User login
Metastatic cancer linked to worse outcomes of COVID-19
Cancer type, stage, and recent treatment may affect outcomes of COVID-19 in cancer patients, according to a study of patients from China.
The data showed that patients with hematologic malignancies and those with metastatic cancers had higher risks of developing severe or critical COVID-19 symptoms, being admitted to the ICU, requiring ventilation, and dying.
On the other hand, patients with nonmetastatic cancer had outcomes comparable to those of noncancer patients with COVID-19.
Similarly, cancer patients who had recently undergone surgery or received immunotherapy were more likely to have poor outcomes, whereas cancer patients treated with radiotherapy had outcomes similar to those of noncancer COVID-19 patients.
Hongbing Cai, MD, of Zhongnan Hospital of Wuhan University in China, presented these results at the AACR virtual meeting I. The results also were published in Cancer Discovery.
Cancer vs. noncancer patients
The study included 105 cancer patients with COVID-19 who were treated from Jan. 1 to Feb. 24, 2020, at 14 hospitals in Wuhan, China. Patients had lung (20.95%), gastrointestinal (12.38%), breast (10.48%), and thyroid cancers (10.48%) as well as hematologic malignancies (8.57%). Dr. Cai and colleagues matched the COVID-19 cancer patients to 536 COVID-19 patients without cancer. Patients were matched by hospital, duration of hospitalization, and age.
“COVID-19 patients with cancer had higher risks of all severe outcomes,” Dr. Cai noted.
Compared with noncancer patients, the cancer patients had a higher risk of:
- Severe or critical COVID-19 symptoms – odds ratio, 2.79 (P < .01).
- Being admitted to the ICU – OR, 2.84 (P < .01).
- Requiring invasive mechanical ventilation – OR, 14 (P < .01).
- Death – OR, 2.34 (P = .03).
Cancer type and stage
Dr. Cai noted that outcomes were the worst among patients with hematologic malignancies and those with metastatic cancer (stage IV).
Compared with patients without cancer, those with hematologic malignancies had a higher risk of:
- Severe/critical symptoms – OR, 10.61 (P < .01).
- ICU admission – OR, 9.66 (P < .01).
- Invasive mechanical ventilation – OR, 38 (P < .01).
- Death – OR, 9.07 (P = .01).
Compared with patients without cancer, those with metastatic cancer had a higher risk of:
- Severe/critical symptoms – OR, 5.97 (P < .01).
- ICU admission – OR, 6.59 (P < 0.01).
- Invasive mechanical ventilation – OR, 55.42 (P < .01).
- Death – OR, 5.58 (P = .01).
On the other hand, outcomes in patients with nonmetastatic cancer were not significantly different from outcomes in patients without cancer (P > .05 for all outcomes).
Cancer treatment
The treatments cancer patients received within 40 days before the onset of COVID-19 symptoms were radiotherapy (12.26%), chemotherapy (14.15%), surgery (7.62%), targeted therapies (3.81%), and immunotherapy (5.71%).
Compared with patients without cancer, those who received immunotherapy had a higher risk of:
- Severe/critical symptoms – OR, 10.61 (P < .01).
- Death – OR, 9.07 (P = .04).
Patients who underwent surgery had a higher risk of:
- Severe/critical symptoms – OR, 8.84 (P < .01).
- ICU admission – OR, 7.24 (P = .02).
- Invasive mechanical ventilation – OR, 44.33 (P < .01).
Conversely, outcomes in cancer patients who received radiotherapy were not significantly different from outcomes in patients without cancer (P > .10 for all).
These results suggest that “postponing surgery should be considered in outbreak areas,” Dr. Cai said, adding that scheduled radiotherapy can go ahead but with “intensive protection and surveillance.”
Dr. Cai said it remains to be seen whether patients with early-stage cancer need to postpone their treatments during the COVID-19 pandemic or whether immunotherapy aggravates severe outcomes in cancer patients with COVID-19. For now, she said, cancer patients should have individualized treatment plans based on their tumor type and stage.
Dr. Cai disclosed no conflicts of interest. This study was supported by the National Natural Science Foundation of China, the Singapore Ministry of Health’s National Medical Research Council, the National Institutes of Health/National Heart, Lung, and Blood Institute, and the Xiu Research Fund.
SOURCE: Cai H. AACR 2020. Patients with cancer appear more vulnerable to SARS-COV-2: A multicenter study during the COVID-19 outbreak; Dai M et al. Cancer Discov. 2020 Apr 28. doi: 10.1158/2159-8290.CD-20-0422.
Cancer type, stage, and recent treatment may affect outcomes of COVID-19 in cancer patients, according to a study of patients from China.
The data showed that patients with hematologic malignancies and those with metastatic cancers had higher risks of developing severe or critical COVID-19 symptoms, being admitted to the ICU, requiring ventilation, and dying.
On the other hand, patients with nonmetastatic cancer had outcomes comparable to those of noncancer patients with COVID-19.
Similarly, cancer patients who had recently undergone surgery or received immunotherapy were more likely to have poor outcomes, whereas cancer patients treated with radiotherapy had outcomes similar to those of noncancer COVID-19 patients.
Hongbing Cai, MD, of Zhongnan Hospital of Wuhan University in China, presented these results at the AACR virtual meeting I. The results also were published in Cancer Discovery.
Cancer vs. noncancer patients
The study included 105 cancer patients with COVID-19 who were treated from Jan. 1 to Feb. 24, 2020, at 14 hospitals in Wuhan, China. Patients had lung (20.95%), gastrointestinal (12.38%), breast (10.48%), and thyroid cancers (10.48%) as well as hematologic malignancies (8.57%). Dr. Cai and colleagues matched the COVID-19 cancer patients to 536 COVID-19 patients without cancer. Patients were matched by hospital, duration of hospitalization, and age.
“COVID-19 patients with cancer had higher risks of all severe outcomes,” Dr. Cai noted.
Compared with noncancer patients, the cancer patients had a higher risk of:
- Severe or critical COVID-19 symptoms – odds ratio, 2.79 (P < .01).
- Being admitted to the ICU – OR, 2.84 (P < .01).
- Requiring invasive mechanical ventilation – OR, 14 (P < .01).
- Death – OR, 2.34 (P = .03).
Cancer type and stage
Dr. Cai noted that outcomes were the worst among patients with hematologic malignancies and those with metastatic cancer (stage IV).
Compared with patients without cancer, those with hematologic malignancies had a higher risk of:
- Severe/critical symptoms – OR, 10.61 (P < .01).
- ICU admission – OR, 9.66 (P < .01).
- Invasive mechanical ventilation – OR, 38 (P < .01).
- Death – OR, 9.07 (P = .01).
Compared with patients without cancer, those with metastatic cancer had a higher risk of:
- Severe/critical symptoms – OR, 5.97 (P < .01).
- ICU admission – OR, 6.59 (P < 0.01).
- Invasive mechanical ventilation – OR, 55.42 (P < .01).
- Death – OR, 5.58 (P = .01).
On the other hand, outcomes in patients with nonmetastatic cancer were not significantly different from outcomes in patients without cancer (P > .05 for all outcomes).
Cancer treatment
The treatments cancer patients received within 40 days before the onset of COVID-19 symptoms were radiotherapy (12.26%), chemotherapy (14.15%), surgery (7.62%), targeted therapies (3.81%), and immunotherapy (5.71%).
Compared with patients without cancer, those who received immunotherapy had a higher risk of:
- Severe/critical symptoms – OR, 10.61 (P < .01).
- Death – OR, 9.07 (P = .04).
Patients who underwent surgery had a higher risk of:
- Severe/critical symptoms – OR, 8.84 (P < .01).
- ICU admission – OR, 7.24 (P = .02).
- Invasive mechanical ventilation – OR, 44.33 (P < .01).
Conversely, outcomes in cancer patients who received radiotherapy were not significantly different from outcomes in patients without cancer (P > .10 for all).
These results suggest that “postponing surgery should be considered in outbreak areas,” Dr. Cai said, adding that scheduled radiotherapy can go ahead but with “intensive protection and surveillance.”
Dr. Cai said it remains to be seen whether patients with early-stage cancer need to postpone their treatments during the COVID-19 pandemic or whether immunotherapy aggravates severe outcomes in cancer patients with COVID-19. For now, she said, cancer patients should have individualized treatment plans based on their tumor type and stage.
Dr. Cai disclosed no conflicts of interest. This study was supported by the National Natural Science Foundation of China, the Singapore Ministry of Health’s National Medical Research Council, the National Institutes of Health/National Heart, Lung, and Blood Institute, and the Xiu Research Fund.
SOURCE: Cai H. AACR 2020. Patients with cancer appear more vulnerable to SARS-COV-2: A multicenter study during the COVID-19 outbreak; Dai M et al. Cancer Discov. 2020 Apr 28. doi: 10.1158/2159-8290.CD-20-0422.
Cancer type, stage, and recent treatment may affect outcomes of COVID-19 in cancer patients, according to a study of patients from China.
The data showed that patients with hematologic malignancies and those with metastatic cancers had higher risks of developing severe or critical COVID-19 symptoms, being admitted to the ICU, requiring ventilation, and dying.
On the other hand, patients with nonmetastatic cancer had outcomes comparable to those of noncancer patients with COVID-19.
Similarly, cancer patients who had recently undergone surgery or received immunotherapy were more likely to have poor outcomes, whereas cancer patients treated with radiotherapy had outcomes similar to those of noncancer COVID-19 patients.
Hongbing Cai, MD, of Zhongnan Hospital of Wuhan University in China, presented these results at the AACR virtual meeting I. The results also were published in Cancer Discovery.
Cancer vs. noncancer patients
The study included 105 cancer patients with COVID-19 who were treated from Jan. 1 to Feb. 24, 2020, at 14 hospitals in Wuhan, China. Patients had lung (20.95%), gastrointestinal (12.38%), breast (10.48%), and thyroid cancers (10.48%) as well as hematologic malignancies (8.57%). Dr. Cai and colleagues matched the COVID-19 cancer patients to 536 COVID-19 patients without cancer. Patients were matched by hospital, duration of hospitalization, and age.
“COVID-19 patients with cancer had higher risks of all severe outcomes,” Dr. Cai noted.
Compared with noncancer patients, the cancer patients had a higher risk of:
- Severe or critical COVID-19 symptoms – odds ratio, 2.79 (P < .01).
- Being admitted to the ICU – OR, 2.84 (P < .01).
- Requiring invasive mechanical ventilation – OR, 14 (P < .01).
- Death – OR, 2.34 (P = .03).
Cancer type and stage
Dr. Cai noted that outcomes were the worst among patients with hematologic malignancies and those with metastatic cancer (stage IV).
Compared with patients without cancer, those with hematologic malignancies had a higher risk of:
- Severe/critical symptoms – OR, 10.61 (P < .01).
- ICU admission – OR, 9.66 (P < .01).
- Invasive mechanical ventilation – OR, 38 (P < .01).
- Death – OR, 9.07 (P = .01).
Compared with patients without cancer, those with metastatic cancer had a higher risk of:
- Severe/critical symptoms – OR, 5.97 (P < .01).
- ICU admission – OR, 6.59 (P < 0.01).
- Invasive mechanical ventilation – OR, 55.42 (P < .01).
- Death – OR, 5.58 (P = .01).
On the other hand, outcomes in patients with nonmetastatic cancer were not significantly different from outcomes in patients without cancer (P > .05 for all outcomes).
Cancer treatment
The treatments cancer patients received within 40 days before the onset of COVID-19 symptoms were radiotherapy (12.26%), chemotherapy (14.15%), surgery (7.62%), targeted therapies (3.81%), and immunotherapy (5.71%).
Compared with patients without cancer, those who received immunotherapy had a higher risk of:
- Severe/critical symptoms – OR, 10.61 (P < .01).
- Death – OR, 9.07 (P = .04).
Patients who underwent surgery had a higher risk of:
- Severe/critical symptoms – OR, 8.84 (P < .01).
- ICU admission – OR, 7.24 (P = .02).
- Invasive mechanical ventilation – OR, 44.33 (P < .01).
Conversely, outcomes in cancer patients who received radiotherapy were not significantly different from outcomes in patients without cancer (P > .10 for all).
These results suggest that “postponing surgery should be considered in outbreak areas,” Dr. Cai said, adding that scheduled radiotherapy can go ahead but with “intensive protection and surveillance.”
Dr. Cai said it remains to be seen whether patients with early-stage cancer need to postpone their treatments during the COVID-19 pandemic or whether immunotherapy aggravates severe outcomes in cancer patients with COVID-19. For now, she said, cancer patients should have individualized treatment plans based on their tumor type and stage.
Dr. Cai disclosed no conflicts of interest. This study was supported by the National Natural Science Foundation of China, the Singapore Ministry of Health’s National Medical Research Council, the National Institutes of Health/National Heart, Lung, and Blood Institute, and the Xiu Research Fund.
SOURCE: Cai H. AACR 2020. Patients with cancer appear more vulnerable to SARS-COV-2: A multicenter study during the COVID-19 outbreak; Dai M et al. Cancer Discov. 2020 Apr 28. doi: 10.1158/2159-8290.CD-20-0422.
FROM AACR 2020
What is the significance of isolated tumor cells in endometrial cancer?
Over the past decade gynecologic oncology surgeons have increasingly adopted the technique of sentinel lymph node (SLN) biopsy to stage endometrial cancer. This is supported by evidence that selective removal of the few lymph nodes which are the first to drain the uterus can accurately detect metastatic disease, sparing the patient a complete lymphadenectomy and its associated risks, such as lymphedema.1 The proposed benefits of SLN biopsy are not just its ability to spare the patient removal of dozens of unnecessary lymph nodes, but also the ability to improve upon the detection of previously unrecognized nodal metastases in locations not routinely sampled by lymphadenectomy and by identifying very-low-volume metastatic disease. This is beneficial only, however, if that previously overlooked low-volume disease is clinically significant.
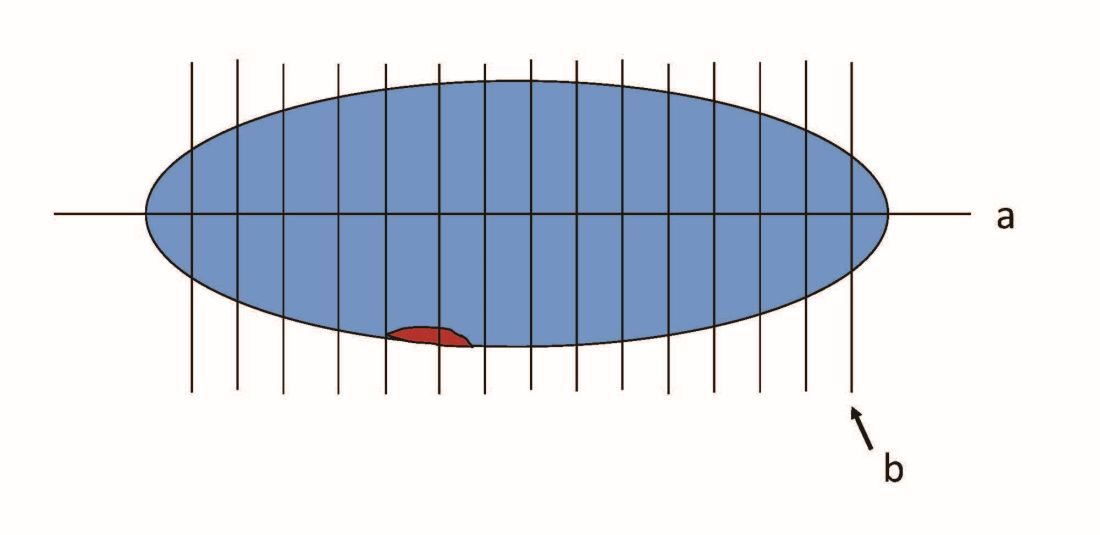
When pathologists evaluate lymph nodes as part of conventional lymphadenectomy, they typically bivalve the lymph node and evaluate with hematoxylin and eosin (H&E) stains. This technique is capable of detecting metastatic lesions greater than 2 mm, but can miss low-volume disease. In contrast, pathologists process SLNs with much finer sectioning (no greater than 2 mm), and, if the node is larger than 4 mm, they will section it perpendicular to the long axis in a bread-loaf fashion. It is not feasible to perform this ultrasectioning on the large numbers of lymph nodes of a complete lymphadenectomy specimen, but when applied to an SLN it allows pathologists to detect much smaller metastatic foci, the so-called “micrometastases” that are between 0.2 and 2 mm in size, and which typically arise in the subcapsular region of the node. The graphic depicts how a traditional longitudinal cut (a) might miss the micrometastasis that could be identified on the finer perpendicular cuts of ultra-sectioning (b). In addition to the ultrasectioning of the node into smaller slices, the pathologist performs additional immunohistochemistry stains for cytokeratin on sentinel nodes which appear negative on preliminary H&E stains. This allows the pathologist to identify even smaller clusters of malignant cells that are less than 0.2 mm, or individual cancer cells, so-called “isolated tumor cells” (ITCs) as shown in the photo. Most SLN series identify that approximately half of their “positive” lymph nodes are low-volume disease (micrometastases and ITCs). ITCs make up the majority of these cases, typically three-quarters.
Clinicians might be reassured by the discovery of low-volume metastatic disease, perceiving that the added attention afforded by the SLN approach helped them to identify metastases that might otherwise have been missed and therefore not treated. This is because node-positive (stage IIIC) disease is not cured by surgery or radiation alone and requires the addition of chemotherapy for survival benefit.2 Alternatively, there is no clear survival benefit derived from treating stage I high/intermediate cancers with chemotherapy, and therefore, the prescription of chemotherapy hinges upon reliable identification of extrauterine disease on pathology.3
It would make sense that if SLNs are more effective in identifying metastatic disease, clinicians who practice SLN biopsy would identify it more of the time. This appears to be the case with a trend towards upstaging in patients who undergo SLN biopsy, compared with those undergoing complete lymphadenectomy.4 It should also follow that if this increased detection of metastatic disease was clinically relevant, we would observe a corresponding improvement in survival outcomes. If not, then the additional identification of low-volume disease may not be value added: imparting toxicity of adjuvant therapy without survival benefit.
Micrometastases (foci sized 0.2-2 mm) are not a new phenomenon to the SLN era. Low-volume lesions were occasionally detected with routine nodal processing and H&E stains. Attention wasn’t paid to nodal volume categorization in pathology reports prior to the SLN era. These were usually reported collectively as stage IIIC disease. It would make sense to continue to approach micrometastases in a manner similar to what we have always done, recognizing that it may represent a continuum of nodal macrometastases. In contrast, ITCs are rarely detected with routine pathologic processing. Perhaps they are less within a continuum of nodal metastases, and more within the continuum of lymphovascular space invasion. We know that ITCs are significantly associated with the cofinding of this uterine phenomenon, which itself is considered a significant risk factor for local recurrence.5
Series have consistently shown the outcomes of women with ITCs to be favorable, compared with those with micrometastases or macrometastases.5,6 However, most retrospective series that evaluated the outcomes of patients with respect to volume of metastatic disease have high rates of treatment of ITCs with chemotherapy, radiotherapy, or both.6 This may mask and confuse whether there is any intrinsically favorable prognostic virtue of ITCs, compared with larger metastatic foci. When ITCs are untreated, it would appear that the rates and patterns of recurrence appear similar to those with negative SLNs, with the caveat that these series all include small numbers.5,7 This would suggest that women with ITCs do not need additional therapy beyond what would be prescribed for their uterine risk factors.
Further supporting the notion that ITCs have more favorable prognosis is that, while SLN biopsy is associated with a higher detection of nodal metastatic disease, it is not necessarily associated with improved survival when compared with complete lymphadenectomy in retrospective series.8 This suggests that finding and treating ITCs may not positively affect outcomes. Or possibly it is a result of inadequate statistical power to show a small benefit should one exist. It is especially difficult to differentiate micrometastases and ITCs with respect to treatment outcomes. Given that ITCs make up the majority of low-volume nodal disease detected through the SLN technique, any potential benefit of increased capture and treatment of the more substantial micrometastases is not likely to be captured. As a result, most series tend to lump patients with micrometastases with those with ITCs in their analysis of patient outcomes. This may be a mistake.
Clearly more research needs to be performed to definitively address the clinical significance of ITCs. While it would be ideal to conduct a prospective trial in which patients with ITCs are randomized to therapy or observation, in reality the scope of such a trial makes it impractical. ITCs are detected in only approximately 5% of all the patients with endometrial cancer, and given that outcomes for this group are, in general, good, it would require enrollment of tens of thousands of patients to establish a statistically satisfactory result. Therefore it is likely that we will need to rely on the results of large retrospective, population-based, observational series to determine if the identification and treatment of ITCs adds value and superior outcomes to patients. In addition, we are making leaps in better understanding the molecular profile of endometrial cancers and how we might incorporate this data with histology and staging results to create treatment algorithms, much like what has been developed for breast cancer. This is likely where the future lies in interpreting the results of staging. In the meantime, it seems reasonable to collect the data regarding volume of metastatic disease including the presence of ITCs, making shared treatment decisions with the patient regarding the addition of adjuvant therapy, recognizing that
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no conflicts of interest to declare. Email her at [email protected].
References
1. Lancet Oncol. 2017 Mar;18(3):384-92.
2. J Clin Oncol. 2006 Jan 1;24(1):36-44.
3. J Clin Oncol. 2019 Jul 20;37(21):1810-8.
4. Clin Transl Oncol. 2019. doi: 10.1007/s12094-019-02249-x.
5. Gynecol Oncol. 2017 Aug;146(2):240-6.
6. Ann Surg Oncol. 2016 May;23(5):1653-9.
7. Gynecol Oncol. 2019 Jun;153(3):496-9.
8. Gynecol Oncol. 2018 Nov;151(2):235-42.
Over the past decade gynecologic oncology surgeons have increasingly adopted the technique of sentinel lymph node (SLN) biopsy to stage endometrial cancer. This is supported by evidence that selective removal of the few lymph nodes which are the first to drain the uterus can accurately detect metastatic disease, sparing the patient a complete lymphadenectomy and its associated risks, such as lymphedema.1 The proposed benefits of SLN biopsy are not just its ability to spare the patient removal of dozens of unnecessary lymph nodes, but also the ability to improve upon the detection of previously unrecognized nodal metastases in locations not routinely sampled by lymphadenectomy and by identifying very-low-volume metastatic disease. This is beneficial only, however, if that previously overlooked low-volume disease is clinically significant.

When pathologists evaluate lymph nodes as part of conventional lymphadenectomy, they typically bivalve the lymph node and evaluate with hematoxylin and eosin (H&E) stains. This technique is capable of detecting metastatic lesions greater than 2 mm, but can miss low-volume disease. In contrast, pathologists process SLNs with much finer sectioning (no greater than 2 mm), and, if the node is larger than 4 mm, they will section it perpendicular to the long axis in a bread-loaf fashion. It is not feasible to perform this ultrasectioning on the large numbers of lymph nodes of a complete lymphadenectomy specimen, but when applied to an SLN it allows pathologists to detect much smaller metastatic foci, the so-called “micrometastases” that are between 0.2 and 2 mm in size, and which typically arise in the subcapsular region of the node. The graphic depicts how a traditional longitudinal cut (a) might miss the micrometastasis that could be identified on the finer perpendicular cuts of ultra-sectioning (b). In addition to the ultrasectioning of the node into smaller slices, the pathologist performs additional immunohistochemistry stains for cytokeratin on sentinel nodes which appear negative on preliminary H&E stains. This allows the pathologist to identify even smaller clusters of malignant cells that are less than 0.2 mm, or individual cancer cells, so-called “isolated tumor cells” (ITCs) as shown in the photo. Most SLN series identify that approximately half of their “positive” lymph nodes are low-volume disease (micrometastases and ITCs). ITCs make up the majority of these cases, typically three-quarters.
Clinicians might be reassured by the discovery of low-volume metastatic disease, perceiving that the added attention afforded by the SLN approach helped them to identify metastases that might otherwise have been missed and therefore not treated. This is because node-positive (stage IIIC) disease is not cured by surgery or radiation alone and requires the addition of chemotherapy for survival benefit.2 Alternatively, there is no clear survival benefit derived from treating stage I high/intermediate cancers with chemotherapy, and therefore, the prescription of chemotherapy hinges upon reliable identification of extrauterine disease on pathology.3
It would make sense that if SLNs are more effective in identifying metastatic disease, clinicians who practice SLN biopsy would identify it more of the time. This appears to be the case with a trend towards upstaging in patients who undergo SLN biopsy, compared with those undergoing complete lymphadenectomy.4 It should also follow that if this increased detection of metastatic disease was clinically relevant, we would observe a corresponding improvement in survival outcomes. If not, then the additional identification of low-volume disease may not be value added: imparting toxicity of adjuvant therapy without survival benefit.
Micrometastases (foci sized 0.2-2 mm) are not a new phenomenon to the SLN era. Low-volume lesions were occasionally detected with routine nodal processing and H&E stains. Attention wasn’t paid to nodal volume categorization in pathology reports prior to the SLN era. These were usually reported collectively as stage IIIC disease. It would make sense to continue to approach micrometastases in a manner similar to what we have always done, recognizing that it may represent a continuum of nodal macrometastases. In contrast, ITCs are rarely detected with routine pathologic processing. Perhaps they are less within a continuum of nodal metastases, and more within the continuum of lymphovascular space invasion. We know that ITCs are significantly associated with the cofinding of this uterine phenomenon, which itself is considered a significant risk factor for local recurrence.5
Series have consistently shown the outcomes of women with ITCs to be favorable, compared with those with micrometastases or macrometastases.5,6 However, most retrospective series that evaluated the outcomes of patients with respect to volume of metastatic disease have high rates of treatment of ITCs with chemotherapy, radiotherapy, or both.6 This may mask and confuse whether there is any intrinsically favorable prognostic virtue of ITCs, compared with larger metastatic foci. When ITCs are untreated, it would appear that the rates and patterns of recurrence appear similar to those with negative SLNs, with the caveat that these series all include small numbers.5,7 This would suggest that women with ITCs do not need additional therapy beyond what would be prescribed for their uterine risk factors.
Further supporting the notion that ITCs have more favorable prognosis is that, while SLN biopsy is associated with a higher detection of nodal metastatic disease, it is not necessarily associated with improved survival when compared with complete lymphadenectomy in retrospective series.8 This suggests that finding and treating ITCs may not positively affect outcomes. Or possibly it is a result of inadequate statistical power to show a small benefit should one exist. It is especially difficult to differentiate micrometastases and ITCs with respect to treatment outcomes. Given that ITCs make up the majority of low-volume nodal disease detected through the SLN technique, any potential benefit of increased capture and treatment of the more substantial micrometastases is not likely to be captured. As a result, most series tend to lump patients with micrometastases with those with ITCs in their analysis of patient outcomes. This may be a mistake.
Clearly more research needs to be performed to definitively address the clinical significance of ITCs. While it would be ideal to conduct a prospective trial in which patients with ITCs are randomized to therapy or observation, in reality the scope of such a trial makes it impractical. ITCs are detected in only approximately 5% of all the patients with endometrial cancer, and given that outcomes for this group are, in general, good, it would require enrollment of tens of thousands of patients to establish a statistically satisfactory result. Therefore it is likely that we will need to rely on the results of large retrospective, population-based, observational series to determine if the identification and treatment of ITCs adds value and superior outcomes to patients. In addition, we are making leaps in better understanding the molecular profile of endometrial cancers and how we might incorporate this data with histology and staging results to create treatment algorithms, much like what has been developed for breast cancer. This is likely where the future lies in interpreting the results of staging. In the meantime, it seems reasonable to collect the data regarding volume of metastatic disease including the presence of ITCs, making shared treatment decisions with the patient regarding the addition of adjuvant therapy, recognizing that
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no conflicts of interest to declare. Email her at [email protected].
References
1. Lancet Oncol. 2017 Mar;18(3):384-92.
2. J Clin Oncol. 2006 Jan 1;24(1):36-44.
3. J Clin Oncol. 2019 Jul 20;37(21):1810-8.
4. Clin Transl Oncol. 2019. doi: 10.1007/s12094-019-02249-x.
5. Gynecol Oncol. 2017 Aug;146(2):240-6.
6. Ann Surg Oncol. 2016 May;23(5):1653-9.
7. Gynecol Oncol. 2019 Jun;153(3):496-9.
8. Gynecol Oncol. 2018 Nov;151(2):235-42.
Over the past decade gynecologic oncology surgeons have increasingly adopted the technique of sentinel lymph node (SLN) biopsy to stage endometrial cancer. This is supported by evidence that selective removal of the few lymph nodes which are the first to drain the uterus can accurately detect metastatic disease, sparing the patient a complete lymphadenectomy and its associated risks, such as lymphedema.1 The proposed benefits of SLN biopsy are not just its ability to spare the patient removal of dozens of unnecessary lymph nodes, but also the ability to improve upon the detection of previously unrecognized nodal metastases in locations not routinely sampled by lymphadenectomy and by identifying very-low-volume metastatic disease. This is beneficial only, however, if that previously overlooked low-volume disease is clinically significant.

When pathologists evaluate lymph nodes as part of conventional lymphadenectomy, they typically bivalve the lymph node and evaluate with hematoxylin and eosin (H&E) stains. This technique is capable of detecting metastatic lesions greater than 2 mm, but can miss low-volume disease. In contrast, pathologists process SLNs with much finer sectioning (no greater than 2 mm), and, if the node is larger than 4 mm, they will section it perpendicular to the long axis in a bread-loaf fashion. It is not feasible to perform this ultrasectioning on the large numbers of lymph nodes of a complete lymphadenectomy specimen, but when applied to an SLN it allows pathologists to detect much smaller metastatic foci, the so-called “micrometastases” that are between 0.2 and 2 mm in size, and which typically arise in the subcapsular region of the node. The graphic depicts how a traditional longitudinal cut (a) might miss the micrometastasis that could be identified on the finer perpendicular cuts of ultra-sectioning (b). In addition to the ultrasectioning of the node into smaller slices, the pathologist performs additional immunohistochemistry stains for cytokeratin on sentinel nodes which appear negative on preliminary H&E stains. This allows the pathologist to identify even smaller clusters of malignant cells that are less than 0.2 mm, or individual cancer cells, so-called “isolated tumor cells” (ITCs) as shown in the photo. Most SLN series identify that approximately half of their “positive” lymph nodes are low-volume disease (micrometastases and ITCs). ITCs make up the majority of these cases, typically three-quarters.
Clinicians might be reassured by the discovery of low-volume metastatic disease, perceiving that the added attention afforded by the SLN approach helped them to identify metastases that might otherwise have been missed and therefore not treated. This is because node-positive (stage IIIC) disease is not cured by surgery or radiation alone and requires the addition of chemotherapy for survival benefit.2 Alternatively, there is no clear survival benefit derived from treating stage I high/intermediate cancers with chemotherapy, and therefore, the prescription of chemotherapy hinges upon reliable identification of extrauterine disease on pathology.3
It would make sense that if SLNs are more effective in identifying metastatic disease, clinicians who practice SLN biopsy would identify it more of the time. This appears to be the case with a trend towards upstaging in patients who undergo SLN biopsy, compared with those undergoing complete lymphadenectomy.4 It should also follow that if this increased detection of metastatic disease was clinically relevant, we would observe a corresponding improvement in survival outcomes. If not, then the additional identification of low-volume disease may not be value added: imparting toxicity of adjuvant therapy without survival benefit.
Micrometastases (foci sized 0.2-2 mm) are not a new phenomenon to the SLN era. Low-volume lesions were occasionally detected with routine nodal processing and H&E stains. Attention wasn’t paid to nodal volume categorization in pathology reports prior to the SLN era. These were usually reported collectively as stage IIIC disease. It would make sense to continue to approach micrometastases in a manner similar to what we have always done, recognizing that it may represent a continuum of nodal macrometastases. In contrast, ITCs are rarely detected with routine pathologic processing. Perhaps they are less within a continuum of nodal metastases, and more within the continuum of lymphovascular space invasion. We know that ITCs are significantly associated with the cofinding of this uterine phenomenon, which itself is considered a significant risk factor for local recurrence.5
Series have consistently shown the outcomes of women with ITCs to be favorable, compared with those with micrometastases or macrometastases.5,6 However, most retrospective series that evaluated the outcomes of patients with respect to volume of metastatic disease have high rates of treatment of ITCs with chemotherapy, radiotherapy, or both.6 This may mask and confuse whether there is any intrinsically favorable prognostic virtue of ITCs, compared with larger metastatic foci. When ITCs are untreated, it would appear that the rates and patterns of recurrence appear similar to those with negative SLNs, with the caveat that these series all include small numbers.5,7 This would suggest that women with ITCs do not need additional therapy beyond what would be prescribed for their uterine risk factors.
Further supporting the notion that ITCs have more favorable prognosis is that, while SLN biopsy is associated with a higher detection of nodal metastatic disease, it is not necessarily associated with improved survival when compared with complete lymphadenectomy in retrospective series.8 This suggests that finding and treating ITCs may not positively affect outcomes. Or possibly it is a result of inadequate statistical power to show a small benefit should one exist. It is especially difficult to differentiate micrometastases and ITCs with respect to treatment outcomes. Given that ITCs make up the majority of low-volume nodal disease detected through the SLN technique, any potential benefit of increased capture and treatment of the more substantial micrometastases is not likely to be captured. As a result, most series tend to lump patients with micrometastases with those with ITCs in their analysis of patient outcomes. This may be a mistake.
Clearly more research needs to be performed to definitively address the clinical significance of ITCs. While it would be ideal to conduct a prospective trial in which patients with ITCs are randomized to therapy or observation, in reality the scope of such a trial makes it impractical. ITCs are detected in only approximately 5% of all the patients with endometrial cancer, and given that outcomes for this group are, in general, good, it would require enrollment of tens of thousands of patients to establish a statistically satisfactory result. Therefore it is likely that we will need to rely on the results of large retrospective, population-based, observational series to determine if the identification and treatment of ITCs adds value and superior outcomes to patients. In addition, we are making leaps in better understanding the molecular profile of endometrial cancers and how we might incorporate this data with histology and staging results to create treatment algorithms, much like what has been developed for breast cancer. This is likely where the future lies in interpreting the results of staging. In the meantime, it seems reasonable to collect the data regarding volume of metastatic disease including the presence of ITCs, making shared treatment decisions with the patient regarding the addition of adjuvant therapy, recognizing that
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no conflicts of interest to declare. Email her at [email protected].
References
1. Lancet Oncol. 2017 Mar;18(3):384-92.
2. J Clin Oncol. 2006 Jan 1;24(1):36-44.
3. J Clin Oncol. 2019 Jul 20;37(21):1810-8.
4. Clin Transl Oncol. 2019. doi: 10.1007/s12094-019-02249-x.
5. Gynecol Oncol. 2017 Aug;146(2):240-6.
6. Ann Surg Oncol. 2016 May;23(5):1653-9.
7. Gynecol Oncol. 2019 Jun;153(3):496-9.
8. Gynecol Oncol. 2018 Nov;151(2):235-42.
ASCO panel outlines cancer care challenges during COVID-19 pandemic
The COVID-19 pandemic continues to exact a heavy price on cancer patients, cancer care, and clinical trials, an expert panel reported during a presscast.
“Limited data available thus far are sobering: In Italy, about 20% of COVID-related deaths occurred in people with cancer, and, in China, COVID-19 patients who had cancer were about five times more likely than others to die or be placed on a ventilator in an intensive care unit,” said Howard A “Skip” Burris, MD, president of the American Society of Clinical Oncology and president and CEO of the Sarah Cannon Cancer Institute in Nashville, Tenn.
“We also have little evidence on returning COVID-19 patients with cancer. Physicians have to rely on limited data, anecdotal reports, and their own professional expertise” regarding the extent of increased risk to cancer patients with COVID-19, whether to interrupt or modify treatment, and the effects of cancer on recovery from COVID-19 infection, Dr. Burris said during the ASCO-sponsored online presscast.
Care of COVID-free patients
For cancer patients without COVID-19, the picture is equally dim, with the prospect of delayed surgery, chemotherapy, or screening; shortages of medications and equipment needed for critical care; the shift to telemedicine that may increase patient anxiety; and the potential loss of access to innovative therapies through clinical trials, Dr. Burris said.
“We’re concerned that some hospitals have effectively deemed all cancer surgeries to be elective, requiring them to be postponed. For patients with fast-moving or hard-to-treat cancer, this delay may be devastating,” he said.
Dr. Burris also cited concerns about delayed cancer diagnosis. “In a typical month, roughly 150,000 Americans are diagnosed with cancer. But right now, routine screening visits are postponed, and patients with pain or other warning signs may put off a doctor’s visit because of social distancing,” he said.
The pandemic has also exacerbated shortages of sedatives and opioid analgesics required for intubation and mechanical ventilation of patients.
Trials halted or slowed
Dr. Burris also briefly discussed results of a new survey, which were posted online ahead of publication in JCO Oncology Practice. The survey showed that, of 14 academic and 18 community-based cancer programs, 59.4% reported halting screening and/or enrollment for at least some clinical trials and suspending research-based clinical visits except for those where cancer treatment was delivered.
“Half of respondents reported ceasing research-only blood and/or tissue collections,” the authors of the article reported.
“Trial interruptions are devastating news for thousands of patients; in many cases, clinical trials are the best or only appropriate option for care,” Dr. Burris said.
The article authors, led by David Waterhouse, MD, of Oncology Hematology Care in Cincinnati, pointed to a silver lining in the pandemic cloud in the form of opportunities to improve clinical trials going forward.
“Nearly all respondents (90.3%) identified telehealth visits for participants as a potential improvement to clinical trial conduct, and more than three-quarters (77.4%) indicated that remote patient review of symptoms held similar potential,” the authors wrote.
Other potential improvements included remote site visits from trial sponsors and/or contract research organizations, more efficient study enrollment through secure electronic platforms, direct shipment of oral drugs to patients, remote assessments of adverse events, and streamlined data collection.
Lessons from the front lines
Another member of the presscast panel, Melissa Dillmon, MD, of the Harbin Clinic Cancer Center in Rome, Georgia, described the experience of community oncologists during the pandemic.
Her community, located in northeastern Georgia, experienced a COVID-19 outbreak in early March linked to services at two large churches. Community public health authorities issued a shelter-in-place order before the state government issued stay-at-home guidelines and shuttered all but essential business, some of which were allowed by state order to reopen as of April 24.
Dr. Dillmon’s center began screening patients for COVID-19 symptoms at the door, limited visitors or companions, instituted virtual visits and tumor boards, and set up a cancer treatment triage system that would allow essential surgeries to proceed and most infusions to continue, while delaying the start of chemotherapy when possible.
“We have encouraged patients to continue on treatment, especially if treatment is being given with curative intent, or if the cancer is responding well already to treatment,” she said.
The center, located in a community with a high prevalence of comorbidities and high incidence of lung cancer, has seen a sharp decline in colonoscopies, mammograms, and lung scans as patient shelter in place.
“We have great concerns about patients missing their screening lung scans, as this program has already proven to be finding earlier lung cancers that are curable,” Dr. Dillmon said.
A view from Washington state
Another panel member, Gary Lyman, MD, of the Fred Hutchinson Cancer Research Center in Seattle, described the response by the state of Washington, the initial epicenter of the COVID-19 outbreak in the United States.
Following identification of infections in hospitalized patients and at a nursing home in Kirkland, Washington, “our response, which began in early March and progressed through the second and third week in March at the state level, was to restrict large gatherings; progressively, schools were closed; larger businesses closed; and, by March 23, a stay-at-home policy was implemented, and all nonessential businesses were closed,” Dr. Lyman said.
“We believe, based on what has happened since that time, that this has considerably flattened the curve,” he continued.
Lessons from the Washington experience include the need to plan for a long-term disruption or alteration of cancer care, expand COVID-19 testing to all patients coming into hospitals or major clinics, institute aggressive supportive care measures, prepare for subsequent waves of infection, collect and share data, and, for remote or rural areas, identify lifelines to needed resources, Dr. Lyman said.
ASCO resources
Also speaking at the presscast, Jonathan Marron, MD, of Boston Children’s Hospital and Harvard Medical School, Boston, outlined ASCO’s guidance on allocation of scarce resources during the COVID-19 pandemic.
Richard L. Schilsky, MD, ASCO chief medical officer and executive vice president, outlined community-wide collaborations, data initiatives, and online resources for both clinicians and patients.
The COVID-19 pandemic continues to exact a heavy price on cancer patients, cancer care, and clinical trials, an expert panel reported during a presscast.
“Limited data available thus far are sobering: In Italy, about 20% of COVID-related deaths occurred in people with cancer, and, in China, COVID-19 patients who had cancer were about five times more likely than others to die or be placed on a ventilator in an intensive care unit,” said Howard A “Skip” Burris, MD, president of the American Society of Clinical Oncology and president and CEO of the Sarah Cannon Cancer Institute in Nashville, Tenn.
“We also have little evidence on returning COVID-19 patients with cancer. Physicians have to rely on limited data, anecdotal reports, and their own professional expertise” regarding the extent of increased risk to cancer patients with COVID-19, whether to interrupt or modify treatment, and the effects of cancer on recovery from COVID-19 infection, Dr. Burris said during the ASCO-sponsored online presscast.
Care of COVID-free patients
For cancer patients without COVID-19, the picture is equally dim, with the prospect of delayed surgery, chemotherapy, or screening; shortages of medications and equipment needed for critical care; the shift to telemedicine that may increase patient anxiety; and the potential loss of access to innovative therapies through clinical trials, Dr. Burris said.
“We’re concerned that some hospitals have effectively deemed all cancer surgeries to be elective, requiring them to be postponed. For patients with fast-moving or hard-to-treat cancer, this delay may be devastating,” he said.
Dr. Burris also cited concerns about delayed cancer diagnosis. “In a typical month, roughly 150,000 Americans are diagnosed with cancer. But right now, routine screening visits are postponed, and patients with pain or other warning signs may put off a doctor’s visit because of social distancing,” he said.
The pandemic has also exacerbated shortages of sedatives and opioid analgesics required for intubation and mechanical ventilation of patients.
Trials halted or slowed
Dr. Burris also briefly discussed results of a new survey, which were posted online ahead of publication in JCO Oncology Practice. The survey showed that, of 14 academic and 18 community-based cancer programs, 59.4% reported halting screening and/or enrollment for at least some clinical trials and suspending research-based clinical visits except for those where cancer treatment was delivered.
“Half of respondents reported ceasing research-only blood and/or tissue collections,” the authors of the article reported.
“Trial interruptions are devastating news for thousands of patients; in many cases, clinical trials are the best or only appropriate option for care,” Dr. Burris said.
The article authors, led by David Waterhouse, MD, of Oncology Hematology Care in Cincinnati, pointed to a silver lining in the pandemic cloud in the form of opportunities to improve clinical trials going forward.
“Nearly all respondents (90.3%) identified telehealth visits for participants as a potential improvement to clinical trial conduct, and more than three-quarters (77.4%) indicated that remote patient review of symptoms held similar potential,” the authors wrote.
Other potential improvements included remote site visits from trial sponsors and/or contract research organizations, more efficient study enrollment through secure electronic platforms, direct shipment of oral drugs to patients, remote assessments of adverse events, and streamlined data collection.
Lessons from the front lines
Another member of the presscast panel, Melissa Dillmon, MD, of the Harbin Clinic Cancer Center in Rome, Georgia, described the experience of community oncologists during the pandemic.
Her community, located in northeastern Georgia, experienced a COVID-19 outbreak in early March linked to services at two large churches. Community public health authorities issued a shelter-in-place order before the state government issued stay-at-home guidelines and shuttered all but essential business, some of which were allowed by state order to reopen as of April 24.
Dr. Dillmon’s center began screening patients for COVID-19 symptoms at the door, limited visitors or companions, instituted virtual visits and tumor boards, and set up a cancer treatment triage system that would allow essential surgeries to proceed and most infusions to continue, while delaying the start of chemotherapy when possible.
“We have encouraged patients to continue on treatment, especially if treatment is being given with curative intent, or if the cancer is responding well already to treatment,” she said.
The center, located in a community with a high prevalence of comorbidities and high incidence of lung cancer, has seen a sharp decline in colonoscopies, mammograms, and lung scans as patient shelter in place.
“We have great concerns about patients missing their screening lung scans, as this program has already proven to be finding earlier lung cancers that are curable,” Dr. Dillmon said.
A view from Washington state
Another panel member, Gary Lyman, MD, of the Fred Hutchinson Cancer Research Center in Seattle, described the response by the state of Washington, the initial epicenter of the COVID-19 outbreak in the United States.
Following identification of infections in hospitalized patients and at a nursing home in Kirkland, Washington, “our response, which began in early March and progressed through the second and third week in March at the state level, was to restrict large gatherings; progressively, schools were closed; larger businesses closed; and, by March 23, a stay-at-home policy was implemented, and all nonessential businesses were closed,” Dr. Lyman said.
“We believe, based on what has happened since that time, that this has considerably flattened the curve,” he continued.
Lessons from the Washington experience include the need to plan for a long-term disruption or alteration of cancer care, expand COVID-19 testing to all patients coming into hospitals or major clinics, institute aggressive supportive care measures, prepare for subsequent waves of infection, collect and share data, and, for remote or rural areas, identify lifelines to needed resources, Dr. Lyman said.
ASCO resources
Also speaking at the presscast, Jonathan Marron, MD, of Boston Children’s Hospital and Harvard Medical School, Boston, outlined ASCO’s guidance on allocation of scarce resources during the COVID-19 pandemic.
Richard L. Schilsky, MD, ASCO chief medical officer and executive vice president, outlined community-wide collaborations, data initiatives, and online resources for both clinicians and patients.
The COVID-19 pandemic continues to exact a heavy price on cancer patients, cancer care, and clinical trials, an expert panel reported during a presscast.
“Limited data available thus far are sobering: In Italy, about 20% of COVID-related deaths occurred in people with cancer, and, in China, COVID-19 patients who had cancer were about five times more likely than others to die or be placed on a ventilator in an intensive care unit,” said Howard A “Skip” Burris, MD, president of the American Society of Clinical Oncology and president and CEO of the Sarah Cannon Cancer Institute in Nashville, Tenn.
“We also have little evidence on returning COVID-19 patients with cancer. Physicians have to rely on limited data, anecdotal reports, and their own professional expertise” regarding the extent of increased risk to cancer patients with COVID-19, whether to interrupt or modify treatment, and the effects of cancer on recovery from COVID-19 infection, Dr. Burris said during the ASCO-sponsored online presscast.
Care of COVID-free patients
For cancer patients without COVID-19, the picture is equally dim, with the prospect of delayed surgery, chemotherapy, or screening; shortages of medications and equipment needed for critical care; the shift to telemedicine that may increase patient anxiety; and the potential loss of access to innovative therapies through clinical trials, Dr. Burris said.
“We’re concerned that some hospitals have effectively deemed all cancer surgeries to be elective, requiring them to be postponed. For patients with fast-moving or hard-to-treat cancer, this delay may be devastating,” he said.
Dr. Burris also cited concerns about delayed cancer diagnosis. “In a typical month, roughly 150,000 Americans are diagnosed with cancer. But right now, routine screening visits are postponed, and patients with pain or other warning signs may put off a doctor’s visit because of social distancing,” he said.
The pandemic has also exacerbated shortages of sedatives and opioid analgesics required for intubation and mechanical ventilation of patients.
Trials halted or slowed
Dr. Burris also briefly discussed results of a new survey, which were posted online ahead of publication in JCO Oncology Practice. The survey showed that, of 14 academic and 18 community-based cancer programs, 59.4% reported halting screening and/or enrollment for at least some clinical trials and suspending research-based clinical visits except for those where cancer treatment was delivered.
“Half of respondents reported ceasing research-only blood and/or tissue collections,” the authors of the article reported.
“Trial interruptions are devastating news for thousands of patients; in many cases, clinical trials are the best or only appropriate option for care,” Dr. Burris said.
The article authors, led by David Waterhouse, MD, of Oncology Hematology Care in Cincinnati, pointed to a silver lining in the pandemic cloud in the form of opportunities to improve clinical trials going forward.
“Nearly all respondents (90.3%) identified telehealth visits for participants as a potential improvement to clinical trial conduct, and more than three-quarters (77.4%) indicated that remote patient review of symptoms held similar potential,” the authors wrote.
Other potential improvements included remote site visits from trial sponsors and/or contract research organizations, more efficient study enrollment through secure electronic platforms, direct shipment of oral drugs to patients, remote assessments of adverse events, and streamlined data collection.
Lessons from the front lines
Another member of the presscast panel, Melissa Dillmon, MD, of the Harbin Clinic Cancer Center in Rome, Georgia, described the experience of community oncologists during the pandemic.
Her community, located in northeastern Georgia, experienced a COVID-19 outbreak in early March linked to services at two large churches. Community public health authorities issued a shelter-in-place order before the state government issued stay-at-home guidelines and shuttered all but essential business, some of which were allowed by state order to reopen as of April 24.
Dr. Dillmon’s center began screening patients for COVID-19 symptoms at the door, limited visitors or companions, instituted virtual visits and tumor boards, and set up a cancer treatment triage system that would allow essential surgeries to proceed and most infusions to continue, while delaying the start of chemotherapy when possible.
“We have encouraged patients to continue on treatment, especially if treatment is being given with curative intent, or if the cancer is responding well already to treatment,” she said.
The center, located in a community with a high prevalence of comorbidities and high incidence of lung cancer, has seen a sharp decline in colonoscopies, mammograms, and lung scans as patient shelter in place.
“We have great concerns about patients missing their screening lung scans, as this program has already proven to be finding earlier lung cancers that are curable,” Dr. Dillmon said.
A view from Washington state
Another panel member, Gary Lyman, MD, of the Fred Hutchinson Cancer Research Center in Seattle, described the response by the state of Washington, the initial epicenter of the COVID-19 outbreak in the United States.
Following identification of infections in hospitalized patients and at a nursing home in Kirkland, Washington, “our response, which began in early March and progressed through the second and third week in March at the state level, was to restrict large gatherings; progressively, schools were closed; larger businesses closed; and, by March 23, a stay-at-home policy was implemented, and all nonessential businesses were closed,” Dr. Lyman said.
“We believe, based on what has happened since that time, that this has considerably flattened the curve,” he continued.
Lessons from the Washington experience include the need to plan for a long-term disruption or alteration of cancer care, expand COVID-19 testing to all patients coming into hospitals or major clinics, institute aggressive supportive care measures, prepare for subsequent waves of infection, collect and share data, and, for remote or rural areas, identify lifelines to needed resources, Dr. Lyman said.
ASCO resources
Also speaking at the presscast, Jonathan Marron, MD, of Boston Children’s Hospital and Harvard Medical School, Boston, outlined ASCO’s guidance on allocation of scarce resources during the COVID-19 pandemic.
Richard L. Schilsky, MD, ASCO chief medical officer and executive vice president, outlined community-wide collaborations, data initiatives, and online resources for both clinicians and patients.
Will coronavirus restrictions lead to more advanced cancers?
My pathology lab once faced a daily flood of colon polyps, pap smears, and prostate biopsies. Suddenly, our work has dried up. The coronavirus pandemic has cleared out operating rooms and clinics across the country. Endoscopy and radiology suites have gone dark.
Pathology is largely driven by mass screening programs, and the machinery of screening has grinded to a halt during the COVID-19 pandemic. The American Cancer Society currently recommends that “no one should go to a health care facility for routine cancer screening at this time.”
But malignancies are still growing and spreading even though a great deal of medical care is on hold. The most urgent cancer care is still taking place; the risks of delaying treatment for patients with advanced or symptomatic cancer are obvious—these tumors can cause severe pain and life-threatening complications.
But that leaves us with a more complex and uncomfortable question: Will the pause in screening ultimately leave patients with tiny, asymptomatic cancers or precursor lesions worse off? What will a delay mean for those with ductal carcinoma in situ or small breast cancers? What’s the long-term effect of all those dysplastic nevi and early melanoma left unexcised by dermatologists? Perhaps more troubling, what about the spreading kidney cancer that may have turned up as an incidental finding on a CT scan?
COVID-19: A natural experiment
For many years, we’ve been dealing with the other side of the screening question: overdiagnosing and treating cancers that would probably never harm the patient. Overdiagnosis has been on a decades-long rise due to organized screening like PSA testing and mammography, as well as through ad hoc detection from heavier use of medical imaging. All of these have been disrupted by the pandemic.
Because the correlation between medical interventions and cancer overdiagnosis is clear, we can safely assume that overdiagnosis will decline during the pandemic. But what will be the net effect? Early detection of cancer undoubtedly saves some lives, but how many and at what cost has been a seemingly intractable debate.
Until now.
The coronavirus outbreak will be a natural experiment like no other. Economists and epidemiologists love to study “natural experiments” – systemic shocks that shed light on a complex phenomenon.
The unexpected nationwide delay in screening will undoubtedly inform the debate on overdiagnosis. For one, we can learn whether less intensive screening leads to more advanced cancers. Because screening will probably return to normal at different times across the country, we can almost simulate a randomized trial. Will this transformative data be a silver lining to this awful time?
The pressure to ‘fight’
The pandemic has also raised a question about cancer screening that goes beyond data: Why has the loud epidemic of coronavirus so thoroughly trumped cancer’s silent one? To me, the necessary urgency of our coronavirus response stands in stark contrast to the overly aggressive public health messaging used for cancer screening.
The tools used to fight the coronavirus epidemic have been forceful. We’re all diligently washing our hands and staying inside. We’re making sacrifices in our jobs and personal lives to stop the virus’ spread.
Cancer screening has similarly been touted as dogma – an urgent public health intervention that only a fool would turn down. The American Cancer Society once ran an infamous advertisement suggesting that if you decline mammography, you “need more than your breasts examined.” Even today, well-intentioned organizations run cancer screening drives pushing people to pledge to “get screened now.” It is no surprise, then, that I have had patients and family members confide in me that they feel guilty about not pursuing all of their recommended screening tests. The thought of anyone feeling like they caused their own cancer appalls me.
This pressure extends into the clinic. In many practices, primary care doctors are evaluated based on how many patients “comply” with screening recommendations. There seems to be a relentless drive to reach 100% screening penetration. These oversimplified tactics run counter to the shared decision making and informed consent we profess to value in medicine.
The tricky thing about cancer screening is that because most people will never develop the cancer being screened for, we know that most people can also never be helped by it. This doesn’t make screening useless, just as washing your hands can help even if it doesn’t guarantee that you won’t catch coronavirus. We know that some individuals benefit, which we detect at the population level. Overdiagnosis arises in the same way, as a phenomenon detected within populations and not individuals. These aspects of screening are what has led to cancer being viewed as a “societal disease” requiring a uniform response – 100% screening compliance.
Metaphors of war
These assumptions fall apart now that we are facing a real societal disease, an infectious disease outbreak. Coronavirus has made us reflect on what actions individuals should take in order to protect others. But cancer is not a contagion. When we decide whether and how to screen, we make intimate decisions affecting primarily ourselves and our family – not society at large.
Countless articles have been written about the use of metaphor in cancer, perhaps most famously by essayist and breast cancer patient Susan Sontag. Sontag and others have been critical of the rampant use of war metaphors in the cancer community. Wars invoke sacrifice, duty, and suffering. The “battle” against coronavirus really puts the “war on cancer” in perspective. These pandemic weeks have terrified me. I have been willing to do anything to protect myself and others. They’ve also exhausted me. We can’t be at war forever.
When this current war ends, will the “war on cancer” resume unchanged? Screening will no doubt begin again, hopefully improved by data from the coronavirus natural experiment. But I wonder whether we will tolerate the same kinds of public health messages – and whether we should – having now experienced an infectious disease outbreak where our actions as individuals really do have an impact on the health of others.
After feeling helpless, besieged, and even guilt-ridden during the pandemic, I think many people would appreciate regaining a sense of control over other aspects of their health. Cancer screening can save lives, but it’s a choice we should make for ourselves based on an understanding of the trade-offs and our own preferences. When screening restarts, I hope its paternalistic dogma can be replaced by nuanced, empowering tactics more appropriate for peacetime.
Benjamin Mazer, MD, MBA, is an anatomic and clinical pathology resident at Yale with interests in diagnostic surgical pathology, laboratory management, and evidence-based medicine.
This article first appeared on Medscape.com.
My pathology lab once faced a daily flood of colon polyps, pap smears, and prostate biopsies. Suddenly, our work has dried up. The coronavirus pandemic has cleared out operating rooms and clinics across the country. Endoscopy and radiology suites have gone dark.
Pathology is largely driven by mass screening programs, and the machinery of screening has grinded to a halt during the COVID-19 pandemic. The American Cancer Society currently recommends that “no one should go to a health care facility for routine cancer screening at this time.”
But malignancies are still growing and spreading even though a great deal of medical care is on hold. The most urgent cancer care is still taking place; the risks of delaying treatment for patients with advanced or symptomatic cancer are obvious—these tumors can cause severe pain and life-threatening complications.
But that leaves us with a more complex and uncomfortable question: Will the pause in screening ultimately leave patients with tiny, asymptomatic cancers or precursor lesions worse off? What will a delay mean for those with ductal carcinoma in situ or small breast cancers? What’s the long-term effect of all those dysplastic nevi and early melanoma left unexcised by dermatologists? Perhaps more troubling, what about the spreading kidney cancer that may have turned up as an incidental finding on a CT scan?
COVID-19: A natural experiment
For many years, we’ve been dealing with the other side of the screening question: overdiagnosing and treating cancers that would probably never harm the patient. Overdiagnosis has been on a decades-long rise due to organized screening like PSA testing and mammography, as well as through ad hoc detection from heavier use of medical imaging. All of these have been disrupted by the pandemic.
Because the correlation between medical interventions and cancer overdiagnosis is clear, we can safely assume that overdiagnosis will decline during the pandemic. But what will be the net effect? Early detection of cancer undoubtedly saves some lives, but how many and at what cost has been a seemingly intractable debate.
Until now.
The coronavirus outbreak will be a natural experiment like no other. Economists and epidemiologists love to study “natural experiments” – systemic shocks that shed light on a complex phenomenon.
The unexpected nationwide delay in screening will undoubtedly inform the debate on overdiagnosis. For one, we can learn whether less intensive screening leads to more advanced cancers. Because screening will probably return to normal at different times across the country, we can almost simulate a randomized trial. Will this transformative data be a silver lining to this awful time?
The pressure to ‘fight’
The pandemic has also raised a question about cancer screening that goes beyond data: Why has the loud epidemic of coronavirus so thoroughly trumped cancer’s silent one? To me, the necessary urgency of our coronavirus response stands in stark contrast to the overly aggressive public health messaging used for cancer screening.
The tools used to fight the coronavirus epidemic have been forceful. We’re all diligently washing our hands and staying inside. We’re making sacrifices in our jobs and personal lives to stop the virus’ spread.
Cancer screening has similarly been touted as dogma – an urgent public health intervention that only a fool would turn down. The American Cancer Society once ran an infamous advertisement suggesting that if you decline mammography, you “need more than your breasts examined.” Even today, well-intentioned organizations run cancer screening drives pushing people to pledge to “get screened now.” It is no surprise, then, that I have had patients and family members confide in me that they feel guilty about not pursuing all of their recommended screening tests. The thought of anyone feeling like they caused their own cancer appalls me.
This pressure extends into the clinic. In many practices, primary care doctors are evaluated based on how many patients “comply” with screening recommendations. There seems to be a relentless drive to reach 100% screening penetration. These oversimplified tactics run counter to the shared decision making and informed consent we profess to value in medicine.
The tricky thing about cancer screening is that because most people will never develop the cancer being screened for, we know that most people can also never be helped by it. This doesn’t make screening useless, just as washing your hands can help even if it doesn’t guarantee that you won’t catch coronavirus. We know that some individuals benefit, which we detect at the population level. Overdiagnosis arises in the same way, as a phenomenon detected within populations and not individuals. These aspects of screening are what has led to cancer being viewed as a “societal disease” requiring a uniform response – 100% screening compliance.
Metaphors of war
These assumptions fall apart now that we are facing a real societal disease, an infectious disease outbreak. Coronavirus has made us reflect on what actions individuals should take in order to protect others. But cancer is not a contagion. When we decide whether and how to screen, we make intimate decisions affecting primarily ourselves and our family – not society at large.
Countless articles have been written about the use of metaphor in cancer, perhaps most famously by essayist and breast cancer patient Susan Sontag. Sontag and others have been critical of the rampant use of war metaphors in the cancer community. Wars invoke sacrifice, duty, and suffering. The “battle” against coronavirus really puts the “war on cancer” in perspective. These pandemic weeks have terrified me. I have been willing to do anything to protect myself and others. They’ve also exhausted me. We can’t be at war forever.
When this current war ends, will the “war on cancer” resume unchanged? Screening will no doubt begin again, hopefully improved by data from the coronavirus natural experiment. But I wonder whether we will tolerate the same kinds of public health messages – and whether we should – having now experienced an infectious disease outbreak where our actions as individuals really do have an impact on the health of others.
After feeling helpless, besieged, and even guilt-ridden during the pandemic, I think many people would appreciate regaining a sense of control over other aspects of their health. Cancer screening can save lives, but it’s a choice we should make for ourselves based on an understanding of the trade-offs and our own preferences. When screening restarts, I hope its paternalistic dogma can be replaced by nuanced, empowering tactics more appropriate for peacetime.
Benjamin Mazer, MD, MBA, is an anatomic and clinical pathology resident at Yale with interests in diagnostic surgical pathology, laboratory management, and evidence-based medicine.
This article first appeared on Medscape.com.
My pathology lab once faced a daily flood of colon polyps, pap smears, and prostate biopsies. Suddenly, our work has dried up. The coronavirus pandemic has cleared out operating rooms and clinics across the country. Endoscopy and radiology suites have gone dark.
Pathology is largely driven by mass screening programs, and the machinery of screening has grinded to a halt during the COVID-19 pandemic. The American Cancer Society currently recommends that “no one should go to a health care facility for routine cancer screening at this time.”
But malignancies are still growing and spreading even though a great deal of medical care is on hold. The most urgent cancer care is still taking place; the risks of delaying treatment for patients with advanced or symptomatic cancer are obvious—these tumors can cause severe pain and life-threatening complications.
But that leaves us with a more complex and uncomfortable question: Will the pause in screening ultimately leave patients with tiny, asymptomatic cancers or precursor lesions worse off? What will a delay mean for those with ductal carcinoma in situ or small breast cancers? What’s the long-term effect of all those dysplastic nevi and early melanoma left unexcised by dermatologists? Perhaps more troubling, what about the spreading kidney cancer that may have turned up as an incidental finding on a CT scan?
COVID-19: A natural experiment
For many years, we’ve been dealing with the other side of the screening question: overdiagnosing and treating cancers that would probably never harm the patient. Overdiagnosis has been on a decades-long rise due to organized screening like PSA testing and mammography, as well as through ad hoc detection from heavier use of medical imaging. All of these have been disrupted by the pandemic.
Because the correlation between medical interventions and cancer overdiagnosis is clear, we can safely assume that overdiagnosis will decline during the pandemic. But what will be the net effect? Early detection of cancer undoubtedly saves some lives, but how many and at what cost has been a seemingly intractable debate.
Until now.
The coronavirus outbreak will be a natural experiment like no other. Economists and epidemiologists love to study “natural experiments” – systemic shocks that shed light on a complex phenomenon.
The unexpected nationwide delay in screening will undoubtedly inform the debate on overdiagnosis. For one, we can learn whether less intensive screening leads to more advanced cancers. Because screening will probably return to normal at different times across the country, we can almost simulate a randomized trial. Will this transformative data be a silver lining to this awful time?
The pressure to ‘fight’
The pandemic has also raised a question about cancer screening that goes beyond data: Why has the loud epidemic of coronavirus so thoroughly trumped cancer’s silent one? To me, the necessary urgency of our coronavirus response stands in stark contrast to the overly aggressive public health messaging used for cancer screening.
The tools used to fight the coronavirus epidemic have been forceful. We’re all diligently washing our hands and staying inside. We’re making sacrifices in our jobs and personal lives to stop the virus’ spread.
Cancer screening has similarly been touted as dogma – an urgent public health intervention that only a fool would turn down. The American Cancer Society once ran an infamous advertisement suggesting that if you decline mammography, you “need more than your breasts examined.” Even today, well-intentioned organizations run cancer screening drives pushing people to pledge to “get screened now.” It is no surprise, then, that I have had patients and family members confide in me that they feel guilty about not pursuing all of their recommended screening tests. The thought of anyone feeling like they caused their own cancer appalls me.
This pressure extends into the clinic. In many practices, primary care doctors are evaluated based on how many patients “comply” with screening recommendations. There seems to be a relentless drive to reach 100% screening penetration. These oversimplified tactics run counter to the shared decision making and informed consent we profess to value in medicine.
The tricky thing about cancer screening is that because most people will never develop the cancer being screened for, we know that most people can also never be helped by it. This doesn’t make screening useless, just as washing your hands can help even if it doesn’t guarantee that you won’t catch coronavirus. We know that some individuals benefit, which we detect at the population level. Overdiagnosis arises in the same way, as a phenomenon detected within populations and not individuals. These aspects of screening are what has led to cancer being viewed as a “societal disease” requiring a uniform response – 100% screening compliance.
Metaphors of war
These assumptions fall apart now that we are facing a real societal disease, an infectious disease outbreak. Coronavirus has made us reflect on what actions individuals should take in order to protect others. But cancer is not a contagion. When we decide whether and how to screen, we make intimate decisions affecting primarily ourselves and our family – not society at large.
Countless articles have been written about the use of metaphor in cancer, perhaps most famously by essayist and breast cancer patient Susan Sontag. Sontag and others have been critical of the rampant use of war metaphors in the cancer community. Wars invoke sacrifice, duty, and suffering. The “battle” against coronavirus really puts the “war on cancer” in perspective. These pandemic weeks have terrified me. I have been willing to do anything to protect myself and others. They’ve also exhausted me. We can’t be at war forever.
When this current war ends, will the “war on cancer” resume unchanged? Screening will no doubt begin again, hopefully improved by data from the coronavirus natural experiment. But I wonder whether we will tolerate the same kinds of public health messages – and whether we should – having now experienced an infectious disease outbreak where our actions as individuals really do have an impact on the health of others.
After feeling helpless, besieged, and even guilt-ridden during the pandemic, I think many people would appreciate regaining a sense of control over other aspects of their health. Cancer screening can save lives, but it’s a choice we should make for ourselves based on an understanding of the trade-offs and our own preferences. When screening restarts, I hope its paternalistic dogma can be replaced by nuanced, empowering tactics more appropriate for peacetime.
Benjamin Mazer, MD, MBA, is an anatomic and clinical pathology resident at Yale with interests in diagnostic surgical pathology, laboratory management, and evidence-based medicine.
This article first appeared on Medscape.com.
European cancer centers restructure care in the era of COVID-19
Delivering cancer care during the COVID-19 pandemic has proved particularly challenging, as minimizing the risk of infection must be balanced with maintaining optimal outcomes.
Healthcare systems and oncologists have had to reorganize standard oncologic care in order to protect vulnerable patients from exposure to COVID-19 as well as deal with pandemic-related issues of equipment and staffing shortages.
A new article now describes how seven cancer centers in Europe rapidly reorganized their oncologic services and are tackling this crisis, as well as offering guidance to other institutions.
This was a major undertaking, to work out a system where patients can still get care but in a safer manner, explained coauthor Emile Voest, MD, medical director of the Netherlands Cancer Institute in Amsterdam.
“Decisions needed to be taken based on availability of personnel, protective materials, and urgencies,” he told Medscape Medical News. “Because every country had its own speed of development of the COVID pandemic, there were different scenarios in all institutions, but all with a common factor of key expertise on how to de-escalate in a safe manner.”
The article was published April 16 in Nature Medicine.
The Netherlands Cancer Institute (the Netherlands), Karolinska Institute (Sweden), Institute Gustave Roussy (France), Cambridge Cancer Center (United Kingdom), Istituto Nazionale dei Tumori di Milano (Italy), German Cancer Research Center (Germany), and Vall d’Hebron Institute of Oncology (Spain) have been working closely together in a legal entity since 2014, and have created ‘Cancer Core Europe’ (CCE). The goal is to “maximize coherence and critical mass in cancer research,” the authors note.
The consortium represents roughly 60,000 patients with newly diagnosed cancer, delivers approximately 300,000 treatment courses, and conducts about 1.2 million consultations annually, with more than 1,500 ongoing clinical trials. In a joint effort, the centers collected, translated, and compared the guidelines that had been put in place to treat patients with cancer during the COVID-19 pandemic.
Cancer treatment is multidisciplinary and involves many specialties including surgery, radiology, pathology, radiation oncology, and medical oncology. Coordinating care among disciplines is a very complex process, Voest noted.
“Changing treatment also means that you need to reconsider capacities and requirements,” he said. “Hospitals have installed crisis teams that were very good at coordinating these efforts.”
Restructuring care
Cancer care had to be reorganized on multiple levels, and the CCE centers looked at several aspects that needed to be accounted for, to ensure continuity in cancer care.
“The biggest challenge for the NHS and other healthcare systems is the surge of patients requiring oxygen and/or intensive care, and the nature and infectiousness of the virus,” said coauthor Carlos Caldas, MD, FMedSci, professor of cancer medicine at the University of Cambridge, United Kingdom. “In hospitals that are mostly run close to capacity, and where all kinds of patients are treated, this has created major resource and logistical problems.”
For regular clinical activities, the institutions with dedicated cancer centers (German Cancer Research Center, Institute Gustave Roussy, Istituto Nazionale dei Tumori di Milano, and Netherlands Cancer Institute) have attempted to stay COVID-19 free. This policy would in turn help ensure that sufficient clinical and intensive-care capacity could be reserved for critical cancer surgeries or management of treatment-related side effects, and allow hospitals outside of the CCE to transfer patients with cancer to these centers. The general hospitals can then focus on caring for patients with COVID-19, as well as other illnesses/injuries that require inpatient care.
As the CCE centers located within general hospitals (Cambridge Cancer Center, Vall d’Hebron Institute of Oncology and Karolinska Institute) have to admit patients with suspected and positive cases of COVID-19, being “COVID-19 free” was never a realistic or pursued goal.
The authors note that it is the responsibility of all healthcare professionals to ensure patients are not exposed to COVID-19, and this has meant minimizing hospital visits and person-to-person contact. For example, whenever possible, consultations take place via telephone calls or over the Internet, and nonurgent appointments that would require a patient’s physical presence at the clinic have been postponed. Visitors are also not permitted to accompany patients when admitted to the hospital or during procedures.
Standard-of-care treatment regimens have been adapted across all centers to minimize the number of hospital visits and hospitalizations and prevent “anticancer treatment-induced” complications of COVID-19.
To minimize visits and hospitalizations, strategies include converting intravenous treatments to oral or subcutaneous regimens when possible; switching from cytotoxic chemotherapy to a less-toxic approach to minimize the risk of complications requiring hospitalization; or to pause therapies when possible (stable disease reached or better). In addition, nonemergency surgeries have been postponed or replaced by radiotherapy.
To prevent anticancer treatment-induced complications of COVID-19, most centers use the paradigm that the added benefit for tumor control should be weighed against the potential risk for COVID-19–related morbidity and mortality. To prevent or reduce the risk of neutropenia and lymphopenia, for example, all centers have suggested a de-escalation of cytotoxic chemotherapy or targeted treatment strategies, or to forgo second or subsequent lines of palliative treatments if response rates from up-front therapy are low.
Some of these changes may be here to stay, noted Caldas. “One of the positive messages that comes out of this is that, clearly, care can be delivered in a safe and compassionate manner without requiring as many hospital visits as in the pre-COVID-19 era,” he said. “In the future, we will take heed of the COVID-19 experience to improve delivery of cancer care.”
Capacity of facilities
Many healthcare systems have become overwhelmed as the pandemic has intensified, thus making it necessary to prioritize. To prepare for this possibility, CCE centers have established protocols to categorize and prioritize patients for systemic treatment or surgery. While the protocols vary by center, they are comparable with one another as they prioritize on the basis of anticipated treatment outcome, the authors note.
The guidelines in CCE centers unanimously recommend that neoadjuvant therapies and curative surgeries be the top priority, for the times when operating room and/or ICU capacity is limited. As an alternative, neoadjuvant systemic treatments may be initiated or extended to postpone surgery, and other nonsurgical interventions can be considered.
In addition, some centers agree that certain elective surgeries can be safely delayed if backed by scientific evidence. As an example, an 11-week deferment of surgery may be acceptable for patients with rectal cancer after downstaging.
Cancer centers may also need to upscale and downscale quickly, depending on how the pandemic evolves, and many have already outlined scenarios to prepare for increasing or decreasing their capacity using phased approaches.
The Netherlands Cancer Institute, for example, has defined four phases of increasing severity; in Germany, capacity planning has been coordinated among 18 hospitals and the federal ministry of health, in order to prevent shortages of cancer services.
“We note that the optimal downscaling strategies depend on country- and center-specific capacities and preferences,” they write. “Therefore, it is difficult to propose a common schedule, and it will be most effective if hospitals outline their own phase-specific downscaling strategies based on the prioritization schemes and practical handles discussed above.”
Future research
Better strategies will be needed to reduce the impact of COVID-19 in cancer care, and four research priorities were identified to allow for evidence-based adjustments of cancer care protocols while the pandemic continues:
- Collect real-world data about the effects of adjustment and de-escalation of treatment regimens on outcomes
- Determine the incidence of COVID-19 in both the general population and among patients with cancer who have received systemic therapies, with large-scale serological testing
- Develop an epidemiological model that will allow estimates of the cumulative incidence of COVID-19 for a patient with cancer, within a specific time frame
- Determine COVID-19 related morbidity and mortality in patients with cancer who have been treated with systemic therapies and/or granulocyte colony-stimulating factor (G-CSF). Several projects are currently underway, such as the UK Coronavirus Cancer Monitoring Project.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Delivering cancer care during the COVID-19 pandemic has proved particularly challenging, as minimizing the risk of infection must be balanced with maintaining optimal outcomes.
Healthcare systems and oncologists have had to reorganize standard oncologic care in order to protect vulnerable patients from exposure to COVID-19 as well as deal with pandemic-related issues of equipment and staffing shortages.
A new article now describes how seven cancer centers in Europe rapidly reorganized their oncologic services and are tackling this crisis, as well as offering guidance to other institutions.
This was a major undertaking, to work out a system where patients can still get care but in a safer manner, explained coauthor Emile Voest, MD, medical director of the Netherlands Cancer Institute in Amsterdam.
“Decisions needed to be taken based on availability of personnel, protective materials, and urgencies,” he told Medscape Medical News. “Because every country had its own speed of development of the COVID pandemic, there were different scenarios in all institutions, but all with a common factor of key expertise on how to de-escalate in a safe manner.”
The article was published April 16 in Nature Medicine.
The Netherlands Cancer Institute (the Netherlands), Karolinska Institute (Sweden), Institute Gustave Roussy (France), Cambridge Cancer Center (United Kingdom), Istituto Nazionale dei Tumori di Milano (Italy), German Cancer Research Center (Germany), and Vall d’Hebron Institute of Oncology (Spain) have been working closely together in a legal entity since 2014, and have created ‘Cancer Core Europe’ (CCE). The goal is to “maximize coherence and critical mass in cancer research,” the authors note.
The consortium represents roughly 60,000 patients with newly diagnosed cancer, delivers approximately 300,000 treatment courses, and conducts about 1.2 million consultations annually, with more than 1,500 ongoing clinical trials. In a joint effort, the centers collected, translated, and compared the guidelines that had been put in place to treat patients with cancer during the COVID-19 pandemic.
Cancer treatment is multidisciplinary and involves many specialties including surgery, radiology, pathology, radiation oncology, and medical oncology. Coordinating care among disciplines is a very complex process, Voest noted.
“Changing treatment also means that you need to reconsider capacities and requirements,” he said. “Hospitals have installed crisis teams that were very good at coordinating these efforts.”
Restructuring care
Cancer care had to be reorganized on multiple levels, and the CCE centers looked at several aspects that needed to be accounted for, to ensure continuity in cancer care.
“The biggest challenge for the NHS and other healthcare systems is the surge of patients requiring oxygen and/or intensive care, and the nature and infectiousness of the virus,” said coauthor Carlos Caldas, MD, FMedSci, professor of cancer medicine at the University of Cambridge, United Kingdom. “In hospitals that are mostly run close to capacity, and where all kinds of patients are treated, this has created major resource and logistical problems.”
For regular clinical activities, the institutions with dedicated cancer centers (German Cancer Research Center, Institute Gustave Roussy, Istituto Nazionale dei Tumori di Milano, and Netherlands Cancer Institute) have attempted to stay COVID-19 free. This policy would in turn help ensure that sufficient clinical and intensive-care capacity could be reserved for critical cancer surgeries or management of treatment-related side effects, and allow hospitals outside of the CCE to transfer patients with cancer to these centers. The general hospitals can then focus on caring for patients with COVID-19, as well as other illnesses/injuries that require inpatient care.
As the CCE centers located within general hospitals (Cambridge Cancer Center, Vall d’Hebron Institute of Oncology and Karolinska Institute) have to admit patients with suspected and positive cases of COVID-19, being “COVID-19 free” was never a realistic or pursued goal.
The authors note that it is the responsibility of all healthcare professionals to ensure patients are not exposed to COVID-19, and this has meant minimizing hospital visits and person-to-person contact. For example, whenever possible, consultations take place via telephone calls or over the Internet, and nonurgent appointments that would require a patient’s physical presence at the clinic have been postponed. Visitors are also not permitted to accompany patients when admitted to the hospital or during procedures.
Standard-of-care treatment regimens have been adapted across all centers to minimize the number of hospital visits and hospitalizations and prevent “anticancer treatment-induced” complications of COVID-19.
To minimize visits and hospitalizations, strategies include converting intravenous treatments to oral or subcutaneous regimens when possible; switching from cytotoxic chemotherapy to a less-toxic approach to minimize the risk of complications requiring hospitalization; or to pause therapies when possible (stable disease reached or better). In addition, nonemergency surgeries have been postponed or replaced by radiotherapy.
To prevent anticancer treatment-induced complications of COVID-19, most centers use the paradigm that the added benefit for tumor control should be weighed against the potential risk for COVID-19–related morbidity and mortality. To prevent or reduce the risk of neutropenia and lymphopenia, for example, all centers have suggested a de-escalation of cytotoxic chemotherapy or targeted treatment strategies, or to forgo second or subsequent lines of palliative treatments if response rates from up-front therapy are low.
Some of these changes may be here to stay, noted Caldas. “One of the positive messages that comes out of this is that, clearly, care can be delivered in a safe and compassionate manner without requiring as many hospital visits as in the pre-COVID-19 era,” he said. “In the future, we will take heed of the COVID-19 experience to improve delivery of cancer care.”
Capacity of facilities
Many healthcare systems have become overwhelmed as the pandemic has intensified, thus making it necessary to prioritize. To prepare for this possibility, CCE centers have established protocols to categorize and prioritize patients for systemic treatment or surgery. While the protocols vary by center, they are comparable with one another as they prioritize on the basis of anticipated treatment outcome, the authors note.
The guidelines in CCE centers unanimously recommend that neoadjuvant therapies and curative surgeries be the top priority, for the times when operating room and/or ICU capacity is limited. As an alternative, neoadjuvant systemic treatments may be initiated or extended to postpone surgery, and other nonsurgical interventions can be considered.
In addition, some centers agree that certain elective surgeries can be safely delayed if backed by scientific evidence. As an example, an 11-week deferment of surgery may be acceptable for patients with rectal cancer after downstaging.
Cancer centers may also need to upscale and downscale quickly, depending on how the pandemic evolves, and many have already outlined scenarios to prepare for increasing or decreasing their capacity using phased approaches.
The Netherlands Cancer Institute, for example, has defined four phases of increasing severity; in Germany, capacity planning has been coordinated among 18 hospitals and the federal ministry of health, in order to prevent shortages of cancer services.
“We note that the optimal downscaling strategies depend on country- and center-specific capacities and preferences,” they write. “Therefore, it is difficult to propose a common schedule, and it will be most effective if hospitals outline their own phase-specific downscaling strategies based on the prioritization schemes and practical handles discussed above.”
Future research
Better strategies will be needed to reduce the impact of COVID-19 in cancer care, and four research priorities were identified to allow for evidence-based adjustments of cancer care protocols while the pandemic continues:
- Collect real-world data about the effects of adjustment and de-escalation of treatment regimens on outcomes
- Determine the incidence of COVID-19 in both the general population and among patients with cancer who have received systemic therapies, with large-scale serological testing
- Develop an epidemiological model that will allow estimates of the cumulative incidence of COVID-19 for a patient with cancer, within a specific time frame
- Determine COVID-19 related morbidity and mortality in patients with cancer who have been treated with systemic therapies and/or granulocyte colony-stimulating factor (G-CSF). Several projects are currently underway, such as the UK Coronavirus Cancer Monitoring Project.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Delivering cancer care during the COVID-19 pandemic has proved particularly challenging, as minimizing the risk of infection must be balanced with maintaining optimal outcomes.
Healthcare systems and oncologists have had to reorganize standard oncologic care in order to protect vulnerable patients from exposure to COVID-19 as well as deal with pandemic-related issues of equipment and staffing shortages.
A new article now describes how seven cancer centers in Europe rapidly reorganized their oncologic services and are tackling this crisis, as well as offering guidance to other institutions.
This was a major undertaking, to work out a system where patients can still get care but in a safer manner, explained coauthor Emile Voest, MD, medical director of the Netherlands Cancer Institute in Amsterdam.
“Decisions needed to be taken based on availability of personnel, protective materials, and urgencies,” he told Medscape Medical News. “Because every country had its own speed of development of the COVID pandemic, there were different scenarios in all institutions, but all with a common factor of key expertise on how to de-escalate in a safe manner.”
The article was published April 16 in Nature Medicine.
The Netherlands Cancer Institute (the Netherlands), Karolinska Institute (Sweden), Institute Gustave Roussy (France), Cambridge Cancer Center (United Kingdom), Istituto Nazionale dei Tumori di Milano (Italy), German Cancer Research Center (Germany), and Vall d’Hebron Institute of Oncology (Spain) have been working closely together in a legal entity since 2014, and have created ‘Cancer Core Europe’ (CCE). The goal is to “maximize coherence and critical mass in cancer research,” the authors note.
The consortium represents roughly 60,000 patients with newly diagnosed cancer, delivers approximately 300,000 treatment courses, and conducts about 1.2 million consultations annually, with more than 1,500 ongoing clinical trials. In a joint effort, the centers collected, translated, and compared the guidelines that had been put in place to treat patients with cancer during the COVID-19 pandemic.
Cancer treatment is multidisciplinary and involves many specialties including surgery, radiology, pathology, radiation oncology, and medical oncology. Coordinating care among disciplines is a very complex process, Voest noted.
“Changing treatment also means that you need to reconsider capacities and requirements,” he said. “Hospitals have installed crisis teams that were very good at coordinating these efforts.”
Restructuring care
Cancer care had to be reorganized on multiple levels, and the CCE centers looked at several aspects that needed to be accounted for, to ensure continuity in cancer care.
“The biggest challenge for the NHS and other healthcare systems is the surge of patients requiring oxygen and/or intensive care, and the nature and infectiousness of the virus,” said coauthor Carlos Caldas, MD, FMedSci, professor of cancer medicine at the University of Cambridge, United Kingdom. “In hospitals that are mostly run close to capacity, and where all kinds of patients are treated, this has created major resource and logistical problems.”
For regular clinical activities, the institutions with dedicated cancer centers (German Cancer Research Center, Institute Gustave Roussy, Istituto Nazionale dei Tumori di Milano, and Netherlands Cancer Institute) have attempted to stay COVID-19 free. This policy would in turn help ensure that sufficient clinical and intensive-care capacity could be reserved for critical cancer surgeries or management of treatment-related side effects, and allow hospitals outside of the CCE to transfer patients with cancer to these centers. The general hospitals can then focus on caring for patients with COVID-19, as well as other illnesses/injuries that require inpatient care.
As the CCE centers located within general hospitals (Cambridge Cancer Center, Vall d’Hebron Institute of Oncology and Karolinska Institute) have to admit patients with suspected and positive cases of COVID-19, being “COVID-19 free” was never a realistic or pursued goal.
The authors note that it is the responsibility of all healthcare professionals to ensure patients are not exposed to COVID-19, and this has meant minimizing hospital visits and person-to-person contact. For example, whenever possible, consultations take place via telephone calls or over the Internet, and nonurgent appointments that would require a patient’s physical presence at the clinic have been postponed. Visitors are also not permitted to accompany patients when admitted to the hospital or during procedures.
Standard-of-care treatment regimens have been adapted across all centers to minimize the number of hospital visits and hospitalizations and prevent “anticancer treatment-induced” complications of COVID-19.
To minimize visits and hospitalizations, strategies include converting intravenous treatments to oral or subcutaneous regimens when possible; switching from cytotoxic chemotherapy to a less-toxic approach to minimize the risk of complications requiring hospitalization; or to pause therapies when possible (stable disease reached or better). In addition, nonemergency surgeries have been postponed or replaced by radiotherapy.
To prevent anticancer treatment-induced complications of COVID-19, most centers use the paradigm that the added benefit for tumor control should be weighed against the potential risk for COVID-19–related morbidity and mortality. To prevent or reduce the risk of neutropenia and lymphopenia, for example, all centers have suggested a de-escalation of cytotoxic chemotherapy or targeted treatment strategies, or to forgo second or subsequent lines of palliative treatments if response rates from up-front therapy are low.
Some of these changes may be here to stay, noted Caldas. “One of the positive messages that comes out of this is that, clearly, care can be delivered in a safe and compassionate manner without requiring as many hospital visits as in the pre-COVID-19 era,” he said. “In the future, we will take heed of the COVID-19 experience to improve delivery of cancer care.”
Capacity of facilities
Many healthcare systems have become overwhelmed as the pandemic has intensified, thus making it necessary to prioritize. To prepare for this possibility, CCE centers have established protocols to categorize and prioritize patients for systemic treatment or surgery. While the protocols vary by center, they are comparable with one another as they prioritize on the basis of anticipated treatment outcome, the authors note.
The guidelines in CCE centers unanimously recommend that neoadjuvant therapies and curative surgeries be the top priority, for the times when operating room and/or ICU capacity is limited. As an alternative, neoadjuvant systemic treatments may be initiated or extended to postpone surgery, and other nonsurgical interventions can be considered.
In addition, some centers agree that certain elective surgeries can be safely delayed if backed by scientific evidence. As an example, an 11-week deferment of surgery may be acceptable for patients with rectal cancer after downstaging.
Cancer centers may also need to upscale and downscale quickly, depending on how the pandemic evolves, and many have already outlined scenarios to prepare for increasing or decreasing their capacity using phased approaches.
The Netherlands Cancer Institute, for example, has defined four phases of increasing severity; in Germany, capacity planning has been coordinated among 18 hospitals and the federal ministry of health, in order to prevent shortages of cancer services.
“We note that the optimal downscaling strategies depend on country- and center-specific capacities and preferences,” they write. “Therefore, it is difficult to propose a common schedule, and it will be most effective if hospitals outline their own phase-specific downscaling strategies based on the prioritization schemes and practical handles discussed above.”
Future research
Better strategies will be needed to reduce the impact of COVID-19 in cancer care, and four research priorities were identified to allow for evidence-based adjustments of cancer care protocols while the pandemic continues:
- Collect real-world data about the effects of adjustment and de-escalation of treatment regimens on outcomes
- Determine the incidence of COVID-19 in both the general population and among patients with cancer who have received systemic therapies, with large-scale serological testing
- Develop an epidemiological model that will allow estimates of the cumulative incidence of COVID-19 for a patient with cancer, within a specific time frame
- Determine COVID-19 related morbidity and mortality in patients with cancer who have been treated with systemic therapies and/or granulocyte colony-stimulating factor (G-CSF). Several projects are currently underway, such as the UK Coronavirus Cancer Monitoring Project.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
ESMO offers ‘European perspective’ on treating gynecologic cancers during the pandemic
With health care systems becoming increasingly stretched as the COVID-19 pandemic sweeps the globe, the European Society for Medical Oncology (ESMO) has produced practical recommendations for prioritizing the management of cancer patients, including those with gynecologic cancers.
ESMO’s guidelines for cervical, endometrial, and epithelial ovarian cancer delineate which patients should be prioritized for treatment in the face of reduced resources and despite the risk of SARS-CoV-2 infection.
“Many European countries have already sorted their own guidelines, either nationally or through their own societies,” said Jonathan Ledermann, MD, a professor of medical oncology at the University College London Cancer Institute who was involved in developing ESMO’s recommendations for gynecologic cancers.
Dr. Ledermann noted that the British Gynaecological Cancer Society, for example, has published guidance on COVID-19 that reflects U.K. practice.
“ESMO obviously feels a responsibility, from the European perspective, to give some guidance to their membership about the COVID-19 situation in the same way that they would put out guidelines if a new drug became available,” Dr. Ledermann said.
Prioritizing care
All of the ESMO COVID-19 guidelines group cancer patients into high-, medium-, or low-priority categories to ensure that patients who may need the most care will be seen first as hospital services become affected by the pandemic.
Those in the high-priority category are patients whose condition is either immediately life-threatening or clinically unstable or who may benefit greatly from intervention. Those in the low-priority group are patients who may be stable enough to have treatment delayed while the COVID-19 pandemic is ongoing or for whom the benefit of the intervention is low, compared with the risks of SARS-CoV-2 infection.
Those in the medium-priority group are patients whose treatment is noncritical, but for whom delaying treatment for more than 6 weeks could potentially impact the overall outcome or care of the patient.
For all gynecologic cancers covered, the guidelines stress that decisions made by the multidisciplinary team need to be documented, taking the patient’s condition into account, assessing who may be the most vulnerable, and considering the available resources.
High-priority visits
Examples of patients with cervical cancer who are a high priority for outpatient visits, according to the guidelines, include patients who have acute abdominal symptoms, renal obstruction, or complications after surgery or radiotherapy. Persistent and severe symptomatic pelvic or vaginal bleeding is another reason to be categorized as high priority for an outpatient visit, alongside anuria or symptoms of deep vein thrombosis.
New patients with histologically confirmed cervical changes should also be seen as a high priority to stage their cancer, but the guidelines stress that any blood tests and imaging should be done as close to the patient’s home as possible.
Similar recommendations are made for women with endometrial cancer, with those who have potentially unstable symptoms, severe bleeding from their tumors, and signs of venous thromboembolism or anuria being at the highest priority for outpatient visits.
Women with potentially unstable epithelial ovarian cancer – who have acute abdominal pain, intestinal obstruction, or complications after surgery – are also a high priority for an outpatient visit, as are new patients who have symptomatic ascites, pleural effusion, or intestinal obstruction.
Applying guidelines in practice
Knowing that ESMO and other organizations have carefully considered the management of cancer patients specifically in relation to COVID-19 could offer oncologists “a feeling of support and some security when they make difficult decisions,” Dr. Ledermann said.
“With all guidelines, particularly in this sort of situation, we have to be very careful in terms of their interpretation, because what fits one country may not fit another, and what fits one hospital may not necessarily fit another. So they should be taken as guidance rather than prescriptive documents,” Dr. Ledermann said.
As vice president of the European Society for Gynecologic Oncology, Dr. Ledermann noted that ESGO has taken a slightly different approach than ESMO. ESGO decided to collect and post links to existing COVID-19 resources on its website rather than create its own specific recommendations.
ESGO is also producing an expert webinar series, which has, so far, covered the management of ovarian and uterine cancers, giving clinicians the chance to learn from those who have experienced dramatic changes to their services during the COVID-19 pandemic.
Dr. Ledermann has no conflicts of interest.
With health care systems becoming increasingly stretched as the COVID-19 pandemic sweeps the globe, the European Society for Medical Oncology (ESMO) has produced practical recommendations for prioritizing the management of cancer patients, including those with gynecologic cancers.
ESMO’s guidelines for cervical, endometrial, and epithelial ovarian cancer delineate which patients should be prioritized for treatment in the face of reduced resources and despite the risk of SARS-CoV-2 infection.
“Many European countries have already sorted their own guidelines, either nationally or through their own societies,” said Jonathan Ledermann, MD, a professor of medical oncology at the University College London Cancer Institute who was involved in developing ESMO’s recommendations for gynecologic cancers.
Dr. Ledermann noted that the British Gynaecological Cancer Society, for example, has published guidance on COVID-19 that reflects U.K. practice.
“ESMO obviously feels a responsibility, from the European perspective, to give some guidance to their membership about the COVID-19 situation in the same way that they would put out guidelines if a new drug became available,” Dr. Ledermann said.
Prioritizing care
All of the ESMO COVID-19 guidelines group cancer patients into high-, medium-, or low-priority categories to ensure that patients who may need the most care will be seen first as hospital services become affected by the pandemic.
Those in the high-priority category are patients whose condition is either immediately life-threatening or clinically unstable or who may benefit greatly from intervention. Those in the low-priority group are patients who may be stable enough to have treatment delayed while the COVID-19 pandemic is ongoing or for whom the benefit of the intervention is low, compared with the risks of SARS-CoV-2 infection.
Those in the medium-priority group are patients whose treatment is noncritical, but for whom delaying treatment for more than 6 weeks could potentially impact the overall outcome or care of the patient.
For all gynecologic cancers covered, the guidelines stress that decisions made by the multidisciplinary team need to be documented, taking the patient’s condition into account, assessing who may be the most vulnerable, and considering the available resources.
High-priority visits
Examples of patients with cervical cancer who are a high priority for outpatient visits, according to the guidelines, include patients who have acute abdominal symptoms, renal obstruction, or complications after surgery or radiotherapy. Persistent and severe symptomatic pelvic or vaginal bleeding is another reason to be categorized as high priority for an outpatient visit, alongside anuria or symptoms of deep vein thrombosis.
New patients with histologically confirmed cervical changes should also be seen as a high priority to stage their cancer, but the guidelines stress that any blood tests and imaging should be done as close to the patient’s home as possible.
Similar recommendations are made for women with endometrial cancer, with those who have potentially unstable symptoms, severe bleeding from their tumors, and signs of venous thromboembolism or anuria being at the highest priority for outpatient visits.
Women with potentially unstable epithelial ovarian cancer – who have acute abdominal pain, intestinal obstruction, or complications after surgery – are also a high priority for an outpatient visit, as are new patients who have symptomatic ascites, pleural effusion, or intestinal obstruction.
Applying guidelines in practice
Knowing that ESMO and other organizations have carefully considered the management of cancer patients specifically in relation to COVID-19 could offer oncologists “a feeling of support and some security when they make difficult decisions,” Dr. Ledermann said.
“With all guidelines, particularly in this sort of situation, we have to be very careful in terms of their interpretation, because what fits one country may not fit another, and what fits one hospital may not necessarily fit another. So they should be taken as guidance rather than prescriptive documents,” Dr. Ledermann said.
As vice president of the European Society for Gynecologic Oncology, Dr. Ledermann noted that ESGO has taken a slightly different approach than ESMO. ESGO decided to collect and post links to existing COVID-19 resources on its website rather than create its own specific recommendations.
ESGO is also producing an expert webinar series, which has, so far, covered the management of ovarian and uterine cancers, giving clinicians the chance to learn from those who have experienced dramatic changes to their services during the COVID-19 pandemic.
Dr. Ledermann has no conflicts of interest.
With health care systems becoming increasingly stretched as the COVID-19 pandemic sweeps the globe, the European Society for Medical Oncology (ESMO) has produced practical recommendations for prioritizing the management of cancer patients, including those with gynecologic cancers.
ESMO’s guidelines for cervical, endometrial, and epithelial ovarian cancer delineate which patients should be prioritized for treatment in the face of reduced resources and despite the risk of SARS-CoV-2 infection.
“Many European countries have already sorted their own guidelines, either nationally or through their own societies,” said Jonathan Ledermann, MD, a professor of medical oncology at the University College London Cancer Institute who was involved in developing ESMO’s recommendations for gynecologic cancers.
Dr. Ledermann noted that the British Gynaecological Cancer Society, for example, has published guidance on COVID-19 that reflects U.K. practice.
“ESMO obviously feels a responsibility, from the European perspective, to give some guidance to their membership about the COVID-19 situation in the same way that they would put out guidelines if a new drug became available,” Dr. Ledermann said.
Prioritizing care
All of the ESMO COVID-19 guidelines group cancer patients into high-, medium-, or low-priority categories to ensure that patients who may need the most care will be seen first as hospital services become affected by the pandemic.
Those in the high-priority category are patients whose condition is either immediately life-threatening or clinically unstable or who may benefit greatly from intervention. Those in the low-priority group are patients who may be stable enough to have treatment delayed while the COVID-19 pandemic is ongoing or for whom the benefit of the intervention is low, compared with the risks of SARS-CoV-2 infection.
Those in the medium-priority group are patients whose treatment is noncritical, but for whom delaying treatment for more than 6 weeks could potentially impact the overall outcome or care of the patient.
For all gynecologic cancers covered, the guidelines stress that decisions made by the multidisciplinary team need to be documented, taking the patient’s condition into account, assessing who may be the most vulnerable, and considering the available resources.
High-priority visits
Examples of patients with cervical cancer who are a high priority for outpatient visits, according to the guidelines, include patients who have acute abdominal symptoms, renal obstruction, or complications after surgery or radiotherapy. Persistent and severe symptomatic pelvic or vaginal bleeding is another reason to be categorized as high priority for an outpatient visit, alongside anuria or symptoms of deep vein thrombosis.
New patients with histologically confirmed cervical changes should also be seen as a high priority to stage their cancer, but the guidelines stress that any blood tests and imaging should be done as close to the patient’s home as possible.
Similar recommendations are made for women with endometrial cancer, with those who have potentially unstable symptoms, severe bleeding from their tumors, and signs of venous thromboembolism or anuria being at the highest priority for outpatient visits.
Women with potentially unstable epithelial ovarian cancer – who have acute abdominal pain, intestinal obstruction, or complications after surgery – are also a high priority for an outpatient visit, as are new patients who have symptomatic ascites, pleural effusion, or intestinal obstruction.
Applying guidelines in practice
Knowing that ESMO and other organizations have carefully considered the management of cancer patients specifically in relation to COVID-19 could offer oncologists “a feeling of support and some security when they make difficult decisions,” Dr. Ledermann said.
“With all guidelines, particularly in this sort of situation, we have to be very careful in terms of their interpretation, because what fits one country may not fit another, and what fits one hospital may not necessarily fit another. So they should be taken as guidance rather than prescriptive documents,” Dr. Ledermann said.
As vice president of the European Society for Gynecologic Oncology, Dr. Ledermann noted that ESGO has taken a slightly different approach than ESMO. ESGO decided to collect and post links to existing COVID-19 resources on its website rather than create its own specific recommendations.
ESGO is also producing an expert webinar series, which has, so far, covered the management of ovarian and uterine cancers, giving clinicians the chance to learn from those who have experienced dramatic changes to their services during the COVID-19 pandemic.
Dr. Ledermann has no conflicts of interest.
Want to keep cancer patients and providers safe during the pandemic? Here’s how
according to the authors of a special feature article in the Journal of the National Comprehensive Cancer Network.
Prescreening, telemedicine, and limiting procedures top the authors’ list of 10 recommendations for ensuring patient safety in U.S. oncology practices. Assuring appropriate personal proctective equipment (PPE), encouraging telecommuting, and providing wellness/stress management are a few of the ways to look out for health care worker safety during the crisis.
These recommendations were drafted to provide guidance during the rapidly evolving global pandemic that, in some cases, has deluged health care delivery systems and strained the ability of providers to assure safe and effective care, said lead author Pelin Cinar, MD, of the Hellen Diller Family Comprehensive Cancer Center at the University of California, San Francisco.
“I think we have been so overwhelmed that sometimes it’s difficult to get organized in our thought processes,” Dr. Cinar said in an interview. “So this [article] was really trying to provide some structure to each of the different steps that we should be addressing at minimum.”
Screening patients
Prescreening systems are a critical first step to ensure cancer centers are helping control community spread of the virus, according to the article. Whether done by phone or online, prescreening 1-2 days before a patient’s visit can help identify COVID-19 symptoms and exposure history, guiding whether patients need to be evaluated, monitored, or referred to an ED.
Next, screening clinics can help ensure cancer patients with COVID-19 symptoms are evaluated and tested in a unit with dedicated staff, according to the article.
“If symptomatic patients present to the cancer center for treatment after a negative prescreening assessment, they must be provided with a mask and directed to a screening clinic for evaluation and potential testing before moving forward with any cancer-directed therapy,” the article states.
Telemedicine and treatment
Telemedicine visits should be done whenever possible to avoid in-person visits, according to the article. Dr. Cinar said that her center, like other cancer centers, has seen a major uptick in these visits, which are typically done over video. In February, there were a total of 232 video visits at her center, which jumped to 1,702 in March, or an approximate 600% increase.
“Even though we had a relatively robust presence [before the pandemic], we still weren’t at a level where we are now,” Dr. Cinar said.
When it comes to cancer treatment, surgeries and procedures should be limited to essential or urgent cases, and, if possible, chemotherapy and systemic therapy regimens can be modified to allow for fewer visits to the cancer center or infusion center, according to the article.
Transitions to outpatient care can help further reduce the need for in-person visits, while intervals between scans can be increased, or biochemical markers can be used instead of scans.
Protecting providers
Health care workers providing cancer care should be assured appropriate PPE, and websites or other centralized resources should be in place to make sure workers are aware of current PPE guidelines and changes in workflow, according to the article.
The authors note that daily screening tools or temperature checks of symptomatic workers can help decrease the risk of exposure to others. The authors also recommend establishing clear rules for when health care workers with suspected or confirmed COVID-19 should be staying at home and returning to the job.
Telecommuting should be encouraged, with limited staff participating in onsite rotations to further reduce exposure risks, the article states.
Anxiety, insomnia, and distress have been reported among frontline health care workers managing patients with COVID-19, according to the article, which recommends wellness and stress management resources be available as an “invaluable resource” in cancer centers.
“We have to take care of ourselves to be able to take care of others,” Dr. Cinar said. “With PPE, you’re physically protecting yourself, while self-care, stress management, and wellness are also a big component of protecting ourselves.”
The report by Dr. Cinar and colleagues was an invited article from the NCCN Best Practices Committee. One coauthor reported relationships with Abbvie, Adaptive Biotechnologies, Aduro, and several other companies. Dr. Cinar and the remaining authors said they had no relevant conflicts of interest.
SOURCE: Cinar P et al. J Natl Compr Canc Netw. 2020 Apr 15. doi: 10.6004/jnccn.2020.7572.
according to the authors of a special feature article in the Journal of the National Comprehensive Cancer Network.
Prescreening, telemedicine, and limiting procedures top the authors’ list of 10 recommendations for ensuring patient safety in U.S. oncology practices. Assuring appropriate personal proctective equipment (PPE), encouraging telecommuting, and providing wellness/stress management are a few of the ways to look out for health care worker safety during the crisis.
These recommendations were drafted to provide guidance during the rapidly evolving global pandemic that, in some cases, has deluged health care delivery systems and strained the ability of providers to assure safe and effective care, said lead author Pelin Cinar, MD, of the Hellen Diller Family Comprehensive Cancer Center at the University of California, San Francisco.
“I think we have been so overwhelmed that sometimes it’s difficult to get organized in our thought processes,” Dr. Cinar said in an interview. “So this [article] was really trying to provide some structure to each of the different steps that we should be addressing at minimum.”
Screening patients
Prescreening systems are a critical first step to ensure cancer centers are helping control community spread of the virus, according to the article. Whether done by phone or online, prescreening 1-2 days before a patient’s visit can help identify COVID-19 symptoms and exposure history, guiding whether patients need to be evaluated, monitored, or referred to an ED.
Next, screening clinics can help ensure cancer patients with COVID-19 symptoms are evaluated and tested in a unit with dedicated staff, according to the article.
“If symptomatic patients present to the cancer center for treatment after a negative prescreening assessment, they must be provided with a mask and directed to a screening clinic for evaluation and potential testing before moving forward with any cancer-directed therapy,” the article states.
Telemedicine and treatment
Telemedicine visits should be done whenever possible to avoid in-person visits, according to the article. Dr. Cinar said that her center, like other cancer centers, has seen a major uptick in these visits, which are typically done over video. In February, there were a total of 232 video visits at her center, which jumped to 1,702 in March, or an approximate 600% increase.
“Even though we had a relatively robust presence [before the pandemic], we still weren’t at a level where we are now,” Dr. Cinar said.
When it comes to cancer treatment, surgeries and procedures should be limited to essential or urgent cases, and, if possible, chemotherapy and systemic therapy regimens can be modified to allow for fewer visits to the cancer center or infusion center, according to the article.
Transitions to outpatient care can help further reduce the need for in-person visits, while intervals between scans can be increased, or biochemical markers can be used instead of scans.
Protecting providers
Health care workers providing cancer care should be assured appropriate PPE, and websites or other centralized resources should be in place to make sure workers are aware of current PPE guidelines and changes in workflow, according to the article.
The authors note that daily screening tools or temperature checks of symptomatic workers can help decrease the risk of exposure to others. The authors also recommend establishing clear rules for when health care workers with suspected or confirmed COVID-19 should be staying at home and returning to the job.
Telecommuting should be encouraged, with limited staff participating in onsite rotations to further reduce exposure risks, the article states.
Anxiety, insomnia, and distress have been reported among frontline health care workers managing patients with COVID-19, according to the article, which recommends wellness and stress management resources be available as an “invaluable resource” in cancer centers.
“We have to take care of ourselves to be able to take care of others,” Dr. Cinar said. “With PPE, you’re physically protecting yourself, while self-care, stress management, and wellness are also a big component of protecting ourselves.”
The report by Dr. Cinar and colleagues was an invited article from the NCCN Best Practices Committee. One coauthor reported relationships with Abbvie, Adaptive Biotechnologies, Aduro, and several other companies. Dr. Cinar and the remaining authors said they had no relevant conflicts of interest.
SOURCE: Cinar P et al. J Natl Compr Canc Netw. 2020 Apr 15. doi: 10.6004/jnccn.2020.7572.
according to the authors of a special feature article in the Journal of the National Comprehensive Cancer Network.
Prescreening, telemedicine, and limiting procedures top the authors’ list of 10 recommendations for ensuring patient safety in U.S. oncology practices. Assuring appropriate personal proctective equipment (PPE), encouraging telecommuting, and providing wellness/stress management are a few of the ways to look out for health care worker safety during the crisis.
These recommendations were drafted to provide guidance during the rapidly evolving global pandemic that, in some cases, has deluged health care delivery systems and strained the ability of providers to assure safe and effective care, said lead author Pelin Cinar, MD, of the Hellen Diller Family Comprehensive Cancer Center at the University of California, San Francisco.
“I think we have been so overwhelmed that sometimes it’s difficult to get organized in our thought processes,” Dr. Cinar said in an interview. “So this [article] was really trying to provide some structure to each of the different steps that we should be addressing at minimum.”
Screening patients
Prescreening systems are a critical first step to ensure cancer centers are helping control community spread of the virus, according to the article. Whether done by phone or online, prescreening 1-2 days before a patient’s visit can help identify COVID-19 symptoms and exposure history, guiding whether patients need to be evaluated, monitored, or referred to an ED.
Next, screening clinics can help ensure cancer patients with COVID-19 symptoms are evaluated and tested in a unit with dedicated staff, according to the article.
“If symptomatic patients present to the cancer center for treatment after a negative prescreening assessment, they must be provided with a mask and directed to a screening clinic for evaluation and potential testing before moving forward with any cancer-directed therapy,” the article states.
Telemedicine and treatment
Telemedicine visits should be done whenever possible to avoid in-person visits, according to the article. Dr. Cinar said that her center, like other cancer centers, has seen a major uptick in these visits, which are typically done over video. In February, there were a total of 232 video visits at her center, which jumped to 1,702 in March, or an approximate 600% increase.
“Even though we had a relatively robust presence [before the pandemic], we still weren’t at a level where we are now,” Dr. Cinar said.
When it comes to cancer treatment, surgeries and procedures should be limited to essential or urgent cases, and, if possible, chemotherapy and systemic therapy regimens can be modified to allow for fewer visits to the cancer center or infusion center, according to the article.
Transitions to outpatient care can help further reduce the need for in-person visits, while intervals between scans can be increased, or biochemical markers can be used instead of scans.
Protecting providers
Health care workers providing cancer care should be assured appropriate PPE, and websites or other centralized resources should be in place to make sure workers are aware of current PPE guidelines and changes in workflow, according to the article.
The authors note that daily screening tools or temperature checks of symptomatic workers can help decrease the risk of exposure to others. The authors also recommend establishing clear rules for when health care workers with suspected or confirmed COVID-19 should be staying at home and returning to the job.
Telecommuting should be encouraged, with limited staff participating in onsite rotations to further reduce exposure risks, the article states.
Anxiety, insomnia, and distress have been reported among frontline health care workers managing patients with COVID-19, according to the article, which recommends wellness and stress management resources be available as an “invaluable resource” in cancer centers.
“We have to take care of ourselves to be able to take care of others,” Dr. Cinar said. “With PPE, you’re physically protecting yourself, while self-care, stress management, and wellness are also a big component of protecting ourselves.”
The report by Dr. Cinar and colleagues was an invited article from the NCCN Best Practices Committee. One coauthor reported relationships with Abbvie, Adaptive Biotechnologies, Aduro, and several other companies. Dr. Cinar and the remaining authors said they had no relevant conflicts of interest.
SOURCE: Cinar P et al. J Natl Compr Canc Netw. 2020 Apr 15. doi: 10.6004/jnccn.2020.7572.
FROM THE JOURNAL OF THE NATIONAL COMPREHENSIVE CANCER NETWORK
Cancer patients report delays in treatment because of COVID-19
As the COVID-19 pandemic continues, many cancer patients are finding it increasingly difficult to receive the care they need and are facing financial challenges.
Half of the cancer patients and survivors who responded to a recent survey reported changes, delays, or disruptions to the care they were receiving. The survey, with 1,219 respondents, was conducted by the American Cancer Society Cancer Action Network (ACS CAN).
“The circumstances of this virus – from the fact cancer patients are at higher risk of severe complications should they be diagnosed with COVID-19, to the fact many patients are facing serious financial strain caused by the virus’ economic effect – make getting care especially difficult,” Keysha Brooks-Coley, vice president of federal advocacy for ACS CAN, told Medscape Medical News.
Nearly a quarter (24%) of survey respondents reported a delay in care or treatment. The proportion was slightly more (27%) among those currently receiving active treatment.
In addition, 12% (13% in active treatment) stated that not only was their care delayed but that they also have not been told when services would be rescheduled.
As previously reported by Medscape Medical News, many oncology groups have issued new guidelines for cancer care in reaction to the current crisis. These include recommendations to delay cancer treatment in order to avoid exposing cancer patients to the virus.
Half of those in active treatment report disruptions
The survey was initiated by ACS CAN on March 25 and was distributed over a 2-week period. The goal was to gain a better understanding of how COVID-19 was affecting cancer patients and survivors in the United States. Of the 1,219 respondents, half (51%) were cancer patients currently undergoing active treatment.
Among the patients and survivors who were currently in active treatment, 55% reported that there have been changes, delays, or disruptions in their care. The services most frequently affected included in-person provider visits (50%), supportive services (20%), and imaging procedures to monitor tumor growth (20%).
In addition, 8% reported that their treatment, including chemotherapy and immunotherapy, had been affected by the COVID-19 pandemic.
Financial concerns
Almost all of the survey respondents were covered by some type of insurance; 49% had coverage through an employer, 32% were covered by Medicare, 7% had privately purchased insurance, and 4% were covered through Medicaid.
Many cancer patients had already been having difficulty paying for their care, but for a substantial proportion of survey respondents, the COVID-19 pandemic has exacerbated the problem. More than one-third (38%) stated that COVID-19 “has had a notable impact on their financial situation that affects their ability to pay for health care.”
The most common financial problems that were related to access to care include reduced work hours (14%), reduced investment values (11%), having difficulty affording food and supplies because of staying at home to avoid contracting the virus (9%), and becoming unemployed (8%).
A reduction in work hours and job loss were of particular concern to respondents because of the possible effects these would have on their health insurance coverage. Of those who reported that they or a family member living with them had lost a job, 43% had employer-sponsored health insurance. Additionally, 58% of patients or a family member whose working hours had been reduced also had health insurance through their employer
Among the entire cohort, 28% reported that they were worried that the financial impact of COVID-19 would make it difficult to pay for the health care they need as cancer survivors. This concern was highly correlated with income. Almost half (46%) of patients who earned $30,000 or less reported that they were worried, but even in household with incomes over $110,000 per year, 21% were also concerned about the financial impact.
“Now more than ever, patients need to be able to get, keep, and afford health coverage to treat their disease,” commented Brooks-Coley.
Taking action
“ACS CAN is working every day to make clear to Congress and the administration the real and immediate challenges cancer patients and survivors face during this pandemic,” said Brooks-Coley.
With nearly 50 other professional and advocacy groups, ACS CAN has sent letters to congressional leadership and the Secretary of the Department of Health & Human Services asking them to make policy changes that would help patients.
The proposed action points include having insurers allow patients to use providers who are out of network if necessary; waiving site-specific precertification and prior authorization for cancer treatment; utilizing shared decision making between patients and providers in deciding whether to use home infusion without pressure from the insurer; allowing patients to obtain 90-day supplies of medication; increasing funding for state Medicaid programs and assistance for those who have lost employee-sponsored coverage; and improving telehealth services.
“We urge Congress and the administration to keep the needs of cancer patients and survivors in mind as they continue to address the public health crisis,” she said.
This article first appeared on Medscape.com.
As the COVID-19 pandemic continues, many cancer patients are finding it increasingly difficult to receive the care they need and are facing financial challenges.
Half of the cancer patients and survivors who responded to a recent survey reported changes, delays, or disruptions to the care they were receiving. The survey, with 1,219 respondents, was conducted by the American Cancer Society Cancer Action Network (ACS CAN).
“The circumstances of this virus – from the fact cancer patients are at higher risk of severe complications should they be diagnosed with COVID-19, to the fact many patients are facing serious financial strain caused by the virus’ economic effect – make getting care especially difficult,” Keysha Brooks-Coley, vice president of federal advocacy for ACS CAN, told Medscape Medical News.
Nearly a quarter (24%) of survey respondents reported a delay in care or treatment. The proportion was slightly more (27%) among those currently receiving active treatment.
In addition, 12% (13% in active treatment) stated that not only was their care delayed but that they also have not been told when services would be rescheduled.
As previously reported by Medscape Medical News, many oncology groups have issued new guidelines for cancer care in reaction to the current crisis. These include recommendations to delay cancer treatment in order to avoid exposing cancer patients to the virus.
Half of those in active treatment report disruptions
The survey was initiated by ACS CAN on March 25 and was distributed over a 2-week period. The goal was to gain a better understanding of how COVID-19 was affecting cancer patients and survivors in the United States. Of the 1,219 respondents, half (51%) were cancer patients currently undergoing active treatment.
Among the patients and survivors who were currently in active treatment, 55% reported that there have been changes, delays, or disruptions in their care. The services most frequently affected included in-person provider visits (50%), supportive services (20%), and imaging procedures to monitor tumor growth (20%).
In addition, 8% reported that their treatment, including chemotherapy and immunotherapy, had been affected by the COVID-19 pandemic.
Financial concerns
Almost all of the survey respondents were covered by some type of insurance; 49% had coverage through an employer, 32% were covered by Medicare, 7% had privately purchased insurance, and 4% were covered through Medicaid.
Many cancer patients had already been having difficulty paying for their care, but for a substantial proportion of survey respondents, the COVID-19 pandemic has exacerbated the problem. More than one-third (38%) stated that COVID-19 “has had a notable impact on their financial situation that affects their ability to pay for health care.”
The most common financial problems that were related to access to care include reduced work hours (14%), reduced investment values (11%), having difficulty affording food and supplies because of staying at home to avoid contracting the virus (9%), and becoming unemployed (8%).
A reduction in work hours and job loss were of particular concern to respondents because of the possible effects these would have on their health insurance coverage. Of those who reported that they or a family member living with them had lost a job, 43% had employer-sponsored health insurance. Additionally, 58% of patients or a family member whose working hours had been reduced also had health insurance through their employer
Among the entire cohort, 28% reported that they were worried that the financial impact of COVID-19 would make it difficult to pay for the health care they need as cancer survivors. This concern was highly correlated with income. Almost half (46%) of patients who earned $30,000 or less reported that they were worried, but even in household with incomes over $110,000 per year, 21% were also concerned about the financial impact.
“Now more than ever, patients need to be able to get, keep, and afford health coverage to treat their disease,” commented Brooks-Coley.
Taking action
“ACS CAN is working every day to make clear to Congress and the administration the real and immediate challenges cancer patients and survivors face during this pandemic,” said Brooks-Coley.
With nearly 50 other professional and advocacy groups, ACS CAN has sent letters to congressional leadership and the Secretary of the Department of Health & Human Services asking them to make policy changes that would help patients.
The proposed action points include having insurers allow patients to use providers who are out of network if necessary; waiving site-specific precertification and prior authorization for cancer treatment; utilizing shared decision making between patients and providers in deciding whether to use home infusion without pressure from the insurer; allowing patients to obtain 90-day supplies of medication; increasing funding for state Medicaid programs and assistance for those who have lost employee-sponsored coverage; and improving telehealth services.
“We urge Congress and the administration to keep the needs of cancer patients and survivors in mind as they continue to address the public health crisis,” she said.
This article first appeared on Medscape.com.
As the COVID-19 pandemic continues, many cancer patients are finding it increasingly difficult to receive the care they need and are facing financial challenges.
Half of the cancer patients and survivors who responded to a recent survey reported changes, delays, or disruptions to the care they were receiving. The survey, with 1,219 respondents, was conducted by the American Cancer Society Cancer Action Network (ACS CAN).
“The circumstances of this virus – from the fact cancer patients are at higher risk of severe complications should they be diagnosed with COVID-19, to the fact many patients are facing serious financial strain caused by the virus’ economic effect – make getting care especially difficult,” Keysha Brooks-Coley, vice president of federal advocacy for ACS CAN, told Medscape Medical News.
Nearly a quarter (24%) of survey respondents reported a delay in care or treatment. The proportion was slightly more (27%) among those currently receiving active treatment.
In addition, 12% (13% in active treatment) stated that not only was their care delayed but that they also have not been told when services would be rescheduled.
As previously reported by Medscape Medical News, many oncology groups have issued new guidelines for cancer care in reaction to the current crisis. These include recommendations to delay cancer treatment in order to avoid exposing cancer patients to the virus.
Half of those in active treatment report disruptions
The survey was initiated by ACS CAN on March 25 and was distributed over a 2-week period. The goal was to gain a better understanding of how COVID-19 was affecting cancer patients and survivors in the United States. Of the 1,219 respondents, half (51%) were cancer patients currently undergoing active treatment.
Among the patients and survivors who were currently in active treatment, 55% reported that there have been changes, delays, or disruptions in their care. The services most frequently affected included in-person provider visits (50%), supportive services (20%), and imaging procedures to monitor tumor growth (20%).
In addition, 8% reported that their treatment, including chemotherapy and immunotherapy, had been affected by the COVID-19 pandemic.
Financial concerns
Almost all of the survey respondents were covered by some type of insurance; 49% had coverage through an employer, 32% were covered by Medicare, 7% had privately purchased insurance, and 4% were covered through Medicaid.
Many cancer patients had already been having difficulty paying for their care, but for a substantial proportion of survey respondents, the COVID-19 pandemic has exacerbated the problem. More than one-third (38%) stated that COVID-19 “has had a notable impact on their financial situation that affects their ability to pay for health care.”
The most common financial problems that were related to access to care include reduced work hours (14%), reduced investment values (11%), having difficulty affording food and supplies because of staying at home to avoid contracting the virus (9%), and becoming unemployed (8%).
A reduction in work hours and job loss were of particular concern to respondents because of the possible effects these would have on their health insurance coverage. Of those who reported that they or a family member living with them had lost a job, 43% had employer-sponsored health insurance. Additionally, 58% of patients or a family member whose working hours had been reduced also had health insurance through their employer
Among the entire cohort, 28% reported that they were worried that the financial impact of COVID-19 would make it difficult to pay for the health care they need as cancer survivors. This concern was highly correlated with income. Almost half (46%) of patients who earned $30,000 or less reported that they were worried, but even in household with incomes over $110,000 per year, 21% were also concerned about the financial impact.
“Now more than ever, patients need to be able to get, keep, and afford health coverage to treat their disease,” commented Brooks-Coley.
Taking action
“ACS CAN is working every day to make clear to Congress and the administration the real and immediate challenges cancer patients and survivors face during this pandemic,” said Brooks-Coley.
With nearly 50 other professional and advocacy groups, ACS CAN has sent letters to congressional leadership and the Secretary of the Department of Health & Human Services asking them to make policy changes that would help patients.
The proposed action points include having insurers allow patients to use providers who are out of network if necessary; waiving site-specific precertification and prior authorization for cancer treatment; utilizing shared decision making between patients and providers in deciding whether to use home infusion without pressure from the insurer; allowing patients to obtain 90-day supplies of medication; increasing funding for state Medicaid programs and assistance for those who have lost employee-sponsored coverage; and improving telehealth services.
“We urge Congress and the administration to keep the needs of cancer patients and survivors in mind as they continue to address the public health crisis,” she said.
This article first appeared on Medscape.com.
Update confirms survival benefit with trastuzumab in uterine serous carcinoma
Adding trastuzumab to carboplatin/paclitaxel improved survival in patients with advanced or recurrent HER2/Neu-positive uterine serous carcinoma (USC), according to an updated analysis from a phase 2 trial.
At a median follow-up of 25.9 months, the median progression-free survival (PFS) was 12.9 months in patients who received trastuzumab plus carboplatin/paclitaxel and 8.0 months in patients who received only carboplatin/paclitaxel. The median overall survival (OS) was 29.6 months and 24.4 months, respectively.
Amanda Nickles Fader, MD, of Johns Hopkins Medicine, Baltimore, and colleagues reported these findings in an abstract that was slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled because of the COVID-19 pandemic.
Confirmed benefit
The phase 2 trial was designed to assess whether trastuzumab, a humanized monoclonal antibody that targets HER2/neu – a growth factor receptor found in almost all USC cases and overexpressed in 30% of cases – would improve survival in patients with USC, Dr. Nickles Fader explained in an interview. She noted that trastuzumab has been shown to provide benefit in breast cancer patients with HER2/neu overexpression.
“[U]terine serous carcinoma ... is a very aggressive high-grade endometrial cancer subtype that is associated with really poor clinical outcomes and significant mortality,” Dr. Nickles Fader said. “It represents fewer than 10% of all uterine cancer cases, but it actually accounts for a disproportionate 40% of all deaths from uterine cancer.”
The overall survival among USC patients is about 45%, compared with 91% for more common lower-grade types of uterine cancers, she added.
“The conventional treatments for uterine serous carcinoma include surgery and then chemotherapy, but we’ve only really gotten so far by using a sort of one-size-fits-all treatment philosophy,” Dr. Nickles Fader said.
Based on preliminary findings from the current trial (J Clin Oncol. 2018 Jul 10;36[20]:2044-51), trastuzumab plus carboplatin/paclitaxel is now recognized as an alternative standard in treating advanced or recurrent HER2/Neu-positive USC, and this updated analysis confirms the benefits of adding trastuzumab, she said.
PFS, OS, and toxicity
There were 58 evaluable patients with primary stage III-IV or recurrent USC who were randomized to receive six cycles of carboplatin/paclitaxel alone or in combination with intravenous trastuzumab given until toxicity or progression.
The median PFS at a median follow-up of 25.9 months “very significantly favored” the trastuzumab arm, Dr. Nickles Fader said. The median PFS was 12.9 months in the trastuzumab arm and 8.0 months in the carboplatin/paclitaxel arm (hazard ratio, 0.46; P = .005).
In the 41 patients undergoing primary treatment, the median PFS was 17.7 months in the trastuzumab arm and 9.3 months in the control arm (HR, 0.44; P = .015). In the 17 patients with recurrent disease, the median PFS was 9.2 months and 7.0 months, respectively (HR, 0.12; P = .004).
“We were very pleased to see that there was also an overall survival benefit of about 5 months in the trastuzumab arm, compared to the control arm,” Dr. Nickles Fader said. The median OS was 29.6 months and 24.4 months, respectively (HR, 0.58; P = .046).
The PFS and OS benefit was “particularly striking” in the stage III-IV patients, according to Dr. Nickles Fader and colleagues. In this subgroup, the median OS was not reached in the trastuzumab arm, and it was 25.4 months in the control arm (HR, 0.49; P = .041).
Long-term toxicity did not differ between the treatment arms.
Applications and next steps
“The take-home message here is women should be tested if they have this subtype,” Dr. Nickles Fader said. “If they’re newly diagnosed, they should be tested for the HER2/neu receptor, and if [it is overexpressed] and they have advanced disease, we do recommend treatment with not only the conventional treatment, but with trastuzumab added to that, because that’s where we saw the most benefit.”
This is the only trial that has ever shown a major PFS and OS difference with combination targeted therapy and conventional chemotherapy in USC, Dr. Nickles Fader noted.
“So it was really exciting to see that,” she said, adding that a “much larger cooperative group trial” is being designed by the National Cancer Institute and NRG Oncology Group to look at this approach in HER2-positive, advanced-stage uterine cancers. The trial will include patients with USC, but it will extend to other uterine cancer types as well.
“We’re looking at different combinations of anti-HER2 therapies to sort of validate the results of this trial, but also to study this in other tumors that are HER2 positive,” Dr. Nickles Fader explained.
She also stressed the importance of addressing racial disparities in survival among women with USC, as African American women have higher rates of USC and related mortality than do other groups.
“It’s going to be important to look at not only molecular targets and improving survival but also racial inequalities and potentially epigenetics to really improve survival across the board,” Dr. Nickles Fader said.
She reported having no disclosures. The trial was sponsored by Yale University in collaboration with Genentech.
SOURCE: Nickles Fader A et al. SGO 2020, Abstract 12.
Adding trastuzumab to carboplatin/paclitaxel improved survival in patients with advanced or recurrent HER2/Neu-positive uterine serous carcinoma (USC), according to an updated analysis from a phase 2 trial.
At a median follow-up of 25.9 months, the median progression-free survival (PFS) was 12.9 months in patients who received trastuzumab plus carboplatin/paclitaxel and 8.0 months in patients who received only carboplatin/paclitaxel. The median overall survival (OS) was 29.6 months and 24.4 months, respectively.
Amanda Nickles Fader, MD, of Johns Hopkins Medicine, Baltimore, and colleagues reported these findings in an abstract that was slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled because of the COVID-19 pandemic.
Confirmed benefit
The phase 2 trial was designed to assess whether trastuzumab, a humanized monoclonal antibody that targets HER2/neu – a growth factor receptor found in almost all USC cases and overexpressed in 30% of cases – would improve survival in patients with USC, Dr. Nickles Fader explained in an interview. She noted that trastuzumab has been shown to provide benefit in breast cancer patients with HER2/neu overexpression.
“[U]terine serous carcinoma ... is a very aggressive high-grade endometrial cancer subtype that is associated with really poor clinical outcomes and significant mortality,” Dr. Nickles Fader said. “It represents fewer than 10% of all uterine cancer cases, but it actually accounts for a disproportionate 40% of all deaths from uterine cancer.”
The overall survival among USC patients is about 45%, compared with 91% for more common lower-grade types of uterine cancers, she added.
“The conventional treatments for uterine serous carcinoma include surgery and then chemotherapy, but we’ve only really gotten so far by using a sort of one-size-fits-all treatment philosophy,” Dr. Nickles Fader said.
Based on preliminary findings from the current trial (J Clin Oncol. 2018 Jul 10;36[20]:2044-51), trastuzumab plus carboplatin/paclitaxel is now recognized as an alternative standard in treating advanced or recurrent HER2/Neu-positive USC, and this updated analysis confirms the benefits of adding trastuzumab, she said.
PFS, OS, and toxicity
There were 58 evaluable patients with primary stage III-IV or recurrent USC who were randomized to receive six cycles of carboplatin/paclitaxel alone or in combination with intravenous trastuzumab given until toxicity or progression.
The median PFS at a median follow-up of 25.9 months “very significantly favored” the trastuzumab arm, Dr. Nickles Fader said. The median PFS was 12.9 months in the trastuzumab arm and 8.0 months in the carboplatin/paclitaxel arm (hazard ratio, 0.46; P = .005).
In the 41 patients undergoing primary treatment, the median PFS was 17.7 months in the trastuzumab arm and 9.3 months in the control arm (HR, 0.44; P = .015). In the 17 patients with recurrent disease, the median PFS was 9.2 months and 7.0 months, respectively (HR, 0.12; P = .004).
“We were very pleased to see that there was also an overall survival benefit of about 5 months in the trastuzumab arm, compared to the control arm,” Dr. Nickles Fader said. The median OS was 29.6 months and 24.4 months, respectively (HR, 0.58; P = .046).
The PFS and OS benefit was “particularly striking” in the stage III-IV patients, according to Dr. Nickles Fader and colleagues. In this subgroup, the median OS was not reached in the trastuzumab arm, and it was 25.4 months in the control arm (HR, 0.49; P = .041).
Long-term toxicity did not differ between the treatment arms.
Applications and next steps
“The take-home message here is women should be tested if they have this subtype,” Dr. Nickles Fader said. “If they’re newly diagnosed, they should be tested for the HER2/neu receptor, and if [it is overexpressed] and they have advanced disease, we do recommend treatment with not only the conventional treatment, but with trastuzumab added to that, because that’s where we saw the most benefit.”
This is the only trial that has ever shown a major PFS and OS difference with combination targeted therapy and conventional chemotherapy in USC, Dr. Nickles Fader noted.
“So it was really exciting to see that,” she said, adding that a “much larger cooperative group trial” is being designed by the National Cancer Institute and NRG Oncology Group to look at this approach in HER2-positive, advanced-stage uterine cancers. The trial will include patients with USC, but it will extend to other uterine cancer types as well.
“We’re looking at different combinations of anti-HER2 therapies to sort of validate the results of this trial, but also to study this in other tumors that are HER2 positive,” Dr. Nickles Fader explained.
She also stressed the importance of addressing racial disparities in survival among women with USC, as African American women have higher rates of USC and related mortality than do other groups.
“It’s going to be important to look at not only molecular targets and improving survival but also racial inequalities and potentially epigenetics to really improve survival across the board,” Dr. Nickles Fader said.
She reported having no disclosures. The trial was sponsored by Yale University in collaboration with Genentech.
SOURCE: Nickles Fader A et al. SGO 2020, Abstract 12.
Adding trastuzumab to carboplatin/paclitaxel improved survival in patients with advanced or recurrent HER2/Neu-positive uterine serous carcinoma (USC), according to an updated analysis from a phase 2 trial.
At a median follow-up of 25.9 months, the median progression-free survival (PFS) was 12.9 months in patients who received trastuzumab plus carboplatin/paclitaxel and 8.0 months in patients who received only carboplatin/paclitaxel. The median overall survival (OS) was 29.6 months and 24.4 months, respectively.
Amanda Nickles Fader, MD, of Johns Hopkins Medicine, Baltimore, and colleagues reported these findings in an abstract that was slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled because of the COVID-19 pandemic.
Confirmed benefit
The phase 2 trial was designed to assess whether trastuzumab, a humanized monoclonal antibody that targets HER2/neu – a growth factor receptor found in almost all USC cases and overexpressed in 30% of cases – would improve survival in patients with USC, Dr. Nickles Fader explained in an interview. She noted that trastuzumab has been shown to provide benefit in breast cancer patients with HER2/neu overexpression.
“[U]terine serous carcinoma ... is a very aggressive high-grade endometrial cancer subtype that is associated with really poor clinical outcomes and significant mortality,” Dr. Nickles Fader said. “It represents fewer than 10% of all uterine cancer cases, but it actually accounts for a disproportionate 40% of all deaths from uterine cancer.”
The overall survival among USC patients is about 45%, compared with 91% for more common lower-grade types of uterine cancers, she added.
“The conventional treatments for uterine serous carcinoma include surgery and then chemotherapy, but we’ve only really gotten so far by using a sort of one-size-fits-all treatment philosophy,” Dr. Nickles Fader said.
Based on preliminary findings from the current trial (J Clin Oncol. 2018 Jul 10;36[20]:2044-51), trastuzumab plus carboplatin/paclitaxel is now recognized as an alternative standard in treating advanced or recurrent HER2/Neu-positive USC, and this updated analysis confirms the benefits of adding trastuzumab, she said.
PFS, OS, and toxicity
There were 58 evaluable patients with primary stage III-IV or recurrent USC who were randomized to receive six cycles of carboplatin/paclitaxel alone or in combination with intravenous trastuzumab given until toxicity or progression.
The median PFS at a median follow-up of 25.9 months “very significantly favored” the trastuzumab arm, Dr. Nickles Fader said. The median PFS was 12.9 months in the trastuzumab arm and 8.0 months in the carboplatin/paclitaxel arm (hazard ratio, 0.46; P = .005).
In the 41 patients undergoing primary treatment, the median PFS was 17.7 months in the trastuzumab arm and 9.3 months in the control arm (HR, 0.44; P = .015). In the 17 patients with recurrent disease, the median PFS was 9.2 months and 7.0 months, respectively (HR, 0.12; P = .004).
“We were very pleased to see that there was also an overall survival benefit of about 5 months in the trastuzumab arm, compared to the control arm,” Dr. Nickles Fader said. The median OS was 29.6 months and 24.4 months, respectively (HR, 0.58; P = .046).
The PFS and OS benefit was “particularly striking” in the stage III-IV patients, according to Dr. Nickles Fader and colleagues. In this subgroup, the median OS was not reached in the trastuzumab arm, and it was 25.4 months in the control arm (HR, 0.49; P = .041).
Long-term toxicity did not differ between the treatment arms.
Applications and next steps
“The take-home message here is women should be tested if they have this subtype,” Dr. Nickles Fader said. “If they’re newly diagnosed, they should be tested for the HER2/neu receptor, and if [it is overexpressed] and they have advanced disease, we do recommend treatment with not only the conventional treatment, but with trastuzumab added to that, because that’s where we saw the most benefit.”
This is the only trial that has ever shown a major PFS and OS difference with combination targeted therapy and conventional chemotherapy in USC, Dr. Nickles Fader noted.
“So it was really exciting to see that,” she said, adding that a “much larger cooperative group trial” is being designed by the National Cancer Institute and NRG Oncology Group to look at this approach in HER2-positive, advanced-stage uterine cancers. The trial will include patients with USC, but it will extend to other uterine cancer types as well.
“We’re looking at different combinations of anti-HER2 therapies to sort of validate the results of this trial, but also to study this in other tumors that are HER2 positive,” Dr. Nickles Fader explained.
She also stressed the importance of addressing racial disparities in survival among women with USC, as African American women have higher rates of USC and related mortality than do other groups.
“It’s going to be important to look at not only molecular targets and improving survival but also racial inequalities and potentially epigenetics to really improve survival across the board,” Dr. Nickles Fader said.
She reported having no disclosures. The trial was sponsored by Yale University in collaboration with Genentech.
SOURCE: Nickles Fader A et al. SGO 2020, Abstract 12.
FROM SGO 2020
Cancer care ‘transformed in space of a month’ because of pandemic
, the most “revolutionary” being a deep dive into telehealth, predicts Deborah Schrag, MD, MPH, a medical oncologist specializing in gastrointestinal cancers at the Dana Farber Cancer Institute in Boston, Massachusetts.
“In the space of a month, approaches and accepted norms of cancer care delivery have been transformed of necessity,” Schrag and colleagues write in an article published in JAMA on April 13.
“Most of these changes would not have occurred without the pandemic,” they add. They predict that some changes will last after the crisis is over.
“None of us want to be thrown in the deep end.... On the other hand, sometimes it works,” Schrag told Medscape Medical News.
“The in-person visit between patient and physician has been upended,” she said.
“I don’t think there’s any going back to the way it was before because cancer patients won’t stand for it,” she said. “They’re not going to drive in to get the results of a blood test.
“I think that on balance, of course, there are situations where you need eye-to-eye contact. No one wants to have an initial oncology meeting by telehealth – doctors or patients – that’s ridiculous,” she said. “But for follow-up visits, patients are now going to be more demanding, and doctors will be more willing.”
The “essential empathy” of oncologists can still “transcend the new physical barriers presented by masks and telehealth,” Schrag and colleagues comment.
“Doctors are figuring out how to deliver empathy by Zoom,” she told Medscape Medical News. “It’s not the same, but we all convey empathy to our elderly relatives over the phone.”
Pandemic impact on oncology
While the crisis has affected all of medicine – dismantling how care is delivered and forcing clinicians to make difficult decisions regarding triage – the fact that some cancers present an immediate threat to survival means that oncology “provides a lens into the major shifts currently underway in clinical care,” Schrag and colleagues write.
They illustrate the point by highlighting systemic chemotherapy, which is provided to a large proportion of patients with advanced cancer. The pandemic has tipped the risk-benefit ratio away from treatments that have a marginal effect on quality or quantity of life, they note. It has forced an “elimination of low-value treatments that were identified by the Choosing Wisely campaign,” the authors write. Up to now, the uptake of recommendations to eliminate these treatments has been slow.
“For example, for most metastatic solid tumors, chemotherapy beyond the third regimen does not improve survival for more than a few weeks; therefore, oncologists are advising supportive care instead. For patients receiving adjuvant therapy for curable cancers, delaying initiation or abbreviating the number of cycles is appropriate. Oncologists are postponing initiation of adjuvant chemotherapy for some estrogen receptor–negative stage II breast cancers by 8 weeks and administering 6 rather than 12 cycles of adjuvant chemotherapy for stage III colorectal cancers,” Schrag and colleagues write.
On the other hand, even in the epicenters of the pandemic, thus far, oncologists are still delivering cancer treatments that have the potential to cure and cannot safely be delayed, they point out. “This includes most patients with new diagnoses of acute leukemia, high-grade lymphoma, and those with chemotherapy-responsive tumors such as testicular, ovarian, and small cell lung cancer. Despite the risks, oncologists are not modifying such treatments because these cancers are likely more lethal than COVID-19.”
It’s the cancer patients who fall in between these two extremes who pose the biggest treatment challenge during this crisis – the patients for whom a delay would have “moderate clinically important adverse influence on quality of life or survival.” In these cases, oncologists are “prescribing marginally less effective regimens that have lower risk of precipitating hospitalization,” the authors note.
These treatments include the use of “white cell growth factor, more stringent neutrophil counts for proceeding with a next cycle of therapy, and omitting use of steroids to manage nausea.” In addition, where possible, oncologists are substituting oral agents for intravenous agents and “myriad other modifications to minimize visits and hospitalizations.”
Most hospitals and outpatient infusion centers now prohibit visitors from accompanying patients, and oncologists are prioritizing conversations with patients about advance directives, healthcare proxies, and end-of-life care preferences. Yet, even here, telehealth offers a new, enhanced layer to those conversations by enabling families to gather with their loved one and the doctor, she said.
This article first appeared on Medscape.com.
, the most “revolutionary” being a deep dive into telehealth, predicts Deborah Schrag, MD, MPH, a medical oncologist specializing in gastrointestinal cancers at the Dana Farber Cancer Institute in Boston, Massachusetts.
“In the space of a month, approaches and accepted norms of cancer care delivery have been transformed of necessity,” Schrag and colleagues write in an article published in JAMA on April 13.
“Most of these changes would not have occurred without the pandemic,” they add. They predict that some changes will last after the crisis is over.
“None of us want to be thrown in the deep end.... On the other hand, sometimes it works,” Schrag told Medscape Medical News.
“The in-person visit between patient and physician has been upended,” she said.
“I don’t think there’s any going back to the way it was before because cancer patients won’t stand for it,” she said. “They’re not going to drive in to get the results of a blood test.
“I think that on balance, of course, there are situations where you need eye-to-eye contact. No one wants to have an initial oncology meeting by telehealth – doctors or patients – that’s ridiculous,” she said. “But for follow-up visits, patients are now going to be more demanding, and doctors will be more willing.”
The “essential empathy” of oncologists can still “transcend the new physical barriers presented by masks and telehealth,” Schrag and colleagues comment.
“Doctors are figuring out how to deliver empathy by Zoom,” she told Medscape Medical News. “It’s not the same, but we all convey empathy to our elderly relatives over the phone.”
Pandemic impact on oncology
While the crisis has affected all of medicine – dismantling how care is delivered and forcing clinicians to make difficult decisions regarding triage – the fact that some cancers present an immediate threat to survival means that oncology “provides a lens into the major shifts currently underway in clinical care,” Schrag and colleagues write.
They illustrate the point by highlighting systemic chemotherapy, which is provided to a large proportion of patients with advanced cancer. The pandemic has tipped the risk-benefit ratio away from treatments that have a marginal effect on quality or quantity of life, they note. It has forced an “elimination of low-value treatments that were identified by the Choosing Wisely campaign,” the authors write. Up to now, the uptake of recommendations to eliminate these treatments has been slow.
“For example, for most metastatic solid tumors, chemotherapy beyond the third regimen does not improve survival for more than a few weeks; therefore, oncologists are advising supportive care instead. For patients receiving adjuvant therapy for curable cancers, delaying initiation or abbreviating the number of cycles is appropriate. Oncologists are postponing initiation of adjuvant chemotherapy for some estrogen receptor–negative stage II breast cancers by 8 weeks and administering 6 rather than 12 cycles of adjuvant chemotherapy for stage III colorectal cancers,” Schrag and colleagues write.
On the other hand, even in the epicenters of the pandemic, thus far, oncologists are still delivering cancer treatments that have the potential to cure and cannot safely be delayed, they point out. “This includes most patients with new diagnoses of acute leukemia, high-grade lymphoma, and those with chemotherapy-responsive tumors such as testicular, ovarian, and small cell lung cancer. Despite the risks, oncologists are not modifying such treatments because these cancers are likely more lethal than COVID-19.”
It’s the cancer patients who fall in between these two extremes who pose the biggest treatment challenge during this crisis – the patients for whom a delay would have “moderate clinically important adverse influence on quality of life or survival.” In these cases, oncologists are “prescribing marginally less effective regimens that have lower risk of precipitating hospitalization,” the authors note.
These treatments include the use of “white cell growth factor, more stringent neutrophil counts for proceeding with a next cycle of therapy, and omitting use of steroids to manage nausea.” In addition, where possible, oncologists are substituting oral agents for intravenous agents and “myriad other modifications to minimize visits and hospitalizations.”
Most hospitals and outpatient infusion centers now prohibit visitors from accompanying patients, and oncologists are prioritizing conversations with patients about advance directives, healthcare proxies, and end-of-life care preferences. Yet, even here, telehealth offers a new, enhanced layer to those conversations by enabling families to gather with their loved one and the doctor, she said.
This article first appeared on Medscape.com.
, the most “revolutionary” being a deep dive into telehealth, predicts Deborah Schrag, MD, MPH, a medical oncologist specializing in gastrointestinal cancers at the Dana Farber Cancer Institute in Boston, Massachusetts.
“In the space of a month, approaches and accepted norms of cancer care delivery have been transformed of necessity,” Schrag and colleagues write in an article published in JAMA on April 13.
“Most of these changes would not have occurred without the pandemic,” they add. They predict that some changes will last after the crisis is over.
“None of us want to be thrown in the deep end.... On the other hand, sometimes it works,” Schrag told Medscape Medical News.
“The in-person visit between patient and physician has been upended,” she said.
“I don’t think there’s any going back to the way it was before because cancer patients won’t stand for it,” she said. “They’re not going to drive in to get the results of a blood test.
“I think that on balance, of course, there are situations where you need eye-to-eye contact. No one wants to have an initial oncology meeting by telehealth – doctors or patients – that’s ridiculous,” she said. “But for follow-up visits, patients are now going to be more demanding, and doctors will be more willing.”
The “essential empathy” of oncologists can still “transcend the new physical barriers presented by masks and telehealth,” Schrag and colleagues comment.
“Doctors are figuring out how to deliver empathy by Zoom,” she told Medscape Medical News. “It’s not the same, but we all convey empathy to our elderly relatives over the phone.”
Pandemic impact on oncology
While the crisis has affected all of medicine – dismantling how care is delivered and forcing clinicians to make difficult decisions regarding triage – the fact that some cancers present an immediate threat to survival means that oncology “provides a lens into the major shifts currently underway in clinical care,” Schrag and colleagues write.
They illustrate the point by highlighting systemic chemotherapy, which is provided to a large proportion of patients with advanced cancer. The pandemic has tipped the risk-benefit ratio away from treatments that have a marginal effect on quality or quantity of life, they note. It has forced an “elimination of low-value treatments that were identified by the Choosing Wisely campaign,” the authors write. Up to now, the uptake of recommendations to eliminate these treatments has been slow.
“For example, for most metastatic solid tumors, chemotherapy beyond the third regimen does not improve survival for more than a few weeks; therefore, oncologists are advising supportive care instead. For patients receiving adjuvant therapy for curable cancers, delaying initiation or abbreviating the number of cycles is appropriate. Oncologists are postponing initiation of adjuvant chemotherapy for some estrogen receptor–negative stage II breast cancers by 8 weeks and administering 6 rather than 12 cycles of adjuvant chemotherapy for stage III colorectal cancers,” Schrag and colleagues write.
On the other hand, even in the epicenters of the pandemic, thus far, oncologists are still delivering cancer treatments that have the potential to cure and cannot safely be delayed, they point out. “This includes most patients with new diagnoses of acute leukemia, high-grade lymphoma, and those with chemotherapy-responsive tumors such as testicular, ovarian, and small cell lung cancer. Despite the risks, oncologists are not modifying such treatments because these cancers are likely more lethal than COVID-19.”
It’s the cancer patients who fall in between these two extremes who pose the biggest treatment challenge during this crisis – the patients for whom a delay would have “moderate clinically important adverse influence on quality of life or survival.” In these cases, oncologists are “prescribing marginally less effective regimens that have lower risk of precipitating hospitalization,” the authors note.
These treatments include the use of “white cell growth factor, more stringent neutrophil counts for proceeding with a next cycle of therapy, and omitting use of steroids to manage nausea.” In addition, where possible, oncologists are substituting oral agents for intravenous agents and “myriad other modifications to minimize visits and hospitalizations.”
Most hospitals and outpatient infusion centers now prohibit visitors from accompanying patients, and oncologists are prioritizing conversations with patients about advance directives, healthcare proxies, and end-of-life care preferences. Yet, even here, telehealth offers a new, enhanced layer to those conversations by enabling families to gather with their loved one and the doctor, she said.
This article first appeared on Medscape.com.