User login
Adolescent lap band removal rate swells by 5 years
LOS ANGELES – An increasing number of adolescents are undergoing gastric band removal after 2 years post operation, a prospective, longitudinal study shows.
“At 2 years most bands are still in place, with 96% of patients having them. After this point, however, multiple bands are removed each year, demonstrating that 2 years perhaps is only the tip of the iceberg,” Dr. Christine Schad said at Obesity Week.
Indeed, the number of adolescents with bands in place reduced to 87%, 76%, and 53% at years 3, 4, and 5 of follow-up. After 5 years, patients continued to undergo band removal.
Like their adult counterparts, adolescents underwent band removal secondary to weight loss failure, reflux esophagitis, and refractory gastric prolapse, Dr. Schad of Morgan Stanley Children’s Hospital, New York-Presbyterian Columbia University Medical Center, New York, said.
Weight loss seemed to plateau over time among the 79 evaluable adolescents, with less than 39% of patients able to lose more than 50% of their excess body weight over the 5-year study.
“Although gastric banding can be performed safely, 2 years seems inadequate to evaluate efficacy,” she said.
The use of adjustable gastric banding rose rapidly after Food and Drug Administration approval in 2001, thanks to low perioperative morbidity, reversibility, and good early results.
Gastric banding has fallen sharply, however, with recent adult studies showing a high incidence of weight loss failure, weight regain, and device-related complications.
Previous studies have reported on the safety of laparoscopic adjustable gastric banding in adolescents; however, these studies are limited to 3-year follow-up at most, Dr. Schad said at the meeting presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery.
The investigators enrolled 137 morbidly obese adolescents, aged 14-18 years, who underwent laparoscopic adjustable gastric banding from 2006 to 2011. The current analysis included patients with at least 5 years follow-up and patients who had band removal at any point or who did not survive to study end. There were two deaths. The remaining patients had not reached the 5-year follow-up mark or still had their bands in place.
The 79 evaluable patients had a preoperative weight of 138 kg, body mass index of 49.3 kg/m2, and excess body weight of 47.2%. At the time of surgery, their average age was 16.9 years, 71% were female, 43% Hispanic, 36.7% white, and 16.5% black.
Even though gastric banding is declining, the results are important because there has been little information about adolescents, and in some parts of the country, gastric banding may be the only available option, session comoderator Dr. Robert Carpenter of Scott & White Healthcare in Temple, Tex., said in an interview.
“The other issue is that there are a lot of pediatricians that only want their patients to have nonstapled, nondivided operations,” he said. “If that’s the case, and we now know that perhaps for adolescents there is a 30%, 40%, 50% conversion and/or failure rate, then we are putting these kids at an extreme risk.”
Oftentimes, these adolescents also won’t have an opportunity for another operation.
“Many insurance companies that they’ll transition to away from their parents will actually have a complete exclusion for bariatric surgery or they have a onetime, lifetime operative opportunity,” Dr. Carpenter said. “So, if that’s been burned, it’s burned.”
LOS ANGELES – An increasing number of adolescents are undergoing gastric band removal after 2 years post operation, a prospective, longitudinal study shows.
“At 2 years most bands are still in place, with 96% of patients having them. After this point, however, multiple bands are removed each year, demonstrating that 2 years perhaps is only the tip of the iceberg,” Dr. Christine Schad said at Obesity Week.
Indeed, the number of adolescents with bands in place reduced to 87%, 76%, and 53% at years 3, 4, and 5 of follow-up. After 5 years, patients continued to undergo band removal.
Like their adult counterparts, adolescents underwent band removal secondary to weight loss failure, reflux esophagitis, and refractory gastric prolapse, Dr. Schad of Morgan Stanley Children’s Hospital, New York-Presbyterian Columbia University Medical Center, New York, said.
Weight loss seemed to plateau over time among the 79 evaluable adolescents, with less than 39% of patients able to lose more than 50% of their excess body weight over the 5-year study.
“Although gastric banding can be performed safely, 2 years seems inadequate to evaluate efficacy,” she said.
The use of adjustable gastric banding rose rapidly after Food and Drug Administration approval in 2001, thanks to low perioperative morbidity, reversibility, and good early results.
Gastric banding has fallen sharply, however, with recent adult studies showing a high incidence of weight loss failure, weight regain, and device-related complications.
Previous studies have reported on the safety of laparoscopic adjustable gastric banding in adolescents; however, these studies are limited to 3-year follow-up at most, Dr. Schad said at the meeting presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery.
The investigators enrolled 137 morbidly obese adolescents, aged 14-18 years, who underwent laparoscopic adjustable gastric banding from 2006 to 2011. The current analysis included patients with at least 5 years follow-up and patients who had band removal at any point or who did not survive to study end. There were two deaths. The remaining patients had not reached the 5-year follow-up mark or still had their bands in place.
The 79 evaluable patients had a preoperative weight of 138 kg, body mass index of 49.3 kg/m2, and excess body weight of 47.2%. At the time of surgery, their average age was 16.9 years, 71% were female, 43% Hispanic, 36.7% white, and 16.5% black.
Even though gastric banding is declining, the results are important because there has been little information about adolescents, and in some parts of the country, gastric banding may be the only available option, session comoderator Dr. Robert Carpenter of Scott & White Healthcare in Temple, Tex., said in an interview.
“The other issue is that there are a lot of pediatricians that only want their patients to have nonstapled, nondivided operations,” he said. “If that’s the case, and we now know that perhaps for adolescents there is a 30%, 40%, 50% conversion and/or failure rate, then we are putting these kids at an extreme risk.”
Oftentimes, these adolescents also won’t have an opportunity for another operation.
“Many insurance companies that they’ll transition to away from their parents will actually have a complete exclusion for bariatric surgery or they have a onetime, lifetime operative opportunity,” Dr. Carpenter said. “So, if that’s been burned, it’s burned.”
LOS ANGELES – An increasing number of adolescents are undergoing gastric band removal after 2 years post operation, a prospective, longitudinal study shows.
“At 2 years most bands are still in place, with 96% of patients having them. After this point, however, multiple bands are removed each year, demonstrating that 2 years perhaps is only the tip of the iceberg,” Dr. Christine Schad said at Obesity Week.
Indeed, the number of adolescents with bands in place reduced to 87%, 76%, and 53% at years 3, 4, and 5 of follow-up. After 5 years, patients continued to undergo band removal.
Like their adult counterparts, adolescents underwent band removal secondary to weight loss failure, reflux esophagitis, and refractory gastric prolapse, Dr. Schad of Morgan Stanley Children’s Hospital, New York-Presbyterian Columbia University Medical Center, New York, said.
Weight loss seemed to plateau over time among the 79 evaluable adolescents, with less than 39% of patients able to lose more than 50% of their excess body weight over the 5-year study.
“Although gastric banding can be performed safely, 2 years seems inadequate to evaluate efficacy,” she said.
The use of adjustable gastric banding rose rapidly after Food and Drug Administration approval in 2001, thanks to low perioperative morbidity, reversibility, and good early results.
Gastric banding has fallen sharply, however, with recent adult studies showing a high incidence of weight loss failure, weight regain, and device-related complications.
Previous studies have reported on the safety of laparoscopic adjustable gastric banding in adolescents; however, these studies are limited to 3-year follow-up at most, Dr. Schad said at the meeting presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery.
The investigators enrolled 137 morbidly obese adolescents, aged 14-18 years, who underwent laparoscopic adjustable gastric banding from 2006 to 2011. The current analysis included patients with at least 5 years follow-up and patients who had band removal at any point or who did not survive to study end. There were two deaths. The remaining patients had not reached the 5-year follow-up mark or still had their bands in place.
The 79 evaluable patients had a preoperative weight of 138 kg, body mass index of 49.3 kg/m2, and excess body weight of 47.2%. At the time of surgery, their average age was 16.9 years, 71% were female, 43% Hispanic, 36.7% white, and 16.5% black.
Even though gastric banding is declining, the results are important because there has been little information about adolescents, and in some parts of the country, gastric banding may be the only available option, session comoderator Dr. Robert Carpenter of Scott & White Healthcare in Temple, Tex., said in an interview.
“The other issue is that there are a lot of pediatricians that only want their patients to have nonstapled, nondivided operations,” he said. “If that’s the case, and we now know that perhaps for adolescents there is a 30%, 40%, 50% conversion and/or failure rate, then we are putting these kids at an extreme risk.”
Oftentimes, these adolescents also won’t have an opportunity for another operation.
“Many insurance companies that they’ll transition to away from their parents will actually have a complete exclusion for bariatric surgery or they have a onetime, lifetime operative opportunity,” Dr. Carpenter said. “So, if that’s been burned, it’s burned.”
AT OBESITY WEEK 2015
Key clinical point: Adolescents undergo laparoscopic adjustable gastric band removal at increasing numbers after 2 years post operation.
Major finding: The percentage of bands in place was 96% at 2 years, declining to 87%, 76%, and 53% at years 3, 4, and 5.
Data source: Prospective, longitudinal study in 79 adolescents.
Disclosures: Dr. Schad reported having no disclosures.
Robotic surgery progress: Is resistance futile?
Despite the lack of a clear-cut cost-effectiveness case, it appears that the use of robotics in surgery is on its way to becoming the norm.
What may help make that cost-effectiveness case is time, as the hardware becomes cheaper and the technology becomes more widespread.
“I think there are many surgeons who are doing robotic surgery who truly believe that the current of state of the art will bear only a partial resemblance to the state of the art 5-10 years from now,” Dr. Henry Pitt, chief quality officer of the Temple University Health System, Philadelphia, said in an interview. “And part of the persistence by many of the robotic surgeons, while they fully understand that what they are doing is not cost effective now, is that they believe that the next few generations of this technology will become the state of the art and will become cost effective.”
Dr. Pitt, an ACS Fellow, compared the evolution of robotic surgery to that of computers and the application of Moore’s Law, by which the technology advances rapidly while the price eventually comes down.
Spread of robot-assisted surgery
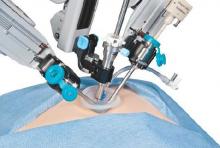
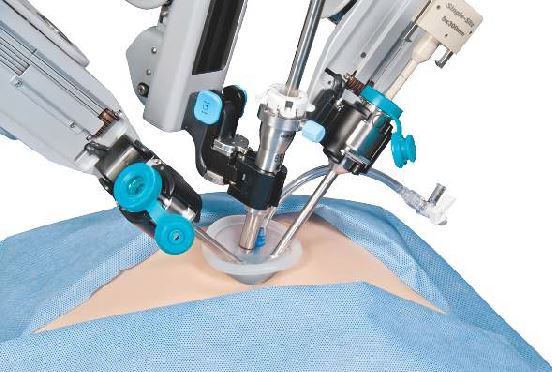
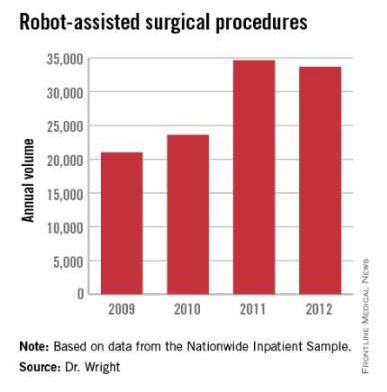
Research by the surgical robot industry and independent investigators shows that use of robotics is on an upward climb. A presentation at the 2015 American College of Surgeons Clinical Congress reveals that, based on retrospective review of the Nationwide Inpatient Sample from 2009-2012 using the ICD-9 code that identifies robot-assisted procedures, there were 113,022 robot-assisted procedures in that period.
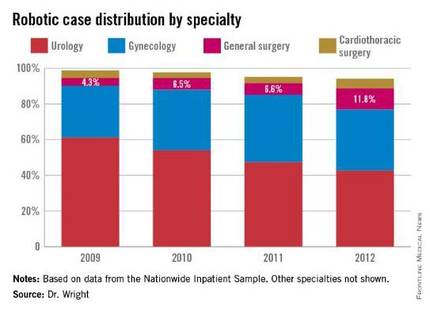
In 2009, there were 21,006 procedures, with the volume growing to 33,713 in 2012. Perhaps more telling about the use of robotics is that it is not confined to specialties with a proven track record such as gynecology and urology. The study, led by Dr. G. Paul Wright of Grand Rapids Medical Education Partners and Michigan State University, shows an increase in robotics use in general surgery procedures by 4.5-fold from 2009-2012, with steady increases each year. General surgery accounted for 11.8% of robot-assisted cases in 2012, Dr. Wright and colleagues reported.
Dr. Wright suggested a number of factors contributing to the “surprising” growth of robotics in general surgery.
“I think the growth is stimulated, at least in part, by market forces,” he said in an interview. “For patients who are the consumers of health care, they are often drawn to [surgeons] whom they perceive to be on the cutting edge. Robotic surgery certainly offers that intrigue. With greater access to the technology, if a patient is seeking a robotic surgery that you do not offer as a surgeon, they can likely find that elsewhere, potentially at your competitor. Another driving force is potentially the ease of suturing with the robot which more resembles open surgery while advanced laparoscopic suturing in tight spaces is a more limited skill set among general surgeons.”
Dr. Wright added that “some of the stigma of robotic surgery is dissolving among surgeons as they become more familiar with the technology. I also believe the market forces will help drive down costs in robotics which may help to increase utilization as well.”
Industry touts cost effectiveness
And while Dr. Pitt is looking forward, the industry is energetically promoting the cost effectiveness of robotics today. Intuitive Surgical Inc., manufacturer of the da Vinci surgery robot presented in a recent press briefing data culled from literature found on PubMed showing that robotic surgery, at least with their product, is cost effective now (view the report at http://goo.gl/AfAuT3). But in recent years, the large number of clinical trials comparing robotic vs. laparoscopic surgery for colorectal disease, for example, is equivocal. For many procedures, trials appear to show similar between laparoscopic and robot-assisted approaches, with the main factors in play being length of operation time, hospital stay, and surgical site infection.
According to Intuitive Surgical’s manager of clinical economics, Andrew Yiu, a review of peer-reviewed articles that featured nationwide cohorts of comparisons and included comparisons to both open and laparoscopic procedures outcomes shows improvements across a variety of procedures in terms of hospital stay, lower conversion rates, reduced complications, reduced 30-day readmissions, and improved patient satisfaction, though outcomes were more pronounced in some areas compared with others. Highlighting one procedure in particular – benign hysterectomy – Mr. Yiu noted that rates of minor and major complications associated with robotic vs. laparoscopic surgery were relatively similar but that the conversion rate to open surgery from laparoscopic surgery was 3.9%, compared with 0.9% for da Vinci–assisted surgery.
Overall, Intuitive Surgical calculated the potential savings on the episode of care with the da Vinci system at $1,087 vs. laparoscopic procedures and $2,996 over open surgery.
The company is currently promoting its da Vinci IX Surgical System. Cleared by the Food and Drug Administration in April 2014, Intuitive Surgical says that this latest version has broader capabilities than earlier generations and has been optimized for complex, multiquadrant surgeries.
Costs and other trade-offs
In some ways, robot-assisted surgery is following the same path as laparoscopic surgery, observed Dr. Tyler Hughes of the department of general surgery at McPherson (Kan.) Hospital.
“Certainly, we had the issue back in the late ‘80s and early ‘90s of laparoscopy,” Dr. Hughes said in an interview. “Was it cost effective? Because at the time $30,000 or $40,000 for a laparoscopic set-up seemed like a horse-choking amount of money. However, it converted a 5-day in-the-hospital experience to a 4-hour day-surgery experience. The nonclinical benefits to the patient were so enormous that it took the whole place by storm.”
Dr. Hughes, an ACS Fellow, suggested that the leap in savings elsewhere in the episode of care when using laparoscopic surgery over open surgery is not being mirrored in the robotic space and suggested that money should be spent in other areas of need.
“Cost is going to choke the future if we’re not careful. We need nurses. We need infrastructure. We need to be careful to buy things that really make a big difference,” he said.
In a study presented by at the ACS Clinical Congress on a cost-benefit analysis of robotic vs. laparoscopic colectomy, Anastasia Postoev, a fourth-year medical student at Caribbean Medical University in Willemstad, Curacao and her colleagues found little difference in clinical outcomes but higher costs as a result of added time needed to prepare the robot for surgery. The study showed that the potential up-front costs can be $1 to $2 million for the robot in addition to ongoing costs related to training and maintenance. Dr. Hughes said. “It is a terrific marketing tool in the sense that you can certainly attract a lot of people to a hospital with robotic surgery, but whether or not those outcomes are truly different and the cost of those outcomes are justifiable is yet to be determined.”
The ease of use of robotics, however, could end up being a double-edged sword, particularly if there are areas of the country where robotics may not be as pervasive, or in cases when a conversion to open surgery might be required.
“It is a real concern that trainees are being taught robotic surgery for what is not really needed to be robotic in the mainstream,” Dr. Hughes said. “Then they come out with less experience with straight stick laparoscopy and we’ve certainly seen lots of problems in the laparoscopic range for people once they get to the point that they have to convert to open. We really don’t have a large volume of trainee experience in complex open surgery. That’s a big problem. Robotics certainly isn’t going to make that a lot better.”
[email protected]
Despite the lack of a clear-cut cost-effectiveness case, it appears that the use of robotics in surgery is on its way to becoming the norm.
What may help make that cost-effectiveness case is time, as the hardware becomes cheaper and the technology becomes more widespread.
“I think there are many surgeons who are doing robotic surgery who truly believe that the current of state of the art will bear only a partial resemblance to the state of the art 5-10 years from now,” Dr. Henry Pitt, chief quality officer of the Temple University Health System, Philadelphia, said in an interview. “And part of the persistence by many of the robotic surgeons, while they fully understand that what they are doing is not cost effective now, is that they believe that the next few generations of this technology will become the state of the art and will become cost effective.”
Dr. Pitt, an ACS Fellow, compared the evolution of robotic surgery to that of computers and the application of Moore’s Law, by which the technology advances rapidly while the price eventually comes down.
Spread of robot-assisted surgery
Research by the surgical robot industry and independent investigators shows that use of robotics is on an upward climb. A presentation at the 2015 American College of Surgeons Clinical Congress reveals that, based on retrospective review of the Nationwide Inpatient Sample from 2009-2012 using the ICD-9 code that identifies robot-assisted procedures, there were 113,022 robot-assisted procedures in that period.
In 2009, there were 21,006 procedures, with the volume growing to 33,713 in 2012. Perhaps more telling about the use of robotics is that it is not confined to specialties with a proven track record such as gynecology and urology. The study, led by Dr. G. Paul Wright of Grand Rapids Medical Education Partners and Michigan State University, shows an increase in robotics use in general surgery procedures by 4.5-fold from 2009-2012, with steady increases each year. General surgery accounted for 11.8% of robot-assisted cases in 2012, Dr. Wright and colleagues reported.

Dr. Wright suggested a number of factors contributing to the “surprising” growth of robotics in general surgery.
“I think the growth is stimulated, at least in part, by market forces,” he said in an interview. “For patients who are the consumers of health care, they are often drawn to [surgeons] whom they perceive to be on the cutting edge. Robotic surgery certainly offers that intrigue. With greater access to the technology, if a patient is seeking a robotic surgery that you do not offer as a surgeon, they can likely find that elsewhere, potentially at your competitor. Another driving force is potentially the ease of suturing with the robot which more resembles open surgery while advanced laparoscopic suturing in tight spaces is a more limited skill set among general surgeons.”
Dr. Wright added that “some of the stigma of robotic surgery is dissolving among surgeons as they become more familiar with the technology. I also believe the market forces will help drive down costs in robotics which may help to increase utilization as well.”
Industry touts cost effectiveness
And while Dr. Pitt is looking forward, the industry is energetically promoting the cost effectiveness of robotics today. Intuitive Surgical Inc., manufacturer of the da Vinci surgery robot presented in a recent press briefing data culled from literature found on PubMed showing that robotic surgery, at least with their product, is cost effective now (view the report at http://goo.gl/AfAuT3). But in recent years, the large number of clinical trials comparing robotic vs. laparoscopic surgery for colorectal disease, for example, is equivocal. For many procedures, trials appear to show similar between laparoscopic and robot-assisted approaches, with the main factors in play being length of operation time, hospital stay, and surgical site infection.
According to Intuitive Surgical’s manager of clinical economics, Andrew Yiu, a review of peer-reviewed articles that featured nationwide cohorts of comparisons and included comparisons to both open and laparoscopic procedures outcomes shows improvements across a variety of procedures in terms of hospital stay, lower conversion rates, reduced complications, reduced 30-day readmissions, and improved patient satisfaction, though outcomes were more pronounced in some areas compared with others. Highlighting one procedure in particular – benign hysterectomy – Mr. Yiu noted that rates of minor and major complications associated with robotic vs. laparoscopic surgery were relatively similar but that the conversion rate to open surgery from laparoscopic surgery was 3.9%, compared with 0.9% for da Vinci–assisted surgery.

Overall, Intuitive Surgical calculated the potential savings on the episode of care with the da Vinci system at $1,087 vs. laparoscopic procedures and $2,996 over open surgery.
The company is currently promoting its da Vinci IX Surgical System. Cleared by the Food and Drug Administration in April 2014, Intuitive Surgical says that this latest version has broader capabilities than earlier generations and has been optimized for complex, multiquadrant surgeries.
Costs and other trade-offs
In some ways, robot-assisted surgery is following the same path as laparoscopic surgery, observed Dr. Tyler Hughes of the department of general surgery at McPherson (Kan.) Hospital.
“Certainly, we had the issue back in the late ‘80s and early ‘90s of laparoscopy,” Dr. Hughes said in an interview. “Was it cost effective? Because at the time $30,000 or $40,000 for a laparoscopic set-up seemed like a horse-choking amount of money. However, it converted a 5-day in-the-hospital experience to a 4-hour day-surgery experience. The nonclinical benefits to the patient were so enormous that it took the whole place by storm.”
Dr. Hughes, an ACS Fellow, suggested that the leap in savings elsewhere in the episode of care when using laparoscopic surgery over open surgery is not being mirrored in the robotic space and suggested that money should be spent in other areas of need.
“Cost is going to choke the future if we’re not careful. We need nurses. We need infrastructure. We need to be careful to buy things that really make a big difference,” he said.
In a study presented by at the ACS Clinical Congress on a cost-benefit analysis of robotic vs. laparoscopic colectomy, Anastasia Postoev, a fourth-year medical student at Caribbean Medical University in Willemstad, Curacao and her colleagues found little difference in clinical outcomes but higher costs as a result of added time needed to prepare the robot for surgery. The study showed that the potential up-front costs can be $1 to $2 million for the robot in addition to ongoing costs related to training and maintenance. Dr. Hughes said. “It is a terrific marketing tool in the sense that you can certainly attract a lot of people to a hospital with robotic surgery, but whether or not those outcomes are truly different and the cost of those outcomes are justifiable is yet to be determined.”
The ease of use of robotics, however, could end up being a double-edged sword, particularly if there are areas of the country where robotics may not be as pervasive, or in cases when a conversion to open surgery might be required.
“It is a real concern that trainees are being taught robotic surgery for what is not really needed to be robotic in the mainstream,” Dr. Hughes said. “Then they come out with less experience with straight stick laparoscopy and we’ve certainly seen lots of problems in the laparoscopic range for people once they get to the point that they have to convert to open. We really don’t have a large volume of trainee experience in complex open surgery. That’s a big problem. Robotics certainly isn’t going to make that a lot better.”
[email protected]
Despite the lack of a clear-cut cost-effectiveness case, it appears that the use of robotics in surgery is on its way to becoming the norm.
What may help make that cost-effectiveness case is time, as the hardware becomes cheaper and the technology becomes more widespread.
“I think there are many surgeons who are doing robotic surgery who truly believe that the current of state of the art will bear only a partial resemblance to the state of the art 5-10 years from now,” Dr. Henry Pitt, chief quality officer of the Temple University Health System, Philadelphia, said in an interview. “And part of the persistence by many of the robotic surgeons, while they fully understand that what they are doing is not cost effective now, is that they believe that the next few generations of this technology will become the state of the art and will become cost effective.”
Dr. Pitt, an ACS Fellow, compared the evolution of robotic surgery to that of computers and the application of Moore’s Law, by which the technology advances rapidly while the price eventually comes down.
Spread of robot-assisted surgery
Research by the surgical robot industry and independent investigators shows that use of robotics is on an upward climb. A presentation at the 2015 American College of Surgeons Clinical Congress reveals that, based on retrospective review of the Nationwide Inpatient Sample from 2009-2012 using the ICD-9 code that identifies robot-assisted procedures, there were 113,022 robot-assisted procedures in that period.
In 2009, there were 21,006 procedures, with the volume growing to 33,713 in 2012. Perhaps more telling about the use of robotics is that it is not confined to specialties with a proven track record such as gynecology and urology. The study, led by Dr. G. Paul Wright of Grand Rapids Medical Education Partners and Michigan State University, shows an increase in robotics use in general surgery procedures by 4.5-fold from 2009-2012, with steady increases each year. General surgery accounted for 11.8% of robot-assisted cases in 2012, Dr. Wright and colleagues reported.

Dr. Wright suggested a number of factors contributing to the “surprising” growth of robotics in general surgery.
“I think the growth is stimulated, at least in part, by market forces,” he said in an interview. “For patients who are the consumers of health care, they are often drawn to [surgeons] whom they perceive to be on the cutting edge. Robotic surgery certainly offers that intrigue. With greater access to the technology, if a patient is seeking a robotic surgery that you do not offer as a surgeon, they can likely find that elsewhere, potentially at your competitor. Another driving force is potentially the ease of suturing with the robot which more resembles open surgery while advanced laparoscopic suturing in tight spaces is a more limited skill set among general surgeons.”
Dr. Wright added that “some of the stigma of robotic surgery is dissolving among surgeons as they become more familiar with the technology. I also believe the market forces will help drive down costs in robotics which may help to increase utilization as well.”
Industry touts cost effectiveness
And while Dr. Pitt is looking forward, the industry is energetically promoting the cost effectiveness of robotics today. Intuitive Surgical Inc., manufacturer of the da Vinci surgery robot presented in a recent press briefing data culled from literature found on PubMed showing that robotic surgery, at least with their product, is cost effective now (view the report at http://goo.gl/AfAuT3). But in recent years, the large number of clinical trials comparing robotic vs. laparoscopic surgery for colorectal disease, for example, is equivocal. For many procedures, trials appear to show similar between laparoscopic and robot-assisted approaches, with the main factors in play being length of operation time, hospital stay, and surgical site infection.
According to Intuitive Surgical’s manager of clinical economics, Andrew Yiu, a review of peer-reviewed articles that featured nationwide cohorts of comparisons and included comparisons to both open and laparoscopic procedures outcomes shows improvements across a variety of procedures in terms of hospital stay, lower conversion rates, reduced complications, reduced 30-day readmissions, and improved patient satisfaction, though outcomes were more pronounced in some areas compared with others. Highlighting one procedure in particular – benign hysterectomy – Mr. Yiu noted that rates of minor and major complications associated with robotic vs. laparoscopic surgery were relatively similar but that the conversion rate to open surgery from laparoscopic surgery was 3.9%, compared with 0.9% for da Vinci–assisted surgery.

Overall, Intuitive Surgical calculated the potential savings on the episode of care with the da Vinci system at $1,087 vs. laparoscopic procedures and $2,996 over open surgery.
The company is currently promoting its da Vinci IX Surgical System. Cleared by the Food and Drug Administration in April 2014, Intuitive Surgical says that this latest version has broader capabilities than earlier generations and has been optimized for complex, multiquadrant surgeries.
Costs and other trade-offs
In some ways, robot-assisted surgery is following the same path as laparoscopic surgery, observed Dr. Tyler Hughes of the department of general surgery at McPherson (Kan.) Hospital.
“Certainly, we had the issue back in the late ‘80s and early ‘90s of laparoscopy,” Dr. Hughes said in an interview. “Was it cost effective? Because at the time $30,000 or $40,000 for a laparoscopic set-up seemed like a horse-choking amount of money. However, it converted a 5-day in-the-hospital experience to a 4-hour day-surgery experience. The nonclinical benefits to the patient were so enormous that it took the whole place by storm.”
Dr. Hughes, an ACS Fellow, suggested that the leap in savings elsewhere in the episode of care when using laparoscopic surgery over open surgery is not being mirrored in the robotic space and suggested that money should be spent in other areas of need.
“Cost is going to choke the future if we’re not careful. We need nurses. We need infrastructure. We need to be careful to buy things that really make a big difference,” he said.
In a study presented by at the ACS Clinical Congress on a cost-benefit analysis of robotic vs. laparoscopic colectomy, Anastasia Postoev, a fourth-year medical student at Caribbean Medical University in Willemstad, Curacao and her colleagues found little difference in clinical outcomes but higher costs as a result of added time needed to prepare the robot for surgery. The study showed that the potential up-front costs can be $1 to $2 million for the robot in addition to ongoing costs related to training and maintenance. Dr. Hughes said. “It is a terrific marketing tool in the sense that you can certainly attract a lot of people to a hospital with robotic surgery, but whether or not those outcomes are truly different and the cost of those outcomes are justifiable is yet to be determined.”
The ease of use of robotics, however, could end up being a double-edged sword, particularly if there are areas of the country where robotics may not be as pervasive, or in cases when a conversion to open surgery might be required.
“It is a real concern that trainees are being taught robotic surgery for what is not really needed to be robotic in the mainstream,” Dr. Hughes said. “Then they come out with less experience with straight stick laparoscopy and we’ve certainly seen lots of problems in the laparoscopic range for people once they get to the point that they have to convert to open. We really don’t have a large volume of trainee experience in complex open surgery. That’s a big problem. Robotics certainly isn’t going to make that a lot better.”
[email protected]
CDC to celebrate best blood clot prevention strategies
The Centers for Disease Control and Prevention has launched a program to honor hospitals, health systems, and managed care organizations that have implemented effective strategies to prevent health care–associated blood clots.
The HA-VTE Prevention Challenge invites provider organizations around the world to submit evidence of demonstrated successful use of venous thromboembolism (VTE) prevention strategies and interventions. VTE leads to approximately 100,000 premature deaths in the United States every year, according to the CDC, yet as many as 70% of HA-VTEs are preventable, although fewer than half of hospital patients receive appropriate prevention. Indeed, about half of all blood clots happen after a recent hospital stay or surgery.
“Doctors and nurses in hospitals and other health care settings can save lives by implementing the best practices discovered through this challenge,” Dr. Tom Frieden, CDC director, said in a statement. “Tell us about what you are doing and what’s helping prevent blood clots, so we can advance science and save lives together.”
The purpose of the challenge is to highlight the systems, processes, and staffing that contribute to exceptional VTE prevention, according to the CDC. Processes may include the implementation of protocols, risk assessments, and the use of health information technology and clinical decision support tools. Seven of the highest scoring U.S. non-federal hospitals, multihospital systems, hospital networks, and managed care organizations will be recognized as HA-VTE Prevention Champions and will receive a cash award of $10,000 each. Winning submissions from U.S. federal and international entities will be eligible for nonmonetary recognition.
The CDC will accept submissions from Nov. 2, 2015, until Jan. 10, 2016. Winners will be announced in March 2016.
For more information, visit the HA-VTE Prevention Challenge website.
On Twitter: @richpizzi
The Centers for Disease Control and Prevention has launched a program to honor hospitals, health systems, and managed care organizations that have implemented effective strategies to prevent health care–associated blood clots.
The HA-VTE Prevention Challenge invites provider organizations around the world to submit evidence of demonstrated successful use of venous thromboembolism (VTE) prevention strategies and interventions. VTE leads to approximately 100,000 premature deaths in the United States every year, according to the CDC, yet as many as 70% of HA-VTEs are preventable, although fewer than half of hospital patients receive appropriate prevention. Indeed, about half of all blood clots happen after a recent hospital stay or surgery.
“Doctors and nurses in hospitals and other health care settings can save lives by implementing the best practices discovered through this challenge,” Dr. Tom Frieden, CDC director, said in a statement. “Tell us about what you are doing and what’s helping prevent blood clots, so we can advance science and save lives together.”
The purpose of the challenge is to highlight the systems, processes, and staffing that contribute to exceptional VTE prevention, according to the CDC. Processes may include the implementation of protocols, risk assessments, and the use of health information technology and clinical decision support tools. Seven of the highest scoring U.S. non-federal hospitals, multihospital systems, hospital networks, and managed care organizations will be recognized as HA-VTE Prevention Champions and will receive a cash award of $10,000 each. Winning submissions from U.S. federal and international entities will be eligible for nonmonetary recognition.
The CDC will accept submissions from Nov. 2, 2015, until Jan. 10, 2016. Winners will be announced in March 2016.
For more information, visit the HA-VTE Prevention Challenge website.
On Twitter: @richpizzi
The Centers for Disease Control and Prevention has launched a program to honor hospitals, health systems, and managed care organizations that have implemented effective strategies to prevent health care–associated blood clots.
The HA-VTE Prevention Challenge invites provider organizations around the world to submit evidence of demonstrated successful use of venous thromboembolism (VTE) prevention strategies and interventions. VTE leads to approximately 100,000 premature deaths in the United States every year, according to the CDC, yet as many as 70% of HA-VTEs are preventable, although fewer than half of hospital patients receive appropriate prevention. Indeed, about half of all blood clots happen after a recent hospital stay or surgery.
“Doctors and nurses in hospitals and other health care settings can save lives by implementing the best practices discovered through this challenge,” Dr. Tom Frieden, CDC director, said in a statement. “Tell us about what you are doing and what’s helping prevent blood clots, so we can advance science and save lives together.”
The purpose of the challenge is to highlight the systems, processes, and staffing that contribute to exceptional VTE prevention, according to the CDC. Processes may include the implementation of protocols, risk assessments, and the use of health information technology and clinical decision support tools. Seven of the highest scoring U.S. non-federal hospitals, multihospital systems, hospital networks, and managed care organizations will be recognized as HA-VTE Prevention Champions and will receive a cash award of $10,000 each. Winning submissions from U.S. federal and international entities will be eligible for nonmonetary recognition.
The CDC will accept submissions from Nov. 2, 2015, until Jan. 10, 2016. Winners will be announced in March 2016.
For more information, visit the HA-VTE Prevention Challenge website.
On Twitter: @richpizzi
Magnetic sphincter device clicks in real-world GERD patients
CHICAGO – Patients receiving the LINX magnetic sphincter device for gastroesophageal reflux disease (GERD) at a community hospital have outcomes comparable with those achieved at the best academic centers, a study suggests.
“This is a safe and effective operation that, importantly, is very reproducible in the community setting,” Dr. F. Paul “Tripp” Buckley III said at the annual clinical congress of the American College of Surgeons. “Unlike a Nissen [fundoplication] where you have to have 50 [surgeries completed] to be considered an expert and then do over 35 a year to continue to have great outcomes, this is a highly teachable event and can be employed out in the community with little hesitation.”
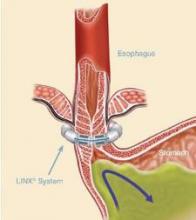
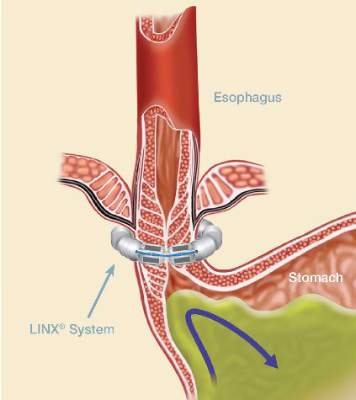
The magnetic sphincter augmentation device (LINX, Torax Medical) was approved in the United States in 2012 for the treatment of GERD and consists of a series of interlinked titanium-encased magnets implanted laparoscopically around the esophagus. The magnets augment a weak lower esophageal sphincter and avoid some of the complications of fundoplication, said Dr. Buckley, who disclosed serving as a proctor and speaker for Torax.
In the 5-year study leading to its approval, 64% of patients achieved the primary outcome of normalization of esophageal acid exposure or at least a 50% reduction in exposure at 1 year after sphincter augmentation and 93% of patients cut their use of proton-pump inhibitors (PPIs) by at least 50%. Six serious adverse events occurred (N Engl J Med. 2013;368:719-27).
Dr. Buckley, of Scott & White Health in Round Rock, Tex., and his associates evaluated their first 102 patients undergoing magnetic sphincter augmentation under general anesthesia at two community hospitals. GERD health-related quality of life (GERD-HRQL) scores were compared before and after surgery and overall results were compared with the clinical trial data. The highest possible total GERD-HRQL score is 75 (worst symptoms) and lowest score 0 (no symptoms).
The community and clinical trial cohorts were similar in age (median, 54 vs. 53 years), sex (both 52% male), median body mass index (both 28 kg/m2), and PPI use (98% vs. 100%), which was a requirement for trial entry.
After patients in the community underwent magnetic sphincter augmentation, PPI use decreased from 98% to 8%, median GERD-HRQL scores declined from 27 to 5, and patient satisfaction with GERD rose from 8% to 84%, which fell short of the satisfaction rate in the clinical trial (84% vs. 94%; P = .05), Dr. Buckley said.
The reduction in PPI use, however, was similar with that reported in the clinical trial, as was the percentage achieving at least 50% improvement in GERD-HRQL scores (86% vs. 92%), and operative times (median 49 vs. 36 minutes), he noted.
Device removal was rare at 1% in the community vs. 6% in the trial (P = .06).
Lower rates of dilation were noted in the community (9% vs. 19%; P = .04), perhaps because of refinements in technique and postoperative management, Dr. Buckley said.
“We know with this device you have to eat normally after implantation so that it will open and close within the scar capsule that’s naturally going to form,” he noted. “You have some patients that scar very tightly and you’ve got other patients that have a little bit of pain with eating and then suddenly they’re back on a liquid diet, which is a death knell for the success of this operation. So you really have to talk with these patients. I tell folks, ‘This is not a fix-it-and-forget-it type of operation.’ You’re going to get to know these patients pretty well, particularly in the early postoperative period.”
Final results recently reported with 5 years of follow-up in the clinical trial showed that 85% of patients were free from daily dependence on PPIs, heartburn was reduced from 89% to 12%, and moderate or severe regurgitation declined from 57% to 1.2%. No migrations or malfunctions occurred (J Laparoendosc Adv Surg Tech A. 2015 Oct;25[10]:787-92).
“One key aspect of this operation, and we think one of the reasons for its success and lack of migrations of the device, is that we keep the phrenoesophogeal ligament intact, so you are never in the mediastinum,” Dr. Buckley said. “For those of us who do a lot of Nissens, you end up blowing through it every time. But here when you keep it intact, you can roll it up on the distal esophagus and you can really reduce the hiatal hernia pretty significantly, even pretty large ones.”
A 3-cm cutoff was used for hiatal hernias in the trial and is recommended for early adoption in the community. A study is planned looking at hernias greater than 3 cm with no upper limit, where the minimal dissection method cannot reduce the hernia and full dissection is required, and the only requirement is that the patient have normal esophageal motility, he said.
Discussant Dr. Douglas Smink from Brigham and Women’s Hospital in Boston, said, “[There is a] lot of interest in this procedure obviously and it’s really nice to see what it’s like outside of the trials and to see your data is very similar.”
He went on to ask whether Dr. Buckley is still recommending fundoplication and, if so, in whom.
The LINX device has supplanted a classic Nissen fundoplication as the first-line operation in patients with good esophageal motility, although the device is not entirely covered by insurance, Dr. Buckley said.
“Basically, I tell patients I think it’s a marginally better operation. It’s not leaps and bounds, but it’s a marginally better operation,” he said, adding that though there has been concern about erosions, their erosion rate is zero and thus far, only seven erosions out of nearly 4,000 devices placed.
CHICAGO – Patients receiving the LINX magnetic sphincter device for gastroesophageal reflux disease (GERD) at a community hospital have outcomes comparable with those achieved at the best academic centers, a study suggests.
“This is a safe and effective operation that, importantly, is very reproducible in the community setting,” Dr. F. Paul “Tripp” Buckley III said at the annual clinical congress of the American College of Surgeons. “Unlike a Nissen [fundoplication] where you have to have 50 [surgeries completed] to be considered an expert and then do over 35 a year to continue to have great outcomes, this is a highly teachable event and can be employed out in the community with little hesitation.”
The magnetic sphincter augmentation device (LINX, Torax Medical) was approved in the United States in 2012 for the treatment of GERD and consists of a series of interlinked titanium-encased magnets implanted laparoscopically around the esophagus. The magnets augment a weak lower esophageal sphincter and avoid some of the complications of fundoplication, said Dr. Buckley, who disclosed serving as a proctor and speaker for Torax.
In the 5-year study leading to its approval, 64% of patients achieved the primary outcome of normalization of esophageal acid exposure or at least a 50% reduction in exposure at 1 year after sphincter augmentation and 93% of patients cut their use of proton-pump inhibitors (PPIs) by at least 50%. Six serious adverse events occurred (N Engl J Med. 2013;368:719-27).
Dr. Buckley, of Scott & White Health in Round Rock, Tex., and his associates evaluated their first 102 patients undergoing magnetic sphincter augmentation under general anesthesia at two community hospitals. GERD health-related quality of life (GERD-HRQL) scores were compared before and after surgery and overall results were compared with the clinical trial data. The highest possible total GERD-HRQL score is 75 (worst symptoms) and lowest score 0 (no symptoms).
The community and clinical trial cohorts were similar in age (median, 54 vs. 53 years), sex (both 52% male), median body mass index (both 28 kg/m2), and PPI use (98% vs. 100%), which was a requirement for trial entry.
After patients in the community underwent magnetic sphincter augmentation, PPI use decreased from 98% to 8%, median GERD-HRQL scores declined from 27 to 5, and patient satisfaction with GERD rose from 8% to 84%, which fell short of the satisfaction rate in the clinical trial (84% vs. 94%; P = .05), Dr. Buckley said.
The reduction in PPI use, however, was similar with that reported in the clinical trial, as was the percentage achieving at least 50% improvement in GERD-HRQL scores (86% vs. 92%), and operative times (median 49 vs. 36 minutes), he noted.
Device removal was rare at 1% in the community vs. 6% in the trial (P = .06).
Lower rates of dilation were noted in the community (9% vs. 19%; P = .04), perhaps because of refinements in technique and postoperative management, Dr. Buckley said.
“We know with this device you have to eat normally after implantation so that it will open and close within the scar capsule that’s naturally going to form,” he noted. “You have some patients that scar very tightly and you’ve got other patients that have a little bit of pain with eating and then suddenly they’re back on a liquid diet, which is a death knell for the success of this operation. So you really have to talk with these patients. I tell folks, ‘This is not a fix-it-and-forget-it type of operation.’ You’re going to get to know these patients pretty well, particularly in the early postoperative period.”
Final results recently reported with 5 years of follow-up in the clinical trial showed that 85% of patients were free from daily dependence on PPIs, heartburn was reduced from 89% to 12%, and moderate or severe regurgitation declined from 57% to 1.2%. No migrations or malfunctions occurred (J Laparoendosc Adv Surg Tech A. 2015 Oct;25[10]:787-92).
“One key aspect of this operation, and we think one of the reasons for its success and lack of migrations of the device, is that we keep the phrenoesophogeal ligament intact, so you are never in the mediastinum,” Dr. Buckley said. “For those of us who do a lot of Nissens, you end up blowing through it every time. But here when you keep it intact, you can roll it up on the distal esophagus and you can really reduce the hiatal hernia pretty significantly, even pretty large ones.”
A 3-cm cutoff was used for hiatal hernias in the trial and is recommended for early adoption in the community. A study is planned looking at hernias greater than 3 cm with no upper limit, where the minimal dissection method cannot reduce the hernia and full dissection is required, and the only requirement is that the patient have normal esophageal motility, he said.
Discussant Dr. Douglas Smink from Brigham and Women’s Hospital in Boston, said, “[There is a] lot of interest in this procedure obviously and it’s really nice to see what it’s like outside of the trials and to see your data is very similar.”
He went on to ask whether Dr. Buckley is still recommending fundoplication and, if so, in whom.
The LINX device has supplanted a classic Nissen fundoplication as the first-line operation in patients with good esophageal motility, although the device is not entirely covered by insurance, Dr. Buckley said.
“Basically, I tell patients I think it’s a marginally better operation. It’s not leaps and bounds, but it’s a marginally better operation,” he said, adding that though there has been concern about erosions, their erosion rate is zero and thus far, only seven erosions out of nearly 4,000 devices placed.
CHICAGO – Patients receiving the LINX magnetic sphincter device for gastroesophageal reflux disease (GERD) at a community hospital have outcomes comparable with those achieved at the best academic centers, a study suggests.
“This is a safe and effective operation that, importantly, is very reproducible in the community setting,” Dr. F. Paul “Tripp” Buckley III said at the annual clinical congress of the American College of Surgeons. “Unlike a Nissen [fundoplication] where you have to have 50 [surgeries completed] to be considered an expert and then do over 35 a year to continue to have great outcomes, this is a highly teachable event and can be employed out in the community with little hesitation.”
The magnetic sphincter augmentation device (LINX, Torax Medical) was approved in the United States in 2012 for the treatment of GERD and consists of a series of interlinked titanium-encased magnets implanted laparoscopically around the esophagus. The magnets augment a weak lower esophageal sphincter and avoid some of the complications of fundoplication, said Dr. Buckley, who disclosed serving as a proctor and speaker for Torax.
In the 5-year study leading to its approval, 64% of patients achieved the primary outcome of normalization of esophageal acid exposure or at least a 50% reduction in exposure at 1 year after sphincter augmentation and 93% of patients cut their use of proton-pump inhibitors (PPIs) by at least 50%. Six serious adverse events occurred (N Engl J Med. 2013;368:719-27).
Dr. Buckley, of Scott & White Health in Round Rock, Tex., and his associates evaluated their first 102 patients undergoing magnetic sphincter augmentation under general anesthesia at two community hospitals. GERD health-related quality of life (GERD-HRQL) scores were compared before and after surgery and overall results were compared with the clinical trial data. The highest possible total GERD-HRQL score is 75 (worst symptoms) and lowest score 0 (no symptoms).
The community and clinical trial cohorts were similar in age (median, 54 vs. 53 years), sex (both 52% male), median body mass index (both 28 kg/m2), and PPI use (98% vs. 100%), which was a requirement for trial entry.
After patients in the community underwent magnetic sphincter augmentation, PPI use decreased from 98% to 8%, median GERD-HRQL scores declined from 27 to 5, and patient satisfaction with GERD rose from 8% to 84%, which fell short of the satisfaction rate in the clinical trial (84% vs. 94%; P = .05), Dr. Buckley said.
The reduction in PPI use, however, was similar with that reported in the clinical trial, as was the percentage achieving at least 50% improvement in GERD-HRQL scores (86% vs. 92%), and operative times (median 49 vs. 36 minutes), he noted.
Device removal was rare at 1% in the community vs. 6% in the trial (P = .06).
Lower rates of dilation were noted in the community (9% vs. 19%; P = .04), perhaps because of refinements in technique and postoperative management, Dr. Buckley said.
“We know with this device you have to eat normally after implantation so that it will open and close within the scar capsule that’s naturally going to form,” he noted. “You have some patients that scar very tightly and you’ve got other patients that have a little bit of pain with eating and then suddenly they’re back on a liquid diet, which is a death knell for the success of this operation. So you really have to talk with these patients. I tell folks, ‘This is not a fix-it-and-forget-it type of operation.’ You’re going to get to know these patients pretty well, particularly in the early postoperative period.”
Final results recently reported with 5 years of follow-up in the clinical trial showed that 85% of patients were free from daily dependence on PPIs, heartburn was reduced from 89% to 12%, and moderate or severe regurgitation declined from 57% to 1.2%. No migrations or malfunctions occurred (J Laparoendosc Adv Surg Tech A. 2015 Oct;25[10]:787-92).
“One key aspect of this operation, and we think one of the reasons for its success and lack of migrations of the device, is that we keep the phrenoesophogeal ligament intact, so you are never in the mediastinum,” Dr. Buckley said. “For those of us who do a lot of Nissens, you end up blowing through it every time. But here when you keep it intact, you can roll it up on the distal esophagus and you can really reduce the hiatal hernia pretty significantly, even pretty large ones.”
A 3-cm cutoff was used for hiatal hernias in the trial and is recommended for early adoption in the community. A study is planned looking at hernias greater than 3 cm with no upper limit, where the minimal dissection method cannot reduce the hernia and full dissection is required, and the only requirement is that the patient have normal esophageal motility, he said.
Discussant Dr. Douglas Smink from Brigham and Women’s Hospital in Boston, said, “[There is a] lot of interest in this procedure obviously and it’s really nice to see what it’s like outside of the trials and to see your data is very similar.”
He went on to ask whether Dr. Buckley is still recommending fundoplication and, if so, in whom.
The LINX device has supplanted a classic Nissen fundoplication as the first-line operation in patients with good esophageal motility, although the device is not entirely covered by insurance, Dr. Buckley said.
“Basically, I tell patients I think it’s a marginally better operation. It’s not leaps and bounds, but it’s a marginally better operation,” he said, adding that though there has been concern about erosions, their erosion rate is zero and thus far, only seven erosions out of nearly 4,000 devices placed.
AT THE ACS CLINICAL CONGRESS
Key clinical point: Magnetic sphincter augmentation in the community setting provides results comparable with those seen in clinical trials.
Major finding: PPI use decreased from 98% to 8% after magnetic sphincter augmentation.
Data source: Prospective study in 102 patients with gastroesophageal reflux disease.
Disclosures: Dr. Buckley reported serving as a proctor and speaker for Torax Medical, which markets the Linx system.
Study: High rate of medical errors in postop drug administrations
Medication errors or adverse drug events after surgery occur in as many as one in twenty perioperative medication administrations, according to data published online in the journal Anesthesiology.
A prospective observational study of 277 surgical operations and 3,671 medication administrations found 193 cases (5.3%) involved a medication error or adverse drug event, nearly four-fifths (79.3%) of which were preventable and 68.9% of which were serious (Anesthesiology. 2015 Oct. doi:10.1097/ALN.0000000000000904).
Among the 51 medication errors that led to adverse reactions, nearly half were the result of inappropriate medication doses and 31.4% were due to omitted medications or failure to act, but the most common overall error type was a labeling error.
The medications most commonly associated with errors were propofol, phenylephrine, and fentanyl, and operations greater than 6 hours in duration or with 13 or more medication administrations were associated with a significantly greater risk of errors.
“Examples of technology-based interventions [to minimize perioperative MEs and/or ADEs] include bar code–assisted syringe labeling systems, point-of-care bar code–assisted anesthesia documentation systems, specific drug decision support, and alerts,” wrote the study’s lead author Dr. Karen C. Nanji of Massachusetts General Hospital in Boston, and her coauthors.
The study was supported by the Doctors Company Foundation and the National Institute of General Medical Sciences of the National Institutes of Health. One coauthor – Dr. David Bates – declared financial interests in medical decision support software, as well as funding and positions with a variety of medical technology companies.
Medication errors or adverse drug events after surgery occur in as many as one in twenty perioperative medication administrations, according to data published online in the journal Anesthesiology.
A prospective observational study of 277 surgical operations and 3,671 medication administrations found 193 cases (5.3%) involved a medication error or adverse drug event, nearly four-fifths (79.3%) of which were preventable and 68.9% of which were serious (Anesthesiology. 2015 Oct. doi:10.1097/ALN.0000000000000904).
Among the 51 medication errors that led to adverse reactions, nearly half were the result of inappropriate medication doses and 31.4% were due to omitted medications or failure to act, but the most common overall error type was a labeling error.
The medications most commonly associated with errors were propofol, phenylephrine, and fentanyl, and operations greater than 6 hours in duration or with 13 or more medication administrations were associated with a significantly greater risk of errors.
“Examples of technology-based interventions [to minimize perioperative MEs and/or ADEs] include bar code–assisted syringe labeling systems, point-of-care bar code–assisted anesthesia documentation systems, specific drug decision support, and alerts,” wrote the study’s lead author Dr. Karen C. Nanji of Massachusetts General Hospital in Boston, and her coauthors.
The study was supported by the Doctors Company Foundation and the National Institute of General Medical Sciences of the National Institutes of Health. One coauthor – Dr. David Bates – declared financial interests in medical decision support software, as well as funding and positions with a variety of medical technology companies.
Medication errors or adverse drug events after surgery occur in as many as one in twenty perioperative medication administrations, according to data published online in the journal Anesthesiology.
A prospective observational study of 277 surgical operations and 3,671 medication administrations found 193 cases (5.3%) involved a medication error or adverse drug event, nearly four-fifths (79.3%) of which were preventable and 68.9% of which were serious (Anesthesiology. 2015 Oct. doi:10.1097/ALN.0000000000000904).
Among the 51 medication errors that led to adverse reactions, nearly half were the result of inappropriate medication doses and 31.4% were due to omitted medications or failure to act, but the most common overall error type was a labeling error.
The medications most commonly associated with errors were propofol, phenylephrine, and fentanyl, and operations greater than 6 hours in duration or with 13 or more medication administrations were associated with a significantly greater risk of errors.
“Examples of technology-based interventions [to minimize perioperative MEs and/or ADEs] include bar code–assisted syringe labeling systems, point-of-care bar code–assisted anesthesia documentation systems, specific drug decision support, and alerts,” wrote the study’s lead author Dr. Karen C. Nanji of Massachusetts General Hospital in Boston, and her coauthors.
The study was supported by the Doctors Company Foundation and the National Institute of General Medical Sciences of the National Institutes of Health. One coauthor – Dr. David Bates – declared financial interests in medical decision support software, as well as funding and positions with a variety of medical technology companies.
FROM ANESTHESIOLOGY
Key clinical point: One in twenty perioperative medication administrations may involve a medication error and/or adverse drug event.
Major finding: Nearly four-fifths of perioperative medication errors or adverse events are preventable.
Data source: A prospective observational study of 277 surgical operations and 3,671 medication administrations.
Disclosures: The study was supported by the Doctors Company Foundation and the National Institute of General Medical Sciences of the National Institutes of Health. One coauthor – Dr. David Bates – declared financial interests in medical decision support software, as well as funding and positions with a variety of medical technology companies.
Concurrent mesh herniorrhaphy with bowel surgery doesn’t up morbidity
Concurrent colorectal surgery and mesh herniorrhaphy can be done safely, according to findings from a case-matched study based on American College of Surgeons National Surgical Quality Improvement Program data.
“Our study suggests that mesh repair for [ventral hernia] in the setting of [colorectal surgery] does not worsen 30-day postoperative outcomes,” wrote Dr. Cigdem Benlice and colleagues of the Cleveland Clinic. The study was published in the American Journal of Surgery (2015; 210[4]:766-771]
These two operations are undertaken concurrently in part to save patients from further procedures and anesthesia, but the practice is limited by safety concerns. The complications anticipated include intra-abdominal adhesions, chronic draining sinus, chronic enteric fistula, chronic wound infection, and mesh migration. Any one of these complications could entail further surgical interventions.
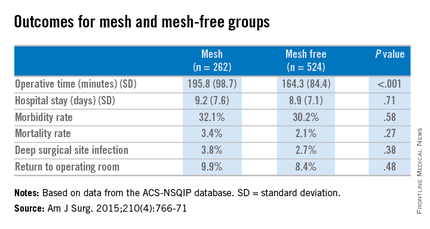
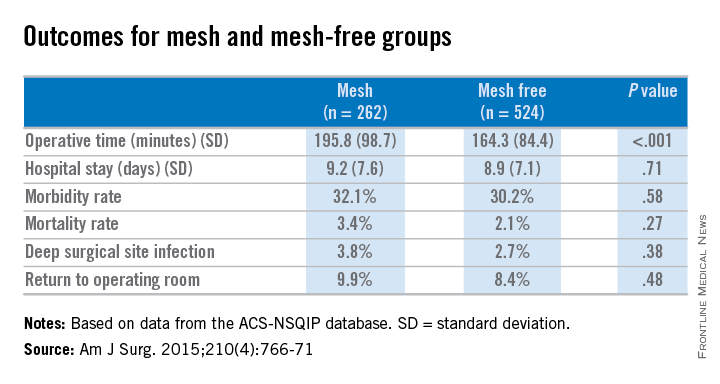
Dr. Benlice and her colleagues used the national, validated, risk-adjusted American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database to look at short-term outcomes after concurrent ventral hernia repair (VHR) and colorectal surgery (CS), and the risk factors associated with complications. Data on 2,250 patients having CS and VHR operations simultaneously from 2005 to 2010 were reviewed. Patients having simultaneous VHR with mesh and CS (262) were matched closely with those having both procedures but without mesh (524). The case-matching criteria included diagnosis, type of bowel procedure, and American Society of Anesthesiologists (ASA) class, and follow-up was limited to 30 days.
In a comparison of the mesh and nonmesh groups, morbidity rates were found to be similar (32% vs. 30%, P = .58) as were mortality rates (3.4% vs. 2.1%, P = .27). The rate of deep surgical site infections (3.8% vs. 2.7%, P = .38) were similar between the two groups, and wound disruptions (3.1% vs. 3.1%) rates were the same. The length of hospital stay was also comparable. Mean operating time, however, proved longer in patients who had hernia repair using mesh (196 minutes vs. 164 minutes, P less than .001).
A multivariate analysis showed that open colorectal procedures, III or IV ASA class, smoking, and preoperative open wound or wound infection were significant and independent risk factors (odds ratios, 2.67, 2.51, 2.06, and 2.27, respectively) for surgical site infection.
The limitations of this study reflect some of the limitations of the ACS-NSQIP database. Long-term follow-up, hernia size, type of mesh used, and type of technique used in the operation could not be assessed using the available data. In addition, further analysis of the subgroups of patients with surgical site infections would be needed to examine the impact of mesh (and types of mesh) use on clean-contaminated cases. Researchers noted that at the Cleveland Clinic, type of mesh (biologic vs. nonbiologic) used has been found to have no impact on rates of wound and mesh infection and hernia recurrence, but they were unable to do this kind of analysis with the available data for simultaneous bowel and hernia repair surgery.
The researchers concluded, “the large cohort and strict inclusion, exclusion, and case-matching criteria strengthen the clinical value of this study. When performed simultaneously with CS, VHR with mesh can be performed without increasing perioperative and short-term postoperative morbidity.”
The researchers had no relevant disclosures.
Concurrent colorectal surgery and mesh herniorrhaphy can be done safely, according to findings from a case-matched study based on American College of Surgeons National Surgical Quality Improvement Program data.
“Our study suggests that mesh repair for [ventral hernia] in the setting of [colorectal surgery] does not worsen 30-day postoperative outcomes,” wrote Dr. Cigdem Benlice and colleagues of the Cleveland Clinic. The study was published in the American Journal of Surgery (2015; 210[4]:766-771]
These two operations are undertaken concurrently in part to save patients from further procedures and anesthesia, but the practice is limited by safety concerns. The complications anticipated include intra-abdominal adhesions, chronic draining sinus, chronic enteric fistula, chronic wound infection, and mesh migration. Any one of these complications could entail further surgical interventions.

Dr. Benlice and her colleagues used the national, validated, risk-adjusted American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database to look at short-term outcomes after concurrent ventral hernia repair (VHR) and colorectal surgery (CS), and the risk factors associated with complications. Data on 2,250 patients having CS and VHR operations simultaneously from 2005 to 2010 were reviewed. Patients having simultaneous VHR with mesh and CS (262) were matched closely with those having both procedures but without mesh (524). The case-matching criteria included diagnosis, type of bowel procedure, and American Society of Anesthesiologists (ASA) class, and follow-up was limited to 30 days.
In a comparison of the mesh and nonmesh groups, morbidity rates were found to be similar (32% vs. 30%, P = .58) as were mortality rates (3.4% vs. 2.1%, P = .27). The rate of deep surgical site infections (3.8% vs. 2.7%, P = .38) were similar between the two groups, and wound disruptions (3.1% vs. 3.1%) rates were the same. The length of hospital stay was also comparable. Mean operating time, however, proved longer in patients who had hernia repair using mesh (196 minutes vs. 164 minutes, P less than .001).
A multivariate analysis showed that open colorectal procedures, III or IV ASA class, smoking, and preoperative open wound or wound infection were significant and independent risk factors (odds ratios, 2.67, 2.51, 2.06, and 2.27, respectively) for surgical site infection.
The limitations of this study reflect some of the limitations of the ACS-NSQIP database. Long-term follow-up, hernia size, type of mesh used, and type of technique used in the operation could not be assessed using the available data. In addition, further analysis of the subgroups of patients with surgical site infections would be needed to examine the impact of mesh (and types of mesh) use on clean-contaminated cases. Researchers noted that at the Cleveland Clinic, type of mesh (biologic vs. nonbiologic) used has been found to have no impact on rates of wound and mesh infection and hernia recurrence, but they were unable to do this kind of analysis with the available data for simultaneous bowel and hernia repair surgery.
The researchers concluded, “the large cohort and strict inclusion, exclusion, and case-matching criteria strengthen the clinical value of this study. When performed simultaneously with CS, VHR with mesh can be performed without increasing perioperative and short-term postoperative morbidity.”
The researchers had no relevant disclosures.
Concurrent colorectal surgery and mesh herniorrhaphy can be done safely, according to findings from a case-matched study based on American College of Surgeons National Surgical Quality Improvement Program data.
“Our study suggests that mesh repair for [ventral hernia] in the setting of [colorectal surgery] does not worsen 30-day postoperative outcomes,” wrote Dr. Cigdem Benlice and colleagues of the Cleveland Clinic. The study was published in the American Journal of Surgery (2015; 210[4]:766-771]
These two operations are undertaken concurrently in part to save patients from further procedures and anesthesia, but the practice is limited by safety concerns. The complications anticipated include intra-abdominal adhesions, chronic draining sinus, chronic enteric fistula, chronic wound infection, and mesh migration. Any one of these complications could entail further surgical interventions.

Dr. Benlice and her colleagues used the national, validated, risk-adjusted American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database to look at short-term outcomes after concurrent ventral hernia repair (VHR) and colorectal surgery (CS), and the risk factors associated with complications. Data on 2,250 patients having CS and VHR operations simultaneously from 2005 to 2010 were reviewed. Patients having simultaneous VHR with mesh and CS (262) were matched closely with those having both procedures but without mesh (524). The case-matching criteria included diagnosis, type of bowel procedure, and American Society of Anesthesiologists (ASA) class, and follow-up was limited to 30 days.
In a comparison of the mesh and nonmesh groups, morbidity rates were found to be similar (32% vs. 30%, P = .58) as were mortality rates (3.4% vs. 2.1%, P = .27). The rate of deep surgical site infections (3.8% vs. 2.7%, P = .38) were similar between the two groups, and wound disruptions (3.1% vs. 3.1%) rates were the same. The length of hospital stay was also comparable. Mean operating time, however, proved longer in patients who had hernia repair using mesh (196 minutes vs. 164 minutes, P less than .001).
A multivariate analysis showed that open colorectal procedures, III or IV ASA class, smoking, and preoperative open wound or wound infection were significant and independent risk factors (odds ratios, 2.67, 2.51, 2.06, and 2.27, respectively) for surgical site infection.
The limitations of this study reflect some of the limitations of the ACS-NSQIP database. Long-term follow-up, hernia size, type of mesh used, and type of technique used in the operation could not be assessed using the available data. In addition, further analysis of the subgroups of patients with surgical site infections would be needed to examine the impact of mesh (and types of mesh) use on clean-contaminated cases. Researchers noted that at the Cleveland Clinic, type of mesh (biologic vs. nonbiologic) used has been found to have no impact on rates of wound and mesh infection and hernia recurrence, but they were unable to do this kind of analysis with the available data for simultaneous bowel and hernia repair surgery.
The researchers concluded, “the large cohort and strict inclusion, exclusion, and case-matching criteria strengthen the clinical value of this study. When performed simultaneously with CS, VHR with mesh can be performed without increasing perioperative and short-term postoperative morbidity.”
The researchers had no relevant disclosures.
FROM THE AMERICAN JOURNAL OF SURGERY
Key clinical point: Simultaneous bowel and hernia surgery with mesh are comparable to a nonmesh operation in terms of postoperative morbidity.
Major finding: In a comparison of the mesh and nonmesh groups, morbidity rates were found to be similar (32% vs. 30%, P = .27) as was the rate of deep surgical site infections (3.8% vs. 2.7%, P = .38).
Data source: ACS-NSQIP data on case-matched patients having concurrent bowel and hernia surgery, with mesh (262) and without mesh (524).
Disclosures: The researchers had no relevant disclosures.
Early TIPS tied to mortality reduction in esophageal bleeds
HONOLULU – Early use of a transjugular intrahepatic portosystemic shunt (TIPS) is associated with substantial reductions in mortality, according to an analysis of a national inpatient database.
Based on this study, “early use of TIPS, together with patient and physician education on current guidelines and protocols, should continue to be a priority to improve patient outcomes” in patients with hepatic cirrhosis and risk of recurrent esophageal variceal bleeds, reported Dr. Basile Njei, a gastroenterology fellow at Yale University, New Haven, Conn.
In this study, the Nationwide Inpatient Sample database was queried by ICD-9 codes to identify patients with esophageal variceal bleeding treated between the years 2000 and 2010. The goal was to compare early use of TIPS, defined as TIPS administered within 72 hours of the bleeding, relative to rescue TIPS, defined as TIPS after two or more episodes of bleeding or one bleeding episode followed by another endoscopic intervention, such as balloon tamponade or surgery.
Over the period of study, a Poisson regression analysis used to control for multiple variables associated any TIPS utilization with an inverse association with overall mortality, producing a relative risk of 0.88 (95% confidence interval, 0.83-0.92). In the context of timing of TIPS, in-hospital mortality fell from 5.6% for those who received rescue TIPS to 1.5% in those who underwent early TIPS.
On multivariate analysis, an advantage was observed for early TIPS relative to rescue TIPS for in-hospital mortality (RR, 0.85; P less than .01), in-hospital rebleeding (RR, 0.57; P less than .01), and length of hospital stay (RR, 0.87; P less than .01). Rates of sepsis (RR, 0.83; P = .32) and hepatic encephalopathy (RR, 0.87; P = .22) were not significantly lower in the early TIPS group, but they were also not increased. For early TIPS versus no TIPS, the advantages on multivariate analysis were similar for both in-hospital deaths (RR, 0.87; P less than .01) and in-hospital rebleeding (RR, 0.57; P less than .01), but no advantage was seen for length of stay for TIPS versus no TIPS (RR, 0.99; P = .18).
Overall, there was a steady decline in mortality associated with esophageal variceal bleeding over the period of evaluation, falling incrementally over time from 656 deaths per 100,000 hospitalizations in 2000 to 412 deaths per 100,000 in 2010. This 37.2% reduction was statistically significant (P less than .01). The reduction in mortality was inversely associated with an increasing use of TIPS over the study period.
The data from this analysis are consistent with a multicenter randomized trial conducted several years ago in Europe (N Engl J Med. 2010;362:2370-9). In that study 63 patients with hepatic cirrhosis and acute variceal bleeding who had been treated with vasoactive drugs plus endoscopic therapy were randomized to early TIPS or rescue TIPS. At 1 year, 86% of those in the early TIPS group were alive versus 61% (P = .01) of those randomized to receive TIPS as a rescue strategy.
Relative to the previous study, the key finding of this study is that early TIPS “is associated with significant short-term reductions in rebleeding and mortality without a significant increase in encephalopathy in real world U.S. clinical practice,” according to Dr. Njei. It substantiates the European study and encourages a protocol that emphasizes early TIPS, particularly in those with a high risk of repeat esophageal variceal bleeding.
In the discussion that followed the presentation of these results at the annual meeting of the American College of Gastroenterology, the moderator, Dr. Paul Y. Kwo, medical director of liver transplantation, Indiana University, Indianapolis, pointed out, that some of those in the rescue TIPS group might simply have been poor candidates for this intervention. Although he praised the methodology of this study, which won the 2015 ACG Fellows-In-Training Award, he questioned whether rescue TIPS was a last resort salvage therapy in those initially considered poor risks for TIPS. Dr. Njei responded that the multivariate analysis was specifically designed to control for variables such as risk status to diminish this potential bias. Indeed, he said he believes TIPS is underemployed.
“The relatively small percentage of eligible cases receiving early TIPS suggests that there is room for further improvement in the treatment of patients with decompensated cirrhosis and esophageal variceal bleeding,” Dr. Njei concluded.
Dr. Njei reported that he had no relevant financial relationships to disclose.
HONOLULU – Early use of a transjugular intrahepatic portosystemic shunt (TIPS) is associated with substantial reductions in mortality, according to an analysis of a national inpatient database.
Based on this study, “early use of TIPS, together with patient and physician education on current guidelines and protocols, should continue to be a priority to improve patient outcomes” in patients with hepatic cirrhosis and risk of recurrent esophageal variceal bleeds, reported Dr. Basile Njei, a gastroenterology fellow at Yale University, New Haven, Conn.
In this study, the Nationwide Inpatient Sample database was queried by ICD-9 codes to identify patients with esophageal variceal bleeding treated between the years 2000 and 2010. The goal was to compare early use of TIPS, defined as TIPS administered within 72 hours of the bleeding, relative to rescue TIPS, defined as TIPS after two or more episodes of bleeding or one bleeding episode followed by another endoscopic intervention, such as balloon tamponade or surgery.
Over the period of study, a Poisson regression analysis used to control for multiple variables associated any TIPS utilization with an inverse association with overall mortality, producing a relative risk of 0.88 (95% confidence interval, 0.83-0.92). In the context of timing of TIPS, in-hospital mortality fell from 5.6% for those who received rescue TIPS to 1.5% in those who underwent early TIPS.
On multivariate analysis, an advantage was observed for early TIPS relative to rescue TIPS for in-hospital mortality (RR, 0.85; P less than .01), in-hospital rebleeding (RR, 0.57; P less than .01), and length of hospital stay (RR, 0.87; P less than .01). Rates of sepsis (RR, 0.83; P = .32) and hepatic encephalopathy (RR, 0.87; P = .22) were not significantly lower in the early TIPS group, but they were also not increased. For early TIPS versus no TIPS, the advantages on multivariate analysis were similar for both in-hospital deaths (RR, 0.87; P less than .01) and in-hospital rebleeding (RR, 0.57; P less than .01), but no advantage was seen for length of stay for TIPS versus no TIPS (RR, 0.99; P = .18).
Overall, there was a steady decline in mortality associated with esophageal variceal bleeding over the period of evaluation, falling incrementally over time from 656 deaths per 100,000 hospitalizations in 2000 to 412 deaths per 100,000 in 2010. This 37.2% reduction was statistically significant (P less than .01). The reduction in mortality was inversely associated with an increasing use of TIPS over the study period.
The data from this analysis are consistent with a multicenter randomized trial conducted several years ago in Europe (N Engl J Med. 2010;362:2370-9). In that study 63 patients with hepatic cirrhosis and acute variceal bleeding who had been treated with vasoactive drugs plus endoscopic therapy were randomized to early TIPS or rescue TIPS. At 1 year, 86% of those in the early TIPS group were alive versus 61% (P = .01) of those randomized to receive TIPS as a rescue strategy.
Relative to the previous study, the key finding of this study is that early TIPS “is associated with significant short-term reductions in rebleeding and mortality without a significant increase in encephalopathy in real world U.S. clinical practice,” according to Dr. Njei. It substantiates the European study and encourages a protocol that emphasizes early TIPS, particularly in those with a high risk of repeat esophageal variceal bleeding.
In the discussion that followed the presentation of these results at the annual meeting of the American College of Gastroenterology, the moderator, Dr. Paul Y. Kwo, medical director of liver transplantation, Indiana University, Indianapolis, pointed out, that some of those in the rescue TIPS group might simply have been poor candidates for this intervention. Although he praised the methodology of this study, which won the 2015 ACG Fellows-In-Training Award, he questioned whether rescue TIPS was a last resort salvage therapy in those initially considered poor risks for TIPS. Dr. Njei responded that the multivariate analysis was specifically designed to control for variables such as risk status to diminish this potential bias. Indeed, he said he believes TIPS is underemployed.
“The relatively small percentage of eligible cases receiving early TIPS suggests that there is room for further improvement in the treatment of patients with decompensated cirrhosis and esophageal variceal bleeding,” Dr. Njei concluded.
Dr. Njei reported that he had no relevant financial relationships to disclose.
HONOLULU – Early use of a transjugular intrahepatic portosystemic shunt (TIPS) is associated with substantial reductions in mortality, according to an analysis of a national inpatient database.
Based on this study, “early use of TIPS, together with patient and physician education on current guidelines and protocols, should continue to be a priority to improve patient outcomes” in patients with hepatic cirrhosis and risk of recurrent esophageal variceal bleeds, reported Dr. Basile Njei, a gastroenterology fellow at Yale University, New Haven, Conn.
In this study, the Nationwide Inpatient Sample database was queried by ICD-9 codes to identify patients with esophageal variceal bleeding treated between the years 2000 and 2010. The goal was to compare early use of TIPS, defined as TIPS administered within 72 hours of the bleeding, relative to rescue TIPS, defined as TIPS after two or more episodes of bleeding or one bleeding episode followed by another endoscopic intervention, such as balloon tamponade or surgery.
Over the period of study, a Poisson regression analysis used to control for multiple variables associated any TIPS utilization with an inverse association with overall mortality, producing a relative risk of 0.88 (95% confidence interval, 0.83-0.92). In the context of timing of TIPS, in-hospital mortality fell from 5.6% for those who received rescue TIPS to 1.5% in those who underwent early TIPS.
On multivariate analysis, an advantage was observed for early TIPS relative to rescue TIPS for in-hospital mortality (RR, 0.85; P less than .01), in-hospital rebleeding (RR, 0.57; P less than .01), and length of hospital stay (RR, 0.87; P less than .01). Rates of sepsis (RR, 0.83; P = .32) and hepatic encephalopathy (RR, 0.87; P = .22) were not significantly lower in the early TIPS group, but they were also not increased. For early TIPS versus no TIPS, the advantages on multivariate analysis were similar for both in-hospital deaths (RR, 0.87; P less than .01) and in-hospital rebleeding (RR, 0.57; P less than .01), but no advantage was seen for length of stay for TIPS versus no TIPS (RR, 0.99; P = .18).
Overall, there was a steady decline in mortality associated with esophageal variceal bleeding over the period of evaluation, falling incrementally over time from 656 deaths per 100,000 hospitalizations in 2000 to 412 deaths per 100,000 in 2010. This 37.2% reduction was statistically significant (P less than .01). The reduction in mortality was inversely associated with an increasing use of TIPS over the study period.
The data from this analysis are consistent with a multicenter randomized trial conducted several years ago in Europe (N Engl J Med. 2010;362:2370-9). In that study 63 patients with hepatic cirrhosis and acute variceal bleeding who had been treated with vasoactive drugs plus endoscopic therapy were randomized to early TIPS or rescue TIPS. At 1 year, 86% of those in the early TIPS group were alive versus 61% (P = .01) of those randomized to receive TIPS as a rescue strategy.
Relative to the previous study, the key finding of this study is that early TIPS “is associated with significant short-term reductions in rebleeding and mortality without a significant increase in encephalopathy in real world U.S. clinical practice,” according to Dr. Njei. It substantiates the European study and encourages a protocol that emphasizes early TIPS, particularly in those with a high risk of repeat esophageal variceal bleeding.
In the discussion that followed the presentation of these results at the annual meeting of the American College of Gastroenterology, the moderator, Dr. Paul Y. Kwo, medical director of liver transplantation, Indiana University, Indianapolis, pointed out, that some of those in the rescue TIPS group might simply have been poor candidates for this intervention. Although he praised the methodology of this study, which won the 2015 ACG Fellows-In-Training Award, he questioned whether rescue TIPS was a last resort salvage therapy in those initially considered poor risks for TIPS. Dr. Njei responded that the multivariate analysis was specifically designed to control for variables such as risk status to diminish this potential bias. Indeed, he said he believes TIPS is underemployed.
“The relatively small percentage of eligible cases receiving early TIPS suggests that there is room for further improvement in the treatment of patients with decompensated cirrhosis and esophageal variceal bleeding,” Dr. Njei concluded.
Dr. Njei reported that he had no relevant financial relationships to disclose.
AT ACG 2015
Key clinical point:Early use of a transjugular intrahepatic portosystemic shunt to reduce the risk of esophageal variceal rebleeding is associated with reduced mortality.
Major finding: In those receiving early TIPS (TIPS administered within 72 hours of the bleeding) mortality was 1.5% vs. 5.6% for those receiving TIPS as rescue therapy.
Data source: A retrospective evaluation of a national inpatient database.
Disclosures: Dr. Njei reported that he had no relevant financial relationships to disclose.
Frail elders at high mortality risk in the year following surgery
SAN DIEGO – Frail elderly patients face a significantly increased risk of mortality in the year after undergoing major elective noncardiac surgery, a large study from Canada showed.
“The current literature on perioperative frailty clearly shows that being frail before surgery substantially increases your risk of adverse postoperative outcomes,” Dr. Daniel I. McIsaac said in an interview prior to the annual meeting of the American Society of Anesthesiologists, where the study was presented. “In fact, frailty may underlie a lot of the associations between advanced age and adverse postoperative outcomes. Frailty increases in prevalence with increasing age, and as we all know, the population is aging. Therefore, we expect to see an increasing number of frail patients coming for surgery.”
In an effort to determine the risk of 1-year mortality in frail elderly patients having major elective surgery, the researchers used population-based health administrative data in Ontario, to identify 202,811 patients over the age of 65 who had intermediate- to high-risk elective noncardiac surgery between 2002 and 2012. They used the Johns Hopkins Adjusted Clinical Groups (ACG) frailty indicator and captured all deaths that occurred within 1 year of surgery. Proportional hazards regression models adjusted for age, gender, and socioeconomic status were used to evaluate the impact of frailty on 1-year postoperative mortality.
Of the 202,811 patients, 6,289 (3.1%) were frail, reported Dr. McIsaac of the department of anesthesiology at the University of Ottawa. The 1-year postoperative mortality was 13.6% among frail patients, compared with 4.8% of nonfrail patients, for an adjusted hazard ratio of 2.23. Mortality was higher among frail patients for all types of surgery, compared with their nonfrail counterparts, with the exception of pancreaticoduodenectomy. Frailty had the strongest impact on the risk of mortality after total joint arthroplasty (adjusted hazard ratio of 3.79 for hip replacement and adjusted HR of 2.68 for knee replacement).
The risk of postoperative mortality for frail patients was much higher than for nonfrail patients in the early time period after surgery, especially during the first postoperative week. “Depending on how you control for other variables, a frail patient was 13-35 times more likely to die in the week after surgery than a nonfrail patient of the same age having the same surgery,” said Dr. McIsaac, who is also a staff anesthesiologist at the Ottawa Hospital. “This makes a lot of sense; frail patients are vulnerable to stressors, and surgery puts an enormous physiological stress on even healthy patients. Future work clearly needs to focus [on] addressing this high-risk time in the immediate postoperative period.”
He acknowledged certain limitations of the study, including its reliance on health administrative data and the fact that frailty “is a challenging exposure to study because there are a plethora of instruments that can be used to call someone frail. We used a validated set of frailty-defining diagnoses that have been shown to identify people with multidimensional frailty. That said, you can’t necessarily generalize our findings to patients identified as frail using other instruments.”
The findings, Dr. McIsaac concluded, suggest that clinicians should focus on identifying frail patients prior to surgery, “support them to ensure that they are more likely to derive benefit from surgery than harm, and focus on optimizing their care after surgery to address this early mortality risk.”
The study was funded by departments of anesthesiology at the University of Ottawa and at the Ottawa Hospital. Dr. McIsaac reported having no financial disclosures.
SAN DIEGO – Frail elderly patients face a significantly increased risk of mortality in the year after undergoing major elective noncardiac surgery, a large study from Canada showed.
“The current literature on perioperative frailty clearly shows that being frail before surgery substantially increases your risk of adverse postoperative outcomes,” Dr. Daniel I. McIsaac said in an interview prior to the annual meeting of the American Society of Anesthesiologists, where the study was presented. “In fact, frailty may underlie a lot of the associations between advanced age and adverse postoperative outcomes. Frailty increases in prevalence with increasing age, and as we all know, the population is aging. Therefore, we expect to see an increasing number of frail patients coming for surgery.”
In an effort to determine the risk of 1-year mortality in frail elderly patients having major elective surgery, the researchers used population-based health administrative data in Ontario, to identify 202,811 patients over the age of 65 who had intermediate- to high-risk elective noncardiac surgery between 2002 and 2012. They used the Johns Hopkins Adjusted Clinical Groups (ACG) frailty indicator and captured all deaths that occurred within 1 year of surgery. Proportional hazards regression models adjusted for age, gender, and socioeconomic status were used to evaluate the impact of frailty on 1-year postoperative mortality.
Of the 202,811 patients, 6,289 (3.1%) were frail, reported Dr. McIsaac of the department of anesthesiology at the University of Ottawa. The 1-year postoperative mortality was 13.6% among frail patients, compared with 4.8% of nonfrail patients, for an adjusted hazard ratio of 2.23. Mortality was higher among frail patients for all types of surgery, compared with their nonfrail counterparts, with the exception of pancreaticoduodenectomy. Frailty had the strongest impact on the risk of mortality after total joint arthroplasty (adjusted hazard ratio of 3.79 for hip replacement and adjusted HR of 2.68 for knee replacement).
The risk of postoperative mortality for frail patients was much higher than for nonfrail patients in the early time period after surgery, especially during the first postoperative week. “Depending on how you control for other variables, a frail patient was 13-35 times more likely to die in the week after surgery than a nonfrail patient of the same age having the same surgery,” said Dr. McIsaac, who is also a staff anesthesiologist at the Ottawa Hospital. “This makes a lot of sense; frail patients are vulnerable to stressors, and surgery puts an enormous physiological stress on even healthy patients. Future work clearly needs to focus [on] addressing this high-risk time in the immediate postoperative period.”
He acknowledged certain limitations of the study, including its reliance on health administrative data and the fact that frailty “is a challenging exposure to study because there are a plethora of instruments that can be used to call someone frail. We used a validated set of frailty-defining diagnoses that have been shown to identify people with multidimensional frailty. That said, you can’t necessarily generalize our findings to patients identified as frail using other instruments.”
The findings, Dr. McIsaac concluded, suggest that clinicians should focus on identifying frail patients prior to surgery, “support them to ensure that they are more likely to derive benefit from surgery than harm, and focus on optimizing their care after surgery to address this early mortality risk.”
The study was funded by departments of anesthesiology at the University of Ottawa and at the Ottawa Hospital. Dr. McIsaac reported having no financial disclosures.
SAN DIEGO – Frail elderly patients face a significantly increased risk of mortality in the year after undergoing major elective noncardiac surgery, a large study from Canada showed.
“The current literature on perioperative frailty clearly shows that being frail before surgery substantially increases your risk of adverse postoperative outcomes,” Dr. Daniel I. McIsaac said in an interview prior to the annual meeting of the American Society of Anesthesiologists, where the study was presented. “In fact, frailty may underlie a lot of the associations between advanced age and adverse postoperative outcomes. Frailty increases in prevalence with increasing age, and as we all know, the population is aging. Therefore, we expect to see an increasing number of frail patients coming for surgery.”
In an effort to determine the risk of 1-year mortality in frail elderly patients having major elective surgery, the researchers used population-based health administrative data in Ontario, to identify 202,811 patients over the age of 65 who had intermediate- to high-risk elective noncardiac surgery between 2002 and 2012. They used the Johns Hopkins Adjusted Clinical Groups (ACG) frailty indicator and captured all deaths that occurred within 1 year of surgery. Proportional hazards regression models adjusted for age, gender, and socioeconomic status were used to evaluate the impact of frailty on 1-year postoperative mortality.
Of the 202,811 patients, 6,289 (3.1%) were frail, reported Dr. McIsaac of the department of anesthesiology at the University of Ottawa. The 1-year postoperative mortality was 13.6% among frail patients, compared with 4.8% of nonfrail patients, for an adjusted hazard ratio of 2.23. Mortality was higher among frail patients for all types of surgery, compared with their nonfrail counterparts, with the exception of pancreaticoduodenectomy. Frailty had the strongest impact on the risk of mortality after total joint arthroplasty (adjusted hazard ratio of 3.79 for hip replacement and adjusted HR of 2.68 for knee replacement).
The risk of postoperative mortality for frail patients was much higher than for nonfrail patients in the early time period after surgery, especially during the first postoperative week. “Depending on how you control for other variables, a frail patient was 13-35 times more likely to die in the week after surgery than a nonfrail patient of the same age having the same surgery,” said Dr. McIsaac, who is also a staff anesthesiologist at the Ottawa Hospital. “This makes a lot of sense; frail patients are vulnerable to stressors, and surgery puts an enormous physiological stress on even healthy patients. Future work clearly needs to focus [on] addressing this high-risk time in the immediate postoperative period.”
He acknowledged certain limitations of the study, including its reliance on health administrative data and the fact that frailty “is a challenging exposure to study because there are a plethora of instruments that can be used to call someone frail. We used a validated set of frailty-defining diagnoses that have been shown to identify people with multidimensional frailty. That said, you can’t necessarily generalize our findings to patients identified as frail using other instruments.”
The findings, Dr. McIsaac concluded, suggest that clinicians should focus on identifying frail patients prior to surgery, “support them to ensure that they are more likely to derive benefit from surgery than harm, and focus on optimizing their care after surgery to address this early mortality risk.”
The study was funded by departments of anesthesiology at the University of Ottawa and at the Ottawa Hospital. Dr. McIsaac reported having no financial disclosures.
AT THE ASA ANNUAL MEETING
Key clinical point: Frail elderly patients face an increased risk of mortality within 1 year of undergoing noncardiac surgery.
Major finding: The 1-year postoperative mortality was 13.6% among frail patients, compared with 4.8% of nonfrail patients, for an adjusted hazard ratio of 2.23.
Data source: A study of 202,811 patients over the age of 65 years who underwent noncardiac surgery between 2002 and 2012.
Disclosures: The study was funded by departments of anesthesiology at the University of Ottawa and at The Ottawa Hospital. Dr. McIsaac reported having no financial disclosures.
Psychiatrists’ role in bariatric surgery important
Obesity has been officially declared a global epidemic by the World Health Organization. In virtually every region of the world, obesity’s adverse health effects are a well-documented public health crisis that affects people of all ages. In light of poor long-term weight management reported in most traditional diet strategies, attention has shifted to gastric bypass surgery as an effective treatment option for obesity.
Bariatric weight loss procedures are associated with numerous health benefits in patients: dramatic weight loss, rapid improvement in blood glucose levels, blood pressure stabilization, and resolution of obesity-related orthopedic problems. It’s not surprising, then, that gastric bypass surgery is a life-changing event for many patients.
Recently however, it has become clear that “life-changing” interventions do not always mean happy endings. Alongside these encouraging postsurgical outcomes are alarming counterintuitive reports of increased self-harm events. A recent study published in JAMA Surgery by Junaid A. Bhatti, Ph.D., of the department of evaluative clinical sciences, Sunnybrook Research Institute, Toronto, and the department of surgery, University of Toronto, and colleagues reported an increase in suicide attempts among postoperative gastric bypass patients (JAMA Surg. 2015 Oct 7. doi: 10.1001/jamasurg.2015.3414). The study conducted at Sunnybrook found that the rate of self-harm emergencies from attempted suicide was four times higher in these patients, compared with the general population.
Most gastric bypass surgery programs require a pre-op mental health evaluation. This is not enough, according to Dr. Peter F. Crookes, associate professor* of Bariatric Surgery at Keck Hospital of the University of Southern California, Los Angeles. Dr. Crookes’s clinical experiences and my (Dr. Baron’s) 20-plus years of conducting psychiatric evaluations on bypass patients have revealed more vexing issues regarding psychiatric illnesses occurring in morbidly obese patients. The requirement has been established for presurgical psychiatric assessment, but long-term post-surgical emotional and behavioral challenges need to be evaluated as well. In addition to primary mental illness, obese patients are at risk for stress-related exacerbations of preexisting psychopathology. After a body-altering surgical procedure like gastric bypass, maladaptive coping strategies are likely to complicate a patient’s physical AND emotional long-term recovery.
Many factors play into the development of these psychiatric symptoms, not the least of which are coping and emotional support systems. Literature on obesity and psychiatry has revealed connections between weight status and issues, such as childhood trauma, especially sexual abuse in childhood. While some patients claim that their weight loss surgery was the best thing they ever did for themselves, other patients have reported to me after significant weight loss: “I feel like a fat person trapped in a skinny body.” This surgery is not just body altering but can be identity altering. Patients also have reported relationship issues after significant weight loss. Relationship dynamics for both partners can become strained as a result of perceived changes in sexual attractiveness.
Given the short- and long-term consequences, it is essential for psychiatrists and other mental health care providers to work closely with the bypass surgical team at 1 month, 3 months, and 1-year postsurgical follow-up visits. These follow-ups are particularly important for patients who experience depression, relationship stress, or worsened psychiatric symptoms. In 2004, Dr. Crookes coauthored a then controversial study concerning bariatric surgery for obese patients with a formal diagnosis of schizophrenia or schizoaffective disorder (Obes Surg. 2004 Mar;14[3]:349-52). Historically, bariatric surgeons had avoided operating on these patients. However, his study showed that when psychotic symptoms were controlled postoperatively, these patients’ weight loss results were comparable to those of nonpsychotic patients.
Quality of life after gastric bypass depends on much more than a decrease in the number on the scale or clothing label. These reports demonstrate that psychiatrists play an important role in the overall biopsychosocial outcome of the patient.
Dr. Baron is professor of clinical psychiatry and interim chair of the department of psychiatry at the University of Southern California, Los Angeles. He also serves as director of the Global Center for Exercise, Psychiatry and Sports at USC. Ms. In addition, Dr. Baron is former chair of the department of psychiatry at Temple University, Philadelphia, where he directed the psychiatric component of the Bariatric Surgery program. Ms. Uno is a third-year medical student at USC.
*Correction, 10/29/2015: An earlier version of this story misstated the title of Dr. Crookes.
Obesity has been officially declared a global epidemic by the World Health Organization. In virtually every region of the world, obesity’s adverse health effects are a well-documented public health crisis that affects people of all ages. In light of poor long-term weight management reported in most traditional diet strategies, attention has shifted to gastric bypass surgery as an effective treatment option for obesity.
Bariatric weight loss procedures are associated with numerous health benefits in patients: dramatic weight loss, rapid improvement in blood glucose levels, blood pressure stabilization, and resolution of obesity-related orthopedic problems. It’s not surprising, then, that gastric bypass surgery is a life-changing event for many patients.
Recently however, it has become clear that “life-changing” interventions do not always mean happy endings. Alongside these encouraging postsurgical outcomes are alarming counterintuitive reports of increased self-harm events. A recent study published in JAMA Surgery by Junaid A. Bhatti, Ph.D., of the department of evaluative clinical sciences, Sunnybrook Research Institute, Toronto, and the department of surgery, University of Toronto, and colleagues reported an increase in suicide attempts among postoperative gastric bypass patients (JAMA Surg. 2015 Oct 7. doi: 10.1001/jamasurg.2015.3414). The study conducted at Sunnybrook found that the rate of self-harm emergencies from attempted suicide was four times higher in these patients, compared with the general population.
Most gastric bypass surgery programs require a pre-op mental health evaluation. This is not enough, according to Dr. Peter F. Crookes, associate professor* of Bariatric Surgery at Keck Hospital of the University of Southern California, Los Angeles. Dr. Crookes’s clinical experiences and my (Dr. Baron’s) 20-plus years of conducting psychiatric evaluations on bypass patients have revealed more vexing issues regarding psychiatric illnesses occurring in morbidly obese patients. The requirement has been established for presurgical psychiatric assessment, but long-term post-surgical emotional and behavioral challenges need to be evaluated as well. In addition to primary mental illness, obese patients are at risk for stress-related exacerbations of preexisting psychopathology. After a body-altering surgical procedure like gastric bypass, maladaptive coping strategies are likely to complicate a patient’s physical AND emotional long-term recovery.
Many factors play into the development of these psychiatric symptoms, not the least of which are coping and emotional support systems. Literature on obesity and psychiatry has revealed connections between weight status and issues, such as childhood trauma, especially sexual abuse in childhood. While some patients claim that their weight loss surgery was the best thing they ever did for themselves, other patients have reported to me after significant weight loss: “I feel like a fat person trapped in a skinny body.” This surgery is not just body altering but can be identity altering. Patients also have reported relationship issues after significant weight loss. Relationship dynamics for both partners can become strained as a result of perceived changes in sexual attractiveness.
Given the short- and long-term consequences, it is essential for psychiatrists and other mental health care providers to work closely with the bypass surgical team at 1 month, 3 months, and 1-year postsurgical follow-up visits. These follow-ups are particularly important for patients who experience depression, relationship stress, or worsened psychiatric symptoms. In 2004, Dr. Crookes coauthored a then controversial study concerning bariatric surgery for obese patients with a formal diagnosis of schizophrenia or schizoaffective disorder (Obes Surg. 2004 Mar;14[3]:349-52). Historically, bariatric surgeons had avoided operating on these patients. However, his study showed that when psychotic symptoms were controlled postoperatively, these patients’ weight loss results were comparable to those of nonpsychotic patients.
Quality of life after gastric bypass depends on much more than a decrease in the number on the scale or clothing label. These reports demonstrate that psychiatrists play an important role in the overall biopsychosocial outcome of the patient.
Dr. Baron is professor of clinical psychiatry and interim chair of the department of psychiatry at the University of Southern California, Los Angeles. He also serves as director of the Global Center for Exercise, Psychiatry and Sports at USC. Ms. In addition, Dr. Baron is former chair of the department of psychiatry at Temple University, Philadelphia, where he directed the psychiatric component of the Bariatric Surgery program. Ms. Uno is a third-year medical student at USC.
*Correction, 10/29/2015: An earlier version of this story misstated the title of Dr. Crookes.
Obesity has been officially declared a global epidemic by the World Health Organization. In virtually every region of the world, obesity’s adverse health effects are a well-documented public health crisis that affects people of all ages. In light of poor long-term weight management reported in most traditional diet strategies, attention has shifted to gastric bypass surgery as an effective treatment option for obesity.
Bariatric weight loss procedures are associated with numerous health benefits in patients: dramatic weight loss, rapid improvement in blood glucose levels, blood pressure stabilization, and resolution of obesity-related orthopedic problems. It’s not surprising, then, that gastric bypass surgery is a life-changing event for many patients.
Recently however, it has become clear that “life-changing” interventions do not always mean happy endings. Alongside these encouraging postsurgical outcomes are alarming counterintuitive reports of increased self-harm events. A recent study published in JAMA Surgery by Junaid A. Bhatti, Ph.D., of the department of evaluative clinical sciences, Sunnybrook Research Institute, Toronto, and the department of surgery, University of Toronto, and colleagues reported an increase in suicide attempts among postoperative gastric bypass patients (JAMA Surg. 2015 Oct 7. doi: 10.1001/jamasurg.2015.3414). The study conducted at Sunnybrook found that the rate of self-harm emergencies from attempted suicide was four times higher in these patients, compared with the general population.
Most gastric bypass surgery programs require a pre-op mental health evaluation. This is not enough, according to Dr. Peter F. Crookes, associate professor* of Bariatric Surgery at Keck Hospital of the University of Southern California, Los Angeles. Dr. Crookes’s clinical experiences and my (Dr. Baron’s) 20-plus years of conducting psychiatric evaluations on bypass patients have revealed more vexing issues regarding psychiatric illnesses occurring in morbidly obese patients. The requirement has been established for presurgical psychiatric assessment, but long-term post-surgical emotional and behavioral challenges need to be evaluated as well. In addition to primary mental illness, obese patients are at risk for stress-related exacerbations of preexisting psychopathology. After a body-altering surgical procedure like gastric bypass, maladaptive coping strategies are likely to complicate a patient’s physical AND emotional long-term recovery.
Many factors play into the development of these psychiatric symptoms, not the least of which are coping and emotional support systems. Literature on obesity and psychiatry has revealed connections between weight status and issues, such as childhood trauma, especially sexual abuse in childhood. While some patients claim that their weight loss surgery was the best thing they ever did for themselves, other patients have reported to me after significant weight loss: “I feel like a fat person trapped in a skinny body.” This surgery is not just body altering but can be identity altering. Patients also have reported relationship issues after significant weight loss. Relationship dynamics for both partners can become strained as a result of perceived changes in sexual attractiveness.
Given the short- and long-term consequences, it is essential for psychiatrists and other mental health care providers to work closely with the bypass surgical team at 1 month, 3 months, and 1-year postsurgical follow-up visits. These follow-ups are particularly important for patients who experience depression, relationship stress, or worsened psychiatric symptoms. In 2004, Dr. Crookes coauthored a then controversial study concerning bariatric surgery for obese patients with a formal diagnosis of schizophrenia or schizoaffective disorder (Obes Surg. 2004 Mar;14[3]:349-52). Historically, bariatric surgeons had avoided operating on these patients. However, his study showed that when psychotic symptoms were controlled postoperatively, these patients’ weight loss results were comparable to those of nonpsychotic patients.
Quality of life after gastric bypass depends on much more than a decrease in the number on the scale or clothing label. These reports demonstrate that psychiatrists play an important role in the overall biopsychosocial outcome of the patient.
Dr. Baron is professor of clinical psychiatry and interim chair of the department of psychiatry at the University of Southern California, Los Angeles. He also serves as director of the Global Center for Exercise, Psychiatry and Sports at USC. Ms. In addition, Dr. Baron is former chair of the department of psychiatry at Temple University, Philadelphia, where he directed the psychiatric component of the Bariatric Surgery program. Ms. Uno is a third-year medical student at USC.
*Correction, 10/29/2015: An earlier version of this story misstated the title of Dr. Crookes.
ACS: Less pneumonia, fewer deaths with ketamine for rib fracture pain
CHICAGO – Ketamine is a safe and simple alternative to epidural anesthesia for pain control in the setting of multiple rib fractures, investigators from the Jacobi Medical Center in the Bronx (N.Y.) concluded after reviewing their experience with the drug.
Epidural analgesia has been the standard for controlling pain after multiple rib fractures, but epidurals are sometimes contraindicated in trauma, especially with back and neck injuries. There’s also a bleeding risk, and the need for an on-call anesthesia team to place them, something not all hospitals have.
Those problems – and the success Jacobi surgeons reported with ketamine pain control after thoracotomy – led the hospital to switch to a ketamine-based rib fracture protocol in 2007.
“As far as we know, we are the only people doing this routinely for multiple rib fractures,” Dr. Joelle Getrajdman, a second-year surgery resident at the medical center, said at the annual clinical congress of the American College of Surgeons.
Patients there with two or more rib fractures get a low-dose peripheral intravenous infusion of ketamine 0.05 mg/kg per hour while in the ICU and step-down unit, along with other pain medications as indicated. The hospital discontinues ketamine once patients leave the step-down unit to prevent diversion for illicit use.
To see how well the protocol has worked, the investigators reviewed all 128 adult trauma patients who received ketamine for multiple rib fractures from 2007 to 2014.
These patients were 60 years old on average, with a median of six rib fractures, many of them bilateral. Almost half had injury severity scores above 15, and most had chest Abbreviated Injury Scores of at least 3. Pneumo- and hemothoraces were common. Patients spent a mean of 6 days in the surgical ICU and 13 days in the hospital.
Along with ketamine, almost all had a morphine or hydromorphone (Dilaudid) patient-controlled analgesia (PCA) pumps, more than half received IV ketorolac (Toradol), and about 40% IV Tylenol. Only 14% had paravertebral or intercostal blocks.
Fourteen patients (10.9%) developed pneumonia, and four (3.1%) died, which compares favorably with outcomes in patients receiving epidurals. Historically, epidural analgesia for multiple traumatic rib fractures has been associated with about an 18% pneumonia rate, and about 9% mortality.
Ketamine side effects were minimal; none of the patients had hallucinations or tachycardia, and three (2.3%) were hypotensive on the drug.
Jacobi’s database did not record how many times patients used their PCA pumps, so the study did not have a direct measure of pain control. The investigators plan to look into this question prospectively.
Even so, when patients hurt from rib fractures, they breathe shallowly, which puts them at risk for pneumonia and death. “We have lower rates” of both than with epidurals, “so you could extrapolate that we must be controlling pain better,” Dr. Getrajdman said.
Ketamine at high doses is an anesthetic, but at lower doses it’s an antagonist of N-methyl-D-aspartate (NMDA), and a mild opioid receptor agonist, “so we can give patients the same amount of morphine but achieve a higher analgesic effect,” she said.
Ketamine had been the subject of intense interest in recent years for pain control in a wide variety of settings, as well as for psychiatric and other problems. Clinicaltrials.gov currently lists 138 open investigations of the drug.
Among them is a randomized trial from the Medical College of Wisconsin pitting ketamine against placebo for rib fracture pain following blunt trauma.
Dr. Getrajdman has no disclosures, and there was no outside funding for the work.
CHICAGO – Ketamine is a safe and simple alternative to epidural anesthesia for pain control in the setting of multiple rib fractures, investigators from the Jacobi Medical Center in the Bronx (N.Y.) concluded after reviewing their experience with the drug.
Epidural analgesia has been the standard for controlling pain after multiple rib fractures, but epidurals are sometimes contraindicated in trauma, especially with back and neck injuries. There’s also a bleeding risk, and the need for an on-call anesthesia team to place them, something not all hospitals have.
Those problems – and the success Jacobi surgeons reported with ketamine pain control after thoracotomy – led the hospital to switch to a ketamine-based rib fracture protocol in 2007.
“As far as we know, we are the only people doing this routinely for multiple rib fractures,” Dr. Joelle Getrajdman, a second-year surgery resident at the medical center, said at the annual clinical congress of the American College of Surgeons.
Patients there with two or more rib fractures get a low-dose peripheral intravenous infusion of ketamine 0.05 mg/kg per hour while in the ICU and step-down unit, along with other pain medications as indicated. The hospital discontinues ketamine once patients leave the step-down unit to prevent diversion for illicit use.
To see how well the protocol has worked, the investigators reviewed all 128 adult trauma patients who received ketamine for multiple rib fractures from 2007 to 2014.
These patients were 60 years old on average, with a median of six rib fractures, many of them bilateral. Almost half had injury severity scores above 15, and most had chest Abbreviated Injury Scores of at least 3. Pneumo- and hemothoraces were common. Patients spent a mean of 6 days in the surgical ICU and 13 days in the hospital.
Along with ketamine, almost all had a morphine or hydromorphone (Dilaudid) patient-controlled analgesia (PCA) pumps, more than half received IV ketorolac (Toradol), and about 40% IV Tylenol. Only 14% had paravertebral or intercostal blocks.
Fourteen patients (10.9%) developed pneumonia, and four (3.1%) died, which compares favorably with outcomes in patients receiving epidurals. Historically, epidural analgesia for multiple traumatic rib fractures has been associated with about an 18% pneumonia rate, and about 9% mortality.
Ketamine side effects were minimal; none of the patients had hallucinations or tachycardia, and three (2.3%) were hypotensive on the drug.
Jacobi’s database did not record how many times patients used their PCA pumps, so the study did not have a direct measure of pain control. The investigators plan to look into this question prospectively.
Even so, when patients hurt from rib fractures, they breathe shallowly, which puts them at risk for pneumonia and death. “We have lower rates” of both than with epidurals, “so you could extrapolate that we must be controlling pain better,” Dr. Getrajdman said.
Ketamine at high doses is an anesthetic, but at lower doses it’s an antagonist of N-methyl-D-aspartate (NMDA), and a mild opioid receptor agonist, “so we can give patients the same amount of morphine but achieve a higher analgesic effect,” she said.
Ketamine had been the subject of intense interest in recent years for pain control in a wide variety of settings, as well as for psychiatric and other problems. Clinicaltrials.gov currently lists 138 open investigations of the drug.
Among them is a randomized trial from the Medical College of Wisconsin pitting ketamine against placebo for rib fracture pain following blunt trauma.
Dr. Getrajdman has no disclosures, and there was no outside funding for the work.
CHICAGO – Ketamine is a safe and simple alternative to epidural anesthesia for pain control in the setting of multiple rib fractures, investigators from the Jacobi Medical Center in the Bronx (N.Y.) concluded after reviewing their experience with the drug.
Epidural analgesia has been the standard for controlling pain after multiple rib fractures, but epidurals are sometimes contraindicated in trauma, especially with back and neck injuries. There’s also a bleeding risk, and the need for an on-call anesthesia team to place them, something not all hospitals have.
Those problems – and the success Jacobi surgeons reported with ketamine pain control after thoracotomy – led the hospital to switch to a ketamine-based rib fracture protocol in 2007.
“As far as we know, we are the only people doing this routinely for multiple rib fractures,” Dr. Joelle Getrajdman, a second-year surgery resident at the medical center, said at the annual clinical congress of the American College of Surgeons.
Patients there with two or more rib fractures get a low-dose peripheral intravenous infusion of ketamine 0.05 mg/kg per hour while in the ICU and step-down unit, along with other pain medications as indicated. The hospital discontinues ketamine once patients leave the step-down unit to prevent diversion for illicit use.
To see how well the protocol has worked, the investigators reviewed all 128 adult trauma patients who received ketamine for multiple rib fractures from 2007 to 2014.
These patients were 60 years old on average, with a median of six rib fractures, many of them bilateral. Almost half had injury severity scores above 15, and most had chest Abbreviated Injury Scores of at least 3. Pneumo- and hemothoraces were common. Patients spent a mean of 6 days in the surgical ICU and 13 days in the hospital.
Along with ketamine, almost all had a morphine or hydromorphone (Dilaudid) patient-controlled analgesia (PCA) pumps, more than half received IV ketorolac (Toradol), and about 40% IV Tylenol. Only 14% had paravertebral or intercostal blocks.
Fourteen patients (10.9%) developed pneumonia, and four (3.1%) died, which compares favorably with outcomes in patients receiving epidurals. Historically, epidural analgesia for multiple traumatic rib fractures has been associated with about an 18% pneumonia rate, and about 9% mortality.
Ketamine side effects were minimal; none of the patients had hallucinations or tachycardia, and three (2.3%) were hypotensive on the drug.
Jacobi’s database did not record how many times patients used their PCA pumps, so the study did not have a direct measure of pain control. The investigators plan to look into this question prospectively.
Even so, when patients hurt from rib fractures, they breathe shallowly, which puts them at risk for pneumonia and death. “We have lower rates” of both than with epidurals, “so you could extrapolate that we must be controlling pain better,” Dr. Getrajdman said.
Ketamine at high doses is an anesthetic, but at lower doses it’s an antagonist of N-methyl-D-aspartate (NMDA), and a mild opioid receptor agonist, “so we can give patients the same amount of morphine but achieve a higher analgesic effect,” she said.
Ketamine had been the subject of intense interest in recent years for pain control in a wide variety of settings, as well as for psychiatric and other problems. Clinicaltrials.gov currently lists 138 open investigations of the drug.
Among them is a randomized trial from the Medical College of Wisconsin pitting ketamine against placebo for rib fracture pain following blunt trauma.
Dr. Getrajdman has no disclosures, and there was no outside funding for the work.
AT THE ACS CLINICAL CONGRESS
Key clinical point: Patients with multiple rib fractures treated with ketamine for pain control had less risk of pneumonia and death than did patients receiving epidural for pain.
Major finding: Overall, 14 ketamine patients (10.9%) developed pneumonia, and four (3.1%) died.
Data source: Review of 128 rib fracture patients.
Disclosures: The lead investigator has no disclosures, and there was no outside funding for the work.