User login
Higher death rate seen in cancer patients with nosocomial COVID-19
, according to researchers.
In an observational study of patients with COVID-19 and cancer, 19% of patients had COVID-19 acquired during a non-COVID-related hospital stay, and 81% had community-acquired COVID-19.
At a median follow-up of 23 days, the overall mortality rate was 28%. However, the all-cause mortality rate in patients with nosocomial COVID-19 was more than double that of patients with community-acquired COVID-19, at 47% and 23%, respectively.
Arielle Elkrief, MD, of the University of Montreal, reported these results during the AACR virtual meeting: COVID-19 and Cancer.
“This is the first report that describes a high rate of hospital-acquired COVID-19 in patients with cancer, at a rate of 19%,” Dr. Elkrief said. “This was associated with high mortality in both univariate and multivariate analyses.”
The study included 250 adults and 3 children with COVID-19 and cancer who were identified between March 3 and May 23, 2020. They ranged in age from 4 to 95 years, but the median age was 73 years.
All patients had either laboratory-confirmed (95%) or presumed COVID-19 (5%) and invasive cancer. The most common cancer types were similar to those seen in the general population. Lung and breast cancer were the most common, followed by lymphoma, prostate cancer, and colorectal cancer. Most patients were on active anticancer therapy, most often chemotherapy.
Most patients (n = 236) were residents of Quebec, but 17 patients were residents of British Columbia.
“It is important to note that Quebec was one of the most heavily affected areas in North America at the time of the study,” Dr. Elkrief said.
Outcomes by group
There were 206 patients (81%) who had community-acquired COVID-19 and 47 (19%) who had nosocomial COVID-19. The two groups were similar with respect to sex, performance status, and cancer stage. A small trend toward more patients on active therapy was seen in the nosocomial group, but the difference did not reach statistical significance.
The median overall survival was 27 days in the nosocomial group and 71 days in the community-acquired group (hazard ratio, 2.2; P = .002).
A multivariate analysis showed that nosocomial infection was “strongly and independently associated with death,” Dr. Elkrief said. “Other risk factors for poor prognosis included age, poor [performance] status, and advanced stage of cancer.”
There were no significant differences between the hospital-acquired and community-acquired groups for other outcomes, including oxygen requirements (43% and 47%, respectively), ICU admission (13% and 11%), need for mechanical ventilation (6% and 5%), or length of stay (median, 9.5 days and 8.5 days).
The low rate of ICU admission, considering the mortality rate of 28%, “could reflect that patients with cancer are less likely to be admitted to the ICU,” Dr. Elkrief noted.
Applying the findings to practice
The findings reinforce the importance of adherence to stringent infection control guidelines to protect vulnerable patients, such as those with cancer, Dr. Elkrief said.
In ambulatory settings, this means decreasing in-person visits through increased use of teleconsultations, and for those who need to be seen in person, screening for symptoms or use of polymerase chain reaction testing should be used when resources are available, she said.
“Similar principles apply to chemotherapy treatment units,” Dr. Elkrief said. She added that staff must avoid cross-contamination between COVID and COVID-free zones, and that “dedicated personnel and equipment should be maintained and separate between these two zones.
“Adequate protective personal equipment and strict hand hygiene protocols are also of utmost importance,” Dr. Elkrief said. “The threat of COVID-19 is not behind us, and so we continue to enforce these strategies to protect our patients.”
Session moderator Gypsyamber D’Souza, PhD, an infectious disease epidemiologist at Johns Hopkins University in Baltimore, raised the question of whether the high nosocomial infection and death rate in this study was related to patients having more severe disease because of underlying comorbidities.
Dr. Elkrief explained that the overall mortality rate was indeed higher than the 13% reported in other studies, and it may reflect an overrepresentation of hospitalized or more severely ill patients in the cohort.
However, the investigators made every effort to include all patients with both cancer and COVID-19 by using systematic screening of inpatient and outpatients lists and registries.
Further, the multivariate analysis included both inpatients and outpatients and adjusted for known negative prognostic factors for COVID-19 outcomes. These included increasing age, poor performance status, and different comorbidities.
The finding that nosocomial infection was an independent predictor of death “pushed us to look at nosocomial infection as a new independent risk factor,” Dr. Elkrief said.
Dr. Elkrief reported grant support from AstraZeneca. Dr. D’Souza did not report any disclosures.
SOURCE: Elkrief A et al. AACR: COVID and Cancer, Abstract S12-01.
, according to researchers.
In an observational study of patients with COVID-19 and cancer, 19% of patients had COVID-19 acquired during a non-COVID-related hospital stay, and 81% had community-acquired COVID-19.
At a median follow-up of 23 days, the overall mortality rate was 28%. However, the all-cause mortality rate in patients with nosocomial COVID-19 was more than double that of patients with community-acquired COVID-19, at 47% and 23%, respectively.
Arielle Elkrief, MD, of the University of Montreal, reported these results during the AACR virtual meeting: COVID-19 and Cancer.
“This is the first report that describes a high rate of hospital-acquired COVID-19 in patients with cancer, at a rate of 19%,” Dr. Elkrief said. “This was associated with high mortality in both univariate and multivariate analyses.”
The study included 250 adults and 3 children with COVID-19 and cancer who were identified between March 3 and May 23, 2020. They ranged in age from 4 to 95 years, but the median age was 73 years.
All patients had either laboratory-confirmed (95%) or presumed COVID-19 (5%) and invasive cancer. The most common cancer types were similar to those seen in the general population. Lung and breast cancer were the most common, followed by lymphoma, prostate cancer, and colorectal cancer. Most patients were on active anticancer therapy, most often chemotherapy.
Most patients (n = 236) were residents of Quebec, but 17 patients were residents of British Columbia.
“It is important to note that Quebec was one of the most heavily affected areas in North America at the time of the study,” Dr. Elkrief said.
Outcomes by group
There were 206 patients (81%) who had community-acquired COVID-19 and 47 (19%) who had nosocomial COVID-19. The two groups were similar with respect to sex, performance status, and cancer stage. A small trend toward more patients on active therapy was seen in the nosocomial group, but the difference did not reach statistical significance.
The median overall survival was 27 days in the nosocomial group and 71 days in the community-acquired group (hazard ratio, 2.2; P = .002).
A multivariate analysis showed that nosocomial infection was “strongly and independently associated with death,” Dr. Elkrief said. “Other risk factors for poor prognosis included age, poor [performance] status, and advanced stage of cancer.”
There were no significant differences between the hospital-acquired and community-acquired groups for other outcomes, including oxygen requirements (43% and 47%, respectively), ICU admission (13% and 11%), need for mechanical ventilation (6% and 5%), or length of stay (median, 9.5 days and 8.5 days).
The low rate of ICU admission, considering the mortality rate of 28%, “could reflect that patients with cancer are less likely to be admitted to the ICU,” Dr. Elkrief noted.
Applying the findings to practice
The findings reinforce the importance of adherence to stringent infection control guidelines to protect vulnerable patients, such as those with cancer, Dr. Elkrief said.
In ambulatory settings, this means decreasing in-person visits through increased use of teleconsultations, and for those who need to be seen in person, screening for symptoms or use of polymerase chain reaction testing should be used when resources are available, she said.
“Similar principles apply to chemotherapy treatment units,” Dr. Elkrief said. She added that staff must avoid cross-contamination between COVID and COVID-free zones, and that “dedicated personnel and equipment should be maintained and separate between these two zones.
“Adequate protective personal equipment and strict hand hygiene protocols are also of utmost importance,” Dr. Elkrief said. “The threat of COVID-19 is not behind us, and so we continue to enforce these strategies to protect our patients.”
Session moderator Gypsyamber D’Souza, PhD, an infectious disease epidemiologist at Johns Hopkins University in Baltimore, raised the question of whether the high nosocomial infection and death rate in this study was related to patients having more severe disease because of underlying comorbidities.
Dr. Elkrief explained that the overall mortality rate was indeed higher than the 13% reported in other studies, and it may reflect an overrepresentation of hospitalized or more severely ill patients in the cohort.
However, the investigators made every effort to include all patients with both cancer and COVID-19 by using systematic screening of inpatient and outpatients lists and registries.
Further, the multivariate analysis included both inpatients and outpatients and adjusted for known negative prognostic factors for COVID-19 outcomes. These included increasing age, poor performance status, and different comorbidities.
The finding that nosocomial infection was an independent predictor of death “pushed us to look at nosocomial infection as a new independent risk factor,” Dr. Elkrief said.
Dr. Elkrief reported grant support from AstraZeneca. Dr. D’Souza did not report any disclosures.
SOURCE: Elkrief A et al. AACR: COVID and Cancer, Abstract S12-01.
, according to researchers.
In an observational study of patients with COVID-19 and cancer, 19% of patients had COVID-19 acquired during a non-COVID-related hospital stay, and 81% had community-acquired COVID-19.
At a median follow-up of 23 days, the overall mortality rate was 28%. However, the all-cause mortality rate in patients with nosocomial COVID-19 was more than double that of patients with community-acquired COVID-19, at 47% and 23%, respectively.
Arielle Elkrief, MD, of the University of Montreal, reported these results during the AACR virtual meeting: COVID-19 and Cancer.
“This is the first report that describes a high rate of hospital-acquired COVID-19 in patients with cancer, at a rate of 19%,” Dr. Elkrief said. “This was associated with high mortality in both univariate and multivariate analyses.”
The study included 250 adults and 3 children with COVID-19 and cancer who were identified between March 3 and May 23, 2020. They ranged in age from 4 to 95 years, but the median age was 73 years.
All patients had either laboratory-confirmed (95%) or presumed COVID-19 (5%) and invasive cancer. The most common cancer types were similar to those seen in the general population. Lung and breast cancer were the most common, followed by lymphoma, prostate cancer, and colorectal cancer. Most patients were on active anticancer therapy, most often chemotherapy.
Most patients (n = 236) were residents of Quebec, but 17 patients were residents of British Columbia.
“It is important to note that Quebec was one of the most heavily affected areas in North America at the time of the study,” Dr. Elkrief said.
Outcomes by group
There were 206 patients (81%) who had community-acquired COVID-19 and 47 (19%) who had nosocomial COVID-19. The two groups were similar with respect to sex, performance status, and cancer stage. A small trend toward more patients on active therapy was seen in the nosocomial group, but the difference did not reach statistical significance.
The median overall survival was 27 days in the nosocomial group and 71 days in the community-acquired group (hazard ratio, 2.2; P = .002).
A multivariate analysis showed that nosocomial infection was “strongly and independently associated with death,” Dr. Elkrief said. “Other risk factors for poor prognosis included age, poor [performance] status, and advanced stage of cancer.”
There were no significant differences between the hospital-acquired and community-acquired groups for other outcomes, including oxygen requirements (43% and 47%, respectively), ICU admission (13% and 11%), need for mechanical ventilation (6% and 5%), or length of stay (median, 9.5 days and 8.5 days).
The low rate of ICU admission, considering the mortality rate of 28%, “could reflect that patients with cancer are less likely to be admitted to the ICU,” Dr. Elkrief noted.
Applying the findings to practice
The findings reinforce the importance of adherence to stringent infection control guidelines to protect vulnerable patients, such as those with cancer, Dr. Elkrief said.
In ambulatory settings, this means decreasing in-person visits through increased use of teleconsultations, and for those who need to be seen in person, screening for symptoms or use of polymerase chain reaction testing should be used when resources are available, she said.
“Similar principles apply to chemotherapy treatment units,” Dr. Elkrief said. She added that staff must avoid cross-contamination between COVID and COVID-free zones, and that “dedicated personnel and equipment should be maintained and separate between these two zones.
“Adequate protective personal equipment and strict hand hygiene protocols are also of utmost importance,” Dr. Elkrief said. “The threat of COVID-19 is not behind us, and so we continue to enforce these strategies to protect our patients.”
Session moderator Gypsyamber D’Souza, PhD, an infectious disease epidemiologist at Johns Hopkins University in Baltimore, raised the question of whether the high nosocomial infection and death rate in this study was related to patients having more severe disease because of underlying comorbidities.
Dr. Elkrief explained that the overall mortality rate was indeed higher than the 13% reported in other studies, and it may reflect an overrepresentation of hospitalized or more severely ill patients in the cohort.
However, the investigators made every effort to include all patients with both cancer and COVID-19 by using systematic screening of inpatient and outpatients lists and registries.
Further, the multivariate analysis included both inpatients and outpatients and adjusted for known negative prognostic factors for COVID-19 outcomes. These included increasing age, poor performance status, and different comorbidities.
The finding that nosocomial infection was an independent predictor of death “pushed us to look at nosocomial infection as a new independent risk factor,” Dr. Elkrief said.
Dr. Elkrief reported grant support from AstraZeneca. Dr. D’Souza did not report any disclosures.
SOURCE: Elkrief A et al. AACR: COVID and Cancer, Abstract S12-01.
FROM AACR: COVID-19 AND CANCER
Robotic renal surgery bests open partial nephrectomy
RAPN was associated with a 61% decrease in intraoperative complications and a 71% decrease in overall complications in the IRON study.
Alessandro Larcher, MD, of San Raffaele Hospital and the Urological Research Institute in Milan, presented results from IRON during a live poster session at the virtual annual congress of the European Association of Urology.
The IRON study was performed in nine high-volume centers and involved 3,468 patients with renal cell cancer. Patients were recruited if they had a localized renal cell mass (cT1-2) with no nodal involvement or metastases. There were 2,405 patients who underwent RAPN and 1,063 who underwent OPN.
Intraoperative complications occurred in 5.7% of patients who underwent RAPN and in 9.3% of those who underwent OPN. Overall complications occurred in 33% and 18%, respectively (P < .001 for both).
“The complication profile was invariably in favor of robot-assisted surgery,” Dr. Larcher observed.
Patients who underwent RAPN had less estimated median blood loss (150 mL vs. 180 mL, P < .001) as well as lower rates of hemorrhagic complications (6.4% vs. 9%, P < .01) and urinary leakage (0.8% vs. 4.6%, P < .01).
The operative time was longer with RAPN than with OPN, at a median of 150 minutes and 120 minutes, respectively (P < .001). However, patients remained in the hospital for less time with RAPN than with OPN, at a median of 4 days and 6 days, respectively (P < .01).
RAPN was associated with fewer surgical complications than OPN according to the Clavien-Dindo system. Grade 2 or higher complications occurred in 12% and 20% of patients, respectively (P < .001). Grade 3 or higher complications occurred in 4% and 6.1%, respectively (P < .001).
“The benefit with respect to the complication risk reduction in the case of robot-assisted surgery was not affected by the tumor complexity, by the dimension of the mass, the comorbidities of the patients, or the baseline renal function,” Dr. Larcher said. “[T]he advantage after robot-assisted surgery is consistent regardless of all these features.”
Early renal function was better after OPN, but there was no significant difference between the two groups at 1 year of follow-up. The median ischemia time was 15 minutes with OPN and 16 minutes with RAPN (P < .001).
Postoperatively, the median estimated glomerular filtration rate was 78 mL/min/1.73m2 with OPN and 76 mL/min/1.73m2 with RAPN (P < .001). At 1 year, the median estimated glomerular filtration rate was 68 and 71 mL/min/1.73m2, respectively (P = .5).
Dr. Larcher noted that there was no difference between RAPN and OPN in terms of 5-year oncologic outcomes. Local recurrence occurred in 1.6% and 2.1% of patients, respectively (P = .06); systemic progression was seen in 1.8% and 4.5%, respectively (P = .5); and clinical progression was observed in 3.2% and 6.6%, respectively (P = .9).
“[IRON is] a really powerful study. It’s one of those studies that kind of has to be done,” said Ben Challacombe, MBBS, a consultant urological surgeon at Guy’s Hospital and St. Thomas’ Hospital in London who chaired the poster session during which these findings were presented.
Dr. Challacombe, who specializes in the treatment of kidney and prostatic disease using robotic surgery, noted that about 75% of procedures in the United Kingdom are now being performed with robotic assistance and queried what percentage of procedures should still be done by open surgery.
“I would turn it,” Dr. Larcher said. “What is the percentage of surgeons that should use one technique or the other?” In the IRON study, as well as other studies, surgical expertise, training, and center volumes were important.
“What the data are telling us is that those who are really confident in robotic surgeries can achieve even better outcomes, also in very complex cases,” Dr. Larcher said. “I think it’s not any longer dependent on the tumor factors. The answer to the question is only determined by human factors.”
The IRON study was supported by a grant from Intuitive. Dr. Larcher declared no conflicts of interest. Dr. Challacombe did not present any disclosures.
SOURCE: Larcher A et al. EAU20, Abstract 30. Eur Urol Open Sci 2020;19(Suppl 2):e142.
RAPN was associated with a 61% decrease in intraoperative complications and a 71% decrease in overall complications in the IRON study.
Alessandro Larcher, MD, of San Raffaele Hospital and the Urological Research Institute in Milan, presented results from IRON during a live poster session at the virtual annual congress of the European Association of Urology.
The IRON study was performed in nine high-volume centers and involved 3,468 patients with renal cell cancer. Patients were recruited if they had a localized renal cell mass (cT1-2) with no nodal involvement or metastases. There were 2,405 patients who underwent RAPN and 1,063 who underwent OPN.
Intraoperative complications occurred in 5.7% of patients who underwent RAPN and in 9.3% of those who underwent OPN. Overall complications occurred in 33% and 18%, respectively (P < .001 for both).
“The complication profile was invariably in favor of robot-assisted surgery,” Dr. Larcher observed.
Patients who underwent RAPN had less estimated median blood loss (150 mL vs. 180 mL, P < .001) as well as lower rates of hemorrhagic complications (6.4% vs. 9%, P < .01) and urinary leakage (0.8% vs. 4.6%, P < .01).
The operative time was longer with RAPN than with OPN, at a median of 150 minutes and 120 minutes, respectively (P < .001). However, patients remained in the hospital for less time with RAPN than with OPN, at a median of 4 days and 6 days, respectively (P < .01).
RAPN was associated with fewer surgical complications than OPN according to the Clavien-Dindo system. Grade 2 or higher complications occurred in 12% and 20% of patients, respectively (P < .001). Grade 3 or higher complications occurred in 4% and 6.1%, respectively (P < .001).
“The benefit with respect to the complication risk reduction in the case of robot-assisted surgery was not affected by the tumor complexity, by the dimension of the mass, the comorbidities of the patients, or the baseline renal function,” Dr. Larcher said. “[T]he advantage after robot-assisted surgery is consistent regardless of all these features.”
Early renal function was better after OPN, but there was no significant difference between the two groups at 1 year of follow-up. The median ischemia time was 15 minutes with OPN and 16 minutes with RAPN (P < .001).
Postoperatively, the median estimated glomerular filtration rate was 78 mL/min/1.73m2 with OPN and 76 mL/min/1.73m2 with RAPN (P < .001). At 1 year, the median estimated glomerular filtration rate was 68 and 71 mL/min/1.73m2, respectively (P = .5).
Dr. Larcher noted that there was no difference between RAPN and OPN in terms of 5-year oncologic outcomes. Local recurrence occurred in 1.6% and 2.1% of patients, respectively (P = .06); systemic progression was seen in 1.8% and 4.5%, respectively (P = .5); and clinical progression was observed in 3.2% and 6.6%, respectively (P = .9).
“[IRON is] a really powerful study. It’s one of those studies that kind of has to be done,” said Ben Challacombe, MBBS, a consultant urological surgeon at Guy’s Hospital and St. Thomas’ Hospital in London who chaired the poster session during which these findings were presented.
Dr. Challacombe, who specializes in the treatment of kidney and prostatic disease using robotic surgery, noted that about 75% of procedures in the United Kingdom are now being performed with robotic assistance and queried what percentage of procedures should still be done by open surgery.
“I would turn it,” Dr. Larcher said. “What is the percentage of surgeons that should use one technique or the other?” In the IRON study, as well as other studies, surgical expertise, training, and center volumes were important.
“What the data are telling us is that those who are really confident in robotic surgeries can achieve even better outcomes, also in very complex cases,” Dr. Larcher said. “I think it’s not any longer dependent on the tumor factors. The answer to the question is only determined by human factors.”
The IRON study was supported by a grant from Intuitive. Dr. Larcher declared no conflicts of interest. Dr. Challacombe did not present any disclosures.
SOURCE: Larcher A et al. EAU20, Abstract 30. Eur Urol Open Sci 2020;19(Suppl 2):e142.
RAPN was associated with a 61% decrease in intraoperative complications and a 71% decrease in overall complications in the IRON study.
Alessandro Larcher, MD, of San Raffaele Hospital and the Urological Research Institute in Milan, presented results from IRON during a live poster session at the virtual annual congress of the European Association of Urology.
The IRON study was performed in nine high-volume centers and involved 3,468 patients with renal cell cancer. Patients were recruited if they had a localized renal cell mass (cT1-2) with no nodal involvement or metastases. There were 2,405 patients who underwent RAPN and 1,063 who underwent OPN.
Intraoperative complications occurred in 5.7% of patients who underwent RAPN and in 9.3% of those who underwent OPN. Overall complications occurred in 33% and 18%, respectively (P < .001 for both).
“The complication profile was invariably in favor of robot-assisted surgery,” Dr. Larcher observed.
Patients who underwent RAPN had less estimated median blood loss (150 mL vs. 180 mL, P < .001) as well as lower rates of hemorrhagic complications (6.4% vs. 9%, P < .01) and urinary leakage (0.8% vs. 4.6%, P < .01).
The operative time was longer with RAPN than with OPN, at a median of 150 minutes and 120 minutes, respectively (P < .001). However, patients remained in the hospital for less time with RAPN than with OPN, at a median of 4 days and 6 days, respectively (P < .01).
RAPN was associated with fewer surgical complications than OPN according to the Clavien-Dindo system. Grade 2 or higher complications occurred in 12% and 20% of patients, respectively (P < .001). Grade 3 or higher complications occurred in 4% and 6.1%, respectively (P < .001).
“The benefit with respect to the complication risk reduction in the case of robot-assisted surgery was not affected by the tumor complexity, by the dimension of the mass, the comorbidities of the patients, or the baseline renal function,” Dr. Larcher said. “[T]he advantage after robot-assisted surgery is consistent regardless of all these features.”
Early renal function was better after OPN, but there was no significant difference between the two groups at 1 year of follow-up. The median ischemia time was 15 minutes with OPN and 16 minutes with RAPN (P < .001).
Postoperatively, the median estimated glomerular filtration rate was 78 mL/min/1.73m2 with OPN and 76 mL/min/1.73m2 with RAPN (P < .001). At 1 year, the median estimated glomerular filtration rate was 68 and 71 mL/min/1.73m2, respectively (P = .5).
Dr. Larcher noted that there was no difference between RAPN and OPN in terms of 5-year oncologic outcomes. Local recurrence occurred in 1.6% and 2.1% of patients, respectively (P = .06); systemic progression was seen in 1.8% and 4.5%, respectively (P = .5); and clinical progression was observed in 3.2% and 6.6%, respectively (P = .9).
“[IRON is] a really powerful study. It’s one of those studies that kind of has to be done,” said Ben Challacombe, MBBS, a consultant urological surgeon at Guy’s Hospital and St. Thomas’ Hospital in London who chaired the poster session during which these findings were presented.
Dr. Challacombe, who specializes in the treatment of kidney and prostatic disease using robotic surgery, noted that about 75% of procedures in the United Kingdom are now being performed with robotic assistance and queried what percentage of procedures should still be done by open surgery.
“I would turn it,” Dr. Larcher said. “What is the percentage of surgeons that should use one technique or the other?” In the IRON study, as well as other studies, surgical expertise, training, and center volumes were important.
“What the data are telling us is that those who are really confident in robotic surgeries can achieve even better outcomes, also in very complex cases,” Dr. Larcher said. “I think it’s not any longer dependent on the tumor factors. The answer to the question is only determined by human factors.”
The IRON study was supported by a grant from Intuitive. Dr. Larcher declared no conflicts of interest. Dr. Challacombe did not present any disclosures.
SOURCE: Larcher A et al. EAU20, Abstract 30. Eur Urol Open Sci 2020;19(Suppl 2):e142.
FROM EAU20
CCC19, other registries help define COVID/cancer landscape
Initial results from the CCC19 registry were reported as part of the American Society of Clinical Oncology (ASCO) virtual scientific program and published in The Lancet (Lancet. 2020 Jun 20;395[10241]:1907-18).
The latest data were presented at the AACR virtual meeting: COVID-19 and Cancer by Brian I. Rini, MD, of Vanderbilt University, Nashville, Tenn. They were simultaneously published in Cancer Discovery (Cancer Discov. 2020 Jul 22;CD-20-0941).
The CCC19 registry was launched in March by a few institutions as part of “a grassroots idea ... to collect granular data regarding cancer patients and their outcomes with COVID,” Dr. Rini said.
Within a few months of its inception, the registry had partnered with more than 100 institutions worldwide and accrued data from more than 2,000 patients.
The reports in The Lancet and at ASCO included outcomes for the first 928 patients and showed a 13% mortality rate as well as a fivefold increase in the risk of 30-day mortality among patients with COVID-19 and progressing cancer.
The data also showed an increased mortality risk among older patients, men, former smokers, those with poor performance status, those with multiple comorbidities, and those treated with hydroxychloroquine and azithromycin.
The latest data
The CCC19 registry has grown to include 114 sites worldwide, including major comprehensive cancer centers and community sites. As of June 26, there were 2,749 patients enrolled.
Since the last data were reported, the mortality rate increased from 13% to 16% (versus 5% globally). In addition, the increased mortality risk among non-Hispanic black patients and patients with hematologic malignancies reached statistical significance, Dr. Rini said. He noted that the increase in mortality rate was largely attributable to improved follow-up.
Mechanical ventilation was required in 12% of patients, ICU admission was required in 16%, oxygen was required in 45%, and hospitalization was required in 60%. The composite outcome of death, severe illness requiring hospitalization, ICU admission, or mechanical ventilation was reached in 29% of patients, Dr. Rini said.
Mortality rates across cancer types ranged from 3% to 26%, with thyroid and breast cancer patients having the lowest rates (3% and 8%, respectively), and with lymphoma and lung cancer patients having the highest (22% and 26%, respectively), Dr. Rini said.
He noted that the TERAVOLT registry, a COVID-19 registry for patients with thoracic cancers, also showed a very high mortality rate in this subgroup of patients.
Results from TERAVOLT were reported at the AACR virtual meeting I, presented at ASCO, and published in The Lancet (Lancet Oncol. 2020 Jul;21[7]:914-22). The most recent results showed a mortality rate of nearly 36% and reinforce the high mortality rate seen in lung cancer patients in CCC19, Dr. Rini said.
Increased mortality risk
After adjustment for several demographic and disease characteristics, the updated CCC19 data showed a significantly increased risk of mortality among:
- Older patients (adjusted odds ratio [aOR] per decade of age, 1.52).
- Men (aOR, 1.43).
- Current or former smokers vs. never smokers (aOR, 1.28).
- Patients with Eastern Cooperative Oncology Group performance scores of 1 vs. 0 (aOR of 1.80) or 2 vs. 0 (aOR, 4.22).
- Stable cancer vs. remission (aOR, 1.47).
- Progressive cancer vs. remission (aOR, 2.96).
- Non-Hispanic Black vs. White patients (aOR, 1.56).
- Hematologic malignancies vs. solid tumors (aOR, 1.80).
“Importantly, there were some factors that did not reach statistical significance,” Dr. Rini said. These include obesity (aOR, 1.23), recent surgery (aOR, 1.05), receipt of cytotoxic chemotherapy vs. no chemotherapy (aOR, 1.14), and receipt of noncytotoxic chemotherapy vs. no chemotherapy (aOR, 0.75).
“I think this provides some reassurance that cancer care can and should continue for these patients,” Dr. Rini said.
He noted, however, that in TERAVOLT, chemotherapy with or without other treatment was a risk factor for mortality in lung cancer patients when compared with no chemotherapy (OR, 1.71) and when compared with immunotherapy or targeted therapy (OR, 1.64).
NCCAPS and other registries
Dr. Rini discussed a number of registries looking at outcomes in COVID-19 patients with cancer, and he said the findings to date appear to confirm a higher mortality rate among cancer patients, particularly those with lung cancer.
Several factors are emerging that appear to be related to risk, including both cancer-related and non–cancer-related factors, he added.
The ongoing prospective National Cancer Institute COVID-19 in Cancer Patients Study (NCCAPS) “will provide much needed longitudinal data and, importantly, biospecimen collection in a large cohort of patients who have active cancer and are receiving treatment, said Dr. Rini, who is the study’s protocol chair. NCCAPS is a natural history study in that population, he said.
The planned accrual is about 2,000 patients who will be followed for up to 2 years for data collection, imaging scans, and research specimens.
The use of specimens is “a unique and special part of this study,” Dr. Rini said, explaining that the specimens will be used to look for development of antibodies over time, to describe the trajectory of cytokine abnormalities – especially in patients with more acute inpatient courses – to perform DNA-based genome-wide association studies, and to assess coagulation parameters.
NCCAPS is activated at 546 sties, 10 patients were enrolled as of June 21, and rapid accrual is expected over the next several months, he said.
Gypsyamber D’Souza, PhD, session moderator and an infectious disease epidemiologist at Johns Hopkins University in Baltimore, acknowledged the challenge that registry administrators face when trying to balance the need to get data out against the desire to ask the right questions and to have the right comparison groups, stratification, and analyses, especially amid a crisis like the COVID-19 pandemic.
Dr. Rini said it has indeed been a bit of a struggle with CCC19 to determine what information should be published and when, and what constitutes an important update.
“It’s been a learning experience, and frankly, I think we’re still learning,” he said. “This has been such a unique time in terms of a rush to get data out, balanced against making sure that there’s quality data and that you’re actually answering important questions.”
In fact, a number of ongoing registries “should start to produce great data [that will be presented] at upcoming big conferences,” Dr. Rini said. He added that those data “will help piece together different important aspects of this and different hypotheses, and hopefully complement the clinical data that’s starting to come out.”
The CCC19 registry is sponsored by Vanderbilt-Ingram Cancer Center. Dr. Rini disclosed relationships with Pfizer, Merck, Genentech/Roche, Aveo, AstraZeneca, Bristol Myers Squibb, Exelixis, Synthorx, Peloton, Compugen, Corvus, Surface Oncology, 3DMedicines, Aravive, Alkermes, Arrowhead, and PTC Therapeutics. Dr. D’Souza did not disclose any conflicts.
SOURCE: Rini BI. AACR: COVID-19 and Cancer. Abstract IA26.
Initial results from the CCC19 registry were reported as part of the American Society of Clinical Oncology (ASCO) virtual scientific program and published in The Lancet (Lancet. 2020 Jun 20;395[10241]:1907-18).
The latest data were presented at the AACR virtual meeting: COVID-19 and Cancer by Brian I. Rini, MD, of Vanderbilt University, Nashville, Tenn. They were simultaneously published in Cancer Discovery (Cancer Discov. 2020 Jul 22;CD-20-0941).
The CCC19 registry was launched in March by a few institutions as part of “a grassroots idea ... to collect granular data regarding cancer patients and their outcomes with COVID,” Dr. Rini said.
Within a few months of its inception, the registry had partnered with more than 100 institutions worldwide and accrued data from more than 2,000 patients.
The reports in The Lancet and at ASCO included outcomes for the first 928 patients and showed a 13% mortality rate as well as a fivefold increase in the risk of 30-day mortality among patients with COVID-19 and progressing cancer.
The data also showed an increased mortality risk among older patients, men, former smokers, those with poor performance status, those with multiple comorbidities, and those treated with hydroxychloroquine and azithromycin.
The latest data
The CCC19 registry has grown to include 114 sites worldwide, including major comprehensive cancer centers and community sites. As of June 26, there were 2,749 patients enrolled.
Since the last data were reported, the mortality rate increased from 13% to 16% (versus 5% globally). In addition, the increased mortality risk among non-Hispanic black patients and patients with hematologic malignancies reached statistical significance, Dr. Rini said. He noted that the increase in mortality rate was largely attributable to improved follow-up.
Mechanical ventilation was required in 12% of patients, ICU admission was required in 16%, oxygen was required in 45%, and hospitalization was required in 60%. The composite outcome of death, severe illness requiring hospitalization, ICU admission, or mechanical ventilation was reached in 29% of patients, Dr. Rini said.
Mortality rates across cancer types ranged from 3% to 26%, with thyroid and breast cancer patients having the lowest rates (3% and 8%, respectively), and with lymphoma and lung cancer patients having the highest (22% and 26%, respectively), Dr. Rini said.
He noted that the TERAVOLT registry, a COVID-19 registry for patients with thoracic cancers, also showed a very high mortality rate in this subgroup of patients.
Results from TERAVOLT were reported at the AACR virtual meeting I, presented at ASCO, and published in The Lancet (Lancet Oncol. 2020 Jul;21[7]:914-22). The most recent results showed a mortality rate of nearly 36% and reinforce the high mortality rate seen in lung cancer patients in CCC19, Dr. Rini said.
Increased mortality risk
After adjustment for several demographic and disease characteristics, the updated CCC19 data showed a significantly increased risk of mortality among:
- Older patients (adjusted odds ratio [aOR] per decade of age, 1.52).
- Men (aOR, 1.43).
- Current or former smokers vs. never smokers (aOR, 1.28).
- Patients with Eastern Cooperative Oncology Group performance scores of 1 vs. 0 (aOR of 1.80) or 2 vs. 0 (aOR, 4.22).
- Stable cancer vs. remission (aOR, 1.47).
- Progressive cancer vs. remission (aOR, 2.96).
- Non-Hispanic Black vs. White patients (aOR, 1.56).
- Hematologic malignancies vs. solid tumors (aOR, 1.80).
“Importantly, there were some factors that did not reach statistical significance,” Dr. Rini said. These include obesity (aOR, 1.23), recent surgery (aOR, 1.05), receipt of cytotoxic chemotherapy vs. no chemotherapy (aOR, 1.14), and receipt of noncytotoxic chemotherapy vs. no chemotherapy (aOR, 0.75).
“I think this provides some reassurance that cancer care can and should continue for these patients,” Dr. Rini said.
He noted, however, that in TERAVOLT, chemotherapy with or without other treatment was a risk factor for mortality in lung cancer patients when compared with no chemotherapy (OR, 1.71) and when compared with immunotherapy or targeted therapy (OR, 1.64).
NCCAPS and other registries
Dr. Rini discussed a number of registries looking at outcomes in COVID-19 patients with cancer, and he said the findings to date appear to confirm a higher mortality rate among cancer patients, particularly those with lung cancer.
Several factors are emerging that appear to be related to risk, including both cancer-related and non–cancer-related factors, he added.
The ongoing prospective National Cancer Institute COVID-19 in Cancer Patients Study (NCCAPS) “will provide much needed longitudinal data and, importantly, biospecimen collection in a large cohort of patients who have active cancer and are receiving treatment, said Dr. Rini, who is the study’s protocol chair. NCCAPS is a natural history study in that population, he said.
The planned accrual is about 2,000 patients who will be followed for up to 2 years for data collection, imaging scans, and research specimens.
The use of specimens is “a unique and special part of this study,” Dr. Rini said, explaining that the specimens will be used to look for development of antibodies over time, to describe the trajectory of cytokine abnormalities – especially in patients with more acute inpatient courses – to perform DNA-based genome-wide association studies, and to assess coagulation parameters.
NCCAPS is activated at 546 sties, 10 patients were enrolled as of June 21, and rapid accrual is expected over the next several months, he said.
Gypsyamber D’Souza, PhD, session moderator and an infectious disease epidemiologist at Johns Hopkins University in Baltimore, acknowledged the challenge that registry administrators face when trying to balance the need to get data out against the desire to ask the right questions and to have the right comparison groups, stratification, and analyses, especially amid a crisis like the COVID-19 pandemic.
Dr. Rini said it has indeed been a bit of a struggle with CCC19 to determine what information should be published and when, and what constitutes an important update.
“It’s been a learning experience, and frankly, I think we’re still learning,” he said. “This has been such a unique time in terms of a rush to get data out, balanced against making sure that there’s quality data and that you’re actually answering important questions.”
In fact, a number of ongoing registries “should start to produce great data [that will be presented] at upcoming big conferences,” Dr. Rini said. He added that those data “will help piece together different important aspects of this and different hypotheses, and hopefully complement the clinical data that’s starting to come out.”
The CCC19 registry is sponsored by Vanderbilt-Ingram Cancer Center. Dr. Rini disclosed relationships with Pfizer, Merck, Genentech/Roche, Aveo, AstraZeneca, Bristol Myers Squibb, Exelixis, Synthorx, Peloton, Compugen, Corvus, Surface Oncology, 3DMedicines, Aravive, Alkermes, Arrowhead, and PTC Therapeutics. Dr. D’Souza did not disclose any conflicts.
SOURCE: Rini BI. AACR: COVID-19 and Cancer. Abstract IA26.
Initial results from the CCC19 registry were reported as part of the American Society of Clinical Oncology (ASCO) virtual scientific program and published in The Lancet (Lancet. 2020 Jun 20;395[10241]:1907-18).
The latest data were presented at the AACR virtual meeting: COVID-19 and Cancer by Brian I. Rini, MD, of Vanderbilt University, Nashville, Tenn. They were simultaneously published in Cancer Discovery (Cancer Discov. 2020 Jul 22;CD-20-0941).
The CCC19 registry was launched in March by a few institutions as part of “a grassroots idea ... to collect granular data regarding cancer patients and their outcomes with COVID,” Dr. Rini said.
Within a few months of its inception, the registry had partnered with more than 100 institutions worldwide and accrued data from more than 2,000 patients.
The reports in The Lancet and at ASCO included outcomes for the first 928 patients and showed a 13% mortality rate as well as a fivefold increase in the risk of 30-day mortality among patients with COVID-19 and progressing cancer.
The data also showed an increased mortality risk among older patients, men, former smokers, those with poor performance status, those with multiple comorbidities, and those treated with hydroxychloroquine and azithromycin.
The latest data
The CCC19 registry has grown to include 114 sites worldwide, including major comprehensive cancer centers and community sites. As of June 26, there were 2,749 patients enrolled.
Since the last data were reported, the mortality rate increased from 13% to 16% (versus 5% globally). In addition, the increased mortality risk among non-Hispanic black patients and patients with hematologic malignancies reached statistical significance, Dr. Rini said. He noted that the increase in mortality rate was largely attributable to improved follow-up.
Mechanical ventilation was required in 12% of patients, ICU admission was required in 16%, oxygen was required in 45%, and hospitalization was required in 60%. The composite outcome of death, severe illness requiring hospitalization, ICU admission, or mechanical ventilation was reached in 29% of patients, Dr. Rini said.
Mortality rates across cancer types ranged from 3% to 26%, with thyroid and breast cancer patients having the lowest rates (3% and 8%, respectively), and with lymphoma and lung cancer patients having the highest (22% and 26%, respectively), Dr. Rini said.
He noted that the TERAVOLT registry, a COVID-19 registry for patients with thoracic cancers, also showed a very high mortality rate in this subgroup of patients.
Results from TERAVOLT were reported at the AACR virtual meeting I, presented at ASCO, and published in The Lancet (Lancet Oncol. 2020 Jul;21[7]:914-22). The most recent results showed a mortality rate of nearly 36% and reinforce the high mortality rate seen in lung cancer patients in CCC19, Dr. Rini said.
Increased mortality risk
After adjustment for several demographic and disease characteristics, the updated CCC19 data showed a significantly increased risk of mortality among:
- Older patients (adjusted odds ratio [aOR] per decade of age, 1.52).
- Men (aOR, 1.43).
- Current or former smokers vs. never smokers (aOR, 1.28).
- Patients with Eastern Cooperative Oncology Group performance scores of 1 vs. 0 (aOR of 1.80) or 2 vs. 0 (aOR, 4.22).
- Stable cancer vs. remission (aOR, 1.47).
- Progressive cancer vs. remission (aOR, 2.96).
- Non-Hispanic Black vs. White patients (aOR, 1.56).
- Hematologic malignancies vs. solid tumors (aOR, 1.80).
“Importantly, there were some factors that did not reach statistical significance,” Dr. Rini said. These include obesity (aOR, 1.23), recent surgery (aOR, 1.05), receipt of cytotoxic chemotherapy vs. no chemotherapy (aOR, 1.14), and receipt of noncytotoxic chemotherapy vs. no chemotherapy (aOR, 0.75).
“I think this provides some reassurance that cancer care can and should continue for these patients,” Dr. Rini said.
He noted, however, that in TERAVOLT, chemotherapy with or without other treatment was a risk factor for mortality in lung cancer patients when compared with no chemotherapy (OR, 1.71) and when compared with immunotherapy or targeted therapy (OR, 1.64).
NCCAPS and other registries
Dr. Rini discussed a number of registries looking at outcomes in COVID-19 patients with cancer, and he said the findings to date appear to confirm a higher mortality rate among cancer patients, particularly those with lung cancer.
Several factors are emerging that appear to be related to risk, including both cancer-related and non–cancer-related factors, he added.
The ongoing prospective National Cancer Institute COVID-19 in Cancer Patients Study (NCCAPS) “will provide much needed longitudinal data and, importantly, biospecimen collection in a large cohort of patients who have active cancer and are receiving treatment, said Dr. Rini, who is the study’s protocol chair. NCCAPS is a natural history study in that population, he said.
The planned accrual is about 2,000 patients who will be followed for up to 2 years for data collection, imaging scans, and research specimens.
The use of specimens is “a unique and special part of this study,” Dr. Rini said, explaining that the specimens will be used to look for development of antibodies over time, to describe the trajectory of cytokine abnormalities – especially in patients with more acute inpatient courses – to perform DNA-based genome-wide association studies, and to assess coagulation parameters.
NCCAPS is activated at 546 sties, 10 patients were enrolled as of June 21, and rapid accrual is expected over the next several months, he said.
Gypsyamber D’Souza, PhD, session moderator and an infectious disease epidemiologist at Johns Hopkins University in Baltimore, acknowledged the challenge that registry administrators face when trying to balance the need to get data out against the desire to ask the right questions and to have the right comparison groups, stratification, and analyses, especially amid a crisis like the COVID-19 pandemic.
Dr. Rini said it has indeed been a bit of a struggle with CCC19 to determine what information should be published and when, and what constitutes an important update.
“It’s been a learning experience, and frankly, I think we’re still learning,” he said. “This has been such a unique time in terms of a rush to get data out, balanced against making sure that there’s quality data and that you’re actually answering important questions.”
In fact, a number of ongoing registries “should start to produce great data [that will be presented] at upcoming big conferences,” Dr. Rini said. He added that those data “will help piece together different important aspects of this and different hypotheses, and hopefully complement the clinical data that’s starting to come out.”
The CCC19 registry is sponsored by Vanderbilt-Ingram Cancer Center. Dr. Rini disclosed relationships with Pfizer, Merck, Genentech/Roche, Aveo, AstraZeneca, Bristol Myers Squibb, Exelixis, Synthorx, Peloton, Compugen, Corvus, Surface Oncology, 3DMedicines, Aravive, Alkermes, Arrowhead, and PTC Therapeutics. Dr. D’Souza did not disclose any conflicts.
SOURCE: Rini BI. AACR: COVID-19 and Cancer. Abstract IA26.
FROM AACR: COVID-19 and CANCER
Genetic differences by ancestry shouldn’t impact efficacy of prostate cancer therapies
, according to an analysis published in Clinical Cancer Research.
“[N]o significant differences were seen in clinically actionable DNA repair genes, MSI-high [microsatellite instability–high] status, and tumor mutation burden, suggesting that current therapeutic strategies may be equally beneficial in both populations,” wrote study author Yusuke Koga, of the Boston University, and colleagues.
“Since these findings suggest that the frequency of targetable genetic alterations is similar in patients of predominantly African versus European ancestry, offering comprehensive genomic profiling and biomarker-based therapies to all patients, including African American patients, is a critical component of promoting equity in the management of metastatic prostate cancer,” said Atish D. Choudhury, MD, PhD, of the Dana-Farber Cancer Institute in Boston, who was not involved in this study.
Mr. Koga and colleagues noted that, when compared with European-American men, African American men have a higher incidence of prostate cancer, present with more advanced disease at an earlier age, and have increased mortality. These differences persist even after adjustment for socioeconomic covariates. That raises the question of the role of genetics.
“There is emerging evidence that, across some clinical trials and equal-access health systems, outcomes between AFR [African-American] men and European-American men with prostate cancer are similar,” the investigators wrote. “Although these data suggest that disparities can be ameliorated, there is limited knowledge of the genomic alterations that differ between groups and that could impact clinical outcomes.”
Study details and results
To get a handle on the issue, the investigators performed a meta-analysis of tumors from 250 African American men and 611 European-American men to compare the frequencies of somatic alterations across datasets from the Cancer Genome Atlas, the African Ancestry prostate cancer cohort, and the Memorial Sloan Kettering–Integrated Mutation Profiling of Actionable Cancer Targets panel.
The team also compared prostate cancer sequencing data from a commercial platform, the Foundation Medicine assay, from 436 African-American men and 3,018 European-American men.
In the meta-analysis, mutations in ZFHX3 and focal deletions in ETV3 were more common in tumors from African American men than in tumors from European-American men. Both genes are putative prostate cancer tumor suppressors, the investigators noted.
TP53 mutations, meanwhile, were associated with increasing Gleason scores in both groups, suggesting “that if TP53 mutations are found in low-grade disease, they may potentially indicate a more aggressive clinical trajectory,” the investigators wrote.
In the analysis with the commercial assay, MYC amplifications were more frequent in African American men with metastatic disease, raising “the possibility that MYC amplifications may also contribute to high-risk disease in this population,” the team wrote.
Deletions in PTEN and rearrangements in TMPRSS2-ERG were less frequent in tumors from African American men, but KMT2D truncations and CCND1 amplifications were more frequent.
“Higher expression of CCND1 has been implicated with perineural invasion in prostate cancer, an aggressive histological feature in prostate cancer. Truncating mutations in KMT2D have been reported in both localized and metastatic prostate cancer patients with unclear clinical significance,” the investigators noted.
“The genomic differences seen in genes such as MYC, ZFHX3, PTEN, and TMPRSS2-ERG suggest that different pathways of carcinogenesis may be active in AFR [African American] men, which could lead to further disparities if targeted therapies for some of these alterations become available,” the team wrote.
They noted that the meta-analysis was limited by the fact that some cohorts lacked matched tumors from European-American men, which limited the investigators’ ability to control for differences in region, clinical setting, or sequencing assay. Furthermore, age, tumor stage, and Gleason grade were unavailable in the cohort analyzed with the commercial assay.
This research was funded by the Department of Defense, the National Cancer Institute, and the Prostate Cancer Foundation. Two authors are employees of Foundation Medicine.
SOURCE: Koga Y et al. Clin Cancer Res. 2020 Jul 10. doi: 10.1158/1078-0432.CCR-19-4112.
, according to an analysis published in Clinical Cancer Research.
“[N]o significant differences were seen in clinically actionable DNA repair genes, MSI-high [microsatellite instability–high] status, and tumor mutation burden, suggesting that current therapeutic strategies may be equally beneficial in both populations,” wrote study author Yusuke Koga, of the Boston University, and colleagues.
“Since these findings suggest that the frequency of targetable genetic alterations is similar in patients of predominantly African versus European ancestry, offering comprehensive genomic profiling and biomarker-based therapies to all patients, including African American patients, is a critical component of promoting equity in the management of metastatic prostate cancer,” said Atish D. Choudhury, MD, PhD, of the Dana-Farber Cancer Institute in Boston, who was not involved in this study.
Mr. Koga and colleagues noted that, when compared with European-American men, African American men have a higher incidence of prostate cancer, present with more advanced disease at an earlier age, and have increased mortality. These differences persist even after adjustment for socioeconomic covariates. That raises the question of the role of genetics.
“There is emerging evidence that, across some clinical trials and equal-access health systems, outcomes between AFR [African-American] men and European-American men with prostate cancer are similar,” the investigators wrote. “Although these data suggest that disparities can be ameliorated, there is limited knowledge of the genomic alterations that differ between groups and that could impact clinical outcomes.”
Study details and results
To get a handle on the issue, the investigators performed a meta-analysis of tumors from 250 African American men and 611 European-American men to compare the frequencies of somatic alterations across datasets from the Cancer Genome Atlas, the African Ancestry prostate cancer cohort, and the Memorial Sloan Kettering–Integrated Mutation Profiling of Actionable Cancer Targets panel.
The team also compared prostate cancer sequencing data from a commercial platform, the Foundation Medicine assay, from 436 African-American men and 3,018 European-American men.
In the meta-analysis, mutations in ZFHX3 and focal deletions in ETV3 were more common in tumors from African American men than in tumors from European-American men. Both genes are putative prostate cancer tumor suppressors, the investigators noted.
TP53 mutations, meanwhile, were associated with increasing Gleason scores in both groups, suggesting “that if TP53 mutations are found in low-grade disease, they may potentially indicate a more aggressive clinical trajectory,” the investigators wrote.
In the analysis with the commercial assay, MYC amplifications were more frequent in African American men with metastatic disease, raising “the possibility that MYC amplifications may also contribute to high-risk disease in this population,” the team wrote.
Deletions in PTEN and rearrangements in TMPRSS2-ERG were less frequent in tumors from African American men, but KMT2D truncations and CCND1 amplifications were more frequent.
“Higher expression of CCND1 has been implicated with perineural invasion in prostate cancer, an aggressive histological feature in prostate cancer. Truncating mutations in KMT2D have been reported in both localized and metastatic prostate cancer patients with unclear clinical significance,” the investigators noted.
“The genomic differences seen in genes such as MYC, ZFHX3, PTEN, and TMPRSS2-ERG suggest that different pathways of carcinogenesis may be active in AFR [African American] men, which could lead to further disparities if targeted therapies for some of these alterations become available,” the team wrote.
They noted that the meta-analysis was limited by the fact that some cohorts lacked matched tumors from European-American men, which limited the investigators’ ability to control for differences in region, clinical setting, or sequencing assay. Furthermore, age, tumor stage, and Gleason grade were unavailable in the cohort analyzed with the commercial assay.
This research was funded by the Department of Defense, the National Cancer Institute, and the Prostate Cancer Foundation. Two authors are employees of Foundation Medicine.
SOURCE: Koga Y et al. Clin Cancer Res. 2020 Jul 10. doi: 10.1158/1078-0432.CCR-19-4112.
, according to an analysis published in Clinical Cancer Research.
“[N]o significant differences were seen in clinically actionable DNA repair genes, MSI-high [microsatellite instability–high] status, and tumor mutation burden, suggesting that current therapeutic strategies may be equally beneficial in both populations,” wrote study author Yusuke Koga, of the Boston University, and colleagues.
“Since these findings suggest that the frequency of targetable genetic alterations is similar in patients of predominantly African versus European ancestry, offering comprehensive genomic profiling and biomarker-based therapies to all patients, including African American patients, is a critical component of promoting equity in the management of metastatic prostate cancer,” said Atish D. Choudhury, MD, PhD, of the Dana-Farber Cancer Institute in Boston, who was not involved in this study.
Mr. Koga and colleagues noted that, when compared with European-American men, African American men have a higher incidence of prostate cancer, present with more advanced disease at an earlier age, and have increased mortality. These differences persist even after adjustment for socioeconomic covariates. That raises the question of the role of genetics.
“There is emerging evidence that, across some clinical trials and equal-access health systems, outcomes between AFR [African-American] men and European-American men with prostate cancer are similar,” the investigators wrote. “Although these data suggest that disparities can be ameliorated, there is limited knowledge of the genomic alterations that differ between groups and that could impact clinical outcomes.”
Study details and results
To get a handle on the issue, the investigators performed a meta-analysis of tumors from 250 African American men and 611 European-American men to compare the frequencies of somatic alterations across datasets from the Cancer Genome Atlas, the African Ancestry prostate cancer cohort, and the Memorial Sloan Kettering–Integrated Mutation Profiling of Actionable Cancer Targets panel.
The team also compared prostate cancer sequencing data from a commercial platform, the Foundation Medicine assay, from 436 African-American men and 3,018 European-American men.
In the meta-analysis, mutations in ZFHX3 and focal deletions in ETV3 were more common in tumors from African American men than in tumors from European-American men. Both genes are putative prostate cancer tumor suppressors, the investigators noted.
TP53 mutations, meanwhile, were associated with increasing Gleason scores in both groups, suggesting “that if TP53 mutations are found in low-grade disease, they may potentially indicate a more aggressive clinical trajectory,” the investigators wrote.
In the analysis with the commercial assay, MYC amplifications were more frequent in African American men with metastatic disease, raising “the possibility that MYC amplifications may also contribute to high-risk disease in this population,” the team wrote.
Deletions in PTEN and rearrangements in TMPRSS2-ERG were less frequent in tumors from African American men, but KMT2D truncations and CCND1 amplifications were more frequent.
“Higher expression of CCND1 has been implicated with perineural invasion in prostate cancer, an aggressive histological feature in prostate cancer. Truncating mutations in KMT2D have been reported in both localized and metastatic prostate cancer patients with unclear clinical significance,” the investigators noted.
“The genomic differences seen in genes such as MYC, ZFHX3, PTEN, and TMPRSS2-ERG suggest that different pathways of carcinogenesis may be active in AFR [African American] men, which could lead to further disparities if targeted therapies for some of these alterations become available,” the team wrote.
They noted that the meta-analysis was limited by the fact that some cohorts lacked matched tumors from European-American men, which limited the investigators’ ability to control for differences in region, clinical setting, or sequencing assay. Furthermore, age, tumor stage, and Gleason grade were unavailable in the cohort analyzed with the commercial assay.
This research was funded by the Department of Defense, the National Cancer Institute, and the Prostate Cancer Foundation. Two authors are employees of Foundation Medicine.
SOURCE: Koga Y et al. Clin Cancer Res. 2020 Jul 10. doi: 10.1158/1078-0432.CCR-19-4112.
FROM CLINICAL CANCER RESEARCH
Intravesical BCG dosing frequency ‘critical’ in bladder cancer
The rates of recurrence were 27.1% in the reduced dosing frequency arm and 12% in the standard dosing frequency arm. These results were reported at the virtual annual congress of the European Association of Urology.
More patients in the reduced dosing frequency arm than in the standard dosing frequency arm had a shorter time to recurrence, which was the primary endpoint of the trial.
At 6 months, the rate of recurrence was 18% in the reduced frequency arm and 8% in the standard frequency arm. The gap widened further at both 12 months (24% and 11%, respectively) and 24 months (34% and 15%, respectively). The hazard ratio for time to recurrence was 0.403 in favor of the standard dosing frequency arm.
“The recommended dose and schedule of BCG consists of once-weekly installations during 6 weeks of induction, followed by 3 weeks of maintenance at 3, 6, and 12 months,” observed study investigator Marc-Oliver Grimm, MD, of Jena (Germany) University Hospital.
“BCG instillation is, however, frequently associated with adverse events, which may lead to discontinuation, and several attempts have been made to reduce symptom burden associated with BCG,” he added.
Dr. Grimm presented the recently published findings from NIMBUS (Eur Urol. 2020 May 20;S0302-2838[20]30334-1) alongside some new information from a post hoc analysis.
Trial details
NIMBUS was a randomized, unblinded study of 345 patients with high-grade NMIBC who were recruited over a prolonged period, Dr. Grimm said. The long accrual was caused by a shortage of BCG and meant that the statistical assumptions had to be revised to include fewer patients.
The trial was designed to compare induction consisting of three versus six weekly BCG instillations and maintenance consisting of two versus three weekly BCG instillations at 3, 6, and 12 months. The aim had been to show that a reduced dosing frequency of BCG – 9 rather than 15 instillations – was noninferior to the standard dosing frequency of BCG, Dr. Grimm said. However, that was not the case, and the trial had to be stopped prematurely. In October 2019, the study’s sponsor, the EAU Research Foundation, announced that the trial would end.
Despite its unexpected ending, the trial’s data now fill some knowledge gaps, as pointed out by the discussant for the trial, Peter Black, MD, of the University of British Columbia in Vancouver.
Previous studies, such as the SWOG 8507, EORTC 30962, and CUETO 98013 trials, had shown that maintenance treatment works, but the schedule matters, he said. Results have also shown that the duration of maintenance treatment is less important than the dose of BCG given.
“The NIMBUS trial now tells us that dosing frequency is critical,” Dr. Black said.
Not only did the NIMBUS trial alter the maintenance schedule, it also altered the induction course of BCG instillation.
“The dramatic difference in recurrence-free survival, especially with the large separation of K-M [Kaplan-Meier] curves early on, suggests that this change to induction has had a major impact on the outcomes,” Dr. Black observed.
Post hoc analysis
Dr. Grimm presented a post hoc analysis comparing the rates of recurrence in the NIMBUS trial with rates seen in the EORTC 30962 and CUETO 98013 trials. Dr. Black also compared NIMBUS results to results from the SWOG 8507 trial.
The analysis showed lower rates of recurrence in the standard dose frequency arm in the NIMBUS trial than in the EORTC and CUETO trials at both 12 months (11%, 25%, and 18%, respectively) and 24 months (15%, 32%, and 27%, respectively).
However, as Dr. Black pointed out, the SWOG trial had similar recurrence rates as the NIMBUS trial at 12 months (9% and 11%, respectively) and 24 months (19% and 15%, respectively).
Dr. Grimm suggested that the lower rates of recurrence in the standard dosing arm of NIMBUS versus the other trials might have been because 91% of patients in the NIMBUS trial having undergone repeat transurethral resection for bladder tumor before BCG instillation.
Dr. Black said while this might have had an effect, it was probably not the only answer. While it’s true that the other trials had not considered repeat transurethral resection for bladder tumor, there were other confounding factors that might have been important, from patient selection bias to the use of advanced cystoscopy technologies, he said.
“If we really want to discern differences between surgery and intravesical therapy, we need to focus on CIS [carcinoma in situ] patients. Although this has major implications on feasibility since the patient pool is smaller,” Dr. Black said.
“One final point I’d like to make is that we really need to use these trials to understand the biology of non–muscle invasive bladder cancer,” he said. ”We know that BCG induces a cellular response, and we can measure this, as well as cytokine response. We know that the response builds to a plateau over four to six doses of induction and over two to three doses of maintenance therapy. This is perhaps more rapid in patients with pre-existing BCG immune reactivity. But there is biological rationale for the current six-plus-three protocol, and I think the reduced dose frequency in the NIMBUS trial probably failed to achieve the same immune activation as the established protocol.”
“If we were faced with a BCG shortage, it is better to reduce dose or duration of therapy but not the frequency of dosing,” Dr. Black added.
The NIMBUS trial was sponsored by the EAU Research Foundation. Dr. Grimm disclosed ties to Novartis, Bristol-Myers Squibb, Pfizer, AstraZeneca, and many other pharmaceutical companies. Dr. Black had no conflicts of interests relevant to his comments.
SOURCE: Grimm M-O. EAU20. https://urosource.uroweb.org/resource-centre/EAU20V/212877/Abstract/
The rates of recurrence were 27.1% in the reduced dosing frequency arm and 12% in the standard dosing frequency arm. These results were reported at the virtual annual congress of the European Association of Urology.
More patients in the reduced dosing frequency arm than in the standard dosing frequency arm had a shorter time to recurrence, which was the primary endpoint of the trial.
At 6 months, the rate of recurrence was 18% in the reduced frequency arm and 8% in the standard frequency arm. The gap widened further at both 12 months (24% and 11%, respectively) and 24 months (34% and 15%, respectively). The hazard ratio for time to recurrence was 0.403 in favor of the standard dosing frequency arm.
“The recommended dose and schedule of BCG consists of once-weekly installations during 6 weeks of induction, followed by 3 weeks of maintenance at 3, 6, and 12 months,” observed study investigator Marc-Oliver Grimm, MD, of Jena (Germany) University Hospital.
“BCG instillation is, however, frequently associated with adverse events, which may lead to discontinuation, and several attempts have been made to reduce symptom burden associated with BCG,” he added.
Dr. Grimm presented the recently published findings from NIMBUS (Eur Urol. 2020 May 20;S0302-2838[20]30334-1) alongside some new information from a post hoc analysis.
Trial details
NIMBUS was a randomized, unblinded study of 345 patients with high-grade NMIBC who were recruited over a prolonged period, Dr. Grimm said. The long accrual was caused by a shortage of BCG and meant that the statistical assumptions had to be revised to include fewer patients.
The trial was designed to compare induction consisting of three versus six weekly BCG instillations and maintenance consisting of two versus three weekly BCG instillations at 3, 6, and 12 months. The aim had been to show that a reduced dosing frequency of BCG – 9 rather than 15 instillations – was noninferior to the standard dosing frequency of BCG, Dr. Grimm said. However, that was not the case, and the trial had to be stopped prematurely. In October 2019, the study’s sponsor, the EAU Research Foundation, announced that the trial would end.
Despite its unexpected ending, the trial’s data now fill some knowledge gaps, as pointed out by the discussant for the trial, Peter Black, MD, of the University of British Columbia in Vancouver.
Previous studies, such as the SWOG 8507, EORTC 30962, and CUETO 98013 trials, had shown that maintenance treatment works, but the schedule matters, he said. Results have also shown that the duration of maintenance treatment is less important than the dose of BCG given.
“The NIMBUS trial now tells us that dosing frequency is critical,” Dr. Black said.
Not only did the NIMBUS trial alter the maintenance schedule, it also altered the induction course of BCG instillation.
“The dramatic difference in recurrence-free survival, especially with the large separation of K-M [Kaplan-Meier] curves early on, suggests that this change to induction has had a major impact on the outcomes,” Dr. Black observed.
Post hoc analysis
Dr. Grimm presented a post hoc analysis comparing the rates of recurrence in the NIMBUS trial with rates seen in the EORTC 30962 and CUETO 98013 trials. Dr. Black also compared NIMBUS results to results from the SWOG 8507 trial.
The analysis showed lower rates of recurrence in the standard dose frequency arm in the NIMBUS trial than in the EORTC and CUETO trials at both 12 months (11%, 25%, and 18%, respectively) and 24 months (15%, 32%, and 27%, respectively).
However, as Dr. Black pointed out, the SWOG trial had similar recurrence rates as the NIMBUS trial at 12 months (9% and 11%, respectively) and 24 months (19% and 15%, respectively).
Dr. Grimm suggested that the lower rates of recurrence in the standard dosing arm of NIMBUS versus the other trials might have been because 91% of patients in the NIMBUS trial having undergone repeat transurethral resection for bladder tumor before BCG instillation.
Dr. Black said while this might have had an effect, it was probably not the only answer. While it’s true that the other trials had not considered repeat transurethral resection for bladder tumor, there were other confounding factors that might have been important, from patient selection bias to the use of advanced cystoscopy technologies, he said.
“If we really want to discern differences between surgery and intravesical therapy, we need to focus on CIS [carcinoma in situ] patients. Although this has major implications on feasibility since the patient pool is smaller,” Dr. Black said.
“One final point I’d like to make is that we really need to use these trials to understand the biology of non–muscle invasive bladder cancer,” he said. ”We know that BCG induces a cellular response, and we can measure this, as well as cytokine response. We know that the response builds to a plateau over four to six doses of induction and over two to three doses of maintenance therapy. This is perhaps more rapid in patients with pre-existing BCG immune reactivity. But there is biological rationale for the current six-plus-three protocol, and I think the reduced dose frequency in the NIMBUS trial probably failed to achieve the same immune activation as the established protocol.”
“If we were faced with a BCG shortage, it is better to reduce dose or duration of therapy but not the frequency of dosing,” Dr. Black added.
The NIMBUS trial was sponsored by the EAU Research Foundation. Dr. Grimm disclosed ties to Novartis, Bristol-Myers Squibb, Pfizer, AstraZeneca, and many other pharmaceutical companies. Dr. Black had no conflicts of interests relevant to his comments.
SOURCE: Grimm M-O. EAU20. https://urosource.uroweb.org/resource-centre/EAU20V/212877/Abstract/
The rates of recurrence were 27.1% in the reduced dosing frequency arm and 12% in the standard dosing frequency arm. These results were reported at the virtual annual congress of the European Association of Urology.
More patients in the reduced dosing frequency arm than in the standard dosing frequency arm had a shorter time to recurrence, which was the primary endpoint of the trial.
At 6 months, the rate of recurrence was 18% in the reduced frequency arm and 8% in the standard frequency arm. The gap widened further at both 12 months (24% and 11%, respectively) and 24 months (34% and 15%, respectively). The hazard ratio for time to recurrence was 0.403 in favor of the standard dosing frequency arm.
“The recommended dose and schedule of BCG consists of once-weekly installations during 6 weeks of induction, followed by 3 weeks of maintenance at 3, 6, and 12 months,” observed study investigator Marc-Oliver Grimm, MD, of Jena (Germany) University Hospital.
“BCG instillation is, however, frequently associated with adverse events, which may lead to discontinuation, and several attempts have been made to reduce symptom burden associated with BCG,” he added.
Dr. Grimm presented the recently published findings from NIMBUS (Eur Urol. 2020 May 20;S0302-2838[20]30334-1) alongside some new information from a post hoc analysis.
Trial details
NIMBUS was a randomized, unblinded study of 345 patients with high-grade NMIBC who were recruited over a prolonged period, Dr. Grimm said. The long accrual was caused by a shortage of BCG and meant that the statistical assumptions had to be revised to include fewer patients.
The trial was designed to compare induction consisting of three versus six weekly BCG instillations and maintenance consisting of two versus three weekly BCG instillations at 3, 6, and 12 months. The aim had been to show that a reduced dosing frequency of BCG – 9 rather than 15 instillations – was noninferior to the standard dosing frequency of BCG, Dr. Grimm said. However, that was not the case, and the trial had to be stopped prematurely. In October 2019, the study’s sponsor, the EAU Research Foundation, announced that the trial would end.
Despite its unexpected ending, the trial’s data now fill some knowledge gaps, as pointed out by the discussant for the trial, Peter Black, MD, of the University of British Columbia in Vancouver.
Previous studies, such as the SWOG 8507, EORTC 30962, and CUETO 98013 trials, had shown that maintenance treatment works, but the schedule matters, he said. Results have also shown that the duration of maintenance treatment is less important than the dose of BCG given.
“The NIMBUS trial now tells us that dosing frequency is critical,” Dr. Black said.
Not only did the NIMBUS trial alter the maintenance schedule, it also altered the induction course of BCG instillation.
“The dramatic difference in recurrence-free survival, especially with the large separation of K-M [Kaplan-Meier] curves early on, suggests that this change to induction has had a major impact on the outcomes,” Dr. Black observed.
Post hoc analysis
Dr. Grimm presented a post hoc analysis comparing the rates of recurrence in the NIMBUS trial with rates seen in the EORTC 30962 and CUETO 98013 trials. Dr. Black also compared NIMBUS results to results from the SWOG 8507 trial.
The analysis showed lower rates of recurrence in the standard dose frequency arm in the NIMBUS trial than in the EORTC and CUETO trials at both 12 months (11%, 25%, and 18%, respectively) and 24 months (15%, 32%, and 27%, respectively).
However, as Dr. Black pointed out, the SWOG trial had similar recurrence rates as the NIMBUS trial at 12 months (9% and 11%, respectively) and 24 months (19% and 15%, respectively).
Dr. Grimm suggested that the lower rates of recurrence in the standard dosing arm of NIMBUS versus the other trials might have been because 91% of patients in the NIMBUS trial having undergone repeat transurethral resection for bladder tumor before BCG instillation.
Dr. Black said while this might have had an effect, it was probably not the only answer. While it’s true that the other trials had not considered repeat transurethral resection for bladder tumor, there were other confounding factors that might have been important, from patient selection bias to the use of advanced cystoscopy technologies, he said.
“If we really want to discern differences between surgery and intravesical therapy, we need to focus on CIS [carcinoma in situ] patients. Although this has major implications on feasibility since the patient pool is smaller,” Dr. Black said.
“One final point I’d like to make is that we really need to use these trials to understand the biology of non–muscle invasive bladder cancer,” he said. ”We know that BCG induces a cellular response, and we can measure this, as well as cytokine response. We know that the response builds to a plateau over four to six doses of induction and over two to three doses of maintenance therapy. This is perhaps more rapid in patients with pre-existing BCG immune reactivity. But there is biological rationale for the current six-plus-three protocol, and I think the reduced dose frequency in the NIMBUS trial probably failed to achieve the same immune activation as the established protocol.”
“If we were faced with a BCG shortage, it is better to reduce dose or duration of therapy but not the frequency of dosing,” Dr. Black added.
The NIMBUS trial was sponsored by the EAU Research Foundation. Dr. Grimm disclosed ties to Novartis, Bristol-Myers Squibb, Pfizer, AstraZeneca, and many other pharmaceutical companies. Dr. Black had no conflicts of interests relevant to his comments.
SOURCE: Grimm M-O. EAU20. https://urosource.uroweb.org/resource-centre/EAU20V/212877/Abstract/
FROM EAU20
PSMA PET/CT may be new ‘gold standard’ for prostate cancer staging
The accuracy was 92% for PSMA PET/CT and 65% for CT and bone scintigraphy (P < .001), according to data reported at the virtual annual congress of the European Association of Urology and published in The Lancet.
In addition, PSMA PET/CT had greater effects on treatment. First-line imaging led to treatment changes in 28% of the PSMA PET/CT group and 15% of the CT/bone scan group. Second-line imaging led to treatment changes in 27% and 5% of patients, respectively.
“My strong view is that this is practice-changing data,” said study investigator Michael Hofman, MBBS, of the Peter MacCallum Cancer Centre in Melbourne.
Highly relevant secondary outcomes were included in the study, Dr. Hofman said, and results were all in favor of PSMA PET/CT over conventional imaging.
PSMA PET/CT was associated with a lower rate of equivocal or uncertain findings (7% vs. 23%), and half the radiation dose was needed with PSMA PET/CT (8 mSv vs. 19 mSv). Furthermore, PSMA PET/CT was more accurate when used after CT/bone scan than when CT/bone scan was used after PSMA PET/CT (19% vs. 2%).
“PSMA PET/CT has emerged as a potential new gold standard for imaging prostate cancer,” Dr. Hofman said. The images it can produce were “striking” compared to conventional CT, he added. Pelvic and abdominal metastases that are barely visible on CT were “lighting up very brightly” on PSMA PET/CT, he said.
The study also showed that PSMA PET/CT was superior to CT/bone scans for picking up metastases throughout the body. The detection rate was 91% and 59%, respectively, for pelvic nodal metastases and 95% and 74%, respectively, for distant metastases.
Study details
ProPSMA is a multicenter, phase 3 trial directly comparing PSMA PET/CT and the standard of imaging. Of 339 men assessed for inclusion across 10 centers in Australia, 302 were randomized. They had a median age of 69 years. All patients had high-risk prostate cancer, which was defined as a prostate-specific antigen level of 20 ng/mL, Gleason Grade Group 3-5, or clinical stage T3 or higher. They were all about to undergo either surgery or radiotherapy with the intention of curing their prostate cancer.
PSMA PET/CT was performed using the gallium-68-labelled PSMA-11 tracer, but the results would likely be no different if another tracer were used, Dr. Hofman said in the discussion following his talk.
Of the three available tracers, there were minor differences, mostly in how they were excreted. However, “they’re all extremely good. I’m not sure anyone’s ever going to undertake a head-to-head study comparing them,” Dr. Hofman said.
“Whichever one you can access, at the cheapest cost, I think, is going to be the best one in your center,” he added. “That really does vary geographically, but I really don’t think one is better or worse than the other.”
Praise and criticism
The latest European guidelines acknowledge that PSMA PET/CT is more sensitive for detecting lymph node and bone metastases than the classical workup of abdominopelvic CT and bone scintigraphy, according to invited discussant Matthias Heck, PD Dr. med, of the Technical University of Munich in Germany.
“Molecular imaging using PSMA PET/CT facilitates the detection of small lymph node metastasis, with the size of a few millimeters,” Dr. Heck said.
Although he commended the ProPSMA investigators, Dr. Heck had one criticism of the study design that may have resulted in over-sensitivity of PSMA PET/CT.
“As a urologist, I want to address as a discussion point the low number of histopathologic validation in the ProPSMA study,” he said. “Pelvic lymph node sampling was performed only in 66% of patients treated with radical prostatectomy for high-risk prostate cancer. Hard criteria to define the presence of metastasis were only used in 23% of patients with metastases. Therefore, it is possible that the sensitivity was overestimated by using mainly soft criteria.”
The sensitivity of PSMA PET/CT was 85%, while that of CT/bone scan was 38%. The respective specificities were 98% and 91%.
“What I like most about this study is that, when we perform a PSMA PET/CT, you see the whole body; you don’t see only pelvic lymph nodes,” Dr. Heck said. Since it was not possible to validate distant metastasis by histopathology, he added, this imaging method could clearly help determine the best treatment.
“If we have distant metastasis in the bones or in the lymph nodes outside of the pelvis, it’s clearly unnecessary to direct this patient to undergo local treatment, and we need to think about other treatments,” Dr. Heck said. “Therefore, I think it’s a very important question that is being raised by this study, and we all need to look at the whole body of the patient and not focus only on the pelvic lymph nodes.”
The study was funded by the Prostate Cancer Foundation of Australia. Dr. Hofman said he has no relevant conflicts of interest. Dr. Heck disclosed relationships with Astellas, Janssen, Ipsen, Amgen, Bayer, Heise, Merck, Sanofi, and Takeda.
SOURCES: Hofman M et al. Lancet. March 22, doi: https://doi.org/10.1016/S0140-6736(20)30314-7.
The accuracy was 92% for PSMA PET/CT and 65% for CT and bone scintigraphy (P < .001), according to data reported at the virtual annual congress of the European Association of Urology and published in The Lancet.
In addition, PSMA PET/CT had greater effects on treatment. First-line imaging led to treatment changes in 28% of the PSMA PET/CT group and 15% of the CT/bone scan group. Second-line imaging led to treatment changes in 27% and 5% of patients, respectively.
“My strong view is that this is practice-changing data,” said study investigator Michael Hofman, MBBS, of the Peter MacCallum Cancer Centre in Melbourne.
Highly relevant secondary outcomes were included in the study, Dr. Hofman said, and results were all in favor of PSMA PET/CT over conventional imaging.
PSMA PET/CT was associated with a lower rate of equivocal or uncertain findings (7% vs. 23%), and half the radiation dose was needed with PSMA PET/CT (8 mSv vs. 19 mSv). Furthermore, PSMA PET/CT was more accurate when used after CT/bone scan than when CT/bone scan was used after PSMA PET/CT (19% vs. 2%).
“PSMA PET/CT has emerged as a potential new gold standard for imaging prostate cancer,” Dr. Hofman said. The images it can produce were “striking” compared to conventional CT, he added. Pelvic and abdominal metastases that are barely visible on CT were “lighting up very brightly” on PSMA PET/CT, he said.
The study also showed that PSMA PET/CT was superior to CT/bone scans for picking up metastases throughout the body. The detection rate was 91% and 59%, respectively, for pelvic nodal metastases and 95% and 74%, respectively, for distant metastases.
Study details
ProPSMA is a multicenter, phase 3 trial directly comparing PSMA PET/CT and the standard of imaging. Of 339 men assessed for inclusion across 10 centers in Australia, 302 were randomized. They had a median age of 69 years. All patients had high-risk prostate cancer, which was defined as a prostate-specific antigen level of 20 ng/mL, Gleason Grade Group 3-5, or clinical stage T3 or higher. They were all about to undergo either surgery or radiotherapy with the intention of curing their prostate cancer.
PSMA PET/CT was performed using the gallium-68-labelled PSMA-11 tracer, but the results would likely be no different if another tracer were used, Dr. Hofman said in the discussion following his talk.
Of the three available tracers, there were minor differences, mostly in how they were excreted. However, “they’re all extremely good. I’m not sure anyone’s ever going to undertake a head-to-head study comparing them,” Dr. Hofman said.
“Whichever one you can access, at the cheapest cost, I think, is going to be the best one in your center,” he added. “That really does vary geographically, but I really don’t think one is better or worse than the other.”
Praise and criticism
The latest European guidelines acknowledge that PSMA PET/CT is more sensitive for detecting lymph node and bone metastases than the classical workup of abdominopelvic CT and bone scintigraphy, according to invited discussant Matthias Heck, PD Dr. med, of the Technical University of Munich in Germany.
“Molecular imaging using PSMA PET/CT facilitates the detection of small lymph node metastasis, with the size of a few millimeters,” Dr. Heck said.
Although he commended the ProPSMA investigators, Dr. Heck had one criticism of the study design that may have resulted in over-sensitivity of PSMA PET/CT.
“As a urologist, I want to address as a discussion point the low number of histopathologic validation in the ProPSMA study,” he said. “Pelvic lymph node sampling was performed only in 66% of patients treated with radical prostatectomy for high-risk prostate cancer. Hard criteria to define the presence of metastasis were only used in 23% of patients with metastases. Therefore, it is possible that the sensitivity was overestimated by using mainly soft criteria.”
The sensitivity of PSMA PET/CT was 85%, while that of CT/bone scan was 38%. The respective specificities were 98% and 91%.
“What I like most about this study is that, when we perform a PSMA PET/CT, you see the whole body; you don’t see only pelvic lymph nodes,” Dr. Heck said. Since it was not possible to validate distant metastasis by histopathology, he added, this imaging method could clearly help determine the best treatment.
“If we have distant metastasis in the bones or in the lymph nodes outside of the pelvis, it’s clearly unnecessary to direct this patient to undergo local treatment, and we need to think about other treatments,” Dr. Heck said. “Therefore, I think it’s a very important question that is being raised by this study, and we all need to look at the whole body of the patient and not focus only on the pelvic lymph nodes.”
The study was funded by the Prostate Cancer Foundation of Australia. Dr. Hofman said he has no relevant conflicts of interest. Dr. Heck disclosed relationships with Astellas, Janssen, Ipsen, Amgen, Bayer, Heise, Merck, Sanofi, and Takeda.
SOURCES: Hofman M et al. Lancet. March 22, doi: https://doi.org/10.1016/S0140-6736(20)30314-7.
The accuracy was 92% for PSMA PET/CT and 65% for CT and bone scintigraphy (P < .001), according to data reported at the virtual annual congress of the European Association of Urology and published in The Lancet.
In addition, PSMA PET/CT had greater effects on treatment. First-line imaging led to treatment changes in 28% of the PSMA PET/CT group and 15% of the CT/bone scan group. Second-line imaging led to treatment changes in 27% and 5% of patients, respectively.
“My strong view is that this is practice-changing data,” said study investigator Michael Hofman, MBBS, of the Peter MacCallum Cancer Centre in Melbourne.
Highly relevant secondary outcomes were included in the study, Dr. Hofman said, and results were all in favor of PSMA PET/CT over conventional imaging.
PSMA PET/CT was associated with a lower rate of equivocal or uncertain findings (7% vs. 23%), and half the radiation dose was needed with PSMA PET/CT (8 mSv vs. 19 mSv). Furthermore, PSMA PET/CT was more accurate when used after CT/bone scan than when CT/bone scan was used after PSMA PET/CT (19% vs. 2%).
“PSMA PET/CT has emerged as a potential new gold standard for imaging prostate cancer,” Dr. Hofman said. The images it can produce were “striking” compared to conventional CT, he added. Pelvic and abdominal metastases that are barely visible on CT were “lighting up very brightly” on PSMA PET/CT, he said.
The study also showed that PSMA PET/CT was superior to CT/bone scans for picking up metastases throughout the body. The detection rate was 91% and 59%, respectively, for pelvic nodal metastases and 95% and 74%, respectively, for distant metastases.
Study details
ProPSMA is a multicenter, phase 3 trial directly comparing PSMA PET/CT and the standard of imaging. Of 339 men assessed for inclusion across 10 centers in Australia, 302 were randomized. They had a median age of 69 years. All patients had high-risk prostate cancer, which was defined as a prostate-specific antigen level of 20 ng/mL, Gleason Grade Group 3-5, or clinical stage T3 or higher. They were all about to undergo either surgery or radiotherapy with the intention of curing their prostate cancer.
PSMA PET/CT was performed using the gallium-68-labelled PSMA-11 tracer, but the results would likely be no different if another tracer were used, Dr. Hofman said in the discussion following his talk.
Of the three available tracers, there were minor differences, mostly in how they were excreted. However, “they’re all extremely good. I’m not sure anyone’s ever going to undertake a head-to-head study comparing them,” Dr. Hofman said.
“Whichever one you can access, at the cheapest cost, I think, is going to be the best one in your center,” he added. “That really does vary geographically, but I really don’t think one is better or worse than the other.”
Praise and criticism
The latest European guidelines acknowledge that PSMA PET/CT is more sensitive for detecting lymph node and bone metastases than the classical workup of abdominopelvic CT and bone scintigraphy, according to invited discussant Matthias Heck, PD Dr. med, of the Technical University of Munich in Germany.
“Molecular imaging using PSMA PET/CT facilitates the detection of small lymph node metastasis, with the size of a few millimeters,” Dr. Heck said.
Although he commended the ProPSMA investigators, Dr. Heck had one criticism of the study design that may have resulted in over-sensitivity of PSMA PET/CT.
“As a urologist, I want to address as a discussion point the low number of histopathologic validation in the ProPSMA study,” he said. “Pelvic lymph node sampling was performed only in 66% of patients treated with radical prostatectomy for high-risk prostate cancer. Hard criteria to define the presence of metastasis were only used in 23% of patients with metastases. Therefore, it is possible that the sensitivity was overestimated by using mainly soft criteria.”
The sensitivity of PSMA PET/CT was 85%, while that of CT/bone scan was 38%. The respective specificities were 98% and 91%.
“What I like most about this study is that, when we perform a PSMA PET/CT, you see the whole body; you don’t see only pelvic lymph nodes,” Dr. Heck said. Since it was not possible to validate distant metastasis by histopathology, he added, this imaging method could clearly help determine the best treatment.
“If we have distant metastasis in the bones or in the lymph nodes outside of the pelvis, it’s clearly unnecessary to direct this patient to undergo local treatment, and we need to think about other treatments,” Dr. Heck said. “Therefore, I think it’s a very important question that is being raised by this study, and we all need to look at the whole body of the patient and not focus only on the pelvic lymph nodes.”
The study was funded by the Prostate Cancer Foundation of Australia. Dr. Hofman said he has no relevant conflicts of interest. Dr. Heck disclosed relationships with Astellas, Janssen, Ipsen, Amgen, Bayer, Heise, Merck, Sanofi, and Takeda.
SOURCES: Hofman M et al. Lancet. March 22, doi: https://doi.org/10.1016/S0140-6736(20)30314-7.
FROM EAU20
Cancer patient organizations critically affected by pandemic
The COVID-19 pandemic has disrupted every aspect of cancer care, from diagnosis, treatment, and follow-up to participation in clinical trials, according to a new report that collected responses from cancer patient organizations around the world.
The report includes responses from 157 organizations in 56 countries, representing some 350,000 patients with cancer.
“The COVID-19 global pandemic has quite literally wreaked havoc with all of our lives but especially for cancer patients,” said the report’s author, Frances Reid, MBA, program director, World Ovarian Cancer Coalition.
“To those who have the power or influence to ensure that cancer treatment and services are not set back several years, please listen to those organizations who can articulate clearly the impact on patients, work with them, and act on it as soon as you can,” she added.
The new report, entitled “The Impact of COVID-19 on Cancer Patient Organisations,” was released on June 12. The organizations were surveyed from May 11 to May 25.
Cancer diagnosis
Two-thirds of the organizations surveyed said cancer screening programs had been canceled in their country, and 59% indicated they had seen a drop in urgent referrals for suspected cancer.
Some 44% said that access to pathology services had been reduced. One group in Australia reported that “results of pathology tests are taking longer to be returned. Generally a result would be returned within 48 hours. Since COVID-19, results are taking up to 7 days to be returned.”
As for treatment, 68% of organizations reported delays or cancellations of surgery or other treatments; 58% reported there had been a need to modify treatment protocols; and 48% indicated there had been a drop in participation in clinical trials.
Respondents were also concerned about reported increases in stress, anxiety, and isolation among many cancer patients. “Often at increased risk of infection and serious illness themselves ... many have been required to ‘shield’ from others, totally withdrawing from life outside their homes, thus increasing the already high levels of isolation they feel because of their life-limiting conditions,” the report notes.
In addition, some 60% of the organizations said that the pandemic had increased financial hardship among cancer patients. One US group commented: “Unemployment levels in the States similar to depression era. This has been a real challenge as many have lost insurance as well as jobs.”
Only a minority of respondents reported that cancer care was being offered in hospitals with no special arrangements in place to treat concomitant COVID-19 patients.
On the other hand, only 15% of respondents indicated that patients were being treated in a hospital that was not also caring for COVID-19 patients.
“Cancer will not wait for COVID-19 to pass, if it ever will, and the patient organizations are the key to minimizing the devastating impact [COVID-19 is having] on people with cancer,” Reid emphasized.
“More than ever, the patient/support services should be strengthened,” commented a group from France.
Patient services affected
“Almost all organisations (89%) have had to alter their services for people with cancer,” the report notes.
Two thirds of organizations involved in professional educational activities have had to change their services in some way, either by moving them online or stopping programs altogether, at least temporarily. “Some found that doctors and nurses are too busy with the pandemic to participate, and that their appetite for such activity is also diminished,” the report notes.
The volume of phone calls and emails increased in almost 6 of 10 organizations that provide support services for patients. Compared to prepandemic levels, volume increased by an average of 44%.
The most common queries raised by people with cancer (accounting for 85% of all queries) were questions about the risks of contracting COVID-19 and cancer treatments during the pandemic.
Some of the organizations also commented about how they had been affected. One group from Uganda said: “We had a sudden lockdown and we could not access office to give face to face counselling. We stopped research due to national guidelines on research. We continued giving information via phone and social media especially WhatsApp. We created groups for patients and counsellors to continue interacting.”
A group in Costa Rica reported: “We developed a new program of transfers from their homes to the hospital for cancer patients in chemotherapy and radiotherapy. 200 monthly transfers. We created a virtual community instead of our face-to-face support group, we started in April and we have 108 members, virtual sessions are held every two weeks.”
An organization based in the United States reported that it was “totally revamping our educational programs to be delivered in new ways in an online format ― not just replicating the in-person formats, but reaching out to our community and asking them what they would find the most valuable.”
Impact on fundraising
Almost 9 in 10 organizations raise funds to support their activities, the report notes. “A shocking 79% of organisations say they predict a fall in income over the next 12 months, with a further 16% not sure, leaving only 5% confident of their financial stability.”
Every type of fund-raising has been affected by COVID-19, from grants and major donors to community fund-raising events. Sixty percent of organisations said they were trying to find new ways to raise funds.
However, as one organization in Japan noted: “At the moment we can survive and feel it is unethical to ask the public for money when many are facing dire financial personal circumstances.”
A group from Australia commented: “Fundraising has been extremely difficult due to COVID-19 with distancing laws and no group gatherings as well as the economic downturn. Crisis appeals have been unsuccessful and all outdoor events and major events have been cancelled. In Australia we have had to contend with also the fires earlier in the year where a lot of money was donated to leaving other foundations struggling to get donor support.”
A little more than half (55%) of the organizations surveyed have had to cut costs.
Staffing cuts have been made in 1 in 10 of the organizations surveyed. A similar proportion of organizations have furloughed staff. Many if not all staff from numerous organizations are working from home.
A little more than half of those surveyed either provide funding for research or conduct research themselves, but only one quarter of them indicated there had been no change in their research projects. The others have indicated that they had to either reduce the scope of their research, put it on pause, or stop it altogether.
Three quarters of survey respondents noted that they had engaged in advocacy activities prior to the pandemic, and almost two thirds of them said they had to delay these activities.
Several of the organizations expressed thanks to the survey authors.
“COVID-19 is a global pandemic and cancer patients all around the world have similar worries, concerns and questions ― we are a small/medium organisation working in one country but believe in the power of community and coalitions and so this survey is a very welcome part of looking at this from a greater perspective,” commented one British group.
Reid has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The COVID-19 pandemic has disrupted every aspect of cancer care, from diagnosis, treatment, and follow-up to participation in clinical trials, according to a new report that collected responses from cancer patient organizations around the world.
The report includes responses from 157 organizations in 56 countries, representing some 350,000 patients with cancer.
“The COVID-19 global pandemic has quite literally wreaked havoc with all of our lives but especially for cancer patients,” said the report’s author, Frances Reid, MBA, program director, World Ovarian Cancer Coalition.
“To those who have the power or influence to ensure that cancer treatment and services are not set back several years, please listen to those organizations who can articulate clearly the impact on patients, work with them, and act on it as soon as you can,” she added.
The new report, entitled “The Impact of COVID-19 on Cancer Patient Organisations,” was released on June 12. The organizations were surveyed from May 11 to May 25.
Cancer diagnosis
Two-thirds of the organizations surveyed said cancer screening programs had been canceled in their country, and 59% indicated they had seen a drop in urgent referrals for suspected cancer.
Some 44% said that access to pathology services had been reduced. One group in Australia reported that “results of pathology tests are taking longer to be returned. Generally a result would be returned within 48 hours. Since COVID-19, results are taking up to 7 days to be returned.”
As for treatment, 68% of organizations reported delays or cancellations of surgery or other treatments; 58% reported there had been a need to modify treatment protocols; and 48% indicated there had been a drop in participation in clinical trials.
Respondents were also concerned about reported increases in stress, anxiety, and isolation among many cancer patients. “Often at increased risk of infection and serious illness themselves ... many have been required to ‘shield’ from others, totally withdrawing from life outside their homes, thus increasing the already high levels of isolation they feel because of their life-limiting conditions,” the report notes.
In addition, some 60% of the organizations said that the pandemic had increased financial hardship among cancer patients. One US group commented: “Unemployment levels in the States similar to depression era. This has been a real challenge as many have lost insurance as well as jobs.”
Only a minority of respondents reported that cancer care was being offered in hospitals with no special arrangements in place to treat concomitant COVID-19 patients.
On the other hand, only 15% of respondents indicated that patients were being treated in a hospital that was not also caring for COVID-19 patients.
“Cancer will not wait for COVID-19 to pass, if it ever will, and the patient organizations are the key to minimizing the devastating impact [COVID-19 is having] on people with cancer,” Reid emphasized.
“More than ever, the patient/support services should be strengthened,” commented a group from France.
Patient services affected
“Almost all organisations (89%) have had to alter their services for people with cancer,” the report notes.
Two thirds of organizations involved in professional educational activities have had to change their services in some way, either by moving them online or stopping programs altogether, at least temporarily. “Some found that doctors and nurses are too busy with the pandemic to participate, and that their appetite for such activity is also diminished,” the report notes.
The volume of phone calls and emails increased in almost 6 of 10 organizations that provide support services for patients. Compared to prepandemic levels, volume increased by an average of 44%.
The most common queries raised by people with cancer (accounting for 85% of all queries) were questions about the risks of contracting COVID-19 and cancer treatments during the pandemic.
Some of the organizations also commented about how they had been affected. One group from Uganda said: “We had a sudden lockdown and we could not access office to give face to face counselling. We stopped research due to national guidelines on research. We continued giving information via phone and social media especially WhatsApp. We created groups for patients and counsellors to continue interacting.”
A group in Costa Rica reported: “We developed a new program of transfers from their homes to the hospital for cancer patients in chemotherapy and radiotherapy. 200 monthly transfers. We created a virtual community instead of our face-to-face support group, we started in April and we have 108 members, virtual sessions are held every two weeks.”
An organization based in the United States reported that it was “totally revamping our educational programs to be delivered in new ways in an online format ― not just replicating the in-person formats, but reaching out to our community and asking them what they would find the most valuable.”
Impact on fundraising
Almost 9 in 10 organizations raise funds to support their activities, the report notes. “A shocking 79% of organisations say they predict a fall in income over the next 12 months, with a further 16% not sure, leaving only 5% confident of their financial stability.”
Every type of fund-raising has been affected by COVID-19, from grants and major donors to community fund-raising events. Sixty percent of organisations said they were trying to find new ways to raise funds.
However, as one organization in Japan noted: “At the moment we can survive and feel it is unethical to ask the public for money when many are facing dire financial personal circumstances.”
A group from Australia commented: “Fundraising has been extremely difficult due to COVID-19 with distancing laws and no group gatherings as well as the economic downturn. Crisis appeals have been unsuccessful and all outdoor events and major events have been cancelled. In Australia we have had to contend with also the fires earlier in the year where a lot of money was donated to leaving other foundations struggling to get donor support.”
A little more than half (55%) of the organizations surveyed have had to cut costs.
Staffing cuts have been made in 1 in 10 of the organizations surveyed. A similar proportion of organizations have furloughed staff. Many if not all staff from numerous organizations are working from home.
A little more than half of those surveyed either provide funding for research or conduct research themselves, but only one quarter of them indicated there had been no change in their research projects. The others have indicated that they had to either reduce the scope of their research, put it on pause, or stop it altogether.
Three quarters of survey respondents noted that they had engaged in advocacy activities prior to the pandemic, and almost two thirds of them said they had to delay these activities.
Several of the organizations expressed thanks to the survey authors.
“COVID-19 is a global pandemic and cancer patients all around the world have similar worries, concerns and questions ― we are a small/medium organisation working in one country but believe in the power of community and coalitions and so this survey is a very welcome part of looking at this from a greater perspective,” commented one British group.
Reid has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The COVID-19 pandemic has disrupted every aspect of cancer care, from diagnosis, treatment, and follow-up to participation in clinical trials, according to a new report that collected responses from cancer patient organizations around the world.
The report includes responses from 157 organizations in 56 countries, representing some 350,000 patients with cancer.
“The COVID-19 global pandemic has quite literally wreaked havoc with all of our lives but especially for cancer patients,” said the report’s author, Frances Reid, MBA, program director, World Ovarian Cancer Coalition.
“To those who have the power or influence to ensure that cancer treatment and services are not set back several years, please listen to those organizations who can articulate clearly the impact on patients, work with them, and act on it as soon as you can,” she added.
The new report, entitled “The Impact of COVID-19 on Cancer Patient Organisations,” was released on June 12. The organizations were surveyed from May 11 to May 25.
Cancer diagnosis
Two-thirds of the organizations surveyed said cancer screening programs had been canceled in their country, and 59% indicated they had seen a drop in urgent referrals for suspected cancer.
Some 44% said that access to pathology services had been reduced. One group in Australia reported that “results of pathology tests are taking longer to be returned. Generally a result would be returned within 48 hours. Since COVID-19, results are taking up to 7 days to be returned.”
As for treatment, 68% of organizations reported delays or cancellations of surgery or other treatments; 58% reported there had been a need to modify treatment protocols; and 48% indicated there had been a drop in participation in clinical trials.
Respondents were also concerned about reported increases in stress, anxiety, and isolation among many cancer patients. “Often at increased risk of infection and serious illness themselves ... many have been required to ‘shield’ from others, totally withdrawing from life outside their homes, thus increasing the already high levels of isolation they feel because of their life-limiting conditions,” the report notes.
In addition, some 60% of the organizations said that the pandemic had increased financial hardship among cancer patients. One US group commented: “Unemployment levels in the States similar to depression era. This has been a real challenge as many have lost insurance as well as jobs.”
Only a minority of respondents reported that cancer care was being offered in hospitals with no special arrangements in place to treat concomitant COVID-19 patients.
On the other hand, only 15% of respondents indicated that patients were being treated in a hospital that was not also caring for COVID-19 patients.
“Cancer will not wait for COVID-19 to pass, if it ever will, and the patient organizations are the key to minimizing the devastating impact [COVID-19 is having] on people with cancer,” Reid emphasized.
“More than ever, the patient/support services should be strengthened,” commented a group from France.
Patient services affected
“Almost all organisations (89%) have had to alter their services for people with cancer,” the report notes.
Two thirds of organizations involved in professional educational activities have had to change their services in some way, either by moving them online or stopping programs altogether, at least temporarily. “Some found that doctors and nurses are too busy with the pandemic to participate, and that their appetite for such activity is also diminished,” the report notes.
The volume of phone calls and emails increased in almost 6 of 10 organizations that provide support services for patients. Compared to prepandemic levels, volume increased by an average of 44%.
The most common queries raised by people with cancer (accounting for 85% of all queries) were questions about the risks of contracting COVID-19 and cancer treatments during the pandemic.
Some of the organizations also commented about how they had been affected. One group from Uganda said: “We had a sudden lockdown and we could not access office to give face to face counselling. We stopped research due to national guidelines on research. We continued giving information via phone and social media especially WhatsApp. We created groups for patients and counsellors to continue interacting.”
A group in Costa Rica reported: “We developed a new program of transfers from their homes to the hospital for cancer patients in chemotherapy and radiotherapy. 200 monthly transfers. We created a virtual community instead of our face-to-face support group, we started in April and we have 108 members, virtual sessions are held every two weeks.”
An organization based in the United States reported that it was “totally revamping our educational programs to be delivered in new ways in an online format ― not just replicating the in-person formats, but reaching out to our community and asking them what they would find the most valuable.”
Impact on fundraising
Almost 9 in 10 organizations raise funds to support their activities, the report notes. “A shocking 79% of organisations say they predict a fall in income over the next 12 months, with a further 16% not sure, leaving only 5% confident of their financial stability.”
Every type of fund-raising has been affected by COVID-19, from grants and major donors to community fund-raising events. Sixty percent of organisations said they were trying to find new ways to raise funds.
However, as one organization in Japan noted: “At the moment we can survive and feel it is unethical to ask the public for money when many are facing dire financial personal circumstances.”
A group from Australia commented: “Fundraising has been extremely difficult due to COVID-19 with distancing laws and no group gatherings as well as the economic downturn. Crisis appeals have been unsuccessful and all outdoor events and major events have been cancelled. In Australia we have had to contend with also the fires earlier in the year where a lot of money was donated to leaving other foundations struggling to get donor support.”
A little more than half (55%) of the organizations surveyed have had to cut costs.
Staffing cuts have been made in 1 in 10 of the organizations surveyed. A similar proportion of organizations have furloughed staff. Many if not all staff from numerous organizations are working from home.
A little more than half of those surveyed either provide funding for research or conduct research themselves, but only one quarter of them indicated there had been no change in their research projects. The others have indicated that they had to either reduce the scope of their research, put it on pause, or stop it altogether.
Three quarters of survey respondents noted that they had engaged in advocacy activities prior to the pandemic, and almost two thirds of them said they had to delay these activities.
Several of the organizations expressed thanks to the survey authors.
“COVID-19 is a global pandemic and cancer patients all around the world have similar worries, concerns and questions ― we are a small/medium organisation working in one country but believe in the power of community and coalitions and so this survey is a very welcome part of looking at this from a greater perspective,” commented one British group.
Reid has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
MRI reliably identifies significant prostate cancer
Prostate cancers that are missed on multiparametric (mp) MRI are small and “not life-threatening,” according to an analysis of data from the Prostate MR Imaging Study (PROMIS).
“Our work suggests that MRI scans of the prostate appear to deliver crucial information about a man’s risk of dying from prostate cancer, even before he has a biopsy,” said Joseph Norris, BM BS, from University College London.
“This may mean that we can finally move prostate cancer to a position in which we can use imaging as the primary tool to direct further investigations, treatment, and prediction of risk,” he told Medscape Medical News.
This is “a position that all other solid organ cancers have reached,” said Norris, who will present the findings at the upcoming virtual European Association of Urology 2020 Congress.
All 576 PROMIS participants underwent an mpMRI scan, a transrectal ultrasonography (TRUS)–guided biopsy, and a template prostate mapping (TPM) biopsy taken at 5-mm intervals across the entire prostate.
PROMIS researchers previously showed that mpMRI had a 93% sensitivity for clinically significant cancer, whereas TRUS biopsy had only a 48% sensitivity, as reported by Medscape Medical News. And they concluded that the use of mpMRI as a first-line diagnostic tool could prevent 27% of all biopsies, which can have serious adverse effects, such as pain, urinary problems, infection, bleeding, and erectile dysfunction.
However, in their study looking at the accuracy of mpMRI and TRUS biopsy, the researchers did not investigate the severity of the 7% of cancers that mpMRI missed. “What if those missed cancers are, in fact, aggressive? That’s what we set out to examine,” Norris explained.
So he and his colleagues conducted a post ad hoc analysis of the PROMIS participants in whom clinically significant cancer had been detected with TPM biopsy to see which of those cancers had been detected with mpMRI. The findings were published online in European Urology.
Cancers met the strict definition of clinically significant if they had a Gleason score of at least 4+3 for a tumor of any length, or a maximum cancer core length (MCCL) greater than 6 mm for a cancer of any grade. They met the less-strict definition if they had a Gleason score of at least 3+4 for a tumor of any length, or a MCCL greater than 4 mm for a cancer of any grade.
In PROMIS, TPM biopsy detected 230 cancers that met the strict definition of clinically significant and 331 that met the less-strict definition.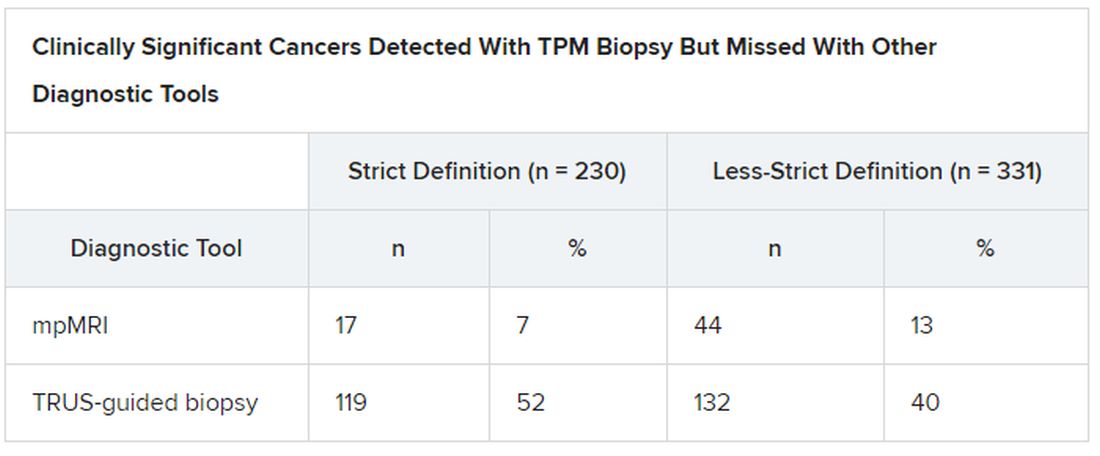
Overall Gleason scores were significantly lower for the 17 strict-definition cancers not detected with 1.5 T mpMRI than for those detected with mpMRI (P = .0007), as were maximum Gleason scores (P < .0001).
Median MCCL was 3 mm shorter for all 17 tumors missed with mpMRI than for those detected with mpMRI (5 vs 8 mm; P < .0001).
mpMRI detected all tumors identified on TPM biopsy that had an overall Gleason score greater than 3+4 (Gleason grades 3 to 5) or a maximum Gleason score greater than 4+3 (Gleason grades 4 and 5).
“This finding is important, given that in PROMIS, no men with an overall Gleason score of 4+3 had cancer missed by MRI, indicating that actually MRI may be able to identify all truly significant cancers,” said Norris.
Adding PSA Density Threshold
To further assess cancers missed on mpMRI, the researchers looked at prostate-specific antigen (PSA) density, calculated as total PSA level (ng/mL) divided by prostate volume (mL).
“We found that if we applied a threshold PSA density to men with normal-looking MRI scans, we could reduce the proportion of missed significant cancer to just 5%. This is exciting; it means we can make MRI an even more effective test for prostate cancer in a very simple way,” Norris reported.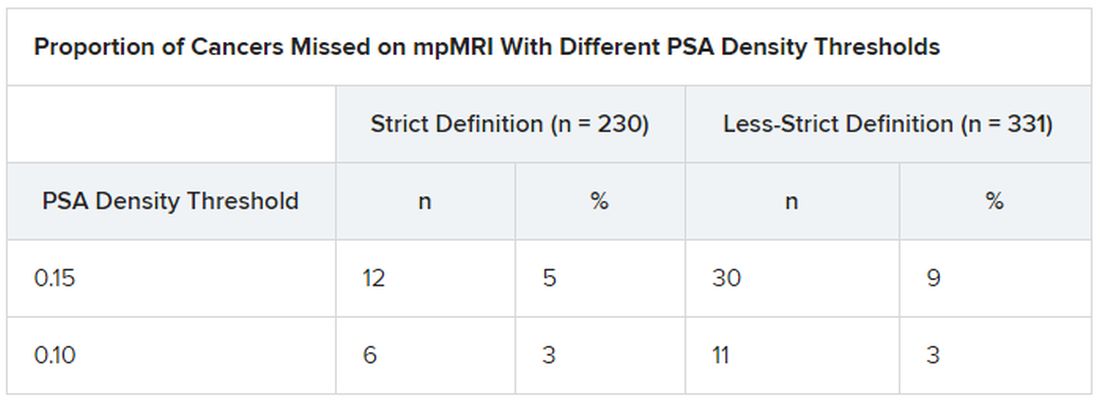
“These data show that no highly aggressive prostate cancers were missed by MRI, either at the level of the whole prostate or at the individual needle level,” said Norris. This should lead to positive outcomes in the long term.
And since the PROMIS data were gathered, MRI technology has improved, he said. The “MRI scanners in PROMIS were 1.5 Tesla,” whereas today’s machines are 3.0 T, which could increase the detection of significant prostate cancer.
In fact, “our analysis here potentially overestimates the amount of undetected disease,” he noted.
Prostate cancer that is not clinically significant is often monitored with active surveillance, so “invisible” cancers missed on mpMRI could actually be looked at in a positive light, he explained.
Variation in technique, interpretation
But the quality of care when it comes to the diagnosis of prostate cancer is not equal everywhere, said Gerald Andriole, MD, from the Washington School of Medicine in St. Louis, Missouri.
“When you get an MRI in a center that doesn’t do a lot of prostate cancer testing, you may not have the best software and you may have a radiologist who is not that experienced,” he told Medscape Medical News. Specialized cancer centers of excellence tend to do a great job finding prostate cancer, “but other centers have high significant-miss rates or high overcall rates.”
“The elephant in the room remains the considerable variation in technique and interobserver interpretation of prostate mpMRI,” write Steven Monda, MD, and Marc Dall’Era, MD, both from UC Davis Health in Sacramento, California, in an editorial that accompanies the new PROMIS analysis.
“These problems must be addressed and remedied in each institution before relying on results from PROMIS to drive changes in clinical practice,” they add.
Norris, Andriole, Monda, and Dall’Era have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Prostate cancers that are missed on multiparametric (mp) MRI are small and “not life-threatening,” according to an analysis of data from the Prostate MR Imaging Study (PROMIS).
“Our work suggests that MRI scans of the prostate appear to deliver crucial information about a man’s risk of dying from prostate cancer, even before he has a biopsy,” said Joseph Norris, BM BS, from University College London.
“This may mean that we can finally move prostate cancer to a position in which we can use imaging as the primary tool to direct further investigations, treatment, and prediction of risk,” he told Medscape Medical News.
This is “a position that all other solid organ cancers have reached,” said Norris, who will present the findings at the upcoming virtual European Association of Urology 2020 Congress.
All 576 PROMIS participants underwent an mpMRI scan, a transrectal ultrasonography (TRUS)–guided biopsy, and a template prostate mapping (TPM) biopsy taken at 5-mm intervals across the entire prostate.
PROMIS researchers previously showed that mpMRI had a 93% sensitivity for clinically significant cancer, whereas TRUS biopsy had only a 48% sensitivity, as reported by Medscape Medical News. And they concluded that the use of mpMRI as a first-line diagnostic tool could prevent 27% of all biopsies, which can have serious adverse effects, such as pain, urinary problems, infection, bleeding, and erectile dysfunction.
However, in their study looking at the accuracy of mpMRI and TRUS biopsy, the researchers did not investigate the severity of the 7% of cancers that mpMRI missed. “What if those missed cancers are, in fact, aggressive? That’s what we set out to examine,” Norris explained.
So he and his colleagues conducted a post ad hoc analysis of the PROMIS participants in whom clinically significant cancer had been detected with TPM biopsy to see which of those cancers had been detected with mpMRI. The findings were published online in European Urology.
Cancers met the strict definition of clinically significant if they had a Gleason score of at least 4+3 for a tumor of any length, or a maximum cancer core length (MCCL) greater than 6 mm for a cancer of any grade. They met the less-strict definition if they had a Gleason score of at least 3+4 for a tumor of any length, or a MCCL greater than 4 mm for a cancer of any grade.
In PROMIS, TPM biopsy detected 230 cancers that met the strict definition of clinically significant and 331 that met the less-strict definition.
Overall Gleason scores were significantly lower for the 17 strict-definition cancers not detected with 1.5 T mpMRI than for those detected with mpMRI (P = .0007), as were maximum Gleason scores (P < .0001).
Median MCCL was 3 mm shorter for all 17 tumors missed with mpMRI than for those detected with mpMRI (5 vs 8 mm; P < .0001).
mpMRI detected all tumors identified on TPM biopsy that had an overall Gleason score greater than 3+4 (Gleason grades 3 to 5) or a maximum Gleason score greater than 4+3 (Gleason grades 4 and 5).
“This finding is important, given that in PROMIS, no men with an overall Gleason score of 4+3 had cancer missed by MRI, indicating that actually MRI may be able to identify all truly significant cancers,” said Norris.
Adding PSA Density Threshold
To further assess cancers missed on mpMRI, the researchers looked at prostate-specific antigen (PSA) density, calculated as total PSA level (ng/mL) divided by prostate volume (mL).
“We found that if we applied a threshold PSA density to men with normal-looking MRI scans, we could reduce the proportion of missed significant cancer to just 5%. This is exciting; it means we can make MRI an even more effective test for prostate cancer in a very simple way,” Norris reported.
“These data show that no highly aggressive prostate cancers were missed by MRI, either at the level of the whole prostate or at the individual needle level,” said Norris. This should lead to positive outcomes in the long term.
And since the PROMIS data were gathered, MRI technology has improved, he said. The “MRI scanners in PROMIS were 1.5 Tesla,” whereas today’s machines are 3.0 T, which could increase the detection of significant prostate cancer.
In fact, “our analysis here potentially overestimates the amount of undetected disease,” he noted.
Prostate cancer that is not clinically significant is often monitored with active surveillance, so “invisible” cancers missed on mpMRI could actually be looked at in a positive light, he explained.
Variation in technique, interpretation
But the quality of care when it comes to the diagnosis of prostate cancer is not equal everywhere, said Gerald Andriole, MD, from the Washington School of Medicine in St. Louis, Missouri.
“When you get an MRI in a center that doesn’t do a lot of prostate cancer testing, you may not have the best software and you may have a radiologist who is not that experienced,” he told Medscape Medical News. Specialized cancer centers of excellence tend to do a great job finding prostate cancer, “but other centers have high significant-miss rates or high overcall rates.”
“The elephant in the room remains the considerable variation in technique and interobserver interpretation of prostate mpMRI,” write Steven Monda, MD, and Marc Dall’Era, MD, both from UC Davis Health in Sacramento, California, in an editorial that accompanies the new PROMIS analysis.
“These problems must be addressed and remedied in each institution before relying on results from PROMIS to drive changes in clinical practice,” they add.
Norris, Andriole, Monda, and Dall’Era have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Prostate cancers that are missed on multiparametric (mp) MRI are small and “not life-threatening,” according to an analysis of data from the Prostate MR Imaging Study (PROMIS).
“Our work suggests that MRI scans of the prostate appear to deliver crucial information about a man’s risk of dying from prostate cancer, even before he has a biopsy,” said Joseph Norris, BM BS, from University College London.
“This may mean that we can finally move prostate cancer to a position in which we can use imaging as the primary tool to direct further investigations, treatment, and prediction of risk,” he told Medscape Medical News.
This is “a position that all other solid organ cancers have reached,” said Norris, who will present the findings at the upcoming virtual European Association of Urology 2020 Congress.
All 576 PROMIS participants underwent an mpMRI scan, a transrectal ultrasonography (TRUS)–guided biopsy, and a template prostate mapping (TPM) biopsy taken at 5-mm intervals across the entire prostate.
PROMIS researchers previously showed that mpMRI had a 93% sensitivity for clinically significant cancer, whereas TRUS biopsy had only a 48% sensitivity, as reported by Medscape Medical News. And they concluded that the use of mpMRI as a first-line diagnostic tool could prevent 27% of all biopsies, which can have serious adverse effects, such as pain, urinary problems, infection, bleeding, and erectile dysfunction.
However, in their study looking at the accuracy of mpMRI and TRUS biopsy, the researchers did not investigate the severity of the 7% of cancers that mpMRI missed. “What if those missed cancers are, in fact, aggressive? That’s what we set out to examine,” Norris explained.
So he and his colleagues conducted a post ad hoc analysis of the PROMIS participants in whom clinically significant cancer had been detected with TPM biopsy to see which of those cancers had been detected with mpMRI. The findings were published online in European Urology.
Cancers met the strict definition of clinically significant if they had a Gleason score of at least 4+3 for a tumor of any length, or a maximum cancer core length (MCCL) greater than 6 mm for a cancer of any grade. They met the less-strict definition if they had a Gleason score of at least 3+4 for a tumor of any length, or a MCCL greater than 4 mm for a cancer of any grade.
In PROMIS, TPM biopsy detected 230 cancers that met the strict definition of clinically significant and 331 that met the less-strict definition.
Overall Gleason scores were significantly lower for the 17 strict-definition cancers not detected with 1.5 T mpMRI than for those detected with mpMRI (P = .0007), as were maximum Gleason scores (P < .0001).
Median MCCL was 3 mm shorter for all 17 tumors missed with mpMRI than for those detected with mpMRI (5 vs 8 mm; P < .0001).
mpMRI detected all tumors identified on TPM biopsy that had an overall Gleason score greater than 3+4 (Gleason grades 3 to 5) or a maximum Gleason score greater than 4+3 (Gleason grades 4 and 5).
“This finding is important, given that in PROMIS, no men with an overall Gleason score of 4+3 had cancer missed by MRI, indicating that actually MRI may be able to identify all truly significant cancers,” said Norris.
Adding PSA Density Threshold
To further assess cancers missed on mpMRI, the researchers looked at prostate-specific antigen (PSA) density, calculated as total PSA level (ng/mL) divided by prostate volume (mL).
“We found that if we applied a threshold PSA density to men with normal-looking MRI scans, we could reduce the proportion of missed significant cancer to just 5%. This is exciting; it means we can make MRI an even more effective test for prostate cancer in a very simple way,” Norris reported.
“These data show that no highly aggressive prostate cancers were missed by MRI, either at the level of the whole prostate or at the individual needle level,” said Norris. This should lead to positive outcomes in the long term.
And since the PROMIS data were gathered, MRI technology has improved, he said. The “MRI scanners in PROMIS were 1.5 Tesla,” whereas today’s machines are 3.0 T, which could increase the detection of significant prostate cancer.
In fact, “our analysis here potentially overestimates the amount of undetected disease,” he noted.
Prostate cancer that is not clinically significant is often monitored with active surveillance, so “invisible” cancers missed on mpMRI could actually be looked at in a positive light, he explained.
Variation in technique, interpretation
But the quality of care when it comes to the diagnosis of prostate cancer is not equal everywhere, said Gerald Andriole, MD, from the Washington School of Medicine in St. Louis, Missouri.
“When you get an MRI in a center that doesn’t do a lot of prostate cancer testing, you may not have the best software and you may have a radiologist who is not that experienced,” he told Medscape Medical News. Specialized cancer centers of excellence tend to do a great job finding prostate cancer, “but other centers have high significant-miss rates or high overcall rates.”
“The elephant in the room remains the considerable variation in technique and interobserver interpretation of prostate mpMRI,” write Steven Monda, MD, and Marc Dall’Era, MD, both from UC Davis Health in Sacramento, California, in an editorial that accompanies the new PROMIS analysis.
“These problems must be addressed and remedied in each institution before relying on results from PROMIS to drive changes in clinical practice,” they add.
Norris, Andriole, Monda, and Dall’Era have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Analysis of early onset cancers suggests need for genetic testing
according to a presentation at the AACR virtual meeting II.
Investigators analyzed blood samples from 1,201 patients who were aged 18-39 years when diagnosed with a solid tumor malignancy.
In this group, there were 877 patients with early onset cancers, defined as cancers for which 39 years of age is greater than 1 standard deviation below the mean age of diagnosis for the cancer type.
The remaining 324 patients had young adult cancers, defined as cancers for which 39 years of age is less than 1 standard deviation below the mean age of diagnosis.
The most common early onset cancers were breast, colorectal, kidney, pancreas, and ovarian cancer.
The most common young adult cancers were sarcoma, brain cancer, and testicular cancer, as expected, said investigator Zsofia K. Stadler, MD, of Memorial Sloan Kettering Cancer Center in New York.
Dr. Stadler and colleagues performed next-generation sequencing of the patient samples using a panel of up to 88 genes previously implicated in cancer predisposition. This revealed a significantly higher prevalence of germline mutations in patients with early onset cancers than in those with young adult cancers – 21% and 13%, respectively (P = .002).
In patients with only high- and moderate-risk cancer susceptibility genes, the prevalence was 15% in the early onset group and 10% in the young adult group (P = .01). “Among the early onset cancer group, pancreas, breast, and kidney cancer patients harbored the highest rates of germline mutations,” Dr. Stadler said, noting that the spectrum of mutated genes differed in early onset and young adult cancer patients.
“In early onset patients, the most commonly mutated genes were BRCA1 and BRCA2 [4.9%], Lynch syndrome genes [2.2%], ATM [1.6%], and CHECK2 [1.7%],” Dr. Stadler said. “On the other hand, in young adults, TP53 mutations [2.2%], and SDHA and SDHB mutations dominated [1.9%], with the majority of mutations occurring in sarcoma patients.”
These findings suggest the prevalence of inherited cancer susceptibility syndromes in young adults with cancer is not uniform.
“We found a very high prevalence of germline mutations in young patients with cancer types that typically present at later ages,” Dr. Stadler said, referring to the early onset patients.
Conversely, the young adult cancer patients had a prevalence and spectrum of mutations more similar to what is seen in pediatric cancer populations, she noted.
The findings are surprising, according to AACR past president Elaine R. Mardis, PhD, of The Ohio State University in Columbus.
Dr. Mardis said the results show that, in young adults with early onset cancers, “the germline prevalence of these mutations is significantly higher than we had previously thought.”
“Although representing only about 4% of all cancers, young adults with cancer ... face unique challenges,” Dr. Stadler said. “Identifying whether a young patient’s cancer occurred in the setting of an inherited cancer predisposition syndrome is especially important in this patient population.”
Such knowledge “can significantly impact the risk of second primary cancers and the need for increased surveillance measures or even risk-reducing surgeries,” Dr. Stadler explained. She added that it can also have implications for identifying at-risk family members, such as younger siblings or children who should pursue genetic testing and appropriate prevention measures.
“Our results suggest that, among patients with early onset cancer, the increased prevalence of germline mutations supports a role for genetic testing, irrespective of tumor type,” Dr. Stadler said.
This study was partially funded by the Precision, Interception and Prevention Program, the Robert and Katie Niehaus Center for Inherited Cancer Genomics, the Marie-Josee and Henry R. Kravis Center for Molecular Oncology, and a National Cancer Institute Cancer Center Core Grant. Dr. Stadler reported that an immediate family member serves as a consultant in ophthalmology for Allergan, Adverum Biotechnologies, Alimera Sciences, BioMarin, Fortress Biotech, Genentech/Roche, Novartis, Optos, Regeneron, Regenxbio, and Spark Therapeutics. Dr. Mardis disclosed relationships with Qiagen NV, Pact Pharma LLC, Moderna Inc., and Interpreta LLC.
SOURCE: Stadler Z et al. AACR 2020, Abstract 1122.
according to a presentation at the AACR virtual meeting II.
Investigators analyzed blood samples from 1,201 patients who were aged 18-39 years when diagnosed with a solid tumor malignancy.
In this group, there were 877 patients with early onset cancers, defined as cancers for which 39 years of age is greater than 1 standard deviation below the mean age of diagnosis for the cancer type.
The remaining 324 patients had young adult cancers, defined as cancers for which 39 years of age is less than 1 standard deviation below the mean age of diagnosis.
The most common early onset cancers were breast, colorectal, kidney, pancreas, and ovarian cancer.
The most common young adult cancers were sarcoma, brain cancer, and testicular cancer, as expected, said investigator Zsofia K. Stadler, MD, of Memorial Sloan Kettering Cancer Center in New York.
Dr. Stadler and colleagues performed next-generation sequencing of the patient samples using a panel of up to 88 genes previously implicated in cancer predisposition. This revealed a significantly higher prevalence of germline mutations in patients with early onset cancers than in those with young adult cancers – 21% and 13%, respectively (P = .002).
In patients with only high- and moderate-risk cancer susceptibility genes, the prevalence was 15% in the early onset group and 10% in the young adult group (P = .01). “Among the early onset cancer group, pancreas, breast, and kidney cancer patients harbored the highest rates of germline mutations,” Dr. Stadler said, noting that the spectrum of mutated genes differed in early onset and young adult cancer patients.
“In early onset patients, the most commonly mutated genes were BRCA1 and BRCA2 [4.9%], Lynch syndrome genes [2.2%], ATM [1.6%], and CHECK2 [1.7%],” Dr. Stadler said. “On the other hand, in young adults, TP53 mutations [2.2%], and SDHA and SDHB mutations dominated [1.9%], with the majority of mutations occurring in sarcoma patients.”
These findings suggest the prevalence of inherited cancer susceptibility syndromes in young adults with cancer is not uniform.
“We found a very high prevalence of germline mutations in young patients with cancer types that typically present at later ages,” Dr. Stadler said, referring to the early onset patients.
Conversely, the young adult cancer patients had a prevalence and spectrum of mutations more similar to what is seen in pediatric cancer populations, she noted.
The findings are surprising, according to AACR past president Elaine R. Mardis, PhD, of The Ohio State University in Columbus.
Dr. Mardis said the results show that, in young adults with early onset cancers, “the germline prevalence of these mutations is significantly higher than we had previously thought.”
“Although representing only about 4% of all cancers, young adults with cancer ... face unique challenges,” Dr. Stadler said. “Identifying whether a young patient’s cancer occurred in the setting of an inherited cancer predisposition syndrome is especially important in this patient population.”
Such knowledge “can significantly impact the risk of second primary cancers and the need for increased surveillance measures or even risk-reducing surgeries,” Dr. Stadler explained. She added that it can also have implications for identifying at-risk family members, such as younger siblings or children who should pursue genetic testing and appropriate prevention measures.
“Our results suggest that, among patients with early onset cancer, the increased prevalence of germline mutations supports a role for genetic testing, irrespective of tumor type,” Dr. Stadler said.
This study was partially funded by the Precision, Interception and Prevention Program, the Robert and Katie Niehaus Center for Inherited Cancer Genomics, the Marie-Josee and Henry R. Kravis Center for Molecular Oncology, and a National Cancer Institute Cancer Center Core Grant. Dr. Stadler reported that an immediate family member serves as a consultant in ophthalmology for Allergan, Adverum Biotechnologies, Alimera Sciences, BioMarin, Fortress Biotech, Genentech/Roche, Novartis, Optos, Regeneron, Regenxbio, and Spark Therapeutics. Dr. Mardis disclosed relationships with Qiagen NV, Pact Pharma LLC, Moderna Inc., and Interpreta LLC.
SOURCE: Stadler Z et al. AACR 2020, Abstract 1122.
according to a presentation at the AACR virtual meeting II.
Investigators analyzed blood samples from 1,201 patients who were aged 18-39 years when diagnosed with a solid tumor malignancy.
In this group, there were 877 patients with early onset cancers, defined as cancers for which 39 years of age is greater than 1 standard deviation below the mean age of diagnosis for the cancer type.
The remaining 324 patients had young adult cancers, defined as cancers for which 39 years of age is less than 1 standard deviation below the mean age of diagnosis.
The most common early onset cancers were breast, colorectal, kidney, pancreas, and ovarian cancer.
The most common young adult cancers were sarcoma, brain cancer, and testicular cancer, as expected, said investigator Zsofia K. Stadler, MD, of Memorial Sloan Kettering Cancer Center in New York.
Dr. Stadler and colleagues performed next-generation sequencing of the patient samples using a panel of up to 88 genes previously implicated in cancer predisposition. This revealed a significantly higher prevalence of germline mutations in patients with early onset cancers than in those with young adult cancers – 21% and 13%, respectively (P = .002).
In patients with only high- and moderate-risk cancer susceptibility genes, the prevalence was 15% in the early onset group and 10% in the young adult group (P = .01). “Among the early onset cancer group, pancreas, breast, and kidney cancer patients harbored the highest rates of germline mutations,” Dr. Stadler said, noting that the spectrum of mutated genes differed in early onset and young adult cancer patients.
“In early onset patients, the most commonly mutated genes were BRCA1 and BRCA2 [4.9%], Lynch syndrome genes [2.2%], ATM [1.6%], and CHECK2 [1.7%],” Dr. Stadler said. “On the other hand, in young adults, TP53 mutations [2.2%], and SDHA and SDHB mutations dominated [1.9%], with the majority of mutations occurring in sarcoma patients.”
These findings suggest the prevalence of inherited cancer susceptibility syndromes in young adults with cancer is not uniform.
“We found a very high prevalence of germline mutations in young patients with cancer types that typically present at later ages,” Dr. Stadler said, referring to the early onset patients.
Conversely, the young adult cancer patients had a prevalence and spectrum of mutations more similar to what is seen in pediatric cancer populations, she noted.
The findings are surprising, according to AACR past president Elaine R. Mardis, PhD, of The Ohio State University in Columbus.
Dr. Mardis said the results show that, in young adults with early onset cancers, “the germline prevalence of these mutations is significantly higher than we had previously thought.”
“Although representing only about 4% of all cancers, young adults with cancer ... face unique challenges,” Dr. Stadler said. “Identifying whether a young patient’s cancer occurred in the setting of an inherited cancer predisposition syndrome is especially important in this patient population.”
Such knowledge “can significantly impact the risk of second primary cancers and the need for increased surveillance measures or even risk-reducing surgeries,” Dr. Stadler explained. She added that it can also have implications for identifying at-risk family members, such as younger siblings or children who should pursue genetic testing and appropriate prevention measures.
“Our results suggest that, among patients with early onset cancer, the increased prevalence of germline mutations supports a role for genetic testing, irrespective of tumor type,” Dr. Stadler said.
This study was partially funded by the Precision, Interception and Prevention Program, the Robert and Katie Niehaus Center for Inherited Cancer Genomics, the Marie-Josee and Henry R. Kravis Center for Molecular Oncology, and a National Cancer Institute Cancer Center Core Grant. Dr. Stadler reported that an immediate family member serves as a consultant in ophthalmology for Allergan, Adverum Biotechnologies, Alimera Sciences, BioMarin, Fortress Biotech, Genentech/Roche, Novartis, Optos, Regeneron, Regenxbio, and Spark Therapeutics. Dr. Mardis disclosed relationships with Qiagen NV, Pact Pharma LLC, Moderna Inc., and Interpreta LLC.
SOURCE: Stadler Z et al. AACR 2020, Abstract 1122.
FROM AACR 2020
AI markers can predict progression, survival in prostate cancer
An AI-based Gleason score – derived from 7,267 digitized biopsy slides, pathology reports, and clinical data from patient electronic medical records – was calculated for each of 599 prostate cancer patients.
The AI scores were compared with pathologists’ Gleason scores, which were obtained from pathology reports for each of the patients.
The two scores were “highly correlated,” according to investigators. The area under the curve (AUC) for the 7-year mortality rate was 0.667 for the AI-based scores and 0.659 for the pathologists’ scores.
The investigators also found that markers extracted using AI-based algorithms could predict disease progression in patients with low- and higher-grade disease.
Daphna Laifenfeld, PhD, chief scientific officer of Ibex Medical Analytics in Tel Aviv, reported these results in a poster at the AACR virtual meeting II. Ibex Medical Analytics is the company that developed the AI-based algorithms and Gleason score (the Ibex score).
In addition to comparing the Ibex Gleason scores with pathologists’ scores, Dr. Laifenfeld and colleagues sought to “develop AI markers – computational features extracted from slides using AI-based algorithms – that can predict disease progression in low-, and separately, higher-grade patients.”
Information extracted using the algorithms included Gleason scores; perineural invasion; and other characteristics such as inflammation, high-grade prostatic intraepithelial neoplasia, and atrophy.
“We used data ... to address each aim, analyzing hundreds of patients in each comparison, and employed logistic regression to develop the predictive models,” Dr. Laifenfeld said.
Of the 357 patients evaluated, 180 had low-grade disease, defined by a prebiopsy prostate-specific antigen (PSA) level less than 10 ng/mL (Gleason group 1), and 177 patients had higher-grade disease (Gleason group 2 or higher).
Gleason group 1 patients were considered to have progressed if they developed higher-grade cancer, underwent prostatectomy, or if their cancer had metastasized. Gleason group 2 and above patients were considered to have progressed if their cancer metastasized or if they had a postprostatectomy PSA level greater than 4 ng/ml.
In Gleason group 1 patients, combining multiple features from the pathology report with prebiopsy PSA levels was shown to predict disease progression better than prebiopsy PSA levels alone (AUC, 0.687).
“Importantly, AI markers that combine features automatically extracted by Ibex with prebiopsy PSA levels are even better associated with progression (AUC, 0.748),” Dr. Laifenfeld said.
Similarly, in the Gleason group 2 and above patients, the AI markers that combine Ibex-extracted features with prebiopsy PSA levels were also highly associated with progression (AUC, 0.862 vs. AUC, 0.77 for the non–Ibex-based approach) and can be used for patient stratification, Dr. Laifenfeld said.
“For each patient, we can predict whether or not their disease will progress,” she said. “[T]his type of stratification can then be used to support clinical disease management decisions, and [it can be used] in the course of drug development for patient stratification and trial enrichment strategies.”
Dr. Laifenfeld and some coinvestigators are employed by Ibex Medical Analytics.
SOURCE: Laifenfeld D et al. AACR 2020, Abstract 867.
An AI-based Gleason score – derived from 7,267 digitized biopsy slides, pathology reports, and clinical data from patient electronic medical records – was calculated for each of 599 prostate cancer patients.
The AI scores were compared with pathologists’ Gleason scores, which were obtained from pathology reports for each of the patients.
The two scores were “highly correlated,” according to investigators. The area under the curve (AUC) for the 7-year mortality rate was 0.667 for the AI-based scores and 0.659 for the pathologists’ scores.
The investigators also found that markers extracted using AI-based algorithms could predict disease progression in patients with low- and higher-grade disease.
Daphna Laifenfeld, PhD, chief scientific officer of Ibex Medical Analytics in Tel Aviv, reported these results in a poster at the AACR virtual meeting II. Ibex Medical Analytics is the company that developed the AI-based algorithms and Gleason score (the Ibex score).
In addition to comparing the Ibex Gleason scores with pathologists’ scores, Dr. Laifenfeld and colleagues sought to “develop AI markers – computational features extracted from slides using AI-based algorithms – that can predict disease progression in low-, and separately, higher-grade patients.”
Information extracted using the algorithms included Gleason scores; perineural invasion; and other characteristics such as inflammation, high-grade prostatic intraepithelial neoplasia, and atrophy.
“We used data ... to address each aim, analyzing hundreds of patients in each comparison, and employed logistic regression to develop the predictive models,” Dr. Laifenfeld said.
Of the 357 patients evaluated, 180 had low-grade disease, defined by a prebiopsy prostate-specific antigen (PSA) level less than 10 ng/mL (Gleason group 1), and 177 patients had higher-grade disease (Gleason group 2 or higher).
Gleason group 1 patients were considered to have progressed if they developed higher-grade cancer, underwent prostatectomy, or if their cancer had metastasized. Gleason group 2 and above patients were considered to have progressed if their cancer metastasized or if they had a postprostatectomy PSA level greater than 4 ng/ml.
In Gleason group 1 patients, combining multiple features from the pathology report with prebiopsy PSA levels was shown to predict disease progression better than prebiopsy PSA levels alone (AUC, 0.687).
“Importantly, AI markers that combine features automatically extracted by Ibex with prebiopsy PSA levels are even better associated with progression (AUC, 0.748),” Dr. Laifenfeld said.
Similarly, in the Gleason group 2 and above patients, the AI markers that combine Ibex-extracted features with prebiopsy PSA levels were also highly associated with progression (AUC, 0.862 vs. AUC, 0.77 for the non–Ibex-based approach) and can be used for patient stratification, Dr. Laifenfeld said.
“For each patient, we can predict whether or not their disease will progress,” she said. “[T]his type of stratification can then be used to support clinical disease management decisions, and [it can be used] in the course of drug development for patient stratification and trial enrichment strategies.”
Dr. Laifenfeld and some coinvestigators are employed by Ibex Medical Analytics.
SOURCE: Laifenfeld D et al. AACR 2020, Abstract 867.
An AI-based Gleason score – derived from 7,267 digitized biopsy slides, pathology reports, and clinical data from patient electronic medical records – was calculated for each of 599 prostate cancer patients.
The AI scores were compared with pathologists’ Gleason scores, which were obtained from pathology reports for each of the patients.
The two scores were “highly correlated,” according to investigators. The area under the curve (AUC) for the 7-year mortality rate was 0.667 for the AI-based scores and 0.659 for the pathologists’ scores.
The investigators also found that markers extracted using AI-based algorithms could predict disease progression in patients with low- and higher-grade disease.
Daphna Laifenfeld, PhD, chief scientific officer of Ibex Medical Analytics in Tel Aviv, reported these results in a poster at the AACR virtual meeting II. Ibex Medical Analytics is the company that developed the AI-based algorithms and Gleason score (the Ibex score).
In addition to comparing the Ibex Gleason scores with pathologists’ scores, Dr. Laifenfeld and colleagues sought to “develop AI markers – computational features extracted from slides using AI-based algorithms – that can predict disease progression in low-, and separately, higher-grade patients.”
Information extracted using the algorithms included Gleason scores; perineural invasion; and other characteristics such as inflammation, high-grade prostatic intraepithelial neoplasia, and atrophy.
“We used data ... to address each aim, analyzing hundreds of patients in each comparison, and employed logistic regression to develop the predictive models,” Dr. Laifenfeld said.
Of the 357 patients evaluated, 180 had low-grade disease, defined by a prebiopsy prostate-specific antigen (PSA) level less than 10 ng/mL (Gleason group 1), and 177 patients had higher-grade disease (Gleason group 2 or higher).
Gleason group 1 patients were considered to have progressed if they developed higher-grade cancer, underwent prostatectomy, or if their cancer had metastasized. Gleason group 2 and above patients were considered to have progressed if their cancer metastasized or if they had a postprostatectomy PSA level greater than 4 ng/ml.
In Gleason group 1 patients, combining multiple features from the pathology report with prebiopsy PSA levels was shown to predict disease progression better than prebiopsy PSA levels alone (AUC, 0.687).
“Importantly, AI markers that combine features automatically extracted by Ibex with prebiopsy PSA levels are even better associated with progression (AUC, 0.748),” Dr. Laifenfeld said.
Similarly, in the Gleason group 2 and above patients, the AI markers that combine Ibex-extracted features with prebiopsy PSA levels were also highly associated with progression (AUC, 0.862 vs. AUC, 0.77 for the non–Ibex-based approach) and can be used for patient stratification, Dr. Laifenfeld said.
“For each patient, we can predict whether or not their disease will progress,” she said. “[T]his type of stratification can then be used to support clinical disease management decisions, and [it can be used] in the course of drug development for patient stratification and trial enrichment strategies.”
Dr. Laifenfeld and some coinvestigators are employed by Ibex Medical Analytics.
SOURCE: Laifenfeld D et al. AACR 2020, Abstract 867.
FROM AACR 2020