User login
Complete resection tied to improved survival in low-grade serous ovarian cancer
NATIONAL HARBOR, MD. – Surgical resection to the point of no residual macroscopic disease significantly improved survival among patients with low-grade serous ovarian carcinoma, based on the findings of a large multicenter retrospective cohort study.
Adjuvant platinum-based therapy, however, did not appear to boost survival in the analysis, Tamayaa May, MD, MSc, said at the annual meeting of the Society of Gynecologic Oncology. “Genotyping and targeted sequencing of low-grade serous ovarian carcinoma often identifies actionable mutations, and treatment with MEK-based combination therapy might be a viable therapeutic strategy in patients with KRAS or NRAS mutations,” added Dr. May of Princess Margaret Cancer Center at the University of Toronto. She and her associates plan to examine more subgroups to determine if genomic alterations predict systemic response, she said.
Low-grade (Silverberg grade 1) serous ovarian tumors are slow growing but tend to resist chemotherapy, making optimal debulking a crucial part of treatment. In past studies, debulking that eliminated macroscopic evidence of disease was associated with a median survival time of about 115 months, compared with about 43 months if patients had residual disease, Dr. May noted.
To further explore outcomes after surgical resection, and to help clarify the role of systemic platinum-based therapy in low-grade serous ovarian carcinoma, she and her associates analyzed clinical data from 714 patients with low-grade serous ovarian carcinomas, including 40 from her institution and 674 from the Ovarian Cancer Association Consortium Registry. Most (60%) patients had stage III disease at diagnosis.
Complete data on surgical outcomes were available for 382 patients, of whom 202 (53%) had residual macroscopic disease and 43% did not. Among 439 patients with complete treatment data, 170 (39%) received first-line platinum-based chemotherapy. For the 391 patients with complete data on progression-free survival (PFS), the median follow-up was 4.9 years and median PFS was 3.1 years (95% confidence interval, 2.6-4.5 years). Residual macroscopic disease correlated with shorter PFS (P less than .001), as did higher tumor stage and baseline CA125 (P less than .001), but platinum-based therapy did not (P = .1).
A multivariable analysis of 333 patients confirmed these findings, Dr. May said. Independent correlates of death or disease progression included residual macroscopic disease (hazard ratio, 2.38; 95% CI, 1.68-3.37; P less than .001), older age (HR, 1.15; 95% CI, 1.02-1.30; P = .02), and stage III (HR, 3.28; 95% CI, 1.87-5.76; P less than .001) or stage IV disease (HR, 5.68; 95% CI, 2.73-11.83; P less than .001), compared with stage I disease. In contrast, platinum-based therapy did not correlate with survival (HR, 0.94; 95% CI, 0.69-1.28; P = .69).
The overall survival analysis also linked mortality with higher tumor stage, increased baseline CA125, and residual disease (P less than .001 for each association), but not with platinum-based therapy (P = .2). The multivariable analysis independently tied mortality to older age (HR, 1.25; P less than .001), stage III (HR, 2.31; P = .006) or IV disease (HR, 3.86; P less than .001), and residual disease (HR, 2.53; P less than .001), but not to platinum-based therapy (HR, 1.05; P = .77).
Data consistency and completeness were issues in this study: most notably, 45% of patients had no PFS data, Dr. May commented. Nonetheless, this type of large retrospective multicenter analysis is one of the best ways to study rare tumors, including low-grade serous ovarian carcinoma, she said.
The Ovarian Cancer Research Fund provides financial support for OCAC. Dr. May reported having no conflicts of interest.
NATIONAL HARBOR, MD. – Surgical resection to the point of no residual macroscopic disease significantly improved survival among patients with low-grade serous ovarian carcinoma, based on the findings of a large multicenter retrospective cohort study.
Adjuvant platinum-based therapy, however, did not appear to boost survival in the analysis, Tamayaa May, MD, MSc, said at the annual meeting of the Society of Gynecologic Oncology. “Genotyping and targeted sequencing of low-grade serous ovarian carcinoma often identifies actionable mutations, and treatment with MEK-based combination therapy might be a viable therapeutic strategy in patients with KRAS or NRAS mutations,” added Dr. May of Princess Margaret Cancer Center at the University of Toronto. She and her associates plan to examine more subgroups to determine if genomic alterations predict systemic response, she said.
Low-grade (Silverberg grade 1) serous ovarian tumors are slow growing but tend to resist chemotherapy, making optimal debulking a crucial part of treatment. In past studies, debulking that eliminated macroscopic evidence of disease was associated with a median survival time of about 115 months, compared with about 43 months if patients had residual disease, Dr. May noted.
To further explore outcomes after surgical resection, and to help clarify the role of systemic platinum-based therapy in low-grade serous ovarian carcinoma, she and her associates analyzed clinical data from 714 patients with low-grade serous ovarian carcinomas, including 40 from her institution and 674 from the Ovarian Cancer Association Consortium Registry. Most (60%) patients had stage III disease at diagnosis.
Complete data on surgical outcomes were available for 382 patients, of whom 202 (53%) had residual macroscopic disease and 43% did not. Among 439 patients with complete treatment data, 170 (39%) received first-line platinum-based chemotherapy. For the 391 patients with complete data on progression-free survival (PFS), the median follow-up was 4.9 years and median PFS was 3.1 years (95% confidence interval, 2.6-4.5 years). Residual macroscopic disease correlated with shorter PFS (P less than .001), as did higher tumor stage and baseline CA125 (P less than .001), but platinum-based therapy did not (P = .1).
A multivariable analysis of 333 patients confirmed these findings, Dr. May said. Independent correlates of death or disease progression included residual macroscopic disease (hazard ratio, 2.38; 95% CI, 1.68-3.37; P less than .001), older age (HR, 1.15; 95% CI, 1.02-1.30; P = .02), and stage III (HR, 3.28; 95% CI, 1.87-5.76; P less than .001) or stage IV disease (HR, 5.68; 95% CI, 2.73-11.83; P less than .001), compared with stage I disease. In contrast, platinum-based therapy did not correlate with survival (HR, 0.94; 95% CI, 0.69-1.28; P = .69).
The overall survival analysis also linked mortality with higher tumor stage, increased baseline CA125, and residual disease (P less than .001 for each association), but not with platinum-based therapy (P = .2). The multivariable analysis independently tied mortality to older age (HR, 1.25; P less than .001), stage III (HR, 2.31; P = .006) or IV disease (HR, 3.86; P less than .001), and residual disease (HR, 2.53; P less than .001), but not to platinum-based therapy (HR, 1.05; P = .77).
Data consistency and completeness were issues in this study: most notably, 45% of patients had no PFS data, Dr. May commented. Nonetheless, this type of large retrospective multicenter analysis is one of the best ways to study rare tumors, including low-grade serous ovarian carcinoma, she said.
The Ovarian Cancer Research Fund provides financial support for OCAC. Dr. May reported having no conflicts of interest.
NATIONAL HARBOR, MD. – Surgical resection to the point of no residual macroscopic disease significantly improved survival among patients with low-grade serous ovarian carcinoma, based on the findings of a large multicenter retrospective cohort study.
Adjuvant platinum-based therapy, however, did not appear to boost survival in the analysis, Tamayaa May, MD, MSc, said at the annual meeting of the Society of Gynecologic Oncology. “Genotyping and targeted sequencing of low-grade serous ovarian carcinoma often identifies actionable mutations, and treatment with MEK-based combination therapy might be a viable therapeutic strategy in patients with KRAS or NRAS mutations,” added Dr. May of Princess Margaret Cancer Center at the University of Toronto. She and her associates plan to examine more subgroups to determine if genomic alterations predict systemic response, she said.
Low-grade (Silverberg grade 1) serous ovarian tumors are slow growing but tend to resist chemotherapy, making optimal debulking a crucial part of treatment. In past studies, debulking that eliminated macroscopic evidence of disease was associated with a median survival time of about 115 months, compared with about 43 months if patients had residual disease, Dr. May noted.
To further explore outcomes after surgical resection, and to help clarify the role of systemic platinum-based therapy in low-grade serous ovarian carcinoma, she and her associates analyzed clinical data from 714 patients with low-grade serous ovarian carcinomas, including 40 from her institution and 674 from the Ovarian Cancer Association Consortium Registry. Most (60%) patients had stage III disease at diagnosis.
Complete data on surgical outcomes were available for 382 patients, of whom 202 (53%) had residual macroscopic disease and 43% did not. Among 439 patients with complete treatment data, 170 (39%) received first-line platinum-based chemotherapy. For the 391 patients with complete data on progression-free survival (PFS), the median follow-up was 4.9 years and median PFS was 3.1 years (95% confidence interval, 2.6-4.5 years). Residual macroscopic disease correlated with shorter PFS (P less than .001), as did higher tumor stage and baseline CA125 (P less than .001), but platinum-based therapy did not (P = .1).
A multivariable analysis of 333 patients confirmed these findings, Dr. May said. Independent correlates of death or disease progression included residual macroscopic disease (hazard ratio, 2.38; 95% CI, 1.68-3.37; P less than .001), older age (HR, 1.15; 95% CI, 1.02-1.30; P = .02), and stage III (HR, 3.28; 95% CI, 1.87-5.76; P less than .001) or stage IV disease (HR, 5.68; 95% CI, 2.73-11.83; P less than .001), compared with stage I disease. In contrast, platinum-based therapy did not correlate with survival (HR, 0.94; 95% CI, 0.69-1.28; P = .69).
The overall survival analysis also linked mortality with higher tumor stage, increased baseline CA125, and residual disease (P less than .001 for each association), but not with platinum-based therapy (P = .2). The multivariable analysis independently tied mortality to older age (HR, 1.25; P less than .001), stage III (HR, 2.31; P = .006) or IV disease (HR, 3.86; P less than .001), and residual disease (HR, 2.53; P less than .001), but not to platinum-based therapy (HR, 1.05; P = .77).
Data consistency and completeness were issues in this study: most notably, 45% of patients had no PFS data, Dr. May commented. Nonetheless, this type of large retrospective multicenter analysis is one of the best ways to study rare tumors, including low-grade serous ovarian carcinoma, she said.
The Ovarian Cancer Research Fund provides financial support for OCAC. Dr. May reported having no conflicts of interest.
AT THE ANNUAL MEETING ON WOMEN’S CANCER
Key clinical point: Unlike platinum-based chemotherapy, resection to the point of no residual disease was associated with improved survival among patients with low-grade serous ovarian carcinoma.
Major finding: Independent correlates of death or disease progression included residual macroscopic disease (hazard ratio, 2.38; P less than .001), older age (HR, 1.15; P = .02), and stage III (HR, 3.28; P less than .001) or stage IV (HR, 5.68; P less than .001) disease, compared with stage I disease. Platinum-based therapy was not associated with improved survival (HR, 0.94; P = 69).
Data source: A retrospective cohort study of 714 patients with low-grade (Silverberg grade 1) serous tumors from the Ovarian Cancer Association Consortium Registry.
Disclosures: The Ovarian Cancer Research Fund provides financial support for OCAC. Dr. May reported having no conflicts of interest.
VIDEO: Pain and impaired QOL persist after open endometrial cancer surgery
NATIONAL HARBOR, MD. – Patient-reported outcomes from a prospective cohort study of minimally invasive versus open surgery for women with endometrial cancer showed that the disability from open surgery persisted for longer than had previously been recognized. Further, for a subset of patients, impairment in sexual functioning was significant, and persistent, regardless of the type of surgery.
At 3 weeks after surgery, patients who had open surgery had greater pain as measured by the Brief Pain Inventory (minimally important difference greater than 1, P = .0004). By 3 months post surgery, responses on the Functional Assessment of Cancer Therapy–General were still significantly lower for the open-surgery group, compared with the minimally invasive group (P = .0011).
Although patients’ pain and overall state of health were better at 3 weeks post surgery, regardless of whether women had open, laparoscopic, or robotic surgery, the reduced overall quality of life experienced by patients who had open surgery persisted.
“What was a bit different from other studies … is that we found that this is maintained even at 3 months, and it was clinically and statistically different,” Sarah Ferguson, MD, said in a video interview at the annual meeting of the Society of Gynecologic Oncology. “So that was really, I think, an interesting finding, that this doesn’t just impact the very short term. Three months is a fairly long time after a primary surgery, and [it’s] important for women to know this.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Patients in the eight-center study had histologically confirmed clinical stage I or II endometrial cancer. The open-surgery arm of the study involved 106 patients, and 414 had minimally invasive surgery (168 laparascopic, 246 robotic).
The robotic and laparoscopic arms showed no statistically significant differences for any patient-reported outcome, even after adjusting for potentially confounding variables, said Dr. Ferguson of Princess Margaret Cancer Centre at the University of Toronto. Accordingly, investigators compared both minimally invasive arms grouped together against open surgery.
Overall, about 80% of patients completed the quality-of-life questionnaires. The response rate for the sexual-functioning questionnaires, however, was much lower, ranging from about a quarter to a half of the participants.
When Dr. Ferguson and her colleagues examined the characteristics of the patients who did complete the sexual-functioning questionnaires, they found that these women were more likely to be younger, partnered, premenopausal and sexually active at the time of diagnosis. Both of the surgical groups “met the clinical cutoff for sexual dysfunction” on the Female Sexual Function Index questionnaire, she said.
For the sexual function questionnaires, differences between the open and minimally invasive groups were not significant at any time point throughout the 26 weeks that patients were studied. “Though it’s a small population, I think these results are important,” said Dr. Ferguson. “These variables may be helpful for us to target patients in our practice, or in future studies, who require intervention.”
Though the study was not randomized, Dr. Ferguson said that the baseline characteristics were similar between groups, and the investigators’ intention-to-treat analysis used a statistical model that adjusted for many potential confounding variables.
Dr. Ferguson reported having no conflicts of interest.
[email protected]
On Twitter @karioakes
NATIONAL HARBOR, MD. – Patient-reported outcomes from a prospective cohort study of minimally invasive versus open surgery for women with endometrial cancer showed that the disability from open surgery persisted for longer than had previously been recognized. Further, for a subset of patients, impairment in sexual functioning was significant, and persistent, regardless of the type of surgery.
At 3 weeks after surgery, patients who had open surgery had greater pain as measured by the Brief Pain Inventory (minimally important difference greater than 1, P = .0004). By 3 months post surgery, responses on the Functional Assessment of Cancer Therapy–General were still significantly lower for the open-surgery group, compared with the minimally invasive group (P = .0011).
Although patients’ pain and overall state of health were better at 3 weeks post surgery, regardless of whether women had open, laparoscopic, or robotic surgery, the reduced overall quality of life experienced by patients who had open surgery persisted.
“What was a bit different from other studies … is that we found that this is maintained even at 3 months, and it was clinically and statistically different,” Sarah Ferguson, MD, said in a video interview at the annual meeting of the Society of Gynecologic Oncology. “So that was really, I think, an interesting finding, that this doesn’t just impact the very short term. Three months is a fairly long time after a primary surgery, and [it’s] important for women to know this.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Patients in the eight-center study had histologically confirmed clinical stage I or II endometrial cancer. The open-surgery arm of the study involved 106 patients, and 414 had minimally invasive surgery (168 laparascopic, 246 robotic).
The robotic and laparoscopic arms showed no statistically significant differences for any patient-reported outcome, even after adjusting for potentially confounding variables, said Dr. Ferguson of Princess Margaret Cancer Centre at the University of Toronto. Accordingly, investigators compared both minimally invasive arms grouped together against open surgery.
Overall, about 80% of patients completed the quality-of-life questionnaires. The response rate for the sexual-functioning questionnaires, however, was much lower, ranging from about a quarter to a half of the participants.
When Dr. Ferguson and her colleagues examined the characteristics of the patients who did complete the sexual-functioning questionnaires, they found that these women were more likely to be younger, partnered, premenopausal and sexually active at the time of diagnosis. Both of the surgical groups “met the clinical cutoff for sexual dysfunction” on the Female Sexual Function Index questionnaire, she said.
For the sexual function questionnaires, differences between the open and minimally invasive groups were not significant at any time point throughout the 26 weeks that patients were studied. “Though it’s a small population, I think these results are important,” said Dr. Ferguson. “These variables may be helpful for us to target patients in our practice, or in future studies, who require intervention.”
Though the study was not randomized, Dr. Ferguson said that the baseline characteristics were similar between groups, and the investigators’ intention-to-treat analysis used a statistical model that adjusted for many potential confounding variables.
Dr. Ferguson reported having no conflicts of interest.
[email protected]
On Twitter @karioakes
NATIONAL HARBOR, MD. – Patient-reported outcomes from a prospective cohort study of minimally invasive versus open surgery for women with endometrial cancer showed that the disability from open surgery persisted for longer than had previously been recognized. Further, for a subset of patients, impairment in sexual functioning was significant, and persistent, regardless of the type of surgery.
At 3 weeks after surgery, patients who had open surgery had greater pain as measured by the Brief Pain Inventory (minimally important difference greater than 1, P = .0004). By 3 months post surgery, responses on the Functional Assessment of Cancer Therapy–General were still significantly lower for the open-surgery group, compared with the minimally invasive group (P = .0011).
Although patients’ pain and overall state of health were better at 3 weeks post surgery, regardless of whether women had open, laparoscopic, or robotic surgery, the reduced overall quality of life experienced by patients who had open surgery persisted.
“What was a bit different from other studies … is that we found that this is maintained even at 3 months, and it was clinically and statistically different,” Sarah Ferguson, MD, said in a video interview at the annual meeting of the Society of Gynecologic Oncology. “So that was really, I think, an interesting finding, that this doesn’t just impact the very short term. Three months is a fairly long time after a primary surgery, and [it’s] important for women to know this.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Patients in the eight-center study had histologically confirmed clinical stage I or II endometrial cancer. The open-surgery arm of the study involved 106 patients, and 414 had minimally invasive surgery (168 laparascopic, 246 robotic).
The robotic and laparoscopic arms showed no statistically significant differences for any patient-reported outcome, even after adjusting for potentially confounding variables, said Dr. Ferguson of Princess Margaret Cancer Centre at the University of Toronto. Accordingly, investigators compared both minimally invasive arms grouped together against open surgery.
Overall, about 80% of patients completed the quality-of-life questionnaires. The response rate for the sexual-functioning questionnaires, however, was much lower, ranging from about a quarter to a half of the participants.
When Dr. Ferguson and her colleagues examined the characteristics of the patients who did complete the sexual-functioning questionnaires, they found that these women were more likely to be younger, partnered, premenopausal and sexually active at the time of diagnosis. Both of the surgical groups “met the clinical cutoff for sexual dysfunction” on the Female Sexual Function Index questionnaire, she said.
For the sexual function questionnaires, differences between the open and minimally invasive groups were not significant at any time point throughout the 26 weeks that patients were studied. “Though it’s a small population, I think these results are important,” said Dr. Ferguson. “These variables may be helpful for us to target patients in our practice, or in future studies, who require intervention.”
Though the study was not randomized, Dr. Ferguson said that the baseline characteristics were similar between groups, and the investigators’ intention-to-treat analysis used a statistical model that adjusted for many potential confounding variables.
Dr. Ferguson reported having no conflicts of interest.
[email protected]
On Twitter @karioakes
AT THE ANNUAL MEETING ON WOMEN'S CANCER
Isolated tumor cells did not predict progression in endometrial cancer
NATIONAL HARBOR, MD. – Patients with endometrial cancer should not receive adjuvant chemotherapy or radiotherapy solely because they have isolated tumor cells in their sentinel lymph nodes, Marie Plante, MD, said during an oral presentation at the annual meeting of the Society of Gynecologic Oncology.
In a single-center prospective cohort study, about 96% of patients with endometrial cancer were alive and progression free at 3 years, a rate which resembles those reported for node-negative patients, said Dr. Plante of Laval University, Quebec City. Moreover, all 10 patients who did not receive adjuvant therapy remained alive and progression free during follow-up, she said. “Patients with isolated tumor cells carry an excellent prognosis,” she added. “Adjuvant treatment should be tailored based on uterine factors and histology and not solely on the presence of isolated tumor cells in sentinel lymph nodes.”
Pathologic ultrastaging has boosted the detection of low-volume metastases, which comprise anywhere from 35% to 63% of nodal metastases in patients with endometrial cancer. Clinicians continue to debate management when this low-volume disease consists of isolated tumor cells (ITC), defined as fewer than 200 carcinoma cells found singly or in small clusters. Finding ITC in endometrial cancer is uncommon, and few studies have examined this subgroup, Dr. Plante noted.
She and her associates evaluated 519 patients who underwent hysterectomy, salpingo-oophorectomy, lymphadenectomy, or sentinel lymph node mapping for endometrial cancer at their center between 2010 and 2015. Pathologic ultrastaging identified 31 patients with ITC (6%), of whom 11 patients received adjuvant chemotherapy, 14 received pelvic radiation therapy, and 10 underwent only brachytherapy or observation, with some patients receiving more than one treatment. Another 54 patients in the cohort had metastatic disease, including 43 patients with macrometastasis and 11 with micrometastasis.
Stage, not treatment, predicted progression-free survival (PFS), Dr. Plante emphasized. After a median follow-up period of 29 months, the estimated 3-year rate of PFS was significantly better among patients with ITC (96%), node-negative disease (88%), or micrometastasis (86%) than among those with macrometastasis (59%; P = .001), even though macrometastasis patients received significantly more chemotherapy (P = .0001).
Rates of PFS did not statistically differ between the ITC and node-negative groups, Dr. Plante noted. The single recurrence in an ITC patient involved a 7 cm carcinosarcoma that recurred despite adjuvant chemotherapy and radiation therapy. There were no recurrences among patients with endometrioid histology.
Among ITC patients who received no adjuvant treatment, half had stage IA endometrial cancer and half were stage IB, half were grade 1 and half were grade 2, all had endometrioid histology, and seven (70%) had evidence of lymphovascular space invasion, Dr. Plante said. All remained alive and progression free at follow-up.
Ultrastaging should only be performed if a sentinel lymph node is negative on initial hematoxylin and eosin stain and if there is myoinvasion, commented Nadeem R. Abu-Rustum, MD, chief of the gynecology service at Memorial Sloan Kettering Cancer Center, New York, who was not involved in the study. “Ultrastaging increases positive-node detection by about 8%,” he said during the scientific plenary session at the conference. “Finding positive nodes can change management, and we have to be careful not to overtreat.”
Ongoing research is examining the topography and anatomic location of ITC in sentinel lymph nodes, Dr. Abu-Rustum said. In the meantime, he advised clinicians to consider any ultrastaging result of ITC in context. “When modeling the risk of ITCs, don’t look at them in isolation. Don’t be ‘node-centric,’ ” he advised. “Look at the uterine factors and the overall bigger picture.”
Dr. Plante did not acknowledge external funding sources. Dr. Plante and Dr. Abu-Rustum reported having no conflicts of interest.
NATIONAL HARBOR, MD. – Patients with endometrial cancer should not receive adjuvant chemotherapy or radiotherapy solely because they have isolated tumor cells in their sentinel lymph nodes, Marie Plante, MD, said during an oral presentation at the annual meeting of the Society of Gynecologic Oncology.
In a single-center prospective cohort study, about 96% of patients with endometrial cancer were alive and progression free at 3 years, a rate which resembles those reported for node-negative patients, said Dr. Plante of Laval University, Quebec City. Moreover, all 10 patients who did not receive adjuvant therapy remained alive and progression free during follow-up, she said. “Patients with isolated tumor cells carry an excellent prognosis,” she added. “Adjuvant treatment should be tailored based on uterine factors and histology and not solely on the presence of isolated tumor cells in sentinel lymph nodes.”
Pathologic ultrastaging has boosted the detection of low-volume metastases, which comprise anywhere from 35% to 63% of nodal metastases in patients with endometrial cancer. Clinicians continue to debate management when this low-volume disease consists of isolated tumor cells (ITC), defined as fewer than 200 carcinoma cells found singly or in small clusters. Finding ITC in endometrial cancer is uncommon, and few studies have examined this subgroup, Dr. Plante noted.
She and her associates evaluated 519 patients who underwent hysterectomy, salpingo-oophorectomy, lymphadenectomy, or sentinel lymph node mapping for endometrial cancer at their center between 2010 and 2015. Pathologic ultrastaging identified 31 patients with ITC (6%), of whom 11 patients received adjuvant chemotherapy, 14 received pelvic radiation therapy, and 10 underwent only brachytherapy or observation, with some patients receiving more than one treatment. Another 54 patients in the cohort had metastatic disease, including 43 patients with macrometastasis and 11 with micrometastasis.
Stage, not treatment, predicted progression-free survival (PFS), Dr. Plante emphasized. After a median follow-up period of 29 months, the estimated 3-year rate of PFS was significantly better among patients with ITC (96%), node-negative disease (88%), or micrometastasis (86%) than among those with macrometastasis (59%; P = .001), even though macrometastasis patients received significantly more chemotherapy (P = .0001).
Rates of PFS did not statistically differ between the ITC and node-negative groups, Dr. Plante noted. The single recurrence in an ITC patient involved a 7 cm carcinosarcoma that recurred despite adjuvant chemotherapy and radiation therapy. There were no recurrences among patients with endometrioid histology.
Among ITC patients who received no adjuvant treatment, half had stage IA endometrial cancer and half were stage IB, half were grade 1 and half were grade 2, all had endometrioid histology, and seven (70%) had evidence of lymphovascular space invasion, Dr. Plante said. All remained alive and progression free at follow-up.
Ultrastaging should only be performed if a sentinel lymph node is negative on initial hematoxylin and eosin stain and if there is myoinvasion, commented Nadeem R. Abu-Rustum, MD, chief of the gynecology service at Memorial Sloan Kettering Cancer Center, New York, who was not involved in the study. “Ultrastaging increases positive-node detection by about 8%,” he said during the scientific plenary session at the conference. “Finding positive nodes can change management, and we have to be careful not to overtreat.”
Ongoing research is examining the topography and anatomic location of ITC in sentinel lymph nodes, Dr. Abu-Rustum said. In the meantime, he advised clinicians to consider any ultrastaging result of ITC in context. “When modeling the risk of ITCs, don’t look at them in isolation. Don’t be ‘node-centric,’ ” he advised. “Look at the uterine factors and the overall bigger picture.”
Dr. Plante did not acknowledge external funding sources. Dr. Plante and Dr. Abu-Rustum reported having no conflicts of interest.
NATIONAL HARBOR, MD. – Patients with endometrial cancer should not receive adjuvant chemotherapy or radiotherapy solely because they have isolated tumor cells in their sentinel lymph nodes, Marie Plante, MD, said during an oral presentation at the annual meeting of the Society of Gynecologic Oncology.
In a single-center prospective cohort study, about 96% of patients with endometrial cancer were alive and progression free at 3 years, a rate which resembles those reported for node-negative patients, said Dr. Plante of Laval University, Quebec City. Moreover, all 10 patients who did not receive adjuvant therapy remained alive and progression free during follow-up, she said. “Patients with isolated tumor cells carry an excellent prognosis,” she added. “Adjuvant treatment should be tailored based on uterine factors and histology and not solely on the presence of isolated tumor cells in sentinel lymph nodes.”
Pathologic ultrastaging has boosted the detection of low-volume metastases, which comprise anywhere from 35% to 63% of nodal metastases in patients with endometrial cancer. Clinicians continue to debate management when this low-volume disease consists of isolated tumor cells (ITC), defined as fewer than 200 carcinoma cells found singly or in small clusters. Finding ITC in endometrial cancer is uncommon, and few studies have examined this subgroup, Dr. Plante noted.
She and her associates evaluated 519 patients who underwent hysterectomy, salpingo-oophorectomy, lymphadenectomy, or sentinel lymph node mapping for endometrial cancer at their center between 2010 and 2015. Pathologic ultrastaging identified 31 patients with ITC (6%), of whom 11 patients received adjuvant chemotherapy, 14 received pelvic radiation therapy, and 10 underwent only brachytherapy or observation, with some patients receiving more than one treatment. Another 54 patients in the cohort had metastatic disease, including 43 patients with macrometastasis and 11 with micrometastasis.
Stage, not treatment, predicted progression-free survival (PFS), Dr. Plante emphasized. After a median follow-up period of 29 months, the estimated 3-year rate of PFS was significantly better among patients with ITC (96%), node-negative disease (88%), or micrometastasis (86%) than among those with macrometastasis (59%; P = .001), even though macrometastasis patients received significantly more chemotherapy (P = .0001).
Rates of PFS did not statistically differ between the ITC and node-negative groups, Dr. Plante noted. The single recurrence in an ITC patient involved a 7 cm carcinosarcoma that recurred despite adjuvant chemotherapy and radiation therapy. There were no recurrences among patients with endometrioid histology.
Among ITC patients who received no adjuvant treatment, half had stage IA endometrial cancer and half were stage IB, half were grade 1 and half were grade 2, all had endometrioid histology, and seven (70%) had evidence of lymphovascular space invasion, Dr. Plante said. All remained alive and progression free at follow-up.
Ultrastaging should only be performed if a sentinel lymph node is negative on initial hematoxylin and eosin stain and if there is myoinvasion, commented Nadeem R. Abu-Rustum, MD, chief of the gynecology service at Memorial Sloan Kettering Cancer Center, New York, who was not involved in the study. “Ultrastaging increases positive-node detection by about 8%,” he said during the scientific plenary session at the conference. “Finding positive nodes can change management, and we have to be careful not to overtreat.”
Ongoing research is examining the topography and anatomic location of ITC in sentinel lymph nodes, Dr. Abu-Rustum said. In the meantime, he advised clinicians to consider any ultrastaging result of ITC in context. “When modeling the risk of ITCs, don’t look at them in isolation. Don’t be ‘node-centric,’ ” he advised. “Look at the uterine factors and the overall bigger picture.”
Dr. Plante did not acknowledge external funding sources. Dr. Plante and Dr. Abu-Rustum reported having no conflicts of interest.
AT THE ANNUAL MEETING ON WOMEN’S CANCER
Key clinical point: For patients with endometrial cancer, isolated tumor cells in sentinel lymph nodes did not lead to disease progression and were not an indication for adjuvant treatments.
Major finding: At 3 years, the estimated rate of progression-free survival was 100% among patients who underwent only pelvic brachytherapy or observation.
Data source: A single-center prospective study of 519 patients undergoing hysterectomy, salpingo-oophorectomy, lymphadenectomy, and sentinel lymph node mapping for endometrial cancer, including 31 patients with isolated tumor cells identified in sentinel lymph nodes.
Disclosures: Dr. Plante did not report having external funding sources. Dr. Plante and Dr. Abu-Rustum had no disclosures.
Postoperative pain in women with preexisting chronic pain
Chronic pain disorders have reached epidemic levels in the United States, with the Institute of Medicine reporting more than 100 million Americans affected and health care costs more than $500 billion annually.1 Although many pain disorders are confined to the abdomen or pelvis (chronic pelvic pain, vulvodynia, irritable bowel syndrome, and bladder pain syndrome), others present with global symptoms (fibromyalgia and chronic fatigue syndrome). Women are more likely to be diagnosed with a chronic pain condition and more likely to seek treatment for chronic pain, including undergoing a surgical intervention. In fact, chronic pelvic pain alone affects upward of 20% of women in the United States, and, of the 400,000 hysterectomies performed each year (54.2%, abdominal; 16.7%, vaginal; and 16.8%, laparoscopic/robotic assisted), approximately 15% are for chronic pain.2
Neurobiology of pain
Perioperative pain control, specifically in women with preexisting pain disorders, can provide an additional challenge. Unlike acute pain, chronic pain (lasting more than 6 months) is associated with an amplified pain response of the central nervous system. This abnormal pain processing, known as centralization of pain, may result in a decrease of the inhibitory pain pathways and/or an increase of the amplification pathways, often augmenting the pain response of the original peripheral insult, specifically surgery. Because of these physiologic changes, a multimodal approach to perioperative pain should be offered, especially in women with preexisting pain. The approach ideally ought to target the different mechanisms of actions in both the peripheral and central nervous systems to provide an overall reduction in pain perception.
Preoperative visit
Perhaps the most underutilized opportunity to optimize postoperative pain is a proactive, preoperative approach. Preoperative education, including goal setting of postoperative pain expectations, has been associated with a significant reduction in postoperative opioid use, less preoperative anxiety, and a decreased length of surgical stay.3 While it is unknown exactly when this should be provided to the patient in the treatment course, it should occur prior to the day of surgery to allow for appropriate intervention.
The use of a shared decision-making model between the clinician and the chronic pain patient in the development of a pain management plan has been highly successful in improving pain outcomes in the outpatient setting.4 A similar method can be applied to the preoperative course as well. A detailed history (including the use of an opioid risk assessment tool) allows the clinician to identify patients at risk for opioid misuse and abuse. This is also an opportunity to review a plan for an opioid taper with the patient and the prescriber, if the postoperative plan includes opioid reduction/cessation. The preoperative visit may be an opportunity to adjust centrally acting medications (antidepressants, anticonvulsants) before surgery or to reduce the dose or frequency of high-risk medications, such as benzodiazepines.
Perioperative strategy
One of the most impactful ways for us, as surgeons, to reduce tissue injury and decrease pain from surgery is by offering a minimally invasive approach. The benefits of minimally invasive surgery are well established, resulting in improved perioperative pain control, decreased blood loss, lower infection rates, decreased length of hospital stay, and a faster recovery, compared with laparotomy. Because patients with chronic pain disorders are at increased risk of greater acute postoperative pain and have an elevated risk for the development of chronic postsurgical pain, a minimally invasive surgical approach should be prioritized, when available.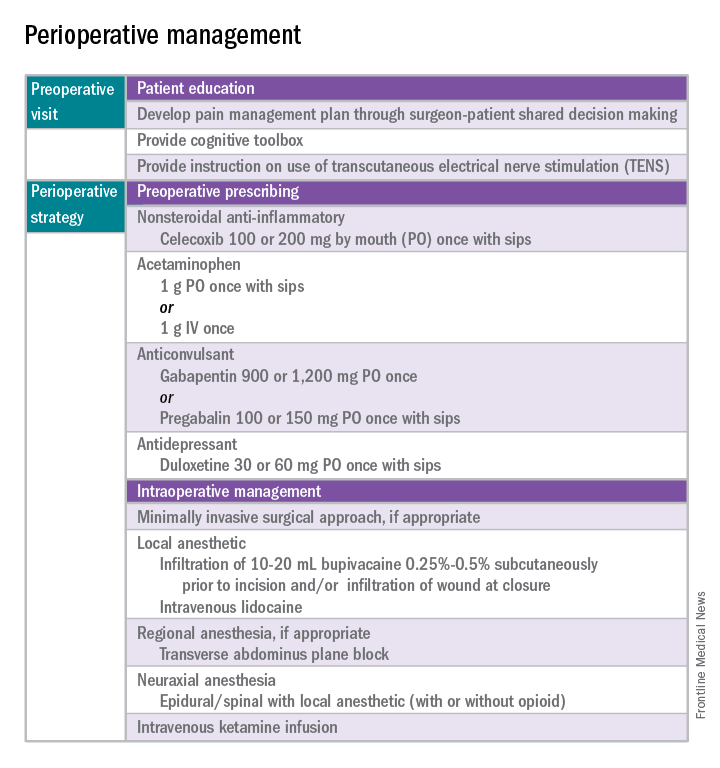
Perioperative multimodal drug therapy is associated with significant decreases in opioid consumption and reductions in acute postoperative pain.6 Recently, a multidisciplinary expert panel from the American Pain Society devised an evidence-based clinical practice guideline for postoperative pain.7 While there is no consensus as to the best regimen specific to gynecologic surgery, the general principles are similar across disciplines.
The postoperative period
Opioid-tolerant patients may experience greater pain during the first 24 hours postoperatively and require an increase in opioids, compared with opioid-naive patients.8 In the event that a postoperative patient does not respond as expected to the usual course, that patient should be evaluated for barriers to routine postoperative care, such as a surgical complication, opioid tolerance, or psychological distress. Surgeons should be aggressive with pain management immediately after surgery, even in the opioid-tolerant patient, and make short-term adjustments as needed based on the pain response. These patients will require pain medications beyond their baseline dose. Additionally, if an opioid taper is not planned in a chronic opioid user, work with the patient and the long-term opioid prescriber in restarting baseline opioid therapy outside of the acute surgical window. 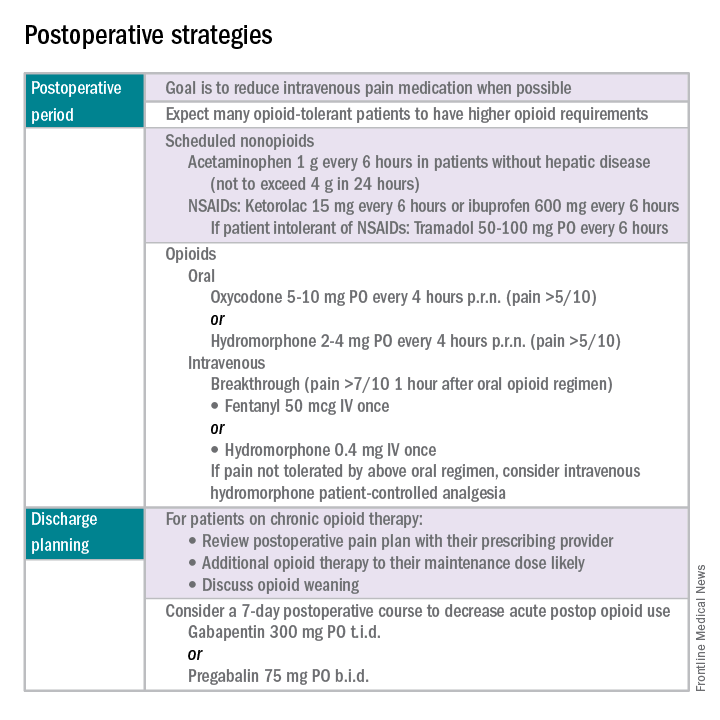
References
1. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press, 2011.
2. Obstet Gynecol. 2013 Aug;122(2 Pt 1):233-41.
3. N Engl J Med. 1964 Apr 16;270:825-7.
4. J Pain Symptom Manage. 1999 Jul;18(1):38-48.
5. Pain. 2010 Dec;151(3):694-702.
6. Anesthesiology. 2005 Dec;103(6):1296-304.
7. J Pain. 2016 Feb;17(2):131-57.
8. Pharmacotherapy. 2008 Dec;28(12):1453-60.
Dr. Carey is the director of minimally invasive gynecologic surgery at the University of North Carolina at Chapel Hill, and specializes in the medical and surgical management of pelvic pain disorders. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC–Chapel Hill. They reported having no relevant financial disclosures.
Chronic pain disorders have reached epidemic levels in the United States, with the Institute of Medicine reporting more than 100 million Americans affected and health care costs more than $500 billion annually.1 Although many pain disorders are confined to the abdomen or pelvis (chronic pelvic pain, vulvodynia, irritable bowel syndrome, and bladder pain syndrome), others present with global symptoms (fibromyalgia and chronic fatigue syndrome). Women are more likely to be diagnosed with a chronic pain condition and more likely to seek treatment for chronic pain, including undergoing a surgical intervention. In fact, chronic pelvic pain alone affects upward of 20% of women in the United States, and, of the 400,000 hysterectomies performed each year (54.2%, abdominal; 16.7%, vaginal; and 16.8%, laparoscopic/robotic assisted), approximately 15% are for chronic pain.2
Neurobiology of pain
Perioperative pain control, specifically in women with preexisting pain disorders, can provide an additional challenge. Unlike acute pain, chronic pain (lasting more than 6 months) is associated with an amplified pain response of the central nervous system. This abnormal pain processing, known as centralization of pain, may result in a decrease of the inhibitory pain pathways and/or an increase of the amplification pathways, often augmenting the pain response of the original peripheral insult, specifically surgery. Because of these physiologic changes, a multimodal approach to perioperative pain should be offered, especially in women with preexisting pain. The approach ideally ought to target the different mechanisms of actions in both the peripheral and central nervous systems to provide an overall reduction in pain perception.
Preoperative visit
Perhaps the most underutilized opportunity to optimize postoperative pain is a proactive, preoperative approach. Preoperative education, including goal setting of postoperative pain expectations, has been associated with a significant reduction in postoperative opioid use, less preoperative anxiety, and a decreased length of surgical stay.3 While it is unknown exactly when this should be provided to the patient in the treatment course, it should occur prior to the day of surgery to allow for appropriate intervention.
The use of a shared decision-making model between the clinician and the chronic pain patient in the development of a pain management plan has been highly successful in improving pain outcomes in the outpatient setting.4 A similar method can be applied to the preoperative course as well. A detailed history (including the use of an opioid risk assessment tool) allows the clinician to identify patients at risk for opioid misuse and abuse. This is also an opportunity to review a plan for an opioid taper with the patient and the prescriber, if the postoperative plan includes opioid reduction/cessation. The preoperative visit may be an opportunity to adjust centrally acting medications (antidepressants, anticonvulsants) before surgery or to reduce the dose or frequency of high-risk medications, such as benzodiazepines.
Perioperative strategy
One of the most impactful ways for us, as surgeons, to reduce tissue injury and decrease pain from surgery is by offering a minimally invasive approach. The benefits of minimally invasive surgery are well established, resulting in improved perioperative pain control, decreased blood loss, lower infection rates, decreased length of hospital stay, and a faster recovery, compared with laparotomy. Because patients with chronic pain disorders are at increased risk of greater acute postoperative pain and have an elevated risk for the development of chronic postsurgical pain, a minimally invasive surgical approach should be prioritized, when available.
Perioperative multimodal drug therapy is associated with significant decreases in opioid consumption and reductions in acute postoperative pain.6 Recently, a multidisciplinary expert panel from the American Pain Society devised an evidence-based clinical practice guideline for postoperative pain.7 While there is no consensus as to the best regimen specific to gynecologic surgery, the general principles are similar across disciplines.
The postoperative period
Opioid-tolerant patients may experience greater pain during the first 24 hours postoperatively and require an increase in opioids, compared with opioid-naive patients.8 In the event that a postoperative patient does not respond as expected to the usual course, that patient should be evaluated for barriers to routine postoperative care, such as a surgical complication, opioid tolerance, or psychological distress. Surgeons should be aggressive with pain management immediately after surgery, even in the opioid-tolerant patient, and make short-term adjustments as needed based on the pain response. These patients will require pain medications beyond their baseline dose. Additionally, if an opioid taper is not planned in a chronic opioid user, work with the patient and the long-term opioid prescriber in restarting baseline opioid therapy outside of the acute surgical window. 
References
1. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press, 2011.
2. Obstet Gynecol. 2013 Aug;122(2 Pt 1):233-41.
3. N Engl J Med. 1964 Apr 16;270:825-7.
4. J Pain Symptom Manage. 1999 Jul;18(1):38-48.
5. Pain. 2010 Dec;151(3):694-702.
6. Anesthesiology. 2005 Dec;103(6):1296-304.
7. J Pain. 2016 Feb;17(2):131-57.
8. Pharmacotherapy. 2008 Dec;28(12):1453-60.
Dr. Carey is the director of minimally invasive gynecologic surgery at the University of North Carolina at Chapel Hill, and specializes in the medical and surgical management of pelvic pain disorders. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC–Chapel Hill. They reported having no relevant financial disclosures.
Chronic pain disorders have reached epidemic levels in the United States, with the Institute of Medicine reporting more than 100 million Americans affected and health care costs more than $500 billion annually.1 Although many pain disorders are confined to the abdomen or pelvis (chronic pelvic pain, vulvodynia, irritable bowel syndrome, and bladder pain syndrome), others present with global symptoms (fibromyalgia and chronic fatigue syndrome). Women are more likely to be diagnosed with a chronic pain condition and more likely to seek treatment for chronic pain, including undergoing a surgical intervention. In fact, chronic pelvic pain alone affects upward of 20% of women in the United States, and, of the 400,000 hysterectomies performed each year (54.2%, abdominal; 16.7%, vaginal; and 16.8%, laparoscopic/robotic assisted), approximately 15% are for chronic pain.2
Neurobiology of pain
Perioperative pain control, specifically in women with preexisting pain disorders, can provide an additional challenge. Unlike acute pain, chronic pain (lasting more than 6 months) is associated with an amplified pain response of the central nervous system. This abnormal pain processing, known as centralization of pain, may result in a decrease of the inhibitory pain pathways and/or an increase of the amplification pathways, often augmenting the pain response of the original peripheral insult, specifically surgery. Because of these physiologic changes, a multimodal approach to perioperative pain should be offered, especially in women with preexisting pain. The approach ideally ought to target the different mechanisms of actions in both the peripheral and central nervous systems to provide an overall reduction in pain perception.
Preoperative visit
Perhaps the most underutilized opportunity to optimize postoperative pain is a proactive, preoperative approach. Preoperative education, including goal setting of postoperative pain expectations, has been associated with a significant reduction in postoperative opioid use, less preoperative anxiety, and a decreased length of surgical stay.3 While it is unknown exactly when this should be provided to the patient in the treatment course, it should occur prior to the day of surgery to allow for appropriate intervention.
The use of a shared decision-making model between the clinician and the chronic pain patient in the development of a pain management plan has been highly successful in improving pain outcomes in the outpatient setting.4 A similar method can be applied to the preoperative course as well. A detailed history (including the use of an opioid risk assessment tool) allows the clinician to identify patients at risk for opioid misuse and abuse. This is also an opportunity to review a plan for an opioid taper with the patient and the prescriber, if the postoperative plan includes opioid reduction/cessation. The preoperative visit may be an opportunity to adjust centrally acting medications (antidepressants, anticonvulsants) before surgery or to reduce the dose or frequency of high-risk medications, such as benzodiazepines.
Perioperative strategy
One of the most impactful ways for us, as surgeons, to reduce tissue injury and decrease pain from surgery is by offering a minimally invasive approach. The benefits of minimally invasive surgery are well established, resulting in improved perioperative pain control, decreased blood loss, lower infection rates, decreased length of hospital stay, and a faster recovery, compared with laparotomy. Because patients with chronic pain disorders are at increased risk of greater acute postoperative pain and have an elevated risk for the development of chronic postsurgical pain, a minimally invasive surgical approach should be prioritized, when available.
Perioperative multimodal drug therapy is associated with significant decreases in opioid consumption and reductions in acute postoperative pain.6 Recently, a multidisciplinary expert panel from the American Pain Society devised an evidence-based clinical practice guideline for postoperative pain.7 While there is no consensus as to the best regimen specific to gynecologic surgery, the general principles are similar across disciplines.
The postoperative period
Opioid-tolerant patients may experience greater pain during the first 24 hours postoperatively and require an increase in opioids, compared with opioid-naive patients.8 In the event that a postoperative patient does not respond as expected to the usual course, that patient should be evaluated for barriers to routine postoperative care, such as a surgical complication, opioid tolerance, or psychological distress. Surgeons should be aggressive with pain management immediately after surgery, even in the opioid-tolerant patient, and make short-term adjustments as needed based on the pain response. These patients will require pain medications beyond their baseline dose. Additionally, if an opioid taper is not planned in a chronic opioid user, work with the patient and the long-term opioid prescriber in restarting baseline opioid therapy outside of the acute surgical window. 
References
1. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press, 2011.
2. Obstet Gynecol. 2013 Aug;122(2 Pt 1):233-41.
3. N Engl J Med. 1964 Apr 16;270:825-7.
4. J Pain Symptom Manage. 1999 Jul;18(1):38-48.
5. Pain. 2010 Dec;151(3):694-702.
6. Anesthesiology. 2005 Dec;103(6):1296-304.
7. J Pain. 2016 Feb;17(2):131-57.
8. Pharmacotherapy. 2008 Dec;28(12):1453-60.
Dr. Carey is the director of minimally invasive gynecologic surgery at the University of North Carolina at Chapel Hill, and specializes in the medical and surgical management of pelvic pain disorders. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC–Chapel Hill. They reported having no relevant financial disclosures.
Vascular surgeons underutilize palliative care planning
Investment in advanced palliative care planning has the potential to improve the quality of care for vascular surgery patients, according to investigators from Oregon Health and Science University, Portland.
Dale G. Wilson, MD, and his colleagues performed a retrospective review of electronic medical records for 111 patients, who died while on the vascular surgery service at the OHSU Hospital during 2005-2014.
Almost three-quarters (73%) of patients were transitioned to palliative care; of those, 14% presented with an advanced directive, and 28% received a palliative care consultation (JAMA Surg. 2017;152[2]:183-90. doi: 10.1001/jamasurg.2016.3970).
While palliative care services are increasing in hospitals, accounting for 4% of annual hospital admissions in 2012 according to the study, they are not implemented consistently. “Many teams from various specialties care for patients at end of life; however, we still do not know what prompts end-of-life discussions,” Dr. Wilson said. “There is still no consensus on when to involve palliative services in the care of critically ill patients.”
While the decision to advise a consultation is “variable and physician dependent,” the type of treatment required may help identify when consultations are appropriate.
Of the 14 patients who did not choose comfort care, 11 (79%) required CPR. Additionally, all had to be taken to the operating room and required mechanical ventilation.
Of 81 patients who chose palliative care, 31 did so despite potential medical options. These patients were older – average age, 77 years, as compared with 68 years for patients who did not choose comfort care – with 8 of the 31 (26%) presenting an advanced directive, compared with only 7 of 83 patients (8%) for those who did not receive palliative care.
Dr. Wilson and his colleagues found that patients who chose palliative care were more likely to have received a palliative care consultation, as well: 10 of 31 patients who chose comfort care received a consultation, as opposed to 1 of 83 who chose comfort care but did not receive a consultation.
The nature of the vascular surgery service calls for early efforts to gather information regarding patients’ views on end-of-life care, Dr. Wilson said, noting that 73% of patients studied were admitted emergently and 87% underwent surgery, leaving little time for patients to express their wishes.
“Because the events associated with withdrawal of care are often not anticipated, we argue that all vascular surgical patients should have an advance directive, and perhaps, those at particular high risk should have a preoperative palliative care consultation,” Dr. Wilson wrote.
Limitations to the study included the data abstraction, which was performed by a single unblinded physician. Researchers also gathered patients’ reasons for transitioning to comfort care retrospectively.
The low rate of palliative care consultations found in this study mirrors my own experience, as does the feeling of urgency to shed more light on the issue. The biggest hurdle surgeons face when it comes to palliative care consultations is that, in their minds, seeking these meetings is associated with immediate death care. Many surgeons are shy about bringing palliative care specialists on board because approaching families can be daunting.
Family members who do not know enough about comfort care can be upset by the idea. Addressing this misunderstanding is crucial. Consultations are not just conversations about hospice care but can be emotional and spiritual experiences that prepare both the family and the patient for alternative options when surgical intervention cannot guarantee a good quality of life. I would encourage surgeons to be more proactive and less defensive about comfort care . Luckily, understanding the importance of this issue among professionals is growing.
When I approach these situations, it’s important for me to have a full understanding of what families and patients usually expect. Decisions should not be based on how bad things are now but on the future. What was the patient’s last year like? What is the best-case scenario for moving forward on a proposed intervention? What will the patient’s quality of life be? Answering these questions helps the patient understand his or her situation, without diminishing a surgeon’s ability. If you are honest, the family will usually come to the conclusion that they do not want to subject the patient to ultimately unnecessary treatment.
Palliative care services help patients and their families deal with pain beyond the physical symptoms. Dealing with pain, depression, or delirium is only a part of comfort care – coping with a sense of hopelessness, family disruption, or feelings of guilt also can be a part and, significantly, a part that surgeons are not trained to diagnose or treat.
With more than 70 surgeons certified in hospice care and a growing number of fellowships in palliative care, I am extremely optimistic in the progress we have made and will continue to make.
Geoffrey Dunn, MD, FACS, is the medical director of the Palliative Care Consultation Service at UPMC Hamot Medical Center, Erie, Penn. He currently is Community Editor for the Pain and Palliative Care Community for the ACS’s web portal.
The low rate of palliative care consultations found in this study mirrors my own experience, as does the feeling of urgency to shed more light on the issue. The biggest hurdle surgeons face when it comes to palliative care consultations is that, in their minds, seeking these meetings is associated with immediate death care. Many surgeons are shy about bringing palliative care specialists on board because approaching families can be daunting.
Family members who do not know enough about comfort care can be upset by the idea. Addressing this misunderstanding is crucial. Consultations are not just conversations about hospice care but can be emotional and spiritual experiences that prepare both the family and the patient for alternative options when surgical intervention cannot guarantee a good quality of life. I would encourage surgeons to be more proactive and less defensive about comfort care . Luckily, understanding the importance of this issue among professionals is growing.
When I approach these situations, it’s important for me to have a full understanding of what families and patients usually expect. Decisions should not be based on how bad things are now but on the future. What was the patient’s last year like? What is the best-case scenario for moving forward on a proposed intervention? What will the patient’s quality of life be? Answering these questions helps the patient understand his or her situation, without diminishing a surgeon’s ability. If you are honest, the family will usually come to the conclusion that they do not want to subject the patient to ultimately unnecessary treatment.
Palliative care services help patients and their families deal with pain beyond the physical symptoms. Dealing with pain, depression, or delirium is only a part of comfort care – coping with a sense of hopelessness, family disruption, or feelings of guilt also can be a part and, significantly, a part that surgeons are not trained to diagnose or treat.
With more than 70 surgeons certified in hospice care and a growing number of fellowships in palliative care, I am extremely optimistic in the progress we have made and will continue to make.
Geoffrey Dunn, MD, FACS, is the medical director of the Palliative Care Consultation Service at UPMC Hamot Medical Center, Erie, Penn. He currently is Community Editor for the Pain and Palliative Care Community for the ACS’s web portal.
The low rate of palliative care consultations found in this study mirrors my own experience, as does the feeling of urgency to shed more light on the issue. The biggest hurdle surgeons face when it comes to palliative care consultations is that, in their minds, seeking these meetings is associated with immediate death care. Many surgeons are shy about bringing palliative care specialists on board because approaching families can be daunting.
Family members who do not know enough about comfort care can be upset by the idea. Addressing this misunderstanding is crucial. Consultations are not just conversations about hospice care but can be emotional and spiritual experiences that prepare both the family and the patient for alternative options when surgical intervention cannot guarantee a good quality of life. I would encourage surgeons to be more proactive and less defensive about comfort care . Luckily, understanding the importance of this issue among professionals is growing.
When I approach these situations, it’s important for me to have a full understanding of what families and patients usually expect. Decisions should not be based on how bad things are now but on the future. What was the patient’s last year like? What is the best-case scenario for moving forward on a proposed intervention? What will the patient’s quality of life be? Answering these questions helps the patient understand his or her situation, without diminishing a surgeon’s ability. If you are honest, the family will usually come to the conclusion that they do not want to subject the patient to ultimately unnecessary treatment.
Palliative care services help patients and their families deal with pain beyond the physical symptoms. Dealing with pain, depression, or delirium is only a part of comfort care – coping with a sense of hopelessness, family disruption, or feelings of guilt also can be a part and, significantly, a part that surgeons are not trained to diagnose or treat.
With more than 70 surgeons certified in hospice care and a growing number of fellowships in palliative care, I am extremely optimistic in the progress we have made and will continue to make.
Geoffrey Dunn, MD, FACS, is the medical director of the Palliative Care Consultation Service at UPMC Hamot Medical Center, Erie, Penn. He currently is Community Editor for the Pain and Palliative Care Community for the ACS’s web portal.
Investment in advanced palliative care planning has the potential to improve the quality of care for vascular surgery patients, according to investigators from Oregon Health and Science University, Portland.
Dale G. Wilson, MD, and his colleagues performed a retrospective review of electronic medical records for 111 patients, who died while on the vascular surgery service at the OHSU Hospital during 2005-2014.
Almost three-quarters (73%) of patients were transitioned to palliative care; of those, 14% presented with an advanced directive, and 28% received a palliative care consultation (JAMA Surg. 2017;152[2]:183-90. doi: 10.1001/jamasurg.2016.3970).
While palliative care services are increasing in hospitals, accounting for 4% of annual hospital admissions in 2012 according to the study, they are not implemented consistently. “Many teams from various specialties care for patients at end of life; however, we still do not know what prompts end-of-life discussions,” Dr. Wilson said. “There is still no consensus on when to involve palliative services in the care of critically ill patients.”
While the decision to advise a consultation is “variable and physician dependent,” the type of treatment required may help identify when consultations are appropriate.
Of the 14 patients who did not choose comfort care, 11 (79%) required CPR. Additionally, all had to be taken to the operating room and required mechanical ventilation.
Of 81 patients who chose palliative care, 31 did so despite potential medical options. These patients were older – average age, 77 years, as compared with 68 years for patients who did not choose comfort care – with 8 of the 31 (26%) presenting an advanced directive, compared with only 7 of 83 patients (8%) for those who did not receive palliative care.
Dr. Wilson and his colleagues found that patients who chose palliative care were more likely to have received a palliative care consultation, as well: 10 of 31 patients who chose comfort care received a consultation, as opposed to 1 of 83 who chose comfort care but did not receive a consultation.
The nature of the vascular surgery service calls for early efforts to gather information regarding patients’ views on end-of-life care, Dr. Wilson said, noting that 73% of patients studied were admitted emergently and 87% underwent surgery, leaving little time for patients to express their wishes.
“Because the events associated with withdrawal of care are often not anticipated, we argue that all vascular surgical patients should have an advance directive, and perhaps, those at particular high risk should have a preoperative palliative care consultation,” Dr. Wilson wrote.
Limitations to the study included the data abstraction, which was performed by a single unblinded physician. Researchers also gathered patients’ reasons for transitioning to comfort care retrospectively.
Investment in advanced palliative care planning has the potential to improve the quality of care for vascular surgery patients, according to investigators from Oregon Health and Science University, Portland.
Dale G. Wilson, MD, and his colleagues performed a retrospective review of electronic medical records for 111 patients, who died while on the vascular surgery service at the OHSU Hospital during 2005-2014.
Almost three-quarters (73%) of patients were transitioned to palliative care; of those, 14% presented with an advanced directive, and 28% received a palliative care consultation (JAMA Surg. 2017;152[2]:183-90. doi: 10.1001/jamasurg.2016.3970).
While palliative care services are increasing in hospitals, accounting for 4% of annual hospital admissions in 2012 according to the study, they are not implemented consistently. “Many teams from various specialties care for patients at end of life; however, we still do not know what prompts end-of-life discussions,” Dr. Wilson said. “There is still no consensus on when to involve palliative services in the care of critically ill patients.”
While the decision to advise a consultation is “variable and physician dependent,” the type of treatment required may help identify when consultations are appropriate.
Of the 14 patients who did not choose comfort care, 11 (79%) required CPR. Additionally, all had to be taken to the operating room and required mechanical ventilation.
Of 81 patients who chose palliative care, 31 did so despite potential medical options. These patients were older – average age, 77 years, as compared with 68 years for patients who did not choose comfort care – with 8 of the 31 (26%) presenting an advanced directive, compared with only 7 of 83 patients (8%) for those who did not receive palliative care.
Dr. Wilson and his colleagues found that patients who chose palliative care were more likely to have received a palliative care consultation, as well: 10 of 31 patients who chose comfort care received a consultation, as opposed to 1 of 83 who chose comfort care but did not receive a consultation.
The nature of the vascular surgery service calls for early efforts to gather information regarding patients’ views on end-of-life care, Dr. Wilson said, noting that 73% of patients studied were admitted emergently and 87% underwent surgery, leaving little time for patients to express their wishes.
“Because the events associated with withdrawal of care are often not anticipated, we argue that all vascular surgical patients should have an advance directive, and perhaps, those at particular high risk should have a preoperative palliative care consultation,” Dr. Wilson wrote.
Limitations to the study included the data abstraction, which was performed by a single unblinded physician. Researchers also gathered patients’ reasons for transitioning to comfort care retrospectively.
FROM JAMA SURGERY
Key clinical point:
Major finding: Of the 111 patients studied, 81 died on palliative care, but only 15 presented an advanced directive.
Data source: A retrospective cohort study of the records of patients aged 18-99 years who died in the vascular surgery service at Oregon Health and Science University Hospital from 2005-2014.
Disclosures: The authors reported no financial disclosures.
Diagnostic laparoscopy identifies ovarian cancers amenable to PCS
For women with suspected advanced epithelial ovarian cancer, diagnostic laparoscopy can help to distinguish between patients who could benefit from primary cytoreductive surgery (PCS) and those who might have better outcomes with neoadjuvant chemotherapy and interval cytoreductive surgery, according to investigators in the Netherlands.
In a randomized controlled trial exploring whether initial diagnostic laparoscopy could spare some patients from undergoing futile PCS, the investigators found that only 10% of patients assigned to diagnostic laparoscopy prior to PCS underwent a subsequent futile laparotomy, defined as residual disease greater than 1 cm following surgery. In contrast, 39% of women assigned to primary PCS had disease that might have been better treated by chemotherapy and interval surgery,
“In women with a plan for PCS, these data suggest that performance of diagnostic laparoscopy first is reasonable and that if cytoreduction to [less than] 1 cm of residual disease seems feasible, to proceed with PCS,” wrote Marrije R. Buist, MD of Academic Medical Center, Amsterdam, and colleagues.
Among women with International Federation of Gynecology and Obstetrics (FIGO) stage IIIC to IV epithelial ovarian cancer, survival depends largely on the ability of surgery to either completely remove disease, or to leave at best less than 1 cm of residual disease. However, aggressive surgery in patients with more extensive disease is associated with significant morbidities, the authors noted.
“If at PCS, extensive disease is present, surgery could be ceased, and neoadjuvant chemotherapy with interval surgery could be a good alternative treatment. Therefore, the identification of patients with extensive disease who are likely to have [more than] 1 cm of residual tumor after PCS, defined as a futile laparotomy, is important,” they wrote.
To test this idea, the investigators, from eight cancer centers in the Netherlands, enrolled 201 patients with suspected FIGO stage IIB ovarian cancer or higher, and randomly assigned them to undergo either initial diagnostic laparoscopy or PCS.
They found that 10 of the 102 patients (10%) assigned to diagnostic laparoscopy went on to undergo PCS that revealed residual disease greater than 1 cm, compared with 39 of the 99 patients (39%) assigned to PCS. This difference translated into a relative risk for futile laparotomy of 0.25 for diagnostic laparoscopy compared with PCS (P less than .001).
Only 3 (3%) patients in the diagnostic laparoscopy group went on to have both PCS and interval surgery, compared with 28 (28%) patients initially assigned to PCS (P less than .001).
The Dutch Organization for Health Research and Development supported the study. All but one coauthor reported having no potential conflicts of interest.
For women with suspected advanced epithelial ovarian cancer, diagnostic laparoscopy can help to distinguish between patients who could benefit from primary cytoreductive surgery (PCS) and those who might have better outcomes with neoadjuvant chemotherapy and interval cytoreductive surgery, according to investigators in the Netherlands.
In a randomized controlled trial exploring whether initial diagnostic laparoscopy could spare some patients from undergoing futile PCS, the investigators found that only 10% of patients assigned to diagnostic laparoscopy prior to PCS underwent a subsequent futile laparotomy, defined as residual disease greater than 1 cm following surgery. In contrast, 39% of women assigned to primary PCS had disease that might have been better treated by chemotherapy and interval surgery,
“In women with a plan for PCS, these data suggest that performance of diagnostic laparoscopy first is reasonable and that if cytoreduction to [less than] 1 cm of residual disease seems feasible, to proceed with PCS,” wrote Marrije R. Buist, MD of Academic Medical Center, Amsterdam, and colleagues.
Among women with International Federation of Gynecology and Obstetrics (FIGO) stage IIIC to IV epithelial ovarian cancer, survival depends largely on the ability of surgery to either completely remove disease, or to leave at best less than 1 cm of residual disease. However, aggressive surgery in patients with more extensive disease is associated with significant morbidities, the authors noted.
“If at PCS, extensive disease is present, surgery could be ceased, and neoadjuvant chemotherapy with interval surgery could be a good alternative treatment. Therefore, the identification of patients with extensive disease who are likely to have [more than] 1 cm of residual tumor after PCS, defined as a futile laparotomy, is important,” they wrote.
To test this idea, the investigators, from eight cancer centers in the Netherlands, enrolled 201 patients with suspected FIGO stage IIB ovarian cancer or higher, and randomly assigned them to undergo either initial diagnostic laparoscopy or PCS.
They found that 10 of the 102 patients (10%) assigned to diagnostic laparoscopy went on to undergo PCS that revealed residual disease greater than 1 cm, compared with 39 of the 99 patients (39%) assigned to PCS. This difference translated into a relative risk for futile laparotomy of 0.25 for diagnostic laparoscopy compared with PCS (P less than .001).
Only 3 (3%) patients in the diagnostic laparoscopy group went on to have both PCS and interval surgery, compared with 28 (28%) patients initially assigned to PCS (P less than .001).
The Dutch Organization for Health Research and Development supported the study. All but one coauthor reported having no potential conflicts of interest.
For women with suspected advanced epithelial ovarian cancer, diagnostic laparoscopy can help to distinguish between patients who could benefit from primary cytoreductive surgery (PCS) and those who might have better outcomes with neoadjuvant chemotherapy and interval cytoreductive surgery, according to investigators in the Netherlands.
In a randomized controlled trial exploring whether initial diagnostic laparoscopy could spare some patients from undergoing futile PCS, the investigators found that only 10% of patients assigned to diagnostic laparoscopy prior to PCS underwent a subsequent futile laparotomy, defined as residual disease greater than 1 cm following surgery. In contrast, 39% of women assigned to primary PCS had disease that might have been better treated by chemotherapy and interval surgery,
“In women with a plan for PCS, these data suggest that performance of diagnostic laparoscopy first is reasonable and that if cytoreduction to [less than] 1 cm of residual disease seems feasible, to proceed with PCS,” wrote Marrije R. Buist, MD of Academic Medical Center, Amsterdam, and colleagues.
Among women with International Federation of Gynecology and Obstetrics (FIGO) stage IIIC to IV epithelial ovarian cancer, survival depends largely on the ability of surgery to either completely remove disease, or to leave at best less than 1 cm of residual disease. However, aggressive surgery in patients with more extensive disease is associated with significant morbidities, the authors noted.
“If at PCS, extensive disease is present, surgery could be ceased, and neoadjuvant chemotherapy with interval surgery could be a good alternative treatment. Therefore, the identification of patients with extensive disease who are likely to have [more than] 1 cm of residual tumor after PCS, defined as a futile laparotomy, is important,” they wrote.
To test this idea, the investigators, from eight cancer centers in the Netherlands, enrolled 201 patients with suspected FIGO stage IIB ovarian cancer or higher, and randomly assigned them to undergo either initial diagnostic laparoscopy or PCS.
They found that 10 of the 102 patients (10%) assigned to diagnostic laparoscopy went on to undergo PCS that revealed residual disease greater than 1 cm, compared with 39 of the 99 patients (39%) assigned to PCS. This difference translated into a relative risk for futile laparotomy of 0.25 for diagnostic laparoscopy compared with PCS (P less than .001).
Only 3 (3%) patients in the diagnostic laparoscopy group went on to have both PCS and interval surgery, compared with 28 (28%) patients initially assigned to PCS (P less than .001).
The Dutch Organization for Health Research and Development supported the study. All but one coauthor reported having no potential conflicts of interest.
Key clinical point: Diagnostic laparoscopy can help to identify patients with advanced ovarian cancer who can best benefit from primary surgery or chemotherapy.
Major finding: Ten percent of women assigned to diagnostic laparoscopy underwent futile laparotomy, vs. 39% assigned to primary cytoreductive surgery.
Data source: Randomized controlled trial of 201 women with suspected FIGO stage IIB or greater disease.
Disclosures The Dutch Organization for Health Research and Development supported the study. All but one coauthor reported having no potential conflicts of interest.
Preventing surgical site infections in hysterectomy
Surgical site infections are a major source of patient morbidity. They are also an important quality metric for surgeons and hospital systems, and are increasingly being linked to reimbursement.
They occur in approximately 2% of the 600,000 women undergoing hysterectomy in the United States each year. The U.S. Centers for Disease Control and Prevention defines surgical site infection (SSI) as an infection that occurs within 30 days of a procedure in the part of the body where the surgery took place. Most SSIs are superficial incisional, but they also include deep incisional or organ or space infections.
Classification
The incidence of SSI varies according to the classification of the wound, as defined by the National Academy of Sciences.1 Most hysterectomies are classified as clean-contaminated wounds because they involve entry into the mucosa of the genitourinary tract. However, hysterectomy with contamination of bowel flora, or in the setting of acute infection (such as suppurative pelvic inflammatory disease) are considered a contaminated wound class, and are associated with even higher rates of SSI.
Risk factors
The risk factors associated with SSI are both modifiable and unmodifiable. Broadly speaking, they include increased risk to endogenous flora (e.g., wound classification), increased exposure to exogenous flora (e.g., inadequate protection of a wound from external pathogens), and impairment of the body’s immune mechanisms to prevent and overcome infection (e.g., hypothermia and hypoglycemia).
Unmodifiable risk factors include increasing age, a history of radiation exposure, vascular disease, and a history of prior SSIs. Modifiable risk factors include obesity, tobacco use, immunosuppressive medications, hypoalbuminemia, route of hysterectomy, hair removal, preoperative infections (such as bacterial vaginosis), surgical scrub, skin and vaginal preparation, antimicrobial prophylaxis (inappropriate choice or timing, inadequate dosing or redosing), operative time, blood transfusion, surgical skill, and operating room characteristics (ventilation, increased OR traffic, and sterilization of surgical equipment).
Antimicrobial prophylaxis
The CDC and the American College of Obstetricians and Gynecologists (ACOG) have provided clear guidelines regarding methods to reduce SSI in hysterectomy.3,4 There is strong evidence for using antimicrobial prophylaxis for hysterectomy.
It is important that physicians confirm the validity of beta-lactam allergies with patients because there are higher rates of SSI with the use of non–beta-lactam regimens, even those endorsed by the CDC and ACOG.5
Antibiotics should be administered within 1 hour of skin incision, and ideally within 30 minutes. They should be discontinued within 24 hours. Dosing should be adjusted to weight, and antimicrobials should be redosed for long procedures (at intervals of two half-lives), and for increased blood loss.
Skin preparation
Hair removal should be avoided unless necessary for technical reasons. If it is required, it should be performed outside of the operative space using clippers, not razors. For patients colonized with methicillin-resistant S. aureus, there is supporting evidence for pretreatment with mupirocin ointment to the nares, and chlorhexidine showers for 5-10 days. Patients who have bacterial vaginosis should be treated before surgery to decrease the rate of vaginal cuff SSI.

If there is a planned or potential gastrointestinal procedure as part of the hysterectomy, the surgeon should consider using an impervious plastic wound protector in place of, or in addition to, other retractors. Preoperative oral antimicrobials with mechanical bowel preparation have been associated with decreased SSIs; however, this benefit is not observed with mechanical bowel preparation alone.
Wound closure
Surgical technique and wound closure techniques also impact SSI. Minimally invasive and vaginal hysterectomy routes are preferred, as these are associated with the lowest rates of SSI. Antimicrobial-impregnated suture materials appear to be unnecessary. Surgeons should ensure that there is delicate handling of tissues and closure of dead spaces. If the subcutaneous fat space depth measures more than 2.5 cm, it should be reapproximated with a rapidly-absorbing suture material.
Use of electrosurgery versus a scalpel when creating the incision does not appear to influence infection rates, nor does use of staples versus subcuticular suture during closure.7
Using a dilute iodine lavage in the subcutaneous space, opening a sterile closing tray, and having surgeons change gloves prior to skin closure should be considered. The CDC recommends keeping the skin dressing in place for 24 hours postoperatively.
Other strategies
Hyperglycemia is associated with impaired neutrophil response, and therefore blood glucose should be controlled before surgery (hemoglobin A1c levels of less than 7% preoperatively) and immediately postoperatively (less than 180 mg/dL within 18-24 hours after the end of anesthesia).
It is also important to minimize perioperative hypothermia (less than 35.5° F), as this also impairs the body’s immune response. Keeping operative room ambient temperatures higher, minimizing incision size, warming CO2 gas in minimally invasive procedures, warming fluids, and using extrinsic body warmers can help achieve this.
Excessive blood loss should be minimized because blood transfusion is associated with impaired macrophage function and increased risk for SSI.
In addition to teamwide (including nonsurgeon) strict adherence to hand hygiene, OR personnel should avoid unnecessary operating room traffic. Hospital officials should ensure that the facility’s ventilator systems are well maintained and that there is care and maintenance of air handlers.
Many strategies can be employed perioperatively to decrease SSI rates for hysterectomy. We advocate for a protocol-based approach (known as “bundling” strategies) to achieve consistency of practice and to maximize surgeon and institutional improvements in SSI rates. This is similar to the approach outlined in a recent consensus statement from the Council on Patient Safety in Women’s Health Care.8
A comprehensive multidisciplinary approach throughout the perioperative period is necessary. It is imperative that good communication exist with patients regarding SSIs after hysterectomy and how patients, surgeons, and hospitals can together minimize the risks of SSIs.
References
1. Altemeier WA. “Manual on Control of Infection in Surgical Patients” (Philadelphia: Lippincott Williams & Wilkins, 1984).
2. Rev Infect Dis. 1991 Sep-Oct;13(Suppl 10):S821-41.
3. Infect Control Hosp Epidemiol. 2014 Jun;35(6):605-27.
4. Obstet Gynecol. 2009 May;113(5):1180-9.
5. Obstet Gynecol. 2016 Feb;127(2):321-9.
6. Am J Obstet Gynecol. 2005 Feb;192(2):422-5.
7. J Gastrointest Surg. 2016 Dec;20(12):2083-92.
8. Obstet Gynecol. 2016 Dec 7. doi: 10.1097/AOG.0000000000001751.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Jackson-Moore is an associate professor in gynecologic oncology at UNC. They reported having no relevant financial disclosures.
Surgical site infections are a major source of patient morbidity. They are also an important quality metric for surgeons and hospital systems, and are increasingly being linked to reimbursement.
They occur in approximately 2% of the 600,000 women undergoing hysterectomy in the United States each year. The U.S. Centers for Disease Control and Prevention defines surgical site infection (SSI) as an infection that occurs within 30 days of a procedure in the part of the body where the surgery took place. Most SSIs are superficial incisional, but they also include deep incisional or organ or space infections.
Classification
The incidence of SSI varies according to the classification of the wound, as defined by the National Academy of Sciences.1 Most hysterectomies are classified as clean-contaminated wounds because they involve entry into the mucosa of the genitourinary tract. However, hysterectomy with contamination of bowel flora, or in the setting of acute infection (such as suppurative pelvic inflammatory disease) are considered a contaminated wound class, and are associated with even higher rates of SSI.
Risk factors
The risk factors associated with SSI are both modifiable and unmodifiable. Broadly speaking, they include increased risk to endogenous flora (e.g., wound classification), increased exposure to exogenous flora (e.g., inadequate protection of a wound from external pathogens), and impairment of the body’s immune mechanisms to prevent and overcome infection (e.g., hypothermia and hypoglycemia).
Unmodifiable risk factors include increasing age, a history of radiation exposure, vascular disease, and a history of prior SSIs. Modifiable risk factors include obesity, tobacco use, immunosuppressive medications, hypoalbuminemia, route of hysterectomy, hair removal, preoperative infections (such as bacterial vaginosis), surgical scrub, skin and vaginal preparation, antimicrobial prophylaxis (inappropriate choice or timing, inadequate dosing or redosing), operative time, blood transfusion, surgical skill, and operating room characteristics (ventilation, increased OR traffic, and sterilization of surgical equipment).
Antimicrobial prophylaxis
The CDC and the American College of Obstetricians and Gynecologists (ACOG) have provided clear guidelines regarding methods to reduce SSI in hysterectomy.3,4 There is strong evidence for using antimicrobial prophylaxis for hysterectomy.
It is important that physicians confirm the validity of beta-lactam allergies with patients because there are higher rates of SSI with the use of non–beta-lactam regimens, even those endorsed by the CDC and ACOG.5
Antibiotics should be administered within 1 hour of skin incision, and ideally within 30 minutes. They should be discontinued within 24 hours. Dosing should be adjusted to weight, and antimicrobials should be redosed for long procedures (at intervals of two half-lives), and for increased blood loss.
Skin preparation
Hair removal should be avoided unless necessary for technical reasons. If it is required, it should be performed outside of the operative space using clippers, not razors. For patients colonized with methicillin-resistant S. aureus, there is supporting evidence for pretreatment with mupirocin ointment to the nares, and chlorhexidine showers for 5-10 days. Patients who have bacterial vaginosis should be treated before surgery to decrease the rate of vaginal cuff SSI.

If there is a planned or potential gastrointestinal procedure as part of the hysterectomy, the surgeon should consider using an impervious plastic wound protector in place of, or in addition to, other retractors. Preoperative oral antimicrobials with mechanical bowel preparation have been associated with decreased SSIs; however, this benefit is not observed with mechanical bowel preparation alone.
Wound closure
Surgical technique and wound closure techniques also impact SSI. Minimally invasive and vaginal hysterectomy routes are preferred, as these are associated with the lowest rates of SSI. Antimicrobial-impregnated suture materials appear to be unnecessary. Surgeons should ensure that there is delicate handling of tissues and closure of dead spaces. If the subcutaneous fat space depth measures more than 2.5 cm, it should be reapproximated with a rapidly-absorbing suture material.
Use of electrosurgery versus a scalpel when creating the incision does not appear to influence infection rates, nor does use of staples versus subcuticular suture during closure.7
Using a dilute iodine lavage in the subcutaneous space, opening a sterile closing tray, and having surgeons change gloves prior to skin closure should be considered. The CDC recommends keeping the skin dressing in place for 24 hours postoperatively.
Other strategies
Hyperglycemia is associated with impaired neutrophil response, and therefore blood glucose should be controlled before surgery (hemoglobin A1c levels of less than 7% preoperatively) and immediately postoperatively (less than 180 mg/dL within 18-24 hours after the end of anesthesia).
It is also important to minimize perioperative hypothermia (less than 35.5° F), as this also impairs the body’s immune response. Keeping operative room ambient temperatures higher, minimizing incision size, warming CO2 gas in minimally invasive procedures, warming fluids, and using extrinsic body warmers can help achieve this.
Excessive blood loss should be minimized because blood transfusion is associated with impaired macrophage function and increased risk for SSI.
In addition to teamwide (including nonsurgeon) strict adherence to hand hygiene, OR personnel should avoid unnecessary operating room traffic. Hospital officials should ensure that the facility’s ventilator systems are well maintained and that there is care and maintenance of air handlers.
Many strategies can be employed perioperatively to decrease SSI rates for hysterectomy. We advocate for a protocol-based approach (known as “bundling” strategies) to achieve consistency of practice and to maximize surgeon and institutional improvements in SSI rates. This is similar to the approach outlined in a recent consensus statement from the Council on Patient Safety in Women’s Health Care.8
A comprehensive multidisciplinary approach throughout the perioperative period is necessary. It is imperative that good communication exist with patients regarding SSIs after hysterectomy and how patients, surgeons, and hospitals can together minimize the risks of SSIs.
References
1. Altemeier WA. “Manual on Control of Infection in Surgical Patients” (Philadelphia: Lippincott Williams & Wilkins, 1984).
2. Rev Infect Dis. 1991 Sep-Oct;13(Suppl 10):S821-41.
3. Infect Control Hosp Epidemiol. 2014 Jun;35(6):605-27.
4. Obstet Gynecol. 2009 May;113(5):1180-9.
5. Obstet Gynecol. 2016 Feb;127(2):321-9.
6. Am J Obstet Gynecol. 2005 Feb;192(2):422-5.
7. J Gastrointest Surg. 2016 Dec;20(12):2083-92.
8. Obstet Gynecol. 2016 Dec 7. doi: 10.1097/AOG.0000000000001751.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Jackson-Moore is an associate professor in gynecologic oncology at UNC. They reported having no relevant financial disclosures.
Surgical site infections are a major source of patient morbidity. They are also an important quality metric for surgeons and hospital systems, and are increasingly being linked to reimbursement.
They occur in approximately 2% of the 600,000 women undergoing hysterectomy in the United States each year. The U.S. Centers for Disease Control and Prevention defines surgical site infection (SSI) as an infection that occurs within 30 days of a procedure in the part of the body where the surgery took place. Most SSIs are superficial incisional, but they also include deep incisional or organ or space infections.
Classification
The incidence of SSI varies according to the classification of the wound, as defined by the National Academy of Sciences.1 Most hysterectomies are classified as clean-contaminated wounds because they involve entry into the mucosa of the genitourinary tract. However, hysterectomy with contamination of bowel flora, or in the setting of acute infection (such as suppurative pelvic inflammatory disease) are considered a contaminated wound class, and are associated with even higher rates of SSI.
Risk factors
The risk factors associated with SSI are both modifiable and unmodifiable. Broadly speaking, they include increased risk to endogenous flora (e.g., wound classification), increased exposure to exogenous flora (e.g., inadequate protection of a wound from external pathogens), and impairment of the body’s immune mechanisms to prevent and overcome infection (e.g., hypothermia and hypoglycemia).
Unmodifiable risk factors include increasing age, a history of radiation exposure, vascular disease, and a history of prior SSIs. Modifiable risk factors include obesity, tobacco use, immunosuppressive medications, hypoalbuminemia, route of hysterectomy, hair removal, preoperative infections (such as bacterial vaginosis), surgical scrub, skin and vaginal preparation, antimicrobial prophylaxis (inappropriate choice or timing, inadequate dosing or redosing), operative time, blood transfusion, surgical skill, and operating room characteristics (ventilation, increased OR traffic, and sterilization of surgical equipment).
Antimicrobial prophylaxis
The CDC and the American College of Obstetricians and Gynecologists (ACOG) have provided clear guidelines regarding methods to reduce SSI in hysterectomy.3,4 There is strong evidence for using antimicrobial prophylaxis for hysterectomy.
It is important that physicians confirm the validity of beta-lactam allergies with patients because there are higher rates of SSI with the use of non–beta-lactam regimens, even those endorsed by the CDC and ACOG.5
Antibiotics should be administered within 1 hour of skin incision, and ideally within 30 minutes. They should be discontinued within 24 hours. Dosing should be adjusted to weight, and antimicrobials should be redosed for long procedures (at intervals of two half-lives), and for increased blood loss.
Skin preparation
Hair removal should be avoided unless necessary for technical reasons. If it is required, it should be performed outside of the operative space using clippers, not razors. For patients colonized with methicillin-resistant S. aureus, there is supporting evidence for pretreatment with mupirocin ointment to the nares, and chlorhexidine showers for 5-10 days. Patients who have bacterial vaginosis should be treated before surgery to decrease the rate of vaginal cuff SSI.

If there is a planned or potential gastrointestinal procedure as part of the hysterectomy, the surgeon should consider using an impervious plastic wound protector in place of, or in addition to, other retractors. Preoperative oral antimicrobials with mechanical bowel preparation have been associated with decreased SSIs; however, this benefit is not observed with mechanical bowel preparation alone.
Wound closure
Surgical technique and wound closure techniques also impact SSI. Minimally invasive and vaginal hysterectomy routes are preferred, as these are associated with the lowest rates of SSI. Antimicrobial-impregnated suture materials appear to be unnecessary. Surgeons should ensure that there is delicate handling of tissues and closure of dead spaces. If the subcutaneous fat space depth measures more than 2.5 cm, it should be reapproximated with a rapidly-absorbing suture material.
Use of electrosurgery versus a scalpel when creating the incision does not appear to influence infection rates, nor does use of staples versus subcuticular suture during closure.7
Using a dilute iodine lavage in the subcutaneous space, opening a sterile closing tray, and having surgeons change gloves prior to skin closure should be considered. The CDC recommends keeping the skin dressing in place for 24 hours postoperatively.
Other strategies
Hyperglycemia is associated with impaired neutrophil response, and therefore blood glucose should be controlled before surgery (hemoglobin A1c levels of less than 7% preoperatively) and immediately postoperatively (less than 180 mg/dL within 18-24 hours after the end of anesthesia).
It is also important to minimize perioperative hypothermia (less than 35.5° F), as this also impairs the body’s immune response. Keeping operative room ambient temperatures higher, minimizing incision size, warming CO2 gas in minimally invasive procedures, warming fluids, and using extrinsic body warmers can help achieve this.
Excessive blood loss should be minimized because blood transfusion is associated with impaired macrophage function and increased risk for SSI.
In addition to teamwide (including nonsurgeon) strict adherence to hand hygiene, OR personnel should avoid unnecessary operating room traffic. Hospital officials should ensure that the facility’s ventilator systems are well maintained and that there is care and maintenance of air handlers.
Many strategies can be employed perioperatively to decrease SSI rates for hysterectomy. We advocate for a protocol-based approach (known as “bundling” strategies) to achieve consistency of practice and to maximize surgeon and institutional improvements in SSI rates. This is similar to the approach outlined in a recent consensus statement from the Council on Patient Safety in Women’s Health Care.8
A comprehensive multidisciplinary approach throughout the perioperative period is necessary. It is imperative that good communication exist with patients regarding SSIs after hysterectomy and how patients, surgeons, and hospitals can together minimize the risks of SSIs.
References
1. Altemeier WA. “Manual on Control of Infection in Surgical Patients” (Philadelphia: Lippincott Williams & Wilkins, 1984).
2. Rev Infect Dis. 1991 Sep-Oct;13(Suppl 10):S821-41.
3. Infect Control Hosp Epidemiol. 2014 Jun;35(6):605-27.
4. Obstet Gynecol. 2009 May;113(5):1180-9.
5. Obstet Gynecol. 2016 Feb;127(2):321-9.
6. Am J Obstet Gynecol. 2005 Feb;192(2):422-5.
7. J Gastrointest Surg. 2016 Dec;20(12):2083-92.
8. Obstet Gynecol. 2016 Dec 7. doi: 10.1097/AOG.0000000000001751.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Jackson-Moore is an associate professor in gynecologic oncology at UNC. They reported having no relevant financial disclosures.
Four-part ‘safety bundle’ targets gynecologic surgery infections
In a new consensus safety bundle designed to reduce the frequency of infections related to gynecologic surgery, an expert panel is calling for preoperative evaluation of infection risk in every patient and surgical timeouts in every case.
The bundle, from the Council on Patient Safety in Women & Mother’s Health Care, seeks to compile existing guidelines and evidence-based recommendations in a way that can be easily implemented with whatever resources an individual institution has available.
The safety bundle covers four domains:
1. Readiness (every facility). The statement calls for standardized preoperative and postoperative care instructions and clearly defined roles for each surgical team member.
Standards should also be established regarding skin preparation, use of prophylactic antibiotics (terminate them within 24 hours after surgery completion unless medical indications are present), and temperature, such as ambient operating room temperature.
“Although the effect of temperature maintenance on surgical site infection is not definitive,” the consensus statement says, “there is no denying other benefits of normothermia; foremost among these is overall patient satisfaction and comfort.”
2. Recognition and prevention (every patient). Every patient should undergo a preoperative evaluation of infection risk based on blood glucose level, body mass index, immunodeficiency, methicillin-resistant Staphylococcus aureus (MRSA) status, nutritional status, and smoking status.
3. Response (every case). Develop “timeouts” during operations, as mandated by the Joint Commission, to address antibiotic dosage and timing, and reassess risk for infection following the procedure based on the length of surgery and blood loss.
4. Reporting and systems learning (every facility). Develop “huddles” – brief team meetings of less than 15 minutes – for high-risk patients. Surgeons and hospital officials should also create a system to report and analyze data about surgical site infections and share physician-specific infection data with all surgeons as part of ongoing professional practice evaluation.
The statement appeared in Obstetrics & Gynecology (2017;129:50-61) and was published concurrently in Anesthesia & Analgesia, the Journal of Obstetric, Gynecologic & Neonatal Nursing, and the AANA Journal.
The authors reported having no potential conflicts of interest.
In a new consensus safety bundle designed to reduce the frequency of infections related to gynecologic surgery, an expert panel is calling for preoperative evaluation of infection risk in every patient and surgical timeouts in every case.
The bundle, from the Council on Patient Safety in Women & Mother’s Health Care, seeks to compile existing guidelines and evidence-based recommendations in a way that can be easily implemented with whatever resources an individual institution has available.
The safety bundle covers four domains:
1. Readiness (every facility). The statement calls for standardized preoperative and postoperative care instructions and clearly defined roles for each surgical team member.
Standards should also be established regarding skin preparation, use of prophylactic antibiotics (terminate them within 24 hours after surgery completion unless medical indications are present), and temperature, such as ambient operating room temperature.
“Although the effect of temperature maintenance on surgical site infection is not definitive,” the consensus statement says, “there is no denying other benefits of normothermia; foremost among these is overall patient satisfaction and comfort.”
2. Recognition and prevention (every patient). Every patient should undergo a preoperative evaluation of infection risk based on blood glucose level, body mass index, immunodeficiency, methicillin-resistant Staphylococcus aureus (MRSA) status, nutritional status, and smoking status.
3. Response (every case). Develop “timeouts” during operations, as mandated by the Joint Commission, to address antibiotic dosage and timing, and reassess risk for infection following the procedure based on the length of surgery and blood loss.
4. Reporting and systems learning (every facility). Develop “huddles” – brief team meetings of less than 15 minutes – for high-risk patients. Surgeons and hospital officials should also create a system to report and analyze data about surgical site infections and share physician-specific infection data with all surgeons as part of ongoing professional practice evaluation.
The statement appeared in Obstetrics & Gynecology (2017;129:50-61) and was published concurrently in Anesthesia & Analgesia, the Journal of Obstetric, Gynecologic & Neonatal Nursing, and the AANA Journal.
The authors reported having no potential conflicts of interest.
In a new consensus safety bundle designed to reduce the frequency of infections related to gynecologic surgery, an expert panel is calling for preoperative evaluation of infection risk in every patient and surgical timeouts in every case.
The bundle, from the Council on Patient Safety in Women & Mother’s Health Care, seeks to compile existing guidelines and evidence-based recommendations in a way that can be easily implemented with whatever resources an individual institution has available.
The safety bundle covers four domains:
1. Readiness (every facility). The statement calls for standardized preoperative and postoperative care instructions and clearly defined roles for each surgical team member.
Standards should also be established regarding skin preparation, use of prophylactic antibiotics (terminate them within 24 hours after surgery completion unless medical indications are present), and temperature, such as ambient operating room temperature.
“Although the effect of temperature maintenance on surgical site infection is not definitive,” the consensus statement says, “there is no denying other benefits of normothermia; foremost among these is overall patient satisfaction and comfort.”
2. Recognition and prevention (every patient). Every patient should undergo a preoperative evaluation of infection risk based on blood glucose level, body mass index, immunodeficiency, methicillin-resistant Staphylococcus aureus (MRSA) status, nutritional status, and smoking status.
3. Response (every case). Develop “timeouts” during operations, as mandated by the Joint Commission, to address antibiotic dosage and timing, and reassess risk for infection following the procedure based on the length of surgery and blood loss.
4. Reporting and systems learning (every facility). Develop “huddles” – brief team meetings of less than 15 minutes – for high-risk patients. Surgeons and hospital officials should also create a system to report and analyze data about surgical site infections and share physician-specific infection data with all surgeons as part of ongoing professional practice evaluation.
The statement appeared in Obstetrics & Gynecology (2017;129:50-61) and was published concurrently in Anesthesia & Analgesia, the Journal of Obstetric, Gynecologic & Neonatal Nursing, and the AANA Journal.
The authors reported having no potential conflicts of interest.
FROM OBSTETRICS & GYNECOLOGY
New-onset pain rare after Essure placement
ORLANDO – While some women report new-onset pelvic pain after placement of an Essure sterilization device, results of a retrospective study suggest this pain is actually associated with placement of the device in about 1% of cases.
Among 1,430 women who had an Essure micro-insert (Bayer) placed at a tertiary care hospital in Canada from June 2002 to June 2013, 62 secondary surgeries were performed, including some for removal of fallopian tubes and removal of the device.
In total, 27 patients reported new-onset pelvic pain after Essure placement and another 11 reported worsening of previous pain. Upon further workup, 15 of the 27 women in the new-onset pain group had another possible explanation for their pain, including surgical or pathology findings of endometriosis or adenomyosis. The investigators concluded there was a link between the pain and the device in just 12 (0.8%) of the women.
Among these dozen patients, the investigators linked the pain in eight women to perforation or migration of the Essure device. Investigators found no other obvious cause for the new-onset pain in the remaining four patients and attributed it to the Essure device.
Set realistic expectations, take a comprehensive pain history, and reassure women when they report post-Essure placement pain, James Robinson, MD, a minimally invasive gynecologic surgeon at Medstar Washington Hospital in Washington, D.C., advised at the meeting, which was sponsored by AAGL.
Dr. Robinson pointed out that there is no standardized approach to managing women with complaints of pain or guidelines on how best to remove the device. Imaging to confirm proper placement and to rule out other sources of pelvic pain, followed by medical or surgical management as warranted, can be effective strategies.
“There is less science here – it’s more the art of medicine, I think,” he said.
Dr. Robinson filled in as a presenter for one of the study coauthors, John A. Thiel, MD, of the University of Saskatchewan, Saskatoon, who was unable to attend the conference.
The study by Dr. Thiel and his colleagues suggests a thorough examination will typically reveal other reasons for pelvic pain and rules out the Essure device as the cause, Dr. Robinson said. The full findings of the study are published in the Journal of Minimally Invasive Gynecology (2016 Nov-Dec;23[7]:1158-1162).
Does removal help?
In a recently published case series of 29 women who had their Essure device removed laparoscopically because of pain, 23 reported relief following excision (Contraception. 2016 Aug;94[2]:190-2).
“Again, a subset had misplaced inserts or had another condition such as endometriosis,” Dr. Robinson said.
The majority of women whose pain resolved with removal of the devices reported their pain early on, so there is an important takeaway from this,” Dr. Robinson said. “We need to listen to our patients when they report pain shortly after device placement … and respond to that.”
Dr. Robinson advised physicians to be ready to surgically remove the device if that is warranted. “I think a lot of people doing these procedures are not comfortable taking their patient back to the operating room or don’t know who to send them to,” he said. “If you are going to place the device, you should be able to take it out or know someone who can.”
The bigger picture
Even though the Essure device is not frequently the cause of pelvic pain, physicians needs to be aware that some patients are likely to assume that it is.
Dr. Robinson pointed to a case in which a woman who had the Essure micro-insert placed 7 years earlier presented with a complaint of a bilateral tingling sensation over the course of 6 months. Online research led her to suspect Essure as the cause of her symptoms. However, on further investigation, it turned out she had relatively high levels of lead in her system from leaky pipes in her home. “It wasn’t an Essure issue,” he said. “But because it’s out there, people will jump to the conclusion that the foreign body is likely the cause of their problem.”
Ob.gyns. should become familiar with websites such as essureproblems.webs.com, which chronicle problems patients have reported with the device, he said.
When talking to patients, start with informed consent and listen to their concerns, Dr. Robinson advised. As part of the counseling about Essure permanent birth control, discuss the risks and benefits of alternatives, such as laparoscopic tubal ligation, long-acting reversible contraception, and vasectomy.
“I took the time to listen to the FDA hearing in Sept 2015 and … it moved me to listen to those patients, and I’ve been a huge advocate of Essure sterilization. I felt for a while I would never do another tubal ligation,” Dr. Robinson said. “But when you listen to patients who have real complaints, what sticks out in your mind is so many of these people are upset because no one took them seriously and listened to them.”
ORLANDO – While some women report new-onset pelvic pain after placement of an Essure sterilization device, results of a retrospective study suggest this pain is actually associated with placement of the device in about 1% of cases.
Among 1,430 women who had an Essure micro-insert (Bayer) placed at a tertiary care hospital in Canada from June 2002 to June 2013, 62 secondary surgeries were performed, including some for removal of fallopian tubes and removal of the device.
In total, 27 patients reported new-onset pelvic pain after Essure placement and another 11 reported worsening of previous pain. Upon further workup, 15 of the 27 women in the new-onset pain group had another possible explanation for their pain, including surgical or pathology findings of endometriosis or adenomyosis. The investigators concluded there was a link between the pain and the device in just 12 (0.8%) of the women.
Among these dozen patients, the investigators linked the pain in eight women to perforation or migration of the Essure device. Investigators found no other obvious cause for the new-onset pain in the remaining four patients and attributed it to the Essure device.
Set realistic expectations, take a comprehensive pain history, and reassure women when they report post-Essure placement pain, James Robinson, MD, a minimally invasive gynecologic surgeon at Medstar Washington Hospital in Washington, D.C., advised at the meeting, which was sponsored by AAGL.
Dr. Robinson pointed out that there is no standardized approach to managing women with complaints of pain or guidelines on how best to remove the device. Imaging to confirm proper placement and to rule out other sources of pelvic pain, followed by medical or surgical management as warranted, can be effective strategies.
“There is less science here – it’s more the art of medicine, I think,” he said.
Dr. Robinson filled in as a presenter for one of the study coauthors, John A. Thiel, MD, of the University of Saskatchewan, Saskatoon, who was unable to attend the conference.
The study by Dr. Thiel and his colleagues suggests a thorough examination will typically reveal other reasons for pelvic pain and rules out the Essure device as the cause, Dr. Robinson said. The full findings of the study are published in the Journal of Minimally Invasive Gynecology (2016 Nov-Dec;23[7]:1158-1162).
Does removal help?
In a recently published case series of 29 women who had their Essure device removed laparoscopically because of pain, 23 reported relief following excision (Contraception. 2016 Aug;94[2]:190-2).
“Again, a subset had misplaced inserts or had another condition such as endometriosis,” Dr. Robinson said.
The majority of women whose pain resolved with removal of the devices reported their pain early on, so there is an important takeaway from this,” Dr. Robinson said. “We need to listen to our patients when they report pain shortly after device placement … and respond to that.”
Dr. Robinson advised physicians to be ready to surgically remove the device if that is warranted. “I think a lot of people doing these procedures are not comfortable taking their patient back to the operating room or don’t know who to send them to,” he said. “If you are going to place the device, you should be able to take it out or know someone who can.”
The bigger picture
Even though the Essure device is not frequently the cause of pelvic pain, physicians needs to be aware that some patients are likely to assume that it is.
Dr. Robinson pointed to a case in which a woman who had the Essure micro-insert placed 7 years earlier presented with a complaint of a bilateral tingling sensation over the course of 6 months. Online research led her to suspect Essure as the cause of her symptoms. However, on further investigation, it turned out she had relatively high levels of lead in her system from leaky pipes in her home. “It wasn’t an Essure issue,” he said. “But because it’s out there, people will jump to the conclusion that the foreign body is likely the cause of their problem.”
Ob.gyns. should become familiar with websites such as essureproblems.webs.com, which chronicle problems patients have reported with the device, he said.
When talking to patients, start with informed consent and listen to their concerns, Dr. Robinson advised. As part of the counseling about Essure permanent birth control, discuss the risks and benefits of alternatives, such as laparoscopic tubal ligation, long-acting reversible contraception, and vasectomy.
“I took the time to listen to the FDA hearing in Sept 2015 and … it moved me to listen to those patients, and I’ve been a huge advocate of Essure sterilization. I felt for a while I would never do another tubal ligation,” Dr. Robinson said. “But when you listen to patients who have real complaints, what sticks out in your mind is so many of these people are upset because no one took them seriously and listened to them.”
ORLANDO – While some women report new-onset pelvic pain after placement of an Essure sterilization device, results of a retrospective study suggest this pain is actually associated with placement of the device in about 1% of cases.
Among 1,430 women who had an Essure micro-insert (Bayer) placed at a tertiary care hospital in Canada from June 2002 to June 2013, 62 secondary surgeries were performed, including some for removal of fallopian tubes and removal of the device.
In total, 27 patients reported new-onset pelvic pain after Essure placement and another 11 reported worsening of previous pain. Upon further workup, 15 of the 27 women in the new-onset pain group had another possible explanation for their pain, including surgical or pathology findings of endometriosis or adenomyosis. The investigators concluded there was a link between the pain and the device in just 12 (0.8%) of the women.
Among these dozen patients, the investigators linked the pain in eight women to perforation or migration of the Essure device. Investigators found no other obvious cause for the new-onset pain in the remaining four patients and attributed it to the Essure device.
Set realistic expectations, take a comprehensive pain history, and reassure women when they report post-Essure placement pain, James Robinson, MD, a minimally invasive gynecologic surgeon at Medstar Washington Hospital in Washington, D.C., advised at the meeting, which was sponsored by AAGL.
Dr. Robinson pointed out that there is no standardized approach to managing women with complaints of pain or guidelines on how best to remove the device. Imaging to confirm proper placement and to rule out other sources of pelvic pain, followed by medical or surgical management as warranted, can be effective strategies.
“There is less science here – it’s more the art of medicine, I think,” he said.
Dr. Robinson filled in as a presenter for one of the study coauthors, John A. Thiel, MD, of the University of Saskatchewan, Saskatoon, who was unable to attend the conference.
The study by Dr. Thiel and his colleagues suggests a thorough examination will typically reveal other reasons for pelvic pain and rules out the Essure device as the cause, Dr. Robinson said. The full findings of the study are published in the Journal of Minimally Invasive Gynecology (2016 Nov-Dec;23[7]:1158-1162).
Does removal help?
In a recently published case series of 29 women who had their Essure device removed laparoscopically because of pain, 23 reported relief following excision (Contraception. 2016 Aug;94[2]:190-2).
“Again, a subset had misplaced inserts or had another condition such as endometriosis,” Dr. Robinson said.
The majority of women whose pain resolved with removal of the devices reported their pain early on, so there is an important takeaway from this,” Dr. Robinson said. “We need to listen to our patients when they report pain shortly after device placement … and respond to that.”
Dr. Robinson advised physicians to be ready to surgically remove the device if that is warranted. “I think a lot of people doing these procedures are not comfortable taking their patient back to the operating room or don’t know who to send them to,” he said. “If you are going to place the device, you should be able to take it out or know someone who can.”
The bigger picture
Even though the Essure device is not frequently the cause of pelvic pain, physicians needs to be aware that some patients are likely to assume that it is.
Dr. Robinson pointed to a case in which a woman who had the Essure micro-insert placed 7 years earlier presented with a complaint of a bilateral tingling sensation over the course of 6 months. Online research led her to suspect Essure as the cause of her symptoms. However, on further investigation, it turned out she had relatively high levels of lead in her system from leaky pipes in her home. “It wasn’t an Essure issue,” he said. “But because it’s out there, people will jump to the conclusion that the foreign body is likely the cause of their problem.”
Ob.gyns. should become familiar with websites such as essureproblems.webs.com, which chronicle problems patients have reported with the device, he said.
When talking to patients, start with informed consent and listen to their concerns, Dr. Robinson advised. As part of the counseling about Essure permanent birth control, discuss the risks and benefits of alternatives, such as laparoscopic tubal ligation, long-acting reversible contraception, and vasectomy.
“I took the time to listen to the FDA hearing in Sept 2015 and … it moved me to listen to those patients, and I’ve been a huge advocate of Essure sterilization. I felt for a while I would never do another tubal ligation,” Dr. Robinson said. “But when you listen to patients who have real complaints, what sticks out in your mind is so many of these people are upset because no one took them seriously and listened to them.”
Key clinical point:
Major finding: Of 1,430 women who had the Essure inserts placed, just 12 had new-onset pain that was found to be related to the device.
Data source: Retrospective cohort study of 1,430 women treated at a tertiary care hospital in Canada.
Disclosures: Dr. Robinson reported having no financial disclosures.
Tips for avoiding nerve injuries in gynecologic surgery
Upper- and lower-extremity injuries can occur during gynecologic surgery. The incidence of lower-extremity injury is 1.1%-1.9% and upper-extremity injuries can occur in 0.16% of cases.1-5 Fortunately, most of the injuries are transient, sensory injuries that resolve spontaneously. However, a small percentage of injuries result in long-term sequelae.
The pathophysiology of the nerve injuries can be mechanistically separated into three categories: neuropraxia, axonotmesis, and neurotmesis. Neuropraxia results from nerve demyelination at the site of injury because of compression and typically resolves within weeks to months as the nerve is remyelinated. Axonotmesis results from severe compression with axon damage. This may take up to a year to resolve as axonal regeneration proceeds at the rate of 1 mm per day. This can be separated into second and third degree and refers to the severity of damage and the resultant persistent deficit. Neurotmesis results from complete transection and is associated with a poor prognosis without reparative surgery.
Brachial plexus
Stretch injury is the most common reason for a brachial plexus injury. This can occur if the arm board is extended to greater than 90 degrees from the patient’s torso or if the patient’s arm falls off of the arm board. Careful positioning and securing the patient’s arm on the arm board before draping can avoid this injury. A brachial plexus injury can also occur if shoulder braces are placed too laterally during minimally invasive surgery. Radial nerve injuries can occur if there is too much pressure on the humerus during positioning. Ulnar injuries arise from pressure placed on the medial aspect of the elbow.
Tip #1: When tucking a patient’s arm for minimally invasive surgery, appropriate padding should be placed around the elbow and wrist, and the arm should be in the “thumbs-up” position.
Tip #2: Shoulder blocks should be placed over the acromioclavicular (AC) joint.
Lumbosacral plexus
The femoral nerve is the nerve most commonly injured during gynecologic surgery and this usually occurs because of compression of the nerve from the lateral blades of self-retaining retractors. One study showed an 8% incidence of injury from self-retaining retractors, compared with less than 1% when the retractors were not used.6 The femoral nerve can also be stretched when patients are placed in the lithotomy position and the hip is hyperflexed.
As with brachial injury prevention, patients should be positioned prior to draping and care must be taken to not hyperflex or externally rotate the hip during minimally invasive surgical procedures. With the introduction of robot-assisted surgery, care must be taken when docking the robot and surgeons must resist excessive movement of the stirrups.
Tip #3: During laparotomy, surgeons should use the shortest blades that allow for adequate visualization and check the blades during the procedure to ensure that excessive pressure is not placed on the psoas muscle. Consider intermittently releasing the pressure on the lateral blades during other portions of the procedure.
Tip #4: Make sure the stirrups are at the same height and that the leg is in line with the patient’s contralateral shoulder.
Obturator nerve injuries can occur during retroperitoneal dissection for pelvic lymphadenectomy (obturator nodes) and can be either a transection or a cautery injury. It can also be injured during urogynecologic procedures including paravaginal defect repairs and during the placement of transobturator tapes.
The sciatic nerve and its branch, the common peroneal, are generally injured because of excessive stretch or pressure. Both nerves can be injured from hyperflexion of the thigh and the common peroneal can suffer a pressure injury as it courses around the lateral head of the fibula. Therefore, care during lithotomy positioning with both candy cane and Allen stirrups is critical during vaginal surgery.
Tip #5: Ensure that the lateral fibula is not touching the stirrup or that padding is placed between the fibular head and the stirrup.
The ilioinguinal and iliohypogastric nerves are typically injured via suture entrapment from low transverse skin incisions, though laparoscopic injury has also been reported. The incidence after a Pfannenstiel incision is about 3.7%.7
Tip #6: Avoid extending the low transverse incision beyond the lateral margin of the rectus muscle, and do not extend the fascial closure suture more than 1.5 cm from the lateral edge of the fascial incision to avoid catching the nerve with the suture.
The pudendal nerve is most commonly injured during vaginal procedures such as sacrospinous fixation. Pain is typically worse when seated.
The genitofemoral nerve is typically injured during retroperitoneal lymph node dissection, particularly the external iliac nodes. The nerve is small and runs lateral to the external iliac artery. It can suffer cautery and transection injuries. Usually, the paresthesias over the mons pubis, labia majora, and medial inner thigh are temporary.
Tip #7: Care should be taken to identify and spare the nerve during retroperitoneal dissection or external iliac node removal.
Nerve injuries during gynecologic surgery are common and are a significant cause of potential morbidity. While occasionally unavoidable and inherent to the surgical procedure, many times the injury could be prevented with proper attention and care to patient positioning and retractor use. Gynecologists should be aware of the risks and have a through understanding of the anatomy. However, should an injury occur, the patient can be reassured that most are self-limited and full recovery is generally expected. In a prospective study, the median time to resolution of symptoms was 31.5 days (range, 1 day to 6 months).5
References
1. The Obstetrician & Gynaecologist 2014;16:29-36.
2. Gynecol Oncol. 1988 Nov;31(3):462-6.
3. Fertil Steril. 1993 Oct;60(4):729-32.
4. J Minim Invasive Gynecol. 2007 Sep-Oct;14(5):664-72.
5. Am J Obstet Gynecol. 2009 Nov;201(5):531.e1-7.
6. Eur J Obstet Gynecol Reprod Biol. 1985 Dec;20(6):385-92.
7. Obstet Gynecol. 2008 Apr;111(4):839-46.
Dr. Gehrig is professor and director of gynecologic oncology at the University of North Carolina at Chapel Hill. She reported having no financial disclosures relevant to this column. Email her at [email protected].
Upper- and lower-extremity injuries can occur during gynecologic surgery. The incidence of lower-extremity injury is 1.1%-1.9% and upper-extremity injuries can occur in 0.16% of cases.1-5 Fortunately, most of the injuries are transient, sensory injuries that resolve spontaneously. However, a small percentage of injuries result in long-term sequelae.
The pathophysiology of the nerve injuries can be mechanistically separated into three categories: neuropraxia, axonotmesis, and neurotmesis. Neuropraxia results from nerve demyelination at the site of injury because of compression and typically resolves within weeks to months as the nerve is remyelinated. Axonotmesis results from severe compression with axon damage. This may take up to a year to resolve as axonal regeneration proceeds at the rate of 1 mm per day. This can be separated into second and third degree and refers to the severity of damage and the resultant persistent deficit. Neurotmesis results from complete transection and is associated with a poor prognosis without reparative surgery.
Brachial plexus
Stretch injury is the most common reason for a brachial plexus injury. This can occur if the arm board is extended to greater than 90 degrees from the patient’s torso or if the patient’s arm falls off of the arm board. Careful positioning and securing the patient’s arm on the arm board before draping can avoid this injury. A brachial plexus injury can also occur if shoulder braces are placed too laterally during minimally invasive surgery. Radial nerve injuries can occur if there is too much pressure on the humerus during positioning. Ulnar injuries arise from pressure placed on the medial aspect of the elbow.
Tip #1: When tucking a patient’s arm for minimally invasive surgery, appropriate padding should be placed around the elbow and wrist, and the arm should be in the “thumbs-up” position.
Tip #2: Shoulder blocks should be placed over the acromioclavicular (AC) joint.
Lumbosacral plexus
The femoral nerve is the nerve most commonly injured during gynecologic surgery and this usually occurs because of compression of the nerve from the lateral blades of self-retaining retractors. One study showed an 8% incidence of injury from self-retaining retractors, compared with less than 1% when the retractors were not used.6 The femoral nerve can also be stretched when patients are placed in the lithotomy position and the hip is hyperflexed.
As with brachial injury prevention, patients should be positioned prior to draping and care must be taken to not hyperflex or externally rotate the hip during minimally invasive surgical procedures. With the introduction of robot-assisted surgery, care must be taken when docking the robot and surgeons must resist excessive movement of the stirrups.
Tip #3: During laparotomy, surgeons should use the shortest blades that allow for adequate visualization and check the blades during the procedure to ensure that excessive pressure is not placed on the psoas muscle. Consider intermittently releasing the pressure on the lateral blades during other portions of the procedure.
Tip #4: Make sure the stirrups are at the same height and that the leg is in line with the patient’s contralateral shoulder.
Obturator nerve injuries can occur during retroperitoneal dissection for pelvic lymphadenectomy (obturator nodes) and can be either a transection or a cautery injury. It can also be injured during urogynecologic procedures including paravaginal defect repairs and during the placement of transobturator tapes.
The sciatic nerve and its branch, the common peroneal, are generally injured because of excessive stretch or pressure. Both nerves can be injured from hyperflexion of the thigh and the common peroneal can suffer a pressure injury as it courses around the lateral head of the fibula. Therefore, care during lithotomy positioning with both candy cane and Allen stirrups is critical during vaginal surgery.
Tip #5: Ensure that the lateral fibula is not touching the stirrup or that padding is placed between the fibular head and the stirrup.
The ilioinguinal and iliohypogastric nerves are typically injured via suture entrapment from low transverse skin incisions, though laparoscopic injury has also been reported. The incidence after a Pfannenstiel incision is about 3.7%.7
Tip #6: Avoid extending the low transverse incision beyond the lateral margin of the rectus muscle, and do not extend the fascial closure suture more than 1.5 cm from the lateral edge of the fascial incision to avoid catching the nerve with the suture.
The pudendal nerve is most commonly injured during vaginal procedures such as sacrospinous fixation. Pain is typically worse when seated.
The genitofemoral nerve is typically injured during retroperitoneal lymph node dissection, particularly the external iliac nodes. The nerve is small and runs lateral to the external iliac artery. It can suffer cautery and transection injuries. Usually, the paresthesias over the mons pubis, labia majora, and medial inner thigh are temporary.
Tip #7: Care should be taken to identify and spare the nerve during retroperitoneal dissection or external iliac node removal.
Nerve injuries during gynecologic surgery are common and are a significant cause of potential morbidity. While occasionally unavoidable and inherent to the surgical procedure, many times the injury could be prevented with proper attention and care to patient positioning and retractor use. Gynecologists should be aware of the risks and have a through understanding of the anatomy. However, should an injury occur, the patient can be reassured that most are self-limited and full recovery is generally expected. In a prospective study, the median time to resolution of symptoms was 31.5 days (range, 1 day to 6 months).5
References
1. The Obstetrician & Gynaecologist 2014;16:29-36.
2. Gynecol Oncol. 1988 Nov;31(3):462-6.
3. Fertil Steril. 1993 Oct;60(4):729-32.
4. J Minim Invasive Gynecol. 2007 Sep-Oct;14(5):664-72.
5. Am J Obstet Gynecol. 2009 Nov;201(5):531.e1-7.
6. Eur J Obstet Gynecol Reprod Biol. 1985 Dec;20(6):385-92.
7. Obstet Gynecol. 2008 Apr;111(4):839-46.
Dr. Gehrig is professor and director of gynecologic oncology at the University of North Carolina at Chapel Hill. She reported having no financial disclosures relevant to this column. Email her at [email protected].
Upper- and lower-extremity injuries can occur during gynecologic surgery. The incidence of lower-extremity injury is 1.1%-1.9% and upper-extremity injuries can occur in 0.16% of cases.1-5 Fortunately, most of the injuries are transient, sensory injuries that resolve spontaneously. However, a small percentage of injuries result in long-term sequelae.
The pathophysiology of the nerve injuries can be mechanistically separated into three categories: neuropraxia, axonotmesis, and neurotmesis. Neuropraxia results from nerve demyelination at the site of injury because of compression and typically resolves within weeks to months as the nerve is remyelinated. Axonotmesis results from severe compression with axon damage. This may take up to a year to resolve as axonal regeneration proceeds at the rate of 1 mm per day. This can be separated into second and third degree and refers to the severity of damage and the resultant persistent deficit. Neurotmesis results from complete transection and is associated with a poor prognosis without reparative surgery.
Brachial plexus
Stretch injury is the most common reason for a brachial plexus injury. This can occur if the arm board is extended to greater than 90 degrees from the patient’s torso or if the patient’s arm falls off of the arm board. Careful positioning and securing the patient’s arm on the arm board before draping can avoid this injury. A brachial plexus injury can also occur if shoulder braces are placed too laterally during minimally invasive surgery. Radial nerve injuries can occur if there is too much pressure on the humerus during positioning. Ulnar injuries arise from pressure placed on the medial aspect of the elbow.
Tip #1: When tucking a patient’s arm for minimally invasive surgery, appropriate padding should be placed around the elbow and wrist, and the arm should be in the “thumbs-up” position.
Tip #2: Shoulder blocks should be placed over the acromioclavicular (AC) joint.
Lumbosacral plexus
The femoral nerve is the nerve most commonly injured during gynecologic surgery and this usually occurs because of compression of the nerve from the lateral blades of self-retaining retractors. One study showed an 8% incidence of injury from self-retaining retractors, compared with less than 1% when the retractors were not used.6 The femoral nerve can also be stretched when patients are placed in the lithotomy position and the hip is hyperflexed.
As with brachial injury prevention, patients should be positioned prior to draping and care must be taken to not hyperflex or externally rotate the hip during minimally invasive surgical procedures. With the introduction of robot-assisted surgery, care must be taken when docking the robot and surgeons must resist excessive movement of the stirrups.
Tip #3: During laparotomy, surgeons should use the shortest blades that allow for adequate visualization and check the blades during the procedure to ensure that excessive pressure is not placed on the psoas muscle. Consider intermittently releasing the pressure on the lateral blades during other portions of the procedure.
Tip #4: Make sure the stirrups are at the same height and that the leg is in line with the patient’s contralateral shoulder.
Obturator nerve injuries can occur during retroperitoneal dissection for pelvic lymphadenectomy (obturator nodes) and can be either a transection or a cautery injury. It can also be injured during urogynecologic procedures including paravaginal defect repairs and during the placement of transobturator tapes.
The sciatic nerve and its branch, the common peroneal, are generally injured because of excessive stretch or pressure. Both nerves can be injured from hyperflexion of the thigh and the common peroneal can suffer a pressure injury as it courses around the lateral head of the fibula. Therefore, care during lithotomy positioning with both candy cane and Allen stirrups is critical during vaginal surgery.
Tip #5: Ensure that the lateral fibula is not touching the stirrup or that padding is placed between the fibular head and the stirrup.
The ilioinguinal and iliohypogastric nerves are typically injured via suture entrapment from low transverse skin incisions, though laparoscopic injury has also been reported. The incidence after a Pfannenstiel incision is about 3.7%.7
Tip #6: Avoid extending the low transverse incision beyond the lateral margin of the rectus muscle, and do not extend the fascial closure suture more than 1.5 cm from the lateral edge of the fascial incision to avoid catching the nerve with the suture.
The pudendal nerve is most commonly injured during vaginal procedures such as sacrospinous fixation. Pain is typically worse when seated.
The genitofemoral nerve is typically injured during retroperitoneal lymph node dissection, particularly the external iliac nodes. The nerve is small and runs lateral to the external iliac artery. It can suffer cautery and transection injuries. Usually, the paresthesias over the mons pubis, labia majora, and medial inner thigh are temporary.
Tip #7: Care should be taken to identify and spare the nerve during retroperitoneal dissection or external iliac node removal.
Nerve injuries during gynecologic surgery are common and are a significant cause of potential morbidity. While occasionally unavoidable and inherent to the surgical procedure, many times the injury could be prevented with proper attention and care to patient positioning and retractor use. Gynecologists should be aware of the risks and have a through understanding of the anatomy. However, should an injury occur, the patient can be reassured that most are self-limited and full recovery is generally expected. In a prospective study, the median time to resolution of symptoms was 31.5 days (range, 1 day to 6 months).5
References
1. The Obstetrician & Gynaecologist 2014;16:29-36.
2. Gynecol Oncol. 1988 Nov;31(3):462-6.
3. Fertil Steril. 1993 Oct;60(4):729-32.
4. J Minim Invasive Gynecol. 2007 Sep-Oct;14(5):664-72.
5. Am J Obstet Gynecol. 2009 Nov;201(5):531.e1-7.
6. Eur J Obstet Gynecol Reprod Biol. 1985 Dec;20(6):385-92.
7. Obstet Gynecol. 2008 Apr;111(4):839-46.
Dr. Gehrig is professor and director of gynecologic oncology at the University of North Carolina at Chapel Hill. She reported having no financial disclosures relevant to this column. Email her at [email protected].