User login
First guideline on NGS testing in cancer, from ESMO
Recommendations on the use of next-generation sequencing (NGS) tests for patients with metastatic cancer have been issued by the European Society for Medical Oncology, the first recommendations of their kind to be published by any medical society.
“Until now, there were no recommendations from scientific societies on how to use this technique in daily clinical practice to profile metastatic cancers,” Fernanda Mosele, MD, medical oncologist, Gustave Roussy, Villejuif, France, said in a statement.
NGS testing is already used extensively in oncology, particularly in metastatic cancer, she noted. The technology is used to assess the sequence of DNA in genes from a tumor tissue sample. Numerous genes can be quickly sequenced at the same time at relatively low cost. The results provide information on mutations that are present, which, in turn, helps with deciding which treatments to use, including drugs targeting the identified mutations.
“Our intent is that they [the guidelines] will unify decision-making about how NGS should be used for patients with metastatic cancer,” Dr. Mosele said.
The recommendations were published online August 25 in Annals of Oncology.
Overall, ESMO recommends the use of tumor multigene NGS for non–small cell lung cancer (NSCLC), prostate cancer, ovarian cancer, and cholangiocarcinoma.
For other cancers, the authors said that NGS is not recommended in clinical practice but could be used for research purposes.
However, patients should be informed that it is unlikely that test results would benefit them much personally.
Physicians and patients may decide together to subject the tumor to mutational testing using a large panel of genes, provided testing doesn’t burden the health care system with additional costs.
“This recommendation acknowledges that a small number of patients could benefit from a drug because they have a rare mutation,” Joaquin Mateo, MD, chair of the ESMO working group, said in a statement.
“So beyond the cancers in which everyone should receive NGS, there is room for physicians and patients to discuss the pros and cons of ordering these tests,” he added.
ESMO also does not recommend the use of off-label drugs matched to any genomic alteration detected by NGS unless an access program and a decisional procedure have been developed, either regionally or nationally.
No need for NGS testing of other cancers
In contrast to NSCLC, “there is currently no need to perform tumor multigene NGS for patients with mBC [metastatic breast cancer] in the context of daily practice,” ESMO stated.
This is largely because somatic sequencing cannot fully substitute for germline testing for BRCA status, and other mutations, such as HER2, can be detected using immunohistochemistry (IHC).
The same can be said for patients with metastatic gastric cancer, inasmuch as detection of alterations can and should be done using cheaper testing methods, ESMO pointed out.
However, ESMO members still emphasized that it’s important to include patients with metastatic breast cancer in molecular screening programs as well as in clinical trials testing targeted agents.
Similarly, there is no need to test metastatic colorectal cancer (mCRC) using multigene NGS in daily practice, inasmuch as most level 1 alterations in mCRC can be determined by IHC or PCR.
However, NGS can be considered as an alternative to PCR-based tests in mCRC, provided NGS is not associated with additional cost.
ESMO again recommended that research centers include mCRC patients in molecular screening programs in order for them to have access to innovative clinical trial agents.
As for advanced prostate cancer, ESMO does recommend that clinicians perform NGS on tissue samples to assess the tumor’s mutational status, at least for the presence of BRCA1 and BRCA2 mutations, when patients have access to the poly (ADP-ribose) polymerase inhibitors for treatment.
The authors cautioned, however, that this strategy is unlikely to be cost-effective, so larger panels should be used only when there are specific agreements with payers.
Multigene NGS is also not recommended for patients with advanced pancreatic ductal adenocarcinoma (PDAC), although ESMO points out that it is the role of research centers to propose multigene sequencing for these patients in the context of molecular screening programs.
This is again to facilitate access to innovative drugs for these patients.
Similar to recommendations for patients with advanced PDAC, patients with advanced hepatocellular carcinoma (HCC) do not need to have tumor multigene NGS either.
Considering the high unmet needs of HCC patients, ESMO feels that research centers should propose multigene sequencing to patients with advanced HCC in the context of molecular screening programs.
In contrast, ESMO recommended that tumor multigene NGS be used to detect actionable alterations in patients with advanced cholangiocarcinoma.
Again, they predict that this strategy is unlikely to be cost-effective, so larger panels should only be used if a specific agreement is in place with payers.
ESMO also assessed the frequency of level 1 alterations in less frequent tumor types, including ovarian cancers. Because BRCA1 and BRCA2 somatic mutations in ovarian tumors have been associated with increased response to the PARP inhibitors, the use of multigene NGS is justified with this malignancy, ESMO states.
The authors also recommend that tumor mutational burden be determined in cervical cancer, moderately differentiated neuroendocrine tumors, salivary cancers, vulvar cancer, and thyroid cancers.
Dr. Mosele has disclosed no relevant financial relationships. Many coauthors have relationships with the pharmaceutical industry, as listed in the article.
This article first appeared on Medscape.com.
Recommendations on the use of next-generation sequencing (NGS) tests for patients with metastatic cancer have been issued by the European Society for Medical Oncology, the first recommendations of their kind to be published by any medical society.
“Until now, there were no recommendations from scientific societies on how to use this technique in daily clinical practice to profile metastatic cancers,” Fernanda Mosele, MD, medical oncologist, Gustave Roussy, Villejuif, France, said in a statement.
NGS testing is already used extensively in oncology, particularly in metastatic cancer, she noted. The technology is used to assess the sequence of DNA in genes from a tumor tissue sample. Numerous genes can be quickly sequenced at the same time at relatively low cost. The results provide information on mutations that are present, which, in turn, helps with deciding which treatments to use, including drugs targeting the identified mutations.
“Our intent is that they [the guidelines] will unify decision-making about how NGS should be used for patients with metastatic cancer,” Dr. Mosele said.
The recommendations were published online August 25 in Annals of Oncology.
Overall, ESMO recommends the use of tumor multigene NGS for non–small cell lung cancer (NSCLC), prostate cancer, ovarian cancer, and cholangiocarcinoma.
For other cancers, the authors said that NGS is not recommended in clinical practice but could be used for research purposes.
However, patients should be informed that it is unlikely that test results would benefit them much personally.
Physicians and patients may decide together to subject the tumor to mutational testing using a large panel of genes, provided testing doesn’t burden the health care system with additional costs.
“This recommendation acknowledges that a small number of patients could benefit from a drug because they have a rare mutation,” Joaquin Mateo, MD, chair of the ESMO working group, said in a statement.
“So beyond the cancers in which everyone should receive NGS, there is room for physicians and patients to discuss the pros and cons of ordering these tests,” he added.
ESMO also does not recommend the use of off-label drugs matched to any genomic alteration detected by NGS unless an access program and a decisional procedure have been developed, either regionally or nationally.
No need for NGS testing of other cancers
In contrast to NSCLC, “there is currently no need to perform tumor multigene NGS for patients with mBC [metastatic breast cancer] in the context of daily practice,” ESMO stated.
This is largely because somatic sequencing cannot fully substitute for germline testing for BRCA status, and other mutations, such as HER2, can be detected using immunohistochemistry (IHC).
The same can be said for patients with metastatic gastric cancer, inasmuch as detection of alterations can and should be done using cheaper testing methods, ESMO pointed out.
However, ESMO members still emphasized that it’s important to include patients with metastatic breast cancer in molecular screening programs as well as in clinical trials testing targeted agents.
Similarly, there is no need to test metastatic colorectal cancer (mCRC) using multigene NGS in daily practice, inasmuch as most level 1 alterations in mCRC can be determined by IHC or PCR.
However, NGS can be considered as an alternative to PCR-based tests in mCRC, provided NGS is not associated with additional cost.
ESMO again recommended that research centers include mCRC patients in molecular screening programs in order for them to have access to innovative clinical trial agents.
As for advanced prostate cancer, ESMO does recommend that clinicians perform NGS on tissue samples to assess the tumor’s mutational status, at least for the presence of BRCA1 and BRCA2 mutations, when patients have access to the poly (ADP-ribose) polymerase inhibitors for treatment.
The authors cautioned, however, that this strategy is unlikely to be cost-effective, so larger panels should be used only when there are specific agreements with payers.
Multigene NGS is also not recommended for patients with advanced pancreatic ductal adenocarcinoma (PDAC), although ESMO points out that it is the role of research centers to propose multigene sequencing for these patients in the context of molecular screening programs.
This is again to facilitate access to innovative drugs for these patients.
Similar to recommendations for patients with advanced PDAC, patients with advanced hepatocellular carcinoma (HCC) do not need to have tumor multigene NGS either.
Considering the high unmet needs of HCC patients, ESMO feels that research centers should propose multigene sequencing to patients with advanced HCC in the context of molecular screening programs.
In contrast, ESMO recommended that tumor multigene NGS be used to detect actionable alterations in patients with advanced cholangiocarcinoma.
Again, they predict that this strategy is unlikely to be cost-effective, so larger panels should only be used if a specific agreement is in place with payers.
ESMO also assessed the frequency of level 1 alterations in less frequent tumor types, including ovarian cancers. Because BRCA1 and BRCA2 somatic mutations in ovarian tumors have been associated with increased response to the PARP inhibitors, the use of multigene NGS is justified with this malignancy, ESMO states.
The authors also recommend that tumor mutational burden be determined in cervical cancer, moderately differentiated neuroendocrine tumors, salivary cancers, vulvar cancer, and thyroid cancers.
Dr. Mosele has disclosed no relevant financial relationships. Many coauthors have relationships with the pharmaceutical industry, as listed in the article.
This article first appeared on Medscape.com.
Recommendations on the use of next-generation sequencing (NGS) tests for patients with metastatic cancer have been issued by the European Society for Medical Oncology, the first recommendations of their kind to be published by any medical society.
“Until now, there were no recommendations from scientific societies on how to use this technique in daily clinical practice to profile metastatic cancers,” Fernanda Mosele, MD, medical oncologist, Gustave Roussy, Villejuif, France, said in a statement.
NGS testing is already used extensively in oncology, particularly in metastatic cancer, she noted. The technology is used to assess the sequence of DNA in genes from a tumor tissue sample. Numerous genes can be quickly sequenced at the same time at relatively low cost. The results provide information on mutations that are present, which, in turn, helps with deciding which treatments to use, including drugs targeting the identified mutations.
“Our intent is that they [the guidelines] will unify decision-making about how NGS should be used for patients with metastatic cancer,” Dr. Mosele said.
The recommendations were published online August 25 in Annals of Oncology.
Overall, ESMO recommends the use of tumor multigene NGS for non–small cell lung cancer (NSCLC), prostate cancer, ovarian cancer, and cholangiocarcinoma.
For other cancers, the authors said that NGS is not recommended in clinical practice but could be used for research purposes.
However, patients should be informed that it is unlikely that test results would benefit them much personally.
Physicians and patients may decide together to subject the tumor to mutational testing using a large panel of genes, provided testing doesn’t burden the health care system with additional costs.
“This recommendation acknowledges that a small number of patients could benefit from a drug because they have a rare mutation,” Joaquin Mateo, MD, chair of the ESMO working group, said in a statement.
“So beyond the cancers in which everyone should receive NGS, there is room for physicians and patients to discuss the pros and cons of ordering these tests,” he added.
ESMO also does not recommend the use of off-label drugs matched to any genomic alteration detected by NGS unless an access program and a decisional procedure have been developed, either regionally or nationally.
No need for NGS testing of other cancers
In contrast to NSCLC, “there is currently no need to perform tumor multigene NGS for patients with mBC [metastatic breast cancer] in the context of daily practice,” ESMO stated.
This is largely because somatic sequencing cannot fully substitute for germline testing for BRCA status, and other mutations, such as HER2, can be detected using immunohistochemistry (IHC).
The same can be said for patients with metastatic gastric cancer, inasmuch as detection of alterations can and should be done using cheaper testing methods, ESMO pointed out.
However, ESMO members still emphasized that it’s important to include patients with metastatic breast cancer in molecular screening programs as well as in clinical trials testing targeted agents.
Similarly, there is no need to test metastatic colorectal cancer (mCRC) using multigene NGS in daily practice, inasmuch as most level 1 alterations in mCRC can be determined by IHC or PCR.
However, NGS can be considered as an alternative to PCR-based tests in mCRC, provided NGS is not associated with additional cost.
ESMO again recommended that research centers include mCRC patients in molecular screening programs in order for them to have access to innovative clinical trial agents.
As for advanced prostate cancer, ESMO does recommend that clinicians perform NGS on tissue samples to assess the tumor’s mutational status, at least for the presence of BRCA1 and BRCA2 mutations, when patients have access to the poly (ADP-ribose) polymerase inhibitors for treatment.
The authors cautioned, however, that this strategy is unlikely to be cost-effective, so larger panels should be used only when there are specific agreements with payers.
Multigene NGS is also not recommended for patients with advanced pancreatic ductal adenocarcinoma (PDAC), although ESMO points out that it is the role of research centers to propose multigene sequencing for these patients in the context of molecular screening programs.
This is again to facilitate access to innovative drugs for these patients.
Similar to recommendations for patients with advanced PDAC, patients with advanced hepatocellular carcinoma (HCC) do not need to have tumor multigene NGS either.
Considering the high unmet needs of HCC patients, ESMO feels that research centers should propose multigene sequencing to patients with advanced HCC in the context of molecular screening programs.
In contrast, ESMO recommended that tumor multigene NGS be used to detect actionable alterations in patients with advanced cholangiocarcinoma.
Again, they predict that this strategy is unlikely to be cost-effective, so larger panels should only be used if a specific agreement is in place with payers.
ESMO also assessed the frequency of level 1 alterations in less frequent tumor types, including ovarian cancers. Because BRCA1 and BRCA2 somatic mutations in ovarian tumors have been associated with increased response to the PARP inhibitors, the use of multigene NGS is justified with this malignancy, ESMO states.
The authors also recommend that tumor mutational burden be determined in cervical cancer, moderately differentiated neuroendocrine tumors, salivary cancers, vulvar cancer, and thyroid cancers.
Dr. Mosele has disclosed no relevant financial relationships. Many coauthors have relationships with the pharmaceutical industry, as listed in the article.
This article first appeared on Medscape.com.
Immunotherapy should not be withheld because of sex, age, or PS
The improvement in survival in many cancer types that is seen with immune checkpoint inhibitors (ICIs), when compared to control therapies, is not affected by the patient’s sex, age, or Eastern Cooperative Oncology Group (ECOG) performance status (PS), according to a new meta-analysis.
Therefore, treatment with these immunotherapies should not be withheld on the basis of these factors, the authors concluded.
Asked whether there have been such instances of withholding ICIs, lead author Yucai Wang, MD, PhD, Mayo Clinic, Rochester, Minnesota, told Medscape Medical News: “We did this study solely based on scientific questions we had and not because we were seeing any bias at the moment in the use of ICIs.
“And we saw that the survival benefits were very similar across all of the categories [we analyzed], with a survival benefit of about 20% from immunotherapy across the board, which is clinically meaningful,” he added.
The study was published online August 7 in JAMA Network Open.
“The comparable survival advantage between patients of different sex, age, and ECOG PS may encourage more patients to receive ICI treatment regardless of cancer types, lines of therapy, agents of immunotherapy, and intervention therapies,” the authors commented.
Wang noted that there have been conflicting reports in the literature suggesting that male patients may benefit more from immunotherapy than female patients and that older patients may benefit more from the same treatment than younger patients.
However, there are also suggestions in the literature that women experience a stronger immune response than men and that, with aging, the immune system generally undergoes immunosenescence.
In addition, the PS of oncology patients has been implicated in how well patients respond to immunotherapy.
Wang noted that the findings of past studies have contradicted each other.
Findings of the Meta-Analysis
The meta-analysis included 37 randomized clinical trials that involved a total of 23,760 patients with a variety of advanced cancers. “Most of the trials were phase 3 (n = 34) and conduced for subsequent lines of therapy (n = 22),” the authors explained.
The most common cancers treated with an ICI were non–small cell lung cancer and melanoma.
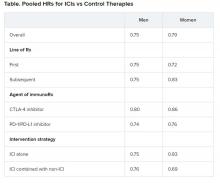
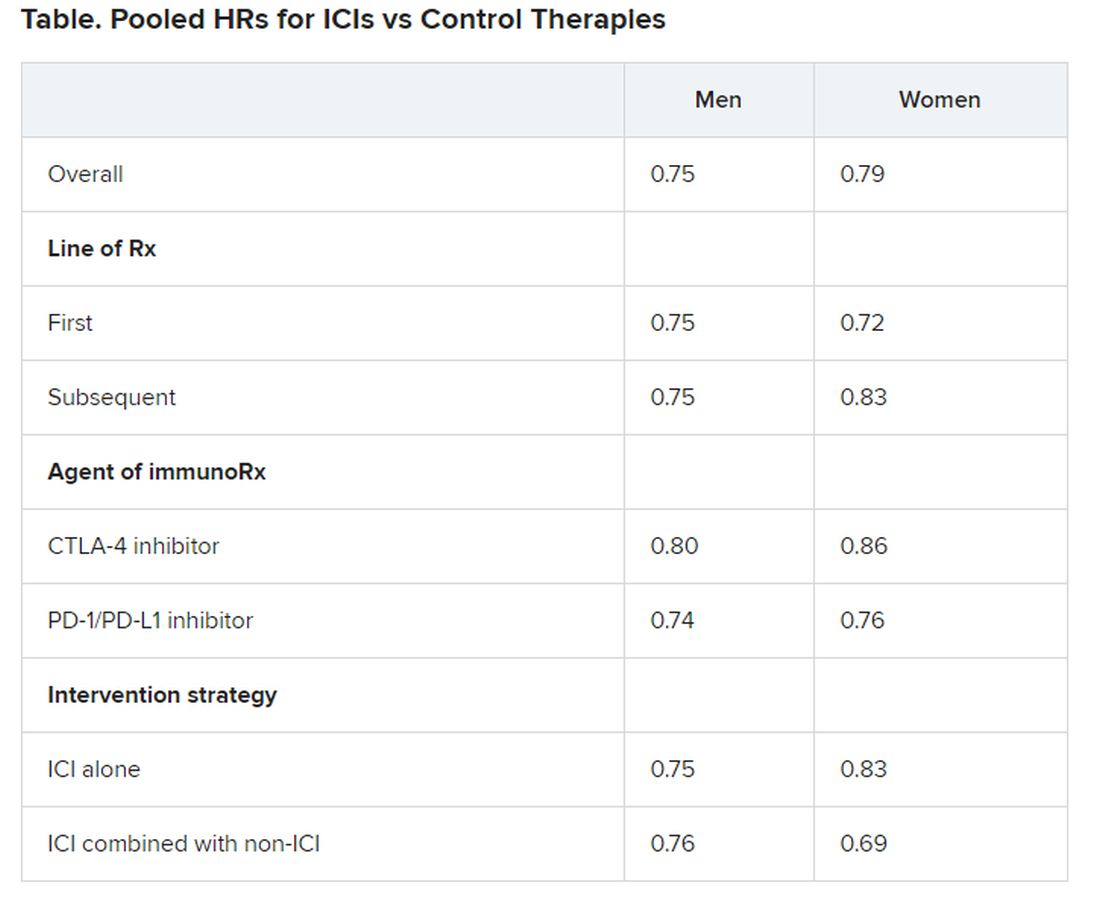
Pooled overall survival (OS) hazard ratios (HRs) were calculated on the basis of sex, age (younger than 65 years and 65 years and older), and an ECOG PS of 0 and 1 or higher.
Responses were stratified on the basis of cancer type, line of therapy, the ICI used, and the immunotherapy strategy used in the ICI arm.
Most of the drugs evaluated were PD-1 and PD-L1 inhibitors. The specific drugs assessed included ipilimumab, tremelimumab, nivolumab, pembrolizumab, atezolizumab, durvalumab, and avelumab.
A total of 32 trials that involved more than 20,000 patients reported HRs for death according to the patients’ sex. Thirty-four trials that involved more than 21,000 patients reported HRs for death according to patients’ age, and 30 trials that involved more than 19,000 patients reported HRs for death according to patients’ ECOG PS.
No significant differences in OS benefit were seen by cancer type, line of therapy, agent of immunotherapy, or intervention strategy, the investigators pointed out.
There were also no differences in survival benefit associated with immunotherapy vs control therapies for patients with an ECOG PS of 0 and an ECOG PS of 1 or greater. The OS benefit was 0.81 for those with an ECOG PS of 0 and 0.79 for those with an ECOG PS of 1 or greater.
Wang has disclosed no relevant financial relationships.
This article first appeared on Medscape.com .
The improvement in survival in many cancer types that is seen with immune checkpoint inhibitors (ICIs), when compared to control therapies, is not affected by the patient’s sex, age, or Eastern Cooperative Oncology Group (ECOG) performance status (PS), according to a new meta-analysis.
Therefore, treatment with these immunotherapies should not be withheld on the basis of these factors, the authors concluded.
Asked whether there have been such instances of withholding ICIs, lead author Yucai Wang, MD, PhD, Mayo Clinic, Rochester, Minnesota, told Medscape Medical News: “We did this study solely based on scientific questions we had and not because we were seeing any bias at the moment in the use of ICIs.
“And we saw that the survival benefits were very similar across all of the categories [we analyzed], with a survival benefit of about 20% from immunotherapy across the board, which is clinically meaningful,” he added.
The study was published online August 7 in JAMA Network Open.
“The comparable survival advantage between patients of different sex, age, and ECOG PS may encourage more patients to receive ICI treatment regardless of cancer types, lines of therapy, agents of immunotherapy, and intervention therapies,” the authors commented.
Wang noted that there have been conflicting reports in the literature suggesting that male patients may benefit more from immunotherapy than female patients and that older patients may benefit more from the same treatment than younger patients.
However, there are also suggestions in the literature that women experience a stronger immune response than men and that, with aging, the immune system generally undergoes immunosenescence.
In addition, the PS of oncology patients has been implicated in how well patients respond to immunotherapy.
Wang noted that the findings of past studies have contradicted each other.
Findings of the Meta-Analysis
The meta-analysis included 37 randomized clinical trials that involved a total of 23,760 patients with a variety of advanced cancers. “Most of the trials were phase 3 (n = 34) and conduced for subsequent lines of therapy (n = 22),” the authors explained.
The most common cancers treated with an ICI were non–small cell lung cancer and melanoma.
Pooled overall survival (OS) hazard ratios (HRs) were calculated on the basis of sex, age (younger than 65 years and 65 years and older), and an ECOG PS of 0 and 1 or higher.
Responses were stratified on the basis of cancer type, line of therapy, the ICI used, and the immunotherapy strategy used in the ICI arm.
Most of the drugs evaluated were PD-1 and PD-L1 inhibitors. The specific drugs assessed included ipilimumab, tremelimumab, nivolumab, pembrolizumab, atezolizumab, durvalumab, and avelumab.
A total of 32 trials that involved more than 20,000 patients reported HRs for death according to the patients’ sex. Thirty-four trials that involved more than 21,000 patients reported HRs for death according to patients’ age, and 30 trials that involved more than 19,000 patients reported HRs for death according to patients’ ECOG PS.
No significant differences in OS benefit were seen by cancer type, line of therapy, agent of immunotherapy, or intervention strategy, the investigators pointed out.
There were also no differences in survival benefit associated with immunotherapy vs control therapies for patients with an ECOG PS of 0 and an ECOG PS of 1 or greater. The OS benefit was 0.81 for those with an ECOG PS of 0 and 0.79 for those with an ECOG PS of 1 or greater.
Wang has disclosed no relevant financial relationships.
This article first appeared on Medscape.com .
The improvement in survival in many cancer types that is seen with immune checkpoint inhibitors (ICIs), when compared to control therapies, is not affected by the patient’s sex, age, or Eastern Cooperative Oncology Group (ECOG) performance status (PS), according to a new meta-analysis.
Therefore, treatment with these immunotherapies should not be withheld on the basis of these factors, the authors concluded.
Asked whether there have been such instances of withholding ICIs, lead author Yucai Wang, MD, PhD, Mayo Clinic, Rochester, Minnesota, told Medscape Medical News: “We did this study solely based on scientific questions we had and not because we were seeing any bias at the moment in the use of ICIs.
“And we saw that the survival benefits were very similar across all of the categories [we analyzed], with a survival benefit of about 20% from immunotherapy across the board, which is clinically meaningful,” he added.
The study was published online August 7 in JAMA Network Open.
“The comparable survival advantage between patients of different sex, age, and ECOG PS may encourage more patients to receive ICI treatment regardless of cancer types, lines of therapy, agents of immunotherapy, and intervention therapies,” the authors commented.
Wang noted that there have been conflicting reports in the literature suggesting that male patients may benefit more from immunotherapy than female patients and that older patients may benefit more from the same treatment than younger patients.
However, there are also suggestions in the literature that women experience a stronger immune response than men and that, with aging, the immune system generally undergoes immunosenescence.
In addition, the PS of oncology patients has been implicated in how well patients respond to immunotherapy.
Wang noted that the findings of past studies have contradicted each other.
Findings of the Meta-Analysis
The meta-analysis included 37 randomized clinical trials that involved a total of 23,760 patients with a variety of advanced cancers. “Most of the trials were phase 3 (n = 34) and conduced for subsequent lines of therapy (n = 22),” the authors explained.
The most common cancers treated with an ICI were non–small cell lung cancer and melanoma.
Pooled overall survival (OS) hazard ratios (HRs) were calculated on the basis of sex, age (younger than 65 years and 65 years and older), and an ECOG PS of 0 and 1 or higher.
Responses were stratified on the basis of cancer type, line of therapy, the ICI used, and the immunotherapy strategy used in the ICI arm.
Most of the drugs evaluated were PD-1 and PD-L1 inhibitors. The specific drugs assessed included ipilimumab, tremelimumab, nivolumab, pembrolizumab, atezolizumab, durvalumab, and avelumab.
A total of 32 trials that involved more than 20,000 patients reported HRs for death according to the patients’ sex. Thirty-four trials that involved more than 21,000 patients reported HRs for death according to patients’ age, and 30 trials that involved more than 19,000 patients reported HRs for death according to patients’ ECOG PS.
No significant differences in OS benefit were seen by cancer type, line of therapy, agent of immunotherapy, or intervention strategy, the investigators pointed out.
There were also no differences in survival benefit associated with immunotherapy vs control therapies for patients with an ECOG PS of 0 and an ECOG PS of 1 or greater. The OS benefit was 0.81 for those with an ECOG PS of 0 and 0.79 for those with an ECOG PS of 1 or greater.
Wang has disclosed no relevant financial relationships.
This article first appeared on Medscape.com .
Aspirin may accelerate cancer progression in older adults
Aspirin may accelerate the progression of advanced cancers and lead to an earlier death as a result, new data from the ASPREE study suggest.
The results showed that patients 65 years and older who started taking daily low-dose aspirin had a 19% higher chance of being diagnosed with metastatic cancer, a 22% higher chance of being diagnosed with a stage 4 tumor, and a 31% increased risk of death from stage 4 cancer, when compared with patients who took a placebo.
John J. McNeil, MBBS, PhD, of Monash University in Melbourne, Australia, and colleagues detailed these findings in the Journal of the National Cancer Institute.
“If confirmed, the clinical implications of these findings could be important for the use of aspirin in an older population,” the authors wrote.
When results of the ASPREE study were first reported in 2018, they “raised important concerns,” Ernest Hawk, MD, and Karen Colbert Maresso wrote in an editorial related to the current publication.
“Unlike ARRIVE, ASCEND, and nearly all prior primary prevention CVD [cardiovascular disease] trials of aspirin, ASPREE surprisingly demonstrated increased all-cause mortality in the aspirin group, which appeared to be driven largely by an increase in cancer-related deaths,” wrote the editorialists, who are both from the University of Texas MD Anderson Cancer Center in Houston.
Even though the ASPREE investigators have now taken a deeper dive into their data, the findings “neither explain nor alleviate the concerns raised by the initial ASPREE report,” the editorialists noted.
ASPREE design and results
ASPREE is a multicenter, double-blind trial of 19,114 older adults living in Australia (n = 16,703) or the United States (n = 2,411). Most patients were 70 years or older at baseline. However, the U.S. group also included patients 65 years and older who were racial/ethnic minorities (n = 564).
Patients were randomized to receive 100 mg of enteric-coated aspirin daily (n = 9,525) or matching placebo (n = 9,589) from March 2010 through December 2014.
At inclusion, all participants were free from cardiovascular disease, dementia, or physical disability. A previous history of cancer was not used to exclude participants, and 19.1% of patients had cancer at randomization. Most patients (89%) had not used aspirin regularly before entering the trial.
At a median follow-up of 4.7 years, there were 981 incident cancer events in the aspirin-treated group and 952 in the placebo-treated group, with an overall incident cancer rate of 10.1%.
Of the 1,933 patients with newly diagnosed cancer, 65.7% had a localized cancer, 18.8% had a new metastatic cancer, 5.8% had metastatic disease from an existing cancer, and 9.7% had a new hematologic or lymphatic cancer.
A quarter of cancer patients (n = 495) died as a result of their malignancy, with 52 dying from a cancer they already had at randomization.
Aspirin was not associated with the risk of first incident cancer diagnosis or incident localized cancer diagnosis. The hazard ratios were 1.04 for all incident cancers (95% confidence interval, 0.95-1.14) and 0.99 for incident localized cancers (95% CI, 0.89-1.11).
However, aspirin was associated with an increased risk of metastatic cancer and cancer presenting at stage 4. The HR for metastatic cancer was 1.19 (95% CI, 1.00-1.43), and the HR for newly diagnosed stage 4 cancer was 1.22 (95% CI, 1.02-1.45).
Furthermore, “an increased progression to death was observed amongst those randomized to aspirin, regardless of whether the initial cancer presentation had been localized or metastatic,” the investigators wrote.
The HRs for death were 1.35 for all cancers (95% CI, 1.13-1.61), 1.47 for localized cancers (95% CI, 1.07-2.02), and 1.30 for metastatic cancers (95% CI, 1.03-1.63).
“Deaths were particularly high among those on aspirin who were diagnosed with advanced solid cancers,” study author Andrew Chan, MD, of Massachusetts General Hospital in Boston, said in a press statement.
Indeed, HRs for death in patients with solid tumors presenting at stage 3 and 4 were a respective 2.11 (95% CI, 1.03-4.33) and 1.31 (95% CI, 1.04-1.64). This suggests a possible adverse effect of aspirin on the growth of cancers once they have already developed in older adults, Dr. Chan said.
Where does that leave aspirin for cancer prevention?
“Although these results suggest that we should be cautious about starting aspirin therapy in otherwise healthy older adults, this does not mean that individuals who are already taking aspirin – particularly if they began taking it at a younger age – should stop their aspirin regimen,” Dr. Chan said.
There are decades of data supporting the use of daily aspirin to prevent multiple cancer types, particularly colorectal cancer, in individuals under the age of 70 years. In a recent meta-analysis, for example, regular aspirin use was linked to a 27% reduced risk for colorectal cancer, a 33% reduced risk for squamous cell esophageal cancer, a 39% decreased risk for adenocarcinoma of the esophagus and gastric cardia, a 36% decreased risk for stomach cancer, a 38% decreased risk for hepatobiliary tract cancer, and a 22% decreased risk for pancreatic cancer.
While these figures are mostly based on observational and case-control studies, it “reaffirms the fact that, overall, when you look at all of the ages, that there is still a benefit of aspirin for cancer,” John Cuzick, PhD, of Queen Mary University of London (England), said in an interview.
In fact, the meta-analysis goes as far as suggesting that perhaps the dose of aspirin being used is too low, with the authors noting that there was a 35% risk reduction in colorectal cancer with a dose of 325 mg daily. That’s a new finding, Dr. Cuzick said.
He noted that the ASPREE study largely consists of patients 70 years of age or older, and the authors “draw some conclusions which we can’t ignore about potential safety.”
One of the safety concerns is the increased risk for gastrointestinal bleeding, which is why Dr. Cuzick and colleagues previously recommended caution in the use of aspirin to prevent cancer in elderly patients. The group published a study in 2015 that suggested a benefit of taking aspirin daily for 5-10 years in patients aged 50-65 years, but the risk/benefit ratio was unclear for patients 70 years and older.
The ASPREE data now add to those uncertainties and suggest “there may be some side effects that we do not understand,” Dr. Cuzick said.
“I’m still optimistic that aspirin is going to be important for cancer prevention, but probably focusing on ages 50-70,” he added. “[The ASPREE data] reinforce the caution that we have to take in terms of trying to understand what the side effects are and what’s going on at these older ages.”
Dr. Cuzick is currently leading the AsCaP Project, an international effort to better understand why aspirin might work in preventing some cancer types but not others. AsCaP is supported by Cancer Research UK and also includes Dr. Chan among the researchers attempting to find out which patients may benefit the most from aspirin and which may be at greater risk of adverse effects.
The ASPREE trial was funded by grants from the National Institute on Aging, the National Cancer Institute, the National Health and Medical Research Council of Australia, Monash University, and the Victorian Cancer Agency. Several ASPREE investigators disclosed financial relationships with Bayer Pharma. The editorialists had no conflicts of interest. Dr. Cuzick has been an advisory board member for Bayer in the past.
SOURCE: McNeil J et al. J Natl Cancer Inst. 2020 Aug 11. doi: 10.1093/jnci/djaa114.
Aspirin may accelerate the progression of advanced cancers and lead to an earlier death as a result, new data from the ASPREE study suggest.
The results showed that patients 65 years and older who started taking daily low-dose aspirin had a 19% higher chance of being diagnosed with metastatic cancer, a 22% higher chance of being diagnosed with a stage 4 tumor, and a 31% increased risk of death from stage 4 cancer, when compared with patients who took a placebo.
John J. McNeil, MBBS, PhD, of Monash University in Melbourne, Australia, and colleagues detailed these findings in the Journal of the National Cancer Institute.
“If confirmed, the clinical implications of these findings could be important for the use of aspirin in an older population,” the authors wrote.
When results of the ASPREE study were first reported in 2018, they “raised important concerns,” Ernest Hawk, MD, and Karen Colbert Maresso wrote in an editorial related to the current publication.
“Unlike ARRIVE, ASCEND, and nearly all prior primary prevention CVD [cardiovascular disease] trials of aspirin, ASPREE surprisingly demonstrated increased all-cause mortality in the aspirin group, which appeared to be driven largely by an increase in cancer-related deaths,” wrote the editorialists, who are both from the University of Texas MD Anderson Cancer Center in Houston.
Even though the ASPREE investigators have now taken a deeper dive into their data, the findings “neither explain nor alleviate the concerns raised by the initial ASPREE report,” the editorialists noted.
ASPREE design and results
ASPREE is a multicenter, double-blind trial of 19,114 older adults living in Australia (n = 16,703) or the United States (n = 2,411). Most patients were 70 years or older at baseline. However, the U.S. group also included patients 65 years and older who were racial/ethnic minorities (n = 564).
Patients were randomized to receive 100 mg of enteric-coated aspirin daily (n = 9,525) or matching placebo (n = 9,589) from March 2010 through December 2014.
At inclusion, all participants were free from cardiovascular disease, dementia, or physical disability. A previous history of cancer was not used to exclude participants, and 19.1% of patients had cancer at randomization. Most patients (89%) had not used aspirin regularly before entering the trial.
At a median follow-up of 4.7 years, there were 981 incident cancer events in the aspirin-treated group and 952 in the placebo-treated group, with an overall incident cancer rate of 10.1%.
Of the 1,933 patients with newly diagnosed cancer, 65.7% had a localized cancer, 18.8% had a new metastatic cancer, 5.8% had metastatic disease from an existing cancer, and 9.7% had a new hematologic or lymphatic cancer.
A quarter of cancer patients (n = 495) died as a result of their malignancy, with 52 dying from a cancer they already had at randomization.
Aspirin was not associated with the risk of first incident cancer diagnosis or incident localized cancer diagnosis. The hazard ratios were 1.04 for all incident cancers (95% confidence interval, 0.95-1.14) and 0.99 for incident localized cancers (95% CI, 0.89-1.11).
However, aspirin was associated with an increased risk of metastatic cancer and cancer presenting at stage 4. The HR for metastatic cancer was 1.19 (95% CI, 1.00-1.43), and the HR for newly diagnosed stage 4 cancer was 1.22 (95% CI, 1.02-1.45).
Furthermore, “an increased progression to death was observed amongst those randomized to aspirin, regardless of whether the initial cancer presentation had been localized or metastatic,” the investigators wrote.
The HRs for death were 1.35 for all cancers (95% CI, 1.13-1.61), 1.47 for localized cancers (95% CI, 1.07-2.02), and 1.30 for metastatic cancers (95% CI, 1.03-1.63).
“Deaths were particularly high among those on aspirin who were diagnosed with advanced solid cancers,” study author Andrew Chan, MD, of Massachusetts General Hospital in Boston, said in a press statement.
Indeed, HRs for death in patients with solid tumors presenting at stage 3 and 4 were a respective 2.11 (95% CI, 1.03-4.33) and 1.31 (95% CI, 1.04-1.64). This suggests a possible adverse effect of aspirin on the growth of cancers once they have already developed in older adults, Dr. Chan said.
Where does that leave aspirin for cancer prevention?
“Although these results suggest that we should be cautious about starting aspirin therapy in otherwise healthy older adults, this does not mean that individuals who are already taking aspirin – particularly if they began taking it at a younger age – should stop their aspirin regimen,” Dr. Chan said.
There are decades of data supporting the use of daily aspirin to prevent multiple cancer types, particularly colorectal cancer, in individuals under the age of 70 years. In a recent meta-analysis, for example, regular aspirin use was linked to a 27% reduced risk for colorectal cancer, a 33% reduced risk for squamous cell esophageal cancer, a 39% decreased risk for adenocarcinoma of the esophagus and gastric cardia, a 36% decreased risk for stomach cancer, a 38% decreased risk for hepatobiliary tract cancer, and a 22% decreased risk for pancreatic cancer.
While these figures are mostly based on observational and case-control studies, it “reaffirms the fact that, overall, when you look at all of the ages, that there is still a benefit of aspirin for cancer,” John Cuzick, PhD, of Queen Mary University of London (England), said in an interview.
In fact, the meta-analysis goes as far as suggesting that perhaps the dose of aspirin being used is too low, with the authors noting that there was a 35% risk reduction in colorectal cancer with a dose of 325 mg daily. That’s a new finding, Dr. Cuzick said.
He noted that the ASPREE study largely consists of patients 70 years of age or older, and the authors “draw some conclusions which we can’t ignore about potential safety.”
One of the safety concerns is the increased risk for gastrointestinal bleeding, which is why Dr. Cuzick and colleagues previously recommended caution in the use of aspirin to prevent cancer in elderly patients. The group published a study in 2015 that suggested a benefit of taking aspirin daily for 5-10 years in patients aged 50-65 years, but the risk/benefit ratio was unclear for patients 70 years and older.
The ASPREE data now add to those uncertainties and suggest “there may be some side effects that we do not understand,” Dr. Cuzick said.
“I’m still optimistic that aspirin is going to be important for cancer prevention, but probably focusing on ages 50-70,” he added. “[The ASPREE data] reinforce the caution that we have to take in terms of trying to understand what the side effects are and what’s going on at these older ages.”
Dr. Cuzick is currently leading the AsCaP Project, an international effort to better understand why aspirin might work in preventing some cancer types but not others. AsCaP is supported by Cancer Research UK and also includes Dr. Chan among the researchers attempting to find out which patients may benefit the most from aspirin and which may be at greater risk of adverse effects.
The ASPREE trial was funded by grants from the National Institute on Aging, the National Cancer Institute, the National Health and Medical Research Council of Australia, Monash University, and the Victorian Cancer Agency. Several ASPREE investigators disclosed financial relationships with Bayer Pharma. The editorialists had no conflicts of interest. Dr. Cuzick has been an advisory board member for Bayer in the past.
SOURCE: McNeil J et al. J Natl Cancer Inst. 2020 Aug 11. doi: 10.1093/jnci/djaa114.
Aspirin may accelerate the progression of advanced cancers and lead to an earlier death as a result, new data from the ASPREE study suggest.
The results showed that patients 65 years and older who started taking daily low-dose aspirin had a 19% higher chance of being diagnosed with metastatic cancer, a 22% higher chance of being diagnosed with a stage 4 tumor, and a 31% increased risk of death from stage 4 cancer, when compared with patients who took a placebo.
John J. McNeil, MBBS, PhD, of Monash University in Melbourne, Australia, and colleagues detailed these findings in the Journal of the National Cancer Institute.
“If confirmed, the clinical implications of these findings could be important for the use of aspirin in an older population,” the authors wrote.
When results of the ASPREE study were first reported in 2018, they “raised important concerns,” Ernest Hawk, MD, and Karen Colbert Maresso wrote in an editorial related to the current publication.
“Unlike ARRIVE, ASCEND, and nearly all prior primary prevention CVD [cardiovascular disease] trials of aspirin, ASPREE surprisingly demonstrated increased all-cause mortality in the aspirin group, which appeared to be driven largely by an increase in cancer-related deaths,” wrote the editorialists, who are both from the University of Texas MD Anderson Cancer Center in Houston.
Even though the ASPREE investigators have now taken a deeper dive into their data, the findings “neither explain nor alleviate the concerns raised by the initial ASPREE report,” the editorialists noted.
ASPREE design and results
ASPREE is a multicenter, double-blind trial of 19,114 older adults living in Australia (n = 16,703) or the United States (n = 2,411). Most patients were 70 years or older at baseline. However, the U.S. group also included patients 65 years and older who were racial/ethnic minorities (n = 564).
Patients were randomized to receive 100 mg of enteric-coated aspirin daily (n = 9,525) or matching placebo (n = 9,589) from March 2010 through December 2014.
At inclusion, all participants were free from cardiovascular disease, dementia, or physical disability. A previous history of cancer was not used to exclude participants, and 19.1% of patients had cancer at randomization. Most patients (89%) had not used aspirin regularly before entering the trial.
At a median follow-up of 4.7 years, there were 981 incident cancer events in the aspirin-treated group and 952 in the placebo-treated group, with an overall incident cancer rate of 10.1%.
Of the 1,933 patients with newly diagnosed cancer, 65.7% had a localized cancer, 18.8% had a new metastatic cancer, 5.8% had metastatic disease from an existing cancer, and 9.7% had a new hematologic or lymphatic cancer.
A quarter of cancer patients (n = 495) died as a result of their malignancy, with 52 dying from a cancer they already had at randomization.
Aspirin was not associated with the risk of first incident cancer diagnosis or incident localized cancer diagnosis. The hazard ratios were 1.04 for all incident cancers (95% confidence interval, 0.95-1.14) and 0.99 for incident localized cancers (95% CI, 0.89-1.11).
However, aspirin was associated with an increased risk of metastatic cancer and cancer presenting at stage 4. The HR for metastatic cancer was 1.19 (95% CI, 1.00-1.43), and the HR for newly diagnosed stage 4 cancer was 1.22 (95% CI, 1.02-1.45).
Furthermore, “an increased progression to death was observed amongst those randomized to aspirin, regardless of whether the initial cancer presentation had been localized or metastatic,” the investigators wrote.
The HRs for death were 1.35 for all cancers (95% CI, 1.13-1.61), 1.47 for localized cancers (95% CI, 1.07-2.02), and 1.30 for metastatic cancers (95% CI, 1.03-1.63).
“Deaths were particularly high among those on aspirin who were diagnosed with advanced solid cancers,” study author Andrew Chan, MD, of Massachusetts General Hospital in Boston, said in a press statement.
Indeed, HRs for death in patients with solid tumors presenting at stage 3 and 4 were a respective 2.11 (95% CI, 1.03-4.33) and 1.31 (95% CI, 1.04-1.64). This suggests a possible adverse effect of aspirin on the growth of cancers once they have already developed in older adults, Dr. Chan said.
Where does that leave aspirin for cancer prevention?
“Although these results suggest that we should be cautious about starting aspirin therapy in otherwise healthy older adults, this does not mean that individuals who are already taking aspirin – particularly if they began taking it at a younger age – should stop their aspirin regimen,” Dr. Chan said.
There are decades of data supporting the use of daily aspirin to prevent multiple cancer types, particularly colorectal cancer, in individuals under the age of 70 years. In a recent meta-analysis, for example, regular aspirin use was linked to a 27% reduced risk for colorectal cancer, a 33% reduced risk for squamous cell esophageal cancer, a 39% decreased risk for adenocarcinoma of the esophagus and gastric cardia, a 36% decreased risk for stomach cancer, a 38% decreased risk for hepatobiliary tract cancer, and a 22% decreased risk for pancreatic cancer.
While these figures are mostly based on observational and case-control studies, it “reaffirms the fact that, overall, when you look at all of the ages, that there is still a benefit of aspirin for cancer,” John Cuzick, PhD, of Queen Mary University of London (England), said in an interview.
In fact, the meta-analysis goes as far as suggesting that perhaps the dose of aspirin being used is too low, with the authors noting that there was a 35% risk reduction in colorectal cancer with a dose of 325 mg daily. That’s a new finding, Dr. Cuzick said.
He noted that the ASPREE study largely consists of patients 70 years of age or older, and the authors “draw some conclusions which we can’t ignore about potential safety.”
One of the safety concerns is the increased risk for gastrointestinal bleeding, which is why Dr. Cuzick and colleagues previously recommended caution in the use of aspirin to prevent cancer in elderly patients. The group published a study in 2015 that suggested a benefit of taking aspirin daily for 5-10 years in patients aged 50-65 years, but the risk/benefit ratio was unclear for patients 70 years and older.
The ASPREE data now add to those uncertainties and suggest “there may be some side effects that we do not understand,” Dr. Cuzick said.
“I’m still optimistic that aspirin is going to be important for cancer prevention, but probably focusing on ages 50-70,” he added. “[The ASPREE data] reinforce the caution that we have to take in terms of trying to understand what the side effects are and what’s going on at these older ages.”
Dr. Cuzick is currently leading the AsCaP Project, an international effort to better understand why aspirin might work in preventing some cancer types but not others. AsCaP is supported by Cancer Research UK and also includes Dr. Chan among the researchers attempting to find out which patients may benefit the most from aspirin and which may be at greater risk of adverse effects.
The ASPREE trial was funded by grants from the National Institute on Aging, the National Cancer Institute, the National Health and Medical Research Council of Australia, Monash University, and the Victorian Cancer Agency. Several ASPREE investigators disclosed financial relationships with Bayer Pharma. The editorialists had no conflicts of interest. Dr. Cuzick has been an advisory board member for Bayer in the past.
SOURCE: McNeil J et al. J Natl Cancer Inst. 2020 Aug 11. doi: 10.1093/jnci/djaa114.
FROM JOURNAL OF THE NATIONAL CANCER INSTITUTE
Age, smoking among leading cancer risk factors for SLE patients
A new study has quantified cancer risk factors in patients with systemic lupus erythematosus, including smoking and the use of certain medications.
“As expected, older age was associated with cancer overall, as well as with the most common cancer subtypes,” wrote Sasha Bernatsky, MD, PhD, of McGill University, Montreal, and coauthors. The study was published in Arthritis Care & Research.
To determine the risk of cancer in people with clinically confirmed incident systemic lupus erythematosus (SLE), the researchers analyzed data from 1,668 newly diagnosed lupus patients with at least one follow-up visit. All patients were enrolled in the Systemic Lupus International Collaborating Clinics inception cohort from across 33 different centers in North America, Europe, and Asia. A total of 89% (n = 1,480) were women, and 49% (n = 824) were white. The average follow-up period was 9 years.
Of the 1,668 SLE patients, 65 developed some type of cancer. The cancers included 15 breast;, 10 nonmelanoma skin; 7 lung; 6 hematologic, 6 prostate; 5 melanoma; 3 cervical; 3 renal; 2 gastric; 2 head and neck; 2 thyroid; and 1 rectal, sarcoma, thymoma, or uterine. No patient had more than one type, and the mean age of the cancer patients at time of SLE diagnosis was 45.6 (standard deviation, 14.5).
Almost half of the 65 cancers occurred in past or current smokers, including all of the lung cancers, while only 33% of patients without cancers smoked prior to baseline. After univariate analysis, characteristics associated with a higher risk of all cancers included older age at SLE diagnosis (adjusted hazard ratio, 1.05; 95% confidence interval, 1.03-1.06), White race/ethnicity (aHR 1.34; 95% CI, 0.76-2.37), and smoking (aHR 1.21; 95% CI, 0.73-2.01).
After multivariate analysis, the two characteristics most associated with increased cancer risk were older age at SLE diagnosis and being male. The analyses also confirmed that older age was a risk factor for breast cancer (aHR 1.06; 95% CI, 1.02-1.10) and nonmelanoma skin cancer (aHR, 1.06; 95% CI, 1.02-1.11), while use of antimalarial drugs was associated with a lower risk of both breast (aHR, 0.28; 95% CI, 0.09-0.90) and nonmelanoma skin (aHR, 0.23; 95% CI, 0.05-0.95) cancers. For lung cancer, the highest risk factor was smoking 15 or more cigarettes a day (aHR, 6.64; 95% CI, 1.43-30.9); for hematologic cancers, it was being in the top quartile of SLE disease activity (aHR, 7.14; 95% CI, 1.13-45.3).
The authors acknowledged their study’s limitations, including the small number of cancers overall and purposefully not comparing cancer risk in SLE patients with risk in the general population. Although their methods – “physicians recording events at annual visits, confirmed by review of charts” – were recognized as very suitable for the current analysis, they noted that a broader comparison would “potentially be problematic due to differential misclassification error” in cancer registry data.
Two of the study’s authors reported potential conflicts of interest, including receiving grants and consulting and personal fees from various pharmaceutical companies. No other potential conflicts were reported.
SOURCE: Bernatsky S et al. Arthritis Care Res. 2020 Aug 19. doi: 10.1002/acr.24425.
A new study has quantified cancer risk factors in patients with systemic lupus erythematosus, including smoking and the use of certain medications.
“As expected, older age was associated with cancer overall, as well as with the most common cancer subtypes,” wrote Sasha Bernatsky, MD, PhD, of McGill University, Montreal, and coauthors. The study was published in Arthritis Care & Research.
To determine the risk of cancer in people with clinically confirmed incident systemic lupus erythematosus (SLE), the researchers analyzed data from 1,668 newly diagnosed lupus patients with at least one follow-up visit. All patients were enrolled in the Systemic Lupus International Collaborating Clinics inception cohort from across 33 different centers in North America, Europe, and Asia. A total of 89% (n = 1,480) were women, and 49% (n = 824) were white. The average follow-up period was 9 years.
Of the 1,668 SLE patients, 65 developed some type of cancer. The cancers included 15 breast;, 10 nonmelanoma skin; 7 lung; 6 hematologic, 6 prostate; 5 melanoma; 3 cervical; 3 renal; 2 gastric; 2 head and neck; 2 thyroid; and 1 rectal, sarcoma, thymoma, or uterine. No patient had more than one type, and the mean age of the cancer patients at time of SLE diagnosis was 45.6 (standard deviation, 14.5).
Almost half of the 65 cancers occurred in past or current smokers, including all of the lung cancers, while only 33% of patients without cancers smoked prior to baseline. After univariate analysis, characteristics associated with a higher risk of all cancers included older age at SLE diagnosis (adjusted hazard ratio, 1.05; 95% confidence interval, 1.03-1.06), White race/ethnicity (aHR 1.34; 95% CI, 0.76-2.37), and smoking (aHR 1.21; 95% CI, 0.73-2.01).
After multivariate analysis, the two characteristics most associated with increased cancer risk were older age at SLE diagnosis and being male. The analyses also confirmed that older age was a risk factor for breast cancer (aHR 1.06; 95% CI, 1.02-1.10) and nonmelanoma skin cancer (aHR, 1.06; 95% CI, 1.02-1.11), while use of antimalarial drugs was associated with a lower risk of both breast (aHR, 0.28; 95% CI, 0.09-0.90) and nonmelanoma skin (aHR, 0.23; 95% CI, 0.05-0.95) cancers. For lung cancer, the highest risk factor was smoking 15 or more cigarettes a day (aHR, 6.64; 95% CI, 1.43-30.9); for hematologic cancers, it was being in the top quartile of SLE disease activity (aHR, 7.14; 95% CI, 1.13-45.3).
The authors acknowledged their study’s limitations, including the small number of cancers overall and purposefully not comparing cancer risk in SLE patients with risk in the general population. Although their methods – “physicians recording events at annual visits, confirmed by review of charts” – were recognized as very suitable for the current analysis, they noted that a broader comparison would “potentially be problematic due to differential misclassification error” in cancer registry data.
Two of the study’s authors reported potential conflicts of interest, including receiving grants and consulting and personal fees from various pharmaceutical companies. No other potential conflicts were reported.
SOURCE: Bernatsky S et al. Arthritis Care Res. 2020 Aug 19. doi: 10.1002/acr.24425.
A new study has quantified cancer risk factors in patients with systemic lupus erythematosus, including smoking and the use of certain medications.
“As expected, older age was associated with cancer overall, as well as with the most common cancer subtypes,” wrote Sasha Bernatsky, MD, PhD, of McGill University, Montreal, and coauthors. The study was published in Arthritis Care & Research.
To determine the risk of cancer in people with clinically confirmed incident systemic lupus erythematosus (SLE), the researchers analyzed data from 1,668 newly diagnosed lupus patients with at least one follow-up visit. All patients were enrolled in the Systemic Lupus International Collaborating Clinics inception cohort from across 33 different centers in North America, Europe, and Asia. A total of 89% (n = 1,480) were women, and 49% (n = 824) were white. The average follow-up period was 9 years.
Of the 1,668 SLE patients, 65 developed some type of cancer. The cancers included 15 breast;, 10 nonmelanoma skin; 7 lung; 6 hematologic, 6 prostate; 5 melanoma; 3 cervical; 3 renal; 2 gastric; 2 head and neck; 2 thyroid; and 1 rectal, sarcoma, thymoma, or uterine. No patient had more than one type, and the mean age of the cancer patients at time of SLE diagnosis was 45.6 (standard deviation, 14.5).
Almost half of the 65 cancers occurred in past or current smokers, including all of the lung cancers, while only 33% of patients without cancers smoked prior to baseline. After univariate analysis, characteristics associated with a higher risk of all cancers included older age at SLE diagnosis (adjusted hazard ratio, 1.05; 95% confidence interval, 1.03-1.06), White race/ethnicity (aHR 1.34; 95% CI, 0.76-2.37), and smoking (aHR 1.21; 95% CI, 0.73-2.01).
After multivariate analysis, the two characteristics most associated with increased cancer risk were older age at SLE diagnosis and being male. The analyses also confirmed that older age was a risk factor for breast cancer (aHR 1.06; 95% CI, 1.02-1.10) and nonmelanoma skin cancer (aHR, 1.06; 95% CI, 1.02-1.11), while use of antimalarial drugs was associated with a lower risk of both breast (aHR, 0.28; 95% CI, 0.09-0.90) and nonmelanoma skin (aHR, 0.23; 95% CI, 0.05-0.95) cancers. For lung cancer, the highest risk factor was smoking 15 or more cigarettes a day (aHR, 6.64; 95% CI, 1.43-30.9); for hematologic cancers, it was being in the top quartile of SLE disease activity (aHR, 7.14; 95% CI, 1.13-45.3).
The authors acknowledged their study’s limitations, including the small number of cancers overall and purposefully not comparing cancer risk in SLE patients with risk in the general population. Although their methods – “physicians recording events at annual visits, confirmed by review of charts” – were recognized as very suitable for the current analysis, they noted that a broader comparison would “potentially be problematic due to differential misclassification error” in cancer registry data.
Two of the study’s authors reported potential conflicts of interest, including receiving grants and consulting and personal fees from various pharmaceutical companies. No other potential conflicts were reported.
SOURCE: Bernatsky S et al. Arthritis Care Res. 2020 Aug 19. doi: 10.1002/acr.24425.
FROM ARTHRITIS CARE & RESEARCH
New hormonal medical treatment is an important advance for AUB caused by uterine fibroids
Uterine leiomyomata (fibroids) are the most common pelvic tumor diagnosed in women.1 Women with symptomatic fibroids often report abnormal uterine bleeding (AUB) and pelvic cramping, fullness, or pain. Fibroids also may cause frequency of urination and contribute to fertility and pregnancy problems. Treatment options for the AUB caused by fibroids include, but are not limited to, hysterectomy, myomectomy, uterine artery embolization, endometrial ablation, insertion of a levonorgestrel intrauterine device, focused ultrasound surgery, radiofrequency ablation, leuprolide acetate, and elagolix plus low-dose hormone add-back (Oriahnn; AbbVie, North Chicago, Illinois).1 Oriahnn is the most recent addition to our treatment armamentarium for fibroids and represents the first US Food and Drug Administration (FDA)-approved long-term hormonal option for AUB caused by fibroids.
Gene dysregulation contributes to fibroid development
Most uterine fibroids are clonal tumors, which develop following a somatic mutation in a precursor uterine myocyte. The somatic mutation causes gene dysregulation that stimulates cell growth resulting in a benign tumor mass. The majority of fibroids contain a mutation in one of the following 6 genes: mediator complex subunit 12 (MED12), high mobility group AT-hook (HMGA2 or HMGA1), RAD51B, fumarate hydratase (FH), collagen type IV, alpha 5 chain (COL4A5), or collagen type IV alpha 6 chain (COL4A6).2
Gene dysregulation in fibroids may arise following chromothripsis of the uterine myocyte genome
Chromothripsis is a catastrophic intracellular genetic event in which one or more chromosomes are broken and reassemble in a new nucleic acid sequence, producing a derivative chromosome that contains complex genetic rearrangements.3 Chromothripsis is believed to occur frequently in uterine myocytes. It is unknown why uterine myocytes are susceptible to chromothripsis,3 or why a catastrophic intracellular event such as chromothripsis results in preferential mutations in the 6 genes that are associated with myoma formation.
Estrogen and progesterone influence fibroid size and cell activity
Although uterine fibroids are clonal tumors containing broken genes, they are also exquisitely responsive to estradiol and progesterone. Estradiol and progesterone play an important role in regulating fibroid size and function.4 Estrogen stimulates uterine fibroids to increase in size. In a hypoestrogenic state, uterine fibroids decrease in size. In addition, a hypoestrogenic state results in an atrophic endometrium and thereby reduces AUB. For women with uterine fibroids and AUB, a reversible hypoestrogenic state can be induced either with a parenteral GnRH-agonist analogue (leuprolide) or an oral GnRH-antagonist (elagolix). Both leuprolide and elagolix are approved for the treatment of uterine fibroids (see below).
Surprisingly, progesterone stimulates cell division in normal uterine myocytes and fibroid cells.5 In the luteal phase of the menstrual cycle, uterine myocyte mitoses are more frequent than in the follicular phase. In addition, synthetic progestins appear to maintain fibroid size in a hypoestrogenic environment. In one randomized trial, women with uterine fibroids treated with leuprolide acetate plus a placebo pill for 24 weeks had a 51% reduction in uterine volume as measured by ultrasound.6 Women with uterine fibroids treated with leuprolide acetate plus the synthetic progestin, oral medroxyprogesterone acetate 20 mg daily, had only a 15% reduction in uterine volume.6 This finding suggests that synthetic progestins partially block the decrease in uterine volume that occurs in a hypoestrogenic state.
Further evidence that progesterone plays a role in fibroid biology is the observation that treatment of women with uterine fibroids with the antiprogestin ulipristal decreases fibroid size and reduces AUB.7-9 Ulipristal was approved for the treatment of fibroids in many countries but not the United States. Reports of severe, life-threatening liver injury—some necessitating liver transplantation—among women using ulipristal prompted the European Medicines Agency (EMA) in 2020 to recommend that women stop taking ulipristal. In addition, the EMA recommended that no woman should initiate ulipristal treatment at this time.10
Continue to: Leuprolide acetate...
Leuprolide acetate
Leuprolide acetate is a peptide GnRH-agonist analogue. Initiation of leuprolide treatment stimulates gonadotropin release, but with chronic administration pituitary secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) decreases, resulting in reduced ovarian follicular activity, anovulation, and low serum concentration of estradiol and progesterone. Leuprolide treatment concomitant with iron therapy is approved by the FDA for improving red blood cell volume prior to surgery in women with fibroids, AUB, and anemia.11 Among women with fibroids, AUB, and anemia, after 12 weeks of treatment, the hemoglobin concentration was ≥12 g/dL in 79% treated with leuprolide plus iron and 56% treated with iron alone.11 The FDA recommends limiting preoperative leuprolide treatment to no more than 3 months. The approved leuprolide regimens are a maximum of 3 monthly injections of leuprolide 3.75 mg or a single injection of leuprolide 11.25 mg. Leuprolide treatment prior to hysterectomy surgery for uterine fibroids usually will result in a decrease in uterine size and may facilitate vaginal hysterectomy.
Elagolix plus estradiol plus norethindrone acetate (Oriahnn)
GnRH analogues cause a hypoestrogenic state resulting in adverse effects, including moderate to severe hot flashes and a reduction in bone mineral density. One approach to reducing the unwanted effects of hot flashes and decreased bone density is to combine a GnRH analogue with low-dose steroid hormone add-back therapy. Combining a GnRH analogue with low-dose steroid hormone add-back permits long-term treatment of AUB caused by fibroids, with few hot flashes and a minimal decrease in bone mineral density. The FDA recently has approved the combination of elagolix plus low-dose estradiol and norethindrone acetate (Oriahnn) for the long-term treatment of AUB caused by fibroids.
Elagolix is a nonpeptide oral GnRH antagonist that reduces pituitary secretion of LH and FSH, resulting in a decrease in ovarian follicular activity, anovulation, and low serum concentration of estradiol and progesterone. Unlike leuprolide, which causes an initial increase in LH and FSH secretion, the initiation of elagolix treatment causes an immediate and sustained reduction in LH and FSH secretion. Combining elagolix with a low dose of estradiol and norethindrone acetate reduces the side effects of hot flashes and decreased bone density. Clinical trials have reported that the combination of elagolix (300 mg) twice daily plus estradiol (1 mg) and norethindrone acetate (0.5 mg) once daily is an effective long-term treatment of AUB caused by uterine fibroids.
To study the efficacy of elagolix (alone or with estrogen-progestin add-back therapy) for the treatment of AUB caused by uterine fibroids, two identical trials were performed,12 in which 790 women participated. The participants had a mean age of 42 years and were documented to have heavy menstrual bleeding (>80 mL blood loss per cycle) and ultrasound-diagnosed uterine fibroids. The participants were randomized to one of 3 groups:
- elagolix (300 mg twice daily) plus low-dose steroid add-back (1 mg estradiol and 0.5 mg norethindrone acetate once daily),
- elagolix 300 mg twice daily with no steroid add-back (elagolix alone), or
- placebo for 6 months.12
Menstrual blood loss was quantified using the alkaline hematin method on collected sanitary products. The primary endpoint was menstrual blood loss <80 mL per cycle as well as a ≥50% reduction in quantified blood loss from baseline during the final month of treatment. At 6 months, the percentage of women achieving the primary endpoint in the first trial was 84% (elagolix alone), 69% (elagolix plus add-back), and 9% (placebo). Mean changes from baseline in lumbar spine bone density were −2.95% (elagolix alone), −0.76% (elagolix plus add-back), and −0.21% (placebo). The percentage of women reporting hot flashes was 64% in the elagolix group, 20% in the elagolix plus low-dose steroid add-back group, and 9% in the placebo group. Results were similar in the second trial.12
The initial trials were extended to 12 months with two groups: elagolix 300 mg twice daily plus low-dose hormone add-back with 1 mg estradiol and 0.5 mg norethindrone acetate once daily (n = 218) or elagolix 300 mg twice daily (elagolix alone) (n = 98).13 Following 12 months of treatment, heavy menstrual bleeding was controlled in 88% and 89% of women treated with elagolix plus add-back and elagolix alone, respectively. Amenorrhea was reported by 65% of the women in the elagolix plus add-back group. Compared with baseline bone density, at the end of 12 months of treatment, bone mineral density in the lumbar spine was reduced by -1.5% and -4.8% in the women treated with elagolix plus add-back and elagolix alone, respectively. Compared with baseline bone density, at 1 year following completion of treatment, bone mineral density in the lumbar spine was reduced by -0.6% and -2.0% in the women treated with elagolix plus add-back and elagolix alone, respectively. Similar trends were observed in total hip and femoral neck bone density. During treatment with elagolix plus add-back, adverse effects were modest, including hot flushes (6%), night sweats (3.2%), headache (5.5%), and nausea (4.1%). Two women developed liver transaminase levels >3 times the upper limit of normal, resulting in one woman discontinuing treatment.13
Continue to: Contraindications to Oriahnn include known allergies...
Contraindications to Oriahnn include known allergies to the components of the medication (including the yellow dye tartrazine); high risk of arterial, venous thrombotic or thromboembolic disorders; pregnancy; known osteoporosis; current breast cancer or other hormonally-sensitive malignancies; known liver disease; and concurrent use of organic anion transporting polypeptide 1B1 inhibitors, which includes many HIV antiviral medications.14 Undiagnosed AUB is a contraindication, and all women prescribed Oriahnn should have endometrial sampling before initiating treatment. Oriahnn should not be used for more than 24 months due to the risk of irreversible bone loss.14 Systemic estrogen and progestin combinations, a component of Oriahnn, increases the risk for pulmonary embolism, deep vein thrombosis, stroke, and myocardial infarction, especially in women at increased risk for these events (such as women >35 years who smoke cigarettes and women with uncontrolled hypertension).14 In two studies there was a higher incidence of depression, depressed mood, and/or tearfulness in women taking Oriahnn (3%) compared with those taking a placebo (1%).14 The FDA recommends promptly evaluating women with depressive symptoms to determine the risks of initiating and continuing Oriahnn therapy. In two studies there was a higher risk of reported alopecia among women taking Oriahnn (3.5%) compared with placebo (1%).14
It should be noted that elagolix is approved for the treatment of pelvic pain caused by endometriosis at a dose of 150 mg daily for 24 months or 200 mg twice daily for 6 months. The elagolix dose for the treatment of AUB caused by fibroids is 300 mg twice daily for up to 24 months, necessitating the addition of low-dose estradiol-norethindrone add-back to reduce the frequency and severity of hot flashes and minimize the loss of bone density. Norethindrone acetate also protects the endometrium from the stimulatory effect of estradiol, reducing the risk of developing endometrial hyperplasia and cancer. Oriahnn is formulated as two different capsules. A yellow and white capsule contains elagolix 300 mg plus estradiol 1 mg and norethindrone acetate 0.5 mg to be taken in the morning, and a blue and white capsule contains elagolix 300 mg to be taken in the evening.
AUB caused by fibroids is a common problem in gyn practice
There are many procedural interventions that are effective in reducing AUB caused by fibroids. However, prior to the approval of Oriahnn there were no hormonal medications that were FDA approved for the long-term treatment of AUB caused by fibroids. Hence, Oriahnn represents an important advance in the hormonal treatment of AUB caused by fibroids and expands the treatment options available to our patients. ●
Black women are more likely to develop fibroids and experience more severe fibroid symptoms. Obstetrician-gynecologists are experts in the diagnosis and treatment of fibroids. We play a key role in partnering with Black women to reduce fibroid disease burden.
Factors that increase the risk of developing fibroids include: increasing age, Black race, nulliparity, early menarche (<10 years of age), obesity, and consumption of red meat.1 The Nurses Health Study II is the largest prospective study of the factors that influence fibroid development.2 A total of 95,061 premenopausal nurses aged 25 to 44 years were followed from September 1989 through May 1993. Review of a sample of medical records demonstrated that the nurses participating in the study were reliable reporters of whether or not they had been diagnosed with fibroids. Based on a report of an ultrasound or hysterectomy diagnosis, the incidence rate for fibroids increased with age. Incidence rate per 1,000 women-years was 4.3 (age 25 to 29 years), 9.0 (30 to 34 years), 14.7 (age 35 to 39 years), and 22.5 (40 to 44 years). Compared with White race, Black race (but not Hispanic ethnicity or Asian race) was associated with an increased incidence of fibroids. Incidence rate per 1,000 women-years was 12.5 (White race), 37.9 (Black race), 14.5 (Hispanic ethnicity), and 10.4 (Asian race). The risk of developing fibroids was 3.25 times (95% CI, 2.71 to 3.88) greater among Black compared with White women after controlling for body mass index, age at first birth, years since last birth, history of infertility, age at first oral contraceptive use, marital status, and current alcohol use.2
Other epidemiology studies also report an increased incidence of fibroids among Black women.3,4 The size of the uterus, the size and number of fibroids, and the severity of fibroid symptoms are greater among Black versus White women.5,6 The molecular factors that increase fibroid incidence among Black women are unknown. Given the burden of fibroid disease among Black women, obstetrician-gynecologists are best positioned to ensure early diagnosis and to develop an effective follow-up and treatment plan for affected women.
References
1. Stewart EA, Laughlin-Tommaso SK, Catherino WH, et al. Uterine fibroids. Nat Rev Dis Primers. 2016;2:16043.
2. Marshall LM, Spiegelman D, Barbieri RL, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90:967-973.
3. Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
4. Brett KM, Marsh JV, Madans JH. Epidemiology of hysterectomy in the United States: demographic and reproductive factors in a nationally representative sample. J Womens Health. 1997;6:309-316.
5. Peddada SD, Laughlin SK, Miner K, et al. Growth of uterine leiomyomata among premenopausal black and white women. Proc Natl Acad Sci USA. 2008;105:1988719892.
6. Huyck KL, Panhuysen CI, Cuenco KT, et al. The impact of race as a risk factor for symptom severity and age at diagnosis of uterine leiomyomata among affected sisters. Am J Obstet Gynecol. 2008;198:168.e1-e9.
- Stewart EA. Uterine fibroids. N Engl J Med. 2015;372:1646-1655.
- Mehine M, Makinen N, Heinonen HR, et al. Genomics of uterine leiomyomas: insights from high-throughput sequencing. Fertil Steril. 2014;102:621-629.
- Mehine M, Kaasinen E, Makinen N, et al. Characterization of uterine leiomyomas by whole-genome sequencing. N Engl J Med. 2013;369:43-53.
- Moravek MB, Bulun SE. Endocrinology of uterine fibroids: steroid hormones, stem cells and genetic contribution. Curr Opin Obstet Gynecol. 2015;27:276-283.
- Rein MS. Advances in uterine leiomyoma research: the progesterone hypothesis. Environ Health Perspect. 2000;108(suppl 5):791-793.
- Friedman AJ, Barbieri RL, Doubilet PM, et al. A randomized double-blind trial of a gonadotropin-releasing hormone agonist (leuprolide) with or without medroxyprogesterone acetate in the treatment of leiomyomata uteri. Fertil Steril. 1988;49:404-409.
- Donnez J, Hudecek R, Donnez O, et al. Efficacy and safety of repeated use of ulipristal acetate in uterine fibroids. Fertil Steril. 2015;103:519-527.
- Donnez J, Tatarchuk TF, Bouchard P, et al. Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med. 2012;366:409-420.
- Donnez J, Tomaszewski J, Vazquez F, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med. 2012;366:421-432.
- European Medicines Agency. Suspension of ulipristal acetate for uterine fibroids during ongoing EMA review of liver injury risk. March 13, 2020. https://www.ema.europa.eu/en/news/suspension-ulipristal-acetate-uterine-fibroids-during-ongoing-ema-review-liver-injury-risk#:~:text=EMA's%20safety%20committee%20(PRAC)%20has,the%20EU%20during%20the%20review. Accessed July 24, 2020.
- Lupron Depot [package insert]. Osaka, Japan: Takeda; Revised March 2012.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
- Simon JA, Al-Hendy A, Archer DF, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326.
- Oriahnn [package insert]. North Chicago, IL: AbbVie; 2020.
Uterine leiomyomata (fibroids) are the most common pelvic tumor diagnosed in women.1 Women with symptomatic fibroids often report abnormal uterine bleeding (AUB) and pelvic cramping, fullness, or pain. Fibroids also may cause frequency of urination and contribute to fertility and pregnancy problems. Treatment options for the AUB caused by fibroids include, but are not limited to, hysterectomy, myomectomy, uterine artery embolization, endometrial ablation, insertion of a levonorgestrel intrauterine device, focused ultrasound surgery, radiofrequency ablation, leuprolide acetate, and elagolix plus low-dose hormone add-back (Oriahnn; AbbVie, North Chicago, Illinois).1 Oriahnn is the most recent addition to our treatment armamentarium for fibroids and represents the first US Food and Drug Administration (FDA)-approved long-term hormonal option for AUB caused by fibroids.
Gene dysregulation contributes to fibroid development
Most uterine fibroids are clonal tumors, which develop following a somatic mutation in a precursor uterine myocyte. The somatic mutation causes gene dysregulation that stimulates cell growth resulting in a benign tumor mass. The majority of fibroids contain a mutation in one of the following 6 genes: mediator complex subunit 12 (MED12), high mobility group AT-hook (HMGA2 or HMGA1), RAD51B, fumarate hydratase (FH), collagen type IV, alpha 5 chain (COL4A5), or collagen type IV alpha 6 chain (COL4A6).2
Gene dysregulation in fibroids may arise following chromothripsis of the uterine myocyte genome
Chromothripsis is a catastrophic intracellular genetic event in which one or more chromosomes are broken and reassemble in a new nucleic acid sequence, producing a derivative chromosome that contains complex genetic rearrangements.3 Chromothripsis is believed to occur frequently in uterine myocytes. It is unknown why uterine myocytes are susceptible to chromothripsis,3 or why a catastrophic intracellular event such as chromothripsis results in preferential mutations in the 6 genes that are associated with myoma formation.
Estrogen and progesterone influence fibroid size and cell activity
Although uterine fibroids are clonal tumors containing broken genes, they are also exquisitely responsive to estradiol and progesterone. Estradiol and progesterone play an important role in regulating fibroid size and function.4 Estrogen stimulates uterine fibroids to increase in size. In a hypoestrogenic state, uterine fibroids decrease in size. In addition, a hypoestrogenic state results in an atrophic endometrium and thereby reduces AUB. For women with uterine fibroids and AUB, a reversible hypoestrogenic state can be induced either with a parenteral GnRH-agonist analogue (leuprolide) or an oral GnRH-antagonist (elagolix). Both leuprolide and elagolix are approved for the treatment of uterine fibroids (see below).
Surprisingly, progesterone stimulates cell division in normal uterine myocytes and fibroid cells.5 In the luteal phase of the menstrual cycle, uterine myocyte mitoses are more frequent than in the follicular phase. In addition, synthetic progestins appear to maintain fibroid size in a hypoestrogenic environment. In one randomized trial, women with uterine fibroids treated with leuprolide acetate plus a placebo pill for 24 weeks had a 51% reduction in uterine volume as measured by ultrasound.6 Women with uterine fibroids treated with leuprolide acetate plus the synthetic progestin, oral medroxyprogesterone acetate 20 mg daily, had only a 15% reduction in uterine volume.6 This finding suggests that synthetic progestins partially block the decrease in uterine volume that occurs in a hypoestrogenic state.
Further evidence that progesterone plays a role in fibroid biology is the observation that treatment of women with uterine fibroids with the antiprogestin ulipristal decreases fibroid size and reduces AUB.7-9 Ulipristal was approved for the treatment of fibroids in many countries but not the United States. Reports of severe, life-threatening liver injury—some necessitating liver transplantation—among women using ulipristal prompted the European Medicines Agency (EMA) in 2020 to recommend that women stop taking ulipristal. In addition, the EMA recommended that no woman should initiate ulipristal treatment at this time.10
Continue to: Leuprolide acetate...
Leuprolide acetate
Leuprolide acetate is a peptide GnRH-agonist analogue. Initiation of leuprolide treatment stimulates gonadotropin release, but with chronic administration pituitary secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) decreases, resulting in reduced ovarian follicular activity, anovulation, and low serum concentration of estradiol and progesterone. Leuprolide treatment concomitant with iron therapy is approved by the FDA for improving red blood cell volume prior to surgery in women with fibroids, AUB, and anemia.11 Among women with fibroids, AUB, and anemia, after 12 weeks of treatment, the hemoglobin concentration was ≥12 g/dL in 79% treated with leuprolide plus iron and 56% treated with iron alone.11 The FDA recommends limiting preoperative leuprolide treatment to no more than 3 months. The approved leuprolide regimens are a maximum of 3 monthly injections of leuprolide 3.75 mg or a single injection of leuprolide 11.25 mg. Leuprolide treatment prior to hysterectomy surgery for uterine fibroids usually will result in a decrease in uterine size and may facilitate vaginal hysterectomy.
Elagolix plus estradiol plus norethindrone acetate (Oriahnn)
GnRH analogues cause a hypoestrogenic state resulting in adverse effects, including moderate to severe hot flashes and a reduction in bone mineral density. One approach to reducing the unwanted effects of hot flashes and decreased bone density is to combine a GnRH analogue with low-dose steroid hormone add-back therapy. Combining a GnRH analogue with low-dose steroid hormone add-back permits long-term treatment of AUB caused by fibroids, with few hot flashes and a minimal decrease in bone mineral density. The FDA recently has approved the combination of elagolix plus low-dose estradiol and norethindrone acetate (Oriahnn) for the long-term treatment of AUB caused by fibroids.
Elagolix is a nonpeptide oral GnRH antagonist that reduces pituitary secretion of LH and FSH, resulting in a decrease in ovarian follicular activity, anovulation, and low serum concentration of estradiol and progesterone. Unlike leuprolide, which causes an initial increase in LH and FSH secretion, the initiation of elagolix treatment causes an immediate and sustained reduction in LH and FSH secretion. Combining elagolix with a low dose of estradiol and norethindrone acetate reduces the side effects of hot flashes and decreased bone density. Clinical trials have reported that the combination of elagolix (300 mg) twice daily plus estradiol (1 mg) and norethindrone acetate (0.5 mg) once daily is an effective long-term treatment of AUB caused by uterine fibroids.
To study the efficacy of elagolix (alone or with estrogen-progestin add-back therapy) for the treatment of AUB caused by uterine fibroids, two identical trials were performed,12 in which 790 women participated. The participants had a mean age of 42 years and were documented to have heavy menstrual bleeding (>80 mL blood loss per cycle) and ultrasound-diagnosed uterine fibroids. The participants were randomized to one of 3 groups:
- elagolix (300 mg twice daily) plus low-dose steroid add-back (1 mg estradiol and 0.5 mg norethindrone acetate once daily),
- elagolix 300 mg twice daily with no steroid add-back (elagolix alone), or
- placebo for 6 months.12
Menstrual blood loss was quantified using the alkaline hematin method on collected sanitary products. The primary endpoint was menstrual blood loss <80 mL per cycle as well as a ≥50% reduction in quantified blood loss from baseline during the final month of treatment. At 6 months, the percentage of women achieving the primary endpoint in the first trial was 84% (elagolix alone), 69% (elagolix plus add-back), and 9% (placebo). Mean changes from baseline in lumbar spine bone density were −2.95% (elagolix alone), −0.76% (elagolix plus add-back), and −0.21% (placebo). The percentage of women reporting hot flashes was 64% in the elagolix group, 20% in the elagolix plus low-dose steroid add-back group, and 9% in the placebo group. Results were similar in the second trial.12
The initial trials were extended to 12 months with two groups: elagolix 300 mg twice daily plus low-dose hormone add-back with 1 mg estradiol and 0.5 mg norethindrone acetate once daily (n = 218) or elagolix 300 mg twice daily (elagolix alone) (n = 98).13 Following 12 months of treatment, heavy menstrual bleeding was controlled in 88% and 89% of women treated with elagolix plus add-back and elagolix alone, respectively. Amenorrhea was reported by 65% of the women in the elagolix plus add-back group. Compared with baseline bone density, at the end of 12 months of treatment, bone mineral density in the lumbar spine was reduced by -1.5% and -4.8% in the women treated with elagolix plus add-back and elagolix alone, respectively. Compared with baseline bone density, at 1 year following completion of treatment, bone mineral density in the lumbar spine was reduced by -0.6% and -2.0% in the women treated with elagolix plus add-back and elagolix alone, respectively. Similar trends were observed in total hip and femoral neck bone density. During treatment with elagolix plus add-back, adverse effects were modest, including hot flushes (6%), night sweats (3.2%), headache (5.5%), and nausea (4.1%). Two women developed liver transaminase levels >3 times the upper limit of normal, resulting in one woman discontinuing treatment.13
Continue to: Contraindications to Oriahnn include known allergies...
Contraindications to Oriahnn include known allergies to the components of the medication (including the yellow dye tartrazine); high risk of arterial, venous thrombotic or thromboembolic disorders; pregnancy; known osteoporosis; current breast cancer or other hormonally-sensitive malignancies; known liver disease; and concurrent use of organic anion transporting polypeptide 1B1 inhibitors, which includes many HIV antiviral medications.14 Undiagnosed AUB is a contraindication, and all women prescribed Oriahnn should have endometrial sampling before initiating treatment. Oriahnn should not be used for more than 24 months due to the risk of irreversible bone loss.14 Systemic estrogen and progestin combinations, a component of Oriahnn, increases the risk for pulmonary embolism, deep vein thrombosis, stroke, and myocardial infarction, especially in women at increased risk for these events (such as women >35 years who smoke cigarettes and women with uncontrolled hypertension).14 In two studies there was a higher incidence of depression, depressed mood, and/or tearfulness in women taking Oriahnn (3%) compared with those taking a placebo (1%).14 The FDA recommends promptly evaluating women with depressive symptoms to determine the risks of initiating and continuing Oriahnn therapy. In two studies there was a higher risk of reported alopecia among women taking Oriahnn (3.5%) compared with placebo (1%).14
It should be noted that elagolix is approved for the treatment of pelvic pain caused by endometriosis at a dose of 150 mg daily for 24 months or 200 mg twice daily for 6 months. The elagolix dose for the treatment of AUB caused by fibroids is 300 mg twice daily for up to 24 months, necessitating the addition of low-dose estradiol-norethindrone add-back to reduce the frequency and severity of hot flashes and minimize the loss of bone density. Norethindrone acetate also protects the endometrium from the stimulatory effect of estradiol, reducing the risk of developing endometrial hyperplasia and cancer. Oriahnn is formulated as two different capsules. A yellow and white capsule contains elagolix 300 mg plus estradiol 1 mg and norethindrone acetate 0.5 mg to be taken in the morning, and a blue and white capsule contains elagolix 300 mg to be taken in the evening.
AUB caused by fibroids is a common problem in gyn practice
There are many procedural interventions that are effective in reducing AUB caused by fibroids. However, prior to the approval of Oriahnn there were no hormonal medications that were FDA approved for the long-term treatment of AUB caused by fibroids. Hence, Oriahnn represents an important advance in the hormonal treatment of AUB caused by fibroids and expands the treatment options available to our patients. ●
Black women are more likely to develop fibroids and experience more severe fibroid symptoms. Obstetrician-gynecologists are experts in the diagnosis and treatment of fibroids. We play a key role in partnering with Black women to reduce fibroid disease burden.
Factors that increase the risk of developing fibroids include: increasing age, Black race, nulliparity, early menarche (<10 years of age), obesity, and consumption of red meat.1 The Nurses Health Study II is the largest prospective study of the factors that influence fibroid development.2 A total of 95,061 premenopausal nurses aged 25 to 44 years were followed from September 1989 through May 1993. Review of a sample of medical records demonstrated that the nurses participating in the study were reliable reporters of whether or not they had been diagnosed with fibroids. Based on a report of an ultrasound or hysterectomy diagnosis, the incidence rate for fibroids increased with age. Incidence rate per 1,000 women-years was 4.3 (age 25 to 29 years), 9.0 (30 to 34 years), 14.7 (age 35 to 39 years), and 22.5 (40 to 44 years). Compared with White race, Black race (but not Hispanic ethnicity or Asian race) was associated with an increased incidence of fibroids. Incidence rate per 1,000 women-years was 12.5 (White race), 37.9 (Black race), 14.5 (Hispanic ethnicity), and 10.4 (Asian race). The risk of developing fibroids was 3.25 times (95% CI, 2.71 to 3.88) greater among Black compared with White women after controlling for body mass index, age at first birth, years since last birth, history of infertility, age at first oral contraceptive use, marital status, and current alcohol use.2
Other epidemiology studies also report an increased incidence of fibroids among Black women.3,4 The size of the uterus, the size and number of fibroids, and the severity of fibroid symptoms are greater among Black versus White women.5,6 The molecular factors that increase fibroid incidence among Black women are unknown. Given the burden of fibroid disease among Black women, obstetrician-gynecologists are best positioned to ensure early diagnosis and to develop an effective follow-up and treatment plan for affected women.
References
1. Stewart EA, Laughlin-Tommaso SK, Catherino WH, et al. Uterine fibroids. Nat Rev Dis Primers. 2016;2:16043.
2. Marshall LM, Spiegelman D, Barbieri RL, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90:967-973.
3. Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
4. Brett KM, Marsh JV, Madans JH. Epidemiology of hysterectomy in the United States: demographic and reproductive factors in a nationally representative sample. J Womens Health. 1997;6:309-316.
5. Peddada SD, Laughlin SK, Miner K, et al. Growth of uterine leiomyomata among premenopausal black and white women. Proc Natl Acad Sci USA. 2008;105:1988719892.
6. Huyck KL, Panhuysen CI, Cuenco KT, et al. The impact of race as a risk factor for symptom severity and age at diagnosis of uterine leiomyomata among affected sisters. Am J Obstet Gynecol. 2008;198:168.e1-e9.
Uterine leiomyomata (fibroids) are the most common pelvic tumor diagnosed in women.1 Women with symptomatic fibroids often report abnormal uterine bleeding (AUB) and pelvic cramping, fullness, or pain. Fibroids also may cause frequency of urination and contribute to fertility and pregnancy problems. Treatment options for the AUB caused by fibroids include, but are not limited to, hysterectomy, myomectomy, uterine artery embolization, endometrial ablation, insertion of a levonorgestrel intrauterine device, focused ultrasound surgery, radiofrequency ablation, leuprolide acetate, and elagolix plus low-dose hormone add-back (Oriahnn; AbbVie, North Chicago, Illinois).1 Oriahnn is the most recent addition to our treatment armamentarium for fibroids and represents the first US Food and Drug Administration (FDA)-approved long-term hormonal option for AUB caused by fibroids.
Gene dysregulation contributes to fibroid development
Most uterine fibroids are clonal tumors, which develop following a somatic mutation in a precursor uterine myocyte. The somatic mutation causes gene dysregulation that stimulates cell growth resulting in a benign tumor mass. The majority of fibroids contain a mutation in one of the following 6 genes: mediator complex subunit 12 (MED12), high mobility group AT-hook (HMGA2 or HMGA1), RAD51B, fumarate hydratase (FH), collagen type IV, alpha 5 chain (COL4A5), or collagen type IV alpha 6 chain (COL4A6).2
Gene dysregulation in fibroids may arise following chromothripsis of the uterine myocyte genome
Chromothripsis is a catastrophic intracellular genetic event in which one or more chromosomes are broken and reassemble in a new nucleic acid sequence, producing a derivative chromosome that contains complex genetic rearrangements.3 Chromothripsis is believed to occur frequently in uterine myocytes. It is unknown why uterine myocytes are susceptible to chromothripsis,3 or why a catastrophic intracellular event such as chromothripsis results in preferential mutations in the 6 genes that are associated with myoma formation.
Estrogen and progesterone influence fibroid size and cell activity
Although uterine fibroids are clonal tumors containing broken genes, they are also exquisitely responsive to estradiol and progesterone. Estradiol and progesterone play an important role in regulating fibroid size and function.4 Estrogen stimulates uterine fibroids to increase in size. In a hypoestrogenic state, uterine fibroids decrease in size. In addition, a hypoestrogenic state results in an atrophic endometrium and thereby reduces AUB. For women with uterine fibroids and AUB, a reversible hypoestrogenic state can be induced either with a parenteral GnRH-agonist analogue (leuprolide) or an oral GnRH-antagonist (elagolix). Both leuprolide and elagolix are approved for the treatment of uterine fibroids (see below).
Surprisingly, progesterone stimulates cell division in normal uterine myocytes and fibroid cells.5 In the luteal phase of the menstrual cycle, uterine myocyte mitoses are more frequent than in the follicular phase. In addition, synthetic progestins appear to maintain fibroid size in a hypoestrogenic environment. In one randomized trial, women with uterine fibroids treated with leuprolide acetate plus a placebo pill for 24 weeks had a 51% reduction in uterine volume as measured by ultrasound.6 Women with uterine fibroids treated with leuprolide acetate plus the synthetic progestin, oral medroxyprogesterone acetate 20 mg daily, had only a 15% reduction in uterine volume.6 This finding suggests that synthetic progestins partially block the decrease in uterine volume that occurs in a hypoestrogenic state.
Further evidence that progesterone plays a role in fibroid biology is the observation that treatment of women with uterine fibroids with the antiprogestin ulipristal decreases fibroid size and reduces AUB.7-9 Ulipristal was approved for the treatment of fibroids in many countries but not the United States. Reports of severe, life-threatening liver injury—some necessitating liver transplantation—among women using ulipristal prompted the European Medicines Agency (EMA) in 2020 to recommend that women stop taking ulipristal. In addition, the EMA recommended that no woman should initiate ulipristal treatment at this time.10
Continue to: Leuprolide acetate...
Leuprolide acetate
Leuprolide acetate is a peptide GnRH-agonist analogue. Initiation of leuprolide treatment stimulates gonadotropin release, but with chronic administration pituitary secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) decreases, resulting in reduced ovarian follicular activity, anovulation, and low serum concentration of estradiol and progesterone. Leuprolide treatment concomitant with iron therapy is approved by the FDA for improving red blood cell volume prior to surgery in women with fibroids, AUB, and anemia.11 Among women with fibroids, AUB, and anemia, after 12 weeks of treatment, the hemoglobin concentration was ≥12 g/dL in 79% treated with leuprolide plus iron and 56% treated with iron alone.11 The FDA recommends limiting preoperative leuprolide treatment to no more than 3 months. The approved leuprolide regimens are a maximum of 3 monthly injections of leuprolide 3.75 mg or a single injection of leuprolide 11.25 mg. Leuprolide treatment prior to hysterectomy surgery for uterine fibroids usually will result in a decrease in uterine size and may facilitate vaginal hysterectomy.
Elagolix plus estradiol plus norethindrone acetate (Oriahnn)
GnRH analogues cause a hypoestrogenic state resulting in adverse effects, including moderate to severe hot flashes and a reduction in bone mineral density. One approach to reducing the unwanted effects of hot flashes and decreased bone density is to combine a GnRH analogue with low-dose steroid hormone add-back therapy. Combining a GnRH analogue with low-dose steroid hormone add-back permits long-term treatment of AUB caused by fibroids, with few hot flashes and a minimal decrease in bone mineral density. The FDA recently has approved the combination of elagolix plus low-dose estradiol and norethindrone acetate (Oriahnn) for the long-term treatment of AUB caused by fibroids.
Elagolix is a nonpeptide oral GnRH antagonist that reduces pituitary secretion of LH and FSH, resulting in a decrease in ovarian follicular activity, anovulation, and low serum concentration of estradiol and progesterone. Unlike leuprolide, which causes an initial increase in LH and FSH secretion, the initiation of elagolix treatment causes an immediate and sustained reduction in LH and FSH secretion. Combining elagolix with a low dose of estradiol and norethindrone acetate reduces the side effects of hot flashes and decreased bone density. Clinical trials have reported that the combination of elagolix (300 mg) twice daily plus estradiol (1 mg) and norethindrone acetate (0.5 mg) once daily is an effective long-term treatment of AUB caused by uterine fibroids.
To study the efficacy of elagolix (alone or with estrogen-progestin add-back therapy) for the treatment of AUB caused by uterine fibroids, two identical trials were performed,12 in which 790 women participated. The participants had a mean age of 42 years and were documented to have heavy menstrual bleeding (>80 mL blood loss per cycle) and ultrasound-diagnosed uterine fibroids. The participants were randomized to one of 3 groups:
- elagolix (300 mg twice daily) plus low-dose steroid add-back (1 mg estradiol and 0.5 mg norethindrone acetate once daily),
- elagolix 300 mg twice daily with no steroid add-back (elagolix alone), or
- placebo for 6 months.12
Menstrual blood loss was quantified using the alkaline hematin method on collected sanitary products. The primary endpoint was menstrual blood loss <80 mL per cycle as well as a ≥50% reduction in quantified blood loss from baseline during the final month of treatment. At 6 months, the percentage of women achieving the primary endpoint in the first trial was 84% (elagolix alone), 69% (elagolix plus add-back), and 9% (placebo). Mean changes from baseline in lumbar spine bone density were −2.95% (elagolix alone), −0.76% (elagolix plus add-back), and −0.21% (placebo). The percentage of women reporting hot flashes was 64% in the elagolix group, 20% in the elagolix plus low-dose steroid add-back group, and 9% in the placebo group. Results were similar in the second trial.12
The initial trials were extended to 12 months with two groups: elagolix 300 mg twice daily plus low-dose hormone add-back with 1 mg estradiol and 0.5 mg norethindrone acetate once daily (n = 218) or elagolix 300 mg twice daily (elagolix alone) (n = 98).13 Following 12 months of treatment, heavy menstrual bleeding was controlled in 88% and 89% of women treated with elagolix plus add-back and elagolix alone, respectively. Amenorrhea was reported by 65% of the women in the elagolix plus add-back group. Compared with baseline bone density, at the end of 12 months of treatment, bone mineral density in the lumbar spine was reduced by -1.5% and -4.8% in the women treated with elagolix plus add-back and elagolix alone, respectively. Compared with baseline bone density, at 1 year following completion of treatment, bone mineral density in the lumbar spine was reduced by -0.6% and -2.0% in the women treated with elagolix plus add-back and elagolix alone, respectively. Similar trends were observed in total hip and femoral neck bone density. During treatment with elagolix plus add-back, adverse effects were modest, including hot flushes (6%), night sweats (3.2%), headache (5.5%), and nausea (4.1%). Two women developed liver transaminase levels >3 times the upper limit of normal, resulting in one woman discontinuing treatment.13
Continue to: Contraindications to Oriahnn include known allergies...
Contraindications to Oriahnn include known allergies to the components of the medication (including the yellow dye tartrazine); high risk of arterial, venous thrombotic or thromboembolic disorders; pregnancy; known osteoporosis; current breast cancer or other hormonally-sensitive malignancies; known liver disease; and concurrent use of organic anion transporting polypeptide 1B1 inhibitors, which includes many HIV antiviral medications.14 Undiagnosed AUB is a contraindication, and all women prescribed Oriahnn should have endometrial sampling before initiating treatment. Oriahnn should not be used for more than 24 months due to the risk of irreversible bone loss.14 Systemic estrogen and progestin combinations, a component of Oriahnn, increases the risk for pulmonary embolism, deep vein thrombosis, stroke, and myocardial infarction, especially in women at increased risk for these events (such as women >35 years who smoke cigarettes and women with uncontrolled hypertension).14 In two studies there was a higher incidence of depression, depressed mood, and/or tearfulness in women taking Oriahnn (3%) compared with those taking a placebo (1%).14 The FDA recommends promptly evaluating women with depressive symptoms to determine the risks of initiating and continuing Oriahnn therapy. In two studies there was a higher risk of reported alopecia among women taking Oriahnn (3.5%) compared with placebo (1%).14
It should be noted that elagolix is approved for the treatment of pelvic pain caused by endometriosis at a dose of 150 mg daily for 24 months or 200 mg twice daily for 6 months. The elagolix dose for the treatment of AUB caused by fibroids is 300 mg twice daily for up to 24 months, necessitating the addition of low-dose estradiol-norethindrone add-back to reduce the frequency and severity of hot flashes and minimize the loss of bone density. Norethindrone acetate also protects the endometrium from the stimulatory effect of estradiol, reducing the risk of developing endometrial hyperplasia and cancer. Oriahnn is formulated as two different capsules. A yellow and white capsule contains elagolix 300 mg plus estradiol 1 mg and norethindrone acetate 0.5 mg to be taken in the morning, and a blue and white capsule contains elagolix 300 mg to be taken in the evening.
AUB caused by fibroids is a common problem in gyn practice
There are many procedural interventions that are effective in reducing AUB caused by fibroids. However, prior to the approval of Oriahnn there were no hormonal medications that were FDA approved for the long-term treatment of AUB caused by fibroids. Hence, Oriahnn represents an important advance in the hormonal treatment of AUB caused by fibroids and expands the treatment options available to our patients. ●
Black women are more likely to develop fibroids and experience more severe fibroid symptoms. Obstetrician-gynecologists are experts in the diagnosis and treatment of fibroids. We play a key role in partnering with Black women to reduce fibroid disease burden.
Factors that increase the risk of developing fibroids include: increasing age, Black race, nulliparity, early menarche (<10 years of age), obesity, and consumption of red meat.1 The Nurses Health Study II is the largest prospective study of the factors that influence fibroid development.2 A total of 95,061 premenopausal nurses aged 25 to 44 years were followed from September 1989 through May 1993. Review of a sample of medical records demonstrated that the nurses participating in the study were reliable reporters of whether or not they had been diagnosed with fibroids. Based on a report of an ultrasound or hysterectomy diagnosis, the incidence rate for fibroids increased with age. Incidence rate per 1,000 women-years was 4.3 (age 25 to 29 years), 9.0 (30 to 34 years), 14.7 (age 35 to 39 years), and 22.5 (40 to 44 years). Compared with White race, Black race (but not Hispanic ethnicity or Asian race) was associated with an increased incidence of fibroids. Incidence rate per 1,000 women-years was 12.5 (White race), 37.9 (Black race), 14.5 (Hispanic ethnicity), and 10.4 (Asian race). The risk of developing fibroids was 3.25 times (95% CI, 2.71 to 3.88) greater among Black compared with White women after controlling for body mass index, age at first birth, years since last birth, history of infertility, age at first oral contraceptive use, marital status, and current alcohol use.2
Other epidemiology studies also report an increased incidence of fibroids among Black women.3,4 The size of the uterus, the size and number of fibroids, and the severity of fibroid symptoms are greater among Black versus White women.5,6 The molecular factors that increase fibroid incidence among Black women are unknown. Given the burden of fibroid disease among Black women, obstetrician-gynecologists are best positioned to ensure early diagnosis and to develop an effective follow-up and treatment plan for affected women.
References
1. Stewart EA, Laughlin-Tommaso SK, Catherino WH, et al. Uterine fibroids. Nat Rev Dis Primers. 2016;2:16043.
2. Marshall LM, Spiegelman D, Barbieri RL, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90:967-973.
3. Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
4. Brett KM, Marsh JV, Madans JH. Epidemiology of hysterectomy in the United States: demographic and reproductive factors in a nationally representative sample. J Womens Health. 1997;6:309-316.
5. Peddada SD, Laughlin SK, Miner K, et al. Growth of uterine leiomyomata among premenopausal black and white women. Proc Natl Acad Sci USA. 2008;105:1988719892.
6. Huyck KL, Panhuysen CI, Cuenco KT, et al. The impact of race as a risk factor for symptom severity and age at diagnosis of uterine leiomyomata among affected sisters. Am J Obstet Gynecol. 2008;198:168.e1-e9.
- Stewart EA. Uterine fibroids. N Engl J Med. 2015;372:1646-1655.
- Mehine M, Makinen N, Heinonen HR, et al. Genomics of uterine leiomyomas: insights from high-throughput sequencing. Fertil Steril. 2014;102:621-629.
- Mehine M, Kaasinen E, Makinen N, et al. Characterization of uterine leiomyomas by whole-genome sequencing. N Engl J Med. 2013;369:43-53.
- Moravek MB, Bulun SE. Endocrinology of uterine fibroids: steroid hormones, stem cells and genetic contribution. Curr Opin Obstet Gynecol. 2015;27:276-283.
- Rein MS. Advances in uterine leiomyoma research: the progesterone hypothesis. Environ Health Perspect. 2000;108(suppl 5):791-793.
- Friedman AJ, Barbieri RL, Doubilet PM, et al. A randomized double-blind trial of a gonadotropin-releasing hormone agonist (leuprolide) with or without medroxyprogesterone acetate in the treatment of leiomyomata uteri. Fertil Steril. 1988;49:404-409.
- Donnez J, Hudecek R, Donnez O, et al. Efficacy and safety of repeated use of ulipristal acetate in uterine fibroids. Fertil Steril. 2015;103:519-527.
- Donnez J, Tatarchuk TF, Bouchard P, et al. Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med. 2012;366:409-420.
- Donnez J, Tomaszewski J, Vazquez F, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med. 2012;366:421-432.
- European Medicines Agency. Suspension of ulipristal acetate for uterine fibroids during ongoing EMA review of liver injury risk. March 13, 2020. https://www.ema.europa.eu/en/news/suspension-ulipristal-acetate-uterine-fibroids-during-ongoing-ema-review-liver-injury-risk#:~:text=EMA's%20safety%20committee%20(PRAC)%20has,the%20EU%20during%20the%20review. Accessed July 24, 2020.
- Lupron Depot [package insert]. Osaka, Japan: Takeda; Revised March 2012.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
- Simon JA, Al-Hendy A, Archer DF, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326.
- Oriahnn [package insert]. North Chicago, IL: AbbVie; 2020.
- Stewart EA. Uterine fibroids. N Engl J Med. 2015;372:1646-1655.
- Mehine M, Makinen N, Heinonen HR, et al. Genomics of uterine leiomyomas: insights from high-throughput sequencing. Fertil Steril. 2014;102:621-629.
- Mehine M, Kaasinen E, Makinen N, et al. Characterization of uterine leiomyomas by whole-genome sequencing. N Engl J Med. 2013;369:43-53.
- Moravek MB, Bulun SE. Endocrinology of uterine fibroids: steroid hormones, stem cells and genetic contribution. Curr Opin Obstet Gynecol. 2015;27:276-283.
- Rein MS. Advances in uterine leiomyoma research: the progesterone hypothesis. Environ Health Perspect. 2000;108(suppl 5):791-793.
- Friedman AJ, Barbieri RL, Doubilet PM, et al. A randomized double-blind trial of a gonadotropin-releasing hormone agonist (leuprolide) with or without medroxyprogesterone acetate in the treatment of leiomyomata uteri. Fertil Steril. 1988;49:404-409.
- Donnez J, Hudecek R, Donnez O, et al. Efficacy and safety of repeated use of ulipristal acetate in uterine fibroids. Fertil Steril. 2015;103:519-527.
- Donnez J, Tatarchuk TF, Bouchard P, et al. Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med. 2012;366:409-420.
- Donnez J, Tomaszewski J, Vazquez F, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med. 2012;366:421-432.
- European Medicines Agency. Suspension of ulipristal acetate for uterine fibroids during ongoing EMA review of liver injury risk. March 13, 2020. https://www.ema.europa.eu/en/news/suspension-ulipristal-acetate-uterine-fibroids-during-ongoing-ema-review-liver-injury-risk#:~:text=EMA's%20safety%20committee%20(PRAC)%20has,the%20EU%20during%20the%20review. Accessed July 24, 2020.
- Lupron Depot [package insert]. Osaka, Japan: Takeda; Revised March 2012.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
- Simon JA, Al-Hendy A, Archer DF, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326.
- Oriahnn [package insert]. North Chicago, IL: AbbVie; 2020.
COVID-19 impact: Less chemo, immune checkpoint inhibitors, and steroids
While neoadjuvant treatment recommendations were not strongly affected by the pandemic, about half of oncologists reported increased hesitancy over recommending frontline chemotherapy for metastatic disease, and a vast majority said they would recommend second- or third-line chemotherapy less often in the metastatic setting.
Most oncologists said they did not perform routine COVID-19 testing via reverse transcriptase–polymerase chain reaction (RT-PCR) before treating cancer patients. In fact, only 3% said they performed COVID-19 RT-PCR testing routinely.
Yüksel Ürün, MD, of Ankara (Turkey) University, and colleagues reported these findings in JCO Global Oncology.
The goal of the survey was to “understand readiness measures taken by oncologists to protect patients and health care workers from the novel coronavirus (COVID-19) and how their clinical decision-making was influenced by the pandemic,” the authors wrote.
The online survey was conducted among 343 oncologists from 28 countries. Responses were collected anonymously, a majority (71%) from university or academic centers, with 95% received between April 1 and April 29, 2020.
Use of telemedicine was common (80%) among respondents, as was use of surgical masks (90%) and personal protective equipment in general.
Only 33% of respondents described using N95 masks. However, the proportion of oncologists who had access to N95 masks while caring for patients known to have COVID-19, especially while doing invasive procedures such as intubation, bronchoscopy, and any airway-related manipulations, was not captured by the survey.
COVID testing and cancer treatment
Most respondents (58%) said they did not perform routine COVID-19 RT-PCR testing prior to administering systemic cancer treatment, with 39% stating they performed RT-PCR tests in selected patients, and 3% saying they performed such testing in all patients.
The survey indicated that hormonal treatments, tyrosine kinase inhibitors, and bone-modifying agents were considered relatively safe, but cytotoxic chemotherapy and immune therapies were not.
Nearly all oncologists said the pandemic would cause them to make no change to their recommendations regarding hormone therapy, and nearly 80% said they would make no changes regarding tyrosine kinase inhibitors or bone-modifying agents.
However, more than 90% of respondents said they would recommend cytotoxic chemotherapy less often, about 70% said they would recommend corticosteroids less often, and around 50% said they would recommend anti–programmed death-1/PD-ligand 1 or anti–cytotoxic T-lymphocyte–associated protein 4 antibodies less often.
The pandemic made most respondents more reluctant to recommend second- or third-line chemotherapy in the metastatic setting. About 80% and 70% of respondents, respectively, would recommend second- or third-line chemotherapy less often.
However, first-line chemotherapy for metastatic disease, as well as adjuvant and neoadjuvant therapy, were less affected. About 30% of respondents said they would recommend neoadjuvant therapy less often, and 50%-55% would recommend adjuvant therapy or frontline chemotherapy for metastatic disease less often.
Most respondents (78%) said they would use granulocyte colony–stimulating factor (G-CSF) more frequently during the pandemic.
The factors most likely to affect oncologists’ treatment decisions were patient age (81%) and concomitant disease (92%). Additionally, 80% of respondents’ treatment decisions were influenced by Eastern Cooperative Oncology Group performance status of 2 or higher, or the presence of chronic obstructive pulmonary disease.
Interpretation and implications
“These results highlight that, even in the early phases of COVID-19 – during which there was considerable uncertainty – basic core principles were guideposts for oncologists,” observed Aly-Khan Lalani, MD, of Juravinski Cancer Centre and McMaster University, Hamilton, Ont., who was not involved in this study.
“For example, [oncologists were] prioritizing strategies for treatments with the largest expected impact and carefully tailoring treatment according to patient comorbidities and performance status,” Dr. Lalani said.
Another oncologist who was not involved in the study expressed concern over reductions in adjuvant therapy supported by half of oncologists surveyed.
“Although benefits may be marginal in some cases, these are curative settings and especially warrant careful individual-level risk/benefit discussions,” said Kartik Sehgal, MD, of Dana-Farber Cancer Institute/Brigham and Women’s Hospital in Boston.
His concern extended as well to the small proportion (3%) of oncologists testing for COVID-19 in all patients. “Systematic testing is the need of the hour,” Dr. Sehgal said.
In their discussion of the findings, Dr. Ürün and colleagues noted a lack of consensus on monoclonal antibody and immunotherapy safety among surveyed oncologists. The steroids needed to manage severe immune-mediated toxicity with immune checkpoint inhibitors has led to some prescribing reluctance during the pandemic.
Immunosuppressive properties of immune checkpoint inhibitors also raise concern that they can increase COVID-19 severity. Studies are few, and findings to date are inconsistent with respect to the effect of immune checkpoint inhibitors on COVID-19 clinical course. However, a recently presented study suggested that immune checkpoint inhibitors do not increase the risk of death among cancer patients with COVID-19 (AACR: COVID-19 and Cancer, Abstract S02-01).
Dr. Ürün and colleagues noted that greater COVID-19 severity has been shown in patients with performance status greater than 1, hematologic malignancies, lung cancer, stage IV metastatic disease, chemotherapy within the prior 3 months, cancer treatment in the last 14 days, and the presence of chronic obstructive pulmonary disease. Nonmetastatic cancer has not been shown to affect COVID-19 severity, however.
Dr. Ürün and colleagues also underscored the need for research evidence to balance potential reductions in neutropenic complications with G-CSF (and therefore, reduced hospitalizations) with a theoretical risk of G-CSF–mediated pulmonary injury through its stimulation of an excessive immune response.
Finally, the authors urged oncologists to evaluate each proposed therapy’s risk/benefit ratio on an individual patient basis, and the team tasked the oncology community with gathering comprehensive, rigorous data.
There was no funding source declared for this study. Dr. Ürün and colleagues disclosed various relationships with many pharmaceutical companies, which included receiving research funding. Dr. Sehgal and Dr. Lalani reported no relevant conflicts.
SOURCE: Ürün Y et al. JCO Glob Oncol. 2020 Aug;6:1248-57.
While neoadjuvant treatment recommendations were not strongly affected by the pandemic, about half of oncologists reported increased hesitancy over recommending frontline chemotherapy for metastatic disease, and a vast majority said they would recommend second- or third-line chemotherapy less often in the metastatic setting.
Most oncologists said they did not perform routine COVID-19 testing via reverse transcriptase–polymerase chain reaction (RT-PCR) before treating cancer patients. In fact, only 3% said they performed COVID-19 RT-PCR testing routinely.
Yüksel Ürün, MD, of Ankara (Turkey) University, and colleagues reported these findings in JCO Global Oncology.
The goal of the survey was to “understand readiness measures taken by oncologists to protect patients and health care workers from the novel coronavirus (COVID-19) and how their clinical decision-making was influenced by the pandemic,” the authors wrote.
The online survey was conducted among 343 oncologists from 28 countries. Responses were collected anonymously, a majority (71%) from university or academic centers, with 95% received between April 1 and April 29, 2020.
Use of telemedicine was common (80%) among respondents, as was use of surgical masks (90%) and personal protective equipment in general.
Only 33% of respondents described using N95 masks. However, the proportion of oncologists who had access to N95 masks while caring for patients known to have COVID-19, especially while doing invasive procedures such as intubation, bronchoscopy, and any airway-related manipulations, was not captured by the survey.
COVID testing and cancer treatment
Most respondents (58%) said they did not perform routine COVID-19 RT-PCR testing prior to administering systemic cancer treatment, with 39% stating they performed RT-PCR tests in selected patients, and 3% saying they performed such testing in all patients.
The survey indicated that hormonal treatments, tyrosine kinase inhibitors, and bone-modifying agents were considered relatively safe, but cytotoxic chemotherapy and immune therapies were not.
Nearly all oncologists said the pandemic would cause them to make no change to their recommendations regarding hormone therapy, and nearly 80% said they would make no changes regarding tyrosine kinase inhibitors or bone-modifying agents.
However, more than 90% of respondents said they would recommend cytotoxic chemotherapy less often, about 70% said they would recommend corticosteroids less often, and around 50% said they would recommend anti–programmed death-1/PD-ligand 1 or anti–cytotoxic T-lymphocyte–associated protein 4 antibodies less often.
The pandemic made most respondents more reluctant to recommend second- or third-line chemotherapy in the metastatic setting. About 80% and 70% of respondents, respectively, would recommend second- or third-line chemotherapy less often.
However, first-line chemotherapy for metastatic disease, as well as adjuvant and neoadjuvant therapy, were less affected. About 30% of respondents said they would recommend neoadjuvant therapy less often, and 50%-55% would recommend adjuvant therapy or frontline chemotherapy for metastatic disease less often.
Most respondents (78%) said they would use granulocyte colony–stimulating factor (G-CSF) more frequently during the pandemic.
The factors most likely to affect oncologists’ treatment decisions were patient age (81%) and concomitant disease (92%). Additionally, 80% of respondents’ treatment decisions were influenced by Eastern Cooperative Oncology Group performance status of 2 or higher, or the presence of chronic obstructive pulmonary disease.
Interpretation and implications
“These results highlight that, even in the early phases of COVID-19 – during which there was considerable uncertainty – basic core principles were guideposts for oncologists,” observed Aly-Khan Lalani, MD, of Juravinski Cancer Centre and McMaster University, Hamilton, Ont., who was not involved in this study.
“For example, [oncologists were] prioritizing strategies for treatments with the largest expected impact and carefully tailoring treatment according to patient comorbidities and performance status,” Dr. Lalani said.
Another oncologist who was not involved in the study expressed concern over reductions in adjuvant therapy supported by half of oncologists surveyed.
“Although benefits may be marginal in some cases, these are curative settings and especially warrant careful individual-level risk/benefit discussions,” said Kartik Sehgal, MD, of Dana-Farber Cancer Institute/Brigham and Women’s Hospital in Boston.
His concern extended as well to the small proportion (3%) of oncologists testing for COVID-19 in all patients. “Systematic testing is the need of the hour,” Dr. Sehgal said.
In their discussion of the findings, Dr. Ürün and colleagues noted a lack of consensus on monoclonal antibody and immunotherapy safety among surveyed oncologists. The steroids needed to manage severe immune-mediated toxicity with immune checkpoint inhibitors has led to some prescribing reluctance during the pandemic.
Immunosuppressive properties of immune checkpoint inhibitors also raise concern that they can increase COVID-19 severity. Studies are few, and findings to date are inconsistent with respect to the effect of immune checkpoint inhibitors on COVID-19 clinical course. However, a recently presented study suggested that immune checkpoint inhibitors do not increase the risk of death among cancer patients with COVID-19 (AACR: COVID-19 and Cancer, Abstract S02-01).
Dr. Ürün and colleagues noted that greater COVID-19 severity has been shown in patients with performance status greater than 1, hematologic malignancies, lung cancer, stage IV metastatic disease, chemotherapy within the prior 3 months, cancer treatment in the last 14 days, and the presence of chronic obstructive pulmonary disease. Nonmetastatic cancer has not been shown to affect COVID-19 severity, however.
Dr. Ürün and colleagues also underscored the need for research evidence to balance potential reductions in neutropenic complications with G-CSF (and therefore, reduced hospitalizations) with a theoretical risk of G-CSF–mediated pulmonary injury through its stimulation of an excessive immune response.
Finally, the authors urged oncologists to evaluate each proposed therapy’s risk/benefit ratio on an individual patient basis, and the team tasked the oncology community with gathering comprehensive, rigorous data.
There was no funding source declared for this study. Dr. Ürün and colleagues disclosed various relationships with many pharmaceutical companies, which included receiving research funding. Dr. Sehgal and Dr. Lalani reported no relevant conflicts.
SOURCE: Ürün Y et al. JCO Glob Oncol. 2020 Aug;6:1248-57.
While neoadjuvant treatment recommendations were not strongly affected by the pandemic, about half of oncologists reported increased hesitancy over recommending frontline chemotherapy for metastatic disease, and a vast majority said they would recommend second- or third-line chemotherapy less often in the metastatic setting.
Most oncologists said they did not perform routine COVID-19 testing via reverse transcriptase–polymerase chain reaction (RT-PCR) before treating cancer patients. In fact, only 3% said they performed COVID-19 RT-PCR testing routinely.
Yüksel Ürün, MD, of Ankara (Turkey) University, and colleagues reported these findings in JCO Global Oncology.
The goal of the survey was to “understand readiness measures taken by oncologists to protect patients and health care workers from the novel coronavirus (COVID-19) and how their clinical decision-making was influenced by the pandemic,” the authors wrote.
The online survey was conducted among 343 oncologists from 28 countries. Responses were collected anonymously, a majority (71%) from university or academic centers, with 95% received between April 1 and April 29, 2020.
Use of telemedicine was common (80%) among respondents, as was use of surgical masks (90%) and personal protective equipment in general.
Only 33% of respondents described using N95 masks. However, the proportion of oncologists who had access to N95 masks while caring for patients known to have COVID-19, especially while doing invasive procedures such as intubation, bronchoscopy, and any airway-related manipulations, was not captured by the survey.
COVID testing and cancer treatment
Most respondents (58%) said they did not perform routine COVID-19 RT-PCR testing prior to administering systemic cancer treatment, with 39% stating they performed RT-PCR tests in selected patients, and 3% saying they performed such testing in all patients.
The survey indicated that hormonal treatments, tyrosine kinase inhibitors, and bone-modifying agents were considered relatively safe, but cytotoxic chemotherapy and immune therapies were not.
Nearly all oncologists said the pandemic would cause them to make no change to their recommendations regarding hormone therapy, and nearly 80% said they would make no changes regarding tyrosine kinase inhibitors or bone-modifying agents.
However, more than 90% of respondents said they would recommend cytotoxic chemotherapy less often, about 70% said they would recommend corticosteroids less often, and around 50% said they would recommend anti–programmed death-1/PD-ligand 1 or anti–cytotoxic T-lymphocyte–associated protein 4 antibodies less often.
The pandemic made most respondents more reluctant to recommend second- or third-line chemotherapy in the metastatic setting. About 80% and 70% of respondents, respectively, would recommend second- or third-line chemotherapy less often.
However, first-line chemotherapy for metastatic disease, as well as adjuvant and neoadjuvant therapy, were less affected. About 30% of respondents said they would recommend neoadjuvant therapy less often, and 50%-55% would recommend adjuvant therapy or frontline chemotherapy for metastatic disease less often.
Most respondents (78%) said they would use granulocyte colony–stimulating factor (G-CSF) more frequently during the pandemic.
The factors most likely to affect oncologists’ treatment decisions were patient age (81%) and concomitant disease (92%). Additionally, 80% of respondents’ treatment decisions were influenced by Eastern Cooperative Oncology Group performance status of 2 or higher, or the presence of chronic obstructive pulmonary disease.
Interpretation and implications
“These results highlight that, even in the early phases of COVID-19 – during which there was considerable uncertainty – basic core principles were guideposts for oncologists,” observed Aly-Khan Lalani, MD, of Juravinski Cancer Centre and McMaster University, Hamilton, Ont., who was not involved in this study.
“For example, [oncologists were] prioritizing strategies for treatments with the largest expected impact and carefully tailoring treatment according to patient comorbidities and performance status,” Dr. Lalani said.
Another oncologist who was not involved in the study expressed concern over reductions in adjuvant therapy supported by half of oncologists surveyed.
“Although benefits may be marginal in some cases, these are curative settings and especially warrant careful individual-level risk/benefit discussions,” said Kartik Sehgal, MD, of Dana-Farber Cancer Institute/Brigham and Women’s Hospital in Boston.
His concern extended as well to the small proportion (3%) of oncologists testing for COVID-19 in all patients. “Systematic testing is the need of the hour,” Dr. Sehgal said.
In their discussion of the findings, Dr. Ürün and colleagues noted a lack of consensus on monoclonal antibody and immunotherapy safety among surveyed oncologists. The steroids needed to manage severe immune-mediated toxicity with immune checkpoint inhibitors has led to some prescribing reluctance during the pandemic.
Immunosuppressive properties of immune checkpoint inhibitors also raise concern that they can increase COVID-19 severity. Studies are few, and findings to date are inconsistent with respect to the effect of immune checkpoint inhibitors on COVID-19 clinical course. However, a recently presented study suggested that immune checkpoint inhibitors do not increase the risk of death among cancer patients with COVID-19 (AACR: COVID-19 and Cancer, Abstract S02-01).
Dr. Ürün and colleagues noted that greater COVID-19 severity has been shown in patients with performance status greater than 1, hematologic malignancies, lung cancer, stage IV metastatic disease, chemotherapy within the prior 3 months, cancer treatment in the last 14 days, and the presence of chronic obstructive pulmonary disease. Nonmetastatic cancer has not been shown to affect COVID-19 severity, however.
Dr. Ürün and colleagues also underscored the need for research evidence to balance potential reductions in neutropenic complications with G-CSF (and therefore, reduced hospitalizations) with a theoretical risk of G-CSF–mediated pulmonary injury through its stimulation of an excessive immune response.
Finally, the authors urged oncologists to evaluate each proposed therapy’s risk/benefit ratio on an individual patient basis, and the team tasked the oncology community with gathering comprehensive, rigorous data.
There was no funding source declared for this study. Dr. Ürün and colleagues disclosed various relationships with many pharmaceutical companies, which included receiving research funding. Dr. Sehgal and Dr. Lalani reported no relevant conflicts.
SOURCE: Ürün Y et al. JCO Glob Oncol. 2020 Aug;6:1248-57.
FROM JCO GLOBAL ONCOLOGY
AI improves diagnostic accuracy in cervical cancer
This could allow some women to avoid surgery and be treated with chemotherapy instead, suggested researchers.
The model mined tumor information from pelvic sagittal contrast-enhanced T1-weighted MRIs and combined this with clinical MRI lymph node status.
It was 90.62% sensitive and 87.16% specific for predicting lymph node metastases (LNMs) in a validation cohort of women who underwent surgery for cervical cancer.
The area under the curve was 0.933. The approach was significantly associated with disease-free survival (hazard ratio, 4.59; 95% confidence interval, 2.04-10.31; P < .001).
The study was published online on July 24 in JAMA Network Open.
“The findings of this study suggest that deep learning can be used as a preoperative noninvasive tool to diagnose lymph node metastasis in cervical cancer ... This model might be used preoperatively to help gynecologists make decisions,” said investigators led by Qingxia Wu, PhD, of the Northeastern University College of Medicine and Biomedical Information Engineering in Shenyang, China.
“Studies like these suggest that deep learning has the potential to improve the way we care for our patients,” but there’s much to be done “before these types of algorithms will be commonplace,” commented Christiaan Rees, MD, PhD, an internal medicine resident at Brigham and Women’s Hospital, Boston, who has a doctorate in quantitative biomedical sciences.
Next steps include repeated validation across multiple control groups, he said in an interview, as well as “finding ways to effectively integrate these tools into the radiologist’s day-to-day practice. One possibility would be for direct integration of the algorithm into the electronic health record.”
Accurate prediction could lead to skipping surgery
Chemotherapy, rather than surgery, is an option for women with positive lymph nodes (LNs), so accurate prediction can help them avoid an operation and its risks, the authors said.
The problem is that “the traditional methods for assessing LN status in cervical cancer, which rely mainly on assessing the size of LNs on MRI, have limited sensitivity in diagnosing LNM in cervical cancer and might lead to inappropriate treatment decisions,” they wrote.
“Although sentinel LN dissection ... shows good sensitivity and specificity, its application is limited by available facilities and experts,” the team said.
DL is an advanced form of artificial intelligence in which a computer program continuously improves on a given task as it incorporates more data – in Dr. Wu’s study, more than 14 million images. Deep learning has recently shown promise in several imaging tasks, such as diagnosing Alzheimer’s disease and screening for breast cancer.
Once adapted for cervical cancer, DL “does not require precise tumor delineation, making it an easy-to-use method in clinical practice. In many tumor analysis tasks, DL outperforms traditional radiomic features,” the team noted.
The study involved 479 women – 338 during model development, and 141 in the validation cohorts. The mean age of the participants was 49.1 years. They had undergone radical hysterectomy with pelvic lymphadenectomy for stage IB-IIB cervical cancer within 2 weeks of a pelvic MRI. Pathology reports were used to check the accuracy of the model’s predictions.
Specificity, sensitivity, and area under the curve were a little better in the study’s development cohort than its validation group, for whom median disease-free survival was 23 months versus 31 months among the patients in the development cohort. Nodes were positive on lymphadenectomy in a little more than 20% of women in both groups.
Incorporation of both intratumoral and peritumoral regions on contrast-enhanced T1-weighted MRIs versus axial T2-weighted and axial diffusion-weighted imaging, produced the highest sensitivity. Adding MRI-LN status – defined as positive when the short-axis diameter of the largest LN on MRI was ≥1 cm – improved the model’s specificity.
To understand how the model reached its conclusions, the team analyzed how it extracted features from tumor images. “In the shallow convolution layers, the DL model extracted simple tumor edge features ... while in deeper convolution layers, it extracted complex tumor texture information ... In the last convolution layer, the DL model extracted high-level abstract features (the fourteenth layer). Although these high-level features were so intricate that they were hard to interpret by general gross observation, they were associated with LN status,” the investigators said.
The team notes that “both intratumoral and peritumoral regions were necessary for the DL model to make decisions,” which “can probably be explained by the fact that higher lymphatic vessel density in peritumoral regions might lead to higher regional LNM.”
Commenting on the study, Dr. Rees said that “the authors did a [good] job of essentially deconstructing their neural network to see what the algorithm was actually picking up on to make its decision.
“One of the nice features of deep learning is that once the algorithm has been developed and validated, the end user doesn’t need any experience in deep learning in order to use it,” he added.
Even so, “while these resources can be incredibly powerful tools, they should not function in a vacuum without human judgment,” Dr. Rees said.
The work was funded by the National Natural Science Foundation of China, among others. The investigators have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
This could allow some women to avoid surgery and be treated with chemotherapy instead, suggested researchers.
The model mined tumor information from pelvic sagittal contrast-enhanced T1-weighted MRIs and combined this with clinical MRI lymph node status.
It was 90.62% sensitive and 87.16% specific for predicting lymph node metastases (LNMs) in a validation cohort of women who underwent surgery for cervical cancer.
The area under the curve was 0.933. The approach was significantly associated with disease-free survival (hazard ratio, 4.59; 95% confidence interval, 2.04-10.31; P < .001).
The study was published online on July 24 in JAMA Network Open.
“The findings of this study suggest that deep learning can be used as a preoperative noninvasive tool to diagnose lymph node metastasis in cervical cancer ... This model might be used preoperatively to help gynecologists make decisions,” said investigators led by Qingxia Wu, PhD, of the Northeastern University College of Medicine and Biomedical Information Engineering in Shenyang, China.
“Studies like these suggest that deep learning has the potential to improve the way we care for our patients,” but there’s much to be done “before these types of algorithms will be commonplace,” commented Christiaan Rees, MD, PhD, an internal medicine resident at Brigham and Women’s Hospital, Boston, who has a doctorate in quantitative biomedical sciences.
Next steps include repeated validation across multiple control groups, he said in an interview, as well as “finding ways to effectively integrate these tools into the radiologist’s day-to-day practice. One possibility would be for direct integration of the algorithm into the electronic health record.”
Accurate prediction could lead to skipping surgery
Chemotherapy, rather than surgery, is an option for women with positive lymph nodes (LNs), so accurate prediction can help them avoid an operation and its risks, the authors said.
The problem is that “the traditional methods for assessing LN status in cervical cancer, which rely mainly on assessing the size of LNs on MRI, have limited sensitivity in diagnosing LNM in cervical cancer and might lead to inappropriate treatment decisions,” they wrote.
“Although sentinel LN dissection ... shows good sensitivity and specificity, its application is limited by available facilities and experts,” the team said.
DL is an advanced form of artificial intelligence in which a computer program continuously improves on a given task as it incorporates more data – in Dr. Wu’s study, more than 14 million images. Deep learning has recently shown promise in several imaging tasks, such as diagnosing Alzheimer’s disease and screening for breast cancer.
Once adapted for cervical cancer, DL “does not require precise tumor delineation, making it an easy-to-use method in clinical practice. In many tumor analysis tasks, DL outperforms traditional radiomic features,” the team noted.
The study involved 479 women – 338 during model development, and 141 in the validation cohorts. The mean age of the participants was 49.1 years. They had undergone radical hysterectomy with pelvic lymphadenectomy for stage IB-IIB cervical cancer within 2 weeks of a pelvic MRI. Pathology reports were used to check the accuracy of the model’s predictions.
Specificity, sensitivity, and area under the curve were a little better in the study’s development cohort than its validation group, for whom median disease-free survival was 23 months versus 31 months among the patients in the development cohort. Nodes were positive on lymphadenectomy in a little more than 20% of women in both groups.
Incorporation of both intratumoral and peritumoral regions on contrast-enhanced T1-weighted MRIs versus axial T2-weighted and axial diffusion-weighted imaging, produced the highest sensitivity. Adding MRI-LN status – defined as positive when the short-axis diameter of the largest LN on MRI was ≥1 cm – improved the model’s specificity.
To understand how the model reached its conclusions, the team analyzed how it extracted features from tumor images. “In the shallow convolution layers, the DL model extracted simple tumor edge features ... while in deeper convolution layers, it extracted complex tumor texture information ... In the last convolution layer, the DL model extracted high-level abstract features (the fourteenth layer). Although these high-level features were so intricate that they were hard to interpret by general gross observation, they were associated with LN status,” the investigators said.
The team notes that “both intratumoral and peritumoral regions were necessary for the DL model to make decisions,” which “can probably be explained by the fact that higher lymphatic vessel density in peritumoral regions might lead to higher regional LNM.”
Commenting on the study, Dr. Rees said that “the authors did a [good] job of essentially deconstructing their neural network to see what the algorithm was actually picking up on to make its decision.
“One of the nice features of deep learning is that once the algorithm has been developed and validated, the end user doesn’t need any experience in deep learning in order to use it,” he added.
Even so, “while these resources can be incredibly powerful tools, they should not function in a vacuum without human judgment,” Dr. Rees said.
The work was funded by the National Natural Science Foundation of China, among others. The investigators have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
This could allow some women to avoid surgery and be treated with chemotherapy instead, suggested researchers.
The model mined tumor information from pelvic sagittal contrast-enhanced T1-weighted MRIs and combined this with clinical MRI lymph node status.
It was 90.62% sensitive and 87.16% specific for predicting lymph node metastases (LNMs) in a validation cohort of women who underwent surgery for cervical cancer.
The area under the curve was 0.933. The approach was significantly associated with disease-free survival (hazard ratio, 4.59; 95% confidence interval, 2.04-10.31; P < .001).
The study was published online on July 24 in JAMA Network Open.
“The findings of this study suggest that deep learning can be used as a preoperative noninvasive tool to diagnose lymph node metastasis in cervical cancer ... This model might be used preoperatively to help gynecologists make decisions,” said investigators led by Qingxia Wu, PhD, of the Northeastern University College of Medicine and Biomedical Information Engineering in Shenyang, China.
“Studies like these suggest that deep learning has the potential to improve the way we care for our patients,” but there’s much to be done “before these types of algorithms will be commonplace,” commented Christiaan Rees, MD, PhD, an internal medicine resident at Brigham and Women’s Hospital, Boston, who has a doctorate in quantitative biomedical sciences.
Next steps include repeated validation across multiple control groups, he said in an interview, as well as “finding ways to effectively integrate these tools into the radiologist’s day-to-day practice. One possibility would be for direct integration of the algorithm into the electronic health record.”
Accurate prediction could lead to skipping surgery
Chemotherapy, rather than surgery, is an option for women with positive lymph nodes (LNs), so accurate prediction can help them avoid an operation and its risks, the authors said.
The problem is that “the traditional methods for assessing LN status in cervical cancer, which rely mainly on assessing the size of LNs on MRI, have limited sensitivity in diagnosing LNM in cervical cancer and might lead to inappropriate treatment decisions,” they wrote.
“Although sentinel LN dissection ... shows good sensitivity and specificity, its application is limited by available facilities and experts,” the team said.
DL is an advanced form of artificial intelligence in which a computer program continuously improves on a given task as it incorporates more data – in Dr. Wu’s study, more than 14 million images. Deep learning has recently shown promise in several imaging tasks, such as diagnosing Alzheimer’s disease and screening for breast cancer.
Once adapted for cervical cancer, DL “does not require precise tumor delineation, making it an easy-to-use method in clinical practice. In many tumor analysis tasks, DL outperforms traditional radiomic features,” the team noted.
The study involved 479 women – 338 during model development, and 141 in the validation cohorts. The mean age of the participants was 49.1 years. They had undergone radical hysterectomy with pelvic lymphadenectomy for stage IB-IIB cervical cancer within 2 weeks of a pelvic MRI. Pathology reports were used to check the accuracy of the model’s predictions.
Specificity, sensitivity, and area under the curve were a little better in the study’s development cohort than its validation group, for whom median disease-free survival was 23 months versus 31 months among the patients in the development cohort. Nodes were positive on lymphadenectomy in a little more than 20% of women in both groups.
Incorporation of both intratumoral and peritumoral regions on contrast-enhanced T1-weighted MRIs versus axial T2-weighted and axial diffusion-weighted imaging, produced the highest sensitivity. Adding MRI-LN status – defined as positive when the short-axis diameter of the largest LN on MRI was ≥1 cm – improved the model’s specificity.
To understand how the model reached its conclusions, the team analyzed how it extracted features from tumor images. “In the shallow convolution layers, the DL model extracted simple tumor edge features ... while in deeper convolution layers, it extracted complex tumor texture information ... In the last convolution layer, the DL model extracted high-level abstract features (the fourteenth layer). Although these high-level features were so intricate that they were hard to interpret by general gross observation, they were associated with LN status,” the investigators said.
The team notes that “both intratumoral and peritumoral regions were necessary for the DL model to make decisions,” which “can probably be explained by the fact that higher lymphatic vessel density in peritumoral regions might lead to higher regional LNM.”
Commenting on the study, Dr. Rees said that “the authors did a [good] job of essentially deconstructing their neural network to see what the algorithm was actually picking up on to make its decision.
“One of the nice features of deep learning is that once the algorithm has been developed and validated, the end user doesn’t need any experience in deep learning in order to use it,” he added.
Even so, “while these resources can be incredibly powerful tools, they should not function in a vacuum without human judgment,” Dr. Rees said.
The work was funded by the National Natural Science Foundation of China, among others. The investigators have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Artificial intelligence matches cancer genotypes to patient phenotypes
Precision medicine is driven by technologies such as rapid genome sequencing and artificial intelligence (AI), according to a presentation at the AACR virtual meeting II.
AI can be applied to the sequencing information derived from advanced cancers to make highly personalized treatment recommendations for patients, said Olivier Elemento, PhD, of Weill Cornell Medicine, New York.
Dr. Elemento described such work during the opening plenary session of the meeting.
Dr. Elemento advocated for whole-genome sequencing (WGS) of metastatic sites, as it can reveal “branched evolution” as tumors progress from localized to metastatic (Nat Genet. 2016 Dec;48[12]:1490-9).
The metastases share common mutations with the primaries from which they arise but also develop their own mutational profiles, which facilitate site-of-origin-agnostic, predictive treatment choices.
As examples, Dr. Elemento mentioned HER2 amplification found in a patient with urothelial cancer (J Natl Compr Canc Netw. 2019 Mar 1;17[3]:194-200) and a patient with uterine serous carcinoma (Gynecol Oncol Rep. 2019 Feb 21;28:54-7), both of whom experienced long-lasting remissions to HER2-targeted therapy.
Dr. Elemento also noted that WGS can reveal complex structural variants in lung adenocarcinomas that lack alterations in the RTK/RAS/RAF pathway (unpublished data).
Application of machine learning
One study suggested that microRNA expression and machine learning can be used to identify malignant thyroid lesions (Clin Cancer Res. 2012 Apr 1;18[7]:2032-8). The approach diagnosed malignant lesions with 90% accuracy, 100% sensitivity, and 86% specificity.
Dr. Elemento and colleagues used a similar approach to predict response to immunotherapy in melanoma (unpublished data).
The idea was to mine the cancer genome and transcriptome, allowing for identification of signals from neoantigens, immune gene expression, immune cell composition, and T-cell receptor repertoires, Dr. Elemento said. Integrating these signals with clinical outcome data via machine learning technology enabled the researchers to predict immunotherapy response in malignant melanoma with nearly 90% accuracy.
AI and image analysis
Studies have indicated that AI can be applied to medical images to improve diagnosis and treatment. The approach has been shown to:
- Facilitate correct diagnoses of malignant skin lesions (Nature. 2017 Feb 2;542[7639]:115-8).
- Distinguish lung adenocarcinoma from squamous cell cancer with 100% accuracy (EBioMedicine. 2018 Jan;27:317-28).
- Recognize distinct breast cancer subtypes (ductal, lobular, mucinous, papillary) and biomarkers (bioRxiv 242818. doi: 10.1101/242818; EBioMedicine. 2018 Jan;27:317-28)
- Predict mesothelioma prognosis (Nat Med. 2019 Oct;25[10]:1519-25).
- Predict prostate biopsy results (unpublished data) and calculate Gleason scores that can predict survival in prostate cancer patients (AACR 2020, Abstract 867).
Drug development through applied AI
In another study, Dr. Elemento and colleagues used a Bayesian machine learning approach to predict targets of molecules without a known mechanism of action (Nat Commun. 2019 Nov 19;10[1]:5221).
The method involved using data on gene expression profiles, cell line viability, side effects in animals, and structures of the molecules. The researchers applied this method to a large library of orphan small molecules and found it could predict targets in about 40% of cases.
Of 24 AI-predicted microtubule-targeting molecules, 14 depolymerized microtubules in the lab. Five of these molecules were effective in cell lines that were resistant to other microtubule-targeted drugs.
Dr. Elemento went on to describe how Oncoceutics was developing an antineoplastic agent called ONC201, but the company lacked information about the agent’s target. Using AI, the target was identified as dopamine receptor 2 (DRD2; Clin Cancer Res. 2019 Apr 1;25[7]:2305-13).
With that information, Oncoceutics initiated trials of ONC201 in tumors expressing high levels of DRD2, including a highly resistant glioma (J Neurooncol. 2019 Oct;145[1]:97-105). Responses were seen, and ONC201 is now being tested against other DRD2-expressing cancers.
Challenges to acknowledge
Potential benefits of AI in the clinic are exciting, but there are many bench-to-bedside challenges.
A clinically obvious example of AI’s applications is radiographic image analysis. There is no biologic rationale for our RECIST “cut values” for partial response, minimal response, and stable disease.
If AI can measure subtle changes on imaging that correlate with tumor biology (i.e., radiomics), we stand a better chance of predicting treatment outcomes than we can with conventional measurements of shrinkage of arbitrarily selected “target lesions.”
A tremendous amount of work is needed to build the required large image banks. During that time, AI will only improve – and without the human risks of fatigue, inconsistency, or burnout.
Those human frailties notwithstanding, AI cannot substitute for the key discussions between patient and clinician regarding goals of care, trade-offs of risks and benefits, and shared decision-making regarding management options.
At least initially (but painfully), complex technologies like WGS and digital image analysis via AI may further disadvantage patients who are medically disadvantaged by geography or socioeconomic circumstances.
In the discussion period, AACR President Antoni Ribas, MD, of University of California, Los Angeles, asked whether AI can simulate crosstalk between gene pathways so that unique treatment combinations can be identified. Dr. Elemento said those simulations are the subject of ongoing investigation.
The theme of the opening plenary session at the AACR virtual meeting II was “Turning Science into Life-Saving Care.” Applications of AI to optimize personalized use of genomics, digital image analysis, and drug development show great promise for being among the technologies that can help to realize AACR’s thematic vision.
Dr. Elemento disclosed relationships with Volastra Therapeutics, OneThree Biotech, Owkin, Freenome, Genetic Intelligence, Acuamark Diagnostics, Eli Lilly, Janssen, and Sanofi.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Precision medicine is driven by technologies such as rapid genome sequencing and artificial intelligence (AI), according to a presentation at the AACR virtual meeting II.
AI can be applied to the sequencing information derived from advanced cancers to make highly personalized treatment recommendations for patients, said Olivier Elemento, PhD, of Weill Cornell Medicine, New York.
Dr. Elemento described such work during the opening plenary session of the meeting.
Dr. Elemento advocated for whole-genome sequencing (WGS) of metastatic sites, as it can reveal “branched evolution” as tumors progress from localized to metastatic (Nat Genet. 2016 Dec;48[12]:1490-9).
The metastases share common mutations with the primaries from which they arise but also develop their own mutational profiles, which facilitate site-of-origin-agnostic, predictive treatment choices.
As examples, Dr. Elemento mentioned HER2 amplification found in a patient with urothelial cancer (J Natl Compr Canc Netw. 2019 Mar 1;17[3]:194-200) and a patient with uterine serous carcinoma (Gynecol Oncol Rep. 2019 Feb 21;28:54-7), both of whom experienced long-lasting remissions to HER2-targeted therapy.
Dr. Elemento also noted that WGS can reveal complex structural variants in lung adenocarcinomas that lack alterations in the RTK/RAS/RAF pathway (unpublished data).
Application of machine learning
One study suggested that microRNA expression and machine learning can be used to identify malignant thyroid lesions (Clin Cancer Res. 2012 Apr 1;18[7]:2032-8). The approach diagnosed malignant lesions with 90% accuracy, 100% sensitivity, and 86% specificity.
Dr. Elemento and colleagues used a similar approach to predict response to immunotherapy in melanoma (unpublished data).
The idea was to mine the cancer genome and transcriptome, allowing for identification of signals from neoantigens, immune gene expression, immune cell composition, and T-cell receptor repertoires, Dr. Elemento said. Integrating these signals with clinical outcome data via machine learning technology enabled the researchers to predict immunotherapy response in malignant melanoma with nearly 90% accuracy.
AI and image analysis
Studies have indicated that AI can be applied to medical images to improve diagnosis and treatment. The approach has been shown to:
- Facilitate correct diagnoses of malignant skin lesions (Nature. 2017 Feb 2;542[7639]:115-8).
- Distinguish lung adenocarcinoma from squamous cell cancer with 100% accuracy (EBioMedicine. 2018 Jan;27:317-28).
- Recognize distinct breast cancer subtypes (ductal, lobular, mucinous, papillary) and biomarkers (bioRxiv 242818. doi: 10.1101/242818; EBioMedicine. 2018 Jan;27:317-28)
- Predict mesothelioma prognosis (Nat Med. 2019 Oct;25[10]:1519-25).
- Predict prostate biopsy results (unpublished data) and calculate Gleason scores that can predict survival in prostate cancer patients (AACR 2020, Abstract 867).
Drug development through applied AI
In another study, Dr. Elemento and colleagues used a Bayesian machine learning approach to predict targets of molecules without a known mechanism of action (Nat Commun. 2019 Nov 19;10[1]:5221).
The method involved using data on gene expression profiles, cell line viability, side effects in animals, and structures of the molecules. The researchers applied this method to a large library of orphan small molecules and found it could predict targets in about 40% of cases.
Of 24 AI-predicted microtubule-targeting molecules, 14 depolymerized microtubules in the lab. Five of these molecules were effective in cell lines that were resistant to other microtubule-targeted drugs.
Dr. Elemento went on to describe how Oncoceutics was developing an antineoplastic agent called ONC201, but the company lacked information about the agent’s target. Using AI, the target was identified as dopamine receptor 2 (DRD2; Clin Cancer Res. 2019 Apr 1;25[7]:2305-13).
With that information, Oncoceutics initiated trials of ONC201 in tumors expressing high levels of DRD2, including a highly resistant glioma (J Neurooncol. 2019 Oct;145[1]:97-105). Responses were seen, and ONC201 is now being tested against other DRD2-expressing cancers.
Challenges to acknowledge
Potential benefits of AI in the clinic are exciting, but there are many bench-to-bedside challenges.
A clinically obvious example of AI’s applications is radiographic image analysis. There is no biologic rationale for our RECIST “cut values” for partial response, minimal response, and stable disease.
If AI can measure subtle changes on imaging that correlate with tumor biology (i.e., radiomics), we stand a better chance of predicting treatment outcomes than we can with conventional measurements of shrinkage of arbitrarily selected “target lesions.”
A tremendous amount of work is needed to build the required large image banks. During that time, AI will only improve – and without the human risks of fatigue, inconsistency, or burnout.
Those human frailties notwithstanding, AI cannot substitute for the key discussions between patient and clinician regarding goals of care, trade-offs of risks and benefits, and shared decision-making regarding management options.
At least initially (but painfully), complex technologies like WGS and digital image analysis via AI may further disadvantage patients who are medically disadvantaged by geography or socioeconomic circumstances.
In the discussion period, AACR President Antoni Ribas, MD, of University of California, Los Angeles, asked whether AI can simulate crosstalk between gene pathways so that unique treatment combinations can be identified. Dr. Elemento said those simulations are the subject of ongoing investigation.
The theme of the opening plenary session at the AACR virtual meeting II was “Turning Science into Life-Saving Care.” Applications of AI to optimize personalized use of genomics, digital image analysis, and drug development show great promise for being among the technologies that can help to realize AACR’s thematic vision.
Dr. Elemento disclosed relationships with Volastra Therapeutics, OneThree Biotech, Owkin, Freenome, Genetic Intelligence, Acuamark Diagnostics, Eli Lilly, Janssen, and Sanofi.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Precision medicine is driven by technologies such as rapid genome sequencing and artificial intelligence (AI), according to a presentation at the AACR virtual meeting II.
AI can be applied to the sequencing information derived from advanced cancers to make highly personalized treatment recommendations for patients, said Olivier Elemento, PhD, of Weill Cornell Medicine, New York.
Dr. Elemento described such work during the opening plenary session of the meeting.
Dr. Elemento advocated for whole-genome sequencing (WGS) of metastatic sites, as it can reveal “branched evolution” as tumors progress from localized to metastatic (Nat Genet. 2016 Dec;48[12]:1490-9).
The metastases share common mutations with the primaries from which they arise but also develop their own mutational profiles, which facilitate site-of-origin-agnostic, predictive treatment choices.
As examples, Dr. Elemento mentioned HER2 amplification found in a patient with urothelial cancer (J Natl Compr Canc Netw. 2019 Mar 1;17[3]:194-200) and a patient with uterine serous carcinoma (Gynecol Oncol Rep. 2019 Feb 21;28:54-7), both of whom experienced long-lasting remissions to HER2-targeted therapy.
Dr. Elemento also noted that WGS can reveal complex structural variants in lung adenocarcinomas that lack alterations in the RTK/RAS/RAF pathway (unpublished data).
Application of machine learning
One study suggested that microRNA expression and machine learning can be used to identify malignant thyroid lesions (Clin Cancer Res. 2012 Apr 1;18[7]:2032-8). The approach diagnosed malignant lesions with 90% accuracy, 100% sensitivity, and 86% specificity.
Dr. Elemento and colleagues used a similar approach to predict response to immunotherapy in melanoma (unpublished data).
The idea was to mine the cancer genome and transcriptome, allowing for identification of signals from neoantigens, immune gene expression, immune cell composition, and T-cell receptor repertoires, Dr. Elemento said. Integrating these signals with clinical outcome data via machine learning technology enabled the researchers to predict immunotherapy response in malignant melanoma with nearly 90% accuracy.
AI and image analysis
Studies have indicated that AI can be applied to medical images to improve diagnosis and treatment. The approach has been shown to:
- Facilitate correct diagnoses of malignant skin lesions (Nature. 2017 Feb 2;542[7639]:115-8).
- Distinguish lung adenocarcinoma from squamous cell cancer with 100% accuracy (EBioMedicine. 2018 Jan;27:317-28).
- Recognize distinct breast cancer subtypes (ductal, lobular, mucinous, papillary) and biomarkers (bioRxiv 242818. doi: 10.1101/242818; EBioMedicine. 2018 Jan;27:317-28)
- Predict mesothelioma prognosis (Nat Med. 2019 Oct;25[10]:1519-25).
- Predict prostate biopsy results (unpublished data) and calculate Gleason scores that can predict survival in prostate cancer patients (AACR 2020, Abstract 867).
Drug development through applied AI
In another study, Dr. Elemento and colleagues used a Bayesian machine learning approach to predict targets of molecules without a known mechanism of action (Nat Commun. 2019 Nov 19;10[1]:5221).
The method involved using data on gene expression profiles, cell line viability, side effects in animals, and structures of the molecules. The researchers applied this method to a large library of orphan small molecules and found it could predict targets in about 40% of cases.
Of 24 AI-predicted microtubule-targeting molecules, 14 depolymerized microtubules in the lab. Five of these molecules were effective in cell lines that were resistant to other microtubule-targeted drugs.
Dr. Elemento went on to describe how Oncoceutics was developing an antineoplastic agent called ONC201, but the company lacked information about the agent’s target. Using AI, the target was identified as dopamine receptor 2 (DRD2; Clin Cancer Res. 2019 Apr 1;25[7]:2305-13).
With that information, Oncoceutics initiated trials of ONC201 in tumors expressing high levels of DRD2, including a highly resistant glioma (J Neurooncol. 2019 Oct;145[1]:97-105). Responses were seen, and ONC201 is now being tested against other DRD2-expressing cancers.
Challenges to acknowledge
Potential benefits of AI in the clinic are exciting, but there are many bench-to-bedside challenges.
A clinically obvious example of AI’s applications is radiographic image analysis. There is no biologic rationale for our RECIST “cut values” for partial response, minimal response, and stable disease.
If AI can measure subtle changes on imaging that correlate with tumor biology (i.e., radiomics), we stand a better chance of predicting treatment outcomes than we can with conventional measurements of shrinkage of arbitrarily selected “target lesions.”
A tremendous amount of work is needed to build the required large image banks. During that time, AI will only improve – and without the human risks of fatigue, inconsistency, or burnout.
Those human frailties notwithstanding, AI cannot substitute for the key discussions between patient and clinician regarding goals of care, trade-offs of risks and benefits, and shared decision-making regarding management options.
At least initially (but painfully), complex technologies like WGS and digital image analysis via AI may further disadvantage patients who are medically disadvantaged by geography or socioeconomic circumstances.
In the discussion period, AACR President Antoni Ribas, MD, of University of California, Los Angeles, asked whether AI can simulate crosstalk between gene pathways so that unique treatment combinations can be identified. Dr. Elemento said those simulations are the subject of ongoing investigation.
The theme of the opening plenary session at the AACR virtual meeting II was “Turning Science into Life-Saving Care.” Applications of AI to optimize personalized use of genomics, digital image analysis, and drug development show great promise for being among the technologies that can help to realize AACR’s thematic vision.
Dr. Elemento disclosed relationships with Volastra Therapeutics, OneThree Biotech, Owkin, Freenome, Genetic Intelligence, Acuamark Diagnostics, Eli Lilly, Janssen, and Sanofi.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
FROM AACR 2020
Hepatitis screening now for all patients with cancer on therapy
All patients with cancer who are candidates for systemic anticancer therapy should be screened for hepatitis B virus (HBV) infection prior to or at the start of therapy, according to an updated provisional clinical opinion (PCO) from the American Society of Clinical Oncology.
“This is a new approach [that] will actively take system changes ... but it will ultimately be safer for patients – and that is crucial,” commented Jessica P. Hwang, MD, MPH, cochair of the American Society of Clinical Oncology HBV Screening Expert Panel and the first author of the PCO.
Uptake of this universal screening approach would streamline testing protocols and identify more patients at risk for HBV reactivation who should receive prophylactic antiviral therapy, Dr. Hwang said in an interview.
The PCO calls for antiviral prophylaxis during and for at least 12 months after therapy for those with chronic HBV infection who are receiving any systemic anticancer treatment and for those with have had HBV in the past and are receiving any therapies that pose a risk for HBV reactivation.
“Hepatitis B reactivation can cause really terrible outcomes, like organ failure and even death,” Dr. Hwang, who is also a professor at the University of Texas MD Anderson Cancer Center, Houston, commented in an interview.
“This whole [issue of] reactivation and adverse outcomes with anticancer therapies is completely preventable with good planning, good communication, comanagement with specialists, and antiviral therapy and monitoring,” she added.
The updated opinion was published online July 27 in the Journal of Clinical Oncology.
It was developed in response to new data that call into question the previously recommended risk-adaptive approach to HBV screening of cancer patients, say the authors.
ASCO PCOs are developed “to provide timely clinical guidance” on the basis of emerging practice-changing information. This is the second update to follow the initial HBV screening PCO, published in 2010. In the absence of clear consensus because of limited data, the original PCO called for a risk-based approach to screening. A 2015 update extended the recommendation for screening to patients starting anti-CD20 therapy or who are to undergo stem cell transplant and to those with risk factors for HBV exposure.
The current update provides “a clinically pragmatic approach to HBV screening and management” that is based on the latest findings, say the authors. These include findings from a multicenter prospective cohort study of more than 3000 patients. In that study, 21% of patients with chronic HBV had no known risk factors for the infection. In another large prospective observational cohort study, led by Dr. Hwang, which included more than 2100 patients with cancer, 90% had one or more significant risk factors for HBV infection, making selective screening “inefficient and impractical,” she said.
“The results of these two studies suggest that a universal screening approach, its potential harms (e.g., patient and clinician anxiety about management, financial burden associated with antiviral therapy) notwithstanding, is the most efficient, clinically pragmatic approach to HBV screening in persons anticipating systemic anticancer treatment,” the authors comment.
The screening recommended in the PCO requires three tests: hepatitis B surface antigen (HBsAg), core antibody total immunoglobulin or IgG, and antibody to HBsAg tests.
Anticancer therapy should not be delayed pending the results, they write.
Planning for monitoring and long-term prophylaxis for chronic HBV infection should involve a clinician experienced in HBV management, the authors write. Management of those with past infection should be individualized. Alternatively, patients with past infection can be carefully monitored rather than given prophylactic treatment, as long as frequent and consistent follow-up is possible to allow for rapid initiation of antiviral therapy in the event of reactivation, they say.
Hormonal therapy without systemic anticancer therapy is not likely to lead to HBV reactivation in patients with chronic or past infection; antiviral therapy and management of these patients should follow relevant national HBV guidelines, they note.
Challenges in implementing universal HBV screening
The expert panel acknowledges the challenges associated with implementation of universal HBV screening as recommended in their report and notes that electronic health record–based approaches that use alerts to prompt screening have demonstrated success. In one study of high-risk primary care patients, an EHR alert system significantly increased testing rates (odds ratio, 2.64 in comparison with a control group without alerts), and another study that used a simple “sticky-note” alert system to promote referral of HBsAg patients to hepatologists increased referrals from 28% to 73%.
In a cancer population, a “comprehensive set of multimodal interventions,” including pharmacy staff checks for screening prior to anti-CD20 therapy administration and electronic medication order reviews to assess for appropriate testing and treatment before anti-CD20 therapy, increased testing rates to greater than 90% and antiviral prophylaxis rates to more than 80%.
A study of 965 patients in Taiwan showed that a computer-assisted reminder system that prompted for testing prior to ordering anticancer therapy increased screening from 8% to 86% but was less effective for improving the rates of antiviral prophylaxis for those who tested positive for HBV, particularly among physicians treating patients with nonhematologic malignancies.
“Future studies will be needed to make universal HBV screening and linkage to care efficient and systematic, likely based in EHR systems,” the panel says. The authors note that “[o]ngoing studies of HBV tests such as ultrasensitive HBsAg, HBV RNA, and hepatitis B core antigen are being studied and may be useful in predicting risk of HBV reactivation.”
The panel also identified a research gap related to HBV reactivation risks “for the growing list of agents that deplete or modulate B cells.” It notes a need for additional research on the cost-effectiveness of HBV screening. The results of prior cost analyses have been inconsistent and vary with respect to the population studied. For example, universal screening and antiviral prophylaxis approaches have been shown to be cost-effective for patients with hematologic malignancies and high HBV reactivation risk but are less so for patients with solid tumors and lower reactivation risk, they explain.
Dr. Hwang said that not one of the more than 2100 patients in her HBV screening cohort study encountered problems with receiving insurance payment for their HBV screening.
“That’s a really strong statement that insurance payers are accepting of this kind of preventative service,” she said.
Expert panel cochair Andrew Artz, MD, commented that there is now greater acceptance of the need for HBV screening across medical specialties.
“There’s growing consensus among hepatologists, infectious disease specialists, oncologists, and HBV specialists that we need to do a better job of finding patients with hepatitis B [who are] about to receive immunocompromising treatment,” Dr. Artz said in an interview.
Dr. Artz is director of the Program for Aging and Blood Cancers and deputy director of the Center for Cancer and Aging at City of Hope Comprehensive Cancer Center, Duarte, California.
He suggested that the growing acceptance is due in part to the increasing number of anticancer therapies available and the resulting increase in the likelihood of patients receiving therapies that could cause reactivation.
More therapies – and more lines of therapy – could mean greater risk, he explained. He said that testing is easy and that universal screening is the simplest approach to determining who needs it. “There’s no question we will have to change practice,” Dr. Artz said in an interview. “But this is easier than the previous approach that essentially wasn’t being followed because it was too difficult to follow and patients were being missed.”
Most clinicians will appreciate having an approach that’s easier to follow, Dr. Artz predicted.
If there’s a challenge it will be in developing partnerships with HBV specialists, particularly in rural areas. In areas where there is a paucity of subspecialists, oncologists will have to “take some ownership of the issue,” as they often do in such settings, he said.
However, with support from pharmacists, administrators, and others in embracing this guidance, implementation can take place at a systems level rather than an individual clinician level, he added.
The recommendations in this updated PCO were all rated as “strong,” with the exception of the recommendation on hormonal therapy in the absence of systemic anticancer therapy, which was rated as “moderate.” All were based on “informal consensus,” with the exception of the key recommendation for universal HBV screening – use of three specific tests – which was “evidence based.”
The expert panel agreed that the benefits outweigh the harms for each recommendation in the update.
Dr. Hwang received research funding to her institution from Gilead Sciences and Merck Sharp & Dohme. She also has a relationship with the Asian Health Foundation. Dr. Artz received research funding from Miltenyi Biotec. All expert panel members’ disclosures are available in the PCO update.
This article first appeared on Medscape.com.
All patients with cancer who are candidates for systemic anticancer therapy should be screened for hepatitis B virus (HBV) infection prior to or at the start of therapy, according to an updated provisional clinical opinion (PCO) from the American Society of Clinical Oncology.
“This is a new approach [that] will actively take system changes ... but it will ultimately be safer for patients – and that is crucial,” commented Jessica P. Hwang, MD, MPH, cochair of the American Society of Clinical Oncology HBV Screening Expert Panel and the first author of the PCO.
Uptake of this universal screening approach would streamline testing protocols and identify more patients at risk for HBV reactivation who should receive prophylactic antiviral therapy, Dr. Hwang said in an interview.
The PCO calls for antiviral prophylaxis during and for at least 12 months after therapy for those with chronic HBV infection who are receiving any systemic anticancer treatment and for those with have had HBV in the past and are receiving any therapies that pose a risk for HBV reactivation.
“Hepatitis B reactivation can cause really terrible outcomes, like organ failure and even death,” Dr. Hwang, who is also a professor at the University of Texas MD Anderson Cancer Center, Houston, commented in an interview.
“This whole [issue of] reactivation and adverse outcomes with anticancer therapies is completely preventable with good planning, good communication, comanagement with specialists, and antiviral therapy and monitoring,” she added.
The updated opinion was published online July 27 in the Journal of Clinical Oncology.
It was developed in response to new data that call into question the previously recommended risk-adaptive approach to HBV screening of cancer patients, say the authors.
ASCO PCOs are developed “to provide timely clinical guidance” on the basis of emerging practice-changing information. This is the second update to follow the initial HBV screening PCO, published in 2010. In the absence of clear consensus because of limited data, the original PCO called for a risk-based approach to screening. A 2015 update extended the recommendation for screening to patients starting anti-CD20 therapy or who are to undergo stem cell transplant and to those with risk factors for HBV exposure.
The current update provides “a clinically pragmatic approach to HBV screening and management” that is based on the latest findings, say the authors. These include findings from a multicenter prospective cohort study of more than 3000 patients. In that study, 21% of patients with chronic HBV had no known risk factors for the infection. In another large prospective observational cohort study, led by Dr. Hwang, which included more than 2100 patients with cancer, 90% had one or more significant risk factors for HBV infection, making selective screening “inefficient and impractical,” she said.
“The results of these two studies suggest that a universal screening approach, its potential harms (e.g., patient and clinician anxiety about management, financial burden associated with antiviral therapy) notwithstanding, is the most efficient, clinically pragmatic approach to HBV screening in persons anticipating systemic anticancer treatment,” the authors comment.
The screening recommended in the PCO requires three tests: hepatitis B surface antigen (HBsAg), core antibody total immunoglobulin or IgG, and antibody to HBsAg tests.
Anticancer therapy should not be delayed pending the results, they write.
Planning for monitoring and long-term prophylaxis for chronic HBV infection should involve a clinician experienced in HBV management, the authors write. Management of those with past infection should be individualized. Alternatively, patients with past infection can be carefully monitored rather than given prophylactic treatment, as long as frequent and consistent follow-up is possible to allow for rapid initiation of antiviral therapy in the event of reactivation, they say.
Hormonal therapy without systemic anticancer therapy is not likely to lead to HBV reactivation in patients with chronic or past infection; antiviral therapy and management of these patients should follow relevant national HBV guidelines, they note.
Challenges in implementing universal HBV screening
The expert panel acknowledges the challenges associated with implementation of universal HBV screening as recommended in their report and notes that electronic health record–based approaches that use alerts to prompt screening have demonstrated success. In one study of high-risk primary care patients, an EHR alert system significantly increased testing rates (odds ratio, 2.64 in comparison with a control group without alerts), and another study that used a simple “sticky-note” alert system to promote referral of HBsAg patients to hepatologists increased referrals from 28% to 73%.
In a cancer population, a “comprehensive set of multimodal interventions,” including pharmacy staff checks for screening prior to anti-CD20 therapy administration and electronic medication order reviews to assess for appropriate testing and treatment before anti-CD20 therapy, increased testing rates to greater than 90% and antiviral prophylaxis rates to more than 80%.
A study of 965 patients in Taiwan showed that a computer-assisted reminder system that prompted for testing prior to ordering anticancer therapy increased screening from 8% to 86% but was less effective for improving the rates of antiviral prophylaxis for those who tested positive for HBV, particularly among physicians treating patients with nonhematologic malignancies.
“Future studies will be needed to make universal HBV screening and linkage to care efficient and systematic, likely based in EHR systems,” the panel says. The authors note that “[o]ngoing studies of HBV tests such as ultrasensitive HBsAg, HBV RNA, and hepatitis B core antigen are being studied and may be useful in predicting risk of HBV reactivation.”
The panel also identified a research gap related to HBV reactivation risks “for the growing list of agents that deplete or modulate B cells.” It notes a need for additional research on the cost-effectiveness of HBV screening. The results of prior cost analyses have been inconsistent and vary with respect to the population studied. For example, universal screening and antiviral prophylaxis approaches have been shown to be cost-effective for patients with hematologic malignancies and high HBV reactivation risk but are less so for patients with solid tumors and lower reactivation risk, they explain.
Dr. Hwang said that not one of the more than 2100 patients in her HBV screening cohort study encountered problems with receiving insurance payment for their HBV screening.
“That’s a really strong statement that insurance payers are accepting of this kind of preventative service,” she said.
Expert panel cochair Andrew Artz, MD, commented that there is now greater acceptance of the need for HBV screening across medical specialties.
“There’s growing consensus among hepatologists, infectious disease specialists, oncologists, and HBV specialists that we need to do a better job of finding patients with hepatitis B [who are] about to receive immunocompromising treatment,” Dr. Artz said in an interview.
Dr. Artz is director of the Program for Aging and Blood Cancers and deputy director of the Center for Cancer and Aging at City of Hope Comprehensive Cancer Center, Duarte, California.
He suggested that the growing acceptance is due in part to the increasing number of anticancer therapies available and the resulting increase in the likelihood of patients receiving therapies that could cause reactivation.
More therapies – and more lines of therapy – could mean greater risk, he explained. He said that testing is easy and that universal screening is the simplest approach to determining who needs it. “There’s no question we will have to change practice,” Dr. Artz said in an interview. “But this is easier than the previous approach that essentially wasn’t being followed because it was too difficult to follow and patients were being missed.”
Most clinicians will appreciate having an approach that’s easier to follow, Dr. Artz predicted.
If there’s a challenge it will be in developing partnerships with HBV specialists, particularly in rural areas. In areas where there is a paucity of subspecialists, oncologists will have to “take some ownership of the issue,” as they often do in such settings, he said.
However, with support from pharmacists, administrators, and others in embracing this guidance, implementation can take place at a systems level rather than an individual clinician level, he added.
The recommendations in this updated PCO were all rated as “strong,” with the exception of the recommendation on hormonal therapy in the absence of systemic anticancer therapy, which was rated as “moderate.” All were based on “informal consensus,” with the exception of the key recommendation for universal HBV screening – use of three specific tests – which was “evidence based.”
The expert panel agreed that the benefits outweigh the harms for each recommendation in the update.
Dr. Hwang received research funding to her institution from Gilead Sciences and Merck Sharp & Dohme. She also has a relationship with the Asian Health Foundation. Dr. Artz received research funding from Miltenyi Biotec. All expert panel members’ disclosures are available in the PCO update.
This article first appeared on Medscape.com.
All patients with cancer who are candidates for systemic anticancer therapy should be screened for hepatitis B virus (HBV) infection prior to or at the start of therapy, according to an updated provisional clinical opinion (PCO) from the American Society of Clinical Oncology.
“This is a new approach [that] will actively take system changes ... but it will ultimately be safer for patients – and that is crucial,” commented Jessica P. Hwang, MD, MPH, cochair of the American Society of Clinical Oncology HBV Screening Expert Panel and the first author of the PCO.
Uptake of this universal screening approach would streamline testing protocols and identify more patients at risk for HBV reactivation who should receive prophylactic antiviral therapy, Dr. Hwang said in an interview.
The PCO calls for antiviral prophylaxis during and for at least 12 months after therapy for those with chronic HBV infection who are receiving any systemic anticancer treatment and for those with have had HBV in the past and are receiving any therapies that pose a risk for HBV reactivation.
“Hepatitis B reactivation can cause really terrible outcomes, like organ failure and even death,” Dr. Hwang, who is also a professor at the University of Texas MD Anderson Cancer Center, Houston, commented in an interview.
“This whole [issue of] reactivation and adverse outcomes with anticancer therapies is completely preventable with good planning, good communication, comanagement with specialists, and antiviral therapy and monitoring,” she added.
The updated opinion was published online July 27 in the Journal of Clinical Oncology.
It was developed in response to new data that call into question the previously recommended risk-adaptive approach to HBV screening of cancer patients, say the authors.
ASCO PCOs are developed “to provide timely clinical guidance” on the basis of emerging practice-changing information. This is the second update to follow the initial HBV screening PCO, published in 2010. In the absence of clear consensus because of limited data, the original PCO called for a risk-based approach to screening. A 2015 update extended the recommendation for screening to patients starting anti-CD20 therapy or who are to undergo stem cell transplant and to those with risk factors for HBV exposure.
The current update provides “a clinically pragmatic approach to HBV screening and management” that is based on the latest findings, say the authors. These include findings from a multicenter prospective cohort study of more than 3000 patients. In that study, 21% of patients with chronic HBV had no known risk factors for the infection. In another large prospective observational cohort study, led by Dr. Hwang, which included more than 2100 patients with cancer, 90% had one or more significant risk factors for HBV infection, making selective screening “inefficient and impractical,” she said.
“The results of these two studies suggest that a universal screening approach, its potential harms (e.g., patient and clinician anxiety about management, financial burden associated with antiviral therapy) notwithstanding, is the most efficient, clinically pragmatic approach to HBV screening in persons anticipating systemic anticancer treatment,” the authors comment.
The screening recommended in the PCO requires three tests: hepatitis B surface antigen (HBsAg), core antibody total immunoglobulin or IgG, and antibody to HBsAg tests.
Anticancer therapy should not be delayed pending the results, they write.
Planning for monitoring and long-term prophylaxis for chronic HBV infection should involve a clinician experienced in HBV management, the authors write. Management of those with past infection should be individualized. Alternatively, patients with past infection can be carefully monitored rather than given prophylactic treatment, as long as frequent and consistent follow-up is possible to allow for rapid initiation of antiviral therapy in the event of reactivation, they say.
Hormonal therapy without systemic anticancer therapy is not likely to lead to HBV reactivation in patients with chronic or past infection; antiviral therapy and management of these patients should follow relevant national HBV guidelines, they note.
Challenges in implementing universal HBV screening
The expert panel acknowledges the challenges associated with implementation of universal HBV screening as recommended in their report and notes that electronic health record–based approaches that use alerts to prompt screening have demonstrated success. In one study of high-risk primary care patients, an EHR alert system significantly increased testing rates (odds ratio, 2.64 in comparison with a control group without alerts), and another study that used a simple “sticky-note” alert system to promote referral of HBsAg patients to hepatologists increased referrals from 28% to 73%.
In a cancer population, a “comprehensive set of multimodal interventions,” including pharmacy staff checks for screening prior to anti-CD20 therapy administration and electronic medication order reviews to assess for appropriate testing and treatment before anti-CD20 therapy, increased testing rates to greater than 90% and antiviral prophylaxis rates to more than 80%.
A study of 965 patients in Taiwan showed that a computer-assisted reminder system that prompted for testing prior to ordering anticancer therapy increased screening from 8% to 86% but was less effective for improving the rates of antiviral prophylaxis for those who tested positive for HBV, particularly among physicians treating patients with nonhematologic malignancies.
“Future studies will be needed to make universal HBV screening and linkage to care efficient and systematic, likely based in EHR systems,” the panel says. The authors note that “[o]ngoing studies of HBV tests such as ultrasensitive HBsAg, HBV RNA, and hepatitis B core antigen are being studied and may be useful in predicting risk of HBV reactivation.”
The panel also identified a research gap related to HBV reactivation risks “for the growing list of agents that deplete or modulate B cells.” It notes a need for additional research on the cost-effectiveness of HBV screening. The results of prior cost analyses have been inconsistent and vary with respect to the population studied. For example, universal screening and antiviral prophylaxis approaches have been shown to be cost-effective for patients with hematologic malignancies and high HBV reactivation risk but are less so for patients with solid tumors and lower reactivation risk, they explain.
Dr. Hwang said that not one of the more than 2100 patients in her HBV screening cohort study encountered problems with receiving insurance payment for their HBV screening.
“That’s a really strong statement that insurance payers are accepting of this kind of preventative service,” she said.
Expert panel cochair Andrew Artz, MD, commented that there is now greater acceptance of the need for HBV screening across medical specialties.
“There’s growing consensus among hepatologists, infectious disease specialists, oncologists, and HBV specialists that we need to do a better job of finding patients with hepatitis B [who are] about to receive immunocompromising treatment,” Dr. Artz said in an interview.
Dr. Artz is director of the Program for Aging and Blood Cancers and deputy director of the Center for Cancer and Aging at City of Hope Comprehensive Cancer Center, Duarte, California.
He suggested that the growing acceptance is due in part to the increasing number of anticancer therapies available and the resulting increase in the likelihood of patients receiving therapies that could cause reactivation.
More therapies – and more lines of therapy – could mean greater risk, he explained. He said that testing is easy and that universal screening is the simplest approach to determining who needs it. “There’s no question we will have to change practice,” Dr. Artz said in an interview. “But this is easier than the previous approach that essentially wasn’t being followed because it was too difficult to follow and patients were being missed.”
Most clinicians will appreciate having an approach that’s easier to follow, Dr. Artz predicted.
If there’s a challenge it will be in developing partnerships with HBV specialists, particularly in rural areas. In areas where there is a paucity of subspecialists, oncologists will have to “take some ownership of the issue,” as they often do in such settings, he said.
However, with support from pharmacists, administrators, and others in embracing this guidance, implementation can take place at a systems level rather than an individual clinician level, he added.
The recommendations in this updated PCO were all rated as “strong,” with the exception of the recommendation on hormonal therapy in the absence of systemic anticancer therapy, which was rated as “moderate.” All were based on “informal consensus,” with the exception of the key recommendation for universal HBV screening – use of three specific tests – which was “evidence based.”
The expert panel agreed that the benefits outweigh the harms for each recommendation in the update.
Dr. Hwang received research funding to her institution from Gilead Sciences and Merck Sharp & Dohme. She also has a relationship with the Asian Health Foundation. Dr. Artz received research funding from Miltenyi Biotec. All expert panel members’ disclosures are available in the PCO update.
This article first appeared on Medscape.com.
ASCO says ‘no’ to home infusions of cancer treatment, with exceptions
in a new policy statement issued July 31.
At the same time, it supports exceptions: namely, when individual physicians and patients, having jointly discussed risks and benefits, agree to have treatments administered in the home.
The new policy is limited to intravenous infusions of anticancer agents such as chemotherapy, monoclonal antibodies, and other drugs — administered by health care personnel. It does not refer to injections.
The policy was prompted by regulatory flexibilities from the Centers for Medicare & Medicaid Services made in response to the accelerating COVID-19 pandemic. “Among these flexibilities were new provisions that enabled providers to deliver care in a setting most appropriate – and safest – for individual patient circumstances,” which has “opened the path for potential increases in use of home infusion for anticancer therapy,” says ASCO.
“We’re not ready to endorse [chemo at home] as a general policy until we have evidence that it’s safe. At the same time, the policy gives physicians and patients autonomy to respond to whatever situation they find themselves in,” Stephen Grubbs, MD, ASCO’s senior director of clinical affairs, said in an interview.
“Antineoplastic drugs are effective at treating cancer but can be extremely toxic to normal human cells,” reads the statement, which was written by a group of about 25 professionals, including Grubbs and other ASCO staff as well as independent advisers.
“There is a paucity of evidence directly comparing the safety of chemotherapy infusions in the home and outpatient settings,” the ASCO policy explains.
ASCO’s policy acknowledges that there are data “from other countries demonstrating that ... home infusion can be safe, well-tolerated, and may be preferred by some patients.” But such data are limited and only apply “to certain circumstances and for specific agents,” it adds.
One US cancer center (in Philadelphia) already has an established chemo-at-home program and has seen an increase in its use during the pandemic, as reported by Medscape Medical News. Approached for comment, Justin Bekelman, MD, director of the Penn Center for Cancer Care Innovation in Philadelphia, interpreted the new ASCO policy in a positive light.
“Physicians at the Abramson Cancer Center of the University of Pennsylvania and ASCO agree – home-based cancer therapy with oncologist oversight and well-designed safety protocols can be a safe option for patients with cancer,” he said in a statement.
ASCO says its existing safety standards “may be difficult to satisfy in the home infusion context,” including for safely resolving life-threatening emergencies.
Grubbs said that in the worst-case scenario, such as anaphylaxis, “you can die from [it] if you don’t manage it quickly and properly.”
“When I was practicing, we always had a physician present right next to the infusion area because these are severe reactions that happen very quickly,” he said, adding that “several a year” occurred when he practiced full-time.
Also, chemotherapy spills are a “big deal” in the home, as clean-up may be complex and difficult, added Grubbs.
Data from ASCO’s PracticeNET program show that in the first months (March and April) of the COVID-19 pandemic, chemotherapy visits to infusion suites were not reduced in a dataset of 16 US practices, he noted. However, there are exceptions and variance based on location, Grubbs said, such as “hot spots” including New York City in April.
While the pandemic has no end in sight, ASCO issued a set of six recommendations for use of anticancer therapies infused in the home. First, they call for independent, publicly funded research to evaluate the safety and effectiveness of home infusion of anticancer therapy.
Next in importance, ASCO wants the current temporary regulation change from CMS due to the pandemic to end.
“CMS should not extend the temporary flexibility related to home infusion for Part B cancer drugs that was approved as part of their response to the public health emergency,” they state.
Even before the pandemic, changes were afoot. Under the 21st Century Cures Act, which was passed in 2019 and will be implemented in 2021, CMS instituted a permanent home infusion therapy services benefit, which includes anticancer therapies. It “remains to be seen what, if any, shift away from outpatient infusion facilities will occur,” observes ASCO in its policy statement.
This article first appeared on Medscape.com.
in a new policy statement issued July 31.
At the same time, it supports exceptions: namely, when individual physicians and patients, having jointly discussed risks and benefits, agree to have treatments administered in the home.
The new policy is limited to intravenous infusions of anticancer agents such as chemotherapy, monoclonal antibodies, and other drugs — administered by health care personnel. It does not refer to injections.
The policy was prompted by regulatory flexibilities from the Centers for Medicare & Medicaid Services made in response to the accelerating COVID-19 pandemic. “Among these flexibilities were new provisions that enabled providers to deliver care in a setting most appropriate – and safest – for individual patient circumstances,” which has “opened the path for potential increases in use of home infusion for anticancer therapy,” says ASCO.
“We’re not ready to endorse [chemo at home] as a general policy until we have evidence that it’s safe. At the same time, the policy gives physicians and patients autonomy to respond to whatever situation they find themselves in,” Stephen Grubbs, MD, ASCO’s senior director of clinical affairs, said in an interview.
“Antineoplastic drugs are effective at treating cancer but can be extremely toxic to normal human cells,” reads the statement, which was written by a group of about 25 professionals, including Grubbs and other ASCO staff as well as independent advisers.
“There is a paucity of evidence directly comparing the safety of chemotherapy infusions in the home and outpatient settings,” the ASCO policy explains.
ASCO’s policy acknowledges that there are data “from other countries demonstrating that ... home infusion can be safe, well-tolerated, and may be preferred by some patients.” But such data are limited and only apply “to certain circumstances and for specific agents,” it adds.
One US cancer center (in Philadelphia) already has an established chemo-at-home program and has seen an increase in its use during the pandemic, as reported by Medscape Medical News. Approached for comment, Justin Bekelman, MD, director of the Penn Center for Cancer Care Innovation in Philadelphia, interpreted the new ASCO policy in a positive light.
“Physicians at the Abramson Cancer Center of the University of Pennsylvania and ASCO agree – home-based cancer therapy with oncologist oversight and well-designed safety protocols can be a safe option for patients with cancer,” he said in a statement.
ASCO says its existing safety standards “may be difficult to satisfy in the home infusion context,” including for safely resolving life-threatening emergencies.
Grubbs said that in the worst-case scenario, such as anaphylaxis, “you can die from [it] if you don’t manage it quickly and properly.”
“When I was practicing, we always had a physician present right next to the infusion area because these are severe reactions that happen very quickly,” he said, adding that “several a year” occurred when he practiced full-time.
Also, chemotherapy spills are a “big deal” in the home, as clean-up may be complex and difficult, added Grubbs.
Data from ASCO’s PracticeNET program show that in the first months (March and April) of the COVID-19 pandemic, chemotherapy visits to infusion suites were not reduced in a dataset of 16 US practices, he noted. However, there are exceptions and variance based on location, Grubbs said, such as “hot spots” including New York City in April.
While the pandemic has no end in sight, ASCO issued a set of six recommendations for use of anticancer therapies infused in the home. First, they call for independent, publicly funded research to evaluate the safety and effectiveness of home infusion of anticancer therapy.
Next in importance, ASCO wants the current temporary regulation change from CMS due to the pandemic to end.
“CMS should not extend the temporary flexibility related to home infusion for Part B cancer drugs that was approved as part of their response to the public health emergency,” they state.
Even before the pandemic, changes were afoot. Under the 21st Century Cures Act, which was passed in 2019 and will be implemented in 2021, CMS instituted a permanent home infusion therapy services benefit, which includes anticancer therapies. It “remains to be seen what, if any, shift away from outpatient infusion facilities will occur,” observes ASCO in its policy statement.
This article first appeared on Medscape.com.
in a new policy statement issued July 31.
At the same time, it supports exceptions: namely, when individual physicians and patients, having jointly discussed risks and benefits, agree to have treatments administered in the home.
The new policy is limited to intravenous infusions of anticancer agents such as chemotherapy, monoclonal antibodies, and other drugs — administered by health care personnel. It does not refer to injections.
The policy was prompted by regulatory flexibilities from the Centers for Medicare & Medicaid Services made in response to the accelerating COVID-19 pandemic. “Among these flexibilities were new provisions that enabled providers to deliver care in a setting most appropriate – and safest – for individual patient circumstances,” which has “opened the path for potential increases in use of home infusion for anticancer therapy,” says ASCO.
“We’re not ready to endorse [chemo at home] as a general policy until we have evidence that it’s safe. At the same time, the policy gives physicians and patients autonomy to respond to whatever situation they find themselves in,” Stephen Grubbs, MD, ASCO’s senior director of clinical affairs, said in an interview.
“Antineoplastic drugs are effective at treating cancer but can be extremely toxic to normal human cells,” reads the statement, which was written by a group of about 25 professionals, including Grubbs and other ASCO staff as well as independent advisers.
“There is a paucity of evidence directly comparing the safety of chemotherapy infusions in the home and outpatient settings,” the ASCO policy explains.
ASCO’s policy acknowledges that there are data “from other countries demonstrating that ... home infusion can be safe, well-tolerated, and may be preferred by some patients.” But such data are limited and only apply “to certain circumstances and for specific agents,” it adds.
One US cancer center (in Philadelphia) already has an established chemo-at-home program and has seen an increase in its use during the pandemic, as reported by Medscape Medical News. Approached for comment, Justin Bekelman, MD, director of the Penn Center for Cancer Care Innovation in Philadelphia, interpreted the new ASCO policy in a positive light.
“Physicians at the Abramson Cancer Center of the University of Pennsylvania and ASCO agree – home-based cancer therapy with oncologist oversight and well-designed safety protocols can be a safe option for patients with cancer,” he said in a statement.
ASCO says its existing safety standards “may be difficult to satisfy in the home infusion context,” including for safely resolving life-threatening emergencies.
Grubbs said that in the worst-case scenario, such as anaphylaxis, “you can die from [it] if you don’t manage it quickly and properly.”
“When I was practicing, we always had a physician present right next to the infusion area because these are severe reactions that happen very quickly,” he said, adding that “several a year” occurred when he practiced full-time.
Also, chemotherapy spills are a “big deal” in the home, as clean-up may be complex and difficult, added Grubbs.
Data from ASCO’s PracticeNET program show that in the first months (March and April) of the COVID-19 pandemic, chemotherapy visits to infusion suites were not reduced in a dataset of 16 US practices, he noted. However, there are exceptions and variance based on location, Grubbs said, such as “hot spots” including New York City in April.
While the pandemic has no end in sight, ASCO issued a set of six recommendations for use of anticancer therapies infused in the home. First, they call for independent, publicly funded research to evaluate the safety and effectiveness of home infusion of anticancer therapy.
Next in importance, ASCO wants the current temporary regulation change from CMS due to the pandemic to end.
“CMS should not extend the temporary flexibility related to home infusion for Part B cancer drugs that was approved as part of their response to the public health emergency,” they state.
Even before the pandemic, changes were afoot. Under the 21st Century Cures Act, which was passed in 2019 and will be implemented in 2021, CMS instituted a permanent home infusion therapy services benefit, which includes anticancer therapies. It “remains to be seen what, if any, shift away from outpatient infusion facilities will occur,” observes ASCO in its policy statement.
This article first appeared on Medscape.com.