User login
Adjuvant capecitabine shown a less punishing option after NPC chemoradiation
Adjuvant capecitabine is an effective and better tolerated alternative to chemotherapy following chemoradiation for locoregionally advanced nasopharyngeal carcinoma, according to two phase 3 trials from China.
Despite using different dosing strategies, 3-year failure-free survival approached 90% in both trials, comparable to the standard approach with adjuvant platinum doublets, but with better compliance.
The study teams were looking for “a way to make adjuvant therapy more tolerable,” said oncologist Herbert Loong, MBBS, clinical associate professor at the Chinese University of Hong Kong, who discussed both trials after they were presented at the American Society for Clinical Oncology annual meeting.
Compliance is low with platinum doublets because of the toxicity. About 60%-70% of patients can tolerate 2-3 cycles after chemoradiation, and about a third can’t even start because of the toll chemoradiation took on their bodies, he said.
Induction chemotherapy is an alternative, but patients seem to derive more benefit form adjuvant therapy after chemoradiation. Indeed, “given the fact that we may now have a well established, well tolerated adjuvant therapy for our patients” – capecitabine – “the role of induction therapy” is in question, Dr. Loong said.
Metronomic dosing
One trial randomized 204 patients to metronomic dosing of capecitabine, 650 mg/m2 twice daily for 1 year within 12 to 16 weeks after the last radiation, and 202 others to observation.
The idea of metronomic dosing was to give a lower dose of the agent over a longer period to reduce side effects and perhaps improve efficacy.
Subjects had high-risk stage III to IVA disease with no locoregional disease or distant metastasis after chemoradiotherapy. Almost 80% in both arms also had induction chemotherapy, most commonly docetaxel and cisplatin.
Almost three-quarters of people in the treatment arm complied with capecitabine for an entire year.
At a median follow-up of 38 months, 3-year failure-free survival was 85.3% with metronomic capecitabine vs. 75.7% with observation (hazard ratio, .50, P = .002). Three-year overall survival was 93.3% with capecitabine and 88.6% with observation (HR .44, P = .018).
The incidence of grade 3/4 adverse events was 17.4% with metronomic capecitabine vs. 5.5% with observation. Hand-foot syndrome was the most common complication, occurring in 9% of capecitabine subjects. There were no treatment-related deaths, and there was no clinically meaningful deterioration in quality of life associated with treatment.
The approach “significantly improved” outcomes, “with a manageable safety profile and no compromise to quality of life ... Metronomic adjuvant capecitabine can be an option for first-line treatment in this high-risk” group, concluded investigators led by Jun Ma, MD, professor of radiation oncology and deputy president of Sun Yat-sen University Cancer Center in Guangzhou, China.
Standard dosing
The second trial used standard dosing. The researchers randomized 90 subjects to capecitabine 1,000 mg/m2 twice daily for 14 days in eight 21-day cycles after chemoradiation; 90 subjects were randomized to observation. None of the subjects received induction chemotherapy.
Patients had stage III-IVb disease plus at least one high-risk feature, such as high Epstein Barr virus DNA titers. The majority of patients in both arms had three or more high risk features.
Over 80% of capecitabine subjects completed all eight cycles of treatment.
At a median follow-up of 44.8 months, 3-year failure-free survival was 87.7% with capecitabine vs. 73.3% in the control arm (HR .52, P = .037). Three-year overall survival was 92.6% with capecitabine vs. 88.9% with observation, which wasn’t statistically significant.
The incidence of acute grade 3/4 adverse events was 57.8% with standard dose capecitabine. Rates of hand foot syndrome, mucositis, anemia, and other problems were substantially higher than with observation.
For high-risk patients unable to tolerate chemotherapy, adjuvant capecitabine is a “suitable alternative treatment option,” said lead investigator Jingjing Miao, MD, of the department of nasopharyngeal carcinoma at Sun Yat-sen University Cancer Center, Guangzhou, China.
The trials were funded by Sun Yat-sen University and others. The investigators had no disclosures. Dr. Loong is an adviser, speaker, and/or researcher for a number of companies, including Novartis, Pfizer, and Lilly.
Adjuvant capecitabine is an effective and better tolerated alternative to chemotherapy following chemoradiation for locoregionally advanced nasopharyngeal carcinoma, according to two phase 3 trials from China.
Despite using different dosing strategies, 3-year failure-free survival approached 90% in both trials, comparable to the standard approach with adjuvant platinum doublets, but with better compliance.
The study teams were looking for “a way to make adjuvant therapy more tolerable,” said oncologist Herbert Loong, MBBS, clinical associate professor at the Chinese University of Hong Kong, who discussed both trials after they were presented at the American Society for Clinical Oncology annual meeting.
Compliance is low with platinum doublets because of the toxicity. About 60%-70% of patients can tolerate 2-3 cycles after chemoradiation, and about a third can’t even start because of the toll chemoradiation took on their bodies, he said.
Induction chemotherapy is an alternative, but patients seem to derive more benefit form adjuvant therapy after chemoradiation. Indeed, “given the fact that we may now have a well established, well tolerated adjuvant therapy for our patients” – capecitabine – “the role of induction therapy” is in question, Dr. Loong said.
Metronomic dosing
One trial randomized 204 patients to metronomic dosing of capecitabine, 650 mg/m2 twice daily for 1 year within 12 to 16 weeks after the last radiation, and 202 others to observation.
The idea of metronomic dosing was to give a lower dose of the agent over a longer period to reduce side effects and perhaps improve efficacy.
Subjects had high-risk stage III to IVA disease with no locoregional disease or distant metastasis after chemoradiotherapy. Almost 80% in both arms also had induction chemotherapy, most commonly docetaxel and cisplatin.
Almost three-quarters of people in the treatment arm complied with capecitabine for an entire year.
At a median follow-up of 38 months, 3-year failure-free survival was 85.3% with metronomic capecitabine vs. 75.7% with observation (hazard ratio, .50, P = .002). Three-year overall survival was 93.3% with capecitabine and 88.6% with observation (HR .44, P = .018).
The incidence of grade 3/4 adverse events was 17.4% with metronomic capecitabine vs. 5.5% with observation. Hand-foot syndrome was the most common complication, occurring in 9% of capecitabine subjects. There were no treatment-related deaths, and there was no clinically meaningful deterioration in quality of life associated with treatment.
The approach “significantly improved” outcomes, “with a manageable safety profile and no compromise to quality of life ... Metronomic adjuvant capecitabine can be an option for first-line treatment in this high-risk” group, concluded investigators led by Jun Ma, MD, professor of radiation oncology and deputy president of Sun Yat-sen University Cancer Center in Guangzhou, China.
Standard dosing
The second trial used standard dosing. The researchers randomized 90 subjects to capecitabine 1,000 mg/m2 twice daily for 14 days in eight 21-day cycles after chemoradiation; 90 subjects were randomized to observation. None of the subjects received induction chemotherapy.
Patients had stage III-IVb disease plus at least one high-risk feature, such as high Epstein Barr virus DNA titers. The majority of patients in both arms had three or more high risk features.
Over 80% of capecitabine subjects completed all eight cycles of treatment.
At a median follow-up of 44.8 months, 3-year failure-free survival was 87.7% with capecitabine vs. 73.3% in the control arm (HR .52, P = .037). Three-year overall survival was 92.6% with capecitabine vs. 88.9% with observation, which wasn’t statistically significant.
The incidence of acute grade 3/4 adverse events was 57.8% with standard dose capecitabine. Rates of hand foot syndrome, mucositis, anemia, and other problems were substantially higher than with observation.
For high-risk patients unable to tolerate chemotherapy, adjuvant capecitabine is a “suitable alternative treatment option,” said lead investigator Jingjing Miao, MD, of the department of nasopharyngeal carcinoma at Sun Yat-sen University Cancer Center, Guangzhou, China.
The trials were funded by Sun Yat-sen University and others. The investigators had no disclosures. Dr. Loong is an adviser, speaker, and/or researcher for a number of companies, including Novartis, Pfizer, and Lilly.
Adjuvant capecitabine is an effective and better tolerated alternative to chemotherapy following chemoradiation for locoregionally advanced nasopharyngeal carcinoma, according to two phase 3 trials from China.
Despite using different dosing strategies, 3-year failure-free survival approached 90% in both trials, comparable to the standard approach with adjuvant platinum doublets, but with better compliance.
The study teams were looking for “a way to make adjuvant therapy more tolerable,” said oncologist Herbert Loong, MBBS, clinical associate professor at the Chinese University of Hong Kong, who discussed both trials after they were presented at the American Society for Clinical Oncology annual meeting.
Compliance is low with platinum doublets because of the toxicity. About 60%-70% of patients can tolerate 2-3 cycles after chemoradiation, and about a third can’t even start because of the toll chemoradiation took on their bodies, he said.
Induction chemotherapy is an alternative, but patients seem to derive more benefit form adjuvant therapy after chemoradiation. Indeed, “given the fact that we may now have a well established, well tolerated adjuvant therapy for our patients” – capecitabine – “the role of induction therapy” is in question, Dr. Loong said.
Metronomic dosing
One trial randomized 204 patients to metronomic dosing of capecitabine, 650 mg/m2 twice daily for 1 year within 12 to 16 weeks after the last radiation, and 202 others to observation.
The idea of metronomic dosing was to give a lower dose of the agent over a longer period to reduce side effects and perhaps improve efficacy.
Subjects had high-risk stage III to IVA disease with no locoregional disease or distant metastasis after chemoradiotherapy. Almost 80% in both arms also had induction chemotherapy, most commonly docetaxel and cisplatin.
Almost three-quarters of people in the treatment arm complied with capecitabine for an entire year.
At a median follow-up of 38 months, 3-year failure-free survival was 85.3% with metronomic capecitabine vs. 75.7% with observation (hazard ratio, .50, P = .002). Three-year overall survival was 93.3% with capecitabine and 88.6% with observation (HR .44, P = .018).
The incidence of grade 3/4 adverse events was 17.4% with metronomic capecitabine vs. 5.5% with observation. Hand-foot syndrome was the most common complication, occurring in 9% of capecitabine subjects. There were no treatment-related deaths, and there was no clinically meaningful deterioration in quality of life associated with treatment.
The approach “significantly improved” outcomes, “with a manageable safety profile and no compromise to quality of life ... Metronomic adjuvant capecitabine can be an option for first-line treatment in this high-risk” group, concluded investigators led by Jun Ma, MD, professor of radiation oncology and deputy president of Sun Yat-sen University Cancer Center in Guangzhou, China.
Standard dosing
The second trial used standard dosing. The researchers randomized 90 subjects to capecitabine 1,000 mg/m2 twice daily for 14 days in eight 21-day cycles after chemoradiation; 90 subjects were randomized to observation. None of the subjects received induction chemotherapy.
Patients had stage III-IVb disease plus at least one high-risk feature, such as high Epstein Barr virus DNA titers. The majority of patients in both arms had three or more high risk features.
Over 80% of capecitabine subjects completed all eight cycles of treatment.
At a median follow-up of 44.8 months, 3-year failure-free survival was 87.7% with capecitabine vs. 73.3% in the control arm (HR .52, P = .037). Three-year overall survival was 92.6% with capecitabine vs. 88.9% with observation, which wasn’t statistically significant.
The incidence of acute grade 3/4 adverse events was 57.8% with standard dose capecitabine. Rates of hand foot syndrome, mucositis, anemia, and other problems were substantially higher than with observation.
For high-risk patients unable to tolerate chemotherapy, adjuvant capecitabine is a “suitable alternative treatment option,” said lead investigator Jingjing Miao, MD, of the department of nasopharyngeal carcinoma at Sun Yat-sen University Cancer Center, Guangzhou, China.
The trials were funded by Sun Yat-sen University and others. The investigators had no disclosures. Dr. Loong is an adviser, speaker, and/or researcher for a number of companies, including Novartis, Pfizer, and Lilly.
FROM ASCO 2021
Cabozantinib gains ground for salvage in differentiated thyroid cancer
The vascular endothelial growth factor receptor 2 blocker cabozantinib prolonged progression-free survival in radioiodine-refractory differentiated thyroid cancer following progression on first-line VEGFR inhibitors in a phase 3 trial from cabozantinib maker, Exelixis.
After a median follow up of 6.2 months, the 125 subjects on cabozantinib 60 mg every day had not reached median progression-free survival, but among the 62 randomized to placebo, mPFS was only 1.9 months.
The results led the Food and Drug Administration to grant cabozantinib breakthrough therapy status for salvage after progression on lenvatinib or sorafenib, the two VEGFR inhibitors approved for first-line treatment in radioiodine refractory differentiated thyroid cancer. Cabozantinib already carries a first-line indication for progressive, metastatic medullary thyroid cancer.
“We were all so excited to have sorafenib and lenvatinib,” but patients need another option after they develop resistance. “Cabozantinib is positioned to be the next in line,” said lead investigator Marcia Brose, MD, PhD, a thyroid specialist and professor at the University of Pennsylvania, Philadelphia, who presented the findings at the American Society of Clinical Oncology Annual Meeting.
The trial discussant, medical oncologist Nicole Chau, MD, clinical associate professor at the University of British Columbia, Vancouver, said the results “support cabozantinib as a potential second- or third-line” option, but its impact on quality of life and financial toxicity “should be evaluated.”
Subjects in the trial – dubbed COSMIC-311 – had locally advanced or metastatic disease that had progressed during or after first-line VEGFR treatment, including about 40% with lenvatinib, almost 40% with sorafenib, and over 20% with both.
The median age in the study was 66 years, and 55% of the subjects were women. Bone and liver metastases were more common in the cabozantinib arm.
Promising results
The robust of mPFS benefit (hazard ratio, 0.22; P < .0001) held across subgroups. The mPFS in the placebo arm of 1.9 months demonstrates that “these patients [progress rapidly], so you have to be ready with the next thing in line” to start it quickly, Dr. Brose said.
Overall survival favored cabozantinib (HR, 0.54) but didn’t reach statistical significance perhaps because placebo patients were allowed to cross over to cabozantinib after progression.
The overall response rate was 15% with cabozantinib versus no responders with placebo, which also didn’t meet the study’s criteria for clinical significance. Even so, the disease control rate of 60% with cabozantinib versus 27% with placebo, “is clinically meaningful in this heavily pretreated population,” Dr. Chau said.
Adverse events
Safety was consistent with previous reports and included diarrhea in 51% of cabozantinib subjects, hand-foot skin reaction in 46%, hypertension in 28%, fatigue in 27%, and nausea in 24%, all substantially higher than with placebo. Over half of cabozantinib subjects had grade 3-4 adverse events versus 26% on placebo. There were no treatment related deaths.
Adverse events led to dose reductions or holds in the majority of cabozantinib subjects, but only 5% discontinued the drug; the number might have been higher with longer follow-up, Dr. Chau said.
Genotype-targeted therapy is an option for patients with fusion alterations, but whether it should come before or after VEGFR inhibition is unclear, Dr. Brose noted.
Biomarkers to help with such treatment decisions will become “increasingly important as we move beyond the era of single-agent” VEGFR inhibitors, Dr. Chau said.
The study was funded by cabozantinib maker Exelixis, and three investigators were employees. Dr. Brose disclosed research funding and honoraria from the company, and is an adviser. Dr. Chau has no involvement with Exelixis.
The vascular endothelial growth factor receptor 2 blocker cabozantinib prolonged progression-free survival in radioiodine-refractory differentiated thyroid cancer following progression on first-line VEGFR inhibitors in a phase 3 trial from cabozantinib maker, Exelixis.
After a median follow up of 6.2 months, the 125 subjects on cabozantinib 60 mg every day had not reached median progression-free survival, but among the 62 randomized to placebo, mPFS was only 1.9 months.
The results led the Food and Drug Administration to grant cabozantinib breakthrough therapy status for salvage after progression on lenvatinib or sorafenib, the two VEGFR inhibitors approved for first-line treatment in radioiodine refractory differentiated thyroid cancer. Cabozantinib already carries a first-line indication for progressive, metastatic medullary thyroid cancer.
“We were all so excited to have sorafenib and lenvatinib,” but patients need another option after they develop resistance. “Cabozantinib is positioned to be the next in line,” said lead investigator Marcia Brose, MD, PhD, a thyroid specialist and professor at the University of Pennsylvania, Philadelphia, who presented the findings at the American Society of Clinical Oncology Annual Meeting.
The trial discussant, medical oncologist Nicole Chau, MD, clinical associate professor at the University of British Columbia, Vancouver, said the results “support cabozantinib as a potential second- or third-line” option, but its impact on quality of life and financial toxicity “should be evaluated.”
Subjects in the trial – dubbed COSMIC-311 – had locally advanced or metastatic disease that had progressed during or after first-line VEGFR treatment, including about 40% with lenvatinib, almost 40% with sorafenib, and over 20% with both.
The median age in the study was 66 years, and 55% of the subjects were women. Bone and liver metastases were more common in the cabozantinib arm.
Promising results
The robust of mPFS benefit (hazard ratio, 0.22; P < .0001) held across subgroups. The mPFS in the placebo arm of 1.9 months demonstrates that “these patients [progress rapidly], so you have to be ready with the next thing in line” to start it quickly, Dr. Brose said.
Overall survival favored cabozantinib (HR, 0.54) but didn’t reach statistical significance perhaps because placebo patients were allowed to cross over to cabozantinib after progression.
The overall response rate was 15% with cabozantinib versus no responders with placebo, which also didn’t meet the study’s criteria for clinical significance. Even so, the disease control rate of 60% with cabozantinib versus 27% with placebo, “is clinically meaningful in this heavily pretreated population,” Dr. Chau said.
Adverse events
Safety was consistent with previous reports and included diarrhea in 51% of cabozantinib subjects, hand-foot skin reaction in 46%, hypertension in 28%, fatigue in 27%, and nausea in 24%, all substantially higher than with placebo. Over half of cabozantinib subjects had grade 3-4 adverse events versus 26% on placebo. There were no treatment related deaths.
Adverse events led to dose reductions or holds in the majority of cabozantinib subjects, but only 5% discontinued the drug; the number might have been higher with longer follow-up, Dr. Chau said.
Genotype-targeted therapy is an option for patients with fusion alterations, but whether it should come before or after VEGFR inhibition is unclear, Dr. Brose noted.
Biomarkers to help with such treatment decisions will become “increasingly important as we move beyond the era of single-agent” VEGFR inhibitors, Dr. Chau said.
The study was funded by cabozantinib maker Exelixis, and three investigators were employees. Dr. Brose disclosed research funding and honoraria from the company, and is an adviser. Dr. Chau has no involvement with Exelixis.
The vascular endothelial growth factor receptor 2 blocker cabozantinib prolonged progression-free survival in radioiodine-refractory differentiated thyroid cancer following progression on first-line VEGFR inhibitors in a phase 3 trial from cabozantinib maker, Exelixis.
After a median follow up of 6.2 months, the 125 subjects on cabozantinib 60 mg every day had not reached median progression-free survival, but among the 62 randomized to placebo, mPFS was only 1.9 months.
The results led the Food and Drug Administration to grant cabozantinib breakthrough therapy status for salvage after progression on lenvatinib or sorafenib, the two VEGFR inhibitors approved for first-line treatment in radioiodine refractory differentiated thyroid cancer. Cabozantinib already carries a first-line indication for progressive, metastatic medullary thyroid cancer.
“We were all so excited to have sorafenib and lenvatinib,” but patients need another option after they develop resistance. “Cabozantinib is positioned to be the next in line,” said lead investigator Marcia Brose, MD, PhD, a thyroid specialist and professor at the University of Pennsylvania, Philadelphia, who presented the findings at the American Society of Clinical Oncology Annual Meeting.
The trial discussant, medical oncologist Nicole Chau, MD, clinical associate professor at the University of British Columbia, Vancouver, said the results “support cabozantinib as a potential second- or third-line” option, but its impact on quality of life and financial toxicity “should be evaluated.”
Subjects in the trial – dubbed COSMIC-311 – had locally advanced or metastatic disease that had progressed during or after first-line VEGFR treatment, including about 40% with lenvatinib, almost 40% with sorafenib, and over 20% with both.
The median age in the study was 66 years, and 55% of the subjects were women. Bone and liver metastases were more common in the cabozantinib arm.
Promising results
The robust of mPFS benefit (hazard ratio, 0.22; P < .0001) held across subgroups. The mPFS in the placebo arm of 1.9 months demonstrates that “these patients [progress rapidly], so you have to be ready with the next thing in line” to start it quickly, Dr. Brose said.
Overall survival favored cabozantinib (HR, 0.54) but didn’t reach statistical significance perhaps because placebo patients were allowed to cross over to cabozantinib after progression.
The overall response rate was 15% with cabozantinib versus no responders with placebo, which also didn’t meet the study’s criteria for clinical significance. Even so, the disease control rate of 60% with cabozantinib versus 27% with placebo, “is clinically meaningful in this heavily pretreated population,” Dr. Chau said.
Adverse events
Safety was consistent with previous reports and included diarrhea in 51% of cabozantinib subjects, hand-foot skin reaction in 46%, hypertension in 28%, fatigue in 27%, and nausea in 24%, all substantially higher than with placebo. Over half of cabozantinib subjects had grade 3-4 adverse events versus 26% on placebo. There were no treatment related deaths.
Adverse events led to dose reductions or holds in the majority of cabozantinib subjects, but only 5% discontinued the drug; the number might have been higher with longer follow-up, Dr. Chau said.
Genotype-targeted therapy is an option for patients with fusion alterations, but whether it should come before or after VEGFR inhibition is unclear, Dr. Brose noted.
Biomarkers to help with such treatment decisions will become “increasingly important as we move beyond the era of single-agent” VEGFR inhibitors, Dr. Chau said.
The study was funded by cabozantinib maker Exelixis, and three investigators were employees. Dr. Brose disclosed research funding and honoraria from the company, and is an adviser. Dr. Chau has no involvement with Exelixis.
FROM ASCO 2021
New drug toripalimab improves survival in nasopharyngeal cancer
A new immunotherapy, toripalimab, has the potential to change practice in the treatment of nasopharyngeal carcinoma (NPC), say experts.
The drug is a monoclonal antibody that blocks programmed cell death protein 1 (PD-1), developed in China and recently approved there for the third-line treatment of NPC, among other indications. The U.S. Food and Drug Administration has granted it a breakthrough therapy designation for recurrent/metastatic NPC, as well as fast-track and orphan drug status for other tumor types.
New results show that when toripalimab was added onto chemotherapy with gemcitabine and cisplatin in the first line for recurrent or metastatic nasopharyngeal carcinoma, there was a significant improvement in both progression-free survival and overall survival.
The results come from the phase 3 trial dubbed JUPITER-02 and will be presented at the plenary session of the American Society of Clinical Oncology annual meeting this Sunday; some details were released earlier at a press briefing
The trial randomly assigned 146 patients to toripalimab and 143 to placebo on a background of gemcitabine and cisplatin, the current standard of care for recurrent/metastatic NPC.
Median progression-free survival (PFS) was 11.7 months with toripalimab vs. 8 months with placebo, a significant improvement (hazard ratio, 0.52; 95% confidence interval, 0.36-0.74. P = .0003). Overall survival was not mature at reporting but favored toripalimab with 25 deaths versus 39 in the placebo group, a 40% risk reduction (P = .0462).
The results “support the use of toripalimab in combination with [gemcitabine and cisplatin] as a new standard of care for first-line treatment of recurrent or metastatic nasopharyngeal carcinoma,” said lead investigator and medical oncologist Rui-Hua Xu, MD, PhD, of the Sun Yat-sen University Cancer Center in Guangzhou, China.
Potential to change practice
The significance of the study is that it used immunotherapy in the first-line setting for NPC instead of the second line where it’s frequently used today, commented Jared Weiss, MD, an associate professor of oncology and a head and neck cancer specialist at the University of North Carolina, Chapel Hill.
If FDA approves toripalimab for the indication, it “would change [first-line] standard of care to the triplet regimen,” he said in an interview.
The discussant for this presentation, Julie Gralow, MD, agreed. “This is one of the first studies in metastatic or recurrent NPC to show a benefit” for combining a PD-1 inhibitor with chemotherapy.
“With FDA approval, these findings should prove practice-changing,” said Dr. Gralow, a professor of breast medical oncology at the University of Washington, Seattle, and ASCO’s chief medical officer.
Toripalimab, dosed at 240 mg in the trial, or placebo were administered with gemcitabine and cisplatin every 3 weeks for up to 6 cycles, followed by toripalimab or placebo maintenance every 3 weeks until disease progression, intolerable toxicity, or completion of 2 years of treatment.
The overall response rate was 77.4% with toripalimab and 66.4% with placebo, and the median duration of response in the toripalimab group was 10 months vs. 5.7 months with placebo.
One-year PFS was 49.4% with toripalimab versus 27.9% with placebo; improved PFS was observed with toripalimab across PD-L1 subgroups.
Grade 3 or worse adverse events occurred in slightly less than 90% of both groups, with fatal adverse events occurring in slightly less than 3% in both.
Adverse events leading to discontinuation occurred in 7.5% of the study group and 4.9% on placebo. As expected with immunotherapy, immune-related adverse events such as hypothyroidism were more common with toripalimab (39.7% vs. 18.9%), as were grade 3 or worse immune-related adverse events (7.5% vs. 0.7%).
At interim analysis in May 2020, the median duration treatment was 39 weeks in the toripalimab group and 36 weeks in the placebo group.
The trial was conducted in China, Taiwan, and Singapore.
JUPITER-02 was funded by Shanghai Junshi Bioscience. Investigator disclosures weren’t reported. Dr. Weiss said he had no relevant disclosures. Dr. Gralow is an advisor for a number of companies, including Genentech, Novartis, and Roche.
A version of this article first appeared on Medscape.com.
A new immunotherapy, toripalimab, has the potential to change practice in the treatment of nasopharyngeal carcinoma (NPC), say experts.
The drug is a monoclonal antibody that blocks programmed cell death protein 1 (PD-1), developed in China and recently approved there for the third-line treatment of NPC, among other indications. The U.S. Food and Drug Administration has granted it a breakthrough therapy designation for recurrent/metastatic NPC, as well as fast-track and orphan drug status for other tumor types.
New results show that when toripalimab was added onto chemotherapy with gemcitabine and cisplatin in the first line for recurrent or metastatic nasopharyngeal carcinoma, there was a significant improvement in both progression-free survival and overall survival.
The results come from the phase 3 trial dubbed JUPITER-02 and will be presented at the plenary session of the American Society of Clinical Oncology annual meeting this Sunday; some details were released earlier at a press briefing
The trial randomly assigned 146 patients to toripalimab and 143 to placebo on a background of gemcitabine and cisplatin, the current standard of care for recurrent/metastatic NPC.
Median progression-free survival (PFS) was 11.7 months with toripalimab vs. 8 months with placebo, a significant improvement (hazard ratio, 0.52; 95% confidence interval, 0.36-0.74. P = .0003). Overall survival was not mature at reporting but favored toripalimab with 25 deaths versus 39 in the placebo group, a 40% risk reduction (P = .0462).
The results “support the use of toripalimab in combination with [gemcitabine and cisplatin] as a new standard of care for first-line treatment of recurrent or metastatic nasopharyngeal carcinoma,” said lead investigator and medical oncologist Rui-Hua Xu, MD, PhD, of the Sun Yat-sen University Cancer Center in Guangzhou, China.
Potential to change practice
The significance of the study is that it used immunotherapy in the first-line setting for NPC instead of the second line where it’s frequently used today, commented Jared Weiss, MD, an associate professor of oncology and a head and neck cancer specialist at the University of North Carolina, Chapel Hill.
If FDA approves toripalimab for the indication, it “would change [first-line] standard of care to the triplet regimen,” he said in an interview.
The discussant for this presentation, Julie Gralow, MD, agreed. “This is one of the first studies in metastatic or recurrent NPC to show a benefit” for combining a PD-1 inhibitor with chemotherapy.
“With FDA approval, these findings should prove practice-changing,” said Dr. Gralow, a professor of breast medical oncology at the University of Washington, Seattle, and ASCO’s chief medical officer.
Toripalimab, dosed at 240 mg in the trial, or placebo were administered with gemcitabine and cisplatin every 3 weeks for up to 6 cycles, followed by toripalimab or placebo maintenance every 3 weeks until disease progression, intolerable toxicity, or completion of 2 years of treatment.
The overall response rate was 77.4% with toripalimab and 66.4% with placebo, and the median duration of response in the toripalimab group was 10 months vs. 5.7 months with placebo.
One-year PFS was 49.4% with toripalimab versus 27.9% with placebo; improved PFS was observed with toripalimab across PD-L1 subgroups.
Grade 3 or worse adverse events occurred in slightly less than 90% of both groups, with fatal adverse events occurring in slightly less than 3% in both.
Adverse events leading to discontinuation occurred in 7.5% of the study group and 4.9% on placebo. As expected with immunotherapy, immune-related adverse events such as hypothyroidism were more common with toripalimab (39.7% vs. 18.9%), as were grade 3 or worse immune-related adverse events (7.5% vs. 0.7%).
At interim analysis in May 2020, the median duration treatment was 39 weeks in the toripalimab group and 36 weeks in the placebo group.
The trial was conducted in China, Taiwan, and Singapore.
JUPITER-02 was funded by Shanghai Junshi Bioscience. Investigator disclosures weren’t reported. Dr. Weiss said he had no relevant disclosures. Dr. Gralow is an advisor for a number of companies, including Genentech, Novartis, and Roche.
A version of this article first appeared on Medscape.com.
A new immunotherapy, toripalimab, has the potential to change practice in the treatment of nasopharyngeal carcinoma (NPC), say experts.
The drug is a monoclonal antibody that blocks programmed cell death protein 1 (PD-1), developed in China and recently approved there for the third-line treatment of NPC, among other indications. The U.S. Food and Drug Administration has granted it a breakthrough therapy designation for recurrent/metastatic NPC, as well as fast-track and orphan drug status for other tumor types.
New results show that when toripalimab was added onto chemotherapy with gemcitabine and cisplatin in the first line for recurrent or metastatic nasopharyngeal carcinoma, there was a significant improvement in both progression-free survival and overall survival.
The results come from the phase 3 trial dubbed JUPITER-02 and will be presented at the plenary session of the American Society of Clinical Oncology annual meeting this Sunday; some details were released earlier at a press briefing
The trial randomly assigned 146 patients to toripalimab and 143 to placebo on a background of gemcitabine and cisplatin, the current standard of care for recurrent/metastatic NPC.
Median progression-free survival (PFS) was 11.7 months with toripalimab vs. 8 months with placebo, a significant improvement (hazard ratio, 0.52; 95% confidence interval, 0.36-0.74. P = .0003). Overall survival was not mature at reporting but favored toripalimab with 25 deaths versus 39 in the placebo group, a 40% risk reduction (P = .0462).
The results “support the use of toripalimab in combination with [gemcitabine and cisplatin] as a new standard of care for first-line treatment of recurrent or metastatic nasopharyngeal carcinoma,” said lead investigator and medical oncologist Rui-Hua Xu, MD, PhD, of the Sun Yat-sen University Cancer Center in Guangzhou, China.
Potential to change practice
The significance of the study is that it used immunotherapy in the first-line setting for NPC instead of the second line where it’s frequently used today, commented Jared Weiss, MD, an associate professor of oncology and a head and neck cancer specialist at the University of North Carolina, Chapel Hill.
If FDA approves toripalimab for the indication, it “would change [first-line] standard of care to the triplet regimen,” he said in an interview.
The discussant for this presentation, Julie Gralow, MD, agreed. “This is one of the first studies in metastatic or recurrent NPC to show a benefit” for combining a PD-1 inhibitor with chemotherapy.
“With FDA approval, these findings should prove practice-changing,” said Dr. Gralow, a professor of breast medical oncology at the University of Washington, Seattle, and ASCO’s chief medical officer.
Toripalimab, dosed at 240 mg in the trial, or placebo were administered with gemcitabine and cisplatin every 3 weeks for up to 6 cycles, followed by toripalimab or placebo maintenance every 3 weeks until disease progression, intolerable toxicity, or completion of 2 years of treatment.
The overall response rate was 77.4% with toripalimab and 66.4% with placebo, and the median duration of response in the toripalimab group was 10 months vs. 5.7 months with placebo.
One-year PFS was 49.4% with toripalimab versus 27.9% with placebo; improved PFS was observed with toripalimab across PD-L1 subgroups.
Grade 3 or worse adverse events occurred in slightly less than 90% of both groups, with fatal adverse events occurring in slightly less than 3% in both.
Adverse events leading to discontinuation occurred in 7.5% of the study group and 4.9% on placebo. As expected with immunotherapy, immune-related adverse events such as hypothyroidism were more common with toripalimab (39.7% vs. 18.9%), as were grade 3 or worse immune-related adverse events (7.5% vs. 0.7%).
At interim analysis in May 2020, the median duration treatment was 39 weeks in the toripalimab group and 36 weeks in the placebo group.
The trial was conducted in China, Taiwan, and Singapore.
JUPITER-02 was funded by Shanghai Junshi Bioscience. Investigator disclosures weren’t reported. Dr. Weiss said he had no relevant disclosures. Dr. Gralow is an advisor for a number of companies, including Genentech, Novartis, and Roche.
A version of this article first appeared on Medscape.com.
Photobiomodulation reduced acute radiodermatitis severity in head and neck cancer patients
The delivery of , according to results from the first randomized study of its kind.
“The use of light therapy-based applications for cancer therapy-related adverse events has steadily increased in the past 40 years,” lead study author Jolien Robijns, MSc, PhD, told this news organization during the annual conference of the American Society for Laser Medicine and Surgery. “The most well-known and studied indication of photobiomodulation therapy in supportive cancer care is oral mucositis,” she said, referring to a recent systematic review, which found that based on the available evidence, PBMT is an effective therapy for the prevention of oral mucositis, using well-defined PBM parameters in specific patient populations. “Various internationally well-recognized health organizations in oncology recommend PBMT to prevent and manage oral mucositis,” she added.
Based on the wound-healing and anti-inflammatory properties of PBMT, several studies have investigated its use for the prevention and management of acute radiodermatitis (ARD) since the 1990s, said Dr. Robijns, a postdoctoral researcher at Limburg Clinical Research Center in Hasselt, Belgium. Under the supervision of Jeroen Mebis, MD, PhD, at the Limburg Oncologic Laser Institute, she and her colleagues have been conducting clinical research on PBMT and ARD since 2014, with successful results. In 2020 they published a narrative review, which showed that based on nine clinical trials, PBMT could effectively reduce the incidence of severe ARD, decrease accompanying pain, and improve patients’ quality of life.
For the current study, known as the DERMISHEAD trial and published online March 9, 2021, in Radiotherapy and Oncology, investigators at Limburg Oncology Center at Jessa Hospital in Hasselt, and Hasselt University, recruited head and neck cancer patients who underwent bilateral radiotherapy with or without chemotherapy, for a total dose of 30-35 x 2 Gy . All patients received standard skin care combined with two PBMT or sham sessions twice per week during the complete course of RT, which resulted in 14 total sessions.
As described in the Radiotherapy and Oncology study, the commercially available device used for PBMT “consists of two laser diodes with different wavelengths (808-905 nm), peak powers (1.1-25 W), and emission modes (continuous and pulsed). Both diodes work simultaneously and synchronously with coincident propagation axes (average radiant power 3.3 W). The energy density (fluence) was set at 4 J/cm2 based on earlier recommendations and on our clinical experience.” A blinded study nurse used Radiation Therapy Oncology Group criteria to evaluate the skin reactions.
After 303 patients were initially assessed for eligibility, 46 patients were enrolled in DERMISHEAD (18 in the placebo group and 28 in the PBMT group). At the end of radiotherapy, 77.8% of patients in the placebo group had a grade 2 or 3 skin reaction, compared with 28.6% of patients in the PBMT group (P = .001).
“The DERMISHEAD trial proved that PBMT significantly reduces the severity of ARD,” Dr. Robijns said. “Thereby, it improves the patients’ quality of life during their radiotherapy course. The trial supports the further implementation of PBM in the supportive care of cancer patients undergoing radiotherapy.”
The results are similar to those in the TRANSDERMIS trial, in which Dr. Robijns and her colleagues used PMBT to treat breast cancer patients.
“However, an interesting difference is that the percentage decrease in severe ARD was higher in the DERMISHEAD trial than in the TRANSDERMIS trial: 49% vs. 23%, respectively,” she noted. “This difference can be rationalized because in total, more control head and neck cancer patients developed grade 3 ARD than did control breast cancer patients (17% vs. 5%). A possible explanation of this finding can be related to the difference in treatment regimens and radiotherapy parameters between the two trials.”
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Conn., who was asked to comment on the study, said that acute radiation dermatitis “can be very painful and distressing to patients, and over time, the skin changes can create long-term problems. Prevention of acute and chronic radiation dermatitis is worthwhile, particularly for patients at risk.”
This study, she added, “shows a benefit of photobiomodulation therapy as a potential preventative treatment. Notably, patients did not always follow up appropriately for the therapy, and the authors said that it is yet another thing that patients need to keep track of, in addition to their cancer therapy visits. Thus, optimally, it would be useful to have a biomarker of which patients would most benefit from treatments that prevent/potentiate radiation dermatitis.”
Dr. Robijns acknowledged certain limitations of the trial, including its small sample size and the scarcity of clinical trials on PBM and acute radiation dermatitis. “More studies are needed,” she said. “Future studies should focus on randomized controlled study designs with well-described and complete PBMT parameters in a larger and more diverse patient population. This would enable the implementation of PBM in the field of ARD and supportive cancer care, which would enhance wound care management and improve the patient’s quality of life.”
This work won a “best of clinical applications” abstract award from the ASLMS.
The research is part of the Limburg Clinical Research Center UHasselt-ZOL-Jessa, financially supported by the foundation Limburg Sterk Merk, province of Limburg, Flemish Government, Hasselt University, Ziekenhuis Oost-Limburg, and Jessa Hospital. The research is also funded by Kom op tegen Kanker (Stand up to Cancer), the Flemish Cancer Society, Limburgs Kankerfonds, and ASA Srl. Dr. Robijns reported having no financial disclosures.
The delivery of , according to results from the first randomized study of its kind.
“The use of light therapy-based applications for cancer therapy-related adverse events has steadily increased in the past 40 years,” lead study author Jolien Robijns, MSc, PhD, told this news organization during the annual conference of the American Society for Laser Medicine and Surgery. “The most well-known and studied indication of photobiomodulation therapy in supportive cancer care is oral mucositis,” she said, referring to a recent systematic review, which found that based on the available evidence, PBMT is an effective therapy for the prevention of oral mucositis, using well-defined PBM parameters in specific patient populations. “Various internationally well-recognized health organizations in oncology recommend PBMT to prevent and manage oral mucositis,” she added.
Based on the wound-healing and anti-inflammatory properties of PBMT, several studies have investigated its use for the prevention and management of acute radiodermatitis (ARD) since the 1990s, said Dr. Robijns, a postdoctoral researcher at Limburg Clinical Research Center in Hasselt, Belgium. Under the supervision of Jeroen Mebis, MD, PhD, at the Limburg Oncologic Laser Institute, she and her colleagues have been conducting clinical research on PBMT and ARD since 2014, with successful results. In 2020 they published a narrative review, which showed that based on nine clinical trials, PBMT could effectively reduce the incidence of severe ARD, decrease accompanying pain, and improve patients’ quality of life.
For the current study, known as the DERMISHEAD trial and published online March 9, 2021, in Radiotherapy and Oncology, investigators at Limburg Oncology Center at Jessa Hospital in Hasselt, and Hasselt University, recruited head and neck cancer patients who underwent bilateral radiotherapy with or without chemotherapy, for a total dose of 30-35 x 2 Gy . All patients received standard skin care combined with two PBMT or sham sessions twice per week during the complete course of RT, which resulted in 14 total sessions.
As described in the Radiotherapy and Oncology study, the commercially available device used for PBMT “consists of two laser diodes with different wavelengths (808-905 nm), peak powers (1.1-25 W), and emission modes (continuous and pulsed). Both diodes work simultaneously and synchronously with coincident propagation axes (average radiant power 3.3 W). The energy density (fluence) was set at 4 J/cm2 based on earlier recommendations and on our clinical experience.” A blinded study nurse used Radiation Therapy Oncology Group criteria to evaluate the skin reactions.
After 303 patients were initially assessed for eligibility, 46 patients were enrolled in DERMISHEAD (18 in the placebo group and 28 in the PBMT group). At the end of radiotherapy, 77.8% of patients in the placebo group had a grade 2 or 3 skin reaction, compared with 28.6% of patients in the PBMT group (P = .001).
“The DERMISHEAD trial proved that PBMT significantly reduces the severity of ARD,” Dr. Robijns said. “Thereby, it improves the patients’ quality of life during their radiotherapy course. The trial supports the further implementation of PBM in the supportive care of cancer patients undergoing radiotherapy.”
The results are similar to those in the TRANSDERMIS trial, in which Dr. Robijns and her colleagues used PMBT to treat breast cancer patients.
“However, an interesting difference is that the percentage decrease in severe ARD was higher in the DERMISHEAD trial than in the TRANSDERMIS trial: 49% vs. 23%, respectively,” she noted. “This difference can be rationalized because in total, more control head and neck cancer patients developed grade 3 ARD than did control breast cancer patients (17% vs. 5%). A possible explanation of this finding can be related to the difference in treatment regimens and radiotherapy parameters between the two trials.”
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Conn., who was asked to comment on the study, said that acute radiation dermatitis “can be very painful and distressing to patients, and over time, the skin changes can create long-term problems. Prevention of acute and chronic radiation dermatitis is worthwhile, particularly for patients at risk.”
This study, she added, “shows a benefit of photobiomodulation therapy as a potential preventative treatment. Notably, patients did not always follow up appropriately for the therapy, and the authors said that it is yet another thing that patients need to keep track of, in addition to their cancer therapy visits. Thus, optimally, it would be useful to have a biomarker of which patients would most benefit from treatments that prevent/potentiate radiation dermatitis.”
Dr. Robijns acknowledged certain limitations of the trial, including its small sample size and the scarcity of clinical trials on PBM and acute radiation dermatitis. “More studies are needed,” she said. “Future studies should focus on randomized controlled study designs with well-described and complete PBMT parameters in a larger and more diverse patient population. This would enable the implementation of PBM in the field of ARD and supportive cancer care, which would enhance wound care management and improve the patient’s quality of life.”
This work won a “best of clinical applications” abstract award from the ASLMS.
The research is part of the Limburg Clinical Research Center UHasselt-ZOL-Jessa, financially supported by the foundation Limburg Sterk Merk, province of Limburg, Flemish Government, Hasselt University, Ziekenhuis Oost-Limburg, and Jessa Hospital. The research is also funded by Kom op tegen Kanker (Stand up to Cancer), the Flemish Cancer Society, Limburgs Kankerfonds, and ASA Srl. Dr. Robijns reported having no financial disclosures.
The delivery of , according to results from the first randomized study of its kind.
“The use of light therapy-based applications for cancer therapy-related adverse events has steadily increased in the past 40 years,” lead study author Jolien Robijns, MSc, PhD, told this news organization during the annual conference of the American Society for Laser Medicine and Surgery. “The most well-known and studied indication of photobiomodulation therapy in supportive cancer care is oral mucositis,” she said, referring to a recent systematic review, which found that based on the available evidence, PBMT is an effective therapy for the prevention of oral mucositis, using well-defined PBM parameters in specific patient populations. “Various internationally well-recognized health organizations in oncology recommend PBMT to prevent and manage oral mucositis,” she added.
Based on the wound-healing and anti-inflammatory properties of PBMT, several studies have investigated its use for the prevention and management of acute radiodermatitis (ARD) since the 1990s, said Dr. Robijns, a postdoctoral researcher at Limburg Clinical Research Center in Hasselt, Belgium. Under the supervision of Jeroen Mebis, MD, PhD, at the Limburg Oncologic Laser Institute, she and her colleagues have been conducting clinical research on PBMT and ARD since 2014, with successful results. In 2020 they published a narrative review, which showed that based on nine clinical trials, PBMT could effectively reduce the incidence of severe ARD, decrease accompanying pain, and improve patients’ quality of life.
For the current study, known as the DERMISHEAD trial and published online March 9, 2021, in Radiotherapy and Oncology, investigators at Limburg Oncology Center at Jessa Hospital in Hasselt, and Hasselt University, recruited head and neck cancer patients who underwent bilateral radiotherapy with or without chemotherapy, for a total dose of 30-35 x 2 Gy . All patients received standard skin care combined with two PBMT or sham sessions twice per week during the complete course of RT, which resulted in 14 total sessions.
As described in the Radiotherapy and Oncology study, the commercially available device used for PBMT “consists of two laser diodes with different wavelengths (808-905 nm), peak powers (1.1-25 W), and emission modes (continuous and pulsed). Both diodes work simultaneously and synchronously with coincident propagation axes (average radiant power 3.3 W). The energy density (fluence) was set at 4 J/cm2 based on earlier recommendations and on our clinical experience.” A blinded study nurse used Radiation Therapy Oncology Group criteria to evaluate the skin reactions.
After 303 patients were initially assessed for eligibility, 46 patients were enrolled in DERMISHEAD (18 in the placebo group and 28 in the PBMT group). At the end of radiotherapy, 77.8% of patients in the placebo group had a grade 2 or 3 skin reaction, compared with 28.6% of patients in the PBMT group (P = .001).
“The DERMISHEAD trial proved that PBMT significantly reduces the severity of ARD,” Dr. Robijns said. “Thereby, it improves the patients’ quality of life during their radiotherapy course. The trial supports the further implementation of PBM in the supportive care of cancer patients undergoing radiotherapy.”
The results are similar to those in the TRANSDERMIS trial, in which Dr. Robijns and her colleagues used PMBT to treat breast cancer patients.
“However, an interesting difference is that the percentage decrease in severe ARD was higher in the DERMISHEAD trial than in the TRANSDERMIS trial: 49% vs. 23%, respectively,” she noted. “This difference can be rationalized because in total, more control head and neck cancer patients developed grade 3 ARD than did control breast cancer patients (17% vs. 5%). A possible explanation of this finding can be related to the difference in treatment regimens and radiotherapy parameters between the two trials.”
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Conn., who was asked to comment on the study, said that acute radiation dermatitis “can be very painful and distressing to patients, and over time, the skin changes can create long-term problems. Prevention of acute and chronic radiation dermatitis is worthwhile, particularly for patients at risk.”
This study, she added, “shows a benefit of photobiomodulation therapy as a potential preventative treatment. Notably, patients did not always follow up appropriately for the therapy, and the authors said that it is yet another thing that patients need to keep track of, in addition to their cancer therapy visits. Thus, optimally, it would be useful to have a biomarker of which patients would most benefit from treatments that prevent/potentiate radiation dermatitis.”
Dr. Robijns acknowledged certain limitations of the trial, including its small sample size and the scarcity of clinical trials on PBM and acute radiation dermatitis. “More studies are needed,” she said. “Future studies should focus on randomized controlled study designs with well-described and complete PBMT parameters in a larger and more diverse patient population. This would enable the implementation of PBM in the field of ARD and supportive cancer care, which would enhance wound care management and improve the patient’s quality of life.”
This work won a “best of clinical applications” abstract award from the ASLMS.
The research is part of the Limburg Clinical Research Center UHasselt-ZOL-Jessa, financially supported by the foundation Limburg Sterk Merk, province of Limburg, Flemish Government, Hasselt University, Ziekenhuis Oost-Limburg, and Jessa Hospital. The research is also funded by Kom op tegen Kanker (Stand up to Cancer), the Flemish Cancer Society, Limburgs Kankerfonds, and ASA Srl. Dr. Robijns reported having no financial disclosures.
FROM ASLMS 2021
Impact of an Oral Antineoplastic Renewal Clinic on Medication Possession Ratio and Cost-Savings
Evaluation of oral antineoplastic agent (OAN) adherence patterns have identified correlations between nonadherence or over-adherence and poorer disease-related outcomes. Multiple studies have focused on imatinib use in chronic myeloid leukemia (CML) due to its continuous, long-term use. A study by Ganesan and colleagues found that nonadherence to imatinib showed a significant decrease in 5-year event-free survival between 76.7% of adherent participants compared with 59.8% of nonadherent participants.1 This study found that 44% of patients who were adherent to imatinib achieved complete cytogenetic response vs only 26% of patients who were nonadherent. In another study of imatinib for CML, major molecular response (MMR) was strongly correlated with adherence and no patients with adherence < 80% were able to achieve MMR.2 Similarly, in studies of tamoxifen for breast cancer, < 80% adherence resulted in a 10% decrease in survival when compared to those who were more adherent.3,4
In addition to the clinical implications of nonadherence, there can be a significant cost associated with suboptimal use of these medications. The price of a single dose of OAN medication may cost as much as $440.5
The benefits of multidisciplinary care teams have been identified in many studies.6,7 While studies are limited in oncology, pharmacists provide vital contributions to the oncology multidisciplinary team when managing OANs as these health care professionals have expert knowledge of the medications, potential adverse events (AEs), and necessary monitoring parameters.8 In one study, patients seen by the pharmacist-led oral chemotherapy management program experienced improved clinical outcomes and response to therapy when compared with preintervention patients (early molecular response, 88.9% vs 54.8%, P = .01; major molecular response, 83.3% vs 57.6%, P = .06).9 During the study, 318 AEs were reported, leading to 235 pharmacist interventions to ameliorate AEs and improve adherence.
The primary objective of this study was to measure the impact of a pharmacist-driven OAN renewal clinic on medication adherence. The secondary objective was to estimate cost-savings of this new service.
Methods
Prior to July 2014, several limitations were identified related to OAN prescribing and monitoring at the Richard L. Roudebush Veterans Affairs Medical Center in Indianapolis, Indiana (RLRVAMC). The prescription ordering process relied primarily on the patient to initiate refills, rather than the prescriber OAN prescriptions also lacked consistency for number of refills or quantities dispensed. Furthermore, ordering of antineoplastic products was not limited to hematology/oncology providers. Patients were identified with significant supply on hand at the time of medication discontinuation, creating concerns for medication waste, tolerability, and nonadherence.
As a result, opportunities were identified to improve the prescribing process, recommended monitoring, toxicity and tolerability evaluation, medication reconciliation, and medication adherence. In July of 2014, the RLRVAMC adopted a new chemotherapy order entry system capable of restricting prescriptions to hematology/oncology providers and limiting dispensed quantities and refill amounts. A comprehensive pharmacist driven OAN renewal clinic was implemented on September 1, 2014 with the goal of improving long-term adherence and tolerability, in addition to minimizing medication waste.
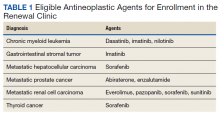
Patients were eligible for enrollment in the clinic if they had a cancer diagnosis and were concomitantly prescribed an OAN outlined in Table 1. All eligible patients were automatically enrolled in the clinic when they were deemed stable on their OAN by a hematology/oncology pharmacy specialist. Stability was defined as ≤ Grade 1 symptoms associated with the toxicities of OAN therapy managed with or without intervention as defined by the Common Terminology Criteria for Adverse Events (CTCAE) version 4.03. Once enrolled in the renewal clinic, patients were called by an oncology pharmacy resident (PGY2) 1 week prior to any OAN refill due date. Patients were asked a series of 5 adherence and tolerability questions (Table 2) to evaluate renewal criteria for approval or need for further evaluation. These questions were developed based on targeted information and published reports on monitoring adherence.10,11 Criteria for renewal included: < 10% self-reported missed doses of the OAN during the previous dispensing period, no hospitalizations or emergency department visits since most recent hematology/oncology provider appointment, no changes to concomitant medication therapies, and no new or worsening medication-related AEs. Patients meeting all criteria were given a 30-day supply of OAN. Prescribing, dispensing, and delivery of OAN were facilitated by the pharmacist. Patient cases that did not meet criteria for renewal were escalated to the hematology/oncology provider or oncology clinical pharmacy specialist for further evaluation.
Study Design and Setting
This was a pre/post retrospective cohort, quality improvement study of patients enrolled in the RLRVAMC OAN pharmacist renewal clinic. The study was deemed exempt from institutional review board (IRB) by the US Department of Veterans Affairs (VA) Research and Development Department.
Study Population
Patients were included in the preimplementation group if they had received at least 2 prescriptions of an eligible OAN. Therapy for the preimplementation group was required to be a monthly duration > 21 days and between the dates of September 1, 2013 and August 31, 2014. Patients were included in the postimplementation group if they had received at least 2 prescriptions of the studied OANs between September 1, 2014 and January 31, 2015. Patients were excluded if they had filled < 2 prescriptions of OAN; were managed by a non-VA oncologist or hematologist; or received an OAN other than those listed in Table 1.
Data Collection
For all patients in both the pre- and postimplementation cohorts, a standardized data collection tool was used to collect the following via electronic health record review by a PGY2 oncology resident: age, race, gender, oral antineoplastic agent, refill dates, days’ supply, estimated unit cost per dose cancer diagnosis, distance from the RLRVAMC, copay status, presence of hospitalizations/ED visits/dosage reductions, discontinuation rates, reasons for discontinuation, and total number of current prescriptions. The presence or absence of dosage reductions were collected to identify concerns for tolerability, but only the original dose for the preimplementation group and dosage at time of clinic enrollment for the postimplementation group was included in the analysis.
Outcomes and Statistical Analyses
The primary outcome was medication adherence defined as the median medication possession ratio (MPR) before and after implementation of the clinic. Secondary outcomes included the proportion of patients who were adherent from before implementation to after implementation and estimated cost-savings of this clinic after implementation. MPR was used to estimate medication adherence by taking the cumulative day supply of medication on hand divided by the number of days on therapy.12 Number of days on therapy was determined by taking the difference on the start date of the new medication regimen and the discontinuation date of the same regimen. Patients were grouped by adherence into one of the following categories: < 0.8, 0.8 to 0.89, 0.9 to 1, and > 1.1. Patients were considered adherent if they reported taking ≥ 90% (MPR ≥ 0.9) of prescribed doses, adopted from the study by Anderson and colleagues.12 A patient with an MPR > 1, likely due to filling prior to the anticipated refill date, was considered 100% adherent (MPR = 1). If a patient switched OAN during the study, both agents were included as separate entities.
A conservative estimate of cost-savings was made by multiplying the RLRVAMC cost per unit of medication at time of initial prescription fill by the number of units taken each day multiplied by the total days’ supply on hand at time of therapy discontinuation. Patients with an MPR < 1 at time of therapy discontinuation were assumed to have zero remaining units on hand and zero cost savings was estimated. Waste, for purposes of cost-savings, was calculated for all MPR values > 1. Additional supply anticipated to be on hand from dose reductions was not included in the estimated cost of unused medication.
Descriptive statistics compared demographic characteristics between the pre- and postimplementation groups. MPR data were not normally distributed, which required the use of nonparametric Mann-Whitney U tests to compare pre- and postMPRs. Pearson χ2 compared the proportion of adherent patients between groups while descriptive statistics were used to estimate cost savings. Significance was determined based on a P value < .05. IBM SPSS Statistics software was used for all statistical analyses. As this was a complete sample of all eligible subjects, no sample size calculation was performed.
Results
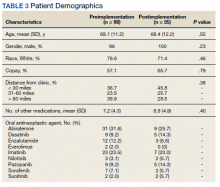
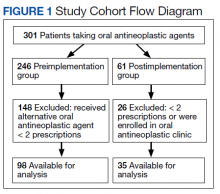
In the preimplementation period, 246 patients received an OAN and 61 patients received an OAN in the postimplementation period (Figure 1). Of the 246 patients in the preimplementation period, 98 were eligible and included in the preimplementation group. Similarly, of the 61 patients in the postimplementation period, 35 patients met inclusion criteria for the postimplementation group. The study population was predominantly male with an average age of approximately 70 years in both groups (Table 3). More than 70% of the population in each group was White. No statistically significant differences between groups were identified. The most commonly prescribed OAN in the preimplementation group were abiraterone, imatinib, and enzalutamide (Table 3). In the postimplementation group, the most commonly prescribed agents were abiraterone, imatinib, pazopanib, and dasatinib. No significant differences were observed in prescribing of individual agents between the pre- and postimplementation groups or other characteristics that may affect adherence including patient copay status, number of concomitant medications, and driving distance from the RLRVAMC.
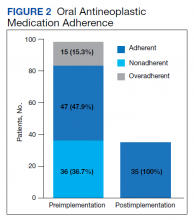
Thirty-six (36.7%) patients in the preimplementation group were considered nonadherent (MPR < 0.9) and 18 (18.4%) had an MPR < 0.8. Fifteen (15.3%) patients in the preimplementation clinic were considered overadherent (MPR > 1.1). Forty-seven (47.9%) patients in the preimplementation group were considered adherent (MPR 0.9 - 1.1) while all 35 (100%) patients in the postimplementation group were considered adherent (MPR 0.9 - 1.1). No non- or overadherent patients were identified in the postimplementation group (Figure 2). The median MPR for all patients in the preimplementation group was 0.94 compared with 1.06 (P < .001) in the postimplementation group.
Thirty-five (35.7%) patients had therapy discontinued or held in the preimplementation group compared with 2 (5.7%) patients in the postimplementation group (P < .001). Reasons for discontinuation in the preimplementation group included disease progression (n = 27), death (n = 3), lost to follow up (n = 2), and intolerability of therapy (n = 3). Both patients that discontinued therapy in the postimplementation group did so due to disease progression. Of the 35 patients who had their OAN discontinued or held in the preimplementation group, 14 patients had excess supply on hand at time of discontinuation. The estimated value of the unused medication was $37,890. Nine (25%) of the 35 patients who discontinued therapy had a dosage reduction during the course of therapy and the additional supply was not included in the cost estimate. Similarly, 1 of the 2 patients in the postimplementation group had their OAN discontinued during study. The cost of oversupply of medication at the time of therapy discontinuation was estimated at $1,555. No patients in the postimplementation group had dose reductions. After implementation of the OAN renewal clinic, the total cost savings between pre ($37,890) and postimplementation ($1,555) groups was $36,355.
Discussion
OANs are widely used therapies, with more than 25 million doses administered per year in the United States alone.12 The use of these agents will continue to grow as more targeted agents become available and patients request more convenient treatment options. The role for hematology/oncology clinical pharmacy services must adapt to this increased usage of OANs, including increasing pharmacist involvement in medication education, adherence and tolerability assessments, and proactive drug interaction monitoring.However, additional research is needed to determine optimal management strategies.
Our study aimed to compare OAN adherence among patients at a tertiary care VA hospital before and after implementation of a renewal clinic. The preimplementation population had a median MPR of 0.94 compared with 1.06 in the postimplementation group (P < .001). Although an ideal MPR is 1.0, we aimed for a slightly higher MPR to allow a supply buffer in the event of prescription delivery delays, as more than 90% of prescriptions are mailed to patients from a regional mail-order pharmacy. Importantly, the median MPRs do not adequately convey the impact from this clinic. The proportion of patients who were considered adherent to OANs increased from 47.9% in the preimplementation to 100% in the postimplementation period. These finding suggest that the clinical pharmacist role to assess and encourage adherence through monitoring tolerability of these OANs improved the overall medication taking experience of these patients.
Upon initial evaluation of adherence pre- and postimplementation, median adherence rates in both groups appeared to be above goal at 0.94 and 1.06 respectively. Patients in the postimplementation group intentionally received a 5- to 7-day supply buffer to account for potential prescription delivery delays due to holidays and inclement weather. This would indicate that the patients in the postimplementation group would have 15% oversupply due to the 5-day supply buffer. After correcting for patients with confounding reasons for excess (dose reductions, breaks in treatment, etc.), the median MPR in the prerefill clinic group decreased to 0.9 and the MPR in the postrefill clinic group increased slightly to 1.08. Although the median adherence rate in both the pre- and postimplementation groups were above goal of 0.90, 36% of the patients in the preimplementation group were considered nonadherent (MPR < 0.9) compared with no patients in the postimplementation group. Therefore, our intervention to improve patient adherence appeared to be beneficial at our institution.
In addition to improving adherence, one of the goals of the renewal clinic was to minimize excess supply at the time of therapy discontinuation. This was accomplished by aligning medication fills with medical visits and objective monitoring, as well as limiting supply to no more than 30 days. Of the patients in the postimplementation group, only 1 patient had remaining medication at the time of therapy discontinuation compared with 14 patients in the preimplementation group. The estimated cost savings from excess supply was $36,335. Limiting the amount of unused supply not only saves money for the patient and the institution, but also decreases opportunity for improper hazardous waste disposal and unnecessary exposure of hazardous materials to others.
Our results show the pharmacist intervention in the coordination of renewals improved adherence, minimized medication waste, and saved money. The cost of pharmacist time participating in the refill clinic was not calculated. Each visit was completed in approximately 5 minutes, with subsequent documentation and coordination taking an additional 5 to 10 minutes. During the launch of this service, the oncology pharmacy resident provided all coverage of the clinic. Oversite of the resident was provided by hematology/oncology clinical pharmacy specialists. We have continued to utilize pharmacy resident coverage since that time to meet education needs and keep the estimated cost per visit low. Another option in the case that pharmacy residents are not available would be utilization of a pharmacy technician, intern, or professional student to conduct the adherence and tolerability phone assessments. Our escalation protocol allows intervention by clinical pharmacy specialist and/or other health care providers when necessary. Trainees have only required basic training on how to use the protocol.
Limitations
Due to this study’s retrospective design, an inherent limitation is dependence on prescriber and refill records for documentation of initiation and discontinuation dates. Therefore, only the association of impact of pharmacist intervention on medication adherence can be determined as opposed to causation. We did not take into account discrepancies in day supply secondary to ‘held’ therapies, dose reductions, or doses supplied during an inpatient admission, which may alter estimates of MPR and cost-savings data. Patients in the postimplementation group intentionally received a 5 to 7-day supply buffer to account for potential prescription delivery delays due to holidays and inclement weather. This would indicate that the patients in the postimplementation group would have 15% oversupply due to the 5-day supply buffer, thereby skewing MPR values. This study did not account for cost avoidance resulting from early identification and management of toxicity. Finally, the postimplementation data only spans 4 months and a longer duration of time is needed to more accurately determine sustainability of renewal clinic interventions and provide comprehensive evaluation of cost-avoidance.
Conclusion
Implementation of an OAN renewal clinic was associated with an increase in MPR, improved proportion of patients considered adherent, and an estimated $36,335 cost-savings. However, prospective evaluation and a longer study duration are needed to determine causality of improved adherence and cost-savings associated with a pharmacist-driven OAN renewal clinic.
1. Ganesan P, Sagar TG, Dubashi B, et al. Nonadherence to imatinib adversely affects event free survival in chronic phase chronic myeloid leukemia. Am J Hematol 2011; 86: 471-474. doi:10.1002/ajh.22019
2. Marin D, Bazeos A, Mahon FX, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol 2010; 28: 2381-2388. doi:10.1200/JCO.2009.26.3087
3. McCowan C, Shearer J, Donnan PT, et al. Cohort study examining tamoxifen adherence and its relationship to mortality in women with breast cancer. Br J Cancer 2008; 99: 1763-1768. doi:10.1038/sj.bjc.6604758
4. Lexicomp Online. Sunitinib. Hudson, Ohio: Lexi-Comp, Inc; August 20, 2019.
5. Babiker A, El Husseini M, Al Nemri A, et al. Health care professional development: Working as a team to improve patient care. Sudan J Paediatr. 2014;14(2):9-16.
6. Spence MM, Makarem AF, Reyes SL, et al. Evaluation of an outpatient pharmacy clinical services program on adherence and clinical outcomes among patients with diabetes and/or coronary artery disease. J Manag Care Spec Pharm. 2014;20(10):1036-1045. doi:10.18553/jmcp.2014.20.10.1036
7. Holle LM, Puri S, Clement JM. Physician-pharmacist collaboration for oral chemotherapy monitoring: Insights from an academic genitourinary oncology practice. J Oncol Pharm Pract 2015; doi:10.1177/1078155215581524
8. Muluneh B, Schneider M, Faso A, et al. Improved Adherence Rates and Clinical Outcomes of an Integrated, Closed-Loop, Pharmacist-Led Oral Chemotherapy Management Program. Journal of Oncology Practice. 2018;14(6):371-333. doi:10.1200/JOP.17.00039.
9. Font R, Espinas JA, Gil-Gil M, et al. Prescription refill, patient self-report and physician report in assessing adherence to oral endocrine therapy in early breast cancer patients: a retrospective cohort study in Catalonia, Spain. British Journal of Cancer. 2012 ;107(8):1249-1256. doi:10.1038/bjc.2012.389.
10. Anderson KR, Chambers CR, Lam N, et al. Medication adherence among adults prescribed imatinib, dasatinib, or nilotinib for the treatment of chronic myeloid leukemia. J Oncol Pharm Practice. 2015;21(1):19–25. doi:10.1177/1078155213520261
11. Weingart SN, Brown E, Bach PB, et al. NCCN Task Force Report: oral chemotherapy. J Natl Compr Canc Netw. 2008;6(3): S1-S14.
Evaluation of oral antineoplastic agent (OAN) adherence patterns have identified correlations between nonadherence or over-adherence and poorer disease-related outcomes. Multiple studies have focused on imatinib use in chronic myeloid leukemia (CML) due to its continuous, long-term use. A study by Ganesan and colleagues found that nonadherence to imatinib showed a significant decrease in 5-year event-free survival between 76.7% of adherent participants compared with 59.8% of nonadherent participants.1 This study found that 44% of patients who were adherent to imatinib achieved complete cytogenetic response vs only 26% of patients who were nonadherent. In another study of imatinib for CML, major molecular response (MMR) was strongly correlated with adherence and no patients with adherence < 80% were able to achieve MMR.2 Similarly, in studies of tamoxifen for breast cancer, < 80% adherence resulted in a 10% decrease in survival when compared to those who were more adherent.3,4
In addition to the clinical implications of nonadherence, there can be a significant cost associated with suboptimal use of these medications. The price of a single dose of OAN medication may cost as much as $440.5
The benefits of multidisciplinary care teams have been identified in many studies.6,7 While studies are limited in oncology, pharmacists provide vital contributions to the oncology multidisciplinary team when managing OANs as these health care professionals have expert knowledge of the medications, potential adverse events (AEs), and necessary monitoring parameters.8 In one study, patients seen by the pharmacist-led oral chemotherapy management program experienced improved clinical outcomes and response to therapy when compared with preintervention patients (early molecular response, 88.9% vs 54.8%, P = .01; major molecular response, 83.3% vs 57.6%, P = .06).9 During the study, 318 AEs were reported, leading to 235 pharmacist interventions to ameliorate AEs and improve adherence.
The primary objective of this study was to measure the impact of a pharmacist-driven OAN renewal clinic on medication adherence. The secondary objective was to estimate cost-savings of this new service.
Methods
Prior to July 2014, several limitations were identified related to OAN prescribing and monitoring at the Richard L. Roudebush Veterans Affairs Medical Center in Indianapolis, Indiana (RLRVAMC). The prescription ordering process relied primarily on the patient to initiate refills, rather than the prescriber OAN prescriptions also lacked consistency for number of refills or quantities dispensed. Furthermore, ordering of antineoplastic products was not limited to hematology/oncology providers. Patients were identified with significant supply on hand at the time of medication discontinuation, creating concerns for medication waste, tolerability, and nonadherence.
As a result, opportunities were identified to improve the prescribing process, recommended monitoring, toxicity and tolerability evaluation, medication reconciliation, and medication adherence. In July of 2014, the RLRVAMC adopted a new chemotherapy order entry system capable of restricting prescriptions to hematology/oncology providers and limiting dispensed quantities and refill amounts. A comprehensive pharmacist driven OAN renewal clinic was implemented on September 1, 2014 with the goal of improving long-term adherence and tolerability, in addition to minimizing medication waste.
Patients were eligible for enrollment in the clinic if they had a cancer diagnosis and were concomitantly prescribed an OAN outlined in Table 1. All eligible patients were automatically enrolled in the clinic when they were deemed stable on their OAN by a hematology/oncology pharmacy specialist. Stability was defined as ≤ Grade 1 symptoms associated with the toxicities of OAN therapy managed with or without intervention as defined by the Common Terminology Criteria for Adverse Events (CTCAE) version 4.03. Once enrolled in the renewal clinic, patients were called by an oncology pharmacy resident (PGY2) 1 week prior to any OAN refill due date. Patients were asked a series of 5 adherence and tolerability questions (Table 2) to evaluate renewal criteria for approval or need for further evaluation. These questions were developed based on targeted information and published reports on monitoring adherence.10,11 Criteria for renewal included: < 10% self-reported missed doses of the OAN during the previous dispensing period, no hospitalizations or emergency department visits since most recent hematology/oncology provider appointment, no changes to concomitant medication therapies, and no new or worsening medication-related AEs. Patients meeting all criteria were given a 30-day supply of OAN. Prescribing, dispensing, and delivery of OAN were facilitated by the pharmacist. Patient cases that did not meet criteria for renewal were escalated to the hematology/oncology provider or oncology clinical pharmacy specialist for further evaluation.
Study Design and Setting
This was a pre/post retrospective cohort, quality improvement study of patients enrolled in the RLRVAMC OAN pharmacist renewal clinic. The study was deemed exempt from institutional review board (IRB) by the US Department of Veterans Affairs (VA) Research and Development Department.
Study Population
Patients were included in the preimplementation group if they had received at least 2 prescriptions of an eligible OAN. Therapy for the preimplementation group was required to be a monthly duration > 21 days and between the dates of September 1, 2013 and August 31, 2014. Patients were included in the postimplementation group if they had received at least 2 prescriptions of the studied OANs between September 1, 2014 and January 31, 2015. Patients were excluded if they had filled < 2 prescriptions of OAN; were managed by a non-VA oncologist or hematologist; or received an OAN other than those listed in Table 1.
Data Collection
For all patients in both the pre- and postimplementation cohorts, a standardized data collection tool was used to collect the following via electronic health record review by a PGY2 oncology resident: age, race, gender, oral antineoplastic agent, refill dates, days’ supply, estimated unit cost per dose cancer diagnosis, distance from the RLRVAMC, copay status, presence of hospitalizations/ED visits/dosage reductions, discontinuation rates, reasons for discontinuation, and total number of current prescriptions. The presence or absence of dosage reductions were collected to identify concerns for tolerability, but only the original dose for the preimplementation group and dosage at time of clinic enrollment for the postimplementation group was included in the analysis.
Outcomes and Statistical Analyses
The primary outcome was medication adherence defined as the median medication possession ratio (MPR) before and after implementation of the clinic. Secondary outcomes included the proportion of patients who were adherent from before implementation to after implementation and estimated cost-savings of this clinic after implementation. MPR was used to estimate medication adherence by taking the cumulative day supply of medication on hand divided by the number of days on therapy.12 Number of days on therapy was determined by taking the difference on the start date of the new medication regimen and the discontinuation date of the same regimen. Patients were grouped by adherence into one of the following categories: < 0.8, 0.8 to 0.89, 0.9 to 1, and > 1.1. Patients were considered adherent if they reported taking ≥ 90% (MPR ≥ 0.9) of prescribed doses, adopted from the study by Anderson and colleagues.12 A patient with an MPR > 1, likely due to filling prior to the anticipated refill date, was considered 100% adherent (MPR = 1). If a patient switched OAN during the study, both agents were included as separate entities.
A conservative estimate of cost-savings was made by multiplying the RLRVAMC cost per unit of medication at time of initial prescription fill by the number of units taken each day multiplied by the total days’ supply on hand at time of therapy discontinuation. Patients with an MPR < 1 at time of therapy discontinuation were assumed to have zero remaining units on hand and zero cost savings was estimated. Waste, for purposes of cost-savings, was calculated for all MPR values > 1. Additional supply anticipated to be on hand from dose reductions was not included in the estimated cost of unused medication.
Descriptive statistics compared demographic characteristics between the pre- and postimplementation groups. MPR data were not normally distributed, which required the use of nonparametric Mann-Whitney U tests to compare pre- and postMPRs. Pearson χ2 compared the proportion of adherent patients between groups while descriptive statistics were used to estimate cost savings. Significance was determined based on a P value < .05. IBM SPSS Statistics software was used for all statistical analyses. As this was a complete sample of all eligible subjects, no sample size calculation was performed.
Results
In the preimplementation period, 246 patients received an OAN and 61 patients received an OAN in the postimplementation period (Figure 1). Of the 246 patients in the preimplementation period, 98 were eligible and included in the preimplementation group. Similarly, of the 61 patients in the postimplementation period, 35 patients met inclusion criteria for the postimplementation group. The study population was predominantly male with an average age of approximately 70 years in both groups (Table 3). More than 70% of the population in each group was White. No statistically significant differences between groups were identified. The most commonly prescribed OAN in the preimplementation group were abiraterone, imatinib, and enzalutamide (Table 3). In the postimplementation group, the most commonly prescribed agents were abiraterone, imatinib, pazopanib, and dasatinib. No significant differences were observed in prescribing of individual agents between the pre- and postimplementation groups or other characteristics that may affect adherence including patient copay status, number of concomitant medications, and driving distance from the RLRVAMC.
Thirty-six (36.7%) patients in the preimplementation group were considered nonadherent (MPR < 0.9) and 18 (18.4%) had an MPR < 0.8. Fifteen (15.3%) patients in the preimplementation clinic were considered overadherent (MPR > 1.1). Forty-seven (47.9%) patients in the preimplementation group were considered adherent (MPR 0.9 - 1.1) while all 35 (100%) patients in the postimplementation group were considered adherent (MPR 0.9 - 1.1). No non- or overadherent patients were identified in the postimplementation group (Figure 2). The median MPR for all patients in the preimplementation group was 0.94 compared with 1.06 (P < .001) in the postimplementation group.
Thirty-five (35.7%) patients had therapy discontinued or held in the preimplementation group compared with 2 (5.7%) patients in the postimplementation group (P < .001). Reasons for discontinuation in the preimplementation group included disease progression (n = 27), death (n = 3), lost to follow up (n = 2), and intolerability of therapy (n = 3). Both patients that discontinued therapy in the postimplementation group did so due to disease progression. Of the 35 patients who had their OAN discontinued or held in the preimplementation group, 14 patients had excess supply on hand at time of discontinuation. The estimated value of the unused medication was $37,890. Nine (25%) of the 35 patients who discontinued therapy had a dosage reduction during the course of therapy and the additional supply was not included in the cost estimate. Similarly, 1 of the 2 patients in the postimplementation group had their OAN discontinued during study. The cost of oversupply of medication at the time of therapy discontinuation was estimated at $1,555. No patients in the postimplementation group had dose reductions. After implementation of the OAN renewal clinic, the total cost savings between pre ($37,890) and postimplementation ($1,555) groups was $36,355.
Discussion
OANs are widely used therapies, with more than 25 million doses administered per year in the United States alone.12 The use of these agents will continue to grow as more targeted agents become available and patients request more convenient treatment options. The role for hematology/oncology clinical pharmacy services must adapt to this increased usage of OANs, including increasing pharmacist involvement in medication education, adherence and tolerability assessments, and proactive drug interaction monitoring.However, additional research is needed to determine optimal management strategies.
Our study aimed to compare OAN adherence among patients at a tertiary care VA hospital before and after implementation of a renewal clinic. The preimplementation population had a median MPR of 0.94 compared with 1.06 in the postimplementation group (P < .001). Although an ideal MPR is 1.0, we aimed for a slightly higher MPR to allow a supply buffer in the event of prescription delivery delays, as more than 90% of prescriptions are mailed to patients from a regional mail-order pharmacy. Importantly, the median MPRs do not adequately convey the impact from this clinic. The proportion of patients who were considered adherent to OANs increased from 47.9% in the preimplementation to 100% in the postimplementation period. These finding suggest that the clinical pharmacist role to assess and encourage adherence through monitoring tolerability of these OANs improved the overall medication taking experience of these patients.
Upon initial evaluation of adherence pre- and postimplementation, median adherence rates in both groups appeared to be above goal at 0.94 and 1.06 respectively. Patients in the postimplementation group intentionally received a 5- to 7-day supply buffer to account for potential prescription delivery delays due to holidays and inclement weather. This would indicate that the patients in the postimplementation group would have 15% oversupply due to the 5-day supply buffer. After correcting for patients with confounding reasons for excess (dose reductions, breaks in treatment, etc.), the median MPR in the prerefill clinic group decreased to 0.9 and the MPR in the postrefill clinic group increased slightly to 1.08. Although the median adherence rate in both the pre- and postimplementation groups were above goal of 0.90, 36% of the patients in the preimplementation group were considered nonadherent (MPR < 0.9) compared with no patients in the postimplementation group. Therefore, our intervention to improve patient adherence appeared to be beneficial at our institution.
In addition to improving adherence, one of the goals of the renewal clinic was to minimize excess supply at the time of therapy discontinuation. This was accomplished by aligning medication fills with medical visits and objective monitoring, as well as limiting supply to no more than 30 days. Of the patients in the postimplementation group, only 1 patient had remaining medication at the time of therapy discontinuation compared with 14 patients in the preimplementation group. The estimated cost savings from excess supply was $36,335. Limiting the amount of unused supply not only saves money for the patient and the institution, but also decreases opportunity for improper hazardous waste disposal and unnecessary exposure of hazardous materials to others.
Our results show the pharmacist intervention in the coordination of renewals improved adherence, minimized medication waste, and saved money. The cost of pharmacist time participating in the refill clinic was not calculated. Each visit was completed in approximately 5 minutes, with subsequent documentation and coordination taking an additional 5 to 10 minutes. During the launch of this service, the oncology pharmacy resident provided all coverage of the clinic. Oversite of the resident was provided by hematology/oncology clinical pharmacy specialists. We have continued to utilize pharmacy resident coverage since that time to meet education needs and keep the estimated cost per visit low. Another option in the case that pharmacy residents are not available would be utilization of a pharmacy technician, intern, or professional student to conduct the adherence and tolerability phone assessments. Our escalation protocol allows intervention by clinical pharmacy specialist and/or other health care providers when necessary. Trainees have only required basic training on how to use the protocol.
Limitations
Due to this study’s retrospective design, an inherent limitation is dependence on prescriber and refill records for documentation of initiation and discontinuation dates. Therefore, only the association of impact of pharmacist intervention on medication adherence can be determined as opposed to causation. We did not take into account discrepancies in day supply secondary to ‘held’ therapies, dose reductions, or doses supplied during an inpatient admission, which may alter estimates of MPR and cost-savings data. Patients in the postimplementation group intentionally received a 5 to 7-day supply buffer to account for potential prescription delivery delays due to holidays and inclement weather. This would indicate that the patients in the postimplementation group would have 15% oversupply due to the 5-day supply buffer, thereby skewing MPR values. This study did not account for cost avoidance resulting from early identification and management of toxicity. Finally, the postimplementation data only spans 4 months and a longer duration of time is needed to more accurately determine sustainability of renewal clinic interventions and provide comprehensive evaluation of cost-avoidance.
Conclusion
Implementation of an OAN renewal clinic was associated with an increase in MPR, improved proportion of patients considered adherent, and an estimated $36,335 cost-savings. However, prospective evaluation and a longer study duration are needed to determine causality of improved adherence and cost-savings associated with a pharmacist-driven OAN renewal clinic.
Evaluation of oral antineoplastic agent (OAN) adherence patterns have identified correlations between nonadherence or over-adherence and poorer disease-related outcomes. Multiple studies have focused on imatinib use in chronic myeloid leukemia (CML) due to its continuous, long-term use. A study by Ganesan and colleagues found that nonadherence to imatinib showed a significant decrease in 5-year event-free survival between 76.7% of adherent participants compared with 59.8% of nonadherent participants.1 This study found that 44% of patients who were adherent to imatinib achieved complete cytogenetic response vs only 26% of patients who were nonadherent. In another study of imatinib for CML, major molecular response (MMR) was strongly correlated with adherence and no patients with adherence < 80% were able to achieve MMR.2 Similarly, in studies of tamoxifen for breast cancer, < 80% adherence resulted in a 10% decrease in survival when compared to those who were more adherent.3,4
In addition to the clinical implications of nonadherence, there can be a significant cost associated with suboptimal use of these medications. The price of a single dose of OAN medication may cost as much as $440.5
The benefits of multidisciplinary care teams have been identified in many studies.6,7 While studies are limited in oncology, pharmacists provide vital contributions to the oncology multidisciplinary team when managing OANs as these health care professionals have expert knowledge of the medications, potential adverse events (AEs), and necessary monitoring parameters.8 In one study, patients seen by the pharmacist-led oral chemotherapy management program experienced improved clinical outcomes and response to therapy when compared with preintervention patients (early molecular response, 88.9% vs 54.8%, P = .01; major molecular response, 83.3% vs 57.6%, P = .06).9 During the study, 318 AEs were reported, leading to 235 pharmacist interventions to ameliorate AEs and improve adherence.
The primary objective of this study was to measure the impact of a pharmacist-driven OAN renewal clinic on medication adherence. The secondary objective was to estimate cost-savings of this new service.
Methods
Prior to July 2014, several limitations were identified related to OAN prescribing and monitoring at the Richard L. Roudebush Veterans Affairs Medical Center in Indianapolis, Indiana (RLRVAMC). The prescription ordering process relied primarily on the patient to initiate refills, rather than the prescriber OAN prescriptions also lacked consistency for number of refills or quantities dispensed. Furthermore, ordering of antineoplastic products was not limited to hematology/oncology providers. Patients were identified with significant supply on hand at the time of medication discontinuation, creating concerns for medication waste, tolerability, and nonadherence.
As a result, opportunities were identified to improve the prescribing process, recommended monitoring, toxicity and tolerability evaluation, medication reconciliation, and medication adherence. In July of 2014, the RLRVAMC adopted a new chemotherapy order entry system capable of restricting prescriptions to hematology/oncology providers and limiting dispensed quantities and refill amounts. A comprehensive pharmacist driven OAN renewal clinic was implemented on September 1, 2014 with the goal of improving long-term adherence and tolerability, in addition to minimizing medication waste.
Patients were eligible for enrollment in the clinic if they had a cancer diagnosis and were concomitantly prescribed an OAN outlined in Table 1. All eligible patients were automatically enrolled in the clinic when they were deemed stable on their OAN by a hematology/oncology pharmacy specialist. Stability was defined as ≤ Grade 1 symptoms associated with the toxicities of OAN therapy managed with or without intervention as defined by the Common Terminology Criteria for Adverse Events (CTCAE) version 4.03. Once enrolled in the renewal clinic, patients were called by an oncology pharmacy resident (PGY2) 1 week prior to any OAN refill due date. Patients were asked a series of 5 adherence and tolerability questions (Table 2) to evaluate renewal criteria for approval or need for further evaluation. These questions were developed based on targeted information and published reports on monitoring adherence.10,11 Criteria for renewal included: < 10% self-reported missed doses of the OAN during the previous dispensing period, no hospitalizations or emergency department visits since most recent hematology/oncology provider appointment, no changes to concomitant medication therapies, and no new or worsening medication-related AEs. Patients meeting all criteria were given a 30-day supply of OAN. Prescribing, dispensing, and delivery of OAN were facilitated by the pharmacist. Patient cases that did not meet criteria for renewal were escalated to the hematology/oncology provider or oncology clinical pharmacy specialist for further evaluation.
Study Design and Setting
This was a pre/post retrospective cohort, quality improvement study of patients enrolled in the RLRVAMC OAN pharmacist renewal clinic. The study was deemed exempt from institutional review board (IRB) by the US Department of Veterans Affairs (VA) Research and Development Department.
Study Population
Patients were included in the preimplementation group if they had received at least 2 prescriptions of an eligible OAN. Therapy for the preimplementation group was required to be a monthly duration > 21 days and between the dates of September 1, 2013 and August 31, 2014. Patients were included in the postimplementation group if they had received at least 2 prescriptions of the studied OANs between September 1, 2014 and January 31, 2015. Patients were excluded if they had filled < 2 prescriptions of OAN; were managed by a non-VA oncologist or hematologist; or received an OAN other than those listed in Table 1.
Data Collection
For all patients in both the pre- and postimplementation cohorts, a standardized data collection tool was used to collect the following via electronic health record review by a PGY2 oncology resident: age, race, gender, oral antineoplastic agent, refill dates, days’ supply, estimated unit cost per dose cancer diagnosis, distance from the RLRVAMC, copay status, presence of hospitalizations/ED visits/dosage reductions, discontinuation rates, reasons for discontinuation, and total number of current prescriptions. The presence or absence of dosage reductions were collected to identify concerns for tolerability, but only the original dose for the preimplementation group and dosage at time of clinic enrollment for the postimplementation group was included in the analysis.
Outcomes and Statistical Analyses
The primary outcome was medication adherence defined as the median medication possession ratio (MPR) before and after implementation of the clinic. Secondary outcomes included the proportion of patients who were adherent from before implementation to after implementation and estimated cost-savings of this clinic after implementation. MPR was used to estimate medication adherence by taking the cumulative day supply of medication on hand divided by the number of days on therapy.12 Number of days on therapy was determined by taking the difference on the start date of the new medication regimen and the discontinuation date of the same regimen. Patients were grouped by adherence into one of the following categories: < 0.8, 0.8 to 0.89, 0.9 to 1, and > 1.1. Patients were considered adherent if they reported taking ≥ 90% (MPR ≥ 0.9) of prescribed doses, adopted from the study by Anderson and colleagues.12 A patient with an MPR > 1, likely due to filling prior to the anticipated refill date, was considered 100% adherent (MPR = 1). If a patient switched OAN during the study, both agents were included as separate entities.
A conservative estimate of cost-savings was made by multiplying the RLRVAMC cost per unit of medication at time of initial prescription fill by the number of units taken each day multiplied by the total days’ supply on hand at time of therapy discontinuation. Patients with an MPR < 1 at time of therapy discontinuation were assumed to have zero remaining units on hand and zero cost savings was estimated. Waste, for purposes of cost-savings, was calculated for all MPR values > 1. Additional supply anticipated to be on hand from dose reductions was not included in the estimated cost of unused medication.
Descriptive statistics compared demographic characteristics between the pre- and postimplementation groups. MPR data were not normally distributed, which required the use of nonparametric Mann-Whitney U tests to compare pre- and postMPRs. Pearson χ2 compared the proportion of adherent patients between groups while descriptive statistics were used to estimate cost savings. Significance was determined based on a P value < .05. IBM SPSS Statistics software was used for all statistical analyses. As this was a complete sample of all eligible subjects, no sample size calculation was performed.
Results
In the preimplementation period, 246 patients received an OAN and 61 patients received an OAN in the postimplementation period (Figure 1). Of the 246 patients in the preimplementation period, 98 were eligible and included in the preimplementation group. Similarly, of the 61 patients in the postimplementation period, 35 patients met inclusion criteria for the postimplementation group. The study population was predominantly male with an average age of approximately 70 years in both groups (Table 3). More than 70% of the population in each group was White. No statistically significant differences between groups were identified. The most commonly prescribed OAN in the preimplementation group were abiraterone, imatinib, and enzalutamide (Table 3). In the postimplementation group, the most commonly prescribed agents were abiraterone, imatinib, pazopanib, and dasatinib. No significant differences were observed in prescribing of individual agents between the pre- and postimplementation groups or other characteristics that may affect adherence including patient copay status, number of concomitant medications, and driving distance from the RLRVAMC.
Thirty-six (36.7%) patients in the preimplementation group were considered nonadherent (MPR < 0.9) and 18 (18.4%) had an MPR < 0.8. Fifteen (15.3%) patients in the preimplementation clinic were considered overadherent (MPR > 1.1). Forty-seven (47.9%) patients in the preimplementation group were considered adherent (MPR 0.9 - 1.1) while all 35 (100%) patients in the postimplementation group were considered adherent (MPR 0.9 - 1.1). No non- or overadherent patients were identified in the postimplementation group (Figure 2). The median MPR for all patients in the preimplementation group was 0.94 compared with 1.06 (P < .001) in the postimplementation group.
Thirty-five (35.7%) patients had therapy discontinued or held in the preimplementation group compared with 2 (5.7%) patients in the postimplementation group (P < .001). Reasons for discontinuation in the preimplementation group included disease progression (n = 27), death (n = 3), lost to follow up (n = 2), and intolerability of therapy (n = 3). Both patients that discontinued therapy in the postimplementation group did so due to disease progression. Of the 35 patients who had their OAN discontinued or held in the preimplementation group, 14 patients had excess supply on hand at time of discontinuation. The estimated value of the unused medication was $37,890. Nine (25%) of the 35 patients who discontinued therapy had a dosage reduction during the course of therapy and the additional supply was not included in the cost estimate. Similarly, 1 of the 2 patients in the postimplementation group had their OAN discontinued during study. The cost of oversupply of medication at the time of therapy discontinuation was estimated at $1,555. No patients in the postimplementation group had dose reductions. After implementation of the OAN renewal clinic, the total cost savings between pre ($37,890) and postimplementation ($1,555) groups was $36,355.
Discussion
OANs are widely used therapies, with more than 25 million doses administered per year in the United States alone.12 The use of these agents will continue to grow as more targeted agents become available and patients request more convenient treatment options. The role for hematology/oncology clinical pharmacy services must adapt to this increased usage of OANs, including increasing pharmacist involvement in medication education, adherence and tolerability assessments, and proactive drug interaction monitoring.However, additional research is needed to determine optimal management strategies.
Our study aimed to compare OAN adherence among patients at a tertiary care VA hospital before and after implementation of a renewal clinic. The preimplementation population had a median MPR of 0.94 compared with 1.06 in the postimplementation group (P < .001). Although an ideal MPR is 1.0, we aimed for a slightly higher MPR to allow a supply buffer in the event of prescription delivery delays, as more than 90% of prescriptions are mailed to patients from a regional mail-order pharmacy. Importantly, the median MPRs do not adequately convey the impact from this clinic. The proportion of patients who were considered adherent to OANs increased from 47.9% in the preimplementation to 100% in the postimplementation period. These finding suggest that the clinical pharmacist role to assess and encourage adherence through monitoring tolerability of these OANs improved the overall medication taking experience of these patients.
Upon initial evaluation of adherence pre- and postimplementation, median adherence rates in both groups appeared to be above goal at 0.94 and 1.06 respectively. Patients in the postimplementation group intentionally received a 5- to 7-day supply buffer to account for potential prescription delivery delays due to holidays and inclement weather. This would indicate that the patients in the postimplementation group would have 15% oversupply due to the 5-day supply buffer. After correcting for patients with confounding reasons for excess (dose reductions, breaks in treatment, etc.), the median MPR in the prerefill clinic group decreased to 0.9 and the MPR in the postrefill clinic group increased slightly to 1.08. Although the median adherence rate in both the pre- and postimplementation groups were above goal of 0.90, 36% of the patients in the preimplementation group were considered nonadherent (MPR < 0.9) compared with no patients in the postimplementation group. Therefore, our intervention to improve patient adherence appeared to be beneficial at our institution.
In addition to improving adherence, one of the goals of the renewal clinic was to minimize excess supply at the time of therapy discontinuation. This was accomplished by aligning medication fills with medical visits and objective monitoring, as well as limiting supply to no more than 30 days. Of the patients in the postimplementation group, only 1 patient had remaining medication at the time of therapy discontinuation compared with 14 patients in the preimplementation group. The estimated cost savings from excess supply was $36,335. Limiting the amount of unused supply not only saves money for the patient and the institution, but also decreases opportunity for improper hazardous waste disposal and unnecessary exposure of hazardous materials to others.
Our results show the pharmacist intervention in the coordination of renewals improved adherence, minimized medication waste, and saved money. The cost of pharmacist time participating in the refill clinic was not calculated. Each visit was completed in approximately 5 minutes, with subsequent documentation and coordination taking an additional 5 to 10 minutes. During the launch of this service, the oncology pharmacy resident provided all coverage of the clinic. Oversite of the resident was provided by hematology/oncology clinical pharmacy specialists. We have continued to utilize pharmacy resident coverage since that time to meet education needs and keep the estimated cost per visit low. Another option in the case that pharmacy residents are not available would be utilization of a pharmacy technician, intern, or professional student to conduct the adherence and tolerability phone assessments. Our escalation protocol allows intervention by clinical pharmacy specialist and/or other health care providers when necessary. Trainees have only required basic training on how to use the protocol.
Limitations
Due to this study’s retrospective design, an inherent limitation is dependence on prescriber and refill records for documentation of initiation and discontinuation dates. Therefore, only the association of impact of pharmacist intervention on medication adherence can be determined as opposed to causation. We did not take into account discrepancies in day supply secondary to ‘held’ therapies, dose reductions, or doses supplied during an inpatient admission, which may alter estimates of MPR and cost-savings data. Patients in the postimplementation group intentionally received a 5 to 7-day supply buffer to account for potential prescription delivery delays due to holidays and inclement weather. This would indicate that the patients in the postimplementation group would have 15% oversupply due to the 5-day supply buffer, thereby skewing MPR values. This study did not account for cost avoidance resulting from early identification and management of toxicity. Finally, the postimplementation data only spans 4 months and a longer duration of time is needed to more accurately determine sustainability of renewal clinic interventions and provide comprehensive evaluation of cost-avoidance.
Conclusion
Implementation of an OAN renewal clinic was associated with an increase in MPR, improved proportion of patients considered adherent, and an estimated $36,335 cost-savings. However, prospective evaluation and a longer study duration are needed to determine causality of improved adherence and cost-savings associated with a pharmacist-driven OAN renewal clinic.
1. Ganesan P, Sagar TG, Dubashi B, et al. Nonadherence to imatinib adversely affects event free survival in chronic phase chronic myeloid leukemia. Am J Hematol 2011; 86: 471-474. doi:10.1002/ajh.22019
2. Marin D, Bazeos A, Mahon FX, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol 2010; 28: 2381-2388. doi:10.1200/JCO.2009.26.3087
3. McCowan C, Shearer J, Donnan PT, et al. Cohort study examining tamoxifen adherence and its relationship to mortality in women with breast cancer. Br J Cancer 2008; 99: 1763-1768. doi:10.1038/sj.bjc.6604758
4. Lexicomp Online. Sunitinib. Hudson, Ohio: Lexi-Comp, Inc; August 20, 2019.
5. Babiker A, El Husseini M, Al Nemri A, et al. Health care professional development: Working as a team to improve patient care. Sudan J Paediatr. 2014;14(2):9-16.
6. Spence MM, Makarem AF, Reyes SL, et al. Evaluation of an outpatient pharmacy clinical services program on adherence and clinical outcomes among patients with diabetes and/or coronary artery disease. J Manag Care Spec Pharm. 2014;20(10):1036-1045. doi:10.18553/jmcp.2014.20.10.1036
7. Holle LM, Puri S, Clement JM. Physician-pharmacist collaboration for oral chemotherapy monitoring: Insights from an academic genitourinary oncology practice. J Oncol Pharm Pract 2015; doi:10.1177/1078155215581524
8. Muluneh B, Schneider M, Faso A, et al. Improved Adherence Rates and Clinical Outcomes of an Integrated, Closed-Loop, Pharmacist-Led Oral Chemotherapy Management Program. Journal of Oncology Practice. 2018;14(6):371-333. doi:10.1200/JOP.17.00039.
9. Font R, Espinas JA, Gil-Gil M, et al. Prescription refill, patient self-report and physician report in assessing adherence to oral endocrine therapy in early breast cancer patients: a retrospective cohort study in Catalonia, Spain. British Journal of Cancer. 2012 ;107(8):1249-1256. doi:10.1038/bjc.2012.389.
10. Anderson KR, Chambers CR, Lam N, et al. Medication adherence among adults prescribed imatinib, dasatinib, or nilotinib for the treatment of chronic myeloid leukemia. J Oncol Pharm Practice. 2015;21(1):19–25. doi:10.1177/1078155213520261
11. Weingart SN, Brown E, Bach PB, et al. NCCN Task Force Report: oral chemotherapy. J Natl Compr Canc Netw. 2008;6(3): S1-S14.
1. Ganesan P, Sagar TG, Dubashi B, et al. Nonadherence to imatinib adversely affects event free survival in chronic phase chronic myeloid leukemia. Am J Hematol 2011; 86: 471-474. doi:10.1002/ajh.22019
2. Marin D, Bazeos A, Mahon FX, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol 2010; 28: 2381-2388. doi:10.1200/JCO.2009.26.3087
3. McCowan C, Shearer J, Donnan PT, et al. Cohort study examining tamoxifen adherence and its relationship to mortality in women with breast cancer. Br J Cancer 2008; 99: 1763-1768. doi:10.1038/sj.bjc.6604758
4. Lexicomp Online. Sunitinib. Hudson, Ohio: Lexi-Comp, Inc; August 20, 2019.
5. Babiker A, El Husseini M, Al Nemri A, et al. Health care professional development: Working as a team to improve patient care. Sudan J Paediatr. 2014;14(2):9-16.
6. Spence MM, Makarem AF, Reyes SL, et al. Evaluation of an outpatient pharmacy clinical services program on adherence and clinical outcomes among patients with diabetes and/or coronary artery disease. J Manag Care Spec Pharm. 2014;20(10):1036-1045. doi:10.18553/jmcp.2014.20.10.1036
7. Holle LM, Puri S, Clement JM. Physician-pharmacist collaboration for oral chemotherapy monitoring: Insights from an academic genitourinary oncology practice. J Oncol Pharm Pract 2015; doi:10.1177/1078155215581524
8. Muluneh B, Schneider M, Faso A, et al. Improved Adherence Rates and Clinical Outcomes of an Integrated, Closed-Loop, Pharmacist-Led Oral Chemotherapy Management Program. Journal of Oncology Practice. 2018;14(6):371-333. doi:10.1200/JOP.17.00039.
9. Font R, Espinas JA, Gil-Gil M, et al. Prescription refill, patient self-report and physician report in assessing adherence to oral endocrine therapy in early breast cancer patients: a retrospective cohort study in Catalonia, Spain. British Journal of Cancer. 2012 ;107(8):1249-1256. doi:10.1038/bjc.2012.389.
10. Anderson KR, Chambers CR, Lam N, et al. Medication adherence among adults prescribed imatinib, dasatinib, or nilotinib for the treatment of chronic myeloid leukemia. J Oncol Pharm Practice. 2015;21(1):19–25. doi:10.1177/1078155213520261
11. Weingart SN, Brown E, Bach PB, et al. NCCN Task Force Report: oral chemotherapy. J Natl Compr Canc Netw. 2008;6(3): S1-S14.
Study supports intensifying chemoradiotherapy for head and neck cancer
Of the 16 treatment options compared and ranked, HFCRT topped the list for overall survival, event-free survival, locoregional control, and cancer-specific death.
The results also suggested that taxane-based induction chemotherapy followed by locoregional therapy, especially with concomitant chemotherapy, “is another good option in selected patients with a good performance status and minor comorbidities,” according to investigator Claire Petit, MD, PhD, of Centre hospitalier de l’Université de Montréal in Canada, and colleagues.
The investigators concluded that further intensifying chemoradiotherapy with these approaches “could improve outcomes over chemoradiotherapy.”
The findings, published in The Lancet Oncology, “could help to guide clinical decision-making in locally advanced head and neck cancer with a high risk of locoregional failure, especially human papillomavirus–negative tumours,” the authors wrote.
However, Jared Weiss, MD, of the University of North Carolina, Chapel Hill, cautioned that this “study is an individual patient data network meta-analysis, not a randomized controlled trial. As the authors note, it can help frame existing data but cannot define standard of care.”
Still, “it does support the efficacy of two commonly considered intensification strategies for high-risk patients – hyperfractionation of the radiation and the addition of preceding induction chemotherapy. Both of these intensifications substantially increase the time commitment from the patient, and many patients find this unacceptable. But, for select patients, hyperfractionation and induction chemotherapy have a role and may be considered for patients at high risk of treatment failure,” Dr. Weiss said.
Study details
The goal of this study was to find the best option among many chemoradiation approaches for head and neck cancer. The investigators pulled together and reanalyzed individual patient data from recently updated meta-analyses.
The current analysis included 115 randomized trials that enrolled patients between Jan. 1, 1980, and April 30, 2012. This encompassed 28,978 patients with 20,579 progression events and 19,253 deaths over a median follow-up of 6.6 years.
Treatments were ranked by P score, with higher scores indicating more effective therapies.
For overall survival, HFCRT had a P score of 97%. The hazard ratio (HR) was 0.63 for the comparison with locoregional therapy alone (surgery, radiotherapy, or both). The absolute benefit at 5 years, compared with locoregional therapy alone, was 16.7% with HFCRT.
The P score for the second most effective treatment option – induction chemotherapy with taxane, cisplatin, and fluorouracil followed by locoregional therapy (ICTaxPF-LRT) – was 89%, with a hazard ratio of 0.69 and an absolute benefit at 5 years of 13.4%, versus locoregional therapy.
The HR of HFCRT versus the accepted standard of care worldwide – locoregional therapy with concomitant platinum-based chemotherapy and radiotherapy (CLRTP) – was 0.82 in favor of HFCRT for overall survival and 0.80 for event-free survival.
For overall survival, the P score for CLRTP was 78%. Three other treatment options had a better P score than CLRTP but not a better HR (0.77). These included ICTaxPF-LRT (P score, 89%; HR, 0.69), accelerated radiotherapy with concomitant chemotherapy (P score, 82%; HR, 0.75), and ICTaxPF-LRT followed by CLRTP (P score, 80%; HR, 0.75).
In the end, the investigators found “superiority of HFCRT over other treatments,” but noted it can be difficult to implement HFCRT in the era of intensity-modulated radiotherapy for head and neck cancer. Even so, HFCRT “could be considered as an option for tertiary centres with a high throughput of patients,” the investigators wrote.
The team noted that one of the limitations of this study is that cancer care has improved substantially since the very earliest trials that were included in the analysis. This introduces potential confounders, including that patients in older trials might have been understaged so that even an experimental local therapy would have been less effective.
Toxicity wasn’t part of this analysis but must be taken into account when making therapeutic decisions, “especially because HFCRT and induction chemotherapy based on taxane, cisplatin, and fluorouracil are known to be toxic,” the investigators wrote.
This research was funded by the French Institut National du Cancer, French Ligue Nationale Contre le Cancer, and Fondation ARC. The authors disclosed relationships with numerous companies, including AbbVie, Lilly, and Merck. Dr. Weiss did not report any relevant conflicts.
Of the 16 treatment options compared and ranked, HFCRT topped the list for overall survival, event-free survival, locoregional control, and cancer-specific death.
The results also suggested that taxane-based induction chemotherapy followed by locoregional therapy, especially with concomitant chemotherapy, “is another good option in selected patients with a good performance status and minor comorbidities,” according to investigator Claire Petit, MD, PhD, of Centre hospitalier de l’Université de Montréal in Canada, and colleagues.
The investigators concluded that further intensifying chemoradiotherapy with these approaches “could improve outcomes over chemoradiotherapy.”
The findings, published in The Lancet Oncology, “could help to guide clinical decision-making in locally advanced head and neck cancer with a high risk of locoregional failure, especially human papillomavirus–negative tumours,” the authors wrote.
However, Jared Weiss, MD, of the University of North Carolina, Chapel Hill, cautioned that this “study is an individual patient data network meta-analysis, not a randomized controlled trial. As the authors note, it can help frame existing data but cannot define standard of care.”
Still, “it does support the efficacy of two commonly considered intensification strategies for high-risk patients – hyperfractionation of the radiation and the addition of preceding induction chemotherapy. Both of these intensifications substantially increase the time commitment from the patient, and many patients find this unacceptable. But, for select patients, hyperfractionation and induction chemotherapy have a role and may be considered for patients at high risk of treatment failure,” Dr. Weiss said.
Study details
The goal of this study was to find the best option among many chemoradiation approaches for head and neck cancer. The investigators pulled together and reanalyzed individual patient data from recently updated meta-analyses.
The current analysis included 115 randomized trials that enrolled patients between Jan. 1, 1980, and April 30, 2012. This encompassed 28,978 patients with 20,579 progression events and 19,253 deaths over a median follow-up of 6.6 years.
Treatments were ranked by P score, with higher scores indicating more effective therapies.
For overall survival, HFCRT had a P score of 97%. The hazard ratio (HR) was 0.63 for the comparison with locoregional therapy alone (surgery, radiotherapy, or both). The absolute benefit at 5 years, compared with locoregional therapy alone, was 16.7% with HFCRT.
The P score for the second most effective treatment option – induction chemotherapy with taxane, cisplatin, and fluorouracil followed by locoregional therapy (ICTaxPF-LRT) – was 89%, with a hazard ratio of 0.69 and an absolute benefit at 5 years of 13.4%, versus locoregional therapy.
The HR of HFCRT versus the accepted standard of care worldwide – locoregional therapy with concomitant platinum-based chemotherapy and radiotherapy (CLRTP) – was 0.82 in favor of HFCRT for overall survival and 0.80 for event-free survival.
For overall survival, the P score for CLRTP was 78%. Three other treatment options had a better P score than CLRTP but not a better HR (0.77). These included ICTaxPF-LRT (P score, 89%; HR, 0.69), accelerated radiotherapy with concomitant chemotherapy (P score, 82%; HR, 0.75), and ICTaxPF-LRT followed by CLRTP (P score, 80%; HR, 0.75).
In the end, the investigators found “superiority of HFCRT over other treatments,” but noted it can be difficult to implement HFCRT in the era of intensity-modulated radiotherapy for head and neck cancer. Even so, HFCRT “could be considered as an option for tertiary centres with a high throughput of patients,” the investigators wrote.
The team noted that one of the limitations of this study is that cancer care has improved substantially since the very earliest trials that were included in the analysis. This introduces potential confounders, including that patients in older trials might have been understaged so that even an experimental local therapy would have been less effective.
Toxicity wasn’t part of this analysis but must be taken into account when making therapeutic decisions, “especially because HFCRT and induction chemotherapy based on taxane, cisplatin, and fluorouracil are known to be toxic,” the investigators wrote.
This research was funded by the French Institut National du Cancer, French Ligue Nationale Contre le Cancer, and Fondation ARC. The authors disclosed relationships with numerous companies, including AbbVie, Lilly, and Merck. Dr. Weiss did not report any relevant conflicts.
Of the 16 treatment options compared and ranked, HFCRT topped the list for overall survival, event-free survival, locoregional control, and cancer-specific death.
The results also suggested that taxane-based induction chemotherapy followed by locoregional therapy, especially with concomitant chemotherapy, “is another good option in selected patients with a good performance status and minor comorbidities,” according to investigator Claire Petit, MD, PhD, of Centre hospitalier de l’Université de Montréal in Canada, and colleagues.
The investigators concluded that further intensifying chemoradiotherapy with these approaches “could improve outcomes over chemoradiotherapy.”
The findings, published in The Lancet Oncology, “could help to guide clinical decision-making in locally advanced head and neck cancer with a high risk of locoregional failure, especially human papillomavirus–negative tumours,” the authors wrote.
However, Jared Weiss, MD, of the University of North Carolina, Chapel Hill, cautioned that this “study is an individual patient data network meta-analysis, not a randomized controlled trial. As the authors note, it can help frame existing data but cannot define standard of care.”
Still, “it does support the efficacy of two commonly considered intensification strategies for high-risk patients – hyperfractionation of the radiation and the addition of preceding induction chemotherapy. Both of these intensifications substantially increase the time commitment from the patient, and many patients find this unacceptable. But, for select patients, hyperfractionation and induction chemotherapy have a role and may be considered for patients at high risk of treatment failure,” Dr. Weiss said.
Study details
The goal of this study was to find the best option among many chemoradiation approaches for head and neck cancer. The investigators pulled together and reanalyzed individual patient data from recently updated meta-analyses.
The current analysis included 115 randomized trials that enrolled patients between Jan. 1, 1980, and April 30, 2012. This encompassed 28,978 patients with 20,579 progression events and 19,253 deaths over a median follow-up of 6.6 years.
Treatments were ranked by P score, with higher scores indicating more effective therapies.
For overall survival, HFCRT had a P score of 97%. The hazard ratio (HR) was 0.63 for the comparison with locoregional therapy alone (surgery, radiotherapy, or both). The absolute benefit at 5 years, compared with locoregional therapy alone, was 16.7% with HFCRT.
The P score for the second most effective treatment option – induction chemotherapy with taxane, cisplatin, and fluorouracil followed by locoregional therapy (ICTaxPF-LRT) – was 89%, with a hazard ratio of 0.69 and an absolute benefit at 5 years of 13.4%, versus locoregional therapy.
The HR of HFCRT versus the accepted standard of care worldwide – locoregional therapy with concomitant platinum-based chemotherapy and radiotherapy (CLRTP) – was 0.82 in favor of HFCRT for overall survival and 0.80 for event-free survival.
For overall survival, the P score for CLRTP was 78%. Three other treatment options had a better P score than CLRTP but not a better HR (0.77). These included ICTaxPF-LRT (P score, 89%; HR, 0.69), accelerated radiotherapy with concomitant chemotherapy (P score, 82%; HR, 0.75), and ICTaxPF-LRT followed by CLRTP (P score, 80%; HR, 0.75).
In the end, the investigators found “superiority of HFCRT over other treatments,” but noted it can be difficult to implement HFCRT in the era of intensity-modulated radiotherapy for head and neck cancer. Even so, HFCRT “could be considered as an option for tertiary centres with a high throughput of patients,” the investigators wrote.
The team noted that one of the limitations of this study is that cancer care has improved substantially since the very earliest trials that were included in the analysis. This introduces potential confounders, including that patients in older trials might have been understaged so that even an experimental local therapy would have been less effective.
Toxicity wasn’t part of this analysis but must be taken into account when making therapeutic decisions, “especially because HFCRT and induction chemotherapy based on taxane, cisplatin, and fluorouracil are known to be toxic,” the investigators wrote.
This research was funded by the French Institut National du Cancer, French Ligue Nationale Contre le Cancer, and Fondation ARC. The authors disclosed relationships with numerous companies, including AbbVie, Lilly, and Merck. Dr. Weiss did not report any relevant conflicts.
FROM THE LANCET ONCOLOGY
The power and promise of social media in oncology
Mark A. Lewis, MD, explained to the COSMO meeting audience how storytelling on social media can educate and engage patients, advocates, and professional colleagues – advancing knowledge, dispelling misinformation, and promoting clinical research.
Dr. Lewis, an oncologist at Intermountain Healthcare in Salt Lake City, reflected on the bifid roles of oncologists as scientists engaged in life-long learning and humanists who can internalize and appreciate the unique character and circumstances of their patients.
Patients who have serious illnesses are necessarily aggregated by statistics. However, in an essay published in 2011, Dr. Lewis noted that “each individual patient partakes in a unique, irreproducible experiment where n = 1” (J Clin Oncol. 2011 Aug 1;29[22]:3103-4).
Dr. Lewis highlighted the duality of individual data points on a survival curve as descriptors of common disease trajectories and treatment effects. However, those data points also conceal important narratives regarding the most highly valued aspects of the doctor-patient relationship and the impact of cancer treatment on patients’ lives.
In referring to the futuristic essay “Ars Brevis,” Dr. Lewis contrasted the humanism of oncology specialists in the present day with the fictional image of data-regurgitating robots programmed to maximize the efficiency of each patient encounter (J Clin Oncol. 2013 May 10;31[14]:1792-4).
Dr. Lewis reminded attendees that to practice medicine without using both “head and heart” undermines the inherent nature of medical care.
Unfortunately, that perspective may not match the public perception of oncologists. Dr. Lewis described his experience of typing “oncologists are” into an Internet search engine and seeing the auto-complete function prompt words such as “criminals,” “evil,” “murderers,” and “confused.”
Obviously, it is hard to establish a trusting patient-doctor relationship if that is the prima facie perception of the oncology specialty.
Dispelling myths and creating community via social media
A primary goal of consultation with a newly-diagnosed cancer patient is for the patient to feel that the oncologist will be there to take care of them, regardless of what the future holds.
Dr. Lewis has found that social media can potentially extend that feeling to a global community of patients, caregivers, and others seeking information relevant to a cancer diagnosis. He believes that oncologists have an opportunity to dispel myths and fears by being attentive to the real-life concerns of patients.
Dr. Lewis took advantage of this opportunity when he underwent a Whipple procedure (pancreaticoduodenectomy) for a pancreatic neuroendocrine tumor. He and the hospital’s media services staff “live-tweeted” his surgery and recovery.
With those tweets, Dr. Lewis demystified each step of a major surgical procedure. From messages he received on social media, Dr. Lewis knows he made the decision to have a Whipple procedure more acceptable to other patients.
His personal medical experience notwithstanding, Dr. Lewis acknowledged that every patient’s circumstances are unique.
Oncologists cannot possibly empathize with every circumstance. However, when they show sensitivity to personal elements of the cancer experience, they shed light on the complicated role they play in patient care and can facilitate good decision-making among patients across the globe.
Social media for professional development and patient care
The publication of his 2011 essay was gratifying for Dr. Lewis, but the finite number of comments he received thereafter illustrated the rather limited audience that traditional academic publications have and the laborious process for subsequent interaction (J Clin Oncol. 2011 Aug 1;29[22]:3103-4).
First as an observer and later as a participant on social media, Dr. Lewis appreciated that teaching points and publications can be amplified by global distribution and the potential for informal bidirectional communication.
Social media platforms enable physicians to connect with a larger audience through participative communication, in which users develop, share, and react to content (N Engl J Med. 2009 Aug 13;361[7]:649-51).
Dr. Lewis reflected on how oncologists are challenged to sort through the thousands of oncology-focused publications annually. Through social media, one can see the studies on which the experts are commenting and appreciate the nuances that contextualize the results. Focused interactions with renowned doctors, at regular intervals, require little formality.
Online journal clubs enable the sharing of ideas, opinions, multimedia resources, and references across institutional and international borders (J Gen Intern Med. 2014 Oct;29[10]:1317-8).
Social media in oncology: Accomplishments and promise
The development of broadband Internet, wireless connectivity, and social media for peer-to-peer and general communication are among the major technological advances that have transformed medical communication.
As an organization, COSMO aims to describe, understand, and improve the use of social media to increase the penetration of evidence-based guidelines and research insights into clinical practice (Future Oncol. 2017 Jun;13[15]:1281-5).
At the inaugural COSMO meeting, areas of progress since COSMO’s inception in 2015 were highlighted, including:
- The involvement of cancer professionals and advocates in multiple distinctive platforms.
- The development of hashtag libraries to aggregate interest groups and topics.
- The refinement of strategies for engaging advocates with attention to inclusiveness.
- A steady trajectory of growth in tweeting at scientific conferences.
An overarching theme of the COSMO meeting was “authenticity,” a virtue that is easy to admire but requires conscious, consistent effort to achieve.
Disclosure of conflicts of interest and avoiding using social media simply as a recruitment tool for clinical trials are basic components of accurate self-representation.
In addition, Dr. Lewis advocated for sharing personal experiences in a component of social media posts so oncologists can show humanity as a feature of their professional online identity and inherent nature.
Dr. Lewis disclosed consultancy with Medscape/WebMD, which are owned by the same parent company as MDedge. He also disclosed relationships with Foundation Medicine, Natera, Exelixis, QED, HalioDX, and Ipsen.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Mark A. Lewis, MD, explained to the COSMO meeting audience how storytelling on social media can educate and engage patients, advocates, and professional colleagues – advancing knowledge, dispelling misinformation, and promoting clinical research.
Dr. Lewis, an oncologist at Intermountain Healthcare in Salt Lake City, reflected on the bifid roles of oncologists as scientists engaged in life-long learning and humanists who can internalize and appreciate the unique character and circumstances of their patients.
Patients who have serious illnesses are necessarily aggregated by statistics. However, in an essay published in 2011, Dr. Lewis noted that “each individual patient partakes in a unique, irreproducible experiment where n = 1” (J Clin Oncol. 2011 Aug 1;29[22]:3103-4).
Dr. Lewis highlighted the duality of individual data points on a survival curve as descriptors of common disease trajectories and treatment effects. However, those data points also conceal important narratives regarding the most highly valued aspects of the doctor-patient relationship and the impact of cancer treatment on patients’ lives.
In referring to the futuristic essay “Ars Brevis,” Dr. Lewis contrasted the humanism of oncology specialists in the present day with the fictional image of data-regurgitating robots programmed to maximize the efficiency of each patient encounter (J Clin Oncol. 2013 May 10;31[14]:1792-4).
Dr. Lewis reminded attendees that to practice medicine without using both “head and heart” undermines the inherent nature of medical care.
Unfortunately, that perspective may not match the public perception of oncologists. Dr. Lewis described his experience of typing “oncologists are” into an Internet search engine and seeing the auto-complete function prompt words such as “criminals,” “evil,” “murderers,” and “confused.”
Obviously, it is hard to establish a trusting patient-doctor relationship if that is the prima facie perception of the oncology specialty.
Dispelling myths and creating community via social media
A primary goal of consultation with a newly-diagnosed cancer patient is for the patient to feel that the oncologist will be there to take care of them, regardless of what the future holds.
Dr. Lewis has found that social media can potentially extend that feeling to a global community of patients, caregivers, and others seeking information relevant to a cancer diagnosis. He believes that oncologists have an opportunity to dispel myths and fears by being attentive to the real-life concerns of patients.
Dr. Lewis took advantage of this opportunity when he underwent a Whipple procedure (pancreaticoduodenectomy) for a pancreatic neuroendocrine tumor. He and the hospital’s media services staff “live-tweeted” his surgery and recovery.
With those tweets, Dr. Lewis demystified each step of a major surgical procedure. From messages he received on social media, Dr. Lewis knows he made the decision to have a Whipple procedure more acceptable to other patients.
His personal medical experience notwithstanding, Dr. Lewis acknowledged that every patient’s circumstances are unique.
Oncologists cannot possibly empathize with every circumstance. However, when they show sensitivity to personal elements of the cancer experience, they shed light on the complicated role they play in patient care and can facilitate good decision-making among patients across the globe.
Social media for professional development and patient care
The publication of his 2011 essay was gratifying for Dr. Lewis, but the finite number of comments he received thereafter illustrated the rather limited audience that traditional academic publications have and the laborious process for subsequent interaction (J Clin Oncol. 2011 Aug 1;29[22]:3103-4).
First as an observer and later as a participant on social media, Dr. Lewis appreciated that teaching points and publications can be amplified by global distribution and the potential for informal bidirectional communication.
Social media platforms enable physicians to connect with a larger audience through participative communication, in which users develop, share, and react to content (N Engl J Med. 2009 Aug 13;361[7]:649-51).
Dr. Lewis reflected on how oncologists are challenged to sort through the thousands of oncology-focused publications annually. Through social media, one can see the studies on which the experts are commenting and appreciate the nuances that contextualize the results. Focused interactions with renowned doctors, at regular intervals, require little formality.
Online journal clubs enable the sharing of ideas, opinions, multimedia resources, and references across institutional and international borders (J Gen Intern Med. 2014 Oct;29[10]:1317-8).
Social media in oncology: Accomplishments and promise
The development of broadband Internet, wireless connectivity, and social media for peer-to-peer and general communication are among the major technological advances that have transformed medical communication.
As an organization, COSMO aims to describe, understand, and improve the use of social media to increase the penetration of evidence-based guidelines and research insights into clinical practice (Future Oncol. 2017 Jun;13[15]:1281-5).
At the inaugural COSMO meeting, areas of progress since COSMO’s inception in 2015 were highlighted, including:
- The involvement of cancer professionals and advocates in multiple distinctive platforms.
- The development of hashtag libraries to aggregate interest groups and topics.
- The refinement of strategies for engaging advocates with attention to inclusiveness.
- A steady trajectory of growth in tweeting at scientific conferences.
An overarching theme of the COSMO meeting was “authenticity,” a virtue that is easy to admire but requires conscious, consistent effort to achieve.
Disclosure of conflicts of interest and avoiding using social media simply as a recruitment tool for clinical trials are basic components of accurate self-representation.
In addition, Dr. Lewis advocated for sharing personal experiences in a component of social media posts so oncologists can show humanity as a feature of their professional online identity and inherent nature.
Dr. Lewis disclosed consultancy with Medscape/WebMD, which are owned by the same parent company as MDedge. He also disclosed relationships with Foundation Medicine, Natera, Exelixis, QED, HalioDX, and Ipsen.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Mark A. Lewis, MD, explained to the COSMO meeting audience how storytelling on social media can educate and engage patients, advocates, and professional colleagues – advancing knowledge, dispelling misinformation, and promoting clinical research.
Dr. Lewis, an oncologist at Intermountain Healthcare in Salt Lake City, reflected on the bifid roles of oncologists as scientists engaged in life-long learning and humanists who can internalize and appreciate the unique character and circumstances of their patients.
Patients who have serious illnesses are necessarily aggregated by statistics. However, in an essay published in 2011, Dr. Lewis noted that “each individual patient partakes in a unique, irreproducible experiment where n = 1” (J Clin Oncol. 2011 Aug 1;29[22]:3103-4).
Dr. Lewis highlighted the duality of individual data points on a survival curve as descriptors of common disease trajectories and treatment effects. However, those data points also conceal important narratives regarding the most highly valued aspects of the doctor-patient relationship and the impact of cancer treatment on patients’ lives.
In referring to the futuristic essay “Ars Brevis,” Dr. Lewis contrasted the humanism of oncology specialists in the present day with the fictional image of data-regurgitating robots programmed to maximize the efficiency of each patient encounter (J Clin Oncol. 2013 May 10;31[14]:1792-4).
Dr. Lewis reminded attendees that to practice medicine without using both “head and heart” undermines the inherent nature of medical care.
Unfortunately, that perspective may not match the public perception of oncologists. Dr. Lewis described his experience of typing “oncologists are” into an Internet search engine and seeing the auto-complete function prompt words such as “criminals,” “evil,” “murderers,” and “confused.”
Obviously, it is hard to establish a trusting patient-doctor relationship if that is the prima facie perception of the oncology specialty.
Dispelling myths and creating community via social media
A primary goal of consultation with a newly-diagnosed cancer patient is for the patient to feel that the oncologist will be there to take care of them, regardless of what the future holds.
Dr. Lewis has found that social media can potentially extend that feeling to a global community of patients, caregivers, and others seeking information relevant to a cancer diagnosis. He believes that oncologists have an opportunity to dispel myths and fears by being attentive to the real-life concerns of patients.
Dr. Lewis took advantage of this opportunity when he underwent a Whipple procedure (pancreaticoduodenectomy) for a pancreatic neuroendocrine tumor. He and the hospital’s media services staff “live-tweeted” his surgery and recovery.
With those tweets, Dr. Lewis demystified each step of a major surgical procedure. From messages he received on social media, Dr. Lewis knows he made the decision to have a Whipple procedure more acceptable to other patients.
His personal medical experience notwithstanding, Dr. Lewis acknowledged that every patient’s circumstances are unique.
Oncologists cannot possibly empathize with every circumstance. However, when they show sensitivity to personal elements of the cancer experience, they shed light on the complicated role they play in patient care and can facilitate good decision-making among patients across the globe.
Social media for professional development and patient care
The publication of his 2011 essay was gratifying for Dr. Lewis, but the finite number of comments he received thereafter illustrated the rather limited audience that traditional academic publications have and the laborious process for subsequent interaction (J Clin Oncol. 2011 Aug 1;29[22]:3103-4).
First as an observer and later as a participant on social media, Dr. Lewis appreciated that teaching points and publications can be amplified by global distribution and the potential for informal bidirectional communication.
Social media platforms enable physicians to connect with a larger audience through participative communication, in which users develop, share, and react to content (N Engl J Med. 2009 Aug 13;361[7]:649-51).
Dr. Lewis reflected on how oncologists are challenged to sort through the thousands of oncology-focused publications annually. Through social media, one can see the studies on which the experts are commenting and appreciate the nuances that contextualize the results. Focused interactions with renowned doctors, at regular intervals, require little formality.
Online journal clubs enable the sharing of ideas, opinions, multimedia resources, and references across institutional and international borders (J Gen Intern Med. 2014 Oct;29[10]:1317-8).
Social media in oncology: Accomplishments and promise
The development of broadband Internet, wireless connectivity, and social media for peer-to-peer and general communication are among the major technological advances that have transformed medical communication.
As an organization, COSMO aims to describe, understand, and improve the use of social media to increase the penetration of evidence-based guidelines and research insights into clinical practice (Future Oncol. 2017 Jun;13[15]:1281-5).
At the inaugural COSMO meeting, areas of progress since COSMO’s inception in 2015 were highlighted, including:
- The involvement of cancer professionals and advocates in multiple distinctive platforms.
- The development of hashtag libraries to aggregate interest groups and topics.
- The refinement of strategies for engaging advocates with attention to inclusiveness.
- A steady trajectory of growth in tweeting at scientific conferences.
An overarching theme of the COSMO meeting was “authenticity,” a virtue that is easy to admire but requires conscious, consistent effort to achieve.
Disclosure of conflicts of interest and avoiding using social media simply as a recruitment tool for clinical trials are basic components of accurate self-representation.
In addition, Dr. Lewis advocated for sharing personal experiences in a component of social media posts so oncologists can show humanity as a feature of their professional online identity and inherent nature.
Dr. Lewis disclosed consultancy with Medscape/WebMD, which are owned by the same parent company as MDedge. He also disclosed relationships with Foundation Medicine, Natera, Exelixis, QED, HalioDX, and Ipsen.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
FROM COSMO 2021
Hyperprogression on immunotherapy: When outcomes are much worse
Immunotherapy with checkpoint inhibitors has ushered in a new era of cancer therapy, with some patients showing dramatic responses and significantly better outcomes than with other therapies across many cancer types. But some patients do worse, sometimes much worse.
A subset of patients who undergo immunotherapy experience unexpected, rapid disease progression, with a dramatic acceleration of disease trajectory. They also have a shorter progression-free survival and overall survival than would have been expected.
This has been described as hyperprogression and has been termed “hyperprogressive disease” (HPD). It has been seen in a variety of cancers; the incidence ranges from 4% to 29% in the studies reported to date.
There has been some debate over whether this is a real phenomenon or whether it is part of the natural course of disease.
HPD is a “provocative phenomenon,” wrote the authors of a recent commentary entitled “Hyperprogression and Immunotherapy: Fact, Fiction, or Alternative Fact?”
“This phenomenon has polarized oncologists who debate that this could still reflect the natural history of the disease,” said the author of another commentary.
But the tide is now turning toward acceptance of HPD, said Kartik Sehgal, MD, an oncologist at Dana-Farber Cancer Institute and Harvard University, both in Boston.
“With publication of multiple clinical reports of different cancer types worldwide, hyperprogression is now accepted by most oncologists to be a true phenomenon rather than natural progression of disease,” Dr. Sehgal said.
He authored an invited commentary in JAMA Network Openabout one of the latest meta-analyses (JAMA Netw Open. 2021;4[3]:e211136) to investigate HPD during immunotherapy. One of the biggest issues is that the studies that have reported on HPD have been retrospective, with a lack of comparator groups and a lack of a standardized definition of hyperprogression. Dr. Sehgal emphasized the need to study hyperprogression in well-designed prospective studies.
Existing data on HPD
HPD was described as “a new pattern of progression” seen in patients undergoing immune checkpoint inhibitor therapy in a 2017 article published in Clinical Cancer Research. Authors Stephane Champiat, MD, PhD, of Institut Gustave Roussy, Universite Paris Saclay, Villejuif, France, and colleagues cited “anecdotal occurrences” of HPD among patients in phase 1 trials of anti–PD-1/PD-L1 agents.
In that study, HPD was defined by tumor growth rate ratio. The incidence was 9% among 213 patients.
The findings raised concerns about treating elderly patients with anti–PD-1/PD-L1 monotherapy, according to the authors, who called for further study.
That same year, Roberto Ferrara, MD, and colleagues from the Insitut Gustave Roussy reported additional data indicating an incidence of HPD of 16% among 333 patients with non–small cell lung cancer who underwent immunotherapy at eight centers from 2012 to 2017. The findings, which were presented at the 2017 World Conference on Lung Cancer and reported at the time by this news organization, also showed that the incidence of HPD was higher with immunotherapy than with single-agent chemotherapy (5%).
Median overall survival (OS) was just 3.4 months among those with HPD, compared with 13 months in the overall study population – worse, even, than the median 5.4-month OS observed among patients with progressive disease who received immunotherapy.
In the wake of these findings, numerous researchers have attempted to better define HPD, its incidence, and patient factors associated with developing HPD while undergoing immunotherapy.
However, there is little so far to show for those efforts, Vivek Subbiah, MD, of the University of Texas MD Anderson Cancer Center, Houston, said in an interview.
“Many questions remain to be answered,” said Dr. Subbiah, clinical medical director of the Clinical Center for Targeted Therapy in the division of cancer medicine at MD Anderson. He was the senior author of the “Fact, Fiction, or Alternative Fact?” commentary.
Work is underway to elucidate biological mechanisms. Some groups have implicated the Fc region of antibodies. Another group has reported EGFR and MDM2/MDM4 amplifications in patients with HPD, Dr. Subbiah and colleagues noted.
Other “proposed contributing pathological mechanisms include modulation of tumor immune microenvironment through macrophages and regulatory T cells as well as activation of oncogenic signaling pathways,” noted Dr. Sehgal.
Both groups of authors emphasize the urgent need for prospective studies.
It is imperative to confirm underlying biology, predict which patients are at risk, and identify therapeutic directions for patients who experience HPD, Dr. Subbiah said.
The main challenge is defining HPD, he added. Definitions that have been proposed include tumor growth at least two times greater than in control persons, a 15% increase in tumor burden in a set period, and disease progression of 50% from the first evaluation before treatment, he said.
The recent meta-analysis by Hyo Jung Park, MD, PhD, and colleagues, which Dr. Sehgal addressed in his invited commentary, highlights the many approaches used for defining HPD.
Depending on the definition used, the incidence of HPD across 24 studies involving more than 3,100 patients ranged from 5.9% to 43.1%.
“Hyperprogressive disease could be overestimated or underestimated based on current assessment,” Dr. Park and colleagues concluded. They highlighted the importance of “establishing uniform and clinically relevant criteria based on currently available evidence.”
Steps for solving the HPD mystery
“I think we need to come up with consensus criteria for an HPD definition. We need a unified definition,” Dr. Subbiah said. “We also need to design prospective studies to prove or disprove the immunotherapy-HPD association.”
Prospective registries with independent review of patients with suspected immunotherapy-related HPD would be useful for assessing the true incidence and the biology of HPD among patients undergoing immunotherapy, he suggested.
“We need to know the immunologic signals of HPD. This can give us an idea if patients can be prospectively identified for being at risk,” he said. “We also need to know what to do if they are at risk.”
Dr. Sehgal also called for consensus on an HPD definition, with input from a multidisciplinary group that includes “colleagues from radiology, medical oncology, radiation oncology. Getting expertise from different disciplines would be helpful,” he said.
Dr. Park and colleagues suggested several key requirements for an optimal HP definition, such as the inclusion of multiple variables for measuring tumor growth acceleration, “sufficiently quantitative” criteria for determining time to failure, and establishment of a standardized measure of tumor growth acceleration.
The agreed-upon definition of HPD could be applied to patients in a prospective registry and to existing trial data, Dr. Sehgal said.
“Eventually, the goal of this exercise is to [determine] how we can help our patients the best, having a biomarker that can at least inform us in terms of being aware and being proactive in terms of looking for this ... so that interventions can be brought on earlier,” he said.
“If we know what may be a biological mechanism, we can design trials that are designed to look at how to overcome that HPD,” he said.
Dr. Sehgal said he believes HPD is triggered in some way by treatment, including immunotherapy, chemotherapy, and targeted therapy, but perhaps in different ways for each.
He estimated the true incidence of immunotherapy-related HPD will be in the 9%-10% range.
“This is a substantial number of patients, so it’s important that we try to understand this phenomenon, using, again, uniform criteria,” he said.
Current treatment decision-making
Until more is known, Dr. Sehgal said he considers the potential risk factors when treating patients with immunotherapy.
For example, the presence of MDM2 or MDM4 amplification on a genomic profile may factor into his treatment decision-making when it comes to using immunotherapy or immunotherapy in combination with chemotherapy, he said.
“Is that the only factor that is going to make me choose one thing or another? No,” Dr. Sehgal said. However, he said it would make him more “proactive in making sure the patient is doing clinically okay” and in determining when to obtain on-treatment imaging studies.
Dr. Subbiah emphasized the relative benefit of immunotherapy, noting that survival with chemotherapy for many difficult-to-treat cancers in the relapsed/refractory metastatic setting is less than 2 years.
Immunotherapy with checkpoint inhibitors has allowed some of these patients to live longer (with survival reported to be more than 10 years for patients with metastatic melanoma).
“Immunotherapy has been a game changer; it has been transformative in the lives of these patients,” Dr. Subbiah said. “So unless there is any other contraindication, the benefit of receiving immunotherapy for an approved indication far outweighs the risk of HPD.”
A version of this article first appeared on Medscape.com.
Immunotherapy with checkpoint inhibitors has ushered in a new era of cancer therapy, with some patients showing dramatic responses and significantly better outcomes than with other therapies across many cancer types. But some patients do worse, sometimes much worse.
A subset of patients who undergo immunotherapy experience unexpected, rapid disease progression, with a dramatic acceleration of disease trajectory. They also have a shorter progression-free survival and overall survival than would have been expected.
This has been described as hyperprogression and has been termed “hyperprogressive disease” (HPD). It has been seen in a variety of cancers; the incidence ranges from 4% to 29% in the studies reported to date.
There has been some debate over whether this is a real phenomenon or whether it is part of the natural course of disease.
HPD is a “provocative phenomenon,” wrote the authors of a recent commentary entitled “Hyperprogression and Immunotherapy: Fact, Fiction, or Alternative Fact?”
“This phenomenon has polarized oncologists who debate that this could still reflect the natural history of the disease,” said the author of another commentary.
But the tide is now turning toward acceptance of HPD, said Kartik Sehgal, MD, an oncologist at Dana-Farber Cancer Institute and Harvard University, both in Boston.
“With publication of multiple clinical reports of different cancer types worldwide, hyperprogression is now accepted by most oncologists to be a true phenomenon rather than natural progression of disease,” Dr. Sehgal said.
He authored an invited commentary in JAMA Network Openabout one of the latest meta-analyses (JAMA Netw Open. 2021;4[3]:e211136) to investigate HPD during immunotherapy. One of the biggest issues is that the studies that have reported on HPD have been retrospective, with a lack of comparator groups and a lack of a standardized definition of hyperprogression. Dr. Sehgal emphasized the need to study hyperprogression in well-designed prospective studies.
Existing data on HPD
HPD was described as “a new pattern of progression” seen in patients undergoing immune checkpoint inhibitor therapy in a 2017 article published in Clinical Cancer Research. Authors Stephane Champiat, MD, PhD, of Institut Gustave Roussy, Universite Paris Saclay, Villejuif, France, and colleagues cited “anecdotal occurrences” of HPD among patients in phase 1 trials of anti–PD-1/PD-L1 agents.
In that study, HPD was defined by tumor growth rate ratio. The incidence was 9% among 213 patients.
The findings raised concerns about treating elderly patients with anti–PD-1/PD-L1 monotherapy, according to the authors, who called for further study.
That same year, Roberto Ferrara, MD, and colleagues from the Insitut Gustave Roussy reported additional data indicating an incidence of HPD of 16% among 333 patients with non–small cell lung cancer who underwent immunotherapy at eight centers from 2012 to 2017. The findings, which were presented at the 2017 World Conference on Lung Cancer and reported at the time by this news organization, also showed that the incidence of HPD was higher with immunotherapy than with single-agent chemotherapy (5%).
Median overall survival (OS) was just 3.4 months among those with HPD, compared with 13 months in the overall study population – worse, even, than the median 5.4-month OS observed among patients with progressive disease who received immunotherapy.
In the wake of these findings, numerous researchers have attempted to better define HPD, its incidence, and patient factors associated with developing HPD while undergoing immunotherapy.
However, there is little so far to show for those efforts, Vivek Subbiah, MD, of the University of Texas MD Anderson Cancer Center, Houston, said in an interview.
“Many questions remain to be answered,” said Dr. Subbiah, clinical medical director of the Clinical Center for Targeted Therapy in the division of cancer medicine at MD Anderson. He was the senior author of the “Fact, Fiction, or Alternative Fact?” commentary.
Work is underway to elucidate biological mechanisms. Some groups have implicated the Fc region of antibodies. Another group has reported EGFR and MDM2/MDM4 amplifications in patients with HPD, Dr. Subbiah and colleagues noted.
Other “proposed contributing pathological mechanisms include modulation of tumor immune microenvironment through macrophages and regulatory T cells as well as activation of oncogenic signaling pathways,” noted Dr. Sehgal.
Both groups of authors emphasize the urgent need for prospective studies.
It is imperative to confirm underlying biology, predict which patients are at risk, and identify therapeutic directions for patients who experience HPD, Dr. Subbiah said.
The main challenge is defining HPD, he added. Definitions that have been proposed include tumor growth at least two times greater than in control persons, a 15% increase in tumor burden in a set period, and disease progression of 50% from the first evaluation before treatment, he said.
The recent meta-analysis by Hyo Jung Park, MD, PhD, and colleagues, which Dr. Sehgal addressed in his invited commentary, highlights the many approaches used for defining HPD.
Depending on the definition used, the incidence of HPD across 24 studies involving more than 3,100 patients ranged from 5.9% to 43.1%.
“Hyperprogressive disease could be overestimated or underestimated based on current assessment,” Dr. Park and colleagues concluded. They highlighted the importance of “establishing uniform and clinically relevant criteria based on currently available evidence.”
Steps for solving the HPD mystery
“I think we need to come up with consensus criteria for an HPD definition. We need a unified definition,” Dr. Subbiah said. “We also need to design prospective studies to prove or disprove the immunotherapy-HPD association.”
Prospective registries with independent review of patients with suspected immunotherapy-related HPD would be useful for assessing the true incidence and the biology of HPD among patients undergoing immunotherapy, he suggested.
“We need to know the immunologic signals of HPD. This can give us an idea if patients can be prospectively identified for being at risk,” he said. “We also need to know what to do if they are at risk.”
Dr. Sehgal also called for consensus on an HPD definition, with input from a multidisciplinary group that includes “colleagues from radiology, medical oncology, radiation oncology. Getting expertise from different disciplines would be helpful,” he said.
Dr. Park and colleagues suggested several key requirements for an optimal HP definition, such as the inclusion of multiple variables for measuring tumor growth acceleration, “sufficiently quantitative” criteria for determining time to failure, and establishment of a standardized measure of tumor growth acceleration.
The agreed-upon definition of HPD could be applied to patients in a prospective registry and to existing trial data, Dr. Sehgal said.
“Eventually, the goal of this exercise is to [determine] how we can help our patients the best, having a biomarker that can at least inform us in terms of being aware and being proactive in terms of looking for this ... so that interventions can be brought on earlier,” he said.
“If we know what may be a biological mechanism, we can design trials that are designed to look at how to overcome that HPD,” he said.
Dr. Sehgal said he believes HPD is triggered in some way by treatment, including immunotherapy, chemotherapy, and targeted therapy, but perhaps in different ways for each.
He estimated the true incidence of immunotherapy-related HPD will be in the 9%-10% range.
“This is a substantial number of patients, so it’s important that we try to understand this phenomenon, using, again, uniform criteria,” he said.
Current treatment decision-making
Until more is known, Dr. Sehgal said he considers the potential risk factors when treating patients with immunotherapy.
For example, the presence of MDM2 or MDM4 amplification on a genomic profile may factor into his treatment decision-making when it comes to using immunotherapy or immunotherapy in combination with chemotherapy, he said.
“Is that the only factor that is going to make me choose one thing or another? No,” Dr. Sehgal said. However, he said it would make him more “proactive in making sure the patient is doing clinically okay” and in determining when to obtain on-treatment imaging studies.
Dr. Subbiah emphasized the relative benefit of immunotherapy, noting that survival with chemotherapy for many difficult-to-treat cancers in the relapsed/refractory metastatic setting is less than 2 years.
Immunotherapy with checkpoint inhibitors has allowed some of these patients to live longer (with survival reported to be more than 10 years for patients with metastatic melanoma).
“Immunotherapy has been a game changer; it has been transformative in the lives of these patients,” Dr. Subbiah said. “So unless there is any other contraindication, the benefit of receiving immunotherapy for an approved indication far outweighs the risk of HPD.”
A version of this article first appeared on Medscape.com.
Immunotherapy with checkpoint inhibitors has ushered in a new era of cancer therapy, with some patients showing dramatic responses and significantly better outcomes than with other therapies across many cancer types. But some patients do worse, sometimes much worse.
A subset of patients who undergo immunotherapy experience unexpected, rapid disease progression, with a dramatic acceleration of disease trajectory. They also have a shorter progression-free survival and overall survival than would have been expected.
This has been described as hyperprogression and has been termed “hyperprogressive disease” (HPD). It has been seen in a variety of cancers; the incidence ranges from 4% to 29% in the studies reported to date.
There has been some debate over whether this is a real phenomenon or whether it is part of the natural course of disease.
HPD is a “provocative phenomenon,” wrote the authors of a recent commentary entitled “Hyperprogression and Immunotherapy: Fact, Fiction, or Alternative Fact?”
“This phenomenon has polarized oncologists who debate that this could still reflect the natural history of the disease,” said the author of another commentary.
But the tide is now turning toward acceptance of HPD, said Kartik Sehgal, MD, an oncologist at Dana-Farber Cancer Institute and Harvard University, both in Boston.
“With publication of multiple clinical reports of different cancer types worldwide, hyperprogression is now accepted by most oncologists to be a true phenomenon rather than natural progression of disease,” Dr. Sehgal said.
He authored an invited commentary in JAMA Network Openabout one of the latest meta-analyses (JAMA Netw Open. 2021;4[3]:e211136) to investigate HPD during immunotherapy. One of the biggest issues is that the studies that have reported on HPD have been retrospective, with a lack of comparator groups and a lack of a standardized definition of hyperprogression. Dr. Sehgal emphasized the need to study hyperprogression in well-designed prospective studies.
Existing data on HPD
HPD was described as “a new pattern of progression” seen in patients undergoing immune checkpoint inhibitor therapy in a 2017 article published in Clinical Cancer Research. Authors Stephane Champiat, MD, PhD, of Institut Gustave Roussy, Universite Paris Saclay, Villejuif, France, and colleagues cited “anecdotal occurrences” of HPD among patients in phase 1 trials of anti–PD-1/PD-L1 agents.
In that study, HPD was defined by tumor growth rate ratio. The incidence was 9% among 213 patients.
The findings raised concerns about treating elderly patients with anti–PD-1/PD-L1 monotherapy, according to the authors, who called for further study.
That same year, Roberto Ferrara, MD, and colleagues from the Insitut Gustave Roussy reported additional data indicating an incidence of HPD of 16% among 333 patients with non–small cell lung cancer who underwent immunotherapy at eight centers from 2012 to 2017. The findings, which were presented at the 2017 World Conference on Lung Cancer and reported at the time by this news organization, also showed that the incidence of HPD was higher with immunotherapy than with single-agent chemotherapy (5%).
Median overall survival (OS) was just 3.4 months among those with HPD, compared with 13 months in the overall study population – worse, even, than the median 5.4-month OS observed among patients with progressive disease who received immunotherapy.
In the wake of these findings, numerous researchers have attempted to better define HPD, its incidence, and patient factors associated with developing HPD while undergoing immunotherapy.
However, there is little so far to show for those efforts, Vivek Subbiah, MD, of the University of Texas MD Anderson Cancer Center, Houston, said in an interview.
“Many questions remain to be answered,” said Dr. Subbiah, clinical medical director of the Clinical Center for Targeted Therapy in the division of cancer medicine at MD Anderson. He was the senior author of the “Fact, Fiction, or Alternative Fact?” commentary.
Work is underway to elucidate biological mechanisms. Some groups have implicated the Fc region of antibodies. Another group has reported EGFR and MDM2/MDM4 amplifications in patients with HPD, Dr. Subbiah and colleagues noted.
Other “proposed contributing pathological mechanisms include modulation of tumor immune microenvironment through macrophages and regulatory T cells as well as activation of oncogenic signaling pathways,” noted Dr. Sehgal.
Both groups of authors emphasize the urgent need for prospective studies.
It is imperative to confirm underlying biology, predict which patients are at risk, and identify therapeutic directions for patients who experience HPD, Dr. Subbiah said.
The main challenge is defining HPD, he added. Definitions that have been proposed include tumor growth at least two times greater than in control persons, a 15% increase in tumor burden in a set period, and disease progression of 50% from the first evaluation before treatment, he said.
The recent meta-analysis by Hyo Jung Park, MD, PhD, and colleagues, which Dr. Sehgal addressed in his invited commentary, highlights the many approaches used for defining HPD.
Depending on the definition used, the incidence of HPD across 24 studies involving more than 3,100 patients ranged from 5.9% to 43.1%.
“Hyperprogressive disease could be overestimated or underestimated based on current assessment,” Dr. Park and colleagues concluded. They highlighted the importance of “establishing uniform and clinically relevant criteria based on currently available evidence.”
Steps for solving the HPD mystery
“I think we need to come up with consensus criteria for an HPD definition. We need a unified definition,” Dr. Subbiah said. “We also need to design prospective studies to prove or disprove the immunotherapy-HPD association.”
Prospective registries with independent review of patients with suspected immunotherapy-related HPD would be useful for assessing the true incidence and the biology of HPD among patients undergoing immunotherapy, he suggested.
“We need to know the immunologic signals of HPD. This can give us an idea if patients can be prospectively identified for being at risk,” he said. “We also need to know what to do if they are at risk.”
Dr. Sehgal also called for consensus on an HPD definition, with input from a multidisciplinary group that includes “colleagues from radiology, medical oncology, radiation oncology. Getting expertise from different disciplines would be helpful,” he said.
Dr. Park and colleagues suggested several key requirements for an optimal HP definition, such as the inclusion of multiple variables for measuring tumor growth acceleration, “sufficiently quantitative” criteria for determining time to failure, and establishment of a standardized measure of tumor growth acceleration.
The agreed-upon definition of HPD could be applied to patients in a prospective registry and to existing trial data, Dr. Sehgal said.
“Eventually, the goal of this exercise is to [determine] how we can help our patients the best, having a biomarker that can at least inform us in terms of being aware and being proactive in terms of looking for this ... so that interventions can be brought on earlier,” he said.
“If we know what may be a biological mechanism, we can design trials that are designed to look at how to overcome that HPD,” he said.
Dr. Sehgal said he believes HPD is triggered in some way by treatment, including immunotherapy, chemotherapy, and targeted therapy, but perhaps in different ways for each.
He estimated the true incidence of immunotherapy-related HPD will be in the 9%-10% range.
“This is a substantial number of patients, so it’s important that we try to understand this phenomenon, using, again, uniform criteria,” he said.
Current treatment decision-making
Until more is known, Dr. Sehgal said he considers the potential risk factors when treating patients with immunotherapy.
For example, the presence of MDM2 or MDM4 amplification on a genomic profile may factor into his treatment decision-making when it comes to using immunotherapy or immunotherapy in combination with chemotherapy, he said.
“Is that the only factor that is going to make me choose one thing or another? No,” Dr. Sehgal said. However, he said it would make him more “proactive in making sure the patient is doing clinically okay” and in determining when to obtain on-treatment imaging studies.
Dr. Subbiah emphasized the relative benefit of immunotherapy, noting that survival with chemotherapy for many difficult-to-treat cancers in the relapsed/refractory metastatic setting is less than 2 years.
Immunotherapy with checkpoint inhibitors has allowed some of these patients to live longer (with survival reported to be more than 10 years for patients with metastatic melanoma).
“Immunotherapy has been a game changer; it has been transformative in the lives of these patients,” Dr. Subbiah said. “So unless there is any other contraindication, the benefit of receiving immunotherapy for an approved indication far outweighs the risk of HPD.”
A version of this article first appeared on Medscape.com.
Lobaplatin deemed ‘promising alternative’ to cisplatin in nasopharyngeal carcinoma
Lobaplatin-based induction plus chemoradiation was as effective as, and safer than, a cisplatin-based regimen in a phase 3 trial of more than 500 patients with advanced nasopharyngeal carcinoma.
The lobaplatin regimen proved to be a “promising alternative regimen to cisplatin-based treatment,” Xing Lv, MD, of Sun Yat-sen University Cancer Centre in Guangzhou, China, and colleagues wrote in The Lancet Oncology.
A lobaplatin-based regimen might be particularly attractive when cisplatin is contraindicated, according to authors of a related editorial.
Given the encouraging results in this trial, lobaplatin might even “overtake carboplatin” in cisplatin-ineligible patients, wrote the editorialists, Stefano Cavalieri, MD, and Lisa Licitra, MD, both of Fondazione IRCCS Istituto Nazionale dei Tumori di Milano in Italy.
“Furthermore, the optimal treatment compliance in patients receiving lobaplatin [in the trial] suggests that this agent might be a good candidate for research into escalation strategies,” the editorialists wrote.
Study rationale
Cisplatin-based induction followed by concurrent chemoradiotherapy is a standard treatment option in the United States, recommended by National Comprehensive Cancer Network guidelines for stage II-IVB nasopharyngeal carcinoma.
However, cisplatin is associated with poor treatment compliance due to significant hematologic and nonhematologic side effects. Cisplatin also requires increased hydration for renal protection, increasing the risk of fluid overload.
“Safer and more effective platinum drugs are needed for the treatment of nasopharyngeal carcinoma,” the investigators wrote.
Carboplatin is an alternative available in the United States, but “the evidence supporting equivalence between cisplatin with carboplatin is still controversial” for nasopharyngeal cancer, the editorialists wrote.
Study details
The phase 3 study enrolled 502 patients from five hospitals in China. The patients had previously untreated, non-keratinizing stage III–IVB nasopharyngeal carcinoma and a Karnofsky performance status score of at least 70. The study excluded patients older than 60 years of age, and adequate renal, hematologic, and hepatic function were required.
Half of patients (n = 252) were randomized to induction with lobaplatin and fluorouracil for two cycles followed by concurrent therapy with lobaplatin for two cycles plus intensity-modulated radiotherapy. The other half of patients (n = 250) were randomized to cisplatin-fluorouracil induction for two cycles, followed by intensity-modulated radiotherapy plus two cycles of cisplatin.
The investigators opted for two-cycle chemotherapy instead of three cycles after observing good activity and safety with two cycles in a phase 2 trial.
Treatment compliance was better in the lobaplatin arm, with 91% of those patients completing two cycles of concurrent chemotherapy, compared with 84% of patients in the cisplatin arm.
The 5-year progression-free survival rate was 75% in the lobaplatin arm and 75.5% in the cisplatin arm in the intention-to-treat population (P noninferiority = .007).
In the per-protocol population, the 5-year progression-free survival rates were 74.8% with lobaplatin and 76.4% with cisplatin (P noninferiority = .016).
The most common grade 3-4 adverse events were mucositis (41% in the lobaplatin arm and 40% in the cisplatin arm), leukopenia (16% and 23%, respectively), and neutropenia (10% and 24%, respectively).
Grade 1-2 nephrotoxicity, nausea, vomiting, and weight loss were significantly less common with lobaplatin (P < .0001 for all).
Questions about generalizability
The editorialists urged caution about generalizing the study results to a wider population.
Study subjects were younger and had no major comorbidities as well as good renal function, which is not always the case with nasopharyngeal carcinoma. In addition, although antiemesis prophylaxis was the standard of care for the study period (2013-2015), guidelines have since been updated to included more intense premedication, which likely would have reduced nausea, vomiting, weight loss, and possibly nephrotoxicity, especially in the cisplatin group.
Induction chemotherapy consisted of cisplatin and fluorouracil, whereas current standards for induction chemotherapy are cisplatin plus gemcitabine or docetaxel plus cisplatin and fluorouracil, the editorialists noted.
As for the two-cycle approach, it’s known that concomitant chemoradiotherapy with two cycles of cisplatin in head and neck cancer “provides similar outcomes to concomitant chemoradiotherapy with three cycles, but only in cases of accelerated intensity-modulated radiotherapy,” not with the standard fractionation used in the study (30-32 fractions, 5 days per week), the editorialists wrote.
The study was funded by the National Science and Technology Pillar Program, International Cooperation Project of Science and Technology Program of Guangdong Province, Planned Science and Technology Project of Guangdong Province, and Cultivation Foundation for the Junior Teachers at Sun Yat-sen University. The investigators and Dr. Cavalieri declared no competing interests. Dr. Licitra disclosed relationships AstraZeneca, Bristol Myers Squibb, and Hoffmann-La Roche.
Lobaplatin-based induction plus chemoradiation was as effective as, and safer than, a cisplatin-based regimen in a phase 3 trial of more than 500 patients with advanced nasopharyngeal carcinoma.
The lobaplatin regimen proved to be a “promising alternative regimen to cisplatin-based treatment,” Xing Lv, MD, of Sun Yat-sen University Cancer Centre in Guangzhou, China, and colleagues wrote in The Lancet Oncology.
A lobaplatin-based regimen might be particularly attractive when cisplatin is contraindicated, according to authors of a related editorial.
Given the encouraging results in this trial, lobaplatin might even “overtake carboplatin” in cisplatin-ineligible patients, wrote the editorialists, Stefano Cavalieri, MD, and Lisa Licitra, MD, both of Fondazione IRCCS Istituto Nazionale dei Tumori di Milano in Italy.
“Furthermore, the optimal treatment compliance in patients receiving lobaplatin [in the trial] suggests that this agent might be a good candidate for research into escalation strategies,” the editorialists wrote.
Study rationale
Cisplatin-based induction followed by concurrent chemoradiotherapy is a standard treatment option in the United States, recommended by National Comprehensive Cancer Network guidelines for stage II-IVB nasopharyngeal carcinoma.
However, cisplatin is associated with poor treatment compliance due to significant hematologic and nonhematologic side effects. Cisplatin also requires increased hydration for renal protection, increasing the risk of fluid overload.
“Safer and more effective platinum drugs are needed for the treatment of nasopharyngeal carcinoma,” the investigators wrote.
Carboplatin is an alternative available in the United States, but “the evidence supporting equivalence between cisplatin with carboplatin is still controversial” for nasopharyngeal cancer, the editorialists wrote.
Study details
The phase 3 study enrolled 502 patients from five hospitals in China. The patients had previously untreated, non-keratinizing stage III–IVB nasopharyngeal carcinoma and a Karnofsky performance status score of at least 70. The study excluded patients older than 60 years of age, and adequate renal, hematologic, and hepatic function were required.
Half of patients (n = 252) were randomized to induction with lobaplatin and fluorouracil for two cycles followed by concurrent therapy with lobaplatin for two cycles plus intensity-modulated radiotherapy. The other half of patients (n = 250) were randomized to cisplatin-fluorouracil induction for two cycles, followed by intensity-modulated radiotherapy plus two cycles of cisplatin.
The investigators opted for two-cycle chemotherapy instead of three cycles after observing good activity and safety with two cycles in a phase 2 trial.
Treatment compliance was better in the lobaplatin arm, with 91% of those patients completing two cycles of concurrent chemotherapy, compared with 84% of patients in the cisplatin arm.
The 5-year progression-free survival rate was 75% in the lobaplatin arm and 75.5% in the cisplatin arm in the intention-to-treat population (P noninferiority = .007).
In the per-protocol population, the 5-year progression-free survival rates were 74.8% with lobaplatin and 76.4% with cisplatin (P noninferiority = .016).
The most common grade 3-4 adverse events were mucositis (41% in the lobaplatin arm and 40% in the cisplatin arm), leukopenia (16% and 23%, respectively), and neutropenia (10% and 24%, respectively).
Grade 1-2 nephrotoxicity, nausea, vomiting, and weight loss were significantly less common with lobaplatin (P < .0001 for all).
Questions about generalizability
The editorialists urged caution about generalizing the study results to a wider population.
Study subjects were younger and had no major comorbidities as well as good renal function, which is not always the case with nasopharyngeal carcinoma. In addition, although antiemesis prophylaxis was the standard of care for the study period (2013-2015), guidelines have since been updated to included more intense premedication, which likely would have reduced nausea, vomiting, weight loss, and possibly nephrotoxicity, especially in the cisplatin group.
Induction chemotherapy consisted of cisplatin and fluorouracil, whereas current standards for induction chemotherapy are cisplatin plus gemcitabine or docetaxel plus cisplatin and fluorouracil, the editorialists noted.
As for the two-cycle approach, it’s known that concomitant chemoradiotherapy with two cycles of cisplatin in head and neck cancer “provides similar outcomes to concomitant chemoradiotherapy with three cycles, but only in cases of accelerated intensity-modulated radiotherapy,” not with the standard fractionation used in the study (30-32 fractions, 5 days per week), the editorialists wrote.
The study was funded by the National Science and Technology Pillar Program, International Cooperation Project of Science and Technology Program of Guangdong Province, Planned Science and Technology Project of Guangdong Province, and Cultivation Foundation for the Junior Teachers at Sun Yat-sen University. The investigators and Dr. Cavalieri declared no competing interests. Dr. Licitra disclosed relationships AstraZeneca, Bristol Myers Squibb, and Hoffmann-La Roche.
Lobaplatin-based induction plus chemoradiation was as effective as, and safer than, a cisplatin-based regimen in a phase 3 trial of more than 500 patients with advanced nasopharyngeal carcinoma.
The lobaplatin regimen proved to be a “promising alternative regimen to cisplatin-based treatment,” Xing Lv, MD, of Sun Yat-sen University Cancer Centre in Guangzhou, China, and colleagues wrote in The Lancet Oncology.
A lobaplatin-based regimen might be particularly attractive when cisplatin is contraindicated, according to authors of a related editorial.
Given the encouraging results in this trial, lobaplatin might even “overtake carboplatin” in cisplatin-ineligible patients, wrote the editorialists, Stefano Cavalieri, MD, and Lisa Licitra, MD, both of Fondazione IRCCS Istituto Nazionale dei Tumori di Milano in Italy.
“Furthermore, the optimal treatment compliance in patients receiving lobaplatin [in the trial] suggests that this agent might be a good candidate for research into escalation strategies,” the editorialists wrote.
Study rationale
Cisplatin-based induction followed by concurrent chemoradiotherapy is a standard treatment option in the United States, recommended by National Comprehensive Cancer Network guidelines for stage II-IVB nasopharyngeal carcinoma.
However, cisplatin is associated with poor treatment compliance due to significant hematologic and nonhematologic side effects. Cisplatin also requires increased hydration for renal protection, increasing the risk of fluid overload.
“Safer and more effective platinum drugs are needed for the treatment of nasopharyngeal carcinoma,” the investigators wrote.
Carboplatin is an alternative available in the United States, but “the evidence supporting equivalence between cisplatin with carboplatin is still controversial” for nasopharyngeal cancer, the editorialists wrote.
Study details
The phase 3 study enrolled 502 patients from five hospitals in China. The patients had previously untreated, non-keratinizing stage III–IVB nasopharyngeal carcinoma and a Karnofsky performance status score of at least 70. The study excluded patients older than 60 years of age, and adequate renal, hematologic, and hepatic function were required.
Half of patients (n = 252) were randomized to induction with lobaplatin and fluorouracil for two cycles followed by concurrent therapy with lobaplatin for two cycles plus intensity-modulated radiotherapy. The other half of patients (n = 250) were randomized to cisplatin-fluorouracil induction for two cycles, followed by intensity-modulated radiotherapy plus two cycles of cisplatin.
The investigators opted for two-cycle chemotherapy instead of three cycles after observing good activity and safety with two cycles in a phase 2 trial.
Treatment compliance was better in the lobaplatin arm, with 91% of those patients completing two cycles of concurrent chemotherapy, compared with 84% of patients in the cisplatin arm.
The 5-year progression-free survival rate was 75% in the lobaplatin arm and 75.5% in the cisplatin arm in the intention-to-treat population (P noninferiority = .007).
In the per-protocol population, the 5-year progression-free survival rates were 74.8% with lobaplatin and 76.4% with cisplatin (P noninferiority = .016).
The most common grade 3-4 adverse events were mucositis (41% in the lobaplatin arm and 40% in the cisplatin arm), leukopenia (16% and 23%, respectively), and neutropenia (10% and 24%, respectively).
Grade 1-2 nephrotoxicity, nausea, vomiting, and weight loss were significantly less common with lobaplatin (P < .0001 for all).
Questions about generalizability
The editorialists urged caution about generalizing the study results to a wider population.
Study subjects were younger and had no major comorbidities as well as good renal function, which is not always the case with nasopharyngeal carcinoma. In addition, although antiemesis prophylaxis was the standard of care for the study period (2013-2015), guidelines have since been updated to included more intense premedication, which likely would have reduced nausea, vomiting, weight loss, and possibly nephrotoxicity, especially in the cisplatin group.
Induction chemotherapy consisted of cisplatin and fluorouracil, whereas current standards for induction chemotherapy are cisplatin plus gemcitabine or docetaxel plus cisplatin and fluorouracil, the editorialists noted.
As for the two-cycle approach, it’s known that concomitant chemoradiotherapy with two cycles of cisplatin in head and neck cancer “provides similar outcomes to concomitant chemoradiotherapy with three cycles, but only in cases of accelerated intensity-modulated radiotherapy,” not with the standard fractionation used in the study (30-32 fractions, 5 days per week), the editorialists wrote.
The study was funded by the National Science and Technology Pillar Program, International Cooperation Project of Science and Technology Program of Guangdong Province, Planned Science and Technology Project of Guangdong Province, and Cultivation Foundation for the Junior Teachers at Sun Yat-sen University. The investigators and Dr. Cavalieri declared no competing interests. Dr. Licitra disclosed relationships AstraZeneca, Bristol Myers Squibb, and Hoffmann-La Roche.
FROM THE LANCET ONCOLOGY
Personalized cancer vaccine shows early promise across tumor types
The vaccine, PGV-001, was given to 13 patients with solid tumors or multiple myeloma who had a high risk of recurrence after surgery or autologous stem cell transplant.
At last follow-up, four patients were still alive without evidence of disease and had not received subsequent therapy, four were alive and receiving therapy, three had died, and two were lost to follow-up.
Thomas Marron, MD, PhD , of Mount Sinai in New York presented these results in a poster at the American Association for Cancer Research Annual Meeting 2021: Week 1 ( Abstract LB048 ). Data in the abstract differ from the data presented.
“While cancer immunotherapy has revolutionized the treatment of cancer, we know that the majority of patients fail to achieve significant clinical response,” Dr. Marron said during his presentation. “One reason for this may be due to lack of preexisting primed T-cell response needed for PD-1 blockade to have a significant effect. To address this, personalized neoantigen vaccines may help prime an improved immune response against tumor cells.”
With this in mind, Dr. Marron and colleagues developed PGV-001, a vaccine consisting of patient-specific synthetic neoantigen peptides given to patients in the adjuvant setting.
Creating a personalized vaccine
The researchers synthesized PGV-001 for 15 patients with advanced malignancies. The patients first underwent tumor and germline DNA sequencing as well as HLA typing. Bulk RNA sequencing was performed on patients’ tumors as well.
Then, the researchers used a computational pipeline called OpenVax to identify candidate neoantigens. This pipeline, developed at Mount Sinai, identified and prioritized candidate neoantigens using predicted MHC class I binding affinity and neoantigen abundance.
OpenVax identified an average of 71.5 neoantigens per patient (range, 7-193). The goal was to synthesize a maximum of 10 peptides per patient, but two patients did not have an adequate number of neoantigens.
Vaccine administration
The peptides were administered over the course of 27 weeks along with poly-ICLC and a tetanus helper peptide. Before receiving their vaccine doses, patients with solid tumors had undergone curative-intent surgery, and those with multiple myeloma had undergone autologous stem cell transplant.
“Most experimental personalized cancer vaccines are administered in the metastatic setting, but prior research indicates that immunotherapies tend to be more effective in patients who have less cancer spread,” principal investigator Nina Bhardwaj, MD, PhD , of Mount Sinai, explained in a press release .
“We have, therefore, developed a neoantigen vaccine that is administered after standard-of-care adjuvant therapy, such as surgery in solid tumors and bone marrow transplant in multiple myeloma, when patients have minimal, typically microscopic, residual disease.”
Feasibility, safety, and immunogenicity
PGV-001 was synthesized for 15 patients and administered to 13 of them. Six of the 13 patients had head and neck squamous cell carcinoma, three had multiple myeloma, two had non–small cell lung cancer, one had breast cancer, and one had urothelial carcinoma.
Eleven patients received all 10 intended doses, and two patients received at least 8 doses.
“The vaccine was well tolerated, with only half of patients experiencing mild, grade 1 adverse events,” Dr. Marron said.
Transient injection site reactions occurred in four patients, and grade 1 fever was reported in one patient.
Immune monitoring is ongoing, but an initial analysis in one patient showed “robust responses” in CD4 and CD8 T cells by intracellular cytokine staining for interferon-gamma, tumor necrosis factor–alpha, and interleukin-2 after in vitro expansion in the presence of vaccine antigens, according to the researchers.
Dr. Marron noted that robust T-cell reactivity was seen at the completion of all 10 doses but was not seen after the 6th dose, and this supports the need for a prolonged dosing schedule.
Survival and subsequent therapy
At a mean follow-up of 880 days, four patients had no evidence of disease and had not received subsequent therapy. This includes one patient with stage IIIA non–small cell lung cancer, one with stage IVA HER-2 positive breast cancer, one with stage II urothelial carcinoma, and one with multiple myeloma.
Four patients were alive and receiving subsequent lines of therapy. Two of these patients had significant responses to anti–PD-1 therapy.
Three patients have died, two of whom had documented recurrence of their malignancy. The last two patients were lost to follow-up without documented recurrence.
“Our results demonstrate that the OpenVax pipeline is a viable approach to generate a safe, personalized cancer vaccine, which could potentially be used to treat a range of tumor types,” Dr. Bhardwaj said.
Trials combining neoantigens identified with the OpenVax platform are ongoing in patients with urothelial carcinoma and glioblastoma multiforme, Dr. Marron said.
The current study ( NCT02721043 ) is sponsored by Dr. Bhardwaj. Dr. Marron and Dr. Bhardwaj reported having no disclosures. Their colleagues disclosed relationships with Bristol Myers Squibb, Sema4, and Related Sciences.
The vaccine, PGV-001, was given to 13 patients with solid tumors or multiple myeloma who had a high risk of recurrence after surgery or autologous stem cell transplant.
At last follow-up, four patients were still alive without evidence of disease and had not received subsequent therapy, four were alive and receiving therapy, three had died, and two were lost to follow-up.
Thomas Marron, MD, PhD , of Mount Sinai in New York presented these results in a poster at the American Association for Cancer Research Annual Meeting 2021: Week 1 ( Abstract LB048 ). Data in the abstract differ from the data presented.
“While cancer immunotherapy has revolutionized the treatment of cancer, we know that the majority of patients fail to achieve significant clinical response,” Dr. Marron said during his presentation. “One reason for this may be due to lack of preexisting primed T-cell response needed for PD-1 blockade to have a significant effect. To address this, personalized neoantigen vaccines may help prime an improved immune response against tumor cells.”
With this in mind, Dr. Marron and colleagues developed PGV-001, a vaccine consisting of patient-specific synthetic neoantigen peptides given to patients in the adjuvant setting.
Creating a personalized vaccine
The researchers synthesized PGV-001 for 15 patients with advanced malignancies. The patients first underwent tumor and germline DNA sequencing as well as HLA typing. Bulk RNA sequencing was performed on patients’ tumors as well.
Then, the researchers used a computational pipeline called OpenVax to identify candidate neoantigens. This pipeline, developed at Mount Sinai, identified and prioritized candidate neoantigens using predicted MHC class I binding affinity and neoantigen abundance.
OpenVax identified an average of 71.5 neoantigens per patient (range, 7-193). The goal was to synthesize a maximum of 10 peptides per patient, but two patients did not have an adequate number of neoantigens.
Vaccine administration
The peptides were administered over the course of 27 weeks along with poly-ICLC and a tetanus helper peptide. Before receiving their vaccine doses, patients with solid tumors had undergone curative-intent surgery, and those with multiple myeloma had undergone autologous stem cell transplant.
“Most experimental personalized cancer vaccines are administered in the metastatic setting, but prior research indicates that immunotherapies tend to be more effective in patients who have less cancer spread,” principal investigator Nina Bhardwaj, MD, PhD , of Mount Sinai, explained in a press release .
“We have, therefore, developed a neoantigen vaccine that is administered after standard-of-care adjuvant therapy, such as surgery in solid tumors and bone marrow transplant in multiple myeloma, when patients have minimal, typically microscopic, residual disease.”
Feasibility, safety, and immunogenicity
PGV-001 was synthesized for 15 patients and administered to 13 of them. Six of the 13 patients had head and neck squamous cell carcinoma, three had multiple myeloma, two had non–small cell lung cancer, one had breast cancer, and one had urothelial carcinoma.
Eleven patients received all 10 intended doses, and two patients received at least 8 doses.
“The vaccine was well tolerated, with only half of patients experiencing mild, grade 1 adverse events,” Dr. Marron said.
Transient injection site reactions occurred in four patients, and grade 1 fever was reported in one patient.
Immune monitoring is ongoing, but an initial analysis in one patient showed “robust responses” in CD4 and CD8 T cells by intracellular cytokine staining for interferon-gamma, tumor necrosis factor–alpha, and interleukin-2 after in vitro expansion in the presence of vaccine antigens, according to the researchers.
Dr. Marron noted that robust T-cell reactivity was seen at the completion of all 10 doses but was not seen after the 6th dose, and this supports the need for a prolonged dosing schedule.
Survival and subsequent therapy
At a mean follow-up of 880 days, four patients had no evidence of disease and had not received subsequent therapy. This includes one patient with stage IIIA non–small cell lung cancer, one with stage IVA HER-2 positive breast cancer, one with stage II urothelial carcinoma, and one with multiple myeloma.
Four patients were alive and receiving subsequent lines of therapy. Two of these patients had significant responses to anti–PD-1 therapy.
Three patients have died, two of whom had documented recurrence of their malignancy. The last two patients were lost to follow-up without documented recurrence.
“Our results demonstrate that the OpenVax pipeline is a viable approach to generate a safe, personalized cancer vaccine, which could potentially be used to treat a range of tumor types,” Dr. Bhardwaj said.
Trials combining neoantigens identified with the OpenVax platform are ongoing in patients with urothelial carcinoma and glioblastoma multiforme, Dr. Marron said.
The current study ( NCT02721043 ) is sponsored by Dr. Bhardwaj. Dr. Marron and Dr. Bhardwaj reported having no disclosures. Their colleagues disclosed relationships with Bristol Myers Squibb, Sema4, and Related Sciences.
The vaccine, PGV-001, was given to 13 patients with solid tumors or multiple myeloma who had a high risk of recurrence after surgery or autologous stem cell transplant.
At last follow-up, four patients were still alive without evidence of disease and had not received subsequent therapy, four were alive and receiving therapy, three had died, and two were lost to follow-up.
Thomas Marron, MD, PhD , of Mount Sinai in New York presented these results in a poster at the American Association for Cancer Research Annual Meeting 2021: Week 1 ( Abstract LB048 ). Data in the abstract differ from the data presented.
“While cancer immunotherapy has revolutionized the treatment of cancer, we know that the majority of patients fail to achieve significant clinical response,” Dr. Marron said during his presentation. “One reason for this may be due to lack of preexisting primed T-cell response needed for PD-1 blockade to have a significant effect. To address this, personalized neoantigen vaccines may help prime an improved immune response against tumor cells.”
With this in mind, Dr. Marron and colleagues developed PGV-001, a vaccine consisting of patient-specific synthetic neoantigen peptides given to patients in the adjuvant setting.
Creating a personalized vaccine
The researchers synthesized PGV-001 for 15 patients with advanced malignancies. The patients first underwent tumor and germline DNA sequencing as well as HLA typing. Bulk RNA sequencing was performed on patients’ tumors as well.
Then, the researchers used a computational pipeline called OpenVax to identify candidate neoantigens. This pipeline, developed at Mount Sinai, identified and prioritized candidate neoantigens using predicted MHC class I binding affinity and neoantigen abundance.
OpenVax identified an average of 71.5 neoantigens per patient (range, 7-193). The goal was to synthesize a maximum of 10 peptides per patient, but two patients did not have an adequate number of neoantigens.
Vaccine administration
The peptides were administered over the course of 27 weeks along with poly-ICLC and a tetanus helper peptide. Before receiving their vaccine doses, patients with solid tumors had undergone curative-intent surgery, and those with multiple myeloma had undergone autologous stem cell transplant.
“Most experimental personalized cancer vaccines are administered in the metastatic setting, but prior research indicates that immunotherapies tend to be more effective in patients who have less cancer spread,” principal investigator Nina Bhardwaj, MD, PhD , of Mount Sinai, explained in a press release .
“We have, therefore, developed a neoantigen vaccine that is administered after standard-of-care adjuvant therapy, such as surgery in solid tumors and bone marrow transplant in multiple myeloma, when patients have minimal, typically microscopic, residual disease.”
Feasibility, safety, and immunogenicity
PGV-001 was synthesized for 15 patients and administered to 13 of them. Six of the 13 patients had head and neck squamous cell carcinoma, three had multiple myeloma, two had non–small cell lung cancer, one had breast cancer, and one had urothelial carcinoma.
Eleven patients received all 10 intended doses, and two patients received at least 8 doses.
“The vaccine was well tolerated, with only half of patients experiencing mild, grade 1 adverse events,” Dr. Marron said.
Transient injection site reactions occurred in four patients, and grade 1 fever was reported in one patient.
Immune monitoring is ongoing, but an initial analysis in one patient showed “robust responses” in CD4 and CD8 T cells by intracellular cytokine staining for interferon-gamma, tumor necrosis factor–alpha, and interleukin-2 after in vitro expansion in the presence of vaccine antigens, according to the researchers.
Dr. Marron noted that robust T-cell reactivity was seen at the completion of all 10 doses but was not seen after the 6th dose, and this supports the need for a prolonged dosing schedule.
Survival and subsequent therapy
At a mean follow-up of 880 days, four patients had no evidence of disease and had not received subsequent therapy. This includes one patient with stage IIIA non–small cell lung cancer, one with stage IVA HER-2 positive breast cancer, one with stage II urothelial carcinoma, and one with multiple myeloma.
Four patients were alive and receiving subsequent lines of therapy. Two of these patients had significant responses to anti–PD-1 therapy.
Three patients have died, two of whom had documented recurrence of their malignancy. The last two patients were lost to follow-up without documented recurrence.
“Our results demonstrate that the OpenVax pipeline is a viable approach to generate a safe, personalized cancer vaccine, which could potentially be used to treat a range of tumor types,” Dr. Bhardwaj said.
Trials combining neoantigens identified with the OpenVax platform are ongoing in patients with urothelial carcinoma and glioblastoma multiforme, Dr. Marron said.
The current study ( NCT02721043 ) is sponsored by Dr. Bhardwaj. Dr. Marron and Dr. Bhardwaj reported having no disclosures. Their colleagues disclosed relationships with Bristol Myers Squibb, Sema4, and Related Sciences.
FROM AACR 2021