User login
SUDs rates highest in head, neck, and gastric cancer survivors
.
The association between cancer and substance use is well known, but data on the prevalence of different substance use disorders (SUDs) in different types of cancer are limited, Katie F. Jones, PhD, of the VA Boston Healthcare System, and colleagues, wrote in their paper.
“Substance use and use disorders are on the rise in general and among older adults, who represent the majority of people diagnosed with cancer, and SUDs have significant potential to complicate cancer care and negatively impact cancer outcomes,” corresponding author Devon K. Check, PhD, of Duke University, Durham, N.C., said in an interview. “We thought it was important to understand whether SUDs are more common with certain types of cancer. We can use that information to guide resources toward populations where interventions to integrate SUD treatment and cancer treatment are most needed,” he said. “In addition, because different SUDs (opioid use disorder, alcohol use disorder) might complicate cancer treatment in different ways and necessitate different types of interventions, we thought it was important to understand the distribution of specific disorders,” he explained.
In the cross-sectional study published in JAMA Oncology, the researchers reviewed data from 6,101 adult cancer survivors who participated in the National Survey of Drug Use and Health (NSDUH) between 2015 and 2020.
The study population included survivors of solid tumor cancers. SUD was defined as meeting at least one of four criteria for substance abuse or at least 3 of 6 criteria for dependence based on the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) criteria.
Overall, 3.83% of the participants met criteria for SUD. Survivors of head and neck cancers and survivors of gastric and esophageal cancers had the highest rates of SUDs (approximately 9%), followed by cervical cancer and melanoma survivors (approximately 6%).
Alcohol use disorder was the most common SUD both overall (2.8%) and among survivors of head and neck cancers, cervical cancers, and melanoma.
Cannabis use disorder was the most prevalent SUD among esophageal and gastric cancer survivors (approximately 9%).
The prevalence of SUDs overall and within the past year (active) was approximately 4%, but the prevalence of active SUDs was significantly higher for those with head and neck cancers and cervical cancer (18.73% and 15.70%, respectively). However, the distribution of specific SUDs was different in the newly diagnosed patients. Sedative use disorder took the top spot as the most common SUD for head and neck cancer survivors (9.81%), while alcohol use disorder was the most common SUD among cervical cancer survivors (10.49%).
Limitations and Implications
The findings were limited by several factors, including the nature of the study population and the data source, said Dr. Check.
“The average prevalence of SUD (or the prevalence across cancer types) was lower than we might have expected,” but the results make sense given the mainly older and female study population, he said. SUDs are less common among older adults compared with younger adults and among women compared with men, and the study’s data source (NSDUH) has been shown in other research to underestimate the prevalence of opioid use disorder, he added.
“Otherwise, the study findings were generally consistent with what we would expect,” Dr. Check said in an interview. “For example, alcohol use disorder is the most common SUD in the general U.S. population, and that was true for our study population of cancer survivors as well. In addition, SUD prevalence was higher in cancers such as cervical cancer and head and neck cancers that are causally linked to alcohol and/or tobacco use,” he said.
Integrated care is needed
“Among people diagnosed with certain types of cancers, including cervical and head and neck cancers, the estimated prevalence of SUD is similar to those [with] medical comorbidities such as diabetes and cardiopulmonary conditions,” said Dr. Check. “Within the field, there is an increasing emphasis on ensuring that people diagnosed with cancer have access to integrated care for their comorbid medical conditions. Similar efforts for people who concurrently manage cancer and SUD are largely absent but critically needed; these efforts should prioritize cancer populations where SUD prevalence is high,” he said.
Looking ahead, “We need to understand more about the specific challenges that arise at the intersection of cancer and SUD so we can design interventions and programs to better support both patients who concurrently manage cancer and SUD and the clinicians who care for them,” Dr. Check added.
Recognize risk factors
“It is very important to study overall substance use disorders in patients with cancer, because understanding the risks of developing these issues after treatment helps us develop approaches to best support these patients following their cancer therapies,” Henry S. Park, MD, a radiation oncologist at Yale University, New Haven, Connecticut, said in an interview.
The current study findings “are generally consistent with my experience and intuition, but it is still helpful to see the actual data,” said Dr. Park, who was not involved in the study. “This may be partially because of the baseline elevated risk of preexisting SUDs for certain patients from the higher-prevalence disease sites. However, it may also be related to the intense side effects that survivors of some types of cancers, such as head and neck cancer, gastroesophageal cancer, and cervical cancer, may experience soon after treatment, and even chronically long after treatment,” he said.
Individualize risk assessment
“Ultimately, clinicians should be aware that not all patients with cancer are the same, and that the majority do not necessarily develop SUDs,” Dr. Park said in an interview. “We should be careful to treat symptoms appropriately, and not withhold therapies purely because of an elevated risk of developing SUDs. However, there are some patients who are at higher risk of SUDs who will need extra support and care from physicians, advanced practice providers, nutritionists, social workers, psychologists, dietitians, and survivorship clinics, both in the short-term and long-term,” he emphasized.
As for additional research, “more work needs to be done on which particular patients within each disease subset are most likely to develop SUDs,” said Dr. Park. “Most importantly, once we identify our high-risk group as reliably as possible, we will have to study interventions that rely on supporting and partnering with patients to decrease the risk of developing SUDs as much as possible, while adequately treating residual symptoms and quality-of-life effects following cancer treatment,” he said.
The study received no outside funding. Dr. Check disclosed grants from Duke University during the study period and grants from the National Institutes of Health and AstraZeneca unrelated to the current study. Dr. Park had no financial conflicts to disclose.
.
The association between cancer and substance use is well known, but data on the prevalence of different substance use disorders (SUDs) in different types of cancer are limited, Katie F. Jones, PhD, of the VA Boston Healthcare System, and colleagues, wrote in their paper.
“Substance use and use disorders are on the rise in general and among older adults, who represent the majority of people diagnosed with cancer, and SUDs have significant potential to complicate cancer care and negatively impact cancer outcomes,” corresponding author Devon K. Check, PhD, of Duke University, Durham, N.C., said in an interview. “We thought it was important to understand whether SUDs are more common with certain types of cancer. We can use that information to guide resources toward populations where interventions to integrate SUD treatment and cancer treatment are most needed,” he said. “In addition, because different SUDs (opioid use disorder, alcohol use disorder) might complicate cancer treatment in different ways and necessitate different types of interventions, we thought it was important to understand the distribution of specific disorders,” he explained.
In the cross-sectional study published in JAMA Oncology, the researchers reviewed data from 6,101 adult cancer survivors who participated in the National Survey of Drug Use and Health (NSDUH) between 2015 and 2020.
The study population included survivors of solid tumor cancers. SUD was defined as meeting at least one of four criteria for substance abuse or at least 3 of 6 criteria for dependence based on the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) criteria.
Overall, 3.83% of the participants met criteria for SUD. Survivors of head and neck cancers and survivors of gastric and esophageal cancers had the highest rates of SUDs (approximately 9%), followed by cervical cancer and melanoma survivors (approximately 6%).
Alcohol use disorder was the most common SUD both overall (2.8%) and among survivors of head and neck cancers, cervical cancers, and melanoma.
Cannabis use disorder was the most prevalent SUD among esophageal and gastric cancer survivors (approximately 9%).
The prevalence of SUDs overall and within the past year (active) was approximately 4%, but the prevalence of active SUDs was significantly higher for those with head and neck cancers and cervical cancer (18.73% and 15.70%, respectively). However, the distribution of specific SUDs was different in the newly diagnosed patients. Sedative use disorder took the top spot as the most common SUD for head and neck cancer survivors (9.81%), while alcohol use disorder was the most common SUD among cervical cancer survivors (10.49%).
Limitations and Implications
The findings were limited by several factors, including the nature of the study population and the data source, said Dr. Check.
“The average prevalence of SUD (or the prevalence across cancer types) was lower than we might have expected,” but the results make sense given the mainly older and female study population, he said. SUDs are less common among older adults compared with younger adults and among women compared with men, and the study’s data source (NSDUH) has been shown in other research to underestimate the prevalence of opioid use disorder, he added.
“Otherwise, the study findings were generally consistent with what we would expect,” Dr. Check said in an interview. “For example, alcohol use disorder is the most common SUD in the general U.S. population, and that was true for our study population of cancer survivors as well. In addition, SUD prevalence was higher in cancers such as cervical cancer and head and neck cancers that are causally linked to alcohol and/or tobacco use,” he said.
Integrated care is needed
“Among people diagnosed with certain types of cancers, including cervical and head and neck cancers, the estimated prevalence of SUD is similar to those [with] medical comorbidities such as diabetes and cardiopulmonary conditions,” said Dr. Check. “Within the field, there is an increasing emphasis on ensuring that people diagnosed with cancer have access to integrated care for their comorbid medical conditions. Similar efforts for people who concurrently manage cancer and SUD are largely absent but critically needed; these efforts should prioritize cancer populations where SUD prevalence is high,” he said.
Looking ahead, “We need to understand more about the specific challenges that arise at the intersection of cancer and SUD so we can design interventions and programs to better support both patients who concurrently manage cancer and SUD and the clinicians who care for them,” Dr. Check added.
Recognize risk factors
“It is very important to study overall substance use disorders in patients with cancer, because understanding the risks of developing these issues after treatment helps us develop approaches to best support these patients following their cancer therapies,” Henry S. Park, MD, a radiation oncologist at Yale University, New Haven, Connecticut, said in an interview.
The current study findings “are generally consistent with my experience and intuition, but it is still helpful to see the actual data,” said Dr. Park, who was not involved in the study. “This may be partially because of the baseline elevated risk of preexisting SUDs for certain patients from the higher-prevalence disease sites. However, it may also be related to the intense side effects that survivors of some types of cancers, such as head and neck cancer, gastroesophageal cancer, and cervical cancer, may experience soon after treatment, and even chronically long after treatment,” he said.
Individualize risk assessment
“Ultimately, clinicians should be aware that not all patients with cancer are the same, and that the majority do not necessarily develop SUDs,” Dr. Park said in an interview. “We should be careful to treat symptoms appropriately, and not withhold therapies purely because of an elevated risk of developing SUDs. However, there are some patients who are at higher risk of SUDs who will need extra support and care from physicians, advanced practice providers, nutritionists, social workers, psychologists, dietitians, and survivorship clinics, both in the short-term and long-term,” he emphasized.
As for additional research, “more work needs to be done on which particular patients within each disease subset are most likely to develop SUDs,” said Dr. Park. “Most importantly, once we identify our high-risk group as reliably as possible, we will have to study interventions that rely on supporting and partnering with patients to decrease the risk of developing SUDs as much as possible, while adequately treating residual symptoms and quality-of-life effects following cancer treatment,” he said.
The study received no outside funding. Dr. Check disclosed grants from Duke University during the study period and grants from the National Institutes of Health and AstraZeneca unrelated to the current study. Dr. Park had no financial conflicts to disclose.
.
The association between cancer and substance use is well known, but data on the prevalence of different substance use disorders (SUDs) in different types of cancer are limited, Katie F. Jones, PhD, of the VA Boston Healthcare System, and colleagues, wrote in their paper.
“Substance use and use disorders are on the rise in general and among older adults, who represent the majority of people diagnosed with cancer, and SUDs have significant potential to complicate cancer care and negatively impact cancer outcomes,” corresponding author Devon K. Check, PhD, of Duke University, Durham, N.C., said in an interview. “We thought it was important to understand whether SUDs are more common with certain types of cancer. We can use that information to guide resources toward populations where interventions to integrate SUD treatment and cancer treatment are most needed,” he said. “In addition, because different SUDs (opioid use disorder, alcohol use disorder) might complicate cancer treatment in different ways and necessitate different types of interventions, we thought it was important to understand the distribution of specific disorders,” he explained.
In the cross-sectional study published in JAMA Oncology, the researchers reviewed data from 6,101 adult cancer survivors who participated in the National Survey of Drug Use and Health (NSDUH) between 2015 and 2020.
The study population included survivors of solid tumor cancers. SUD was defined as meeting at least one of four criteria for substance abuse or at least 3 of 6 criteria for dependence based on the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) criteria.
Overall, 3.83% of the participants met criteria for SUD. Survivors of head and neck cancers and survivors of gastric and esophageal cancers had the highest rates of SUDs (approximately 9%), followed by cervical cancer and melanoma survivors (approximately 6%).
Alcohol use disorder was the most common SUD both overall (2.8%) and among survivors of head and neck cancers, cervical cancers, and melanoma.
Cannabis use disorder was the most prevalent SUD among esophageal and gastric cancer survivors (approximately 9%).
The prevalence of SUDs overall and within the past year (active) was approximately 4%, but the prevalence of active SUDs was significantly higher for those with head and neck cancers and cervical cancer (18.73% and 15.70%, respectively). However, the distribution of specific SUDs was different in the newly diagnosed patients. Sedative use disorder took the top spot as the most common SUD for head and neck cancer survivors (9.81%), while alcohol use disorder was the most common SUD among cervical cancer survivors (10.49%).
Limitations and Implications
The findings were limited by several factors, including the nature of the study population and the data source, said Dr. Check.
“The average prevalence of SUD (or the prevalence across cancer types) was lower than we might have expected,” but the results make sense given the mainly older and female study population, he said. SUDs are less common among older adults compared with younger adults and among women compared with men, and the study’s data source (NSDUH) has been shown in other research to underestimate the prevalence of opioid use disorder, he added.
“Otherwise, the study findings were generally consistent with what we would expect,” Dr. Check said in an interview. “For example, alcohol use disorder is the most common SUD in the general U.S. population, and that was true for our study population of cancer survivors as well. In addition, SUD prevalence was higher in cancers such as cervical cancer and head and neck cancers that are causally linked to alcohol and/or tobacco use,” he said.
Integrated care is needed
“Among people diagnosed with certain types of cancers, including cervical and head and neck cancers, the estimated prevalence of SUD is similar to those [with] medical comorbidities such as diabetes and cardiopulmonary conditions,” said Dr. Check. “Within the field, there is an increasing emphasis on ensuring that people diagnosed with cancer have access to integrated care for their comorbid medical conditions. Similar efforts for people who concurrently manage cancer and SUD are largely absent but critically needed; these efforts should prioritize cancer populations where SUD prevalence is high,” he said.
Looking ahead, “We need to understand more about the specific challenges that arise at the intersection of cancer and SUD so we can design interventions and programs to better support both patients who concurrently manage cancer and SUD and the clinicians who care for them,” Dr. Check added.
Recognize risk factors
“It is very important to study overall substance use disorders in patients with cancer, because understanding the risks of developing these issues after treatment helps us develop approaches to best support these patients following their cancer therapies,” Henry S. Park, MD, a radiation oncologist at Yale University, New Haven, Connecticut, said in an interview.
The current study findings “are generally consistent with my experience and intuition, but it is still helpful to see the actual data,” said Dr. Park, who was not involved in the study. “This may be partially because of the baseline elevated risk of preexisting SUDs for certain patients from the higher-prevalence disease sites. However, it may also be related to the intense side effects that survivors of some types of cancers, such as head and neck cancer, gastroesophageal cancer, and cervical cancer, may experience soon after treatment, and even chronically long after treatment,” he said.
Individualize risk assessment
“Ultimately, clinicians should be aware that not all patients with cancer are the same, and that the majority do not necessarily develop SUDs,” Dr. Park said in an interview. “We should be careful to treat symptoms appropriately, and not withhold therapies purely because of an elevated risk of developing SUDs. However, there are some patients who are at higher risk of SUDs who will need extra support and care from physicians, advanced practice providers, nutritionists, social workers, psychologists, dietitians, and survivorship clinics, both in the short-term and long-term,” he emphasized.
As for additional research, “more work needs to be done on which particular patients within each disease subset are most likely to develop SUDs,” said Dr. Park. “Most importantly, once we identify our high-risk group as reliably as possible, we will have to study interventions that rely on supporting and partnering with patients to decrease the risk of developing SUDs as much as possible, while adequately treating residual symptoms and quality-of-life effects following cancer treatment,” he said.
The study received no outside funding. Dr. Check disclosed grants from Duke University during the study period and grants from the National Institutes of Health and AstraZeneca unrelated to the current study. Dr. Park had no financial conflicts to disclose.
FROM JAMA ONCOLOGY
HPV Vax Tied to Lower Odds of Cervical Lesion Progression
TOPLINE:
Among women with cervical intraepithelial neoplasia grade 2 (CIN2), vaccination against human papillomavirus (HPV) before age 20 is associated with lower odds of progression.
METHODOLOGY:
- Researchers analyzed data from 7904 women in Denmark who were undergoing active surveillance for CIN2 between 2007 and 2020.
- CIN2 lesions on their own. Removing them can increase the risk for during subsequent pregnancies, the researchers noted.
- Nearly half of the women had received at least one dose of an HPV vaccine at least 1 year before the diagnosis of cervical dysplasia.
TAKEAWAY:
- During 28 months of follow-up, the risk for progression was 22.9% for women vaccinated before age 15, 31.5% for women vaccinated between ages 15 and 20, and 37.6% for women who were not vaccinated.
- Women vaccinated before age 15 had a 35% lower risk for progression than unvaccinated women, after adjusting for cytology, income, and education (adjusted relative risk, 0.65; 95% CI, 0.57-0.75).
- Cervical cancer developed in 0.37% of the unvaccinated women and 0.13% of the vaccinated women.
- All cases of cervical cancer in the vaccinated group occurred in women who received the vaccine after age 20.
IN PRACTICE:
“These findings suggest that HPV vaccination status may be used to identify women at higher risk for progression, thereby enabling risk stratification at the time of CIN2 diagnosis,” the researchers wrote.
SOURCE:
Louise Krog, BscMed, with Aarhus University, Aarhus, Denmark, was the corresponding author of the study. The research was published online in the American Journal of Obstetrics & Gynecology.
LIMITATIONS:
The study authors had limited information about potential confounders such as smoking, immunosuppressive conditions, and the age at which patients became sexually active.
DISCLOSURES:
The study was funded by the Danish Cancer Society, the Carpenter Axel Kastrup-Nielsen’s Memorial Fund, and the Dagmar Marshall’s Fund. Co-authors disclosed ties to AstraZeneca, Roche, and Hologic.
A version of this article appeared on Medscape.com.
TOPLINE:
Among women with cervical intraepithelial neoplasia grade 2 (CIN2), vaccination against human papillomavirus (HPV) before age 20 is associated with lower odds of progression.
METHODOLOGY:
- Researchers analyzed data from 7904 women in Denmark who were undergoing active surveillance for CIN2 between 2007 and 2020.
- CIN2 lesions on their own. Removing them can increase the risk for during subsequent pregnancies, the researchers noted.
- Nearly half of the women had received at least one dose of an HPV vaccine at least 1 year before the diagnosis of cervical dysplasia.
TAKEAWAY:
- During 28 months of follow-up, the risk for progression was 22.9% for women vaccinated before age 15, 31.5% for women vaccinated between ages 15 and 20, and 37.6% for women who were not vaccinated.
- Women vaccinated before age 15 had a 35% lower risk for progression than unvaccinated women, after adjusting for cytology, income, and education (adjusted relative risk, 0.65; 95% CI, 0.57-0.75).
- Cervical cancer developed in 0.37% of the unvaccinated women and 0.13% of the vaccinated women.
- All cases of cervical cancer in the vaccinated group occurred in women who received the vaccine after age 20.
IN PRACTICE:
“These findings suggest that HPV vaccination status may be used to identify women at higher risk for progression, thereby enabling risk stratification at the time of CIN2 diagnosis,” the researchers wrote.
SOURCE:
Louise Krog, BscMed, with Aarhus University, Aarhus, Denmark, was the corresponding author of the study. The research was published online in the American Journal of Obstetrics & Gynecology.
LIMITATIONS:
The study authors had limited information about potential confounders such as smoking, immunosuppressive conditions, and the age at which patients became sexually active.
DISCLOSURES:
The study was funded by the Danish Cancer Society, the Carpenter Axel Kastrup-Nielsen’s Memorial Fund, and the Dagmar Marshall’s Fund. Co-authors disclosed ties to AstraZeneca, Roche, and Hologic.
A version of this article appeared on Medscape.com.
TOPLINE:
Among women with cervical intraepithelial neoplasia grade 2 (CIN2), vaccination against human papillomavirus (HPV) before age 20 is associated with lower odds of progression.
METHODOLOGY:
- Researchers analyzed data from 7904 women in Denmark who were undergoing active surveillance for CIN2 between 2007 and 2020.
- CIN2 lesions on their own. Removing them can increase the risk for during subsequent pregnancies, the researchers noted.
- Nearly half of the women had received at least one dose of an HPV vaccine at least 1 year before the diagnosis of cervical dysplasia.
TAKEAWAY:
- During 28 months of follow-up, the risk for progression was 22.9% for women vaccinated before age 15, 31.5% for women vaccinated between ages 15 and 20, and 37.6% for women who were not vaccinated.
- Women vaccinated before age 15 had a 35% lower risk for progression than unvaccinated women, after adjusting for cytology, income, and education (adjusted relative risk, 0.65; 95% CI, 0.57-0.75).
- Cervical cancer developed in 0.37% of the unvaccinated women and 0.13% of the vaccinated women.
- All cases of cervical cancer in the vaccinated group occurred in women who received the vaccine after age 20.
IN PRACTICE:
“These findings suggest that HPV vaccination status may be used to identify women at higher risk for progression, thereby enabling risk stratification at the time of CIN2 diagnosis,” the researchers wrote.
SOURCE:
Louise Krog, BscMed, with Aarhus University, Aarhus, Denmark, was the corresponding author of the study. The research was published online in the American Journal of Obstetrics & Gynecology.
LIMITATIONS:
The study authors had limited information about potential confounders such as smoking, immunosuppressive conditions, and the age at which patients became sexually active.
DISCLOSURES:
The study was funded by the Danish Cancer Society, the Carpenter Axel Kastrup-Nielsen’s Memorial Fund, and the Dagmar Marshall’s Fund. Co-authors disclosed ties to AstraZeneca, Roche, and Hologic.
A version of this article appeared on Medscape.com.
Optimal Follow-up After Fertility-Sparing Cervical Cancer Surgery
TOPLINE:
METHODOLOGY:
- Among patients with early-stage cervical cancer, the optimal follow-up strategy to detect recurrence after fertility-sparing surgery remains unclear. The authors wanted to find out if follow-up could be tailored to the patient’s risk for recurrence instead of using the current inefficient one-size-fits-all approach.
- The retrospective cohort study, which used data from the Netherlands Cancer Registry and the Dutch Nationwide Pathology Databank, included 1462 patients aged 18-40 years with early-stage cervical cancer who received fertility-sparing surgery (large loop excision of the transformation zone, conization, or trachelectomy) between 2000 and 2020.
- The primary endpoint was the cumulative incidence of recurrent cervical intraepithelial neoplasia grade 2 or worse (CIN2+), including recurrent cervical cancer.
- The authors stratified the likelihood of recurrence by cytology and high-risk HPV results at the first follow-up visit within 12 months of fertility-sparing surgery; they also compared the cumulative incidence of recurrence — the number of new cases divided by all at-risk individuals over a specific interval — at four timepoints in 2 years (6, 12, 18, and 24 months).
TAKEAWAY:
- Overall, the 10-year recurrence-free survival for CIN2+ was 89.3%. Patients with high-grade cytology at the first follow-up had worse 10-year recurrence-free survival for CIN2+ (43.1%) than those who had normal (92.1%) and low-grade cytology (84.6%). Similarly for HPV status, patients positive for high-risk HPV at the first follow-up had worse 10-year recurrence-free survival rates for CIN2+ (73.6%) than those negative for high-risk HPV (91.1%).
- Patients negative for both high-risk HPV and high-grade cytology 6-24 months after fertility-sparing surgery had a cumulative incidence of recurrence of 0.0%-0.7% within 6 months of follow-up compared with 0.0%-33.3% among patients negative for high-risk HPV but who had high-grade cytology.
- By contrast, patients positive for high-risk HPV but not high-grade cytology had a cumulative incidence of recurrence of 0.0%-15.4% within 6 months of any follow-up visit compared with 50.0%-100.0% among those with both high-risk HPV and high-grade cytology.
- Patients who remained free of high-risk HPV and high-grade cytology at their 6-month and 12-month follow-ups had no disease recurrence over the next 6 months.
IN PRACTICE:
“Patients who are negative for high-risk HPV with normal or low-grade cytology at 6-24 months after fertility-sparing surgery could be offered a prolonged follow-up interval of 6 months,” the authors concluded, adding that this “group comprises 80% of all patients receiving fertility-sparing surgery.”
“Reducing the number of follow-up visits, and subsequently the number of follow-up tests, in patients with low risk for recurrence on the basis of co-testing has the potential to substantially reduce healthcare costs,” the authors explained.
SOURCE:
The study, led by Teska N. Schuurman, MD, of the Netherlands Cancer Institute, Amsterdam, was published in the December 2023 issue of The Lancet Oncology.
LIMITATIONS:
The retrospective design of the study meant that analysis was limited to available records, so data on patients’ symptoms, physical examinations, or colposcopic findings were not available. Follow-up biopsies, considered the gold standard for diagnosing recurrence, are not routine in the Netherlands, so recurrence could have been underreported.
DISCLOSURES:
The authors declared no competing interests.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Among patients with early-stage cervical cancer, the optimal follow-up strategy to detect recurrence after fertility-sparing surgery remains unclear. The authors wanted to find out if follow-up could be tailored to the patient’s risk for recurrence instead of using the current inefficient one-size-fits-all approach.
- The retrospective cohort study, which used data from the Netherlands Cancer Registry and the Dutch Nationwide Pathology Databank, included 1462 patients aged 18-40 years with early-stage cervical cancer who received fertility-sparing surgery (large loop excision of the transformation zone, conization, or trachelectomy) between 2000 and 2020.
- The primary endpoint was the cumulative incidence of recurrent cervical intraepithelial neoplasia grade 2 or worse (CIN2+), including recurrent cervical cancer.
- The authors stratified the likelihood of recurrence by cytology and high-risk HPV results at the first follow-up visit within 12 months of fertility-sparing surgery; they also compared the cumulative incidence of recurrence — the number of new cases divided by all at-risk individuals over a specific interval — at four timepoints in 2 years (6, 12, 18, and 24 months).
TAKEAWAY:
- Overall, the 10-year recurrence-free survival for CIN2+ was 89.3%. Patients with high-grade cytology at the first follow-up had worse 10-year recurrence-free survival for CIN2+ (43.1%) than those who had normal (92.1%) and low-grade cytology (84.6%). Similarly for HPV status, patients positive for high-risk HPV at the first follow-up had worse 10-year recurrence-free survival rates for CIN2+ (73.6%) than those negative for high-risk HPV (91.1%).
- Patients negative for both high-risk HPV and high-grade cytology 6-24 months after fertility-sparing surgery had a cumulative incidence of recurrence of 0.0%-0.7% within 6 months of follow-up compared with 0.0%-33.3% among patients negative for high-risk HPV but who had high-grade cytology.
- By contrast, patients positive for high-risk HPV but not high-grade cytology had a cumulative incidence of recurrence of 0.0%-15.4% within 6 months of any follow-up visit compared with 50.0%-100.0% among those with both high-risk HPV and high-grade cytology.
- Patients who remained free of high-risk HPV and high-grade cytology at their 6-month and 12-month follow-ups had no disease recurrence over the next 6 months.
IN PRACTICE:
“Patients who are negative for high-risk HPV with normal or low-grade cytology at 6-24 months after fertility-sparing surgery could be offered a prolonged follow-up interval of 6 months,” the authors concluded, adding that this “group comprises 80% of all patients receiving fertility-sparing surgery.”
“Reducing the number of follow-up visits, and subsequently the number of follow-up tests, in patients with low risk for recurrence on the basis of co-testing has the potential to substantially reduce healthcare costs,” the authors explained.
SOURCE:
The study, led by Teska N. Schuurman, MD, of the Netherlands Cancer Institute, Amsterdam, was published in the December 2023 issue of The Lancet Oncology.
LIMITATIONS:
The retrospective design of the study meant that analysis was limited to available records, so data on patients’ symptoms, physical examinations, or colposcopic findings were not available. Follow-up biopsies, considered the gold standard for diagnosing recurrence, are not routine in the Netherlands, so recurrence could have been underreported.
DISCLOSURES:
The authors declared no competing interests.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Among patients with early-stage cervical cancer, the optimal follow-up strategy to detect recurrence after fertility-sparing surgery remains unclear. The authors wanted to find out if follow-up could be tailored to the patient’s risk for recurrence instead of using the current inefficient one-size-fits-all approach.
- The retrospective cohort study, which used data from the Netherlands Cancer Registry and the Dutch Nationwide Pathology Databank, included 1462 patients aged 18-40 years with early-stage cervical cancer who received fertility-sparing surgery (large loop excision of the transformation zone, conization, or trachelectomy) between 2000 and 2020.
- The primary endpoint was the cumulative incidence of recurrent cervical intraepithelial neoplasia grade 2 or worse (CIN2+), including recurrent cervical cancer.
- The authors stratified the likelihood of recurrence by cytology and high-risk HPV results at the first follow-up visit within 12 months of fertility-sparing surgery; they also compared the cumulative incidence of recurrence — the number of new cases divided by all at-risk individuals over a specific interval — at four timepoints in 2 years (6, 12, 18, and 24 months).
TAKEAWAY:
- Overall, the 10-year recurrence-free survival for CIN2+ was 89.3%. Patients with high-grade cytology at the first follow-up had worse 10-year recurrence-free survival for CIN2+ (43.1%) than those who had normal (92.1%) and low-grade cytology (84.6%). Similarly for HPV status, patients positive for high-risk HPV at the first follow-up had worse 10-year recurrence-free survival rates for CIN2+ (73.6%) than those negative for high-risk HPV (91.1%).
- Patients negative for both high-risk HPV and high-grade cytology 6-24 months after fertility-sparing surgery had a cumulative incidence of recurrence of 0.0%-0.7% within 6 months of follow-up compared with 0.0%-33.3% among patients negative for high-risk HPV but who had high-grade cytology.
- By contrast, patients positive for high-risk HPV but not high-grade cytology had a cumulative incidence of recurrence of 0.0%-15.4% within 6 months of any follow-up visit compared with 50.0%-100.0% among those with both high-risk HPV and high-grade cytology.
- Patients who remained free of high-risk HPV and high-grade cytology at their 6-month and 12-month follow-ups had no disease recurrence over the next 6 months.
IN PRACTICE:
“Patients who are negative for high-risk HPV with normal or low-grade cytology at 6-24 months after fertility-sparing surgery could be offered a prolonged follow-up interval of 6 months,” the authors concluded, adding that this “group comprises 80% of all patients receiving fertility-sparing surgery.”
“Reducing the number of follow-up visits, and subsequently the number of follow-up tests, in patients with low risk for recurrence on the basis of co-testing has the potential to substantially reduce healthcare costs,” the authors explained.
SOURCE:
The study, led by Teska N. Schuurman, MD, of the Netherlands Cancer Institute, Amsterdam, was published in the December 2023 issue of The Lancet Oncology.
LIMITATIONS:
The retrospective design of the study meant that analysis was limited to available records, so data on patients’ symptoms, physical examinations, or colposcopic findings were not available. Follow-up biopsies, considered the gold standard for diagnosing recurrence, are not routine in the Netherlands, so recurrence could have been underreported.
DISCLOSURES:
The authors declared no competing interests.
A version of this article appeared on Medscape.com.
ASCO details how to manage ongoing cancer drug shortage
As of November 30, the US Food and Drug Administration lists 16 commonly used oncology drugs currently in shortage, including methotrexate, capecitabine, vinblastine, carboplatin, and cisplatin, along with another 13 discontinued agents.
The ASCO guidance, which is updated regularly on ASCO’s drug shortage website, covers dozens of clinical situations involving breast, gastrointestinal, genitourinary, gynecologic, thoracic, and head & neck cancers, as well as Hodgkin lymphoma.
The recommendations, published earlier in JCO Oncology Practice, represent the work of a Drug Shortages Advisory Group with over 40 oncologists, ethicists, and patient advocates brought together by ASCO in collaboration with the Society for Gynecologic Oncology.
In the guidance, the advisory group also provides some context about why these shortage issues have persisted, including a paucity of generic options, quality control issues, and reluctance among manufacturers to produce older drugs with slim profit margins.
And “while ASCO continues to work to address the root causes of the shortages, this guidance document aims to support clinicians, as they navigate the complexities of treatment planning amid the drug shortage, and patients with cancer who are already enduring physical and emotional hardships,” the advisory group writes.
The overall message in the guidance: conserve oncology drugs in limited supply to use when needed most.
The recommendations highlight alternative regimens, when available, and what to do in situations when there are no alternatives, advice that has become particularly relevant for the oncology workhorses cisplatin and carboplatin.
More generally, when ranges of acceptable doses and dose frequencies exist for drugs in short supply, clinicians should opt for the lowest dose at the longest interval. Dose rounding and multi-use vials should also be used to eliminate waste, and alternatives should be used whenever possible. If an alternative agent with similar efficacy and safety is available, the agent in limited supply should not be ordered.
In certain settings where no reasonable alternatives to platinum regimens exist, the advisory group recommends patients travel to where platinum agents are available. The group noted this strategy specifically for patients with non–small cell lung cancer or testicular germ cell cancers, but also acknowledged that this option “may cause additional financial toxicity, hardship, and distress.”
Other, more granular advice includes holding carboplatin in reserve for patients with early-stage triple-negative breast cancer on neoadjuvant therapy who don’t respond well to upfront doxorubicin, cyclophosphamide, and pembrolizumab.
In addition to providing strategies to manage the ongoing cancer drug shortages, ASCO advises counseling for patients and clinicians struggling with the “psychological or moral distress” from the ongoing shortages.
“Unfortunately, drug shortages place the patient and the provider in a challenging situation, possibly resulting in inferior outcomes, delayed or denied care, and increased adverse events,” the advisory group writes. “ASCO will continue to respond to the oncology drug shortage crisis through policy and advocacy efforts, provide ethical guidance for allocation and prioritization decisions, and maintain shortage-specific clinical guidance as long as necessary.”
A version of this article appeared on Medscape.com.
As of November 30, the US Food and Drug Administration lists 16 commonly used oncology drugs currently in shortage, including methotrexate, capecitabine, vinblastine, carboplatin, and cisplatin, along with another 13 discontinued agents.
The ASCO guidance, which is updated regularly on ASCO’s drug shortage website, covers dozens of clinical situations involving breast, gastrointestinal, genitourinary, gynecologic, thoracic, and head & neck cancers, as well as Hodgkin lymphoma.
The recommendations, published earlier in JCO Oncology Practice, represent the work of a Drug Shortages Advisory Group with over 40 oncologists, ethicists, and patient advocates brought together by ASCO in collaboration with the Society for Gynecologic Oncology.
In the guidance, the advisory group also provides some context about why these shortage issues have persisted, including a paucity of generic options, quality control issues, and reluctance among manufacturers to produce older drugs with slim profit margins.
And “while ASCO continues to work to address the root causes of the shortages, this guidance document aims to support clinicians, as they navigate the complexities of treatment planning amid the drug shortage, and patients with cancer who are already enduring physical and emotional hardships,” the advisory group writes.
The overall message in the guidance: conserve oncology drugs in limited supply to use when needed most.
The recommendations highlight alternative regimens, when available, and what to do in situations when there are no alternatives, advice that has become particularly relevant for the oncology workhorses cisplatin and carboplatin.
More generally, when ranges of acceptable doses and dose frequencies exist for drugs in short supply, clinicians should opt for the lowest dose at the longest interval. Dose rounding and multi-use vials should also be used to eliminate waste, and alternatives should be used whenever possible. If an alternative agent with similar efficacy and safety is available, the agent in limited supply should not be ordered.
In certain settings where no reasonable alternatives to platinum regimens exist, the advisory group recommends patients travel to where platinum agents are available. The group noted this strategy specifically for patients with non–small cell lung cancer or testicular germ cell cancers, but also acknowledged that this option “may cause additional financial toxicity, hardship, and distress.”
Other, more granular advice includes holding carboplatin in reserve for patients with early-stage triple-negative breast cancer on neoadjuvant therapy who don’t respond well to upfront doxorubicin, cyclophosphamide, and pembrolizumab.
In addition to providing strategies to manage the ongoing cancer drug shortages, ASCO advises counseling for patients and clinicians struggling with the “psychological or moral distress” from the ongoing shortages.
“Unfortunately, drug shortages place the patient and the provider in a challenging situation, possibly resulting in inferior outcomes, delayed or denied care, and increased adverse events,” the advisory group writes. “ASCO will continue to respond to the oncology drug shortage crisis through policy and advocacy efforts, provide ethical guidance for allocation and prioritization decisions, and maintain shortage-specific clinical guidance as long as necessary.”
A version of this article appeared on Medscape.com.
As of November 30, the US Food and Drug Administration lists 16 commonly used oncology drugs currently in shortage, including methotrexate, capecitabine, vinblastine, carboplatin, and cisplatin, along with another 13 discontinued agents.
The ASCO guidance, which is updated regularly on ASCO’s drug shortage website, covers dozens of clinical situations involving breast, gastrointestinal, genitourinary, gynecologic, thoracic, and head & neck cancers, as well as Hodgkin lymphoma.
The recommendations, published earlier in JCO Oncology Practice, represent the work of a Drug Shortages Advisory Group with over 40 oncologists, ethicists, and patient advocates brought together by ASCO in collaboration with the Society for Gynecologic Oncology.
In the guidance, the advisory group also provides some context about why these shortage issues have persisted, including a paucity of generic options, quality control issues, and reluctance among manufacturers to produce older drugs with slim profit margins.
And “while ASCO continues to work to address the root causes of the shortages, this guidance document aims to support clinicians, as they navigate the complexities of treatment planning amid the drug shortage, and patients with cancer who are already enduring physical and emotional hardships,” the advisory group writes.
The overall message in the guidance: conserve oncology drugs in limited supply to use when needed most.
The recommendations highlight alternative regimens, when available, and what to do in situations when there are no alternatives, advice that has become particularly relevant for the oncology workhorses cisplatin and carboplatin.
More generally, when ranges of acceptable doses and dose frequencies exist for drugs in short supply, clinicians should opt for the lowest dose at the longest interval. Dose rounding and multi-use vials should also be used to eliminate waste, and alternatives should be used whenever possible. If an alternative agent with similar efficacy and safety is available, the agent in limited supply should not be ordered.
In certain settings where no reasonable alternatives to platinum regimens exist, the advisory group recommends patients travel to where platinum agents are available. The group noted this strategy specifically for patients with non–small cell lung cancer or testicular germ cell cancers, but also acknowledged that this option “may cause additional financial toxicity, hardship, and distress.”
Other, more granular advice includes holding carboplatin in reserve for patients with early-stage triple-negative breast cancer on neoadjuvant therapy who don’t respond well to upfront doxorubicin, cyclophosphamide, and pembrolizumab.
In addition to providing strategies to manage the ongoing cancer drug shortages, ASCO advises counseling for patients and clinicians struggling with the “psychological or moral distress” from the ongoing shortages.
“Unfortunately, drug shortages place the patient and the provider in a challenging situation, possibly resulting in inferior outcomes, delayed or denied care, and increased adverse events,” the advisory group writes. “ASCO will continue to respond to the oncology drug shortage crisis through policy and advocacy efforts, provide ethical guidance for allocation and prioritization decisions, and maintain shortage-specific clinical guidance as long as necessary.”
A version of this article appeared on Medscape.com.
FROM JCO ONCOLOGY PRACTICE
2023 Update on cervical disease
Cervical cancer was the most common cancer killer of persons with a cervix in the early 1900s in the United States. Widespread adoption of the Pap test in the mid-20th century followed by large-scale outreach through programs such as the National Breast and Cervical Cancer Early Detection Program have dramatically reduced deaths from cervical cancer. The development of a highly effective vaccine that targets human papillomavirus (HPV), the virus implicated in all cervical cancers, has made prevention even more accessible and attainable. Primary prevention with HPV vaccination in conjunction with regular screening as recommended by current guidelines is the most effective way we can prevent cervical cancer.
Despite these advances, the incidence and death rates from cervical cancer have plateaued over the last decade.1 Additionally, many fear that due to the poor attendance at screening visits since the beginning of the COVID-19 pandemic, the incidence might further rise in the United States.2 Among those in the United States diagnosed with cervical cancer, more than 50% have not been screened in over 5 years or had their abnormal results not managed as recommended by current guidelines, suggesting that operational and access issues are contributors to incident cervical cancer. In addition, HPV vaccination rates have increased only slightly from year to year. According to the most recent data from the Centers for Disease Control and Prevention (CDC), coverage with 1 or more doses of HPV vaccine in 2021 increased only by 1.8% and has stagnated, with administration to about 75% of those for whom it is recommended.3 The plateauing will limit our ability to eradicate cervical cancer in the United States, permitting death from a largely preventable disease.
Establishing the framework for the eradication of cervical cancer
The World Health Organization (WHO) adopted a global strategy called the Cervical Cancer Elimination Initiative in August 2020. This initiative is a multipronged effort that focuses on vaccination (90% of girls fully vaccinated by age 15), screening (70% of women screened by age 35 with an effective test and again at age 45), and treatment (90% treatment of precancer and 90% management of women with invasive cancer).4
These are the numbers we need to achieve if all countries are to reach a cervical cancer incidence of less than 4 per 100,000 persons with a cervix. The WHO further suggests that each country should meet the “90-70-90” targets by 2030 if we are to achieve the low incidence by the turn of the century.4 To date, few regions of the world have achieved these goals, and sadly the United States is not among them.
In response to this call to action, many medical and policymaking organizations are taking inventory and implementing strategies to achieve the WHO 2030 targets for cervical cancer eradication. In the United States, the Society of Gynecologic Oncology (SGO; www.sgo.org), the American Society for Colposcopy and Cervical Pathology (ASCCP; www.ASCCP.org), the American College of Obstetricians and Gynecologists (ACOG; www.acog.org), the American Cancer Society (ACS; www.cancer.org), and many others have initiated programs in a collaborative esprit de corps with the aim of eradicating this deadly disease.
In this Update, we review several studies with evidence of screening and management strategies that show promise of accelerating the eradication of cervical cancer.
Continue to: Transitioning to primary HPV screening in the United States...
Transitioning to primary HPV screening in the United States
Downs LS Jr, Nayar R, Gerndt J, et al; American Cancer Society Primary HPV Screening Initiative Steering Committee. Implementation in action: collaborating on the transition to primary HPV screening for cervical cancer in the United States. CA Cancer J Clin. 2023;73:458-460.
The American Cancer Society released an updated cervical cancer screening guideline in July 2020 that recommended testing for HPV as the preferred strategy. Reasons behind the change, moving away from a Pap test as part of the initial screen, are:
- increased sensitivity of primary HPV testing when compared with conventional cervical cytology (Pap test)
- improved risk stratification to identify who is at risk for cervical cancer now and in the future
- improved efficiency in identifying those who need colposcopy, thus limiting unnecessary procedures without increasing the risk of false-negative tests, thereby missing cervical precancer or invasive cancer.
Some countries with organized screening programs have already made the switch. Self-sampling for HPV is currently being considered for an approved use in the United States, further improving access to screening for cervical cancer when the initial step can be completed by the patient at home or simplified in nontraditional health care settings.2
ACS initiative created to address barriers to primary HPV testing
Challenges to primary HPV testing remain, including laboratory implementation, payment, and operationalizing clinical workflow (for example, HPV testing with reflex cytology instead of cytology with reflex HPV testing).5 There are undoubtedly other unforeseen barriers in the current US health care environment.
In a recent commentary, Downs and colleagues described how the ACS has convened the Primary HPV Screening Initiative (PHSI), nested under the ACS National Roundtable on Cervical Cancer, which is charged with identifying critical barriers to, and opportunities for, transitioning to primary HPV screening.5 The deliverable will be a roadmap with tools and recommendations to support health systems, laboratories, providers, patients, and payers as they make this evolution.
Work groups will develop resources
Patients, particularly those who have had routine cervical cancer screening over their lifetime, also will be curious about the changes in recommendations. The Provider Needs Workgroup within the PHSI structure will develop tools and patient education materials regarding the data, workflow, benefits, and safety of this new paradigm for cervical cancer screening.
Laboratories that process and interpret tests likely will bear the heaviest load of changes. For example, not all commercially available HPV tests in the United States are approved by the US Food and Drug Administration (FDA) for primary HPV testing. Some sites will need to adapt their equipment to ensure adherence to FDA-approved tests. Laboratory workflows will need to be altered for aliquots to be tested for HPV first, and the remainder for cytology. Quality assurance and accreditation requirements for testing will need modifications, and further efforts will be needed to ensure sufficient numbers of trained cytopathologists, whose workforce is rapidly declining, for processing and reading cervical cytology.
In addition, payment for HPV testing alone, without the need for a Pap test, might not be supported by payers that support safety-net providers and sites, who arguably serve the most vulnerable patients and those most at risk for cervical cancer. Collaboration across medical professionals, societies, payers, and policymakers will provide a critical infrastructure to make the change in the most seamless fashion and limit the harm from missed opportunities for screening.
HPV testing as the primary screen for cervical cancer is now recommended in guidelines due to improved sensitivity and improved efficiency when compared with other methods of screening. Implementation of this new workflow for clinicians and labs will require collaboration across multiple stakeholders.
Continue to: The quest for a “molecular Pap”: Dual-stain testing as a predictor of high-grade CIN...
The quest for a “molecular Pap”: Dual-stain testing as a predictor of high-grade CIN
Magkana M, Mentzelopoulou P, Magkana E, et al. p16/Ki-67 Dual staining is a reliable biomarker for risk stratification for patients with borderline/mild cytology in cervical cancer screening. Anticancer Res. 2022;42:2599-2606.
Stanczuk G, Currie H, Forson W, et al. Clinical performance of triage strategies for Hr-HPV-positive women; a longitudinal evaluation of cytology, p16/K-67 dual stain cytology, and HPV16/18 genotyping. Cancer Epidemiol Biomarkers Prev. 2022;31:1492-1498.
One new technology that was recently FDA approved and recommended for management of abnormal cervical cancer screening testing is dual-stain (DS) testing. Dual-stain testing is a cytology-based test that evaluates the concurrent expression of p16, a tumor suppressor protein upregulated in HPV oncogenesis, and Ki-67, a cell proliferation marker.6,7 Two recent studies have showcased the outstanding clinical performance of DS testing and triage strategies that incorporate DS testing.
Higher specificity, fewer colposcopies needed with DS testing
Magkana and colleagues prospectively evaluated patients with atypical squamous cells of undetermined significance (ASCUS), low-grade squamous intraepithelial lesion (LSIL), or negative for intraepithelial lesion or malignancy (NILM) cytology referred for colposcopy, and they compared p16/Ki-67 DS testing with high-risk HPV (HR-HPV) testing for the detection of cervical intraepithelial neoplasia grade 2 or worse (CIN 2+); comparable sensitivities for CIN 2+ detection were seen (97.3% and 98.7%, respectively).8
Dual-stain testing exhibited higher specificity at 99.3% compared with HR-HPV testing at 52.2%. Incorporating DS testing into triage strategies also led to fewer colposcopies needed to detect CIN 2+ compared with current ASCCP guidelines that use traditional cervical cancer screening algorithms.
DS cytology strategy had the highest sensitivity for CIN 2+ detection
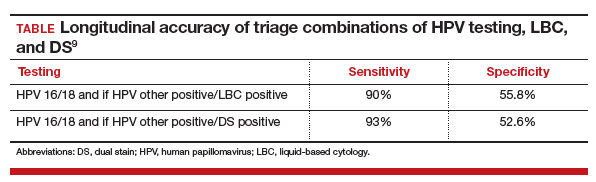
An additional study by Stanczuk and colleagues evaluated triage strategies in a cohort of HR-HPV positive patients who participated in the Scottish Papillomavirus Dumfries and Galloway study with HPV 16/18 genotyping (HPV 16/18), liquid-based cytology (LBC), and p16/Ki-67 DS cytology.9 Of these 3 triage strategies, DS cytology had the highest sensitivity for the detection of CIN 2+, at 77.7% (with a specificity of 74.2%), performance that is arguably better than cytology.
When evaluated in sequence as part of a triage strategy after HPV primary screening, HPV 16/18–positive patients reflexed to DS testing showed a similar sensitivity as those who would be triaged with LBC (TABLE).9
DS testing’s potential
These studies add to the growing body of literature that supports the use of DS testing in cervical cancer screening management guidelines and that are being incorporated into currently existing workflows. Furthermore, with advancements in digital imaging and machine learning, DS testing holds the potential for a high throughput, reproducible, and accurate risk stratification that can replace the current reliance on cytology, furthering the potential for a fully molecular Pap test.10,11
The introduction of p16/Ki-67 dual-stain testing has the potential to allow us to safely move away from a traditional Pap test for cervical cancer screening by allowing for more accurate and reliable identification of high-risk lesions with a molecular test that can be automated and have a high throughput.
Continue to: Cervical cancer screening in women older than age 65: Is there benefit?...
Cervical cancer screening in women older than age 65: Is there benefit?
Firtina Tuncer S, Tuncer HA. Cervical cancer screening in women aged older than 65 years. J Low Genit Tract Dis. 2023;27:207-211.
Booth BB, Tranberg M, Gustafson LW, et al. Risk of cervical intraepithelial neoplasia grade 2 or worse in women aged ≥ 69 referred to colposcopy due to an HPV-positive screening test. BMC Cancer. 2023;23:405.
Current guidelines in the United States recommend that cervical cancer screening for all persons with a cervix end at age 65. These age restrictions were a change in guidelines updated in 2012 and endorsed by the US Preventive Services Task Force.12,13 Evidence suggests that because of high likelihood of regression and slow progression of disease, risks of screening prior to age 21 outweigh its benefits. With primary HPV testing, the age at screening debut is 25 for the same reasons.14 In people with a history of CIN 2+, active surveillance should continue for at least 25 years with HPV-based screening regardless of age. In the absence of a history of CIN 2+, however, the data to support discontinuation of screening after age 65 are less clear.
HPV positivity found to be most substantial risk for CIN 2+
In a study published this year in the Journal of Lower Genital Tract Disease, Firtina Tuncer and colleagues described their experience extending “routine screening” in patients older than 65 years.15 Data including cervical cytology, HPV test results, biopsy findings, and endocervical curettage results were collected, and abnormal findings were managed according to the 2012 and 2019 ASCCP guidelines.
When compared with negative HPV testing and normal cytology, the authors found that HPV positivity and abnormal cytology increased the risk of CIN 2+(odds ratio [OR], 136.1 and 13.1, respectively). Patients whose screening prior to age 65 had been insufficient or demonstrated CIN 2+ in the preceding 10 years were similarly more likely to have findings of CIN 2+ (OR, 9.7 when compared with HPV-negative controls).
The authors concluded that, among persons with a cervix older than age 65, previous screening and abnormal cytology were important in risk stratifications for CIN 2+; however, HPV positivity conferred the most substantial risk.
Study finds cervical dysplasia is prevalent in older populations
It has been suggested that screening for cervical cancer should continue beyond age 65 as cytology-based screening may have decreased sensitivity in older patients, which may contribute to the higher rates of advanced-stage diagnoses and cancer-related death in this population.16,17
Authors of an observational study conducted in Denmark invited persons with a cervix aged 69 and older to have one additional HPV-based screening test, and they referred them for colposcopy if HPV positive or in the presence of ASCUS or greater cytology.18 Among the 191 patients with HPV-positive results, 20% were found to have a diagnosis of CIN 2+, and 24.4% had CIN 2+ detected at another point in the study period. Notably, most patients diagnosed with CIN 2+ had no abnormalities visualized on colposcopy, and the majority of biopsies taken (65.8%) did not contain the transitional zone.
Biopsies underestimated CIN 2+ in 17.9% of cases compared with loop electrosurgical excision procedure (LEEP). These findings suggest both that high-grade cervical dysplasia is prevalent in an older population and that older populations may be susceptible to false-negative results. They also further support the use of HPV-based screening.
There are risk factors overscreening and underscreening that impact decision making regarding restricting screening to persons with a cervix younger than age 65. As more data become available, and as the population ages, it will be essential to closely examine the incidence of and trends in cervical cancer to determine appropriate patterns of screening.
Harnessing the immune system to improve survival rates in recurrent cervical cancer
Colombo N, Dubot C, Lorusso D, et al; KEYNOTE-826 Investigators. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385:1856-1867.
Unfortunately, most clinical trials for recurrent or metastatic cervical cancer are negative trials or have results that show limited impact on disease outcomes. Currently, cervical cancer is treated with multiple agents, including platinum-based chemotherapy and bevacizumab, a medication that targets vascular growth. Despite these usually very effective drugs given in combination to cervical cancer patients, long-term survival remains low. Over the past few decades, many trials have been designed to help patients with this terrible disease, but few have shown significant promise.
Immune checkpoint inhibitors, such as pembrolizumab, have revolutionized care for many cancers. Checkpoint inhibitors block the proteins that cause a tumor to remain undetected by the immune system’s army of T cells. By blocking these proteins, the cancer cells can then be recognized by the immune system as foreign. Several studies have concluded that including immune checkpoint inhibitors in the comprehensive regimen for recurrent cervical cancer improves survival.
Addition of pembrolizumab increased survival
Investigators in the phase 3 double-blinded KEYNOTE-826 trial evaluated whether or not the addition of pembrolizumab to standard of care improved progression-free and overall survival in advanced, recurrent, or persistent cervical cancer.19 As part of the evaluation, the investigators measured the protein that turns off the immune system’s ability to recognize tumors, anti-programmed cell death protein-1 (PD-1).
Compared with placebo, the investigators found that, regardless of PD-1 status, the addition of pembrolizumab immunotherapy to the standard regimen increased progression-free survival and overall survival without any significantly increased adverse effects or safety concerns (FIGURE).19 At 1 year after treatment, more patients who received pembrolizumab were still alive regardless of PD-1 status, and their responses lasted longer. The most profound improvements were seen in patients whose tumors exhibited high expression of PD-L1, the target of pembrolizumab and many other immune checkpoint inhibitors.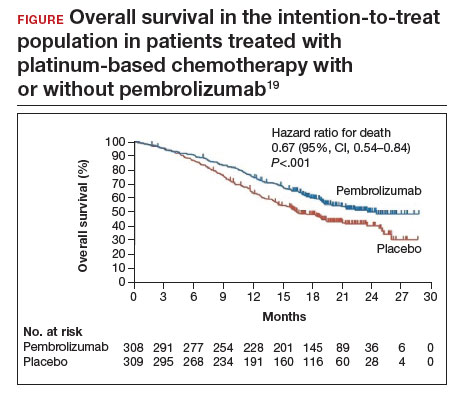
Despite these promising results, more studies are needed to find additional therapeutic targets and treatments. Using the immune system to fight cancer represents a promising step toward the ultimate goal of cervical cancer eradication. ●
Metastatic cervical cancer can be a devastating disease that cannot be treated surgically and therefore has limited treatment options that have curative intent. Immune checkpoint inhibition via pembrolizumab opens new avenues for treatment and is a huge step forward toward the goal of cervical cancer eradication.
- US Cancer Statistics Working Group. US Cancer Statistics Data Visualizations Tool, based on 2022 submission data (1999-2020). US Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute. June 2023. Accessed October 9, 2023. https://gis.cdc.gov/Cancer/USCS/#/Trends/
- Einstein MH, Zhou N, Gabor L, et al. Primary human papillomavirus testing and other new technologies for cervical cancer screening. Obstet Gynecol. September 14, 2023. doi:10.1097/AOG.0000000000005393
- Pingali C, Yankey D, Elam-Evans LD, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years—United States, 2020. MMWR Morbid Mortal Weekly Rep. 2021;70:1183-1190.
- Cervical cancer elimination initiative. World Health Organization. 2023. Accessed October 10, 2023. https ://www.who.int/initiatives/cervical-cancer-eliminationinitiative#cms
- Downs LS Jr, Nayar R, Gerndt J, et al; American Cancer Society Primary HPV Screening Initiative Steering Committee. Implementation in action: collaborating on the transition to primary HPV screening for cervical cancer in the United States. CA Cancer J Clin. 2023;73:458-460.
- Wentzensen N, Fetterman B, Castle PE, et al. p16/Ki-67 Dual stain cytology for detection of cervical precancer in HPV-positive women. J Natl Cancer Inst. 2015;107:djv257.
- Ikenberg H, Bergeron C, Schmidt D, et al; PALMS Study Group. Screening for cervical cancer precursors with p16 /Ki-67 dual-stained cytology: results of the PALMS study. J Natl Cancer Inst. 2013;105:1550-1557.
- Magkana M, Mentzelopoulou P, Magkana E, et al. p16/Ki-67 Dual staining is a reliable biomarker for risk stratification for patients with borderline/mild cytology in cervical cancer screening. Anticancer Res. 2022;42:2599-2606.
- Stanczuk G, Currie H, Forson W, et al. Clinical performance of triage strategies for Hr-HPV-positive women; a longitudinal evaluation of cytology, p16/K-67 dual stain cytology, and HPV16/18 genotyping. Cancer Epidemiol Biomarkers Prev. 2022;31:1492-1498.
- Wright TC Jr, Stoler MH, Behrens CM, et al. Interlaboratory variation in the performance of liquid-based cytology: insights from the ATHENA trial. Int J Cancer. 2014;134: 1835-1843.
- Wentzensen N, Lahrmann B, Clarke MA, et al. Accuracy and efficiency of deep-learning-based automation of dual stain cytology in cervical cancer screening. J Natl Cancer Inst. 2021;113:72-79.
- Massad LS, Einstein MH, Huh WK, et al; 2012 ASCCP Consensus Guidelines Conference. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol. 2013;121:829-846.
- Moyer VA; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156: 880-891, W312.
- Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346.
- Firtina Tuncer S, Tuncer HA. Cervical cancer screening in women aged older than 65 years. J Low Genit Tract Dis. 2023;27:207-211.
- Hammer A, Hee L, Blaakaer J, et al. Temporal patterns of cervical cancer screening among Danish women 55 years and older diagnosed with cervical cancer. J Low Genit Tract Dis. 2018;22:1-7.
- Hammer A, Soegaard V, Maimburg RD, et al. Cervical cancer screening history prior to a diagnosis of cervical cancer in Danish women aged 60 years and older—A national cohort study. Cancer Med. 2019;8:418-427.
- Booth BB, Tranberg M, Gustafson LW, et al. Risk of cervical intraepithelial neoplasia grade 2 or worse in women aged ≥ 69 referred to colposcopy due to an HPV-positive screening test. BMC Cancer. 2023;23:405.
- Colombo N, Dubot C, Lorusso D, et al; KEYNOTE-826 Investigators. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385:1856-1867.

Cervical cancer was the most common cancer killer of persons with a cervix in the early 1900s in the United States. Widespread adoption of the Pap test in the mid-20th century followed by large-scale outreach through programs such as the National Breast and Cervical Cancer Early Detection Program have dramatically reduced deaths from cervical cancer. The development of a highly effective vaccine that targets human papillomavirus (HPV), the virus implicated in all cervical cancers, has made prevention even more accessible and attainable. Primary prevention with HPV vaccination in conjunction with regular screening as recommended by current guidelines is the most effective way we can prevent cervical cancer.
Despite these advances, the incidence and death rates from cervical cancer have plateaued over the last decade.1 Additionally, many fear that due to the poor attendance at screening visits since the beginning of the COVID-19 pandemic, the incidence might further rise in the United States.2 Among those in the United States diagnosed with cervical cancer, more than 50% have not been screened in over 5 years or had their abnormal results not managed as recommended by current guidelines, suggesting that operational and access issues are contributors to incident cervical cancer. In addition, HPV vaccination rates have increased only slightly from year to year. According to the most recent data from the Centers for Disease Control and Prevention (CDC), coverage with 1 or more doses of HPV vaccine in 2021 increased only by 1.8% and has stagnated, with administration to about 75% of those for whom it is recommended.3 The plateauing will limit our ability to eradicate cervical cancer in the United States, permitting death from a largely preventable disease.
Establishing the framework for the eradication of cervical cancer
The World Health Organization (WHO) adopted a global strategy called the Cervical Cancer Elimination Initiative in August 2020. This initiative is a multipronged effort that focuses on vaccination (90% of girls fully vaccinated by age 15), screening (70% of women screened by age 35 with an effective test and again at age 45), and treatment (90% treatment of precancer and 90% management of women with invasive cancer).4
These are the numbers we need to achieve if all countries are to reach a cervical cancer incidence of less than 4 per 100,000 persons with a cervix. The WHO further suggests that each country should meet the “90-70-90” targets by 2030 if we are to achieve the low incidence by the turn of the century.4 To date, few regions of the world have achieved these goals, and sadly the United States is not among them.
In response to this call to action, many medical and policymaking organizations are taking inventory and implementing strategies to achieve the WHO 2030 targets for cervical cancer eradication. In the United States, the Society of Gynecologic Oncology (SGO; www.sgo.org), the American Society for Colposcopy and Cervical Pathology (ASCCP; www.ASCCP.org), the American College of Obstetricians and Gynecologists (ACOG; www.acog.org), the American Cancer Society (ACS; www.cancer.org), and many others have initiated programs in a collaborative esprit de corps with the aim of eradicating this deadly disease.
In this Update, we review several studies with evidence of screening and management strategies that show promise of accelerating the eradication of cervical cancer.
Continue to: Transitioning to primary HPV screening in the United States...
Transitioning to primary HPV screening in the United States
Downs LS Jr, Nayar R, Gerndt J, et al; American Cancer Society Primary HPV Screening Initiative Steering Committee. Implementation in action: collaborating on the transition to primary HPV screening for cervical cancer in the United States. CA Cancer J Clin. 2023;73:458-460.
The American Cancer Society released an updated cervical cancer screening guideline in July 2020 that recommended testing for HPV as the preferred strategy. Reasons behind the change, moving away from a Pap test as part of the initial screen, are:
- increased sensitivity of primary HPV testing when compared with conventional cervical cytology (Pap test)
- improved risk stratification to identify who is at risk for cervical cancer now and in the future
- improved efficiency in identifying those who need colposcopy, thus limiting unnecessary procedures without increasing the risk of false-negative tests, thereby missing cervical precancer or invasive cancer.
Some countries with organized screening programs have already made the switch. Self-sampling for HPV is currently being considered for an approved use in the United States, further improving access to screening for cervical cancer when the initial step can be completed by the patient at home or simplified in nontraditional health care settings.2
ACS initiative created to address barriers to primary HPV testing
Challenges to primary HPV testing remain, including laboratory implementation, payment, and operationalizing clinical workflow (for example, HPV testing with reflex cytology instead of cytology with reflex HPV testing).5 There are undoubtedly other unforeseen barriers in the current US health care environment.
In a recent commentary, Downs and colleagues described how the ACS has convened the Primary HPV Screening Initiative (PHSI), nested under the ACS National Roundtable on Cervical Cancer, which is charged with identifying critical barriers to, and opportunities for, transitioning to primary HPV screening.5 The deliverable will be a roadmap with tools and recommendations to support health systems, laboratories, providers, patients, and payers as they make this evolution.
Work groups will develop resources
Patients, particularly those who have had routine cervical cancer screening over their lifetime, also will be curious about the changes in recommendations. The Provider Needs Workgroup within the PHSI structure will develop tools and patient education materials regarding the data, workflow, benefits, and safety of this new paradigm for cervical cancer screening.
Laboratories that process and interpret tests likely will bear the heaviest load of changes. For example, not all commercially available HPV tests in the United States are approved by the US Food and Drug Administration (FDA) for primary HPV testing. Some sites will need to adapt their equipment to ensure adherence to FDA-approved tests. Laboratory workflows will need to be altered for aliquots to be tested for HPV first, and the remainder for cytology. Quality assurance and accreditation requirements for testing will need modifications, and further efforts will be needed to ensure sufficient numbers of trained cytopathologists, whose workforce is rapidly declining, for processing and reading cervical cytology.
In addition, payment for HPV testing alone, without the need for a Pap test, might not be supported by payers that support safety-net providers and sites, who arguably serve the most vulnerable patients and those most at risk for cervical cancer. Collaboration across medical professionals, societies, payers, and policymakers will provide a critical infrastructure to make the change in the most seamless fashion and limit the harm from missed opportunities for screening.
HPV testing as the primary screen for cervical cancer is now recommended in guidelines due to improved sensitivity and improved efficiency when compared with other methods of screening. Implementation of this new workflow for clinicians and labs will require collaboration across multiple stakeholders.
Continue to: The quest for a “molecular Pap”: Dual-stain testing as a predictor of high-grade CIN...
The quest for a “molecular Pap”: Dual-stain testing as a predictor of high-grade CIN
Magkana M, Mentzelopoulou P, Magkana E, et al. p16/Ki-67 Dual staining is a reliable biomarker for risk stratification for patients with borderline/mild cytology in cervical cancer screening. Anticancer Res. 2022;42:2599-2606.
Stanczuk G, Currie H, Forson W, et al. Clinical performance of triage strategies for Hr-HPV-positive women; a longitudinal evaluation of cytology, p16/K-67 dual stain cytology, and HPV16/18 genotyping. Cancer Epidemiol Biomarkers Prev. 2022;31:1492-1498.
One new technology that was recently FDA approved and recommended for management of abnormal cervical cancer screening testing is dual-stain (DS) testing. Dual-stain testing is a cytology-based test that evaluates the concurrent expression of p16, a tumor suppressor protein upregulated in HPV oncogenesis, and Ki-67, a cell proliferation marker.6,7 Two recent studies have showcased the outstanding clinical performance of DS testing and triage strategies that incorporate DS testing.
Higher specificity, fewer colposcopies needed with DS testing
Magkana and colleagues prospectively evaluated patients with atypical squamous cells of undetermined significance (ASCUS), low-grade squamous intraepithelial lesion (LSIL), or negative for intraepithelial lesion or malignancy (NILM) cytology referred for colposcopy, and they compared p16/Ki-67 DS testing with high-risk HPV (HR-HPV) testing for the detection of cervical intraepithelial neoplasia grade 2 or worse (CIN 2+); comparable sensitivities for CIN 2+ detection were seen (97.3% and 98.7%, respectively).8
Dual-stain testing exhibited higher specificity at 99.3% compared with HR-HPV testing at 52.2%. Incorporating DS testing into triage strategies also led to fewer colposcopies needed to detect CIN 2+ compared with current ASCCP guidelines that use traditional cervical cancer screening algorithms.
DS cytology strategy had the highest sensitivity for CIN 2+ detection
An additional study by Stanczuk and colleagues evaluated triage strategies in a cohort of HR-HPV positive patients who participated in the Scottish Papillomavirus Dumfries and Galloway study with HPV 16/18 genotyping (HPV 16/18), liquid-based cytology (LBC), and p16/Ki-67 DS cytology.9 Of these 3 triage strategies, DS cytology had the highest sensitivity for the detection of CIN 2+, at 77.7% (with a specificity of 74.2%), performance that is arguably better than cytology.
When evaluated in sequence as part of a triage strategy after HPV primary screening, HPV 16/18–positive patients reflexed to DS testing showed a similar sensitivity as those who would be triaged with LBC (TABLE).9

DS testing’s potential
These studies add to the growing body of literature that supports the use of DS testing in cervical cancer screening management guidelines and that are being incorporated into currently existing workflows. Furthermore, with advancements in digital imaging and machine learning, DS testing holds the potential for a high throughput, reproducible, and accurate risk stratification that can replace the current reliance on cytology, furthering the potential for a fully molecular Pap test.10,11
The introduction of p16/Ki-67 dual-stain testing has the potential to allow us to safely move away from a traditional Pap test for cervical cancer screening by allowing for more accurate and reliable identification of high-risk lesions with a molecular test that can be automated and have a high throughput.
Continue to: Cervical cancer screening in women older than age 65: Is there benefit?...
Cervical cancer screening in women older than age 65: Is there benefit?
Firtina Tuncer S, Tuncer HA. Cervical cancer screening in women aged older than 65 years. J Low Genit Tract Dis. 2023;27:207-211.
Booth BB, Tranberg M, Gustafson LW, et al. Risk of cervical intraepithelial neoplasia grade 2 or worse in women aged ≥ 69 referred to colposcopy due to an HPV-positive screening test. BMC Cancer. 2023;23:405.
Current guidelines in the United States recommend that cervical cancer screening for all persons with a cervix end at age 65. These age restrictions were a change in guidelines updated in 2012 and endorsed by the US Preventive Services Task Force.12,13 Evidence suggests that because of high likelihood of regression and slow progression of disease, risks of screening prior to age 21 outweigh its benefits. With primary HPV testing, the age at screening debut is 25 for the same reasons.14 In people with a history of CIN 2+, active surveillance should continue for at least 25 years with HPV-based screening regardless of age. In the absence of a history of CIN 2+, however, the data to support discontinuation of screening after age 65 are less clear.
HPV positivity found to be most substantial risk for CIN 2+
In a study published this year in the Journal of Lower Genital Tract Disease, Firtina Tuncer and colleagues described their experience extending “routine screening” in patients older than 65 years.15 Data including cervical cytology, HPV test results, biopsy findings, and endocervical curettage results were collected, and abnormal findings were managed according to the 2012 and 2019 ASCCP guidelines.
When compared with negative HPV testing and normal cytology, the authors found that HPV positivity and abnormal cytology increased the risk of CIN 2+(odds ratio [OR], 136.1 and 13.1, respectively). Patients whose screening prior to age 65 had been insufficient or demonstrated CIN 2+ in the preceding 10 years were similarly more likely to have findings of CIN 2+ (OR, 9.7 when compared with HPV-negative controls).
The authors concluded that, among persons with a cervix older than age 65, previous screening and abnormal cytology were important in risk stratifications for CIN 2+; however, HPV positivity conferred the most substantial risk.
Study finds cervical dysplasia is prevalent in older populations
It has been suggested that screening for cervical cancer should continue beyond age 65 as cytology-based screening may have decreased sensitivity in older patients, which may contribute to the higher rates of advanced-stage diagnoses and cancer-related death in this population.16,17
Authors of an observational study conducted in Denmark invited persons with a cervix aged 69 and older to have one additional HPV-based screening test, and they referred them for colposcopy if HPV positive or in the presence of ASCUS or greater cytology.18 Among the 191 patients with HPV-positive results, 20% were found to have a diagnosis of CIN 2+, and 24.4% had CIN 2+ detected at another point in the study period. Notably, most patients diagnosed with CIN 2+ had no abnormalities visualized on colposcopy, and the majority of biopsies taken (65.8%) did not contain the transitional zone.
Biopsies underestimated CIN 2+ in 17.9% of cases compared with loop electrosurgical excision procedure (LEEP). These findings suggest both that high-grade cervical dysplasia is prevalent in an older population and that older populations may be susceptible to false-negative results. They also further support the use of HPV-based screening.
There are risk factors overscreening and underscreening that impact decision making regarding restricting screening to persons with a cervix younger than age 65. As more data become available, and as the population ages, it will be essential to closely examine the incidence of and trends in cervical cancer to determine appropriate patterns of screening.
Harnessing the immune system to improve survival rates in recurrent cervical cancer
Colombo N, Dubot C, Lorusso D, et al; KEYNOTE-826 Investigators. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385:1856-1867.
Unfortunately, most clinical trials for recurrent or metastatic cervical cancer are negative trials or have results that show limited impact on disease outcomes. Currently, cervical cancer is treated with multiple agents, including platinum-based chemotherapy and bevacizumab, a medication that targets vascular growth. Despite these usually very effective drugs given in combination to cervical cancer patients, long-term survival remains low. Over the past few decades, many trials have been designed to help patients with this terrible disease, but few have shown significant promise.
Immune checkpoint inhibitors, such as pembrolizumab, have revolutionized care for many cancers. Checkpoint inhibitors block the proteins that cause a tumor to remain undetected by the immune system’s army of T cells. By blocking these proteins, the cancer cells can then be recognized by the immune system as foreign. Several studies have concluded that including immune checkpoint inhibitors in the comprehensive regimen for recurrent cervical cancer improves survival.
Addition of pembrolizumab increased survival
Investigators in the phase 3 double-blinded KEYNOTE-826 trial evaluated whether or not the addition of pembrolizumab to standard of care improved progression-free and overall survival in advanced, recurrent, or persistent cervical cancer.19 As part of the evaluation, the investigators measured the protein that turns off the immune system’s ability to recognize tumors, anti-programmed cell death protein-1 (PD-1).
Compared with placebo, the investigators found that, regardless of PD-1 status, the addition of pembrolizumab immunotherapy to the standard regimen increased progression-free survival and overall survival without any significantly increased adverse effects or safety concerns (FIGURE).19 At 1 year after treatment, more patients who received pembrolizumab were still alive regardless of PD-1 status, and their responses lasted longer. The most profound improvements were seen in patients whose tumors exhibited high expression of PD-L1, the target of pembrolizumab and many other immune checkpoint inhibitors.
Despite these promising results, more studies are needed to find additional therapeutic targets and treatments. Using the immune system to fight cancer represents a promising step toward the ultimate goal of cervical cancer eradication. ●
Metastatic cervical cancer can be a devastating disease that cannot be treated surgically and therefore has limited treatment options that have curative intent. Immune checkpoint inhibition via pembrolizumab opens new avenues for treatment and is a huge step forward toward the goal of cervical cancer eradication.

Cervical cancer was the most common cancer killer of persons with a cervix in the early 1900s in the United States. Widespread adoption of the Pap test in the mid-20th century followed by large-scale outreach through programs such as the National Breast and Cervical Cancer Early Detection Program have dramatically reduced deaths from cervical cancer. The development of a highly effective vaccine that targets human papillomavirus (HPV), the virus implicated in all cervical cancers, has made prevention even more accessible and attainable. Primary prevention with HPV vaccination in conjunction with regular screening as recommended by current guidelines is the most effective way we can prevent cervical cancer.
Despite these advances, the incidence and death rates from cervical cancer have plateaued over the last decade.1 Additionally, many fear that due to the poor attendance at screening visits since the beginning of the COVID-19 pandemic, the incidence might further rise in the United States.2 Among those in the United States diagnosed with cervical cancer, more than 50% have not been screened in over 5 years or had their abnormal results not managed as recommended by current guidelines, suggesting that operational and access issues are contributors to incident cervical cancer. In addition, HPV vaccination rates have increased only slightly from year to year. According to the most recent data from the Centers for Disease Control and Prevention (CDC), coverage with 1 or more doses of HPV vaccine in 2021 increased only by 1.8% and has stagnated, with administration to about 75% of those for whom it is recommended.3 The plateauing will limit our ability to eradicate cervical cancer in the United States, permitting death from a largely preventable disease.
Establishing the framework for the eradication of cervical cancer
The World Health Organization (WHO) adopted a global strategy called the Cervical Cancer Elimination Initiative in August 2020. This initiative is a multipronged effort that focuses on vaccination (90% of girls fully vaccinated by age 15), screening (70% of women screened by age 35 with an effective test and again at age 45), and treatment (90% treatment of precancer and 90% management of women with invasive cancer).4
These are the numbers we need to achieve if all countries are to reach a cervical cancer incidence of less than 4 per 100,000 persons with a cervix. The WHO further suggests that each country should meet the “90-70-90” targets by 2030 if we are to achieve the low incidence by the turn of the century.4 To date, few regions of the world have achieved these goals, and sadly the United States is not among them.
In response to this call to action, many medical and policymaking organizations are taking inventory and implementing strategies to achieve the WHO 2030 targets for cervical cancer eradication. In the United States, the Society of Gynecologic Oncology (SGO; www.sgo.org), the American Society for Colposcopy and Cervical Pathology (ASCCP; www.ASCCP.org), the American College of Obstetricians and Gynecologists (ACOG; www.acog.org), the American Cancer Society (ACS; www.cancer.org), and many others have initiated programs in a collaborative esprit de corps with the aim of eradicating this deadly disease.
In this Update, we review several studies with evidence of screening and management strategies that show promise of accelerating the eradication of cervical cancer.
Continue to: Transitioning to primary HPV screening in the United States...
Transitioning to primary HPV screening in the United States
Downs LS Jr, Nayar R, Gerndt J, et al; American Cancer Society Primary HPV Screening Initiative Steering Committee. Implementation in action: collaborating on the transition to primary HPV screening for cervical cancer in the United States. CA Cancer J Clin. 2023;73:458-460.
The American Cancer Society released an updated cervical cancer screening guideline in July 2020 that recommended testing for HPV as the preferred strategy. Reasons behind the change, moving away from a Pap test as part of the initial screen, are:
- increased sensitivity of primary HPV testing when compared with conventional cervical cytology (Pap test)
- improved risk stratification to identify who is at risk for cervical cancer now and in the future
- improved efficiency in identifying those who need colposcopy, thus limiting unnecessary procedures without increasing the risk of false-negative tests, thereby missing cervical precancer or invasive cancer.
Some countries with organized screening programs have already made the switch. Self-sampling for HPV is currently being considered for an approved use in the United States, further improving access to screening for cervical cancer when the initial step can be completed by the patient at home or simplified in nontraditional health care settings.2
ACS initiative created to address barriers to primary HPV testing
Challenges to primary HPV testing remain, including laboratory implementation, payment, and operationalizing clinical workflow (for example, HPV testing with reflex cytology instead of cytology with reflex HPV testing).5 There are undoubtedly other unforeseen barriers in the current US health care environment.
In a recent commentary, Downs and colleagues described how the ACS has convened the Primary HPV Screening Initiative (PHSI), nested under the ACS National Roundtable on Cervical Cancer, which is charged with identifying critical barriers to, and opportunities for, transitioning to primary HPV screening.5 The deliverable will be a roadmap with tools and recommendations to support health systems, laboratories, providers, patients, and payers as they make this evolution.
Work groups will develop resources
Patients, particularly those who have had routine cervical cancer screening over their lifetime, also will be curious about the changes in recommendations. The Provider Needs Workgroup within the PHSI structure will develop tools and patient education materials regarding the data, workflow, benefits, and safety of this new paradigm for cervical cancer screening.
Laboratories that process and interpret tests likely will bear the heaviest load of changes. For example, not all commercially available HPV tests in the United States are approved by the US Food and Drug Administration (FDA) for primary HPV testing. Some sites will need to adapt their equipment to ensure adherence to FDA-approved tests. Laboratory workflows will need to be altered for aliquots to be tested for HPV first, and the remainder for cytology. Quality assurance and accreditation requirements for testing will need modifications, and further efforts will be needed to ensure sufficient numbers of trained cytopathologists, whose workforce is rapidly declining, for processing and reading cervical cytology.
In addition, payment for HPV testing alone, without the need for a Pap test, might not be supported by payers that support safety-net providers and sites, who arguably serve the most vulnerable patients and those most at risk for cervical cancer. Collaboration across medical professionals, societies, payers, and policymakers will provide a critical infrastructure to make the change in the most seamless fashion and limit the harm from missed opportunities for screening.
HPV testing as the primary screen for cervical cancer is now recommended in guidelines due to improved sensitivity and improved efficiency when compared with other methods of screening. Implementation of this new workflow for clinicians and labs will require collaboration across multiple stakeholders.
Continue to: The quest for a “molecular Pap”: Dual-stain testing as a predictor of high-grade CIN...
The quest for a “molecular Pap”: Dual-stain testing as a predictor of high-grade CIN
Magkana M, Mentzelopoulou P, Magkana E, et al. p16/Ki-67 Dual staining is a reliable biomarker for risk stratification for patients with borderline/mild cytology in cervical cancer screening. Anticancer Res. 2022;42:2599-2606.
Stanczuk G, Currie H, Forson W, et al. Clinical performance of triage strategies for Hr-HPV-positive women; a longitudinal evaluation of cytology, p16/K-67 dual stain cytology, and HPV16/18 genotyping. Cancer Epidemiol Biomarkers Prev. 2022;31:1492-1498.
One new technology that was recently FDA approved and recommended for management of abnormal cervical cancer screening testing is dual-stain (DS) testing. Dual-stain testing is a cytology-based test that evaluates the concurrent expression of p16, a tumor suppressor protein upregulated in HPV oncogenesis, and Ki-67, a cell proliferation marker.6,7 Two recent studies have showcased the outstanding clinical performance of DS testing and triage strategies that incorporate DS testing.
Higher specificity, fewer colposcopies needed with DS testing
Magkana and colleagues prospectively evaluated patients with atypical squamous cells of undetermined significance (ASCUS), low-grade squamous intraepithelial lesion (LSIL), or negative for intraepithelial lesion or malignancy (NILM) cytology referred for colposcopy, and they compared p16/Ki-67 DS testing with high-risk HPV (HR-HPV) testing for the detection of cervical intraepithelial neoplasia grade 2 or worse (CIN 2+); comparable sensitivities for CIN 2+ detection were seen (97.3% and 98.7%, respectively).8
Dual-stain testing exhibited higher specificity at 99.3% compared with HR-HPV testing at 52.2%. Incorporating DS testing into triage strategies also led to fewer colposcopies needed to detect CIN 2+ compared with current ASCCP guidelines that use traditional cervical cancer screening algorithms.
DS cytology strategy had the highest sensitivity for CIN 2+ detection
An additional study by Stanczuk and colleagues evaluated triage strategies in a cohort of HR-HPV positive patients who participated in the Scottish Papillomavirus Dumfries and Galloway study with HPV 16/18 genotyping (HPV 16/18), liquid-based cytology (LBC), and p16/Ki-67 DS cytology.9 Of these 3 triage strategies, DS cytology had the highest sensitivity for the detection of CIN 2+, at 77.7% (with a specificity of 74.2%), performance that is arguably better than cytology.
When evaluated in sequence as part of a triage strategy after HPV primary screening, HPV 16/18–positive patients reflexed to DS testing showed a similar sensitivity as those who would be triaged with LBC (TABLE).9

DS testing’s potential
These studies add to the growing body of literature that supports the use of DS testing in cervical cancer screening management guidelines and that are being incorporated into currently existing workflows. Furthermore, with advancements in digital imaging and machine learning, DS testing holds the potential for a high throughput, reproducible, and accurate risk stratification that can replace the current reliance on cytology, furthering the potential for a fully molecular Pap test.10,11
The introduction of p16/Ki-67 dual-stain testing has the potential to allow us to safely move away from a traditional Pap test for cervical cancer screening by allowing for more accurate and reliable identification of high-risk lesions with a molecular test that can be automated and have a high throughput.
Continue to: Cervical cancer screening in women older than age 65: Is there benefit?...
Cervical cancer screening in women older than age 65: Is there benefit?
Firtina Tuncer S, Tuncer HA. Cervical cancer screening in women aged older than 65 years. J Low Genit Tract Dis. 2023;27:207-211.
Booth BB, Tranberg M, Gustafson LW, et al. Risk of cervical intraepithelial neoplasia grade 2 or worse in women aged ≥ 69 referred to colposcopy due to an HPV-positive screening test. BMC Cancer. 2023;23:405.
Current guidelines in the United States recommend that cervical cancer screening for all persons with a cervix end at age 65. These age restrictions were a change in guidelines updated in 2012 and endorsed by the US Preventive Services Task Force.12,13 Evidence suggests that because of high likelihood of regression and slow progression of disease, risks of screening prior to age 21 outweigh its benefits. With primary HPV testing, the age at screening debut is 25 for the same reasons.14 In people with a history of CIN 2+, active surveillance should continue for at least 25 years with HPV-based screening regardless of age. In the absence of a history of CIN 2+, however, the data to support discontinuation of screening after age 65 are less clear.
HPV positivity found to be most substantial risk for CIN 2+
In a study published this year in the Journal of Lower Genital Tract Disease, Firtina Tuncer and colleagues described their experience extending “routine screening” in patients older than 65 years.15 Data including cervical cytology, HPV test results, biopsy findings, and endocervical curettage results were collected, and abnormal findings were managed according to the 2012 and 2019 ASCCP guidelines.
When compared with negative HPV testing and normal cytology, the authors found that HPV positivity and abnormal cytology increased the risk of CIN 2+(odds ratio [OR], 136.1 and 13.1, respectively). Patients whose screening prior to age 65 had been insufficient or demonstrated CIN 2+ in the preceding 10 years were similarly more likely to have findings of CIN 2+ (OR, 9.7 when compared with HPV-negative controls).
The authors concluded that, among persons with a cervix older than age 65, previous screening and abnormal cytology were important in risk stratifications for CIN 2+; however, HPV positivity conferred the most substantial risk.
Study finds cervical dysplasia is prevalent in older populations
It has been suggested that screening for cervical cancer should continue beyond age 65 as cytology-based screening may have decreased sensitivity in older patients, which may contribute to the higher rates of advanced-stage diagnoses and cancer-related death in this population.16,17
Authors of an observational study conducted in Denmark invited persons with a cervix aged 69 and older to have one additional HPV-based screening test, and they referred them for colposcopy if HPV positive or in the presence of ASCUS or greater cytology.18 Among the 191 patients with HPV-positive results, 20% were found to have a diagnosis of CIN 2+, and 24.4% had CIN 2+ detected at another point in the study period. Notably, most patients diagnosed with CIN 2+ had no abnormalities visualized on colposcopy, and the majority of biopsies taken (65.8%) did not contain the transitional zone.
Biopsies underestimated CIN 2+ in 17.9% of cases compared with loop electrosurgical excision procedure (LEEP). These findings suggest both that high-grade cervical dysplasia is prevalent in an older population and that older populations may be susceptible to false-negative results. They also further support the use of HPV-based screening.
There are risk factors overscreening and underscreening that impact decision making regarding restricting screening to persons with a cervix younger than age 65. As more data become available, and as the population ages, it will be essential to closely examine the incidence of and trends in cervical cancer to determine appropriate patterns of screening.
Harnessing the immune system to improve survival rates in recurrent cervical cancer
Colombo N, Dubot C, Lorusso D, et al; KEYNOTE-826 Investigators. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385:1856-1867.
Unfortunately, most clinical trials for recurrent or metastatic cervical cancer are negative trials or have results that show limited impact on disease outcomes. Currently, cervical cancer is treated with multiple agents, including platinum-based chemotherapy and bevacizumab, a medication that targets vascular growth. Despite these usually very effective drugs given in combination to cervical cancer patients, long-term survival remains low. Over the past few decades, many trials have been designed to help patients with this terrible disease, but few have shown significant promise.
Immune checkpoint inhibitors, such as pembrolizumab, have revolutionized care for many cancers. Checkpoint inhibitors block the proteins that cause a tumor to remain undetected by the immune system’s army of T cells. By blocking these proteins, the cancer cells can then be recognized by the immune system as foreign. Several studies have concluded that including immune checkpoint inhibitors in the comprehensive regimen for recurrent cervical cancer improves survival.
Addition of pembrolizumab increased survival
Investigators in the phase 3 double-blinded KEYNOTE-826 trial evaluated whether or not the addition of pembrolizumab to standard of care improved progression-free and overall survival in advanced, recurrent, or persistent cervical cancer.19 As part of the evaluation, the investigators measured the protein that turns off the immune system’s ability to recognize tumors, anti-programmed cell death protein-1 (PD-1).
Compared with placebo, the investigators found that, regardless of PD-1 status, the addition of pembrolizumab immunotherapy to the standard regimen increased progression-free survival and overall survival without any significantly increased adverse effects or safety concerns (FIGURE).19 At 1 year after treatment, more patients who received pembrolizumab were still alive regardless of PD-1 status, and their responses lasted longer. The most profound improvements were seen in patients whose tumors exhibited high expression of PD-L1, the target of pembrolizumab and many other immune checkpoint inhibitors.
Despite these promising results, more studies are needed to find additional therapeutic targets and treatments. Using the immune system to fight cancer represents a promising step toward the ultimate goal of cervical cancer eradication. ●
Metastatic cervical cancer can be a devastating disease that cannot be treated surgically and therefore has limited treatment options that have curative intent. Immune checkpoint inhibition via pembrolizumab opens new avenues for treatment and is a huge step forward toward the goal of cervical cancer eradication.
- US Cancer Statistics Working Group. US Cancer Statistics Data Visualizations Tool, based on 2022 submission data (1999-2020). US Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute. June 2023. Accessed October 9, 2023. https://gis.cdc.gov/Cancer/USCS/#/Trends/
- Einstein MH, Zhou N, Gabor L, et al. Primary human papillomavirus testing and other new technologies for cervical cancer screening. Obstet Gynecol. September 14, 2023. doi:10.1097/AOG.0000000000005393
- Pingali C, Yankey D, Elam-Evans LD, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years—United States, 2020. MMWR Morbid Mortal Weekly Rep. 2021;70:1183-1190.
- Cervical cancer elimination initiative. World Health Organization. 2023. Accessed October 10, 2023. https ://www.who.int/initiatives/cervical-cancer-eliminationinitiative#cms
- Downs LS Jr, Nayar R, Gerndt J, et al; American Cancer Society Primary HPV Screening Initiative Steering Committee. Implementation in action: collaborating on the transition to primary HPV screening for cervical cancer in the United States. CA Cancer J Clin. 2023;73:458-460.
- Wentzensen N, Fetterman B, Castle PE, et al. p16/Ki-67 Dual stain cytology for detection of cervical precancer in HPV-positive women. J Natl Cancer Inst. 2015;107:djv257.
- Ikenberg H, Bergeron C, Schmidt D, et al; PALMS Study Group. Screening for cervical cancer precursors with p16 /Ki-67 dual-stained cytology: results of the PALMS study. J Natl Cancer Inst. 2013;105:1550-1557.
- Magkana M, Mentzelopoulou P, Magkana E, et al. p16/Ki-67 Dual staining is a reliable biomarker for risk stratification for patients with borderline/mild cytology in cervical cancer screening. Anticancer Res. 2022;42:2599-2606.
- Stanczuk G, Currie H, Forson W, et al. Clinical performance of triage strategies for Hr-HPV-positive women; a longitudinal evaluation of cytology, p16/K-67 dual stain cytology, and HPV16/18 genotyping. Cancer Epidemiol Biomarkers Prev. 2022;31:1492-1498.
- Wright TC Jr, Stoler MH, Behrens CM, et al. Interlaboratory variation in the performance of liquid-based cytology: insights from the ATHENA trial. Int J Cancer. 2014;134: 1835-1843.
- Wentzensen N, Lahrmann B, Clarke MA, et al. Accuracy and efficiency of deep-learning-based automation of dual stain cytology in cervical cancer screening. J Natl Cancer Inst. 2021;113:72-79.
- Massad LS, Einstein MH, Huh WK, et al; 2012 ASCCP Consensus Guidelines Conference. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol. 2013;121:829-846.
- Moyer VA; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156: 880-891, W312.
- Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346.
- Firtina Tuncer S, Tuncer HA. Cervical cancer screening in women aged older than 65 years. J Low Genit Tract Dis. 2023;27:207-211.
- Hammer A, Hee L, Blaakaer J, et al. Temporal patterns of cervical cancer screening among Danish women 55 years and older diagnosed with cervical cancer. J Low Genit Tract Dis. 2018;22:1-7.
- Hammer A, Soegaard V, Maimburg RD, et al. Cervical cancer screening history prior to a diagnosis of cervical cancer in Danish women aged 60 years and older—A national cohort study. Cancer Med. 2019;8:418-427.
- Booth BB, Tranberg M, Gustafson LW, et al. Risk of cervical intraepithelial neoplasia grade 2 or worse in women aged ≥ 69 referred to colposcopy due to an HPV-positive screening test. BMC Cancer. 2023;23:405.
- Colombo N, Dubot C, Lorusso D, et al; KEYNOTE-826 Investigators. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385:1856-1867.
- US Cancer Statistics Working Group. US Cancer Statistics Data Visualizations Tool, based on 2022 submission data (1999-2020). US Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute. June 2023. Accessed October 9, 2023. https://gis.cdc.gov/Cancer/USCS/#/Trends/
- Einstein MH, Zhou N, Gabor L, et al. Primary human papillomavirus testing and other new technologies for cervical cancer screening. Obstet Gynecol. September 14, 2023. doi:10.1097/AOG.0000000000005393
- Pingali C, Yankey D, Elam-Evans LD, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years—United States, 2020. MMWR Morbid Mortal Weekly Rep. 2021;70:1183-1190.
- Cervical cancer elimination initiative. World Health Organization. 2023. Accessed October 10, 2023. https ://www.who.int/initiatives/cervical-cancer-eliminationinitiative#cms
- Downs LS Jr, Nayar R, Gerndt J, et al; American Cancer Society Primary HPV Screening Initiative Steering Committee. Implementation in action: collaborating on the transition to primary HPV screening for cervical cancer in the United States. CA Cancer J Clin. 2023;73:458-460.
- Wentzensen N, Fetterman B, Castle PE, et al. p16/Ki-67 Dual stain cytology for detection of cervical precancer in HPV-positive women. J Natl Cancer Inst. 2015;107:djv257.
- Ikenberg H, Bergeron C, Schmidt D, et al; PALMS Study Group. Screening for cervical cancer precursors with p16 /Ki-67 dual-stained cytology: results of the PALMS study. J Natl Cancer Inst. 2013;105:1550-1557.
- Magkana M, Mentzelopoulou P, Magkana E, et al. p16/Ki-67 Dual staining is a reliable biomarker for risk stratification for patients with borderline/mild cytology in cervical cancer screening. Anticancer Res. 2022;42:2599-2606.
- Stanczuk G, Currie H, Forson W, et al. Clinical performance of triage strategies for Hr-HPV-positive women; a longitudinal evaluation of cytology, p16/K-67 dual stain cytology, and HPV16/18 genotyping. Cancer Epidemiol Biomarkers Prev. 2022;31:1492-1498.
- Wright TC Jr, Stoler MH, Behrens CM, et al. Interlaboratory variation in the performance of liquid-based cytology: insights from the ATHENA trial. Int J Cancer. 2014;134: 1835-1843.
- Wentzensen N, Lahrmann B, Clarke MA, et al. Accuracy and efficiency of deep-learning-based automation of dual stain cytology in cervical cancer screening. J Natl Cancer Inst. 2021;113:72-79.
- Massad LS, Einstein MH, Huh WK, et al; 2012 ASCCP Consensus Guidelines Conference. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol. 2013;121:829-846.
- Moyer VA; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156: 880-891, W312.
- Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346.
- Firtina Tuncer S, Tuncer HA. Cervical cancer screening in women aged older than 65 years. J Low Genit Tract Dis. 2023;27:207-211.
- Hammer A, Hee L, Blaakaer J, et al. Temporal patterns of cervical cancer screening among Danish women 55 years and older diagnosed with cervical cancer. J Low Genit Tract Dis. 2018;22:1-7.
- Hammer A, Soegaard V, Maimburg RD, et al. Cervical cancer screening history prior to a diagnosis of cervical cancer in Danish women aged 60 years and older—A national cohort study. Cancer Med. 2019;8:418-427.
- Booth BB, Tranberg M, Gustafson LW, et al. Risk of cervical intraepithelial neoplasia grade 2 or worse in women aged ≥ 69 referred to colposcopy due to an HPV-positive screening test. BMC Cancer. 2023;23:405.
- Colombo N, Dubot C, Lorusso D, et al; KEYNOTE-826 Investigators. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385:1856-1867.
Later-line tisotumab vedotin shows survival benefit in metastatic cervical CA
which was presented at the European Society of Medical Oncology (ESMO) Congress 2023.
Median overall survival (OS) was 11.5 months among 253 women randomized to tisotumab vedotin (TV) monotherapy versus 9.5 months among 249 randomized to investigators’ choice of chemotherapy, a 30% reduction in the risk of death (P = .0038).
Median progression-free survival (PFS) was 4.2 months with TV versus 2.9 months with chemotherapy (P < .0001), but survival benefits were not statistically significant in a number of subgroups.
Nonetheless, “tisotumab vedotin should be considered as a potential new standard of care for patients who have progressed after first-line systemic therapy,” said lead investigator Ignace Vergote, MD, PhD, a gynecologic oncologist and researcher at the Catholic University of Leuven, Belgium, who presented the findings.
New and emerging options
The trial serves as the confirmation the Food and Drug Administration required when it gave TV accelerated approval in 2021 for recurrent or metastatic cervical cancer (r/m CC) that’s progressed during or after first-line treatment, an approval based on response rates in an earlier phase 2 trial, the InnovaTV 204 study.
TV is the only antibody-drug conjugate approved for the indication, but another agent is also under investigation, the anti-PD-1 cemiplimab. It’s not yet approved for r/m CC, but it is approved in the United States for locally advanced/metastatic basal cell carcinoma, cutaneous squamous cell carcinoma, and non–small cell lung cancer.
Cemiplimab outcomes were similar to TV’s in a phase 3 trial following progression on first-line treatment without anti-PD-1 therapy, with a median OS of 12 months with cemiplimab versus 8.5 months with investigators’ choice of chemotherapy.
Pembrolizumab is also approved as monotherapy for r/m CC for PD-L1 positive women after progression on or during first-line treatment based on response outcomes, not survival.
The question now is how to pick among the various options, said Krishnansu Tewari, MD, a gynecologic oncologist and researcher at the University of California, Irvine, who discussed InnovaTV 301 at the meeting.
In the second line for r/m CC, “we can hypothetically consider” TV monotherapy; pembrolizumab in PD-L1-positive women not previously exposed to a checkpoint inhibitor (CPI); cemiplimab in women not previously exposed to a CPI, and perhaps TV plus pembrolizumab, also in women new to CPIs.
It remains particularly unclear at the moment how to select between TV and cemiplimab monotherapy, if cemiplimab is approved for the indication.
One difference is that unlike in the cemiplimab trial, 28.1% of women treated with TV in the phase 3 trial had been on an anti-PD-(L)1 in the first line. However, although PFS benefits were statistically significant for TV after checkpoint inhibitor exposure, OS benefit was not.
Regarding cost, TV was administered at 2 mg/kg every 3 weeks in Innova; 40 mg costs around $7,000.
Cemiplimab was dosed at 350 mg every 3 weeks in its trial; a single dose costs over $10,000.
Subgroups fall short of statistical significance
In InnovaTV 301, 12-month OS was about 48.7% with TV versus 35% with chemotherapy; 6-month PFS was 30.4% with TV versus 18.9%.
The PFS benefit with TV did not reach statistical significance among the 35.2% of women who had not been treated with bevacizumab in the first-line, and there was no OS benefit or trend to benefit (HR 1.0) for them.
In addition to women previously treated with an anti-PD-1, OS benefits with TV were not statistically significant among the 54.2% of women with baseline performance scores of 0; the 36.8% with adeno or adenosquamous carcinoma, and the 62.8% who had been on one prior systemic regimen instead two.
Women in the trial were a median of 50 years old, and fewer than 7% were from the United States. Investigator choice of chemotherapy included topotecan, vinorelbine, gemcitabine, irinotecan, or pemetrexed.
The rate of grade 3 or higher adverse events was 29.2% with TV and 45.2% with chemotherapy.
The known side effects of TV were all higher than in the chemotherapy arm, including grade 3 or worse peripheral neuropathy (5.2%), ocular events (3.2%), and bleeding (0.8%).
The study was funded in part by Genmab and SeaGen, the companies co-developing TV. Dr. Vergote is an adviser to both companies and many others. Dr. Tewari is an adviser/consultant, researcher, and speaker for SeaGen and Genmab as well as for Merck, AstraZeneca, and other companies.
which was presented at the European Society of Medical Oncology (ESMO) Congress 2023.
Median overall survival (OS) was 11.5 months among 253 women randomized to tisotumab vedotin (TV) monotherapy versus 9.5 months among 249 randomized to investigators’ choice of chemotherapy, a 30% reduction in the risk of death (P = .0038).
Median progression-free survival (PFS) was 4.2 months with TV versus 2.9 months with chemotherapy (P < .0001), but survival benefits were not statistically significant in a number of subgroups.
Nonetheless, “tisotumab vedotin should be considered as a potential new standard of care for patients who have progressed after first-line systemic therapy,” said lead investigator Ignace Vergote, MD, PhD, a gynecologic oncologist and researcher at the Catholic University of Leuven, Belgium, who presented the findings.
New and emerging options
The trial serves as the confirmation the Food and Drug Administration required when it gave TV accelerated approval in 2021 for recurrent or metastatic cervical cancer (r/m CC) that’s progressed during or after first-line treatment, an approval based on response rates in an earlier phase 2 trial, the InnovaTV 204 study.
TV is the only antibody-drug conjugate approved for the indication, but another agent is also under investigation, the anti-PD-1 cemiplimab. It’s not yet approved for r/m CC, but it is approved in the United States for locally advanced/metastatic basal cell carcinoma, cutaneous squamous cell carcinoma, and non–small cell lung cancer.
Cemiplimab outcomes were similar to TV’s in a phase 3 trial following progression on first-line treatment without anti-PD-1 therapy, with a median OS of 12 months with cemiplimab versus 8.5 months with investigators’ choice of chemotherapy.
Pembrolizumab is also approved as monotherapy for r/m CC for PD-L1 positive women after progression on or during first-line treatment based on response outcomes, not survival.
The question now is how to pick among the various options, said Krishnansu Tewari, MD, a gynecologic oncologist and researcher at the University of California, Irvine, who discussed InnovaTV 301 at the meeting.
In the second line for r/m CC, “we can hypothetically consider” TV monotherapy; pembrolizumab in PD-L1-positive women not previously exposed to a checkpoint inhibitor (CPI); cemiplimab in women not previously exposed to a CPI, and perhaps TV plus pembrolizumab, also in women new to CPIs.
It remains particularly unclear at the moment how to select between TV and cemiplimab monotherapy, if cemiplimab is approved for the indication.
One difference is that unlike in the cemiplimab trial, 28.1% of women treated with TV in the phase 3 trial had been on an anti-PD-(L)1 in the first line. However, although PFS benefits were statistically significant for TV after checkpoint inhibitor exposure, OS benefit was not.
Regarding cost, TV was administered at 2 mg/kg every 3 weeks in Innova; 40 mg costs around $7,000.
Cemiplimab was dosed at 350 mg every 3 weeks in its trial; a single dose costs over $10,000.
Subgroups fall short of statistical significance
In InnovaTV 301, 12-month OS was about 48.7% with TV versus 35% with chemotherapy; 6-month PFS was 30.4% with TV versus 18.9%.
The PFS benefit with TV did not reach statistical significance among the 35.2% of women who had not been treated with bevacizumab in the first-line, and there was no OS benefit or trend to benefit (HR 1.0) for them.
In addition to women previously treated with an anti-PD-1, OS benefits with TV were not statistically significant among the 54.2% of women with baseline performance scores of 0; the 36.8% with adeno or adenosquamous carcinoma, and the 62.8% who had been on one prior systemic regimen instead two.
Women in the trial were a median of 50 years old, and fewer than 7% were from the United States. Investigator choice of chemotherapy included topotecan, vinorelbine, gemcitabine, irinotecan, or pemetrexed.
The rate of grade 3 or higher adverse events was 29.2% with TV and 45.2% with chemotherapy.
The known side effects of TV were all higher than in the chemotherapy arm, including grade 3 or worse peripheral neuropathy (5.2%), ocular events (3.2%), and bleeding (0.8%).
The study was funded in part by Genmab and SeaGen, the companies co-developing TV. Dr. Vergote is an adviser to both companies and many others. Dr. Tewari is an adviser/consultant, researcher, and speaker for SeaGen and Genmab as well as for Merck, AstraZeneca, and other companies.
which was presented at the European Society of Medical Oncology (ESMO) Congress 2023.
Median overall survival (OS) was 11.5 months among 253 women randomized to tisotumab vedotin (TV) monotherapy versus 9.5 months among 249 randomized to investigators’ choice of chemotherapy, a 30% reduction in the risk of death (P = .0038).
Median progression-free survival (PFS) was 4.2 months with TV versus 2.9 months with chemotherapy (P < .0001), but survival benefits were not statistically significant in a number of subgroups.
Nonetheless, “tisotumab vedotin should be considered as a potential new standard of care for patients who have progressed after first-line systemic therapy,” said lead investigator Ignace Vergote, MD, PhD, a gynecologic oncologist and researcher at the Catholic University of Leuven, Belgium, who presented the findings.
New and emerging options
The trial serves as the confirmation the Food and Drug Administration required when it gave TV accelerated approval in 2021 for recurrent or metastatic cervical cancer (r/m CC) that’s progressed during or after first-line treatment, an approval based on response rates in an earlier phase 2 trial, the InnovaTV 204 study.
TV is the only antibody-drug conjugate approved for the indication, but another agent is also under investigation, the anti-PD-1 cemiplimab. It’s not yet approved for r/m CC, but it is approved in the United States for locally advanced/metastatic basal cell carcinoma, cutaneous squamous cell carcinoma, and non–small cell lung cancer.
Cemiplimab outcomes were similar to TV’s in a phase 3 trial following progression on first-line treatment without anti-PD-1 therapy, with a median OS of 12 months with cemiplimab versus 8.5 months with investigators’ choice of chemotherapy.
Pembrolizumab is also approved as monotherapy for r/m CC for PD-L1 positive women after progression on or during first-line treatment based on response outcomes, not survival.
The question now is how to pick among the various options, said Krishnansu Tewari, MD, a gynecologic oncologist and researcher at the University of California, Irvine, who discussed InnovaTV 301 at the meeting.
In the second line for r/m CC, “we can hypothetically consider” TV monotherapy; pembrolizumab in PD-L1-positive women not previously exposed to a checkpoint inhibitor (CPI); cemiplimab in women not previously exposed to a CPI, and perhaps TV plus pembrolizumab, also in women new to CPIs.
It remains particularly unclear at the moment how to select between TV and cemiplimab monotherapy, if cemiplimab is approved for the indication.
One difference is that unlike in the cemiplimab trial, 28.1% of women treated with TV in the phase 3 trial had been on an anti-PD-(L)1 in the first line. However, although PFS benefits were statistically significant for TV after checkpoint inhibitor exposure, OS benefit was not.
Regarding cost, TV was administered at 2 mg/kg every 3 weeks in Innova; 40 mg costs around $7,000.
Cemiplimab was dosed at 350 mg every 3 weeks in its trial; a single dose costs over $10,000.
Subgroups fall short of statistical significance
In InnovaTV 301, 12-month OS was about 48.7% with TV versus 35% with chemotherapy; 6-month PFS was 30.4% with TV versus 18.9%.
The PFS benefit with TV did not reach statistical significance among the 35.2% of women who had not been treated with bevacizumab in the first-line, and there was no OS benefit or trend to benefit (HR 1.0) for them.
In addition to women previously treated with an anti-PD-1, OS benefits with TV were not statistically significant among the 54.2% of women with baseline performance scores of 0; the 36.8% with adeno or adenosquamous carcinoma, and the 62.8% who had been on one prior systemic regimen instead two.
Women in the trial were a median of 50 years old, and fewer than 7% were from the United States. Investigator choice of chemotherapy included topotecan, vinorelbine, gemcitabine, irinotecan, or pemetrexed.
The rate of grade 3 or higher adverse events was 29.2% with TV and 45.2% with chemotherapy.
The known side effects of TV were all higher than in the chemotherapy arm, including grade 3 or worse peripheral neuropathy (5.2%), ocular events (3.2%), and bleeding (0.8%).
The study was funded in part by Genmab and SeaGen, the companies co-developing TV. Dr. Vergote is an adviser to both companies and many others. Dr. Tewari is an adviser/consultant, researcher, and speaker for SeaGen and Genmab as well as for Merck, AstraZeneca, and other companies.
FROM ESMO 2023
Induction chemotherapy in first line improves survival for locally advanced cervical cancer
and should be considered the new standard of care, according to Mary McCormack, MBBS, PhD, a gynecologic and breast oncologist at the University College Hospital, London.
Dr. McCormack was the lead investigator on a phase 3 trial called INTERLACE that tested the approach against stand-alone chemoradiation – the current standard of care – in 500 women, majority in the United Kingdom and Mexico.
She made her comments after presenting the results at the annual meeting of the European Society for Medical Oncology.
The 250 women randomized to induction chemotherapy before chemoradiation (CRT) had a 35% improvement in progression-free survival (PFS), with a 5-year PFS of 73% versus 64% among 250 randomized to CRT alone. Likewise, overall survival (OS) improved 39% in the induction group, with a 5-year OS of 80% versus 72% among women who went straight to CRT.
Induction chemotherapy consisted of 6 weekly doses of carboplatin AUC2 and paclitaxel 80 mg/m2 followed by CRT within 7 days. CRT consisted of 5 weekly doses of cisplatin 40 mg/m2 plus external beam radiotherapy and brachytherapy. Compliance in both arms was high.
“Induction chemotherapy with weekly paclitaxel and carboplatin delivered immediately before chemoradiotherapy should be considered the new standard in locally advanced cervical cancer, and [it] is feasible across diverse healthcare settings,” Dr. McCormack said.
Study discussant Krishnansu Tewari, MD, a gynecologic oncologist at the University of California, Irvine, was impressed by the results.
“This is the first phase 3 randomized trial in locally advanced cervical cancer that has shown [an overall] survival benefit in over 2 decades. Physicians taking care of these patients could consider induction chemotherapy ... tomorrow morning,” he said.
Dr. Tewari brought up how to incorporate the findings with another trial presented earlier at the meeting, KEYNOTE-A18.
KEYNOTE-A18 added pembrolizumab to CRT, which resulted in substantially better PFS and a strong trend towards better OS that could reach statistical significance with additional follow-up.
Both trials are “practice changing” for locally advanced cervical cancer. “I think we are ready for a paradigm shift,” Dr. Tewari said.
He noted a limit in the INTERLACE presentation was that outcomes were not broken down by tumor stage.
Over three-quarters of the women had stage 2 disease; 9% had stage 1 disease, and only 14% had stage 3B or 4A tumors. Almost 60% of the women were node negative.
It’s unclear at this point if women who have node-negative stage 1B3 or stage 2A-B disease “really need induction chemotherapy. I would think that those patients are probably curable by standard chemoradiation plus brachytherapy, and that the real [benefit would be] for stage 3B and 4A patients,” he said.
The median age in the study was 46 years, and 82% of the women had squamous cell tumors.
Grade 3/4 adverse events were higher in the induction arm, 59% versus 48%, driven mostly by a higher incidence of neutropenia and other hematologic adverse events with induction.
One woman died of adverse events in the induction arm and two died in the CRT-alone arm.
Local and pelvic relapse rates were equal in both groups at 16%, but total distant relapses were lower with induction chemotherapy, 12% versus 20%, over a median follow-up of 64 months.
The work was funded by Cancer Research UK. Dr. McCormack is a consultant for AstraZeneca, Eisai, and GSK, and disclosed honoraria/meeting expenses from Daiicho Sankyo, Roche, and Medscape, the publisher of this article. Among other industry ties, Dr. Tewari is an advisor/consultant, researcher, and speaker for Merck, SeaGen, and AstraZeneca.
and should be considered the new standard of care, according to Mary McCormack, MBBS, PhD, a gynecologic and breast oncologist at the University College Hospital, London.
Dr. McCormack was the lead investigator on a phase 3 trial called INTERLACE that tested the approach against stand-alone chemoradiation – the current standard of care – in 500 women, majority in the United Kingdom and Mexico.
She made her comments after presenting the results at the annual meeting of the European Society for Medical Oncology.
The 250 women randomized to induction chemotherapy before chemoradiation (CRT) had a 35% improvement in progression-free survival (PFS), with a 5-year PFS of 73% versus 64% among 250 randomized to CRT alone. Likewise, overall survival (OS) improved 39% in the induction group, with a 5-year OS of 80% versus 72% among women who went straight to CRT.
Induction chemotherapy consisted of 6 weekly doses of carboplatin AUC2 and paclitaxel 80 mg/m2 followed by CRT within 7 days. CRT consisted of 5 weekly doses of cisplatin 40 mg/m2 plus external beam radiotherapy and brachytherapy. Compliance in both arms was high.
“Induction chemotherapy with weekly paclitaxel and carboplatin delivered immediately before chemoradiotherapy should be considered the new standard in locally advanced cervical cancer, and [it] is feasible across diverse healthcare settings,” Dr. McCormack said.
Study discussant Krishnansu Tewari, MD, a gynecologic oncologist at the University of California, Irvine, was impressed by the results.
“This is the first phase 3 randomized trial in locally advanced cervical cancer that has shown [an overall] survival benefit in over 2 decades. Physicians taking care of these patients could consider induction chemotherapy ... tomorrow morning,” he said.
Dr. Tewari brought up how to incorporate the findings with another trial presented earlier at the meeting, KEYNOTE-A18.
KEYNOTE-A18 added pembrolizumab to CRT, which resulted in substantially better PFS and a strong trend towards better OS that could reach statistical significance with additional follow-up.
Both trials are “practice changing” for locally advanced cervical cancer. “I think we are ready for a paradigm shift,” Dr. Tewari said.
He noted a limit in the INTERLACE presentation was that outcomes were not broken down by tumor stage.
Over three-quarters of the women had stage 2 disease; 9% had stage 1 disease, and only 14% had stage 3B or 4A tumors. Almost 60% of the women were node negative.
It’s unclear at this point if women who have node-negative stage 1B3 or stage 2A-B disease “really need induction chemotherapy. I would think that those patients are probably curable by standard chemoradiation plus brachytherapy, and that the real [benefit would be] for stage 3B and 4A patients,” he said.
The median age in the study was 46 years, and 82% of the women had squamous cell tumors.
Grade 3/4 adverse events were higher in the induction arm, 59% versus 48%, driven mostly by a higher incidence of neutropenia and other hematologic adverse events with induction.
One woman died of adverse events in the induction arm and two died in the CRT-alone arm.
Local and pelvic relapse rates were equal in both groups at 16%, but total distant relapses were lower with induction chemotherapy, 12% versus 20%, over a median follow-up of 64 months.
The work was funded by Cancer Research UK. Dr. McCormack is a consultant for AstraZeneca, Eisai, and GSK, and disclosed honoraria/meeting expenses from Daiicho Sankyo, Roche, and Medscape, the publisher of this article. Among other industry ties, Dr. Tewari is an advisor/consultant, researcher, and speaker for Merck, SeaGen, and AstraZeneca.
and should be considered the new standard of care, according to Mary McCormack, MBBS, PhD, a gynecologic and breast oncologist at the University College Hospital, London.
Dr. McCormack was the lead investigator on a phase 3 trial called INTERLACE that tested the approach against stand-alone chemoradiation – the current standard of care – in 500 women, majority in the United Kingdom and Mexico.
She made her comments after presenting the results at the annual meeting of the European Society for Medical Oncology.
The 250 women randomized to induction chemotherapy before chemoradiation (CRT) had a 35% improvement in progression-free survival (PFS), with a 5-year PFS of 73% versus 64% among 250 randomized to CRT alone. Likewise, overall survival (OS) improved 39% in the induction group, with a 5-year OS of 80% versus 72% among women who went straight to CRT.
Induction chemotherapy consisted of 6 weekly doses of carboplatin AUC2 and paclitaxel 80 mg/m2 followed by CRT within 7 days. CRT consisted of 5 weekly doses of cisplatin 40 mg/m2 plus external beam radiotherapy and brachytherapy. Compliance in both arms was high.
“Induction chemotherapy with weekly paclitaxel and carboplatin delivered immediately before chemoradiotherapy should be considered the new standard in locally advanced cervical cancer, and [it] is feasible across diverse healthcare settings,” Dr. McCormack said.
Study discussant Krishnansu Tewari, MD, a gynecologic oncologist at the University of California, Irvine, was impressed by the results.
“This is the first phase 3 randomized trial in locally advanced cervical cancer that has shown [an overall] survival benefit in over 2 decades. Physicians taking care of these patients could consider induction chemotherapy ... tomorrow morning,” he said.
Dr. Tewari brought up how to incorporate the findings with another trial presented earlier at the meeting, KEYNOTE-A18.
KEYNOTE-A18 added pembrolizumab to CRT, which resulted in substantially better PFS and a strong trend towards better OS that could reach statistical significance with additional follow-up.
Both trials are “practice changing” for locally advanced cervical cancer. “I think we are ready for a paradigm shift,” Dr. Tewari said.
He noted a limit in the INTERLACE presentation was that outcomes were not broken down by tumor stage.
Over three-quarters of the women had stage 2 disease; 9% had stage 1 disease, and only 14% had stage 3B or 4A tumors. Almost 60% of the women were node negative.
It’s unclear at this point if women who have node-negative stage 1B3 or stage 2A-B disease “really need induction chemotherapy. I would think that those patients are probably curable by standard chemoradiation plus brachytherapy, and that the real [benefit would be] for stage 3B and 4A patients,” he said.
The median age in the study was 46 years, and 82% of the women had squamous cell tumors.
Grade 3/4 adverse events were higher in the induction arm, 59% versus 48%, driven mostly by a higher incidence of neutropenia and other hematologic adverse events with induction.
One woman died of adverse events in the induction arm and two died in the CRT-alone arm.
Local and pelvic relapse rates were equal in both groups at 16%, but total distant relapses were lower with induction chemotherapy, 12% versus 20%, over a median follow-up of 64 months.
The work was funded by Cancer Research UK. Dr. McCormack is a consultant for AstraZeneca, Eisai, and GSK, and disclosed honoraria/meeting expenses from Daiicho Sankyo, Roche, and Medscape, the publisher of this article. Among other industry ties, Dr. Tewari is an advisor/consultant, researcher, and speaker for Merck, SeaGen, and AstraZeneca.
FROM ESMO CONGRESS 2023
Chemo-immunotherapy good, adding a PARP inhibitor better in endometrial cancer?
MADRID – Research presented at the European Society for Medical Oncology (ESMO) Annual Meeting 2023 underline the benefit of adding immunotherapy to chemotherapy in advanced or recurrent endometrial cancer, and question whether adding the PARP inhibitor olaparib to the chemo-immunotherapy combination could provide further benefit.
In the AtTEnd trial, presented on Oct. 21, more than 550 patients with advanced newly diagnosed or recurrent disease were randomized to the antiprogrammed death–ligand 1 (PD-L1) antibody atezolizumab (Tecentriq) or placebo plus chemotherapy followed by maintenance atezolizumab or placebo.
– 28.1% vs. 17% at 2 years. The PFS benefit was much more pronounced among patients with mismatch repair-deficient (dMMR) disease – 50.4% vs. 16% at 2 years. Mismatch repair-deficient disease patients receiving atezolizumab also demonstrated an early overall survival benefit, according to findings from the interim analysis.
In the DUO-E trial, presented during the same Oct. 21 session, nearly 720 patients with newly diagnosed advanced or recurrent endometrial cancer were randomized to one of three groups: Chemotherapy alone with maintenance placebo, chemotherapy plus durvalumab (Imfinzi) with maintenance durvalumab, or chemotherapy plus durvalumab with maintenance durvalumab and the PARP inhibitor olaparib.
The results, published simultaneously in the Journal of Clinical Oncology, showed that adding durvalumab to chemotherapy followed by maintenance durvalumab with or without olaparib led to a significant improvement in PFS, compared with chemotherapy alone. As in the AtTEnd trial, this PFS was also more pronounced in dMMR patients.
Overall, Andrés Cervantes, MD, PhD, from the University of Valencia, Spain, and president of ESMO, explained that this research marks “very positive data for women with gynecological cancers,” with immunotherapy now incorporated into the standard of care.
However, an expert questioned whether the DUO-E trial clearly demonstrated the benefit of adding olaparib to immuno- and chemotherapy and whether certain subsets of patients may be more likely to benefit from the PARP inhibitor.
Inside AtTEnd
A growing body of research has shown that single agent immunotherapy is effective in treating endometrial cancer, particularly in tumors with dMMR, and that immunotherapy and chemotherapy may have a synergistic effect.
David S. P. Tan, MD, PhD, National University Cancer Institute, Singapore, who was not involved in the studies, commented that “the molecular classification of endometrial cancer is now leading us to areas that we didn’t think before [were] possible.”
The rationale for combining immunotherapy with chemotherapy, Dr. Tan explained, is that “the cytotoxicity you get from chemotherapy is partly dependent on immune activity within the tumor, and so it makes sense” to combine them.
This approach was borne out by recent positive PFS results from the NRG-GY018 trial of pembrolizumab plus chemotherapy in advanced endometrial cancer as well as from the RUBY trial of dostarlimab in primary advanced or recurrent disease.
To further investigate this chemo-immunotherapy strategy, the AtTEnd team enrolled patients with newly diagnosed or recurrent stage III-IV disease who had received no prior systemic chemotherapy for recurrence within the previous 6 months.
Overall, 551 patients from 89 sites across 10 countries were randomized to standard first-line chemotherapy – carboplatin plus paclitaxel – with either atezolizumab or placebo, followed by maintenance atezolizumab or placebo, which continued until confirmed disease progression.
The median age in the intention-to-treat population was 64-67 years. Nearly 23% of patients had dMMR tumors, and 67.2% had recurrent disease.
The baseline characteristics were well balanced and distributed between arms in the dMMR and all-comers population, said Nicoletta Colombo, MD, University of Milan–Bicocca, European Institute of Oncology Istituto di Ricovero e Cura a Carattere Scientifico, Italy, who presented the findings at ESMO.
Over a median follow up of 26.2 months, Dr. Colombo and colleagues observed a statistically significant improvement in PFS in the dMMR arm in favor of atezolizumab (hazard ratio, 0.36; P = .0005). At 2 years, 50.4% of patients receiving the immunotherapy were progression-free, compared with 16.0% in the placebo arm.
In all-comers, the PFS improvement with atezolizumab was less pronounced but remained significant (HR, 0.74; P = .0219).
A secondary analysis revealed, among dMMR patients, atezolizumab was associated with an overall survival advantage over placebo (HR, 0.41), with 75% of patients still alive at 2 years vs. 54.2% in the placebo arm. Dr. Colombo also noted a “clear trend” for improved overall survival with atezolizumab as well (HR, 0.82; P = .0483), but no PFS or overall survival benefit was seen with atezolizumab in MMR proficient (pMMR) patients.
Dr. Colombo said the safety profile of atezolizumab plus chemotherapy was “manageable,” with no differences in the rates of “major side effects,” although there was an increase in the rate of treatment-related grade ≥ 3 adverse events in the atezolizumab group (25.8% vs. 14.1%).
Dr. Tan noted that the AtTEnd trial revealed comparable results to earlier trials in this space but underlined that the survival curves in the interim analysis revealed a “red zone” of dMMR patients who do not respond to the combination and in whom immunotherapy is “not sufficient.”
Alongside this, Dr. Tan flagged a “blue zone” of dMMR patients who plateaued in both PFS and overall survival after 2 years. The question for these patients at this point is whether they need to continue immunotherapy beyond 24 months, he said.
But overall, Dr. Tan noted, the AtTEnd data “continue to validate practice-changing therapy for dMMR endometrial cancer patients” with immunotherapy plus chemotherapy, with the lack of benefit in pMMR disease underscoring an “unmet medical need.”
Inside DUO-E
The burning question, however, was whether adding a PARP inhibitor to immunotherapy and chemotherapy would boost the survival outcomes further.
The DUO-E trial involved patients with newly diagnosed stage III/IV or recurrent endometrial cancer who had not received systematic therapy for advanced disease and were naive to both PARP inhibitors and immune-mediated therapy.
Overall, 718 patients were randomized to one of three arms: Chemotherapy alone followed by maintenance placebo, chemotherapy plus durvalumab with maintenance durvalumab, or chemotherapy plus durvalumab with maintenance durvalumab plus olaparib.
Maintenance was continued until disease progression or unacceptable toxicity, or the patients met another discontinuation criteria.
About half of patients were newly diagnosed, half had recurrent disease, and approximately one-fifth had dMMR disease, said Shannon Westin, MD, from the University of Texas MD Anderson Cancer Center, Houston, who presented the findings.
Compared with placebo plus chemotherapy, patients in both the durvalumab alone and durvalumab plus olaparib arms experienced a significant improvement in PFS (HR, 0.71; P = .003; and HR, 0.55; P < .0001, respectively).
This effect was amplified in dMMR patients with durvalumab (HR, 0.42) as well as with durvalumab plus olaparib (HR, 0.41).
In pMMR patients, PFS benefit was stronger in the durvalumab-olaparib arm vs. durvalumab (HR, 0.57 vs. 0.77).
Although the overall survival analysis remains exploratory, Dr. Westin noted a trend toward better overall survival in the two treatment arms vs. placebo (HR, 0.77 with durvalumab, and HR, 0.59 with durvalumab plus olaparib).
However, adding olaparib to the equation increased the rate of grade ≥ 3 adverse events – 67.2% vs. 54.9% with durvalumab and 56.4% with chemotherapy alone in the overall analysis. The addition of olaparib also led to treatment discontinuation in 24.4% of patients vs. 20.9% in the durvalumab arm and 18.6% in the chemotherapy alone arm.
Domenica Lorusso, MD, PhD, who was not involved in the study, commented that the marginal PFS benefit of adding olaparib in DUO-E is “not surprising” because the bar set by immunotherapy is “so high in this population that it’s very difficult” to go any higher.
But the results in pMMR patients reveal “a clear additional benefit” to olaparib, said Dr. Lorusso, from Fondazione IRCCS Istituto Nazionale dei Tumori, Milan.
“The main limitation of the trial,” she continued, “is that it was not powered to make a formal comparison between the two experimental arms.”
So, what then is the added benefit of olaparib? “Unfortunately, that remains an unanswered question,” Dr. Lorusso said.
AtTEnd was sponsored by the Mario Negri Institute for Pharmacological Research.
DUO-E was funded by AstraZeneca.
Dr. Colombo declares relationships with AstraZeneca, Clovis Oncology, Esai, GSK, Immunogen, Mersana, MSD/Merck, Nuvation Bio, OncXerna, Pieris, Roche, and Novocure.
Dr. Tan declares relationships with AstraZeneca, Karyopharm Therapeutics, Bayer, Roche, MSD, Genmab, Esai, PMV, BioNTech, Ellipses Pharma, Boehringer Ingelheim, Merck Serono, Takeda, and Clovis.
Dr. Westin declares relationships with AstraZeneca, Avenge Bio, Bayer, Bio-Path, Clovis, Genentech/Roche, GSK, Jazz Pharmaceuticals, Mereo, Novartis, Nuvectis, and Zentalis; and consulting and advisory roles for AstraZeneca, Caris, Clovis, Eisai, EQRx, Genentech/Roche, Gilead, GSK, Immunocore, ImmunoGen, Lilly, Merck, Mersana, Mereo, NGM Bio, Nuvectis, Seagen, Verastem, Vincerx, Zentalis, and ZielBio.
Dr. Lorusso declares relationships with PharmaMar, Merck Serono, Novartis, AstraZeneca, Clovis, Tesaro/GSK, Genmab, Immunogen, and Roche.
A version of this article first appeared on Medscape.com.
MADRID – Research presented at the European Society for Medical Oncology (ESMO) Annual Meeting 2023 underline the benefit of adding immunotherapy to chemotherapy in advanced or recurrent endometrial cancer, and question whether adding the PARP inhibitor olaparib to the chemo-immunotherapy combination could provide further benefit.
In the AtTEnd trial, presented on Oct. 21, more than 550 patients with advanced newly diagnosed or recurrent disease were randomized to the antiprogrammed death–ligand 1 (PD-L1) antibody atezolizumab (Tecentriq) or placebo plus chemotherapy followed by maintenance atezolizumab or placebo.
– 28.1% vs. 17% at 2 years. The PFS benefit was much more pronounced among patients with mismatch repair-deficient (dMMR) disease – 50.4% vs. 16% at 2 years. Mismatch repair-deficient disease patients receiving atezolizumab also demonstrated an early overall survival benefit, according to findings from the interim analysis.
In the DUO-E trial, presented during the same Oct. 21 session, nearly 720 patients with newly diagnosed advanced or recurrent endometrial cancer were randomized to one of three groups: Chemotherapy alone with maintenance placebo, chemotherapy plus durvalumab (Imfinzi) with maintenance durvalumab, or chemotherapy plus durvalumab with maintenance durvalumab and the PARP inhibitor olaparib.
The results, published simultaneously in the Journal of Clinical Oncology, showed that adding durvalumab to chemotherapy followed by maintenance durvalumab with or without olaparib led to a significant improvement in PFS, compared with chemotherapy alone. As in the AtTEnd trial, this PFS was also more pronounced in dMMR patients.
Overall, Andrés Cervantes, MD, PhD, from the University of Valencia, Spain, and president of ESMO, explained that this research marks “very positive data for women with gynecological cancers,” with immunotherapy now incorporated into the standard of care.
However, an expert questioned whether the DUO-E trial clearly demonstrated the benefit of adding olaparib to immuno- and chemotherapy and whether certain subsets of patients may be more likely to benefit from the PARP inhibitor.
Inside AtTEnd
A growing body of research has shown that single agent immunotherapy is effective in treating endometrial cancer, particularly in tumors with dMMR, and that immunotherapy and chemotherapy may have a synergistic effect.
David S. P. Tan, MD, PhD, National University Cancer Institute, Singapore, who was not involved in the studies, commented that “the molecular classification of endometrial cancer is now leading us to areas that we didn’t think before [were] possible.”
The rationale for combining immunotherapy with chemotherapy, Dr. Tan explained, is that “the cytotoxicity you get from chemotherapy is partly dependent on immune activity within the tumor, and so it makes sense” to combine them.
This approach was borne out by recent positive PFS results from the NRG-GY018 trial of pembrolizumab plus chemotherapy in advanced endometrial cancer as well as from the RUBY trial of dostarlimab in primary advanced or recurrent disease.
To further investigate this chemo-immunotherapy strategy, the AtTEnd team enrolled patients with newly diagnosed or recurrent stage III-IV disease who had received no prior systemic chemotherapy for recurrence within the previous 6 months.
Overall, 551 patients from 89 sites across 10 countries were randomized to standard first-line chemotherapy – carboplatin plus paclitaxel – with either atezolizumab or placebo, followed by maintenance atezolizumab or placebo, which continued until confirmed disease progression.
The median age in the intention-to-treat population was 64-67 years. Nearly 23% of patients had dMMR tumors, and 67.2% had recurrent disease.
The baseline characteristics were well balanced and distributed between arms in the dMMR and all-comers population, said Nicoletta Colombo, MD, University of Milan–Bicocca, European Institute of Oncology Istituto di Ricovero e Cura a Carattere Scientifico, Italy, who presented the findings at ESMO.
Over a median follow up of 26.2 months, Dr. Colombo and colleagues observed a statistically significant improvement in PFS in the dMMR arm in favor of atezolizumab (hazard ratio, 0.36; P = .0005). At 2 years, 50.4% of patients receiving the immunotherapy were progression-free, compared with 16.0% in the placebo arm.
In all-comers, the PFS improvement with atezolizumab was less pronounced but remained significant (HR, 0.74; P = .0219).
A secondary analysis revealed, among dMMR patients, atezolizumab was associated with an overall survival advantage over placebo (HR, 0.41), with 75% of patients still alive at 2 years vs. 54.2% in the placebo arm. Dr. Colombo also noted a “clear trend” for improved overall survival with atezolizumab as well (HR, 0.82; P = .0483), but no PFS or overall survival benefit was seen with atezolizumab in MMR proficient (pMMR) patients.
Dr. Colombo said the safety profile of atezolizumab plus chemotherapy was “manageable,” with no differences in the rates of “major side effects,” although there was an increase in the rate of treatment-related grade ≥ 3 adverse events in the atezolizumab group (25.8% vs. 14.1%).
Dr. Tan noted that the AtTEnd trial revealed comparable results to earlier trials in this space but underlined that the survival curves in the interim analysis revealed a “red zone” of dMMR patients who do not respond to the combination and in whom immunotherapy is “not sufficient.”
Alongside this, Dr. Tan flagged a “blue zone” of dMMR patients who plateaued in both PFS and overall survival after 2 years. The question for these patients at this point is whether they need to continue immunotherapy beyond 24 months, he said.
But overall, Dr. Tan noted, the AtTEnd data “continue to validate practice-changing therapy for dMMR endometrial cancer patients” with immunotherapy plus chemotherapy, with the lack of benefit in pMMR disease underscoring an “unmet medical need.”
Inside DUO-E
The burning question, however, was whether adding a PARP inhibitor to immunotherapy and chemotherapy would boost the survival outcomes further.
The DUO-E trial involved patients with newly diagnosed stage III/IV or recurrent endometrial cancer who had not received systematic therapy for advanced disease and were naive to both PARP inhibitors and immune-mediated therapy.
Overall, 718 patients were randomized to one of three arms: Chemotherapy alone followed by maintenance placebo, chemotherapy plus durvalumab with maintenance durvalumab, or chemotherapy plus durvalumab with maintenance durvalumab plus olaparib.
Maintenance was continued until disease progression or unacceptable toxicity, or the patients met another discontinuation criteria.
About half of patients were newly diagnosed, half had recurrent disease, and approximately one-fifth had dMMR disease, said Shannon Westin, MD, from the University of Texas MD Anderson Cancer Center, Houston, who presented the findings.
Compared with placebo plus chemotherapy, patients in both the durvalumab alone and durvalumab plus olaparib arms experienced a significant improvement in PFS (HR, 0.71; P = .003; and HR, 0.55; P < .0001, respectively).
This effect was amplified in dMMR patients with durvalumab (HR, 0.42) as well as with durvalumab plus olaparib (HR, 0.41).
In pMMR patients, PFS benefit was stronger in the durvalumab-olaparib arm vs. durvalumab (HR, 0.57 vs. 0.77).
Although the overall survival analysis remains exploratory, Dr. Westin noted a trend toward better overall survival in the two treatment arms vs. placebo (HR, 0.77 with durvalumab, and HR, 0.59 with durvalumab plus olaparib).
However, adding olaparib to the equation increased the rate of grade ≥ 3 adverse events – 67.2% vs. 54.9% with durvalumab and 56.4% with chemotherapy alone in the overall analysis. The addition of olaparib also led to treatment discontinuation in 24.4% of patients vs. 20.9% in the durvalumab arm and 18.6% in the chemotherapy alone arm.
Domenica Lorusso, MD, PhD, who was not involved in the study, commented that the marginal PFS benefit of adding olaparib in DUO-E is “not surprising” because the bar set by immunotherapy is “so high in this population that it’s very difficult” to go any higher.
But the results in pMMR patients reveal “a clear additional benefit” to olaparib, said Dr. Lorusso, from Fondazione IRCCS Istituto Nazionale dei Tumori, Milan.
“The main limitation of the trial,” she continued, “is that it was not powered to make a formal comparison between the two experimental arms.”
So, what then is the added benefit of olaparib? “Unfortunately, that remains an unanswered question,” Dr. Lorusso said.
AtTEnd was sponsored by the Mario Negri Institute for Pharmacological Research.
DUO-E was funded by AstraZeneca.
Dr. Colombo declares relationships with AstraZeneca, Clovis Oncology, Esai, GSK, Immunogen, Mersana, MSD/Merck, Nuvation Bio, OncXerna, Pieris, Roche, and Novocure.
Dr. Tan declares relationships with AstraZeneca, Karyopharm Therapeutics, Bayer, Roche, MSD, Genmab, Esai, PMV, BioNTech, Ellipses Pharma, Boehringer Ingelheim, Merck Serono, Takeda, and Clovis.
Dr. Westin declares relationships with AstraZeneca, Avenge Bio, Bayer, Bio-Path, Clovis, Genentech/Roche, GSK, Jazz Pharmaceuticals, Mereo, Novartis, Nuvectis, and Zentalis; and consulting and advisory roles for AstraZeneca, Caris, Clovis, Eisai, EQRx, Genentech/Roche, Gilead, GSK, Immunocore, ImmunoGen, Lilly, Merck, Mersana, Mereo, NGM Bio, Nuvectis, Seagen, Verastem, Vincerx, Zentalis, and ZielBio.
Dr. Lorusso declares relationships with PharmaMar, Merck Serono, Novartis, AstraZeneca, Clovis, Tesaro/GSK, Genmab, Immunogen, and Roche.
A version of this article first appeared on Medscape.com.
MADRID – Research presented at the European Society for Medical Oncology (ESMO) Annual Meeting 2023 underline the benefit of adding immunotherapy to chemotherapy in advanced or recurrent endometrial cancer, and question whether adding the PARP inhibitor olaparib to the chemo-immunotherapy combination could provide further benefit.
In the AtTEnd trial, presented on Oct. 21, more than 550 patients with advanced newly diagnosed or recurrent disease were randomized to the antiprogrammed death–ligand 1 (PD-L1) antibody atezolizumab (Tecentriq) or placebo plus chemotherapy followed by maintenance atezolizumab or placebo.
– 28.1% vs. 17% at 2 years. The PFS benefit was much more pronounced among patients with mismatch repair-deficient (dMMR) disease – 50.4% vs. 16% at 2 years. Mismatch repair-deficient disease patients receiving atezolizumab also demonstrated an early overall survival benefit, according to findings from the interim analysis.
In the DUO-E trial, presented during the same Oct. 21 session, nearly 720 patients with newly diagnosed advanced or recurrent endometrial cancer were randomized to one of three groups: Chemotherapy alone with maintenance placebo, chemotherapy plus durvalumab (Imfinzi) with maintenance durvalumab, or chemotherapy plus durvalumab with maintenance durvalumab and the PARP inhibitor olaparib.
The results, published simultaneously in the Journal of Clinical Oncology, showed that adding durvalumab to chemotherapy followed by maintenance durvalumab with or without olaparib led to a significant improvement in PFS, compared with chemotherapy alone. As in the AtTEnd trial, this PFS was also more pronounced in dMMR patients.
Overall, Andrés Cervantes, MD, PhD, from the University of Valencia, Spain, and president of ESMO, explained that this research marks “very positive data for women with gynecological cancers,” with immunotherapy now incorporated into the standard of care.
However, an expert questioned whether the DUO-E trial clearly demonstrated the benefit of adding olaparib to immuno- and chemotherapy and whether certain subsets of patients may be more likely to benefit from the PARP inhibitor.
Inside AtTEnd
A growing body of research has shown that single agent immunotherapy is effective in treating endometrial cancer, particularly in tumors with dMMR, and that immunotherapy and chemotherapy may have a synergistic effect.
David S. P. Tan, MD, PhD, National University Cancer Institute, Singapore, who was not involved in the studies, commented that “the molecular classification of endometrial cancer is now leading us to areas that we didn’t think before [were] possible.”
The rationale for combining immunotherapy with chemotherapy, Dr. Tan explained, is that “the cytotoxicity you get from chemotherapy is partly dependent on immune activity within the tumor, and so it makes sense” to combine them.
This approach was borne out by recent positive PFS results from the NRG-GY018 trial of pembrolizumab plus chemotherapy in advanced endometrial cancer as well as from the RUBY trial of dostarlimab in primary advanced or recurrent disease.
To further investigate this chemo-immunotherapy strategy, the AtTEnd team enrolled patients with newly diagnosed or recurrent stage III-IV disease who had received no prior systemic chemotherapy for recurrence within the previous 6 months.
Overall, 551 patients from 89 sites across 10 countries were randomized to standard first-line chemotherapy – carboplatin plus paclitaxel – with either atezolizumab or placebo, followed by maintenance atezolizumab or placebo, which continued until confirmed disease progression.
The median age in the intention-to-treat population was 64-67 years. Nearly 23% of patients had dMMR tumors, and 67.2% had recurrent disease.
The baseline characteristics were well balanced and distributed between arms in the dMMR and all-comers population, said Nicoletta Colombo, MD, University of Milan–Bicocca, European Institute of Oncology Istituto di Ricovero e Cura a Carattere Scientifico, Italy, who presented the findings at ESMO.
Over a median follow up of 26.2 months, Dr. Colombo and colleagues observed a statistically significant improvement in PFS in the dMMR arm in favor of atezolizumab (hazard ratio, 0.36; P = .0005). At 2 years, 50.4% of patients receiving the immunotherapy were progression-free, compared with 16.0% in the placebo arm.
In all-comers, the PFS improvement with atezolizumab was less pronounced but remained significant (HR, 0.74; P = .0219).
A secondary analysis revealed, among dMMR patients, atezolizumab was associated with an overall survival advantage over placebo (HR, 0.41), with 75% of patients still alive at 2 years vs. 54.2% in the placebo arm. Dr. Colombo also noted a “clear trend” for improved overall survival with atezolizumab as well (HR, 0.82; P = .0483), but no PFS or overall survival benefit was seen with atezolizumab in MMR proficient (pMMR) patients.
Dr. Colombo said the safety profile of atezolizumab plus chemotherapy was “manageable,” with no differences in the rates of “major side effects,” although there was an increase in the rate of treatment-related grade ≥ 3 adverse events in the atezolizumab group (25.8% vs. 14.1%).
Dr. Tan noted that the AtTEnd trial revealed comparable results to earlier trials in this space but underlined that the survival curves in the interim analysis revealed a “red zone” of dMMR patients who do not respond to the combination and in whom immunotherapy is “not sufficient.”
Alongside this, Dr. Tan flagged a “blue zone” of dMMR patients who plateaued in both PFS and overall survival after 2 years. The question for these patients at this point is whether they need to continue immunotherapy beyond 24 months, he said.
But overall, Dr. Tan noted, the AtTEnd data “continue to validate practice-changing therapy for dMMR endometrial cancer patients” with immunotherapy plus chemotherapy, with the lack of benefit in pMMR disease underscoring an “unmet medical need.”
Inside DUO-E
The burning question, however, was whether adding a PARP inhibitor to immunotherapy and chemotherapy would boost the survival outcomes further.
The DUO-E trial involved patients with newly diagnosed stage III/IV or recurrent endometrial cancer who had not received systematic therapy for advanced disease and were naive to both PARP inhibitors and immune-mediated therapy.
Overall, 718 patients were randomized to one of three arms: Chemotherapy alone followed by maintenance placebo, chemotherapy plus durvalumab with maintenance durvalumab, or chemotherapy plus durvalumab with maintenance durvalumab plus olaparib.
Maintenance was continued until disease progression or unacceptable toxicity, or the patients met another discontinuation criteria.
About half of patients were newly diagnosed, half had recurrent disease, and approximately one-fifth had dMMR disease, said Shannon Westin, MD, from the University of Texas MD Anderson Cancer Center, Houston, who presented the findings.
Compared with placebo plus chemotherapy, patients in both the durvalumab alone and durvalumab plus olaparib arms experienced a significant improvement in PFS (HR, 0.71; P = .003; and HR, 0.55; P < .0001, respectively).
This effect was amplified in dMMR patients with durvalumab (HR, 0.42) as well as with durvalumab plus olaparib (HR, 0.41).
In pMMR patients, PFS benefit was stronger in the durvalumab-olaparib arm vs. durvalumab (HR, 0.57 vs. 0.77).
Although the overall survival analysis remains exploratory, Dr. Westin noted a trend toward better overall survival in the two treatment arms vs. placebo (HR, 0.77 with durvalumab, and HR, 0.59 with durvalumab plus olaparib).
However, adding olaparib to the equation increased the rate of grade ≥ 3 adverse events – 67.2% vs. 54.9% with durvalumab and 56.4% with chemotherapy alone in the overall analysis. The addition of olaparib also led to treatment discontinuation in 24.4% of patients vs. 20.9% in the durvalumab arm and 18.6% in the chemotherapy alone arm.
Domenica Lorusso, MD, PhD, who was not involved in the study, commented that the marginal PFS benefit of adding olaparib in DUO-E is “not surprising” because the bar set by immunotherapy is “so high in this population that it’s very difficult” to go any higher.
But the results in pMMR patients reveal “a clear additional benefit” to olaparib, said Dr. Lorusso, from Fondazione IRCCS Istituto Nazionale dei Tumori, Milan.
“The main limitation of the trial,” she continued, “is that it was not powered to make a formal comparison between the two experimental arms.”
So, what then is the added benefit of olaparib? “Unfortunately, that remains an unanswered question,” Dr. Lorusso said.
AtTEnd was sponsored by the Mario Negri Institute for Pharmacological Research.
DUO-E was funded by AstraZeneca.
Dr. Colombo declares relationships with AstraZeneca, Clovis Oncology, Esai, GSK, Immunogen, Mersana, MSD/Merck, Nuvation Bio, OncXerna, Pieris, Roche, and Novocure.
Dr. Tan declares relationships with AstraZeneca, Karyopharm Therapeutics, Bayer, Roche, MSD, Genmab, Esai, PMV, BioNTech, Ellipses Pharma, Boehringer Ingelheim, Merck Serono, Takeda, and Clovis.
Dr. Westin declares relationships with AstraZeneca, Avenge Bio, Bayer, Bio-Path, Clovis, Genentech/Roche, GSK, Jazz Pharmaceuticals, Mereo, Novartis, Nuvectis, and Zentalis; and consulting and advisory roles for AstraZeneca, Caris, Clovis, Eisai, EQRx, Genentech/Roche, Gilead, GSK, Immunocore, ImmunoGen, Lilly, Merck, Mersana, Mereo, NGM Bio, Nuvectis, Seagen, Verastem, Vincerx, Zentalis, and ZielBio.
Dr. Lorusso declares relationships with PharmaMar, Merck Serono, Novartis, AstraZeneca, Clovis, Tesaro/GSK, Genmab, Immunogen, and Roche.
A version of this article first appeared on Medscape.com.
FROM ESMO 2023
Does first-line pembrolizumab add-on improve PFS in high-risk cervical cancer?
Pembrolizumab (Keytruda) improved progression-free survival when added to standard concurrent chemoradiotherapy in the first-line for newly diagnosed, locally advanced cervical cancer in the KEYNOTE-A18 trial, according to a study presented at the annual meeting of the European Society for Medical Oncology.
The study “supports pembrolizumab plus chemoradiotherapy as a new potential standard of care” in the first-line setting for high-risk, locally advanced cervical cancer, said lead investigator Domenica Lorusso, MD, PhD, a gynecologic oncologist at the Catholic University of Rome, who reported the findings at the meeting.
The results of the trial “are compelling, especially considering newly diagnosed patients with high-risk locally advanced cervical cancer have not seen an advance in treatment options in 20 years,” she said in a press release from pembrolizumab maker Merck.
Trial data are under review at the Food and Drug Administration as part of Merck’s application for a first-line indication for pembrolizumab added to concurrent chemoradiotherapy in newly diagnosed patients with high-risk, locally advanced cervical cancer; the agency’s decision is expected in Jan. 2024.
Pembrolizumab already carries indications for persistent, recurrent, or metastatic cervical cancer.
Women in the trial were new to treatment and had either stage 1B2-2B disease with lymph node involvement or stage 3-4A disease; almost 85% had squamous cell cancer. About half the women were White; 28% were Asian, and about 2% were Black. About 5% of subjects were PD-L1 negative.
Overall, 529 women were randomized to 200 mg pembrolizumab every 3 weeks for five cycles with concurrent chemoradiotherapy (CCRT); they then received pembrolizumab 400 mg every 6 weeks for 15 cycles; 531 were randomized to placebo with CCRT, followed by 15 6-week placebo cycles.
CCRT included five cycles of cisplatin 40 mg/m2 every week for 5-6 weeks plus external beam radiotherapy followed by brachytherapy.
Two-year progression-free survival was 57.3% with placebo but 67.8% with pembrolizumab add-on, a 30% reduction in the risk of progression (P = .002).
On subgroup analysis, pembrolizumab’s PFS benefit was not statistically significant for White women (hazard ratio, 0.83; 95% confidence interval, 0.59-1.15) and women with stage 1B2 to 2B disease (HR, 0.91; 95% CI, 0.63-1.31), among others.
Although OS is not yet mature, 80.8% of placebo subjects but 87.2% of pembrolizumab women were alive at 2 years, a 27% drop in the risk of death (95% CI, 0.49-1.07).
At the meeting, Bradley Monk, MD, a gynecologic oncologist at the University of Arizona, Phoenix, who was also the study discussant, noted that “the magnitude of the benefit here is difficult to interpret because 55% of the patients [were] still on treatment” in the interim analysis, but the difference “is substantial enough for us to have confidence.”
Rates of grade 3/4 treatment-related adverse events were 60.6% in the placebo group and 67% with pembrolizumab, with anemia, nausea, and diarrhea the most common.
Grade 3/4 immune-mediated adverse events occurred in 1.1% of placebo and 4.2% of pembrolizumab subjects; hypothyroidism was the most common with pembrolizumab.
Protocol amendments in the trial included a change from PFS assessment by blinded, independent, central review to investigator assessment.
In the press release, Dr. Monk, said the results “demonstrate that, by moving an immunotherapy regimen to earlier stages of cervical cancer, we have the potential to improve outcomes for these patients compared to the current standard of care.”
The study was funded by Merck, maker of pembrolizumab. Investigators reported wide-ranging ties to the company, including Dr. Lorusso, who reported honoraria from Merck as well as ties to other companies. Dr. Monk also had deep industry ties, including being a speaker and consultant for Merck and reporting honoraria from the company.
Pembrolizumab (Keytruda) improved progression-free survival when added to standard concurrent chemoradiotherapy in the first-line for newly diagnosed, locally advanced cervical cancer in the KEYNOTE-A18 trial, according to a study presented at the annual meeting of the European Society for Medical Oncology.
The study “supports pembrolizumab plus chemoradiotherapy as a new potential standard of care” in the first-line setting for high-risk, locally advanced cervical cancer, said lead investigator Domenica Lorusso, MD, PhD, a gynecologic oncologist at the Catholic University of Rome, who reported the findings at the meeting.
The results of the trial “are compelling, especially considering newly diagnosed patients with high-risk locally advanced cervical cancer have not seen an advance in treatment options in 20 years,” she said in a press release from pembrolizumab maker Merck.
Trial data are under review at the Food and Drug Administration as part of Merck’s application for a first-line indication for pembrolizumab added to concurrent chemoradiotherapy in newly diagnosed patients with high-risk, locally advanced cervical cancer; the agency’s decision is expected in Jan. 2024.
Pembrolizumab already carries indications for persistent, recurrent, or metastatic cervical cancer.
Women in the trial were new to treatment and had either stage 1B2-2B disease with lymph node involvement or stage 3-4A disease; almost 85% had squamous cell cancer. About half the women were White; 28% were Asian, and about 2% were Black. About 5% of subjects were PD-L1 negative.
Overall, 529 women were randomized to 200 mg pembrolizumab every 3 weeks for five cycles with concurrent chemoradiotherapy (CCRT); they then received pembrolizumab 400 mg every 6 weeks for 15 cycles; 531 were randomized to placebo with CCRT, followed by 15 6-week placebo cycles.
CCRT included five cycles of cisplatin 40 mg/m2 every week for 5-6 weeks plus external beam radiotherapy followed by brachytherapy.
Two-year progression-free survival was 57.3% with placebo but 67.8% with pembrolizumab add-on, a 30% reduction in the risk of progression (P = .002).
On subgroup analysis, pembrolizumab’s PFS benefit was not statistically significant for White women (hazard ratio, 0.83; 95% confidence interval, 0.59-1.15) and women with stage 1B2 to 2B disease (HR, 0.91; 95% CI, 0.63-1.31), among others.
Although OS is not yet mature, 80.8% of placebo subjects but 87.2% of pembrolizumab women were alive at 2 years, a 27% drop in the risk of death (95% CI, 0.49-1.07).
At the meeting, Bradley Monk, MD, a gynecologic oncologist at the University of Arizona, Phoenix, who was also the study discussant, noted that “the magnitude of the benefit here is difficult to interpret because 55% of the patients [were] still on treatment” in the interim analysis, but the difference “is substantial enough for us to have confidence.”
Rates of grade 3/4 treatment-related adverse events were 60.6% in the placebo group and 67% with pembrolizumab, with anemia, nausea, and diarrhea the most common.
Grade 3/4 immune-mediated adverse events occurred in 1.1% of placebo and 4.2% of pembrolizumab subjects; hypothyroidism was the most common with pembrolizumab.
Protocol amendments in the trial included a change from PFS assessment by blinded, independent, central review to investigator assessment.
In the press release, Dr. Monk, said the results “demonstrate that, by moving an immunotherapy regimen to earlier stages of cervical cancer, we have the potential to improve outcomes for these patients compared to the current standard of care.”
The study was funded by Merck, maker of pembrolizumab. Investigators reported wide-ranging ties to the company, including Dr. Lorusso, who reported honoraria from Merck as well as ties to other companies. Dr. Monk also had deep industry ties, including being a speaker and consultant for Merck and reporting honoraria from the company.
Pembrolizumab (Keytruda) improved progression-free survival when added to standard concurrent chemoradiotherapy in the first-line for newly diagnosed, locally advanced cervical cancer in the KEYNOTE-A18 trial, according to a study presented at the annual meeting of the European Society for Medical Oncology.
The study “supports pembrolizumab plus chemoradiotherapy as a new potential standard of care” in the first-line setting for high-risk, locally advanced cervical cancer, said lead investigator Domenica Lorusso, MD, PhD, a gynecologic oncologist at the Catholic University of Rome, who reported the findings at the meeting.
The results of the trial “are compelling, especially considering newly diagnosed patients with high-risk locally advanced cervical cancer have not seen an advance in treatment options in 20 years,” she said in a press release from pembrolizumab maker Merck.
Trial data are under review at the Food and Drug Administration as part of Merck’s application for a first-line indication for pembrolizumab added to concurrent chemoradiotherapy in newly diagnosed patients with high-risk, locally advanced cervical cancer; the agency’s decision is expected in Jan. 2024.
Pembrolizumab already carries indications for persistent, recurrent, or metastatic cervical cancer.
Women in the trial were new to treatment and had either stage 1B2-2B disease with lymph node involvement or stage 3-4A disease; almost 85% had squamous cell cancer. About half the women were White; 28% were Asian, and about 2% were Black. About 5% of subjects were PD-L1 negative.
Overall, 529 women were randomized to 200 mg pembrolizumab every 3 weeks for five cycles with concurrent chemoradiotherapy (CCRT); they then received pembrolizumab 400 mg every 6 weeks for 15 cycles; 531 were randomized to placebo with CCRT, followed by 15 6-week placebo cycles.
CCRT included five cycles of cisplatin 40 mg/m2 every week for 5-6 weeks plus external beam radiotherapy followed by brachytherapy.
Two-year progression-free survival was 57.3% with placebo but 67.8% with pembrolizumab add-on, a 30% reduction in the risk of progression (P = .002).
On subgroup analysis, pembrolizumab’s PFS benefit was not statistically significant for White women (hazard ratio, 0.83; 95% confidence interval, 0.59-1.15) and women with stage 1B2 to 2B disease (HR, 0.91; 95% CI, 0.63-1.31), among others.
Although OS is not yet mature, 80.8% of placebo subjects but 87.2% of pembrolizumab women were alive at 2 years, a 27% drop in the risk of death (95% CI, 0.49-1.07).
At the meeting, Bradley Monk, MD, a gynecologic oncologist at the University of Arizona, Phoenix, who was also the study discussant, noted that “the magnitude of the benefit here is difficult to interpret because 55% of the patients [were] still on treatment” in the interim analysis, but the difference “is substantial enough for us to have confidence.”
Rates of grade 3/4 treatment-related adverse events were 60.6% in the placebo group and 67% with pembrolizumab, with anemia, nausea, and diarrhea the most common.
Grade 3/4 immune-mediated adverse events occurred in 1.1% of placebo and 4.2% of pembrolizumab subjects; hypothyroidism was the most common with pembrolizumab.
Protocol amendments in the trial included a change from PFS assessment by blinded, independent, central review to investigator assessment.
In the press release, Dr. Monk, said the results “demonstrate that, by moving an immunotherapy regimen to earlier stages of cervical cancer, we have the potential to improve outcomes for these patients compared to the current standard of care.”
The study was funded by Merck, maker of pembrolizumab. Investigators reported wide-ranging ties to the company, including Dr. Lorusso, who reported honoraria from Merck as well as ties to other companies. Dr. Monk also had deep industry ties, including being a speaker and consultant for Merck and reporting honoraria from the company.
FROM ESMO CONGRESS 2023
What is the future for multicancer early-detection tests?
Suzette Delaloge, MD, MSc, oncologist, breast cancer specialist, and director of the individualized cancer prevention program (Interception) at the Gustave Roussy Institute in Villejuif, France, looks into these “liquid biopsies” and shares her reservations about their potential marketing, especially to the organized care plans.
Question: What are the general principles underpinning these MCED tests?
Suzette Delaloge, MD, MSc: Despite their specificities, the general idea is to detect certain cancer markers in various body fluids (blood, urine, saliva, etc.), for example, molecules released by cancer cells (cytokines, inflammatory proteins, leptin, etc.) or distinctive features of the DNA in tumor cells. In blood, these molecules can be found in plasma or in serum. In urine, it’s more about detecting kidney, bladder, and urinary tract cancers.
Q: What sort of time frame are we looking at for these MCED tests to be used in routine practice?
Dr. Delaloge: They first appeared around 10 years ago. Development of these tests has intensified in recent years. There are numerous research laboratories, both public and private, that are developing different early-detection tests for cancer.
Some of these development processes are about to come to an end and are expected to be in regular, concrete use within 5-10 years. For the most advanced developments, the main biologic material researched and analyzed is DNA from cancer cells. We all have fragments of DNA from dead cells in our plasma (apoptosis), but cancer cells release more of these than others, and most importantly, their DNA has distinctive characteristics. The idea is to develop tests capable of detecting these characteristics.
Liquid biopsies based on genomic biomarkers could make MCED a reality, especially for cancers for which there is no standard screening process. But at this stage of the research, there are limitations, including low sensitivity for detecting stage I cancers in validation studies and an increased risk for overdiagnosis.
Q: What specific set of characteristics are the most advanced approaches based on?
Dr. Delaloge: They’re based on the analysis of DNA methylation, a biological process by which CH3 methyl groups are added to the DNA molecule and that determines gene expression. This phenomenon differs depending on whether the cell is cancerous. Among the tests currently under development making use of this specific characteristic is the Galleri test, which is the most advanced of them all.
A previous British National Health Service study, SYMPLIFY, which was published in 2023 by researchers at the University of Oxford, was conducted in symptomatic patients attending a health center. It offers promising results in a diagnostic situation. It has nothing at all to do with screening here. A large, randomized English study, NHS-Galleri, is underway, this time involving the general population, with the aim of assessing the potential benefit of the same test as screening in 140,000 people between ages 50 and 77 years.
In the SYMPLIFY study, which was carried out in symptomatic patients attending a health center, the Galleri MCED test had a positive predictive value of 75.5%, a negative predictive value of 97.6%, a sensitivity of 66.3%, and a specificity of 98.4%. Sensitivity increased with age and cancer stage from 24.2% at stage I to 95.3% at stage IV. For cases for which a cancer signal was detected in patients with cancer, the prediction of the original site of the cancer by the MCED test was accurate in 85.2% of cases. This large-scale prospective evaluation of an MCED diagnostic test confirms its feasibility in a symptomatic population but is not yet sufficiently accurate to “confirm or rule out the presence of cancer.” According to the authors, “in cases in which the MCED test detects a cancer signal in this context, the probability of a diagnosis of cancer being made is considerably higher and may identify cancers at sites other than those suspected during the initial referral phase, thus reducing delays in diagnosis.” A negative test means a lower likelihood of cancer but not so low that proper investigation can be ruled out. Further tests will be needed to optimize use of a negative predictive value.
Q: Does MCED testing concern all types of cancer?
Dr. Delaloge: The Galleri test is based on full profiling of DNA methylation. This allows for early diagnosis of cancer even before it can be seen on imaging tests. The issue with these tests is that they aren’t that good at early diagnosis of the most common types of cancer (breast, colorectal, cervical, etc.) for which we already have more efficient means such as the fecal immunochemical test for colorectal cancer, mammography, HPV testing, and so on.
These blood tests would thus not be aimed at replacing routine screening but rather at screening asymptomatic individuals or those with nonspecific signs for cancers for which we have few or no screening measures and which are on the rise, such as deep tumors and cancer diagnosed at a late stage, namely pancreas, bile duct, ovarian, esophageal, lung, stomach, etc.
The results from the studies published are promising, but others are underway to confirm the benefit of these MCEDs. The challenge is to identify cancer at an early stage, at a stage where it will be easier to cure the patient and control its growth using treatments that are less onerous for the patient and that have fewer aftereffects but not at the expense of a massive increase in overdiagnosis, as seen with prostate-specific antigen levels in prostate cancer a few years ago!
Q: What would be the focus of these MCED tests?
Dr. Delaloge: We must be alert to the risk for the market development of MCED tests. For now, they are mostly, especially the Galleri test, developed in the general population to screen for types of cancer that could not be detected in any other way but also because it’s the most financially beneficial situation. The designers want to position themselves in the general population, regardless of whether this means they’ll have to test hundreds of people to find one for whom the test is beneficial. What’s more, developing tests in isolation, without considering their place in ad hoc treatment pathways, is not realistic. It’s likely that some of these tests will be marketed within the next 10 years, but the health care systems destined to receive them are not remotely ready to do so.
Q: An even more recent publication, from late July 2023, is even more exciting in relation to early detection of lung cancer using circulating DNA sequencing. What are your thoughts on it?
Dr. Delaloge: Initially overtaken by other technologies in favor of MCED approaches, DNA sequencing as a technique to detect somatic mutations seems to have reentered the competition with this new-generation research. The authors published some very interesting results, especially for stage I lung cancer with a very high sensitivity of 75%. [Editor’s note: A machine-learning model using genome-wide mutational profiles combined with other features and followed by CT imaging detected more than 90% of patients with lung cancer, including those with stage I and II disease.]
This research illustrates the difficulty of providing high performance while covering a broad range of cancers. Here, the good results mainly concern lung cancer. Researchers and health care authorities must be alert to ensuring that MCED tests prove themselves in terms of sensitivity and specificity in responding to a medical need and in their impact on specific mortality. This craze for MCED tests must not hinder the development of “single-cancer” technologies that may be much better for detecting specific cancers. This recent publication is interesting in this respect, because this sequencing test seems to be particularly good at detecting lung cancer.
Q: Another approach used in MCED tests is based on analyzing the size of DNA fragments in the blood. Can you explain how this works?
Dr. Delaloge: When cancer is not present, the size of DNA fragments in cells is much more homogeneous. Here also, the benefit of MCED based on this technique rests on the very early detection of cancers that are less common than those for which we already have good screening methods available.
Other approaches, still at the experimental stage, detect certain proteins, certain inflammatory molecules, RNA, etc. But for many researchers, the future will involve pairing tests on the basis of circulating DNA in the blood with the detection of specific molecules indicating the presence of cancer to obtain early screening tests that are even more effective or that possibly even allow us to identify an appropriate treatment at an early stage.
The development of a simple test based on a blood draw that allows us to screen early for all cancers and that would replace all current screening measures is, therefore, not imminent, although it could potentially be on the horizon in years to come. Alongside this, an important issue is the benefit of cancer screening in the general population vs. in a targeted population with a specific risk. The latter option is in development but requires an individualized screening pathway based on blood testing and current screening methods: imaging, etc. It also depends on an individual’s cancer risk profile such as age, personal and family medical history, genetic predisposition, and so on.
According to recent modeling, these multicancer tests could theoretically prevent a minimum of 2,000 deaths from cancer per 100,000 people between ages 50 and 79 years screened per year (17% fewer deaths from cancer per year).
This article was translated from the Medscape French Edition. A version appeared on Medscape.com.
Suzette Delaloge, MD, MSc, oncologist, breast cancer specialist, and director of the individualized cancer prevention program (Interception) at the Gustave Roussy Institute in Villejuif, France, looks into these “liquid biopsies” and shares her reservations about their potential marketing, especially to the organized care plans.
Question: What are the general principles underpinning these MCED tests?
Suzette Delaloge, MD, MSc: Despite their specificities, the general idea is to detect certain cancer markers in various body fluids (blood, urine, saliva, etc.), for example, molecules released by cancer cells (cytokines, inflammatory proteins, leptin, etc.) or distinctive features of the DNA in tumor cells. In blood, these molecules can be found in plasma or in serum. In urine, it’s more about detecting kidney, bladder, and urinary tract cancers.
Q: What sort of time frame are we looking at for these MCED tests to be used in routine practice?
Dr. Delaloge: They first appeared around 10 years ago. Development of these tests has intensified in recent years. There are numerous research laboratories, both public and private, that are developing different early-detection tests for cancer.
Some of these development processes are about to come to an end and are expected to be in regular, concrete use within 5-10 years. For the most advanced developments, the main biologic material researched and analyzed is DNA from cancer cells. We all have fragments of DNA from dead cells in our plasma (apoptosis), but cancer cells release more of these than others, and most importantly, their DNA has distinctive characteristics. The idea is to develop tests capable of detecting these characteristics.
Liquid biopsies based on genomic biomarkers could make MCED a reality, especially for cancers for which there is no standard screening process. But at this stage of the research, there are limitations, including low sensitivity for detecting stage I cancers in validation studies and an increased risk for overdiagnosis.
Q: What specific set of characteristics are the most advanced approaches based on?
Dr. Delaloge: They’re based on the analysis of DNA methylation, a biological process by which CH3 methyl groups are added to the DNA molecule and that determines gene expression. This phenomenon differs depending on whether the cell is cancerous. Among the tests currently under development making use of this specific characteristic is the Galleri test, which is the most advanced of them all.
A previous British National Health Service study, SYMPLIFY, which was published in 2023 by researchers at the University of Oxford, was conducted in symptomatic patients attending a health center. It offers promising results in a diagnostic situation. It has nothing at all to do with screening here. A large, randomized English study, NHS-Galleri, is underway, this time involving the general population, with the aim of assessing the potential benefit of the same test as screening in 140,000 people between ages 50 and 77 years.
In the SYMPLIFY study, which was carried out in symptomatic patients attending a health center, the Galleri MCED test had a positive predictive value of 75.5%, a negative predictive value of 97.6%, a sensitivity of 66.3%, and a specificity of 98.4%. Sensitivity increased with age and cancer stage from 24.2% at stage I to 95.3% at stage IV. For cases for which a cancer signal was detected in patients with cancer, the prediction of the original site of the cancer by the MCED test was accurate in 85.2% of cases. This large-scale prospective evaluation of an MCED diagnostic test confirms its feasibility in a symptomatic population but is not yet sufficiently accurate to “confirm or rule out the presence of cancer.” According to the authors, “in cases in which the MCED test detects a cancer signal in this context, the probability of a diagnosis of cancer being made is considerably higher and may identify cancers at sites other than those suspected during the initial referral phase, thus reducing delays in diagnosis.” A negative test means a lower likelihood of cancer but not so low that proper investigation can be ruled out. Further tests will be needed to optimize use of a negative predictive value.
Q: Does MCED testing concern all types of cancer?
Dr. Delaloge: The Galleri test is based on full profiling of DNA methylation. This allows for early diagnosis of cancer even before it can be seen on imaging tests. The issue with these tests is that they aren’t that good at early diagnosis of the most common types of cancer (breast, colorectal, cervical, etc.) for which we already have more efficient means such as the fecal immunochemical test for colorectal cancer, mammography, HPV testing, and so on.
These blood tests would thus not be aimed at replacing routine screening but rather at screening asymptomatic individuals or those with nonspecific signs for cancers for which we have few or no screening measures and which are on the rise, such as deep tumors and cancer diagnosed at a late stage, namely pancreas, bile duct, ovarian, esophageal, lung, stomach, etc.
The results from the studies published are promising, but others are underway to confirm the benefit of these MCEDs. The challenge is to identify cancer at an early stage, at a stage where it will be easier to cure the patient and control its growth using treatments that are less onerous for the patient and that have fewer aftereffects but not at the expense of a massive increase in overdiagnosis, as seen with prostate-specific antigen levels in prostate cancer a few years ago!
Q: What would be the focus of these MCED tests?
Dr. Delaloge: We must be alert to the risk for the market development of MCED tests. For now, they are mostly, especially the Galleri test, developed in the general population to screen for types of cancer that could not be detected in any other way but also because it’s the most financially beneficial situation. The designers want to position themselves in the general population, regardless of whether this means they’ll have to test hundreds of people to find one for whom the test is beneficial. What’s more, developing tests in isolation, without considering their place in ad hoc treatment pathways, is not realistic. It’s likely that some of these tests will be marketed within the next 10 years, but the health care systems destined to receive them are not remotely ready to do so.
Q: An even more recent publication, from late July 2023, is even more exciting in relation to early detection of lung cancer using circulating DNA sequencing. What are your thoughts on it?
Dr. Delaloge: Initially overtaken by other technologies in favor of MCED approaches, DNA sequencing as a technique to detect somatic mutations seems to have reentered the competition with this new-generation research. The authors published some very interesting results, especially for stage I lung cancer with a very high sensitivity of 75%. [Editor’s note: A machine-learning model using genome-wide mutational profiles combined with other features and followed by CT imaging detected more than 90% of patients with lung cancer, including those with stage I and II disease.]
This research illustrates the difficulty of providing high performance while covering a broad range of cancers. Here, the good results mainly concern lung cancer. Researchers and health care authorities must be alert to ensuring that MCED tests prove themselves in terms of sensitivity and specificity in responding to a medical need and in their impact on specific mortality. This craze for MCED tests must not hinder the development of “single-cancer” technologies that may be much better for detecting specific cancers. This recent publication is interesting in this respect, because this sequencing test seems to be particularly good at detecting lung cancer.
Q: Another approach used in MCED tests is based on analyzing the size of DNA fragments in the blood. Can you explain how this works?
Dr. Delaloge: When cancer is not present, the size of DNA fragments in cells is much more homogeneous. Here also, the benefit of MCED based on this technique rests on the very early detection of cancers that are less common than those for which we already have good screening methods available.
Other approaches, still at the experimental stage, detect certain proteins, certain inflammatory molecules, RNA, etc. But for many researchers, the future will involve pairing tests on the basis of circulating DNA in the blood with the detection of specific molecules indicating the presence of cancer to obtain early screening tests that are even more effective or that possibly even allow us to identify an appropriate treatment at an early stage.
The development of a simple test based on a blood draw that allows us to screen early for all cancers and that would replace all current screening measures is, therefore, not imminent, although it could potentially be on the horizon in years to come. Alongside this, an important issue is the benefit of cancer screening in the general population vs. in a targeted population with a specific risk. The latter option is in development but requires an individualized screening pathway based on blood testing and current screening methods: imaging, etc. It also depends on an individual’s cancer risk profile such as age, personal and family medical history, genetic predisposition, and so on.
According to recent modeling, these multicancer tests could theoretically prevent a minimum of 2,000 deaths from cancer per 100,000 people between ages 50 and 79 years screened per year (17% fewer deaths from cancer per year).
This article was translated from the Medscape French Edition. A version appeared on Medscape.com.
Suzette Delaloge, MD, MSc, oncologist, breast cancer specialist, and director of the individualized cancer prevention program (Interception) at the Gustave Roussy Institute in Villejuif, France, looks into these “liquid biopsies” and shares her reservations about their potential marketing, especially to the organized care plans.
Question: What are the general principles underpinning these MCED tests?
Suzette Delaloge, MD, MSc: Despite their specificities, the general idea is to detect certain cancer markers in various body fluids (blood, urine, saliva, etc.), for example, molecules released by cancer cells (cytokines, inflammatory proteins, leptin, etc.) or distinctive features of the DNA in tumor cells. In blood, these molecules can be found in plasma or in serum. In urine, it’s more about detecting kidney, bladder, and urinary tract cancers.
Q: What sort of time frame are we looking at for these MCED tests to be used in routine practice?
Dr. Delaloge: They first appeared around 10 years ago. Development of these tests has intensified in recent years. There are numerous research laboratories, both public and private, that are developing different early-detection tests for cancer.
Some of these development processes are about to come to an end and are expected to be in regular, concrete use within 5-10 years. For the most advanced developments, the main biologic material researched and analyzed is DNA from cancer cells. We all have fragments of DNA from dead cells in our plasma (apoptosis), but cancer cells release more of these than others, and most importantly, their DNA has distinctive characteristics. The idea is to develop tests capable of detecting these characteristics.
Liquid biopsies based on genomic biomarkers could make MCED a reality, especially for cancers for which there is no standard screening process. But at this stage of the research, there are limitations, including low sensitivity for detecting stage I cancers in validation studies and an increased risk for overdiagnosis.
Q: What specific set of characteristics are the most advanced approaches based on?
Dr. Delaloge: They’re based on the analysis of DNA methylation, a biological process by which CH3 methyl groups are added to the DNA molecule and that determines gene expression. This phenomenon differs depending on whether the cell is cancerous. Among the tests currently under development making use of this specific characteristic is the Galleri test, which is the most advanced of them all.
A previous British National Health Service study, SYMPLIFY, which was published in 2023 by researchers at the University of Oxford, was conducted in symptomatic patients attending a health center. It offers promising results in a diagnostic situation. It has nothing at all to do with screening here. A large, randomized English study, NHS-Galleri, is underway, this time involving the general population, with the aim of assessing the potential benefit of the same test as screening in 140,000 people between ages 50 and 77 years.
In the SYMPLIFY study, which was carried out in symptomatic patients attending a health center, the Galleri MCED test had a positive predictive value of 75.5%, a negative predictive value of 97.6%, a sensitivity of 66.3%, and a specificity of 98.4%. Sensitivity increased with age and cancer stage from 24.2% at stage I to 95.3% at stage IV. For cases for which a cancer signal was detected in patients with cancer, the prediction of the original site of the cancer by the MCED test was accurate in 85.2% of cases. This large-scale prospective evaluation of an MCED diagnostic test confirms its feasibility in a symptomatic population but is not yet sufficiently accurate to “confirm or rule out the presence of cancer.” According to the authors, “in cases in which the MCED test detects a cancer signal in this context, the probability of a diagnosis of cancer being made is considerably higher and may identify cancers at sites other than those suspected during the initial referral phase, thus reducing delays in diagnosis.” A negative test means a lower likelihood of cancer but not so low that proper investigation can be ruled out. Further tests will be needed to optimize use of a negative predictive value.
Q: Does MCED testing concern all types of cancer?
Dr. Delaloge: The Galleri test is based on full profiling of DNA methylation. This allows for early diagnosis of cancer even before it can be seen on imaging tests. The issue with these tests is that they aren’t that good at early diagnosis of the most common types of cancer (breast, colorectal, cervical, etc.) for which we already have more efficient means such as the fecal immunochemical test for colorectal cancer, mammography, HPV testing, and so on.
These blood tests would thus not be aimed at replacing routine screening but rather at screening asymptomatic individuals or those with nonspecific signs for cancers for which we have few or no screening measures and which are on the rise, such as deep tumors and cancer diagnosed at a late stage, namely pancreas, bile duct, ovarian, esophageal, lung, stomach, etc.
The results from the studies published are promising, but others are underway to confirm the benefit of these MCEDs. The challenge is to identify cancer at an early stage, at a stage where it will be easier to cure the patient and control its growth using treatments that are less onerous for the patient and that have fewer aftereffects but not at the expense of a massive increase in overdiagnosis, as seen with prostate-specific antigen levels in prostate cancer a few years ago!
Q: What would be the focus of these MCED tests?
Dr. Delaloge: We must be alert to the risk for the market development of MCED tests. For now, they are mostly, especially the Galleri test, developed in the general population to screen for types of cancer that could not be detected in any other way but also because it’s the most financially beneficial situation. The designers want to position themselves in the general population, regardless of whether this means they’ll have to test hundreds of people to find one for whom the test is beneficial. What’s more, developing tests in isolation, without considering their place in ad hoc treatment pathways, is not realistic. It’s likely that some of these tests will be marketed within the next 10 years, but the health care systems destined to receive them are not remotely ready to do so.
Q: An even more recent publication, from late July 2023, is even more exciting in relation to early detection of lung cancer using circulating DNA sequencing. What are your thoughts on it?
Dr. Delaloge: Initially overtaken by other technologies in favor of MCED approaches, DNA sequencing as a technique to detect somatic mutations seems to have reentered the competition with this new-generation research. The authors published some very interesting results, especially for stage I lung cancer with a very high sensitivity of 75%. [Editor’s note: A machine-learning model using genome-wide mutational profiles combined with other features and followed by CT imaging detected more than 90% of patients with lung cancer, including those with stage I and II disease.]
This research illustrates the difficulty of providing high performance while covering a broad range of cancers. Here, the good results mainly concern lung cancer. Researchers and health care authorities must be alert to ensuring that MCED tests prove themselves in terms of sensitivity and specificity in responding to a medical need and in their impact on specific mortality. This craze for MCED tests must not hinder the development of “single-cancer” technologies that may be much better for detecting specific cancers. This recent publication is interesting in this respect, because this sequencing test seems to be particularly good at detecting lung cancer.
Q: Another approach used in MCED tests is based on analyzing the size of DNA fragments in the blood. Can you explain how this works?
Dr. Delaloge: When cancer is not present, the size of DNA fragments in cells is much more homogeneous. Here also, the benefit of MCED based on this technique rests on the very early detection of cancers that are less common than those for which we already have good screening methods available.
Other approaches, still at the experimental stage, detect certain proteins, certain inflammatory molecules, RNA, etc. But for many researchers, the future will involve pairing tests on the basis of circulating DNA in the blood with the detection of specific molecules indicating the presence of cancer to obtain early screening tests that are even more effective or that possibly even allow us to identify an appropriate treatment at an early stage.
The development of a simple test based on a blood draw that allows us to screen early for all cancers and that would replace all current screening measures is, therefore, not imminent, although it could potentially be on the horizon in years to come. Alongside this, an important issue is the benefit of cancer screening in the general population vs. in a targeted population with a specific risk. The latter option is in development but requires an individualized screening pathway based on blood testing and current screening methods: imaging, etc. It also depends on an individual’s cancer risk profile such as age, personal and family medical history, genetic predisposition, and so on.
According to recent modeling, these multicancer tests could theoretically prevent a minimum of 2,000 deaths from cancer per 100,000 people between ages 50 and 79 years screened per year (17% fewer deaths from cancer per year).
This article was translated from the Medscape French Edition. A version appeared on Medscape.com.