User login
Prescribed opioids increase pneumonia risk in patients with, without HIV
Prescribed opioids were associated with an increase in community-acquired pneumonia in patients with and without HIV infection, according to results of a large database study.
People living with HIV (PLWH) appeared to have a greater community-acquired pneumonia (CAP) risk at lower opioid doses and particularly with immunosuppressive opioids compared with uninfected patients, although the difference was not significant, E. Jennifer Edelman, MD, of Yale University, New Haven, Conn., and her colleagues wrote in JAMA Internal Medicine.
The researchers performed a nested case-control study comprising 25,392 participants (98.9% men; mean age, 55 years) in the Veterans Aging Cohort Study from Jan. 1, 2000, through Dec. 31, 2012.
Dr. Edelman and her colleagues compared the characteristics of 4,246 CAP cases with those of 21,146 uninfected controls in the sample. They also compared cases and controls by HIV status. They ran bivariate and multivariate analysis to estimate odds ratios for CAP risk associated with opioid exposure. In addition, the researchers ran models stratified by HIV status and formally checked for an interaction between prescribed opioid characteristics and HIV status.
In unadjusted logistic regression, prescribed opioids were associated with increased odds of CAP, with the greatest risk observed with currently prescribed opioids, compared with past prescribed opioids or no opioids.
Prescribed opioids remained associated with CAP in the adjusted models for past unknown or nonimmunosuppressive (adjusted OR, 1.24; 95% confidence interval, 1.09-1.40) and past immunosuppressive opioid use (aOR, 1.42; 95% CI, 1.21-1.67).
For currently prescribed opioids, nonimmunosuppressive or unknown, the aOR was 1.23 (95% CI, 1.03-1.48). For currently prescribed immunosuppressive opioids, the aOR was 3.18 (95% CI, 2.44-4.14).
The researchers also found evidence of a dose-response effect such that currently prescribed high-dose opioids were associated with the greatest CAP risk, followed by medium- and then by low-dose opioids, whether immunosuppressive or not.
With regard to the effect of HIV status in stratified, adjusted analyses, CAP risk tended to be greater among PLWH with current prescribed opioids, especially immunosuppressive opioids, compared with uninfected patients. However, the overall interaction term for opioid × HIV status was not significant (P = .36).
Although the researchers stated that a limitation of their study was an inability to prove causality or rule out respiratory depression (vs. immunosuppression) as the cause of the increased CAP risk, “the observed effects of opioid immunosuppressive properties and CAP risk lend support to our hypothesis that opioids have clinically relevant immunosuppressive properties.”
Dr. Edelman and her colleagues cited several limitations. For example, they were not able to determine whether patients took their prescribed medications appropriately and assess whether the patients took nonmedically prescribed opioids. Also, because men made up such a large portion of the study population, it is unclear whether the results are generalizable to women.
Nevertheless, the study “adds to growing evidence of potential medical harms associated with prescribed opioids,” they wrote.
“Health care professionals should be aware of this additional CAP risk when they prescribe opioids, and future studies should investigate the effects of opioids prescribed for longer durations and on other immune-related outcomes,” wrote Dr. Edelman and her colleagues. “Understanding whether mitigating the risk of prescribed opioids for CAP is possible by using a lower dose and nonimmunosuppressive opioids awaits further study.”
However, without such data, when prescribed opioids are warranted, physicians should attempt to modify other factors known to affect CAP risk, including smoking and lack of vaccination, Dr. Edelman and her colleagues concluded.
Several U.S. government agencies and Yale University provided funding for the study. The authors reported that they had no conflicts.
SOURCE: Edelman EJ et al. JAMA Intern Med. 2019 Jan 7. doi: 10.1001/jamainternmed.2018.6101.
Prescribed opioids were associated with an increase in community-acquired pneumonia in patients with and without HIV infection, according to results of a large database study.
People living with HIV (PLWH) appeared to have a greater community-acquired pneumonia (CAP) risk at lower opioid doses and particularly with immunosuppressive opioids compared with uninfected patients, although the difference was not significant, E. Jennifer Edelman, MD, of Yale University, New Haven, Conn., and her colleagues wrote in JAMA Internal Medicine.
The researchers performed a nested case-control study comprising 25,392 participants (98.9% men; mean age, 55 years) in the Veterans Aging Cohort Study from Jan. 1, 2000, through Dec. 31, 2012.
Dr. Edelman and her colleagues compared the characteristics of 4,246 CAP cases with those of 21,146 uninfected controls in the sample. They also compared cases and controls by HIV status. They ran bivariate and multivariate analysis to estimate odds ratios for CAP risk associated with opioid exposure. In addition, the researchers ran models stratified by HIV status and formally checked for an interaction between prescribed opioid characteristics and HIV status.
In unadjusted logistic regression, prescribed opioids were associated with increased odds of CAP, with the greatest risk observed with currently prescribed opioids, compared with past prescribed opioids or no opioids.
Prescribed opioids remained associated with CAP in the adjusted models for past unknown or nonimmunosuppressive (adjusted OR, 1.24; 95% confidence interval, 1.09-1.40) and past immunosuppressive opioid use (aOR, 1.42; 95% CI, 1.21-1.67).
For currently prescribed opioids, nonimmunosuppressive or unknown, the aOR was 1.23 (95% CI, 1.03-1.48). For currently prescribed immunosuppressive opioids, the aOR was 3.18 (95% CI, 2.44-4.14).
The researchers also found evidence of a dose-response effect such that currently prescribed high-dose opioids were associated with the greatest CAP risk, followed by medium- and then by low-dose opioids, whether immunosuppressive or not.
With regard to the effect of HIV status in stratified, adjusted analyses, CAP risk tended to be greater among PLWH with current prescribed opioids, especially immunosuppressive opioids, compared with uninfected patients. However, the overall interaction term for opioid × HIV status was not significant (P = .36).
Although the researchers stated that a limitation of their study was an inability to prove causality or rule out respiratory depression (vs. immunosuppression) as the cause of the increased CAP risk, “the observed effects of opioid immunosuppressive properties and CAP risk lend support to our hypothesis that opioids have clinically relevant immunosuppressive properties.”
Dr. Edelman and her colleagues cited several limitations. For example, they were not able to determine whether patients took their prescribed medications appropriately and assess whether the patients took nonmedically prescribed opioids. Also, because men made up such a large portion of the study population, it is unclear whether the results are generalizable to women.
Nevertheless, the study “adds to growing evidence of potential medical harms associated with prescribed opioids,” they wrote.
“Health care professionals should be aware of this additional CAP risk when they prescribe opioids, and future studies should investigate the effects of opioids prescribed for longer durations and on other immune-related outcomes,” wrote Dr. Edelman and her colleagues. “Understanding whether mitigating the risk of prescribed opioids for CAP is possible by using a lower dose and nonimmunosuppressive opioids awaits further study.”
However, without such data, when prescribed opioids are warranted, physicians should attempt to modify other factors known to affect CAP risk, including smoking and lack of vaccination, Dr. Edelman and her colleagues concluded.
Several U.S. government agencies and Yale University provided funding for the study. The authors reported that they had no conflicts.
SOURCE: Edelman EJ et al. JAMA Intern Med. 2019 Jan 7. doi: 10.1001/jamainternmed.2018.6101.
Prescribed opioids were associated with an increase in community-acquired pneumonia in patients with and without HIV infection, according to results of a large database study.
People living with HIV (PLWH) appeared to have a greater community-acquired pneumonia (CAP) risk at lower opioid doses and particularly with immunosuppressive opioids compared with uninfected patients, although the difference was not significant, E. Jennifer Edelman, MD, of Yale University, New Haven, Conn., and her colleagues wrote in JAMA Internal Medicine.
The researchers performed a nested case-control study comprising 25,392 participants (98.9% men; mean age, 55 years) in the Veterans Aging Cohort Study from Jan. 1, 2000, through Dec. 31, 2012.
Dr. Edelman and her colleagues compared the characteristics of 4,246 CAP cases with those of 21,146 uninfected controls in the sample. They also compared cases and controls by HIV status. They ran bivariate and multivariate analysis to estimate odds ratios for CAP risk associated with opioid exposure. In addition, the researchers ran models stratified by HIV status and formally checked for an interaction between prescribed opioid characteristics and HIV status.
In unadjusted logistic regression, prescribed opioids were associated with increased odds of CAP, with the greatest risk observed with currently prescribed opioids, compared with past prescribed opioids or no opioids.
Prescribed opioids remained associated with CAP in the adjusted models for past unknown or nonimmunosuppressive (adjusted OR, 1.24; 95% confidence interval, 1.09-1.40) and past immunosuppressive opioid use (aOR, 1.42; 95% CI, 1.21-1.67).
For currently prescribed opioids, nonimmunosuppressive or unknown, the aOR was 1.23 (95% CI, 1.03-1.48). For currently prescribed immunosuppressive opioids, the aOR was 3.18 (95% CI, 2.44-4.14).
The researchers also found evidence of a dose-response effect such that currently prescribed high-dose opioids were associated with the greatest CAP risk, followed by medium- and then by low-dose opioids, whether immunosuppressive or not.
With regard to the effect of HIV status in stratified, adjusted analyses, CAP risk tended to be greater among PLWH with current prescribed opioids, especially immunosuppressive opioids, compared with uninfected patients. However, the overall interaction term for opioid × HIV status was not significant (P = .36).
Although the researchers stated that a limitation of their study was an inability to prove causality or rule out respiratory depression (vs. immunosuppression) as the cause of the increased CAP risk, “the observed effects of opioid immunosuppressive properties and CAP risk lend support to our hypothesis that opioids have clinically relevant immunosuppressive properties.”
Dr. Edelman and her colleagues cited several limitations. For example, they were not able to determine whether patients took their prescribed medications appropriately and assess whether the patients took nonmedically prescribed opioids. Also, because men made up such a large portion of the study population, it is unclear whether the results are generalizable to women.
Nevertheless, the study “adds to growing evidence of potential medical harms associated with prescribed opioids,” they wrote.
“Health care professionals should be aware of this additional CAP risk when they prescribe opioids, and future studies should investigate the effects of opioids prescribed for longer durations and on other immune-related outcomes,” wrote Dr. Edelman and her colleagues. “Understanding whether mitigating the risk of prescribed opioids for CAP is possible by using a lower dose and nonimmunosuppressive opioids awaits further study.”
However, without such data, when prescribed opioids are warranted, physicians should attempt to modify other factors known to affect CAP risk, including smoking and lack of vaccination, Dr. Edelman and her colleagues concluded.
Several U.S. government agencies and Yale University provided funding for the study. The authors reported that they had no conflicts.
SOURCE: Edelman EJ et al. JAMA Intern Med. 2019 Jan 7. doi: 10.1001/jamainternmed.2018.6101.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Prescribed opioids, especially those with immunosuppressive properties, are associated with increased community-acquired pneumonia risk.
Major finding: For currently prescribed immunosuppressive opioids, the adjusted odds ratio for community-acquired pneumonia was 3.18 (95% confidence interval, 2.44-4.14).
Study details: A nested case-control study of 25,392 patients in the Veterans Aging Cohort Study from Jan. 1, 2000, through Dec. 31, 2012.
Disclosures: Funding was provided by a variety of government organizations and Yale University, New Haven, Conn. The authors reported that they had no conflicts.
Source: Edelman EJ et al. JAMA Intern Med. 2019 Jan 7. doi: 10.1001/jamainternmed.2018.6101.
HBV, HCV, HIV testing of new cancer patients advised
Oncologists should consider testing all patients with newly diagnosed cancers for infection with the hepatitis B and C viruses, a multicenter team has recommended.
A prospective study of hepatitis B virus (HBV), hepatitis C virus (HCV), and HIV infections among 3,051 patients with newly diagnosed cancers showed that 6.5% of patients tested positive for previous HBV and 0.6% had chronic HBV infection. In addition, 2.4% of patients were positive for HCV, and 1.1% for HIV infections, reported Scott D. Ramsey, MD, PhD, from the Fred Hutchinson Cancer Research Center in Seattle, and colleagues.
“Many patients had no known risk factors for infection, suggesting that current risk-based models for screening may be insufficient. Thus, we believe our results warrant consideration of universal testing of patients with newly diagnosed cancer for HBV and HCV infection, particularly if such an approach is shown to be cost effective,” they wrote in JAMA Oncology.
The investigators noted that patients with undiagnosed hepatitis and/or HIV infections could transmit them to unsuspecting caregivers, adding that “with effective treatments available, not screening for these viruses misses an opportunity to reduce future morbidity associated with these infections and to avoid viral reactivation during treatment, with resulting morbidity and mortality.”
To estimate the prevalence of the infections in patients with newly diagnosed cancers, investigators looked at a cohort of 3,051 patients with a cancer diagnosis made within the previous 120 days at nine academic medical centers and nine community oncology centers representing a total of 41 cancer clinics affiliated with the SWOG Cancer Research Network (formerly the Southwest Oncology Group).
The median patient age was 60.6 years. Female patients constitute 60.4% of the sample; 18.1% were black, and 18.3% were of Hispanic heritage.
Of 3,050 patients for whom HBV testing results were available, 6.5% (197) were positive for previous HBV infection, compared with an estimated U.S. population prevalence of 4.7%. In addition, 0.6% (19 patients) were found to have chronic HBV, compared with an estimated 0.3% US population prevalence.
HCV infections were detected in 2.4% (71 of 2990 patients), compared with an estimated population prevalence of 1.3%, and HIV infections were detected in 1.1%, compared with a background estimated population prevalence of 0.3%.
In all, 32 patients were diagnosed with viral infections by testing performed for the study, including 8 patients with chronic HBV, 22 with HCV, and 2 with HIV.
Additionally, the authors found that 4 patients with chronic HBV, 23 with HCV, and 7 with HIV had no identifiable risk factors.
The highest prevalence of infections occurred among patients with liver cancer, nonliver and noncolorectal cancers of the gastrointestinal tract, head and neck cancers, lung cancers, and prostate cancer. A finding of viral positivity changed the treatment plan in only 8% of all infected patients, however.
“Given that most HIV-infected patients in our study knew their viral status, the yield of universal HIV testing among patients with newly diagnosed cancer may likely be low. Although age-directed screening is recommended for HIV and HCV, uptake rates in primary care are variable and low overall,” Dr. Ramsey and his colleagues wrote.
The study was supported by grants from the National Cancer Institute. Dr. Ramsey and several co-authors reported receiving NCI grants, and multiple co-authors reported grants and/or consulting fees from various companies.
SOURCE: Ramsey SD et al. JAMA Oncol. 2019 Jan 17. doi: 10.1001/jamaoncol.2018.6437.
Oncologists should consider testing all patients with newly diagnosed cancers for infection with the hepatitis B and C viruses, a multicenter team has recommended.
A prospective study of hepatitis B virus (HBV), hepatitis C virus (HCV), and HIV infections among 3,051 patients with newly diagnosed cancers showed that 6.5% of patients tested positive for previous HBV and 0.6% had chronic HBV infection. In addition, 2.4% of patients were positive for HCV, and 1.1% for HIV infections, reported Scott D. Ramsey, MD, PhD, from the Fred Hutchinson Cancer Research Center in Seattle, and colleagues.
“Many patients had no known risk factors for infection, suggesting that current risk-based models for screening may be insufficient. Thus, we believe our results warrant consideration of universal testing of patients with newly diagnosed cancer for HBV and HCV infection, particularly if such an approach is shown to be cost effective,” they wrote in JAMA Oncology.
The investigators noted that patients with undiagnosed hepatitis and/or HIV infections could transmit them to unsuspecting caregivers, adding that “with effective treatments available, not screening for these viruses misses an opportunity to reduce future morbidity associated with these infections and to avoid viral reactivation during treatment, with resulting morbidity and mortality.”
To estimate the prevalence of the infections in patients with newly diagnosed cancers, investigators looked at a cohort of 3,051 patients with a cancer diagnosis made within the previous 120 days at nine academic medical centers and nine community oncology centers representing a total of 41 cancer clinics affiliated with the SWOG Cancer Research Network (formerly the Southwest Oncology Group).
The median patient age was 60.6 years. Female patients constitute 60.4% of the sample; 18.1% were black, and 18.3% were of Hispanic heritage.
Of 3,050 patients for whom HBV testing results were available, 6.5% (197) were positive for previous HBV infection, compared with an estimated U.S. population prevalence of 4.7%. In addition, 0.6% (19 patients) were found to have chronic HBV, compared with an estimated 0.3% US population prevalence.
HCV infections were detected in 2.4% (71 of 2990 patients), compared with an estimated population prevalence of 1.3%, and HIV infections were detected in 1.1%, compared with a background estimated population prevalence of 0.3%.
In all, 32 patients were diagnosed with viral infections by testing performed for the study, including 8 patients with chronic HBV, 22 with HCV, and 2 with HIV.
Additionally, the authors found that 4 patients with chronic HBV, 23 with HCV, and 7 with HIV had no identifiable risk factors.
The highest prevalence of infections occurred among patients with liver cancer, nonliver and noncolorectal cancers of the gastrointestinal tract, head and neck cancers, lung cancers, and prostate cancer. A finding of viral positivity changed the treatment plan in only 8% of all infected patients, however.
“Given that most HIV-infected patients in our study knew their viral status, the yield of universal HIV testing among patients with newly diagnosed cancer may likely be low. Although age-directed screening is recommended for HIV and HCV, uptake rates in primary care are variable and low overall,” Dr. Ramsey and his colleagues wrote.
The study was supported by grants from the National Cancer Institute. Dr. Ramsey and several co-authors reported receiving NCI grants, and multiple co-authors reported grants and/or consulting fees from various companies.
SOURCE: Ramsey SD et al. JAMA Oncol. 2019 Jan 17. doi: 10.1001/jamaoncol.2018.6437.
Oncologists should consider testing all patients with newly diagnosed cancers for infection with the hepatitis B and C viruses, a multicenter team has recommended.
A prospective study of hepatitis B virus (HBV), hepatitis C virus (HCV), and HIV infections among 3,051 patients with newly diagnosed cancers showed that 6.5% of patients tested positive for previous HBV and 0.6% had chronic HBV infection. In addition, 2.4% of patients were positive for HCV, and 1.1% for HIV infections, reported Scott D. Ramsey, MD, PhD, from the Fred Hutchinson Cancer Research Center in Seattle, and colleagues.
“Many patients had no known risk factors for infection, suggesting that current risk-based models for screening may be insufficient. Thus, we believe our results warrant consideration of universal testing of patients with newly diagnosed cancer for HBV and HCV infection, particularly if such an approach is shown to be cost effective,” they wrote in JAMA Oncology.
The investigators noted that patients with undiagnosed hepatitis and/or HIV infections could transmit them to unsuspecting caregivers, adding that “with effective treatments available, not screening for these viruses misses an opportunity to reduce future morbidity associated with these infections and to avoid viral reactivation during treatment, with resulting morbidity and mortality.”
To estimate the prevalence of the infections in patients with newly diagnosed cancers, investigators looked at a cohort of 3,051 patients with a cancer diagnosis made within the previous 120 days at nine academic medical centers and nine community oncology centers representing a total of 41 cancer clinics affiliated with the SWOG Cancer Research Network (formerly the Southwest Oncology Group).
The median patient age was 60.6 years. Female patients constitute 60.4% of the sample; 18.1% were black, and 18.3% were of Hispanic heritage.
Of 3,050 patients for whom HBV testing results were available, 6.5% (197) were positive for previous HBV infection, compared with an estimated U.S. population prevalence of 4.7%. In addition, 0.6% (19 patients) were found to have chronic HBV, compared with an estimated 0.3% US population prevalence.
HCV infections were detected in 2.4% (71 of 2990 patients), compared with an estimated population prevalence of 1.3%, and HIV infections were detected in 1.1%, compared with a background estimated population prevalence of 0.3%.
In all, 32 patients were diagnosed with viral infections by testing performed for the study, including 8 patients with chronic HBV, 22 with HCV, and 2 with HIV.
Additionally, the authors found that 4 patients with chronic HBV, 23 with HCV, and 7 with HIV had no identifiable risk factors.
The highest prevalence of infections occurred among patients with liver cancer, nonliver and noncolorectal cancers of the gastrointestinal tract, head and neck cancers, lung cancers, and prostate cancer. A finding of viral positivity changed the treatment plan in only 8% of all infected patients, however.
“Given that most HIV-infected patients in our study knew their viral status, the yield of universal HIV testing among patients with newly diagnosed cancer may likely be low. Although age-directed screening is recommended for HIV and HCV, uptake rates in primary care are variable and low overall,” Dr. Ramsey and his colleagues wrote.
The study was supported by grants from the National Cancer Institute. Dr. Ramsey and several co-authors reported receiving NCI grants, and multiple co-authors reported grants and/or consulting fees from various companies.
SOURCE: Ramsey SD et al. JAMA Oncol. 2019 Jan 17. doi: 10.1001/jamaoncol.2018.6437.
FROM JAMA ONCOLOGY
Key clinical point: Patients with newly diagnosed cancers should be screened for viral infections that may pose a transmission risk or could be reactivated by cancer therapies.
Major finding: Infection rates of HBV, HCV, and HIV in patients with newly diagnosed cancers were 6.5%, 2.4%, and 1.1%, respectively.
Study details: Prospective study of viral infections in 3,051 patients with a diagnosis of cancer within the previous 120 days.
Disclosures: The study was supported by grants from the National Cancer Institute. Dr. Ramsey and several coauthors reported receiving NCI grants, and multiple coauthors reported grants and/or consulting fees from various companies.
Source: Ramsey SD et al. JAMA Oncology. 2019 Jan 17. doi: 10.1001/jamaoncol.2018.6437.
Chronic infections such as HCV, HIV, and TB cause unique problems for psoriasis patients
In a review of therapeutic issues for psoriasis patients who have such chronic infections as hepatitis, HIV, or latent tuberculosis infection (LTBI) or those who fall into the category of special populations (pregnant women or children), significant concerns were directly tied to the mode of action of the drugs involved.
In particular, “Most systemic agents for psoriasis are immunosuppressive, which poses a unique treatment challenge in patients with psoriasis with chronic infections because they are already immunosuppressed,” according to Shivani B. Kaushik, MD, a resident in the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York, and her colleague Mark G. Lebwohl, MD, professor and system chair of the department.
For example, the reviewers detailed a report of hepatitis B virus (HBV) and hepatitis C virus (HCV) reactivation in patients with psoriasis who were taking biologics. Virus reactivation was noted in 2/175 patients who were positive for anti-HBc antibody, 3/97 patients with HCV infection, and 8/40 patients who were positive for HBsAg (the surface antigen of HBV). From this, they concluded that “biologics pose minimal risk for viral reactivation in patients with anti-HCV or anti-HBc antibodies, but they are of considerable risk in HBsAg-positive patients.” (J Amer Acad Derm. 2019 Jan;80:43-53).
Giving a specific example, Dr. Kaushik and her colleague pointed out that the safety of ustekinumab in patients with psoriasis with concurrent HCV and HBV infection was not clear. Viral reactivation and hepatocellular cancer were reported in one of four patients with HCV and in two of seven HBsAg-positive patients; and yet, another study showed that the successful use of ustekinumab for psoriasis had no impact on liver function or viral load in a patient with coexisting HCV.
Overall, “Patients should not be treated with immunosuppressive therapies during the acute stage. However, biologic treatment can be initiated in patients with chronic or resolved hepatitis under close monitoring and collaboration with a gastroenterologist,” the researchers stated.
In addition, they pointed out that methotrexate, another commonly prescribed drug for psoriasis, is absolutely contraindicated, although the use of cyclosporine remains controversial for those patients who are HCV-antibody positive.
“Most systemic agents used in psoriasis are immunosuppressive and require appropriate screening, monitoring, and prophylaxis when used in [psoriasis] patients with chronic infections, such as hepatitis, HIV, and LTBI,” the authors concluded.
The authors reported receiving funding from a number of pharmaceutical companies.
SOURCE: Kaushik BS et al. J Amer Acad Derm. 2019;80:43-53.
In a review of therapeutic issues for psoriasis patients who have such chronic infections as hepatitis, HIV, or latent tuberculosis infection (LTBI) or those who fall into the category of special populations (pregnant women or children), significant concerns were directly tied to the mode of action of the drugs involved.
In particular, “Most systemic agents for psoriasis are immunosuppressive, which poses a unique treatment challenge in patients with psoriasis with chronic infections because they are already immunosuppressed,” according to Shivani B. Kaushik, MD, a resident in the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York, and her colleague Mark G. Lebwohl, MD, professor and system chair of the department.
For example, the reviewers detailed a report of hepatitis B virus (HBV) and hepatitis C virus (HCV) reactivation in patients with psoriasis who were taking biologics. Virus reactivation was noted in 2/175 patients who were positive for anti-HBc antibody, 3/97 patients with HCV infection, and 8/40 patients who were positive for HBsAg (the surface antigen of HBV). From this, they concluded that “biologics pose minimal risk for viral reactivation in patients with anti-HCV or anti-HBc antibodies, but they are of considerable risk in HBsAg-positive patients.” (J Amer Acad Derm. 2019 Jan;80:43-53).
Giving a specific example, Dr. Kaushik and her colleague pointed out that the safety of ustekinumab in patients with psoriasis with concurrent HCV and HBV infection was not clear. Viral reactivation and hepatocellular cancer were reported in one of four patients with HCV and in two of seven HBsAg-positive patients; and yet, another study showed that the successful use of ustekinumab for psoriasis had no impact on liver function or viral load in a patient with coexisting HCV.
Overall, “Patients should not be treated with immunosuppressive therapies during the acute stage. However, biologic treatment can be initiated in patients with chronic or resolved hepatitis under close monitoring and collaboration with a gastroenterologist,” the researchers stated.
In addition, they pointed out that methotrexate, another commonly prescribed drug for psoriasis, is absolutely contraindicated, although the use of cyclosporine remains controversial for those patients who are HCV-antibody positive.
“Most systemic agents used in psoriasis are immunosuppressive and require appropriate screening, monitoring, and prophylaxis when used in [psoriasis] patients with chronic infections, such as hepatitis, HIV, and LTBI,” the authors concluded.
The authors reported receiving funding from a number of pharmaceutical companies.
SOURCE: Kaushik BS et al. J Amer Acad Derm. 2019;80:43-53.
In a review of therapeutic issues for psoriasis patients who have such chronic infections as hepatitis, HIV, or latent tuberculosis infection (LTBI) or those who fall into the category of special populations (pregnant women or children), significant concerns were directly tied to the mode of action of the drugs involved.
In particular, “Most systemic agents for psoriasis are immunosuppressive, which poses a unique treatment challenge in patients with psoriasis with chronic infections because they are already immunosuppressed,” according to Shivani B. Kaushik, MD, a resident in the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York, and her colleague Mark G. Lebwohl, MD, professor and system chair of the department.
For example, the reviewers detailed a report of hepatitis B virus (HBV) and hepatitis C virus (HCV) reactivation in patients with psoriasis who were taking biologics. Virus reactivation was noted in 2/175 patients who were positive for anti-HBc antibody, 3/97 patients with HCV infection, and 8/40 patients who were positive for HBsAg (the surface antigen of HBV). From this, they concluded that “biologics pose minimal risk for viral reactivation in patients with anti-HCV or anti-HBc antibodies, but they are of considerable risk in HBsAg-positive patients.” (J Amer Acad Derm. 2019 Jan;80:43-53).
Giving a specific example, Dr. Kaushik and her colleague pointed out that the safety of ustekinumab in patients with psoriasis with concurrent HCV and HBV infection was not clear. Viral reactivation and hepatocellular cancer were reported in one of four patients with HCV and in two of seven HBsAg-positive patients; and yet, another study showed that the successful use of ustekinumab for psoriasis had no impact on liver function or viral load in a patient with coexisting HCV.
Overall, “Patients should not be treated with immunosuppressive therapies during the acute stage. However, biologic treatment can be initiated in patients with chronic or resolved hepatitis under close monitoring and collaboration with a gastroenterologist,” the researchers stated.
In addition, they pointed out that methotrexate, another commonly prescribed drug for psoriasis, is absolutely contraindicated, although the use of cyclosporine remains controversial for those patients who are HCV-antibody positive.
“Most systemic agents used in psoriasis are immunosuppressive and require appropriate screening, monitoring, and prophylaxis when used in [psoriasis] patients with chronic infections, such as hepatitis, HIV, and LTBI,” the authors concluded.
The authors reported receiving funding from a number of pharmaceutical companies.
SOURCE: Kaushik BS et al. J Amer Acad Derm. 2019;80:43-53.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Check for neuromyelitis optica spectrum disorder in suspect HIV patients
HIV-associated neuromyelitis optica spectrum disorder (NMOSD) is a recently recognized entity and high index of suspicion is needed to diagnose these patients, according to Thomas Mathew, MD, and his colleagues at St. John’s Medical College Hospital, Bengaluru, India.
“NMOSD can be associated with a wide range of autoimmune diseases but clinicians rarely diagnose NMOSD in cases of HIV infection and HIV-associated NMOSD is rarely mentioned in the conventional classification of NMOSD,” they stated.
Dr. Mathew and his colleagues reported the results of a case study they made of six cases of HIV-NMOSD identified from the literature and 1 HIV-infected patient from a registry for NMOSD that they had established, which had a total of 25 patients with the condition.
There were four men and three women in the study, ranging from 8 years to 49 years of age. The duration of HIV infection in these patients ranged from newly detected to 15 years, according to the report, published in Multiple Sclerosis and Related Disorders (2019 Jan;27:289-93).
Optic neuritis followed by myelitis was the commonest presentation, occurring in five of the seven patients. Of these, six patients were assayed for anti–aquaporin 4 antibodies, which are considered a serological marker of neuromyelitis optica; three patients were positive and three were negative.
All patients received immunomodulatory treatment. Five of the seven patients had a poor recovery from acute attacks, but no patient had further relapses while on immunomodulatory treatment and antiretroviral therapy.
Dr. Mathew and his colleagues suggested that all patients with HIV infection presenting with optic neuritis or/and myelitis, should have their anti–aquaporin 4 antibody status checked and in all patients of NMOSD, HIV infection should be ruled out.
“Prognosis of these patients is variable; residual neurological deficits were common but treatment prevented further attacks. Increased awareness of this association will lead to earlier diagnosis, early treatment and prevention of disability,” the researchers concluded.
The authors reported that they had no conflicts.
SOURCE: Mathew T et al. Mult Scler Relat Disord. 2019 Jan;27:289-93.
HIV-associated neuromyelitis optica spectrum disorder (NMOSD) is a recently recognized entity and high index of suspicion is needed to diagnose these patients, according to Thomas Mathew, MD, and his colleagues at St. John’s Medical College Hospital, Bengaluru, India.
“NMOSD can be associated with a wide range of autoimmune diseases but clinicians rarely diagnose NMOSD in cases of HIV infection and HIV-associated NMOSD is rarely mentioned in the conventional classification of NMOSD,” they stated.
Dr. Mathew and his colleagues reported the results of a case study they made of six cases of HIV-NMOSD identified from the literature and 1 HIV-infected patient from a registry for NMOSD that they had established, which had a total of 25 patients with the condition.
There were four men and three women in the study, ranging from 8 years to 49 years of age. The duration of HIV infection in these patients ranged from newly detected to 15 years, according to the report, published in Multiple Sclerosis and Related Disorders (2019 Jan;27:289-93).
Optic neuritis followed by myelitis was the commonest presentation, occurring in five of the seven patients. Of these, six patients were assayed for anti–aquaporin 4 antibodies, which are considered a serological marker of neuromyelitis optica; three patients were positive and three were negative.
All patients received immunomodulatory treatment. Five of the seven patients had a poor recovery from acute attacks, but no patient had further relapses while on immunomodulatory treatment and antiretroviral therapy.
Dr. Mathew and his colleagues suggested that all patients with HIV infection presenting with optic neuritis or/and myelitis, should have their anti–aquaporin 4 antibody status checked and in all patients of NMOSD, HIV infection should be ruled out.
“Prognosis of these patients is variable; residual neurological deficits were common but treatment prevented further attacks. Increased awareness of this association will lead to earlier diagnosis, early treatment and prevention of disability,” the researchers concluded.
The authors reported that they had no conflicts.
SOURCE: Mathew T et al. Mult Scler Relat Disord. 2019 Jan;27:289-93.
HIV-associated neuromyelitis optica spectrum disorder (NMOSD) is a recently recognized entity and high index of suspicion is needed to diagnose these patients, according to Thomas Mathew, MD, and his colleagues at St. John’s Medical College Hospital, Bengaluru, India.
“NMOSD can be associated with a wide range of autoimmune diseases but clinicians rarely diagnose NMOSD in cases of HIV infection and HIV-associated NMOSD is rarely mentioned in the conventional classification of NMOSD,” they stated.
Dr. Mathew and his colleagues reported the results of a case study they made of six cases of HIV-NMOSD identified from the literature and 1 HIV-infected patient from a registry for NMOSD that they had established, which had a total of 25 patients with the condition.
There were four men and three women in the study, ranging from 8 years to 49 years of age. The duration of HIV infection in these patients ranged from newly detected to 15 years, according to the report, published in Multiple Sclerosis and Related Disorders (2019 Jan;27:289-93).
Optic neuritis followed by myelitis was the commonest presentation, occurring in five of the seven patients. Of these, six patients were assayed for anti–aquaporin 4 antibodies, which are considered a serological marker of neuromyelitis optica; three patients were positive and three were negative.
All patients received immunomodulatory treatment. Five of the seven patients had a poor recovery from acute attacks, but no patient had further relapses while on immunomodulatory treatment and antiretroviral therapy.
Dr. Mathew and his colleagues suggested that all patients with HIV infection presenting with optic neuritis or/and myelitis, should have their anti–aquaporin 4 antibody status checked and in all patients of NMOSD, HIV infection should be ruled out.
“Prognosis of these patients is variable; residual neurological deficits were common but treatment prevented further attacks. Increased awareness of this association will lead to earlier diagnosis, early treatment and prevention of disability,” the researchers concluded.
The authors reported that they had no conflicts.
SOURCE: Mathew T et al. Mult Scler Relat Disord. 2019 Jan;27:289-93.
FROM MULTIPLE SCLEROSIS AND RELATED DISORDERS
T marneffei Infection: Risk Extends to Patients Without HIV
Patients with HIV/AIDs are vulnerable to Talaromyces marneffei (T marneffei) infection, formerly penicilliosis. But in recent years more cases have been seen in patients not infected with HIV, too. Most cases originate in Southeast Asia: 10% of patients with AIDS in Hong Kong and 30% of patients in North Thailand, for example, have T marneffei infections. But patients with AIDS and penicilliosis travel, as do other immunocompromised patients. Thus, the first reported case of a patient with long-standing pulmonary sarcoidosis who developed T marneffei infection may have significance for clinicians caring for people with, or without, HIV.
Most patients with T marneffei infection have fever, weight loss, and malaise. Subcutaneous abscesses and papulelike ulcers are common (sometimes the lesions are very small). Anemia, hepatosplenomegaly, lymphadenopathy, and diarrhea also are relatively common. However, while cough is a notable symptom, pneumonia is rare—even though the organism is inhaled.
The patient in this report, a native of Cangnan County (an endemic fungal area) in Southeast China, was admitted to the hospital with a 3-week history of daily hyperpyrexia and coughing sputum. When antibiotics did not help, a fungal culture revealed why: He had T marneffei infection. The clinicians say the preexisting pulmonary sarcoidosis covered the clinical features of T marneffei and initially misled them.
After 3 months of antifungal treatment, the patient’s physical condition improved. And the lung lesions were “markedly absorbed” after 3 months. The respiratory signs and skin lesions disappeared gradually after 8 days of treatment.
T marneffei infection is fatal if untreated. Early diagnosis and treatment with antifungals can be life saving.
Source:
Yu X, Miao K, Zhou C, et al. BMC Infect Dis. 2018;18(1):390.
Patients with HIV/AIDs are vulnerable to Talaromyces marneffei (T marneffei) infection, formerly penicilliosis. But in recent years more cases have been seen in patients not infected with HIV, too. Most cases originate in Southeast Asia: 10% of patients with AIDS in Hong Kong and 30% of patients in North Thailand, for example, have T marneffei infections. But patients with AIDS and penicilliosis travel, as do other immunocompromised patients. Thus, the first reported case of a patient with long-standing pulmonary sarcoidosis who developed T marneffei infection may have significance for clinicians caring for people with, or without, HIV.
Most patients with T marneffei infection have fever, weight loss, and malaise. Subcutaneous abscesses and papulelike ulcers are common (sometimes the lesions are very small). Anemia, hepatosplenomegaly, lymphadenopathy, and diarrhea also are relatively common. However, while cough is a notable symptom, pneumonia is rare—even though the organism is inhaled.
The patient in this report, a native of Cangnan County (an endemic fungal area) in Southeast China, was admitted to the hospital with a 3-week history of daily hyperpyrexia and coughing sputum. When antibiotics did not help, a fungal culture revealed why: He had T marneffei infection. The clinicians say the preexisting pulmonary sarcoidosis covered the clinical features of T marneffei and initially misled them.
After 3 months of antifungal treatment, the patient’s physical condition improved. And the lung lesions were “markedly absorbed” after 3 months. The respiratory signs and skin lesions disappeared gradually after 8 days of treatment.
T marneffei infection is fatal if untreated. Early diagnosis and treatment with antifungals can be life saving.
Source:
Yu X, Miao K, Zhou C, et al. BMC Infect Dis. 2018;18(1):390.
Patients with HIV/AIDs are vulnerable to Talaromyces marneffei (T marneffei) infection, formerly penicilliosis. But in recent years more cases have been seen in patients not infected with HIV, too. Most cases originate in Southeast Asia: 10% of patients with AIDS in Hong Kong and 30% of patients in North Thailand, for example, have T marneffei infections. But patients with AIDS and penicilliosis travel, as do other immunocompromised patients. Thus, the first reported case of a patient with long-standing pulmonary sarcoidosis who developed T marneffei infection may have significance for clinicians caring for people with, or without, HIV.
Most patients with T marneffei infection have fever, weight loss, and malaise. Subcutaneous abscesses and papulelike ulcers are common (sometimes the lesions are very small). Anemia, hepatosplenomegaly, lymphadenopathy, and diarrhea also are relatively common. However, while cough is a notable symptom, pneumonia is rare—even though the organism is inhaled.
The patient in this report, a native of Cangnan County (an endemic fungal area) in Southeast China, was admitted to the hospital with a 3-week history of daily hyperpyrexia and coughing sputum. When antibiotics did not help, a fungal culture revealed why: He had T marneffei infection. The clinicians say the preexisting pulmonary sarcoidosis covered the clinical features of T marneffei and initially misled them.
After 3 months of antifungal treatment, the patient’s physical condition improved. And the lung lesions were “markedly absorbed” after 3 months. The respiratory signs and skin lesions disappeared gradually after 8 days of treatment.
T marneffei infection is fatal if untreated. Early diagnosis and treatment with antifungals can be life saving.
Source:
Yu X, Miao K, Zhou C, et al. BMC Infect Dis. 2018;18(1):390.
People with HIV still at increased cardiovascular risk
CHICAGO – HIV infection remained linked with an increased risk for developing a cardiovascular disease event among U.S. patients, even in a recent era of antiretroviral therapy.
U.S. health insurance beneficiaries diagnosed with an HIV infection and likely put on antiretroviral therapy sometime during 2011-2015 had a statistically significant, 21% increased risk for the combination of MIs, coronary revascularizations, stroke, and lower-extremity peripheral artery disease (PAD) in a case-control, retrospective analysis, Robert S. Rosenson, MD, said in a poster he presented at the American Heart Association scientific sessions.
“We looked at a contemporary population of people with HIV treated with antiretroviral therapy, and we looked at stroke and lower-extremity PAD [peripheral artery disease] as well as MI, while most prior studies only looked at MIs,” noted Dr. Rosenson, a professor of medicine and director of cardiometabolic disorders at the Icahn School of Medicine at Mount Sinai Medical Center in New York.
The analysis found no significant differences in outcomes that linked with the specific type of antiretroviral therapy patients received. The most commonly used antiretroviral drug was a non–nucleoside reverse transcriptase inhibitor, taken by about 80% of the HIV-infected patients, Dr. Rosenson said. The 2011-2015 period examined in the study largely predated the more recent era, when integrase strand transfer inhibitor drugs have increasingly become the core agent for treating HIV infection.
Another key finding in the study was that a scant 19% of the people infected with HIV received statin treatment, and only 4% were on a high-intensity dosage. The 2018 guideline on cholesterol management identifies HIV infection as one of several “risk enhancers” that boost a person’s cardiovascular disease (CVD) risk and intensify their need for statin treatment (Circulation. 2018 Nov 10. doi: 10.1161/CIR.0000000000000625).
“Hopefully use of statins will increase in people with HIV, but of course we need evidence because so far the evidence does not show benefit,” he noted. In the data Dr. Rosenson reported, the HIV-infected patients who received a statin had roughly the same elevated risk for a CVD event as did HIV-infected patients who did not get a statin.
His study used data from a U.S. commercial database that combined Medicare patients with patients covered by commercial insurers. The analysis identified 82,426 people presumed recently infected by HIV based on either a hospitalization discharge with a diagnostic code for HIV or after filling at least two prescriptions for an antiretroviral drug during January 2011–June 2015. The researchers matched these cases on a 4:1 basis with 329,704 controls from the database matched by age, sex, and year for their index date. The total study cohort averaged about 45 years old, but the people infected by HIV averaged a couple of years older and also had at baseline an increased prevalence of several CVD risk factors and comorbidities. The people with HIV had a more than threefold higher rate of tobacco use, chronic kidney disease, and liver disease, and double the rate of diagnosed depression.
In a multivariate analysis that controlled for many demographic, social, and clinical variables, the results showed that the HIV-infected people had statistically significant higher rates of every individual element in the CVD composite. They had a 26% higher rate of MIs, a 17% higher rate of MIs plus coronary revascularization, a 30% higher rate of stroke, and a doubled rate of lower-extremity PAD.
SOURCE: Rosenson RS et al. Circulation. 2018 Nov 6;138[suppl 1]:A14410.
CHICAGO – HIV infection remained linked with an increased risk for developing a cardiovascular disease event among U.S. patients, even in a recent era of antiretroviral therapy.
U.S. health insurance beneficiaries diagnosed with an HIV infection and likely put on antiretroviral therapy sometime during 2011-2015 had a statistically significant, 21% increased risk for the combination of MIs, coronary revascularizations, stroke, and lower-extremity peripheral artery disease (PAD) in a case-control, retrospective analysis, Robert S. Rosenson, MD, said in a poster he presented at the American Heart Association scientific sessions.
“We looked at a contemporary population of people with HIV treated with antiretroviral therapy, and we looked at stroke and lower-extremity PAD [peripheral artery disease] as well as MI, while most prior studies only looked at MIs,” noted Dr. Rosenson, a professor of medicine and director of cardiometabolic disorders at the Icahn School of Medicine at Mount Sinai Medical Center in New York.
The analysis found no significant differences in outcomes that linked with the specific type of antiretroviral therapy patients received. The most commonly used antiretroviral drug was a non–nucleoside reverse transcriptase inhibitor, taken by about 80% of the HIV-infected patients, Dr. Rosenson said. The 2011-2015 period examined in the study largely predated the more recent era, when integrase strand transfer inhibitor drugs have increasingly become the core agent for treating HIV infection.
Another key finding in the study was that a scant 19% of the people infected with HIV received statin treatment, and only 4% were on a high-intensity dosage. The 2018 guideline on cholesterol management identifies HIV infection as one of several “risk enhancers” that boost a person’s cardiovascular disease (CVD) risk and intensify their need for statin treatment (Circulation. 2018 Nov 10. doi: 10.1161/CIR.0000000000000625).
“Hopefully use of statins will increase in people with HIV, but of course we need evidence because so far the evidence does not show benefit,” he noted. In the data Dr. Rosenson reported, the HIV-infected patients who received a statin had roughly the same elevated risk for a CVD event as did HIV-infected patients who did not get a statin.
His study used data from a U.S. commercial database that combined Medicare patients with patients covered by commercial insurers. The analysis identified 82,426 people presumed recently infected by HIV based on either a hospitalization discharge with a diagnostic code for HIV or after filling at least two prescriptions for an antiretroviral drug during January 2011–June 2015. The researchers matched these cases on a 4:1 basis with 329,704 controls from the database matched by age, sex, and year for their index date. The total study cohort averaged about 45 years old, but the people infected by HIV averaged a couple of years older and also had at baseline an increased prevalence of several CVD risk factors and comorbidities. The people with HIV had a more than threefold higher rate of tobacco use, chronic kidney disease, and liver disease, and double the rate of diagnosed depression.
In a multivariate analysis that controlled for many demographic, social, and clinical variables, the results showed that the HIV-infected people had statistically significant higher rates of every individual element in the CVD composite. They had a 26% higher rate of MIs, a 17% higher rate of MIs plus coronary revascularization, a 30% higher rate of stroke, and a doubled rate of lower-extremity PAD.
SOURCE: Rosenson RS et al. Circulation. 2018 Nov 6;138[suppl 1]:A14410.
CHICAGO – HIV infection remained linked with an increased risk for developing a cardiovascular disease event among U.S. patients, even in a recent era of antiretroviral therapy.
U.S. health insurance beneficiaries diagnosed with an HIV infection and likely put on antiretroviral therapy sometime during 2011-2015 had a statistically significant, 21% increased risk for the combination of MIs, coronary revascularizations, stroke, and lower-extremity peripheral artery disease (PAD) in a case-control, retrospective analysis, Robert S. Rosenson, MD, said in a poster he presented at the American Heart Association scientific sessions.
“We looked at a contemporary population of people with HIV treated with antiretroviral therapy, and we looked at stroke and lower-extremity PAD [peripheral artery disease] as well as MI, while most prior studies only looked at MIs,” noted Dr. Rosenson, a professor of medicine and director of cardiometabolic disorders at the Icahn School of Medicine at Mount Sinai Medical Center in New York.
The analysis found no significant differences in outcomes that linked with the specific type of antiretroviral therapy patients received. The most commonly used antiretroviral drug was a non–nucleoside reverse transcriptase inhibitor, taken by about 80% of the HIV-infected patients, Dr. Rosenson said. The 2011-2015 period examined in the study largely predated the more recent era, when integrase strand transfer inhibitor drugs have increasingly become the core agent for treating HIV infection.
Another key finding in the study was that a scant 19% of the people infected with HIV received statin treatment, and only 4% were on a high-intensity dosage. The 2018 guideline on cholesterol management identifies HIV infection as one of several “risk enhancers” that boost a person’s cardiovascular disease (CVD) risk and intensify their need for statin treatment (Circulation. 2018 Nov 10. doi: 10.1161/CIR.0000000000000625).
“Hopefully use of statins will increase in people with HIV, but of course we need evidence because so far the evidence does not show benefit,” he noted. In the data Dr. Rosenson reported, the HIV-infected patients who received a statin had roughly the same elevated risk for a CVD event as did HIV-infected patients who did not get a statin.
His study used data from a U.S. commercial database that combined Medicare patients with patients covered by commercial insurers. The analysis identified 82,426 people presumed recently infected by HIV based on either a hospitalization discharge with a diagnostic code for HIV or after filling at least two prescriptions for an antiretroviral drug during January 2011–June 2015. The researchers matched these cases on a 4:1 basis with 329,704 controls from the database matched by age, sex, and year for their index date. The total study cohort averaged about 45 years old, but the people infected by HIV averaged a couple of years older and also had at baseline an increased prevalence of several CVD risk factors and comorbidities. The people with HIV had a more than threefold higher rate of tobacco use, chronic kidney disease, and liver disease, and double the rate of diagnosed depression.
In a multivariate analysis that controlled for many demographic, social, and clinical variables, the results showed that the HIV-infected people had statistically significant higher rates of every individual element in the CVD composite. They had a 26% higher rate of MIs, a 17% higher rate of MIs plus coronary revascularization, a 30% higher rate of stroke, and a doubled rate of lower-extremity PAD.
SOURCE: Rosenson RS et al. Circulation. 2018 Nov 6;138[suppl 1]:A14410.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point: U.S. insurance beneficiaries newly diagnosed with HIV had a significantly higher rate of CVD events than people without HIV.
Major finding: The adjusted rate of cardiovascular disease events was 21% higher in people infected with HIV, compared with matched, uninfected people.
Study details: A retrospective, case control study of 412,130 U.S. health insurance beneficiaries.
Disclosures: The study received partial funding from Amgen. Dr. Rosenson has received honoraria from Amgen, Akcaa, and Kowa; he has been an advisor to Amgen, Regeneron, and Sanofi; and he has received research funding from Amgen, Akcaa, AstraZeneca, and The Medicines Company.
Source: Rosenson RS et al. Circulation. 2018 Nov 6;138[suppl 1]:A14410.
HIV prevention: Mandating insurance coverage of PrEP
Pre-exposure prophylaxis (PrEP) for HIV is valuable enough for the federal government to mandate insurance coverage, a group of experts told the personal finance website WalletHub, but individuals who are at risk for infection may be missing out for other reasons.
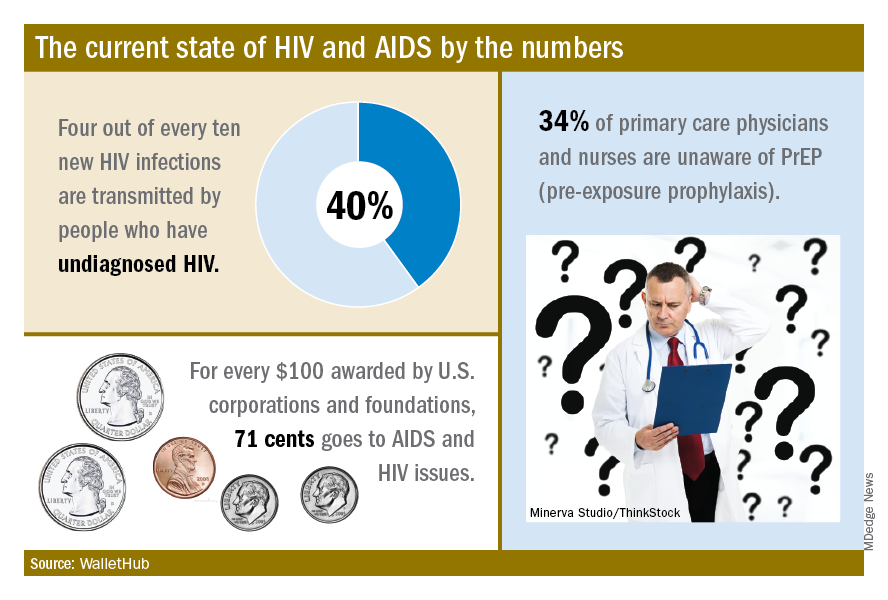
The effectiveness of PrEP is clear, those experts said, but 34% of primary care physicians and nurses in the United States are unaware of the preventive regimen, according to the WalletHub report, which also noted that the majority of Americans with AIDS (61%) are not seeing a specialist.
“Even among [men who have sex with men] in the U.S., coverage is only about 10%, which is abysmal. We can and need to do better. If we don’t pay now, we’ll pay later,” Steffanie Strathdee, PhD, associate dean of global health sciences and Harold Simon Professor at the University of California, San Diego, told WalletHub.
Those taking PrEP have a 90% chance of avoiding HIV infection, the report noted.
“Making PrEP available to all is a giant step forward in the fight against HIV. Mandating this critical prevention be covered by all insurance plans makes it part of mainstream medicine and will only increase its use and help prevent HIV acquisition in exposed populations. I can’t think of other low-risk, high-reward prophylaxis for a lifelong disease,” said Sharon Nachman, MD, professor of pediatrics and associate dean for research at the State University of New York at Stony Brook.
To get PrEP covered, the U.S. Preventive Services Task Force needs to act, explained Gerald M. Oppenheimer, PhD, MPH, of the department of health policy and management at the City University of New York.
“Under the Affordable Care Act, if the [USPSTF] finds that PrEP serves as an effective prevention to disease and gives it a grade of A or B, all insurers must offer it free. That, of course, may lead to an increase in premiums. This is another example of pharmaceutical companies charging high prices in the U.S., compared to what other countries pay, and cries out for an amendment to Medicare Part D, allowing the federal government to negotiate lower drug prices,” he said.
Pre-exposure prophylaxis (PrEP) for HIV is valuable enough for the federal government to mandate insurance coverage, a group of experts told the personal finance website WalletHub, but individuals who are at risk for infection may be missing out for other reasons.

The effectiveness of PrEP is clear, those experts said, but 34% of primary care physicians and nurses in the United States are unaware of the preventive regimen, according to the WalletHub report, which also noted that the majority of Americans with AIDS (61%) are not seeing a specialist.
“Even among [men who have sex with men] in the U.S., coverage is only about 10%, which is abysmal. We can and need to do better. If we don’t pay now, we’ll pay later,” Steffanie Strathdee, PhD, associate dean of global health sciences and Harold Simon Professor at the University of California, San Diego, told WalletHub.
Those taking PrEP have a 90% chance of avoiding HIV infection, the report noted.
“Making PrEP available to all is a giant step forward in the fight against HIV. Mandating this critical prevention be covered by all insurance plans makes it part of mainstream medicine and will only increase its use and help prevent HIV acquisition in exposed populations. I can’t think of other low-risk, high-reward prophylaxis for a lifelong disease,” said Sharon Nachman, MD, professor of pediatrics and associate dean for research at the State University of New York at Stony Brook.
To get PrEP covered, the U.S. Preventive Services Task Force needs to act, explained Gerald M. Oppenheimer, PhD, MPH, of the department of health policy and management at the City University of New York.
“Under the Affordable Care Act, if the [USPSTF] finds that PrEP serves as an effective prevention to disease and gives it a grade of A or B, all insurers must offer it free. That, of course, may lead to an increase in premiums. This is another example of pharmaceutical companies charging high prices in the U.S., compared to what other countries pay, and cries out for an amendment to Medicare Part D, allowing the federal government to negotiate lower drug prices,” he said.
Pre-exposure prophylaxis (PrEP) for HIV is valuable enough for the federal government to mandate insurance coverage, a group of experts told the personal finance website WalletHub, but individuals who are at risk for infection may be missing out for other reasons.

The effectiveness of PrEP is clear, those experts said, but 34% of primary care physicians and nurses in the United States are unaware of the preventive regimen, according to the WalletHub report, which also noted that the majority of Americans with AIDS (61%) are not seeing a specialist.
“Even among [men who have sex with men] in the U.S., coverage is only about 10%, which is abysmal. We can and need to do better. If we don’t pay now, we’ll pay later,” Steffanie Strathdee, PhD, associate dean of global health sciences and Harold Simon Professor at the University of California, San Diego, told WalletHub.
Those taking PrEP have a 90% chance of avoiding HIV infection, the report noted.
“Making PrEP available to all is a giant step forward in the fight against HIV. Mandating this critical prevention be covered by all insurance plans makes it part of mainstream medicine and will only increase its use and help prevent HIV acquisition in exposed populations. I can’t think of other low-risk, high-reward prophylaxis for a lifelong disease,” said Sharon Nachman, MD, professor of pediatrics and associate dean for research at the State University of New York at Stony Brook.
To get PrEP covered, the U.S. Preventive Services Task Force needs to act, explained Gerald M. Oppenheimer, PhD, MPH, of the department of health policy and management at the City University of New York.
“Under the Affordable Care Act, if the [USPSTF] finds that PrEP serves as an effective prevention to disease and gives it a grade of A or B, all insurers must offer it free. That, of course, may lead to an increase in premiums. This is another example of pharmaceutical companies charging high prices in the U.S., compared to what other countries pay, and cries out for an amendment to Medicare Part D, allowing the federal government to negotiate lower drug prices,” he said.
Missed HIV screening opportunities found among subsequently infected youth
In the year prior to HIV diagnosis, there were high rates of missed opportunities for HIV testing and sexual history documentation, according to a retrospective study of youth with HIV aged 14-26 years who were treated at an HIV clinic. These results demonstrate a failed need for routine HIV screening and counseling in adolescents, according to Nellie Riendeau Lazar, MPH, of Children’s Hospital of Philadelphia, and her colleagues.
The researchers retrospectively identified 301 subjects between January 2009 and April 2015 who met their study criteria. A total of 58 of these (19%) had at least one visit in the care network in the year prior to diagnosis and their entry into the adolescent HIV clinic, and they were analyzed for missed diagnosis. The adolescent HIV clinic is part of a large care network in the Philadelphia area that includes a pediatric emergency department and a tertiary care hospital. At the time of the study, there were 31 primary care sites, according to the authors.
The mean age of the subjects in the study was 17. The majority (80%) were young men, African-American (93%), and men who have sex with men (81%). There were no significant differences seen in demographics between those with and without prior visits in the health system (J Adolesc Health. 2018;63:799-802).
The 58 subjects were seen in 179 health care visits in the year prior to their diagnosis: 56% outpatient, 40% emergency department, and 4% inpatient visits. Only 59% of these visits had any documentation of sexual history and “the overwhelming majority of those noting sexual activity included no other information,” such as number of partners, sex of partners, or condom use, according to the researchers.
Among the total cohort, 183 of 301 had never had an HIV test prior to their first positive test, even though 26% had been seen in the care network in the 3 years prior to their diagnosis. Among the 58 in the missed opportunity analysis, only 48% had HIV testing, even though 88% (51) had documented symptoms in their visits that could have been consistent with acute infection.
“Our findings support the most recent guidelines from the Centers for Disease Control and Prevention, American Academy of Pediatrics (AAP), and United States Preventive Services Task Force (USPSTF), recommending routine HIV screening for all adolescents, regardless of risk,” the researchers stated. “Adolescents may not always disclose sexual activity during routine assessment, and provider level barriers limit the reach of risk-based testing algorithms,” they added.
The authors reported that they had no conflicts of interest.
SOURCE: Lazar NR et al. J Adolesc Health. 2018;63:799-802.
In the year prior to HIV diagnosis, there were high rates of missed opportunities for HIV testing and sexual history documentation, according to a retrospective study of youth with HIV aged 14-26 years who were treated at an HIV clinic. These results demonstrate a failed need for routine HIV screening and counseling in adolescents, according to Nellie Riendeau Lazar, MPH, of Children’s Hospital of Philadelphia, and her colleagues.
The researchers retrospectively identified 301 subjects between January 2009 and April 2015 who met their study criteria. A total of 58 of these (19%) had at least one visit in the care network in the year prior to diagnosis and their entry into the adolescent HIV clinic, and they were analyzed for missed diagnosis. The adolescent HIV clinic is part of a large care network in the Philadelphia area that includes a pediatric emergency department and a tertiary care hospital. At the time of the study, there were 31 primary care sites, according to the authors.
The mean age of the subjects in the study was 17. The majority (80%) were young men, African-American (93%), and men who have sex with men (81%). There were no significant differences seen in demographics between those with and without prior visits in the health system (J Adolesc Health. 2018;63:799-802).
The 58 subjects were seen in 179 health care visits in the year prior to their diagnosis: 56% outpatient, 40% emergency department, and 4% inpatient visits. Only 59% of these visits had any documentation of sexual history and “the overwhelming majority of those noting sexual activity included no other information,” such as number of partners, sex of partners, or condom use, according to the researchers.
Among the total cohort, 183 of 301 had never had an HIV test prior to their first positive test, even though 26% had been seen in the care network in the 3 years prior to their diagnosis. Among the 58 in the missed opportunity analysis, only 48% had HIV testing, even though 88% (51) had documented symptoms in their visits that could have been consistent with acute infection.
“Our findings support the most recent guidelines from the Centers for Disease Control and Prevention, American Academy of Pediatrics (AAP), and United States Preventive Services Task Force (USPSTF), recommending routine HIV screening for all adolescents, regardless of risk,” the researchers stated. “Adolescents may not always disclose sexual activity during routine assessment, and provider level barriers limit the reach of risk-based testing algorithms,” they added.
The authors reported that they had no conflicts of interest.
SOURCE: Lazar NR et al. J Adolesc Health. 2018;63:799-802.
In the year prior to HIV diagnosis, there were high rates of missed opportunities for HIV testing and sexual history documentation, according to a retrospective study of youth with HIV aged 14-26 years who were treated at an HIV clinic. These results demonstrate a failed need for routine HIV screening and counseling in adolescents, according to Nellie Riendeau Lazar, MPH, of Children’s Hospital of Philadelphia, and her colleagues.
The researchers retrospectively identified 301 subjects between January 2009 and April 2015 who met their study criteria. A total of 58 of these (19%) had at least one visit in the care network in the year prior to diagnosis and their entry into the adolescent HIV clinic, and they were analyzed for missed diagnosis. The adolescent HIV clinic is part of a large care network in the Philadelphia area that includes a pediatric emergency department and a tertiary care hospital. At the time of the study, there were 31 primary care sites, according to the authors.
The mean age of the subjects in the study was 17. The majority (80%) were young men, African-American (93%), and men who have sex with men (81%). There were no significant differences seen in demographics between those with and without prior visits in the health system (J Adolesc Health. 2018;63:799-802).
The 58 subjects were seen in 179 health care visits in the year prior to their diagnosis: 56% outpatient, 40% emergency department, and 4% inpatient visits. Only 59% of these visits had any documentation of sexual history and “the overwhelming majority of those noting sexual activity included no other information,” such as number of partners, sex of partners, or condom use, according to the researchers.
Among the total cohort, 183 of 301 had never had an HIV test prior to their first positive test, even though 26% had been seen in the care network in the 3 years prior to their diagnosis. Among the 58 in the missed opportunity analysis, only 48% had HIV testing, even though 88% (51) had documented symptoms in their visits that could have been consistent with acute infection.
“Our findings support the most recent guidelines from the Centers for Disease Control and Prevention, American Academy of Pediatrics (AAP), and United States Preventive Services Task Force (USPSTF), recommending routine HIV screening for all adolescents, regardless of risk,” the researchers stated. “Adolescents may not always disclose sexual activity during routine assessment, and provider level barriers limit the reach of risk-based testing algorithms,” they added.
The authors reported that they had no conflicts of interest.
SOURCE: Lazar NR et al. J Adolesc Health. 2018;63:799-802.
FROM THE JOURNAL OF ADOLESCENT HEALTH
Key clinical point: Only 51% of youth with symptoms suggesting acute retroviral syndrome were tested.
Major finding: HIV testing was performed in only 48% of the subjects seen in the year prior to their diagnosis.
Study details: Retrospective review of subjects with HIV aged 14-26 years, comparing those with and without HIV screening within the year prior to diagnosis.
Disclosures: The authors reported that they had no conflicts of interest.
Source: Lazar NR et al. J Adolesc Health. 2018;63:799-802.
Temixys plus other antiretrovirals approved for HIV-1
The Food and Drug Administration has approved the combination of lamivudine (3TC) and tenofovir disoproxil fumarate (TDF) known as Temixys for treatment of HIV-1 when used with other antiretrovirals. The approval is for adult and pediatric patients with HIV-1 who weigh at least 35 kg.
The approval is based on data through 144 weeks in a double-blind, active-controlled, multicenter trial in 600 antiretroviral-naive patients. The trial compared TDF/3TC plus efavirenz (EFV) with 3TC/EFV plus stavudine (d4T). The results showed similar responses at 144 weeks between both groups: 62% of patients taking TDF/3TC/EFV and 58% of patients taking d4T/3TC/EFV achieved and maintained fewer than 50 copies/mL of HIV-1 RNA.
The most common adverse events include headache, pain, depression, rash, and diarrhea. Prior to initiating treatment, patients should be tested for hepatitis B virus because there have been reports of 3TC-resistant strains of hepatitis B virus associated with treatment of HIV-1 with 3TC-containing regimens in coinfected patients. Patients should also be tested for estimated creatinine clearance, urine glucose, and urine protein because TDF/3TC is not recommended for patients with renal impairment.
The full prescribing information can be found on the FDA website.
The Food and Drug Administration has approved the combination of lamivudine (3TC) and tenofovir disoproxil fumarate (TDF) known as Temixys for treatment of HIV-1 when used with other antiretrovirals. The approval is for adult and pediatric patients with HIV-1 who weigh at least 35 kg.
The approval is based on data through 144 weeks in a double-blind, active-controlled, multicenter trial in 600 antiretroviral-naive patients. The trial compared TDF/3TC plus efavirenz (EFV) with 3TC/EFV plus stavudine (d4T). The results showed similar responses at 144 weeks between both groups: 62% of patients taking TDF/3TC/EFV and 58% of patients taking d4T/3TC/EFV achieved and maintained fewer than 50 copies/mL of HIV-1 RNA.
The most common adverse events include headache, pain, depression, rash, and diarrhea. Prior to initiating treatment, patients should be tested for hepatitis B virus because there have been reports of 3TC-resistant strains of hepatitis B virus associated with treatment of HIV-1 with 3TC-containing regimens in coinfected patients. Patients should also be tested for estimated creatinine clearance, urine glucose, and urine protein because TDF/3TC is not recommended for patients with renal impairment.
The full prescribing information can be found on the FDA website.
The Food and Drug Administration has approved the combination of lamivudine (3TC) and tenofovir disoproxil fumarate (TDF) known as Temixys for treatment of HIV-1 when used with other antiretrovirals. The approval is for adult and pediatric patients with HIV-1 who weigh at least 35 kg.
The approval is based on data through 144 weeks in a double-blind, active-controlled, multicenter trial in 600 antiretroviral-naive patients. The trial compared TDF/3TC plus efavirenz (EFV) with 3TC/EFV plus stavudine (d4T). The results showed similar responses at 144 weeks between both groups: 62% of patients taking TDF/3TC/EFV and 58% of patients taking d4T/3TC/EFV achieved and maintained fewer than 50 copies/mL of HIV-1 RNA.
The most common adverse events include headache, pain, depression, rash, and diarrhea. Prior to initiating treatment, patients should be tested for hepatitis B virus because there have been reports of 3TC-resistant strains of hepatitis B virus associated with treatment of HIV-1 with 3TC-containing regimens in coinfected patients. Patients should also be tested for estimated creatinine clearance, urine glucose, and urine protein because TDF/3TC is not recommended for patients with renal impairment.
The full prescribing information can be found on the FDA website.
Promising Results From Anti-HIV Combination Treatment
Reliable treatment with broadly neutralizing antibodies—bNAbs—could change the future for people living with HIV. But studies have found that infusions of a single bNAb did not suppress HIV because some patients developed resistance.
Rockefeller University researchers, however, theorized that combining multiple antibodies that target distinct regions of HIV would both suppress the virus and prevent resistance. So in an NIH-supported pilot study, researhcers recruited 15 volunteers whose HIV was suppressed with antiretroviral treatment (ART) and who were sensitive to 3BNC117 and 10-1074, both potent bNAbs.
Participants received infusions of both bNAbs, stopped taking ART 2 days later, and received additional infusions 3 and 6 weeks later.
Of the 11 people who completed the study, 9 maintained viral suppression without ART for an average of 15 weeks, until the amount of bNAbs in their bodies fell below protective levels. In 2 of the 9, virus was controlled through the end of the 30-week follow-up period. The remaining 2 participants were found to harbor HIV resistant to at least 1 bNAb and experienced viral rebound before 12 weeks after stopping ART.
The researchers are enrolling people with HIV in a larger study to determine an optimal regimen of bNAbs.
Reliable treatment with broadly neutralizing antibodies—bNAbs—could change the future for people living with HIV. But studies have found that infusions of a single bNAb did not suppress HIV because some patients developed resistance.
Rockefeller University researchers, however, theorized that combining multiple antibodies that target distinct regions of HIV would both suppress the virus and prevent resistance. So in an NIH-supported pilot study, researhcers recruited 15 volunteers whose HIV was suppressed with antiretroviral treatment (ART) and who were sensitive to 3BNC117 and 10-1074, both potent bNAbs.
Participants received infusions of both bNAbs, stopped taking ART 2 days later, and received additional infusions 3 and 6 weeks later.
Of the 11 people who completed the study, 9 maintained viral suppression without ART for an average of 15 weeks, until the amount of bNAbs in their bodies fell below protective levels. In 2 of the 9, virus was controlled through the end of the 30-week follow-up period. The remaining 2 participants were found to harbor HIV resistant to at least 1 bNAb and experienced viral rebound before 12 weeks after stopping ART.
The researchers are enrolling people with HIV in a larger study to determine an optimal regimen of bNAbs.
Reliable treatment with broadly neutralizing antibodies—bNAbs—could change the future for people living with HIV. But studies have found that infusions of a single bNAb did not suppress HIV because some patients developed resistance.
Rockefeller University researchers, however, theorized that combining multiple antibodies that target distinct regions of HIV would both suppress the virus and prevent resistance. So in an NIH-supported pilot study, researhcers recruited 15 volunteers whose HIV was suppressed with antiretroviral treatment (ART) and who were sensitive to 3BNC117 and 10-1074, both potent bNAbs.
Participants received infusions of both bNAbs, stopped taking ART 2 days later, and received additional infusions 3 and 6 weeks later.
Of the 11 people who completed the study, 9 maintained viral suppression without ART for an average of 15 weeks, until the amount of bNAbs in their bodies fell below protective levels. In 2 of the 9, virus was controlled through the end of the 30-week follow-up period. The remaining 2 participants were found to harbor HIV resistant to at least 1 bNAb and experienced viral rebound before 12 weeks after stopping ART.
The researchers are enrolling people with HIV in a larger study to determine an optimal regimen of bNAbs.