User login
Growth in early life may predict early puberty
Faster gains in weight, length or height, or body mass index in the first 5 years of life were associated with an earlier onset of puberty in boys and girls, based on data from a cohort study of more than 7,000 children.
In recent decades, clinicians and parents have raised concerns about an earlier onset of puberty in children in the United States and other countries, Izzudin M. Aris, PhD, of Harvard Medical School, Boston, and colleagues wrote.
“Children with earlier pubertal onset not only may be at increased risk for long-term chronic diseases, but also may experience adverse consequences during adolescence, including psychosocial difficulties and dysmetabolism,” they said. However, the effect of growth in the first 5 years of life on pubertal onset has not been well studied.
In a study published in JAMA Network Open, the researchers identified 7,495 children from 36 cohorts participating in the Environmental Influences on Child Health Outcomes program from Jan. 1, 1986, to Dec. 31, 2015.
The study population included 3,772 girls and 3,723 boys; 60% reported as White, 23% as Black, 15% as Hispanic, 12% as one of the following: American Indian or Alaska Native, Native Hawaiian or Pacific Islander, multiple races, or other race. Most (84.1%) were born during or after the year 2000.
The primary outcome was the pubertal growth spurt, also known as age at peak height velocity (APHV). The researchers measured growth at 3 age periods in the first 5 years (early infancy, late infancy, and early childhood) and estimated rates of weight, length or height, and body mass index (BMI) gain. Secondary outcomes included self-reported pubic hair staging and scores on the Pubertal Development Scale.
Overall, weight and length or height gain velocities declined in the first 5 years of life, and boys had faster gains in early infancy, compared with girls.
APHV was negatively correlated with puberty scores and Tanner staging for pubic hair development in both boys and girls, while puberty score was positively correlated with Tanner staging for pubic hair in both sexes.
After controlling for maternal and child confounders including maternal age at delivery, maternal education level, and year of birth, faster gains in weight, length or height, or BMI at each of the three measurement periods in early life was associated with earlier APHV in boys. No effect was noted for race, maternal education level, or birth year.
In girls, faster gains in weight, length, or height, only at the latest measurement period (early childhood) were associated with younger APHV. No associations with APHV occurred for velocities of BMI gain at any age period in girls, the researchers noted. However, age at menarche was positively correlated with early APHV and negatively correlated with puberty score and Tanner staging for pubic hair.
The findings support previous studies of associations between child growth and pubertal onset, the researchers wrote. The mechanisms of action are many, and have not been explained, the researchers wrote in their discussion of the findings.
“We speculate that insulinlike growth factor 1 may be a factor in the associations observed in the present study, either directly or indirectly through sex steroid synthesis and secretion. Alternatively, in girls, androgens and adipokines may be factors in the observed associations for pubic hair staging and menarche, respectively,” they said. Genetics and other factors including social factors, environmental exposures, diet, and physical activity also affect growth in early life.
The study findings were limited by several factors including the use of child-reported measures of pubic hair staging and parent reports of pubertal scores, with the potential for error and misclassification, the researchers noted. Other limitations include a lack of data on maternal age at menarche and the use of weight-for-length rather than BMI for children younger than 2 years.
However, the results were strengthened by the large sample size, long-term follow-up, and especially the use of a nationally representative contemporary cohort that addresses gaps in the current literature from later time periods. The results support the associations of sex-specific early pubertal onset in children with faster growth early in life. “In the long term, results of the present study may inform future research that aims to develop and/or test preventive interventions to optimize nutrition, environmental exposures, physical activity, and other behaviors related to growth during these age periods,” they concluded.
Time and timing limit practical application of results
The current study addresses two issues that are ongoing concerns for clinicians, specifically, the rise in obesity in childhood and its potential link to an earlier age of entry into puberty, M. Susan Jay, MD, of the Medical College of Wisconsin, Milwaukee, said in an interview.
“Authors in prior studies have suggested that earlier puberty, and indeed earlier menarche, in females may be associated with the potential of long-term health issues,” Dr. Jay noted. “It has also been suggested that both early maturing females and males may be impacted psychosocially. Others have suggested that the pathways through puberty are key and environmental factors as well as nutrition can have an impact on adolescence as well as health consequences later in life.”
The current study is important because it focused on children born in the present era of the obesity epidemic, while earlier studies were conducted on a group in the 1960s-1980s. “This study suggests that there are sex-specific associations of faster growth and earlier entry into puberty,” Dr. Jay said.
“While it is exciting to consider closer monitoring of pubertal progression in pediatric settings, often patients and families do not present in a timely manner for assessment,” she said. “Also, the authors suggest that preventive support may be offered to children who are traversing puberty at earlier ages. However, given the current stress on practices with COVID as well as stress on providers offering clinical services, identifying supportive interventions may be a stretch at best for practitioners already burdened by clinical and administrative demands.
“Ongoing studies are needed to address the knowledge gaps that exist in the arena of pubertal onset and growth during childhood across life periods,” said Dr. Jay. “In the long term, the present study may help direct research that could focus on preventive interventions to optimize nutrition, physical activity, environmental exposures, and other factors that intersect growth during infancy through early childhood, which may hasten early pubertal development’s later sequelae in adulthood.”
The study was supported by various grants to the researchers from the Environmental Influences on Child Health Outcomes program, Office of the Director, National Institutes of Health, as well as the Colorado Clinical and Translational Sciences Institute, University of Colorado at Denver. Lead author Dr. Aris had no financial conflicts to disclose. Dr. Jay had no conflicts to disclose and serves on the editorial advisory board of Pediatric News.
Faster gains in weight, length or height, or body mass index in the first 5 years of life were associated with an earlier onset of puberty in boys and girls, based on data from a cohort study of more than 7,000 children.
In recent decades, clinicians and parents have raised concerns about an earlier onset of puberty in children in the United States and other countries, Izzudin M. Aris, PhD, of Harvard Medical School, Boston, and colleagues wrote.
“Children with earlier pubertal onset not only may be at increased risk for long-term chronic diseases, but also may experience adverse consequences during adolescence, including psychosocial difficulties and dysmetabolism,” they said. However, the effect of growth in the first 5 years of life on pubertal onset has not been well studied.
In a study published in JAMA Network Open, the researchers identified 7,495 children from 36 cohorts participating in the Environmental Influences on Child Health Outcomes program from Jan. 1, 1986, to Dec. 31, 2015.
The study population included 3,772 girls and 3,723 boys; 60% reported as White, 23% as Black, 15% as Hispanic, 12% as one of the following: American Indian or Alaska Native, Native Hawaiian or Pacific Islander, multiple races, or other race. Most (84.1%) were born during or after the year 2000.
The primary outcome was the pubertal growth spurt, also known as age at peak height velocity (APHV). The researchers measured growth at 3 age periods in the first 5 years (early infancy, late infancy, and early childhood) and estimated rates of weight, length or height, and body mass index (BMI) gain. Secondary outcomes included self-reported pubic hair staging and scores on the Pubertal Development Scale.
Overall, weight and length or height gain velocities declined in the first 5 years of life, and boys had faster gains in early infancy, compared with girls.
APHV was negatively correlated with puberty scores and Tanner staging for pubic hair development in both boys and girls, while puberty score was positively correlated with Tanner staging for pubic hair in both sexes.
After controlling for maternal and child confounders including maternal age at delivery, maternal education level, and year of birth, faster gains in weight, length or height, or BMI at each of the three measurement periods in early life was associated with earlier APHV in boys. No effect was noted for race, maternal education level, or birth year.
In girls, faster gains in weight, length, or height, only at the latest measurement period (early childhood) were associated with younger APHV. No associations with APHV occurred for velocities of BMI gain at any age period in girls, the researchers noted. However, age at menarche was positively correlated with early APHV and negatively correlated with puberty score and Tanner staging for pubic hair.
The findings support previous studies of associations between child growth and pubertal onset, the researchers wrote. The mechanisms of action are many, and have not been explained, the researchers wrote in their discussion of the findings.
“We speculate that insulinlike growth factor 1 may be a factor in the associations observed in the present study, either directly or indirectly through sex steroid synthesis and secretion. Alternatively, in girls, androgens and adipokines may be factors in the observed associations for pubic hair staging and menarche, respectively,” they said. Genetics and other factors including social factors, environmental exposures, diet, and physical activity also affect growth in early life.
The study findings were limited by several factors including the use of child-reported measures of pubic hair staging and parent reports of pubertal scores, with the potential for error and misclassification, the researchers noted. Other limitations include a lack of data on maternal age at menarche and the use of weight-for-length rather than BMI for children younger than 2 years.
However, the results were strengthened by the large sample size, long-term follow-up, and especially the use of a nationally representative contemporary cohort that addresses gaps in the current literature from later time periods. The results support the associations of sex-specific early pubertal onset in children with faster growth early in life. “In the long term, results of the present study may inform future research that aims to develop and/or test preventive interventions to optimize nutrition, environmental exposures, physical activity, and other behaviors related to growth during these age periods,” they concluded.
Time and timing limit practical application of results
The current study addresses two issues that are ongoing concerns for clinicians, specifically, the rise in obesity in childhood and its potential link to an earlier age of entry into puberty, M. Susan Jay, MD, of the Medical College of Wisconsin, Milwaukee, said in an interview.
“Authors in prior studies have suggested that earlier puberty, and indeed earlier menarche, in females may be associated with the potential of long-term health issues,” Dr. Jay noted. “It has also been suggested that both early maturing females and males may be impacted psychosocially. Others have suggested that the pathways through puberty are key and environmental factors as well as nutrition can have an impact on adolescence as well as health consequences later in life.”
The current study is important because it focused on children born in the present era of the obesity epidemic, while earlier studies were conducted on a group in the 1960s-1980s. “This study suggests that there are sex-specific associations of faster growth and earlier entry into puberty,” Dr. Jay said.
“While it is exciting to consider closer monitoring of pubertal progression in pediatric settings, often patients and families do not present in a timely manner for assessment,” she said. “Also, the authors suggest that preventive support may be offered to children who are traversing puberty at earlier ages. However, given the current stress on practices with COVID as well as stress on providers offering clinical services, identifying supportive interventions may be a stretch at best for practitioners already burdened by clinical and administrative demands.
“Ongoing studies are needed to address the knowledge gaps that exist in the arena of pubertal onset and growth during childhood across life periods,” said Dr. Jay. “In the long term, the present study may help direct research that could focus on preventive interventions to optimize nutrition, physical activity, environmental exposures, and other factors that intersect growth during infancy through early childhood, which may hasten early pubertal development’s later sequelae in adulthood.”
The study was supported by various grants to the researchers from the Environmental Influences on Child Health Outcomes program, Office of the Director, National Institutes of Health, as well as the Colorado Clinical and Translational Sciences Institute, University of Colorado at Denver. Lead author Dr. Aris had no financial conflicts to disclose. Dr. Jay had no conflicts to disclose and serves on the editorial advisory board of Pediatric News.
Faster gains in weight, length or height, or body mass index in the first 5 years of life were associated with an earlier onset of puberty in boys and girls, based on data from a cohort study of more than 7,000 children.
In recent decades, clinicians and parents have raised concerns about an earlier onset of puberty in children in the United States and other countries, Izzudin M. Aris, PhD, of Harvard Medical School, Boston, and colleagues wrote.
“Children with earlier pubertal onset not only may be at increased risk for long-term chronic diseases, but also may experience adverse consequences during adolescence, including psychosocial difficulties and dysmetabolism,” they said. However, the effect of growth in the first 5 years of life on pubertal onset has not been well studied.
In a study published in JAMA Network Open, the researchers identified 7,495 children from 36 cohorts participating in the Environmental Influences on Child Health Outcomes program from Jan. 1, 1986, to Dec. 31, 2015.
The study population included 3,772 girls and 3,723 boys; 60% reported as White, 23% as Black, 15% as Hispanic, 12% as one of the following: American Indian or Alaska Native, Native Hawaiian or Pacific Islander, multiple races, or other race. Most (84.1%) were born during or after the year 2000.
The primary outcome was the pubertal growth spurt, also known as age at peak height velocity (APHV). The researchers measured growth at 3 age periods in the first 5 years (early infancy, late infancy, and early childhood) and estimated rates of weight, length or height, and body mass index (BMI) gain. Secondary outcomes included self-reported pubic hair staging and scores on the Pubertal Development Scale.
Overall, weight and length or height gain velocities declined in the first 5 years of life, and boys had faster gains in early infancy, compared with girls.
APHV was negatively correlated with puberty scores and Tanner staging for pubic hair development in both boys and girls, while puberty score was positively correlated with Tanner staging for pubic hair in both sexes.
After controlling for maternal and child confounders including maternal age at delivery, maternal education level, and year of birth, faster gains in weight, length or height, or BMI at each of the three measurement periods in early life was associated with earlier APHV in boys. No effect was noted for race, maternal education level, or birth year.
In girls, faster gains in weight, length, or height, only at the latest measurement period (early childhood) were associated with younger APHV. No associations with APHV occurred for velocities of BMI gain at any age period in girls, the researchers noted. However, age at menarche was positively correlated with early APHV and negatively correlated with puberty score and Tanner staging for pubic hair.
The findings support previous studies of associations between child growth and pubertal onset, the researchers wrote. The mechanisms of action are many, and have not been explained, the researchers wrote in their discussion of the findings.
“We speculate that insulinlike growth factor 1 may be a factor in the associations observed in the present study, either directly or indirectly through sex steroid synthesis and secretion. Alternatively, in girls, androgens and adipokines may be factors in the observed associations for pubic hair staging and menarche, respectively,” they said. Genetics and other factors including social factors, environmental exposures, diet, and physical activity also affect growth in early life.
The study findings were limited by several factors including the use of child-reported measures of pubic hair staging and parent reports of pubertal scores, with the potential for error and misclassification, the researchers noted. Other limitations include a lack of data on maternal age at menarche and the use of weight-for-length rather than BMI for children younger than 2 years.
However, the results were strengthened by the large sample size, long-term follow-up, and especially the use of a nationally representative contemporary cohort that addresses gaps in the current literature from later time periods. The results support the associations of sex-specific early pubertal onset in children with faster growth early in life. “In the long term, results of the present study may inform future research that aims to develop and/or test preventive interventions to optimize nutrition, environmental exposures, physical activity, and other behaviors related to growth during these age periods,” they concluded.
Time and timing limit practical application of results
The current study addresses two issues that are ongoing concerns for clinicians, specifically, the rise in obesity in childhood and its potential link to an earlier age of entry into puberty, M. Susan Jay, MD, of the Medical College of Wisconsin, Milwaukee, said in an interview.
“Authors in prior studies have suggested that earlier puberty, and indeed earlier menarche, in females may be associated with the potential of long-term health issues,” Dr. Jay noted. “It has also been suggested that both early maturing females and males may be impacted psychosocially. Others have suggested that the pathways through puberty are key and environmental factors as well as nutrition can have an impact on adolescence as well as health consequences later in life.”
The current study is important because it focused on children born in the present era of the obesity epidemic, while earlier studies were conducted on a group in the 1960s-1980s. “This study suggests that there are sex-specific associations of faster growth and earlier entry into puberty,” Dr. Jay said.
“While it is exciting to consider closer monitoring of pubertal progression in pediatric settings, often patients and families do not present in a timely manner for assessment,” she said. “Also, the authors suggest that preventive support may be offered to children who are traversing puberty at earlier ages. However, given the current stress on practices with COVID as well as stress on providers offering clinical services, identifying supportive interventions may be a stretch at best for practitioners already burdened by clinical and administrative demands.
“Ongoing studies are needed to address the knowledge gaps that exist in the arena of pubertal onset and growth during childhood across life periods,” said Dr. Jay. “In the long term, the present study may help direct research that could focus on preventive interventions to optimize nutrition, physical activity, environmental exposures, and other factors that intersect growth during infancy through early childhood, which may hasten early pubertal development’s later sequelae in adulthood.”
The study was supported by various grants to the researchers from the Environmental Influences on Child Health Outcomes program, Office of the Director, National Institutes of Health, as well as the Colorado Clinical and Translational Sciences Institute, University of Colorado at Denver. Lead author Dr. Aris had no financial conflicts to disclose. Dr. Jay had no conflicts to disclose and serves on the editorial advisory board of Pediatric News.
FROM JAMA NETWORK OPEN
Heavy cannabis use tied to less diabetes in women
Women who used marijuana (cannabis) at least four times in the previous month (heavy users) were less likely to have type 2 diabetes than women who were light users or nonusers, in a nationally representative U.S. observational study.
In contrast, there were no differences in the prevalence of type 2 diabetes in men who were light or heavy cannabis users versus nonusers.
These findings are based on data from the 2013-2018 National Health and Nutrition Examination Survey (NHANES), whereby participants self-reported their cannabis use.
The study by Ayobami S. Ogunsola, MD, MPH, a graduate student at Texas A&M University, College Station, and colleagues was recently published in Cannabis and Cannabinoid Research.
What do the findings mean?
Although overall findings linking cannabis use and diabetes have been inconsistent, the gender differences in the current study are consistent with animal studies and some clinical studies, senior author Ibraheem M. Karaye, MD, MPH, said in an interview.
However, these gender differences need to be confirmed, and “we strongly recommend that more biological or biochemical studies be conducted that could actually tell us the mechanisms,” said Dr. Karaye, an assistant professor in the department of population health, Hofstra University, Hempstead, N.Y.
“It’s indisputable that medical marijuana has some medical benefits,” he added. “Women [who use cannabis] have been shown to lose more weight than men, for example.”
“If women [cannabis users] are less likely to develop diabetes or more likely to express improvement of symptoms of diabetes,” he noted, “this means that hyperglycemic medications that are being prescribed should be watched scrupulously. Otherwise, there is a risk that [women] may overrespond.”
That is, Dr. Karaye continued, women “may be at risk of developing hypoglycemia because the cannabis is acting synergistically with the regular drug that is being used to treat the diabetes.”
U.S. clinicians, especially in states with legalized medical marijuana, need to be aware of the potential synergy.
“One would have to consider the patient as a whole,” he stressed. “For example, a woman that uses medical marijuana may actually respond differently to hyperglycemic medication.”
Conflicting reports explained by sex differences?
Evidence on whether cannabis use is linked with type 2 diabetes is limited and conflicting, the researchers wrote. They hypothesized that these conflicting findings might be explained by sex differences.
To “help inform current diabetes prevention and mitigation efforts,” they investigated sex differences in cannabis use and prevalence of type 2 diabetes in 15,602 men and women in the 2013-2014, 2015-2016, and 2017-2018 NHANES surveys.
Participants were classified as having type 2 diabetes if they had a physician’s diagnosis; a 2-hour plasma glucose of at least 200 mg/dL (in a glucose tolerance test); fasting blood glucose of at least 126 mg/dL; or A1c of at least 6.5%.
About half of respondents were women (52%) and close to half (44%) were age 18-39.
More than a third (38%) had a body mass index (BMI) of at least 30 kg/m2, indicating obesity.
Roughly 1 in 10 had a diagnosis of type 2 diabetes (13.5%) or A1c of at least 6.5% (9.8%).
Close to a fifth smoked cigarettes (16%). Similarly, 14.5% used cannabis at least four times a week, 3.3% used it less often, and the rest did not use it. Half of participants were not physically active (49%).
Just over half had at least a college education (55%).
Heavy cannabis users were more likely to be younger than age 40 (57% of men, 57% of women), college graduates (54% of men, 63% of women), cigarette smokers (79% of men, 83% of women), and physically inactive (39% of men, 49% of women).
Among women, heavy cannabis users were 49% less likely to have type 2 diabetes than nonusers, after adjusting for age, sex, race/ethnicity, educational level, physical activity, tobacco use, alcohol use, marital status, difficulty walking, employment status, income, and BMI (adjusted odds ratio, 0.51; 95% confidence interval, 0.31-0.84).
There were no significant differences between light cannabis users versus nonusers and diabetes prevalence in women, or between light or heavy cannabis users versus nonusers and diabetes prevalence in men.
Limitations, yet biologically plausible
The researchers acknowledged several study limitations.
They do not know how long participants had used marijuana. The men and women may have underreported their cannabis use, especially in states where medical marijuana was not legal, and the NHANES data did not specify whether the cannabis was recreational or medicinal.
The study may have been underpowered to detect a smaller difference in men who used versus did not use marijuana.
And importantly, this was an observational study (a snapshot at one point in time), so it cannot say whether the heavy cannabis use in women caused a decreased likelihood of diabetes.
Nevertheless, the inverse association between cannabis use and presence of type 2 diabetes is biologically plausible, Dr. Ogunsola and colleagues wrote.
The two major cannabis compounds, cannabidiol and delta-9-tetrahydrocannabinol, stimulate CBD1 and CBD2 receptors in the central and peripheral nervous systems, respectively. And “activation of the CBD1 receptor increases insulin secretion, glucagon, and somatostatin, and activates metabolic processes in fat and skeletal muscles – mechanisms that improve glucose disposal,” they explained.
The researchers speculated that the sex differences they found for this association may be caused by differences in sex hormones, or the endocannabinoid system, or fat deposits.
Therefore, “additional studies are needed to investigate the sex-based heterogeneity reported in this study and to elucidate potential mechanisms for the observation,” they concluded.
The study did not receive any funding and the researchers have no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Women who used marijuana (cannabis) at least four times in the previous month (heavy users) were less likely to have type 2 diabetes than women who were light users or nonusers, in a nationally representative U.S. observational study.
In contrast, there were no differences in the prevalence of type 2 diabetes in men who were light or heavy cannabis users versus nonusers.
These findings are based on data from the 2013-2018 National Health and Nutrition Examination Survey (NHANES), whereby participants self-reported their cannabis use.
The study by Ayobami S. Ogunsola, MD, MPH, a graduate student at Texas A&M University, College Station, and colleagues was recently published in Cannabis and Cannabinoid Research.
What do the findings mean?
Although overall findings linking cannabis use and diabetes have been inconsistent, the gender differences in the current study are consistent with animal studies and some clinical studies, senior author Ibraheem M. Karaye, MD, MPH, said in an interview.
However, these gender differences need to be confirmed, and “we strongly recommend that more biological or biochemical studies be conducted that could actually tell us the mechanisms,” said Dr. Karaye, an assistant professor in the department of population health, Hofstra University, Hempstead, N.Y.
“It’s indisputable that medical marijuana has some medical benefits,” he added. “Women [who use cannabis] have been shown to lose more weight than men, for example.”
“If women [cannabis users] are less likely to develop diabetes or more likely to express improvement of symptoms of diabetes,” he noted, “this means that hyperglycemic medications that are being prescribed should be watched scrupulously. Otherwise, there is a risk that [women] may overrespond.”
That is, Dr. Karaye continued, women “may be at risk of developing hypoglycemia because the cannabis is acting synergistically with the regular drug that is being used to treat the diabetes.”
U.S. clinicians, especially in states with legalized medical marijuana, need to be aware of the potential synergy.
“One would have to consider the patient as a whole,” he stressed. “For example, a woman that uses medical marijuana may actually respond differently to hyperglycemic medication.”
Conflicting reports explained by sex differences?
Evidence on whether cannabis use is linked with type 2 diabetes is limited and conflicting, the researchers wrote. They hypothesized that these conflicting findings might be explained by sex differences.
To “help inform current diabetes prevention and mitigation efforts,” they investigated sex differences in cannabis use and prevalence of type 2 diabetes in 15,602 men and women in the 2013-2014, 2015-2016, and 2017-2018 NHANES surveys.
Participants were classified as having type 2 diabetes if they had a physician’s diagnosis; a 2-hour plasma glucose of at least 200 mg/dL (in a glucose tolerance test); fasting blood glucose of at least 126 mg/dL; or A1c of at least 6.5%.
About half of respondents were women (52%) and close to half (44%) were age 18-39.
More than a third (38%) had a body mass index (BMI) of at least 30 kg/m2, indicating obesity.
Roughly 1 in 10 had a diagnosis of type 2 diabetes (13.5%) or A1c of at least 6.5% (9.8%).
Close to a fifth smoked cigarettes (16%). Similarly, 14.5% used cannabis at least four times a week, 3.3% used it less often, and the rest did not use it. Half of participants were not physically active (49%).
Just over half had at least a college education (55%).
Heavy cannabis users were more likely to be younger than age 40 (57% of men, 57% of women), college graduates (54% of men, 63% of women), cigarette smokers (79% of men, 83% of women), and physically inactive (39% of men, 49% of women).
Among women, heavy cannabis users were 49% less likely to have type 2 diabetes than nonusers, after adjusting for age, sex, race/ethnicity, educational level, physical activity, tobacco use, alcohol use, marital status, difficulty walking, employment status, income, and BMI (adjusted odds ratio, 0.51; 95% confidence interval, 0.31-0.84).
There were no significant differences between light cannabis users versus nonusers and diabetes prevalence in women, or between light or heavy cannabis users versus nonusers and diabetes prevalence in men.
Limitations, yet biologically plausible
The researchers acknowledged several study limitations.
They do not know how long participants had used marijuana. The men and women may have underreported their cannabis use, especially in states where medical marijuana was not legal, and the NHANES data did not specify whether the cannabis was recreational or medicinal.
The study may have been underpowered to detect a smaller difference in men who used versus did not use marijuana.
And importantly, this was an observational study (a snapshot at one point in time), so it cannot say whether the heavy cannabis use in women caused a decreased likelihood of diabetes.
Nevertheless, the inverse association between cannabis use and presence of type 2 diabetes is biologically plausible, Dr. Ogunsola and colleagues wrote.
The two major cannabis compounds, cannabidiol and delta-9-tetrahydrocannabinol, stimulate CBD1 and CBD2 receptors in the central and peripheral nervous systems, respectively. And “activation of the CBD1 receptor increases insulin secretion, glucagon, and somatostatin, and activates metabolic processes in fat and skeletal muscles – mechanisms that improve glucose disposal,” they explained.
The researchers speculated that the sex differences they found for this association may be caused by differences in sex hormones, or the endocannabinoid system, or fat deposits.
Therefore, “additional studies are needed to investigate the sex-based heterogeneity reported in this study and to elucidate potential mechanisms for the observation,” they concluded.
The study did not receive any funding and the researchers have no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Women who used marijuana (cannabis) at least four times in the previous month (heavy users) were less likely to have type 2 diabetes than women who were light users or nonusers, in a nationally representative U.S. observational study.
In contrast, there were no differences in the prevalence of type 2 diabetes in men who were light or heavy cannabis users versus nonusers.
These findings are based on data from the 2013-2018 National Health and Nutrition Examination Survey (NHANES), whereby participants self-reported their cannabis use.
The study by Ayobami S. Ogunsola, MD, MPH, a graduate student at Texas A&M University, College Station, and colleagues was recently published in Cannabis and Cannabinoid Research.
What do the findings mean?
Although overall findings linking cannabis use and diabetes have been inconsistent, the gender differences in the current study are consistent with animal studies and some clinical studies, senior author Ibraheem M. Karaye, MD, MPH, said in an interview.
However, these gender differences need to be confirmed, and “we strongly recommend that more biological or biochemical studies be conducted that could actually tell us the mechanisms,” said Dr. Karaye, an assistant professor in the department of population health, Hofstra University, Hempstead, N.Y.
“It’s indisputable that medical marijuana has some medical benefits,” he added. “Women [who use cannabis] have been shown to lose more weight than men, for example.”
“If women [cannabis users] are less likely to develop diabetes or more likely to express improvement of symptoms of diabetes,” he noted, “this means that hyperglycemic medications that are being prescribed should be watched scrupulously. Otherwise, there is a risk that [women] may overrespond.”
That is, Dr. Karaye continued, women “may be at risk of developing hypoglycemia because the cannabis is acting synergistically with the regular drug that is being used to treat the diabetes.”
U.S. clinicians, especially in states with legalized medical marijuana, need to be aware of the potential synergy.
“One would have to consider the patient as a whole,” he stressed. “For example, a woman that uses medical marijuana may actually respond differently to hyperglycemic medication.”
Conflicting reports explained by sex differences?
Evidence on whether cannabis use is linked with type 2 diabetes is limited and conflicting, the researchers wrote. They hypothesized that these conflicting findings might be explained by sex differences.
To “help inform current diabetes prevention and mitigation efforts,” they investigated sex differences in cannabis use and prevalence of type 2 diabetes in 15,602 men and women in the 2013-2014, 2015-2016, and 2017-2018 NHANES surveys.
Participants were classified as having type 2 diabetes if they had a physician’s diagnosis; a 2-hour plasma glucose of at least 200 mg/dL (in a glucose tolerance test); fasting blood glucose of at least 126 mg/dL; or A1c of at least 6.5%.
About half of respondents were women (52%) and close to half (44%) were age 18-39.
More than a third (38%) had a body mass index (BMI) of at least 30 kg/m2, indicating obesity.
Roughly 1 in 10 had a diagnosis of type 2 diabetes (13.5%) or A1c of at least 6.5% (9.8%).
Close to a fifth smoked cigarettes (16%). Similarly, 14.5% used cannabis at least four times a week, 3.3% used it less often, and the rest did not use it. Half of participants were not physically active (49%).
Just over half had at least a college education (55%).
Heavy cannabis users were more likely to be younger than age 40 (57% of men, 57% of women), college graduates (54% of men, 63% of women), cigarette smokers (79% of men, 83% of women), and physically inactive (39% of men, 49% of women).
Among women, heavy cannabis users were 49% less likely to have type 2 diabetes than nonusers, after adjusting for age, sex, race/ethnicity, educational level, physical activity, tobacco use, alcohol use, marital status, difficulty walking, employment status, income, and BMI (adjusted odds ratio, 0.51; 95% confidence interval, 0.31-0.84).
There were no significant differences between light cannabis users versus nonusers and diabetes prevalence in women, or between light or heavy cannabis users versus nonusers and diabetes prevalence in men.
Limitations, yet biologically plausible
The researchers acknowledged several study limitations.
They do not know how long participants had used marijuana. The men and women may have underreported their cannabis use, especially in states where medical marijuana was not legal, and the NHANES data did not specify whether the cannabis was recreational or medicinal.
The study may have been underpowered to detect a smaller difference in men who used versus did not use marijuana.
And importantly, this was an observational study (a snapshot at one point in time), so it cannot say whether the heavy cannabis use in women caused a decreased likelihood of diabetes.
Nevertheless, the inverse association between cannabis use and presence of type 2 diabetes is biologically plausible, Dr. Ogunsola and colleagues wrote.
The two major cannabis compounds, cannabidiol and delta-9-tetrahydrocannabinol, stimulate CBD1 and CBD2 receptors in the central and peripheral nervous systems, respectively. And “activation of the CBD1 receptor increases insulin secretion, glucagon, and somatostatin, and activates metabolic processes in fat and skeletal muscles – mechanisms that improve glucose disposal,” they explained.
The researchers speculated that the sex differences they found for this association may be caused by differences in sex hormones, or the endocannabinoid system, or fat deposits.
Therefore, “additional studies are needed to investigate the sex-based heterogeneity reported in this study and to elucidate potential mechanisms for the observation,” they concluded.
The study did not receive any funding and the researchers have no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
FROM CANNABIS AND CANNABINOID RESEARCH
Endocrine Society and others to FDA: Restrict BPA
The chemical is used to make plastics in items such as food containers, pitchers, and inner linings of metal products. Small amounts of BPA can leak into food and beverages.
The petition points to a December 2021 report by the European Food Safety Authority titled: “Re-evaluation of the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs,” which summarizes evidence gathered since 2013.
It concludes that “there is a health concern from BPA exposure for all age groups.” Specific concerns include harm to the immune system and male and female reproductive systems.
Average American exposed to 5,000 times the safe level of BPA
The EFSA established a new “tolerable daily intake” of BPA of 0.04 ng/kg of body weight per day. By contrast, in 2014 the FDA estimated that the mean BPA intake for the U.S. population older than 2 years was 200 ng/kg bw/day and that the 90th percentile for BPA intake was 500 ng/kg of body weight per day.
“Using FDA’s own exposure estimates, the average American is exposed to more than 5000 times the safe level of 0.04 ng BPA/kg [body weight per day] set by the EFSA expert panel. Without a doubt, these values constitute a high health risk and support the conclusion that uses of BPA are not safe ... Given the magnitude of the overexposure, we request an expedited review by FDA,” the petition reads.
In addition to the Endocrine Society, which has long warned about the dangers of endocrine-disrupting chemicals, other signatories to the petition include the Environmental Defense Fund, Breast Cancer Prevention Partners, Clean Water Action/Clean Water Fund, Consumer Reports, Environmental Working Group, Healthy Babies Bright Futures, and the former director of the National Institute of Environmental Health Sciences and National Toxicology Program.
In a statement, Endocrine Society BPA expert Heather Patisaul, PhD, of North Carolina University, Raleigh, said the report’s findings “are extremely concerning and prove the point that even very low levels of BPA exposure can be harmful and lead to issues with reproductive health, breast cancer risk, behavior, and metabolism.”
“The FDA needs to acknowledge the science behind endocrine-disrupting chemicals and act accordingly to protect public health,” she urged.
The FDA is expected to decide within the next few days whether to open a docket to accept comments.
A final decision could take 6 months or longer, an Endocrine Society spokesperson told this news organization.
A version of this article first appeared on Medscape.com.
The chemical is used to make plastics in items such as food containers, pitchers, and inner linings of metal products. Small amounts of BPA can leak into food and beverages.
The petition points to a December 2021 report by the European Food Safety Authority titled: “Re-evaluation of the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs,” which summarizes evidence gathered since 2013.
It concludes that “there is a health concern from BPA exposure for all age groups.” Specific concerns include harm to the immune system and male and female reproductive systems.
Average American exposed to 5,000 times the safe level of BPA
The EFSA established a new “tolerable daily intake” of BPA of 0.04 ng/kg of body weight per day. By contrast, in 2014 the FDA estimated that the mean BPA intake for the U.S. population older than 2 years was 200 ng/kg bw/day and that the 90th percentile for BPA intake was 500 ng/kg of body weight per day.
“Using FDA’s own exposure estimates, the average American is exposed to more than 5000 times the safe level of 0.04 ng BPA/kg [body weight per day] set by the EFSA expert panel. Without a doubt, these values constitute a high health risk and support the conclusion that uses of BPA are not safe ... Given the magnitude of the overexposure, we request an expedited review by FDA,” the petition reads.
In addition to the Endocrine Society, which has long warned about the dangers of endocrine-disrupting chemicals, other signatories to the petition include the Environmental Defense Fund, Breast Cancer Prevention Partners, Clean Water Action/Clean Water Fund, Consumer Reports, Environmental Working Group, Healthy Babies Bright Futures, and the former director of the National Institute of Environmental Health Sciences and National Toxicology Program.
In a statement, Endocrine Society BPA expert Heather Patisaul, PhD, of North Carolina University, Raleigh, said the report’s findings “are extremely concerning and prove the point that even very low levels of BPA exposure can be harmful and lead to issues with reproductive health, breast cancer risk, behavior, and metabolism.”
“The FDA needs to acknowledge the science behind endocrine-disrupting chemicals and act accordingly to protect public health,” she urged.
The FDA is expected to decide within the next few days whether to open a docket to accept comments.
A final decision could take 6 months or longer, an Endocrine Society spokesperson told this news organization.
A version of this article first appeared on Medscape.com.
The chemical is used to make plastics in items such as food containers, pitchers, and inner linings of metal products. Small amounts of BPA can leak into food and beverages.
The petition points to a December 2021 report by the European Food Safety Authority titled: “Re-evaluation of the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs,” which summarizes evidence gathered since 2013.
It concludes that “there is a health concern from BPA exposure for all age groups.” Specific concerns include harm to the immune system and male and female reproductive systems.
Average American exposed to 5,000 times the safe level of BPA
The EFSA established a new “tolerable daily intake” of BPA of 0.04 ng/kg of body weight per day. By contrast, in 2014 the FDA estimated that the mean BPA intake for the U.S. population older than 2 years was 200 ng/kg bw/day and that the 90th percentile for BPA intake was 500 ng/kg of body weight per day.
“Using FDA’s own exposure estimates, the average American is exposed to more than 5000 times the safe level of 0.04 ng BPA/kg [body weight per day] set by the EFSA expert panel. Without a doubt, these values constitute a high health risk and support the conclusion that uses of BPA are not safe ... Given the magnitude of the overexposure, we request an expedited review by FDA,” the petition reads.
In addition to the Endocrine Society, which has long warned about the dangers of endocrine-disrupting chemicals, other signatories to the petition include the Environmental Defense Fund, Breast Cancer Prevention Partners, Clean Water Action/Clean Water Fund, Consumer Reports, Environmental Working Group, Healthy Babies Bright Futures, and the former director of the National Institute of Environmental Health Sciences and National Toxicology Program.
In a statement, Endocrine Society BPA expert Heather Patisaul, PhD, of North Carolina University, Raleigh, said the report’s findings “are extremely concerning and prove the point that even very low levels of BPA exposure can be harmful and lead to issues with reproductive health, breast cancer risk, behavior, and metabolism.”
“The FDA needs to acknowledge the science behind endocrine-disrupting chemicals and act accordingly to protect public health,” she urged.
The FDA is expected to decide within the next few days whether to open a docket to accept comments.
A final decision could take 6 months or longer, an Endocrine Society spokesperson told this news organization.
A version of this article first appeared on Medscape.com.
Anxiety in men tied to risk factors for CVD, diabetes
Among healthy middle-aged men, those who were more anxious were more likely to develop high levels of multiple biomarkers of cardiometabolic risk over a 40-year follow-up in a new study.
“By middle adulthood, higher anxiety levels are associated with stable differences” in biomarkers of risk for coronary artery disease (CAD), stroke, and type 2 diabetes, which “are maintained into older ages,” the researchers wrote.
Anxious individuals “may experience deteriorations in cardiometabolic health earlier in life and remain on a stable trajectory of heightened risk into older ages,” they concluded.
The study, led by Lewina Lee, PhD, was published online Jan. 24, 2022, in the Journal of the American Heart Association.
“Men who had higher levels of anxiety at the beginning of the study had consistently higher biological risk for cardiometabolic disease than less anxious men from midlife into old age,” Dr. Lee, assistant professor of psychiatry, Boston University, summarized in an email.
Clinicians may not screen for heart disease and diabetes, and/or only discuss lifestyle modifications when patients are older or have the first signs of disease, she added.
However, the study findings “suggest that worries and anxiety are associated with preclinical pathophysiological processes that tend to culminate in cardiometabolic disease” and show “the importance of screening for mental health difficulties, such as worries and anxiety, in men as early as in their 30s and 40s,” she stressed.
Since most of the men were White (97%) and veterans (94%), “it would be important for future studies to evaluate if these associations exist among women, people from diverse racial and ethnic groups, and in more socioeconomically varying samples, and to consider how anxiety may relate to the development of cardiometabolic risk in much younger individuals than those in our study,” Dr. Lee said in a press release from the American Heart Association.
“This study adds to the growing body of research that link psychological health to cardiovascular risk,” Glenn N. Levine, MD, who was not involved with this research, told this news organization in an email.
“We know that factors such as depression and stress can increase cardiac risk; this study further supports that anxiety can as well,” added Dr. Levine, chief of cardiology, Michael E. DeBakey Veterans Affairs Medical Center, Houston.
“Everyone experiences some anxiety in their life,” he added. However, “if a provider senses that a patient’s anxiety is far beyond the ‘normal’ that we all have from time to time, and it is seemingly adversely impacting both their psychological and physical health, it would be reasonable to suggest to the patient that it might be useful to speak with a mental health professional, and if the patient is receptive, to then make a formal consultation or referral,” said Dr. Levine, who was writing group chair of a recent AHA Scientific Statement on mind-heart-body connection.
Neuroticism and worry
Several studies have linked anxiety to a greater risk of cardiometabolic disease onset, Dr. Lee and colleagues wrote, but it is unclear if anxious individuals have a steadily worsening risk as they age, or if they have a higher risk in middle age, which stays the same in older age.
To investigate this, they analyzed data from 1561 men who were seen at the VA Boston outpatient clinic and did not have CAD, type 2 diabetes, stroke, or cancer when they enrolled in the Normative Aging Study.
The men had a mean age of 53 years (range, 33-84) in 1975 and were followed until 2015 or until dropout from the study or death.
At baseline, the study participants filled in the Eysenck Personality Inventory, which assesses neuroticism, and also responded to a scale indicating how much they worry about 20 issues (excluding health).
“Neuroticism,” the researchers explained, “is a tendency to perceive experiences as threatening, feel that challenges are uncontrollable, and experience frequent and disproportionately intense negative emotions,” such as fear, anxiety, sadness, and anger, “across many situations.”
“Worry refers to attempts to solve a problem where future outcome is uncertain and potentially positive or negative,” Dr. Lee noted. Although worry can be healthy and lead to constructive solutions, “it may be unhealthy, especially when it becomes uncontrollable and interferes with day-to-day functioning.”
Of note, in 1980, the American Psychiatric Association removed the term neurosis from its diagnostic manual. What was previously called neurosis is included as part of generalized anxiety disorder; GAD also encompasses excessive worry.
Cardiometabolic risk from midlife to old age
The men in the current study had on-site physical examinations every 3-5 years.
The researchers calculated the men’s cardiometabolic risk score (from 0 to 7) by assigning 1 point each for the following: systolic blood pressure greater than 130 mm Hg, diastolic blood pressure greater than 85 mm Hg, total cholesterol of at least 240 mg/dL, triglycerides of at least 150 mg/dL, body mass index of at least 30 kg/m2, glucose of at least 100 mg/dL, and erythrocyte sedimentation rate of at least 14 mm/hour.
Alternatively, patients were assigned a point each for taking medication that could affect these markers (except for body mass index).
Overall, on average, at baseline, the men had a cardiometabolic risk score of 2.9. From age 33-65, this score increased to 3.8, and then it did not increase as much later on.
That is, the cardiometabolic risk score increased by 0.8 per decade until age 65, followed by a slower increase of 0.5 per decade.
At all ages, men with higher levels of neuroticism or worry had a higher cardiometabolic risk score
Each additional standard deviation of neuroticism was associated with a 13% increased risk of having six or more of the seven cardiometabolic risk markers during follow-up, after adjusting for age, demographics, and family history of CAD, but the relationship was attenuated after also adjusting for health behaviors (for example, smoking, alcohol consumption, physical activity, and past-year physician visit at baseline).
Similarly, each additional standard deviation of worry was associated with a 10% increased risk of having six or more of the seven cardiometabolic risk markers during follow-up after the same adjustments, and was also no longer significantly different after the same further adjustments.
The research was supported by grants from the National Institutes of Health and a Senior Research Career Scientist Award from the Office of Research and Development, Department of Veterans Affairs. The Normative Aging Study is a research component of the Massachusetts Veterans Epidemiology Research and Information Center and is supported by the VA Cooperative Studies Program/Epidemiological Research Centers. The study authors and Dr. Levine disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Among healthy middle-aged men, those who were more anxious were more likely to develop high levels of multiple biomarkers of cardiometabolic risk over a 40-year follow-up in a new study.
“By middle adulthood, higher anxiety levels are associated with stable differences” in biomarkers of risk for coronary artery disease (CAD), stroke, and type 2 diabetes, which “are maintained into older ages,” the researchers wrote.
Anxious individuals “may experience deteriorations in cardiometabolic health earlier in life and remain on a stable trajectory of heightened risk into older ages,” they concluded.
The study, led by Lewina Lee, PhD, was published online Jan. 24, 2022, in the Journal of the American Heart Association.
“Men who had higher levels of anxiety at the beginning of the study had consistently higher biological risk for cardiometabolic disease than less anxious men from midlife into old age,” Dr. Lee, assistant professor of psychiatry, Boston University, summarized in an email.
Clinicians may not screen for heart disease and diabetes, and/or only discuss lifestyle modifications when patients are older or have the first signs of disease, she added.
However, the study findings “suggest that worries and anxiety are associated with preclinical pathophysiological processes that tend to culminate in cardiometabolic disease” and show “the importance of screening for mental health difficulties, such as worries and anxiety, in men as early as in their 30s and 40s,” she stressed.
Since most of the men were White (97%) and veterans (94%), “it would be important for future studies to evaluate if these associations exist among women, people from diverse racial and ethnic groups, and in more socioeconomically varying samples, and to consider how anxiety may relate to the development of cardiometabolic risk in much younger individuals than those in our study,” Dr. Lee said in a press release from the American Heart Association.
“This study adds to the growing body of research that link psychological health to cardiovascular risk,” Glenn N. Levine, MD, who was not involved with this research, told this news organization in an email.
“We know that factors such as depression and stress can increase cardiac risk; this study further supports that anxiety can as well,” added Dr. Levine, chief of cardiology, Michael E. DeBakey Veterans Affairs Medical Center, Houston.
“Everyone experiences some anxiety in their life,” he added. However, “if a provider senses that a patient’s anxiety is far beyond the ‘normal’ that we all have from time to time, and it is seemingly adversely impacting both their psychological and physical health, it would be reasonable to suggest to the patient that it might be useful to speak with a mental health professional, and if the patient is receptive, to then make a formal consultation or referral,” said Dr. Levine, who was writing group chair of a recent AHA Scientific Statement on mind-heart-body connection.
Neuroticism and worry
Several studies have linked anxiety to a greater risk of cardiometabolic disease onset, Dr. Lee and colleagues wrote, but it is unclear if anxious individuals have a steadily worsening risk as they age, or if they have a higher risk in middle age, which stays the same in older age.
To investigate this, they analyzed data from 1561 men who were seen at the VA Boston outpatient clinic and did not have CAD, type 2 diabetes, stroke, or cancer when they enrolled in the Normative Aging Study.
The men had a mean age of 53 years (range, 33-84) in 1975 and were followed until 2015 or until dropout from the study or death.
At baseline, the study participants filled in the Eysenck Personality Inventory, which assesses neuroticism, and also responded to a scale indicating how much they worry about 20 issues (excluding health).
“Neuroticism,” the researchers explained, “is a tendency to perceive experiences as threatening, feel that challenges are uncontrollable, and experience frequent and disproportionately intense negative emotions,” such as fear, anxiety, sadness, and anger, “across many situations.”
“Worry refers to attempts to solve a problem where future outcome is uncertain and potentially positive or negative,” Dr. Lee noted. Although worry can be healthy and lead to constructive solutions, “it may be unhealthy, especially when it becomes uncontrollable and interferes with day-to-day functioning.”
Of note, in 1980, the American Psychiatric Association removed the term neurosis from its diagnostic manual. What was previously called neurosis is included as part of generalized anxiety disorder; GAD also encompasses excessive worry.
Cardiometabolic risk from midlife to old age
The men in the current study had on-site physical examinations every 3-5 years.
The researchers calculated the men’s cardiometabolic risk score (from 0 to 7) by assigning 1 point each for the following: systolic blood pressure greater than 130 mm Hg, diastolic blood pressure greater than 85 mm Hg, total cholesterol of at least 240 mg/dL, triglycerides of at least 150 mg/dL, body mass index of at least 30 kg/m2, glucose of at least 100 mg/dL, and erythrocyte sedimentation rate of at least 14 mm/hour.
Alternatively, patients were assigned a point each for taking medication that could affect these markers (except for body mass index).
Overall, on average, at baseline, the men had a cardiometabolic risk score of 2.9. From age 33-65, this score increased to 3.8, and then it did not increase as much later on.
That is, the cardiometabolic risk score increased by 0.8 per decade until age 65, followed by a slower increase of 0.5 per decade.
At all ages, men with higher levels of neuroticism or worry had a higher cardiometabolic risk score
Each additional standard deviation of neuroticism was associated with a 13% increased risk of having six or more of the seven cardiometabolic risk markers during follow-up, after adjusting for age, demographics, and family history of CAD, but the relationship was attenuated after also adjusting for health behaviors (for example, smoking, alcohol consumption, physical activity, and past-year physician visit at baseline).
Similarly, each additional standard deviation of worry was associated with a 10% increased risk of having six or more of the seven cardiometabolic risk markers during follow-up after the same adjustments, and was also no longer significantly different after the same further adjustments.
The research was supported by grants from the National Institutes of Health and a Senior Research Career Scientist Award from the Office of Research and Development, Department of Veterans Affairs. The Normative Aging Study is a research component of the Massachusetts Veterans Epidemiology Research and Information Center and is supported by the VA Cooperative Studies Program/Epidemiological Research Centers. The study authors and Dr. Levine disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Among healthy middle-aged men, those who were more anxious were more likely to develop high levels of multiple biomarkers of cardiometabolic risk over a 40-year follow-up in a new study.
“By middle adulthood, higher anxiety levels are associated with stable differences” in biomarkers of risk for coronary artery disease (CAD), stroke, and type 2 diabetes, which “are maintained into older ages,” the researchers wrote.
Anxious individuals “may experience deteriorations in cardiometabolic health earlier in life and remain on a stable trajectory of heightened risk into older ages,” they concluded.
The study, led by Lewina Lee, PhD, was published online Jan. 24, 2022, in the Journal of the American Heart Association.
“Men who had higher levels of anxiety at the beginning of the study had consistently higher biological risk for cardiometabolic disease than less anxious men from midlife into old age,” Dr. Lee, assistant professor of psychiatry, Boston University, summarized in an email.
Clinicians may not screen for heart disease and diabetes, and/or only discuss lifestyle modifications when patients are older or have the first signs of disease, she added.
However, the study findings “suggest that worries and anxiety are associated with preclinical pathophysiological processes that tend to culminate in cardiometabolic disease” and show “the importance of screening for mental health difficulties, such as worries and anxiety, in men as early as in their 30s and 40s,” she stressed.
Since most of the men were White (97%) and veterans (94%), “it would be important for future studies to evaluate if these associations exist among women, people from diverse racial and ethnic groups, and in more socioeconomically varying samples, and to consider how anxiety may relate to the development of cardiometabolic risk in much younger individuals than those in our study,” Dr. Lee said in a press release from the American Heart Association.
“This study adds to the growing body of research that link psychological health to cardiovascular risk,” Glenn N. Levine, MD, who was not involved with this research, told this news organization in an email.
“We know that factors such as depression and stress can increase cardiac risk; this study further supports that anxiety can as well,” added Dr. Levine, chief of cardiology, Michael E. DeBakey Veterans Affairs Medical Center, Houston.
“Everyone experiences some anxiety in their life,” he added. However, “if a provider senses that a patient’s anxiety is far beyond the ‘normal’ that we all have from time to time, and it is seemingly adversely impacting both their psychological and physical health, it would be reasonable to suggest to the patient that it might be useful to speak with a mental health professional, and if the patient is receptive, to then make a formal consultation or referral,” said Dr. Levine, who was writing group chair of a recent AHA Scientific Statement on mind-heart-body connection.
Neuroticism and worry
Several studies have linked anxiety to a greater risk of cardiometabolic disease onset, Dr. Lee and colleagues wrote, but it is unclear if anxious individuals have a steadily worsening risk as they age, or if they have a higher risk in middle age, which stays the same in older age.
To investigate this, they analyzed data from 1561 men who were seen at the VA Boston outpatient clinic and did not have CAD, type 2 diabetes, stroke, or cancer when they enrolled in the Normative Aging Study.
The men had a mean age of 53 years (range, 33-84) in 1975 and were followed until 2015 or until dropout from the study or death.
At baseline, the study participants filled in the Eysenck Personality Inventory, which assesses neuroticism, and also responded to a scale indicating how much they worry about 20 issues (excluding health).
“Neuroticism,” the researchers explained, “is a tendency to perceive experiences as threatening, feel that challenges are uncontrollable, and experience frequent and disproportionately intense negative emotions,” such as fear, anxiety, sadness, and anger, “across many situations.”
“Worry refers to attempts to solve a problem where future outcome is uncertain and potentially positive or negative,” Dr. Lee noted. Although worry can be healthy and lead to constructive solutions, “it may be unhealthy, especially when it becomes uncontrollable and interferes with day-to-day functioning.”
Of note, in 1980, the American Psychiatric Association removed the term neurosis from its diagnostic manual. What was previously called neurosis is included as part of generalized anxiety disorder; GAD also encompasses excessive worry.
Cardiometabolic risk from midlife to old age
The men in the current study had on-site physical examinations every 3-5 years.
The researchers calculated the men’s cardiometabolic risk score (from 0 to 7) by assigning 1 point each for the following: systolic blood pressure greater than 130 mm Hg, diastolic blood pressure greater than 85 mm Hg, total cholesterol of at least 240 mg/dL, triglycerides of at least 150 mg/dL, body mass index of at least 30 kg/m2, glucose of at least 100 mg/dL, and erythrocyte sedimentation rate of at least 14 mm/hour.
Alternatively, patients were assigned a point each for taking medication that could affect these markers (except for body mass index).
Overall, on average, at baseline, the men had a cardiometabolic risk score of 2.9. From age 33-65, this score increased to 3.8, and then it did not increase as much later on.
That is, the cardiometabolic risk score increased by 0.8 per decade until age 65, followed by a slower increase of 0.5 per decade.
At all ages, men with higher levels of neuroticism or worry had a higher cardiometabolic risk score
Each additional standard deviation of neuroticism was associated with a 13% increased risk of having six or more of the seven cardiometabolic risk markers during follow-up, after adjusting for age, demographics, and family history of CAD, but the relationship was attenuated after also adjusting for health behaviors (for example, smoking, alcohol consumption, physical activity, and past-year physician visit at baseline).
Similarly, each additional standard deviation of worry was associated with a 10% increased risk of having six or more of the seven cardiometabolic risk markers during follow-up after the same adjustments, and was also no longer significantly different after the same further adjustments.
The research was supported by grants from the National Institutes of Health and a Senior Research Career Scientist Award from the Office of Research and Development, Department of Veterans Affairs. The Normative Aging Study is a research component of the Massachusetts Veterans Epidemiology Research and Information Center and is supported by the VA Cooperative Studies Program/Epidemiological Research Centers. The study authors and Dr. Levine disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN HEART ASSOCIATION
Chronic stress accelerates aging: Epigenetic evidence
The increase in cardiovascular disease caused by chronic stress is related to biologic mechanisms (metabolic, hormonal, inflammatory) and to behavioral mechanisms (lifestyle). There is a popular saying that “stress speeds up aging,” which makes sense if we consider the age-old idea that “our age corresponds to that of our arteries.”
The study of the mechanisms of psychosocial risk factors is of major relevance to the creation of the individual and communal preventive strategies that ensure longevity and maintain quality of life.
The following hypotheses were proposed by a group of researchers from Yale University, in New Haven, Conn., in a recent study:
1. Stress is positively associated with accelerated biologic aging, and this relationship will be mediated by stress-related physiologic changes, such as insulin and hypothalamic-pituitary-adrenal (HPA) signaling.
2. Strong factors associated with psychologic resilience will be protective against the negative consequences of stress on aging. (These relationships are predictive, not causative, as this study is cross-sectional.)
The study
In their study, the team assessed 444 adults with no chronic medical conditions or psychiatric disorders who were 18-50 years of age and living in the greater New Haven area. Levels of obesity and alcohol consumption in the study cohort were generally in line with those in a community population, so alcohol use and body mass index were used as covariates to account for their impact on the results.
The team also used the latest “epigenetic clock,” known as GrimAge. In recent years, several methods of determining biologic age have been developed that trace chemical changes in the DNA that are natural to the aging process but occur at different moments in different people. The epigenetic clocks have proved to be better predictors of longevity and health than chronologic age, and GrimAge predicts mortality better than other epigenetic clocks.
Results
1. Cumulative stress was associated with the acceleration of GrimAge and stress-related physiologic measures of adrenal sensitivity (cortisol/ACTH ratio) and insulin resistance (HOMA). After the researchers controlled for demographic and behavioral factors, HOMA was correlated with GrimAge acceleration.
2. Psychologic resilience factors moderated the association between stress and aging, such that with worse regulation of emotions, there was greater stress-related age acceleration, and with stronger regulation of emotions, any significant effect of stress on GrimAge was prevented. Self-control moderated the relationship between stress and insulin resistance, with high self-control blunting this relationship.
3. In the final model, in those with poor emotion regulation, cumulative stress continued to predict additional GrimAge acceleration, even when demographic, physiologic, and behavioral covariates were accounted for.
Implications
These results elegantly demonstrate that cumulative stress is associated with epigenetic aging in a healthy population, and these associations are modified by biobehavioral resilience factors.
Even after adjustment for demographic and behavioral factors – such as smoking, body mass index, race, and income – people with high chronic stress scores showed markers of accelerated aging and physiologic changes, such as increased insulin resistance.
However, individuals with high scores on two psychologic resilience measures – emotion regulation and self-control – were more resilient to the effects of stress on aging and insulin resistance.
These results support the popular notion that
In other words, the greater the psychologic resilience, the more likely the individual is to live a long and healthy life. “We like to feel as if we have some sovereignty over our destiny and, therefore, it is worth emphasizing to people (and healthcare providers) that it is important to invest in mental health,” said one of the study researchers.
With all the stress we face these days, it is essential to remember that there is no health without mental health. Above all, if we can achieve greater psychologic resilience, we will have a better chance of delaying aging.
A version of this article first appeared on Medscape.com.
The increase in cardiovascular disease caused by chronic stress is related to biologic mechanisms (metabolic, hormonal, inflammatory) and to behavioral mechanisms (lifestyle). There is a popular saying that “stress speeds up aging,” which makes sense if we consider the age-old idea that “our age corresponds to that of our arteries.”
The study of the mechanisms of psychosocial risk factors is of major relevance to the creation of the individual and communal preventive strategies that ensure longevity and maintain quality of life.
The following hypotheses were proposed by a group of researchers from Yale University, in New Haven, Conn., in a recent study:
1. Stress is positively associated with accelerated biologic aging, and this relationship will be mediated by stress-related physiologic changes, such as insulin and hypothalamic-pituitary-adrenal (HPA) signaling.
2. Strong factors associated with psychologic resilience will be protective against the negative consequences of stress on aging. (These relationships are predictive, not causative, as this study is cross-sectional.)
The study
In their study, the team assessed 444 adults with no chronic medical conditions or psychiatric disorders who were 18-50 years of age and living in the greater New Haven area. Levels of obesity and alcohol consumption in the study cohort were generally in line with those in a community population, so alcohol use and body mass index were used as covariates to account for their impact on the results.
The team also used the latest “epigenetic clock,” known as GrimAge. In recent years, several methods of determining biologic age have been developed that trace chemical changes in the DNA that are natural to the aging process but occur at different moments in different people. The epigenetic clocks have proved to be better predictors of longevity and health than chronologic age, and GrimAge predicts mortality better than other epigenetic clocks.
Results
1. Cumulative stress was associated with the acceleration of GrimAge and stress-related physiologic measures of adrenal sensitivity (cortisol/ACTH ratio) and insulin resistance (HOMA). After the researchers controlled for demographic and behavioral factors, HOMA was correlated with GrimAge acceleration.
2. Psychologic resilience factors moderated the association between stress and aging, such that with worse regulation of emotions, there was greater stress-related age acceleration, and with stronger regulation of emotions, any significant effect of stress on GrimAge was prevented. Self-control moderated the relationship between stress and insulin resistance, with high self-control blunting this relationship.
3. In the final model, in those with poor emotion regulation, cumulative stress continued to predict additional GrimAge acceleration, even when demographic, physiologic, and behavioral covariates were accounted for.
Implications
These results elegantly demonstrate that cumulative stress is associated with epigenetic aging in a healthy population, and these associations are modified by biobehavioral resilience factors.
Even after adjustment for demographic and behavioral factors – such as smoking, body mass index, race, and income – people with high chronic stress scores showed markers of accelerated aging and physiologic changes, such as increased insulin resistance.
However, individuals with high scores on two psychologic resilience measures – emotion regulation and self-control – were more resilient to the effects of stress on aging and insulin resistance.
These results support the popular notion that
In other words, the greater the psychologic resilience, the more likely the individual is to live a long and healthy life. “We like to feel as if we have some sovereignty over our destiny and, therefore, it is worth emphasizing to people (and healthcare providers) that it is important to invest in mental health,” said one of the study researchers.
With all the stress we face these days, it is essential to remember that there is no health without mental health. Above all, if we can achieve greater psychologic resilience, we will have a better chance of delaying aging.
A version of this article first appeared on Medscape.com.
The increase in cardiovascular disease caused by chronic stress is related to biologic mechanisms (metabolic, hormonal, inflammatory) and to behavioral mechanisms (lifestyle). There is a popular saying that “stress speeds up aging,” which makes sense if we consider the age-old idea that “our age corresponds to that of our arteries.”
The study of the mechanisms of psychosocial risk factors is of major relevance to the creation of the individual and communal preventive strategies that ensure longevity and maintain quality of life.
The following hypotheses were proposed by a group of researchers from Yale University, in New Haven, Conn., in a recent study:
1. Stress is positively associated with accelerated biologic aging, and this relationship will be mediated by stress-related physiologic changes, such as insulin and hypothalamic-pituitary-adrenal (HPA) signaling.
2. Strong factors associated with psychologic resilience will be protective against the negative consequences of stress on aging. (These relationships are predictive, not causative, as this study is cross-sectional.)
The study
In their study, the team assessed 444 adults with no chronic medical conditions or psychiatric disorders who were 18-50 years of age and living in the greater New Haven area. Levels of obesity and alcohol consumption in the study cohort were generally in line with those in a community population, so alcohol use and body mass index were used as covariates to account for their impact on the results.
The team also used the latest “epigenetic clock,” known as GrimAge. In recent years, several methods of determining biologic age have been developed that trace chemical changes in the DNA that are natural to the aging process but occur at different moments in different people. The epigenetic clocks have proved to be better predictors of longevity and health than chronologic age, and GrimAge predicts mortality better than other epigenetic clocks.
Results
1. Cumulative stress was associated with the acceleration of GrimAge and stress-related physiologic measures of adrenal sensitivity (cortisol/ACTH ratio) and insulin resistance (HOMA). After the researchers controlled for demographic and behavioral factors, HOMA was correlated with GrimAge acceleration.
2. Psychologic resilience factors moderated the association between stress and aging, such that with worse regulation of emotions, there was greater stress-related age acceleration, and with stronger regulation of emotions, any significant effect of stress on GrimAge was prevented. Self-control moderated the relationship between stress and insulin resistance, with high self-control blunting this relationship.
3. In the final model, in those with poor emotion regulation, cumulative stress continued to predict additional GrimAge acceleration, even when demographic, physiologic, and behavioral covariates were accounted for.
Implications
These results elegantly demonstrate that cumulative stress is associated with epigenetic aging in a healthy population, and these associations are modified by biobehavioral resilience factors.
Even after adjustment for demographic and behavioral factors – such as smoking, body mass index, race, and income – people with high chronic stress scores showed markers of accelerated aging and physiologic changes, such as increased insulin resistance.
However, individuals with high scores on two psychologic resilience measures – emotion regulation and self-control – were more resilient to the effects of stress on aging and insulin resistance.
These results support the popular notion that
In other words, the greater the psychologic resilience, the more likely the individual is to live a long and healthy life. “We like to feel as if we have some sovereignty over our destiny and, therefore, it is worth emphasizing to people (and healthcare providers) that it is important to invest in mental health,” said one of the study researchers.
With all the stress we face these days, it is essential to remember that there is no health without mental health. Above all, if we can achieve greater psychologic resilience, we will have a better chance of delaying aging.
A version of this article first appeared on Medscape.com.
‘Lucky genes’ may protect against some obesity-related diseases
in a large new genetics study.
That is, people with unfavorable adiposity gene variants had fat stored under the skin throughout the body, but they also had more ectopic fat (fat in the “wrong place”) surrounding the pancreas and liver, which is associated with a higher risk of metabolic diseases such as heart disease and type 2 diabetes.
In contrast, people with favorable adiposity gene variants had more subcutaneous fat (such as a paunch or a double chin).
The study by Susan Martin, PhD, a postdoctoral research associate at the University of Exeter (England) and colleagues, was recently published in eLife.
“Some people have ‘unlucky fat genes,’ meaning they store higher levels of fat everywhere, including under the skin [and around the] liver and pancreas. That’s associated with a higher risk of diseases such as type 2 diabetes,” senior author Hanieh Yaghootkar, MD, PhD, summarized in a press release from the University of Exeter.
“Others are luckier and have genes that mean higher fat under the skin but lower liver fat and a lower risk of diseases like type 2 diabetes,” added Dr. Yaghootkar, from Brunel University London.
Among 37 chronic diseases that are associated with obesity, the researchers found the metabolic effects of adiposity are likely the main cause of the following 11: type 2 diabetes, polycystic ovary syndrome, coronary artery disease, peripheral artery disease, hypertension, stroke, heart failure, atrial fibrillation, chronic kidney disease, renal cancer, and gout.
On the other hand, excess weight itself (such as a heavy load on the joints) rather than a metabolic effect is associated with nine other obesity-related diseases: osteoarthritis, rheumatoid arthritis, osteoporosis, gastro-esophageal reflux disease, gallstones, adult-onset asthma, psoriasis, deep vein thrombosis, and venous thromboembolism.
Good genes no substitute for a healthy lifestyle
“People with more favorable adiposity gene variants are still at risk of the nine diseases” that are not caused by metabolic effects – such as osteoarthritis – but are caused by the effect of excess weight on the joints, another author, Timothy M. Frayling, PhD, stressed.
“People with obesity and unfavorable adiposity gene variants are at higher risk of all 20 diseases because they have the double hit of the excess mechanical effects and the adverse metabolic effects,” Dr. Frayling of the University of Exeter, told this news organization in an email.
The main clinical message, he said, is that “this research helps inform which conditions may respond better to therapies that lower the adverse effects” of risk factors such as high cholesterol and blood glucose levels, “and high blood pressure, even with no weight loss.”
“In contrast, other conditions really require the weight loss.”
“These results emphasize that many people in the community who are of higher body mass index are at risk of multiple chronic conditions that can severely impair their quality of life or cause morbidity or mortality, even if their metabolic parameters appear relatively normal,” the researchers conclude.
“Whilst it’s important that we identify the causes of obesity-related disease, good genes [are] still no substitute for a healthy lifestyle,” Dr. Martin stressed.
“A favorable adiposity will only go so far. If you’re obese, the advice is to still try and shift the excess weight where you can,” she said.
“The authors have conducted a robust and very comprehensive study using Mendelian randomization to disentangle metabolic and nonmetabolic effects of overweight on a long list of disease outcomes,” reviewing editor Edward D. Janus, MD, PhD, of the University of Melbourne summarized.
“This is an important topic and can help us better understand how overweight influences risk of several important outcomes.”
Metabolic and nonmetabolic diseases caused by obesity
The researchers aimed to investigate the effects of adiposity on metabolic and nonmetabolic diseases caused by obesity.
They used data from 176,899 individuals in the FinnGen project in Finland and from over 500,000 individuals in the UK Biobank database.
They performed Mendelian randomization studies to investigate the causal association between BMI, body fat percentage, favorable adiposity alleles, and unfavorable adiposity alleles with 37 disease outcomes.
Of these 37 chronic diseases associated with obesity, 11 diseases were directly related to the metabolic effect of adiposity (where favorable adiposity or unfavorable adiposity gene variants had opposite effects). Nine other diseases were unrelated to the metabolic effects of adiposity.
For most of the remaining diseases – for example, Alzheimer’s disease and different cancers – it was difficult to draw firm conclusions about the respective roles of favorable adiposity and unfavorable adiposity gene variants.
The study was funded by Diabetes UK, the UK Medical Research Council, the World Cancer Research Fund, and the National Cancer Institute. Author disclosures are listed with the article.
A version of this article first appeared on Medscape.com.
in a large new genetics study.
That is, people with unfavorable adiposity gene variants had fat stored under the skin throughout the body, but they also had more ectopic fat (fat in the “wrong place”) surrounding the pancreas and liver, which is associated with a higher risk of metabolic diseases such as heart disease and type 2 diabetes.
In contrast, people with favorable adiposity gene variants had more subcutaneous fat (such as a paunch or a double chin).
The study by Susan Martin, PhD, a postdoctoral research associate at the University of Exeter (England) and colleagues, was recently published in eLife.
“Some people have ‘unlucky fat genes,’ meaning they store higher levels of fat everywhere, including under the skin [and around the] liver and pancreas. That’s associated with a higher risk of diseases such as type 2 diabetes,” senior author Hanieh Yaghootkar, MD, PhD, summarized in a press release from the University of Exeter.
“Others are luckier and have genes that mean higher fat under the skin but lower liver fat and a lower risk of diseases like type 2 diabetes,” added Dr. Yaghootkar, from Brunel University London.
Among 37 chronic diseases that are associated with obesity, the researchers found the metabolic effects of adiposity are likely the main cause of the following 11: type 2 diabetes, polycystic ovary syndrome, coronary artery disease, peripheral artery disease, hypertension, stroke, heart failure, atrial fibrillation, chronic kidney disease, renal cancer, and gout.
On the other hand, excess weight itself (such as a heavy load on the joints) rather than a metabolic effect is associated with nine other obesity-related diseases: osteoarthritis, rheumatoid arthritis, osteoporosis, gastro-esophageal reflux disease, gallstones, adult-onset asthma, psoriasis, deep vein thrombosis, and venous thromboembolism.
Good genes no substitute for a healthy lifestyle
“People with more favorable adiposity gene variants are still at risk of the nine diseases” that are not caused by metabolic effects – such as osteoarthritis – but are caused by the effect of excess weight on the joints, another author, Timothy M. Frayling, PhD, stressed.
“People with obesity and unfavorable adiposity gene variants are at higher risk of all 20 diseases because they have the double hit of the excess mechanical effects and the adverse metabolic effects,” Dr. Frayling of the University of Exeter, told this news organization in an email.
The main clinical message, he said, is that “this research helps inform which conditions may respond better to therapies that lower the adverse effects” of risk factors such as high cholesterol and blood glucose levels, “and high blood pressure, even with no weight loss.”
“In contrast, other conditions really require the weight loss.”
“These results emphasize that many people in the community who are of higher body mass index are at risk of multiple chronic conditions that can severely impair their quality of life or cause morbidity or mortality, even if their metabolic parameters appear relatively normal,” the researchers conclude.
“Whilst it’s important that we identify the causes of obesity-related disease, good genes [are] still no substitute for a healthy lifestyle,” Dr. Martin stressed.
“A favorable adiposity will only go so far. If you’re obese, the advice is to still try and shift the excess weight where you can,” she said.
“The authors have conducted a robust and very comprehensive study using Mendelian randomization to disentangle metabolic and nonmetabolic effects of overweight on a long list of disease outcomes,” reviewing editor Edward D. Janus, MD, PhD, of the University of Melbourne summarized.
“This is an important topic and can help us better understand how overweight influences risk of several important outcomes.”
Metabolic and nonmetabolic diseases caused by obesity
The researchers aimed to investigate the effects of adiposity on metabolic and nonmetabolic diseases caused by obesity.
They used data from 176,899 individuals in the FinnGen project in Finland and from over 500,000 individuals in the UK Biobank database.
They performed Mendelian randomization studies to investigate the causal association between BMI, body fat percentage, favorable adiposity alleles, and unfavorable adiposity alleles with 37 disease outcomes.
Of these 37 chronic diseases associated with obesity, 11 diseases were directly related to the metabolic effect of adiposity (where favorable adiposity or unfavorable adiposity gene variants had opposite effects). Nine other diseases were unrelated to the metabolic effects of adiposity.
For most of the remaining diseases – for example, Alzheimer’s disease and different cancers – it was difficult to draw firm conclusions about the respective roles of favorable adiposity and unfavorable adiposity gene variants.
The study was funded by Diabetes UK, the UK Medical Research Council, the World Cancer Research Fund, and the National Cancer Institute. Author disclosures are listed with the article.
A version of this article first appeared on Medscape.com.
in a large new genetics study.
That is, people with unfavorable adiposity gene variants had fat stored under the skin throughout the body, but they also had more ectopic fat (fat in the “wrong place”) surrounding the pancreas and liver, which is associated with a higher risk of metabolic diseases such as heart disease and type 2 diabetes.
In contrast, people with favorable adiposity gene variants had more subcutaneous fat (such as a paunch or a double chin).
The study by Susan Martin, PhD, a postdoctoral research associate at the University of Exeter (England) and colleagues, was recently published in eLife.
“Some people have ‘unlucky fat genes,’ meaning they store higher levels of fat everywhere, including under the skin [and around the] liver and pancreas. That’s associated with a higher risk of diseases such as type 2 diabetes,” senior author Hanieh Yaghootkar, MD, PhD, summarized in a press release from the University of Exeter.
“Others are luckier and have genes that mean higher fat under the skin but lower liver fat and a lower risk of diseases like type 2 diabetes,” added Dr. Yaghootkar, from Brunel University London.
Among 37 chronic diseases that are associated with obesity, the researchers found the metabolic effects of adiposity are likely the main cause of the following 11: type 2 diabetes, polycystic ovary syndrome, coronary artery disease, peripheral artery disease, hypertension, stroke, heart failure, atrial fibrillation, chronic kidney disease, renal cancer, and gout.
On the other hand, excess weight itself (such as a heavy load on the joints) rather than a metabolic effect is associated with nine other obesity-related diseases: osteoarthritis, rheumatoid arthritis, osteoporosis, gastro-esophageal reflux disease, gallstones, adult-onset asthma, psoriasis, deep vein thrombosis, and venous thromboembolism.
Good genes no substitute for a healthy lifestyle
“People with more favorable adiposity gene variants are still at risk of the nine diseases” that are not caused by metabolic effects – such as osteoarthritis – but are caused by the effect of excess weight on the joints, another author, Timothy M. Frayling, PhD, stressed.
“People with obesity and unfavorable adiposity gene variants are at higher risk of all 20 diseases because they have the double hit of the excess mechanical effects and the adverse metabolic effects,” Dr. Frayling of the University of Exeter, told this news organization in an email.
The main clinical message, he said, is that “this research helps inform which conditions may respond better to therapies that lower the adverse effects” of risk factors such as high cholesterol and blood glucose levels, “and high blood pressure, even with no weight loss.”
“In contrast, other conditions really require the weight loss.”
“These results emphasize that many people in the community who are of higher body mass index are at risk of multiple chronic conditions that can severely impair their quality of life or cause morbidity or mortality, even if their metabolic parameters appear relatively normal,” the researchers conclude.
“Whilst it’s important that we identify the causes of obesity-related disease, good genes [are] still no substitute for a healthy lifestyle,” Dr. Martin stressed.
“A favorable adiposity will only go so far. If you’re obese, the advice is to still try and shift the excess weight where you can,” she said.
“The authors have conducted a robust and very comprehensive study using Mendelian randomization to disentangle metabolic and nonmetabolic effects of overweight on a long list of disease outcomes,” reviewing editor Edward D. Janus, MD, PhD, of the University of Melbourne summarized.
“This is an important topic and can help us better understand how overweight influences risk of several important outcomes.”
Metabolic and nonmetabolic diseases caused by obesity
The researchers aimed to investigate the effects of adiposity on metabolic and nonmetabolic diseases caused by obesity.
They used data from 176,899 individuals in the FinnGen project in Finland and from over 500,000 individuals in the UK Biobank database.
They performed Mendelian randomization studies to investigate the causal association between BMI, body fat percentage, favorable adiposity alleles, and unfavorable adiposity alleles with 37 disease outcomes.
Of these 37 chronic diseases associated with obesity, 11 diseases were directly related to the metabolic effect of adiposity (where favorable adiposity or unfavorable adiposity gene variants had opposite effects). Nine other diseases were unrelated to the metabolic effects of adiposity.
For most of the remaining diseases – for example, Alzheimer’s disease and different cancers – it was difficult to draw firm conclusions about the respective roles of favorable adiposity and unfavorable adiposity gene variants.
The study was funded by Diabetes UK, the UK Medical Research Council, the World Cancer Research Fund, and the National Cancer Institute. Author disclosures are listed with the article.
A version of this article first appeared on Medscape.com.
Men with hypersexual disorder may have oxytocin overload
Men with hypersexual disorder showed higher levels of oxytocin in their blood than did healthy control men without the disorder, in a study with 102 participants.
Hypersexual disorder (HD) is characterized by “excessive and persistent sexual behaviors in relation to various mood states, with an impulsivity component and experienced loss of control,” John Flanagan, MD, of the Karolinska Institutet in Stockholm and colleagues wrote. Although HD is not included as a separate diagnosis in the current DSM, the similar disorder of compulsive sexual behavior is included in the ICD.
Data on the pathophysiology of HD are limited, although a previous study by corresponding author Andreas Chatzittofis, MD, and colleagues showed evidence of neuroendocrine dysregulation in men with HD, and prompted the current study to explore the possible involvement of the oxytocinergic system in HD.
In the current study, published in the Journal of Clinical Endocrinology & Metabolism, the researchers identified 64 men with HD and 38 healthy male controls. The patients were help-seeking men older than 18 years diagnosed with HD who presented to a single center in Sweden during 2013-2014. The men were included in a randomized clinical trial of cognitive-behavioral therapy for HD, and 30 of them participated in a 7-week CBT program.
Oxytocin, secreted by the pituitary gland, is known to play a role in sexual behavior, but has not been examined in HD men, the researchers said. At baseline, the mean plasma oxytocin was 31.0 pM in the HD patients, which was significantly higher than the mean 16.9 pM in healthy controls (P < .001). However, the 30 HD men who underwent CBT showed significant improvement in oxytocin levels, from a mean pretreatment level of 30.5 to a mean posttreatment level of 20.2 pM (P = .0000019).
The study findings were limited by several factors, including the lack of data on oxytocin for a wait list or control group, as well as the inability to control for confounding factors such as diet, physical activity, ethnicity, and stress, and a lack of data on sexual activity prior to oxytocin measurements, the researchers noted.
However, “although there is no clear consensus at this point, previous studies support the use of oxytocin plasma levels as a surrogate variable for [cerebrospinal fluid] oxytocin activity,” the researchers wrote in their discussion. The current study findings support the potential of oxytocin as a biomarker for HD diagnostics and also as a measure of disease severity. Larger studies to confirm the findings, especially those that exclude potential confounders, would be valuable.
Oxytocin may be treatment target
The study is important because of the lack of knowledge regarding the pathophysiology underlying hypersexual disorder, Dr. Chatzittofis of the University of Cyprus, Nicosia, said in an interview. “This is the first study to indicate a role for oxytocin’s involvement” in hypersexual disorder in men. Dr. Chatzittofis led a team in a previous study that showed an association between HD in men and dysregulation of the hypothalamic pituitary adrenal axis.
In the current study, “we discovered that men with compulsive sexual behavior disorder had higher oxytocin levels, compared with healthy men,” said Dr. Chatzittofis, adding that the take-home message for clinicians is the potential of CBT for treatment. “Cognitive-behavior therapy led to a reduction in both hypersexual behavior and oxytocin levels.” The results suggest that oxytocin plays an important role in sex addiction.
Consequently, oxytocin may be a potential drug target for future pharmacologic treatment of hypersexual disorder, he added.
The study was supported by the Swedish Research Council, the Stockholm County Council, and by a partnership between Umeå University and Västerbotten County Council. The researchers had no financial conflicts to disclose.
Men with hypersexual disorder showed higher levels of oxytocin in their blood than did healthy control men without the disorder, in a study with 102 participants.
Hypersexual disorder (HD) is characterized by “excessive and persistent sexual behaviors in relation to various mood states, with an impulsivity component and experienced loss of control,” John Flanagan, MD, of the Karolinska Institutet in Stockholm and colleagues wrote. Although HD is not included as a separate diagnosis in the current DSM, the similar disorder of compulsive sexual behavior is included in the ICD.
Data on the pathophysiology of HD are limited, although a previous study by corresponding author Andreas Chatzittofis, MD, and colleagues showed evidence of neuroendocrine dysregulation in men with HD, and prompted the current study to explore the possible involvement of the oxytocinergic system in HD.
In the current study, published in the Journal of Clinical Endocrinology & Metabolism, the researchers identified 64 men with HD and 38 healthy male controls. The patients were help-seeking men older than 18 years diagnosed with HD who presented to a single center in Sweden during 2013-2014. The men were included in a randomized clinical trial of cognitive-behavioral therapy for HD, and 30 of them participated in a 7-week CBT program.
Oxytocin, secreted by the pituitary gland, is known to play a role in sexual behavior, but has not been examined in HD men, the researchers said. At baseline, the mean plasma oxytocin was 31.0 pM in the HD patients, which was significantly higher than the mean 16.9 pM in healthy controls (P < .001). However, the 30 HD men who underwent CBT showed significant improvement in oxytocin levels, from a mean pretreatment level of 30.5 to a mean posttreatment level of 20.2 pM (P = .0000019).
The study findings were limited by several factors, including the lack of data on oxytocin for a wait list or control group, as well as the inability to control for confounding factors such as diet, physical activity, ethnicity, and stress, and a lack of data on sexual activity prior to oxytocin measurements, the researchers noted.
However, “although there is no clear consensus at this point, previous studies support the use of oxytocin plasma levels as a surrogate variable for [cerebrospinal fluid] oxytocin activity,” the researchers wrote in their discussion. The current study findings support the potential of oxytocin as a biomarker for HD diagnostics and also as a measure of disease severity. Larger studies to confirm the findings, especially those that exclude potential confounders, would be valuable.
Oxytocin may be treatment target
The study is important because of the lack of knowledge regarding the pathophysiology underlying hypersexual disorder, Dr. Chatzittofis of the University of Cyprus, Nicosia, said in an interview. “This is the first study to indicate a role for oxytocin’s involvement” in hypersexual disorder in men. Dr. Chatzittofis led a team in a previous study that showed an association between HD in men and dysregulation of the hypothalamic pituitary adrenal axis.
In the current study, “we discovered that men with compulsive sexual behavior disorder had higher oxytocin levels, compared with healthy men,” said Dr. Chatzittofis, adding that the take-home message for clinicians is the potential of CBT for treatment. “Cognitive-behavior therapy led to a reduction in both hypersexual behavior and oxytocin levels.” The results suggest that oxytocin plays an important role in sex addiction.
Consequently, oxytocin may be a potential drug target for future pharmacologic treatment of hypersexual disorder, he added.
The study was supported by the Swedish Research Council, the Stockholm County Council, and by a partnership between Umeå University and Västerbotten County Council. The researchers had no financial conflicts to disclose.
Men with hypersexual disorder showed higher levels of oxytocin in their blood than did healthy control men without the disorder, in a study with 102 participants.
Hypersexual disorder (HD) is characterized by “excessive and persistent sexual behaviors in relation to various mood states, with an impulsivity component and experienced loss of control,” John Flanagan, MD, of the Karolinska Institutet in Stockholm and colleagues wrote. Although HD is not included as a separate diagnosis in the current DSM, the similar disorder of compulsive sexual behavior is included in the ICD.
Data on the pathophysiology of HD are limited, although a previous study by corresponding author Andreas Chatzittofis, MD, and colleagues showed evidence of neuroendocrine dysregulation in men with HD, and prompted the current study to explore the possible involvement of the oxytocinergic system in HD.
In the current study, published in the Journal of Clinical Endocrinology & Metabolism, the researchers identified 64 men with HD and 38 healthy male controls. The patients were help-seeking men older than 18 years diagnosed with HD who presented to a single center in Sweden during 2013-2014. The men were included in a randomized clinical trial of cognitive-behavioral therapy for HD, and 30 of them participated in a 7-week CBT program.
Oxytocin, secreted by the pituitary gland, is known to play a role in sexual behavior, but has not been examined in HD men, the researchers said. At baseline, the mean plasma oxytocin was 31.0 pM in the HD patients, which was significantly higher than the mean 16.9 pM in healthy controls (P < .001). However, the 30 HD men who underwent CBT showed significant improvement in oxytocin levels, from a mean pretreatment level of 30.5 to a mean posttreatment level of 20.2 pM (P = .0000019).
The study findings were limited by several factors, including the lack of data on oxytocin for a wait list or control group, as well as the inability to control for confounding factors such as diet, physical activity, ethnicity, and stress, and a lack of data on sexual activity prior to oxytocin measurements, the researchers noted.
However, “although there is no clear consensus at this point, previous studies support the use of oxytocin plasma levels as a surrogate variable for [cerebrospinal fluid] oxytocin activity,” the researchers wrote in their discussion. The current study findings support the potential of oxytocin as a biomarker for HD diagnostics and also as a measure of disease severity. Larger studies to confirm the findings, especially those that exclude potential confounders, would be valuable.
Oxytocin may be treatment target
The study is important because of the lack of knowledge regarding the pathophysiology underlying hypersexual disorder, Dr. Chatzittofis of the University of Cyprus, Nicosia, said in an interview. “This is the first study to indicate a role for oxytocin’s involvement” in hypersexual disorder in men. Dr. Chatzittofis led a team in a previous study that showed an association between HD in men and dysregulation of the hypothalamic pituitary adrenal axis.
In the current study, “we discovered that men with compulsive sexual behavior disorder had higher oxytocin levels, compared with healthy men,” said Dr. Chatzittofis, adding that the take-home message for clinicians is the potential of CBT for treatment. “Cognitive-behavior therapy led to a reduction in both hypersexual behavior and oxytocin levels.” The results suggest that oxytocin plays an important role in sex addiction.
Consequently, oxytocin may be a potential drug target for future pharmacologic treatment of hypersexual disorder, he added.
The study was supported by the Swedish Research Council, the Stockholm County Council, and by a partnership between Umeå University and Västerbotten County Council. The researchers had no financial conflicts to disclose.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
Differences in COVID-19 Outcomes Among Patients With Type 1 Diabetes: First vs Later Surges
From Hassenfeld Children’s Hospital at NYU Langone Health, New York, NY (Dr Gallagher), T1D Exchange, Boston, MA (Saketh Rompicherla; Drs Ebekozien, Noor, Odugbesan, and Mungmode; Nicole Rioles, Emma Ospelt), University of Mississippi School of Population Health, Jackson, MS (Dr. Ebekozien), Icahn School of Medicine at Mount Sinai, New York, NY (Drs. Wilkes, O’Malley, and Rapaport), Weill Cornell Medicine, New York, NY (Drs. Antal and Feuer), NYU Long Island School of Medicine, Mineola, NY (Dr. Gabriel), NYU Langone Health, New York, NY (Dr. Golden), Barbara Davis Center, Aurora, CO (Dr. Alonso), Texas Children’s Hospital/Baylor College of Medicine, Houston, TX (Dr. Lyons), Stanford University, Stanford, CA (Dr. Prahalad), Children Mercy Kansas City, MO (Dr. Clements), Indiana University School of Medicine, IN (Dr. Neyman), Rady Children’s Hospital, University of California, San Diego, CA (Dr. Demeterco-Berggren).
Background: Patient outcomes of COVID-19 have improved throughout the pandemic. However, because it is not known whether outcomes of COVID-19 in the type 1 diabetes (T1D) population improved over time, we investigated differences in COVID-19 outcomes for patients with T1D in the United States.
Methods: We analyzed data collected via a registry of patients with T1D and COVID-19 from 56 sites between April 2020 and January 2021. We grouped cases into first surge (April 9, 2020, to July 31, 2020, n = 188) and late surge (August 1, 2020, to January 31, 2021, n = 410), and then compared outcomes between both groups using descriptive statistics and logistic regression models.
Results: Adverse outcomes were more frequent during the first surge, including diabetic ketoacidosis (32% vs 15%, P < .001), severe hypoglycemia (4% vs 1%, P = .04), and hospitalization (52% vs 22%, P < .001). Patients in the first surge were older (28 [SD,18.8] years vs 18.0 [SD, 11.1] years, P < .001), had higher median hemoglobin A1c levels (9.3 [interquartile range {IQR}, 4.0] vs 8.4 (IQR, 2.8), P < .001), and were more likely to use public insurance (107 [57%] vs 154 [38%], P < .001). The odds of hospitalization for adults in the first surge were 5 times higher compared to the late surge (odds ratio, 5.01; 95% CI, 2.11-12.63).
Conclusion: Patients with T1D who presented with COVID-19 during the first surge had a higher proportion of adverse outcomes than those who presented in a later surge.
Keywords: TD1, diabetic ketoacidosis, hypoglycemia.
After the World Health Organization declared the disease caused by the novel coronavirus SARS-CoV-2, COVID-19, a pandemic on March 11, 2020, the Centers for Disease Control and Prevention identified patients with diabetes as high risk for severe illness.1-7 The case-fatality rate for COVID-19 has significantly improved over the past 2 years. Public health measures, less severe COVID-19 variants, increased access to testing, and new treatments for COVID-19 have contributed to improved outcomes.
The T1D Exchange has previously published findings on COVID-19 outcomes for patients with type 1 diabetes (T1D) using data from the T1D COVID-19 Surveillance Registry.8-12 Given improved outcomes in COVID-19 in the general population, we sought to determine if outcomes for cases of COVID-19 reported to this registry changed over time.
Methods
This study was coordinated by the T1D Exchange and approved as nonhuman subject research by the Western Institutional Review Board. All participating centers also obtained local institutional review board approval. No identifiable patient information was collected as part of this noninterventional, cross-sectional study.
The T1D Exchange Multi-center COVID-19 Surveillance Study collected data from endocrinology clinics that completed a retrospective chart review and submitted information to T1D Exchange via an online questionnaire for all patients with T1D at their sites who tested positive for COVID-19.13,14 The questionnaire was administered using the Qualtrics survey platform (www.qualtrics.com version XM) and contained 33 pre-coded and free-text response fields to collect patient and clinical attributes.
Each participating center identified 1 team member for reporting to avoid duplicate case submission. Each submitted case was reviewed for potential errors and incomplete information. The coordinating center verified the number of cases per site for data quality assurance.
Quantitative data were represented as mean (standard deviation) or median (interquartile range). Categorical data were described as the number (percentage) of patients. Summary statistics, including frequency and percentage for categorical variables, were calculated for all patient-related and clinical characteristics. The date August 1, 2021, was selected as the end of the first surge based on a review of national COVID-19 surges.
We used the Fisher’s exact test to assess associations between hospitalization and demographics, HbA1c, diabetes duration, symptoms, and adverse outcomes. In addition, multivariate logistic regression was used to calculate odds ratios (OR). Logistic regression models were used to determine the association between time of surge and hospitalization separately for both the pediatric and adult populations. Each model was adjusted for potential sociodemographic confounders, specifically age, sex, race, insurance, and HbA1c.
All tests were 2-sided, with type 1 error set at 5%. Fisher’s exact test and logistic regression were performed using statistical software R, version 3.6.2 (R Foundation for Statistical Computing).
Results
The characteristics of COVID-19 cases in patients with T1D that were reported early in the pandemic, before August 1, 2020 (first surge), compared with those of cases reported on and after August 1, 2020 (later surges) are shown in Table 1.
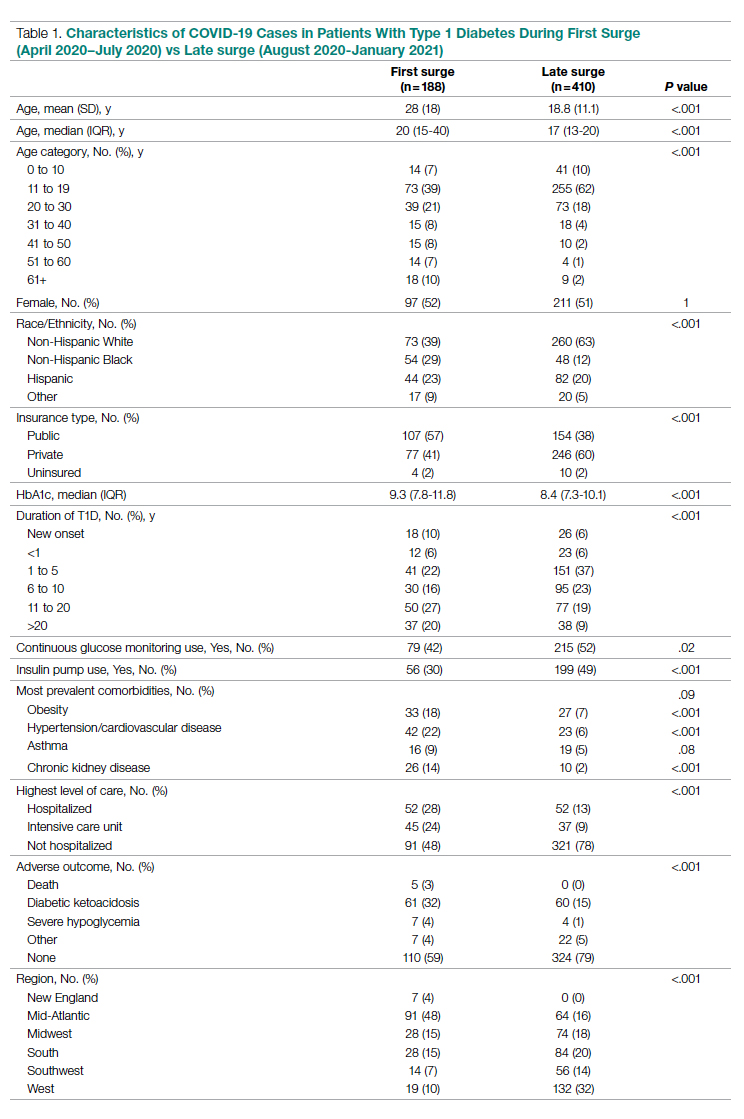
Patients with T1D who presented with COVID-19 during the first surge as compared to the later surges were older (mean age 28 [SD, 18.0] years vs 18.8 [SD, 11.1] years; P < .001) and had a longer duration of diabetes (P < .001). The first-surge group also had more patients with >20 years’ diabetes duration (20% vs 9%, P < .001). Obesity, hypertension, and chronic kidney disease were also more commonly reported in first-surge cases (all P < .001).
There was a significant difference in race and ethnicity reported in the first surge vs the later surge cases, with fewer patients identifying as non-Hispanic White (39% vs, 63%, P < .001) and more patients identifying as non-Hispanic Black (29% vs 12%, P < .001). The groups also differed significantly in terms of insurance type, with more people on public insurance in the first-surge group (57% vs 38%, P < .001). In addition, median HbA1c was higher (9.3% vs 8.4%, P < .001) and continuous glucose monitor and insulin pump use were less common (P = .02 and <.001, respectively) in the early surge.
All symptoms and adverse outcomes were reported more often in the first surge, including diabetic ketoacidosis (DKA; 32% vs 15%; P < .001) and severe hypoglycemia (4% vs 1%, P = .04). Hospitalization (52% vs 13%, P < .001) and ICU admission (24% vs 9%, P < .001) were reported more often in the first-surge group.
Regression Analyses
Table 2 shows the results of logistic regression analyses for hospitalization in the pediatric (≤19 years of age) and adult (>19 years of age) groups, along with the odds of hospitalization during the first vs late surge among COVID-positive people with T1D. Adult patients who tested positive in the first surge were about 5 times more likely to be hospitalized than adults who tested positive for infection in the late surge after adjusting for age, insurance type, sex, race, and HbA1c levels. Pediatric patients also had an increased odds for hospitalization during the first surge, but this increase was not statistically significant.
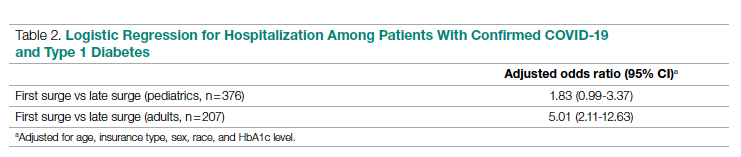
Discussion
Our analysis of COVID-19 cases in patients with T1D reported by diabetes providers across the United States found that adverse outcomes were more prevalent early in the pandemic. There may be a number of reasons for this difference in outcomes between patients who presented in the first surge vs a later surge. First, because testing for COVID-19 was extremely limited and reserved for hospitalized patients early in the pandemic, the first-surge patients with confirmed COVID-19 likely represent a skewed population of higher-acuity patients. This may also explain the relative paucity of cases in younger patients reported early in the pandemic. Second, worse outcomes in the early surge may also have been associated with overwhelmed hospitals in New York City at the start of the outbreak. According to Cummings et al, the abrupt surge of critically ill patients hospitalized with severe acute respiratory distress syndrome initially outpaced their capacity to provide prone-positioning ventilation, which has been expanded since then.15 While there was very little hypertension, cardiovascular disease, or kidney disease reported in the pediatric groups, there was a higher prevalence of obesity in the pediatric group from the mid-Atlantic region. Obesity has been associated with a worse prognosis for COVID-19 illness in children.16 Finally, there were 5 deaths reported in this study, all of which were reported during the first surge. Older age and increased rates of cardiovascular disease and chronic kidney disease in the first surge cases likely contributed to worse outcomes for adults in mid-Atlantic region relative to the other regions. Minority race and the use of public insurance, risk factors for more severe outcomes in all regions, were also more common in cases reported from the mid-Atlantic region.
This study has several limitations. First, it is a cross-sectional study that relies upon voluntary provider reports. Second, availability of COVID-19 testing was limited in all regions in spring 2020. Third, different regions of the country experienced subsequent surges at different times within the reported timeframes in this analysis. Fourth, this report time period does not include the impact of the newer COVID-19 variants. Finally, trends in COVID-19 outcomes were affected by the evolution of care that developed throughout 2020.
Conclusion
Adult patients with T1D and COVID-19 who reported during the first surge had about 5 times higher hospitalization odds than those who presented in a later surge.
Corresponding author: Osagie Ebekozien, MD, MPH, 11 Avenue de Lafayette, Boston, MA 02111; [email protected]
Disclosures: Dr Ebekozien reports receiving research grants from Medtronic Diabetes, Eli Lilly, and Dexcom, and receiving honoraria from Medtronic Diabetes.
1. Barron E, Bakhai C, Kar P, et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 2020;8(10):813-822. doi:10.1016/S2213-8587(20)30272-2
2. Fisher L, Polonsky W, Asuni A, Jolly Y, Hessler D. The early impact of the COVID-19 pandemic on adults with type 1 or type 2 diabetes: A national cohort study. J Diabetes Complications. 2020;34(12):107748. doi:10.1016/j.jdiacomp.2020.107748
3. Holman N, Knighton P, Kar P, et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8(10):823-833. doi:10.1016/S2213-8587(20)30271-0
4. Wargny M, Gourdy P, Ludwig L, et al. Type 1 diabetes in people hospitalized for COVID-19: new insights from the CORONADO study. Diabetes Care. 2020;43(11):e174-e177. doi:10.2337/dc20-1217
5. Gregory JM, Slaughter JC, Duffus SH, et al. COVID-19 severity is tripled in the diabetes community: a prospective analysis of the pandemic’s impact in type 1 and type 2 diabetes. Diabetes Care. 2021;44(2):526-532. doi:10.2337/dc20-2260
6. Cardona-Hernandez R, Cherubini V, Iafusco D, Schiaffini R, Luo X, Maahs DM. Children and youth with diabetes are not at increased risk for hospitalization due to COVID-19. Pediatr Diabetes. 2021;22(2):202-206. doi:10.1111/pedi.13158
7. Maahs DM, Alonso GT, Gallagher MP, Ebekozien O. Comment on Gregory et al. COVID-19 severity is tripled in the diabetes community: a prospective analysis of the pandemic’s impact in type 1 and type 2 diabetes. Diabetes Care. 2021;44:526-532. Diabetes Care. 2021;44(5):e102. doi:10.2337/dc20-3119
8. Ebekozien OA, Noor N, Gallagher MP, Alonso GT. Type 1 diabetes and COVID-19: preliminary findings from a multicenter surveillance study in the US. Diabetes Care. 2020;43(8):e83-e85. doi:10.2337/dc20-1088
9. Beliard K, Ebekozien O, Demeterco-Berggren C, et al. Increased DKA at presentation among newly diagnosed type 1 diabetes patients with or without COVID-19: Data from a multi-site surveillance registry. J Diabetes. 2021;13(3):270-272. doi:10.1111/1753-0407
10. O’Malley G, Ebekozien O, Desimone M, et al. COVID-19 hospitalization in adults with type 1 diabetes: results from the T1D Exchange Multicenter Surveillance study. J Clin Endocrinol Metab. 2021;106(2):e936-e942. doi:10.1210/clinem/dgaa825
11. Ebekozien O, Agarwal S, Noor N, et al. Inequities in diabetic ketoacidosis among patients with type 1 diabetes and COVID-19: data from 52 US clinical centers. J Clin Endocrinol Metab. 2021;106(4):e1755-e1762. doi:10.1210/clinem/dgaa920
12. Alonso GT, Ebekozien O, Gallagher MP, et al. Diabetic ketoacidosis drives COVID-19 related hospitalizations in children with type 1 diabetes. J Diabetes. 2021;13(8):681-687. doi:10.1111/1753-0407.13184
13. Noor N, Ebekozien O, Levin L, et al. Diabetes technology use for management of type 1 diabetes is associated with fewer adverse COVID-19 outcomes: findings from the T1D Exchange COVID-19 Surveillance Registry. Diabetes Care. 2021;44(8):e160-e162. doi:10.2337/dc21-0074
14. Demeterco-Berggren C, Ebekozien O, Rompicherla S, et al. Age and hospitalization risk in people with type 1 diabetes and COVID-19: Data from the T1D Exchange Surveillance Study. J Clin Endocrinol Metab. 2021;dgab668. doi:10.1210/clinem/dgab668
15. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763-1770. doi:10.1016/S0140-6736(20)31189-2
16. Tsankov BK, Allaire JM, Irvine MA, et al. Severe COVID-19 infection and pediatric comorbidities: a systematic review and meta-analysis. Int J Infect Dis. 2021;103:246-256. doi:10.1016/j.ijid.2020.11.163
From Hassenfeld Children’s Hospital at NYU Langone Health, New York, NY (Dr Gallagher), T1D Exchange, Boston, MA (Saketh Rompicherla; Drs Ebekozien, Noor, Odugbesan, and Mungmode; Nicole Rioles, Emma Ospelt), University of Mississippi School of Population Health, Jackson, MS (Dr. Ebekozien), Icahn School of Medicine at Mount Sinai, New York, NY (Drs. Wilkes, O’Malley, and Rapaport), Weill Cornell Medicine, New York, NY (Drs. Antal and Feuer), NYU Long Island School of Medicine, Mineola, NY (Dr. Gabriel), NYU Langone Health, New York, NY (Dr. Golden), Barbara Davis Center, Aurora, CO (Dr. Alonso), Texas Children’s Hospital/Baylor College of Medicine, Houston, TX (Dr. Lyons), Stanford University, Stanford, CA (Dr. Prahalad), Children Mercy Kansas City, MO (Dr. Clements), Indiana University School of Medicine, IN (Dr. Neyman), Rady Children’s Hospital, University of California, San Diego, CA (Dr. Demeterco-Berggren).
Background: Patient outcomes of COVID-19 have improved throughout the pandemic. However, because it is not known whether outcomes of COVID-19 in the type 1 diabetes (T1D) population improved over time, we investigated differences in COVID-19 outcomes for patients with T1D in the United States.
Methods: We analyzed data collected via a registry of patients with T1D and COVID-19 from 56 sites between April 2020 and January 2021. We grouped cases into first surge (April 9, 2020, to July 31, 2020, n = 188) and late surge (August 1, 2020, to January 31, 2021, n = 410), and then compared outcomes between both groups using descriptive statistics and logistic regression models.
Results: Adverse outcomes were more frequent during the first surge, including diabetic ketoacidosis (32% vs 15%, P < .001), severe hypoglycemia (4% vs 1%, P = .04), and hospitalization (52% vs 22%, P < .001). Patients in the first surge were older (28 [SD,18.8] years vs 18.0 [SD, 11.1] years, P < .001), had higher median hemoglobin A1c levels (9.3 [interquartile range {IQR}, 4.0] vs 8.4 (IQR, 2.8), P < .001), and were more likely to use public insurance (107 [57%] vs 154 [38%], P < .001). The odds of hospitalization for adults in the first surge were 5 times higher compared to the late surge (odds ratio, 5.01; 95% CI, 2.11-12.63).
Conclusion: Patients with T1D who presented with COVID-19 during the first surge had a higher proportion of adverse outcomes than those who presented in a later surge.
Keywords: TD1, diabetic ketoacidosis, hypoglycemia.
After the World Health Organization declared the disease caused by the novel coronavirus SARS-CoV-2, COVID-19, a pandemic on March 11, 2020, the Centers for Disease Control and Prevention identified patients with diabetes as high risk for severe illness.1-7 The case-fatality rate for COVID-19 has significantly improved over the past 2 years. Public health measures, less severe COVID-19 variants, increased access to testing, and new treatments for COVID-19 have contributed to improved outcomes.
The T1D Exchange has previously published findings on COVID-19 outcomes for patients with type 1 diabetes (T1D) using data from the T1D COVID-19 Surveillance Registry.8-12 Given improved outcomes in COVID-19 in the general population, we sought to determine if outcomes for cases of COVID-19 reported to this registry changed over time.
Methods
This study was coordinated by the T1D Exchange and approved as nonhuman subject research by the Western Institutional Review Board. All participating centers also obtained local institutional review board approval. No identifiable patient information was collected as part of this noninterventional, cross-sectional study.
The T1D Exchange Multi-center COVID-19 Surveillance Study collected data from endocrinology clinics that completed a retrospective chart review and submitted information to T1D Exchange via an online questionnaire for all patients with T1D at their sites who tested positive for COVID-19.13,14 The questionnaire was administered using the Qualtrics survey platform (www.qualtrics.com version XM) and contained 33 pre-coded and free-text response fields to collect patient and clinical attributes.
Each participating center identified 1 team member for reporting to avoid duplicate case submission. Each submitted case was reviewed for potential errors and incomplete information. The coordinating center verified the number of cases per site for data quality assurance.
Quantitative data were represented as mean (standard deviation) or median (interquartile range). Categorical data were described as the number (percentage) of patients. Summary statistics, including frequency and percentage for categorical variables, were calculated for all patient-related and clinical characteristics. The date August 1, 2021, was selected as the end of the first surge based on a review of national COVID-19 surges.
We used the Fisher’s exact test to assess associations between hospitalization and demographics, HbA1c, diabetes duration, symptoms, and adverse outcomes. In addition, multivariate logistic regression was used to calculate odds ratios (OR). Logistic regression models were used to determine the association between time of surge and hospitalization separately for both the pediatric and adult populations. Each model was adjusted for potential sociodemographic confounders, specifically age, sex, race, insurance, and HbA1c.
All tests were 2-sided, with type 1 error set at 5%. Fisher’s exact test and logistic regression were performed using statistical software R, version 3.6.2 (R Foundation for Statistical Computing).
Results
The characteristics of COVID-19 cases in patients with T1D that were reported early in the pandemic, before August 1, 2020 (first surge), compared with those of cases reported on and after August 1, 2020 (later surges) are shown in Table 1.

Patients with T1D who presented with COVID-19 during the first surge as compared to the later surges were older (mean age 28 [SD, 18.0] years vs 18.8 [SD, 11.1] years; P < .001) and had a longer duration of diabetes (P < .001). The first-surge group also had more patients with >20 years’ diabetes duration (20% vs 9%, P < .001). Obesity, hypertension, and chronic kidney disease were also more commonly reported in first-surge cases (all P < .001).
There was a significant difference in race and ethnicity reported in the first surge vs the later surge cases, with fewer patients identifying as non-Hispanic White (39% vs, 63%, P < .001) and more patients identifying as non-Hispanic Black (29% vs 12%, P < .001). The groups also differed significantly in terms of insurance type, with more people on public insurance in the first-surge group (57% vs 38%, P < .001). In addition, median HbA1c was higher (9.3% vs 8.4%, P < .001) and continuous glucose monitor and insulin pump use were less common (P = .02 and <.001, respectively) in the early surge.
All symptoms and adverse outcomes were reported more often in the first surge, including diabetic ketoacidosis (DKA; 32% vs 15%; P < .001) and severe hypoglycemia (4% vs 1%, P = .04). Hospitalization (52% vs 13%, P < .001) and ICU admission (24% vs 9%, P < .001) were reported more often in the first-surge group.
Regression Analyses
Table 2 shows the results of logistic regression analyses for hospitalization in the pediatric (≤19 years of age) and adult (>19 years of age) groups, along with the odds of hospitalization during the first vs late surge among COVID-positive people with T1D. Adult patients who tested positive in the first surge were about 5 times more likely to be hospitalized than adults who tested positive for infection in the late surge after adjusting for age, insurance type, sex, race, and HbA1c levels. Pediatric patients also had an increased odds for hospitalization during the first surge, but this increase was not statistically significant.

Discussion
Our analysis of COVID-19 cases in patients with T1D reported by diabetes providers across the United States found that adverse outcomes were more prevalent early in the pandemic. There may be a number of reasons for this difference in outcomes between patients who presented in the first surge vs a later surge. First, because testing for COVID-19 was extremely limited and reserved for hospitalized patients early in the pandemic, the first-surge patients with confirmed COVID-19 likely represent a skewed population of higher-acuity patients. This may also explain the relative paucity of cases in younger patients reported early in the pandemic. Second, worse outcomes in the early surge may also have been associated with overwhelmed hospitals in New York City at the start of the outbreak. According to Cummings et al, the abrupt surge of critically ill patients hospitalized with severe acute respiratory distress syndrome initially outpaced their capacity to provide prone-positioning ventilation, which has been expanded since then.15 While there was very little hypertension, cardiovascular disease, or kidney disease reported in the pediatric groups, there was a higher prevalence of obesity in the pediatric group from the mid-Atlantic region. Obesity has been associated with a worse prognosis for COVID-19 illness in children.16 Finally, there were 5 deaths reported in this study, all of which were reported during the first surge. Older age and increased rates of cardiovascular disease and chronic kidney disease in the first surge cases likely contributed to worse outcomes for adults in mid-Atlantic region relative to the other regions. Minority race and the use of public insurance, risk factors for more severe outcomes in all regions, were also more common in cases reported from the mid-Atlantic region.
This study has several limitations. First, it is a cross-sectional study that relies upon voluntary provider reports. Second, availability of COVID-19 testing was limited in all regions in spring 2020. Third, different regions of the country experienced subsequent surges at different times within the reported timeframes in this analysis. Fourth, this report time period does not include the impact of the newer COVID-19 variants. Finally, trends in COVID-19 outcomes were affected by the evolution of care that developed throughout 2020.
Conclusion
Adult patients with T1D and COVID-19 who reported during the first surge had about 5 times higher hospitalization odds than those who presented in a later surge.
Corresponding author: Osagie Ebekozien, MD, MPH, 11 Avenue de Lafayette, Boston, MA 02111; [email protected]
Disclosures: Dr Ebekozien reports receiving research grants from Medtronic Diabetes, Eli Lilly, and Dexcom, and receiving honoraria from Medtronic Diabetes.
From Hassenfeld Children’s Hospital at NYU Langone Health, New York, NY (Dr Gallagher), T1D Exchange, Boston, MA (Saketh Rompicherla; Drs Ebekozien, Noor, Odugbesan, and Mungmode; Nicole Rioles, Emma Ospelt), University of Mississippi School of Population Health, Jackson, MS (Dr. Ebekozien), Icahn School of Medicine at Mount Sinai, New York, NY (Drs. Wilkes, O’Malley, and Rapaport), Weill Cornell Medicine, New York, NY (Drs. Antal and Feuer), NYU Long Island School of Medicine, Mineola, NY (Dr. Gabriel), NYU Langone Health, New York, NY (Dr. Golden), Barbara Davis Center, Aurora, CO (Dr. Alonso), Texas Children’s Hospital/Baylor College of Medicine, Houston, TX (Dr. Lyons), Stanford University, Stanford, CA (Dr. Prahalad), Children Mercy Kansas City, MO (Dr. Clements), Indiana University School of Medicine, IN (Dr. Neyman), Rady Children’s Hospital, University of California, San Diego, CA (Dr. Demeterco-Berggren).
Background: Patient outcomes of COVID-19 have improved throughout the pandemic. However, because it is not known whether outcomes of COVID-19 in the type 1 diabetes (T1D) population improved over time, we investigated differences in COVID-19 outcomes for patients with T1D in the United States.
Methods: We analyzed data collected via a registry of patients with T1D and COVID-19 from 56 sites between April 2020 and January 2021. We grouped cases into first surge (April 9, 2020, to July 31, 2020, n = 188) and late surge (August 1, 2020, to January 31, 2021, n = 410), and then compared outcomes between both groups using descriptive statistics and logistic regression models.
Results: Adverse outcomes were more frequent during the first surge, including diabetic ketoacidosis (32% vs 15%, P < .001), severe hypoglycemia (4% vs 1%, P = .04), and hospitalization (52% vs 22%, P < .001). Patients in the first surge were older (28 [SD,18.8] years vs 18.0 [SD, 11.1] years, P < .001), had higher median hemoglobin A1c levels (9.3 [interquartile range {IQR}, 4.0] vs 8.4 (IQR, 2.8), P < .001), and were more likely to use public insurance (107 [57%] vs 154 [38%], P < .001). The odds of hospitalization for adults in the first surge were 5 times higher compared to the late surge (odds ratio, 5.01; 95% CI, 2.11-12.63).
Conclusion: Patients with T1D who presented with COVID-19 during the first surge had a higher proportion of adverse outcomes than those who presented in a later surge.
Keywords: TD1, diabetic ketoacidosis, hypoglycemia.
After the World Health Organization declared the disease caused by the novel coronavirus SARS-CoV-2, COVID-19, a pandemic on March 11, 2020, the Centers for Disease Control and Prevention identified patients with diabetes as high risk for severe illness.1-7 The case-fatality rate for COVID-19 has significantly improved over the past 2 years. Public health measures, less severe COVID-19 variants, increased access to testing, and new treatments for COVID-19 have contributed to improved outcomes.
The T1D Exchange has previously published findings on COVID-19 outcomes for patients with type 1 diabetes (T1D) using data from the T1D COVID-19 Surveillance Registry.8-12 Given improved outcomes in COVID-19 in the general population, we sought to determine if outcomes for cases of COVID-19 reported to this registry changed over time.
Methods
This study was coordinated by the T1D Exchange and approved as nonhuman subject research by the Western Institutional Review Board. All participating centers also obtained local institutional review board approval. No identifiable patient information was collected as part of this noninterventional, cross-sectional study.
The T1D Exchange Multi-center COVID-19 Surveillance Study collected data from endocrinology clinics that completed a retrospective chart review and submitted information to T1D Exchange via an online questionnaire for all patients with T1D at their sites who tested positive for COVID-19.13,14 The questionnaire was administered using the Qualtrics survey platform (www.qualtrics.com version XM) and contained 33 pre-coded and free-text response fields to collect patient and clinical attributes.
Each participating center identified 1 team member for reporting to avoid duplicate case submission. Each submitted case was reviewed for potential errors and incomplete information. The coordinating center verified the number of cases per site for data quality assurance.
Quantitative data were represented as mean (standard deviation) or median (interquartile range). Categorical data were described as the number (percentage) of patients. Summary statistics, including frequency and percentage for categorical variables, were calculated for all patient-related and clinical characteristics. The date August 1, 2021, was selected as the end of the first surge based on a review of national COVID-19 surges.
We used the Fisher’s exact test to assess associations between hospitalization and demographics, HbA1c, diabetes duration, symptoms, and adverse outcomes. In addition, multivariate logistic regression was used to calculate odds ratios (OR). Logistic regression models were used to determine the association between time of surge and hospitalization separately for both the pediatric and adult populations. Each model was adjusted for potential sociodemographic confounders, specifically age, sex, race, insurance, and HbA1c.
All tests were 2-sided, with type 1 error set at 5%. Fisher’s exact test and logistic regression were performed using statistical software R, version 3.6.2 (R Foundation for Statistical Computing).
Results
The characteristics of COVID-19 cases in patients with T1D that were reported early in the pandemic, before August 1, 2020 (first surge), compared with those of cases reported on and after August 1, 2020 (later surges) are shown in Table 1.

Patients with T1D who presented with COVID-19 during the first surge as compared to the later surges were older (mean age 28 [SD, 18.0] years vs 18.8 [SD, 11.1] years; P < .001) and had a longer duration of diabetes (P < .001). The first-surge group also had more patients with >20 years’ diabetes duration (20% vs 9%, P < .001). Obesity, hypertension, and chronic kidney disease were also more commonly reported in first-surge cases (all P < .001).
There was a significant difference in race and ethnicity reported in the first surge vs the later surge cases, with fewer patients identifying as non-Hispanic White (39% vs, 63%, P < .001) and more patients identifying as non-Hispanic Black (29% vs 12%, P < .001). The groups also differed significantly in terms of insurance type, with more people on public insurance in the first-surge group (57% vs 38%, P < .001). In addition, median HbA1c was higher (9.3% vs 8.4%, P < .001) and continuous glucose monitor and insulin pump use were less common (P = .02 and <.001, respectively) in the early surge.
All symptoms and adverse outcomes were reported more often in the first surge, including diabetic ketoacidosis (DKA; 32% vs 15%; P < .001) and severe hypoglycemia (4% vs 1%, P = .04). Hospitalization (52% vs 13%, P < .001) and ICU admission (24% vs 9%, P < .001) were reported more often in the first-surge group.
Regression Analyses
Table 2 shows the results of logistic regression analyses for hospitalization in the pediatric (≤19 years of age) and adult (>19 years of age) groups, along with the odds of hospitalization during the first vs late surge among COVID-positive people with T1D. Adult patients who tested positive in the first surge were about 5 times more likely to be hospitalized than adults who tested positive for infection in the late surge after adjusting for age, insurance type, sex, race, and HbA1c levels. Pediatric patients also had an increased odds for hospitalization during the first surge, but this increase was not statistically significant.

Discussion
Our analysis of COVID-19 cases in patients with T1D reported by diabetes providers across the United States found that adverse outcomes were more prevalent early in the pandemic. There may be a number of reasons for this difference in outcomes between patients who presented in the first surge vs a later surge. First, because testing for COVID-19 was extremely limited and reserved for hospitalized patients early in the pandemic, the first-surge patients with confirmed COVID-19 likely represent a skewed population of higher-acuity patients. This may also explain the relative paucity of cases in younger patients reported early in the pandemic. Second, worse outcomes in the early surge may also have been associated with overwhelmed hospitals in New York City at the start of the outbreak. According to Cummings et al, the abrupt surge of critically ill patients hospitalized with severe acute respiratory distress syndrome initially outpaced their capacity to provide prone-positioning ventilation, which has been expanded since then.15 While there was very little hypertension, cardiovascular disease, or kidney disease reported in the pediatric groups, there was a higher prevalence of obesity in the pediatric group from the mid-Atlantic region. Obesity has been associated with a worse prognosis for COVID-19 illness in children.16 Finally, there were 5 deaths reported in this study, all of which were reported during the first surge. Older age and increased rates of cardiovascular disease and chronic kidney disease in the first surge cases likely contributed to worse outcomes for adults in mid-Atlantic region relative to the other regions. Minority race and the use of public insurance, risk factors for more severe outcomes in all regions, were also more common in cases reported from the mid-Atlantic region.
This study has several limitations. First, it is a cross-sectional study that relies upon voluntary provider reports. Second, availability of COVID-19 testing was limited in all regions in spring 2020. Third, different regions of the country experienced subsequent surges at different times within the reported timeframes in this analysis. Fourth, this report time period does not include the impact of the newer COVID-19 variants. Finally, trends in COVID-19 outcomes were affected by the evolution of care that developed throughout 2020.
Conclusion
Adult patients with T1D and COVID-19 who reported during the first surge had about 5 times higher hospitalization odds than those who presented in a later surge.
Corresponding author: Osagie Ebekozien, MD, MPH, 11 Avenue de Lafayette, Boston, MA 02111; [email protected]
Disclosures: Dr Ebekozien reports receiving research grants from Medtronic Diabetes, Eli Lilly, and Dexcom, and receiving honoraria from Medtronic Diabetes.
1. Barron E, Bakhai C, Kar P, et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 2020;8(10):813-822. doi:10.1016/S2213-8587(20)30272-2
2. Fisher L, Polonsky W, Asuni A, Jolly Y, Hessler D. The early impact of the COVID-19 pandemic on adults with type 1 or type 2 diabetes: A national cohort study. J Diabetes Complications. 2020;34(12):107748. doi:10.1016/j.jdiacomp.2020.107748
3. Holman N, Knighton P, Kar P, et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8(10):823-833. doi:10.1016/S2213-8587(20)30271-0
4. Wargny M, Gourdy P, Ludwig L, et al. Type 1 diabetes in people hospitalized for COVID-19: new insights from the CORONADO study. Diabetes Care. 2020;43(11):e174-e177. doi:10.2337/dc20-1217
5. Gregory JM, Slaughter JC, Duffus SH, et al. COVID-19 severity is tripled in the diabetes community: a prospective analysis of the pandemic’s impact in type 1 and type 2 diabetes. Diabetes Care. 2021;44(2):526-532. doi:10.2337/dc20-2260
6. Cardona-Hernandez R, Cherubini V, Iafusco D, Schiaffini R, Luo X, Maahs DM. Children and youth with diabetes are not at increased risk for hospitalization due to COVID-19. Pediatr Diabetes. 2021;22(2):202-206. doi:10.1111/pedi.13158
7. Maahs DM, Alonso GT, Gallagher MP, Ebekozien O. Comment on Gregory et al. COVID-19 severity is tripled in the diabetes community: a prospective analysis of the pandemic’s impact in type 1 and type 2 diabetes. Diabetes Care. 2021;44:526-532. Diabetes Care. 2021;44(5):e102. doi:10.2337/dc20-3119
8. Ebekozien OA, Noor N, Gallagher MP, Alonso GT. Type 1 diabetes and COVID-19: preliminary findings from a multicenter surveillance study in the US. Diabetes Care. 2020;43(8):e83-e85. doi:10.2337/dc20-1088
9. Beliard K, Ebekozien O, Demeterco-Berggren C, et al. Increased DKA at presentation among newly diagnosed type 1 diabetes patients with or without COVID-19: Data from a multi-site surveillance registry. J Diabetes. 2021;13(3):270-272. doi:10.1111/1753-0407
10. O’Malley G, Ebekozien O, Desimone M, et al. COVID-19 hospitalization in adults with type 1 diabetes: results from the T1D Exchange Multicenter Surveillance study. J Clin Endocrinol Metab. 2021;106(2):e936-e942. doi:10.1210/clinem/dgaa825
11. Ebekozien O, Agarwal S, Noor N, et al. Inequities in diabetic ketoacidosis among patients with type 1 diabetes and COVID-19: data from 52 US clinical centers. J Clin Endocrinol Metab. 2021;106(4):e1755-e1762. doi:10.1210/clinem/dgaa920
12. Alonso GT, Ebekozien O, Gallagher MP, et al. Diabetic ketoacidosis drives COVID-19 related hospitalizations in children with type 1 diabetes. J Diabetes. 2021;13(8):681-687. doi:10.1111/1753-0407.13184
13. Noor N, Ebekozien O, Levin L, et al. Diabetes technology use for management of type 1 diabetes is associated with fewer adverse COVID-19 outcomes: findings from the T1D Exchange COVID-19 Surveillance Registry. Diabetes Care. 2021;44(8):e160-e162. doi:10.2337/dc21-0074
14. Demeterco-Berggren C, Ebekozien O, Rompicherla S, et al. Age and hospitalization risk in people with type 1 diabetes and COVID-19: Data from the T1D Exchange Surveillance Study. J Clin Endocrinol Metab. 2021;dgab668. doi:10.1210/clinem/dgab668
15. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763-1770. doi:10.1016/S0140-6736(20)31189-2
16. Tsankov BK, Allaire JM, Irvine MA, et al. Severe COVID-19 infection and pediatric comorbidities: a systematic review and meta-analysis. Int J Infect Dis. 2021;103:246-256. doi:10.1016/j.ijid.2020.11.163
1. Barron E, Bakhai C, Kar P, et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 2020;8(10):813-822. doi:10.1016/S2213-8587(20)30272-2
2. Fisher L, Polonsky W, Asuni A, Jolly Y, Hessler D. The early impact of the COVID-19 pandemic on adults with type 1 or type 2 diabetes: A national cohort study. J Diabetes Complications. 2020;34(12):107748. doi:10.1016/j.jdiacomp.2020.107748
3. Holman N, Knighton P, Kar P, et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8(10):823-833. doi:10.1016/S2213-8587(20)30271-0
4. Wargny M, Gourdy P, Ludwig L, et al. Type 1 diabetes in people hospitalized for COVID-19: new insights from the CORONADO study. Diabetes Care. 2020;43(11):e174-e177. doi:10.2337/dc20-1217
5. Gregory JM, Slaughter JC, Duffus SH, et al. COVID-19 severity is tripled in the diabetes community: a prospective analysis of the pandemic’s impact in type 1 and type 2 diabetes. Diabetes Care. 2021;44(2):526-532. doi:10.2337/dc20-2260
6. Cardona-Hernandez R, Cherubini V, Iafusco D, Schiaffini R, Luo X, Maahs DM. Children and youth with diabetes are not at increased risk for hospitalization due to COVID-19. Pediatr Diabetes. 2021;22(2):202-206. doi:10.1111/pedi.13158
7. Maahs DM, Alonso GT, Gallagher MP, Ebekozien O. Comment on Gregory et al. COVID-19 severity is tripled in the diabetes community: a prospective analysis of the pandemic’s impact in type 1 and type 2 diabetes. Diabetes Care. 2021;44:526-532. Diabetes Care. 2021;44(5):e102. doi:10.2337/dc20-3119
8. Ebekozien OA, Noor N, Gallagher MP, Alonso GT. Type 1 diabetes and COVID-19: preliminary findings from a multicenter surveillance study in the US. Diabetes Care. 2020;43(8):e83-e85. doi:10.2337/dc20-1088
9. Beliard K, Ebekozien O, Demeterco-Berggren C, et al. Increased DKA at presentation among newly diagnosed type 1 diabetes patients with or without COVID-19: Data from a multi-site surveillance registry. J Diabetes. 2021;13(3):270-272. doi:10.1111/1753-0407
10. O’Malley G, Ebekozien O, Desimone M, et al. COVID-19 hospitalization in adults with type 1 diabetes: results from the T1D Exchange Multicenter Surveillance study. J Clin Endocrinol Metab. 2021;106(2):e936-e942. doi:10.1210/clinem/dgaa825
11. Ebekozien O, Agarwal S, Noor N, et al. Inequities in diabetic ketoacidosis among patients with type 1 diabetes and COVID-19: data from 52 US clinical centers. J Clin Endocrinol Metab. 2021;106(4):e1755-e1762. doi:10.1210/clinem/dgaa920
12. Alonso GT, Ebekozien O, Gallagher MP, et al. Diabetic ketoacidosis drives COVID-19 related hospitalizations in children with type 1 diabetes. J Diabetes. 2021;13(8):681-687. doi:10.1111/1753-0407.13184
13. Noor N, Ebekozien O, Levin L, et al. Diabetes technology use for management of type 1 diabetes is associated with fewer adverse COVID-19 outcomes: findings from the T1D Exchange COVID-19 Surveillance Registry. Diabetes Care. 2021;44(8):e160-e162. doi:10.2337/dc21-0074
14. Demeterco-Berggren C, Ebekozien O, Rompicherla S, et al. Age and hospitalization risk in people with type 1 diabetes and COVID-19: Data from the T1D Exchange Surveillance Study. J Clin Endocrinol Metab. 2021;dgab668. doi:10.1210/clinem/dgab668
15. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763-1770. doi:10.1016/S0140-6736(20)31189-2
16. Tsankov BK, Allaire JM, Irvine MA, et al. Severe COVID-19 infection and pediatric comorbidities: a systematic review and meta-analysis. Int J Infect Dis. 2021;103:246-256. doi:10.1016/j.ijid.2020.11.163
Vitamin D shows no survival benefit in nondeficient elderly
, including mortality linked to cardiovascular disease, new results from a large, placebo-controlled trial show.
“The take-home message is that routine vitamin D supplementation, irrespective of the dosing regimen, is unlikely to be beneficial in a population with a low prevalence of vitamin D deficiency,” first author Rachel E. Neale, PhD, of the Population Health Department, QIMR Berghofer Medical Research Institute, in Brisbane, Australia, told this news organization.
Despite extensive previous research on vitamin D supplementation, “mortality has not been the primary outcome in any previous large trial of high-dose vitamin D supplementation,” Dr. Neale and coauthors noted. The results, published online in Lancet Diabetes & Endocrinology, are from the D-Health trial.
With more than 20,000 participants, this is the largest intermittent-dosing trial to date, the authors noted. The primary outcome was all-cause mortality.
In an accompanying editorial, Inez Schoenmakers, PhD, noted that “the findings [are] highly relevant for population policy, owing to the study’s population-based design, large scale, and long duration.”
This new “research contributes to the concept that improving vitamin D status with supplementation in a mostly vitamin D-replete older population does not influence all-cause mortality,” Dr. Schoenmakers, of the Faculty of Medicine and Health Sciences, University of East Anglia, Norwich, England, said in an interview.
“This is not dissimilar to research with many other nutrients showing that increasing intake above the adequate intake has no further health benefits,” she added.
D-Health Trial
The D-Health Trial involved 21,315 participants in Australia, enrolled between February 2014 and June 2015, who had not been screened for vitamin D deficiency but were largely considered to be vitamin D replete. They were a mean age of 69.3 years and 54% were men.
Participants were randomized 1:1 to a once-monthly oral vitamin D3 supplementation of 60,000 IU (n = 10,662) or a placebo capsule (n = 10,653).
They were permitted to take up to 2,000 IU/day of supplemental vitamin D in addition to the study protocol and had no history of kidney stones, hypercalcemia, hyperparathyroidism, osteomalacia, or sarcoidosis.
Over a median follow-up of 5.7 years, there were 1,100 deaths: 562 in the vitamin D group (5.3%) and 538 in the placebo group (5.1%). With a hazard ratio (HR) for all-cause mortality of 1.04, the difference was not significant (P = .47).
There were also no significant differences in terms of mortality from cardiovascular disease (HR, 0.96; P = .77), cancer (HR, 1.15; P = .13), or other causes (HR, 0.83; P = .15).
Rates of total adverse events between the two groups, including hypercalcemia and kidney stones, were similar.
An exploratory analysis excluding the first 2 years of follow-up in fact showed a numerically higher hazard ratio for cancer mortality in the vitamin D group versus no supplementation (HR, 1.24; P = .05). However, the authors noted that the effect was “not apparent when the analysis was restricted to deaths that were coded by the study team and not officially coded.”
Nevertheless, “our findings, from a large study in an unscreened population, give pause to earlier reports that vitamin D supplements might reduce cancer mortality,” they underscored.
Retention and adherence in the study were high, each exceeding 80%. Although blood samples were not collected at baseline, samples from 3,943 randomly sampled participants during follow-up showed mean serum 25-hydroxy-vitamin D concentrations of 77 nmol/L in the placebo group and 115 nmol/L in the vitamin D group, both within the normal range of 50-125 nmol/L.
Findings supported by previous research
The trial results are consistent with those of prior large studies and meta-analyses of older adults with a low prevalence of vitamin D deficiency showing that vitamin D3 supplementation, regardless of whether taken daily or monthly, is not likely to have an effect on all-cause mortality.
In the US VITAL trial, recently published in the New England Journal of Medicine, among 25,871 participants administered 2,000 IU/day of vitamin D3 for a median of 5.3 years, there was no reduction in all-cause mortality.
The ViDA trial of 5,110 older adults in New Zealand, published in 2019 in the Journal of Endocrinological Investigation, also showed monthly vitamin D3 supplementation of 100,000 IU for a median of 3.3 years was not associated with a benefit in people who were not deficient.
“In total, the results from the large trials and meta-analyses suggest that routine supplementation of older adults in populations with a low prevalence of vitamin D deficiency is unlikely to reduce the rate of all-cause mortality,” Dr. Neale and colleagues concluded.
Longer-term supplementation beneficial?
The population was limited to older adults and the study had a relatively short follow-up period, which Dr. Neale noted was necessary for pragmatic reasons.
“Our primary outcome was all-cause mortality, so to have sufficient deaths we either needed to study older adults or a much larger sample of younger adults,” she explained.
“However, we felt that [the former] ... had biological justification, as there is evidence that vitamin D plays a role later in the course of a number of diseases, with potential impacts on mortality.”
She noted that recent studies evaluating genetically predicted concentrations of serum 25(OH)D have further shown no link between those levels and all-cause mortality, stroke, or coronary heart disease.
“This confirms the statement that vitamin D is unlikely to be beneficial in people who are not vitamin D deficient, irrespective of whether supplementation occurs over the short or longer term,” Dr. Neale said.
The source of vitamin D, itself, is another consideration, with ongoing speculation of differences in benefits between dietary or supplementation sources versus sunlight exposure.
“Exposure to ultraviolet radiation, for which serum 25(OH)D concentration is a good marker, might confer benefits not mediated by vitamin D,” Dr. Neale and coauthors noted.
They added that the results in the older Australian population “cannot be generalized to populations with a higher prevalence of vitamin D deficiency, or with a greater proportion of people not of White ancestry, than the study population.”
Ten-year mortality rates from the D-Health trial are expected to be reported in the future.
Strategies still needed to address vitamin D deficiency
Further commenting on the findings, Dr. Schoenmakers underscored that “vitamin D deficiency is very common worldwide, [and] more should be done to develop strategies to address the needs of those groups and populations that are at risk of the consequences of vitamin D deficiency.”
That said, the D-Health study is important in helping to distinguish when supplementation may – and may not – be of benefit, she noted.
“This and other research in the past 15 years have contributed to our understanding [of] what the ranges of vitamin D status are [in which] health consequences may be anticipated.”
The D-Health Trial was funded by the National Health and Medical Research Council. Dr. Neale and Dr. Schoenmakers have reported no relevant financial relationships.
version of this article first appeared on Medscape.com.
, including mortality linked to cardiovascular disease, new results from a large, placebo-controlled trial show.
“The take-home message is that routine vitamin D supplementation, irrespective of the dosing regimen, is unlikely to be beneficial in a population with a low prevalence of vitamin D deficiency,” first author Rachel E. Neale, PhD, of the Population Health Department, QIMR Berghofer Medical Research Institute, in Brisbane, Australia, told this news organization.
Despite extensive previous research on vitamin D supplementation, “mortality has not been the primary outcome in any previous large trial of high-dose vitamin D supplementation,” Dr. Neale and coauthors noted. The results, published online in Lancet Diabetes & Endocrinology, are from the D-Health trial.
With more than 20,000 participants, this is the largest intermittent-dosing trial to date, the authors noted. The primary outcome was all-cause mortality.
In an accompanying editorial, Inez Schoenmakers, PhD, noted that “the findings [are] highly relevant for population policy, owing to the study’s population-based design, large scale, and long duration.”
This new “research contributes to the concept that improving vitamin D status with supplementation in a mostly vitamin D-replete older population does not influence all-cause mortality,” Dr. Schoenmakers, of the Faculty of Medicine and Health Sciences, University of East Anglia, Norwich, England, said in an interview.
“This is not dissimilar to research with many other nutrients showing that increasing intake above the adequate intake has no further health benefits,” she added.
D-Health Trial
The D-Health Trial involved 21,315 participants in Australia, enrolled between February 2014 and June 2015, who had not been screened for vitamin D deficiency but were largely considered to be vitamin D replete. They were a mean age of 69.3 years and 54% were men.
Participants were randomized 1:1 to a once-monthly oral vitamin D3 supplementation of 60,000 IU (n = 10,662) or a placebo capsule (n = 10,653).
They were permitted to take up to 2,000 IU/day of supplemental vitamin D in addition to the study protocol and had no history of kidney stones, hypercalcemia, hyperparathyroidism, osteomalacia, or sarcoidosis.
Over a median follow-up of 5.7 years, there were 1,100 deaths: 562 in the vitamin D group (5.3%) and 538 in the placebo group (5.1%). With a hazard ratio (HR) for all-cause mortality of 1.04, the difference was not significant (P = .47).
There were also no significant differences in terms of mortality from cardiovascular disease (HR, 0.96; P = .77), cancer (HR, 1.15; P = .13), or other causes (HR, 0.83; P = .15).
Rates of total adverse events between the two groups, including hypercalcemia and kidney stones, were similar.
An exploratory analysis excluding the first 2 years of follow-up in fact showed a numerically higher hazard ratio for cancer mortality in the vitamin D group versus no supplementation (HR, 1.24; P = .05). However, the authors noted that the effect was “not apparent when the analysis was restricted to deaths that were coded by the study team and not officially coded.”
Nevertheless, “our findings, from a large study in an unscreened population, give pause to earlier reports that vitamin D supplements might reduce cancer mortality,” they underscored.
Retention and adherence in the study were high, each exceeding 80%. Although blood samples were not collected at baseline, samples from 3,943 randomly sampled participants during follow-up showed mean serum 25-hydroxy-vitamin D concentrations of 77 nmol/L in the placebo group and 115 nmol/L in the vitamin D group, both within the normal range of 50-125 nmol/L.
Findings supported by previous research
The trial results are consistent with those of prior large studies and meta-analyses of older adults with a low prevalence of vitamin D deficiency showing that vitamin D3 supplementation, regardless of whether taken daily or monthly, is not likely to have an effect on all-cause mortality.
In the US VITAL trial, recently published in the New England Journal of Medicine, among 25,871 participants administered 2,000 IU/day of vitamin D3 for a median of 5.3 years, there was no reduction in all-cause mortality.
The ViDA trial of 5,110 older adults in New Zealand, published in 2019 in the Journal of Endocrinological Investigation, also showed monthly vitamin D3 supplementation of 100,000 IU for a median of 3.3 years was not associated with a benefit in people who were not deficient.
“In total, the results from the large trials and meta-analyses suggest that routine supplementation of older adults in populations with a low prevalence of vitamin D deficiency is unlikely to reduce the rate of all-cause mortality,” Dr. Neale and colleagues concluded.
Longer-term supplementation beneficial?
The population was limited to older adults and the study had a relatively short follow-up period, which Dr. Neale noted was necessary for pragmatic reasons.
“Our primary outcome was all-cause mortality, so to have sufficient deaths we either needed to study older adults or a much larger sample of younger adults,” she explained.
“However, we felt that [the former] ... had biological justification, as there is evidence that vitamin D plays a role later in the course of a number of diseases, with potential impacts on mortality.”
She noted that recent studies evaluating genetically predicted concentrations of serum 25(OH)D have further shown no link between those levels and all-cause mortality, stroke, or coronary heart disease.
“This confirms the statement that vitamin D is unlikely to be beneficial in people who are not vitamin D deficient, irrespective of whether supplementation occurs over the short or longer term,” Dr. Neale said.
The source of vitamin D, itself, is another consideration, with ongoing speculation of differences in benefits between dietary or supplementation sources versus sunlight exposure.
“Exposure to ultraviolet radiation, for which serum 25(OH)D concentration is a good marker, might confer benefits not mediated by vitamin D,” Dr. Neale and coauthors noted.
They added that the results in the older Australian population “cannot be generalized to populations with a higher prevalence of vitamin D deficiency, or with a greater proportion of people not of White ancestry, than the study population.”
Ten-year mortality rates from the D-Health trial are expected to be reported in the future.
Strategies still needed to address vitamin D deficiency
Further commenting on the findings, Dr. Schoenmakers underscored that “vitamin D deficiency is very common worldwide, [and] more should be done to develop strategies to address the needs of those groups and populations that are at risk of the consequences of vitamin D deficiency.”
That said, the D-Health study is important in helping to distinguish when supplementation may – and may not – be of benefit, she noted.
“This and other research in the past 15 years have contributed to our understanding [of] what the ranges of vitamin D status are [in which] health consequences may be anticipated.”
The D-Health Trial was funded by the National Health and Medical Research Council. Dr. Neale and Dr. Schoenmakers have reported no relevant financial relationships.
version of this article first appeared on Medscape.com.
, including mortality linked to cardiovascular disease, new results from a large, placebo-controlled trial show.
“The take-home message is that routine vitamin D supplementation, irrespective of the dosing regimen, is unlikely to be beneficial in a population with a low prevalence of vitamin D deficiency,” first author Rachel E. Neale, PhD, of the Population Health Department, QIMR Berghofer Medical Research Institute, in Brisbane, Australia, told this news organization.
Despite extensive previous research on vitamin D supplementation, “mortality has not been the primary outcome in any previous large trial of high-dose vitamin D supplementation,” Dr. Neale and coauthors noted. The results, published online in Lancet Diabetes & Endocrinology, are from the D-Health trial.
With more than 20,000 participants, this is the largest intermittent-dosing trial to date, the authors noted. The primary outcome was all-cause mortality.
In an accompanying editorial, Inez Schoenmakers, PhD, noted that “the findings [are] highly relevant for population policy, owing to the study’s population-based design, large scale, and long duration.”
This new “research contributes to the concept that improving vitamin D status with supplementation in a mostly vitamin D-replete older population does not influence all-cause mortality,” Dr. Schoenmakers, of the Faculty of Medicine and Health Sciences, University of East Anglia, Norwich, England, said in an interview.
“This is not dissimilar to research with many other nutrients showing that increasing intake above the adequate intake has no further health benefits,” she added.
D-Health Trial
The D-Health Trial involved 21,315 participants in Australia, enrolled between February 2014 and June 2015, who had not been screened for vitamin D deficiency but were largely considered to be vitamin D replete. They were a mean age of 69.3 years and 54% were men.
Participants were randomized 1:1 to a once-monthly oral vitamin D3 supplementation of 60,000 IU (n = 10,662) or a placebo capsule (n = 10,653).
They were permitted to take up to 2,000 IU/day of supplemental vitamin D in addition to the study protocol and had no history of kidney stones, hypercalcemia, hyperparathyroidism, osteomalacia, or sarcoidosis.
Over a median follow-up of 5.7 years, there were 1,100 deaths: 562 in the vitamin D group (5.3%) and 538 in the placebo group (5.1%). With a hazard ratio (HR) for all-cause mortality of 1.04, the difference was not significant (P = .47).
There were also no significant differences in terms of mortality from cardiovascular disease (HR, 0.96; P = .77), cancer (HR, 1.15; P = .13), or other causes (HR, 0.83; P = .15).
Rates of total adverse events between the two groups, including hypercalcemia and kidney stones, were similar.
An exploratory analysis excluding the first 2 years of follow-up in fact showed a numerically higher hazard ratio for cancer mortality in the vitamin D group versus no supplementation (HR, 1.24; P = .05). However, the authors noted that the effect was “not apparent when the analysis was restricted to deaths that were coded by the study team and not officially coded.”
Nevertheless, “our findings, from a large study in an unscreened population, give pause to earlier reports that vitamin D supplements might reduce cancer mortality,” they underscored.
Retention and adherence in the study were high, each exceeding 80%. Although blood samples were not collected at baseline, samples from 3,943 randomly sampled participants during follow-up showed mean serum 25-hydroxy-vitamin D concentrations of 77 nmol/L in the placebo group and 115 nmol/L in the vitamin D group, both within the normal range of 50-125 nmol/L.
Findings supported by previous research
The trial results are consistent with those of prior large studies and meta-analyses of older adults with a low prevalence of vitamin D deficiency showing that vitamin D3 supplementation, regardless of whether taken daily or monthly, is not likely to have an effect on all-cause mortality.
In the US VITAL trial, recently published in the New England Journal of Medicine, among 25,871 participants administered 2,000 IU/day of vitamin D3 for a median of 5.3 years, there was no reduction in all-cause mortality.
The ViDA trial of 5,110 older adults in New Zealand, published in 2019 in the Journal of Endocrinological Investigation, also showed monthly vitamin D3 supplementation of 100,000 IU for a median of 3.3 years was not associated with a benefit in people who were not deficient.
“In total, the results from the large trials and meta-analyses suggest that routine supplementation of older adults in populations with a low prevalence of vitamin D deficiency is unlikely to reduce the rate of all-cause mortality,” Dr. Neale and colleagues concluded.
Longer-term supplementation beneficial?
The population was limited to older adults and the study had a relatively short follow-up period, which Dr. Neale noted was necessary for pragmatic reasons.
“Our primary outcome was all-cause mortality, so to have sufficient deaths we either needed to study older adults or a much larger sample of younger adults,” she explained.
“However, we felt that [the former] ... had biological justification, as there is evidence that vitamin D plays a role later in the course of a number of diseases, with potential impacts on mortality.”
She noted that recent studies evaluating genetically predicted concentrations of serum 25(OH)D have further shown no link between those levels and all-cause mortality, stroke, or coronary heart disease.
“This confirms the statement that vitamin D is unlikely to be beneficial in people who are not vitamin D deficient, irrespective of whether supplementation occurs over the short or longer term,” Dr. Neale said.
The source of vitamin D, itself, is another consideration, with ongoing speculation of differences in benefits between dietary or supplementation sources versus sunlight exposure.
“Exposure to ultraviolet radiation, for which serum 25(OH)D concentration is a good marker, might confer benefits not mediated by vitamin D,” Dr. Neale and coauthors noted.
They added that the results in the older Australian population “cannot be generalized to populations with a higher prevalence of vitamin D deficiency, or with a greater proportion of people not of White ancestry, than the study population.”
Ten-year mortality rates from the D-Health trial are expected to be reported in the future.
Strategies still needed to address vitamin D deficiency
Further commenting on the findings, Dr. Schoenmakers underscored that “vitamin D deficiency is very common worldwide, [and] more should be done to develop strategies to address the needs of those groups and populations that are at risk of the consequences of vitamin D deficiency.”
That said, the D-Health study is important in helping to distinguish when supplementation may – and may not – be of benefit, she noted.
“This and other research in the past 15 years have contributed to our understanding [of] what the ranges of vitamin D status are [in which] health consequences may be anticipated.”
The D-Health Trial was funded by the National Health and Medical Research Council. Dr. Neale and Dr. Schoenmakers have reported no relevant financial relationships.
version of this article first appeared on Medscape.com.
FROM THE LANCET DIABETES & ENDOCRINOLOGY
FDA okays first tubing-free ‘artificial pancreas’ Omnipod 5
The Food and Drug Administration has cleared the Omnipod 5 Automated Insulin Delivery System (Insulet), the third semiautomated closed-loop insulin delivery system in the United States and the first that is tubing free.
Omnipod 5 is cleared for people aged 6 years and older with type 1 diabetes. The system integrates the tubeless insulin delivery Pods with Dexcom G6 continuous glucose monitors (CGM) and a smartphone app or a separate controller device to automatically adjust insulin to minimize high and low blood glucose levels via SmartAdjust technology.
Within the app is a SmartBolus calculator that receives Dexcom CGM values every 5 minutes and automatically adjusts insulin up or down or pauses it based on predicted values for 60 minutes into the future and the individual’s customized glucose targets.
The Omnipod 5 becomes the third FDA-cleared semiautomated insulin delivery system in the United States, along with systems by Tandem and Medtronic. Others are available outside the United States. All of the currently marketed systems incorporate insulin pumps with tubing, whereas the tubeless Pods are worn directly on the body and changed every 3 days.
In a statement, JDRF, the type 1 diabetes advocacy organization, said: “Authorization of the Insulet Omnipod 5 is a huge win for the type 1 diabetes community. As the first tubeless hybrid closed-loop system to receive FDA clearance, this is a critical step forward in making day-to-day life better for people living with the disease.”
JDRF, which worked with the FDA to establish regulatory pathways for artificial pancreas technology, supported the development of the Omnipod 5 control algorithm through investigators in the JDRF Artificial Pancreas Consortium.
The Omnipod 5 will be available as a pharmacy product. It will be launched soon in limited market release and broadly thereafter.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has cleared the Omnipod 5 Automated Insulin Delivery System (Insulet), the third semiautomated closed-loop insulin delivery system in the United States and the first that is tubing free.
Omnipod 5 is cleared for people aged 6 years and older with type 1 diabetes. The system integrates the tubeless insulin delivery Pods with Dexcom G6 continuous glucose monitors (CGM) and a smartphone app or a separate controller device to automatically adjust insulin to minimize high and low blood glucose levels via SmartAdjust technology.
Within the app is a SmartBolus calculator that receives Dexcom CGM values every 5 minutes and automatically adjusts insulin up or down or pauses it based on predicted values for 60 minutes into the future and the individual’s customized glucose targets.
The Omnipod 5 becomes the third FDA-cleared semiautomated insulin delivery system in the United States, along with systems by Tandem and Medtronic. Others are available outside the United States. All of the currently marketed systems incorporate insulin pumps with tubing, whereas the tubeless Pods are worn directly on the body and changed every 3 days.
In a statement, JDRF, the type 1 diabetes advocacy organization, said: “Authorization of the Insulet Omnipod 5 is a huge win for the type 1 diabetes community. As the first tubeless hybrid closed-loop system to receive FDA clearance, this is a critical step forward in making day-to-day life better for people living with the disease.”
JDRF, which worked with the FDA to establish regulatory pathways for artificial pancreas technology, supported the development of the Omnipod 5 control algorithm through investigators in the JDRF Artificial Pancreas Consortium.
The Omnipod 5 will be available as a pharmacy product. It will be launched soon in limited market release and broadly thereafter.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has cleared the Omnipod 5 Automated Insulin Delivery System (Insulet), the third semiautomated closed-loop insulin delivery system in the United States and the first that is tubing free.
Omnipod 5 is cleared for people aged 6 years and older with type 1 diabetes. The system integrates the tubeless insulin delivery Pods with Dexcom G6 continuous glucose monitors (CGM) and a smartphone app or a separate controller device to automatically adjust insulin to minimize high and low blood glucose levels via SmartAdjust technology.
Within the app is a SmartBolus calculator that receives Dexcom CGM values every 5 minutes and automatically adjusts insulin up or down or pauses it based on predicted values for 60 minutes into the future and the individual’s customized glucose targets.
The Omnipod 5 becomes the third FDA-cleared semiautomated insulin delivery system in the United States, along with systems by Tandem and Medtronic. Others are available outside the United States. All of the currently marketed systems incorporate insulin pumps with tubing, whereas the tubeless Pods are worn directly on the body and changed every 3 days.
In a statement, JDRF, the type 1 diabetes advocacy organization, said: “Authorization of the Insulet Omnipod 5 is a huge win for the type 1 diabetes community. As the first tubeless hybrid closed-loop system to receive FDA clearance, this is a critical step forward in making day-to-day life better for people living with the disease.”
JDRF, which worked with the FDA to establish regulatory pathways for artificial pancreas technology, supported the development of the Omnipod 5 control algorithm through investigators in the JDRF Artificial Pancreas Consortium.
The Omnipod 5 will be available as a pharmacy product. It will be launched soon in limited market release and broadly thereafter.
A version of this article first appeared on Medscape.com.







