User login
A 69-year-old woman with double vision and lower-extremity weakness
A 69-year-old woman was admitted to the hospital with double vision, weakness in the lower extremities, sensory loss, pain, and falls. Her symptoms started with sudden onset of horizontal diplopia 6 weeks before, followed by gradually worsening lower-extremity weakness, as well as ataxia and patchy and bilateral radicular burning leg pain more pronounced on the right. Her medical history included narcolepsy, obstructive sleep apnea, hypertension, hyperlipidemia, and bilateral knee replacements for osteoarthritis.
Neurologic examination showed inability to abduct the right eye, bilateral hip flexion weakness, decreased pinprick response, decreased proprioception, and diminished muscle stretch reflexes in the lower extremities. Magnetic resonance imaging (MRI) of the brain without contrast and magnetic resonance angiography of the brain and carotid arteries showed no evidence of acute stroke. No abnormalities were noted on electrocardiography and echocardiography.
A diagnosis of idiopathic peripheral neuropathy was made, and outpatient physical therapy was recommended. Over the subsequent 2 weeks, her condition declined to the point where she needed a walker. She continued to have worsening leg weakness with falls, prompting hospital readmission.
INITIAL EVALUATION
In addition to her diplopia and weakness, she said she had lost 15 pounds since the onset of symptoms and had experienced symptoms suggesting urinary retention.
Physical examination
Her temperature was 37°C (98.6°F), heart rate 79 beats per minute, blood pressure 117/86 mm Hg, respiratory rate 14 breaths per minute, and oxygen saturation 98% on room air. Examination of the head, neck, heart, lung, abdomen, lymph nodes, and extremities yielded nothing remarkable except for chronic venous changes in the lower extremities.
The neurologic examination showed incomplete lateral gaze bilaterally (cranial nerve VI dysfunction). Strength in the upper extremities was normal. In the legs, the Medical Research Council scale score for proximal muscle strength was 2 to 3 out of 5, and for distal muscles 3 to 4 out of 5, with the right side worse than the left and flexors and extensors affected equally. Muscle stretch reflexes were absent in both lower extremities and the left upper extremity, but intact in the right upper extremity. No abnormal corticospinal tract reflexes were elicited.
Sensory testing revealed diminished pin-prick perception in a length-dependent fashion in the lower extremities, reduced 50% compared with the hands. Gait could not be assessed due to weakness.
Initial laboratory testing
Results of initial laboratory tests—complete blood cell count, complete metabolic panel, erythrocyte sedimentation rate, C-reactive protein, thyroid-stimulating hormone, and hemoglobin A1c—were unremarkable.
FURTHER EVALUATION AND DIFFERENTIAL DIAGNOSIS
1. Which of the following is the most likely diagnosis at this point?
- Cerebral infarction
- Guillain-Barré syndrome
- Progressive polyneuropathy
- Transverse myelitis
- Polyradiculopathy
In the absence of definitive diagnostic tests, all of the above options were considered in the differential diagnosis for this patient.
Cerebral infarction
Although acute-onset diplopia can be explained by brainstem stroke involving cranial nerve nuclei or their projections, the onset of diplopia with progressive bilateral lower-extremity weakness makes stroke unlikely. Flaccid paralysis, areflexia of the lower extremities, and sensory involvement can also be caused by acute anterior spinal artery occlusion leading to spinal cord infarction; however, the deficits are usually maximal at onset.
Guillain-Barré syndrome
The combination of acute-subacute progressive ascending weakness, sensory involvement, and diminished or absent reflexes is typical of Guillain-Barré syndrome. Cranial nerve involvement can overlap with the more typical features of the syndrome. However, most patients reach the nadir of their disease by 4 weeks after initial symptom onset, even without treatment.1 This patient’s condition continued to worsen over 8 weeks. In addition, the asymmetric lower-extremity weakness and sparing of the arms are atypical for Guillain-Barré syndrome.
Given the progression of symptoms, chronic inflammatory demyelinating polyneuropathy is also a consideration, typically presenting as a relapsing or progressive neuropathy in proximal and distal muscles and worsening over at least an 8-week period.2
The initial workup for Guillain-Barré syndrome or chronic inflammatory demyelinating polyneuropathy includes lumbar puncture to assess for albuminocytologic dissociation (elevated protein with normal white blood cell count) in cerebrospinal fluid (CSF), and electromyography (EMG) to assess for neurophysiologic evidence of peripheral nerve demyelination. In Miller-Fisher syndrome, a rare variant of Guillain-Barré syndrome characterized by ataxia, ophthalmoparesis, and areflexia, serum ganglioside antibodies to GQ1b are found in over 90% of patients.3,4 Although MRI of the spine is not necessary to diagnose Guillain-Barré syndrome, it is often done to exclude other causes of lower-extremity weakness such as spinal cord or cauda equina compression that would require urgent neurosurgical consultation. MRI can support the diagnosis of Guillain-Barré syndrome when it reveals enhancement of the spinal nerve roots or cauda equina.
Other polyneuropathies
Polyneuropathy is caused by a variety of diseases that affect the function of peripheral motor, sensory, or autonomic nerves. The differential diagnosis is broad and involves inflammatory diseases (including autoimmune and paraneoplastic causes), hereditary disorders, infection, toxicity, and ischemic and nutritional deficiencies.5 Polyneuropathy can present in a distal-predominant, generalized, or asymmetric pattern involving individual nerve trunks termed “mononeuropathy multiplex,” as in our patient’s presentation. The initial workup includes EMG and a battery of serologic tests. In cases of severe and progressive polyneuropathy, nerve biopsy can assess for the presence of vasculitis, amyloidosis, and paraprotein deposition.
Transverse myelitis
Transverse myelitis is an inflammatory myelopathy that usually presents with acute or subacute weakness of the upper extremities or lower extremities, or both, corresponding to the level of the lesion, hyperreflexia, bladder and bowel dysfunction, spinal level of sensory loss, and autonomic involvement.6 The differential diagnosis of acute myelopathy includes:
- Infection (eg, herpes simplex virus, West Nile virus, Lyme disease, Mycoplasma pneumoniae, human immunodeficiency virus)
- Systemic inflammatory disease (systemic lupus erythematosus, sarcoidosis, Sjögren syndrome, scleroderma, paraneoplastic syndrome)
- Central nervous system demyelinating disease (acute disseminated encephalomyelitis, multiple sclerosis, neuromyelitis optica)
- Vascular malformation (dural arteriovenous fistula)
- Compression due to tumor, bleeding, disc herniation, infection, or abscess.
The workup involves laboratory tests to exclude systemic inflammatory and infectious causes, as well as MRI of the spine with and without contrast to identify a causative lesion. Lumbar puncture and CSF analysis may show pleocytosis, elevated protein concentration, and increased intrathecal immunoglobulin G (IgG) index.7
Although our patient’s presentation with subacute lower-extremity weakness, sensory changes, and bladder dysfunction were consistent with transverse myelitis, her cranial nerve abnormalities would be atypical for it.
Polyradiculopathy
Polyradiculopathy has many possible causes. In the United States, the most common causes are lumbar spondylosis, lumbar canal stenosis, and diabetic polyradiculoneuropathy.
When multiple spinal segments are affected, leptomeningeal disease involving the arachnoid and pia mater should be considered. Causes include malignant invasion, inflammatory cell accumulation, and protein deposition, leading to patchy but widespread dysfunction of spinal nerve roots and cranial nerves. Specific causes are myriad and include carcinomatous meningitis,8 syphilis, tuberculosis, sarcoidosis, and paraproteinemias. CSF and MRI changes are often nonspecific, leading to the need for meningeal biopsy for diagnosis.
CASE CONTINUED
During her hospitalization, our patient developed acute right upper and lower facial weakness consistent with peripheral facial mononeuropathy. Bilateral lower-extremity weakness progressed to disabling paraparesis.
She underwent lumbar puncture and CSF analysis (Table 1). The most notable findings were significant pleocytosis (72% lymphocytic predominance), protein elevation, and elevated IgG index (indicative of elevated intrathecal immunoglobulin synthesis in the central nervous system). Viral, bacterial, and fungal studies were negative. Guillain-Barré syndrome, other polyneuropathies, and spinal cord infarction would not be expected with these CSF features.
Surface EMG demonstrated normal sensory responses, and needle EMG showed chronic and active motor axon loss in the L3 and S1 root distributions, suggesting polyradiculopathy without polyneuropathy. These findings would not be expected in typical acute transverse myelitis but could be seen with spinal cord infarction.
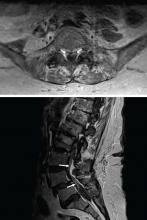
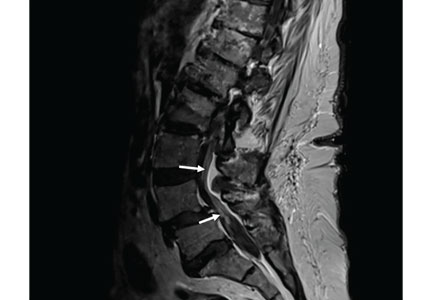
MRI of the entire spine with and without contrast showed cauda equina nerve root thickening and enhancement, especially involving the L5 and S1 roots (Figure 1). The spinal cord appeared normal. These findings further supported polyradiculopathy and a leptomeningeal process.
Further evaluation included chest radiography, erythrocyte sedimentation rate, C-reactive protein, hemoglobin A1c, human immunodeficiency virus testing, antinuclear antibody, antineutrophil cytoplasmic antibody, extractable nuclear antibody, GQ1b antibody, serum and CSF paraneoplastic panels, levels of vitamin B1, B12, and B6, copper, and ceruloplasmin, and a screen for heavy metals. All results were within normal ranges.
ESTABLISHING THE DIAGNOSIS
Serum monoclonal protein analysis with immunofixation revealed IgM kappa monoclonal gammopathy with an IgM level of 1,570 (reference range 53–334 mg/dL) and M-spike 0.75 (0.00 mg/dL), serum free kappa light chains 61.1 (3.30–19.40 mg/L), lambda 9.3 (5.7–26.3 mg/L), and kappa-lambda ratio 6.57 (0.26–1.65).
2. Which is the best next step in this patient’s neurologic evaluation?
- Test CSF angiotensin-converting enzyme level
- CSF cytology
- Meningeal biopsy
- Peripheral nerve biopsy
Given the high suspicion for malignancy, CSF cytology was performed and showed increased numbers of mononuclear chronic inflammatory cells, including a mixture of lymphocytes and monocytes, favoring a reactive lymphoid pleocytosis. Flow cytometry indicated the presence of a monoclonal, CD5- and CD10- negative, B-cell lymphoproliferative disorder. The immunophenotypic findings were not specific for a single diagnosis. The differential diagnosis included marginal zone lymphoma and lymphoplasmacytic lymphoma.
3. Given the presence of serum IgM monoclonal gammopathy in this patient, which is the most likely diagnosis?
- Neurosarcoidosis
- Multiple myeloma
- Waldenström macroglobulinemia
- Carcinomatous meningitis
Study of bone marrow biopsy demonstrated limited bone marrow involvement (1%) by a lymphoproliferative disorder with plasmacytoid features, and DNA testing detected an MYD88 L265P mutation, reported to be present in 90% of patients with Waldenström macroglobulinemia.9 This finding confirmed the diagnosis of Waldenström macroglobulinemia with central nervous system involvement. Our patient began therapy with rituximab and methotrexate, which resulted in some improvement in strength, gait, and vision.
WALDENSTRÖM MACROGLOBULINEMIA AND BING-NEEL SYNDROME
Waldenström macroglobulinemia is a lymphoplasmacytic lymphoma associated with a monoclonal IgM protein.10 It is considered a paraproteinemic disorder, similar to multiple myeloma. The presenting symptoms and complications are related to direct tumor infiltration, hyperviscosity syndrome, and deposition of IgM in various tissues.11,12
Waldenström macroglobulinemia is usually indolent, and treatment is reserved for patients with symptoms.13,14 It includes rituximab, usually in combination with chemotherapy or other targeted agents.15,16
Paraneoplastic antibody-mediated polyneuropathy may occur in these patients. However, the pattern is usually symmetrical clinically, with demyelination on EMG, and is not associated with cranial nerve or meningeal involvement. Management with plasmapheresis, corticosteroids, and intravenous immunoglobulin has not been shown to be effective.17
Involvement of the central nervous system as a complication of Waldenström macroglobulinemia has been described as Bing-Neel syndrome. It can present as diffuse malignant cell infiltration of the leptomeningeal space, white matter, or spinal cord, or in a tumoral form presenting as intraparenchymal masses or nodular lesions. The distinction between the tumoral and diffuse forms is based primarily on imaging findings.18
In a report of 44 patients with Bing-Neel syndrome, 36% presented with the disorder as the initial manifestation of Waldenström macroglobulinemia.18 The primary presenting symptoms were imbalance and gait difficulty (48%) and cranial nerve involvement (36%), which presented as predominantly facial or oculomotor nerve palsy. Cauda equina syndrome with motor involvement (seen in our patient) occurred in 14% of patients. Other presenting symptoms included cognitive impairment, sensory deficits, headache, dysarthria, aphasia, and seizures.
LEARNING POINTS
The differential diagnosis for patients presenting with multifocal neurologic symptoms can be broad, and a systematic approach to the diagnosis is necessary. Localizing the lesion is important in determining the diagnosis for patients presenting with neurologic symptoms. The process of localization begins with taking the history, is further refined during the examination, and is confirmed with diagnostic studies. Atypical presentations of relatively common neurologic diseases such as Guillain-Barré syndrome, transverse myelitis, and peripheral polyneuropathy do occur, but uncommon diagnoses need to be considered when support for the initial diagnosis is lacking.
- Fokke C, van den Berg B, Drenthen J, Walgaard C, van Doorn PA, Jacobs BC. Diagnosis of Guillain-Barre syndrome and validation of Brighton criteria. Brain 2014; 137(Pt 1):33–43. doi:10.1093/brain/awt285
- Mathey EK, Park SB, Hughes RA, et al. Chronic inflammatory demyelinating polyradiculoneuropathy: from pathology to phenotype. J Neurol Neurosurg Psychiatry 2015; 86(9):973–985. doi:10.1136/jnnp-2014-309697
- Chiba A, Kusunoki S, Obata H, Machinami R, Kanazawa I. Serum anti-GQ1b IgG antibody is associated with ophthalmoplegia in Miller Fisher syndrome and Guillain-Barré syndrome: clinical and immunohistochemical studies. Neurology 1993; 43(10):1911–1917. pmid:8413947
- Teener J. Miller Fisher’s syndrome. Semin Neurol 2012; 32(5):512–516. doi:10.1055/s-0033-1334470
- Watson JC, Dyck PJ. Peripheral neuropathy: a practical approach to diagnosis and symptom management. Mayo Clin Proc 2015; 90(7):940–951. doi:10.1016/j.mayocp.2015.05.004
- Greenberg BM. Treatment of acute transverse myelitis and its early complications. Continuum (Minneap Minn) 2011; 17(4):733–743. doi:10.1212/01.CON.0000403792.36161.f5
- West TW. Transverse myelitis—a review of the presentation, diagnosis, and initial management. Discov Med 2013; 16(88):167–177. pmid:24099672
- Le Rhun E, Taillibert S, Chamberlain MC. Carcinomatous meningitis: leptomeningeal metastases in solid tumors. Surg Neurol Int 2013; 4(suppl 4):S265–S288. doi:10.4103/2152-7806.111304
- Treon SP, Xu L, Yang G, et al. MYD88 L265P somatic mutation in Waldenström’s macroglobulinemia. N Engl J Med 2012; 367(9):826–833. doi:10.1056/NEJMoa1200710
- Owen RG, Treon SP, Al-Katib A, et al. Clinicopathological definition of Waldenstrom’s macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom’s Macroglobulinemia. Semin Oncol 2003; 30(2):110–115. doi:10.1053/sonc.2003.50082
- Björkholm M, Johansson E, Papamichael D, et al. Patterns of clinical presentation, treatment, and outcome in patients with Waldenstrom’s macroglobulinemia: a two-institution study. Semin Oncol 2003; 30(2):226–230. doi:10.1053/sonc.2003.50054
- Rison RA, Beydoun SR. Paraproteinemic neuropathy: a practical review. BMC Neurol 2016; 16:13. doi:10.1186/s12883-016-0532-4
- Kyle RA, Benson J, Larson D, et al. IgM monoclonal gammopathy of undetermined significance and smoldering Waldenström’s macroglobulinemia. Clin Lymphoma Myeloma 2009; 9(1):17–18. doi:10.3816/CLM.2009.n.002
- Kyle RA, Benson JT, Larson DR, et al. Progression in smoldering Waldenstrom macroglobulinemia: long-term results. Blood 2012; 119(19):4462–4466. doi:10.1182/blood-2011-10-384768
- Leblond V, Kastritis E, Advani R, et al. Treatment recommendations from the Eighth International Workshop on Waldenström’s macroglobulinemia. Blood 2016; 128(10):1321–1328. doi:10.1182/blood-2016-04-711234
- Kapoor P, Ansell SM, Fonseca R, et al. Diagnosis and management of Waldenström macroglobulinemia: Mayo stratification of macroglobulinemia and risk-adapted therapy (mSMART) guidelines 2016. JAMA Oncol 2017; 3(9):1257–1265. doi:10.1001/jamaoncol.2016.5763
- D’Sa S, Kersten MJ, Castillo JJ, et al. Investigation and management of IgM and Waldenström-associated peripheral neuropathies: recommendations from the IWWM-8 consensus panel. Br J Haematol 2017; 176(5):728–742. doi:10.1111/bjh.14492
- Simon L, Fitsiori A, Lemal R, et al. Bing-Neel syndrome, a rare complication of Waldenström macroglobulinemia: analysis of 44 cases and review of the literature. A study on behalf of the French Innovative Leukemia Organization (FILO). Haematologica 2015; 100(12):1587–1594. doi:10.3324/haematol.2015.133744
A 69-year-old woman was admitted to the hospital with double vision, weakness in the lower extremities, sensory loss, pain, and falls. Her symptoms started with sudden onset of horizontal diplopia 6 weeks before, followed by gradually worsening lower-extremity weakness, as well as ataxia and patchy and bilateral radicular burning leg pain more pronounced on the right. Her medical history included narcolepsy, obstructive sleep apnea, hypertension, hyperlipidemia, and bilateral knee replacements for osteoarthritis.
Neurologic examination showed inability to abduct the right eye, bilateral hip flexion weakness, decreased pinprick response, decreased proprioception, and diminished muscle stretch reflexes in the lower extremities. Magnetic resonance imaging (MRI) of the brain without contrast and magnetic resonance angiography of the brain and carotid arteries showed no evidence of acute stroke. No abnormalities were noted on electrocardiography and echocardiography.
A diagnosis of idiopathic peripheral neuropathy was made, and outpatient physical therapy was recommended. Over the subsequent 2 weeks, her condition declined to the point where she needed a walker. She continued to have worsening leg weakness with falls, prompting hospital readmission.
INITIAL EVALUATION
In addition to her diplopia and weakness, she said she had lost 15 pounds since the onset of symptoms and had experienced symptoms suggesting urinary retention.
Physical examination
Her temperature was 37°C (98.6°F), heart rate 79 beats per minute, blood pressure 117/86 mm Hg, respiratory rate 14 breaths per minute, and oxygen saturation 98% on room air. Examination of the head, neck, heart, lung, abdomen, lymph nodes, and extremities yielded nothing remarkable except for chronic venous changes in the lower extremities.
The neurologic examination showed incomplete lateral gaze bilaterally (cranial nerve VI dysfunction). Strength in the upper extremities was normal. In the legs, the Medical Research Council scale score for proximal muscle strength was 2 to 3 out of 5, and for distal muscles 3 to 4 out of 5, with the right side worse than the left and flexors and extensors affected equally. Muscle stretch reflexes were absent in both lower extremities and the left upper extremity, but intact in the right upper extremity. No abnormal corticospinal tract reflexes were elicited.
Sensory testing revealed diminished pin-prick perception in a length-dependent fashion in the lower extremities, reduced 50% compared with the hands. Gait could not be assessed due to weakness.
Initial laboratory testing
Results of initial laboratory tests—complete blood cell count, complete metabolic panel, erythrocyte sedimentation rate, C-reactive protein, thyroid-stimulating hormone, and hemoglobin A1c—were unremarkable.
FURTHER EVALUATION AND DIFFERENTIAL DIAGNOSIS
1. Which of the following is the most likely diagnosis at this point?
- Cerebral infarction
- Guillain-Barré syndrome
- Progressive polyneuropathy
- Transverse myelitis
- Polyradiculopathy
In the absence of definitive diagnostic tests, all of the above options were considered in the differential diagnosis for this patient.
Cerebral infarction
Although acute-onset diplopia can be explained by brainstem stroke involving cranial nerve nuclei or their projections, the onset of diplopia with progressive bilateral lower-extremity weakness makes stroke unlikely. Flaccid paralysis, areflexia of the lower extremities, and sensory involvement can also be caused by acute anterior spinal artery occlusion leading to spinal cord infarction; however, the deficits are usually maximal at onset.
Guillain-Barré syndrome
The combination of acute-subacute progressive ascending weakness, sensory involvement, and diminished or absent reflexes is typical of Guillain-Barré syndrome. Cranial nerve involvement can overlap with the more typical features of the syndrome. However, most patients reach the nadir of their disease by 4 weeks after initial symptom onset, even without treatment.1 This patient’s condition continued to worsen over 8 weeks. In addition, the asymmetric lower-extremity weakness and sparing of the arms are atypical for Guillain-Barré syndrome.
Given the progression of symptoms, chronic inflammatory demyelinating polyneuropathy is also a consideration, typically presenting as a relapsing or progressive neuropathy in proximal and distal muscles and worsening over at least an 8-week period.2
The initial workup for Guillain-Barré syndrome or chronic inflammatory demyelinating polyneuropathy includes lumbar puncture to assess for albuminocytologic dissociation (elevated protein with normal white blood cell count) in cerebrospinal fluid (CSF), and electromyography (EMG) to assess for neurophysiologic evidence of peripheral nerve demyelination. In Miller-Fisher syndrome, a rare variant of Guillain-Barré syndrome characterized by ataxia, ophthalmoparesis, and areflexia, serum ganglioside antibodies to GQ1b are found in over 90% of patients.3,4 Although MRI of the spine is not necessary to diagnose Guillain-Barré syndrome, it is often done to exclude other causes of lower-extremity weakness such as spinal cord or cauda equina compression that would require urgent neurosurgical consultation. MRI can support the diagnosis of Guillain-Barré syndrome when it reveals enhancement of the spinal nerve roots or cauda equina.
Other polyneuropathies
Polyneuropathy is caused by a variety of diseases that affect the function of peripheral motor, sensory, or autonomic nerves. The differential diagnosis is broad and involves inflammatory diseases (including autoimmune and paraneoplastic causes), hereditary disorders, infection, toxicity, and ischemic and nutritional deficiencies.5 Polyneuropathy can present in a distal-predominant, generalized, or asymmetric pattern involving individual nerve trunks termed “mononeuropathy multiplex,” as in our patient’s presentation. The initial workup includes EMG and a battery of serologic tests. In cases of severe and progressive polyneuropathy, nerve biopsy can assess for the presence of vasculitis, amyloidosis, and paraprotein deposition.
Transverse myelitis
Transverse myelitis is an inflammatory myelopathy that usually presents with acute or subacute weakness of the upper extremities or lower extremities, or both, corresponding to the level of the lesion, hyperreflexia, bladder and bowel dysfunction, spinal level of sensory loss, and autonomic involvement.6 The differential diagnosis of acute myelopathy includes:
- Infection (eg, herpes simplex virus, West Nile virus, Lyme disease, Mycoplasma pneumoniae, human immunodeficiency virus)
- Systemic inflammatory disease (systemic lupus erythematosus, sarcoidosis, Sjögren syndrome, scleroderma, paraneoplastic syndrome)
- Central nervous system demyelinating disease (acute disseminated encephalomyelitis, multiple sclerosis, neuromyelitis optica)
- Vascular malformation (dural arteriovenous fistula)
- Compression due to tumor, bleeding, disc herniation, infection, or abscess.
The workup involves laboratory tests to exclude systemic inflammatory and infectious causes, as well as MRI of the spine with and without contrast to identify a causative lesion. Lumbar puncture and CSF analysis may show pleocytosis, elevated protein concentration, and increased intrathecal immunoglobulin G (IgG) index.7
Although our patient’s presentation with subacute lower-extremity weakness, sensory changes, and bladder dysfunction were consistent with transverse myelitis, her cranial nerve abnormalities would be atypical for it.
Polyradiculopathy
Polyradiculopathy has many possible causes. In the United States, the most common causes are lumbar spondylosis, lumbar canal stenosis, and diabetic polyradiculoneuropathy.
When multiple spinal segments are affected, leptomeningeal disease involving the arachnoid and pia mater should be considered. Causes include malignant invasion, inflammatory cell accumulation, and protein deposition, leading to patchy but widespread dysfunction of spinal nerve roots and cranial nerves. Specific causes are myriad and include carcinomatous meningitis,8 syphilis, tuberculosis, sarcoidosis, and paraproteinemias. CSF and MRI changes are often nonspecific, leading to the need for meningeal biopsy for diagnosis.
CASE CONTINUED
During her hospitalization, our patient developed acute right upper and lower facial weakness consistent with peripheral facial mononeuropathy. Bilateral lower-extremity weakness progressed to disabling paraparesis.
She underwent lumbar puncture and CSF analysis (Table 1). The most notable findings were significant pleocytosis (72% lymphocytic predominance), protein elevation, and elevated IgG index (indicative of elevated intrathecal immunoglobulin synthesis in the central nervous system). Viral, bacterial, and fungal studies were negative. Guillain-Barré syndrome, other polyneuropathies, and spinal cord infarction would not be expected with these CSF features.
Surface EMG demonstrated normal sensory responses, and needle EMG showed chronic and active motor axon loss in the L3 and S1 root distributions, suggesting polyradiculopathy without polyneuropathy. These findings would not be expected in typical acute transverse myelitis but could be seen with spinal cord infarction.
MRI of the entire spine with and without contrast showed cauda equina nerve root thickening and enhancement, especially involving the L5 and S1 roots (Figure 1). The spinal cord appeared normal. These findings further supported polyradiculopathy and a leptomeningeal process.
Further evaluation included chest radiography, erythrocyte sedimentation rate, C-reactive protein, hemoglobin A1c, human immunodeficiency virus testing, antinuclear antibody, antineutrophil cytoplasmic antibody, extractable nuclear antibody, GQ1b antibody, serum and CSF paraneoplastic panels, levels of vitamin B1, B12, and B6, copper, and ceruloplasmin, and a screen for heavy metals. All results were within normal ranges.
ESTABLISHING THE DIAGNOSIS
Serum monoclonal protein analysis with immunofixation revealed IgM kappa monoclonal gammopathy with an IgM level of 1,570 (reference range 53–334 mg/dL) and M-spike 0.75 (0.00 mg/dL), serum free kappa light chains 61.1 (3.30–19.40 mg/L), lambda 9.3 (5.7–26.3 mg/L), and kappa-lambda ratio 6.57 (0.26–1.65).
2. Which is the best next step in this patient’s neurologic evaluation?
- Test CSF angiotensin-converting enzyme level
- CSF cytology
- Meningeal biopsy
- Peripheral nerve biopsy
Given the high suspicion for malignancy, CSF cytology was performed and showed increased numbers of mononuclear chronic inflammatory cells, including a mixture of lymphocytes and monocytes, favoring a reactive lymphoid pleocytosis. Flow cytometry indicated the presence of a monoclonal, CD5- and CD10- negative, B-cell lymphoproliferative disorder. The immunophenotypic findings were not specific for a single diagnosis. The differential diagnosis included marginal zone lymphoma and lymphoplasmacytic lymphoma.
3. Given the presence of serum IgM monoclonal gammopathy in this patient, which is the most likely diagnosis?
- Neurosarcoidosis
- Multiple myeloma
- Waldenström macroglobulinemia
- Carcinomatous meningitis
Study of bone marrow biopsy demonstrated limited bone marrow involvement (1%) by a lymphoproliferative disorder with plasmacytoid features, and DNA testing detected an MYD88 L265P mutation, reported to be present in 90% of patients with Waldenström macroglobulinemia.9 This finding confirmed the diagnosis of Waldenström macroglobulinemia with central nervous system involvement. Our patient began therapy with rituximab and methotrexate, which resulted in some improvement in strength, gait, and vision.
WALDENSTRÖM MACROGLOBULINEMIA AND BING-NEEL SYNDROME
Waldenström macroglobulinemia is a lymphoplasmacytic lymphoma associated with a monoclonal IgM protein.10 It is considered a paraproteinemic disorder, similar to multiple myeloma. The presenting symptoms and complications are related to direct tumor infiltration, hyperviscosity syndrome, and deposition of IgM in various tissues.11,12
Waldenström macroglobulinemia is usually indolent, and treatment is reserved for patients with symptoms.13,14 It includes rituximab, usually in combination with chemotherapy or other targeted agents.15,16
Paraneoplastic antibody-mediated polyneuropathy may occur in these patients. However, the pattern is usually symmetrical clinically, with demyelination on EMG, and is not associated with cranial nerve or meningeal involvement. Management with plasmapheresis, corticosteroids, and intravenous immunoglobulin has not been shown to be effective.17
Involvement of the central nervous system as a complication of Waldenström macroglobulinemia has been described as Bing-Neel syndrome. It can present as diffuse malignant cell infiltration of the leptomeningeal space, white matter, or spinal cord, or in a tumoral form presenting as intraparenchymal masses or nodular lesions. The distinction between the tumoral and diffuse forms is based primarily on imaging findings.18
In a report of 44 patients with Bing-Neel syndrome, 36% presented with the disorder as the initial manifestation of Waldenström macroglobulinemia.18 The primary presenting symptoms were imbalance and gait difficulty (48%) and cranial nerve involvement (36%), which presented as predominantly facial or oculomotor nerve palsy. Cauda equina syndrome with motor involvement (seen in our patient) occurred in 14% of patients. Other presenting symptoms included cognitive impairment, sensory deficits, headache, dysarthria, aphasia, and seizures.
LEARNING POINTS
The differential diagnosis for patients presenting with multifocal neurologic symptoms can be broad, and a systematic approach to the diagnosis is necessary. Localizing the lesion is important in determining the diagnosis for patients presenting with neurologic symptoms. The process of localization begins with taking the history, is further refined during the examination, and is confirmed with diagnostic studies. Atypical presentations of relatively common neurologic diseases such as Guillain-Barré syndrome, transverse myelitis, and peripheral polyneuropathy do occur, but uncommon diagnoses need to be considered when support for the initial diagnosis is lacking.
A 69-year-old woman was admitted to the hospital with double vision, weakness in the lower extremities, sensory loss, pain, and falls. Her symptoms started with sudden onset of horizontal diplopia 6 weeks before, followed by gradually worsening lower-extremity weakness, as well as ataxia and patchy and bilateral radicular burning leg pain more pronounced on the right. Her medical history included narcolepsy, obstructive sleep apnea, hypertension, hyperlipidemia, and bilateral knee replacements for osteoarthritis.
Neurologic examination showed inability to abduct the right eye, bilateral hip flexion weakness, decreased pinprick response, decreased proprioception, and diminished muscle stretch reflexes in the lower extremities. Magnetic resonance imaging (MRI) of the brain without contrast and magnetic resonance angiography of the brain and carotid arteries showed no evidence of acute stroke. No abnormalities were noted on electrocardiography and echocardiography.
A diagnosis of idiopathic peripheral neuropathy was made, and outpatient physical therapy was recommended. Over the subsequent 2 weeks, her condition declined to the point where she needed a walker. She continued to have worsening leg weakness with falls, prompting hospital readmission.
INITIAL EVALUATION
In addition to her diplopia and weakness, she said she had lost 15 pounds since the onset of symptoms and had experienced symptoms suggesting urinary retention.
Physical examination
Her temperature was 37°C (98.6°F), heart rate 79 beats per minute, blood pressure 117/86 mm Hg, respiratory rate 14 breaths per minute, and oxygen saturation 98% on room air. Examination of the head, neck, heart, lung, abdomen, lymph nodes, and extremities yielded nothing remarkable except for chronic venous changes in the lower extremities.
The neurologic examination showed incomplete lateral gaze bilaterally (cranial nerve VI dysfunction). Strength in the upper extremities was normal. In the legs, the Medical Research Council scale score for proximal muscle strength was 2 to 3 out of 5, and for distal muscles 3 to 4 out of 5, with the right side worse than the left and flexors and extensors affected equally. Muscle stretch reflexes were absent in both lower extremities and the left upper extremity, but intact in the right upper extremity. No abnormal corticospinal tract reflexes were elicited.
Sensory testing revealed diminished pin-prick perception in a length-dependent fashion in the lower extremities, reduced 50% compared with the hands. Gait could not be assessed due to weakness.
Initial laboratory testing
Results of initial laboratory tests—complete blood cell count, complete metabolic panel, erythrocyte sedimentation rate, C-reactive protein, thyroid-stimulating hormone, and hemoglobin A1c—were unremarkable.
FURTHER EVALUATION AND DIFFERENTIAL DIAGNOSIS
1. Which of the following is the most likely diagnosis at this point?
- Cerebral infarction
- Guillain-Barré syndrome
- Progressive polyneuropathy
- Transverse myelitis
- Polyradiculopathy
In the absence of definitive diagnostic tests, all of the above options were considered in the differential diagnosis for this patient.
Cerebral infarction
Although acute-onset diplopia can be explained by brainstem stroke involving cranial nerve nuclei or their projections, the onset of diplopia with progressive bilateral lower-extremity weakness makes stroke unlikely. Flaccid paralysis, areflexia of the lower extremities, and sensory involvement can also be caused by acute anterior spinal artery occlusion leading to spinal cord infarction; however, the deficits are usually maximal at onset.
Guillain-Barré syndrome
The combination of acute-subacute progressive ascending weakness, sensory involvement, and diminished or absent reflexes is typical of Guillain-Barré syndrome. Cranial nerve involvement can overlap with the more typical features of the syndrome. However, most patients reach the nadir of their disease by 4 weeks after initial symptom onset, even without treatment.1 This patient’s condition continued to worsen over 8 weeks. In addition, the asymmetric lower-extremity weakness and sparing of the arms are atypical for Guillain-Barré syndrome.
Given the progression of symptoms, chronic inflammatory demyelinating polyneuropathy is also a consideration, typically presenting as a relapsing or progressive neuropathy in proximal and distal muscles and worsening over at least an 8-week period.2
The initial workup for Guillain-Barré syndrome or chronic inflammatory demyelinating polyneuropathy includes lumbar puncture to assess for albuminocytologic dissociation (elevated protein with normal white blood cell count) in cerebrospinal fluid (CSF), and electromyography (EMG) to assess for neurophysiologic evidence of peripheral nerve demyelination. In Miller-Fisher syndrome, a rare variant of Guillain-Barré syndrome characterized by ataxia, ophthalmoparesis, and areflexia, serum ganglioside antibodies to GQ1b are found in over 90% of patients.3,4 Although MRI of the spine is not necessary to diagnose Guillain-Barré syndrome, it is often done to exclude other causes of lower-extremity weakness such as spinal cord or cauda equina compression that would require urgent neurosurgical consultation. MRI can support the diagnosis of Guillain-Barré syndrome when it reveals enhancement of the spinal nerve roots or cauda equina.
Other polyneuropathies
Polyneuropathy is caused by a variety of diseases that affect the function of peripheral motor, sensory, or autonomic nerves. The differential diagnosis is broad and involves inflammatory diseases (including autoimmune and paraneoplastic causes), hereditary disorders, infection, toxicity, and ischemic and nutritional deficiencies.5 Polyneuropathy can present in a distal-predominant, generalized, or asymmetric pattern involving individual nerve trunks termed “mononeuropathy multiplex,” as in our patient’s presentation. The initial workup includes EMG and a battery of serologic tests. In cases of severe and progressive polyneuropathy, nerve biopsy can assess for the presence of vasculitis, amyloidosis, and paraprotein deposition.
Transverse myelitis
Transverse myelitis is an inflammatory myelopathy that usually presents with acute or subacute weakness of the upper extremities or lower extremities, or both, corresponding to the level of the lesion, hyperreflexia, bladder and bowel dysfunction, spinal level of sensory loss, and autonomic involvement.6 The differential diagnosis of acute myelopathy includes:
- Infection (eg, herpes simplex virus, West Nile virus, Lyme disease, Mycoplasma pneumoniae, human immunodeficiency virus)
- Systemic inflammatory disease (systemic lupus erythematosus, sarcoidosis, Sjögren syndrome, scleroderma, paraneoplastic syndrome)
- Central nervous system demyelinating disease (acute disseminated encephalomyelitis, multiple sclerosis, neuromyelitis optica)
- Vascular malformation (dural arteriovenous fistula)
- Compression due to tumor, bleeding, disc herniation, infection, or abscess.
The workup involves laboratory tests to exclude systemic inflammatory and infectious causes, as well as MRI of the spine with and without contrast to identify a causative lesion. Lumbar puncture and CSF analysis may show pleocytosis, elevated protein concentration, and increased intrathecal immunoglobulin G (IgG) index.7
Although our patient’s presentation with subacute lower-extremity weakness, sensory changes, and bladder dysfunction were consistent with transverse myelitis, her cranial nerve abnormalities would be atypical for it.
Polyradiculopathy
Polyradiculopathy has many possible causes. In the United States, the most common causes are lumbar spondylosis, lumbar canal stenosis, and diabetic polyradiculoneuropathy.
When multiple spinal segments are affected, leptomeningeal disease involving the arachnoid and pia mater should be considered. Causes include malignant invasion, inflammatory cell accumulation, and protein deposition, leading to patchy but widespread dysfunction of spinal nerve roots and cranial nerves. Specific causes are myriad and include carcinomatous meningitis,8 syphilis, tuberculosis, sarcoidosis, and paraproteinemias. CSF and MRI changes are often nonspecific, leading to the need for meningeal biopsy for diagnosis.
CASE CONTINUED
During her hospitalization, our patient developed acute right upper and lower facial weakness consistent with peripheral facial mononeuropathy. Bilateral lower-extremity weakness progressed to disabling paraparesis.
She underwent lumbar puncture and CSF analysis (Table 1). The most notable findings were significant pleocytosis (72% lymphocytic predominance), protein elevation, and elevated IgG index (indicative of elevated intrathecal immunoglobulin synthesis in the central nervous system). Viral, bacterial, and fungal studies were negative. Guillain-Barré syndrome, other polyneuropathies, and spinal cord infarction would not be expected with these CSF features.
Surface EMG demonstrated normal sensory responses, and needle EMG showed chronic and active motor axon loss in the L3 and S1 root distributions, suggesting polyradiculopathy without polyneuropathy. These findings would not be expected in typical acute transverse myelitis but could be seen with spinal cord infarction.
MRI of the entire spine with and without contrast showed cauda equina nerve root thickening and enhancement, especially involving the L5 and S1 roots (Figure 1). The spinal cord appeared normal. These findings further supported polyradiculopathy and a leptomeningeal process.
Further evaluation included chest radiography, erythrocyte sedimentation rate, C-reactive protein, hemoglobin A1c, human immunodeficiency virus testing, antinuclear antibody, antineutrophil cytoplasmic antibody, extractable nuclear antibody, GQ1b antibody, serum and CSF paraneoplastic panels, levels of vitamin B1, B12, and B6, copper, and ceruloplasmin, and a screen for heavy metals. All results were within normal ranges.
ESTABLISHING THE DIAGNOSIS
Serum monoclonal protein analysis with immunofixation revealed IgM kappa monoclonal gammopathy with an IgM level of 1,570 (reference range 53–334 mg/dL) and M-spike 0.75 (0.00 mg/dL), serum free kappa light chains 61.1 (3.30–19.40 mg/L), lambda 9.3 (5.7–26.3 mg/L), and kappa-lambda ratio 6.57 (0.26–1.65).
2. Which is the best next step in this patient’s neurologic evaluation?
- Test CSF angiotensin-converting enzyme level
- CSF cytology
- Meningeal biopsy
- Peripheral nerve biopsy
Given the high suspicion for malignancy, CSF cytology was performed and showed increased numbers of mononuclear chronic inflammatory cells, including a mixture of lymphocytes and monocytes, favoring a reactive lymphoid pleocytosis. Flow cytometry indicated the presence of a monoclonal, CD5- and CD10- negative, B-cell lymphoproliferative disorder. The immunophenotypic findings were not specific for a single diagnosis. The differential diagnosis included marginal zone lymphoma and lymphoplasmacytic lymphoma.
3. Given the presence of serum IgM monoclonal gammopathy in this patient, which is the most likely diagnosis?
- Neurosarcoidosis
- Multiple myeloma
- Waldenström macroglobulinemia
- Carcinomatous meningitis
Study of bone marrow biopsy demonstrated limited bone marrow involvement (1%) by a lymphoproliferative disorder with plasmacytoid features, and DNA testing detected an MYD88 L265P mutation, reported to be present in 90% of patients with Waldenström macroglobulinemia.9 This finding confirmed the diagnosis of Waldenström macroglobulinemia with central nervous system involvement. Our patient began therapy with rituximab and methotrexate, which resulted in some improvement in strength, gait, and vision.
WALDENSTRÖM MACROGLOBULINEMIA AND BING-NEEL SYNDROME
Waldenström macroglobulinemia is a lymphoplasmacytic lymphoma associated with a monoclonal IgM protein.10 It is considered a paraproteinemic disorder, similar to multiple myeloma. The presenting symptoms and complications are related to direct tumor infiltration, hyperviscosity syndrome, and deposition of IgM in various tissues.11,12
Waldenström macroglobulinemia is usually indolent, and treatment is reserved for patients with symptoms.13,14 It includes rituximab, usually in combination with chemotherapy or other targeted agents.15,16
Paraneoplastic antibody-mediated polyneuropathy may occur in these patients. However, the pattern is usually symmetrical clinically, with demyelination on EMG, and is not associated with cranial nerve or meningeal involvement. Management with plasmapheresis, corticosteroids, and intravenous immunoglobulin has not been shown to be effective.17
Involvement of the central nervous system as a complication of Waldenström macroglobulinemia has been described as Bing-Neel syndrome. It can present as diffuse malignant cell infiltration of the leptomeningeal space, white matter, or spinal cord, or in a tumoral form presenting as intraparenchymal masses or nodular lesions. The distinction between the tumoral and diffuse forms is based primarily on imaging findings.18
In a report of 44 patients with Bing-Neel syndrome, 36% presented with the disorder as the initial manifestation of Waldenström macroglobulinemia.18 The primary presenting symptoms were imbalance and gait difficulty (48%) and cranial nerve involvement (36%), which presented as predominantly facial or oculomotor nerve palsy. Cauda equina syndrome with motor involvement (seen in our patient) occurred in 14% of patients. Other presenting symptoms included cognitive impairment, sensory deficits, headache, dysarthria, aphasia, and seizures.
LEARNING POINTS
The differential diagnosis for patients presenting with multifocal neurologic symptoms can be broad, and a systematic approach to the diagnosis is necessary. Localizing the lesion is important in determining the diagnosis for patients presenting with neurologic symptoms. The process of localization begins with taking the history, is further refined during the examination, and is confirmed with diagnostic studies. Atypical presentations of relatively common neurologic diseases such as Guillain-Barré syndrome, transverse myelitis, and peripheral polyneuropathy do occur, but uncommon diagnoses need to be considered when support for the initial diagnosis is lacking.
- Fokke C, van den Berg B, Drenthen J, Walgaard C, van Doorn PA, Jacobs BC. Diagnosis of Guillain-Barre syndrome and validation of Brighton criteria. Brain 2014; 137(Pt 1):33–43. doi:10.1093/brain/awt285
- Mathey EK, Park SB, Hughes RA, et al. Chronic inflammatory demyelinating polyradiculoneuropathy: from pathology to phenotype. J Neurol Neurosurg Psychiatry 2015; 86(9):973–985. doi:10.1136/jnnp-2014-309697
- Chiba A, Kusunoki S, Obata H, Machinami R, Kanazawa I. Serum anti-GQ1b IgG antibody is associated with ophthalmoplegia in Miller Fisher syndrome and Guillain-Barré syndrome: clinical and immunohistochemical studies. Neurology 1993; 43(10):1911–1917. pmid:8413947
- Teener J. Miller Fisher’s syndrome. Semin Neurol 2012; 32(5):512–516. doi:10.1055/s-0033-1334470
- Watson JC, Dyck PJ. Peripheral neuropathy: a practical approach to diagnosis and symptom management. Mayo Clin Proc 2015; 90(7):940–951. doi:10.1016/j.mayocp.2015.05.004
- Greenberg BM. Treatment of acute transverse myelitis and its early complications. Continuum (Minneap Minn) 2011; 17(4):733–743. doi:10.1212/01.CON.0000403792.36161.f5
- West TW. Transverse myelitis—a review of the presentation, diagnosis, and initial management. Discov Med 2013; 16(88):167–177. pmid:24099672
- Le Rhun E, Taillibert S, Chamberlain MC. Carcinomatous meningitis: leptomeningeal metastases in solid tumors. Surg Neurol Int 2013; 4(suppl 4):S265–S288. doi:10.4103/2152-7806.111304
- Treon SP, Xu L, Yang G, et al. MYD88 L265P somatic mutation in Waldenström’s macroglobulinemia. N Engl J Med 2012; 367(9):826–833. doi:10.1056/NEJMoa1200710
- Owen RG, Treon SP, Al-Katib A, et al. Clinicopathological definition of Waldenstrom’s macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom’s Macroglobulinemia. Semin Oncol 2003; 30(2):110–115. doi:10.1053/sonc.2003.50082
- Björkholm M, Johansson E, Papamichael D, et al. Patterns of clinical presentation, treatment, and outcome in patients with Waldenstrom’s macroglobulinemia: a two-institution study. Semin Oncol 2003; 30(2):226–230. doi:10.1053/sonc.2003.50054
- Rison RA, Beydoun SR. Paraproteinemic neuropathy: a practical review. BMC Neurol 2016; 16:13. doi:10.1186/s12883-016-0532-4
- Kyle RA, Benson J, Larson D, et al. IgM monoclonal gammopathy of undetermined significance and smoldering Waldenström’s macroglobulinemia. Clin Lymphoma Myeloma 2009; 9(1):17–18. doi:10.3816/CLM.2009.n.002
- Kyle RA, Benson JT, Larson DR, et al. Progression in smoldering Waldenstrom macroglobulinemia: long-term results. Blood 2012; 119(19):4462–4466. doi:10.1182/blood-2011-10-384768
- Leblond V, Kastritis E, Advani R, et al. Treatment recommendations from the Eighth International Workshop on Waldenström’s macroglobulinemia. Blood 2016; 128(10):1321–1328. doi:10.1182/blood-2016-04-711234
- Kapoor P, Ansell SM, Fonseca R, et al. Diagnosis and management of Waldenström macroglobulinemia: Mayo stratification of macroglobulinemia and risk-adapted therapy (mSMART) guidelines 2016. JAMA Oncol 2017; 3(9):1257–1265. doi:10.1001/jamaoncol.2016.5763
- D’Sa S, Kersten MJ, Castillo JJ, et al. Investigation and management of IgM and Waldenström-associated peripheral neuropathies: recommendations from the IWWM-8 consensus panel. Br J Haematol 2017; 176(5):728–742. doi:10.1111/bjh.14492
- Simon L, Fitsiori A, Lemal R, et al. Bing-Neel syndrome, a rare complication of Waldenström macroglobulinemia: analysis of 44 cases and review of the literature. A study on behalf of the French Innovative Leukemia Organization (FILO). Haematologica 2015; 100(12):1587–1594. doi:10.3324/haematol.2015.133744
- Fokke C, van den Berg B, Drenthen J, Walgaard C, van Doorn PA, Jacobs BC. Diagnosis of Guillain-Barre syndrome and validation of Brighton criteria. Brain 2014; 137(Pt 1):33–43. doi:10.1093/brain/awt285
- Mathey EK, Park SB, Hughes RA, et al. Chronic inflammatory demyelinating polyradiculoneuropathy: from pathology to phenotype. J Neurol Neurosurg Psychiatry 2015; 86(9):973–985. doi:10.1136/jnnp-2014-309697
- Chiba A, Kusunoki S, Obata H, Machinami R, Kanazawa I. Serum anti-GQ1b IgG antibody is associated with ophthalmoplegia in Miller Fisher syndrome and Guillain-Barré syndrome: clinical and immunohistochemical studies. Neurology 1993; 43(10):1911–1917. pmid:8413947
- Teener J. Miller Fisher’s syndrome. Semin Neurol 2012; 32(5):512–516. doi:10.1055/s-0033-1334470
- Watson JC, Dyck PJ. Peripheral neuropathy: a practical approach to diagnosis and symptom management. Mayo Clin Proc 2015; 90(7):940–951. doi:10.1016/j.mayocp.2015.05.004
- Greenberg BM. Treatment of acute transverse myelitis and its early complications. Continuum (Minneap Minn) 2011; 17(4):733–743. doi:10.1212/01.CON.0000403792.36161.f5
- West TW. Transverse myelitis—a review of the presentation, diagnosis, and initial management. Discov Med 2013; 16(88):167–177. pmid:24099672
- Le Rhun E, Taillibert S, Chamberlain MC. Carcinomatous meningitis: leptomeningeal metastases in solid tumors. Surg Neurol Int 2013; 4(suppl 4):S265–S288. doi:10.4103/2152-7806.111304
- Treon SP, Xu L, Yang G, et al. MYD88 L265P somatic mutation in Waldenström’s macroglobulinemia. N Engl J Med 2012; 367(9):826–833. doi:10.1056/NEJMoa1200710
- Owen RG, Treon SP, Al-Katib A, et al. Clinicopathological definition of Waldenstrom’s macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom’s Macroglobulinemia. Semin Oncol 2003; 30(2):110–115. doi:10.1053/sonc.2003.50082
- Björkholm M, Johansson E, Papamichael D, et al. Patterns of clinical presentation, treatment, and outcome in patients with Waldenstrom’s macroglobulinemia: a two-institution study. Semin Oncol 2003; 30(2):226–230. doi:10.1053/sonc.2003.50054
- Rison RA, Beydoun SR. Paraproteinemic neuropathy: a practical review. BMC Neurol 2016; 16:13. doi:10.1186/s12883-016-0532-4
- Kyle RA, Benson J, Larson D, et al. IgM monoclonal gammopathy of undetermined significance and smoldering Waldenström’s macroglobulinemia. Clin Lymphoma Myeloma 2009; 9(1):17–18. doi:10.3816/CLM.2009.n.002
- Kyle RA, Benson JT, Larson DR, et al. Progression in smoldering Waldenstrom macroglobulinemia: long-term results. Blood 2012; 119(19):4462–4466. doi:10.1182/blood-2011-10-384768
- Leblond V, Kastritis E, Advani R, et al. Treatment recommendations from the Eighth International Workshop on Waldenström’s macroglobulinemia. Blood 2016; 128(10):1321–1328. doi:10.1182/blood-2016-04-711234
- Kapoor P, Ansell SM, Fonseca R, et al. Diagnosis and management of Waldenström macroglobulinemia: Mayo stratification of macroglobulinemia and risk-adapted therapy (mSMART) guidelines 2016. JAMA Oncol 2017; 3(9):1257–1265. doi:10.1001/jamaoncol.2016.5763
- D’Sa S, Kersten MJ, Castillo JJ, et al. Investigation and management of IgM and Waldenström-associated peripheral neuropathies: recommendations from the IWWM-8 consensus panel. Br J Haematol 2017; 176(5):728–742. doi:10.1111/bjh.14492
- Simon L, Fitsiori A, Lemal R, et al. Bing-Neel syndrome, a rare complication of Waldenström macroglobulinemia: analysis of 44 cases and review of the literature. A study on behalf of the French Innovative Leukemia Organization (FILO). Haematologica 2015; 100(12):1587–1594. doi:10.3324/haematol.2015.133744
Complete blood cell count
To the Editor: The review by May et al1 of 3 neglected numbers in the complete blood cell count (CBC) was a good reminder to look more closely at the results of the CBCs we often order in primary care. I was surprised to see no mention of the red cell distribution width in relation to another cardiovascular disorder—obstructive sleep apnea.2,3 I wonder if the authors would comment on this association?
- May JE, Marques MB, Reddy VVB, Gangaraju R. Three neglected numbers in the CBC: The RDW, MPV, and NRBC count. Cleve Clin J Med 2019; 86(3):167–172. doi:10.3949/ccjm.86a.18072
- Sökücü SN, Karasulu L, Dalar L, Seyhan EC, Altın S. Can red blood cell distribution width predict severity of obstructive sleep apnea syndrome? J Clin Sleep Med 2012; 8(5):521–525. doi:10.5664/jcsm.2146
- Yousef AM, Alkhiary W. The severity of obstructive sleep apnea syndrome is related to red cell distribution width and hematocrit values. J Sleep Disord Ther 2015; 4(2):1000192. doi:10.4172/2167-0277.1000192
To the Editor: The review by May et al1 of 3 neglected numbers in the complete blood cell count (CBC) was a good reminder to look more closely at the results of the CBCs we often order in primary care. I was surprised to see no mention of the red cell distribution width in relation to another cardiovascular disorder—obstructive sleep apnea.2,3 I wonder if the authors would comment on this association?
To the Editor: The review by May et al1 of 3 neglected numbers in the complete blood cell count (CBC) was a good reminder to look more closely at the results of the CBCs we often order in primary care. I was surprised to see no mention of the red cell distribution width in relation to another cardiovascular disorder—obstructive sleep apnea.2,3 I wonder if the authors would comment on this association?
- May JE, Marques MB, Reddy VVB, Gangaraju R. Three neglected numbers in the CBC: The RDW, MPV, and NRBC count. Cleve Clin J Med 2019; 86(3):167–172. doi:10.3949/ccjm.86a.18072
- Sökücü SN, Karasulu L, Dalar L, Seyhan EC, Altın S. Can red blood cell distribution width predict severity of obstructive sleep apnea syndrome? J Clin Sleep Med 2012; 8(5):521–525. doi:10.5664/jcsm.2146
- Yousef AM, Alkhiary W. The severity of obstructive sleep apnea syndrome is related to red cell distribution width and hematocrit values. J Sleep Disord Ther 2015; 4(2):1000192. doi:10.4172/2167-0277.1000192
- May JE, Marques MB, Reddy VVB, Gangaraju R. Three neglected numbers in the CBC: The RDW, MPV, and NRBC count. Cleve Clin J Med 2019; 86(3):167–172. doi:10.3949/ccjm.86a.18072
- Sökücü SN, Karasulu L, Dalar L, Seyhan EC, Altın S. Can red blood cell distribution width predict severity of obstructive sleep apnea syndrome? J Clin Sleep Med 2012; 8(5):521–525. doi:10.5664/jcsm.2146
- Yousef AM, Alkhiary W. The severity of obstructive sleep apnea syndrome is related to red cell distribution width and hematocrit values. J Sleep Disord Ther 2015; 4(2):1000192. doi:10.4172/2167-0277.1000192
In reply: Complete blood cell count
In Reply: We thank Dr. Homler for his question and for highlighting another important disease state, obstructive sleep apnea, in which a high red cell distribution width (RDW) has correlated with disease severity.1,2 The 2 retrospective studies he mentioned indicated that RDW is negatively correlated with metrics such as oxygen saturation, sleep time, and sleep quality. Interestingly, another retrospective study showed that RDW was significantly higher in patients with concurrent obstructive sleep apnea and cardiovascular disease than in patients with obstructive sleep apnea alone, suggesting that the presence of anisocytosis in obstructive sleep apnea may be due to its link to cardiovascular disease.3
Although we focused on cardiovascular disease in our review, RDW has also shown prognostic significance in many other disorders including ischemic stroke,4 pneumonia,5,6 chronic kidney disease,7 and gastrointestinal disorders.8 Collectively, these studies indicate that RDW may serve as a red flag for clinicians, raising concern for increased disease severity and potential adverse outcomes. However, further research is needed to determine if and how RDW monitoring should be used to prompt interventions to improve patient outcomes.
- Sökücü SN, Karasulu L, Dalar L, Seyhan EC, Altın S. Can red blood cell distribution width predict severity of obstructive sleep apnea syndrome? J Clin Sleep Med 2012; 8(5):521–525. doi:10.5664/jcsm.2146
- Yousef AM, Alkhiary W. The severity of obstructive sleep apnea syndrome is related to red cell distribution width and hematocrit values. J Sleep Disord Ther 2015; 4(2):1000192. doi:10.4172/2167-0277.1000192
- Sunnetcioglu A, Gunbatar H, Yildiz H. Red cell distribution width and uric acid in patients with obstructive sleep apnea. Clin Respir J 2018; 12(3):1046–1052. doi:10.1111/crj.12626
- Feng G-H, Li H-P, Li Q-L, Fu Y, Huang R-B. Red blood cell distribution width and ischaemic stroke. Stroke Vasc Neurol 2017; 2(3):172-175. doi:10.1136/svn-2017-000071
- Lee JH, Chung HJ, Kim K, et al. Red cell distribution width as a prognostic marker in patients with community-acquired pneumonia. Am J Emerg Med 2013; 31:72–79. doi:10.1016/j.ajem.2012.06.004
- Miranda SJ. Validity of red cell distribution width as a predictor of clinical outcomes in pediatric patients diagnosed with pneumonia [abstract]. Chest 2017; 152(4 suppl):A843. doi:10.1016/j.chest.2017.08.877
- Kor CT, Hsieh YP, Chang CC, Chiu PF. The prognostic value of interaction between mean corpuscular volume and red cell distribution width in mortality in chronic kidney disease. Sci Rep 2018; 8(1):11870. doi:10.1038/s41598-018-19881-2
- Goyal H, Lippi G, Gjymishka A, et al. Prognostic significance of red blood cell distribution width in gastrointestinal disorders. World J Gastroenterol 2017; 23(27):4879–4891. doi:10.3748/wjg.v23.i27.4879
In Reply: We thank Dr. Homler for his question and for highlighting another important disease state, obstructive sleep apnea, in which a high red cell distribution width (RDW) has correlated with disease severity.1,2 The 2 retrospective studies he mentioned indicated that RDW is negatively correlated with metrics such as oxygen saturation, sleep time, and sleep quality. Interestingly, another retrospective study showed that RDW was significantly higher in patients with concurrent obstructive sleep apnea and cardiovascular disease than in patients with obstructive sleep apnea alone, suggesting that the presence of anisocytosis in obstructive sleep apnea may be due to its link to cardiovascular disease.3
Although we focused on cardiovascular disease in our review, RDW has also shown prognostic significance in many other disorders including ischemic stroke,4 pneumonia,5,6 chronic kidney disease,7 and gastrointestinal disorders.8 Collectively, these studies indicate that RDW may serve as a red flag for clinicians, raising concern for increased disease severity and potential adverse outcomes. However, further research is needed to determine if and how RDW monitoring should be used to prompt interventions to improve patient outcomes.
In Reply: We thank Dr. Homler for his question and for highlighting another important disease state, obstructive sleep apnea, in which a high red cell distribution width (RDW) has correlated with disease severity.1,2 The 2 retrospective studies he mentioned indicated that RDW is negatively correlated with metrics such as oxygen saturation, sleep time, and sleep quality. Interestingly, another retrospective study showed that RDW was significantly higher in patients with concurrent obstructive sleep apnea and cardiovascular disease than in patients with obstructive sleep apnea alone, suggesting that the presence of anisocytosis in obstructive sleep apnea may be due to its link to cardiovascular disease.3
Although we focused on cardiovascular disease in our review, RDW has also shown prognostic significance in many other disorders including ischemic stroke,4 pneumonia,5,6 chronic kidney disease,7 and gastrointestinal disorders.8 Collectively, these studies indicate that RDW may serve as a red flag for clinicians, raising concern for increased disease severity and potential adverse outcomes. However, further research is needed to determine if and how RDW monitoring should be used to prompt interventions to improve patient outcomes.
- Sökücü SN, Karasulu L, Dalar L, Seyhan EC, Altın S. Can red blood cell distribution width predict severity of obstructive sleep apnea syndrome? J Clin Sleep Med 2012; 8(5):521–525. doi:10.5664/jcsm.2146
- Yousef AM, Alkhiary W. The severity of obstructive sleep apnea syndrome is related to red cell distribution width and hematocrit values. J Sleep Disord Ther 2015; 4(2):1000192. doi:10.4172/2167-0277.1000192
- Sunnetcioglu A, Gunbatar H, Yildiz H. Red cell distribution width and uric acid in patients with obstructive sleep apnea. Clin Respir J 2018; 12(3):1046–1052. doi:10.1111/crj.12626
- Feng G-H, Li H-P, Li Q-L, Fu Y, Huang R-B. Red blood cell distribution width and ischaemic stroke. Stroke Vasc Neurol 2017; 2(3):172-175. doi:10.1136/svn-2017-000071
- Lee JH, Chung HJ, Kim K, et al. Red cell distribution width as a prognostic marker in patients with community-acquired pneumonia. Am J Emerg Med 2013; 31:72–79. doi:10.1016/j.ajem.2012.06.004
- Miranda SJ. Validity of red cell distribution width as a predictor of clinical outcomes in pediatric patients diagnosed with pneumonia [abstract]. Chest 2017; 152(4 suppl):A843. doi:10.1016/j.chest.2017.08.877
- Kor CT, Hsieh YP, Chang CC, Chiu PF. The prognostic value of interaction between mean corpuscular volume and red cell distribution width in mortality in chronic kidney disease. Sci Rep 2018; 8(1):11870. doi:10.1038/s41598-018-19881-2
- Goyal H, Lippi G, Gjymishka A, et al. Prognostic significance of red blood cell distribution width in gastrointestinal disorders. World J Gastroenterol 2017; 23(27):4879–4891. doi:10.3748/wjg.v23.i27.4879
- Sökücü SN, Karasulu L, Dalar L, Seyhan EC, Altın S. Can red blood cell distribution width predict severity of obstructive sleep apnea syndrome? J Clin Sleep Med 2012; 8(5):521–525. doi:10.5664/jcsm.2146
- Yousef AM, Alkhiary W. The severity of obstructive sleep apnea syndrome is related to red cell distribution width and hematocrit values. J Sleep Disord Ther 2015; 4(2):1000192. doi:10.4172/2167-0277.1000192
- Sunnetcioglu A, Gunbatar H, Yildiz H. Red cell distribution width and uric acid in patients with obstructive sleep apnea. Clin Respir J 2018; 12(3):1046–1052. doi:10.1111/crj.12626
- Feng G-H, Li H-P, Li Q-L, Fu Y, Huang R-B. Red blood cell distribution width and ischaemic stroke. Stroke Vasc Neurol 2017; 2(3):172-175. doi:10.1136/svn-2017-000071
- Lee JH, Chung HJ, Kim K, et al. Red cell distribution width as a prognostic marker in patients with community-acquired pneumonia. Am J Emerg Med 2013; 31:72–79. doi:10.1016/j.ajem.2012.06.004
- Miranda SJ. Validity of red cell distribution width as a predictor of clinical outcomes in pediatric patients diagnosed with pneumonia [abstract]. Chest 2017; 152(4 suppl):A843. doi:10.1016/j.chest.2017.08.877
- Kor CT, Hsieh YP, Chang CC, Chiu PF. The prognostic value of interaction between mean corpuscular volume and red cell distribution width in mortality in chronic kidney disease. Sci Rep 2018; 8(1):11870. doi:10.1038/s41598-018-19881-2
- Goyal H, Lippi G, Gjymishka A, et al. Prognostic significance of red blood cell distribution width in gastrointestinal disorders. World J Gastroenterol 2017; 23(27):4879–4891. doi:10.3748/wjg.v23.i27.4879
Click for Credit: Biomarkers for VTE risk; Exercise & concussion recovery; more
Here are 5 articles from the June issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Expert: There’s no single treatment for fibromyalgia
To take the posttest, go to: https://bit.ly/2EAI5v1
Expires February 3, 2020
2. Mood and behavior are different targets for irritability in children
To take the posttest, go to: https://bit.ly/2wpLS9X
Expires February 6, 2020
3. Biomarkers predict VTE risk with menopausal oral hormone therapy
To take the posttest, go to: https://bit.ly/2JKEQFC
Expires February 6, 2020
4. Mild aerobic exercise speeds sports concussion recovery
To take the posttest, go to: https://bit.ly/30RuYiE
Expires February 4, 2020
5. For CABG, multiple and single arterial grafts show no survival difference
To take the posttest, go to: https://bit.ly/2wtiCiF
Expires January 31, 2020
Here are 5 articles from the June issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Expert: There’s no single treatment for fibromyalgia
To take the posttest, go to: https://bit.ly/2EAI5v1
Expires February 3, 2020
2. Mood and behavior are different targets for irritability in children
To take the posttest, go to: https://bit.ly/2wpLS9X
Expires February 6, 2020
3. Biomarkers predict VTE risk with menopausal oral hormone therapy
To take the posttest, go to: https://bit.ly/2JKEQFC
Expires February 6, 2020
4. Mild aerobic exercise speeds sports concussion recovery
To take the posttest, go to: https://bit.ly/30RuYiE
Expires February 4, 2020
5. For CABG, multiple and single arterial grafts show no survival difference
To take the posttest, go to: https://bit.ly/2wtiCiF
Expires January 31, 2020
Here are 5 articles from the June issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Expert: There’s no single treatment for fibromyalgia
To take the posttest, go to: https://bit.ly/2EAI5v1
Expires February 3, 2020
2. Mood and behavior are different targets for irritability in children
To take the posttest, go to: https://bit.ly/2wpLS9X
Expires February 6, 2020
3. Biomarkers predict VTE risk with menopausal oral hormone therapy
To take the posttest, go to: https://bit.ly/2JKEQFC
Expires February 6, 2020
4. Mild aerobic exercise speeds sports concussion recovery
To take the posttest, go to: https://bit.ly/30RuYiE
Expires February 4, 2020
5. For CABG, multiple and single arterial grafts show no survival difference
To take the posttest, go to: https://bit.ly/2wtiCiF
Expires January 31, 2020
Studies cast doubt on FDA’s accelerated cancer drug pathway
In the first study, lead investigator Emerson Y. Chen, MD, of Oregon Health & Science University, Portland, and colleagues conducted a retrospective analysis of all drugs approved by the FDA on the basis of response rate – the percentage of patients who experience tumor shrinkage – from Jan. 1, 2006, to Sept. 30, 2018. The data set consisted of 59 oncology drugs with 85 unique indications approved by the FDA for advanced-stage metastatic cancer on the basis of a response rate (RR) endpoint during the study period.
Of the 85 indications, 32 were granted regular approval immediately with limited postmarketing efficacy requirements and 53 (62%) were granted accelerated approval. Of the accelerated approvals, 29 (55%) were later converted to regular approval.
The median RR for the 85 indications was 41%, and the median sample size of such RR trials was 117 patients, according to the analysis published in JAMA Internal Medicine.
Among all approvals, 14 of 85 (16%) had an RR less than 20%, 28 of 85 (33%) had an RR less than 30%, and 40 of 85 (47%) had an RR less than 40%.
Most approved drugs had an RR ranging from 20% to 59%, the study found. Of 81 available indications, the median complete response rate – defined as the percentage of patients with no visible disease and normalization of lymph nodes – was 6%. (Complete response data were not reported for four drug indications.)
The investigators found that many of the drugs studied have remained on the market for years without subsequent confirmatory data. For example, when the accelerated approvals based on RR were converted to full approval, 23 of 29 were made on the basis of surrogate endpoints (progression-free survival or RR), 7 of 29 were made on the basis of RR, and just 6 of 29 were made on the basis of overall survival (OS).
The findings suggest that most cancer drugs approved by the FDA based on RR have less than transformational response rates, and that such indications do not have confirmed clinical benefit, the study authors wrote.
While in some settings, a response can equal prognostic value regarding overall survival, the authors wrote that “the ability of RR to serve as a validated surrogate for OS varies among cancer types and is generally poor.”
In the second study, researchers found that confirmatory trials for only one-fifth of cancer drug indications approved via the FDA’s accelerated approval route demonstrated improvements in overall patient survival.
Lead investigator Bishal Gyawali, MD, PhD, of Queen’s University, Kingston, Ont., and colleagues examined FDA data on recent drugs and indications that received accelerated approval and were later granted full approval.
For their analysis, the investigators reviewed the FDA’s database of postmarketing requirements and commitments, as well as PubMed, to determine the current status of postmarket trials for indications labeled as “ongoing” in the original FDA data.
Of 93 cancer drug indications for which accelerated approval was granted from Dec. 11, 1992, to May 31, 2017, the FDA reported clinical benefit was adequately confirmed in 51 indications. Of these confirmations, 15 demonstrated improvement in overall survival.
In their updated analysis, the investigators determined that confirmatory trials for 19 of the 93 (20%) cancer drug approvals reported an improvement in OS, 19 trials (20%) reported improvement in the same surrogate used in the preapproval trial, and 20 trials (21%) reported improvement in a different surrogate, according to the study, also published in JAMA Internal Medicine.
Additionally, results showed that 5 confirmatory trials were delayed, 10 trials were pending, and 9 trials were ongoing.
For three recent accelerated approvals, the primary endpoints were not met in the confirmatory trials, but one of the indications still received full approval.
The findings raise several concerns about the accelerated cancer drug pathway, including whether the same surrogate efficacy measure should be used as verification of drug benefit, according to the investigators. Conversely, using a different surrogate endpoint than the original measure can cause confusion among physicians and patients about whether the cancer drug improves survival or quality of life, information that is essential in the benefit-risk evaluation for clinical decision making.
That a number of the confirmatory trials examined were delayed or pending emphasize the considerable time that can elapse between drug approval and confirmatory trial completion, they added.
“Timely planning and completion of postmarketing trials is necessary for proper implementation of the accelerated approval pathway, and the FDA should minimize the period during which patients and physicians are using drugs approved through accelerated pathways without rigorous data on their ultimate clinical benefit,” the authors wrote in the analysis.
Dr. Chen, lead author of the RR study, said both studies call into question what criteria is optimal when assessing cancer drug value, while ensuring such measurements are not too high to achieve – preventing useful drugs to market – but also not too low – allowing drugs with marginal benefit into the market.
“There has been tremendous drug development within the oncology space, and it is always important to look back to reassess and see if the process [matches] the original vision so that we can correct any misuse or concerns,” Dr. Chen said in an interview.
Dr. Chen said his study indicates the RR endpoint has been misused in scenarios with low response rate, common cancer, and/or situations with already available therapies. In the study by Dr. Gyawali, the results suggest many drugs approved on the basis of a surrogate endpoint (RR or progression-free survival) ultimately do not demonstrate survival benefit confirmation or patient-reported benefit, Dr. Chen said.
“We hope that readers of these JAMA IM studies and the accompanying commentaries will recognize that there could be a set of guidance criteria from regulatory agencies or oncology organizations to recommend use of surrogate endpoints in special situations: high response rate of the drug, very rare cancer, or highly innovative therapy not yet seen before,” he said. “The use of surrogate endpoints to justify these therapies must also have postmarketing confirmation of survival or patient-reported benefit.”
The study led by Dr. Chen was supported by the Laura and John Arnold Foundation. Dr Chen reported receiving lecture honorarium from Horizon CME; another coauthor reported receiving honorarium from universities, medical centers, and publishers. The study led by Dr. Gyawali was supported by the Arnold Ventures; one of the coauthors reported receiving grant support from the Harvard-MIT Center for Regulatory Science and the Engelberg Foundation, as well as unrelated research funding from the FDA.
SOURCES: Chen EY et al. JAMA Intern Med. 2019 May 28. doi: 10.1001/jamainternmed.2019.0583; Gyawali B et al. JAMA Intern Med. 2019 May 28. doi: 10.1001/jamainternmed.2019.0462.
In the first study, lead investigator Emerson Y. Chen, MD, of Oregon Health & Science University, Portland, and colleagues conducted a retrospective analysis of all drugs approved by the FDA on the basis of response rate – the percentage of patients who experience tumor shrinkage – from Jan. 1, 2006, to Sept. 30, 2018. The data set consisted of 59 oncology drugs with 85 unique indications approved by the FDA for advanced-stage metastatic cancer on the basis of a response rate (RR) endpoint during the study period.
Of the 85 indications, 32 were granted regular approval immediately with limited postmarketing efficacy requirements and 53 (62%) were granted accelerated approval. Of the accelerated approvals, 29 (55%) were later converted to regular approval.
The median RR for the 85 indications was 41%, and the median sample size of such RR trials was 117 patients, according to the analysis published in JAMA Internal Medicine.
Among all approvals, 14 of 85 (16%) had an RR less than 20%, 28 of 85 (33%) had an RR less than 30%, and 40 of 85 (47%) had an RR less than 40%.
Most approved drugs had an RR ranging from 20% to 59%, the study found. Of 81 available indications, the median complete response rate – defined as the percentage of patients with no visible disease and normalization of lymph nodes – was 6%. (Complete response data were not reported for four drug indications.)
The investigators found that many of the drugs studied have remained on the market for years without subsequent confirmatory data. For example, when the accelerated approvals based on RR were converted to full approval, 23 of 29 were made on the basis of surrogate endpoints (progression-free survival or RR), 7 of 29 were made on the basis of RR, and just 6 of 29 were made on the basis of overall survival (OS).
The findings suggest that most cancer drugs approved by the FDA based on RR have less than transformational response rates, and that such indications do not have confirmed clinical benefit, the study authors wrote.
While in some settings, a response can equal prognostic value regarding overall survival, the authors wrote that “the ability of RR to serve as a validated surrogate for OS varies among cancer types and is generally poor.”
In the second study, researchers found that confirmatory trials for only one-fifth of cancer drug indications approved via the FDA’s accelerated approval route demonstrated improvements in overall patient survival.
Lead investigator Bishal Gyawali, MD, PhD, of Queen’s University, Kingston, Ont., and colleagues examined FDA data on recent drugs and indications that received accelerated approval and were later granted full approval.
For their analysis, the investigators reviewed the FDA’s database of postmarketing requirements and commitments, as well as PubMed, to determine the current status of postmarket trials for indications labeled as “ongoing” in the original FDA data.
Of 93 cancer drug indications for which accelerated approval was granted from Dec. 11, 1992, to May 31, 2017, the FDA reported clinical benefit was adequately confirmed in 51 indications. Of these confirmations, 15 demonstrated improvement in overall survival.
In their updated analysis, the investigators determined that confirmatory trials for 19 of the 93 (20%) cancer drug approvals reported an improvement in OS, 19 trials (20%) reported improvement in the same surrogate used in the preapproval trial, and 20 trials (21%) reported improvement in a different surrogate, according to the study, also published in JAMA Internal Medicine.
Additionally, results showed that 5 confirmatory trials were delayed, 10 trials were pending, and 9 trials were ongoing.
For three recent accelerated approvals, the primary endpoints were not met in the confirmatory trials, but one of the indications still received full approval.
The findings raise several concerns about the accelerated cancer drug pathway, including whether the same surrogate efficacy measure should be used as verification of drug benefit, according to the investigators. Conversely, using a different surrogate endpoint than the original measure can cause confusion among physicians and patients about whether the cancer drug improves survival or quality of life, information that is essential in the benefit-risk evaluation for clinical decision making.
That a number of the confirmatory trials examined were delayed or pending emphasize the considerable time that can elapse between drug approval and confirmatory trial completion, they added.
“Timely planning and completion of postmarketing trials is necessary for proper implementation of the accelerated approval pathway, and the FDA should minimize the period during which patients and physicians are using drugs approved through accelerated pathways without rigorous data on their ultimate clinical benefit,” the authors wrote in the analysis.
Dr. Chen, lead author of the RR study, said both studies call into question what criteria is optimal when assessing cancer drug value, while ensuring such measurements are not too high to achieve – preventing useful drugs to market – but also not too low – allowing drugs with marginal benefit into the market.
“There has been tremendous drug development within the oncology space, and it is always important to look back to reassess and see if the process [matches] the original vision so that we can correct any misuse or concerns,” Dr. Chen said in an interview.
Dr. Chen said his study indicates the RR endpoint has been misused in scenarios with low response rate, common cancer, and/or situations with already available therapies. In the study by Dr. Gyawali, the results suggest many drugs approved on the basis of a surrogate endpoint (RR or progression-free survival) ultimately do not demonstrate survival benefit confirmation or patient-reported benefit, Dr. Chen said.
“We hope that readers of these JAMA IM studies and the accompanying commentaries will recognize that there could be a set of guidance criteria from regulatory agencies or oncology organizations to recommend use of surrogate endpoints in special situations: high response rate of the drug, very rare cancer, or highly innovative therapy not yet seen before,” he said. “The use of surrogate endpoints to justify these therapies must also have postmarketing confirmation of survival or patient-reported benefit.”
The study led by Dr. Chen was supported by the Laura and John Arnold Foundation. Dr Chen reported receiving lecture honorarium from Horizon CME; another coauthor reported receiving honorarium from universities, medical centers, and publishers. The study led by Dr. Gyawali was supported by the Arnold Ventures; one of the coauthors reported receiving grant support from the Harvard-MIT Center for Regulatory Science and the Engelberg Foundation, as well as unrelated research funding from the FDA.
SOURCES: Chen EY et al. JAMA Intern Med. 2019 May 28. doi: 10.1001/jamainternmed.2019.0583; Gyawali B et al. JAMA Intern Med. 2019 May 28. doi: 10.1001/jamainternmed.2019.0462.
In the first study, lead investigator Emerson Y. Chen, MD, of Oregon Health & Science University, Portland, and colleagues conducted a retrospective analysis of all drugs approved by the FDA on the basis of response rate – the percentage of patients who experience tumor shrinkage – from Jan. 1, 2006, to Sept. 30, 2018. The data set consisted of 59 oncology drugs with 85 unique indications approved by the FDA for advanced-stage metastatic cancer on the basis of a response rate (RR) endpoint during the study period.
Of the 85 indications, 32 were granted regular approval immediately with limited postmarketing efficacy requirements and 53 (62%) were granted accelerated approval. Of the accelerated approvals, 29 (55%) were later converted to regular approval.
The median RR for the 85 indications was 41%, and the median sample size of such RR trials was 117 patients, according to the analysis published in JAMA Internal Medicine.
Among all approvals, 14 of 85 (16%) had an RR less than 20%, 28 of 85 (33%) had an RR less than 30%, and 40 of 85 (47%) had an RR less than 40%.
Most approved drugs had an RR ranging from 20% to 59%, the study found. Of 81 available indications, the median complete response rate – defined as the percentage of patients with no visible disease and normalization of lymph nodes – was 6%. (Complete response data were not reported for four drug indications.)
The investigators found that many of the drugs studied have remained on the market for years without subsequent confirmatory data. For example, when the accelerated approvals based on RR were converted to full approval, 23 of 29 were made on the basis of surrogate endpoints (progression-free survival or RR), 7 of 29 were made on the basis of RR, and just 6 of 29 were made on the basis of overall survival (OS).
The findings suggest that most cancer drugs approved by the FDA based on RR have less than transformational response rates, and that such indications do not have confirmed clinical benefit, the study authors wrote.
While in some settings, a response can equal prognostic value regarding overall survival, the authors wrote that “the ability of RR to serve as a validated surrogate for OS varies among cancer types and is generally poor.”
In the second study, researchers found that confirmatory trials for only one-fifth of cancer drug indications approved via the FDA’s accelerated approval route demonstrated improvements in overall patient survival.
Lead investigator Bishal Gyawali, MD, PhD, of Queen’s University, Kingston, Ont., and colleagues examined FDA data on recent drugs and indications that received accelerated approval and were later granted full approval.
For their analysis, the investigators reviewed the FDA’s database of postmarketing requirements and commitments, as well as PubMed, to determine the current status of postmarket trials for indications labeled as “ongoing” in the original FDA data.
Of 93 cancer drug indications for which accelerated approval was granted from Dec. 11, 1992, to May 31, 2017, the FDA reported clinical benefit was adequately confirmed in 51 indications. Of these confirmations, 15 demonstrated improvement in overall survival.
In their updated analysis, the investigators determined that confirmatory trials for 19 of the 93 (20%) cancer drug approvals reported an improvement in OS, 19 trials (20%) reported improvement in the same surrogate used in the preapproval trial, and 20 trials (21%) reported improvement in a different surrogate, according to the study, also published in JAMA Internal Medicine.
Additionally, results showed that 5 confirmatory trials were delayed, 10 trials were pending, and 9 trials were ongoing.
For three recent accelerated approvals, the primary endpoints were not met in the confirmatory trials, but one of the indications still received full approval.
The findings raise several concerns about the accelerated cancer drug pathway, including whether the same surrogate efficacy measure should be used as verification of drug benefit, according to the investigators. Conversely, using a different surrogate endpoint than the original measure can cause confusion among physicians and patients about whether the cancer drug improves survival or quality of life, information that is essential in the benefit-risk evaluation for clinical decision making.
That a number of the confirmatory trials examined were delayed or pending emphasize the considerable time that can elapse between drug approval and confirmatory trial completion, they added.
“Timely planning and completion of postmarketing trials is necessary for proper implementation of the accelerated approval pathway, and the FDA should minimize the period during which patients and physicians are using drugs approved through accelerated pathways without rigorous data on their ultimate clinical benefit,” the authors wrote in the analysis.
Dr. Chen, lead author of the RR study, said both studies call into question what criteria is optimal when assessing cancer drug value, while ensuring such measurements are not too high to achieve – preventing useful drugs to market – but also not too low – allowing drugs with marginal benefit into the market.
“There has been tremendous drug development within the oncology space, and it is always important to look back to reassess and see if the process [matches] the original vision so that we can correct any misuse or concerns,” Dr. Chen said in an interview.
Dr. Chen said his study indicates the RR endpoint has been misused in scenarios with low response rate, common cancer, and/or situations with already available therapies. In the study by Dr. Gyawali, the results suggest many drugs approved on the basis of a surrogate endpoint (RR or progression-free survival) ultimately do not demonstrate survival benefit confirmation or patient-reported benefit, Dr. Chen said.
“We hope that readers of these JAMA IM studies and the accompanying commentaries will recognize that there could be a set of guidance criteria from regulatory agencies or oncology organizations to recommend use of surrogate endpoints in special situations: high response rate of the drug, very rare cancer, or highly innovative therapy not yet seen before,” he said. “The use of surrogate endpoints to justify these therapies must also have postmarketing confirmation of survival or patient-reported benefit.”
The study led by Dr. Chen was supported by the Laura and John Arnold Foundation. Dr Chen reported receiving lecture honorarium from Horizon CME; another coauthor reported receiving honorarium from universities, medical centers, and publishers. The study led by Dr. Gyawali was supported by the Arnold Ventures; one of the coauthors reported receiving grant support from the Harvard-MIT Center for Regulatory Science and the Engelberg Foundation, as well as unrelated research funding from the FDA.
SOURCES: Chen EY et al. JAMA Intern Med. 2019 May 28. doi: 10.1001/jamainternmed.2019.0583; Gyawali B et al. JAMA Intern Med. 2019 May 28. doi: 10.1001/jamainternmed.2019.0462.
FROM JAMA INTERNAL MEDICINE
Daratumumab regimen shows benefit in transplant-ineligible myeloma
For patients with newly diagnosed multiple myeloma who are ineligible for autologous stem cell transplantation (ASCT), adding daratumumab to lenalidomide and dexamethasone provides better outcomes than standard therapy alone, based on an interim analysis from the phase 3 MAIA trial.
A greater proportion of patients in the daratumumab group had complete responses and were alive without disease progression after a median follow-up of 28 months, reported lead author Thierry Facon, MD, of the University of Lille (France) and colleagues, who also noted that daratumumab was associated with higher rates of grade 3 or 4 pneumonia, neutropenia, and lymphopenia.
“For patients who are ineligible for stem-cell transplantation, multiagent regimens, including alkylating agents, glucocorticoids, immunomodulatory drugs, proteasome inhibitors, and new agents, are the standard of care,” the investigators wrote in the New England Journal of Medicine.
The findings from MAIA add clarity to the efficacy and safety of daratumumab in this setting, building on previous phase 3 myeloma trials in the same area, such as ALCYONE, CASTOR, and POLLUX, the investigators noted.
MAIA was an open-label, international trial involving 737 patients with newly diagnosed multiple myeloma who were ineligible for ASCT. Patients were randomized in a 1:1 ratio to receive either daratumumab, lenalidomide, and dexamethasone (daratumumab group; n = 368) or lenalidomide and dexamethasone alone (control group; n = 369).
On a 28-day cycle, all patients received oral lenalidomide 25 mg on days 1-21 and oral dexamethasone 40 mg on days 1, 8, 15, and 22. Patients in the daratumumab group received intravenous daratumumab dosed at 16 mg/kg once a week for cycles 1 and 2, every 2 weeks for cycles 3-6, and then every 4 weeks thereafter. Treatment was continued until unacceptable toxic effects or disease progression occurred.
The primary end point was progression-free survival (PFS). Various secondary end points were also evaluated, including time to progression, complete responses, overall survival, and others.
Among the 737 randomized patients, 729 ultimately underwent treatment. The median patient age was 73 years.
Generally, efficacy measures favored adding daratumumab. After a median follow-up of 28.0 months, disease progression or death had occurred in 26.4% of patients in the daratumumab group, compared with 38.8% in the control group.
The median PFS was not reached in the daratumumab group, compared with 31.9 months in the control group. There was a 44% lower risk of disease progression or death among patients who received daratumumab, compared with the control group (hazard ratio, 0.56, P less than .001).
This PFS trend was consistent across most subgroups, including those for sex, age, and race, with the exception of patients with baseline hepatic impairment.
Additional efficacy measures added weight to the apparent benefit of adding daratumumab. For instance, more patients in the daratumumab group achieved a complete response or better (47.6% vs. 24.9%) and were negative for minimum residual disease (24.2% vs. 7.3%).
In terms of safety, more patients in the daratumumab group than the control group developed grade 3 or higher neutropenia (50% vs. 35.3%), lymphopenia (15.1% vs. 10.7%), infections (32.1% vs. 23.3%) or pneumonia (13.7% vs. 7.9%).
In contrast, grade 3 or 4 anemia was less common in the daratumumab group than the control group (11.8% vs. 19.7%). Overall, the rate of serious adverse events was similar for both groups (approximately 63%), as was the rate of adverse events resulting in death (approximately 6%-7%).
“In this trial involving patients with newly diagnosed multiple myeloma who were ineligible for stem-cell transplantation, the addition of daratumumab to lenalidomide and dexamethasone resulted in significantly longer progression-free survival, a higher response rate, an increased depth of response, and a longer duration of response than lenalidomide and dexamethasone alone,” the investigators concluded.
The study was funded by Janssen Research and Development. The investigators reported relationships with Janssen, Celgene, Takeda, Sanofi, and other companies.
SOURCE: Facon T et al. N Engl J Med. 2019;380:2104-15.
The findings from the phase 3 MAIA trial highlight the “superior efficacy” of adding daratumumab to lenalidomide and dexamethasone for patients with newly diagnosed multiple myeloma who are ineligible for stem cell transplantation, Jacob Laubach, MD, commented in an accompanying editorial.
Dr. Laubach noted several important clinical implications of the study findings, including that the use of CD38-targeting monoclonal antibody therapy was associated with a significant improvement in the number of patients who had a complete response to therapy and who were negative for minimal residual disease.
However, with daratumumab as a component of induction and maintenance therapy for patients with multiple myeloma who are ineligible for transplantation, it is important to consider the feasibility of retreatment with CD38-targeting therapy in patients who become resistant to daratumumab-containing regimens.
Jacob Laubach, MD, is at the Dana-Farber Cancer Institute in Boston. He reported having no financial disclosures. He made his remarks in an editorial in the New England Journal of Medicine (2019;380:2172-3).
The findings from the phase 3 MAIA trial highlight the “superior efficacy” of adding daratumumab to lenalidomide and dexamethasone for patients with newly diagnosed multiple myeloma who are ineligible for stem cell transplantation, Jacob Laubach, MD, commented in an accompanying editorial.
Dr. Laubach noted several important clinical implications of the study findings, including that the use of CD38-targeting monoclonal antibody therapy was associated with a significant improvement in the number of patients who had a complete response to therapy and who were negative for minimal residual disease.
However, with daratumumab as a component of induction and maintenance therapy for patients with multiple myeloma who are ineligible for transplantation, it is important to consider the feasibility of retreatment with CD38-targeting therapy in patients who become resistant to daratumumab-containing regimens.
Jacob Laubach, MD, is at the Dana-Farber Cancer Institute in Boston. He reported having no financial disclosures. He made his remarks in an editorial in the New England Journal of Medicine (2019;380:2172-3).
The findings from the phase 3 MAIA trial highlight the “superior efficacy” of adding daratumumab to lenalidomide and dexamethasone for patients with newly diagnosed multiple myeloma who are ineligible for stem cell transplantation, Jacob Laubach, MD, commented in an accompanying editorial.
Dr. Laubach noted several important clinical implications of the study findings, including that the use of CD38-targeting monoclonal antibody therapy was associated with a significant improvement in the number of patients who had a complete response to therapy and who were negative for minimal residual disease.
However, with daratumumab as a component of induction and maintenance therapy for patients with multiple myeloma who are ineligible for transplantation, it is important to consider the feasibility of retreatment with CD38-targeting therapy in patients who become resistant to daratumumab-containing regimens.
Jacob Laubach, MD, is at the Dana-Farber Cancer Institute in Boston. He reported having no financial disclosures. He made his remarks in an editorial in the New England Journal of Medicine (2019;380:2172-3).
For patients with newly diagnosed multiple myeloma who are ineligible for autologous stem cell transplantation (ASCT), adding daratumumab to lenalidomide and dexamethasone provides better outcomes than standard therapy alone, based on an interim analysis from the phase 3 MAIA trial.
A greater proportion of patients in the daratumumab group had complete responses and were alive without disease progression after a median follow-up of 28 months, reported lead author Thierry Facon, MD, of the University of Lille (France) and colleagues, who also noted that daratumumab was associated with higher rates of grade 3 or 4 pneumonia, neutropenia, and lymphopenia.
“For patients who are ineligible for stem-cell transplantation, multiagent regimens, including alkylating agents, glucocorticoids, immunomodulatory drugs, proteasome inhibitors, and new agents, are the standard of care,” the investigators wrote in the New England Journal of Medicine.
The findings from MAIA add clarity to the efficacy and safety of daratumumab in this setting, building on previous phase 3 myeloma trials in the same area, such as ALCYONE, CASTOR, and POLLUX, the investigators noted.
MAIA was an open-label, international trial involving 737 patients with newly diagnosed multiple myeloma who were ineligible for ASCT. Patients were randomized in a 1:1 ratio to receive either daratumumab, lenalidomide, and dexamethasone (daratumumab group; n = 368) or lenalidomide and dexamethasone alone (control group; n = 369).
On a 28-day cycle, all patients received oral lenalidomide 25 mg on days 1-21 and oral dexamethasone 40 mg on days 1, 8, 15, and 22. Patients in the daratumumab group received intravenous daratumumab dosed at 16 mg/kg once a week for cycles 1 and 2, every 2 weeks for cycles 3-6, and then every 4 weeks thereafter. Treatment was continued until unacceptable toxic effects or disease progression occurred.
The primary end point was progression-free survival (PFS). Various secondary end points were also evaluated, including time to progression, complete responses, overall survival, and others.
Among the 737 randomized patients, 729 ultimately underwent treatment. The median patient age was 73 years.
Generally, efficacy measures favored adding daratumumab. After a median follow-up of 28.0 months, disease progression or death had occurred in 26.4% of patients in the daratumumab group, compared with 38.8% in the control group.
The median PFS was not reached in the daratumumab group, compared with 31.9 months in the control group. There was a 44% lower risk of disease progression or death among patients who received daratumumab, compared with the control group (hazard ratio, 0.56, P less than .001).
This PFS trend was consistent across most subgroups, including those for sex, age, and race, with the exception of patients with baseline hepatic impairment.
Additional efficacy measures added weight to the apparent benefit of adding daratumumab. For instance, more patients in the daratumumab group achieved a complete response or better (47.6% vs. 24.9%) and were negative for minimum residual disease (24.2% vs. 7.3%).
In terms of safety, more patients in the daratumumab group than the control group developed grade 3 or higher neutropenia (50% vs. 35.3%), lymphopenia (15.1% vs. 10.7%), infections (32.1% vs. 23.3%) or pneumonia (13.7% vs. 7.9%).
In contrast, grade 3 or 4 anemia was less common in the daratumumab group than the control group (11.8% vs. 19.7%). Overall, the rate of serious adverse events was similar for both groups (approximately 63%), as was the rate of adverse events resulting in death (approximately 6%-7%).
“In this trial involving patients with newly diagnosed multiple myeloma who were ineligible for stem-cell transplantation, the addition of daratumumab to lenalidomide and dexamethasone resulted in significantly longer progression-free survival, a higher response rate, an increased depth of response, and a longer duration of response than lenalidomide and dexamethasone alone,” the investigators concluded.
The study was funded by Janssen Research and Development. The investigators reported relationships with Janssen, Celgene, Takeda, Sanofi, and other companies.
SOURCE: Facon T et al. N Engl J Med. 2019;380:2104-15.
For patients with newly diagnosed multiple myeloma who are ineligible for autologous stem cell transplantation (ASCT), adding daratumumab to lenalidomide and dexamethasone provides better outcomes than standard therapy alone, based on an interim analysis from the phase 3 MAIA trial.
A greater proportion of patients in the daratumumab group had complete responses and were alive without disease progression after a median follow-up of 28 months, reported lead author Thierry Facon, MD, of the University of Lille (France) and colleagues, who also noted that daratumumab was associated with higher rates of grade 3 or 4 pneumonia, neutropenia, and lymphopenia.
“For patients who are ineligible for stem-cell transplantation, multiagent regimens, including alkylating agents, glucocorticoids, immunomodulatory drugs, proteasome inhibitors, and new agents, are the standard of care,” the investigators wrote in the New England Journal of Medicine.
The findings from MAIA add clarity to the efficacy and safety of daratumumab in this setting, building on previous phase 3 myeloma trials in the same area, such as ALCYONE, CASTOR, and POLLUX, the investigators noted.
MAIA was an open-label, international trial involving 737 patients with newly diagnosed multiple myeloma who were ineligible for ASCT. Patients were randomized in a 1:1 ratio to receive either daratumumab, lenalidomide, and dexamethasone (daratumumab group; n = 368) or lenalidomide and dexamethasone alone (control group; n = 369).
On a 28-day cycle, all patients received oral lenalidomide 25 mg on days 1-21 and oral dexamethasone 40 mg on days 1, 8, 15, and 22. Patients in the daratumumab group received intravenous daratumumab dosed at 16 mg/kg once a week for cycles 1 and 2, every 2 weeks for cycles 3-6, and then every 4 weeks thereafter. Treatment was continued until unacceptable toxic effects or disease progression occurred.
The primary end point was progression-free survival (PFS). Various secondary end points were also evaluated, including time to progression, complete responses, overall survival, and others.
Among the 737 randomized patients, 729 ultimately underwent treatment. The median patient age was 73 years.
Generally, efficacy measures favored adding daratumumab. After a median follow-up of 28.0 months, disease progression or death had occurred in 26.4% of patients in the daratumumab group, compared with 38.8% in the control group.
The median PFS was not reached in the daratumumab group, compared with 31.9 months in the control group. There was a 44% lower risk of disease progression or death among patients who received daratumumab, compared with the control group (hazard ratio, 0.56, P less than .001).
This PFS trend was consistent across most subgroups, including those for sex, age, and race, with the exception of patients with baseline hepatic impairment.
Additional efficacy measures added weight to the apparent benefit of adding daratumumab. For instance, more patients in the daratumumab group achieved a complete response or better (47.6% vs. 24.9%) and were negative for minimum residual disease (24.2% vs. 7.3%).
In terms of safety, more patients in the daratumumab group than the control group developed grade 3 or higher neutropenia (50% vs. 35.3%), lymphopenia (15.1% vs. 10.7%), infections (32.1% vs. 23.3%) or pneumonia (13.7% vs. 7.9%).
In contrast, grade 3 or 4 anemia was less common in the daratumumab group than the control group (11.8% vs. 19.7%). Overall, the rate of serious adverse events was similar for both groups (approximately 63%), as was the rate of adverse events resulting in death (approximately 6%-7%).
“In this trial involving patients with newly diagnosed multiple myeloma who were ineligible for stem-cell transplantation, the addition of daratumumab to lenalidomide and dexamethasone resulted in significantly longer progression-free survival, a higher response rate, an increased depth of response, and a longer duration of response than lenalidomide and dexamethasone alone,” the investigators concluded.
The study was funded by Janssen Research and Development. The investigators reported relationships with Janssen, Celgene, Takeda, Sanofi, and other companies.
SOURCE: Facon T et al. N Engl J Med. 2019;380:2104-15.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: For patients with newly diagnosed multiple myeloma who are ineligible for autologous stem cell transplantation, adding daratumumab to lenalidomide and dexamethasone standard therapy provides better outcomes than standard therapy alone.
Major finding: After 28-month follow-up, 26.4% of patients in the daratumumab group had disease progression or died, compared with 38.8% in the control group.
Study details: A randomized, open-label, phase 3 trial involving 737 patients with newly diagnosed multiple myeloma.
Disclosures: The study was funded by Janssen Research and Development. The investigators reported relationships with Janssen, Celgene, Takeda, Sanofi, and other companies.
Source: Facon T et al. N Engl J Med. 2019;380:2104-15.
Genetic analysis identifies prognostic markers in CLL
A genetic analysis of patients with chronic lymphocytic leukemia treated with frontline, rituximab-based regimens found that deletion 11q22 and unmutated IgVH status may predict worse prognosis.
Michaela Spunarova, MD, of Masaryk University, Brno, Czech Republic, and colleagues conducted a genetic analysis of 177 patients with chronic lymphocytic leukemia (CLL). The results of the analysis were published in Leukemia Research.
The study focused on patients with CLL with an intact TP53 gene, looking at recurrently muted genes in CLL, genomic aberrations by fluorescence in situ hybridization, and IgVH status, according to the researchers.
The team analyzed the effects of these mutations on progression-free survival (PFS) following frontline treatment with bendamustine and rituximab (BR) or fludarabine, cyclophosphamide, and rituximab (FCR) therapeutic regimens.
Dr. Spunarova and colleagues used next-generation sequencing to analyze DNA from the patient samples. Data on 11q22, 13q14, trisomy 12, and IgVH mutation status were also considered in the analyses of PFS.
After analysis, the researchers validated that unmutated IgVH status is an indicator of poor prognosis in CLL patients with wild-type TP53 treated with frontline FCR.
When looking at both BR and FCR regimens, a single 11q22 deletion, lacking an ATM mutation on the other allele, resulted in the shortest PFS, at a median of just 16 months.
“Based on our data, special attention should be given to CLL patients harboring a sole 11q22 deletion, with no ATM mutation on the other allele, who manifest particularly short PFS,” they noted.
The researchers acknowledged a key limitation of the study was the small sample size. As a result, the results should be interpreted in a careful manner.
The study was funded by the Ministry of Health of the Czech Republic. The authors reported having no conflicts of interest.
SOURCE: Spunarova M et al. Leuk Res. 2019 Jun;81:75-81.
A genetic analysis of patients with chronic lymphocytic leukemia treated with frontline, rituximab-based regimens found that deletion 11q22 and unmutated IgVH status may predict worse prognosis.
Michaela Spunarova, MD, of Masaryk University, Brno, Czech Republic, and colleagues conducted a genetic analysis of 177 patients with chronic lymphocytic leukemia (CLL). The results of the analysis were published in Leukemia Research.
The study focused on patients with CLL with an intact TP53 gene, looking at recurrently muted genes in CLL, genomic aberrations by fluorescence in situ hybridization, and IgVH status, according to the researchers.
The team analyzed the effects of these mutations on progression-free survival (PFS) following frontline treatment with bendamustine and rituximab (BR) or fludarabine, cyclophosphamide, and rituximab (FCR) therapeutic regimens.
Dr. Spunarova and colleagues used next-generation sequencing to analyze DNA from the patient samples. Data on 11q22, 13q14, trisomy 12, and IgVH mutation status were also considered in the analyses of PFS.
After analysis, the researchers validated that unmutated IgVH status is an indicator of poor prognosis in CLL patients with wild-type TP53 treated with frontline FCR.
When looking at both BR and FCR regimens, a single 11q22 deletion, lacking an ATM mutation on the other allele, resulted in the shortest PFS, at a median of just 16 months.
“Based on our data, special attention should be given to CLL patients harboring a sole 11q22 deletion, with no ATM mutation on the other allele, who manifest particularly short PFS,” they noted.
The researchers acknowledged a key limitation of the study was the small sample size. As a result, the results should be interpreted in a careful manner.
The study was funded by the Ministry of Health of the Czech Republic. The authors reported having no conflicts of interest.
SOURCE: Spunarova M et al. Leuk Res. 2019 Jun;81:75-81.
A genetic analysis of patients with chronic lymphocytic leukemia treated with frontline, rituximab-based regimens found that deletion 11q22 and unmutated IgVH status may predict worse prognosis.
Michaela Spunarova, MD, of Masaryk University, Brno, Czech Republic, and colleagues conducted a genetic analysis of 177 patients with chronic lymphocytic leukemia (CLL). The results of the analysis were published in Leukemia Research.
The study focused on patients with CLL with an intact TP53 gene, looking at recurrently muted genes in CLL, genomic aberrations by fluorescence in situ hybridization, and IgVH status, according to the researchers.
The team analyzed the effects of these mutations on progression-free survival (PFS) following frontline treatment with bendamustine and rituximab (BR) or fludarabine, cyclophosphamide, and rituximab (FCR) therapeutic regimens.
Dr. Spunarova and colleagues used next-generation sequencing to analyze DNA from the patient samples. Data on 11q22, 13q14, trisomy 12, and IgVH mutation status were also considered in the analyses of PFS.
After analysis, the researchers validated that unmutated IgVH status is an indicator of poor prognosis in CLL patients with wild-type TP53 treated with frontline FCR.
When looking at both BR and FCR regimens, a single 11q22 deletion, lacking an ATM mutation on the other allele, resulted in the shortest PFS, at a median of just 16 months.
“Based on our data, special attention should be given to CLL patients harboring a sole 11q22 deletion, with no ATM mutation on the other allele, who manifest particularly short PFS,” they noted.
The researchers acknowledged a key limitation of the study was the small sample size. As a result, the results should be interpreted in a careful manner.
The study was funded by the Ministry of Health of the Czech Republic. The authors reported having no conflicts of interest.
SOURCE: Spunarova M et al. Leuk Res. 2019 Jun;81:75-81.
FROM LEUKEMIA RESEARCH
FDA: Faulty hematology analyzers face class I recall
The Food and Drug Administration is alerting laboratories and providers to a class I recall on Beckman Coulter hematology analyzers because of the potential for inaccurate platelet count results.
A class I recall indicates reasonable probability of serious adverse health consequences or death associated with use, according to the FDA.
The recall is related to the devices’ platelet analyzing function; among other uses, these devices help assess patients fitness for surgery, so a faulty reading on platelet counts could result in increased risk for life-threatening bleeding during a procedure in patients who have unidentified severe thrombocytopenia, according to a statement from the agency.
“Because this may cause serious injury, or even death, to a patient, we are urging health care professionals to be aware of the potential for inaccurate diagnostic results with these analyzers and to take appropriate actions including the use of alternative diagnostic testing or confirming analyzer results with manual scanning or estimate of platelets,” Tim Stenzel, MD, PhD, director of the Office of In Vitro Diagnostics and Radiological Health in the FDA’s Center for Devices and Radiological Health, said in the statement.
The recall applies to the UniCel DxH 800 Coulter Cellular Analysis System, UniCel DxH 600 Coulter Cellular Analysis System, and UniCel DxH 900 Coulter Cellular Analysis System. The faulty devices were first identified in 2018, and the manufacturer released an urgent medical device correction letter at that time. The company has more recently released a software patch for the devices, but the FDA has not yet assessed whether it resolves the problem. The agency has released detailed actions and recommendations related to these devices.
At this time, the FDA is unaware of any serious adverse events that have been directly linked to these devices, but the agency recommends that any events be reported through its MedWatch reporting system.
The Food and Drug Administration is alerting laboratories and providers to a class I recall on Beckman Coulter hematology analyzers because of the potential for inaccurate platelet count results.
A class I recall indicates reasonable probability of serious adverse health consequences or death associated with use, according to the FDA.
The recall is related to the devices’ platelet analyzing function; among other uses, these devices help assess patients fitness for surgery, so a faulty reading on platelet counts could result in increased risk for life-threatening bleeding during a procedure in patients who have unidentified severe thrombocytopenia, according to a statement from the agency.
“Because this may cause serious injury, or even death, to a patient, we are urging health care professionals to be aware of the potential for inaccurate diagnostic results with these analyzers and to take appropriate actions including the use of alternative diagnostic testing or confirming analyzer results with manual scanning or estimate of platelets,” Tim Stenzel, MD, PhD, director of the Office of In Vitro Diagnostics and Radiological Health in the FDA’s Center for Devices and Radiological Health, said in the statement.
The recall applies to the UniCel DxH 800 Coulter Cellular Analysis System, UniCel DxH 600 Coulter Cellular Analysis System, and UniCel DxH 900 Coulter Cellular Analysis System. The faulty devices were first identified in 2018, and the manufacturer released an urgent medical device correction letter at that time. The company has more recently released a software patch for the devices, but the FDA has not yet assessed whether it resolves the problem. The agency has released detailed actions and recommendations related to these devices.
At this time, the FDA is unaware of any serious adverse events that have been directly linked to these devices, but the agency recommends that any events be reported through its MedWatch reporting system.
The Food and Drug Administration is alerting laboratories and providers to a class I recall on Beckman Coulter hematology analyzers because of the potential for inaccurate platelet count results.
A class I recall indicates reasonable probability of serious adverse health consequences or death associated with use, according to the FDA.
The recall is related to the devices’ platelet analyzing function; among other uses, these devices help assess patients fitness for surgery, so a faulty reading on platelet counts could result in increased risk for life-threatening bleeding during a procedure in patients who have unidentified severe thrombocytopenia, according to a statement from the agency.
“Because this may cause serious injury, or even death, to a patient, we are urging health care professionals to be aware of the potential for inaccurate diagnostic results with these analyzers and to take appropriate actions including the use of alternative diagnostic testing or confirming analyzer results with manual scanning or estimate of platelets,” Tim Stenzel, MD, PhD, director of the Office of In Vitro Diagnostics and Radiological Health in the FDA’s Center for Devices and Radiological Health, said in the statement.
The recall applies to the UniCel DxH 800 Coulter Cellular Analysis System, UniCel DxH 600 Coulter Cellular Analysis System, and UniCel DxH 900 Coulter Cellular Analysis System. The faulty devices were first identified in 2018, and the manufacturer released an urgent medical device correction letter at that time. The company has more recently released a software patch for the devices, but the FDA has not yet assessed whether it resolves the problem. The agency has released detailed actions and recommendations related to these devices.
At this time, the FDA is unaware of any serious adverse events that have been directly linked to these devices, but the agency recommends that any events be reported through its MedWatch reporting system.
Call for 2019 AVAHO Abstracts
The AVAHO Program Planning Committee is pleased to announce the call for abstracts for our meeting in Minneapolis, Minnesota, September 20-22, 2019. Abstracts should be submitted online here: https://www.avaho.org/call-for-abstracts.
Abstracts should not exceed 350 words, excluding title and titles should not exceed 300 characters. Illustrations, tables or bullet points are not permitted.
The following categories of abstracts are suitable for submission:
- Research (eg, clinical trials, laboratory studies, translational studies, descriptive studies, qualitative studies)
- Evidence-Based Projects
- Quality Improvement Projects and initiatives
- Clinical Practice (eg, best clinical practice exemplar, case study, disease management, palliative care (non-research), survivorship (nonresearch), symptom management (non-research)
- Program Initiatives (eg, workforce, infrastructure, workflow)
- Projects in Progress
The first author is considered the primary author, is responsible for the content and integrity of the project, and will serve as the contact person by the AVAHO administrator and Planning Committee. Authors may submit more than one abstract; however, they may be first author on only one abstract. At least one author must be a member of AVAHO. One author must present the abstract at the meeting. Accepted abstracts will be published in a special edition of Federal Practitioner.
The electronic poster session was viewed very favorably by attendees last year and will be used again this year. This requires more logistic hurdles and thus, the June 1 submission deadline is firm.
Each poster will be assigned to one of two time slots for presentation. One author must be present to give a brief presentation of the poster. Authors are encouraged to record their poster presentation to provide attendees another option for viewing your poster. This recording does NOT replace the obligation to be present IN PERSON during the assigned poster session. Details regarding this recording will be forthcoming.
All abstracts must be submitted by June 1, 2019. This is a firm deadline. No extensions.
The AVAHO Program Planning Committee is pleased to announce the call for abstracts for our meeting in Minneapolis, Minnesota, September 20-22, 2019. Abstracts should be submitted online here: https://www.avaho.org/call-for-abstracts.
Abstracts should not exceed 350 words, excluding title and titles should not exceed 300 characters. Illustrations, tables or bullet points are not permitted.
The following categories of abstracts are suitable for submission:
- Research (eg, clinical trials, laboratory studies, translational studies, descriptive studies, qualitative studies)
- Evidence-Based Projects
- Quality Improvement Projects and initiatives
- Clinical Practice (eg, best clinical practice exemplar, case study, disease management, palliative care (non-research), survivorship (nonresearch), symptom management (non-research)
- Program Initiatives (eg, workforce, infrastructure, workflow)
- Projects in Progress
The first author is considered the primary author, is responsible for the content and integrity of the project, and will serve as the contact person by the AVAHO administrator and Planning Committee. Authors may submit more than one abstract; however, they may be first author on only one abstract. At least one author must be a member of AVAHO. One author must present the abstract at the meeting. Accepted abstracts will be published in a special edition of Federal Practitioner.
The electronic poster session was viewed very favorably by attendees last year and will be used again this year. This requires more logistic hurdles and thus, the June 1 submission deadline is firm.
Each poster will be assigned to one of two time slots for presentation. One author must be present to give a brief presentation of the poster. Authors are encouraged to record their poster presentation to provide attendees another option for viewing your poster. This recording does NOT replace the obligation to be present IN PERSON during the assigned poster session. Details regarding this recording will be forthcoming.
All abstracts must be submitted by June 1, 2019. This is a firm deadline. No extensions.
The AVAHO Program Planning Committee is pleased to announce the call for abstracts for our meeting in Minneapolis, Minnesota, September 20-22, 2019. Abstracts should be submitted online here: https://www.avaho.org/call-for-abstracts.
Abstracts should not exceed 350 words, excluding title and titles should not exceed 300 characters. Illustrations, tables or bullet points are not permitted.
The following categories of abstracts are suitable for submission:
- Research (eg, clinical trials, laboratory studies, translational studies, descriptive studies, qualitative studies)
- Evidence-Based Projects
- Quality Improvement Projects and initiatives
- Clinical Practice (eg, best clinical practice exemplar, case study, disease management, palliative care (non-research), survivorship (nonresearch), symptom management (non-research)
- Program Initiatives (eg, workforce, infrastructure, workflow)
- Projects in Progress
The first author is considered the primary author, is responsible for the content and integrity of the project, and will serve as the contact person by the AVAHO administrator and Planning Committee. Authors may submit more than one abstract; however, they may be first author on only one abstract. At least one author must be a member of AVAHO. One author must present the abstract at the meeting. Accepted abstracts will be published in a special edition of Federal Practitioner.
The electronic poster session was viewed very favorably by attendees last year and will be used again this year. This requires more logistic hurdles and thus, the June 1 submission deadline is firm.
Each poster will be assigned to one of two time slots for presentation. One author must be present to give a brief presentation of the poster. Authors are encouraged to record their poster presentation to provide attendees another option for viewing your poster. This recording does NOT replace the obligation to be present IN PERSON during the assigned poster session. Details regarding this recording will be forthcoming.
All abstracts must be submitted by June 1, 2019. This is a firm deadline. No extensions.
Family history plays a large role in bleeding disorder diagnosis in women
Disease severity and family history appear to play a significant role in the age of diagnosis for women with congenital bleeding disorders, according to recent survey findings.
A European multinational survey has identified delays in diagnosis and other challenges faced by girls and women with congenital bleeding disorders.
“The aim of this survey, carried out by the European Haemophilia Consortium (EHC), was to provide the patient voice of their lived experiences with congenital bleeding disorders,” wrote Declan Noone of the EHC in Brussels, and colleagues. The findings were published in Haemophilia.
The researchers conducted a survey of 709 girls and women with various congenital bleeding disorders from 32 countries, primarily located in Western Europe. Most respondents were adults, with just 3.8% under age 18 years.
The questionnaire was administered to eligible patients at various hemophilia treatment centers. More than half of respondents were hemophilia carriers and nearly 28% had von Willebrand disease.
The survey explored the effects of bleeding disorders on several activities of daily life, including symptoms, physical activity, and reproductive ability.
After analysis, the researchers found that overall the median age at diagnosis of a bleeding disorder was 16 years (range, 2-28 years) among respondents. Having a family history of a bleeding disorder resulted in a significantly younger median age at diagnosis (6 years; range, 0-26 years) versus those without a family history (17 years; range 5-28 years; P less than .01).
Disease severity also appears to play a role. Women with type 3 von Willebrand disease had a median age of diagnosis of 1 year old, compared with 19.3 years old for type 2 disease (P less than .01).
Respondents reported a substantial disease burden on activities of daily life, especially for women with platelet function disorders and other factor deficiency.
Women without a known family history of a bleeding disorders reported a significantly greater impact on their physical life, social life, and romantic life (P less than .01 for all domains), compared with women with a family history of bleeding disorders.
There were no statistically significant differences across types of bleeding disorders on questions related to reproductive life. However, the researchers reported that “surprisingly,” 25% of women reported that having a bleeding disorder “has had a severe impact on their decision or has prevented them from having children.
“The bleeding symptom of biggest impact on daily life is [heavy menstrual bleeding], reported by 55% of women,” the researchers wrote.
The researchers acknowledged that a key limitation of the survey was the composition of the sample: predominantly of patients from Western Europe. As a result, the findings may not be generalizable to all patient populations.
No funding sources were reported. The authors reported having no conflicts of interest.
SOURCE: Noone D et al. Haemophilia. 2019 Apr 29. doi: 10.1111/hae.13722.
Disease severity and family history appear to play a significant role in the age of diagnosis for women with congenital bleeding disorders, according to recent survey findings.
A European multinational survey has identified delays in diagnosis and other challenges faced by girls and women with congenital bleeding disorders.
“The aim of this survey, carried out by the European Haemophilia Consortium (EHC), was to provide the patient voice of their lived experiences with congenital bleeding disorders,” wrote Declan Noone of the EHC in Brussels, and colleagues. The findings were published in Haemophilia.
The researchers conducted a survey of 709 girls and women with various congenital bleeding disorders from 32 countries, primarily located in Western Europe. Most respondents were adults, with just 3.8% under age 18 years.
The questionnaire was administered to eligible patients at various hemophilia treatment centers. More than half of respondents were hemophilia carriers and nearly 28% had von Willebrand disease.
The survey explored the effects of bleeding disorders on several activities of daily life, including symptoms, physical activity, and reproductive ability.
After analysis, the researchers found that overall the median age at diagnosis of a bleeding disorder was 16 years (range, 2-28 years) among respondents. Having a family history of a bleeding disorder resulted in a significantly younger median age at diagnosis (6 years; range, 0-26 years) versus those without a family history (17 years; range 5-28 years; P less than .01).
Disease severity also appears to play a role. Women with type 3 von Willebrand disease had a median age of diagnosis of 1 year old, compared with 19.3 years old for type 2 disease (P less than .01).
Respondents reported a substantial disease burden on activities of daily life, especially for women with platelet function disorders and other factor deficiency.
Women without a known family history of a bleeding disorders reported a significantly greater impact on their physical life, social life, and romantic life (P less than .01 for all domains), compared with women with a family history of bleeding disorders.
There were no statistically significant differences across types of bleeding disorders on questions related to reproductive life. However, the researchers reported that “surprisingly,” 25% of women reported that having a bleeding disorder “has had a severe impact on their decision or has prevented them from having children.
“The bleeding symptom of biggest impact on daily life is [heavy menstrual bleeding], reported by 55% of women,” the researchers wrote.
The researchers acknowledged that a key limitation of the survey was the composition of the sample: predominantly of patients from Western Europe. As a result, the findings may not be generalizable to all patient populations.
No funding sources were reported. The authors reported having no conflicts of interest.
SOURCE: Noone D et al. Haemophilia. 2019 Apr 29. doi: 10.1111/hae.13722.
Disease severity and family history appear to play a significant role in the age of diagnosis for women with congenital bleeding disorders, according to recent survey findings.
A European multinational survey has identified delays in diagnosis and other challenges faced by girls and women with congenital bleeding disorders.
“The aim of this survey, carried out by the European Haemophilia Consortium (EHC), was to provide the patient voice of their lived experiences with congenital bleeding disorders,” wrote Declan Noone of the EHC in Brussels, and colleagues. The findings were published in Haemophilia.
The researchers conducted a survey of 709 girls and women with various congenital bleeding disorders from 32 countries, primarily located in Western Europe. Most respondents were adults, with just 3.8% under age 18 years.
The questionnaire was administered to eligible patients at various hemophilia treatment centers. More than half of respondents were hemophilia carriers and nearly 28% had von Willebrand disease.
The survey explored the effects of bleeding disorders on several activities of daily life, including symptoms, physical activity, and reproductive ability.
After analysis, the researchers found that overall the median age at diagnosis of a bleeding disorder was 16 years (range, 2-28 years) among respondents. Having a family history of a bleeding disorder resulted in a significantly younger median age at diagnosis (6 years; range, 0-26 years) versus those without a family history (17 years; range 5-28 years; P less than .01).
Disease severity also appears to play a role. Women with type 3 von Willebrand disease had a median age of diagnosis of 1 year old, compared with 19.3 years old for type 2 disease (P less than .01).
Respondents reported a substantial disease burden on activities of daily life, especially for women with platelet function disorders and other factor deficiency.
Women without a known family history of a bleeding disorders reported a significantly greater impact on their physical life, social life, and romantic life (P less than .01 for all domains), compared with women with a family history of bleeding disorders.
There were no statistically significant differences across types of bleeding disorders on questions related to reproductive life. However, the researchers reported that “surprisingly,” 25% of women reported that having a bleeding disorder “has had a severe impact on their decision or has prevented them from having children.
“The bleeding symptom of biggest impact on daily life is [heavy menstrual bleeding], reported by 55% of women,” the researchers wrote.
The researchers acknowledged that a key limitation of the survey was the composition of the sample: predominantly of patients from Western Europe. As a result, the findings may not be generalizable to all patient populations.
No funding sources were reported. The authors reported having no conflicts of interest.
SOURCE: Noone D et al. Haemophilia. 2019 Apr 29. doi: 10.1111/hae.13722.
FROM HAEMOPHILIA