User login
Click for Credit: Long-term antibiotics & stroke, CHD; Postvaccination seizures; more
Here are 5 articles from the November issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Poor response to statins hikes risk of cardiovascular events
To take the posttest, go to: https://bit.ly/2MVHlDR
Expires April 17, 2020
2. Postvaccination febrile seizures are no more severe than other febrile seizures
To take the posttest, go to: https://bit.ly/2VUJzaE
Expires April 19, 2020
3. Hydroxychloroquine adherence in SLE: worse than you thought
To take the posttest, go to: https://bit.ly/2oT00Z9
Expires April 22, 2020
4. Long-term antibiotic use may heighten stroke, CHD risk
To take the posttest, go to: https://bit.ly/2OUUVu5
Expires April 28, 2020
5. Knowledge gaps about long-term osteoporosis drug therapy benefits, risks remain large
To take the posttest, go to: https://bit.ly/2Msgqkb
Expires May 1, 2020
Here are 5 articles from the November issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Poor response to statins hikes risk of cardiovascular events
To take the posttest, go to: https://bit.ly/2MVHlDR
Expires April 17, 2020
2. Postvaccination febrile seizures are no more severe than other febrile seizures
To take the posttest, go to: https://bit.ly/2VUJzaE
Expires April 19, 2020
3. Hydroxychloroquine adherence in SLE: worse than you thought
To take the posttest, go to: https://bit.ly/2oT00Z9
Expires April 22, 2020
4. Long-term antibiotic use may heighten stroke, CHD risk
To take the posttest, go to: https://bit.ly/2OUUVu5
Expires April 28, 2020
5. Knowledge gaps about long-term osteoporosis drug therapy benefits, risks remain large
To take the posttest, go to: https://bit.ly/2Msgqkb
Expires May 1, 2020
Here are 5 articles from the November issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Poor response to statins hikes risk of cardiovascular events
To take the posttest, go to: https://bit.ly/2MVHlDR
Expires April 17, 2020
2. Postvaccination febrile seizures are no more severe than other febrile seizures
To take the posttest, go to: https://bit.ly/2VUJzaE
Expires April 19, 2020
3. Hydroxychloroquine adherence in SLE: worse than you thought
To take the posttest, go to: https://bit.ly/2oT00Z9
Expires April 22, 2020
4. Long-term antibiotic use may heighten stroke, CHD risk
To take the posttest, go to: https://bit.ly/2OUUVu5
Expires April 28, 2020
5. Knowledge gaps about long-term osteoporosis drug therapy benefits, risks remain large
To take the posttest, go to: https://bit.ly/2Msgqkb
Expires May 1, 2020
Severe hypercalcemia in a 54-year-old woman
A morbidly obese 54-year-old woman presented to the emergency department after experiencing generalized abdominal pain for 3 days. She rated the pain as 5 on a scale of 10 and described it as dull, cramping, waxing and waning, not radiating, and not relieved with changes of position—in fact, not alleviated by anything she had tried. Her pain was associated with nausea and 1 episode of vomiting. She also experienced constipation before the onset of pain.
She denied recent trauma, recent travel, diarrhea, fevers, weakness, shortness of breath, chest pain, other muscle pains, or recent changes in diet. She also denied having this pain in the past. She said she had unintentionally lost some weight but was not certain how much. She denied tobacco, alcohol, or illicit drug use. She had no history of surgery.
Her medical history included hypertension, anemia, and uterine fibroids. Her current medications included losartan, hydrochlorothiazide, and albuterol. She had no family history of significant disease.
INITIAL EVALUATION AND MANAGEMENT
On admission, her temperature was 97.8°F (36.6°C), heart rate 100 beats per minute, blood pressure 136/64 mm Hg, respiratory rate 18 breaths per minute, oxygen saturation 97% on room air, weight 130.6 kg, and body mass index 35 kg/m2.
She was alert and oriented to person, place, and time. She was in mild discomfort but no distress. Her lungs were clear to auscultation, with no wheezing or crackles. Heart rate and rhythm were regular, with no extra heart sounds or murmurs. Bowel sounds were normal in all 4 quadrants, with tenderness to palpation of the epigastric area, but with no guarding or rebound tenderness.
Laboratory test results
Notable results of blood testing at presentation were as follows:
- Hemoglobin 8.2 g/dL (reference range 12.3–15.3)
- Hematocrit 26% (41–50)
- Mean corpuscular volume 107 fL (80–100)
- Blood urea nitrogen 33 mg/dL (8–21); 6 months earlier it was 16
- Serum creatinine 3.6 mg/dL (0.58–0.96); 6 months earlier, it was 0.75
- Albumin 3.3 g/dL (3.5–5)
- Calcium 18.4 mg/dL (8.4–10.2); 6 months earlier, it was 9.6
- Corrected calcium 19 mg/dL.
Findings on imaging, electrocardiography
Chest radiography showed no acute cardiopulmonary abnormalities. Abdominal computed tomography without contrast showed no abnormalities within the pancreas and no evidence of inflammation or obstruction. Electrocardiography showed sinus tachycardia.
DIFFERENTIAL DIAGNOSIS
1. Which is the most likely cause of this patient’s symptoms?
- Primary hyperparathyroidism
- Malignancy
- Her drug therapy
- Familial hypercalcemic hypocalciuria
In total, her laboratory results were consistent with macrocytic anemia, severe hypercalcemia, and acute kidney injury, and she had generalized symptoms.
Primary hyperparathyroidism
A main cause of hypercalcemia is primary hyperparathyroidism, and this needs to be ruled out. Benign adenomas are the most common cause of primary hyperparathyroidism, and a risk factor for benign adenoma is exposure to therapeutic levels of radiation.3
In hyperparathyroidism, there is an increased secretion of parathyroid hormone (PTH), which has multiple effects including increased reabsorption of calcium from the urine, increased excretion of phosphate, and increased expression of 1,25-hydroxyvitamin D hydroxylase to activate vitamin D. PTH also stimulates osteoclasts to increase their expression of receptor activator of nuclear factor kappa B ligand (RANKL), which has a downstream effect on osteoclast precursors to cause bone reabsorption.3
Inherited primary hyperparathyroidism tends to present at a younger age, with multiple overactive parathyroid glands.3 Given our patient’s age, inherited primary hyparathyroidism is thus less likely.
Malignancy
The probability that malignancy is causing the hypercalcemia increases with calcium levels greater than 13 mg/dL. Epidemiologically, in hospitalized patients with hypercalcemia, the source tends to be malignancy.4 Typically, patients who develop hypercalcemia from malignancy have a worse prognosis.5
Solid tumors and leukemias can cause hypercalcemia. The mechanisms include humoral factors secreted by the malignancy, local osteolysis due to tumor invasion of bone, and excessive absorption of calcium due to excess vitamin D produced by malignancies.5 The cancers that most frequently cause an increase in calcium resorption are lung cancer, renal cancer, breast cancer, and multiple myeloma.1
Solid tumors with no bone metastasis and non-Hodgkin lymphoma that release PTH-related protein (PTHrP) cause humoral hypercalcemia in malignancy. The patient is typically in an advanced stage of disease. PTHrP increases serum calcium levels by decreasing the kidney’s ability to excrete calcium and by increasing bone turnover. It has no effect on intestinal absorption because of its inability to stimulate activated vitamin D3. Thus, the increase in systemic calcium comes directly from breakdown of bone and inability to excrete the excess.
PTHrP has a unique role in breast cancer: it is released locally in areas where cancer cells have metastasized to bone, but it does not cause a systemic effect. Bone resorption occurs in areas of metastasis and results from an increase in expression of RANKL and RANK in osteoclasts in response to the effects of PTHrP, leading to an increase in the production of osteoclastic cells.1
Tamoxifen, an endocrine therapy often used in breast cancer, also causes a release of bone-reabsorbing factors from tumor cells, which can partially contribute to hypercalcemia.5
Myeloma cells secrete RANKL, which stimulates osteoclastic activity, and they also release interleukin 6 (IL-6) and activating macrophage inflammatory protein alpha. Serum testing usually shows low or normal intact PTH, PTHrP, and 1,25-dihydroxyvitamin D.1
Patients with multiple myeloma have a worse prognosis if they have a high red blood cell distribution width, a condition shown to correlate with malnutrition, leading to deficiencies in vitamin B12 and to poor response to treatment.6 Up to 14% of patients with multiple myeloma have vitamin B12 deficiency.7
Our patient’s recent weight loss and severe hypercalcemia raise suspicion of malignancy. Further, her obesity makes proper routine breast examination difficult and thus increases the chance of undiagnosed breast cancer.8 Her decrease in renal function and her anemia complicated by hypercalcemia also raise suspicion of multiple myeloma.
Hypercalcemia due to drug therapy
Thiazide diuretics, lithium, teriparatide, and vitamin A in excessive amounts can raise the serum calcium concentration.5 Our patient was taking a thiazide for hypertension, but her extremely high calcium level places drug-induced hypercalcemia as the sole cause lower on the differential list.
Familial hypercalcemic hypocalciuria
Familial hypercalcemic hypocalciuria is a rare autosomal-dominant cause of hypercalcemia in which the ability of the body (and especially the kidneys) to sense levels of calcium is impaired, leading to a decrease in excretion of calcium in the urine.3 Very high calcium levels are rare in hypercalcemic hypocalciuria.3 In our patient with a corrected calcium concentration of nearly 19 mg/dL, familial hypercalcemic hypocalciuria is very unlikely to be the cause of the hypercalcemia.
WHAT ARE THE NEXT STEPS IN THE WORKUP?
As hypercalcemia has been confirmed, the intact PTH level should be checked to determine whether the patient’s condition is PTH-mediated. If the PTH level is in the upper range of normal or is minimally elevated, primary hyperparathyroidism is likely. Elevated PTH confirms primary hyperparathyroidism. A low-normal or low intact PTH confirms a non-PTH-mediated process, and once this is confirmed, PTHrP levels should be checked. An elevated PTHrP suggests humoral hypercalcemia of malignancy. Serum protein electrophoresis, urine protein electrophoresis, and a serum light chain assay should be performed to rule out multiple myeloma.
Vitamin D toxicity is associated with high concentrations of 1,25-dihydroxyvitamin D and 25-hydroxyvitamin D metabolites. These levels should be checked in this patient.
Other disorders that cause hypercalcemia are vitamin A toxicity and hyperthyroidism, so vitamin A and thyroid-stimulating hormone levels should also be checked.5
CASE CONTINUED
After further questioning, the patient said that she had had lower back pain about 1 to 2 weeks before coming to the emergency room; her primary care doctor had said the pain was likely from muscle strain. The pain had almost resolved but was still present.
The results of further laboratory testing were as follows:
- Serum PTH 11 pg/mL (15–65)
- PTHrP 3.4 pmol/L (< 2.0)
- Protein electrophoresis showed a monoclonal (M) spike of 0.2 g/dL (0)
- Activated vitamin D < 5 ng/mL (19.9–79.3)
- Vitamin A 7.2 mg/dL (33.1–100)
- Vitamin B12 194 pg/mL (239–931)
- Thyroid-stimulating hormone 1.21 mIU/ L (0.47–4.68
- Free thyroxine 1.27 ng/dL (0.78–2.19)
- Iron 103 µg/dL (37–170)
- Total iron-binding capacity 335 µg/dL (265–497)
- Transferrin 248 mg/dL (206–381)
- Ferritin 66 ng/mL (11.1–264)
- Urine protein (random) 100 mg/dL (0–20)
- Urine microalbumin (random) 5.9 mg/dL (0–1.6)
- Urine creatinine clearance 88.5 mL/min (88–128)
- Urine albumin-creatinine ratio 66.66 mg/g (< 30).
Imaging reports
A nuclear bone scan showed increased bone uptake in the hip and both shoulders, consistent with arthritis, and increased activity in 2 of the lower left ribs, associated with rib fractures secondary to lytic lesions. A skeletal survey at a later date showed multiple well-circumscribed “punched-out” lytic lesions in both forearms and both femurs.
2. What should be the next step in this patient’s management?
- Intravenous (IV) fluids
- Calcitonin
- Bisphosphonate treatment
- Denosumab
- Hemodialysis
Initial treatment of severe hypercalcemia includes the following:
Start IV isotonic fluids at a rate of 150 mL/h (if the patient is making urine) to maintain urine output at more than 100 mL/h. Closely monitor urine output.
Give calcitonin 4 IU/kg in combination with IV fluids to reduce calcium levels within the first 12 to 48 hours of treatment.
Give a bisphosphonate, eg, zoledronic acid 4 mg over 15 minutes, or pamidronate 60 to 90 mg over 2 hours. Zoledronic acid is preferred in malignancy-induced hypercalcemia because it is more potent. Doses should be adjusted in patients with renal failure.
Give denosumab if hypercalcemia is refractory to bisphosphonates, or when bisphosphonates cannot be used in renal failure.9
Hemodialysis is performed in patients who have significant neurologic symptoms irrespective of acute renal insufficiency.
Our patient was started on 0.9% sodium chloride at a rate of 150 mL/h for severe hypercalcemia. Zoledronic acid 4 mg IV was given once. These measures lowered her calcium level and lessened her acute kidney injury.
ADDITIONAL FINDINGS
Urine testing was positive for Bence Jones protein. Immune electrophoresis, performed because of suspicion of multiple myeloma, showed an elevated level of kappa light chains at 806.7 mg/dL (0.33–1.94) and normal lambda light chains at 0.62 mg/dL (0.57–2.63). The immunoglobulin G level was low at 496 mg/dL (610–1,660). In patients with severe hypercalcemia, these results point to a diagnosis of malignancy. Bone marrow aspiration study showed greater than 10% plasma cells, confirming multiple myeloma.
MULTIPLE MYELOMA
The diagnosis of multiple myeloma is based in part on the presence of 10% or more of clonal bone marrow plasma cells10 and of specific end-organ damage (anemia, hypercalcemia, renal insufficiency, or bone lesions).9
Bone marrow clonality can be shown by the ratio of kappa to lambda light chains as detected with immunohistochemistry, immunofluorescence, or flow cytometry.11 The normal ratio is 0.26 to 1.65 for a patient with normal kidney function. In this patient, however, the ratio was 1,301.08 (806.67 kappa to 0.62 lambda), which was extremely out of range. The patient’s bone marrow biopsy results revealed the presence of 15% clonal bone marrow plasma cells.
Multiple myeloma causes osteolytic lesions through increased activation of osteoclast activating factor that stimulates the growth of osteoclast precursors. At the same time, it inhibits osteoblast formation via multiple pathways, including the action of sclerostin.11 Our patient had lytic lesions in 2 left lower ribs and in both forearms and femurs.
Hypercalcemia in multiple myeloma is attributed to 2 main factors: bone breakdown and macrophage overactivation. Multiple myeloma cells increase the release of macrophage inflammatory protein 1-alpha and tumor necrosis factor, which are inflammatory proteins that cause an increase in macrophages, which cause an increase in calcitriol.11 As noted, our patient’s calcium level at presentation was 18.4 mg/dL uncorrected and 18.96 mg/dL corrected.
Cast nephropathy can occur in the distal tubules from the increased free light chains circulating and combining with Tamm-Horsfall protein, which in turn causes obstruction and local inflammation,12 leading to a rise in creatinine levels and resulting in acute kidney injury,12 as in our patient.
TREATMENT CONSIDERATIONS IN MULTIPLE MYELOMA
Our patient was referred to an oncologist for management.
In the management of multiple myeloma, the patient’s quality of life needs to be considered. With the development of new agents to combat the damages of the osteolytic effects, there is hope for improving quality of life.13,14 New agents under study include anabolic agents such as antisclerostin and anti-Dickkopf-1, which promote osteoblastogenesis, leading to bone formation, with the possibility of repairing existing damage.15
TAKE-HOME POINTS
- If hypercalcemia is mild to moderate, consider primary hyperparathyroidism.
- Identify patients with severe symptoms of hypercalcemia such as volume depletion, acute kidney injury, arrhythmia, or seizures.
- Confirm severe cases of hypercalcemia and treat severe cases effectively.
- Severe hypercalcemia may need further investigation into a potential underlying malignancy.
- Sternlicht H, Glezerman IG. Hypercalcemia of malignancy and new treatment options. Ther Clin Risk Manag 2015; 11:1779–1788. doi:10.2147/TCRM.S83681
- Ahmed R, Hashiba K. Reliability of QT intervals as indicators of clinical hypercalcemia. Clin Cardiol 1988; 11(6):395–400. doi:10.1002/clc.4960110607
- Bilezikian JP, Cusano NE, Khan AA, Liu JM, Marcocci C, Bandeira F. Primary hyperparathyroidism. Nat Rev Dis Primers 2016; 2:16033. doi:10.1038/nrdp.2016.33
- Kuchay MS, Kaur P, Mishra SK, Mithal A. The changing profile of hypercalcemia in a tertiary care setting in North India: an 18-month retrospective study. Clin Cases Miner Bone Metab 2017; 14(2):131–135. doi:10.11138/ccmbm/2017.14.1.131
- Rosner MH, Dalkin AC. Onco-nephrology: the pathophysiology and treatment of malignancy-associated hypercalcemia. Clin J Am Soc Nephrol 2012; 7(10):1722–1729. doi:10.2215/CJN.02470312
- Ai L, Mu S, Hu Y. Prognostic role of RDW in hematological malignancies: a systematic review and meta-analysis. Cancer Cell Int 2018; 18:61. doi:10.1186/s12935-018-0558-3
- Baz R, Alemany C, Green R, Hussein MA. Prevalence of vitamin B12 deficiency in patients with plasma cell dyscrasias: a retrospective review. Cancer 2004; 101(4):790–795. doi:10.1002/cncr.20441
- Elmore JG, Carney PA, Abraham LA, et al. The association between obesity and screening mammography accuracy. Arch Intern Med 2004; 164(10):1140–1147. doi:10.1001/archinte.164.10.1140
- Gerecke C, Fuhrmann S, Strifler S, Schmidt-Hieber M, Einsele H, Knop S. The diagnosis and treatment of multiple myeloma. Dtsch Arztebl Int 2016; 113(27–28):470–476. doi:10.3238/arztebl.2016.0470
- Rajkumar SV. Multiple myeloma: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol 2016; 91(7):719–734. doi:10.1002/ajh.24402
- Silbermann R, Roodman GD. Myeloma bone disease: pathophysiology and management. J Bone Oncol 2013; 2(2):59–69. doi:10.1016/j.jbo.2013.04.001
- Doshi M, Lahoti A, Danesh FR, Batuman V, Sanders PW; American Society of Nephrology Onco-Nephrology Forum. Paraprotein-related kidney disease: kidney injury from paraproteins—what determines the site of injury? Clin J Am Soc Nephrol 2016; 11(12):2288–2294. doi:10.2215/CJN.02560316
- Reece D. Update on the initial therapy of multiple myeloma. Am Soc Clin Oncol Educ Book 2013. doi:10.1200/EdBook_AM.2013.33.e307
- Nishida H. Bone-targeted agents in multiple myeloma. Hematol Rep 2018; 10(1):7401. doi:10.4081/hr.2018.7401
- Ring ES, Lawson MA, Snowden JA, Jolley I, Chantry AD. New agents in the treatment of myeloma bone disease. Calcif Tissue Int 2018; 102(2):196–209. doi:10.1007/s00223-017-0351-7
A morbidly obese 54-year-old woman presented to the emergency department after experiencing generalized abdominal pain for 3 days. She rated the pain as 5 on a scale of 10 and described it as dull, cramping, waxing and waning, not radiating, and not relieved with changes of position—in fact, not alleviated by anything she had tried. Her pain was associated with nausea and 1 episode of vomiting. She also experienced constipation before the onset of pain.
She denied recent trauma, recent travel, diarrhea, fevers, weakness, shortness of breath, chest pain, other muscle pains, or recent changes in diet. She also denied having this pain in the past. She said she had unintentionally lost some weight but was not certain how much. She denied tobacco, alcohol, or illicit drug use. She had no history of surgery.
Her medical history included hypertension, anemia, and uterine fibroids. Her current medications included losartan, hydrochlorothiazide, and albuterol. She had no family history of significant disease.
INITIAL EVALUATION AND MANAGEMENT
On admission, her temperature was 97.8°F (36.6°C), heart rate 100 beats per minute, blood pressure 136/64 mm Hg, respiratory rate 18 breaths per minute, oxygen saturation 97% on room air, weight 130.6 kg, and body mass index 35 kg/m2.
She was alert and oriented to person, place, and time. She was in mild discomfort but no distress. Her lungs were clear to auscultation, with no wheezing or crackles. Heart rate and rhythm were regular, with no extra heart sounds or murmurs. Bowel sounds were normal in all 4 quadrants, with tenderness to palpation of the epigastric area, but with no guarding or rebound tenderness.
Laboratory test results
Notable results of blood testing at presentation were as follows:
- Hemoglobin 8.2 g/dL (reference range 12.3–15.3)
- Hematocrit 26% (41–50)
- Mean corpuscular volume 107 fL (80–100)
- Blood urea nitrogen 33 mg/dL (8–21); 6 months earlier it was 16
- Serum creatinine 3.6 mg/dL (0.58–0.96); 6 months earlier, it was 0.75
- Albumin 3.3 g/dL (3.5–5)
- Calcium 18.4 mg/dL (8.4–10.2); 6 months earlier, it was 9.6
- Corrected calcium 19 mg/dL.
Findings on imaging, electrocardiography
Chest radiography showed no acute cardiopulmonary abnormalities. Abdominal computed tomography without contrast showed no abnormalities within the pancreas and no evidence of inflammation or obstruction. Electrocardiography showed sinus tachycardia.
DIFFERENTIAL DIAGNOSIS
1. Which is the most likely cause of this patient’s symptoms?
- Primary hyperparathyroidism
- Malignancy
- Her drug therapy
- Familial hypercalcemic hypocalciuria
In total, her laboratory results were consistent with macrocytic anemia, severe hypercalcemia, and acute kidney injury, and she had generalized symptoms.
Primary hyperparathyroidism
A main cause of hypercalcemia is primary hyperparathyroidism, and this needs to be ruled out. Benign adenomas are the most common cause of primary hyperparathyroidism, and a risk factor for benign adenoma is exposure to therapeutic levels of radiation.3
In hyperparathyroidism, there is an increased secretion of parathyroid hormone (PTH), which has multiple effects including increased reabsorption of calcium from the urine, increased excretion of phosphate, and increased expression of 1,25-hydroxyvitamin D hydroxylase to activate vitamin D. PTH also stimulates osteoclasts to increase their expression of receptor activator of nuclear factor kappa B ligand (RANKL), which has a downstream effect on osteoclast precursors to cause bone reabsorption.3
Inherited primary hyperparathyroidism tends to present at a younger age, with multiple overactive parathyroid glands.3 Given our patient’s age, inherited primary hyparathyroidism is thus less likely.
Malignancy
The probability that malignancy is causing the hypercalcemia increases with calcium levels greater than 13 mg/dL. Epidemiologically, in hospitalized patients with hypercalcemia, the source tends to be malignancy.4 Typically, patients who develop hypercalcemia from malignancy have a worse prognosis.5
Solid tumors and leukemias can cause hypercalcemia. The mechanisms include humoral factors secreted by the malignancy, local osteolysis due to tumor invasion of bone, and excessive absorption of calcium due to excess vitamin D produced by malignancies.5 The cancers that most frequently cause an increase in calcium resorption are lung cancer, renal cancer, breast cancer, and multiple myeloma.1
Solid tumors with no bone metastasis and non-Hodgkin lymphoma that release PTH-related protein (PTHrP) cause humoral hypercalcemia in malignancy. The patient is typically in an advanced stage of disease. PTHrP increases serum calcium levels by decreasing the kidney’s ability to excrete calcium and by increasing bone turnover. It has no effect on intestinal absorption because of its inability to stimulate activated vitamin D3. Thus, the increase in systemic calcium comes directly from breakdown of bone and inability to excrete the excess.
PTHrP has a unique role in breast cancer: it is released locally in areas where cancer cells have metastasized to bone, but it does not cause a systemic effect. Bone resorption occurs in areas of metastasis and results from an increase in expression of RANKL and RANK in osteoclasts in response to the effects of PTHrP, leading to an increase in the production of osteoclastic cells.1
Tamoxifen, an endocrine therapy often used in breast cancer, also causes a release of bone-reabsorbing factors from tumor cells, which can partially contribute to hypercalcemia.5
Myeloma cells secrete RANKL, which stimulates osteoclastic activity, and they also release interleukin 6 (IL-6) and activating macrophage inflammatory protein alpha. Serum testing usually shows low or normal intact PTH, PTHrP, and 1,25-dihydroxyvitamin D.1
Patients with multiple myeloma have a worse prognosis if they have a high red blood cell distribution width, a condition shown to correlate with malnutrition, leading to deficiencies in vitamin B12 and to poor response to treatment.6 Up to 14% of patients with multiple myeloma have vitamin B12 deficiency.7
Our patient’s recent weight loss and severe hypercalcemia raise suspicion of malignancy. Further, her obesity makes proper routine breast examination difficult and thus increases the chance of undiagnosed breast cancer.8 Her decrease in renal function and her anemia complicated by hypercalcemia also raise suspicion of multiple myeloma.
Hypercalcemia due to drug therapy
Thiazide diuretics, lithium, teriparatide, and vitamin A in excessive amounts can raise the serum calcium concentration.5 Our patient was taking a thiazide for hypertension, but her extremely high calcium level places drug-induced hypercalcemia as the sole cause lower on the differential list.
Familial hypercalcemic hypocalciuria
Familial hypercalcemic hypocalciuria is a rare autosomal-dominant cause of hypercalcemia in which the ability of the body (and especially the kidneys) to sense levels of calcium is impaired, leading to a decrease in excretion of calcium in the urine.3 Very high calcium levels are rare in hypercalcemic hypocalciuria.3 In our patient with a corrected calcium concentration of nearly 19 mg/dL, familial hypercalcemic hypocalciuria is very unlikely to be the cause of the hypercalcemia.
WHAT ARE THE NEXT STEPS IN THE WORKUP?
As hypercalcemia has been confirmed, the intact PTH level should be checked to determine whether the patient’s condition is PTH-mediated. If the PTH level is in the upper range of normal or is minimally elevated, primary hyperparathyroidism is likely. Elevated PTH confirms primary hyperparathyroidism. A low-normal or low intact PTH confirms a non-PTH-mediated process, and once this is confirmed, PTHrP levels should be checked. An elevated PTHrP suggests humoral hypercalcemia of malignancy. Serum protein electrophoresis, urine protein electrophoresis, and a serum light chain assay should be performed to rule out multiple myeloma.
Vitamin D toxicity is associated with high concentrations of 1,25-dihydroxyvitamin D and 25-hydroxyvitamin D metabolites. These levels should be checked in this patient.
Other disorders that cause hypercalcemia are vitamin A toxicity and hyperthyroidism, so vitamin A and thyroid-stimulating hormone levels should also be checked.5
CASE CONTINUED
After further questioning, the patient said that she had had lower back pain about 1 to 2 weeks before coming to the emergency room; her primary care doctor had said the pain was likely from muscle strain. The pain had almost resolved but was still present.
The results of further laboratory testing were as follows:
- Serum PTH 11 pg/mL (15–65)
- PTHrP 3.4 pmol/L (< 2.0)
- Protein electrophoresis showed a monoclonal (M) spike of 0.2 g/dL (0)
- Activated vitamin D < 5 ng/mL (19.9–79.3)
- Vitamin A 7.2 mg/dL (33.1–100)
- Vitamin B12 194 pg/mL (239–931)
- Thyroid-stimulating hormone 1.21 mIU/ L (0.47–4.68
- Free thyroxine 1.27 ng/dL (0.78–2.19)
- Iron 103 µg/dL (37–170)
- Total iron-binding capacity 335 µg/dL (265–497)
- Transferrin 248 mg/dL (206–381)
- Ferritin 66 ng/mL (11.1–264)
- Urine protein (random) 100 mg/dL (0–20)
- Urine microalbumin (random) 5.9 mg/dL (0–1.6)
- Urine creatinine clearance 88.5 mL/min (88–128)
- Urine albumin-creatinine ratio 66.66 mg/g (< 30).
Imaging reports
A nuclear bone scan showed increased bone uptake in the hip and both shoulders, consistent with arthritis, and increased activity in 2 of the lower left ribs, associated with rib fractures secondary to lytic lesions. A skeletal survey at a later date showed multiple well-circumscribed “punched-out” lytic lesions in both forearms and both femurs.
2. What should be the next step in this patient’s management?
- Intravenous (IV) fluids
- Calcitonin
- Bisphosphonate treatment
- Denosumab
- Hemodialysis
Initial treatment of severe hypercalcemia includes the following:
Start IV isotonic fluids at a rate of 150 mL/h (if the patient is making urine) to maintain urine output at more than 100 mL/h. Closely monitor urine output.
Give calcitonin 4 IU/kg in combination with IV fluids to reduce calcium levels within the first 12 to 48 hours of treatment.
Give a bisphosphonate, eg, zoledronic acid 4 mg over 15 minutes, or pamidronate 60 to 90 mg over 2 hours. Zoledronic acid is preferred in malignancy-induced hypercalcemia because it is more potent. Doses should be adjusted in patients with renal failure.
Give denosumab if hypercalcemia is refractory to bisphosphonates, or when bisphosphonates cannot be used in renal failure.9
Hemodialysis is performed in patients who have significant neurologic symptoms irrespective of acute renal insufficiency.
Our patient was started on 0.9% sodium chloride at a rate of 150 mL/h for severe hypercalcemia. Zoledronic acid 4 mg IV was given once. These measures lowered her calcium level and lessened her acute kidney injury.
ADDITIONAL FINDINGS
Urine testing was positive for Bence Jones protein. Immune electrophoresis, performed because of suspicion of multiple myeloma, showed an elevated level of kappa light chains at 806.7 mg/dL (0.33–1.94) and normal lambda light chains at 0.62 mg/dL (0.57–2.63). The immunoglobulin G level was low at 496 mg/dL (610–1,660). In patients with severe hypercalcemia, these results point to a diagnosis of malignancy. Bone marrow aspiration study showed greater than 10% plasma cells, confirming multiple myeloma.
MULTIPLE MYELOMA
The diagnosis of multiple myeloma is based in part on the presence of 10% or more of clonal bone marrow plasma cells10 and of specific end-organ damage (anemia, hypercalcemia, renal insufficiency, or bone lesions).9
Bone marrow clonality can be shown by the ratio of kappa to lambda light chains as detected with immunohistochemistry, immunofluorescence, or flow cytometry.11 The normal ratio is 0.26 to 1.65 for a patient with normal kidney function. In this patient, however, the ratio was 1,301.08 (806.67 kappa to 0.62 lambda), which was extremely out of range. The patient’s bone marrow biopsy results revealed the presence of 15% clonal bone marrow plasma cells.
Multiple myeloma causes osteolytic lesions through increased activation of osteoclast activating factor that stimulates the growth of osteoclast precursors. At the same time, it inhibits osteoblast formation via multiple pathways, including the action of sclerostin.11 Our patient had lytic lesions in 2 left lower ribs and in both forearms and femurs.
Hypercalcemia in multiple myeloma is attributed to 2 main factors: bone breakdown and macrophage overactivation. Multiple myeloma cells increase the release of macrophage inflammatory protein 1-alpha and tumor necrosis factor, which are inflammatory proteins that cause an increase in macrophages, which cause an increase in calcitriol.11 As noted, our patient’s calcium level at presentation was 18.4 mg/dL uncorrected and 18.96 mg/dL corrected.
Cast nephropathy can occur in the distal tubules from the increased free light chains circulating and combining with Tamm-Horsfall protein, which in turn causes obstruction and local inflammation,12 leading to a rise in creatinine levels and resulting in acute kidney injury,12 as in our patient.
TREATMENT CONSIDERATIONS IN MULTIPLE MYELOMA
Our patient was referred to an oncologist for management.
In the management of multiple myeloma, the patient’s quality of life needs to be considered. With the development of new agents to combat the damages of the osteolytic effects, there is hope for improving quality of life.13,14 New agents under study include anabolic agents such as antisclerostin and anti-Dickkopf-1, which promote osteoblastogenesis, leading to bone formation, with the possibility of repairing existing damage.15
TAKE-HOME POINTS
- If hypercalcemia is mild to moderate, consider primary hyperparathyroidism.
- Identify patients with severe symptoms of hypercalcemia such as volume depletion, acute kidney injury, arrhythmia, or seizures.
- Confirm severe cases of hypercalcemia and treat severe cases effectively.
- Severe hypercalcemia may need further investigation into a potential underlying malignancy.
A morbidly obese 54-year-old woman presented to the emergency department after experiencing generalized abdominal pain for 3 days. She rated the pain as 5 on a scale of 10 and described it as dull, cramping, waxing and waning, not radiating, and not relieved with changes of position—in fact, not alleviated by anything she had tried. Her pain was associated with nausea and 1 episode of vomiting. She also experienced constipation before the onset of pain.
She denied recent trauma, recent travel, diarrhea, fevers, weakness, shortness of breath, chest pain, other muscle pains, or recent changes in diet. She also denied having this pain in the past. She said she had unintentionally lost some weight but was not certain how much. She denied tobacco, alcohol, or illicit drug use. She had no history of surgery.
Her medical history included hypertension, anemia, and uterine fibroids. Her current medications included losartan, hydrochlorothiazide, and albuterol. She had no family history of significant disease.
INITIAL EVALUATION AND MANAGEMENT
On admission, her temperature was 97.8°F (36.6°C), heart rate 100 beats per minute, blood pressure 136/64 mm Hg, respiratory rate 18 breaths per minute, oxygen saturation 97% on room air, weight 130.6 kg, and body mass index 35 kg/m2.
She was alert and oriented to person, place, and time. She was in mild discomfort but no distress. Her lungs were clear to auscultation, with no wheezing or crackles. Heart rate and rhythm were regular, with no extra heart sounds or murmurs. Bowel sounds were normal in all 4 quadrants, with tenderness to palpation of the epigastric area, but with no guarding or rebound tenderness.
Laboratory test results
Notable results of blood testing at presentation were as follows:
- Hemoglobin 8.2 g/dL (reference range 12.3–15.3)
- Hematocrit 26% (41–50)
- Mean corpuscular volume 107 fL (80–100)
- Blood urea nitrogen 33 mg/dL (8–21); 6 months earlier it was 16
- Serum creatinine 3.6 mg/dL (0.58–0.96); 6 months earlier, it was 0.75
- Albumin 3.3 g/dL (3.5–5)
- Calcium 18.4 mg/dL (8.4–10.2); 6 months earlier, it was 9.6
- Corrected calcium 19 mg/dL.
Findings on imaging, electrocardiography
Chest radiography showed no acute cardiopulmonary abnormalities. Abdominal computed tomography without contrast showed no abnormalities within the pancreas and no evidence of inflammation or obstruction. Electrocardiography showed sinus tachycardia.
DIFFERENTIAL DIAGNOSIS
1. Which is the most likely cause of this patient’s symptoms?
- Primary hyperparathyroidism
- Malignancy
- Her drug therapy
- Familial hypercalcemic hypocalciuria
In total, her laboratory results were consistent with macrocytic anemia, severe hypercalcemia, and acute kidney injury, and she had generalized symptoms.
Primary hyperparathyroidism
A main cause of hypercalcemia is primary hyperparathyroidism, and this needs to be ruled out. Benign adenomas are the most common cause of primary hyperparathyroidism, and a risk factor for benign adenoma is exposure to therapeutic levels of radiation.3
In hyperparathyroidism, there is an increased secretion of parathyroid hormone (PTH), which has multiple effects including increased reabsorption of calcium from the urine, increased excretion of phosphate, and increased expression of 1,25-hydroxyvitamin D hydroxylase to activate vitamin D. PTH also stimulates osteoclasts to increase their expression of receptor activator of nuclear factor kappa B ligand (RANKL), which has a downstream effect on osteoclast precursors to cause bone reabsorption.3
Inherited primary hyperparathyroidism tends to present at a younger age, with multiple overactive parathyroid glands.3 Given our patient’s age, inherited primary hyparathyroidism is thus less likely.
Malignancy
The probability that malignancy is causing the hypercalcemia increases with calcium levels greater than 13 mg/dL. Epidemiologically, in hospitalized patients with hypercalcemia, the source tends to be malignancy.4 Typically, patients who develop hypercalcemia from malignancy have a worse prognosis.5
Solid tumors and leukemias can cause hypercalcemia. The mechanisms include humoral factors secreted by the malignancy, local osteolysis due to tumor invasion of bone, and excessive absorption of calcium due to excess vitamin D produced by malignancies.5 The cancers that most frequently cause an increase in calcium resorption are lung cancer, renal cancer, breast cancer, and multiple myeloma.1
Solid tumors with no bone metastasis and non-Hodgkin lymphoma that release PTH-related protein (PTHrP) cause humoral hypercalcemia in malignancy. The patient is typically in an advanced stage of disease. PTHrP increases serum calcium levels by decreasing the kidney’s ability to excrete calcium and by increasing bone turnover. It has no effect on intestinal absorption because of its inability to stimulate activated vitamin D3. Thus, the increase in systemic calcium comes directly from breakdown of bone and inability to excrete the excess.
PTHrP has a unique role in breast cancer: it is released locally in areas where cancer cells have metastasized to bone, but it does not cause a systemic effect. Bone resorption occurs in areas of metastasis and results from an increase in expression of RANKL and RANK in osteoclasts in response to the effects of PTHrP, leading to an increase in the production of osteoclastic cells.1
Tamoxifen, an endocrine therapy often used in breast cancer, also causes a release of bone-reabsorbing factors from tumor cells, which can partially contribute to hypercalcemia.5
Myeloma cells secrete RANKL, which stimulates osteoclastic activity, and they also release interleukin 6 (IL-6) and activating macrophage inflammatory protein alpha. Serum testing usually shows low or normal intact PTH, PTHrP, and 1,25-dihydroxyvitamin D.1
Patients with multiple myeloma have a worse prognosis if they have a high red blood cell distribution width, a condition shown to correlate with malnutrition, leading to deficiencies in vitamin B12 and to poor response to treatment.6 Up to 14% of patients with multiple myeloma have vitamin B12 deficiency.7
Our patient’s recent weight loss and severe hypercalcemia raise suspicion of malignancy. Further, her obesity makes proper routine breast examination difficult and thus increases the chance of undiagnosed breast cancer.8 Her decrease in renal function and her anemia complicated by hypercalcemia also raise suspicion of multiple myeloma.
Hypercalcemia due to drug therapy
Thiazide diuretics, lithium, teriparatide, and vitamin A in excessive amounts can raise the serum calcium concentration.5 Our patient was taking a thiazide for hypertension, but her extremely high calcium level places drug-induced hypercalcemia as the sole cause lower on the differential list.
Familial hypercalcemic hypocalciuria
Familial hypercalcemic hypocalciuria is a rare autosomal-dominant cause of hypercalcemia in which the ability of the body (and especially the kidneys) to sense levels of calcium is impaired, leading to a decrease in excretion of calcium in the urine.3 Very high calcium levels are rare in hypercalcemic hypocalciuria.3 In our patient with a corrected calcium concentration of nearly 19 mg/dL, familial hypercalcemic hypocalciuria is very unlikely to be the cause of the hypercalcemia.
WHAT ARE THE NEXT STEPS IN THE WORKUP?
As hypercalcemia has been confirmed, the intact PTH level should be checked to determine whether the patient’s condition is PTH-mediated. If the PTH level is in the upper range of normal or is minimally elevated, primary hyperparathyroidism is likely. Elevated PTH confirms primary hyperparathyroidism. A low-normal or low intact PTH confirms a non-PTH-mediated process, and once this is confirmed, PTHrP levels should be checked. An elevated PTHrP suggests humoral hypercalcemia of malignancy. Serum protein electrophoresis, urine protein electrophoresis, and a serum light chain assay should be performed to rule out multiple myeloma.
Vitamin D toxicity is associated with high concentrations of 1,25-dihydroxyvitamin D and 25-hydroxyvitamin D metabolites. These levels should be checked in this patient.
Other disorders that cause hypercalcemia are vitamin A toxicity and hyperthyroidism, so vitamin A and thyroid-stimulating hormone levels should also be checked.5
CASE CONTINUED
After further questioning, the patient said that she had had lower back pain about 1 to 2 weeks before coming to the emergency room; her primary care doctor had said the pain was likely from muscle strain. The pain had almost resolved but was still present.
The results of further laboratory testing were as follows:
- Serum PTH 11 pg/mL (15–65)
- PTHrP 3.4 pmol/L (< 2.0)
- Protein electrophoresis showed a monoclonal (M) spike of 0.2 g/dL (0)
- Activated vitamin D < 5 ng/mL (19.9–79.3)
- Vitamin A 7.2 mg/dL (33.1–100)
- Vitamin B12 194 pg/mL (239–931)
- Thyroid-stimulating hormone 1.21 mIU/ L (0.47–4.68
- Free thyroxine 1.27 ng/dL (0.78–2.19)
- Iron 103 µg/dL (37–170)
- Total iron-binding capacity 335 µg/dL (265–497)
- Transferrin 248 mg/dL (206–381)
- Ferritin 66 ng/mL (11.1–264)
- Urine protein (random) 100 mg/dL (0–20)
- Urine microalbumin (random) 5.9 mg/dL (0–1.6)
- Urine creatinine clearance 88.5 mL/min (88–128)
- Urine albumin-creatinine ratio 66.66 mg/g (< 30).
Imaging reports
A nuclear bone scan showed increased bone uptake in the hip and both shoulders, consistent with arthritis, and increased activity in 2 of the lower left ribs, associated with rib fractures secondary to lytic lesions. A skeletal survey at a later date showed multiple well-circumscribed “punched-out” lytic lesions in both forearms and both femurs.
2. What should be the next step in this patient’s management?
- Intravenous (IV) fluids
- Calcitonin
- Bisphosphonate treatment
- Denosumab
- Hemodialysis
Initial treatment of severe hypercalcemia includes the following:
Start IV isotonic fluids at a rate of 150 mL/h (if the patient is making urine) to maintain urine output at more than 100 mL/h. Closely monitor urine output.
Give calcitonin 4 IU/kg in combination with IV fluids to reduce calcium levels within the first 12 to 48 hours of treatment.
Give a bisphosphonate, eg, zoledronic acid 4 mg over 15 minutes, or pamidronate 60 to 90 mg over 2 hours. Zoledronic acid is preferred in malignancy-induced hypercalcemia because it is more potent. Doses should be adjusted in patients with renal failure.
Give denosumab if hypercalcemia is refractory to bisphosphonates, or when bisphosphonates cannot be used in renal failure.9
Hemodialysis is performed in patients who have significant neurologic symptoms irrespective of acute renal insufficiency.
Our patient was started on 0.9% sodium chloride at a rate of 150 mL/h for severe hypercalcemia. Zoledronic acid 4 mg IV was given once. These measures lowered her calcium level and lessened her acute kidney injury.
ADDITIONAL FINDINGS
Urine testing was positive for Bence Jones protein. Immune electrophoresis, performed because of suspicion of multiple myeloma, showed an elevated level of kappa light chains at 806.7 mg/dL (0.33–1.94) and normal lambda light chains at 0.62 mg/dL (0.57–2.63). The immunoglobulin G level was low at 496 mg/dL (610–1,660). In patients with severe hypercalcemia, these results point to a diagnosis of malignancy. Bone marrow aspiration study showed greater than 10% plasma cells, confirming multiple myeloma.
MULTIPLE MYELOMA
The diagnosis of multiple myeloma is based in part on the presence of 10% or more of clonal bone marrow plasma cells10 and of specific end-organ damage (anemia, hypercalcemia, renal insufficiency, or bone lesions).9
Bone marrow clonality can be shown by the ratio of kappa to lambda light chains as detected with immunohistochemistry, immunofluorescence, or flow cytometry.11 The normal ratio is 0.26 to 1.65 for a patient with normal kidney function. In this patient, however, the ratio was 1,301.08 (806.67 kappa to 0.62 lambda), which was extremely out of range. The patient’s bone marrow biopsy results revealed the presence of 15% clonal bone marrow plasma cells.
Multiple myeloma causes osteolytic lesions through increased activation of osteoclast activating factor that stimulates the growth of osteoclast precursors. At the same time, it inhibits osteoblast formation via multiple pathways, including the action of sclerostin.11 Our patient had lytic lesions in 2 left lower ribs and in both forearms and femurs.
Hypercalcemia in multiple myeloma is attributed to 2 main factors: bone breakdown and macrophage overactivation. Multiple myeloma cells increase the release of macrophage inflammatory protein 1-alpha and tumor necrosis factor, which are inflammatory proteins that cause an increase in macrophages, which cause an increase in calcitriol.11 As noted, our patient’s calcium level at presentation was 18.4 mg/dL uncorrected and 18.96 mg/dL corrected.
Cast nephropathy can occur in the distal tubules from the increased free light chains circulating and combining with Tamm-Horsfall protein, which in turn causes obstruction and local inflammation,12 leading to a rise in creatinine levels and resulting in acute kidney injury,12 as in our patient.
TREATMENT CONSIDERATIONS IN MULTIPLE MYELOMA
Our patient was referred to an oncologist for management.
In the management of multiple myeloma, the patient’s quality of life needs to be considered. With the development of new agents to combat the damages of the osteolytic effects, there is hope for improving quality of life.13,14 New agents under study include anabolic agents such as antisclerostin and anti-Dickkopf-1, which promote osteoblastogenesis, leading to bone formation, with the possibility of repairing existing damage.15
TAKE-HOME POINTS
- If hypercalcemia is mild to moderate, consider primary hyperparathyroidism.
- Identify patients with severe symptoms of hypercalcemia such as volume depletion, acute kidney injury, arrhythmia, or seizures.
- Confirm severe cases of hypercalcemia and treat severe cases effectively.
- Severe hypercalcemia may need further investigation into a potential underlying malignancy.
- Sternlicht H, Glezerman IG. Hypercalcemia of malignancy and new treatment options. Ther Clin Risk Manag 2015; 11:1779–1788. doi:10.2147/TCRM.S83681
- Ahmed R, Hashiba K. Reliability of QT intervals as indicators of clinical hypercalcemia. Clin Cardiol 1988; 11(6):395–400. doi:10.1002/clc.4960110607
- Bilezikian JP, Cusano NE, Khan AA, Liu JM, Marcocci C, Bandeira F. Primary hyperparathyroidism. Nat Rev Dis Primers 2016; 2:16033. doi:10.1038/nrdp.2016.33
- Kuchay MS, Kaur P, Mishra SK, Mithal A. The changing profile of hypercalcemia in a tertiary care setting in North India: an 18-month retrospective study. Clin Cases Miner Bone Metab 2017; 14(2):131–135. doi:10.11138/ccmbm/2017.14.1.131
- Rosner MH, Dalkin AC. Onco-nephrology: the pathophysiology and treatment of malignancy-associated hypercalcemia. Clin J Am Soc Nephrol 2012; 7(10):1722–1729. doi:10.2215/CJN.02470312
- Ai L, Mu S, Hu Y. Prognostic role of RDW in hematological malignancies: a systematic review and meta-analysis. Cancer Cell Int 2018; 18:61. doi:10.1186/s12935-018-0558-3
- Baz R, Alemany C, Green R, Hussein MA. Prevalence of vitamin B12 deficiency in patients with plasma cell dyscrasias: a retrospective review. Cancer 2004; 101(4):790–795. doi:10.1002/cncr.20441
- Elmore JG, Carney PA, Abraham LA, et al. The association between obesity and screening mammography accuracy. Arch Intern Med 2004; 164(10):1140–1147. doi:10.1001/archinte.164.10.1140
- Gerecke C, Fuhrmann S, Strifler S, Schmidt-Hieber M, Einsele H, Knop S. The diagnosis and treatment of multiple myeloma. Dtsch Arztebl Int 2016; 113(27–28):470–476. doi:10.3238/arztebl.2016.0470
- Rajkumar SV. Multiple myeloma: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol 2016; 91(7):719–734. doi:10.1002/ajh.24402
- Silbermann R, Roodman GD. Myeloma bone disease: pathophysiology and management. J Bone Oncol 2013; 2(2):59–69. doi:10.1016/j.jbo.2013.04.001
- Doshi M, Lahoti A, Danesh FR, Batuman V, Sanders PW; American Society of Nephrology Onco-Nephrology Forum. Paraprotein-related kidney disease: kidney injury from paraproteins—what determines the site of injury? Clin J Am Soc Nephrol 2016; 11(12):2288–2294. doi:10.2215/CJN.02560316
- Reece D. Update on the initial therapy of multiple myeloma. Am Soc Clin Oncol Educ Book 2013. doi:10.1200/EdBook_AM.2013.33.e307
- Nishida H. Bone-targeted agents in multiple myeloma. Hematol Rep 2018; 10(1):7401. doi:10.4081/hr.2018.7401
- Ring ES, Lawson MA, Snowden JA, Jolley I, Chantry AD. New agents in the treatment of myeloma bone disease. Calcif Tissue Int 2018; 102(2):196–209. doi:10.1007/s00223-017-0351-7
- Sternlicht H, Glezerman IG. Hypercalcemia of malignancy and new treatment options. Ther Clin Risk Manag 2015; 11:1779–1788. doi:10.2147/TCRM.S83681
- Ahmed R, Hashiba K. Reliability of QT intervals as indicators of clinical hypercalcemia. Clin Cardiol 1988; 11(6):395–400. doi:10.1002/clc.4960110607
- Bilezikian JP, Cusano NE, Khan AA, Liu JM, Marcocci C, Bandeira F. Primary hyperparathyroidism. Nat Rev Dis Primers 2016; 2:16033. doi:10.1038/nrdp.2016.33
- Kuchay MS, Kaur P, Mishra SK, Mithal A. The changing profile of hypercalcemia in a tertiary care setting in North India: an 18-month retrospective study. Clin Cases Miner Bone Metab 2017; 14(2):131–135. doi:10.11138/ccmbm/2017.14.1.131
- Rosner MH, Dalkin AC. Onco-nephrology: the pathophysiology and treatment of malignancy-associated hypercalcemia. Clin J Am Soc Nephrol 2012; 7(10):1722–1729. doi:10.2215/CJN.02470312
- Ai L, Mu S, Hu Y. Prognostic role of RDW in hematological malignancies: a systematic review and meta-analysis. Cancer Cell Int 2018; 18:61. doi:10.1186/s12935-018-0558-3
- Baz R, Alemany C, Green R, Hussein MA. Prevalence of vitamin B12 deficiency in patients with plasma cell dyscrasias: a retrospective review. Cancer 2004; 101(4):790–795. doi:10.1002/cncr.20441
- Elmore JG, Carney PA, Abraham LA, et al. The association between obesity and screening mammography accuracy. Arch Intern Med 2004; 164(10):1140–1147. doi:10.1001/archinte.164.10.1140
- Gerecke C, Fuhrmann S, Strifler S, Schmidt-Hieber M, Einsele H, Knop S. The diagnosis and treatment of multiple myeloma. Dtsch Arztebl Int 2016; 113(27–28):470–476. doi:10.3238/arztebl.2016.0470
- Rajkumar SV. Multiple myeloma: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol 2016; 91(7):719–734. doi:10.1002/ajh.24402
- Silbermann R, Roodman GD. Myeloma bone disease: pathophysiology and management. J Bone Oncol 2013; 2(2):59–69. doi:10.1016/j.jbo.2013.04.001
- Doshi M, Lahoti A, Danesh FR, Batuman V, Sanders PW; American Society of Nephrology Onco-Nephrology Forum. Paraprotein-related kidney disease: kidney injury from paraproteins—what determines the site of injury? Clin J Am Soc Nephrol 2016; 11(12):2288–2294. doi:10.2215/CJN.02560316
- Reece D. Update on the initial therapy of multiple myeloma. Am Soc Clin Oncol Educ Book 2013. doi:10.1200/EdBook_AM.2013.33.e307
- Nishida H. Bone-targeted agents in multiple myeloma. Hematol Rep 2018; 10(1):7401. doi:10.4081/hr.2018.7401
- Ring ES, Lawson MA, Snowden JA, Jolley I, Chantry AD. New agents in the treatment of myeloma bone disease. Calcif Tissue Int 2018; 102(2):196–209. doi:10.1007/s00223-017-0351-7
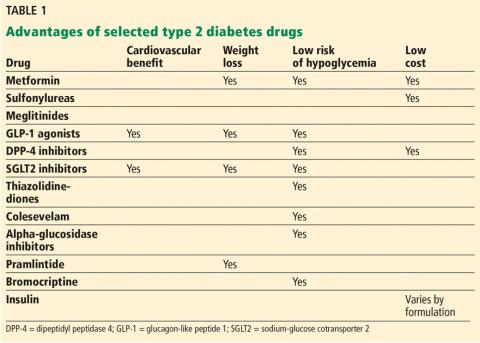
Correction: Diabetes management
Information was omitted from Table 1 on page 596 of the article, Makin V, Lansang MC. Diabetes management: beyond hemoglobin A1c (Cleve Clin J Med 2019; 86[9]:595–600, doi:10.3949/ccjm.86a.18031).
The sodium-glucose cotransporter 2 (SGLT2) inhibitors pose a low risk of hypoglyemia, and that should have been noted in the table. The corrected table appears below and online.
Information was omitted from Table 1 on page 596 of the article, Makin V, Lansang MC. Diabetes management: beyond hemoglobin A1c (Cleve Clin J Med 2019; 86[9]:595–600, doi:10.3949/ccjm.86a.18031).
The sodium-glucose cotransporter 2 (SGLT2) inhibitors pose a low risk of hypoglyemia, and that should have been noted in the table. The corrected table appears below and online.
Information was omitted from Table 1 on page 596 of the article, Makin V, Lansang MC. Diabetes management: beyond hemoglobin A1c (Cleve Clin J Med 2019; 86[9]:595–600, doi:10.3949/ccjm.86a.18031).
The sodium-glucose cotransporter 2 (SGLT2) inhibitors pose a low risk of hypoglyemia, and that should have been noted in the table. The corrected table appears below and online.
Lifestyle program improves chance of spontaneous conception for women with obesity
PHILADELPHIA – Women with obesity who underwent a lifestyle program targeting healthy eating and physical activity were significantly more likely to achieve pregnancy or become spontaneously pregnant, Jean-Patrice Baillargeon, MD, MSc, reported at the annual meeting of the American Society for Reproductive Medicine.
However, women with polycystic ovary syndrome (PCOS) in the study appeared to benefit more than did women without PCOS who participated in the lifestyle program, said Dr. Baillargeon, from the University of Sherbrooke (Que.).
“ Women with PCOS seemed to benefit more from such a program,” said Dr. Baillargeon.
“These benefits occur along with small changes in weight, but important improvements in lifestyle, so lifestyle seems to be more important than weight change here,” he added.
The researchers randomized 130 women to receive the Fit-For-Fertility lifestyle program or usual care for infertility. The lifestyle program consisted of a low-intensity weekly intervention for 6 weeks in which patients met individually with a kinesiologist and nutritionist every week and also attended group sessions each week. Women in the intervention did not receive fertility treatment for the first 6 months while on the lifestyle program, and if they did not conceive during that time, they continued the program in combination with fertility treatments.
Patients were included if they were aged 18-40 years and had either infertility and a body mass index of 30 kg/m2 or greater or PCOS and a BMI of 27 kg/m2 or greater. Researchers excluded women planning to undergo bariatric surgery, women who were already undergoing another lifestyle intervention, and women with severe infertility or who had a male partner with severe infertility for whom in vitro fertilization was their only option for conceiving. Researchers collected data from patients at baseline and every 6 months up to 18 months, with additional visits for pregnant women scheduled at the beginning of pregnancy and at 26 weeks’ gestation. They collected baseline data on age, BMI, waist circumference, fat mass percentage, daily energy expenditure, and food frequency using the Healthy Eating Index (HEI).
Overall, 46 women in the intervention group and 52 women in the control group had a research visit at 6 months or pregnancy research visit at less than 6 months; of these, 33 women in the intervention group (65%) and 35 women in the control group (61%) had PCOS. At baseline, both PCOS and non-PCOS groups were similar; however, women in the PCOS intervention group had a lower BMI than did women without PCOS in the intervention group (37 kg/m2 vs. 41 kg/m2; P less than .05), while women without PCOS in the intervention group had a higher fat mass percentage than did women with PCOS in the intervention group (46% vs. 49%; P less than .05).
With regard to weight loss, there was a 2.4% reduction in weight among all patients in the intervention group, compared with the control group (P = .003), with a 2.7% reduction in weight for the PCOS group (P = .015) and a 1.8% reduction in the non-PCOS group (P = .139). However, there were no significant differences between PCOS status and the lifestyle intervention, said Dr. Baillargeon.
At 6 months, the quality of women’s diets in the combined PCOS and non-PCOS group that participated in the lifestyle program showed significant improvement, compared with control groups (HEI, 18% vs. 5%; P less than .001). The PCOS group on its own showed significant improvement with the intervention (20% vs. 4%; P less than .001), whereas women without PCOS showed a nonsignificant improvement with the intervention (14% vs. 6%; P = .055). Daily energy expenditure improved in all groups that received the intervention, compared with the control groups, but there were no significant between-group differences in energy expenditure.
When analyzing fertility outcomes at 18 months, the pregnancy rate for all patients who received lifestyle interventions was 61%, compared with 39% in the control group (P = .02; number needed to treat, 4.5). In women with PCOS, those who underwent the lifestyle intervention had a pregnancy rate of 58%, compared with 34% in the control group (P = .05; NNT, 4.3); although women without PCOS who participated in the lifestyle program had an improved pregnancy rate over women in the control group, the results were not significant (67% vs. 46%; P = .18; NNT, 4.7).
The researchers also looked at the spontaneous pregnancy rate and found women who received the intervention had nearly three times the rate of spontaneous pregnancy, compared with women in the control group (33% vs. 12%; P = .01), while women with PCOS in the lifestyle program had nearly five times the rate of spontaneous pregnancy, compared with the control group (27% vs. 6%; P = .02). Women without PCOS in the lifestyle program had nearly twice the increased likelihood of spontaneous pregnancy, but the results were not significant (44% vs. 23%; P = .15).
Women with PCOS in the lifestyle program also had a higher live birth rate, compared with women in the control group (55% vs. 31%; P = .05; NNT, 4.3). Although women without PCOS in the lifestyle program (67% vs. 46%; P = .18; NNT, 4.7) and women in the study overall experienced higher live birth rates (51% vs. 37%; P = .14; NNT, 7.0), compared with the control group, these results were not significant, said Dr. Baillargeon.
“Such lifestyle interventions in women with obesity could significantly lower costs of fertility treatments, which is important,” concluded Dr. Baillargeon.
The Fit-For-Fertility program was funded by an unrestricted grant from Ferring.
SOURCE: Baillargeon J-P, et al. ASRM 2019. Abstract O-95.
PHILADELPHIA – Women with obesity who underwent a lifestyle program targeting healthy eating and physical activity were significantly more likely to achieve pregnancy or become spontaneously pregnant, Jean-Patrice Baillargeon, MD, MSc, reported at the annual meeting of the American Society for Reproductive Medicine.
However, women with polycystic ovary syndrome (PCOS) in the study appeared to benefit more than did women without PCOS who participated in the lifestyle program, said Dr. Baillargeon, from the University of Sherbrooke (Que.).
“ Women with PCOS seemed to benefit more from such a program,” said Dr. Baillargeon.
“These benefits occur along with small changes in weight, but important improvements in lifestyle, so lifestyle seems to be more important than weight change here,” he added.
The researchers randomized 130 women to receive the Fit-For-Fertility lifestyle program or usual care for infertility. The lifestyle program consisted of a low-intensity weekly intervention for 6 weeks in which patients met individually with a kinesiologist and nutritionist every week and also attended group sessions each week. Women in the intervention did not receive fertility treatment for the first 6 months while on the lifestyle program, and if they did not conceive during that time, they continued the program in combination with fertility treatments.
Patients were included if they were aged 18-40 years and had either infertility and a body mass index of 30 kg/m2 or greater or PCOS and a BMI of 27 kg/m2 or greater. Researchers excluded women planning to undergo bariatric surgery, women who were already undergoing another lifestyle intervention, and women with severe infertility or who had a male partner with severe infertility for whom in vitro fertilization was their only option for conceiving. Researchers collected data from patients at baseline and every 6 months up to 18 months, with additional visits for pregnant women scheduled at the beginning of pregnancy and at 26 weeks’ gestation. They collected baseline data on age, BMI, waist circumference, fat mass percentage, daily energy expenditure, and food frequency using the Healthy Eating Index (HEI).
Overall, 46 women in the intervention group and 52 women in the control group had a research visit at 6 months or pregnancy research visit at less than 6 months; of these, 33 women in the intervention group (65%) and 35 women in the control group (61%) had PCOS. At baseline, both PCOS and non-PCOS groups were similar; however, women in the PCOS intervention group had a lower BMI than did women without PCOS in the intervention group (37 kg/m2 vs. 41 kg/m2; P less than .05), while women without PCOS in the intervention group had a higher fat mass percentage than did women with PCOS in the intervention group (46% vs. 49%; P less than .05).
With regard to weight loss, there was a 2.4% reduction in weight among all patients in the intervention group, compared with the control group (P = .003), with a 2.7% reduction in weight for the PCOS group (P = .015) and a 1.8% reduction in the non-PCOS group (P = .139). However, there were no significant differences between PCOS status and the lifestyle intervention, said Dr. Baillargeon.
At 6 months, the quality of women’s diets in the combined PCOS and non-PCOS group that participated in the lifestyle program showed significant improvement, compared with control groups (HEI, 18% vs. 5%; P less than .001). The PCOS group on its own showed significant improvement with the intervention (20% vs. 4%; P less than .001), whereas women without PCOS showed a nonsignificant improvement with the intervention (14% vs. 6%; P = .055). Daily energy expenditure improved in all groups that received the intervention, compared with the control groups, but there were no significant between-group differences in energy expenditure.
When analyzing fertility outcomes at 18 months, the pregnancy rate for all patients who received lifestyle interventions was 61%, compared with 39% in the control group (P = .02; number needed to treat, 4.5). In women with PCOS, those who underwent the lifestyle intervention had a pregnancy rate of 58%, compared with 34% in the control group (P = .05; NNT, 4.3); although women without PCOS who participated in the lifestyle program had an improved pregnancy rate over women in the control group, the results were not significant (67% vs. 46%; P = .18; NNT, 4.7).
The researchers also looked at the spontaneous pregnancy rate and found women who received the intervention had nearly three times the rate of spontaneous pregnancy, compared with women in the control group (33% vs. 12%; P = .01), while women with PCOS in the lifestyle program had nearly five times the rate of spontaneous pregnancy, compared with the control group (27% vs. 6%; P = .02). Women without PCOS in the lifestyle program had nearly twice the increased likelihood of spontaneous pregnancy, but the results were not significant (44% vs. 23%; P = .15).
Women with PCOS in the lifestyle program also had a higher live birth rate, compared with women in the control group (55% vs. 31%; P = .05; NNT, 4.3). Although women without PCOS in the lifestyle program (67% vs. 46%; P = .18; NNT, 4.7) and women in the study overall experienced higher live birth rates (51% vs. 37%; P = .14; NNT, 7.0), compared with the control group, these results were not significant, said Dr. Baillargeon.
“Such lifestyle interventions in women with obesity could significantly lower costs of fertility treatments, which is important,” concluded Dr. Baillargeon.
The Fit-For-Fertility program was funded by an unrestricted grant from Ferring.
SOURCE: Baillargeon J-P, et al. ASRM 2019. Abstract O-95.
PHILADELPHIA – Women with obesity who underwent a lifestyle program targeting healthy eating and physical activity were significantly more likely to achieve pregnancy or become spontaneously pregnant, Jean-Patrice Baillargeon, MD, MSc, reported at the annual meeting of the American Society for Reproductive Medicine.
However, women with polycystic ovary syndrome (PCOS) in the study appeared to benefit more than did women without PCOS who participated in the lifestyle program, said Dr. Baillargeon, from the University of Sherbrooke (Que.).
“ Women with PCOS seemed to benefit more from such a program,” said Dr. Baillargeon.
“These benefits occur along with small changes in weight, but important improvements in lifestyle, so lifestyle seems to be more important than weight change here,” he added.
The researchers randomized 130 women to receive the Fit-For-Fertility lifestyle program or usual care for infertility. The lifestyle program consisted of a low-intensity weekly intervention for 6 weeks in which patients met individually with a kinesiologist and nutritionist every week and also attended group sessions each week. Women in the intervention did not receive fertility treatment for the first 6 months while on the lifestyle program, and if they did not conceive during that time, they continued the program in combination with fertility treatments.
Patients were included if they were aged 18-40 years and had either infertility and a body mass index of 30 kg/m2 or greater or PCOS and a BMI of 27 kg/m2 or greater. Researchers excluded women planning to undergo bariatric surgery, women who were already undergoing another lifestyle intervention, and women with severe infertility or who had a male partner with severe infertility for whom in vitro fertilization was their only option for conceiving. Researchers collected data from patients at baseline and every 6 months up to 18 months, with additional visits for pregnant women scheduled at the beginning of pregnancy and at 26 weeks’ gestation. They collected baseline data on age, BMI, waist circumference, fat mass percentage, daily energy expenditure, and food frequency using the Healthy Eating Index (HEI).
Overall, 46 women in the intervention group and 52 women in the control group had a research visit at 6 months or pregnancy research visit at less than 6 months; of these, 33 women in the intervention group (65%) and 35 women in the control group (61%) had PCOS. At baseline, both PCOS and non-PCOS groups were similar; however, women in the PCOS intervention group had a lower BMI than did women without PCOS in the intervention group (37 kg/m2 vs. 41 kg/m2; P less than .05), while women without PCOS in the intervention group had a higher fat mass percentage than did women with PCOS in the intervention group (46% vs. 49%; P less than .05).
With regard to weight loss, there was a 2.4% reduction in weight among all patients in the intervention group, compared with the control group (P = .003), with a 2.7% reduction in weight for the PCOS group (P = .015) and a 1.8% reduction in the non-PCOS group (P = .139). However, there were no significant differences between PCOS status and the lifestyle intervention, said Dr. Baillargeon.
At 6 months, the quality of women’s diets in the combined PCOS and non-PCOS group that participated in the lifestyle program showed significant improvement, compared with control groups (HEI, 18% vs. 5%; P less than .001). The PCOS group on its own showed significant improvement with the intervention (20% vs. 4%; P less than .001), whereas women without PCOS showed a nonsignificant improvement with the intervention (14% vs. 6%; P = .055). Daily energy expenditure improved in all groups that received the intervention, compared with the control groups, but there were no significant between-group differences in energy expenditure.
When analyzing fertility outcomes at 18 months, the pregnancy rate for all patients who received lifestyle interventions was 61%, compared with 39% in the control group (P = .02; number needed to treat, 4.5). In women with PCOS, those who underwent the lifestyle intervention had a pregnancy rate of 58%, compared with 34% in the control group (P = .05; NNT, 4.3); although women without PCOS who participated in the lifestyle program had an improved pregnancy rate over women in the control group, the results were not significant (67% vs. 46%; P = .18; NNT, 4.7).
The researchers also looked at the spontaneous pregnancy rate and found women who received the intervention had nearly three times the rate of spontaneous pregnancy, compared with women in the control group (33% vs. 12%; P = .01), while women with PCOS in the lifestyle program had nearly five times the rate of spontaneous pregnancy, compared with the control group (27% vs. 6%; P = .02). Women without PCOS in the lifestyle program had nearly twice the increased likelihood of spontaneous pregnancy, but the results were not significant (44% vs. 23%; P = .15).
Women with PCOS in the lifestyle program also had a higher live birth rate, compared with women in the control group (55% vs. 31%; P = .05; NNT, 4.3). Although women without PCOS in the lifestyle program (67% vs. 46%; P = .18; NNT, 4.7) and women in the study overall experienced higher live birth rates (51% vs. 37%; P = .14; NNT, 7.0), compared with the control group, these results were not significant, said Dr. Baillargeon.
“Such lifestyle interventions in women with obesity could significantly lower costs of fertility treatments, which is important,” concluded Dr. Baillargeon.
The Fit-For-Fertility program was funded by an unrestricted grant from Ferring.
SOURCE: Baillargeon J-P, et al. ASRM 2019. Abstract O-95.
REPORTING FROM ASRM 2019
Hormone therapy in transgender patients is safe for bone
ORLANDO – according to a presentation at the annual meeting of the American Society for Bone and Mineral Research.
“Hormonal treatment of transgender people is safe with respect to bone,” said Martin den Heijer, MD, PhD, of the VU University Medical Center in Amsterdam.
At baseline, transwomen have lower bone mass than do male reference populations, said Dr. den Heijer, citing a study that found 25 transwomen had less muscle mass (P less than or equal to .001), strength (P less than or equal to .05), and lower BMD at the hip, femoral neck, and spine (P less than .001), compared with 25 cisgender men in a control group and 941 men in a male reference population (Bone. 2013;54[1]:92-7). In a 2019 study from his own group, Dr. den Heijer said the z score in the lumbar spine for 711 transwomen was -0.9 and the incidence of osteoporosis was 14.2%, compared with a z score of 0.0 and 2.4% incidence of osteoporosis in 543 transmen (J Bone Min Res. 2019;34[3]:447-54).
In the prospective European Network for the Investigation of Gender Incongruence (ENIGI) study, researchers examined short-term effects of hormone therapy on BMD in 144 transwomen and 162 transmen who had a normal body mass index and were mostly white. The percentage of patients who reported they were current smokers was between 25% and 30%, and fewer than 10% said they consumed more than seven units of alcohol per week. Transwomen received estradiol (an oral estradiol valerate at a dose of 4 mg/day or an estradiol patch) together with 100 mg/day of cyproterone acetate, and transmen received testosterone in the form of a gel (50 mg/day), intramuscular esters (250 mg every 2-3 weeks), or intramuscular undecanoate at a dose of 1,000 mg every 12 weeks (J Sex Med. 2016;13[6]:994-9).
After 1 year of treatment, there were significant increases in BMD in transwomen in the lumbar spine (3.67%; 95% confidence interval, 3.20%-4.13%), femoral neck (1.86%; 95% CI, 1.41%-2.31%), and total hip (0.97%; 95% CI, 0.62%-1.31%). Transmen also had increased BMD in the lumbar spine (0.86%; 95% CI, 0.38%-1.35%) and total hip (1.04%; 95% CI, 0.64%-1.44%), with a slight decrease in femoral neck BMD (–0.46%; 95% CI, –1.07% to 0.16%).
Dr. den Heijer also discussed the long-term effects of hormone therapy on BMD in the Amsterdam Cohort of Dysphoria (ACOG) study, which consisted of 711 transwomen and 543 transmen and followed some patients out to 2 years, 5 years, and 10 years after beginning hormone therapy (J Sex Med. 2018;15[4]:582-90). Among transwomen, the median age was 33 years, 68.9% had begun hormone therapy, and 75.3% received a gonadectomy; among transmen, the median age was 25 years, 72.9% had begun hormone therapy, and 83.8% received a gonadectomy. Of these patients, dual-energy x-ray absorptiometry data were available for the lumbar spine BMD for 234 transwomen and 236 transmen at 2 years, 174 transwomen and 95 transmen at 5 years, and 102 transwomen and 70 transmen at 10 years.
Although there was no significant mean change in absolute BMD over the 10-year period, the concentration of estradiol in transwomen and transmen affected change in BMD the longer the transperson was receiving hormone therapy: Transwomen who received an estradiol concentration of 118 pmol/L had a decrease of –0.026% at 2 years, –0.044% at 5 years, and –0.009% at 10 years, compared with a dose of 443 pmol/L (+0.044% at 2 years, +0.025% at 5 years, +0.063% at 10 years), whereas transmen also had decreased BMD at the lowest estradiol concentrations of 95 pmol/L (–0.007% at 2 years, –0.024% at 5 years, +0.010% at 10 years), compared with transmen receiving the highest doses of 323 pmol/L (+0.028% at 2 years, +0.002% at 5 years, +0.053% at 10 years). There was no significant change in BMD in either group at any time point with regard to testosterone concentration.
When the investigators linked these patients to a national statistics database in the Netherlands to evaluate fracture incidence (J Bone Miner Res. 2019 Sep 5. doi: 10.1002/jbmr.3862), pairing five cisgender female controls and five cisgender male controls to every transgender patient, the researchers found transwomen had a higher incidence of osteoporotic fracture of the hip, spine, forearm, and humerus (41.8%), compared with cisgender men (26.6%; P = .014) and cisgender women (36.0%; P = .381). There was not enough information in the study to examine fracture information for transmen, Dr. den Heijer said. Transwomen and transmen who experienced a fracture were more likely to be a current smoker and have lower estradiol concentrations than were transwomen and transmen, respectively, who did not have a fracture.
“Attention for lifestyle factors remains important, especially smoking cessation, vitamin D intake, and regular exercise,” Dr. den Heijer said. “It remains important for everybody, but especially for transgender women.”
Dr. den Heijer reported no relevant conflicts of interest.
ORLANDO – according to a presentation at the annual meeting of the American Society for Bone and Mineral Research.
“Hormonal treatment of transgender people is safe with respect to bone,” said Martin den Heijer, MD, PhD, of the VU University Medical Center in Amsterdam.
At baseline, transwomen have lower bone mass than do male reference populations, said Dr. den Heijer, citing a study that found 25 transwomen had less muscle mass (P less than or equal to .001), strength (P less than or equal to .05), and lower BMD at the hip, femoral neck, and spine (P less than .001), compared with 25 cisgender men in a control group and 941 men in a male reference population (Bone. 2013;54[1]:92-7). In a 2019 study from his own group, Dr. den Heijer said the z score in the lumbar spine for 711 transwomen was -0.9 and the incidence of osteoporosis was 14.2%, compared with a z score of 0.0 and 2.4% incidence of osteoporosis in 543 transmen (J Bone Min Res. 2019;34[3]:447-54).
In the prospective European Network for the Investigation of Gender Incongruence (ENIGI) study, researchers examined short-term effects of hormone therapy on BMD in 144 transwomen and 162 transmen who had a normal body mass index and were mostly white. The percentage of patients who reported they were current smokers was between 25% and 30%, and fewer than 10% said they consumed more than seven units of alcohol per week. Transwomen received estradiol (an oral estradiol valerate at a dose of 4 mg/day or an estradiol patch) together with 100 mg/day of cyproterone acetate, and transmen received testosterone in the form of a gel (50 mg/day), intramuscular esters (250 mg every 2-3 weeks), or intramuscular undecanoate at a dose of 1,000 mg every 12 weeks (J Sex Med. 2016;13[6]:994-9).
After 1 year of treatment, there were significant increases in BMD in transwomen in the lumbar spine (3.67%; 95% confidence interval, 3.20%-4.13%), femoral neck (1.86%; 95% CI, 1.41%-2.31%), and total hip (0.97%; 95% CI, 0.62%-1.31%). Transmen also had increased BMD in the lumbar spine (0.86%; 95% CI, 0.38%-1.35%) and total hip (1.04%; 95% CI, 0.64%-1.44%), with a slight decrease in femoral neck BMD (–0.46%; 95% CI, –1.07% to 0.16%).
Dr. den Heijer also discussed the long-term effects of hormone therapy on BMD in the Amsterdam Cohort of Dysphoria (ACOG) study, which consisted of 711 transwomen and 543 transmen and followed some patients out to 2 years, 5 years, and 10 years after beginning hormone therapy (J Sex Med. 2018;15[4]:582-90). Among transwomen, the median age was 33 years, 68.9% had begun hormone therapy, and 75.3% received a gonadectomy; among transmen, the median age was 25 years, 72.9% had begun hormone therapy, and 83.8% received a gonadectomy. Of these patients, dual-energy x-ray absorptiometry data were available for the lumbar spine BMD for 234 transwomen and 236 transmen at 2 years, 174 transwomen and 95 transmen at 5 years, and 102 transwomen and 70 transmen at 10 years.
Although there was no significant mean change in absolute BMD over the 10-year period, the concentration of estradiol in transwomen and transmen affected change in BMD the longer the transperson was receiving hormone therapy: Transwomen who received an estradiol concentration of 118 pmol/L had a decrease of –0.026% at 2 years, –0.044% at 5 years, and –0.009% at 10 years, compared with a dose of 443 pmol/L (+0.044% at 2 years, +0.025% at 5 years, +0.063% at 10 years), whereas transmen also had decreased BMD at the lowest estradiol concentrations of 95 pmol/L (–0.007% at 2 years, –0.024% at 5 years, +0.010% at 10 years), compared with transmen receiving the highest doses of 323 pmol/L (+0.028% at 2 years, +0.002% at 5 years, +0.053% at 10 years). There was no significant change in BMD in either group at any time point with regard to testosterone concentration.
When the investigators linked these patients to a national statistics database in the Netherlands to evaluate fracture incidence (J Bone Miner Res. 2019 Sep 5. doi: 10.1002/jbmr.3862), pairing five cisgender female controls and five cisgender male controls to every transgender patient, the researchers found transwomen had a higher incidence of osteoporotic fracture of the hip, spine, forearm, and humerus (41.8%), compared with cisgender men (26.6%; P = .014) and cisgender women (36.0%; P = .381). There was not enough information in the study to examine fracture information for transmen, Dr. den Heijer said. Transwomen and transmen who experienced a fracture were more likely to be a current smoker and have lower estradiol concentrations than were transwomen and transmen, respectively, who did not have a fracture.
“Attention for lifestyle factors remains important, especially smoking cessation, vitamin D intake, and regular exercise,” Dr. den Heijer said. “It remains important for everybody, but especially for transgender women.”
Dr. den Heijer reported no relevant conflicts of interest.
ORLANDO – according to a presentation at the annual meeting of the American Society for Bone and Mineral Research.
“Hormonal treatment of transgender people is safe with respect to bone,” said Martin den Heijer, MD, PhD, of the VU University Medical Center in Amsterdam.
At baseline, transwomen have lower bone mass than do male reference populations, said Dr. den Heijer, citing a study that found 25 transwomen had less muscle mass (P less than or equal to .001), strength (P less than or equal to .05), and lower BMD at the hip, femoral neck, and spine (P less than .001), compared with 25 cisgender men in a control group and 941 men in a male reference population (Bone. 2013;54[1]:92-7). In a 2019 study from his own group, Dr. den Heijer said the z score in the lumbar spine for 711 transwomen was -0.9 and the incidence of osteoporosis was 14.2%, compared with a z score of 0.0 and 2.4% incidence of osteoporosis in 543 transmen (J Bone Min Res. 2019;34[3]:447-54).
In the prospective European Network for the Investigation of Gender Incongruence (ENIGI) study, researchers examined short-term effects of hormone therapy on BMD in 144 transwomen and 162 transmen who had a normal body mass index and were mostly white. The percentage of patients who reported they were current smokers was between 25% and 30%, and fewer than 10% said they consumed more than seven units of alcohol per week. Transwomen received estradiol (an oral estradiol valerate at a dose of 4 mg/day or an estradiol patch) together with 100 mg/day of cyproterone acetate, and transmen received testosterone in the form of a gel (50 mg/day), intramuscular esters (250 mg every 2-3 weeks), or intramuscular undecanoate at a dose of 1,000 mg every 12 weeks (J Sex Med. 2016;13[6]:994-9).
After 1 year of treatment, there were significant increases in BMD in transwomen in the lumbar spine (3.67%; 95% confidence interval, 3.20%-4.13%), femoral neck (1.86%; 95% CI, 1.41%-2.31%), and total hip (0.97%; 95% CI, 0.62%-1.31%). Transmen also had increased BMD in the lumbar spine (0.86%; 95% CI, 0.38%-1.35%) and total hip (1.04%; 95% CI, 0.64%-1.44%), with a slight decrease in femoral neck BMD (–0.46%; 95% CI, –1.07% to 0.16%).
Dr. den Heijer also discussed the long-term effects of hormone therapy on BMD in the Amsterdam Cohort of Dysphoria (ACOG) study, which consisted of 711 transwomen and 543 transmen and followed some patients out to 2 years, 5 years, and 10 years after beginning hormone therapy (J Sex Med. 2018;15[4]:582-90). Among transwomen, the median age was 33 years, 68.9% had begun hormone therapy, and 75.3% received a gonadectomy; among transmen, the median age was 25 years, 72.9% had begun hormone therapy, and 83.8% received a gonadectomy. Of these patients, dual-energy x-ray absorptiometry data were available for the lumbar spine BMD for 234 transwomen and 236 transmen at 2 years, 174 transwomen and 95 transmen at 5 years, and 102 transwomen and 70 transmen at 10 years.
Although there was no significant mean change in absolute BMD over the 10-year period, the concentration of estradiol in transwomen and transmen affected change in BMD the longer the transperson was receiving hormone therapy: Transwomen who received an estradiol concentration of 118 pmol/L had a decrease of –0.026% at 2 years, –0.044% at 5 years, and –0.009% at 10 years, compared with a dose of 443 pmol/L (+0.044% at 2 years, +0.025% at 5 years, +0.063% at 10 years), whereas transmen also had decreased BMD at the lowest estradiol concentrations of 95 pmol/L (–0.007% at 2 years, –0.024% at 5 years, +0.010% at 10 years), compared with transmen receiving the highest doses of 323 pmol/L (+0.028% at 2 years, +0.002% at 5 years, +0.053% at 10 years). There was no significant change in BMD in either group at any time point with regard to testosterone concentration.
When the investigators linked these patients to a national statistics database in the Netherlands to evaluate fracture incidence (J Bone Miner Res. 2019 Sep 5. doi: 10.1002/jbmr.3862), pairing five cisgender female controls and five cisgender male controls to every transgender patient, the researchers found transwomen had a higher incidence of osteoporotic fracture of the hip, spine, forearm, and humerus (41.8%), compared with cisgender men (26.6%; P = .014) and cisgender women (36.0%; P = .381). There was not enough information in the study to examine fracture information for transmen, Dr. den Heijer said. Transwomen and transmen who experienced a fracture were more likely to be a current smoker and have lower estradiol concentrations than were transwomen and transmen, respectively, who did not have a fracture.
“Attention for lifestyle factors remains important, especially smoking cessation, vitamin D intake, and regular exercise,” Dr. den Heijer said. “It remains important for everybody, but especially for transgender women.”
Dr. den Heijer reported no relevant conflicts of interest.
EXPERT ANALYSIS FROM ASBMR 2019
Dapagliflozin approved for reducing HF hospitalization in diabetes
The Food And Drug Administration has approved the sodium-glucose cotransporter 2 (SGLT2) inhibitor dapagliflozin (Farxiga) for reducing the risk of hospitalization for heart failure in adults with type 2 diabetes and established cardiovascular disease or multiple cardiovascular risk factors, according to a statement from AstraZeneca.
The approval was based on results from the DECLARE-TIMI 58 cardiovascular outcomes trial, which evaluated dapagliflozin in more than 17,000 patients with type 2 diabetes and cardiovascular risk factors or cardiovascular disease. They showed that dapagliflozin significantly reduced the risk of the primary composite endpoint of hospitalization for heart failure by 27%, compared with placebo (2.5% vs. 3.3%; HR, 0.73; 95% confidence interval, 0.61-0.88).
The drug is an oral, once-daily SGLT2 inhibitor initially approved as a monotherapy or combination therapy for glycemic control in adults with type 2 diabetes. It has additional benefits of weight loss and reduction in blood pressure in concert with diet and exercise in the same population.
“,” Ruud Dobber, PhD, executive vice president of the company’s biopharmaceuticals business unit, said in the statement. “This is promising news for the 30 million people living with type 2 diabetes in the U.S., as heart failure is one of the earliest cardiovascular complications for them, before heart attack or stroke. [Dapagliflozin] now offers the opportunity for physicians to act sooner and reduce the risk of hospitalization for heart failure.”
In September, the agency granted dapagliflozin a Fast Track designation to reduce the risk of cardiovascular death, or the worsening of heart failure in adults with heart failure with reduced ejection fraction or preserved ejection fraction, based on the phase 3 DAPA-HF and DELIVER trials. It also gave the drug Fast Track designation to delay the progression of renal failure and prevent CV and renal death in patients with chronic kidney disease based on the phase 3 DAPA-CKD trial, the statement noted.
The Food And Drug Administration has approved the sodium-glucose cotransporter 2 (SGLT2) inhibitor dapagliflozin (Farxiga) for reducing the risk of hospitalization for heart failure in adults with type 2 diabetes and established cardiovascular disease or multiple cardiovascular risk factors, according to a statement from AstraZeneca.
The approval was based on results from the DECLARE-TIMI 58 cardiovascular outcomes trial, which evaluated dapagliflozin in more than 17,000 patients with type 2 diabetes and cardiovascular risk factors or cardiovascular disease. They showed that dapagliflozin significantly reduced the risk of the primary composite endpoint of hospitalization for heart failure by 27%, compared with placebo (2.5% vs. 3.3%; HR, 0.73; 95% confidence interval, 0.61-0.88).
The drug is an oral, once-daily SGLT2 inhibitor initially approved as a monotherapy or combination therapy for glycemic control in adults with type 2 diabetes. It has additional benefits of weight loss and reduction in blood pressure in concert with diet and exercise in the same population.
“,” Ruud Dobber, PhD, executive vice president of the company’s biopharmaceuticals business unit, said in the statement. “This is promising news for the 30 million people living with type 2 diabetes in the U.S., as heart failure is one of the earliest cardiovascular complications for them, before heart attack or stroke. [Dapagliflozin] now offers the opportunity for physicians to act sooner and reduce the risk of hospitalization for heart failure.”
In September, the agency granted dapagliflozin a Fast Track designation to reduce the risk of cardiovascular death, or the worsening of heart failure in adults with heart failure with reduced ejection fraction or preserved ejection fraction, based on the phase 3 DAPA-HF and DELIVER trials. It also gave the drug Fast Track designation to delay the progression of renal failure and prevent CV and renal death in patients with chronic kidney disease based on the phase 3 DAPA-CKD trial, the statement noted.
The Food And Drug Administration has approved the sodium-glucose cotransporter 2 (SGLT2) inhibitor dapagliflozin (Farxiga) for reducing the risk of hospitalization for heart failure in adults with type 2 diabetes and established cardiovascular disease or multiple cardiovascular risk factors, according to a statement from AstraZeneca.
The approval was based on results from the DECLARE-TIMI 58 cardiovascular outcomes trial, which evaluated dapagliflozin in more than 17,000 patients with type 2 diabetes and cardiovascular risk factors or cardiovascular disease. They showed that dapagliflozin significantly reduced the risk of the primary composite endpoint of hospitalization for heart failure by 27%, compared with placebo (2.5% vs. 3.3%; HR, 0.73; 95% confidence interval, 0.61-0.88).
The drug is an oral, once-daily SGLT2 inhibitor initially approved as a monotherapy or combination therapy for glycemic control in adults with type 2 diabetes. It has additional benefits of weight loss and reduction in blood pressure in concert with diet and exercise in the same population.
“,” Ruud Dobber, PhD, executive vice president of the company’s biopharmaceuticals business unit, said in the statement. “This is promising news for the 30 million people living with type 2 diabetes in the U.S., as heart failure is one of the earliest cardiovascular complications for them, before heart attack or stroke. [Dapagliflozin] now offers the opportunity for physicians to act sooner and reduce the risk of hospitalization for heart failure.”
In September, the agency granted dapagliflozin a Fast Track designation to reduce the risk of cardiovascular death, or the worsening of heart failure in adults with heart failure with reduced ejection fraction or preserved ejection fraction, based on the phase 3 DAPA-HF and DELIVER trials. It also gave the drug Fast Track designation to delay the progression of renal failure and prevent CV and renal death in patients with chronic kidney disease based on the phase 3 DAPA-CKD trial, the statement noted.
Certain diabetes drugs may thwart dementia
COPENHAGEN – Selected antidiabetes medications appear to blunt the increased risk of dementia associated with type 2 diabetes, according to a Danish national case control registry study.
This benefit applies to the newer antidiabetic agents – specifically, the dipeptidyl peptidase 4 (DPP4) inhibitors, the glucagon-like peptide 1 (GLP1) analogs, and the sodium-glucose transport protein 2 (SGLT2) inhibitors – and metformin as well, Merete Osler, MD, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
In contrast, neither insulin nor the sulfonylureas showed any signal of a protective effect against development of dementia. In fact, the use of sulfonylureas was associated with a small but statistically significant 7% increased risk, added Dr. Osler, of the University of Copenhagen.
Elsewhere at the meeting, investigators tapped a Swedish national registry to demonstrate that individuals with type 1 diabetes have a sharply reduced risk of developing schizophrenia.
Type 2 diabetes medications and dementia
Dr. Osler and colleagues are among several groups of investigators who have previously shown that patients with type 2 diabetes have an increased risk of dementia.
“This has raised the question of the role of dysregulated glucose metabolism in the development of this neurodegenerative disorder, and the possible effect of antidiabetic medications,” she noted.
To further explore this issue, which links two great ongoing global epidemics, Dr. Osler and coinvestigators conducted a nested case-control study including all 176,250 patients with type 2 diabetes in the comprehensive Danish National Diabetes Register for 1995-2012. The 11,619 patients with type 2 diabetes who received a dementia diagnosis were matched with 46,476 type 2 diabetes patients without dementia. The objective was to determine associations between dementia and ever-use and cumulative dose of antidiabetes drugs, alone and in combination, in logistic regression analyses adjusted for demographics, comorbid conditions, marital status, diabetic complications, and year of dementia diagnosis.
Patients who had ever used metformin had an adjusted 6% reduction in the likelihood of dementia compared with metformin nonusers, a modest but statistically significant difference. Those on a DPP4 inhibitor had a 20% reduction in risk. The GLP1 analogs were associated with a 42% decrease in risk. So were the SGLT2 inhibitors. A dose-response relationship was evident: The higher the cumulative exposure to these agents, the lower the odds of dementia.
Combination therapy is common in type 2 diabetes, so the investigators scrutinized the impact of a variety of multidrug combinations. Combinations including a DPP4 inhibitor or GLP1 analog were also associated with significantly reduced dementia risk.
Records of glycemic control in the form of hemoglobin A1c values were available on only 1,446 type 2 diabetic dementia patients and 4,003 matched controls. An analysis that incorporated this variable showed that the observed anti-dementia effect of selected diabetes drugs was independent of glycemic control, according to Dr. Osler.
The protective effect appeared to extend to both Alzheimer’s disease and vascular dementias, although firm conclusions can’t be drawn on this score because the study was insufficiently powered to address that issue.
Dr. Osler noted that the Danish study confirms a recent Taiwanese study showing an apparent protective effect against dementia for metformin in patients with type 2 diabetes (Aging Dis. 2019 Feb 1;10(1):37-48).
“Ours is the first study on the newer diabetic drugs, so our results need to be confirmed,” she pointed out.
If confirmed, however, it would warrant exploration of these drugs more generally as potential interventions to prevent dementia. That could open a whole new chapter in the remarkable story of the SGLT2 inhibitors, a class of drugs originally developed for treatment of type 2 diabetes but which in major randomized clinical trials later proved to be so effective in the treatment of heart failure that they are now considered cardiology drugs first.
Asked if she thinks these antidiabetes agents have a general neuroprotective effect or, instead, that the observed reduced risk of dementia is a function of patients being treated better early on with modern drugs, the psychiatrist replied, “I think it might be a combination of both, especially because we find different risk estimates between the drugs.”
Dr. Osler reported having no financial conflicts of interest regarding the study, which was funded by the Danish Diabetes Foundation, the Danish Medical Association, and several other foundations.
The full study details were published online shortly before her presentation at ECNP 2019 (Eur J Endocrinol. 2019 Aug 1. pii: EJE-19-0259.R1. doi: 10.1530/EJE-19-0259).
Type 1 diabetes and schizophrenia risk
Kristina Melkersson, MD, PhD, presented a cohort study that utilized Swedish national registries to examine the relationship between type 1 diabetes and schizophrenia. The study comprised 1,745,977 individuals, of whom 10,117 had type 1 diabetes, who were followed for a median of 9.7 and maximum of 18 years from their 13th birthday. During follow-up, 1,280 individuals were diagnosed with schizophrenia and 649 others with schizoaffective disorder. The adjusted risk of schizophrenia was 70% lower in patients with type 1 diabetes. However, there was no difference in the risk of schizoaffective disorder in the type 1 diabetic versus nondiabetic subjects.
The Swedish data confirm the findings of an earlier Finnish national study showing that the risk of schizophrenia is reduced in patients with type 1 diabetes (Arch Gen Psychiatry. 2007 Aug;64(8):894-9). These findings raise the intriguing possibility that autoimmunity somehow figures into the etiology of the psychiatric disorder. Other investigators have previously reported a reduced prevalence of rheumatoid arthritis in patients with schizophrenia, noted Dr. Melkersson of the Karolinska Institute in Stockholm.
She reported having no financial conflicts regarding her study.
SOURCE: Osler M. ECNP Abstract P180. Melkersson K. Abstract 81.
COPENHAGEN – Selected antidiabetes medications appear to blunt the increased risk of dementia associated with type 2 diabetes, according to a Danish national case control registry study.
This benefit applies to the newer antidiabetic agents – specifically, the dipeptidyl peptidase 4 (DPP4) inhibitors, the glucagon-like peptide 1 (GLP1) analogs, and the sodium-glucose transport protein 2 (SGLT2) inhibitors – and metformin as well, Merete Osler, MD, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
In contrast, neither insulin nor the sulfonylureas showed any signal of a protective effect against development of dementia. In fact, the use of sulfonylureas was associated with a small but statistically significant 7% increased risk, added Dr. Osler, of the University of Copenhagen.
Elsewhere at the meeting, investigators tapped a Swedish national registry to demonstrate that individuals with type 1 diabetes have a sharply reduced risk of developing schizophrenia.
Type 2 diabetes medications and dementia
Dr. Osler and colleagues are among several groups of investigators who have previously shown that patients with type 2 diabetes have an increased risk of dementia.
“This has raised the question of the role of dysregulated glucose metabolism in the development of this neurodegenerative disorder, and the possible effect of antidiabetic medications,” she noted.
To further explore this issue, which links two great ongoing global epidemics, Dr. Osler and coinvestigators conducted a nested case-control study including all 176,250 patients with type 2 diabetes in the comprehensive Danish National Diabetes Register for 1995-2012. The 11,619 patients with type 2 diabetes who received a dementia diagnosis were matched with 46,476 type 2 diabetes patients without dementia. The objective was to determine associations between dementia and ever-use and cumulative dose of antidiabetes drugs, alone and in combination, in logistic regression analyses adjusted for demographics, comorbid conditions, marital status, diabetic complications, and year of dementia diagnosis.
Patients who had ever used metformin had an adjusted 6% reduction in the likelihood of dementia compared with metformin nonusers, a modest but statistically significant difference. Those on a DPP4 inhibitor had a 20% reduction in risk. The GLP1 analogs were associated with a 42% decrease in risk. So were the SGLT2 inhibitors. A dose-response relationship was evident: The higher the cumulative exposure to these agents, the lower the odds of dementia.
Combination therapy is common in type 2 diabetes, so the investigators scrutinized the impact of a variety of multidrug combinations. Combinations including a DPP4 inhibitor or GLP1 analog were also associated with significantly reduced dementia risk.
Records of glycemic control in the form of hemoglobin A1c values were available on only 1,446 type 2 diabetic dementia patients and 4,003 matched controls. An analysis that incorporated this variable showed that the observed anti-dementia effect of selected diabetes drugs was independent of glycemic control, according to Dr. Osler.
The protective effect appeared to extend to both Alzheimer’s disease and vascular dementias, although firm conclusions can’t be drawn on this score because the study was insufficiently powered to address that issue.
Dr. Osler noted that the Danish study confirms a recent Taiwanese study showing an apparent protective effect against dementia for metformin in patients with type 2 diabetes (Aging Dis. 2019 Feb 1;10(1):37-48).
“Ours is the first study on the newer diabetic drugs, so our results need to be confirmed,” she pointed out.
If confirmed, however, it would warrant exploration of these drugs more generally as potential interventions to prevent dementia. That could open a whole new chapter in the remarkable story of the SGLT2 inhibitors, a class of drugs originally developed for treatment of type 2 diabetes but which in major randomized clinical trials later proved to be so effective in the treatment of heart failure that they are now considered cardiology drugs first.
Asked if she thinks these antidiabetes agents have a general neuroprotective effect or, instead, that the observed reduced risk of dementia is a function of patients being treated better early on with modern drugs, the psychiatrist replied, “I think it might be a combination of both, especially because we find different risk estimates between the drugs.”
Dr. Osler reported having no financial conflicts of interest regarding the study, which was funded by the Danish Diabetes Foundation, the Danish Medical Association, and several other foundations.
The full study details were published online shortly before her presentation at ECNP 2019 (Eur J Endocrinol. 2019 Aug 1. pii: EJE-19-0259.R1. doi: 10.1530/EJE-19-0259).
Type 1 diabetes and schizophrenia risk
Kristina Melkersson, MD, PhD, presented a cohort study that utilized Swedish national registries to examine the relationship between type 1 diabetes and schizophrenia. The study comprised 1,745,977 individuals, of whom 10,117 had type 1 diabetes, who were followed for a median of 9.7 and maximum of 18 years from their 13th birthday. During follow-up, 1,280 individuals were diagnosed with schizophrenia and 649 others with schizoaffective disorder. The adjusted risk of schizophrenia was 70% lower in patients with type 1 diabetes. However, there was no difference in the risk of schizoaffective disorder in the type 1 diabetic versus nondiabetic subjects.
The Swedish data confirm the findings of an earlier Finnish national study showing that the risk of schizophrenia is reduced in patients with type 1 diabetes (Arch Gen Psychiatry. 2007 Aug;64(8):894-9). These findings raise the intriguing possibility that autoimmunity somehow figures into the etiology of the psychiatric disorder. Other investigators have previously reported a reduced prevalence of rheumatoid arthritis in patients with schizophrenia, noted Dr. Melkersson of the Karolinska Institute in Stockholm.
She reported having no financial conflicts regarding her study.
SOURCE: Osler M. ECNP Abstract P180. Melkersson K. Abstract 81.
COPENHAGEN – Selected antidiabetes medications appear to blunt the increased risk of dementia associated with type 2 diabetes, according to a Danish national case control registry study.
This benefit applies to the newer antidiabetic agents – specifically, the dipeptidyl peptidase 4 (DPP4) inhibitors, the glucagon-like peptide 1 (GLP1) analogs, and the sodium-glucose transport protein 2 (SGLT2) inhibitors – and metformin as well, Merete Osler, MD, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
In contrast, neither insulin nor the sulfonylureas showed any signal of a protective effect against development of dementia. In fact, the use of sulfonylureas was associated with a small but statistically significant 7% increased risk, added Dr. Osler, of the University of Copenhagen.
Elsewhere at the meeting, investigators tapped a Swedish national registry to demonstrate that individuals with type 1 diabetes have a sharply reduced risk of developing schizophrenia.
Type 2 diabetes medications and dementia
Dr. Osler and colleagues are among several groups of investigators who have previously shown that patients with type 2 diabetes have an increased risk of dementia.
“This has raised the question of the role of dysregulated glucose metabolism in the development of this neurodegenerative disorder, and the possible effect of antidiabetic medications,” she noted.
To further explore this issue, which links two great ongoing global epidemics, Dr. Osler and coinvestigators conducted a nested case-control study including all 176,250 patients with type 2 diabetes in the comprehensive Danish National Diabetes Register for 1995-2012. The 11,619 patients with type 2 diabetes who received a dementia diagnosis were matched with 46,476 type 2 diabetes patients without dementia. The objective was to determine associations between dementia and ever-use and cumulative dose of antidiabetes drugs, alone and in combination, in logistic regression analyses adjusted for demographics, comorbid conditions, marital status, diabetic complications, and year of dementia diagnosis.
Patients who had ever used metformin had an adjusted 6% reduction in the likelihood of dementia compared with metformin nonusers, a modest but statistically significant difference. Those on a DPP4 inhibitor had a 20% reduction in risk. The GLP1 analogs were associated with a 42% decrease in risk. So were the SGLT2 inhibitors. A dose-response relationship was evident: The higher the cumulative exposure to these agents, the lower the odds of dementia.
Combination therapy is common in type 2 diabetes, so the investigators scrutinized the impact of a variety of multidrug combinations. Combinations including a DPP4 inhibitor or GLP1 analog were also associated with significantly reduced dementia risk.
Records of glycemic control in the form of hemoglobin A1c values were available on only 1,446 type 2 diabetic dementia patients and 4,003 matched controls. An analysis that incorporated this variable showed that the observed anti-dementia effect of selected diabetes drugs was independent of glycemic control, according to Dr. Osler.
The protective effect appeared to extend to both Alzheimer’s disease and vascular dementias, although firm conclusions can’t be drawn on this score because the study was insufficiently powered to address that issue.
Dr. Osler noted that the Danish study confirms a recent Taiwanese study showing an apparent protective effect against dementia for metformin in patients with type 2 diabetes (Aging Dis. 2019 Feb 1;10(1):37-48).
“Ours is the first study on the newer diabetic drugs, so our results need to be confirmed,” she pointed out.
If confirmed, however, it would warrant exploration of these drugs more generally as potential interventions to prevent dementia. That could open a whole new chapter in the remarkable story of the SGLT2 inhibitors, a class of drugs originally developed for treatment of type 2 diabetes but which in major randomized clinical trials later proved to be so effective in the treatment of heart failure that they are now considered cardiology drugs first.
Asked if she thinks these antidiabetes agents have a general neuroprotective effect or, instead, that the observed reduced risk of dementia is a function of patients being treated better early on with modern drugs, the psychiatrist replied, “I think it might be a combination of both, especially because we find different risk estimates between the drugs.”
Dr. Osler reported having no financial conflicts of interest regarding the study, which was funded by the Danish Diabetes Foundation, the Danish Medical Association, and several other foundations.
The full study details were published online shortly before her presentation at ECNP 2019 (Eur J Endocrinol. 2019 Aug 1. pii: EJE-19-0259.R1. doi: 10.1530/EJE-19-0259).
Type 1 diabetes and schizophrenia risk
Kristina Melkersson, MD, PhD, presented a cohort study that utilized Swedish national registries to examine the relationship between type 1 diabetes and schizophrenia. The study comprised 1,745,977 individuals, of whom 10,117 had type 1 diabetes, who were followed for a median of 9.7 and maximum of 18 years from their 13th birthday. During follow-up, 1,280 individuals were diagnosed with schizophrenia and 649 others with schizoaffective disorder. The adjusted risk of schizophrenia was 70% lower in patients with type 1 diabetes. However, there was no difference in the risk of schizoaffective disorder in the type 1 diabetic versus nondiabetic subjects.
The Swedish data confirm the findings of an earlier Finnish national study showing that the risk of schizophrenia is reduced in patients with type 1 diabetes (Arch Gen Psychiatry. 2007 Aug;64(8):894-9). These findings raise the intriguing possibility that autoimmunity somehow figures into the etiology of the psychiatric disorder. Other investigators have previously reported a reduced prevalence of rheumatoid arthritis in patients with schizophrenia, noted Dr. Melkersson of the Karolinska Institute in Stockholm.
She reported having no financial conflicts regarding her study.
SOURCE: Osler M. ECNP Abstract P180. Melkersson K. Abstract 81.
REPORTING FROM ECNP 2019
Take drug, patient-level factors into account for when to end antiresorptive therapy
LAS VEGAS – according to an overview presented by Marcy B. Bolster, MD.
Recently published studies may help guide decisions about initiating and discontinuing treatment with bisphosphonates or denosumab (Prolia), the antiresorptive therapies. Understanding the ideal duration of bisphosphonate drug holidays “is a work in progress,” Dr. Bolster, from Harvard Medical School in Boston, said at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
No holiday with denosumab
Data indicate that twice yearly denosumab remains safe at 10 years, but studies have found a rapid loss of bone mineral density and an increased risk for vertebral fractures after treatment is discontinued (J Bone Miner Res. 2018 Feb;33[2]:190-8).
“Therefore, it is not appropriate for denosumab to be utilized with a drug holiday. If a patient is placed on denosumab, then consideration needs to be given for what to follow the course of denosumab,” Dr. Bolster said. “It is important to review with our patients the essential scheduled dosing of every 6 months, that the patient should not miss doses, and that we are not going to be able to initiate a drug holiday without starting another medicine.”
Patients likely to require hospitalization may not be good candidates for denosumab therapy because they may not be able to adhere to the dosing regimen, she said.
Denosumab vs. bisphosphonates: Real-world data
Trials have found greater increases in bone mineral density with denosumab, compared with the bisphosphonate drug alendronate, but that finding does not necessarily equate with reduced fracture risk, Dr. Bolster said. A recent population-based study examined fracture risk in approximately 92,000 people over age 50 years. Most were women, and their mean age was 71 years (JAMA Netw Open. 2019 Apr 5;2[4]:e192416).
The researchers compared the incidence of hospitalization for hip fracture among new denosumab users and new alendronate users during the 3 years after starting treatment. At 3 years, hip fractures occurred in 3.7% of the denosumab group and in 3.1% of the alendronate group. The rate of any fracture was 9% for each group. Although the study design had limitations, the analysis found “no difference between denosumab and alendronate in terms of fracture-risk reduction,” Dr. Bolster said. “Both agents are good agents.”
A recent meta-analysis compared fracture risk with denosumab and any bisphosphonate treatment using data from 10 trials that included more than 5,000 patients (J Clin Endocrinol Metab. 2019 May 1;104[5]:1753-65).
At 12 and 24 months, denosumab produced greater increases in bone mineral density at the spine, hip, and femoral neck. “In fact, there was a greater increase in bone density seen in those on denosumab who had had prior bisphosphonate use,” Dr. Bolster said. In 9 out of 10 trials, however, fracture rate did not differ between patients who received denosumab or any bisphosphonate at 12 or 24 months.
Bisphosphonate drug holidays
An increased risk of atypical femoral fracture with long-term bisphosphonate therapy has driven research on the effects of bisphosphonate drug holidays. “When we start a drug holiday, it requires continued close monitoring of the patient’s risk factors,” as well as monitoring whether a new fracture occurs during the holiday, Dr. Bolster said.
“We have very little data to guide the duration of a drug holiday,” she said. One study examined changes in bone density and bone turnover markers during a drug holiday after treatment with oral alendronate or intravenous zoledronic acid (J Bone Miner Res. 2019 May;34[5]:810-6).
The investigators conducted a post hoc analysis of data from the FLEX and HORIZON trials. Although alendronate was used for a longer duration, compared with zoledronic acid (5 years vs. 3 years), alendronate had a more rapid offset of drug effect after 3 years. The difference may relate to compliance rates with oral therapy during the treatment period, Dr. Bolster said.
The study did not examine fracture rates, which is the outcome that ultimately matters at the end of the day, she said.
Data suggest that bisphosphonate holidays are associated with increased risk of hip fracture. An analysis of Medicare data by Curtis et al. found that “hip fracture rates were lowest among those who remained on bisphosphonates,” Dr. Bolster said. Hip fracture rates increased with the length of the drug holiday, and a drug holiday of between 2 and 3 years was associated with 39% increased risk. The analysis included data from more than 156,000 women, about 40% of whom stopped bisphosphonates for more than 6 months. A total of 3,745 hip fractures occurred during follow-up.
Individualize treatment
“Duration of therapy should be individualized to the patient,” Dr. Bolster said. Physicians should assess the patient’s risk factors and take into account fragility fractures before and during treatment, bone density, and comorbidities.
“In terms of duration for drug holiday, does the patient now have osteopenia after treatment?” she said. “It is uncommon for bone density to change significantly during treatment, but occasionally we have a patient who goes from osteoporosis to osteopenia.”
The resumption of treatment should be based on established guidelines and individual patient factors, she said. For some postmenopausal woman, estrogen or raloxifene may not be ideal treatments when resuming therapy because these medications may increase cardiovascular or thrombotic risks. Denosumab may not be a good option for some patients because of the limitations surrounding its ability to be discontinued. The anabolic agents teriparatide and abaloparatide “may be good options to consider after a drug holiday, or even to give to patients during the drug holiday,” Dr. Bolster said. “The drug holiday does not have to be a treatment holiday. It really just needs to be an antiresorptive holiday.”
Dr. Bolster owns stock in Johnson & Johnson and is on an advisory board for Gilead.
Global Academy for Medical Education and this news organization are owned by the same parent company.
LAS VEGAS – according to an overview presented by Marcy B. Bolster, MD.
Recently published studies may help guide decisions about initiating and discontinuing treatment with bisphosphonates or denosumab (Prolia), the antiresorptive therapies. Understanding the ideal duration of bisphosphonate drug holidays “is a work in progress,” Dr. Bolster, from Harvard Medical School in Boston, said at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
No holiday with denosumab
Data indicate that twice yearly denosumab remains safe at 10 years, but studies have found a rapid loss of bone mineral density and an increased risk for vertebral fractures after treatment is discontinued (J Bone Miner Res. 2018 Feb;33[2]:190-8).
“Therefore, it is not appropriate for denosumab to be utilized with a drug holiday. If a patient is placed on denosumab, then consideration needs to be given for what to follow the course of denosumab,” Dr. Bolster said. “It is important to review with our patients the essential scheduled dosing of every 6 months, that the patient should not miss doses, and that we are not going to be able to initiate a drug holiday without starting another medicine.”
Patients likely to require hospitalization may not be good candidates for denosumab therapy because they may not be able to adhere to the dosing regimen, she said.
Denosumab vs. bisphosphonates: Real-world data
Trials have found greater increases in bone mineral density with denosumab, compared with the bisphosphonate drug alendronate, but that finding does not necessarily equate with reduced fracture risk, Dr. Bolster said. A recent population-based study examined fracture risk in approximately 92,000 people over age 50 years. Most were women, and their mean age was 71 years (JAMA Netw Open. 2019 Apr 5;2[4]:e192416).
The researchers compared the incidence of hospitalization for hip fracture among new denosumab users and new alendronate users during the 3 years after starting treatment. At 3 years, hip fractures occurred in 3.7% of the denosumab group and in 3.1% of the alendronate group. The rate of any fracture was 9% for each group. Although the study design had limitations, the analysis found “no difference between denosumab and alendronate in terms of fracture-risk reduction,” Dr. Bolster said. “Both agents are good agents.”
A recent meta-analysis compared fracture risk with denosumab and any bisphosphonate treatment using data from 10 trials that included more than 5,000 patients (J Clin Endocrinol Metab. 2019 May 1;104[5]:1753-65).
At 12 and 24 months, denosumab produced greater increases in bone mineral density at the spine, hip, and femoral neck. “In fact, there was a greater increase in bone density seen in those on denosumab who had had prior bisphosphonate use,” Dr. Bolster said. In 9 out of 10 trials, however, fracture rate did not differ between patients who received denosumab or any bisphosphonate at 12 or 24 months.
Bisphosphonate drug holidays
An increased risk of atypical femoral fracture with long-term bisphosphonate therapy has driven research on the effects of bisphosphonate drug holidays. “When we start a drug holiday, it requires continued close monitoring of the patient’s risk factors,” as well as monitoring whether a new fracture occurs during the holiday, Dr. Bolster said.
“We have very little data to guide the duration of a drug holiday,” she said. One study examined changes in bone density and bone turnover markers during a drug holiday after treatment with oral alendronate or intravenous zoledronic acid (J Bone Miner Res. 2019 May;34[5]:810-6).
The investigators conducted a post hoc analysis of data from the FLEX and HORIZON trials. Although alendronate was used for a longer duration, compared with zoledronic acid (5 years vs. 3 years), alendronate had a more rapid offset of drug effect after 3 years. The difference may relate to compliance rates with oral therapy during the treatment period, Dr. Bolster said.
The study did not examine fracture rates, which is the outcome that ultimately matters at the end of the day, she said.
Data suggest that bisphosphonate holidays are associated with increased risk of hip fracture. An analysis of Medicare data by Curtis et al. found that “hip fracture rates were lowest among those who remained on bisphosphonates,” Dr. Bolster said. Hip fracture rates increased with the length of the drug holiday, and a drug holiday of between 2 and 3 years was associated with 39% increased risk. The analysis included data from more than 156,000 women, about 40% of whom stopped bisphosphonates for more than 6 months. A total of 3,745 hip fractures occurred during follow-up.
Individualize treatment
“Duration of therapy should be individualized to the patient,” Dr. Bolster said. Physicians should assess the patient’s risk factors and take into account fragility fractures before and during treatment, bone density, and comorbidities.
“In terms of duration for drug holiday, does the patient now have osteopenia after treatment?” she said. “It is uncommon for bone density to change significantly during treatment, but occasionally we have a patient who goes from osteoporosis to osteopenia.”
The resumption of treatment should be based on established guidelines and individual patient factors, she said. For some postmenopausal woman, estrogen or raloxifene may not be ideal treatments when resuming therapy because these medications may increase cardiovascular or thrombotic risks. Denosumab may not be a good option for some patients because of the limitations surrounding its ability to be discontinued. The anabolic agents teriparatide and abaloparatide “may be good options to consider after a drug holiday, or even to give to patients during the drug holiday,” Dr. Bolster said. “The drug holiday does not have to be a treatment holiday. It really just needs to be an antiresorptive holiday.”
Dr. Bolster owns stock in Johnson & Johnson and is on an advisory board for Gilead.
Global Academy for Medical Education and this news organization are owned by the same parent company.
LAS VEGAS – according to an overview presented by Marcy B. Bolster, MD.
Recently published studies may help guide decisions about initiating and discontinuing treatment with bisphosphonates or denosumab (Prolia), the antiresorptive therapies. Understanding the ideal duration of bisphosphonate drug holidays “is a work in progress,” Dr. Bolster, from Harvard Medical School in Boston, said at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
No holiday with denosumab
Data indicate that twice yearly denosumab remains safe at 10 years, but studies have found a rapid loss of bone mineral density and an increased risk for vertebral fractures after treatment is discontinued (J Bone Miner Res. 2018 Feb;33[2]:190-8).
“Therefore, it is not appropriate for denosumab to be utilized with a drug holiday. If a patient is placed on denosumab, then consideration needs to be given for what to follow the course of denosumab,” Dr. Bolster said. “It is important to review with our patients the essential scheduled dosing of every 6 months, that the patient should not miss doses, and that we are not going to be able to initiate a drug holiday without starting another medicine.”
Patients likely to require hospitalization may not be good candidates for denosumab therapy because they may not be able to adhere to the dosing regimen, she said.
Denosumab vs. bisphosphonates: Real-world data
Trials have found greater increases in bone mineral density with denosumab, compared with the bisphosphonate drug alendronate, but that finding does not necessarily equate with reduced fracture risk, Dr. Bolster said. A recent population-based study examined fracture risk in approximately 92,000 people over age 50 years. Most were women, and their mean age was 71 years (JAMA Netw Open. 2019 Apr 5;2[4]:e192416).
The researchers compared the incidence of hospitalization for hip fracture among new denosumab users and new alendronate users during the 3 years after starting treatment. At 3 years, hip fractures occurred in 3.7% of the denosumab group and in 3.1% of the alendronate group. The rate of any fracture was 9% for each group. Although the study design had limitations, the analysis found “no difference between denosumab and alendronate in terms of fracture-risk reduction,” Dr. Bolster said. “Both agents are good agents.”
A recent meta-analysis compared fracture risk with denosumab and any bisphosphonate treatment using data from 10 trials that included more than 5,000 patients (J Clin Endocrinol Metab. 2019 May 1;104[5]:1753-65).
At 12 and 24 months, denosumab produced greater increases in bone mineral density at the spine, hip, and femoral neck. “In fact, there was a greater increase in bone density seen in those on denosumab who had had prior bisphosphonate use,” Dr. Bolster said. In 9 out of 10 trials, however, fracture rate did not differ between patients who received denosumab or any bisphosphonate at 12 or 24 months.
Bisphosphonate drug holidays
An increased risk of atypical femoral fracture with long-term bisphosphonate therapy has driven research on the effects of bisphosphonate drug holidays. “When we start a drug holiday, it requires continued close monitoring of the patient’s risk factors,” as well as monitoring whether a new fracture occurs during the holiday, Dr. Bolster said.
“We have very little data to guide the duration of a drug holiday,” she said. One study examined changes in bone density and bone turnover markers during a drug holiday after treatment with oral alendronate or intravenous zoledronic acid (J Bone Miner Res. 2019 May;34[5]:810-6).
The investigators conducted a post hoc analysis of data from the FLEX and HORIZON trials. Although alendronate was used for a longer duration, compared with zoledronic acid (5 years vs. 3 years), alendronate had a more rapid offset of drug effect after 3 years. The difference may relate to compliance rates with oral therapy during the treatment period, Dr. Bolster said.
The study did not examine fracture rates, which is the outcome that ultimately matters at the end of the day, she said.
Data suggest that bisphosphonate holidays are associated with increased risk of hip fracture. An analysis of Medicare data by Curtis et al. found that “hip fracture rates were lowest among those who remained on bisphosphonates,” Dr. Bolster said. Hip fracture rates increased with the length of the drug holiday, and a drug holiday of between 2 and 3 years was associated with 39% increased risk. The analysis included data from more than 156,000 women, about 40% of whom stopped bisphosphonates for more than 6 months. A total of 3,745 hip fractures occurred during follow-up.
Individualize treatment
“Duration of therapy should be individualized to the patient,” Dr. Bolster said. Physicians should assess the patient’s risk factors and take into account fragility fractures before and during treatment, bone density, and comorbidities.
“In terms of duration for drug holiday, does the patient now have osteopenia after treatment?” she said. “It is uncommon for bone density to change significantly during treatment, but occasionally we have a patient who goes from osteoporosis to osteopenia.”
The resumption of treatment should be based on established guidelines and individual patient factors, she said. For some postmenopausal woman, estrogen or raloxifene may not be ideal treatments when resuming therapy because these medications may increase cardiovascular or thrombotic risks. Denosumab may not be a good option for some patients because of the limitations surrounding its ability to be discontinued. The anabolic agents teriparatide and abaloparatide “may be good options to consider after a drug holiday, or even to give to patients during the drug holiday,” Dr. Bolster said. “The drug holiday does not have to be a treatment holiday. It really just needs to be an antiresorptive holiday.”
Dr. Bolster owns stock in Johnson & Johnson and is on an advisory board for Gilead.
Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM PRD 2019
SSRIs may reduce fecundability, live birth rate in reproductive-age women
PHILADELPHIA – Lindsey A. Sjaarda, PhD, reported at the annual meeting of the American Society for Reproductive Medicine.
In addition, women in a subgroup receiving fluoxetine experienced a lower live birth rate and greater incidence of pregnancy loss than women taking other SSRIs, but the results were not statistically significant, said Dr. Sjaarda of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
“[It] is biologically plausible that fluoxetine might have some different effects,” she noted. “It does have some different interaction with [cytochrome] P-450 enzyme activity, and this translates to it having a much longer half-life as well. It’s different in terms of drug metabolism and in its interaction with the hormone biosynthesis pathway.”
Most of the research on antidepressants and SSRIs in pregnancy has focused on the safety of the agents, rather than the effect on pregnancy for women trying to conceive, explained Dr. Sjaarda. Previous research also has shown inconsistent findings for fecundability in women of reproductive age taking SSRIs, and the risk of specific SSRI compounds on pregnancy loss is unclear.
The researchers performed a longitudinal exposure assessment of the Effects of Aspirin in Gestation and Reproduction (EAGeR) trial, which consisted of 1,228 women aged between 18 and 40 years trying to conceive. Patients were included if they had one to two prior pregnancy losses, up to two live births, and had been trying to become pregnant for six menstrual cycles; they were excluded if they had a severe history of mental illness. There were 1,035 women who had no preconception antidepressant exposure and 183 who did have preconception antidepressant exposure.
Patients provided longitudinal urine samples at various time points, including while trying to conceive and in early pregnancy, during the menses phase of each menstrual cycle and at their last menstrual cycle, and at 4 and 8 weeks’ gestation if they become pregnant. The urine samples were collected at home or in clinic; human chorionic gonadotropin levels were measured on the stored samples. The researchers defined pregnancy loss as any kind of loss measured after detecting human chorionic gonadotropin, and they used the patient’s medical record to determine live birth. The fecundability odds ratio was used to estimate the odds of conception in menstrual cycles.
Aypical and tricyclic antidepressants and SSRIs such as sertraline, fluoxetine, and citalopram/escitalopram were analyzed, as well as use of opioids, cannabinoids, and benzodiazepines. In total, 172 women used SSRIs, which represented 94% of the patient group analyzed, said Dr. Sjaarda. “This cohort really represents women who are successfully controlled with first-line agents.”
Patients in both the SSRI and no-antidepressant groups had similar baseline characteristics, but there were differences with regard to body mass index (26 kg/m2 vs. 28 kg/m2), employment status (77% vs. 67%), perceived stress (1.0 vs. 0.9), and opioid exposure (16% vs. 23%).
The researchers found use of any SSRI was associated with a 23% reduction in fecundability, with patients using fluoxetine, sertraline, and citalopram/escitalopram having a similar reduction in fecundability, compared with patients not using SSRIs.
Patients who received SSRIs also had approximately a 53% live birth rate overall. When analyzed by individual SSRI, however, there was a statistically significant reduction in the live birth rate for patients who were using fluoxetine, compared with patients using sertraline and citalopram/escitalopram. “This suggests that there was something besides just reduced fecundability going on with the fluoxetine-exposed women,” said Dr. Sjaarda.
When SSRI use was analyzed with regard to pregnancy loss, there was a generally null effect between women exposed to SSRIs overall versus those not exposed at the time before conception, at last menstrual period, and at 4 or 8 weeks’ gestation. But when grouped by specific SSRI, patients receiving fluoxetine had increased risk of pregnancy loss prior to conception, compared with patients not taking fluoxetine (34% vs. 24%; adjusted risk ratio, 1.41; 95% confidence interval, 0.94-2.12) , as well as at their last menstrual period (34% vs. 24%; adjusted RR, 1.48; 95% CI, 0.98-2.24) and at 4 weeks of pregnancy (31% vs. 22%; adjusted RR, 1.61; 95% CI, 0.94-2.78). “This was about a 40%-60% increase in pregnancy loss, even though the sample size is generally small when you divide it into these groups,” said Dr. Sjaarda.
Mental health care is an important public health and maternal health issue, and SSRIs as a drug class are essential for helping to appropriately manage mental health, noted Dr. Sjaarda.
Because “patients’ disease severities all vary and the reactions to different drugs vary, no one-size-fits-all recommendation can be made for people planning a pregnancy while using SSRIs,” concluded Dr. Sjaarda. “However, we’re hoping that women and their physicians can now consider these new data, which are based on objective and longitudinally measured exposure, as well as prospectively-assessed outcomes for these most common antidepressants and develop to a more informed and individualized plan for women who are trying to conceive and use SSRIs.”
Dr. Sjaarda reported no relevant conflicts of interest.
SOURCE: Sjaarda L et al. ASRM 2019, Abstract O-1.
PHILADELPHIA – Lindsey A. Sjaarda, PhD, reported at the annual meeting of the American Society for Reproductive Medicine.
In addition, women in a subgroup receiving fluoxetine experienced a lower live birth rate and greater incidence of pregnancy loss than women taking other SSRIs, but the results were not statistically significant, said Dr. Sjaarda of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
“[It] is biologically plausible that fluoxetine might have some different effects,” she noted. “It does have some different interaction with [cytochrome] P-450 enzyme activity, and this translates to it having a much longer half-life as well. It’s different in terms of drug metabolism and in its interaction with the hormone biosynthesis pathway.”
Most of the research on antidepressants and SSRIs in pregnancy has focused on the safety of the agents, rather than the effect on pregnancy for women trying to conceive, explained Dr. Sjaarda. Previous research also has shown inconsistent findings for fecundability in women of reproductive age taking SSRIs, and the risk of specific SSRI compounds on pregnancy loss is unclear.
The researchers performed a longitudinal exposure assessment of the Effects of Aspirin in Gestation and Reproduction (EAGeR) trial, which consisted of 1,228 women aged between 18 and 40 years trying to conceive. Patients were included if they had one to two prior pregnancy losses, up to two live births, and had been trying to become pregnant for six menstrual cycles; they were excluded if they had a severe history of mental illness. There were 1,035 women who had no preconception antidepressant exposure and 183 who did have preconception antidepressant exposure.
Patients provided longitudinal urine samples at various time points, including while trying to conceive and in early pregnancy, during the menses phase of each menstrual cycle and at their last menstrual cycle, and at 4 and 8 weeks’ gestation if they become pregnant. The urine samples were collected at home or in clinic; human chorionic gonadotropin levels were measured on the stored samples. The researchers defined pregnancy loss as any kind of loss measured after detecting human chorionic gonadotropin, and they used the patient’s medical record to determine live birth. The fecundability odds ratio was used to estimate the odds of conception in menstrual cycles.
Aypical and tricyclic antidepressants and SSRIs such as sertraline, fluoxetine, and citalopram/escitalopram were analyzed, as well as use of opioids, cannabinoids, and benzodiazepines. In total, 172 women used SSRIs, which represented 94% of the patient group analyzed, said Dr. Sjaarda. “This cohort really represents women who are successfully controlled with first-line agents.”
Patients in both the SSRI and no-antidepressant groups had similar baseline characteristics, but there were differences with regard to body mass index (26 kg/m2 vs. 28 kg/m2), employment status (77% vs. 67%), perceived stress (1.0 vs. 0.9), and opioid exposure (16% vs. 23%).
The researchers found use of any SSRI was associated with a 23% reduction in fecundability, with patients using fluoxetine, sertraline, and citalopram/escitalopram having a similar reduction in fecundability, compared with patients not using SSRIs.
Patients who received SSRIs also had approximately a 53% live birth rate overall. When analyzed by individual SSRI, however, there was a statistically significant reduction in the live birth rate for patients who were using fluoxetine, compared with patients using sertraline and citalopram/escitalopram. “This suggests that there was something besides just reduced fecundability going on with the fluoxetine-exposed women,” said Dr. Sjaarda.
When SSRI use was analyzed with regard to pregnancy loss, there was a generally null effect between women exposed to SSRIs overall versus those not exposed at the time before conception, at last menstrual period, and at 4 or 8 weeks’ gestation. But when grouped by specific SSRI, patients receiving fluoxetine had increased risk of pregnancy loss prior to conception, compared with patients not taking fluoxetine (34% vs. 24%; adjusted risk ratio, 1.41; 95% confidence interval, 0.94-2.12) , as well as at their last menstrual period (34% vs. 24%; adjusted RR, 1.48; 95% CI, 0.98-2.24) and at 4 weeks of pregnancy (31% vs. 22%; adjusted RR, 1.61; 95% CI, 0.94-2.78). “This was about a 40%-60% increase in pregnancy loss, even though the sample size is generally small when you divide it into these groups,” said Dr. Sjaarda.
Mental health care is an important public health and maternal health issue, and SSRIs as a drug class are essential for helping to appropriately manage mental health, noted Dr. Sjaarda.
Because “patients’ disease severities all vary and the reactions to different drugs vary, no one-size-fits-all recommendation can be made for people planning a pregnancy while using SSRIs,” concluded Dr. Sjaarda. “However, we’re hoping that women and their physicians can now consider these new data, which are based on objective and longitudinally measured exposure, as well as prospectively-assessed outcomes for these most common antidepressants and develop to a more informed and individualized plan for women who are trying to conceive and use SSRIs.”
Dr. Sjaarda reported no relevant conflicts of interest.
SOURCE: Sjaarda L et al. ASRM 2019, Abstract O-1.
PHILADELPHIA – Lindsey A. Sjaarda, PhD, reported at the annual meeting of the American Society for Reproductive Medicine.
In addition, women in a subgroup receiving fluoxetine experienced a lower live birth rate and greater incidence of pregnancy loss than women taking other SSRIs, but the results were not statistically significant, said Dr. Sjaarda of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
“[It] is biologically plausible that fluoxetine might have some different effects,” she noted. “It does have some different interaction with [cytochrome] P-450 enzyme activity, and this translates to it having a much longer half-life as well. It’s different in terms of drug metabolism and in its interaction with the hormone biosynthesis pathway.”
Most of the research on antidepressants and SSRIs in pregnancy has focused on the safety of the agents, rather than the effect on pregnancy for women trying to conceive, explained Dr. Sjaarda. Previous research also has shown inconsistent findings for fecundability in women of reproductive age taking SSRIs, and the risk of specific SSRI compounds on pregnancy loss is unclear.
The researchers performed a longitudinal exposure assessment of the Effects of Aspirin in Gestation and Reproduction (EAGeR) trial, which consisted of 1,228 women aged between 18 and 40 years trying to conceive. Patients were included if they had one to two prior pregnancy losses, up to two live births, and had been trying to become pregnant for six menstrual cycles; they were excluded if they had a severe history of mental illness. There were 1,035 women who had no preconception antidepressant exposure and 183 who did have preconception antidepressant exposure.
Patients provided longitudinal urine samples at various time points, including while trying to conceive and in early pregnancy, during the menses phase of each menstrual cycle and at their last menstrual cycle, and at 4 and 8 weeks’ gestation if they become pregnant. The urine samples were collected at home or in clinic; human chorionic gonadotropin levels were measured on the stored samples. The researchers defined pregnancy loss as any kind of loss measured after detecting human chorionic gonadotropin, and they used the patient’s medical record to determine live birth. The fecundability odds ratio was used to estimate the odds of conception in menstrual cycles.
Aypical and tricyclic antidepressants and SSRIs such as sertraline, fluoxetine, and citalopram/escitalopram were analyzed, as well as use of opioids, cannabinoids, and benzodiazepines. In total, 172 women used SSRIs, which represented 94% of the patient group analyzed, said Dr. Sjaarda. “This cohort really represents women who are successfully controlled with first-line agents.”
Patients in both the SSRI and no-antidepressant groups had similar baseline characteristics, but there were differences with regard to body mass index (26 kg/m2 vs. 28 kg/m2), employment status (77% vs. 67%), perceived stress (1.0 vs. 0.9), and opioid exposure (16% vs. 23%).
The researchers found use of any SSRI was associated with a 23% reduction in fecundability, with patients using fluoxetine, sertraline, and citalopram/escitalopram having a similar reduction in fecundability, compared with patients not using SSRIs.
Patients who received SSRIs also had approximately a 53% live birth rate overall. When analyzed by individual SSRI, however, there was a statistically significant reduction in the live birth rate for patients who were using fluoxetine, compared with patients using sertraline and citalopram/escitalopram. “This suggests that there was something besides just reduced fecundability going on with the fluoxetine-exposed women,” said Dr. Sjaarda.
When SSRI use was analyzed with regard to pregnancy loss, there was a generally null effect between women exposed to SSRIs overall versus those not exposed at the time before conception, at last menstrual period, and at 4 or 8 weeks’ gestation. But when grouped by specific SSRI, patients receiving fluoxetine had increased risk of pregnancy loss prior to conception, compared with patients not taking fluoxetine (34% vs. 24%; adjusted risk ratio, 1.41; 95% confidence interval, 0.94-2.12) , as well as at their last menstrual period (34% vs. 24%; adjusted RR, 1.48; 95% CI, 0.98-2.24) and at 4 weeks of pregnancy (31% vs. 22%; adjusted RR, 1.61; 95% CI, 0.94-2.78). “This was about a 40%-60% increase in pregnancy loss, even though the sample size is generally small when you divide it into these groups,” said Dr. Sjaarda.
Mental health care is an important public health and maternal health issue, and SSRIs as a drug class are essential for helping to appropriately manage mental health, noted Dr. Sjaarda.
Because “patients’ disease severities all vary and the reactions to different drugs vary, no one-size-fits-all recommendation can be made for people planning a pregnancy while using SSRIs,” concluded Dr. Sjaarda. “However, we’re hoping that women and their physicians can now consider these new data, which are based on objective and longitudinally measured exposure, as well as prospectively-assessed outcomes for these most common antidepressants and develop to a more informed and individualized plan for women who are trying to conceive and use SSRIs.”
Dr. Sjaarda reported no relevant conflicts of interest.
SOURCE: Sjaarda L et al. ASRM 2019, Abstract O-1.
REPORTING FROM ASRM 2019
Multiple zoledronic acid doses may be needed after stopping denosumab
ORLANDO – A single infusion of zoledronic acid does not completely prevent bone mineral density (BMD) loss regardless of the timing of the infusion in patients with osteopenia who discontinued denosumab (Prolia), according to a speaker at the annual meeting of the American Society for Bone and Mineral Research.
“If you consider a treatment break in patients treated with [denosumab] for a long time, I suggest to aim for a higher BMD, allowing a smaller bone loss when transitioning via [zoledronic acid] to a treatment break, or maybe you need to give more than one infusion of [zoledronic acid],” said Bente Langdahl, MD, PhD, of Aarhus (Denmark) University Hospital during her presentation.
Dr. Langdahl and her colleagues enrolled 60 patients with osteopenia in a 2-year, randomized, open-label interventional study of patients who discontinued denosumab after more than 2 years of use (average treatment, 4.6 years). One group of 20 patients received 5 mg of zoledronic acid at 6 months after their last denosumab injection. Another group of 20 patients underwent monthly monitoring starting at 6 months after their last denosumab injection and received zoledronic acid if their s-carboxy-terminal collagen crosslinks (s-CTX) increased more than 1.26 mcg/L or if they reached 9 months after their last denosumab injection. A third group underwent observation with monthly monitoring starting at 6 months after their last denosumab injection, and they received zoledronic acid if s-CTX increased more than 1.26 mcg/L, BMD loss was 5% or more at any site, they experienced a fragility vertebral or hip fracture, or they reached 12 months after their last denosumab injection. All patients received a readministration of zoledronic acid if their BMD decreased by greater than 5% or if their s-CTX increased more than 1.26 mcg/L after 6 months, 12 months, or 24 months.
The researchers included postmenopausal women and men older than 50 years (mean, 67.7 years), but the majority of patients were women (n = 53) distributed evenly between the 6-month, 9-month, and observation groups. Patients were excluded if they had a low-energy vertebral fracture, had any hip fracture within 12 months, had a T score of less than –2.5 at any site, had received alendronate more than 3 years prior to taking denosumab, used glucocorticoids, had metabolic bone disease, had received hormone replacement therapy, or had cancer.
The observational group patients who met criteria to receive zoledronic acid because of increased s-CTX included one at 1 month after stopping denosumab, two at 2 months, six at 3 months, and one at 4 months. Of six patients who met BMD criteria for treatment at 3 months, one received retreatment 6 months after their first administration of zoledronic acid, and four patients received retreatment at 12 months.
At 2 months, two patients in the 9-month group met s-CTX criteria for treatment, four patients underwent retreatment under BMD criteria 6 months after the first administration of zoledronic acid, and one patient underwent retreatment at 12 months under BMD criteria. In the 6-month group, one patient met s-CTX criteria for retreatment at 6 months, and one patient at 12 months, with five patients meeting BMD criteria for retreatment at 6 months and at 12 months.
Overall, the average bone loss was 4.6% at the lumbar spine and 3.2% for total hip with no clinically significant between-group differences for either site. At 12 months, lumbar spine bone loss was 4.8% in the 6-month group, 4.2% in the 9-month group, and 4.9% in the observational group (P less than or equal to .006), and total hip bone loss was 2.6% in the 6-month group, 3.3% in the 9-month group, and 3.8% in the observational group (P less than or equal to .001).
Although the study followed patients for 2 years after the first zoledronic acid injection, data were available for the first year only, and the study is ongoing, Dr. Langdahl said.
This study was funded in part by Amgen, the Foundation of Vilhelm Pedersen and wife, Aarhus University, the Danish Osteoporosis Society Research Foundation, the P. Carl Petersens Foundation, and the Torkil Steenbeck Foundation. Dr. Langdahl reported receiving research funding from Amgen and Novo Nordisk and is on the advisory board for Amgen, Eli Lilly, and UCB.
SOURCE: Sølling A. ASBMR 2019. Abstract LB-1169
ORLANDO – A single infusion of zoledronic acid does not completely prevent bone mineral density (BMD) loss regardless of the timing of the infusion in patients with osteopenia who discontinued denosumab (Prolia), according to a speaker at the annual meeting of the American Society for Bone and Mineral Research.
“If you consider a treatment break in patients treated with [denosumab] for a long time, I suggest to aim for a higher BMD, allowing a smaller bone loss when transitioning via [zoledronic acid] to a treatment break, or maybe you need to give more than one infusion of [zoledronic acid],” said Bente Langdahl, MD, PhD, of Aarhus (Denmark) University Hospital during her presentation.
Dr. Langdahl and her colleagues enrolled 60 patients with osteopenia in a 2-year, randomized, open-label interventional study of patients who discontinued denosumab after more than 2 years of use (average treatment, 4.6 years). One group of 20 patients received 5 mg of zoledronic acid at 6 months after their last denosumab injection. Another group of 20 patients underwent monthly monitoring starting at 6 months after their last denosumab injection and received zoledronic acid if their s-carboxy-terminal collagen crosslinks (s-CTX) increased more than 1.26 mcg/L or if they reached 9 months after their last denosumab injection. A third group underwent observation with monthly monitoring starting at 6 months after their last denosumab injection, and they received zoledronic acid if s-CTX increased more than 1.26 mcg/L, BMD loss was 5% or more at any site, they experienced a fragility vertebral or hip fracture, or they reached 12 months after their last denosumab injection. All patients received a readministration of zoledronic acid if their BMD decreased by greater than 5% or if their s-CTX increased more than 1.26 mcg/L after 6 months, 12 months, or 24 months.
The researchers included postmenopausal women and men older than 50 years (mean, 67.7 years), but the majority of patients were women (n = 53) distributed evenly between the 6-month, 9-month, and observation groups. Patients were excluded if they had a low-energy vertebral fracture, had any hip fracture within 12 months, had a T score of less than –2.5 at any site, had received alendronate more than 3 years prior to taking denosumab, used glucocorticoids, had metabolic bone disease, had received hormone replacement therapy, or had cancer.
The observational group patients who met criteria to receive zoledronic acid because of increased s-CTX included one at 1 month after stopping denosumab, two at 2 months, six at 3 months, and one at 4 months. Of six patients who met BMD criteria for treatment at 3 months, one received retreatment 6 months after their first administration of zoledronic acid, and four patients received retreatment at 12 months.
At 2 months, two patients in the 9-month group met s-CTX criteria for treatment, four patients underwent retreatment under BMD criteria 6 months after the first administration of zoledronic acid, and one patient underwent retreatment at 12 months under BMD criteria. In the 6-month group, one patient met s-CTX criteria for retreatment at 6 months, and one patient at 12 months, with five patients meeting BMD criteria for retreatment at 6 months and at 12 months.
Overall, the average bone loss was 4.6% at the lumbar spine and 3.2% for total hip with no clinically significant between-group differences for either site. At 12 months, lumbar spine bone loss was 4.8% in the 6-month group, 4.2% in the 9-month group, and 4.9% in the observational group (P less than or equal to .006), and total hip bone loss was 2.6% in the 6-month group, 3.3% in the 9-month group, and 3.8% in the observational group (P less than or equal to .001).
Although the study followed patients for 2 years after the first zoledronic acid injection, data were available for the first year only, and the study is ongoing, Dr. Langdahl said.
This study was funded in part by Amgen, the Foundation of Vilhelm Pedersen and wife, Aarhus University, the Danish Osteoporosis Society Research Foundation, the P. Carl Petersens Foundation, and the Torkil Steenbeck Foundation. Dr. Langdahl reported receiving research funding from Amgen and Novo Nordisk and is on the advisory board for Amgen, Eli Lilly, and UCB.
SOURCE: Sølling A. ASBMR 2019. Abstract LB-1169
ORLANDO – A single infusion of zoledronic acid does not completely prevent bone mineral density (BMD) loss regardless of the timing of the infusion in patients with osteopenia who discontinued denosumab (Prolia), according to a speaker at the annual meeting of the American Society for Bone and Mineral Research.
“If you consider a treatment break in patients treated with [denosumab] for a long time, I suggest to aim for a higher BMD, allowing a smaller bone loss when transitioning via [zoledronic acid] to a treatment break, or maybe you need to give more than one infusion of [zoledronic acid],” said Bente Langdahl, MD, PhD, of Aarhus (Denmark) University Hospital during her presentation.
Dr. Langdahl and her colleagues enrolled 60 patients with osteopenia in a 2-year, randomized, open-label interventional study of patients who discontinued denosumab after more than 2 years of use (average treatment, 4.6 years). One group of 20 patients received 5 mg of zoledronic acid at 6 months after their last denosumab injection. Another group of 20 patients underwent monthly monitoring starting at 6 months after their last denosumab injection and received zoledronic acid if their s-carboxy-terminal collagen crosslinks (s-CTX) increased more than 1.26 mcg/L or if they reached 9 months after their last denosumab injection. A third group underwent observation with monthly monitoring starting at 6 months after their last denosumab injection, and they received zoledronic acid if s-CTX increased more than 1.26 mcg/L, BMD loss was 5% or more at any site, they experienced a fragility vertebral or hip fracture, or they reached 12 months after their last denosumab injection. All patients received a readministration of zoledronic acid if their BMD decreased by greater than 5% or if their s-CTX increased more than 1.26 mcg/L after 6 months, 12 months, or 24 months.
The researchers included postmenopausal women and men older than 50 years (mean, 67.7 years), but the majority of patients were women (n = 53) distributed evenly between the 6-month, 9-month, and observation groups. Patients were excluded if they had a low-energy vertebral fracture, had any hip fracture within 12 months, had a T score of less than –2.5 at any site, had received alendronate more than 3 years prior to taking denosumab, used glucocorticoids, had metabolic bone disease, had received hormone replacement therapy, or had cancer.
The observational group patients who met criteria to receive zoledronic acid because of increased s-CTX included one at 1 month after stopping denosumab, two at 2 months, six at 3 months, and one at 4 months. Of six patients who met BMD criteria for treatment at 3 months, one received retreatment 6 months after their first administration of zoledronic acid, and four patients received retreatment at 12 months.
At 2 months, two patients in the 9-month group met s-CTX criteria for treatment, four patients underwent retreatment under BMD criteria 6 months after the first administration of zoledronic acid, and one patient underwent retreatment at 12 months under BMD criteria. In the 6-month group, one patient met s-CTX criteria for retreatment at 6 months, and one patient at 12 months, with five patients meeting BMD criteria for retreatment at 6 months and at 12 months.
Overall, the average bone loss was 4.6% at the lumbar spine and 3.2% for total hip with no clinically significant between-group differences for either site. At 12 months, lumbar spine bone loss was 4.8% in the 6-month group, 4.2% in the 9-month group, and 4.9% in the observational group (P less than or equal to .006), and total hip bone loss was 2.6% in the 6-month group, 3.3% in the 9-month group, and 3.8% in the observational group (P less than or equal to .001).
Although the study followed patients for 2 years after the first zoledronic acid injection, data were available for the first year only, and the study is ongoing, Dr. Langdahl said.
This study was funded in part by Amgen, the Foundation of Vilhelm Pedersen and wife, Aarhus University, the Danish Osteoporosis Society Research Foundation, the P. Carl Petersens Foundation, and the Torkil Steenbeck Foundation. Dr. Langdahl reported receiving research funding from Amgen and Novo Nordisk and is on the advisory board for Amgen, Eli Lilly, and UCB.
SOURCE: Sølling A. ASBMR 2019. Abstract LB-1169
REPORTING FROM ASBMR 2019