User login
Survey quantifies COVID-19’s impact on oncology
An international survey provides new insights into how COVID-19 has affected, and may continue to affect, the field of oncology.
The survey showed that “COVID-19 has had a major impact on the organization of patient care, on the well-being of caregivers, on continued medical education, and on clinical trial activities in oncology,” stated Guy Jerusalem, MD, PhD, of Centre Hospitalier Universitaire de Liège (Belgium).
Dr. Jerusalem presented these findings at the European Society for Medical Oncology Virtual Congress 2020.
The survey was distributed by 20 oncologists from 10 of the countries most affected by COVID-19. Responses were obtained from 109 oncologists representing centers in 18 countries. The responses were recorded between June 17 and July 14, 2020.
The survey consisted of 95 items intended to evaluate the impact of COVID-19 on the organization of oncologic care. Questions encompassed the capacity and service offered at each center, the magnitude of COVID-19–based care interruptions and the reasons for them, the ensuing challenges faced, interventions implemented, and the estimated harms to patients during the pandemic.
The 109 oncologists surveyed had a median of 20 years of oncology experience. A majority of respondents were men (61.5%), and the median age was 48.5 years.
The respondents had worked predominantly (62.4%) at academic hospitals, with 29.6% at community hospitals. Most respondents worked at general hospitals with an oncology unit (66.1%) rather than a specialized separate cancer center (32.1%).
The most common specialty was breast cancer (60.6%), followed by gastrointestinal cancer (10.1%), urogenital cancer (9.2%), and lung cancer (8.3%).
Impact on treatment
The treatment modalities affected by the pandemic – through cancellations or delays in more than 10% of patients – included surgery (in 34% of centers), chemotherapy (22%), radiotherapy (13.7%), checkpoint inhibitor therapy (9.1%), monoclonal antibodies (9%), and oral targeted therapy (3.7%).
Among oncologists treating breast cancer, cancellations/delays in more than 10% of patients were reported for everolimus (18%), CDK4/6 inhibitors (8.9%), and endocrine therapy (2.2%).
Overall, 34.8% of respondents reported increased use of granulocyte colony–stimulating factor, and 6.4% reported increased use of erythropoietin.
On the other hand, 11.1% of respondents reported a decrease in the use of double immunotherapy, and 21.9% reported decreased use of corticosteroids.
Not only can the immunosuppressive effects of steroid use increase infection risks, Dr. Jerusalem noted, fever suppression can lead to a delayed diagnosis of COVID-19.
“To circumvent potential higher infection risks or greater disease severity, we use lower doses of steroids, but this is not based on studies,” he said.
“Previous exposure to steroids or being on steroids at the time of COVID-19 infection is a detrimental factor for complications and mortality,” commented ESMO President Solange Peters, MD, PhD, of Centre Hospitalier Universitaire Vaudois in Lausanne, Switzerland.
Dr. Peters noted that the observation was based on lung cancer registry findings. Furthermore, because data from smaller outbreaks of other coronavirus infections suggested worse prognosis and increased mortality, steroid use was already feared in the very early days of the COVID-19 pandemic.
Lastly, earlier cessation of palliative treatment was observed in 32.1% of centers, and 64.2% of respondents agreed that undertreatment because of COVID-19 is a major concern.
Dr. Jerusalem noted that the survey data do not explain the early cessation of palliative treatment. “I suspect that many patients died at home rather than alone in institutions because it was the only way they could die with their families around them.”
Telehealth, meetings, and trials
The survey also revealed rationales for the use of teleconsultation, including follow-up (94.5%), oral therapy (92.7%), immunotherapy (57.8%), and chemotherapy (55%).
Most respondents reported more frequent use of virtual meetings for continuing medical education (94%), oncologic team meetings (92%), and tumor boards (82%).
While about 82% of respondents said they were likely to continue the use of telemedicine, 45% said virtual conferences are not an acceptable alternative to live international conferences such as ESMO, Dr. Jerusalem said.
Finally, nearly three-quarters of respondents (72.5%) said all clinical trial activities are or will soon be activated, or never stopped, at their centers. On the other hand, 27.5% of respondents reported that their centers had major protocol violations or deviations, and 37% of respondents said they expect significant reductions in clinical trial activities this year.
Dr. Jerusalem concluded that COVID-19 is having a major, long-term impact on the organization of patient care, caregivers, continued medical education, and clinical trial activities in oncology.
He cautioned that “the risk of a delayed diagnosis of new cancers and economic consequences of COVID-19 on access to health care and cancer treatments have to be carefully evaluated.”
This research was funded by Fondation Léon Fredericq. Dr. Jerusalem disclosed relationships with Novartis, Roche, Lilly, Pfizer, Amgen, Bristol-Myers Squibb, AstraZeneca, Daiichi Sankyo, AbbVie, MedImmune, and Merck. Dr. Peters disclosed relationships with AbbVie, Amgen, AstraZeneca, and many other companies.
SOURCE: Jerusalem G et al. ESMO 2020, Abstract LBA76.
An international survey provides new insights into how COVID-19 has affected, and may continue to affect, the field of oncology.
The survey showed that “COVID-19 has had a major impact on the organization of patient care, on the well-being of caregivers, on continued medical education, and on clinical trial activities in oncology,” stated Guy Jerusalem, MD, PhD, of Centre Hospitalier Universitaire de Liège (Belgium).
Dr. Jerusalem presented these findings at the European Society for Medical Oncology Virtual Congress 2020.
The survey was distributed by 20 oncologists from 10 of the countries most affected by COVID-19. Responses were obtained from 109 oncologists representing centers in 18 countries. The responses were recorded between June 17 and July 14, 2020.
The survey consisted of 95 items intended to evaluate the impact of COVID-19 on the organization of oncologic care. Questions encompassed the capacity and service offered at each center, the magnitude of COVID-19–based care interruptions and the reasons for them, the ensuing challenges faced, interventions implemented, and the estimated harms to patients during the pandemic.
The 109 oncologists surveyed had a median of 20 years of oncology experience. A majority of respondents were men (61.5%), and the median age was 48.5 years.
The respondents had worked predominantly (62.4%) at academic hospitals, with 29.6% at community hospitals. Most respondents worked at general hospitals with an oncology unit (66.1%) rather than a specialized separate cancer center (32.1%).
The most common specialty was breast cancer (60.6%), followed by gastrointestinal cancer (10.1%), urogenital cancer (9.2%), and lung cancer (8.3%).
Impact on treatment
The treatment modalities affected by the pandemic – through cancellations or delays in more than 10% of patients – included surgery (in 34% of centers), chemotherapy (22%), radiotherapy (13.7%), checkpoint inhibitor therapy (9.1%), monoclonal antibodies (9%), and oral targeted therapy (3.7%).
Among oncologists treating breast cancer, cancellations/delays in more than 10% of patients were reported for everolimus (18%), CDK4/6 inhibitors (8.9%), and endocrine therapy (2.2%).
Overall, 34.8% of respondents reported increased use of granulocyte colony–stimulating factor, and 6.4% reported increased use of erythropoietin.
On the other hand, 11.1% of respondents reported a decrease in the use of double immunotherapy, and 21.9% reported decreased use of corticosteroids.
Not only can the immunosuppressive effects of steroid use increase infection risks, Dr. Jerusalem noted, fever suppression can lead to a delayed diagnosis of COVID-19.
“To circumvent potential higher infection risks or greater disease severity, we use lower doses of steroids, but this is not based on studies,” he said.
“Previous exposure to steroids or being on steroids at the time of COVID-19 infection is a detrimental factor for complications and mortality,” commented ESMO President Solange Peters, MD, PhD, of Centre Hospitalier Universitaire Vaudois in Lausanne, Switzerland.
Dr. Peters noted that the observation was based on lung cancer registry findings. Furthermore, because data from smaller outbreaks of other coronavirus infections suggested worse prognosis and increased mortality, steroid use was already feared in the very early days of the COVID-19 pandemic.
Lastly, earlier cessation of palliative treatment was observed in 32.1% of centers, and 64.2% of respondents agreed that undertreatment because of COVID-19 is a major concern.
Dr. Jerusalem noted that the survey data do not explain the early cessation of palliative treatment. “I suspect that many patients died at home rather than alone in institutions because it was the only way they could die with their families around them.”
Telehealth, meetings, and trials
The survey also revealed rationales for the use of teleconsultation, including follow-up (94.5%), oral therapy (92.7%), immunotherapy (57.8%), and chemotherapy (55%).
Most respondents reported more frequent use of virtual meetings for continuing medical education (94%), oncologic team meetings (92%), and tumor boards (82%).
While about 82% of respondents said they were likely to continue the use of telemedicine, 45% said virtual conferences are not an acceptable alternative to live international conferences such as ESMO, Dr. Jerusalem said.
Finally, nearly three-quarters of respondents (72.5%) said all clinical trial activities are or will soon be activated, or never stopped, at their centers. On the other hand, 27.5% of respondents reported that their centers had major protocol violations or deviations, and 37% of respondents said they expect significant reductions in clinical trial activities this year.
Dr. Jerusalem concluded that COVID-19 is having a major, long-term impact on the organization of patient care, caregivers, continued medical education, and clinical trial activities in oncology.
He cautioned that “the risk of a delayed diagnosis of new cancers and economic consequences of COVID-19 on access to health care and cancer treatments have to be carefully evaluated.”
This research was funded by Fondation Léon Fredericq. Dr. Jerusalem disclosed relationships with Novartis, Roche, Lilly, Pfizer, Amgen, Bristol-Myers Squibb, AstraZeneca, Daiichi Sankyo, AbbVie, MedImmune, and Merck. Dr. Peters disclosed relationships with AbbVie, Amgen, AstraZeneca, and many other companies.
SOURCE: Jerusalem G et al. ESMO 2020, Abstract LBA76.
An international survey provides new insights into how COVID-19 has affected, and may continue to affect, the field of oncology.
The survey showed that “COVID-19 has had a major impact on the organization of patient care, on the well-being of caregivers, on continued medical education, and on clinical trial activities in oncology,” stated Guy Jerusalem, MD, PhD, of Centre Hospitalier Universitaire de Liège (Belgium).
Dr. Jerusalem presented these findings at the European Society for Medical Oncology Virtual Congress 2020.
The survey was distributed by 20 oncologists from 10 of the countries most affected by COVID-19. Responses were obtained from 109 oncologists representing centers in 18 countries. The responses were recorded between June 17 and July 14, 2020.
The survey consisted of 95 items intended to evaluate the impact of COVID-19 on the organization of oncologic care. Questions encompassed the capacity and service offered at each center, the magnitude of COVID-19–based care interruptions and the reasons for them, the ensuing challenges faced, interventions implemented, and the estimated harms to patients during the pandemic.
The 109 oncologists surveyed had a median of 20 years of oncology experience. A majority of respondents were men (61.5%), and the median age was 48.5 years.
The respondents had worked predominantly (62.4%) at academic hospitals, with 29.6% at community hospitals. Most respondents worked at general hospitals with an oncology unit (66.1%) rather than a specialized separate cancer center (32.1%).
The most common specialty was breast cancer (60.6%), followed by gastrointestinal cancer (10.1%), urogenital cancer (9.2%), and lung cancer (8.3%).
Impact on treatment
The treatment modalities affected by the pandemic – through cancellations or delays in more than 10% of patients – included surgery (in 34% of centers), chemotherapy (22%), radiotherapy (13.7%), checkpoint inhibitor therapy (9.1%), monoclonal antibodies (9%), and oral targeted therapy (3.7%).
Among oncologists treating breast cancer, cancellations/delays in more than 10% of patients were reported for everolimus (18%), CDK4/6 inhibitors (8.9%), and endocrine therapy (2.2%).
Overall, 34.8% of respondents reported increased use of granulocyte colony–stimulating factor, and 6.4% reported increased use of erythropoietin.
On the other hand, 11.1% of respondents reported a decrease in the use of double immunotherapy, and 21.9% reported decreased use of corticosteroids.
Not only can the immunosuppressive effects of steroid use increase infection risks, Dr. Jerusalem noted, fever suppression can lead to a delayed diagnosis of COVID-19.
“To circumvent potential higher infection risks or greater disease severity, we use lower doses of steroids, but this is not based on studies,” he said.
“Previous exposure to steroids or being on steroids at the time of COVID-19 infection is a detrimental factor for complications and mortality,” commented ESMO President Solange Peters, MD, PhD, of Centre Hospitalier Universitaire Vaudois in Lausanne, Switzerland.
Dr. Peters noted that the observation was based on lung cancer registry findings. Furthermore, because data from smaller outbreaks of other coronavirus infections suggested worse prognosis and increased mortality, steroid use was already feared in the very early days of the COVID-19 pandemic.
Lastly, earlier cessation of palliative treatment was observed in 32.1% of centers, and 64.2% of respondents agreed that undertreatment because of COVID-19 is a major concern.
Dr. Jerusalem noted that the survey data do not explain the early cessation of palliative treatment. “I suspect that many patients died at home rather than alone in institutions because it was the only way they could die with their families around them.”
Telehealth, meetings, and trials
The survey also revealed rationales for the use of teleconsultation, including follow-up (94.5%), oral therapy (92.7%), immunotherapy (57.8%), and chemotherapy (55%).
Most respondents reported more frequent use of virtual meetings for continuing medical education (94%), oncologic team meetings (92%), and tumor boards (82%).
While about 82% of respondents said they were likely to continue the use of telemedicine, 45% said virtual conferences are not an acceptable alternative to live international conferences such as ESMO, Dr. Jerusalem said.
Finally, nearly three-quarters of respondents (72.5%) said all clinical trial activities are or will soon be activated, or never stopped, at their centers. On the other hand, 27.5% of respondents reported that their centers had major protocol violations or deviations, and 37% of respondents said they expect significant reductions in clinical trial activities this year.
Dr. Jerusalem concluded that COVID-19 is having a major, long-term impact on the organization of patient care, caregivers, continued medical education, and clinical trial activities in oncology.
He cautioned that “the risk of a delayed diagnosis of new cancers and economic consequences of COVID-19 on access to health care and cancer treatments have to be carefully evaluated.”
This research was funded by Fondation Léon Fredericq. Dr. Jerusalem disclosed relationships with Novartis, Roche, Lilly, Pfizer, Amgen, Bristol-Myers Squibb, AstraZeneca, Daiichi Sankyo, AbbVie, MedImmune, and Merck. Dr. Peters disclosed relationships with AbbVie, Amgen, AstraZeneca, and many other companies.
SOURCE: Jerusalem G et al. ESMO 2020, Abstract LBA76.
FROM ESMO 2020
COVID-19 prompts ‘democratization’ of cancer trials
The pandemic has taught researchers how to decentralize trials, which should not only improve patient satisfaction but increase trial accrual by providing access to typically underserved populations, Patricia M. LoRusso, DO, of Yale University, New Haven, Conn., said at the meeting.
Dr. LoRusso was one of six panelists who participated in a forum about changes to cancer trials that were prompted by the pandemic. The forum was moderated by Keith T. Flaherty, MD, of Massachusetts General Hospital in Boston.
Dr. Flaherty asked the panelists to explain adjustments their organizations have made in response to the pandemic, discuss accomplishments, and speculate on future challenges and priorities.
Trial, administrative, and patient-care modifications
COVID-19 put some cancer trials on hold. For others, the pandemic forced sponsors and study chairs to reduce trial complexity and identify nonessential aspects of the studies, according to panelist José Baselga, MD, PhD, of AstraZeneca.
Specifically, exploratory objectives were subjugated to patient safety and a focus on the primary endpoints of each trial.
Once the critical data were identified, study chairs were asked to determine whether data could be obtained through technologies that could substitute for face-to-face contact between patients and staff – for example, patient-reported outcome tools and at-home digital monitoring.
Modifications prompted by the pandemic include the following:
- On-site auditing was suspended.
- Oral investigational agents were shipped directly to patients.
- “Remote” informed consent (telephone or video consenting) was permitted.
- Local providers could perform study-related services, with oversight by the research site.
- Minor deviations from the written protocols were allowed, provided the deviations did not affect patient care or data integrity.
“Obviously, the pandemic has been horrible, but what it has allowed us to do, as investigators in the clinical research landscape, … is to change our focus somewhat and realize, first and foremost, the patient is at the center of this,” Dr. LoRusso said.
Operational accomplishments and benefits
The pandemic caused a 40% decline in accrual to studies supported by the National Cancer Institute’s (NCI) Clinical Trials Network (NCTN) from mid-March to early April, according to James H. Doroshow, MD, of NCI.
However, after modifications to administrative and regulatory procedures, accrual to NCTN trials recovered to approximately 80% of prepandemic levels, Dr. Doroshow said.
The pandemic prompted investigators to leverage tools and technology they had not previously used frequently or at all, the panelists pointed out.
Investigators discovered perforce that telehealth could be used for almost all trial-related assessments. In lieu of physical examination, patients could send pictures of rashes and use electronic devices to monitor blood sugar values and vital signs.
Digital radiographic studies were performed at sites that were most convenient for patients, downloaded, and reinterpreted at the study institution. Visiting nurses and neighborhood laboratories enabled less-frequent in-person visits for assessments.
These adjustments have been particularly important for geographically and/or socioeconomically disadvantaged patients, the panelists said.
Overall, there was agreement among the panelists that shared values and trust among regulatory authorities, sponsors, investigators, and clinicians were impressive in their urgency, sincerity, and patient centricity.
“This pandemic … has forced us to think differently and be nimble and creative to our approach to maintaining our overriding goals while at the same time bringing these innovative therapies forward for patients with cancer and other serious and life-threatening diseases as quickly as possible,” said panelist Kristen M. Hege, MD, of Bristol-Myers Squibb.
In fact, Dr. Hege noted, some cancer-related therapies (e.g., BTK inhibitors, JAK inhibitors, and immunomodulatory agents) were “repurposed” rapidly and tested against COVID-related complications.
Streamlining trial regulatory processes
In addition to changing ongoing trials, the pandemic has affected how new research projects are launched.
One new study that came together quickly in response to the pandemic is the NCI COVID-19 in Cancer Patients Study (NCCAPS). NCCAPS is a natural history study with biospecimens and an imaging library. It was approved in just 5 weeks and is active in 650 sites, with “gangbusters” accrual, Dr. Doroshow said.
The rapidness of NCCAPS’ design and implementation should prompt the revision of previously accepted timelines for trial activation and lead to streamlined future processes.
Another project that was launched quickly in response to the pandemic is the COVID-19 evidence accelerator, according to Paul G. Kluetz, MD, of the Food and Drug Administration.
The COVID-19 evidence accelerator integrates real-world evidence into a database to provide investigators and health systems with the ability to gather information, design rapid turnaround queries, and share results. The evidence accelerator can provide study chairs with information that may have relevance to the safety of participants in clinical trials.
Future directions and challenges
The panelists agreed that pandemic-related modifications in processes will not only accelerate trial approval and activation but should facilitate higher study accrual, increase the diversity of protocol participants, and decrease the costs associated with clinical trial conduct.
With that in mind, the NCI is planning randomized clinical trials in which “process A” is compared with “process B,” Dr. Doroshow said. The goal is to determine which modifications are most likely to make trials available to patients without compromising data integrity or patient safety.
“How much less data do you need to have an outcome that will be similar?” Dr. Doroshow asked. “How many fewer visits, how many fewer tests, how much can you save? Physicians, clinical trialists, all of us respond to data, and if you get the same outcome at a third of the cost, then everybody benefits.”
Nonetheless, we will need to be vigilant for unintended vulnerabilities from well-intended efforts, according to Dr. Kluetz. Study chairs, sponsors, and regulatory agencies will need to be attentive to whether there are important differences in scan quality or interpretation, missing data that influence trial outcomes, and so on.
Dr. Hege pointed out that differences among data sources may be less important when treatments generate large effects but may be vitally important when the relative differences among treatments are small.
On a practical level, decentralizing clinical research may negatively impact the finances of tertiary care centers, which could threaten the required infrastructure for clinical trials, a few panelists noted.
The relative balance of NCI-, industry-, and investigator-initiated trials may require adjustment so that research income is adequate to maintain the costs associated with cancer clinical trials.
Shared goals and democratization
The pandemic has required all stakeholders in clinical research to rely on relationships of trust and shared goals, said Caroline Robert, MD, PhD, of Institut Gustave Roussy in Villejuif, France.
Dr. Kluetz summarized those goals as improving trial efficiencies, decreasing patient burden, decentralizing trials, and maintaining trial integrity.
A decentralized clinical trials operational model could lead to better generalizability of study outcomes, normalization of life for patients on studies, and lower costs of trial conduct. As such, decentralization would promote democratization.
Coupled with ongoing efforts to reduce eligibility criteria in cancer trials, the pandemic has brought operational solutions that should be perpetuated and has reminded us of the interlocking and mutually supportive relationships on which clinical research success depends.
Dr. Doroshow and Dr. Kluetz disclosed no conflicts of interest. All other panelists disclosed financial relationships, including employment, with a range of companies.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
SOURCE: Flaherty KT et al. AACR: COVID-19 and Cancer, Regulatory and Operational Implications of Cancer Clinical Trial Changes During COVID-19.
The pandemic has taught researchers how to decentralize trials, which should not only improve patient satisfaction but increase trial accrual by providing access to typically underserved populations, Patricia M. LoRusso, DO, of Yale University, New Haven, Conn., said at the meeting.
Dr. LoRusso was one of six panelists who participated in a forum about changes to cancer trials that were prompted by the pandemic. The forum was moderated by Keith T. Flaherty, MD, of Massachusetts General Hospital in Boston.
Dr. Flaherty asked the panelists to explain adjustments their organizations have made in response to the pandemic, discuss accomplishments, and speculate on future challenges and priorities.
Trial, administrative, and patient-care modifications
COVID-19 put some cancer trials on hold. For others, the pandemic forced sponsors and study chairs to reduce trial complexity and identify nonessential aspects of the studies, according to panelist José Baselga, MD, PhD, of AstraZeneca.
Specifically, exploratory objectives were subjugated to patient safety and a focus on the primary endpoints of each trial.
Once the critical data were identified, study chairs were asked to determine whether data could be obtained through technologies that could substitute for face-to-face contact between patients and staff – for example, patient-reported outcome tools and at-home digital monitoring.
Modifications prompted by the pandemic include the following:
- On-site auditing was suspended.
- Oral investigational agents were shipped directly to patients.
- “Remote” informed consent (telephone or video consenting) was permitted.
- Local providers could perform study-related services, with oversight by the research site.
- Minor deviations from the written protocols were allowed, provided the deviations did not affect patient care or data integrity.
“Obviously, the pandemic has been horrible, but what it has allowed us to do, as investigators in the clinical research landscape, … is to change our focus somewhat and realize, first and foremost, the patient is at the center of this,” Dr. LoRusso said.
Operational accomplishments and benefits
The pandemic caused a 40% decline in accrual to studies supported by the National Cancer Institute’s (NCI) Clinical Trials Network (NCTN) from mid-March to early April, according to James H. Doroshow, MD, of NCI.
However, after modifications to administrative and regulatory procedures, accrual to NCTN trials recovered to approximately 80% of prepandemic levels, Dr. Doroshow said.
The pandemic prompted investigators to leverage tools and technology they had not previously used frequently or at all, the panelists pointed out.
Investigators discovered perforce that telehealth could be used for almost all trial-related assessments. In lieu of physical examination, patients could send pictures of rashes and use electronic devices to monitor blood sugar values and vital signs.
Digital radiographic studies were performed at sites that were most convenient for patients, downloaded, and reinterpreted at the study institution. Visiting nurses and neighborhood laboratories enabled less-frequent in-person visits for assessments.
These adjustments have been particularly important for geographically and/or socioeconomically disadvantaged patients, the panelists said.
Overall, there was agreement among the panelists that shared values and trust among regulatory authorities, sponsors, investigators, and clinicians were impressive in their urgency, sincerity, and patient centricity.
“This pandemic … has forced us to think differently and be nimble and creative to our approach to maintaining our overriding goals while at the same time bringing these innovative therapies forward for patients with cancer and other serious and life-threatening diseases as quickly as possible,” said panelist Kristen M. Hege, MD, of Bristol-Myers Squibb.
In fact, Dr. Hege noted, some cancer-related therapies (e.g., BTK inhibitors, JAK inhibitors, and immunomodulatory agents) were “repurposed” rapidly and tested against COVID-related complications.
Streamlining trial regulatory processes
In addition to changing ongoing trials, the pandemic has affected how new research projects are launched.
One new study that came together quickly in response to the pandemic is the NCI COVID-19 in Cancer Patients Study (NCCAPS). NCCAPS is a natural history study with biospecimens and an imaging library. It was approved in just 5 weeks and is active in 650 sites, with “gangbusters” accrual, Dr. Doroshow said.
The rapidness of NCCAPS’ design and implementation should prompt the revision of previously accepted timelines for trial activation and lead to streamlined future processes.
Another project that was launched quickly in response to the pandemic is the COVID-19 evidence accelerator, according to Paul G. Kluetz, MD, of the Food and Drug Administration.
The COVID-19 evidence accelerator integrates real-world evidence into a database to provide investigators and health systems with the ability to gather information, design rapid turnaround queries, and share results. The evidence accelerator can provide study chairs with information that may have relevance to the safety of participants in clinical trials.
Future directions and challenges
The panelists agreed that pandemic-related modifications in processes will not only accelerate trial approval and activation but should facilitate higher study accrual, increase the diversity of protocol participants, and decrease the costs associated with clinical trial conduct.
With that in mind, the NCI is planning randomized clinical trials in which “process A” is compared with “process B,” Dr. Doroshow said. The goal is to determine which modifications are most likely to make trials available to patients without compromising data integrity or patient safety.
“How much less data do you need to have an outcome that will be similar?” Dr. Doroshow asked. “How many fewer visits, how many fewer tests, how much can you save? Physicians, clinical trialists, all of us respond to data, and if you get the same outcome at a third of the cost, then everybody benefits.”
Nonetheless, we will need to be vigilant for unintended vulnerabilities from well-intended efforts, according to Dr. Kluetz. Study chairs, sponsors, and regulatory agencies will need to be attentive to whether there are important differences in scan quality or interpretation, missing data that influence trial outcomes, and so on.
Dr. Hege pointed out that differences among data sources may be less important when treatments generate large effects but may be vitally important when the relative differences among treatments are small.
On a practical level, decentralizing clinical research may negatively impact the finances of tertiary care centers, which could threaten the required infrastructure for clinical trials, a few panelists noted.
The relative balance of NCI-, industry-, and investigator-initiated trials may require adjustment so that research income is adequate to maintain the costs associated with cancer clinical trials.
Shared goals and democratization
The pandemic has required all stakeholders in clinical research to rely on relationships of trust and shared goals, said Caroline Robert, MD, PhD, of Institut Gustave Roussy in Villejuif, France.
Dr. Kluetz summarized those goals as improving trial efficiencies, decreasing patient burden, decentralizing trials, and maintaining trial integrity.
A decentralized clinical trials operational model could lead to better generalizability of study outcomes, normalization of life for patients on studies, and lower costs of trial conduct. As such, decentralization would promote democratization.
Coupled with ongoing efforts to reduce eligibility criteria in cancer trials, the pandemic has brought operational solutions that should be perpetuated and has reminded us of the interlocking and mutually supportive relationships on which clinical research success depends.
Dr. Doroshow and Dr. Kluetz disclosed no conflicts of interest. All other panelists disclosed financial relationships, including employment, with a range of companies.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
SOURCE: Flaherty KT et al. AACR: COVID-19 and Cancer, Regulatory and Operational Implications of Cancer Clinical Trial Changes During COVID-19.
The pandemic has taught researchers how to decentralize trials, which should not only improve patient satisfaction but increase trial accrual by providing access to typically underserved populations, Patricia M. LoRusso, DO, of Yale University, New Haven, Conn., said at the meeting.
Dr. LoRusso was one of six panelists who participated in a forum about changes to cancer trials that were prompted by the pandemic. The forum was moderated by Keith T. Flaherty, MD, of Massachusetts General Hospital in Boston.
Dr. Flaherty asked the panelists to explain adjustments their organizations have made in response to the pandemic, discuss accomplishments, and speculate on future challenges and priorities.
Trial, administrative, and patient-care modifications
COVID-19 put some cancer trials on hold. For others, the pandemic forced sponsors and study chairs to reduce trial complexity and identify nonessential aspects of the studies, according to panelist José Baselga, MD, PhD, of AstraZeneca.
Specifically, exploratory objectives were subjugated to patient safety and a focus on the primary endpoints of each trial.
Once the critical data were identified, study chairs were asked to determine whether data could be obtained through technologies that could substitute for face-to-face contact between patients and staff – for example, patient-reported outcome tools and at-home digital monitoring.
Modifications prompted by the pandemic include the following:
- On-site auditing was suspended.
- Oral investigational agents were shipped directly to patients.
- “Remote” informed consent (telephone or video consenting) was permitted.
- Local providers could perform study-related services, with oversight by the research site.
- Minor deviations from the written protocols were allowed, provided the deviations did not affect patient care or data integrity.
“Obviously, the pandemic has been horrible, but what it has allowed us to do, as investigators in the clinical research landscape, … is to change our focus somewhat and realize, first and foremost, the patient is at the center of this,” Dr. LoRusso said.
Operational accomplishments and benefits
The pandemic caused a 40% decline in accrual to studies supported by the National Cancer Institute’s (NCI) Clinical Trials Network (NCTN) from mid-March to early April, according to James H. Doroshow, MD, of NCI.
However, after modifications to administrative and regulatory procedures, accrual to NCTN trials recovered to approximately 80% of prepandemic levels, Dr. Doroshow said.
The pandemic prompted investigators to leverage tools and technology they had not previously used frequently or at all, the panelists pointed out.
Investigators discovered perforce that telehealth could be used for almost all trial-related assessments. In lieu of physical examination, patients could send pictures of rashes and use electronic devices to monitor blood sugar values and vital signs.
Digital radiographic studies were performed at sites that were most convenient for patients, downloaded, and reinterpreted at the study institution. Visiting nurses and neighborhood laboratories enabled less-frequent in-person visits for assessments.
These adjustments have been particularly important for geographically and/or socioeconomically disadvantaged patients, the panelists said.
Overall, there was agreement among the panelists that shared values and trust among regulatory authorities, sponsors, investigators, and clinicians were impressive in their urgency, sincerity, and patient centricity.
“This pandemic … has forced us to think differently and be nimble and creative to our approach to maintaining our overriding goals while at the same time bringing these innovative therapies forward for patients with cancer and other serious and life-threatening diseases as quickly as possible,” said panelist Kristen M. Hege, MD, of Bristol-Myers Squibb.
In fact, Dr. Hege noted, some cancer-related therapies (e.g., BTK inhibitors, JAK inhibitors, and immunomodulatory agents) were “repurposed” rapidly and tested against COVID-related complications.
Streamlining trial regulatory processes
In addition to changing ongoing trials, the pandemic has affected how new research projects are launched.
One new study that came together quickly in response to the pandemic is the NCI COVID-19 in Cancer Patients Study (NCCAPS). NCCAPS is a natural history study with biospecimens and an imaging library. It was approved in just 5 weeks and is active in 650 sites, with “gangbusters” accrual, Dr. Doroshow said.
The rapidness of NCCAPS’ design and implementation should prompt the revision of previously accepted timelines for trial activation and lead to streamlined future processes.
Another project that was launched quickly in response to the pandemic is the COVID-19 evidence accelerator, according to Paul G. Kluetz, MD, of the Food and Drug Administration.
The COVID-19 evidence accelerator integrates real-world evidence into a database to provide investigators and health systems with the ability to gather information, design rapid turnaround queries, and share results. The evidence accelerator can provide study chairs with information that may have relevance to the safety of participants in clinical trials.
Future directions and challenges
The panelists agreed that pandemic-related modifications in processes will not only accelerate trial approval and activation but should facilitate higher study accrual, increase the diversity of protocol participants, and decrease the costs associated with clinical trial conduct.
With that in mind, the NCI is planning randomized clinical trials in which “process A” is compared with “process B,” Dr. Doroshow said. The goal is to determine which modifications are most likely to make trials available to patients without compromising data integrity or patient safety.
“How much less data do you need to have an outcome that will be similar?” Dr. Doroshow asked. “How many fewer visits, how many fewer tests, how much can you save? Physicians, clinical trialists, all of us respond to data, and if you get the same outcome at a third of the cost, then everybody benefits.”
Nonetheless, we will need to be vigilant for unintended vulnerabilities from well-intended efforts, according to Dr. Kluetz. Study chairs, sponsors, and regulatory agencies will need to be attentive to whether there are important differences in scan quality or interpretation, missing data that influence trial outcomes, and so on.
Dr. Hege pointed out that differences among data sources may be less important when treatments generate large effects but may be vitally important when the relative differences among treatments are small.
On a practical level, decentralizing clinical research may negatively impact the finances of tertiary care centers, which could threaten the required infrastructure for clinical trials, a few panelists noted.
The relative balance of NCI-, industry-, and investigator-initiated trials may require adjustment so that research income is adequate to maintain the costs associated with cancer clinical trials.
Shared goals and democratization
The pandemic has required all stakeholders in clinical research to rely on relationships of trust and shared goals, said Caroline Robert, MD, PhD, of Institut Gustave Roussy in Villejuif, France.
Dr. Kluetz summarized those goals as improving trial efficiencies, decreasing patient burden, decentralizing trials, and maintaining trial integrity.
A decentralized clinical trials operational model could lead to better generalizability of study outcomes, normalization of life for patients on studies, and lower costs of trial conduct. As such, decentralization would promote democratization.
Coupled with ongoing efforts to reduce eligibility criteria in cancer trials, the pandemic has brought operational solutions that should be perpetuated and has reminded us of the interlocking and mutually supportive relationships on which clinical research success depends.
Dr. Doroshow and Dr. Kluetz disclosed no conflicts of interest. All other panelists disclosed financial relationships, including employment, with a range of companies.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
SOURCE: Flaherty KT et al. AACR: COVID-19 and Cancer, Regulatory and Operational Implications of Cancer Clinical Trial Changes During COVID-19.
FROM AACR: COVID-19 and Cancer
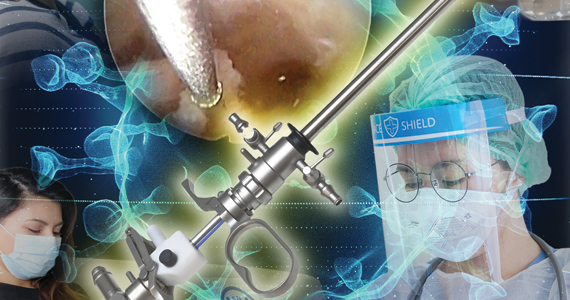
Hysteroscopy and COVID-19: Have recommended techniques changed due to the pandemic?
The emergence of the coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (COVID-19) in December 2019, has resulted in a global pandemic that has challenged the medical community and will continue to represent a public health emergency for the next several months.1 It has rapidly spread globally, infecting many individuals in an unprecedented rate of infection and worldwide reach. On March 11, 2020, the World Health Organization designated COVID-19 as a pandemic. While the majority of infected individuals are asymptomatic or develop only mild symptoms, some have an unfortunate clinical course resulting in multi-organ failure and death.2
It is accepted that the virus mainly spreads during close contact and via respiratory droplets.3 The average time from infection to onset of symptoms ranges from 2 to 14 days, with an average of 5 days.4 Recommended measures to prevent the spread of the infection include social distancing (at least 6 feet from others), meticulous hand hygiene, and wearing a mask covering the mouth and nose when in public.5 Aiming to mitigate the risk of viral dissemination for patients and health care providers, and to preserve hospital resources, all nonessential medical interventions were initially suspended. Recently, the American College of Surgeons in a joint statement with 9 women’s health care societies have provided recommendations on how to resume clinical activities as we recover from the pandemic.6
As we reinitiate clinical activities, gynecologists have been alerted of the potential risk of viral dissemination during gynecologic minimally invasive surgical procedures due to the presence of the virus in blood, stool, and the potential risk of aerosolization of the virus, especially when using smoke-generating devices.7,8 This risk is not limited to intubation and extubation of the airway during anesthesia; the risk also presents itself during other aerosol-generating procedures, such as laparoscopy or robotic surgery.9,10
Hysteroscopy is considered the gold standard procedure for the diagnosis and management of intrauterine pathologies.11 It is frequently performed in an office setting without the use of anesthesia.11,12 It is usually well tolerated, with only a few patients reporting discomfort.12 It allows for immediate treatment (using the “see and treat” approach) while avoiding not only the risk of anesthesia, as stated, but also the need for intubation—which has a high risk of droplet contamination in COVID-19–infected individuals.13

Is there risk of viral dissemination during hysteroscopic procedures?
The novel and rapidly changing nature of the COVID-19 pandemic present many challenges to the gynecologist. Significant concerns have been raised regarding potential risk of viral dissemination during laparoscopic surgery due to aerosolization of viral particles and the presence of the virus in blood and the gastrointestinal tract of infected patients.7 Diagnostic, and some simple, hysteroscopic procedures are commonly performed in an outpatient setting, with the patient awake. Complex hysteroscopic interventions, however, are generally performed in the operating room, typically with the use of general anesthesia. Hysteroscopy has the theoretical risks of viral dissemination when performed in COVID-19–positive patients. Two important questions must be addressed to better understand the potential risk of COVID-19 viral dissemination during hysteroscopic procedures.
Continue to: 1. Is the virus present in the vaginal fluid of women infected with COVID-19?...
1. Is the virus present in the vaginal fluid of women infected with COVID-19?
Recent studies have confirmed the presence of viral particles in urine, feces, blood, and tears in addition to the respiratory tract in patients infected with COVID-19.3,14,15 The presence of the SARS-CoV-2 virus in the female genital system is currently unknown. Previous studies, of other epidemic viral infections, have demonstrated the presence of the virus in the female genital tract in affected patients of Zika virus and Ebola.16,17 However, 2 recent studies have failed to demonstrate the presence of the SARS-CoV-2 virus in the vaginal fluid of pregnant14 and not pregnant18 women with severe COVID-19 infection.
2. Is there risk of viral dissemination during hysteroscopy if using electrosurgery?
There are significant concerns with possible risk of COVID-19 transmission to health care providers in direct contact with infected patients during minimally invasive gynecologic procedures due to direct contamination and aerosolization of the virus.10,19 Current data on COVID-19 transmission during surgery are limited. However, it is important to recognize that viral aerosolization has been documented with other viral diseases, such as human papillomavirus and hepatitis B.20 A recent report called for awareness in the surgical community about the potential risks of COVID-19 viral dissemination during laparoscopic surgery. Among other recommendations, international experts advised minimizing the use of electrosurgery to reduce the creation of surgical plume, decreasing the pneumoperitoneum pressure to minimum levels, and using suction devices in a closed system.21 Although these preventive measures apply to laparoscopic surgery, it is important to consider that hysteroscopy is performed in a unique environment.
During hysteroscopy the uterine cavity is distended with a liquid medium (normal saline or electrolyte-free solutions); this is opposed to gynecologic laparoscopy, in which the peritoneal cavity is distended with carbon dioxide.22 The smoke produced with the use of hysteroscopic electrosurgical instruments generates bubbles that are immediately cooled down to the temperature of the distention media and subsequently dissolve into it. Therefore, there are no bubbles generated during hysteroscopic surgery that are subsequently released into the air. This results in a low risk for viral dissemination during hysteroscopic procedures. Nevertheless, the necessary precautions to minimize the risk of COVID-19 transmission during hysteroscopic intervention are extremely important.
Recommendations for hysteroscopic procedures during the COVID-19 pandemic
We provide our overall recommendations for hysteroscopy, as well as those specific to the office and hospital setting.
Recommendations: General
Limit hysteroscopic procedures to COVID-19–negative patients and to those patients in whom delaying the procedure could result in adverse clinical outcomes.23
Universally screen for potential COVID-19 infection. When possible, a phone interview to triage patients based on their symptoms and infection exposure status should take place before the patient arrives to the health care center. Patients with suspected or confirmed COVID-19 infection who require immediate evaluation should be directed to COVID-19–designated emergency areas.
Universally test for SARS-CoV-2 before procedures performed in the operating room (OR). Using nasopharyngeal swabs for the detection of viral RNA, employing molecular methods such as polymerase chain reaction (PCR), within 48 to 72 hours prior to all OR hysteroscopic procedures is strongly recommended. Adopting this testing strategy will aid to identify asymptomatic SARS-CoV-2‒infected patients, allowing to defer the procedure, if possible, among patients testing positive. If tests are limited, testing only patients scheduled for hysteroscopic procedures in which general or regional anesthesia will be required is acceptable.
Universal SARS-CoV-2 testing of patients undergoing in-office hysteroscopic diagnostic or minor operative procedures without the use of anesthesia is not required.
Limit the presence of a companion. It is understood that visitor policies may vary at the discretion of each institution’s guidelines. Children and individuals over the age of 60 years should not be granted access to the center. Companions will be subjected to the same screening criteria as patients.
Provide for social distancing and other precautionary measures. If more than one patient is scheduled to be at the facility at the same time, ensure that the facility provides adequate space to allow the appropriate social distancing recommendations between patients. Hand sanitizers and facemasks should be available for patients and companions.
Provide PPE for clinicians. All health care providers in close contact with the patient must wear personal protective equipment (PPE), which includes an apron and gown, a surgical mask, eye protection, and gloves. Health care providers should wear PPE deemed appropriate by their regulatory institutions following their local and national guidelines during clinical patient interactions.
Restrict surgical attendees to vital personnel. The participation of learners by physical presence in the office or operating room should be restricted.
Continue to: Recommendations: Office setting...
Recommendations: Office setting
Preprocedural recommendations
- Advise patients to come to the office alone. If the patient requires a companion, a maximum of one adult companion under the age of 60 should be accepted.
- Limit the number of health care team members present in the procedure room.
Intraprocedural recommendations
- Choose the appropriate device(s) that will allow for an effective and fast procedure.
- Use the recommended PPE for all clinicians.
- Limit the movement of staff members in and out of the procedure room.
Postprocedure recommendations
- When more than one case is scheduled to be performed in the same procedure room, allow enough time in between cases to grant a thorough OR decontamination.
- Allow for patients to recover from the procedure in the same room as the procedure took place in order to avoid potential contamination of multiple rooms.
- Expedite patient discharge.
- Follow up after the procedure by phone or telemedicine.
- Use standard endoscope disinfection procedures, as they are effective and should not be modified.
Continue to: Recommendations: Operating room setting...
Recommendations: Operating room setting
Preprocedural recommendations
- Perform adequate patient screening for potential COVID-19 infection. (Screening should be independent of symptoms and not be limited to those with clinical symptoms.)
- Limit the number of health care team members in the operating procedure room.
- To minimize unnecessary staff exposure, have surgeons and staff not needed for intubation remain outside the OR until intubation is completed and leave the OR before extubation.
Intraprocedure recommendations
- Limit personnel in the OR to a minimum.
- Staff should not enter or leave the room during the procedure.
- When possible, use conscious sedation or regional anesthesia to avoid the risk of viral dissemination at the time of intubation/extubation.
- Choose the device that will allow an effective and fast procedure.
- Favor non–smoke-generating devices, such as hysteroscopic scissors, graspers, and tissue retrieval systems.
- Connect active suction to the outflow, especially when using smoke-generating instruments, to facilitate the extraction of surgical smoke.
Postprocedure recommendations
- When more than one case is scheduled to be performed in the same room, allow enough time in between cases to grant a thorough OR decontamination.
- Expedite postprocedure recovery and patient discharge.
- After completion of the procedure, staff should remove scrubs and change into clean clothing.
- Use standard endoscope disinfection procedures, as they are effective and should not be modified.
Conclusions
The COVID-19 pandemic has caused a global health emergency. Our knowledge of this devastating virus is constantly evolving as we continue to fight this overwhelming disease. Theoretical risk of “viral” dissemination is considered extremely low, or negligible, during hysterosocopy. Hysteroscopic procedures in COVID-19–positive patients with life-threatening conditions or in patients in whom delaying the procedure could worsen outcomes should be performed taking appropriate measures. Patients who test negative for COVID-19 (confirmed by PCR) and require hysteroscopic procedures, should be treated using universal precautions. ●
- Al-Shamsi HO, Alhazzani W, Alhuraiji A, et al. A practical approach to the management of cancer patients during the novel coronavirus disease 2019 (COVID-19) pandemic: an international collaborative group. Oncologist. 2020;25:e936-e945.
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. February 24, 2020. doi:10.1001/jama.2020.2648.
- Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843-1844.
- Yu F, Yan L, Wang N, et al. Quantitative detection and viral load analysis of SARS-CoV-2 in infected patients. Clin Infect Dis. 2020;71:793-798.
- Prem K, Liu Y, Russell TW, et al; Centre for the Mathematical Modelling of Infectious Diseases COVID-19 Working Group. The effect of control strategies to reduce social mixing on outcomes of the COVID-19 epidemic in Wuhan, China: a modelling study. Lancet Public Health. 2020;5:e261-e270.
- American College of Surgeons, American Society of Aesthesiologists, Association of periOperative Registered Nurses, American Hospital Association. Joint Statement: Roadmap for resuming elective surgery after COVID-19 pandemic. April 16, 2020. https://www.aorn.org/guidelines/aorn-support/roadmap-for-resuming-elective-surgery-after-covid-19. Accessed August 27, 2020.
- Zhang W, Du RH, Li B, et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9:386-389.
- Mowbray NG, Ansell J, Horwood J, et al. Safe management of surgical smoke in the age of COVID-19. Br J Surg. May 3, 2020. doi: 10.1002/bjs.11679.
- Cohen SL, Liu G, Abrao M, et al. Perspectives on surgery in the time of COVID-19: safety first. J Minim Invasive Gynecol. 2020;27:792-793.
- COVID-19: protecting health-care workers. Lancet. 2020;395:922.
- Salazar CA, Isaacson KB. Office operative hysteroscopy: an update. J Minim Invasive Gynecol. 2018;25:199-208.
- Cicinelli E. Hysteroscopy without anesthesia: review of recent literature. J Minim Invasive Gynecol. 2010;17:703-708.
- Wax RS, Christian MD. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anaesth. 2020;67:568-576.
- Aslan MM, Yuvaci HU, Köse O, et al. SARS-CoV-2 is not present in the vaginal fluid of pregnant women with COVID-19. J Matern Fetal Neonatal Med. 2020:1-3. doi: 10.1080/14767058.2020.1793318.
- Chen Y, Chen L, Deng Q, et al. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J Med Virol. 2020;92:833-840.
- Prisant N, Bujan L, Benichou H, et al. Zika virus in the female genital tract. Lancet Infect Dis. 2016;16:1000-1001.
- Rodriguez LL, De Roo A, Guimard Y, et al. Persistence and genetic stability of Ebola virus during the outbreak in Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis. 1999;179 Suppl 1:S170-S176.
- Qiu L, Liu X, Xiao M, et al. SARS-CoV-2 is not detectable in the vaginal fluid of women with severe COVID-19 infection. Clin Infect Dis. 2020;71:813-817.
- Brat GA, Hersey S, Chhabra K, et al. Protecting surgical teams during the COVID-19 outbreak: a narrative review and clinical considerations. Ann Surg. April 17, 2020. doi: 10.1097/SLA.0000000000003926.
- Kwak HD, Kim SH, Seo YS, et al. Detecting hepatitis B virus in surgical smoke emitted during laparoscopic surgery. Occup Environ Med. 2016;73:857-863.
- Zheng MH, Boni L, Fingerhut A. Minimally invasive surgery and the novel coronavirus outbreak: lessons learned in China and Italy. Ann Surg. 2020;272:e5-e6.
- Catena U. Surgical smoke in hysteroscopic surgery: does it really matter in COVID-19 times? Facts Views Vis Obgyn. 2020;12:67-68.
- Carugno J, Di Spiezio Sardo A, Alonso L, et al. COVID-19 pandemic. Impact on hysteroscopic procedures: a consensus statement from the Global Congress of Hysteroscopy Scientific Committee. J Minim Invasive Gynecol. 2020;27:988-992.
The emergence of the coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (COVID-19) in December 2019, has resulted in a global pandemic that has challenged the medical community and will continue to represent a public health emergency for the next several months.1 It has rapidly spread globally, infecting many individuals in an unprecedented rate of infection and worldwide reach. On March 11, 2020, the World Health Organization designated COVID-19 as a pandemic. While the majority of infected individuals are asymptomatic or develop only mild symptoms, some have an unfortunate clinical course resulting in multi-organ failure and death.2
It is accepted that the virus mainly spreads during close contact and via respiratory droplets.3 The average time from infection to onset of symptoms ranges from 2 to 14 days, with an average of 5 days.4 Recommended measures to prevent the spread of the infection include social distancing (at least 6 feet from others), meticulous hand hygiene, and wearing a mask covering the mouth and nose when in public.5 Aiming to mitigate the risk of viral dissemination for patients and health care providers, and to preserve hospital resources, all nonessential medical interventions were initially suspended. Recently, the American College of Surgeons in a joint statement with 9 women’s health care societies have provided recommendations on how to resume clinical activities as we recover from the pandemic.6
As we reinitiate clinical activities, gynecologists have been alerted of the potential risk of viral dissemination during gynecologic minimally invasive surgical procedures due to the presence of the virus in blood, stool, and the potential risk of aerosolization of the virus, especially when using smoke-generating devices.7,8 This risk is not limited to intubation and extubation of the airway during anesthesia; the risk also presents itself during other aerosol-generating procedures, such as laparoscopy or robotic surgery.9,10
Hysteroscopy is considered the gold standard procedure for the diagnosis and management of intrauterine pathologies.11 It is frequently performed in an office setting without the use of anesthesia.11,12 It is usually well tolerated, with only a few patients reporting discomfort.12 It allows for immediate treatment (using the “see and treat” approach) while avoiding not only the risk of anesthesia, as stated, but also the need for intubation—which has a high risk of droplet contamination in COVID-19–infected individuals.13

Is there risk of viral dissemination during hysteroscopic procedures?
The novel and rapidly changing nature of the COVID-19 pandemic present many challenges to the gynecologist. Significant concerns have been raised regarding potential risk of viral dissemination during laparoscopic surgery due to aerosolization of viral particles and the presence of the virus in blood and the gastrointestinal tract of infected patients.7 Diagnostic, and some simple, hysteroscopic procedures are commonly performed in an outpatient setting, with the patient awake. Complex hysteroscopic interventions, however, are generally performed in the operating room, typically with the use of general anesthesia. Hysteroscopy has the theoretical risks of viral dissemination when performed in COVID-19–positive patients. Two important questions must be addressed to better understand the potential risk of COVID-19 viral dissemination during hysteroscopic procedures.
Continue to: 1. Is the virus present in the vaginal fluid of women infected with COVID-19?...
1. Is the virus present in the vaginal fluid of women infected with COVID-19?
Recent studies have confirmed the presence of viral particles in urine, feces, blood, and tears in addition to the respiratory tract in patients infected with COVID-19.3,14,15 The presence of the SARS-CoV-2 virus in the female genital system is currently unknown. Previous studies, of other epidemic viral infections, have demonstrated the presence of the virus in the female genital tract in affected patients of Zika virus and Ebola.16,17 However, 2 recent studies have failed to demonstrate the presence of the SARS-CoV-2 virus in the vaginal fluid of pregnant14 and not pregnant18 women with severe COVID-19 infection.
2. Is there risk of viral dissemination during hysteroscopy if using electrosurgery?
There are significant concerns with possible risk of COVID-19 transmission to health care providers in direct contact with infected patients during minimally invasive gynecologic procedures due to direct contamination and aerosolization of the virus.10,19 Current data on COVID-19 transmission during surgery are limited. However, it is important to recognize that viral aerosolization has been documented with other viral diseases, such as human papillomavirus and hepatitis B.20 A recent report called for awareness in the surgical community about the potential risks of COVID-19 viral dissemination during laparoscopic surgery. Among other recommendations, international experts advised minimizing the use of electrosurgery to reduce the creation of surgical plume, decreasing the pneumoperitoneum pressure to minimum levels, and using suction devices in a closed system.21 Although these preventive measures apply to laparoscopic surgery, it is important to consider that hysteroscopy is performed in a unique environment.
During hysteroscopy the uterine cavity is distended with a liquid medium (normal saline or electrolyte-free solutions); this is opposed to gynecologic laparoscopy, in which the peritoneal cavity is distended with carbon dioxide.22 The smoke produced with the use of hysteroscopic electrosurgical instruments generates bubbles that are immediately cooled down to the temperature of the distention media and subsequently dissolve into it. Therefore, there are no bubbles generated during hysteroscopic surgery that are subsequently released into the air. This results in a low risk for viral dissemination during hysteroscopic procedures. Nevertheless, the necessary precautions to minimize the risk of COVID-19 transmission during hysteroscopic intervention are extremely important.
Recommendations for hysteroscopic procedures during the COVID-19 pandemic
We provide our overall recommendations for hysteroscopy, as well as those specific to the office and hospital setting.
Recommendations: General
Limit hysteroscopic procedures to COVID-19–negative patients and to those patients in whom delaying the procedure could result in adverse clinical outcomes.23
Universally screen for potential COVID-19 infection. When possible, a phone interview to triage patients based on their symptoms and infection exposure status should take place before the patient arrives to the health care center. Patients with suspected or confirmed COVID-19 infection who require immediate evaluation should be directed to COVID-19–designated emergency areas.
Universally test for SARS-CoV-2 before procedures performed in the operating room (OR). Using nasopharyngeal swabs for the detection of viral RNA, employing molecular methods such as polymerase chain reaction (PCR), within 48 to 72 hours prior to all OR hysteroscopic procedures is strongly recommended. Adopting this testing strategy will aid to identify asymptomatic SARS-CoV-2‒infected patients, allowing to defer the procedure, if possible, among patients testing positive. If tests are limited, testing only patients scheduled for hysteroscopic procedures in which general or regional anesthesia will be required is acceptable.
Universal SARS-CoV-2 testing of patients undergoing in-office hysteroscopic diagnostic or minor operative procedures without the use of anesthesia is not required.
Limit the presence of a companion. It is understood that visitor policies may vary at the discretion of each institution’s guidelines. Children and individuals over the age of 60 years should not be granted access to the center. Companions will be subjected to the same screening criteria as patients.
Provide for social distancing and other precautionary measures. If more than one patient is scheduled to be at the facility at the same time, ensure that the facility provides adequate space to allow the appropriate social distancing recommendations between patients. Hand sanitizers and facemasks should be available for patients and companions.
Provide PPE for clinicians. All health care providers in close contact with the patient must wear personal protective equipment (PPE), which includes an apron and gown, a surgical mask, eye protection, and gloves. Health care providers should wear PPE deemed appropriate by their regulatory institutions following their local and national guidelines during clinical patient interactions.
Restrict surgical attendees to vital personnel. The participation of learners by physical presence in the office or operating room should be restricted.
Continue to: Recommendations: Office setting...
Recommendations: Office setting
Preprocedural recommendations
- Advise patients to come to the office alone. If the patient requires a companion, a maximum of one adult companion under the age of 60 should be accepted.
- Limit the number of health care team members present in the procedure room.
Intraprocedural recommendations
- Choose the appropriate device(s) that will allow for an effective and fast procedure.
- Use the recommended PPE for all clinicians.
- Limit the movement of staff members in and out of the procedure room.
Postprocedure recommendations
- When more than one case is scheduled to be performed in the same procedure room, allow enough time in between cases to grant a thorough OR decontamination.
- Allow for patients to recover from the procedure in the same room as the procedure took place in order to avoid potential contamination of multiple rooms.
- Expedite patient discharge.
- Follow up after the procedure by phone or telemedicine.
- Use standard endoscope disinfection procedures, as they are effective and should not be modified.
Continue to: Recommendations: Operating room setting...
Recommendations: Operating room setting
Preprocedural recommendations
- Perform adequate patient screening for potential COVID-19 infection. (Screening should be independent of symptoms and not be limited to those with clinical symptoms.)
- Limit the number of health care team members in the operating procedure room.
- To minimize unnecessary staff exposure, have surgeons and staff not needed for intubation remain outside the OR until intubation is completed and leave the OR before extubation.
Intraprocedure recommendations
- Limit personnel in the OR to a minimum.
- Staff should not enter or leave the room during the procedure.
- When possible, use conscious sedation or regional anesthesia to avoid the risk of viral dissemination at the time of intubation/extubation.
- Choose the device that will allow an effective and fast procedure.
- Favor non–smoke-generating devices, such as hysteroscopic scissors, graspers, and tissue retrieval systems.
- Connect active suction to the outflow, especially when using smoke-generating instruments, to facilitate the extraction of surgical smoke.
Postprocedure recommendations
- When more than one case is scheduled to be performed in the same room, allow enough time in between cases to grant a thorough OR decontamination.
- Expedite postprocedure recovery and patient discharge.
- After completion of the procedure, staff should remove scrubs and change into clean clothing.
- Use standard endoscope disinfection procedures, as they are effective and should not be modified.
Conclusions
The COVID-19 pandemic has caused a global health emergency. Our knowledge of this devastating virus is constantly evolving as we continue to fight this overwhelming disease. Theoretical risk of “viral” dissemination is considered extremely low, or negligible, during hysterosocopy. Hysteroscopic procedures in COVID-19–positive patients with life-threatening conditions or in patients in whom delaying the procedure could worsen outcomes should be performed taking appropriate measures. Patients who test negative for COVID-19 (confirmed by PCR) and require hysteroscopic procedures, should be treated using universal precautions. ●
The emergence of the coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (COVID-19) in December 2019, has resulted in a global pandemic that has challenged the medical community and will continue to represent a public health emergency for the next several months.1 It has rapidly spread globally, infecting many individuals in an unprecedented rate of infection and worldwide reach. On March 11, 2020, the World Health Organization designated COVID-19 as a pandemic. While the majority of infected individuals are asymptomatic or develop only mild symptoms, some have an unfortunate clinical course resulting in multi-organ failure and death.2
It is accepted that the virus mainly spreads during close contact and via respiratory droplets.3 The average time from infection to onset of symptoms ranges from 2 to 14 days, with an average of 5 days.4 Recommended measures to prevent the spread of the infection include social distancing (at least 6 feet from others), meticulous hand hygiene, and wearing a mask covering the mouth and nose when in public.5 Aiming to mitigate the risk of viral dissemination for patients and health care providers, and to preserve hospital resources, all nonessential medical interventions were initially suspended. Recently, the American College of Surgeons in a joint statement with 9 women’s health care societies have provided recommendations on how to resume clinical activities as we recover from the pandemic.6
As we reinitiate clinical activities, gynecologists have been alerted of the potential risk of viral dissemination during gynecologic minimally invasive surgical procedures due to the presence of the virus in blood, stool, and the potential risk of aerosolization of the virus, especially when using smoke-generating devices.7,8 This risk is not limited to intubation and extubation of the airway during anesthesia; the risk also presents itself during other aerosol-generating procedures, such as laparoscopy or robotic surgery.9,10
Hysteroscopy is considered the gold standard procedure for the diagnosis and management of intrauterine pathologies.11 It is frequently performed in an office setting without the use of anesthesia.11,12 It is usually well tolerated, with only a few patients reporting discomfort.12 It allows for immediate treatment (using the “see and treat” approach) while avoiding not only the risk of anesthesia, as stated, but also the need for intubation—which has a high risk of droplet contamination in COVID-19–infected individuals.13

Is there risk of viral dissemination during hysteroscopic procedures?
The novel and rapidly changing nature of the COVID-19 pandemic present many challenges to the gynecologist. Significant concerns have been raised regarding potential risk of viral dissemination during laparoscopic surgery due to aerosolization of viral particles and the presence of the virus in blood and the gastrointestinal tract of infected patients.7 Diagnostic, and some simple, hysteroscopic procedures are commonly performed in an outpatient setting, with the patient awake. Complex hysteroscopic interventions, however, are generally performed in the operating room, typically with the use of general anesthesia. Hysteroscopy has the theoretical risks of viral dissemination when performed in COVID-19–positive patients. Two important questions must be addressed to better understand the potential risk of COVID-19 viral dissemination during hysteroscopic procedures.
Continue to: 1. Is the virus present in the vaginal fluid of women infected with COVID-19?...
1. Is the virus present in the vaginal fluid of women infected with COVID-19?
Recent studies have confirmed the presence of viral particles in urine, feces, blood, and tears in addition to the respiratory tract in patients infected with COVID-19.3,14,15 The presence of the SARS-CoV-2 virus in the female genital system is currently unknown. Previous studies, of other epidemic viral infections, have demonstrated the presence of the virus in the female genital tract in affected patients of Zika virus and Ebola.16,17 However, 2 recent studies have failed to demonstrate the presence of the SARS-CoV-2 virus in the vaginal fluid of pregnant14 and not pregnant18 women with severe COVID-19 infection.
2. Is there risk of viral dissemination during hysteroscopy if using electrosurgery?
There are significant concerns with possible risk of COVID-19 transmission to health care providers in direct contact with infected patients during minimally invasive gynecologic procedures due to direct contamination and aerosolization of the virus.10,19 Current data on COVID-19 transmission during surgery are limited. However, it is important to recognize that viral aerosolization has been documented with other viral diseases, such as human papillomavirus and hepatitis B.20 A recent report called for awareness in the surgical community about the potential risks of COVID-19 viral dissemination during laparoscopic surgery. Among other recommendations, international experts advised minimizing the use of electrosurgery to reduce the creation of surgical plume, decreasing the pneumoperitoneum pressure to minimum levels, and using suction devices in a closed system.21 Although these preventive measures apply to laparoscopic surgery, it is important to consider that hysteroscopy is performed in a unique environment.
During hysteroscopy the uterine cavity is distended with a liquid medium (normal saline or electrolyte-free solutions); this is opposed to gynecologic laparoscopy, in which the peritoneal cavity is distended with carbon dioxide.22 The smoke produced with the use of hysteroscopic electrosurgical instruments generates bubbles that are immediately cooled down to the temperature of the distention media and subsequently dissolve into it. Therefore, there are no bubbles generated during hysteroscopic surgery that are subsequently released into the air. This results in a low risk for viral dissemination during hysteroscopic procedures. Nevertheless, the necessary precautions to minimize the risk of COVID-19 transmission during hysteroscopic intervention are extremely important.
Recommendations for hysteroscopic procedures during the COVID-19 pandemic
We provide our overall recommendations for hysteroscopy, as well as those specific to the office and hospital setting.
Recommendations: General
Limit hysteroscopic procedures to COVID-19–negative patients and to those patients in whom delaying the procedure could result in adverse clinical outcomes.23
Universally screen for potential COVID-19 infection. When possible, a phone interview to triage patients based on their symptoms and infection exposure status should take place before the patient arrives to the health care center. Patients with suspected or confirmed COVID-19 infection who require immediate evaluation should be directed to COVID-19–designated emergency areas.
Universally test for SARS-CoV-2 before procedures performed in the operating room (OR). Using nasopharyngeal swabs for the detection of viral RNA, employing molecular methods such as polymerase chain reaction (PCR), within 48 to 72 hours prior to all OR hysteroscopic procedures is strongly recommended. Adopting this testing strategy will aid to identify asymptomatic SARS-CoV-2‒infected patients, allowing to defer the procedure, if possible, among patients testing positive. If tests are limited, testing only patients scheduled for hysteroscopic procedures in which general or regional anesthesia will be required is acceptable.
Universal SARS-CoV-2 testing of patients undergoing in-office hysteroscopic diagnostic or minor operative procedures without the use of anesthesia is not required.
Limit the presence of a companion. It is understood that visitor policies may vary at the discretion of each institution’s guidelines. Children and individuals over the age of 60 years should not be granted access to the center. Companions will be subjected to the same screening criteria as patients.
Provide for social distancing and other precautionary measures. If more than one patient is scheduled to be at the facility at the same time, ensure that the facility provides adequate space to allow the appropriate social distancing recommendations between patients. Hand sanitizers and facemasks should be available for patients and companions.
Provide PPE for clinicians. All health care providers in close contact with the patient must wear personal protective equipment (PPE), which includes an apron and gown, a surgical mask, eye protection, and gloves. Health care providers should wear PPE deemed appropriate by their regulatory institutions following their local and national guidelines during clinical patient interactions.
Restrict surgical attendees to vital personnel. The participation of learners by physical presence in the office or operating room should be restricted.
Continue to: Recommendations: Office setting...
Recommendations: Office setting
Preprocedural recommendations
- Advise patients to come to the office alone. If the patient requires a companion, a maximum of one adult companion under the age of 60 should be accepted.
- Limit the number of health care team members present in the procedure room.
Intraprocedural recommendations
- Choose the appropriate device(s) that will allow for an effective and fast procedure.
- Use the recommended PPE for all clinicians.
- Limit the movement of staff members in and out of the procedure room.
Postprocedure recommendations
- When more than one case is scheduled to be performed in the same procedure room, allow enough time in between cases to grant a thorough OR decontamination.
- Allow for patients to recover from the procedure in the same room as the procedure took place in order to avoid potential contamination of multiple rooms.
- Expedite patient discharge.
- Follow up after the procedure by phone or telemedicine.
- Use standard endoscope disinfection procedures, as they are effective and should not be modified.
Continue to: Recommendations: Operating room setting...
Recommendations: Operating room setting
Preprocedural recommendations
- Perform adequate patient screening for potential COVID-19 infection. (Screening should be independent of symptoms and not be limited to those with clinical symptoms.)
- Limit the number of health care team members in the operating procedure room.
- To minimize unnecessary staff exposure, have surgeons and staff not needed for intubation remain outside the OR until intubation is completed and leave the OR before extubation.
Intraprocedure recommendations
- Limit personnel in the OR to a minimum.
- Staff should not enter or leave the room during the procedure.
- When possible, use conscious sedation or regional anesthesia to avoid the risk of viral dissemination at the time of intubation/extubation.
- Choose the device that will allow an effective and fast procedure.
- Favor non–smoke-generating devices, such as hysteroscopic scissors, graspers, and tissue retrieval systems.
- Connect active suction to the outflow, especially when using smoke-generating instruments, to facilitate the extraction of surgical smoke.
Postprocedure recommendations
- When more than one case is scheduled to be performed in the same room, allow enough time in between cases to grant a thorough OR decontamination.
- Expedite postprocedure recovery and patient discharge.
- After completion of the procedure, staff should remove scrubs and change into clean clothing.
- Use standard endoscope disinfection procedures, as they are effective and should not be modified.
Conclusions
The COVID-19 pandemic has caused a global health emergency. Our knowledge of this devastating virus is constantly evolving as we continue to fight this overwhelming disease. Theoretical risk of “viral” dissemination is considered extremely low, or negligible, during hysterosocopy. Hysteroscopic procedures in COVID-19–positive patients with life-threatening conditions or in patients in whom delaying the procedure could worsen outcomes should be performed taking appropriate measures. Patients who test negative for COVID-19 (confirmed by PCR) and require hysteroscopic procedures, should be treated using universal precautions. ●
- Al-Shamsi HO, Alhazzani W, Alhuraiji A, et al. A practical approach to the management of cancer patients during the novel coronavirus disease 2019 (COVID-19) pandemic: an international collaborative group. Oncologist. 2020;25:e936-e945.
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. February 24, 2020. doi:10.1001/jama.2020.2648.
- Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843-1844.
- Yu F, Yan L, Wang N, et al. Quantitative detection and viral load analysis of SARS-CoV-2 in infected patients. Clin Infect Dis. 2020;71:793-798.
- Prem K, Liu Y, Russell TW, et al; Centre for the Mathematical Modelling of Infectious Diseases COVID-19 Working Group. The effect of control strategies to reduce social mixing on outcomes of the COVID-19 epidemic in Wuhan, China: a modelling study. Lancet Public Health. 2020;5:e261-e270.
- American College of Surgeons, American Society of Aesthesiologists, Association of periOperative Registered Nurses, American Hospital Association. Joint Statement: Roadmap for resuming elective surgery after COVID-19 pandemic. April 16, 2020. https://www.aorn.org/guidelines/aorn-support/roadmap-for-resuming-elective-surgery-after-covid-19. Accessed August 27, 2020.
- Zhang W, Du RH, Li B, et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9:386-389.
- Mowbray NG, Ansell J, Horwood J, et al. Safe management of surgical smoke in the age of COVID-19. Br J Surg. May 3, 2020. doi: 10.1002/bjs.11679.
- Cohen SL, Liu G, Abrao M, et al. Perspectives on surgery in the time of COVID-19: safety first. J Minim Invasive Gynecol. 2020;27:792-793.
- COVID-19: protecting health-care workers. Lancet. 2020;395:922.
- Salazar CA, Isaacson KB. Office operative hysteroscopy: an update. J Minim Invasive Gynecol. 2018;25:199-208.
- Cicinelli E. Hysteroscopy without anesthesia: review of recent literature. J Minim Invasive Gynecol. 2010;17:703-708.
- Wax RS, Christian MD. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anaesth. 2020;67:568-576.
- Aslan MM, Yuvaci HU, Köse O, et al. SARS-CoV-2 is not present in the vaginal fluid of pregnant women with COVID-19. J Matern Fetal Neonatal Med. 2020:1-3. doi: 10.1080/14767058.2020.1793318.
- Chen Y, Chen L, Deng Q, et al. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J Med Virol. 2020;92:833-840.
- Prisant N, Bujan L, Benichou H, et al. Zika virus in the female genital tract. Lancet Infect Dis. 2016;16:1000-1001.
- Rodriguez LL, De Roo A, Guimard Y, et al. Persistence and genetic stability of Ebola virus during the outbreak in Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis. 1999;179 Suppl 1:S170-S176.
- Qiu L, Liu X, Xiao M, et al. SARS-CoV-2 is not detectable in the vaginal fluid of women with severe COVID-19 infection. Clin Infect Dis. 2020;71:813-817.
- Brat GA, Hersey S, Chhabra K, et al. Protecting surgical teams during the COVID-19 outbreak: a narrative review and clinical considerations. Ann Surg. April 17, 2020. doi: 10.1097/SLA.0000000000003926.
- Kwak HD, Kim SH, Seo YS, et al. Detecting hepatitis B virus in surgical smoke emitted during laparoscopic surgery. Occup Environ Med. 2016;73:857-863.
- Zheng MH, Boni L, Fingerhut A. Minimally invasive surgery and the novel coronavirus outbreak: lessons learned in China and Italy. Ann Surg. 2020;272:e5-e6.
- Catena U. Surgical smoke in hysteroscopic surgery: does it really matter in COVID-19 times? Facts Views Vis Obgyn. 2020;12:67-68.
- Carugno J, Di Spiezio Sardo A, Alonso L, et al. COVID-19 pandemic. Impact on hysteroscopic procedures: a consensus statement from the Global Congress of Hysteroscopy Scientific Committee. J Minim Invasive Gynecol. 2020;27:988-992.
- Al-Shamsi HO, Alhazzani W, Alhuraiji A, et al. A practical approach to the management of cancer patients during the novel coronavirus disease 2019 (COVID-19) pandemic: an international collaborative group. Oncologist. 2020;25:e936-e945.
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. February 24, 2020. doi:10.1001/jama.2020.2648.
- Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843-1844.
- Yu F, Yan L, Wang N, et al. Quantitative detection and viral load analysis of SARS-CoV-2 in infected patients. Clin Infect Dis. 2020;71:793-798.
- Prem K, Liu Y, Russell TW, et al; Centre for the Mathematical Modelling of Infectious Diseases COVID-19 Working Group. The effect of control strategies to reduce social mixing on outcomes of the COVID-19 epidemic in Wuhan, China: a modelling study. Lancet Public Health. 2020;5:e261-e270.
- American College of Surgeons, American Society of Aesthesiologists, Association of periOperative Registered Nurses, American Hospital Association. Joint Statement: Roadmap for resuming elective surgery after COVID-19 pandemic. April 16, 2020. https://www.aorn.org/guidelines/aorn-support/roadmap-for-resuming-elective-surgery-after-covid-19. Accessed August 27, 2020.
- Zhang W, Du RH, Li B, et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9:386-389.
- Mowbray NG, Ansell J, Horwood J, et al. Safe management of surgical smoke in the age of COVID-19. Br J Surg. May 3, 2020. doi: 10.1002/bjs.11679.
- Cohen SL, Liu G, Abrao M, et al. Perspectives on surgery in the time of COVID-19: safety first. J Minim Invasive Gynecol. 2020;27:792-793.
- COVID-19: protecting health-care workers. Lancet. 2020;395:922.
- Salazar CA, Isaacson KB. Office operative hysteroscopy: an update. J Minim Invasive Gynecol. 2018;25:199-208.
- Cicinelli E. Hysteroscopy without anesthesia: review of recent literature. J Minim Invasive Gynecol. 2010;17:703-708.
- Wax RS, Christian MD. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anaesth. 2020;67:568-576.
- Aslan MM, Yuvaci HU, Köse O, et al. SARS-CoV-2 is not present in the vaginal fluid of pregnant women with COVID-19. J Matern Fetal Neonatal Med. 2020:1-3. doi: 10.1080/14767058.2020.1793318.
- Chen Y, Chen L, Deng Q, et al. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J Med Virol. 2020;92:833-840.
- Prisant N, Bujan L, Benichou H, et al. Zika virus in the female genital tract. Lancet Infect Dis. 2016;16:1000-1001.
- Rodriguez LL, De Roo A, Guimard Y, et al. Persistence and genetic stability of Ebola virus during the outbreak in Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis. 1999;179 Suppl 1:S170-S176.
- Qiu L, Liu X, Xiao M, et al. SARS-CoV-2 is not detectable in the vaginal fluid of women with severe COVID-19 infection. Clin Infect Dis. 2020;71:813-817.
- Brat GA, Hersey S, Chhabra K, et al. Protecting surgical teams during the COVID-19 outbreak: a narrative review and clinical considerations. Ann Surg. April 17, 2020. doi: 10.1097/SLA.0000000000003926.
- Kwak HD, Kim SH, Seo YS, et al. Detecting hepatitis B virus in surgical smoke emitted during laparoscopic surgery. Occup Environ Med. 2016;73:857-863.
- Zheng MH, Boni L, Fingerhut A. Minimally invasive surgery and the novel coronavirus outbreak: lessons learned in China and Italy. Ann Surg. 2020;272:e5-e6.
- Catena U. Surgical smoke in hysteroscopic surgery: does it really matter in COVID-19 times? Facts Views Vis Obgyn. 2020;12:67-68.
- Carugno J, Di Spiezio Sardo A, Alonso L, et al. COVID-19 pandemic. Impact on hysteroscopic procedures: a consensus statement from the Global Congress of Hysteroscopy Scientific Committee. J Minim Invasive Gynecol. 2020;27:988-992.
First U.S. trial to test aerosolized chemotherapy in advanced cancers
A team of U.S. researchers is investigating whether pressurized intraperitoneal aerosolized chemotherapy (PIPAC) can benefit patients with advanced cancer and peritoneal carcinomatosis.
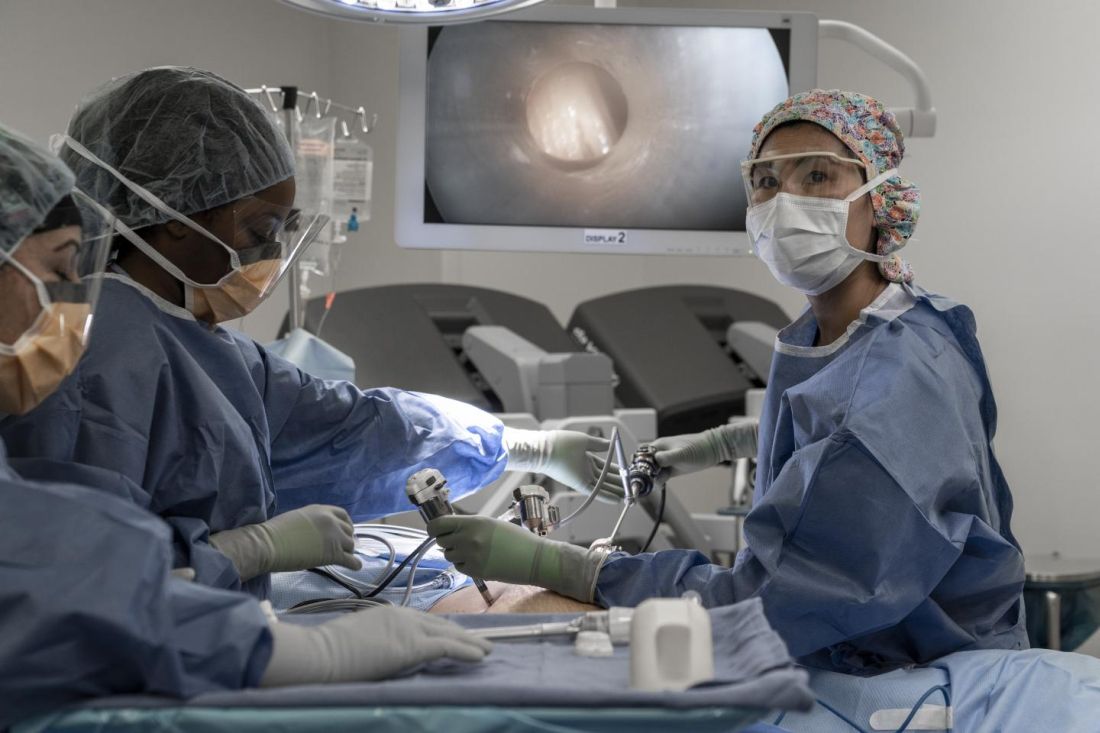
The team’s phase 1 trial is the first in the United States to test PIPAC, and it will enroll patients with ovarian, uterine, colorectal, or gastric cancer who have peritoneal carcinomatosis.
Data from studies outside the United States suggest PIPAC can induce regression of peritoneal carcinomatosis, even in end-stage, therapy-resistant gastric, ovarian, and colorectal cancers (Lancet Oncol. 2019 Jul;20[7]:e368-e377).
The current study (NCT04329494) formally introduces PIPAC to the United States and serves as a launching pad for further investigation into how the treatment should be administered and which types of chemotherapies can be used.
About PIPAC
“PIPAC is a novel therapeutic approach that is minimally invasive, does not require cytoreduction, and can be repeated frequently,” said Thanh Dellinger, MD, a gynecologic oncology surgeon at City of Hope in Duarte, Calif., and co–principal investigator of the phase 1 trial.
“[PIPAC] entails accessing the abdominal cavity using standard laparoscopic techniques and relies on the increased intra-abdominal pressure (15 mm Hg) achieved with laparoscopic surgery, which generates a convective flux that forces aerosolized chemotherapy drugs from the peritoneal cavity into the subperitoneal tissue and overcomes the tumor’s interstitial pressure,” Dr. Dellinger explained in an interview.
“The surgical procedure to deliver PIPAC does not typically cause adhesive disease and allows for repeated delivery of intraperitoneal chemotherapy, objective tumor staging, and response assessment,” she noted.
Dr. Dellinger said the advantages of PIPAC include a minimally invasive approach; no debulking surgery required; deeper uptake of drugs in tumor tissues; wider, more effective drug distribution; fewer toxicities caused by lower drug dosage; repeatable administration; and palliation of peritoneal carcinomatosis symptoms, including abdominal bloating and ascites.
PIPAC achieves a deeper peritoneal nodule penetration of several millimeters with cisplatin, compared to less than 1 mm with heated intraoperative peritoneal chemotherapy (HIPEC) and other intraperitoneal methods, according to Amit Merchea, MD, an assistant professor of surgery at the Mayo Clinic in Jacksonville, Fla.
Dr. Merchea performed the first PIPAC procedure in the United States in December 2019.
Innovative therapies needed
Peritoneal carcinomatosis is often a late-stage manifestation of abdominal cancers and is usually lethal, Dr. Dellinger said. She noted that systemic chemotherapy in the palliative setting is relatively ineffective in patients with peritoneal carcinomatosis because of pharmacokinetic limitations, poor peritoneal drug uptake, and impaired local drug distribution.
“Innovative, effective therapies are urgently needed for people who have ovarian, uterine, gastric, or colorectal cancer with peritoneal carcinomatosis,” Dr. Dellinger said.
“PIPAC is a novel treatment option that has had very favorable and exciting results,” Dr. Merchea said. “It is a potential option for patients when no other treatment options exist, and it is an avenue to provide hope to patients when often they have none.”
Potential candidates for PIPAC include patients who have peritoneal carcinomatosis, have failed other standard therapies, have more than 6 months’ life expectancy, and are not candidates for cytoreduction with HIPEC. There remains very limited data on the use of PIPAC as a neoadjuvant approach to convert patients who were previously unresectable to resectable disease, Dr. Merchea noted.
“To deliver chemotherapy directly to the tumor under pressure allows PIPAC to better penetrate the peritoneal surface and tumor nodules than traditional approaches, such as HIPEC,” Dr. Merchea said. “And the drug distribution at the tissue level is better than what is often achieved by systemic chemotherapy, but without the systemic effects of chemotherapy, such as hair loss. The treatment gives essentially a real-time, quantitative assessment of response by being able to directly assess the tumor via laparoscopic visualization and repeat biopsy.”
“Importantly, patients who undergo PIPAC don’t notice a decrease in their quality of life, and some patients note improvement, particularly with respect to nausea, vomiting, appetite, fatigue, and constipation,” Dr. Merchea said.
Trial details
The phase 1 trial of PIPAC will include a maximum of 24 patients. They will receive treatment every 6 weeks for up to three cycles and be followed for up to 3 years.
Patients with ovarian, uterine, or gastric cancer will undergo PIPAC with cisplatin, followed by doxorubicin. Patients with colorectal cancer will undergo PIPAC with oxaliplatin preceded by leucovorin and fluorouracil for cycles 2 and 3.
The researchers also plan to profile patients’ tumors.
“Tumor samples will be chronologically evaluated with genomics, spatial transcriptomics, pharmacodynamics, and single-cell sequencing throughout a patient’s treatment course, thus elucidating the treatment effects and natural history of peritoneal cancers,” Dr. Dellinger said.
The trial sites include City of Hope, Mayo Clinic in Florida, Northwell Health in New York, and the National Cancer Institute in Maryland.
The trial is sponsored by City of Hope in collaboration with the National Cancer Institute. Dr. Merchea and Dr. Dellinger reported having no conflicts of interest.
A team of U.S. researchers is investigating whether pressurized intraperitoneal aerosolized chemotherapy (PIPAC) can benefit patients with advanced cancer and peritoneal carcinomatosis.

The team’s phase 1 trial is the first in the United States to test PIPAC, and it will enroll patients with ovarian, uterine, colorectal, or gastric cancer who have peritoneal carcinomatosis.
Data from studies outside the United States suggest PIPAC can induce regression of peritoneal carcinomatosis, even in end-stage, therapy-resistant gastric, ovarian, and colorectal cancers (Lancet Oncol. 2019 Jul;20[7]:e368-e377).
The current study (NCT04329494) formally introduces PIPAC to the United States and serves as a launching pad for further investigation into how the treatment should be administered and which types of chemotherapies can be used.
About PIPAC
“PIPAC is a novel therapeutic approach that is minimally invasive, does not require cytoreduction, and can be repeated frequently,” said Thanh Dellinger, MD, a gynecologic oncology surgeon at City of Hope in Duarte, Calif., and co–principal investigator of the phase 1 trial.
“[PIPAC] entails accessing the abdominal cavity using standard laparoscopic techniques and relies on the increased intra-abdominal pressure (15 mm Hg) achieved with laparoscopic surgery, which generates a convective flux that forces aerosolized chemotherapy drugs from the peritoneal cavity into the subperitoneal tissue and overcomes the tumor’s interstitial pressure,” Dr. Dellinger explained in an interview.
“The surgical procedure to deliver PIPAC does not typically cause adhesive disease and allows for repeated delivery of intraperitoneal chemotherapy, objective tumor staging, and response assessment,” she noted.
Dr. Dellinger said the advantages of PIPAC include a minimally invasive approach; no debulking surgery required; deeper uptake of drugs in tumor tissues; wider, more effective drug distribution; fewer toxicities caused by lower drug dosage; repeatable administration; and palliation of peritoneal carcinomatosis symptoms, including abdominal bloating and ascites.
PIPAC achieves a deeper peritoneal nodule penetration of several millimeters with cisplatin, compared to less than 1 mm with heated intraoperative peritoneal chemotherapy (HIPEC) and other intraperitoneal methods, according to Amit Merchea, MD, an assistant professor of surgery at the Mayo Clinic in Jacksonville, Fla.
Dr. Merchea performed the first PIPAC procedure in the United States in December 2019.
Innovative therapies needed
Peritoneal carcinomatosis is often a late-stage manifestation of abdominal cancers and is usually lethal, Dr. Dellinger said. She noted that systemic chemotherapy in the palliative setting is relatively ineffective in patients with peritoneal carcinomatosis because of pharmacokinetic limitations, poor peritoneal drug uptake, and impaired local drug distribution.
“Innovative, effective therapies are urgently needed for people who have ovarian, uterine, gastric, or colorectal cancer with peritoneal carcinomatosis,” Dr. Dellinger said.
“PIPAC is a novel treatment option that has had very favorable and exciting results,” Dr. Merchea said. “It is a potential option for patients when no other treatment options exist, and it is an avenue to provide hope to patients when often they have none.”
Potential candidates for PIPAC include patients who have peritoneal carcinomatosis, have failed other standard therapies, have more than 6 months’ life expectancy, and are not candidates for cytoreduction with HIPEC. There remains very limited data on the use of PIPAC as a neoadjuvant approach to convert patients who were previously unresectable to resectable disease, Dr. Merchea noted.
“To deliver chemotherapy directly to the tumor under pressure allows PIPAC to better penetrate the peritoneal surface and tumor nodules than traditional approaches, such as HIPEC,” Dr. Merchea said. “And the drug distribution at the tissue level is better than what is often achieved by systemic chemotherapy, but without the systemic effects of chemotherapy, such as hair loss. The treatment gives essentially a real-time, quantitative assessment of response by being able to directly assess the tumor via laparoscopic visualization and repeat biopsy.”
“Importantly, patients who undergo PIPAC don’t notice a decrease in their quality of life, and some patients note improvement, particularly with respect to nausea, vomiting, appetite, fatigue, and constipation,” Dr. Merchea said.
Trial details
The phase 1 trial of PIPAC will include a maximum of 24 patients. They will receive treatment every 6 weeks for up to three cycles and be followed for up to 3 years.
Patients with ovarian, uterine, or gastric cancer will undergo PIPAC with cisplatin, followed by doxorubicin. Patients with colorectal cancer will undergo PIPAC with oxaliplatin preceded by leucovorin and fluorouracil for cycles 2 and 3.
The researchers also plan to profile patients’ tumors.
“Tumor samples will be chronologically evaluated with genomics, spatial transcriptomics, pharmacodynamics, and single-cell sequencing throughout a patient’s treatment course, thus elucidating the treatment effects and natural history of peritoneal cancers,” Dr. Dellinger said.
The trial sites include City of Hope, Mayo Clinic in Florida, Northwell Health in New York, and the National Cancer Institute in Maryland.
The trial is sponsored by City of Hope in collaboration with the National Cancer Institute. Dr. Merchea and Dr. Dellinger reported having no conflicts of interest.
A team of U.S. researchers is investigating whether pressurized intraperitoneal aerosolized chemotherapy (PIPAC) can benefit patients with advanced cancer and peritoneal carcinomatosis.

The team’s phase 1 trial is the first in the United States to test PIPAC, and it will enroll patients with ovarian, uterine, colorectal, or gastric cancer who have peritoneal carcinomatosis.
Data from studies outside the United States suggest PIPAC can induce regression of peritoneal carcinomatosis, even in end-stage, therapy-resistant gastric, ovarian, and colorectal cancers (Lancet Oncol. 2019 Jul;20[7]:e368-e377).
The current study (NCT04329494) formally introduces PIPAC to the United States and serves as a launching pad for further investigation into how the treatment should be administered and which types of chemotherapies can be used.
About PIPAC
“PIPAC is a novel therapeutic approach that is minimally invasive, does not require cytoreduction, and can be repeated frequently,” said Thanh Dellinger, MD, a gynecologic oncology surgeon at City of Hope in Duarte, Calif., and co–principal investigator of the phase 1 trial.
“[PIPAC] entails accessing the abdominal cavity using standard laparoscopic techniques and relies on the increased intra-abdominal pressure (15 mm Hg) achieved with laparoscopic surgery, which generates a convective flux that forces aerosolized chemotherapy drugs from the peritoneal cavity into the subperitoneal tissue and overcomes the tumor’s interstitial pressure,” Dr. Dellinger explained in an interview.
“The surgical procedure to deliver PIPAC does not typically cause adhesive disease and allows for repeated delivery of intraperitoneal chemotherapy, objective tumor staging, and response assessment,” she noted.
Dr. Dellinger said the advantages of PIPAC include a minimally invasive approach; no debulking surgery required; deeper uptake of drugs in tumor tissues; wider, more effective drug distribution; fewer toxicities caused by lower drug dosage; repeatable administration; and palliation of peritoneal carcinomatosis symptoms, including abdominal bloating and ascites.
PIPAC achieves a deeper peritoneal nodule penetration of several millimeters with cisplatin, compared to less than 1 mm with heated intraoperative peritoneal chemotherapy (HIPEC) and other intraperitoneal methods, according to Amit Merchea, MD, an assistant professor of surgery at the Mayo Clinic in Jacksonville, Fla.
Dr. Merchea performed the first PIPAC procedure in the United States in December 2019.
Innovative therapies needed
Peritoneal carcinomatosis is often a late-stage manifestation of abdominal cancers and is usually lethal, Dr. Dellinger said. She noted that systemic chemotherapy in the palliative setting is relatively ineffective in patients with peritoneal carcinomatosis because of pharmacokinetic limitations, poor peritoneal drug uptake, and impaired local drug distribution.
“Innovative, effective therapies are urgently needed for people who have ovarian, uterine, gastric, or colorectal cancer with peritoneal carcinomatosis,” Dr. Dellinger said.
“PIPAC is a novel treatment option that has had very favorable and exciting results,” Dr. Merchea said. “It is a potential option for patients when no other treatment options exist, and it is an avenue to provide hope to patients when often they have none.”
Potential candidates for PIPAC include patients who have peritoneal carcinomatosis, have failed other standard therapies, have more than 6 months’ life expectancy, and are not candidates for cytoreduction with HIPEC. There remains very limited data on the use of PIPAC as a neoadjuvant approach to convert patients who were previously unresectable to resectable disease, Dr. Merchea noted.
“To deliver chemotherapy directly to the tumor under pressure allows PIPAC to better penetrate the peritoneal surface and tumor nodules than traditional approaches, such as HIPEC,” Dr. Merchea said. “And the drug distribution at the tissue level is better than what is often achieved by systemic chemotherapy, but without the systemic effects of chemotherapy, such as hair loss. The treatment gives essentially a real-time, quantitative assessment of response by being able to directly assess the tumor via laparoscopic visualization and repeat biopsy.”
“Importantly, patients who undergo PIPAC don’t notice a decrease in their quality of life, and some patients note improvement, particularly with respect to nausea, vomiting, appetite, fatigue, and constipation,” Dr. Merchea said.
Trial details
The phase 1 trial of PIPAC will include a maximum of 24 patients. They will receive treatment every 6 weeks for up to three cycles and be followed for up to 3 years.
Patients with ovarian, uterine, or gastric cancer will undergo PIPAC with cisplatin, followed by doxorubicin. Patients with colorectal cancer will undergo PIPAC with oxaliplatin preceded by leucovorin and fluorouracil for cycles 2 and 3.
The researchers also plan to profile patients’ tumors.
“Tumor samples will be chronologically evaluated with genomics, spatial transcriptomics, pharmacodynamics, and single-cell sequencing throughout a patient’s treatment course, thus elucidating the treatment effects and natural history of peritoneal cancers,” Dr. Dellinger said.
The trial sites include City of Hope, Mayo Clinic in Florida, Northwell Health in New York, and the National Cancer Institute in Maryland.
The trial is sponsored by City of Hope in collaboration with the National Cancer Institute. Dr. Merchea and Dr. Dellinger reported having no conflicts of interest.
Hair dye and cancer study ‘offers some reassurance’
Findings limited to White women in United States
The largest study of its kind has found no positive association between personal use of permanent hair dye and the risk for most cancers and cancer mortality.
The findings come from the Nurses’ Health Study, an ongoing prospective cohort study of more than 117,000 women who have been followed for 36 years and who did not have cancer at baseline.
The findings were published online on September 2 in the BMJ.
The results “offer some reassurance against concerns that personal use of permanent hair dyes might be associated with increased cancer risk or mortality,” write the investigators, with first author Yin Zhang, PhD, of Harvard Medical School, Boston.
The findings, which are limited to White women in the United States, indicate correlation, not causation, the authors emphasize.
Nevertheless, the researchers found an increased risk for some cancers among hair dye users, especially with greater cumulative dose (200 or more uses during the study period). The risk was increased for basal cell carcinoma, breast cancer (specifically, estrogen receptor negative [ER–], progesterone receptor negative [PR–], and hormone receptor negative [ER–, PR–]), and ovarian cancer.
A British expert not involved in the study dismissed these findings. “The reported associations are very weak, and, given the number of associations reported in this manuscript, they are very likely to be chance findings,” commented Paul Pharoah, PhD, professor of cancer epidemiology at the University of Cambridge (England).
“For the cancers where an increase in risk is reported, the results are not compelling. Even if they were real findings, the associations may not be cause-and-effect, and, even if they were causal associations, the magnitude of the effects are so small that any risk would be trivial.
“In short, none of the findings reported in this manuscript suggest that women who use hair dye are putting themselves at increased risk of cancer,” he stated.
A U.S. researcher who has previously coauthored a study suggesting an association between hair dye and breast cancer agreed that the increases in risk reported in this current study are “small.” But they are “of interest,” especially for breast and ovarian cancer, said Alexandra White, PhD, of the National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, N.C.
Hair dyes include compounds that “are not just potential carcinogens but also act as endocrine disruptors,” she said in an interview.
“In both breast and ovarian cancer, we know that hormones play an important part in the etiology ... so it’s biologically plausible that you would see [these associations in the current study],” added Dr. White, who was approached for comment.
However, she added that, even with the “modest” 20%-28% increase in the relative risk for certain breast cancers linked to a heavy cumulative dose of dyes in the current study, “there doesn’t seem to be any strong association with any cancer type.”
But she also pointed out that the most outstanding risk association was among ER–/PR– breast cancers, which are the “most aggressive and difficult to treat,” and thus the new findings are “important.”
Dr. White is the lead author of a 2019 study that received a lot of media attention because it rang an alarm bell about hair dyes and breast cancer risk.
That study concluded that ever using permanent hair dye or hair straighteners was associated with a higher risk for breast cancer than never using them and that this higher risk was especially associated with Black women. However, the study participants were from the prospective Sister Study. The participants in that study had no history of breast cancer, but they each had at least one sister who did. This family history of breast cancer may represent selection bias.
With changes in the 1980s, even safer now?
The study of hair dyes and cancer has “major public health implications” because the use of hair dye is widespread, Dr. Zhang and colleagues write in their article. They estimate that 50% to 80% of women and 10% of men aged 40 years and older in the United States and Europe use hair dye.
Permanent hair dyes “pose the greatest potential concern,” they stated, adding that these account for approximately 80% of hair dyes used in the United States and Europe and an even higher percentage in Asia.
The International Agency for Research on Cancer classifies occupational exposure to hair dyes as probably carcinogenic, but the carcinogenicity resulting from personal use of hair dyes is not classifiable – thus, there is no warning about at-home usage.
Notably, there was “a huge and very important” change in hair dye ingredients in the 1980s after the Food and Drug Administration warned about some chemicals in permanent hair dyes and the cosmetic industry altered their formulas, lead author Dr. Zhang said.
However, the researchers could not analyze use before and after the changes because not enough women reported first use of permanent hair dye after 1980 (only 1890 of 117,200 participants).
“We could expect that the current ingredients should make it safer,” Dr. Zhang said.
Study details
The researchers report that ever-users of permanent hair dyes had no significant increases in risk for solid cancers (n = 20,805; hazard ratio, 0.98, 95% confidence interval, 0.96-1.01) or hematopoietic cancers overall (n = 1,807; HR, 1.00; 95% CI, 0.91-1.10) compared with nonusers.
Additionally, ever-users did not have an increased risk for most specific cancers or cancer-related death (n = 4,860; HR, 0.96; 95% CI, 0.91-1.02).
As noted above, there were some exceptions.
Basal cell carcinoma risk was slightly increased for ever-users (n = 22,560; HR, 1.05; 95% CI, 1.02-1.08). Cumulative dose (a calculation of duration and frequency) was positively associated with risk for ER– breast cancer, PR– breast cancer, ER–/PR– breast cancer, and ovarian cancer, with risk rising in accordance with the total amount of dye.
Notably, at a cumulative dose of ≥200 uses, there was a 20% increase in the relative risk for ER- breast cancer (n = 1521; HR, 1.20; 95% CI, 1.02-1.41; P value for trend, .03). At the same cumulative dose, there was a 28% increase in the relative risk for ER-/PR- breast cancer (n = 1287; HR, 1.28, 95% CI, 1.08-1.52; P value for trend, .006).
In addition, an increased risk for Hodgkin lymphoma was observed, but only for women with naturally dark hair (the calculation was based on 70 women, 24 of whom had dark hair).
In a press statement, senior author Eva Schernhammer, PhD, of Harvard and the Medical University of Vienna, said the results “justify further prospective validation.”
She also explained that there are many variables to consider in this research, including different populations and countries, different susceptibility genotypes, different exposure settings (personal use vs. occupational exposure), and different colors of the permanent hair dyes used (dark dyes vs. light dyes).
Geographic location is a particularly important variable, suggested the study authors.
They pointed out that Europe, but not the United States, banned some individual hair dye ingredients that were considered carcinogenic during both the 1980s and 2000s. One country has even tighter oversight: “The most restrictive regulation of hair dyes exists in Japan, where cosmetic products are considered equivalent to drugs.”
The study was funded by the Centers for Disease Control and Prevention and the National Institute for Occupational Safety and Health. The study authors and Dr. White have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Findings limited to White women in United States
Findings limited to White women in United States
The largest study of its kind has found no positive association between personal use of permanent hair dye and the risk for most cancers and cancer mortality.
The findings come from the Nurses’ Health Study, an ongoing prospective cohort study of more than 117,000 women who have been followed for 36 years and who did not have cancer at baseline.
The findings were published online on September 2 in the BMJ.
The results “offer some reassurance against concerns that personal use of permanent hair dyes might be associated with increased cancer risk or mortality,” write the investigators, with first author Yin Zhang, PhD, of Harvard Medical School, Boston.
The findings, which are limited to White women in the United States, indicate correlation, not causation, the authors emphasize.
Nevertheless, the researchers found an increased risk for some cancers among hair dye users, especially with greater cumulative dose (200 or more uses during the study period). The risk was increased for basal cell carcinoma, breast cancer (specifically, estrogen receptor negative [ER–], progesterone receptor negative [PR–], and hormone receptor negative [ER–, PR–]), and ovarian cancer.
A British expert not involved in the study dismissed these findings. “The reported associations are very weak, and, given the number of associations reported in this manuscript, they are very likely to be chance findings,” commented Paul Pharoah, PhD, professor of cancer epidemiology at the University of Cambridge (England).
“For the cancers where an increase in risk is reported, the results are not compelling. Even if they were real findings, the associations may not be cause-and-effect, and, even if they were causal associations, the magnitude of the effects are so small that any risk would be trivial.
“In short, none of the findings reported in this manuscript suggest that women who use hair dye are putting themselves at increased risk of cancer,” he stated.
A U.S. researcher who has previously coauthored a study suggesting an association between hair dye and breast cancer agreed that the increases in risk reported in this current study are “small.” But they are “of interest,” especially for breast and ovarian cancer, said Alexandra White, PhD, of the National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, N.C.
Hair dyes include compounds that “are not just potential carcinogens but also act as endocrine disruptors,” she said in an interview.
“In both breast and ovarian cancer, we know that hormones play an important part in the etiology ... so it’s biologically plausible that you would see [these associations in the current study],” added Dr. White, who was approached for comment.
However, she added that, even with the “modest” 20%-28% increase in the relative risk for certain breast cancers linked to a heavy cumulative dose of dyes in the current study, “there doesn’t seem to be any strong association with any cancer type.”
But she also pointed out that the most outstanding risk association was among ER–/PR– breast cancers, which are the “most aggressive and difficult to treat,” and thus the new findings are “important.”
Dr. White is the lead author of a 2019 study that received a lot of media attention because it rang an alarm bell about hair dyes and breast cancer risk.
That study concluded that ever using permanent hair dye or hair straighteners was associated with a higher risk for breast cancer than never using them and that this higher risk was especially associated with Black women. However, the study participants were from the prospective Sister Study. The participants in that study had no history of breast cancer, but they each had at least one sister who did. This family history of breast cancer may represent selection bias.
With changes in the 1980s, even safer now?
The study of hair dyes and cancer has “major public health implications” because the use of hair dye is widespread, Dr. Zhang and colleagues write in their article. They estimate that 50% to 80% of women and 10% of men aged 40 years and older in the United States and Europe use hair dye.
Permanent hair dyes “pose the greatest potential concern,” they stated, adding that these account for approximately 80% of hair dyes used in the United States and Europe and an even higher percentage in Asia.
The International Agency for Research on Cancer classifies occupational exposure to hair dyes as probably carcinogenic, but the carcinogenicity resulting from personal use of hair dyes is not classifiable – thus, there is no warning about at-home usage.
Notably, there was “a huge and very important” change in hair dye ingredients in the 1980s after the Food and Drug Administration warned about some chemicals in permanent hair dyes and the cosmetic industry altered their formulas, lead author Dr. Zhang said.
However, the researchers could not analyze use before and after the changes because not enough women reported first use of permanent hair dye after 1980 (only 1890 of 117,200 participants).
“We could expect that the current ingredients should make it safer,” Dr. Zhang said.
Study details
The researchers report that ever-users of permanent hair dyes had no significant increases in risk for solid cancers (n = 20,805; hazard ratio, 0.98, 95% confidence interval, 0.96-1.01) or hematopoietic cancers overall (n = 1,807; HR, 1.00; 95% CI, 0.91-1.10) compared with nonusers.
Additionally, ever-users did not have an increased risk for most specific cancers or cancer-related death (n = 4,860; HR, 0.96; 95% CI, 0.91-1.02).
As noted above, there were some exceptions.
Basal cell carcinoma risk was slightly increased for ever-users (n = 22,560; HR, 1.05; 95% CI, 1.02-1.08). Cumulative dose (a calculation of duration and frequency) was positively associated with risk for ER– breast cancer, PR– breast cancer, ER–/PR– breast cancer, and ovarian cancer, with risk rising in accordance with the total amount of dye.
Notably, at a cumulative dose of ≥200 uses, there was a 20% increase in the relative risk for ER- breast cancer (n = 1521; HR, 1.20; 95% CI, 1.02-1.41; P value for trend, .03). At the same cumulative dose, there was a 28% increase in the relative risk for ER-/PR- breast cancer (n = 1287; HR, 1.28, 95% CI, 1.08-1.52; P value for trend, .006).
In addition, an increased risk for Hodgkin lymphoma was observed, but only for women with naturally dark hair (the calculation was based on 70 women, 24 of whom had dark hair).
In a press statement, senior author Eva Schernhammer, PhD, of Harvard and the Medical University of Vienna, said the results “justify further prospective validation.”
She also explained that there are many variables to consider in this research, including different populations and countries, different susceptibility genotypes, different exposure settings (personal use vs. occupational exposure), and different colors of the permanent hair dyes used (dark dyes vs. light dyes).
Geographic location is a particularly important variable, suggested the study authors.
They pointed out that Europe, but not the United States, banned some individual hair dye ingredients that were considered carcinogenic during both the 1980s and 2000s. One country has even tighter oversight: “The most restrictive regulation of hair dyes exists in Japan, where cosmetic products are considered equivalent to drugs.”
The study was funded by the Centers for Disease Control and Prevention and the National Institute for Occupational Safety and Health. The study authors and Dr. White have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The largest study of its kind has found no positive association between personal use of permanent hair dye and the risk for most cancers and cancer mortality.
The findings come from the Nurses’ Health Study, an ongoing prospective cohort study of more than 117,000 women who have been followed for 36 years and who did not have cancer at baseline.
The findings were published online on September 2 in the BMJ.
The results “offer some reassurance against concerns that personal use of permanent hair dyes might be associated with increased cancer risk or mortality,” write the investigators, with first author Yin Zhang, PhD, of Harvard Medical School, Boston.
The findings, which are limited to White women in the United States, indicate correlation, not causation, the authors emphasize.
Nevertheless, the researchers found an increased risk for some cancers among hair dye users, especially with greater cumulative dose (200 or more uses during the study period). The risk was increased for basal cell carcinoma, breast cancer (specifically, estrogen receptor negative [ER–], progesterone receptor negative [PR–], and hormone receptor negative [ER–, PR–]), and ovarian cancer.
A British expert not involved in the study dismissed these findings. “The reported associations are very weak, and, given the number of associations reported in this manuscript, they are very likely to be chance findings,” commented Paul Pharoah, PhD, professor of cancer epidemiology at the University of Cambridge (England).
“For the cancers where an increase in risk is reported, the results are not compelling. Even if they were real findings, the associations may not be cause-and-effect, and, even if they were causal associations, the magnitude of the effects are so small that any risk would be trivial.
“In short, none of the findings reported in this manuscript suggest that women who use hair dye are putting themselves at increased risk of cancer,” he stated.
A U.S. researcher who has previously coauthored a study suggesting an association between hair dye and breast cancer agreed that the increases in risk reported in this current study are “small.” But they are “of interest,” especially for breast and ovarian cancer, said Alexandra White, PhD, of the National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, N.C.
Hair dyes include compounds that “are not just potential carcinogens but also act as endocrine disruptors,” she said in an interview.
“In both breast and ovarian cancer, we know that hormones play an important part in the etiology ... so it’s biologically plausible that you would see [these associations in the current study],” added Dr. White, who was approached for comment.
However, she added that, even with the “modest” 20%-28% increase in the relative risk for certain breast cancers linked to a heavy cumulative dose of dyes in the current study, “there doesn’t seem to be any strong association with any cancer type.”
But she also pointed out that the most outstanding risk association was among ER–/PR– breast cancers, which are the “most aggressive and difficult to treat,” and thus the new findings are “important.”
Dr. White is the lead author of a 2019 study that received a lot of media attention because it rang an alarm bell about hair dyes and breast cancer risk.
That study concluded that ever using permanent hair dye or hair straighteners was associated with a higher risk for breast cancer than never using them and that this higher risk was especially associated with Black women. However, the study participants were from the prospective Sister Study. The participants in that study had no history of breast cancer, but they each had at least one sister who did. This family history of breast cancer may represent selection bias.
With changes in the 1980s, even safer now?
The study of hair dyes and cancer has “major public health implications” because the use of hair dye is widespread, Dr. Zhang and colleagues write in their article. They estimate that 50% to 80% of women and 10% of men aged 40 years and older in the United States and Europe use hair dye.
Permanent hair dyes “pose the greatest potential concern,” they stated, adding that these account for approximately 80% of hair dyes used in the United States and Europe and an even higher percentage in Asia.
The International Agency for Research on Cancer classifies occupational exposure to hair dyes as probably carcinogenic, but the carcinogenicity resulting from personal use of hair dyes is not classifiable – thus, there is no warning about at-home usage.
Notably, there was “a huge and very important” change in hair dye ingredients in the 1980s after the Food and Drug Administration warned about some chemicals in permanent hair dyes and the cosmetic industry altered their formulas, lead author Dr. Zhang said.
However, the researchers could not analyze use before and after the changes because not enough women reported first use of permanent hair dye after 1980 (only 1890 of 117,200 participants).
“We could expect that the current ingredients should make it safer,” Dr. Zhang said.
Study details
The researchers report that ever-users of permanent hair dyes had no significant increases in risk for solid cancers (n = 20,805; hazard ratio, 0.98, 95% confidence interval, 0.96-1.01) or hematopoietic cancers overall (n = 1,807; HR, 1.00; 95% CI, 0.91-1.10) compared with nonusers.
Additionally, ever-users did not have an increased risk for most specific cancers or cancer-related death (n = 4,860; HR, 0.96; 95% CI, 0.91-1.02).
As noted above, there were some exceptions.
Basal cell carcinoma risk was slightly increased for ever-users (n = 22,560; HR, 1.05; 95% CI, 1.02-1.08). Cumulative dose (a calculation of duration and frequency) was positively associated with risk for ER– breast cancer, PR– breast cancer, ER–/PR– breast cancer, and ovarian cancer, with risk rising in accordance with the total amount of dye.
Notably, at a cumulative dose of ≥200 uses, there was a 20% increase in the relative risk for ER- breast cancer (n = 1521; HR, 1.20; 95% CI, 1.02-1.41; P value for trend, .03). At the same cumulative dose, there was a 28% increase in the relative risk for ER-/PR- breast cancer (n = 1287; HR, 1.28, 95% CI, 1.08-1.52; P value for trend, .006).
In addition, an increased risk for Hodgkin lymphoma was observed, but only for women with naturally dark hair (the calculation was based on 70 women, 24 of whom had dark hair).
In a press statement, senior author Eva Schernhammer, PhD, of Harvard and the Medical University of Vienna, said the results “justify further prospective validation.”
She also explained that there are many variables to consider in this research, including different populations and countries, different susceptibility genotypes, different exposure settings (personal use vs. occupational exposure), and different colors of the permanent hair dyes used (dark dyes vs. light dyes).
Geographic location is a particularly important variable, suggested the study authors.
They pointed out that Europe, but not the United States, banned some individual hair dye ingredients that were considered carcinogenic during both the 1980s and 2000s. One country has even tighter oversight: “The most restrictive regulation of hair dyes exists in Japan, where cosmetic products are considered equivalent to drugs.”
The study was funded by the Centers for Disease Control and Prevention and the National Institute for Occupational Safety and Health. The study authors and Dr. White have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Gene signature may improve prognostication in ovarian cancer
according to a study published in Annals of Oncology.
“Gene expression signature tests for prognosis are available for other cancers, such as breast cancer, and these help with treatment decisions, but no such tests are available for ovarian cancer,” senior investigator Susan J. Ramus, PhD, of Lowy Cancer Research Centre, University of NSW Sydney, commented in an interview.
Dr. Ramus and associates developed and validated their 101-gene expression signature using pretreatment tumor tissue from 3,769 women with high-grade serous ovarian cancer treated on 21 studies.
The investigators found this signature, called OTTA-SPOT (Ovarian Tumor Tissue Analysis Consortium–Stratified Prognosis of Ovarian Tumors), performed well at stratifying women according to overall survival. Median overall survival times ranged from about 2 years for patients in the top quintile of scores to more than 9 years for patients in the bottom quintile.
Moreover, OTTA-SPOT significantly improved prognostication when added to age and stage.
“This tumor test works on formalin-fixed, paraffin-embedded tumors, as collected routinely in clinical practice,” Dr. Ramus noted. “Women predicted to have poor survival using current treatments could be included in clinical trials to rapidly get alternative treatment. Many of the genes included in this test are targets of known drugs, so this information could lead to alternative targeted treatments.
“This test is not ready for routine clinical care yet,” she added. “The next step would be to include this signature as part of a clinical trial. If patients predicted to have poor survival are given alternative treatments that improve their survival, then the test could be included in treatment decisions.”
Study details
Dr. Ramus and colleagues began this work by measuring tumor expression of 513 genes selected via meta-analysis. The team then developed a gene expression assay and a prognostic signature for overall survival, which they trained on tumors from 2,702 women in 15 studies and validated on an independent set of tumors from 1,067 women in 6 studies.
In analyses adjusted for covariates, expression levels of 276 genes were associated with overall survival. The signature with the best prognostic performance contained 101 genes that were enriched in pathways having treatment implications, such as pathways involved in immune response, mitosis, and homologous recombination repair.
Adding the signature to age and stage alone improved prediction of 2- and 5-year overall survival. The area under the curve increased from 0.61 to 0.69 for 2-year overall survival and from 0.62 to 0.75 for 5-year overall survival (with nonoverlapping 95% confidence intervals for 5-year survival).
Each standard deviation increase in the gene expression score was associated with a more than doubling of the risk of death (hazard ratio, 2.35; P < .001).
The median overall survival by gene expression score quintile was 9.5 years for patients in the first quintile, 5.4 years for patients in the second, 3.8 years for patients in the third, 3.2 years for patients in the fourth, and 2.3 years for patients in the fifth.
This study was funded by the National Institutes of Health/National Cancer Institute, the Canadian Institutes for Health Research, and the Department of Defense Ovarian Cancer Research Program. Some of the authors disclosed financial relationships with a range of companies. Dr. Ramus disclosed no conflicts of interest.
SOURCE: Millstein J et al. Ann Oncol. 2020 Sep;31(9):1240-50.
according to a study published in Annals of Oncology.
“Gene expression signature tests for prognosis are available for other cancers, such as breast cancer, and these help with treatment decisions, but no such tests are available for ovarian cancer,” senior investigator Susan J. Ramus, PhD, of Lowy Cancer Research Centre, University of NSW Sydney, commented in an interview.
Dr. Ramus and associates developed and validated their 101-gene expression signature using pretreatment tumor tissue from 3,769 women with high-grade serous ovarian cancer treated on 21 studies.
The investigators found this signature, called OTTA-SPOT (Ovarian Tumor Tissue Analysis Consortium–Stratified Prognosis of Ovarian Tumors), performed well at stratifying women according to overall survival. Median overall survival times ranged from about 2 years for patients in the top quintile of scores to more than 9 years for patients in the bottom quintile.
Moreover, OTTA-SPOT significantly improved prognostication when added to age and stage.
“This tumor test works on formalin-fixed, paraffin-embedded tumors, as collected routinely in clinical practice,” Dr. Ramus noted. “Women predicted to have poor survival using current treatments could be included in clinical trials to rapidly get alternative treatment. Many of the genes included in this test are targets of known drugs, so this information could lead to alternative targeted treatments.
“This test is not ready for routine clinical care yet,” she added. “The next step would be to include this signature as part of a clinical trial. If patients predicted to have poor survival are given alternative treatments that improve their survival, then the test could be included in treatment decisions.”
Study details
Dr. Ramus and colleagues began this work by measuring tumor expression of 513 genes selected via meta-analysis. The team then developed a gene expression assay and a prognostic signature for overall survival, which they trained on tumors from 2,702 women in 15 studies and validated on an independent set of tumors from 1,067 women in 6 studies.
In analyses adjusted for covariates, expression levels of 276 genes were associated with overall survival. The signature with the best prognostic performance contained 101 genes that were enriched in pathways having treatment implications, such as pathways involved in immune response, mitosis, and homologous recombination repair.
Adding the signature to age and stage alone improved prediction of 2- and 5-year overall survival. The area under the curve increased from 0.61 to 0.69 for 2-year overall survival and from 0.62 to 0.75 for 5-year overall survival (with nonoverlapping 95% confidence intervals for 5-year survival).
Each standard deviation increase in the gene expression score was associated with a more than doubling of the risk of death (hazard ratio, 2.35; P < .001).
The median overall survival by gene expression score quintile was 9.5 years for patients in the first quintile, 5.4 years for patients in the second, 3.8 years for patients in the third, 3.2 years for patients in the fourth, and 2.3 years for patients in the fifth.
This study was funded by the National Institutes of Health/National Cancer Institute, the Canadian Institutes for Health Research, and the Department of Defense Ovarian Cancer Research Program. Some of the authors disclosed financial relationships with a range of companies. Dr. Ramus disclosed no conflicts of interest.
SOURCE: Millstein J et al. Ann Oncol. 2020 Sep;31(9):1240-50.
according to a study published in Annals of Oncology.
“Gene expression signature tests for prognosis are available for other cancers, such as breast cancer, and these help with treatment decisions, but no such tests are available for ovarian cancer,” senior investigator Susan J. Ramus, PhD, of Lowy Cancer Research Centre, University of NSW Sydney, commented in an interview.
Dr. Ramus and associates developed and validated their 101-gene expression signature using pretreatment tumor tissue from 3,769 women with high-grade serous ovarian cancer treated on 21 studies.
The investigators found this signature, called OTTA-SPOT (Ovarian Tumor Tissue Analysis Consortium–Stratified Prognosis of Ovarian Tumors), performed well at stratifying women according to overall survival. Median overall survival times ranged from about 2 years for patients in the top quintile of scores to more than 9 years for patients in the bottom quintile.
Moreover, OTTA-SPOT significantly improved prognostication when added to age and stage.
“This tumor test works on formalin-fixed, paraffin-embedded tumors, as collected routinely in clinical practice,” Dr. Ramus noted. “Women predicted to have poor survival using current treatments could be included in clinical trials to rapidly get alternative treatment. Many of the genes included in this test are targets of known drugs, so this information could lead to alternative targeted treatments.
“This test is not ready for routine clinical care yet,” she added. “The next step would be to include this signature as part of a clinical trial. If patients predicted to have poor survival are given alternative treatments that improve their survival, then the test could be included in treatment decisions.”
Study details
Dr. Ramus and colleagues began this work by measuring tumor expression of 513 genes selected via meta-analysis. The team then developed a gene expression assay and a prognostic signature for overall survival, which they trained on tumors from 2,702 women in 15 studies and validated on an independent set of tumors from 1,067 women in 6 studies.
In analyses adjusted for covariates, expression levels of 276 genes were associated with overall survival. The signature with the best prognostic performance contained 101 genes that were enriched in pathways having treatment implications, such as pathways involved in immune response, mitosis, and homologous recombination repair.
Adding the signature to age and stage alone improved prediction of 2- and 5-year overall survival. The area under the curve increased from 0.61 to 0.69 for 2-year overall survival and from 0.62 to 0.75 for 5-year overall survival (with nonoverlapping 95% confidence intervals for 5-year survival).
Each standard deviation increase in the gene expression score was associated with a more than doubling of the risk of death (hazard ratio, 2.35; P < .001).
The median overall survival by gene expression score quintile was 9.5 years for patients in the first quintile, 5.4 years for patients in the second, 3.8 years for patients in the third, 3.2 years for patients in the fourth, and 2.3 years for patients in the fifth.
This study was funded by the National Institutes of Health/National Cancer Institute, the Canadian Institutes for Health Research, and the Department of Defense Ovarian Cancer Research Program. Some of the authors disclosed financial relationships with a range of companies. Dr. Ramus disclosed no conflicts of interest.
SOURCE: Millstein J et al. Ann Oncol. 2020 Sep;31(9):1240-50.
FROM ANNALS OF ONCOLOGY
Identifying ovarian malignancy is not so easy
When an ovarian mass is anticipated or known, following evaluation of a patient’s history and physician examination, imaging via transvaginal and often abdominal ultrasound is the very next step. This evaluation likely will include both gray-scale and color Doppler examination. The initial concern always must be to identify ovarian malignancy.
Despite morphological scoring systems as well as the use of Doppler ultrasonography, there remains a lack of agreement and acceptance. In a 2008 multicenter study, Timmerman and colleagues evaluated 1,066 patients with 1,233 persistent adnexal tumors via transvaginal grayscale and Doppler ultrasound; 73% were benign tumors, and 27% were malignant tumors. Information on 42 gray-scale ultrasound variables and 6 Doppler variables was collected and evaluated to determine which variables had the highest positive predictive value for a malignant tumor and for a benign mass (Ultrasound Obstet Gynecol. 2008 Jun. doi: 10.1002/uog.5365).
Five simple rules were selected that best predict malignancy (M-rules), as follows:
- Irregular solid tumor.
- Ascites.
- At least four papillary projections.
- Irregular multilocular-solid tumor with a greatest diameter greater than or equal to 10 cm.
- Very high color content on Doppler exam.
The following five simple rules suggested that a mass is benign (B-rules):
- Unilocular cyst.
- Largest solid component less than 7 mm.
- Acoustic shadows.
- Smooth multilocular tumor less than 10 cm.
- No detectable blood flow with Doppler exam.
Unfortunately, despite a sensitivity of 93% and specificity of 90%, and a positive and negative predictive value of 80% and 97%, these 10 simple rules were applicable to only 76% of tumors.
To assist those of us who are not gynecologic oncologists and who are often faced with having to determine whether surgery is recommended, I have elicited the expertise of Jubilee Brown, MD, professor and associate director of gynecologic oncology at the Levine Cancer Institute, Carolinas HealthCare System, in Charlotte, N.C., and the current president of the AAGL, to lead us in a review of evaluating an ovarian mass.
Dr. Miller is professor of obstetrics & gynecology in the department of clinical sciences, Rosalind Franklin University, North Chicago, Ill., and director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, both in Illinois. Email him at [email protected].
When an ovarian mass is anticipated or known, following evaluation of a patient’s history and physician examination, imaging via transvaginal and often abdominal ultrasound is the very next step. This evaluation likely will include both gray-scale and color Doppler examination. The initial concern always must be to identify ovarian malignancy.
Despite morphological scoring systems as well as the use of Doppler ultrasonography, there remains a lack of agreement and acceptance. In a 2008 multicenter study, Timmerman and colleagues evaluated 1,066 patients with 1,233 persistent adnexal tumors via transvaginal grayscale and Doppler ultrasound; 73% were benign tumors, and 27% were malignant tumors. Information on 42 gray-scale ultrasound variables and 6 Doppler variables was collected and evaluated to determine which variables had the highest positive predictive value for a malignant tumor and for a benign mass (Ultrasound Obstet Gynecol. 2008 Jun. doi: 10.1002/uog.5365).
Five simple rules were selected that best predict malignancy (M-rules), as follows:
- Irregular solid tumor.
- Ascites.
- At least four papillary projections.
- Irregular multilocular-solid tumor with a greatest diameter greater than or equal to 10 cm.
- Very high color content on Doppler exam.
The following five simple rules suggested that a mass is benign (B-rules):
- Unilocular cyst.
- Largest solid component less than 7 mm.
- Acoustic shadows.
- Smooth multilocular tumor less than 10 cm.
- No detectable blood flow with Doppler exam.
Unfortunately, despite a sensitivity of 93% and specificity of 90%, and a positive and negative predictive value of 80% and 97%, these 10 simple rules were applicable to only 76% of tumors.
To assist those of us who are not gynecologic oncologists and who are often faced with having to determine whether surgery is recommended, I have elicited the expertise of Jubilee Brown, MD, professor and associate director of gynecologic oncology at the Levine Cancer Institute, Carolinas HealthCare System, in Charlotte, N.C., and the current president of the AAGL, to lead us in a review of evaluating an ovarian mass.
Dr. Miller is professor of obstetrics & gynecology in the department of clinical sciences, Rosalind Franklin University, North Chicago, Ill., and director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, both in Illinois. Email him at [email protected].
When an ovarian mass is anticipated or known, following evaluation of a patient’s history and physician examination, imaging via transvaginal and often abdominal ultrasound is the very next step. This evaluation likely will include both gray-scale and color Doppler examination. The initial concern always must be to identify ovarian malignancy.
Despite morphological scoring systems as well as the use of Doppler ultrasonography, there remains a lack of agreement and acceptance. In a 2008 multicenter study, Timmerman and colleagues evaluated 1,066 patients with 1,233 persistent adnexal tumors via transvaginal grayscale and Doppler ultrasound; 73% were benign tumors, and 27% were malignant tumors. Information on 42 gray-scale ultrasound variables and 6 Doppler variables was collected and evaluated to determine which variables had the highest positive predictive value for a malignant tumor and for a benign mass (Ultrasound Obstet Gynecol. 2008 Jun. doi: 10.1002/uog.5365).
Five simple rules were selected that best predict malignancy (M-rules), as follows:
- Irregular solid tumor.
- Ascites.
- At least four papillary projections.
- Irregular multilocular-solid tumor with a greatest diameter greater than or equal to 10 cm.
- Very high color content on Doppler exam.
The following five simple rules suggested that a mass is benign (B-rules):
- Unilocular cyst.
- Largest solid component less than 7 mm.
- Acoustic shadows.
- Smooth multilocular tumor less than 10 cm.
- No detectable blood flow with Doppler exam.
Unfortunately, despite a sensitivity of 93% and specificity of 90%, and a positive and negative predictive value of 80% and 97%, these 10 simple rules were applicable to only 76% of tumors.
To assist those of us who are not gynecologic oncologists and who are often faced with having to determine whether surgery is recommended, I have elicited the expertise of Jubilee Brown, MD, professor and associate director of gynecologic oncology at the Levine Cancer Institute, Carolinas HealthCare System, in Charlotte, N.C., and the current president of the AAGL, to lead us in a review of evaluating an ovarian mass.
Dr. Miller is professor of obstetrics & gynecology in the department of clinical sciences, Rosalind Franklin University, North Chicago, Ill., and director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, both in Illinois. Email him at [email protected].
How to evaluate a suspicious ovarian mass
Ovarian masses are common in women of all ages. It is important not to miss even one ovarian cancer, but we must also identify masses that will resolve on their own over time to avoid overtreatment. These concurrent goals of excluding malignancy while not overtreating patients are the basis for management of the pelvic mass. Additionally, fertility preservation is important when surgery is performed in a reproductive-aged woman.
An ovarian mass may be anything from a simple functional or physiologic cyst to an endometrioma to an epithelial carcinoma, a germ-cell tumor, or a stromal tumor (the latter three of which may metastasize). Across the general population, women have a 5%-10% lifetime risk of needing surgery for a suspected ovarian mass and a 1.4% (1 in 70) risk that this mass is cancerous. The majority of ovarian cysts or masses therefore are benign.
A thorough history – including family history – and physical examination with appropriate laboratory testing and directed imaging are important first steps for the ob.gyn. Fortunately, we have guidelines and criteria governing not only when observation or surgery is warranted but also when patients should be referred to a gynecologic oncologist. By following these guidelines,1 we are able to achieve the best outcomes.
Transvaginal ultrasound
A 2007 groundbreaking study led by Barbara Goff, MD, demonstrated that there are warning signs for ovarian cancer – symptoms that are significantly associated with malignancy. Dr. Goff and her coinvestigators evaluated the charts of hundreds of patients, including about 150 with ovarian cancer, and found that pelvic/abdominal pressure or pain, bloating, increase in abdominal size, and difficulty eating or feeling full were significantly and independently associated with cancer if these symptoms were present for less than a year and occurred at least 12 times per month.2
A pelvic examination is an integral part of evaluating every patient who has such concerns. That said, pelvic exams have limited ability to identify adnexal masses, especially in women who are obese – and that’s where imaging becomes especially important.
Masses generally can be considered simple or complex based on their appearance. A simple cyst is fluid-filled with thin, smooth walls and the absence of solid components or septations; it is significantly more likely to resolve on its own and is less likely to imply malignancy than a complex cyst, especially in a premenopausal woman. A complex cyst is multiseptated and/or solid – possibly with papillary projections – and is more concerning, especially if there is increased, new vascularity. Making this distinction helps us determine the risk of malignancy.
Transvaginal ultrasound (TVUS) is the preferred method for imaging, and our threshold for obtaining a TVUS should be very low. Women who have symptoms or concerns that can’t be attributed to a particular condition, and women in whom a mass can be palpated (even if asymptomatic) should have a TVUS. The imaging modality is cost effective and well tolerated by patients, does not expose the patient to ionizing radiation, and should generally be considered first-line imaging.3,4
Size is not predictive of malignancy, but it is important for determining whether surgery is warranted. In our experience, a mass of 8-10 cm or larger on TVUS is at risk of torsion and is unlikely to resolve on its own, even in a premenopausal woman. While large masses generally require surgery, patients of any age who have simple cysts smaller than 8-10 cm generally can be followed with serial exams and ultrasound; spontaneous regression is common.
Doppler ultrasonography is useful for evaluating blood flow in and around an ovarian mass and can be helpful for confirming suspected characteristics of a mass.
Recent studies from the radiology community have looked at the utility of the resistive index – a measure of the impedance and velocity of blood flow – as a predictor of ovarian malignancy. However, we caution against using Doppler to determine whether a mass is benign or malignant, or to determine the necessity of surgery. An abnormal ovary may have what is considered to be a normal resistive index, and the resistive index of a normal ovary may fall within the abnormal range. Doppler flow can be helpful, but it must be combined with other predictive features, like solid components with flow or papillary projections within a cyst, to define a decision about surgery.4,5
Magnetic resonance imaging can be useful in differentiating a fibroid from an ovarian mass, and a CT scan can be helpful in looking for disseminated disease when ovarian cancer is suspected based on ultrasound imaging, physical and history, and serum markers. A CT is useful, for instance, in a patient whose ovary is distended with ascites or who has upper abdominal complaints and a complex cyst. CT, PET, and MRI are not recommended in the initial evaluation of an ovarian mass.
The utility of serum biomarkers
Cancer antigen 125 (CA-125) testing may be helpful – in combination with other findings – for decision-making regarding the likelihood of malignancy and the need to refer patients. CA-125 is like Doppler in that a normal CA-125 cannot eliminate the possibility of cancer, and an abnormal CA-125 does not in and of itself imply malignancy. It’s far from a perfect cancer screening test.
CA-125 is a protein associated with epithelial ovarian malignancies, the type of ovarian cancer most commonly seen in postmenopausal women with genetic predispositions. Its specificity and positive predictive value are much higher in postmenopausal women than in average-risk premenopausal women (those without a family history or a known mutation that predisposes them to ovarian cancer). Levels of the marker are elevated in association with many nonmalignant conditions in premenopausal women – endometriosis, fibroids, and various inflammatory conditions, for instance – so the marker’s utility in this population is limited.
For women who have a family history of ovarian cancer or a known breast cancer gene 1 (BRCA1) or BRCA2 mutation, there are some data that suggest that monitoring with CA-125 measurements and TVUS may be a good approach to following patients prior to the age at which risk-reducing surgery can best be performed.
In an adolescent girl or a woman of reproductive age, we think less about epithelial cancer and more about germ-cell and stromal tumors. When a solid mass is palpated or visualized on imaging, we therefore will utilize a different set of markers; alpha-fetoprotein, L-lactate dehydrogenase, and beta-HCG, for instance, have much higher specificity than CA-125 does for germ-cell tumors in this age group and may be helpful in the evaluation. Similarly, in cases of a very large mass resembling a mucinous tumor, a carcinoembryonic antigen may be helpful.
A number of proprietary profiling technologies have been developed to determine the risk of a diagnosed mass being malignant. For instance, the OVA1 assay looks at five serum markers and scores the results, and the Risk of Ovarian Malignancy Algorithm (ROMA) combines the results of three serum markers with menopausal status into a numerical score. Both have Food and Drug Administration approval for use in women in whom surgery has been deemed necessary. These panels can be fairly predictive of risk and may be helpful – especially in rural areas – in determining which women should be referred to a gynecologic oncologist for surgery.
It is important to appreciate that an ovarian cyst or mass should never be biopsied or aspirated lest a malignant tumor confined to one ovary be potentially spread to the peritoneum.
Referral to a gynecologic oncologist
Postmenopausal women with a CA-125 greater than 35 U/mL should be referred, as should postmenopausal women with ascites, those with a nodular or fixed pelvic mass, and those with suspected abdominal or distant metastases (per a CT scan, for instance).
In premenopausal women, ascites, a nodular or fixed mass, and evidence of metastases also are reasons for referral to a gynecologic oncologist. CA-125, again, is much more likely to be elevated for reasons other than malignancy and therefore is not as strong a driver for referral as in postmenopausal women. Patients with markedly elevated levels, however, should probably be referred – particularly when other clinical factors also suggest the need for consultation. While there is no evidence-based threshold for CA-125 in premenopausal women, a CA-125 greater than 200 U/mL is a good cutoff for referral.
For any patient, family history of breast and/or ovarian cancer – especially in a first-degree relative – raises the risk of malignancy and should figure prominently into decision-making regarding referral. Criteria for referral are among the points discussed in the ACOG 2016 Practice Bulletin on Evaluation and Management of Adnexal Masses.1
A note on BRCA mutations
As the American College of Obstetricians and Gynecologists says in its practice bulletin, the most important personal risk factor for ovarian cancer is a strong family history of breast or ovarian cancer. Women with such a family history can undergo genetic testing for BRCA mutations and have the opportunity to prevent ovarian cancers when mutations are detected. This simple blood test can save lives.
A modeling study we recently completed – not yet published – shows that it actually would be cost effective to do population screening with BRCA testing performed on every woman at age 30 years.
According to the National Cancer Institute website (last review: 2018), it is estimated that about 44% of women who inherit a BRCA1 mutation, and about 17% of those who inherit a BRAC2 mutation, will develop ovarian cancer by the age of 80 years. By identifying those mutations, women may undergo risk-reducing surgery at designated ages after childbearing is complete and bring their risk down to under 5%.
An international take on managing adnexal masses
- Pelvic ultrasound should include the transvaginal approach. Use Doppler imaging as indicated.
- Although simple ovarian cysts are not precursor lesions to a malignant ovarian cancer, perform a high-quality examination to make sure there are no solid/papillary structures before classifying a cyst as a simple cyst. The risk of progression to malignancy is extremely low, but some follow-up is prudent.
- The most accurate method of characterizing an ovarian mass currently is real-time pattern recognition sonography in the hands of an experienced imager.
- Pattern recognition sonography or a risk model such as the International Ovarian Tumor Analysis (IOTA) Simple Rules can be used to initially characterize an ovarian mass.
- When an ovarian lesion is classified as benign, the patient may be followed conservatively, or if indicated, surgery can be performed by a general gynecologist.
- Serial sonography can be beneficial, but there are limited prospective data to support an exact interval and duration.
- Fewer surgical interventions may result in an increase in sonographic surveillance.
- When an ovarian lesion is considered indeterminate on initial sonography, and after appropriate clinical evaluation, a “second-step” evaluation may include referral to an expert sonologist, serial sonography, application of established risk-prediction models, correlation with serum biomarkers, correlation with MRI, or referral to a gynecologic oncologist for further evaluation.
From the First International Consensus Report on Adnexal Masses: Management Recommendations
Source: Glanc P et al. J Ultrasound Med. 2017 May;36(5):849-63.
Dr. Brown reported that she had received an earlier grant from Aspira Labs, the company that developed the OVA1 assay. Dr. Miller reported that he has no relevant financial disclosures.
References
1. Obstet Gynecol. 2016 Nov. doi: 10.1097/AOG.0000000000001768.
2. Cancer. 2007 Jan 15. doi: 10.1002/cncr.22371.
3. Clin Obstet Gynecol. 2015 Mar. doi: 10.1097/GRF.0000000000000083.
4. Ultrasound Q. 2013 Mar. doi: 10.1097/RUQ.0b013e3182814d9b.
5. Ultrasound Obstet Gynecol. 2008 Jun. doi: 10.1002/uog.5365.
Ovarian masses are common in women of all ages. It is important not to miss even one ovarian cancer, but we must also identify masses that will resolve on their own over time to avoid overtreatment. These concurrent goals of excluding malignancy while not overtreating patients are the basis for management of the pelvic mass. Additionally, fertility preservation is important when surgery is performed in a reproductive-aged woman.
An ovarian mass may be anything from a simple functional or physiologic cyst to an endometrioma to an epithelial carcinoma, a germ-cell tumor, or a stromal tumor (the latter three of which may metastasize). Across the general population, women have a 5%-10% lifetime risk of needing surgery for a suspected ovarian mass and a 1.4% (1 in 70) risk that this mass is cancerous. The majority of ovarian cysts or masses therefore are benign.
A thorough history – including family history – and physical examination with appropriate laboratory testing and directed imaging are important first steps for the ob.gyn. Fortunately, we have guidelines and criteria governing not only when observation or surgery is warranted but also when patients should be referred to a gynecologic oncologist. By following these guidelines,1 we are able to achieve the best outcomes.
Transvaginal ultrasound
A 2007 groundbreaking study led by Barbara Goff, MD, demonstrated that there are warning signs for ovarian cancer – symptoms that are significantly associated with malignancy. Dr. Goff and her coinvestigators evaluated the charts of hundreds of patients, including about 150 with ovarian cancer, and found that pelvic/abdominal pressure or pain, bloating, increase in abdominal size, and difficulty eating or feeling full were significantly and independently associated with cancer if these symptoms were present for less than a year and occurred at least 12 times per month.2
A pelvic examination is an integral part of evaluating every patient who has such concerns. That said, pelvic exams have limited ability to identify adnexal masses, especially in women who are obese – and that’s where imaging becomes especially important.
Masses generally can be considered simple or complex based on their appearance. A simple cyst is fluid-filled with thin, smooth walls and the absence of solid components or septations; it is significantly more likely to resolve on its own and is less likely to imply malignancy than a complex cyst, especially in a premenopausal woman. A complex cyst is multiseptated and/or solid – possibly with papillary projections – and is more concerning, especially if there is increased, new vascularity. Making this distinction helps us determine the risk of malignancy.
Transvaginal ultrasound (TVUS) is the preferred method for imaging, and our threshold for obtaining a TVUS should be very low. Women who have symptoms or concerns that can’t be attributed to a particular condition, and women in whom a mass can be palpated (even if asymptomatic) should have a TVUS. The imaging modality is cost effective and well tolerated by patients, does not expose the patient to ionizing radiation, and should generally be considered first-line imaging.3,4
Size is not predictive of malignancy, but it is important for determining whether surgery is warranted. In our experience, a mass of 8-10 cm or larger on TVUS is at risk of torsion and is unlikely to resolve on its own, even in a premenopausal woman. While large masses generally require surgery, patients of any age who have simple cysts smaller than 8-10 cm generally can be followed with serial exams and ultrasound; spontaneous regression is common.
Doppler ultrasonography is useful for evaluating blood flow in and around an ovarian mass and can be helpful for confirming suspected characteristics of a mass.
Recent studies from the radiology community have looked at the utility of the resistive index – a measure of the impedance and velocity of blood flow – as a predictor of ovarian malignancy. However, we caution against using Doppler to determine whether a mass is benign or malignant, or to determine the necessity of surgery. An abnormal ovary may have what is considered to be a normal resistive index, and the resistive index of a normal ovary may fall within the abnormal range. Doppler flow can be helpful, but it must be combined with other predictive features, like solid components with flow or papillary projections within a cyst, to define a decision about surgery.4,5
Magnetic resonance imaging can be useful in differentiating a fibroid from an ovarian mass, and a CT scan can be helpful in looking for disseminated disease when ovarian cancer is suspected based on ultrasound imaging, physical and history, and serum markers. A CT is useful, for instance, in a patient whose ovary is distended with ascites or who has upper abdominal complaints and a complex cyst. CT, PET, and MRI are not recommended in the initial evaluation of an ovarian mass.
The utility of serum biomarkers
Cancer antigen 125 (CA-125) testing may be helpful – in combination with other findings – for decision-making regarding the likelihood of malignancy and the need to refer patients. CA-125 is like Doppler in that a normal CA-125 cannot eliminate the possibility of cancer, and an abnormal CA-125 does not in and of itself imply malignancy. It’s far from a perfect cancer screening test.
CA-125 is a protein associated with epithelial ovarian malignancies, the type of ovarian cancer most commonly seen in postmenopausal women with genetic predispositions. Its specificity and positive predictive value are much higher in postmenopausal women than in average-risk premenopausal women (those without a family history or a known mutation that predisposes them to ovarian cancer). Levels of the marker are elevated in association with many nonmalignant conditions in premenopausal women – endometriosis, fibroids, and various inflammatory conditions, for instance – so the marker’s utility in this population is limited.
For women who have a family history of ovarian cancer or a known breast cancer gene 1 (BRCA1) or BRCA2 mutation, there are some data that suggest that monitoring with CA-125 measurements and TVUS may be a good approach to following patients prior to the age at which risk-reducing surgery can best be performed.
In an adolescent girl or a woman of reproductive age, we think less about epithelial cancer and more about germ-cell and stromal tumors. When a solid mass is palpated or visualized on imaging, we therefore will utilize a different set of markers; alpha-fetoprotein, L-lactate dehydrogenase, and beta-HCG, for instance, have much higher specificity than CA-125 does for germ-cell tumors in this age group and may be helpful in the evaluation. Similarly, in cases of a very large mass resembling a mucinous tumor, a carcinoembryonic antigen may be helpful.
A number of proprietary profiling technologies have been developed to determine the risk of a diagnosed mass being malignant. For instance, the OVA1 assay looks at five serum markers and scores the results, and the Risk of Ovarian Malignancy Algorithm (ROMA) combines the results of three serum markers with menopausal status into a numerical score. Both have Food and Drug Administration approval for use in women in whom surgery has been deemed necessary. These panels can be fairly predictive of risk and may be helpful – especially in rural areas – in determining which women should be referred to a gynecologic oncologist for surgery.
It is important to appreciate that an ovarian cyst or mass should never be biopsied or aspirated lest a malignant tumor confined to one ovary be potentially spread to the peritoneum.
Referral to a gynecologic oncologist
Postmenopausal women with a CA-125 greater than 35 U/mL should be referred, as should postmenopausal women with ascites, those with a nodular or fixed pelvic mass, and those with suspected abdominal or distant metastases (per a CT scan, for instance).
In premenopausal women, ascites, a nodular or fixed mass, and evidence of metastases also are reasons for referral to a gynecologic oncologist. CA-125, again, is much more likely to be elevated for reasons other than malignancy and therefore is not as strong a driver for referral as in postmenopausal women. Patients with markedly elevated levels, however, should probably be referred – particularly when other clinical factors also suggest the need for consultation. While there is no evidence-based threshold for CA-125 in premenopausal women, a CA-125 greater than 200 U/mL is a good cutoff for referral.
For any patient, family history of breast and/or ovarian cancer – especially in a first-degree relative – raises the risk of malignancy and should figure prominently into decision-making regarding referral. Criteria for referral are among the points discussed in the ACOG 2016 Practice Bulletin on Evaluation and Management of Adnexal Masses.1
A note on BRCA mutations
As the American College of Obstetricians and Gynecologists says in its practice bulletin, the most important personal risk factor for ovarian cancer is a strong family history of breast or ovarian cancer. Women with such a family history can undergo genetic testing for BRCA mutations and have the opportunity to prevent ovarian cancers when mutations are detected. This simple blood test can save lives.
A modeling study we recently completed – not yet published – shows that it actually would be cost effective to do population screening with BRCA testing performed on every woman at age 30 years.
According to the National Cancer Institute website (last review: 2018), it is estimated that about 44% of women who inherit a BRCA1 mutation, and about 17% of those who inherit a BRAC2 mutation, will develop ovarian cancer by the age of 80 years. By identifying those mutations, women may undergo risk-reducing surgery at designated ages after childbearing is complete and bring their risk down to under 5%.
An international take on managing adnexal masses
- Pelvic ultrasound should include the transvaginal approach. Use Doppler imaging as indicated.
- Although simple ovarian cysts are not precursor lesions to a malignant ovarian cancer, perform a high-quality examination to make sure there are no solid/papillary structures before classifying a cyst as a simple cyst. The risk of progression to malignancy is extremely low, but some follow-up is prudent.
- The most accurate method of characterizing an ovarian mass currently is real-time pattern recognition sonography in the hands of an experienced imager.
- Pattern recognition sonography or a risk model such as the International Ovarian Tumor Analysis (IOTA) Simple Rules can be used to initially characterize an ovarian mass.
- When an ovarian lesion is classified as benign, the patient may be followed conservatively, or if indicated, surgery can be performed by a general gynecologist.
- Serial sonography can be beneficial, but there are limited prospective data to support an exact interval and duration.
- Fewer surgical interventions may result in an increase in sonographic surveillance.
- When an ovarian lesion is considered indeterminate on initial sonography, and after appropriate clinical evaluation, a “second-step” evaluation may include referral to an expert sonologist, serial sonography, application of established risk-prediction models, correlation with serum biomarkers, correlation with MRI, or referral to a gynecologic oncologist for further evaluation.
From the First International Consensus Report on Adnexal Masses: Management Recommendations
Source: Glanc P et al. J Ultrasound Med. 2017 May;36(5):849-63.
Dr. Brown reported that she had received an earlier grant from Aspira Labs, the company that developed the OVA1 assay. Dr. Miller reported that he has no relevant financial disclosures.
References
1. Obstet Gynecol. 2016 Nov. doi: 10.1097/AOG.0000000000001768.
2. Cancer. 2007 Jan 15. doi: 10.1002/cncr.22371.
3. Clin Obstet Gynecol. 2015 Mar. doi: 10.1097/GRF.0000000000000083.
4. Ultrasound Q. 2013 Mar. doi: 10.1097/RUQ.0b013e3182814d9b.
5. Ultrasound Obstet Gynecol. 2008 Jun. doi: 10.1002/uog.5365.
Ovarian masses are common in women of all ages. It is important not to miss even one ovarian cancer, but we must also identify masses that will resolve on their own over time to avoid overtreatment. These concurrent goals of excluding malignancy while not overtreating patients are the basis for management of the pelvic mass. Additionally, fertility preservation is important when surgery is performed in a reproductive-aged woman.
An ovarian mass may be anything from a simple functional or physiologic cyst to an endometrioma to an epithelial carcinoma, a germ-cell tumor, or a stromal tumor (the latter three of which may metastasize). Across the general population, women have a 5%-10% lifetime risk of needing surgery for a suspected ovarian mass and a 1.4% (1 in 70) risk that this mass is cancerous. The majority of ovarian cysts or masses therefore are benign.
A thorough history – including family history – and physical examination with appropriate laboratory testing and directed imaging are important first steps for the ob.gyn. Fortunately, we have guidelines and criteria governing not only when observation or surgery is warranted but also when patients should be referred to a gynecologic oncologist. By following these guidelines,1 we are able to achieve the best outcomes.
Transvaginal ultrasound
A 2007 groundbreaking study led by Barbara Goff, MD, demonstrated that there are warning signs for ovarian cancer – symptoms that are significantly associated with malignancy. Dr. Goff and her coinvestigators evaluated the charts of hundreds of patients, including about 150 with ovarian cancer, and found that pelvic/abdominal pressure or pain, bloating, increase in abdominal size, and difficulty eating or feeling full were significantly and independently associated with cancer if these symptoms were present for less than a year and occurred at least 12 times per month.2
A pelvic examination is an integral part of evaluating every patient who has such concerns. That said, pelvic exams have limited ability to identify adnexal masses, especially in women who are obese – and that’s where imaging becomes especially important.
Masses generally can be considered simple or complex based on their appearance. A simple cyst is fluid-filled with thin, smooth walls and the absence of solid components or septations; it is significantly more likely to resolve on its own and is less likely to imply malignancy than a complex cyst, especially in a premenopausal woman. A complex cyst is multiseptated and/or solid – possibly with papillary projections – and is more concerning, especially if there is increased, new vascularity. Making this distinction helps us determine the risk of malignancy.
Transvaginal ultrasound (TVUS) is the preferred method for imaging, and our threshold for obtaining a TVUS should be very low. Women who have symptoms or concerns that can’t be attributed to a particular condition, and women in whom a mass can be palpated (even if asymptomatic) should have a TVUS. The imaging modality is cost effective and well tolerated by patients, does not expose the patient to ionizing radiation, and should generally be considered first-line imaging.3,4
Size is not predictive of malignancy, but it is important for determining whether surgery is warranted. In our experience, a mass of 8-10 cm or larger on TVUS is at risk of torsion and is unlikely to resolve on its own, even in a premenopausal woman. While large masses generally require surgery, patients of any age who have simple cysts smaller than 8-10 cm generally can be followed with serial exams and ultrasound; spontaneous regression is common.
Doppler ultrasonography is useful for evaluating blood flow in and around an ovarian mass and can be helpful for confirming suspected characteristics of a mass.
Recent studies from the radiology community have looked at the utility of the resistive index – a measure of the impedance and velocity of blood flow – as a predictor of ovarian malignancy. However, we caution against using Doppler to determine whether a mass is benign or malignant, or to determine the necessity of surgery. An abnormal ovary may have what is considered to be a normal resistive index, and the resistive index of a normal ovary may fall within the abnormal range. Doppler flow can be helpful, but it must be combined with other predictive features, like solid components with flow or papillary projections within a cyst, to define a decision about surgery.4,5
Magnetic resonance imaging can be useful in differentiating a fibroid from an ovarian mass, and a CT scan can be helpful in looking for disseminated disease when ovarian cancer is suspected based on ultrasound imaging, physical and history, and serum markers. A CT is useful, for instance, in a patient whose ovary is distended with ascites or who has upper abdominal complaints and a complex cyst. CT, PET, and MRI are not recommended in the initial evaluation of an ovarian mass.
The utility of serum biomarkers
Cancer antigen 125 (CA-125) testing may be helpful – in combination with other findings – for decision-making regarding the likelihood of malignancy and the need to refer patients. CA-125 is like Doppler in that a normal CA-125 cannot eliminate the possibility of cancer, and an abnormal CA-125 does not in and of itself imply malignancy. It’s far from a perfect cancer screening test.
CA-125 is a protein associated with epithelial ovarian malignancies, the type of ovarian cancer most commonly seen in postmenopausal women with genetic predispositions. Its specificity and positive predictive value are much higher in postmenopausal women than in average-risk premenopausal women (those without a family history or a known mutation that predisposes them to ovarian cancer). Levels of the marker are elevated in association with many nonmalignant conditions in premenopausal women – endometriosis, fibroids, and various inflammatory conditions, for instance – so the marker’s utility in this population is limited.
For women who have a family history of ovarian cancer or a known breast cancer gene 1 (BRCA1) or BRCA2 mutation, there are some data that suggest that monitoring with CA-125 measurements and TVUS may be a good approach to following patients prior to the age at which risk-reducing surgery can best be performed.
In an adolescent girl or a woman of reproductive age, we think less about epithelial cancer and more about germ-cell and stromal tumors. When a solid mass is palpated or visualized on imaging, we therefore will utilize a different set of markers; alpha-fetoprotein, L-lactate dehydrogenase, and beta-HCG, for instance, have much higher specificity than CA-125 does for germ-cell tumors in this age group and may be helpful in the evaluation. Similarly, in cases of a very large mass resembling a mucinous tumor, a carcinoembryonic antigen may be helpful.
A number of proprietary profiling technologies have been developed to determine the risk of a diagnosed mass being malignant. For instance, the OVA1 assay looks at five serum markers and scores the results, and the Risk of Ovarian Malignancy Algorithm (ROMA) combines the results of three serum markers with menopausal status into a numerical score. Both have Food and Drug Administration approval for use in women in whom surgery has been deemed necessary. These panels can be fairly predictive of risk and may be helpful – especially in rural areas – in determining which women should be referred to a gynecologic oncologist for surgery.
It is important to appreciate that an ovarian cyst or mass should never be biopsied or aspirated lest a malignant tumor confined to one ovary be potentially spread to the peritoneum.
Referral to a gynecologic oncologist
Postmenopausal women with a CA-125 greater than 35 U/mL should be referred, as should postmenopausal women with ascites, those with a nodular or fixed pelvic mass, and those with suspected abdominal or distant metastases (per a CT scan, for instance).
In premenopausal women, ascites, a nodular or fixed mass, and evidence of metastases also are reasons for referral to a gynecologic oncologist. CA-125, again, is much more likely to be elevated for reasons other than malignancy and therefore is not as strong a driver for referral as in postmenopausal women. Patients with markedly elevated levels, however, should probably be referred – particularly when other clinical factors also suggest the need for consultation. While there is no evidence-based threshold for CA-125 in premenopausal women, a CA-125 greater than 200 U/mL is a good cutoff for referral.
For any patient, family history of breast and/or ovarian cancer – especially in a first-degree relative – raises the risk of malignancy and should figure prominently into decision-making regarding referral. Criteria for referral are among the points discussed in the ACOG 2016 Practice Bulletin on Evaluation and Management of Adnexal Masses.1
A note on BRCA mutations
As the American College of Obstetricians and Gynecologists says in its practice bulletin, the most important personal risk factor for ovarian cancer is a strong family history of breast or ovarian cancer. Women with such a family history can undergo genetic testing for BRCA mutations and have the opportunity to prevent ovarian cancers when mutations are detected. This simple blood test can save lives.
A modeling study we recently completed – not yet published – shows that it actually would be cost effective to do population screening with BRCA testing performed on every woman at age 30 years.
According to the National Cancer Institute website (last review: 2018), it is estimated that about 44% of women who inherit a BRCA1 mutation, and about 17% of those who inherit a BRAC2 mutation, will develop ovarian cancer by the age of 80 years. By identifying those mutations, women may undergo risk-reducing surgery at designated ages after childbearing is complete and bring their risk down to under 5%.
An international take on managing adnexal masses
- Pelvic ultrasound should include the transvaginal approach. Use Doppler imaging as indicated.
- Although simple ovarian cysts are not precursor lesions to a malignant ovarian cancer, perform a high-quality examination to make sure there are no solid/papillary structures before classifying a cyst as a simple cyst. The risk of progression to malignancy is extremely low, but some follow-up is prudent.
- The most accurate method of characterizing an ovarian mass currently is real-time pattern recognition sonography in the hands of an experienced imager.
- Pattern recognition sonography or a risk model such as the International Ovarian Tumor Analysis (IOTA) Simple Rules can be used to initially characterize an ovarian mass.
- When an ovarian lesion is classified as benign, the patient may be followed conservatively, or if indicated, surgery can be performed by a general gynecologist.
- Serial sonography can be beneficial, but there are limited prospective data to support an exact interval and duration.
- Fewer surgical interventions may result in an increase in sonographic surveillance.
- When an ovarian lesion is considered indeterminate on initial sonography, and after appropriate clinical evaluation, a “second-step” evaluation may include referral to an expert sonologist, serial sonography, application of established risk-prediction models, correlation with serum biomarkers, correlation with MRI, or referral to a gynecologic oncologist for further evaluation.
From the First International Consensus Report on Adnexal Masses: Management Recommendations
Source: Glanc P et al. J Ultrasound Med. 2017 May;36(5):849-63.
Dr. Brown reported that she had received an earlier grant from Aspira Labs, the company that developed the OVA1 assay. Dr. Miller reported that he has no relevant financial disclosures.
References
1. Obstet Gynecol. 2016 Nov. doi: 10.1097/AOG.0000000000001768.
2. Cancer. 2007 Jan 15. doi: 10.1002/cncr.22371.
3. Clin Obstet Gynecol. 2015 Mar. doi: 10.1097/GRF.0000000000000083.
4. Ultrasound Q. 2013 Mar. doi: 10.1097/RUQ.0b013e3182814d9b.
5. Ultrasound Obstet Gynecol. 2008 Jun. doi: 10.1002/uog.5365.
Molecular developments in treatment of UPSC
Uterine papillary serous carcinoma (UPSC) is an infrequent but deadly form of endometrial cancer comprising 10% of cases but contributing 40% of deaths from the disease. Recurrence rates are high for this disease. Five-year survival is 55% for all patients and only 70% for stage I disease.1 Patterns of recurrence tend to be distant (extrapelvic and extraabdominal) as frequently as they are localized to the pelvis, and metastases and recurrences are unrelated to the extent of uterine disease (such as myometrial invasion). It is for these reasons that the recommended course of adjuvant therapy for this disease is systemic therapy (typically six doses of carboplatin and paclitaxel chemotherapy) with consideration for radiation to the vagina or pelvis to consolidate pelvic and vaginal control.2 This differs from early-stage high/intermediate–risk endometrioid adenocarcinomas, for which adjuvant chemotherapy has not been found to be helpful.
Because of the lower incidence of UPSC, it frequently has been studied alongside endometrioid cell types in clinical trials which explore novel adjuvant therapies. However, UPSC is biologically distinct from endometrioid endometrial cancers, which likely results in inferior clinical responses to conventional interventions. Fortunately we are beginning to better understand UPSC at a molecular level, and advancements are being made in the targeted therapies for these patients that are unique, compared with those applied to other cancer subtypes.
As discussed above, UPSC is a particularly aggressive form of uterine cancer. Histologically it is characterized by a precursor lesion of endometrial glandular dysplasia progressing to endometrial intraepithelial neoplasia (EIC). Histologically it presents with a highly atypical slit-like glandular configuration, which appears similar to serous carcinomas of the fallopian tube and ovary. Molecularly these tumors commonly manifest mutations in tumor protein p53 (TP53) and phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA), which are both genes associated with oncogenic potential.1 While most UPSC tumors have loss of expression in hormone receptors such as estrogen and progesterone, 25%-30% of cases overexpress the tyrosine kinase receptor human epidermal growth factor receptor 2 (HER2).3-5 This has proven to provide an exciting target for therapeutic interventions.
A target for therapeutic intervention
HER2 is a transmembrane receptor which, when activated, signals complex downstream pathways responsible for cellular proliferation, dedifferentiation, and metastasis. In a recent multi-institutional analysis of early-stage UPSC, HER2 overexpression was identified among 25% of cases.4 Approximately 30% of cases of advanced disease manifest overexpression of this biomarker.5 HER2 overexpression (HER2-positive status) is significantly associated with higher rates of recurrence and mortality, even among patients treated with conventional therapies.3 Thus HER2-positive status is obviously an indicator of particularly aggressive disease.
Fortunately this particular biomarker is one for which we have established and developing therapeutics. The humanized monoclonal antibody, trastuzumab, has been highly effective in improving survival for HER2-positive breast cancer.6 More recently, it was studied in a phase 2 trial with carboplatin and paclitaxel chemotherapy for advanced or recurrent HER2-positive UPSC.5 This trial showed that the addition of this targeted therapy to conventional chemotherapy improved recurrence-free survival from 8 months to 12 months, and improved overall survival from 24.4 months to 29.6 months.5
One discovery leads to another treatment
This discovery led to the approval of trastuzumab to be used in addition to chemotherapy for advanced or recurrent disease.2 The most significant effects appear to be among those who have not received prior therapies, with a doubling of progression-free survival among these patients, and a more modest response among patients treated for recurrent, mostly pretreated disease.
Work currently is underway to explore an array of antibody or small-molecule blockades of HER2 in addition to vaccines against the protein or treatment with conjugate compounds in which an antibody to HER2 is paired with a cytotoxic drug able to be internalized into HER2-expressing cells.7 This represents a form of personalized medicine referred to as biomarker-driven targeted therapy, in which therapies are prescribed based on the expression of specific molecular markers (such as HER2 expression) typically in combination with other clinical markers such as surgical staging results, race, age, etc. These approaches can be very effective strategies in rare tumor subtypes with distinct molecular and clinical behaviors.
As previously mentioned, the targeting of HER2 overexpression with trastuzumab has been shown to be highly effective in the treatment of HER2-positive breast cancers where even patients with early-stage disease receive a multimodal therapy approach including antibody, chemotherapy, surgical, and often radiation treatments.6 We are moving towards a similar multimodal comprehensive treatment strategy for UPSC. If it is as successful as it is in breast cancer, it will be long overdue, and desperately necessary given the poor prognosis of this disease for all stages because of the inadequacies of current treatments strategies.
Routine testing of UPSC for HER2 expression is now a part of routine molecular substaging of uterine cancers in the same way we have embraced testing for microsatellite instability and hormone-receptor status. While a diagnosis of HER2 overexpression in UPSC portends a poor prognosis, patients can be reassured that treatment strategies exist that can target this malignant mechanism in advanced disease and more are under further development for early-stage disease.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no relevant financial disclosures. Email her at [email protected].
References
1. Curr Opin Obstet Gynecol. 2010 Feb. doi: 10.1097/GCO.0b013e328334d8a3.
2. National Comprehensive Cancer Network. Uterine Neoplasms (version 2.2020).
3. Cancer 2005 Oct 1. doi: 10.1002/cncr.21308.
4. Gynecol Oncol 2020 doi: 10.1016/j.ygyno.2020.07.016.
5. J Clin Oncol 2018. doi: 10.1200/JCO.2017.76.5966.
6. N Engl J Med 2011. doi: 10.1056/NEJMoa0910383.
7. Discov Med. 2016 Apr;21(116):293-303.
Uterine papillary serous carcinoma (UPSC) is an infrequent but deadly form of endometrial cancer comprising 10% of cases but contributing 40% of deaths from the disease. Recurrence rates are high for this disease. Five-year survival is 55% for all patients and only 70% for stage I disease.1 Patterns of recurrence tend to be distant (extrapelvic and extraabdominal) as frequently as they are localized to the pelvis, and metastases and recurrences are unrelated to the extent of uterine disease (such as myometrial invasion). It is for these reasons that the recommended course of adjuvant therapy for this disease is systemic therapy (typically six doses of carboplatin and paclitaxel chemotherapy) with consideration for radiation to the vagina or pelvis to consolidate pelvic and vaginal control.2 This differs from early-stage high/intermediate–risk endometrioid adenocarcinomas, for which adjuvant chemotherapy has not been found to be helpful.
Because of the lower incidence of UPSC, it frequently has been studied alongside endometrioid cell types in clinical trials which explore novel adjuvant therapies. However, UPSC is biologically distinct from endometrioid endometrial cancers, which likely results in inferior clinical responses to conventional interventions. Fortunately we are beginning to better understand UPSC at a molecular level, and advancements are being made in the targeted therapies for these patients that are unique, compared with those applied to other cancer subtypes.
As discussed above, UPSC is a particularly aggressive form of uterine cancer. Histologically it is characterized by a precursor lesion of endometrial glandular dysplasia progressing to endometrial intraepithelial neoplasia (EIC). Histologically it presents with a highly atypical slit-like glandular configuration, which appears similar to serous carcinomas of the fallopian tube and ovary. Molecularly these tumors commonly manifest mutations in tumor protein p53 (TP53) and phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA), which are both genes associated with oncogenic potential.1 While most UPSC tumors have loss of expression in hormone receptors such as estrogen and progesterone, 25%-30% of cases overexpress the tyrosine kinase receptor human epidermal growth factor receptor 2 (HER2).3-5 This has proven to provide an exciting target for therapeutic interventions.
A target for therapeutic intervention
HER2 is a transmembrane receptor which, when activated, signals complex downstream pathways responsible for cellular proliferation, dedifferentiation, and metastasis. In a recent multi-institutional analysis of early-stage UPSC, HER2 overexpression was identified among 25% of cases.4 Approximately 30% of cases of advanced disease manifest overexpression of this biomarker.5 HER2 overexpression (HER2-positive status) is significantly associated with higher rates of recurrence and mortality, even among patients treated with conventional therapies.3 Thus HER2-positive status is obviously an indicator of particularly aggressive disease.
Fortunately this particular biomarker is one for which we have established and developing therapeutics. The humanized monoclonal antibody, trastuzumab, has been highly effective in improving survival for HER2-positive breast cancer.6 More recently, it was studied in a phase 2 trial with carboplatin and paclitaxel chemotherapy for advanced or recurrent HER2-positive UPSC.5 This trial showed that the addition of this targeted therapy to conventional chemotherapy improved recurrence-free survival from 8 months to 12 months, and improved overall survival from 24.4 months to 29.6 months.5
One discovery leads to another treatment
This discovery led to the approval of trastuzumab to be used in addition to chemotherapy for advanced or recurrent disease.2 The most significant effects appear to be among those who have not received prior therapies, with a doubling of progression-free survival among these patients, and a more modest response among patients treated for recurrent, mostly pretreated disease.
Work currently is underway to explore an array of antibody or small-molecule blockades of HER2 in addition to vaccines against the protein or treatment with conjugate compounds in which an antibody to HER2 is paired with a cytotoxic drug able to be internalized into HER2-expressing cells.7 This represents a form of personalized medicine referred to as biomarker-driven targeted therapy, in which therapies are prescribed based on the expression of specific molecular markers (such as HER2 expression) typically in combination with other clinical markers such as surgical staging results, race, age, etc. These approaches can be very effective strategies in rare tumor subtypes with distinct molecular and clinical behaviors.
As previously mentioned, the targeting of HER2 overexpression with trastuzumab has been shown to be highly effective in the treatment of HER2-positive breast cancers where even patients with early-stage disease receive a multimodal therapy approach including antibody, chemotherapy, surgical, and often radiation treatments.6 We are moving towards a similar multimodal comprehensive treatment strategy for UPSC. If it is as successful as it is in breast cancer, it will be long overdue, and desperately necessary given the poor prognosis of this disease for all stages because of the inadequacies of current treatments strategies.
Routine testing of UPSC for HER2 expression is now a part of routine molecular substaging of uterine cancers in the same way we have embraced testing for microsatellite instability and hormone-receptor status. While a diagnosis of HER2 overexpression in UPSC portends a poor prognosis, patients can be reassured that treatment strategies exist that can target this malignant mechanism in advanced disease and more are under further development for early-stage disease.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no relevant financial disclosures. Email her at [email protected].
References
1. Curr Opin Obstet Gynecol. 2010 Feb. doi: 10.1097/GCO.0b013e328334d8a3.
2. National Comprehensive Cancer Network. Uterine Neoplasms (version 2.2020).
3. Cancer 2005 Oct 1. doi: 10.1002/cncr.21308.
4. Gynecol Oncol 2020 doi: 10.1016/j.ygyno.2020.07.016.
5. J Clin Oncol 2018. doi: 10.1200/JCO.2017.76.5966.
6. N Engl J Med 2011. doi: 10.1056/NEJMoa0910383.
7. Discov Med. 2016 Apr;21(116):293-303.
Uterine papillary serous carcinoma (UPSC) is an infrequent but deadly form of endometrial cancer comprising 10% of cases but contributing 40% of deaths from the disease. Recurrence rates are high for this disease. Five-year survival is 55% for all patients and only 70% for stage I disease.1 Patterns of recurrence tend to be distant (extrapelvic and extraabdominal) as frequently as they are localized to the pelvis, and metastases and recurrences are unrelated to the extent of uterine disease (such as myometrial invasion). It is for these reasons that the recommended course of adjuvant therapy for this disease is systemic therapy (typically six doses of carboplatin and paclitaxel chemotherapy) with consideration for radiation to the vagina or pelvis to consolidate pelvic and vaginal control.2 This differs from early-stage high/intermediate–risk endometrioid adenocarcinomas, for which adjuvant chemotherapy has not been found to be helpful.
Because of the lower incidence of UPSC, it frequently has been studied alongside endometrioid cell types in clinical trials which explore novel adjuvant therapies. However, UPSC is biologically distinct from endometrioid endometrial cancers, which likely results in inferior clinical responses to conventional interventions. Fortunately we are beginning to better understand UPSC at a molecular level, and advancements are being made in the targeted therapies for these patients that are unique, compared with those applied to other cancer subtypes.
As discussed above, UPSC is a particularly aggressive form of uterine cancer. Histologically it is characterized by a precursor lesion of endometrial glandular dysplasia progressing to endometrial intraepithelial neoplasia (EIC). Histologically it presents with a highly atypical slit-like glandular configuration, which appears similar to serous carcinomas of the fallopian tube and ovary. Molecularly these tumors commonly manifest mutations in tumor protein p53 (TP53) and phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA), which are both genes associated with oncogenic potential.1 While most UPSC tumors have loss of expression in hormone receptors such as estrogen and progesterone, 25%-30% of cases overexpress the tyrosine kinase receptor human epidermal growth factor receptor 2 (HER2).3-5 This has proven to provide an exciting target for therapeutic interventions.
A target for therapeutic intervention
HER2 is a transmembrane receptor which, when activated, signals complex downstream pathways responsible for cellular proliferation, dedifferentiation, and metastasis. In a recent multi-institutional analysis of early-stage UPSC, HER2 overexpression was identified among 25% of cases.4 Approximately 30% of cases of advanced disease manifest overexpression of this biomarker.5 HER2 overexpression (HER2-positive status) is significantly associated with higher rates of recurrence and mortality, even among patients treated with conventional therapies.3 Thus HER2-positive status is obviously an indicator of particularly aggressive disease.
Fortunately this particular biomarker is one for which we have established and developing therapeutics. The humanized monoclonal antibody, trastuzumab, has been highly effective in improving survival for HER2-positive breast cancer.6 More recently, it was studied in a phase 2 trial with carboplatin and paclitaxel chemotherapy for advanced or recurrent HER2-positive UPSC.5 This trial showed that the addition of this targeted therapy to conventional chemotherapy improved recurrence-free survival from 8 months to 12 months, and improved overall survival from 24.4 months to 29.6 months.5
One discovery leads to another treatment
This discovery led to the approval of trastuzumab to be used in addition to chemotherapy for advanced or recurrent disease.2 The most significant effects appear to be among those who have not received prior therapies, with a doubling of progression-free survival among these patients, and a more modest response among patients treated for recurrent, mostly pretreated disease.
Work currently is underway to explore an array of antibody or small-molecule blockades of HER2 in addition to vaccines against the protein or treatment with conjugate compounds in which an antibody to HER2 is paired with a cytotoxic drug able to be internalized into HER2-expressing cells.7 This represents a form of personalized medicine referred to as biomarker-driven targeted therapy, in which therapies are prescribed based on the expression of specific molecular markers (such as HER2 expression) typically in combination with other clinical markers such as surgical staging results, race, age, etc. These approaches can be very effective strategies in rare tumor subtypes with distinct molecular and clinical behaviors.
As previously mentioned, the targeting of HER2 overexpression with trastuzumab has been shown to be highly effective in the treatment of HER2-positive breast cancers where even patients with early-stage disease receive a multimodal therapy approach including antibody, chemotherapy, surgical, and often radiation treatments.6 We are moving towards a similar multimodal comprehensive treatment strategy for UPSC. If it is as successful as it is in breast cancer, it will be long overdue, and desperately necessary given the poor prognosis of this disease for all stages because of the inadequacies of current treatments strategies.
Routine testing of UPSC for HER2 expression is now a part of routine molecular substaging of uterine cancers in the same way we have embraced testing for microsatellite instability and hormone-receptor status. While a diagnosis of HER2 overexpression in UPSC portends a poor prognosis, patients can be reassured that treatment strategies exist that can target this malignant mechanism in advanced disease and more are under further development for early-stage disease.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no relevant financial disclosures. Email her at [email protected].
References
1. Curr Opin Obstet Gynecol. 2010 Feb. doi: 10.1097/GCO.0b013e328334d8a3.
2. National Comprehensive Cancer Network. Uterine Neoplasms (version 2.2020).
3. Cancer 2005 Oct 1. doi: 10.1002/cncr.21308.
4. Gynecol Oncol 2020 doi: 10.1016/j.ygyno.2020.07.016.
5. J Clin Oncol 2018. doi: 10.1200/JCO.2017.76.5966.
6. N Engl J Med 2011. doi: 10.1056/NEJMoa0910383.
7. Discov Med. 2016 Apr;21(116):293-303.
VTE, sepsis risk increased among COVID-19 patients with cancer
, according to data from a registry study.
Researchers analyzed data on 5,556 patients with COVID-19 who had an inpatient or emergency encounter at Mount Sinai Health System (MSHS) in New York between March 1 and May 27, 2020. Patients were included in an anonymous MSHS COVID-19 registry.
There were 421 patients who had cancer: 96 with a hematologic malignancy and 325 with solid tumors.
After adjustment for age, gender, and number of comorbidities, the odds ratios for acute VTE and sepsis for patients with cancer (versus those without cancer) were 1.77 and 1.34, respectively. The adjusted odds ratio for mortality in cancer patients was 1.02.
The results remained “relatively consistent” after stratification by solid and nonsolid cancer types, with no significant difference in outcomes between those two groups, and results remained consistent in a propensity-matched model, according to Naomi Alpert, a biostatistician at Icahn School of Medicine at Mount Sinai, New York.
Ms. Alpert reported these findings at the AACR virtual meeting: COVID-19 and Cancer.
She noted that the cancer patients were older than the noncancer patients (mean age, 69.2 years vs. 63.8 years), and cancer patients were more likely to have two or more comorbid conditions (48.2% vs. 30.4%). Cancer patients also had significantly lower hemoglobin levels and red blood cell, platelet, and white blood cell counts (P < .01 for all).
“Low white blood cell count may be one of the reasons for higher risk of sepsis in cancer patients, as it may lead to a higher risk of infection,” Ms. Alpert said. “However, it’s not clear what role cancer therapies play in the risks of COVID-19 morbidity and mortality, so there is still quite a bit to learn.”
In fact, the findings are limited by a lack of information about cancer treatment, as the registry was not designed for that purpose, she noted.
Another study limitation is the short follow-up of a month or less in most patients, due, in part, to the novelty of COVID-19, but also to the lack of information on patients after they left the hospital.
“However, we had a very large sample size, with more than 400 cancer patients included, and, to our knowledge, this is the largest analysis of its kind to be done so far,” Ms. Alpert said. “In the future, it’s going to be very important to assess the effect of cancer therapies on COVID-19 complications and to see if prior therapies had any effect on outcomes.”
Longer follow-up would also be helpful for assessing the chronic effects of COVID-19 on cancer patients over time, she said. “It would be important to see whether some of these elevated risks of venous thromboembolism and sepsis are associated with longer-term mortality risks than what we were able to measure here,” she added.
Asked about the discrepancy between mortality in this study and those of larger registries, such as the COVID-19 and Cancer Consortium (CCC19) and TERAVOLT, Ms. Alpert noted that the current study included only patients who required hospitalization or emergency care.
“Our mortality rate was actually a bit higher than what was reported in some of the other studies,” she said. “We had about a 30% mortality rate in the cancer patients and about 25% for the noncancer patients, so ... we’re sort of looking at a subset of patients who we know are the sickest of the sick, which may explain some of the higher mortality that we’re seeing.”
Ms. Alpert reported having no disclosures.
SOURCE: Alpert N et al. AACR COVID-19 and Cancer, Abstract S12-02.
, according to data from a registry study.
Researchers analyzed data on 5,556 patients with COVID-19 who had an inpatient or emergency encounter at Mount Sinai Health System (MSHS) in New York between March 1 and May 27, 2020. Patients were included in an anonymous MSHS COVID-19 registry.
There were 421 patients who had cancer: 96 with a hematologic malignancy and 325 with solid tumors.
After adjustment for age, gender, and number of comorbidities, the odds ratios for acute VTE and sepsis for patients with cancer (versus those without cancer) were 1.77 and 1.34, respectively. The adjusted odds ratio for mortality in cancer patients was 1.02.
The results remained “relatively consistent” after stratification by solid and nonsolid cancer types, with no significant difference in outcomes between those two groups, and results remained consistent in a propensity-matched model, according to Naomi Alpert, a biostatistician at Icahn School of Medicine at Mount Sinai, New York.
Ms. Alpert reported these findings at the AACR virtual meeting: COVID-19 and Cancer.
She noted that the cancer patients were older than the noncancer patients (mean age, 69.2 years vs. 63.8 years), and cancer patients were more likely to have two or more comorbid conditions (48.2% vs. 30.4%). Cancer patients also had significantly lower hemoglobin levels and red blood cell, platelet, and white blood cell counts (P < .01 for all).
“Low white blood cell count may be one of the reasons for higher risk of sepsis in cancer patients, as it may lead to a higher risk of infection,” Ms. Alpert said. “However, it’s not clear what role cancer therapies play in the risks of COVID-19 morbidity and mortality, so there is still quite a bit to learn.”
In fact, the findings are limited by a lack of information about cancer treatment, as the registry was not designed for that purpose, she noted.
Another study limitation is the short follow-up of a month or less in most patients, due, in part, to the novelty of COVID-19, but also to the lack of information on patients after they left the hospital.
“However, we had a very large sample size, with more than 400 cancer patients included, and, to our knowledge, this is the largest analysis of its kind to be done so far,” Ms. Alpert said. “In the future, it’s going to be very important to assess the effect of cancer therapies on COVID-19 complications and to see if prior therapies had any effect on outcomes.”
Longer follow-up would also be helpful for assessing the chronic effects of COVID-19 on cancer patients over time, she said. “It would be important to see whether some of these elevated risks of venous thromboembolism and sepsis are associated with longer-term mortality risks than what we were able to measure here,” she added.
Asked about the discrepancy between mortality in this study and those of larger registries, such as the COVID-19 and Cancer Consortium (CCC19) and TERAVOLT, Ms. Alpert noted that the current study included only patients who required hospitalization or emergency care.
“Our mortality rate was actually a bit higher than what was reported in some of the other studies,” she said. “We had about a 30% mortality rate in the cancer patients and about 25% for the noncancer patients, so ... we’re sort of looking at a subset of patients who we know are the sickest of the sick, which may explain some of the higher mortality that we’re seeing.”
Ms. Alpert reported having no disclosures.
SOURCE: Alpert N et al. AACR COVID-19 and Cancer, Abstract S12-02.
, according to data from a registry study.
Researchers analyzed data on 5,556 patients with COVID-19 who had an inpatient or emergency encounter at Mount Sinai Health System (MSHS) in New York between March 1 and May 27, 2020. Patients were included in an anonymous MSHS COVID-19 registry.
There were 421 patients who had cancer: 96 with a hematologic malignancy and 325 with solid tumors.
After adjustment for age, gender, and number of comorbidities, the odds ratios for acute VTE and sepsis for patients with cancer (versus those without cancer) were 1.77 and 1.34, respectively. The adjusted odds ratio for mortality in cancer patients was 1.02.
The results remained “relatively consistent” after stratification by solid and nonsolid cancer types, with no significant difference in outcomes between those two groups, and results remained consistent in a propensity-matched model, according to Naomi Alpert, a biostatistician at Icahn School of Medicine at Mount Sinai, New York.
Ms. Alpert reported these findings at the AACR virtual meeting: COVID-19 and Cancer.
She noted that the cancer patients were older than the noncancer patients (mean age, 69.2 years vs. 63.8 years), and cancer patients were more likely to have two or more comorbid conditions (48.2% vs. 30.4%). Cancer patients also had significantly lower hemoglobin levels and red blood cell, platelet, and white blood cell counts (P < .01 for all).
“Low white blood cell count may be one of the reasons for higher risk of sepsis in cancer patients, as it may lead to a higher risk of infection,” Ms. Alpert said. “However, it’s not clear what role cancer therapies play in the risks of COVID-19 morbidity and mortality, so there is still quite a bit to learn.”
In fact, the findings are limited by a lack of information about cancer treatment, as the registry was not designed for that purpose, she noted.
Another study limitation is the short follow-up of a month or less in most patients, due, in part, to the novelty of COVID-19, but also to the lack of information on patients after they left the hospital.
“However, we had a very large sample size, with more than 400 cancer patients included, and, to our knowledge, this is the largest analysis of its kind to be done so far,” Ms. Alpert said. “In the future, it’s going to be very important to assess the effect of cancer therapies on COVID-19 complications and to see if prior therapies had any effect on outcomes.”
Longer follow-up would also be helpful for assessing the chronic effects of COVID-19 on cancer patients over time, she said. “It would be important to see whether some of these elevated risks of venous thromboembolism and sepsis are associated with longer-term mortality risks than what we were able to measure here,” she added.
Asked about the discrepancy between mortality in this study and those of larger registries, such as the COVID-19 and Cancer Consortium (CCC19) and TERAVOLT, Ms. Alpert noted that the current study included only patients who required hospitalization or emergency care.
“Our mortality rate was actually a bit higher than what was reported in some of the other studies,” she said. “We had about a 30% mortality rate in the cancer patients and about 25% for the noncancer patients, so ... we’re sort of looking at a subset of patients who we know are the sickest of the sick, which may explain some of the higher mortality that we’re seeing.”
Ms. Alpert reported having no disclosures.
SOURCE: Alpert N et al. AACR COVID-19 and Cancer, Abstract S12-02.
FROM AACR: COVID-19 AND CANCER