User login
Spreading out daily meals and snacks may boost heart failure survival
CLEVELAND – , an observational study suggests.
The new findings, based primarily on 15 years of data from the National Health and Nutrition Examination Survey (NHANES), may argue against time-restricted diet interventions like intermittent fasting for patients with HF, researchers say.
The study’s nearly 1,000 participants on medical therapy for HF reported a mean daily eating window of 11 hours and daily average of four “eating occasions,” defined as meals or snacks of at least 50 kcal.
A daily eating window of 11 or more hours, compared with less than 11 hours, corresponded to a greater than 40% drop in risk for CV mortality (P = .013) over 5-6 years, reported Hayley E. Billingsley, RD, CEP, Virginia Commonwealth University, Richmond, Va,, at the annual scientific meeting of the Heart Failure Society of America.
The analysis adjusted for caloric intake, daily number of eating occasions, body mass index (BMI), history of CV disease and cancer, diabetes, and a slew of other potential confounders.
Prior evidence, mostly from healthy people, has suggested that extended fasting during the day is associated with less physical activity, Ms. Billingsley said in an interview. So it may be that people with HF who spread out their calorie intake are more active throughout the day.
A longer time window for eating, therefore, may have indirect metabolic benefits and help preserve their lean body mass, possibly reducing CV risk in a patient group at risk for muscle wasting.
The findings add to earlier evidence from Ms. Billingsley’s center that suggests that expanded daily time windows for eating, especially later final food rather than earlier first food, may help boost CV fitness for patients with obesity and HF with preserved ejection fraction.
Intermittent fasting and other practices involving the timing of food intake have been studied for weight loss and metabolic health in mostly healthy people and patients with diabetes, she noted. “But it’s really underexplored in people with established cardiovascular disease.”
On the basis of admittedly “very preliminary” findings, it may be that some patients should not shorten their daily time windows for eating or engage in intermittent fasting, Ms. Billingsley said. It’s probably worth considering, before the approach is recommended, “what their risk is for malnutrition or sarcopenia.”
The current study included 991 persons who entered the NHANES database from 2003 to 2018. The patients self-identified as having HF, reported taking medications commonly prescribed in HF, and provided at least two “reliable” dietary recalls.
The average age of the patients was 68 years, and they had had HF for a mean of 9.5 years; 47% were women, three-fourths were White persons, two thirds had dyslipidemia, and a quarter had a history of cancer.
On average, their first eating occasion of the day was at about 8:30 a.m., and the last occasion was at about 7:30 p.m., for a time window of about 11 hours; daily calorie consumption averaged about 1,830 kcal.
About 52% died over the mean follow-up of 69 months; about 44% of deaths were from CV causes.
In a model adjusted for demographics, BMI, smoking status, times of eating occasions, CV disease, diabetes, and cancer history, the all-cause mortality hazard ratio for time windows ≥ 11 hours vs. < 11 hours was 0.236 (95% confidence interval, 0.07-0.715; P = .011).
The reduction was no longer significant on further adjustment for duration of HF, a score reflecting difficulty walking, nightly hours of sleep (which averaged 7.2 hours), daily number of eating occasions, and caloric intake, Ms. Billingsley reported.
But in the fully adjusted analysis, the HR for CV mortality for the longer vs. shorter time window was 0.368 (95% CI, 0.169-0.803; P = .013).
The issue deserves further exploration in a randomized trial, Ms. Billingsley proposed, perhaps one in which patients with HF wear accelerometers to track daily activity levels. “We’d love to do a pilot study of extending their eating window that really digs into what the mechanism of any benefit might be if we assign them to a longer time window and whether it’s related to physical activity.”
Ms. Billingsley reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
CLEVELAND – , an observational study suggests.
The new findings, based primarily on 15 years of data from the National Health and Nutrition Examination Survey (NHANES), may argue against time-restricted diet interventions like intermittent fasting for patients with HF, researchers say.
The study’s nearly 1,000 participants on medical therapy for HF reported a mean daily eating window of 11 hours and daily average of four “eating occasions,” defined as meals or snacks of at least 50 kcal.
A daily eating window of 11 or more hours, compared with less than 11 hours, corresponded to a greater than 40% drop in risk for CV mortality (P = .013) over 5-6 years, reported Hayley E. Billingsley, RD, CEP, Virginia Commonwealth University, Richmond, Va,, at the annual scientific meeting of the Heart Failure Society of America.
The analysis adjusted for caloric intake, daily number of eating occasions, body mass index (BMI), history of CV disease and cancer, diabetes, and a slew of other potential confounders.
Prior evidence, mostly from healthy people, has suggested that extended fasting during the day is associated with less physical activity, Ms. Billingsley said in an interview. So it may be that people with HF who spread out their calorie intake are more active throughout the day.
A longer time window for eating, therefore, may have indirect metabolic benefits and help preserve their lean body mass, possibly reducing CV risk in a patient group at risk for muscle wasting.
The findings add to earlier evidence from Ms. Billingsley’s center that suggests that expanded daily time windows for eating, especially later final food rather than earlier first food, may help boost CV fitness for patients with obesity and HF with preserved ejection fraction.
Intermittent fasting and other practices involving the timing of food intake have been studied for weight loss and metabolic health in mostly healthy people and patients with diabetes, she noted. “But it’s really underexplored in people with established cardiovascular disease.”
On the basis of admittedly “very preliminary” findings, it may be that some patients should not shorten their daily time windows for eating or engage in intermittent fasting, Ms. Billingsley said. It’s probably worth considering, before the approach is recommended, “what their risk is for malnutrition or sarcopenia.”
The current study included 991 persons who entered the NHANES database from 2003 to 2018. The patients self-identified as having HF, reported taking medications commonly prescribed in HF, and provided at least two “reliable” dietary recalls.
The average age of the patients was 68 years, and they had had HF for a mean of 9.5 years; 47% were women, three-fourths were White persons, two thirds had dyslipidemia, and a quarter had a history of cancer.
On average, their first eating occasion of the day was at about 8:30 a.m., and the last occasion was at about 7:30 p.m., for a time window of about 11 hours; daily calorie consumption averaged about 1,830 kcal.
About 52% died over the mean follow-up of 69 months; about 44% of deaths were from CV causes.
In a model adjusted for demographics, BMI, smoking status, times of eating occasions, CV disease, diabetes, and cancer history, the all-cause mortality hazard ratio for time windows ≥ 11 hours vs. < 11 hours was 0.236 (95% confidence interval, 0.07-0.715; P = .011).
The reduction was no longer significant on further adjustment for duration of HF, a score reflecting difficulty walking, nightly hours of sleep (which averaged 7.2 hours), daily number of eating occasions, and caloric intake, Ms. Billingsley reported.
But in the fully adjusted analysis, the HR for CV mortality for the longer vs. shorter time window was 0.368 (95% CI, 0.169-0.803; P = .013).
The issue deserves further exploration in a randomized trial, Ms. Billingsley proposed, perhaps one in which patients with HF wear accelerometers to track daily activity levels. “We’d love to do a pilot study of extending their eating window that really digs into what the mechanism of any benefit might be if we assign them to a longer time window and whether it’s related to physical activity.”
Ms. Billingsley reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
CLEVELAND – , an observational study suggests.
The new findings, based primarily on 15 years of data from the National Health and Nutrition Examination Survey (NHANES), may argue against time-restricted diet interventions like intermittent fasting for patients with HF, researchers say.
The study’s nearly 1,000 participants on medical therapy for HF reported a mean daily eating window of 11 hours and daily average of four “eating occasions,” defined as meals or snacks of at least 50 kcal.
A daily eating window of 11 or more hours, compared with less than 11 hours, corresponded to a greater than 40% drop in risk for CV mortality (P = .013) over 5-6 years, reported Hayley E. Billingsley, RD, CEP, Virginia Commonwealth University, Richmond, Va,, at the annual scientific meeting of the Heart Failure Society of America.
The analysis adjusted for caloric intake, daily number of eating occasions, body mass index (BMI), history of CV disease and cancer, diabetes, and a slew of other potential confounders.
Prior evidence, mostly from healthy people, has suggested that extended fasting during the day is associated with less physical activity, Ms. Billingsley said in an interview. So it may be that people with HF who spread out their calorie intake are more active throughout the day.
A longer time window for eating, therefore, may have indirect metabolic benefits and help preserve their lean body mass, possibly reducing CV risk in a patient group at risk for muscle wasting.
The findings add to earlier evidence from Ms. Billingsley’s center that suggests that expanded daily time windows for eating, especially later final food rather than earlier first food, may help boost CV fitness for patients with obesity and HF with preserved ejection fraction.
Intermittent fasting and other practices involving the timing of food intake have been studied for weight loss and metabolic health in mostly healthy people and patients with diabetes, she noted. “But it’s really underexplored in people with established cardiovascular disease.”
On the basis of admittedly “very preliminary” findings, it may be that some patients should not shorten their daily time windows for eating or engage in intermittent fasting, Ms. Billingsley said. It’s probably worth considering, before the approach is recommended, “what their risk is for malnutrition or sarcopenia.”
The current study included 991 persons who entered the NHANES database from 2003 to 2018. The patients self-identified as having HF, reported taking medications commonly prescribed in HF, and provided at least two “reliable” dietary recalls.
The average age of the patients was 68 years, and they had had HF for a mean of 9.5 years; 47% were women, three-fourths were White persons, two thirds had dyslipidemia, and a quarter had a history of cancer.
On average, their first eating occasion of the day was at about 8:30 a.m., and the last occasion was at about 7:30 p.m., for a time window of about 11 hours; daily calorie consumption averaged about 1,830 kcal.
About 52% died over the mean follow-up of 69 months; about 44% of deaths were from CV causes.
In a model adjusted for demographics, BMI, smoking status, times of eating occasions, CV disease, diabetes, and cancer history, the all-cause mortality hazard ratio for time windows ≥ 11 hours vs. < 11 hours was 0.236 (95% confidence interval, 0.07-0.715; P = .011).
The reduction was no longer significant on further adjustment for duration of HF, a score reflecting difficulty walking, nightly hours of sleep (which averaged 7.2 hours), daily number of eating occasions, and caloric intake, Ms. Billingsley reported.
But in the fully adjusted analysis, the HR for CV mortality for the longer vs. shorter time window was 0.368 (95% CI, 0.169-0.803; P = .013).
The issue deserves further exploration in a randomized trial, Ms. Billingsley proposed, perhaps one in which patients with HF wear accelerometers to track daily activity levels. “We’d love to do a pilot study of extending their eating window that really digs into what the mechanism of any benefit might be if we assign them to a longer time window and whether it’s related to physical activity.”
Ms. Billingsley reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
AT HFSA 2023
Semaglutide win in HFpEF with obesity regardless of ejection fraction: STEP-HFpEF
CLEVELAND – independently of baseline left-ventricular ejection fraction (LVEF).
The finding comes from a prespecified secondary analysis of the STEP-HFpEF trial of more than 500 nondiabetic patients with obesity and HF with an initial LVEF of 45% or greater.
They suggest that for patients with the obesity phenotype of HFpEF, semaglutide (Wegovy) could potentially join SGLT2 inhibitors on the short list of meds with consistent treatment effects whether LVEF is mildly reduced, preserved, or in the normal range.
That would distinguish the drug, a glucagon-like peptide-1 (GLP-1) receptor agonist, from mineralocorticoid receptor antagonists (MRA), sacubitril-valsartan (Entresto), and other renin-angiotensin-system inhibitors (RASi), whose benefits tend to taper off with rising LVEF.
The patients assigned to semaglutide showed significant improvement in both primary endpoints – change in Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (KCCQ-CSS) and change in body weight at 52 weeks – whether their baseline LVEF was 45%-49%, 50%-59%, or 60% or greater.
Results were similar for improvements in 6-minute walk distance (6MWD) and levels of NT-terminal pro–brain natriuretic peptide (NT-proBNP) and C-reactive protein, observed Javed Butler, MD, when presenting the analysis at the annual meeting of the Heart Failure Society of America, Cleveland.
Dr. Butler, of Baylor Scott and White Research Institute, Dallas, and the University of Mississippi, Jackson, is also lead author of the study, which was published on the same day in the Journal of the American College of Cardiology.
In his presentation, Dr. Butler singled out the NT-proBNP finding as “very meaningful” with respect to understanding potential mechanisms of the drug effects observed in the trial.
For example, people with obesity tend to have lower than average natriuretic peptide levels that “actually go up a bit” when they lose weight, he observed. But in the trial, “we saw a reduction in NT-proBNP in spite of the weight loss,” regardless of LVEF category.
John McMurray, MD, University of Glasgow, the invited discussant for Dr. Butler’s presentation, agreed that it raises the question whether weight loss was the sole semaglutide effect responsible for the improvement in heart failure status and biomarkers. The accompanying NT-proBNP reductions – when the opposite might otherwise have been expected – may point to a possible mechanism of action that is “something more than just weight loss,” he said. “If that were the case, it becomes very important, because it means that this treatment might do good things in non-obese patients or might do good things in patients with other types of heart failure.”
‘Vital reassurance’
More definitive trials are needed “to clarify safety and efficacy of obesity-targeted therapeutics in HF across the ejection fraction spectrum,” according to an accompanying editorial).
Still, the STEP-HFpEF analysis “strengthens the role of GLP-1 [receptor agonists] to ameliorate health status” for patients with obesity and HF with mildly reduced or preserved ejection fraction, write Muthiah Vaduganathan, MD, MPH, and John W. Ostrominski, MD, Brigham and Women’s Hospital and Harvard Medical School, both in Boston.
Its findings “provide vital reassurance” on semaglutide safety and efficacy in HF with below-normal LVEF and “tentatively support the existence of a more general, LVEF-independent, obesity-related HF phenotype capable of favorable modification with incretin-based therapies.”
The lack of heterogeneity in treatment effects across LVEF subgroups “is not surprising,” but “the findings reinforce that the benefits of this therapy in those meeting trial criteria do not vary by left ventricular ejection fraction,” Gregg C. Fonarow, MD, University of California, Los Angeles, Medical Center, said in an interview.
It remains unknown, however, “whether the improvement in health status, functional status, and reduced inflammation” will translate to reduced risk of cardiovascular death or HF hospitalization, said Dr. Fonarow, who isn’t connected to STEP-HFpEF.
It’s a question for future studies, he agreed, whether semaglutide would confer similar benefits for patients with obesity and HF with LVEF less than 45% or in non-obese HF patients.
Dr. McMurray proposed that future GLP-1 receptor agonist heart-failure trials should include non-obese patients to determine whether the effects seen in STEP-HFpEF were due to something more than weight loss. Trials in patients with obesity and HF with reduced LVEF would also be important.
“If it turns out just to be about weight loss, then we need to think about the alternatives,” including diet, exercise, and bariatric surgery but also, potentially, weight-loss drugs other than semaglutide, he said.
No heterogeneity by LVEF
STEP-HFpEF randomly assigned 529 patients free of diabetes with an LVEF greater than or equal to 45%, a body mass index (BMI) of at least 30 kg/m2, and NYHA functional status of 2-4 to either a placebo injection or 2.4-mg semaglutide subcutaneously once a week (the dose used for weight reduction) atop standard care.
As previously reported, those assigned to semaglutide showed significant improvements at 1 year in symptoms and in physical limitation, per changes in KCCQ-CSS, and weight loss, compared with the control group. Their exercise capacity, as measured by 6MWD, also improved.
The more weight patients lost while taking semaglutide, the better their KCCQ-CSS and 6MWD outcomes, a prior secondary analysis suggested. But the STEP-HFpEF researchers said weight loss did not appear to explain all of their gains, compared with usual care.
For the current analysis, the 263 patients assigned to receive semaglutide and 266 control patients were divided into three groups by baseline LVEF and compared for the same outcomes.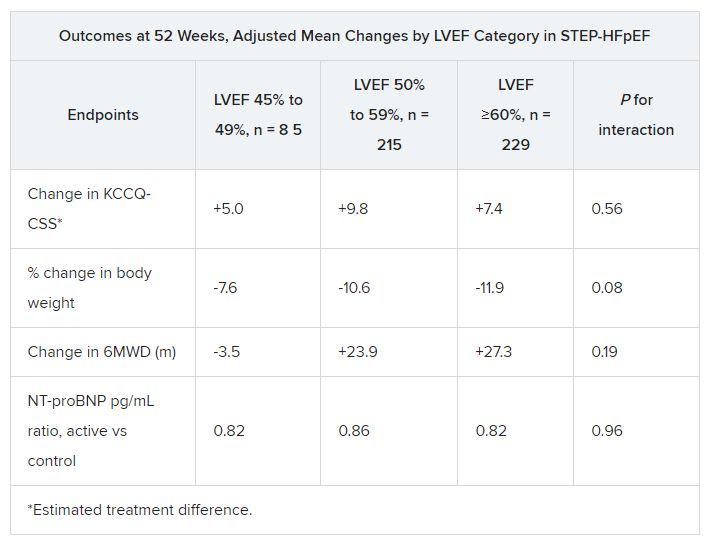
The semaglutide group, compared with control patients, also showed a significantly increased hierarchical composite win ratio, 1.72 (95% CI, 1.37-2.15; P < .001), that was consistent across LVEF categories and that accounted for all-cause mortality, HF events, KCCQ-CSS and 6MWD changes, and change in CRP.
Limitations make it hard to generalize the results, the authors caution. Well over 90% of the participants were White patients, for example, and the overall trial was not powered to show subgroup differences.
Given the many patients with HFpEF who have a cardiometabolic phenotype and are with overweight or obesity, write Dr. Butler and colleagues, their treatment approach “may ultimately include combination therapy with SGLT2 inhibitors and GLP-1 receptor agonists, given their non-overlapping and complementary mechanisms of action.”
Dr. Fonarow noted that both MRAs and sacubitril-valsartan offer clinical benefits for patients with HF and LVEF “in the 41%-60% range” that are evident “across BMI categories.”
So it’s likely, he said, that those medications as well as SGLT2 inhibitors will be used along with GLP-1 receptor agonists for patients with HFpEF and obesity.
STEP-HFpEF was funded by Novo Nordisk. Dr. Butler and the other authors disclose consulting for many companies, a list of which can be found in the report. Dr. Fonarow reports consulting for multiple companies. Dr. McMurray discloses consulting for AstraZeneca. Dr. Ostrominski reports no relevant disclosures. Dr. Vaduganathan discloses receiving grant support, serving on advisory boards, or speaking for multiple companies and serving on committees for studies sponsored by AstraZeneca, Galmed, Novartis, Bayer AG, Occlutech, and Impulse Dynamics.
A version of this article appeared on Medscape.com.
CLEVELAND – independently of baseline left-ventricular ejection fraction (LVEF).
The finding comes from a prespecified secondary analysis of the STEP-HFpEF trial of more than 500 nondiabetic patients with obesity and HF with an initial LVEF of 45% or greater.
They suggest that for patients with the obesity phenotype of HFpEF, semaglutide (Wegovy) could potentially join SGLT2 inhibitors on the short list of meds with consistent treatment effects whether LVEF is mildly reduced, preserved, or in the normal range.
That would distinguish the drug, a glucagon-like peptide-1 (GLP-1) receptor agonist, from mineralocorticoid receptor antagonists (MRA), sacubitril-valsartan (Entresto), and other renin-angiotensin-system inhibitors (RASi), whose benefits tend to taper off with rising LVEF.
The patients assigned to semaglutide showed significant improvement in both primary endpoints – change in Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (KCCQ-CSS) and change in body weight at 52 weeks – whether their baseline LVEF was 45%-49%, 50%-59%, or 60% or greater.
Results were similar for improvements in 6-minute walk distance (6MWD) and levels of NT-terminal pro–brain natriuretic peptide (NT-proBNP) and C-reactive protein, observed Javed Butler, MD, when presenting the analysis at the annual meeting of the Heart Failure Society of America, Cleveland.
Dr. Butler, of Baylor Scott and White Research Institute, Dallas, and the University of Mississippi, Jackson, is also lead author of the study, which was published on the same day in the Journal of the American College of Cardiology.
In his presentation, Dr. Butler singled out the NT-proBNP finding as “very meaningful” with respect to understanding potential mechanisms of the drug effects observed in the trial.
For example, people with obesity tend to have lower than average natriuretic peptide levels that “actually go up a bit” when they lose weight, he observed. But in the trial, “we saw a reduction in NT-proBNP in spite of the weight loss,” regardless of LVEF category.
John McMurray, MD, University of Glasgow, the invited discussant for Dr. Butler’s presentation, agreed that it raises the question whether weight loss was the sole semaglutide effect responsible for the improvement in heart failure status and biomarkers. The accompanying NT-proBNP reductions – when the opposite might otherwise have been expected – may point to a possible mechanism of action that is “something more than just weight loss,” he said. “If that were the case, it becomes very important, because it means that this treatment might do good things in non-obese patients or might do good things in patients with other types of heart failure.”
‘Vital reassurance’
More definitive trials are needed “to clarify safety and efficacy of obesity-targeted therapeutics in HF across the ejection fraction spectrum,” according to an accompanying editorial).
Still, the STEP-HFpEF analysis “strengthens the role of GLP-1 [receptor agonists] to ameliorate health status” for patients with obesity and HF with mildly reduced or preserved ejection fraction, write Muthiah Vaduganathan, MD, MPH, and John W. Ostrominski, MD, Brigham and Women’s Hospital and Harvard Medical School, both in Boston.
Its findings “provide vital reassurance” on semaglutide safety and efficacy in HF with below-normal LVEF and “tentatively support the existence of a more general, LVEF-independent, obesity-related HF phenotype capable of favorable modification with incretin-based therapies.”
The lack of heterogeneity in treatment effects across LVEF subgroups “is not surprising,” but “the findings reinforce that the benefits of this therapy in those meeting trial criteria do not vary by left ventricular ejection fraction,” Gregg C. Fonarow, MD, University of California, Los Angeles, Medical Center, said in an interview.
It remains unknown, however, “whether the improvement in health status, functional status, and reduced inflammation” will translate to reduced risk of cardiovascular death or HF hospitalization, said Dr. Fonarow, who isn’t connected to STEP-HFpEF.
It’s a question for future studies, he agreed, whether semaglutide would confer similar benefits for patients with obesity and HF with LVEF less than 45% or in non-obese HF patients.
Dr. McMurray proposed that future GLP-1 receptor agonist heart-failure trials should include non-obese patients to determine whether the effects seen in STEP-HFpEF were due to something more than weight loss. Trials in patients with obesity and HF with reduced LVEF would also be important.
“If it turns out just to be about weight loss, then we need to think about the alternatives,” including diet, exercise, and bariatric surgery but also, potentially, weight-loss drugs other than semaglutide, he said.
No heterogeneity by LVEF
STEP-HFpEF randomly assigned 529 patients free of diabetes with an LVEF greater than or equal to 45%, a body mass index (BMI) of at least 30 kg/m2, and NYHA functional status of 2-4 to either a placebo injection or 2.4-mg semaglutide subcutaneously once a week (the dose used for weight reduction) atop standard care.
As previously reported, those assigned to semaglutide showed significant improvements at 1 year in symptoms and in physical limitation, per changes in KCCQ-CSS, and weight loss, compared with the control group. Their exercise capacity, as measured by 6MWD, also improved.
The more weight patients lost while taking semaglutide, the better their KCCQ-CSS and 6MWD outcomes, a prior secondary analysis suggested. But the STEP-HFpEF researchers said weight loss did not appear to explain all of their gains, compared with usual care.
For the current analysis, the 263 patients assigned to receive semaglutide and 266 control patients were divided into three groups by baseline LVEF and compared for the same outcomes.
The semaglutide group, compared with control patients, also showed a significantly increased hierarchical composite win ratio, 1.72 (95% CI, 1.37-2.15; P < .001), that was consistent across LVEF categories and that accounted for all-cause mortality, HF events, KCCQ-CSS and 6MWD changes, and change in CRP.
Limitations make it hard to generalize the results, the authors caution. Well over 90% of the participants were White patients, for example, and the overall trial was not powered to show subgroup differences.
Given the many patients with HFpEF who have a cardiometabolic phenotype and are with overweight or obesity, write Dr. Butler and colleagues, their treatment approach “may ultimately include combination therapy with SGLT2 inhibitors and GLP-1 receptor agonists, given their non-overlapping and complementary mechanisms of action.”
Dr. Fonarow noted that both MRAs and sacubitril-valsartan offer clinical benefits for patients with HF and LVEF “in the 41%-60% range” that are evident “across BMI categories.”
So it’s likely, he said, that those medications as well as SGLT2 inhibitors will be used along with GLP-1 receptor agonists for patients with HFpEF and obesity.
STEP-HFpEF was funded by Novo Nordisk. Dr. Butler and the other authors disclose consulting for many companies, a list of which can be found in the report. Dr. Fonarow reports consulting for multiple companies. Dr. McMurray discloses consulting for AstraZeneca. Dr. Ostrominski reports no relevant disclosures. Dr. Vaduganathan discloses receiving grant support, serving on advisory boards, or speaking for multiple companies and serving on committees for studies sponsored by AstraZeneca, Galmed, Novartis, Bayer AG, Occlutech, and Impulse Dynamics.
A version of this article appeared on Medscape.com.
CLEVELAND – independently of baseline left-ventricular ejection fraction (LVEF).
The finding comes from a prespecified secondary analysis of the STEP-HFpEF trial of more than 500 nondiabetic patients with obesity and HF with an initial LVEF of 45% or greater.
They suggest that for patients with the obesity phenotype of HFpEF, semaglutide (Wegovy) could potentially join SGLT2 inhibitors on the short list of meds with consistent treatment effects whether LVEF is mildly reduced, preserved, or in the normal range.
That would distinguish the drug, a glucagon-like peptide-1 (GLP-1) receptor agonist, from mineralocorticoid receptor antagonists (MRA), sacubitril-valsartan (Entresto), and other renin-angiotensin-system inhibitors (RASi), whose benefits tend to taper off with rising LVEF.
The patients assigned to semaglutide showed significant improvement in both primary endpoints – change in Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (KCCQ-CSS) and change in body weight at 52 weeks – whether their baseline LVEF was 45%-49%, 50%-59%, or 60% or greater.
Results were similar for improvements in 6-minute walk distance (6MWD) and levels of NT-terminal pro–brain natriuretic peptide (NT-proBNP) and C-reactive protein, observed Javed Butler, MD, when presenting the analysis at the annual meeting of the Heart Failure Society of America, Cleveland.
Dr. Butler, of Baylor Scott and White Research Institute, Dallas, and the University of Mississippi, Jackson, is also lead author of the study, which was published on the same day in the Journal of the American College of Cardiology.
In his presentation, Dr. Butler singled out the NT-proBNP finding as “very meaningful” with respect to understanding potential mechanisms of the drug effects observed in the trial.
For example, people with obesity tend to have lower than average natriuretic peptide levels that “actually go up a bit” when they lose weight, he observed. But in the trial, “we saw a reduction in NT-proBNP in spite of the weight loss,” regardless of LVEF category.
John McMurray, MD, University of Glasgow, the invited discussant for Dr. Butler’s presentation, agreed that it raises the question whether weight loss was the sole semaglutide effect responsible for the improvement in heart failure status and biomarkers. The accompanying NT-proBNP reductions – when the opposite might otherwise have been expected – may point to a possible mechanism of action that is “something more than just weight loss,” he said. “If that were the case, it becomes very important, because it means that this treatment might do good things in non-obese patients or might do good things in patients with other types of heart failure.”
‘Vital reassurance’
More definitive trials are needed “to clarify safety and efficacy of obesity-targeted therapeutics in HF across the ejection fraction spectrum,” according to an accompanying editorial).
Still, the STEP-HFpEF analysis “strengthens the role of GLP-1 [receptor agonists] to ameliorate health status” for patients with obesity and HF with mildly reduced or preserved ejection fraction, write Muthiah Vaduganathan, MD, MPH, and John W. Ostrominski, MD, Brigham and Women’s Hospital and Harvard Medical School, both in Boston.
Its findings “provide vital reassurance” on semaglutide safety and efficacy in HF with below-normal LVEF and “tentatively support the existence of a more general, LVEF-independent, obesity-related HF phenotype capable of favorable modification with incretin-based therapies.”
The lack of heterogeneity in treatment effects across LVEF subgroups “is not surprising,” but “the findings reinforce that the benefits of this therapy in those meeting trial criteria do not vary by left ventricular ejection fraction,” Gregg C. Fonarow, MD, University of California, Los Angeles, Medical Center, said in an interview.
It remains unknown, however, “whether the improvement in health status, functional status, and reduced inflammation” will translate to reduced risk of cardiovascular death or HF hospitalization, said Dr. Fonarow, who isn’t connected to STEP-HFpEF.
It’s a question for future studies, he agreed, whether semaglutide would confer similar benefits for patients with obesity and HF with LVEF less than 45% or in non-obese HF patients.
Dr. McMurray proposed that future GLP-1 receptor agonist heart-failure trials should include non-obese patients to determine whether the effects seen in STEP-HFpEF were due to something more than weight loss. Trials in patients with obesity and HF with reduced LVEF would also be important.
“If it turns out just to be about weight loss, then we need to think about the alternatives,” including diet, exercise, and bariatric surgery but also, potentially, weight-loss drugs other than semaglutide, he said.
No heterogeneity by LVEF
STEP-HFpEF randomly assigned 529 patients free of diabetes with an LVEF greater than or equal to 45%, a body mass index (BMI) of at least 30 kg/m2, and NYHA functional status of 2-4 to either a placebo injection or 2.4-mg semaglutide subcutaneously once a week (the dose used for weight reduction) atop standard care.
As previously reported, those assigned to semaglutide showed significant improvements at 1 year in symptoms and in physical limitation, per changes in KCCQ-CSS, and weight loss, compared with the control group. Their exercise capacity, as measured by 6MWD, also improved.
The more weight patients lost while taking semaglutide, the better their KCCQ-CSS and 6MWD outcomes, a prior secondary analysis suggested. But the STEP-HFpEF researchers said weight loss did not appear to explain all of their gains, compared with usual care.
For the current analysis, the 263 patients assigned to receive semaglutide and 266 control patients were divided into three groups by baseline LVEF and compared for the same outcomes.
The semaglutide group, compared with control patients, also showed a significantly increased hierarchical composite win ratio, 1.72 (95% CI, 1.37-2.15; P < .001), that was consistent across LVEF categories and that accounted for all-cause mortality, HF events, KCCQ-CSS and 6MWD changes, and change in CRP.
Limitations make it hard to generalize the results, the authors caution. Well over 90% of the participants were White patients, for example, and the overall trial was not powered to show subgroup differences.
Given the many patients with HFpEF who have a cardiometabolic phenotype and are with overweight or obesity, write Dr. Butler and colleagues, their treatment approach “may ultimately include combination therapy with SGLT2 inhibitors and GLP-1 receptor agonists, given their non-overlapping and complementary mechanisms of action.”
Dr. Fonarow noted that both MRAs and sacubitril-valsartan offer clinical benefits for patients with HF and LVEF “in the 41%-60% range” that are evident “across BMI categories.”
So it’s likely, he said, that those medications as well as SGLT2 inhibitors will be used along with GLP-1 receptor agonists for patients with HFpEF and obesity.
STEP-HFpEF was funded by Novo Nordisk. Dr. Butler and the other authors disclose consulting for many companies, a list of which can be found in the report. Dr. Fonarow reports consulting for multiple companies. Dr. McMurray discloses consulting for AstraZeneca. Dr. Ostrominski reports no relevant disclosures. Dr. Vaduganathan discloses receiving grant support, serving on advisory boards, or speaking for multiple companies and serving on committees for studies sponsored by AstraZeneca, Galmed, Novartis, Bayer AG, Occlutech, and Impulse Dynamics.
A version of this article appeared on Medscape.com.
AT HFSA 2023
The how and why of quad therapy in reduced-EF heart failure
It’s as if hospitals, clinicians, and the health care system itself were unprepared for such success as a powerful multiple-drug regimen emerged for hospitalized patients with heart failure with reduced ejection fraction (HFrEF).
Uptake in practice has been sluggish for the management strategy driven by a quartet of medications, each with its own mechanisms of action, started in the hospital simultaneously or in rapid succession over a few days. Key to the regimen, dosages are at least partly uptitrated in the hospital then optimized during close postdischarge follow-up.
The so-called four pillars of medical therapy for HFrEF, defined by a left ventricular ejection fraction (LVEF) of 40% or lower, include an SGLT2 inhibitor, a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and a renin-angiotensin-system (RAS) inhibitor – preferably sacubitril-valsartan (Entresto) or, as a backup, an ACE inhibitor or angiotensin receptor blocker (ARB).
Academic consensus on the strategy is strong. The approach is consistent with heart failure (HF) guidelines on both sides of the Atlantic and is backed by solid trial evidence suggesting striking improvements in survival, readmission risk, and quality of life.
Gregg C. Fonarow, MD, University of California, Los Angeles, said in an interview.
“Yet, when we look at their actual implementation in clinical practice, we’ve seen this slow and variable uptake.”
So, why is that?
The STRONG-HF trial tested a version of the multiple-drug strategy and demonstrated what it could achieve even without a contribution from SGLT2 inhibitors, which weren’t yet indicated for HF. Eligibility for the trial, with more than 1,000 patients, wasn’t dependent on their LVEF.
Patients assigned to early and rapidly sequential initiation of a beta-blocker, an MRA, and a RAS inhibitor, compared with a standard-care control group, benefited with a 34% drop (P = .002) in risk for death or HF readmission over the next 6 months.
Few doubt – and the bulk of evidence suggests – that adding an SGLT2 inhibitor to round out the four-pillar strategy would safely boost its clinical potential in HFrEF.
The strategy’s smooth adoption in practice likely has multiple confounders that include clinical inertia, perceptions of HF medical management as a long-term outpatient process, and the onerous and Kafkaesque systems of care and reimbursement in the United States.
For example, the drug initiation and uptitration process may seem too complex for integration into slow-to-change hospital practices. And there could be a misguided sense that the regimen and follow-up must abide by the same exacting detail and standards set forth in, for example, the STRONG-HF protocol.
But starting hospitalized patients with HFrEF on the quartet of drugs and optimizing their dosages in hospital and after discharge can be simpler and more straightforward than that, Dr. Fonarow and other experts explain.
The academic community’s buy-in is a first step, but broader acceptance is frustrated by an “overwhelming culture of clinical care for heart failure” that encourages a more drawn-out process for adding medications, said Stephen J. Greene, MD, Duke Clinical Research Institute, Durham, N.C. “We need to turn our thinking on its head about heart failure in clinical practice.”
The “dramatic” underuse of the four pillars in the hospital stems in part from “outmoded” treatment algorithms that clinicians are following, Dr. Fonarow said. And they have “no sense of urgency,” sometimes wrongly believing “that it takes months for these medications to ultimately kick in.”
For hospitalized patients with HFrEF, “there is an imperative to overcome these timid algorithms and timid thinking,” he said. They should be on “full quadruple therapy” before discharge.
“And for newly diagnosed outpatients, you should essentially give yourself 7 days to get these drugs on board,” he added, either simultaneously or in “very rapid sequence.”
What’s needed is a “cultural shift” in medicine that “elevates heart failure to the same level of urgency that we have in the care of some other disease states,” agreed Muthiah Vaduganathan, MD, MPH, Brigham and Women’s Hospital and Harvard Medical School, Boston.
Hospital as opportunity
The patient’s 4-7 days in the hospital typically represent a “wonderful opportunity” to initiate all four drug classes in rapid succession and start uptitrations. But most hospitals and other health care settings, Dr. Vaduganathan observed, lack the structure and systems to support the process. Broad application will require “buy-in from multiple parties – from the clinician, from the patient, their caregivers, and their partners as well as the health system.”
Physician awareness and support for the strategy, suggests at least one of these experts, is probably much less of a challenge to its broad adoption than the bewildering mechanics of health care delivery and reimbursement.
“The problem is not education. The problem is the way that our health care system is structured,” said Milton Packer, MD, Baylor Heart and Vascular Institute, Dallas.
For example, sacubitril-valsartan and the SGLT2 inhibitors are still under patent and are far more expensive than longtime generic beta-blockers and MRAs. That means physicians typically spend valuable time pursuing prior authorizations for the brand-name drugs under pressure to eventually discharge the patient because of limits on hospital reimbursement.
Clinicians in the hospital are “almost disincentivized by the system” to implement management plans that call for early and rapid initiation of multiple drugs, Dr. Vaduganathan pointed out.
One change per day
There’s no one formula for carrying out the quadruple drug strategy, Dr. Vaduganathan noted. “I make only a single change per day” to the regimen, such as uptitration or addition of a single agent. That way, tolerability can be evaluated one drug at a time, “and then the following day, I can make the next therapeutic change.”
The order in which the drugs are started mostly does not matter, in contrast to a traditional approach that might have added new drugs in the sequence of their approval for HFrEF or adoption in guidelines. Under that scenario, each successive agent might be fully uptitrated before the next could be brought on board.
Historically, Dr. Packer observed, “you would start with an ACE inhibitor, add a beta-blocker, add an MRA, switch to sacubitril-valsartan, add an SGLT2 inhibitor – and it would take 8 months.” Any prescribed sequence is pointless given the short time frame that is ideal for initiating all the drugs, he said.
Hypothetically, however, there is some rationale for starting them in an order that leverages their unique actions and side effects. For example, Dr. Vaduganathan and others observed, it may be helpful to start an SGLT2 inhibitor and sacubitril-valsartan early in the process, because they can mitigate any hyperkalemia from the subsequent addition of an MRA.
That being said, “I don’t think we have firm evidence that any particular order is more efficacious than another,” Dr. Vaduganathan said. “It’s really about getting patients on all four drugs as quickly as possible, regardless of the sequence.”
Discussions about sequencing the drugs are “a distraction for our field,” Dr. Greene said. In trials, clinical benefit from the multiple-drug regimen has emerged almost right away once the drugs were on board. “The data clearly show that initiating all four, at least at low doses, gives the best bang for your buck and would be a high-yield strategy.”
Best evidence suggests that once all four agents have been started, attention can turn to uptitration, “with the beta-blocker as the higher priority,” Dr. Greene said. “The bottom line is to keep it simple: four drugs, simultaneously or within 1 week, and prioritize initiation at low doses to maximize tolerability.”
The four-drug approach yields survival and rehospitalization benefits even when uptitrations don’t reach prespecified goals, Dr. Fonarow observed. The SGLT2 inhibitors are started and maintained at the same dosage. But for the other three agents, uptitration should aim for the highest well-tolerated level, up to the target, even if the highest tolerated is the initial dosage.
‘Challenging to generalize’
The goal in STRONG-HF was to start and at least partly uptitrate a beta-blocker, an MRA, and sacubitril-valsartan in the hospital and fully optimize their dosages within 2 weeks after discharge. Symptoms and laboratory biomarkers, including natriuretic peptides, were closely monitored at four in-person evaluations during the first 6 outpatient weeks.
But few believe the trial’s intensive drug regimen and postdischarge follow-up, as stipulated in the protocol, would be tolerated by current systems of care and reimbursement.
STRONG-HF “affirms the strategy in a rigorous, well conducted way,” Dr. Vaduganathan said, but would be “challenging to generalize to all health care systems.”
As a result, some in the field are “quick to almost disregard STRONG-HF in its entirety” and consider it “wishful thinking,” Dr. Greene said. Better that providers not become distracted by the precise details of its protocol.
At Duke, he said, “we see all our patients within 1 week of discharge to ensure they’re doing okay in terms of volume status and look for opportunities to escalate their guideline-directed medical therapy.”
But that can be done without in-person visits. A lot of the follow-up and uptitrations, Dr. Greene said, can be achieved by telephone or at virtual appointments in conjunction with regular laboratory testing. “That, I think, really is the path for the future, in this age when clinics are overwhelmed by in-person visits.”
Mildly reduced and preserved EF
STRONG-HF, in which patients were enrolled without regard to ejection fraction, suggests that its rapidly sequential drug regimen and intensive management protocol improves outcomes for patients with HF at any level of LVEF.
Those findings and others, along with DELIVER, EMPEROR-Preserved and other studies, make a tantalizing case for the quadruple drug approach in patients with HF and LVEF >40% – that is, those with mildly reduced (LVEF > 40% to < 50%, HFmrEF) or preserved LVEF > 50%, HFpEF) ejection fraction.
But the case isn’t solid enough to declare the four agents as core therapy for HF and LVEF > 40%, observed Dr. Vaduganathan. Currently, SGLT2 inhibitors “are the only drug class that we are routinely implementing” in HFmrEF and HFpEF.
There have been suggestions of clinical benefit for such patients with sacubitril-valsartan and MRAs, especially in PARAGON-HF and TOPCAT, respectively. The evidence is stronger in HFmrEF than in HFpEF, but in either case it’s weaker than the clear-cut trial support for SGLT2 inhibitors in those HF categories.
Trials also suggest that in HF with LVEF > 40%, clinical benefits from RAS inhibitors and MRAs taper off with increasing ejection fraction, especially into the > 60% range.
In both HFmrEF and HFpEF, “I routinely try to get the patient on an SGLT2 inhibitor rapidly and then treat with some of the other agents on a more individual basis,” Dr. Vaduganathan said. An LVEF in the HFmrEF range, for example, would likely call for the addition of an MRA and sacubitril-valsartan.
Dr. Packer said he would likely recommend all four agents for patients with HF and LVEF up to 60%, which he considers a more appropriate definition of HFrEF. Their clinical benefits appear consistent across that LVEF range, he said, although they thin out somewhat at the higher end.
Evidence supporting the four pillars in HF with LV > 40% and < 60% is weakest for beta-blockers, Dr. Packer noted, so arguably those drugs could be left out of the mix for patients with ejection fractions in that range.
Dr. Fonarow reported ties with Abbott, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Eli Lilly, Johnson & Johnson, Medtronic, Merck, Novartis, and Pfizer. Dr. Greene disclosed ties with Amgen, AstraZeneca, Bayer, Boehringer Ingelheim/Lilly, Bristol-Myers Squibb, Corteria, CSL Vifor, Cytokinetics, Lexicon Merck, Novartis, Pfizer, PharmaIN, Roche Diagnostics, Sanofi, scPharmaceuticals, Tricog Health, and Urovant Pharmaceuticals. Dr. Vaduganathan disclosed ties with American Regent, Amgen, AstraZeneca, Bayer AG, Baxter Healthcare, Boehringer Ingelheim, Chiesi, Cytokinetics, Galmed, Impulse Dynamics, Lexicon Pharmaceuticals, Merck, Novartis, Novo Nordisk, Occlutech, Pharmacosmos, Relypsa, Roche Diagnostics, Sanofi, and Tricog Health. Dr. Packer disclosed relationships with 89bio, AbbVie, Actavis, Amarin, Amgen, AstraZeneca, Attralus, Boehringer Ingelheim, Caladrius, Casana, CSL Behring, Cytokinetics, Imara, Lilly, Medtronic, Moderna, Novartis, Pharmacosmos, Reata, Regeneron, Relypsa, and Salamandra.
A version of this article first appeared on Medscape.com.
It’s as if hospitals, clinicians, and the health care system itself were unprepared for such success as a powerful multiple-drug regimen emerged for hospitalized patients with heart failure with reduced ejection fraction (HFrEF).
Uptake in practice has been sluggish for the management strategy driven by a quartet of medications, each with its own mechanisms of action, started in the hospital simultaneously or in rapid succession over a few days. Key to the regimen, dosages are at least partly uptitrated in the hospital then optimized during close postdischarge follow-up.
The so-called four pillars of medical therapy for HFrEF, defined by a left ventricular ejection fraction (LVEF) of 40% or lower, include an SGLT2 inhibitor, a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and a renin-angiotensin-system (RAS) inhibitor – preferably sacubitril-valsartan (Entresto) or, as a backup, an ACE inhibitor or angiotensin receptor blocker (ARB).
Academic consensus on the strategy is strong. The approach is consistent with heart failure (HF) guidelines on both sides of the Atlantic and is backed by solid trial evidence suggesting striking improvements in survival, readmission risk, and quality of life.
Gregg C. Fonarow, MD, University of California, Los Angeles, said in an interview.
“Yet, when we look at their actual implementation in clinical practice, we’ve seen this slow and variable uptake.”
So, why is that?
The STRONG-HF trial tested a version of the multiple-drug strategy and demonstrated what it could achieve even without a contribution from SGLT2 inhibitors, which weren’t yet indicated for HF. Eligibility for the trial, with more than 1,000 patients, wasn’t dependent on their LVEF.
Patients assigned to early and rapidly sequential initiation of a beta-blocker, an MRA, and a RAS inhibitor, compared with a standard-care control group, benefited with a 34% drop (P = .002) in risk for death or HF readmission over the next 6 months.
Few doubt – and the bulk of evidence suggests – that adding an SGLT2 inhibitor to round out the four-pillar strategy would safely boost its clinical potential in HFrEF.
The strategy’s smooth adoption in practice likely has multiple confounders that include clinical inertia, perceptions of HF medical management as a long-term outpatient process, and the onerous and Kafkaesque systems of care and reimbursement in the United States.
For example, the drug initiation and uptitration process may seem too complex for integration into slow-to-change hospital practices. And there could be a misguided sense that the regimen and follow-up must abide by the same exacting detail and standards set forth in, for example, the STRONG-HF protocol.
But starting hospitalized patients with HFrEF on the quartet of drugs and optimizing their dosages in hospital and after discharge can be simpler and more straightforward than that, Dr. Fonarow and other experts explain.
The academic community’s buy-in is a first step, but broader acceptance is frustrated by an “overwhelming culture of clinical care for heart failure” that encourages a more drawn-out process for adding medications, said Stephen J. Greene, MD, Duke Clinical Research Institute, Durham, N.C. “We need to turn our thinking on its head about heart failure in clinical practice.”
The “dramatic” underuse of the four pillars in the hospital stems in part from “outmoded” treatment algorithms that clinicians are following, Dr. Fonarow said. And they have “no sense of urgency,” sometimes wrongly believing “that it takes months for these medications to ultimately kick in.”
For hospitalized patients with HFrEF, “there is an imperative to overcome these timid algorithms and timid thinking,” he said. They should be on “full quadruple therapy” before discharge.
“And for newly diagnosed outpatients, you should essentially give yourself 7 days to get these drugs on board,” he added, either simultaneously or in “very rapid sequence.”
What’s needed is a “cultural shift” in medicine that “elevates heart failure to the same level of urgency that we have in the care of some other disease states,” agreed Muthiah Vaduganathan, MD, MPH, Brigham and Women’s Hospital and Harvard Medical School, Boston.
Hospital as opportunity
The patient’s 4-7 days in the hospital typically represent a “wonderful opportunity” to initiate all four drug classes in rapid succession and start uptitrations. But most hospitals and other health care settings, Dr. Vaduganathan observed, lack the structure and systems to support the process. Broad application will require “buy-in from multiple parties – from the clinician, from the patient, their caregivers, and their partners as well as the health system.”
Physician awareness and support for the strategy, suggests at least one of these experts, is probably much less of a challenge to its broad adoption than the bewildering mechanics of health care delivery and reimbursement.
“The problem is not education. The problem is the way that our health care system is structured,” said Milton Packer, MD, Baylor Heart and Vascular Institute, Dallas.
For example, sacubitril-valsartan and the SGLT2 inhibitors are still under patent and are far more expensive than longtime generic beta-blockers and MRAs. That means physicians typically spend valuable time pursuing prior authorizations for the brand-name drugs under pressure to eventually discharge the patient because of limits on hospital reimbursement.
Clinicians in the hospital are “almost disincentivized by the system” to implement management plans that call for early and rapid initiation of multiple drugs, Dr. Vaduganathan pointed out.
One change per day
There’s no one formula for carrying out the quadruple drug strategy, Dr. Vaduganathan noted. “I make only a single change per day” to the regimen, such as uptitration or addition of a single agent. That way, tolerability can be evaluated one drug at a time, “and then the following day, I can make the next therapeutic change.”
The order in which the drugs are started mostly does not matter, in contrast to a traditional approach that might have added new drugs in the sequence of their approval for HFrEF or adoption in guidelines. Under that scenario, each successive agent might be fully uptitrated before the next could be brought on board.
Historically, Dr. Packer observed, “you would start with an ACE inhibitor, add a beta-blocker, add an MRA, switch to sacubitril-valsartan, add an SGLT2 inhibitor – and it would take 8 months.” Any prescribed sequence is pointless given the short time frame that is ideal for initiating all the drugs, he said.
Hypothetically, however, there is some rationale for starting them in an order that leverages their unique actions and side effects. For example, Dr. Vaduganathan and others observed, it may be helpful to start an SGLT2 inhibitor and sacubitril-valsartan early in the process, because they can mitigate any hyperkalemia from the subsequent addition of an MRA.
That being said, “I don’t think we have firm evidence that any particular order is more efficacious than another,” Dr. Vaduganathan said. “It’s really about getting patients on all four drugs as quickly as possible, regardless of the sequence.”
Discussions about sequencing the drugs are “a distraction for our field,” Dr. Greene said. In trials, clinical benefit from the multiple-drug regimen has emerged almost right away once the drugs were on board. “The data clearly show that initiating all four, at least at low doses, gives the best bang for your buck and would be a high-yield strategy.”
Best evidence suggests that once all four agents have been started, attention can turn to uptitration, “with the beta-blocker as the higher priority,” Dr. Greene said. “The bottom line is to keep it simple: four drugs, simultaneously or within 1 week, and prioritize initiation at low doses to maximize tolerability.”
The four-drug approach yields survival and rehospitalization benefits even when uptitrations don’t reach prespecified goals, Dr. Fonarow observed. The SGLT2 inhibitors are started and maintained at the same dosage. But for the other three agents, uptitration should aim for the highest well-tolerated level, up to the target, even if the highest tolerated is the initial dosage.
‘Challenging to generalize’
The goal in STRONG-HF was to start and at least partly uptitrate a beta-blocker, an MRA, and sacubitril-valsartan in the hospital and fully optimize their dosages within 2 weeks after discharge. Symptoms and laboratory biomarkers, including natriuretic peptides, were closely monitored at four in-person evaluations during the first 6 outpatient weeks.
But few believe the trial’s intensive drug regimen and postdischarge follow-up, as stipulated in the protocol, would be tolerated by current systems of care and reimbursement.
STRONG-HF “affirms the strategy in a rigorous, well conducted way,” Dr. Vaduganathan said, but would be “challenging to generalize to all health care systems.”
As a result, some in the field are “quick to almost disregard STRONG-HF in its entirety” and consider it “wishful thinking,” Dr. Greene said. Better that providers not become distracted by the precise details of its protocol.
At Duke, he said, “we see all our patients within 1 week of discharge to ensure they’re doing okay in terms of volume status and look for opportunities to escalate their guideline-directed medical therapy.”
But that can be done without in-person visits. A lot of the follow-up and uptitrations, Dr. Greene said, can be achieved by telephone or at virtual appointments in conjunction with regular laboratory testing. “That, I think, really is the path for the future, in this age when clinics are overwhelmed by in-person visits.”
Mildly reduced and preserved EF
STRONG-HF, in which patients were enrolled without regard to ejection fraction, suggests that its rapidly sequential drug regimen and intensive management protocol improves outcomes for patients with HF at any level of LVEF.
Those findings and others, along with DELIVER, EMPEROR-Preserved and other studies, make a tantalizing case for the quadruple drug approach in patients with HF and LVEF >40% – that is, those with mildly reduced (LVEF > 40% to < 50%, HFmrEF) or preserved LVEF > 50%, HFpEF) ejection fraction.
But the case isn’t solid enough to declare the four agents as core therapy for HF and LVEF > 40%, observed Dr. Vaduganathan. Currently, SGLT2 inhibitors “are the only drug class that we are routinely implementing” in HFmrEF and HFpEF.
There have been suggestions of clinical benefit for such patients with sacubitril-valsartan and MRAs, especially in PARAGON-HF and TOPCAT, respectively. The evidence is stronger in HFmrEF than in HFpEF, but in either case it’s weaker than the clear-cut trial support for SGLT2 inhibitors in those HF categories.
Trials also suggest that in HF with LVEF > 40%, clinical benefits from RAS inhibitors and MRAs taper off with increasing ejection fraction, especially into the > 60% range.
In both HFmrEF and HFpEF, “I routinely try to get the patient on an SGLT2 inhibitor rapidly and then treat with some of the other agents on a more individual basis,” Dr. Vaduganathan said. An LVEF in the HFmrEF range, for example, would likely call for the addition of an MRA and sacubitril-valsartan.
Dr. Packer said he would likely recommend all four agents for patients with HF and LVEF up to 60%, which he considers a more appropriate definition of HFrEF. Their clinical benefits appear consistent across that LVEF range, he said, although they thin out somewhat at the higher end.
Evidence supporting the four pillars in HF with LV > 40% and < 60% is weakest for beta-blockers, Dr. Packer noted, so arguably those drugs could be left out of the mix for patients with ejection fractions in that range.
Dr. Fonarow reported ties with Abbott, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Eli Lilly, Johnson & Johnson, Medtronic, Merck, Novartis, and Pfizer. Dr. Greene disclosed ties with Amgen, AstraZeneca, Bayer, Boehringer Ingelheim/Lilly, Bristol-Myers Squibb, Corteria, CSL Vifor, Cytokinetics, Lexicon Merck, Novartis, Pfizer, PharmaIN, Roche Diagnostics, Sanofi, scPharmaceuticals, Tricog Health, and Urovant Pharmaceuticals. Dr. Vaduganathan disclosed ties with American Regent, Amgen, AstraZeneca, Bayer AG, Baxter Healthcare, Boehringer Ingelheim, Chiesi, Cytokinetics, Galmed, Impulse Dynamics, Lexicon Pharmaceuticals, Merck, Novartis, Novo Nordisk, Occlutech, Pharmacosmos, Relypsa, Roche Diagnostics, Sanofi, and Tricog Health. Dr. Packer disclosed relationships with 89bio, AbbVie, Actavis, Amarin, Amgen, AstraZeneca, Attralus, Boehringer Ingelheim, Caladrius, Casana, CSL Behring, Cytokinetics, Imara, Lilly, Medtronic, Moderna, Novartis, Pharmacosmos, Reata, Regeneron, Relypsa, and Salamandra.
A version of this article first appeared on Medscape.com.
It’s as if hospitals, clinicians, and the health care system itself were unprepared for such success as a powerful multiple-drug regimen emerged for hospitalized patients with heart failure with reduced ejection fraction (HFrEF).
Uptake in practice has been sluggish for the management strategy driven by a quartet of medications, each with its own mechanisms of action, started in the hospital simultaneously or in rapid succession over a few days. Key to the regimen, dosages are at least partly uptitrated in the hospital then optimized during close postdischarge follow-up.
The so-called four pillars of medical therapy for HFrEF, defined by a left ventricular ejection fraction (LVEF) of 40% or lower, include an SGLT2 inhibitor, a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and a renin-angiotensin-system (RAS) inhibitor – preferably sacubitril-valsartan (Entresto) or, as a backup, an ACE inhibitor or angiotensin receptor blocker (ARB).
Academic consensus on the strategy is strong. The approach is consistent with heart failure (HF) guidelines on both sides of the Atlantic and is backed by solid trial evidence suggesting striking improvements in survival, readmission risk, and quality of life.
Gregg C. Fonarow, MD, University of California, Los Angeles, said in an interview.
“Yet, when we look at their actual implementation in clinical practice, we’ve seen this slow and variable uptake.”
So, why is that?
The STRONG-HF trial tested a version of the multiple-drug strategy and demonstrated what it could achieve even without a contribution from SGLT2 inhibitors, which weren’t yet indicated for HF. Eligibility for the trial, with more than 1,000 patients, wasn’t dependent on their LVEF.
Patients assigned to early and rapidly sequential initiation of a beta-blocker, an MRA, and a RAS inhibitor, compared with a standard-care control group, benefited with a 34% drop (P = .002) in risk for death or HF readmission over the next 6 months.
Few doubt – and the bulk of evidence suggests – that adding an SGLT2 inhibitor to round out the four-pillar strategy would safely boost its clinical potential in HFrEF.
The strategy’s smooth adoption in practice likely has multiple confounders that include clinical inertia, perceptions of HF medical management as a long-term outpatient process, and the onerous and Kafkaesque systems of care and reimbursement in the United States.
For example, the drug initiation and uptitration process may seem too complex for integration into slow-to-change hospital practices. And there could be a misguided sense that the regimen and follow-up must abide by the same exacting detail and standards set forth in, for example, the STRONG-HF protocol.
But starting hospitalized patients with HFrEF on the quartet of drugs and optimizing their dosages in hospital and after discharge can be simpler and more straightforward than that, Dr. Fonarow and other experts explain.
The academic community’s buy-in is a first step, but broader acceptance is frustrated by an “overwhelming culture of clinical care for heart failure” that encourages a more drawn-out process for adding medications, said Stephen J. Greene, MD, Duke Clinical Research Institute, Durham, N.C. “We need to turn our thinking on its head about heart failure in clinical practice.”
The “dramatic” underuse of the four pillars in the hospital stems in part from “outmoded” treatment algorithms that clinicians are following, Dr. Fonarow said. And they have “no sense of urgency,” sometimes wrongly believing “that it takes months for these medications to ultimately kick in.”
For hospitalized patients with HFrEF, “there is an imperative to overcome these timid algorithms and timid thinking,” he said. They should be on “full quadruple therapy” before discharge.
“And for newly diagnosed outpatients, you should essentially give yourself 7 days to get these drugs on board,” he added, either simultaneously or in “very rapid sequence.”
What’s needed is a “cultural shift” in medicine that “elevates heart failure to the same level of urgency that we have in the care of some other disease states,” agreed Muthiah Vaduganathan, MD, MPH, Brigham and Women’s Hospital and Harvard Medical School, Boston.
Hospital as opportunity
The patient’s 4-7 days in the hospital typically represent a “wonderful opportunity” to initiate all four drug classes in rapid succession and start uptitrations. But most hospitals and other health care settings, Dr. Vaduganathan observed, lack the structure and systems to support the process. Broad application will require “buy-in from multiple parties – from the clinician, from the patient, their caregivers, and their partners as well as the health system.”
Physician awareness and support for the strategy, suggests at least one of these experts, is probably much less of a challenge to its broad adoption than the bewildering mechanics of health care delivery and reimbursement.
“The problem is not education. The problem is the way that our health care system is structured,” said Milton Packer, MD, Baylor Heart and Vascular Institute, Dallas.
For example, sacubitril-valsartan and the SGLT2 inhibitors are still under patent and are far more expensive than longtime generic beta-blockers and MRAs. That means physicians typically spend valuable time pursuing prior authorizations for the brand-name drugs under pressure to eventually discharge the patient because of limits on hospital reimbursement.
Clinicians in the hospital are “almost disincentivized by the system” to implement management plans that call for early and rapid initiation of multiple drugs, Dr. Vaduganathan pointed out.
One change per day
There’s no one formula for carrying out the quadruple drug strategy, Dr. Vaduganathan noted. “I make only a single change per day” to the regimen, such as uptitration or addition of a single agent. That way, tolerability can be evaluated one drug at a time, “and then the following day, I can make the next therapeutic change.”
The order in which the drugs are started mostly does not matter, in contrast to a traditional approach that might have added new drugs in the sequence of their approval for HFrEF or adoption in guidelines. Under that scenario, each successive agent might be fully uptitrated before the next could be brought on board.
Historically, Dr. Packer observed, “you would start with an ACE inhibitor, add a beta-blocker, add an MRA, switch to sacubitril-valsartan, add an SGLT2 inhibitor – and it would take 8 months.” Any prescribed sequence is pointless given the short time frame that is ideal for initiating all the drugs, he said.
Hypothetically, however, there is some rationale for starting them in an order that leverages their unique actions and side effects. For example, Dr. Vaduganathan and others observed, it may be helpful to start an SGLT2 inhibitor and sacubitril-valsartan early in the process, because they can mitigate any hyperkalemia from the subsequent addition of an MRA.
That being said, “I don’t think we have firm evidence that any particular order is more efficacious than another,” Dr. Vaduganathan said. “It’s really about getting patients on all four drugs as quickly as possible, regardless of the sequence.”
Discussions about sequencing the drugs are “a distraction for our field,” Dr. Greene said. In trials, clinical benefit from the multiple-drug regimen has emerged almost right away once the drugs were on board. “The data clearly show that initiating all four, at least at low doses, gives the best bang for your buck and would be a high-yield strategy.”
Best evidence suggests that once all four agents have been started, attention can turn to uptitration, “with the beta-blocker as the higher priority,” Dr. Greene said. “The bottom line is to keep it simple: four drugs, simultaneously or within 1 week, and prioritize initiation at low doses to maximize tolerability.”
The four-drug approach yields survival and rehospitalization benefits even when uptitrations don’t reach prespecified goals, Dr. Fonarow observed. The SGLT2 inhibitors are started and maintained at the same dosage. But for the other three agents, uptitration should aim for the highest well-tolerated level, up to the target, even if the highest tolerated is the initial dosage.
‘Challenging to generalize’
The goal in STRONG-HF was to start and at least partly uptitrate a beta-blocker, an MRA, and sacubitril-valsartan in the hospital and fully optimize their dosages within 2 weeks after discharge. Symptoms and laboratory biomarkers, including natriuretic peptides, were closely monitored at four in-person evaluations during the first 6 outpatient weeks.
But few believe the trial’s intensive drug regimen and postdischarge follow-up, as stipulated in the protocol, would be tolerated by current systems of care and reimbursement.
STRONG-HF “affirms the strategy in a rigorous, well conducted way,” Dr. Vaduganathan said, but would be “challenging to generalize to all health care systems.”
As a result, some in the field are “quick to almost disregard STRONG-HF in its entirety” and consider it “wishful thinking,” Dr. Greene said. Better that providers not become distracted by the precise details of its protocol.
At Duke, he said, “we see all our patients within 1 week of discharge to ensure they’re doing okay in terms of volume status and look for opportunities to escalate their guideline-directed medical therapy.”
But that can be done without in-person visits. A lot of the follow-up and uptitrations, Dr. Greene said, can be achieved by telephone or at virtual appointments in conjunction with regular laboratory testing. “That, I think, really is the path for the future, in this age when clinics are overwhelmed by in-person visits.”
Mildly reduced and preserved EF
STRONG-HF, in which patients were enrolled without regard to ejection fraction, suggests that its rapidly sequential drug regimen and intensive management protocol improves outcomes for patients with HF at any level of LVEF.
Those findings and others, along with DELIVER, EMPEROR-Preserved and other studies, make a tantalizing case for the quadruple drug approach in patients with HF and LVEF >40% – that is, those with mildly reduced (LVEF > 40% to < 50%, HFmrEF) or preserved LVEF > 50%, HFpEF) ejection fraction.
But the case isn’t solid enough to declare the four agents as core therapy for HF and LVEF > 40%, observed Dr. Vaduganathan. Currently, SGLT2 inhibitors “are the only drug class that we are routinely implementing” in HFmrEF and HFpEF.
There have been suggestions of clinical benefit for such patients with sacubitril-valsartan and MRAs, especially in PARAGON-HF and TOPCAT, respectively. The evidence is stronger in HFmrEF than in HFpEF, but in either case it’s weaker than the clear-cut trial support for SGLT2 inhibitors in those HF categories.
Trials also suggest that in HF with LVEF > 40%, clinical benefits from RAS inhibitors and MRAs taper off with increasing ejection fraction, especially into the > 60% range.
In both HFmrEF and HFpEF, “I routinely try to get the patient on an SGLT2 inhibitor rapidly and then treat with some of the other agents on a more individual basis,” Dr. Vaduganathan said. An LVEF in the HFmrEF range, for example, would likely call for the addition of an MRA and sacubitril-valsartan.
Dr. Packer said he would likely recommend all four agents for patients with HF and LVEF up to 60%, which he considers a more appropriate definition of HFrEF. Their clinical benefits appear consistent across that LVEF range, he said, although they thin out somewhat at the higher end.
Evidence supporting the four pillars in HF with LV > 40% and < 60% is weakest for beta-blockers, Dr. Packer noted, so arguably those drugs could be left out of the mix for patients with ejection fractions in that range.
Dr. Fonarow reported ties with Abbott, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Eli Lilly, Johnson & Johnson, Medtronic, Merck, Novartis, and Pfizer. Dr. Greene disclosed ties with Amgen, AstraZeneca, Bayer, Boehringer Ingelheim/Lilly, Bristol-Myers Squibb, Corteria, CSL Vifor, Cytokinetics, Lexicon Merck, Novartis, Pfizer, PharmaIN, Roche Diagnostics, Sanofi, scPharmaceuticals, Tricog Health, and Urovant Pharmaceuticals. Dr. Vaduganathan disclosed ties with American Regent, Amgen, AstraZeneca, Bayer AG, Baxter Healthcare, Boehringer Ingelheim, Chiesi, Cytokinetics, Galmed, Impulse Dynamics, Lexicon Pharmaceuticals, Merck, Novartis, Novo Nordisk, Occlutech, Pharmacosmos, Relypsa, Roche Diagnostics, Sanofi, and Tricog Health. Dr. Packer disclosed relationships with 89bio, AbbVie, Actavis, Amarin, Amgen, AstraZeneca, Attralus, Boehringer Ingelheim, Caladrius, Casana, CSL Behring, Cytokinetics, Imara, Lilly, Medtronic, Moderna, Novartis, Pharmacosmos, Reata, Regeneron, Relypsa, and Salamandra.
A version of this article first appeared on Medscape.com.
History of heart transplant tied to worse pregnancy outcome
TOPLINE:
than do other pregnant women, results of a large study with a nationwide sample suggest.
METHODOLOGY:
- The retrospective cohort study included 2010-2020 information from the Nationwide Readmissions Database (NRD), a large, all-payer administrative dataset that allows for tracking of patient hospital readmissions in the same U.S. state within the same calendar year and includes patient demographics, hospital characteristics, diagnosis and procedure codes (including for cardiac transplants), length of stay, and discharge disposition.
- The primary outcome was nontransfusion SMM which, among other conditions, included acute myocardial infarction, aortic aneurysm, acute renal failure, adult respiratory distress syndrome, amniotic fluid embolism, cardiac arrest/ventricular fibrillation, and heart failure/arrest, during the delivery hospitalization.
- Additional outcomes included rates of all SMMs (including transfusion), a composite cardiovascular SMM (cSMM) outcome that included acute myocardial infarction, aortic aneurysm, cardiac arrest/ventricular fibrillation, cardioversion, and acute heart failure, preterm birth, and readmission rates.
TAKEAWAY:
- From 2010 to 2020, there were 19,399,521 hospital deliveries, of which, 105 were in HT recipients.
- In unadjusted comparisons, rates of all outcomes were higher in HT, compared with non-HT delivery hospitalizations, and after adjusting for age, demographic and facility characteristics, comorbid conditions, and calendar year, HT recipients continued to have higher odds of adverse maternal outcomes. For example, HT recipients had higher rates of nontransfusion SMM (adjusted odds ratio, 28.12; 95% confidence interval, 15.65-50.53), all SMM (aOR, 15.73; 95% CI, 9.17-27.00), cSMM (aOR, 37.7; 95% CI, 17.39-82.01), and preterm birth (aOR, 7.15; 95%, CI 4.75-10.77).
- HT recipients also had longer hospital stays and higher rates of cesarean delivery, although the authors noted that it’s unclear whether this increase was caused by the HT or complications of pregnancy because data were unavailable regarding indication for cesareans.
- Patients with HT were also at increased risk for hospital readmission within the first year after delivery, particularly within the first 6 months, including for HT-related complications, a finding that supports guidelines recommending an initial postpartum visit within 7-14 days of discharge for patients with cardiac conditions, write the authors.
IN PRACTICE:
The findings demonstrate the importance of counseling HT patients at early gestational ages “to provide information about anticipated risks in pregnancy and the postpartum period to allow patients the opportunity to make informed choices regarding their reproductive options,” the authors conclude.
SOURCE:
The study was conducted by Amanda M. Craig, MD, division of maternal fetal medicine, department of obstetrics and gynecology, Duke University Medical Center, Durham, N.C., and colleagues. It was published online in JACC Heart Failure.
LIMITATIONS:
Relying on diagnosis and procedure codes in administrative datasets like NRD may result in underestimation of outcomes. In this study, outcomes were limited to delivery hospitalizations, which may underestimate the true incidence of complications or fail to include pregnancies that didn’t end in a delivery, including pregnancy terminations or spontaneous abortions. Information related to race, ethnicity, hospital regions, and cause of death are not captured in the NRD dataset.
DISCLOSURES:
The authors have no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
than do other pregnant women, results of a large study with a nationwide sample suggest.
METHODOLOGY:
- The retrospective cohort study included 2010-2020 information from the Nationwide Readmissions Database (NRD), a large, all-payer administrative dataset that allows for tracking of patient hospital readmissions in the same U.S. state within the same calendar year and includes patient demographics, hospital characteristics, diagnosis and procedure codes (including for cardiac transplants), length of stay, and discharge disposition.
- The primary outcome was nontransfusion SMM which, among other conditions, included acute myocardial infarction, aortic aneurysm, acute renal failure, adult respiratory distress syndrome, amniotic fluid embolism, cardiac arrest/ventricular fibrillation, and heart failure/arrest, during the delivery hospitalization.
- Additional outcomes included rates of all SMMs (including transfusion), a composite cardiovascular SMM (cSMM) outcome that included acute myocardial infarction, aortic aneurysm, cardiac arrest/ventricular fibrillation, cardioversion, and acute heart failure, preterm birth, and readmission rates.
TAKEAWAY:
- From 2010 to 2020, there were 19,399,521 hospital deliveries, of which, 105 were in HT recipients.
- In unadjusted comparisons, rates of all outcomes were higher in HT, compared with non-HT delivery hospitalizations, and after adjusting for age, demographic and facility characteristics, comorbid conditions, and calendar year, HT recipients continued to have higher odds of adverse maternal outcomes. For example, HT recipients had higher rates of nontransfusion SMM (adjusted odds ratio, 28.12; 95% confidence interval, 15.65-50.53), all SMM (aOR, 15.73; 95% CI, 9.17-27.00), cSMM (aOR, 37.7; 95% CI, 17.39-82.01), and preterm birth (aOR, 7.15; 95%, CI 4.75-10.77).
- HT recipients also had longer hospital stays and higher rates of cesarean delivery, although the authors noted that it’s unclear whether this increase was caused by the HT or complications of pregnancy because data were unavailable regarding indication for cesareans.
- Patients with HT were also at increased risk for hospital readmission within the first year after delivery, particularly within the first 6 months, including for HT-related complications, a finding that supports guidelines recommending an initial postpartum visit within 7-14 days of discharge for patients with cardiac conditions, write the authors.
IN PRACTICE:
The findings demonstrate the importance of counseling HT patients at early gestational ages “to provide information about anticipated risks in pregnancy and the postpartum period to allow patients the opportunity to make informed choices regarding their reproductive options,” the authors conclude.
SOURCE:
The study was conducted by Amanda M. Craig, MD, division of maternal fetal medicine, department of obstetrics and gynecology, Duke University Medical Center, Durham, N.C., and colleagues. It was published online in JACC Heart Failure.
LIMITATIONS:
Relying on diagnosis and procedure codes in administrative datasets like NRD may result in underestimation of outcomes. In this study, outcomes were limited to delivery hospitalizations, which may underestimate the true incidence of complications or fail to include pregnancies that didn’t end in a delivery, including pregnancy terminations or spontaneous abortions. Information related to race, ethnicity, hospital regions, and cause of death are not captured in the NRD dataset.
DISCLOSURES:
The authors have no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
than do other pregnant women, results of a large study with a nationwide sample suggest.
METHODOLOGY:
- The retrospective cohort study included 2010-2020 information from the Nationwide Readmissions Database (NRD), a large, all-payer administrative dataset that allows for tracking of patient hospital readmissions in the same U.S. state within the same calendar year and includes patient demographics, hospital characteristics, diagnosis and procedure codes (including for cardiac transplants), length of stay, and discharge disposition.
- The primary outcome was nontransfusion SMM which, among other conditions, included acute myocardial infarction, aortic aneurysm, acute renal failure, adult respiratory distress syndrome, amniotic fluid embolism, cardiac arrest/ventricular fibrillation, and heart failure/arrest, during the delivery hospitalization.
- Additional outcomes included rates of all SMMs (including transfusion), a composite cardiovascular SMM (cSMM) outcome that included acute myocardial infarction, aortic aneurysm, cardiac arrest/ventricular fibrillation, cardioversion, and acute heart failure, preterm birth, and readmission rates.
TAKEAWAY:
- From 2010 to 2020, there were 19,399,521 hospital deliveries, of which, 105 were in HT recipients.
- In unadjusted comparisons, rates of all outcomes were higher in HT, compared with non-HT delivery hospitalizations, and after adjusting for age, demographic and facility characteristics, comorbid conditions, and calendar year, HT recipients continued to have higher odds of adverse maternal outcomes. For example, HT recipients had higher rates of nontransfusion SMM (adjusted odds ratio, 28.12; 95% confidence interval, 15.65-50.53), all SMM (aOR, 15.73; 95% CI, 9.17-27.00), cSMM (aOR, 37.7; 95% CI, 17.39-82.01), and preterm birth (aOR, 7.15; 95%, CI 4.75-10.77).
- HT recipients also had longer hospital stays and higher rates of cesarean delivery, although the authors noted that it’s unclear whether this increase was caused by the HT or complications of pregnancy because data were unavailable regarding indication for cesareans.
- Patients with HT were also at increased risk for hospital readmission within the first year after delivery, particularly within the first 6 months, including for HT-related complications, a finding that supports guidelines recommending an initial postpartum visit within 7-14 days of discharge for patients with cardiac conditions, write the authors.
IN PRACTICE:
The findings demonstrate the importance of counseling HT patients at early gestational ages “to provide information about anticipated risks in pregnancy and the postpartum period to allow patients the opportunity to make informed choices regarding their reproductive options,” the authors conclude.
SOURCE:
The study was conducted by Amanda M. Craig, MD, division of maternal fetal medicine, department of obstetrics and gynecology, Duke University Medical Center, Durham, N.C., and colleagues. It was published online in JACC Heart Failure.
LIMITATIONS:
Relying on diagnosis and procedure codes in administrative datasets like NRD may result in underestimation of outcomes. In this study, outcomes were limited to delivery hospitalizations, which may underestimate the true incidence of complications or fail to include pregnancies that didn’t end in a delivery, including pregnancy terminations or spontaneous abortions. Information related to race, ethnicity, hospital regions, and cause of death are not captured in the NRD dataset.
DISCLOSURES:
The authors have no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Can a drug for overactive bladder disease prevent progression to heart failure?
TOPLINE:
(pre-HF) structural heart disease who were at risk of developing or worsening HF.
METHODOLOGY:
- Interventions for patients with asymptomatic pre-HF may be important in reducing the incidence of clinically overt HF, including HF with preserved ejection fraction (HFpEF).
- Mirabegron activates the cardiac beta-3 adrenergic receptor, which may offer an alternative activation of the cyclic guanosine monophosphate protein/kinase G (cGMP/PKG) pathway for patients at risk of or with mild HF and protect against worsening left ventricular hypertrophy (LVH) and/or diastolic dysfunction, but few clinical trials have evaluated the effect of mirabegron on cardiovascular outcomes.
- The phase 2b Beta3_LVH trial included 296 patients, some with and some without HF symptoms (mean age, 63 years), at 10 centers in Europe and the United Kingdom. All had an increased LV mass index (LVMI) (≥ 115 g/m2 for men and ≥ 95 g/m2 for women) or end-diastolic wall thickness of ≥ 13 mm in at least one wall segment.
- Patients, many of whom had risk factors, including hypertension, and were receiving cardiovascular therapies, were randomly assigned to receive mirabegron 50 mg/day or placebo and underwent various tests, including cardiac MRI, Doppler echocardiography, and urine and blood sampling for fasting glucose, insulin, hemoglobin A1c, serum lipids, and other measures.
- The two primary endpoints were change in left ventricular mass index (LVMI), expressed in grams per meters squared, and change in diastolic function, assessed as the ratio of peak early transmitral ventricular filling velocity to early diastolic tissue Doppler velocity (E/e´).
TAKEAWAY:
- Neither primary outcome reached statistical significance at 12 months; adjusted differences between groups included a 1.3g/m2 increase in LVMI (95% confidence interval, −0.15 to 2.74; P = .08) and a −0.15 decrease in E/e´ (95% CI, −0.69 to 0.4; P = .60).
- There was no statistically significant effect of mirabegron, in comparison with placebo, on lipids, glycemic control, or insulin sensitivity.
- The effect of mirabegron remained neutral in exploratory subgroup analyses, including age (≤ 65 years or > 65 years at baseline), sex (men or women), body mass index (≤ 30 kg/m2 or > 30 at baseline), presence of type 2 diabetes, atrial fibrillation, beta-blocker use, and geographic region.
- There were no deaths. There was a total of 428 adverse events (AEs), but there were no statistically significant between-group differences in the occurrence of these AEs.
IN PRACTICE:
While this study showed that mirabegron had a neutral effect on LV mass and diastolic function for patients with pre-HF or mild HF, the researchers suggest that longer-term effects of beta-3 adrenergic stimulation on myocardial remodeling and function “need to be tested in patients with established HFpEF, including with recent, more potent agonists.”
SOURCE:
The study was conducted by Jean-Luc Balligand, MD, Institut de Recherche Expérimentale et Clinique, Université Catholique de Louvain, Brussels, and colleagues. It was published online in JAMA Cardiology.
LIMITATIONS:
Inclusion of patients with mild HF and use of a single standard mirabegron dosage (50 mg/day) may have prevented detection of a treatment effect. More advanced techniques than measurements of E/e´, such as cardiac strain, may have been better for assessing early changes in diastolic function. Although missing data and dropouts were relatively infrequent and were compensated for in the study, these remain limitations.
DISCLOSURES:
The study was funded by European Commission Horizon 2020 Framework Programme. Dr. Balligand reported receiving grants from the European Commission during the conduct of the study, grants from Novartis and Daiichi Sankyo outside the submitted work, and consulting fees from Amgen, Novartis, and Daiichi Sankyo outside the submitted work; he also reported being a minor shareholder of Spinovit and serving as a board member for the Wallonia Health and Biotech Cluster, Biowin, and the AstraZeneca Foundation.
A version of this article first appeared on Medscape.com.
TOPLINE:
(pre-HF) structural heart disease who were at risk of developing or worsening HF.
METHODOLOGY:
- Interventions for patients with asymptomatic pre-HF may be important in reducing the incidence of clinically overt HF, including HF with preserved ejection fraction (HFpEF).
- Mirabegron activates the cardiac beta-3 adrenergic receptor, which may offer an alternative activation of the cyclic guanosine monophosphate protein/kinase G (cGMP/PKG) pathway for patients at risk of or with mild HF and protect against worsening left ventricular hypertrophy (LVH) and/or diastolic dysfunction, but few clinical trials have evaluated the effect of mirabegron on cardiovascular outcomes.
- The phase 2b Beta3_LVH trial included 296 patients, some with and some without HF symptoms (mean age, 63 years), at 10 centers in Europe and the United Kingdom. All had an increased LV mass index (LVMI) (≥ 115 g/m2 for men and ≥ 95 g/m2 for women) or end-diastolic wall thickness of ≥ 13 mm in at least one wall segment.
- Patients, many of whom had risk factors, including hypertension, and were receiving cardiovascular therapies, were randomly assigned to receive mirabegron 50 mg/day or placebo and underwent various tests, including cardiac MRI, Doppler echocardiography, and urine and blood sampling for fasting glucose, insulin, hemoglobin A1c, serum lipids, and other measures.
- The two primary endpoints were change in left ventricular mass index (LVMI), expressed in grams per meters squared, and change in diastolic function, assessed as the ratio of peak early transmitral ventricular filling velocity to early diastolic tissue Doppler velocity (E/e´).
TAKEAWAY:
- Neither primary outcome reached statistical significance at 12 months; adjusted differences between groups included a 1.3g/m2 increase in LVMI (95% confidence interval, −0.15 to 2.74; P = .08) and a −0.15 decrease in E/e´ (95% CI, −0.69 to 0.4; P = .60).
- There was no statistically significant effect of mirabegron, in comparison with placebo, on lipids, glycemic control, or insulin sensitivity.
- The effect of mirabegron remained neutral in exploratory subgroup analyses, including age (≤ 65 years or > 65 years at baseline), sex (men or women), body mass index (≤ 30 kg/m2 or > 30 at baseline), presence of type 2 diabetes, atrial fibrillation, beta-blocker use, and geographic region.
- There were no deaths. There was a total of 428 adverse events (AEs), but there were no statistically significant between-group differences in the occurrence of these AEs.
IN PRACTICE:
While this study showed that mirabegron had a neutral effect on LV mass and diastolic function for patients with pre-HF or mild HF, the researchers suggest that longer-term effects of beta-3 adrenergic stimulation on myocardial remodeling and function “need to be tested in patients with established HFpEF, including with recent, more potent agonists.”
SOURCE:
The study was conducted by Jean-Luc Balligand, MD, Institut de Recherche Expérimentale et Clinique, Université Catholique de Louvain, Brussels, and colleagues. It was published online in JAMA Cardiology.
LIMITATIONS:
Inclusion of patients with mild HF and use of a single standard mirabegron dosage (50 mg/day) may have prevented detection of a treatment effect. More advanced techniques than measurements of E/e´, such as cardiac strain, may have been better for assessing early changes in diastolic function. Although missing data and dropouts were relatively infrequent and were compensated for in the study, these remain limitations.
DISCLOSURES:
The study was funded by European Commission Horizon 2020 Framework Programme. Dr. Balligand reported receiving grants from the European Commission during the conduct of the study, grants from Novartis and Daiichi Sankyo outside the submitted work, and consulting fees from Amgen, Novartis, and Daiichi Sankyo outside the submitted work; he also reported being a minor shareholder of Spinovit and serving as a board member for the Wallonia Health and Biotech Cluster, Biowin, and the AstraZeneca Foundation.
A version of this article first appeared on Medscape.com.
TOPLINE:
(pre-HF) structural heart disease who were at risk of developing or worsening HF.
METHODOLOGY:
- Interventions for patients with asymptomatic pre-HF may be important in reducing the incidence of clinically overt HF, including HF with preserved ejection fraction (HFpEF).
- Mirabegron activates the cardiac beta-3 adrenergic receptor, which may offer an alternative activation of the cyclic guanosine monophosphate protein/kinase G (cGMP/PKG) pathway for patients at risk of or with mild HF and protect against worsening left ventricular hypertrophy (LVH) and/or diastolic dysfunction, but few clinical trials have evaluated the effect of mirabegron on cardiovascular outcomes.
- The phase 2b Beta3_LVH trial included 296 patients, some with and some without HF symptoms (mean age, 63 years), at 10 centers in Europe and the United Kingdom. All had an increased LV mass index (LVMI) (≥ 115 g/m2 for men and ≥ 95 g/m2 for women) or end-diastolic wall thickness of ≥ 13 mm in at least one wall segment.
- Patients, many of whom had risk factors, including hypertension, and were receiving cardiovascular therapies, were randomly assigned to receive mirabegron 50 mg/day or placebo and underwent various tests, including cardiac MRI, Doppler echocardiography, and urine and blood sampling for fasting glucose, insulin, hemoglobin A1c, serum lipids, and other measures.
- The two primary endpoints were change in left ventricular mass index (LVMI), expressed in grams per meters squared, and change in diastolic function, assessed as the ratio of peak early transmitral ventricular filling velocity to early diastolic tissue Doppler velocity (E/e´).
TAKEAWAY:
- Neither primary outcome reached statistical significance at 12 months; adjusted differences between groups included a 1.3g/m2 increase in LVMI (95% confidence interval, −0.15 to 2.74; P = .08) and a −0.15 decrease in E/e´ (95% CI, −0.69 to 0.4; P = .60).
- There was no statistically significant effect of mirabegron, in comparison with placebo, on lipids, glycemic control, or insulin sensitivity.
- The effect of mirabegron remained neutral in exploratory subgroup analyses, including age (≤ 65 years or > 65 years at baseline), sex (men or women), body mass index (≤ 30 kg/m2 or > 30 at baseline), presence of type 2 diabetes, atrial fibrillation, beta-blocker use, and geographic region.
- There were no deaths. There was a total of 428 adverse events (AEs), but there were no statistically significant between-group differences in the occurrence of these AEs.
IN PRACTICE:
While this study showed that mirabegron had a neutral effect on LV mass and diastolic function for patients with pre-HF or mild HF, the researchers suggest that longer-term effects of beta-3 adrenergic stimulation on myocardial remodeling and function “need to be tested in patients with established HFpEF, including with recent, more potent agonists.”
SOURCE:
The study was conducted by Jean-Luc Balligand, MD, Institut de Recherche Expérimentale et Clinique, Université Catholique de Louvain, Brussels, and colleagues. It was published online in JAMA Cardiology.
LIMITATIONS:
Inclusion of patients with mild HF and use of a single standard mirabegron dosage (50 mg/day) may have prevented detection of a treatment effect. More advanced techniques than measurements of E/e´, such as cardiac strain, may have been better for assessing early changes in diastolic function. Although missing data and dropouts were relatively infrequent and were compensated for in the study, these remain limitations.
DISCLOSURES:
The study was funded by European Commission Horizon 2020 Framework Programme. Dr. Balligand reported receiving grants from the European Commission during the conduct of the study, grants from Novartis and Daiichi Sankyo outside the submitted work, and consulting fees from Amgen, Novartis, and Daiichi Sankyo outside the submitted work; he also reported being a minor shareholder of Spinovit and serving as a board member for the Wallonia Health and Biotech Cluster, Biowin, and the AstraZeneca Foundation.
A version of this article first appeared on Medscape.com.
FROM JAMA CARDIOLOGY
SGLT2 inhibitors: No benefit or harm in hospitalized COVID-19
A new meta-analysis has shown that SGLT2 inhibitors do not lead to lower 28-day all-cause mortality, compared with usual care or placebo, in patients hospitalized with COVID-19.
However, no major safety issues were identified with the use of SGLT2 inhibitors in these acutely ill patients, the researchers report.
“While these findings do not support the use of SGLT2-inhibitors as standard of care for patients hospitalized with COVID-19, I think the most important take home message here is that the use of these medications appears to be safe even in really acutely ill hospitalized patients,” lead investigator of the meta-analysis, Mikhail Kosiborod, MD, Saint Luke’s Mid America Heart Institute, Kansas City, Mo., concluded.
He said this was important because the list of indications for SGLT2 inhibitors is rapidly growing.
“These medications are being used in more and more patients. And we know that when we discontinue medications in the hospital they frequently don’t get restarted, which can lead to real risks if SGLT2 inhibitors are stopped in patients with heart failure, chronic kidney disease, or diabetes. So, ,” he added.
The new meta-analysis was presented at the recent annual congress of the European Society of Cardiology, held in Amsterdam.
Discussant of the presentation at the ESC Hotline session, Muthiah Vaduganathan, MD, MPH, Brigham and Women’s Hospital, Boston, agreed with Dr. Kosiborod’s interpretation.
“Until today we have had very limited information on the safety of SGLT2-inhibitors in acute illness, as the pivotal trials which established the use of these drugs in diabetes and chronic kidney disease largely excluded patients who were hospitalized,” Dr. Vaduganathan said.
“While the overall results of this meta-analysis are neutral and SGLT2 inhibitors will not be added as drugs to be used in the primary care of patients with COVID-19, it certainly sends a strong message of safety in acutely ill patients,” he added.
Dr. Vaduganathan explained that from the beginning of the COVID-19 pandemic, there was great interest in repurposing established therapies for alternative indications for their use in the management of COVID-19.
“Conditions that strongly predispose to adverse COVID outcomes strongly overlap with established indications for SGLT2-inhibitors. So many wondered whether these drugs may be an ideal treatment candidate for the management of COVID-19. However, there have been many safety concerns about the use of SGLT2-inhibitors in this acute setting, with worries that they may induce hemodynamic changes such an excessive lowering of blood pressure, or metabolic changes such as ketoacidosis in acutely ill patients,” he noted.
The initial DARE-19 study investigating SGLT2-inhibitors in COVID-19, with 1,250 participants, found a 20% reduction in the primary outcome of organ dysfunction or death, but this did not reach statistical significance, and no safety issues were seen. This “intriguing” result led to two further larger trials – the ACTIV-4a and RECOVERY trials, Dr. Vaduganathan reported.
“Those early signals of benefit seen in DARE-19 were largely not substantiated in the ACTIV-4A and RECOVERY trials, or in this new meta-analysis, and now we have this much larger body of evidence and more stable estimates about the efficacy of these drugs in acutely ill COVID-19 patients,” he said.
“But the story that we will all take forward is one of safety. This set of trials was arguably conducted in some of the sickest patients we’ve seen who have been exposed to SGLT2-inhibitors, and they strongly affirm that these agents can be safely continued in the setting of acute illness, with very low rates of ketoacidosis and kidney injury, and there was no prolongation of hospital stay,” he commented.
In his presentation, Dr. Kosiborod explained that treatments targeting COVID-19 pathobiology such as dysregulated immune responses, endothelial damage, microvascular thrombosis, and inflammation have been shown to improve the key outcomes in this patient group.
SGLT2 inhibitors, which modulate similar pathobiology, provide cardiovascular protection and prevent the progression of kidney disease in patients at risk for these events, including those with type 2 diabetes, heart failure, and kidney disease, and may also lead to organ protection in a setting of acute illness such as COVID-19, he noted. However, the role of SGLT2 inhibitors in patients hospitalized with COVID-19 remains uncertain.
To address the need for more definitive efficacy data, the World Health Organization Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group conducted a prospective meta-analysis using data from the three randomized controlled trials, DARE-19, RECOVERY, and ACTIV-4a, evaluating SGLT2 inhibitors in patients hospitalized with COVID-19.
Overall, these trials randomized 6,096 participants: 3,025 to SGLT2 inhibitors and 3,071 to usual care or placebo. The average age of participants ranged between 62 and 73 years across the trials, 39% were women, and 25% had type 2 diabetes.
By 28 days after randomization, all-cause mortality, the primary endpoint, had occurred in 11.6% of the SGLT2-inhibitor patients, compared with 12.4% of those randomized to usual care or placebo, giving an odds ratio of 0.93 (95% confidence interval, 0.79-1.08; P = .33) for SGLT2 inhibitors, with consistency across trials.
Data on in-hospital and 90-day all-cause mortality were only available for two out of three trials (DARE-19 and ACTIV-4a), but the results were similar to the primary endpoint showing nonsignificant trends toward a possible benefit in the SGLT2-inhibitor group.
The results were also similar for the secondary outcomes of progression to acute kidney injury or requirement for dialysis or death, and progression to invasive mechanical ventilation, extracorporeal membrane oxygenation, or death, both assessed at 28 days.
The primary safety outcome of ketoacidosis by 28 days was observed in seven and two patients allocated to SGLT2 inhibitors and usual care or placebo, respectively, and overall, the incidence of reported serious adverse events was balanced between treatment groups.
The RECOVERY trial was supported by grants to the University of Oxford from UK Research and Innovation, the National Institute for Health and Care Research, and Wellcome. The ACTIV-4a platform was sponsored by the National Heart, Lung, and Blood Institute. DARE-19 was an investigator-initiated collaborative trial supported by AstraZeneca. Dr. Kosiborod reported numerous conflicts of interest.
A version of this article first appeared on Medscape.com.
A new meta-analysis has shown that SGLT2 inhibitors do not lead to lower 28-day all-cause mortality, compared with usual care or placebo, in patients hospitalized with COVID-19.
However, no major safety issues were identified with the use of SGLT2 inhibitors in these acutely ill patients, the researchers report.
“While these findings do not support the use of SGLT2-inhibitors as standard of care for patients hospitalized with COVID-19, I think the most important take home message here is that the use of these medications appears to be safe even in really acutely ill hospitalized patients,” lead investigator of the meta-analysis, Mikhail Kosiborod, MD, Saint Luke’s Mid America Heart Institute, Kansas City, Mo., concluded.
He said this was important because the list of indications for SGLT2 inhibitors is rapidly growing.
“These medications are being used in more and more patients. And we know that when we discontinue medications in the hospital they frequently don’t get restarted, which can lead to real risks if SGLT2 inhibitors are stopped in patients with heart failure, chronic kidney disease, or diabetes. So, ,” he added.
The new meta-analysis was presented at the recent annual congress of the European Society of Cardiology, held in Amsterdam.
Discussant of the presentation at the ESC Hotline session, Muthiah Vaduganathan, MD, MPH, Brigham and Women’s Hospital, Boston, agreed with Dr. Kosiborod’s interpretation.
“Until today we have had very limited information on the safety of SGLT2-inhibitors in acute illness, as the pivotal trials which established the use of these drugs in diabetes and chronic kidney disease largely excluded patients who were hospitalized,” Dr. Vaduganathan said.
“While the overall results of this meta-analysis are neutral and SGLT2 inhibitors will not be added as drugs to be used in the primary care of patients with COVID-19, it certainly sends a strong message of safety in acutely ill patients,” he added.
Dr. Vaduganathan explained that from the beginning of the COVID-19 pandemic, there was great interest in repurposing established therapies for alternative indications for their use in the management of COVID-19.
“Conditions that strongly predispose to adverse COVID outcomes strongly overlap with established indications for SGLT2-inhibitors. So many wondered whether these drugs may be an ideal treatment candidate for the management of COVID-19. However, there have been many safety concerns about the use of SGLT2-inhibitors in this acute setting, with worries that they may induce hemodynamic changes such an excessive lowering of blood pressure, or metabolic changes such as ketoacidosis in acutely ill patients,” he noted.
The initial DARE-19 study investigating SGLT2-inhibitors in COVID-19, with 1,250 participants, found a 20% reduction in the primary outcome of organ dysfunction or death, but this did not reach statistical significance, and no safety issues were seen. This “intriguing” result led to two further larger trials – the ACTIV-4a and RECOVERY trials, Dr. Vaduganathan reported.
“Those early signals of benefit seen in DARE-19 were largely not substantiated in the ACTIV-4A and RECOVERY trials, or in this new meta-analysis, and now we have this much larger body of evidence and more stable estimates about the efficacy of these drugs in acutely ill COVID-19 patients,” he said.
“But the story that we will all take forward is one of safety. This set of trials was arguably conducted in some of the sickest patients we’ve seen who have been exposed to SGLT2-inhibitors, and they strongly affirm that these agents can be safely continued in the setting of acute illness, with very low rates of ketoacidosis and kidney injury, and there was no prolongation of hospital stay,” he commented.
In his presentation, Dr. Kosiborod explained that treatments targeting COVID-19 pathobiology such as dysregulated immune responses, endothelial damage, microvascular thrombosis, and inflammation have been shown to improve the key outcomes in this patient group.
SGLT2 inhibitors, which modulate similar pathobiology, provide cardiovascular protection and prevent the progression of kidney disease in patients at risk for these events, including those with type 2 diabetes, heart failure, and kidney disease, and may also lead to organ protection in a setting of acute illness such as COVID-19, he noted. However, the role of SGLT2 inhibitors in patients hospitalized with COVID-19 remains uncertain.
To address the need for more definitive efficacy data, the World Health Organization Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group conducted a prospective meta-analysis using data from the three randomized controlled trials, DARE-19, RECOVERY, and ACTIV-4a, evaluating SGLT2 inhibitors in patients hospitalized with COVID-19.
Overall, these trials randomized 6,096 participants: 3,025 to SGLT2 inhibitors and 3,071 to usual care or placebo. The average age of participants ranged between 62 and 73 years across the trials, 39% were women, and 25% had type 2 diabetes.
By 28 days after randomization, all-cause mortality, the primary endpoint, had occurred in 11.6% of the SGLT2-inhibitor patients, compared with 12.4% of those randomized to usual care or placebo, giving an odds ratio of 0.93 (95% confidence interval, 0.79-1.08; P = .33) for SGLT2 inhibitors, with consistency across trials.
Data on in-hospital and 90-day all-cause mortality were only available for two out of three trials (DARE-19 and ACTIV-4a), but the results were similar to the primary endpoint showing nonsignificant trends toward a possible benefit in the SGLT2-inhibitor group.
The results were also similar for the secondary outcomes of progression to acute kidney injury or requirement for dialysis or death, and progression to invasive mechanical ventilation, extracorporeal membrane oxygenation, or death, both assessed at 28 days.
The primary safety outcome of ketoacidosis by 28 days was observed in seven and two patients allocated to SGLT2 inhibitors and usual care or placebo, respectively, and overall, the incidence of reported serious adverse events was balanced between treatment groups.
The RECOVERY trial was supported by grants to the University of Oxford from UK Research and Innovation, the National Institute for Health and Care Research, and Wellcome. The ACTIV-4a platform was sponsored by the National Heart, Lung, and Blood Institute. DARE-19 was an investigator-initiated collaborative trial supported by AstraZeneca. Dr. Kosiborod reported numerous conflicts of interest.
A version of this article first appeared on Medscape.com.
A new meta-analysis has shown that SGLT2 inhibitors do not lead to lower 28-day all-cause mortality, compared with usual care or placebo, in patients hospitalized with COVID-19.
However, no major safety issues were identified with the use of SGLT2 inhibitors in these acutely ill patients, the researchers report.
“While these findings do not support the use of SGLT2-inhibitors as standard of care for patients hospitalized with COVID-19, I think the most important take home message here is that the use of these medications appears to be safe even in really acutely ill hospitalized patients,” lead investigator of the meta-analysis, Mikhail Kosiborod, MD, Saint Luke’s Mid America Heart Institute, Kansas City, Mo., concluded.
He said this was important because the list of indications for SGLT2 inhibitors is rapidly growing.
“These medications are being used in more and more patients. And we know that when we discontinue medications in the hospital they frequently don’t get restarted, which can lead to real risks if SGLT2 inhibitors are stopped in patients with heart failure, chronic kidney disease, or diabetes. So, ,” he added.
The new meta-analysis was presented at the recent annual congress of the European Society of Cardiology, held in Amsterdam.
Discussant of the presentation at the ESC Hotline session, Muthiah Vaduganathan, MD, MPH, Brigham and Women’s Hospital, Boston, agreed with Dr. Kosiborod’s interpretation.
“Until today we have had very limited information on the safety of SGLT2-inhibitors in acute illness, as the pivotal trials which established the use of these drugs in diabetes and chronic kidney disease largely excluded patients who were hospitalized,” Dr. Vaduganathan said.
“While the overall results of this meta-analysis are neutral and SGLT2 inhibitors will not be added as drugs to be used in the primary care of patients with COVID-19, it certainly sends a strong message of safety in acutely ill patients,” he added.
Dr. Vaduganathan explained that from the beginning of the COVID-19 pandemic, there was great interest in repurposing established therapies for alternative indications for their use in the management of COVID-19.
“Conditions that strongly predispose to adverse COVID outcomes strongly overlap with established indications for SGLT2-inhibitors. So many wondered whether these drugs may be an ideal treatment candidate for the management of COVID-19. However, there have been many safety concerns about the use of SGLT2-inhibitors in this acute setting, with worries that they may induce hemodynamic changes such an excessive lowering of blood pressure, or metabolic changes such as ketoacidosis in acutely ill patients,” he noted.
The initial DARE-19 study investigating SGLT2-inhibitors in COVID-19, with 1,250 participants, found a 20% reduction in the primary outcome of organ dysfunction or death, but this did not reach statistical significance, and no safety issues were seen. This “intriguing” result led to two further larger trials – the ACTIV-4a and RECOVERY trials, Dr. Vaduganathan reported.
“Those early signals of benefit seen in DARE-19 were largely not substantiated in the ACTIV-4A and RECOVERY trials, or in this new meta-analysis, and now we have this much larger body of evidence and more stable estimates about the efficacy of these drugs in acutely ill COVID-19 patients,” he said.
“But the story that we will all take forward is one of safety. This set of trials was arguably conducted in some of the sickest patients we’ve seen who have been exposed to SGLT2-inhibitors, and they strongly affirm that these agents can be safely continued in the setting of acute illness, with very low rates of ketoacidosis and kidney injury, and there was no prolongation of hospital stay,” he commented.
In his presentation, Dr. Kosiborod explained that treatments targeting COVID-19 pathobiology such as dysregulated immune responses, endothelial damage, microvascular thrombosis, and inflammation have been shown to improve the key outcomes in this patient group.
SGLT2 inhibitors, which modulate similar pathobiology, provide cardiovascular protection and prevent the progression of kidney disease in patients at risk for these events, including those with type 2 diabetes, heart failure, and kidney disease, and may also lead to organ protection in a setting of acute illness such as COVID-19, he noted. However, the role of SGLT2 inhibitors in patients hospitalized with COVID-19 remains uncertain.
To address the need for more definitive efficacy data, the World Health Organization Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group conducted a prospective meta-analysis using data from the three randomized controlled trials, DARE-19, RECOVERY, and ACTIV-4a, evaluating SGLT2 inhibitors in patients hospitalized with COVID-19.
Overall, these trials randomized 6,096 participants: 3,025 to SGLT2 inhibitors and 3,071 to usual care or placebo. The average age of participants ranged between 62 and 73 years across the trials, 39% were women, and 25% had type 2 diabetes.
By 28 days after randomization, all-cause mortality, the primary endpoint, had occurred in 11.6% of the SGLT2-inhibitor patients, compared with 12.4% of those randomized to usual care or placebo, giving an odds ratio of 0.93 (95% confidence interval, 0.79-1.08; P = .33) for SGLT2 inhibitors, with consistency across trials.
Data on in-hospital and 90-day all-cause mortality were only available for two out of three trials (DARE-19 and ACTIV-4a), but the results were similar to the primary endpoint showing nonsignificant trends toward a possible benefit in the SGLT2-inhibitor group.
The results were also similar for the secondary outcomes of progression to acute kidney injury or requirement for dialysis or death, and progression to invasive mechanical ventilation, extracorporeal membrane oxygenation, or death, both assessed at 28 days.
The primary safety outcome of ketoacidosis by 28 days was observed in seven and two patients allocated to SGLT2 inhibitors and usual care or placebo, respectively, and overall, the incidence of reported serious adverse events was balanced between treatment groups.
The RECOVERY trial was supported by grants to the University of Oxford from UK Research and Innovation, the National Institute for Health and Care Research, and Wellcome. The ACTIV-4a platform was sponsored by the National Heart, Lung, and Blood Institute. DARE-19 was an investigator-initiated collaborative trial supported by AstraZeneca. Dr. Kosiborod reported numerous conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM ESC CONGRESS 2023
Recent leaps in heart failure therapy spur ESC guideline–focused update
Two years is a long time in the world of heart failure (HF) management, enough to see publication of more than a dozen studies with insights that would supplant and expand key sections of a far-reaching European Society of Cardiology (ESC) clinical practice guideline on HF unveiled in 2021.
“Back in 2021, we had three and a half decades of data to consider,” but recent years have seen “an amazing amount of progress” that has necessitated some adjustments and key additions, including several Class I recommendations, observed Roy S. Gardner, MBChB, MD, Golden Jubilee National Hospital, Clydebank, United Kingdom.
, which Dr. Gardner helped unveil over several days at the annual congress of the European Society of Cardiology, held in Amsterdam.
The new document was also published in the European Heart Journal during the ESC sessions. Dr. Gardner is a co-author on both the 2021 and 2023 documents.
The task force that was charged with the focused update’s development “considered a large number of trials across the spectrum of acute chronic heart failure and the comorbidities associated with it,” Ultimately, it considered only those with “results that would lead to new or changed Class I or Class IIa recommendations,” noted Theresa A. McDonagh, MD, during the ESC sessions.
Dr. McDonagh, of King’s College Hospital, London, chaired the task force and led the document’s list of authors along with Marco Metra, MD, University of Brescia (Italy).
Chronic HF management
The 2021 document’s “beautiful algorithm” on managing HF with reduced ejection fraction, that is HF with an LVEF less than 40%, had helped enshrine the expeditious uptitration of the “four pillars” of drug therapy as a top management goal. That remains unchanged in the focused update, Dr. Gardner noted.
But the new document gives a boost to recommendations for HF with mildly reduced ejection fraction (HFmrEF), characterized by an LVEF greater than 40% to less than 50%. For that, the 2021 document recommended three of the four pillars of HF medical therapy: beta blockers, mineralocorticoid receptor antagonists (MRA), renin-angiotensin system (RAS) inhibitors.
The focused update, however, adds the fourth pillar – SGLT2 inhibitors – to core therapy for both HFmrEF and HF with preserved ejection fraction (HFpEF), the latter defined by an LVEF greater than 50%. Publication of trials supporting those new recommendations had narrowly missed availability for the 2021 document.
EMPEROR-Preserved, for example, was published during the same ESC 2021 sessions that introduced the 2021 guidelines. Its patients with HFpEF (which at the time included patients meeting the current definition of HFmrEF) assigned to the SGLT2 inhibitor empagliflozin (Jardiance) showed a 21% reduction in risk for a composite primary endpoint that was driven by the HF-hospitalization component.
“This wasn’t a fluke finding,” Dr. Gardner said, as the following year saw publication of the DELIVER trial, which resembled EMPEROR-Preserved in design and outcomes using the SGLT2 inhibitor dapagliflozin (Farxiga).
The two trials, backed up by meta-analyses that also included DAPA-HF and other studies, suggested as well that the two SGLT2 inhibitors “work across the spectrum of ejection fraction,” Dr. Gardner said.
The 2023 focused update indicates an SGLT2 inhibitor, either empagliflozin or dapagliflozin, for patients with either HFmrEF or HFpEF to reduce the risk of HF hospitalization or cardiovascular death. Both recommendations are of Class I, level of evidence A.
The new indications make SGLT2 inhibitors and diuretics (as needed for fluid retention) the only drugs for HFmrEF or HFpEF with a Class I recommendation. Previously established “rather weaker” Class IIb recommendation for RAS inhibitors, MRAs, and beta blockers that had been “based on subgroup analyses of neutral trials” remained unchanged in the focused update, Dr. McDonagh noted.
Patients hospitalized with HF
The 2021 guidelines had recommended that patients hospitalized with acute HF be started on evidence-based meds before discharge and that they return for evaluation 1 to 2 weeks after discharge. But the recommendation was unsupported by randomized trials.
That changed with the 2022 publication of STRONG-HF, in which a strategy of early and rapid uptitration of guideline-directed meds, initiated predischarge regardless of LVEF, led to a one-third reduced 6-month risk for death or HF readmission.
Based primarily on STRONG-HF, the focused update recommends “an intensive strategy of initiation and rapid up-titration of evidence-based treatment before discharge and during frequent and careful follow-up visits in the first 6 weeks after hospitalization” to reduce readmission and mortality: Class I, level of evidence B.
“There was a large consensus around this recommendation,” said STRONG-HF principal investigator Alexandre Mebazaa, MD, PhD, a co-author of both the 2021 and 2023 documents. Conducted before the advent of the four pillars of drug therapy, sometimes called quartet therapy, the trial’s requirement for evidence-based meds didn’t include SGLT2 inhibitors.
The new focused update considers the new status of those agents, especially with regard to their benefits independent of LVEF. So, it completed the quartet by adding empagliflozin or dapagliflozin to the agents that should be initiated predischarge, observed Dr. Mebazaa, University Hospitals Saint Louis‐Lariboisière, Paris, at the focused-update’s ESC 2023 sessions.
The new document also follows STRONG-HF with its emphasis on “frequent and careful follow-up” by recommending certain clinical and laboratory evaluations known to be prognostic in HF. They include congestion status, blood pressure, heart rate, natriuretic peptide (NT-proBNP) and potassium levels and estimated glomerular filtration rate.
Dr. Mebazaa stressed the importance of monitoring NT-proBNP after discharge. “What we saw in STRONG-HF is that sometimes the clinical signs do not necessarily tell you that the patient is still congested.”
After discharge, he said, NT-proBNP levels “should only go down.” So, knowing whether NT-proBNP levels “are stable or increasing” during the med optimization process can help guide diuretic dosing.
HF with comorbidities
The new document includes two new Class I recommendations for patients with HF and both type 2 diabetes and chronic kidney disease based on several recent randomized trials and meta-analyses.
The focused update recommends SGLT2 inhibitors as well as the selective, non-steroidal MRA finerenone (Kerendia) in HF patients with CKD and type-2 diabetes. Both Class I recommendations are supported by a level of evidence A.
The SGLT2 indication is based on DAPA-CKD and EMPA-KIDNEY plus meta-analyses that included those trials along with others. The recommendation for finerenone derives from the FIDELIO-DKD and FIGARO-DKD trials and a pooled analysis of the two studies.
The 2023 focused update also accounts for new clinical-trial insights for patients with HF and iron deficiency. The 2021 document featured recommendations for the diagnosis and iron-repletion therapy in such cases, but only as Class IIa or at lower low levels of evidence. The focused update considers more recent studies, especially IRONMAN and some meta-analyses.
The 2023 document indicates intravenous iron supplementation for symptomatic patients with iron deficiency and either HFrEF or HFmrEF to improve symptoms and quality of life (Class I, level of evidence A), and says it should be considered (Class IIa, level of evidence A) to reduce risk for HF hospitalization.
When the task force assembled to plan the 2023 focused update, Dr. Gardner observed, “the first thing we thought about was the nomenclature around the phenotyping of heart failure.”
Although the 2021 guidelines relied fundamentally on the distinctions between HFrEF, HFmrEF, and HFpEF, it had become apparent to some in the field that some meds, especially the SGLT2 inhibitors, were obscuring their LVEF-based boundaries, at least with respect to drug therapy.
The 2023 document’s developers, Dr. Gardner said, seriously considered changing the three categories to two, that is HFrEF and – to account for all other heart failure – HF with normal ejection fraction (HFnEF).
That didn’t happen, although the proposal was popular within the task force. Any changes to the 2021 document would require a 75% consensus on the matter, Dr. Gardner explained. When the task force took a vote on whether to change the nomenclature, he said, 71% favored the proposal.
Disclosures for members of the task force can be found in a supplement to the published 2023 Focused Update.
A version of this article first appeared on Medscape.com.
Two years is a long time in the world of heart failure (HF) management, enough to see publication of more than a dozen studies with insights that would supplant and expand key sections of a far-reaching European Society of Cardiology (ESC) clinical practice guideline on HF unveiled in 2021.
“Back in 2021, we had three and a half decades of data to consider,” but recent years have seen “an amazing amount of progress” that has necessitated some adjustments and key additions, including several Class I recommendations, observed Roy S. Gardner, MBChB, MD, Golden Jubilee National Hospital, Clydebank, United Kingdom.
, which Dr. Gardner helped unveil over several days at the annual congress of the European Society of Cardiology, held in Amsterdam.
The new document was also published in the European Heart Journal during the ESC sessions. Dr. Gardner is a co-author on both the 2021 and 2023 documents.
The task force that was charged with the focused update’s development “considered a large number of trials across the spectrum of acute chronic heart failure and the comorbidities associated with it,” Ultimately, it considered only those with “results that would lead to new or changed Class I or Class IIa recommendations,” noted Theresa A. McDonagh, MD, during the ESC sessions.
Dr. McDonagh, of King’s College Hospital, London, chaired the task force and led the document’s list of authors along with Marco Metra, MD, University of Brescia (Italy).
Chronic HF management
The 2021 document’s “beautiful algorithm” on managing HF with reduced ejection fraction, that is HF with an LVEF less than 40%, had helped enshrine the expeditious uptitration of the “four pillars” of drug therapy as a top management goal. That remains unchanged in the focused update, Dr. Gardner noted.
But the new document gives a boost to recommendations for HF with mildly reduced ejection fraction (HFmrEF), characterized by an LVEF greater than 40% to less than 50%. For that, the 2021 document recommended three of the four pillars of HF medical therapy: beta blockers, mineralocorticoid receptor antagonists (MRA), renin-angiotensin system (RAS) inhibitors.
The focused update, however, adds the fourth pillar – SGLT2 inhibitors – to core therapy for both HFmrEF and HF with preserved ejection fraction (HFpEF), the latter defined by an LVEF greater than 50%. Publication of trials supporting those new recommendations had narrowly missed availability for the 2021 document.
EMPEROR-Preserved, for example, was published during the same ESC 2021 sessions that introduced the 2021 guidelines. Its patients with HFpEF (which at the time included patients meeting the current definition of HFmrEF) assigned to the SGLT2 inhibitor empagliflozin (Jardiance) showed a 21% reduction in risk for a composite primary endpoint that was driven by the HF-hospitalization component.
“This wasn’t a fluke finding,” Dr. Gardner said, as the following year saw publication of the DELIVER trial, which resembled EMPEROR-Preserved in design and outcomes using the SGLT2 inhibitor dapagliflozin (Farxiga).
The two trials, backed up by meta-analyses that also included DAPA-HF and other studies, suggested as well that the two SGLT2 inhibitors “work across the spectrum of ejection fraction,” Dr. Gardner said.
The 2023 focused update indicates an SGLT2 inhibitor, either empagliflozin or dapagliflozin, for patients with either HFmrEF or HFpEF to reduce the risk of HF hospitalization or cardiovascular death. Both recommendations are of Class I, level of evidence A.
The new indications make SGLT2 inhibitors and diuretics (as needed for fluid retention) the only drugs for HFmrEF or HFpEF with a Class I recommendation. Previously established “rather weaker” Class IIb recommendation for RAS inhibitors, MRAs, and beta blockers that had been “based on subgroup analyses of neutral trials” remained unchanged in the focused update, Dr. McDonagh noted.
Patients hospitalized with HF
The 2021 guidelines had recommended that patients hospitalized with acute HF be started on evidence-based meds before discharge and that they return for evaluation 1 to 2 weeks after discharge. But the recommendation was unsupported by randomized trials.
That changed with the 2022 publication of STRONG-HF, in which a strategy of early and rapid uptitration of guideline-directed meds, initiated predischarge regardless of LVEF, led to a one-third reduced 6-month risk for death or HF readmission.
Based primarily on STRONG-HF, the focused update recommends “an intensive strategy of initiation and rapid up-titration of evidence-based treatment before discharge and during frequent and careful follow-up visits in the first 6 weeks after hospitalization” to reduce readmission and mortality: Class I, level of evidence B.
“There was a large consensus around this recommendation,” said STRONG-HF principal investigator Alexandre Mebazaa, MD, PhD, a co-author of both the 2021 and 2023 documents. Conducted before the advent of the four pillars of drug therapy, sometimes called quartet therapy, the trial’s requirement for evidence-based meds didn’t include SGLT2 inhibitors.
The new focused update considers the new status of those agents, especially with regard to their benefits independent of LVEF. So, it completed the quartet by adding empagliflozin or dapagliflozin to the agents that should be initiated predischarge, observed Dr. Mebazaa, University Hospitals Saint Louis‐Lariboisière, Paris, at the focused-update’s ESC 2023 sessions.
The new document also follows STRONG-HF with its emphasis on “frequent and careful follow-up” by recommending certain clinical and laboratory evaluations known to be prognostic in HF. They include congestion status, blood pressure, heart rate, natriuretic peptide (NT-proBNP) and potassium levels and estimated glomerular filtration rate.
Dr. Mebazaa stressed the importance of monitoring NT-proBNP after discharge. “What we saw in STRONG-HF is that sometimes the clinical signs do not necessarily tell you that the patient is still congested.”
After discharge, he said, NT-proBNP levels “should only go down.” So, knowing whether NT-proBNP levels “are stable or increasing” during the med optimization process can help guide diuretic dosing.
HF with comorbidities
The new document includes two new Class I recommendations for patients with HF and both type 2 diabetes and chronic kidney disease based on several recent randomized trials and meta-analyses.
The focused update recommends SGLT2 inhibitors as well as the selective, non-steroidal MRA finerenone (Kerendia) in HF patients with CKD and type-2 diabetes. Both Class I recommendations are supported by a level of evidence A.
The SGLT2 indication is based on DAPA-CKD and EMPA-KIDNEY plus meta-analyses that included those trials along with others. The recommendation for finerenone derives from the FIDELIO-DKD and FIGARO-DKD trials and a pooled analysis of the two studies.
The 2023 focused update also accounts for new clinical-trial insights for patients with HF and iron deficiency. The 2021 document featured recommendations for the diagnosis and iron-repletion therapy in such cases, but only as Class IIa or at lower low levels of evidence. The focused update considers more recent studies, especially IRONMAN and some meta-analyses.
The 2023 document indicates intravenous iron supplementation for symptomatic patients with iron deficiency and either HFrEF or HFmrEF to improve symptoms and quality of life (Class I, level of evidence A), and says it should be considered (Class IIa, level of evidence A) to reduce risk for HF hospitalization.
When the task force assembled to plan the 2023 focused update, Dr. Gardner observed, “the first thing we thought about was the nomenclature around the phenotyping of heart failure.”
Although the 2021 guidelines relied fundamentally on the distinctions between HFrEF, HFmrEF, and HFpEF, it had become apparent to some in the field that some meds, especially the SGLT2 inhibitors, were obscuring their LVEF-based boundaries, at least with respect to drug therapy.
The 2023 document’s developers, Dr. Gardner said, seriously considered changing the three categories to two, that is HFrEF and – to account for all other heart failure – HF with normal ejection fraction (HFnEF).
That didn’t happen, although the proposal was popular within the task force. Any changes to the 2021 document would require a 75% consensus on the matter, Dr. Gardner explained. When the task force took a vote on whether to change the nomenclature, he said, 71% favored the proposal.
Disclosures for members of the task force can be found in a supplement to the published 2023 Focused Update.
A version of this article first appeared on Medscape.com.
Two years is a long time in the world of heart failure (HF) management, enough to see publication of more than a dozen studies with insights that would supplant and expand key sections of a far-reaching European Society of Cardiology (ESC) clinical practice guideline on HF unveiled in 2021.
“Back in 2021, we had three and a half decades of data to consider,” but recent years have seen “an amazing amount of progress” that has necessitated some adjustments and key additions, including several Class I recommendations, observed Roy S. Gardner, MBChB, MD, Golden Jubilee National Hospital, Clydebank, United Kingdom.
, which Dr. Gardner helped unveil over several days at the annual congress of the European Society of Cardiology, held in Amsterdam.
The new document was also published in the European Heart Journal during the ESC sessions. Dr. Gardner is a co-author on both the 2021 and 2023 documents.
The task force that was charged with the focused update’s development “considered a large number of trials across the spectrum of acute chronic heart failure and the comorbidities associated with it,” Ultimately, it considered only those with “results that would lead to new or changed Class I or Class IIa recommendations,” noted Theresa A. McDonagh, MD, during the ESC sessions.
Dr. McDonagh, of King’s College Hospital, London, chaired the task force and led the document’s list of authors along with Marco Metra, MD, University of Brescia (Italy).
Chronic HF management
The 2021 document’s “beautiful algorithm” on managing HF with reduced ejection fraction, that is HF with an LVEF less than 40%, had helped enshrine the expeditious uptitration of the “four pillars” of drug therapy as a top management goal. That remains unchanged in the focused update, Dr. Gardner noted.
But the new document gives a boost to recommendations for HF with mildly reduced ejection fraction (HFmrEF), characterized by an LVEF greater than 40% to less than 50%. For that, the 2021 document recommended three of the four pillars of HF medical therapy: beta blockers, mineralocorticoid receptor antagonists (MRA), renin-angiotensin system (RAS) inhibitors.
The focused update, however, adds the fourth pillar – SGLT2 inhibitors – to core therapy for both HFmrEF and HF with preserved ejection fraction (HFpEF), the latter defined by an LVEF greater than 50%. Publication of trials supporting those new recommendations had narrowly missed availability for the 2021 document.
EMPEROR-Preserved, for example, was published during the same ESC 2021 sessions that introduced the 2021 guidelines. Its patients with HFpEF (which at the time included patients meeting the current definition of HFmrEF) assigned to the SGLT2 inhibitor empagliflozin (Jardiance) showed a 21% reduction in risk for a composite primary endpoint that was driven by the HF-hospitalization component.
“This wasn’t a fluke finding,” Dr. Gardner said, as the following year saw publication of the DELIVER trial, which resembled EMPEROR-Preserved in design and outcomes using the SGLT2 inhibitor dapagliflozin (Farxiga).
The two trials, backed up by meta-analyses that also included DAPA-HF and other studies, suggested as well that the two SGLT2 inhibitors “work across the spectrum of ejection fraction,” Dr. Gardner said.
The 2023 focused update indicates an SGLT2 inhibitor, either empagliflozin or dapagliflozin, for patients with either HFmrEF or HFpEF to reduce the risk of HF hospitalization or cardiovascular death. Both recommendations are of Class I, level of evidence A.
The new indications make SGLT2 inhibitors and diuretics (as needed for fluid retention) the only drugs for HFmrEF or HFpEF with a Class I recommendation. Previously established “rather weaker” Class IIb recommendation for RAS inhibitors, MRAs, and beta blockers that had been “based on subgroup analyses of neutral trials” remained unchanged in the focused update, Dr. McDonagh noted.
Patients hospitalized with HF
The 2021 guidelines had recommended that patients hospitalized with acute HF be started on evidence-based meds before discharge and that they return for evaluation 1 to 2 weeks after discharge. But the recommendation was unsupported by randomized trials.
That changed with the 2022 publication of STRONG-HF, in which a strategy of early and rapid uptitration of guideline-directed meds, initiated predischarge regardless of LVEF, led to a one-third reduced 6-month risk for death or HF readmission.
Based primarily on STRONG-HF, the focused update recommends “an intensive strategy of initiation and rapid up-titration of evidence-based treatment before discharge and during frequent and careful follow-up visits in the first 6 weeks after hospitalization” to reduce readmission and mortality: Class I, level of evidence B.
“There was a large consensus around this recommendation,” said STRONG-HF principal investigator Alexandre Mebazaa, MD, PhD, a co-author of both the 2021 and 2023 documents. Conducted before the advent of the four pillars of drug therapy, sometimes called quartet therapy, the trial’s requirement for evidence-based meds didn’t include SGLT2 inhibitors.
The new focused update considers the new status of those agents, especially with regard to their benefits independent of LVEF. So, it completed the quartet by adding empagliflozin or dapagliflozin to the agents that should be initiated predischarge, observed Dr. Mebazaa, University Hospitals Saint Louis‐Lariboisière, Paris, at the focused-update’s ESC 2023 sessions.
The new document also follows STRONG-HF with its emphasis on “frequent and careful follow-up” by recommending certain clinical and laboratory evaluations known to be prognostic in HF. They include congestion status, blood pressure, heart rate, natriuretic peptide (NT-proBNP) and potassium levels and estimated glomerular filtration rate.
Dr. Mebazaa stressed the importance of monitoring NT-proBNP after discharge. “What we saw in STRONG-HF is that sometimes the clinical signs do not necessarily tell you that the patient is still congested.”
After discharge, he said, NT-proBNP levels “should only go down.” So, knowing whether NT-proBNP levels “are stable or increasing” during the med optimization process can help guide diuretic dosing.
HF with comorbidities
The new document includes two new Class I recommendations for patients with HF and both type 2 diabetes and chronic kidney disease based on several recent randomized trials and meta-analyses.
The focused update recommends SGLT2 inhibitors as well as the selective, non-steroidal MRA finerenone (Kerendia) in HF patients with CKD and type-2 diabetes. Both Class I recommendations are supported by a level of evidence A.
The SGLT2 indication is based on DAPA-CKD and EMPA-KIDNEY plus meta-analyses that included those trials along with others. The recommendation for finerenone derives from the FIDELIO-DKD and FIGARO-DKD trials and a pooled analysis of the two studies.
The 2023 focused update also accounts for new clinical-trial insights for patients with HF and iron deficiency. The 2021 document featured recommendations for the diagnosis and iron-repletion therapy in such cases, but only as Class IIa or at lower low levels of evidence. The focused update considers more recent studies, especially IRONMAN and some meta-analyses.
The 2023 document indicates intravenous iron supplementation for symptomatic patients with iron deficiency and either HFrEF or HFmrEF to improve symptoms and quality of life (Class I, level of evidence A), and says it should be considered (Class IIa, level of evidence A) to reduce risk for HF hospitalization.
When the task force assembled to plan the 2023 focused update, Dr. Gardner observed, “the first thing we thought about was the nomenclature around the phenotyping of heart failure.”
Although the 2021 guidelines relied fundamentally on the distinctions between HFrEF, HFmrEF, and HFpEF, it had become apparent to some in the field that some meds, especially the SGLT2 inhibitors, were obscuring their LVEF-based boundaries, at least with respect to drug therapy.
The 2023 document’s developers, Dr. Gardner said, seriously considered changing the three categories to two, that is HFrEF and – to account for all other heart failure – HF with normal ejection fraction (HFnEF).
That didn’t happen, although the proposal was popular within the task force. Any changes to the 2021 document would require a 75% consensus on the matter, Dr. Gardner explained. When the task force took a vote on whether to change the nomenclature, he said, 71% favored the proposal.
Disclosures for members of the task force can be found in a supplement to the published 2023 Focused Update.
A version of this article first appeared on Medscape.com.
FROM THE ESC CONGRESS 2023
Is AFib ablation the fifth pillar in heart failure care? CASTLE-HTx
Recorded Aug. 28, 2023. This transcript has been edited for clarity.
John M. Mandrola, MD: I’m here at the European Society of Cardiology meeting, and I’m very excited to have two colleagues whom I met at the Western Atrial Fibrillation Symposium (Western AFib) and who presented the CASTLE-HTx study. This is Christian Sohns and Philipp Sommer, and the CASTLE-HTx study is very exciting.
Before I get into that, I really want to introduce the concept of atrial fibrillation in heart failure. I like to say that there are two big populations of patients with atrial fibrillation, and the vast majority can be treated slowly with reassurance and education. There is a group of patients who have heart failure who, when they develop atrial fibrillation, can degenerate rapidly. The CASTLE-HTx study looked at catheter ablation versus medical therapy in patients with advanced heart failure.
Christian, why don’t you tell us the top-line results and what you found.
CASTLE-HTx key findings
Christian Sohns, MD, PhD: Thanks, first of all, for mentioning this special cohort of patients in end-stage heart failure, which is very important. The endpoint of the study was a composite of death from any cause or left ventricular assist device (LVAD) implantation and heart transplantation. These are very hard, strong clinical endpoints, not the rate of rehospitalization or something like that.
Catheter ablation was superior to medical therapy alone in terms of this composite endpoint. That was driven by cardiovascular death and all-cause mortality, which highlights the fact that you should always consider atrial fibrillation ablation in the end-stage heart failure cohort. The findings were driven by the fact that we saw left ventricular reverse remodeling and the reduction of atrial fibrillation in these patients.
Dr. Mandrola: Tell me about how it came about. It was conducted at your center. Who were these patients?
Philipp Sommer, MD: As one of the biggest centers for heart transplantations all over Europe, with roughly 100 transplants per year, we had many patients being referred to our center with the questions of whether those patients are eligible for a heart transplantation. Not all of the patients in our study were listed for a transplant, but all of them were admitted in that end-stage heart failure status to evaluate their eligibility for transplant.
If we look at the baseline data of those patients, they had an ejection fraction of 29%. They had a 6-minute walk test as a functional capacity parameter of around 300 m. Approximately two thirds of them were New York Heart Association class III and IV, which is significantly worse than what we saw in the previous studies dealing with heart failure patients.
I think overall, if you also look at NT-proBNP levels, this is a really sick patient population where some people might doubt if they should admit and refer those patients for an ablation procedure. Therefore, it’s really interesting and fascinating to see the results.
Dr. Mandrola: I did read in the manuscript, and I heard from you, that these were recruited as outpatients. So they were stable outpatients who were referred to the center for consideration of an LVAD or transplant?
Dr. Sohns: The definition of stability is very difficult in these patients because they have hospital stays, they have a history of drug therapy, and they have a history of interventions also behind them – not atrial fibrillation ablation, but others. I think these patients are referred because the referring physicians are done with the case. They can no longer offer any option to the patients other than surgical treatment, assist device, pump implantation, or transplantation.
If you look at the guidelines, they do not comment on atrial fibrillation ablation in this cohort of patients. Also, they have different recommendations between the American societies and the European societies regarding what is end-stage heart failure and how to treat these patients. Therefore, it was a big benefit of CASTLE-HTx that we randomized a cohort of patients with advanced end-stage heart failure.
How can AFib ablation have such big, early effects?
Dr. Mandrola: These are very clinically significant findings, with large effect sizes and very early separation of the Kaplan-Meier curves. How do you explain how dramatic an effect that is, and how early of an effect?
Dr. Sommer: That’s one of the key questions at the end of the day. I think our job basically was to provide the data and to ensure that the data are clean and that it’s all perfectly done. The interpretation of these data is really kind of difficult, although we do not have the 100% perfect and obvious explanation why the curves separated so early. Our view on that is that we are talking about a pretty fragile patient population, so little differences like having a tachyarrhythmia of 110 day in, day out or being in sinus rhythm of 60 can make a huge difference. That’s obviously pretty early.
The one that remains in tachyarrhythmia will deteriorate and will require an LVAD after a couple of months, and the one that you may keep in sinus rhythm, even with reduced atrial fibrillation burden – not zero, but reduced atrial fibrillation burden – and improved LV function, all of a sudden this patient will still remain on a low level of being stable, but he or she will remain stable and will not require any surgical interventions for the next 1.5-2 years. If we can manage to do this, just postponing the natural cause of the disease, I think that is a great benefit for the patient.
Dr. Mandrola: One of the things that comes up in our center is that I look at some of these patients and think, there’s no way I can put this patient under general anesthetic and do all of this. Your ablation procedure wasn’t that extensive, was it?
Dr. Sohns: On the one hand, no. On the other hand, yes. You need to take into consideration that it has been performed by experienced physicians with experience in heart failure treatment and atrial fibrillation in heart transplantation centers, though it›s not sure that we can transfer these results one-to-one to all other centers in the world.
It is very clear that we have almost no major complications in these patients. We were able to do these ablation procedures without general anesthesia. We have 60% of patients who had pulmonary vein isolation only and 40% of patients who have PVI and additional therapy. We have a procedure duration of almost 90 minutes during radiofrequency ablation.
We have different categories. When you talk about the different patient cohorts, we also see different stages of myocardial tissue damage, which will be part of another publication for sure. It is, in part, surprising how normal some of the atria were despite having a volume of 180 mL, but they had no fibrosis. That was very interesting.
Dr. Mandrola: How did the persistent vs paroxysmal atrial fibrillation sort out? Were these mostly patients with persistent atrial fibrillation?
Dr. Sommer: Two-thirds were persistent. It would be expected in this patient population that you would not find so many paroxysmal cases. I think it›s very important what Christian was just mentioning that when we discussed the trial design, we were anticipating problems with the sedation, for example. With the follow-up of those procedures, would they decompensate because of the fluid that you have to deliver during such a procedure.
We were quite surprised at the end of the day that the procedures were quite straightforward. Fortunately, we had no major complications. I think there were four complications in the 100 ablated patients. I think we were really positive about how the procedures turned out.
I should mention that one of the exclusion criteria was a left atrial diameter of about 60 mm. The huge ones may be very diseased, and maybe the hopeless ones were excluded from the study. Below 60 mm, we did the ablation.
Rhythm control
Dr. Mandrola: One of my colleagues, who is even more skeptical than me, wanted me to ask you, why wouldn’t you take a patient with persistent atrial fibrillation who had heart failure and just cardiovert and use amiodarone and try and maintain sinus rhythm that way?
Dr. Sohns: It is important to mention that 50% of the patients have already had amiodarone before they were randomized and enrolled for the trial. It might bring you a couple of minutes or a couple of hours [of relief], but the patients would get recurrence.
It was very interesting also, and this is in line with the data from Jason Andrade, who demonstrated that we were able to reduce the percentage of patients with persistent atrial fibrillation to paroxysmal. We did a down-staging of the underlying disease. This is not possible with cardioversion or drugs, for example.
Dr. Sommer: What I really like about that question and that comment is the idea that rhythm control in this subset of patients obviously has a role and an importance. It may be a cardioversion initially, giving amiodarone if they didn’t have that before, and you can keep the patient in sinus rhythm with this therapy, I think we’re reaching the same goal.
I think the critical point to get into the mind of physicians who treat heart failure is that sinus rhythm is beneficial, however you get there. Ablation, of course, as in other studies, is the most powerful tool to get there. Cardioversion can be a really good thing to do; you just have to think about it and consider it.
Dr. Mandrola: I do want to say to everybody that there is a tension sometimes between the heart failure community and the electrophysiology community. I think the ideal situation is that we work together, because I think that we can help with the maintenance of sinus rhythm. The control group mortality at 1 year was 20%, and I’ve heard people say that that’s not advanced heart failure. Advanced heart failure patients have much higher mortality than that. My colleague who is a heart failure specialist was criticizing a selection bias in picking the best patients. How would you answer that?
Dr. Sohns: There are data available from Eurotransplant, for example, that the waiting list mortality is 18%, so I think we are almost in line with this 20% mortality in this conservative group. You cannot generalize it. All these patients have different histories. We have 60% dilated cardiomyopathy and 40% ischemic cardiomyopathy. I think it is a very representative group in contrast to your friend who suggests that it is not.
Dr. Sommer: What I like about the discussion is that some approach us to say that the mortality in the control group is much too high – like, what are you doing with those patients that you create so many endpoints? Then others say that it’s not high enough because that is not end-stage heart failure. Come on! We have a patient cohort that is very well described and very well characterized.
If the label is end-stage heart failure, advanced heart failure, or whatever, they are sicker than the patients that we had in earlier trials. The patients that we treated were mostly excluded from all other trials. We opened the door. We found a clear result. I think everyone can see whatever you like to see.
Dr. Mandrola: What would your take-home message be after having done this trial design, the trial was conducted in your single center, and you come up with these amazing results? What would your message be to the whole community?
Dr. Sohns: Taking into consideration how severely sick these patients are, I can just repeat it: They are one step away from death, more or less, or from surgical intervention that can prolong their life. You should also consider that there are options like atrial fibrillation ablation that can buy time, postpone the natural course, or even in some patients replace the destination therapy. Therefore, in my opinion the next guidelines should recommend that every patient should carefully be checked for sinus rhythm before bringing these patients into the environment of transplantation.
Dr. Sommer: My interpretation is that we have to try to bring into physicians’ minds that besides a well-established and well-documented effect of drug therapy with the fabulous four, we may now have the fabulous five, including an ablation option for patients with atrial fibrillation.
Dr. Mandrola is a clinical electrophysiologist at Baptist Medical Associates, Louisville, Ky. Dr. Sohns is deputy director of the Heart and Diabetes Center NRW, Ruhr University Bochum, Bad Oeynhausen, Germany. Dr. Sommer is professor of cardiology at the Heart and Diabetes Center NRW. Dr. Mandrola reported no conflicts of interest. Dr. Sohns reported receiving research funding from Else Kröner–Fresenius–Stiftung. Dr. Sommer reported consulting with Abbott, Biosense Webster, Boston Scientific, and Medtronic USA.
A version of this article first appeared on Medscape.com.
Recorded Aug. 28, 2023. This transcript has been edited for clarity.
John M. Mandrola, MD: I’m here at the European Society of Cardiology meeting, and I’m very excited to have two colleagues whom I met at the Western Atrial Fibrillation Symposium (Western AFib) and who presented the CASTLE-HTx study. This is Christian Sohns and Philipp Sommer, and the CASTLE-HTx study is very exciting.
Before I get into that, I really want to introduce the concept of atrial fibrillation in heart failure. I like to say that there are two big populations of patients with atrial fibrillation, and the vast majority can be treated slowly with reassurance and education. There is a group of patients who have heart failure who, when they develop atrial fibrillation, can degenerate rapidly. The CASTLE-HTx study looked at catheter ablation versus medical therapy in patients with advanced heart failure.
Christian, why don’t you tell us the top-line results and what you found.
CASTLE-HTx key findings
Christian Sohns, MD, PhD: Thanks, first of all, for mentioning this special cohort of patients in end-stage heart failure, which is very important. The endpoint of the study was a composite of death from any cause or left ventricular assist device (LVAD) implantation and heart transplantation. These are very hard, strong clinical endpoints, not the rate of rehospitalization or something like that.
Catheter ablation was superior to medical therapy alone in terms of this composite endpoint. That was driven by cardiovascular death and all-cause mortality, which highlights the fact that you should always consider atrial fibrillation ablation in the end-stage heart failure cohort. The findings were driven by the fact that we saw left ventricular reverse remodeling and the reduction of atrial fibrillation in these patients.
Dr. Mandrola: Tell me about how it came about. It was conducted at your center. Who were these patients?
Philipp Sommer, MD: As one of the biggest centers for heart transplantations all over Europe, with roughly 100 transplants per year, we had many patients being referred to our center with the questions of whether those patients are eligible for a heart transplantation. Not all of the patients in our study were listed for a transplant, but all of them were admitted in that end-stage heart failure status to evaluate their eligibility for transplant.
If we look at the baseline data of those patients, they had an ejection fraction of 29%. They had a 6-minute walk test as a functional capacity parameter of around 300 m. Approximately two thirds of them were New York Heart Association class III and IV, which is significantly worse than what we saw in the previous studies dealing with heart failure patients.
I think overall, if you also look at NT-proBNP levels, this is a really sick patient population where some people might doubt if they should admit and refer those patients for an ablation procedure. Therefore, it’s really interesting and fascinating to see the results.
Dr. Mandrola: I did read in the manuscript, and I heard from you, that these were recruited as outpatients. So they were stable outpatients who were referred to the center for consideration of an LVAD or transplant?
Dr. Sohns: The definition of stability is very difficult in these patients because they have hospital stays, they have a history of drug therapy, and they have a history of interventions also behind them – not atrial fibrillation ablation, but others. I think these patients are referred because the referring physicians are done with the case. They can no longer offer any option to the patients other than surgical treatment, assist device, pump implantation, or transplantation.
If you look at the guidelines, they do not comment on atrial fibrillation ablation in this cohort of patients. Also, they have different recommendations between the American societies and the European societies regarding what is end-stage heart failure and how to treat these patients. Therefore, it was a big benefit of CASTLE-HTx that we randomized a cohort of patients with advanced end-stage heart failure.
How can AFib ablation have such big, early effects?
Dr. Mandrola: These are very clinically significant findings, with large effect sizes and very early separation of the Kaplan-Meier curves. How do you explain how dramatic an effect that is, and how early of an effect?
Dr. Sommer: That’s one of the key questions at the end of the day. I think our job basically was to provide the data and to ensure that the data are clean and that it’s all perfectly done. The interpretation of these data is really kind of difficult, although we do not have the 100% perfect and obvious explanation why the curves separated so early. Our view on that is that we are talking about a pretty fragile patient population, so little differences like having a tachyarrhythmia of 110 day in, day out or being in sinus rhythm of 60 can make a huge difference. That’s obviously pretty early.
The one that remains in tachyarrhythmia will deteriorate and will require an LVAD after a couple of months, and the one that you may keep in sinus rhythm, even with reduced atrial fibrillation burden – not zero, but reduced atrial fibrillation burden – and improved LV function, all of a sudden this patient will still remain on a low level of being stable, but he or she will remain stable and will not require any surgical interventions for the next 1.5-2 years. If we can manage to do this, just postponing the natural cause of the disease, I think that is a great benefit for the patient.
Dr. Mandrola: One of the things that comes up in our center is that I look at some of these patients and think, there’s no way I can put this patient under general anesthetic and do all of this. Your ablation procedure wasn’t that extensive, was it?
Dr. Sohns: On the one hand, no. On the other hand, yes. You need to take into consideration that it has been performed by experienced physicians with experience in heart failure treatment and atrial fibrillation in heart transplantation centers, though it›s not sure that we can transfer these results one-to-one to all other centers in the world.
It is very clear that we have almost no major complications in these patients. We were able to do these ablation procedures without general anesthesia. We have 60% of patients who had pulmonary vein isolation only and 40% of patients who have PVI and additional therapy. We have a procedure duration of almost 90 minutes during radiofrequency ablation.
We have different categories. When you talk about the different patient cohorts, we also see different stages of myocardial tissue damage, which will be part of another publication for sure. It is, in part, surprising how normal some of the atria were despite having a volume of 180 mL, but they had no fibrosis. That was very interesting.
Dr. Mandrola: How did the persistent vs paroxysmal atrial fibrillation sort out? Were these mostly patients with persistent atrial fibrillation?
Dr. Sommer: Two-thirds were persistent. It would be expected in this patient population that you would not find so many paroxysmal cases. I think it›s very important what Christian was just mentioning that when we discussed the trial design, we were anticipating problems with the sedation, for example. With the follow-up of those procedures, would they decompensate because of the fluid that you have to deliver during such a procedure.
We were quite surprised at the end of the day that the procedures were quite straightforward. Fortunately, we had no major complications. I think there were four complications in the 100 ablated patients. I think we were really positive about how the procedures turned out.
I should mention that one of the exclusion criteria was a left atrial diameter of about 60 mm. The huge ones may be very diseased, and maybe the hopeless ones were excluded from the study. Below 60 mm, we did the ablation.
Rhythm control
Dr. Mandrola: One of my colleagues, who is even more skeptical than me, wanted me to ask you, why wouldn’t you take a patient with persistent atrial fibrillation who had heart failure and just cardiovert and use amiodarone and try and maintain sinus rhythm that way?
Dr. Sohns: It is important to mention that 50% of the patients have already had amiodarone before they were randomized and enrolled for the trial. It might bring you a couple of minutes or a couple of hours [of relief], but the patients would get recurrence.
It was very interesting also, and this is in line with the data from Jason Andrade, who demonstrated that we were able to reduce the percentage of patients with persistent atrial fibrillation to paroxysmal. We did a down-staging of the underlying disease. This is not possible with cardioversion or drugs, for example.
Dr. Sommer: What I really like about that question and that comment is the idea that rhythm control in this subset of patients obviously has a role and an importance. It may be a cardioversion initially, giving amiodarone if they didn’t have that before, and you can keep the patient in sinus rhythm with this therapy, I think we’re reaching the same goal.
I think the critical point to get into the mind of physicians who treat heart failure is that sinus rhythm is beneficial, however you get there. Ablation, of course, as in other studies, is the most powerful tool to get there. Cardioversion can be a really good thing to do; you just have to think about it and consider it.
Dr. Mandrola: I do want to say to everybody that there is a tension sometimes between the heart failure community and the electrophysiology community. I think the ideal situation is that we work together, because I think that we can help with the maintenance of sinus rhythm. The control group mortality at 1 year was 20%, and I’ve heard people say that that’s not advanced heart failure. Advanced heart failure patients have much higher mortality than that. My colleague who is a heart failure specialist was criticizing a selection bias in picking the best patients. How would you answer that?
Dr. Sohns: There are data available from Eurotransplant, for example, that the waiting list mortality is 18%, so I think we are almost in line with this 20% mortality in this conservative group. You cannot generalize it. All these patients have different histories. We have 60% dilated cardiomyopathy and 40% ischemic cardiomyopathy. I think it is a very representative group in contrast to your friend who suggests that it is not.
Dr. Sommer: What I like about the discussion is that some approach us to say that the mortality in the control group is much too high – like, what are you doing with those patients that you create so many endpoints? Then others say that it’s not high enough because that is not end-stage heart failure. Come on! We have a patient cohort that is very well described and very well characterized.
If the label is end-stage heart failure, advanced heart failure, or whatever, they are sicker than the patients that we had in earlier trials. The patients that we treated were mostly excluded from all other trials. We opened the door. We found a clear result. I think everyone can see whatever you like to see.
Dr. Mandrola: What would your take-home message be after having done this trial design, the trial was conducted in your single center, and you come up with these amazing results? What would your message be to the whole community?
Dr. Sohns: Taking into consideration how severely sick these patients are, I can just repeat it: They are one step away from death, more or less, or from surgical intervention that can prolong their life. You should also consider that there are options like atrial fibrillation ablation that can buy time, postpone the natural course, or even in some patients replace the destination therapy. Therefore, in my opinion the next guidelines should recommend that every patient should carefully be checked for sinus rhythm before bringing these patients into the environment of transplantation.
Dr. Sommer: My interpretation is that we have to try to bring into physicians’ minds that besides a well-established and well-documented effect of drug therapy with the fabulous four, we may now have the fabulous five, including an ablation option for patients with atrial fibrillation.
Dr. Mandrola is a clinical electrophysiologist at Baptist Medical Associates, Louisville, Ky. Dr. Sohns is deputy director of the Heart and Diabetes Center NRW, Ruhr University Bochum, Bad Oeynhausen, Germany. Dr. Sommer is professor of cardiology at the Heart and Diabetes Center NRW. Dr. Mandrola reported no conflicts of interest. Dr. Sohns reported receiving research funding from Else Kröner–Fresenius–Stiftung. Dr. Sommer reported consulting with Abbott, Biosense Webster, Boston Scientific, and Medtronic USA.
A version of this article first appeared on Medscape.com.
Recorded Aug. 28, 2023. This transcript has been edited for clarity.
John M. Mandrola, MD: I’m here at the European Society of Cardiology meeting, and I’m very excited to have two colleagues whom I met at the Western Atrial Fibrillation Symposium (Western AFib) and who presented the CASTLE-HTx study. This is Christian Sohns and Philipp Sommer, and the CASTLE-HTx study is very exciting.
Before I get into that, I really want to introduce the concept of atrial fibrillation in heart failure. I like to say that there are two big populations of patients with atrial fibrillation, and the vast majority can be treated slowly with reassurance and education. There is a group of patients who have heart failure who, when they develop atrial fibrillation, can degenerate rapidly. The CASTLE-HTx study looked at catheter ablation versus medical therapy in patients with advanced heart failure.
Christian, why don’t you tell us the top-line results and what you found.
CASTLE-HTx key findings
Christian Sohns, MD, PhD: Thanks, first of all, for mentioning this special cohort of patients in end-stage heart failure, which is very important. The endpoint of the study was a composite of death from any cause or left ventricular assist device (LVAD) implantation and heart transplantation. These are very hard, strong clinical endpoints, not the rate of rehospitalization or something like that.
Catheter ablation was superior to medical therapy alone in terms of this composite endpoint. That was driven by cardiovascular death and all-cause mortality, which highlights the fact that you should always consider atrial fibrillation ablation in the end-stage heart failure cohort. The findings were driven by the fact that we saw left ventricular reverse remodeling and the reduction of atrial fibrillation in these patients.
Dr. Mandrola: Tell me about how it came about. It was conducted at your center. Who were these patients?
Philipp Sommer, MD: As one of the biggest centers for heart transplantations all over Europe, with roughly 100 transplants per year, we had many patients being referred to our center with the questions of whether those patients are eligible for a heart transplantation. Not all of the patients in our study were listed for a transplant, but all of them were admitted in that end-stage heart failure status to evaluate their eligibility for transplant.
If we look at the baseline data of those patients, they had an ejection fraction of 29%. They had a 6-minute walk test as a functional capacity parameter of around 300 m. Approximately two thirds of them were New York Heart Association class III and IV, which is significantly worse than what we saw in the previous studies dealing with heart failure patients.
I think overall, if you also look at NT-proBNP levels, this is a really sick patient population where some people might doubt if they should admit and refer those patients for an ablation procedure. Therefore, it’s really interesting and fascinating to see the results.
Dr. Mandrola: I did read in the manuscript, and I heard from you, that these were recruited as outpatients. So they were stable outpatients who were referred to the center for consideration of an LVAD or transplant?
Dr. Sohns: The definition of stability is very difficult in these patients because they have hospital stays, they have a history of drug therapy, and they have a history of interventions also behind them – not atrial fibrillation ablation, but others. I think these patients are referred because the referring physicians are done with the case. They can no longer offer any option to the patients other than surgical treatment, assist device, pump implantation, or transplantation.
If you look at the guidelines, they do not comment on atrial fibrillation ablation in this cohort of patients. Also, they have different recommendations between the American societies and the European societies regarding what is end-stage heart failure and how to treat these patients. Therefore, it was a big benefit of CASTLE-HTx that we randomized a cohort of patients with advanced end-stage heart failure.
How can AFib ablation have such big, early effects?
Dr. Mandrola: These are very clinically significant findings, with large effect sizes and very early separation of the Kaplan-Meier curves. How do you explain how dramatic an effect that is, and how early of an effect?
Dr. Sommer: That’s one of the key questions at the end of the day. I think our job basically was to provide the data and to ensure that the data are clean and that it’s all perfectly done. The interpretation of these data is really kind of difficult, although we do not have the 100% perfect and obvious explanation why the curves separated so early. Our view on that is that we are talking about a pretty fragile patient population, so little differences like having a tachyarrhythmia of 110 day in, day out or being in sinus rhythm of 60 can make a huge difference. That’s obviously pretty early.
The one that remains in tachyarrhythmia will deteriorate and will require an LVAD after a couple of months, and the one that you may keep in sinus rhythm, even with reduced atrial fibrillation burden – not zero, but reduced atrial fibrillation burden – and improved LV function, all of a sudden this patient will still remain on a low level of being stable, but he or she will remain stable and will not require any surgical interventions for the next 1.5-2 years. If we can manage to do this, just postponing the natural cause of the disease, I think that is a great benefit for the patient.
Dr. Mandrola: One of the things that comes up in our center is that I look at some of these patients and think, there’s no way I can put this patient under general anesthetic and do all of this. Your ablation procedure wasn’t that extensive, was it?
Dr. Sohns: On the one hand, no. On the other hand, yes. You need to take into consideration that it has been performed by experienced physicians with experience in heart failure treatment and atrial fibrillation in heart transplantation centers, though it›s not sure that we can transfer these results one-to-one to all other centers in the world.
It is very clear that we have almost no major complications in these patients. We were able to do these ablation procedures without general anesthesia. We have 60% of patients who had pulmonary vein isolation only and 40% of patients who have PVI and additional therapy. We have a procedure duration of almost 90 minutes during radiofrequency ablation.
We have different categories. When you talk about the different patient cohorts, we also see different stages of myocardial tissue damage, which will be part of another publication for sure. It is, in part, surprising how normal some of the atria were despite having a volume of 180 mL, but they had no fibrosis. That was very interesting.
Dr. Mandrola: How did the persistent vs paroxysmal atrial fibrillation sort out? Were these mostly patients with persistent atrial fibrillation?
Dr. Sommer: Two-thirds were persistent. It would be expected in this patient population that you would not find so many paroxysmal cases. I think it›s very important what Christian was just mentioning that when we discussed the trial design, we were anticipating problems with the sedation, for example. With the follow-up of those procedures, would they decompensate because of the fluid that you have to deliver during such a procedure.
We were quite surprised at the end of the day that the procedures were quite straightforward. Fortunately, we had no major complications. I think there were four complications in the 100 ablated patients. I think we were really positive about how the procedures turned out.
I should mention that one of the exclusion criteria was a left atrial diameter of about 60 mm. The huge ones may be very diseased, and maybe the hopeless ones were excluded from the study. Below 60 mm, we did the ablation.
Rhythm control
Dr. Mandrola: One of my colleagues, who is even more skeptical than me, wanted me to ask you, why wouldn’t you take a patient with persistent atrial fibrillation who had heart failure and just cardiovert and use amiodarone and try and maintain sinus rhythm that way?
Dr. Sohns: It is important to mention that 50% of the patients have already had amiodarone before they were randomized and enrolled for the trial. It might bring you a couple of minutes or a couple of hours [of relief], but the patients would get recurrence.
It was very interesting also, and this is in line with the data from Jason Andrade, who demonstrated that we were able to reduce the percentage of patients with persistent atrial fibrillation to paroxysmal. We did a down-staging of the underlying disease. This is not possible with cardioversion or drugs, for example.
Dr. Sommer: What I really like about that question and that comment is the idea that rhythm control in this subset of patients obviously has a role and an importance. It may be a cardioversion initially, giving amiodarone if they didn’t have that before, and you can keep the patient in sinus rhythm with this therapy, I think we’re reaching the same goal.
I think the critical point to get into the mind of physicians who treat heart failure is that sinus rhythm is beneficial, however you get there. Ablation, of course, as in other studies, is the most powerful tool to get there. Cardioversion can be a really good thing to do; you just have to think about it and consider it.
Dr. Mandrola: I do want to say to everybody that there is a tension sometimes between the heart failure community and the electrophysiology community. I think the ideal situation is that we work together, because I think that we can help with the maintenance of sinus rhythm. The control group mortality at 1 year was 20%, and I’ve heard people say that that’s not advanced heart failure. Advanced heart failure patients have much higher mortality than that. My colleague who is a heart failure specialist was criticizing a selection bias in picking the best patients. How would you answer that?
Dr. Sohns: There are data available from Eurotransplant, for example, that the waiting list mortality is 18%, so I think we are almost in line with this 20% mortality in this conservative group. You cannot generalize it. All these patients have different histories. We have 60% dilated cardiomyopathy and 40% ischemic cardiomyopathy. I think it is a very representative group in contrast to your friend who suggests that it is not.
Dr. Sommer: What I like about the discussion is that some approach us to say that the mortality in the control group is much too high – like, what are you doing with those patients that you create so many endpoints? Then others say that it’s not high enough because that is not end-stage heart failure. Come on! We have a patient cohort that is very well described and very well characterized.
If the label is end-stage heart failure, advanced heart failure, or whatever, they are sicker than the patients that we had in earlier trials. The patients that we treated were mostly excluded from all other trials. We opened the door. We found a clear result. I think everyone can see whatever you like to see.
Dr. Mandrola: What would your take-home message be after having done this trial design, the trial was conducted in your single center, and you come up with these amazing results? What would your message be to the whole community?
Dr. Sohns: Taking into consideration how severely sick these patients are, I can just repeat it: They are one step away from death, more or less, or from surgical intervention that can prolong their life. You should also consider that there are options like atrial fibrillation ablation that can buy time, postpone the natural course, or even in some patients replace the destination therapy. Therefore, in my opinion the next guidelines should recommend that every patient should carefully be checked for sinus rhythm before bringing these patients into the environment of transplantation.
Dr. Sommer: My interpretation is that we have to try to bring into physicians’ minds that besides a well-established and well-documented effect of drug therapy with the fabulous four, we may now have the fabulous five, including an ablation option for patients with atrial fibrillation.
Dr. Mandrola is a clinical electrophysiologist at Baptist Medical Associates, Louisville, Ky. Dr. Sohns is deputy director of the Heart and Diabetes Center NRW, Ruhr University Bochum, Bad Oeynhausen, Germany. Dr. Sommer is professor of cardiology at the Heart and Diabetes Center NRW. Dr. Mandrola reported no conflicts of interest. Dr. Sohns reported receiving research funding from Else Kröner–Fresenius–Stiftung. Dr. Sommer reported consulting with Abbott, Biosense Webster, Boston Scientific, and Medtronic USA.
A version of this article first appeared on Medscape.com.
Heart failure guidelines update: What the ESC got right
This transcript has been edited for clarity.
This is my usual blog, except I am here from the absolutely beautiful city of Amsterdam, where the annual congress of the European Society of Cardiology has been going on.
SGLT2 inhibitors for HFpEF and HFrEF
I’m going to review very briefly the 2023 focused update to the ESC heart failure guidelines. Theresa McDonagh was the first author of this and of the previous ESC or European guidelines. These are a little bit different than the American guidelines, which were presented in 2022. We know that we need an update. The Europeans have gotten ahead of us, and now we have the European update, which I find incredibly well written and it really highlights the areas that I think the takeaways are for the clinicians.
First, we have been seeing now for several years – since 2018 – the benefits of the sodium-glucose cotransporter 2 (SGLT2) inhibitors. Every time we lift the veil on something, there they are in a positive light. We have learned about heart failure with reduced ejection fraction (HFrEF) for both empagliflozin and dapagliflozin. There are very similar results. One population may be enriched with a little of this and a little of that, but the basic messages are the same. In HFrEF, both of these drugs improve outcomes and it happens quickly. You don’t have to wait 1 or 2 years to see this. Within months, and actually within days, you start to see the curves split apart statistically.
The next logical ground was heart failure with preserved ejection fraction (HFpEF). The definition, when we started the HFpEF trials, was 45% or greater. I want the audience to realize that, in the midst of all these trials, we came out – we meaning the American Heart Association, the American College of Cardiology, and the Heart Failure Society – with the new definition of heart failure, which said that true HFpEF is 50% or greater. That in-between zone of 40%-50% or 41%-49% is mRF, or mid-range, what I call middle of the road. I think the Europeans have really emphasized that to us. I believe that those patients really behave much more like a HFrEF population.
Now that we have very positive findings with the SGLT2 inhibitors, both dapagliflozin and empagliflozin, in HFpEF – defined, as I said, as 40% or 45% or greater, not necessarily 50% – with excellent point estimates that just line up, one on top of the other. It doesn’t matter if patients have diabetes or not; the results are exactly the same.
This has been so promising that I am not surprised that the Europeans elevated the SGLT2 inhibitors to a class 1A indication. In the United States in 2022, we thought we were really way ahead by calling it a class 2A indication. Well, now it’s a class 1A indication in Europe, and I have a feeling that the AHA and the ACC are going to start talking about an update because the data are so strong.
Now, we even have data on initiating these drugs in the hospital. EMPULSE was a very large trial about the benefits of starting these drugs in the hospital. You do not have to wait until the patient is in the outpatient setting. You can start it in the hospital.
When? I have no specific day that I start it. I used to try to do a good diuresis first, get the patients somewhat decongested, and then start it. I don’t want to deprive the patients of the benefits of these drugs that happen very early by waiting until the patients are in the outpatient setting.
In the United States, we’ve had some issues with coverage of some of these drugs. In my institution, we now have both on the formulary, and I pick the drug depending upon the patient’s coverage. Medicare pretty much covers most of them. If the patient is older but not yet a Medicare patient, they may have a very large copay. I advise you to get your offices or your health system to look into this so that, when you give the prescription to the patient, whether they’re leaving the hospital or are now in your clinics, they can actually get the drug.
Finerenone and intravenous iron
There is an additional recommendation in these guidelines for finerenone, the mineralocorticoid receptor agonist that I’ve discussed before, that has some really promising data on type 2 diabetes with chronic kidney disease. They have called that a class 1A indication for finerenone. I think there is more to come.
One more: the iron deficiency. Giving intravenous iron actually does improve symptoms and quality of life. I have seen this in my own patients, so I have been very diligent at looking for iron deficiency.
It is a new era. We have more tools, obviously, for our patients. It means one more drug, and that’s always a challenge. We’ve already been doing the pillars of care. This is the fourth pillar of care, but now with a class 1A indication.
Take a look. They’re easy to read. Dr. McDonagh is the first author, and I think they’ve been extremely well done.
Dr. Piña is professor of medicine at Thomas Jefferson University Hospital in Philadelphia. She is a heart failure and cardiac transplantation expert. She disclosed serving as an adviser/consultant to the FDA’s Center for Devices and Radiological Health and has been a volunteer for the American Heart Association since 1982.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
This is my usual blog, except I am here from the absolutely beautiful city of Amsterdam, where the annual congress of the European Society of Cardiology has been going on.
SGLT2 inhibitors for HFpEF and HFrEF
I’m going to review very briefly the 2023 focused update to the ESC heart failure guidelines. Theresa McDonagh was the first author of this and of the previous ESC or European guidelines. These are a little bit different than the American guidelines, which were presented in 2022. We know that we need an update. The Europeans have gotten ahead of us, and now we have the European update, which I find incredibly well written and it really highlights the areas that I think the takeaways are for the clinicians.
First, we have been seeing now for several years – since 2018 – the benefits of the sodium-glucose cotransporter 2 (SGLT2) inhibitors. Every time we lift the veil on something, there they are in a positive light. We have learned about heart failure with reduced ejection fraction (HFrEF) for both empagliflozin and dapagliflozin. There are very similar results. One population may be enriched with a little of this and a little of that, but the basic messages are the same. In HFrEF, both of these drugs improve outcomes and it happens quickly. You don’t have to wait 1 or 2 years to see this. Within months, and actually within days, you start to see the curves split apart statistically.
The next logical ground was heart failure with preserved ejection fraction (HFpEF). The definition, when we started the HFpEF trials, was 45% or greater. I want the audience to realize that, in the midst of all these trials, we came out – we meaning the American Heart Association, the American College of Cardiology, and the Heart Failure Society – with the new definition of heart failure, which said that true HFpEF is 50% or greater. That in-between zone of 40%-50% or 41%-49% is mRF, or mid-range, what I call middle of the road. I think the Europeans have really emphasized that to us. I believe that those patients really behave much more like a HFrEF population.
Now that we have very positive findings with the SGLT2 inhibitors, both dapagliflozin and empagliflozin, in HFpEF – defined, as I said, as 40% or 45% or greater, not necessarily 50% – with excellent point estimates that just line up, one on top of the other. It doesn’t matter if patients have diabetes or not; the results are exactly the same.
This has been so promising that I am not surprised that the Europeans elevated the SGLT2 inhibitors to a class 1A indication. In the United States in 2022, we thought we were really way ahead by calling it a class 2A indication. Well, now it’s a class 1A indication in Europe, and I have a feeling that the AHA and the ACC are going to start talking about an update because the data are so strong.
Now, we even have data on initiating these drugs in the hospital. EMPULSE was a very large trial about the benefits of starting these drugs in the hospital. You do not have to wait until the patient is in the outpatient setting. You can start it in the hospital.
When? I have no specific day that I start it. I used to try to do a good diuresis first, get the patients somewhat decongested, and then start it. I don’t want to deprive the patients of the benefits of these drugs that happen very early by waiting until the patients are in the outpatient setting.
In the United States, we’ve had some issues with coverage of some of these drugs. In my institution, we now have both on the formulary, and I pick the drug depending upon the patient’s coverage. Medicare pretty much covers most of them. If the patient is older but not yet a Medicare patient, they may have a very large copay. I advise you to get your offices or your health system to look into this so that, when you give the prescription to the patient, whether they’re leaving the hospital or are now in your clinics, they can actually get the drug.
Finerenone and intravenous iron
There is an additional recommendation in these guidelines for finerenone, the mineralocorticoid receptor agonist that I’ve discussed before, that has some really promising data on type 2 diabetes with chronic kidney disease. They have called that a class 1A indication for finerenone. I think there is more to come.
One more: the iron deficiency. Giving intravenous iron actually does improve symptoms and quality of life. I have seen this in my own patients, so I have been very diligent at looking for iron deficiency.
It is a new era. We have more tools, obviously, for our patients. It means one more drug, and that’s always a challenge. We’ve already been doing the pillars of care. This is the fourth pillar of care, but now with a class 1A indication.
Take a look. They’re easy to read. Dr. McDonagh is the first author, and I think they’ve been extremely well done.
Dr. Piña is professor of medicine at Thomas Jefferson University Hospital in Philadelphia. She is a heart failure and cardiac transplantation expert. She disclosed serving as an adviser/consultant to the FDA’s Center for Devices and Radiological Health and has been a volunteer for the American Heart Association since 1982.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
This is my usual blog, except I am here from the absolutely beautiful city of Amsterdam, where the annual congress of the European Society of Cardiology has been going on.
SGLT2 inhibitors for HFpEF and HFrEF
I’m going to review very briefly the 2023 focused update to the ESC heart failure guidelines. Theresa McDonagh was the first author of this and of the previous ESC or European guidelines. These are a little bit different than the American guidelines, which were presented in 2022. We know that we need an update. The Europeans have gotten ahead of us, and now we have the European update, which I find incredibly well written and it really highlights the areas that I think the takeaways are for the clinicians.
First, we have been seeing now for several years – since 2018 – the benefits of the sodium-glucose cotransporter 2 (SGLT2) inhibitors. Every time we lift the veil on something, there they are in a positive light. We have learned about heart failure with reduced ejection fraction (HFrEF) for both empagliflozin and dapagliflozin. There are very similar results. One population may be enriched with a little of this and a little of that, but the basic messages are the same. In HFrEF, both of these drugs improve outcomes and it happens quickly. You don’t have to wait 1 or 2 years to see this. Within months, and actually within days, you start to see the curves split apart statistically.
The next logical ground was heart failure with preserved ejection fraction (HFpEF). The definition, when we started the HFpEF trials, was 45% or greater. I want the audience to realize that, in the midst of all these trials, we came out – we meaning the American Heart Association, the American College of Cardiology, and the Heart Failure Society – with the new definition of heart failure, which said that true HFpEF is 50% or greater. That in-between zone of 40%-50% or 41%-49% is mRF, or mid-range, what I call middle of the road. I think the Europeans have really emphasized that to us. I believe that those patients really behave much more like a HFrEF population.
Now that we have very positive findings with the SGLT2 inhibitors, both dapagliflozin and empagliflozin, in HFpEF – defined, as I said, as 40% or 45% or greater, not necessarily 50% – with excellent point estimates that just line up, one on top of the other. It doesn’t matter if patients have diabetes or not; the results are exactly the same.
This has been so promising that I am not surprised that the Europeans elevated the SGLT2 inhibitors to a class 1A indication. In the United States in 2022, we thought we were really way ahead by calling it a class 2A indication. Well, now it’s a class 1A indication in Europe, and I have a feeling that the AHA and the ACC are going to start talking about an update because the data are so strong.
Now, we even have data on initiating these drugs in the hospital. EMPULSE was a very large trial about the benefits of starting these drugs in the hospital. You do not have to wait until the patient is in the outpatient setting. You can start it in the hospital.
When? I have no specific day that I start it. I used to try to do a good diuresis first, get the patients somewhat decongested, and then start it. I don’t want to deprive the patients of the benefits of these drugs that happen very early by waiting until the patients are in the outpatient setting.
In the United States, we’ve had some issues with coverage of some of these drugs. In my institution, we now have both on the formulary, and I pick the drug depending upon the patient’s coverage. Medicare pretty much covers most of them. If the patient is older but not yet a Medicare patient, they may have a very large copay. I advise you to get your offices or your health system to look into this so that, when you give the prescription to the patient, whether they’re leaving the hospital or are now in your clinics, they can actually get the drug.
Finerenone and intravenous iron
There is an additional recommendation in these guidelines for finerenone, the mineralocorticoid receptor agonist that I’ve discussed before, that has some really promising data on type 2 diabetes with chronic kidney disease. They have called that a class 1A indication for finerenone. I think there is more to come.
One more: the iron deficiency. Giving intravenous iron actually does improve symptoms and quality of life. I have seen this in my own patients, so I have been very diligent at looking for iron deficiency.
It is a new era. We have more tools, obviously, for our patients. It means one more drug, and that’s always a challenge. We’ve already been doing the pillars of care. This is the fourth pillar of care, but now with a class 1A indication.
Take a look. They’re easy to read. Dr. McDonagh is the first author, and I think they’ve been extremely well done.
Dr. Piña is professor of medicine at Thomas Jefferson University Hospital in Philadelphia. She is a heart failure and cardiac transplantation expert. She disclosed serving as an adviser/consultant to the FDA’s Center for Devices and Radiological Health and has been a volunteer for the American Heart Association since 1982.
A version of this article appeared on Medscape.com.
Navigating chronic cough in primary care
Chronic cough took center stage at the European Respiratory Society Congress session titled “Conditions We Are Just Dealing With the Tip of the Iceberg in Primary Care: Frequently Mismanaged Conditions in Primary Health Care.”
“When it comes to chronic cough, general practitioners often feel lost,” Miguel Román Rodríguez, family doctor and an associate professor of family medicine at the University of the Balearic Islands, Palma, Mallorca, Spain, and one of the chairs of the session, said to this news organization.
“GPs are central in diagnosing conditions like chronic cough. We bring something that the specialists don’t bring: the knowledge of the context, of the family, the longitudinal history,” echoed the second chair of the session, Hilary Pinnock, family physician and professor of primary care respiratory medicine at the University of Edinburgh.
Understanding the multifaceted nature of chronic cough
Imran Satia, an assistant professor at McMaster University, Hamilton, Ont., guided attendees at the Milan, Italy, meeting through a comprehensive exploration of chronic cough. The first issue he addressed was the definition of the condition, emphasizing that it is defined by its duration, with chronic cough typically lasting for more than 8 weeks. Prof. Satia pointed out common associations of chronic cough, including asthma, nasal disease, and reflux disease.
Delving into epidemiology, he cited a meta-analysis indicating a global prevalence of approximately 10% in the adult population, with significant regional variability: from 18.1% in Australia to 2.3% in Africa. Notably, the Canadian Longitudinal Study on Aging found an overall prevalence of 16% at baseline. “The most common risk factor was smoke, but even in nonsmokers the prevalence reached 10%,” Prof. Satia added, highlighting that it increased with age and changed depending on location. “The most common associated comorbidities were heart failure and hypertension, but also conditions related to chronic pain, mood, and anxiety,” he explained.
Mental health was identified as a crucial factor in chronic cough, with psychological distress and depressive symptoms emerging as risk factors for developing chronic cough over the next 3 years, contributing to a 20% increased risk.
Effective management strategies
Prof. Satia proposed the use of algorithms to aid in the management of patients with chronic cough in primary care. He introduced a Canadian algorithm that offers specific recommendations for both primary and secondary care.
The algorithm’s primary care assessment, step 1, includes a comprehensive evaluation of the cough history (duration, severity, triggers, nature, location); cardiorespiratory, gastrointestinal, and nasal symptoms; and use of angiotensin-converting enzyme inhibitors and smoking status. Essential diagnostic tests, such as chest radiography (to check for structural disease), complete blood cell count, and spirometry (with or without bronchodilator reversibility), were emphasized. Urgent referral criteria encompassed symptoms like hemoptysis, weight loss, fever, or abnormal chest radiography findings.
“When checking for cough history, GPs should always consider factors like the presence of dry or productive cough, mental health, presence of chronic pain, stroke, and swallowing,” said Prof. Satia, stressing the importance of documenting the impact of chronic cough on quality of life, work life, social life, and family life. “This is something that doctors sometimes do not ask about. They may think that these are not major problems, but acknowledging their importance can help the patient,” he added.
Step 2 of the algorithm focuses on treatment options tailored to specific diagnoses, such as asthma or chronic obstructive pulmonary disease. Prof. Satia urged caution, emphasizing that treatment should only be initiated when evidence of these conditions is present. Additionally, he encouraged early consideration of cough hypersensitivity syndrome when patients exhibit coughing in response to low levels of mechanical stimulation.
Current treatments and future prospects
Prof. Satia presented an overview of existing treatments for chronic cough, outlining their respective advantages and disadvantages. For instance, speech therapy is a patient-led approach with no side effects but entails challenges related to access, costs, and patient motivation. On the other hand, low-dose morphine offers rapid relief but is associated with issues like nausea, stigma, and constipation.
Looking ahead, Prof. Satia shared the results of COUGH-1 and COUGH-2, pivotal phase 3 trials evaluating the oral, peripherally acting P2X3-receptor antagonist gefapixant. This drug, currently approved in Switzerland and Japan, demonstrated a significant reduction in cough frequency, compared with placebo, with rapid and sustained effects. “The estimated relative reduction for 45 mg was 18.45% in COUGH-1 (12 weeks) and 14.64% in COUGH-2 (24 weeks). Of note, cough reduction is very quick and sustained with gefapixant, but a 40% reduction is observed in the placebo arm,” commented Prof. Satia.
Experts unanimously stressed the importance for specialists and GPs of effective communication in managing chronic cough, involving both patients and their families.
“As GPs, we are crucial to manage the common problems, but we are also crucial to spot the needle in the haystack: this is extremely difficult and challenging, and we need support from our colleagues,” Dr. Pinnock concluded.
Prof. Satia reported funding from Merck MSD, AstraZeneca, and GSK; consulting fees from Merck MSD, Genentech, and Respiplus; and speaker fees from AstraZeneca, GSK, and Merck MSD.
A version of this article first appeared on Medscape.com.
Chronic cough took center stage at the European Respiratory Society Congress session titled “Conditions We Are Just Dealing With the Tip of the Iceberg in Primary Care: Frequently Mismanaged Conditions in Primary Health Care.”
“When it comes to chronic cough, general practitioners often feel lost,” Miguel Román Rodríguez, family doctor and an associate professor of family medicine at the University of the Balearic Islands, Palma, Mallorca, Spain, and one of the chairs of the session, said to this news organization.
“GPs are central in diagnosing conditions like chronic cough. We bring something that the specialists don’t bring: the knowledge of the context, of the family, the longitudinal history,” echoed the second chair of the session, Hilary Pinnock, family physician and professor of primary care respiratory medicine at the University of Edinburgh.
Understanding the multifaceted nature of chronic cough
Imran Satia, an assistant professor at McMaster University, Hamilton, Ont., guided attendees at the Milan, Italy, meeting through a comprehensive exploration of chronic cough. The first issue he addressed was the definition of the condition, emphasizing that it is defined by its duration, with chronic cough typically lasting for more than 8 weeks. Prof. Satia pointed out common associations of chronic cough, including asthma, nasal disease, and reflux disease.
Delving into epidemiology, he cited a meta-analysis indicating a global prevalence of approximately 10% in the adult population, with significant regional variability: from 18.1% in Australia to 2.3% in Africa. Notably, the Canadian Longitudinal Study on Aging found an overall prevalence of 16% at baseline. “The most common risk factor was smoke, but even in nonsmokers the prevalence reached 10%,” Prof. Satia added, highlighting that it increased with age and changed depending on location. “The most common associated comorbidities were heart failure and hypertension, but also conditions related to chronic pain, mood, and anxiety,” he explained.
Mental health was identified as a crucial factor in chronic cough, with psychological distress and depressive symptoms emerging as risk factors for developing chronic cough over the next 3 years, contributing to a 20% increased risk.
Effective management strategies
Prof. Satia proposed the use of algorithms to aid in the management of patients with chronic cough in primary care. He introduced a Canadian algorithm that offers specific recommendations for both primary and secondary care.
The algorithm’s primary care assessment, step 1, includes a comprehensive evaluation of the cough history (duration, severity, triggers, nature, location); cardiorespiratory, gastrointestinal, and nasal symptoms; and use of angiotensin-converting enzyme inhibitors and smoking status. Essential diagnostic tests, such as chest radiography (to check for structural disease), complete blood cell count, and spirometry (with or without bronchodilator reversibility), were emphasized. Urgent referral criteria encompassed symptoms like hemoptysis, weight loss, fever, or abnormal chest radiography findings.
“When checking for cough history, GPs should always consider factors like the presence of dry or productive cough, mental health, presence of chronic pain, stroke, and swallowing,” said Prof. Satia, stressing the importance of documenting the impact of chronic cough on quality of life, work life, social life, and family life. “This is something that doctors sometimes do not ask about. They may think that these are not major problems, but acknowledging their importance can help the patient,” he added.
Step 2 of the algorithm focuses on treatment options tailored to specific diagnoses, such as asthma or chronic obstructive pulmonary disease. Prof. Satia urged caution, emphasizing that treatment should only be initiated when evidence of these conditions is present. Additionally, he encouraged early consideration of cough hypersensitivity syndrome when patients exhibit coughing in response to low levels of mechanical stimulation.
Current treatments and future prospects
Prof. Satia presented an overview of existing treatments for chronic cough, outlining their respective advantages and disadvantages. For instance, speech therapy is a patient-led approach with no side effects but entails challenges related to access, costs, and patient motivation. On the other hand, low-dose morphine offers rapid relief but is associated with issues like nausea, stigma, and constipation.
Looking ahead, Prof. Satia shared the results of COUGH-1 and COUGH-2, pivotal phase 3 trials evaluating the oral, peripherally acting P2X3-receptor antagonist gefapixant. This drug, currently approved in Switzerland and Japan, demonstrated a significant reduction in cough frequency, compared with placebo, with rapid and sustained effects. “The estimated relative reduction for 45 mg was 18.45% in COUGH-1 (12 weeks) and 14.64% in COUGH-2 (24 weeks). Of note, cough reduction is very quick and sustained with gefapixant, but a 40% reduction is observed in the placebo arm,” commented Prof. Satia.
Experts unanimously stressed the importance for specialists and GPs of effective communication in managing chronic cough, involving both patients and their families.
“As GPs, we are crucial to manage the common problems, but we are also crucial to spot the needle in the haystack: this is extremely difficult and challenging, and we need support from our colleagues,” Dr. Pinnock concluded.
Prof. Satia reported funding from Merck MSD, AstraZeneca, and GSK; consulting fees from Merck MSD, Genentech, and Respiplus; and speaker fees from AstraZeneca, GSK, and Merck MSD.
A version of this article first appeared on Medscape.com.
Chronic cough took center stage at the European Respiratory Society Congress session titled “Conditions We Are Just Dealing With the Tip of the Iceberg in Primary Care: Frequently Mismanaged Conditions in Primary Health Care.”
“When it comes to chronic cough, general practitioners often feel lost,” Miguel Román Rodríguez, family doctor and an associate professor of family medicine at the University of the Balearic Islands, Palma, Mallorca, Spain, and one of the chairs of the session, said to this news organization.
“GPs are central in diagnosing conditions like chronic cough. We bring something that the specialists don’t bring: the knowledge of the context, of the family, the longitudinal history,” echoed the second chair of the session, Hilary Pinnock, family physician and professor of primary care respiratory medicine at the University of Edinburgh.
Understanding the multifaceted nature of chronic cough
Imran Satia, an assistant professor at McMaster University, Hamilton, Ont., guided attendees at the Milan, Italy, meeting through a comprehensive exploration of chronic cough. The first issue he addressed was the definition of the condition, emphasizing that it is defined by its duration, with chronic cough typically lasting for more than 8 weeks. Prof. Satia pointed out common associations of chronic cough, including asthma, nasal disease, and reflux disease.
Delving into epidemiology, he cited a meta-analysis indicating a global prevalence of approximately 10% in the adult population, with significant regional variability: from 18.1% in Australia to 2.3% in Africa. Notably, the Canadian Longitudinal Study on Aging found an overall prevalence of 16% at baseline. “The most common risk factor was smoke, but even in nonsmokers the prevalence reached 10%,” Prof. Satia added, highlighting that it increased with age and changed depending on location. “The most common associated comorbidities were heart failure and hypertension, but also conditions related to chronic pain, mood, and anxiety,” he explained.
Mental health was identified as a crucial factor in chronic cough, with psychological distress and depressive symptoms emerging as risk factors for developing chronic cough over the next 3 years, contributing to a 20% increased risk.
Effective management strategies
Prof. Satia proposed the use of algorithms to aid in the management of patients with chronic cough in primary care. He introduced a Canadian algorithm that offers specific recommendations for both primary and secondary care.
The algorithm’s primary care assessment, step 1, includes a comprehensive evaluation of the cough history (duration, severity, triggers, nature, location); cardiorespiratory, gastrointestinal, and nasal symptoms; and use of angiotensin-converting enzyme inhibitors and smoking status. Essential diagnostic tests, such as chest radiography (to check for structural disease), complete blood cell count, and spirometry (with or without bronchodilator reversibility), were emphasized. Urgent referral criteria encompassed symptoms like hemoptysis, weight loss, fever, or abnormal chest radiography findings.
“When checking for cough history, GPs should always consider factors like the presence of dry or productive cough, mental health, presence of chronic pain, stroke, and swallowing,” said Prof. Satia, stressing the importance of documenting the impact of chronic cough on quality of life, work life, social life, and family life. “This is something that doctors sometimes do not ask about. They may think that these are not major problems, but acknowledging their importance can help the patient,” he added.
Step 2 of the algorithm focuses on treatment options tailored to specific diagnoses, such as asthma or chronic obstructive pulmonary disease. Prof. Satia urged caution, emphasizing that treatment should only be initiated when evidence of these conditions is present. Additionally, he encouraged early consideration of cough hypersensitivity syndrome when patients exhibit coughing in response to low levels of mechanical stimulation.
Current treatments and future prospects
Prof. Satia presented an overview of existing treatments for chronic cough, outlining their respective advantages and disadvantages. For instance, speech therapy is a patient-led approach with no side effects but entails challenges related to access, costs, and patient motivation. On the other hand, low-dose morphine offers rapid relief but is associated with issues like nausea, stigma, and constipation.
Looking ahead, Prof. Satia shared the results of COUGH-1 and COUGH-2, pivotal phase 3 trials evaluating the oral, peripherally acting P2X3-receptor antagonist gefapixant. This drug, currently approved in Switzerland and Japan, demonstrated a significant reduction in cough frequency, compared with placebo, with rapid and sustained effects. “The estimated relative reduction for 45 mg was 18.45% in COUGH-1 (12 weeks) and 14.64% in COUGH-2 (24 weeks). Of note, cough reduction is very quick and sustained with gefapixant, but a 40% reduction is observed in the placebo arm,” commented Prof. Satia.
Experts unanimously stressed the importance for specialists and GPs of effective communication in managing chronic cough, involving both patients and their families.
“As GPs, we are crucial to manage the common problems, but we are also crucial to spot the needle in the haystack: this is extremely difficult and challenging, and we need support from our colleagues,” Dr. Pinnock concluded.
Prof. Satia reported funding from Merck MSD, AstraZeneca, and GSK; consulting fees from Merck MSD, Genentech, and Respiplus; and speaker fees from AstraZeneca, GSK, and Merck MSD.
A version of this article first appeared on Medscape.com.
FROM ERS 2023