User login
Concurrent Beau Lines, Onychomadesis, and Retronychia Following Scurvy
Beau lines are palpable transverse depressions on the dorsal aspect of the nail plate that result from a temporary slowing of nail plate production by the proximal nail matrix. Onychomadesis is a separation of the proximal nail plate from the distal nail plate leading to shedding of the nail. It occurs due to a complete growth arrest in the nail matrix and is thought to be on a continuum with Beau lines. The etiologies of these 2 conditions overlap and include trauma, inflammatory diseases, systemic illnesses, hereditary conditions, and infections.1-5 In almost all cases of both conditions, normal nail plate production ensues upon identification and removal of the inciting agent or recuperation from the causal illness.3,4,6 Beau lines will move distally as the nail grows out and can be clipped. In onychomadesis, the affected nails will be shed with time. Resolution of these nail defects can be estimated from average nail growth rates (1 mm/mo for fingernails and 2–3 mm/mo for toenails).7
Retronychia is defined as a proximal ingrowing of the nail plate into the ventral surface of the proximal nail fold.4,6 It is thought to occur via vertical progression of the nail plate into the proximal nail fold, repetitive nail matrix trauma, or shearing forces, resulting in inflammation that leads to nail plate stacking.8,9 Although conservative treatment using topical corticosteroids may be attempted, proximal nail plate avulsion typically is required for treatment.10
Braswell et al1 suggested a unifying hypothesis for a common pathophysiologic basis for these 3 conditions; that is, nail matrix injury results in slowing and/or cessation of nail plate production, followed by recommencement of nail plate production by the nail matrix after removal of the insult. We report a case of a patient presenting with concurrent Beau lines, onychomadesis, and retronychia following scurvy, thus supporting the hypothesis that these 3 nail conditions lie on a continuum.
Case Report
A 41-year-old woman with a history of thyroiditis, gastroesophageal reflux disease, endometriosis, osteoarthritis, gastric ulcer, pancreatitis, fatty liver, and polycystic ovarian syndrome presented with lines on the toenails and no growth of the right second toenail of several months’ duration. She denied any pain or prior trauma to the nails, participation in sports activities, or wearing tight or high-heeled shoes. She had presented 6 months prior for evaluation of perifollicular erythema on the posterior thighs, legs, and abdomen, as well as gingival bleeding.11 At that time, one of the authors (S.R.L.) found that she was vitamin C deficient, and a diagnosis of scurvy was made. The rash and gingival bleeding resolved with vitamin C supplementation.11
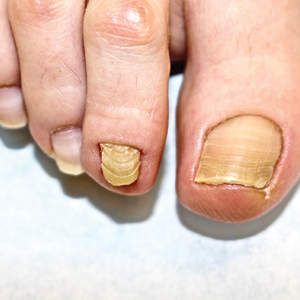
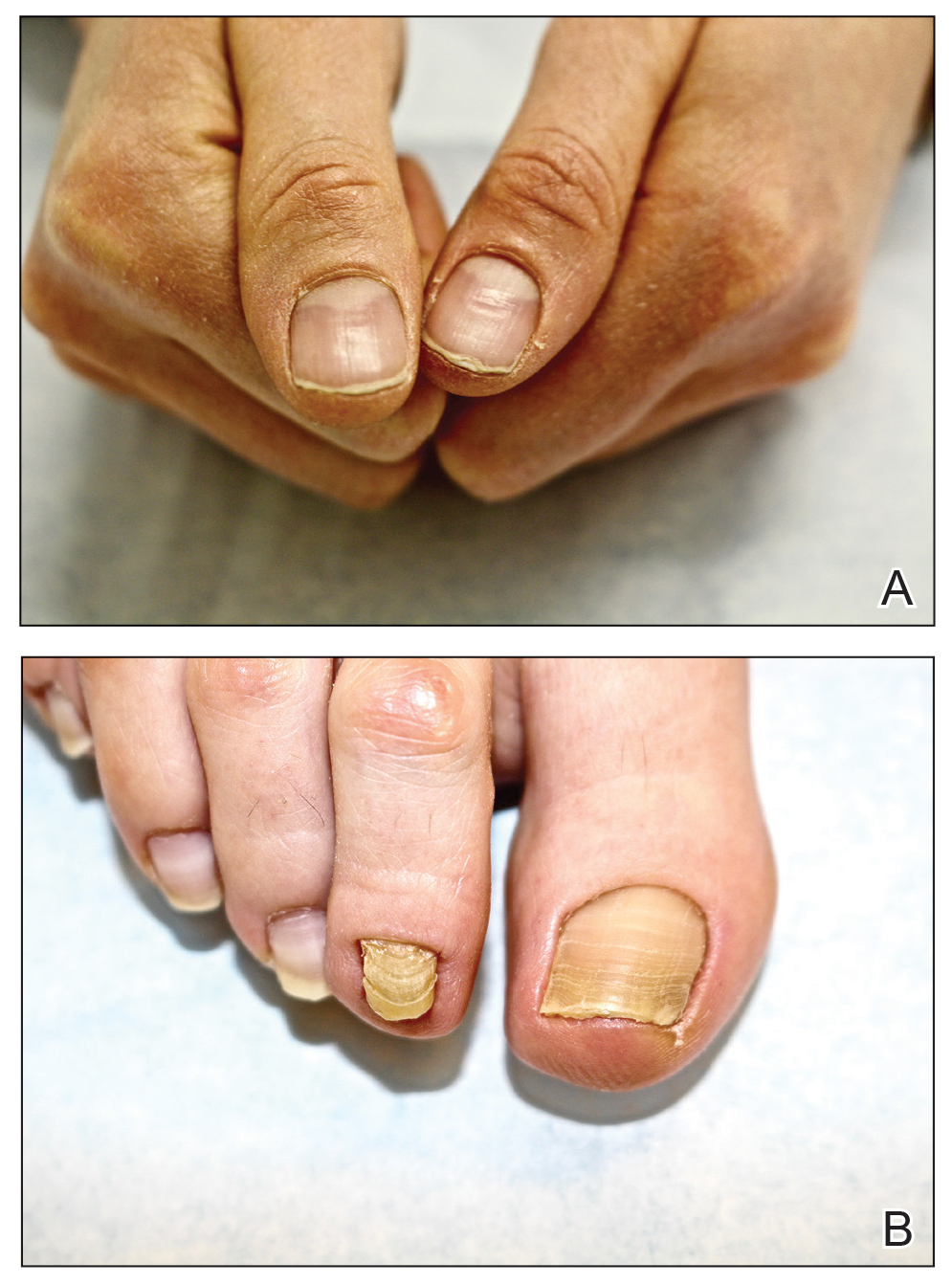
At the current presentation, physical examination revealed transverse grooves involving several fingernails but most evident on the left thumbnail (Figure, A). The grooves did not span the entire breadth of the nail, which was consistent with Beau lines. Several toenails had parallel transverse grooves spanning the entire width of the nail plate such that the proximal nail plate was discontinuous with the distal nail plate, which was consistent with onychomadesis (Figure, B). The right second toenail was yellow and thickened with layered nail plates, indicative of retronychia (Figure, B). Histopathology of a nail plate clipping from the right second toenail was negative for fungal hyphae, and a radiograph was negative for bony changes or exostosis.
Comment
The nail matrix is responsible for nail plate production, and the newly formed nail plate then moves outward over the nail bed. It is hypothesized that the pathophysiologic basis for Beau lines, onychomadesis, and retronychia lies on a continuum such that all 3 conditions are caused by an insult to the nail matrix that results in slowing and/or halting of nail plate growth. Beau lines result from slowing or disruption in cell growth from the nail matrix, whereas onychomadesis is associated with a complete halt in nail plate production.1,3 In retronychia, the new nail growing from the matrix pushes the old one upward, interrupting the longitudinal growth of the nail and leading to nail plate stacking.10
Our patient presented with concurrent Beau lines, onychomadesis, and retronychia. Although Beau lines and onychomadesis have been reported together in some instances,12-14 retronychia is not commonly reported with either of these conditions. The exact incidence of each condition has not been studied, but Beau lines are relatively common, onychomadesis is less common, and retronychia is seen infrequently; therefore, the concurrent presentation of these 3 conditions in the same patient is exceedingly rare. Thus, it was most likely that one etiology accounted for all 3 nail findings.
Because the patient had been diagnosed with scurvy 6 months prior to presentation, we hypothesized that the associated vitamin C deficiency caused a systemic insult to the nail matrix, which resulted in cessation of nail growth. The mechanism of nail matrix arrest in the setting of systemic disease is thought to be due to inhibition of cellular proliferation or a change in the quality of the newly manufactured nail plate, which becomes thinner and more dystrophic.15 Vitamin C (ascorbic acid) deficiency causes scurvy, which is characterized by cutaneous signs such as perifollicular hemorrhage and purpura, corkscrew hairs, bruising, gingivitis, arthralgia, and impaired wound healing.16 These clinical manifestations are due to impaired collagen synthesis and disordered connective tissue. Ascorbic acid also is involved in fatty acid transport, neurotransmitter synthesis, prostaglandin metabolism, and nitric oxide synthesis.17 Ascorbic acid has not been studied for its role in nail plate synthesis18; however, given the role that ascorbic acid plays in a myriad of biologic processes, the deficiency associated with scurvy likely had a considerable systemic effect in our patient that halted nail plate synthesis and resulted in the concurrent presentation of Beau lines, onychomadesis, and retronychia.
- Braswell MA, Daniel CR III, Brodell RT. Beau lines, onychomadesis, and retronychia: a unifying hypothesis. J Am Acad Dermatol. 2015;73:849-855.
- Lipner SR. Onychomadesis following a fish pedicure. JAMA Dermatol. 2018;154:1091-1092.
- Bettoli V, Zauli S, Toni G, et al. Onychomadesis following hand, foot, and mouth disease: a case report from Italy and review of the literature. Int J Dermatol. 2013;52:728-730.
- Lawry M, Daniel CR III. Nails in systemic disease. In: Scher RK, Daniel CR III, eds. Nails: Diagnosis, Therapy, Surgery. 3rd ed. Oxford, England: Elsevier Saunders; 2005:147-176.
- Lipner SR, Scher RK. Evaluation of nail lines: color and shape hold clues. Cleve Clin J Med. 2016;83:385.
- Rich P. Nail signs and symptoms. In: Scher RK, Daniel CR III, eds. Nails: Diagnosis, Therapy, Surgery. 3rd ed. Oxford, England: Elsevier Saunders; 2005:1-6.
- Lipner SR, Scher RK. Nail growth evaluation and factors affecting nail growth. In: Humbert P, Fanian F, Maibach H, et al, eds. Agache’s Measuring the Skin. Cham, Switzerland: Springer; 2017:1-15.
- de Berker DA, Richert B, Duhard E, et al. Retronychia: proximal ingrowing of the nail plate. J Am Acad Dermatol. 2008;58:978-983.
- Wortsman X, Wortsman J, Guerrero R, et al. Anatomical changes in retronychia and onychomadesis detected using ultrasound. Dermatol Surg. 2010;36:1615-1620.
- Piraccini BM, Richert B, de Berker DA, et al. Retronychia in children, adolescents, and young adults: a case series. J Am Acad Dermatol. 2014;70:388-390.
- Lipner S. A classic case of scurvy. Lancet. 2018;392:431.
- Jacobsen L, Zimmerman S, Lohr J. Nail findings in hand-foot-and-mouth disease. Pediatr Infect Dis J. 2015;34:449-450.
- Damevska K, Gocev G, Pollozhani N, et al. Onychomadesis following cutaneous vasculitis. Acta Dermatovenerol Croat. 2017;25:77-79.
- Clementz GC, Mancini AJ. Nail matrix arrest following hand‐foot‐mouth disease: a report of five children. Pediatr Dermatol. 2000;17:7-11.
- Weismann K. J.H.S Beau and his descriptions of transverse depressions on nails. Br J Dermatol. 1977;97:571-572.
- Abdullah M, Jamil RT, Attia FN. Vitamin C (ascorbic acid). Treasure Island, FL: StatPearls Publishing; 2019. https://www.ncbi.nlm.nih.gov/books/NBK499877/. Updated October 21, 2019. Accessed February 24, 2020.
- Pazirandeh S, Burns DL. Overview of water-soluble vitamins. UpToDate. https://www.uptodate.com/contents/overview-of-water-soluble-vitamins. Updated January 29, 2020. Accessed February 24, 2020.
- Scheinfeld N, Dahdah MJ, Scher RK. Vitamins and minerals: their role in nail health and disease. J Drugs Dermatol. 2007;6:782-787.
Beau lines are palpable transverse depressions on the dorsal aspect of the nail plate that result from a temporary slowing of nail plate production by the proximal nail matrix. Onychomadesis is a separation of the proximal nail plate from the distal nail plate leading to shedding of the nail. It occurs due to a complete growth arrest in the nail matrix and is thought to be on a continuum with Beau lines. The etiologies of these 2 conditions overlap and include trauma, inflammatory diseases, systemic illnesses, hereditary conditions, and infections.1-5 In almost all cases of both conditions, normal nail plate production ensues upon identification and removal of the inciting agent or recuperation from the causal illness.3,4,6 Beau lines will move distally as the nail grows out and can be clipped. In onychomadesis, the affected nails will be shed with time. Resolution of these nail defects can be estimated from average nail growth rates (1 mm/mo for fingernails and 2–3 mm/mo for toenails).7
Retronychia is defined as a proximal ingrowing of the nail plate into the ventral surface of the proximal nail fold.4,6 It is thought to occur via vertical progression of the nail plate into the proximal nail fold, repetitive nail matrix trauma, or shearing forces, resulting in inflammation that leads to nail plate stacking.8,9 Although conservative treatment using topical corticosteroids may be attempted, proximal nail plate avulsion typically is required for treatment.10
Braswell et al1 suggested a unifying hypothesis for a common pathophysiologic basis for these 3 conditions; that is, nail matrix injury results in slowing and/or cessation of nail plate production, followed by recommencement of nail plate production by the nail matrix after removal of the insult. We report a case of a patient presenting with concurrent Beau lines, onychomadesis, and retronychia following scurvy, thus supporting the hypothesis that these 3 nail conditions lie on a continuum.
Case Report
A 41-year-old woman with a history of thyroiditis, gastroesophageal reflux disease, endometriosis, osteoarthritis, gastric ulcer, pancreatitis, fatty liver, and polycystic ovarian syndrome presented with lines on the toenails and no growth of the right second toenail of several months’ duration. She denied any pain or prior trauma to the nails, participation in sports activities, or wearing tight or high-heeled shoes. She had presented 6 months prior for evaluation of perifollicular erythema on the posterior thighs, legs, and abdomen, as well as gingival bleeding.11 At that time, one of the authors (S.R.L.) found that she was vitamin C deficient, and a diagnosis of scurvy was made. The rash and gingival bleeding resolved with vitamin C supplementation.11
At the current presentation, physical examination revealed transverse grooves involving several fingernails but most evident on the left thumbnail (Figure, A). The grooves did not span the entire breadth of the nail, which was consistent with Beau lines. Several toenails had parallel transverse grooves spanning the entire width of the nail plate such that the proximal nail plate was discontinuous with the distal nail plate, which was consistent with onychomadesis (Figure, B). The right second toenail was yellow and thickened with layered nail plates, indicative of retronychia (Figure, B). Histopathology of a nail plate clipping from the right second toenail was negative for fungal hyphae, and a radiograph was negative for bony changes or exostosis.

Comment
The nail matrix is responsible for nail plate production, and the newly formed nail plate then moves outward over the nail bed. It is hypothesized that the pathophysiologic basis for Beau lines, onychomadesis, and retronychia lies on a continuum such that all 3 conditions are caused by an insult to the nail matrix that results in slowing and/or halting of nail plate growth. Beau lines result from slowing or disruption in cell growth from the nail matrix, whereas onychomadesis is associated with a complete halt in nail plate production.1,3 In retronychia, the new nail growing from the matrix pushes the old one upward, interrupting the longitudinal growth of the nail and leading to nail plate stacking.10
Our patient presented with concurrent Beau lines, onychomadesis, and retronychia. Although Beau lines and onychomadesis have been reported together in some instances,12-14 retronychia is not commonly reported with either of these conditions. The exact incidence of each condition has not been studied, but Beau lines are relatively common, onychomadesis is less common, and retronychia is seen infrequently; therefore, the concurrent presentation of these 3 conditions in the same patient is exceedingly rare. Thus, it was most likely that one etiology accounted for all 3 nail findings.
Because the patient had been diagnosed with scurvy 6 months prior to presentation, we hypothesized that the associated vitamin C deficiency caused a systemic insult to the nail matrix, which resulted in cessation of nail growth. The mechanism of nail matrix arrest in the setting of systemic disease is thought to be due to inhibition of cellular proliferation or a change in the quality of the newly manufactured nail plate, which becomes thinner and more dystrophic.15 Vitamin C (ascorbic acid) deficiency causes scurvy, which is characterized by cutaneous signs such as perifollicular hemorrhage and purpura, corkscrew hairs, bruising, gingivitis, arthralgia, and impaired wound healing.16 These clinical manifestations are due to impaired collagen synthesis and disordered connective tissue. Ascorbic acid also is involved in fatty acid transport, neurotransmitter synthesis, prostaglandin metabolism, and nitric oxide synthesis.17 Ascorbic acid has not been studied for its role in nail plate synthesis18; however, given the role that ascorbic acid plays in a myriad of biologic processes, the deficiency associated with scurvy likely had a considerable systemic effect in our patient that halted nail plate synthesis and resulted in the concurrent presentation of Beau lines, onychomadesis, and retronychia.
Beau lines are palpable transverse depressions on the dorsal aspect of the nail plate that result from a temporary slowing of nail plate production by the proximal nail matrix. Onychomadesis is a separation of the proximal nail plate from the distal nail plate leading to shedding of the nail. It occurs due to a complete growth arrest in the nail matrix and is thought to be on a continuum with Beau lines. The etiologies of these 2 conditions overlap and include trauma, inflammatory diseases, systemic illnesses, hereditary conditions, and infections.1-5 In almost all cases of both conditions, normal nail plate production ensues upon identification and removal of the inciting agent or recuperation from the causal illness.3,4,6 Beau lines will move distally as the nail grows out and can be clipped. In onychomadesis, the affected nails will be shed with time. Resolution of these nail defects can be estimated from average nail growth rates (1 mm/mo for fingernails and 2–3 mm/mo for toenails).7
Retronychia is defined as a proximal ingrowing of the nail plate into the ventral surface of the proximal nail fold.4,6 It is thought to occur via vertical progression of the nail plate into the proximal nail fold, repetitive nail matrix trauma, or shearing forces, resulting in inflammation that leads to nail plate stacking.8,9 Although conservative treatment using topical corticosteroids may be attempted, proximal nail plate avulsion typically is required for treatment.10
Braswell et al1 suggested a unifying hypothesis for a common pathophysiologic basis for these 3 conditions; that is, nail matrix injury results in slowing and/or cessation of nail plate production, followed by recommencement of nail plate production by the nail matrix after removal of the insult. We report a case of a patient presenting with concurrent Beau lines, onychomadesis, and retronychia following scurvy, thus supporting the hypothesis that these 3 nail conditions lie on a continuum.
Case Report
A 41-year-old woman with a history of thyroiditis, gastroesophageal reflux disease, endometriosis, osteoarthritis, gastric ulcer, pancreatitis, fatty liver, and polycystic ovarian syndrome presented with lines on the toenails and no growth of the right second toenail of several months’ duration. She denied any pain or prior trauma to the nails, participation in sports activities, or wearing tight or high-heeled shoes. She had presented 6 months prior for evaluation of perifollicular erythema on the posterior thighs, legs, and abdomen, as well as gingival bleeding.11 At that time, one of the authors (S.R.L.) found that she was vitamin C deficient, and a diagnosis of scurvy was made. The rash and gingival bleeding resolved with vitamin C supplementation.11
At the current presentation, physical examination revealed transverse grooves involving several fingernails but most evident on the left thumbnail (Figure, A). The grooves did not span the entire breadth of the nail, which was consistent with Beau lines. Several toenails had parallel transverse grooves spanning the entire width of the nail plate such that the proximal nail plate was discontinuous with the distal nail plate, which was consistent with onychomadesis (Figure, B). The right second toenail was yellow and thickened with layered nail plates, indicative of retronychia (Figure, B). Histopathology of a nail plate clipping from the right second toenail was negative for fungal hyphae, and a radiograph was negative for bony changes or exostosis.

Comment
The nail matrix is responsible for nail plate production, and the newly formed nail plate then moves outward over the nail bed. It is hypothesized that the pathophysiologic basis for Beau lines, onychomadesis, and retronychia lies on a continuum such that all 3 conditions are caused by an insult to the nail matrix that results in slowing and/or halting of nail plate growth. Beau lines result from slowing or disruption in cell growth from the nail matrix, whereas onychomadesis is associated with a complete halt in nail plate production.1,3 In retronychia, the new nail growing from the matrix pushes the old one upward, interrupting the longitudinal growth of the nail and leading to nail plate stacking.10
Our patient presented with concurrent Beau lines, onychomadesis, and retronychia. Although Beau lines and onychomadesis have been reported together in some instances,12-14 retronychia is not commonly reported with either of these conditions. The exact incidence of each condition has not been studied, but Beau lines are relatively common, onychomadesis is less common, and retronychia is seen infrequently; therefore, the concurrent presentation of these 3 conditions in the same patient is exceedingly rare. Thus, it was most likely that one etiology accounted for all 3 nail findings.
Because the patient had been diagnosed with scurvy 6 months prior to presentation, we hypothesized that the associated vitamin C deficiency caused a systemic insult to the nail matrix, which resulted in cessation of nail growth. The mechanism of nail matrix arrest in the setting of systemic disease is thought to be due to inhibition of cellular proliferation or a change in the quality of the newly manufactured nail plate, which becomes thinner and more dystrophic.15 Vitamin C (ascorbic acid) deficiency causes scurvy, which is characterized by cutaneous signs such as perifollicular hemorrhage and purpura, corkscrew hairs, bruising, gingivitis, arthralgia, and impaired wound healing.16 These clinical manifestations are due to impaired collagen synthesis and disordered connective tissue. Ascorbic acid also is involved in fatty acid transport, neurotransmitter synthesis, prostaglandin metabolism, and nitric oxide synthesis.17 Ascorbic acid has not been studied for its role in nail plate synthesis18; however, given the role that ascorbic acid plays in a myriad of biologic processes, the deficiency associated with scurvy likely had a considerable systemic effect in our patient that halted nail plate synthesis and resulted in the concurrent presentation of Beau lines, onychomadesis, and retronychia.
- Braswell MA, Daniel CR III, Brodell RT. Beau lines, onychomadesis, and retronychia: a unifying hypothesis. J Am Acad Dermatol. 2015;73:849-855.
- Lipner SR. Onychomadesis following a fish pedicure. JAMA Dermatol. 2018;154:1091-1092.
- Bettoli V, Zauli S, Toni G, et al. Onychomadesis following hand, foot, and mouth disease: a case report from Italy and review of the literature. Int J Dermatol. 2013;52:728-730.
- Lawry M, Daniel CR III. Nails in systemic disease. In: Scher RK, Daniel CR III, eds. Nails: Diagnosis, Therapy, Surgery. 3rd ed. Oxford, England: Elsevier Saunders; 2005:147-176.
- Lipner SR, Scher RK. Evaluation of nail lines: color and shape hold clues. Cleve Clin J Med. 2016;83:385.
- Rich P. Nail signs and symptoms. In: Scher RK, Daniel CR III, eds. Nails: Diagnosis, Therapy, Surgery. 3rd ed. Oxford, England: Elsevier Saunders; 2005:1-6.
- Lipner SR, Scher RK. Nail growth evaluation and factors affecting nail growth. In: Humbert P, Fanian F, Maibach H, et al, eds. Agache’s Measuring the Skin. Cham, Switzerland: Springer; 2017:1-15.
- de Berker DA, Richert B, Duhard E, et al. Retronychia: proximal ingrowing of the nail plate. J Am Acad Dermatol. 2008;58:978-983.
- Wortsman X, Wortsman J, Guerrero R, et al. Anatomical changes in retronychia and onychomadesis detected using ultrasound. Dermatol Surg. 2010;36:1615-1620.
- Piraccini BM, Richert B, de Berker DA, et al. Retronychia in children, adolescents, and young adults: a case series. J Am Acad Dermatol. 2014;70:388-390.
- Lipner S. A classic case of scurvy. Lancet. 2018;392:431.
- Jacobsen L, Zimmerman S, Lohr J. Nail findings in hand-foot-and-mouth disease. Pediatr Infect Dis J. 2015;34:449-450.
- Damevska K, Gocev G, Pollozhani N, et al. Onychomadesis following cutaneous vasculitis. Acta Dermatovenerol Croat. 2017;25:77-79.
- Clementz GC, Mancini AJ. Nail matrix arrest following hand‐foot‐mouth disease: a report of five children. Pediatr Dermatol. 2000;17:7-11.
- Weismann K. J.H.S Beau and his descriptions of transverse depressions on nails. Br J Dermatol. 1977;97:571-572.
- Abdullah M, Jamil RT, Attia FN. Vitamin C (ascorbic acid). Treasure Island, FL: StatPearls Publishing; 2019. https://www.ncbi.nlm.nih.gov/books/NBK499877/. Updated October 21, 2019. Accessed February 24, 2020.
- Pazirandeh S, Burns DL. Overview of water-soluble vitamins. UpToDate. https://www.uptodate.com/contents/overview-of-water-soluble-vitamins. Updated January 29, 2020. Accessed February 24, 2020.
- Scheinfeld N, Dahdah MJ, Scher RK. Vitamins and minerals: their role in nail health and disease. J Drugs Dermatol. 2007;6:782-787.
- Braswell MA, Daniel CR III, Brodell RT. Beau lines, onychomadesis, and retronychia: a unifying hypothesis. J Am Acad Dermatol. 2015;73:849-855.
- Lipner SR. Onychomadesis following a fish pedicure. JAMA Dermatol. 2018;154:1091-1092.
- Bettoli V, Zauli S, Toni G, et al. Onychomadesis following hand, foot, and mouth disease: a case report from Italy and review of the literature. Int J Dermatol. 2013;52:728-730.
- Lawry M, Daniel CR III. Nails in systemic disease. In: Scher RK, Daniel CR III, eds. Nails: Diagnosis, Therapy, Surgery. 3rd ed. Oxford, England: Elsevier Saunders; 2005:147-176.
- Lipner SR, Scher RK. Evaluation of nail lines: color and shape hold clues. Cleve Clin J Med. 2016;83:385.
- Rich P. Nail signs and symptoms. In: Scher RK, Daniel CR III, eds. Nails: Diagnosis, Therapy, Surgery. 3rd ed. Oxford, England: Elsevier Saunders; 2005:1-6.
- Lipner SR, Scher RK. Nail growth evaluation and factors affecting nail growth. In: Humbert P, Fanian F, Maibach H, et al, eds. Agache’s Measuring the Skin. Cham, Switzerland: Springer; 2017:1-15.
- de Berker DA, Richert B, Duhard E, et al. Retronychia: proximal ingrowing of the nail plate. J Am Acad Dermatol. 2008;58:978-983.
- Wortsman X, Wortsman J, Guerrero R, et al. Anatomical changes in retronychia and onychomadesis detected using ultrasound. Dermatol Surg. 2010;36:1615-1620.
- Piraccini BM, Richert B, de Berker DA, et al. Retronychia in children, adolescents, and young adults: a case series. J Am Acad Dermatol. 2014;70:388-390.
- Lipner S. A classic case of scurvy. Lancet. 2018;392:431.
- Jacobsen L, Zimmerman S, Lohr J. Nail findings in hand-foot-and-mouth disease. Pediatr Infect Dis J. 2015;34:449-450.
- Damevska K, Gocev G, Pollozhani N, et al. Onychomadesis following cutaneous vasculitis. Acta Dermatovenerol Croat. 2017;25:77-79.
- Clementz GC, Mancini AJ. Nail matrix arrest following hand‐foot‐mouth disease: a report of five children. Pediatr Dermatol. 2000;17:7-11.
- Weismann K. J.H.S Beau and his descriptions of transverse depressions on nails. Br J Dermatol. 1977;97:571-572.
- Abdullah M, Jamil RT, Attia FN. Vitamin C (ascorbic acid). Treasure Island, FL: StatPearls Publishing; 2019. https://www.ncbi.nlm.nih.gov/books/NBK499877/. Updated October 21, 2019. Accessed February 24, 2020.
- Pazirandeh S, Burns DL. Overview of water-soluble vitamins. UpToDate. https://www.uptodate.com/contents/overview-of-water-soluble-vitamins. Updated January 29, 2020. Accessed February 24, 2020.
- Scheinfeld N, Dahdah MJ, Scher RK. Vitamins and minerals: their role in nail health and disease. J Drugs Dermatol. 2007;6:782-787.
Practice Points
- Beau lines, onychomadesis, and retronychia are nail conditions with distinct clinical findings.
- Beau lines and onychomadesis may be seen concurrently following trauma, inflammatory diseases, systemic illnesses, hereditary conditions, and infections.
- Retronychia shares a common pathophysiology with Beau lines and onychomadesis, and all reflect slowing or cessation of nail plate production.
What’s Eating You? Human Body Lice (Pediculus humanus corporis)
Epidemiology and Transmission
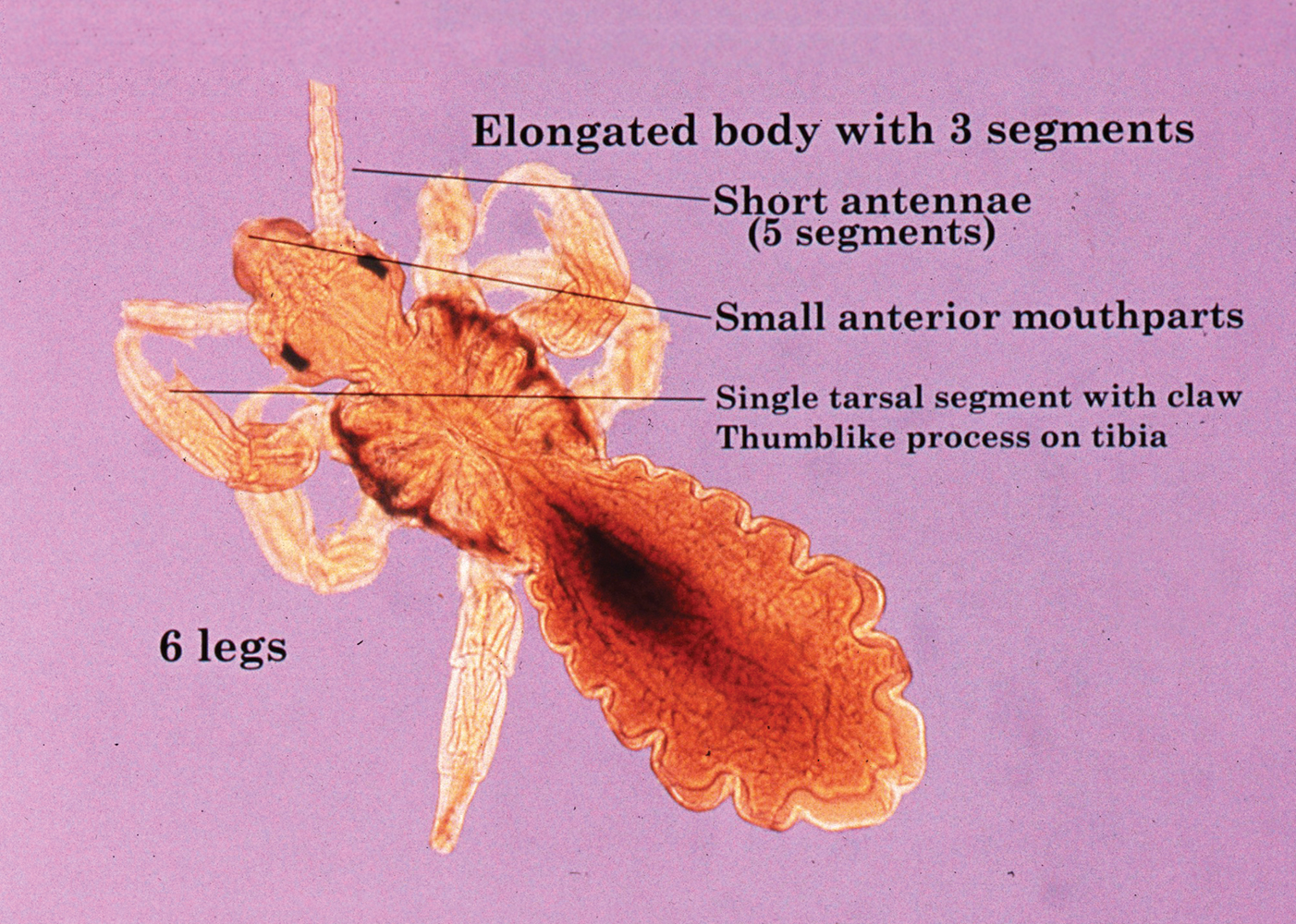
Pediculus humanus corporis, commonly known as the human body louse, is one in a family of 3 ectoparasites of the same suborder that also encompasses pubic lice (Phthirus pubis) and head lice (Pediculus humanus capitis). Adults are approximately 2 mm in size, with the same life cycle as head lice (Figure 1). They require blood meals roughly 5 times per day and cannot survive longer than 2 days without feeding.1 Although similar in structure to head lice, body lice differ behaviorally in that they do not reside on their human host’s body; instead, they infest the host’s clothing, localizing to seams (Figure 2), and migrate to the host for blood meals. In fact, based on this behavior, genetic analysis of early human body lice has been used to postulate when clothing was first used by humans as well as to determine early human migration patterns.2,3

Although clinicians in developed countries may be less familiar with body lice compared to their counterparts, body lice nevertheless remain a global health concern in impoverished, densely populated areas, as well as in homeless populations due to poor hygiene. Transmission frequently occurs via physical contact with an affected individual and his/her personal items (eg, linens) via fomites.4,5 Body louse infestation is more prevalent in homeless individuals who sleep outside vs in shelters; a history of pubic lice and lack of regular bathing have been reported as additional risk factors.6 Outbreaks have been noted in the wake of natural disasters, in the setting of political upheavals, and in refugee camps, as well as in individuals seeking political asylum.7 Unlike head and pubic lice, body lice can serve as vectors for infectious diseases including Rickettsia prowazekii (epidemic typhus), Borrelia recurrentis (louse-borne relapsing fever), Bartonella quintana (trench fever), and Yersinia pestis (plague).5,8,9 Several Acinetobacter species were isolated from nearly one-third of collected body louse specimens in a French study.10 Additionally, serology for B quintana was found to be positive in up to 30% of cases in one United States urban homeless population.4
Clinical Manifestations
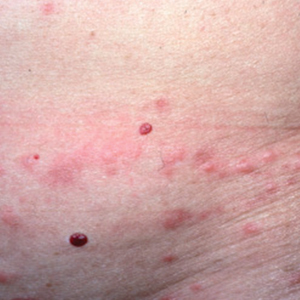
Patients often present with generalized pruritus, usually considerably more severe than with P humanus capitis, with lesions concentrated on the trunk.11 In addition to often impetiginized, self-inflicted excoriations, feeding sites may present as erythematous macules (Figure 3), papules, or papular urticaria with a central hemorrhagic punctum. Extensive infestation also can manifest as the colloquial vagabond disease, characterized by postinflammatory hyperpigmentation and thickening of the involved skin. Remarkably, patients also may present with considerable iron-deficiency anemia secondary to high parasite load and large volume blood feeding. Multiple case reports have demonstrated associated morbidity.12-14 The differential diagnosis for pediculosis may include scabies, lichen simplex chronicus, and eczematous dermatitis, though the clinician should prudently consider whether both scabies and pediculosis may be present, as coexistence is possible.4,15

Diagnosis
Diagnosis can be reached by visualizing adult lice, nymphs, or viable nits on the body or more commonly within inner clothing seams; nits also fluoresce under Wood light.15 Although dermoscopy has proven useful for increased sensitivity and differentiation between viable and hatched nits, the insects also can be viewed with the unaided eye.16
Treatment: New Concerns and Strategies
The mainstay of treatment for body lice has long consisted of thorough washing and drying of all clothing and linens in a hot dryer. Treatment can be augmented with the addition of pharmacotherapy, plus antibiotics as warranted for louse-borne disease. Pharmacologic intervention often is used in cases of mass infestation and is similar to head lice.
Options for head lice include topical permethrin, malathion, lindane, spinosad, benzyl alcohol, and ivermectin. Pyrethroids, derived from the chrysanthemum, generally are considered safe for human use with a side-effect profile limited to irritation and allergy17; however, neurotoxicity and leukemia are clinical concerns, with an association more recently shown between large-volume use of pyrethroids and acute lymphoblastic leukemia.18,19 Use of lindane is not recommended due to a greater potential for central nervous system neurotoxicity, manifested by seizures, with repeated large surface application. Malathion is problematic due to the risk for mucosal irritation, flammability of some formulations, and theoretical organophosphate poisoning, as its mechanism of action involves inhibition of acetylcholinesterase.15 However, in the context of head lice treatment, a randomized controlled trial reported no incidence of acetylcholinesterase inhibition.20 Spinosad, manufactured from the soil bacterium Saccharopolyspora spinosa, functions similarly by interfering with the nicotinic acetylcholine receptor and also carries a risk for skin irritation.21 Among all the treatment options, we prefer benzyl alcohol, particularly in the context of resistance, as it is effective via a physical mechanism of action and lacks notable neurotoxic effects to the host. Use of benzyl alcohol is approved for patients as young as 6 months; it functions by asphyxiating the lice via paralysis of the respiratory spiracle with occlusion by inert ingredients. Itching, episodic numbness, and scalp or mucosal irritation are possible complications of treatment.22
Treatment resistance of body lice has increased in recent years, warranting exploration of additional management strategies. Moreover, developing resistance to lindane and malathion has been reported.23 Resistance to pyrethroids has been attributed to mutations in a voltage-gated sodium channel, one of which was universally present in the sampling of a single population.24 A randomized controlled trial showed that off-label oral ivermectin 400 μg/kg was superior to malathion lotion 0.5% in difficult-to-treat cases of head lice25; utility of oral ivermectin also has been reported in body lice.26 In vitro studies also have shown promise for pursuing synergistic treatment of body lice with both ivermectin and antibiotics.27
A novel primary prophylaxis approach for at-risk homeless individuals recently utilized permethrin-impregnated underwear. Although the intervention provided short-term infestation improvement, longer-term use did not show improvement from placebo and also increased prevalence of permethrin-resistant haplotypes.2
- Veracx A, Raoult D. Biology and genetics of human head and body lice. Trends Parasitol. 2012;28:563-571.
- Kittler R, Kayser M, Stoneking M. Molecular evolution of Pediculus humanus and the origin of clothing. Curr Biol. 2003;13:1414-1417.
- Drali R, Mumcuoglu KY, Yesilyurt G, et al. Studies of ancient lice reveal unsuspected past migrations of vectors. Am J Trop Med Hyg. 2015;93:623-625.
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826.
- Feldmeier H, Heukelbach J. Epidermal parasitic skin diseases: a neglected category of poverty-associated plagues. Bull World Health Organ. 2009;87:152-159.
- Arnaud A, Chosidow O, Detrez MA, et al. Prevalence of scabies and Pediculosis corporis among homeless people in the Paris region: results from two randomized cross-sectional surveys (HYTPEAC study). Br J Dermatol. 2016;174:104-112.
- Hytonen J, Khawaja T, Gronroos JO, et al. Louse-borne relapsing fever in Finland in two asylum seekers from Somalia. APMIS. 2017;125:59-62.
- Nordmann T, Feldt T, Bosselmann M, et al. Outbreak of louse-borne relapsing fever among urban dwellers in Arsi Zone, Central Ethiopia, from July to November 2016. Am J Trop Med Hyg. 2018;98:1599-1602.
- Louni M, Mana N, Bitam I, et al. Body lice of homeless people reveal the presence of several emerging bacterial pathogens in northern Algeria. PLoS Negl Trop Dis. 2018;12:E0006397.
- Candy K, Amanzougaghene N, Izri A, et al. Molecular survey of head and body lice, Pediculus humanus, in France. Vector Borne Zoonotic Dis. 2018;18:243-251.
Bolognia JL, Schaffer JV, Cerroni L. Dermatology. 4th ed. Elsevier Limited; 2018. - Nara A, Nagai H, Yamaguchi R, et al. An unusual autopsy case of lethal hypothermia exacerbated by body lice-induced severe anemia. Int J Legal Med. 2016;130:765-769.
- Althomali SA, Alzubaidi LM, Alkhaldi DM. Severe iron deficiency anaemia associated with heavy lice infestation in a young woman [published online November 5, 2015]. BMJ Case Rep. doi:10.1136/bcr-2015-212207.
- Hau V, Muhi-Iddin N. A ghost covered in lice: a case of severe blood loss with long-standing heavy pediculosis capitis infestation [published online December 19, 2014]. BMJ Case Rep. doi:10.1136/bcr-2014-206623.
- Diaz JH. Lice (Pediculosis). In: Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 9th ed. New York, NY: Elsevier; 2020:3482-3486.
- Martins LG, Bernardes Filho F, Quaresma MV, et al. Dermoscopy applied to pediculosis corporis diagnosis. An Bras Dermatol. 2014;89:513-514.
- Devore CD, Schutze GE; Council on School Health and Committee on Infectious Diseases, American Academy of Pediatrics. Head lice. Pediatrics. 2015;135:E1355-E1365.
- Shafer TJ, Meyer DA, Crofton KM. Developmental neurotoxicity of pyrethroid insecticides: critical review and future research needs. Environ Health Perspect. 2005;113:123-136.
- Ding G, Shi R, Gao Y, et al. Pyrethroid pesticide exposure and risk of childhood acute lymphocytic leukemia in Shanghai. Environ Sci Technol. 2012;46:13480-13487.
- Meinking TL, Vicaria M, Eyerdam DH, et al. A randomized, investigator-blinded, time-ranging study of the comparative efficacy of 0.5% malathion gel versus Ovide Lotion (0.5% malathion) or Nix Crème Rinse (1% permethrin) used as labeled, for the treatment of head lice. Pediatr Dermatol. 2007;24:405-411.
- McCormack PL. Spinosad: in pediculosis capitis. Am J Clin Dermatol. 2011;12:349-353.
- Meinking TL, Villar ME, Vicaria M, et al. The clinical trials supporting benzyl alcohol lotion 5% (Ulesfia): a safe and effective topical treatment for head lice (pediculosis humanus capitis). Pediatr Dermatol. 2010;27:19-24.
- Lebwohl M, Clark L, Levitt J. Therapy for head lice based on life cycle, resistance, and safety considerations. Pediatrics. 2007;119:965-974
- Drali R, Benkouiten S, Badiaga S, et al. Detection of a knockdown resistance mutation associated with permethrin resistance in the body louse Pediculus humanus corporis by use of melting curve analysis genotyping. J Clin Microbiol. 2012;50:2229-2233.
- Chosidow O, Giraudeau B, Cottrell J, et al. Oral ivermectin versus malathion lotion for difficult-to-treat head lice. N Engl J Med. 2010;362:896-905.
- Foucault C, Ranque S, Badiaga S, et al. Oral ivermectin in the treatment of body lice. J Infect Dis. 2006;193:474-476.
- Sangaré AK, Doumbo OK, Raoult D. Management and treatment of human lice [published online July 27, 2016]. Biomed Res Int. doi:10.1155/2016/8962685.
- Benkouiten S, Drali R, Badiaga S, et al. Effect of permethrin-impregnated underwear on body lice in sheltered homeless persons: a randomized controlled trial. JAMA Dermatol. 2014;150:273-279.
Epidemiology and Transmission
Pediculus humanus corporis, commonly known as the human body louse, is one in a family of 3 ectoparasites of the same suborder that also encompasses pubic lice (Phthirus pubis) and head lice (Pediculus humanus capitis). Adults are approximately 2 mm in size, with the same life cycle as head lice (Figure 1). They require blood meals roughly 5 times per day and cannot survive longer than 2 days without feeding.1 Although similar in structure to head lice, body lice differ behaviorally in that they do not reside on their human host’s body; instead, they infest the host’s clothing, localizing to seams (Figure 2), and migrate to the host for blood meals. In fact, based on this behavior, genetic analysis of early human body lice has been used to postulate when clothing was first used by humans as well as to determine early human migration patterns.2,3


Although clinicians in developed countries may be less familiar with body lice compared to their counterparts, body lice nevertheless remain a global health concern in impoverished, densely populated areas, as well as in homeless populations due to poor hygiene. Transmission frequently occurs via physical contact with an affected individual and his/her personal items (eg, linens) via fomites.4,5 Body louse infestation is more prevalent in homeless individuals who sleep outside vs in shelters; a history of pubic lice and lack of regular bathing have been reported as additional risk factors.6 Outbreaks have been noted in the wake of natural disasters, in the setting of political upheavals, and in refugee camps, as well as in individuals seeking political asylum.7 Unlike head and pubic lice, body lice can serve as vectors for infectious diseases including Rickettsia prowazekii (epidemic typhus), Borrelia recurrentis (louse-borne relapsing fever), Bartonella quintana (trench fever), and Yersinia pestis (plague).5,8,9 Several Acinetobacter species were isolated from nearly one-third of collected body louse specimens in a French study.10 Additionally, serology for B quintana was found to be positive in up to 30% of cases in one United States urban homeless population.4
Clinical Manifestations
Patients often present with generalized pruritus, usually considerably more severe than with P humanus capitis, with lesions concentrated on the trunk.11 In addition to often impetiginized, self-inflicted excoriations, feeding sites may present as erythematous macules (Figure 3), papules, or papular urticaria with a central hemorrhagic punctum. Extensive infestation also can manifest as the colloquial vagabond disease, characterized by postinflammatory hyperpigmentation and thickening of the involved skin. Remarkably, patients also may present with considerable iron-deficiency anemia secondary to high parasite load and large volume blood feeding. Multiple case reports have demonstrated associated morbidity.12-14 The differential diagnosis for pediculosis may include scabies, lichen simplex chronicus, and eczematous dermatitis, though the clinician should prudently consider whether both scabies and pediculosis may be present, as coexistence is possible.4,15

Diagnosis
Diagnosis can be reached by visualizing adult lice, nymphs, or viable nits on the body or more commonly within inner clothing seams; nits also fluoresce under Wood light.15 Although dermoscopy has proven useful for increased sensitivity and differentiation between viable and hatched nits, the insects also can be viewed with the unaided eye.16
Treatment: New Concerns and Strategies
The mainstay of treatment for body lice has long consisted of thorough washing and drying of all clothing and linens in a hot dryer. Treatment can be augmented with the addition of pharmacotherapy, plus antibiotics as warranted for louse-borne disease. Pharmacologic intervention often is used in cases of mass infestation and is similar to head lice.
Options for head lice include topical permethrin, malathion, lindane, spinosad, benzyl alcohol, and ivermectin. Pyrethroids, derived from the chrysanthemum, generally are considered safe for human use with a side-effect profile limited to irritation and allergy17; however, neurotoxicity and leukemia are clinical concerns, with an association more recently shown between large-volume use of pyrethroids and acute lymphoblastic leukemia.18,19 Use of lindane is not recommended due to a greater potential for central nervous system neurotoxicity, manifested by seizures, with repeated large surface application. Malathion is problematic due to the risk for mucosal irritation, flammability of some formulations, and theoretical organophosphate poisoning, as its mechanism of action involves inhibition of acetylcholinesterase.15 However, in the context of head lice treatment, a randomized controlled trial reported no incidence of acetylcholinesterase inhibition.20 Spinosad, manufactured from the soil bacterium Saccharopolyspora spinosa, functions similarly by interfering with the nicotinic acetylcholine receptor and also carries a risk for skin irritation.21 Among all the treatment options, we prefer benzyl alcohol, particularly in the context of resistance, as it is effective via a physical mechanism of action and lacks notable neurotoxic effects to the host. Use of benzyl alcohol is approved for patients as young as 6 months; it functions by asphyxiating the lice via paralysis of the respiratory spiracle with occlusion by inert ingredients. Itching, episodic numbness, and scalp or mucosal irritation are possible complications of treatment.22
Treatment resistance of body lice has increased in recent years, warranting exploration of additional management strategies. Moreover, developing resistance to lindane and malathion has been reported.23 Resistance to pyrethroids has been attributed to mutations in a voltage-gated sodium channel, one of which was universally present in the sampling of a single population.24 A randomized controlled trial showed that off-label oral ivermectin 400 μg/kg was superior to malathion lotion 0.5% in difficult-to-treat cases of head lice25; utility of oral ivermectin also has been reported in body lice.26 In vitro studies also have shown promise for pursuing synergistic treatment of body lice with both ivermectin and antibiotics.27
A novel primary prophylaxis approach for at-risk homeless individuals recently utilized permethrin-impregnated underwear. Although the intervention provided short-term infestation improvement, longer-term use did not show improvement from placebo and also increased prevalence of permethrin-resistant haplotypes.2
Epidemiology and Transmission
Pediculus humanus corporis, commonly known as the human body louse, is one in a family of 3 ectoparasites of the same suborder that also encompasses pubic lice (Phthirus pubis) and head lice (Pediculus humanus capitis). Adults are approximately 2 mm in size, with the same life cycle as head lice (Figure 1). They require blood meals roughly 5 times per day and cannot survive longer than 2 days without feeding.1 Although similar in structure to head lice, body lice differ behaviorally in that they do not reside on their human host’s body; instead, they infest the host’s clothing, localizing to seams (Figure 2), and migrate to the host for blood meals. In fact, based on this behavior, genetic analysis of early human body lice has been used to postulate when clothing was first used by humans as well as to determine early human migration patterns.2,3


Although clinicians in developed countries may be less familiar with body lice compared to their counterparts, body lice nevertheless remain a global health concern in impoverished, densely populated areas, as well as in homeless populations due to poor hygiene. Transmission frequently occurs via physical contact with an affected individual and his/her personal items (eg, linens) via fomites.4,5 Body louse infestation is more prevalent in homeless individuals who sleep outside vs in shelters; a history of pubic lice and lack of regular bathing have been reported as additional risk factors.6 Outbreaks have been noted in the wake of natural disasters, in the setting of political upheavals, and in refugee camps, as well as in individuals seeking political asylum.7 Unlike head and pubic lice, body lice can serve as vectors for infectious diseases including Rickettsia prowazekii (epidemic typhus), Borrelia recurrentis (louse-borne relapsing fever), Bartonella quintana (trench fever), and Yersinia pestis (plague).5,8,9 Several Acinetobacter species were isolated from nearly one-third of collected body louse specimens in a French study.10 Additionally, serology for B quintana was found to be positive in up to 30% of cases in one United States urban homeless population.4
Clinical Manifestations
Patients often present with generalized pruritus, usually considerably more severe than with P humanus capitis, with lesions concentrated on the trunk.11 In addition to often impetiginized, self-inflicted excoriations, feeding sites may present as erythematous macules (Figure 3), papules, or papular urticaria with a central hemorrhagic punctum. Extensive infestation also can manifest as the colloquial vagabond disease, characterized by postinflammatory hyperpigmentation and thickening of the involved skin. Remarkably, patients also may present with considerable iron-deficiency anemia secondary to high parasite load and large volume blood feeding. Multiple case reports have demonstrated associated morbidity.12-14 The differential diagnosis for pediculosis may include scabies, lichen simplex chronicus, and eczematous dermatitis, though the clinician should prudently consider whether both scabies and pediculosis may be present, as coexistence is possible.4,15

Diagnosis
Diagnosis can be reached by visualizing adult lice, nymphs, or viable nits on the body or more commonly within inner clothing seams; nits also fluoresce under Wood light.15 Although dermoscopy has proven useful for increased sensitivity and differentiation between viable and hatched nits, the insects also can be viewed with the unaided eye.16
Treatment: New Concerns and Strategies
The mainstay of treatment for body lice has long consisted of thorough washing and drying of all clothing and linens in a hot dryer. Treatment can be augmented with the addition of pharmacotherapy, plus antibiotics as warranted for louse-borne disease. Pharmacologic intervention often is used in cases of mass infestation and is similar to head lice.
Options for head lice include topical permethrin, malathion, lindane, spinosad, benzyl alcohol, and ivermectin. Pyrethroids, derived from the chrysanthemum, generally are considered safe for human use with a side-effect profile limited to irritation and allergy17; however, neurotoxicity and leukemia are clinical concerns, with an association more recently shown between large-volume use of pyrethroids and acute lymphoblastic leukemia.18,19 Use of lindane is not recommended due to a greater potential for central nervous system neurotoxicity, manifested by seizures, with repeated large surface application. Malathion is problematic due to the risk for mucosal irritation, flammability of some formulations, and theoretical organophosphate poisoning, as its mechanism of action involves inhibition of acetylcholinesterase.15 However, in the context of head lice treatment, a randomized controlled trial reported no incidence of acetylcholinesterase inhibition.20 Spinosad, manufactured from the soil bacterium Saccharopolyspora spinosa, functions similarly by interfering with the nicotinic acetylcholine receptor and also carries a risk for skin irritation.21 Among all the treatment options, we prefer benzyl alcohol, particularly in the context of resistance, as it is effective via a physical mechanism of action and lacks notable neurotoxic effects to the host. Use of benzyl alcohol is approved for patients as young as 6 months; it functions by asphyxiating the lice via paralysis of the respiratory spiracle with occlusion by inert ingredients. Itching, episodic numbness, and scalp or mucosal irritation are possible complications of treatment.22
Treatment resistance of body lice has increased in recent years, warranting exploration of additional management strategies. Moreover, developing resistance to lindane and malathion has been reported.23 Resistance to pyrethroids has been attributed to mutations in a voltage-gated sodium channel, one of which was universally present in the sampling of a single population.24 A randomized controlled trial showed that off-label oral ivermectin 400 μg/kg was superior to malathion lotion 0.5% in difficult-to-treat cases of head lice25; utility of oral ivermectin also has been reported in body lice.26 In vitro studies also have shown promise for pursuing synergistic treatment of body lice with both ivermectin and antibiotics.27
A novel primary prophylaxis approach for at-risk homeless individuals recently utilized permethrin-impregnated underwear. Although the intervention provided short-term infestation improvement, longer-term use did not show improvement from placebo and also increased prevalence of permethrin-resistant haplotypes.2
- Veracx A, Raoult D. Biology and genetics of human head and body lice. Trends Parasitol. 2012;28:563-571.
- Kittler R, Kayser M, Stoneking M. Molecular evolution of Pediculus humanus and the origin of clothing. Curr Biol. 2003;13:1414-1417.
- Drali R, Mumcuoglu KY, Yesilyurt G, et al. Studies of ancient lice reveal unsuspected past migrations of vectors. Am J Trop Med Hyg. 2015;93:623-625.
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826.
- Feldmeier H, Heukelbach J. Epidermal parasitic skin diseases: a neglected category of poverty-associated plagues. Bull World Health Organ. 2009;87:152-159.
- Arnaud A, Chosidow O, Detrez MA, et al. Prevalence of scabies and Pediculosis corporis among homeless people in the Paris region: results from two randomized cross-sectional surveys (HYTPEAC study). Br J Dermatol. 2016;174:104-112.
- Hytonen J, Khawaja T, Gronroos JO, et al. Louse-borne relapsing fever in Finland in two asylum seekers from Somalia. APMIS. 2017;125:59-62.
- Nordmann T, Feldt T, Bosselmann M, et al. Outbreak of louse-borne relapsing fever among urban dwellers in Arsi Zone, Central Ethiopia, from July to November 2016. Am J Trop Med Hyg. 2018;98:1599-1602.
- Louni M, Mana N, Bitam I, et al. Body lice of homeless people reveal the presence of several emerging bacterial pathogens in northern Algeria. PLoS Negl Trop Dis. 2018;12:E0006397.
- Candy K, Amanzougaghene N, Izri A, et al. Molecular survey of head and body lice, Pediculus humanus, in France. Vector Borne Zoonotic Dis. 2018;18:243-251.
Bolognia JL, Schaffer JV, Cerroni L. Dermatology. 4th ed. Elsevier Limited; 2018. - Nara A, Nagai H, Yamaguchi R, et al. An unusual autopsy case of lethal hypothermia exacerbated by body lice-induced severe anemia. Int J Legal Med. 2016;130:765-769.
- Althomali SA, Alzubaidi LM, Alkhaldi DM. Severe iron deficiency anaemia associated with heavy lice infestation in a young woman [published online November 5, 2015]. BMJ Case Rep. doi:10.1136/bcr-2015-212207.
- Hau V, Muhi-Iddin N. A ghost covered in lice: a case of severe blood loss with long-standing heavy pediculosis capitis infestation [published online December 19, 2014]. BMJ Case Rep. doi:10.1136/bcr-2014-206623.
- Diaz JH. Lice (Pediculosis). In: Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 9th ed. New York, NY: Elsevier; 2020:3482-3486.
- Martins LG, Bernardes Filho F, Quaresma MV, et al. Dermoscopy applied to pediculosis corporis diagnosis. An Bras Dermatol. 2014;89:513-514.
- Devore CD, Schutze GE; Council on School Health and Committee on Infectious Diseases, American Academy of Pediatrics. Head lice. Pediatrics. 2015;135:E1355-E1365.
- Shafer TJ, Meyer DA, Crofton KM. Developmental neurotoxicity of pyrethroid insecticides: critical review and future research needs. Environ Health Perspect. 2005;113:123-136.
- Ding G, Shi R, Gao Y, et al. Pyrethroid pesticide exposure and risk of childhood acute lymphocytic leukemia in Shanghai. Environ Sci Technol. 2012;46:13480-13487.
- Meinking TL, Vicaria M, Eyerdam DH, et al. A randomized, investigator-blinded, time-ranging study of the comparative efficacy of 0.5% malathion gel versus Ovide Lotion (0.5% malathion) or Nix Crème Rinse (1% permethrin) used as labeled, for the treatment of head lice. Pediatr Dermatol. 2007;24:405-411.
- McCormack PL. Spinosad: in pediculosis capitis. Am J Clin Dermatol. 2011;12:349-353.
- Meinking TL, Villar ME, Vicaria M, et al. The clinical trials supporting benzyl alcohol lotion 5% (Ulesfia): a safe and effective topical treatment for head lice (pediculosis humanus capitis). Pediatr Dermatol. 2010;27:19-24.
- Lebwohl M, Clark L, Levitt J. Therapy for head lice based on life cycle, resistance, and safety considerations. Pediatrics. 2007;119:965-974
- Drali R, Benkouiten S, Badiaga S, et al. Detection of a knockdown resistance mutation associated with permethrin resistance in the body louse Pediculus humanus corporis by use of melting curve analysis genotyping. J Clin Microbiol. 2012;50:2229-2233.
- Chosidow O, Giraudeau B, Cottrell J, et al. Oral ivermectin versus malathion lotion for difficult-to-treat head lice. N Engl J Med. 2010;362:896-905.
- Foucault C, Ranque S, Badiaga S, et al. Oral ivermectin in the treatment of body lice. J Infect Dis. 2006;193:474-476.
- Sangaré AK, Doumbo OK, Raoult D. Management and treatment of human lice [published online July 27, 2016]. Biomed Res Int. doi:10.1155/2016/8962685.
- Benkouiten S, Drali R, Badiaga S, et al. Effect of permethrin-impregnated underwear on body lice in sheltered homeless persons: a randomized controlled trial. JAMA Dermatol. 2014;150:273-279.
- Veracx A, Raoult D. Biology and genetics of human head and body lice. Trends Parasitol. 2012;28:563-571.
- Kittler R, Kayser M, Stoneking M. Molecular evolution of Pediculus humanus and the origin of clothing. Curr Biol. 2003;13:1414-1417.
- Drali R, Mumcuoglu KY, Yesilyurt G, et al. Studies of ancient lice reveal unsuspected past migrations of vectors. Am J Trop Med Hyg. 2015;93:623-625.
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826.
- Feldmeier H, Heukelbach J. Epidermal parasitic skin diseases: a neglected category of poverty-associated plagues. Bull World Health Organ. 2009;87:152-159.
- Arnaud A, Chosidow O, Detrez MA, et al. Prevalence of scabies and Pediculosis corporis among homeless people in the Paris region: results from two randomized cross-sectional surveys (HYTPEAC study). Br J Dermatol. 2016;174:104-112.
- Hytonen J, Khawaja T, Gronroos JO, et al. Louse-borne relapsing fever in Finland in two asylum seekers from Somalia. APMIS. 2017;125:59-62.
- Nordmann T, Feldt T, Bosselmann M, et al. Outbreak of louse-borne relapsing fever among urban dwellers in Arsi Zone, Central Ethiopia, from July to November 2016. Am J Trop Med Hyg. 2018;98:1599-1602.
- Louni M, Mana N, Bitam I, et al. Body lice of homeless people reveal the presence of several emerging bacterial pathogens in northern Algeria. PLoS Negl Trop Dis. 2018;12:E0006397.
- Candy K, Amanzougaghene N, Izri A, et al. Molecular survey of head and body lice, Pediculus humanus, in France. Vector Borne Zoonotic Dis. 2018;18:243-251.
Bolognia JL, Schaffer JV, Cerroni L. Dermatology. 4th ed. Elsevier Limited; 2018. - Nara A, Nagai H, Yamaguchi R, et al. An unusual autopsy case of lethal hypothermia exacerbated by body lice-induced severe anemia. Int J Legal Med. 2016;130:765-769.
- Althomali SA, Alzubaidi LM, Alkhaldi DM. Severe iron deficiency anaemia associated with heavy lice infestation in a young woman [published online November 5, 2015]. BMJ Case Rep. doi:10.1136/bcr-2015-212207.
- Hau V, Muhi-Iddin N. A ghost covered in lice: a case of severe blood loss with long-standing heavy pediculosis capitis infestation [published online December 19, 2014]. BMJ Case Rep. doi:10.1136/bcr-2014-206623.
- Diaz JH. Lice (Pediculosis). In: Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 9th ed. New York, NY: Elsevier; 2020:3482-3486.
- Martins LG, Bernardes Filho F, Quaresma MV, et al. Dermoscopy applied to pediculosis corporis diagnosis. An Bras Dermatol. 2014;89:513-514.
- Devore CD, Schutze GE; Council on School Health and Committee on Infectious Diseases, American Academy of Pediatrics. Head lice. Pediatrics. 2015;135:E1355-E1365.
- Shafer TJ, Meyer DA, Crofton KM. Developmental neurotoxicity of pyrethroid insecticides: critical review and future research needs. Environ Health Perspect. 2005;113:123-136.
- Ding G, Shi R, Gao Y, et al. Pyrethroid pesticide exposure and risk of childhood acute lymphocytic leukemia in Shanghai. Environ Sci Technol. 2012;46:13480-13487.
- Meinking TL, Vicaria M, Eyerdam DH, et al. A randomized, investigator-blinded, time-ranging study of the comparative efficacy of 0.5% malathion gel versus Ovide Lotion (0.5% malathion) or Nix Crème Rinse (1% permethrin) used as labeled, for the treatment of head lice. Pediatr Dermatol. 2007;24:405-411.
- McCormack PL. Spinosad: in pediculosis capitis. Am J Clin Dermatol. 2011;12:349-353.
- Meinking TL, Villar ME, Vicaria M, et al. The clinical trials supporting benzyl alcohol lotion 5% (Ulesfia): a safe and effective topical treatment for head lice (pediculosis humanus capitis). Pediatr Dermatol. 2010;27:19-24.
- Lebwohl M, Clark L, Levitt J. Therapy for head lice based on life cycle, resistance, and safety considerations. Pediatrics. 2007;119:965-974
- Drali R, Benkouiten S, Badiaga S, et al. Detection of a knockdown resistance mutation associated with permethrin resistance in the body louse Pediculus humanus corporis by use of melting curve analysis genotyping. J Clin Microbiol. 2012;50:2229-2233.
- Chosidow O, Giraudeau B, Cottrell J, et al. Oral ivermectin versus malathion lotion for difficult-to-treat head lice. N Engl J Med. 2010;362:896-905.
- Foucault C, Ranque S, Badiaga S, et al. Oral ivermectin in the treatment of body lice. J Infect Dis. 2006;193:474-476.
- Sangaré AK, Doumbo OK, Raoult D. Management and treatment of human lice [published online July 27, 2016]. Biomed Res Int. doi:10.1155/2016/8962685.
- Benkouiten S, Drali R, Badiaga S, et al. Effect of permethrin-impregnated underwear on body lice in sheltered homeless persons: a randomized controlled trial. JAMA Dermatol. 2014;150:273-279.
Practice Points
- Body lice reside in clothing, particularly folds and seams, and migrate to the host for blood meals. To evaluate for infestation, the clinician should not only look at the skin but also closely examine the patient’s clothing. Clothes also are a target for treatment via washing in hot water.
- Due to observed and theoretical adverse effects of other chemical treatments, benzyl alcohol is the authors’ choice for treatment of head lice.
- Oral ivermectin is a promising future treatment for body lice.
Persistent Chlorotrichosis With Chronic Sun Exposure
To the Editor:
Chlorotrichosis, or green hair discoloration, is a dermatologic condition secondary to copper deposition on the hair. It most often is seen among swimmers who have prolonged exposure to chlorinated pools. The classic patient has predisposing chemical, heat, or mechanical damage to the hair shaft and usually lighter-colored hair.1-3 We present a case of chlorotrichosis in a young brunette patient who did not have predisposing factors except for chronic sun exposure.
A 13-year-old healthy adolescent girl with brown hair presented with persistent green hair for 2 years (Figure 1A). She had first noted hair discoloration after swimming in a neighbor’s chlorinated outdoor pool during summertime but experienced year-round persistence even without swimming. She denied any history of typical risk factors for hair damage, including exposure to hair dye or bleach, styling products, heat, or mechanical damage from excessive brushing. Her sister had blonde hair with a history of similar activities and exposures, and although she did style her hair with heat, she did not develop hair discoloration. The patient lived in a newer home, and prior tap water testing did not show elevated levels of copper. She admitted to strictly wearing her hair down at all times, including during strenuous activity and swimming. Excessive teasing at school prompted her mother to seek advice from hair salons. Bleaching test strips of hair reportedly caused paradoxical intensification of green, and the patient declined recommendations for red hair dye. The patient also tried Internet-based suggestions such as topically applying crushed aspirin, lemon juice, tea tree oil, and clarifying shampoos, which all failed to result in notable improvement.
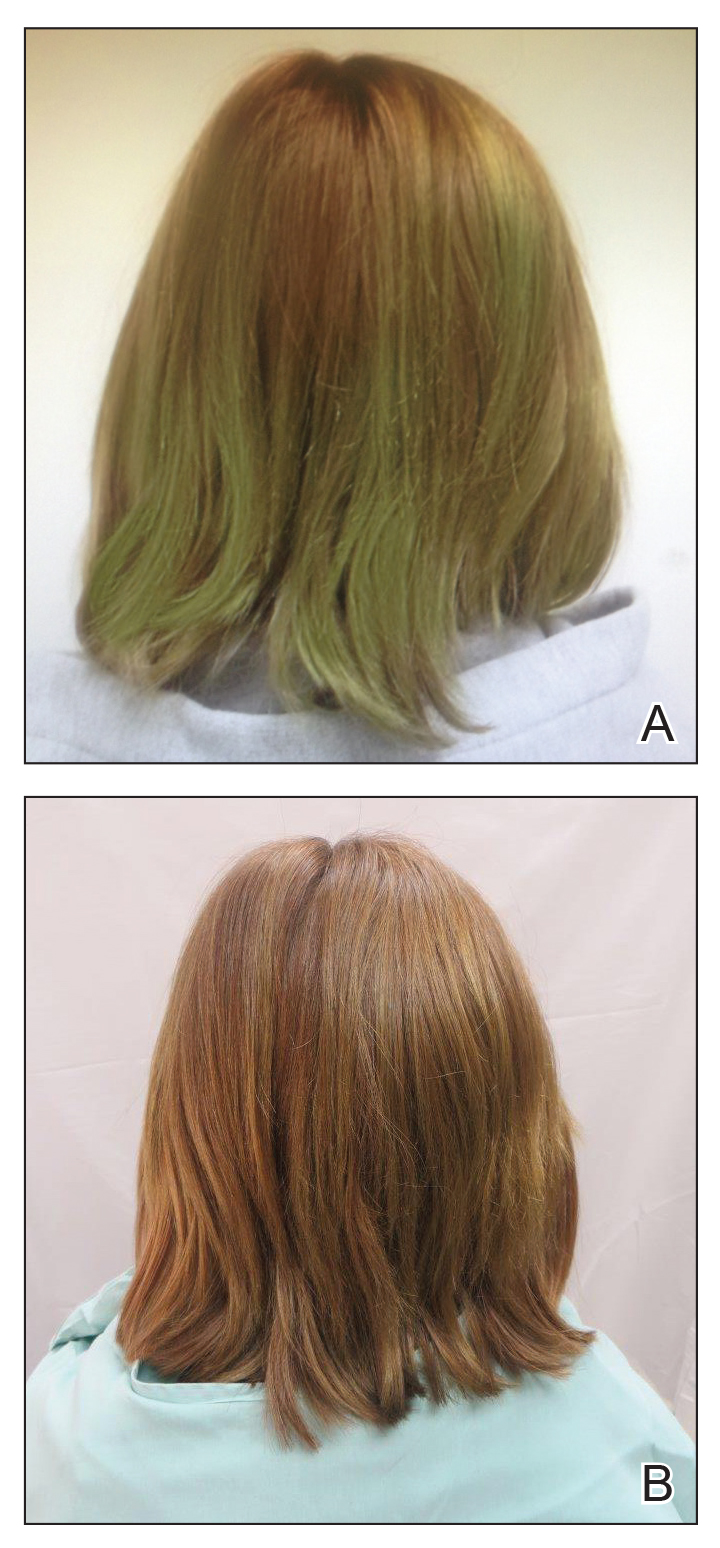
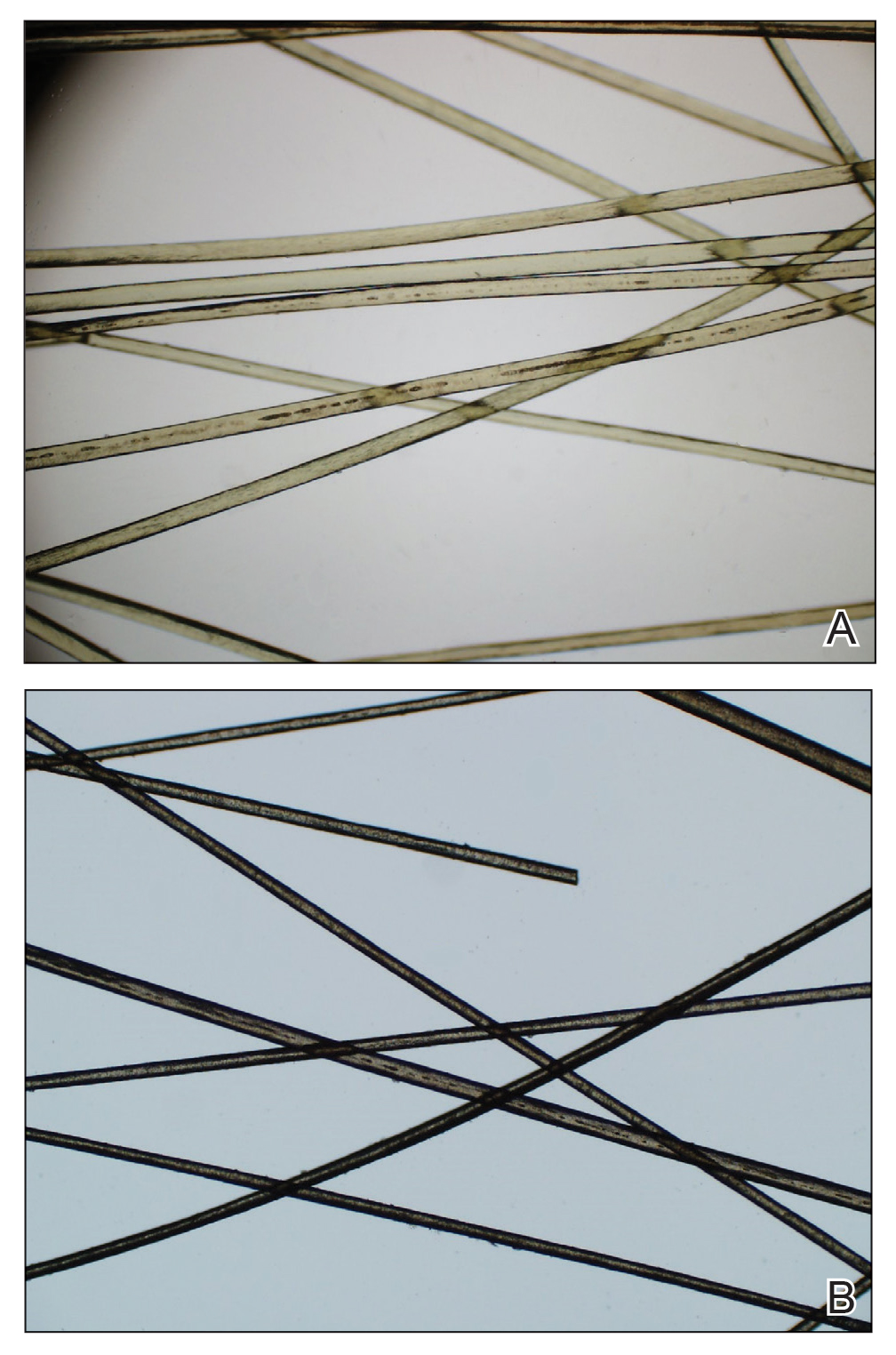
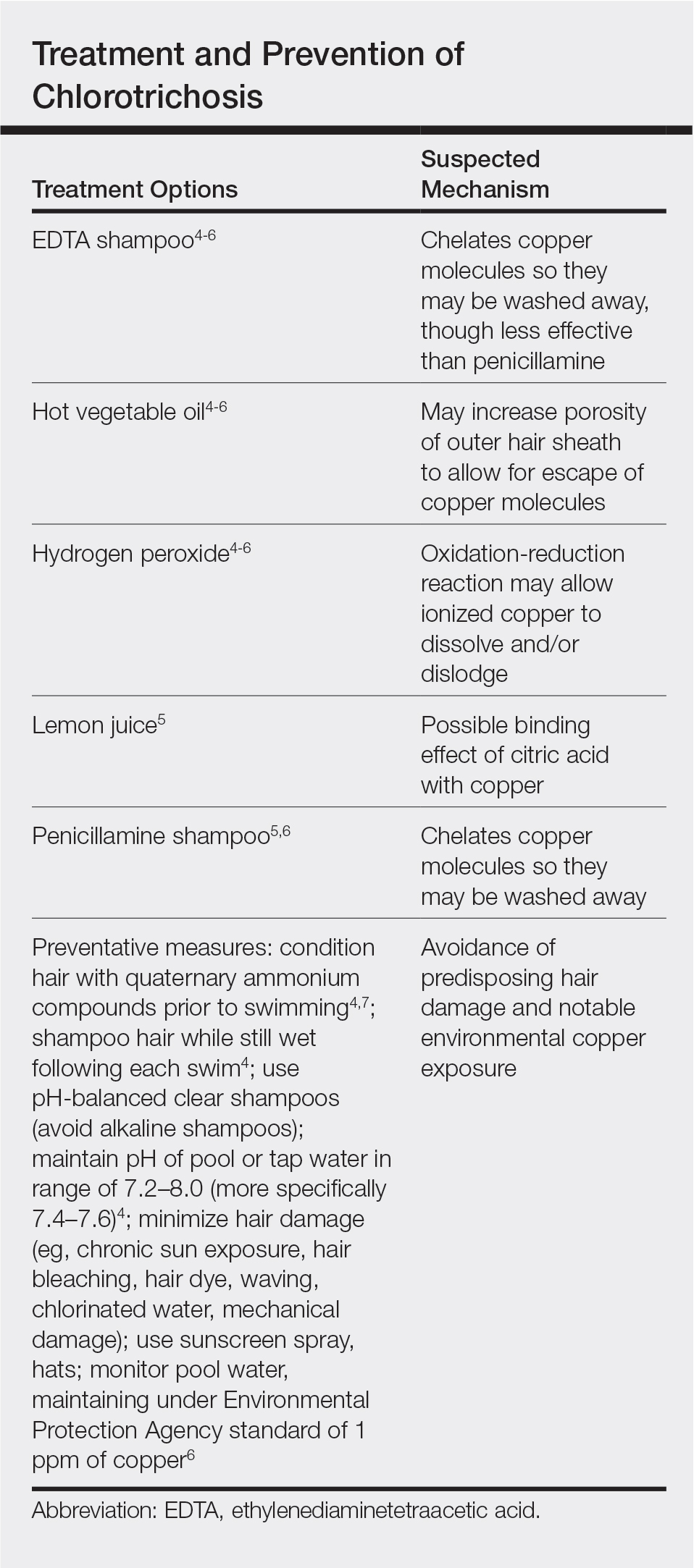
Physical examination revealed a sun-exposed distribution of ashy green hair that was worse at the distal hair ends and completely spared the roots. Trichoscopy of discolored hair (Figure 2A) revealed diffuse cuticle thinning, whereas unaffected hair appeared normal (Figure 2B). Because the patient reported slight improvement with tea tree oil, treatment was initiated with twice-weekly hot vegetable oil treatments applied for 20 minutes, which ultimately proved unsuccessful. Penicillamine shampoo (250-mg capsule of penicillamine into 5-mL purified water and 5-mL pH-balanced clear shampoo) was then recommended. At 3-month follow-up, the patient exhibited notable improvement of the hair discoloration, with only mild persistence at the distal ends of sun-damaged hair, visible only under fluorescent lighting (Figure 1B). Our recommendations thereafter were focused on prevention (Table).
The source of exogenous copper in chlorotrichosis commonly is tap water flowing through copper pipes or swimming pools rich in chlorine and copper-containing algaecides.2,4,8 The acidity of tap water is thought to cause the release of copper from the pipes.2,5 Such acidity could result from the effects of acid rain on water reservoirs or from water additives such as fluoride2 or those used in decalcification systems.5 Additionally, the attachment of electrical grounds to copper piping can cause copper to solubilize through an electric current, increasing water levels of copper.3 Although low pH facilitates copper solubility, high pH within the hair facilitates copper precipitation, which is quickly followed by adhesion to anionic molecules within hair shafts. Therefore, it is postulated that chlorotrichosis may persist in insufficiently rinsed hair with residual alkaline shampoo.6
Beyond pH flux in the induction of chlorotrichosis, other environmental agents have been suspected to play a role. A case report of green hair in a black patient following use of selenium sulfide 2.5% shampoo identified hair damage from tinea capitis infection as predisposing to chlorotrichosis.9 Other reports have cited tar shampoo and industrial exposure to cobalt, nickel, brass, mercury, or chromium as causative factors.2,3,6,7 Interestingly, green hair discoloration also has been observed in the metabolic disorder phenylketonuria.1
Few individuals exposed to elevated levels of copper will develop chlorotrichosis, which emphasizes the critical role of predisposing hair damage in its pathogenesis. With violation of the hair cuticle, chlorine can crystallize and copper can adhere to the hair shaft.10 Bleaching and waving of the hair also appear to alter the composition of keratin by increasing the number of cysteic acid and similar anionic sulfonate groups, which can bind copper.8
Although not harmful, chlorotrichosis may be aesthetically undesirable and lead to considerable social ostracism. Without intrinsic hair defects or obvious differences in predisposing factors, the question was raised as to why our patient, as a brunette, experienced dramatic hair discoloration while her blonde sister was entirely unaffected. We postulated that our patient’s persistent green hair may have been due to her unique predisposition to extensive sun-induced and mechanical hair damage because of her unwavering tendency to wear her hair down at all times. A variety of treatments of variable reported efficacy have been proposed (Table); fortunately, if treatments fail, the discoloration resolves with hair growth.
This case is unique in that it presented in a brunette patient with seemingly minimal hair damage with an unaffected blonde-haired sibling and with persistence over years. Furthermore, it lends credence to the use of penicillamine shampoo in treating chlorotrichosis, even in particularly difficult cases in which other treatments have failed.
- Holmes LB, Goldsmith LA. The man with green hair [letter]. N Engl J Med. 1974;291:1037.
Lampe RM, Henderson AL, Hansen GH. Green hair. JAMA. 1977;237:2092. - Nordlund JJ, Hartley C, Fister J. On the cause of green hair. Arch Dermatol. 1977;113:1700.
- Goldschmidt H. Green hair. Arch Dermatol. 1979;115:1288.
- Hinz T, Klingmuller K, Bieber T, et al. The mystery of green hair. Eur J Dermatol. 2009;19:409-410.
- Mascaro JM Jr, Ferrando J, Fontarnau R, et al. Green hair. Cutis. 1995;56:37-40.
- Bhat GR, Lukenbach ER, Kennedy RR, et al. The green hair problem: a preliminary investigation. J Soc Cosmet Chem. 1979;30:1-8.
- Blanc D, Zultak M, Rochefort A, et al. Green hair: clinical, chemical and epidemiologic study. apropos of a case. Ann Dermatol Venereol. 1988;115:807-812.
- Fitzgerald EA, Purcell SM, Goldman HM. Green hair discoloration due to selenium sulfide. Int J Dermatol. 1997;36:238-239.
- Fair NB, Gupta BS. The chlorine-hair interaction. II. effect of chlorination at varied pH levels on hair properties. J Soc Cosmet Chem. 1987;38:371-384.
To the Editor:
Chlorotrichosis, or green hair discoloration, is a dermatologic condition secondary to copper deposition on the hair. It most often is seen among swimmers who have prolonged exposure to chlorinated pools. The classic patient has predisposing chemical, heat, or mechanical damage to the hair shaft and usually lighter-colored hair.1-3 We present a case of chlorotrichosis in a young brunette patient who did not have predisposing factors except for chronic sun exposure.
A 13-year-old healthy adolescent girl with brown hair presented with persistent green hair for 2 years (Figure 1A). She had first noted hair discoloration after swimming in a neighbor’s chlorinated outdoor pool during summertime but experienced year-round persistence even without swimming. She denied any history of typical risk factors for hair damage, including exposure to hair dye or bleach, styling products, heat, or mechanical damage from excessive brushing. Her sister had blonde hair with a history of similar activities and exposures, and although she did style her hair with heat, she did not develop hair discoloration. The patient lived in a newer home, and prior tap water testing did not show elevated levels of copper. She admitted to strictly wearing her hair down at all times, including during strenuous activity and swimming. Excessive teasing at school prompted her mother to seek advice from hair salons. Bleaching test strips of hair reportedly caused paradoxical intensification of green, and the patient declined recommendations for red hair dye. The patient also tried Internet-based suggestions such as topically applying crushed aspirin, lemon juice, tea tree oil, and clarifying shampoos, which all failed to result in notable improvement.

Physical examination revealed a sun-exposed distribution of ashy green hair that was worse at the distal hair ends and completely spared the roots. Trichoscopy of discolored hair (Figure 2A) revealed diffuse cuticle thinning, whereas unaffected hair appeared normal (Figure 2B). Because the patient reported slight improvement with tea tree oil, treatment was initiated with twice-weekly hot vegetable oil treatments applied for 20 minutes, which ultimately proved unsuccessful. Penicillamine shampoo (250-mg capsule of penicillamine into 5-mL purified water and 5-mL pH-balanced clear shampoo) was then recommended. At 3-month follow-up, the patient exhibited notable improvement of the hair discoloration, with only mild persistence at the distal ends of sun-damaged hair, visible only under fluorescent lighting (Figure 1B). Our recommendations thereafter were focused on prevention (Table).

The source of exogenous copper in chlorotrichosis commonly is tap water flowing through copper pipes or swimming pools rich in chlorine and copper-containing algaecides.2,4,8 The acidity of tap water is thought to cause the release of copper from the pipes.2,5 Such acidity could result from the effects of acid rain on water reservoirs or from water additives such as fluoride2 or those used in decalcification systems.5 Additionally, the attachment of electrical grounds to copper piping can cause copper to solubilize through an electric current, increasing water levels of copper.3 Although low pH facilitates copper solubility, high pH within the hair facilitates copper precipitation, which is quickly followed by adhesion to anionic molecules within hair shafts. Therefore, it is postulated that chlorotrichosis may persist in insufficiently rinsed hair with residual alkaline shampoo.6
Beyond pH flux in the induction of chlorotrichosis, other environmental agents have been suspected to play a role. A case report of green hair in a black patient following use of selenium sulfide 2.5% shampoo identified hair damage from tinea capitis infection as predisposing to chlorotrichosis.9 Other reports have cited tar shampoo and industrial exposure to cobalt, nickel, brass, mercury, or chromium as causative factors.2,3,6,7 Interestingly, green hair discoloration also has been observed in the metabolic disorder phenylketonuria.1
Few individuals exposed to elevated levels of copper will develop chlorotrichosis, which emphasizes the critical role of predisposing hair damage in its pathogenesis. With violation of the hair cuticle, chlorine can crystallize and copper can adhere to the hair shaft.10 Bleaching and waving of the hair also appear to alter the composition of keratin by increasing the number of cysteic acid and similar anionic sulfonate groups, which can bind copper.8
Although not harmful, chlorotrichosis may be aesthetically undesirable and lead to considerable social ostracism. Without intrinsic hair defects or obvious differences in predisposing factors, the question was raised as to why our patient, as a brunette, experienced dramatic hair discoloration while her blonde sister was entirely unaffected. We postulated that our patient’s persistent green hair may have been due to her unique predisposition to extensive sun-induced and mechanical hair damage because of her unwavering tendency to wear her hair down at all times. A variety of treatments of variable reported efficacy have been proposed (Table); fortunately, if treatments fail, the discoloration resolves with hair growth.

This case is unique in that it presented in a brunette patient with seemingly minimal hair damage with an unaffected blonde-haired sibling and with persistence over years. Furthermore, it lends credence to the use of penicillamine shampoo in treating chlorotrichosis, even in particularly difficult cases in which other treatments have failed.
To the Editor:
Chlorotrichosis, or green hair discoloration, is a dermatologic condition secondary to copper deposition on the hair. It most often is seen among swimmers who have prolonged exposure to chlorinated pools. The classic patient has predisposing chemical, heat, or mechanical damage to the hair shaft and usually lighter-colored hair.1-3 We present a case of chlorotrichosis in a young brunette patient who did not have predisposing factors except for chronic sun exposure.
A 13-year-old healthy adolescent girl with brown hair presented with persistent green hair for 2 years (Figure 1A). She had first noted hair discoloration after swimming in a neighbor’s chlorinated outdoor pool during summertime but experienced year-round persistence even without swimming. She denied any history of typical risk factors for hair damage, including exposure to hair dye or bleach, styling products, heat, or mechanical damage from excessive brushing. Her sister had blonde hair with a history of similar activities and exposures, and although she did style her hair with heat, she did not develop hair discoloration. The patient lived in a newer home, and prior tap water testing did not show elevated levels of copper. She admitted to strictly wearing her hair down at all times, including during strenuous activity and swimming. Excessive teasing at school prompted her mother to seek advice from hair salons. Bleaching test strips of hair reportedly caused paradoxical intensification of green, and the patient declined recommendations for red hair dye. The patient also tried Internet-based suggestions such as topically applying crushed aspirin, lemon juice, tea tree oil, and clarifying shampoos, which all failed to result in notable improvement.

Physical examination revealed a sun-exposed distribution of ashy green hair that was worse at the distal hair ends and completely spared the roots. Trichoscopy of discolored hair (Figure 2A) revealed diffuse cuticle thinning, whereas unaffected hair appeared normal (Figure 2B). Because the patient reported slight improvement with tea tree oil, treatment was initiated with twice-weekly hot vegetable oil treatments applied for 20 minutes, which ultimately proved unsuccessful. Penicillamine shampoo (250-mg capsule of penicillamine into 5-mL purified water and 5-mL pH-balanced clear shampoo) was then recommended. At 3-month follow-up, the patient exhibited notable improvement of the hair discoloration, with only mild persistence at the distal ends of sun-damaged hair, visible only under fluorescent lighting (Figure 1B). Our recommendations thereafter were focused on prevention (Table).

The source of exogenous copper in chlorotrichosis commonly is tap water flowing through copper pipes or swimming pools rich in chlorine and copper-containing algaecides.2,4,8 The acidity of tap water is thought to cause the release of copper from the pipes.2,5 Such acidity could result from the effects of acid rain on water reservoirs or from water additives such as fluoride2 or those used in decalcification systems.5 Additionally, the attachment of electrical grounds to copper piping can cause copper to solubilize through an electric current, increasing water levels of copper.3 Although low pH facilitates copper solubility, high pH within the hair facilitates copper precipitation, which is quickly followed by adhesion to anionic molecules within hair shafts. Therefore, it is postulated that chlorotrichosis may persist in insufficiently rinsed hair with residual alkaline shampoo.6
Beyond pH flux in the induction of chlorotrichosis, other environmental agents have been suspected to play a role. A case report of green hair in a black patient following use of selenium sulfide 2.5% shampoo identified hair damage from tinea capitis infection as predisposing to chlorotrichosis.9 Other reports have cited tar shampoo and industrial exposure to cobalt, nickel, brass, mercury, or chromium as causative factors.2,3,6,7 Interestingly, green hair discoloration also has been observed in the metabolic disorder phenylketonuria.1
Few individuals exposed to elevated levels of copper will develop chlorotrichosis, which emphasizes the critical role of predisposing hair damage in its pathogenesis. With violation of the hair cuticle, chlorine can crystallize and copper can adhere to the hair shaft.10 Bleaching and waving of the hair also appear to alter the composition of keratin by increasing the number of cysteic acid and similar anionic sulfonate groups, which can bind copper.8
Although not harmful, chlorotrichosis may be aesthetically undesirable and lead to considerable social ostracism. Without intrinsic hair defects or obvious differences in predisposing factors, the question was raised as to why our patient, as a brunette, experienced dramatic hair discoloration while her blonde sister was entirely unaffected. We postulated that our patient’s persistent green hair may have been due to her unique predisposition to extensive sun-induced and mechanical hair damage because of her unwavering tendency to wear her hair down at all times. A variety of treatments of variable reported efficacy have been proposed (Table); fortunately, if treatments fail, the discoloration resolves with hair growth.

This case is unique in that it presented in a brunette patient with seemingly minimal hair damage with an unaffected blonde-haired sibling and with persistence over years. Furthermore, it lends credence to the use of penicillamine shampoo in treating chlorotrichosis, even in particularly difficult cases in which other treatments have failed.
- Holmes LB, Goldsmith LA. The man with green hair [letter]. N Engl J Med. 1974;291:1037.
Lampe RM, Henderson AL, Hansen GH. Green hair. JAMA. 1977;237:2092. - Nordlund JJ, Hartley C, Fister J. On the cause of green hair. Arch Dermatol. 1977;113:1700.
- Goldschmidt H. Green hair. Arch Dermatol. 1979;115:1288.
- Hinz T, Klingmuller K, Bieber T, et al. The mystery of green hair. Eur J Dermatol. 2009;19:409-410.
- Mascaro JM Jr, Ferrando J, Fontarnau R, et al. Green hair. Cutis. 1995;56:37-40.
- Bhat GR, Lukenbach ER, Kennedy RR, et al. The green hair problem: a preliminary investigation. J Soc Cosmet Chem. 1979;30:1-8.
- Blanc D, Zultak M, Rochefort A, et al. Green hair: clinical, chemical and epidemiologic study. apropos of a case. Ann Dermatol Venereol. 1988;115:807-812.
- Fitzgerald EA, Purcell SM, Goldman HM. Green hair discoloration due to selenium sulfide. Int J Dermatol. 1997;36:238-239.
- Fair NB, Gupta BS. The chlorine-hair interaction. II. effect of chlorination at varied pH levels on hair properties. J Soc Cosmet Chem. 1987;38:371-384.
- Holmes LB, Goldsmith LA. The man with green hair [letter]. N Engl J Med. 1974;291:1037.
Lampe RM, Henderson AL, Hansen GH. Green hair. JAMA. 1977;237:2092. - Nordlund JJ, Hartley C, Fister J. On the cause of green hair. Arch Dermatol. 1977;113:1700.
- Goldschmidt H. Green hair. Arch Dermatol. 1979;115:1288.
- Hinz T, Klingmuller K, Bieber T, et al. The mystery of green hair. Eur J Dermatol. 2009;19:409-410.
- Mascaro JM Jr, Ferrando J, Fontarnau R, et al. Green hair. Cutis. 1995;56:37-40.
- Bhat GR, Lukenbach ER, Kennedy RR, et al. The green hair problem: a preliminary investigation. J Soc Cosmet Chem. 1979;30:1-8.
- Blanc D, Zultak M, Rochefort A, et al. Green hair: clinical, chemical and epidemiologic study. apropos of a case. Ann Dermatol Venereol. 1988;115:807-812.
- Fitzgerald EA, Purcell SM, Goldman HM. Green hair discoloration due to selenium sulfide. Int J Dermatol. 1997;36:238-239.
- Fair NB, Gupta BS. The chlorine-hair interaction. II. effect of chlorination at varied pH levels on hair properties. J Soc Cosmet Chem. 1987;38:371-384.
Practice Points
- Chlorotrichosis is the deposition of copper onto hair, which causes a green discoloration and most commonly occurs in blonde patients with excessive exposure to chlorinated water.
- Hair cuticle damage from hair care practices, such as use of heat or chemicals, can predispose patients to the development of chlorotrichosis.
- Although a number of treatments have been proposed, the use of penicillamine shampoo seems to be particularly effective and works via chelation of the adherent copper molecules.
Data back botulinum toxin for facial flushing, androgenetic alopecia
LAHAINA, HAWAII – The list of Mark Rubin, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
There are data to support these uses, and there are data associating botulinum toxin treatment with improvement in depression, which suggest the effect may not be necessarily be related to improvement in appearance, said Dr. Rubin, who is in private practice in Beverly Hills, Calif., and is associate professor of dermatology at the University of California, San Diego.
Facial flushing: Very few people use botulinum toxin for facial flushing, but Dr. Rubin, who is among those who do not, described the data as “impressive.” Several trials, he noted, have found that very small doses can significantly reduce the amount of facial erythema, including an average 45% reduction after 60 days in one trial of 24 women (Acta Med Iran. 2016 Jul;54[7]:454-7).
In another study of 25 patients with facial erythema related to rosacea who were treated with 14-45 units intradermally to the nasal tip, bridge, and alae, there were statistically significant improvements in erythema 1, 2, and 3 months after treatment among the 15 with complete data (Dermatol Surg. 2015 Jan;41 Suppl 1:S9-16).
“If you’re using very small doses and they’re intradermal, there really is minimal risk you’re going to have a problem by inadvertently affecting musculature” in these patients, Dr. Rubin commented.
In another study of 9 patients with rosacea, treatment with incobotulinumtoxinA was associated with a significant reduction in erythema, papules, pustules, and telangiectasias, up to 15 weeks, compared with saline. The treatment patients also experienced less burning and stinging that did those who received saline (J Drugs Dermatol. 2017 Jun 1;16[6]:549-54.)
Menopausal hot flashes: Dr. Rubin described one study of 60 patients with severe hot flashes that compared saline with botulinum toxin, injected in 40 sites (2 units per site), including the neck, hairline, scalp, and chest. At 60 days’ follow-up, those treated with botulinum toxin had a significant reduction in sweating and in the number and severity of hot flashes; these women also had improved mood in terms of depression and irritability (Dermatol Surg. 2011 Nov;37[11]:1579-83).
Androgenetic alopecia: In a 60-week study of 50 men with androgenetic alopecia (Hamilton ratings of II-IV), 150 units of botulinum toxin A was injected into the scalp muscles (temporalis, frontalis, periauricular, and occipital), and repeated 6 months later (Plast Reconstr Surg. 2010 Nov;126[5]:246e-8e). Among the 40 patients who completed the trial, 75% had a response, and from baseline to 48 weeks, there was an 18% increase in mean hair counts in a 2 cm area, and a“profound” 39% reduction in hair loss (as measured by hair counts on the pillow in the morning), Dr. Rubin noted.
“Presumably, this is because if you’re relaxing the scalp muscles you’re getting increased blood flow into the scalp,” including increased oxygenation, which decreases the conversion of testosterone to dihydrotestosterone and increases the conversion of testosterone to estradiol, he said.
In another study, 8 of 10 patients with androgenic alopecia has “good to excellent” results 24 weeks after botulinum toxin injections with 5 units per site at 30 sites. Referring to the increasing popularity of platelet-rich plasma (PRP) injections for male pattern alopecia, Dr. Rubin said that in his opinion “PRP certainly doesn’t do any better” than botulinum toxin for male pattern alopecia and is a much more involved injection, “so this is definitely something worth considering if you have more people coming into your practice thinking about injections for male pattern alopecia.”
Pore size and sebum production: A 2019 review of published studies of botulinum toxin A looking at the effect on sebum and pore size, Dr. Rubin said, found that most studies “suggest it does actually reduce pore size and sebum production” (J Cosmet Dermatol. 2019 Apr;18[2]:451-7).
This can be considered an option for those patients concerned about pore size, who are not satisfied with results of retinoid or laser treatment, he commented. This approach may not have an effect in all patients, so he advised first treating a small trial area, and photographing patients to record their level of improvement. “It’s rarely profound, but it’s additive, it’s one more thing you can do.”
Depression: These data include a study of 30 patients with major depression, half who received one onabotulinumtoxinA injection in the glabellar area as adjunctive treatment of depression. After 6 weeks, those who were treated had an average of 47% reduction in depression scores on the Hamilton Depression Rating Scale, compared with an average 9% reduction among those on placebo (J Psychiatr Res. 2012 May;46[5]:574-81). Two recent studies have had similar results, according to Dr. Rubin.
Results of another study, he said, raise the question of whether patients are less depressed because they are pleased with the cosmetic effects or if there is another explanation (J Am Acad Dermatol. 2016 Jan;74[1]:171-3.e1). The study, which included 59 patients with depression treated in the glabellar areas with botulinum toxin injections, found no association between severity of the furrows and degree of depression or between the degree of furrow correction and degree of relief from depression after treatment. “So the patients who had the most improvement were not necessarily the ones who were the least depressed afterwards,” he said.
These data imply that something else may be occurring that is not necessarily muscle related, he said.
Dr. Rubin said he had no relevant disclosures. SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – The list of Mark Rubin, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
There are data to support these uses, and there are data associating botulinum toxin treatment with improvement in depression, which suggest the effect may not be necessarily be related to improvement in appearance, said Dr. Rubin, who is in private practice in Beverly Hills, Calif., and is associate professor of dermatology at the University of California, San Diego.
Facial flushing: Very few people use botulinum toxin for facial flushing, but Dr. Rubin, who is among those who do not, described the data as “impressive.” Several trials, he noted, have found that very small doses can significantly reduce the amount of facial erythema, including an average 45% reduction after 60 days in one trial of 24 women (Acta Med Iran. 2016 Jul;54[7]:454-7).
In another study of 25 patients with facial erythema related to rosacea who were treated with 14-45 units intradermally to the nasal tip, bridge, and alae, there were statistically significant improvements in erythema 1, 2, and 3 months after treatment among the 15 with complete data (Dermatol Surg. 2015 Jan;41 Suppl 1:S9-16).
“If you’re using very small doses and they’re intradermal, there really is minimal risk you’re going to have a problem by inadvertently affecting musculature” in these patients, Dr. Rubin commented.
In another study of 9 patients with rosacea, treatment with incobotulinumtoxinA was associated with a significant reduction in erythema, papules, pustules, and telangiectasias, up to 15 weeks, compared with saline. The treatment patients also experienced less burning and stinging that did those who received saline (J Drugs Dermatol. 2017 Jun 1;16[6]:549-54.)
Menopausal hot flashes: Dr. Rubin described one study of 60 patients with severe hot flashes that compared saline with botulinum toxin, injected in 40 sites (2 units per site), including the neck, hairline, scalp, and chest. At 60 days’ follow-up, those treated with botulinum toxin had a significant reduction in sweating and in the number and severity of hot flashes; these women also had improved mood in terms of depression and irritability (Dermatol Surg. 2011 Nov;37[11]:1579-83).
Androgenetic alopecia: In a 60-week study of 50 men with androgenetic alopecia (Hamilton ratings of II-IV), 150 units of botulinum toxin A was injected into the scalp muscles (temporalis, frontalis, periauricular, and occipital), and repeated 6 months later (Plast Reconstr Surg. 2010 Nov;126[5]:246e-8e). Among the 40 patients who completed the trial, 75% had a response, and from baseline to 48 weeks, there was an 18% increase in mean hair counts in a 2 cm area, and a“profound” 39% reduction in hair loss (as measured by hair counts on the pillow in the morning), Dr. Rubin noted.
“Presumably, this is because if you’re relaxing the scalp muscles you’re getting increased blood flow into the scalp,” including increased oxygenation, which decreases the conversion of testosterone to dihydrotestosterone and increases the conversion of testosterone to estradiol, he said.
In another study, 8 of 10 patients with androgenic alopecia has “good to excellent” results 24 weeks after botulinum toxin injections with 5 units per site at 30 sites. Referring to the increasing popularity of platelet-rich plasma (PRP) injections for male pattern alopecia, Dr. Rubin said that in his opinion “PRP certainly doesn’t do any better” than botulinum toxin for male pattern alopecia and is a much more involved injection, “so this is definitely something worth considering if you have more people coming into your practice thinking about injections for male pattern alopecia.”
Pore size and sebum production: A 2019 review of published studies of botulinum toxin A looking at the effect on sebum and pore size, Dr. Rubin said, found that most studies “suggest it does actually reduce pore size and sebum production” (J Cosmet Dermatol. 2019 Apr;18[2]:451-7).
This can be considered an option for those patients concerned about pore size, who are not satisfied with results of retinoid or laser treatment, he commented. This approach may not have an effect in all patients, so he advised first treating a small trial area, and photographing patients to record their level of improvement. “It’s rarely profound, but it’s additive, it’s one more thing you can do.”
Depression: These data include a study of 30 patients with major depression, half who received one onabotulinumtoxinA injection in the glabellar area as adjunctive treatment of depression. After 6 weeks, those who were treated had an average of 47% reduction in depression scores on the Hamilton Depression Rating Scale, compared with an average 9% reduction among those on placebo (J Psychiatr Res. 2012 May;46[5]:574-81). Two recent studies have had similar results, according to Dr. Rubin.
Results of another study, he said, raise the question of whether patients are less depressed because they are pleased with the cosmetic effects or if there is another explanation (J Am Acad Dermatol. 2016 Jan;74[1]:171-3.e1). The study, which included 59 patients with depression treated in the glabellar areas with botulinum toxin injections, found no association between severity of the furrows and degree of depression or between the degree of furrow correction and degree of relief from depression after treatment. “So the patients who had the most improvement were not necessarily the ones who were the least depressed afterwards,” he said.
These data imply that something else may be occurring that is not necessarily muscle related, he said.
Dr. Rubin said he had no relevant disclosures. SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – The list of Mark Rubin, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
There are data to support these uses, and there are data associating botulinum toxin treatment with improvement in depression, which suggest the effect may not be necessarily be related to improvement in appearance, said Dr. Rubin, who is in private practice in Beverly Hills, Calif., and is associate professor of dermatology at the University of California, San Diego.
Facial flushing: Very few people use botulinum toxin for facial flushing, but Dr. Rubin, who is among those who do not, described the data as “impressive.” Several trials, he noted, have found that very small doses can significantly reduce the amount of facial erythema, including an average 45% reduction after 60 days in one trial of 24 women (Acta Med Iran. 2016 Jul;54[7]:454-7).
In another study of 25 patients with facial erythema related to rosacea who were treated with 14-45 units intradermally to the nasal tip, bridge, and alae, there were statistically significant improvements in erythema 1, 2, and 3 months after treatment among the 15 with complete data (Dermatol Surg. 2015 Jan;41 Suppl 1:S9-16).
“If you’re using very small doses and they’re intradermal, there really is minimal risk you’re going to have a problem by inadvertently affecting musculature” in these patients, Dr. Rubin commented.
In another study of 9 patients with rosacea, treatment with incobotulinumtoxinA was associated with a significant reduction in erythema, papules, pustules, and telangiectasias, up to 15 weeks, compared with saline. The treatment patients also experienced less burning and stinging that did those who received saline (J Drugs Dermatol. 2017 Jun 1;16[6]:549-54.)
Menopausal hot flashes: Dr. Rubin described one study of 60 patients with severe hot flashes that compared saline with botulinum toxin, injected in 40 sites (2 units per site), including the neck, hairline, scalp, and chest. At 60 days’ follow-up, those treated with botulinum toxin had a significant reduction in sweating and in the number and severity of hot flashes; these women also had improved mood in terms of depression and irritability (Dermatol Surg. 2011 Nov;37[11]:1579-83).
Androgenetic alopecia: In a 60-week study of 50 men with androgenetic alopecia (Hamilton ratings of II-IV), 150 units of botulinum toxin A was injected into the scalp muscles (temporalis, frontalis, periauricular, and occipital), and repeated 6 months later (Plast Reconstr Surg. 2010 Nov;126[5]:246e-8e). Among the 40 patients who completed the trial, 75% had a response, and from baseline to 48 weeks, there was an 18% increase in mean hair counts in a 2 cm area, and a“profound” 39% reduction in hair loss (as measured by hair counts on the pillow in the morning), Dr. Rubin noted.
“Presumably, this is because if you’re relaxing the scalp muscles you’re getting increased blood flow into the scalp,” including increased oxygenation, which decreases the conversion of testosterone to dihydrotestosterone and increases the conversion of testosterone to estradiol, he said.
In another study, 8 of 10 patients with androgenic alopecia has “good to excellent” results 24 weeks after botulinum toxin injections with 5 units per site at 30 sites. Referring to the increasing popularity of platelet-rich plasma (PRP) injections for male pattern alopecia, Dr. Rubin said that in his opinion “PRP certainly doesn’t do any better” than botulinum toxin for male pattern alopecia and is a much more involved injection, “so this is definitely something worth considering if you have more people coming into your practice thinking about injections for male pattern alopecia.”
Pore size and sebum production: A 2019 review of published studies of botulinum toxin A looking at the effect on sebum and pore size, Dr. Rubin said, found that most studies “suggest it does actually reduce pore size and sebum production” (J Cosmet Dermatol. 2019 Apr;18[2]:451-7).
This can be considered an option for those patients concerned about pore size, who are not satisfied with results of retinoid or laser treatment, he commented. This approach may not have an effect in all patients, so he advised first treating a small trial area, and photographing patients to record their level of improvement. “It’s rarely profound, but it’s additive, it’s one more thing you can do.”
Depression: These data include a study of 30 patients with major depression, half who received one onabotulinumtoxinA injection in the glabellar area as adjunctive treatment of depression. After 6 weeks, those who were treated had an average of 47% reduction in depression scores on the Hamilton Depression Rating Scale, compared with an average 9% reduction among those on placebo (J Psychiatr Res. 2012 May;46[5]:574-81). Two recent studies have had similar results, according to Dr. Rubin.
Results of another study, he said, raise the question of whether patients are less depressed because they are pleased with the cosmetic effects or if there is another explanation (J Am Acad Dermatol. 2016 Jan;74[1]:171-3.e1). The study, which included 59 patients with depression treated in the glabellar areas with botulinum toxin injections, found no association between severity of the furrows and degree of depression or between the degree of furrow correction and degree of relief from depression after treatment. “So the patients who had the most improvement were not necessarily the ones who were the least depressed afterwards,” he said.
These data imply that something else may be occurring that is not necessarily muscle related, he said.
Dr. Rubin said he had no relevant disclosures. SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Nail dystrophy and nail plate thinning
At a follow-up visit, a biopsy of the skin on the fingertips was performed, which showed lichenoid lymphocytic inflammatory infiltrate with associated hyperkeratosis, hypergranulosis, and acanthosis.
No fungal elements were seen. The findings were consistent with lichen planus.
The patient was started on hydroxychloroquine. It was recommended she start a 6-week course of oral prednisone, but the mother was opposed to systemic treatment because of potential side effects.
She continued topical betamethasone without much change. Topical tacrolimus later was recommended to use on off days of betamethasone, which led to no improvement. Narrow-band UVB also was started with minimal improvement. Unfortunately,
Nail lichen planus (NLP) in children is not a common condition.1 In a recent series from Chiheb et al., NLP was reported in 90 patients, of which 40% were children; a quarter of the patients reported having extracutaneous involvement as well.2 In another childhood LP series,14 % of the children presented with nail disease.3 It can be a severe disease that, if not treated aggressively, may lead to destruction of the nail bed. This condition seems to be more prevalent in boys than girls and more prevalent in African American children.3 Unfortunately, in this patient’s case, the mother was hesitant to use systemic therapy and aggressive treatment was delayed.
Possible but not clear associations with autoimmune conditions such as vitiligo, autoimmune thyroiditis, myasthenia gravis, alopecia areata, thymoma, autoimmune polyendocrinopathy, atopic dermatitis, and lichen nitidus have been described in children with LP.
The clinical characteristics of NLP include nail plate thinning with longitudinal ridging and fissuring, with or without pterygium; trachyonychia; and erythema of the lunula when the nail matrix is involved. When the nail bed is affected, the patient can present with onycholysis with or without subungual hyperkeratosis and violaceous hue of the nail bed.4 NLP can have three different clinical presentations described by Tosti et al., which include typical NLP, 20‐nail dystrophy (trachyonychia), and idiopathic nail atrophy. Idiopathic nail atrophy is described solely in children as an acute and rapid progression that leads to destruction of the nail within months, which appears to be the clinical presentation in our patient.
The differential diagnosis of nail dystrophy in children includes infectious processes such as onychomycosis, especially when children present with onycholysis and subungual hyperkeratosis. Because of this, it is recommended to perform a nail culture or submit a sample of nail clippings for microscopic evaluation to confirm the diagnosis of onychomycosis prior to starting systemic therapy in children. Fingernail involvement without toenail involvement is an unusual presentation of onychomycosis.
Twenty-nail dystrophy – also known as trachyonychia – can be caused by several inflammatory skin conditions such as lichen planus, psoriasis, eczema, pemphigus vulgaris, and alopecia areata. Clinically, there is uniformly monomorphic thinning of the nail plate with longitudinal ridging without splitting or pterygium.1 This is a benign condition and should not cause scarring. About 10% of the cases of 20-nail dystrophy are caused by lichen planus.
Nail psoriasis is characterized by nail pitting, oil spots on the nail plate, leukonychia, subungual hyperkeratosis, and onycholysis, as well as nail crumbling, which were not seen in our patient. Although her initial presentation was of 20-nail dystrophy, which also can be a presentation of nail psoriasis, its rapid evolution with associated nail atrophy and pterygium make it unlikely to be psoriasis in this particular patient.
Patients with pachyonychia congenita – which is a genetic disorder or keratinization caused by mutations on several genes encoding keratin such as K6a, K16, K17, K6b, and possibly K6c – present with nail thickening (pachyonychia) and discoloration of the nails, as well as pincer nails. These patients also present with oral leukokeratosis and focal palmoplantar keratoderma.
The main treatment of lichen planus is potent topical corticosteroids.
For nail disease, topical treatment may not be effective and systemic treatment may be necessary. Systemic corticosteroids have been used in several pediatric series varying from a short course given at a dose of 1- 2 mg/kg per day for 2 weeks to a longer 3-month course followed by tapering.3 There are several protocols of intramuscular triamcinolone at a dose of 0.5 mg/kg in children in once a month injections for about 3 months that have been reported successful with minimal side effects.1 Other medications reported useful in patients with NLP include dapsone and acitretin. Other treatment options include narrow-band UVB and PUVA.3
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at [email protected].
References
1. Arch Dermatol. 2001 Aug;137(8):1027-32.
2. Ann Dermatol Venereol. 2015 Jan;142(1):21-5.
3. Pediatr Dermatol. 2014 Jan-Feb;31(1):59-67.
4. Dermatological diseases, in “Nails: Diagnosis, Therapy, and Surgery,” 3rd ed. (Oxford: Elsevier Saunders, 2005, p. 105).
At a follow-up visit, a biopsy of the skin on the fingertips was performed, which showed lichenoid lymphocytic inflammatory infiltrate with associated hyperkeratosis, hypergranulosis, and acanthosis.
No fungal elements were seen. The findings were consistent with lichen planus.
The patient was started on hydroxychloroquine. It was recommended she start a 6-week course of oral prednisone, but the mother was opposed to systemic treatment because of potential side effects.
She continued topical betamethasone without much change. Topical tacrolimus later was recommended to use on off days of betamethasone, which led to no improvement. Narrow-band UVB also was started with minimal improvement. Unfortunately,
Nail lichen planus (NLP) in children is not a common condition.1 In a recent series from Chiheb et al., NLP was reported in 90 patients, of which 40% were children; a quarter of the patients reported having extracutaneous involvement as well.2 In another childhood LP series,14 % of the children presented with nail disease.3 It can be a severe disease that, if not treated aggressively, may lead to destruction of the nail bed. This condition seems to be more prevalent in boys than girls and more prevalent in African American children.3 Unfortunately, in this patient’s case, the mother was hesitant to use systemic therapy and aggressive treatment was delayed.
Possible but not clear associations with autoimmune conditions such as vitiligo, autoimmune thyroiditis, myasthenia gravis, alopecia areata, thymoma, autoimmune polyendocrinopathy, atopic dermatitis, and lichen nitidus have been described in children with LP.
The clinical characteristics of NLP include nail plate thinning with longitudinal ridging and fissuring, with or without pterygium; trachyonychia; and erythema of the lunula when the nail matrix is involved. When the nail bed is affected, the patient can present with onycholysis with or without subungual hyperkeratosis and violaceous hue of the nail bed.4 NLP can have three different clinical presentations described by Tosti et al., which include typical NLP, 20‐nail dystrophy (trachyonychia), and idiopathic nail atrophy. Idiopathic nail atrophy is described solely in children as an acute and rapid progression that leads to destruction of the nail within months, which appears to be the clinical presentation in our patient.
The differential diagnosis of nail dystrophy in children includes infectious processes such as onychomycosis, especially when children present with onycholysis and subungual hyperkeratosis. Because of this, it is recommended to perform a nail culture or submit a sample of nail clippings for microscopic evaluation to confirm the diagnosis of onychomycosis prior to starting systemic therapy in children. Fingernail involvement without toenail involvement is an unusual presentation of onychomycosis.
Twenty-nail dystrophy – also known as trachyonychia – can be caused by several inflammatory skin conditions such as lichen planus, psoriasis, eczema, pemphigus vulgaris, and alopecia areata. Clinically, there is uniformly monomorphic thinning of the nail plate with longitudinal ridging without splitting or pterygium.1 This is a benign condition and should not cause scarring. About 10% of the cases of 20-nail dystrophy are caused by lichen planus.
Nail psoriasis is characterized by nail pitting, oil spots on the nail plate, leukonychia, subungual hyperkeratosis, and onycholysis, as well as nail crumbling, which were not seen in our patient. Although her initial presentation was of 20-nail dystrophy, which also can be a presentation of nail psoriasis, its rapid evolution with associated nail atrophy and pterygium make it unlikely to be psoriasis in this particular patient.
Patients with pachyonychia congenita – which is a genetic disorder or keratinization caused by mutations on several genes encoding keratin such as K6a, K16, K17, K6b, and possibly K6c – present with nail thickening (pachyonychia) and discoloration of the nails, as well as pincer nails. These patients also present with oral leukokeratosis and focal palmoplantar keratoderma.
The main treatment of lichen planus is potent topical corticosteroids.
For nail disease, topical treatment may not be effective and systemic treatment may be necessary. Systemic corticosteroids have been used in several pediatric series varying from a short course given at a dose of 1- 2 mg/kg per day for 2 weeks to a longer 3-month course followed by tapering.3 There are several protocols of intramuscular triamcinolone at a dose of 0.5 mg/kg in children in once a month injections for about 3 months that have been reported successful with minimal side effects.1 Other medications reported useful in patients with NLP include dapsone and acitretin. Other treatment options include narrow-band UVB and PUVA.3
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at [email protected].
References
1. Arch Dermatol. 2001 Aug;137(8):1027-32.
2. Ann Dermatol Venereol. 2015 Jan;142(1):21-5.
3. Pediatr Dermatol. 2014 Jan-Feb;31(1):59-67.
4. Dermatological diseases, in “Nails: Diagnosis, Therapy, and Surgery,” 3rd ed. (Oxford: Elsevier Saunders, 2005, p. 105).
At a follow-up visit, a biopsy of the skin on the fingertips was performed, which showed lichenoid lymphocytic inflammatory infiltrate with associated hyperkeratosis, hypergranulosis, and acanthosis.
No fungal elements were seen. The findings were consistent with lichen planus.
The patient was started on hydroxychloroquine. It was recommended she start a 6-week course of oral prednisone, but the mother was opposed to systemic treatment because of potential side effects.
She continued topical betamethasone without much change. Topical tacrolimus later was recommended to use on off days of betamethasone, which led to no improvement. Narrow-band UVB also was started with minimal improvement. Unfortunately,
Nail lichen planus (NLP) in children is not a common condition.1 In a recent series from Chiheb et al., NLP was reported in 90 patients, of which 40% were children; a quarter of the patients reported having extracutaneous involvement as well.2 In another childhood LP series,14 % of the children presented with nail disease.3 It can be a severe disease that, if not treated aggressively, may lead to destruction of the nail bed. This condition seems to be more prevalent in boys than girls and more prevalent in African American children.3 Unfortunately, in this patient’s case, the mother was hesitant to use systemic therapy and aggressive treatment was delayed.
Possible but not clear associations with autoimmune conditions such as vitiligo, autoimmune thyroiditis, myasthenia gravis, alopecia areata, thymoma, autoimmune polyendocrinopathy, atopic dermatitis, and lichen nitidus have been described in children with LP.
The clinical characteristics of NLP include nail plate thinning with longitudinal ridging and fissuring, with or without pterygium; trachyonychia; and erythema of the lunula when the nail matrix is involved. When the nail bed is affected, the patient can present with onycholysis with or without subungual hyperkeratosis and violaceous hue of the nail bed.4 NLP can have three different clinical presentations described by Tosti et al., which include typical NLP, 20‐nail dystrophy (trachyonychia), and idiopathic nail atrophy. Idiopathic nail atrophy is described solely in children as an acute and rapid progression that leads to destruction of the nail within months, which appears to be the clinical presentation in our patient.
The differential diagnosis of nail dystrophy in children includes infectious processes such as onychomycosis, especially when children present with onycholysis and subungual hyperkeratosis. Because of this, it is recommended to perform a nail culture or submit a sample of nail clippings for microscopic evaluation to confirm the diagnosis of onychomycosis prior to starting systemic therapy in children. Fingernail involvement without toenail involvement is an unusual presentation of onychomycosis.
Twenty-nail dystrophy – also known as trachyonychia – can be caused by several inflammatory skin conditions such as lichen planus, psoriasis, eczema, pemphigus vulgaris, and alopecia areata. Clinically, there is uniformly monomorphic thinning of the nail plate with longitudinal ridging without splitting or pterygium.1 This is a benign condition and should not cause scarring. About 10% of the cases of 20-nail dystrophy are caused by lichen planus.
Nail psoriasis is characterized by nail pitting, oil spots on the nail plate, leukonychia, subungual hyperkeratosis, and onycholysis, as well as nail crumbling, which were not seen in our patient. Although her initial presentation was of 20-nail dystrophy, which also can be a presentation of nail psoriasis, its rapid evolution with associated nail atrophy and pterygium make it unlikely to be psoriasis in this particular patient.
Patients with pachyonychia congenita – which is a genetic disorder or keratinization caused by mutations on several genes encoding keratin such as K6a, K16, K17, K6b, and possibly K6c – present with nail thickening (pachyonychia) and discoloration of the nails, as well as pincer nails. These patients also present with oral leukokeratosis and focal palmoplantar keratoderma.
The main treatment of lichen planus is potent topical corticosteroids.
For nail disease, topical treatment may not be effective and systemic treatment may be necessary. Systemic corticosteroids have been used in several pediatric series varying from a short course given at a dose of 1- 2 mg/kg per day for 2 weeks to a longer 3-month course followed by tapering.3 There are several protocols of intramuscular triamcinolone at a dose of 0.5 mg/kg in children in once a month injections for about 3 months that have been reported successful with minimal side effects.1 Other medications reported useful in patients with NLP include dapsone and acitretin. Other treatment options include narrow-band UVB and PUVA.3
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at [email protected].
References
1. Arch Dermatol. 2001 Aug;137(8):1027-32.
2. Ann Dermatol Venereol. 2015 Jan;142(1):21-5.
3. Pediatr Dermatol. 2014 Jan-Feb;31(1):59-67.
4. Dermatological diseases, in “Nails: Diagnosis, Therapy, and Surgery,” 3rd ed. (Oxford: Elsevier Saunders, 2005, p. 105).
An 8-year-old female child comes to our pediatric dermatology clinic for evaluation of onychomycosis on her fingernails. The mother stated the child started developing funny-looking nails 1 year prior to the visit. It started with only two fingernails affected and now has spread to all her fingernails. Her toenails are not involved.
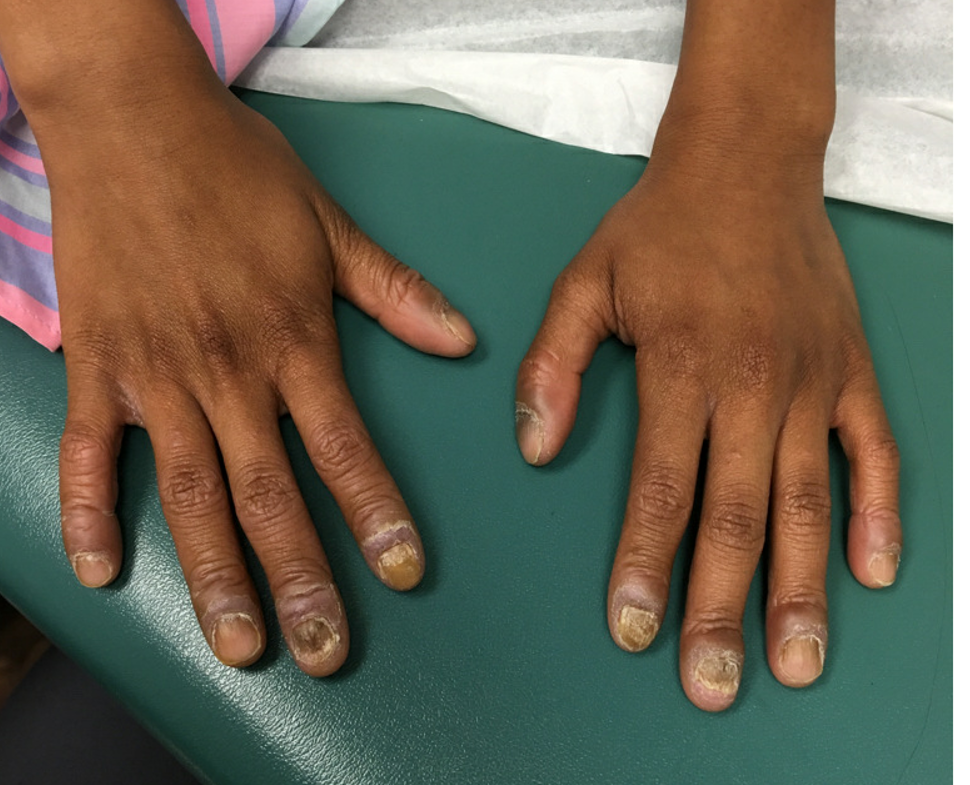
She denied any pain or itching. She initially was treated with topical antifungal medications as well as tea tree oil, apple cider vinegar, and a 6-week course of oral griseofulvin without any improvement. Her nails progressively have gotten much worse. She has no history of atopic dermatitis or any other skin conditions. She denied any joint pain, sun sensitivity, hair loss, or any other symptoms. The mother denied any family history of nail fungus, ringworm, psoriasis, or eczema.
She likes to play basketball and enjoys arts and crafts. She has a cat and a dog; neither of them have any skin problems.
On physical examination, there is nail dystrophy with nail plate thinning and longitudinal fissuring of all fingernails but not of the toenails. She also has hyperpigmented violaceous plaques on the surrounding periungual skin. There are no other skin lesions, and there are no oral or genital lesions. There is no scalp involvement or hair loss. At follow-up several months later, she had complete destruction of the nail plate with scar formation.
A fungal culture was performed, as well as microscopic analysis of the nail with periodic acid fast and giemsa stains, which showed no fungal organisms.
She initially was treated with topical betamethasone twice a day for 6 weeks and then 2 weeks on and 2 weeks off without much change.
Effect of In-Office Samples on Dermatologists’ Prescribing Habits: A Retrospective Review
Over the years, there has been growing concern about the relationship between physicians and pharmaceutical companies. Many studies have demonstrated that pharmaceutical interactions and incentives can influence physicians’ prescribing habits.1-3 As a result, many academic centers have adopted policies that attempt to limit the pharmaceutical industry’s influence on faculty and in-training physicians. Although these policies can vary greatly, they generally limit access of pharmaceutical representatives to providers and restrict pharmaceutical samples.4,5 This policy shift has even been reported in private practice.6
At the heart of the matter is the question: What really influences physicians to write a prescription for a particular medication? Is it cost, efficacy, or representatives pushing a product? Prior studies illustrate that generic medications are equivalent to their brand-name counterparts. In fact, current regulations require no more than 5% to 7% difference in bioequivalence.7-9 Although most generic medications are bioequivalent, it may not be universal.10
Garrison and Levin11 distributed a survey to US-based prescribers in family practice, psychiatry, and internal medicine and found that prescribers deemed patient response and success as the highest priority when determining which drugs to prescribe. In contrast, drug representatives and free samples only slightly contributed.11 Considering the minimum duration for efficacy of a medication such as an antidepressant vs a topical steroid, this pattern may differ with samples in dermatologic settings. Interestingly, another survey concluded that samples were associated with “sticky” prescribing habits, noting that physicians would prescribe a brand-name medication after using a sample, despite increased cost to the patient.12 Further, it has been suggested that recipients of free samples may experience increased costs in the long run, which contrasts a stated goal of affordability to patients.12,13
Physician interaction with pharmaceutical companies begins as early as medical school,14 with physicians reporting interactions as often as 4 times each month.14-18 Interactions can include meetings with pharmaceutical representatives, sponsored meals, gifts, continuing medical education sponsorship, funding for travel, pharmaceutical representative speakers, research funding, and drug samples.3
A 2014 study reported that prescribing habits are influenced by the free drug samples provided by nongeneric pharmaceutical companies.19 Nationally, the number of brand-name and branded generic medications constitute 79% of prescriptions, yet together they only comprise 17% of medications prescribed at an academic medical clinic that does not provide samples. The number of medications with samples being prescribed by dermatologists increased by 15% over 9 years, which may correlate with the wider availability of medication samples, more specifically an increase in branded generic samples.19 This potential interaction is the reason why institutions question the current influence of pharmaceutical companies. Samples may appear convenient, allowing a patient to test the medication prior to committing; however, with brand-name samples being provided to the physician, he/she may become more inclined to prescribe the branded medication.12,15,19-22 Because brand-name medications are more expensive than generic medications, this practice can increase the cost of health care.13 One study found that over 1 year, the overuse of nongeneric medications led to a loss of potential savings throughout 49 states, equating to $229 million just through Medicaid; interestingly, it was noted that in some states, a maximum reimbursement is set by Medicaid, regardless of whether the generic or branded medication is dispensed. The authors also noted variability in the potential savings by state, which may be a function of the state-by-state maximum reimbursements for certain medications.23 Another study on oral combination medications estimated Medicare spending on branded drugs relative to the cost if generic combinations had been purchased instead. This study examined branded medications for which the active components were available as over-the-counter (OTC), generic, or same-class generic, and the authors estimated that $925 million could have been saved in 2016 by purchasing a generic substitute.24 The overuse of nongeneric medications when generic alternatives are available becomes an issue that not only financially impacts patients but all taxpayers. However, this pattern may differ if limited only to dermatologic medications, which was not the focus of the prior studies.
To limit conflicts of interest in interactions with the pharmaceutical, medical device, and biotechnology industries, the University of South Florida (USF) Morsani College of Medicine (COM)(Tampa, Florida) implemented its own set of regulations that eliminated in-office pharmaceutical samples, in addition to other restrictions. This study aimed to investigate if there was a change in the prescribing habits of academic dermatologists after their medical school implemented these new policies.
We hypothesized that the number of brand-name drugs prescribed by physicians in the Department of Dermatology & Cutaneous Surgery would change following USF Morsani COM pharmaceutical policy changes. We sought to determine how physician prescribing practices within the Department of Dermatology & Cutaneous Surgery changed following USF Morsani COM pharmaceutical policy changes.
Methods
Data Collection
A retrospective review of medical records was conducted to investigate the effect of the USF Morsani COM pharmaceutical policy changes on physician prescribing practices within the Department of Dermatology & Cutaneous Surgery. Medical records of patients seen for common dermatology diagnoses before (January 1, 2010, to May 30, 2010) and after (August 1, 2011, to December 31, 2011) the pharmaceutical policy changes were reviewed, and all medications prescribed were recorded. Data were collected from medical records within the USF Health electronic medical record system and included visits with each of the department’s 3 attending dermatologists. The diagnoses included in the study—acne vulgaris, atopic dermatitis, onychomycosis, psoriasis, and rosacea—were chosen because in-office samples were available. Prescribing data from the first 100 consecutive medical records were collected from each time period, and a medical record was included only if it contained at least 1 of the following diagnoses: acne vulgaris, atopic dermatitis, onychomycosis, psoriasis, or rosacea. The assessment and plan of each progress note were reviewed, and the exact medication name and associated diagnosis were recorded for each prescription. Subsequently, each medication was reviewed and placed in 1 of 3 categories: brand name, generic, and OTC. The total number of prescriptions for each diagnosis (per visit/note); the specific number of brand, generic, and OTC medications prescribed (per visit/note); and the percentage of brand, generic, and OTC medications prescribed (per visit/note and per diagnosis in total) were calculated. To ensure only intended medications were included, each medication recorded in the medical record note was cross-referenced with the prescribed medication in the electronic medical record. The primary objective of this study was to capture the prescribing physician’s intent as proxied by the pattern of prescription. Thus, changes made in prescriptions after the initial plan—whether insurance related or otherwise—were not relevant to this investigation.
The data were collected to compare the percentage of brand vs generic or OTC prescriptions per diagnosis to see if there was a difference in the prescribing habits before and after the pharmaceutical policy changes. Of note, several other pieces of data were collected from each medical record, including age, race, class of insurance (ie, Medicare, Medicaid, private health maintenance organization, private preferred provider organization), subtype diagnoses, and whether the prescription was new or a refill. The information gathered from the written record on the assessment and plan was verified using prescriptions ordered in the Allscripts electronic record, and any difference was noted. No identifying information that could be used to easily identify study participants was recorded.
Differences in prescribing habits across diagnoses before and after the policy changes were ascertained using a Fisher exact test and were further assessed using a mixed effects ordinal logistic regression model that accounted for within-provider clustering and baseline patient characteristics. An ordinal model was chosen to recognize differences in average cost among brand-name, generic, and OTC medications.
Results
In total, 200 medical records were collected. For the period analyzed before the policy change, 252 brand-name medications were prescribed compared to 231 prescribed for the period analyzed after the policy changes. There was insufficient evidence of an overall difference in brand-name medications prescribed before and after the policy changes (P=.145; Fisher exact test)(Table 1). There also was insufficient evidence of an overall difference in generic prescriptions, which totaled 153 before and 134 after the policy changes (P=.872; Fisher exact test)(Table 2). Over-the-counter prescriptions totaled 49 before and 69 after the policy changes. There was insufficient evidence of an overall difference before and after the policy changes for OTC medications (P=.192; Fisher exact test)(Table 3).

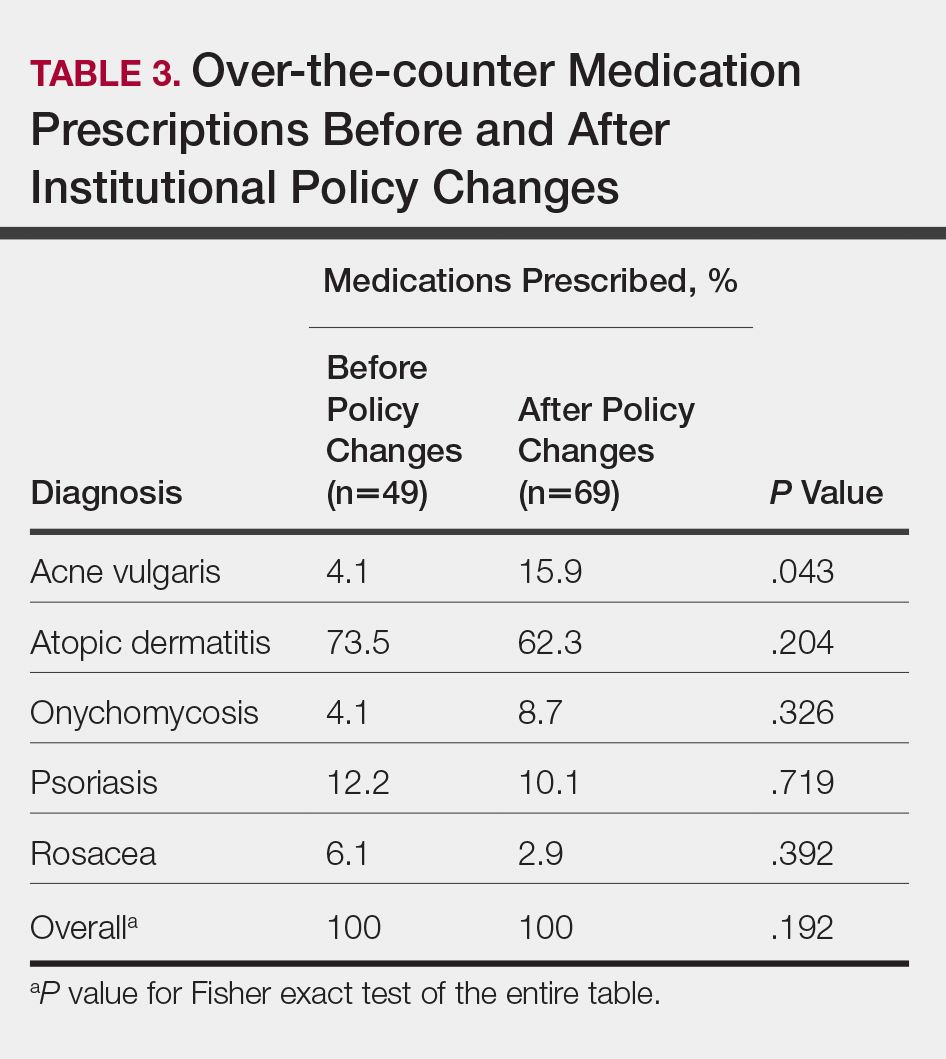
Comment
Although some medical institutions are diligently working to limit the potential influence pharmaceutical companies have on physician prescribing habits,4,5,25 the effect on physician prescribing habits is only now being established.15 Prior studies12,19,21 have found evidence that medication samples may lead to overuse of brand-name medications, but these findings do not hold true for the USF dermatologists included in this study, perhaps due to the difference in pharmaceutical company interactions or physicians maintaining prior prescription habits that were unrelated to the policy. Although this study focused on policy changes for in-office samples, prior studies either included other forms of interaction21 or did not include samples.22
Pharmaceutical samples allow patients to try a medication before committing to a long-term course of treatment with a particular medication, which has utility for physicians and patients. Although brand-name prescriptions may cost more, a trial period may assist the patient in deciding whether the medication is worth purchasing. Furthermore, physicians may feel more comfortable prescribing a medication once the individual patient has demonstrated a benefit from the sample, which may be particularly true in a specialty such as dermatology in which many branded topical medications contain a different vehicle than generic formulations, resulting in notable variations in active medication delivery and efficacy. Given the higher cost of branded topical medications, proving efficacy in patients through samples can provide a useful tool to the physician to determine the need for a branded formulation.
The benefits described are subjective but should not be disregarded. Although Hurley et al19 found that the number of brand-name medications prescribed increases as more samples are given out, our study demonstrated that after eliminating medication samples, there was no significant difference in the percentage of brand-name medications prescribed compared to generic and OTC medications.
Physician education concerning the price of each brand-name medication prescribed in office may be one method of reducing the amount of such prescriptions. Physicians generally are uninformed of the cost of the medications being prescribed26 and may not recognize the financial burden one medication may have compared to its alternative. However, educating physicians will empower them to make the conscious decision to prefer or not prefer a brand-name medication. With some generic medications shown to have a difference in bioequivalence compared to their brand-name counterparts, a physician may find more success prescribing the brand-name medications, regardless of pharmaceutical company influence, which is an alternative solution to policy changes that eliminate samples entirely. Although this study found insufficient evidence that removing samples decreases brand-name medication prescriptions, it is imperative that solutions are established to reduce the country’s increasing burden of medical costs.
Possible shortfalls of this study include the short period of time between which prepolicy data and postpolicy data were collected. It is possible that providers did not have enough time to adjust their prescribing habits or that providers would not have changed a prescribing pattern or preference simply because of a policy change. Future studies could allow a time period greater than 2 years to compare prepolicy and postpolicy prescribing habits, or a future study might make comparisons of prescriber patterns at different institutions that have different policies. Another possible shortfall is that providers and patients were limited to those at the Department of Dermatology & Cutaneous Surgery at the USF Morsani COM. Although this study has found insufficient evidence of a difference in prescribing habits, it may be beneficial to conduct a larger study that encompasses multiple academic institutions with similar policy changes. Most importantly, this study only investigated the influence of in-office pharmaceutical samples on prescribing patterns. This study did not look at the many other ways in which providers may be influenced by pharmaceutical companies, which likely is a significant confounding variable in this study. Continued additional studies that specifically examine other methods through which providers may be influenced would be helpful in further examining the many ways in which physician prescription habits are influenced.
Conclusion
Changes in pharmaceutical policy in 2011 at USF Morsani COM specifically banned in-office samples. The totality of evidence in this study shows modest observational evidence of a change in the postpolicy odds relative to prepolicy odds, but the data also are compatible with no change between prescribing habits before and after the policy changes. Further study is needed to fully understand this relationship.
- Sondergaard J, Vach K, Kragstrup J, et al. Impact of pharmaceutical representative visits on GPs’ drug preferences. Fam Pract. 2009;26:204-209.
- Jelinek GA, Neate SL. The influence of the pharmaceutical industry in medicine. J Law Med. 2009;17:216-223.
- Wazana A. Physicians and the pharmaceutical industry: is a gift ever just a gift? JAMA. 2000;283:373-380.
- Coleman DL. Establishing policies for the relationship between industry and clinicians: lessons learned from two academic health centers. Acad Med. 2008;83:882-887.
- Coleman DL, Kazdin AE, Miller LA, et al. Guidelines for interactions between clinical faculty and the pharmaceutical industry: one medical school’s approach. Acad Med. 2006;81:154-160.
- Evans D, Hartung DM, Beasley D, et al. Breaking up is hard to do: lessons learned from a pharma-free practice transformation. J Am Board Fam Med. 2013;26:332-338.
- Davit BM, Nwakama PE, Buehler GJ, et al. Comparing generic and innovator drugs: a review of 12 years of bioequivalence data from the United States Food and Drug Administration. Ann Pharmacother. 2009;43:1583-1597.
- Kesselheim AS, Misono AS, Lee JL, et al. Clinical equivalence of generic and brand-name drugs used in cardiovascular disease: a systematic review and meta-analysis. JAMA. 2008;300:2514-2526.
- McCormack J, Chmelicek JT. Generic versus brand name: the other drug war. Can Fam Physician. 2014;60:911.
- Borgheini G. The bioequivalence and therapeutic efficacy of generic versus brand-name psychoactive drugs. Clin Ther. 2003;25:1578-1592.
- Garrison GD, Levin GM. Factors affecting prescribing of the newer antidepressants. Ann Pharmacother. 2000;34:10-14.
- Rafique S, Sarwar W, Rashid A, et al. Influence of free drug samples on prescribing by physicians: a cross sectional survey. J Pak Med Assoc. 2017;67:465-467.
- Alexander GC, Zhang J, Basu A. Characteristics of patients receiving pharmaceutical samples and association between sample receipt and out-of-pocket prescription costs. Med Care. 2008;46:394-402.
- Hodges B. Interactions with the pharmaceutical industry: experiences and attitudes of psychiatry residents, interns and clerks. CMAJ. 1995;153:553-559.
- Brotzman GL, Mark DH. The effect on resident attitudes of regulatory policies regarding pharmaceutical representative activities. J Gen Intern Med. 1993;8:130-134.
- Keim SM, Sanders AB, Witzke DB, et al. Beliefs and practices of emergency medicine faculty and residents regarding professional interactions with the biomedical industry. Ann Emerg Med. 1993;22:1576-1581.
- Thomson AN, Craig BJ, Barham PM. Attitudes of general practitioners in New Zealand to pharmaceutical representatives. Br J Gen Pract. 1994;44:220-223.
- Ziegler MG, Lew P, Singer BC. The accuracy of drug information from pharmaceutical sales representatives. JAMA. 1995;273:1296-1298.
- Hurley MP, Stafford RS, Lane AT. Characterizing the relationship between free drug samples and prescription patterns for acne vulgaris and rosacea. JAMA Dermatol. 2014;150:487-493.
- Lexchin J. Interactions between physicians and the pharmaceutical industry: what does the literature say? CMAJ. 1993;149:1401-1407.
- Lieb K, Scheurich A. Contact between doctors and the pharmaceutical industry, their perceptions, and the effects on prescribing habits. PLoS One. 2014;9:e110130.
- Spurling GK, Mansfield PR, Montgomery BD, et al. Information from pharmaceutical companies and the quality, quantity, and cost of physicians’ prescribing: a systematic review. PLoS Med. 2010;7:e1000352.
- Fischer MA, Avorn J. Economic consequences of underuse of generic drugs: evidence from Medicaid and implications for prescription drug benefit plans. Health Serv Res. 2003;38:1051-1064.
- Sacks CA, Lee CC, Kesselheim AS, et al. Medicare spending on brand-name combination medications vs their generic constituents. JAMA. 2018;320:650-656.
- Brennan TA, Rothman DJ, Blank L, et al. Health industry practices that create conflicts of interest: a policy proposal for academic medical centers. JAMA. 2006;295:429-433.
- Allan GM, Lexchin J, Wiebe N. Physician awareness of drug cost: a systematic review. PLoS Med. 2007;4:e283.
Over the years, there has been growing concern about the relationship between physicians and pharmaceutical companies. Many studies have demonstrated that pharmaceutical interactions and incentives can influence physicians’ prescribing habits.1-3 As a result, many academic centers have adopted policies that attempt to limit the pharmaceutical industry’s influence on faculty and in-training physicians. Although these policies can vary greatly, they generally limit access of pharmaceutical representatives to providers and restrict pharmaceutical samples.4,5 This policy shift has even been reported in private practice.6
At the heart of the matter is the question: What really influences physicians to write a prescription for a particular medication? Is it cost, efficacy, or representatives pushing a product? Prior studies illustrate that generic medications are equivalent to their brand-name counterparts. In fact, current regulations require no more than 5% to 7% difference in bioequivalence.7-9 Although most generic medications are bioequivalent, it may not be universal.10
Garrison and Levin11 distributed a survey to US-based prescribers in family practice, psychiatry, and internal medicine and found that prescribers deemed patient response and success as the highest priority when determining which drugs to prescribe. In contrast, drug representatives and free samples only slightly contributed.11 Considering the minimum duration for efficacy of a medication such as an antidepressant vs a topical steroid, this pattern may differ with samples in dermatologic settings. Interestingly, another survey concluded that samples were associated with “sticky” prescribing habits, noting that physicians would prescribe a brand-name medication after using a sample, despite increased cost to the patient.12 Further, it has been suggested that recipients of free samples may experience increased costs in the long run, which contrasts a stated goal of affordability to patients.12,13
Physician interaction with pharmaceutical companies begins as early as medical school,14 with physicians reporting interactions as often as 4 times each month.14-18 Interactions can include meetings with pharmaceutical representatives, sponsored meals, gifts, continuing medical education sponsorship, funding for travel, pharmaceutical representative speakers, research funding, and drug samples.3
A 2014 study reported that prescribing habits are influenced by the free drug samples provided by nongeneric pharmaceutical companies.19 Nationally, the number of brand-name and branded generic medications constitute 79% of prescriptions, yet together they only comprise 17% of medications prescribed at an academic medical clinic that does not provide samples. The number of medications with samples being prescribed by dermatologists increased by 15% over 9 years, which may correlate with the wider availability of medication samples, more specifically an increase in branded generic samples.19 This potential interaction is the reason why institutions question the current influence of pharmaceutical companies. Samples may appear convenient, allowing a patient to test the medication prior to committing; however, with brand-name samples being provided to the physician, he/she may become more inclined to prescribe the branded medication.12,15,19-22 Because brand-name medications are more expensive than generic medications, this practice can increase the cost of health care.13 One study found that over 1 year, the overuse of nongeneric medications led to a loss of potential savings throughout 49 states, equating to $229 million just through Medicaid; interestingly, it was noted that in some states, a maximum reimbursement is set by Medicaid, regardless of whether the generic or branded medication is dispensed. The authors also noted variability in the potential savings by state, which may be a function of the state-by-state maximum reimbursements for certain medications.23 Another study on oral combination medications estimated Medicare spending on branded drugs relative to the cost if generic combinations had been purchased instead. This study examined branded medications for which the active components were available as over-the-counter (OTC), generic, or same-class generic, and the authors estimated that $925 million could have been saved in 2016 by purchasing a generic substitute.24 The overuse of nongeneric medications when generic alternatives are available becomes an issue that not only financially impacts patients but all taxpayers. However, this pattern may differ if limited only to dermatologic medications, which was not the focus of the prior studies.
To limit conflicts of interest in interactions with the pharmaceutical, medical device, and biotechnology industries, the University of South Florida (USF) Morsani College of Medicine (COM)(Tampa, Florida) implemented its own set of regulations that eliminated in-office pharmaceutical samples, in addition to other restrictions. This study aimed to investigate if there was a change in the prescribing habits of academic dermatologists after their medical school implemented these new policies.
We hypothesized that the number of brand-name drugs prescribed by physicians in the Department of Dermatology & Cutaneous Surgery would change following USF Morsani COM pharmaceutical policy changes. We sought to determine how physician prescribing practices within the Department of Dermatology & Cutaneous Surgery changed following USF Morsani COM pharmaceutical policy changes.
Methods
Data Collection
A retrospective review of medical records was conducted to investigate the effect of the USF Morsani COM pharmaceutical policy changes on physician prescribing practices within the Department of Dermatology & Cutaneous Surgery. Medical records of patients seen for common dermatology diagnoses before (January 1, 2010, to May 30, 2010) and after (August 1, 2011, to December 31, 2011) the pharmaceutical policy changes were reviewed, and all medications prescribed were recorded. Data were collected from medical records within the USF Health electronic medical record system and included visits with each of the department’s 3 attending dermatologists. The diagnoses included in the study—acne vulgaris, atopic dermatitis, onychomycosis, psoriasis, and rosacea—were chosen because in-office samples were available. Prescribing data from the first 100 consecutive medical records were collected from each time period, and a medical record was included only if it contained at least 1 of the following diagnoses: acne vulgaris, atopic dermatitis, onychomycosis, psoriasis, or rosacea. The assessment and plan of each progress note were reviewed, and the exact medication name and associated diagnosis were recorded for each prescription. Subsequently, each medication was reviewed and placed in 1 of 3 categories: brand name, generic, and OTC. The total number of prescriptions for each diagnosis (per visit/note); the specific number of brand, generic, and OTC medications prescribed (per visit/note); and the percentage of brand, generic, and OTC medications prescribed (per visit/note and per diagnosis in total) were calculated. To ensure only intended medications were included, each medication recorded in the medical record note was cross-referenced with the prescribed medication in the electronic medical record. The primary objective of this study was to capture the prescribing physician’s intent as proxied by the pattern of prescription. Thus, changes made in prescriptions after the initial plan—whether insurance related or otherwise—were not relevant to this investigation.
The data were collected to compare the percentage of brand vs generic or OTC prescriptions per diagnosis to see if there was a difference in the prescribing habits before and after the pharmaceutical policy changes. Of note, several other pieces of data were collected from each medical record, including age, race, class of insurance (ie, Medicare, Medicaid, private health maintenance organization, private preferred provider organization), subtype diagnoses, and whether the prescription was new or a refill. The information gathered from the written record on the assessment and plan was verified using prescriptions ordered in the Allscripts electronic record, and any difference was noted. No identifying information that could be used to easily identify study participants was recorded.
Differences in prescribing habits across diagnoses before and after the policy changes were ascertained using a Fisher exact test and were further assessed using a mixed effects ordinal logistic regression model that accounted for within-provider clustering and baseline patient characteristics. An ordinal model was chosen to recognize differences in average cost among brand-name, generic, and OTC medications.
Results
In total, 200 medical records were collected. For the period analyzed before the policy change, 252 brand-name medications were prescribed compared to 231 prescribed for the period analyzed after the policy changes. There was insufficient evidence of an overall difference in brand-name medications prescribed before and after the policy changes (P=.145; Fisher exact test)(Table 1). There also was insufficient evidence of an overall difference in generic prescriptions, which totaled 153 before and 134 after the policy changes (P=.872; Fisher exact test)(Table 2). Over-the-counter prescriptions totaled 49 before and 69 after the policy changes. There was insufficient evidence of an overall difference before and after the policy changes for OTC medications (P=.192; Fisher exact test)(Table 3).




Comment
Although some medical institutions are diligently working to limit the potential influence pharmaceutical companies have on physician prescribing habits,4,5,25 the effect on physician prescribing habits is only now being established.15 Prior studies12,19,21 have found evidence that medication samples may lead to overuse of brand-name medications, but these findings do not hold true for the USF dermatologists included in this study, perhaps due to the difference in pharmaceutical company interactions or physicians maintaining prior prescription habits that were unrelated to the policy. Although this study focused on policy changes for in-office samples, prior studies either included other forms of interaction21 or did not include samples.22
Pharmaceutical samples allow patients to try a medication before committing to a long-term course of treatment with a particular medication, which has utility for physicians and patients. Although brand-name prescriptions may cost more, a trial period may assist the patient in deciding whether the medication is worth purchasing. Furthermore, physicians may feel more comfortable prescribing a medication once the individual patient has demonstrated a benefit from the sample, which may be particularly true in a specialty such as dermatology in which many branded topical medications contain a different vehicle than generic formulations, resulting in notable variations in active medication delivery and efficacy. Given the higher cost of branded topical medications, proving efficacy in patients through samples can provide a useful tool to the physician to determine the need for a branded formulation.
The benefits described are subjective but should not be disregarded. Although Hurley et al19 found that the number of brand-name medications prescribed increases as more samples are given out, our study demonstrated that after eliminating medication samples, there was no significant difference in the percentage of brand-name medications prescribed compared to generic and OTC medications.
Physician education concerning the price of each brand-name medication prescribed in office may be one method of reducing the amount of such prescriptions. Physicians generally are uninformed of the cost of the medications being prescribed26 and may not recognize the financial burden one medication may have compared to its alternative. However, educating physicians will empower them to make the conscious decision to prefer or not prefer a brand-name medication. With some generic medications shown to have a difference in bioequivalence compared to their brand-name counterparts, a physician may find more success prescribing the brand-name medications, regardless of pharmaceutical company influence, which is an alternative solution to policy changes that eliminate samples entirely. Although this study found insufficient evidence that removing samples decreases brand-name medication prescriptions, it is imperative that solutions are established to reduce the country’s increasing burden of medical costs.
Possible shortfalls of this study include the short period of time between which prepolicy data and postpolicy data were collected. It is possible that providers did not have enough time to adjust their prescribing habits or that providers would not have changed a prescribing pattern or preference simply because of a policy change. Future studies could allow a time period greater than 2 years to compare prepolicy and postpolicy prescribing habits, or a future study might make comparisons of prescriber patterns at different institutions that have different policies. Another possible shortfall is that providers and patients were limited to those at the Department of Dermatology & Cutaneous Surgery at the USF Morsani COM. Although this study has found insufficient evidence of a difference in prescribing habits, it may be beneficial to conduct a larger study that encompasses multiple academic institutions with similar policy changes. Most importantly, this study only investigated the influence of in-office pharmaceutical samples on prescribing patterns. This study did not look at the many other ways in which providers may be influenced by pharmaceutical companies, which likely is a significant confounding variable in this study. Continued additional studies that specifically examine other methods through which providers may be influenced would be helpful in further examining the many ways in which physician prescription habits are influenced.
Conclusion
Changes in pharmaceutical policy in 2011 at USF Morsani COM specifically banned in-office samples. The totality of evidence in this study shows modest observational evidence of a change in the postpolicy odds relative to prepolicy odds, but the data also are compatible with no change between prescribing habits before and after the policy changes. Further study is needed to fully understand this relationship.
Over the years, there has been growing concern about the relationship between physicians and pharmaceutical companies. Many studies have demonstrated that pharmaceutical interactions and incentives can influence physicians’ prescribing habits.1-3 As a result, many academic centers have adopted policies that attempt to limit the pharmaceutical industry’s influence on faculty and in-training physicians. Although these policies can vary greatly, they generally limit access of pharmaceutical representatives to providers and restrict pharmaceutical samples.4,5 This policy shift has even been reported in private practice.6
At the heart of the matter is the question: What really influences physicians to write a prescription for a particular medication? Is it cost, efficacy, or representatives pushing a product? Prior studies illustrate that generic medications are equivalent to their brand-name counterparts. In fact, current regulations require no more than 5% to 7% difference in bioequivalence.7-9 Although most generic medications are bioequivalent, it may not be universal.10
Garrison and Levin11 distributed a survey to US-based prescribers in family practice, psychiatry, and internal medicine and found that prescribers deemed patient response and success as the highest priority when determining which drugs to prescribe. In contrast, drug representatives and free samples only slightly contributed.11 Considering the minimum duration for efficacy of a medication such as an antidepressant vs a topical steroid, this pattern may differ with samples in dermatologic settings. Interestingly, another survey concluded that samples were associated with “sticky” prescribing habits, noting that physicians would prescribe a brand-name medication after using a sample, despite increased cost to the patient.12 Further, it has been suggested that recipients of free samples may experience increased costs in the long run, which contrasts a stated goal of affordability to patients.12,13
Physician interaction with pharmaceutical companies begins as early as medical school,14 with physicians reporting interactions as often as 4 times each month.14-18 Interactions can include meetings with pharmaceutical representatives, sponsored meals, gifts, continuing medical education sponsorship, funding for travel, pharmaceutical representative speakers, research funding, and drug samples.3
A 2014 study reported that prescribing habits are influenced by the free drug samples provided by nongeneric pharmaceutical companies.19 Nationally, the number of brand-name and branded generic medications constitute 79% of prescriptions, yet together they only comprise 17% of medications prescribed at an academic medical clinic that does not provide samples. The number of medications with samples being prescribed by dermatologists increased by 15% over 9 years, which may correlate with the wider availability of medication samples, more specifically an increase in branded generic samples.19 This potential interaction is the reason why institutions question the current influence of pharmaceutical companies. Samples may appear convenient, allowing a patient to test the medication prior to committing; however, with brand-name samples being provided to the physician, he/she may become more inclined to prescribe the branded medication.12,15,19-22 Because brand-name medications are more expensive than generic medications, this practice can increase the cost of health care.13 One study found that over 1 year, the overuse of nongeneric medications led to a loss of potential savings throughout 49 states, equating to $229 million just through Medicaid; interestingly, it was noted that in some states, a maximum reimbursement is set by Medicaid, regardless of whether the generic or branded medication is dispensed. The authors also noted variability in the potential savings by state, which may be a function of the state-by-state maximum reimbursements for certain medications.23 Another study on oral combination medications estimated Medicare spending on branded drugs relative to the cost if generic combinations had been purchased instead. This study examined branded medications for which the active components were available as over-the-counter (OTC), generic, or same-class generic, and the authors estimated that $925 million could have been saved in 2016 by purchasing a generic substitute.24 The overuse of nongeneric medications when generic alternatives are available becomes an issue that not only financially impacts patients but all taxpayers. However, this pattern may differ if limited only to dermatologic medications, which was not the focus of the prior studies.
To limit conflicts of interest in interactions with the pharmaceutical, medical device, and biotechnology industries, the University of South Florida (USF) Morsani College of Medicine (COM)(Tampa, Florida) implemented its own set of regulations that eliminated in-office pharmaceutical samples, in addition to other restrictions. This study aimed to investigate if there was a change in the prescribing habits of academic dermatologists after their medical school implemented these new policies.
We hypothesized that the number of brand-name drugs prescribed by physicians in the Department of Dermatology & Cutaneous Surgery would change following USF Morsani COM pharmaceutical policy changes. We sought to determine how physician prescribing practices within the Department of Dermatology & Cutaneous Surgery changed following USF Morsani COM pharmaceutical policy changes.
Methods
Data Collection
A retrospective review of medical records was conducted to investigate the effect of the USF Morsani COM pharmaceutical policy changes on physician prescribing practices within the Department of Dermatology & Cutaneous Surgery. Medical records of patients seen for common dermatology diagnoses before (January 1, 2010, to May 30, 2010) and after (August 1, 2011, to December 31, 2011) the pharmaceutical policy changes were reviewed, and all medications prescribed were recorded. Data were collected from medical records within the USF Health electronic medical record system and included visits with each of the department’s 3 attending dermatologists. The diagnoses included in the study—acne vulgaris, atopic dermatitis, onychomycosis, psoriasis, and rosacea—were chosen because in-office samples were available. Prescribing data from the first 100 consecutive medical records were collected from each time period, and a medical record was included only if it contained at least 1 of the following diagnoses: acne vulgaris, atopic dermatitis, onychomycosis, psoriasis, or rosacea. The assessment and plan of each progress note were reviewed, and the exact medication name and associated diagnosis were recorded for each prescription. Subsequently, each medication was reviewed and placed in 1 of 3 categories: brand name, generic, and OTC. The total number of prescriptions for each diagnosis (per visit/note); the specific number of brand, generic, and OTC medications prescribed (per visit/note); and the percentage of brand, generic, and OTC medications prescribed (per visit/note and per diagnosis in total) were calculated. To ensure only intended medications were included, each medication recorded in the medical record note was cross-referenced with the prescribed medication in the electronic medical record. The primary objective of this study was to capture the prescribing physician’s intent as proxied by the pattern of prescription. Thus, changes made in prescriptions after the initial plan—whether insurance related or otherwise—were not relevant to this investigation.
The data were collected to compare the percentage of brand vs generic or OTC prescriptions per diagnosis to see if there was a difference in the prescribing habits before and after the pharmaceutical policy changes. Of note, several other pieces of data were collected from each medical record, including age, race, class of insurance (ie, Medicare, Medicaid, private health maintenance organization, private preferred provider organization), subtype diagnoses, and whether the prescription was new or a refill. The information gathered from the written record on the assessment and plan was verified using prescriptions ordered in the Allscripts electronic record, and any difference was noted. No identifying information that could be used to easily identify study participants was recorded.
Differences in prescribing habits across diagnoses before and after the policy changes were ascertained using a Fisher exact test and were further assessed using a mixed effects ordinal logistic regression model that accounted for within-provider clustering and baseline patient characteristics. An ordinal model was chosen to recognize differences in average cost among brand-name, generic, and OTC medications.
Results
In total, 200 medical records were collected. For the period analyzed before the policy change, 252 brand-name medications were prescribed compared to 231 prescribed for the period analyzed after the policy changes. There was insufficient evidence of an overall difference in brand-name medications prescribed before and after the policy changes (P=.145; Fisher exact test)(Table 1). There also was insufficient evidence of an overall difference in generic prescriptions, which totaled 153 before and 134 after the policy changes (P=.872; Fisher exact test)(Table 2). Over-the-counter prescriptions totaled 49 before and 69 after the policy changes. There was insufficient evidence of an overall difference before and after the policy changes for OTC medications (P=.192; Fisher exact test)(Table 3).




Comment
Although some medical institutions are diligently working to limit the potential influence pharmaceutical companies have on physician prescribing habits,4,5,25 the effect on physician prescribing habits is only now being established.15 Prior studies12,19,21 have found evidence that medication samples may lead to overuse of brand-name medications, but these findings do not hold true for the USF dermatologists included in this study, perhaps due to the difference in pharmaceutical company interactions or physicians maintaining prior prescription habits that were unrelated to the policy. Although this study focused on policy changes for in-office samples, prior studies either included other forms of interaction21 or did not include samples.22
Pharmaceutical samples allow patients to try a medication before committing to a long-term course of treatment with a particular medication, which has utility for physicians and patients. Although brand-name prescriptions may cost more, a trial period may assist the patient in deciding whether the medication is worth purchasing. Furthermore, physicians may feel more comfortable prescribing a medication once the individual patient has demonstrated a benefit from the sample, which may be particularly true in a specialty such as dermatology in which many branded topical medications contain a different vehicle than generic formulations, resulting in notable variations in active medication delivery and efficacy. Given the higher cost of branded topical medications, proving efficacy in patients through samples can provide a useful tool to the physician to determine the need for a branded formulation.
The benefits described are subjective but should not be disregarded. Although Hurley et al19 found that the number of brand-name medications prescribed increases as more samples are given out, our study demonstrated that after eliminating medication samples, there was no significant difference in the percentage of brand-name medications prescribed compared to generic and OTC medications.
Physician education concerning the price of each brand-name medication prescribed in office may be one method of reducing the amount of such prescriptions. Physicians generally are uninformed of the cost of the medications being prescribed26 and may not recognize the financial burden one medication may have compared to its alternative. However, educating physicians will empower them to make the conscious decision to prefer or not prefer a brand-name medication. With some generic medications shown to have a difference in bioequivalence compared to their brand-name counterparts, a physician may find more success prescribing the brand-name medications, regardless of pharmaceutical company influence, which is an alternative solution to policy changes that eliminate samples entirely. Although this study found insufficient evidence that removing samples decreases brand-name medication prescriptions, it is imperative that solutions are established to reduce the country’s increasing burden of medical costs.
Possible shortfalls of this study include the short period of time between which prepolicy data and postpolicy data were collected. It is possible that providers did not have enough time to adjust their prescribing habits or that providers would not have changed a prescribing pattern or preference simply because of a policy change. Future studies could allow a time period greater than 2 years to compare prepolicy and postpolicy prescribing habits, or a future study might make comparisons of prescriber patterns at different institutions that have different policies. Another possible shortfall is that providers and patients were limited to those at the Department of Dermatology & Cutaneous Surgery at the USF Morsani COM. Although this study has found insufficient evidence of a difference in prescribing habits, it may be beneficial to conduct a larger study that encompasses multiple academic institutions with similar policy changes. Most importantly, this study only investigated the influence of in-office pharmaceutical samples on prescribing patterns. This study did not look at the many other ways in which providers may be influenced by pharmaceutical companies, which likely is a significant confounding variable in this study. Continued additional studies that specifically examine other methods through which providers may be influenced would be helpful in further examining the many ways in which physician prescription habits are influenced.
Conclusion
Changes in pharmaceutical policy in 2011 at USF Morsani COM specifically banned in-office samples. The totality of evidence in this study shows modest observational evidence of a change in the postpolicy odds relative to prepolicy odds, but the data also are compatible with no change between prescribing habits before and after the policy changes. Further study is needed to fully understand this relationship.
- Sondergaard J, Vach K, Kragstrup J, et al. Impact of pharmaceutical representative visits on GPs’ drug preferences. Fam Pract. 2009;26:204-209.
- Jelinek GA, Neate SL. The influence of the pharmaceutical industry in medicine. J Law Med. 2009;17:216-223.
- Wazana A. Physicians and the pharmaceutical industry: is a gift ever just a gift? JAMA. 2000;283:373-380.
- Coleman DL. Establishing policies for the relationship between industry and clinicians: lessons learned from two academic health centers. Acad Med. 2008;83:882-887.
- Coleman DL, Kazdin AE, Miller LA, et al. Guidelines for interactions between clinical faculty and the pharmaceutical industry: one medical school’s approach. Acad Med. 2006;81:154-160.
- Evans D, Hartung DM, Beasley D, et al. Breaking up is hard to do: lessons learned from a pharma-free practice transformation. J Am Board Fam Med. 2013;26:332-338.
- Davit BM, Nwakama PE, Buehler GJ, et al. Comparing generic and innovator drugs: a review of 12 years of bioequivalence data from the United States Food and Drug Administration. Ann Pharmacother. 2009;43:1583-1597.
- Kesselheim AS, Misono AS, Lee JL, et al. Clinical equivalence of generic and brand-name drugs used in cardiovascular disease: a systematic review and meta-analysis. JAMA. 2008;300:2514-2526.
- McCormack J, Chmelicek JT. Generic versus brand name: the other drug war. Can Fam Physician. 2014;60:911.
- Borgheini G. The bioequivalence and therapeutic efficacy of generic versus brand-name psychoactive drugs. Clin Ther. 2003;25:1578-1592.
- Garrison GD, Levin GM. Factors affecting prescribing of the newer antidepressants. Ann Pharmacother. 2000;34:10-14.
- Rafique S, Sarwar W, Rashid A, et al. Influence of free drug samples on prescribing by physicians: a cross sectional survey. J Pak Med Assoc. 2017;67:465-467.
- Alexander GC, Zhang J, Basu A. Characteristics of patients receiving pharmaceutical samples and association between sample receipt and out-of-pocket prescription costs. Med Care. 2008;46:394-402.
- Hodges B. Interactions with the pharmaceutical industry: experiences and attitudes of psychiatry residents, interns and clerks. CMAJ. 1995;153:553-559.
- Brotzman GL, Mark DH. The effect on resident attitudes of regulatory policies regarding pharmaceutical representative activities. J Gen Intern Med. 1993;8:130-134.
- Keim SM, Sanders AB, Witzke DB, et al. Beliefs and practices of emergency medicine faculty and residents regarding professional interactions with the biomedical industry. Ann Emerg Med. 1993;22:1576-1581.
- Thomson AN, Craig BJ, Barham PM. Attitudes of general practitioners in New Zealand to pharmaceutical representatives. Br J Gen Pract. 1994;44:220-223.
- Ziegler MG, Lew P, Singer BC. The accuracy of drug information from pharmaceutical sales representatives. JAMA. 1995;273:1296-1298.
- Hurley MP, Stafford RS, Lane AT. Characterizing the relationship between free drug samples and prescription patterns for acne vulgaris and rosacea. JAMA Dermatol. 2014;150:487-493.
- Lexchin J. Interactions between physicians and the pharmaceutical industry: what does the literature say? CMAJ. 1993;149:1401-1407.
- Lieb K, Scheurich A. Contact between doctors and the pharmaceutical industry, their perceptions, and the effects on prescribing habits. PLoS One. 2014;9:e110130.
- Spurling GK, Mansfield PR, Montgomery BD, et al. Information from pharmaceutical companies and the quality, quantity, and cost of physicians’ prescribing: a systematic review. PLoS Med. 2010;7:e1000352.
- Fischer MA, Avorn J. Economic consequences of underuse of generic drugs: evidence from Medicaid and implications for prescription drug benefit plans. Health Serv Res. 2003;38:1051-1064.
- Sacks CA, Lee CC, Kesselheim AS, et al. Medicare spending on brand-name combination medications vs their generic constituents. JAMA. 2018;320:650-656.
- Brennan TA, Rothman DJ, Blank L, et al. Health industry practices that create conflicts of interest: a policy proposal for academic medical centers. JAMA. 2006;295:429-433.
- Allan GM, Lexchin J, Wiebe N. Physician awareness of drug cost: a systematic review. PLoS Med. 2007;4:e283.
- Sondergaard J, Vach K, Kragstrup J, et al. Impact of pharmaceutical representative visits on GPs’ drug preferences. Fam Pract. 2009;26:204-209.
- Jelinek GA, Neate SL. The influence of the pharmaceutical industry in medicine. J Law Med. 2009;17:216-223.
- Wazana A. Physicians and the pharmaceutical industry: is a gift ever just a gift? JAMA. 2000;283:373-380.
- Coleman DL. Establishing policies for the relationship between industry and clinicians: lessons learned from two academic health centers. Acad Med. 2008;83:882-887.
- Coleman DL, Kazdin AE, Miller LA, et al. Guidelines for interactions between clinical faculty and the pharmaceutical industry: one medical school’s approach. Acad Med. 2006;81:154-160.
- Evans D, Hartung DM, Beasley D, et al. Breaking up is hard to do: lessons learned from a pharma-free practice transformation. J Am Board Fam Med. 2013;26:332-338.
- Davit BM, Nwakama PE, Buehler GJ, et al. Comparing generic and innovator drugs: a review of 12 years of bioequivalence data from the United States Food and Drug Administration. Ann Pharmacother. 2009;43:1583-1597.
- Kesselheim AS, Misono AS, Lee JL, et al. Clinical equivalence of generic and brand-name drugs used in cardiovascular disease: a systematic review and meta-analysis. JAMA. 2008;300:2514-2526.
- McCormack J, Chmelicek JT. Generic versus brand name: the other drug war. Can Fam Physician. 2014;60:911.
- Borgheini G. The bioequivalence and therapeutic efficacy of generic versus brand-name psychoactive drugs. Clin Ther. 2003;25:1578-1592.
- Garrison GD, Levin GM. Factors affecting prescribing of the newer antidepressants. Ann Pharmacother. 2000;34:10-14.
- Rafique S, Sarwar W, Rashid A, et al. Influence of free drug samples on prescribing by physicians: a cross sectional survey. J Pak Med Assoc. 2017;67:465-467.
- Alexander GC, Zhang J, Basu A. Characteristics of patients receiving pharmaceutical samples and association between sample receipt and out-of-pocket prescription costs. Med Care. 2008;46:394-402.
- Hodges B. Interactions with the pharmaceutical industry: experiences and attitudes of psychiatry residents, interns and clerks. CMAJ. 1995;153:553-559.
- Brotzman GL, Mark DH. The effect on resident attitudes of regulatory policies regarding pharmaceutical representative activities. J Gen Intern Med. 1993;8:130-134.
- Keim SM, Sanders AB, Witzke DB, et al. Beliefs and practices of emergency medicine faculty and residents regarding professional interactions with the biomedical industry. Ann Emerg Med. 1993;22:1576-1581.
- Thomson AN, Craig BJ, Barham PM. Attitudes of general practitioners in New Zealand to pharmaceutical representatives. Br J Gen Pract. 1994;44:220-223.
- Ziegler MG, Lew P, Singer BC. The accuracy of drug information from pharmaceutical sales representatives. JAMA. 1995;273:1296-1298.
- Hurley MP, Stafford RS, Lane AT. Characterizing the relationship between free drug samples and prescription patterns for acne vulgaris and rosacea. JAMA Dermatol. 2014;150:487-493.
- Lexchin J. Interactions between physicians and the pharmaceutical industry: what does the literature say? CMAJ. 1993;149:1401-1407.
- Lieb K, Scheurich A. Contact between doctors and the pharmaceutical industry, their perceptions, and the effects on prescribing habits. PLoS One. 2014;9:e110130.
- Spurling GK, Mansfield PR, Montgomery BD, et al. Information from pharmaceutical companies and the quality, quantity, and cost of physicians’ prescribing: a systematic review. PLoS Med. 2010;7:e1000352.
- Fischer MA, Avorn J. Economic consequences of underuse of generic drugs: evidence from Medicaid and implications for prescription drug benefit plans. Health Serv Res. 2003;38:1051-1064.
- Sacks CA, Lee CC, Kesselheim AS, et al. Medicare spending on brand-name combination medications vs their generic constituents. JAMA. 2018;320:650-656.
- Brennan TA, Rothman DJ, Blank L, et al. Health industry practices that create conflicts of interest: a policy proposal for academic medical centers. JAMA. 2006;295:429-433.
- Allan GM, Lexchin J, Wiebe N. Physician awareness of drug cost: a systematic review. PLoS Med. 2007;4:e283.
Practice Points
- There has been growing concern that pharmaceutical interactions and incentives can influence physicians’ prescribing habits.
- Many academic centers have adopted policies that attempt to limit the pharmaceutical industry’s influence on faculty and in-training physicians.
- This study aimed to investigate if there was a change in the prescribing habits of academic dermatologists after the medical school implemented new policies that banned in-office samples.
Transillumination for Improved Diagnosis of Digital Myxoid Cysts
Practice Gap
Myxoid cysts are among the most common space-occupying lesions involving the nail unit. Their etiology has not been fully elucidated, but these cysts likely form due to leakage of synovial fluid following trauma or chronic wear and tear. They are highly associated with osteoarthritis and typically are found in close proximity to the distal interphalangeal joints.1 Myxoid cysts often extend into the eponychium, where mechanical stress on the nail matrix may lead to nail dystrophy, most commonly resulting in a longitudinal groove in the nail plate (Figure, A). The presence of multiple myxoid cysts is not uncommon. Differentiation of this lesion from other nodules of the digits, including epidermoid cysts, acquired digital fibrokeratomas, and giant cell tendon sheath tumors often is challenging without a biopsy.

Technique
The normal nail unit transmits light to some extent, and masses may be identified by how easily they transmit light relative to the adjacent skin. Solid tumors of the nail unit, such as acquired digital fibrokeratomas and giant cell tendon sheath tumors, will not transmit light, while myxoid cysts transmit light easily. A dermatoscope can be used to project light from the dorsal digit through the nail unit. The area occupied by the myxoid cyst will appear bright compared to the surrounding skin (Figure, B). Drainage of the lesion using an 18-gauge needle yielded a clear jellylike fluid that was consistent with a myxoid cyst. This technique aids in localizing and characterizing the myxoid cyst for treatment or drainage. Physician assessment of transillumination has been shown to demonstrate clinical accuracy and high intraobserver reliability in differentiating between cystic and solid tumors.2
Practice Implications
Transillumination is a valuable technique that may aid dermatologists in both the diagnosis and subsequent treatment of myxoid cysts. Location is important to consider when choosing a treatment option. Although lower recurrence rates are achieved with nail surgery, permanent nail dystrophy is likely when cysts are in close proximity to the nail matrix.3 When multiple cysts are present, only the largest may be apparent. Transillumination can guide the physician in achieving more accurate and thorough drainage of the cyst contents, negating the need for more costly imaging modalities. Dermatologists may utilize transillumination as a rapid and economical diagnostic method for space-occupying lesions involving the nail unit.
- Dermatol Surg. 2008;34:364-369.
- Erne HC, Gardner TR, Strauch RJ. Transillumination of hand tumors: a cadaver study to evaluate accuracy and intraobserver reliability. Hand (N Y). 2011;6:390-393.
- Fritz GR, Stern PJ, Dickey M. Complications following mucous cyst excision. J Hand Surg Br. 1997;22:222-225.
Practice Gap
Myxoid cysts are among the most common space-occupying lesions involving the nail unit. Their etiology has not been fully elucidated, but these cysts likely form due to leakage of synovial fluid following trauma or chronic wear and tear. They are highly associated with osteoarthritis and typically are found in close proximity to the distal interphalangeal joints.1 Myxoid cysts often extend into the eponychium, where mechanical stress on the nail matrix may lead to nail dystrophy, most commonly resulting in a longitudinal groove in the nail plate (Figure, A). The presence of multiple myxoid cysts is not uncommon. Differentiation of this lesion from other nodules of the digits, including epidermoid cysts, acquired digital fibrokeratomas, and giant cell tendon sheath tumors often is challenging without a biopsy.

Technique
The normal nail unit transmits light to some extent, and masses may be identified by how easily they transmit light relative to the adjacent skin. Solid tumors of the nail unit, such as acquired digital fibrokeratomas and giant cell tendon sheath tumors, will not transmit light, while myxoid cysts transmit light easily. A dermatoscope can be used to project light from the dorsal digit through the nail unit. The area occupied by the myxoid cyst will appear bright compared to the surrounding skin (Figure, B). Drainage of the lesion using an 18-gauge needle yielded a clear jellylike fluid that was consistent with a myxoid cyst. This technique aids in localizing and characterizing the myxoid cyst for treatment or drainage. Physician assessment of transillumination has been shown to demonstrate clinical accuracy and high intraobserver reliability in differentiating between cystic and solid tumors.2
Practice Implications
Transillumination is a valuable technique that may aid dermatologists in both the diagnosis and subsequent treatment of myxoid cysts. Location is important to consider when choosing a treatment option. Although lower recurrence rates are achieved with nail surgery, permanent nail dystrophy is likely when cysts are in close proximity to the nail matrix.3 When multiple cysts are present, only the largest may be apparent. Transillumination can guide the physician in achieving more accurate and thorough drainage of the cyst contents, negating the need for more costly imaging modalities. Dermatologists may utilize transillumination as a rapid and economical diagnostic method for space-occupying lesions involving the nail unit.
Practice Gap
Myxoid cysts are among the most common space-occupying lesions involving the nail unit. Their etiology has not been fully elucidated, but these cysts likely form due to leakage of synovial fluid following trauma or chronic wear and tear. They are highly associated with osteoarthritis and typically are found in close proximity to the distal interphalangeal joints.1 Myxoid cysts often extend into the eponychium, where mechanical stress on the nail matrix may lead to nail dystrophy, most commonly resulting in a longitudinal groove in the nail plate (Figure, A). The presence of multiple myxoid cysts is not uncommon. Differentiation of this lesion from other nodules of the digits, including epidermoid cysts, acquired digital fibrokeratomas, and giant cell tendon sheath tumors often is challenging without a biopsy.

Technique
The normal nail unit transmits light to some extent, and masses may be identified by how easily they transmit light relative to the adjacent skin. Solid tumors of the nail unit, such as acquired digital fibrokeratomas and giant cell tendon sheath tumors, will not transmit light, while myxoid cysts transmit light easily. A dermatoscope can be used to project light from the dorsal digit through the nail unit. The area occupied by the myxoid cyst will appear bright compared to the surrounding skin (Figure, B). Drainage of the lesion using an 18-gauge needle yielded a clear jellylike fluid that was consistent with a myxoid cyst. This technique aids in localizing and characterizing the myxoid cyst for treatment or drainage. Physician assessment of transillumination has been shown to demonstrate clinical accuracy and high intraobserver reliability in differentiating between cystic and solid tumors.2
Practice Implications
Transillumination is a valuable technique that may aid dermatologists in both the diagnosis and subsequent treatment of myxoid cysts. Location is important to consider when choosing a treatment option. Although lower recurrence rates are achieved with nail surgery, permanent nail dystrophy is likely when cysts are in close proximity to the nail matrix.3 When multiple cysts are present, only the largest may be apparent. Transillumination can guide the physician in achieving more accurate and thorough drainage of the cyst contents, negating the need for more costly imaging modalities. Dermatologists may utilize transillumination as a rapid and economical diagnostic method for space-occupying lesions involving the nail unit.
- Dermatol Surg. 2008;34:364-369.
- Erne HC, Gardner TR, Strauch RJ. Transillumination of hand tumors: a cadaver study to evaluate accuracy and intraobserver reliability. Hand (N Y). 2011;6:390-393.
- Fritz GR, Stern PJ, Dickey M. Complications following mucous cyst excision. J Hand Surg Br. 1997;22:222-225.
- Dermatol Surg. 2008;34:364-369.
- Erne HC, Gardner TR, Strauch RJ. Transillumination of hand tumors: a cadaver study to evaluate accuracy and intraobserver reliability. Hand (N Y). 2011;6:390-393.
- Fritz GR, Stern PJ, Dickey M. Complications following mucous cyst excision. J Hand Surg Br. 1997;22:222-225.
Subungual Hemorrhage From an Epidermal Growth Factor Receptor Inhibitor
To the Editor:
The epidermal growth factor receptor (EGFR) signaling pathway plays a role in the differentiation, proliferation, and survival of several cell types.1 Erlotinib is an EGFR inhibitor that targets aberrant cells that overexpress this receptor and has been used in the treatment of various solid malignant tumors.2,3 Common dermatologic side effects associated with EGFR inhibitors include papulopustular rash, xeroderma, and paronychia.2,3 We present a unique finding of subungual hemorrhage of the thumbnails in a patient taking erlotinib.
A 50-year-old man presented with acute-onset tenderness and discoloration of the thumbnails of 1 week’s duration. There was no preceding trauma or history of similar symptoms. His medical history was notable for recurrent lung adenocarcinoma with EGFR L858R mutation. Erlotinib therapy was initiated 5 weeks prior to symptom onset. He developed notable xeroderma of the palms and soles that preceded nail changes by a few days. He completed treatment with carboplatin and pemetrexed 16 months prior to relapse after paclitaxel failed due to a severe allergic reaction. There were no nail symptoms during that time. The patient did not have a documented coagulation disorder and was not on any known medications that would predispose him to bleeding. Physical examination demonstrated subungual hemorrhage of the thumbnails with tenderness on palpation (Figure). There was no evidence of periungual changes or nail plate abnormality. All other nails appeared normal. Laboratory test results showed normal platelets. Supportive therapeutic measures were recommended, and the patient was advised to avoid trauma to the nails.
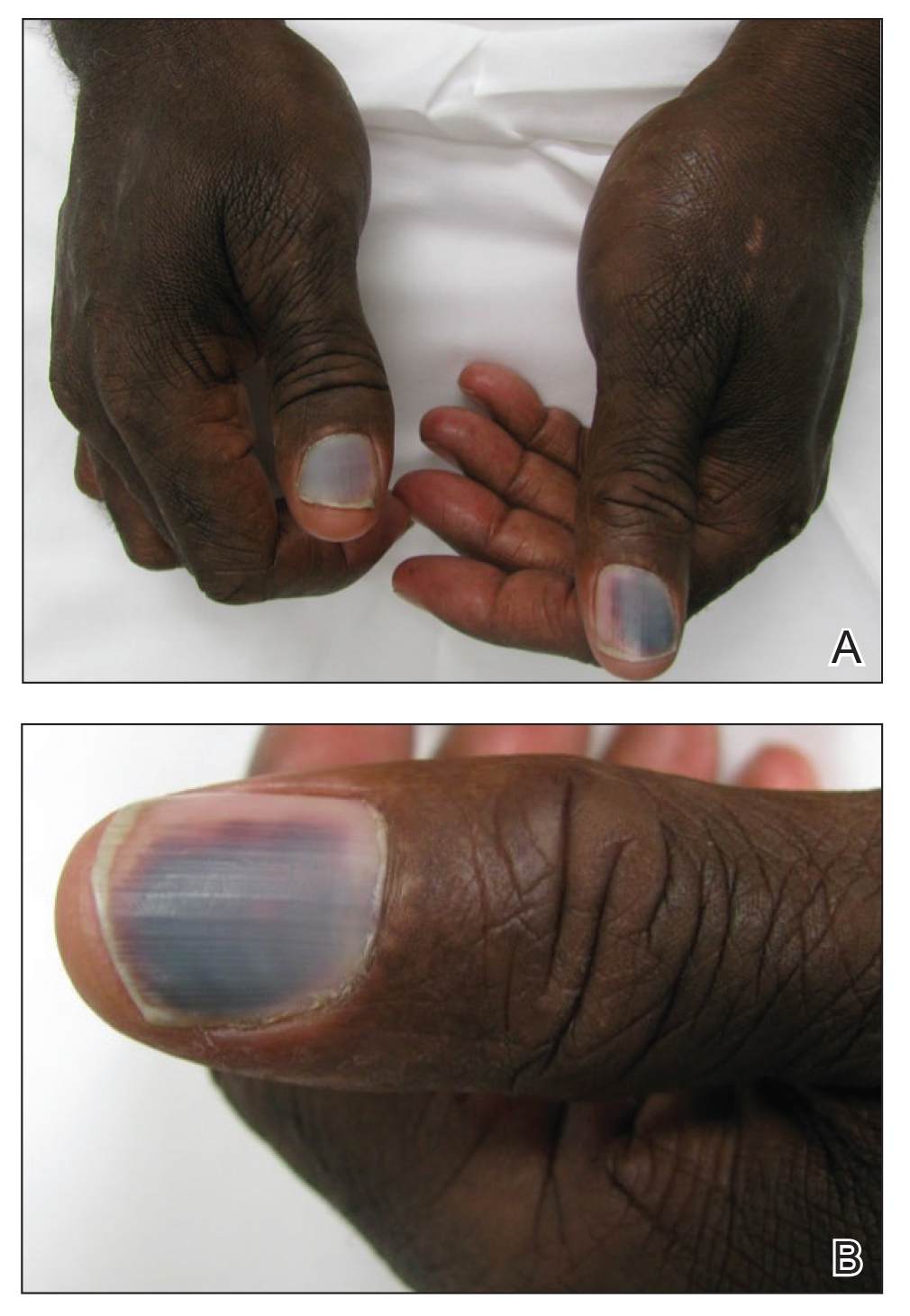
Nail toxicities reported with EGFR inhibitors include paronychia, periungual pyogenic granulomas, and ingrown nails.1-3 Inflammation of the nail bed also can lead to secondary nail changes, such as onychodystrophy or onycholysis.2 Subungual hemorrhage has been reported as a side effect of taxanes, anticoagulants, anthracyclines, anti-inflammatory agents, and retinoids.4,5
The pathogenesis of nail toxicity secondary to EGFR inhibitors is not entirely clear. Symptoms commonly occur several weeks to months after therapy initiation.6 Epidermal growth factor receptor inhibitors disrupt proliferation and promote apoptosis of keratinocytes that is thought to enhance fragility of the periungual skin and nail plate.1,3 Under the influence of EGFR inhibition, a proinflammatory microenvironment in the skin is created through a type I interferon response leading to tissue damage.7 These changes may predispose patients to develop subungual hemorrhage in response to repeated nail microtrauma. Subungual asymptomatic splinter hemorrhage is a nail finding described in patients treated with the multikinase inhibitors sorafenib and sunitinib. Splinter hemorrhages of the nails are thought to be secondary to capillary microinjuries of the digits that cannot be repaired due to inhibition of vascular EGFRs.4
The time course of erlotinib administration and the simultaneous onset of xeroderma, a known side effect of the drug, in our patient are consistent with other cases.6 Subungual hemorrhage, which the patient reported observing only days after the onset of xeroderma, provides increased support that the anti-EGFR medication was likely responsible for both side effects concurrently. Bilateral involvement of the thumbs makes trauma as an inciting event unlikely.
Incidence of nail changes secondary to anti-EGFR drugs are likely underestimated and underreported.3 Subungual hemorrhage should be considered as an additional, less common nail side effect of EGFR inhibitors that clinicians and patients may encounter. Improved awareness and understanding of nail toxicities associated with EGFR inhibitors may offer better insight into the pathogenesis of these side effects and management options.
- Piraccini BM, Alessandrini A. Drug-related nail disease. Clin Dermatol. 2013;31:618-626.
- Kiyohara Y, Yamazaki N, Kishi A. Erlotinib-related skin toxicities: treatment strategies in patients with metastatic non-small cell lung cancer. J Am Acad Dermatol. 2013;69:463-472.
- Minisini AM, Tosti A, Sobrero AF, et al. Taxane-induced nail changes: incidence, clinical presentation and outcome. Ann Oncol. 2003;333-337.
- Garden BC, Wu S, Lacouture ME. The risk of nail changes with epidermal growth factor receptor inhibitors: a systematic review of the literature and meta-analysis. J Am Acad Dermatol. 2012;67:400-408.
- Fox LP. Nail toxicity associated with epidermal growth factor receptor inhibitor therapy. J Am Acad Dermatol. 2007;56:460-465.
- Chen KL, Lin CC, Cho YT, et al. Comparison of skin toxic effects associated with gefitinib, erlotinib or afatinib treatment for non-small cell lung cancer. JAMA Dermatol. 2016;152:340-342.
- Lulli D, Carbone ML, Pastore S. Epidermal growth factor receptor inhibitors trigger a type I interferon response in human skin. Oncotarget. 2016;7:47777-47793.
To the Editor:
The epidermal growth factor receptor (EGFR) signaling pathway plays a role in the differentiation, proliferation, and survival of several cell types.1 Erlotinib is an EGFR inhibitor that targets aberrant cells that overexpress this receptor and has been used in the treatment of various solid malignant tumors.2,3 Common dermatologic side effects associated with EGFR inhibitors include papulopustular rash, xeroderma, and paronychia.2,3 We present a unique finding of subungual hemorrhage of the thumbnails in a patient taking erlotinib.
A 50-year-old man presented with acute-onset tenderness and discoloration of the thumbnails of 1 week’s duration. There was no preceding trauma or history of similar symptoms. His medical history was notable for recurrent lung adenocarcinoma with EGFR L858R mutation. Erlotinib therapy was initiated 5 weeks prior to symptom onset. He developed notable xeroderma of the palms and soles that preceded nail changes by a few days. He completed treatment with carboplatin and pemetrexed 16 months prior to relapse after paclitaxel failed due to a severe allergic reaction. There were no nail symptoms during that time. The patient did not have a documented coagulation disorder and was not on any known medications that would predispose him to bleeding. Physical examination demonstrated subungual hemorrhage of the thumbnails with tenderness on palpation (Figure). There was no evidence of periungual changes or nail plate abnormality. All other nails appeared normal. Laboratory test results showed normal platelets. Supportive therapeutic measures were recommended, and the patient was advised to avoid trauma to the nails.

Nail toxicities reported with EGFR inhibitors include paronychia, periungual pyogenic granulomas, and ingrown nails.1-3 Inflammation of the nail bed also can lead to secondary nail changes, such as onychodystrophy or onycholysis.2 Subungual hemorrhage has been reported as a side effect of taxanes, anticoagulants, anthracyclines, anti-inflammatory agents, and retinoids.4,5
The pathogenesis of nail toxicity secondary to EGFR inhibitors is not entirely clear. Symptoms commonly occur several weeks to months after therapy initiation.6 Epidermal growth factor receptor inhibitors disrupt proliferation and promote apoptosis of keratinocytes that is thought to enhance fragility of the periungual skin and nail plate.1,3 Under the influence of EGFR inhibition, a proinflammatory microenvironment in the skin is created through a type I interferon response leading to tissue damage.7 These changes may predispose patients to develop subungual hemorrhage in response to repeated nail microtrauma. Subungual asymptomatic splinter hemorrhage is a nail finding described in patients treated with the multikinase inhibitors sorafenib and sunitinib. Splinter hemorrhages of the nails are thought to be secondary to capillary microinjuries of the digits that cannot be repaired due to inhibition of vascular EGFRs.4
The time course of erlotinib administration and the simultaneous onset of xeroderma, a known side effect of the drug, in our patient are consistent with other cases.6 Subungual hemorrhage, which the patient reported observing only days after the onset of xeroderma, provides increased support that the anti-EGFR medication was likely responsible for both side effects concurrently. Bilateral involvement of the thumbs makes trauma as an inciting event unlikely.
Incidence of nail changes secondary to anti-EGFR drugs are likely underestimated and underreported.3 Subungual hemorrhage should be considered as an additional, less common nail side effect of EGFR inhibitors that clinicians and patients may encounter. Improved awareness and understanding of nail toxicities associated with EGFR inhibitors may offer better insight into the pathogenesis of these side effects and management options.
To the Editor:
The epidermal growth factor receptor (EGFR) signaling pathway plays a role in the differentiation, proliferation, and survival of several cell types.1 Erlotinib is an EGFR inhibitor that targets aberrant cells that overexpress this receptor and has been used in the treatment of various solid malignant tumors.2,3 Common dermatologic side effects associated with EGFR inhibitors include papulopustular rash, xeroderma, and paronychia.2,3 We present a unique finding of subungual hemorrhage of the thumbnails in a patient taking erlotinib.
A 50-year-old man presented with acute-onset tenderness and discoloration of the thumbnails of 1 week’s duration. There was no preceding trauma or history of similar symptoms. His medical history was notable for recurrent lung adenocarcinoma with EGFR L858R mutation. Erlotinib therapy was initiated 5 weeks prior to symptom onset. He developed notable xeroderma of the palms and soles that preceded nail changes by a few days. He completed treatment with carboplatin and pemetrexed 16 months prior to relapse after paclitaxel failed due to a severe allergic reaction. There were no nail symptoms during that time. The patient did not have a documented coagulation disorder and was not on any known medications that would predispose him to bleeding. Physical examination demonstrated subungual hemorrhage of the thumbnails with tenderness on palpation (Figure). There was no evidence of periungual changes or nail plate abnormality. All other nails appeared normal. Laboratory test results showed normal platelets. Supportive therapeutic measures were recommended, and the patient was advised to avoid trauma to the nails.

Nail toxicities reported with EGFR inhibitors include paronychia, periungual pyogenic granulomas, and ingrown nails.1-3 Inflammation of the nail bed also can lead to secondary nail changes, such as onychodystrophy or onycholysis.2 Subungual hemorrhage has been reported as a side effect of taxanes, anticoagulants, anthracyclines, anti-inflammatory agents, and retinoids.4,5
The pathogenesis of nail toxicity secondary to EGFR inhibitors is not entirely clear. Symptoms commonly occur several weeks to months after therapy initiation.6 Epidermal growth factor receptor inhibitors disrupt proliferation and promote apoptosis of keratinocytes that is thought to enhance fragility of the periungual skin and nail plate.1,3 Under the influence of EGFR inhibition, a proinflammatory microenvironment in the skin is created through a type I interferon response leading to tissue damage.7 These changes may predispose patients to develop subungual hemorrhage in response to repeated nail microtrauma. Subungual asymptomatic splinter hemorrhage is a nail finding described in patients treated with the multikinase inhibitors sorafenib and sunitinib. Splinter hemorrhages of the nails are thought to be secondary to capillary microinjuries of the digits that cannot be repaired due to inhibition of vascular EGFRs.4
The time course of erlotinib administration and the simultaneous onset of xeroderma, a known side effect of the drug, in our patient are consistent with other cases.6 Subungual hemorrhage, which the patient reported observing only days after the onset of xeroderma, provides increased support that the anti-EGFR medication was likely responsible for both side effects concurrently. Bilateral involvement of the thumbs makes trauma as an inciting event unlikely.
Incidence of nail changes secondary to anti-EGFR drugs are likely underestimated and underreported.3 Subungual hemorrhage should be considered as an additional, less common nail side effect of EGFR inhibitors that clinicians and patients may encounter. Improved awareness and understanding of nail toxicities associated with EGFR inhibitors may offer better insight into the pathogenesis of these side effects and management options.
- Piraccini BM, Alessandrini A. Drug-related nail disease. Clin Dermatol. 2013;31:618-626.
- Kiyohara Y, Yamazaki N, Kishi A. Erlotinib-related skin toxicities: treatment strategies in patients with metastatic non-small cell lung cancer. J Am Acad Dermatol. 2013;69:463-472.
- Minisini AM, Tosti A, Sobrero AF, et al. Taxane-induced nail changes: incidence, clinical presentation and outcome. Ann Oncol. 2003;333-337.
- Garden BC, Wu S, Lacouture ME. The risk of nail changes with epidermal growth factor receptor inhibitors: a systematic review of the literature and meta-analysis. J Am Acad Dermatol. 2012;67:400-408.
- Fox LP. Nail toxicity associated with epidermal growth factor receptor inhibitor therapy. J Am Acad Dermatol. 2007;56:460-465.
- Chen KL, Lin CC, Cho YT, et al. Comparison of skin toxic effects associated with gefitinib, erlotinib or afatinib treatment for non-small cell lung cancer. JAMA Dermatol. 2016;152:340-342.
- Lulli D, Carbone ML, Pastore S. Epidermal growth factor receptor inhibitors trigger a type I interferon response in human skin. Oncotarget. 2016;7:47777-47793.
- Piraccini BM, Alessandrini A. Drug-related nail disease. Clin Dermatol. 2013;31:618-626.
- Kiyohara Y, Yamazaki N, Kishi A. Erlotinib-related skin toxicities: treatment strategies in patients with metastatic non-small cell lung cancer. J Am Acad Dermatol. 2013;69:463-472.
- Minisini AM, Tosti A, Sobrero AF, et al. Taxane-induced nail changes: incidence, clinical presentation and outcome. Ann Oncol. 2003;333-337.
- Garden BC, Wu S, Lacouture ME. The risk of nail changes with epidermal growth factor receptor inhibitors: a systematic review of the literature and meta-analysis. J Am Acad Dermatol. 2012;67:400-408.
- Fox LP. Nail toxicity associated with epidermal growth factor receptor inhibitor therapy. J Am Acad Dermatol. 2007;56:460-465.
- Chen KL, Lin CC, Cho YT, et al. Comparison of skin toxic effects associated with gefitinib, erlotinib or afatinib treatment for non-small cell lung cancer. JAMA Dermatol. 2016;152:340-342.
- Lulli D, Carbone ML, Pastore S. Epidermal growth factor receptor inhibitors trigger a type I interferon response in human skin. Oncotarget. 2016;7:47777-47793.
Practice Points
- Subungual hemorrhage is a potential adverse side effect of epidermal growth factor receptor inhibitors.
- Epidermal growth factor receptor inhibition may lead to enhanced fragility of the periungual skin and nail plate as well as a proinflammatory microenvironment in the skin, predisposing patients to nail toxicity.
Remote-Onset Alopecia Areata Attributed to Ipilimumab
Cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) is a key co-stimulatory receptor expressed on activated T cells that negatively regulates T-cell activation.1-3 It exerts its effects in part by the prevention of IL-2 transcription and inhibition of cell-cycle progression.4 Cytotoxic T-lymphocyte–associated antigen 4 also is expressed by a subset of CD25+CD4+ regulatory T cells (Tregs), where it plays a role in immune tolerance.5 Blockade has demonstrated antitumor activity as well as immune activation, and CTLA-4 dysregulation has been implicated in autoimmune diseases such as alopecia areata (AA).6
Ipilimumab is a fully humanized monoclonal antibody against CTLA-4 and one of a growing class of immune checkpoint inhibitor therapies for metastatic melanoma. Phase 2 and 3 clinical trials have shown an improved survival effect of ipilimumab in patients with advanced melanoma,7-10 with 3-year survival rates ranging from 20.8% to 46.5%.10,11 The US Food and Drug Administration approved ipilimumab in 2011 for treatment of unresectable or metastatic melanoma.12 The most common toxicities of ipilimumab are immune-related adverse effects (irAEs), which represent loss of tolerance to self-antigens.13 Immune-related adverse effects occur in 64.2% of patients,14 with severe or life-threatening irAEs in 17.8% of patients.14 Rates of irAEs appear dose dependent but consistent across increased doses.15 Cutaneous irAEs occur in more than 47% of patients16 and commonly manifest as pruritus with or without a diffuse morbilliform rash,10,17 though less common skin reactions, including vitiligo, vasculitis, and Stevens-Johnson syndrome/toxic epidermal necrolysis, have been documented.9,18
Generalized AA and its more widespread variant, alopecia universalis, have been reported as adverse effects of ipilimumab monotherapy in 2 prior cases in the English-language literature (Table).17,19 Alopecia areata also has been attributed to combination immune checkpoint inhibitor therapy.20,21 We report a case of AA attributable to ipilimumab monotherapy that was localized exclusively to the scalp and remote in onset following treatment.
Case Report
An 88-year-old man with pT3bpN3 nodular melanoma of the back demonstrated multiple lung metastases by positron emission tomography–computed tomography. Lactate dehydrogenase was within reference range, and his Eastern Cooperative Oncology Group performance status was 0 (fully active). One month later, he was started on ipilimumab 3 mg/kg intravenous infusion every 3 weeks for a total of 4 doses. At approximately week 6, his course was complicated by mild fatigue, a faintly erythematous morbilliform rash, and mild pruritus, with laboratory evidence of subclinical hyperthyroidism. Follow-up positron emission tomography–computed tomography at the conclusion of treatment demonstrated complete regression of previously noted hypermetabolic foci. His symptoms and subclinical hyperthyroidism resolved several months later.
Seventeen months after completion of ipilimumab therapy (at age 90 years), the patient’s barber noted new-onset hair loss on the right occipital scalp. Physical examination demonstrated a well-circumscribed patch of nonscarring alopecia (approximately 6 cm) that was clinically consistent with AA (Figure). There were no associated symptoms or other involved areas of hair loss. He denied any personal or family history of AA. The patient’s melanoma has remained in remission to date.
Comment
This case is unique in that AA was localized to a single circumscribed patch on the scalp and occurred nearly 1.5 years after treatment with ipilimumab, which may indicate a robust blockade of CTLA-4 given the remote development of autoimmunity in the setting of persistent remission of melanoma. Although the appearance of AA may be coincidental, onset at 90 years of age would be unusual. The mean age of onset of AA has been reported between 25.2 and 36.3 years,22,23 and its incidence in men older than 60 years is only 6.4 per 100,000 person-years.24
Although AA is a rare irAE of CTLA-4 blockade, the disease has been increasingly linked to CTLA-4 dysregulation in both animal models and humans.6,25,26 A genome-wide association study of 1054 patients with AA and 3278 controls implicated several genes controlling activation and proliferation of Tregs, including CTLA-4.27 More specifically, single-nucleotide polymorphisms of the CTLA-4 gene were found to be associated with AA in a study of 1196 unrelated patients and 1280 controls,28 and Megiorni et al
Given the role of CTLA-4 dysregulation in the pathogenesis of AA, the very low rates of AA in ipilimumab are somewhat surprising, which may represent a reporting bias. Alternatively, there may be sufficient Treg activity to prevent high rates of AA at a lower ipilimumab dose of 3 mg/kg but insufficient activity to prevent development of other irAEs. With US Food and Drug Administration approval of ipilimumab at a higher dose of 10 mg/kg for use as adjuvant therapy for stage III melanomas,12 less common cutaneous irAEs such as AA may be seen with increased frequency. Clinicians planning ipilimumab therapy should discuss this side effect and other potential irAEs with their patients before initiation of treatment.
- Brunet JF, Denizot F, Luciani MF, et al. A new member of the immunoglobulin superfamily--CTLA-4. Nature. 1987;328:267-270.
- Scalapino KJ, Daikh DI. CTLA-4: a key regulatory point in the control of autoimmune disease. Immunol Rev. 2008;223:143-155.
- Buchbinder E, Hodi FS. Cytotoxic T lymphocyte antigen-4 and immune checkpoint blockade. J Clin Invest. 2015;125:3377-3383.
- Brunner MC, Chambers CA, Chan FK, et al. CTLA-4-mediated inhibition of early events of T cell proliferation. J Immunol. 1999;162:5813-5820.
- Takahashi T, Tagami T, Yamazaki S, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303-310.
- Carroll JM, McElwee KJ, E King L, et al. Gene array profiling and immunomodulation studies define a cell-mediated immune response underlying the pathogenesis of alopecia areata in a mouse model and humans. J Invest Dermatol. 2002;119:392-402.
- Weber J, Thompson JA, Hamid O, et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res. 2009;15:5591-5598.
- O’Day SJ, Maio M, Chiarion-Sileni V, et al. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Ann Oncol. 2010;21:1712-1717.
- Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-723.
- Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517-2526.
- Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16:522-530.
- Yervoy (ipilimumab)[package insert]. Princeton, NJ: Bristol-Myers Squibb; 2019.
- Weber J. Review: anti-CTLA-4 antibody ipilimumab: case studies of clinical response and immune-related adverse events. Oncologist. 2007;12:864-872.
- Ibrahim RA, Berman DM, DePril V, et al. Ipilimumab safety profile: summary of findings from completed trials in advanced melanoma [abstract]. J Clin Oncol. 2011;29(suppl):8583.
- Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155-164.
- Kähler KC, Hauschild A. Treatment and side effect management of CTLA-4 antibody therapy in metastatic melanoma. J Dtsch Dermatol Ges. 2011;9:277-286.
- Jaber SH, Cowen EW, Haworth LR, et al. Skin reactions in a subset of patients with stage IV melanoma treated with anti-cytotoxic T-lymphocyte antigen 4 monoclonal antibody as a single agent. Arch Dermatol. 2006;142:166-172.
- Voskens CJ, Goldinger SM, Loquai C, et al. The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PLoS One. 2013;8:E537545.
- Assi H, Wilson KS. Immune toxicities and long remission duration after ipilimumab therapy for metastatic melanoma: two illustrative cases. Curr Oncol. 2013;20:E165-E169.
- Zarbo A, Belum VR, Sibaud V, et al. Immune-related alopecia (areata and universalis) in cancer patients receiving immune checkpoint inhibitors. Br J Dermatol. 2017;176:1649-1652.
- Lakhmiri M, Cavelier-Balloy B, Lacoste C, et al. Nivolumab-induced alopecia areata: a reversible factor of good prognosis? JAAD Case Rep. 2018;4:761-765.
- Tan E, Tay YK, Goh CL, et al. The pattern and profile of alopecia areata in Singapore–a study of 219 Asians. Int J Dermatol. 2002;41:748-753.
- Goh C, Finkel M, Christos PJ, et al. Profile of 513 patients with alopecia areata: associations of disease subtypes with atopy, autoimmune disease and positive family history. J Eur Acad Dermatol Venereol. 2006;20:1055-1060.
- Mirzoyev SA, Schrum AG, Davis MD, et al. Lifetime incidence risk of alopecia areata estimated at 2.1% by Rochester Epidemiology Project, 1990-2009. J Invest Dermatol. 2014;134:1141-1142.
- Zöller M, McElwee KJ, Engel P, et al. Transient CD44 variant isoform expression and reduction in CD4(+)/CD25(+) regulatory T cells in C3H/HeJ mice with alopecia areata. J Invest Dermatol. 2002;118:983-992.
- Zöller M, McElwee KJ, Vitacolonna M, et al. The progressive state, in contrast to the stable or regressive state of alopecia areata, is reflected in peripheral blood mononuclear cells. Exp Dermatol. 2004;13:435-444.
- Petukhova L, Duvic M, Hordinsky M, et al. Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature. 2010;466:113-117.
- John KK, Brockschmidt FF, Redler S, et al. Genetic variants in CTLA4 are strongly associated with alopecia areata. J Invest Dermatol. 2011;131:1169-1172.
- Megiorni F, Mora B, Maxia C, et al. Cytotoxic T-lymphocyte antigen 4 (CTLA4) +49AG and CT60 gene polymorphisms in alopecia areata: a case-control association study in the Italian population. Arch Dermatol Res. 2013;305:665-670
Cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) is a key co-stimulatory receptor expressed on activated T cells that negatively regulates T-cell activation.1-3 It exerts its effects in part by the prevention of IL-2 transcription and inhibition of cell-cycle progression.4 Cytotoxic T-lymphocyte–associated antigen 4 also is expressed by a subset of CD25+CD4+ regulatory T cells (Tregs), where it plays a role in immune tolerance.5 Blockade has demonstrated antitumor activity as well as immune activation, and CTLA-4 dysregulation has been implicated in autoimmune diseases such as alopecia areata (AA).6
Ipilimumab is a fully humanized monoclonal antibody against CTLA-4 and one of a growing class of immune checkpoint inhibitor therapies for metastatic melanoma. Phase 2 and 3 clinical trials have shown an improved survival effect of ipilimumab in patients with advanced melanoma,7-10 with 3-year survival rates ranging from 20.8% to 46.5%.10,11 The US Food and Drug Administration approved ipilimumab in 2011 for treatment of unresectable or metastatic melanoma.12 The most common toxicities of ipilimumab are immune-related adverse effects (irAEs), which represent loss of tolerance to self-antigens.13 Immune-related adverse effects occur in 64.2% of patients,14 with severe or life-threatening irAEs in 17.8% of patients.14 Rates of irAEs appear dose dependent but consistent across increased doses.15 Cutaneous irAEs occur in more than 47% of patients16 and commonly manifest as pruritus with or without a diffuse morbilliform rash,10,17 though less common skin reactions, including vitiligo, vasculitis, and Stevens-Johnson syndrome/toxic epidermal necrolysis, have been documented.9,18
Generalized AA and its more widespread variant, alopecia universalis, have been reported as adverse effects of ipilimumab monotherapy in 2 prior cases in the English-language literature (Table).17,19 Alopecia areata also has been attributed to combination immune checkpoint inhibitor therapy.20,21 We report a case of AA attributable to ipilimumab monotherapy that was localized exclusively to the scalp and remote in onset following treatment.

Case Report
An 88-year-old man with pT3bpN3 nodular melanoma of the back demonstrated multiple lung metastases by positron emission tomography–computed tomography. Lactate dehydrogenase was within reference range, and his Eastern Cooperative Oncology Group performance status was 0 (fully active). One month later, he was started on ipilimumab 3 mg/kg intravenous infusion every 3 weeks for a total of 4 doses. At approximately week 6, his course was complicated by mild fatigue, a faintly erythematous morbilliform rash, and mild pruritus, with laboratory evidence of subclinical hyperthyroidism. Follow-up positron emission tomography–computed tomography at the conclusion of treatment demonstrated complete regression of previously noted hypermetabolic foci. His symptoms and subclinical hyperthyroidism resolved several months later.
Seventeen months after completion of ipilimumab therapy (at age 90 years), the patient’s barber noted new-onset hair loss on the right occipital scalp. Physical examination demonstrated a well-circumscribed patch of nonscarring alopecia (approximately 6 cm) that was clinically consistent with AA (Figure). There were no associated symptoms or other involved areas of hair loss. He denied any personal or family history of AA. The patient’s melanoma has remained in remission to date.

Comment
This case is unique in that AA was localized to a single circumscribed patch on the scalp and occurred nearly 1.5 years after treatment with ipilimumab, which may indicate a robust blockade of CTLA-4 given the remote development of autoimmunity in the setting of persistent remission of melanoma. Although the appearance of AA may be coincidental, onset at 90 years of age would be unusual. The mean age of onset of AA has been reported between 25.2 and 36.3 years,22,23 and its incidence in men older than 60 years is only 6.4 per 100,000 person-years.24
Although AA is a rare irAE of CTLA-4 blockade, the disease has been increasingly linked to CTLA-4 dysregulation in both animal models and humans.6,25,26 A genome-wide association study of 1054 patients with AA and 3278 controls implicated several genes controlling activation and proliferation of Tregs, including CTLA-4.27 More specifically, single-nucleotide polymorphisms of the CTLA-4 gene were found to be associated with AA in a study of 1196 unrelated patients and 1280 controls,28 and Megiorni et al
Given the role of CTLA-4 dysregulation in the pathogenesis of AA, the very low rates of AA in ipilimumab are somewhat surprising, which may represent a reporting bias. Alternatively, there may be sufficient Treg activity to prevent high rates of AA at a lower ipilimumab dose of 3 mg/kg but insufficient activity to prevent development of other irAEs. With US Food and Drug Administration approval of ipilimumab at a higher dose of 10 mg/kg for use as adjuvant therapy for stage III melanomas,12 less common cutaneous irAEs such as AA may be seen with increased frequency. Clinicians planning ipilimumab therapy should discuss this side effect and other potential irAEs with their patients before initiation of treatment.
Cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) is a key co-stimulatory receptor expressed on activated T cells that negatively regulates T-cell activation.1-3 It exerts its effects in part by the prevention of IL-2 transcription and inhibition of cell-cycle progression.4 Cytotoxic T-lymphocyte–associated antigen 4 also is expressed by a subset of CD25+CD4+ regulatory T cells (Tregs), where it plays a role in immune tolerance.5 Blockade has demonstrated antitumor activity as well as immune activation, and CTLA-4 dysregulation has been implicated in autoimmune diseases such as alopecia areata (AA).6
Ipilimumab is a fully humanized monoclonal antibody against CTLA-4 and one of a growing class of immune checkpoint inhibitor therapies for metastatic melanoma. Phase 2 and 3 clinical trials have shown an improved survival effect of ipilimumab in patients with advanced melanoma,7-10 with 3-year survival rates ranging from 20.8% to 46.5%.10,11 The US Food and Drug Administration approved ipilimumab in 2011 for treatment of unresectable or metastatic melanoma.12 The most common toxicities of ipilimumab are immune-related adverse effects (irAEs), which represent loss of tolerance to self-antigens.13 Immune-related adverse effects occur in 64.2% of patients,14 with severe or life-threatening irAEs in 17.8% of patients.14 Rates of irAEs appear dose dependent but consistent across increased doses.15 Cutaneous irAEs occur in more than 47% of patients16 and commonly manifest as pruritus with or without a diffuse morbilliform rash,10,17 though less common skin reactions, including vitiligo, vasculitis, and Stevens-Johnson syndrome/toxic epidermal necrolysis, have been documented.9,18
Generalized AA and its more widespread variant, alopecia universalis, have been reported as adverse effects of ipilimumab monotherapy in 2 prior cases in the English-language literature (Table).17,19 Alopecia areata also has been attributed to combination immune checkpoint inhibitor therapy.20,21 We report a case of AA attributable to ipilimumab monotherapy that was localized exclusively to the scalp and remote in onset following treatment.

Case Report
An 88-year-old man with pT3bpN3 nodular melanoma of the back demonstrated multiple lung metastases by positron emission tomography–computed tomography. Lactate dehydrogenase was within reference range, and his Eastern Cooperative Oncology Group performance status was 0 (fully active). One month later, he was started on ipilimumab 3 mg/kg intravenous infusion every 3 weeks for a total of 4 doses. At approximately week 6, his course was complicated by mild fatigue, a faintly erythematous morbilliform rash, and mild pruritus, with laboratory evidence of subclinical hyperthyroidism. Follow-up positron emission tomography–computed tomography at the conclusion of treatment demonstrated complete regression of previously noted hypermetabolic foci. His symptoms and subclinical hyperthyroidism resolved several months later.
Seventeen months after completion of ipilimumab therapy (at age 90 years), the patient’s barber noted new-onset hair loss on the right occipital scalp. Physical examination demonstrated a well-circumscribed patch of nonscarring alopecia (approximately 6 cm) that was clinically consistent with AA (Figure). There were no associated symptoms or other involved areas of hair loss. He denied any personal or family history of AA. The patient’s melanoma has remained in remission to date.

Comment
This case is unique in that AA was localized to a single circumscribed patch on the scalp and occurred nearly 1.5 years after treatment with ipilimumab, which may indicate a robust blockade of CTLA-4 given the remote development of autoimmunity in the setting of persistent remission of melanoma. Although the appearance of AA may be coincidental, onset at 90 years of age would be unusual. The mean age of onset of AA has been reported between 25.2 and 36.3 years,22,23 and its incidence in men older than 60 years is only 6.4 per 100,000 person-years.24
Although AA is a rare irAE of CTLA-4 blockade, the disease has been increasingly linked to CTLA-4 dysregulation in both animal models and humans.6,25,26 A genome-wide association study of 1054 patients with AA and 3278 controls implicated several genes controlling activation and proliferation of Tregs, including CTLA-4.27 More specifically, single-nucleotide polymorphisms of the CTLA-4 gene were found to be associated with AA in a study of 1196 unrelated patients and 1280 controls,28 and Megiorni et al
Given the role of CTLA-4 dysregulation in the pathogenesis of AA, the very low rates of AA in ipilimumab are somewhat surprising, which may represent a reporting bias. Alternatively, there may be sufficient Treg activity to prevent high rates of AA at a lower ipilimumab dose of 3 mg/kg but insufficient activity to prevent development of other irAEs. With US Food and Drug Administration approval of ipilimumab at a higher dose of 10 mg/kg for use as adjuvant therapy for stage III melanomas,12 less common cutaneous irAEs such as AA may be seen with increased frequency. Clinicians planning ipilimumab therapy should discuss this side effect and other potential irAEs with their patients before initiation of treatment.
- Brunet JF, Denizot F, Luciani MF, et al. A new member of the immunoglobulin superfamily--CTLA-4. Nature. 1987;328:267-270.
- Scalapino KJ, Daikh DI. CTLA-4: a key regulatory point in the control of autoimmune disease. Immunol Rev. 2008;223:143-155.
- Buchbinder E, Hodi FS. Cytotoxic T lymphocyte antigen-4 and immune checkpoint blockade. J Clin Invest. 2015;125:3377-3383.
- Brunner MC, Chambers CA, Chan FK, et al. CTLA-4-mediated inhibition of early events of T cell proliferation. J Immunol. 1999;162:5813-5820.
- Takahashi T, Tagami T, Yamazaki S, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303-310.
- Carroll JM, McElwee KJ, E King L, et al. Gene array profiling and immunomodulation studies define a cell-mediated immune response underlying the pathogenesis of alopecia areata in a mouse model and humans. J Invest Dermatol. 2002;119:392-402.
- Weber J, Thompson JA, Hamid O, et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res. 2009;15:5591-5598.
- O’Day SJ, Maio M, Chiarion-Sileni V, et al. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Ann Oncol. 2010;21:1712-1717.
- Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-723.
- Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517-2526.
- Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16:522-530.
- Yervoy (ipilimumab)[package insert]. Princeton, NJ: Bristol-Myers Squibb; 2019.
- Weber J. Review: anti-CTLA-4 antibody ipilimumab: case studies of clinical response and immune-related adverse events. Oncologist. 2007;12:864-872.
- Ibrahim RA, Berman DM, DePril V, et al. Ipilimumab safety profile: summary of findings from completed trials in advanced melanoma [abstract]. J Clin Oncol. 2011;29(suppl):8583.
- Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155-164.
- Kähler KC, Hauschild A. Treatment and side effect management of CTLA-4 antibody therapy in metastatic melanoma. J Dtsch Dermatol Ges. 2011;9:277-286.
- Jaber SH, Cowen EW, Haworth LR, et al. Skin reactions in a subset of patients with stage IV melanoma treated with anti-cytotoxic T-lymphocyte antigen 4 monoclonal antibody as a single agent. Arch Dermatol. 2006;142:166-172.
- Voskens CJ, Goldinger SM, Loquai C, et al. The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PLoS One. 2013;8:E537545.
- Assi H, Wilson KS. Immune toxicities and long remission duration after ipilimumab therapy for metastatic melanoma: two illustrative cases. Curr Oncol. 2013;20:E165-E169.
- Zarbo A, Belum VR, Sibaud V, et al. Immune-related alopecia (areata and universalis) in cancer patients receiving immune checkpoint inhibitors. Br J Dermatol. 2017;176:1649-1652.
- Lakhmiri M, Cavelier-Balloy B, Lacoste C, et al. Nivolumab-induced alopecia areata: a reversible factor of good prognosis? JAAD Case Rep. 2018;4:761-765.
- Tan E, Tay YK, Goh CL, et al. The pattern and profile of alopecia areata in Singapore–a study of 219 Asians. Int J Dermatol. 2002;41:748-753.
- Goh C, Finkel M, Christos PJ, et al. Profile of 513 patients with alopecia areata: associations of disease subtypes with atopy, autoimmune disease and positive family history. J Eur Acad Dermatol Venereol. 2006;20:1055-1060.
- Mirzoyev SA, Schrum AG, Davis MD, et al. Lifetime incidence risk of alopecia areata estimated at 2.1% by Rochester Epidemiology Project, 1990-2009. J Invest Dermatol. 2014;134:1141-1142.
- Zöller M, McElwee KJ, Engel P, et al. Transient CD44 variant isoform expression and reduction in CD4(+)/CD25(+) regulatory T cells in C3H/HeJ mice with alopecia areata. J Invest Dermatol. 2002;118:983-992.
- Zöller M, McElwee KJ, Vitacolonna M, et al. The progressive state, in contrast to the stable or regressive state of alopecia areata, is reflected in peripheral blood mononuclear cells. Exp Dermatol. 2004;13:435-444.
- Petukhova L, Duvic M, Hordinsky M, et al. Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature. 2010;466:113-117.
- John KK, Brockschmidt FF, Redler S, et al. Genetic variants in CTLA4 are strongly associated with alopecia areata. J Invest Dermatol. 2011;131:1169-1172.
- Megiorni F, Mora B, Maxia C, et al. Cytotoxic T-lymphocyte antigen 4 (CTLA4) +49AG and CT60 gene polymorphisms in alopecia areata: a case-control association study in the Italian population. Arch Dermatol Res. 2013;305:665-670
- Brunet JF, Denizot F, Luciani MF, et al. A new member of the immunoglobulin superfamily--CTLA-4. Nature. 1987;328:267-270.
- Scalapino KJ, Daikh DI. CTLA-4: a key regulatory point in the control of autoimmune disease. Immunol Rev. 2008;223:143-155.
- Buchbinder E, Hodi FS. Cytotoxic T lymphocyte antigen-4 and immune checkpoint blockade. J Clin Invest. 2015;125:3377-3383.
- Brunner MC, Chambers CA, Chan FK, et al. CTLA-4-mediated inhibition of early events of T cell proliferation. J Immunol. 1999;162:5813-5820.
- Takahashi T, Tagami T, Yamazaki S, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303-310.
- Carroll JM, McElwee KJ, E King L, et al. Gene array profiling and immunomodulation studies define a cell-mediated immune response underlying the pathogenesis of alopecia areata in a mouse model and humans. J Invest Dermatol. 2002;119:392-402.
- Weber J, Thompson JA, Hamid O, et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res. 2009;15:5591-5598.
- O’Day SJ, Maio M, Chiarion-Sileni V, et al. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Ann Oncol. 2010;21:1712-1717.
- Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-723.
- Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517-2526.
- Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16:522-530.
- Yervoy (ipilimumab)[package insert]. Princeton, NJ: Bristol-Myers Squibb; 2019.
- Weber J. Review: anti-CTLA-4 antibody ipilimumab: case studies of clinical response and immune-related adverse events. Oncologist. 2007;12:864-872.
- Ibrahim RA, Berman DM, DePril V, et al. Ipilimumab safety profile: summary of findings from completed trials in advanced melanoma [abstract]. J Clin Oncol. 2011;29(suppl):8583.
- Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155-164.
- Kähler KC, Hauschild A. Treatment and side effect management of CTLA-4 antibody therapy in metastatic melanoma. J Dtsch Dermatol Ges. 2011;9:277-286.
- Jaber SH, Cowen EW, Haworth LR, et al. Skin reactions in a subset of patients with stage IV melanoma treated with anti-cytotoxic T-lymphocyte antigen 4 monoclonal antibody as a single agent. Arch Dermatol. 2006;142:166-172.
- Voskens CJ, Goldinger SM, Loquai C, et al. The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PLoS One. 2013;8:E537545.
- Assi H, Wilson KS. Immune toxicities and long remission duration after ipilimumab therapy for metastatic melanoma: two illustrative cases. Curr Oncol. 2013;20:E165-E169.
- Zarbo A, Belum VR, Sibaud V, et al. Immune-related alopecia (areata and universalis) in cancer patients receiving immune checkpoint inhibitors. Br J Dermatol. 2017;176:1649-1652.
- Lakhmiri M, Cavelier-Balloy B, Lacoste C, et al. Nivolumab-induced alopecia areata: a reversible factor of good prognosis? JAAD Case Rep. 2018;4:761-765.
- Tan E, Tay YK, Goh CL, et al. The pattern and profile of alopecia areata in Singapore–a study of 219 Asians. Int J Dermatol. 2002;41:748-753.
- Goh C, Finkel M, Christos PJ, et al. Profile of 513 patients with alopecia areata: associations of disease subtypes with atopy, autoimmune disease and positive family history. J Eur Acad Dermatol Venereol. 2006;20:1055-1060.
- Mirzoyev SA, Schrum AG, Davis MD, et al. Lifetime incidence risk of alopecia areata estimated at 2.1% by Rochester Epidemiology Project, 1990-2009. J Invest Dermatol. 2014;134:1141-1142.
- Zöller M, McElwee KJ, Engel P, et al. Transient CD44 variant isoform expression and reduction in CD4(+)/CD25(+) regulatory T cells in C3H/HeJ mice with alopecia areata. J Invest Dermatol. 2002;118:983-992.
- Zöller M, McElwee KJ, Vitacolonna M, et al. The progressive state, in contrast to the stable or regressive state of alopecia areata, is reflected in peripheral blood mononuclear cells. Exp Dermatol. 2004;13:435-444.
- Petukhova L, Duvic M, Hordinsky M, et al. Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature. 2010;466:113-117.
- John KK, Brockschmidt FF, Redler S, et al. Genetic variants in CTLA4 are strongly associated with alopecia areata. J Invest Dermatol. 2011;131:1169-1172.
- Megiorni F, Mora B, Maxia C, et al. Cytotoxic T-lymphocyte antigen 4 (CTLA4) +49AG and CT60 gene polymorphisms in alopecia areata: a case-control association study in the Italian population. Arch Dermatol Res. 2013;305:665-670
Practice Points
- Cutaneous immune-related adverse effects (irAEs) are among the most common adverse effects of ipilimumab, a fully humanized monoclonal antibody directed against cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) used to treat advanced-stage melanoma.
- Alopecia areata is a rarely reported irAE, but its connection to CTLA-4 dysregulation may mean that clinicians see an increased incidence at higher ipilimumab doses.
Updates in Our Understanding of Central Centrifugal Cicatricial Alopecia
It has been more than 50 years since central centrifugal cicatricial alopecia (CCCA) was first defined by LoPresti and colleagues1 as hot comb alopecia. Fifty years later, we are only just starting to understand the pathogenesis of CCCA and its systemic implications.
Then and Now
The use of hot combs, a metal device used to straighten naturally curly hair, was ubiquitous in the households of black women in the 1960s. It is no surprise then that this styling process was labeled as the culprit of this disease that affects black women almost exclusively. As the use of hot combs waned but the prevalence of CCCA persisted, its name evolved to chemically induced alopecia—an ode to the popular styling product of the 1990s, the chemical relaxer—and eventually CCCA, a name that reflects its clinical progression and histologic findings.2
Since then, research has explored the association with systemic diseases, some noting increased rates of type 2 diabetes mellitus and thyroid disease, and more recently, an increased rate of fibroids in affected patients.3,4
Clues to Pathogenesis
Compared to other primary cicatricial alopecias, CCCA is unique in that active progression is difficult to detect. Symptoms, such as pruritus, often are minimal or absent, rendering clinical assessment quite difficult.5 Unlike other forms of scarring hair loss, fibrosis, not inflammation, is the predominant clinical feature. The clinical presentation is not unlike a group of disorders termed fibroproliferative disorders, which includes systemic sclerosis, uterine fibroids, atherosclerosis, and keloids, among others. It has been postulated that diseases of aberrant scarring are more common in black individuals due to the protective effect profibrotic alleles have against endemic helminthic infections of sub-Saharan infections, including oncocerciasis.6
A recent study showed an increased expression of fibroproliferative genes, particularly those implicated in other fibroproliferative disorders, in affected scalp of patients with CCCA.7 Most notably, an expression in gene overlap was noted between fibroids and CCCA in this study, though the relationship between these two diseases needs to be further explored.
Gene Variants Identified in CCCA
More recently, a new study has identified a gene variant of peptidyl arginine deiminase 3, PADI3, that is present in approximately one-quarter of studied patients with CCCA.8PADI3 plays a role in hair shaft formation and has been implicated in another hair disorder, uncombable hair syndrome, though the latter presents in children, improves with age, and is not associated with a scarring phenotype.9 However, this study has provided greater insight into our understanding of CCCA by establishing a possible genetic predisposition in patients affected with this disease.8
What’s Next for CCCA?
For years, many patients with CCCA have been turned away with few answers and left thinking that it is their own styling habits that have led to their hair loss,
The future is bright for CCCA. Although our understanding of CCCA is still in its infancy, it is my hope that with greater understanding of this disease will come greater empathy for our patients.
- LoPresti P, Papa CM, Kligman AM. Hot comb alopecia. Arch Dermatol. 1968;98:234-238.
- Gathers RC, Lim HW. Central centrifugal cicatricial alopecia: past, present, and future. J Am Acad Dermatol. 2009;60:660-668.
- Kyei A, Bergfeld WF, Piliang M, et al. Medical and environmental risk factors for the development of central centrifugal cicatricial alopecia: a population study. Arch Dermatol. 2011;147:909-914.
- Dina Y, Okoye GA, Aguh C. Association of uterine leiomyomas with central centrifugal cicatricial alopecia. JAMA Dermatol. 2018;154:213-214.
- Whiting DA, Olsen EA. Central centrifugal cicatricial alopecia. Dermatol Ther. 2008;21:268-278.
- Hellwege JN, Torstenson ES, Russell SB, et al. Evidence of selection as a cause for racial disparities in fibroproliferative disease. PLoS One. 2017;12:e0182791. doi:10.1371/journal.pone.0182791.
- Aguh C, Dina Y, Talbot CC Jr, et al. Fibroproliferative genes are preferentially expressed in central centrifugal cicatricial alopecia. J Am Acad Dermatol. 2018;79:904.e1-912.e1.
- Malki L, Sarig O, Romano MT, et al. Variant PADI3 in central centrifugal cicatricial alopecia. N Engl J Med. 2019;380:833-841.
- Matis WL, Baden H, Green R, et al. Uncombable-hair syndrome. Pediatr Dermatol. 1987;4:215-219.
It has been more than 50 years since central centrifugal cicatricial alopecia (CCCA) was first defined by LoPresti and colleagues1 as hot comb alopecia. Fifty years later, we are only just starting to understand the pathogenesis of CCCA and its systemic implications.
Then and Now
The use of hot combs, a metal device used to straighten naturally curly hair, was ubiquitous in the households of black women in the 1960s. It is no surprise then that this styling process was labeled as the culprit of this disease that affects black women almost exclusively. As the use of hot combs waned but the prevalence of CCCA persisted, its name evolved to chemically induced alopecia—an ode to the popular styling product of the 1990s, the chemical relaxer—and eventually CCCA, a name that reflects its clinical progression and histologic findings.2
Since then, research has explored the association with systemic diseases, some noting increased rates of type 2 diabetes mellitus and thyroid disease, and more recently, an increased rate of fibroids in affected patients.3,4
Clues to Pathogenesis
Compared to other primary cicatricial alopecias, CCCA is unique in that active progression is difficult to detect. Symptoms, such as pruritus, often are minimal or absent, rendering clinical assessment quite difficult.5 Unlike other forms of scarring hair loss, fibrosis, not inflammation, is the predominant clinical feature. The clinical presentation is not unlike a group of disorders termed fibroproliferative disorders, which includes systemic sclerosis, uterine fibroids, atherosclerosis, and keloids, among others. It has been postulated that diseases of aberrant scarring are more common in black individuals due to the protective effect profibrotic alleles have against endemic helminthic infections of sub-Saharan infections, including oncocerciasis.6
A recent study showed an increased expression of fibroproliferative genes, particularly those implicated in other fibroproliferative disorders, in affected scalp of patients with CCCA.7 Most notably, an expression in gene overlap was noted between fibroids and CCCA in this study, though the relationship between these two diseases needs to be further explored.
Gene Variants Identified in CCCA
More recently, a new study has identified a gene variant of peptidyl arginine deiminase 3, PADI3, that is present in approximately one-quarter of studied patients with CCCA.8PADI3 plays a role in hair shaft formation and has been implicated in another hair disorder, uncombable hair syndrome, though the latter presents in children, improves with age, and is not associated with a scarring phenotype.9 However, this study has provided greater insight into our understanding of CCCA by establishing a possible genetic predisposition in patients affected with this disease.8
What’s Next for CCCA?
For years, many patients with CCCA have been turned away with few answers and left thinking that it is their own styling habits that have led to their hair loss,
The future is bright for CCCA. Although our understanding of CCCA is still in its infancy, it is my hope that with greater understanding of this disease will come greater empathy for our patients.
It has been more than 50 years since central centrifugal cicatricial alopecia (CCCA) was first defined by LoPresti and colleagues1 as hot comb alopecia. Fifty years later, we are only just starting to understand the pathogenesis of CCCA and its systemic implications.
Then and Now
The use of hot combs, a metal device used to straighten naturally curly hair, was ubiquitous in the households of black women in the 1960s. It is no surprise then that this styling process was labeled as the culprit of this disease that affects black women almost exclusively. As the use of hot combs waned but the prevalence of CCCA persisted, its name evolved to chemically induced alopecia—an ode to the popular styling product of the 1990s, the chemical relaxer—and eventually CCCA, a name that reflects its clinical progression and histologic findings.2
Since then, research has explored the association with systemic diseases, some noting increased rates of type 2 diabetes mellitus and thyroid disease, and more recently, an increased rate of fibroids in affected patients.3,4
Clues to Pathogenesis
Compared to other primary cicatricial alopecias, CCCA is unique in that active progression is difficult to detect. Symptoms, such as pruritus, often are minimal or absent, rendering clinical assessment quite difficult.5 Unlike other forms of scarring hair loss, fibrosis, not inflammation, is the predominant clinical feature. The clinical presentation is not unlike a group of disorders termed fibroproliferative disorders, which includes systemic sclerosis, uterine fibroids, atherosclerosis, and keloids, among others. It has been postulated that diseases of aberrant scarring are more common in black individuals due to the protective effect profibrotic alleles have against endemic helminthic infections of sub-Saharan infections, including oncocerciasis.6
A recent study showed an increased expression of fibroproliferative genes, particularly those implicated in other fibroproliferative disorders, in affected scalp of patients with CCCA.7 Most notably, an expression in gene overlap was noted between fibroids and CCCA in this study, though the relationship between these two diseases needs to be further explored.
Gene Variants Identified in CCCA
More recently, a new study has identified a gene variant of peptidyl arginine deiminase 3, PADI3, that is present in approximately one-quarter of studied patients with CCCA.8PADI3 plays a role in hair shaft formation and has been implicated in another hair disorder, uncombable hair syndrome, though the latter presents in children, improves with age, and is not associated with a scarring phenotype.9 However, this study has provided greater insight into our understanding of CCCA by establishing a possible genetic predisposition in patients affected with this disease.8
What’s Next for CCCA?
For years, many patients with CCCA have been turned away with few answers and left thinking that it is their own styling habits that have led to their hair loss,
The future is bright for CCCA. Although our understanding of CCCA is still in its infancy, it is my hope that with greater understanding of this disease will come greater empathy for our patients.
- LoPresti P, Papa CM, Kligman AM. Hot comb alopecia. Arch Dermatol. 1968;98:234-238.
- Gathers RC, Lim HW. Central centrifugal cicatricial alopecia: past, present, and future. J Am Acad Dermatol. 2009;60:660-668.
- Kyei A, Bergfeld WF, Piliang M, et al. Medical and environmental risk factors for the development of central centrifugal cicatricial alopecia: a population study. Arch Dermatol. 2011;147:909-914.
- Dina Y, Okoye GA, Aguh C. Association of uterine leiomyomas with central centrifugal cicatricial alopecia. JAMA Dermatol. 2018;154:213-214.
- Whiting DA, Olsen EA. Central centrifugal cicatricial alopecia. Dermatol Ther. 2008;21:268-278.
- Hellwege JN, Torstenson ES, Russell SB, et al. Evidence of selection as a cause for racial disparities in fibroproliferative disease. PLoS One. 2017;12:e0182791. doi:10.1371/journal.pone.0182791.
- Aguh C, Dina Y, Talbot CC Jr, et al. Fibroproliferative genes are preferentially expressed in central centrifugal cicatricial alopecia. J Am Acad Dermatol. 2018;79:904.e1-912.e1.
- Malki L, Sarig O, Romano MT, et al. Variant PADI3 in central centrifugal cicatricial alopecia. N Engl J Med. 2019;380:833-841.
- Matis WL, Baden H, Green R, et al. Uncombable-hair syndrome. Pediatr Dermatol. 1987;4:215-219.
- LoPresti P, Papa CM, Kligman AM. Hot comb alopecia. Arch Dermatol. 1968;98:234-238.
- Gathers RC, Lim HW. Central centrifugal cicatricial alopecia: past, present, and future. J Am Acad Dermatol. 2009;60:660-668.
- Kyei A, Bergfeld WF, Piliang M, et al. Medical and environmental risk factors for the development of central centrifugal cicatricial alopecia: a population study. Arch Dermatol. 2011;147:909-914.
- Dina Y, Okoye GA, Aguh C. Association of uterine leiomyomas with central centrifugal cicatricial alopecia. JAMA Dermatol. 2018;154:213-214.
- Whiting DA, Olsen EA. Central centrifugal cicatricial alopecia. Dermatol Ther. 2008;21:268-278.
- Hellwege JN, Torstenson ES, Russell SB, et al. Evidence of selection as a cause for racial disparities in fibroproliferative disease. PLoS One. 2017;12:e0182791. doi:10.1371/journal.pone.0182791.
- Aguh C, Dina Y, Talbot CC Jr, et al. Fibroproliferative genes are preferentially expressed in central centrifugal cicatricial alopecia. J Am Acad Dermatol. 2018;79:904.e1-912.e1.
- Malki L, Sarig O, Romano MT, et al. Variant PADI3 in central centrifugal cicatricial alopecia. N Engl J Med. 2019;380:833-841.
- Matis WL, Baden H, Green R, et al. Uncombable-hair syndrome. Pediatr Dermatol. 1987;4:215-219.