User login
Compulsivity contributes to poor outcomes in body-focused repetitive behaviors
Although body-focused repetitive behaviors (BFRBs), specifically trichotillomania and skin-picking disorder, are similar in clinical presentation to aspects of obsessive-compulsive disorder (OCD), the role of compulsivity in TTM and SPD has not been well studied, wrote Jon E. Grant, MD, of the University of Chicago and colleagues.
In a study published in the Journal of Psychiatric Research, the authors recruited 69 women and 22 men who met DSM-5 criteria for TTM and SPD. Participants completed diagnostic interviews, symptom inventories, and measures of disability/functioning. Compulsivity was measured using the 15-item Cambridge-Chicago Compulsivity Trait Scale (CHI-T). The average age of the participants was 30.9 years; 48 had TTM, 37 had SPD, and 2 had both conditions.
Overall, total CHI-T scores were significantly correlated with worse disability and quality of life, based on the Quality of Life Inventory (P = .0278) and the Sheehan Disability Scale (P = .0085) but not with severity of TTM or SPD symptoms. TTM and SPD symptoms were assessed using the Massachusetts General Hospital Hair Pulling Scale and the Skin Picking Symptom Symptom Assessment Scale.
“In the current study, we did not find a link between conventional symptom severity measures for BFRBs and disability or quality of life, whereas trans-diagnostic compulsivity did correlate with these clinically important parameters,” the researchers wrote in their discussion. “These findings might suggest the current symptom measures for BFRBs are not including an important aspect of the disease and that a fuller understanding of these symptoms requires measurement of compulsivity. Including validated measures of compulsivity in clinical trials of therapy or medication would also seem to be important for future work,” they said.
The study findings were limited by several factors including the use of a community sample that may not generalize to a clinical setting, the researchers noted. Other limitations include the cross-sectional design, which prevents conclusions about causality, the lack of a control group, and the relatively small sample size, they said.
However, the study is the first known to use a validated compulsivity measure to assess BFRBs, and the results suggest a clinically relevant impact of compulsivity on both psychosocial dysfunction and poor quality of life in this patient population, with possible implications for treatment, the researchers wrote.
The study received no outside funding. Lead author Dr. Grant disclosed research grants from Otsuka and Biohaven Pharmaceuticals, yearly compensation from Springer Publishing for acting as editor in chief of the Journal of Gambling Studies, and royalties from Oxford University Press, American Psychiatric Publishing, Norton Press, and McGraw Hill.
Although body-focused repetitive behaviors (BFRBs), specifically trichotillomania and skin-picking disorder, are similar in clinical presentation to aspects of obsessive-compulsive disorder (OCD), the role of compulsivity in TTM and SPD has not been well studied, wrote Jon E. Grant, MD, of the University of Chicago and colleagues.
In a study published in the Journal of Psychiatric Research, the authors recruited 69 women and 22 men who met DSM-5 criteria for TTM and SPD. Participants completed diagnostic interviews, symptom inventories, and measures of disability/functioning. Compulsivity was measured using the 15-item Cambridge-Chicago Compulsivity Trait Scale (CHI-T). The average age of the participants was 30.9 years; 48 had TTM, 37 had SPD, and 2 had both conditions.
Overall, total CHI-T scores were significantly correlated with worse disability and quality of life, based on the Quality of Life Inventory (P = .0278) and the Sheehan Disability Scale (P = .0085) but not with severity of TTM or SPD symptoms. TTM and SPD symptoms were assessed using the Massachusetts General Hospital Hair Pulling Scale and the Skin Picking Symptom Symptom Assessment Scale.
“In the current study, we did not find a link between conventional symptom severity measures for BFRBs and disability or quality of life, whereas trans-diagnostic compulsivity did correlate with these clinically important parameters,” the researchers wrote in their discussion. “These findings might suggest the current symptom measures for BFRBs are not including an important aspect of the disease and that a fuller understanding of these symptoms requires measurement of compulsivity. Including validated measures of compulsivity in clinical trials of therapy or medication would also seem to be important for future work,” they said.
The study findings were limited by several factors including the use of a community sample that may not generalize to a clinical setting, the researchers noted. Other limitations include the cross-sectional design, which prevents conclusions about causality, the lack of a control group, and the relatively small sample size, they said.
However, the study is the first known to use a validated compulsivity measure to assess BFRBs, and the results suggest a clinically relevant impact of compulsivity on both psychosocial dysfunction and poor quality of life in this patient population, with possible implications for treatment, the researchers wrote.
The study received no outside funding. Lead author Dr. Grant disclosed research grants from Otsuka and Biohaven Pharmaceuticals, yearly compensation from Springer Publishing for acting as editor in chief of the Journal of Gambling Studies, and royalties from Oxford University Press, American Psychiatric Publishing, Norton Press, and McGraw Hill.
Although body-focused repetitive behaviors (BFRBs), specifically trichotillomania and skin-picking disorder, are similar in clinical presentation to aspects of obsessive-compulsive disorder (OCD), the role of compulsivity in TTM and SPD has not been well studied, wrote Jon E. Grant, MD, of the University of Chicago and colleagues.
In a study published in the Journal of Psychiatric Research, the authors recruited 69 women and 22 men who met DSM-5 criteria for TTM and SPD. Participants completed diagnostic interviews, symptom inventories, and measures of disability/functioning. Compulsivity was measured using the 15-item Cambridge-Chicago Compulsivity Trait Scale (CHI-T). The average age of the participants was 30.9 years; 48 had TTM, 37 had SPD, and 2 had both conditions.
Overall, total CHI-T scores were significantly correlated with worse disability and quality of life, based on the Quality of Life Inventory (P = .0278) and the Sheehan Disability Scale (P = .0085) but not with severity of TTM or SPD symptoms. TTM and SPD symptoms were assessed using the Massachusetts General Hospital Hair Pulling Scale and the Skin Picking Symptom Symptom Assessment Scale.
“In the current study, we did not find a link between conventional symptom severity measures for BFRBs and disability or quality of life, whereas trans-diagnostic compulsivity did correlate with these clinically important parameters,” the researchers wrote in their discussion. “These findings might suggest the current symptom measures for BFRBs are not including an important aspect of the disease and that a fuller understanding of these symptoms requires measurement of compulsivity. Including validated measures of compulsivity in clinical trials of therapy or medication would also seem to be important for future work,” they said.
The study findings were limited by several factors including the use of a community sample that may not generalize to a clinical setting, the researchers noted. Other limitations include the cross-sectional design, which prevents conclusions about causality, the lack of a control group, and the relatively small sample size, they said.
However, the study is the first known to use a validated compulsivity measure to assess BFRBs, and the results suggest a clinically relevant impact of compulsivity on both psychosocial dysfunction and poor quality of life in this patient population, with possible implications for treatment, the researchers wrote.
The study received no outside funding. Lead author Dr. Grant disclosed research grants from Otsuka and Biohaven Pharmaceuticals, yearly compensation from Springer Publishing for acting as editor in chief of the Journal of Gambling Studies, and royalties from Oxford University Press, American Psychiatric Publishing, Norton Press, and McGraw Hill.
FROM THE JOURNAL OF PSYCHIATRIC RESEARCH
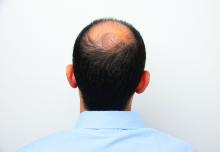
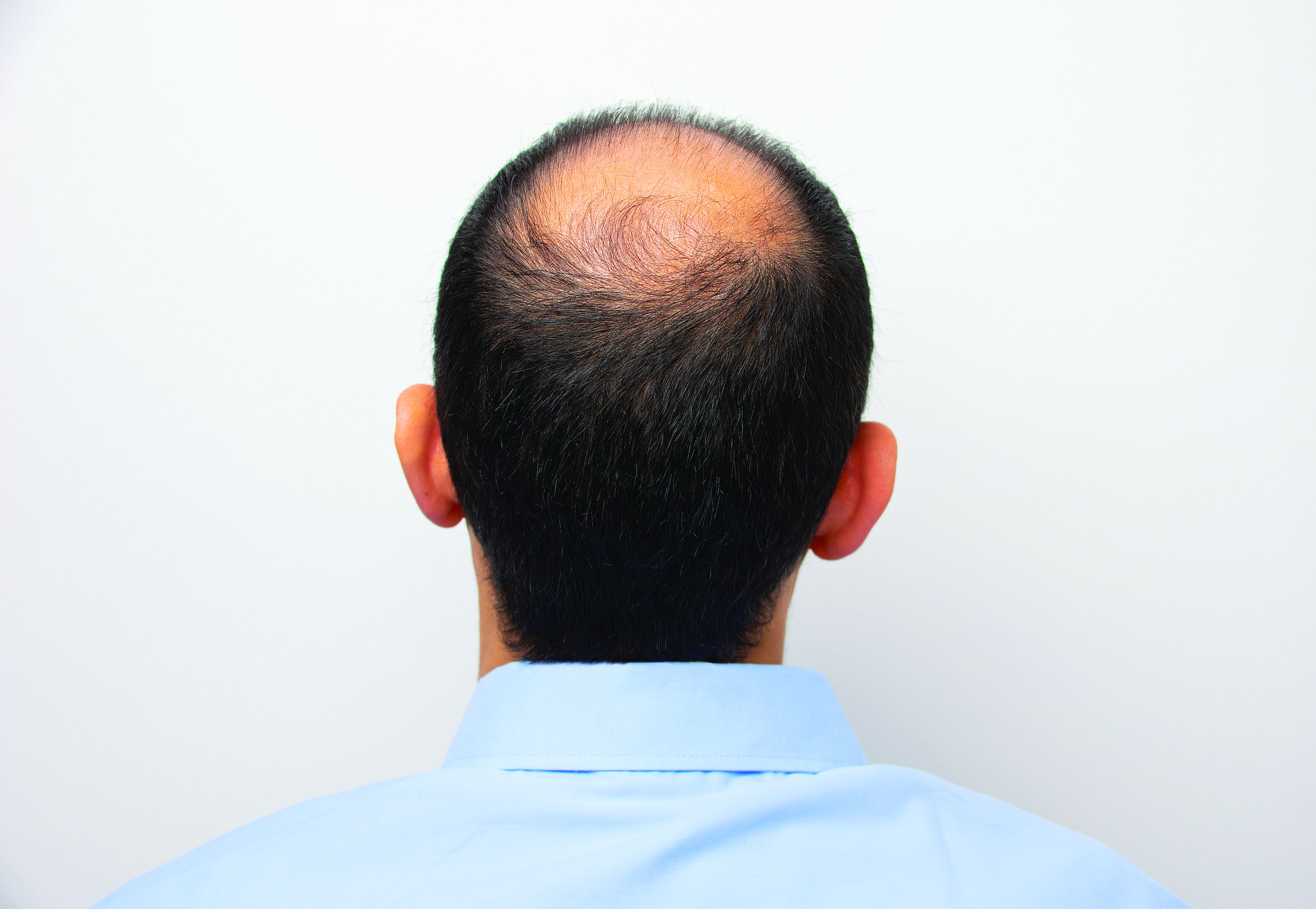
Baricitinib’s approval for alopecia areata: Considerations for starting patients on treatment
On June 13, the FDA approved baricitinib, a Janus kinase inhibitor (Olumiant, Lilly), for severe AA, and two other options may not be far behind. Pfizer and Concert Pharmaceuticals have JAK inhibitors in late-stage development for AA. JAK inhibitors, including baricitinib, are already on the market for treating rheumatoid arthritis and other autoimmune diseases.
Meanwhile, dermatologists have been fielding calls from hopeful patients and sorting out who should get the treatment, how to advise patients on risks and benefits, and what tests should be used before and after starting treatment.
Uptake for new systemic drugs, such as biologics, can be slow in dermatology, noted Adam Friedman, MD, professor and chair of dermatology, George Washington University, Washington, as some doctors like to stick with what they know.
He told this news organization that he hopes that uptake for baricitinib is quicker, as it is the only approved oral systemic treatment for patients with severe alopecia areata, which affects about 300,000 people a year in the United States. Other treatments, including steroid injections in the scalp, have lacked efficacy and convenience.
Beyond the physical effects, the mental toll of patchy hair clumps and missing brows and lashes can be devastating for patients with alopecia areata.
Fielding patient inquiries
Word of the FDA approval spread fast, and calls and emails are coming into dermatologists’ offices and clinics from interested patients.
Physicians should be ready for patients with any kind of hair loss, not just severe alopecia areata, to ask about the drug, Dr. Friedman said. Some patients contacting him don’t fit the indication, which “highlights how disabling hair loss” is for people, considering that, in general, “people see this and think it is for them.”
Baricitinib is not a new drug, but a drug with a new indication. It had already been approved for treating moderate to severe RA in patients who have had an inadequate response to one or more tumor necrosis factor blockers, and for treating COVID-19 in certain hospitalized adults.
Boxed warning
Patients may ask about the boxed warning in the baricitinib label about the increased risk for serious infections, mortality, malignancy, major adverse cardiovascular events, and thrombosis.
Natasha A. Mesinkovska, MD, PhD, an investigator in the clinical trials that led to FDA approval of baricitinib and the chief scientific officer at the National Alopecia Areata Foundation, told this news organization that several aspects of the label are important to point out.
One is that the warning is for all the JAK inhibitors used to treat RA and other inflammatory conditions, not just baricitinib. Also, the warning is based mostly on data on patients with RA who, she noted, have substantial comorbidities and have been taking toxic immunosuppressive medications. The RA population is also typically many years older than the alopecia areata population.
“Whether the warnings apply to the alopecia areata patients is as yet unclear,” said Dr. Mesinkovska, who is also an associate professor of dermatology at the University of California, Irvine.
Patients are also asking about how well it works.
In one of the two trials that led up to the FDA approval, which enrolled patients with at least 50% scalp hair loss for over 6 months, 22% of the patients who received 2 mg of baricitinib and 35% of those who received 4 mg saw adequate hair coverage (at least 80%) at week 36, compared with 5% on placebo. In the second trial, 17% of those who received 2 mg and 32% who received 4 mg saw adequate hair coverage, compared with 3% on placebo.
Common side effects associated with baricitinib, according to the FDA, are lower respiratory tract infections, headache, acne, high cholesterol, increased creatinine phosphokinase, urinary tract infection, liver enzyme elevations, folliculitis, fatigue, nausea, genital yeast infections, anemia, neutropenia, abdominal pain, herpes zoster (shingles), and weight gain.
The risk-benefit discussions with patients should also include potential benefits beyond hair regrowth on the scalp. Loss of hair in the ears and nose can affect hearing and allergies, Dr. Mesinkovska said.
“About 30%-50% with alopecia areata, depending on age group or part of the world, will have allergies,” she said.
Patients should also know that baricitinib will need to be taken “for a very long time,” Dr. Mesinkovska noted. It’s possible that could be forever and that stopping the medication at any point may result in hair falling out again, she says, but duration will vary from case to case.
The good news is that it has been well tolerated. “We give a lot of medications for acne like doxycycline and other antibiotics and people have more stomach problems and angst with those than with [baricitinib],” she said.
Regrowth takes time
Benjamin Ungar, MD, a dermatologist at the Alopecia Center of Excellence at Mount Sinai, New York, told this news organization that an important message for patients is that hair regrowth takes time. For some other skin conditions, patients start treatment and see almost instant improvement.
“That is not the case for alopecia areata,” he said. “The expectation is that it will take months for regrowth in general.”
He said he hasn’t started prescribing baricitinib yet, but plans to do so soon.
“Obviously, I’ll have conversations with patients about it, but it’s a medication I’m going to be using, definitely. I have no reservations,” Dr. Ungar said.
After initial testing, physicians may find that some patients might not be ideal candidates, he added. People with liver disease, a history of blood clots, abnormal blood counts, or low neutrophils are among those who may not be the best candidates for baricitinib.
For most with severe alopecia areata, though, baricitinib provides hope.
“Treatment options have been not readily available, often inaccessible, ineffective, often dangerous,” he said. “There’s a treatment now that can be accessed, generally is safe and is effective for many people.”
Be up front with patients about the unknown
Additionally, it’s important to tell patients what is not yet known, the experts interviewed say.
“Alopecia areata is a chronic disease. We don’t have long-term data on the patient population yet,” Dr. Friedman said.
Also unknown is how easy it will be for physicians to get insurance to reimburse for baricitinib, which, at the end of June, was priced at about $5,000 a month for the 4-mg dose. FDA approval was important in that regard. Previously, some claims had been rejected for drugs used off label for AA.
“We dermatologists know how much it affects patients,” Dr. Mesinkovska said. “As long as we stick by what we know and convey to insurers how much it affects people’s lives, they should cover it.”
Another unknown is what other drugs can be taken with baricitinib. In clinical trials, it was used alone, she said. Currently, concomitant use of other immune suppressants – such as methotrexate or prednisone – is not recommended. But it remains to be seen what other medications will be safe to use at the same time as more long-term data are available.
Lynne J. Goldberg, MD, professor of dermatology, pathology, and laboratory medicine, Boston University, and director of the Hair Clinic at Boston Medical Center, said that she received a slew of emails from patients asking about baricitinib, but most of them did not have alopecia areata and were not candidates for this treatment.
She said that nurses in her clinic have been instructed on what to tell patients about which patients the drug is meant to treat, side effects, and benefits.
Access won’t be immediate
Dr. Goldberg said the drug’s approval does not mean immediate access. The patient has to come in, discuss the treatment, and get lab tests first. “It’s not a casual drug. This is a potent immunosuppressant drug. You need lab tests and once you start it you need blood tests every 3 months to stay on it.”
Those tests may vary by physician, but people will generally need a standard blood count and a comprehensive metabolic panel and lipid panel. “There’s nothing esoteric,” she said.
She added that physicians will need to check for presence of infections including tuberculosis and hepatitis B and C before prescribing, just as they would before they start prescribing a biologic.
“You don’t want to reactivate something,” she noted.
But, Dr. Goldberg added, the benefits for all who have been either living with only patches of hair or no hair or who put on a wig or hat every day are “life changing.”
Dr. Mesinkovska is on the advisory boards and runs trials for Eli Lilly, Pfizer, and Concert Pharmaceuticals. Dr. Friedman, Dr. Goldberg, and Dr. Ungar reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
On June 13, the FDA approved baricitinib, a Janus kinase inhibitor (Olumiant, Lilly), for severe AA, and two other options may not be far behind. Pfizer and Concert Pharmaceuticals have JAK inhibitors in late-stage development for AA. JAK inhibitors, including baricitinib, are already on the market for treating rheumatoid arthritis and other autoimmune diseases.
Meanwhile, dermatologists have been fielding calls from hopeful patients and sorting out who should get the treatment, how to advise patients on risks and benefits, and what tests should be used before and after starting treatment.
Uptake for new systemic drugs, such as biologics, can be slow in dermatology, noted Adam Friedman, MD, professor and chair of dermatology, George Washington University, Washington, as some doctors like to stick with what they know.
He told this news organization that he hopes that uptake for baricitinib is quicker, as it is the only approved oral systemic treatment for patients with severe alopecia areata, which affects about 300,000 people a year in the United States. Other treatments, including steroid injections in the scalp, have lacked efficacy and convenience.
Beyond the physical effects, the mental toll of patchy hair clumps and missing brows and lashes can be devastating for patients with alopecia areata.
Fielding patient inquiries
Word of the FDA approval spread fast, and calls and emails are coming into dermatologists’ offices and clinics from interested patients.
Physicians should be ready for patients with any kind of hair loss, not just severe alopecia areata, to ask about the drug, Dr. Friedman said. Some patients contacting him don’t fit the indication, which “highlights how disabling hair loss” is for people, considering that, in general, “people see this and think it is for them.”
Baricitinib is not a new drug, but a drug with a new indication. It had already been approved for treating moderate to severe RA in patients who have had an inadequate response to one or more tumor necrosis factor blockers, and for treating COVID-19 in certain hospitalized adults.
Boxed warning
Patients may ask about the boxed warning in the baricitinib label about the increased risk for serious infections, mortality, malignancy, major adverse cardiovascular events, and thrombosis.
Natasha A. Mesinkovska, MD, PhD, an investigator in the clinical trials that led to FDA approval of baricitinib and the chief scientific officer at the National Alopecia Areata Foundation, told this news organization that several aspects of the label are important to point out.
One is that the warning is for all the JAK inhibitors used to treat RA and other inflammatory conditions, not just baricitinib. Also, the warning is based mostly on data on patients with RA who, she noted, have substantial comorbidities and have been taking toxic immunosuppressive medications. The RA population is also typically many years older than the alopecia areata population.
“Whether the warnings apply to the alopecia areata patients is as yet unclear,” said Dr. Mesinkovska, who is also an associate professor of dermatology at the University of California, Irvine.
Patients are also asking about how well it works.
In one of the two trials that led up to the FDA approval, which enrolled patients with at least 50% scalp hair loss for over 6 months, 22% of the patients who received 2 mg of baricitinib and 35% of those who received 4 mg saw adequate hair coverage (at least 80%) at week 36, compared with 5% on placebo. In the second trial, 17% of those who received 2 mg and 32% who received 4 mg saw adequate hair coverage, compared with 3% on placebo.
Common side effects associated with baricitinib, according to the FDA, are lower respiratory tract infections, headache, acne, high cholesterol, increased creatinine phosphokinase, urinary tract infection, liver enzyme elevations, folliculitis, fatigue, nausea, genital yeast infections, anemia, neutropenia, abdominal pain, herpes zoster (shingles), and weight gain.
The risk-benefit discussions with patients should also include potential benefits beyond hair regrowth on the scalp. Loss of hair in the ears and nose can affect hearing and allergies, Dr. Mesinkovska said.
“About 30%-50% with alopecia areata, depending on age group or part of the world, will have allergies,” she said.
Patients should also know that baricitinib will need to be taken “for a very long time,” Dr. Mesinkovska noted. It’s possible that could be forever and that stopping the medication at any point may result in hair falling out again, she says, but duration will vary from case to case.
The good news is that it has been well tolerated. “We give a lot of medications for acne like doxycycline and other antibiotics and people have more stomach problems and angst with those than with [baricitinib],” she said.
Regrowth takes time
Benjamin Ungar, MD, a dermatologist at the Alopecia Center of Excellence at Mount Sinai, New York, told this news organization that an important message for patients is that hair regrowth takes time. For some other skin conditions, patients start treatment and see almost instant improvement.
“That is not the case for alopecia areata,” he said. “The expectation is that it will take months for regrowth in general.”
He said he hasn’t started prescribing baricitinib yet, but plans to do so soon.
“Obviously, I’ll have conversations with patients about it, but it’s a medication I’m going to be using, definitely. I have no reservations,” Dr. Ungar said.
After initial testing, physicians may find that some patients might not be ideal candidates, he added. People with liver disease, a history of blood clots, abnormal blood counts, or low neutrophils are among those who may not be the best candidates for baricitinib.
For most with severe alopecia areata, though, baricitinib provides hope.
“Treatment options have been not readily available, often inaccessible, ineffective, often dangerous,” he said. “There’s a treatment now that can be accessed, generally is safe and is effective for many people.”
Be up front with patients about the unknown
Additionally, it’s important to tell patients what is not yet known, the experts interviewed say.
“Alopecia areata is a chronic disease. We don’t have long-term data on the patient population yet,” Dr. Friedman said.
Also unknown is how easy it will be for physicians to get insurance to reimburse for baricitinib, which, at the end of June, was priced at about $5,000 a month for the 4-mg dose. FDA approval was important in that regard. Previously, some claims had been rejected for drugs used off label for AA.
“We dermatologists know how much it affects patients,” Dr. Mesinkovska said. “As long as we stick by what we know and convey to insurers how much it affects people’s lives, they should cover it.”
Another unknown is what other drugs can be taken with baricitinib. In clinical trials, it was used alone, she said. Currently, concomitant use of other immune suppressants – such as methotrexate or prednisone – is not recommended. But it remains to be seen what other medications will be safe to use at the same time as more long-term data are available.
Lynne J. Goldberg, MD, professor of dermatology, pathology, and laboratory medicine, Boston University, and director of the Hair Clinic at Boston Medical Center, said that she received a slew of emails from patients asking about baricitinib, but most of them did not have alopecia areata and were not candidates for this treatment.
She said that nurses in her clinic have been instructed on what to tell patients about which patients the drug is meant to treat, side effects, and benefits.
Access won’t be immediate
Dr. Goldberg said the drug’s approval does not mean immediate access. The patient has to come in, discuss the treatment, and get lab tests first. “It’s not a casual drug. This is a potent immunosuppressant drug. You need lab tests and once you start it you need blood tests every 3 months to stay on it.”
Those tests may vary by physician, but people will generally need a standard blood count and a comprehensive metabolic panel and lipid panel. “There’s nothing esoteric,” she said.
She added that physicians will need to check for presence of infections including tuberculosis and hepatitis B and C before prescribing, just as they would before they start prescribing a biologic.
“You don’t want to reactivate something,” she noted.
But, Dr. Goldberg added, the benefits for all who have been either living with only patches of hair or no hair or who put on a wig or hat every day are “life changing.”
Dr. Mesinkovska is on the advisory boards and runs trials for Eli Lilly, Pfizer, and Concert Pharmaceuticals. Dr. Friedman, Dr. Goldberg, and Dr. Ungar reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
On June 13, the FDA approved baricitinib, a Janus kinase inhibitor (Olumiant, Lilly), for severe AA, and two other options may not be far behind. Pfizer and Concert Pharmaceuticals have JAK inhibitors in late-stage development for AA. JAK inhibitors, including baricitinib, are already on the market for treating rheumatoid arthritis and other autoimmune diseases.
Meanwhile, dermatologists have been fielding calls from hopeful patients and sorting out who should get the treatment, how to advise patients on risks and benefits, and what tests should be used before and after starting treatment.
Uptake for new systemic drugs, such as biologics, can be slow in dermatology, noted Adam Friedman, MD, professor and chair of dermatology, George Washington University, Washington, as some doctors like to stick with what they know.
He told this news organization that he hopes that uptake for baricitinib is quicker, as it is the only approved oral systemic treatment for patients with severe alopecia areata, which affects about 300,000 people a year in the United States. Other treatments, including steroid injections in the scalp, have lacked efficacy and convenience.
Beyond the physical effects, the mental toll of patchy hair clumps and missing brows and lashes can be devastating for patients with alopecia areata.
Fielding patient inquiries
Word of the FDA approval spread fast, and calls and emails are coming into dermatologists’ offices and clinics from interested patients.
Physicians should be ready for patients with any kind of hair loss, not just severe alopecia areata, to ask about the drug, Dr. Friedman said. Some patients contacting him don’t fit the indication, which “highlights how disabling hair loss” is for people, considering that, in general, “people see this and think it is for them.”
Baricitinib is not a new drug, but a drug with a new indication. It had already been approved for treating moderate to severe RA in patients who have had an inadequate response to one or more tumor necrosis factor blockers, and for treating COVID-19 in certain hospitalized adults.
Boxed warning
Patients may ask about the boxed warning in the baricitinib label about the increased risk for serious infections, mortality, malignancy, major adverse cardiovascular events, and thrombosis.
Natasha A. Mesinkovska, MD, PhD, an investigator in the clinical trials that led to FDA approval of baricitinib and the chief scientific officer at the National Alopecia Areata Foundation, told this news organization that several aspects of the label are important to point out.
One is that the warning is for all the JAK inhibitors used to treat RA and other inflammatory conditions, not just baricitinib. Also, the warning is based mostly on data on patients with RA who, she noted, have substantial comorbidities and have been taking toxic immunosuppressive medications. The RA population is also typically many years older than the alopecia areata population.
“Whether the warnings apply to the alopecia areata patients is as yet unclear,” said Dr. Mesinkovska, who is also an associate professor of dermatology at the University of California, Irvine.
Patients are also asking about how well it works.
In one of the two trials that led up to the FDA approval, which enrolled patients with at least 50% scalp hair loss for over 6 months, 22% of the patients who received 2 mg of baricitinib and 35% of those who received 4 mg saw adequate hair coverage (at least 80%) at week 36, compared with 5% on placebo. In the second trial, 17% of those who received 2 mg and 32% who received 4 mg saw adequate hair coverage, compared with 3% on placebo.
Common side effects associated with baricitinib, according to the FDA, are lower respiratory tract infections, headache, acne, high cholesterol, increased creatinine phosphokinase, urinary tract infection, liver enzyme elevations, folliculitis, fatigue, nausea, genital yeast infections, anemia, neutropenia, abdominal pain, herpes zoster (shingles), and weight gain.
The risk-benefit discussions with patients should also include potential benefits beyond hair regrowth on the scalp. Loss of hair in the ears and nose can affect hearing and allergies, Dr. Mesinkovska said.
“About 30%-50% with alopecia areata, depending on age group or part of the world, will have allergies,” she said.
Patients should also know that baricitinib will need to be taken “for a very long time,” Dr. Mesinkovska noted. It’s possible that could be forever and that stopping the medication at any point may result in hair falling out again, she says, but duration will vary from case to case.
The good news is that it has been well tolerated. “We give a lot of medications for acne like doxycycline and other antibiotics and people have more stomach problems and angst with those than with [baricitinib],” she said.
Regrowth takes time
Benjamin Ungar, MD, a dermatologist at the Alopecia Center of Excellence at Mount Sinai, New York, told this news organization that an important message for patients is that hair regrowth takes time. For some other skin conditions, patients start treatment and see almost instant improvement.
“That is not the case for alopecia areata,” he said. “The expectation is that it will take months for regrowth in general.”
He said he hasn’t started prescribing baricitinib yet, but plans to do so soon.
“Obviously, I’ll have conversations with patients about it, but it’s a medication I’m going to be using, definitely. I have no reservations,” Dr. Ungar said.
After initial testing, physicians may find that some patients might not be ideal candidates, he added. People with liver disease, a history of blood clots, abnormal blood counts, or low neutrophils are among those who may not be the best candidates for baricitinib.
For most with severe alopecia areata, though, baricitinib provides hope.
“Treatment options have been not readily available, often inaccessible, ineffective, often dangerous,” he said. “There’s a treatment now that can be accessed, generally is safe and is effective for many people.”
Be up front with patients about the unknown
Additionally, it’s important to tell patients what is not yet known, the experts interviewed say.
“Alopecia areata is a chronic disease. We don’t have long-term data on the patient population yet,” Dr. Friedman said.
Also unknown is how easy it will be for physicians to get insurance to reimburse for baricitinib, which, at the end of June, was priced at about $5,000 a month for the 4-mg dose. FDA approval was important in that regard. Previously, some claims had been rejected for drugs used off label for AA.
“We dermatologists know how much it affects patients,” Dr. Mesinkovska said. “As long as we stick by what we know and convey to insurers how much it affects people’s lives, they should cover it.”
Another unknown is what other drugs can be taken with baricitinib. In clinical trials, it was used alone, she said. Currently, concomitant use of other immune suppressants – such as methotrexate or prednisone – is not recommended. But it remains to be seen what other medications will be safe to use at the same time as more long-term data are available.
Lynne J. Goldberg, MD, professor of dermatology, pathology, and laboratory medicine, Boston University, and director of the Hair Clinic at Boston Medical Center, said that she received a slew of emails from patients asking about baricitinib, but most of them did not have alopecia areata and were not candidates for this treatment.
She said that nurses in her clinic have been instructed on what to tell patients about which patients the drug is meant to treat, side effects, and benefits.
Access won’t be immediate
Dr. Goldberg said the drug’s approval does not mean immediate access. The patient has to come in, discuss the treatment, and get lab tests first. “It’s not a casual drug. This is a potent immunosuppressant drug. You need lab tests and once you start it you need blood tests every 3 months to stay on it.”
Those tests may vary by physician, but people will generally need a standard blood count and a comprehensive metabolic panel and lipid panel. “There’s nothing esoteric,” she said.
She added that physicians will need to check for presence of infections including tuberculosis and hepatitis B and C before prescribing, just as they would before they start prescribing a biologic.
“You don’t want to reactivate something,” she noted.
But, Dr. Goldberg added, the benefits for all who have been either living with only patches of hair or no hair or who put on a wig or hat every day are “life changing.”
Dr. Mesinkovska is on the advisory boards and runs trials for Eli Lilly, Pfizer, and Concert Pharmaceuticals. Dr. Friedman, Dr. Goldberg, and Dr. Ungar reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Hair disorder treatments are evolving
“No matter who the patient is, whether a child, adolescent, or adult, the key to figuring out hair disease is getting a good history,” Maria Hordinsky, MD, professor and chair of the department of dermatology at the University of Minnesota, Minneapolis, said at the Medscape Live Women’s and Pediatric Dermatology Seminar.
. She also urged physicians and other health care providers to use the electronic medical record and to be thorough in documenting information – noting nutrition, hair care habits, supplement use, and other details.
Lab tests should be selected based on that history, she said. For instance, low iron stores can be associated with hair shedding; and thyroid function studies might be needed.
Other highlights of her presentation included comments on different types of alopecia, and some new treatment approaches:
Androgenetic alopecia. In a meta-analysis and systematic review published in 2017, all treatments tested (2% and 5% minoxidil in men, 1 mg finasteride in men, 2% minoxidil in women, and low-level laser light therapy in men) were superior to placebo. Several photobiomodulation (PBM) devices (also known as low-level laser light) for home use have been cleared for androgenetic alopecia by the Food and Drug Administration; a clinician’s guide, published in 2018, provides information on these devices.
Hair and hormones. Combination therapy for female-pattern hair loss – low-dose minoxidil and spironolactone – is important to know about, she said, adding there are data from an observational pilot study supporting this treatment. Women should not become pregnant while on this treatment, Dr. Hordinsky cautioned.
PRP (platelet rich plasma). This treatment for hair loss can be costly, she cautioned, as it’s viewed as a cosmetic technique, “but it actually can work rather well.”
Hair regrowth measures. Traditionally, measures center on global assessment, the patient’s self-assessment, investigator assessment, and an independent photo review. Enter the dermatoscope. “We can now get pictures as a baseline. Patients can see, and also see the health of their scalp,” and if treatments make it look better or worse, she noted.
Alopecia areata (AA). Patients and families need to be made aware that this is an autoimmune disease that can recur, and if it does recur, the extent of hair loss is not predictable. According to Dr. Hordinsky, the most widely used tool to halt disease activity has been treatment with a corticosteroid (topical, intralesional, oral, or even intravenous corticosteroids).
Clinical trials and publications from 2018 to 2020 have triggered interest in off-label use and further studies of JAK inhibitors for treating AA, which include baricitinib, ruxolitinib, and tofacitinib. At the American Academy of Dermatology meeting in March 2022, results of the ALLEGRO phase 2b/3 trial found that the JAK inhibitor ritlecitinib (50 mg or 20 mg daily, with or without a 200-mg loading dose), was efficacious in adults and adolescents with AA, compared with placebo, with no safety concerns noted. “This looks to be very, very promising,” she said, “and also very safe.” Two phase 3 trials of baricitinib also presented at the same meeting found it was superior to placebo for hair regrowth in adults with severe AA at 36 weeks. (On June 13, shortly after Dr. Hordinsky spoke at the meeting, the FDA approved baricitinib for treating AA in adults, making this the first systemic treatment to be approved for AA).
Research on topical JAK inhibitors for AA has been disappointing, Dr. Hordinsky said.
Alopecia areata and atopic dermatitis. For patients with both AA and AD, dupilumab may provide relief, she said. She referred to a recently published phase 2a trial in patients with AA (including some with both AA and AD), which found that Severity of Alopecia Tool (SALT) scores improved after 48 weeks of treatment, with higher response rates among those with baseline IgE levels of 200 IU/mL or higher. “If your patient has both, and their immunoglobulin-E level is greater than 200, then they may be a good candidate for dupilumab and both diseases may respond,” she said.
Scalp symptoms. It can be challenging when patients complain of itch, pain, or burning on the scalp, but have no obvious skin disease, Dr. Hordinsky said. Her tips: Some of these patients may be experiencing scalp symptoms secondary to a neuropathy; others may have mast cell degranulation, but for others, the basis of the symptoms may be unclear. Special nerve studies may be needed. For relief, a trial of antihistamines or topical or oral gabapentin may be needed, she said.
Frontal fibrosing alopecia (FFA). This condition, first described in postmenopausal women, is now reported in men and in younger women. While sunscreen has been suspected, there are no good data that have proven that link, she said. Cosmetics are also considered a possible culprit. For treatment, “the first thing we try to do is treat the inflammation,” Dr. Hordinsky said. Treatment options include topical high-potency corticosteroids, intralesional steroids, and topical nonsteroid anti-inflammatory creams (tier 1); hydroxychloroquine, low-dose antibiotics, and acitretin (tier 2); and cyclosporin and mycophenolate mofetil (tier 3).
In an observational study of mostly women with FFA, she noted, treatment with dutasteride was more effective than commonly used systemic treatments.
“Don’t forget to address the psychosocial needs of the hair loss patient,” Dr. Hordinsky advised. “Hair loss patients are very distressed, and you have to learn how to be fast and nimble and address those needs.” Working with a behavioral health specialist or therapist can help, she said.
She also recommended directing patients to appropriate organizations such as the National Alopecia Areata Foundation and the Scarring Alopecia Foundation, as well as conferences, such as the upcoming NAAF conference in Washington. “These organizations do give good information that should complement what you are doing.”
Medscape Live and this news organization are owned by the same parent company. Dr. Hordinsky reported no disclosures.
“No matter who the patient is, whether a child, adolescent, or adult, the key to figuring out hair disease is getting a good history,” Maria Hordinsky, MD, professor and chair of the department of dermatology at the University of Minnesota, Minneapolis, said at the Medscape Live Women’s and Pediatric Dermatology Seminar.
. She also urged physicians and other health care providers to use the electronic medical record and to be thorough in documenting information – noting nutrition, hair care habits, supplement use, and other details.
Lab tests should be selected based on that history, she said. For instance, low iron stores can be associated with hair shedding; and thyroid function studies might be needed.
Other highlights of her presentation included comments on different types of alopecia, and some new treatment approaches:
Androgenetic alopecia. In a meta-analysis and systematic review published in 2017, all treatments tested (2% and 5% minoxidil in men, 1 mg finasteride in men, 2% minoxidil in women, and low-level laser light therapy in men) were superior to placebo. Several photobiomodulation (PBM) devices (also known as low-level laser light) for home use have been cleared for androgenetic alopecia by the Food and Drug Administration; a clinician’s guide, published in 2018, provides information on these devices.
Hair and hormones. Combination therapy for female-pattern hair loss – low-dose minoxidil and spironolactone – is important to know about, she said, adding there are data from an observational pilot study supporting this treatment. Women should not become pregnant while on this treatment, Dr. Hordinsky cautioned.
PRP (platelet rich plasma). This treatment for hair loss can be costly, she cautioned, as it’s viewed as a cosmetic technique, “but it actually can work rather well.”
Hair regrowth measures. Traditionally, measures center on global assessment, the patient’s self-assessment, investigator assessment, and an independent photo review. Enter the dermatoscope. “We can now get pictures as a baseline. Patients can see, and also see the health of their scalp,” and if treatments make it look better or worse, she noted.
Alopecia areata (AA). Patients and families need to be made aware that this is an autoimmune disease that can recur, and if it does recur, the extent of hair loss is not predictable. According to Dr. Hordinsky, the most widely used tool to halt disease activity has been treatment with a corticosteroid (topical, intralesional, oral, or even intravenous corticosteroids).
Clinical trials and publications from 2018 to 2020 have triggered interest in off-label use and further studies of JAK inhibitors for treating AA, which include baricitinib, ruxolitinib, and tofacitinib. At the American Academy of Dermatology meeting in March 2022, results of the ALLEGRO phase 2b/3 trial found that the JAK inhibitor ritlecitinib (50 mg or 20 mg daily, with or without a 200-mg loading dose), was efficacious in adults and adolescents with AA, compared with placebo, with no safety concerns noted. “This looks to be very, very promising,” she said, “and also very safe.” Two phase 3 trials of baricitinib also presented at the same meeting found it was superior to placebo for hair regrowth in adults with severe AA at 36 weeks. (On June 13, shortly after Dr. Hordinsky spoke at the meeting, the FDA approved baricitinib for treating AA in adults, making this the first systemic treatment to be approved for AA).
Research on topical JAK inhibitors for AA has been disappointing, Dr. Hordinsky said.
Alopecia areata and atopic dermatitis. For patients with both AA and AD, dupilumab may provide relief, she said. She referred to a recently published phase 2a trial in patients with AA (including some with both AA and AD), which found that Severity of Alopecia Tool (SALT) scores improved after 48 weeks of treatment, with higher response rates among those with baseline IgE levels of 200 IU/mL or higher. “If your patient has both, and their immunoglobulin-E level is greater than 200, then they may be a good candidate for dupilumab and both diseases may respond,” she said.
Scalp symptoms. It can be challenging when patients complain of itch, pain, or burning on the scalp, but have no obvious skin disease, Dr. Hordinsky said. Her tips: Some of these patients may be experiencing scalp symptoms secondary to a neuropathy; others may have mast cell degranulation, but for others, the basis of the symptoms may be unclear. Special nerve studies may be needed. For relief, a trial of antihistamines or topical or oral gabapentin may be needed, she said.
Frontal fibrosing alopecia (FFA). This condition, first described in postmenopausal women, is now reported in men and in younger women. While sunscreen has been suspected, there are no good data that have proven that link, she said. Cosmetics are also considered a possible culprit. For treatment, “the first thing we try to do is treat the inflammation,” Dr. Hordinsky said. Treatment options include topical high-potency corticosteroids, intralesional steroids, and topical nonsteroid anti-inflammatory creams (tier 1); hydroxychloroquine, low-dose antibiotics, and acitretin (tier 2); and cyclosporin and mycophenolate mofetil (tier 3).
In an observational study of mostly women with FFA, she noted, treatment with dutasteride was more effective than commonly used systemic treatments.
“Don’t forget to address the psychosocial needs of the hair loss patient,” Dr. Hordinsky advised. “Hair loss patients are very distressed, and you have to learn how to be fast and nimble and address those needs.” Working with a behavioral health specialist or therapist can help, she said.
She also recommended directing patients to appropriate organizations such as the National Alopecia Areata Foundation and the Scarring Alopecia Foundation, as well as conferences, such as the upcoming NAAF conference in Washington. “These organizations do give good information that should complement what you are doing.”
Medscape Live and this news organization are owned by the same parent company. Dr. Hordinsky reported no disclosures.
“No matter who the patient is, whether a child, adolescent, or adult, the key to figuring out hair disease is getting a good history,” Maria Hordinsky, MD, professor and chair of the department of dermatology at the University of Minnesota, Minneapolis, said at the Medscape Live Women’s and Pediatric Dermatology Seminar.
. She also urged physicians and other health care providers to use the electronic medical record and to be thorough in documenting information – noting nutrition, hair care habits, supplement use, and other details.
Lab tests should be selected based on that history, she said. For instance, low iron stores can be associated with hair shedding; and thyroid function studies might be needed.
Other highlights of her presentation included comments on different types of alopecia, and some new treatment approaches:
Androgenetic alopecia. In a meta-analysis and systematic review published in 2017, all treatments tested (2% and 5% minoxidil in men, 1 mg finasteride in men, 2% minoxidil in women, and low-level laser light therapy in men) were superior to placebo. Several photobiomodulation (PBM) devices (also known as low-level laser light) for home use have been cleared for androgenetic alopecia by the Food and Drug Administration; a clinician’s guide, published in 2018, provides information on these devices.
Hair and hormones. Combination therapy for female-pattern hair loss – low-dose minoxidil and spironolactone – is important to know about, she said, adding there are data from an observational pilot study supporting this treatment. Women should not become pregnant while on this treatment, Dr. Hordinsky cautioned.
PRP (platelet rich plasma). This treatment for hair loss can be costly, she cautioned, as it’s viewed as a cosmetic technique, “but it actually can work rather well.”
Hair regrowth measures. Traditionally, measures center on global assessment, the patient’s self-assessment, investigator assessment, and an independent photo review. Enter the dermatoscope. “We can now get pictures as a baseline. Patients can see, and also see the health of their scalp,” and if treatments make it look better or worse, she noted.
Alopecia areata (AA). Patients and families need to be made aware that this is an autoimmune disease that can recur, and if it does recur, the extent of hair loss is not predictable. According to Dr. Hordinsky, the most widely used tool to halt disease activity has been treatment with a corticosteroid (topical, intralesional, oral, or even intravenous corticosteroids).
Clinical trials and publications from 2018 to 2020 have triggered interest in off-label use and further studies of JAK inhibitors for treating AA, which include baricitinib, ruxolitinib, and tofacitinib. At the American Academy of Dermatology meeting in March 2022, results of the ALLEGRO phase 2b/3 trial found that the JAK inhibitor ritlecitinib (50 mg or 20 mg daily, with or without a 200-mg loading dose), was efficacious in adults and adolescents with AA, compared with placebo, with no safety concerns noted. “This looks to be very, very promising,” she said, “and also very safe.” Two phase 3 trials of baricitinib also presented at the same meeting found it was superior to placebo for hair regrowth in adults with severe AA at 36 weeks. (On June 13, shortly after Dr. Hordinsky spoke at the meeting, the FDA approved baricitinib for treating AA in adults, making this the first systemic treatment to be approved for AA).
Research on topical JAK inhibitors for AA has been disappointing, Dr. Hordinsky said.
Alopecia areata and atopic dermatitis. For patients with both AA and AD, dupilumab may provide relief, she said. She referred to a recently published phase 2a trial in patients with AA (including some with both AA and AD), which found that Severity of Alopecia Tool (SALT) scores improved after 48 weeks of treatment, with higher response rates among those with baseline IgE levels of 200 IU/mL or higher. “If your patient has both, and their immunoglobulin-E level is greater than 200, then they may be a good candidate for dupilumab and both diseases may respond,” she said.
Scalp symptoms. It can be challenging when patients complain of itch, pain, or burning on the scalp, but have no obvious skin disease, Dr. Hordinsky said. Her tips: Some of these patients may be experiencing scalp symptoms secondary to a neuropathy; others may have mast cell degranulation, but for others, the basis of the symptoms may be unclear. Special nerve studies may be needed. For relief, a trial of antihistamines or topical or oral gabapentin may be needed, she said.
Frontal fibrosing alopecia (FFA). This condition, first described in postmenopausal women, is now reported in men and in younger women. While sunscreen has been suspected, there are no good data that have proven that link, she said. Cosmetics are also considered a possible culprit. For treatment, “the first thing we try to do is treat the inflammation,” Dr. Hordinsky said. Treatment options include topical high-potency corticosteroids, intralesional steroids, and topical nonsteroid anti-inflammatory creams (tier 1); hydroxychloroquine, low-dose antibiotics, and acitretin (tier 2); and cyclosporin and mycophenolate mofetil (tier 3).
In an observational study of mostly women with FFA, she noted, treatment with dutasteride was more effective than commonly used systemic treatments.
“Don’t forget to address the psychosocial needs of the hair loss patient,” Dr. Hordinsky advised. “Hair loss patients are very distressed, and you have to learn how to be fast and nimble and address those needs.” Working with a behavioral health specialist or therapist can help, she said.
She also recommended directing patients to appropriate organizations such as the National Alopecia Areata Foundation and the Scarring Alopecia Foundation, as well as conferences, such as the upcoming NAAF conference in Washington. “These organizations do give good information that should complement what you are doing.”
Medscape Live and this news organization are owned by the same parent company. Dr. Hordinsky reported no disclosures.
FROM MEDSCAPELIVE WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR
FDA OKs first systemic treatment for alopecia areata
.
The disorder with the hallmark signs of patchy baldness affects more than 300,000 people in the United States each year. In patients with the autoimmune disorder, the body attacks its own hair follicles and hair falls out, often in clumps. In February, the FDA granted priority review for baricitinib in adults with severe AA.
Baricitinib (Olumiant) is a Janus kinase (JAK) inhibitor, which blocks the activity of one or more enzymes, interfering with the pathway that leads to inflammation.
The FDA reports the most common side effects include upper respiratory tract infections, headache, acne, hyperlipidemia, increase of creatinine phosphokinase, urinary tract infection, elevated liver enzymes, inflammation of hair follicles, fatigue, lower respiratory tract infections, nausea, Candida infections, anemia, neutropenia, abdominal pain, herpes zoster (shingles), and weight gain. The labeling for baricitinib includes a boxed warning for serious infections, mortality, malignancy, major adverse cardiovascular events, and thrombosis.
Evidence from two trials led to announcement
The decision came after review of the results from two randomized, double-blind, placebo-controlled trials (BRAVE AA-1 and BRAVE AA-2) with patients who had at least 50% scalp hair loss as measured by the Severity of Alopecia Tool (SALT score) for more than 6 months.
Patients in these trials got either a placebo, 2 mg of baricitinib, or 4 mg of baricitinib every day. The primary endpoint for both trials was the proportion of patients who achieved at least 80% scalp hair coverage at week 36.
In BRAVE AA-1, 22% of the 184 patients who received 2 mg of baricitinib and 35% of the 281 patients who received 4 mg of baricitinib achieved at least 80% scalp hair coverage, compared with 5% of the 189 patients in the placebo group.
In BRAVE AA-2, 17% of the 156 patients who received 2 mg of baricitinib and 32% of the 234 patients who received 4 mg achieved at least 80% scalp hair coverage, compared with 3% of the 156 patients in the placebo group.
The results were reported at the annual meeting of the American Academy of Dermatology meeting in March.
Baricitinib was originally approved in 2018 as a treatment for adult patients with moderately to severely active rheumatoid arthritis who have had an inadequate response to one or more tumor necrosis factor (TNF)–blockers. It is also approved for treating COVID-19 in certain hospitalized adults.
Two other companies, Pfizer and Concert Pharmaceuticals, have JAK inhibitors in late-stage development for AA. The drugs are already on the market for treating rheumatoid arthritis and other autoimmune diseases. FDA approval is important for insurance coverage of the drugs, which have a list price of nearly $2,500 a month, according to The New York Times.
Until now, the only treatments for moderate to severe AA approved by the FDA have been intralesional steroid injections, contact sensitization, and systemic immunosuppressants, but they have demonstrated limited efficacy, are inconvenient for patients to take, and have been unsuitable for use long term.
“Today’s approval will help fulfill a significant unmet need for patients with severe alopecia areata,” Kendall Marcus, MD, director of the Division of Dermatology and Dentistry in the FDA’s Center for Drug Evaluation and Research, said in the press release.
As Medscape reported last month, The European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) has recommended approval of baricitinib for adults with severe AA.
AA received widespread international attention earlier this year at the Academy Awards ceremony, when actor Will Smith walked from the audience up onto the stage and slapped comedian Chris Rock in the face after he directed a joke at Mr. Smith’s wife, Jada Pinkett Smith, about her shaved head. Mrs. Pinkett Smith has AA and has been public about her struggles with the disease.
A version of this article first appeared on Medscape.com.
.
The disorder with the hallmark signs of patchy baldness affects more than 300,000 people in the United States each year. In patients with the autoimmune disorder, the body attacks its own hair follicles and hair falls out, often in clumps. In February, the FDA granted priority review for baricitinib in adults with severe AA.
Baricitinib (Olumiant) is a Janus kinase (JAK) inhibitor, which blocks the activity of one or more enzymes, interfering with the pathway that leads to inflammation.
The FDA reports the most common side effects include upper respiratory tract infections, headache, acne, hyperlipidemia, increase of creatinine phosphokinase, urinary tract infection, elevated liver enzymes, inflammation of hair follicles, fatigue, lower respiratory tract infections, nausea, Candida infections, anemia, neutropenia, abdominal pain, herpes zoster (shingles), and weight gain. The labeling for baricitinib includes a boxed warning for serious infections, mortality, malignancy, major adverse cardiovascular events, and thrombosis.
Evidence from two trials led to announcement
The decision came after review of the results from two randomized, double-blind, placebo-controlled trials (BRAVE AA-1 and BRAVE AA-2) with patients who had at least 50% scalp hair loss as measured by the Severity of Alopecia Tool (SALT score) for more than 6 months.
Patients in these trials got either a placebo, 2 mg of baricitinib, or 4 mg of baricitinib every day. The primary endpoint for both trials was the proportion of patients who achieved at least 80% scalp hair coverage at week 36.
In BRAVE AA-1, 22% of the 184 patients who received 2 mg of baricitinib and 35% of the 281 patients who received 4 mg of baricitinib achieved at least 80% scalp hair coverage, compared with 5% of the 189 patients in the placebo group.
In BRAVE AA-2, 17% of the 156 patients who received 2 mg of baricitinib and 32% of the 234 patients who received 4 mg achieved at least 80% scalp hair coverage, compared with 3% of the 156 patients in the placebo group.
The results were reported at the annual meeting of the American Academy of Dermatology meeting in March.
Baricitinib was originally approved in 2018 as a treatment for adult patients with moderately to severely active rheumatoid arthritis who have had an inadequate response to one or more tumor necrosis factor (TNF)–blockers. It is also approved for treating COVID-19 in certain hospitalized adults.
Two other companies, Pfizer and Concert Pharmaceuticals, have JAK inhibitors in late-stage development for AA. The drugs are already on the market for treating rheumatoid arthritis and other autoimmune diseases. FDA approval is important for insurance coverage of the drugs, which have a list price of nearly $2,500 a month, according to The New York Times.
Until now, the only treatments for moderate to severe AA approved by the FDA have been intralesional steroid injections, contact sensitization, and systemic immunosuppressants, but they have demonstrated limited efficacy, are inconvenient for patients to take, and have been unsuitable for use long term.
“Today’s approval will help fulfill a significant unmet need for patients with severe alopecia areata,” Kendall Marcus, MD, director of the Division of Dermatology and Dentistry in the FDA’s Center for Drug Evaluation and Research, said in the press release.
As Medscape reported last month, The European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) has recommended approval of baricitinib for adults with severe AA.
AA received widespread international attention earlier this year at the Academy Awards ceremony, when actor Will Smith walked from the audience up onto the stage and slapped comedian Chris Rock in the face after he directed a joke at Mr. Smith’s wife, Jada Pinkett Smith, about her shaved head. Mrs. Pinkett Smith has AA and has been public about her struggles with the disease.
A version of this article first appeared on Medscape.com.
.
The disorder with the hallmark signs of patchy baldness affects more than 300,000 people in the United States each year. In patients with the autoimmune disorder, the body attacks its own hair follicles and hair falls out, often in clumps. In February, the FDA granted priority review for baricitinib in adults with severe AA.
Baricitinib (Olumiant) is a Janus kinase (JAK) inhibitor, which blocks the activity of one or more enzymes, interfering with the pathway that leads to inflammation.
The FDA reports the most common side effects include upper respiratory tract infections, headache, acne, hyperlipidemia, increase of creatinine phosphokinase, urinary tract infection, elevated liver enzymes, inflammation of hair follicles, fatigue, lower respiratory tract infections, nausea, Candida infections, anemia, neutropenia, abdominal pain, herpes zoster (shingles), and weight gain. The labeling for baricitinib includes a boxed warning for serious infections, mortality, malignancy, major adverse cardiovascular events, and thrombosis.
Evidence from two trials led to announcement
The decision came after review of the results from two randomized, double-blind, placebo-controlled trials (BRAVE AA-1 and BRAVE AA-2) with patients who had at least 50% scalp hair loss as measured by the Severity of Alopecia Tool (SALT score) for more than 6 months.
Patients in these trials got either a placebo, 2 mg of baricitinib, or 4 mg of baricitinib every day. The primary endpoint for both trials was the proportion of patients who achieved at least 80% scalp hair coverage at week 36.
In BRAVE AA-1, 22% of the 184 patients who received 2 mg of baricitinib and 35% of the 281 patients who received 4 mg of baricitinib achieved at least 80% scalp hair coverage, compared with 5% of the 189 patients in the placebo group.
In BRAVE AA-2, 17% of the 156 patients who received 2 mg of baricitinib and 32% of the 234 patients who received 4 mg achieved at least 80% scalp hair coverage, compared with 3% of the 156 patients in the placebo group.
The results were reported at the annual meeting of the American Academy of Dermatology meeting in March.
Baricitinib was originally approved in 2018 as a treatment for adult patients with moderately to severely active rheumatoid arthritis who have had an inadequate response to one or more tumor necrosis factor (TNF)–blockers. It is also approved for treating COVID-19 in certain hospitalized adults.
Two other companies, Pfizer and Concert Pharmaceuticals, have JAK inhibitors in late-stage development for AA. The drugs are already on the market for treating rheumatoid arthritis and other autoimmune diseases. FDA approval is important for insurance coverage of the drugs, which have a list price of nearly $2,500 a month, according to The New York Times.
Until now, the only treatments for moderate to severe AA approved by the FDA have been intralesional steroid injections, contact sensitization, and systemic immunosuppressants, but they have demonstrated limited efficacy, are inconvenient for patients to take, and have been unsuitable for use long term.
“Today’s approval will help fulfill a significant unmet need for patients with severe alopecia areata,” Kendall Marcus, MD, director of the Division of Dermatology and Dentistry in the FDA’s Center for Drug Evaluation and Research, said in the press release.
As Medscape reported last month, The European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) has recommended approval of baricitinib for adults with severe AA.
AA received widespread international attention earlier this year at the Academy Awards ceremony, when actor Will Smith walked from the audience up onto the stage and slapped comedian Chris Rock in the face after he directed a joke at Mr. Smith’s wife, Jada Pinkett Smith, about her shaved head. Mrs. Pinkett Smith has AA and has been public about her struggles with the disease.
A version of this article first appeared on Medscape.com.
Acute Alopecia Associated With Albendazole Toxicosis
To the Editor:
Albendazole is a commonly prescribed anthelmintic that typically is well tolerated. Its broadest application is in developing countries that have a high rate of endemic nematode infection.1,2 Albendazole belongs to the benzimidazole class of anthelmintic chemotherapeutic agents that function by inhibiting microtubule dynamics, resulting in cytotoxic antimitotic effects.3 Benzimidazoles (eg, albendazole, mebendazole) have a binding affinity for helminthic β-tubulin that is 25- to 400-times greater than their binding affinity for the mammalian counterpart.4 Consequently, benzimidazoles generally are afforded a very broad therapeutic index for helminthic infection.
A 53-year-old man presented to the emergency department (ED) after an episode of syncope and sudden hair loss. At presentation he had a fever (temperature, 103 °F [39.4 °C]), a heart rate of 120 bpm, and pancytopenia (white blood cell count, 0.4×103/μL [reference range, 4.0–10.0×103/μL]; hemoglobin, 7.0 g/dL [reference range, 11.2–15.7 g/dL]; platelet count, 100
The patient reported severe gastrointestinal (GI) distress and diarrhea for the last year as well as a 25-lb weight loss. He discussed his belief that his GI symptoms were due to a parasite he had acquired the year prior; however, he reported that an exhaustive outpatient GI workup had been negative. Two weeks before presentation to our ED, the patient presented to another ED with stomach upset and was given a dose of albendazole. Perceiving alleviation of his symptoms, he purchased 2 bottles of veterinary albendazole online and consumed 113,000 mg—approximately 300 times the standard dose of 400 mg.
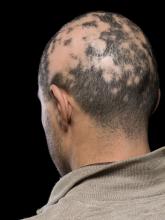
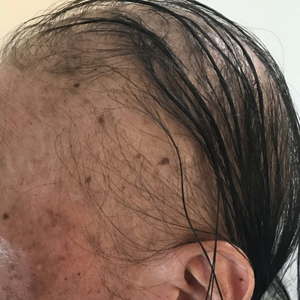
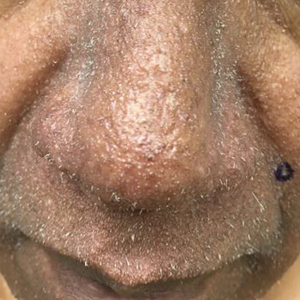
A dermatologic examination in our ED demonstrated reticulated violaceous patches on the face and severe alopecia with preferential sparing of the occipital scalp (Figure 1). Photographs taken by the patient on his phone from a week prior to presentation showed no facial dyschromia or signs of hair loss. A punch biopsy of the chin demonstrated perivascular and perifollicular dermatitis with eosinophils, most consistent with a drug reaction.
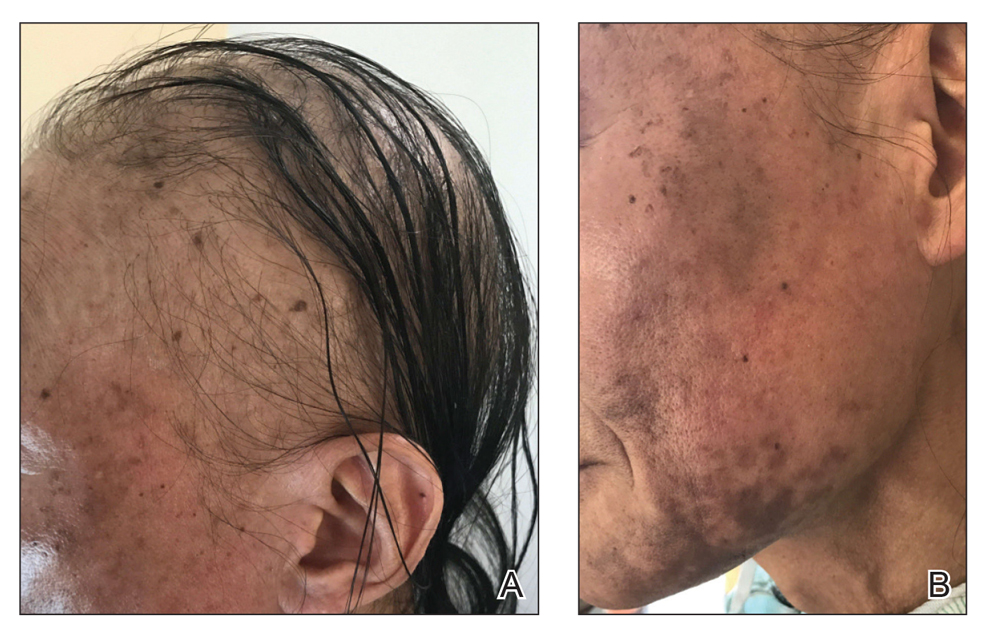
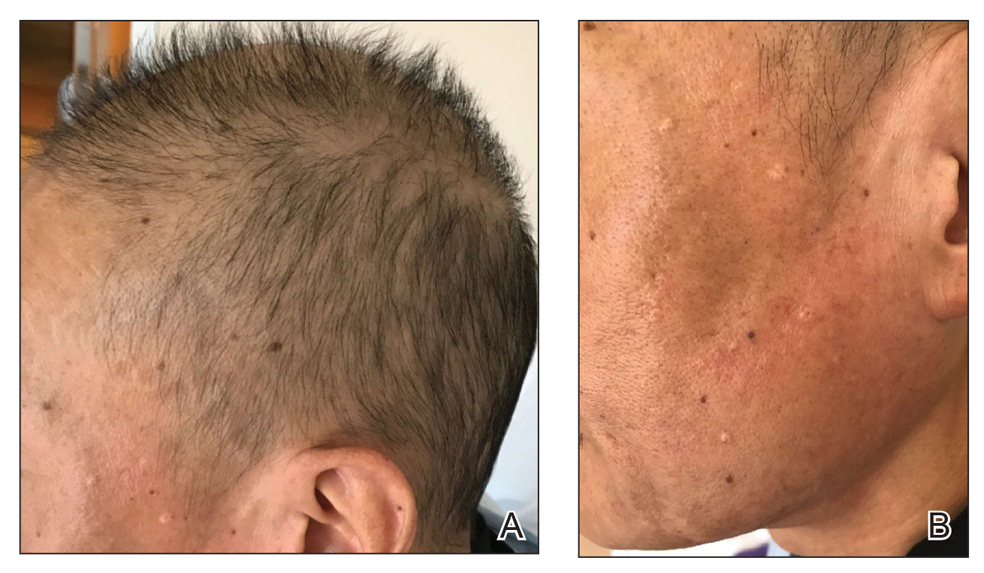
The patient received broad-spectrum antibiotics and supportive care. Blood count parameters normalized, and his hair began to regrow within 2 weeks after albendazole discontinuation (Figure 2).
Our patient exhibited symptoms of tachycardia, pancytopenia, and acute massive hair loss with preferential sparing of the occipital and posterior hair line; this pattern of hair loss is classic in men with chemotherapy-induced anagen effluvium.5 Conventional chemotherapeutics include taxanes and Vinca alkaloids, both of which bind mammalian β-tubulin and commonly induce anagen effluvium.
Our patient’s toxicosis syndrome was strikingly similar to common adverse effects in patients treated with conventional chemotherapeutics, including aplastic anemia with severe neutropenia and anagen effluvium.6,7 This adverse effect profile suggests that albendazole exerts an effect on mammalian β-tubulin that is similar to conventional chemotherapy when albendazole is ingested in a massive quantity.
Other reports of albendazole-induced alopecia describe an idiosyncratic, dose-dependent telogen effluvium.8-10 Conventional chemotherapy uncommonly might induce telogen effluvium when given below a threshold necessary to induce anagen effluvium. In those cases, follicular matrix keratinocytes are disrupted without complete follicular fracture and attempt to repair the damaged elongating follicle before entering the telogen phase.7 This observed phenomenon and the inherent susceptibility of matrix keratinocytes to antimicrotubule agents might explain why a therapeutic dose of albendazole has been associated with telogen effluvium in certain individuals.
Our case of albendazole-related toxicosis of this magnitude is unique. Ghias et al11 reported a case of abendazole-induced anagen effluvium. Future reports might clarify whether this toxicosis syndrome is typical or atypical in massive albendazole overdose.
- Keiser J, Utzinger J. Efficacy of current drugs against soil-transmitted helminth infections: systematic review and meta-analysis. JAMA. 2008;299:1937-1948. doi:10.1001/jama.299.16.1937
- Bethony J, Brooker S, Albonico M, et al. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521-1532. doi:10.1016/S0140-6736(06)68653-4
- Lanusse CE, Prichard RK. Clinical pharmacokinetics and metabolism of benzimidazole anthelmintics in ruminants. Drug Metab Rev. 1993;25:235-279. doi:10.3109/03602539308993977
- Page SW. Antiparasitic drugs. In: Maddison JE, Church DB, Page SW, eds. Small Animal Clinical Pharmacology. 2nd ed. W.B. Saunders; 2008:198-260.
- Yun SJ, Kim S-J. Hair loss pattern due to chemotherapy-induced anagen effluvium: a cross-sectional observation. Dermatology. 2007;215:36-40. doi:10.1159/000102031
- de Weger VA, Beijnen JH, Schellens JHM. Cellular and clinical pharmacology of the taxanes docetaxel and paclitaxel—a review. Anticancer Drugs. 2014;25:488-494. doi:10.1097/CAD.0000000000000093
- Paus R, Haslam IS, Sharov AA, et al. Pathobiology of chemotherapy-induced hair loss. Lancet Oncol. 2013;14:E50-E59. doi:10.1016/S1470-2045(12)70553-3
- Imamkuliev KD, Alekseev VG, Dovgalev AS, et al. A case of alopecia in a patient with hydatid disease treated with Nemozole (albendazole)[in Russian]. Med Parazitol (Mosk). 2013:48-50.
- Tas A, Köklü S, Celik H. Loss of body hair as a side effect of albendazole. Wien Klin Wochenschr. 2012;124:220. doi:10.1007/s00508-011-0112-y
- Pilar García-Muret M, Sitjas D, Tuneu L, et al. Telogen effluvium associated with albendazole therapy. Int J Dermatol. 1990;29:669-670. doi:10.1111/j.1365-4362.1990.tb02597.x
- Ghias M, Amin B, Kutner A. Albendazole-induced anagen effluvium. JAAD Case Rep. 2020;6:54-56.
To the Editor:
Albendazole is a commonly prescribed anthelmintic that typically is well tolerated. Its broadest application is in developing countries that have a high rate of endemic nematode infection.1,2 Albendazole belongs to the benzimidazole class of anthelmintic chemotherapeutic agents that function by inhibiting microtubule dynamics, resulting in cytotoxic antimitotic effects.3 Benzimidazoles (eg, albendazole, mebendazole) have a binding affinity for helminthic β-tubulin that is 25- to 400-times greater than their binding affinity for the mammalian counterpart.4 Consequently, benzimidazoles generally are afforded a very broad therapeutic index for helminthic infection.
A 53-year-old man presented to the emergency department (ED) after an episode of syncope and sudden hair loss. At presentation he had a fever (temperature, 103 °F [39.4 °C]), a heart rate of 120 bpm, and pancytopenia (white blood cell count, 0.4×103/μL [reference range, 4.0–10.0×103/μL]; hemoglobin, 7.0 g/dL [reference range, 11.2–15.7 g/dL]; platelet count, 100
The patient reported severe gastrointestinal (GI) distress and diarrhea for the last year as well as a 25-lb weight loss. He discussed his belief that his GI symptoms were due to a parasite he had acquired the year prior; however, he reported that an exhaustive outpatient GI workup had been negative. Two weeks before presentation to our ED, the patient presented to another ED with stomach upset and was given a dose of albendazole. Perceiving alleviation of his symptoms, he purchased 2 bottles of veterinary albendazole online and consumed 113,000 mg—approximately 300 times the standard dose of 400 mg.
A dermatologic examination in our ED demonstrated reticulated violaceous patches on the face and severe alopecia with preferential sparing of the occipital scalp (Figure 1). Photographs taken by the patient on his phone from a week prior to presentation showed no facial dyschromia or signs of hair loss. A punch biopsy of the chin demonstrated perivascular and perifollicular dermatitis with eosinophils, most consistent with a drug reaction.

The patient received broad-spectrum antibiotics and supportive care. Blood count parameters normalized, and his hair began to regrow within 2 weeks after albendazole discontinuation (Figure 2).

Our patient exhibited symptoms of tachycardia, pancytopenia, and acute massive hair loss with preferential sparing of the occipital and posterior hair line; this pattern of hair loss is classic in men with chemotherapy-induced anagen effluvium.5 Conventional chemotherapeutics include taxanes and Vinca alkaloids, both of which bind mammalian β-tubulin and commonly induce anagen effluvium.
Our patient’s toxicosis syndrome was strikingly similar to common adverse effects in patients treated with conventional chemotherapeutics, including aplastic anemia with severe neutropenia and anagen effluvium.6,7 This adverse effect profile suggests that albendazole exerts an effect on mammalian β-tubulin that is similar to conventional chemotherapy when albendazole is ingested in a massive quantity.
Other reports of albendazole-induced alopecia describe an idiosyncratic, dose-dependent telogen effluvium.8-10 Conventional chemotherapy uncommonly might induce telogen effluvium when given below a threshold necessary to induce anagen effluvium. In those cases, follicular matrix keratinocytes are disrupted without complete follicular fracture and attempt to repair the damaged elongating follicle before entering the telogen phase.7 This observed phenomenon and the inherent susceptibility of matrix keratinocytes to antimicrotubule agents might explain why a therapeutic dose of albendazole has been associated with telogen effluvium in certain individuals.
Our case of albendazole-related toxicosis of this magnitude is unique. Ghias et al11 reported a case of abendazole-induced anagen effluvium. Future reports might clarify whether this toxicosis syndrome is typical or atypical in massive albendazole overdose.
To the Editor:
Albendazole is a commonly prescribed anthelmintic that typically is well tolerated. Its broadest application is in developing countries that have a high rate of endemic nematode infection.1,2 Albendazole belongs to the benzimidazole class of anthelmintic chemotherapeutic agents that function by inhibiting microtubule dynamics, resulting in cytotoxic antimitotic effects.3 Benzimidazoles (eg, albendazole, mebendazole) have a binding affinity for helminthic β-tubulin that is 25- to 400-times greater than their binding affinity for the mammalian counterpart.4 Consequently, benzimidazoles generally are afforded a very broad therapeutic index for helminthic infection.
A 53-year-old man presented to the emergency department (ED) after an episode of syncope and sudden hair loss. At presentation he had a fever (temperature, 103 °F [39.4 °C]), a heart rate of 120 bpm, and pancytopenia (white blood cell count, 0.4×103/μL [reference range, 4.0–10.0×103/μL]; hemoglobin, 7.0 g/dL [reference range, 11.2–15.7 g/dL]; platelet count, 100
The patient reported severe gastrointestinal (GI) distress and diarrhea for the last year as well as a 25-lb weight loss. He discussed his belief that his GI symptoms were due to a parasite he had acquired the year prior; however, he reported that an exhaustive outpatient GI workup had been negative. Two weeks before presentation to our ED, the patient presented to another ED with stomach upset and was given a dose of albendazole. Perceiving alleviation of his symptoms, he purchased 2 bottles of veterinary albendazole online and consumed 113,000 mg—approximately 300 times the standard dose of 400 mg.
A dermatologic examination in our ED demonstrated reticulated violaceous patches on the face and severe alopecia with preferential sparing of the occipital scalp (Figure 1). Photographs taken by the patient on his phone from a week prior to presentation showed no facial dyschromia or signs of hair loss. A punch biopsy of the chin demonstrated perivascular and perifollicular dermatitis with eosinophils, most consistent with a drug reaction.

The patient received broad-spectrum antibiotics and supportive care. Blood count parameters normalized, and his hair began to regrow within 2 weeks after albendazole discontinuation (Figure 2).

Our patient exhibited symptoms of tachycardia, pancytopenia, and acute massive hair loss with preferential sparing of the occipital and posterior hair line; this pattern of hair loss is classic in men with chemotherapy-induced anagen effluvium.5 Conventional chemotherapeutics include taxanes and Vinca alkaloids, both of which bind mammalian β-tubulin and commonly induce anagen effluvium.
Our patient’s toxicosis syndrome was strikingly similar to common adverse effects in patients treated with conventional chemotherapeutics, including aplastic anemia with severe neutropenia and anagen effluvium.6,7 This adverse effect profile suggests that albendazole exerts an effect on mammalian β-tubulin that is similar to conventional chemotherapy when albendazole is ingested in a massive quantity.
Other reports of albendazole-induced alopecia describe an idiosyncratic, dose-dependent telogen effluvium.8-10 Conventional chemotherapy uncommonly might induce telogen effluvium when given below a threshold necessary to induce anagen effluvium. In those cases, follicular matrix keratinocytes are disrupted without complete follicular fracture and attempt to repair the damaged elongating follicle before entering the telogen phase.7 This observed phenomenon and the inherent susceptibility of matrix keratinocytes to antimicrotubule agents might explain why a therapeutic dose of albendazole has been associated with telogen effluvium in certain individuals.
Our case of albendazole-related toxicosis of this magnitude is unique. Ghias et al11 reported a case of abendazole-induced anagen effluvium. Future reports might clarify whether this toxicosis syndrome is typical or atypical in massive albendazole overdose.
- Keiser J, Utzinger J. Efficacy of current drugs against soil-transmitted helminth infections: systematic review and meta-analysis. JAMA. 2008;299:1937-1948. doi:10.1001/jama.299.16.1937
- Bethony J, Brooker S, Albonico M, et al. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521-1532. doi:10.1016/S0140-6736(06)68653-4
- Lanusse CE, Prichard RK. Clinical pharmacokinetics and metabolism of benzimidazole anthelmintics in ruminants. Drug Metab Rev. 1993;25:235-279. doi:10.3109/03602539308993977
- Page SW. Antiparasitic drugs. In: Maddison JE, Church DB, Page SW, eds. Small Animal Clinical Pharmacology. 2nd ed. W.B. Saunders; 2008:198-260.
- Yun SJ, Kim S-J. Hair loss pattern due to chemotherapy-induced anagen effluvium: a cross-sectional observation. Dermatology. 2007;215:36-40. doi:10.1159/000102031
- de Weger VA, Beijnen JH, Schellens JHM. Cellular and clinical pharmacology of the taxanes docetaxel and paclitaxel—a review. Anticancer Drugs. 2014;25:488-494. doi:10.1097/CAD.0000000000000093
- Paus R, Haslam IS, Sharov AA, et al. Pathobiology of chemotherapy-induced hair loss. Lancet Oncol. 2013;14:E50-E59. doi:10.1016/S1470-2045(12)70553-3
- Imamkuliev KD, Alekseev VG, Dovgalev AS, et al. A case of alopecia in a patient with hydatid disease treated with Nemozole (albendazole)[in Russian]. Med Parazitol (Mosk). 2013:48-50.
- Tas A, Köklü S, Celik H. Loss of body hair as a side effect of albendazole. Wien Klin Wochenschr. 2012;124:220. doi:10.1007/s00508-011-0112-y
- Pilar García-Muret M, Sitjas D, Tuneu L, et al. Telogen effluvium associated with albendazole therapy. Int J Dermatol. 1990;29:669-670. doi:10.1111/j.1365-4362.1990.tb02597.x
- Ghias M, Amin B, Kutner A. Albendazole-induced anagen effluvium. JAAD Case Rep. 2020;6:54-56.
- Keiser J, Utzinger J. Efficacy of current drugs against soil-transmitted helminth infections: systematic review and meta-analysis. JAMA. 2008;299:1937-1948. doi:10.1001/jama.299.16.1937
- Bethony J, Brooker S, Albonico M, et al. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521-1532. doi:10.1016/S0140-6736(06)68653-4
- Lanusse CE, Prichard RK. Clinical pharmacokinetics and metabolism of benzimidazole anthelmintics in ruminants. Drug Metab Rev. 1993;25:235-279. doi:10.3109/03602539308993977
- Page SW. Antiparasitic drugs. In: Maddison JE, Church DB, Page SW, eds. Small Animal Clinical Pharmacology. 2nd ed. W.B. Saunders; 2008:198-260.
- Yun SJ, Kim S-J. Hair loss pattern due to chemotherapy-induced anagen effluvium: a cross-sectional observation. Dermatology. 2007;215:36-40. doi:10.1159/000102031
- de Weger VA, Beijnen JH, Schellens JHM. Cellular and clinical pharmacology of the taxanes docetaxel and paclitaxel—a review. Anticancer Drugs. 2014;25:488-494. doi:10.1097/CAD.0000000000000093
- Paus R, Haslam IS, Sharov AA, et al. Pathobiology of chemotherapy-induced hair loss. Lancet Oncol. 2013;14:E50-E59. doi:10.1016/S1470-2045(12)70553-3
- Imamkuliev KD, Alekseev VG, Dovgalev AS, et al. A case of alopecia in a patient with hydatid disease treated with Nemozole (albendazole)[in Russian]. Med Parazitol (Mosk). 2013:48-50.
- Tas A, Köklü S, Celik H. Loss of body hair as a side effect of albendazole. Wien Klin Wochenschr. 2012;124:220. doi:10.1007/s00508-011-0112-y
- Pilar García-Muret M, Sitjas D, Tuneu L, et al. Telogen effluvium associated with albendazole therapy. Int J Dermatol. 1990;29:669-670. doi:10.1111/j.1365-4362.1990.tb02597.x
- Ghias M, Amin B, Kutner A. Albendazole-induced anagen effluvium. JAAD Case Rep. 2020;6:54-56.
PRACTICE POINTS
- Albendazole functions by inhibiting microtubule dynamics and has a remarkably greater binding affinity for helminthic β-tubulin than for its mammalian counterpart.
- An uncommon adverse effect of albendazole at therapeutic dosing is a dose-dependent telogen effluvium in susceptible persons, likely caused by the inherent susceptibility of follicular matrix keratinocytes to antimicrotubule agents.
- Massive albendazole overdose can cause anagen effluvium and myelosuppression similar to the effects of conventional chemotherapy.
European committee recommends approval of baricitinib for severe alopecia areata
The European Medicines Agency’s ( (AA).
The development, which was announced in a May 20, 2022, press release from the manufacturer, Eli Lilly and Incyte, marks the first step toward European regulatory approval of baricitinib (Olumiant) for patients with severe AA, and it is now referred to the European Commission for final action. A decision is expected within the next 2 months.
The committee based its positive opinion on the results of the phase 3 BRAVE-AA1 and BRAVE-AA2 trials, recently published in the New England Journal of Medicine, which evaluated the efficacy and safety of baricitinib in 1,200 patients with severe AA, according to the press release. The primary endpoint was the proportion of patients achieving a Severity of Alopecia Tool (SALT) score of ≤20 at week 36. In both studies, 1 out of 3 patients treated with baricitinib 4-mg achieved 80% or more scalp hair coverage, compared with 1 out of 20 patients and 1 out of 50 patients taking placebo in BRAVE-AA1 and BRAVE-AA2, respectively (P ≤ .001 for all comparisons to placebo).
According to safety profile information from the phase 3 BRAVE-AA clinical program, few patients discontinued treatment because of adverse events (2.6% or less across both studies), and most treatment-emergent adverse events were mild or moderate in severity.
In February 2022, the Food and Drug Administration granted priority review for baricitinib in adults with severe AA. Lilly expects additional regulatory decisions in the United States and Japan in 2022.
Baricitinib is approved in the United States as a treatment for adults with moderate to severe rheumatoid arthritis. Prescribing information can be viewed here.
The European Medicines Agency’s ( (AA).
The development, which was announced in a May 20, 2022, press release from the manufacturer, Eli Lilly and Incyte, marks the first step toward European regulatory approval of baricitinib (Olumiant) for patients with severe AA, and it is now referred to the European Commission for final action. A decision is expected within the next 2 months.
The committee based its positive opinion on the results of the phase 3 BRAVE-AA1 and BRAVE-AA2 trials, recently published in the New England Journal of Medicine, which evaluated the efficacy and safety of baricitinib in 1,200 patients with severe AA, according to the press release. The primary endpoint was the proportion of patients achieving a Severity of Alopecia Tool (SALT) score of ≤20 at week 36. In both studies, 1 out of 3 patients treated with baricitinib 4-mg achieved 80% or more scalp hair coverage, compared with 1 out of 20 patients and 1 out of 50 patients taking placebo in BRAVE-AA1 and BRAVE-AA2, respectively (P ≤ .001 for all comparisons to placebo).
According to safety profile information from the phase 3 BRAVE-AA clinical program, few patients discontinued treatment because of adverse events (2.6% or less across both studies), and most treatment-emergent adverse events were mild or moderate in severity.
In February 2022, the Food and Drug Administration granted priority review for baricitinib in adults with severe AA. Lilly expects additional regulatory decisions in the United States and Japan in 2022.
Baricitinib is approved in the United States as a treatment for adults with moderate to severe rheumatoid arthritis. Prescribing information can be viewed here.
The European Medicines Agency’s ( (AA).
The development, which was announced in a May 20, 2022, press release from the manufacturer, Eli Lilly and Incyte, marks the first step toward European regulatory approval of baricitinib (Olumiant) for patients with severe AA, and it is now referred to the European Commission for final action. A decision is expected within the next 2 months.
The committee based its positive opinion on the results of the phase 3 BRAVE-AA1 and BRAVE-AA2 trials, recently published in the New England Journal of Medicine, which evaluated the efficacy and safety of baricitinib in 1,200 patients with severe AA, according to the press release. The primary endpoint was the proportion of patients achieving a Severity of Alopecia Tool (SALT) score of ≤20 at week 36. In both studies, 1 out of 3 patients treated with baricitinib 4-mg achieved 80% or more scalp hair coverage, compared with 1 out of 20 patients and 1 out of 50 patients taking placebo in BRAVE-AA1 and BRAVE-AA2, respectively (P ≤ .001 for all comparisons to placebo).
According to safety profile information from the phase 3 BRAVE-AA clinical program, few patients discontinued treatment because of adverse events (2.6% or less across both studies), and most treatment-emergent adverse events were mild or moderate in severity.
In February 2022, the Food and Drug Administration granted priority review for baricitinib in adults with severe AA. Lilly expects additional regulatory decisions in the United States and Japan in 2022.
Baricitinib is approved in the United States as a treatment for adults with moderate to severe rheumatoid arthritis. Prescribing information can be viewed here.
Facial Follicular Spicules: A Rare Cutaneous Presentation of Trichodysplasia Spinulosa
To the Editor:
A 57-year-old man with hypertension, dyslipidemia, and congestive heart failure presented with a disfiguring eruption comprised of asymptomatic papules on the face that appeared 12 months post–heart transplantation. Immunosuppressive medications included mycophenolic acid and tacrolimus ointment (FK506). The pinpoint papules spread from the central face to the ears, arms, and legs. Physical examination revealed multiple 0.5- to 1-mm flesh-colored papules over the glabella, nose, nasolabial folds, philtrum, chin, ears, arms, and legs sparing the trunk. The initial appearance of the facial rash resembled the surface of a nutmeg grater with central white spiny excrescences overlying fine papules (spinulosism)(Figure 1). In addition, eyebrow alopecia was present.
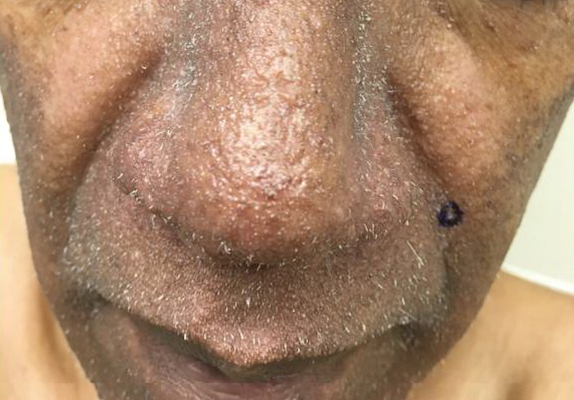
A 3-mm punch biopsy of a papule with a central spine was performed on the left thigh. Microscopic examination revealed marked dilatation of anagen hair follicles with a proliferation of haphazard inner root sheath cells replacing the follicular lumen. Hair shafts were absent, and plugged infundibula were observed (Figure 2). The inner root sheath keratinocytes were enlarged and dystrophic with deeply eosinophilic trichohyalin granules (Figure 3). The epidermis, outer root sheath epithelium, and eccrine structures were unremarkable.
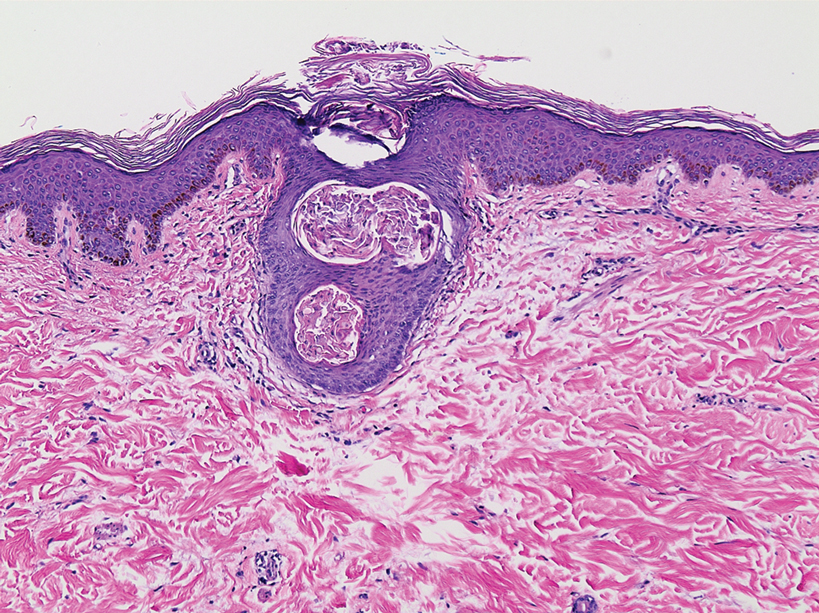
Transmission electron microscopy (TEM) confirmed the presence of intranuclear viral inclusions within affected inner root sheath keratinocytes composed of nonenveloped icosahedral viral particles measuring 33 to 38 nm in diameter (Figure 4). These findings morphologically were consistent with a polyomavirus. No intracytoplasmic or extracellular viral particles were identified. The clinical history, physical examination, histopathology, and electron microscopy features strongly supported the diagnosis of trichodysplasia spinulosa (TS) despite insufficient material being retrieved for polymerase chain reaction identification.
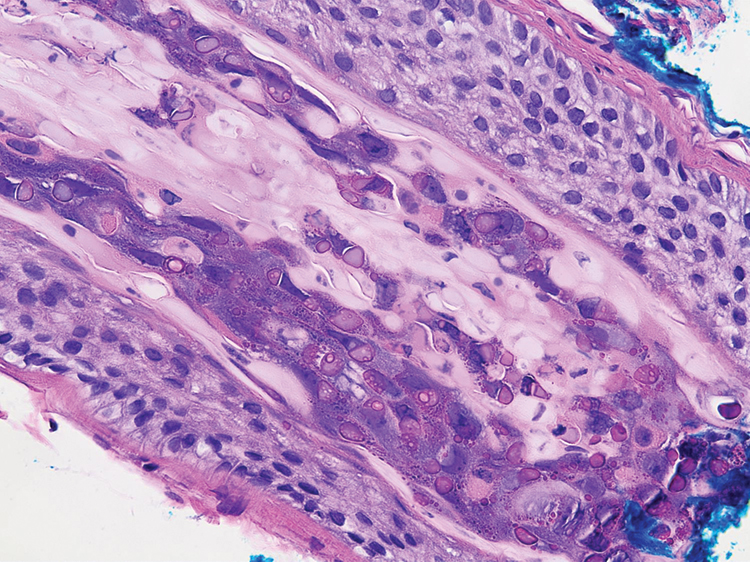
Trichodysplasia spinulosa was first described by Haycox et al1 in 1999. The authors suggested a viral etiology. Eleven years later, TS-associated polyomavirus (TSPyV) was identified by van der Meijden et al.2 Follicular keratinocytes are the specific target for TSPyV.3 Evidence has been presented suggesting that TS is caused by a primary infection or reactivation of TSPyV in the setting of immunosuppression.4,5
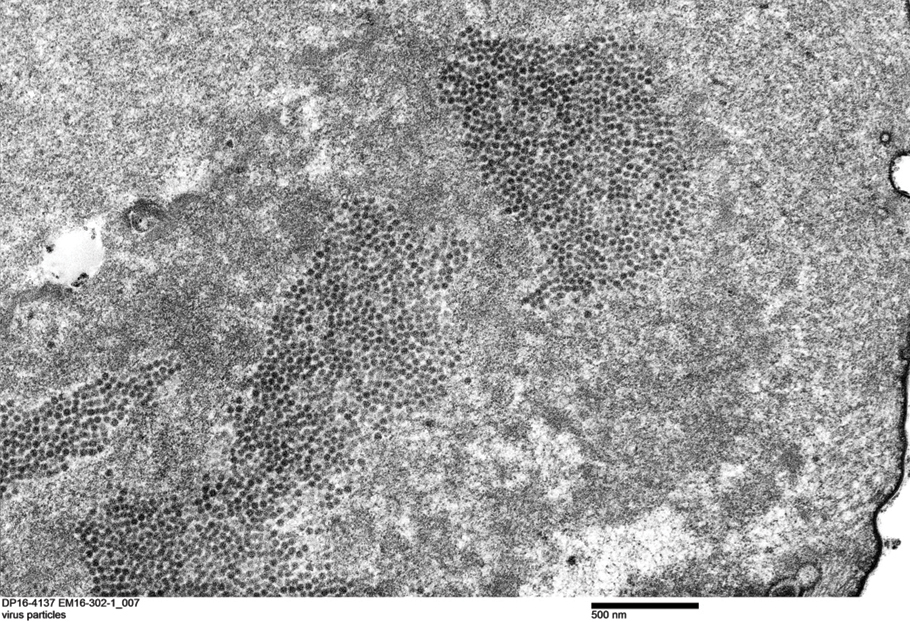
Patients with TS present with papular eruptions that appear on the central face with spiny excrescences and various degrees of alopecia involving the eyebrows or eyelashes. Histopathologic features include distended hair follicles with expansion of inner root sheath cells, eosinophilic trichohyalin granules, and the absence of hair shafts. The viral protein can be verified through immunohistochemistry TSPyV VP1 staining that demonstrates co-localization with trichohyalin. Viral particles also can be visualized as 35- to 38-nm intranuclear particles with an organized crystalloid morphology on TEM.6,7 The negative polymerase chain reaction in our patient could be the result of suboptimal template DNA concentration extracted from the limited amount of tissue remaining in the block after hematoxylin and eosin staining.
The clinical differential diagnosis of central facial spinulosism includes the follicular spicules of multiple myeloma (FSMM). In fact, FSMM and TS can only be differentiated after obtaining a blood profile and bone marrow biopsy that excludes the diagnosis of FSMM. A history of immunosuppression typically suggests TS. Histopathology often is equivocal in FSMM8; however, TEM reveals viral particles (TSPyV) in TS. Transmission electron microscopy in FSMM demonstrates fibrillary structures arranged in a paracrystalline configuration with unknown significance instead of viral particles. Despite the absence of viral particles on TEM, a low mean copy number of Merkel cell polyomavirus was isolated from a patient with FSMM who responded dramatically to treatment with topical cidofovir gel 1%.8 In addition to treating the underlying multiple myeloma in FSMM, topical cidofovir gel 1% also may have a role in treatment of these patients, suggesting a possible viral rather than simply paraneoplastic etiology of FSMM. Therefore, polyomavirus infection should be considered in the initial workup of any patient with fine facial follicular spicules.
The most effective management of TS in transplant recipients is to reduce immunosuppression to the lowest level possible without jeopardizing the transplanted organ.9 In our case, reduction of immunosuppressive drugs was not possible. In fact, immunosuppression in our patient was increased following evidence of early rejection of the heart transplant. Although manual extraction of the keratin spicules resulted in considerable improvement in a similar facial eruption in a patient with pediatric pre–B-cell acute lymphoblastic leukemia developing TS,10 it is impossible to apply this approach to patients such as ours who have thousands of tiny lesions. Fortunately, custom-compounded cidofovir gel 1% applied twice daily to the patient’s face and ears for 4 weeks led to near-complete clearance at follow-up (Figure 5). Due to the high cost of the medication (approaching $700 for one tube), our patient applied this medication to the face only several times weekly with excellent improvement. Thus, it appears that it is possible to suppress this virus with topical medication alone.

Polyomavirus infection should be considered in patients presenting with fine follicular spiny papules, especially those who are immunosuppressed. The possibility of coexisting multiple myeloma should be excluded.
Acknowledgment—We sincerely thank Glenn A. Hoskins (Jackson, Mississippi), the electron microscopy technologist, for the detection of viral particles and the electron microscope photographs.
- Haycox CL, Kim S, Fleckman P, et al. Trichodysplasia spinulosa: a newly described folliculocentric viral infection in an immunocompromised host. J Investig Dermatol Symp Proc. 1999;4:268-271.
- van der Meijden E, Janssens RWA, Lauber C, et al. Discovery of a new human polyomavirus associated with trichodysplasia spinulosa in an immunocompromized patient. PLoS Pathog. 2010;6:E1001024.
- Rouanet J, Aubin F, Gaboriaud P, et al. Trichodysplasia spinulosa: a polyomavirus infection specifically targeting follicular keratinocytes in immunocompromised patients. Br J Dermatol. 2016;174:629-632.
- van der Meijden E, Kazem S, Burgers MM, et al. Seroprevalence of trichodysplasia spinulosa-associated polyomavirus. Emerg Infect Dis. 2011;17:1355-1363.
- van der Meijden E, Horváth B, Nijland M, et al. Primary polyomavirus infection, not reactivation, as the cause of trichodysplasia spinulosa in immunocompromised patients. J Infect Dis. 2017;215:1080-1084.
- Fischer MK, Kao GF, Nguyen HP, et al. Specific detection of trichodysplasia spinulosa-associated polyomavirus DNA in skin and renal allograft tissues in a patient with trichodysplasia spinulosa. Arch Dermatol. 2012;148:726-733.
- Kazem S, van der Meijden E, Feltkamp MC. The trichodysplasia spinulosa-associated polyomavirus: virological background and clinical implications. APMIS. 2013;121:770-782.
- van Boheemen S, Jones T, Muhlemann B, et al. Cidofovir gel as treatment of follicular spicules in multiple myeloma. JAMA Dermatol. 2015;151:82-84.
- DeCrescenzo AJ, Philips RC, Wilkerson MG. Trichodysplasia spinulosa: a rare complication of immunosuppression. JAAD Case Rep. 2016;2:307-309.
- Barton M, Lockhart S, Sidbury R, et al. Trichodysplasia spinulosa in a 7-year-old boy managed using physical extraction of keratin spicules. Pediatr Dermatol. 2017;34:E74-E76.
To the Editor:
A 57-year-old man with hypertension, dyslipidemia, and congestive heart failure presented with a disfiguring eruption comprised of asymptomatic papules on the face that appeared 12 months post–heart transplantation. Immunosuppressive medications included mycophenolic acid and tacrolimus ointment (FK506). The pinpoint papules spread from the central face to the ears, arms, and legs. Physical examination revealed multiple 0.5- to 1-mm flesh-colored papules over the glabella, nose, nasolabial folds, philtrum, chin, ears, arms, and legs sparing the trunk. The initial appearance of the facial rash resembled the surface of a nutmeg grater with central white spiny excrescences overlying fine papules (spinulosism)(Figure 1). In addition, eyebrow alopecia was present.

A 3-mm punch biopsy of a papule with a central spine was performed on the left thigh. Microscopic examination revealed marked dilatation of anagen hair follicles with a proliferation of haphazard inner root sheath cells replacing the follicular lumen. Hair shafts were absent, and plugged infundibula were observed (Figure 2). The inner root sheath keratinocytes were enlarged and dystrophic with deeply eosinophilic trichohyalin granules (Figure 3). The epidermis, outer root sheath epithelium, and eccrine structures were unremarkable.

Transmission electron microscopy (TEM) confirmed the presence of intranuclear viral inclusions within affected inner root sheath keratinocytes composed of nonenveloped icosahedral viral particles measuring 33 to 38 nm in diameter (Figure 4). These findings morphologically were consistent with a polyomavirus. No intracytoplasmic or extracellular viral particles were identified. The clinical history, physical examination, histopathology, and electron microscopy features strongly supported the diagnosis of trichodysplasia spinulosa (TS) despite insufficient material being retrieved for polymerase chain reaction identification.

Trichodysplasia spinulosa was first described by Haycox et al1 in 1999. The authors suggested a viral etiology. Eleven years later, TS-associated polyomavirus (TSPyV) was identified by van der Meijden et al.2 Follicular keratinocytes are the specific target for TSPyV.3 Evidence has been presented suggesting that TS is caused by a primary infection or reactivation of TSPyV in the setting of immunosuppression.4,5

Patients with TS present with papular eruptions that appear on the central face with spiny excrescences and various degrees of alopecia involving the eyebrows or eyelashes. Histopathologic features include distended hair follicles with expansion of inner root sheath cells, eosinophilic trichohyalin granules, and the absence of hair shafts. The viral protein can be verified through immunohistochemistry TSPyV VP1 staining that demonstrates co-localization with trichohyalin. Viral particles also can be visualized as 35- to 38-nm intranuclear particles with an organized crystalloid morphology on TEM.6,7 The negative polymerase chain reaction in our patient could be the result of suboptimal template DNA concentration extracted from the limited amount of tissue remaining in the block after hematoxylin and eosin staining.
The clinical differential diagnosis of central facial spinulosism includes the follicular spicules of multiple myeloma (FSMM). In fact, FSMM and TS can only be differentiated after obtaining a blood profile and bone marrow biopsy that excludes the diagnosis of FSMM. A history of immunosuppression typically suggests TS. Histopathology often is equivocal in FSMM8; however, TEM reveals viral particles (TSPyV) in TS. Transmission electron microscopy in FSMM demonstrates fibrillary structures arranged in a paracrystalline configuration with unknown significance instead of viral particles. Despite the absence of viral particles on TEM, a low mean copy number of Merkel cell polyomavirus was isolated from a patient with FSMM who responded dramatically to treatment with topical cidofovir gel 1%.8 In addition to treating the underlying multiple myeloma in FSMM, topical cidofovir gel 1% also may have a role in treatment of these patients, suggesting a possible viral rather than simply paraneoplastic etiology of FSMM. Therefore, polyomavirus infection should be considered in the initial workup of any patient with fine facial follicular spicules.
The most effective management of TS in transplant recipients is to reduce immunosuppression to the lowest level possible without jeopardizing the transplanted organ.9 In our case, reduction of immunosuppressive drugs was not possible. In fact, immunosuppression in our patient was increased following evidence of early rejection of the heart transplant. Although manual extraction of the keratin spicules resulted in considerable improvement in a similar facial eruption in a patient with pediatric pre–B-cell acute lymphoblastic leukemia developing TS,10 it is impossible to apply this approach to patients such as ours who have thousands of tiny lesions. Fortunately, custom-compounded cidofovir gel 1% applied twice daily to the patient’s face and ears for 4 weeks led to near-complete clearance at follow-up (Figure 5). Due to the high cost of the medication (approaching $700 for one tube), our patient applied this medication to the face only several times weekly with excellent improvement. Thus, it appears that it is possible to suppress this virus with topical medication alone.

Polyomavirus infection should be considered in patients presenting with fine follicular spiny papules, especially those who are immunosuppressed. The possibility of coexisting multiple myeloma should be excluded.
Acknowledgment—We sincerely thank Glenn A. Hoskins (Jackson, Mississippi), the electron microscopy technologist, for the detection of viral particles and the electron microscope photographs.
To the Editor:
A 57-year-old man with hypertension, dyslipidemia, and congestive heart failure presented with a disfiguring eruption comprised of asymptomatic papules on the face that appeared 12 months post–heart transplantation. Immunosuppressive medications included mycophenolic acid and tacrolimus ointment (FK506). The pinpoint papules spread from the central face to the ears, arms, and legs. Physical examination revealed multiple 0.5- to 1-mm flesh-colored papules over the glabella, nose, nasolabial folds, philtrum, chin, ears, arms, and legs sparing the trunk. The initial appearance of the facial rash resembled the surface of a nutmeg grater with central white spiny excrescences overlying fine papules (spinulosism)(Figure 1). In addition, eyebrow alopecia was present.

A 3-mm punch biopsy of a papule with a central spine was performed on the left thigh. Microscopic examination revealed marked dilatation of anagen hair follicles with a proliferation of haphazard inner root sheath cells replacing the follicular lumen. Hair shafts were absent, and plugged infundibula were observed (Figure 2). The inner root sheath keratinocytes were enlarged and dystrophic with deeply eosinophilic trichohyalin granules (Figure 3). The epidermis, outer root sheath epithelium, and eccrine structures were unremarkable.

Transmission electron microscopy (TEM) confirmed the presence of intranuclear viral inclusions within affected inner root sheath keratinocytes composed of nonenveloped icosahedral viral particles measuring 33 to 38 nm in diameter (Figure 4). These findings morphologically were consistent with a polyomavirus. No intracytoplasmic or extracellular viral particles were identified. The clinical history, physical examination, histopathology, and electron microscopy features strongly supported the diagnosis of trichodysplasia spinulosa (TS) despite insufficient material being retrieved for polymerase chain reaction identification.

Trichodysplasia spinulosa was first described by Haycox et al1 in 1999. The authors suggested a viral etiology. Eleven years later, TS-associated polyomavirus (TSPyV) was identified by van der Meijden et al.2 Follicular keratinocytes are the specific target for TSPyV.3 Evidence has been presented suggesting that TS is caused by a primary infection or reactivation of TSPyV in the setting of immunosuppression.4,5

Patients with TS present with papular eruptions that appear on the central face with spiny excrescences and various degrees of alopecia involving the eyebrows or eyelashes. Histopathologic features include distended hair follicles with expansion of inner root sheath cells, eosinophilic trichohyalin granules, and the absence of hair shafts. The viral protein can be verified through immunohistochemistry TSPyV VP1 staining that demonstrates co-localization with trichohyalin. Viral particles also can be visualized as 35- to 38-nm intranuclear particles with an organized crystalloid morphology on TEM.6,7 The negative polymerase chain reaction in our patient could be the result of suboptimal template DNA concentration extracted from the limited amount of tissue remaining in the block after hematoxylin and eosin staining.
The clinical differential diagnosis of central facial spinulosism includes the follicular spicules of multiple myeloma (FSMM). In fact, FSMM and TS can only be differentiated after obtaining a blood profile and bone marrow biopsy that excludes the diagnosis of FSMM. A history of immunosuppression typically suggests TS. Histopathology often is equivocal in FSMM8; however, TEM reveals viral particles (TSPyV) in TS. Transmission electron microscopy in FSMM demonstrates fibrillary structures arranged in a paracrystalline configuration with unknown significance instead of viral particles. Despite the absence of viral particles on TEM, a low mean copy number of Merkel cell polyomavirus was isolated from a patient with FSMM who responded dramatically to treatment with topical cidofovir gel 1%.8 In addition to treating the underlying multiple myeloma in FSMM, topical cidofovir gel 1% also may have a role in treatment of these patients, suggesting a possible viral rather than simply paraneoplastic etiology of FSMM. Therefore, polyomavirus infection should be considered in the initial workup of any patient with fine facial follicular spicules.
The most effective management of TS in transplant recipients is to reduce immunosuppression to the lowest level possible without jeopardizing the transplanted organ.9 In our case, reduction of immunosuppressive drugs was not possible. In fact, immunosuppression in our patient was increased following evidence of early rejection of the heart transplant. Although manual extraction of the keratin spicules resulted in considerable improvement in a similar facial eruption in a patient with pediatric pre–B-cell acute lymphoblastic leukemia developing TS,10 it is impossible to apply this approach to patients such as ours who have thousands of tiny lesions. Fortunately, custom-compounded cidofovir gel 1% applied twice daily to the patient’s face and ears for 4 weeks led to near-complete clearance at follow-up (Figure 5). Due to the high cost of the medication (approaching $700 for one tube), our patient applied this medication to the face only several times weekly with excellent improvement. Thus, it appears that it is possible to suppress this virus with topical medication alone.

Polyomavirus infection should be considered in patients presenting with fine follicular spiny papules, especially those who are immunosuppressed. The possibility of coexisting multiple myeloma should be excluded.
Acknowledgment—We sincerely thank Glenn A. Hoskins (Jackson, Mississippi), the electron microscopy technologist, for the detection of viral particles and the electron microscope photographs.
- Haycox CL, Kim S, Fleckman P, et al. Trichodysplasia spinulosa: a newly described folliculocentric viral infection in an immunocompromised host. J Investig Dermatol Symp Proc. 1999;4:268-271.
- van der Meijden E, Janssens RWA, Lauber C, et al. Discovery of a new human polyomavirus associated with trichodysplasia spinulosa in an immunocompromized patient. PLoS Pathog. 2010;6:E1001024.
- Rouanet J, Aubin F, Gaboriaud P, et al. Trichodysplasia spinulosa: a polyomavirus infection specifically targeting follicular keratinocytes in immunocompromised patients. Br J Dermatol. 2016;174:629-632.
- van der Meijden E, Kazem S, Burgers MM, et al. Seroprevalence of trichodysplasia spinulosa-associated polyomavirus. Emerg Infect Dis. 2011;17:1355-1363.
- van der Meijden E, Horváth B, Nijland M, et al. Primary polyomavirus infection, not reactivation, as the cause of trichodysplasia spinulosa in immunocompromised patients. J Infect Dis. 2017;215:1080-1084.
- Fischer MK, Kao GF, Nguyen HP, et al. Specific detection of trichodysplasia spinulosa-associated polyomavirus DNA in skin and renal allograft tissues in a patient with trichodysplasia spinulosa. Arch Dermatol. 2012;148:726-733.
- Kazem S, van der Meijden E, Feltkamp MC. The trichodysplasia spinulosa-associated polyomavirus: virological background and clinical implications. APMIS. 2013;121:770-782.
- van Boheemen S, Jones T, Muhlemann B, et al. Cidofovir gel as treatment of follicular spicules in multiple myeloma. JAMA Dermatol. 2015;151:82-84.
- DeCrescenzo AJ, Philips RC, Wilkerson MG. Trichodysplasia spinulosa: a rare complication of immunosuppression. JAAD Case Rep. 2016;2:307-309.
- Barton M, Lockhart S, Sidbury R, et al. Trichodysplasia spinulosa in a 7-year-old boy managed using physical extraction of keratin spicules. Pediatr Dermatol. 2017;34:E74-E76.
- Haycox CL, Kim S, Fleckman P, et al. Trichodysplasia spinulosa: a newly described folliculocentric viral infection in an immunocompromised host. J Investig Dermatol Symp Proc. 1999;4:268-271.
- van der Meijden E, Janssens RWA, Lauber C, et al. Discovery of a new human polyomavirus associated with trichodysplasia spinulosa in an immunocompromized patient. PLoS Pathog. 2010;6:E1001024.
- Rouanet J, Aubin F, Gaboriaud P, et al. Trichodysplasia spinulosa: a polyomavirus infection specifically targeting follicular keratinocytes in immunocompromised patients. Br J Dermatol. 2016;174:629-632.
- van der Meijden E, Kazem S, Burgers MM, et al. Seroprevalence of trichodysplasia spinulosa-associated polyomavirus. Emerg Infect Dis. 2011;17:1355-1363.
- van der Meijden E, Horváth B, Nijland M, et al. Primary polyomavirus infection, not reactivation, as the cause of trichodysplasia spinulosa in immunocompromised patients. J Infect Dis. 2017;215:1080-1084.
- Fischer MK, Kao GF, Nguyen HP, et al. Specific detection of trichodysplasia spinulosa-associated polyomavirus DNA in skin and renal allograft tissues in a patient with trichodysplasia spinulosa. Arch Dermatol. 2012;148:726-733.
- Kazem S, van der Meijden E, Feltkamp MC. The trichodysplasia spinulosa-associated polyomavirus: virological background and clinical implications. APMIS. 2013;121:770-782.
- van Boheemen S, Jones T, Muhlemann B, et al. Cidofovir gel as treatment of follicular spicules in multiple myeloma. JAMA Dermatol. 2015;151:82-84.
- DeCrescenzo AJ, Philips RC, Wilkerson MG. Trichodysplasia spinulosa: a rare complication of immunosuppression. JAAD Case Rep. 2016;2:307-309.
- Barton M, Lockhart S, Sidbury R, et al. Trichodysplasia spinulosa in a 7-year-old boy managed using physical extraction of keratin spicules. Pediatr Dermatol. 2017;34:E74-E76.
Practice Points
- Trichodysplasia spinulosa (TS) is a rare skin disease caused by primary TS-associated polyomavirus (TSPyV) infecting follicular keratinocytes in immunocompromised patients.
- Trichodysplasia spinulosa typically presents with papular eruptions that appear on the central face with spiny excrescences and various degrees of alopecia involving the eyebrows or eyelashes.
- The viral protein can be verified through immunohistochemistry TSPyV major capsid protein VP1 staining or can be visualized on transmission electron microscopy.
- Follicular spicules of multiple myeloma should be ruled out before initiating treatment with cidofovir gel 1% for TS.
Hair loss: Consider a patient’s supplement use
BOSTON – .
This is an important question because patients consider supplements as “natural and healthy,” not as drugs or chemicals, Wilma F. Bergfeld, MD, said at the annual meeting of the American Academy of Dermatology.
Some of these products contain botanicals, which are not always safe, added Dr. Bergfeld, professor of dermatology and pathology at the Cleveland Clinic. “They have many activities, and they are being touted as having some activity in helping the hair or enhancing hair growth,” including having 5-alpha-reductase inhibitors as an ingredient. “Saw palmetto is probably the most common one, but there are a host of natural ingredients that are being put into these supplements, including those that promote androgen induction, as well as antioxidants and anti-inflammatories.”
In the opinion of Dr. Bergfeld, a nutrition-focused physical assessment should include an examination of the scalp and all hairy areas. “It’s also important to see the symmetry and shape of hair growth or hair loss areas, the distribution, hair color, the thickness and texture of the hair fibers,” she added.
Besides asking about what supplements patients are taking, other questions to ask during the visit include: Are you noticing more hair on your brush, pillow, and shoulders, or in the shower? Do you think your hair is thinning? What are your medical problems? Have you experienced rapid weight loss? Have you started any new medications? What medication(s) are you on? What foods do you eat? Do you have a family history of hair loss?
Possible causes of hair loss or changes include environmental factors, stress, hormonal changes, medications, and nutrition.
Common ingredients contained in healthy hair supplements include biotin, folic acid, L-cysteine, L-methionine, MSM (methylsulfonylmethane), vitamin B complex, and vitamins A, C, D, and E. “Vitamin D and A are associated on the hair follicle receptor sites, and they balance each other, so if one is down the other is usually down,” said Dr. Bergfeld, who directs Cleveland Clinic’s hair disorders clinic and its dermatopathology program. Other important ingredients include iron, zinc, manganese, amino acids including L-Lysine, and fatty acids.
Iron deficiency is a known cause of hair loss. “The absorption of iron relies on vitamin C and sometimes lysine,” she said. Red meat has a high iron content and since many patients are restricting red meat intake, “they do need to think about that.” Zinc deficiency is less common in Western countries, she continued, “but when you find it, it’s revolutionary because if they’re shedding hair and their hair character is changing, often some supplementation will do the trick. But remember: Zinc is not only an anti-inflammatory, it’s also an antiandrogen. It has 5-alpha-reductase inhibitor capabilities.”.
Dr. Bergfeld noted that biotin, also known as vitamin B7 and found in many foods, is used in many vitamin supplements marketed for hair loss. The recommended daily allowance (RDA) is 30 mcg/day in adults but the amount in hair supplements can be up to 650% of RDA. “Biotin at high levels is believed to be safe, but can interfere with troponin and other lab testing,” she cautioned. “This can lead to dangerous false laboratory results.”
To date, insufficient data exist to recommend supplementation with zinc, riboflavin, folic acid, or vitamin B12 for hair loss, “but they may help in cases of deficiency,” said Dr. Bergfeld, a past president of the American Hair Research Society. The use of vitamin E and biotin supplementation is not supported in the literature for treating androgenetic alopecia or telogen effluvium. Excessive vitamin A (not beta carotene) and selenium can contribute to hair loss and studies have shown a relationship between androgenetic alopecia and low vitamin D levels. “Vitamin D should be supplemented if serum levels are low, but more studies are needed to determine the effect of iron and zinc supplementation” in patients with androgenetic alopecia, she said.
While there are not enough data to support a recommendation for supplementation of folic or B12 for alopecia, she said, “vitamin B12 deficiency may occur in androgenetic alopecia patients, associated with pernicious anemia.”
She added that the use biotin supplementation for the treatment of androgenetic alopecia is not supported by available data, and “it is also unclear if selenium plays a role in this disease.”
Dr. Bergfeld reported having no disclosures related to her presentation.
BOSTON – .
This is an important question because patients consider supplements as “natural and healthy,” not as drugs or chemicals, Wilma F. Bergfeld, MD, said at the annual meeting of the American Academy of Dermatology.
Some of these products contain botanicals, which are not always safe, added Dr. Bergfeld, professor of dermatology and pathology at the Cleveland Clinic. “They have many activities, and they are being touted as having some activity in helping the hair or enhancing hair growth,” including having 5-alpha-reductase inhibitors as an ingredient. “Saw palmetto is probably the most common one, but there are a host of natural ingredients that are being put into these supplements, including those that promote androgen induction, as well as antioxidants and anti-inflammatories.”
In the opinion of Dr. Bergfeld, a nutrition-focused physical assessment should include an examination of the scalp and all hairy areas. “It’s also important to see the symmetry and shape of hair growth or hair loss areas, the distribution, hair color, the thickness and texture of the hair fibers,” she added.
Besides asking about what supplements patients are taking, other questions to ask during the visit include: Are you noticing more hair on your brush, pillow, and shoulders, or in the shower? Do you think your hair is thinning? What are your medical problems? Have you experienced rapid weight loss? Have you started any new medications? What medication(s) are you on? What foods do you eat? Do you have a family history of hair loss?
Possible causes of hair loss or changes include environmental factors, stress, hormonal changes, medications, and nutrition.
Common ingredients contained in healthy hair supplements include biotin, folic acid, L-cysteine, L-methionine, MSM (methylsulfonylmethane), vitamin B complex, and vitamins A, C, D, and E. “Vitamin D and A are associated on the hair follicle receptor sites, and they balance each other, so if one is down the other is usually down,” said Dr. Bergfeld, who directs Cleveland Clinic’s hair disorders clinic and its dermatopathology program. Other important ingredients include iron, zinc, manganese, amino acids including L-Lysine, and fatty acids.
Iron deficiency is a known cause of hair loss. “The absorption of iron relies on vitamin C and sometimes lysine,” she said. Red meat has a high iron content and since many patients are restricting red meat intake, “they do need to think about that.” Zinc deficiency is less common in Western countries, she continued, “but when you find it, it’s revolutionary because if they’re shedding hair and their hair character is changing, often some supplementation will do the trick. But remember: Zinc is not only an anti-inflammatory, it’s also an antiandrogen. It has 5-alpha-reductase inhibitor capabilities.”.
Dr. Bergfeld noted that biotin, also known as vitamin B7 and found in many foods, is used in many vitamin supplements marketed for hair loss. The recommended daily allowance (RDA) is 30 mcg/day in adults but the amount in hair supplements can be up to 650% of RDA. “Biotin at high levels is believed to be safe, but can interfere with troponin and other lab testing,” she cautioned. “This can lead to dangerous false laboratory results.”
To date, insufficient data exist to recommend supplementation with zinc, riboflavin, folic acid, or vitamin B12 for hair loss, “but they may help in cases of deficiency,” said Dr. Bergfeld, a past president of the American Hair Research Society. The use of vitamin E and biotin supplementation is not supported in the literature for treating androgenetic alopecia or telogen effluvium. Excessive vitamin A (not beta carotene) and selenium can contribute to hair loss and studies have shown a relationship between androgenetic alopecia and low vitamin D levels. “Vitamin D should be supplemented if serum levels are low, but more studies are needed to determine the effect of iron and zinc supplementation” in patients with androgenetic alopecia, she said.
While there are not enough data to support a recommendation for supplementation of folic or B12 for alopecia, she said, “vitamin B12 deficiency may occur in androgenetic alopecia patients, associated with pernicious anemia.”
She added that the use biotin supplementation for the treatment of androgenetic alopecia is not supported by available data, and “it is also unclear if selenium plays a role in this disease.”
Dr. Bergfeld reported having no disclosures related to her presentation.
BOSTON – .
This is an important question because patients consider supplements as “natural and healthy,” not as drugs or chemicals, Wilma F. Bergfeld, MD, said at the annual meeting of the American Academy of Dermatology.
Some of these products contain botanicals, which are not always safe, added Dr. Bergfeld, professor of dermatology and pathology at the Cleveland Clinic. “They have many activities, and they are being touted as having some activity in helping the hair or enhancing hair growth,” including having 5-alpha-reductase inhibitors as an ingredient. “Saw palmetto is probably the most common one, but there are a host of natural ingredients that are being put into these supplements, including those that promote androgen induction, as well as antioxidants and anti-inflammatories.”
In the opinion of Dr. Bergfeld, a nutrition-focused physical assessment should include an examination of the scalp and all hairy areas. “It’s also important to see the symmetry and shape of hair growth or hair loss areas, the distribution, hair color, the thickness and texture of the hair fibers,” she added.
Besides asking about what supplements patients are taking, other questions to ask during the visit include: Are you noticing more hair on your brush, pillow, and shoulders, or in the shower? Do you think your hair is thinning? What are your medical problems? Have you experienced rapid weight loss? Have you started any new medications? What medication(s) are you on? What foods do you eat? Do you have a family history of hair loss?
Possible causes of hair loss or changes include environmental factors, stress, hormonal changes, medications, and nutrition.
Common ingredients contained in healthy hair supplements include biotin, folic acid, L-cysteine, L-methionine, MSM (methylsulfonylmethane), vitamin B complex, and vitamins A, C, D, and E. “Vitamin D and A are associated on the hair follicle receptor sites, and they balance each other, so if one is down the other is usually down,” said Dr. Bergfeld, who directs Cleveland Clinic’s hair disorders clinic and its dermatopathology program. Other important ingredients include iron, zinc, manganese, amino acids including L-Lysine, and fatty acids.
Iron deficiency is a known cause of hair loss. “The absorption of iron relies on vitamin C and sometimes lysine,” she said. Red meat has a high iron content and since many patients are restricting red meat intake, “they do need to think about that.” Zinc deficiency is less common in Western countries, she continued, “but when you find it, it’s revolutionary because if they’re shedding hair and their hair character is changing, often some supplementation will do the trick. But remember: Zinc is not only an anti-inflammatory, it’s also an antiandrogen. It has 5-alpha-reductase inhibitor capabilities.”.
Dr. Bergfeld noted that biotin, also known as vitamin B7 and found in many foods, is used in many vitamin supplements marketed for hair loss. The recommended daily allowance (RDA) is 30 mcg/day in adults but the amount in hair supplements can be up to 650% of RDA. “Biotin at high levels is believed to be safe, but can interfere with troponin and other lab testing,” she cautioned. “This can lead to dangerous false laboratory results.”
To date, insufficient data exist to recommend supplementation with zinc, riboflavin, folic acid, or vitamin B12 for hair loss, “but they may help in cases of deficiency,” said Dr. Bergfeld, a past president of the American Hair Research Society. The use of vitamin E and biotin supplementation is not supported in the literature for treating androgenetic alopecia or telogen effluvium. Excessive vitamin A (not beta carotene) and selenium can contribute to hair loss and studies have shown a relationship between androgenetic alopecia and low vitamin D levels. “Vitamin D should be supplemented if serum levels are low, but more studies are needed to determine the effect of iron and zinc supplementation” in patients with androgenetic alopecia, she said.
While there are not enough data to support a recommendation for supplementation of folic or B12 for alopecia, she said, “vitamin B12 deficiency may occur in androgenetic alopecia patients, associated with pernicious anemia.”
She added that the use biotin supplementation for the treatment of androgenetic alopecia is not supported by available data, and “it is also unclear if selenium plays a role in this disease.”
Dr. Bergfeld reported having no disclosures related to her presentation.
AT AAD 22
Trichotillomania: What you should know about this common hair-pulling disorder
Trichotillomania is a chronic psychiatric disorder that causes people to repeatedly pull out their own hair. Not only does it result in alopecia with no other underlying causes but it can have significant psychosocial ramifications and rare, but serious, complications. Though the reported prevalence rates are up to approximately 2%, it’s probable that you’ll come upon a patient suffering with this disorder at your practice, if you haven’t already.
To find out more about the best methods for diagnosing and treating this disorder, we spoke with Jon E. Grant, JD, MD, MPH, a leading trichotillomania researcher and part of the department of psychiatry and behavioral neuroscience at the University of Chicago.
Defining trichotillomania
What were the earliest descriptions of trichotillomania in medical literature?
The first real discussion of it probably goes back to Hippocrates, but from a modern medical perspective, discussion began in the 19th century with reports from the French dermatologist François Hallopeau.
They didn’t really call them disorders then – it was long before the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) – but they described this in young men who kept pulling their hair for unclear reasons. These early case reports don’t provide a lot of psychological perspective, but they seem consistent with what we see now.
What are the diagnostic criteria for trichotillomania?
The current DSM-5 criteria are recurrent pulling out of hair, an inability to stop it, the pulling resulting in some noticeable thinning or hair loss, and that it causes some level of distress or some type of impairment in functioning.
At what age do most people experience an onset of symptoms?
Generally speaking, it’s in early adolescence, post puberty, around 12-15 years of age. Having said that, we do see children as young as 1-2 years who are pulling their hair, and we occasionally see somebody far older who is doing it for the first time, a sort of geriatric onset.
Overlap and differences with other disorders
You’ve written that although trichotillomania is grouped with obsessive-compulsive disorder (OCD) in the DSM-5, the thinking around that has recently shifted. Why is that?
At first, it was noticed that many of these people pulled their hair repetitively in an almost ritualized manner, perhaps every night before bed. That looked like a compulsion of OCD.
When DSM-5 came out in 2013, they grouped it with OCD. Yet people shifted to thinking that it’s kind of a cousin of OCD because it has this compulsive quality but doesn’t really have obsessive thinking that drives it. Many people just pull their hair. They’re not even always aware of it: sometimes yes, sometimes no.
We know that it has some links to OCD. You’ll see more OCD in folks with trichotillomania, but it clearly is not just the same as OCD. One of the biggest pieces of evidence for that is that our first-line treatment for OCD – a selective serotonin reuptake inhibitor antidepressant – does not really help hair pulling.
Having said that, if people are looking for help with trichotillomania, they often are best served by therapists and doctors who have a familiarity with OCD and have kept it on their radar over the past couple of decades.
How does trichotillomania overlap with skin picking disorder, which is another condition that you’ve closely researched?
It does have some overlap with skin picking in the sense that it often seems familial. For example, the mother may pull her hair and child picks their skin.
It also has a fair amount of comorbidity with skin picking. Many people who pull will pick a little bit or did at some point. Many people who pick pulled their hair at some point. It seems closely related to nail biting as well.
Studies have also shown that one of the things that runs in the histories of most families of people with trichotillomania might be substance abuse – alcohol or drug addiction.
All of this has led people to believe that there might be subtypes of trichotillomania: one that’s more like an OCD and one that’s more like an addiction. That’s similar to the debate with other mental health conditions, that there are probably multiple types of depression, multiple types of schizophrenia.
Is there a component of this that could be defined as self-harm?
That’s been its own debate. It doesn’t seem to have the same developmental trajectory that we see with self-harm, or even some of the personality features.
However, there may be a small segment of folks with trichotillomania that might more appropriately fit that category. For example, those with family histories of trauma, higher rates of posttraumatic stress disorder, or borderline personality. But it wouldn’t be the majority.
The problem is, if you look at some of the pediatrician data, they often group picking, pulling, and cutting. I think that’s far too all-inclusive.
A gap in clinician education
Are adolescent patients likely to self-report this behavior, or is it something that physicians need to suss out for themselves?
Clearly, if child psychologists, psychiatrists, or pediatricians see young people with patches of alopecia – eyebrows or eyelashes missing, head hair with spots – in addition to a dermatologic assessment, they should simply ask, “Do you pull your hair?”
But it’s interesting that with the internet, young people are much more likely to disclose and actually come forward and tell their parents that they think they have trichotillomania.
I also hear from a lot of the adolescents that they have to educate their doctors about trichotillomania because so often physicians don’t know much about it and will assume that it’s self-injury or just a symptom of anxiety. It’s a little bit of a flip from what we might have seen 20 years ago.
I’ve seen several patients who’ve said, basically, “I’m tired of no professionals seeming to know about this. I shouldn’t have to be educating my doctors about this.” I tell them that I completely agree. It’s a shame because if a doctor doesn’t know about it, then how can they get the appropriate care?
What are the complications that accompany trichotillomania?
A small percentage, maybe about 10%, will ingest their hair, much like people who bite and swallow their fingernails. The concern there is that because hair is nondigestible, it could create an intestinal plug that could rupture and be potentially life-threatening. That makes it all the more important to ask those who pull their hair what they do with the hair once they pull it.
However, with most people, the real problem is with self-esteem. Young people may not want to socialize, go on dates, or do other things they would normally do because of it. In adults, you may find that they’re far more educated than their job allows but don’t want to go to an interview because they don’t want to have somebody sit there and look at them and notice that perhaps they don’t have any eyebrows, or that they’re wearing a wig. Those psychosocial implications are huge for so many people.
Treatment options
In a 2021 study, you showed that nearly one-quarter of people with trichotillomania do naturally recover from it. What characteristics do they seem to have?
It’s interesting because we see natural recovery across many mental health problems: alcohol addition, gambling, OCD. The question then becomes why is that some people can seemingly just stop doing a behavior? Can we learn from those people?
We did see that those who naturally recovered were less likely to have some other mental health comorbidities. It seems like when you have other things such as skin picking or OCD plus trichotillomania, that it probably speaks to something that perhaps synergistically is keeping it going. But this is just a first study; learning how to harness and understand it is the next step.
What’s the goal of treating trichotillomania?
The desired goal is zero pulling. The realistic goal is more likely significantly reduced pulling that then leads to greater function in life, greater quality-of-life.
One doesn’t have to go from 100 to 0 in order to do that. I always tell people that maybe every now and then, every few months, when something is going on in life, you might find yourself pulling a hair or two. That’s okay. If you’re not pulling every day and it’s significantly reduced, we’ll call that a success. I think that setting reasonable goals at this point is really important.
And what would the treatment pathway look like for most patients?
The standard approach is probably some type of habit-reversal therapy, of which there have been many variants over the years. It involves doing something different with your hand, identifying the triggers that may set you off, and then doing something in response to those triggers that is not pulling and might neutralize whatever that anxious or stressed feeling is. That could be different with each person.
At this point, there is no drug approved by the U.S. Food and Drug Administration for trichotillomania. Our best approaches have included N-acetylcysteine, a glutamate modulator, which we’ve done research in.
That’s kind of a go-to option for people because its side-effect profile is generally innocuous. The data show that it could be beneficial in many people with very few, if any, side effects. That would be one “medication,” although it’s actually an over-the-counter vitamin. But we’re constantly looking for better and better treatments.
Do you have any final advice for clinicians or researchers?
Given how common it is, I don’t think clinicians should just see it as an innocuous little habit that people should be able to stop on their own. Clinicians should educate themselves about trichotillomania and know where the person should get the appropriate care.
From the research perspective, given the fact that we see this in animals of multiple species – that they overgroom – this seems to be deeply ingrained in us as animals. So when it comes to the underlying neuroscience, people should pay more attention because it probably has a lot to do with our understanding of habit and compulsive behaviors. It arguably can cut across a lot of different behaviors.
A version of this article first appeared on Medscape.com.
Trichotillomania is a chronic psychiatric disorder that causes people to repeatedly pull out their own hair. Not only does it result in alopecia with no other underlying causes but it can have significant psychosocial ramifications and rare, but serious, complications. Though the reported prevalence rates are up to approximately 2%, it’s probable that you’ll come upon a patient suffering with this disorder at your practice, if you haven’t already.
To find out more about the best methods for diagnosing and treating this disorder, we spoke with Jon E. Grant, JD, MD, MPH, a leading trichotillomania researcher and part of the department of psychiatry and behavioral neuroscience at the University of Chicago.
Defining trichotillomania
What were the earliest descriptions of trichotillomania in medical literature?
The first real discussion of it probably goes back to Hippocrates, but from a modern medical perspective, discussion began in the 19th century with reports from the French dermatologist François Hallopeau.
They didn’t really call them disorders then – it was long before the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) – but they described this in young men who kept pulling their hair for unclear reasons. These early case reports don’t provide a lot of psychological perspective, but they seem consistent with what we see now.
What are the diagnostic criteria for trichotillomania?
The current DSM-5 criteria are recurrent pulling out of hair, an inability to stop it, the pulling resulting in some noticeable thinning or hair loss, and that it causes some level of distress or some type of impairment in functioning.
At what age do most people experience an onset of symptoms?
Generally speaking, it’s in early adolescence, post puberty, around 12-15 years of age. Having said that, we do see children as young as 1-2 years who are pulling their hair, and we occasionally see somebody far older who is doing it for the first time, a sort of geriatric onset.
Overlap and differences with other disorders
You’ve written that although trichotillomania is grouped with obsessive-compulsive disorder (OCD) in the DSM-5, the thinking around that has recently shifted. Why is that?
At first, it was noticed that many of these people pulled their hair repetitively in an almost ritualized manner, perhaps every night before bed. That looked like a compulsion of OCD.
When DSM-5 came out in 2013, they grouped it with OCD. Yet people shifted to thinking that it’s kind of a cousin of OCD because it has this compulsive quality but doesn’t really have obsessive thinking that drives it. Many people just pull their hair. They’re not even always aware of it: sometimes yes, sometimes no.
We know that it has some links to OCD. You’ll see more OCD in folks with trichotillomania, but it clearly is not just the same as OCD. One of the biggest pieces of evidence for that is that our first-line treatment for OCD – a selective serotonin reuptake inhibitor antidepressant – does not really help hair pulling.
Having said that, if people are looking for help with trichotillomania, they often are best served by therapists and doctors who have a familiarity with OCD and have kept it on their radar over the past couple of decades.
How does trichotillomania overlap with skin picking disorder, which is another condition that you’ve closely researched?
It does have some overlap with skin picking in the sense that it often seems familial. For example, the mother may pull her hair and child picks their skin.
It also has a fair amount of comorbidity with skin picking. Many people who pull will pick a little bit or did at some point. Many people who pick pulled their hair at some point. It seems closely related to nail biting as well.
Studies have also shown that one of the things that runs in the histories of most families of people with trichotillomania might be substance abuse – alcohol or drug addiction.
All of this has led people to believe that there might be subtypes of trichotillomania: one that’s more like an OCD and one that’s more like an addiction. That’s similar to the debate with other mental health conditions, that there are probably multiple types of depression, multiple types of schizophrenia.
Is there a component of this that could be defined as self-harm?
That’s been its own debate. It doesn’t seem to have the same developmental trajectory that we see with self-harm, or even some of the personality features.
However, there may be a small segment of folks with trichotillomania that might more appropriately fit that category. For example, those with family histories of trauma, higher rates of posttraumatic stress disorder, or borderline personality. But it wouldn’t be the majority.
The problem is, if you look at some of the pediatrician data, they often group picking, pulling, and cutting. I think that’s far too all-inclusive.
A gap in clinician education
Are adolescent patients likely to self-report this behavior, or is it something that physicians need to suss out for themselves?
Clearly, if child psychologists, psychiatrists, or pediatricians see young people with patches of alopecia – eyebrows or eyelashes missing, head hair with spots – in addition to a dermatologic assessment, they should simply ask, “Do you pull your hair?”
But it’s interesting that with the internet, young people are much more likely to disclose and actually come forward and tell their parents that they think they have trichotillomania.
I also hear from a lot of the adolescents that they have to educate their doctors about trichotillomania because so often physicians don’t know much about it and will assume that it’s self-injury or just a symptom of anxiety. It’s a little bit of a flip from what we might have seen 20 years ago.
I’ve seen several patients who’ve said, basically, “I’m tired of no professionals seeming to know about this. I shouldn’t have to be educating my doctors about this.” I tell them that I completely agree. It’s a shame because if a doctor doesn’t know about it, then how can they get the appropriate care?
What are the complications that accompany trichotillomania?
A small percentage, maybe about 10%, will ingest their hair, much like people who bite and swallow their fingernails. The concern there is that because hair is nondigestible, it could create an intestinal plug that could rupture and be potentially life-threatening. That makes it all the more important to ask those who pull their hair what they do with the hair once they pull it.
However, with most people, the real problem is with self-esteem. Young people may not want to socialize, go on dates, or do other things they would normally do because of it. In adults, you may find that they’re far more educated than their job allows but don’t want to go to an interview because they don’t want to have somebody sit there and look at them and notice that perhaps they don’t have any eyebrows, or that they’re wearing a wig. Those psychosocial implications are huge for so many people.
Treatment options
In a 2021 study, you showed that nearly one-quarter of people with trichotillomania do naturally recover from it. What characteristics do they seem to have?
It’s interesting because we see natural recovery across many mental health problems: alcohol addition, gambling, OCD. The question then becomes why is that some people can seemingly just stop doing a behavior? Can we learn from those people?
We did see that those who naturally recovered were less likely to have some other mental health comorbidities. It seems like when you have other things such as skin picking or OCD plus trichotillomania, that it probably speaks to something that perhaps synergistically is keeping it going. But this is just a first study; learning how to harness and understand it is the next step.
What’s the goal of treating trichotillomania?
The desired goal is zero pulling. The realistic goal is more likely significantly reduced pulling that then leads to greater function in life, greater quality-of-life.
One doesn’t have to go from 100 to 0 in order to do that. I always tell people that maybe every now and then, every few months, when something is going on in life, you might find yourself pulling a hair or two. That’s okay. If you’re not pulling every day and it’s significantly reduced, we’ll call that a success. I think that setting reasonable goals at this point is really important.
And what would the treatment pathway look like for most patients?
The standard approach is probably some type of habit-reversal therapy, of which there have been many variants over the years. It involves doing something different with your hand, identifying the triggers that may set you off, and then doing something in response to those triggers that is not pulling and might neutralize whatever that anxious or stressed feeling is. That could be different with each person.
At this point, there is no drug approved by the U.S. Food and Drug Administration for trichotillomania. Our best approaches have included N-acetylcysteine, a glutamate modulator, which we’ve done research in.
That’s kind of a go-to option for people because its side-effect profile is generally innocuous. The data show that it could be beneficial in many people with very few, if any, side effects. That would be one “medication,” although it’s actually an over-the-counter vitamin. But we’re constantly looking for better and better treatments.
Do you have any final advice for clinicians or researchers?
Given how common it is, I don’t think clinicians should just see it as an innocuous little habit that people should be able to stop on their own. Clinicians should educate themselves about trichotillomania and know where the person should get the appropriate care.
From the research perspective, given the fact that we see this in animals of multiple species – that they overgroom – this seems to be deeply ingrained in us as animals. So when it comes to the underlying neuroscience, people should pay more attention because it probably has a lot to do with our understanding of habit and compulsive behaviors. It arguably can cut across a lot of different behaviors.
A version of this article first appeared on Medscape.com.
Trichotillomania is a chronic psychiatric disorder that causes people to repeatedly pull out their own hair. Not only does it result in alopecia with no other underlying causes but it can have significant psychosocial ramifications and rare, but serious, complications. Though the reported prevalence rates are up to approximately 2%, it’s probable that you’ll come upon a patient suffering with this disorder at your practice, if you haven’t already.
To find out more about the best methods for diagnosing and treating this disorder, we spoke with Jon E. Grant, JD, MD, MPH, a leading trichotillomania researcher and part of the department of psychiatry and behavioral neuroscience at the University of Chicago.
Defining trichotillomania
What were the earliest descriptions of trichotillomania in medical literature?
The first real discussion of it probably goes back to Hippocrates, but from a modern medical perspective, discussion began in the 19th century with reports from the French dermatologist François Hallopeau.
They didn’t really call them disorders then – it was long before the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) – but they described this in young men who kept pulling their hair for unclear reasons. These early case reports don’t provide a lot of psychological perspective, but they seem consistent with what we see now.
What are the diagnostic criteria for trichotillomania?
The current DSM-5 criteria are recurrent pulling out of hair, an inability to stop it, the pulling resulting in some noticeable thinning or hair loss, and that it causes some level of distress or some type of impairment in functioning.
At what age do most people experience an onset of symptoms?
Generally speaking, it’s in early adolescence, post puberty, around 12-15 years of age. Having said that, we do see children as young as 1-2 years who are pulling their hair, and we occasionally see somebody far older who is doing it for the first time, a sort of geriatric onset.
Overlap and differences with other disorders
You’ve written that although trichotillomania is grouped with obsessive-compulsive disorder (OCD) in the DSM-5, the thinking around that has recently shifted. Why is that?
At first, it was noticed that many of these people pulled their hair repetitively in an almost ritualized manner, perhaps every night before bed. That looked like a compulsion of OCD.
When DSM-5 came out in 2013, they grouped it with OCD. Yet people shifted to thinking that it’s kind of a cousin of OCD because it has this compulsive quality but doesn’t really have obsessive thinking that drives it. Many people just pull their hair. They’re not even always aware of it: sometimes yes, sometimes no.
We know that it has some links to OCD. You’ll see more OCD in folks with trichotillomania, but it clearly is not just the same as OCD. One of the biggest pieces of evidence for that is that our first-line treatment for OCD – a selective serotonin reuptake inhibitor antidepressant – does not really help hair pulling.
Having said that, if people are looking for help with trichotillomania, they often are best served by therapists and doctors who have a familiarity with OCD and have kept it on their radar over the past couple of decades.
How does trichotillomania overlap with skin picking disorder, which is another condition that you’ve closely researched?
It does have some overlap with skin picking in the sense that it often seems familial. For example, the mother may pull her hair and child picks their skin.
It also has a fair amount of comorbidity with skin picking. Many people who pull will pick a little bit or did at some point. Many people who pick pulled their hair at some point. It seems closely related to nail biting as well.
Studies have also shown that one of the things that runs in the histories of most families of people with trichotillomania might be substance abuse – alcohol or drug addiction.
All of this has led people to believe that there might be subtypes of trichotillomania: one that’s more like an OCD and one that’s more like an addiction. That’s similar to the debate with other mental health conditions, that there are probably multiple types of depression, multiple types of schizophrenia.
Is there a component of this that could be defined as self-harm?
That’s been its own debate. It doesn’t seem to have the same developmental trajectory that we see with self-harm, or even some of the personality features.
However, there may be a small segment of folks with trichotillomania that might more appropriately fit that category. For example, those with family histories of trauma, higher rates of posttraumatic stress disorder, or borderline personality. But it wouldn’t be the majority.
The problem is, if you look at some of the pediatrician data, they often group picking, pulling, and cutting. I think that’s far too all-inclusive.
A gap in clinician education
Are adolescent patients likely to self-report this behavior, or is it something that physicians need to suss out for themselves?
Clearly, if child psychologists, psychiatrists, or pediatricians see young people with patches of alopecia – eyebrows or eyelashes missing, head hair with spots – in addition to a dermatologic assessment, they should simply ask, “Do you pull your hair?”
But it’s interesting that with the internet, young people are much more likely to disclose and actually come forward and tell their parents that they think they have trichotillomania.
I also hear from a lot of the adolescents that they have to educate their doctors about trichotillomania because so often physicians don’t know much about it and will assume that it’s self-injury or just a symptom of anxiety. It’s a little bit of a flip from what we might have seen 20 years ago.
I’ve seen several patients who’ve said, basically, “I’m tired of no professionals seeming to know about this. I shouldn’t have to be educating my doctors about this.” I tell them that I completely agree. It’s a shame because if a doctor doesn’t know about it, then how can they get the appropriate care?
What are the complications that accompany trichotillomania?
A small percentage, maybe about 10%, will ingest their hair, much like people who bite and swallow their fingernails. The concern there is that because hair is nondigestible, it could create an intestinal plug that could rupture and be potentially life-threatening. That makes it all the more important to ask those who pull their hair what they do with the hair once they pull it.
However, with most people, the real problem is with self-esteem. Young people may not want to socialize, go on dates, or do other things they would normally do because of it. In adults, you may find that they’re far more educated than their job allows but don’t want to go to an interview because they don’t want to have somebody sit there and look at them and notice that perhaps they don’t have any eyebrows, or that they’re wearing a wig. Those psychosocial implications are huge for so many people.
Treatment options
In a 2021 study, you showed that nearly one-quarter of people with trichotillomania do naturally recover from it. What characteristics do they seem to have?
It’s interesting because we see natural recovery across many mental health problems: alcohol addition, gambling, OCD. The question then becomes why is that some people can seemingly just stop doing a behavior? Can we learn from those people?
We did see that those who naturally recovered were less likely to have some other mental health comorbidities. It seems like when you have other things such as skin picking or OCD plus trichotillomania, that it probably speaks to something that perhaps synergistically is keeping it going. But this is just a first study; learning how to harness and understand it is the next step.
What’s the goal of treating trichotillomania?
The desired goal is zero pulling. The realistic goal is more likely significantly reduced pulling that then leads to greater function in life, greater quality-of-life.
One doesn’t have to go from 100 to 0 in order to do that. I always tell people that maybe every now and then, every few months, when something is going on in life, you might find yourself pulling a hair or two. That’s okay. If you’re not pulling every day and it’s significantly reduced, we’ll call that a success. I think that setting reasonable goals at this point is really important.
And what would the treatment pathway look like for most patients?
The standard approach is probably some type of habit-reversal therapy, of which there have been many variants over the years. It involves doing something different with your hand, identifying the triggers that may set you off, and then doing something in response to those triggers that is not pulling and might neutralize whatever that anxious or stressed feeling is. That could be different with each person.
At this point, there is no drug approved by the U.S. Food and Drug Administration for trichotillomania. Our best approaches have included N-acetylcysteine, a glutamate modulator, which we’ve done research in.
That’s kind of a go-to option for people because its side-effect profile is generally innocuous. The data show that it could be beneficial in many people with very few, if any, side effects. That would be one “medication,” although it’s actually an over-the-counter vitamin. But we’re constantly looking for better and better treatments.
Do you have any final advice for clinicians or researchers?
Given how common it is, I don’t think clinicians should just see it as an innocuous little habit that people should be able to stop on their own. Clinicians should educate themselves about trichotillomania and know where the person should get the appropriate care.
From the research perspective, given the fact that we see this in animals of multiple species – that they overgroom – this seems to be deeply ingrained in us as animals. So when it comes to the underlying neuroscience, people should pay more attention because it probably has a lot to do with our understanding of habit and compulsive behaviors. It arguably can cut across a lot of different behaviors.
A version of this article first appeared on Medscape.com.
Oscars fight highlights for many the toll alopecia may carry
The Academy Awards ceremony on March 27 is a buzzing topic of conversation.
Troy Kotsur became the first deaf man to win an Oscar – and the highly coveted best supporting actor award, at that.
But it was what happened afterward that arguably stole the show.
Viewers and audience members alike watched in awe as actor Will Smith marched on stage and struck award presenter and comedian Chris Rock in the face after he directed a joke at Smith’s wife, Jada Pinkett Smith, for her shaved head.
and can lead to feelings of depression or mental illness.
About 700,000 people in the United States have alopecia areata, according to a 2020 study. Of them, slightly more than half are women, and more than 77% are White.
Shortly after the awards show, the Los Angeles Police Department released a statement saying it was aware of the incident and Mr. Rock had not pressed charges against Mr. Smith.
The incident set social media ablaze, and strong sentiments were heard from those who have been personally affected by alopecia.
Illness is never funny
Mr. Rock’s comment can be triggering to the millions who have been affected by hair loss, said Carolyn Goh, MD, a dermatologist at UCLA Health.
“As someone with alopecia myself, I consider it a microaggression,” Dr. Goh said. “I’ve experienced many similar comments. These build up over time and wear us down.”
One U.K.-based Instagram user, Kitty Dry, said the expression on Ms. Pinkett Smith’s face represented the hurt felt by so many with this condition.
“I want to preface this post by saying that in no way do I condone any sort of violence, but thank you Will Smith,” said Ms. Dry, 23, who was diagnosed with alopecia universalis after losing all her hair in 12 weeks.
“That slap was for anyone with alopecia who has ever been at the butt of an unwanted joke, comment or stare,” Ms. Dry said.
Others posted comments raising awareness of the tragic passing of Rio Allred, a 12-year-old girl with alopecia who recently died by suicide.
Rio Allred is said to have endured serious bullying at school, with classmates pulling off her wig and smacking her head, according to the Canadian Alopecia Areata Foundation.
It’s common for those who have hair loss conditions to feel helpless, and sometimes confused, said Amy McMichael, MD, a professor and chair of the dermatology department at Wake Forest University, Winston-Salem, N.C. That’s why it’s critical for those people to see a board-certified dermatologist, so they know they are not alone.
“As dermatologists, we can not only diagnose the type of alopecia, but we can also render treatment,” Dr. McMichael said.
Alopecia awareness
Dermatologists can also help connect patients to organizations that address the physical and emotional struggles of those who have hair loss, such as the National Alopecia Areata Foundation and the Scarring Alopecia Foundation, Dr. McMichael said.
She hopes the event shows people the “many faces of hair loss” and shows that these conditions can happen to people of all ages, ethnicities, and genders.
The National Alopecia Areata Foundation calls what happened at the Oscars a “teachable” moment.
“We encourage both our community and the broader public to learn more about alopecia areata so we can end the stigma around this disease,” the organization said in a statement.
Dr. Goh said that anyone with hair loss should feel free to explore potential medical causes and, if needed, seek out mental health treatment, too.
A version of this article first appeared on WebMD.com.
The Academy Awards ceremony on March 27 is a buzzing topic of conversation.
Troy Kotsur became the first deaf man to win an Oscar – and the highly coveted best supporting actor award, at that.
But it was what happened afterward that arguably stole the show.
Viewers and audience members alike watched in awe as actor Will Smith marched on stage and struck award presenter and comedian Chris Rock in the face after he directed a joke at Smith’s wife, Jada Pinkett Smith, for her shaved head.
and can lead to feelings of depression or mental illness.
About 700,000 people in the United States have alopecia areata, according to a 2020 study. Of them, slightly more than half are women, and more than 77% are White.
Shortly after the awards show, the Los Angeles Police Department released a statement saying it was aware of the incident and Mr. Rock had not pressed charges against Mr. Smith.
The incident set social media ablaze, and strong sentiments were heard from those who have been personally affected by alopecia.
Illness is never funny
Mr. Rock’s comment can be triggering to the millions who have been affected by hair loss, said Carolyn Goh, MD, a dermatologist at UCLA Health.
“As someone with alopecia myself, I consider it a microaggression,” Dr. Goh said. “I’ve experienced many similar comments. These build up over time and wear us down.”
One U.K.-based Instagram user, Kitty Dry, said the expression on Ms. Pinkett Smith’s face represented the hurt felt by so many with this condition.
“I want to preface this post by saying that in no way do I condone any sort of violence, but thank you Will Smith,” said Ms. Dry, 23, who was diagnosed with alopecia universalis after losing all her hair in 12 weeks.
“That slap was for anyone with alopecia who has ever been at the butt of an unwanted joke, comment or stare,” Ms. Dry said.
Others posted comments raising awareness of the tragic passing of Rio Allred, a 12-year-old girl with alopecia who recently died by suicide.
Rio Allred is said to have endured serious bullying at school, with classmates pulling off her wig and smacking her head, according to the Canadian Alopecia Areata Foundation.
It’s common for those who have hair loss conditions to feel helpless, and sometimes confused, said Amy McMichael, MD, a professor and chair of the dermatology department at Wake Forest University, Winston-Salem, N.C. That’s why it’s critical for those people to see a board-certified dermatologist, so they know they are not alone.
“As dermatologists, we can not only diagnose the type of alopecia, but we can also render treatment,” Dr. McMichael said.
Alopecia awareness
Dermatologists can also help connect patients to organizations that address the physical and emotional struggles of those who have hair loss, such as the National Alopecia Areata Foundation and the Scarring Alopecia Foundation, Dr. McMichael said.
She hopes the event shows people the “many faces of hair loss” and shows that these conditions can happen to people of all ages, ethnicities, and genders.
The National Alopecia Areata Foundation calls what happened at the Oscars a “teachable” moment.
“We encourage both our community and the broader public to learn more about alopecia areata so we can end the stigma around this disease,” the organization said in a statement.
Dr. Goh said that anyone with hair loss should feel free to explore potential medical causes and, if needed, seek out mental health treatment, too.
A version of this article first appeared on WebMD.com.
The Academy Awards ceremony on March 27 is a buzzing topic of conversation.
Troy Kotsur became the first deaf man to win an Oscar – and the highly coveted best supporting actor award, at that.
But it was what happened afterward that arguably stole the show.
Viewers and audience members alike watched in awe as actor Will Smith marched on stage and struck award presenter and comedian Chris Rock in the face after he directed a joke at Smith’s wife, Jada Pinkett Smith, for her shaved head.
and can lead to feelings of depression or mental illness.
About 700,000 people in the United States have alopecia areata, according to a 2020 study. Of them, slightly more than half are women, and more than 77% are White.
Shortly after the awards show, the Los Angeles Police Department released a statement saying it was aware of the incident and Mr. Rock had not pressed charges against Mr. Smith.
The incident set social media ablaze, and strong sentiments were heard from those who have been personally affected by alopecia.
Illness is never funny
Mr. Rock’s comment can be triggering to the millions who have been affected by hair loss, said Carolyn Goh, MD, a dermatologist at UCLA Health.
“As someone with alopecia myself, I consider it a microaggression,” Dr. Goh said. “I’ve experienced many similar comments. These build up over time and wear us down.”
One U.K.-based Instagram user, Kitty Dry, said the expression on Ms. Pinkett Smith’s face represented the hurt felt by so many with this condition.
“I want to preface this post by saying that in no way do I condone any sort of violence, but thank you Will Smith,” said Ms. Dry, 23, who was diagnosed with alopecia universalis after losing all her hair in 12 weeks.
“That slap was for anyone with alopecia who has ever been at the butt of an unwanted joke, comment or stare,” Ms. Dry said.
Others posted comments raising awareness of the tragic passing of Rio Allred, a 12-year-old girl with alopecia who recently died by suicide.
Rio Allred is said to have endured serious bullying at school, with classmates pulling off her wig and smacking her head, according to the Canadian Alopecia Areata Foundation.
It’s common for those who have hair loss conditions to feel helpless, and sometimes confused, said Amy McMichael, MD, a professor and chair of the dermatology department at Wake Forest University, Winston-Salem, N.C. That’s why it’s critical for those people to see a board-certified dermatologist, so they know they are not alone.
“As dermatologists, we can not only diagnose the type of alopecia, but we can also render treatment,” Dr. McMichael said.
Alopecia awareness
Dermatologists can also help connect patients to organizations that address the physical and emotional struggles of those who have hair loss, such as the National Alopecia Areata Foundation and the Scarring Alopecia Foundation, Dr. McMichael said.
She hopes the event shows people the “many faces of hair loss” and shows that these conditions can happen to people of all ages, ethnicities, and genders.
The National Alopecia Areata Foundation calls what happened at the Oscars a “teachable” moment.
“We encourage both our community and the broader public to learn more about alopecia areata so we can end the stigma around this disease,” the organization said in a statement.
Dr. Goh said that anyone with hair loss should feel free to explore potential medical causes and, if needed, seek out mental health treatment, too.
A version of this article first appeared on WebMD.com.