User login
FDA scrutinizes cancer therapies granted accelerated approval
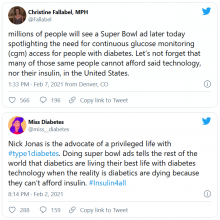
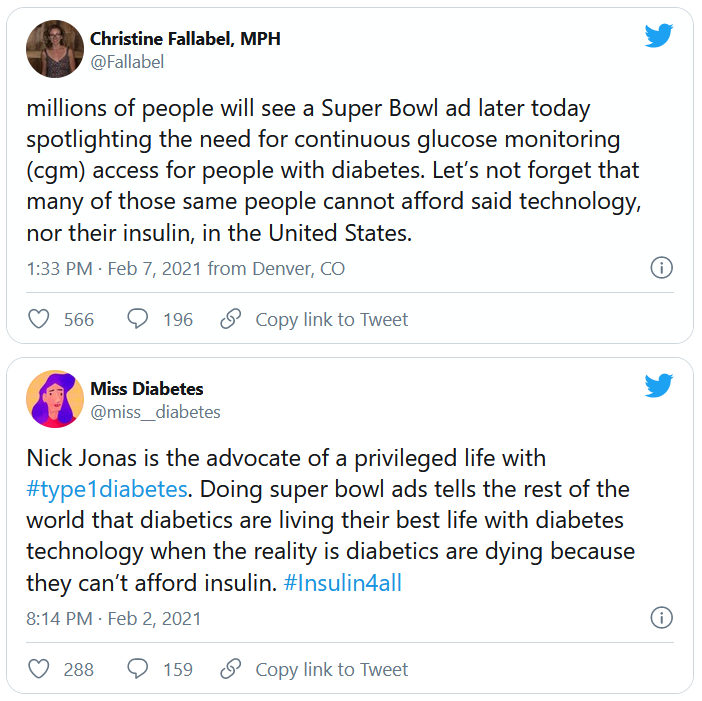
U.S. regulators are stepping up scrutiny of therapies that were granted an accelerated approval to treat cancers on the basis of surrogate endpoints but have failed to show clinical or survival benefits upon more extensive testing.
At issue are a number of cancer indications for immunotherapies. Four have already been withdrawn (voluntarily by the manufacturer), and six more will be reviewed at an upcoming meeting.
In recent years, the US Food and Drug Administration has granted accelerated approvals to oncology medicines on the basis of evidence that suggests a benefit for patients. Examples of such evidence relate to response rates and estimates of tumor shrinkage. But these approvals are granted on the condition that the manufacturer conducts larger clinical trials that show clinical benefit, including benefit in overall survival.
Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence, has argued that the point of these conditional approvals is to find acceptable surrogate markers to allow people with “desperate illnesses” to have access to potentially helpful drugs while work continues to determine the drug’s actual benefit to patients.
Oncologists are now questioning whether the FDA has become too lenient in its approach, Daniel A. Goldstein, MD, a senior physician in medical oncology and internal medicine at the Rabin Medical Center, Petah Tikva, Israel, told this news organization.
“The main two things you want from a cancer drug is to live longer and live a higher quality of life,” said Goldstein. “But these endpoints that they’ve been using over the past few years are not really giving us confidence that these drugs are actually going to help to live longer or better.”
Dr. Pazdur said the FDA will consider withdrawing its accelerated approvals when results of further studies do not confirm expected benefit for patients.
“This is like the pendulum has swung as far as it was going to swing and now is on the backswing,” said Dr. Goldstein, also of the department of health policy and management at the University of North Carolina at Chapel Hill. “You could call this a watershed moment.”
Although there’s near universal interest in allowing people with advanced cancer access to promising medicines, there’s also rising concern about exposing patients needlessly to costly drugs with potentially tough side effects. That may prompt a shift in the standards U.S. regulators apply to cancer medicines, Dr. Goldstein said.
Indications withdrawn and under review
In a meeting scheduled for April 27-29, the FDA’s Oncologic Drugs Advisory Committee will review indications granted through the accelerated approval process for three immunotherapies: pembrolizumab (Keytruda), atezolizumab (Tecentriq), and nivolumab (Opdivo).
It is part of an industry-wide evaluation of accelerated approvals for cancer indications in which confirmatory trials did not confirm clinical benefit, the FDA noted.
The process has already led to voluntary withdrawals of four cancer indications by the manufacturers, including one indication each for pembrolizumab, atezolizumab, and nivolumab, and one for durvalumab (Imfinzi).
All of these immunotherapies are approved for numerous cancer indications, and they all remain on the market. It is only the U.S. approvals for particular cancer indications that have been withdrawn.
In the past, olaratumab (Lartruvo) was withdrawn from the market altogether. The FDA granted accelerated approval of the drug for soft tissue sarcoma, but clinical benefit was not confirmed in a phase 3 trial.
Issue highlighted by Dr. Prasad and Dr. Gyawali
In recent years, much of the attention on accelerated approvals was spurred by the work of a few researchers, particularly Vinay Prasad, MD, MPH, associate professor in the department of epidemiology and biostatistics, University of California, San Francisco, and Bishal Gyawali, MD, PhD, from Queen’s University Cancer Research Institute, Kingston, Ont. (Both are regular contributors to the oncology section of this news organization.)
Dr. Goldstein made this point in a tweet about the FDA’s announcement of the April ODAC meetings:
“Well done to @oncology_bg and @VPrasadMDMPH among others for highlighting in their papers that the FDA wasn’t properly evaluating accelerated approval drugs.
FDA have listened.
And I thought that the impact of academia was limited!”
Dr. Prasad has made the case for closer scrutiny of accelerated approvals in a number of journal articles and in his 2020 book, “Malignant: How Bad Policy and Bad Evidence Harm People with Cancer,” published by Johns Hopkins University Press.
The book includes highlights of a 2016 article published in Mayo Clinic Proceedings that focused on surrogate endpoints used for FDA approvals. In the article, Dr. Prasad and his coauthor report that they did not find formal analyses of the strength of the surrogate-survival correlation in 14 of 25 cases of accelerated approvals (56%) and in 11 of 30 traditional approvals (37%).
“Our results were concerning. They imply that many surrogates are based on little more than a gut feeling. You might rationalize that and argue a gut feeling is the same as ‘reasonably likely to predict,’ but no reasonable person could think a gut feeling means established,” Dr. Prasad writes in his book. “Our result suggests the FDA is using surrogate endpoints far beyond what may be fair or reasonable.”
Dr. Gyawali has argued that the process by which the FDA assesses cancer drugs for approvals has undergone a profound shift. He has most recently remarked on this in an October 2020 commentary on Medscape.
“Until the recent floodgate of approvals based on response rates from single-arm trials, the majority of cancer therapy decisions were supported by evidence generated from randomized controlled trials (RCTs),” Dr. Gyawali wrote. “The evidence base to support clinical decisions in managing therapeutic side effects has been comparatively sparse.”
Accelerated approval to improve access
The FDA has struggled for about 2 decades with questions of where to set the bar on evidence for promising cancer drugs.
The agency’s accelerated approval program for drugs began in 1992. During the first decade, the focus was largely on medicines related to HIV.
In the early 2000s, oncology drugs began to dominate the program.
Dr. Pazdur has presided over the FDA’s marked changes regarding the use of surrogate markers when weighing whether to allow sales of cancer medicines. Formerly a professor at the University of Texas MD Anderson Cancer Center, Houston, Dr. Pazdur joined the FDA as director of the Division of Oncology Drug Products in 1999.
Soon after his appointment, he had to field inquiries from pharmaceutical companies about how much evidence they needed to receive accelerated approvals.
Early on, he publicly expressed impatience about the drugmakers’ approach. “The purpose of accelerated approval was not accelerated drug company profits,” Dr. Padzur said at a 2004 ODAC meeting.
Rather, the point is to allow access to potentially helpful drugs while work continues to determine their actual benefit to patients, he explained.
“It wasn’t a license to do less, less, less, and less to a point now that we may be getting companies that are coming in” intent on determining the minimum evidence the FDA will take, Dr. Pazdur said. “It shouldn’t be what is the lowest. It is what is a sufficient amount to give patients and physicians a real understanding of what their drug will do.”
In a 2016 interview with The New York Times, Dr. Pazdur said that his views on cancer drug approvals have evolved with time. He described himself as being “on a jihad to streamline the review process and get things out the door faster.”
“I have evolved from regulator to regulator-advocate,” Dr. Pazdur told the newspaper.
His attitude reflected his personal experience in losing his wife to ovarian cancer in 2015, as well as shifts in science and law. In 2012, Congress passed a law that gave the FDA new resources to speed medicines for life-threatening diseases to market. In addition, advances in genetics appeared to be making some medications more effective and easier to test, Dr. Pazdur said in The New York Times interview.
Withdrawals seen as sign of success
Since the program’s inception, only 6% of accelerated approvals for oncology indications have been withdrawn, the FDA said.
It would be a sign that the program is working if the April meetings lead to further withdrawals of indications that have been granted accelerated approval, Julie R. Gralow, MD, chief medical officer of the American Society of Clinical Oncology, said in an interview with this news organization.
“It shouldn’t be seen as a failure,” Dr. Gralow said.
In her own practice at the Fred Hutchinson Cancer Research Center, Seattle, she has seen the value of emerging therapies for patients fighting advanced cancers. During her 25 years of clinical practice in an academic setting, she has gained access to drugs through single-patient investigative new drug applications.
However, this path is not an option for many patients who undergo treatment in facilities other than academic centers, she commented. She noted that the accelerated approval process is a way to expand access to emerging medicines, but she sees a need for caution in the use of drugs that have been given only this conditional approval. She emphasizes that such drugs may be suitable only for certain patients.
“I would say that, for metastatic patients, patients with incurable disease, we are willing to take some risk,” Dr. Gralow said. “We don’t have other options. They can’t wait the years that it would take to get a drug approved.”
One such patient is David Mitchell, who serves as the consumer representative on ODAC. He told this news organization that he is taking three drugs for multiple myeloma that received accelerated approvals: pomalidomide, bortezomib, and daratumumab.
“I want the FDA to have the option to approve drugs in an accelerated pathway, because as a patient taking three drugs granted accelerated approval, I’m benefiting – I’ve lived the benefit,” Mr. Mitchell said, “and I want other patients to have the opportunity to have that benefit.”
He believes that the FDA’s approach regarding accelerated approvals serves to get potentially beneficial medicines to patients who have few options and also fulfills the FDA’s mandate to protect the public from treatments that have little benefit but can cause harm.
Accelerated approval also offers needed flexibility to drugmakers as they develop more specifically targeted drugs for diseases that affect relatively few people, such as multiple myeloma, he said. “As the targeting of your therapies gets tighter and for smaller groups of patients, you have a harder time following the traditional model,” such as conducting large, double-blind, placebo-controlled trials that may indicate increased overall survival, he said.
“To me, this is the way the FDA intended it to work,” he added. “It’s going to offer the accelerated approval based on a surrogate endpoint for a safe drug, but it’s going to require the confirmatory trial, and if the confirmatory trial fails, it will pull the drug off the market.”
Some medicines that have received accelerated approvals may ultimately be found not to benefit patients, Mr. Mitchell acknowledged. But people in his situation, whose disease has progressed despite treatments, may want to take that risk, he added.
Four cancer indications recently withdrawn voluntarily by the manufacturer
- December 2020: Nivolumab for the treatment of patients with metastatic small cell lung cancer with progression after platinum-based chemotherapy and at least one other line of therapy (Bristol Myers Squibb).
- February 2021: Durvalumab for the treatment of patients with locally advanced or metastatic urothelial carcinoma whose disease has progressed during or following platinum-based chemotherapy or within 12 months of neoadjuvant or adjuvant platinum-containing chemotherapy (AstraZeneca).
- March 2021: Pembrolizumab for the treatment of patients with metastatic small cell lung cancer with disease progression on or after platinum-based chemotherapy and at least one other prior line of therapy (Merck).
- March 2021: Atezolizumab for treatment of patients with locally advanced or metastatic urothelial carcinoma who experience disease progression during or following platinum-containing atezolizumab chemotherapy or disease progression within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy (Genentech).
Six cancer indications under review at the April 2021 ODAC meeting
- Atezolizumab indicated in combination with protein-bound for the treatment of adults with unresectable locally advanced or metastatic triple-negative whose tumors express PD-L1 (PD-L1 stained tumor-infiltrating immune cells of any intensity covering ≥1% of the tumor area), as determined by an FDA-approved test.
- Atezolizumab indicated for patients with locally advanced or metastatic urothelial carcinoma who are not eligible for cisplatin-containing chemotherapy.
- Pembrolizumab indicated for the treatment of patients with locally advanced or metastatic urothelial carcinoma who are not eligible for cisplatin-containing chemotherapy.
- Pembrolizumab indicated for the treatment of patients with recurrent locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma whose tumors express PD-L1 (Combined Positive Score ≥1), as determined by an FDA-approved test, with disease progression on or after two or more prior lines of therapy including fluoropyrimidine- and platinum-containing chemotherapy and if appropriate, HER2/neu-targeted therapy.
- Pembrolizumab indicated for the treatment of patients with who have been previously treated with .
- Nivolumab indicated as a single agent for the treatment of patients with hepatocellular carcinoma who have been previously treated with sorafenib.
A version of this article first appeared on Medscape.com.
U.S. regulators are stepping up scrutiny of therapies that were granted an accelerated approval to treat cancers on the basis of surrogate endpoints but have failed to show clinical or survival benefits upon more extensive testing.
At issue are a number of cancer indications for immunotherapies. Four have already been withdrawn (voluntarily by the manufacturer), and six more will be reviewed at an upcoming meeting.
In recent years, the US Food and Drug Administration has granted accelerated approvals to oncology medicines on the basis of evidence that suggests a benefit for patients. Examples of such evidence relate to response rates and estimates of tumor shrinkage. But these approvals are granted on the condition that the manufacturer conducts larger clinical trials that show clinical benefit, including benefit in overall survival.
Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence, has argued that the point of these conditional approvals is to find acceptable surrogate markers to allow people with “desperate illnesses” to have access to potentially helpful drugs while work continues to determine the drug’s actual benefit to patients.
Oncologists are now questioning whether the FDA has become too lenient in its approach, Daniel A. Goldstein, MD, a senior physician in medical oncology and internal medicine at the Rabin Medical Center, Petah Tikva, Israel, told this news organization.
“The main two things you want from a cancer drug is to live longer and live a higher quality of life,” said Goldstein. “But these endpoints that they’ve been using over the past few years are not really giving us confidence that these drugs are actually going to help to live longer or better.”
Dr. Pazdur said the FDA will consider withdrawing its accelerated approvals when results of further studies do not confirm expected benefit for patients.
“This is like the pendulum has swung as far as it was going to swing and now is on the backswing,” said Dr. Goldstein, also of the department of health policy and management at the University of North Carolina at Chapel Hill. “You could call this a watershed moment.”
Although there’s near universal interest in allowing people with advanced cancer access to promising medicines, there’s also rising concern about exposing patients needlessly to costly drugs with potentially tough side effects. That may prompt a shift in the standards U.S. regulators apply to cancer medicines, Dr. Goldstein said.
Indications withdrawn and under review
In a meeting scheduled for April 27-29, the FDA’s Oncologic Drugs Advisory Committee will review indications granted through the accelerated approval process for three immunotherapies: pembrolizumab (Keytruda), atezolizumab (Tecentriq), and nivolumab (Opdivo).
It is part of an industry-wide evaluation of accelerated approvals for cancer indications in which confirmatory trials did not confirm clinical benefit, the FDA noted.
The process has already led to voluntary withdrawals of four cancer indications by the manufacturers, including one indication each for pembrolizumab, atezolizumab, and nivolumab, and one for durvalumab (Imfinzi).
All of these immunotherapies are approved for numerous cancer indications, and they all remain on the market. It is only the U.S. approvals for particular cancer indications that have been withdrawn.
In the past, olaratumab (Lartruvo) was withdrawn from the market altogether. The FDA granted accelerated approval of the drug for soft tissue sarcoma, but clinical benefit was not confirmed in a phase 3 trial.
Issue highlighted by Dr. Prasad and Dr. Gyawali
In recent years, much of the attention on accelerated approvals was spurred by the work of a few researchers, particularly Vinay Prasad, MD, MPH, associate professor in the department of epidemiology and biostatistics, University of California, San Francisco, and Bishal Gyawali, MD, PhD, from Queen’s University Cancer Research Institute, Kingston, Ont. (Both are regular contributors to the oncology section of this news organization.)
Dr. Goldstein made this point in a tweet about the FDA’s announcement of the April ODAC meetings:
“Well done to @oncology_bg and @VPrasadMDMPH among others for highlighting in their papers that the FDA wasn’t properly evaluating accelerated approval drugs.
FDA have listened.
And I thought that the impact of academia was limited!”
Dr. Prasad has made the case for closer scrutiny of accelerated approvals in a number of journal articles and in his 2020 book, “Malignant: How Bad Policy and Bad Evidence Harm People with Cancer,” published by Johns Hopkins University Press.
The book includes highlights of a 2016 article published in Mayo Clinic Proceedings that focused on surrogate endpoints used for FDA approvals. In the article, Dr. Prasad and his coauthor report that they did not find formal analyses of the strength of the surrogate-survival correlation in 14 of 25 cases of accelerated approvals (56%) and in 11 of 30 traditional approvals (37%).
“Our results were concerning. They imply that many surrogates are based on little more than a gut feeling. You might rationalize that and argue a gut feeling is the same as ‘reasonably likely to predict,’ but no reasonable person could think a gut feeling means established,” Dr. Prasad writes in his book. “Our result suggests the FDA is using surrogate endpoints far beyond what may be fair or reasonable.”
Dr. Gyawali has argued that the process by which the FDA assesses cancer drugs for approvals has undergone a profound shift. He has most recently remarked on this in an October 2020 commentary on Medscape.
“Until the recent floodgate of approvals based on response rates from single-arm trials, the majority of cancer therapy decisions were supported by evidence generated from randomized controlled trials (RCTs),” Dr. Gyawali wrote. “The evidence base to support clinical decisions in managing therapeutic side effects has been comparatively sparse.”
Accelerated approval to improve access
The FDA has struggled for about 2 decades with questions of where to set the bar on evidence for promising cancer drugs.
The agency’s accelerated approval program for drugs began in 1992. During the first decade, the focus was largely on medicines related to HIV.
In the early 2000s, oncology drugs began to dominate the program.
Dr. Pazdur has presided over the FDA’s marked changes regarding the use of surrogate markers when weighing whether to allow sales of cancer medicines. Formerly a professor at the University of Texas MD Anderson Cancer Center, Houston, Dr. Pazdur joined the FDA as director of the Division of Oncology Drug Products in 1999.
Soon after his appointment, he had to field inquiries from pharmaceutical companies about how much evidence they needed to receive accelerated approvals.
Early on, he publicly expressed impatience about the drugmakers’ approach. “The purpose of accelerated approval was not accelerated drug company profits,” Dr. Padzur said at a 2004 ODAC meeting.
Rather, the point is to allow access to potentially helpful drugs while work continues to determine their actual benefit to patients, he explained.
“It wasn’t a license to do less, less, less, and less to a point now that we may be getting companies that are coming in” intent on determining the minimum evidence the FDA will take, Dr. Pazdur said. “It shouldn’t be what is the lowest. It is what is a sufficient amount to give patients and physicians a real understanding of what their drug will do.”
In a 2016 interview with The New York Times, Dr. Pazdur said that his views on cancer drug approvals have evolved with time. He described himself as being “on a jihad to streamline the review process and get things out the door faster.”
“I have evolved from regulator to regulator-advocate,” Dr. Pazdur told the newspaper.
His attitude reflected his personal experience in losing his wife to ovarian cancer in 2015, as well as shifts in science and law. In 2012, Congress passed a law that gave the FDA new resources to speed medicines for life-threatening diseases to market. In addition, advances in genetics appeared to be making some medications more effective and easier to test, Dr. Pazdur said in The New York Times interview.
Withdrawals seen as sign of success
Since the program’s inception, only 6% of accelerated approvals for oncology indications have been withdrawn, the FDA said.
It would be a sign that the program is working if the April meetings lead to further withdrawals of indications that have been granted accelerated approval, Julie R. Gralow, MD, chief medical officer of the American Society of Clinical Oncology, said in an interview with this news organization.
“It shouldn’t be seen as a failure,” Dr. Gralow said.
In her own practice at the Fred Hutchinson Cancer Research Center, Seattle, she has seen the value of emerging therapies for patients fighting advanced cancers. During her 25 years of clinical practice in an academic setting, she has gained access to drugs through single-patient investigative new drug applications.
However, this path is not an option for many patients who undergo treatment in facilities other than academic centers, she commented. She noted that the accelerated approval process is a way to expand access to emerging medicines, but she sees a need for caution in the use of drugs that have been given only this conditional approval. She emphasizes that such drugs may be suitable only for certain patients.
“I would say that, for metastatic patients, patients with incurable disease, we are willing to take some risk,” Dr. Gralow said. “We don’t have other options. They can’t wait the years that it would take to get a drug approved.”
One such patient is David Mitchell, who serves as the consumer representative on ODAC. He told this news organization that he is taking three drugs for multiple myeloma that received accelerated approvals: pomalidomide, bortezomib, and daratumumab.
“I want the FDA to have the option to approve drugs in an accelerated pathway, because as a patient taking three drugs granted accelerated approval, I’m benefiting – I’ve lived the benefit,” Mr. Mitchell said, “and I want other patients to have the opportunity to have that benefit.”
He believes that the FDA’s approach regarding accelerated approvals serves to get potentially beneficial medicines to patients who have few options and also fulfills the FDA’s mandate to protect the public from treatments that have little benefit but can cause harm.
Accelerated approval also offers needed flexibility to drugmakers as they develop more specifically targeted drugs for diseases that affect relatively few people, such as multiple myeloma, he said. “As the targeting of your therapies gets tighter and for smaller groups of patients, you have a harder time following the traditional model,” such as conducting large, double-blind, placebo-controlled trials that may indicate increased overall survival, he said.
“To me, this is the way the FDA intended it to work,” he added. “It’s going to offer the accelerated approval based on a surrogate endpoint for a safe drug, but it’s going to require the confirmatory trial, and if the confirmatory trial fails, it will pull the drug off the market.”
Some medicines that have received accelerated approvals may ultimately be found not to benefit patients, Mr. Mitchell acknowledged. But people in his situation, whose disease has progressed despite treatments, may want to take that risk, he added.
Four cancer indications recently withdrawn voluntarily by the manufacturer
- December 2020: Nivolumab for the treatment of patients with metastatic small cell lung cancer with progression after platinum-based chemotherapy and at least one other line of therapy (Bristol Myers Squibb).
- February 2021: Durvalumab for the treatment of patients with locally advanced or metastatic urothelial carcinoma whose disease has progressed during or following platinum-based chemotherapy or within 12 months of neoadjuvant or adjuvant platinum-containing chemotherapy (AstraZeneca).
- March 2021: Pembrolizumab for the treatment of patients with metastatic small cell lung cancer with disease progression on or after platinum-based chemotherapy and at least one other prior line of therapy (Merck).
- March 2021: Atezolizumab for treatment of patients with locally advanced or metastatic urothelial carcinoma who experience disease progression during or following platinum-containing atezolizumab chemotherapy or disease progression within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy (Genentech).
Six cancer indications under review at the April 2021 ODAC meeting
- Atezolizumab indicated in combination with protein-bound for the treatment of adults with unresectable locally advanced or metastatic triple-negative whose tumors express PD-L1 (PD-L1 stained tumor-infiltrating immune cells of any intensity covering ≥1% of the tumor area), as determined by an FDA-approved test.
- Atezolizumab indicated for patients with locally advanced or metastatic urothelial carcinoma who are not eligible for cisplatin-containing chemotherapy.
- Pembrolizumab indicated for the treatment of patients with locally advanced or metastatic urothelial carcinoma who are not eligible for cisplatin-containing chemotherapy.
- Pembrolizumab indicated for the treatment of patients with recurrent locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma whose tumors express PD-L1 (Combined Positive Score ≥1), as determined by an FDA-approved test, with disease progression on or after two or more prior lines of therapy including fluoropyrimidine- and platinum-containing chemotherapy and if appropriate, HER2/neu-targeted therapy.
- Pembrolizumab indicated for the treatment of patients with who have been previously treated with .
- Nivolumab indicated as a single agent for the treatment of patients with hepatocellular carcinoma who have been previously treated with sorafenib.
A version of this article first appeared on Medscape.com.
U.S. regulators are stepping up scrutiny of therapies that were granted an accelerated approval to treat cancers on the basis of surrogate endpoints but have failed to show clinical or survival benefits upon more extensive testing.
At issue are a number of cancer indications for immunotherapies. Four have already been withdrawn (voluntarily by the manufacturer), and six more will be reviewed at an upcoming meeting.
In recent years, the US Food and Drug Administration has granted accelerated approvals to oncology medicines on the basis of evidence that suggests a benefit for patients. Examples of such evidence relate to response rates and estimates of tumor shrinkage. But these approvals are granted on the condition that the manufacturer conducts larger clinical trials that show clinical benefit, including benefit in overall survival.
Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence, has argued that the point of these conditional approvals is to find acceptable surrogate markers to allow people with “desperate illnesses” to have access to potentially helpful drugs while work continues to determine the drug’s actual benefit to patients.
Oncologists are now questioning whether the FDA has become too lenient in its approach, Daniel A. Goldstein, MD, a senior physician in medical oncology and internal medicine at the Rabin Medical Center, Petah Tikva, Israel, told this news organization.
“The main two things you want from a cancer drug is to live longer and live a higher quality of life,” said Goldstein. “But these endpoints that they’ve been using over the past few years are not really giving us confidence that these drugs are actually going to help to live longer or better.”
Dr. Pazdur said the FDA will consider withdrawing its accelerated approvals when results of further studies do not confirm expected benefit for patients.
“This is like the pendulum has swung as far as it was going to swing and now is on the backswing,” said Dr. Goldstein, also of the department of health policy and management at the University of North Carolina at Chapel Hill. “You could call this a watershed moment.”
Although there’s near universal interest in allowing people with advanced cancer access to promising medicines, there’s also rising concern about exposing patients needlessly to costly drugs with potentially tough side effects. That may prompt a shift in the standards U.S. regulators apply to cancer medicines, Dr. Goldstein said.
Indications withdrawn and under review
In a meeting scheduled for April 27-29, the FDA’s Oncologic Drugs Advisory Committee will review indications granted through the accelerated approval process for three immunotherapies: pembrolizumab (Keytruda), atezolizumab (Tecentriq), and nivolumab (Opdivo).
It is part of an industry-wide evaluation of accelerated approvals for cancer indications in which confirmatory trials did not confirm clinical benefit, the FDA noted.
The process has already led to voluntary withdrawals of four cancer indications by the manufacturers, including one indication each for pembrolizumab, atezolizumab, and nivolumab, and one for durvalumab (Imfinzi).
All of these immunotherapies are approved for numerous cancer indications, and they all remain on the market. It is only the U.S. approvals for particular cancer indications that have been withdrawn.
In the past, olaratumab (Lartruvo) was withdrawn from the market altogether. The FDA granted accelerated approval of the drug for soft tissue sarcoma, but clinical benefit was not confirmed in a phase 3 trial.
Issue highlighted by Dr. Prasad and Dr. Gyawali
In recent years, much of the attention on accelerated approvals was spurred by the work of a few researchers, particularly Vinay Prasad, MD, MPH, associate professor in the department of epidemiology and biostatistics, University of California, San Francisco, and Bishal Gyawali, MD, PhD, from Queen’s University Cancer Research Institute, Kingston, Ont. (Both are regular contributors to the oncology section of this news organization.)
Dr. Goldstein made this point in a tweet about the FDA’s announcement of the April ODAC meetings:
“Well done to @oncology_bg and @VPrasadMDMPH among others for highlighting in their papers that the FDA wasn’t properly evaluating accelerated approval drugs.
FDA have listened.
And I thought that the impact of academia was limited!”
Dr. Prasad has made the case for closer scrutiny of accelerated approvals in a number of journal articles and in his 2020 book, “Malignant: How Bad Policy and Bad Evidence Harm People with Cancer,” published by Johns Hopkins University Press.
The book includes highlights of a 2016 article published in Mayo Clinic Proceedings that focused on surrogate endpoints used for FDA approvals. In the article, Dr. Prasad and his coauthor report that they did not find formal analyses of the strength of the surrogate-survival correlation in 14 of 25 cases of accelerated approvals (56%) and in 11 of 30 traditional approvals (37%).
“Our results were concerning. They imply that many surrogates are based on little more than a gut feeling. You might rationalize that and argue a gut feeling is the same as ‘reasonably likely to predict,’ but no reasonable person could think a gut feeling means established,” Dr. Prasad writes in his book. “Our result suggests the FDA is using surrogate endpoints far beyond what may be fair or reasonable.”
Dr. Gyawali has argued that the process by which the FDA assesses cancer drugs for approvals has undergone a profound shift. He has most recently remarked on this in an October 2020 commentary on Medscape.
“Until the recent floodgate of approvals based on response rates from single-arm trials, the majority of cancer therapy decisions were supported by evidence generated from randomized controlled trials (RCTs),” Dr. Gyawali wrote. “The evidence base to support clinical decisions in managing therapeutic side effects has been comparatively sparse.”
Accelerated approval to improve access
The FDA has struggled for about 2 decades with questions of where to set the bar on evidence for promising cancer drugs.
The agency’s accelerated approval program for drugs began in 1992. During the first decade, the focus was largely on medicines related to HIV.
In the early 2000s, oncology drugs began to dominate the program.
Dr. Pazdur has presided over the FDA’s marked changes regarding the use of surrogate markers when weighing whether to allow sales of cancer medicines. Formerly a professor at the University of Texas MD Anderson Cancer Center, Houston, Dr. Pazdur joined the FDA as director of the Division of Oncology Drug Products in 1999.
Soon after his appointment, he had to field inquiries from pharmaceutical companies about how much evidence they needed to receive accelerated approvals.
Early on, he publicly expressed impatience about the drugmakers’ approach. “The purpose of accelerated approval was not accelerated drug company profits,” Dr. Padzur said at a 2004 ODAC meeting.
Rather, the point is to allow access to potentially helpful drugs while work continues to determine their actual benefit to patients, he explained.
“It wasn’t a license to do less, less, less, and less to a point now that we may be getting companies that are coming in” intent on determining the minimum evidence the FDA will take, Dr. Pazdur said. “It shouldn’t be what is the lowest. It is what is a sufficient amount to give patients and physicians a real understanding of what their drug will do.”
In a 2016 interview with The New York Times, Dr. Pazdur said that his views on cancer drug approvals have evolved with time. He described himself as being “on a jihad to streamline the review process and get things out the door faster.”
“I have evolved from regulator to regulator-advocate,” Dr. Pazdur told the newspaper.
His attitude reflected his personal experience in losing his wife to ovarian cancer in 2015, as well as shifts in science and law. In 2012, Congress passed a law that gave the FDA new resources to speed medicines for life-threatening diseases to market. In addition, advances in genetics appeared to be making some medications more effective and easier to test, Dr. Pazdur said in The New York Times interview.
Withdrawals seen as sign of success
Since the program’s inception, only 6% of accelerated approvals for oncology indications have been withdrawn, the FDA said.
It would be a sign that the program is working if the April meetings lead to further withdrawals of indications that have been granted accelerated approval, Julie R. Gralow, MD, chief medical officer of the American Society of Clinical Oncology, said in an interview with this news organization.
“It shouldn’t be seen as a failure,” Dr. Gralow said.
In her own practice at the Fred Hutchinson Cancer Research Center, Seattle, she has seen the value of emerging therapies for patients fighting advanced cancers. During her 25 years of clinical practice in an academic setting, she has gained access to drugs through single-patient investigative new drug applications.
However, this path is not an option for many patients who undergo treatment in facilities other than academic centers, she commented. She noted that the accelerated approval process is a way to expand access to emerging medicines, but she sees a need for caution in the use of drugs that have been given only this conditional approval. She emphasizes that such drugs may be suitable only for certain patients.
“I would say that, for metastatic patients, patients with incurable disease, we are willing to take some risk,” Dr. Gralow said. “We don’t have other options. They can’t wait the years that it would take to get a drug approved.”
One such patient is David Mitchell, who serves as the consumer representative on ODAC. He told this news organization that he is taking three drugs for multiple myeloma that received accelerated approvals: pomalidomide, bortezomib, and daratumumab.
“I want the FDA to have the option to approve drugs in an accelerated pathway, because as a patient taking three drugs granted accelerated approval, I’m benefiting – I’ve lived the benefit,” Mr. Mitchell said, “and I want other patients to have the opportunity to have that benefit.”
He believes that the FDA’s approach regarding accelerated approvals serves to get potentially beneficial medicines to patients who have few options and also fulfills the FDA’s mandate to protect the public from treatments that have little benefit but can cause harm.
Accelerated approval also offers needed flexibility to drugmakers as they develop more specifically targeted drugs for diseases that affect relatively few people, such as multiple myeloma, he said. “As the targeting of your therapies gets tighter and for smaller groups of patients, you have a harder time following the traditional model,” such as conducting large, double-blind, placebo-controlled trials that may indicate increased overall survival, he said.
“To me, this is the way the FDA intended it to work,” he added. “It’s going to offer the accelerated approval based on a surrogate endpoint for a safe drug, but it’s going to require the confirmatory trial, and if the confirmatory trial fails, it will pull the drug off the market.”
Some medicines that have received accelerated approvals may ultimately be found not to benefit patients, Mr. Mitchell acknowledged. But people in his situation, whose disease has progressed despite treatments, may want to take that risk, he added.
Four cancer indications recently withdrawn voluntarily by the manufacturer
- December 2020: Nivolumab for the treatment of patients with metastatic small cell lung cancer with progression after platinum-based chemotherapy and at least one other line of therapy (Bristol Myers Squibb).
- February 2021: Durvalumab for the treatment of patients with locally advanced or metastatic urothelial carcinoma whose disease has progressed during or following platinum-based chemotherapy or within 12 months of neoadjuvant or adjuvant platinum-containing chemotherapy (AstraZeneca).
- March 2021: Pembrolizumab for the treatment of patients with metastatic small cell lung cancer with disease progression on or after platinum-based chemotherapy and at least one other prior line of therapy (Merck).
- March 2021: Atezolizumab for treatment of patients with locally advanced or metastatic urothelial carcinoma who experience disease progression during or following platinum-containing atezolizumab chemotherapy or disease progression within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy (Genentech).
Six cancer indications under review at the April 2021 ODAC meeting
- Atezolizumab indicated in combination with protein-bound for the treatment of adults with unresectable locally advanced or metastatic triple-negative whose tumors express PD-L1 (PD-L1 stained tumor-infiltrating immune cells of any intensity covering ≥1% of the tumor area), as determined by an FDA-approved test.
- Atezolizumab indicated for patients with locally advanced or metastatic urothelial carcinoma who are not eligible for cisplatin-containing chemotherapy.
- Pembrolizumab indicated for the treatment of patients with locally advanced or metastatic urothelial carcinoma who are not eligible for cisplatin-containing chemotherapy.
- Pembrolizumab indicated for the treatment of patients with recurrent locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma whose tumors express PD-L1 (Combined Positive Score ≥1), as determined by an FDA-approved test, with disease progression on or after two or more prior lines of therapy including fluoropyrimidine- and platinum-containing chemotherapy and if appropriate, HER2/neu-targeted therapy.
- Pembrolizumab indicated for the treatment of patients with who have been previously treated with .
- Nivolumab indicated as a single agent for the treatment of patients with hepatocellular carcinoma who have been previously treated with sorafenib.
A version of this article first appeared on Medscape.com.
A return of holism? It never left osteopathic medicine
I enjoyed Dr. Jonas’s article, “A new model of care to return holism to family medicine” (J Fam Pract. 2020;69:493-498).
However, I wanted to point out that for more than 100 years the concept of the patient-centered medical home, and the outgrowth of that, has been part of osteopathic medical education, founded by A.T. Still, MD, in the 1800s.
Congratulations to the allopathic medicine profession for recognizing its significance.
Steven Shapiro, DO
Fenton, MI
I enjoyed Dr. Jonas’s article, “A new model of care to return holism to family medicine” (J Fam Pract. 2020;69:493-498).
However, I wanted to point out that for more than 100 years the concept of the patient-centered medical home, and the outgrowth of that, has been part of osteopathic medical education, founded by A.T. Still, MD, in the 1800s.
Congratulations to the allopathic medicine profession for recognizing its significance.
Steven Shapiro, DO
Fenton, MI
I enjoyed Dr. Jonas’s article, “A new model of care to return holism to family medicine” (J Fam Pract. 2020;69:493-498).
However, I wanted to point out that for more than 100 years the concept of the patient-centered medical home, and the outgrowth of that, has been part of osteopathic medical education, founded by A.T. Still, MD, in the 1800s.
Congratulations to the allopathic medicine profession for recognizing its significance.
Steven Shapiro, DO
Fenton, MI
Is there liability if you don’t test for BRCA?
CASE Young woman with family history of breast cancer detects lump
Two weeks after noting a lump on her breast when her cat happened to jump on her in that spot, a 28-year-old woman (G0) went to her primary care provider. She was referred to her gynecologist; breast imaging, ultrasonography, and mammography were obtained, with microcalcifications noted. A fine needle aspiration diagnosed intraductal malignancy. The surgical breast tissue specimen was estrogen receptor (ER)- and progestogen receptor (PR)-positive and HER2-negative. Other tumor markers were obtained, including carcinoembryonic antigen, and tissue polypeptide specific antigen, p53, cathepsin D, cyclin E, and nestin, but results were not available.
With regard to family history, the woman’s mother and maternal grandmother had a history of breast cancer. The patient and her family underwent gene testing. The patient was found to be BRCA1- and BRCA2-positive; her mother was BRCA1-positive, an older sister was BRCA2-positive, and her grandmother was not tested.
The question arose in light of her family history as to why she was not tested for BRCA and appropriately counseled by her gynecologist prior to the cancer diagnosis. Litigation was initiated. While the case did not go forward regarding litigation, it is indeed a case in point. (Please note that this is a hypothetical case. It is based on a composite of several cases.)
Medical considerations
Breast cancer is the most common type of cancer affecting women in the Western world.1 Advances in clinical testing for gene mutations have escalated and allowed for identification of patients at increased risk for breast and ovarian cancer. Along with these advances come professional liability risk. After looking at the medical considerations for BRCA1 and 2 testing, we will consider a number of important legal issues. In the view of some commentators, the failure to diagnose genetic mutations in patients predisposed to cancer is “poised to become the next wave of medical professional liability lawsuits.”2
BRCA1 and BRCA2 genes provide tumor suppressor proteins, and assessment for mutations is recommended for individuals at high risk for breast and/or ovarian cancer; mutations in BRCA genes cause DNA damage, which increases the chance of developing cancer. The other way to look at it is, BRCA1 and 2 are tumor suppressor genes that are integrally involved with DNA damage control. Once there is a mutation, it adversely affects the beneficial effects of the gene. Mutations in these genes account for 5% to 10% of all hereditary breast cancers.3 Of note, men with BRCA2 are at increased risk for prostate cancer.
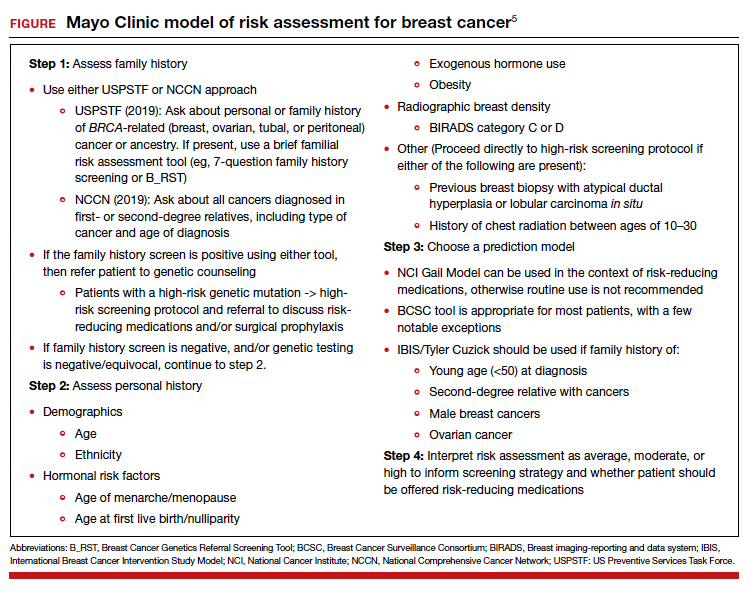
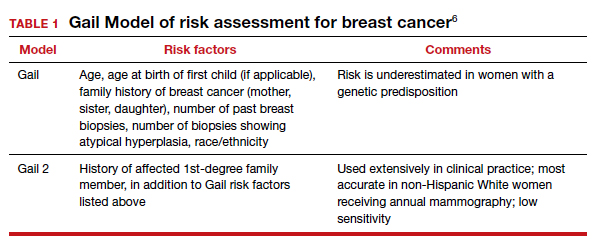
A patient who presents to her gynecologist stating that there is a family history of breast cancer, without knowledge of genetic components, presents a challenge (and a medicolegal risk) for the provider to assess. Prediction models have been used to determine specific patient risk for carrying a genetic mutation with resultant breast cancer development.4 Risk prediction models do not appear to be a good answer to predicting who is more likely to develop breast or ovarian cancer, however. A Mayo model may assist (FIGURE).5 Clinicians should also be aware of other models of risk assessment, including the Gail Model (TABLE 1).6
Continue to: Guidelines for genetic testing...
Guidelines for genetic testing
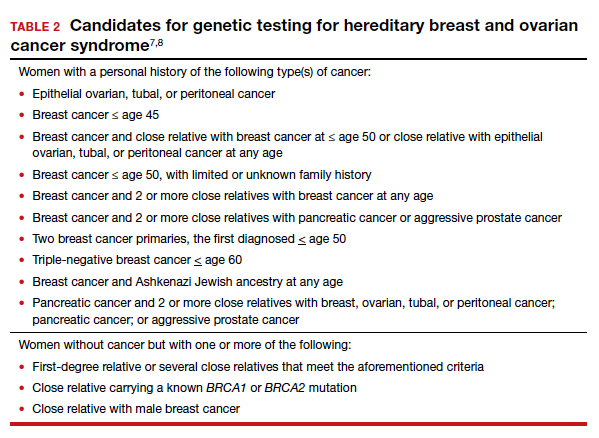
The American College of Obstetricians and Gynecologists states that patient medical history and family history are paramount in obtaining information regarding risk for breast and ovarian cancer. First- and second-degree relatives are allocated to this category. Information regarding age of diagnosis, maternal and paternal lineage, and ethnic background can imply a need for genetic testing (TABLE 2).7,8 A number of genetics national organizations have participated in recommendations and include the American College of Medical Genetics and Genomics, the National Society for Genetic Counselors, and the Society of Gynecologic Oncology.7
The question always surfaces, could the clinical outcome of the cancer when diagnosed have been changed if screening were undertaken, with earlier diagnosis, or prevented with prophylactic mastectomy, and changed the end result. In addition, it is well known that breast augmentation mammoplasty alters the ability to accurately evaluate mammograms. Patients considering this type of plastic surgery, ideally, should be counselled accordingly.9
Bottom line, we as clinicians must be cognizant of both ACOG and United States Preventive Services Task Force (USPSTF) recommendations regarding screening and gene testing for women considered high risk for breast cancer based on family history.7
Legal considerations
The case presented demonstrates that the discovery of the BRCA1 and BRCA2 genes, and reliable tests for determining the existence of the genes, brought with them legal issues as well as medical advantages. We look at professional liability (malpractice) questions this technology raises, and then consider the outcome of the hypothetical case. (BRCA is used here to apply broadly—not only to BRCA1 and 2 but also to PALB2, CHEK2, and similar genetic abnormalities.)
To date, the most visible BRCA legal issues covered in cases and law reviews have focused more on patent law than malpractice. The most important of these was a decision of the US Supreme Court in Association for Molecular Pathology v Myriad Genetics.10 The US Patent Office was granting patents to companies finding useful, naturally occurring segments of human DNA, and had granted Myriad several patents on BRCA1 and BRCA2 genes. This patent policy had the potential to seriously interfere with broad scientific use of these genes.11 Fortunately, the Supreme Court stepped in and unanimously invalidated such patents. It held that a “naturally occurring DNA segment is a product of nature and not patent eligible merely because it has been isolated.” The Court noted, “Finding the location of the BRCA1 and BRCA2 genes does not render the genes patent eligible ‘new . . . composition[s] of matter.’”8 The Court did allow the patenting of tests for specific gene structures, and artificial changes in naturally occurring genes.
Malpractice and BRCA
While the BRCA patent wars have lingered, the potential for a significant increase in BRCA-related malpractice cases is of increasing concern. Like most malpractice liability, these new claims are based on very old principles of negligence.12 To prevail, the plaintiff (ordinarily, an injured patient) must demonstrate 4 things:
- A duty. That is, the physician owed a duty to the injured party. Usually (but not always) that requires a professional relationship between the physician and the person injured.
- A breach of that duty. Malpractice liability is based on the fact that the physician did something that a reasonably careful physician (generally, of the same specialty) would not have done, or that the physician failed to do something that a reasonable physician would have done. This usually means that the profession itself sees what the physician did (or did not do) as medically inappropriate. In medical malpractice cases, that is ordinarily measured by what the usual or common practice is among prudent physicians. In rare circumstances, courts have found the standard practice of a profession to be negligent. Where, for example, it was custom for a professional not to give an eye pressure test to anyone under age 40, a court found that common standard to be inappropriate.13 In the words of Judge Learned Hand (speaking about a different case), “a whole calling may have unduly lagged in the adoption of new and available devices. It never may set its own tests.”14 Underlying negligence is a cost-benefit analysis (discussed below).
- Damages. There must have been some damage that courts recognize, usually loss of money or opportunity to work, the cost of care, pain and suffering, or loss of enjoyment/quality of life. In malpractice, many states now recognize the “loss of chance” or the “loss of a chance.” That means, if a “physician negligently fails to diagnose a curable disease, and the patient is harmed by the disease, the physician should be liable for causing the ‘loss of a chance of a cure.’”15 (Delay in diagnosis is the most common reason for claims in breast cancer care.)16
- Causation. The breach of duty (negligence) must have caused the damages. The causation must have been reasonably close. If a driver drives through a stop sign, or a physician misreads a test, and someone is injured but there is no connection between the negligence and the injury, there is not tort liability.
The 4 elements of malpractice just described are raised in some way in the possible liability associated with BRCA testing. We next look at the ways in which liability may arise from that testing (or lack of it).
Underlying much of the following discussion is the “cost-benefit” consideration noted above. This concept is that the total cost (financial and health) of testing should be compared with the value of the benefits of testing, taking into account the probabilities that the testing will result in better health outcomes. BRCA testing, for example, is essentially cost-free in terms of physical risk. Its financial cost, while not trivial, is not great, and it is commonly covered by health insurance.17 In terms of benefits, the testing has the potential for providing critical information in making treatment decisions for a meaningful percentage of patients and their families. There are many ways of analyzing the liability risks of genetic malpractice,7,18 and the following is intended to discuss some of the greatest risks related to BRCA testing.
Continue to: Areas of liability...
Areas of liability
The failure to recommend a test. The circumstances in which BRCA testing should be undertaken are set out by professional organizations (noted above). These recommendations are not static, however. They change from time to time. Given the potential harm caused by the failure to test in relevant circumstances, malpractice liability is certainly a possibility when the failure to recommend a test to a patient results in a cancer that might have been prevented had the genetic problem been identified in a timely manner. The circumstances in which testing should be considered continue to change, placing an obligation on clinicians to stay well informed of changing genetic understandings. Another risk is that one specialist may assume that it is the job of another specialist to order the test. Whatever the cause of the failure to test, or unnecessary delay in testing, it appears to be the primary basis for BRCA liability.
The failure to properly interpret a test. Any test that is misinterpreted may lead to harm for the patient. A false negative, of course, may mean that preventive treatment that could have been undertaken will be foregone, as a “loss of a chance.” On the other hand, a false positive can lead to radical, unnecessary surgery or treatment. If a misinterpretation occurred because of carelessness by the testing organization, or confusion by a practitioner, there is a likelihood of negligence.19
A different form of “misinterpretation” could be reasonable—and not negligent. Advances in scientific-medical understanding may result in the outcome of tests being reconsidered and changed. That has been the case with genetic testing and breast cancer. The availability of multiple breast cancer SNPs (single nucleotide polymorphisms), and combining this information with other risk factors for example, results in a polygenic risk score that may be at odds with the level of risk from earlier testing.20,21 This naturally leads to the question of when later, updated testing should be recommended to look for a better current interpretation.22,23
The failure to act on BRCA test results. Testing is of no value, of course, if the results are not used properly. Test results or analyses that are not sent to the proper physicians, or are somehow ignored when properly directed, is a “never” event—it should never happen. It almost always would be considered negligence, and if the patient were injured, could lead to liability. Amazingly, one study found that, in genetic testing liability cases, nearly 20% of the claims arose from failure to return test results to patients.24 In addition, when a patient is found to be BRCA-positive, there is an obligation to discuss the options for dealing with the increased risk associated with the gene mutation(s), as well as to recommend the prudent course of action or to refer the patient to someone who will have that discussion.
Informed consent to the patient. BRCA testing requires informed consent. The physical risks of the testing process are minimal, of course, but it carries a number of other emotional and family risks. The informed consent process is an invitation to an honest discussion between clinicians and patients. It should be an opportunity to discuss what the testing is, and is not, and what the test may mean for treatment. It may also be an opportunity to discuss the implications for other members of the patient’s family (noted below).
One element of informed consent is a discussion of the consequences of failure to consent, or to undertake one of the alternatives. In the case of BRCA testing, this is especially important in cases in which a patient expresses a hesitancy to be tested with an “I’d rather not know philosophy.” Although clinicians should not practice law, some patient concerns about discrimination may be addressed by the protection that the federal Genetic Information Nondiscrimination Act (GINA) and other laws provide (which prohibit insurance and employment discrimination based on genetic information). A good source of information about GINA and related nondiscrimination laws is provided by the National Human Genome Research Institute.25 In addition, the National Institutes of Health has a website that may be helpful to many patients26 (and a much more complex site for health professionals).27 At the same time, courts have resisted plaintiffs/patients who have tried to use informed consent as a way of suing for failure to offer genetic testing.28,29
The failure to refer. In some cases, a patient should be formally referred for genetics consultation. The considerations here are similar to other circumstances in modern, fast developing medical practice that require special sensitivity to those occasions in which a patient will benefit from additional expertise. It is a principle that the AMA Council on Ethical and Judicial Affairs has expressed this way: “In the absence of adequate expertise in pretest and posttest counseling, a physician should refer the patient to an appropriate specialist.”30 The failure to refer, when that deviates from acceptable practice, may result in liability.
Informing others. BRCA testing is an area of medicine in which results may be of great significance not only to the patient but also to the patient’s family.31 Physicians should counsel patients on the importance of informing relatives about relevant results and “should make themselves available to assist patients in communicating with relatives to discuss opportunities for counseling and testing, as appropriate.”30 The question may arise, however, of whether in some circumstances physicians should go a step further in ensuring relatives receive important information regarding their loved one’s health.32 The law has been reluctant to impose liability to “third parties” (someone not a patient). Duties usually arise through the physician-patient relationship. There are exceptions. Perhaps the best known has been the obligation of mental health professionals to take action to protect third parties from patients who have made believable threats against identifiable victims.33 There are indications that some courts could find, in extreme circumstances, a “duty to warn” nonpatients in some instances where it is essential to inform third parties that they should receive a specific form of genetic testing.34,35 Such a duty would, of course, have to protect the privacy rights of the patient to the maximum extent possible. A general duty of this type has not been established widely, but may be part of the future.
Continue to: Was there liability in our example case?...
Was there liability in our example case?
The hypothetical case provided above suggests that there could be liability. Routine medical history by the primary care physician would have produced the fact that the patient’s mother, sister, and maternal grandmother had breast cancer. That would clearly have put her in a category of those who should have received genetic testing. Yet, she was not tested until after her cancer was found. From the limited facts we have, it appears that this timeline of events would have been outside accepted practice—and negligent. The case was not pursued by the patient, however, and this may represent the current state of liability for BRCA issues.
The extent of liability seems to be significant
Our discussion of liability suggests that there is significant potential for BRCA testing negligence within practice, and that the damages in these cases could be substantial. Yet the predicted “tsunami” of malpractice lawsuits related to genetic testing has not appeared.36,37 One study of cases in the United States (through 2016) found a “slowly rising tide” of liability cases instead of a tsunami,24 as the number of claims made was low. On the other hand, the payments where damages were awarded were an order of magnitude larger than other malpractice cases—a mean of $5.3 million and median of $2 million. This is compared with mean values in the range of $275,000 to $600,000 in other areas of malpractice.
The majority of the genetic malpractice cases involve prenatal and newborn testing, and diagnosis/susceptibility/pharmacogenomic accounting for about 25% of cases. In terms of type of errors claimed, approximately 50% were diagnostic-interpretation errors, 30% failure to offer testing, nearly 20% failure to return test results to the patients, and a few remaining cases of failure to properly treat in light of genetic testing.24
Despite a few very large payments, however, the fact remains that there is a surprisingly low number of genetics malpractice cases. Gary Marchant and colleagues suggest that several reasons may account for this:
- the clinical implementation of genetic science has been slower than expected
- the lack of expertise of many physicians in genetic science
- expert witnesses have sometimes been hard to find
- the lack of understanding by plaintiffs’ attorneys of genetic malpractice
- potential plaintiffs’ lack of understanding of the nature of genetic testing and the harms resulting from genetic negligence.17,24,37
The tide is slowly coming in
By all appearances, there is every reason to think that genetic malpractice will be increasing, and that the recent past of much higher damages per claim paid in the genetics area will be part of that tide. The National Human Genome Research LawSeq project has suggested a number of useful ways of dealing with the liability issues.18 In addition to the BRCA issues that we have considered in this article for ObGyns, there are other critical issues of prenatal and newborn genetic testing.38 But those are topics for another day. ●
- Sevilla C, Moatti JP, Reynier CJ, et al. Testing for BRCA1 mutations: a cost-effective analysis. Europ J Human Genetics. 2002;10:599-606.
- Cotton V, Kirkpatrick D. Failure to recommend genetic counseling in breast cancer: is the next wave of medical professional liability lawsuits? Contemp OB/GYN. June 1, 2017.
- Suryavanshi M, Kumar D, Panigrahi M, et al. Detection of false positive mutations in BRCA gene by next generation sequencing. Fam Cancer. 2017;16:311-317.
- Black L, Knoppers B, Avard D, et al. Legal liability and the uncertain nature of risk prediction: the case of breast cancer risk prediction models. Public Health Genomics. 2012;15:335-340.
- McClintock A, Gollab A, Laya M. Breast cancer risk assessment, a step-wise approach for primary care physicians on the front lines of shared decision making. Mayo Clin Proc. 2020;95:1268-1275.
- National Cancer Institute. The Breast Cancer Risk Assessment Tool. https://bcrisktool.cancer.gov/. Accessed February 25, 2021.
- Neff J, Richardson G, Phelps J. Legal liabilities associated with hereditary breast and ovarian cancers. J Reprod Med. 2020;65:227-230.
- American College of Obstetricians and Gynecologists. Practice Bulletin No 182: hereditary breast and ovarian cancer syndrome. Obstet Gynecol. 2017;130:e110-e126.
- Sá dos Reis C, Gremion I, and Meystre NR. Study of breast implants mammography examinations for identification of suitable image quality criteria. Insights Imaging. 2020;11:3.
- Association for Molecular Pathology v Myriad Genetics, 569 U.S. 576 (2013).
- Smith SR. The Supreme Court 2012-2013: dogs, DNA, and DOMA. Register Rep. 2013;39(Fall):26-33.
- Bal BS. An introduction to medical malpractice in the United States. Clin Orthop Relat Res. 2009;467:339-347.
- Helling v Carey, 83 Wn.2d 514, 519 P.2d 981 (1974).
- The T.J. Hooper, 60 F.2d 737, 740 (2d Cir.1932), cert. denied 287 U.S. 662 (1932).
- Fischer DA. Tort recovery for loss of a chance. Wake Forest L Rev. 2001;36:605-655.
- Murphy BL, Ray-Zack MD, Reddy PN, et al. Breast cancer litigation in the 21st century. Ann Surg Oncol. 2018;25:2939- 2947.
- Prince AE. Prevention for those who can pay: insurance reimbursement of genetic-based preventive interventions in the liminal state between health and disease. J Law Biosci. 2015;2:365-395.
- Marchant G, Barnes M, Evans JP, et al; LawSeq Liability Task Force. From genetics to genomics: facing the liability implications in clinical care. J Law Med Ethics. 2020;48:11-43.
- Complaint, Held v Ambry Genetics Corp., No. 15-CV-8683, 2015 WL 6750024 (S.D.N.Y. Nov. 4, 2015); Order of Dismissal, Held v Ambry Genetics Corp., No. 15-CV-8683, (S.D.N.Y. Dec. 6, 2016).
- Pederson HJ. Breast cancer risk assessment and treatment: current concepts in genetics and genomics. Contemp OB/ GYN. 2017; 62:A1-A4.
- Pederson HJ. Who needs breast cancer genetics testing? OBG Manag. 2018;30:34-39.
- Roberts JL, Foulkes A. Genetic duties. William Mary L Rev. 2020;62:143-212.
- Thorogood A, Cook-Deegan R, Knoppers B. Public variant databases: liability? Genet Med. 2017;19:838–841.
- Marchant G, Lindor R. Genomic malpractice: an emerging tide or gentle ripple? Food Drug Law J. 2018;73:1-37.
- National Human Genome Research Institute. Genetic discrimination. https://www.genome.gov/about-genomics /policy-issues/Genetic-Discrimination. Updated September 16, 2020. Accessed February 25, 2021.
- National Cancer Institute. BRCA mutations: cancer risk and genetic testing. https://www.cancer.gov/about-cancer /causes-prevention/genetics/brca-fact-sheet. Reviewed November 19, 2020. Accessed February 25, 2021.
- National Cancer Institute. Genetics of breast and gynecologic cancers (PDQ®)–Health Professional Version. https://www .cancer.gov/types/breast/hp/breast-ovarian-genetics-pdq. Updated February 12, 2021. Accessed February 25, 2021.
- Reed v Campagnolo, 630 A.2d 1145, 1152–54 (Md. 1993).
- Munro v Regents of Univ. of Cal.,263 Cal. Rptr. 878, 885, 988 (1989).
- AMA Council on Ethical and Judicial Affairs. AMA Code of Medical Ethics’ opinions on genetic testing. Opinion 2.131. 2009;11:683-685. https://journalofethics.ama-assn .org/article/ama-code-medical-ethics-opinions-genetictesting/2009-09.
- Gilbar R, Barnoy S. Disclosing genetic test results to the patient’ relatives: how does the law influence clinical practice? J Law Technol Policy. 2019;125-168.
- Song K. Warning third parties of genetic risks in the era of personalized medicine. U.C. Davis L Rev. 2016;49:1987-2018.
- Tarasoff v Regents of the University of California, 551 P.2d 334, 131 Cal. Rptr. 14 (Cal. 1976).
- Safer v Estate of Pack, 677 A.2d 1188 (N.J. App. 1996), cert. denied, 683 A.2d 1163 (N.J. 1996).
- Pate v Threlkel, 661 So.2d 278 (Fla. 1995).
- Rothstein MA. Liability issues in pharmacogenomics. Louisiana L Rev. 2005;66:117-124.
- Marchant G, Lindor R. Personalized medicine and genetic malpractice. Genet Med. 2013;15:921-922.
- Westbrook M. Transforming the physician’s standard of care in the context of whole genome sequencing technologies: finding guidance in best practice standards. Saint Louis U J Health Law Policy. 2015;9:111-148.
CASE Young woman with family history of breast cancer detects lump
Two weeks after noting a lump on her breast when her cat happened to jump on her in that spot, a 28-year-old woman (G0) went to her primary care provider. She was referred to her gynecologist; breast imaging, ultrasonography, and mammography were obtained, with microcalcifications noted. A fine needle aspiration diagnosed intraductal malignancy. The surgical breast tissue specimen was estrogen receptor (ER)- and progestogen receptor (PR)-positive and HER2-negative. Other tumor markers were obtained, including carcinoembryonic antigen, and tissue polypeptide specific antigen, p53, cathepsin D, cyclin E, and nestin, but results were not available.
With regard to family history, the woman’s mother and maternal grandmother had a history of breast cancer. The patient and her family underwent gene testing. The patient was found to be BRCA1- and BRCA2-positive; her mother was BRCA1-positive, an older sister was BRCA2-positive, and her grandmother was not tested.
The question arose in light of her family history as to why she was not tested for BRCA and appropriately counseled by her gynecologist prior to the cancer diagnosis. Litigation was initiated. While the case did not go forward regarding litigation, it is indeed a case in point. (Please note that this is a hypothetical case. It is based on a composite of several cases.)
Medical considerations
Breast cancer is the most common type of cancer affecting women in the Western world.1 Advances in clinical testing for gene mutations have escalated and allowed for identification of patients at increased risk for breast and ovarian cancer. Along with these advances come professional liability risk. After looking at the medical considerations for BRCA1 and 2 testing, we will consider a number of important legal issues. In the view of some commentators, the failure to diagnose genetic mutations in patients predisposed to cancer is “poised to become the next wave of medical professional liability lawsuits.”2
BRCA1 and BRCA2 genes provide tumor suppressor proteins, and assessment for mutations is recommended for individuals at high risk for breast and/or ovarian cancer; mutations in BRCA genes cause DNA damage, which increases the chance of developing cancer. The other way to look at it is, BRCA1 and 2 are tumor suppressor genes that are integrally involved with DNA damage control. Once there is a mutation, it adversely affects the beneficial effects of the gene. Mutations in these genes account for 5% to 10% of all hereditary breast cancers.3 Of note, men with BRCA2 are at increased risk for prostate cancer.
A patient who presents to her gynecologist stating that there is a family history of breast cancer, without knowledge of genetic components, presents a challenge (and a medicolegal risk) for the provider to assess. Prediction models have been used to determine specific patient risk for carrying a genetic mutation with resultant breast cancer development.4 Risk prediction models do not appear to be a good answer to predicting who is more likely to develop breast or ovarian cancer, however. A Mayo model may assist (FIGURE).5 Clinicians should also be aware of other models of risk assessment, including the Gail Model (TABLE 1).6


Continue to: Guidelines for genetic testing...
Guidelines for genetic testing
The American College of Obstetricians and Gynecologists states that patient medical history and family history are paramount in obtaining information regarding risk for breast and ovarian cancer. First- and second-degree relatives are allocated to this category. Information regarding age of diagnosis, maternal and paternal lineage, and ethnic background can imply a need for genetic testing (TABLE 2).7,8 A number of genetics national organizations have participated in recommendations and include the American College of Medical Genetics and Genomics, the National Society for Genetic Counselors, and the Society of Gynecologic Oncology.7

The question always surfaces, could the clinical outcome of the cancer when diagnosed have been changed if screening were undertaken, with earlier diagnosis, or prevented with prophylactic mastectomy, and changed the end result. In addition, it is well known that breast augmentation mammoplasty alters the ability to accurately evaluate mammograms. Patients considering this type of plastic surgery, ideally, should be counselled accordingly.9
Bottom line, we as clinicians must be cognizant of both ACOG and United States Preventive Services Task Force (USPSTF) recommendations regarding screening and gene testing for women considered high risk for breast cancer based on family history.7
Legal considerations
The case presented demonstrates that the discovery of the BRCA1 and BRCA2 genes, and reliable tests for determining the existence of the genes, brought with them legal issues as well as medical advantages. We look at professional liability (malpractice) questions this technology raises, and then consider the outcome of the hypothetical case. (BRCA is used here to apply broadly—not only to BRCA1 and 2 but also to PALB2, CHEK2, and similar genetic abnormalities.)
To date, the most visible BRCA legal issues covered in cases and law reviews have focused more on patent law than malpractice. The most important of these was a decision of the US Supreme Court in Association for Molecular Pathology v Myriad Genetics.10 The US Patent Office was granting patents to companies finding useful, naturally occurring segments of human DNA, and had granted Myriad several patents on BRCA1 and BRCA2 genes. This patent policy had the potential to seriously interfere with broad scientific use of these genes.11 Fortunately, the Supreme Court stepped in and unanimously invalidated such patents. It held that a “naturally occurring DNA segment is a product of nature and not patent eligible merely because it has been isolated.” The Court noted, “Finding the location of the BRCA1 and BRCA2 genes does not render the genes patent eligible ‘new . . . composition[s] of matter.’”8 The Court did allow the patenting of tests for specific gene structures, and artificial changes in naturally occurring genes.
Malpractice and BRCA
While the BRCA patent wars have lingered, the potential for a significant increase in BRCA-related malpractice cases is of increasing concern. Like most malpractice liability, these new claims are based on very old principles of negligence.12 To prevail, the plaintiff (ordinarily, an injured patient) must demonstrate 4 things:
- A duty. That is, the physician owed a duty to the injured party. Usually (but not always) that requires a professional relationship between the physician and the person injured.
- A breach of that duty. Malpractice liability is based on the fact that the physician did something that a reasonably careful physician (generally, of the same specialty) would not have done, or that the physician failed to do something that a reasonable physician would have done. This usually means that the profession itself sees what the physician did (or did not do) as medically inappropriate. In medical malpractice cases, that is ordinarily measured by what the usual or common practice is among prudent physicians. In rare circumstances, courts have found the standard practice of a profession to be negligent. Where, for example, it was custom for a professional not to give an eye pressure test to anyone under age 40, a court found that common standard to be inappropriate.13 In the words of Judge Learned Hand (speaking about a different case), “a whole calling may have unduly lagged in the adoption of new and available devices. It never may set its own tests.”14 Underlying negligence is a cost-benefit analysis (discussed below).
- Damages. There must have been some damage that courts recognize, usually loss of money or opportunity to work, the cost of care, pain and suffering, or loss of enjoyment/quality of life. In malpractice, many states now recognize the “loss of chance” or the “loss of a chance.” That means, if a “physician negligently fails to diagnose a curable disease, and the patient is harmed by the disease, the physician should be liable for causing the ‘loss of a chance of a cure.’”15 (Delay in diagnosis is the most common reason for claims in breast cancer care.)16
- Causation. The breach of duty (negligence) must have caused the damages. The causation must have been reasonably close. If a driver drives through a stop sign, or a physician misreads a test, and someone is injured but there is no connection between the negligence and the injury, there is not tort liability.
The 4 elements of malpractice just described are raised in some way in the possible liability associated with BRCA testing. We next look at the ways in which liability may arise from that testing (or lack of it).
Underlying much of the following discussion is the “cost-benefit” consideration noted above. This concept is that the total cost (financial and health) of testing should be compared with the value of the benefits of testing, taking into account the probabilities that the testing will result in better health outcomes. BRCA testing, for example, is essentially cost-free in terms of physical risk. Its financial cost, while not trivial, is not great, and it is commonly covered by health insurance.17 In terms of benefits, the testing has the potential for providing critical information in making treatment decisions for a meaningful percentage of patients and their families. There are many ways of analyzing the liability risks of genetic malpractice,7,18 and the following is intended to discuss some of the greatest risks related to BRCA testing.
Continue to: Areas of liability...
Areas of liability
The failure to recommend a test. The circumstances in which BRCA testing should be undertaken are set out by professional organizations (noted above). These recommendations are not static, however. They change from time to time. Given the potential harm caused by the failure to test in relevant circumstances, malpractice liability is certainly a possibility when the failure to recommend a test to a patient results in a cancer that might have been prevented had the genetic problem been identified in a timely manner. The circumstances in which testing should be considered continue to change, placing an obligation on clinicians to stay well informed of changing genetic understandings. Another risk is that one specialist may assume that it is the job of another specialist to order the test. Whatever the cause of the failure to test, or unnecessary delay in testing, it appears to be the primary basis for BRCA liability.
The failure to properly interpret a test. Any test that is misinterpreted may lead to harm for the patient. A false negative, of course, may mean that preventive treatment that could have been undertaken will be foregone, as a “loss of a chance.” On the other hand, a false positive can lead to radical, unnecessary surgery or treatment. If a misinterpretation occurred because of carelessness by the testing organization, or confusion by a practitioner, there is a likelihood of negligence.19
A different form of “misinterpretation” could be reasonable—and not negligent. Advances in scientific-medical understanding may result in the outcome of tests being reconsidered and changed. That has been the case with genetic testing and breast cancer. The availability of multiple breast cancer SNPs (single nucleotide polymorphisms), and combining this information with other risk factors for example, results in a polygenic risk score that may be at odds with the level of risk from earlier testing.20,21 This naturally leads to the question of when later, updated testing should be recommended to look for a better current interpretation.22,23
The failure to act on BRCA test results. Testing is of no value, of course, if the results are not used properly. Test results or analyses that are not sent to the proper physicians, or are somehow ignored when properly directed, is a “never” event—it should never happen. It almost always would be considered negligence, and if the patient were injured, could lead to liability. Amazingly, one study found that, in genetic testing liability cases, nearly 20% of the claims arose from failure to return test results to patients.24 In addition, when a patient is found to be BRCA-positive, there is an obligation to discuss the options for dealing with the increased risk associated with the gene mutation(s), as well as to recommend the prudent course of action or to refer the patient to someone who will have that discussion.
Informed consent to the patient. BRCA testing requires informed consent. The physical risks of the testing process are minimal, of course, but it carries a number of other emotional and family risks. The informed consent process is an invitation to an honest discussion between clinicians and patients. It should be an opportunity to discuss what the testing is, and is not, and what the test may mean for treatment. It may also be an opportunity to discuss the implications for other members of the patient’s family (noted below).
One element of informed consent is a discussion of the consequences of failure to consent, or to undertake one of the alternatives. In the case of BRCA testing, this is especially important in cases in which a patient expresses a hesitancy to be tested with an “I’d rather not know philosophy.” Although clinicians should not practice law, some patient concerns about discrimination may be addressed by the protection that the federal Genetic Information Nondiscrimination Act (GINA) and other laws provide (which prohibit insurance and employment discrimination based on genetic information). A good source of information about GINA and related nondiscrimination laws is provided by the National Human Genome Research Institute.25 In addition, the National Institutes of Health has a website that may be helpful to many patients26 (and a much more complex site for health professionals).27 At the same time, courts have resisted plaintiffs/patients who have tried to use informed consent as a way of suing for failure to offer genetic testing.28,29
The failure to refer. In some cases, a patient should be formally referred for genetics consultation. The considerations here are similar to other circumstances in modern, fast developing medical practice that require special sensitivity to those occasions in which a patient will benefit from additional expertise. It is a principle that the AMA Council on Ethical and Judicial Affairs has expressed this way: “In the absence of adequate expertise in pretest and posttest counseling, a physician should refer the patient to an appropriate specialist.”30 The failure to refer, when that deviates from acceptable practice, may result in liability.
Informing others. BRCA testing is an area of medicine in which results may be of great significance not only to the patient but also to the patient’s family.31 Physicians should counsel patients on the importance of informing relatives about relevant results and “should make themselves available to assist patients in communicating with relatives to discuss opportunities for counseling and testing, as appropriate.”30 The question may arise, however, of whether in some circumstances physicians should go a step further in ensuring relatives receive important information regarding their loved one’s health.32 The law has been reluctant to impose liability to “third parties” (someone not a patient). Duties usually arise through the physician-patient relationship. There are exceptions. Perhaps the best known has been the obligation of mental health professionals to take action to protect third parties from patients who have made believable threats against identifiable victims.33 There are indications that some courts could find, in extreme circumstances, a “duty to warn” nonpatients in some instances where it is essential to inform third parties that they should receive a specific form of genetic testing.34,35 Such a duty would, of course, have to protect the privacy rights of the patient to the maximum extent possible. A general duty of this type has not been established widely, but may be part of the future.
Continue to: Was there liability in our example case?...
Was there liability in our example case?
The hypothetical case provided above suggests that there could be liability. Routine medical history by the primary care physician would have produced the fact that the patient’s mother, sister, and maternal grandmother had breast cancer. That would clearly have put her in a category of those who should have received genetic testing. Yet, she was not tested until after her cancer was found. From the limited facts we have, it appears that this timeline of events would have been outside accepted practice—and negligent. The case was not pursued by the patient, however, and this may represent the current state of liability for BRCA issues.
The extent of liability seems to be significant
Our discussion of liability suggests that there is significant potential for BRCA testing negligence within practice, and that the damages in these cases could be substantial. Yet the predicted “tsunami” of malpractice lawsuits related to genetic testing has not appeared.36,37 One study of cases in the United States (through 2016) found a “slowly rising tide” of liability cases instead of a tsunami,24 as the number of claims made was low. On the other hand, the payments where damages were awarded were an order of magnitude larger than other malpractice cases—a mean of $5.3 million and median of $2 million. This is compared with mean values in the range of $275,000 to $600,000 in other areas of malpractice.
The majority of the genetic malpractice cases involve prenatal and newborn testing, and diagnosis/susceptibility/pharmacogenomic accounting for about 25% of cases. In terms of type of errors claimed, approximately 50% were diagnostic-interpretation errors, 30% failure to offer testing, nearly 20% failure to return test results to the patients, and a few remaining cases of failure to properly treat in light of genetic testing.24
Despite a few very large payments, however, the fact remains that there is a surprisingly low number of genetics malpractice cases. Gary Marchant and colleagues suggest that several reasons may account for this:
- the clinical implementation of genetic science has been slower than expected
- the lack of expertise of many physicians in genetic science
- expert witnesses have sometimes been hard to find
- the lack of understanding by plaintiffs’ attorneys of genetic malpractice
- potential plaintiffs’ lack of understanding of the nature of genetic testing and the harms resulting from genetic negligence.17,24,37
The tide is slowly coming in
By all appearances, there is every reason to think that genetic malpractice will be increasing, and that the recent past of much higher damages per claim paid in the genetics area will be part of that tide. The National Human Genome Research LawSeq project has suggested a number of useful ways of dealing with the liability issues.18 In addition to the BRCA issues that we have considered in this article for ObGyns, there are other critical issues of prenatal and newborn genetic testing.38 But those are topics for another day. ●
CASE Young woman with family history of breast cancer detects lump
Two weeks after noting a lump on her breast when her cat happened to jump on her in that spot, a 28-year-old woman (G0) went to her primary care provider. She was referred to her gynecologist; breast imaging, ultrasonography, and mammography were obtained, with microcalcifications noted. A fine needle aspiration diagnosed intraductal malignancy. The surgical breast tissue specimen was estrogen receptor (ER)- and progestogen receptor (PR)-positive and HER2-negative. Other tumor markers were obtained, including carcinoembryonic antigen, and tissue polypeptide specific antigen, p53, cathepsin D, cyclin E, and nestin, but results were not available.
With regard to family history, the woman’s mother and maternal grandmother had a history of breast cancer. The patient and her family underwent gene testing. The patient was found to be BRCA1- and BRCA2-positive; her mother was BRCA1-positive, an older sister was BRCA2-positive, and her grandmother was not tested.
The question arose in light of her family history as to why she was not tested for BRCA and appropriately counseled by her gynecologist prior to the cancer diagnosis. Litigation was initiated. While the case did not go forward regarding litigation, it is indeed a case in point. (Please note that this is a hypothetical case. It is based on a composite of several cases.)
Medical considerations
Breast cancer is the most common type of cancer affecting women in the Western world.1 Advances in clinical testing for gene mutations have escalated and allowed for identification of patients at increased risk for breast and ovarian cancer. Along with these advances come professional liability risk. After looking at the medical considerations for BRCA1 and 2 testing, we will consider a number of important legal issues. In the view of some commentators, the failure to diagnose genetic mutations in patients predisposed to cancer is “poised to become the next wave of medical professional liability lawsuits.”2
BRCA1 and BRCA2 genes provide tumor suppressor proteins, and assessment for mutations is recommended for individuals at high risk for breast and/or ovarian cancer; mutations in BRCA genes cause DNA damage, which increases the chance of developing cancer. The other way to look at it is, BRCA1 and 2 are tumor suppressor genes that are integrally involved with DNA damage control. Once there is a mutation, it adversely affects the beneficial effects of the gene. Mutations in these genes account for 5% to 10% of all hereditary breast cancers.3 Of note, men with BRCA2 are at increased risk for prostate cancer.
A patient who presents to her gynecologist stating that there is a family history of breast cancer, without knowledge of genetic components, presents a challenge (and a medicolegal risk) for the provider to assess. Prediction models have been used to determine specific patient risk for carrying a genetic mutation with resultant breast cancer development.4 Risk prediction models do not appear to be a good answer to predicting who is more likely to develop breast or ovarian cancer, however. A Mayo model may assist (FIGURE).5 Clinicians should also be aware of other models of risk assessment, including the Gail Model (TABLE 1).6


Continue to: Guidelines for genetic testing...
Guidelines for genetic testing
The American College of Obstetricians and Gynecologists states that patient medical history and family history are paramount in obtaining information regarding risk for breast and ovarian cancer. First- and second-degree relatives are allocated to this category. Information regarding age of diagnosis, maternal and paternal lineage, and ethnic background can imply a need for genetic testing (TABLE 2).7,8 A number of genetics national organizations have participated in recommendations and include the American College of Medical Genetics and Genomics, the National Society for Genetic Counselors, and the Society of Gynecologic Oncology.7

The question always surfaces, could the clinical outcome of the cancer when diagnosed have been changed if screening were undertaken, with earlier diagnosis, or prevented with prophylactic mastectomy, and changed the end result. In addition, it is well known that breast augmentation mammoplasty alters the ability to accurately evaluate mammograms. Patients considering this type of plastic surgery, ideally, should be counselled accordingly.9
Bottom line, we as clinicians must be cognizant of both ACOG and United States Preventive Services Task Force (USPSTF) recommendations regarding screening and gene testing for women considered high risk for breast cancer based on family history.7
Legal considerations
The case presented demonstrates that the discovery of the BRCA1 and BRCA2 genes, and reliable tests for determining the existence of the genes, brought with them legal issues as well as medical advantages. We look at professional liability (malpractice) questions this technology raises, and then consider the outcome of the hypothetical case. (BRCA is used here to apply broadly—not only to BRCA1 and 2 but also to PALB2, CHEK2, and similar genetic abnormalities.)
To date, the most visible BRCA legal issues covered in cases and law reviews have focused more on patent law than malpractice. The most important of these was a decision of the US Supreme Court in Association for Molecular Pathology v Myriad Genetics.10 The US Patent Office was granting patents to companies finding useful, naturally occurring segments of human DNA, and had granted Myriad several patents on BRCA1 and BRCA2 genes. This patent policy had the potential to seriously interfere with broad scientific use of these genes.11 Fortunately, the Supreme Court stepped in and unanimously invalidated such patents. It held that a “naturally occurring DNA segment is a product of nature and not patent eligible merely because it has been isolated.” The Court noted, “Finding the location of the BRCA1 and BRCA2 genes does not render the genes patent eligible ‘new . . . composition[s] of matter.’”8 The Court did allow the patenting of tests for specific gene structures, and artificial changes in naturally occurring genes.
Malpractice and BRCA
While the BRCA patent wars have lingered, the potential for a significant increase in BRCA-related malpractice cases is of increasing concern. Like most malpractice liability, these new claims are based on very old principles of negligence.12 To prevail, the plaintiff (ordinarily, an injured patient) must demonstrate 4 things:
- A duty. That is, the physician owed a duty to the injured party. Usually (but not always) that requires a professional relationship between the physician and the person injured.
- A breach of that duty. Malpractice liability is based on the fact that the physician did something that a reasonably careful physician (generally, of the same specialty) would not have done, or that the physician failed to do something that a reasonable physician would have done. This usually means that the profession itself sees what the physician did (or did not do) as medically inappropriate. In medical malpractice cases, that is ordinarily measured by what the usual or common practice is among prudent physicians. In rare circumstances, courts have found the standard practice of a profession to be negligent. Where, for example, it was custom for a professional not to give an eye pressure test to anyone under age 40, a court found that common standard to be inappropriate.13 In the words of Judge Learned Hand (speaking about a different case), “a whole calling may have unduly lagged in the adoption of new and available devices. It never may set its own tests.”14 Underlying negligence is a cost-benefit analysis (discussed below).
- Damages. There must have been some damage that courts recognize, usually loss of money or opportunity to work, the cost of care, pain and suffering, or loss of enjoyment/quality of life. In malpractice, many states now recognize the “loss of chance” or the “loss of a chance.” That means, if a “physician negligently fails to diagnose a curable disease, and the patient is harmed by the disease, the physician should be liable for causing the ‘loss of a chance of a cure.’”15 (Delay in diagnosis is the most common reason for claims in breast cancer care.)16
- Causation. The breach of duty (negligence) must have caused the damages. The causation must have been reasonably close. If a driver drives through a stop sign, or a physician misreads a test, and someone is injured but there is no connection between the negligence and the injury, there is not tort liability.
The 4 elements of malpractice just described are raised in some way in the possible liability associated with BRCA testing. We next look at the ways in which liability may arise from that testing (or lack of it).
Underlying much of the following discussion is the “cost-benefit” consideration noted above. This concept is that the total cost (financial and health) of testing should be compared with the value of the benefits of testing, taking into account the probabilities that the testing will result in better health outcomes. BRCA testing, for example, is essentially cost-free in terms of physical risk. Its financial cost, while not trivial, is not great, and it is commonly covered by health insurance.17 In terms of benefits, the testing has the potential for providing critical information in making treatment decisions for a meaningful percentage of patients and their families. There are many ways of analyzing the liability risks of genetic malpractice,7,18 and the following is intended to discuss some of the greatest risks related to BRCA testing.
Continue to: Areas of liability...
Areas of liability
The failure to recommend a test. The circumstances in which BRCA testing should be undertaken are set out by professional organizations (noted above). These recommendations are not static, however. They change from time to time. Given the potential harm caused by the failure to test in relevant circumstances, malpractice liability is certainly a possibility when the failure to recommend a test to a patient results in a cancer that might have been prevented had the genetic problem been identified in a timely manner. The circumstances in which testing should be considered continue to change, placing an obligation on clinicians to stay well informed of changing genetic understandings. Another risk is that one specialist may assume that it is the job of another specialist to order the test. Whatever the cause of the failure to test, or unnecessary delay in testing, it appears to be the primary basis for BRCA liability.
The failure to properly interpret a test. Any test that is misinterpreted may lead to harm for the patient. A false negative, of course, may mean that preventive treatment that could have been undertaken will be foregone, as a “loss of a chance.” On the other hand, a false positive can lead to radical, unnecessary surgery or treatment. If a misinterpretation occurred because of carelessness by the testing organization, or confusion by a practitioner, there is a likelihood of negligence.19
A different form of “misinterpretation” could be reasonable—and not negligent. Advances in scientific-medical understanding may result in the outcome of tests being reconsidered and changed. That has been the case with genetic testing and breast cancer. The availability of multiple breast cancer SNPs (single nucleotide polymorphisms), and combining this information with other risk factors for example, results in a polygenic risk score that may be at odds with the level of risk from earlier testing.20,21 This naturally leads to the question of when later, updated testing should be recommended to look for a better current interpretation.22,23
The failure to act on BRCA test results. Testing is of no value, of course, if the results are not used properly. Test results or analyses that are not sent to the proper physicians, or are somehow ignored when properly directed, is a “never” event—it should never happen. It almost always would be considered negligence, and if the patient were injured, could lead to liability. Amazingly, one study found that, in genetic testing liability cases, nearly 20% of the claims arose from failure to return test results to patients.24 In addition, when a patient is found to be BRCA-positive, there is an obligation to discuss the options for dealing with the increased risk associated with the gene mutation(s), as well as to recommend the prudent course of action or to refer the patient to someone who will have that discussion.
Informed consent to the patient. BRCA testing requires informed consent. The physical risks of the testing process are minimal, of course, but it carries a number of other emotional and family risks. The informed consent process is an invitation to an honest discussion between clinicians and patients. It should be an opportunity to discuss what the testing is, and is not, and what the test may mean for treatment. It may also be an opportunity to discuss the implications for other members of the patient’s family (noted below).
One element of informed consent is a discussion of the consequences of failure to consent, or to undertake one of the alternatives. In the case of BRCA testing, this is especially important in cases in which a patient expresses a hesitancy to be tested with an “I’d rather not know philosophy.” Although clinicians should not practice law, some patient concerns about discrimination may be addressed by the protection that the federal Genetic Information Nondiscrimination Act (GINA) and other laws provide (which prohibit insurance and employment discrimination based on genetic information). A good source of information about GINA and related nondiscrimination laws is provided by the National Human Genome Research Institute.25 In addition, the National Institutes of Health has a website that may be helpful to many patients26 (and a much more complex site for health professionals).27 At the same time, courts have resisted plaintiffs/patients who have tried to use informed consent as a way of suing for failure to offer genetic testing.28,29
The failure to refer. In some cases, a patient should be formally referred for genetics consultation. The considerations here are similar to other circumstances in modern, fast developing medical practice that require special sensitivity to those occasions in which a patient will benefit from additional expertise. It is a principle that the AMA Council on Ethical and Judicial Affairs has expressed this way: “In the absence of adequate expertise in pretest and posttest counseling, a physician should refer the patient to an appropriate specialist.”30 The failure to refer, when that deviates from acceptable practice, may result in liability.
Informing others. BRCA testing is an area of medicine in which results may be of great significance not only to the patient but also to the patient’s family.31 Physicians should counsel patients on the importance of informing relatives about relevant results and “should make themselves available to assist patients in communicating with relatives to discuss opportunities for counseling and testing, as appropriate.”30 The question may arise, however, of whether in some circumstances physicians should go a step further in ensuring relatives receive important information regarding their loved one’s health.32 The law has been reluctant to impose liability to “third parties” (someone not a patient). Duties usually arise through the physician-patient relationship. There are exceptions. Perhaps the best known has been the obligation of mental health professionals to take action to protect third parties from patients who have made believable threats against identifiable victims.33 There are indications that some courts could find, in extreme circumstances, a “duty to warn” nonpatients in some instances where it is essential to inform third parties that they should receive a specific form of genetic testing.34,35 Such a duty would, of course, have to protect the privacy rights of the patient to the maximum extent possible. A general duty of this type has not been established widely, but may be part of the future.
Continue to: Was there liability in our example case?...
Was there liability in our example case?
The hypothetical case provided above suggests that there could be liability. Routine medical history by the primary care physician would have produced the fact that the patient’s mother, sister, and maternal grandmother had breast cancer. That would clearly have put her in a category of those who should have received genetic testing. Yet, she was not tested until after her cancer was found. From the limited facts we have, it appears that this timeline of events would have been outside accepted practice—and negligent. The case was not pursued by the patient, however, and this may represent the current state of liability for BRCA issues.
The extent of liability seems to be significant
Our discussion of liability suggests that there is significant potential for BRCA testing negligence within practice, and that the damages in these cases could be substantial. Yet the predicted “tsunami” of malpractice lawsuits related to genetic testing has not appeared.36,37 One study of cases in the United States (through 2016) found a “slowly rising tide” of liability cases instead of a tsunami,24 as the number of claims made was low. On the other hand, the payments where damages were awarded were an order of magnitude larger than other malpractice cases—a mean of $5.3 million and median of $2 million. This is compared with mean values in the range of $275,000 to $600,000 in other areas of malpractice.
The majority of the genetic malpractice cases involve prenatal and newborn testing, and diagnosis/susceptibility/pharmacogenomic accounting for about 25% of cases. In terms of type of errors claimed, approximately 50% were diagnostic-interpretation errors, 30% failure to offer testing, nearly 20% failure to return test results to the patients, and a few remaining cases of failure to properly treat in light of genetic testing.24
Despite a few very large payments, however, the fact remains that there is a surprisingly low number of genetics malpractice cases. Gary Marchant and colleagues suggest that several reasons may account for this:
- the clinical implementation of genetic science has been slower than expected
- the lack of expertise of many physicians in genetic science
- expert witnesses have sometimes been hard to find
- the lack of understanding by plaintiffs’ attorneys of genetic malpractice
- potential plaintiffs’ lack of understanding of the nature of genetic testing and the harms resulting from genetic negligence.17,24,37
The tide is slowly coming in
By all appearances, there is every reason to think that genetic malpractice will be increasing, and that the recent past of much higher damages per claim paid in the genetics area will be part of that tide. The National Human Genome Research LawSeq project has suggested a number of useful ways of dealing with the liability issues.18 In addition to the BRCA issues that we have considered in this article for ObGyns, there are other critical issues of prenatal and newborn genetic testing.38 But those are topics for another day. ●
- Sevilla C, Moatti JP, Reynier CJ, et al. Testing for BRCA1 mutations: a cost-effective analysis. Europ J Human Genetics. 2002;10:599-606.
- Cotton V, Kirkpatrick D. Failure to recommend genetic counseling in breast cancer: is the next wave of medical professional liability lawsuits? Contemp OB/GYN. June 1, 2017.
- Suryavanshi M, Kumar D, Panigrahi M, et al. Detection of false positive mutations in BRCA gene by next generation sequencing. Fam Cancer. 2017;16:311-317.
- Black L, Knoppers B, Avard D, et al. Legal liability and the uncertain nature of risk prediction: the case of breast cancer risk prediction models. Public Health Genomics. 2012;15:335-340.
- McClintock A, Gollab A, Laya M. Breast cancer risk assessment, a step-wise approach for primary care physicians on the front lines of shared decision making. Mayo Clin Proc. 2020;95:1268-1275.
- National Cancer Institute. The Breast Cancer Risk Assessment Tool. https://bcrisktool.cancer.gov/. Accessed February 25, 2021.
- Neff J, Richardson G, Phelps J. Legal liabilities associated with hereditary breast and ovarian cancers. J Reprod Med. 2020;65:227-230.
- American College of Obstetricians and Gynecologists. Practice Bulletin No 182: hereditary breast and ovarian cancer syndrome. Obstet Gynecol. 2017;130:e110-e126.
- Sá dos Reis C, Gremion I, and Meystre NR. Study of breast implants mammography examinations for identification of suitable image quality criteria. Insights Imaging. 2020;11:3.
- Association for Molecular Pathology v Myriad Genetics, 569 U.S. 576 (2013).
- Smith SR. The Supreme Court 2012-2013: dogs, DNA, and DOMA. Register Rep. 2013;39(Fall):26-33.
- Bal BS. An introduction to medical malpractice in the United States. Clin Orthop Relat Res. 2009;467:339-347.
- Helling v Carey, 83 Wn.2d 514, 519 P.2d 981 (1974).
- The T.J. Hooper, 60 F.2d 737, 740 (2d Cir.1932), cert. denied 287 U.S. 662 (1932).
- Fischer DA. Tort recovery for loss of a chance. Wake Forest L Rev. 2001;36:605-655.
- Murphy BL, Ray-Zack MD, Reddy PN, et al. Breast cancer litigation in the 21st century. Ann Surg Oncol. 2018;25:2939- 2947.
- Prince AE. Prevention for those who can pay: insurance reimbursement of genetic-based preventive interventions in the liminal state between health and disease. J Law Biosci. 2015;2:365-395.
- Marchant G, Barnes M, Evans JP, et al; LawSeq Liability Task Force. From genetics to genomics: facing the liability implications in clinical care. J Law Med Ethics. 2020;48:11-43.
- Complaint, Held v Ambry Genetics Corp., No. 15-CV-8683, 2015 WL 6750024 (S.D.N.Y. Nov. 4, 2015); Order of Dismissal, Held v Ambry Genetics Corp., No. 15-CV-8683, (S.D.N.Y. Dec. 6, 2016).
- Pederson HJ. Breast cancer risk assessment and treatment: current concepts in genetics and genomics. Contemp OB/ GYN. 2017; 62:A1-A4.
- Pederson HJ. Who needs breast cancer genetics testing? OBG Manag. 2018;30:34-39.
- Roberts JL, Foulkes A. Genetic duties. William Mary L Rev. 2020;62:143-212.
- Thorogood A, Cook-Deegan R, Knoppers B. Public variant databases: liability? Genet Med. 2017;19:838–841.
- Marchant G, Lindor R. Genomic malpractice: an emerging tide or gentle ripple? Food Drug Law J. 2018;73:1-37.
- National Human Genome Research Institute. Genetic discrimination. https://www.genome.gov/about-genomics /policy-issues/Genetic-Discrimination. Updated September 16, 2020. Accessed February 25, 2021.
- National Cancer Institute. BRCA mutations: cancer risk and genetic testing. https://www.cancer.gov/about-cancer /causes-prevention/genetics/brca-fact-sheet. Reviewed November 19, 2020. Accessed February 25, 2021.
- National Cancer Institute. Genetics of breast and gynecologic cancers (PDQ®)–Health Professional Version. https://www .cancer.gov/types/breast/hp/breast-ovarian-genetics-pdq. Updated February 12, 2021. Accessed February 25, 2021.
- Reed v Campagnolo, 630 A.2d 1145, 1152–54 (Md. 1993).
- Munro v Regents of Univ. of Cal.,263 Cal. Rptr. 878, 885, 988 (1989).
- AMA Council on Ethical and Judicial Affairs. AMA Code of Medical Ethics’ opinions on genetic testing. Opinion 2.131. 2009;11:683-685. https://journalofethics.ama-assn .org/article/ama-code-medical-ethics-opinions-genetictesting/2009-09.
- Gilbar R, Barnoy S. Disclosing genetic test results to the patient’ relatives: how does the law influence clinical practice? J Law Technol Policy. 2019;125-168.
- Song K. Warning third parties of genetic risks in the era of personalized medicine. U.C. Davis L Rev. 2016;49:1987-2018.
- Tarasoff v Regents of the University of California, 551 P.2d 334, 131 Cal. Rptr. 14 (Cal. 1976).
- Safer v Estate of Pack, 677 A.2d 1188 (N.J. App. 1996), cert. denied, 683 A.2d 1163 (N.J. 1996).
- Pate v Threlkel, 661 So.2d 278 (Fla. 1995).
- Rothstein MA. Liability issues in pharmacogenomics. Louisiana L Rev. 2005;66:117-124.
- Marchant G, Lindor R. Personalized medicine and genetic malpractice. Genet Med. 2013;15:921-922.
- Westbrook M. Transforming the physician’s standard of care in the context of whole genome sequencing technologies: finding guidance in best practice standards. Saint Louis U J Health Law Policy. 2015;9:111-148.
- Sevilla C, Moatti JP, Reynier CJ, et al. Testing for BRCA1 mutations: a cost-effective analysis. Europ J Human Genetics. 2002;10:599-606.
- Cotton V, Kirkpatrick D. Failure to recommend genetic counseling in breast cancer: is the next wave of medical professional liability lawsuits? Contemp OB/GYN. June 1, 2017.
- Suryavanshi M, Kumar D, Panigrahi M, et al. Detection of false positive mutations in BRCA gene by next generation sequencing. Fam Cancer. 2017;16:311-317.
- Black L, Knoppers B, Avard D, et al. Legal liability and the uncertain nature of risk prediction: the case of breast cancer risk prediction models. Public Health Genomics. 2012;15:335-340.
- McClintock A, Gollab A, Laya M. Breast cancer risk assessment, a step-wise approach for primary care physicians on the front lines of shared decision making. Mayo Clin Proc. 2020;95:1268-1275.
- National Cancer Institute. The Breast Cancer Risk Assessment Tool. https://bcrisktool.cancer.gov/. Accessed February 25, 2021.
- Neff J, Richardson G, Phelps J. Legal liabilities associated with hereditary breast and ovarian cancers. J Reprod Med. 2020;65:227-230.
- American College of Obstetricians and Gynecologists. Practice Bulletin No 182: hereditary breast and ovarian cancer syndrome. Obstet Gynecol. 2017;130:e110-e126.
- Sá dos Reis C, Gremion I, and Meystre NR. Study of breast implants mammography examinations for identification of suitable image quality criteria. Insights Imaging. 2020;11:3.
- Association for Molecular Pathology v Myriad Genetics, 569 U.S. 576 (2013).
- Smith SR. The Supreme Court 2012-2013: dogs, DNA, and DOMA. Register Rep. 2013;39(Fall):26-33.
- Bal BS. An introduction to medical malpractice in the United States. Clin Orthop Relat Res. 2009;467:339-347.
- Helling v Carey, 83 Wn.2d 514, 519 P.2d 981 (1974).
- The T.J. Hooper, 60 F.2d 737, 740 (2d Cir.1932), cert. denied 287 U.S. 662 (1932).
- Fischer DA. Tort recovery for loss of a chance. Wake Forest L Rev. 2001;36:605-655.
- Murphy BL, Ray-Zack MD, Reddy PN, et al. Breast cancer litigation in the 21st century. Ann Surg Oncol. 2018;25:2939- 2947.
- Prince AE. Prevention for those who can pay: insurance reimbursement of genetic-based preventive interventions in the liminal state between health and disease. J Law Biosci. 2015;2:365-395.
- Marchant G, Barnes M, Evans JP, et al; LawSeq Liability Task Force. From genetics to genomics: facing the liability implications in clinical care. J Law Med Ethics. 2020;48:11-43.
- Complaint, Held v Ambry Genetics Corp., No. 15-CV-8683, 2015 WL 6750024 (S.D.N.Y. Nov. 4, 2015); Order of Dismissal, Held v Ambry Genetics Corp., No. 15-CV-8683, (S.D.N.Y. Dec. 6, 2016).
- Pederson HJ. Breast cancer risk assessment and treatment: current concepts in genetics and genomics. Contemp OB/ GYN. 2017; 62:A1-A4.
- Pederson HJ. Who needs breast cancer genetics testing? OBG Manag. 2018;30:34-39.
- Roberts JL, Foulkes A. Genetic duties. William Mary L Rev. 2020;62:143-212.
- Thorogood A, Cook-Deegan R, Knoppers B. Public variant databases: liability? Genet Med. 2017;19:838–841.
- Marchant G, Lindor R. Genomic malpractice: an emerging tide or gentle ripple? Food Drug Law J. 2018;73:1-37.
- National Human Genome Research Institute. Genetic discrimination. https://www.genome.gov/about-genomics /policy-issues/Genetic-Discrimination. Updated September 16, 2020. Accessed February 25, 2021.
- National Cancer Institute. BRCA mutations: cancer risk and genetic testing. https://www.cancer.gov/about-cancer /causes-prevention/genetics/brca-fact-sheet. Reviewed November 19, 2020. Accessed February 25, 2021.
- National Cancer Institute. Genetics of breast and gynecologic cancers (PDQ®)–Health Professional Version. https://www .cancer.gov/types/breast/hp/breast-ovarian-genetics-pdq. Updated February 12, 2021. Accessed February 25, 2021.
- Reed v Campagnolo, 630 A.2d 1145, 1152–54 (Md. 1993).
- Munro v Regents of Univ. of Cal.,263 Cal. Rptr. 878, 885, 988 (1989).
- AMA Council on Ethical and Judicial Affairs. AMA Code of Medical Ethics’ opinions on genetic testing. Opinion 2.131. 2009;11:683-685. https://journalofethics.ama-assn .org/article/ama-code-medical-ethics-opinions-genetictesting/2009-09.
- Gilbar R, Barnoy S. Disclosing genetic test results to the patient’ relatives: how does the law influence clinical practice? J Law Technol Policy. 2019;125-168.
- Song K. Warning third parties of genetic risks in the era of personalized medicine. U.C. Davis L Rev. 2016;49:1987-2018.
- Tarasoff v Regents of the University of California, 551 P.2d 334, 131 Cal. Rptr. 14 (Cal. 1976).
- Safer v Estate of Pack, 677 A.2d 1188 (N.J. App. 1996), cert. denied, 683 A.2d 1163 (N.J. 1996).
- Pate v Threlkel, 661 So.2d 278 (Fla. 1995).
- Rothstein MA. Liability issues in pharmacogenomics. Louisiana L Rev. 2005;66:117-124.
- Marchant G, Lindor R. Personalized medicine and genetic malpractice. Genet Med. 2013;15:921-922.
- Westbrook M. Transforming the physician’s standard of care in the context of whole genome sequencing technologies: finding guidance in best practice standards. Saint Louis U J Health Law Policy. 2015;9:111-148.
FDA authorizes first molecular at-home, OTC COVID-19 test
The U.S. Food and Drug Administration has granted emergency use authorization (EUA) for the Cue COVID-19 Test for Home and Over The Counter Use (Cue OTC Test, Cue Health).
The Cue OTC Test is the first molecular diagnostic test available to consumers without a prescription.
The test detects genetic material from SARS-CoV-2 present in the nostrils and delivers results in about 20 minutes to the user’s mobile smart device via the Cue Health app.
In testing, the Cue OTC Test correctly identified 96% of positive nasal swab samples from individuals known to have symptoms and correctly identified 100% of positive samples from individuals without symptoms.
The test is intended for use in people aged 2 years and older with and without symptoms.
“With this authorization, consumers can purchase and self-administer one of the easiest, fastest, and most accurate tests without a prescription,” Clint Sever, cofounder and chief product officer of Cue Health, said in a news release.
“This FDA authorization will help us improve patient outcomes with a solution that provides the accuracy of central lab tests, with the speed and accessibility required to address emergent global health issues,” he said.
Cue Health expects to produce more than 100,000 single-use test kits per day by this summer. Dena Cook, the company’s chief communications officer, told this news organization that the company hasn’t announced pricing information yet, but the price will be “comparable” to other price points and other products on the market.
“The FDA continues to prioritize the availability of more at-home testing options in response to the pandemic,” Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, said in a statement.
“Cue COVID-19 Test for Home and Over-the-Counter Use provides access to accurate and reliable testing at home, without a prescription. The FDA will continue to work collaboratively with test developers to advance effective testing options for doctors, clinicians, and the public,” he said.
In June, the FDA granted an EUA to Cue Health’s COVID-19 test for use in clinical and point-of-care settings.
The test is currently being used in hospitals, physicians’ offices, and dental clinics, as well as schools, essential businesses, nursing homes, and other congregate-care facilities. The test is also being distributed through a program led by the U.S. Department of Defense and the U.S. Department of Health & Human Services across several states.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has granted emergency use authorization (EUA) for the Cue COVID-19 Test for Home and Over The Counter Use (Cue OTC Test, Cue Health).
The Cue OTC Test is the first molecular diagnostic test available to consumers without a prescription.
The test detects genetic material from SARS-CoV-2 present in the nostrils and delivers results in about 20 minutes to the user’s mobile smart device via the Cue Health app.
In testing, the Cue OTC Test correctly identified 96% of positive nasal swab samples from individuals known to have symptoms and correctly identified 100% of positive samples from individuals without symptoms.
The test is intended for use in people aged 2 years and older with and without symptoms.
“With this authorization, consumers can purchase and self-administer one of the easiest, fastest, and most accurate tests without a prescription,” Clint Sever, cofounder and chief product officer of Cue Health, said in a news release.
“This FDA authorization will help us improve patient outcomes with a solution that provides the accuracy of central lab tests, with the speed and accessibility required to address emergent global health issues,” he said.
Cue Health expects to produce more than 100,000 single-use test kits per day by this summer. Dena Cook, the company’s chief communications officer, told this news organization that the company hasn’t announced pricing information yet, but the price will be “comparable” to other price points and other products on the market.
“The FDA continues to prioritize the availability of more at-home testing options in response to the pandemic,” Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, said in a statement.
“Cue COVID-19 Test for Home and Over-the-Counter Use provides access to accurate and reliable testing at home, without a prescription. The FDA will continue to work collaboratively with test developers to advance effective testing options for doctors, clinicians, and the public,” he said.
In June, the FDA granted an EUA to Cue Health’s COVID-19 test for use in clinical and point-of-care settings.
The test is currently being used in hospitals, physicians’ offices, and dental clinics, as well as schools, essential businesses, nursing homes, and other congregate-care facilities. The test is also being distributed through a program led by the U.S. Department of Defense and the U.S. Department of Health & Human Services across several states.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has granted emergency use authorization (EUA) for the Cue COVID-19 Test for Home and Over The Counter Use (Cue OTC Test, Cue Health).
The Cue OTC Test is the first molecular diagnostic test available to consumers without a prescription.
The test detects genetic material from SARS-CoV-2 present in the nostrils and delivers results in about 20 minutes to the user’s mobile smart device via the Cue Health app.
In testing, the Cue OTC Test correctly identified 96% of positive nasal swab samples from individuals known to have symptoms and correctly identified 100% of positive samples from individuals without symptoms.
The test is intended for use in people aged 2 years and older with and without symptoms.
“With this authorization, consumers can purchase and self-administer one of the easiest, fastest, and most accurate tests without a prescription,” Clint Sever, cofounder and chief product officer of Cue Health, said in a news release.
“This FDA authorization will help us improve patient outcomes with a solution that provides the accuracy of central lab tests, with the speed and accessibility required to address emergent global health issues,” he said.
Cue Health expects to produce more than 100,000 single-use test kits per day by this summer. Dena Cook, the company’s chief communications officer, told this news organization that the company hasn’t announced pricing information yet, but the price will be “comparable” to other price points and other products on the market.
“The FDA continues to prioritize the availability of more at-home testing options in response to the pandemic,” Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, said in a statement.
“Cue COVID-19 Test for Home and Over-the-Counter Use provides access to accurate and reliable testing at home, without a prescription. The FDA will continue to work collaboratively with test developers to advance effective testing options for doctors, clinicians, and the public,” he said.
In June, the FDA granted an EUA to Cue Health’s COVID-19 test for use in clinical and point-of-care settings.
The test is currently being used in hospitals, physicians’ offices, and dental clinics, as well as schools, essential businesses, nursing homes, and other congregate-care facilities. The test is also being distributed through a program led by the U.S. Department of Defense and the U.S. Department of Health & Human Services across several states.
A version of this article first appeared on Medscape.com.
Comparison of Shave and Punch Biopsy Utilization Among Dermatology Practices
In 2019, the 2 Current Procedural Terminology (CPT) codes for skin biopsies (11100 and 11101) were replaced with 6 new CPT codes that specify biopsy technique and associated procedural complexity. 1,2 Prior to the coding changes, all biopsies were reimbursed at the same payment level, but a punch biopsy (11104; national nonfacility Medicare payment, $133.29) is now reimbursed more than a shave biopsy (11102; national nonfacility Medicare payment, $106.42). 3 We sought to evaluate whether the decrease in reimbursement for shave biopsies and concurrent increase in reimbursement for punch biopsies led to a shift from shave to punch biopsy utilization.
Methods
We examined shave and punch biopsies submitted for pathologic examination at Brigham and Women’s Hospital, Massachusetts General Hospital, and Massachusetts General Physician’s Organization (all in Boston, Massachusetts), and Penn Medicine, University of Pennsylvania Health System (Philadelphia, Pennsylvania), in May 2018 vs May 2019 (four months after new codes were implemented). This study was approved by Partners HealthCare (Boston, Massachusetts) and the University of Pennsylvania institutional review boards.
We included shave and punch biopsies of skin performed by physician dermatologists and mid-level providers (ie, physician assistants, nurse practitioners) at dermatology practices. All biopsies performed by a technique other than shave or punch, unspecified biopsy type, consultation cases, nonskin biopsies (eg, mucosa), and biopsies performed at nondermatology practices were excluded. We also excluded biopsies by providers who were not present during both study periods to account for provider mix.
Statistical Analysis
To evaluate for changes in the ratio of shave to punch biopsy utilization over time, we performed χ2 tests. Because care practices may differ between private and academic settings as well as between physicians and mid-level providers, we performed subgroup analyses by practice setting and provider type.4
Results
We identified 11,785 biopsies (12.11% of which were punch) submitted for pathologic examination in May 2018 compared to 11,291 biopsies (12.08% of which were punch) in May 2019 (Table). The overall use of punch biopsies relative to shave biopsies did not change between the years. There was a relative decrease in punch biopsy use among academic practices (−1.88%; P=.032) and a relative increase in punch biopsy use among private practices (+0.90%; P=.043). Provider type was not associated with differing utilization of biopsy type.
Comment
Overall, there was not a considerable shift in utilization behavior from shave to punch biopsies after the introduction of new coding changes. However, our study does demonstrate a small yet significant increase in punch biopsy utilization among private practices, and a decrease among academic practices. Although the change in biopsy utilization behavior is small in magnitude, it may have a substantial impact when extrapolated to behavior across the entire United States.
We were unable to assess additional factors, such as clinical diagnosis, body site, and cosmetic concerns, that may impact biopsy type selection in this study. Although we included multiple study sites to improve generalizability, our findings may not be representative of all biopsies performed in the dermatology setting. The baseline difference in relative punch biopsy use in academic vs private practices may reflect differences in patient populations and chief concerns, but assuming these features are stable over a 1-year time period, our findings should remain valid. Future studies should focus on qualitative evaluations of physician decision-making and evaluate whether similar trends persist over time.
Conclusion
Skin biopsy utilization trends among differing practice and provider types should continue to be monitored to assess for longitudinal trends in utilization within the context of updated billing codes and associated reimbursements.
- Grider D. 2019 CPT® coding for skin biopsies. ICD10 monitor website. September 17, 2018. Updated January 7, 2019. Accessed February 17, 2021. https://www.icd10monitor.com/2019-cpt-coding-for-skin-biopsies 2.
- Tongdee E, Siegel DM, Markowitz O. New diagnostic procedure codes and reimbursement. Cutis. 2019;103:208-211.
- Search the physician fee schedule. Centers for Medicare & Medicaid Services website. Updated January 20, 2021. Accessed February 17, 2021. https://www.cms.gov/medicare/physician-fee-schedule/search
- Zhang M, Zippin J, Kaffenberger B. Trends and scope of dermatology procedures billed by advanced practice professionals from 2012 through 2015. JAMA Dermatol. 2018;154:1040-1044.
In 2019, the 2 Current Procedural Terminology (CPT) codes for skin biopsies (11100 and 11101) were replaced with 6 new CPT codes that specify biopsy technique and associated procedural complexity. 1,2 Prior to the coding changes, all biopsies were reimbursed at the same payment level, but a punch biopsy (11104; national nonfacility Medicare payment, $133.29) is now reimbursed more than a shave biopsy (11102; national nonfacility Medicare payment, $106.42). 3 We sought to evaluate whether the decrease in reimbursement for shave biopsies and concurrent increase in reimbursement for punch biopsies led to a shift from shave to punch biopsy utilization.
Methods
We examined shave and punch biopsies submitted for pathologic examination at Brigham and Women’s Hospital, Massachusetts General Hospital, and Massachusetts General Physician’s Organization (all in Boston, Massachusetts), and Penn Medicine, University of Pennsylvania Health System (Philadelphia, Pennsylvania), in May 2018 vs May 2019 (four months after new codes were implemented). This study was approved by Partners HealthCare (Boston, Massachusetts) and the University of Pennsylvania institutional review boards.
We included shave and punch biopsies of skin performed by physician dermatologists and mid-level providers (ie, physician assistants, nurse practitioners) at dermatology practices. All biopsies performed by a technique other than shave or punch, unspecified biopsy type, consultation cases, nonskin biopsies (eg, mucosa), and biopsies performed at nondermatology practices were excluded. We also excluded biopsies by providers who were not present during both study periods to account for provider mix.
Statistical Analysis
To evaluate for changes in the ratio of shave to punch biopsy utilization over time, we performed χ2 tests. Because care practices may differ between private and academic settings as well as between physicians and mid-level providers, we performed subgroup analyses by practice setting and provider type.4
Results
We identified 11,785 biopsies (12.11% of which were punch) submitted for pathologic examination in May 2018 compared to 11,291 biopsies (12.08% of which were punch) in May 2019 (Table). The overall use of punch biopsies relative to shave biopsies did not change between the years. There was a relative decrease in punch biopsy use among academic practices (−1.88%; P=.032) and a relative increase in punch biopsy use among private practices (+0.90%; P=.043). Provider type was not associated with differing utilization of biopsy type.

Comment
Overall, there was not a considerable shift in utilization behavior from shave to punch biopsies after the introduction of new coding changes. However, our study does demonstrate a small yet significant increase in punch biopsy utilization among private practices, and a decrease among academic practices. Although the change in biopsy utilization behavior is small in magnitude, it may have a substantial impact when extrapolated to behavior across the entire United States.
We were unable to assess additional factors, such as clinical diagnosis, body site, and cosmetic concerns, that may impact biopsy type selection in this study. Although we included multiple study sites to improve generalizability, our findings may not be representative of all biopsies performed in the dermatology setting. The baseline difference in relative punch biopsy use in academic vs private practices may reflect differences in patient populations and chief concerns, but assuming these features are stable over a 1-year time period, our findings should remain valid. Future studies should focus on qualitative evaluations of physician decision-making and evaluate whether similar trends persist over time.
Conclusion
Skin biopsy utilization trends among differing practice and provider types should continue to be monitored to assess for longitudinal trends in utilization within the context of updated billing codes and associated reimbursements.
In 2019, the 2 Current Procedural Terminology (CPT) codes for skin biopsies (11100 and 11101) were replaced with 6 new CPT codes that specify biopsy technique and associated procedural complexity. 1,2 Prior to the coding changes, all biopsies were reimbursed at the same payment level, but a punch biopsy (11104; national nonfacility Medicare payment, $133.29) is now reimbursed more than a shave biopsy (11102; national nonfacility Medicare payment, $106.42). 3 We sought to evaluate whether the decrease in reimbursement for shave biopsies and concurrent increase in reimbursement for punch biopsies led to a shift from shave to punch biopsy utilization.
Methods
We examined shave and punch biopsies submitted for pathologic examination at Brigham and Women’s Hospital, Massachusetts General Hospital, and Massachusetts General Physician’s Organization (all in Boston, Massachusetts), and Penn Medicine, University of Pennsylvania Health System (Philadelphia, Pennsylvania), in May 2018 vs May 2019 (four months after new codes were implemented). This study was approved by Partners HealthCare (Boston, Massachusetts) and the University of Pennsylvania institutional review boards.
We included shave and punch biopsies of skin performed by physician dermatologists and mid-level providers (ie, physician assistants, nurse practitioners) at dermatology practices. All biopsies performed by a technique other than shave or punch, unspecified biopsy type, consultation cases, nonskin biopsies (eg, mucosa), and biopsies performed at nondermatology practices were excluded. We also excluded biopsies by providers who were not present during both study periods to account for provider mix.
Statistical Analysis
To evaluate for changes in the ratio of shave to punch biopsy utilization over time, we performed χ2 tests. Because care practices may differ between private and academic settings as well as between physicians and mid-level providers, we performed subgroup analyses by practice setting and provider type.4
Results
We identified 11,785 biopsies (12.11% of which were punch) submitted for pathologic examination in May 2018 compared to 11,291 biopsies (12.08% of which were punch) in May 2019 (Table). The overall use of punch biopsies relative to shave biopsies did not change between the years. There was a relative decrease in punch biopsy use among academic practices (−1.88%; P=.032) and a relative increase in punch biopsy use among private practices (+0.90%; P=.043). Provider type was not associated with differing utilization of biopsy type.

Comment
Overall, there was not a considerable shift in utilization behavior from shave to punch biopsies after the introduction of new coding changes. However, our study does demonstrate a small yet significant increase in punch biopsy utilization among private practices, and a decrease among academic practices. Although the change in biopsy utilization behavior is small in magnitude, it may have a substantial impact when extrapolated to behavior across the entire United States.
We were unable to assess additional factors, such as clinical diagnosis, body site, and cosmetic concerns, that may impact biopsy type selection in this study. Although we included multiple study sites to improve generalizability, our findings may not be representative of all biopsies performed in the dermatology setting. The baseline difference in relative punch biopsy use in academic vs private practices may reflect differences in patient populations and chief concerns, but assuming these features are stable over a 1-year time period, our findings should remain valid. Future studies should focus on qualitative evaluations of physician decision-making and evaluate whether similar trends persist over time.
Conclusion
Skin biopsy utilization trends among differing practice and provider types should continue to be monitored to assess for longitudinal trends in utilization within the context of updated billing codes and associated reimbursements.
- Grider D. 2019 CPT® coding for skin biopsies. ICD10 monitor website. September 17, 2018. Updated January 7, 2019. Accessed February 17, 2021. https://www.icd10monitor.com/2019-cpt-coding-for-skin-biopsies 2.
- Tongdee E, Siegel DM, Markowitz O. New diagnostic procedure codes and reimbursement. Cutis. 2019;103:208-211.
- Search the physician fee schedule. Centers for Medicare & Medicaid Services website. Updated January 20, 2021. Accessed February 17, 2021. https://www.cms.gov/medicare/physician-fee-schedule/search
- Zhang M, Zippin J, Kaffenberger B. Trends and scope of dermatology procedures billed by advanced practice professionals from 2012 through 2015. JAMA Dermatol. 2018;154:1040-1044.
- Grider D. 2019 CPT® coding for skin biopsies. ICD10 monitor website. September 17, 2018. Updated January 7, 2019. Accessed February 17, 2021. https://www.icd10monitor.com/2019-cpt-coding-for-skin-biopsies 2.
- Tongdee E, Siegel DM, Markowitz O. New diagnostic procedure codes and reimbursement. Cutis. 2019;103:208-211.
- Search the physician fee schedule. Centers for Medicare & Medicaid Services website. Updated January 20, 2021. Accessed February 17, 2021. https://www.cms.gov/medicare/physician-fee-schedule/search
- Zhang M, Zippin J, Kaffenberger B. Trends and scope of dermatology procedures billed by advanced practice professionals from 2012 through 2015. JAMA Dermatol. 2018;154:1040-1044.
Practice Points
- Dermatologists should be aware that skin biopsy billing codes and reimbursements were changed in 2019 to reflect their level of complexity, which may impact how often each type of biopsy is used.
- Even small shifts in biopsy utilization behavior among dermatologists in the context of reimbursement changes can have a large impact on net reimbursements.
CDC chief lays out attack plan for COVID variants
earlier this week.
As part of JAMA’s Q&A series with JAMA editor in chief Howard Bauchner, MD, Dr. Walensky referenced the blueprint she coathored with Anthony Fauci, MD, the nation’s top infectious disease expert, and Henry T. Walke, MD, MPH, of the CDC, which was published on Feb. 17 in JAMA.
In the viewpoint article, they explain that the Department of Health & Human Services has established the SARS-CoV-2 Interagency Group to improve coordination among the CDC, the National Institutes of Health, the Food and Drug Administration, the Biomedical Advanced Research and Development Authority, the Department of Agriculture, and the Department of Defense.
Dr. Walensky said the first objective is to reinforce vigilance regarding public health mitigation strategies to decrease the amount of virus that’s circulating.
As part of that strategy, she said, the CDC strongly urges against nonessential travel.
In addition, public health leaders are working on a surveillance system to better understand the SARS-CoV-2 variants. That will take ramping up genome sequencing of the SARS-CoV-2 virus and ensuring that sampling is geographically representative.
She said the CDC is partnering with state health labs to obtain about 750 samples every week and is teaming up with commercial labs and academic centers to obtain an interim target of 6,000 samples per week.
She acknowledged the United States “is not where we need to be” with sequencing but has come a long way since January. At that time, they were sequencing 250 samples every week; they are currently sequencing thousands each week.
Data analysis is another concern: “We need to be able to understand at the basic science level what the information means,” Dr. Walensky said.
Researchers aren’t sure how the variants might affect use of convalescent plasma or monoclonal antibody treatments. It is expected that 5% of persons who are vaccinated against COVID-19 will nevertheless contract the disease. Sequencing will help answer whether such persons who have been vaccinated and who subsequently contract the virus are among those 5% or whether have been infected by a variant that evades the vaccine.
Accelerating vaccine administration globally and in the United States is essential, Dr. Walensky said.
As of Feb. 17, 56 million doses had been administered in the United States.
Top three threats
She updated the numbers on the three biggest variant threats.
Regarding B.1.1.7, which originated in the United Kingdom, she said: “So far, we’ve had over 1,200 cases in 41 states.” She noted that the variant is likely to be about 50% more transmissible and 30% to 50% more virulent.
“So far, it looks like that strain doesn’t have any real decrease in susceptibility to our vaccines,” she said.
The strain from South Africa (B.1.351) has been found in 19 cases in the United States.
The P.1. variant, which originated in Brazil, has been identified in two cases in two states.
Outlook for March and April
Dr. Bauchner asked Dr. Walensky what she envisions for March and April. He noted that public optimism is high in light of the continued reductions in COVID-19 case numbers, hospitalizations, and deaths, as well as the fact that warmer weather is coming and that more vaccinations are on the horizon.
“While I really am hopeful for what could happen in March and April,” Dr. Walensky said, “I really do know that this could go bad so fast. We saw it in November. We saw it in December.”
CDC models have projected that, by March, the more transmissible B.1.1.7 strain is likely to be the dominant strain, she reiterated.
“I worry that it will be spring, and we will all have had enough,” Dr. Walensky said. She noted that some states are already relaxing mask mandates.
“Around that time, life will look and feel a little better, and the motivation for those who might be vaccine hesitant may be diminished,” she said.
Dr. Bauchner also asked her to weigh in on whether a third vaccine, from Johnson & Johnson (J&J), may soon gain FDA emergency-use authorization – and whether its lower expected efficacy rate may result in a tiered system of vaccinations, with higher-risk populations receiving the more efficacious vaccines.
Dr. Walensky said more data are needed before that question can be answered.
“It may very well be that the data point us to the best populations in which to use this vaccine,” she said.
In phase 3 data, the J&J vaccine was shown to be 72% effective in the United States for moderate to severe disease.
Dr. Walensky said it’s important to remember that the projected efficacy for that vaccine is higher than that for the flu shot as well as many other vaccines currently in use for other diseases.
She said it also has several advantages. The vaccine has less-stringent storage requirements, requires just one dose, and protects against hospitalization and death, although it’s less efficacious in protecting against contracting the disease.
“I think many people would opt to get that one if they could get it sooner,” she said.
A version of this article first appeared on Medscape.com.
earlier this week.
As part of JAMA’s Q&A series with JAMA editor in chief Howard Bauchner, MD, Dr. Walensky referenced the blueprint she coathored with Anthony Fauci, MD, the nation’s top infectious disease expert, and Henry T. Walke, MD, MPH, of the CDC, which was published on Feb. 17 in JAMA.
In the viewpoint article, they explain that the Department of Health & Human Services has established the SARS-CoV-2 Interagency Group to improve coordination among the CDC, the National Institutes of Health, the Food and Drug Administration, the Biomedical Advanced Research and Development Authority, the Department of Agriculture, and the Department of Defense.
Dr. Walensky said the first objective is to reinforce vigilance regarding public health mitigation strategies to decrease the amount of virus that’s circulating.
As part of that strategy, she said, the CDC strongly urges against nonessential travel.
In addition, public health leaders are working on a surveillance system to better understand the SARS-CoV-2 variants. That will take ramping up genome sequencing of the SARS-CoV-2 virus and ensuring that sampling is geographically representative.
She said the CDC is partnering with state health labs to obtain about 750 samples every week and is teaming up with commercial labs and academic centers to obtain an interim target of 6,000 samples per week.
She acknowledged the United States “is not where we need to be” with sequencing but has come a long way since January. At that time, they were sequencing 250 samples every week; they are currently sequencing thousands each week.
Data analysis is another concern: “We need to be able to understand at the basic science level what the information means,” Dr. Walensky said.
Researchers aren’t sure how the variants might affect use of convalescent plasma or monoclonal antibody treatments. It is expected that 5% of persons who are vaccinated against COVID-19 will nevertheless contract the disease. Sequencing will help answer whether such persons who have been vaccinated and who subsequently contract the virus are among those 5% or whether have been infected by a variant that evades the vaccine.
Accelerating vaccine administration globally and in the United States is essential, Dr. Walensky said.
As of Feb. 17, 56 million doses had been administered in the United States.
Top three threats
She updated the numbers on the three biggest variant threats.
Regarding B.1.1.7, which originated in the United Kingdom, she said: “So far, we’ve had over 1,200 cases in 41 states.” She noted that the variant is likely to be about 50% more transmissible and 30% to 50% more virulent.
“So far, it looks like that strain doesn’t have any real decrease in susceptibility to our vaccines,” she said.
The strain from South Africa (B.1.351) has been found in 19 cases in the United States.
The P.1. variant, which originated in Brazil, has been identified in two cases in two states.
Outlook for March and April
Dr. Bauchner asked Dr. Walensky what she envisions for March and April. He noted that public optimism is high in light of the continued reductions in COVID-19 case numbers, hospitalizations, and deaths, as well as the fact that warmer weather is coming and that more vaccinations are on the horizon.
“While I really am hopeful for what could happen in March and April,” Dr. Walensky said, “I really do know that this could go bad so fast. We saw it in November. We saw it in December.”
CDC models have projected that, by March, the more transmissible B.1.1.7 strain is likely to be the dominant strain, she reiterated.
“I worry that it will be spring, and we will all have had enough,” Dr. Walensky said. She noted that some states are already relaxing mask mandates.
“Around that time, life will look and feel a little better, and the motivation for those who might be vaccine hesitant may be diminished,” she said.
Dr. Bauchner also asked her to weigh in on whether a third vaccine, from Johnson & Johnson (J&J), may soon gain FDA emergency-use authorization – and whether its lower expected efficacy rate may result in a tiered system of vaccinations, with higher-risk populations receiving the more efficacious vaccines.
Dr. Walensky said more data are needed before that question can be answered.
“It may very well be that the data point us to the best populations in which to use this vaccine,” she said.
In phase 3 data, the J&J vaccine was shown to be 72% effective in the United States for moderate to severe disease.
Dr. Walensky said it’s important to remember that the projected efficacy for that vaccine is higher than that for the flu shot as well as many other vaccines currently in use for other diseases.
She said it also has several advantages. The vaccine has less-stringent storage requirements, requires just one dose, and protects against hospitalization and death, although it’s less efficacious in protecting against contracting the disease.
“I think many people would opt to get that one if they could get it sooner,” she said.
A version of this article first appeared on Medscape.com.
earlier this week.
As part of JAMA’s Q&A series with JAMA editor in chief Howard Bauchner, MD, Dr. Walensky referenced the blueprint she coathored with Anthony Fauci, MD, the nation’s top infectious disease expert, and Henry T. Walke, MD, MPH, of the CDC, which was published on Feb. 17 in JAMA.
In the viewpoint article, they explain that the Department of Health & Human Services has established the SARS-CoV-2 Interagency Group to improve coordination among the CDC, the National Institutes of Health, the Food and Drug Administration, the Biomedical Advanced Research and Development Authority, the Department of Agriculture, and the Department of Defense.
Dr. Walensky said the first objective is to reinforce vigilance regarding public health mitigation strategies to decrease the amount of virus that’s circulating.
As part of that strategy, she said, the CDC strongly urges against nonessential travel.
In addition, public health leaders are working on a surveillance system to better understand the SARS-CoV-2 variants. That will take ramping up genome sequencing of the SARS-CoV-2 virus and ensuring that sampling is geographically representative.
She said the CDC is partnering with state health labs to obtain about 750 samples every week and is teaming up with commercial labs and academic centers to obtain an interim target of 6,000 samples per week.
She acknowledged the United States “is not where we need to be” with sequencing but has come a long way since January. At that time, they were sequencing 250 samples every week; they are currently sequencing thousands each week.
Data analysis is another concern: “We need to be able to understand at the basic science level what the information means,” Dr. Walensky said.
Researchers aren’t sure how the variants might affect use of convalescent plasma or monoclonal antibody treatments. It is expected that 5% of persons who are vaccinated against COVID-19 will nevertheless contract the disease. Sequencing will help answer whether such persons who have been vaccinated and who subsequently contract the virus are among those 5% or whether have been infected by a variant that evades the vaccine.
Accelerating vaccine administration globally and in the United States is essential, Dr. Walensky said.
As of Feb. 17, 56 million doses had been administered in the United States.
Top three threats
She updated the numbers on the three biggest variant threats.
Regarding B.1.1.7, which originated in the United Kingdom, she said: “So far, we’ve had over 1,200 cases in 41 states.” She noted that the variant is likely to be about 50% more transmissible and 30% to 50% more virulent.
“So far, it looks like that strain doesn’t have any real decrease in susceptibility to our vaccines,” she said.
The strain from South Africa (B.1.351) has been found in 19 cases in the United States.
The P.1. variant, which originated in Brazil, has been identified in two cases in two states.
Outlook for March and April
Dr. Bauchner asked Dr. Walensky what she envisions for March and April. He noted that public optimism is high in light of the continued reductions in COVID-19 case numbers, hospitalizations, and deaths, as well as the fact that warmer weather is coming and that more vaccinations are on the horizon.
“While I really am hopeful for what could happen in March and April,” Dr. Walensky said, “I really do know that this could go bad so fast. We saw it in November. We saw it in December.”
CDC models have projected that, by March, the more transmissible B.1.1.7 strain is likely to be the dominant strain, she reiterated.
“I worry that it will be spring, and we will all have had enough,” Dr. Walensky said. She noted that some states are already relaxing mask mandates.
“Around that time, life will look and feel a little better, and the motivation for those who might be vaccine hesitant may be diminished,” she said.
Dr. Bauchner also asked her to weigh in on whether a third vaccine, from Johnson & Johnson (J&J), may soon gain FDA emergency-use authorization – and whether its lower expected efficacy rate may result in a tiered system of vaccinations, with higher-risk populations receiving the more efficacious vaccines.
Dr. Walensky said more data are needed before that question can be answered.
“It may very well be that the data point us to the best populations in which to use this vaccine,” she said.
In phase 3 data, the J&J vaccine was shown to be 72% effective in the United States for moderate to severe disease.
Dr. Walensky said it’s important to remember that the projected efficacy for that vaccine is higher than that for the flu shot as well as many other vaccines currently in use for other diseases.
She said it also has several advantages. The vaccine has less-stringent storage requirements, requires just one dose, and protects against hospitalization and death, although it’s less efficacious in protecting against contracting the disease.
“I think many people would opt to get that one if they could get it sooner,” she said.
A version of this article first appeared on Medscape.com.
Super Bowl ad for diabetes device prompts debate
A commercial for the continuous glucose monitor (CGM) Dexcom G6 shown during the Super Bowl has provoked strong reactions in the diabetes community, both positive and negative.
The 30-second ad, which aired between the first two quarters of the American football game yesterday, features singer-songwriter-actor Nick Jonas, who has type 1 diabetes. During the ad, Mr. Jonas asks – with so much technology available today, including drones that deliver packages and self-driving cars – why are people with diabetes still pricking their fingers to test their blood sugar?
Mr. Jonas goes on to demonstrate the Dexcom G6 smartphone glucose app as it displays three different glucose levels including two trending upward, explaining: “It shows your glucose right in your phone, and where it’s heading, without fingersticks. Finally, technology that makes it easier to manage our diabetes.”
Diabetes type or insulin treatment are not mentioned in the ad, despite the fact that most insurance plans typically only cover CGMs for people with type 1 diabetes and sometimes for those with type 2 diabetes who take multiple daily insulin doses (given the risk for hypoglycemia).
Ad prompts mixed reaction on social media
Reactions rolled in on Twitter after the ad debuted Feb. 2, and then again after it aired during the game.
Some people who have type 1 diabetes themselves or have children with the disease who use the product were thrilled.
“Thanks to @NickJonas for his advocacy on T1. My 11-year old has been on the Dexcom for 3 weeks. For a newly diagnosed kid, it removes a lot of anxiety (and for his parents, too!) Plus, he is thrilled his meter has a Super Bowl commercial!” tweeted @KatisJewell.
Another positive tweet, from @rturnerroy, read: “@nickjonas Thank you for bringing representation to #type1diabetes. And hey #Dexcom, you’re the best.”
But many others were critical, both of Jonas and Dexcom. @hb_herrick tweeted: “Diabetes awareness is fantastic. Dexcom being able to afford Nick Jonas for a #SuperBowl commercial is not. This is a health care product. Make it more affordable for those who need it.”
Another Twitter user, @universeofdust, tweeted: “Feeling ambivalent about the #Dexcom ad tbh. I love the awareness & representation. But also not a big fan of dexcom spending $5.5 mill+ to make the CGM seem like this ~cool & trendy~ thing when many type 1s can’t afford their insulin, let alone a CGM.”
And @andricheli wrote: “Only people lucky enough to have excellent insurance and be able to afford the out-of-pocket costs have access. Many others do not.”
And in another tweet the same user said, “The #Dexcom is an amazing device. It’s literally lifesaving and life extending. But it’s also very expensive and not available to everyone. Maybe instead of spending $5 mil on a Super Bowl ad, @dexcom should spend that on getting Dex into the handle of people who need it.”
Others, including @1hitwonderdate, criticized Mr. Jonas directly, asking him: “As someone who has struggled with diabetes and is trying to support themselves along with millions of others, why not use this platform to help those who can’t afford their supplies or are rationing them?!”
Dexcom and Jonas’ organization respond
This news organization reached out to both Dexcom and to Beyond Type 1, a nonprofit organization cofounded by Mr. Jonas, for comment. Both emailed responses.
Regarding the intended audience for the ad, Dexcom acknowledged that it hoped to reach a much wider group than just people with type 1 diabetes or even just insulin users.
“We believe our CGM technology has the ability to empower any person with diabetes and significantly improve their treatment and quality of life, whether they are using insulin or not,” the company said, adding that the ad was also aimed at “loved ones, caregivers, and even health care professionals who need to know about this technology.”
According to Dexcom, the G6 is covered by 99% of commercial insurance in the United States, in addition to Medicare, and by Medicaid in more than 40 states. Over 70% of Dexcom patients with pharmacy coverage in the United States pay under $60 per month for CGM, and a third pay $0 out-of-pocket.
“That said, we know there’s more to be done to improve access, and we are working with several partners to broaden access to Dexcom CGM, especially for people with type 2 diabetes not on mealtime insulin,” the company noted.
Beyond Type 1 responded to the criticisms about Mr. Jonas personally, noting that the celebrity is, in fact, heavily involved in advocacy.
“Nick was involved in the launch of GetInsulin.org this past October,” they said. “GetInsulin.org is a tool created by Beyond Type 1 to connect people with diabetes in the United States to the insulin access and affordability options that match their unique circumstances. ... Beyond Type 1 will continue driving awareness of short-term solutions related to insulin access and affordability while fighting for systemic change.”
The organization “is also advocating for systemic payment policies that will make devices less expensive and avoid the same pitfalls (and rising prices) as the drug pricing system in the U.S.”
Mr. Jonas himself appears aware of the concerns.
Is 2021’s most expensive Super Bowl ad justified?
Meanwhile, in a piece in Esquire, Dave Holmes, who has type 1 diabetes, weighs up the pros and cons of the ad.
He writes: “While Jonas makes it look fun and easy to use a Dexcom G6 – a program to just get with like you would a drone or LED eyelashes – the process of acquiring one is complicated and often very expensive, even for people with good insurance. Which makes the year’s most expensive ad buy, for a product that only a small percentage of the U.S. population needs, confusing to me and others.”
Mr. Holmes also spoke with Craig Stubing, founder of the Beta Cell Foundation, a nonprofit that aims to educate and empower those with type 1 diabetes.
“Spending all this money on an ad, when people’s lives are at stake. I don’t know if offensive is the right word, but it seems out of touch with the reality that their patients are facing,” Mr. Stubing told Mr. Holmes.
A version of this article first appeared on Medscape.com.
A commercial for the continuous glucose monitor (CGM) Dexcom G6 shown during the Super Bowl has provoked strong reactions in the diabetes community, both positive and negative.
The 30-second ad, which aired between the first two quarters of the American football game yesterday, features singer-songwriter-actor Nick Jonas, who has type 1 diabetes. During the ad, Mr. Jonas asks – with so much technology available today, including drones that deliver packages and self-driving cars – why are people with diabetes still pricking their fingers to test their blood sugar?
Mr. Jonas goes on to demonstrate the Dexcom G6 smartphone glucose app as it displays three different glucose levels including two trending upward, explaining: “It shows your glucose right in your phone, and where it’s heading, without fingersticks. Finally, technology that makes it easier to manage our diabetes.”
Diabetes type or insulin treatment are not mentioned in the ad, despite the fact that most insurance plans typically only cover CGMs for people with type 1 diabetes and sometimes for those with type 2 diabetes who take multiple daily insulin doses (given the risk for hypoglycemia).
Ad prompts mixed reaction on social media
Reactions rolled in on Twitter after the ad debuted Feb. 2, and then again after it aired during the game.
Some people who have type 1 diabetes themselves or have children with the disease who use the product were thrilled.
“Thanks to @NickJonas for his advocacy on T1. My 11-year old has been on the Dexcom for 3 weeks. For a newly diagnosed kid, it removes a lot of anxiety (and for his parents, too!) Plus, he is thrilled his meter has a Super Bowl commercial!” tweeted @KatisJewell.
Another positive tweet, from @rturnerroy, read: “@nickjonas Thank you for bringing representation to #type1diabetes. And hey #Dexcom, you’re the best.”
But many others were critical, both of Jonas and Dexcom. @hb_herrick tweeted: “Diabetes awareness is fantastic. Dexcom being able to afford Nick Jonas for a #SuperBowl commercial is not. This is a health care product. Make it more affordable for those who need it.”
Another Twitter user, @universeofdust, tweeted: “Feeling ambivalent about the #Dexcom ad tbh. I love the awareness & representation. But also not a big fan of dexcom spending $5.5 mill+ to make the CGM seem like this ~cool & trendy~ thing when many type 1s can’t afford their insulin, let alone a CGM.”
And @andricheli wrote: “Only people lucky enough to have excellent insurance and be able to afford the out-of-pocket costs have access. Many others do not.”
And in another tweet the same user said, “The #Dexcom is an amazing device. It’s literally lifesaving and life extending. But it’s also very expensive and not available to everyone. Maybe instead of spending $5 mil on a Super Bowl ad, @dexcom should spend that on getting Dex into the handle of people who need it.”
Others, including @1hitwonderdate, criticized Mr. Jonas directly, asking him: “As someone who has struggled with diabetes and is trying to support themselves along with millions of others, why not use this platform to help those who can’t afford their supplies or are rationing them?!”
Dexcom and Jonas’ organization respond
This news organization reached out to both Dexcom and to Beyond Type 1, a nonprofit organization cofounded by Mr. Jonas, for comment. Both emailed responses.
Regarding the intended audience for the ad, Dexcom acknowledged that it hoped to reach a much wider group than just people with type 1 diabetes or even just insulin users.
“We believe our CGM technology has the ability to empower any person with diabetes and significantly improve their treatment and quality of life, whether they are using insulin or not,” the company said, adding that the ad was also aimed at “loved ones, caregivers, and even health care professionals who need to know about this technology.”
According to Dexcom, the G6 is covered by 99% of commercial insurance in the United States, in addition to Medicare, and by Medicaid in more than 40 states. Over 70% of Dexcom patients with pharmacy coverage in the United States pay under $60 per month for CGM, and a third pay $0 out-of-pocket.
“That said, we know there’s more to be done to improve access, and we are working with several partners to broaden access to Dexcom CGM, especially for people with type 2 diabetes not on mealtime insulin,” the company noted.
Beyond Type 1 responded to the criticisms about Mr. Jonas personally, noting that the celebrity is, in fact, heavily involved in advocacy.
“Nick was involved in the launch of GetInsulin.org this past October,” they said. “GetInsulin.org is a tool created by Beyond Type 1 to connect people with diabetes in the United States to the insulin access and affordability options that match their unique circumstances. ... Beyond Type 1 will continue driving awareness of short-term solutions related to insulin access and affordability while fighting for systemic change.”
The organization “is also advocating for systemic payment policies that will make devices less expensive and avoid the same pitfalls (and rising prices) as the drug pricing system in the U.S.”
Mr. Jonas himself appears aware of the concerns.
Is 2021’s most expensive Super Bowl ad justified?
Meanwhile, in a piece in Esquire, Dave Holmes, who has type 1 diabetes, weighs up the pros and cons of the ad.
He writes: “While Jonas makes it look fun and easy to use a Dexcom G6 – a program to just get with like you would a drone or LED eyelashes – the process of acquiring one is complicated and often very expensive, even for people with good insurance. Which makes the year’s most expensive ad buy, for a product that only a small percentage of the U.S. population needs, confusing to me and others.”
Mr. Holmes also spoke with Craig Stubing, founder of the Beta Cell Foundation, a nonprofit that aims to educate and empower those with type 1 diabetes.
“Spending all this money on an ad, when people’s lives are at stake. I don’t know if offensive is the right word, but it seems out of touch with the reality that their patients are facing,” Mr. Stubing told Mr. Holmes.
A version of this article first appeared on Medscape.com.
A commercial for the continuous glucose monitor (CGM) Dexcom G6 shown during the Super Bowl has provoked strong reactions in the diabetes community, both positive and negative.
The 30-second ad, which aired between the first two quarters of the American football game yesterday, features singer-songwriter-actor Nick Jonas, who has type 1 diabetes. During the ad, Mr. Jonas asks – with so much technology available today, including drones that deliver packages and self-driving cars – why are people with diabetes still pricking their fingers to test their blood sugar?
Mr. Jonas goes on to demonstrate the Dexcom G6 smartphone glucose app as it displays three different glucose levels including two trending upward, explaining: “It shows your glucose right in your phone, and where it’s heading, without fingersticks. Finally, technology that makes it easier to manage our diabetes.”
Diabetes type or insulin treatment are not mentioned in the ad, despite the fact that most insurance plans typically only cover CGMs for people with type 1 diabetes and sometimes for those with type 2 diabetes who take multiple daily insulin doses (given the risk for hypoglycemia).
Ad prompts mixed reaction on social media
Reactions rolled in on Twitter after the ad debuted Feb. 2, and then again after it aired during the game.
Some people who have type 1 diabetes themselves or have children with the disease who use the product were thrilled.
“Thanks to @NickJonas for his advocacy on T1. My 11-year old has been on the Dexcom for 3 weeks. For a newly diagnosed kid, it removes a lot of anxiety (and for his parents, too!) Plus, he is thrilled his meter has a Super Bowl commercial!” tweeted @KatisJewell.
Another positive tweet, from @rturnerroy, read: “@nickjonas Thank you for bringing representation to #type1diabetes. And hey #Dexcom, you’re the best.”
But many others were critical, both of Jonas and Dexcom. @hb_herrick tweeted: “Diabetes awareness is fantastic. Dexcom being able to afford Nick Jonas for a #SuperBowl commercial is not. This is a health care product. Make it more affordable for those who need it.”
Another Twitter user, @universeofdust, tweeted: “Feeling ambivalent about the #Dexcom ad tbh. I love the awareness & representation. But also not a big fan of dexcom spending $5.5 mill+ to make the CGM seem like this ~cool & trendy~ thing when many type 1s can’t afford their insulin, let alone a CGM.”
And @andricheli wrote: “Only people lucky enough to have excellent insurance and be able to afford the out-of-pocket costs have access. Many others do not.”
And in another tweet the same user said, “The #Dexcom is an amazing device. It’s literally lifesaving and life extending. But it’s also very expensive and not available to everyone. Maybe instead of spending $5 mil on a Super Bowl ad, @dexcom should spend that on getting Dex into the handle of people who need it.”
Others, including @1hitwonderdate, criticized Mr. Jonas directly, asking him: “As someone who has struggled with diabetes and is trying to support themselves along with millions of others, why not use this platform to help those who can’t afford their supplies or are rationing them?!”
Dexcom and Jonas’ organization respond
This news organization reached out to both Dexcom and to Beyond Type 1, a nonprofit organization cofounded by Mr. Jonas, for comment. Both emailed responses.
Regarding the intended audience for the ad, Dexcom acknowledged that it hoped to reach a much wider group than just people with type 1 diabetes or even just insulin users.
“We believe our CGM technology has the ability to empower any person with diabetes and significantly improve their treatment and quality of life, whether they are using insulin or not,” the company said, adding that the ad was also aimed at “loved ones, caregivers, and even health care professionals who need to know about this technology.”
According to Dexcom, the G6 is covered by 99% of commercial insurance in the United States, in addition to Medicare, and by Medicaid in more than 40 states. Over 70% of Dexcom patients with pharmacy coverage in the United States pay under $60 per month for CGM, and a third pay $0 out-of-pocket.
“That said, we know there’s more to be done to improve access, and we are working with several partners to broaden access to Dexcom CGM, especially for people with type 2 diabetes not on mealtime insulin,” the company noted.
Beyond Type 1 responded to the criticisms about Mr. Jonas personally, noting that the celebrity is, in fact, heavily involved in advocacy.
“Nick was involved in the launch of GetInsulin.org this past October,” they said. “GetInsulin.org is a tool created by Beyond Type 1 to connect people with diabetes in the United States to the insulin access and affordability options that match their unique circumstances. ... Beyond Type 1 will continue driving awareness of short-term solutions related to insulin access and affordability while fighting for systemic change.”
The organization “is also advocating for systemic payment policies that will make devices less expensive and avoid the same pitfalls (and rising prices) as the drug pricing system in the U.S.”
Mr. Jonas himself appears aware of the concerns.
Is 2021’s most expensive Super Bowl ad justified?
Meanwhile, in a piece in Esquire, Dave Holmes, who has type 1 diabetes, weighs up the pros and cons of the ad.
He writes: “While Jonas makes it look fun and easy to use a Dexcom G6 – a program to just get with like you would a drone or LED eyelashes – the process of acquiring one is complicated and often very expensive, even for people with good insurance. Which makes the year’s most expensive ad buy, for a product that only a small percentage of the U.S. population needs, confusing to me and others.”
Mr. Holmes also spoke with Craig Stubing, founder of the Beta Cell Foundation, a nonprofit that aims to educate and empower those with type 1 diabetes.
“Spending all this money on an ad, when people’s lives are at stake. I don’t know if offensive is the right word, but it seems out of touch with the reality that their patients are facing,” Mr. Stubing told Mr. Holmes.
A version of this article first appeared on Medscape.com.
Burnout rates in ICU staff fueled by shortages, overtime
Health care professionals working in critical care settings have been overburdened because of the plethora of COVID-19 cases, which has led to symptoms of burnout in both physicians and nurses, findings from a new study show.
“Overburdening ICU professionals during an extended period of time leads to burnout,” said lead study author Niek Kok, MSc, of IQ healthcare, Radboud University Medical Center, Radboud Institute for Health Sciences, Nijmegen, the Netherlands. “All ICU professionals are at the risk of this, and in our study, the incidence of physicians experiencing burnout was significantly higher than that of nurses in June 2020.”
This burnout can be explained by conditions caused by the pandemic, he noted, such as the scarcity of staff and resources and having to work with colleagues who were not qualified to work in critical care but who were there out of necessity.
Mr. Kok presented the findings of the study at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
Burnout highest among critical care physicians
The ICU can be a stressful environment for both patients and health care personnel, and burnout is not uncommon among ICU clinicians. However, COVID-19 has amplified the degree of burnout being experienced by clinicians working in this setting. Critical care physicians now top the list of physicians experiencing burnout, at 51%, up from 44% last year, according to the Medscape report ‘Death by 1000 Thousand Cuts’: Physician Burnout and Suicide Report 2021.
The Medscape Nurse Career Satisfaction Report 2020, while not restricted to those working in critical care, also reported higher rates of burnout, compared with the prepandemic period. The percentage of nurses reporting being “very burned out” prior to the pandemic was 4%. Six months into the pandemic, that percentage soared to 18%.
In this study, Mr. Kok and colleagues examined the prevalence and incidence of burnout symptoms and moral distress in health care professionals working in the ICU, both before and during the COVID-19 pandemic.
“When the COVID-19 pandemic surfaced in the Netherlands, the health care professionals in our hospitals were motivated to do everything they could to provide the best care possible,” said Mr. Kok. “Many of the ICU professionals immediately realized that they would have to work longer hours.”
However, the health care professionals that he spoke with did have mixed feelings. Some were afraid of being infected with the virus, while others said that “it was very interesting times for them and that gave them extra motivation to do the work.
“Some physicians [and] the WHO warned that COVID-19 is not going to weathered by a heroic sprint – it is an arduous marathon that is going to go hand in hand with burnout symptoms,” Mr. Kok added. “It will eat away at our qualified ICU staff.”
Before and after data on burnout
It was widely believed that the COVID-19 pandemic would increase burnout symptoms, as had been demonstrated in studies of previous pandemics. However, Mr. Kok emphasized that there are no before and after measurements that transcend cross-sectional designs.
“The claim [has been] that it increases burnout – but there are no assessments of how it progresses in ICU professionals through time,” he said. “So what we really need is a comparison [of] before and after the pandemic.”
It is quite difficult to obtain this type of information because disruptive events like the COVID-19 pandemic cannot be predicted, he said. Thus, it is challenging to get a baseline measurement. But Mr. Kok pointed out that the study has both “before and after” measurements.
“By coincidence really, we had baseline data to measure the impact of the COVID-19 pandemic and had information that was collected before the pandemic,” he said.
In January 2020, a study began looking at the effects of ethics meetings on moral distress in ICU professionals. Data had been collected on moral distress and burnout on ICU professionals in December 2019. The first COVID-19 cases appeared in the Netherlands in February 2020.
A follow-up study was then conducted in May and June 2020, several months into the pandemic.
The longitudinal open cohort study included all ICU personnel who were working in five units within a single university medical center, plus another adult ICU that was based in a separate teaching hospital.
A total of 352 health care professionals responded to a baseline survey in October through December 2019, and then 233 responded to a follow-up survey sent in May and June 2020. The authors measured burnout symptoms and moral distress with the Maslach Burnout Inventory and the Moral Distress Scale, respectively.
Findings
The overall prevalence of burnout symptoms was 23.0% prior to the pandemic, and that jumped to 36.1% at post-peak time. Higher rates of burnout were reported by nurses (38.0%) than physicians (28.6%).
However, the incidence rate of new burnout cases was higher among physicians, compared with nurses (26.7% vs 21.9%). Not surprisingly, a higher prevalence of burnout symptoms was observed in the post-peak period for all clinicians (odds ratio, 1.83; 95% confidence interval, 1.32-2.53), and was higher for nurses (odds ratio, 1.77; 95% confidence interval, 1.03-3.04), for those working overtime (OR, 2.11; 95% CI, 1.48-3.02), and for personnel who directly engaged in patient care (OR, 1.87; 95% CI, 1.35-2.60).
Physicians in general were much more likely to develop burnout symptoms related to the pandemic, compared with nurses (OR, 3.56; 95% CI, 1.06-12.21).
When looking at findings on moral distress, Kok pointed out that it often arises in situations when the health care professional knows the right thing to do but is prevented from doing so. “Morally distressful situations all rose from December to June,” said Mr. Kok. “Scarcity was the most distressing. The other was where colleagues were perceived to be less skilled, and this had to do with the recruitment of people from outside of the ICU to provide care.”
Moral distress from scarcity and unskilled colleagues were both significantly related to burnout, he noted.
In the final model, working in a COVID-19 unit, stress from scarcity of resources and people, stress from unskilled colleagues, and stress from unsafe conditions were all related to burnout. “The stress of physicians was significantly higher,” said Kok. “Even though nurses had higher baseline burnout, it became less pronounced in June 2020. This indicates that burnout was significantly higher in physicians.”
Thus, Mr. Kok and colleagues concluded that overburdening ICU professionals during an extended period of time leads to burnout, and all ICU workers are at risk.
Burnout rates higher in physicians
Weighing in on the study, Greg S. Martin, MD, FCCP, professor of medicine in the division of pulmonary, allergy, critical care and sleep medicine, Emory University, Atlanta, noted that the differences observed between physicians and nurses may have to do with the fact that “nurses have been smoldering all along and experiencing higher rates of burnout.
“They may have adapted better to the pandemic conditions, since they are more used to working overtime and short staffed, and spending far more time at the bedside,” he said. “Because of the volume of patients, physicians may be spending more hours doing patient care and are experiencing more burnout.”
For physicians, this may be a more significant change in the workload, as well as the complexity of the situation because of the pandemic. “Many things layer into it, such as [the fact] that there are no families present to give patients support, the complexity of care of these patients, and things like lack of PPE,” Dr. Martin said.
The study did not differentiate among physician groups, so it is unclear if the affected physicians were residents, fellows, or more senior staff. “Residents are often quite busy already, and don’t usually have the capacity to add more to their schedules, and maybe attendings were having to spend more time doing patient care,” Dr. Martin said. “In the United States, at least some personnel were restricted from working with COVID-19 patients. Medical students were removed in many places as well as nonessential staff, so that may have also added to their burnout.”
The study was conducted in the Netherlands, so there may be differences in the work environment, responsibilities of nurses vs. physicians, staffing, and so on. “But it still shows that burnout is very real among doctors and nurses working in the ICU in pandemic conditions,” he said.
Health care professionals working in critical care settings have been overburdened because of the plethora of COVID-19 cases, which has led to symptoms of burnout in both physicians and nurses, findings from a new study show.
“Overburdening ICU professionals during an extended period of time leads to burnout,” said lead study author Niek Kok, MSc, of IQ healthcare, Radboud University Medical Center, Radboud Institute for Health Sciences, Nijmegen, the Netherlands. “All ICU professionals are at the risk of this, and in our study, the incidence of physicians experiencing burnout was significantly higher than that of nurses in June 2020.”
This burnout can be explained by conditions caused by the pandemic, he noted, such as the scarcity of staff and resources and having to work with colleagues who were not qualified to work in critical care but who were there out of necessity.
Mr. Kok presented the findings of the study at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
Burnout highest among critical care physicians
The ICU can be a stressful environment for both patients and health care personnel, and burnout is not uncommon among ICU clinicians. However, COVID-19 has amplified the degree of burnout being experienced by clinicians working in this setting. Critical care physicians now top the list of physicians experiencing burnout, at 51%, up from 44% last year, according to the Medscape report ‘Death by 1000 Thousand Cuts’: Physician Burnout and Suicide Report 2021.
The Medscape Nurse Career Satisfaction Report 2020, while not restricted to those working in critical care, also reported higher rates of burnout, compared with the prepandemic period. The percentage of nurses reporting being “very burned out” prior to the pandemic was 4%. Six months into the pandemic, that percentage soared to 18%.
In this study, Mr. Kok and colleagues examined the prevalence and incidence of burnout symptoms and moral distress in health care professionals working in the ICU, both before and during the COVID-19 pandemic.
“When the COVID-19 pandemic surfaced in the Netherlands, the health care professionals in our hospitals were motivated to do everything they could to provide the best care possible,” said Mr. Kok. “Many of the ICU professionals immediately realized that they would have to work longer hours.”
However, the health care professionals that he spoke with did have mixed feelings. Some were afraid of being infected with the virus, while others said that “it was very interesting times for them and that gave them extra motivation to do the work.
“Some physicians [and] the WHO warned that COVID-19 is not going to weathered by a heroic sprint – it is an arduous marathon that is going to go hand in hand with burnout symptoms,” Mr. Kok added. “It will eat away at our qualified ICU staff.”
Before and after data on burnout
It was widely believed that the COVID-19 pandemic would increase burnout symptoms, as had been demonstrated in studies of previous pandemics. However, Mr. Kok emphasized that there are no before and after measurements that transcend cross-sectional designs.
“The claim [has been] that it increases burnout – but there are no assessments of how it progresses in ICU professionals through time,” he said. “So what we really need is a comparison [of] before and after the pandemic.”
It is quite difficult to obtain this type of information because disruptive events like the COVID-19 pandemic cannot be predicted, he said. Thus, it is challenging to get a baseline measurement. But Mr. Kok pointed out that the study has both “before and after” measurements.
“By coincidence really, we had baseline data to measure the impact of the COVID-19 pandemic and had information that was collected before the pandemic,” he said.
In January 2020, a study began looking at the effects of ethics meetings on moral distress in ICU professionals. Data had been collected on moral distress and burnout on ICU professionals in December 2019. The first COVID-19 cases appeared in the Netherlands in February 2020.
A follow-up study was then conducted in May and June 2020, several months into the pandemic.
The longitudinal open cohort study included all ICU personnel who were working in five units within a single university medical center, plus another adult ICU that was based in a separate teaching hospital.
A total of 352 health care professionals responded to a baseline survey in October through December 2019, and then 233 responded to a follow-up survey sent in May and June 2020. The authors measured burnout symptoms and moral distress with the Maslach Burnout Inventory and the Moral Distress Scale, respectively.
Findings
The overall prevalence of burnout symptoms was 23.0% prior to the pandemic, and that jumped to 36.1% at post-peak time. Higher rates of burnout were reported by nurses (38.0%) than physicians (28.6%).
However, the incidence rate of new burnout cases was higher among physicians, compared with nurses (26.7% vs 21.9%). Not surprisingly, a higher prevalence of burnout symptoms was observed in the post-peak period for all clinicians (odds ratio, 1.83; 95% confidence interval, 1.32-2.53), and was higher for nurses (odds ratio, 1.77; 95% confidence interval, 1.03-3.04), for those working overtime (OR, 2.11; 95% CI, 1.48-3.02), and for personnel who directly engaged in patient care (OR, 1.87; 95% CI, 1.35-2.60).
Physicians in general were much more likely to develop burnout symptoms related to the pandemic, compared with nurses (OR, 3.56; 95% CI, 1.06-12.21).
When looking at findings on moral distress, Kok pointed out that it often arises in situations when the health care professional knows the right thing to do but is prevented from doing so. “Morally distressful situations all rose from December to June,” said Mr. Kok. “Scarcity was the most distressing. The other was where colleagues were perceived to be less skilled, and this had to do with the recruitment of people from outside of the ICU to provide care.”
Moral distress from scarcity and unskilled colleagues were both significantly related to burnout, he noted.
In the final model, working in a COVID-19 unit, stress from scarcity of resources and people, stress from unskilled colleagues, and stress from unsafe conditions were all related to burnout. “The stress of physicians was significantly higher,” said Kok. “Even though nurses had higher baseline burnout, it became less pronounced in June 2020. This indicates that burnout was significantly higher in physicians.”
Thus, Mr. Kok and colleagues concluded that overburdening ICU professionals during an extended period of time leads to burnout, and all ICU workers are at risk.
Burnout rates higher in physicians
Weighing in on the study, Greg S. Martin, MD, FCCP, professor of medicine in the division of pulmonary, allergy, critical care and sleep medicine, Emory University, Atlanta, noted that the differences observed between physicians and nurses may have to do with the fact that “nurses have been smoldering all along and experiencing higher rates of burnout.
“They may have adapted better to the pandemic conditions, since they are more used to working overtime and short staffed, and spending far more time at the bedside,” he said. “Because of the volume of patients, physicians may be spending more hours doing patient care and are experiencing more burnout.”
For physicians, this may be a more significant change in the workload, as well as the complexity of the situation because of the pandemic. “Many things layer into it, such as [the fact] that there are no families present to give patients support, the complexity of care of these patients, and things like lack of PPE,” Dr. Martin said.
The study did not differentiate among physician groups, so it is unclear if the affected physicians were residents, fellows, or more senior staff. “Residents are often quite busy already, and don’t usually have the capacity to add more to their schedules, and maybe attendings were having to spend more time doing patient care,” Dr. Martin said. “In the United States, at least some personnel were restricted from working with COVID-19 patients. Medical students were removed in many places as well as nonessential staff, so that may have also added to their burnout.”
The study was conducted in the Netherlands, so there may be differences in the work environment, responsibilities of nurses vs. physicians, staffing, and so on. “But it still shows that burnout is very real among doctors and nurses working in the ICU in pandemic conditions,” he said.
Health care professionals working in critical care settings have been overburdened because of the plethora of COVID-19 cases, which has led to symptoms of burnout in both physicians and nurses, findings from a new study show.
“Overburdening ICU professionals during an extended period of time leads to burnout,” said lead study author Niek Kok, MSc, of IQ healthcare, Radboud University Medical Center, Radboud Institute for Health Sciences, Nijmegen, the Netherlands. “All ICU professionals are at the risk of this, and in our study, the incidence of physicians experiencing burnout was significantly higher than that of nurses in June 2020.”
This burnout can be explained by conditions caused by the pandemic, he noted, such as the scarcity of staff and resources and having to work with colleagues who were not qualified to work in critical care but who were there out of necessity.
Mr. Kok presented the findings of the study at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
Burnout highest among critical care physicians
The ICU can be a stressful environment for both patients and health care personnel, and burnout is not uncommon among ICU clinicians. However, COVID-19 has amplified the degree of burnout being experienced by clinicians working in this setting. Critical care physicians now top the list of physicians experiencing burnout, at 51%, up from 44% last year, according to the Medscape report ‘Death by 1000 Thousand Cuts’: Physician Burnout and Suicide Report 2021.
The Medscape Nurse Career Satisfaction Report 2020, while not restricted to those working in critical care, also reported higher rates of burnout, compared with the prepandemic period. The percentage of nurses reporting being “very burned out” prior to the pandemic was 4%. Six months into the pandemic, that percentage soared to 18%.
In this study, Mr. Kok and colleagues examined the prevalence and incidence of burnout symptoms and moral distress in health care professionals working in the ICU, both before and during the COVID-19 pandemic.
“When the COVID-19 pandemic surfaced in the Netherlands, the health care professionals in our hospitals were motivated to do everything they could to provide the best care possible,” said Mr. Kok. “Many of the ICU professionals immediately realized that they would have to work longer hours.”
However, the health care professionals that he spoke with did have mixed feelings. Some were afraid of being infected with the virus, while others said that “it was very interesting times for them and that gave them extra motivation to do the work.
“Some physicians [and] the WHO warned that COVID-19 is not going to weathered by a heroic sprint – it is an arduous marathon that is going to go hand in hand with burnout symptoms,” Mr. Kok added. “It will eat away at our qualified ICU staff.”
Before and after data on burnout
It was widely believed that the COVID-19 pandemic would increase burnout symptoms, as had been demonstrated in studies of previous pandemics. However, Mr. Kok emphasized that there are no before and after measurements that transcend cross-sectional designs.
“The claim [has been] that it increases burnout – but there are no assessments of how it progresses in ICU professionals through time,” he said. “So what we really need is a comparison [of] before and after the pandemic.”
It is quite difficult to obtain this type of information because disruptive events like the COVID-19 pandemic cannot be predicted, he said. Thus, it is challenging to get a baseline measurement. But Mr. Kok pointed out that the study has both “before and after” measurements.
“By coincidence really, we had baseline data to measure the impact of the COVID-19 pandemic and had information that was collected before the pandemic,” he said.
In January 2020, a study began looking at the effects of ethics meetings on moral distress in ICU professionals. Data had been collected on moral distress and burnout on ICU professionals in December 2019. The first COVID-19 cases appeared in the Netherlands in February 2020.
A follow-up study was then conducted in May and June 2020, several months into the pandemic.
The longitudinal open cohort study included all ICU personnel who were working in five units within a single university medical center, plus another adult ICU that was based in a separate teaching hospital.
A total of 352 health care professionals responded to a baseline survey in October through December 2019, and then 233 responded to a follow-up survey sent in May and June 2020. The authors measured burnout symptoms and moral distress with the Maslach Burnout Inventory and the Moral Distress Scale, respectively.
Findings
The overall prevalence of burnout symptoms was 23.0% prior to the pandemic, and that jumped to 36.1% at post-peak time. Higher rates of burnout were reported by nurses (38.0%) than physicians (28.6%).
However, the incidence rate of new burnout cases was higher among physicians, compared with nurses (26.7% vs 21.9%). Not surprisingly, a higher prevalence of burnout symptoms was observed in the post-peak period for all clinicians (odds ratio, 1.83; 95% confidence interval, 1.32-2.53), and was higher for nurses (odds ratio, 1.77; 95% confidence interval, 1.03-3.04), for those working overtime (OR, 2.11; 95% CI, 1.48-3.02), and for personnel who directly engaged in patient care (OR, 1.87; 95% CI, 1.35-2.60).
Physicians in general were much more likely to develop burnout symptoms related to the pandemic, compared with nurses (OR, 3.56; 95% CI, 1.06-12.21).
When looking at findings on moral distress, Kok pointed out that it often arises in situations when the health care professional knows the right thing to do but is prevented from doing so. “Morally distressful situations all rose from December to June,” said Mr. Kok. “Scarcity was the most distressing. The other was where colleagues were perceived to be less skilled, and this had to do with the recruitment of people from outside of the ICU to provide care.”
Moral distress from scarcity and unskilled colleagues were both significantly related to burnout, he noted.
In the final model, working in a COVID-19 unit, stress from scarcity of resources and people, stress from unskilled colleagues, and stress from unsafe conditions were all related to burnout. “The stress of physicians was significantly higher,” said Kok. “Even though nurses had higher baseline burnout, it became less pronounced in June 2020. This indicates that burnout was significantly higher in physicians.”
Thus, Mr. Kok and colleagues concluded that overburdening ICU professionals during an extended period of time leads to burnout, and all ICU workers are at risk.
Burnout rates higher in physicians
Weighing in on the study, Greg S. Martin, MD, FCCP, professor of medicine in the division of pulmonary, allergy, critical care and sleep medicine, Emory University, Atlanta, noted that the differences observed between physicians and nurses may have to do with the fact that “nurses have been smoldering all along and experiencing higher rates of burnout.
“They may have adapted better to the pandemic conditions, since they are more used to working overtime and short staffed, and spending far more time at the bedside,” he said. “Because of the volume of patients, physicians may be spending more hours doing patient care and are experiencing more burnout.”
For physicians, this may be a more significant change in the workload, as well as the complexity of the situation because of the pandemic. “Many things layer into it, such as [the fact] that there are no families present to give patients support, the complexity of care of these patients, and things like lack of PPE,” Dr. Martin said.
The study did not differentiate among physician groups, so it is unclear if the affected physicians were residents, fellows, or more senior staff. “Residents are often quite busy already, and don’t usually have the capacity to add more to their schedules, and maybe attendings were having to spend more time doing patient care,” Dr. Martin said. “In the United States, at least some personnel were restricted from working with COVID-19 patients. Medical students were removed in many places as well as nonessential staff, so that may have also added to their burnout.”
The study was conducted in the Netherlands, so there may be differences in the work environment, responsibilities of nurses vs. physicians, staffing, and so on. “But it still shows that burnout is very real among doctors and nurses working in the ICU in pandemic conditions,” he said.
FROM CCC50
Mask mandates reduced COVID-19 hospitalizations
States that implemented mask mandates in 2020 saw a decline in the growth of COVID-19 hospitalizations between March and October 2020, according to a new study published Feb. 5 in the CDC’s Morbidity and Mortality Weekly Report.
Hospitalization growth rates declined by 5.5 percentage points for adults between ages 18-64 about 3 weeks after the mandates were implemented, compared with climbing growth rates in the 4 weeks before mandates.
CDC Director Rochelle Walensky said she was pleased to see the results, but that it’s “too early” to tell whether President Joe Biden’s recent mask orders have had an effect on cases and hospitalizations in 2021.
“We’re going to be watching the mask data very carefully,” she said during a news briefing with the White House COVID-19 Response Team on Feb. 5. “I think it’s probably still a bit too early to tell, but I’m encouraged with the decrease in case rates right now.”
In another study published Feb. 5 in the Morbidity and Mortality Weekly Report, trained observers tracked mask use at six universities with mask mandates between September and November 2020. Overall, observers reported that about 92% of people wore masks correctly indoors, which varied based on the type of mask.
About 97% of people used N95 masks correctly, compared with 92% who used cloth masks, and 79% who used bandanas, scarves, or neck gaiters. Cloth masks were most common, and bandanas and scarves were least common.
The Biden administration is considering whether to send masks directly to American households to encourage people to wear them, according to NBC News. The White House COVID-19 Response Team is debating the logistics of mailing out masks, including how many to send and what the mask material would be, the news outlet reported.
Wisconsin Gov. Tony Evers reissued a new statewide mask mandate on Feb. 4, just an hour after the Republican-controlled legislature voted to repeal his previous mandate, according to The Associated Press. Gov. Evers said his priority is to keep people safe and that wearing a mask is the easiest way to do so.
“If the legislature keeps playing politics and we don’t keep wearing masks, we’re going to see more preventable deaths,” he said. “It’s going to take even longer to get our state and our economy back on track.”
A version of this article first appeared on WebMD.com.
States that implemented mask mandates in 2020 saw a decline in the growth of COVID-19 hospitalizations between March and October 2020, according to a new study published Feb. 5 in the CDC’s Morbidity and Mortality Weekly Report.
Hospitalization growth rates declined by 5.5 percentage points for adults between ages 18-64 about 3 weeks after the mandates were implemented, compared with climbing growth rates in the 4 weeks before mandates.
CDC Director Rochelle Walensky said she was pleased to see the results, but that it’s “too early” to tell whether President Joe Biden’s recent mask orders have had an effect on cases and hospitalizations in 2021.
“We’re going to be watching the mask data very carefully,” she said during a news briefing with the White House COVID-19 Response Team on Feb. 5. “I think it’s probably still a bit too early to tell, but I’m encouraged with the decrease in case rates right now.”
In another study published Feb. 5 in the Morbidity and Mortality Weekly Report, trained observers tracked mask use at six universities with mask mandates between September and November 2020. Overall, observers reported that about 92% of people wore masks correctly indoors, which varied based on the type of mask.
About 97% of people used N95 masks correctly, compared with 92% who used cloth masks, and 79% who used bandanas, scarves, or neck gaiters. Cloth masks were most common, and bandanas and scarves were least common.
The Biden administration is considering whether to send masks directly to American households to encourage people to wear them, according to NBC News. The White House COVID-19 Response Team is debating the logistics of mailing out masks, including how many to send and what the mask material would be, the news outlet reported.
Wisconsin Gov. Tony Evers reissued a new statewide mask mandate on Feb. 4, just an hour after the Republican-controlled legislature voted to repeal his previous mandate, according to The Associated Press. Gov. Evers said his priority is to keep people safe and that wearing a mask is the easiest way to do so.
“If the legislature keeps playing politics and we don’t keep wearing masks, we’re going to see more preventable deaths,” he said. “It’s going to take even longer to get our state and our economy back on track.”
A version of this article first appeared on WebMD.com.
States that implemented mask mandates in 2020 saw a decline in the growth of COVID-19 hospitalizations between March and October 2020, according to a new study published Feb. 5 in the CDC’s Morbidity and Mortality Weekly Report.
Hospitalization growth rates declined by 5.5 percentage points for adults between ages 18-64 about 3 weeks after the mandates were implemented, compared with climbing growth rates in the 4 weeks before mandates.
CDC Director Rochelle Walensky said she was pleased to see the results, but that it’s “too early” to tell whether President Joe Biden’s recent mask orders have had an effect on cases and hospitalizations in 2021.
“We’re going to be watching the mask data very carefully,” she said during a news briefing with the White House COVID-19 Response Team on Feb. 5. “I think it’s probably still a bit too early to tell, but I’m encouraged with the decrease in case rates right now.”
In another study published Feb. 5 in the Morbidity and Mortality Weekly Report, trained observers tracked mask use at six universities with mask mandates between September and November 2020. Overall, observers reported that about 92% of people wore masks correctly indoors, which varied based on the type of mask.
About 97% of people used N95 masks correctly, compared with 92% who used cloth masks, and 79% who used bandanas, scarves, or neck gaiters. Cloth masks were most common, and bandanas and scarves were least common.
The Biden administration is considering whether to send masks directly to American households to encourage people to wear them, according to NBC News. The White House COVID-19 Response Team is debating the logistics of mailing out masks, including how many to send and what the mask material would be, the news outlet reported.
Wisconsin Gov. Tony Evers reissued a new statewide mask mandate on Feb. 4, just an hour after the Republican-controlled legislature voted to repeal his previous mandate, according to The Associated Press. Gov. Evers said his priority is to keep people safe and that wearing a mask is the easiest way to do so.
“If the legislature keeps playing politics and we don’t keep wearing masks, we’re going to see more preventable deaths,” he said. “It’s going to take even longer to get our state and our economy back on track.”
A version of this article first appeared on WebMD.com.
FDA curbs use of COVID-19 convalescent plasma, citing new data
The Food and Drug Administration has revised its emergency use authorization for COVID-19 convalescent plasma on the basis of the latest available data.
The revision states that only high-titer COVID-19 convalescent plasma can be used and only in hospitalized patients who are early in the disease course and those with impaired humoral immunity who cannot produce an adequate antibody response.
The revisions stem from new clinical trial data analyzed or reported since the original EUA was issued in August 2020. The original EUA did not have these restrictions.
“This and other changes to the EUA represent important updates to the use of convalescent plasma for the treatment of COVID-19 patients,” Peter Marks, MD, PhD, director, FDA Center for Biologics Evaluation and Research, said in a statement announcing the revisions.
“COVID-19 convalescent plasma used according to the revised EUA may have efficacy, and its known and potential benefits outweigh its known and potential risks,” the FDA said.
The agency said it revoked use of low-titer COVID-19 convalescent plasma on the basis of new data from clinical trials, including randomized, controlled trials, that have failed to demonstrate that low-titer convalescent plasma may be effective in the treatment of hospitalized patients with COVID-19.
The FDA’s updated fact sheet for health care providers on the use of COVID-19 convalescent plasma also notes that transfusion of COVID-19 convalescent plasma late in the disease course, following respiratory failure requiring intubation and mechanical ventilation, hasn’t been found to have clinical benefit.
The revised EUA also includes several additional tests that can be used to manufacture COVID-19 convalescent plasma.
“With this update, nine tests are now included in the EUA for testing plasma donations for anti-SARS-CoV-2 antibodies as a manufacturing step to determine suitability before release,” the FDA said.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has revised its emergency use authorization for COVID-19 convalescent plasma on the basis of the latest available data.
The revision states that only high-titer COVID-19 convalescent plasma can be used and only in hospitalized patients who are early in the disease course and those with impaired humoral immunity who cannot produce an adequate antibody response.
The revisions stem from new clinical trial data analyzed or reported since the original EUA was issued in August 2020. The original EUA did not have these restrictions.
“This and other changes to the EUA represent important updates to the use of convalescent plasma for the treatment of COVID-19 patients,” Peter Marks, MD, PhD, director, FDA Center for Biologics Evaluation and Research, said in a statement announcing the revisions.
“COVID-19 convalescent plasma used according to the revised EUA may have efficacy, and its known and potential benefits outweigh its known and potential risks,” the FDA said.
The agency said it revoked use of low-titer COVID-19 convalescent plasma on the basis of new data from clinical trials, including randomized, controlled trials, that have failed to demonstrate that low-titer convalescent plasma may be effective in the treatment of hospitalized patients with COVID-19.
The FDA’s updated fact sheet for health care providers on the use of COVID-19 convalescent plasma also notes that transfusion of COVID-19 convalescent plasma late in the disease course, following respiratory failure requiring intubation and mechanical ventilation, hasn’t been found to have clinical benefit.
The revised EUA also includes several additional tests that can be used to manufacture COVID-19 convalescent plasma.
“With this update, nine tests are now included in the EUA for testing plasma donations for anti-SARS-CoV-2 antibodies as a manufacturing step to determine suitability before release,” the FDA said.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has revised its emergency use authorization for COVID-19 convalescent plasma on the basis of the latest available data.
The revision states that only high-titer COVID-19 convalescent plasma can be used and only in hospitalized patients who are early in the disease course and those with impaired humoral immunity who cannot produce an adequate antibody response.
The revisions stem from new clinical trial data analyzed or reported since the original EUA was issued in August 2020. The original EUA did not have these restrictions.
“This and other changes to the EUA represent important updates to the use of convalescent plasma for the treatment of COVID-19 patients,” Peter Marks, MD, PhD, director, FDA Center for Biologics Evaluation and Research, said in a statement announcing the revisions.
“COVID-19 convalescent plasma used according to the revised EUA may have efficacy, and its known and potential benefits outweigh its known and potential risks,” the FDA said.
The agency said it revoked use of low-titer COVID-19 convalescent plasma on the basis of new data from clinical trials, including randomized, controlled trials, that have failed to demonstrate that low-titer convalescent plasma may be effective in the treatment of hospitalized patients with COVID-19.
The FDA’s updated fact sheet for health care providers on the use of COVID-19 convalescent plasma also notes that transfusion of COVID-19 convalescent plasma late in the disease course, following respiratory failure requiring intubation and mechanical ventilation, hasn’t been found to have clinical benefit.
The revised EUA also includes several additional tests that can be used to manufacture COVID-19 convalescent plasma.
“With this update, nine tests are now included in the EUA for testing plasma donations for anti-SARS-CoV-2 antibodies as a manufacturing step to determine suitability before release,” the FDA said.
A version of this article first appeared on Medscape.com.