse administered the child’s morning insulin. Since school closed, the patient had been responsible for administering this dose of insulin while the parents worked outside the home. Despite close and frequent communication between the patient’s endocrinology team and the family, the patient’s glucose and ketone levels began to rise. The parent administered repeated boluses of insulin at home in an attempt to avoid the perceived exposure risk associated with an ED visit. On presentation to the PED, the patient was profoundly altered, with a pH of 7.0. When transfer to a tertiary care center was recommended, the patient’s parent expressed persistent concerns about COVID-19 exposure in the larger hospital, although ultimately consent to transfer was given.
A third case from this summer provides an example of a different type of patient affected by COVID-19: the neonate whose birth circumstances were altered due to the virus. A 3-day-old, full-term infant presented to the ED with hypothermia after PCP referral. The parents had considered both home birth and hospital delivery earlier in the pregnancy, ultimately opting for home birth due to concerns about COVID-19 exposure in the hospital. The pregnancy and delivery were uncomplicated. The neonate did not receive the first hepatitis B vaccine, erythromycin eye ointment, or vitamin K after delivery. In the first 3 days of life, the patient had voided once and stooled once per day. The patient’s mother, inexperienced with breastfeeding and without access to a lactation consultant, was unsure about latch or emptying of her breasts. At the first pediatrician visit, the infant was noted to be hypothermic to 35°C, intermittently bradycardic to the 80s, and with diminished arousal. In the PED, a full sepsis work-up was initiated. Though multiple attempts were made by different providers, only a minimal amount of blood could be drawn, presumably due to dehydration. Of note, the neonate received vitamin K subcutaneously prior to lumbar puncture.
Pediatricians across the country have gone to great lengths to protect their patients and to provide high-quality care both inside and outside the office during this unprecedented time. Nevertheless, these 3 cases illustrate the detrimental effects of COVID-19 on the delivery of pediatric health care. The first 2 cases in particular demonstrate the limitations of even close and consistent phone and televisit follow-up. Telehealth has provided a lifeline for patients and families during the pandemic, and, in most cases, has provided an excellent temporary substitution for office visits. There are, however, limitations to care without physical evaluation. Had the children in the first 2 cases been evaluated in person sooner, they may have been referred to a higher level of care more expediently. Likewise, in all 3 cases, parental reservations about exposing their children to COVID-19 through a trip to the hospital, however well-intentioned, likely played a role in the eventual severity of illness with which each child presented to the hospital.
If we are encountering children in the PED with severe illness due to delayed presentation to care, what about the children we aren’t seeing? As COVID-19 cases rise daily in the United States, we must be aware of the possibility of ED avoidance. We propose a multimodal approach to combat this dangerous phenomenon. Inpatient and ED-based pediatricians must maintain clear and open lines of communication with outpatient colleagues so that we can partner in considering which cases warrant prompt ED evaluation, even in the midst of a pandemic. All pediatricians must remind families that our hospitals remain open and ready to treat children safely. We must promote community awareness of the numerous safety precautions we take every day so that patients and families can feel comfortable seeking care at the hospital; the message of ED and hospital safety must be even more robust for caregivers of our particularly vulnerable children. As always, how we communicate with patients and their families matters. Validating and addressing concerns about COVID-19 exposure, while providing reassurance about the safety of our hospitals, could save children’s lives.
Acknowledgment: Thank you to Dr. Cynthia Mollen and Dr. Kathy Shaw for their reviews of the manuscript.
Corresponding author: Regina L. Toto, MD, Department of Pediatrics, The Children’s Hospital of Philadelphia, 3401 Civic Center Blvd., Philadelphia, PA 19104; [email protected].
Financial disclosures: None.
Keywords: coronavirus; pediatric; children; access to care; emergency department.
1. Riphagen S, Gomez X, Gonzalez-Martinez C, et al. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607-1608.
2. Wong LE, Hawkins JE, Langness S, et al. Where are all the patients? addressing COVID-19 fear to encourage sick patients to seek emergency care. NEJM Catalyst. 2020. doi:10.1056/CAT.20.0193
3. Moroni F, Gramegna M, Ajello S, et al. Collateral damage: medical care avoidance behavior among patients with acute coronary syndrome during the COVID-19 pandemic. JACC. 2020. doi:10.1016/j.jaccas.2020.04.010
4. Deerberg-Wittram J, Knothe C. Do not stay home: we are ready for you. NEJM Catalyst. 2020. doi:10.1056/CAT.20.0146
5. Woolf SH, Chapman DA, Sabo RT, et al. Excess deaths From COVID-19 and other causes, March-April 2020. JAMA. 2020. doi:10.1001.jama.2020.11787
6. Lazzerini M, Barbi E, Apicella A, et al. Delayed access or provision of care in Italy resulting from fear of COVID-19. Lancet Child Adolesc Health. 2020;4:E10-1.
7. Happle C, Dopfer C, Wetzke M, et al. Covid-19 related reduction in paediatric emergency healthcare utilization--a concerning trend. BMC Pediatrics. [under review]. 2020. doi:10.21203/rs.3.rs-2
1. Riphagen S, Gomez X, Gonzalez-Martinez C, et al. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607-1608.
2. Wong LE, Hawkins JE, Langness S, et al. Where are all the patients? addressing COVID-19 fear to encourage sick patients to seek emergency care. NEJM Catalyst. 2020. doi:10.1056/CAT.20.0193
3. Moroni F, Gramegna M, Ajello S, et al. Collateral damage: medical care avoidance behavior among patients with acute coronary syndrome during the COVID-19 pandemic. JACC. 2020. doi:10.1016/j.jaccas.2020.04.010
4. Deerberg-Wittram J, Knothe C. Do not stay home: we are ready for you. NEJM Catalyst. 2020. doi:10.1056/CAT.20.0146
5. Woolf SH, Chapman DA, Sabo RT, et al. Excess deaths From COVID-19 and other causes, March-April 2020. JAMA. 2020. doi:10.1001.jama.2020.11787
6. Lazzerini M, Barbi E, Apicella A, et al. Delayed access or provision of care in Italy resulting from fear of COVID-19. Lancet Child Adolesc Health. 2020;4:E10-1.
7. Happle C, Dopfer C, Wetzke M, et al. Covid-19 related reduction in paediatric emergency healthcare utilization--a concerning trend. BMC Pediatrics. [under review]. 2020. doi:10.21203/rs.3.rs-2
Procalcitonin-Guided Antibiotic Discontinuation: An Antimicrobial Stewardship Initiative to Assist Providers
From Western Michigan University, Homer Stryker MD School of Medicine, Kalamazoo, MI (Dr. Vaillant and Dr. Kavanaugh), Ferris State University, Grand Rapids, MI (Dr. Mersfelder), and Bronson Methodist Hospital, Kalamazoo, MI (Dr. Maynard).
Abstract
- Background: Procalcitonin has emerged as an important marker of sepsis and lung infections of bacterial origin. The role of procalcitonin in guiding antibiotic stewardship in lower respiratory tract infections and sepsis has been extensively studied, and use of this biomarker has been shown to decrease antibiotic usage in clinical trials. We sought to evaluate the impact of a pharmacist-driven initiative regarding discontinuation of antibiotics utilizing procalcitonin levels at a community teaching hospital.
- Methods: We retrospectively gathered baseline data on adult patients admitted to a community teaching hospital who were 18 years of age and older, under the care of an inpatient service, and had a single procalcitonin level < 0.25 mcg/L obtained during admission. We then prospectively identified an intervention group of similar patients using a web-based, real-time clinical surveillance system. When a low procalcitonin level was identified in the intervention group, the participating clinical pharmacists screened for antibiotic use and the indication(s), determined whether the antibiotic could be discontinued based on the low procalcitonin level and the absence of another indication for antibiotics, and, when appropriate, contacted the patient’s health care provider via telephone to discuss possible antibiotic discontinuation. The total antibiotic treatment duration was compared between the baseline and intervention groups.
- Results: A total of 172 patients were included in this study (86 in each group). The duration of antibiotic use was not significantly different between the baseline (3.14 ± 4.04 days) and the intervention (3.34 ± 2.8 days) groups (P = 0.1083). Other patient demographics did not influence antibiotic duration.
- Conclusion: Our study did not demonstrate a difference in total antibiotic treatment duration with the utilization of procalcitonin and an oral communication intervention made by a clinical pharmacist at a community-based teaching hospital. Outside of clinical trials, and in the absence of an algorithmic approach, procalcitonin has not consistently been shown to aid in the diagnosis and treatment of infectious diseases. It is important to have a comprehensive antimicrobial stewardship program to reduce antibiotic use and effectively use laboratory values.
Keywords: antibiotic use; bacterial infection; biomarkers; procalcitonin.
Procalcitonin is the precursor of the hormone calcitonin, which is normally produced in the parafollicular cells of the thyroid gland under physiological conditions.1 However, procalcitonin is also released in response to a proinflammatory stimulus, especially that of bacterial origin.1 The source of the procalcitonin surge seen during proinflammatory states is not the parafollicular cells of the thyroid, but rather the neuroendocrine cells of the lung and intestine.1 Stimulants of procalcitonin in these scenarios include bacterial endotoxin, tumor necrosis factor, and interleukin-6.1,2 Due to these observations, procalcitonin has emerged as an important marker of sepsis and lung infections of bacterial origin.3
The role of procalcitonin in guiding antibiotic stewardship in lower respiratory tract infections and sepsis has been extensively studied.4,5 Various randomized controlled trials have shown that antibiotic stewardship guided by procalcitonin levels resulted in lower rates of antibiotic initiation and shorter duration of antibiotic use.4-6 Similar results were obtained in prospective studies evaluating its role in patients with chronic obstructive pulmonary disease and sepsis.7,8 Based on these data, protocol-driven procalcitonin-guided antibiotic stewardship appears beneficial.
Many of these studies employed rigorous protocols. Studies of procalcitonin use in a so-called real-world setting, in which the provider can order and use procalcitonin levels without the use of protocols, are limited. The objective of our study was to evaluate the impact of a pharmacist-driven initiative on discontinuing antibiotics, if indicated, utilizing single procalcitonin measurement results of < 0.25 mcg/L at a community teaching hospital.
Methods
Our study utilized a 2-phase approach. The first phase was a retrospective chart review to establish baseline data regarding adult inpatients with a low procalcitonin level; these patients were randomly selected over a 1-year period (2017). Patients were included if they were 18 years of age or older, under the care of an inpatient service, and had a single procalcitonin level < 0.25 mcg/L obtained during their admission. Patients admitted to the intensive care unit were excluded. In the second phase, we prospectively identified similar patients admitted between January and March 2018 using a web-based, real-time clinical surveillance system. When patients with low procalcitonin levels were identified, 2 participating clinical pharmacists screened for antibiotic use and indication. If it was determined that the antibiotic could be discontinued as a result of the low procalcitonin level and no additional indication for antibiotics was present, the pharmacist contacted the patient’s health care provider via telephone to discuss possible antibiotic discontinuation. Data collected before and after the intervention included total antibiotic treatment duration, white blood cell count, maximum temperature, age, and procalcitonin level.
A sample size of 86 was calculated to provide an alpha of 0.05 and a power of 0.8. A nonparametric Wilcoxon 2-sample test was used to test for a difference in duration of antibiotic treatment between the baseline and intervention groups. A nonparametric test was used due to right-skewed data. All patients were included in the group analysis, regardless of antibiotic use, as the procalcitonin level may have been used in the decision to initiate antibiotics, and this is more representative of a real-world application of the test. This allowed for detection of a significant decrease of 2 days in antibiotic duration post intervention, with a 10% margin to compensate for potential missing data. Data from 86 patients obtained prior to the pharmacist intervention acted as a control comparison group. Statistical analysis was performed using SAS 9.4.
Results
A total of 172 patients were included in this study: 86 patients prior to the intervention, and 86 after implementation. Baseline demographics, laboratory values, vitals, and principal diagnoses for both groups are shown in Table 1 and Table 2. The most common indications for procalcitonin measurement were pneumonia (45.9%), chronic obstructive pulmonary disease (15.7%), and sepsis (14.5%). The remaining diagnoses were encephalopathy, fever and leukocytosis, skin and soft tissue infection, urinary tract infection or pyelonephritis, bone and joint infection, meningitis, intra-abdominal infection, and asthma exacerbation.
Antibiotic therapy was initiated in 68% of the patients overall, 59% in the baseline group and 76% in the intervention group. The duration of antibiotic use was not significantly different between the baseline (3.14 ± 4.04 days) and intervention (3.34 ± 2.8 days) groups (P = 0.1083). Furthermore, antibiotic treatment duration did not vary significantly with patient age, white blood cell count, maximum temperature, or procalcitonin level in either group. Although there was no difference in total antibiotic treatment duration, a post-hoc analysis revealed a 0.6-day decrease in the interval between the date of procalcitonin measurement and the stop date of antibiotics in the intervention group. The average time from admission to obtaining a procalcitonin level was 3 days in the baseline group and 2 days in the intervention group.
Discussion
Our study did not demonstrate a difference in total antibiotic treatment duration with procalcitonin measurement and an oral communication intervention made by a clinical pharmacist at a community teaching hospital with a well-established antimicrobial stewardship program. This may be due to several factors. First, the providers did not receive ongoing education regarding the appropriate use or interpretation of procalcitonin. The procalcitonin result in the electronic health record references the risk for progression to severe sepsis and/or septic shock, but does not indicate how to use procalcitonin as an aid in antibiotic decision-making. However, a recent study in patients with lower respiratory tract infections treated by providers who had been educated on the use of procalcitonin failed to find a reduction in total antibiotic use.9 Second, our study included hospital-wide use of procalcitonin, and was not limited to infections for which procalcitonin use has the strongest evidence (eg, upper respiratory tract infections, pneumonia, sepsis). Thus, providers may have been less likely to use protocolized guidelines. Last, we did not limit the data on antibiotic duration to patients with a procalcitonin level obtained within a defined time frame from antibiotic initiation or time of admission, and some patients had procalcitonin levels measured several days into their hospital stay. While this is likely to have skewed the data in favor of longer antibiotic treatment courses, it also represents a more realistic way in which this laboratory test is being used. Our post-hoc finding of earlier discontinuation of antibiotics after procalcitonin measurement suggests that our intervention may have influenced the decision to discontinue antibiotics. Such an effect may be augmented if procalcitonin is measured earlier in a hospital admission.
Previous studies have also failed to show that the use of procalcitonin decreased duration of antibiotics.9,10 In the aforementioned study regarding real-world outcomes in patients with lower respiratory tract infections, antibiotic duration was not reduced, despite provider education.9 A large observational study that evaluated real-world outcomes in intensive care unit patients did not find decreased antibiotic use or improved outcomes with procalcitonin use.10 With these large studies evaluating the 2 most common infectious diseases for which procalcitonin has previously been found to have clinical benefit, it is important for institutions to re-evaluate how procalcitonin is being utilized by providers. Furthermore, institutions should explore ways to optimize procalcitonin use and decrease unnecessary health care costs. Notably, the current community-acquired pneumonia guidelines recommend against routine use of procalcitonin.11
Conclusion
Outside of clinical trials, and in the absence of an algorithmic approach, procalcitonin has not consistently been shown to aid in the diagnosis or treatment of infectious diseases. It is important to have a comprehensive antimicrobial stewardship program that includes an algorithmic protocol to promote appropriate laboratory testing and reduce total antibiotic use. In addition to improved communication with providers, other interventions need to be investigated to effectively use this biomarker or limit its use.
Acknowledgment: The authors thank the Western Michigan University Department of Epidemiology and Biostatistics for their assistance in preparing this article.
Corresponding author: James Vaillant, MD, Western Michigan University, Homer Stryker MD School of Medicine, 1000 Oakland Drive, Kalamazoo, MI, 49008; [email protected].
Financial disclosures: None.
1. Maruna P, Nedelníková K, Gürlich R. Physiology and genetics of procalcitonin. Physiol Res. 2000;(49 suppl 1):S57-S61.
2. Becker KL, Snider R, Nylen ES. Procalcitonin in sepsis and systemic inflammation: a harmful biomarker and a therapeutic target. Br J Pharmacol. 2010;159:253-264.
3. Vijayan AL, Vanimaya RS, Saikant R, et al. Procalcitonin: a promising diagnostic marker for sepsis and antibiotic therapy. J Intensive Care. 2017;5:51.
4. Hey J, Thompson-Leduc P, Kirson NY, et al. Procalcitonin guidance in patients with lower respiratory tract infections: A systematic review and meta-analysis. Clin Chem Lab Med. 2018;56:1200-1209.
5. Schuetz P, Wirz Y, Sager R, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2017;10:CD007498.
6. Huang HB, Peng JM, Weng L, et al. Procalcitonin-guided antibiotic therapy in intensive care unit patients: a systematic review and meta-analysis. Ann Intensive Care. 2017;7:114.
7. Stolz D, Christ-Crain M, Bingisser R, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest. 2007;131:9-19.
8. Prkno A, Wacker C, Brunkhorst FM, Schlattmann P. Procalcitonin-guided therapy in intensive care unit patients with severe sepsis and septic shock—a systematic review and meta-analysis. Crit Care. 2013;17:R291.
9. Huang DT, Yealy DM, Filbin MR, et al. Procalcitonin-guided use of antibiotics for lower respiratory tract infections. N Engl J Med. 2018;379:236-249.
10. Chu DC, Mehta AB, Walkey AJ. Practice patterns and outcomes associated with procalcitonin use in critically ill patients with sepsis. Clin Infect Dis. 2017;64:1509-1515.
11. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45-e67.
From Western Michigan University, Homer Stryker MD School of Medicine, Kalamazoo, MI (Dr. Vaillant and Dr. Kavanaugh), Ferris State University, Grand Rapids, MI (Dr. Mersfelder), and Bronson Methodist Hospital, Kalamazoo, MI (Dr. Maynard).
Abstract
- Background: Procalcitonin has emerged as an important marker of sepsis and lung infections of bacterial origin. The role of procalcitonin in guiding antibiotic stewardship in lower respiratory tract infections and sepsis has been extensively studied, and use of this biomarker has been shown to decrease antibiotic usage in clinical trials. We sought to evaluate the impact of a pharmacist-driven initiative regarding discontinuation of antibiotics utilizing procalcitonin levels at a community teaching hospital.
- Methods: We retrospectively gathered baseline data on adult patients admitted to a community teaching hospital who were 18 years of age and older, under the care of an inpatient service, and had a single procalcitonin level < 0.25 mcg/L obtained during admission. We then prospectively identified an intervention group of similar patients using a web-based, real-time clinical surveillance system. When a low procalcitonin level was identified in the intervention group, the participating clinical pharmacists screened for antibiotic use and the indication(s), determined whether the antibiotic could be discontinued based on the low procalcitonin level and the absence of another indication for antibiotics, and, when appropriate, contacted the patient’s health care provider via telephone to discuss possible antibiotic discontinuation. The total antibiotic treatment duration was compared between the baseline and intervention groups.
- Results: A total of 172 patients were included in this study (86 in each group). The duration of antibiotic use was not significantly different between the baseline (3.14 ± 4.04 days) and the intervention (3.34 ± 2.8 days) groups (P = 0.1083). Other patient demographics did not influence antibiotic duration.
- Conclusion: Our study did not demonstrate a difference in total antibiotic treatment duration with the utilization of procalcitonin and an oral communication intervention made by a clinical pharmacist at a community-based teaching hospital. Outside of clinical trials, and in the absence of an algorithmic approach, procalcitonin has not consistently been shown to aid in the diagnosis and treatment of infectious diseases. It is important to have a comprehensive antimicrobial stewardship program to reduce antibiotic use and effectively use laboratory values.
Keywords: antibiotic use; bacterial infection; biomarkers; procalcitonin.
Procalcitonin is the precursor of the hormone calcitonin, which is normally produced in the parafollicular cells of the thyroid gland under physiological conditions.1 However, procalcitonin is also released in response to a proinflammatory stimulus, especially that of bacterial origin.1 The source of the procalcitonin surge seen during proinflammatory states is not the parafollicular cells of the thyroid, but rather the neuroendocrine cells of the lung and intestine.1 Stimulants of procalcitonin in these scenarios include bacterial endotoxin, tumor necrosis factor, and interleukin-6.1,2 Due to these observations, procalcitonin has emerged as an important marker of sepsis and lung infections of bacterial origin.3
The role of procalcitonin in guiding antibiotic stewardship in lower respiratory tract infections and sepsis has been extensively studied.4,5 Various randomized controlled trials have shown that antibiotic stewardship guided by procalcitonin levels resulted in lower rates of antibiotic initiation and shorter duration of antibiotic use.4-6 Similar results were obtained in prospective studies evaluating its role in patients with chronic obstructive pulmonary disease and sepsis.7,8 Based on these data, protocol-driven procalcitonin-guided antibiotic stewardship appears beneficial.
Many of these studies employed rigorous protocols. Studies of procalcitonin use in a so-called real-world setting, in which the provider can order and use procalcitonin levels without the use of protocols, are limited. The objective of our study was to evaluate the impact of a pharmacist-driven initiative on discontinuing antibiotics, if indicated, utilizing single procalcitonin measurement results of < 0.25 mcg/L at a community teaching hospital.
Methods
Our study utilized a 2-phase approach. The first phase was a retrospective chart review to establish baseline data regarding adult inpatients with a low procalcitonin level; these patients were randomly selected over a 1-year period (2017). Patients were included if they were 18 years of age or older, under the care of an inpatient service, and had a single procalcitonin level < 0.25 mcg/L obtained during their admission. Patients admitted to the intensive care unit were excluded. In the second phase, we prospectively identified similar patients admitted between January and March 2018 using a web-based, real-time clinical surveillance system. When patients with low procalcitonin levels were identified, 2 participating clinical pharmacists screened for antibiotic use and indication. If it was determined that the antibiotic could be discontinued as a result of the low procalcitonin level and no additional indication for antibiotics was present, the pharmacist contacted the patient’s health care provider via telephone to discuss possible antibiotic discontinuation. Data collected before and after the intervention included total antibiotic treatment duration, white blood cell count, maximum temperature, age, and procalcitonin level.
A sample size of 86 was calculated to provide an alpha of 0.05 and a power of 0.8. A nonparametric Wilcoxon 2-sample test was used to test for a difference in duration of antibiotic treatment between the baseline and intervention groups. A nonparametric test was used due to right-skewed data. All patients were included in the group analysis, regardless of antibiotic use, as the procalcitonin level may have been used in the decision to initiate antibiotics, and this is more representative of a real-world application of the test. This allowed for detection of a significant decrease of 2 days in antibiotic duration post intervention, with a 10% margin to compensate for potential missing data. Data from 86 patients obtained prior to the pharmacist intervention acted as a control comparison group. Statistical analysis was performed using SAS 9.4.
Results
A total of 172 patients were included in this study: 86 patients prior to the intervention, and 86 after implementation. Baseline demographics, laboratory values, vitals, and principal diagnoses for both groups are shown in Table 1 and Table 2. The most common indications for procalcitonin measurement were pneumonia (45.9%), chronic obstructive pulmonary disease (15.7%), and sepsis (14.5%). The remaining diagnoses were encephalopathy, fever and leukocytosis, skin and soft tissue infection, urinary tract infection or pyelonephritis, bone and joint infection, meningitis, intra-abdominal infection, and asthma exacerbation.
Antibiotic therapy was initiated in 68% of the patients overall, 59% in the baseline group and 76% in the intervention group. The duration of antibiotic use was not significantly different between the baseline (3.14 ± 4.04 days) and intervention (3.34 ± 2.8 days) groups (P = 0.1083). Furthermore, antibiotic treatment duration did not vary significantly with patient age, white blood cell count, maximum temperature, or procalcitonin level in either group. Although there was no difference in total antibiotic treatment duration, a post-hoc analysis revealed a 0.6-day decrease in the interval between the date of procalcitonin measurement and the stop date of antibiotics in the intervention group. The average time from admission to obtaining a procalcitonin level was 3 days in the baseline group and 2 days in the intervention group.
Discussion
Our study did not demonstrate a difference in total antibiotic treatment duration with procalcitonin measurement and an oral communication intervention made by a clinical pharmacist at a community teaching hospital with a well-established antimicrobial stewardship program. This may be due to several factors. First, the providers did not receive ongoing education regarding the appropriate use or interpretation of procalcitonin. The procalcitonin result in the electronic health record references the risk for progression to severe sepsis and/or septic shock, but does not indicate how to use procalcitonin as an aid in antibiotic decision-making. However, a recent study in patients with lower respiratory tract infections treated by providers who had been educated on the use of procalcitonin failed to find a reduction in total antibiotic use.9 Second, our study included hospital-wide use of procalcitonin, and was not limited to infections for which procalcitonin use has the strongest evidence (eg, upper respiratory tract infections, pneumonia, sepsis). Thus, providers may have been less likely to use protocolized guidelines. Last, we did not limit the data on antibiotic duration to patients with a procalcitonin level obtained within a defined time frame from antibiotic initiation or time of admission, and some patients had procalcitonin levels measured several days into their hospital stay. While this is likely to have skewed the data in favor of longer antibiotic treatment courses, it also represents a more realistic way in which this laboratory test is being used. Our post-hoc finding of earlier discontinuation of antibiotics after procalcitonin measurement suggests that our intervention may have influenced the decision to discontinue antibiotics. Such an effect may be augmented if procalcitonin is measured earlier in a hospital admission.
Previous studies have also failed to show that the use of procalcitonin decreased duration of antibiotics.9,10 In the aforementioned study regarding real-world outcomes in patients with lower respiratory tract infections, antibiotic duration was not reduced, despite provider education.9 A large observational study that evaluated real-world outcomes in intensive care unit patients did not find decreased antibiotic use or improved outcomes with procalcitonin use.10 With these large studies evaluating the 2 most common infectious diseases for which procalcitonin has previously been found to have clinical benefit, it is important for institutions to re-evaluate how procalcitonin is being utilized by providers. Furthermore, institutions should explore ways to optimize procalcitonin use and decrease unnecessary health care costs. Notably, the current community-acquired pneumonia guidelines recommend against routine use of procalcitonin.11
Conclusion
Outside of clinical trials, and in the absence of an algorithmic approach, procalcitonin has not consistently been shown to aid in the diagnosis or treatment of infectious diseases. It is important to have a comprehensive antimicrobial stewardship program that includes an algorithmic protocol to promote appropriate laboratory testing and reduce total antibiotic use. In addition to improved communication with providers, other interventions need to be investigated to effectively use this biomarker or limit its use.
Acknowledgment: The authors thank the Western Michigan University Department of Epidemiology and Biostatistics for their assistance in preparing this article.
Corresponding author: James Vaillant, MD, Western Michigan University, Homer Stryker MD School of Medicine, 1000 Oakland Drive, Kalamazoo, MI, 49008; [email protected].
Financial disclosures: None.
From Western Michigan University, Homer Stryker MD School of Medicine, Kalamazoo, MI (Dr. Vaillant and Dr. Kavanaugh), Ferris State University, Grand Rapids, MI (Dr. Mersfelder), and Bronson Methodist Hospital, Kalamazoo, MI (Dr. Maynard).
Abstract
- Background: Procalcitonin has emerged as an important marker of sepsis and lung infections of bacterial origin. The role of procalcitonin in guiding antibiotic stewardship in lower respiratory tract infections and sepsis has been extensively studied, and use of this biomarker has been shown to decrease antibiotic usage in clinical trials. We sought to evaluate the impact of a pharmacist-driven initiative regarding discontinuation of antibiotics utilizing procalcitonin levels at a community teaching hospital.
- Methods: We retrospectively gathered baseline data on adult patients admitted to a community teaching hospital who were 18 years of age and older, under the care of an inpatient service, and had a single procalcitonin level < 0.25 mcg/L obtained during admission. We then prospectively identified an intervention group of similar patients using a web-based, real-time clinical surveillance system. When a low procalcitonin level was identified in the intervention group, the participating clinical pharmacists screened for antibiotic use and the indication(s), determined whether the antibiotic could be discontinued based on the low procalcitonin level and the absence of another indication for antibiotics, and, when appropriate, contacted the patient’s health care provider via telephone to discuss possible antibiotic discontinuation. The total antibiotic treatment duration was compared between the baseline and intervention groups.
- Results: A total of 172 patients were included in this study (86 in each group). The duration of antibiotic use was not significantly different between the baseline (3.14 ± 4.04 days) and the intervention (3.34 ± 2.8 days) groups (P = 0.1083). Other patient demographics did not influence antibiotic duration.
- Conclusion: Our study did not demonstrate a difference in total antibiotic treatment duration with the utilization of procalcitonin and an oral communication intervention made by a clinical pharmacist at a community-based teaching hospital. Outside of clinical trials, and in the absence of an algorithmic approach, procalcitonin has not consistently been shown to aid in the diagnosis and treatment of infectious diseases. It is important to have a comprehensive antimicrobial stewardship program to reduce antibiotic use and effectively use laboratory values.
Keywords: antibiotic use; bacterial infection; biomarkers; procalcitonin.
Procalcitonin is the precursor of the hormone calcitonin, which is normally produced in the parafollicular cells of the thyroid gland under physiological conditions.1 However, procalcitonin is also released in response to a proinflammatory stimulus, especially that of bacterial origin.1 The source of the procalcitonin surge seen during proinflammatory states is not the parafollicular cells of the thyroid, but rather the neuroendocrine cells of the lung and intestine.1 Stimulants of procalcitonin in these scenarios include bacterial endotoxin, tumor necrosis factor, and interleukin-6.1,2 Due to these observations, procalcitonin has emerged as an important marker of sepsis and lung infections of bacterial origin.3
The role of procalcitonin in guiding antibiotic stewardship in lower respiratory tract infections and sepsis has been extensively studied.4,5 Various randomized controlled trials have shown that antibiotic stewardship guided by procalcitonin levels resulted in lower rates of antibiotic initiation and shorter duration of antibiotic use.4-6 Similar results were obtained in prospective studies evaluating its role in patients with chronic obstructive pulmonary disease and sepsis.7,8 Based on these data, protocol-driven procalcitonin-guided antibiotic stewardship appears beneficial.
Many of these studies employed rigorous protocols. Studies of procalcitonin use in a so-called real-world setting, in which the provider can order and use procalcitonin levels without the use of protocols, are limited. The objective of our study was to evaluate the impact of a pharmacist-driven initiative on discontinuing antibiotics, if indicated, utilizing single procalcitonin measurement results of < 0.25 mcg/L at a community teaching hospital.
Methods
Our study utilized a 2-phase approach. The first phase was a retrospective chart review to establish baseline data regarding adult inpatients with a low procalcitonin level; these patients were randomly selected over a 1-year period (2017). Patients were included if they were 18 years of age or older, under the care of an inpatient service, and had a single procalcitonin level < 0.25 mcg/L obtained during their admission. Patients admitted to the intensive care unit were excluded. In the second phase, we prospectively identified similar patients admitted between January and March 2018 using a web-based, real-time clinical surveillance system. When patients with low procalcitonin levels were identified, 2 participating clinical pharmacists screened for antibiotic use and indication. If it was determined that the antibiotic could be discontinued as a result of the low procalcitonin level and no additional indication for antibiotics was present, the pharmacist contacted the patient’s health care provider via telephone to discuss possible antibiotic discontinuation. Data collected before and after the intervention included total antibiotic treatment duration, white blood cell count, maximum temperature, age, and procalcitonin level.
A sample size of 86 was calculated to provide an alpha of 0.05 and a power of 0.8. A nonparametric Wilcoxon 2-sample test was used to test for a difference in duration of antibiotic treatment between the baseline and intervention groups. A nonparametric test was used due to right-skewed data. All patients were included in the group analysis, regardless of antibiotic use, as the procalcitonin level may have been used in the decision to initiate antibiotics, and this is more representative of a real-world application of the test. This allowed for detection of a significant decrease of 2 days in antibiotic duration post intervention, with a 10% margin to compensate for potential missing data. Data from 86 patients obtained prior to the pharmacist intervention acted as a control comparison group. Statistical analysis was performed using SAS 9.4.
Results
A total of 172 patients were included in this study: 86 patients prior to the intervention, and 86 after implementation. Baseline demographics, laboratory values, vitals, and principal diagnoses for both groups are shown in Table 1 and Table 2. The most common indications for procalcitonin measurement were pneumonia (45.9%), chronic obstructive pulmonary disease (15.7%), and sepsis (14.5%). The remaining diagnoses were encephalopathy, fever and leukocytosis, skin and soft tissue infection, urinary tract infection or pyelonephritis, bone and joint infection, meningitis, intra-abdominal infection, and asthma exacerbation.
Antibiotic therapy was initiated in 68% of the patients overall, 59% in the baseline group and 76% in the intervention group. The duration of antibiotic use was not significantly different between the baseline (3.14 ± 4.04 days) and intervention (3.34 ± 2.8 days) groups (P = 0.1083). Furthermore, antibiotic treatment duration did not vary significantly with patient age, white blood cell count, maximum temperature, or procalcitonin level in either group. Although there was no difference in total antibiotic treatment duration, a post-hoc analysis revealed a 0.6-day decrease in the interval between the date of procalcitonin measurement and the stop date of antibiotics in the intervention group. The average time from admission to obtaining a procalcitonin level was 3 days in the baseline group and 2 days in the intervention group.
Discussion
Our study did not demonstrate a difference in total antibiotic treatment duration with procalcitonin measurement and an oral communication intervention made by a clinical pharmacist at a community teaching hospital with a well-established antimicrobial stewardship program. This may be due to several factors. First, the providers did not receive ongoing education regarding the appropriate use or interpretation of procalcitonin. The procalcitonin result in the electronic health record references the risk for progression to severe sepsis and/or septic shock, but does not indicate how to use procalcitonin as an aid in antibiotic decision-making. However, a recent study in patients with lower respiratory tract infections treated by providers who had been educated on the use of procalcitonin failed to find a reduction in total antibiotic use.9 Second, our study included hospital-wide use of procalcitonin, and was not limited to infections for which procalcitonin use has the strongest evidence (eg, upper respiratory tract infections, pneumonia, sepsis). Thus, providers may have been less likely to use protocolized guidelines. Last, we did not limit the data on antibiotic duration to patients with a procalcitonin level obtained within a defined time frame from antibiotic initiation or time of admission, and some patients had procalcitonin levels measured several days into their hospital stay. While this is likely to have skewed the data in favor of longer antibiotic treatment courses, it also represents a more realistic way in which this laboratory test is being used. Our post-hoc finding of earlier discontinuation of antibiotics after procalcitonin measurement suggests that our intervention may have influenced the decision to discontinue antibiotics. Such an effect may be augmented if procalcitonin is measured earlier in a hospital admission.
Previous studies have also failed to show that the use of procalcitonin decreased duration of antibiotics.9,10 In the aforementioned study regarding real-world outcomes in patients with lower respiratory tract infections, antibiotic duration was not reduced, despite provider education.9 A large observational study that evaluated real-world outcomes in intensive care unit patients did not find decreased antibiotic use or improved outcomes with procalcitonin use.10 With these large studies evaluating the 2 most common infectious diseases for which procalcitonin has previously been found to have clinical benefit, it is important for institutions to re-evaluate how procalcitonin is being utilized by providers. Furthermore, institutions should explore ways to optimize procalcitonin use and decrease unnecessary health care costs. Notably, the current community-acquired pneumonia guidelines recommend against routine use of procalcitonin.11
Conclusion
Outside of clinical trials, and in the absence of an algorithmic approach, procalcitonin has not consistently been shown to aid in the diagnosis or treatment of infectious diseases. It is important to have a comprehensive antimicrobial stewardship program that includes an algorithmic protocol to promote appropriate laboratory testing and reduce total antibiotic use. In addition to improved communication with providers, other interventions need to be investigated to effectively use this biomarker or limit its use.
Acknowledgment: The authors thank the Western Michigan University Department of Epidemiology and Biostatistics for their assistance in preparing this article.
Corresponding author: James Vaillant, MD, Western Michigan University, Homer Stryker MD School of Medicine, 1000 Oakland Drive, Kalamazoo, MI, 49008; [email protected].
Financial disclosures: None.
1. Maruna P, Nedelníková K, Gürlich R. Physiology and genetics of procalcitonin. Physiol Res. 2000;(49 suppl 1):S57-S61.
2. Becker KL, Snider R, Nylen ES. Procalcitonin in sepsis and systemic inflammation: a harmful biomarker and a therapeutic target. Br J Pharmacol. 2010;159:253-264.
3. Vijayan AL, Vanimaya RS, Saikant R, et al. Procalcitonin: a promising diagnostic marker for sepsis and antibiotic therapy. J Intensive Care. 2017;5:51.
4. Hey J, Thompson-Leduc P, Kirson NY, et al. Procalcitonin guidance in patients with lower respiratory tract infections: A systematic review and meta-analysis. Clin Chem Lab Med. 2018;56:1200-1209.
5. Schuetz P, Wirz Y, Sager R, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2017;10:CD007498.
6. Huang HB, Peng JM, Weng L, et al. Procalcitonin-guided antibiotic therapy in intensive care unit patients: a systematic review and meta-analysis. Ann Intensive Care. 2017;7:114.
7. Stolz D, Christ-Crain M, Bingisser R, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest. 2007;131:9-19.
8. Prkno A, Wacker C, Brunkhorst FM, Schlattmann P. Procalcitonin-guided therapy in intensive care unit patients with severe sepsis and septic shock—a systematic review and meta-analysis. Crit Care. 2013;17:R291.
9. Huang DT, Yealy DM, Filbin MR, et al. Procalcitonin-guided use of antibiotics for lower respiratory tract infections. N Engl J Med. 2018;379:236-249.
10. Chu DC, Mehta AB, Walkey AJ. Practice patterns and outcomes associated with procalcitonin use in critically ill patients with sepsis. Clin Infect Dis. 2017;64:1509-1515.
11. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45-e67.
1. Maruna P, Nedelníková K, Gürlich R. Physiology and genetics of procalcitonin. Physiol Res. 2000;(49 suppl 1):S57-S61.
2. Becker KL, Snider R, Nylen ES. Procalcitonin in sepsis and systemic inflammation: a harmful biomarker and a therapeutic target. Br J Pharmacol. 2010;159:253-264.
3. Vijayan AL, Vanimaya RS, Saikant R, et al. Procalcitonin: a promising diagnostic marker for sepsis and antibiotic therapy. J Intensive Care. 2017;5:51.
4. Hey J, Thompson-Leduc P, Kirson NY, et al. Procalcitonin guidance in patients with lower respiratory tract infections: A systematic review and meta-analysis. Clin Chem Lab Med. 2018;56:1200-1209.
5. Schuetz P, Wirz Y, Sager R, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2017;10:CD007498.
6. Huang HB, Peng JM, Weng L, et al. Procalcitonin-guided antibiotic therapy in intensive care unit patients: a systematic review and meta-analysis. Ann Intensive Care. 2017;7:114.
7. Stolz D, Christ-Crain M, Bingisser R, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest. 2007;131:9-19.
8. Prkno A, Wacker C, Brunkhorst FM, Schlattmann P. Procalcitonin-guided therapy in intensive care unit patients with severe sepsis and septic shock—a systematic review and meta-analysis. Crit Care. 2013;17:R291.
9. Huang DT, Yealy DM, Filbin MR, et al. Procalcitonin-guided use of antibiotics for lower respiratory tract infections. N Engl J Med. 2018;379:236-249.
10. Chu DC, Mehta AB, Walkey AJ. Practice patterns and outcomes associated with procalcitonin use in critically ill patients with sepsis. Clin Infect Dis. 2017;64:1509-1515.
11. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45-e67.
Atypical Features of COVID-19: A Literature Review
From the University of Florida College of Medicine, Division of Infectious Diseases and Global Medicine, Gainesville, FL.
Abstract
- Objective: To review current reports on atypical manifestations of coronavirus disease 2019 (COVID-19).
- Methods: Review of the literature.
- Results: Evidence regarding atypical features of COVID-19 is accumulating. SARS-CoV-2 can infect human cells that express the angiotensin-converting enzyme 2 receptor, which would allow for a broad spectrum of illnesses affecting the renal, cardiac, and gastrointestinal organ systems. Neurologic, cutaneous, and musculoskeletal manifestations have also been reported. The potential for SARS-CoV-2 to induce a hypercoagulable state provides another avenue for the virus to indirectly damage various organ systems, as evidenced by reports of cerebrovascular disease, myocardial injury, and a chilblain-like rash in patients with COVID-19.
- Conclusion: Because the signs and symptoms of COVID-19 may occur with varying frequency across populations, it is important to keep differentials broad when assessing patients with a clinical illness that may indeed be COVID-19.
Keywords: coronavirus; severe acute respiratory syndrome coronavirus-2; SARS-CoV-2; pandemic.
Coronavirus disease 2019 (COVID-19), the syndrome caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), was first reported in Wuhan, China, in early December 2019.1 Since then, the virus has spread quickly around the world, with the World Health Organization (WHO) declaring the coronavirus outbreak a global pandemic on March 11, 2020. As of May 21, 2020, more than 5,000,000 cases of COVID-19 have been confirmed, and more than 328,000 deaths related to COVID-19 have been reported globally.2 These numbers are expected to increase, due to the reproduction number (R0) of SARS-CoV-2. R0 represents the number of new infections generated by an infectious person in a totally naïve population.3 The WHO estimates that the R0 of SARS-CoV-2 is 1.95, with other estimates ranging from 1.4 to 6.49.3 To control the pathogen, the R0 needs to be brought under a value of 1.
A fundamental tool in lowering the R0 is prompt testing and isolation of those who display signs and symptoms of infection. SARS-CoV-2 is still a novel pathogen about which we know relatively little. The common symptoms of COVID-19 are now well known—including fever, fatigue, anorexia, cough, and shortness of breath—but atypical manifestations of this viral continue to be reported and described. To help clinicians across specialties and settings identify patients with possible infection, we have summarized findings from current reports on COVID-19 manifestations involving the renal, cardiac, gastrointestinal (GI), and other organ systems.
Renal
During the 2003 SARS-CoV-1 outbreak, acute kidney injury (AKI) was an uncommon complication of the infection, but early reports suggest that AKI may occur more commonly with COVID-19.4 In a study of 193 patients with laboratory-confirmed COVID-19 treated in 3 Chinese hospitals, 59% presented with proteinuria, 44% with hematuria, 14% with increased blood urea nitrogen, and 10% with increased levels of serum creatinine.4 These markers, indicative of AKI, may be associated with increased mortality. Among this cohort, those with AKI had a mortality risk 5.3 times higher than those who did not have AKI.4 The pathophysiology of renal disease in COVID-19 may be related to dehydration or inflammatory mediators, causing decreased renal perfusion and cytokine storm, but evidence also suggests that SARS-CoV-2 is able to directly infect kidney cells.5 The virus infects cells by using angiotensin-converting enzyme 2 (ACE2) on the cell membrane as a cell entry receptor; ACE2 is expressed on the kidney, heart, and GI cells, and this may allow SARS-CoV-2 to directly infect and damage these organs. Other potential mechanisms of renal injury include overproduction of proinflammatory cytokines and administration of nephrotoxic drugs. No matter the mechanism, however, increased serum creatinine and blood urea nitrogen correlate with an increased likelihood of requiring intensive care unit (ICU) admission.6 Therefore, clinicians should carefully monitor renal function in patients with COVID-19.
Cardiac
In a report of 138 Chinese patients hospitalized for COVID-19, 36 required ICU admission: 44.4% of these had arrhythmias and 22.2% had developed acute cardiac injury.6 In addition, the cardiac cell injury biomarker troponin I was more likely to be elevated in ICU patients.6 A study of 21 patients admitted to the ICU in Washington State found elevated levels of brain natriuretic peptide.7 These biomarkers reflect the presence of myocardial stress, but do not necessarily indicate direct myocardial infection. Case reports of fulminant myocarditis in those with COVID-19 have begun to surface, however.8,9 An examination of 68 deaths in persons with COVID-19 concluded that 7% were caused by myocarditis with circulatory failure.10
The pathophysiology of myocardial injury in COVID-19 is likely multifactorial. This includes increased inflammatory mediators, hypoxemia, and metabolic changes that can directly damage myocardial tissue. These factors can also exacerbate comorbid conditions, such as coronary artery disease, leading to ischemia and dysfunction of preexisting electrical conduction abnormalities. However, pathologic evidence of myocarditis and the presence of the ACE2 receptor, which may be a mediator of cardiac function, on cardiac muscle cells suggest that SARS-CoV-2 is capable of directly infecting and damaging myocardial cells. Other proposed mechanisms include infection-mediated downregulation of ACE2, causing cardiac dysfunction, or thrombus formation.11 Although respiratory failure is the most common source of advanced illness in COVID-19 patients, myocarditis and arrhythmias can be life-threatening manifestations of the disease.
Gastrointestinal
As noted, ACE2 is expressed in the GI tract. In 73 patients hospitalized for COVID-19, 53.4% tested positive for SARS-CoV-2 RNA in stool, and 23.4% continued to have RNA-positive stool samples even after their respiratory samples tested negative.12 These findings suggest the potential for SARS-CoV-2 to spread through fecal-oral transmission in those who are asymptomatic, pre-symptomatic, or symptomatic. This mode of transmission has yet to be determined conclusively, and more research is needed. However, GI symptoms have been reported in persons with COVID-19. Among 138 hospitalized patients, 10.1% had complaints of diarrhea and nausea and 3.6% reported vomiting.6 Those who reported nausea and diarrhea noted that they developed these symptoms 1 to 2 days before they developed fever.6 Also, among a cohort of 1099 Chinese patients with COVID-19, 3.8% complained of diarrhea.13 Although diarrhea does not occur in a majority of patients, GI complaints, such as nausea, vomiting, or diarrhea, should raise clinical suspicion for COVID-19, and in known areas of active transmission, testing of patients with GI symptoms is likely warranted.
Ocular
Ocular manifestations of COVID-19 are now being described, and should be taken into consideration when examining a patient. In a study of 38 patients with COVID-19 from Hubei province, China, 31.6% had ocular findings consistent with conjunctivitis, including conjunctival hyperemia, chemosis, epiphora, and increased ocular secretions.14 SARS-CoV-2 was detected in conjunctival and nasopharyngeal samples in 2 patients from this cohort. Conjunctival congestion was reported in a cohort of 1099 patients with COVID-19 treated at multiple centers throughout China, but at a much lower incidence, approximately 0.8%.13 Because SARS-CoV-2 can cause conjunctival disease and has been detected in samples from the external surface of the eye, it appears the virus is transmissible from tears or contact with the eye itself.
Neurologic
Common reported neurologic symptoms include dizziness, headache, impaired consciousness, ataxia, and cerebrovascular events. In a cohort of 214 patients from Wuhan, China, 36.4% had some form of neurological insult.15 These symptoms were more common in those with severe illness (P = 0.02).15 Two interesting neurologic symptoms that have been described are anosmia (loss of smell) and ageusia (loss of taste), which are being found primarily in tandem. It is still unclear how many people with COVID-19 are experiencing these symptoms, but a report from Italy estimates 19.4% of 320 patients examined had chemosensory dysfunction.16 The aforementioned report from Wuhan, China, found that 5.1% had anosmia and 5.6% had ageusia.15 The presence of anosmia/ageusia in some patients suggests that SARS-CoV-2 may enter the central nervous system (CNS) through a retrograde neuronal route.15 In addition, a case report from Japan described a 24-year-old man who presented with meningitis/encephalitis and had SARS-CoV-2 RNA present in his cerebrospinal fluid, showing that SARS-CoV-2 can penetrate into the CNS.17
SARS-CoV-2 may also have an association with Guillain–Barré syndrome, as this condition was reported in 5 patients from 3 hospitals in Northern Italy.18 The symptoms of Guillain–Barré syndrome presented 5 to 10 days after the typical COVID-19 symptoms, and evolved over 36 hours to 4 days afterwards. Four of the 5 patients experienced flaccid tetraparesis or tetraplegia, and 3 required mechanical ventilation.18
Another possible cause of neurologic injury in COVID-19 is damage to endothelial cells in cerebral blood vessels, causing thrombus formation and possibly increasing the risk of acute ischemic stroke.15,19 Supporting this mechanism of injury, significantly lower platelet counts were noted in patients with CNS symptoms (P = 0.005).15 Other hematological impacts of COVID-19 have been reported, particularly hypercoagulability, as evidenced by elevated D-dimer levels.13,20 This hypercoagulable state is linked to overproduction of proinflammatory cytokines (cytokine storm), leading to dysregulation of coagulation pathways and reduced concentrations of anticoagulants, such as protein C, antithrombin III, and tissue factor pathway inhibitor.21
Cutaneous
Cutaneous findings emerging in persons with COVID-19 demonstrate features of small-vessel and capillary occlusion, including erythematous skin eruptions and petechial rash. One report from Italy noted that 20.4% of patients with COVID-19 (n = 88) had a cutaneous finding, with a cutaneous manifestation developing in 8 at the onset of illness and in 10 following hospital admission.22 Fourteen patients had an erythematous rash, primarily on the trunk, with 3 patients having a diffuse urticarial appearing rash, and 1 patient developing vesicles.22 The severity of illness did not appear to correlate with the cutaneous manifestation, and the lesions healed within a few days.
One case report described a patient from Bangkok who was thought to be suffering from dengue fever, but was found to have SARS-CoV-2 infection. He initially presented with skin rash and petechiae, and later developed respiratory disease.23
Other dermatologic findings of COVID-19 resemble chilblains disease, colloquially referred to as “COVID toes.” Two women, 27 and 35 years old, presented to a dermatology clinic in Qatar with a chief complaint of skin rash, described as red-purple papules on the dorsal aspects of the fingers bilaterally.22 Both patients had an unremarkable medical and drug history, but recent travel to the United Kingdom dictated SARS-CoV-2 screening, which was positive.24 An Italian case report describes a 23-year-old man who tested positive for SARS-CoV-2 and had violaceous plaques on an erythematous background on his feet, without any lesions on his hands.25 Since chilblains is less common in the warmer months and these events correspond with the COVID-19 pandemic, SARS-CoV-2 infection is the suspected etiology. The pathophysiology of these lesions is unclear, and more research is needed. As more data become available, we may see cutaneous manifestations in patients with COVID-19 similar to those commonly reported with other viral infectious processes.
Musculoskeletal
Of 138 patients hospitalized in Wuhan, China, for COVID-19, 34.8% presented with myalgia; the presence of myalgia does not appear to be correlated with an increased likelihood of ICU admission.6 Myalgia or arthralgia was also reported in 14.9% among the cohort of 1099 COVID-19 patients in China.13 These musculoskeletal symptoms are described among large muscle groups found in the extremities, trunk, and back, and should raise suspicion in patients who present with other signs and symptoms concerning for COVID-19.
Conclusion
Evidence regarding atypical features of COVID-19 is accumulating. SARS-CoV-2 can infect a human cells that express the ACE2 receptor, which would allow for a broad spectrum of illnesses. The potential for SARS-CoV-2 to induce a hypercoagulable state allows it to indirectly damage various organ systems,20 leading to cerebrovascular disease, myocardial injury, and a chilblain-like rash. Clinicians must be aware of these unique features, as early recognition of persons who present with COVID-19 will allow for prompt testing, institution of infection control and isolation practices, and treatment, as needed, among those infected. Also, this is a pandemic involving a novel virus affecting different populations throughout the world, and these signs and symptoms may occur with varying frequency across populations. Therefore, it is important to keep differentials broad when assessing patients with a clinical illness that may indeed be COVID-19.
Corresponding author: Norman L. Beatty, MD, [email protected].
Financial disclosures: None.
1. WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020 [press release]. World Health Organization; March 11, 2020.
2. Coronavirus COVID-19 Global Cases by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. Johns Hopkins CSSE. https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6 Accessed May 15, 2020.
3. Liu Y, Gayle AA, Wilder-Smith A, Rocklöv J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Travel Med. 2020;27(2):taaa021. doi:10.1093/jtm/taaa021
4. Li Z, Wu M, Guo J, et al. Caution on kidney dysfunctions of 2019-nCoV patients. medRxiv preprint. doi: 10.1101/2020.02.08.20021212
5. Li W, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450-454. doi: 10.1038/nature02145.
6. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. doi:10.1001/jama.2020.1585
7. Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323:1612‐1614. doi:10.1001/jama.2020.4326
8. Chen C, Zhou Y, Wang DW. SARS-CoV-2: a potential novel etiology of fulminant myocarditis. Herz. 2020;45:230-232. doi: 10.1007/s00059-020-04909-z
9. Hu H, Ma F, Wei X, Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. 2020 Mar 16;ehaa190. doi: 10.1093/eurheartj/ehaa190
10. Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846-848. doi:10.1007/s00134-020-05991-x
11. Akhmerov A, Marban E. COVID-19 and the heart. Circ Res. 2020;126:1443-1455. doi:10.1161/CIRCRESAHA.120.317055
12. Xiao F, Tang M, Zheng X, et al. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158:1831-1833. doi: 10.1053/j.gastro.2020.02.055
13. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1078-1720. doi: 10.1056/NEJMoa2002032
14. Wu P, Duan F, Luo C, et al. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020 Mar 31;e201291. doi: 10.1001/jamaophthalmol.2020.1291
15. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020 Apr 10. doi: 10.1001/jamaneurol.2020.1127
16. Vaira LA, Salzano G, Deiana G, De Riu G. Anosmia and ageusia: common findings in COVID-19 patients. Laryngoscope. 2020 Apr 1. doi: 10.1002/lary.28692
17. Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS-coronavirus-2. Int J Infect Dis. 2020;94:55-58. doi: 10.1016/j.ijid.2020.03.062
18. Toscano G, Palmerini F, Ravaglia S, et al. Guillain–Barré syndrome associated with SARS-CoV-2. N Engl J Med. 2020 Apr 17;NEJMc2009191. doi:10.1056/nejmc2009191
19. Dafer RM, Osteraas ND, Biller J. Acute stroke care in the coronavirus disease 2019 pandemic. J Stroke Cerebrovascular Dis. 2020 Apr 17:104881. doi: 10.1016/j.jstrokecerebrovasdis.2020.104881
20. Terpos E, Ntanasis-Stathopoulos I, Elalamy I, et al. Hematological findings and complications of COVID-19. Am J Hematol. 2020;10.1002/ajh.25829. doi:10.1002/ajh.25829
21. Jose RJ, Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. 2020;S2213-2600(20)30216-2. doi:10.1016/S2213-2600(20)30216-2
22. Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020 Mar 26. doi: 10.1111/jdv.16387
23. Joob B, Wiwanitkit V. COVID-19 can present with a rash and be mistaken for dengue. J Am Acad Dermatol. 2020;82(5):e177. doi: 10.1016/j.jaad.2020.03.036
24. Alramthan A, Aldaraji W. A Case of COVID‐19 presenting in clinical picture resembling chilblains disease. First report from the Middle East. Clin Exp Dermatol. 2020 Apr 17. doi: 10.1111/ced.14243
25. Kolivras A, Dehavay F, Delplace D, et al. Coronavirus (COVID-19) infection–induced chilblains: a case report with histopathologic findings. JAAD Case Rep. 2020 Apr 18. doi: 10.1016/j.jdcr.2020.04.011
From the University of Florida College of Medicine, Division of Infectious Diseases and Global Medicine, Gainesville, FL.
Abstract
- Objective: To review current reports on atypical manifestations of coronavirus disease 2019 (COVID-19).
- Methods: Review of the literature.
- Results: Evidence regarding atypical features of COVID-19 is accumulating. SARS-CoV-2 can infect human cells that express the angiotensin-converting enzyme 2 receptor, which would allow for a broad spectrum of illnesses affecting the renal, cardiac, and gastrointestinal organ systems. Neurologic, cutaneous, and musculoskeletal manifestations have also been reported. The potential for SARS-CoV-2 to induce a hypercoagulable state provides another avenue for the virus to indirectly damage various organ systems, as evidenced by reports of cerebrovascular disease, myocardial injury, and a chilblain-like rash in patients with COVID-19.
- Conclusion: Because the signs and symptoms of COVID-19 may occur with varying frequency across populations, it is important to keep differentials broad when assessing patients with a clinical illness that may indeed be COVID-19.
Keywords: coronavirus; severe acute respiratory syndrome coronavirus-2; SARS-CoV-2; pandemic.
Coronavirus disease 2019 (COVID-19), the syndrome caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), was first reported in Wuhan, China, in early December 2019.1 Since then, the virus has spread quickly around the world, with the World Health Organization (WHO) declaring the coronavirus outbreak a global pandemic on March 11, 2020. As of May 21, 2020, more than 5,000,000 cases of COVID-19 have been confirmed, and more than 328,000 deaths related to COVID-19 have been reported globally.2 These numbers are expected to increase, due to the reproduction number (R0) of SARS-CoV-2. R0 represents the number of new infections generated by an infectious person in a totally naïve population.3 The WHO estimates that the R0 of SARS-CoV-2 is 1.95, with other estimates ranging from 1.4 to 6.49.3 To control the pathogen, the R0 needs to be brought under a value of 1.
A fundamental tool in lowering the R0 is prompt testing and isolation of those who display signs and symptoms of infection. SARS-CoV-2 is still a novel pathogen about which we know relatively little. The common symptoms of COVID-19 are now well known—including fever, fatigue, anorexia, cough, and shortness of breath—but atypical manifestations of this viral continue to be reported and described. To help clinicians across specialties and settings identify patients with possible infection, we have summarized findings from current reports on COVID-19 manifestations involving the renal, cardiac, gastrointestinal (GI), and other organ systems.
Renal
During the 2003 SARS-CoV-1 outbreak, acute kidney injury (AKI) was an uncommon complication of the infection, but early reports suggest that AKI may occur more commonly with COVID-19.4 In a study of 193 patients with laboratory-confirmed COVID-19 treated in 3 Chinese hospitals, 59% presented with proteinuria, 44% with hematuria, 14% with increased blood urea nitrogen, and 10% with increased levels of serum creatinine.4 These markers, indicative of AKI, may be associated with increased mortality. Among this cohort, those with AKI had a mortality risk 5.3 times higher than those who did not have AKI.4 The pathophysiology of renal disease in COVID-19 may be related to dehydration or inflammatory mediators, causing decreased renal perfusion and cytokine storm, but evidence also suggests that SARS-CoV-2 is able to directly infect kidney cells.5 The virus infects cells by using angiotensin-converting enzyme 2 (ACE2) on the cell membrane as a cell entry receptor; ACE2 is expressed on the kidney, heart, and GI cells, and this may allow SARS-CoV-2 to directly infect and damage these organs. Other potential mechanisms of renal injury include overproduction of proinflammatory cytokines and administration of nephrotoxic drugs. No matter the mechanism, however, increased serum creatinine and blood urea nitrogen correlate with an increased likelihood of requiring intensive care unit (ICU) admission.6 Therefore, clinicians should carefully monitor renal function in patients with COVID-19.
Cardiac
In a report of 138 Chinese patients hospitalized for COVID-19, 36 required ICU admission: 44.4% of these had arrhythmias and 22.2% had developed acute cardiac injury.6 In addition, the cardiac cell injury biomarker troponin I was more likely to be elevated in ICU patients.6 A study of 21 patients admitted to the ICU in Washington State found elevated levels of brain natriuretic peptide.7 These biomarkers reflect the presence of myocardial stress, but do not necessarily indicate direct myocardial infection. Case reports of fulminant myocarditis in those with COVID-19 have begun to surface, however.8,9 An examination of 68 deaths in persons with COVID-19 concluded that 7% were caused by myocarditis with circulatory failure.10
The pathophysiology of myocardial injury in COVID-19 is likely multifactorial. This includes increased inflammatory mediators, hypoxemia, and metabolic changes that can directly damage myocardial tissue. These factors can also exacerbate comorbid conditions, such as coronary artery disease, leading to ischemia and dysfunction of preexisting electrical conduction abnormalities. However, pathologic evidence of myocarditis and the presence of the ACE2 receptor, which may be a mediator of cardiac function, on cardiac muscle cells suggest that SARS-CoV-2 is capable of directly infecting and damaging myocardial cells. Other proposed mechanisms include infection-mediated downregulation of ACE2, causing cardiac dysfunction, or thrombus formation.11 Although respiratory failure is the most common source of advanced illness in COVID-19 patients, myocarditis and arrhythmias can be life-threatening manifestations of the disease.
Gastrointestinal
As noted, ACE2 is expressed in the GI tract. In 73 patients hospitalized for COVID-19, 53.4% tested positive for SARS-CoV-2 RNA in stool, and 23.4% continued to have RNA-positive stool samples even after their respiratory samples tested negative.12 These findings suggest the potential for SARS-CoV-2 to spread through fecal-oral transmission in those who are asymptomatic, pre-symptomatic, or symptomatic. This mode of transmission has yet to be determined conclusively, and more research is needed. However, GI symptoms have been reported in persons with COVID-19. Among 138 hospitalized patients, 10.1% had complaints of diarrhea and nausea and 3.6% reported vomiting.6 Those who reported nausea and diarrhea noted that they developed these symptoms 1 to 2 days before they developed fever.6 Also, among a cohort of 1099 Chinese patients with COVID-19, 3.8% complained of diarrhea.13 Although diarrhea does not occur in a majority of patients, GI complaints, such as nausea, vomiting, or diarrhea, should raise clinical suspicion for COVID-19, and in known areas of active transmission, testing of patients with GI symptoms is likely warranted.
Ocular
Ocular manifestations of COVID-19 are now being described, and should be taken into consideration when examining a patient. In a study of 38 patients with COVID-19 from Hubei province, China, 31.6% had ocular findings consistent with conjunctivitis, including conjunctival hyperemia, chemosis, epiphora, and increased ocular secretions.14 SARS-CoV-2 was detected in conjunctival and nasopharyngeal samples in 2 patients from this cohort. Conjunctival congestion was reported in a cohort of 1099 patients with COVID-19 treated at multiple centers throughout China, but at a much lower incidence, approximately 0.8%.13 Because SARS-CoV-2 can cause conjunctival disease and has been detected in samples from the external surface of the eye, it appears the virus is transmissible from tears or contact with the eye itself.
Neurologic
Common reported neurologic symptoms include dizziness, headache, impaired consciousness, ataxia, and cerebrovascular events. In a cohort of 214 patients from Wuhan, China, 36.4% had some form of neurological insult.15 These symptoms were more common in those with severe illness (P = 0.02).15 Two interesting neurologic symptoms that have been described are anosmia (loss of smell) and ageusia (loss of taste), which are being found primarily in tandem. It is still unclear how many people with COVID-19 are experiencing these symptoms, but a report from Italy estimates 19.4% of 320 patients examined had chemosensory dysfunction.16 The aforementioned report from Wuhan, China, found that 5.1% had anosmia and 5.6% had ageusia.15 The presence of anosmia/ageusia in some patients suggests that SARS-CoV-2 may enter the central nervous system (CNS) through a retrograde neuronal route.15 In addition, a case report from Japan described a 24-year-old man who presented with meningitis/encephalitis and had SARS-CoV-2 RNA present in his cerebrospinal fluid, showing that SARS-CoV-2 can penetrate into the CNS.17
SARS-CoV-2 may also have an association with Guillain–Barré syndrome, as this condition was reported in 5 patients from 3 hospitals in Northern Italy.18 The symptoms of Guillain–Barré syndrome presented 5 to 10 days after the typical COVID-19 symptoms, and evolved over 36 hours to 4 days afterwards. Four of the 5 patients experienced flaccid tetraparesis or tetraplegia, and 3 required mechanical ventilation.18
Another possible cause of neurologic injury in COVID-19 is damage to endothelial cells in cerebral blood vessels, causing thrombus formation and possibly increasing the risk of acute ischemic stroke.15,19 Supporting this mechanism of injury, significantly lower platelet counts were noted in patients with CNS symptoms (P = 0.005).15 Other hematological impacts of COVID-19 have been reported, particularly hypercoagulability, as evidenced by elevated D-dimer levels.13,20 This hypercoagulable state is linked to overproduction of proinflammatory cytokines (cytokine storm), leading to dysregulation of coagulation pathways and reduced concentrations of anticoagulants, such as protein C, antithrombin III, and tissue factor pathway inhibitor.21
Cutaneous
Cutaneous findings emerging in persons with COVID-19 demonstrate features of small-vessel and capillary occlusion, including erythematous skin eruptions and petechial rash. One report from Italy noted that 20.4% of patients with COVID-19 (n = 88) had a cutaneous finding, with a cutaneous manifestation developing in 8 at the onset of illness and in 10 following hospital admission.22 Fourteen patients had an erythematous rash, primarily on the trunk, with 3 patients having a diffuse urticarial appearing rash, and 1 patient developing vesicles.22 The severity of illness did not appear to correlate with the cutaneous manifestation, and the lesions healed within a few days.
One case report described a patient from Bangkok who was thought to be suffering from dengue fever, but was found to have SARS-CoV-2 infection. He initially presented with skin rash and petechiae, and later developed respiratory disease.23
Other dermatologic findings of COVID-19 resemble chilblains disease, colloquially referred to as “COVID toes.” Two women, 27 and 35 years old, presented to a dermatology clinic in Qatar with a chief complaint of skin rash, described as red-purple papules on the dorsal aspects of the fingers bilaterally.22 Both patients had an unremarkable medical and drug history, but recent travel to the United Kingdom dictated SARS-CoV-2 screening, which was positive.24 An Italian case report describes a 23-year-old man who tested positive for SARS-CoV-2 and had violaceous plaques on an erythematous background on his feet, without any lesions on his hands.25 Since chilblains is less common in the warmer months and these events correspond with the COVID-19 pandemic, SARS-CoV-2 infection is the suspected etiology. The pathophysiology of these lesions is unclear, and more research is needed. As more data become available, we may see cutaneous manifestations in patients with COVID-19 similar to those commonly reported with other viral infectious processes.
Musculoskeletal
Of 138 patients hospitalized in Wuhan, China, for COVID-19, 34.8% presented with myalgia; the presence of myalgia does not appear to be correlated with an increased likelihood of ICU admission.6 Myalgia or arthralgia was also reported in 14.9% among the cohort of 1099 COVID-19 patients in China.13 These musculoskeletal symptoms are described among large muscle groups found in the extremities, trunk, and back, and should raise suspicion in patients who present with other signs and symptoms concerning for COVID-19.
Conclusion
Evidence regarding atypical features of COVID-19 is accumulating. SARS-CoV-2 can infect a human cells that express the ACE2 receptor, which would allow for a broad spectrum of illnesses. The potential for SARS-CoV-2 to induce a hypercoagulable state allows it to indirectly damage various organ systems,20 leading to cerebrovascular disease, myocardial injury, and a chilblain-like rash. Clinicians must be aware of these unique features, as early recognition of persons who present with COVID-19 will allow for prompt testing, institution of infection control and isolation practices, and treatment, as needed, among those infected. Also, this is a pandemic involving a novel virus affecting different populations throughout the world, and these signs and symptoms may occur with varying frequency across populations. Therefore, it is important to keep differentials broad when assessing patients with a clinical illness that may indeed be COVID-19.
Corresponding author: Norman L. Beatty, MD, [email protected].
Financial disclosures: None.
From the University of Florida College of Medicine, Division of Infectious Diseases and Global Medicine, Gainesville, FL.
Abstract
- Objective: To review current reports on atypical manifestations of coronavirus disease 2019 (COVID-19).
- Methods: Review of the literature.
- Results: Evidence regarding atypical features of COVID-19 is accumulating. SARS-CoV-2 can infect human cells that express the angiotensin-converting enzyme 2 receptor, which would allow for a broad spectrum of illnesses affecting the renal, cardiac, and gastrointestinal organ systems. Neurologic, cutaneous, and musculoskeletal manifestations have also been reported. The potential for SARS-CoV-2 to induce a hypercoagulable state provides another avenue for the virus to indirectly damage various organ systems, as evidenced by reports of cerebrovascular disease, myocardial injury, and a chilblain-like rash in patients with COVID-19.
- Conclusion: Because the signs and symptoms of COVID-19 may occur with varying frequency across populations, it is important to keep differentials broad when assessing patients with a clinical illness that may indeed be COVID-19.
Keywords: coronavirus; severe acute respiratory syndrome coronavirus-2; SARS-CoV-2; pandemic.
Coronavirus disease 2019 (COVID-19), the syndrome caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), was first reported in Wuhan, China, in early December 2019.1 Since then, the virus has spread quickly around the world, with the World Health Organization (WHO) declaring the coronavirus outbreak a global pandemic on March 11, 2020. As of May 21, 2020, more than 5,000,000 cases of COVID-19 have been confirmed, and more than 328,000 deaths related to COVID-19 have been reported globally.2 These numbers are expected to increase, due to the reproduction number (R0) of SARS-CoV-2. R0 represents the number of new infections generated by an infectious person in a totally naïve population.3 The WHO estimates that the R0 of SARS-CoV-2 is 1.95, with other estimates ranging from 1.4 to 6.49.3 To control the pathogen, the R0 needs to be brought under a value of 1.
A fundamental tool in lowering the R0 is prompt testing and isolation of those who display signs and symptoms of infection. SARS-CoV-2 is still a novel pathogen about which we know relatively little. The common symptoms of COVID-19 are now well known—including fever, fatigue, anorexia, cough, and shortness of breath—but atypical manifestations of this viral continue to be reported and described. To help clinicians across specialties and settings identify patients with possible infection, we have summarized findings from current reports on COVID-19 manifestations involving the renal, cardiac, gastrointestinal (GI), and other organ systems.
Renal
During the 2003 SARS-CoV-1 outbreak, acute kidney injury (AKI) was an uncommon complication of the infection, but early reports suggest that AKI may occur more commonly with COVID-19.4 In a study of 193 patients with laboratory-confirmed COVID-19 treated in 3 Chinese hospitals, 59% presented with proteinuria, 44% with hematuria, 14% with increased blood urea nitrogen, and 10% with increased levels of serum creatinine.4 These markers, indicative of AKI, may be associated with increased mortality. Among this cohort, those with AKI had a mortality risk 5.3 times higher than those who did not have AKI.4 The pathophysiology of renal disease in COVID-19 may be related to dehydration or inflammatory mediators, causing decreased renal perfusion and cytokine storm, but evidence also suggests that SARS-CoV-2 is able to directly infect kidney cells.5 The virus infects cells by using angiotensin-converting enzyme 2 (ACE2) on the cell membrane as a cell entry receptor; ACE2 is expressed on the kidney, heart, and GI cells, and this may allow SARS-CoV-2 to directly infect and damage these organs. Other potential mechanisms of renal injury include overproduction of proinflammatory cytokines and administration of nephrotoxic drugs. No matter the mechanism, however, increased serum creatinine and blood urea nitrogen correlate with an increased likelihood of requiring intensive care unit (ICU) admission.6 Therefore, clinicians should carefully monitor renal function in patients with COVID-19.
Cardiac
In a report of 138 Chinese patients hospitalized for COVID-19, 36 required ICU admission: 44.4% of these had arrhythmias and 22.2% had developed acute cardiac injury.6 In addition, the cardiac cell injury biomarker troponin I was more likely to be elevated in ICU patients.6 A study of 21 patients admitted to the ICU in Washington State found elevated levels of brain natriuretic peptide.7 These biomarkers reflect the presence of myocardial stress, but do not necessarily indicate direct myocardial infection. Case reports of fulminant myocarditis in those with COVID-19 have begun to surface, however.8,9 An examination of 68 deaths in persons with COVID-19 concluded that 7% were caused by myocarditis with circulatory failure.10
The pathophysiology of myocardial injury in COVID-19 is likely multifactorial. This includes increased inflammatory mediators, hypoxemia, and metabolic changes that can directly damage myocardial tissue. These factors can also exacerbate comorbid conditions, such as coronary artery disease, leading to ischemia and dysfunction of preexisting electrical conduction abnormalities. However, pathologic evidence of myocarditis and the presence of the ACE2 receptor, which may be a mediator of cardiac function, on cardiac muscle cells suggest that SARS-CoV-2 is capable of directly infecting and damaging myocardial cells. Other proposed mechanisms include infection-mediated downregulation of ACE2, causing cardiac dysfunction, or thrombus formation.11 Although respiratory failure is the most common source of advanced illness in COVID-19 patients, myocarditis and arrhythmias can be life-threatening manifestations of the disease.
Gastrointestinal
As noted, ACE2 is expressed in the GI tract. In 73 patients hospitalized for COVID-19, 53.4% tested positive for SARS-CoV-2 RNA in stool, and 23.4% continued to have RNA-positive stool samples even after their respiratory samples tested negative.12 These findings suggest the potential for SARS-CoV-2 to spread through fecal-oral transmission in those who are asymptomatic, pre-symptomatic, or symptomatic. This mode of transmission has yet to be determined conclusively, and more research is needed. However, GI symptoms have been reported in persons with COVID-19. Among 138 hospitalized patients, 10.1% had complaints of diarrhea and nausea and 3.6% reported vomiting.6 Those who reported nausea and diarrhea noted that they developed these symptoms 1 to 2 days before they developed fever.6 Also, among a cohort of 1099 Chinese patients with COVID-19, 3.8% complained of diarrhea.13 Although diarrhea does not occur in a majority of patients, GI complaints, such as nausea, vomiting, or diarrhea, should raise clinical suspicion for COVID-19, and in known areas of active transmission, testing of patients with GI symptoms is likely warranted.
Ocular
Ocular manifestations of COVID-19 are now being described, and should be taken into consideration when examining a patient. In a study of 38 patients with COVID-19 from Hubei province, China, 31.6% had ocular findings consistent with conjunctivitis, including conjunctival hyperemia, chemosis, epiphora, and increased ocular secretions.14 SARS-CoV-2 was detected in conjunctival and nasopharyngeal samples in 2 patients from this cohort. Conjunctival congestion was reported in a cohort of 1099 patients with COVID-19 treated at multiple centers throughout China, but at a much lower incidence, approximately 0.8%.13 Because SARS-CoV-2 can cause conjunctival disease and has been detected in samples from the external surface of the eye, it appears the virus is transmissible from tears or contact with the eye itself.
Neurologic
Common reported neurologic symptoms include dizziness, headache, impaired consciousness, ataxia, and cerebrovascular events. In a cohort of 214 patients from Wuhan, China, 36.4% had some form of neurological insult.15 These symptoms were more common in those with severe illness (P = 0.02).15 Two interesting neurologic symptoms that have been described are anosmia (loss of smell) and ageusia (loss of taste), which are being found primarily in tandem. It is still unclear how many people with COVID-19 are experiencing these symptoms, but a report from Italy estimates 19.4% of 320 patients examined had chemosensory dysfunction.16 The aforementioned report from Wuhan, China, found that 5.1% had anosmia and 5.6% had ageusia.15 The presence of anosmia/ageusia in some patients suggests that SARS-CoV-2 may enter the central nervous system (CNS) through a retrograde neuronal route.15 In addition, a case report from Japan described a 24-year-old man who presented with meningitis/encephalitis and had SARS-CoV-2 RNA present in his cerebrospinal fluid, showing that SARS-CoV-2 can penetrate into the CNS.17
SARS-CoV-2 may also have an association with Guillain–Barré syndrome, as this condition was reported in 5 patients from 3 hospitals in Northern Italy.18 The symptoms of Guillain–Barré syndrome presented 5 to 10 days after the typical COVID-19 symptoms, and evolved over 36 hours to 4 days afterwards. Four of the 5 patients experienced flaccid tetraparesis or tetraplegia, and 3 required mechanical ventilation.18
Another possible cause of neurologic injury in COVID-19 is damage to endothelial cells in cerebral blood vessels, causing thrombus formation and possibly increasing the risk of acute ischemic stroke.15,19 Supporting this mechanism of injury, significantly lower platelet counts were noted in patients with CNS symptoms (P = 0.005).15 Other hematological impacts of COVID-19 have been reported, particularly hypercoagulability, as evidenced by elevated D-dimer levels.13,20 This hypercoagulable state is linked to overproduction of proinflammatory cytokines (cytokine storm), leading to dysregulation of coagulation pathways and reduced concentrations of anticoagulants, such as protein C, antithrombin III, and tissue factor pathway inhibitor.21
Cutaneous
Cutaneous findings emerging in persons with COVID-19 demonstrate features of small-vessel and capillary occlusion, including erythematous skin eruptions and petechial rash. One report from Italy noted that 20.4% of patients with COVID-19 (n = 88) had a cutaneous finding, with a cutaneous manifestation developing in 8 at the onset of illness and in 10 following hospital admission.22 Fourteen patients had an erythematous rash, primarily on the trunk, with 3 patients having a diffuse urticarial appearing rash, and 1 patient developing vesicles.22 The severity of illness did not appear to correlate with the cutaneous manifestation, and the lesions healed within a few days.
One case report described a patient from Bangkok who was thought to be suffering from dengue fever, but was found to have SARS-CoV-2 infection. He initially presented with skin rash and petechiae, and later developed respiratory disease.23
Other dermatologic findings of COVID-19 resemble chilblains disease, colloquially referred to as “COVID toes.” Two women, 27 and 35 years old, presented to a dermatology clinic in Qatar with a chief complaint of skin rash, described as red-purple papules on the dorsal aspects of the fingers bilaterally.22 Both patients had an unremarkable medical and drug history, but recent travel to the United Kingdom dictated SARS-CoV-2 screening, which was positive.24 An Italian case report describes a 23-year-old man who tested positive for SARS-CoV-2 and had violaceous plaques on an erythematous background on his feet, without any lesions on his hands.25 Since chilblains is less common in the warmer months and these events correspond with the COVID-19 pandemic, SARS-CoV-2 infection is the suspected etiology. The pathophysiology of these lesions is unclear, and more research is needed. As more data become available, we may see cutaneous manifestations in patients with COVID-19 similar to those commonly reported with other viral infectious processes.
Musculoskeletal
Of 138 patients hospitalized in Wuhan, China, for COVID-19, 34.8% presented with myalgia; the presence of myalgia does not appear to be correlated with an increased likelihood of ICU admission.6 Myalgia or arthralgia was also reported in 14.9% among the cohort of 1099 COVID-19 patients in China.13 These musculoskeletal symptoms are described among large muscle groups found in the extremities, trunk, and back, and should raise suspicion in patients who present with other signs and symptoms concerning for COVID-19.
Conclusion
Evidence regarding atypical features of COVID-19 is accumulating. SARS-CoV-2 can infect a human cells that express the ACE2 receptor, which would allow for a broad spectrum of illnesses. The potential for SARS-CoV-2 to induce a hypercoagulable state allows it to indirectly damage various organ systems,20 leading to cerebrovascular disease, myocardial injury, and a chilblain-like rash. Clinicians must be aware of these unique features, as early recognition of persons who present with COVID-19 will allow for prompt testing, institution of infection control and isolation practices, and treatment, as needed, among those infected. Also, this is a pandemic involving a novel virus affecting different populations throughout the world, and these signs and symptoms may occur with varying frequency across populations. Therefore, it is important to keep differentials broad when assessing patients with a clinical illness that may indeed be COVID-19.
Corresponding author: Norman L. Beatty, MD, [email protected].
Financial disclosures: None.
1. WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020 [press release]. World Health Organization; March 11, 2020.
2. Coronavirus COVID-19 Global Cases by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. Johns Hopkins CSSE. https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6 Accessed May 15, 2020.
3. Liu Y, Gayle AA, Wilder-Smith A, Rocklöv J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Travel Med. 2020;27(2):taaa021. doi:10.1093/jtm/taaa021
4. Li Z, Wu M, Guo J, et al. Caution on kidney dysfunctions of 2019-nCoV patients. medRxiv preprint. doi: 10.1101/2020.02.08.20021212
5. Li W, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450-454. doi: 10.1038/nature02145.
6. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. doi:10.1001/jama.2020.1585
7. Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323:1612‐1614. doi:10.1001/jama.2020.4326
8. Chen C, Zhou Y, Wang DW. SARS-CoV-2: a potential novel etiology of fulminant myocarditis. Herz. 2020;45:230-232. doi: 10.1007/s00059-020-04909-z
9. Hu H, Ma F, Wei X, Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. 2020 Mar 16;ehaa190. doi: 10.1093/eurheartj/ehaa190
10. Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846-848. doi:10.1007/s00134-020-05991-x
11. Akhmerov A, Marban E. COVID-19 and the heart. Circ Res. 2020;126:1443-1455. doi:10.1161/CIRCRESAHA.120.317055
12. Xiao F, Tang M, Zheng X, et al. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158:1831-1833. doi: 10.1053/j.gastro.2020.02.055
13. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1078-1720. doi: 10.1056/NEJMoa2002032
14. Wu P, Duan F, Luo C, et al. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020 Mar 31;e201291. doi: 10.1001/jamaophthalmol.2020.1291
15. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020 Apr 10. doi: 10.1001/jamaneurol.2020.1127
16. Vaira LA, Salzano G, Deiana G, De Riu G. Anosmia and ageusia: common findings in COVID-19 patients. Laryngoscope. 2020 Apr 1. doi: 10.1002/lary.28692
17. Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS-coronavirus-2. Int J Infect Dis. 2020;94:55-58. doi: 10.1016/j.ijid.2020.03.062
18. Toscano G, Palmerini F, Ravaglia S, et al. Guillain–Barré syndrome associated with SARS-CoV-2. N Engl J Med. 2020 Apr 17;NEJMc2009191. doi:10.1056/nejmc2009191
19. Dafer RM, Osteraas ND, Biller J. Acute stroke care in the coronavirus disease 2019 pandemic. J Stroke Cerebrovascular Dis. 2020 Apr 17:104881. doi: 10.1016/j.jstrokecerebrovasdis.2020.104881
20. Terpos E, Ntanasis-Stathopoulos I, Elalamy I, et al. Hematological findings and complications of COVID-19. Am J Hematol. 2020;10.1002/ajh.25829. doi:10.1002/ajh.25829
21. Jose RJ, Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. 2020;S2213-2600(20)30216-2. doi:10.1016/S2213-2600(20)30216-2
22. Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020 Mar 26. doi: 10.1111/jdv.16387
23. Joob B, Wiwanitkit V. COVID-19 can present with a rash and be mistaken for dengue. J Am Acad Dermatol. 2020;82(5):e177. doi: 10.1016/j.jaad.2020.03.036
24. Alramthan A, Aldaraji W. A Case of COVID‐19 presenting in clinical picture resembling chilblains disease. First report from the Middle East. Clin Exp Dermatol. 2020 Apr 17. doi: 10.1111/ced.14243
25. Kolivras A, Dehavay F, Delplace D, et al. Coronavirus (COVID-19) infection–induced chilblains: a case report with histopathologic findings. JAAD Case Rep. 2020 Apr 18. doi: 10.1016/j.jdcr.2020.04.011
1. WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020 [press release]. World Health Organization; March 11, 2020.
2. Coronavirus COVID-19 Global Cases by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. Johns Hopkins CSSE. https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6 Accessed May 15, 2020.
3. Liu Y, Gayle AA, Wilder-Smith A, Rocklöv J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Travel Med. 2020;27(2):taaa021. doi:10.1093/jtm/taaa021
4. Li Z, Wu M, Guo J, et al. Caution on kidney dysfunctions of 2019-nCoV patients. medRxiv preprint. doi: 10.1101/2020.02.08.20021212
5. Li W, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450-454. doi: 10.1038/nature02145.
6. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. doi:10.1001/jama.2020.1585
7. Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323:1612‐1614. doi:10.1001/jama.2020.4326
8. Chen C, Zhou Y, Wang DW. SARS-CoV-2: a potential novel etiology of fulminant myocarditis. Herz. 2020;45:230-232. doi: 10.1007/s00059-020-04909-z
9. Hu H, Ma F, Wei X, Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. 2020 Mar 16;ehaa190. doi: 10.1093/eurheartj/ehaa190
10. Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846-848. doi:10.1007/s00134-020-05991-x
11. Akhmerov A, Marban E. COVID-19 and the heart. Circ Res. 2020;126:1443-1455. doi:10.1161/CIRCRESAHA.120.317055
12. Xiao F, Tang M, Zheng X, et al. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158:1831-1833. doi: 10.1053/j.gastro.2020.02.055
13. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1078-1720. doi: 10.1056/NEJMoa2002032
14. Wu P, Duan F, Luo C, et al. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020 Mar 31;e201291. doi: 10.1001/jamaophthalmol.2020.1291
15. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020 Apr 10. doi: 10.1001/jamaneurol.2020.1127
16. Vaira LA, Salzano G, Deiana G, De Riu G. Anosmia and ageusia: common findings in COVID-19 patients. Laryngoscope. 2020 Apr 1. doi: 10.1002/lary.28692
17. Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS-coronavirus-2. Int J Infect Dis. 2020;94:55-58. doi: 10.1016/j.ijid.2020.03.062
18. Toscano G, Palmerini F, Ravaglia S, et al. Guillain–Barré syndrome associated with SARS-CoV-2. N Engl J Med. 2020 Apr 17;NEJMc2009191. doi:10.1056/nejmc2009191
19. Dafer RM, Osteraas ND, Biller J. Acute stroke care in the coronavirus disease 2019 pandemic. J Stroke Cerebrovascular Dis. 2020 Apr 17:104881. doi: 10.1016/j.jstrokecerebrovasdis.2020.104881
20. Terpos E, Ntanasis-Stathopoulos I, Elalamy I, et al. Hematological findings and complications of COVID-19. Am J Hematol. 2020;10.1002/ajh.25829. doi:10.1002/ajh.25829
21. Jose RJ, Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. 2020;S2213-2600(20)30216-2. doi:10.1016/S2213-2600(20)30216-2
22. Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020 Mar 26. doi: 10.1111/jdv.16387
23. Joob B, Wiwanitkit V. COVID-19 can present with a rash and be mistaken for dengue. J Am Acad Dermatol. 2020;82(5):e177. doi: 10.1016/j.jaad.2020.03.036
24. Alramthan A, Aldaraji W. A Case of COVID‐19 presenting in clinical picture resembling chilblains disease. First report from the Middle East. Clin Exp Dermatol. 2020 Apr 17. doi: 10.1111/ced.14243
25. Kolivras A, Dehavay F, Delplace D, et al. Coronavirus (COVID-19) infection–induced chilblains: a case report with histopathologic findings. JAAD Case Rep. 2020 Apr 18. doi: 10.1016/j.jdcr.2020.04.011
Use of an Electronic Alert Tool to Prevent Readmissions Following Coronary Artery Bypass Graft Surgery
From the University of North Carolina at Wilmington School of Nursing (Dr. Smith and Dr. Turrise), the New Hanover Regional Medical Center Heart Center (Mr. Jordan), the Coastal Carolinas Health Alliance and Coastal Connect Health Information Exchange (Ms. Robertson), and Coastal Thoracic Surgical Associates (Dr. Kane), Wilmington, NC.
Abstract
Objective: Cardiothoracic (CT) surgeons at our medical center were not receiving timely notification when their coronary artery bypass graft (CABG) surgery patients were admitted to the medical center or to other hospitals. The CT surgical team worked with a health alliance in southeastern North Carolina to implement health information exchange (HIE) real-time electronic notifications for their CABG patients who presented to the hospital’s emergency department (ED) or any ED affiliated with the medical center. The alert tool notifies team members about patient encounters, driving timely clinical engagement.
Methods: The CT team provided the HIE team with the names of CABG surgery patients, which were loaded into the alert tool. When a patient on the list presented to the hospital ED or its affiliates, the alert tool sent a real-time electronic notification to the Cardiac Surgical Services nurse coordinator. This intervention prompted the assessment and disposition of CABG patients, while in the ED, by the CT surgical team.
Results: Over a 16-month period (September 2017-December 2018), the names of 614 post-CABG patients were input into the HIE for tracking. Of these patients, 47 were treated and discharged from the ED; 31 were admitted for observation; 44 were readmitted for inpatient care; and 492 did not have a qualifying event requiring a notification alert. Following implementation of this practice change, the 30-day readmission rate for patients who underwent CABG at our institution decreased from 10% to 7.2%.
Conclusion: Utilizing a real-time alert tool resulted in immediate notification of the CT team when 1 of their patients presented to the ED. This afforded the CT surgical team an opportunity to intervene in the care of their patients, which in turn led to improved quality of care, physician communication and collaboration, and patient outcomes, such as preventable 30-day readmissions.
Keywords: electronic health record; real-time electronic notification; CABG; process improvement.
Unplanned 30-day hospital readmissions of patients who have undergone coronary artery bypass graft (CABG) surgery contribute to higher overall health care costs. CABG is 1 of the conditions/procedures that the Centers for Medicare and Medicaid Services (CMS) monitors for excess readmissions.1 Readmission rates for CABG-related conditions at 30 days post-surgery are reported to be between 16% and 20% for US hospitals.2 Readmissions are not only financially costly, but also have been associated with worse patient outcomes and decreased patient satisfaction.3 Common diagnoses for post-CABG admission include atrial fibrillation, pleural effusion, and wound infection.
The facility where this project was implemented had a 10% post-CABG admission rate for patients across all payers. While this rate is below the national average of 13.2%, the cardiothoracic (CT) surgical team was not being notified in a timely manner when their post-CABG patients were readmitted. The Lean team used the A3 problem-solving process to develop strategies that would reduce these readmissions and improve the care of their patients.
We explored the use of electronic alerts in managing post-CABG patients by conducting a literature search using the terms electronic alerts in patient care, patient engagement in the emergency department, electronic alerts in CABG, real-time notifications to prevent readmission, and CABG readmission. Databases searched were PubMed, Google Scholar, Cumulative Index of Nursing and Allied Health Literature, ProQuest, and ScienceDirect. This search resulted in studies focused on the use of electronic health record (EHR) alerts as a clinical decision-support tool; for example, patient demographic and assessment data are entered into the EHR, and the clinician is prompted with “performance” recommendations (eg, consider electrocardiogram and aspirin).4 In a paper by Engelman and Benjamin,5 the authors discuss the importance of the engaged physician and note that, in their emergency department (ED), an electronic notification is sent when a postoperative patient presents; however, the notification goes to the inpatient service for timely review and disposition. There was no literature that discussed the use of an electronic alert tool as a real-time patient engagement strategy that resulted in a practice change specific to the CT surgical team.
Our process improvement project focused on alerting the CT surgical team when a post-CABG patient presented to the ED, allowing them to evaluate the patient in real time and determine whether the chief complaint was related to the CABG and whether further evaluation by the CT surgeon was required. Specifically, we wanted to determine whether a real-time electronic alert that notified the CT surgical team about post-op CABG patients presenting to the ED would result in timely patient engagement, avoidance of readmissions, and an enhanced patient experience. During this project, alerts were sent to the CT surgical team notifying them of a post-CABG patient presenting to the ED or being directly admitted from home on physician orders, a provider’s office, or inpatient rehabilitation; however, the focus of this article is specifically on the notification regarding post-CABG patients presenting to the ED.
Prior to implementing the electronic notification project, the team developed and implemented several internal and external readmission reduction and prevention strategies for CABG patients. An in-house strategy involved a process whereby patients would receive their discharge medications prior to being discharged from the hospital post-CABG, thereby avoiding potential delays in the patient obtaining medications. When examining post-CABG patient readmissions, the primary conditions that led to readmission were fluid overload, pleural effusion, and atrial fibrillation. As such, a second in-house strategy was developed for post-CABG patients presenting to the ED with atrial fibrillation. The newly established protocol allowed patients to be monitored and treated in the cardiac observation unit. In addition, external strategies, including an outpatient furosemide protocol for home health nurses and an outpatient thoracentesis program and order set, were established (eg, for patients with congestive heart failure, shortness of breath).
Methods
Setting
The regional medical center where this project was implemented is the ninth largest hospital in North Carolina and the largest county-owned public hospital in the state. It is a tertiary care center and teaching hospital with 3 hospital campuses and 855 licensed beds. The medical center was included in the 100 Safecare Hospitals list by the Safecare Group; received a grade “A” Hospital Safety Score from the Leapfrog Group; and is 1 of America’s Top 100 Hospitals for Patient Experience.
Real-Time Notification Project
A regional hospital alliance in southeastern North Carolina established a health information exchange (HIE) with its member hospitals and office-based physicians to enable electronic exchange of patient information to improve quality, safety, and efficiency in health care delivery. Our medical center is part of this alliance. The HIE is a digital platform that facilitates the sharing of information between disparate connected EHR systems, and offers a portal for practices and hospitals to access patient information across North Carolina, South Carolina (via SC HIE), and nationwide (select dialysis centers). More specifically, approved providers and team members are able to access, in real time, patient-care encounter documents from other care settings (eg, acute, post-acute, ambulatory) via the HIE. Additionally, approved care entities can query-retrieve web portal information to support patient outcome improvement strategies. A partnership discussion highlighted the opportunity to utilize the HIE’s capabilities, such as real-time notification, to facilitate workflow (eg, when a patient presents to the ED, the HIE can provide access to health information at the point of care). In this capacity, the alert tool notifies care team members about patient encounters to drive timely clinical engagement for care transitions.
In January 2017, we began discussions on using the HIE to facilitate real-time electronic tracking in the Cardiac Surgical Services department at our medical center. Persons involved in these discussions included the cardiovascular (CV) team (comprised of case managers, department managers and coordinators, program coordinators, administrators, and support services [eg, pre-admission testing and home health staff]) and CT surgeons. At that time, CABG readmissions were manually tracked, and the real-time notification tool was being used in other departments (eg, in case management for tracking readmissions). The entire team was part of the initial decision meeting to pursue this possibility. The CV team reached consensus in June 2017 and proposed extending the use of the alert tool to the post-CABG population presenting to the ED (or any ED affiliated with the medical center) or admitted directly to the medical center.
The HIE staff met with the Cardiac Surgical Services team to tailor and develop the logistics of the project, such as who would be notified and how. The goals of the project were to support appropriate care intervention, reduce preventable hospital readmissions, and improve quality of care through enhanced provider communication and engagement. To achieve these goals, on the day of discharge the Cardiac Surgical Services coordinator provided the HIE team with the names of patients who had undergone CABG surgery. This patient list was loaded into the alert tool and continually updated. At 31 days, patient names were removed from the list. When a patient on the list presented to the hospital ED, the alert tool sent 2 real-time electronic notifications, an email and a text message, to the Cardiac Surgical Services coordinator, noting that a patient event occurred. Personal information was not included in the alert in order to protect patient information and comply with Health Insurance Portability and Accountability Act regulations.
The alert prompted the Cardiac Surgical Services coordinator to securely access patient information to identify and, if necessary, visit the patient. Then, based on the information gathered by the Cardiac Surgical Services coordinator, a Situation-Background-Assessment-Recommendation report was relayed to the CT surgeon, who then determined whether intervention by the CT surgical team was warranted. This process, on average, took approximately 30 minutes to complete. This was a key change in processes, one that allowed post-CABG patients to be seen by the CT surgical team while in the ED. If the issue was related to the CABG surgery, the CT surgeons could then determine an appropriate course of action, including admission or implementation of another protocol, such as the home furosemide protocol. For patients directly admitted, the surgeon contacted the admitting provider to discuss the level of care required (ie, observation or inpatient admission and treatment).
Biweekly CV team meetings were conducted during the implementation of the real-time notification alert tool. At each meeting, updates were provided on notifications received, patients who were missed by the notification process, and how well the real-time alerts were working to enhance care and appropriate disposition.
Measurements
Clinical performance data included total notifications, total number of ED visits, ED disposition (inpatient admission, observation, discharge), total number of direct admissions, direct admissions to observation, direct inpatient admissions, and patients missed by the notification process (eg, due to data entry errors, omissions of information [suffix of junior or senior], as well as programming bugs). Finally, the number of observation admissions converted to inpatient admissions was collected and further analyzed to inform needed process changes.
The Cardiac Surgical Services coordinator collected, entered, and maintained data using Excel. Data were obtained from the EHR, recorded in Excel, and analyzed using basic descriptive statistics in an ongoing fashion. Particular attention was focused on problems with the notification process (eg, patients being missed due to errors in data entry) and summarizing information to keep the Cardiac Surgical Services team updated on the progress of the process improvement. This project did not require staff protections or considerations, and because this was not a research study Institutional Review Board approval was not required.
Results
This practice change was implemented in September 2017 and led to improvements in care quality, as evidenced by improved physician communication and collaboration. In the 16-month period from implementation through December 2018, the names of 614 post-CABG patients were input into the HIE for tracking. Of these patients, 47 were treated and discharged from the ED; 31 were admitted for observation; and 44 were readmitted for inpatient care. The remaining 492 patients did not have a qualifying event requiring a notification alert. Clinical performance data from this period included 70 ED visits, 21 direct admissions, 19 direct admissions to observation, 5 patients missed by the notification process, and 4 observation admissions converted to inpatient admissions. A reduction in the CABG readmission rate from 10% in September 2017 to 7.2% in December 2018 was also noted.
Discussion
The aim of this process improvement project was to determine whether a real-time electronic alert that notified the CT surgical team about post-op CABG patients presenting to the ED would result in timely patient engagement, avoidance of readmissions, and an enhanced patient experience. This practice change has been successful, following 16 months of implementation and process refinement. Integrating a real-time electronic alert with a supporting action plan and care protocols resulted in timely patient engagement and avoidance of readmission of post-CABG patients.
Early notification of possible post-CABG readmissions became a standard-of-care process within the Cardiac Surgical Services department, with expansion to all CT post-op patients. Leveraging HIE technology to support quality improvement processes was also viewed by other departments as relevant and beneficial. For example, the hospital stroke and orthopedic-spine teams established their own processes for receiving real-time alerts.
There were several lessons learned during this project. First, gaining 100% physician buy-in to collaborative communication proved to be critical to the project’s success. The CV team was surprised by the length of time (approximately 8-10 months) it took for the practice change to be adopted by the physicians. In part, some of this delay in adoption resulted from medical staff turnover, primarily in the medical resident training rotations. Collaborative communication was key. The CT surgeons spoke with ED leadership and hospitalist services to explain the readmission reduction project and the use of an electronic alert tool. The CT surgeons also communicated to the ED physicians, hospitalists, and cardiologists that the Cardiac Surgical Services coordinator would be involved in the process and discussions regarding patientss care. Additionally, the CT surgeons authored the furosemide protocol and then committed to its use in the home health setting, further highlighting the role of collaborative communication in avoiding readmissions.
Another key step in this quality improvement project was determining who should receive the alert notifications. At the onset of the project, all notifications were sent to 1 person, the Cardiac Surgical Services coordinator. While this seemed logical in the initial stage of the project, it was unsustainable, as the receipt of the alert and the subsequent notification of the CT surgeon depended on 1 person and their availability. Approximately 10 months into the project, the notification process was further refined, with the cardiovascular intensive care unit charge nurse becoming the point of contact for the alerts. The Cardiac Surgical Services coordinator, in collaboration with nursing leaders and CT surgeons, completed a Lean Standard Work template outlining the major steps and the associated responsibilities (for the cardiovascular intensive care unit charge nurse, CT surgeon and on-call surgeon, Cardiac Surgical Services coordinator) in the process of receiving notifications, collecting patient assessment data, and reporting notifications to the CT surgeons.
Establishing adequate support mechanisms during a practice change is also important. For instance, we had to dedicate personnel time for data collection and analysis and involve additional nursing or other qualified personnel in the new process to avoid depending on a single person for the project’s success. Additional considerations were establishing criteria for surgeon notification and defining an appropriate time frame for notification (eg, urgent versus next-day notifications). We accomplished these activities approximately 10 months into the project, after it became apparent at CV team meeting discussions that further clarification of criteria and timelines was needed.
Some aspects of the project unfolded as planned, while others presented opportunities for improvement. For example, the alert notification process worked as envisioned; however, as previously mentioned, the process needed to be more inclusive to ensure there is always a charge nurse on duty to receive the alert notification, rather than just the Cardiac Surgical Services coordinator, who may not always be at the hospital. The outpatient thoracentesis program was well planned and effectively implemented. This program provided an avenue for patients who had symptoms of pleural effusion to be treated in an outpatient setting, rather than requiring an inpatient stay. Opportunities for improvement included addressing the inconsistent use of the home health furosemide protocol (developed in 2016), and the need for continued interprofessional and interdepartmental communication and coordination. For example, we had to inform the ED physicians and staff who rotate or are new to the ED about established processes and protocols in place for managing post-CABG patients who present to the ED.
The primary limitation of this project was the inability to measure the enhanced patient experience, which was 1 of the stated project goals. This goal became secondary because of more pressing issues, specifically, interorganizational collaboration (eg, hospital EHR, HIE, and CT surgical team) and tailoring the functionality of the electronic alert tool to the project. Developing and implementing measures of enhanced patient experience were not feasible during this implementation. Additionally, because this was not a research study, it was not possible to determine cause and effect or to control for confounders, such as a sicker, older cohort with more comorbid conditions, during the comparison period. Finally, although this process improvement project was conducted at a regional medical center that is the only facility performing CABG within the region, patients may have presented to another facility for an event that led to a readmission. Because readmissions to other facilities could not be captured, it is possible that the actual readmission rate was higher than the rate reported here.
Conclusions and Implications
Utilizing a real-time alert from the HIE to the CT surgical team resulted in CT surgeons being immediately made aware when their patients presented to the ED, allowing the CT surgical team the opportunity to intervene, as appropriate, in the care of their patients. Furthermore, this real-time notification and intervention resulted in timely patient engagement and, in some cases, avoidance of readmissions. Currently, patients are monitored for readmission within 30 days of discharge. In the future, the time will expand to 91 days, in preparation for participation in the CMS bundle payment program for CABG surgery.
This practice change can be used in organizations that do not have or participate in a HIE. In fact, these real-time alert applications may be available through an EHR already in use within the organization. The use of the alert requires collaborative communication and having supporting protocols in place to guide decision-making and care of post-CABG patients presenting to the ED.
There appears to be a gap in the literature discussing the use of an electronic alert tool as a real-time patient engagement strategy for post-CABG patients presenting to the ED. As such, this project contributes important results and lessons learned for other hospital service lines/departments that might consider implementing a similar process. Next steps include designing and conducting methodologically rigorous research studies based on this process improvement project to examine mortality rates as an outcome, and designing a more specific measure of patient experience, as the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey only provides hospital-level data.
Corresponding author: Stephanie D. Smith, PhD, RN, UNCW School of Nursing, 601 South College Road, Wilmington, NC 28403; [email protected].
Funding disclosures: None.
1. Hannan EL, Zhong Y, Lahey SJ, et al. 30-day readmissions after coronary artery bypass graft surgery in New York State. JACC Cardiovasc Interv. 2011;4:569-576.
2. Feng TR, White R, Gaber-Baylis L, et al. Coronary artery bypass graft readmission rates and risk factors- A retrospective cohort study. Int J Surg. 2018;54 (Part A):7-17.
3. Donndorf P, Kaminski A. “Return to sender” or “consider it done”?! The importance of reducing hospital readmission after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2017;154:1298-1299.
4. Sequist TD, Morong SM, Marston A, et al. Electronic risk alerts to improve primary care management of chest pain: A randomized, controlled trial. J Gen Intern Med. 2012;27:438-444.
5. Engelman D, Benjamin EM. Physician engagement: The “secret sauce” to success in bundled health care. Am J Med Qual. 2018;33:100-102.
From the University of North Carolina at Wilmington School of Nursing (Dr. Smith and Dr. Turrise), the New Hanover Regional Medical Center Heart Center (Mr. Jordan), the Coastal Carolinas Health Alliance and Coastal Connect Health Information Exchange (Ms. Robertson), and Coastal Thoracic Surgical Associates (Dr. Kane), Wilmington, NC.
Abstract
Objective: Cardiothoracic (CT) surgeons at our medical center were not receiving timely notification when their coronary artery bypass graft (CABG) surgery patients were admitted to the medical center or to other hospitals. The CT surgical team worked with a health alliance in southeastern North Carolina to implement health information exchange (HIE) real-time electronic notifications for their CABG patients who presented to the hospital’s emergency department (ED) or any ED affiliated with the medical center. The alert tool notifies team members about patient encounters, driving timely clinical engagement.
Methods: The CT team provided the HIE team with the names of CABG surgery patients, which were loaded into the alert tool. When a patient on the list presented to the hospital ED or its affiliates, the alert tool sent a real-time electronic notification to the Cardiac Surgical Services nurse coordinator. This intervention prompted the assessment and disposition of CABG patients, while in the ED, by the CT surgical team.
Results: Over a 16-month period (September 2017-December 2018), the names of 614 post-CABG patients were input into the HIE for tracking. Of these patients, 47 were treated and discharged from the ED; 31 were admitted for observation; 44 were readmitted for inpatient care; and 492 did not have a qualifying event requiring a notification alert. Following implementation of this practice change, the 30-day readmission rate for patients who underwent CABG at our institution decreased from 10% to 7.2%.
Conclusion: Utilizing a real-time alert tool resulted in immediate notification of the CT team when 1 of their patients presented to the ED. This afforded the CT surgical team an opportunity to intervene in the care of their patients, which in turn led to improved quality of care, physician communication and collaboration, and patient outcomes, such as preventable 30-day readmissions.
Keywords: electronic health record; real-time electronic notification; CABG; process improvement.
Unplanned 30-day hospital readmissions of patients who have undergone coronary artery bypass graft (CABG) surgery contribute to higher overall health care costs. CABG is 1 of the conditions/procedures that the Centers for Medicare and Medicaid Services (CMS) monitors for excess readmissions.1 Readmission rates for CABG-related conditions at 30 days post-surgery are reported to be between 16% and 20% for US hospitals.2 Readmissions are not only financially costly, but also have been associated with worse patient outcomes and decreased patient satisfaction.3 Common diagnoses for post-CABG admission include atrial fibrillation, pleural effusion, and wound infection.
The facility where this project was implemented had a 10% post-CABG admission rate for patients across all payers. While this rate is below the national average of 13.2%, the cardiothoracic (CT) surgical team was not being notified in a timely manner when their post-CABG patients were readmitted. The Lean team used the A3 problem-solving process to develop strategies that would reduce these readmissions and improve the care of their patients.
We explored the use of electronic alerts in managing post-CABG patients by conducting a literature search using the terms electronic alerts in patient care, patient engagement in the emergency department, electronic alerts in CABG, real-time notifications to prevent readmission, and CABG readmission. Databases searched were PubMed, Google Scholar, Cumulative Index of Nursing and Allied Health Literature, ProQuest, and ScienceDirect. This search resulted in studies focused on the use of electronic health record (EHR) alerts as a clinical decision-support tool; for example, patient demographic and assessment data are entered into the EHR, and the clinician is prompted with “performance” recommendations (eg, consider electrocardiogram and aspirin).4 In a paper by Engelman and Benjamin,5 the authors discuss the importance of the engaged physician and note that, in their emergency department (ED), an electronic notification is sent when a postoperative patient presents; however, the notification goes to the inpatient service for timely review and disposition. There was no literature that discussed the use of an electronic alert tool as a real-time patient engagement strategy that resulted in a practice change specific to the CT surgical team.
Our process improvement project focused on alerting the CT surgical team when a post-CABG patient presented to the ED, allowing them to evaluate the patient in real time and determine whether the chief complaint was related to the CABG and whether further evaluation by the CT surgeon was required. Specifically, we wanted to determine whether a real-time electronic alert that notified the CT surgical team about post-op CABG patients presenting to the ED would result in timely patient engagement, avoidance of readmissions, and an enhanced patient experience. During this project, alerts were sent to the CT surgical team notifying them of a post-CABG patient presenting to the ED or being directly admitted from home on physician orders, a provider’s office, or inpatient rehabilitation; however, the focus of this article is specifically on the notification regarding post-CABG patients presenting to the ED.
Prior to implementing the electronic notification project, the team developed and implemented several internal and external readmission reduction and prevention strategies for CABG patients. An in-house strategy involved a process whereby patients would receive their discharge medications prior to being discharged from the hospital post-CABG, thereby avoiding potential delays in the patient obtaining medications. When examining post-CABG patient readmissions, the primary conditions that led to readmission were fluid overload, pleural effusion, and atrial fibrillation. As such, a second in-house strategy was developed for post-CABG patients presenting to the ED with atrial fibrillation. The newly established protocol allowed patients to be monitored and treated in the cardiac observation unit. In addition, external strategies, including an outpatient furosemide protocol for home health nurses and an outpatient thoracentesis program and order set, were established (eg, for patients with congestive heart failure, shortness of breath).
Methods
Setting
The regional medical center where this project was implemented is the ninth largest hospital in North Carolina and the largest county-owned public hospital in the state. It is a tertiary care center and teaching hospital with 3 hospital campuses and 855 licensed beds. The medical center was included in the 100 Safecare Hospitals list by the Safecare Group; received a grade “A” Hospital Safety Score from the Leapfrog Group; and is 1 of America’s Top 100 Hospitals for Patient Experience.
Real-Time Notification Project
A regional hospital alliance in southeastern North Carolina established a health information exchange (HIE) with its member hospitals and office-based physicians to enable electronic exchange of patient information to improve quality, safety, and efficiency in health care delivery. Our medical center is part of this alliance. The HIE is a digital platform that facilitates the sharing of information between disparate connected EHR systems, and offers a portal for practices and hospitals to access patient information across North Carolina, South Carolina (via SC HIE), and nationwide (select dialysis centers). More specifically, approved providers and team members are able to access, in real time, patient-care encounter documents from other care settings (eg, acute, post-acute, ambulatory) via the HIE. Additionally, approved care entities can query-retrieve web portal information to support patient outcome improvement strategies. A partnership discussion highlighted the opportunity to utilize the HIE’s capabilities, such as real-time notification, to facilitate workflow (eg, when a patient presents to the ED, the HIE can provide access to health information at the point of care). In this capacity, the alert tool notifies care team members about patient encounters to drive timely clinical engagement for care transitions.
In January 2017, we began discussions on using the HIE to facilitate real-time electronic tracking in the Cardiac Surgical Services department at our medical center. Persons involved in these discussions included the cardiovascular (CV) team (comprised of case managers, department managers and coordinators, program coordinators, administrators, and support services [eg, pre-admission testing and home health staff]) and CT surgeons. At that time, CABG readmissions were manually tracked, and the real-time notification tool was being used in other departments (eg, in case management for tracking readmissions). The entire team was part of the initial decision meeting to pursue this possibility. The CV team reached consensus in June 2017 and proposed extending the use of the alert tool to the post-CABG population presenting to the ED (or any ED affiliated with the medical center) or admitted directly to the medical center.
The HIE staff met with the Cardiac Surgical Services team to tailor and develop the logistics of the project, such as who would be notified and how. The goals of the project were to support appropriate care intervention, reduce preventable hospital readmissions, and improve quality of care through enhanced provider communication and engagement. To achieve these goals, on the day of discharge the Cardiac Surgical Services coordinator provided the HIE team with the names of patients who had undergone CABG surgery. This patient list was loaded into the alert tool and continually updated. At 31 days, patient names were removed from the list. When a patient on the list presented to the hospital ED, the alert tool sent 2 real-time electronic notifications, an email and a text message, to the Cardiac Surgical Services coordinator, noting that a patient event occurred. Personal information was not included in the alert in order to protect patient information and comply with Health Insurance Portability and Accountability Act regulations.
The alert prompted the Cardiac Surgical Services coordinator to securely access patient information to identify and, if necessary, visit the patient. Then, based on the information gathered by the Cardiac Surgical Services coordinator, a Situation-Background-Assessment-Recommendation report was relayed to the CT surgeon, who then determined whether intervention by the CT surgical team was warranted. This process, on average, took approximately 30 minutes to complete. This was a key change in processes, one that allowed post-CABG patients to be seen by the CT surgical team while in the ED. If the issue was related to the CABG surgery, the CT surgeons could then determine an appropriate course of action, including admission or implementation of another protocol, such as the home furosemide protocol. For patients directly admitted, the surgeon contacted the admitting provider to discuss the level of care required (ie, observation or inpatient admission and treatment).
Biweekly CV team meetings were conducted during the implementation of the real-time notification alert tool. At each meeting, updates were provided on notifications received, patients who were missed by the notification process, and how well the real-time alerts were working to enhance care and appropriate disposition.
Measurements
Clinical performance data included total notifications, total number of ED visits, ED disposition (inpatient admission, observation, discharge), total number of direct admissions, direct admissions to observation, direct inpatient admissions, and patients missed by the notification process (eg, due to data entry errors, omissions of information [suffix of junior or senior], as well as programming bugs). Finally, the number of observation admissions converted to inpatient admissions was collected and further analyzed to inform needed process changes.
The Cardiac Surgical Services coordinator collected, entered, and maintained data using Excel. Data were obtained from the EHR, recorded in Excel, and analyzed using basic descriptive statistics in an ongoing fashion. Particular attention was focused on problems with the notification process (eg, patients being missed due to errors in data entry) and summarizing information to keep the Cardiac Surgical Services team updated on the progress of the process improvement. This project did not require staff protections or considerations, and because this was not a research study Institutional Review Board approval was not required.
Results
This practice change was implemented in September 2017 and led to improvements in care quality, as evidenced by improved physician communication and collaboration. In the 16-month period from implementation through December 2018, the names of 614 post-CABG patients were input into the HIE for tracking. Of these patients, 47 were treated and discharged from the ED; 31 were admitted for observation; and 44 were readmitted for inpatient care. The remaining 492 patients did not have a qualifying event requiring a notification alert. Clinical performance data from this period included 70 ED visits, 21 direct admissions, 19 direct admissions to observation, 5 patients missed by the notification process, and 4 observation admissions converted to inpatient admissions. A reduction in the CABG readmission rate from 10% in September 2017 to 7.2% in December 2018 was also noted.
Discussion
The aim of this process improvement project was to determine whether a real-time electronic alert that notified the CT surgical team about post-op CABG patients presenting to the ED would result in timely patient engagement, avoidance of readmissions, and an enhanced patient experience. This practice change has been successful, following 16 months of implementation and process refinement. Integrating a real-time electronic alert with a supporting action plan and care protocols resulted in timely patient engagement and avoidance of readmission of post-CABG patients.
Early notification of possible post-CABG readmissions became a standard-of-care process within the Cardiac Surgical Services department, with expansion to all CT post-op patients. Leveraging HIE technology to support quality improvement processes was also viewed by other departments as relevant and beneficial. For example, the hospital stroke and orthopedic-spine teams established their own processes for receiving real-time alerts.
There were several lessons learned during this project. First, gaining 100% physician buy-in to collaborative communication proved to be critical to the project’s success. The CV team was surprised by the length of time (approximately 8-10 months) it took for the practice change to be adopted by the physicians. In part, some of this delay in adoption resulted from medical staff turnover, primarily in the medical resident training rotations. Collaborative communication was key. The CT surgeons spoke with ED leadership and hospitalist services to explain the readmission reduction project and the use of an electronic alert tool. The CT surgeons also communicated to the ED physicians, hospitalists, and cardiologists that the Cardiac Surgical Services coordinator would be involved in the process and discussions regarding patientss care. Additionally, the CT surgeons authored the furosemide protocol and then committed to its use in the home health setting, further highlighting the role of collaborative communication in avoiding readmissions.
Another key step in this quality improvement project was determining who should receive the alert notifications. At the onset of the project, all notifications were sent to 1 person, the Cardiac Surgical Services coordinator. While this seemed logical in the initial stage of the project, it was unsustainable, as the receipt of the alert and the subsequent notification of the CT surgeon depended on 1 person and their availability. Approximately 10 months into the project, the notification process was further refined, with the cardiovascular intensive care unit charge nurse becoming the point of contact for the alerts. The Cardiac Surgical Services coordinator, in collaboration with nursing leaders and CT surgeons, completed a Lean Standard Work template outlining the major steps and the associated responsibilities (for the cardiovascular intensive care unit charge nurse, CT surgeon and on-call surgeon, Cardiac Surgical Services coordinator) in the process of receiving notifications, collecting patient assessment data, and reporting notifications to the CT surgeons.
Establishing adequate support mechanisms during a practice change is also important. For instance, we had to dedicate personnel time for data collection and analysis and involve additional nursing or other qualified personnel in the new process to avoid depending on a single person for the project’s success. Additional considerations were establishing criteria for surgeon notification and defining an appropriate time frame for notification (eg, urgent versus next-day notifications). We accomplished these activities approximately 10 months into the project, after it became apparent at CV team meeting discussions that further clarification of criteria and timelines was needed.
Some aspects of the project unfolded as planned, while others presented opportunities for improvement. For example, the alert notification process worked as envisioned; however, as previously mentioned, the process needed to be more inclusive to ensure there is always a charge nurse on duty to receive the alert notification, rather than just the Cardiac Surgical Services coordinator, who may not always be at the hospital. The outpatient thoracentesis program was well planned and effectively implemented. This program provided an avenue for patients who had symptoms of pleural effusion to be treated in an outpatient setting, rather than requiring an inpatient stay. Opportunities for improvement included addressing the inconsistent use of the home health furosemide protocol (developed in 2016), and the need for continued interprofessional and interdepartmental communication and coordination. For example, we had to inform the ED physicians and staff who rotate or are new to the ED about established processes and protocols in place for managing post-CABG patients who present to the ED.
The primary limitation of this project was the inability to measure the enhanced patient experience, which was 1 of the stated project goals. This goal became secondary because of more pressing issues, specifically, interorganizational collaboration (eg, hospital EHR, HIE, and CT surgical team) and tailoring the functionality of the electronic alert tool to the project. Developing and implementing measures of enhanced patient experience were not feasible during this implementation. Additionally, because this was not a research study, it was not possible to determine cause and effect or to control for confounders, such as a sicker, older cohort with more comorbid conditions, during the comparison period. Finally, although this process improvement project was conducted at a regional medical center that is the only facility performing CABG within the region, patients may have presented to another facility for an event that led to a readmission. Because readmissions to other facilities could not be captured, it is possible that the actual readmission rate was higher than the rate reported here.
Conclusions and Implications
Utilizing a real-time alert from the HIE to the CT surgical team resulted in CT surgeons being immediately made aware when their patients presented to the ED, allowing the CT surgical team the opportunity to intervene, as appropriate, in the care of their patients. Furthermore, this real-time notification and intervention resulted in timely patient engagement and, in some cases, avoidance of readmissions. Currently, patients are monitored for readmission within 30 days of discharge. In the future, the time will expand to 91 days, in preparation for participation in the CMS bundle payment program for CABG surgery.
This practice change can be used in organizations that do not have or participate in a HIE. In fact, these real-time alert applications may be available through an EHR already in use within the organization. The use of the alert requires collaborative communication and having supporting protocols in place to guide decision-making and care of post-CABG patients presenting to the ED.
There appears to be a gap in the literature discussing the use of an electronic alert tool as a real-time patient engagement strategy for post-CABG patients presenting to the ED. As such, this project contributes important results and lessons learned for other hospital service lines/departments that might consider implementing a similar process. Next steps include designing and conducting methodologically rigorous research studies based on this process improvement project to examine mortality rates as an outcome, and designing a more specific measure of patient experience, as the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey only provides hospital-level data.
Corresponding author: Stephanie D. Smith, PhD, RN, UNCW School of Nursing, 601 South College Road, Wilmington, NC 28403; [email protected].
Funding disclosures: None.
From the University of North Carolina at Wilmington School of Nursing (Dr. Smith and Dr. Turrise), the New Hanover Regional Medical Center Heart Center (Mr. Jordan), the Coastal Carolinas Health Alliance and Coastal Connect Health Information Exchange (Ms. Robertson), and Coastal Thoracic Surgical Associates (Dr. Kane), Wilmington, NC.
Abstract
Objective: Cardiothoracic (CT) surgeons at our medical center were not receiving timely notification when their coronary artery bypass graft (CABG) surgery patients were admitted to the medical center or to other hospitals. The CT surgical team worked with a health alliance in southeastern North Carolina to implement health information exchange (HIE) real-time electronic notifications for their CABG patients who presented to the hospital’s emergency department (ED) or any ED affiliated with the medical center. The alert tool notifies team members about patient encounters, driving timely clinical engagement.
Methods: The CT team provided the HIE team with the names of CABG surgery patients, which were loaded into the alert tool. When a patient on the list presented to the hospital ED or its affiliates, the alert tool sent a real-time electronic notification to the Cardiac Surgical Services nurse coordinator. This intervention prompted the assessment and disposition of CABG patients, while in the ED, by the CT surgical team.
Results: Over a 16-month period (September 2017-December 2018), the names of 614 post-CABG patients were input into the HIE for tracking. Of these patients, 47 were treated and discharged from the ED; 31 were admitted for observation; 44 were readmitted for inpatient care; and 492 did not have a qualifying event requiring a notification alert. Following implementation of this practice change, the 30-day readmission rate for patients who underwent CABG at our institution decreased from 10% to 7.2%.
Conclusion: Utilizing a real-time alert tool resulted in immediate notification of the CT team when 1 of their patients presented to the ED. This afforded the CT surgical team an opportunity to intervene in the care of their patients, which in turn led to improved quality of care, physician communication and collaboration, and patient outcomes, such as preventable 30-day readmissions.
Keywords: electronic health record; real-time electronic notification; CABG; process improvement.
Unplanned 30-day hospital readmissions of patients who have undergone coronary artery bypass graft (CABG) surgery contribute to higher overall health care costs. CABG is 1 of the conditions/procedures that the Centers for Medicare and Medicaid Services (CMS) monitors for excess readmissions.1 Readmission rates for CABG-related conditions at 30 days post-surgery are reported to be between 16% and 20% for US hospitals.2 Readmissions are not only financially costly, but also have been associated with worse patient outcomes and decreased patient satisfaction.3 Common diagnoses for post-CABG admission include atrial fibrillation, pleural effusion, and wound infection.
The facility where this project was implemented had a 10% post-CABG admission rate for patients across all payers. While this rate is below the national average of 13.2%, the cardiothoracic (CT) surgical team was not being notified in a timely manner when their post-CABG patients were readmitted. The Lean team used the A3 problem-solving process to develop strategies that would reduce these readmissions and improve the care of their patients.
We explored the use of electronic alerts in managing post-CABG patients by conducting a literature search using the terms electronic alerts in patient care, patient engagement in the emergency department, electronic alerts in CABG, real-time notifications to prevent readmission, and CABG readmission. Databases searched were PubMed, Google Scholar, Cumulative Index of Nursing and Allied Health Literature, ProQuest, and ScienceDirect. This search resulted in studies focused on the use of electronic health record (EHR) alerts as a clinical decision-support tool; for example, patient demographic and assessment data are entered into the EHR, and the clinician is prompted with “performance” recommendations (eg, consider electrocardiogram and aspirin).4 In a paper by Engelman and Benjamin,5 the authors discuss the importance of the engaged physician and note that, in their emergency department (ED), an electronic notification is sent when a postoperative patient presents; however, the notification goes to the inpatient service for timely review and disposition. There was no literature that discussed the use of an electronic alert tool as a real-time patient engagement strategy that resulted in a practice change specific to the CT surgical team.
Our process improvement project focused on alerting the CT surgical team when a post-CABG patient presented to the ED, allowing them to evaluate the patient in real time and determine whether the chief complaint was related to the CABG and whether further evaluation by the CT surgeon was required. Specifically, we wanted to determine whether a real-time electronic alert that notified the CT surgical team about post-op CABG patients presenting to the ED would result in timely patient engagement, avoidance of readmissions, and an enhanced patient experience. During this project, alerts were sent to the CT surgical team notifying them of a post-CABG patient presenting to the ED or being directly admitted from home on physician orders, a provider’s office, or inpatient rehabilitation; however, the focus of this article is specifically on the notification regarding post-CABG patients presenting to the ED.
Prior to implementing the electronic notification project, the team developed and implemented several internal and external readmission reduction and prevention strategies for CABG patients. An in-house strategy involved a process whereby patients would receive their discharge medications prior to being discharged from the hospital post-CABG, thereby avoiding potential delays in the patient obtaining medications. When examining post-CABG patient readmissions, the primary conditions that led to readmission were fluid overload, pleural effusion, and atrial fibrillation. As such, a second in-house strategy was developed for post-CABG patients presenting to the ED with atrial fibrillation. The newly established protocol allowed patients to be monitored and treated in the cardiac observation unit. In addition, external strategies, including an outpatient furosemide protocol for home health nurses and an outpatient thoracentesis program and order set, were established (eg, for patients with congestive heart failure, shortness of breath).
Methods
Setting
The regional medical center where this project was implemented is the ninth largest hospital in North Carolina and the largest county-owned public hospital in the state. It is a tertiary care center and teaching hospital with 3 hospital campuses and 855 licensed beds. The medical center was included in the 100 Safecare Hospitals list by the Safecare Group; received a grade “A” Hospital Safety Score from the Leapfrog Group; and is 1 of America’s Top 100 Hospitals for Patient Experience.
Real-Time Notification Project
A regional hospital alliance in southeastern North Carolina established a health information exchange (HIE) with its member hospitals and office-based physicians to enable electronic exchange of patient information to improve quality, safety, and efficiency in health care delivery. Our medical center is part of this alliance. The HIE is a digital platform that facilitates the sharing of information between disparate connected EHR systems, and offers a portal for practices and hospitals to access patient information across North Carolina, South Carolina (via SC HIE), and nationwide (select dialysis centers). More specifically, approved providers and team members are able to access, in real time, patient-care encounter documents from other care settings (eg, acute, post-acute, ambulatory) via the HIE. Additionally, approved care entities can query-retrieve web portal information to support patient outcome improvement strategies. A partnership discussion highlighted the opportunity to utilize the HIE’s capabilities, such as real-time notification, to facilitate workflow (eg, when a patient presents to the ED, the HIE can provide access to health information at the point of care). In this capacity, the alert tool notifies care team members about patient encounters to drive timely clinical engagement for care transitions.
In January 2017, we began discussions on using the HIE to facilitate real-time electronic tracking in the Cardiac Surgical Services department at our medical center. Persons involved in these discussions included the cardiovascular (CV) team (comprised of case managers, department managers and coordinators, program coordinators, administrators, and support services [eg, pre-admission testing and home health staff]) and CT surgeons. At that time, CABG readmissions were manually tracked, and the real-time notification tool was being used in other departments (eg, in case management for tracking readmissions). The entire team was part of the initial decision meeting to pursue this possibility. The CV team reached consensus in June 2017 and proposed extending the use of the alert tool to the post-CABG population presenting to the ED (or any ED affiliated with the medical center) or admitted directly to the medical center.
The HIE staff met with the Cardiac Surgical Services team to tailor and develop the logistics of the project, such as who would be notified and how. The goals of the project were to support appropriate care intervention, reduce preventable hospital readmissions, and improve quality of care through enhanced provider communication and engagement. To achieve these goals, on the day of discharge the Cardiac Surgical Services coordinator provided the HIE team with the names of patients who had undergone CABG surgery. This patient list was loaded into the alert tool and continually updated. At 31 days, patient names were removed from the list. When a patient on the list presented to the hospital ED, the alert tool sent 2 real-time electronic notifications, an email and a text message, to the Cardiac Surgical Services coordinator, noting that a patient event occurred. Personal information was not included in the alert in order to protect patient information and comply with Health Insurance Portability and Accountability Act regulations.
The alert prompted the Cardiac Surgical Services coordinator to securely access patient information to identify and, if necessary, visit the patient. Then, based on the information gathered by the Cardiac Surgical Services coordinator, a Situation-Background-Assessment-Recommendation report was relayed to the CT surgeon, who then determined whether intervention by the CT surgical team was warranted. This process, on average, took approximately 30 minutes to complete. This was a key change in processes, one that allowed post-CABG patients to be seen by the CT surgical team while in the ED. If the issue was related to the CABG surgery, the CT surgeons could then determine an appropriate course of action, including admission or implementation of another protocol, such as the home furosemide protocol. For patients directly admitted, the surgeon contacted the admitting provider to discuss the level of care required (ie, observation or inpatient admission and treatment).
Biweekly CV team meetings were conducted during the implementation of the real-time notification alert tool. At each meeting, updates were provided on notifications received, patients who were missed by the notification process, and how well the real-time alerts were working to enhance care and appropriate disposition.
Measurements
Clinical performance data included total notifications, total number of ED visits, ED disposition (inpatient admission, observation, discharge), total number of direct admissions, direct admissions to observation, direct inpatient admissions, and patients missed by the notification process (eg, due to data entry errors, omissions of information [suffix of junior or senior], as well as programming bugs). Finally, the number of observation admissions converted to inpatient admissions was collected and further analyzed to inform needed process changes.
The Cardiac Surgical Services coordinator collected, entered, and maintained data using Excel. Data were obtained from the EHR, recorded in Excel, and analyzed using basic descriptive statistics in an ongoing fashion. Particular attention was focused on problems with the notification process (eg, patients being missed due to errors in data entry) and summarizing information to keep the Cardiac Surgical Services team updated on the progress of the process improvement. This project did not require staff protections or considerations, and because this was not a research study Institutional Review Board approval was not required.
Results
This practice change was implemented in September 2017 and led to improvements in care quality, as evidenced by improved physician communication and collaboration. In the 16-month period from implementation through December 2018, the names of 614 post-CABG patients were input into the HIE for tracking. Of these patients, 47 were treated and discharged from the ED; 31 were admitted for observation; and 44 were readmitted for inpatient care. The remaining 492 patients did not have a qualifying event requiring a notification alert. Clinical performance data from this period included 70 ED visits, 21 direct admissions, 19 direct admissions to observation, 5 patients missed by the notification process, and 4 observation admissions converted to inpatient admissions. A reduction in the CABG readmission rate from 10% in September 2017 to 7.2% in December 2018 was also noted.
Discussion
The aim of this process improvement project was to determine whether a real-time electronic alert that notified the CT surgical team about post-op CABG patients presenting to the ED would result in timely patient engagement, avoidance of readmissions, and an enhanced patient experience. This practice change has been successful, following 16 months of implementation and process refinement. Integrating a real-time electronic alert with a supporting action plan and care protocols resulted in timely patient engagement and avoidance of readmission of post-CABG patients.
Early notification of possible post-CABG readmissions became a standard-of-care process within the Cardiac Surgical Services department, with expansion to all CT post-op patients. Leveraging HIE technology to support quality improvement processes was also viewed by other departments as relevant and beneficial. For example, the hospital stroke and orthopedic-spine teams established their own processes for receiving real-time alerts.
There were several lessons learned during this project. First, gaining 100% physician buy-in to collaborative communication proved to be critical to the project’s success. The CV team was surprised by the length of time (approximately 8-10 months) it took for the practice change to be adopted by the physicians. In part, some of this delay in adoption resulted from medical staff turnover, primarily in the medical resident training rotations. Collaborative communication was key. The CT surgeons spoke with ED leadership and hospitalist services to explain the readmission reduction project and the use of an electronic alert tool. The CT surgeons also communicated to the ED physicians, hospitalists, and cardiologists that the Cardiac Surgical Services coordinator would be involved in the process and discussions regarding patientss care. Additionally, the CT surgeons authored the furosemide protocol and then committed to its use in the home health setting, further highlighting the role of collaborative communication in avoiding readmissions.
Another key step in this quality improvement project was determining who should receive the alert notifications. At the onset of the project, all notifications were sent to 1 person, the Cardiac Surgical Services coordinator. While this seemed logical in the initial stage of the project, it was unsustainable, as the receipt of the alert and the subsequent notification of the CT surgeon depended on 1 person and their availability. Approximately 10 months into the project, the notification process was further refined, with the cardiovascular intensive care unit charge nurse becoming the point of contact for the alerts. The Cardiac Surgical Services coordinator, in collaboration with nursing leaders and CT surgeons, completed a Lean Standard Work template outlining the major steps and the associated responsibilities (for the cardiovascular intensive care unit charge nurse, CT surgeon and on-call surgeon, Cardiac Surgical Services coordinator) in the process of receiving notifications, collecting patient assessment data, and reporting notifications to the CT surgeons.
Establishing adequate support mechanisms during a practice change is also important. For instance, we had to dedicate personnel time for data collection and analysis and involve additional nursing or other qualified personnel in the new process to avoid depending on a single person for the project’s success. Additional considerations were establishing criteria for surgeon notification and defining an appropriate time frame for notification (eg, urgent versus next-day notifications). We accomplished these activities approximately 10 months into the project, after it became apparent at CV team meeting discussions that further clarification of criteria and timelines was needed.
Some aspects of the project unfolded as planned, while others presented opportunities for improvement. For example, the alert notification process worked as envisioned; however, as previously mentioned, the process needed to be more inclusive to ensure there is always a charge nurse on duty to receive the alert notification, rather than just the Cardiac Surgical Services coordinator, who may not always be at the hospital. The outpatient thoracentesis program was well planned and effectively implemented. This program provided an avenue for patients who had symptoms of pleural effusion to be treated in an outpatient setting, rather than requiring an inpatient stay. Opportunities for improvement included addressing the inconsistent use of the home health furosemide protocol (developed in 2016), and the need for continued interprofessional and interdepartmental communication and coordination. For example, we had to inform the ED physicians and staff who rotate or are new to the ED about established processes and protocols in place for managing post-CABG patients who present to the ED.
The primary limitation of this project was the inability to measure the enhanced patient experience, which was 1 of the stated project goals. This goal became secondary because of more pressing issues, specifically, interorganizational collaboration (eg, hospital EHR, HIE, and CT surgical team) and tailoring the functionality of the electronic alert tool to the project. Developing and implementing measures of enhanced patient experience were not feasible during this implementation. Additionally, because this was not a research study, it was not possible to determine cause and effect or to control for confounders, such as a sicker, older cohort with more comorbid conditions, during the comparison period. Finally, although this process improvement project was conducted at a regional medical center that is the only facility performing CABG within the region, patients may have presented to another facility for an event that led to a readmission. Because readmissions to other facilities could not be captured, it is possible that the actual readmission rate was higher than the rate reported here.
Conclusions and Implications
Utilizing a real-time alert from the HIE to the CT surgical team resulted in CT surgeons being immediately made aware when their patients presented to the ED, allowing the CT surgical team the opportunity to intervene, as appropriate, in the care of their patients. Furthermore, this real-time notification and intervention resulted in timely patient engagement and, in some cases, avoidance of readmissions. Currently, patients are monitored for readmission within 30 days of discharge. In the future, the time will expand to 91 days, in preparation for participation in the CMS bundle payment program for CABG surgery.
This practice change can be used in organizations that do not have or participate in a HIE. In fact, these real-time alert applications may be available through an EHR already in use within the organization. The use of the alert requires collaborative communication and having supporting protocols in place to guide decision-making and care of post-CABG patients presenting to the ED.
There appears to be a gap in the literature discussing the use of an electronic alert tool as a real-time patient engagement strategy for post-CABG patients presenting to the ED. As such, this project contributes important results and lessons learned for other hospital service lines/departments that might consider implementing a similar process. Next steps include designing and conducting methodologically rigorous research studies based on this process improvement project to examine mortality rates as an outcome, and designing a more specific measure of patient experience, as the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey only provides hospital-level data.
Corresponding author: Stephanie D. Smith, PhD, RN, UNCW School of Nursing, 601 South College Road, Wilmington, NC 28403; [email protected].
Funding disclosures: None.
1. Hannan EL, Zhong Y, Lahey SJ, et al. 30-day readmissions after coronary artery bypass graft surgery in New York State. JACC Cardiovasc Interv. 2011;4:569-576.
2. Feng TR, White R, Gaber-Baylis L, et al. Coronary artery bypass graft readmission rates and risk factors- A retrospective cohort study. Int J Surg. 2018;54 (Part A):7-17.
3. Donndorf P, Kaminski A. “Return to sender” or “consider it done”?! The importance of reducing hospital readmission after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2017;154:1298-1299.
4. Sequist TD, Morong SM, Marston A, et al. Electronic risk alerts to improve primary care management of chest pain: A randomized, controlled trial. J Gen Intern Med. 2012;27:438-444.
5. Engelman D, Benjamin EM. Physician engagement: The “secret sauce” to success in bundled health care. Am J Med Qual. 2018;33:100-102.
1. Hannan EL, Zhong Y, Lahey SJ, et al. 30-day readmissions after coronary artery bypass graft surgery in New York State. JACC Cardiovasc Interv. 2011;4:569-576.
2. Feng TR, White R, Gaber-Baylis L, et al. Coronary artery bypass graft readmission rates and risk factors- A retrospective cohort study. Int J Surg. 2018;54 (Part A):7-17.
3. Donndorf P, Kaminski A. “Return to sender” or “consider it done”?! The importance of reducing hospital readmission after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2017;154:1298-1299.
4. Sequist TD, Morong SM, Marston A, et al. Electronic risk alerts to improve primary care management of chest pain: A randomized, controlled trial. J Gen Intern Med. 2012;27:438-444.
5. Engelman D, Benjamin EM. Physician engagement: The “secret sauce” to success in bundled health care. Am J Med Qual. 2018;33:100-102.
AAP adds specifics to policy on abusive head trauma
the American Academy of Pediatrics said in an updated policy statement.
Abusive head trauma (AHT) is fatal in approximately one-quarter of cases in infants during the first year of life, and less-obvious clinical signs such as vomiting and fussiness often are missed, wrote Sandeep K. Narang, MD, JD, of Northwestern University, Chicago, and colleagues on the AAP Council on Child Abuse and Neglect.
In a policy statement published in Pediatrics, the AAP cautioned physicians to remain vigilant for signs that are common in AHT cases. In particular, bruising on the torso, ears, and neck in children aged younger than 4 years, or any bruising in infants younger than 4 months should be a red flag. In addition, the most recent data indicate that apnea and retinal hemorrhages are more common in cases of abuse than in accidental injuries. The AAP also recommends a skeletal survey in suspected AHT for children younger than 2 years to identify occult fractures.
“Oral injuries in infants, such as frenulum tears, may also accompany or precede AHT,” Dr. Narang and associates said.
In addition, secondary brain injury as a result of AHT can lead to poor outcomes that may be observed. “Almost 70% of survivors of AHT have some degree of lasting neurologic impairment, including static encephalopathy, intellectual disability, cerebral palsy, cortical blindness, seizure disorders, behavior problems, and learning disabilities,” according to the statement.
Endocrine dysfunction also is common in children with a history of AHT, but might not present until years later, the authors noted.
When AHT is suspected in a patient, the policy statement recommends that a subspecialist in child abuse pediatrics or in related areas including radiology, ophthalmology, neurosurgery, neurology, and general pediatric surgery “should also be consulted when necessary to ensure a complete and accurate evaluation.”
Although falls from a height of 1.5 m or 5 feet often are used as an explanation for AHT injuries, “numerous lines of clinical research have clarified the extreme rarity of short falls as a cause of severe neurologic injury or death in young infants,” Dr. Narang and associates wrote.
Other recommendations in the updated policy encourage use of the term “abusive head trauma” in medical communications, as well as encourage caregivers to serve as a medical home for survivors of AHT or refer them to medical homes for rehabilitation and monitoring. Parents and caregivers may need to be educated about the dangers of shaking or striking an infant, shown safe ways to manage a crying baby, and given tools to manage their own stress and frustration.
Physicians are legally required to report suspected cases of child abuse or neglect, and should be prepared to educate stakeholders if you are called on to work with legal and child protective services about the science behind AHT.
“The role of the pediatric practitioner is not to apportion blame or investigate potential criminal activity but to identify the medical problem, evaluate and treat the child’s injuries, and offer honest medical information to parents, families, investigators, and attorneys and/or judges,” Dr. Narang and associates wrote.
This policy statement updates the previous policy statement issued in 2009 and affirmed in 2013. The policy had no external funding, and the authors had no financial conflicts to disclose. Dr. Narang, Amanda Fingarson, DO, and James Lukefahr, MD, have served as paid expert witnesses/consultants in cases of abusive head trauma in infants and children.
SOURCE: Narang SK et al. Pediatrics. 2020 Mar 23. doi: 10.1542/peds.2020-0203.
the American Academy of Pediatrics said in an updated policy statement.
Abusive head trauma (AHT) is fatal in approximately one-quarter of cases in infants during the first year of life, and less-obvious clinical signs such as vomiting and fussiness often are missed, wrote Sandeep K. Narang, MD, JD, of Northwestern University, Chicago, and colleagues on the AAP Council on Child Abuse and Neglect.
In a policy statement published in Pediatrics, the AAP cautioned physicians to remain vigilant for signs that are common in AHT cases. In particular, bruising on the torso, ears, and neck in children aged younger than 4 years, or any bruising in infants younger than 4 months should be a red flag. In addition, the most recent data indicate that apnea and retinal hemorrhages are more common in cases of abuse than in accidental injuries. The AAP also recommends a skeletal survey in suspected AHT for children younger than 2 years to identify occult fractures.
“Oral injuries in infants, such as frenulum tears, may also accompany or precede AHT,” Dr. Narang and associates said.
In addition, secondary brain injury as a result of AHT can lead to poor outcomes that may be observed. “Almost 70% of survivors of AHT have some degree of lasting neurologic impairment, including static encephalopathy, intellectual disability, cerebral palsy, cortical blindness, seizure disorders, behavior problems, and learning disabilities,” according to the statement.
Endocrine dysfunction also is common in children with a history of AHT, but might not present until years later, the authors noted.
When AHT is suspected in a patient, the policy statement recommends that a subspecialist in child abuse pediatrics or in related areas including radiology, ophthalmology, neurosurgery, neurology, and general pediatric surgery “should also be consulted when necessary to ensure a complete and accurate evaluation.”
Although falls from a height of 1.5 m or 5 feet often are used as an explanation for AHT injuries, “numerous lines of clinical research have clarified the extreme rarity of short falls as a cause of severe neurologic injury or death in young infants,” Dr. Narang and associates wrote.
Other recommendations in the updated policy encourage use of the term “abusive head trauma” in medical communications, as well as encourage caregivers to serve as a medical home for survivors of AHT or refer them to medical homes for rehabilitation and monitoring. Parents and caregivers may need to be educated about the dangers of shaking or striking an infant, shown safe ways to manage a crying baby, and given tools to manage their own stress and frustration.
Physicians are legally required to report suspected cases of child abuse or neglect, and should be prepared to educate stakeholders if you are called on to work with legal and child protective services about the science behind AHT.
“The role of the pediatric practitioner is not to apportion blame or investigate potential criminal activity but to identify the medical problem, evaluate and treat the child’s injuries, and offer honest medical information to parents, families, investigators, and attorneys and/or judges,” Dr. Narang and associates wrote.
This policy statement updates the previous policy statement issued in 2009 and affirmed in 2013. The policy had no external funding, and the authors had no financial conflicts to disclose. Dr. Narang, Amanda Fingarson, DO, and James Lukefahr, MD, have served as paid expert witnesses/consultants in cases of abusive head trauma in infants and children.
SOURCE: Narang SK et al. Pediatrics. 2020 Mar 23. doi: 10.1542/peds.2020-0203.
the American Academy of Pediatrics said in an updated policy statement.
Abusive head trauma (AHT) is fatal in approximately one-quarter of cases in infants during the first year of life, and less-obvious clinical signs such as vomiting and fussiness often are missed, wrote Sandeep K. Narang, MD, JD, of Northwestern University, Chicago, and colleagues on the AAP Council on Child Abuse and Neglect.
In a policy statement published in Pediatrics, the AAP cautioned physicians to remain vigilant for signs that are common in AHT cases. In particular, bruising on the torso, ears, and neck in children aged younger than 4 years, or any bruising in infants younger than 4 months should be a red flag. In addition, the most recent data indicate that apnea and retinal hemorrhages are more common in cases of abuse than in accidental injuries. The AAP also recommends a skeletal survey in suspected AHT for children younger than 2 years to identify occult fractures.
“Oral injuries in infants, such as frenulum tears, may also accompany or precede AHT,” Dr. Narang and associates said.
In addition, secondary brain injury as a result of AHT can lead to poor outcomes that may be observed. “Almost 70% of survivors of AHT have some degree of lasting neurologic impairment, including static encephalopathy, intellectual disability, cerebral palsy, cortical blindness, seizure disorders, behavior problems, and learning disabilities,” according to the statement.
Endocrine dysfunction also is common in children with a history of AHT, but might not present until years later, the authors noted.
When AHT is suspected in a patient, the policy statement recommends that a subspecialist in child abuse pediatrics or in related areas including radiology, ophthalmology, neurosurgery, neurology, and general pediatric surgery “should also be consulted when necessary to ensure a complete and accurate evaluation.”
Although falls from a height of 1.5 m or 5 feet often are used as an explanation for AHT injuries, “numerous lines of clinical research have clarified the extreme rarity of short falls as a cause of severe neurologic injury or death in young infants,” Dr. Narang and associates wrote.
Other recommendations in the updated policy encourage use of the term “abusive head trauma” in medical communications, as well as encourage caregivers to serve as a medical home for survivors of AHT or refer them to medical homes for rehabilitation and monitoring. Parents and caregivers may need to be educated about the dangers of shaking or striking an infant, shown safe ways to manage a crying baby, and given tools to manage their own stress and frustration.
Physicians are legally required to report suspected cases of child abuse or neglect, and should be prepared to educate stakeholders if you are called on to work with legal and child protective services about the science behind AHT.
“The role of the pediatric practitioner is not to apportion blame or investigate potential criminal activity but to identify the medical problem, evaluate and treat the child’s injuries, and offer honest medical information to parents, families, investigators, and attorneys and/or judges,” Dr. Narang and associates wrote.
This policy statement updates the previous policy statement issued in 2009 and affirmed in 2013. The policy had no external funding, and the authors had no financial conflicts to disclose. Dr. Narang, Amanda Fingarson, DO, and James Lukefahr, MD, have served as paid expert witnesses/consultants in cases of abusive head trauma in infants and children.
SOURCE: Narang SK et al. Pediatrics. 2020 Mar 23. doi: 10.1542/peds.2020-0203.
FROM PEDIATRICS