User login
Hair loss: Consider a patient’s supplement use
BOSTON – .
This is an important question because patients consider supplements as “natural and healthy,” not as drugs or chemicals, Wilma F. Bergfeld, MD, said at the annual meeting of the American Academy of Dermatology.
Some of these products contain botanicals, which are not always safe, added Dr. Bergfeld, professor of dermatology and pathology at the Cleveland Clinic. “They have many activities, and they are being touted as having some activity in helping the hair or enhancing hair growth,” including having 5-alpha-reductase inhibitors as an ingredient. “Saw palmetto is probably the most common one, but there are a host of natural ingredients that are being put into these supplements, including those that promote androgen induction, as well as antioxidants and anti-inflammatories.”
In the opinion of Dr. Bergfeld, a nutrition-focused physical assessment should include an examination of the scalp and all hairy areas. “It’s also important to see the symmetry and shape of hair growth or hair loss areas, the distribution, hair color, the thickness and texture of the hair fibers,” she added.
Besides asking about what supplements patients are taking, other questions to ask during the visit include: Are you noticing more hair on your brush, pillow, and shoulders, or in the shower? Do you think your hair is thinning? What are your medical problems? Have you experienced rapid weight loss? Have you started any new medications? What medication(s) are you on? What foods do you eat? Do you have a family history of hair loss?
Possible causes of hair loss or changes include environmental factors, stress, hormonal changes, medications, and nutrition.
Common ingredients contained in healthy hair supplements include biotin, folic acid, L-cysteine, L-methionine, MSM (methylsulfonylmethane), vitamin B complex, and vitamins A, C, D, and E. “Vitamin D and A are associated on the hair follicle receptor sites, and they balance each other, so if one is down the other is usually down,” said Dr. Bergfeld, who directs Cleveland Clinic’s hair disorders clinic and its dermatopathology program. Other important ingredients include iron, zinc, manganese, amino acids including L-Lysine, and fatty acids.
Iron deficiency is a known cause of hair loss. “The absorption of iron relies on vitamin C and sometimes lysine,” she said. Red meat has a high iron content and since many patients are restricting red meat intake, “they do need to think about that.” Zinc deficiency is less common in Western countries, she continued, “but when you find it, it’s revolutionary because if they’re shedding hair and their hair character is changing, often some supplementation will do the trick. But remember: Zinc is not only an anti-inflammatory, it’s also an antiandrogen. It has 5-alpha-reductase inhibitor capabilities.”.
Dr. Bergfeld noted that biotin, also known as vitamin B7 and found in many foods, is used in many vitamin supplements marketed for hair loss. The recommended daily allowance (RDA) is 30 mcg/day in adults but the amount in hair supplements can be up to 650% of RDA. “Biotin at high levels is believed to be safe, but can interfere with troponin and other lab testing,” she cautioned. “This can lead to dangerous false laboratory results.”
To date, insufficient data exist to recommend supplementation with zinc, riboflavin, folic acid, or vitamin B12 for hair loss, “but they may help in cases of deficiency,” said Dr. Bergfeld, a past president of the American Hair Research Society. The use of vitamin E and biotin supplementation is not supported in the literature for treating androgenetic alopecia or telogen effluvium. Excessive vitamin A (not beta carotene) and selenium can contribute to hair loss and studies have shown a relationship between androgenetic alopecia and low vitamin D levels. “Vitamin D should be supplemented if serum levels are low, but more studies are needed to determine the effect of iron and zinc supplementation” in patients with androgenetic alopecia, she said.
While there are not enough data to support a recommendation for supplementation of folic or B12 for alopecia, she said, “vitamin B12 deficiency may occur in androgenetic alopecia patients, associated with pernicious anemia.”
She added that the use biotin supplementation for the treatment of androgenetic alopecia is not supported by available data, and “it is also unclear if selenium plays a role in this disease.”
Dr. Bergfeld reported having no disclosures related to her presentation.
BOSTON – .
This is an important question because patients consider supplements as “natural and healthy,” not as drugs or chemicals, Wilma F. Bergfeld, MD, said at the annual meeting of the American Academy of Dermatology.
Some of these products contain botanicals, which are not always safe, added Dr. Bergfeld, professor of dermatology and pathology at the Cleveland Clinic. “They have many activities, and they are being touted as having some activity in helping the hair or enhancing hair growth,” including having 5-alpha-reductase inhibitors as an ingredient. “Saw palmetto is probably the most common one, but there are a host of natural ingredients that are being put into these supplements, including those that promote androgen induction, as well as antioxidants and anti-inflammatories.”
In the opinion of Dr. Bergfeld, a nutrition-focused physical assessment should include an examination of the scalp and all hairy areas. “It’s also important to see the symmetry and shape of hair growth or hair loss areas, the distribution, hair color, the thickness and texture of the hair fibers,” she added.
Besides asking about what supplements patients are taking, other questions to ask during the visit include: Are you noticing more hair on your brush, pillow, and shoulders, or in the shower? Do you think your hair is thinning? What are your medical problems? Have you experienced rapid weight loss? Have you started any new medications? What medication(s) are you on? What foods do you eat? Do you have a family history of hair loss?
Possible causes of hair loss or changes include environmental factors, stress, hormonal changes, medications, and nutrition.
Common ingredients contained in healthy hair supplements include biotin, folic acid, L-cysteine, L-methionine, MSM (methylsulfonylmethane), vitamin B complex, and vitamins A, C, D, and E. “Vitamin D and A are associated on the hair follicle receptor sites, and they balance each other, so if one is down the other is usually down,” said Dr. Bergfeld, who directs Cleveland Clinic’s hair disorders clinic and its dermatopathology program. Other important ingredients include iron, zinc, manganese, amino acids including L-Lysine, and fatty acids.
Iron deficiency is a known cause of hair loss. “The absorption of iron relies on vitamin C and sometimes lysine,” she said. Red meat has a high iron content and since many patients are restricting red meat intake, “they do need to think about that.” Zinc deficiency is less common in Western countries, she continued, “but when you find it, it’s revolutionary because if they’re shedding hair and their hair character is changing, often some supplementation will do the trick. But remember: Zinc is not only an anti-inflammatory, it’s also an antiandrogen. It has 5-alpha-reductase inhibitor capabilities.”.
Dr. Bergfeld noted that biotin, also known as vitamin B7 and found in many foods, is used in many vitamin supplements marketed for hair loss. The recommended daily allowance (RDA) is 30 mcg/day in adults but the amount in hair supplements can be up to 650% of RDA. “Biotin at high levels is believed to be safe, but can interfere with troponin and other lab testing,” she cautioned. “This can lead to dangerous false laboratory results.”
To date, insufficient data exist to recommend supplementation with zinc, riboflavin, folic acid, or vitamin B12 for hair loss, “but they may help in cases of deficiency,” said Dr. Bergfeld, a past president of the American Hair Research Society. The use of vitamin E and biotin supplementation is not supported in the literature for treating androgenetic alopecia or telogen effluvium. Excessive vitamin A (not beta carotene) and selenium can contribute to hair loss and studies have shown a relationship between androgenetic alopecia and low vitamin D levels. “Vitamin D should be supplemented if serum levels are low, but more studies are needed to determine the effect of iron and zinc supplementation” in patients with androgenetic alopecia, she said.
While there are not enough data to support a recommendation for supplementation of folic or B12 for alopecia, she said, “vitamin B12 deficiency may occur in androgenetic alopecia patients, associated with pernicious anemia.”
She added that the use biotin supplementation for the treatment of androgenetic alopecia is not supported by available data, and “it is also unclear if selenium plays a role in this disease.”
Dr. Bergfeld reported having no disclosures related to her presentation.
BOSTON – .
This is an important question because patients consider supplements as “natural and healthy,” not as drugs or chemicals, Wilma F. Bergfeld, MD, said at the annual meeting of the American Academy of Dermatology.
Some of these products contain botanicals, which are not always safe, added Dr. Bergfeld, professor of dermatology and pathology at the Cleveland Clinic. “They have many activities, and they are being touted as having some activity in helping the hair or enhancing hair growth,” including having 5-alpha-reductase inhibitors as an ingredient. “Saw palmetto is probably the most common one, but there are a host of natural ingredients that are being put into these supplements, including those that promote androgen induction, as well as antioxidants and anti-inflammatories.”
In the opinion of Dr. Bergfeld, a nutrition-focused physical assessment should include an examination of the scalp and all hairy areas. “It’s also important to see the symmetry and shape of hair growth or hair loss areas, the distribution, hair color, the thickness and texture of the hair fibers,” she added.
Besides asking about what supplements patients are taking, other questions to ask during the visit include: Are you noticing more hair on your brush, pillow, and shoulders, or in the shower? Do you think your hair is thinning? What are your medical problems? Have you experienced rapid weight loss? Have you started any new medications? What medication(s) are you on? What foods do you eat? Do you have a family history of hair loss?
Possible causes of hair loss or changes include environmental factors, stress, hormonal changes, medications, and nutrition.
Common ingredients contained in healthy hair supplements include biotin, folic acid, L-cysteine, L-methionine, MSM (methylsulfonylmethane), vitamin B complex, and vitamins A, C, D, and E. “Vitamin D and A are associated on the hair follicle receptor sites, and they balance each other, so if one is down the other is usually down,” said Dr. Bergfeld, who directs Cleveland Clinic’s hair disorders clinic and its dermatopathology program. Other important ingredients include iron, zinc, manganese, amino acids including L-Lysine, and fatty acids.
Iron deficiency is a known cause of hair loss. “The absorption of iron relies on vitamin C and sometimes lysine,” she said. Red meat has a high iron content and since many patients are restricting red meat intake, “they do need to think about that.” Zinc deficiency is less common in Western countries, she continued, “but when you find it, it’s revolutionary because if they’re shedding hair and their hair character is changing, often some supplementation will do the trick. But remember: Zinc is not only an anti-inflammatory, it’s also an antiandrogen. It has 5-alpha-reductase inhibitor capabilities.”.
Dr. Bergfeld noted that biotin, also known as vitamin B7 and found in many foods, is used in many vitamin supplements marketed for hair loss. The recommended daily allowance (RDA) is 30 mcg/day in adults but the amount in hair supplements can be up to 650% of RDA. “Biotin at high levels is believed to be safe, but can interfere with troponin and other lab testing,” she cautioned. “This can lead to dangerous false laboratory results.”
To date, insufficient data exist to recommend supplementation with zinc, riboflavin, folic acid, or vitamin B12 for hair loss, “but they may help in cases of deficiency,” said Dr. Bergfeld, a past president of the American Hair Research Society. The use of vitamin E and biotin supplementation is not supported in the literature for treating androgenetic alopecia or telogen effluvium. Excessive vitamin A (not beta carotene) and selenium can contribute to hair loss and studies have shown a relationship between androgenetic alopecia and low vitamin D levels. “Vitamin D should be supplemented if serum levels are low, but more studies are needed to determine the effect of iron and zinc supplementation” in patients with androgenetic alopecia, she said.
While there are not enough data to support a recommendation for supplementation of folic or B12 for alopecia, she said, “vitamin B12 deficiency may occur in androgenetic alopecia patients, associated with pernicious anemia.”
She added that the use biotin supplementation for the treatment of androgenetic alopecia is not supported by available data, and “it is also unclear if selenium plays a role in this disease.”
Dr. Bergfeld reported having no disclosures related to her presentation.
AT AAD 22
Trichotillomania: What you should know about this common hair-pulling disorder
Trichotillomania is a chronic psychiatric disorder that causes people to repeatedly pull out their own hair. Not only does it result in alopecia with no other underlying causes but it can have significant psychosocial ramifications and rare, but serious, complications. Though the reported prevalence rates are up to approximately 2%, it’s probable that you’ll come upon a patient suffering with this disorder at your practice, if you haven’t already.
To find out more about the best methods for diagnosing and treating this disorder, we spoke with Jon E. Grant, JD, MD, MPH, a leading trichotillomania researcher and part of the department of psychiatry and behavioral neuroscience at the University of Chicago.
Defining trichotillomania
What were the earliest descriptions of trichotillomania in medical literature?
The first real discussion of it probably goes back to Hippocrates, but from a modern medical perspective, discussion began in the 19th century with reports from the French dermatologist François Hallopeau.
They didn’t really call them disorders then – it was long before the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) – but they described this in young men who kept pulling their hair for unclear reasons. These early case reports don’t provide a lot of psychological perspective, but they seem consistent with what we see now.
What are the diagnostic criteria for trichotillomania?
The current DSM-5 criteria are recurrent pulling out of hair, an inability to stop it, the pulling resulting in some noticeable thinning or hair loss, and that it causes some level of distress or some type of impairment in functioning.
At what age do most people experience an onset of symptoms?
Generally speaking, it’s in early adolescence, post puberty, around 12-15 years of age. Having said that, we do see children as young as 1-2 years who are pulling their hair, and we occasionally see somebody far older who is doing it for the first time, a sort of geriatric onset.
Overlap and differences with other disorders
You’ve written that although trichotillomania is grouped with obsessive-compulsive disorder (OCD) in the DSM-5, the thinking around that has recently shifted. Why is that?
At first, it was noticed that many of these people pulled their hair repetitively in an almost ritualized manner, perhaps every night before bed. That looked like a compulsion of OCD.
When DSM-5 came out in 2013, they grouped it with OCD. Yet people shifted to thinking that it’s kind of a cousin of OCD because it has this compulsive quality but doesn’t really have obsessive thinking that drives it. Many people just pull their hair. They’re not even always aware of it: sometimes yes, sometimes no.
We know that it has some links to OCD. You’ll see more OCD in folks with trichotillomania, but it clearly is not just the same as OCD. One of the biggest pieces of evidence for that is that our first-line treatment for OCD – a selective serotonin reuptake inhibitor antidepressant – does not really help hair pulling.
Having said that, if people are looking for help with trichotillomania, they often are best served by therapists and doctors who have a familiarity with OCD and have kept it on their radar over the past couple of decades.
How does trichotillomania overlap with skin picking disorder, which is another condition that you’ve closely researched?
It does have some overlap with skin picking in the sense that it often seems familial. For example, the mother may pull her hair and child picks their skin.
It also has a fair amount of comorbidity with skin picking. Many people who pull will pick a little bit or did at some point. Many people who pick pulled their hair at some point. It seems closely related to nail biting as well.
Studies have also shown that one of the things that runs in the histories of most families of people with trichotillomania might be substance abuse – alcohol or drug addiction.
All of this has led people to believe that there might be subtypes of trichotillomania: one that’s more like an OCD and one that’s more like an addiction. That’s similar to the debate with other mental health conditions, that there are probably multiple types of depression, multiple types of schizophrenia.
Is there a component of this that could be defined as self-harm?
That’s been its own debate. It doesn’t seem to have the same developmental trajectory that we see with self-harm, or even some of the personality features.
However, there may be a small segment of folks with trichotillomania that might more appropriately fit that category. For example, those with family histories of trauma, higher rates of posttraumatic stress disorder, or borderline personality. But it wouldn’t be the majority.
The problem is, if you look at some of the pediatrician data, they often group picking, pulling, and cutting. I think that’s far too all-inclusive.
A gap in clinician education
Are adolescent patients likely to self-report this behavior, or is it something that physicians need to suss out for themselves?
Clearly, if child psychologists, psychiatrists, or pediatricians see young people with patches of alopecia – eyebrows or eyelashes missing, head hair with spots – in addition to a dermatologic assessment, they should simply ask, “Do you pull your hair?”
But it’s interesting that with the internet, young people are much more likely to disclose and actually come forward and tell their parents that they think they have trichotillomania.
I also hear from a lot of the adolescents that they have to educate their doctors about trichotillomania because so often physicians don’t know much about it and will assume that it’s self-injury or just a symptom of anxiety. It’s a little bit of a flip from what we might have seen 20 years ago.
I’ve seen several patients who’ve said, basically, “I’m tired of no professionals seeming to know about this. I shouldn’t have to be educating my doctors about this.” I tell them that I completely agree. It’s a shame because if a doctor doesn’t know about it, then how can they get the appropriate care?
What are the complications that accompany trichotillomania?
A small percentage, maybe about 10%, will ingest their hair, much like people who bite and swallow their fingernails. The concern there is that because hair is nondigestible, it could create an intestinal plug that could rupture and be potentially life-threatening. That makes it all the more important to ask those who pull their hair what they do with the hair once they pull it.
However, with most people, the real problem is with self-esteem. Young people may not want to socialize, go on dates, or do other things they would normally do because of it. In adults, you may find that they’re far more educated than their job allows but don’t want to go to an interview because they don’t want to have somebody sit there and look at them and notice that perhaps they don’t have any eyebrows, or that they’re wearing a wig. Those psychosocial implications are huge for so many people.
Treatment options
In a 2021 study, you showed that nearly one-quarter of people with trichotillomania do naturally recover from it. What characteristics do they seem to have?
It’s interesting because we see natural recovery across many mental health problems: alcohol addition, gambling, OCD. The question then becomes why is that some people can seemingly just stop doing a behavior? Can we learn from those people?
We did see that those who naturally recovered were less likely to have some other mental health comorbidities. It seems like when you have other things such as skin picking or OCD plus trichotillomania, that it probably speaks to something that perhaps synergistically is keeping it going. But this is just a first study; learning how to harness and understand it is the next step.
What’s the goal of treating trichotillomania?
The desired goal is zero pulling. The realistic goal is more likely significantly reduced pulling that then leads to greater function in life, greater quality-of-life.
One doesn’t have to go from 100 to 0 in order to do that. I always tell people that maybe every now and then, every few months, when something is going on in life, you might find yourself pulling a hair or two. That’s okay. If you’re not pulling every day and it’s significantly reduced, we’ll call that a success. I think that setting reasonable goals at this point is really important.
And what would the treatment pathway look like for most patients?
The standard approach is probably some type of habit-reversal therapy, of which there have been many variants over the years. It involves doing something different with your hand, identifying the triggers that may set you off, and then doing something in response to those triggers that is not pulling and might neutralize whatever that anxious or stressed feeling is. That could be different with each person.
At this point, there is no drug approved by the U.S. Food and Drug Administration for trichotillomania. Our best approaches have included N-acetylcysteine, a glutamate modulator, which we’ve done research in.
That’s kind of a go-to option for people because its side-effect profile is generally innocuous. The data show that it could be beneficial in many people with very few, if any, side effects. That would be one “medication,” although it’s actually an over-the-counter vitamin. But we’re constantly looking for better and better treatments.
Do you have any final advice for clinicians or researchers?
Given how common it is, I don’t think clinicians should just see it as an innocuous little habit that people should be able to stop on their own. Clinicians should educate themselves about trichotillomania and know where the person should get the appropriate care.
From the research perspective, given the fact that we see this in animals of multiple species – that they overgroom – this seems to be deeply ingrained in us as animals. So when it comes to the underlying neuroscience, people should pay more attention because it probably has a lot to do with our understanding of habit and compulsive behaviors. It arguably can cut across a lot of different behaviors.
A version of this article first appeared on Medscape.com.
Trichotillomania is a chronic psychiatric disorder that causes people to repeatedly pull out their own hair. Not only does it result in alopecia with no other underlying causes but it can have significant psychosocial ramifications and rare, but serious, complications. Though the reported prevalence rates are up to approximately 2%, it’s probable that you’ll come upon a patient suffering with this disorder at your practice, if you haven’t already.
To find out more about the best methods for diagnosing and treating this disorder, we spoke with Jon E. Grant, JD, MD, MPH, a leading trichotillomania researcher and part of the department of psychiatry and behavioral neuroscience at the University of Chicago.
Defining trichotillomania
What were the earliest descriptions of trichotillomania in medical literature?
The first real discussion of it probably goes back to Hippocrates, but from a modern medical perspective, discussion began in the 19th century with reports from the French dermatologist François Hallopeau.
They didn’t really call them disorders then – it was long before the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) – but they described this in young men who kept pulling their hair for unclear reasons. These early case reports don’t provide a lot of psychological perspective, but they seem consistent with what we see now.
What are the diagnostic criteria for trichotillomania?
The current DSM-5 criteria are recurrent pulling out of hair, an inability to stop it, the pulling resulting in some noticeable thinning or hair loss, and that it causes some level of distress or some type of impairment in functioning.
At what age do most people experience an onset of symptoms?
Generally speaking, it’s in early adolescence, post puberty, around 12-15 years of age. Having said that, we do see children as young as 1-2 years who are pulling their hair, and we occasionally see somebody far older who is doing it for the first time, a sort of geriatric onset.
Overlap and differences with other disorders
You’ve written that although trichotillomania is grouped with obsessive-compulsive disorder (OCD) in the DSM-5, the thinking around that has recently shifted. Why is that?
At first, it was noticed that many of these people pulled their hair repetitively in an almost ritualized manner, perhaps every night before bed. That looked like a compulsion of OCD.
When DSM-5 came out in 2013, they grouped it with OCD. Yet people shifted to thinking that it’s kind of a cousin of OCD because it has this compulsive quality but doesn’t really have obsessive thinking that drives it. Many people just pull their hair. They’re not even always aware of it: sometimes yes, sometimes no.
We know that it has some links to OCD. You’ll see more OCD in folks with trichotillomania, but it clearly is not just the same as OCD. One of the biggest pieces of evidence for that is that our first-line treatment for OCD – a selective serotonin reuptake inhibitor antidepressant – does not really help hair pulling.
Having said that, if people are looking for help with trichotillomania, they often are best served by therapists and doctors who have a familiarity with OCD and have kept it on their radar over the past couple of decades.
How does trichotillomania overlap with skin picking disorder, which is another condition that you’ve closely researched?
It does have some overlap with skin picking in the sense that it often seems familial. For example, the mother may pull her hair and child picks their skin.
It also has a fair amount of comorbidity with skin picking. Many people who pull will pick a little bit or did at some point. Many people who pick pulled their hair at some point. It seems closely related to nail biting as well.
Studies have also shown that one of the things that runs in the histories of most families of people with trichotillomania might be substance abuse – alcohol or drug addiction.
All of this has led people to believe that there might be subtypes of trichotillomania: one that’s more like an OCD and one that’s more like an addiction. That’s similar to the debate with other mental health conditions, that there are probably multiple types of depression, multiple types of schizophrenia.
Is there a component of this that could be defined as self-harm?
That’s been its own debate. It doesn’t seem to have the same developmental trajectory that we see with self-harm, or even some of the personality features.
However, there may be a small segment of folks with trichotillomania that might more appropriately fit that category. For example, those with family histories of trauma, higher rates of posttraumatic stress disorder, or borderline personality. But it wouldn’t be the majority.
The problem is, if you look at some of the pediatrician data, they often group picking, pulling, and cutting. I think that’s far too all-inclusive.
A gap in clinician education
Are adolescent patients likely to self-report this behavior, or is it something that physicians need to suss out for themselves?
Clearly, if child psychologists, psychiatrists, or pediatricians see young people with patches of alopecia – eyebrows or eyelashes missing, head hair with spots – in addition to a dermatologic assessment, they should simply ask, “Do you pull your hair?”
But it’s interesting that with the internet, young people are much more likely to disclose and actually come forward and tell their parents that they think they have trichotillomania.
I also hear from a lot of the adolescents that they have to educate their doctors about trichotillomania because so often physicians don’t know much about it and will assume that it’s self-injury or just a symptom of anxiety. It’s a little bit of a flip from what we might have seen 20 years ago.
I’ve seen several patients who’ve said, basically, “I’m tired of no professionals seeming to know about this. I shouldn’t have to be educating my doctors about this.” I tell them that I completely agree. It’s a shame because if a doctor doesn’t know about it, then how can they get the appropriate care?
What are the complications that accompany trichotillomania?
A small percentage, maybe about 10%, will ingest their hair, much like people who bite and swallow their fingernails. The concern there is that because hair is nondigestible, it could create an intestinal plug that could rupture and be potentially life-threatening. That makes it all the more important to ask those who pull their hair what they do with the hair once they pull it.
However, with most people, the real problem is with self-esteem. Young people may not want to socialize, go on dates, or do other things they would normally do because of it. In adults, you may find that they’re far more educated than their job allows but don’t want to go to an interview because they don’t want to have somebody sit there and look at them and notice that perhaps they don’t have any eyebrows, or that they’re wearing a wig. Those psychosocial implications are huge for so many people.
Treatment options
In a 2021 study, you showed that nearly one-quarter of people with trichotillomania do naturally recover from it. What characteristics do they seem to have?
It’s interesting because we see natural recovery across many mental health problems: alcohol addition, gambling, OCD. The question then becomes why is that some people can seemingly just stop doing a behavior? Can we learn from those people?
We did see that those who naturally recovered were less likely to have some other mental health comorbidities. It seems like when you have other things such as skin picking or OCD plus trichotillomania, that it probably speaks to something that perhaps synergistically is keeping it going. But this is just a first study; learning how to harness and understand it is the next step.
What’s the goal of treating trichotillomania?
The desired goal is zero pulling. The realistic goal is more likely significantly reduced pulling that then leads to greater function in life, greater quality-of-life.
One doesn’t have to go from 100 to 0 in order to do that. I always tell people that maybe every now and then, every few months, when something is going on in life, you might find yourself pulling a hair or two. That’s okay. If you’re not pulling every day and it’s significantly reduced, we’ll call that a success. I think that setting reasonable goals at this point is really important.
And what would the treatment pathway look like for most patients?
The standard approach is probably some type of habit-reversal therapy, of which there have been many variants over the years. It involves doing something different with your hand, identifying the triggers that may set you off, and then doing something in response to those triggers that is not pulling and might neutralize whatever that anxious or stressed feeling is. That could be different with each person.
At this point, there is no drug approved by the U.S. Food and Drug Administration for trichotillomania. Our best approaches have included N-acetylcysteine, a glutamate modulator, which we’ve done research in.
That’s kind of a go-to option for people because its side-effect profile is generally innocuous. The data show that it could be beneficial in many people with very few, if any, side effects. That would be one “medication,” although it’s actually an over-the-counter vitamin. But we’re constantly looking for better and better treatments.
Do you have any final advice for clinicians or researchers?
Given how common it is, I don’t think clinicians should just see it as an innocuous little habit that people should be able to stop on their own. Clinicians should educate themselves about trichotillomania and know where the person should get the appropriate care.
From the research perspective, given the fact that we see this in animals of multiple species – that they overgroom – this seems to be deeply ingrained in us as animals. So when it comes to the underlying neuroscience, people should pay more attention because it probably has a lot to do with our understanding of habit and compulsive behaviors. It arguably can cut across a lot of different behaviors.
A version of this article first appeared on Medscape.com.
Trichotillomania is a chronic psychiatric disorder that causes people to repeatedly pull out their own hair. Not only does it result in alopecia with no other underlying causes but it can have significant psychosocial ramifications and rare, but serious, complications. Though the reported prevalence rates are up to approximately 2%, it’s probable that you’ll come upon a patient suffering with this disorder at your practice, if you haven’t already.
To find out more about the best methods for diagnosing and treating this disorder, we spoke with Jon E. Grant, JD, MD, MPH, a leading trichotillomania researcher and part of the department of psychiatry and behavioral neuroscience at the University of Chicago.
Defining trichotillomania
What were the earliest descriptions of trichotillomania in medical literature?
The first real discussion of it probably goes back to Hippocrates, but from a modern medical perspective, discussion began in the 19th century with reports from the French dermatologist François Hallopeau.
They didn’t really call them disorders then – it was long before the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) – but they described this in young men who kept pulling their hair for unclear reasons. These early case reports don’t provide a lot of psychological perspective, but they seem consistent with what we see now.
What are the diagnostic criteria for trichotillomania?
The current DSM-5 criteria are recurrent pulling out of hair, an inability to stop it, the pulling resulting in some noticeable thinning or hair loss, and that it causes some level of distress or some type of impairment in functioning.
At what age do most people experience an onset of symptoms?
Generally speaking, it’s in early adolescence, post puberty, around 12-15 years of age. Having said that, we do see children as young as 1-2 years who are pulling their hair, and we occasionally see somebody far older who is doing it for the first time, a sort of geriatric onset.
Overlap and differences with other disorders
You’ve written that although trichotillomania is grouped with obsessive-compulsive disorder (OCD) in the DSM-5, the thinking around that has recently shifted. Why is that?
At first, it was noticed that many of these people pulled their hair repetitively in an almost ritualized manner, perhaps every night before bed. That looked like a compulsion of OCD.
When DSM-5 came out in 2013, they grouped it with OCD. Yet people shifted to thinking that it’s kind of a cousin of OCD because it has this compulsive quality but doesn’t really have obsessive thinking that drives it. Many people just pull their hair. They’re not even always aware of it: sometimes yes, sometimes no.
We know that it has some links to OCD. You’ll see more OCD in folks with trichotillomania, but it clearly is not just the same as OCD. One of the biggest pieces of evidence for that is that our first-line treatment for OCD – a selective serotonin reuptake inhibitor antidepressant – does not really help hair pulling.
Having said that, if people are looking for help with trichotillomania, they often are best served by therapists and doctors who have a familiarity with OCD and have kept it on their radar over the past couple of decades.
How does trichotillomania overlap with skin picking disorder, which is another condition that you’ve closely researched?
It does have some overlap with skin picking in the sense that it often seems familial. For example, the mother may pull her hair and child picks their skin.
It also has a fair amount of comorbidity with skin picking. Many people who pull will pick a little bit or did at some point. Many people who pick pulled their hair at some point. It seems closely related to nail biting as well.
Studies have also shown that one of the things that runs in the histories of most families of people with trichotillomania might be substance abuse – alcohol or drug addiction.
All of this has led people to believe that there might be subtypes of trichotillomania: one that’s more like an OCD and one that’s more like an addiction. That’s similar to the debate with other mental health conditions, that there are probably multiple types of depression, multiple types of schizophrenia.
Is there a component of this that could be defined as self-harm?
That’s been its own debate. It doesn’t seem to have the same developmental trajectory that we see with self-harm, or even some of the personality features.
However, there may be a small segment of folks with trichotillomania that might more appropriately fit that category. For example, those with family histories of trauma, higher rates of posttraumatic stress disorder, or borderline personality. But it wouldn’t be the majority.
The problem is, if you look at some of the pediatrician data, they often group picking, pulling, and cutting. I think that’s far too all-inclusive.
A gap in clinician education
Are adolescent patients likely to self-report this behavior, or is it something that physicians need to suss out for themselves?
Clearly, if child psychologists, psychiatrists, or pediatricians see young people with patches of alopecia – eyebrows or eyelashes missing, head hair with spots – in addition to a dermatologic assessment, they should simply ask, “Do you pull your hair?”
But it’s interesting that with the internet, young people are much more likely to disclose and actually come forward and tell their parents that they think they have trichotillomania.
I also hear from a lot of the adolescents that they have to educate their doctors about trichotillomania because so often physicians don’t know much about it and will assume that it’s self-injury or just a symptom of anxiety. It’s a little bit of a flip from what we might have seen 20 years ago.
I’ve seen several patients who’ve said, basically, “I’m tired of no professionals seeming to know about this. I shouldn’t have to be educating my doctors about this.” I tell them that I completely agree. It’s a shame because if a doctor doesn’t know about it, then how can they get the appropriate care?
What are the complications that accompany trichotillomania?
A small percentage, maybe about 10%, will ingest their hair, much like people who bite and swallow their fingernails. The concern there is that because hair is nondigestible, it could create an intestinal plug that could rupture and be potentially life-threatening. That makes it all the more important to ask those who pull their hair what they do with the hair once they pull it.
However, with most people, the real problem is with self-esteem. Young people may not want to socialize, go on dates, or do other things they would normally do because of it. In adults, you may find that they’re far more educated than their job allows but don’t want to go to an interview because they don’t want to have somebody sit there and look at them and notice that perhaps they don’t have any eyebrows, or that they’re wearing a wig. Those psychosocial implications are huge for so many people.
Treatment options
In a 2021 study, you showed that nearly one-quarter of people with trichotillomania do naturally recover from it. What characteristics do they seem to have?
It’s interesting because we see natural recovery across many mental health problems: alcohol addition, gambling, OCD. The question then becomes why is that some people can seemingly just stop doing a behavior? Can we learn from those people?
We did see that those who naturally recovered were less likely to have some other mental health comorbidities. It seems like when you have other things such as skin picking or OCD plus trichotillomania, that it probably speaks to something that perhaps synergistically is keeping it going. But this is just a first study; learning how to harness and understand it is the next step.
What’s the goal of treating trichotillomania?
The desired goal is zero pulling. The realistic goal is more likely significantly reduced pulling that then leads to greater function in life, greater quality-of-life.
One doesn’t have to go from 100 to 0 in order to do that. I always tell people that maybe every now and then, every few months, when something is going on in life, you might find yourself pulling a hair or two. That’s okay. If you’re not pulling every day and it’s significantly reduced, we’ll call that a success. I think that setting reasonable goals at this point is really important.
And what would the treatment pathway look like for most patients?
The standard approach is probably some type of habit-reversal therapy, of which there have been many variants over the years. It involves doing something different with your hand, identifying the triggers that may set you off, and then doing something in response to those triggers that is not pulling and might neutralize whatever that anxious or stressed feeling is. That could be different with each person.
At this point, there is no drug approved by the U.S. Food and Drug Administration for trichotillomania. Our best approaches have included N-acetylcysteine, a glutamate modulator, which we’ve done research in.
That’s kind of a go-to option for people because its side-effect profile is generally innocuous. The data show that it could be beneficial in many people with very few, if any, side effects. That would be one “medication,” although it’s actually an over-the-counter vitamin. But we’re constantly looking for better and better treatments.
Do you have any final advice for clinicians or researchers?
Given how common it is, I don’t think clinicians should just see it as an innocuous little habit that people should be able to stop on their own. Clinicians should educate themselves about trichotillomania and know where the person should get the appropriate care.
From the research perspective, given the fact that we see this in animals of multiple species – that they overgroom – this seems to be deeply ingrained in us as animals. So when it comes to the underlying neuroscience, people should pay more attention because it probably has a lot to do with our understanding of habit and compulsive behaviors. It arguably can cut across a lot of different behaviors.
A version of this article first appeared on Medscape.com.
Oscars fight highlights for many the toll alopecia may carry
The Academy Awards ceremony on March 27 is a buzzing topic of conversation.
Troy Kotsur became the first deaf man to win an Oscar – and the highly coveted best supporting actor award, at that.
But it was what happened afterward that arguably stole the show.
Viewers and audience members alike watched in awe as actor Will Smith marched on stage and struck award presenter and comedian Chris Rock in the face after he directed a joke at Smith’s wife, Jada Pinkett Smith, for her shaved head.
and can lead to feelings of depression or mental illness.
About 700,000 people in the United States have alopecia areata, according to a 2020 study. Of them, slightly more than half are women, and more than 77% are White.
Shortly after the awards show, the Los Angeles Police Department released a statement saying it was aware of the incident and Mr. Rock had not pressed charges against Mr. Smith.
The incident set social media ablaze, and strong sentiments were heard from those who have been personally affected by alopecia.
Illness is never funny
Mr. Rock’s comment can be triggering to the millions who have been affected by hair loss, said Carolyn Goh, MD, a dermatologist at UCLA Health.
“As someone with alopecia myself, I consider it a microaggression,” Dr. Goh said. “I’ve experienced many similar comments. These build up over time and wear us down.”
One U.K.-based Instagram user, Kitty Dry, said the expression on Ms. Pinkett Smith’s face represented the hurt felt by so many with this condition.
“I want to preface this post by saying that in no way do I condone any sort of violence, but thank you Will Smith,” said Ms. Dry, 23, who was diagnosed with alopecia universalis after losing all her hair in 12 weeks.
“That slap was for anyone with alopecia who has ever been at the butt of an unwanted joke, comment or stare,” Ms. Dry said.
Others posted comments raising awareness of the tragic passing of Rio Allred, a 12-year-old girl with alopecia who recently died by suicide.
Rio Allred is said to have endured serious bullying at school, with classmates pulling off her wig and smacking her head, according to the Canadian Alopecia Areata Foundation.
It’s common for those who have hair loss conditions to feel helpless, and sometimes confused, said Amy McMichael, MD, a professor and chair of the dermatology department at Wake Forest University, Winston-Salem, N.C. That’s why it’s critical for those people to see a board-certified dermatologist, so they know they are not alone.
“As dermatologists, we can not only diagnose the type of alopecia, but we can also render treatment,” Dr. McMichael said.
Alopecia awareness
Dermatologists can also help connect patients to organizations that address the physical and emotional struggles of those who have hair loss, such as the National Alopecia Areata Foundation and the Scarring Alopecia Foundation, Dr. McMichael said.
She hopes the event shows people the “many faces of hair loss” and shows that these conditions can happen to people of all ages, ethnicities, and genders.
The National Alopecia Areata Foundation calls what happened at the Oscars a “teachable” moment.
“We encourage both our community and the broader public to learn more about alopecia areata so we can end the stigma around this disease,” the organization said in a statement.
Dr. Goh said that anyone with hair loss should feel free to explore potential medical causes and, if needed, seek out mental health treatment, too.
A version of this article first appeared on WebMD.com.
The Academy Awards ceremony on March 27 is a buzzing topic of conversation.
Troy Kotsur became the first deaf man to win an Oscar – and the highly coveted best supporting actor award, at that.
But it was what happened afterward that arguably stole the show.
Viewers and audience members alike watched in awe as actor Will Smith marched on stage and struck award presenter and comedian Chris Rock in the face after he directed a joke at Smith’s wife, Jada Pinkett Smith, for her shaved head.
and can lead to feelings of depression or mental illness.
About 700,000 people in the United States have alopecia areata, according to a 2020 study. Of them, slightly more than half are women, and more than 77% are White.
Shortly after the awards show, the Los Angeles Police Department released a statement saying it was aware of the incident and Mr. Rock had not pressed charges against Mr. Smith.
The incident set social media ablaze, and strong sentiments were heard from those who have been personally affected by alopecia.
Illness is never funny
Mr. Rock’s comment can be triggering to the millions who have been affected by hair loss, said Carolyn Goh, MD, a dermatologist at UCLA Health.
“As someone with alopecia myself, I consider it a microaggression,” Dr. Goh said. “I’ve experienced many similar comments. These build up over time and wear us down.”
One U.K.-based Instagram user, Kitty Dry, said the expression on Ms. Pinkett Smith’s face represented the hurt felt by so many with this condition.
“I want to preface this post by saying that in no way do I condone any sort of violence, but thank you Will Smith,” said Ms. Dry, 23, who was diagnosed with alopecia universalis after losing all her hair in 12 weeks.
“That slap was for anyone with alopecia who has ever been at the butt of an unwanted joke, comment or stare,” Ms. Dry said.
Others posted comments raising awareness of the tragic passing of Rio Allred, a 12-year-old girl with alopecia who recently died by suicide.
Rio Allred is said to have endured serious bullying at school, with classmates pulling off her wig and smacking her head, according to the Canadian Alopecia Areata Foundation.
It’s common for those who have hair loss conditions to feel helpless, and sometimes confused, said Amy McMichael, MD, a professor and chair of the dermatology department at Wake Forest University, Winston-Salem, N.C. That’s why it’s critical for those people to see a board-certified dermatologist, so they know they are not alone.
“As dermatologists, we can not only diagnose the type of alopecia, but we can also render treatment,” Dr. McMichael said.
Alopecia awareness
Dermatologists can also help connect patients to organizations that address the physical and emotional struggles of those who have hair loss, such as the National Alopecia Areata Foundation and the Scarring Alopecia Foundation, Dr. McMichael said.
She hopes the event shows people the “many faces of hair loss” and shows that these conditions can happen to people of all ages, ethnicities, and genders.
The National Alopecia Areata Foundation calls what happened at the Oscars a “teachable” moment.
“We encourage both our community and the broader public to learn more about alopecia areata so we can end the stigma around this disease,” the organization said in a statement.
Dr. Goh said that anyone with hair loss should feel free to explore potential medical causes and, if needed, seek out mental health treatment, too.
A version of this article first appeared on WebMD.com.
The Academy Awards ceremony on March 27 is a buzzing topic of conversation.
Troy Kotsur became the first deaf man to win an Oscar – and the highly coveted best supporting actor award, at that.
But it was what happened afterward that arguably stole the show.
Viewers and audience members alike watched in awe as actor Will Smith marched on stage and struck award presenter and comedian Chris Rock in the face after he directed a joke at Smith’s wife, Jada Pinkett Smith, for her shaved head.
and can lead to feelings of depression or mental illness.
About 700,000 people in the United States have alopecia areata, according to a 2020 study. Of them, slightly more than half are women, and more than 77% are White.
Shortly after the awards show, the Los Angeles Police Department released a statement saying it was aware of the incident and Mr. Rock had not pressed charges against Mr. Smith.
The incident set social media ablaze, and strong sentiments were heard from those who have been personally affected by alopecia.
Illness is never funny
Mr. Rock’s comment can be triggering to the millions who have been affected by hair loss, said Carolyn Goh, MD, a dermatologist at UCLA Health.
“As someone with alopecia myself, I consider it a microaggression,” Dr. Goh said. “I’ve experienced many similar comments. These build up over time and wear us down.”
One U.K.-based Instagram user, Kitty Dry, said the expression on Ms. Pinkett Smith’s face represented the hurt felt by so many with this condition.
“I want to preface this post by saying that in no way do I condone any sort of violence, but thank you Will Smith,” said Ms. Dry, 23, who was diagnosed with alopecia universalis after losing all her hair in 12 weeks.
“That slap was for anyone with alopecia who has ever been at the butt of an unwanted joke, comment or stare,” Ms. Dry said.
Others posted comments raising awareness of the tragic passing of Rio Allred, a 12-year-old girl with alopecia who recently died by suicide.
Rio Allred is said to have endured serious bullying at school, with classmates pulling off her wig and smacking her head, according to the Canadian Alopecia Areata Foundation.
It’s common for those who have hair loss conditions to feel helpless, and sometimes confused, said Amy McMichael, MD, a professor and chair of the dermatology department at Wake Forest University, Winston-Salem, N.C. That’s why it’s critical for those people to see a board-certified dermatologist, so they know they are not alone.
“As dermatologists, we can not only diagnose the type of alopecia, but we can also render treatment,” Dr. McMichael said.
Alopecia awareness
Dermatologists can also help connect patients to organizations that address the physical and emotional struggles of those who have hair loss, such as the National Alopecia Areata Foundation and the Scarring Alopecia Foundation, Dr. McMichael said.
She hopes the event shows people the “many faces of hair loss” and shows that these conditions can happen to people of all ages, ethnicities, and genders.
The National Alopecia Areata Foundation calls what happened at the Oscars a “teachable” moment.
“We encourage both our community and the broader public to learn more about alopecia areata so we can end the stigma around this disease,” the organization said in a statement.
Dr. Goh said that anyone with hair loss should feel free to explore potential medical causes and, if needed, seek out mental health treatment, too.
A version of this article first appeared on WebMD.com.
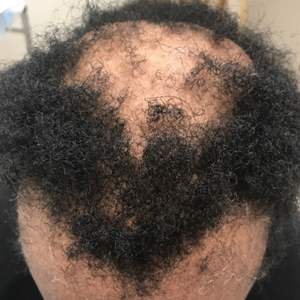
Clinical clarity grows about toenail disorder, experts report
BOSTON – The main commonly leading to the wrong therapy and no resolution to the problem, according to an expert update at the annual meeting of the American Academy of Dermatology.
Misinterpretation of the yellow discoloration, a common feature of retronychia, means “many patients are maintained on antifungal therapy for years and years with no change in their condition,” reported Phoebe Rich, MD, director of the Nail Disorders Clinic, Oregon Health & Science University, Portland.
Infection is not commonly involved in retronychia, but importantly, antifungals and antibiotics “have no role in treating the underlying disorder,” Dr. Rich said.
The term retronychia and its description is only about 20 years old, according to Dr. Rich, who cited work by David A. de Berker, MBBS, PhD, a consultant dermatologist at University Hospitals in Bristol, England. His publication on this disorder appeared in 1999, with a more detailed description published about 10 years later.
Recently, the body of literature on this disorder has been growing, contributing to an increasing consensus about etiology, diagnosis, and treatments to consider in the context of causes and severity, Dr. Rich said.
Some but not all patients have abnormal formation of the nail bed, increasing susceptibility to retronychia, but trauma or microtrauma typically serve as a trigger in most cases. Dancing, high heels, steel-toed shoes, and other sources of trauma to the toes are implicated.
Whether or not patients have an inherent susceptibility, injury separates the existing nail from the matrix and nail bed so that newly forming nail begins to grow under the nail rather continuing to push out the old nail.
Susceptibility is increased substantially in individuals with a shortened nail bed, according to Dr. Rich. In severe cases, when there is simply inadequate nail bed for the nail growth to attach, recurrence is common or even inevitable. Even when the nail is removed and regrowth appears normal at the end of a year, those patients with very short nail beds cannot count on a cure.
“Due to the slow growth of nails, it might take 2 or 3 years for the problem to recur,” Dr. Rich cautioned. For this reason, cure rates reported for the various interventions at 1 year might not predict longer-term benefit.
Retronychia is usually a clinical diagnosis based on the presence of the increased bulk of the toenail when overlapping nails cannot be seen. This is not necessarily a single overgrowth. In some cases, multiple layers of nails are stacked one on top of the other. Xanthonychia (yellow nail) is usually present.
“The layering might not be visible without removing the nail,” said Dr. Rich, explaining one reason that the diagnosis is sometimes missed. Ultrasound is a noninvasive means to confirm the problem, although Rich warned that imaging is not necessarily reimbursed.
“There is no diagnosis by histopathology, so it cannot be confirmed with biopsy,” Dr. Rich said.
Treatments range from conservative strategies, particularly topical or intralesional steroids in mild cases, to more invasive procedures such as clipping of the nail plate or surgical avulsion. All can be effective when used appropriately, according to Dr. Rich.
“The more invasive procedures are the more effective, but the caveat is they are also associated with more complications,” said Dr. Rich, citing, for example, the risk of nail dystrophies. Because of the increasing number of studies, the relative benefits and risks of retronychia treatment have now been summarized in a recent review. Dr. Rich suggested the review is one of the most recent and detailed evaluations of the topic that “I encourage everyone to read.”
Despite progress in describing retronychia, Dr. Rich said that there might be more to learn about risk. In particular, she cited the work of Dana W. Stern, MD, a specialist in nail disorders who is in private practice in New York. Dr. Stern is pursuing a hypothesis that at least some cases are caused by potentially targetable biomechanical issues.
“I have observed that many of the younger patients in my practice with retronychia seem to have atypical foot anatomy,” Dr. Stern said in an interview. “I am collecting cases and hoping to explore this issue in more depth.”
She said that foot anatomy in relationship to retronychia has not been adequately evaluated.
“In my review of the literature, I could not find a single study that showed imagery of the feet,” she said. She is considering a collaboration with others, including Rich, to explore this as a factor in retronychia.
Asked about risk of misdiagnosis, Dr. Stern reiterated some of the points made by Dr. Rich. In particular, she agreed that discolored nails alone should not be a reason to initiate antimycotic therapy without considering the possibility of retronychia.
“So many providers are not familiar with the diagnosis, and only 50% of yellow thickened nails are in fact onychomycosis,” she said. “We end up seeing a plethora of patients [with retronychia] who are unfortunately misdiagnosed for years.”
Dr. Rich reported financial relationships with numerous pharmaceutical companies. Dr. Stern reported a financial relationship with Rare Beauty Brands. Neither Dr. Rich nor Dr. Stern said they had any disclosures related to this topic.
A version of this article first appeared on Medscape.com.
BOSTON – The main commonly leading to the wrong therapy and no resolution to the problem, according to an expert update at the annual meeting of the American Academy of Dermatology.
Misinterpretation of the yellow discoloration, a common feature of retronychia, means “many patients are maintained on antifungal therapy for years and years with no change in their condition,” reported Phoebe Rich, MD, director of the Nail Disorders Clinic, Oregon Health & Science University, Portland.
Infection is not commonly involved in retronychia, but importantly, antifungals and antibiotics “have no role in treating the underlying disorder,” Dr. Rich said.
The term retronychia and its description is only about 20 years old, according to Dr. Rich, who cited work by David A. de Berker, MBBS, PhD, a consultant dermatologist at University Hospitals in Bristol, England. His publication on this disorder appeared in 1999, with a more detailed description published about 10 years later.
Recently, the body of literature on this disorder has been growing, contributing to an increasing consensus about etiology, diagnosis, and treatments to consider in the context of causes and severity, Dr. Rich said.
Some but not all patients have abnormal formation of the nail bed, increasing susceptibility to retronychia, but trauma or microtrauma typically serve as a trigger in most cases. Dancing, high heels, steel-toed shoes, and other sources of trauma to the toes are implicated.
Whether or not patients have an inherent susceptibility, injury separates the existing nail from the matrix and nail bed so that newly forming nail begins to grow under the nail rather continuing to push out the old nail.
Susceptibility is increased substantially in individuals with a shortened nail bed, according to Dr. Rich. In severe cases, when there is simply inadequate nail bed for the nail growth to attach, recurrence is common or even inevitable. Even when the nail is removed and regrowth appears normal at the end of a year, those patients with very short nail beds cannot count on a cure.
“Due to the slow growth of nails, it might take 2 or 3 years for the problem to recur,” Dr. Rich cautioned. For this reason, cure rates reported for the various interventions at 1 year might not predict longer-term benefit.
Retronychia is usually a clinical diagnosis based on the presence of the increased bulk of the toenail when overlapping nails cannot be seen. This is not necessarily a single overgrowth. In some cases, multiple layers of nails are stacked one on top of the other. Xanthonychia (yellow nail) is usually present.
“The layering might not be visible without removing the nail,” said Dr. Rich, explaining one reason that the diagnosis is sometimes missed. Ultrasound is a noninvasive means to confirm the problem, although Rich warned that imaging is not necessarily reimbursed.
“There is no diagnosis by histopathology, so it cannot be confirmed with biopsy,” Dr. Rich said.
Treatments range from conservative strategies, particularly topical or intralesional steroids in mild cases, to more invasive procedures such as clipping of the nail plate or surgical avulsion. All can be effective when used appropriately, according to Dr. Rich.
“The more invasive procedures are the more effective, but the caveat is they are also associated with more complications,” said Dr. Rich, citing, for example, the risk of nail dystrophies. Because of the increasing number of studies, the relative benefits and risks of retronychia treatment have now been summarized in a recent review. Dr. Rich suggested the review is one of the most recent and detailed evaluations of the topic that “I encourage everyone to read.”
Despite progress in describing retronychia, Dr. Rich said that there might be more to learn about risk. In particular, she cited the work of Dana W. Stern, MD, a specialist in nail disorders who is in private practice in New York. Dr. Stern is pursuing a hypothesis that at least some cases are caused by potentially targetable biomechanical issues.
“I have observed that many of the younger patients in my practice with retronychia seem to have atypical foot anatomy,” Dr. Stern said in an interview. “I am collecting cases and hoping to explore this issue in more depth.”
She said that foot anatomy in relationship to retronychia has not been adequately evaluated.
“In my review of the literature, I could not find a single study that showed imagery of the feet,” she said. She is considering a collaboration with others, including Rich, to explore this as a factor in retronychia.
Asked about risk of misdiagnosis, Dr. Stern reiterated some of the points made by Dr. Rich. In particular, she agreed that discolored nails alone should not be a reason to initiate antimycotic therapy without considering the possibility of retronychia.
“So many providers are not familiar with the diagnosis, and only 50% of yellow thickened nails are in fact onychomycosis,” she said. “We end up seeing a plethora of patients [with retronychia] who are unfortunately misdiagnosed for years.”
Dr. Rich reported financial relationships with numerous pharmaceutical companies. Dr. Stern reported a financial relationship with Rare Beauty Brands. Neither Dr. Rich nor Dr. Stern said they had any disclosures related to this topic.
A version of this article first appeared on Medscape.com.
BOSTON – The main commonly leading to the wrong therapy and no resolution to the problem, according to an expert update at the annual meeting of the American Academy of Dermatology.
Misinterpretation of the yellow discoloration, a common feature of retronychia, means “many patients are maintained on antifungal therapy for years and years with no change in their condition,” reported Phoebe Rich, MD, director of the Nail Disorders Clinic, Oregon Health & Science University, Portland.
Infection is not commonly involved in retronychia, but importantly, antifungals and antibiotics “have no role in treating the underlying disorder,” Dr. Rich said.
The term retronychia and its description is only about 20 years old, according to Dr. Rich, who cited work by David A. de Berker, MBBS, PhD, a consultant dermatologist at University Hospitals in Bristol, England. His publication on this disorder appeared in 1999, with a more detailed description published about 10 years later.
Recently, the body of literature on this disorder has been growing, contributing to an increasing consensus about etiology, diagnosis, and treatments to consider in the context of causes and severity, Dr. Rich said.
Some but not all patients have abnormal formation of the nail bed, increasing susceptibility to retronychia, but trauma or microtrauma typically serve as a trigger in most cases. Dancing, high heels, steel-toed shoes, and other sources of trauma to the toes are implicated.
Whether or not patients have an inherent susceptibility, injury separates the existing nail from the matrix and nail bed so that newly forming nail begins to grow under the nail rather continuing to push out the old nail.
Susceptibility is increased substantially in individuals with a shortened nail bed, according to Dr. Rich. In severe cases, when there is simply inadequate nail bed for the nail growth to attach, recurrence is common or even inevitable. Even when the nail is removed and regrowth appears normal at the end of a year, those patients with very short nail beds cannot count on a cure.
“Due to the slow growth of nails, it might take 2 or 3 years for the problem to recur,” Dr. Rich cautioned. For this reason, cure rates reported for the various interventions at 1 year might not predict longer-term benefit.
Retronychia is usually a clinical diagnosis based on the presence of the increased bulk of the toenail when overlapping nails cannot be seen. This is not necessarily a single overgrowth. In some cases, multiple layers of nails are stacked one on top of the other. Xanthonychia (yellow nail) is usually present.
“The layering might not be visible without removing the nail,” said Dr. Rich, explaining one reason that the diagnosis is sometimes missed. Ultrasound is a noninvasive means to confirm the problem, although Rich warned that imaging is not necessarily reimbursed.
“There is no diagnosis by histopathology, so it cannot be confirmed with biopsy,” Dr. Rich said.
Treatments range from conservative strategies, particularly topical or intralesional steroids in mild cases, to more invasive procedures such as clipping of the nail plate or surgical avulsion. All can be effective when used appropriately, according to Dr. Rich.
“The more invasive procedures are the more effective, but the caveat is they are also associated with more complications,” said Dr. Rich, citing, for example, the risk of nail dystrophies. Because of the increasing number of studies, the relative benefits and risks of retronychia treatment have now been summarized in a recent review. Dr. Rich suggested the review is one of the most recent and detailed evaluations of the topic that “I encourage everyone to read.”
Despite progress in describing retronychia, Dr. Rich said that there might be more to learn about risk. In particular, she cited the work of Dana W. Stern, MD, a specialist in nail disorders who is in private practice in New York. Dr. Stern is pursuing a hypothesis that at least some cases are caused by potentially targetable biomechanical issues.
“I have observed that many of the younger patients in my practice with retronychia seem to have atypical foot anatomy,” Dr. Stern said in an interview. “I am collecting cases and hoping to explore this issue in more depth.”
She said that foot anatomy in relationship to retronychia has not been adequately evaluated.
“In my review of the literature, I could not find a single study that showed imagery of the feet,” she said. She is considering a collaboration with others, including Rich, to explore this as a factor in retronychia.
Asked about risk of misdiagnosis, Dr. Stern reiterated some of the points made by Dr. Rich. In particular, she agreed that discolored nails alone should not be a reason to initiate antimycotic therapy without considering the possibility of retronychia.
“So many providers are not familiar with the diagnosis, and only 50% of yellow thickened nails are in fact onychomycosis,” she said. “We end up seeing a plethora of patients [with retronychia] who are unfortunately misdiagnosed for years.”
Dr. Rich reported financial relationships with numerous pharmaceutical companies. Dr. Stern reported a financial relationship with Rare Beauty Brands. Neither Dr. Rich nor Dr. Stern said they had any disclosures related to this topic.
A version of this article first appeared on Medscape.com.
AT AAD 2022
New trial data show hair growth in more alopecia areata patients
BOSTON – according to updated results from two phase 3 trials presented at the annual meeting of the American Academy of Dermatology.
The results indicate improved response rates and hair growth among trial participants, said Brett King, MD, PhD, an associate professor of dermatology at Yale University, New Haven, Conn. He is the lead author of the analyses and presented the research.
Dr. King presented 36-week results from the clinical trials at the 2021 annual meeting of the European Academy of Dermatology and Venereology. The same results were also published March 26, 2022, in the New England Journal of Medicine.
“Every bit of data we’ve had is hugely important,” Dr. King said in an interview. “Every time we add 16 weeks of data across hundreds of patients, we are making a huge step forward toward the goal of [Food and Drug Administration approval for a medication for alopecia areata.”
All patients enrolled in the two trials, called BRAVE-AA1 and BRAVE-AA2, had severe alopecia areata, defined as a Severity of Alopecia Tool (SALT) score of at least 50, meaning 50% or less scalp coverage. The score ranges from 0 (no hair loss) to 100 (complete hair loss). The primary endpoint was a SALT score of 20 or less (80% scalp hair coverage).
The researchers pooled data from both clinical trials, with a combined enrollment of 1,200, for the 52-week results presented at the meeting. The placebo group stopped at 36 weeks, and these patients were randomly reassigned to either the 4-mg or 2-mg once-daily baricitinib treatment groups.
At baseline, patients enrolled in the trial had a mean SALT score of 85.5. After 52 weeks, 39.0% of patients who received 4 mg of baricitinib had at least 80% scalp coverage. Of this group, nearly three out of four (74.1%) had at least 90% scalp coverage, or a SALT score of 10 or less.
In patients who received 2 mg of baricitinib, 22.6% had a SALT score of 20 or less 20 (at least 80% scalp hair coverage) at 52 weeks, and two-thirds of that group (67.5%) had at least 90% scalp hair coverage at 52 weeks.
Comparatively, at 36 weeks, 35.2% of participants in BRAVE-AA1 and 32.5% of participants in BRAVE-AA2 receiving 4 mg of baricitinib had at least 80% scalp coverage. In the group taking the lower dose, 21.7% and 17.3% of patients in the BRAVE-AA1 and BRAVE-AA2 trials, respectively, had achieved at least 80% scalp coverage at 36 weeks. (These percentages differ slightly from the NEJM article because of a different analysis of missing data, Dr. King said. For comparison of both 36- and 52-week results, the percentages from the EADV are used above.)
The results indicate that 5% more patients reached the primary endpoint in the additional 16 weeks of the trial, Dr. King said.
Alopecia areata is an autoimmune condition where immune cells attack hair follicles, causing the hair to fall out, and is associated with emotional and psychological distress. Any hair follicle can be attacked, but they are rarely destroyed, so hair can regrow.
"Many underestimate the impact of this autoimmune hair loss condition," Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, told this news organization. He was not involved with the trial. "The burden of the disease, which certainly is an emotional but also a physical one, definitely needs to be addressed with indicated FDA-approved drugs," he noted, which is the goal of these trials.
The BRAVE-AA1 and BRAVE-AA2 trials focused on scalp hair regrowth.
Eyebrow and eyelash growth, secondary outcomes, also improved between 36 and 52 weeks in both groups, calculated using the proportion of participants who had achieved full regrowth or regrowth with minimal gaps. At 36 weeks, about 31%-35% of patients who received 4 mg of baricitinib regrew eyebrow and eyelash hair. By 52 weeks, more than two out of five patients regrew eyebrow (44.1%) and eyelash (45.3%) hair.
“It’s a fantastic achievement and a major step forward in alopecia areata, especially for patients with the most severe and refractory cases,” said Arash Mostaghimi, MD, MPH, the director of inpatient dermatology at Brigham and Women’s Hospital in Boston, Massachusetts. Dr. Mostaghimi is on the advisory board for Eli Lilly, which manufactures baricitinib, and Brigham and Women’s was one of the clinical sites of the trial.
While dermatologists have been aware of how JAK inhibitors can affect hair regrowth in alopecia patients, they have been using these drugs off label, Dr. Friedman said. Therefore, these drugs are expensive and more difficult to access. These trials provide "data that proves the efficacy and safety of [baricitinib] under the umbrella of the FDA portal," he added, which will hopefully lead to an approved indication for alopecia areata, so it can be more accessible to patients.
Adverse events at 52 weeks were consistent with data from 36 weeks, which found that none of these adverse events occurred in more than 10% of participants. The most common adverse events were headache, acne, and increases in muscle-related blood markers. The most common infections reported were pneumonia, herpes zoster, and urinary tract infection.
In February 2022, the FDA granted priority review for baricitinib for the treatment of severe alopecia areata. Lilly expects a regulatory decision by the end of 2022, they said in a press release.
Lilly provided funding for the BRAVE-AA1 and BRAVE-AA2 trials. Dr. King reported financial relationships with Aclaris, Arena Pharmaceuticals, Bristol-Myers Squibb, Concert Pharmaceutics, Dermavant, Lilly, Pfizer, Regeneron, Sanofi Genzyme, and Viela Bio. Dr. Mostaghimi has reported serving on an advisory board for Lilly. Dr. Friedman reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
*This article was updated on 3/28/2022 to include Dr. Friedman's comments, and on 3/31/2022 to correct the statement regarding adverse events reported in the study
BOSTON – according to updated results from two phase 3 trials presented at the annual meeting of the American Academy of Dermatology.
The results indicate improved response rates and hair growth among trial participants, said Brett King, MD, PhD, an associate professor of dermatology at Yale University, New Haven, Conn. He is the lead author of the analyses and presented the research.
Dr. King presented 36-week results from the clinical trials at the 2021 annual meeting of the European Academy of Dermatology and Venereology. The same results were also published March 26, 2022, in the New England Journal of Medicine.
“Every bit of data we’ve had is hugely important,” Dr. King said in an interview. “Every time we add 16 weeks of data across hundreds of patients, we are making a huge step forward toward the goal of [Food and Drug Administration approval for a medication for alopecia areata.”
All patients enrolled in the two trials, called BRAVE-AA1 and BRAVE-AA2, had severe alopecia areata, defined as a Severity of Alopecia Tool (SALT) score of at least 50, meaning 50% or less scalp coverage. The score ranges from 0 (no hair loss) to 100 (complete hair loss). The primary endpoint was a SALT score of 20 or less (80% scalp hair coverage).
The researchers pooled data from both clinical trials, with a combined enrollment of 1,200, for the 52-week results presented at the meeting. The placebo group stopped at 36 weeks, and these patients were randomly reassigned to either the 4-mg or 2-mg once-daily baricitinib treatment groups.
At baseline, patients enrolled in the trial had a mean SALT score of 85.5. After 52 weeks, 39.0% of patients who received 4 mg of baricitinib had at least 80% scalp coverage. Of this group, nearly three out of four (74.1%) had at least 90% scalp coverage, or a SALT score of 10 or less.
In patients who received 2 mg of baricitinib, 22.6% had a SALT score of 20 or less 20 (at least 80% scalp hair coverage) at 52 weeks, and two-thirds of that group (67.5%) had at least 90% scalp hair coverage at 52 weeks.
Comparatively, at 36 weeks, 35.2% of participants in BRAVE-AA1 and 32.5% of participants in BRAVE-AA2 receiving 4 mg of baricitinib had at least 80% scalp coverage. In the group taking the lower dose, 21.7% and 17.3% of patients in the BRAVE-AA1 and BRAVE-AA2 trials, respectively, had achieved at least 80% scalp coverage at 36 weeks. (These percentages differ slightly from the NEJM article because of a different analysis of missing data, Dr. King said. For comparison of both 36- and 52-week results, the percentages from the EADV are used above.)
The results indicate that 5% more patients reached the primary endpoint in the additional 16 weeks of the trial, Dr. King said.
Alopecia areata is an autoimmune condition where immune cells attack hair follicles, causing the hair to fall out, and is associated with emotional and psychological distress. Any hair follicle can be attacked, but they are rarely destroyed, so hair can regrow.
"Many underestimate the impact of this autoimmune hair loss condition," Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, told this news organization. He was not involved with the trial. "The burden of the disease, which certainly is an emotional but also a physical one, definitely needs to be addressed with indicated FDA-approved drugs," he noted, which is the goal of these trials.
The BRAVE-AA1 and BRAVE-AA2 trials focused on scalp hair regrowth.
Eyebrow and eyelash growth, secondary outcomes, also improved between 36 and 52 weeks in both groups, calculated using the proportion of participants who had achieved full regrowth or regrowth with minimal gaps. At 36 weeks, about 31%-35% of patients who received 4 mg of baricitinib regrew eyebrow and eyelash hair. By 52 weeks, more than two out of five patients regrew eyebrow (44.1%) and eyelash (45.3%) hair.
“It’s a fantastic achievement and a major step forward in alopecia areata, especially for patients with the most severe and refractory cases,” said Arash Mostaghimi, MD, MPH, the director of inpatient dermatology at Brigham and Women’s Hospital in Boston, Massachusetts. Dr. Mostaghimi is on the advisory board for Eli Lilly, which manufactures baricitinib, and Brigham and Women’s was one of the clinical sites of the trial.
While dermatologists have been aware of how JAK inhibitors can affect hair regrowth in alopecia patients, they have been using these drugs off label, Dr. Friedman said. Therefore, these drugs are expensive and more difficult to access. These trials provide "data that proves the efficacy and safety of [baricitinib] under the umbrella of the FDA portal," he added, which will hopefully lead to an approved indication for alopecia areata, so it can be more accessible to patients.
Adverse events at 52 weeks were consistent with data from 36 weeks, which found that none of these adverse events occurred in more than 10% of participants. The most common adverse events were headache, acne, and increases in muscle-related blood markers. The most common infections reported were pneumonia, herpes zoster, and urinary tract infection.
In February 2022, the FDA granted priority review for baricitinib for the treatment of severe alopecia areata. Lilly expects a regulatory decision by the end of 2022, they said in a press release.
Lilly provided funding for the BRAVE-AA1 and BRAVE-AA2 trials. Dr. King reported financial relationships with Aclaris, Arena Pharmaceuticals, Bristol-Myers Squibb, Concert Pharmaceutics, Dermavant, Lilly, Pfizer, Regeneron, Sanofi Genzyme, and Viela Bio. Dr. Mostaghimi has reported serving on an advisory board for Lilly. Dr. Friedman reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
*This article was updated on 3/28/2022 to include Dr. Friedman's comments, and on 3/31/2022 to correct the statement regarding adverse events reported in the study
BOSTON – according to updated results from two phase 3 trials presented at the annual meeting of the American Academy of Dermatology.
The results indicate improved response rates and hair growth among trial participants, said Brett King, MD, PhD, an associate professor of dermatology at Yale University, New Haven, Conn. He is the lead author of the analyses and presented the research.
Dr. King presented 36-week results from the clinical trials at the 2021 annual meeting of the European Academy of Dermatology and Venereology. The same results were also published March 26, 2022, in the New England Journal of Medicine.
“Every bit of data we’ve had is hugely important,” Dr. King said in an interview. “Every time we add 16 weeks of data across hundreds of patients, we are making a huge step forward toward the goal of [Food and Drug Administration approval for a medication for alopecia areata.”
All patients enrolled in the two trials, called BRAVE-AA1 and BRAVE-AA2, had severe alopecia areata, defined as a Severity of Alopecia Tool (SALT) score of at least 50, meaning 50% or less scalp coverage. The score ranges from 0 (no hair loss) to 100 (complete hair loss). The primary endpoint was a SALT score of 20 or less (80% scalp hair coverage).
The researchers pooled data from both clinical trials, with a combined enrollment of 1,200, for the 52-week results presented at the meeting. The placebo group stopped at 36 weeks, and these patients were randomly reassigned to either the 4-mg or 2-mg once-daily baricitinib treatment groups.
At baseline, patients enrolled in the trial had a mean SALT score of 85.5. After 52 weeks, 39.0% of patients who received 4 mg of baricitinib had at least 80% scalp coverage. Of this group, nearly three out of four (74.1%) had at least 90% scalp coverage, or a SALT score of 10 or less.
In patients who received 2 mg of baricitinib, 22.6% had a SALT score of 20 or less 20 (at least 80% scalp hair coverage) at 52 weeks, and two-thirds of that group (67.5%) had at least 90% scalp hair coverage at 52 weeks.
Comparatively, at 36 weeks, 35.2% of participants in BRAVE-AA1 and 32.5% of participants in BRAVE-AA2 receiving 4 mg of baricitinib had at least 80% scalp coverage. In the group taking the lower dose, 21.7% and 17.3% of patients in the BRAVE-AA1 and BRAVE-AA2 trials, respectively, had achieved at least 80% scalp coverage at 36 weeks. (These percentages differ slightly from the NEJM article because of a different analysis of missing data, Dr. King said. For comparison of both 36- and 52-week results, the percentages from the EADV are used above.)
The results indicate that 5% more patients reached the primary endpoint in the additional 16 weeks of the trial, Dr. King said.
Alopecia areata is an autoimmune condition where immune cells attack hair follicles, causing the hair to fall out, and is associated with emotional and psychological distress. Any hair follicle can be attacked, but they are rarely destroyed, so hair can regrow.
"Many underestimate the impact of this autoimmune hair loss condition," Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, told this news organization. He was not involved with the trial. "The burden of the disease, which certainly is an emotional but also a physical one, definitely needs to be addressed with indicated FDA-approved drugs," he noted, which is the goal of these trials.
The BRAVE-AA1 and BRAVE-AA2 trials focused on scalp hair regrowth.
Eyebrow and eyelash growth, secondary outcomes, also improved between 36 and 52 weeks in both groups, calculated using the proportion of participants who had achieved full regrowth or regrowth with minimal gaps. At 36 weeks, about 31%-35% of patients who received 4 mg of baricitinib regrew eyebrow and eyelash hair. By 52 weeks, more than two out of five patients regrew eyebrow (44.1%) and eyelash (45.3%) hair.
“It’s a fantastic achievement and a major step forward in alopecia areata, especially for patients with the most severe and refractory cases,” said Arash Mostaghimi, MD, MPH, the director of inpatient dermatology at Brigham and Women’s Hospital in Boston, Massachusetts. Dr. Mostaghimi is on the advisory board for Eli Lilly, which manufactures baricitinib, and Brigham and Women’s was one of the clinical sites of the trial.
While dermatologists have been aware of how JAK inhibitors can affect hair regrowth in alopecia patients, they have been using these drugs off label, Dr. Friedman said. Therefore, these drugs are expensive and more difficult to access. These trials provide "data that proves the efficacy and safety of [baricitinib] under the umbrella of the FDA portal," he added, which will hopefully lead to an approved indication for alopecia areata, so it can be more accessible to patients.
Adverse events at 52 weeks were consistent with data from 36 weeks, which found that none of these adverse events occurred in more than 10% of participants. The most common adverse events were headache, acne, and increases in muscle-related blood markers. The most common infections reported were pneumonia, herpes zoster, and urinary tract infection.
In February 2022, the FDA granted priority review for baricitinib for the treatment of severe alopecia areata. Lilly expects a regulatory decision by the end of 2022, they said in a press release.
Lilly provided funding for the BRAVE-AA1 and BRAVE-AA2 trials. Dr. King reported financial relationships with Aclaris, Arena Pharmaceuticals, Bristol-Myers Squibb, Concert Pharmaceutics, Dermavant, Lilly, Pfizer, Regeneron, Sanofi Genzyme, and Viela Bio. Dr. Mostaghimi has reported serving on an advisory board for Lilly. Dr. Friedman reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
*This article was updated on 3/28/2022 to include Dr. Friedman's comments, and on 3/31/2022 to correct the statement regarding adverse events reported in the study
AT AAD 2022
Platelet-rich plasma for hair regrowth requires art and science
or administer the highly technique-dependent treatment, which creates plenty of room for suboptimal results, according to several experts at the annual meeting of the American Academy of Dermatology.
“The process is the product,” emphasized Terrence Keaney, MD, clinical associate professor at George Washington University, Washington, as well as cofounder of SkinDC, a private practice in Arlington, Va. He characterized PRP as a “growth factor cytokine cocktail,” for which relative benefits are fully dependent on the ingredients.
In other words, the efficacy of PRP is mostly dependent on the multiple steps in which blood drawn from a patient is separated into its components, processed to create a platelet-rich product, and then administered to the patient by injection or in conjunction with microneedles. While the goal is a platelet concentration two- to fivefold greater than that found in whole blood, this is not as straightforward as it sounds.
Many PRP device kits available
“There are a ton of [centrifuge] devices on the market and a lot of differences in the methodology in optimizing the platelet concentration,” Dr. Keaney explained. In addition, there are numerous proprietary collection tubes using different types of anticoagulants and different separator gels that also play a role in the goal of optimizing a platelet-rich and readily activated product.
“Recognize that each step in the preparation of PRP introduces a source of variation that affects the composition and efficacy of the final product,” said Steven Krueger, MD, who is completing his residency in dermatology at the University of Massachusetts, Worcester, but who has become an expert in the field. He contributed a chapter on this topic in the recently published book, Aesthetic Clinician’s Guide to Platelet Rich Plasma.
The importance of technique is reflected in inconsistent results from published controlled trials. Unfortunately, the authors of many studies have failed to provide details of their protocol. Ultimately, Dr. Krueger said this lack of clarity among available protocols has created a serious obstacle for establishing which steps are important and how to move the field forward.
Dr. Keaney agreed. Because of the frequent lack of details about how PRP was processed in available studies, the effort to draw conclusions about the experiences at different centers is like “comparing apples to oranges.”
“What is the ideal dose and concentrate? We don’t know,” Dr. Keaney said.
The first centrifuge device to receive regulatory approval was developed for orthopedic indications more than 20 years ago. There are now at least 20 centrifuge devices with 510K Food and Drug Administration clearance for separating blood components to produce PRP. The 510K designation means that they are “substantially equivalent” to an already approved device, but Dr. Krueger cautioned that their use in preparing PRP for treatment of hair loss remains off label.
Substandard devices are marketed
In the rapidly expanding world of PRP, there is also a growing array of PRP kits. Some of these kits have been cleared by the FDA but others have not. Dr. Krueger warned that collection tubes are being marketed that are substandard imitations of better-established products. He specifically cautioned against do-it-yourself PRP kits, which are likely to be less effective for isolating platelets and can also be contaminated with pyogenes that cause infection.
“Please use an FDA-cleared kit,” he said, warning that the risk of failing to do so is not just associated with lack of efficacy but also a significant risk of serious adverse events.
Of the centrifuge devices, both Dr. Krueger and Dr. Keaney generally recommend single-spin over double-spin devices, particularly at centers with a limited volume of PRP-based hair loss interventions. These are generally simpler.
Once the PRP has been properly prepared, the efficacy of PRP upon application can also be influenced by strategies for activation. Although the exact mechanism of PRP in stimulating hair growth is incompletely defined, the role of platelets in releasing growth factors is believed to be critical. There are a number of methods to stimulate platelets upon administration, such as exposure to endogenous collagen or thrombin or exogenous chemicals, such as calcium chloride, but again, techniques differ and the optimal approach is unknown.
One concern is the recent and largely unregulated growth of regenerative cell and tissue products for treating a large array of clinical disorders or cosmetic issues, according to Dr. Keaney. He warned of a “wild, wild west mentality” that has attracted providers with inadequate training and experience. In turn, this is now attracting the attention of the FDA as well as those involved in enforcing FDA directives.
“There is definitely more scrutiny of regenerative products,” he said, noting that he is careful about how he markets PRP. While it is reasonable to offer this off-label treatment as an in-office procedure, he noted that it is illegal to advertise off-label products. He reported that he has become more prudent when including this option among hair regrowth services provided in his practice.
Omer E. Ibrahim, MD, a dermatologist affiliated with Chicago Cosmetic Surgery and Dermatology, agreed. While he also feels there is good evidence to support PRP as a hair loss treatment option, particularly for androgenic alopecia, he also expressed caution about promoting this approach in exclusion of other options.
“Patients ask me for a PRP consultation, but there is no such thing as a PRP consultation in my practice,” Dr. Ibrahim said. He incorporates PRP into other strategies. “I stress that it is one part of a multipronged approach,” he added.
Dr. Ibrahim has reported financial relationships with Alastin Skincare, Allergan, Eclipse Medical, Galderma USA, and Revision Skincare. Dr. Keaney has reported financial relationships with Allergan, DermTech, Evolus, Galderma USA, Merz Aesthetics, Revance Therapeutics, and Syneron Candela. Dr. Krueger has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
or administer the highly technique-dependent treatment, which creates plenty of room for suboptimal results, according to several experts at the annual meeting of the American Academy of Dermatology.
“The process is the product,” emphasized Terrence Keaney, MD, clinical associate professor at George Washington University, Washington, as well as cofounder of SkinDC, a private practice in Arlington, Va. He characterized PRP as a “growth factor cytokine cocktail,” for which relative benefits are fully dependent on the ingredients.
In other words, the efficacy of PRP is mostly dependent on the multiple steps in which blood drawn from a patient is separated into its components, processed to create a platelet-rich product, and then administered to the patient by injection or in conjunction with microneedles. While the goal is a platelet concentration two- to fivefold greater than that found in whole blood, this is not as straightforward as it sounds.
Many PRP device kits available
“There are a ton of [centrifuge] devices on the market and a lot of differences in the methodology in optimizing the platelet concentration,” Dr. Keaney explained. In addition, there are numerous proprietary collection tubes using different types of anticoagulants and different separator gels that also play a role in the goal of optimizing a platelet-rich and readily activated product.
“Recognize that each step in the preparation of PRP introduces a source of variation that affects the composition and efficacy of the final product,” said Steven Krueger, MD, who is completing his residency in dermatology at the University of Massachusetts, Worcester, but who has become an expert in the field. He contributed a chapter on this topic in the recently published book, Aesthetic Clinician’s Guide to Platelet Rich Plasma.
The importance of technique is reflected in inconsistent results from published controlled trials. Unfortunately, the authors of many studies have failed to provide details of their protocol. Ultimately, Dr. Krueger said this lack of clarity among available protocols has created a serious obstacle for establishing which steps are important and how to move the field forward.
Dr. Keaney agreed. Because of the frequent lack of details about how PRP was processed in available studies, the effort to draw conclusions about the experiences at different centers is like “comparing apples to oranges.”
“What is the ideal dose and concentrate? We don’t know,” Dr. Keaney said.
The first centrifuge device to receive regulatory approval was developed for orthopedic indications more than 20 years ago. There are now at least 20 centrifuge devices with 510K Food and Drug Administration clearance for separating blood components to produce PRP. The 510K designation means that they are “substantially equivalent” to an already approved device, but Dr. Krueger cautioned that their use in preparing PRP for treatment of hair loss remains off label.
Substandard devices are marketed
In the rapidly expanding world of PRP, there is also a growing array of PRP kits. Some of these kits have been cleared by the FDA but others have not. Dr. Krueger warned that collection tubes are being marketed that are substandard imitations of better-established products. He specifically cautioned against do-it-yourself PRP kits, which are likely to be less effective for isolating platelets and can also be contaminated with pyogenes that cause infection.
“Please use an FDA-cleared kit,” he said, warning that the risk of failing to do so is not just associated with lack of efficacy but also a significant risk of serious adverse events.
Of the centrifuge devices, both Dr. Krueger and Dr. Keaney generally recommend single-spin over double-spin devices, particularly at centers with a limited volume of PRP-based hair loss interventions. These are generally simpler.
Once the PRP has been properly prepared, the efficacy of PRP upon application can also be influenced by strategies for activation. Although the exact mechanism of PRP in stimulating hair growth is incompletely defined, the role of platelets in releasing growth factors is believed to be critical. There are a number of methods to stimulate platelets upon administration, such as exposure to endogenous collagen or thrombin or exogenous chemicals, such as calcium chloride, but again, techniques differ and the optimal approach is unknown.
One concern is the recent and largely unregulated growth of regenerative cell and tissue products for treating a large array of clinical disorders or cosmetic issues, according to Dr. Keaney. He warned of a “wild, wild west mentality” that has attracted providers with inadequate training and experience. In turn, this is now attracting the attention of the FDA as well as those involved in enforcing FDA directives.
“There is definitely more scrutiny of regenerative products,” he said, noting that he is careful about how he markets PRP. While it is reasonable to offer this off-label treatment as an in-office procedure, he noted that it is illegal to advertise off-label products. He reported that he has become more prudent when including this option among hair regrowth services provided in his practice.
Omer E. Ibrahim, MD, a dermatologist affiliated with Chicago Cosmetic Surgery and Dermatology, agreed. While he also feels there is good evidence to support PRP as a hair loss treatment option, particularly for androgenic alopecia, he also expressed caution about promoting this approach in exclusion of other options.
“Patients ask me for a PRP consultation, but there is no such thing as a PRP consultation in my practice,” Dr. Ibrahim said. He incorporates PRP into other strategies. “I stress that it is one part of a multipronged approach,” he added.
Dr. Ibrahim has reported financial relationships with Alastin Skincare, Allergan, Eclipse Medical, Galderma USA, and Revision Skincare. Dr. Keaney has reported financial relationships with Allergan, DermTech, Evolus, Galderma USA, Merz Aesthetics, Revance Therapeutics, and Syneron Candela. Dr. Krueger has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
or administer the highly technique-dependent treatment, which creates plenty of room for suboptimal results, according to several experts at the annual meeting of the American Academy of Dermatology.
“The process is the product,” emphasized Terrence Keaney, MD, clinical associate professor at George Washington University, Washington, as well as cofounder of SkinDC, a private practice in Arlington, Va. He characterized PRP as a “growth factor cytokine cocktail,” for which relative benefits are fully dependent on the ingredients.
In other words, the efficacy of PRP is mostly dependent on the multiple steps in which blood drawn from a patient is separated into its components, processed to create a platelet-rich product, and then administered to the patient by injection or in conjunction with microneedles. While the goal is a platelet concentration two- to fivefold greater than that found in whole blood, this is not as straightforward as it sounds.
Many PRP device kits available
“There are a ton of [centrifuge] devices on the market and a lot of differences in the methodology in optimizing the platelet concentration,” Dr. Keaney explained. In addition, there are numerous proprietary collection tubes using different types of anticoagulants and different separator gels that also play a role in the goal of optimizing a platelet-rich and readily activated product.
“Recognize that each step in the preparation of PRP introduces a source of variation that affects the composition and efficacy of the final product,” said Steven Krueger, MD, who is completing his residency in dermatology at the University of Massachusetts, Worcester, but who has become an expert in the field. He contributed a chapter on this topic in the recently published book, Aesthetic Clinician’s Guide to Platelet Rich Plasma.
The importance of technique is reflected in inconsistent results from published controlled trials. Unfortunately, the authors of many studies have failed to provide details of their protocol. Ultimately, Dr. Krueger said this lack of clarity among available protocols has created a serious obstacle for establishing which steps are important and how to move the field forward.
Dr. Keaney agreed. Because of the frequent lack of details about how PRP was processed in available studies, the effort to draw conclusions about the experiences at different centers is like “comparing apples to oranges.”
“What is the ideal dose and concentrate? We don’t know,” Dr. Keaney said.
The first centrifuge device to receive regulatory approval was developed for orthopedic indications more than 20 years ago. There are now at least 20 centrifuge devices with 510K Food and Drug Administration clearance for separating blood components to produce PRP. The 510K designation means that they are “substantially equivalent” to an already approved device, but Dr. Krueger cautioned that their use in preparing PRP for treatment of hair loss remains off label.
Substandard devices are marketed
In the rapidly expanding world of PRP, there is also a growing array of PRP kits. Some of these kits have been cleared by the FDA but others have not. Dr. Krueger warned that collection tubes are being marketed that are substandard imitations of better-established products. He specifically cautioned against do-it-yourself PRP kits, which are likely to be less effective for isolating platelets and can also be contaminated with pyogenes that cause infection.
“Please use an FDA-cleared kit,” he said, warning that the risk of failing to do so is not just associated with lack of efficacy but also a significant risk of serious adverse events.
Of the centrifuge devices, both Dr. Krueger and Dr. Keaney generally recommend single-spin over double-spin devices, particularly at centers with a limited volume of PRP-based hair loss interventions. These are generally simpler.
Once the PRP has been properly prepared, the efficacy of PRP upon application can also be influenced by strategies for activation. Although the exact mechanism of PRP in stimulating hair growth is incompletely defined, the role of platelets in releasing growth factors is believed to be critical. There are a number of methods to stimulate platelets upon administration, such as exposure to endogenous collagen or thrombin or exogenous chemicals, such as calcium chloride, but again, techniques differ and the optimal approach is unknown.
One concern is the recent and largely unregulated growth of regenerative cell and tissue products for treating a large array of clinical disorders or cosmetic issues, according to Dr. Keaney. He warned of a “wild, wild west mentality” that has attracted providers with inadequate training and experience. In turn, this is now attracting the attention of the FDA as well as those involved in enforcing FDA directives.
“There is definitely more scrutiny of regenerative products,” he said, noting that he is careful about how he markets PRP. While it is reasonable to offer this off-label treatment as an in-office procedure, he noted that it is illegal to advertise off-label products. He reported that he has become more prudent when including this option among hair regrowth services provided in his practice.
Omer E. Ibrahim, MD, a dermatologist affiliated with Chicago Cosmetic Surgery and Dermatology, agreed. While he also feels there is good evidence to support PRP as a hair loss treatment option, particularly for androgenic alopecia, he also expressed caution about promoting this approach in exclusion of other options.
“Patients ask me for a PRP consultation, but there is no such thing as a PRP consultation in my practice,” Dr. Ibrahim said. He incorporates PRP into other strategies. “I stress that it is one part of a multipronged approach,” he added.
Dr. Ibrahim has reported financial relationships with Alastin Skincare, Allergan, Eclipse Medical, Galderma USA, and Revision Skincare. Dr. Keaney has reported financial relationships with Allergan, DermTech, Evolus, Galderma USA, Merz Aesthetics, Revance Therapeutics, and Syneron Candela. Dr. Krueger has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT AAD 2022
COVID-19–alopecia areata link? Review doesn’t find much evidence
A new
If there is a connection, it’s likely not a strong one, said study author Rachel E. Christensen, a graduate student at Rutgers Robert Wood Johnson Medical School, in an interview. “Based on the reported number of cases following COVID-19, alopecia areata appears to be low on the list of common skin manifestations of COVID-19,” she said. Of 402 articles screened from three databases in the review, only 11 were identified as related to alopecia areata (AA) and COVID-19, and only 9 of those met the study inclusion criteria. “This number alone highlights the very low number of published articles investigating this connection.”
The review was published in JAAD International.
While COVID-19 has been linked to a variety of skin conditions, a 2021 South Korean study of 7,958 cases and 218,779 controls found no connection between infection and AA even after covariates such as age, gender, and income level were taken into account. In a letter to the editor published in 2020, dermatologists in Turkey reported that the percentage of patients with AA at the dermatology outpatient clinic jumped from 0.97% in May 2019 to 1.48% in May 2020. The number of patients in each group wasn’t reported.
Systematic review
The investigators launched the systematic review to gain a wider perspective, although there are still limitations. On the one hand, Ms. Christensen said, “we do know that COVID-19, like other viruses, has been linked to various dermatological disorders.”
However, “it is difficult to tease apart whether any worsening of alopecia areata we see following COVID-19 is due to the virus itself or the increased psychological burden related to the infection or to the pandemic in general,” she said. Indeed, the authors of the report in Turkey attributed the rise in cases to stress.
For the review, the researchers analyzed studies from Italy (four), Turkey (two), Brazil (one), the United States (one), and Poland (one).
Six of the studies reported cases of new-onset AA following COVID-19 infection (seven cases; average age, 37 years; females, three). Another study was a retrospective review of 32 patients with preexisting AA who developed COVID-19; none experienced significant worsening of AA within 6 months.
The review also included a study based on a survey of 389 patients with AA. The investigators found that, at a median 2.14 months after infection, 44% of those who had COVID-19 vs. 12% of those who were COVID negative had a relapse. Finally, a case report noted a patient with preexisting AA whose condition worsened following COVID infection.
The findings suggest that AA “could be a dermatological manifestation of COVID-19, with cases most often appearing 1-2 months following infection,” the authors wrote. “However, the heterogeneity of study designs and high proportion of case reports make it challenging to draw any conclusion.”
In an interview, dermatologist Brett King, MD, PhD, of the department of dermatology, Yale University, New Haven, Conn., said the review findings suggest that “there is little concern of alopecia areata following COVID infection.
Does new-onset AA happen, and are there exacerbations of preexisting disease related to COVID infection? Probably yes, but rarely.”
However, he noted that another form of alopecia, telogen effluvium (TE), is more common after COVID-19 infection. According to Dr. King, who was not involved with the systematic review, TE is typically time-limited, compared with AA’s more common chronic waxing-and-waning course.
“Distinguishing TE and AA is usually straightforward because AA typically presents with well-circumscribed patches of hair loss,” such as circular patches, “while TE manifests as diffuse hair loss,” he explained. “Rarely, however, AA does manifest diffuse hair loss without patches, similar to TE. In those cases, it may be difficult to distinguish them. A biopsy may be helpful if there is a question of the diagnosis.”
No study funding is reported. The review authors and Dr. King report no relevant disclosures.
A new
If there is a connection, it’s likely not a strong one, said study author Rachel E. Christensen, a graduate student at Rutgers Robert Wood Johnson Medical School, in an interview. “Based on the reported number of cases following COVID-19, alopecia areata appears to be low on the list of common skin manifestations of COVID-19,” she said. Of 402 articles screened from three databases in the review, only 11 were identified as related to alopecia areata (AA) and COVID-19, and only 9 of those met the study inclusion criteria. “This number alone highlights the very low number of published articles investigating this connection.”
The review was published in JAAD International.
While COVID-19 has been linked to a variety of skin conditions, a 2021 South Korean study of 7,958 cases and 218,779 controls found no connection between infection and AA even after covariates such as age, gender, and income level were taken into account. In a letter to the editor published in 2020, dermatologists in Turkey reported that the percentage of patients with AA at the dermatology outpatient clinic jumped from 0.97% in May 2019 to 1.48% in May 2020. The number of patients in each group wasn’t reported.
Systematic review
The investigators launched the systematic review to gain a wider perspective, although there are still limitations. On the one hand, Ms. Christensen said, “we do know that COVID-19, like other viruses, has been linked to various dermatological disorders.”
However, “it is difficult to tease apart whether any worsening of alopecia areata we see following COVID-19 is due to the virus itself or the increased psychological burden related to the infection or to the pandemic in general,” she said. Indeed, the authors of the report in Turkey attributed the rise in cases to stress.
For the review, the researchers analyzed studies from Italy (four), Turkey (two), Brazil (one), the United States (one), and Poland (one).
Six of the studies reported cases of new-onset AA following COVID-19 infection (seven cases; average age, 37 years; females, three). Another study was a retrospective review of 32 patients with preexisting AA who developed COVID-19; none experienced significant worsening of AA within 6 months.
The review also included a study based on a survey of 389 patients with AA. The investigators found that, at a median 2.14 months after infection, 44% of those who had COVID-19 vs. 12% of those who were COVID negative had a relapse. Finally, a case report noted a patient with preexisting AA whose condition worsened following COVID infection.
The findings suggest that AA “could be a dermatological manifestation of COVID-19, with cases most often appearing 1-2 months following infection,” the authors wrote. “However, the heterogeneity of study designs and high proportion of case reports make it challenging to draw any conclusion.”
In an interview, dermatologist Brett King, MD, PhD, of the department of dermatology, Yale University, New Haven, Conn., said the review findings suggest that “there is little concern of alopecia areata following COVID infection.
Does new-onset AA happen, and are there exacerbations of preexisting disease related to COVID infection? Probably yes, but rarely.”
However, he noted that another form of alopecia, telogen effluvium (TE), is more common after COVID-19 infection. According to Dr. King, who was not involved with the systematic review, TE is typically time-limited, compared with AA’s more common chronic waxing-and-waning course.
“Distinguishing TE and AA is usually straightforward because AA typically presents with well-circumscribed patches of hair loss,” such as circular patches, “while TE manifests as diffuse hair loss,” he explained. “Rarely, however, AA does manifest diffuse hair loss without patches, similar to TE. In those cases, it may be difficult to distinguish them. A biopsy may be helpful if there is a question of the diagnosis.”
No study funding is reported. The review authors and Dr. King report no relevant disclosures.
A new
If there is a connection, it’s likely not a strong one, said study author Rachel E. Christensen, a graduate student at Rutgers Robert Wood Johnson Medical School, in an interview. “Based on the reported number of cases following COVID-19, alopecia areata appears to be low on the list of common skin manifestations of COVID-19,” she said. Of 402 articles screened from three databases in the review, only 11 were identified as related to alopecia areata (AA) and COVID-19, and only 9 of those met the study inclusion criteria. “This number alone highlights the very low number of published articles investigating this connection.”
The review was published in JAAD International.
While COVID-19 has been linked to a variety of skin conditions, a 2021 South Korean study of 7,958 cases and 218,779 controls found no connection between infection and AA even after covariates such as age, gender, and income level were taken into account. In a letter to the editor published in 2020, dermatologists in Turkey reported that the percentage of patients with AA at the dermatology outpatient clinic jumped from 0.97% in May 2019 to 1.48% in May 2020. The number of patients in each group wasn’t reported.
Systematic review
The investigators launched the systematic review to gain a wider perspective, although there are still limitations. On the one hand, Ms. Christensen said, “we do know that COVID-19, like other viruses, has been linked to various dermatological disorders.”
However, “it is difficult to tease apart whether any worsening of alopecia areata we see following COVID-19 is due to the virus itself or the increased psychological burden related to the infection or to the pandemic in general,” she said. Indeed, the authors of the report in Turkey attributed the rise in cases to stress.
For the review, the researchers analyzed studies from Italy (four), Turkey (two), Brazil (one), the United States (one), and Poland (one).
Six of the studies reported cases of new-onset AA following COVID-19 infection (seven cases; average age, 37 years; females, three). Another study was a retrospective review of 32 patients with preexisting AA who developed COVID-19; none experienced significant worsening of AA within 6 months.
The review also included a study based on a survey of 389 patients with AA. The investigators found that, at a median 2.14 months after infection, 44% of those who had COVID-19 vs. 12% of those who were COVID negative had a relapse. Finally, a case report noted a patient with preexisting AA whose condition worsened following COVID infection.
The findings suggest that AA “could be a dermatological manifestation of COVID-19, with cases most often appearing 1-2 months following infection,” the authors wrote. “However, the heterogeneity of study designs and high proportion of case reports make it challenging to draw any conclusion.”
In an interview, dermatologist Brett King, MD, PhD, of the department of dermatology, Yale University, New Haven, Conn., said the review findings suggest that “there is little concern of alopecia areata following COVID infection.
Does new-onset AA happen, and are there exacerbations of preexisting disease related to COVID infection? Probably yes, but rarely.”
However, he noted that another form of alopecia, telogen effluvium (TE), is more common after COVID-19 infection. According to Dr. King, who was not involved with the systematic review, TE is typically time-limited, compared with AA’s more common chronic waxing-and-waning course.
“Distinguishing TE and AA is usually straightforward because AA typically presents with well-circumscribed patches of hair loss,” such as circular patches, “while TE manifests as diffuse hair loss,” he explained. “Rarely, however, AA does manifest diffuse hair loss without patches, similar to TE. In those cases, it may be difficult to distinguish them. A biopsy may be helpful if there is a question of the diagnosis.”
No study funding is reported. The review authors and Dr. King report no relevant disclosures.
FROM JAAD INTERNATIONAL
Oral tofacitinib produces hair regrowth in children with alopecia areata
and published in Pediatric Dermatology.
The 11 pediatric patients, ages 8-18 years, all with a diagnosis of AA, were treated with tofacitinib. Eight patients, or nearly 73%, experienced hair regrowth, while the other three (27.3%) did not, as the investigators reported in the retrospective chart review.
“A success rate of 73% is very good,” said lead author Cory A. Dunnick, MD, professor of dermatology and director of clinical trials at the University of Colorado at Denver, Aurora. No serious adverse events occurred, and adverse events of any kind were limited, the researchers found.
“It is important to get information into the literature about potential treatments for severe alopecia areata because there is no [Food and Drug Administration]–approved therapy at the present time,” Dr. Dunnick told this news organization. Patients’ insurance plans often deny non–FDA-approved therapies unless there are data to support their use.
The researchers found no correlation between the dose, duration of treatment, or the presence of comorbidities and clinical response.
Oral tofacitinib has been shown to be effective and well tolerated for AA in adults, the researchers said. They referred to recent studies that have used JAK inhibitors, including tofacitinib, “in an effort to inhibit T-cell activation and halt disease progression in adult and pediatric patients” with AA.
Study details
Of the 11 patients evaluated, 6 had alopecia universalis, 1 had alopecia totalis, and 4 had patchy AA. Concomitant medical conditions known to be associated with AA affected four patients. These included atopic dermatitis, autoimmune hypothyroidism, and asthma. One patient reported having two brothers with AA.
The median disease duration was 6 years. “In my experience, JAK inhibitors are less effective for patients with longstanding – more than 10 years – alopecia totalis or alopecia universalis,” Dr. Dunnick said.
Previously, patients had been given methotrexate, oral prednisone, intralesional triamcinolone, topical corticosteroids, and topical diphenylcyclopropenone. During treatment with tofacitinib, 5 of the 11 patients also received topical steroid treatment.
The study was a retrospective chart review, so dosing was not standardized, the researchers said. Most took 5-10 mg twice daily. Median treatment time was 32 months, with a range of 5-39 months.
Patients with a complete or near complete clinical response were categorized as responders; subjectively, these were the patients who had persistent hair regrowth over more than 50% of affected areas. Five patients had complete regrowth of hair on the scalp, eyebrows, and body during treatment. Others had incomplete responses. For instance, one patient had improved growth of eyelashes and eyebrows but not on the scalp. Once the medication was increased to 15 mg daily, the patient had complete regrowth of body hair, eyelashes, and eyebrows but slow regrowth on the scalp after 1 year of treatment.
“Patients are very happy with regrowth of their hair,” Dr. Dunnick said, noting that severe AA affects self-esteem and quality of life.
Other research
In a retrospective study that looked at the effects of oral tofacitinib given to 14 preadolescent patients with AA, 9 experienced “clinically significant improvement” in their Severity of Alopecia Tool score. Three had complete remission, and seven (63.6%) had more than a 50% improvement in the score.
Mechanisms, concerns
The researchers of the current study explained that interferon signaling activity through the JAK pathways is a key mediator of the inflammation and cytotoxic T-cell response in AA. That modulation of the signaling may decrease disease progression, as the results of the current chart review suggest.
A main concern, the researchers wrote, is the potential for significant adverse events. Although this chart review did not find any, the researchers did see some transient lab abnormalities. One study found lab abnormalities in such measures as triglycerides and cholesterol.
Asked to comment on the study results, Brett King, MD, PHD, associate professor of dermatology at Yale University, New Haven, Conn., said that the study “is an important addition to a series of articles dating back to 2017 showing efficacy of tofacitinib in the pediatric age group.” The results are similar to those of previous studies, “showing that severe AA can be treated effectively with tofacitinib. Cumulatively, there is significant data to support treatment of this age group with JAK inhibitors,” he said.
At the 2021 European Academy of Dermatology and Venereology meeting, Dr. King presented the results of two phase 3 studies, which found that treatment with the oral JAK inhibitor baricitinib resulted in substantial hair growth in adults with AA. He and colleagues have also reported positive results of tofacitinib in treating AA in four children ages 8-10, with alopecia totalis and alopecia universalis, and in adolescents with AA.
Currently, three large, randomized, phase 3 clinical trials of other JAK inhibitors for AA are underway – ritlecitinib, baricitinib, and ruxolitinib – and the ritlecitinib trial includes adolescents (ages 12 years and older). “It is the results of these trials that we eagerly await, because FDA approval will bring greater access to these treatments,” Dr. King said.
Dr. Dunnick has disclosed no relevant financial relationships. Dr. King has served on advisory boards and/or is a consultant and/or a clinical trial investigator for AbbVie, Bristol-Myers Squibb, Concert Pharmaceuticals, Eli Lilly, Incyte, Pfizer, and others. He is on speaker bureaus for AbbVie, Incyte, Pfizer, and others.
A version of this article first appeared on Medscape.com.
and published in Pediatric Dermatology.
The 11 pediatric patients, ages 8-18 years, all with a diagnosis of AA, were treated with tofacitinib. Eight patients, or nearly 73%, experienced hair regrowth, while the other three (27.3%) did not, as the investigators reported in the retrospective chart review.
“A success rate of 73% is very good,” said lead author Cory A. Dunnick, MD, professor of dermatology and director of clinical trials at the University of Colorado at Denver, Aurora. No serious adverse events occurred, and adverse events of any kind were limited, the researchers found.
“It is important to get information into the literature about potential treatments for severe alopecia areata because there is no [Food and Drug Administration]–approved therapy at the present time,” Dr. Dunnick told this news organization. Patients’ insurance plans often deny non–FDA-approved therapies unless there are data to support their use.
The researchers found no correlation between the dose, duration of treatment, or the presence of comorbidities and clinical response.
Oral tofacitinib has been shown to be effective and well tolerated for AA in adults, the researchers said. They referred to recent studies that have used JAK inhibitors, including tofacitinib, “in an effort to inhibit T-cell activation and halt disease progression in adult and pediatric patients” with AA.
Study details
Of the 11 patients evaluated, 6 had alopecia universalis, 1 had alopecia totalis, and 4 had patchy AA. Concomitant medical conditions known to be associated with AA affected four patients. These included atopic dermatitis, autoimmune hypothyroidism, and asthma. One patient reported having two brothers with AA.
The median disease duration was 6 years. “In my experience, JAK inhibitors are less effective for patients with longstanding – more than 10 years – alopecia totalis or alopecia universalis,” Dr. Dunnick said.
Previously, patients had been given methotrexate, oral prednisone, intralesional triamcinolone, topical corticosteroids, and topical diphenylcyclopropenone. During treatment with tofacitinib, 5 of the 11 patients also received topical steroid treatment.
The study was a retrospective chart review, so dosing was not standardized, the researchers said. Most took 5-10 mg twice daily. Median treatment time was 32 months, with a range of 5-39 months.
Patients with a complete or near complete clinical response were categorized as responders; subjectively, these were the patients who had persistent hair regrowth over more than 50% of affected areas. Five patients had complete regrowth of hair on the scalp, eyebrows, and body during treatment. Others had incomplete responses. For instance, one patient had improved growth of eyelashes and eyebrows but not on the scalp. Once the medication was increased to 15 mg daily, the patient had complete regrowth of body hair, eyelashes, and eyebrows but slow regrowth on the scalp after 1 year of treatment.
“Patients are very happy with regrowth of their hair,” Dr. Dunnick said, noting that severe AA affects self-esteem and quality of life.
Other research
In a retrospective study that looked at the effects of oral tofacitinib given to 14 preadolescent patients with AA, 9 experienced “clinically significant improvement” in their Severity of Alopecia Tool score. Three had complete remission, and seven (63.6%) had more than a 50% improvement in the score.
Mechanisms, concerns
The researchers of the current study explained that interferon signaling activity through the JAK pathways is a key mediator of the inflammation and cytotoxic T-cell response in AA. That modulation of the signaling may decrease disease progression, as the results of the current chart review suggest.
A main concern, the researchers wrote, is the potential for significant adverse events. Although this chart review did not find any, the researchers did see some transient lab abnormalities. One study found lab abnormalities in such measures as triglycerides and cholesterol.
Asked to comment on the study results, Brett King, MD, PHD, associate professor of dermatology at Yale University, New Haven, Conn., said that the study “is an important addition to a series of articles dating back to 2017 showing efficacy of tofacitinib in the pediatric age group.” The results are similar to those of previous studies, “showing that severe AA can be treated effectively with tofacitinib. Cumulatively, there is significant data to support treatment of this age group with JAK inhibitors,” he said.
At the 2021 European Academy of Dermatology and Venereology meeting, Dr. King presented the results of two phase 3 studies, which found that treatment with the oral JAK inhibitor baricitinib resulted in substantial hair growth in adults with AA. He and colleagues have also reported positive results of tofacitinib in treating AA in four children ages 8-10, with alopecia totalis and alopecia universalis, and in adolescents with AA.
Currently, three large, randomized, phase 3 clinical trials of other JAK inhibitors for AA are underway – ritlecitinib, baricitinib, and ruxolitinib – and the ritlecitinib trial includes adolescents (ages 12 years and older). “It is the results of these trials that we eagerly await, because FDA approval will bring greater access to these treatments,” Dr. King said.
Dr. Dunnick has disclosed no relevant financial relationships. Dr. King has served on advisory boards and/or is a consultant and/or a clinical trial investigator for AbbVie, Bristol-Myers Squibb, Concert Pharmaceuticals, Eli Lilly, Incyte, Pfizer, and others. He is on speaker bureaus for AbbVie, Incyte, Pfizer, and others.
A version of this article first appeared on Medscape.com.
and published in Pediatric Dermatology.
The 11 pediatric patients, ages 8-18 years, all with a diagnosis of AA, were treated with tofacitinib. Eight patients, or nearly 73%, experienced hair regrowth, while the other three (27.3%) did not, as the investigators reported in the retrospective chart review.
“A success rate of 73% is very good,” said lead author Cory A. Dunnick, MD, professor of dermatology and director of clinical trials at the University of Colorado at Denver, Aurora. No serious adverse events occurred, and adverse events of any kind were limited, the researchers found.
“It is important to get information into the literature about potential treatments for severe alopecia areata because there is no [Food and Drug Administration]–approved therapy at the present time,” Dr. Dunnick told this news organization. Patients’ insurance plans often deny non–FDA-approved therapies unless there are data to support their use.
The researchers found no correlation between the dose, duration of treatment, or the presence of comorbidities and clinical response.
Oral tofacitinib has been shown to be effective and well tolerated for AA in adults, the researchers said. They referred to recent studies that have used JAK inhibitors, including tofacitinib, “in an effort to inhibit T-cell activation and halt disease progression in adult and pediatric patients” with AA.
Study details
Of the 11 patients evaluated, 6 had alopecia universalis, 1 had alopecia totalis, and 4 had patchy AA. Concomitant medical conditions known to be associated with AA affected four patients. These included atopic dermatitis, autoimmune hypothyroidism, and asthma. One patient reported having two brothers with AA.
The median disease duration was 6 years. “In my experience, JAK inhibitors are less effective for patients with longstanding – more than 10 years – alopecia totalis or alopecia universalis,” Dr. Dunnick said.
Previously, patients had been given methotrexate, oral prednisone, intralesional triamcinolone, topical corticosteroids, and topical diphenylcyclopropenone. During treatment with tofacitinib, 5 of the 11 patients also received topical steroid treatment.
The study was a retrospective chart review, so dosing was not standardized, the researchers said. Most took 5-10 mg twice daily. Median treatment time was 32 months, with a range of 5-39 months.
Patients with a complete or near complete clinical response were categorized as responders; subjectively, these were the patients who had persistent hair regrowth over more than 50% of affected areas. Five patients had complete regrowth of hair on the scalp, eyebrows, and body during treatment. Others had incomplete responses. For instance, one patient had improved growth of eyelashes and eyebrows but not on the scalp. Once the medication was increased to 15 mg daily, the patient had complete regrowth of body hair, eyelashes, and eyebrows but slow regrowth on the scalp after 1 year of treatment.
“Patients are very happy with regrowth of their hair,” Dr. Dunnick said, noting that severe AA affects self-esteem and quality of life.
Other research
In a retrospective study that looked at the effects of oral tofacitinib given to 14 preadolescent patients with AA, 9 experienced “clinically significant improvement” in their Severity of Alopecia Tool score. Three had complete remission, and seven (63.6%) had more than a 50% improvement in the score.
Mechanisms, concerns
The researchers of the current study explained that interferon signaling activity through the JAK pathways is a key mediator of the inflammation and cytotoxic T-cell response in AA. That modulation of the signaling may decrease disease progression, as the results of the current chart review suggest.
A main concern, the researchers wrote, is the potential for significant adverse events. Although this chart review did not find any, the researchers did see some transient lab abnormalities. One study found lab abnormalities in such measures as triglycerides and cholesterol.
Asked to comment on the study results, Brett King, MD, PHD, associate professor of dermatology at Yale University, New Haven, Conn., said that the study “is an important addition to a series of articles dating back to 2017 showing efficacy of tofacitinib in the pediatric age group.” The results are similar to those of previous studies, “showing that severe AA can be treated effectively with tofacitinib. Cumulatively, there is significant data to support treatment of this age group with JAK inhibitors,” he said.
At the 2021 European Academy of Dermatology and Venereology meeting, Dr. King presented the results of two phase 3 studies, which found that treatment with the oral JAK inhibitor baricitinib resulted in substantial hair growth in adults with AA. He and colleagues have also reported positive results of tofacitinib in treating AA in four children ages 8-10, with alopecia totalis and alopecia universalis, and in adolescents with AA.
Currently, three large, randomized, phase 3 clinical trials of other JAK inhibitors for AA are underway – ritlecitinib, baricitinib, and ruxolitinib – and the ritlecitinib trial includes adolescents (ages 12 years and older). “It is the results of these trials that we eagerly await, because FDA approval will bring greater access to these treatments,” Dr. King said.
Dr. Dunnick has disclosed no relevant financial relationships. Dr. King has served on advisory boards and/or is a consultant and/or a clinical trial investigator for AbbVie, Bristol-Myers Squibb, Concert Pharmaceuticals, Eli Lilly, Incyte, Pfizer, and others. He is on speaker bureaus for AbbVie, Incyte, Pfizer, and others.
A version of this article first appeared on Medscape.com.
FROM PEDIATRIC DERMATOLOGY
Male alopecia agents ranked by efficacy in meta-analysis
While up to 90% of men experience AGA in their lifetime, only three therapies are currently approved for treatment of the condition by the Food and Drug Administration – topical minoxidil, oral finasteride 1 mg, and low-level light therapy.
However, with common use of off-label oral minoxidil, as well as oral dutasteride and higher doses of oral finasteride, the latter two being 5-alpha reductase inhibitors, Aditya K. Gupta, MD, PhD, of Mediprobe Research, in London, Ont., and colleagues sought to compare the data on the three agents. Their results were published in JAMA Dermatology.
They note that, while there have been recent comparisons between oral and topical minoxidil, “to our knowledge no study has determined the comparative effectiveness of these 2 [formulations] with that of local and systemic dutasteride and finasteride.”
For the meta-analysis, the authors identified 23 studies meeting their criteria, involving patients with mean ages ranging from 22.8 to 41.8 years.
For the primary endpoint of the greatest increases in total hair count at 24 weeks, the analysis showed the 0.5-mg/day dose of dutasteride topped the list, with significantly greater efficacy, compared with 1 mg/day of finasteride (mean difference, 7.1 hairs per cm2).
The 0.5-mg/d dutasteride dose also showed higher efficacy than oral minoxidil at 0.25 mg/day (mean difference, 23.7 hairs per cm2) and 5 mg/day (mean difference, 15.0 hairs per cm2) and topical minoxidil at 2% (mean difference, 8.5 hairs per cm2).
For the secondary endpoint of the greatest increase in terminal hair count at 24 weeks, the 5-mg/day dose of minoxidil had significantly greater efficacy compared with the 0.25-mg/day dose of the drug, as well as with minoxidil’s 2% and 5% topical formulations.
The minoxidil 5-mg/day dose was also significantly more effective than 1 mg/day of finasteride for terminal hair count at 24 weeks.
In longer-term outcomes at 48 weeks, the greatest increase in total hair count at 48 weeks was observed with 5 mg/day of finasteride, which was significantly more effective, compared with 2% topical minoxidil.
And the greatest increase in terminal hair count at 48 weeks was observed with 1 mg/day of oral finasteride, which was significantly more effective than 2% as well as 5% topical minoxidil.
Based on the results, the authors ranked the agents in a decreasing order of efficacy: 0.5 mg/day of oral dutasteride, 5 mg/day of oral finasteride, 5 mg/day of oral minoxidil, 1 mg/day of oral finasteride, 5% topical minoxidil, 2% topical minoxidil, and 0.25 mg/day of oral minoxidil.
Commenting on the analysis in an accompanying editorial, Kathie P. Huang, MD, of the department of dermatology, Brigham and Women’s Hospital, Boston, and Maryanne M. Senna, MD, of the department of dermatology, Massachusetts General Hospital, Boston, said the results, in general, are consistent with their experiences, noting that 2% minoxidil is typically not used in men.
They noted that, “although topical minoxidil ranked higher than the very-low-dose 0.25 mg oral minoxidil, our personal experience is that oral minoxidil at doses of 1.25 mg to 5 mg are far superior to topical minoxidil for treating AGA.”
Adverse event considerations important
Importantly, however, strong consideration needs to be given to adverse-event profiles, as well as patient comorbidities in selecting agents, the editorial authors asserted.
With 1 mg finasteride, for instance, potential adverse events include decreased libido, erectile dysfunction, decreased ejaculatory volume, reduction in sperm count, testicular pain, depression, and gynecomastia, they noted.
And while finasteride appears to be associated with a decreased risk of prostate cancer, those receiving the drug who do develop prostate cancer may be diagnosed with higher-grade prostate cancer; however, that “might be related to tissue sampling artifact,” the editorial authors said.
Less has been published on dutasteride’s adverse-event profile, and that, in itself, is a concern.
Overall, “as more direct-to-consumer companies treating male AGA emerge, it is especially important that the potential risks of these medications be made clear to patients,” they added.
Further commenting on the analysis to this news organization, Antonella Tosti, MD, the Fredric Brandt Endowed Professor of Dermatology and Cutaneous Surgery at the University of Miami, said the study offers some important insights – and caveats.
“I think this is a very interesting study, but you have to consider what works for your patients,” she said.
Dr. Tosti noted that the 5-mg dose of minoxidil is a concern in terms of side effects. “That dose is pretty high and could feasibly cause some hypertrichosis, which can be a concern to men as well as women.”
She agrees that the lack of data on side effects with dutasteride is also a concern, especially in light of some of the known side effects with other agents.
“That’s why I don’t use it very much in younger patients – because I’m afraid it could potentially affect their fertility,” Dr. Tosti said.
In general, Dr. Tosti said she finds a combination of agents provides the best results, as many clinicians use.
“I find dutasteride (0.5 mg/day) plus oral minoxidil (1-2.5 mg/day) plus topical 5% minoxidil is the best combination,” she said.
The authors and Dr. Tosti disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
While up to 90% of men experience AGA in their lifetime, only three therapies are currently approved for treatment of the condition by the Food and Drug Administration – topical minoxidil, oral finasteride 1 mg, and low-level light therapy.
However, with common use of off-label oral minoxidil, as well as oral dutasteride and higher doses of oral finasteride, the latter two being 5-alpha reductase inhibitors, Aditya K. Gupta, MD, PhD, of Mediprobe Research, in London, Ont., and colleagues sought to compare the data on the three agents. Their results were published in JAMA Dermatology.
They note that, while there have been recent comparisons between oral and topical minoxidil, “to our knowledge no study has determined the comparative effectiveness of these 2 [formulations] with that of local and systemic dutasteride and finasteride.”
For the meta-analysis, the authors identified 23 studies meeting their criteria, involving patients with mean ages ranging from 22.8 to 41.8 years.
For the primary endpoint of the greatest increases in total hair count at 24 weeks, the analysis showed the 0.5-mg/day dose of dutasteride topped the list, with significantly greater efficacy, compared with 1 mg/day of finasteride (mean difference, 7.1 hairs per cm2).
The 0.5-mg/d dutasteride dose also showed higher efficacy than oral minoxidil at 0.25 mg/day (mean difference, 23.7 hairs per cm2) and 5 mg/day (mean difference, 15.0 hairs per cm2) and topical minoxidil at 2% (mean difference, 8.5 hairs per cm2).
For the secondary endpoint of the greatest increase in terminal hair count at 24 weeks, the 5-mg/day dose of minoxidil had significantly greater efficacy compared with the 0.25-mg/day dose of the drug, as well as with minoxidil’s 2% and 5% topical formulations.
The minoxidil 5-mg/day dose was also significantly more effective than 1 mg/day of finasteride for terminal hair count at 24 weeks.
In longer-term outcomes at 48 weeks, the greatest increase in total hair count at 48 weeks was observed with 5 mg/day of finasteride, which was significantly more effective, compared with 2% topical minoxidil.
And the greatest increase in terminal hair count at 48 weeks was observed with 1 mg/day of oral finasteride, which was significantly more effective than 2% as well as 5% topical minoxidil.
Based on the results, the authors ranked the agents in a decreasing order of efficacy: 0.5 mg/day of oral dutasteride, 5 mg/day of oral finasteride, 5 mg/day of oral minoxidil, 1 mg/day of oral finasteride, 5% topical minoxidil, 2% topical minoxidil, and 0.25 mg/day of oral minoxidil.
Commenting on the analysis in an accompanying editorial, Kathie P. Huang, MD, of the department of dermatology, Brigham and Women’s Hospital, Boston, and Maryanne M. Senna, MD, of the department of dermatology, Massachusetts General Hospital, Boston, said the results, in general, are consistent with their experiences, noting that 2% minoxidil is typically not used in men.
They noted that, “although topical minoxidil ranked higher than the very-low-dose 0.25 mg oral minoxidil, our personal experience is that oral minoxidil at doses of 1.25 mg to 5 mg are far superior to topical minoxidil for treating AGA.”
Adverse event considerations important
Importantly, however, strong consideration needs to be given to adverse-event profiles, as well as patient comorbidities in selecting agents, the editorial authors asserted.
With 1 mg finasteride, for instance, potential adverse events include decreased libido, erectile dysfunction, decreased ejaculatory volume, reduction in sperm count, testicular pain, depression, and gynecomastia, they noted.
And while finasteride appears to be associated with a decreased risk of prostate cancer, those receiving the drug who do develop prostate cancer may be diagnosed with higher-grade prostate cancer; however, that “might be related to tissue sampling artifact,” the editorial authors said.
Less has been published on dutasteride’s adverse-event profile, and that, in itself, is a concern.
Overall, “as more direct-to-consumer companies treating male AGA emerge, it is especially important that the potential risks of these medications be made clear to patients,” they added.
Further commenting on the analysis to this news organization, Antonella Tosti, MD, the Fredric Brandt Endowed Professor of Dermatology and Cutaneous Surgery at the University of Miami, said the study offers some important insights – and caveats.
“I think this is a very interesting study, but you have to consider what works for your patients,” she said.
Dr. Tosti noted that the 5-mg dose of minoxidil is a concern in terms of side effects. “That dose is pretty high and could feasibly cause some hypertrichosis, which can be a concern to men as well as women.”
She agrees that the lack of data on side effects with dutasteride is also a concern, especially in light of some of the known side effects with other agents.
“That’s why I don’t use it very much in younger patients – because I’m afraid it could potentially affect their fertility,” Dr. Tosti said.
In general, Dr. Tosti said she finds a combination of agents provides the best results, as many clinicians use.
“I find dutasteride (0.5 mg/day) plus oral minoxidil (1-2.5 mg/day) plus topical 5% minoxidil is the best combination,” she said.
The authors and Dr. Tosti disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
While up to 90% of men experience AGA in their lifetime, only three therapies are currently approved for treatment of the condition by the Food and Drug Administration – topical minoxidil, oral finasteride 1 mg, and low-level light therapy.
However, with common use of off-label oral minoxidil, as well as oral dutasteride and higher doses of oral finasteride, the latter two being 5-alpha reductase inhibitors, Aditya K. Gupta, MD, PhD, of Mediprobe Research, in London, Ont., and colleagues sought to compare the data on the three agents. Their results were published in JAMA Dermatology.
They note that, while there have been recent comparisons between oral and topical minoxidil, “to our knowledge no study has determined the comparative effectiveness of these 2 [formulations] with that of local and systemic dutasteride and finasteride.”
For the meta-analysis, the authors identified 23 studies meeting their criteria, involving patients with mean ages ranging from 22.8 to 41.8 years.
For the primary endpoint of the greatest increases in total hair count at 24 weeks, the analysis showed the 0.5-mg/day dose of dutasteride topped the list, with significantly greater efficacy, compared with 1 mg/day of finasteride (mean difference, 7.1 hairs per cm2).
The 0.5-mg/d dutasteride dose also showed higher efficacy than oral minoxidil at 0.25 mg/day (mean difference, 23.7 hairs per cm2) and 5 mg/day (mean difference, 15.0 hairs per cm2) and topical minoxidil at 2% (mean difference, 8.5 hairs per cm2).
For the secondary endpoint of the greatest increase in terminal hair count at 24 weeks, the 5-mg/day dose of minoxidil had significantly greater efficacy compared with the 0.25-mg/day dose of the drug, as well as with minoxidil’s 2% and 5% topical formulations.
The minoxidil 5-mg/day dose was also significantly more effective than 1 mg/day of finasteride for terminal hair count at 24 weeks.
In longer-term outcomes at 48 weeks, the greatest increase in total hair count at 48 weeks was observed with 5 mg/day of finasteride, which was significantly more effective, compared with 2% topical minoxidil.
And the greatest increase in terminal hair count at 48 weeks was observed with 1 mg/day of oral finasteride, which was significantly more effective than 2% as well as 5% topical minoxidil.
Based on the results, the authors ranked the agents in a decreasing order of efficacy: 0.5 mg/day of oral dutasteride, 5 mg/day of oral finasteride, 5 mg/day of oral minoxidil, 1 mg/day of oral finasteride, 5% topical minoxidil, 2% topical minoxidil, and 0.25 mg/day of oral minoxidil.
Commenting on the analysis in an accompanying editorial, Kathie P. Huang, MD, of the department of dermatology, Brigham and Women’s Hospital, Boston, and Maryanne M. Senna, MD, of the department of dermatology, Massachusetts General Hospital, Boston, said the results, in general, are consistent with their experiences, noting that 2% minoxidil is typically not used in men.
They noted that, “although topical minoxidil ranked higher than the very-low-dose 0.25 mg oral minoxidil, our personal experience is that oral minoxidil at doses of 1.25 mg to 5 mg are far superior to topical minoxidil for treating AGA.”
Adverse event considerations important
Importantly, however, strong consideration needs to be given to adverse-event profiles, as well as patient comorbidities in selecting agents, the editorial authors asserted.
With 1 mg finasteride, for instance, potential adverse events include decreased libido, erectile dysfunction, decreased ejaculatory volume, reduction in sperm count, testicular pain, depression, and gynecomastia, they noted.
And while finasteride appears to be associated with a decreased risk of prostate cancer, those receiving the drug who do develop prostate cancer may be diagnosed with higher-grade prostate cancer; however, that “might be related to tissue sampling artifact,” the editorial authors said.
Less has been published on dutasteride’s adverse-event profile, and that, in itself, is a concern.
Overall, “as more direct-to-consumer companies treating male AGA emerge, it is especially important that the potential risks of these medications be made clear to patients,” they added.
Further commenting on the analysis to this news organization, Antonella Tosti, MD, the Fredric Brandt Endowed Professor of Dermatology and Cutaneous Surgery at the University of Miami, said the study offers some important insights – and caveats.
“I think this is a very interesting study, but you have to consider what works for your patients,” she said.
Dr. Tosti noted that the 5-mg dose of minoxidil is a concern in terms of side effects. “That dose is pretty high and could feasibly cause some hypertrichosis, which can be a concern to men as well as women.”
She agrees that the lack of data on side effects with dutasteride is also a concern, especially in light of some of the known side effects with other agents.
“That’s why I don’t use it very much in younger patients – because I’m afraid it could potentially affect their fertility,” Dr. Tosti said.
In general, Dr. Tosti said she finds a combination of agents provides the best results, as many clinicians use.
“I find dutasteride (0.5 mg/day) plus oral minoxidil (1-2.5 mg/day) plus topical 5% minoxidil is the best combination,” she said.
The authors and Dr. Tosti disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA DERMATOLOGY
Hairstyling Practices to Prevent Hair Damage and Alopecia in Women of African Descent
Central centrifugal cicatricial alopecia (CCCA), traction alopecia, and acquired proximal trichorrhexis nodosa are 3 forms of alopecia that disproportionately affect women of African descent.1 Central centrifugal cicatricial alopecia is characterized by a shiny smooth patch of hair loss over the vertex of the scalp that spreads centrifugally (Figure 1).1-4 Traction alopecia results from prolonged or repeated tension on the hair root that causes mechanical damage, hair loss, and shortening of hairs along the frontotemporal line (the so-called fringe sign)(Figure 2).1,3,5 Acquired proximal trichorrhexis nodosa, a result of trauma, is identified by a substantial number of hairs breaking off midshaft during a hair pull test.1 By understanding the unique structural properties and grooming methods of hair in women of African descent, physicians can better manage and stop the progression of hair loss before it becomes permanent.1,4,5

The characterization of hair between and within ethnic groups is challenging and lies on a spectrum.6,7 Many early studies broadly differentiated hair in 3 ethnic subgroups: African, Asian, and Caucasian6-8; older descriptions of hair texture also included terms such as straight, wavy, curly, and kinky.6 However, defining hair texture should be based on an approach that is more objective than an inaccurate ethnicity-based classification or the use of subjective, ill-defined, and overlapping descriptive terms.7 The segmentation tree analysis method (STAM) is an objective classification system that, when applied to hair, yields 8 curl-type groups (I=straight; VIII=tightly curly) based on curve diameter, curl index, number of waves, and twists.6-9 (We discuss the “tightly coiled” [group VII] through “tight, interwoven small curls” [group VIII] groups in the STAM classification of hair.)
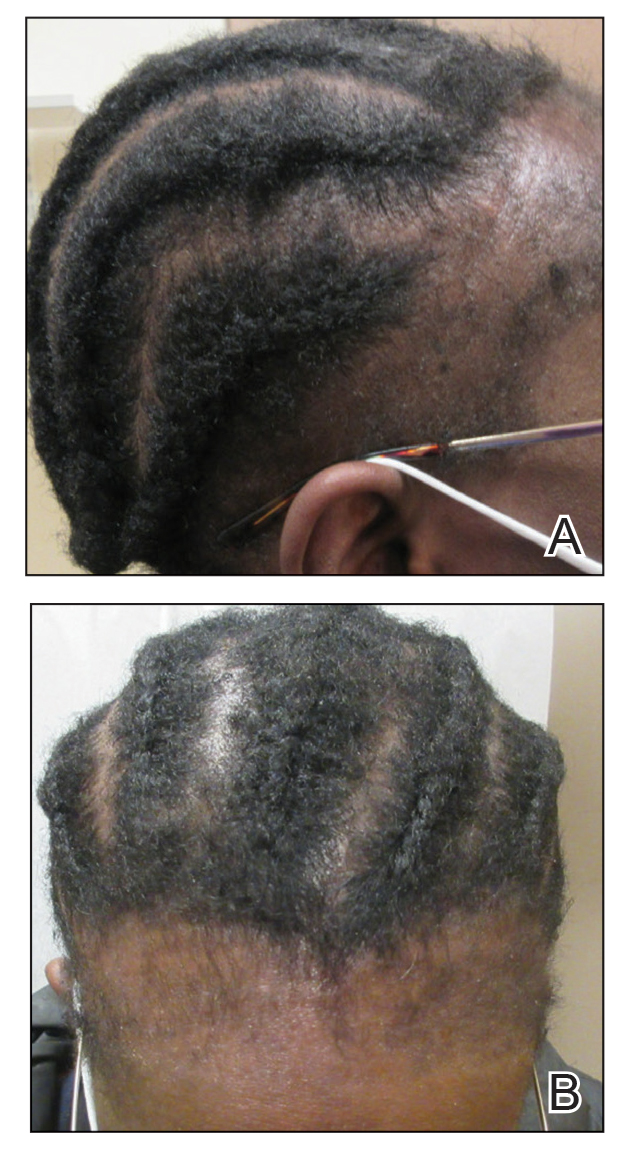
Highly textured hair has been found to be more susceptible to breakage than other hair types because of an increased percentage of spirals and relatively fewer elastic fibers anchoring hair follicles to the dermis.1-4,10,11 In a cross-section, the hair shaft of individuals of African descent tends to be more elliptical and kidney shaped than the hair shaft of Asian individuals, which is round and has a large diameter, and the hair shaft of Caucasian individuals, which structurally lies between African and Asian hair.1,2,4,11 This axial asymmetry and section size contributes to points of lower tensile strength and increased fragility, which are exacerbated by everyday combing and grooming. Curvature of the hair follicle leads to the characteristic curly and spiral nature of African hair, which can lead to increased knotting.2,4
Practice Gap
Among women of African descent, a variety of hairstyles and hair treatments frequently are employed to allow for ease of management and self-expression.1 Many of these practices have been implicated as risk factors for alopecia. Simply advising patients to avoid tight hairstyles is ineffective because tension is subjective and difficult to quantify.5 Furthermore, it might be unreasonable to ask a patient to discontinue a hairstyle or treatment when they are unaware of less damaging alternatives.3,5
We provide an overview of hairstyles for patients who have highly textured hair so that physicians can better identify high-risk hairstyles and provide individualized recommendations for safer alternatives.1,3,5
Techniques for Hair Straightening
Traditional thermal straightening uses a hot comb or flat iron1,2,4,12 to temporarily disrupt hydrogen bonds within the hair shafts, which is reversible with exposure to moisture.1,2,4,5 Patients repeat this process every 1 or 2 weeks to offset the effects of normal perspiration and environmental humidity.5,12 Thermal straightening techniques can lead to increased fragility of the hair shaft and loss of tensile strength.11
Alternate methods of hair straightening use lye (sodium hydroxide) or nonlye (lithium and guanidine hydroxide) “relaxers” to permanently disrupt hydrogen and disulfide bonds in the hair shaft, which can damage and weaken hair.1-5,11,12 Touch-ups to the roots often are performed every 6 to 8 weeks.1,2
Chemical relaxers historically have been associated with CCCA but have not been definitively implicated as causative.2,3,4,13 Most studies have not demonstrated a statistically significant association between chemical relaxers and CCCA because, with a few exceptions,13 studies have either been based on surveys or have not employed trichoscopy or scalp biopsy. In one of those studies, patients with CCCA were determined to be 12.37 times more likely to have used a chemical relaxer in the past (P<.001).13 In another study of 39 women in Nigeria, those who had frequent and prolonged use of a chemical relaxer developed scarring alopecia more often than those who did not use a chemical relaxer (P<.0001). However, it is now known that the pathogenesis of CCCA may be related to an upregulation in genes implicated in fibroproliferative disorders (FPDs), a group of conditions characterized by aberrant wound healing, low-grade inflammation and irritation, and excessive fibrosis.14 They include systemic sclerosis, keloids, atherosclerosis, and uterine fibroids. The risk for certain FPDs is increased in individuals of African descent, and this increased risk is thought to be secondary to the protective effect that profibrotic alleles offer against helminths found in sub-Saharan Africa. A study of 5 patients with biopsy-proven CCCA found that there was increased expression of platelet-derived growth factor gene, PDGF; collagen I gene, COL I; collagen III gene, COL III; matrix metallopeptidase 1 gene, MMP1; matrix metallopeptidase 2 gene, MMP2; matrix metallopeptidase 7 gene, MMP7; and matrix metallopeptidase 9 gene, MMP9, in an affected scalp compared with an unaffected scalp.14 Still, chemical relaxers weaken the hair shaft and follicle structure, increasing the possibility of hair breakage and allowing for inflammation and trauma to render negative follicular effects.3,13
The following interventions can be recommended to patients who thermally or chemically treat their hair to prevent hair damage:
- Decrease the frequency of thermal straightening.
- Use lower heat settings on flat irons and blow-dryers.
- Thermally straighten only clean dry hair.
- Regularly trim split ends.
- Use moisturizing shampoos and conditioners.
- Have a trained professional apply a chemical relaxer, if affordable.
- Consider decreasing (1) the frequency of chemical relaxer touch-up (to every 8 to 10 weeks) and (2) the overall manipulation of hair. There is a fine balance between not treating often enough and treating too often: The transition point between chemically processed hair and grown-out roots is a high-tension breakage point.
- Apply a thick protective emollient (known as scalp basing) to the scalp before applying a relaxer1,5; this protects the scalp from irritation.
Techniques for Braids, Weaves, and Twists
Braids and cornrows, sewn-in or glued-on extensions and weaves, and twists are popular hairstyles. When applied improperly, however, they also can lead to alopecia.1-5,11,12 When braids are too tight, the patient might complain of headache. Characteristic tenting—hair pulled so tight that the scalp is raised—might be observed.3,5 Twists are achieved by interlocking 2 pieces of hair, which are held together by styling gel.1,4 When twists remain over many months, hair eventually knots or tangles into a permanent locking pattern (also known as dreadlocks, dreads, or locs).1,2,4 In some cases, the persistent weight of dreadlocks results in hair breakage.1,3,5
The following recommendations can be made to patients who style their hair with braids or cornrows, extensions or weaves, twists, or dreadlocks:
- Apply these styles with as little traction as possible.
- Change the direction in which braids and cornrows are styled frequently to avoid constant tension over the same areas.
- Opt for larger-diameter braids and twists.
- Leave these styles in place no longer than 2 or 3 months; consider removing extensions and weaves every 3 or 4 weeks.
- Remove extensions and weaves if they cause pain or irritation.
- Avoid the use of glue; opt for loosely sewn-in extensions and weaves.
- Consider the alternative of crochet braiding; this is a protective way to apply extensions to hair and can be worn straight, curly, braided, or twisted.5,12
Techniques for Other Hairstyling Practices
Low-hanging ponytails or buns, wigs, and natural hairstyles generally are considered safe when applied correctly.1,5 The following recommendations can be made to patients who have a low-hanging ponytail, bun, wig, or other natural hairstyle:
- Before a wig is applied, hold the hair against the scalp with a cotton, nylon, or satin wig cap and with clips, tapes, or bonds. Because satin does not cause constant friction or absorb moisture, it is the safest material for a wig cap.5
- Achieve a natural hairstyle by cutting off chemically processed hair and allowing hair to grow out.5
- Hair that has not been thermally or chemically processed better withstands the stresses of traction, pulling, and brushing.5
- For women with natural hair, wash hair at least every 2 weeks and moisturize frequently.5,12
- Caution patients that adding synthetic or human hair (ie, extensions, weaves) to any hairstyle to increase volume or length using glue or sewing techniques1-4,11 can cause problems. The extra weight and tension of extensions and weaves can lead to alopecia. Glue can trigger an irritant or allergic reaction, especially in women who have a latex allergy.1,4,5,11
Practice Implications
Women of African descent might be more susceptible to alopecia because of the distinctive structural properties of their hair and the various hair treatments and styles they often employ. Physicians should be knowledgeable when counseling these patients on their hair care practices. It also is important to understand that it might not be feasible for a patient to completely discontinue a hair treatment or style. In that situation, be prepared to make recommendations for safer hairstyling practices.
- Callender VD, McMichael AJ, Cohen GF. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17:164-176. doi:10.1111/j.1396-0296.2004.04017.x
- Herskovitz I, Miteva M. Central centrifugal cicatricial alopecia: challenges and solutions. Clin Cosmet Investig Dermatol. 2016;9:175-181. doi:10.2147/CCID.S100816
- Tanus A, Oliveira CCC, Villarreal DJ, et al. Black women’s hair: the main scalp dermatoses and aesthetic practices in women of African ethnicity. An Bras Dermatol. 2015;90:450-465. doi:10.1590/abd1806-4841.20152845
- Gathers RC, Lim HW. Central centrifugal cicatricial alopecia: past, present, and future. J Am Acad Dermatol. 2009;60:660-668. doi:10.1016/j.jaad.2008.09.066
- Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia (TA). J Am Acad Dermatol. 2016;75:606-611. doi:10.1016/j.jaad.2016.02.1162
- Loussouarn G, Garcel A-L, Lozano I, et al. Worldwide diversity of hair curliness: a new method of assessment. Int J Dermatol. 2007;46(suppl 1):2-6. doi:10.1111/j.1365-4632.2007.03453.x
- De la Mettrie R, Saint-Léger D, Loussouarn G, et al. Shape variability and classification of human hair: a worldwide approach. Hum Biol. 2007;79:265-281. doi:10.1353/hub.2007.0045
- Takahashi T. Unique hair properties that emerge from combinations of multiple races. Cosmetics. 2019;6:36. https://doi.org/10.3390/cosmetics6020036
- Cloete E, Khumalo NP, Ngoepe MN. The what, why and how of curly hair: a review. Proc Math Phys Eng Sci. 2019;475:20190516. doi:10.1098/rspa.2019.0516
- Westgate GE, Ginger RS, Green MR. The biology and genetics of curly hair. Exp Dermatol. 2017;26:483-490. doi:10.1111/exd.13347
- McMichael AJ. Ethnic hair update: past and present. J Am Acad Dermatol. 2003;48(6 suppl):S127-S133. doi:10.1067/mjd.2003.278
- Roseborough IE, McMichael AJ. Hair care practices in African-American patients. Semin Cutan Med Surg. 2009;28:103-108. doi:10.1016/j.sder.2009.04.007
- Narasimman M, De Bedout V, Castillo DE, et al. Increased association between previous pregnancies and use of chemical relaxers in 74 women with central centrifugal cicatricial alopecia. Int J Trichology. 2020;12:176-181. doi:10.4103/ijt.ijt_37_20
- Aguh C, Dina Y, Talbot CC Jr, et al. Fibroproliferative genes are preferentially expressed in central centrifugal cicatricial alopecia. J Am Acad Dermatol. 2018;79:904-912.e901. doi:10.1016/j.jaad.2018.05.1257
Central centrifugal cicatricial alopecia (CCCA), traction alopecia, and acquired proximal trichorrhexis nodosa are 3 forms of alopecia that disproportionately affect women of African descent.1 Central centrifugal cicatricial alopecia is characterized by a shiny smooth patch of hair loss over the vertex of the scalp that spreads centrifugally (Figure 1).1-4 Traction alopecia results from prolonged or repeated tension on the hair root that causes mechanical damage, hair loss, and shortening of hairs along the frontotemporal line (the so-called fringe sign)(Figure 2).1,3,5 Acquired proximal trichorrhexis nodosa, a result of trauma, is identified by a substantial number of hairs breaking off midshaft during a hair pull test.1 By understanding the unique structural properties and grooming methods of hair in women of African descent, physicians can better manage and stop the progression of hair loss before it becomes permanent.1,4,5

The characterization of hair between and within ethnic groups is challenging and lies on a spectrum.6,7 Many early studies broadly differentiated hair in 3 ethnic subgroups: African, Asian, and Caucasian6-8; older descriptions of hair texture also included terms such as straight, wavy, curly, and kinky.6 However, defining hair texture should be based on an approach that is more objective than an inaccurate ethnicity-based classification or the use of subjective, ill-defined, and overlapping descriptive terms.7 The segmentation tree analysis method (STAM) is an objective classification system that, when applied to hair, yields 8 curl-type groups (I=straight; VIII=tightly curly) based on curve diameter, curl index, number of waves, and twists.6-9 (We discuss the “tightly coiled” [group VII] through “tight, interwoven small curls” [group VIII] groups in the STAM classification of hair.)

Highly textured hair has been found to be more susceptible to breakage than other hair types because of an increased percentage of spirals and relatively fewer elastic fibers anchoring hair follicles to the dermis.1-4,10,11 In a cross-section, the hair shaft of individuals of African descent tends to be more elliptical and kidney shaped than the hair shaft of Asian individuals, which is round and has a large diameter, and the hair shaft of Caucasian individuals, which structurally lies between African and Asian hair.1,2,4,11 This axial asymmetry and section size contributes to points of lower tensile strength and increased fragility, which are exacerbated by everyday combing and grooming. Curvature of the hair follicle leads to the characteristic curly and spiral nature of African hair, which can lead to increased knotting.2,4
Practice Gap
Among women of African descent, a variety of hairstyles and hair treatments frequently are employed to allow for ease of management and self-expression.1 Many of these practices have been implicated as risk factors for alopecia. Simply advising patients to avoid tight hairstyles is ineffective because tension is subjective and difficult to quantify.5 Furthermore, it might be unreasonable to ask a patient to discontinue a hairstyle or treatment when they are unaware of less damaging alternatives.3,5
We provide an overview of hairstyles for patients who have highly textured hair so that physicians can better identify high-risk hairstyles and provide individualized recommendations for safer alternatives.1,3,5
Techniques for Hair Straightening
Traditional thermal straightening uses a hot comb or flat iron1,2,4,12 to temporarily disrupt hydrogen bonds within the hair shafts, which is reversible with exposure to moisture.1,2,4,5 Patients repeat this process every 1 or 2 weeks to offset the effects of normal perspiration and environmental humidity.5,12 Thermal straightening techniques can lead to increased fragility of the hair shaft and loss of tensile strength.11
Alternate methods of hair straightening use lye (sodium hydroxide) or nonlye (lithium and guanidine hydroxide) “relaxers” to permanently disrupt hydrogen and disulfide bonds in the hair shaft, which can damage and weaken hair.1-5,11,12 Touch-ups to the roots often are performed every 6 to 8 weeks.1,2
Chemical relaxers historically have been associated with CCCA but have not been definitively implicated as causative.2,3,4,13 Most studies have not demonstrated a statistically significant association between chemical relaxers and CCCA because, with a few exceptions,13 studies have either been based on surveys or have not employed trichoscopy or scalp biopsy. In one of those studies, patients with CCCA were determined to be 12.37 times more likely to have used a chemical relaxer in the past (P<.001).13 In another study of 39 women in Nigeria, those who had frequent and prolonged use of a chemical relaxer developed scarring alopecia more often than those who did not use a chemical relaxer (P<.0001). However, it is now known that the pathogenesis of CCCA may be related to an upregulation in genes implicated in fibroproliferative disorders (FPDs), a group of conditions characterized by aberrant wound healing, low-grade inflammation and irritation, and excessive fibrosis.14 They include systemic sclerosis, keloids, atherosclerosis, and uterine fibroids. The risk for certain FPDs is increased in individuals of African descent, and this increased risk is thought to be secondary to the protective effect that profibrotic alleles offer against helminths found in sub-Saharan Africa. A study of 5 patients with biopsy-proven CCCA found that there was increased expression of platelet-derived growth factor gene, PDGF; collagen I gene, COL I; collagen III gene, COL III; matrix metallopeptidase 1 gene, MMP1; matrix metallopeptidase 2 gene, MMP2; matrix metallopeptidase 7 gene, MMP7; and matrix metallopeptidase 9 gene, MMP9, in an affected scalp compared with an unaffected scalp.14 Still, chemical relaxers weaken the hair shaft and follicle structure, increasing the possibility of hair breakage and allowing for inflammation and trauma to render negative follicular effects.3,13
The following interventions can be recommended to patients who thermally or chemically treat their hair to prevent hair damage:
- Decrease the frequency of thermal straightening.
- Use lower heat settings on flat irons and blow-dryers.
- Thermally straighten only clean dry hair.
- Regularly trim split ends.
- Use moisturizing shampoos and conditioners.
- Have a trained professional apply a chemical relaxer, if affordable.
- Consider decreasing (1) the frequency of chemical relaxer touch-up (to every 8 to 10 weeks) and (2) the overall manipulation of hair. There is a fine balance between not treating often enough and treating too often: The transition point between chemically processed hair and grown-out roots is a high-tension breakage point.
- Apply a thick protective emollient (known as scalp basing) to the scalp before applying a relaxer1,5; this protects the scalp from irritation.
Techniques for Braids, Weaves, and Twists
Braids and cornrows, sewn-in or glued-on extensions and weaves, and twists are popular hairstyles. When applied improperly, however, they also can lead to alopecia.1-5,11,12 When braids are too tight, the patient might complain of headache. Characteristic tenting—hair pulled so tight that the scalp is raised—might be observed.3,5 Twists are achieved by interlocking 2 pieces of hair, which are held together by styling gel.1,4 When twists remain over many months, hair eventually knots or tangles into a permanent locking pattern (also known as dreadlocks, dreads, or locs).1,2,4 In some cases, the persistent weight of dreadlocks results in hair breakage.1,3,5
The following recommendations can be made to patients who style their hair with braids or cornrows, extensions or weaves, twists, or dreadlocks:
- Apply these styles with as little traction as possible.
- Change the direction in which braids and cornrows are styled frequently to avoid constant tension over the same areas.
- Opt for larger-diameter braids and twists.
- Leave these styles in place no longer than 2 or 3 months; consider removing extensions and weaves every 3 or 4 weeks.
- Remove extensions and weaves if they cause pain or irritation.
- Avoid the use of glue; opt for loosely sewn-in extensions and weaves.
- Consider the alternative of crochet braiding; this is a protective way to apply extensions to hair and can be worn straight, curly, braided, or twisted.5,12
Techniques for Other Hairstyling Practices
Low-hanging ponytails or buns, wigs, and natural hairstyles generally are considered safe when applied correctly.1,5 The following recommendations can be made to patients who have a low-hanging ponytail, bun, wig, or other natural hairstyle:
- Before a wig is applied, hold the hair against the scalp with a cotton, nylon, or satin wig cap and with clips, tapes, or bonds. Because satin does not cause constant friction or absorb moisture, it is the safest material for a wig cap.5
- Achieve a natural hairstyle by cutting off chemically processed hair and allowing hair to grow out.5
- Hair that has not been thermally or chemically processed better withstands the stresses of traction, pulling, and brushing.5
- For women with natural hair, wash hair at least every 2 weeks and moisturize frequently.5,12
- Caution patients that adding synthetic or human hair (ie, extensions, weaves) to any hairstyle to increase volume or length using glue or sewing techniques1-4,11 can cause problems. The extra weight and tension of extensions and weaves can lead to alopecia. Glue can trigger an irritant or allergic reaction, especially in women who have a latex allergy.1,4,5,11
Practice Implications
Women of African descent might be more susceptible to alopecia because of the distinctive structural properties of their hair and the various hair treatments and styles they often employ. Physicians should be knowledgeable when counseling these patients on their hair care practices. It also is important to understand that it might not be feasible for a patient to completely discontinue a hair treatment or style. In that situation, be prepared to make recommendations for safer hairstyling practices.
Central centrifugal cicatricial alopecia (CCCA), traction alopecia, and acquired proximal trichorrhexis nodosa are 3 forms of alopecia that disproportionately affect women of African descent.1 Central centrifugal cicatricial alopecia is characterized by a shiny smooth patch of hair loss over the vertex of the scalp that spreads centrifugally (Figure 1).1-4 Traction alopecia results from prolonged or repeated tension on the hair root that causes mechanical damage, hair loss, and shortening of hairs along the frontotemporal line (the so-called fringe sign)(Figure 2).1,3,5 Acquired proximal trichorrhexis nodosa, a result of trauma, is identified by a substantial number of hairs breaking off midshaft during a hair pull test.1 By understanding the unique structural properties and grooming methods of hair in women of African descent, physicians can better manage and stop the progression of hair loss before it becomes permanent.1,4,5

The characterization of hair between and within ethnic groups is challenging and lies on a spectrum.6,7 Many early studies broadly differentiated hair in 3 ethnic subgroups: African, Asian, and Caucasian6-8; older descriptions of hair texture also included terms such as straight, wavy, curly, and kinky.6 However, defining hair texture should be based on an approach that is more objective than an inaccurate ethnicity-based classification or the use of subjective, ill-defined, and overlapping descriptive terms.7 The segmentation tree analysis method (STAM) is an objective classification system that, when applied to hair, yields 8 curl-type groups (I=straight; VIII=tightly curly) based on curve diameter, curl index, number of waves, and twists.6-9 (We discuss the “tightly coiled” [group VII] through “tight, interwoven small curls” [group VIII] groups in the STAM classification of hair.)

Highly textured hair has been found to be more susceptible to breakage than other hair types because of an increased percentage of spirals and relatively fewer elastic fibers anchoring hair follicles to the dermis.1-4,10,11 In a cross-section, the hair shaft of individuals of African descent tends to be more elliptical and kidney shaped than the hair shaft of Asian individuals, which is round and has a large diameter, and the hair shaft of Caucasian individuals, which structurally lies between African and Asian hair.1,2,4,11 This axial asymmetry and section size contributes to points of lower tensile strength and increased fragility, which are exacerbated by everyday combing and grooming. Curvature of the hair follicle leads to the characteristic curly and spiral nature of African hair, which can lead to increased knotting.2,4
Practice Gap
Among women of African descent, a variety of hairstyles and hair treatments frequently are employed to allow for ease of management and self-expression.1 Many of these practices have been implicated as risk factors for alopecia. Simply advising patients to avoid tight hairstyles is ineffective because tension is subjective and difficult to quantify.5 Furthermore, it might be unreasonable to ask a patient to discontinue a hairstyle or treatment when they are unaware of less damaging alternatives.3,5
We provide an overview of hairstyles for patients who have highly textured hair so that physicians can better identify high-risk hairstyles and provide individualized recommendations for safer alternatives.1,3,5
Techniques for Hair Straightening
Traditional thermal straightening uses a hot comb or flat iron1,2,4,12 to temporarily disrupt hydrogen bonds within the hair shafts, which is reversible with exposure to moisture.1,2,4,5 Patients repeat this process every 1 or 2 weeks to offset the effects of normal perspiration and environmental humidity.5,12 Thermal straightening techniques can lead to increased fragility of the hair shaft and loss of tensile strength.11
Alternate methods of hair straightening use lye (sodium hydroxide) or nonlye (lithium and guanidine hydroxide) “relaxers” to permanently disrupt hydrogen and disulfide bonds in the hair shaft, which can damage and weaken hair.1-5,11,12 Touch-ups to the roots often are performed every 6 to 8 weeks.1,2
Chemical relaxers historically have been associated with CCCA but have not been definitively implicated as causative.2,3,4,13 Most studies have not demonstrated a statistically significant association between chemical relaxers and CCCA because, with a few exceptions,13 studies have either been based on surveys or have not employed trichoscopy or scalp biopsy. In one of those studies, patients with CCCA were determined to be 12.37 times more likely to have used a chemical relaxer in the past (P<.001).13 In another study of 39 women in Nigeria, those who had frequent and prolonged use of a chemical relaxer developed scarring alopecia more often than those who did not use a chemical relaxer (P<.0001). However, it is now known that the pathogenesis of CCCA may be related to an upregulation in genes implicated in fibroproliferative disorders (FPDs), a group of conditions characterized by aberrant wound healing, low-grade inflammation and irritation, and excessive fibrosis.14 They include systemic sclerosis, keloids, atherosclerosis, and uterine fibroids. The risk for certain FPDs is increased in individuals of African descent, and this increased risk is thought to be secondary to the protective effect that profibrotic alleles offer against helminths found in sub-Saharan Africa. A study of 5 patients with biopsy-proven CCCA found that there was increased expression of platelet-derived growth factor gene, PDGF; collagen I gene, COL I; collagen III gene, COL III; matrix metallopeptidase 1 gene, MMP1; matrix metallopeptidase 2 gene, MMP2; matrix metallopeptidase 7 gene, MMP7; and matrix metallopeptidase 9 gene, MMP9, in an affected scalp compared with an unaffected scalp.14 Still, chemical relaxers weaken the hair shaft and follicle structure, increasing the possibility of hair breakage and allowing for inflammation and trauma to render negative follicular effects.3,13
The following interventions can be recommended to patients who thermally or chemically treat their hair to prevent hair damage:
- Decrease the frequency of thermal straightening.
- Use lower heat settings on flat irons and blow-dryers.
- Thermally straighten only clean dry hair.
- Regularly trim split ends.
- Use moisturizing shampoos and conditioners.
- Have a trained professional apply a chemical relaxer, if affordable.
- Consider decreasing (1) the frequency of chemical relaxer touch-up (to every 8 to 10 weeks) and (2) the overall manipulation of hair. There is a fine balance between not treating often enough and treating too often: The transition point between chemically processed hair and grown-out roots is a high-tension breakage point.
- Apply a thick protective emollient (known as scalp basing) to the scalp before applying a relaxer1,5; this protects the scalp from irritation.
Techniques for Braids, Weaves, and Twists
Braids and cornrows, sewn-in or glued-on extensions and weaves, and twists are popular hairstyles. When applied improperly, however, they also can lead to alopecia.1-5,11,12 When braids are too tight, the patient might complain of headache. Characteristic tenting—hair pulled so tight that the scalp is raised—might be observed.3,5 Twists are achieved by interlocking 2 pieces of hair, which are held together by styling gel.1,4 When twists remain over many months, hair eventually knots or tangles into a permanent locking pattern (also known as dreadlocks, dreads, or locs).1,2,4 In some cases, the persistent weight of dreadlocks results in hair breakage.1,3,5
The following recommendations can be made to patients who style their hair with braids or cornrows, extensions or weaves, twists, or dreadlocks:
- Apply these styles with as little traction as possible.
- Change the direction in which braids and cornrows are styled frequently to avoid constant tension over the same areas.
- Opt for larger-diameter braids and twists.
- Leave these styles in place no longer than 2 or 3 months; consider removing extensions and weaves every 3 or 4 weeks.
- Remove extensions and weaves if they cause pain or irritation.
- Avoid the use of glue; opt for loosely sewn-in extensions and weaves.
- Consider the alternative of crochet braiding; this is a protective way to apply extensions to hair and can be worn straight, curly, braided, or twisted.5,12
Techniques for Other Hairstyling Practices
Low-hanging ponytails or buns, wigs, and natural hairstyles generally are considered safe when applied correctly.1,5 The following recommendations can be made to patients who have a low-hanging ponytail, bun, wig, or other natural hairstyle:
- Before a wig is applied, hold the hair against the scalp with a cotton, nylon, or satin wig cap and with clips, tapes, or bonds. Because satin does not cause constant friction or absorb moisture, it is the safest material for a wig cap.5
- Achieve a natural hairstyle by cutting off chemically processed hair and allowing hair to grow out.5
- Hair that has not been thermally or chemically processed better withstands the stresses of traction, pulling, and brushing.5
- For women with natural hair, wash hair at least every 2 weeks and moisturize frequently.5,12
- Caution patients that adding synthetic or human hair (ie, extensions, weaves) to any hairstyle to increase volume or length using glue or sewing techniques1-4,11 can cause problems. The extra weight and tension of extensions and weaves can lead to alopecia. Glue can trigger an irritant or allergic reaction, especially in women who have a latex allergy.1,4,5,11
Practice Implications
Women of African descent might be more susceptible to alopecia because of the distinctive structural properties of their hair and the various hair treatments and styles they often employ. Physicians should be knowledgeable when counseling these patients on their hair care practices. It also is important to understand that it might not be feasible for a patient to completely discontinue a hair treatment or style. In that situation, be prepared to make recommendations for safer hairstyling practices.
- Callender VD, McMichael AJ, Cohen GF. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17:164-176. doi:10.1111/j.1396-0296.2004.04017.x
- Herskovitz I, Miteva M. Central centrifugal cicatricial alopecia: challenges and solutions. Clin Cosmet Investig Dermatol. 2016;9:175-181. doi:10.2147/CCID.S100816
- Tanus A, Oliveira CCC, Villarreal DJ, et al. Black women’s hair: the main scalp dermatoses and aesthetic practices in women of African ethnicity. An Bras Dermatol. 2015;90:450-465. doi:10.1590/abd1806-4841.20152845
- Gathers RC, Lim HW. Central centrifugal cicatricial alopecia: past, present, and future. J Am Acad Dermatol. 2009;60:660-668. doi:10.1016/j.jaad.2008.09.066
- Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia (TA). J Am Acad Dermatol. 2016;75:606-611. doi:10.1016/j.jaad.2016.02.1162
- Loussouarn G, Garcel A-L, Lozano I, et al. Worldwide diversity of hair curliness: a new method of assessment. Int J Dermatol. 2007;46(suppl 1):2-6. doi:10.1111/j.1365-4632.2007.03453.x
- De la Mettrie R, Saint-Léger D, Loussouarn G, et al. Shape variability and classification of human hair: a worldwide approach. Hum Biol. 2007;79:265-281. doi:10.1353/hub.2007.0045
- Takahashi T. Unique hair properties that emerge from combinations of multiple races. Cosmetics. 2019;6:36. https://doi.org/10.3390/cosmetics6020036
- Cloete E, Khumalo NP, Ngoepe MN. The what, why and how of curly hair: a review. Proc Math Phys Eng Sci. 2019;475:20190516. doi:10.1098/rspa.2019.0516
- Westgate GE, Ginger RS, Green MR. The biology and genetics of curly hair. Exp Dermatol. 2017;26:483-490. doi:10.1111/exd.13347
- McMichael AJ. Ethnic hair update: past and present. J Am Acad Dermatol. 2003;48(6 suppl):S127-S133. doi:10.1067/mjd.2003.278
- Roseborough IE, McMichael AJ. Hair care practices in African-American patients. Semin Cutan Med Surg. 2009;28:103-108. doi:10.1016/j.sder.2009.04.007
- Narasimman M, De Bedout V, Castillo DE, et al. Increased association between previous pregnancies and use of chemical relaxers in 74 women with central centrifugal cicatricial alopecia. Int J Trichology. 2020;12:176-181. doi:10.4103/ijt.ijt_37_20
- Aguh C, Dina Y, Talbot CC Jr, et al. Fibroproliferative genes are preferentially expressed in central centrifugal cicatricial alopecia. J Am Acad Dermatol. 2018;79:904-912.e901. doi:10.1016/j.jaad.2018.05.1257
- Callender VD, McMichael AJ, Cohen GF. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17:164-176. doi:10.1111/j.1396-0296.2004.04017.x
- Herskovitz I, Miteva M. Central centrifugal cicatricial alopecia: challenges and solutions. Clin Cosmet Investig Dermatol. 2016;9:175-181. doi:10.2147/CCID.S100816
- Tanus A, Oliveira CCC, Villarreal DJ, et al. Black women’s hair: the main scalp dermatoses and aesthetic practices in women of African ethnicity. An Bras Dermatol. 2015;90:450-465. doi:10.1590/abd1806-4841.20152845
- Gathers RC, Lim HW. Central centrifugal cicatricial alopecia: past, present, and future. J Am Acad Dermatol. 2009;60:660-668. doi:10.1016/j.jaad.2008.09.066
- Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia (TA). J Am Acad Dermatol. 2016;75:606-611. doi:10.1016/j.jaad.2016.02.1162
- Loussouarn G, Garcel A-L, Lozano I, et al. Worldwide diversity of hair curliness: a new method of assessment. Int J Dermatol. 2007;46(suppl 1):2-6. doi:10.1111/j.1365-4632.2007.03453.x
- De la Mettrie R, Saint-Léger D, Loussouarn G, et al. Shape variability and classification of human hair: a worldwide approach. Hum Biol. 2007;79:265-281. doi:10.1353/hub.2007.0045
- Takahashi T. Unique hair properties that emerge from combinations of multiple races. Cosmetics. 2019;6:36. https://doi.org/10.3390/cosmetics6020036
- Cloete E, Khumalo NP, Ngoepe MN. The what, why and how of curly hair: a review. Proc Math Phys Eng Sci. 2019;475:20190516. doi:10.1098/rspa.2019.0516
- Westgate GE, Ginger RS, Green MR. The biology and genetics of curly hair. Exp Dermatol. 2017;26:483-490. doi:10.1111/exd.13347
- McMichael AJ. Ethnic hair update: past and present. J Am Acad Dermatol. 2003;48(6 suppl):S127-S133. doi:10.1067/mjd.2003.278
- Roseborough IE, McMichael AJ. Hair care practices in African-American patients. Semin Cutan Med Surg. 2009;28:103-108. doi:10.1016/j.sder.2009.04.007
- Narasimman M, De Bedout V, Castillo DE, et al. Increased association between previous pregnancies and use of chemical relaxers in 74 women with central centrifugal cicatricial alopecia. Int J Trichology. 2020;12:176-181. doi:10.4103/ijt.ijt_37_20
- Aguh C, Dina Y, Talbot CC Jr, et al. Fibroproliferative genes are preferentially expressed in central centrifugal cicatricial alopecia. J Am Acad Dermatol. 2018;79:904-912.e901. doi:10.1016/j.jaad.2018.05.1257