User login
Analysis of Education on Nail Conditions at the American Academy of Dermatology Annual Meetings
To the Editor:
The diagnosis and treatment of nail conditions are necessary competencies for board-certified dermatologists, but appropriate education often is lacking.1 The American Academy of Dermatology (AAD) annual meeting is one of the largest and most highly attended dermatology educational conferences worldwide. We sought to determine the number of hours dedicated to nail-related topics at the AAD annual meetings from 2013 to 2019.
We accessed programs from the AAD annual meetings archive online (https://www.aad.org/meetings/previous-meetings-archive), and we used hair and psoriasis content for comparison. Event titles and descriptions were searched for nail-related content (using search terms nail, onychia, and onycho), hair-related content (hair, alopecia, trichosis, hirsutism), and psoriasis content (psoriasis). Data acquired for each event included the date, hours, title, and event type (eg, forum, course, focus session, symposium, discussion group, workshop, plenary session).
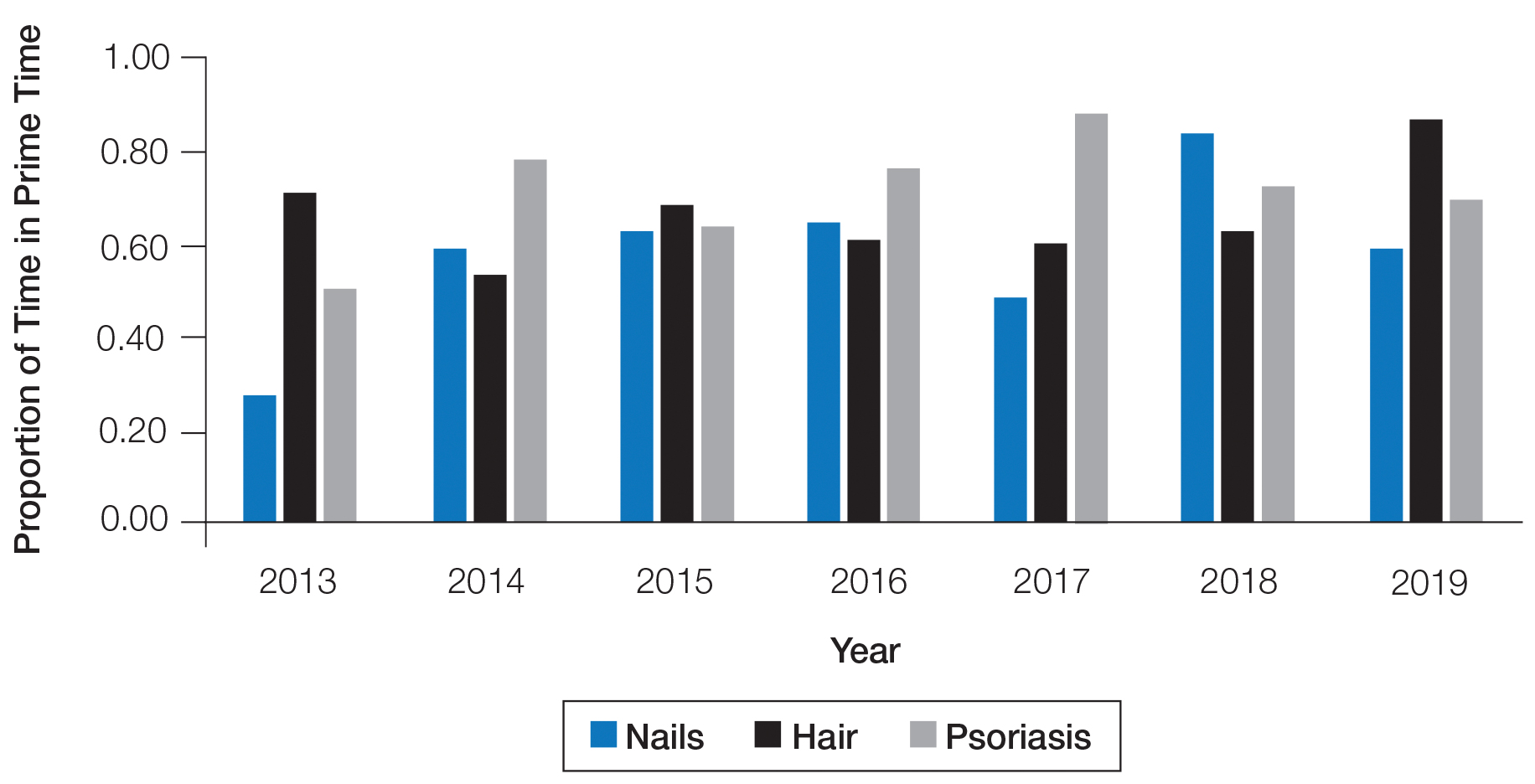
The number of hours dedicated to nail education consistently lagged behind those related to hair and psoriasis content during the study period (Figure 1). According to the AAD, the conference runs Friday to Tuesday with higher attendance Friday to Sunday (Tim Moses, personal communication, July 9, 2019). Lectures during the weekend are likely to have a broader reach than lectures on Monday and Tuesday. The proportion of nail content during weekend prime time slots was similar to that of hair and psoriasis (Figure 2). Plenary sessions often are presented by renowned experts on hot topics in dermatology. Notably, hair (2014-2015) and psoriasis (2015-2017) content were represented in the plenary sessions during the study period, while nail content was not featured.

Our study shows that nail-related education was underrepresented at the AAD annual meetings from 2013 to 2019 compared to hair- and psoriasis-related content. Educational gaps in the diagnosis of fignail conditions previously have been delineated, and prioritization of instruction on nail disease pathology and diagnostic procedures has been recommended to improve patient care.1 The majority of nail unit melanomas are diagnosed at late stages, which has been attributed to deficiencies in clinical knowledge and failure to perform or inadequate biopsy techniques.2 Notably, a survey of third-year dermatology residents (N=240) assessing experience in procedural dermatology showed that 58% performed 10 or fewer nail procedures and 30% did not feel competent in performing nail surgery.3 Furthermore, a survey examining the management of longitudinal melanonychia among attending and resident dermatologists (N=402) found that 62% of residents and 28% of total respondents were not confident in managing melanonychia.4
A limitation of this study was the lack of online data available for AAD annual meetings before 2013, so we were unable to characterize any long-term trends. Furthermore, we were unable to assess the educational reach of these sessions, as data on attendance are lacking.
This study demonstrates a paucity of nail-related content at the AAD annual meetings. The introduction of the “Hands-on: Nail Surgery” in 2015 is an important step forward to diminish the knowledge gap in the diagnosis of various nail diseases and malignancies. We recommend increasing the number of hours and overall content of didactic nail sessions at the AAD annual meeting to further the knowledge and procedural skills of dermatologists in caring for patients with nail disorders.
- Hare AQ, R ich P. Clinical and educational gaps in diagnosis of nail disorders. Dermatol Clin. 2016;34:269-273.
- Tan KB, Moncrieff M, Thompson JF, et al. Subungual melanoma: a study of 124 cases highlighting features of early lesions, potential pitfalls in diagnosis, and guidelines for histologic reporting. Am J Surg Pathol. 2007;31:1902-1912.
- Lee EH, Nehal KS, Dusza SW, et al. Procedural dermatology training during dermatology residency: a survey of third-year dermatology residents. J Am Acad Dermatol. 2011;64:475-483.
- Halteh P, Scher R, Artis A, et al. A survey-based study of management of longitudinal melanonychia amongst attending and resident dermatologists. J Am Acad Dermatol. 2017;76:994-996.
To the Editor:
The diagnosis and treatment of nail conditions are necessary competencies for board-certified dermatologists, but appropriate education often is lacking.1 The American Academy of Dermatology (AAD) annual meeting is one of the largest and most highly attended dermatology educational conferences worldwide. We sought to determine the number of hours dedicated to nail-related topics at the AAD annual meetings from 2013 to 2019.
We accessed programs from the AAD annual meetings archive online (https://www.aad.org/meetings/previous-meetings-archive), and we used hair and psoriasis content for comparison. Event titles and descriptions were searched for nail-related content (using search terms nail, onychia, and onycho), hair-related content (hair, alopecia, trichosis, hirsutism), and psoriasis content (psoriasis). Data acquired for each event included the date, hours, title, and event type (eg, forum, course, focus session, symposium, discussion group, workshop, plenary session).
The number of hours dedicated to nail education consistently lagged behind those related to hair and psoriasis content during the study period (Figure 1). According to the AAD, the conference runs Friday to Tuesday with higher attendance Friday to Sunday (Tim Moses, personal communication, July 9, 2019). Lectures during the weekend are likely to have a broader reach than lectures on Monday and Tuesday. The proportion of nail content during weekend prime time slots was similar to that of hair and psoriasis (Figure 2). Plenary sessions often are presented by renowned experts on hot topics in dermatology. Notably, hair (2014-2015) and psoriasis (2015-2017) content were represented in the plenary sessions during the study period, while nail content was not featured.


Our study shows that nail-related education was underrepresented at the AAD annual meetings from 2013 to 2019 compared to hair- and psoriasis-related content. Educational gaps in the diagnosis of fignail conditions previously have been delineated, and prioritization of instruction on nail disease pathology and diagnostic procedures has been recommended to improve patient care.1 The majority of nail unit melanomas are diagnosed at late stages, which has been attributed to deficiencies in clinical knowledge and failure to perform or inadequate biopsy techniques.2 Notably, a survey of third-year dermatology residents (N=240) assessing experience in procedural dermatology showed that 58% performed 10 or fewer nail procedures and 30% did not feel competent in performing nail surgery.3 Furthermore, a survey examining the management of longitudinal melanonychia among attending and resident dermatologists (N=402) found that 62% of residents and 28% of total respondents were not confident in managing melanonychia.4
A limitation of this study was the lack of online data available for AAD annual meetings before 2013, so we were unable to characterize any long-term trends. Furthermore, we were unable to assess the educational reach of these sessions, as data on attendance are lacking.
This study demonstrates a paucity of nail-related content at the AAD annual meetings. The introduction of the “Hands-on: Nail Surgery” in 2015 is an important step forward to diminish the knowledge gap in the diagnosis of various nail diseases and malignancies. We recommend increasing the number of hours and overall content of didactic nail sessions at the AAD annual meeting to further the knowledge and procedural skills of dermatologists in caring for patients with nail disorders.
To the Editor:
The diagnosis and treatment of nail conditions are necessary competencies for board-certified dermatologists, but appropriate education often is lacking.1 The American Academy of Dermatology (AAD) annual meeting is one of the largest and most highly attended dermatology educational conferences worldwide. We sought to determine the number of hours dedicated to nail-related topics at the AAD annual meetings from 2013 to 2019.
We accessed programs from the AAD annual meetings archive online (https://www.aad.org/meetings/previous-meetings-archive), and we used hair and psoriasis content for comparison. Event titles and descriptions were searched for nail-related content (using search terms nail, onychia, and onycho), hair-related content (hair, alopecia, trichosis, hirsutism), and psoriasis content (psoriasis). Data acquired for each event included the date, hours, title, and event type (eg, forum, course, focus session, symposium, discussion group, workshop, plenary session).
The number of hours dedicated to nail education consistently lagged behind those related to hair and psoriasis content during the study period (Figure 1). According to the AAD, the conference runs Friday to Tuesday with higher attendance Friday to Sunday (Tim Moses, personal communication, July 9, 2019). Lectures during the weekend are likely to have a broader reach than lectures on Monday and Tuesday. The proportion of nail content during weekend prime time slots was similar to that of hair and psoriasis (Figure 2). Plenary sessions often are presented by renowned experts on hot topics in dermatology. Notably, hair (2014-2015) and psoriasis (2015-2017) content were represented in the plenary sessions during the study period, while nail content was not featured.


Our study shows that nail-related education was underrepresented at the AAD annual meetings from 2013 to 2019 compared to hair- and psoriasis-related content. Educational gaps in the diagnosis of fignail conditions previously have been delineated, and prioritization of instruction on nail disease pathology and diagnostic procedures has been recommended to improve patient care.1 The majority of nail unit melanomas are diagnosed at late stages, which has been attributed to deficiencies in clinical knowledge and failure to perform or inadequate biopsy techniques.2 Notably, a survey of third-year dermatology residents (N=240) assessing experience in procedural dermatology showed that 58% performed 10 or fewer nail procedures and 30% did not feel competent in performing nail surgery.3 Furthermore, a survey examining the management of longitudinal melanonychia among attending and resident dermatologists (N=402) found that 62% of residents and 28% of total respondents were not confident in managing melanonychia.4
A limitation of this study was the lack of online data available for AAD annual meetings before 2013, so we were unable to characterize any long-term trends. Furthermore, we were unable to assess the educational reach of these sessions, as data on attendance are lacking.
This study demonstrates a paucity of nail-related content at the AAD annual meetings. The introduction of the “Hands-on: Nail Surgery” in 2015 is an important step forward to diminish the knowledge gap in the diagnosis of various nail diseases and malignancies. We recommend increasing the number of hours and overall content of didactic nail sessions at the AAD annual meeting to further the knowledge and procedural skills of dermatologists in caring for patients with nail disorders.
- Hare AQ, R ich P. Clinical and educational gaps in diagnosis of nail disorders. Dermatol Clin. 2016;34:269-273.
- Tan KB, Moncrieff M, Thompson JF, et al. Subungual melanoma: a study of 124 cases highlighting features of early lesions, potential pitfalls in diagnosis, and guidelines for histologic reporting. Am J Surg Pathol. 2007;31:1902-1912.
- Lee EH, Nehal KS, Dusza SW, et al. Procedural dermatology training during dermatology residency: a survey of third-year dermatology residents. J Am Acad Dermatol. 2011;64:475-483.
- Halteh P, Scher R, Artis A, et al. A survey-based study of management of longitudinal melanonychia amongst attending and resident dermatologists. J Am Acad Dermatol. 2017;76:994-996.
- Hare AQ, R ich P. Clinical and educational gaps in diagnosis of nail disorders. Dermatol Clin. 2016;34:269-273.
- Tan KB, Moncrieff M, Thompson JF, et al. Subungual melanoma: a study of 124 cases highlighting features of early lesions, potential pitfalls in diagnosis, and guidelines for histologic reporting. Am J Surg Pathol. 2007;31:1902-1912.
- Lee EH, Nehal KS, Dusza SW, et al. Procedural dermatology training during dermatology residency: a survey of third-year dermatology residents. J Am Acad Dermatol. 2011;64:475-483.
- Halteh P, Scher R, Artis A, et al. A survey-based study of management of longitudinal melanonychia amongst attending and resident dermatologists. J Am Acad Dermatol. 2017;76:994-996.
Practice Points
- Diagnosis and treatment of nail conditions are necessary competencies for board-certified dermatologists, but appropriate education often is lacking.
- We recommend increasing the number of hours and overall content of didactic nail sessions at the American Academy of Dermatology annual meeting to further the knowledge and procedural skills of dermatologists caring for patients with nail disorders.
Evidence on spironolactone safety, COVID-19 reassuring for acne patients
according to a report in the Journal of the American Academy of Dermatology.
The virus needs androgens to infect cells, and uses androgen-dependent transmembrane protease serine 2 to prime viral protein spikes to anchor onto ACE2 receptors. Without that step, the virus can’t enter cells. Androgens are the only known activator in humans, so androgen blockers like spironolactone probably short-circuit the process, said the report’s lead author Carlos Wambier, MD, PhD, of the department of dermatology at Brown University, Providence, R.I (J Am Acad Dermatol. 2020 Apr 10. doi: 10.1016/j.jaad.2020.04.032).
The lack of androgens could be a possible explanation as to why mortality is so rare among children with COVID-19, and why fatalities among men are higher than among women with COVID-19, he said in an interview.
There are a lot of androgen blocker candidates, but he said spironolactone – a mainstay of acne treatment – might well be the best for the pandemic because of its concomitant lung and heart benefits.
The message counters a post on Instagram in March from a New York City dermatologist in private practice, Ellen Marmur, MD, that raised a question about spironolactone. Concerned about the situation in New York, she reviewed the literature and found a 2005 study that reported that macrophages drawn from 10 heart failure patients had increased ACE2 activity and increased messenger RNA expression after the subjects had been on spironolactone 25 mg per day for a month.
In an interview, she said she has been sharing her concerns with patients on spironolactone and offering them an alternative, such as minocycline, until this issue is better elucidated. To date, she has had one young patient who declined to switch to another treatment, and about six patients who were comfortable switching to another treatment for 1-2 months. She said that she is “clearly cautious yet uncertain about the influence of chronic spironolactone for acne on COVID infection in acne patients,” and that eventually she would be interested in seeing retrospective data on outcomes of patients on spironolactone for hypertension versus acne during the pandemic.
Dr. Marmur’s post was spread on social media and was picked up by a few online news outlets.
In an interview, Adam Friedman, MD, professor and interim chair of dermatology at George Washington University, Washington, said he’s been addressing concerns about spironolactone in educational webinars because of it.
He tells his audience that “you can’t make any claims” for COVID-19 based on the 2005 study. It was a small clinical study in heart failure patients and only assessed ACE2 expression on macrophages, not respiratory, cardiac, or mesangial cells, which are the relevant locations for viral invasion and damage. In fact, there are studies showing that spironolactone reduced ACE2 in renal mesangial cells. Also of note, spironolactone has been used with no indication of virus risk since the 1950s, he pointed out. The American Academy of Dermatology has not said to stop spironolactone.
At least one study is underway to see if spironolactone is beneficial: 100 mg twice a day for 5 days is being pitted against placebo in Turkey among people hospitalized with acute respiratory distress. The study will evaluate the effect of spironolactone on oxygenation.
“There’s no evidence to show spironolactone can increase mortality levels,” Dr. Wambier said. He is using it more now in patients with acne – a sign of androgen hyperactivity – convinced that it will protect against COVID-19. He even started his sister on it to help with androgenic hair loss, and maybe the virus.
Observations in Spain – increased prevalence of androgenic alopecia among hospitalized patients – support the androgen link; 29 of 41 men (71%) hospitalized with bilateral pneumonia had male pattern baldness, which was severe in 16 (39%), according to a recent report (J Cosmet Dermatol. 2020 Apr 16. doi: 10.1111/jocd.13443). The expected prevalence in a similar age-matched population is 31%-53%.
“Based on the scientific rationale combined with this preliminary observation, we believe investigating the potential association between androgens and COVID‐19 disease severity warrants further merit,” concluded the authors, who included Dr. Wambier, and other dermatologists from the United States, as well as Spain, Australia, Croatia, and Switzerland. “If such an association is confirmed, antiandrogens could be evaluated as a potential treatment for COVID‐19 infection,” they wrote.
The numbers are holding up in a larger series from three Spanish hospitals, and also showing a greater prevalence of androgenic hair loss among hospitalized women, Dr. Wambier said in the interview.
Authors of the two studies include an employee of Applied Biology. No conflicts were declared in the Journal of Cosmetic Dermatology study; no disclosures were listed in the JAAD study. Dr. Friedman had no disclosures.
according to a report in the Journal of the American Academy of Dermatology.
The virus needs androgens to infect cells, and uses androgen-dependent transmembrane protease serine 2 to prime viral protein spikes to anchor onto ACE2 receptors. Without that step, the virus can’t enter cells. Androgens are the only known activator in humans, so androgen blockers like spironolactone probably short-circuit the process, said the report’s lead author Carlos Wambier, MD, PhD, of the department of dermatology at Brown University, Providence, R.I (J Am Acad Dermatol. 2020 Apr 10. doi: 10.1016/j.jaad.2020.04.032).
The lack of androgens could be a possible explanation as to why mortality is so rare among children with COVID-19, and why fatalities among men are higher than among women with COVID-19, he said in an interview.
There are a lot of androgen blocker candidates, but he said spironolactone – a mainstay of acne treatment – might well be the best for the pandemic because of its concomitant lung and heart benefits.
The message counters a post on Instagram in March from a New York City dermatologist in private practice, Ellen Marmur, MD, that raised a question about spironolactone. Concerned about the situation in New York, she reviewed the literature and found a 2005 study that reported that macrophages drawn from 10 heart failure patients had increased ACE2 activity and increased messenger RNA expression after the subjects had been on spironolactone 25 mg per day for a month.
In an interview, she said she has been sharing her concerns with patients on spironolactone and offering them an alternative, such as minocycline, until this issue is better elucidated. To date, she has had one young patient who declined to switch to another treatment, and about six patients who were comfortable switching to another treatment for 1-2 months. She said that she is “clearly cautious yet uncertain about the influence of chronic spironolactone for acne on COVID infection in acne patients,” and that eventually she would be interested in seeing retrospective data on outcomes of patients on spironolactone for hypertension versus acne during the pandemic.
Dr. Marmur’s post was spread on social media and was picked up by a few online news outlets.
In an interview, Adam Friedman, MD, professor and interim chair of dermatology at George Washington University, Washington, said he’s been addressing concerns about spironolactone in educational webinars because of it.
He tells his audience that “you can’t make any claims” for COVID-19 based on the 2005 study. It was a small clinical study in heart failure patients and only assessed ACE2 expression on macrophages, not respiratory, cardiac, or mesangial cells, which are the relevant locations for viral invasion and damage. In fact, there are studies showing that spironolactone reduced ACE2 in renal mesangial cells. Also of note, spironolactone has been used with no indication of virus risk since the 1950s, he pointed out. The American Academy of Dermatology has not said to stop spironolactone.
At least one study is underway to see if spironolactone is beneficial: 100 mg twice a day for 5 days is being pitted against placebo in Turkey among people hospitalized with acute respiratory distress. The study will evaluate the effect of spironolactone on oxygenation.
“There’s no evidence to show spironolactone can increase mortality levels,” Dr. Wambier said. He is using it more now in patients with acne – a sign of androgen hyperactivity – convinced that it will protect against COVID-19. He even started his sister on it to help with androgenic hair loss, and maybe the virus.
Observations in Spain – increased prevalence of androgenic alopecia among hospitalized patients – support the androgen link; 29 of 41 men (71%) hospitalized with bilateral pneumonia had male pattern baldness, which was severe in 16 (39%), according to a recent report (J Cosmet Dermatol. 2020 Apr 16. doi: 10.1111/jocd.13443). The expected prevalence in a similar age-matched population is 31%-53%.
“Based on the scientific rationale combined with this preliminary observation, we believe investigating the potential association between androgens and COVID‐19 disease severity warrants further merit,” concluded the authors, who included Dr. Wambier, and other dermatologists from the United States, as well as Spain, Australia, Croatia, and Switzerland. “If such an association is confirmed, antiandrogens could be evaluated as a potential treatment for COVID‐19 infection,” they wrote.
The numbers are holding up in a larger series from three Spanish hospitals, and also showing a greater prevalence of androgenic hair loss among hospitalized women, Dr. Wambier said in the interview.
Authors of the two studies include an employee of Applied Biology. No conflicts were declared in the Journal of Cosmetic Dermatology study; no disclosures were listed in the JAAD study. Dr. Friedman had no disclosures.
according to a report in the Journal of the American Academy of Dermatology.
The virus needs androgens to infect cells, and uses androgen-dependent transmembrane protease serine 2 to prime viral protein spikes to anchor onto ACE2 receptors. Without that step, the virus can’t enter cells. Androgens are the only known activator in humans, so androgen blockers like spironolactone probably short-circuit the process, said the report’s lead author Carlos Wambier, MD, PhD, of the department of dermatology at Brown University, Providence, R.I (J Am Acad Dermatol. 2020 Apr 10. doi: 10.1016/j.jaad.2020.04.032).
The lack of androgens could be a possible explanation as to why mortality is so rare among children with COVID-19, and why fatalities among men are higher than among women with COVID-19, he said in an interview.
There are a lot of androgen blocker candidates, but he said spironolactone – a mainstay of acne treatment – might well be the best for the pandemic because of its concomitant lung and heart benefits.
The message counters a post on Instagram in March from a New York City dermatologist in private practice, Ellen Marmur, MD, that raised a question about spironolactone. Concerned about the situation in New York, she reviewed the literature and found a 2005 study that reported that macrophages drawn from 10 heart failure patients had increased ACE2 activity and increased messenger RNA expression after the subjects had been on spironolactone 25 mg per day for a month.
In an interview, she said she has been sharing her concerns with patients on spironolactone and offering them an alternative, such as minocycline, until this issue is better elucidated. To date, she has had one young patient who declined to switch to another treatment, and about six patients who were comfortable switching to another treatment for 1-2 months. She said that she is “clearly cautious yet uncertain about the influence of chronic spironolactone for acne on COVID infection in acne patients,” and that eventually she would be interested in seeing retrospective data on outcomes of patients on spironolactone for hypertension versus acne during the pandemic.
Dr. Marmur’s post was spread on social media and was picked up by a few online news outlets.
In an interview, Adam Friedman, MD, professor and interim chair of dermatology at George Washington University, Washington, said he’s been addressing concerns about spironolactone in educational webinars because of it.
He tells his audience that “you can’t make any claims” for COVID-19 based on the 2005 study. It was a small clinical study in heart failure patients and only assessed ACE2 expression on macrophages, not respiratory, cardiac, or mesangial cells, which are the relevant locations for viral invasion and damage. In fact, there are studies showing that spironolactone reduced ACE2 in renal mesangial cells. Also of note, spironolactone has been used with no indication of virus risk since the 1950s, he pointed out. The American Academy of Dermatology has not said to stop spironolactone.
At least one study is underway to see if spironolactone is beneficial: 100 mg twice a day for 5 days is being pitted against placebo in Turkey among people hospitalized with acute respiratory distress. The study will evaluate the effect of spironolactone on oxygenation.
“There’s no evidence to show spironolactone can increase mortality levels,” Dr. Wambier said. He is using it more now in patients with acne – a sign of androgen hyperactivity – convinced that it will protect against COVID-19. He even started his sister on it to help with androgenic hair loss, and maybe the virus.
Observations in Spain – increased prevalence of androgenic alopecia among hospitalized patients – support the androgen link; 29 of 41 men (71%) hospitalized with bilateral pneumonia had male pattern baldness, which was severe in 16 (39%), according to a recent report (J Cosmet Dermatol. 2020 Apr 16. doi: 10.1111/jocd.13443). The expected prevalence in a similar age-matched population is 31%-53%.
“Based on the scientific rationale combined with this preliminary observation, we believe investigating the potential association between androgens and COVID‐19 disease severity warrants further merit,” concluded the authors, who included Dr. Wambier, and other dermatologists from the United States, as well as Spain, Australia, Croatia, and Switzerland. “If such an association is confirmed, antiandrogens could be evaluated as a potential treatment for COVID‐19 infection,” they wrote.
The numbers are holding up in a larger series from three Spanish hospitals, and also showing a greater prevalence of androgenic hair loss among hospitalized women, Dr. Wambier said in the interview.
Authors of the two studies include an employee of Applied Biology. No conflicts were declared in the Journal of Cosmetic Dermatology study; no disclosures were listed in the JAAD study. Dr. Friedman had no disclosures.
Lichen Planopilaris in a Patient Treated With Bexarotene for Lymphomatoid Papulosis
To the Editor:
Lymphomatoid papulosis is a rare chronic skin disorder characterized by recurrent, self-healing crops of papulonodular eruptions, often resembling cutaneous T-cell lymphoma.1 Oral bexarotene, a retinoid X receptor–selective retinoid, can be used to control the disease.2,3 Lichen planopilaris (LPP) is a type of cicatricial alopecia characterized by irreversible hair loss, perifollicular inflammation, and follicular hyperkeratosis, commonly affecting the scalp vertex in adults.4 We report a case of a patient with lymphomatoid papulosis who was treated with bexarotene and subsequently developed LPP. We also discuss a proposed mechanism by which bexarotene may have influenced the onset of LPP.
A 35-year-old woman who was previously healthy initially presented with recurrent pruritic papular eruptions on the flank, axillae, and groin of several months’ duration. The lesions appeared as 2-mm, flat-topped, violaceous papules. The patient had no known drug allergies, no medical or family history of skin disease, and was only taking 3000 mg/d of omega-3 fatty acids (fish oil). Histopathologic examination of a biopsy specimen from the inner thigh showed enlarged, atypical, dermal lymphocytes that were CD30+ (Figure 1). These findings were consistent with lymphomatoid papulosis. As she had undergone tubal ligation several years prior, she was prescribed oral bexarotene 300 mg once daily in addition to triamcinolone cream 0.1% twice daily, as needed. Symptoms were well controlled on this regimen.
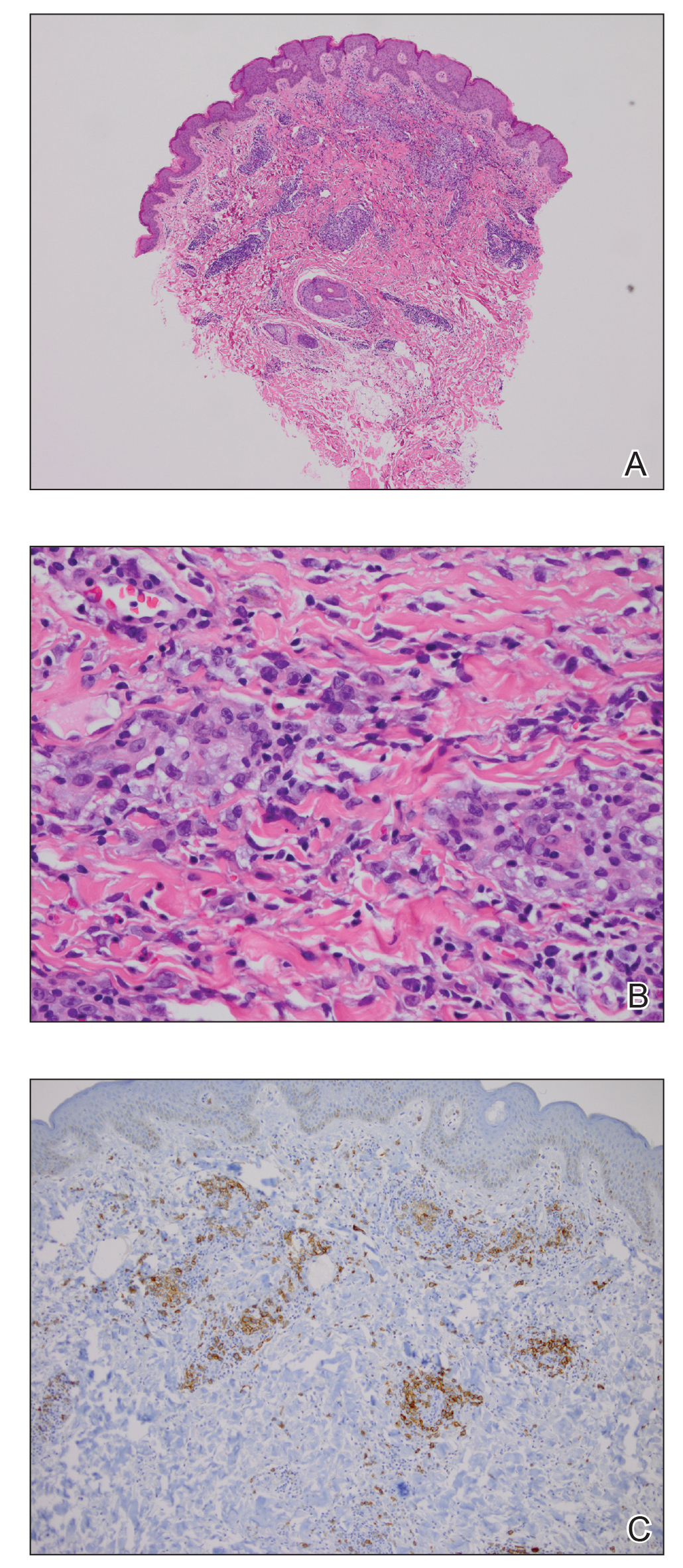
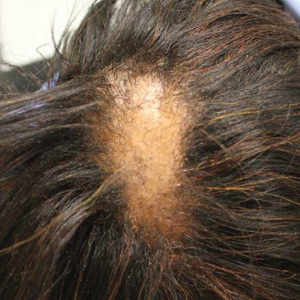
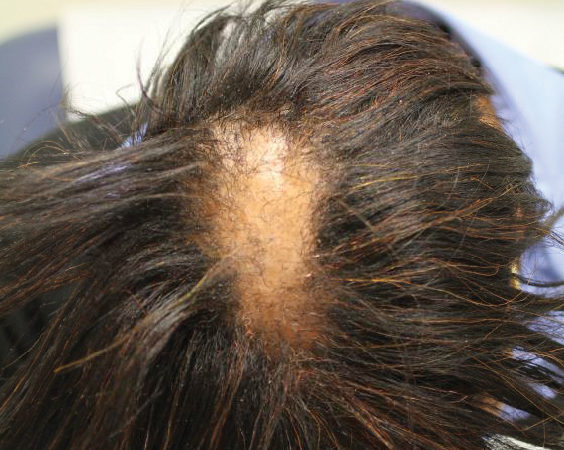
Six months later the patient returned, presenting with a new central patch of scarring alopecia on the vertex of the scalp (Figure 2). Adjacent to the area of hair loss were areas of prominent perifollicular scale that were slightly violaceous in color. Two 4-mm punch biopsies of the scalp showed dermal scarring with perifollicular lamellar fibrosis surrounded by a rim of lymphoplasmacytic inflammation (Figure 3). Sebaceous glands were found to be reduced in number. These findings were consistent with cicatricial alopecia, which was further classified as LPP in conjunction with the clinical findings. No CD30+ lymphocytes were identified in these specimens.
Baseline fasting triglycerides were 123 mg/dL (desirable: <150 mg/dL; borderline: 150–199 mg/dL; high: ≥200 mg/dL) and were stable over the first 4 months on bexarotene. After 5 months of therapy, the triglycerides increased to a high of 255 mg/dL, which corresponded with the onset of LPP. She was treated for the hypertriglyceridemia with omega-3 fatty acids (fish oil), and subsequent triglyceride levels have normalized and been stable. Her alopecia has not progressed but is persistent. She continues to have central hypothyroidism due to bexarotene and is on levothyroxine. The lymphomatoid papulosis also remains stable with no signs of progression to cutaneous T-cell lymphoma.
Although the exact mechanism of LPP is not fully understood, studies have suggested that cellular lipid metabolism may be responsible for the inflammation of the pilosebaceous unit.4-11 Hyperlipidemia is the most common side effect of oral bexarotene, typically occurring within the first 2 to 4 weeks of treatment.3,12 Considering the insights into the role of lipid regulation on LPP pathogenesis, it is reasonable to suspect that the dyslipidemia caused by bexarotene may have triggered the onset of LPP in our patient. The patient’s lipid values mostly remained within reference range throughout the course of treatment, though she did have elevation of triglycerides around the onset of LPP. Dyslipidemia has been reported in patients with lichen planus but not in patients with LPP. One case-control study showed no dyslipidemia in patients with LPP, but the triglyceride levels were not tracked over time and patients had varying durations since onset of disease at presentation.9-11,13 In our case, we were fortunate to have this information, and it may suggest an interaction between lipid dysregulation and the development of LPP. It would be interesting to explore this further in a larger patient population and to evaluate if control of dyslipidemia reduces progression of disease as it appears to have done for our patient.
- Karp DL, Horn TD. Lymphomatoid papulosis. J Am Acad Dermatol. 1994;30:379-395; quiz 396-398.
- Krathen RA, Ward S, Duvic M. Bexarotene is a new treatment option for lymphomatoid papulosis. Dermatology. 2003;206:142-147.
- Targretin (bexarotene) capsule [package insert]. St. Petersburg, FL: Cardinal Health; 2003. http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=63656f64-e240-4855-8df9-ca1655863735. Accessed April 9, 2020.
- Assouly P, Reygagne P. Lichen planopilaris: update on diagnosis and treatment. Semin Cutan Med Surg. 2009;28:3-10.
- Dogra S, Sarangal R. What’s new in cicatricial alopecia? Indian J Dermatol Venereol Leprol. 2013;79:576-90.
- Zheng Y, Eilertsen KJ, Ge L, et al. Scd1 is expressed in sebaceous glands and is disrupted in the asebia mouse. Nat Genet. 1999;23:268-270.
- Sundberg JP, Boggess D, Sundberg BA, et al. Asebia-2J (Scd1(ab2J)): a new allele and a model for scarring alopecia. Am J Pathol. 2000;156:2067-2075.
- Karnik P, Tekeste Z, McCormick TS, et al. Hair follicle stem cell-specific PPARgamma deletion causes scarring alopecia. J Invest Dermatol. 2009;129:1243-157.
- López-Jornet P, Camacho-Alonso F, Rodríguez-Martínes MA. Alterations in serum lipid profile patterns in oral lichen planus: a cross-sectional study. Am J Clin Dermatol. 2012;13:399-404.
- Arias-Santiago S, Buendía-Eisman A, Aneiros-Fernández J, et al. Lipid levels in patients with lichen planus: a case-control study. J Eur Acad Dermatol Venereol. 2011;25:1398-1401.
- Dreiher J, Shapiro J, Cohen AD. Lichen planus and dyslipidaemia: a case-control study. Br J Dermatol. 2009;161:626-629.
- de Vries-van der Weij J, de Haan W, Hu L, et al. Bexarotene induces dyslipidemia by increased very low-density lipoprotein production and cholesteryl ester transfer protein-mediated reduction of high-density lipoprotein. Endocrinology. 2009;150:2368-2375.
- Conic RRZ, Piliang M, Bergfeld W, et al. Association of lichen planopilaris with dyslipidemia. JAMA Dermatol. 2018;154:1088-1089.
To the Editor:
Lymphomatoid papulosis is a rare chronic skin disorder characterized by recurrent, self-healing crops of papulonodular eruptions, often resembling cutaneous T-cell lymphoma.1 Oral bexarotene, a retinoid X receptor–selective retinoid, can be used to control the disease.2,3 Lichen planopilaris (LPP) is a type of cicatricial alopecia characterized by irreversible hair loss, perifollicular inflammation, and follicular hyperkeratosis, commonly affecting the scalp vertex in adults.4 We report a case of a patient with lymphomatoid papulosis who was treated with bexarotene and subsequently developed LPP. We also discuss a proposed mechanism by which bexarotene may have influenced the onset of LPP.
A 35-year-old woman who was previously healthy initially presented with recurrent pruritic papular eruptions on the flank, axillae, and groin of several months’ duration. The lesions appeared as 2-mm, flat-topped, violaceous papules. The patient had no known drug allergies, no medical or family history of skin disease, and was only taking 3000 mg/d of omega-3 fatty acids (fish oil). Histopathologic examination of a biopsy specimen from the inner thigh showed enlarged, atypical, dermal lymphocytes that were CD30+ (Figure 1). These findings were consistent with lymphomatoid papulosis. As she had undergone tubal ligation several years prior, she was prescribed oral bexarotene 300 mg once daily in addition to triamcinolone cream 0.1% twice daily, as needed. Symptoms were well controlled on this regimen.

Six months later the patient returned, presenting with a new central patch of scarring alopecia on the vertex of the scalp (Figure 2). Adjacent to the area of hair loss were areas of prominent perifollicular scale that were slightly violaceous in color. Two 4-mm punch biopsies of the scalp showed dermal scarring with perifollicular lamellar fibrosis surrounded by a rim of lymphoplasmacytic inflammation (Figure 3). Sebaceous glands were found to be reduced in number. These findings were consistent with cicatricial alopecia, which was further classified as LPP in conjunction with the clinical findings. No CD30+ lymphocytes were identified in these specimens.


Baseline fasting triglycerides were 123 mg/dL (desirable: <150 mg/dL; borderline: 150–199 mg/dL; high: ≥200 mg/dL) and were stable over the first 4 months on bexarotene. After 5 months of therapy, the triglycerides increased to a high of 255 mg/dL, which corresponded with the onset of LPP. She was treated for the hypertriglyceridemia with omega-3 fatty acids (fish oil), and subsequent triglyceride levels have normalized and been stable. Her alopecia has not progressed but is persistent. She continues to have central hypothyroidism due to bexarotene and is on levothyroxine. The lymphomatoid papulosis also remains stable with no signs of progression to cutaneous T-cell lymphoma.
Although the exact mechanism of LPP is not fully understood, studies have suggested that cellular lipid metabolism may be responsible for the inflammation of the pilosebaceous unit.4-11 Hyperlipidemia is the most common side effect of oral bexarotene, typically occurring within the first 2 to 4 weeks of treatment.3,12 Considering the insights into the role of lipid regulation on LPP pathogenesis, it is reasonable to suspect that the dyslipidemia caused by bexarotene may have triggered the onset of LPP in our patient. The patient’s lipid values mostly remained within reference range throughout the course of treatment, though she did have elevation of triglycerides around the onset of LPP. Dyslipidemia has been reported in patients with lichen planus but not in patients with LPP. One case-control study showed no dyslipidemia in patients with LPP, but the triglyceride levels were not tracked over time and patients had varying durations since onset of disease at presentation.9-11,13 In our case, we were fortunate to have this information, and it may suggest an interaction between lipid dysregulation and the development of LPP. It would be interesting to explore this further in a larger patient population and to evaluate if control of dyslipidemia reduces progression of disease as it appears to have done for our patient.
To the Editor:
Lymphomatoid papulosis is a rare chronic skin disorder characterized by recurrent, self-healing crops of papulonodular eruptions, often resembling cutaneous T-cell lymphoma.1 Oral bexarotene, a retinoid X receptor–selective retinoid, can be used to control the disease.2,3 Lichen planopilaris (LPP) is a type of cicatricial alopecia characterized by irreversible hair loss, perifollicular inflammation, and follicular hyperkeratosis, commonly affecting the scalp vertex in adults.4 We report a case of a patient with lymphomatoid papulosis who was treated with bexarotene and subsequently developed LPP. We also discuss a proposed mechanism by which bexarotene may have influenced the onset of LPP.
A 35-year-old woman who was previously healthy initially presented with recurrent pruritic papular eruptions on the flank, axillae, and groin of several months’ duration. The lesions appeared as 2-mm, flat-topped, violaceous papules. The patient had no known drug allergies, no medical or family history of skin disease, and was only taking 3000 mg/d of omega-3 fatty acids (fish oil). Histopathologic examination of a biopsy specimen from the inner thigh showed enlarged, atypical, dermal lymphocytes that were CD30+ (Figure 1). These findings were consistent with lymphomatoid papulosis. As she had undergone tubal ligation several years prior, she was prescribed oral bexarotene 300 mg once daily in addition to triamcinolone cream 0.1% twice daily, as needed. Symptoms were well controlled on this regimen.

Six months later the patient returned, presenting with a new central patch of scarring alopecia on the vertex of the scalp (Figure 2). Adjacent to the area of hair loss were areas of prominent perifollicular scale that were slightly violaceous in color. Two 4-mm punch biopsies of the scalp showed dermal scarring with perifollicular lamellar fibrosis surrounded by a rim of lymphoplasmacytic inflammation (Figure 3). Sebaceous glands were found to be reduced in number. These findings were consistent with cicatricial alopecia, which was further classified as LPP in conjunction with the clinical findings. No CD30+ lymphocytes were identified in these specimens.


Baseline fasting triglycerides were 123 mg/dL (desirable: <150 mg/dL; borderline: 150–199 mg/dL; high: ≥200 mg/dL) and were stable over the first 4 months on bexarotene. After 5 months of therapy, the triglycerides increased to a high of 255 mg/dL, which corresponded with the onset of LPP. She was treated for the hypertriglyceridemia with omega-3 fatty acids (fish oil), and subsequent triglyceride levels have normalized and been stable. Her alopecia has not progressed but is persistent. She continues to have central hypothyroidism due to bexarotene and is on levothyroxine. The lymphomatoid papulosis also remains stable with no signs of progression to cutaneous T-cell lymphoma.
Although the exact mechanism of LPP is not fully understood, studies have suggested that cellular lipid metabolism may be responsible for the inflammation of the pilosebaceous unit.4-11 Hyperlipidemia is the most common side effect of oral bexarotene, typically occurring within the first 2 to 4 weeks of treatment.3,12 Considering the insights into the role of lipid regulation on LPP pathogenesis, it is reasonable to suspect that the dyslipidemia caused by bexarotene may have triggered the onset of LPP in our patient. The patient’s lipid values mostly remained within reference range throughout the course of treatment, though she did have elevation of triglycerides around the onset of LPP. Dyslipidemia has been reported in patients with lichen planus but not in patients with LPP. One case-control study showed no dyslipidemia in patients with LPP, but the triglyceride levels were not tracked over time and patients had varying durations since onset of disease at presentation.9-11,13 In our case, we were fortunate to have this information, and it may suggest an interaction between lipid dysregulation and the development of LPP. It would be interesting to explore this further in a larger patient population and to evaluate if control of dyslipidemia reduces progression of disease as it appears to have done for our patient.
- Karp DL, Horn TD. Lymphomatoid papulosis. J Am Acad Dermatol. 1994;30:379-395; quiz 396-398.
- Krathen RA, Ward S, Duvic M. Bexarotene is a new treatment option for lymphomatoid papulosis. Dermatology. 2003;206:142-147.
- Targretin (bexarotene) capsule [package insert]. St. Petersburg, FL: Cardinal Health; 2003. http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=63656f64-e240-4855-8df9-ca1655863735. Accessed April 9, 2020.
- Assouly P, Reygagne P. Lichen planopilaris: update on diagnosis and treatment. Semin Cutan Med Surg. 2009;28:3-10.
- Dogra S, Sarangal R. What’s new in cicatricial alopecia? Indian J Dermatol Venereol Leprol. 2013;79:576-90.
- Zheng Y, Eilertsen KJ, Ge L, et al. Scd1 is expressed in sebaceous glands and is disrupted in the asebia mouse. Nat Genet. 1999;23:268-270.
- Sundberg JP, Boggess D, Sundberg BA, et al. Asebia-2J (Scd1(ab2J)): a new allele and a model for scarring alopecia. Am J Pathol. 2000;156:2067-2075.
- Karnik P, Tekeste Z, McCormick TS, et al. Hair follicle stem cell-specific PPARgamma deletion causes scarring alopecia. J Invest Dermatol. 2009;129:1243-157.
- López-Jornet P, Camacho-Alonso F, Rodríguez-Martínes MA. Alterations in serum lipid profile patterns in oral lichen planus: a cross-sectional study. Am J Clin Dermatol. 2012;13:399-404.
- Arias-Santiago S, Buendía-Eisman A, Aneiros-Fernández J, et al. Lipid levels in patients with lichen planus: a case-control study. J Eur Acad Dermatol Venereol. 2011;25:1398-1401.
- Dreiher J, Shapiro J, Cohen AD. Lichen planus and dyslipidaemia: a case-control study. Br J Dermatol. 2009;161:626-629.
- de Vries-van der Weij J, de Haan W, Hu L, et al. Bexarotene induces dyslipidemia by increased very low-density lipoprotein production and cholesteryl ester transfer protein-mediated reduction of high-density lipoprotein. Endocrinology. 2009;150:2368-2375.
- Conic RRZ, Piliang M, Bergfeld W, et al. Association of lichen planopilaris with dyslipidemia. JAMA Dermatol. 2018;154:1088-1089.
- Karp DL, Horn TD. Lymphomatoid papulosis. J Am Acad Dermatol. 1994;30:379-395; quiz 396-398.
- Krathen RA, Ward S, Duvic M. Bexarotene is a new treatment option for lymphomatoid papulosis. Dermatology. 2003;206:142-147.
- Targretin (bexarotene) capsule [package insert]. St. Petersburg, FL: Cardinal Health; 2003. http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=63656f64-e240-4855-8df9-ca1655863735. Accessed April 9, 2020.
- Assouly P, Reygagne P. Lichen planopilaris: update on diagnosis and treatment. Semin Cutan Med Surg. 2009;28:3-10.
- Dogra S, Sarangal R. What’s new in cicatricial alopecia? Indian J Dermatol Venereol Leprol. 2013;79:576-90.
- Zheng Y, Eilertsen KJ, Ge L, et al. Scd1 is expressed in sebaceous glands and is disrupted in the asebia mouse. Nat Genet. 1999;23:268-270.
- Sundberg JP, Boggess D, Sundberg BA, et al. Asebia-2J (Scd1(ab2J)): a new allele and a model for scarring alopecia. Am J Pathol. 2000;156:2067-2075.
- Karnik P, Tekeste Z, McCormick TS, et al. Hair follicle stem cell-specific PPARgamma deletion causes scarring alopecia. J Invest Dermatol. 2009;129:1243-157.
- López-Jornet P, Camacho-Alonso F, Rodríguez-Martínes MA. Alterations in serum lipid profile patterns in oral lichen planus: a cross-sectional study. Am J Clin Dermatol. 2012;13:399-404.
- Arias-Santiago S, Buendía-Eisman A, Aneiros-Fernández J, et al. Lipid levels in patients with lichen planus: a case-control study. J Eur Acad Dermatol Venereol. 2011;25:1398-1401.
- Dreiher J, Shapiro J, Cohen AD. Lichen planus and dyslipidaemia: a case-control study. Br J Dermatol. 2009;161:626-629.
- de Vries-van der Weij J, de Haan W, Hu L, et al. Bexarotene induces dyslipidemia by increased very low-density lipoprotein production and cholesteryl ester transfer protein-mediated reduction of high-density lipoprotein. Endocrinology. 2009;150:2368-2375.
- Conic RRZ, Piliang M, Bergfeld W, et al. Association of lichen planopilaris with dyslipidemia. JAMA Dermatol. 2018;154:1088-1089.
Practice Points
- Oral retinoids may be associated with development of lichen planopilaris (LPP).
- Hypertriglyceridemia may be associated with onset of LPP.
Cutaneous Metastases From Esophageal Adenocarcinoma on the Scalp
To the Editor:
A 59-year-old man presented with a lesion on the right frontal scalp of 4 months’ duration and a lesion on the left frontal scalp of 1 month’s duration. Both lesions were tender, bleeding, nonhealing, and growing in size. The patient reported no improvement with the use of triple antibiotic ointment. He denied any associated symptoms or trauma to the affected areas. He had a history of stage IV esophageal adenocarcinoma that initially had been surgically removed 6 years prior but metastasized to the lungs and bone. The patient subsequently underwent treatment with FOLFOX (folinic acid, fluorouracil, oxaliplatin), trastuzumab, and radiation therapy.
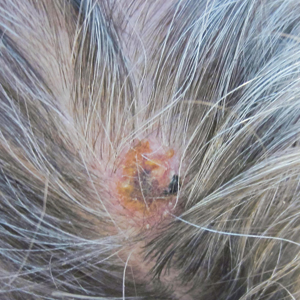
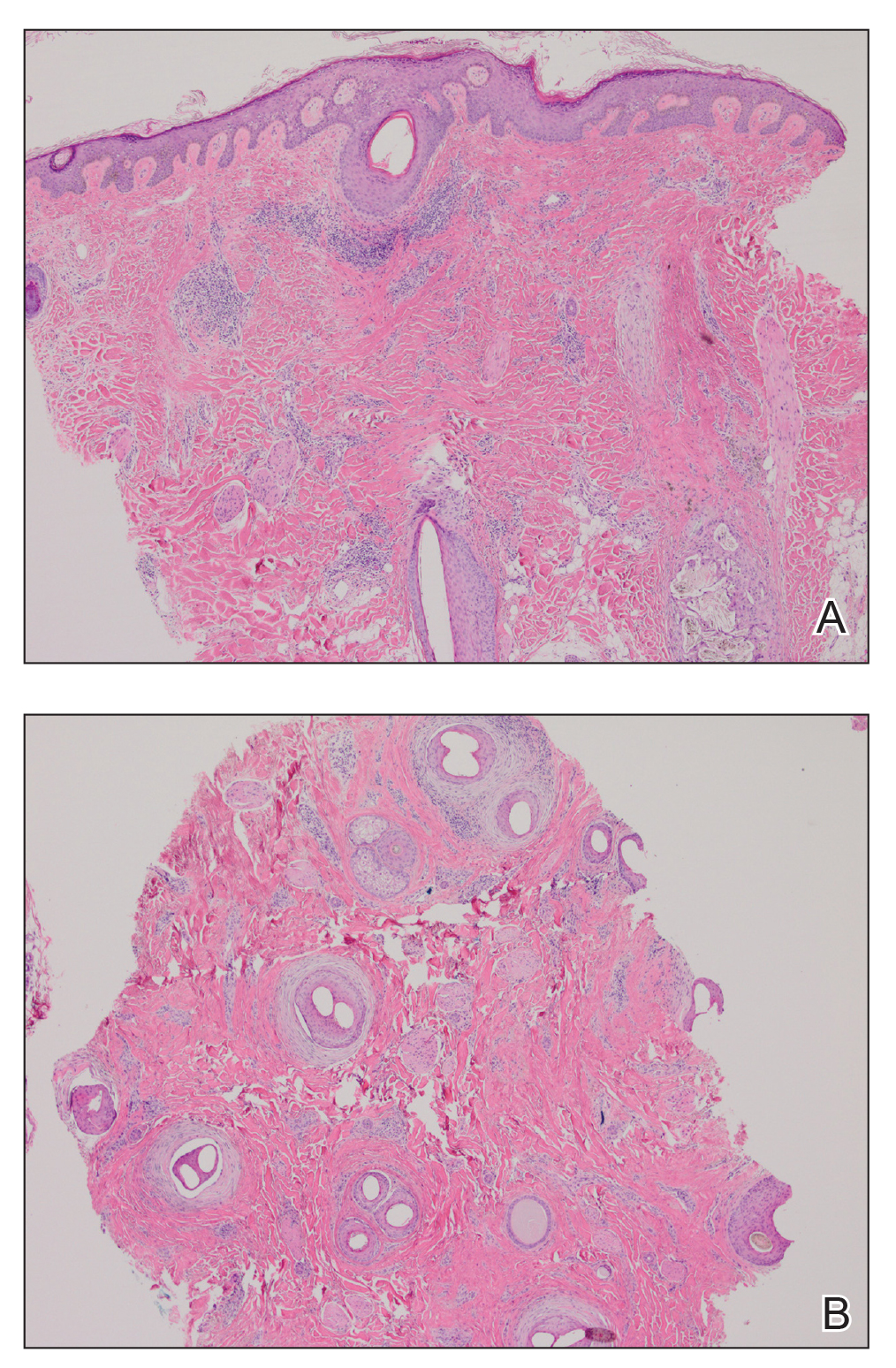
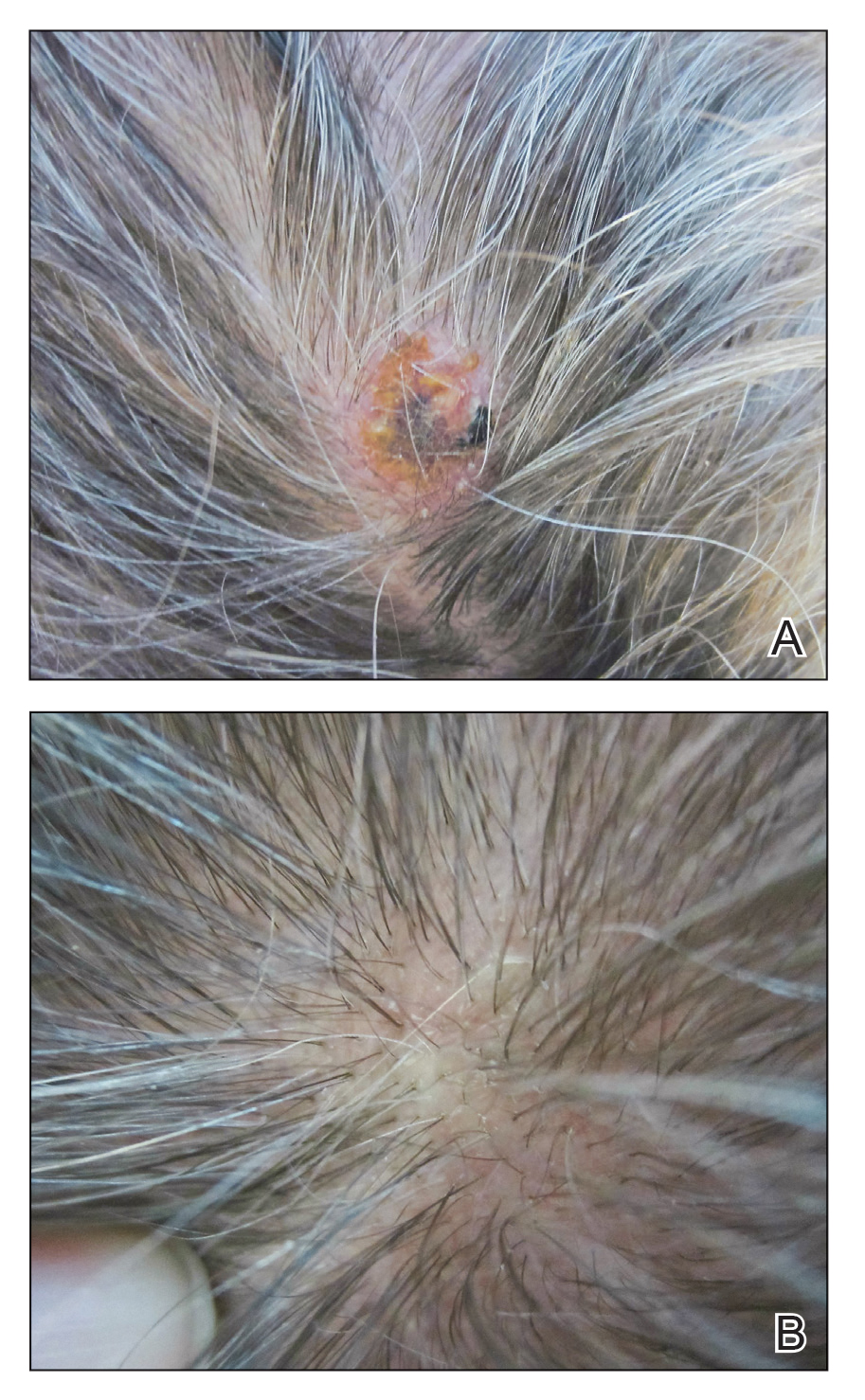
Physical examination revealed a hyperkeratotic pink nodule with a central erosion and crust on the right frontal scalp measuring 1.5×2 cm in diameter (Figure 1A). The left frontal scalp lesion was a smooth pearly papule measuring 5×5 mm in diameter (Figure 1B). The differential diagnosis included basal cell carcinoma, squamous cell carcinoma, and cutaneous metastases from esophageal adenocarcinoma. Shave biopsies were taken of both scalp lesions.
Histologic examination of both scalp lesions demonstrated a dermal gland-forming neoplasm with an infiltrative distribution that was comprised of irregular cribriform glands containing cellular debris (Figure 2). The cells of interest were enlarged and contained pleomorphic crowded nuclei that formed aberrant mitotic division figures. Both biopsies were positive for cytokeratin 7 and negative for cytokeratin 20 and CDX2. The final diagnosis for both scalp lesions was poorly differentiated adenocarcinoma, which was most suggestive of cutaneous metastases of the patient’s known esophageal adenocarcinoma. Given further metastasis, the patient was ultimately switched to ramucirumab and paclitaxel per oncology.
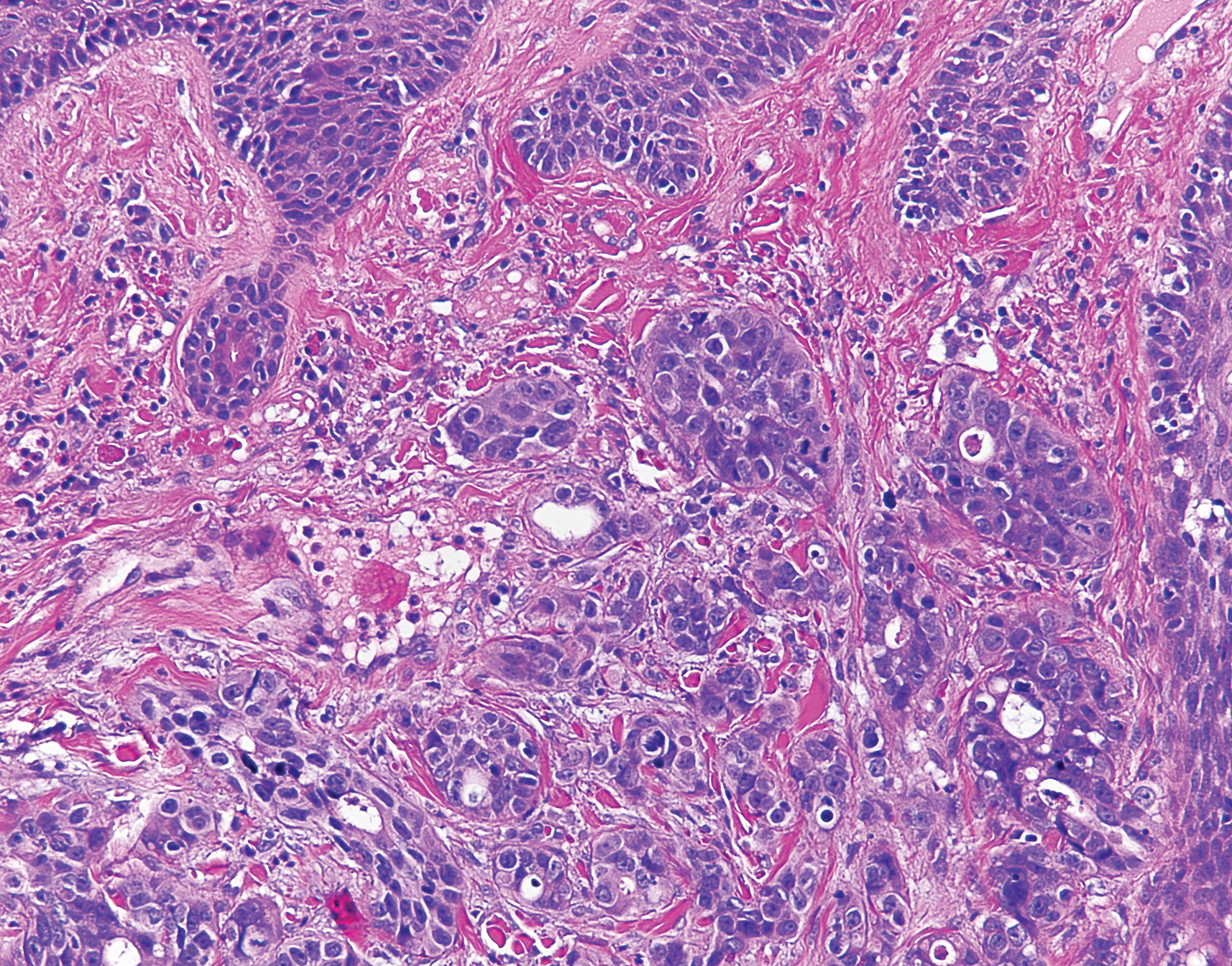
Esophageal carcinoma is the eighth most common cause of death related to cancer worldwide. Adenocarcinoma is the most prevalent histologic type of esophageal carcinoma, with an incidence as high as 5.69 per 100,000 individuals in the United States.1 Internal malignancies that lead to cutaneous metastases are not uncommon; however, the literature is limited on cutaneous scalp metastases from esophageal cancer. Cutaneous metastases secondary to internal malignancies present in less than 10% of overall cases; tend to derive from the breasts, lungs, and large bowel; and usually present in the sixth to seventh decades of life.2 Further, roughly 1% of all skin metastases originate from the esophagus.3 When there are cutaneous metastases to the scalp, they often arise from breast carcinomas and renal cell carcinomas.4,5 Rarely does esophageal cancer spread to the scalp.2,6-9 When cutaneous metastases originate from the esophagus, multiple cancers such as squamous cell carcinomas, mucoepidermoid carcinomas, small cell carcinomas, and adenocarcinomas can be the etiology of origin.10 Metastases originating from esophageal carcinomas frequently are diagnosed in the abdominal lymph nodes (45%), liver (35%), lungs (20%), cervical/supraclavicular lymph nodes (18%), bones (9%), adrenals (5%), peritoneum (2%), brain (2%), stomach (1%), pancreas (1%), pleura (1%), skin/body wall (1%), pericardium (1%), and spleen (1%).3 Additionally, multiple cutaneous scalp metastases from esophageal adenocarcinoma have been reported,7,9 as were seen in our case.
The clinical appearance of cutaneous scalp metastases has been described as inflammatory papules, indurated plaques, or nodules,2 which is consistent with our case, though the spectrum of presentation is admittedly broad. Histopathology of lesions characteristically shows prominent intraluminal necrotic cellular debris, which is common for adenocarcinomas of the gastrointestinal tract.7 However, utilizing immunohistochemical stains to detect specific antigens within tumor cells allows for better specificity of the tumor origin. More specifically, cytokeratin 7 and cytokeratin 20 are stained in esophageal metaplasia, such as Barrett esophagus, rather than in intestinal metaplasia inside the stomach.2,11 Therefore, discerning the location of the adenocarcinoma proves fruitful when using cytokeratin 7 and cytokeratin 20. Although CDX2 is an additional marker that can be used for gastrointestinal adenocarcinomas with decent sensitivity and specificity, it can still be expressed in mucinous ovarian carcinomas and urinary bladder adenocarcinomas.12 In our patient, the strong reactivity of cytokeratin 7 in addition to the characteristic morphology in both presenting biopsies was sufficient to make the diagnosis of cutaneous metastasis of esophageal adenocarcinoma to the scalp.
Our case highlights multiple cutaneous metastases of the scalp from a primary esophageal adenocarcinoma. Although cutaneous scalp metastasis of esophageal adenocarcinoma is rare, it is essential to provide a full-body skin examination, including the scalp, in patients with a history of esophageal cancer and to biopsy any suspicious nodules or plaques. The 1-year survival rate after diagnosis of esophageal carcinoma is less than 50%, and the 5-year survival rate is less than 10%.13 Identifying cutaneous metastasis of an esophageal adenocarcinoma can either change the staging of the cancer (if it was the first distant metastasis noted) or indicate an insufficient response to treatment in a patient with known metastatic disease, prompting a potential change in treatment.7
This case illustrates a rare site of metastasis of a fairly common cancer and highlights the histopathology and accompanying immunohistochemical stains that can be useful in diagnosis as well as the spectrum of its clinical presentation.
- Melhado R, Alderson D, Tucker O. The changing face of esophageal cancer. Cancers (Basel). 2010;2:1379-1404.
- Park JM, Kim DS, Oh SH, et al. A case of esophageal adenocarcinoma metastasized to the scalp [published online May 31, 2009]. Ann Dermatol. 2009;21:164-167.
- Quint LE, Hepburn LM, Francis IR, et al. Incidence and distribution of distant metastases from newly diagnosed esophageal carcinoma. Cancer. 1995;76:1120.
- Dobson C, Tagor V, Myint A, et al. Telangiectatic metastatic breast carcinoma in face and scalp mimicking cutaneous angiosarcoma. J Am Acad Dermatol. 2003;48:635-636.
- Riter H, Ghobrial I. Renal cell carcinoma with acrometastasis and scalp metastasis. Mayo Clin Proc. 2004;79:76.
- Roh EK, Nord R, Jukic DM. Scalp metastasis from esophageal adenocarcinoma. Cutis. 2006;77:106.
- Doumit G, Abouhassan W, Piliang M, et al. Scalp metastasis from esophageal adenocarcinoma: comparative histopathology dictates surgical approach. Ann Plast Surg. 2011;71:60-62.
- Roy AD, Sherparpa M, Prasad PR, et al. Scalp metastasis of gastro-esophageal junction adenocarcinoma: a rare occurrence. 2014;8:159-160.
- Stein R, Spencer J. Painful cutaneous metastases from esophageal carcinoma. Cutis. 2002;70:230.
- Schwartz RA. Cutaneous metastatic disease. J Am Acad Dermatol. 1995;33(2 pt 1):161-182.
- Ormsby AH, Goldblum JR, Rice TW, et al. Cytokeratin subsets can reliably distinguish Barrett’s esophagus from intestinal metaplasia of the stomach. Hum Pathol. 1999;30:288-294.
- Werling RW, Yaziji H, Bacchi CE, et al. CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin: an immunohistochemical survey of 476 primary and metastatic carcinomas. Am J Surg Pathol. 2003;27:303-310.
- Smith KJ, Williams J, Skelton H. Metastatic adenocarcinoma of the esophagus to the skin: new patterns of tumor recurrence and alternate treatments for palliation. J Cutan Pathol. 2001;28:425-431.
To the Editor:
A 59-year-old man presented with a lesion on the right frontal scalp of 4 months’ duration and a lesion on the left frontal scalp of 1 month’s duration. Both lesions were tender, bleeding, nonhealing, and growing in size. The patient reported no improvement with the use of triple antibiotic ointment. He denied any associated symptoms or trauma to the affected areas. He had a history of stage IV esophageal adenocarcinoma that initially had been surgically removed 6 years prior but metastasized to the lungs and bone. The patient subsequently underwent treatment with FOLFOX (folinic acid, fluorouracil, oxaliplatin), trastuzumab, and radiation therapy.
Physical examination revealed a hyperkeratotic pink nodule with a central erosion and crust on the right frontal scalp measuring 1.5×2 cm in diameter (Figure 1A). The left frontal scalp lesion was a smooth pearly papule measuring 5×5 mm in diameter (Figure 1B). The differential diagnosis included basal cell carcinoma, squamous cell carcinoma, and cutaneous metastases from esophageal adenocarcinoma. Shave biopsies were taken of both scalp lesions.

Histologic examination of both scalp lesions demonstrated a dermal gland-forming neoplasm with an infiltrative distribution that was comprised of irregular cribriform glands containing cellular debris (Figure 2). The cells of interest were enlarged and contained pleomorphic crowded nuclei that formed aberrant mitotic division figures. Both biopsies were positive for cytokeratin 7 and negative for cytokeratin 20 and CDX2. The final diagnosis for both scalp lesions was poorly differentiated adenocarcinoma, which was most suggestive of cutaneous metastases of the patient’s known esophageal adenocarcinoma. Given further metastasis, the patient was ultimately switched to ramucirumab and paclitaxel per oncology.

Esophageal carcinoma is the eighth most common cause of death related to cancer worldwide. Adenocarcinoma is the most prevalent histologic type of esophageal carcinoma, with an incidence as high as 5.69 per 100,000 individuals in the United States.1 Internal malignancies that lead to cutaneous metastases are not uncommon; however, the literature is limited on cutaneous scalp metastases from esophageal cancer. Cutaneous metastases secondary to internal malignancies present in less than 10% of overall cases; tend to derive from the breasts, lungs, and large bowel; and usually present in the sixth to seventh decades of life.2 Further, roughly 1% of all skin metastases originate from the esophagus.3 When there are cutaneous metastases to the scalp, they often arise from breast carcinomas and renal cell carcinomas.4,5 Rarely does esophageal cancer spread to the scalp.2,6-9 When cutaneous metastases originate from the esophagus, multiple cancers such as squamous cell carcinomas, mucoepidermoid carcinomas, small cell carcinomas, and adenocarcinomas can be the etiology of origin.10 Metastases originating from esophageal carcinomas frequently are diagnosed in the abdominal lymph nodes (45%), liver (35%), lungs (20%), cervical/supraclavicular lymph nodes (18%), bones (9%), adrenals (5%), peritoneum (2%), brain (2%), stomach (1%), pancreas (1%), pleura (1%), skin/body wall (1%), pericardium (1%), and spleen (1%).3 Additionally, multiple cutaneous scalp metastases from esophageal adenocarcinoma have been reported,7,9 as were seen in our case.
The clinical appearance of cutaneous scalp metastases has been described as inflammatory papules, indurated plaques, or nodules,2 which is consistent with our case, though the spectrum of presentation is admittedly broad. Histopathology of lesions characteristically shows prominent intraluminal necrotic cellular debris, which is common for adenocarcinomas of the gastrointestinal tract.7 However, utilizing immunohistochemical stains to detect specific antigens within tumor cells allows for better specificity of the tumor origin. More specifically, cytokeratin 7 and cytokeratin 20 are stained in esophageal metaplasia, such as Barrett esophagus, rather than in intestinal metaplasia inside the stomach.2,11 Therefore, discerning the location of the adenocarcinoma proves fruitful when using cytokeratin 7 and cytokeratin 20. Although CDX2 is an additional marker that can be used for gastrointestinal adenocarcinomas with decent sensitivity and specificity, it can still be expressed in mucinous ovarian carcinomas and urinary bladder adenocarcinomas.12 In our patient, the strong reactivity of cytokeratin 7 in addition to the characteristic morphology in both presenting biopsies was sufficient to make the diagnosis of cutaneous metastasis of esophageal adenocarcinoma to the scalp.
Our case highlights multiple cutaneous metastases of the scalp from a primary esophageal adenocarcinoma. Although cutaneous scalp metastasis of esophageal adenocarcinoma is rare, it is essential to provide a full-body skin examination, including the scalp, in patients with a history of esophageal cancer and to biopsy any suspicious nodules or plaques. The 1-year survival rate after diagnosis of esophageal carcinoma is less than 50%, and the 5-year survival rate is less than 10%.13 Identifying cutaneous metastasis of an esophageal adenocarcinoma can either change the staging of the cancer (if it was the first distant metastasis noted) or indicate an insufficient response to treatment in a patient with known metastatic disease, prompting a potential change in treatment.7
This case illustrates a rare site of metastasis of a fairly common cancer and highlights the histopathology and accompanying immunohistochemical stains that can be useful in diagnosis as well as the spectrum of its clinical presentation.
To the Editor:
A 59-year-old man presented with a lesion on the right frontal scalp of 4 months’ duration and a lesion on the left frontal scalp of 1 month’s duration. Both lesions were tender, bleeding, nonhealing, and growing in size. The patient reported no improvement with the use of triple antibiotic ointment. He denied any associated symptoms or trauma to the affected areas. He had a history of stage IV esophageal adenocarcinoma that initially had been surgically removed 6 years prior but metastasized to the lungs and bone. The patient subsequently underwent treatment with FOLFOX (folinic acid, fluorouracil, oxaliplatin), trastuzumab, and radiation therapy.
Physical examination revealed a hyperkeratotic pink nodule with a central erosion and crust on the right frontal scalp measuring 1.5×2 cm in diameter (Figure 1A). The left frontal scalp lesion was a smooth pearly papule measuring 5×5 mm in diameter (Figure 1B). The differential diagnosis included basal cell carcinoma, squamous cell carcinoma, and cutaneous metastases from esophageal adenocarcinoma. Shave biopsies were taken of both scalp lesions.

Histologic examination of both scalp lesions demonstrated a dermal gland-forming neoplasm with an infiltrative distribution that was comprised of irregular cribriform glands containing cellular debris (Figure 2). The cells of interest were enlarged and contained pleomorphic crowded nuclei that formed aberrant mitotic division figures. Both biopsies were positive for cytokeratin 7 and negative for cytokeratin 20 and CDX2. The final diagnosis for both scalp lesions was poorly differentiated adenocarcinoma, which was most suggestive of cutaneous metastases of the patient’s known esophageal adenocarcinoma. Given further metastasis, the patient was ultimately switched to ramucirumab and paclitaxel per oncology.

Esophageal carcinoma is the eighth most common cause of death related to cancer worldwide. Adenocarcinoma is the most prevalent histologic type of esophageal carcinoma, with an incidence as high as 5.69 per 100,000 individuals in the United States.1 Internal malignancies that lead to cutaneous metastases are not uncommon; however, the literature is limited on cutaneous scalp metastases from esophageal cancer. Cutaneous metastases secondary to internal malignancies present in less than 10% of overall cases; tend to derive from the breasts, lungs, and large bowel; and usually present in the sixth to seventh decades of life.2 Further, roughly 1% of all skin metastases originate from the esophagus.3 When there are cutaneous metastases to the scalp, they often arise from breast carcinomas and renal cell carcinomas.4,5 Rarely does esophageal cancer spread to the scalp.2,6-9 When cutaneous metastases originate from the esophagus, multiple cancers such as squamous cell carcinomas, mucoepidermoid carcinomas, small cell carcinomas, and adenocarcinomas can be the etiology of origin.10 Metastases originating from esophageal carcinomas frequently are diagnosed in the abdominal lymph nodes (45%), liver (35%), lungs (20%), cervical/supraclavicular lymph nodes (18%), bones (9%), adrenals (5%), peritoneum (2%), brain (2%), stomach (1%), pancreas (1%), pleura (1%), skin/body wall (1%), pericardium (1%), and spleen (1%).3 Additionally, multiple cutaneous scalp metastases from esophageal adenocarcinoma have been reported,7,9 as were seen in our case.
The clinical appearance of cutaneous scalp metastases has been described as inflammatory papules, indurated plaques, or nodules,2 which is consistent with our case, though the spectrum of presentation is admittedly broad. Histopathology of lesions characteristically shows prominent intraluminal necrotic cellular debris, which is common for adenocarcinomas of the gastrointestinal tract.7 However, utilizing immunohistochemical stains to detect specific antigens within tumor cells allows for better specificity of the tumor origin. More specifically, cytokeratin 7 and cytokeratin 20 are stained in esophageal metaplasia, such as Barrett esophagus, rather than in intestinal metaplasia inside the stomach.2,11 Therefore, discerning the location of the adenocarcinoma proves fruitful when using cytokeratin 7 and cytokeratin 20. Although CDX2 is an additional marker that can be used for gastrointestinal adenocarcinomas with decent sensitivity and specificity, it can still be expressed in mucinous ovarian carcinomas and urinary bladder adenocarcinomas.12 In our patient, the strong reactivity of cytokeratin 7 in addition to the characteristic morphology in both presenting biopsies was sufficient to make the diagnosis of cutaneous metastasis of esophageal adenocarcinoma to the scalp.
Our case highlights multiple cutaneous metastases of the scalp from a primary esophageal adenocarcinoma. Although cutaneous scalp metastasis of esophageal adenocarcinoma is rare, it is essential to provide a full-body skin examination, including the scalp, in patients with a history of esophageal cancer and to biopsy any suspicious nodules or plaques. The 1-year survival rate after diagnosis of esophageal carcinoma is less than 50%, and the 5-year survival rate is less than 10%.13 Identifying cutaneous metastasis of an esophageal adenocarcinoma can either change the staging of the cancer (if it was the first distant metastasis noted) or indicate an insufficient response to treatment in a patient with known metastatic disease, prompting a potential change in treatment.7
This case illustrates a rare site of metastasis of a fairly common cancer and highlights the histopathology and accompanying immunohistochemical stains that can be useful in diagnosis as well as the spectrum of its clinical presentation.
- Melhado R, Alderson D, Tucker O. The changing face of esophageal cancer. Cancers (Basel). 2010;2:1379-1404.
- Park JM, Kim DS, Oh SH, et al. A case of esophageal adenocarcinoma metastasized to the scalp [published online May 31, 2009]. Ann Dermatol. 2009;21:164-167.
- Quint LE, Hepburn LM, Francis IR, et al. Incidence and distribution of distant metastases from newly diagnosed esophageal carcinoma. Cancer. 1995;76:1120.
- Dobson C, Tagor V, Myint A, et al. Telangiectatic metastatic breast carcinoma in face and scalp mimicking cutaneous angiosarcoma. J Am Acad Dermatol. 2003;48:635-636.
- Riter H, Ghobrial I. Renal cell carcinoma with acrometastasis and scalp metastasis. Mayo Clin Proc. 2004;79:76.
- Roh EK, Nord R, Jukic DM. Scalp metastasis from esophageal adenocarcinoma. Cutis. 2006;77:106.
- Doumit G, Abouhassan W, Piliang M, et al. Scalp metastasis from esophageal adenocarcinoma: comparative histopathology dictates surgical approach. Ann Plast Surg. 2011;71:60-62.
- Roy AD, Sherparpa M, Prasad PR, et al. Scalp metastasis of gastro-esophageal junction adenocarcinoma: a rare occurrence. 2014;8:159-160.
- Stein R, Spencer J. Painful cutaneous metastases from esophageal carcinoma. Cutis. 2002;70:230.
- Schwartz RA. Cutaneous metastatic disease. J Am Acad Dermatol. 1995;33(2 pt 1):161-182.
- Ormsby AH, Goldblum JR, Rice TW, et al. Cytokeratin subsets can reliably distinguish Barrett’s esophagus from intestinal metaplasia of the stomach. Hum Pathol. 1999;30:288-294.
- Werling RW, Yaziji H, Bacchi CE, et al. CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin: an immunohistochemical survey of 476 primary and metastatic carcinomas. Am J Surg Pathol. 2003;27:303-310.
- Smith KJ, Williams J, Skelton H. Metastatic adenocarcinoma of the esophagus to the skin: new patterns of tumor recurrence and alternate treatments for palliation. J Cutan Pathol. 2001;28:425-431.
- Melhado R, Alderson D, Tucker O. The changing face of esophageal cancer. Cancers (Basel). 2010;2:1379-1404.
- Park JM, Kim DS, Oh SH, et al. A case of esophageal adenocarcinoma metastasized to the scalp [published online May 31, 2009]. Ann Dermatol. 2009;21:164-167.
- Quint LE, Hepburn LM, Francis IR, et al. Incidence and distribution of distant metastases from newly diagnosed esophageal carcinoma. Cancer. 1995;76:1120.
- Dobson C, Tagor V, Myint A, et al. Telangiectatic metastatic breast carcinoma in face and scalp mimicking cutaneous angiosarcoma. J Am Acad Dermatol. 2003;48:635-636.
- Riter H, Ghobrial I. Renal cell carcinoma with acrometastasis and scalp metastasis. Mayo Clin Proc. 2004;79:76.
- Roh EK, Nord R, Jukic DM. Scalp metastasis from esophageal adenocarcinoma. Cutis. 2006;77:106.
- Doumit G, Abouhassan W, Piliang M, et al. Scalp metastasis from esophageal adenocarcinoma: comparative histopathology dictates surgical approach. Ann Plast Surg. 2011;71:60-62.
- Roy AD, Sherparpa M, Prasad PR, et al. Scalp metastasis of gastro-esophageal junction adenocarcinoma: a rare occurrence. 2014;8:159-160.
- Stein R, Spencer J. Painful cutaneous metastases from esophageal carcinoma. Cutis. 2002;70:230.
- Schwartz RA. Cutaneous metastatic disease. J Am Acad Dermatol. 1995;33(2 pt 1):161-182.
- Ormsby AH, Goldblum JR, Rice TW, et al. Cytokeratin subsets can reliably distinguish Barrett’s esophagus from intestinal metaplasia of the stomach. Hum Pathol. 1999;30:288-294.
- Werling RW, Yaziji H, Bacchi CE, et al. CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin: an immunohistochemical survey of 476 primary and metastatic carcinomas. Am J Surg Pathol. 2003;27:303-310.
- Smith KJ, Williams J, Skelton H. Metastatic adenocarcinoma of the esophagus to the skin: new patterns of tumor recurrence and alternate treatments for palliation. J Cutan Pathol. 2001;28:425-431.
Practice Points
- In the setting of underlying esophageal adenocarcinoma, metastatic spread to the scalp should be considered in the differential diagnosis for any suspicious scalp lesions.
- Coupling histopathology with immunohistochemical stains may aid in the diagnosis for cutaneous metastasis of esophageal adenocarcinoma.
Smartphones: Dermatologic Impact of the Digital Age
Over the last decade, the use of mobile phones has changed drastically with the advent of more technologically advanced smartphones.1 Mobile phones are no longer used primarily as devices for talking but rather for text messaging, reading the news, drafting emails, browsing websites, and connecting with others on social media. Considering the increased utility and popularity of social media along with the greater reliance on smartphones, individuals in the United States and worldwide are undoubtedly spending more time on their handheld devices.2 With the increase in use and overuse of smartphones, many aspects of society and health are likely affected. Many celebrities who frequently post on social media platforms also have alluded to or directly discussed changes in their dermatologic health secondary to their increased use of smartphones.3 Numerous studies have investigated the positive and negative effects of smartphone use on various musculoskeletal conditions of the upper extremities4,5 and the social effects of smartphone use on behavior and child development.6,7 Lee et al8 studied the effects of smartphone use on upper extremity muscle pain and activity in relation to 1- or 2-handed operation. In this study, Lee et al8 measured the muscle activity and tenderness in 10 women aged 20 to 22 years after a series of timed periods of smartphone use. They concluded that smartphone use resulted in greater muscle activity and tenderness, especially in 1-handed use compared to 2-handed use.8 Inal et al9 investigated smartphone overuse effects on hand strength and function in 102 college students and discovered that smartphone overuse was correlated with decreased pinch strength, increased median nerve cross-sectional area, and pain in the first digits.9
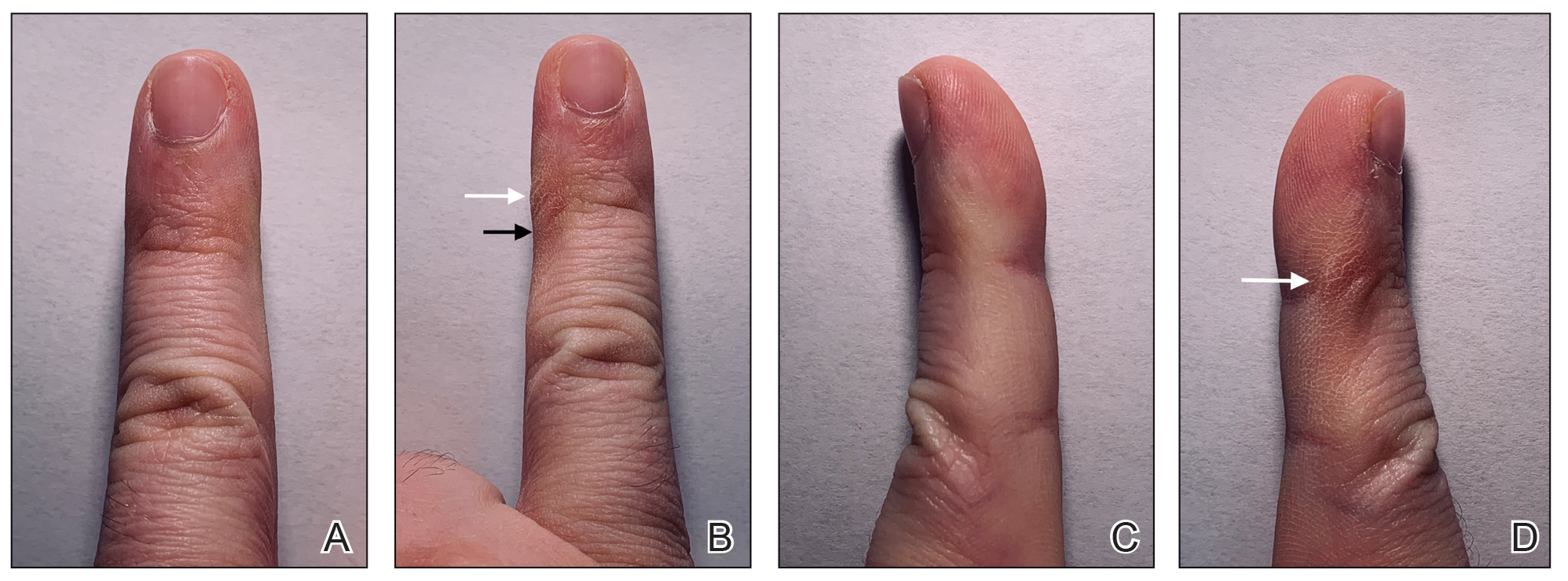
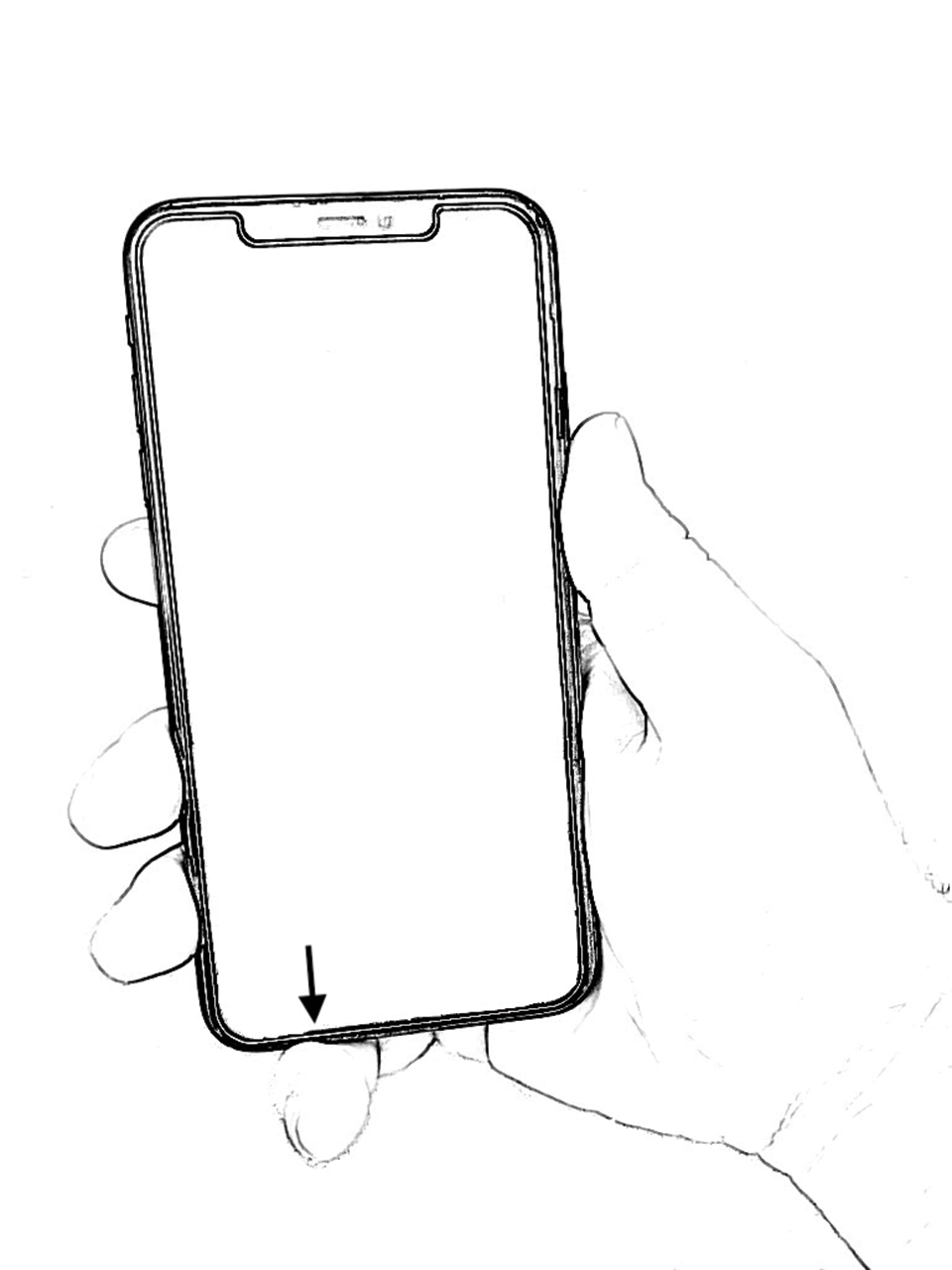
However, few articles have been published investigating skin changes to the digits in relation to smartphone use (Figure 1). In a PubMed search of articles indexed for MEDLINE using the terms smartphone, phone, cell phone, electronic device, handheld device, fifth digit, or skin changes, the authors were unable to find any studies in the literature that involved smartphone use and skin changes to the digits. Based on informal clinical observation and personal experiences, we hypothesized that changes to the fifth digit, likely due to holding a smartphone, would be prevalent and would correlate with amount of time spent on smartphones per day (Figure 2). We also were interested in investigating any other potential correlations with changes to the fifth digit, such as type of smartphone used.
Methods
The study used a cross-sectional design. From September 2018 to December 2018, 374 individuals 18 years or older were recruited to complete a 5-minute anonymous survey online. Using email referrals and social media, participants were presented with a link to a Google survey that only allowed 1 submission per account. On the first page of the survey, participants were presented with a letter explaining that completion of the survey was entirely voluntary, participants were free to withdraw from the study at any time, and participants were providing consent in completing the survey. The protocol was determined to be exempt by the institutional review board at Nova Southeastern University (Fort Lauderdale, Florida) in September 2018.
Survey Design
A 20-item survey was designed to measure the amount of time spent using smartphones per day, classify the type of phone used, and quantify skin changes noticed by each respondent. Demographic information for each respondent also was gathered using the survey. The survey was pilot tested to ensure that respondents were able to understand the items.
One item asked if respondents owned a handheld smartphone. Two items assessed how much time was spent on smartphones per day (ie, <1 hour, 1–2 hours, 2–3 hours, 3–4 hours, 4–5 hours, >5 hours) and the type of smartphone used (ie, Apple iPhone, Samsung Galaxy, Google Pixel, Huawei, LG, other). Six items assessed skin changes to the digits, namely the fifth digit (eg, Do you notice any changes to your fifth digit [pinky finger] that would likely be contributed to how you hold your smartphone, such as divot, callus, bruise, wound, misalignment, bend?). Eleven items were used to collect basic demographic information, including age, sex, legal marital status, ethnicity, race, annual household income, highest-earned educational degree, current employment status, health insurance status, and state of residence.
Statistical Analysis
All data were analyzed using IBM SPSS Statistics 23. The association between changes to the fifth digit and time spent on the phone, hand dominance, and socioeconomic factors (ie, age,
Results
The mean age of the 374 respondents was 33.8 years (range, 18–72 years). One hundred nine respondents were men (29.1%), 262 were women (70.1%), and 3 did not specify (0.8%). Two hundred thirty-four respondents (62.6%) were single, 271 (72.5%) were white, 171 (45.7%) had a bachelor’s degree, and174 (46.5%) were employed full time. Annual household income was normally distributed among the respondents, with 28 (7.5%) earning less than $10,000 per year, 130 (34.8%) earning $10,000 to$49,999 per year, 136 (36.4%) earning $50,000 to $99,999 per year, 52 (13.9%) earning $100,000 to$149,999 per year, and 28 (7.5%) earning more than $150,000 per year. The demographic characteristics of the respondents are presented in Table 1.

Eighty-five (22.7%) respondents admitted to changes to the fifth digit that they associated with holding a smartphone, whereas 289 (77.3%) reported no changes. When asked about the average amount of time spent on their smartphone per day, 17 (4.5%) respondents answered less than 1 hour, 70 (18.7%) answered 1 to 2 hours, 69 (18.4%) answered 2 to 3 hours, 77 (20.6%) answered 3 to 4 hours, 57 (15.2%) answered 4 to 5 hours, and 84 (22.5%) answered more than 5 hours. One hundred ninety-nine (53.2%) respondents indicated they used an Apple iPhone, 95 (25.4%) used a Samsung Galaxy phone, 9 (2.4%) used a Google Pixel phone, 3 (0.8%) used a Huawei phone, 23 (6.1%) used an LG phone, and 45 (12.0%) used another type of smartphone. The characteristics of smartphone use as reported by the respondents are presented in Table 2.

Comment
Consistent with our hypothesis, changes to the fifth digit were prevalent in the surveyed population, with 85 (22.7%) respondents admitting to changes to their fifth digit from holding a smartphone. The changes to the fifth digit were described as 1 or more of the following: divot (impression), callus (skin thickening), bruise, wound, misalignment, or bending. Most respondents who noted skin changes on the survey endorsed changes consistent with calluses and/or divots. These changes can be described as scaly, lichenified, well-demarcated papules or plaques with variable overlying hyperpigmentation and surrounding erythema. In cases with resulting chronic indentations of the skin, one also would observe localized sclerosis, atrophy, and/or induration of the area, which we found to be less prevalent than expected considering the popularity and notable reliance on smartphones.2
The most commonly reported chronic skin changes to the fifth digit are similar to those of lichen simplex chronicus and/or exogenous lobular panniculitis, which can be both symptomatically and cosmetically troubling for a patient. Functional impairment in movement of the fifth digit may result from the overlying lichenification and induration, as well as from lipoatrophy of the underlying traumatized subcutaneous fat, especially if the affected area is overlying the proximal interphalangeal joint of the fifth digit. These resulting alterations in the skin of the fifth digit also may be cosmetically displeasing to the patient.
On histology, we would expect similar changes to that of lichen simplex chronicus—compact hyperkeratosis and hypergranulosis—and/or an exogenous lobular panniculitis. Lobular panniculitis demonstrates necrosis of the fat lobule; vacuolated spaces; and lipomembranous changes such as fatty cystic degeneration with feathery eosinophilic material in an arabesque pattern, which has been described as frost on a windowpane, or a ferning pattern at the edge of the lipid vacuole.10
We also were correct in our hypothesis that prevalence of changes to the fifth digit correlate with amount of time spent on smartphones per day. Bivariate and multivariate logistic regression analysis showed that a change to the fifth digit was not significantly associated with hand dominance or socioeconomic factors (ie, age, sex, legal marital status, ethnicity, race, annual household income, highest-earned educational degree, current employment status, health insurance status, and state of residence). Controlling for all other factors, the only factor that significantly increased the odds of experiencing a change to the fifth digit was the amount of time spent on the phone per day. The respondents who spent more than 5 hours per day on their phones had 5-times greater odds of experiencing a change to their fifth digit compared with respondents who spent less than 1 hour per day on their phones (P=.045).
Although no other correlations with changes to the fifth digit, such as type of smartphone used, were found in our study, future studies should continue to investigate other potential factors that play a role in smartphone use changing the appearance and function of the digits. Our lack of significant correlations with changes to the fifth digit could be attributed to a small sample size and other possible factors, such as the frequent design changes of smartphones by manufacturers. Our study also is limited by the possibility of other factors contributing to these observed skin changes. Although we have anecdotally observed these skin changes and have hypothesized that smartphones are the culprit, other causes, such as holding certain tools, could lead to these skin changes. In addition, there are many different ways to hold a smartphone, and certain hand positionings may be more or less prone to skin changes described in our study. Various accessories, such as cases and gripping devices, also may change the way smartphones are held and would skew the results of our survey. Future studies could examine different ways smartphones are held, how various accessories affect these skin changes, and the size or model of phones that make these skin changes more or less prevalent.
Conclusion
Our study is an initial step in uncovering a possible phenomenon of smartphone use affecting the digits, namely the fifth digit. Our findings demonstrate that the amount of time spent on the phone per day significantly increases the odds of experiencing a change to the fifth digit. We expect these potential skin changes as well as other musculoskeletal changes to increase in prevalence as daily smartphone use continues to increase. With the lack of studies investigating skin changes to the digits in relation to smartphone use, future studies are needed to verify our results and confirm the presence of this issue.
- Ko PH, Hwang YH, Liang HW. Influence of smartphone use styles on typing performance and biomechanical exposure. Ergonomics. 2015;59:821-828.
- Chang J, Choi B, Tjolleng A, et al. Effects of button position on a soft keyboard: muscle activity, touch time, and discomfort in two-thumb text entry. Appl Ergon. 2017;60:282-292.
- Park JH, Christman MP, Linos E, et al. Dermatology on Instagram: an analysis of hashtags. J Drugs Dermatol. 2018;17:482-484.
- Algar L, Valdes K. Using smartphone applications as hand therapy interventions. J Hand Ther. 2014;27:254-257.
- Megna, M, Gisonni P, Napolitano M, et al. The effect of smartphone addiction on hand joints in psoriatic patients: an ultrasound-based study. J Eur Acad Dermatol Venereol. 2017;32:73-78.
- Christensen MA, Bettencourt L, Kaye L, et al. Direct measurements of smartphone screen-time: relationships with demographics and sleep. PLoS One. 2016;11:E0165331.
- Lemola S, Perkinson-Gloor N, Brand S, et al. Adolescents’ electronic media use at night, sleep disturbance, and depressive symptoms in the smartphone age. J Youth Adolesc. 2014;44:405-418.
- Lee M, Hong Y, Lee S, et al. The effects of smartphone use on upper extremity muscle activity and pain threshold. J Phys Ther Sci. 2015;27:1743-1745.
- Inal EE, Demirci K, Çetintürk A, et al. Effects of smartphone overuse on hand function, pinch strength, and the median nerve. Muscle Nerve. 2015;52:183-188.
- Elston D, Ferringer T, Ko C, et al. Dermatopathology. 3rd ed. New York, NY: Elsevier Health Sciences; 2018.
Over the last decade, the use of mobile phones has changed drastically with the advent of more technologically advanced smartphones.1 Mobile phones are no longer used primarily as devices for talking but rather for text messaging, reading the news, drafting emails, browsing websites, and connecting with others on social media. Considering the increased utility and popularity of social media along with the greater reliance on smartphones, individuals in the United States and worldwide are undoubtedly spending more time on their handheld devices.2 With the increase in use and overuse of smartphones, many aspects of society and health are likely affected. Many celebrities who frequently post on social media platforms also have alluded to or directly discussed changes in their dermatologic health secondary to their increased use of smartphones.3 Numerous studies have investigated the positive and negative effects of smartphone use on various musculoskeletal conditions of the upper extremities4,5 and the social effects of smartphone use on behavior and child development.6,7 Lee et al8 studied the effects of smartphone use on upper extremity muscle pain and activity in relation to 1- or 2-handed operation. In this study, Lee et al8 measured the muscle activity and tenderness in 10 women aged 20 to 22 years after a series of timed periods of smartphone use. They concluded that smartphone use resulted in greater muscle activity and tenderness, especially in 1-handed use compared to 2-handed use.8 Inal et al9 investigated smartphone overuse effects on hand strength and function in 102 college students and discovered that smartphone overuse was correlated with decreased pinch strength, increased median nerve cross-sectional area, and pain in the first digits.9
However, few articles have been published investigating skin changes to the digits in relation to smartphone use (Figure 1). In a PubMed search of articles indexed for MEDLINE using the terms smartphone, phone, cell phone, electronic device, handheld device, fifth digit, or skin changes, the authors were unable to find any studies in the literature that involved smartphone use and skin changes to the digits. Based on informal clinical observation and personal experiences, we hypothesized that changes to the fifth digit, likely due to holding a smartphone, would be prevalent and would correlate with amount of time spent on smartphones per day (Figure 2). We also were interested in investigating any other potential correlations with changes to the fifth digit, such as type of smartphone used.


Methods
The study used a cross-sectional design. From September 2018 to December 2018, 374 individuals 18 years or older were recruited to complete a 5-minute anonymous survey online. Using email referrals and social media, participants were presented with a link to a Google survey that only allowed 1 submission per account. On the first page of the survey, participants were presented with a letter explaining that completion of the survey was entirely voluntary, participants were free to withdraw from the study at any time, and participants were providing consent in completing the survey. The protocol was determined to be exempt by the institutional review board at Nova Southeastern University (Fort Lauderdale, Florida) in September 2018.
Survey Design
A 20-item survey was designed to measure the amount of time spent using smartphones per day, classify the type of phone used, and quantify skin changes noticed by each respondent. Demographic information for each respondent also was gathered using the survey. The survey was pilot tested to ensure that respondents were able to understand the items.
One item asked if respondents owned a handheld smartphone. Two items assessed how much time was spent on smartphones per day (ie, <1 hour, 1–2 hours, 2–3 hours, 3–4 hours, 4–5 hours, >5 hours) and the type of smartphone used (ie, Apple iPhone, Samsung Galaxy, Google Pixel, Huawei, LG, other). Six items assessed skin changes to the digits, namely the fifth digit (eg, Do you notice any changes to your fifth digit [pinky finger] that would likely be contributed to how you hold your smartphone, such as divot, callus, bruise, wound, misalignment, bend?). Eleven items were used to collect basic demographic information, including age, sex, legal marital status, ethnicity, race, annual household income, highest-earned educational degree, current employment status, health insurance status, and state of residence.
Statistical Analysis
All data were analyzed using IBM SPSS Statistics 23. The association between changes to the fifth digit and time spent on the phone, hand dominance, and socioeconomic factors (ie, age,
Results
The mean age of the 374 respondents was 33.8 years (range, 18–72 years). One hundred nine respondents were men (29.1%), 262 were women (70.1%), and 3 did not specify (0.8%). Two hundred thirty-four respondents (62.6%) were single, 271 (72.5%) were white, 171 (45.7%) had a bachelor’s degree, and174 (46.5%) were employed full time. Annual household income was normally distributed among the respondents, with 28 (7.5%) earning less than $10,000 per year, 130 (34.8%) earning $10,000 to$49,999 per year, 136 (36.4%) earning $50,000 to $99,999 per year, 52 (13.9%) earning $100,000 to$149,999 per year, and 28 (7.5%) earning more than $150,000 per year. The demographic characteristics of the respondents are presented in Table 1.

Eighty-five (22.7%) respondents admitted to changes to the fifth digit that they associated with holding a smartphone, whereas 289 (77.3%) reported no changes. When asked about the average amount of time spent on their smartphone per day, 17 (4.5%) respondents answered less than 1 hour, 70 (18.7%) answered 1 to 2 hours, 69 (18.4%) answered 2 to 3 hours, 77 (20.6%) answered 3 to 4 hours, 57 (15.2%) answered 4 to 5 hours, and 84 (22.5%) answered more than 5 hours. One hundred ninety-nine (53.2%) respondents indicated they used an Apple iPhone, 95 (25.4%) used a Samsung Galaxy phone, 9 (2.4%) used a Google Pixel phone, 3 (0.8%) used a Huawei phone, 23 (6.1%) used an LG phone, and 45 (12.0%) used another type of smartphone. The characteristics of smartphone use as reported by the respondents are presented in Table 2.

Comment
Consistent with our hypothesis, changes to the fifth digit were prevalent in the surveyed population, with 85 (22.7%) respondents admitting to changes to their fifth digit from holding a smartphone. The changes to the fifth digit were described as 1 or more of the following: divot (impression), callus (skin thickening), bruise, wound, misalignment, or bending. Most respondents who noted skin changes on the survey endorsed changes consistent with calluses and/or divots. These changes can be described as scaly, lichenified, well-demarcated papules or plaques with variable overlying hyperpigmentation and surrounding erythema. In cases with resulting chronic indentations of the skin, one also would observe localized sclerosis, atrophy, and/or induration of the area, which we found to be less prevalent than expected considering the popularity and notable reliance on smartphones.2
The most commonly reported chronic skin changes to the fifth digit are similar to those of lichen simplex chronicus and/or exogenous lobular panniculitis, which can be both symptomatically and cosmetically troubling for a patient. Functional impairment in movement of the fifth digit may result from the overlying lichenification and induration, as well as from lipoatrophy of the underlying traumatized subcutaneous fat, especially if the affected area is overlying the proximal interphalangeal joint of the fifth digit. These resulting alterations in the skin of the fifth digit also may be cosmetically displeasing to the patient.
On histology, we would expect similar changes to that of lichen simplex chronicus—compact hyperkeratosis and hypergranulosis—and/or an exogenous lobular panniculitis. Lobular panniculitis demonstrates necrosis of the fat lobule; vacuolated spaces; and lipomembranous changes such as fatty cystic degeneration with feathery eosinophilic material in an arabesque pattern, which has been described as frost on a windowpane, or a ferning pattern at the edge of the lipid vacuole.10
We also were correct in our hypothesis that prevalence of changes to the fifth digit correlate with amount of time spent on smartphones per day. Bivariate and multivariate logistic regression analysis showed that a change to the fifth digit was not significantly associated with hand dominance or socioeconomic factors (ie, age, sex, legal marital status, ethnicity, race, annual household income, highest-earned educational degree, current employment status, health insurance status, and state of residence). Controlling for all other factors, the only factor that significantly increased the odds of experiencing a change to the fifth digit was the amount of time spent on the phone per day. The respondents who spent more than 5 hours per day on their phones had 5-times greater odds of experiencing a change to their fifth digit compared with respondents who spent less than 1 hour per day on their phones (P=.045).
Although no other correlations with changes to the fifth digit, such as type of smartphone used, were found in our study, future studies should continue to investigate other potential factors that play a role in smartphone use changing the appearance and function of the digits. Our lack of significant correlations with changes to the fifth digit could be attributed to a small sample size and other possible factors, such as the frequent design changes of smartphones by manufacturers. Our study also is limited by the possibility of other factors contributing to these observed skin changes. Although we have anecdotally observed these skin changes and have hypothesized that smartphones are the culprit, other causes, such as holding certain tools, could lead to these skin changes. In addition, there are many different ways to hold a smartphone, and certain hand positionings may be more or less prone to skin changes described in our study. Various accessories, such as cases and gripping devices, also may change the way smartphones are held and would skew the results of our survey. Future studies could examine different ways smartphones are held, how various accessories affect these skin changes, and the size or model of phones that make these skin changes more or less prevalent.
Conclusion
Our study is an initial step in uncovering a possible phenomenon of smartphone use affecting the digits, namely the fifth digit. Our findings demonstrate that the amount of time spent on the phone per day significantly increases the odds of experiencing a change to the fifth digit. We expect these potential skin changes as well as other musculoskeletal changes to increase in prevalence as daily smartphone use continues to increase. With the lack of studies investigating skin changes to the digits in relation to smartphone use, future studies are needed to verify our results and confirm the presence of this issue.
Over the last decade, the use of mobile phones has changed drastically with the advent of more technologically advanced smartphones.1 Mobile phones are no longer used primarily as devices for talking but rather for text messaging, reading the news, drafting emails, browsing websites, and connecting with others on social media. Considering the increased utility and popularity of social media along with the greater reliance on smartphones, individuals in the United States and worldwide are undoubtedly spending more time on their handheld devices.2 With the increase in use and overuse of smartphones, many aspects of society and health are likely affected. Many celebrities who frequently post on social media platforms also have alluded to or directly discussed changes in their dermatologic health secondary to their increased use of smartphones.3 Numerous studies have investigated the positive and negative effects of smartphone use on various musculoskeletal conditions of the upper extremities4,5 and the social effects of smartphone use on behavior and child development.6,7 Lee et al8 studied the effects of smartphone use on upper extremity muscle pain and activity in relation to 1- or 2-handed operation. In this study, Lee et al8 measured the muscle activity and tenderness in 10 women aged 20 to 22 years after a series of timed periods of smartphone use. They concluded that smartphone use resulted in greater muscle activity and tenderness, especially in 1-handed use compared to 2-handed use.8 Inal et al9 investigated smartphone overuse effects on hand strength and function in 102 college students and discovered that smartphone overuse was correlated with decreased pinch strength, increased median nerve cross-sectional area, and pain in the first digits.9
However, few articles have been published investigating skin changes to the digits in relation to smartphone use (Figure 1). In a PubMed search of articles indexed for MEDLINE using the terms smartphone, phone, cell phone, electronic device, handheld device, fifth digit, or skin changes, the authors were unable to find any studies in the literature that involved smartphone use and skin changes to the digits. Based on informal clinical observation and personal experiences, we hypothesized that changes to the fifth digit, likely due to holding a smartphone, would be prevalent and would correlate with amount of time spent on smartphones per day (Figure 2). We also were interested in investigating any other potential correlations with changes to the fifth digit, such as type of smartphone used.


Methods
The study used a cross-sectional design. From September 2018 to December 2018, 374 individuals 18 years or older were recruited to complete a 5-minute anonymous survey online. Using email referrals and social media, participants were presented with a link to a Google survey that only allowed 1 submission per account. On the first page of the survey, participants were presented with a letter explaining that completion of the survey was entirely voluntary, participants were free to withdraw from the study at any time, and participants were providing consent in completing the survey. The protocol was determined to be exempt by the institutional review board at Nova Southeastern University (Fort Lauderdale, Florida) in September 2018.
Survey Design
A 20-item survey was designed to measure the amount of time spent using smartphones per day, classify the type of phone used, and quantify skin changes noticed by each respondent. Demographic information for each respondent also was gathered using the survey. The survey was pilot tested to ensure that respondents were able to understand the items.
One item asked if respondents owned a handheld smartphone. Two items assessed how much time was spent on smartphones per day (ie, <1 hour, 1–2 hours, 2–3 hours, 3–4 hours, 4–5 hours, >5 hours) and the type of smartphone used (ie, Apple iPhone, Samsung Galaxy, Google Pixel, Huawei, LG, other). Six items assessed skin changes to the digits, namely the fifth digit (eg, Do you notice any changes to your fifth digit [pinky finger] that would likely be contributed to how you hold your smartphone, such as divot, callus, bruise, wound, misalignment, bend?). Eleven items were used to collect basic demographic information, including age, sex, legal marital status, ethnicity, race, annual household income, highest-earned educational degree, current employment status, health insurance status, and state of residence.
Statistical Analysis
All data were analyzed using IBM SPSS Statistics 23. The association between changes to the fifth digit and time spent on the phone, hand dominance, and socioeconomic factors (ie, age,
Results
The mean age of the 374 respondents was 33.8 years (range, 18–72 years). One hundred nine respondents were men (29.1%), 262 were women (70.1%), and 3 did not specify (0.8%). Two hundred thirty-four respondents (62.6%) were single, 271 (72.5%) were white, 171 (45.7%) had a bachelor’s degree, and174 (46.5%) were employed full time. Annual household income was normally distributed among the respondents, with 28 (7.5%) earning less than $10,000 per year, 130 (34.8%) earning $10,000 to$49,999 per year, 136 (36.4%) earning $50,000 to $99,999 per year, 52 (13.9%) earning $100,000 to$149,999 per year, and 28 (7.5%) earning more than $150,000 per year. The demographic characteristics of the respondents are presented in Table 1.

Eighty-five (22.7%) respondents admitted to changes to the fifth digit that they associated with holding a smartphone, whereas 289 (77.3%) reported no changes. When asked about the average amount of time spent on their smartphone per day, 17 (4.5%) respondents answered less than 1 hour, 70 (18.7%) answered 1 to 2 hours, 69 (18.4%) answered 2 to 3 hours, 77 (20.6%) answered 3 to 4 hours, 57 (15.2%) answered 4 to 5 hours, and 84 (22.5%) answered more than 5 hours. One hundred ninety-nine (53.2%) respondents indicated they used an Apple iPhone, 95 (25.4%) used a Samsung Galaxy phone, 9 (2.4%) used a Google Pixel phone, 3 (0.8%) used a Huawei phone, 23 (6.1%) used an LG phone, and 45 (12.0%) used another type of smartphone. The characteristics of smartphone use as reported by the respondents are presented in Table 2.

Comment
Consistent with our hypothesis, changes to the fifth digit were prevalent in the surveyed population, with 85 (22.7%) respondents admitting to changes to their fifth digit from holding a smartphone. The changes to the fifth digit were described as 1 or more of the following: divot (impression), callus (skin thickening), bruise, wound, misalignment, or bending. Most respondents who noted skin changes on the survey endorsed changes consistent with calluses and/or divots. These changes can be described as scaly, lichenified, well-demarcated papules or plaques with variable overlying hyperpigmentation and surrounding erythema. In cases with resulting chronic indentations of the skin, one also would observe localized sclerosis, atrophy, and/or induration of the area, which we found to be less prevalent than expected considering the popularity and notable reliance on smartphones.2
The most commonly reported chronic skin changes to the fifth digit are similar to those of lichen simplex chronicus and/or exogenous lobular panniculitis, which can be both symptomatically and cosmetically troubling for a patient. Functional impairment in movement of the fifth digit may result from the overlying lichenification and induration, as well as from lipoatrophy of the underlying traumatized subcutaneous fat, especially if the affected area is overlying the proximal interphalangeal joint of the fifth digit. These resulting alterations in the skin of the fifth digit also may be cosmetically displeasing to the patient.
On histology, we would expect similar changes to that of lichen simplex chronicus—compact hyperkeratosis and hypergranulosis—and/or an exogenous lobular panniculitis. Lobular panniculitis demonstrates necrosis of the fat lobule; vacuolated spaces; and lipomembranous changes such as fatty cystic degeneration with feathery eosinophilic material in an arabesque pattern, which has been described as frost on a windowpane, or a ferning pattern at the edge of the lipid vacuole.10
We also were correct in our hypothesis that prevalence of changes to the fifth digit correlate with amount of time spent on smartphones per day. Bivariate and multivariate logistic regression analysis showed that a change to the fifth digit was not significantly associated with hand dominance or socioeconomic factors (ie, age, sex, legal marital status, ethnicity, race, annual household income, highest-earned educational degree, current employment status, health insurance status, and state of residence). Controlling for all other factors, the only factor that significantly increased the odds of experiencing a change to the fifth digit was the amount of time spent on the phone per day. The respondents who spent more than 5 hours per day on their phones had 5-times greater odds of experiencing a change to their fifth digit compared with respondents who spent less than 1 hour per day on their phones (P=.045).
Although no other correlations with changes to the fifth digit, such as type of smartphone used, were found in our study, future studies should continue to investigate other potential factors that play a role in smartphone use changing the appearance and function of the digits. Our lack of significant correlations with changes to the fifth digit could be attributed to a small sample size and other possible factors, such as the frequent design changes of smartphones by manufacturers. Our study also is limited by the possibility of other factors contributing to these observed skin changes. Although we have anecdotally observed these skin changes and have hypothesized that smartphones are the culprit, other causes, such as holding certain tools, could lead to these skin changes. In addition, there are many different ways to hold a smartphone, and certain hand positionings may be more or less prone to skin changes described in our study. Various accessories, such as cases and gripping devices, also may change the way smartphones are held and would skew the results of our survey. Future studies could examine different ways smartphones are held, how various accessories affect these skin changes, and the size or model of phones that make these skin changes more or less prevalent.
Conclusion
Our study is an initial step in uncovering a possible phenomenon of smartphone use affecting the digits, namely the fifth digit. Our findings demonstrate that the amount of time spent on the phone per day significantly increases the odds of experiencing a change to the fifth digit. We expect these potential skin changes as well as other musculoskeletal changes to increase in prevalence as daily smartphone use continues to increase. With the lack of studies investigating skin changes to the digits in relation to smartphone use, future studies are needed to verify our results and confirm the presence of this issue.
- Ko PH, Hwang YH, Liang HW. Influence of smartphone use styles on typing performance and biomechanical exposure. Ergonomics. 2015;59:821-828.
- Chang J, Choi B, Tjolleng A, et al. Effects of button position on a soft keyboard: muscle activity, touch time, and discomfort in two-thumb text entry. Appl Ergon. 2017;60:282-292.
- Park JH, Christman MP, Linos E, et al. Dermatology on Instagram: an analysis of hashtags. J Drugs Dermatol. 2018;17:482-484.
- Algar L, Valdes K. Using smartphone applications as hand therapy interventions. J Hand Ther. 2014;27:254-257.
- Megna, M, Gisonni P, Napolitano M, et al. The effect of smartphone addiction on hand joints in psoriatic patients: an ultrasound-based study. J Eur Acad Dermatol Venereol. 2017;32:73-78.
- Christensen MA, Bettencourt L, Kaye L, et al. Direct measurements of smartphone screen-time: relationships with demographics and sleep. PLoS One. 2016;11:E0165331.
- Lemola S, Perkinson-Gloor N, Brand S, et al. Adolescents’ electronic media use at night, sleep disturbance, and depressive symptoms in the smartphone age. J Youth Adolesc. 2014;44:405-418.
- Lee M, Hong Y, Lee S, et al. The effects of smartphone use on upper extremity muscle activity and pain threshold. J Phys Ther Sci. 2015;27:1743-1745.
- Inal EE, Demirci K, Çetintürk A, et al. Effects of smartphone overuse on hand function, pinch strength, and the median nerve. Muscle Nerve. 2015;52:183-188.
- Elston D, Ferringer T, Ko C, et al. Dermatopathology. 3rd ed. New York, NY: Elsevier Health Sciences; 2018.
- Ko PH, Hwang YH, Liang HW. Influence of smartphone use styles on typing performance and biomechanical exposure. Ergonomics. 2015;59:821-828.
- Chang J, Choi B, Tjolleng A, et al. Effects of button position on a soft keyboard: muscle activity, touch time, and discomfort in two-thumb text entry. Appl Ergon. 2017;60:282-292.
- Park JH, Christman MP, Linos E, et al. Dermatology on Instagram: an analysis of hashtags. J Drugs Dermatol. 2018;17:482-484.
- Algar L, Valdes K. Using smartphone applications as hand therapy interventions. J Hand Ther. 2014;27:254-257.
- Megna, M, Gisonni P, Napolitano M, et al. The effect of smartphone addiction on hand joints in psoriatic patients: an ultrasound-based study. J Eur Acad Dermatol Venereol. 2017;32:73-78.
- Christensen MA, Bettencourt L, Kaye L, et al. Direct measurements of smartphone screen-time: relationships with demographics and sleep. PLoS One. 2016;11:E0165331.
- Lemola S, Perkinson-Gloor N, Brand S, et al. Adolescents’ electronic media use at night, sleep disturbance, and depressive symptoms in the smartphone age. J Youth Adolesc. 2014;44:405-418.
- Lee M, Hong Y, Lee S, et al. The effects of smartphone use on upper extremity muscle activity and pain threshold. J Phys Ther Sci. 2015;27:1743-1745.
- Inal EE, Demirci K, Çetintürk A, et al. Effects of smartphone overuse on hand function, pinch strength, and the median nerve. Muscle Nerve. 2015;52:183-188.
- Elston D, Ferringer T, Ko C, et al. Dermatopathology. 3rd ed. New York, NY: Elsevier Health Sciences; 2018.
Practice Points
- The amount of time spent on a smartphone was found to directly correlate with skin changes to the fifth digit.
- Skin changes to the fifth digit were mostly reported to be divots (impressions) or calluses.
Hair Care Products Used by Women of African Descent: Review of Ingredients
In the African American and African communities, information regarding the care and treatment of hair and skin often is obtained from relatives as well as Internet videos and bloggers.1 Moreover, fewer than half of African American women surveyed believe that their physician understands African American hair.2 In addition to proficiency in the diagnosis and treatment of hair and scalp disorders in this population, dermatologists must be aware of common hair and scalp beliefs, misconceptions, care, and product use to ensure culturally competent interactions and treatment.
When a patient of African descent refers to their hair as “natural,” he/she is referring to its texture compared with hair that is chemically treated with straighteners (ie, “relaxed” or “permed” hair). Natural hair refers to hair that has not been altered with chemical treatments that permanently break and re-form disulfide bonds of the hair.1 In 2003, it was estimated that 80% of African American women treated their hair with a chemical relaxer.3 However, this preference has changed over the last decade, with a larger percentage of African American women choosing to wear a natural hairstyle.4
Regardless of preferred hairstyle, a multitude of products can be used to obtain and maintain the particular style. According to US Food and Drug Administration regulations, a product’s ingredients must appear on an information panel in descending order of predominance. Additionally, products must be accurately labeled without misleading information. However, one study found that hair care products commonly used by African American women contain mixtures of endocrine-disrupting chemicals, and 84% of detected chemicals are not listed on the label.5
Properties of Hair Care Products
Women of African descent use hair grooming products for cleansing and moisturizing the hair and scalp, detangling, and styling. Products to achieve these goals comprise shampoos, leave-in and rinse-out conditioners, creams, pomades, oils, and gels. In August 2018 we performed a Google search of the most popular hair care products used for natural hair and chemically relaxed African American hair. Key terms used in our search included popular natural hair products, best natural hair products, top natural hair products, products for permed hair, shampoos for permed hair, conditioner for permed hair, popular detanglers for African American hair, popular products for natural hair, detanglers used for permed hair, gels for relaxed hair, moisturizers for relaxed hair, gels for natural hair, and popular moisturizers for African American hair. We reviewed all websites generated by the search and compared the most popular brands, compiled a list of products, and reviewed them for availability in 2 beauty supply stores in Philadelphia, Pennsylvania; 1 Walmart in Hershey, Pennsylvania; and 1 Walmart in Willow Grove, Pennsylvania. Of the 80 products identified, we selected 57 products to be reviewed for ingredients based on which ones were most commonly seen in search results. Table 1 highlights several randomly chosen popular hair care products used by African American women to familiarize dermatologists with specific products and manufacturers.
Tightly coiled hair, common among women of African descent, is considered fragile because of decreased water content and tensile strength.6 Fragility is exacerbated by manipulation during styling, excessive heat, and harsh shampoos that strip the hair of moisture, as well as chemical treatments that lead to protein deficiency.4,6,7 Because tightly coiled hair is naturally dry and fragile, women of African descent have a particular preference for products that reduce hair dryness and breakage, which has led to the popularity of sulfate-free shampoos that minimize loss of moisture in hair; moisturizers, oils, and conditioners also are used to enhance moisture retention in hair. Conditioners also provide protein substances that can help strengthen hair.4
Consumers’ concerns about the inclusion of potentially harmful ingredients have resulted in reformulation of many products. Our review of products demonstrated that natural hair consumers used fewer products containing silicones, parabens, and sulfates, compared to consumers with chemically relaxed hair. Another tool used by manufacturers to address these concerns is the inclusion of an additional label to distinguish the product as sulfate free, silicone free, paraben free, petroleum free, or a combination of these terms. Although many patients believe that there are “good” and “bad” products, they should be made aware that there are pros and cons of ingredients frequently found in hair-grooming products. Popular ingredients in hair care products include sulfates, cationic surfactants and cationic polymers, silicone, oils, and parabens.
Sulfates
Sulfates are anion detergents in shampoo that remove sebum from the scalp and hair. The number of sulfates in a shampoo positively correlates to cleansing strength.1 However, sulfates can cause excessive sebum removal and lead to hair that is hard, rough, dull, and prone to tangle and breakage.6 Sulfates also dissolve oil on the hair, causing additional dryness and breakage.7
There are a variety of sulfate compounds with different sebum-removal capabilities. Lauryl sulfates are commonly used in shampoos for oily hair. Tightly coiled hair that has been overly cleansed with these ingredients can become exceedingly dry and unmanageable, which explains why products with lauryl sulfates are avoided. Table 1 includes only 1 product containing lauryl sulfate (Pantene Pro-V Gold Series Shampoo). Patients using a lauryl sulfate–containing shampoo can select a product that also contains a conditioning agent in the formulation.6 Alternatively, sulfate-free shampoos that contain surfactants with less detergency can be used.8 There are no published studies of the cleansing ability of sulfate-free shampoos or their effects on hair shaft fragility.9
At the opposite end of the spectrum is sodium laureth sulfate, commonly used as a primary detergent in shampoos designed for normal to dry hair.10 Sodium laureth sulfate, which provides excellent cleansing and leaves the hair better moisturized and manageable compared to lauryl sulfates,10 is a common ingredient in the products in Table 1 (ApHogee Deep Moisture Shampoo, Pantene Pro-V Gold Series Shampoo, and Pantene Pro-V Truly Relaxed Moisturizing Shampoo).
An ingredient that might be confused for a sulfate is behentrimonium methosulfate, a cationic quaternary ammonium salt that is not used to cleanse the hair, unlike sodium lauryl sulfate and sodium laureth sulfate, but serves as an antistatic conditioning agent to keep hair moisturized and frizz free.11 Behentrimonium methosulfate is found in conditioners and detanglers in Table 1 (The Mane Choice Green Tea & Carrot Conditioning Mask, Kinky-Curly Knot Today, Miss Jessie’s Leave-In Condish, SheaMoisture Raw Shea Butter Extra-Moisture Detangler, Mielle Pomegranate & Honey Leave-In Conditioner). Patients should be informed that behentrimonium methosulfate is not water soluble, which suggests that it can lead to buildup of residue.
Cationic Surfactants and Cationic Polymers
Cationic surfactants and cationic polymers are found in many hair products and improve manageability by softening and detangling hair.6,10 Hair consists of negatively charged keratin proteins7 that electrostatically attract the positively charged polar group of cationic surfactants and cationic polymers. These surfactants and polymers then adhere to and normalize hair surface charges, resulting in improved texture and reduced friction between strands.6 For African American patients with natural hair, cationic surfactants and polymers help to maintain curl patterns and assist in detangling.6 Polyquaternium is a cationic polymer that is found in several products in Table 1 (Carol’s Daughter Black Vanilla Moisture & Shine Sulfate-Free Shampoo, OGX Nourishing Coconut Milk Shampoo, ApHogee Deep Moisture Shampoo, Pantene Pro-V Gold Series Shampoo, Neutrogena Triple Moisture Silk Touch Leave-In Conditioner, Creme of Nature Argan Oil Strength & Shine Leave-in Conditioner, and John Frieda Frizz Ease Daily Nourishment Leave-In Conditioner).
The surfactants triethanolamine and tetrasodium ethylenediaminetetraacetic acid (EDTA) are ingredients in some styling gels and have been reported as potential carcinogens.12 However, there are inadequate human or animal data to support the carcinogenicity of either ingredient at this time. Of note, tetrasodium EDTA has been reported to increase the penetration of other chemicals through the skin, which might lead to toxicity.12
Silicone
Silicone agents can be found in a variety of hair care products, including shampoos, detanglers, hair conditioners, leave-in conditioners, and moisturizers. Of the 22 products listed in Table 1, silicones are found in 14 products. Common silicones include dimethicone, amodimethicone, cyclopentasiloxane, and dimethiconol. Silicones form hydrophobic films that create smoothness and shine.6,8 Silicone-containing products help reduce frizz and provide protection against breakage and heat damage in chemically relaxed hair.6,7 For patients with natural hair, silicones aid in hair detangling.
Frequent use of silicone products can result in residue buildup due to the insolubility of silicone in water. Preventatively, some products include water-soluble silicones with the same benefits, such as silicones with the prefixes PPG- or PEG-, laurylmethicone copolyol, and dimethicone copolyol.7 Dimethicone copolyol was found in 1 of our reviewed products (OGX Nourishing Coconut Milk Shampoo); 10 products in Table 1 contain ingredients with the prefixes PPG- or PEG-. Several products in our review contain both water-soluble and water-insoluble silicones (eg, Creme of Nature Argan Oil Strength & Shine Leave-In Conditioner).
Oils
Oils in hair care products prevent hair breakage by coating the hair shaft and sealing in moisture. There are various types of oils in hair care products. Essential oils are volatile liquid-aroma substances derived most commonly from plants through dry or steam distillation or by other mechanical processes.13 Essential oils are used to seal and moisturize the hair and often are used to produce fragrance in hair products.6 Examples of essential oils that are ingredients in cosmetics include tea tree oil (TTO), peppermint oil, rosemary oil, and thyme oil. Vegetable oils can be used to dilute essential oils because essential oils can irritate skin.14
Tea tree oil is an essential oil obtained through steam distillation of the leaves of the coastal tree Melaleuca alternifolia. The molecule terpinen-4-ol is a major component of TTO thought to exhibit antiseptic and anti-inflammatory properties.15 Pazyar et al16 reviewed several studies that propose the use of TTO to treat acne vulgaris, seborrheic dermatitis, and chronic gingivitis. Although this herbal oil seemingly has many possible dermatologic applications, dermatologists should be aware that reports have linked TTO to allergic contact dermatitis due to 1,8-cineole, another constituent of TTO.17 Tea tree oil is an ingredient in several of the hair care products that we reviewed. With growing patient interest in the benefits of TTO, further research is necessary to establish guidelines on its use for seborrheic dermatitis.
Castor oil is a vegetable oil pressed from the seeds of the castor oil plant. Its primary fatty acid group—ricinoleic acid—along with certain salts and esters function primarily as skin-conditioning agents, emulsion stabilizers, and surfactants in cosmetic products.18 Jamaican black castor oil is a popular moisturizing oil in the African American natural hair community. It differs in color from standard castor oil because of the manner in which the oil is processed. Anecdotally, it is sometimes advertised as a hair growth serum; some patients admit to applying Jamaican black castor oil on the scalp as self-treatment of alopecia. The basis for such claims might stem from research showing that ricinoleic acid exhibits anti-inflammatory and analgesic properties in some mice and guinea pig models with repeated topical application.17 Scientific evidence does not, however, support claims that castor oil or Jamaican black castor oil can treat alopecia.
Mineral oils have a lubricant base and are refined from petroleum crude oils. The composition of crude oil varies; to remove impurities, it must undergo treatment with different degrees of refinement. When products are highly treated, the result is a substantially decreased level of impurities.19 Although they are beneficial in coating the hair shaft and preventing hair damage, consumers tend to avoid products containing mineral oil because of its carcinogenic potential if untreated or mildly treated.20
Although cosmetics with mineral oils are highly treated, a study showed that mineral oil is the largest contaminant in the human body, with cosmetics being a possible source.21 Studies also have revealed that mineral oils do not prevent hair breakage compared to other oils, such as essential oils and coconut oil.22,23 Many consumers therefore choose to avoid mineral oil because alternative oils exist that are beneficial in preventing hair damage but do not present carcinogenic risk. An example of a mineral oil–free product in Table 1 is Mizani Coconut Souffle Light Moisturizing Hairdress. Only 8 of the 57 products we reviewed did not contain oil, including the following 5 included in Table 1: Carol’s Daughter Black Vanilla Moisture & Shine Sulfate-Free Shampoo, Miss Jessie’s Leave-In Condish, Kinky-Curly Knot Today (although this product did have behentrimonium made from rapeseed oil), Herbal Essences Hello Hydration Moisturizing Conditioner, and ampro Pro Styl Protein Styling Gel.
Parabens
Parabens are preservatives used to prevent growth of pathogens in and prevent decomposition of cosmetic products. Parabens have attracted a lot of criticism because of their possible link to breast cancer.24 In vitro and in vivo studies of parabens have demonstrated weak estrogenic activity that increased proportionally with increased length and branching of alkyl side chains. In vivo animal studies demonstrated weak estrogenic activity—100,000-fold less potent than 17β-estradiol.25 Ongoing research examines the relationship between the estrogenic properties of parabens, endocrine disruption, and cancer in human breast epithelial cells.5,24 The Cosmetic Ingredient Review and the US Food and Drug Administration uphold that parabens are safe to use in cosmetics.26 Several products that include parabens are listed in Table 1 (ApHogee Deep Moisture Shampoo, Neutrogena Triple Moisture Silk Touch Leave-In Conditioner, John Frieda Frizz Ease Daily Nourishment Leave-In Conditioner, and ampro Pro Styl Protein Styling Gel).
Our Recommendations
Table 2 (although not exhaustive) includes the authors’ recommendations of hair care products for individuals of African descent. Dermatologists should discuss the pros and cons of the use of products with ingredients that have controversial health effects, namely parabens, triethanolamine, tetrasodium EDTA, and mineral oils. Our recommendations do not include products that contain the prior ingredients. For many women of African descent, their hair type and therefore product use changes with the season, health of their hair, and normal changes to hair throughout their lifetime. There is no magic product for all: Each patient has specific individual styling preferences and a distinctive hair type. Decisions about which products to use can be guided with the assistance of a dermatologist but will ultimately be left up to the patient.

Conclusion
Given the array of hair and scalp care products, it is helpful for dermatologists to become familiar with several of the most popular ingredients and commonly used products. It might be helpful to ask patients which products they use and which ones have been effective for their unique hair concerns. Thus, you become armed with a catalogue of product recommendations for your patients.
- Taylor S, Kelly AP, Lim HW, et al. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. New York, NY: McGraw-Hill; 2009.
- Gathers RC, Mahan MG. African American women, hair care, and health barriers. J Clin Aesthet Dermatol. 2014;7:26-29.
- Quinn CR, Quinn TM, Kelly AP. Hair care practices in African American women. Cutis. 2003;72:280-282, 285-289.
- Griffin M, Lenzy Y. Contemporary African-American hair care practices. Pract Dermatol. http://practicaldermatology.com/2015/05/contemporary-african-american-hair-care-practices/. May 2015. Accessed March 19, 2020.
- Helm JS, Nishioka M, Brody JG, et al. Measurement of endocrine disrupting and asthma-associated chemicals in hair products used by black women. Environ Res. 2018;165:448-458.
- Crawford K, Hernandez C. A review of hair care products for black individuals. Cutis. 2014;93:289-293.
- Bosley RE, Daveluy S. A primer to natural hair care practices in black patients. Cutis. 2015;95:78-80, 106.
- Cline A, Uwakwe L, McMichael A. No sulfates, no parabens, and the “no-poo” method: a new patient perspective on common shampoo ingredients. Cutis. 2018;101:22-26.
- Gavazzoni Dias MFR. Hair cosmetics: an overview. Int J Trichology. 2015;7:2-15.
- Draelos ZD. Essentials of hair care often neglected: hair cleansing.Int J Trichology. 2010;2:24-29.
- Becker L, Bergfeld W, Belsito D, et al. Safety assessment of trimoniums as used in cosmetics. Int J Toxicol. 2012;31(6 suppl):296S-341S.
- National Center for Biotechnology Information. PubChem Database. Edetate sodium, CID=6144. https://pubchem.ncbi.nlm.nih.gov/compound/EDTA_
tetrasodium#section=FDA-Requirements. Accessed March 19, 2020. - Lanigan RS, Yamarik TA. Final report on the safety assessment of EDTA, calcium disodium EDTA, diammonium EDTA, dipotassium EDTA, disodium EDTA, TEA-EDTA, tetrasodium EDTA, tripotassium EDTA, trisodium EDTA, HEDTA, and trisodium HEDTA. Int J Toxicol. 2002;21(suppl 2):95-142.
- Vasireddy L, Bingle LEH, Davies MS. Antimicrobial activity of essential oils against multidrug-resistant clinical isolates of the Burkholderia cepacia complex. PLoS One. 2018;13:e0201835.
- Mondello F, De Bernardis F, Girolamo A, et al. In vivo activity of terpinen-4-ol, the main bioactive component of Melaleuca alternifolia Cheel (tea tree) oil against azole-susceptible and -resistant human pathogenic Candida species. BMC Infect Dis. 2006;6:158.
- Pazyar N, Yaghoobi R, Bagherani N, et al. A review of applications of tea tree oil in dermatology. Int J Dermatol. 2013;52:784-790.
- Selvaag E, Eriksen B, Thune P. Contact allergy due to tea tree oil and cross-sensitization to colophony. Contact Dermatitis. 1994;31:124-125.
- Vieira C, Fetzer S, Sauer SK, et al. Pro- and anti-inflammatory actions of ricinoleic acid: similarities and differences with capsaicin. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:87-95.
- International Agency for Research on Cancer, IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Polynuclear Aromatic Hydrocarbons, Part 2, Carbon Blacks, Mineral Oils (Lubricant Base Oils and Derived Products) and Sorne Nitroarenes. Vol 33. Lyon, France: International Agency for Research on Cancer; April 1984. https://monographs.iarc.fr/wp-content/uploads/2018/06/mono33.pdf. Accessed March 19, 2020.
- Vieira C, Evangelista S, Cirillo R, et al. Effect of ricinoleic acid in acute and subchronic experimental models of inflammation. Mediators Inflamm. 2000;9:223-228.
- Concin N, Hofstetter G, Plattner B, et al. Evidence for cosmetics as a source of mineral oil contamination in women. J Womens Health (Larchmt). 2011;20:1713-1719.
- Biedermann M, Barp L, Kornauth C, et al. Mineral oil in human tissues, part II: characterization of the accumulated hydrocarbons by comprehensive two-dimensional gas chromatography. Sci Total Environ. 2015;506-507:644-655.
- Ruetsch SB, Kamath YK, Rele AS, et al. Secondary ion mass spectrometric investigation of penetration of coconut and mineral oils into human hair fibers: relevance to hair damage. J Cosmet Sci. 2001;52:169-184.
- Darbre PD, Aljarrah A, Miller WR, et al. Concentrations of parabens in human breast tumours. J Appl Toxicol. 2004;24:5-13.
- Routledge EJ, Parker J, Odum J, et al. Some alkyl hydroxy benzoate preservatives (parabens) are estrogenic. Toxicol Appl Pharmacol. 1998;153:12-19.
- Centers for Disease Control and Prevention. Parabens factsheet. https://www.cdc.gov/biomonitoring/Parabens_FactSheet.html. Updated April 7, 2017. Accessed March 19, 2020.
In the African American and African communities, information regarding the care and treatment of hair and skin often is obtained from relatives as well as Internet videos and bloggers.1 Moreover, fewer than half of African American women surveyed believe that their physician understands African American hair.2 In addition to proficiency in the diagnosis and treatment of hair and scalp disorders in this population, dermatologists must be aware of common hair and scalp beliefs, misconceptions, care, and product use to ensure culturally competent interactions and treatment.
When a patient of African descent refers to their hair as “natural,” he/she is referring to its texture compared with hair that is chemically treated with straighteners (ie, “relaxed” or “permed” hair). Natural hair refers to hair that has not been altered with chemical treatments that permanently break and re-form disulfide bonds of the hair.1 In 2003, it was estimated that 80% of African American women treated their hair with a chemical relaxer.3 However, this preference has changed over the last decade, with a larger percentage of African American women choosing to wear a natural hairstyle.4
Regardless of preferred hairstyle, a multitude of products can be used to obtain and maintain the particular style. According to US Food and Drug Administration regulations, a product’s ingredients must appear on an information panel in descending order of predominance. Additionally, products must be accurately labeled without misleading information. However, one study found that hair care products commonly used by African American women contain mixtures of endocrine-disrupting chemicals, and 84% of detected chemicals are not listed on the label.5
Properties of Hair Care Products
Women of African descent use hair grooming products for cleansing and moisturizing the hair and scalp, detangling, and styling. Products to achieve these goals comprise shampoos, leave-in and rinse-out conditioners, creams, pomades, oils, and gels. In August 2018 we performed a Google search of the most popular hair care products used for natural hair and chemically relaxed African American hair. Key terms used in our search included popular natural hair products, best natural hair products, top natural hair products, products for permed hair, shampoos for permed hair, conditioner for permed hair, popular detanglers for African American hair, popular products for natural hair, detanglers used for permed hair, gels for relaxed hair, moisturizers for relaxed hair, gels for natural hair, and popular moisturizers for African American hair. We reviewed all websites generated by the search and compared the most popular brands, compiled a list of products, and reviewed them for availability in 2 beauty supply stores in Philadelphia, Pennsylvania; 1 Walmart in Hershey, Pennsylvania; and 1 Walmart in Willow Grove, Pennsylvania. Of the 80 products identified, we selected 57 products to be reviewed for ingredients based on which ones were most commonly seen in search results. Table 1 highlights several randomly chosen popular hair care products used by African American women to familiarize dermatologists with specific products and manufacturers.

Tightly coiled hair, common among women of African descent, is considered fragile because of decreased water content and tensile strength.6 Fragility is exacerbated by manipulation during styling, excessive heat, and harsh shampoos that strip the hair of moisture, as well as chemical treatments that lead to protein deficiency.4,6,7 Because tightly coiled hair is naturally dry and fragile, women of African descent have a particular preference for products that reduce hair dryness and breakage, which has led to the popularity of sulfate-free shampoos that minimize loss of moisture in hair; moisturizers, oils, and conditioners also are used to enhance moisture retention in hair. Conditioners also provide protein substances that can help strengthen hair.4
Consumers’ concerns about the inclusion of potentially harmful ingredients have resulted in reformulation of many products. Our review of products demonstrated that natural hair consumers used fewer products containing silicones, parabens, and sulfates, compared to consumers with chemically relaxed hair. Another tool used by manufacturers to address these concerns is the inclusion of an additional label to distinguish the product as sulfate free, silicone free, paraben free, petroleum free, or a combination of these terms. Although many patients believe that there are “good” and “bad” products, they should be made aware that there are pros and cons of ingredients frequently found in hair-grooming products. Popular ingredients in hair care products include sulfates, cationic surfactants and cationic polymers, silicone, oils, and parabens.
Sulfates
Sulfates are anion detergents in shampoo that remove sebum from the scalp and hair. The number of sulfates in a shampoo positively correlates to cleansing strength.1 However, sulfates can cause excessive sebum removal and lead to hair that is hard, rough, dull, and prone to tangle and breakage.6 Sulfates also dissolve oil on the hair, causing additional dryness and breakage.7
There are a variety of sulfate compounds with different sebum-removal capabilities. Lauryl sulfates are commonly used in shampoos for oily hair. Tightly coiled hair that has been overly cleansed with these ingredients can become exceedingly dry and unmanageable, which explains why products with lauryl sulfates are avoided. Table 1 includes only 1 product containing lauryl sulfate (Pantene Pro-V Gold Series Shampoo). Patients using a lauryl sulfate–containing shampoo can select a product that also contains a conditioning agent in the formulation.6 Alternatively, sulfate-free shampoos that contain surfactants with less detergency can be used.8 There are no published studies of the cleansing ability of sulfate-free shampoos or their effects on hair shaft fragility.9
At the opposite end of the spectrum is sodium laureth sulfate, commonly used as a primary detergent in shampoos designed for normal to dry hair.10 Sodium laureth sulfate, which provides excellent cleansing and leaves the hair better moisturized and manageable compared to lauryl sulfates,10 is a common ingredient in the products in Table 1 (ApHogee Deep Moisture Shampoo, Pantene Pro-V Gold Series Shampoo, and Pantene Pro-V Truly Relaxed Moisturizing Shampoo).
An ingredient that might be confused for a sulfate is behentrimonium methosulfate, a cationic quaternary ammonium salt that is not used to cleanse the hair, unlike sodium lauryl sulfate and sodium laureth sulfate, but serves as an antistatic conditioning agent to keep hair moisturized and frizz free.11 Behentrimonium methosulfate is found in conditioners and detanglers in Table 1 (The Mane Choice Green Tea & Carrot Conditioning Mask, Kinky-Curly Knot Today, Miss Jessie’s Leave-In Condish, SheaMoisture Raw Shea Butter Extra-Moisture Detangler, Mielle Pomegranate & Honey Leave-In Conditioner). Patients should be informed that behentrimonium methosulfate is not water soluble, which suggests that it can lead to buildup of residue.
Cationic Surfactants and Cationic Polymers
Cationic surfactants and cationic polymers are found in many hair products and improve manageability by softening and detangling hair.6,10 Hair consists of negatively charged keratin proteins7 that electrostatically attract the positively charged polar group of cationic surfactants and cationic polymers. These surfactants and polymers then adhere to and normalize hair surface charges, resulting in improved texture and reduced friction between strands.6 For African American patients with natural hair, cationic surfactants and polymers help to maintain curl patterns and assist in detangling.6 Polyquaternium is a cationic polymer that is found in several products in Table 1 (Carol’s Daughter Black Vanilla Moisture & Shine Sulfate-Free Shampoo, OGX Nourishing Coconut Milk Shampoo, ApHogee Deep Moisture Shampoo, Pantene Pro-V Gold Series Shampoo, Neutrogena Triple Moisture Silk Touch Leave-In Conditioner, Creme of Nature Argan Oil Strength & Shine Leave-in Conditioner, and John Frieda Frizz Ease Daily Nourishment Leave-In Conditioner).
The surfactants triethanolamine and tetrasodium ethylenediaminetetraacetic acid (EDTA) are ingredients in some styling gels and have been reported as potential carcinogens.12 However, there are inadequate human or animal data to support the carcinogenicity of either ingredient at this time. Of note, tetrasodium EDTA has been reported to increase the penetration of other chemicals through the skin, which might lead to toxicity.12
Silicone
Silicone agents can be found in a variety of hair care products, including shampoos, detanglers, hair conditioners, leave-in conditioners, and moisturizers. Of the 22 products listed in Table 1, silicones are found in 14 products. Common silicones include dimethicone, amodimethicone, cyclopentasiloxane, and dimethiconol. Silicones form hydrophobic films that create smoothness and shine.6,8 Silicone-containing products help reduce frizz and provide protection against breakage and heat damage in chemically relaxed hair.6,7 For patients with natural hair, silicones aid in hair detangling.
Frequent use of silicone products can result in residue buildup due to the insolubility of silicone in water. Preventatively, some products include water-soluble silicones with the same benefits, such as silicones with the prefixes PPG- or PEG-, laurylmethicone copolyol, and dimethicone copolyol.7 Dimethicone copolyol was found in 1 of our reviewed products (OGX Nourishing Coconut Milk Shampoo); 10 products in Table 1 contain ingredients with the prefixes PPG- or PEG-. Several products in our review contain both water-soluble and water-insoluble silicones (eg, Creme of Nature Argan Oil Strength & Shine Leave-In Conditioner).
Oils
Oils in hair care products prevent hair breakage by coating the hair shaft and sealing in moisture. There are various types of oils in hair care products. Essential oils are volatile liquid-aroma substances derived most commonly from plants through dry or steam distillation or by other mechanical processes.13 Essential oils are used to seal and moisturize the hair and often are used to produce fragrance in hair products.6 Examples of essential oils that are ingredients in cosmetics include tea tree oil (TTO), peppermint oil, rosemary oil, and thyme oil. Vegetable oils can be used to dilute essential oils because essential oils can irritate skin.14
Tea tree oil is an essential oil obtained through steam distillation of the leaves of the coastal tree Melaleuca alternifolia. The molecule terpinen-4-ol is a major component of TTO thought to exhibit antiseptic and anti-inflammatory properties.15 Pazyar et al16 reviewed several studies that propose the use of TTO to treat acne vulgaris, seborrheic dermatitis, and chronic gingivitis. Although this herbal oil seemingly has many possible dermatologic applications, dermatologists should be aware that reports have linked TTO to allergic contact dermatitis due to 1,8-cineole, another constituent of TTO.17 Tea tree oil is an ingredient in several of the hair care products that we reviewed. With growing patient interest in the benefits of TTO, further research is necessary to establish guidelines on its use for seborrheic dermatitis.
Castor oil is a vegetable oil pressed from the seeds of the castor oil plant. Its primary fatty acid group—ricinoleic acid—along with certain salts and esters function primarily as skin-conditioning agents, emulsion stabilizers, and surfactants in cosmetic products.18 Jamaican black castor oil is a popular moisturizing oil in the African American natural hair community. It differs in color from standard castor oil because of the manner in which the oil is processed. Anecdotally, it is sometimes advertised as a hair growth serum; some patients admit to applying Jamaican black castor oil on the scalp as self-treatment of alopecia. The basis for such claims might stem from research showing that ricinoleic acid exhibits anti-inflammatory and analgesic properties in some mice and guinea pig models with repeated topical application.17 Scientific evidence does not, however, support claims that castor oil or Jamaican black castor oil can treat alopecia.
Mineral oils have a lubricant base and are refined from petroleum crude oils. The composition of crude oil varies; to remove impurities, it must undergo treatment with different degrees of refinement. When products are highly treated, the result is a substantially decreased level of impurities.19 Although they are beneficial in coating the hair shaft and preventing hair damage, consumers tend to avoid products containing mineral oil because of its carcinogenic potential if untreated or mildly treated.20
Although cosmetics with mineral oils are highly treated, a study showed that mineral oil is the largest contaminant in the human body, with cosmetics being a possible source.21 Studies also have revealed that mineral oils do not prevent hair breakage compared to other oils, such as essential oils and coconut oil.22,23 Many consumers therefore choose to avoid mineral oil because alternative oils exist that are beneficial in preventing hair damage but do not present carcinogenic risk. An example of a mineral oil–free product in Table 1 is Mizani Coconut Souffle Light Moisturizing Hairdress. Only 8 of the 57 products we reviewed did not contain oil, including the following 5 included in Table 1: Carol’s Daughter Black Vanilla Moisture & Shine Sulfate-Free Shampoo, Miss Jessie’s Leave-In Condish, Kinky-Curly Knot Today (although this product did have behentrimonium made from rapeseed oil), Herbal Essences Hello Hydration Moisturizing Conditioner, and ampro Pro Styl Protein Styling Gel.
Parabens
Parabens are preservatives used to prevent growth of pathogens in and prevent decomposition of cosmetic products. Parabens have attracted a lot of criticism because of their possible link to breast cancer.24 In vitro and in vivo studies of parabens have demonstrated weak estrogenic activity that increased proportionally with increased length and branching of alkyl side chains. In vivo animal studies demonstrated weak estrogenic activity—100,000-fold less potent than 17β-estradiol.25 Ongoing research examines the relationship between the estrogenic properties of parabens, endocrine disruption, and cancer in human breast epithelial cells.5,24 The Cosmetic Ingredient Review and the US Food and Drug Administration uphold that parabens are safe to use in cosmetics.26 Several products that include parabens are listed in Table 1 (ApHogee Deep Moisture Shampoo, Neutrogena Triple Moisture Silk Touch Leave-In Conditioner, John Frieda Frizz Ease Daily Nourishment Leave-In Conditioner, and ampro Pro Styl Protein Styling Gel).
Our Recommendations
Table 2 (although not exhaustive) includes the authors’ recommendations of hair care products for individuals of African descent. Dermatologists should discuss the pros and cons of the use of products with ingredients that have controversial health effects, namely parabens, triethanolamine, tetrasodium EDTA, and mineral oils. Our recommendations do not include products that contain the prior ingredients. For many women of African descent, their hair type and therefore product use changes with the season, health of their hair, and normal changes to hair throughout their lifetime. There is no magic product for all: Each patient has specific individual styling preferences and a distinctive hair type. Decisions about which products to use can be guided with the assistance of a dermatologist but will ultimately be left up to the patient.

Conclusion
Given the array of hair and scalp care products, it is helpful for dermatologists to become familiar with several of the most popular ingredients and commonly used products. It might be helpful to ask patients which products they use and which ones have been effective for their unique hair concerns. Thus, you become armed with a catalogue of product recommendations for your patients.
In the African American and African communities, information regarding the care and treatment of hair and skin often is obtained from relatives as well as Internet videos and bloggers.1 Moreover, fewer than half of African American women surveyed believe that their physician understands African American hair.2 In addition to proficiency in the diagnosis and treatment of hair and scalp disorders in this population, dermatologists must be aware of common hair and scalp beliefs, misconceptions, care, and product use to ensure culturally competent interactions and treatment.
When a patient of African descent refers to their hair as “natural,” he/she is referring to its texture compared with hair that is chemically treated with straighteners (ie, “relaxed” or “permed” hair). Natural hair refers to hair that has not been altered with chemical treatments that permanently break and re-form disulfide bonds of the hair.1 In 2003, it was estimated that 80% of African American women treated their hair with a chemical relaxer.3 However, this preference has changed over the last decade, with a larger percentage of African American women choosing to wear a natural hairstyle.4
Regardless of preferred hairstyle, a multitude of products can be used to obtain and maintain the particular style. According to US Food and Drug Administration regulations, a product’s ingredients must appear on an information panel in descending order of predominance. Additionally, products must be accurately labeled without misleading information. However, one study found that hair care products commonly used by African American women contain mixtures of endocrine-disrupting chemicals, and 84% of detected chemicals are not listed on the label.5
Properties of Hair Care Products
Women of African descent use hair grooming products for cleansing and moisturizing the hair and scalp, detangling, and styling. Products to achieve these goals comprise shampoos, leave-in and rinse-out conditioners, creams, pomades, oils, and gels. In August 2018 we performed a Google search of the most popular hair care products used for natural hair and chemically relaxed African American hair. Key terms used in our search included popular natural hair products, best natural hair products, top natural hair products, products for permed hair, shampoos for permed hair, conditioner for permed hair, popular detanglers for African American hair, popular products for natural hair, detanglers used for permed hair, gels for relaxed hair, moisturizers for relaxed hair, gels for natural hair, and popular moisturizers for African American hair. We reviewed all websites generated by the search and compared the most popular brands, compiled a list of products, and reviewed them for availability in 2 beauty supply stores in Philadelphia, Pennsylvania; 1 Walmart in Hershey, Pennsylvania; and 1 Walmart in Willow Grove, Pennsylvania. Of the 80 products identified, we selected 57 products to be reviewed for ingredients based on which ones were most commonly seen in search results. Table 1 highlights several randomly chosen popular hair care products used by African American women to familiarize dermatologists with specific products and manufacturers.

Tightly coiled hair, common among women of African descent, is considered fragile because of decreased water content and tensile strength.6 Fragility is exacerbated by manipulation during styling, excessive heat, and harsh shampoos that strip the hair of moisture, as well as chemical treatments that lead to protein deficiency.4,6,7 Because tightly coiled hair is naturally dry and fragile, women of African descent have a particular preference for products that reduce hair dryness and breakage, which has led to the popularity of sulfate-free shampoos that minimize loss of moisture in hair; moisturizers, oils, and conditioners also are used to enhance moisture retention in hair. Conditioners also provide protein substances that can help strengthen hair.4
Consumers’ concerns about the inclusion of potentially harmful ingredients have resulted in reformulation of many products. Our review of products demonstrated that natural hair consumers used fewer products containing silicones, parabens, and sulfates, compared to consumers with chemically relaxed hair. Another tool used by manufacturers to address these concerns is the inclusion of an additional label to distinguish the product as sulfate free, silicone free, paraben free, petroleum free, or a combination of these terms. Although many patients believe that there are “good” and “bad” products, they should be made aware that there are pros and cons of ingredients frequently found in hair-grooming products. Popular ingredients in hair care products include sulfates, cationic surfactants and cationic polymers, silicone, oils, and parabens.
Sulfates
Sulfates are anion detergents in shampoo that remove sebum from the scalp and hair. The number of sulfates in a shampoo positively correlates to cleansing strength.1 However, sulfates can cause excessive sebum removal and lead to hair that is hard, rough, dull, and prone to tangle and breakage.6 Sulfates also dissolve oil on the hair, causing additional dryness and breakage.7
There are a variety of sulfate compounds with different sebum-removal capabilities. Lauryl sulfates are commonly used in shampoos for oily hair. Tightly coiled hair that has been overly cleansed with these ingredients can become exceedingly dry and unmanageable, which explains why products with lauryl sulfates are avoided. Table 1 includes only 1 product containing lauryl sulfate (Pantene Pro-V Gold Series Shampoo). Patients using a lauryl sulfate–containing shampoo can select a product that also contains a conditioning agent in the formulation.6 Alternatively, sulfate-free shampoos that contain surfactants with less detergency can be used.8 There are no published studies of the cleansing ability of sulfate-free shampoos or their effects on hair shaft fragility.9
At the opposite end of the spectrum is sodium laureth sulfate, commonly used as a primary detergent in shampoos designed for normal to dry hair.10 Sodium laureth sulfate, which provides excellent cleansing and leaves the hair better moisturized and manageable compared to lauryl sulfates,10 is a common ingredient in the products in Table 1 (ApHogee Deep Moisture Shampoo, Pantene Pro-V Gold Series Shampoo, and Pantene Pro-V Truly Relaxed Moisturizing Shampoo).
An ingredient that might be confused for a sulfate is behentrimonium methosulfate, a cationic quaternary ammonium salt that is not used to cleanse the hair, unlike sodium lauryl sulfate and sodium laureth sulfate, but serves as an antistatic conditioning agent to keep hair moisturized and frizz free.11 Behentrimonium methosulfate is found in conditioners and detanglers in Table 1 (The Mane Choice Green Tea & Carrot Conditioning Mask, Kinky-Curly Knot Today, Miss Jessie’s Leave-In Condish, SheaMoisture Raw Shea Butter Extra-Moisture Detangler, Mielle Pomegranate & Honey Leave-In Conditioner). Patients should be informed that behentrimonium methosulfate is not water soluble, which suggests that it can lead to buildup of residue.
Cationic Surfactants and Cationic Polymers
Cationic surfactants and cationic polymers are found in many hair products and improve manageability by softening and detangling hair.6,10 Hair consists of negatively charged keratin proteins7 that electrostatically attract the positively charged polar group of cationic surfactants and cationic polymers. These surfactants and polymers then adhere to and normalize hair surface charges, resulting in improved texture and reduced friction between strands.6 For African American patients with natural hair, cationic surfactants and polymers help to maintain curl patterns and assist in detangling.6 Polyquaternium is a cationic polymer that is found in several products in Table 1 (Carol’s Daughter Black Vanilla Moisture & Shine Sulfate-Free Shampoo, OGX Nourishing Coconut Milk Shampoo, ApHogee Deep Moisture Shampoo, Pantene Pro-V Gold Series Shampoo, Neutrogena Triple Moisture Silk Touch Leave-In Conditioner, Creme of Nature Argan Oil Strength & Shine Leave-in Conditioner, and John Frieda Frizz Ease Daily Nourishment Leave-In Conditioner).
The surfactants triethanolamine and tetrasodium ethylenediaminetetraacetic acid (EDTA) are ingredients in some styling gels and have been reported as potential carcinogens.12 However, there are inadequate human or animal data to support the carcinogenicity of either ingredient at this time. Of note, tetrasodium EDTA has been reported to increase the penetration of other chemicals through the skin, which might lead to toxicity.12
Silicone
Silicone agents can be found in a variety of hair care products, including shampoos, detanglers, hair conditioners, leave-in conditioners, and moisturizers. Of the 22 products listed in Table 1, silicones are found in 14 products. Common silicones include dimethicone, amodimethicone, cyclopentasiloxane, and dimethiconol. Silicones form hydrophobic films that create smoothness and shine.6,8 Silicone-containing products help reduce frizz and provide protection against breakage and heat damage in chemically relaxed hair.6,7 For patients with natural hair, silicones aid in hair detangling.
Frequent use of silicone products can result in residue buildup due to the insolubility of silicone in water. Preventatively, some products include water-soluble silicones with the same benefits, such as silicones with the prefixes PPG- or PEG-, laurylmethicone copolyol, and dimethicone copolyol.7 Dimethicone copolyol was found in 1 of our reviewed products (OGX Nourishing Coconut Milk Shampoo); 10 products in Table 1 contain ingredients with the prefixes PPG- or PEG-. Several products in our review contain both water-soluble and water-insoluble silicones (eg, Creme of Nature Argan Oil Strength & Shine Leave-In Conditioner).
Oils
Oils in hair care products prevent hair breakage by coating the hair shaft and sealing in moisture. There are various types of oils in hair care products. Essential oils are volatile liquid-aroma substances derived most commonly from plants through dry or steam distillation or by other mechanical processes.13 Essential oils are used to seal and moisturize the hair and often are used to produce fragrance in hair products.6 Examples of essential oils that are ingredients in cosmetics include tea tree oil (TTO), peppermint oil, rosemary oil, and thyme oil. Vegetable oils can be used to dilute essential oils because essential oils can irritate skin.14
Tea tree oil is an essential oil obtained through steam distillation of the leaves of the coastal tree Melaleuca alternifolia. The molecule terpinen-4-ol is a major component of TTO thought to exhibit antiseptic and anti-inflammatory properties.15 Pazyar et al16 reviewed several studies that propose the use of TTO to treat acne vulgaris, seborrheic dermatitis, and chronic gingivitis. Although this herbal oil seemingly has many possible dermatologic applications, dermatologists should be aware that reports have linked TTO to allergic contact dermatitis due to 1,8-cineole, another constituent of TTO.17 Tea tree oil is an ingredient in several of the hair care products that we reviewed. With growing patient interest in the benefits of TTO, further research is necessary to establish guidelines on its use for seborrheic dermatitis.
Castor oil is a vegetable oil pressed from the seeds of the castor oil plant. Its primary fatty acid group—ricinoleic acid—along with certain salts and esters function primarily as skin-conditioning agents, emulsion stabilizers, and surfactants in cosmetic products.18 Jamaican black castor oil is a popular moisturizing oil in the African American natural hair community. It differs in color from standard castor oil because of the manner in which the oil is processed. Anecdotally, it is sometimes advertised as a hair growth serum; some patients admit to applying Jamaican black castor oil on the scalp as self-treatment of alopecia. The basis for such claims might stem from research showing that ricinoleic acid exhibits anti-inflammatory and analgesic properties in some mice and guinea pig models with repeated topical application.17 Scientific evidence does not, however, support claims that castor oil or Jamaican black castor oil can treat alopecia.
Mineral oils have a lubricant base and are refined from petroleum crude oils. The composition of crude oil varies; to remove impurities, it must undergo treatment with different degrees of refinement. When products are highly treated, the result is a substantially decreased level of impurities.19 Although they are beneficial in coating the hair shaft and preventing hair damage, consumers tend to avoid products containing mineral oil because of its carcinogenic potential if untreated or mildly treated.20
Although cosmetics with mineral oils are highly treated, a study showed that mineral oil is the largest contaminant in the human body, with cosmetics being a possible source.21 Studies also have revealed that mineral oils do not prevent hair breakage compared to other oils, such as essential oils and coconut oil.22,23 Many consumers therefore choose to avoid mineral oil because alternative oils exist that are beneficial in preventing hair damage but do not present carcinogenic risk. An example of a mineral oil–free product in Table 1 is Mizani Coconut Souffle Light Moisturizing Hairdress. Only 8 of the 57 products we reviewed did not contain oil, including the following 5 included in Table 1: Carol’s Daughter Black Vanilla Moisture & Shine Sulfate-Free Shampoo, Miss Jessie’s Leave-In Condish, Kinky-Curly Knot Today (although this product did have behentrimonium made from rapeseed oil), Herbal Essences Hello Hydration Moisturizing Conditioner, and ampro Pro Styl Protein Styling Gel.
Parabens
Parabens are preservatives used to prevent growth of pathogens in and prevent decomposition of cosmetic products. Parabens have attracted a lot of criticism because of their possible link to breast cancer.24 In vitro and in vivo studies of parabens have demonstrated weak estrogenic activity that increased proportionally with increased length and branching of alkyl side chains. In vivo animal studies demonstrated weak estrogenic activity—100,000-fold less potent than 17β-estradiol.25 Ongoing research examines the relationship between the estrogenic properties of parabens, endocrine disruption, and cancer in human breast epithelial cells.5,24 The Cosmetic Ingredient Review and the US Food and Drug Administration uphold that parabens are safe to use in cosmetics.26 Several products that include parabens are listed in Table 1 (ApHogee Deep Moisture Shampoo, Neutrogena Triple Moisture Silk Touch Leave-In Conditioner, John Frieda Frizz Ease Daily Nourishment Leave-In Conditioner, and ampro Pro Styl Protein Styling Gel).
Our Recommendations
Table 2 (although not exhaustive) includes the authors’ recommendations of hair care products for individuals of African descent. Dermatologists should discuss the pros and cons of the use of products with ingredients that have controversial health effects, namely parabens, triethanolamine, tetrasodium EDTA, and mineral oils. Our recommendations do not include products that contain the prior ingredients. For many women of African descent, their hair type and therefore product use changes with the season, health of their hair, and normal changes to hair throughout their lifetime. There is no magic product for all: Each patient has specific individual styling preferences and a distinctive hair type. Decisions about which products to use can be guided with the assistance of a dermatologist but will ultimately be left up to the patient.

Conclusion
Given the array of hair and scalp care products, it is helpful for dermatologists to become familiar with several of the most popular ingredients and commonly used products. It might be helpful to ask patients which products they use and which ones have been effective for their unique hair concerns. Thus, you become armed with a catalogue of product recommendations for your patients.
- Taylor S, Kelly AP, Lim HW, et al. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. New York, NY: McGraw-Hill; 2009.
- Gathers RC, Mahan MG. African American women, hair care, and health barriers. J Clin Aesthet Dermatol. 2014;7:26-29.
- Quinn CR, Quinn TM, Kelly AP. Hair care practices in African American women. Cutis. 2003;72:280-282, 285-289.
- Griffin M, Lenzy Y. Contemporary African-American hair care practices. Pract Dermatol. http://practicaldermatology.com/2015/05/contemporary-african-american-hair-care-practices/. May 2015. Accessed March 19, 2020.
- Helm JS, Nishioka M, Brody JG, et al. Measurement of endocrine disrupting and asthma-associated chemicals in hair products used by black women. Environ Res. 2018;165:448-458.
- Crawford K, Hernandez C. A review of hair care products for black individuals. Cutis. 2014;93:289-293.
- Bosley RE, Daveluy S. A primer to natural hair care practices in black patients. Cutis. 2015;95:78-80, 106.
- Cline A, Uwakwe L, McMichael A. No sulfates, no parabens, and the “no-poo” method: a new patient perspective on common shampoo ingredients. Cutis. 2018;101:22-26.
- Gavazzoni Dias MFR. Hair cosmetics: an overview. Int J Trichology. 2015;7:2-15.
- Draelos ZD. Essentials of hair care often neglected: hair cleansing.Int J Trichology. 2010;2:24-29.
- Becker L, Bergfeld W, Belsito D, et al. Safety assessment of trimoniums as used in cosmetics. Int J Toxicol. 2012;31(6 suppl):296S-341S.
- National Center for Biotechnology Information. PubChem Database. Edetate sodium, CID=6144. https://pubchem.ncbi.nlm.nih.gov/compound/EDTA_
tetrasodium#section=FDA-Requirements. Accessed March 19, 2020. - Lanigan RS, Yamarik TA. Final report on the safety assessment of EDTA, calcium disodium EDTA, diammonium EDTA, dipotassium EDTA, disodium EDTA, TEA-EDTA, tetrasodium EDTA, tripotassium EDTA, trisodium EDTA, HEDTA, and trisodium HEDTA. Int J Toxicol. 2002;21(suppl 2):95-142.
- Vasireddy L, Bingle LEH, Davies MS. Antimicrobial activity of essential oils against multidrug-resistant clinical isolates of the Burkholderia cepacia complex. PLoS One. 2018;13:e0201835.
- Mondello F, De Bernardis F, Girolamo A, et al. In vivo activity of terpinen-4-ol, the main bioactive component of Melaleuca alternifolia Cheel (tea tree) oil against azole-susceptible and -resistant human pathogenic Candida species. BMC Infect Dis. 2006;6:158.
- Pazyar N, Yaghoobi R, Bagherani N, et al. A review of applications of tea tree oil in dermatology. Int J Dermatol. 2013;52:784-790.
- Selvaag E, Eriksen B, Thune P. Contact allergy due to tea tree oil and cross-sensitization to colophony. Contact Dermatitis. 1994;31:124-125.
- Vieira C, Fetzer S, Sauer SK, et al. Pro- and anti-inflammatory actions of ricinoleic acid: similarities and differences with capsaicin. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:87-95.
- International Agency for Research on Cancer, IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Polynuclear Aromatic Hydrocarbons, Part 2, Carbon Blacks, Mineral Oils (Lubricant Base Oils and Derived Products) and Sorne Nitroarenes. Vol 33. Lyon, France: International Agency for Research on Cancer; April 1984. https://monographs.iarc.fr/wp-content/uploads/2018/06/mono33.pdf. Accessed March 19, 2020.
- Vieira C, Evangelista S, Cirillo R, et al. Effect of ricinoleic acid in acute and subchronic experimental models of inflammation. Mediators Inflamm. 2000;9:223-228.
- Concin N, Hofstetter G, Plattner B, et al. Evidence for cosmetics as a source of mineral oil contamination in women. J Womens Health (Larchmt). 2011;20:1713-1719.
- Biedermann M, Barp L, Kornauth C, et al. Mineral oil in human tissues, part II: characterization of the accumulated hydrocarbons by comprehensive two-dimensional gas chromatography. Sci Total Environ. 2015;506-507:644-655.
- Ruetsch SB, Kamath YK, Rele AS, et al. Secondary ion mass spectrometric investigation of penetration of coconut and mineral oils into human hair fibers: relevance to hair damage. J Cosmet Sci. 2001;52:169-184.
- Darbre PD, Aljarrah A, Miller WR, et al. Concentrations of parabens in human breast tumours. J Appl Toxicol. 2004;24:5-13.
- Routledge EJ, Parker J, Odum J, et al. Some alkyl hydroxy benzoate preservatives (parabens) are estrogenic. Toxicol Appl Pharmacol. 1998;153:12-19.
- Centers for Disease Control and Prevention. Parabens factsheet. https://www.cdc.gov/biomonitoring/Parabens_FactSheet.html. Updated April 7, 2017. Accessed March 19, 2020.
- Taylor S, Kelly AP, Lim HW, et al. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. New York, NY: McGraw-Hill; 2009.
- Gathers RC, Mahan MG. African American women, hair care, and health barriers. J Clin Aesthet Dermatol. 2014;7:26-29.
- Quinn CR, Quinn TM, Kelly AP. Hair care practices in African American women. Cutis. 2003;72:280-282, 285-289.
- Griffin M, Lenzy Y. Contemporary African-American hair care practices. Pract Dermatol. http://practicaldermatology.com/2015/05/contemporary-african-american-hair-care-practices/. May 2015. Accessed March 19, 2020.
- Helm JS, Nishioka M, Brody JG, et al. Measurement of endocrine disrupting and asthma-associated chemicals in hair products used by black women. Environ Res. 2018;165:448-458.
- Crawford K, Hernandez C. A review of hair care products for black individuals. Cutis. 2014;93:289-293.
- Bosley RE, Daveluy S. A primer to natural hair care practices in black patients. Cutis. 2015;95:78-80, 106.
- Cline A, Uwakwe L, McMichael A. No sulfates, no parabens, and the “no-poo” method: a new patient perspective on common shampoo ingredients. Cutis. 2018;101:22-26.
- Gavazzoni Dias MFR. Hair cosmetics: an overview. Int J Trichology. 2015;7:2-15.
- Draelos ZD. Essentials of hair care often neglected: hair cleansing.Int J Trichology. 2010;2:24-29.
- Becker L, Bergfeld W, Belsito D, et al. Safety assessment of trimoniums as used in cosmetics. Int J Toxicol. 2012;31(6 suppl):296S-341S.
- National Center for Biotechnology Information. PubChem Database. Edetate sodium, CID=6144. https://pubchem.ncbi.nlm.nih.gov/compound/EDTA_
tetrasodium#section=FDA-Requirements. Accessed March 19, 2020. - Lanigan RS, Yamarik TA. Final report on the safety assessment of EDTA, calcium disodium EDTA, diammonium EDTA, dipotassium EDTA, disodium EDTA, TEA-EDTA, tetrasodium EDTA, tripotassium EDTA, trisodium EDTA, HEDTA, and trisodium HEDTA. Int J Toxicol. 2002;21(suppl 2):95-142.
- Vasireddy L, Bingle LEH, Davies MS. Antimicrobial activity of essential oils against multidrug-resistant clinical isolates of the Burkholderia cepacia complex. PLoS One. 2018;13:e0201835.
- Mondello F, De Bernardis F, Girolamo A, et al. In vivo activity of terpinen-4-ol, the main bioactive component of Melaleuca alternifolia Cheel (tea tree) oil against azole-susceptible and -resistant human pathogenic Candida species. BMC Infect Dis. 2006;6:158.
- Pazyar N, Yaghoobi R, Bagherani N, et al. A review of applications of tea tree oil in dermatology. Int J Dermatol. 2013;52:784-790.
- Selvaag E, Eriksen B, Thune P. Contact allergy due to tea tree oil and cross-sensitization to colophony. Contact Dermatitis. 1994;31:124-125.
- Vieira C, Fetzer S, Sauer SK, et al. Pro- and anti-inflammatory actions of ricinoleic acid: similarities and differences with capsaicin. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:87-95.
- International Agency for Research on Cancer, IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Polynuclear Aromatic Hydrocarbons, Part 2, Carbon Blacks, Mineral Oils (Lubricant Base Oils and Derived Products) and Sorne Nitroarenes. Vol 33. Lyon, France: International Agency for Research on Cancer; April 1984. https://monographs.iarc.fr/wp-content/uploads/2018/06/mono33.pdf. Accessed March 19, 2020.
- Vieira C, Evangelista S, Cirillo R, et al. Effect of ricinoleic acid in acute and subchronic experimental models of inflammation. Mediators Inflamm. 2000;9:223-228.
- Concin N, Hofstetter G, Plattner B, et al. Evidence for cosmetics as a source of mineral oil contamination in women. J Womens Health (Larchmt). 2011;20:1713-1719.
- Biedermann M, Barp L, Kornauth C, et al. Mineral oil in human tissues, part II: characterization of the accumulated hydrocarbons by comprehensive two-dimensional gas chromatography. Sci Total Environ. 2015;506-507:644-655.
- Ruetsch SB, Kamath YK, Rele AS, et al. Secondary ion mass spectrometric investigation of penetration of coconut and mineral oils into human hair fibers: relevance to hair damage. J Cosmet Sci. 2001;52:169-184.
- Darbre PD, Aljarrah A, Miller WR, et al. Concentrations of parabens in human breast tumours. J Appl Toxicol. 2004;24:5-13.
- Routledge EJ, Parker J, Odum J, et al. Some alkyl hydroxy benzoate preservatives (parabens) are estrogenic. Toxicol Appl Pharmacol. 1998;153:12-19.
- Centers for Disease Control and Prevention. Parabens factsheet. https://www.cdc.gov/biomonitoring/Parabens_FactSheet.html. Updated April 7, 2017. Accessed March 19, 2020.
Practice Points
- Dermatologists must be aware of common hair and scalp beliefs, misconceptions, care, and product use to ensure culturally competent patient interactions and treatment.
- Common ingredients in popular hair care products used by African Americans include sulfates, cationic surfactants and polymers, silicone, oils, and parabens.
Study finds spironolactone doesn’t boost breast cancer recurrence
in a large retrospective study, Chapman Wei said in a in a virtual meeting held by the George Washington University department of dermatology in Washington. The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled due to the COVID-19 pandemic.
Spironolactone is an aldosterone antagonist and heart failure medication that, because of its peripheral antiandrogen effects, is often used off-label to treat female androgenetic hair loss. Although it has been available for nigh on half a century and has a well-established favorable safety profile, with no indication of carcinogenic effects, little is known about its use in treating alopecia in breast cancer survivors on endocrine therapies, where there has been a theoretic possibility that the drug’s antiandrogen effects could promote breast cancer recurrence.
Not so, said Mr. Wei, from George Washington University.
He presented a retrospective, propensity score–matched, case-control study that used the Humana Insurance database. The initial comparison was between 746 women who went on spironolactone after their breast cancer diagnosis versus 28,400 female breast cancer patients who didn’t take the drug. The primary outcome was recurrent breast cancer within 2 years after diagnosis.
“We chose 2 years because most breast cancer relapses occur within that time,” Mr. Wei explained.
In the initial unadjusted between-group comparison, the breast cancer recurrence rate was 16.5% in the spironolactone group, significantly higher than the 12.8% rate in more than 28,000 controls. However, in a comparison between the spironolactone group and 746 controls extensively propensity score–matched for acne, hypertension, hirsutism, smoking, illicit drug use, heart failure, primary aldosteronism, and other potential confounding variables, there was no significant difference between spironolactone users and controls, with 2-year breast cancer recurrence rates of 16.5% and 15.8%, respectively.
In a multivariate Cox regression analysis, the stand-out finding was that alcohol abuse was independently associated with a 2.3-fold increased risk of breast cancer recurrence.
Mr. Wei noted that these findings confirm those in a recent literature review by investigators at Memorial Sloan Kettering Cancer Center in New York who found no increase in estrogen levels with spironolactone and no heightened risk of female breast cancer while on the drug in three studies totaling 49,298 patients.
“Spironolactone has the potential to be used as a relatively safe systemic treatment option for the management of [endocrine therapy–induced alopecia] in female breast cancer patients and survivors on endocrine therapies who respond poorly to monotherapy with topical minoxidil,” the Sloan Kettering researchers declared (Breast Cancer Res Treat. 2019 Feb;174[1]:15-26).
Mr. Wei reported having no financial conflicts regarding his unfunded study.
in a large retrospective study, Chapman Wei said in a in a virtual meeting held by the George Washington University department of dermatology in Washington. The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled due to the COVID-19 pandemic.
Spironolactone is an aldosterone antagonist and heart failure medication that, because of its peripheral antiandrogen effects, is often used off-label to treat female androgenetic hair loss. Although it has been available for nigh on half a century and has a well-established favorable safety profile, with no indication of carcinogenic effects, little is known about its use in treating alopecia in breast cancer survivors on endocrine therapies, where there has been a theoretic possibility that the drug’s antiandrogen effects could promote breast cancer recurrence.
Not so, said Mr. Wei, from George Washington University.
He presented a retrospective, propensity score–matched, case-control study that used the Humana Insurance database. The initial comparison was between 746 women who went on spironolactone after their breast cancer diagnosis versus 28,400 female breast cancer patients who didn’t take the drug. The primary outcome was recurrent breast cancer within 2 years after diagnosis.
“We chose 2 years because most breast cancer relapses occur within that time,” Mr. Wei explained.
In the initial unadjusted between-group comparison, the breast cancer recurrence rate was 16.5% in the spironolactone group, significantly higher than the 12.8% rate in more than 28,000 controls. However, in a comparison between the spironolactone group and 746 controls extensively propensity score–matched for acne, hypertension, hirsutism, smoking, illicit drug use, heart failure, primary aldosteronism, and other potential confounding variables, there was no significant difference between spironolactone users and controls, with 2-year breast cancer recurrence rates of 16.5% and 15.8%, respectively.
In a multivariate Cox regression analysis, the stand-out finding was that alcohol abuse was independently associated with a 2.3-fold increased risk of breast cancer recurrence.
Mr. Wei noted that these findings confirm those in a recent literature review by investigators at Memorial Sloan Kettering Cancer Center in New York who found no increase in estrogen levels with spironolactone and no heightened risk of female breast cancer while on the drug in three studies totaling 49,298 patients.
“Spironolactone has the potential to be used as a relatively safe systemic treatment option for the management of [endocrine therapy–induced alopecia] in female breast cancer patients and survivors on endocrine therapies who respond poorly to monotherapy with topical minoxidil,” the Sloan Kettering researchers declared (Breast Cancer Res Treat. 2019 Feb;174[1]:15-26).
Mr. Wei reported having no financial conflicts regarding his unfunded study.
in a large retrospective study, Chapman Wei said in a in a virtual meeting held by the George Washington University department of dermatology in Washington. The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled due to the COVID-19 pandemic.
Spironolactone is an aldosterone antagonist and heart failure medication that, because of its peripheral antiandrogen effects, is often used off-label to treat female androgenetic hair loss. Although it has been available for nigh on half a century and has a well-established favorable safety profile, with no indication of carcinogenic effects, little is known about its use in treating alopecia in breast cancer survivors on endocrine therapies, where there has been a theoretic possibility that the drug’s antiandrogen effects could promote breast cancer recurrence.
Not so, said Mr. Wei, from George Washington University.
He presented a retrospective, propensity score–matched, case-control study that used the Humana Insurance database. The initial comparison was between 746 women who went on spironolactone after their breast cancer diagnosis versus 28,400 female breast cancer patients who didn’t take the drug. The primary outcome was recurrent breast cancer within 2 years after diagnosis.
“We chose 2 years because most breast cancer relapses occur within that time,” Mr. Wei explained.
In the initial unadjusted between-group comparison, the breast cancer recurrence rate was 16.5% in the spironolactone group, significantly higher than the 12.8% rate in more than 28,000 controls. However, in a comparison between the spironolactone group and 746 controls extensively propensity score–matched for acne, hypertension, hirsutism, smoking, illicit drug use, heart failure, primary aldosteronism, and other potential confounding variables, there was no significant difference between spironolactone users and controls, with 2-year breast cancer recurrence rates of 16.5% and 15.8%, respectively.
In a multivariate Cox regression analysis, the stand-out finding was that alcohol abuse was independently associated with a 2.3-fold increased risk of breast cancer recurrence.
Mr. Wei noted that these findings confirm those in a recent literature review by investigators at Memorial Sloan Kettering Cancer Center in New York who found no increase in estrogen levels with spironolactone and no heightened risk of female breast cancer while on the drug in three studies totaling 49,298 patients.
“Spironolactone has the potential to be used as a relatively safe systemic treatment option for the management of [endocrine therapy–induced alopecia] in female breast cancer patients and survivors on endocrine therapies who respond poorly to monotherapy with topical minoxidil,” the Sloan Kettering researchers declared (Breast Cancer Res Treat. 2019 Feb;174[1]:15-26).
Mr. Wei reported having no financial conflicts regarding his unfunded study.
CD4 cells implicated in pathology of CCCA
in the lymphocytic inflammatory infiltrate, according to a histopathological study of biopsy specimens.
“Evaluation of the T-cell infiltrate may be a useful way to distinguish CCCA from lichen planopilaris or frontal fibrosing alopecia in some cases when histopathological features alone cannot be used to definitely distinguish between them,” reported Alexandra Flamm, MD, Ata Moshiri, MD, and coauthors from the departments of dermatology and pathology, University of Pennsylvania, Philadelphia.
The histopathological features of CCCA have been characterized previously, but the goal of this study was to go further in piecing together the pathophysiology, they noted.
Horizontal sections of 4-mm punch biopsy specimens were examined from 18 black women with a known diagnosis of CCCA. Both affected and unaffected follicles were evaluated with attention to the number and percentage of CD1a+ Langerhans cells, CD3+, CD4+, and CD8+ lymphocytes.
In this series, the lymphocytic infiltrate in both the affected and unaffected follicles was predominantly composed of CD4+ cells. The perifollicular ratio for CD4+ to CD8+ cells in affected follicles was 5.3:1. It was only modestly lower in unaffected follicles (4.3:1) and in the intrafollicular space of affected follicles (2.5:1).
Affected follicles had a higher number of CD1a+ Langerhans cells than unaffected follicles. This finding suggests, as others have hypothesized, that the antigen-presenting Langerhans cells draw lymphocytes to the follicle, according to the investigators. Elevated numbers of Langerhans cells have also been reported in other forms of scarring alopecia, such as lichen planopilaris (LPP).
In the case of CCCA, CD1a+ Langerhans cells appear to localize to the hair follicle in response to stimulus such as an injury. The CD4+ cells that follow the Langerhans cells participate in an inflammatory reaction that drives follicle destruction. In addition to this damage and scarring, the inflammatory response is also likely to be disrupting the blood supply.
“Fibroplasia associated with follicular scarring displaces blood vessels away from the outer root sheath epithelium,” the authors explained. Ultimately, “the mucinous fibroplasia and perifollicular fibrosis may disrupt and fragment blood vessels in the fibrous sheath, leaving only small clusters of vessels more distant to the keratinocytes in the outer root sheath.”
Prior studies of scarring alopecia diseases, including LLP, frontal fibrosing alopecia (FFA), and keratosis follicularis spinulosa decalvans (KFSD), have typically described a predominantly CD8+ lymphocytic infiltrate. The evidence from this study that the infiltrate is CD4+ predominant in CCCA supports the conclusion that the pathophysiologic features of this type of alopecia are unique, according to the authors.
Work by others has associated CCCA with mutations in the PAD13 gene, which suggests a defect in the formation of hair shaft structure, but this may speak to susceptibility but not the mechanism of hair follicle damage. Rather, this study suggests that it is the concentration of a CD4+ predominant lymphocytic infiltrate in the perifollicular space that induces the pathological events.
For determining the fundamental cause of CCCA, “it will be important to determine what recruits the Langerhans cells to affected follicles,” the investigators suggested. Meanwhile, they expressed hope that the progress being made into decoding the pathogenesis of CCCA will lead to novel therapeutic strategies.
The authors did not list any disclosures. The funding source was listed as the Center for Scientific Review (Grant/Award).
SOURCE: Flamm A et al. J Cutan Pathol. 2020 Feb 18.doi: 10.1111/cup.13666.
in the lymphocytic inflammatory infiltrate, according to a histopathological study of biopsy specimens.
“Evaluation of the T-cell infiltrate may be a useful way to distinguish CCCA from lichen planopilaris or frontal fibrosing alopecia in some cases when histopathological features alone cannot be used to definitely distinguish between them,” reported Alexandra Flamm, MD, Ata Moshiri, MD, and coauthors from the departments of dermatology and pathology, University of Pennsylvania, Philadelphia.
The histopathological features of CCCA have been characterized previously, but the goal of this study was to go further in piecing together the pathophysiology, they noted.
Horizontal sections of 4-mm punch biopsy specimens were examined from 18 black women with a known diagnosis of CCCA. Both affected and unaffected follicles were evaluated with attention to the number and percentage of CD1a+ Langerhans cells, CD3+, CD4+, and CD8+ lymphocytes.
In this series, the lymphocytic infiltrate in both the affected and unaffected follicles was predominantly composed of CD4+ cells. The perifollicular ratio for CD4+ to CD8+ cells in affected follicles was 5.3:1. It was only modestly lower in unaffected follicles (4.3:1) and in the intrafollicular space of affected follicles (2.5:1).
Affected follicles had a higher number of CD1a+ Langerhans cells than unaffected follicles. This finding suggests, as others have hypothesized, that the antigen-presenting Langerhans cells draw lymphocytes to the follicle, according to the investigators. Elevated numbers of Langerhans cells have also been reported in other forms of scarring alopecia, such as lichen planopilaris (LPP).
In the case of CCCA, CD1a+ Langerhans cells appear to localize to the hair follicle in response to stimulus such as an injury. The CD4+ cells that follow the Langerhans cells participate in an inflammatory reaction that drives follicle destruction. In addition to this damage and scarring, the inflammatory response is also likely to be disrupting the blood supply.
“Fibroplasia associated with follicular scarring displaces blood vessels away from the outer root sheath epithelium,” the authors explained. Ultimately, “the mucinous fibroplasia and perifollicular fibrosis may disrupt and fragment blood vessels in the fibrous sheath, leaving only small clusters of vessels more distant to the keratinocytes in the outer root sheath.”
Prior studies of scarring alopecia diseases, including LLP, frontal fibrosing alopecia (FFA), and keratosis follicularis spinulosa decalvans (KFSD), have typically described a predominantly CD8+ lymphocytic infiltrate. The evidence from this study that the infiltrate is CD4+ predominant in CCCA supports the conclusion that the pathophysiologic features of this type of alopecia are unique, according to the authors.
Work by others has associated CCCA with mutations in the PAD13 gene, which suggests a defect in the formation of hair shaft structure, but this may speak to susceptibility but not the mechanism of hair follicle damage. Rather, this study suggests that it is the concentration of a CD4+ predominant lymphocytic infiltrate in the perifollicular space that induces the pathological events.
For determining the fundamental cause of CCCA, “it will be important to determine what recruits the Langerhans cells to affected follicles,” the investigators suggested. Meanwhile, they expressed hope that the progress being made into decoding the pathogenesis of CCCA will lead to novel therapeutic strategies.
The authors did not list any disclosures. The funding source was listed as the Center for Scientific Review (Grant/Award).
SOURCE: Flamm A et al. J Cutan Pathol. 2020 Feb 18.doi: 10.1111/cup.13666.
in the lymphocytic inflammatory infiltrate, according to a histopathological study of biopsy specimens.
“Evaluation of the T-cell infiltrate may be a useful way to distinguish CCCA from lichen planopilaris or frontal fibrosing alopecia in some cases when histopathological features alone cannot be used to definitely distinguish between them,” reported Alexandra Flamm, MD, Ata Moshiri, MD, and coauthors from the departments of dermatology and pathology, University of Pennsylvania, Philadelphia.
The histopathological features of CCCA have been characterized previously, but the goal of this study was to go further in piecing together the pathophysiology, they noted.
Horizontal sections of 4-mm punch biopsy specimens were examined from 18 black women with a known diagnosis of CCCA. Both affected and unaffected follicles were evaluated with attention to the number and percentage of CD1a+ Langerhans cells, CD3+, CD4+, and CD8+ lymphocytes.
In this series, the lymphocytic infiltrate in both the affected and unaffected follicles was predominantly composed of CD4+ cells. The perifollicular ratio for CD4+ to CD8+ cells in affected follicles was 5.3:1. It was only modestly lower in unaffected follicles (4.3:1) and in the intrafollicular space of affected follicles (2.5:1).
Affected follicles had a higher number of CD1a+ Langerhans cells than unaffected follicles. This finding suggests, as others have hypothesized, that the antigen-presenting Langerhans cells draw lymphocytes to the follicle, according to the investigators. Elevated numbers of Langerhans cells have also been reported in other forms of scarring alopecia, such as lichen planopilaris (LPP).
In the case of CCCA, CD1a+ Langerhans cells appear to localize to the hair follicle in response to stimulus such as an injury. The CD4+ cells that follow the Langerhans cells participate in an inflammatory reaction that drives follicle destruction. In addition to this damage and scarring, the inflammatory response is also likely to be disrupting the blood supply.
“Fibroplasia associated with follicular scarring displaces blood vessels away from the outer root sheath epithelium,” the authors explained. Ultimately, “the mucinous fibroplasia and perifollicular fibrosis may disrupt and fragment blood vessels in the fibrous sheath, leaving only small clusters of vessels more distant to the keratinocytes in the outer root sheath.”
Prior studies of scarring alopecia diseases, including LLP, frontal fibrosing alopecia (FFA), and keratosis follicularis spinulosa decalvans (KFSD), have typically described a predominantly CD8+ lymphocytic infiltrate. The evidence from this study that the infiltrate is CD4+ predominant in CCCA supports the conclusion that the pathophysiologic features of this type of alopecia are unique, according to the authors.
Work by others has associated CCCA with mutations in the PAD13 gene, which suggests a defect in the formation of hair shaft structure, but this may speak to susceptibility but not the mechanism of hair follicle damage. Rather, this study suggests that it is the concentration of a CD4+ predominant lymphocytic infiltrate in the perifollicular space that induces the pathological events.
For determining the fundamental cause of CCCA, “it will be important to determine what recruits the Langerhans cells to affected follicles,” the investigators suggested. Meanwhile, they expressed hope that the progress being made into decoding the pathogenesis of CCCA will lead to novel therapeutic strategies.
The authors did not list any disclosures. The funding source was listed as the Center for Scientific Review (Grant/Award).
SOURCE: Flamm A et al. J Cutan Pathol. 2020 Feb 18.doi: 10.1111/cup.13666.
‘Natural is not always good’ when it comes to treatments for alopecia
ORLANDO – Biotin is the most popular consumer supplement for alopecia and highly popular online, but should patients be taking it?
Patients may want something “natural” to treat their hair loss, but “natural is not always good,” Amy McMichael, MD, professor and chair of the department of dermatology at Wake Forest Baptist Health, Winston-Salem, N.C., said at the ODAC Dermatology, Aesthetic, & Surgical Conference.
Dosages of commercially available biotin supplements can vary significantly, with some doses as high as 10,000 mcg, making supraphysiological dosing possible. Dr. McMichael said that, not only is biotin unlikely to help with a patient’s alopecia, but a high intake of biotin can interfere with certain assays that use streptavidin-biotin capture techniques (Clin Chem Lab Med. 2017 May 1;55[6]:817-25). This could present a problem for a patient who experiences an MI or has a thyroid disorder where a high level of biotin could affect lab results, she noted.
Dr. McMichael advises patients that there is a
Questioning side effects of 5-alpha reductase inhibitors
Research in the early 2010s associated the 5-alpha reductase inhibitor finasteride with persistent sexual side effects and depression. But a later meta-analysis of the Prostate Cancer Prevention Trial and other trials did not find evidence of persistent sexual side effects or depression in men on finasteride, and the authors said that double-blind, placebo-controlled studies were needed (J Clin Aesthet Dermatol. 2014 Dec;7[12]:51-5).
However, two meta-analyses of 34 clinical trials published in 2015 found that none of the clinical trials evaluating finasteride treatment in patients with androgenic alopecia had accurate safety reporting (JAMA Dermatol. 2015 Jun;151[6]:600-6). Another study published by the same group in 2017 found that 0.8% of men aged 16-42 years to exposed to a 5-alpha reductase inhibitor developed persistent erectile dysfunction after a longer duration of exposure (median, 1,534 days). compared with a shorter duration of exposure (PeerJ. 2017 Mar 9;5:e3020).
The bottom line when considering use of 5-alpha reductase inhibitors in men is to discuss the outlier data on persistent sexual dysfunction in the studies, ask patients whether they have a history of sexual dysfunction and depression, and then only treat appropriate patients with no such history, Dr. McMichael said.
Use of 5-alpha reductase inhibitors also appears to be related to an increased risk of type 2 diabetes in men with benign prostatic hyperplasia, according to more recent data. In a study of patients in the U.K. Clinical Practice Research Datalink and Taiwanese National Health Insurance Research Database who received dutasteride, finasteride, or the alpha blocker tamsulosin, there was a slightly increased risk of type 2 diabetes among those who took the two 5-alpha reductase inhibitors, compared with those on tamsulosin (BMJ. 2019;365:l1204). In light of these results, Dr. McMichael advised clinicians to be aware of these risks and to consider screening patients for type 2 diabetes and ask them about their family history, but the results shouldn’t affect patients at risk for type 2 diabetes or metabolic disorder.
JAK inhibitors for alopecia areata
Within the past few years, promising results for Janus kinase (JAK) inhibitors like tofacitinib and ruxolitinib for alopecia areata have been reported, but they are currently not a first-line therapy, Dr. McMichael said. Consider methotrexate first in older adolescents and adults with more than 50% hair loss, followed by a JAK inhibitor if there is no improvement or methotrexate is not tolerated well.
Clinicians can consider enrolling their patients in a clinical trial to give them access to JAK inhibitors as a treatment option, she noted, and if a trial is not available, it may be worth appealing to an insurance company using an article titled “Alopecia areata is a medical disease” coauthored by Dr. McMichael and others (Am Acad Dermatol. 2018 Apr;78[4]:832-4). After two denials by insurance, the manufacturer’s patient assistance program (Xelsource) may be helpful in obtaining tofacitinib (Xeljanz) through a letter and references. There are adolescent data on JAK inhibitors for alopecia, but “absolutely no data” in very young children, so prior to adolescence, she would not recommend this treatment. In a study of 13 adolescents with alopecia areata, totalis, or universalis, those treated with tofacitinib for a mean of 6.5 months, 9 had significant hair regrowth with treatment and adverse events were mild (J Am Acad Dermatol. 2017 Jan;76[1]:29-32).
Dr. McMichael reports being an investigator for Allergan, Intendis, Proctor & Gamble, Samumed, Cassiopia, Concert, Aclaris, and Incyte; and is a consultant for Johnson & Johnson, Proctor & Gamble, Allergan, Bayer, Galderma, Incyte, Samumed, Aclaris, Anacor, Pfizer, Nutrafol, Bioniz, and Almirall.
ORLANDO – Biotin is the most popular consumer supplement for alopecia and highly popular online, but should patients be taking it?
Patients may want something “natural” to treat their hair loss, but “natural is not always good,” Amy McMichael, MD, professor and chair of the department of dermatology at Wake Forest Baptist Health, Winston-Salem, N.C., said at the ODAC Dermatology, Aesthetic, & Surgical Conference.
Dosages of commercially available biotin supplements can vary significantly, with some doses as high as 10,000 mcg, making supraphysiological dosing possible. Dr. McMichael said that, not only is biotin unlikely to help with a patient’s alopecia, but a high intake of biotin can interfere with certain assays that use streptavidin-biotin capture techniques (Clin Chem Lab Med. 2017 May 1;55[6]:817-25). This could present a problem for a patient who experiences an MI or has a thyroid disorder where a high level of biotin could affect lab results, she noted.
Dr. McMichael advises patients that there is a
Questioning side effects of 5-alpha reductase inhibitors
Research in the early 2010s associated the 5-alpha reductase inhibitor finasteride with persistent sexual side effects and depression. But a later meta-analysis of the Prostate Cancer Prevention Trial and other trials did not find evidence of persistent sexual side effects or depression in men on finasteride, and the authors said that double-blind, placebo-controlled studies were needed (J Clin Aesthet Dermatol. 2014 Dec;7[12]:51-5).
However, two meta-analyses of 34 clinical trials published in 2015 found that none of the clinical trials evaluating finasteride treatment in patients with androgenic alopecia had accurate safety reporting (JAMA Dermatol. 2015 Jun;151[6]:600-6). Another study published by the same group in 2017 found that 0.8% of men aged 16-42 years to exposed to a 5-alpha reductase inhibitor developed persistent erectile dysfunction after a longer duration of exposure (median, 1,534 days). compared with a shorter duration of exposure (PeerJ. 2017 Mar 9;5:e3020).
The bottom line when considering use of 5-alpha reductase inhibitors in men is to discuss the outlier data on persistent sexual dysfunction in the studies, ask patients whether they have a history of sexual dysfunction and depression, and then only treat appropriate patients with no such history, Dr. McMichael said.
Use of 5-alpha reductase inhibitors also appears to be related to an increased risk of type 2 diabetes in men with benign prostatic hyperplasia, according to more recent data. In a study of patients in the U.K. Clinical Practice Research Datalink and Taiwanese National Health Insurance Research Database who received dutasteride, finasteride, or the alpha blocker tamsulosin, there was a slightly increased risk of type 2 diabetes among those who took the two 5-alpha reductase inhibitors, compared with those on tamsulosin (BMJ. 2019;365:l1204). In light of these results, Dr. McMichael advised clinicians to be aware of these risks and to consider screening patients for type 2 diabetes and ask them about their family history, but the results shouldn’t affect patients at risk for type 2 diabetes or metabolic disorder.
JAK inhibitors for alopecia areata
Within the past few years, promising results for Janus kinase (JAK) inhibitors like tofacitinib and ruxolitinib for alopecia areata have been reported, but they are currently not a first-line therapy, Dr. McMichael said. Consider methotrexate first in older adolescents and adults with more than 50% hair loss, followed by a JAK inhibitor if there is no improvement or methotrexate is not tolerated well.
Clinicians can consider enrolling their patients in a clinical trial to give them access to JAK inhibitors as a treatment option, she noted, and if a trial is not available, it may be worth appealing to an insurance company using an article titled “Alopecia areata is a medical disease” coauthored by Dr. McMichael and others (Am Acad Dermatol. 2018 Apr;78[4]:832-4). After two denials by insurance, the manufacturer’s patient assistance program (Xelsource) may be helpful in obtaining tofacitinib (Xeljanz) through a letter and references. There are adolescent data on JAK inhibitors for alopecia, but “absolutely no data” in very young children, so prior to adolescence, she would not recommend this treatment. In a study of 13 adolescents with alopecia areata, totalis, or universalis, those treated with tofacitinib for a mean of 6.5 months, 9 had significant hair regrowth with treatment and adverse events were mild (J Am Acad Dermatol. 2017 Jan;76[1]:29-32).
Dr. McMichael reports being an investigator for Allergan, Intendis, Proctor & Gamble, Samumed, Cassiopia, Concert, Aclaris, and Incyte; and is a consultant for Johnson & Johnson, Proctor & Gamble, Allergan, Bayer, Galderma, Incyte, Samumed, Aclaris, Anacor, Pfizer, Nutrafol, Bioniz, and Almirall.
ORLANDO – Biotin is the most popular consumer supplement for alopecia and highly popular online, but should patients be taking it?
Patients may want something “natural” to treat their hair loss, but “natural is not always good,” Amy McMichael, MD, professor and chair of the department of dermatology at Wake Forest Baptist Health, Winston-Salem, N.C., said at the ODAC Dermatology, Aesthetic, & Surgical Conference.
Dosages of commercially available biotin supplements can vary significantly, with some doses as high as 10,000 mcg, making supraphysiological dosing possible. Dr. McMichael said that, not only is biotin unlikely to help with a patient’s alopecia, but a high intake of biotin can interfere with certain assays that use streptavidin-biotin capture techniques (Clin Chem Lab Med. 2017 May 1;55[6]:817-25). This could present a problem for a patient who experiences an MI or has a thyroid disorder where a high level of biotin could affect lab results, she noted.
Dr. McMichael advises patients that there is a
Questioning side effects of 5-alpha reductase inhibitors
Research in the early 2010s associated the 5-alpha reductase inhibitor finasteride with persistent sexual side effects and depression. But a later meta-analysis of the Prostate Cancer Prevention Trial and other trials did not find evidence of persistent sexual side effects or depression in men on finasteride, and the authors said that double-blind, placebo-controlled studies were needed (J Clin Aesthet Dermatol. 2014 Dec;7[12]:51-5).
However, two meta-analyses of 34 clinical trials published in 2015 found that none of the clinical trials evaluating finasteride treatment in patients with androgenic alopecia had accurate safety reporting (JAMA Dermatol. 2015 Jun;151[6]:600-6). Another study published by the same group in 2017 found that 0.8% of men aged 16-42 years to exposed to a 5-alpha reductase inhibitor developed persistent erectile dysfunction after a longer duration of exposure (median, 1,534 days). compared with a shorter duration of exposure (PeerJ. 2017 Mar 9;5:e3020).
The bottom line when considering use of 5-alpha reductase inhibitors in men is to discuss the outlier data on persistent sexual dysfunction in the studies, ask patients whether they have a history of sexual dysfunction and depression, and then only treat appropriate patients with no such history, Dr. McMichael said.
Use of 5-alpha reductase inhibitors also appears to be related to an increased risk of type 2 diabetes in men with benign prostatic hyperplasia, according to more recent data. In a study of patients in the U.K. Clinical Practice Research Datalink and Taiwanese National Health Insurance Research Database who received dutasteride, finasteride, or the alpha blocker tamsulosin, there was a slightly increased risk of type 2 diabetes among those who took the two 5-alpha reductase inhibitors, compared with those on tamsulosin (BMJ. 2019;365:l1204). In light of these results, Dr. McMichael advised clinicians to be aware of these risks and to consider screening patients for type 2 diabetes and ask them about their family history, but the results shouldn’t affect patients at risk for type 2 diabetes or metabolic disorder.
JAK inhibitors for alopecia areata
Within the past few years, promising results for Janus kinase (JAK) inhibitors like tofacitinib and ruxolitinib for alopecia areata have been reported, but they are currently not a first-line therapy, Dr. McMichael said. Consider methotrexate first in older adolescents and adults with more than 50% hair loss, followed by a JAK inhibitor if there is no improvement or methotrexate is not tolerated well.
Clinicians can consider enrolling their patients in a clinical trial to give them access to JAK inhibitors as a treatment option, she noted, and if a trial is not available, it may be worth appealing to an insurance company using an article titled “Alopecia areata is a medical disease” coauthored by Dr. McMichael and others (Am Acad Dermatol. 2018 Apr;78[4]:832-4). After two denials by insurance, the manufacturer’s patient assistance program (Xelsource) may be helpful in obtaining tofacitinib (Xeljanz) through a letter and references. There are adolescent data on JAK inhibitors for alopecia, but “absolutely no data” in very young children, so prior to adolescence, she would not recommend this treatment. In a study of 13 adolescents with alopecia areata, totalis, or universalis, those treated with tofacitinib for a mean of 6.5 months, 9 had significant hair regrowth with treatment and adverse events were mild (J Am Acad Dermatol. 2017 Jan;76[1]:29-32).
Dr. McMichael reports being an investigator for Allergan, Intendis, Proctor & Gamble, Samumed, Cassiopia, Concert, Aclaris, and Incyte; and is a consultant for Johnson & Johnson, Proctor & Gamble, Allergan, Bayer, Galderma, Incyte, Samumed, Aclaris, Anacor, Pfizer, Nutrafol, Bioniz, and Almirall.
EXPERT ANALYSIS FROM ODAC 2020
Evaluating the Impact and Educational Value of YouTube Videos on Nail Biopsy Procedures
To the Editor:
Nail biopsy is an important surgical procedure for diagnosis of nail pathology. YouTube has become a potential instrument for physicians and patients to learn about medical procedures.1,2 However, the sources, content, and quality of the information available have not been fully studied or characterized. Our objective was to analyze the efficiency of information and quality of YouTube videos on nail biopsies. We hypothesized that the quality of patient education and physician training videos would be unrepresentative on YouTube.
The term nail biopsy was searched on January 29, 2019, and filtered by relevance and rating using the default YouTube algorithm. Data were collected from the top 40 hits for the search term and filter coupling. All videos were viewed and sorted for nail biopsy procedures, and then those videos were categorized as being produced by a physician or other health care provider. The US medical board status of each physician videographer was determined using the American Board of Medical Specialties database.3 DISCERN criteria for assessing consumer health care information4 were used to interpret the videos by researchers (S.I. and S.R.L.) in this study.
From the queried search term collection, only 10 videos (1,023,423 combined views) were analyzed in this study (eTable). Although the other resulting videos were tagged as nail biopsy, they were excluded due to irrelevant content (eg, patient blogs, PowerPoints, nail avulsions). The mean age of the videos was 4 years (range, 4 days to 11 years), with a mean video length of 3.30 minutes (range, 49 seconds to 9.03 minutes). Four of 10 videos were performed for longitudinal melanonychia, and 5 of 10 videos were performed for melanonychia, clinically consistent with subungual hematoma. Dermatologists, plastic surgeons, and podiatrists produced the majority of the nail biopsy videos. The overall mean DISCERN rating was 1.60/5.00 (range, 1–3), meaning that the quality of content on nail biopsies was poor. This low DISCERN score signifies poor consumer health information. Video 2 (published in 2015) received a DISCERN score of 2 and received almost 1 million views, which is likely because the specific channel has a well-established subscriber pool (4.9 million subscribers). The highest DISCERN score of 3, demonstrating a tangential shave biopsy, was given to video 4 (published in 2010) and only received about 17,400 views (56 subscribers). Additionally, many videos lacked important information about the procedure. For instance, only 3 of 10 videos demonstrated the anesthetic technique and only 5 videos showed repair methods.
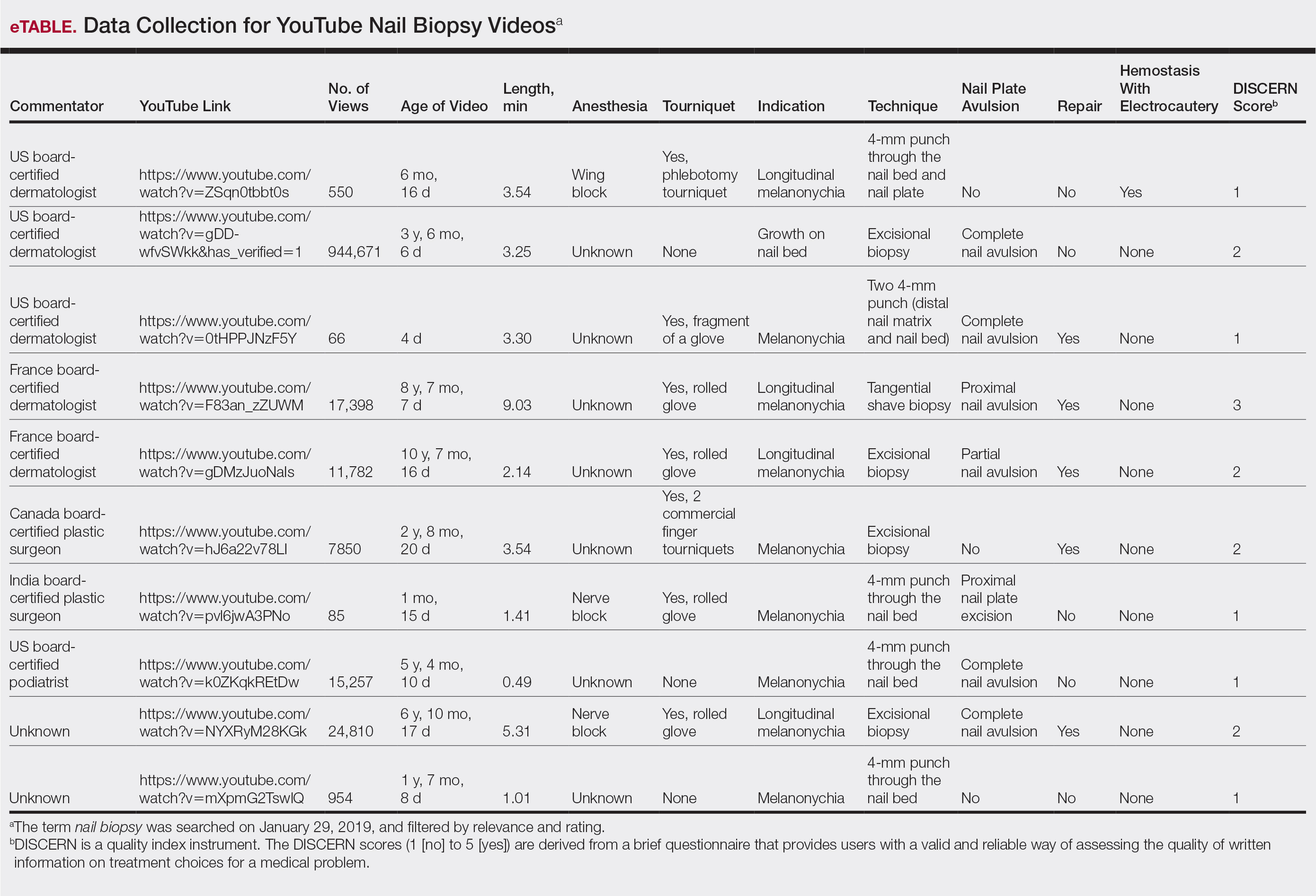
YouTube is an electronic learning source for general information; however, the content and quality of information on nail biopsy is not updated, reliable, or abundant. Patients undergoing nail biopsies are unlikely to find reliable and comprehensible information on YouTube; thus, there is a strong need for patient education in this area. In addition, physicians who did not learn to perform a nail biopsy during training are unlikely to learn this procedure from YouTube. Therefore, there is an unmet need for an outlet that will provide updated reliable content on nail biopsies geared toward both patients and physicians.
- Kwok TM, Singla AA, Phang K, et al. YouTube as a source of patient information for varicose vein treatment options. J Vasc Surg Venous Lymphat Disord. 2017;5:238-243.
- Ward B, Ward M, Nicheporuck A, et al. Assessment of YouTube as an informative resource on facial plastic surgery procedures. JAMA Facial Plastic Surgery. 2019;21:75-76.
- American Board of Medical Specialties. Certification Matters. https://www.certificationmatters.org. Accessed February 7, 2020.
- The DISCERN Instrument. DISCERN Online. http://www.discern.org.uk/discern_instrument.php. Published October 1999. Accessed February 7, 2020.
To the Editor:
Nail biopsy is an important surgical procedure for diagnosis of nail pathology. YouTube has become a potential instrument for physicians and patients to learn about medical procedures.1,2 However, the sources, content, and quality of the information available have not been fully studied or characterized. Our objective was to analyze the efficiency of information and quality of YouTube videos on nail biopsies. We hypothesized that the quality of patient education and physician training videos would be unrepresentative on YouTube.
The term nail biopsy was searched on January 29, 2019, and filtered by relevance and rating using the default YouTube algorithm. Data were collected from the top 40 hits for the search term and filter coupling. All videos were viewed and sorted for nail biopsy procedures, and then those videos were categorized as being produced by a physician or other health care provider. The US medical board status of each physician videographer was determined using the American Board of Medical Specialties database.3 DISCERN criteria for assessing consumer health care information4 were used to interpret the videos by researchers (S.I. and S.R.L.) in this study.
From the queried search term collection, only 10 videos (1,023,423 combined views) were analyzed in this study (eTable). Although the other resulting videos were tagged as nail biopsy, they were excluded due to irrelevant content (eg, patient blogs, PowerPoints, nail avulsions). The mean age of the videos was 4 years (range, 4 days to 11 years), with a mean video length of 3.30 minutes (range, 49 seconds to 9.03 minutes). Four of 10 videos were performed for longitudinal melanonychia, and 5 of 10 videos were performed for melanonychia, clinically consistent with subungual hematoma. Dermatologists, plastic surgeons, and podiatrists produced the majority of the nail biopsy videos. The overall mean DISCERN rating was 1.60/5.00 (range, 1–3), meaning that the quality of content on nail biopsies was poor. This low DISCERN score signifies poor consumer health information. Video 2 (published in 2015) received a DISCERN score of 2 and received almost 1 million views, which is likely because the specific channel has a well-established subscriber pool (4.9 million subscribers). The highest DISCERN score of 3, demonstrating a tangential shave biopsy, was given to video 4 (published in 2010) and only received about 17,400 views (56 subscribers). Additionally, many videos lacked important information about the procedure. For instance, only 3 of 10 videos demonstrated the anesthetic technique and only 5 videos showed repair methods.

YouTube is an electronic learning source for general information; however, the content and quality of information on nail biopsy is not updated, reliable, or abundant. Patients undergoing nail biopsies are unlikely to find reliable and comprehensible information on YouTube; thus, there is a strong need for patient education in this area. In addition, physicians who did not learn to perform a nail biopsy during training are unlikely to learn this procedure from YouTube. Therefore, there is an unmet need for an outlet that will provide updated reliable content on nail biopsies geared toward both patients and physicians.
To the Editor:
Nail biopsy is an important surgical procedure for diagnosis of nail pathology. YouTube has become a potential instrument for physicians and patients to learn about medical procedures.1,2 However, the sources, content, and quality of the information available have not been fully studied or characterized. Our objective was to analyze the efficiency of information and quality of YouTube videos on nail biopsies. We hypothesized that the quality of patient education and physician training videos would be unrepresentative on YouTube.
The term nail biopsy was searched on January 29, 2019, and filtered by relevance and rating using the default YouTube algorithm. Data were collected from the top 40 hits for the search term and filter coupling. All videos were viewed and sorted for nail biopsy procedures, and then those videos were categorized as being produced by a physician or other health care provider. The US medical board status of each physician videographer was determined using the American Board of Medical Specialties database.3 DISCERN criteria for assessing consumer health care information4 were used to interpret the videos by researchers (S.I. and S.R.L.) in this study.
From the queried search term collection, only 10 videos (1,023,423 combined views) were analyzed in this study (eTable). Although the other resulting videos were tagged as nail biopsy, they were excluded due to irrelevant content (eg, patient blogs, PowerPoints, nail avulsions). The mean age of the videos was 4 years (range, 4 days to 11 years), with a mean video length of 3.30 minutes (range, 49 seconds to 9.03 minutes). Four of 10 videos were performed for longitudinal melanonychia, and 5 of 10 videos were performed for melanonychia, clinically consistent with subungual hematoma. Dermatologists, plastic surgeons, and podiatrists produced the majority of the nail biopsy videos. The overall mean DISCERN rating was 1.60/5.00 (range, 1–3), meaning that the quality of content on nail biopsies was poor. This low DISCERN score signifies poor consumer health information. Video 2 (published in 2015) received a DISCERN score of 2 and received almost 1 million views, which is likely because the specific channel has a well-established subscriber pool (4.9 million subscribers). The highest DISCERN score of 3, demonstrating a tangential shave biopsy, was given to video 4 (published in 2010) and only received about 17,400 views (56 subscribers). Additionally, many videos lacked important information about the procedure. For instance, only 3 of 10 videos demonstrated the anesthetic technique and only 5 videos showed repair methods.

YouTube is an electronic learning source for general information; however, the content and quality of information on nail biopsy is not updated, reliable, or abundant. Patients undergoing nail biopsies are unlikely to find reliable and comprehensible information on YouTube; thus, there is a strong need for patient education in this area. In addition, physicians who did not learn to perform a nail biopsy during training are unlikely to learn this procedure from YouTube. Therefore, there is an unmet need for an outlet that will provide updated reliable content on nail biopsies geared toward both patients and physicians.
- Kwok TM, Singla AA, Phang K, et al. YouTube as a source of patient information for varicose vein treatment options. J Vasc Surg Venous Lymphat Disord. 2017;5:238-243.
- Ward B, Ward M, Nicheporuck A, et al. Assessment of YouTube as an informative resource on facial plastic surgery procedures. JAMA Facial Plastic Surgery. 2019;21:75-76.
- American Board of Medical Specialties. Certification Matters. https://www.certificationmatters.org. Accessed February 7, 2020.
- The DISCERN Instrument. DISCERN Online. http://www.discern.org.uk/discern_instrument.php. Published October 1999. Accessed February 7, 2020.
- Kwok TM, Singla AA, Phang K, et al. YouTube as a source of patient information for varicose vein treatment options. J Vasc Surg Venous Lymphat Disord. 2017;5:238-243.
- Ward B, Ward M, Nicheporuck A, et al. Assessment of YouTube as an informative resource on facial plastic surgery procedures. JAMA Facial Plastic Surgery. 2019;21:75-76.
- American Board of Medical Specialties. Certification Matters. https://www.certificationmatters.org. Accessed February 7, 2020.
- The DISCERN Instrument. DISCERN Online. http://www.discern.org.uk/discern_instrument.php. Published October 1999. Accessed February 7, 2020.
Practice Points
- A nail biopsy is sometimes necessary for histopathologic confirmation of a clinical diagnosis.
- YouTube has become a potential educational platform for physicians and patients to learn about nail biopsy procedures.
- Physicians and patients interested in learning more about nail biopsies are unlikely to find reliable and comprehensible information on YouTube; therefore, there is a need for updated reliable video content on nail biopsies geared toward both physicians and patients.