User login
Chaplains play important part of integrated palliative care
WASHINGTON – Hospital chaplains are key partners in meeting the needs of palliative care patients and, as such, have much to offer psychiatry, according to an expert.
“I think we should work with chaplains,” Dr. Laura B. Dunn, professor of psychiatry and behavioral sciences at Stanford (Calif.) University, said in an interview at the annual meeting of the American Association for Geriatric Psychiatry. “It’s very helpful to know what they do, and for them to know what we do in terms of diagnosing and using a biopsychosocial model.”
Dr. Dunn said the relegation of chaplaincy and spiritual care in medicine has been unfortunate. After all, the level of a patient’s spiritual health is an inherent aspect of quality of life in palliative care, particularly for those over age 60 who often want help reconciling with loved ones, data collected by Dr. Dunn show. Viewing those patients in terms of their core needs rather than seeking a psychiatric diagnosis can make them more satisfied with their overall palliative care experience, she said.
How is spiritual health measured? There are few models and even fewer empirical studies, but Dr. Dunn said in addition learning how to deliver pastoral care, professional chaplains are trained to assess, intervene, and observe outcomes in spiritual health.
To begin with, spirituality is defined not in terms of “religiosity” but more as following an ethical path, similar to the idea of the Golden Rule or the ethic of reciprocity: Love your neighbor as yourself, in other words. The spiritual maturity to follow such a path requires the ability to love oneself that is balanced with a connection to others, and God, “if your belief includes God,” said Dr. Dunn, who pointed out that chaplaincy programs for atheists also exist.
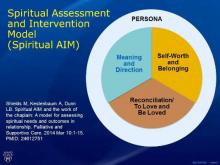
By observing a person’s behavior and conversing with him or her, paying close attention to the person’s attributions of blame, if any, and expressions of chief concerns, a chaplain will assess where on the continuum that person is in three key components of spiritual health: the need for meaning and direction, a sense of self-worth and belonging, and an ability to love and be loved. This latter component often is facilitated through seeking reconciliation when relationships are broken.
In the face of crisis, such as with terminal illness, one of those needs typically supersedes the others. This is what is known as the person’s “core spiritual need,” said Dr. Dunn, who also serves director of the university’s Geriatric Psychiatry Fellowship Training Program, and has extensive research and clinical experience evaluating and managing older adults with mood, anxiety, and cognitive disorders. As part of her research, she and her colleagues have developed a schematic called the Spiritual AIM (Spiritual Assessment and Intervention Model), which depicts those three concerns in relation to one another (Palliat Support Care. 2015 Feb;13:75-89).
Chaplains also are trained to rely on their own feelings about people, similar to the way in which psychiatrists use countertransference. “It is not intrapersonal; it is interpersonal,” Dr. Dunn said. “Healing happens in relationship, and the focus is on that relationship.”
Chaplains undergo standardized clinical pastoral training programs predicated on a combination of theological reflection and psychological theory, plus critique from professional peers and students.
“Their model is very similar to what we [geriatric psychiatrists] do when we assess a patient,” Dr. Dunn said. “We might be more structured trying to ferret out a diagnosis, but we’re assessing all the time.”
Depending on which aspect of spiritual health is most lacking, the chaplain will choose the role of either a “guide” to help with balancing the need for meaning and direction, a “valuer” to help restore feelings of worth and belonging, or a “truth-teller” who will explore with the patient ways he or she might have contributed to broken relationships, and actions the patient might take in order to heal them. This is all done within the context of an interdisciplinary team, Dr. Dunn said.
She and a team of researchers, including a chaplain, conducted a study of 31 advanced-stage adult cancer patients in an outpatient palliative care service, that measured self-reported changes in their spiritual, psychological, and physical symptoms both before and after Spiritual AIM sessions with a chaplain.
The need for balancing one of the three key components of spiritual health was determined by the chaplain to be fairly equal across the cohort, although just more than half of patients younger than 60 years of age struggled more with self-worth and belonging (P = .030). Those over 60 years were equally concerned with either reconciliation or meaning and direction (P less than .05). Two-thirds of the cohort were women, although a relationship between gender and spiritual assessment needs was not determined. Two-thirds also identified themselves as Christian, just over one-third were Jewish, and the rest identified as either Buddhist or nondenominational.
Using a variety of validated palliative care scales, such as the Edmonton Symptom Assessment Syndrome (ESAS) for physical complaints, the Center for Epidemiologic Studies Short Depression Scale, the Mini Mental Adjustment to Cancer scale (Mini-MAC), and the positive and negative RCOPE questionnaire for religious coping, among other scales, Dr. Dunn and her associates found that the change in baseline of overall spiritual health after intervention from the chaplain improved slightly in most measures. Mean baseline scores for the ESAS went from 25 to 24.4 post-intervention. The difference between baseline depression scores fell from 4.2 to 4.1.
Mini-MAC scores improved, particularly in “fighting spirit” and levels of fatalism (P = .084 and P = .036, respectively). In addition, maladaptive coping skills also improved (P = .018).The findings have helped Dr. Dunn in her work as a geriatric psychiatrist, especially when treating cancer patients, or in settings where there is not as much time for a full clinical assessment. “I think of patients in terms of their core needs and what I can do right now to help patients meet those needs.” Dr. Dunn cited, as an example, patients who feel like they don’t belong and are lonely. “If they’re in an assisted living home, can I get them to enter [the communal space]? That’s very different than thinking of them in terms only of depression.”
On Twitter @whitneymcknight
One reason chaplains are such a key part of the palliative care team is that they are seen by patients from a different perspective, Dr. Gurprit S. Lamba said. “Chaplains lend their ears, act as friends, and provide company to these patients,” Dr. Lamba said in an interview. “Discussing spiritual/religious matters helps these individuals cope better. Chaplains can provide more meaningful services with knowledge about different cultures, doctrines, and traditions, so that they can tailor their meetings accordingly.
“Studies have shown that psychiatrists are less religious and show less religious affiliation than their patients and than the population in general. As Dr. Dunn mentioned, chaplaincy programs for atheists also exist. Psychiatrists, with chaplains, can work with any individual to improve and optimize treatment. The critical part lies in assessment of patients’ religious views and spirituality along with their families’ opinions before offering meeting with chaplains.”
Dr. Lamba is a geriatric psychiatrist affiliated with BayRidge Hospital in Lynn, Mass.
One reason chaplains are such a key part of the palliative care team is that they are seen by patients from a different perspective, Dr. Gurprit S. Lamba said. “Chaplains lend their ears, act as friends, and provide company to these patients,” Dr. Lamba said in an interview. “Discussing spiritual/religious matters helps these individuals cope better. Chaplains can provide more meaningful services with knowledge about different cultures, doctrines, and traditions, so that they can tailor their meetings accordingly.
“Studies have shown that psychiatrists are less religious and show less religious affiliation than their patients and than the population in general. As Dr. Dunn mentioned, chaplaincy programs for atheists also exist. Psychiatrists, with chaplains, can work with any individual to improve and optimize treatment. The critical part lies in assessment of patients’ religious views and spirituality along with their families’ opinions before offering meeting with chaplains.”
Dr. Lamba is a geriatric psychiatrist affiliated with BayRidge Hospital in Lynn, Mass.
One reason chaplains are such a key part of the palliative care team is that they are seen by patients from a different perspective, Dr. Gurprit S. Lamba said. “Chaplains lend their ears, act as friends, and provide company to these patients,” Dr. Lamba said in an interview. “Discussing spiritual/religious matters helps these individuals cope better. Chaplains can provide more meaningful services with knowledge about different cultures, doctrines, and traditions, so that they can tailor their meetings accordingly.
“Studies have shown that psychiatrists are less religious and show less religious affiliation than their patients and than the population in general. As Dr. Dunn mentioned, chaplaincy programs for atheists also exist. Psychiatrists, with chaplains, can work with any individual to improve and optimize treatment. The critical part lies in assessment of patients’ religious views and spirituality along with their families’ opinions before offering meeting with chaplains.”
Dr. Lamba is a geriatric psychiatrist affiliated with BayRidge Hospital in Lynn, Mass.
WASHINGTON – Hospital chaplains are key partners in meeting the needs of palliative care patients and, as such, have much to offer psychiatry, according to an expert.
“I think we should work with chaplains,” Dr. Laura B. Dunn, professor of psychiatry and behavioral sciences at Stanford (Calif.) University, said in an interview at the annual meeting of the American Association for Geriatric Psychiatry. “It’s very helpful to know what they do, and for them to know what we do in terms of diagnosing and using a biopsychosocial model.”
Dr. Dunn said the relegation of chaplaincy and spiritual care in medicine has been unfortunate. After all, the level of a patient’s spiritual health is an inherent aspect of quality of life in palliative care, particularly for those over age 60 who often want help reconciling with loved ones, data collected by Dr. Dunn show. Viewing those patients in terms of their core needs rather than seeking a psychiatric diagnosis can make them more satisfied with their overall palliative care experience, she said.
How is spiritual health measured? There are few models and even fewer empirical studies, but Dr. Dunn said in addition learning how to deliver pastoral care, professional chaplains are trained to assess, intervene, and observe outcomes in spiritual health.
To begin with, spirituality is defined not in terms of “religiosity” but more as following an ethical path, similar to the idea of the Golden Rule or the ethic of reciprocity: Love your neighbor as yourself, in other words. The spiritual maturity to follow such a path requires the ability to love oneself that is balanced with a connection to others, and God, “if your belief includes God,” said Dr. Dunn, who pointed out that chaplaincy programs for atheists also exist.
By observing a person’s behavior and conversing with him or her, paying close attention to the person’s attributions of blame, if any, and expressions of chief concerns, a chaplain will assess where on the continuum that person is in three key components of spiritual health: the need for meaning and direction, a sense of self-worth and belonging, and an ability to love and be loved. This latter component often is facilitated through seeking reconciliation when relationships are broken.
In the face of crisis, such as with terminal illness, one of those needs typically supersedes the others. This is what is known as the person’s “core spiritual need,” said Dr. Dunn, who also serves director of the university’s Geriatric Psychiatry Fellowship Training Program, and has extensive research and clinical experience evaluating and managing older adults with mood, anxiety, and cognitive disorders. As part of her research, she and her colleagues have developed a schematic called the Spiritual AIM (Spiritual Assessment and Intervention Model), which depicts those three concerns in relation to one another (Palliat Support Care. 2015 Feb;13:75-89).
Chaplains also are trained to rely on their own feelings about people, similar to the way in which psychiatrists use countertransference. “It is not intrapersonal; it is interpersonal,” Dr. Dunn said. “Healing happens in relationship, and the focus is on that relationship.”
Chaplains undergo standardized clinical pastoral training programs predicated on a combination of theological reflection and psychological theory, plus critique from professional peers and students.
“Their model is very similar to what we [geriatric psychiatrists] do when we assess a patient,” Dr. Dunn said. “We might be more structured trying to ferret out a diagnosis, but we’re assessing all the time.”
Depending on which aspect of spiritual health is most lacking, the chaplain will choose the role of either a “guide” to help with balancing the need for meaning and direction, a “valuer” to help restore feelings of worth and belonging, or a “truth-teller” who will explore with the patient ways he or she might have contributed to broken relationships, and actions the patient might take in order to heal them. This is all done within the context of an interdisciplinary team, Dr. Dunn said.
She and a team of researchers, including a chaplain, conducted a study of 31 advanced-stage adult cancer patients in an outpatient palliative care service, that measured self-reported changes in their spiritual, psychological, and physical symptoms both before and after Spiritual AIM sessions with a chaplain.
The need for balancing one of the three key components of spiritual health was determined by the chaplain to be fairly equal across the cohort, although just more than half of patients younger than 60 years of age struggled more with self-worth and belonging (P = .030). Those over 60 years were equally concerned with either reconciliation or meaning and direction (P less than .05). Two-thirds of the cohort were women, although a relationship between gender and spiritual assessment needs was not determined. Two-thirds also identified themselves as Christian, just over one-third were Jewish, and the rest identified as either Buddhist or nondenominational.
Using a variety of validated palliative care scales, such as the Edmonton Symptom Assessment Syndrome (ESAS) for physical complaints, the Center for Epidemiologic Studies Short Depression Scale, the Mini Mental Adjustment to Cancer scale (Mini-MAC), and the positive and negative RCOPE questionnaire for religious coping, among other scales, Dr. Dunn and her associates found that the change in baseline of overall spiritual health after intervention from the chaplain improved slightly in most measures. Mean baseline scores for the ESAS went from 25 to 24.4 post-intervention. The difference between baseline depression scores fell from 4.2 to 4.1.
Mini-MAC scores improved, particularly in “fighting spirit” and levels of fatalism (P = .084 and P = .036, respectively). In addition, maladaptive coping skills also improved (P = .018).The findings have helped Dr. Dunn in her work as a geriatric psychiatrist, especially when treating cancer patients, or in settings where there is not as much time for a full clinical assessment. “I think of patients in terms of their core needs and what I can do right now to help patients meet those needs.” Dr. Dunn cited, as an example, patients who feel like they don’t belong and are lonely. “If they’re in an assisted living home, can I get them to enter [the communal space]? That’s very different than thinking of them in terms only of depression.”
On Twitter @whitneymcknight
WASHINGTON – Hospital chaplains are key partners in meeting the needs of palliative care patients and, as such, have much to offer psychiatry, according to an expert.
“I think we should work with chaplains,” Dr. Laura B. Dunn, professor of psychiatry and behavioral sciences at Stanford (Calif.) University, said in an interview at the annual meeting of the American Association for Geriatric Psychiatry. “It’s very helpful to know what they do, and for them to know what we do in terms of diagnosing and using a biopsychosocial model.”
Dr. Dunn said the relegation of chaplaincy and spiritual care in medicine has been unfortunate. After all, the level of a patient’s spiritual health is an inherent aspect of quality of life in palliative care, particularly for those over age 60 who often want help reconciling with loved ones, data collected by Dr. Dunn show. Viewing those patients in terms of their core needs rather than seeking a psychiatric diagnosis can make them more satisfied with their overall palliative care experience, she said.
How is spiritual health measured? There are few models and even fewer empirical studies, but Dr. Dunn said in addition learning how to deliver pastoral care, professional chaplains are trained to assess, intervene, and observe outcomes in spiritual health.
To begin with, spirituality is defined not in terms of “religiosity” but more as following an ethical path, similar to the idea of the Golden Rule or the ethic of reciprocity: Love your neighbor as yourself, in other words. The spiritual maturity to follow such a path requires the ability to love oneself that is balanced with a connection to others, and God, “if your belief includes God,” said Dr. Dunn, who pointed out that chaplaincy programs for atheists also exist.
By observing a person’s behavior and conversing with him or her, paying close attention to the person’s attributions of blame, if any, and expressions of chief concerns, a chaplain will assess where on the continuum that person is in three key components of spiritual health: the need for meaning and direction, a sense of self-worth and belonging, and an ability to love and be loved. This latter component often is facilitated through seeking reconciliation when relationships are broken.
In the face of crisis, such as with terminal illness, one of those needs typically supersedes the others. This is what is known as the person’s “core spiritual need,” said Dr. Dunn, who also serves director of the university’s Geriatric Psychiatry Fellowship Training Program, and has extensive research and clinical experience evaluating and managing older adults with mood, anxiety, and cognitive disorders. As part of her research, she and her colleagues have developed a schematic called the Spiritual AIM (Spiritual Assessment and Intervention Model), which depicts those three concerns in relation to one another (Palliat Support Care. 2015 Feb;13:75-89).
Chaplains also are trained to rely on their own feelings about people, similar to the way in which psychiatrists use countertransference. “It is not intrapersonal; it is interpersonal,” Dr. Dunn said. “Healing happens in relationship, and the focus is on that relationship.”
Chaplains undergo standardized clinical pastoral training programs predicated on a combination of theological reflection and psychological theory, plus critique from professional peers and students.
“Their model is very similar to what we [geriatric psychiatrists] do when we assess a patient,” Dr. Dunn said. “We might be more structured trying to ferret out a diagnosis, but we’re assessing all the time.”
Depending on which aspect of spiritual health is most lacking, the chaplain will choose the role of either a “guide” to help with balancing the need for meaning and direction, a “valuer” to help restore feelings of worth and belonging, or a “truth-teller” who will explore with the patient ways he or she might have contributed to broken relationships, and actions the patient might take in order to heal them. This is all done within the context of an interdisciplinary team, Dr. Dunn said.
She and a team of researchers, including a chaplain, conducted a study of 31 advanced-stage adult cancer patients in an outpatient palliative care service, that measured self-reported changes in their spiritual, psychological, and physical symptoms both before and after Spiritual AIM sessions with a chaplain.
The need for balancing one of the three key components of spiritual health was determined by the chaplain to be fairly equal across the cohort, although just more than half of patients younger than 60 years of age struggled more with self-worth and belonging (P = .030). Those over 60 years were equally concerned with either reconciliation or meaning and direction (P less than .05). Two-thirds of the cohort were women, although a relationship between gender and spiritual assessment needs was not determined. Two-thirds also identified themselves as Christian, just over one-third were Jewish, and the rest identified as either Buddhist or nondenominational.
Using a variety of validated palliative care scales, such as the Edmonton Symptom Assessment Syndrome (ESAS) for physical complaints, the Center for Epidemiologic Studies Short Depression Scale, the Mini Mental Adjustment to Cancer scale (Mini-MAC), and the positive and negative RCOPE questionnaire for religious coping, among other scales, Dr. Dunn and her associates found that the change in baseline of overall spiritual health after intervention from the chaplain improved slightly in most measures. Mean baseline scores for the ESAS went from 25 to 24.4 post-intervention. The difference between baseline depression scores fell from 4.2 to 4.1.
Mini-MAC scores improved, particularly in “fighting spirit” and levels of fatalism (P = .084 and P = .036, respectively). In addition, maladaptive coping skills also improved (P = .018).The findings have helped Dr. Dunn in her work as a geriatric psychiatrist, especially when treating cancer patients, or in settings where there is not as much time for a full clinical assessment. “I think of patients in terms of their core needs and what I can do right now to help patients meet those needs.” Dr. Dunn cited, as an example, patients who feel like they don’t belong and are lonely. “If they’re in an assisted living home, can I get them to enter [the communal space]? That’s very different than thinking of them in terms only of depression.”
On Twitter @whitneymcknight
EXPERT ANALYSIS FROM AAGP 2016
Gynecologic cancer patients underutilize advance care directives
SAN DIEGO – Fewer than half of gynecologic oncology patients surveyed at a major cancer center had completed advance care directives regarding their preferences for end-of-life care, and most of those who did had no copy of the documents in their medical records, Dr. Alaina J. Brown reported at the annual meeting of the Society of Gynecologic Oncology.
“These findings indicate there is room to improve advance directive planning documentation in our patient population,” said Dr. Brown, a fellow in gynecologic oncology and reproductive medicine at the University of Texas MD Anderson Cancer Center in Houston.
“Providers must identify and address barriers to advance care planning documentation in order to assist patients in achieving their end-of-life care goals ... I think we need to focus on educating ourselves and becoming proactive about trying to have these conversations earlier in treatment instead of when a patient is quite ill and we know that they’re going to pass away within the next week or so,” she added.
One barrier has recently been overcome by Medicare’s new policy – effective beginning this past January – of providing reimbursement for advance care planning as a separate and billable service.
In addition, Dr. Brown’s survey of 110 gynecologic oncology patients identified two significant psychological barriers to advance care planning: high levels of death anxiety and a feeling of distress that symptoms and/or treatment side effects are interfering with daily activities and relationships.
The survey showed that while 75% of the patients were familiar with advance care directives such as a living will or medical power of attorney, only 49% of subjects had actually completed those documents, and a mere 18% had a copy of an advance care directive in their medical record.
Half of the subjects had recurrent cancer, the rest were visiting the gynecologic oncology service for active surveillance. Only a minority of those with recurrent cancer had completed advance care directives.
Study participants completed two validated, self-administered questionnaire surveys: the 19-item MD Anderson Symptom Inventory (MDASI), which assesses patient-reported disease symptoms and treatment side effects during the previous 24 hours, and the 15-item Templer’s Death Anxiety Scale.
The mean MDASI Interference score, a measure of overall symptom distress and the impact of symptoms on daily life, was significantly higher in gynecologic oncology patients who hadn’t completed advance directives than in those who had. Similarly, patients who hadn’t completed advance directives scored significantly higher on the death anxiety metric.
“Patients with recurrent disease and those with increased disease symptom burden and death anxiety should be targeted for advance care planning discussions, as they may be less likely to engage in advance care planning activities,” Dr. Brown concluded.
She noted that prior research in other medical fields has shown that holding early planning discussions about end-of-life issues improves the likelihood that a patient’s final wishes will be honored, reduces utilization of hospital resources at the end of life, and reduces distress among the patient and family members. It’s important for gynecologic oncologists to step forward in this area because they are in a unique position: they often manage a cancer patient’s surgical care as well as chemotherapy and then later assist in the transition to end of life, she added.
At the conference session on palliative care where Dr. Brown presented her findings, audience members said the 49% completion rate for advance care directives that she found in her study was actually quite impressive; at many gynecologic oncology services the rates are in the 20%-25% range. The audience consensus was that much of the blame for the low rates of advance care planning documentation in their field belongs on the shoulders of gynecologic oncologists themselves.
“I would say that it’s entirely our fault,” declared session codirector Dr. Stephanie Blank of New York University.
Dr. Brown said as a result of her survey findings, she and her colleagues are working to change the institutional practice at MD Anderson such that completion of advance care directive planning directives with documentation in the medical record becomes a quality-of-care goal within the first few patient visits.
“In the past we had a social worker come to those patients who checked off a box on a form in the waiting room; now we’re trying to be more proactive about having a provider engage the patients early on,” she explained.
She reported having no financial conflicts of interest regarding her study.
SAN DIEGO – Fewer than half of gynecologic oncology patients surveyed at a major cancer center had completed advance care directives regarding their preferences for end-of-life care, and most of those who did had no copy of the documents in their medical records, Dr. Alaina J. Brown reported at the annual meeting of the Society of Gynecologic Oncology.
“These findings indicate there is room to improve advance directive planning documentation in our patient population,” said Dr. Brown, a fellow in gynecologic oncology and reproductive medicine at the University of Texas MD Anderson Cancer Center in Houston.
“Providers must identify and address barriers to advance care planning documentation in order to assist patients in achieving their end-of-life care goals ... I think we need to focus on educating ourselves and becoming proactive about trying to have these conversations earlier in treatment instead of when a patient is quite ill and we know that they’re going to pass away within the next week or so,” she added.
One barrier has recently been overcome by Medicare’s new policy – effective beginning this past January – of providing reimbursement for advance care planning as a separate and billable service.
In addition, Dr. Brown’s survey of 110 gynecologic oncology patients identified two significant psychological barriers to advance care planning: high levels of death anxiety and a feeling of distress that symptoms and/or treatment side effects are interfering with daily activities and relationships.
The survey showed that while 75% of the patients were familiar with advance care directives such as a living will or medical power of attorney, only 49% of subjects had actually completed those documents, and a mere 18% had a copy of an advance care directive in their medical record.
Half of the subjects had recurrent cancer, the rest were visiting the gynecologic oncology service for active surveillance. Only a minority of those with recurrent cancer had completed advance care directives.
Study participants completed two validated, self-administered questionnaire surveys: the 19-item MD Anderson Symptom Inventory (MDASI), which assesses patient-reported disease symptoms and treatment side effects during the previous 24 hours, and the 15-item Templer’s Death Anxiety Scale.
The mean MDASI Interference score, a measure of overall symptom distress and the impact of symptoms on daily life, was significantly higher in gynecologic oncology patients who hadn’t completed advance directives than in those who had. Similarly, patients who hadn’t completed advance directives scored significantly higher on the death anxiety metric.
“Patients with recurrent disease and those with increased disease symptom burden and death anxiety should be targeted for advance care planning discussions, as they may be less likely to engage in advance care planning activities,” Dr. Brown concluded.
She noted that prior research in other medical fields has shown that holding early planning discussions about end-of-life issues improves the likelihood that a patient’s final wishes will be honored, reduces utilization of hospital resources at the end of life, and reduces distress among the patient and family members. It’s important for gynecologic oncologists to step forward in this area because they are in a unique position: they often manage a cancer patient’s surgical care as well as chemotherapy and then later assist in the transition to end of life, she added.
At the conference session on palliative care where Dr. Brown presented her findings, audience members said the 49% completion rate for advance care directives that she found in her study was actually quite impressive; at many gynecologic oncology services the rates are in the 20%-25% range. The audience consensus was that much of the blame for the low rates of advance care planning documentation in their field belongs on the shoulders of gynecologic oncologists themselves.
“I would say that it’s entirely our fault,” declared session codirector Dr. Stephanie Blank of New York University.
Dr. Brown said as a result of her survey findings, she and her colleagues are working to change the institutional practice at MD Anderson such that completion of advance care directive planning directives with documentation in the medical record becomes a quality-of-care goal within the first few patient visits.
“In the past we had a social worker come to those patients who checked off a box on a form in the waiting room; now we’re trying to be more proactive about having a provider engage the patients early on,” she explained.
She reported having no financial conflicts of interest regarding her study.
SAN DIEGO – Fewer than half of gynecologic oncology patients surveyed at a major cancer center had completed advance care directives regarding their preferences for end-of-life care, and most of those who did had no copy of the documents in their medical records, Dr. Alaina J. Brown reported at the annual meeting of the Society of Gynecologic Oncology.
“These findings indicate there is room to improve advance directive planning documentation in our patient population,” said Dr. Brown, a fellow in gynecologic oncology and reproductive medicine at the University of Texas MD Anderson Cancer Center in Houston.
“Providers must identify and address barriers to advance care planning documentation in order to assist patients in achieving their end-of-life care goals ... I think we need to focus on educating ourselves and becoming proactive about trying to have these conversations earlier in treatment instead of when a patient is quite ill and we know that they’re going to pass away within the next week or so,” she added.
One barrier has recently been overcome by Medicare’s new policy – effective beginning this past January – of providing reimbursement for advance care planning as a separate and billable service.
In addition, Dr. Brown’s survey of 110 gynecologic oncology patients identified two significant psychological barriers to advance care planning: high levels of death anxiety and a feeling of distress that symptoms and/or treatment side effects are interfering with daily activities and relationships.
The survey showed that while 75% of the patients were familiar with advance care directives such as a living will or medical power of attorney, only 49% of subjects had actually completed those documents, and a mere 18% had a copy of an advance care directive in their medical record.
Half of the subjects had recurrent cancer, the rest were visiting the gynecologic oncology service for active surveillance. Only a minority of those with recurrent cancer had completed advance care directives.
Study participants completed two validated, self-administered questionnaire surveys: the 19-item MD Anderson Symptom Inventory (MDASI), which assesses patient-reported disease symptoms and treatment side effects during the previous 24 hours, and the 15-item Templer’s Death Anxiety Scale.
The mean MDASI Interference score, a measure of overall symptom distress and the impact of symptoms on daily life, was significantly higher in gynecologic oncology patients who hadn’t completed advance directives than in those who had. Similarly, patients who hadn’t completed advance directives scored significantly higher on the death anxiety metric.
“Patients with recurrent disease and those with increased disease symptom burden and death anxiety should be targeted for advance care planning discussions, as they may be less likely to engage in advance care planning activities,” Dr. Brown concluded.
She noted that prior research in other medical fields has shown that holding early planning discussions about end-of-life issues improves the likelihood that a patient’s final wishes will be honored, reduces utilization of hospital resources at the end of life, and reduces distress among the patient and family members. It’s important for gynecologic oncologists to step forward in this area because they are in a unique position: they often manage a cancer patient’s surgical care as well as chemotherapy and then later assist in the transition to end of life, she added.
At the conference session on palliative care where Dr. Brown presented her findings, audience members said the 49% completion rate for advance care directives that she found in her study was actually quite impressive; at many gynecologic oncology services the rates are in the 20%-25% range. The audience consensus was that much of the blame for the low rates of advance care planning documentation in their field belongs on the shoulders of gynecologic oncologists themselves.
“I would say that it’s entirely our fault,” declared session codirector Dr. Stephanie Blank of New York University.
Dr. Brown said as a result of her survey findings, she and her colleagues are working to change the institutional practice at MD Anderson such that completion of advance care directive planning directives with documentation in the medical record becomes a quality-of-care goal within the first few patient visits.
“In the past we had a social worker come to those patients who checked off a box on a form in the waiting room; now we’re trying to be more proactive about having a provider engage the patients early on,” she explained.
She reported having no financial conflicts of interest regarding her study.
AT THE ANNUAL MEETING ON WOMEN’S CANCER
Key clinical point: Gynecologic oncologists aren’t doing well at helping their patients create advance care directives in a timely way.
Major finding: Fewer than one in five gynecologic oncology patients surveyed had an advance care directive included in the medical chart.
Data source: Survey of 110 gynecologic oncology patients at a major cancer center to examine the relationship between completion of advance care directives and patients’ levels of death anxiety and symptom burden.
Disclosures: The presenter reported having no financial conflicts regarding her study, which was conducted free of commercial support.
How physicians can reverse the opioid crisis
In about 2002, Dr. Gary Franklin realized the state of Washington might have a problem.
A big problem.
A state resident who’d suffered a back sprain and filed a workers’ compensation claim died 2 years later – not from heart disease or cancer or stroke, but from an unintentional prescription opioid overdose, recalled Dr. Franklin, medical director of the Washington State Department of Labor and Industries.
“I had never seen anything so sad,” he said.
The case prompted the neurologist and his colleagues to review Washington state workers’ compensation claims. What they uncovered was a local trend that would explode into a national scourge: a marked increase in opioid poisonings among Washington state residents with everyday aches and pains who, in the past, would never have been prescribed opioids.
The gateway drug turned out to be oxycodone (OxyContin), which was heavily marketed at the time as a safe choice for pain relief with little abuse potential. Purdue Pharma has since paid a $600 million federal fine for deceptive marketing.
“This is the worst man-made epidemic in modern medical history,” said Dr. Franklin, also a research professor at the University of Washington, Seattle. “It was made by modern medicine, and it’s up to modern medicine to turn it around.”
For the United States to recover from the opioid crisis, Dr. Franklin said, the medical community must reduce oral opioid prescriptions for noncancer pain. Others interviewed for this story said doctors also have to overcome their aversion to in-office addiction treatment, and find new options for everyday chronic pain.
The first step is “to forget everything you were told in 1999,” said Dr. Franklin. That includes the notions that addiction is rare, opioids are indicated for noncancer chronic pain, and doses should be increased if patients become tolerant.
Those messages led to overprescribing, which in turn “led to an oversupply problem that’s feeding misuse and diversion. It’s only recently that it has become a heroin problem; the vast majority of heroin users these days start on prescription opioids,” he said.
The Washington workers’ comp claims data triggered “a complete rethinking of our approach to chronic pain and a shift to other treatment strategies,” said Dr. David Tauben, chief of pain medicine at the University of Washington, Seattle.
In 2007, Washington became one of the first states to issue opioid treatment guidelines, which are updated regularly. Among other steps, prescribers were urged to limit doses and durations.
Since then, the state has seen a nearly 40% reduction in prescription opioid poisonings. “We also found that with dose reductions” for back pain, headaches, and similar noncancer issues, “pain subsides, function improves, and patient satisfaction” goes up, said Dr. Tauben, who was involved in creating the guidelines.
Clamping down, pushing back
The Centers for Disease Control and Prevention in March released similar guidelines, including a suggested 3-day limit for acute pain prescriptions and a cap of 90 morphine milligram equivalents per day for chronic noncancer pain – the amount in a single 60-mg oxycodone tablet.
Meanwhile, Food and Drug Administration officials are planning a regulatory overhaul to address opioid approval, labeling, and prescribing concerns. In many places, doctors are also facing new opioid training requirements.
The Washington state experience suggests that such efforts are likely to help reverse the opioid crisis.
It’s not about getting rid of the drugs, explained Dr. Gail D’Onofrio, chair of emergency medicine at Yale University in New Haven, Conn.
“Opioids are really good for certain things,” especially cancer pain and, for a few days, acute pain. But “we’ve kind of lost our way,” Dr. D’Onofrio said. “We don’t need to give people 3 months of narcotics for a knee replacement” or 3 weeks of narcotics for a wisdom tooth extraction.
“We are all guilty” of overprescribing, and “just like everywhere else, we’ve seen the problems; every year, it’s getting worse,” she added. In response, “we are changing how we use opioids, adapting the guidelines from the CDC and other groups,” and tailoring them to different services.
At the Yale emergency department, oral opioid prescriptions are now generally limited to 3 days, except for renal colic patients, who might get a few days more. “We do not fill opioid scripts and don’t reorder them for patients.” Instead, “we talk to the prescriber and tell them what’s going on,” Dr. D’Onofrio explained.
Yale’s not alone in cutting back. After years of growth, U.S. oral opioid sales appear to be declining. In fact, in some quarters, there’s concern the clampdown will go too far.
The CDC received more than 4,300 comments about the draft version of its guidelines. Some patients were worried about losing access to drugs that have helped them. And, while supportive of the goals, some professional groups questioned the evidence behind the proposals and worry about undertreatment of pain, among other issues.
“The problem with a lot of the guidelines is that they’re all around limiting prescribing. They don’t really tell doctors what to do instead,” said Dr. Peter Friedmann, an addiction treatment specialist in Springfield, Mass., and chief research officer for Baystate Health.
An alternative for chronic pain
For noncancer chronic pain, recent evidence supports multimodal therapy. Opioids might bring temporary relief, but “throwing drugs at people isn’t going to solve the problem,” said Dr. Tauben, the University of Washington pain expert.
“Multimodal therapy” means focusing more on the burden of pain instead of its intensity, with team-based care. Reducing the burden – anxiety, sleeplessness, reduced mobility, and other problems – seems to reduce the significance and intensity of pain to the point where it can be managed, if needed, with nonsteroidal anti-inflammatory drugs (NSAIDs), trigger-point injections, and other nonopioid options.
Depending on the patient’s needs, primary care physicians might find themselves coordinating services from psychologists, physical therapists, social workers, or others.
For the approach to work, the impact of pain has to be accurately gauged, along with underlying psychological or social issues; to save time, the University of Washington has patients complete an online survey prior to their office visit.
There are national efforts underway to support the approach, and a growing recognition that “by doing it right, you save downstream costs. Primary care must get involved; that’s where chronic pain presents,” Dr. Tauben said.
Batting cleanup
There’s a role for primary care when patients are hooked on opioids, too. Requests for early refills and higher doses are a clue.
“Given the stigma, a lot of doctors don’t want to deal with addiction, but we have to deal with it. We need to move addiction treatment into the mainstream of what we do in medicine,” Dr. Friedmann said. “These patients are no more or no less challenging than any other patients we deal with; the only way doctors are going to find that out is by starting to manage some of them.”
He estimates that about 60% of his patients do well on buprenorphine, a sublingual, partial opioid agonist that blocks the effects of full agonists and dulls withdrawal symptoms. Incorporating it into practice “is not something you need to figure out yourself,” Dr. Friedmann noted. There are training programs and people who can help.
There simply aren’t enough methadone clinics to handle the current situation, especially in suburbs and rural areas where drug dealers have found a new market for heroin. Another option, abstinence programs, “have contributed to the problem of overdose;” people lose their tolerance, reuse, and die, he said.
Buprenorphine treatment might soon get easier. The FDA is expected to make an approval decision soon on probuphine, a matchstick-size subdermal implant that delivers buprenorphine continuously for 6 months.
Dr. Friedmann disclosed relationships with Alkermes, Inavir, and Orexo. The other doctors had no relevant disclosures.
In about 2002, Dr. Gary Franklin realized the state of Washington might have a problem.
A big problem.
A state resident who’d suffered a back sprain and filed a workers’ compensation claim died 2 years later – not from heart disease or cancer or stroke, but from an unintentional prescription opioid overdose, recalled Dr. Franklin, medical director of the Washington State Department of Labor and Industries.
“I had never seen anything so sad,” he said.
The case prompted the neurologist and his colleagues to review Washington state workers’ compensation claims. What they uncovered was a local trend that would explode into a national scourge: a marked increase in opioid poisonings among Washington state residents with everyday aches and pains who, in the past, would never have been prescribed opioids.
The gateway drug turned out to be oxycodone (OxyContin), which was heavily marketed at the time as a safe choice for pain relief with little abuse potential. Purdue Pharma has since paid a $600 million federal fine for deceptive marketing.
“This is the worst man-made epidemic in modern medical history,” said Dr. Franklin, also a research professor at the University of Washington, Seattle. “It was made by modern medicine, and it’s up to modern medicine to turn it around.”
For the United States to recover from the opioid crisis, Dr. Franklin said, the medical community must reduce oral opioid prescriptions for noncancer pain. Others interviewed for this story said doctors also have to overcome their aversion to in-office addiction treatment, and find new options for everyday chronic pain.
The first step is “to forget everything you were told in 1999,” said Dr. Franklin. That includes the notions that addiction is rare, opioids are indicated for noncancer chronic pain, and doses should be increased if patients become tolerant.
Those messages led to overprescribing, which in turn “led to an oversupply problem that’s feeding misuse and diversion. It’s only recently that it has become a heroin problem; the vast majority of heroin users these days start on prescription opioids,” he said.
The Washington workers’ comp claims data triggered “a complete rethinking of our approach to chronic pain and a shift to other treatment strategies,” said Dr. David Tauben, chief of pain medicine at the University of Washington, Seattle.
In 2007, Washington became one of the first states to issue opioid treatment guidelines, which are updated regularly. Among other steps, prescribers were urged to limit doses and durations.
Since then, the state has seen a nearly 40% reduction in prescription opioid poisonings. “We also found that with dose reductions” for back pain, headaches, and similar noncancer issues, “pain subsides, function improves, and patient satisfaction” goes up, said Dr. Tauben, who was involved in creating the guidelines.
Clamping down, pushing back
The Centers for Disease Control and Prevention in March released similar guidelines, including a suggested 3-day limit for acute pain prescriptions and a cap of 90 morphine milligram equivalents per day for chronic noncancer pain – the amount in a single 60-mg oxycodone tablet.
Meanwhile, Food and Drug Administration officials are planning a regulatory overhaul to address opioid approval, labeling, and prescribing concerns. In many places, doctors are also facing new opioid training requirements.
The Washington state experience suggests that such efforts are likely to help reverse the opioid crisis.
It’s not about getting rid of the drugs, explained Dr. Gail D’Onofrio, chair of emergency medicine at Yale University in New Haven, Conn.
“Opioids are really good for certain things,” especially cancer pain and, for a few days, acute pain. But “we’ve kind of lost our way,” Dr. D’Onofrio said. “We don’t need to give people 3 months of narcotics for a knee replacement” or 3 weeks of narcotics for a wisdom tooth extraction.
“We are all guilty” of overprescribing, and “just like everywhere else, we’ve seen the problems; every year, it’s getting worse,” she added. In response, “we are changing how we use opioids, adapting the guidelines from the CDC and other groups,” and tailoring them to different services.
At the Yale emergency department, oral opioid prescriptions are now generally limited to 3 days, except for renal colic patients, who might get a few days more. “We do not fill opioid scripts and don’t reorder them for patients.” Instead, “we talk to the prescriber and tell them what’s going on,” Dr. D’Onofrio explained.
Yale’s not alone in cutting back. After years of growth, U.S. oral opioid sales appear to be declining. In fact, in some quarters, there’s concern the clampdown will go too far.
The CDC received more than 4,300 comments about the draft version of its guidelines. Some patients were worried about losing access to drugs that have helped them. And, while supportive of the goals, some professional groups questioned the evidence behind the proposals and worry about undertreatment of pain, among other issues.
“The problem with a lot of the guidelines is that they’re all around limiting prescribing. They don’t really tell doctors what to do instead,” said Dr. Peter Friedmann, an addiction treatment specialist in Springfield, Mass., and chief research officer for Baystate Health.
An alternative for chronic pain
For noncancer chronic pain, recent evidence supports multimodal therapy. Opioids might bring temporary relief, but “throwing drugs at people isn’t going to solve the problem,” said Dr. Tauben, the University of Washington pain expert.
“Multimodal therapy” means focusing more on the burden of pain instead of its intensity, with team-based care. Reducing the burden – anxiety, sleeplessness, reduced mobility, and other problems – seems to reduce the significance and intensity of pain to the point where it can be managed, if needed, with nonsteroidal anti-inflammatory drugs (NSAIDs), trigger-point injections, and other nonopioid options.
Depending on the patient’s needs, primary care physicians might find themselves coordinating services from psychologists, physical therapists, social workers, or others.
For the approach to work, the impact of pain has to be accurately gauged, along with underlying psychological or social issues; to save time, the University of Washington has patients complete an online survey prior to their office visit.
There are national efforts underway to support the approach, and a growing recognition that “by doing it right, you save downstream costs. Primary care must get involved; that’s where chronic pain presents,” Dr. Tauben said.
Batting cleanup
There’s a role for primary care when patients are hooked on opioids, too. Requests for early refills and higher doses are a clue.
“Given the stigma, a lot of doctors don’t want to deal with addiction, but we have to deal with it. We need to move addiction treatment into the mainstream of what we do in medicine,” Dr. Friedmann said. “These patients are no more or no less challenging than any other patients we deal with; the only way doctors are going to find that out is by starting to manage some of them.”
He estimates that about 60% of his patients do well on buprenorphine, a sublingual, partial opioid agonist that blocks the effects of full agonists and dulls withdrawal symptoms. Incorporating it into practice “is not something you need to figure out yourself,” Dr. Friedmann noted. There are training programs and people who can help.
There simply aren’t enough methadone clinics to handle the current situation, especially in suburbs and rural areas where drug dealers have found a new market for heroin. Another option, abstinence programs, “have contributed to the problem of overdose;” people lose their tolerance, reuse, and die, he said.
Buprenorphine treatment might soon get easier. The FDA is expected to make an approval decision soon on probuphine, a matchstick-size subdermal implant that delivers buprenorphine continuously for 6 months.
Dr. Friedmann disclosed relationships with Alkermes, Inavir, and Orexo. The other doctors had no relevant disclosures.
In about 2002, Dr. Gary Franklin realized the state of Washington might have a problem.
A big problem.
A state resident who’d suffered a back sprain and filed a workers’ compensation claim died 2 years later – not from heart disease or cancer or stroke, but from an unintentional prescription opioid overdose, recalled Dr. Franklin, medical director of the Washington State Department of Labor and Industries.
“I had never seen anything so sad,” he said.
The case prompted the neurologist and his colleagues to review Washington state workers’ compensation claims. What they uncovered was a local trend that would explode into a national scourge: a marked increase in opioid poisonings among Washington state residents with everyday aches and pains who, in the past, would never have been prescribed opioids.
The gateway drug turned out to be oxycodone (OxyContin), which was heavily marketed at the time as a safe choice for pain relief with little abuse potential. Purdue Pharma has since paid a $600 million federal fine for deceptive marketing.
“This is the worst man-made epidemic in modern medical history,” said Dr. Franklin, also a research professor at the University of Washington, Seattle. “It was made by modern medicine, and it’s up to modern medicine to turn it around.”
For the United States to recover from the opioid crisis, Dr. Franklin said, the medical community must reduce oral opioid prescriptions for noncancer pain. Others interviewed for this story said doctors also have to overcome their aversion to in-office addiction treatment, and find new options for everyday chronic pain.
The first step is “to forget everything you were told in 1999,” said Dr. Franklin. That includes the notions that addiction is rare, opioids are indicated for noncancer chronic pain, and doses should be increased if patients become tolerant.
Those messages led to overprescribing, which in turn “led to an oversupply problem that’s feeding misuse and diversion. It’s only recently that it has become a heroin problem; the vast majority of heroin users these days start on prescription opioids,” he said.
The Washington workers’ comp claims data triggered “a complete rethinking of our approach to chronic pain and a shift to other treatment strategies,” said Dr. David Tauben, chief of pain medicine at the University of Washington, Seattle.
In 2007, Washington became one of the first states to issue opioid treatment guidelines, which are updated regularly. Among other steps, prescribers were urged to limit doses and durations.
Since then, the state has seen a nearly 40% reduction in prescription opioid poisonings. “We also found that with dose reductions” for back pain, headaches, and similar noncancer issues, “pain subsides, function improves, and patient satisfaction” goes up, said Dr. Tauben, who was involved in creating the guidelines.
Clamping down, pushing back
The Centers for Disease Control and Prevention in March released similar guidelines, including a suggested 3-day limit for acute pain prescriptions and a cap of 90 morphine milligram equivalents per day for chronic noncancer pain – the amount in a single 60-mg oxycodone tablet.
Meanwhile, Food and Drug Administration officials are planning a regulatory overhaul to address opioid approval, labeling, and prescribing concerns. In many places, doctors are also facing new opioid training requirements.
The Washington state experience suggests that such efforts are likely to help reverse the opioid crisis.
It’s not about getting rid of the drugs, explained Dr. Gail D’Onofrio, chair of emergency medicine at Yale University in New Haven, Conn.
“Opioids are really good for certain things,” especially cancer pain and, for a few days, acute pain. But “we’ve kind of lost our way,” Dr. D’Onofrio said. “We don’t need to give people 3 months of narcotics for a knee replacement” or 3 weeks of narcotics for a wisdom tooth extraction.
“We are all guilty” of overprescribing, and “just like everywhere else, we’ve seen the problems; every year, it’s getting worse,” she added. In response, “we are changing how we use opioids, adapting the guidelines from the CDC and other groups,” and tailoring them to different services.
At the Yale emergency department, oral opioid prescriptions are now generally limited to 3 days, except for renal colic patients, who might get a few days more. “We do not fill opioid scripts and don’t reorder them for patients.” Instead, “we talk to the prescriber and tell them what’s going on,” Dr. D’Onofrio explained.
Yale’s not alone in cutting back. After years of growth, U.S. oral opioid sales appear to be declining. In fact, in some quarters, there’s concern the clampdown will go too far.
The CDC received more than 4,300 comments about the draft version of its guidelines. Some patients were worried about losing access to drugs that have helped them. And, while supportive of the goals, some professional groups questioned the evidence behind the proposals and worry about undertreatment of pain, among other issues.
“The problem with a lot of the guidelines is that they’re all around limiting prescribing. They don’t really tell doctors what to do instead,” said Dr. Peter Friedmann, an addiction treatment specialist in Springfield, Mass., and chief research officer for Baystate Health.
An alternative for chronic pain
For noncancer chronic pain, recent evidence supports multimodal therapy. Opioids might bring temporary relief, but “throwing drugs at people isn’t going to solve the problem,” said Dr. Tauben, the University of Washington pain expert.
“Multimodal therapy” means focusing more on the burden of pain instead of its intensity, with team-based care. Reducing the burden – anxiety, sleeplessness, reduced mobility, and other problems – seems to reduce the significance and intensity of pain to the point where it can be managed, if needed, with nonsteroidal anti-inflammatory drugs (NSAIDs), trigger-point injections, and other nonopioid options.
Depending on the patient’s needs, primary care physicians might find themselves coordinating services from psychologists, physical therapists, social workers, or others.
For the approach to work, the impact of pain has to be accurately gauged, along with underlying psychological or social issues; to save time, the University of Washington has patients complete an online survey prior to their office visit.
There are national efforts underway to support the approach, and a growing recognition that “by doing it right, you save downstream costs. Primary care must get involved; that’s where chronic pain presents,” Dr. Tauben said.
Batting cleanup
There’s a role for primary care when patients are hooked on opioids, too. Requests for early refills and higher doses are a clue.
“Given the stigma, a lot of doctors don’t want to deal with addiction, but we have to deal with it. We need to move addiction treatment into the mainstream of what we do in medicine,” Dr. Friedmann said. “These patients are no more or no less challenging than any other patients we deal with; the only way doctors are going to find that out is by starting to manage some of them.”
He estimates that about 60% of his patients do well on buprenorphine, a sublingual, partial opioid agonist that blocks the effects of full agonists and dulls withdrawal symptoms. Incorporating it into practice “is not something you need to figure out yourself,” Dr. Friedmann noted. There are training programs and people who can help.
There simply aren’t enough methadone clinics to handle the current situation, especially in suburbs and rural areas where drug dealers have found a new market for heroin. Another option, abstinence programs, “have contributed to the problem of overdose;” people lose their tolerance, reuse, and die, he said.
Buprenorphine treatment might soon get easier. The FDA is expected to make an approval decision soon on probuphine, a matchstick-size subdermal implant that delivers buprenorphine continuously for 6 months.
Dr. Friedmann disclosed relationships with Alkermes, Inavir, and Orexo. The other doctors had no relevant disclosures.
Robotic Surgery for Older Cancer Patients
A review by researchers from Hôpital Sud, Rennes, France, and McGill University, Montreal showed that surgery by robot—rather than traditional open surgery—may improve outcomes in elderly patients with pelvic cancer, however the research is sparse. For instance, only 6 published studies specifically address surgery in the elderly with endometrial cancer, the most common gynecologic malignancy in the western world. However, because surgery is challenging for these often-frail patients, minimally invasive (or minimal access) surgery could be the answer. When comparing robotics to standard laparoscopy in treating endometrial cancer, the data suggest significantly less blood loss, reduced operative time, and higher node counts.
Related: A Team Approach to Nonmelanotic Skin Cancer Procedures
Similar to many others, the researchers’ academic center was slow to adopt minimally invasive standard (MIS) laparoscopy. But within 2 years of the introduction of robotic surgery in 2007, more than 95% of patients requiring surgery to treat endometrial cancer undergo MIS. Now, the surgery is offered to each operable patient unless the cancer cannot be extracted intact or in a 15-cm diameter endobag via the vagina.
Related: Solid-Organ Transplant Recipients May Be at Greater Risk for Cancer
The researchers note that the MIS procedure has some unique risks. For example, once the patient is “docked” to the robot, the Trendelenburg position can’t be reversed without undocking. This may adversely affect the respiratory and cardiovascular system. Despite case reports about such adverse effects, though, the researchers say most data support the safety of robotic surgery in the elderly. Moreover, the newest versions of robots allow for multiple quadrant access without the need to undock.
Related:Gene Expression Signatures in Breast Cancer: A Surgical Oncologist’s Perspective
The oncologic safety of robotic surgery seems similar to that of open surgery or laparoscopic surgery. After 2 years of follow-up in 1 study, rates of progression-free survival were similar for the different methods. The researchers advise surgeons to work toward improving preoperative frailty assessments that will help tailor the right surgery for the right subgroup of elderly patients.
Lavoué V, Gotlieb W. Cancers (Basel). 2016;8(1):E12.
doi: 10.3390/cancers8010012.
A review by researchers from Hôpital Sud, Rennes, France, and McGill University, Montreal showed that surgery by robot—rather than traditional open surgery—may improve outcomes in elderly patients with pelvic cancer, however the research is sparse. For instance, only 6 published studies specifically address surgery in the elderly with endometrial cancer, the most common gynecologic malignancy in the western world. However, because surgery is challenging for these often-frail patients, minimally invasive (or minimal access) surgery could be the answer. When comparing robotics to standard laparoscopy in treating endometrial cancer, the data suggest significantly less blood loss, reduced operative time, and higher node counts.
Related: A Team Approach to Nonmelanotic Skin Cancer Procedures
Similar to many others, the researchers’ academic center was slow to adopt minimally invasive standard (MIS) laparoscopy. But within 2 years of the introduction of robotic surgery in 2007, more than 95% of patients requiring surgery to treat endometrial cancer undergo MIS. Now, the surgery is offered to each operable patient unless the cancer cannot be extracted intact or in a 15-cm diameter endobag via the vagina.
Related: Solid-Organ Transplant Recipients May Be at Greater Risk for Cancer
The researchers note that the MIS procedure has some unique risks. For example, once the patient is “docked” to the robot, the Trendelenburg position can’t be reversed without undocking. This may adversely affect the respiratory and cardiovascular system. Despite case reports about such adverse effects, though, the researchers say most data support the safety of robotic surgery in the elderly. Moreover, the newest versions of robots allow for multiple quadrant access without the need to undock.
Related:Gene Expression Signatures in Breast Cancer: A Surgical Oncologist’s Perspective
The oncologic safety of robotic surgery seems similar to that of open surgery or laparoscopic surgery. After 2 years of follow-up in 1 study, rates of progression-free survival were similar for the different methods. The researchers advise surgeons to work toward improving preoperative frailty assessments that will help tailor the right surgery for the right subgroup of elderly patients.
Lavoué V, Gotlieb W. Cancers (Basel). 2016;8(1):E12.
doi: 10.3390/cancers8010012.
A review by researchers from Hôpital Sud, Rennes, France, and McGill University, Montreal showed that surgery by robot—rather than traditional open surgery—may improve outcomes in elderly patients with pelvic cancer, however the research is sparse. For instance, only 6 published studies specifically address surgery in the elderly with endometrial cancer, the most common gynecologic malignancy in the western world. However, because surgery is challenging for these often-frail patients, minimally invasive (or minimal access) surgery could be the answer. When comparing robotics to standard laparoscopy in treating endometrial cancer, the data suggest significantly less blood loss, reduced operative time, and higher node counts.
Related: A Team Approach to Nonmelanotic Skin Cancer Procedures
Similar to many others, the researchers’ academic center was slow to adopt minimally invasive standard (MIS) laparoscopy. But within 2 years of the introduction of robotic surgery in 2007, more than 95% of patients requiring surgery to treat endometrial cancer undergo MIS. Now, the surgery is offered to each operable patient unless the cancer cannot be extracted intact or in a 15-cm diameter endobag via the vagina.
Related: Solid-Organ Transplant Recipients May Be at Greater Risk for Cancer
The researchers note that the MIS procedure has some unique risks. For example, once the patient is “docked” to the robot, the Trendelenburg position can’t be reversed without undocking. This may adversely affect the respiratory and cardiovascular system. Despite case reports about such adverse effects, though, the researchers say most data support the safety of robotic surgery in the elderly. Moreover, the newest versions of robots allow for multiple quadrant access without the need to undock.
Related:Gene Expression Signatures in Breast Cancer: A Surgical Oncologist’s Perspective
The oncologic safety of robotic surgery seems similar to that of open surgery or laparoscopic surgery. After 2 years of follow-up in 1 study, rates of progression-free survival were similar for the different methods. The researchers advise surgeons to work toward improving preoperative frailty assessments that will help tailor the right surgery for the right subgroup of elderly patients.
Lavoué V, Gotlieb W. Cancers (Basel). 2016;8(1):E12.
doi: 10.3390/cancers8010012.
New CDC opioid guideline targets overprescribing for chronic pain
Nonopioid therapy is the preferred approach for managing chronic pain outside of active cancer, palliative, and end-of-life care, according to a new guideline released today by the Centers for Disease Control and Prevention.
The 12 recommendations included in the guideline center around this principle and two others: using the lowest possible effective dosage when opioids are used, and exercising caution and monitoring patients closely when prescribing opioids.
Specifically, the guideline states that “clinicians should consider opioid therapy only if expected benefits for both pain and function are anticipated to outweigh risks to the patient,” and that “treatment should be combined with nonpharmacologic and nonopioid therapy, as appropriate.”
The guideline also addresses steps to take before starting or continuing opioid therapy, and drug selection, dosage, duration, follow-up, and discontinuation. Recommendations for assessing risk and addressing harms of opioid use are also included.
The CDC developed the guideline as part of the U.S. government’s urgent response to the epidemic of overdose deaths, which has been fueled by a quadrupling of the prescribing and sales of opioids since 1999, according to a CDC press statement. The guideline’s purpose is to help prevent opioid misuse and overdose.
“The CDC Guideline for Prescribing Opioids for Chronic Pain, United States, 2016 will help primary care providers ensure the safest and most effective treatment for their patients,” according to the statement. The CDC’s director, Dr. Tom Frieden, noted that “overprescribing opioids – largely for chronic pain – is a key driver of America’s drug-overdose epidemic.”
In a CDC teleconference marking the release of the guideline, Dr. Frieden said it has become increasingly clear that opioids “carry substantial risks but only uncertain benefits, especially compared with other treatments for chronic pain.
“Beginning treatment with an opioid is a momentous decision, and it should only be done with full understanding by both the clinician and the patient of the substantial risks and uncertain benefits involved,” Dr. Frieden said. He added that he knows of no other medication “that’s routinely used for a nonfatal condition [and] that kills patients so frequently.
“With more than 250 million prescriptions written each year, it’s so important that doctors understand that any one of those prescriptions could potentially end a patient’s life,” he cautioned.
A 2015 study showed that 1 of every 550 patients treated with opioids for noncancer pain – and 1 of 32 who received the highest doses (more than 200 morphine milligram equivalents per day) – died within 2.5 years of the first prescription.
Dr. Frieden noted that opioids do have a place when the potential benefits outweigh the potential harms. “But for most patients – the vast majority of patients – the risks will outweigh the benefits,” he said.
The opioid epidemic is one of the most pressing public health issues in the United States today, said Sylvia M. Burwell, secretary of the Department of Health & Human Services. A year ago, she announced an HHS initiative to reduce prescription opioid and heroin-related drug overdose, death, and dependence.
“Last year, more Americans died from drug overdoses than car crashes,” Ms. Burwell said during the teleconference, noting that families across the nation and from all walks of life have been affected.
Combating the opioid epidemic is a national priority, she said, and the CDC guideline will help in that effort.
“We believe this guideline will help health care professionals provide safer and more effective care for patients dealing with chronic pain, and we also believe it will help these providers drive down the rates of opioid use disorder, overdose, and ... death,” she said.
The American Medical Association greeted the guideline with cautious support.
“While we are largely supportive of the guidelines, we remain concerned about the evidence base informing some of the recommendations,” noted Dr. Patrice A. Harris, chair-elect of the AMA board and chair of the AMA Task Force to Reduce Opioid Abuse, in a statement.
The AMA also cited potential conflicts between the guideline and product labeling and state laws, as well as obstacles such as insurance coverage limits on nonpharmacologic treatments.
“If these guidelines help reduce the deaths resulting from opioids, they will prove to be valuable,” Dr. Harris said in the statement. “If they produce unintended consequences, we will need to mitigate them.”
Of note, the guideline stresses the right of patients with chronic pain to receive safe and effective pain management, and focuses on giving primary care providers – who account for about half of all opioid prescriptions – a road map for providing such pain management by increasing the use of effective nonopioid and nonpharmacologic therapies.
It was developed through a “rigorous scientific process using the best available scientific evidence, consulting with experts, and listening to comments from the public and partner organizations,” according to the CDC statement. The organization “is dedicated to working with partners to improve the evidence base and will refine the recommendations as new research becomes available.
”In conjunction with the release of the guideline, the CDC has provided a checklist for prescribing opioids for chronic pain, and a website with additional tools for implementing the recommendations within the guideline.
The CDC's opioid recommendations
The Centers for Disease Control and Prevention’s new opioid prescription guideline includes 12 recommendations. Here they are, modified slightly for style:
1. Nonpharmacologic therapy and nonopioid pharmacologic therapy are preferred for chronic pain. Providers should only consider adding opioid therapy if expected benefits for both pain and function are anticipated to outweigh risks.
2. Before starting opioid therapy for chronic pain, providers should establish treatment goals with all patients, including realistic goals for pain and function. Providers should not initiate opioid therapy without consideration of how therapy will be discontinued if unsuccessful. Providers should continue opioid therapy only if there is clinically meaningful improvement in pain and function.
3. Before starting and periodically during opioid therapy, providers should discuss with patients known risks and realistic benefits of opioid therapy, and patient and provider responsibilities for managing therapy.
4. When starting opioid therapy for chronic pain, providers should prescribe immediate-release opioids instead of extended-release/long-acting opioids.
5. When opioids are started, providers should prescribe the lowest effective dosage. Providers should use caution when prescribing opioids at any dosage, should implement additional precautions when increasing dosage to 50 or more morphine milligram equivalents (MME) per day, and generally should avoid increasing dosage to 90 or more MME per day.
6. When opioids are used for acute pain, providers should prescribe the lowest effective dose of immediate-release opioids. Three or fewer days often will be sufficient.
7. Providers should evaluate the benefits and harms with patients within 1-4 weeks of starting opioid therapy for chronic pain or of dose escalation. They should reevaluate continued therapy’s benefits and harms every 3 months or more frequently. If continued therapy’s benefits do not outweigh harms, providers should work with patients to reduce dosages or discontinue opioids.
8. During therapy, providers should evaluate risk factors for opioid-related harm. Providers should incorporate into the management plan strategies to mitigate risk, including considering offering naloxone when factors that increase risk for opioid overdose – such as history of overdose, history of substance use disorder, or higher opioid dosage (50 MME or more) – are present.
9. Providers should review the patient’s history of controlled substance prescriptions using state prescription drug monitoring program (PDMP) data to determine whether the patient is receiving high opioid dosages or dangerous combinations that put him or her at high risk for overdose. Providers should review PDMP data when starting opioid therapy for chronic pain and periodically during opioid therapy for chronic pain, ranging from every prescription to every 3 months.
10. When prescribing opioids for chronic pain, providers should use urine drug testing before starting opioid therapy and consider urine drug testing at least annually to assess for prescribed medications, as well as other controlled prescription drugs and illicit drugs.
11. Providers should avoid concurrent prescriptions of opioid pain medication and benzodiazepines whenever possible.
12. Providers should offer or arrange evidence-based treatment (usually medication-assisted treatment with buprenorphine or methadone in combination with behavioral therapies) for patients with opioid use disorder.
M. Alexander Otto contributed to this article.
Nonopioid therapy is the preferred approach for managing chronic pain outside of active cancer, palliative, and end-of-life care, according to a new guideline released today by the Centers for Disease Control and Prevention.
The 12 recommendations included in the guideline center around this principle and two others: using the lowest possible effective dosage when opioids are used, and exercising caution and monitoring patients closely when prescribing opioids.
Specifically, the guideline states that “clinicians should consider opioid therapy only if expected benefits for both pain and function are anticipated to outweigh risks to the patient,” and that “treatment should be combined with nonpharmacologic and nonopioid therapy, as appropriate.”
The guideline also addresses steps to take before starting or continuing opioid therapy, and drug selection, dosage, duration, follow-up, and discontinuation. Recommendations for assessing risk and addressing harms of opioid use are also included.
The CDC developed the guideline as part of the U.S. government’s urgent response to the epidemic of overdose deaths, which has been fueled by a quadrupling of the prescribing and sales of opioids since 1999, according to a CDC press statement. The guideline’s purpose is to help prevent opioid misuse and overdose.
“The CDC Guideline for Prescribing Opioids for Chronic Pain, United States, 2016 will help primary care providers ensure the safest and most effective treatment for their patients,” according to the statement. The CDC’s director, Dr. Tom Frieden, noted that “overprescribing opioids – largely for chronic pain – is a key driver of America’s drug-overdose epidemic.”
In a CDC teleconference marking the release of the guideline, Dr. Frieden said it has become increasingly clear that opioids “carry substantial risks but only uncertain benefits, especially compared with other treatments for chronic pain.
“Beginning treatment with an opioid is a momentous decision, and it should only be done with full understanding by both the clinician and the patient of the substantial risks and uncertain benefits involved,” Dr. Frieden said. He added that he knows of no other medication “that’s routinely used for a nonfatal condition [and] that kills patients so frequently.
“With more than 250 million prescriptions written each year, it’s so important that doctors understand that any one of those prescriptions could potentially end a patient’s life,” he cautioned.
A 2015 study showed that 1 of every 550 patients treated with opioids for noncancer pain – and 1 of 32 who received the highest doses (more than 200 morphine milligram equivalents per day) – died within 2.5 years of the first prescription.
Dr. Frieden noted that opioids do have a place when the potential benefits outweigh the potential harms. “But for most patients – the vast majority of patients – the risks will outweigh the benefits,” he said.
The opioid epidemic is one of the most pressing public health issues in the United States today, said Sylvia M. Burwell, secretary of the Department of Health & Human Services. A year ago, she announced an HHS initiative to reduce prescription opioid and heroin-related drug overdose, death, and dependence.
“Last year, more Americans died from drug overdoses than car crashes,” Ms. Burwell said during the teleconference, noting that families across the nation and from all walks of life have been affected.
Combating the opioid epidemic is a national priority, she said, and the CDC guideline will help in that effort.
“We believe this guideline will help health care professionals provide safer and more effective care for patients dealing with chronic pain, and we also believe it will help these providers drive down the rates of opioid use disorder, overdose, and ... death,” she said.
The American Medical Association greeted the guideline with cautious support.
“While we are largely supportive of the guidelines, we remain concerned about the evidence base informing some of the recommendations,” noted Dr. Patrice A. Harris, chair-elect of the AMA board and chair of the AMA Task Force to Reduce Opioid Abuse, in a statement.
The AMA also cited potential conflicts between the guideline and product labeling and state laws, as well as obstacles such as insurance coverage limits on nonpharmacologic treatments.
“If these guidelines help reduce the deaths resulting from opioids, they will prove to be valuable,” Dr. Harris said in the statement. “If they produce unintended consequences, we will need to mitigate them.”
Of note, the guideline stresses the right of patients with chronic pain to receive safe and effective pain management, and focuses on giving primary care providers – who account for about half of all opioid prescriptions – a road map for providing such pain management by increasing the use of effective nonopioid and nonpharmacologic therapies.
It was developed through a “rigorous scientific process using the best available scientific evidence, consulting with experts, and listening to comments from the public and partner organizations,” according to the CDC statement. The organization “is dedicated to working with partners to improve the evidence base and will refine the recommendations as new research becomes available.
”In conjunction with the release of the guideline, the CDC has provided a checklist for prescribing opioids for chronic pain, and a website with additional tools for implementing the recommendations within the guideline.
The CDC's opioid recommendations
The Centers for Disease Control and Prevention’s new opioid prescription guideline includes 12 recommendations. Here they are, modified slightly for style:
1. Nonpharmacologic therapy and nonopioid pharmacologic therapy are preferred for chronic pain. Providers should only consider adding opioid therapy if expected benefits for both pain and function are anticipated to outweigh risks.
2. Before starting opioid therapy for chronic pain, providers should establish treatment goals with all patients, including realistic goals for pain and function. Providers should not initiate opioid therapy without consideration of how therapy will be discontinued if unsuccessful. Providers should continue opioid therapy only if there is clinically meaningful improvement in pain and function.
3. Before starting and periodically during opioid therapy, providers should discuss with patients known risks and realistic benefits of opioid therapy, and patient and provider responsibilities for managing therapy.
4. When starting opioid therapy for chronic pain, providers should prescribe immediate-release opioids instead of extended-release/long-acting opioids.
5. When opioids are started, providers should prescribe the lowest effective dosage. Providers should use caution when prescribing opioids at any dosage, should implement additional precautions when increasing dosage to 50 or more morphine milligram equivalents (MME) per day, and generally should avoid increasing dosage to 90 or more MME per day.
6. When opioids are used for acute pain, providers should prescribe the lowest effective dose of immediate-release opioids. Three or fewer days often will be sufficient.
7. Providers should evaluate the benefits and harms with patients within 1-4 weeks of starting opioid therapy for chronic pain or of dose escalation. They should reevaluate continued therapy’s benefits and harms every 3 months or more frequently. If continued therapy’s benefits do not outweigh harms, providers should work with patients to reduce dosages or discontinue opioids.
8. During therapy, providers should evaluate risk factors for opioid-related harm. Providers should incorporate into the management plan strategies to mitigate risk, including considering offering naloxone when factors that increase risk for opioid overdose – such as history of overdose, history of substance use disorder, or higher opioid dosage (50 MME or more) – are present.
9. Providers should review the patient’s history of controlled substance prescriptions using state prescription drug monitoring program (PDMP) data to determine whether the patient is receiving high opioid dosages or dangerous combinations that put him or her at high risk for overdose. Providers should review PDMP data when starting opioid therapy for chronic pain and periodically during opioid therapy for chronic pain, ranging from every prescription to every 3 months.
10. When prescribing opioids for chronic pain, providers should use urine drug testing before starting opioid therapy and consider urine drug testing at least annually to assess for prescribed medications, as well as other controlled prescription drugs and illicit drugs.
11. Providers should avoid concurrent prescriptions of opioid pain medication and benzodiazepines whenever possible.
12. Providers should offer or arrange evidence-based treatment (usually medication-assisted treatment with buprenorphine or methadone in combination with behavioral therapies) for patients with opioid use disorder.
M. Alexander Otto contributed to this article.
Nonopioid therapy is the preferred approach for managing chronic pain outside of active cancer, palliative, and end-of-life care, according to a new guideline released today by the Centers for Disease Control and Prevention.
The 12 recommendations included in the guideline center around this principle and two others: using the lowest possible effective dosage when opioids are used, and exercising caution and monitoring patients closely when prescribing opioids.
Specifically, the guideline states that “clinicians should consider opioid therapy only if expected benefits for both pain and function are anticipated to outweigh risks to the patient,” and that “treatment should be combined with nonpharmacologic and nonopioid therapy, as appropriate.”
The guideline also addresses steps to take before starting or continuing opioid therapy, and drug selection, dosage, duration, follow-up, and discontinuation. Recommendations for assessing risk and addressing harms of opioid use are also included.
The CDC developed the guideline as part of the U.S. government’s urgent response to the epidemic of overdose deaths, which has been fueled by a quadrupling of the prescribing and sales of opioids since 1999, according to a CDC press statement. The guideline’s purpose is to help prevent opioid misuse and overdose.
“The CDC Guideline for Prescribing Opioids for Chronic Pain, United States, 2016 will help primary care providers ensure the safest and most effective treatment for their patients,” according to the statement. The CDC’s director, Dr. Tom Frieden, noted that “overprescribing opioids – largely for chronic pain – is a key driver of America’s drug-overdose epidemic.”
In a CDC teleconference marking the release of the guideline, Dr. Frieden said it has become increasingly clear that opioids “carry substantial risks but only uncertain benefits, especially compared with other treatments for chronic pain.
“Beginning treatment with an opioid is a momentous decision, and it should only be done with full understanding by both the clinician and the patient of the substantial risks and uncertain benefits involved,” Dr. Frieden said. He added that he knows of no other medication “that’s routinely used for a nonfatal condition [and] that kills patients so frequently.
“With more than 250 million prescriptions written each year, it’s so important that doctors understand that any one of those prescriptions could potentially end a patient’s life,” he cautioned.
A 2015 study showed that 1 of every 550 patients treated with opioids for noncancer pain – and 1 of 32 who received the highest doses (more than 200 morphine milligram equivalents per day) – died within 2.5 years of the first prescription.
Dr. Frieden noted that opioids do have a place when the potential benefits outweigh the potential harms. “But for most patients – the vast majority of patients – the risks will outweigh the benefits,” he said.
The opioid epidemic is one of the most pressing public health issues in the United States today, said Sylvia M. Burwell, secretary of the Department of Health & Human Services. A year ago, she announced an HHS initiative to reduce prescription opioid and heroin-related drug overdose, death, and dependence.
“Last year, more Americans died from drug overdoses than car crashes,” Ms. Burwell said during the teleconference, noting that families across the nation and from all walks of life have been affected.
Combating the opioid epidemic is a national priority, she said, and the CDC guideline will help in that effort.
“We believe this guideline will help health care professionals provide safer and more effective care for patients dealing with chronic pain, and we also believe it will help these providers drive down the rates of opioid use disorder, overdose, and ... death,” she said.
The American Medical Association greeted the guideline with cautious support.
“While we are largely supportive of the guidelines, we remain concerned about the evidence base informing some of the recommendations,” noted Dr. Patrice A. Harris, chair-elect of the AMA board and chair of the AMA Task Force to Reduce Opioid Abuse, in a statement.
The AMA also cited potential conflicts between the guideline and product labeling and state laws, as well as obstacles such as insurance coverage limits on nonpharmacologic treatments.
“If these guidelines help reduce the deaths resulting from opioids, they will prove to be valuable,” Dr. Harris said in the statement. “If they produce unintended consequences, we will need to mitigate them.”
Of note, the guideline stresses the right of patients with chronic pain to receive safe and effective pain management, and focuses on giving primary care providers – who account for about half of all opioid prescriptions – a road map for providing such pain management by increasing the use of effective nonopioid and nonpharmacologic therapies.
It was developed through a “rigorous scientific process using the best available scientific evidence, consulting with experts, and listening to comments from the public and partner organizations,” according to the CDC statement. The organization “is dedicated to working with partners to improve the evidence base and will refine the recommendations as new research becomes available.
”In conjunction with the release of the guideline, the CDC has provided a checklist for prescribing opioids for chronic pain, and a website with additional tools for implementing the recommendations within the guideline.
The CDC's opioid recommendations
The Centers for Disease Control and Prevention’s new opioid prescription guideline includes 12 recommendations. Here they are, modified slightly for style:
1. Nonpharmacologic therapy and nonopioid pharmacologic therapy are preferred for chronic pain. Providers should only consider adding opioid therapy if expected benefits for both pain and function are anticipated to outweigh risks.
2. Before starting opioid therapy for chronic pain, providers should establish treatment goals with all patients, including realistic goals for pain and function. Providers should not initiate opioid therapy without consideration of how therapy will be discontinued if unsuccessful. Providers should continue opioid therapy only if there is clinically meaningful improvement in pain and function.
3. Before starting and periodically during opioid therapy, providers should discuss with patients known risks and realistic benefits of opioid therapy, and patient and provider responsibilities for managing therapy.
4. When starting opioid therapy for chronic pain, providers should prescribe immediate-release opioids instead of extended-release/long-acting opioids.
5. When opioids are started, providers should prescribe the lowest effective dosage. Providers should use caution when prescribing opioids at any dosage, should implement additional precautions when increasing dosage to 50 or more morphine milligram equivalents (MME) per day, and generally should avoid increasing dosage to 90 or more MME per day.
6. When opioids are used for acute pain, providers should prescribe the lowest effective dose of immediate-release opioids. Three or fewer days often will be sufficient.
7. Providers should evaluate the benefits and harms with patients within 1-4 weeks of starting opioid therapy for chronic pain or of dose escalation. They should reevaluate continued therapy’s benefits and harms every 3 months or more frequently. If continued therapy’s benefits do not outweigh harms, providers should work with patients to reduce dosages or discontinue opioids.
8. During therapy, providers should evaluate risk factors for opioid-related harm. Providers should incorporate into the management plan strategies to mitigate risk, including considering offering naloxone when factors that increase risk for opioid overdose – such as history of overdose, history of substance use disorder, or higher opioid dosage (50 MME or more) – are present.
9. Providers should review the patient’s history of controlled substance prescriptions using state prescription drug monitoring program (PDMP) data to determine whether the patient is receiving high opioid dosages or dangerous combinations that put him or her at high risk for overdose. Providers should review PDMP data when starting opioid therapy for chronic pain and periodically during opioid therapy for chronic pain, ranging from every prescription to every 3 months.
10. When prescribing opioids for chronic pain, providers should use urine drug testing before starting opioid therapy and consider urine drug testing at least annually to assess for prescribed medications, as well as other controlled prescription drugs and illicit drugs.
11. Providers should avoid concurrent prescriptions of opioid pain medication and benzodiazepines whenever possible.
12. Providers should offer or arrange evidence-based treatment (usually medication-assisted treatment with buprenorphine or methadone in combination with behavioral therapies) for patients with opioid use disorder.
M. Alexander Otto contributed to this article.
The Cost of Oncology Drugs: A Pharmacy Perspective, Part I
Health care costs are the fastest growing financial segment of the U.S. economy. The Centers for Medicare and Medicaid Services (CMS) estimates health care spending in the U.S. will increase from $3.0 trillion in 2014 to $5.4 trillion by 2024.1 About 19.3% of the U.S. gross domestic product is consumed by health care, which is twice that of any other country in the world. It is often stated that the increasing cost of health care is the most significant financial threat to the U.S. economy. The cost of medications, including those for treating cancer, is the leading cause of increased health care spending.2
The cost of cancer care is the most rapidly increasing component of U.S. health care spending and will increase from $125 billion in 2010 to an estimated $158 billion in 2020, a 27% increase.3 Most experts agree that the current escalation of costs is unsustainable and, if left unchecked, will have a devastating effect on the quality of health care and an increasing negative financial impact on individuals, businesses, and government. However, that discussion is outside the scope of this article.
The affordability of health care has become a major concern for most Americans. During the recent U.S. financial crisis, most of the focus was on the bursting of the housing bubble, plummeting real estate prices, the loss of jobs, and the failure of large financial institutions. However, medical bills were still the leading cause of personal bankruptcies during this period. In 2007, 62% of personal bankruptcies in the U.S. were due to medical costs, and 78% of those bankruptcies involved patients who had health insurance at the beginning of their illness.4
The cost of prescription medications is causing financial difficulties for many patients, especially elderly.
Americans who have multiple chronic medical conditions and live on fixed incomes. A recently released survey by the nonpartisan Kaiser Family Foundation found that the high cost of prescription medications, especially those to treat serious medical conditions such as cancer, is the top health concern of 77% of those Americans polled.5 In this environment, oncology providers face many challenges in their obligation to treat cancer patients in a cost-effective manner.
This article will appear in 2 parts. Part 1 will focus on the emerging discussion of the financial impact of high-cost drugs in the U.S. The drivers of increasing oncology drug costs will also be reviewed. Part 2 will focus on the challenges of high cost medications in the VA and the role the VA Pharmacy Benefits Management (PBM) Service has in evaluating new oncology agents. Clinical guidance tools designed to aid the clinician in the cost-effective use of these agents and results of a nationwide survey of VA oncology pharmacists regarding the use of cost-containment strategies will also be presented.
Background
When discussing the value of targeted therapies, it is useful to define both targeted therapy and value. A targeted therapy is a type of treatment using drugs or other substances to identify and attack cancer cells with less harm to normal cells, according to the National Cancer Institute. 6 Some targeted therapies block the action of certain enzymes, proteins, or other molecules involved in the growth and spread of cancer cells (the molecular target). Other types of targeted therapies help the immune system kill cancer cells or deliver toxic substances directly to cancer cells and kill them.
Targeted therapy may have fewer adverse effects (AEs) than do other types of cancer treatment. Most targeted therapies are either small molecules or monoclonal antibodies. Although imatinib, released in 2001, is the drug that coined the phrase targeted therapy, many drugs released earlier, such as rituximab, can be considered targeted therapies due to their specific, or targeted, mechanism of action.
Value is the price an object will bring in an open and competitive, or free, market as determined by the consumer. To put the definition of value in simpler terms, Warren Buffet has been quoted as saying, “Cost is what you pay, value is what you get.” The oncology market is not entirely free and open. Market price is determined by the manufacturer, entry into the market is regulated by the FDA, purchasers (like the VA and the Centers for Medicare and Medicaid Services) have only limited ability to negotiate prices, and refusing to pay for life-saving or life-prolonging medications often is not an option. As costs for oncology drugs rapidly increase, the cost-benefit ratio, or value, is being increasingly debated. When comparing the clinical benefits these agents provide with cost, the perception of value is highly subjective and can change significantly based on who is paying the bill.
Questioning High-Cost Drugs
Charles Moertel and colleagues published a landmark trial 25 years ago, which reported that treatment with fluorouracil and levamisole for 1 year decreased the death rate of patients with stage C (stage III) colon cancer by 33% following curative surgery.7 Although this trial was clinically significant, there was as much discussion about the high cost of levamisole (Ergamisol) tablets as there
was about its clinical benefit for patients.
In a 1991 letter to the New England Journal of Medicine, Rossof and colleagues questioned the high cost of the levamisole in the treatment regimen.8 Rossof and colleagues were surprised at the drug’s price on approval, about $5 for each tablet, and detailed their concerns on how this price was determined. “On the basis of the cost to a veterinarian, the calculated cost of a hypothetical 50-mg tablet should be in the range of 3 to 6 cents,” they argued. The total cost to the patient of 1 year of treament was nearly $1,200. Their conclusion was that “…the price chosen for the new American consumer is far too high and requires justification by the manufacturer.”
A reply from Janssen Pharmaceutica, the drug’s manufacturer, offered many justifications for the price.8 According to the company, Ergamisol was supplied free to 5,000 research patients prior to FDA approval. It was also given for free to indigent patients. The company also insisted that its pricing compared favorably with its competitors, such as zidovudine, octreotide, newer generation nonsteroidal anti-inflammatories, and antihypertension drugs. “Drug pricing includes additional expensive research, physician education, compassionate use programs, and ensuring high-quality control. Janssen scientists studied immunomodulating effect of Ergamisol for 25 years with no financial return. Drug development is high-risk, so companies must be able to derive a reasonable return on sales.”8
The cost of levamisole was $1,200 per year in 1991, and after adjustment for inflation would cost about $1,988 in 2015, or $166 per month. If these prices caused outrage in 1990, it is easy to see how current prices of well over $10,000 per month for therapies, which often render small clinical benefits, can seem outrageous by comparison.
Public Debate Over Cancer Drug Prices
In the U.S., about 1.66 million patients will be diagnosed with cancer in 2015.9 Although about 30% to 40% of these patients will be effectively cured, only 3% to 4% will be cured using pharmacotherapy (usually traditional chemotherapy) as a sole modality. Therefore, the use of oncology drugs by the vast majority of cancer patients is not to cure but to control or palliate patients with advanced cancer. It is important to note that the cost of most curative regimens is cheap compared with many medications used for advanced disease. Until a few years ago, discussion of the high costs of cancer treatment was rarely made public due to the devastating nature of cancer. However, with the rapid price increases and relatively disappointing clinical benefits of the many new drugs entering the market, the question of value can no longer be ignored. Many authors havepresented commentaries and strategies addressing the issues
surrounding the high cost of cancer drugs.10-15
It was a groundbreaking 2012 letter to the New York Times that brought the issue to public attention.16 Dr. Peter Bach and his colleagues at Memorial Sloan Kettering Cancer Center announced they would not purchase a “phenomenally expensive new cancer drug” for their patients, calling their decision a no-brainer. The drug, ziv-afilbercept (Zaltrap), was twice the price of a similar drug, bevacizumab (Avastin), but was no more efficacious in the treatment of metastatic colorectal cancer. Bach and colleagues went on to say how high drug prices are having a potentially devastating financial impact on patients and that laws protect drug manufacturers to set drug prices at what they feel the market will bear.
Considering the value of cancer treatments is now actively encouraged. To that point, the American Society of Clinical Oncology (ASCO) has recently published a groundbreaking paper entitled “A Conceptual Framework to Assess the Value of Cancer Treatment Options.”17 This tool, which is still in development, will allow oncologists to quantify clinical benefit, toxicity, and out-of-pocket drug costs so patients can compare treatment options with cost as a consideration.
The financial burden put on patients has become the driving force for drug cost reform. In an attempt to control their costs, third-party payers have increased the cost burden for patients by demanding larger copays and other out-of-pocket expenses for medications. It is felt that requiring patients to have more “skin in the game” would force them to make treatment decisions based on cost. Unfortunately, this approach may lead to devastating financial consequences for patients.18-20 The overwhelming emotions patients experience following the diagnosis of cancer make it difficult to focus on the financial impact of treatment recommendations. In addition, many oncologists are not comfortable, or even capable, of discussing costs so patients can make financially informed treatment decisions.14 Unfortunately for patients, “shopping for health care” has very little in common with shopping for a car, television sets, or any other commodity.
The VA Health Care System
The VA is government-sponsored health care and is therefore unique in the U.S. health care environment. The VA might be considered a form of “socialized medicine” that operates under a different economic model than do private health care systems. The treatment of VA patients for common diseases is based on nationally accepted evidence-based guidelines, which allow the best care in a cost-effective manner. For the treatment of cancer, the use of expensive therapies must be made in the context of the finite resources allocated for the treatment of all veterans within the system.
The VA provides lifelong free or minimal cost health care to eligible veterans. For veterans receiving care within the VA, out-of-pocket expenses are considerably less than for non-VA patients. Current medication copays range from free to $9 per month for all medications, regardless of acquisition cost. This is in stark contrast to the private sector, where patients must often pay large, percentage- based copays for oncology medications, which can reach several thousand dollars per month. VA patients are not subject to percentage-based copays; therefore, they are not a financial stakeholder in the treatment
decision process.
Prior to 1995, the VA was a much criticized and poorly performing health care system that had experienced significant budget cuts, forcing many veterans to lose their benefits and seek care outside the VA. Beginning in 1995 with the creation of PBM, a remarkable transformation occurred that modernized and transformed the VA into a system that consistently outperforms the private sector in quality of care, patient safety, and patient satisfaction while maintaining low overall costs. The role of the VA PBM was to develop and maintain the National Drug Formulary, create clinical guidance documents, and manage drug costs and use.
Part 2 of this article will more closely examine the high cost of cancer drugs. It will also discuss the role of VA PBM and other VA efforts to control cost
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here for the digital edition.
1. Centers for Medicare and Medicaid. National health expenditure projections 2014-2024 Table 01. Centers for Medicare and Medicaid Website. https://www.cms.gov/research-statistics-data-and-systems/statistics-trends-and-reports/nationalhealthexpenddata/nationalhealthaccountsprojected.html. Updated July 30, 2015. Accessed January 11, 2016.
2. Bach PB. Limits of Medicare’s ability to control rising spending on cancer drugs. N Engl J Med. 2009;360(6):626-633.
3. Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer in the United States: 2010-2020. J Natl Cancer Inst. 2011;103(2):117-128.
4. Himmelstein DU, Thorne D, Warren E, Woolhandler S. Medical bankruptcy in the United States, 2007: results of a national study. Am J Med. 2009;122(8):741-746.
5. The Henry J. Kaiser Family Foundation. Prescription drug costs remain atop the public’s national health care agenda, well ahead of Affordable Care Act revisions and repeal [press release]. Kaiser Family Foundation Website. http://kff.org/health-costs/press-release/prescription-drug-costs-remain-atop-the-publics-national-health-care-agenda-well-ahead-of-affordable-care-act-revisions-and-repeal. Published October 28, 2015. Accessed January 11, 2016.
6. National Cancer Institute (NCI). NCI dictionary of cancer terms: targeted therapy. National Cancer Institute Website. http://www.cancer.gov/publications/dictionaries/cancer-terms?cdrid=270742. Accessed January 11, 2016.
7. Moertel CG, Fleming TR, Macdonald JS, et al. Levamisole and fluorouracil for adjuvant therapy resected colon carcinoma. N Engl J Med. 1990;322(6):352-358.
8. Rossof AH, Philpot TR, Bunch RS, Letcher J. The high cost of levamisole for humans. N Engl J Med. 1991;324(10):701-702.
9. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5-29.
10. Nadler E, Eckert B, Neumann PJ. Do oncologists believe new cancer drugs offer good value? Oncologist. 2006;11(2):90-95.
11. Hillner BE, Smith TJ. Efficacy does not necessarily translate into cost effectiveness: a case study of the challenges associated with 21st century cancer drug pricing. J Clin Oncol. 2009;27(13):2111-2113.
12. Neumann PJ, Weinstein MC. Legislating against use of cost-effectiveness information. N Engl J Med. 2010;363(16):1495-1497.
13. Elkin EB, Bach PB. Cancer’s next frontier: addressing high and increasing costs. JAMA. 2010;303(11):1086-1087.
14. Smith TJ, Hillner BE. Bending the cost curve in cancer care. N Engl J Med. 2011;364(21):2060-2065.
15. Siddiqui M, Rajkumar SV. The high cost of cancer drugs and what we can do
about it. Mayo Clin Proc. 2012;87(10):935-943.
16. Bach PB, Saltz LB, Wittes RE. In cancer care, cost matters [op-ed]. New York Times. October 14, 2012.
17. Schnipper LE, Davidson NE, Wollins DS, et al; American Society of Clinical Oncology. American Society of Clinical Oncology statement: a conceptual framework to assess the value of cancer treatment options. J Clin Oncol. 2015;33(23): 2563-2577.
18. Zafar SY, Peppercorn JM, Schrag D, et al. The financial toxicity of cancer treatment: a pilot study assessing out-of-pocket expenses and the insured cancer patient’s experience. Oncologist. 2013;18(4):381-390.
19. Fenn KM, Evans SB, McCorkle R, et al. Impact of financial burden of cancer on
survivors’ quality of life. J Oncol Prac. 2014;10(5):332-338.
20. Zafar SY, McNeil RB, Thomas CM, Lathan CS, Ayanian JZ, Provenzale D. Population-based assessment of cancer survivors’ financial burden and quality of life: a prospective cohort study. J Oncol Pract. 2015;11(2):145-150.
Note: Page numbers differ between the print issue and digital edition.
Health care costs are the fastest growing financial segment of the U.S. economy. The Centers for Medicare and Medicaid Services (CMS) estimates health care spending in the U.S. will increase from $3.0 trillion in 2014 to $5.4 trillion by 2024.1 About 19.3% of the U.S. gross domestic product is consumed by health care, which is twice that of any other country in the world. It is often stated that the increasing cost of health care is the most significant financial threat to the U.S. economy. The cost of medications, including those for treating cancer, is the leading cause of increased health care spending.2
The cost of cancer care is the most rapidly increasing component of U.S. health care spending and will increase from $125 billion in 2010 to an estimated $158 billion in 2020, a 27% increase.3 Most experts agree that the current escalation of costs is unsustainable and, if left unchecked, will have a devastating effect on the quality of health care and an increasing negative financial impact on individuals, businesses, and government. However, that discussion is outside the scope of this article.
The affordability of health care has become a major concern for most Americans. During the recent U.S. financial crisis, most of the focus was on the bursting of the housing bubble, plummeting real estate prices, the loss of jobs, and the failure of large financial institutions. However, medical bills were still the leading cause of personal bankruptcies during this period. In 2007, 62% of personal bankruptcies in the U.S. were due to medical costs, and 78% of those bankruptcies involved patients who had health insurance at the beginning of their illness.4
The cost of prescription medications is causing financial difficulties for many patients, especially elderly.
Americans who have multiple chronic medical conditions and live on fixed incomes. A recently released survey by the nonpartisan Kaiser Family Foundation found that the high cost of prescription medications, especially those to treat serious medical conditions such as cancer, is the top health concern of 77% of those Americans polled.5 In this environment, oncology providers face many challenges in their obligation to treat cancer patients in a cost-effective manner.
This article will appear in 2 parts. Part 1 will focus on the emerging discussion of the financial impact of high-cost drugs in the U.S. The drivers of increasing oncology drug costs will also be reviewed. Part 2 will focus on the challenges of high cost medications in the VA and the role the VA Pharmacy Benefits Management (PBM) Service has in evaluating new oncology agents. Clinical guidance tools designed to aid the clinician in the cost-effective use of these agents and results of a nationwide survey of VA oncology pharmacists regarding the use of cost-containment strategies will also be presented.
Background
When discussing the value of targeted therapies, it is useful to define both targeted therapy and value. A targeted therapy is a type of treatment using drugs or other substances to identify and attack cancer cells with less harm to normal cells, according to the National Cancer Institute. 6 Some targeted therapies block the action of certain enzymes, proteins, or other molecules involved in the growth and spread of cancer cells (the molecular target). Other types of targeted therapies help the immune system kill cancer cells or deliver toxic substances directly to cancer cells and kill them.
Targeted therapy may have fewer adverse effects (AEs) than do other types of cancer treatment. Most targeted therapies are either small molecules or monoclonal antibodies. Although imatinib, released in 2001, is the drug that coined the phrase targeted therapy, many drugs released earlier, such as rituximab, can be considered targeted therapies due to their specific, or targeted, mechanism of action.
Value is the price an object will bring in an open and competitive, or free, market as determined by the consumer. To put the definition of value in simpler terms, Warren Buffet has been quoted as saying, “Cost is what you pay, value is what you get.” The oncology market is not entirely free and open. Market price is determined by the manufacturer, entry into the market is regulated by the FDA, purchasers (like the VA and the Centers for Medicare and Medicaid Services) have only limited ability to negotiate prices, and refusing to pay for life-saving or life-prolonging medications often is not an option. As costs for oncology drugs rapidly increase, the cost-benefit ratio, or value, is being increasingly debated. When comparing the clinical benefits these agents provide with cost, the perception of value is highly subjective and can change significantly based on who is paying the bill.
Questioning High-Cost Drugs
Charles Moertel and colleagues published a landmark trial 25 years ago, which reported that treatment with fluorouracil and levamisole for 1 year decreased the death rate of patients with stage C (stage III) colon cancer by 33% following curative surgery.7 Although this trial was clinically significant, there was as much discussion about the high cost of levamisole (Ergamisol) tablets as there
was about its clinical benefit for patients.
In a 1991 letter to the New England Journal of Medicine, Rossof and colleagues questioned the high cost of the levamisole in the treatment regimen.8 Rossof and colleagues were surprised at the drug’s price on approval, about $5 for each tablet, and detailed their concerns on how this price was determined. “On the basis of the cost to a veterinarian, the calculated cost of a hypothetical 50-mg tablet should be in the range of 3 to 6 cents,” they argued. The total cost to the patient of 1 year of treament was nearly $1,200. Their conclusion was that “…the price chosen for the new American consumer is far too high and requires justification by the manufacturer.”
A reply from Janssen Pharmaceutica, the drug’s manufacturer, offered many justifications for the price.8 According to the company, Ergamisol was supplied free to 5,000 research patients prior to FDA approval. It was also given for free to indigent patients. The company also insisted that its pricing compared favorably with its competitors, such as zidovudine, octreotide, newer generation nonsteroidal anti-inflammatories, and antihypertension drugs. “Drug pricing includes additional expensive research, physician education, compassionate use programs, and ensuring high-quality control. Janssen scientists studied immunomodulating effect of Ergamisol for 25 years with no financial return. Drug development is high-risk, so companies must be able to derive a reasonable return on sales.”8
The cost of levamisole was $1,200 per year in 1991, and after adjustment for inflation would cost about $1,988 in 2015, or $166 per month. If these prices caused outrage in 1990, it is easy to see how current prices of well over $10,000 per month for therapies, which often render small clinical benefits, can seem outrageous by comparison.
Public Debate Over Cancer Drug Prices
In the U.S., about 1.66 million patients will be diagnosed with cancer in 2015.9 Although about 30% to 40% of these patients will be effectively cured, only 3% to 4% will be cured using pharmacotherapy (usually traditional chemotherapy) as a sole modality. Therefore, the use of oncology drugs by the vast majority of cancer patients is not to cure but to control or palliate patients with advanced cancer. It is important to note that the cost of most curative regimens is cheap compared with many medications used for advanced disease. Until a few years ago, discussion of the high costs of cancer treatment was rarely made public due to the devastating nature of cancer. However, with the rapid price increases and relatively disappointing clinical benefits of the many new drugs entering the market, the question of value can no longer be ignored. Many authors havepresented commentaries and strategies addressing the issues
surrounding the high cost of cancer drugs.10-15
It was a groundbreaking 2012 letter to the New York Times that brought the issue to public attention.16 Dr. Peter Bach and his colleagues at Memorial Sloan Kettering Cancer Center announced they would not purchase a “phenomenally expensive new cancer drug” for their patients, calling their decision a no-brainer. The drug, ziv-afilbercept (Zaltrap), was twice the price of a similar drug, bevacizumab (Avastin), but was no more efficacious in the treatment of metastatic colorectal cancer. Bach and colleagues went on to say how high drug prices are having a potentially devastating financial impact on patients and that laws protect drug manufacturers to set drug prices at what they feel the market will bear.
Considering the value of cancer treatments is now actively encouraged. To that point, the American Society of Clinical Oncology (ASCO) has recently published a groundbreaking paper entitled “A Conceptual Framework to Assess the Value of Cancer Treatment Options.”17 This tool, which is still in development, will allow oncologists to quantify clinical benefit, toxicity, and out-of-pocket drug costs so patients can compare treatment options with cost as a consideration.
The financial burden put on patients has become the driving force for drug cost reform. In an attempt to control their costs, third-party payers have increased the cost burden for patients by demanding larger copays and other out-of-pocket expenses for medications. It is felt that requiring patients to have more “skin in the game” would force them to make treatment decisions based on cost. Unfortunately, this approach may lead to devastating financial consequences for patients.18-20 The overwhelming emotions patients experience following the diagnosis of cancer make it difficult to focus on the financial impact of treatment recommendations. In addition, many oncologists are not comfortable, or even capable, of discussing costs so patients can make financially informed treatment decisions.14 Unfortunately for patients, “shopping for health care” has very little in common with shopping for a car, television sets, or any other commodity.
The VA Health Care System
The VA is government-sponsored health care and is therefore unique in the U.S. health care environment. The VA might be considered a form of “socialized medicine” that operates under a different economic model than do private health care systems. The treatment of VA patients for common diseases is based on nationally accepted evidence-based guidelines, which allow the best care in a cost-effective manner. For the treatment of cancer, the use of expensive therapies must be made in the context of the finite resources allocated for the treatment of all veterans within the system.
The VA provides lifelong free or minimal cost health care to eligible veterans. For veterans receiving care within the VA, out-of-pocket expenses are considerably less than for non-VA patients. Current medication copays range from free to $9 per month for all medications, regardless of acquisition cost. This is in stark contrast to the private sector, where patients must often pay large, percentage- based copays for oncology medications, which can reach several thousand dollars per month. VA patients are not subject to percentage-based copays; therefore, they are not a financial stakeholder in the treatment
decision process.
Prior to 1995, the VA was a much criticized and poorly performing health care system that had experienced significant budget cuts, forcing many veterans to lose their benefits and seek care outside the VA. Beginning in 1995 with the creation of PBM, a remarkable transformation occurred that modernized and transformed the VA into a system that consistently outperforms the private sector in quality of care, patient safety, and patient satisfaction while maintaining low overall costs. The role of the VA PBM was to develop and maintain the National Drug Formulary, create clinical guidance documents, and manage drug costs and use.
Part 2 of this article will more closely examine the high cost of cancer drugs. It will also discuss the role of VA PBM and other VA efforts to control cost
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here for the digital edition.
Health care costs are the fastest growing financial segment of the U.S. economy. The Centers for Medicare and Medicaid Services (CMS) estimates health care spending in the U.S. will increase from $3.0 trillion in 2014 to $5.4 trillion by 2024.1 About 19.3% of the U.S. gross domestic product is consumed by health care, which is twice that of any other country in the world. It is often stated that the increasing cost of health care is the most significant financial threat to the U.S. economy. The cost of medications, including those for treating cancer, is the leading cause of increased health care spending.2
The cost of cancer care is the most rapidly increasing component of U.S. health care spending and will increase from $125 billion in 2010 to an estimated $158 billion in 2020, a 27% increase.3 Most experts agree that the current escalation of costs is unsustainable and, if left unchecked, will have a devastating effect on the quality of health care and an increasing negative financial impact on individuals, businesses, and government. However, that discussion is outside the scope of this article.
The affordability of health care has become a major concern for most Americans. During the recent U.S. financial crisis, most of the focus was on the bursting of the housing bubble, plummeting real estate prices, the loss of jobs, and the failure of large financial institutions. However, medical bills were still the leading cause of personal bankruptcies during this period. In 2007, 62% of personal bankruptcies in the U.S. were due to medical costs, and 78% of those bankruptcies involved patients who had health insurance at the beginning of their illness.4
The cost of prescription medications is causing financial difficulties for many patients, especially elderly.
Americans who have multiple chronic medical conditions and live on fixed incomes. A recently released survey by the nonpartisan Kaiser Family Foundation found that the high cost of prescription medications, especially those to treat serious medical conditions such as cancer, is the top health concern of 77% of those Americans polled.5 In this environment, oncology providers face many challenges in their obligation to treat cancer patients in a cost-effective manner.
This article will appear in 2 parts. Part 1 will focus on the emerging discussion of the financial impact of high-cost drugs in the U.S. The drivers of increasing oncology drug costs will also be reviewed. Part 2 will focus on the challenges of high cost medications in the VA and the role the VA Pharmacy Benefits Management (PBM) Service has in evaluating new oncology agents. Clinical guidance tools designed to aid the clinician in the cost-effective use of these agents and results of a nationwide survey of VA oncology pharmacists regarding the use of cost-containment strategies will also be presented.
Background
When discussing the value of targeted therapies, it is useful to define both targeted therapy and value. A targeted therapy is a type of treatment using drugs or other substances to identify and attack cancer cells with less harm to normal cells, according to the National Cancer Institute. 6 Some targeted therapies block the action of certain enzymes, proteins, or other molecules involved in the growth and spread of cancer cells (the molecular target). Other types of targeted therapies help the immune system kill cancer cells or deliver toxic substances directly to cancer cells and kill them.
Targeted therapy may have fewer adverse effects (AEs) than do other types of cancer treatment. Most targeted therapies are either small molecules or monoclonal antibodies. Although imatinib, released in 2001, is the drug that coined the phrase targeted therapy, many drugs released earlier, such as rituximab, can be considered targeted therapies due to their specific, or targeted, mechanism of action.
Value is the price an object will bring in an open and competitive, or free, market as determined by the consumer. To put the definition of value in simpler terms, Warren Buffet has been quoted as saying, “Cost is what you pay, value is what you get.” The oncology market is not entirely free and open. Market price is determined by the manufacturer, entry into the market is regulated by the FDA, purchasers (like the VA and the Centers for Medicare and Medicaid Services) have only limited ability to negotiate prices, and refusing to pay for life-saving or life-prolonging medications often is not an option. As costs for oncology drugs rapidly increase, the cost-benefit ratio, or value, is being increasingly debated. When comparing the clinical benefits these agents provide with cost, the perception of value is highly subjective and can change significantly based on who is paying the bill.
Questioning High-Cost Drugs
Charles Moertel and colleagues published a landmark trial 25 years ago, which reported that treatment with fluorouracil and levamisole for 1 year decreased the death rate of patients with stage C (stage III) colon cancer by 33% following curative surgery.7 Although this trial was clinically significant, there was as much discussion about the high cost of levamisole (Ergamisol) tablets as there
was about its clinical benefit for patients.
In a 1991 letter to the New England Journal of Medicine, Rossof and colleagues questioned the high cost of the levamisole in the treatment regimen.8 Rossof and colleagues were surprised at the drug’s price on approval, about $5 for each tablet, and detailed their concerns on how this price was determined. “On the basis of the cost to a veterinarian, the calculated cost of a hypothetical 50-mg tablet should be in the range of 3 to 6 cents,” they argued. The total cost to the patient of 1 year of treament was nearly $1,200. Their conclusion was that “…the price chosen for the new American consumer is far too high and requires justification by the manufacturer.”
A reply from Janssen Pharmaceutica, the drug’s manufacturer, offered many justifications for the price.8 According to the company, Ergamisol was supplied free to 5,000 research patients prior to FDA approval. It was also given for free to indigent patients. The company also insisted that its pricing compared favorably with its competitors, such as zidovudine, octreotide, newer generation nonsteroidal anti-inflammatories, and antihypertension drugs. “Drug pricing includes additional expensive research, physician education, compassionate use programs, and ensuring high-quality control. Janssen scientists studied immunomodulating effect of Ergamisol for 25 years with no financial return. Drug development is high-risk, so companies must be able to derive a reasonable return on sales.”8
The cost of levamisole was $1,200 per year in 1991, and after adjustment for inflation would cost about $1,988 in 2015, or $166 per month. If these prices caused outrage in 1990, it is easy to see how current prices of well over $10,000 per month for therapies, which often render small clinical benefits, can seem outrageous by comparison.
Public Debate Over Cancer Drug Prices
In the U.S., about 1.66 million patients will be diagnosed with cancer in 2015.9 Although about 30% to 40% of these patients will be effectively cured, only 3% to 4% will be cured using pharmacotherapy (usually traditional chemotherapy) as a sole modality. Therefore, the use of oncology drugs by the vast majority of cancer patients is not to cure but to control or palliate patients with advanced cancer. It is important to note that the cost of most curative regimens is cheap compared with many medications used for advanced disease. Until a few years ago, discussion of the high costs of cancer treatment was rarely made public due to the devastating nature of cancer. However, with the rapid price increases and relatively disappointing clinical benefits of the many new drugs entering the market, the question of value can no longer be ignored. Many authors havepresented commentaries and strategies addressing the issues
surrounding the high cost of cancer drugs.10-15
It was a groundbreaking 2012 letter to the New York Times that brought the issue to public attention.16 Dr. Peter Bach and his colleagues at Memorial Sloan Kettering Cancer Center announced they would not purchase a “phenomenally expensive new cancer drug” for their patients, calling their decision a no-brainer. The drug, ziv-afilbercept (Zaltrap), was twice the price of a similar drug, bevacizumab (Avastin), but was no more efficacious in the treatment of metastatic colorectal cancer. Bach and colleagues went on to say how high drug prices are having a potentially devastating financial impact on patients and that laws protect drug manufacturers to set drug prices at what they feel the market will bear.
Considering the value of cancer treatments is now actively encouraged. To that point, the American Society of Clinical Oncology (ASCO) has recently published a groundbreaking paper entitled “A Conceptual Framework to Assess the Value of Cancer Treatment Options.”17 This tool, which is still in development, will allow oncologists to quantify clinical benefit, toxicity, and out-of-pocket drug costs so patients can compare treatment options with cost as a consideration.
The financial burden put on patients has become the driving force for drug cost reform. In an attempt to control their costs, third-party payers have increased the cost burden for patients by demanding larger copays and other out-of-pocket expenses for medications. It is felt that requiring patients to have more “skin in the game” would force them to make treatment decisions based on cost. Unfortunately, this approach may lead to devastating financial consequences for patients.18-20 The overwhelming emotions patients experience following the diagnosis of cancer make it difficult to focus on the financial impact of treatment recommendations. In addition, many oncologists are not comfortable, or even capable, of discussing costs so patients can make financially informed treatment decisions.14 Unfortunately for patients, “shopping for health care” has very little in common with shopping for a car, television sets, or any other commodity.
The VA Health Care System
The VA is government-sponsored health care and is therefore unique in the U.S. health care environment. The VA might be considered a form of “socialized medicine” that operates under a different economic model than do private health care systems. The treatment of VA patients for common diseases is based on nationally accepted evidence-based guidelines, which allow the best care in a cost-effective manner. For the treatment of cancer, the use of expensive therapies must be made in the context of the finite resources allocated for the treatment of all veterans within the system.
The VA provides lifelong free or minimal cost health care to eligible veterans. For veterans receiving care within the VA, out-of-pocket expenses are considerably less than for non-VA patients. Current medication copays range from free to $9 per month for all medications, regardless of acquisition cost. This is in stark contrast to the private sector, where patients must often pay large, percentage- based copays for oncology medications, which can reach several thousand dollars per month. VA patients are not subject to percentage-based copays; therefore, they are not a financial stakeholder in the treatment
decision process.
Prior to 1995, the VA was a much criticized and poorly performing health care system that had experienced significant budget cuts, forcing many veterans to lose their benefits and seek care outside the VA. Beginning in 1995 with the creation of PBM, a remarkable transformation occurred that modernized and transformed the VA into a system that consistently outperforms the private sector in quality of care, patient safety, and patient satisfaction while maintaining low overall costs. The role of the VA PBM was to develop and maintain the National Drug Formulary, create clinical guidance documents, and manage drug costs and use.
Part 2 of this article will more closely examine the high cost of cancer drugs. It will also discuss the role of VA PBM and other VA efforts to control cost
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here for the digital edition.
1. Centers for Medicare and Medicaid. National health expenditure projections 2014-2024 Table 01. Centers for Medicare and Medicaid Website. https://www.cms.gov/research-statistics-data-and-systems/statistics-trends-and-reports/nationalhealthexpenddata/nationalhealthaccountsprojected.html. Updated July 30, 2015. Accessed January 11, 2016.
2. Bach PB. Limits of Medicare’s ability to control rising spending on cancer drugs. N Engl J Med. 2009;360(6):626-633.
3. Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer in the United States: 2010-2020. J Natl Cancer Inst. 2011;103(2):117-128.
4. Himmelstein DU, Thorne D, Warren E, Woolhandler S. Medical bankruptcy in the United States, 2007: results of a national study. Am J Med. 2009;122(8):741-746.
5. The Henry J. Kaiser Family Foundation. Prescription drug costs remain atop the public’s national health care agenda, well ahead of Affordable Care Act revisions and repeal [press release]. Kaiser Family Foundation Website. http://kff.org/health-costs/press-release/prescription-drug-costs-remain-atop-the-publics-national-health-care-agenda-well-ahead-of-affordable-care-act-revisions-and-repeal. Published October 28, 2015. Accessed January 11, 2016.
6. National Cancer Institute (NCI). NCI dictionary of cancer terms: targeted therapy. National Cancer Institute Website. http://www.cancer.gov/publications/dictionaries/cancer-terms?cdrid=270742. Accessed January 11, 2016.
7. Moertel CG, Fleming TR, Macdonald JS, et al. Levamisole and fluorouracil for adjuvant therapy resected colon carcinoma. N Engl J Med. 1990;322(6):352-358.
8. Rossof AH, Philpot TR, Bunch RS, Letcher J. The high cost of levamisole for humans. N Engl J Med. 1991;324(10):701-702.
9. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5-29.
10. Nadler E, Eckert B, Neumann PJ. Do oncologists believe new cancer drugs offer good value? Oncologist. 2006;11(2):90-95.
11. Hillner BE, Smith TJ. Efficacy does not necessarily translate into cost effectiveness: a case study of the challenges associated with 21st century cancer drug pricing. J Clin Oncol. 2009;27(13):2111-2113.
12. Neumann PJ, Weinstein MC. Legislating against use of cost-effectiveness information. N Engl J Med. 2010;363(16):1495-1497.
13. Elkin EB, Bach PB. Cancer’s next frontier: addressing high and increasing costs. JAMA. 2010;303(11):1086-1087.
14. Smith TJ, Hillner BE. Bending the cost curve in cancer care. N Engl J Med. 2011;364(21):2060-2065.
15. Siddiqui M, Rajkumar SV. The high cost of cancer drugs and what we can do
about it. Mayo Clin Proc. 2012;87(10):935-943.
16. Bach PB, Saltz LB, Wittes RE. In cancer care, cost matters [op-ed]. New York Times. October 14, 2012.
17. Schnipper LE, Davidson NE, Wollins DS, et al; American Society of Clinical Oncology. American Society of Clinical Oncology statement: a conceptual framework to assess the value of cancer treatment options. J Clin Oncol. 2015;33(23): 2563-2577.
18. Zafar SY, Peppercorn JM, Schrag D, et al. The financial toxicity of cancer treatment: a pilot study assessing out-of-pocket expenses and the insured cancer patient’s experience. Oncologist. 2013;18(4):381-390.
19. Fenn KM, Evans SB, McCorkle R, et al. Impact of financial burden of cancer on
survivors’ quality of life. J Oncol Prac. 2014;10(5):332-338.
20. Zafar SY, McNeil RB, Thomas CM, Lathan CS, Ayanian JZ, Provenzale D. Population-based assessment of cancer survivors’ financial burden and quality of life: a prospective cohort study. J Oncol Pract. 2015;11(2):145-150.
Note: Page numbers differ between the print issue and digital edition.
1. Centers for Medicare and Medicaid. National health expenditure projections 2014-2024 Table 01. Centers for Medicare and Medicaid Website. https://www.cms.gov/research-statistics-data-and-systems/statistics-trends-and-reports/nationalhealthexpenddata/nationalhealthaccountsprojected.html. Updated July 30, 2015. Accessed January 11, 2016.
2. Bach PB. Limits of Medicare’s ability to control rising spending on cancer drugs. N Engl J Med. 2009;360(6):626-633.
3. Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer in the United States: 2010-2020. J Natl Cancer Inst. 2011;103(2):117-128.
4. Himmelstein DU, Thorne D, Warren E, Woolhandler S. Medical bankruptcy in the United States, 2007: results of a national study. Am J Med. 2009;122(8):741-746.
5. The Henry J. Kaiser Family Foundation. Prescription drug costs remain atop the public’s national health care agenda, well ahead of Affordable Care Act revisions and repeal [press release]. Kaiser Family Foundation Website. http://kff.org/health-costs/press-release/prescription-drug-costs-remain-atop-the-publics-national-health-care-agenda-well-ahead-of-affordable-care-act-revisions-and-repeal. Published October 28, 2015. Accessed January 11, 2016.
6. National Cancer Institute (NCI). NCI dictionary of cancer terms: targeted therapy. National Cancer Institute Website. http://www.cancer.gov/publications/dictionaries/cancer-terms?cdrid=270742. Accessed January 11, 2016.
7. Moertel CG, Fleming TR, Macdonald JS, et al. Levamisole and fluorouracil for adjuvant therapy resected colon carcinoma. N Engl J Med. 1990;322(6):352-358.
8. Rossof AH, Philpot TR, Bunch RS, Letcher J. The high cost of levamisole for humans. N Engl J Med. 1991;324(10):701-702.
9. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5-29.
10. Nadler E, Eckert B, Neumann PJ. Do oncologists believe new cancer drugs offer good value? Oncologist. 2006;11(2):90-95.
11. Hillner BE, Smith TJ. Efficacy does not necessarily translate into cost effectiveness: a case study of the challenges associated with 21st century cancer drug pricing. J Clin Oncol. 2009;27(13):2111-2113.
12. Neumann PJ, Weinstein MC. Legislating against use of cost-effectiveness information. N Engl J Med. 2010;363(16):1495-1497.
13. Elkin EB, Bach PB. Cancer’s next frontier: addressing high and increasing costs. JAMA. 2010;303(11):1086-1087.
14. Smith TJ, Hillner BE. Bending the cost curve in cancer care. N Engl J Med. 2011;364(21):2060-2065.
15. Siddiqui M, Rajkumar SV. The high cost of cancer drugs and what we can do
about it. Mayo Clin Proc. 2012;87(10):935-943.
16. Bach PB, Saltz LB, Wittes RE. In cancer care, cost matters [op-ed]. New York Times. October 14, 2012.
17. Schnipper LE, Davidson NE, Wollins DS, et al; American Society of Clinical Oncology. American Society of Clinical Oncology statement: a conceptual framework to assess the value of cancer treatment options. J Clin Oncol. 2015;33(23): 2563-2577.
18. Zafar SY, Peppercorn JM, Schrag D, et al. The financial toxicity of cancer treatment: a pilot study assessing out-of-pocket expenses and the insured cancer patient’s experience. Oncologist. 2013;18(4):381-390.
19. Fenn KM, Evans SB, McCorkle R, et al. Impact of financial burden of cancer on
survivors’ quality of life. J Oncol Prac. 2014;10(5):332-338.
20. Zafar SY, McNeil RB, Thomas CM, Lathan CS, Ayanian JZ, Provenzale D. Population-based assessment of cancer survivors’ financial burden and quality of life: a prospective cohort study. J Oncol Pract. 2015;11(2):145-150.
Note: Page numbers differ between the print issue and digital edition.
Implementation of a Precision Oncology Program as an Exemplar of a Learning Health Care System in the VA
Traditional research methods, well suited for scientific discovery and drug development, fall short of providing health care systems with pragmatic information in 2 important ways: Current funding and institutions cannot support comparative effectiveness studies in sufficient numbers to answer the plethora of important clinical questions that confront health care providers (HCPs). The resultant knowledge gap manifests in treatment variability based on clinician impression rather than on direct evidence. A second equally important deficiency is the inability to make full use of the knowledge acquired in treating past patients to determine the best treatment option for the current patient.
Digitization of medical records, creation of health care system corporate data warehouses, and state-of-the-art analytical tools already allow for this revolutionary approach to patient care. Obstructing progress, however, is a lack of understanding by health care system managers and HCPs of the capability of the approach, and unfamiliarity with the requisite informatics by traditional medical researchers. Furthermore the regulatory approach is tilted against the reuse of medical record data for learning and toward strict adherence to patient confidentiality.
Note: Page numbers differ between the print issue and digital edition.
Traditional research methods, well suited for scientific discovery and drug development, fall short of providing health care systems with pragmatic information in 2 important ways: Current funding and institutions cannot support comparative effectiveness studies in sufficient numbers to answer the plethora of important clinical questions that confront health care providers (HCPs). The resultant knowledge gap manifests in treatment variability based on clinician impression rather than on direct evidence. A second equally important deficiency is the inability to make full use of the knowledge acquired in treating past patients to determine the best treatment option for the current patient.
Digitization of medical records, creation of health care system corporate data warehouses, and state-of-the-art analytical tools already allow for this revolutionary approach to patient care. Obstructing progress, however, is a lack of understanding by health care system managers and HCPs of the capability of the approach, and unfamiliarity with the requisite informatics by traditional medical researchers. Furthermore the regulatory approach is tilted against the reuse of medical record data for learning and toward strict adherence to patient confidentiality.
Traditional research methods, well suited for scientific discovery and drug development, fall short of providing health care systems with pragmatic information in 2 important ways: Current funding and institutions cannot support comparative effectiveness studies in sufficient numbers to answer the plethora of important clinical questions that confront health care providers (HCPs). The resultant knowledge gap manifests in treatment variability based on clinician impression rather than on direct evidence. A second equally important deficiency is the inability to make full use of the knowledge acquired in treating past patients to determine the best treatment option for the current patient.
Digitization of medical records, creation of health care system corporate data warehouses, and state-of-the-art analytical tools already allow for this revolutionary approach to patient care. Obstructing progress, however, is a lack of understanding by health care system managers and HCPs of the capability of the approach, and unfamiliarity with the requisite informatics by traditional medical researchers. Furthermore the regulatory approach is tilted against the reuse of medical record data for learning and toward strict adherence to patient confidentiality.
Note: Page numbers differ between the print issue and digital edition.
Note: Page numbers differ between the print issue and digital edition.
A Patient Navigation Model for Veterans Traveling for Cancer Care
The VHA has a unique responsibility to provide excellent, patient-centered care to the veterans who have served the U.S. long after their active military service has ended. For veterans diagnosed with cancer, the physical, mental, and financial consequences can pose significant hardships and create barriers to obtaining timely and efficient health care. The need to travel for cancer care, sometimes for long distances over long periods, adds an additional disparity and puts veterans at higher risk for delays in care. Cancer care navigation teams (CCNTs) were established at the VA Puget Sound Health Care System (VAPSHCS) in Seattle, Washington, and throughout the Veterans Integrated Service Network, region 20 (VISN 20), which consists of a large geographical area that includes Alaska, Washington, Oregon, Idaho and one county in both Montana and California. These teams use an interdisciplinary approach to providing personalized assistance, support, and resources to veterans with cancer and their families who require travel for cancer care.
The CCNTs identify and minimize clinical and psychosocial barriers throughout the cancer care continuum. Although structured to address the unique needs and barriers of the veteran population within the VA, CCNT may also be used as a model for patients receiving cancer care within other complex and decentralized health care systems.
Patient Navigation in Cancer Care
The term navigation in the context of cancer care originated in 1990 at Harlem Hospital Center in New York City. The term described an intervention to address barriers to care experienced by a population of low income African American women with breast cancer. By applying patient navigation in addition to offering free and low-cost breast cancer screening and exams for high-risk patients, the 5-year survival rate in this disadvantaged population of women increased from 39% to 70%.1
Since then, navigation programs in cancer care have been adopted in health care settings around the world. Many different models have been described within the literature.2-5 Patient navigation is perhaps best recognized as a means to decrease health disparities by addressing barriers to health care, which may include lack of insurance, poverty, medical or psychiatric comorbidities, low health literacy, food insecurity, and homelessness. By identifying and addressing these barriers to care in high-risk populations, patient navigation programs have demonstrated positive outcomes, including improvement in cancer screening rates, timeliness of care, medication adherence, and patient satisfaction.6-10 Although there is a large amount of literature on navigation in cancer care, there is minimal literature that focuses on navigation in the veteran population and health care system.
Barriers to Cancer Care
The VA is a national health care system composed of community clinics, hospitals, and major referral centers that deliver comprehensive health care to veterans. For veterans diagnosed with cancer, the physical, mental, and financial consequences can pose significant hardships and create barriers to obtaining timely, efficient health care. Research studies have documented significant differences among veterans receiving health care through the VHA compared with veterans who receive health care from other sources. Veterans enrolled at the VA are more likely to be poorer, older, African American, less well educated, unemployed or underemployed, lack social support, and in poorer physical and mental health compared with the general population or with veterans who do not use VA health care.11-13 Such health disparities have been linked to delays in timely access to health care.11
In a study comparing an age-adjusted ambulatory care population with veterans receiving care at the VA, VA patients were also found to be 3 times more likely to have ever been diagnosed with cancer.12 Exposures to carcinogens during their military service, such as Agent Orange, may contribute to this difference.14 Veterans have higher rates of posttraumatic stress disorder (PTSD) and other mental health disorders from military combat experiences or other traumas; these conditions can be exacerbated by the distress of a cancer diagnosis.15-17
Veterans requiring specialty care, such as cancer-related care, are referred within the VISN and may need to travel long distances in to access these specialty providers. Continuity of care is challenged during cancer diagnosis, staging, treatment, and surveillance when some aspects of care may be completed at geographically distant sites or by community providers if unavailable through the local VA. Appointments for care occur within each specialty service, and staff and clinic availability limit scheduling. There are no formal mechanisms for coordinating visits for efficiency or minimizing travel burden. The electronic medical record (EMR) at the VA can be helpful in accessing information from remote locations but does not easily integrate medical information from different facilities. Clinical data, such as recommendations for follow-up care, may take time and patience to access.
These challenges to the delivery of timely, efficient, patient-centered cancer care were documented in a cancer needs assessment performed in 2012 across VISN 20 (Figure 1). In response, a 3-year pilot program was initiated to implement a network of CCNTs in 8 VA facilities across the region.
Planning and Implementation
The VAPSHCS is a major referral center for cancer care that serves veterans living in VISN 20. On average, about 1,000 new cancers are diagnosed, and VAPSHCS sees 2,000 unique veterans for cancer care annually (Figure 2).One-quarter of these veterans are from out of state. For veterans living in Washington, nearly half traveled 50 miles or more to access cancer services at VAPSHCS. VA Puget Sound implemented its CCNT in the fall of 2014 and consists of an advanced practice registered nurse practitioner (ARNP), registered nurse (RN), social worker (SW), and program support assistant (PSA).
Veterans in identified priority cohorts thought to be at highest risk for barriers to cancer care are enrolled in navigation services. These priority groups include those veterans referred from another regional VA facility, those living more than 100 miles from the VAPSHCS, those referred for multimodality care (eg, surgery with neoadjuvant chemoradiation), and those with significant psychosocial barriers to care. Veterans are identified by the CCNT through a formal consult, notification from the CCNT at another VA facility, a cancer conference, a review of pathology results, and in some cases by veteran self-referral.
As it develops further capacity, CCNT will add other high-risk groups. Ideally, CCNT will eventually be a resource all veterans referred to VAPSHCS for cancer care, so all veterans may be assessed for potential barriers to care and be provided with much needed support and resources.
The CCNT is proactive and systematic in its navigation processes. Where possible, CCNT members are cross-trained to provide role coverage. The team reviews medical records for veterans actively enrolled in CCNT services weekly, to identify new barriers to care and address them in a timely manner. A robust data tracking system (created using a relational database) allows for storage of updated patient information and assigns tasks within the team, tracks upcoming appointments to support coordination, identifies travel and lodging needs, and assures follow-up care is completed. It also generates lists used for routine rounding on patient groups, treatment summary reports, and survivorship care plans.
The CCNT uses standardized assessment tools, including a navigation intake form, the National Comprehensive Cancer Network (NCCN) Distress Thermometer, and a functional assessment. Communication is an essential part of the navigation team, which addresses veteran’s identified needs by conducting weekly rounds within the interdisciplinary team to share information and collaborate.
The team has weekly telephone calls with its CCNTs from referring facilities to discuss veterans at all stages of the cancer continuum and facilitate transfer of information between facilities and providers, including needed diagnostic services and follow-up recommendations. The CCNT also facilitates communication with PSHCS specialty services by actively participating in multidisciplinary rounds and cancer conference.
Finally, although the CCNT follows individual veterans, the team also recognizes its role in identifying and addressing system barriers to cancer care. Collaborating with its partners within the facility and across the network, the team has improved access to services, created teaching tools that can be shared across disciplines, and implemented new procedures and policies to meet the American College of Surgeons Commission on Cancer accreditation standards and improve the cancer care system as a whole.
VAPSHCS Cancer Navigation Model
The VAPSHCS cancer navigation model is divided into 4 main processes based on the cancer care continuum. To illustrate this navigation model, this paper follows the journey of a 57-year-old male veteran referred to PSHCS with newly diagnosed head and neck cancer. He is divorced, with very little social support and lives in a remote area about 60 miles from his primary VA facility and more than 400 miles from PSHCS. His case was presented at the PSHCS facility cancer conference, where concurrent chemotherapy and radiation was recommended. This particular treatment consists of daily radiation and weekly chemotherapy over 6 to 7 weeks. The CCNT staff recognized that this veteran met criteria for navigation, entered him in the tracking database, and notified his referring facility CCNT of the plan of care.
Preconsult
Prior to veterans traveling to VAPSHCS for a new diagnosis or suspicion of cancer, the first goal is to identify any potential barriers to travel. It is a financial burden for many veterans to travel, and in the past, travel has prevented veterans from attending their specialty consult appointments. It is the role of the CCNT PSA to contact the veteran by telephone, introduce their services, provide education about available travel and lodging benefits, and schedule a visit with the CCNT RN to coincide with the veteran’s scheduled other specialty appointments.
In this case, the CCNT PSA contacted the veteran with information about the VAPSHCS, placed a lodging consult to arrange hotel accommodations for the veteran while in Seattle, and provided information regarding transportation from the hotel to the VA. The CCNT also identified that the veteran required a radiation oncology consultation and dental evaluation to proceed with a treatment plan. To decrease travel burden with additional trips to Seattle, the PSA contacted these specialty services to schedule the appointments. The PSA then assembled and mailed a packet of information to the veteran, which included details about how to pack and prepare for the trip, a facility map, and a hotel shuttle schedule.
Consult Visit and Planning
When veterans arrive at VAPSHCS, the CCNT RN meets them and completes an intake form. This standardized questionnaire identifies potential barriers to cancer care and supports the need for referrals to services, such as a dietitian, chaplain, palliative care, social work, physical and occupational therapy, travel, or lodging.
During this visit, the CCNT RN also asks the veteran to complete a NCCN Distress Thermometer. This thermometer assessment tool screens for physical, emotional, and practical needs that are specific to cancer. In this particular veteran’s situation, the distress level was 7 out of 10 (a score of 4 or greater triggersan automatic consult to social work once the results are entered in the EMR). Based on the outcomes information obtained from the intake form and NCCN Distress Thermometer, the CCNT RN made referrals to SW, chaplain services, and the oncology dietitian.
During the CCNT RN visit, nurse identified that the veteran’s financial situation had changed significantly resulting in less income and causing financial distress. The veteran was encouraged to complete an updated benefit renewal form with the SW that would likely eliminate his required copays for medical visits and prescription medications during the 6 weeks of chemotherapy and radiation. This need was communicated to the CCNT SW. The RN provided the veteran with information about VA resources to support him during cancer treatment, including meal options and support groups for both veterans and caregivers. They discussed the likely plan of care, including disease progress, information on prescribed drugs, dental evaluation and extractions as needed, placement of a feeding tube and a central line, and gave the veteran written brochures to review at his convenience. The RN also reviewed the logistics of a prolonged stay for the recommended course of chemotherapy and radiation.
During the initial CCNT intake process, the RN identified that the veteran would be without a caregiver and would be staying alone in lodging throughout his cancer treatment. The RN then completed a functional assessment of safety risks while lodging alone during this extended time. This brief questionnaire identifies any deficits in a veteran’s activity of daily living that may influence safety while lodging alone. The assessment is documented in the EMR, and if any concerns are identified, these are discussed with the veteran and a team of medical providers. If necessary, interventions are put into place before the veteran’s return for treatment. Potential safeguards may include obtaining safety equipment (eg, walker and bath chair), identifying an appropriate caregiver, or referring the veteran to a skilled nursing facility for the duration of treatment.
Following the veteran’s consultation visits, he went home with a return date 2 weeks later to start treatment. The VAPSHCS CCNT discussed the plan of care with his local CCNT, which facilitated placement of his feeding tube and addressed other symptom management concerns. The local CCNT SW completed advanced directives with the veteran and coordinated his travel back to VAPSHCS to begin treatment.
During Treatment
Veterans traveling from other VA facilities are away from their primary care providers (PCPs) for a number of weeks. Other specialty providers see a veteran during cancer treatment; however, the CCNT ARNP supports primary care needs while the veteran is away from their home VA facility. The ARNP is able to address chronic or acute medical issues before the start of treatment to prevent delays in cancer care.
Once the veteran returned to VAPSHCS to initiate therapy, the CCNT ARNP completed a history and physical examination to identify and address any active medical problems and document past medical history and current medication list in the EMR. This provides easy access to a thorough and complete baseline to both the oncology and radiation oncology providers. The ARNP examination revealed a new neck wound on the veteran, likely related to his cancer, and an urgent consult was placed to wound care. The otolaryngology, oncology, and radiation oncology departments were alerted to this development so they could assess the patient and adjust treatment plans as necessary. The veteran also required a refill of his blood pressure medication and had a number of questions regarding his upcoming treatment, which were addressed during the visit.
Within the first 2 days of the veteran’s return, he was scheduled to meet with the CCNT SW who reviewed and documented his advanced directive within the system, assessed his distress, provided therapeutic counseling, and completed the health benefit renewal form. Given the veteran’s financial status, the SW was able to help him apply for financial hardship to cover the costs of the care he had already received and assisted him with securing an appointment with the Social Security Administration (SSA) for disability benefits. The CCNT SW then helped the veteran complete a phone interview with the SSA and complete the application process. The SW also helped him complete the application for VA service-connected compensation and pension disability benefits.
Throughout his treatment course, the CCNT continued to be a resource for the veteran. Because he had PTSD and was uncomfortable attending support groups, the CCNT SW met with him weekly to provide counseling and psychosocial support. He stopped by the CCNT office on several occasions to report how he was doing, and the team provided assistance in obtaining supplies for his feeding tube and managing a complication that arose with his lodging. In preparation for his treatment completion and return home, the VAPSHCS CCNT communicated with his local CCNT to describe follow-up needs and ensure appropriate medical visits were scheduled. His travel home was arranged by the VAPSHCS PSA.
Treatment Completion
Before leaving VAPSHCS, the veteran was scheduled and seen in the clinic by the ARNP, where he received a written comprehensive treatment summary. The summary documented his cancer diagnosis, treatment, complications, and recommendations for follow-up care. He had the opportunity to ask questions about his treatment, and a clinical assessment was made for adverse effects. Appropriate interventions also were identified and addressed. A comprehensive treatment summary note was documented in the EMR and sent to his PCP and other medical specialists at his home facility to assure continuity of care.
The VAPSHCS CCNT continued to communicate weekly with the veteran’s home CCNT following his return, to ensure he received appropriate follow-up care and addressed questions and needs that arose. The veteran’s home CCNT continued to monitor the veteran for 1 year post treatment and communicate with VAPSHCS CCNT.
Conclusion
The VA is in a unique position to meet the needs of veterans by providing comprehensive care with sensitivity to military culture, access to a range of complicated benefits awarded to veterans, particularly those with servicerelated exposures or injuries, and specialists in diagnosis and treatment of physical and mental consequences of their service. Patient navigation helps ensure veterans can access these services, maintain continuity of care despite referrals across large geographic regions, and receive support while receiving cancer treatment at the VA.
Use of an interdisciplinary team, including an ARNP, RN, SW, and PSA is vital to fully address the wide range of physical, psychosocial, and practical barriers to care that a veteran may experience. Since September 2014, PSHCS has enrolled more than 500 veterans with CCNT, and nearly 200 are actively being followed and provided with navigation services at any given time (Figure 3). By proactively identifying and addressing barriers to care, the advocacy provided by CCNT has averted patient safety risks, made better use of limited veteran and VA resources, and provided patient-centered care to veterans.
Evaluation is currently underway to measure the impact of the program and develop metrics for the CCNT. Given the needs of the patient population, the team hopes to see further expansion of CCNT in order to reach more risk groups. Institutional support and funding for patient navigation should be a high priority as the VA strives to provide excellent, patient-centered care.
Acknowledgements
The authors would like first and foremost to give a special thank-you to the veterans for their service to our country. In addition, the authors would like to thank champions for the cancer care navigation team, including Dr. Daniel Wu, chief of oncology; and Dr. Peter Wu, cancer committee chair and surgical oncologist, and Sandra Solomon, nurse manager of the Cancer Care Clinic and inpatient cancer unit at VA Puget Sound Health Care System; Dr. Carol Sprague, staff physician and clinical lead VISN 20 Cancer Care Platform, Judy McConnachie, MPH, administrative director, Clinical Business Intelligence Northwest Innovation Center, VA Portland Health Care System in Portland, Oregon; and Tracy Weistreich, PhD, RN, associate director Patient Care Services at VA Roseburg Healthcare System in Roseburg, Oregon; and the VISN 20 Executive Cancer Care Platform Advisory Board.
The authors would also like to acknowledge all the VISN 20 network cancer care navigation teams at the following sites: Anchorage, Alaska; Boise, Idaho; Portland, Oregon; Roseburg, Oregon; Spokane, Washington; Walla Walla, Washington; and White City, Oregon. Team members at each site have been an integral part of the development and success of the VAPSHCS CCNT.
The authors are also grateful to all of the nurse coordinators and providers within all the specialty services at Puget Sound Health Care Systems, including oncology, radiation oncology, cancer care, otolaryngology, general surgery, palliative care, dental and primary care, for their collaboration with veteran care.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to continue reading.
1. Freeman HP. Patient navigation as a targeted intervention: for patients at high risk
for delays in cancer care. Cancer. 2015;121(22):3930-3932.
2. Moy B, Chabner BA. Patient navigator programs, cancer disparities, and the patient protection and affordable care act. Oncologist. 2011;16(7):926-929.
3. Meade CD, Wells KJ, Arevalo M, et al. Lay navigator model for impacting cancer health disparities. J Cancer Educ. 2014;29(3):449-457.
4. Fillion L, Cook S, Veillette AM, et al. Professional navigation: a comparative study of two Canadian models. Can Oncol Nurs J. 2012;22(4):257-277.
5. Lairson DR, Huo J, Ricks KA, Savas L, Fernández ME. The cost of implementing a 2-1-1 call center-based cancer control navigator program. Eval Program Plann. 2013:39:51-56.
6. Percac-Lima S, Cronin PR, Ryan DP, Chabner BA, Daly E, Kimball AB. Patient navigation based on predictive modeling decreases no-show rates in cancer care. Cancer. 2015;121(10):1662-1670.
7. Percac-Lima S, Ashburner JM, McCarthy AM, Piawah S, Atlas SJ. Patient navigation
to improve follow-up of abnormal mammograms among disadvantaged women. J Womens Health (Larchmt). 2015;24(2):138-143.
8. Ladabaum U, Mannalithara A, Jandorf L, Itzkowitz SH. Cost-effectiveness of patient navigation to increase adherence with screening colonoscopy among minority
individuals. Cancer. 2015;121(7):1088-1097.
9. Baliski C, McGahan CE, Liberto CM, et al. Influence of nurse navigation on wait times for breast cancer care in a Canadian regional cancer center. Am J Surg. 2014;207(5):686-691.
10. Hoffman JH, LaVerda NL, Young HA, et al. Patient navigation significantly reduces delays in breast cancer diagnosis in the District of Columbia. Cancer Epidemiol Biomarkers Prev. 2012;1(10):1655-1663
11. Kazis LE, Miller DR, Clark J, et al. Health-related quality of life in patients served by the Department of Veterans Affairs: results from the Veterans Health Study. Arch Intern Med. 1998;158(6):626-632.
12. Rogers WH, Kazis LE, Miller DR, et al. Comparing the health status of VA and non-VA ambulatory patients: the veterans health and medical outcome studies. J Ambul Care Manage. 2004;27(3):249-262.
13. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257.
14. Institute of Medicine (US) Committee to Review the Health Effects in Vietnam Veterans of Exposure to Herbicides. Veterans and Agent Orange: Health Effects of Herbicides Used in Vietnam. Washington, DC: National Academies Press; 1994.
15. Wachen JS, Patidar SM, Mulligan EA, Naik AD, Moye J. Cancer-related PTSD symptoms in a veteran sample: association with age, combat PTSD, and quality of life. Psychooncology. 2014;23(8):921-927.
16. Mulligan EA, Wachen JS, Naik AD, Gosian J, Moye J. Cancer as a criterion a traumatic stressor for veterans: prevalence and correlates. Psychol Trauma. 2014;6(suppl 1):S73-S81.
17. Dobie DJ, Kivlahan DR, Maynard C, Bush KR, Davis TM, Bradley KA. Posttraumatic stress disorder in female veterans: association with self-reported health problems and functional impairment. Arch Intern Med. 2004;164(4):394-400.
Note: Page numbers differ between the print issue and digital edition.
The VHA has a unique responsibility to provide excellent, patient-centered care to the veterans who have served the U.S. long after their active military service has ended. For veterans diagnosed with cancer, the physical, mental, and financial consequences can pose significant hardships and create barriers to obtaining timely and efficient health care. The need to travel for cancer care, sometimes for long distances over long periods, adds an additional disparity and puts veterans at higher risk for delays in care. Cancer care navigation teams (CCNTs) were established at the VA Puget Sound Health Care System (VAPSHCS) in Seattle, Washington, and throughout the Veterans Integrated Service Network, region 20 (VISN 20), which consists of a large geographical area that includes Alaska, Washington, Oregon, Idaho and one county in both Montana and California. These teams use an interdisciplinary approach to providing personalized assistance, support, and resources to veterans with cancer and their families who require travel for cancer care.
The CCNTs identify and minimize clinical and psychosocial barriers throughout the cancer care continuum. Although structured to address the unique needs and barriers of the veteran population within the VA, CCNT may also be used as a model for patients receiving cancer care within other complex and decentralized health care systems.
Patient Navigation in Cancer Care
The term navigation in the context of cancer care originated in 1990 at Harlem Hospital Center in New York City. The term described an intervention to address barriers to care experienced by a population of low income African American women with breast cancer. By applying patient navigation in addition to offering free and low-cost breast cancer screening and exams for high-risk patients, the 5-year survival rate in this disadvantaged population of women increased from 39% to 70%.1
Since then, navigation programs in cancer care have been adopted in health care settings around the world. Many different models have been described within the literature.2-5 Patient navigation is perhaps best recognized as a means to decrease health disparities by addressing barriers to health care, which may include lack of insurance, poverty, medical or psychiatric comorbidities, low health literacy, food insecurity, and homelessness. By identifying and addressing these barriers to care in high-risk populations, patient navigation programs have demonstrated positive outcomes, including improvement in cancer screening rates, timeliness of care, medication adherence, and patient satisfaction.6-10 Although there is a large amount of literature on navigation in cancer care, there is minimal literature that focuses on navigation in the veteran population and health care system.
Barriers to Cancer Care
The VA is a national health care system composed of community clinics, hospitals, and major referral centers that deliver comprehensive health care to veterans. For veterans diagnosed with cancer, the physical, mental, and financial consequences can pose significant hardships and create barriers to obtaining timely, efficient health care. Research studies have documented significant differences among veterans receiving health care through the VHA compared with veterans who receive health care from other sources. Veterans enrolled at the VA are more likely to be poorer, older, African American, less well educated, unemployed or underemployed, lack social support, and in poorer physical and mental health compared with the general population or with veterans who do not use VA health care.11-13 Such health disparities have been linked to delays in timely access to health care.11
In a study comparing an age-adjusted ambulatory care population with veterans receiving care at the VA, VA patients were also found to be 3 times more likely to have ever been diagnosed with cancer.12 Exposures to carcinogens during their military service, such as Agent Orange, may contribute to this difference.14 Veterans have higher rates of posttraumatic stress disorder (PTSD) and other mental health disorders from military combat experiences or other traumas; these conditions can be exacerbated by the distress of a cancer diagnosis.15-17
Veterans requiring specialty care, such as cancer-related care, are referred within the VISN and may need to travel long distances in to access these specialty providers. Continuity of care is challenged during cancer diagnosis, staging, treatment, and surveillance when some aspects of care may be completed at geographically distant sites or by community providers if unavailable through the local VA. Appointments for care occur within each specialty service, and staff and clinic availability limit scheduling. There are no formal mechanisms for coordinating visits for efficiency or minimizing travel burden. The electronic medical record (EMR) at the VA can be helpful in accessing information from remote locations but does not easily integrate medical information from different facilities. Clinical data, such as recommendations for follow-up care, may take time and patience to access.
These challenges to the delivery of timely, efficient, patient-centered cancer care were documented in a cancer needs assessment performed in 2012 across VISN 20 (Figure 1). In response, a 3-year pilot program was initiated to implement a network of CCNTs in 8 VA facilities across the region.
Planning and Implementation
The VAPSHCS is a major referral center for cancer care that serves veterans living in VISN 20. On average, about 1,000 new cancers are diagnosed, and VAPSHCS sees 2,000 unique veterans for cancer care annually (Figure 2).One-quarter of these veterans are from out of state. For veterans living in Washington, nearly half traveled 50 miles or more to access cancer services at VAPSHCS. VA Puget Sound implemented its CCNT in the fall of 2014 and consists of an advanced practice registered nurse practitioner (ARNP), registered nurse (RN), social worker (SW), and program support assistant (PSA).
Veterans in identified priority cohorts thought to be at highest risk for barriers to cancer care are enrolled in navigation services. These priority groups include those veterans referred from another regional VA facility, those living more than 100 miles from the VAPSHCS, those referred for multimodality care (eg, surgery with neoadjuvant chemoradiation), and those with significant psychosocial barriers to care. Veterans are identified by the CCNT through a formal consult, notification from the CCNT at another VA facility, a cancer conference, a review of pathology results, and in some cases by veteran self-referral.
As it develops further capacity, CCNT will add other high-risk groups. Ideally, CCNT will eventually be a resource all veterans referred to VAPSHCS for cancer care, so all veterans may be assessed for potential barriers to care and be provided with much needed support and resources.
The CCNT is proactive and systematic in its navigation processes. Where possible, CCNT members are cross-trained to provide role coverage. The team reviews medical records for veterans actively enrolled in CCNT services weekly, to identify new barriers to care and address them in a timely manner. A robust data tracking system (created using a relational database) allows for storage of updated patient information and assigns tasks within the team, tracks upcoming appointments to support coordination, identifies travel and lodging needs, and assures follow-up care is completed. It also generates lists used for routine rounding on patient groups, treatment summary reports, and survivorship care plans.
The CCNT uses standardized assessment tools, including a navigation intake form, the National Comprehensive Cancer Network (NCCN) Distress Thermometer, and a functional assessment. Communication is an essential part of the navigation team, which addresses veteran’s identified needs by conducting weekly rounds within the interdisciplinary team to share information and collaborate.
The team has weekly telephone calls with its CCNTs from referring facilities to discuss veterans at all stages of the cancer continuum and facilitate transfer of information between facilities and providers, including needed diagnostic services and follow-up recommendations. The CCNT also facilitates communication with PSHCS specialty services by actively participating in multidisciplinary rounds and cancer conference.
Finally, although the CCNT follows individual veterans, the team also recognizes its role in identifying and addressing system barriers to cancer care. Collaborating with its partners within the facility and across the network, the team has improved access to services, created teaching tools that can be shared across disciplines, and implemented new procedures and policies to meet the American College of Surgeons Commission on Cancer accreditation standards and improve the cancer care system as a whole.
VAPSHCS Cancer Navigation Model
The VAPSHCS cancer navigation model is divided into 4 main processes based on the cancer care continuum. To illustrate this navigation model, this paper follows the journey of a 57-year-old male veteran referred to PSHCS with newly diagnosed head and neck cancer. He is divorced, with very little social support and lives in a remote area about 60 miles from his primary VA facility and more than 400 miles from PSHCS. His case was presented at the PSHCS facility cancer conference, where concurrent chemotherapy and radiation was recommended. This particular treatment consists of daily radiation and weekly chemotherapy over 6 to 7 weeks. The CCNT staff recognized that this veteran met criteria for navigation, entered him in the tracking database, and notified his referring facility CCNT of the plan of care.
Preconsult
Prior to veterans traveling to VAPSHCS for a new diagnosis or suspicion of cancer, the first goal is to identify any potential barriers to travel. It is a financial burden for many veterans to travel, and in the past, travel has prevented veterans from attending their specialty consult appointments. It is the role of the CCNT PSA to contact the veteran by telephone, introduce their services, provide education about available travel and lodging benefits, and schedule a visit with the CCNT RN to coincide with the veteran’s scheduled other specialty appointments.
In this case, the CCNT PSA contacted the veteran with information about the VAPSHCS, placed a lodging consult to arrange hotel accommodations for the veteran while in Seattle, and provided information regarding transportation from the hotel to the VA. The CCNT also identified that the veteran required a radiation oncology consultation and dental evaluation to proceed with a treatment plan. To decrease travel burden with additional trips to Seattle, the PSA contacted these specialty services to schedule the appointments. The PSA then assembled and mailed a packet of information to the veteran, which included details about how to pack and prepare for the trip, a facility map, and a hotel shuttle schedule.
Consult Visit and Planning
When veterans arrive at VAPSHCS, the CCNT RN meets them and completes an intake form. This standardized questionnaire identifies potential barriers to cancer care and supports the need for referrals to services, such as a dietitian, chaplain, palliative care, social work, physical and occupational therapy, travel, or lodging.
During this visit, the CCNT RN also asks the veteran to complete a NCCN Distress Thermometer. This thermometer assessment tool screens for physical, emotional, and practical needs that are specific to cancer. In this particular veteran’s situation, the distress level was 7 out of 10 (a score of 4 or greater triggersan automatic consult to social work once the results are entered in the EMR). Based on the outcomes information obtained from the intake form and NCCN Distress Thermometer, the CCNT RN made referrals to SW, chaplain services, and the oncology dietitian.
During the CCNT RN visit, nurse identified that the veteran’s financial situation had changed significantly resulting in less income and causing financial distress. The veteran was encouraged to complete an updated benefit renewal form with the SW that would likely eliminate his required copays for medical visits and prescription medications during the 6 weeks of chemotherapy and radiation. This need was communicated to the CCNT SW. The RN provided the veteran with information about VA resources to support him during cancer treatment, including meal options and support groups for both veterans and caregivers. They discussed the likely plan of care, including disease progress, information on prescribed drugs, dental evaluation and extractions as needed, placement of a feeding tube and a central line, and gave the veteran written brochures to review at his convenience. The RN also reviewed the logistics of a prolonged stay for the recommended course of chemotherapy and radiation.
During the initial CCNT intake process, the RN identified that the veteran would be without a caregiver and would be staying alone in lodging throughout his cancer treatment. The RN then completed a functional assessment of safety risks while lodging alone during this extended time. This brief questionnaire identifies any deficits in a veteran’s activity of daily living that may influence safety while lodging alone. The assessment is documented in the EMR, and if any concerns are identified, these are discussed with the veteran and a team of medical providers. If necessary, interventions are put into place before the veteran’s return for treatment. Potential safeguards may include obtaining safety equipment (eg, walker and bath chair), identifying an appropriate caregiver, or referring the veteran to a skilled nursing facility for the duration of treatment.
Following the veteran’s consultation visits, he went home with a return date 2 weeks later to start treatment. The VAPSHCS CCNT discussed the plan of care with his local CCNT, which facilitated placement of his feeding tube and addressed other symptom management concerns. The local CCNT SW completed advanced directives with the veteran and coordinated his travel back to VAPSHCS to begin treatment.
During Treatment
Veterans traveling from other VA facilities are away from their primary care providers (PCPs) for a number of weeks. Other specialty providers see a veteran during cancer treatment; however, the CCNT ARNP supports primary care needs while the veteran is away from their home VA facility. The ARNP is able to address chronic or acute medical issues before the start of treatment to prevent delays in cancer care.
Once the veteran returned to VAPSHCS to initiate therapy, the CCNT ARNP completed a history and physical examination to identify and address any active medical problems and document past medical history and current medication list in the EMR. This provides easy access to a thorough and complete baseline to both the oncology and radiation oncology providers. The ARNP examination revealed a new neck wound on the veteran, likely related to his cancer, and an urgent consult was placed to wound care. The otolaryngology, oncology, and radiation oncology departments were alerted to this development so they could assess the patient and adjust treatment plans as necessary. The veteran also required a refill of his blood pressure medication and had a number of questions regarding his upcoming treatment, which were addressed during the visit.
Within the first 2 days of the veteran’s return, he was scheduled to meet with the CCNT SW who reviewed and documented his advanced directive within the system, assessed his distress, provided therapeutic counseling, and completed the health benefit renewal form. Given the veteran’s financial status, the SW was able to help him apply for financial hardship to cover the costs of the care he had already received and assisted him with securing an appointment with the Social Security Administration (SSA) for disability benefits. The CCNT SW then helped the veteran complete a phone interview with the SSA and complete the application process. The SW also helped him complete the application for VA service-connected compensation and pension disability benefits.
Throughout his treatment course, the CCNT continued to be a resource for the veteran. Because he had PTSD and was uncomfortable attending support groups, the CCNT SW met with him weekly to provide counseling and psychosocial support. He stopped by the CCNT office on several occasions to report how he was doing, and the team provided assistance in obtaining supplies for his feeding tube and managing a complication that arose with his lodging. In preparation for his treatment completion and return home, the VAPSHCS CCNT communicated with his local CCNT to describe follow-up needs and ensure appropriate medical visits were scheduled. His travel home was arranged by the VAPSHCS PSA.
Treatment Completion
Before leaving VAPSHCS, the veteran was scheduled and seen in the clinic by the ARNP, where he received a written comprehensive treatment summary. The summary documented his cancer diagnosis, treatment, complications, and recommendations for follow-up care. He had the opportunity to ask questions about his treatment, and a clinical assessment was made for adverse effects. Appropriate interventions also were identified and addressed. A comprehensive treatment summary note was documented in the EMR and sent to his PCP and other medical specialists at his home facility to assure continuity of care.
The VAPSHCS CCNT continued to communicate weekly with the veteran’s home CCNT following his return, to ensure he received appropriate follow-up care and addressed questions and needs that arose. The veteran’s home CCNT continued to monitor the veteran for 1 year post treatment and communicate with VAPSHCS CCNT.
Conclusion
The VA is in a unique position to meet the needs of veterans by providing comprehensive care with sensitivity to military culture, access to a range of complicated benefits awarded to veterans, particularly those with servicerelated exposures or injuries, and specialists in diagnosis and treatment of physical and mental consequences of their service. Patient navigation helps ensure veterans can access these services, maintain continuity of care despite referrals across large geographic regions, and receive support while receiving cancer treatment at the VA.
Use of an interdisciplinary team, including an ARNP, RN, SW, and PSA is vital to fully address the wide range of physical, psychosocial, and practical barriers to care that a veteran may experience. Since September 2014, PSHCS has enrolled more than 500 veterans with CCNT, and nearly 200 are actively being followed and provided with navigation services at any given time (Figure 3). By proactively identifying and addressing barriers to care, the advocacy provided by CCNT has averted patient safety risks, made better use of limited veteran and VA resources, and provided patient-centered care to veterans.
Evaluation is currently underway to measure the impact of the program and develop metrics for the CCNT. Given the needs of the patient population, the team hopes to see further expansion of CCNT in order to reach more risk groups. Institutional support and funding for patient navigation should be a high priority as the VA strives to provide excellent, patient-centered care.
Acknowledgements
The authors would like first and foremost to give a special thank-you to the veterans for their service to our country. In addition, the authors would like to thank champions for the cancer care navigation team, including Dr. Daniel Wu, chief of oncology; and Dr. Peter Wu, cancer committee chair and surgical oncologist, and Sandra Solomon, nurse manager of the Cancer Care Clinic and inpatient cancer unit at VA Puget Sound Health Care System; Dr. Carol Sprague, staff physician and clinical lead VISN 20 Cancer Care Platform, Judy McConnachie, MPH, administrative director, Clinical Business Intelligence Northwest Innovation Center, VA Portland Health Care System in Portland, Oregon; and Tracy Weistreich, PhD, RN, associate director Patient Care Services at VA Roseburg Healthcare System in Roseburg, Oregon; and the VISN 20 Executive Cancer Care Platform Advisory Board.
The authors would also like to acknowledge all the VISN 20 network cancer care navigation teams at the following sites: Anchorage, Alaska; Boise, Idaho; Portland, Oregon; Roseburg, Oregon; Spokane, Washington; Walla Walla, Washington; and White City, Oregon. Team members at each site have been an integral part of the development and success of the VAPSHCS CCNT.
The authors are also grateful to all of the nurse coordinators and providers within all the specialty services at Puget Sound Health Care Systems, including oncology, radiation oncology, cancer care, otolaryngology, general surgery, palliative care, dental and primary care, for their collaboration with veteran care.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to continue reading.
The VHA has a unique responsibility to provide excellent, patient-centered care to the veterans who have served the U.S. long after their active military service has ended. For veterans diagnosed with cancer, the physical, mental, and financial consequences can pose significant hardships and create barriers to obtaining timely and efficient health care. The need to travel for cancer care, sometimes for long distances over long periods, adds an additional disparity and puts veterans at higher risk for delays in care. Cancer care navigation teams (CCNTs) were established at the VA Puget Sound Health Care System (VAPSHCS) in Seattle, Washington, and throughout the Veterans Integrated Service Network, region 20 (VISN 20), which consists of a large geographical area that includes Alaska, Washington, Oregon, Idaho and one county in both Montana and California. These teams use an interdisciplinary approach to providing personalized assistance, support, and resources to veterans with cancer and their families who require travel for cancer care.
The CCNTs identify and minimize clinical and psychosocial barriers throughout the cancer care continuum. Although structured to address the unique needs and barriers of the veteran population within the VA, CCNT may also be used as a model for patients receiving cancer care within other complex and decentralized health care systems.
Patient Navigation in Cancer Care
The term navigation in the context of cancer care originated in 1990 at Harlem Hospital Center in New York City. The term described an intervention to address barriers to care experienced by a population of low income African American women with breast cancer. By applying patient navigation in addition to offering free and low-cost breast cancer screening and exams for high-risk patients, the 5-year survival rate in this disadvantaged population of women increased from 39% to 70%.1
Since then, navigation programs in cancer care have been adopted in health care settings around the world. Many different models have been described within the literature.2-5 Patient navigation is perhaps best recognized as a means to decrease health disparities by addressing barriers to health care, which may include lack of insurance, poverty, medical or psychiatric comorbidities, low health literacy, food insecurity, and homelessness. By identifying and addressing these barriers to care in high-risk populations, patient navigation programs have demonstrated positive outcomes, including improvement in cancer screening rates, timeliness of care, medication adherence, and patient satisfaction.6-10 Although there is a large amount of literature on navigation in cancer care, there is minimal literature that focuses on navigation in the veteran population and health care system.
Barriers to Cancer Care
The VA is a national health care system composed of community clinics, hospitals, and major referral centers that deliver comprehensive health care to veterans. For veterans diagnosed with cancer, the physical, mental, and financial consequences can pose significant hardships and create barriers to obtaining timely, efficient health care. Research studies have documented significant differences among veterans receiving health care through the VHA compared with veterans who receive health care from other sources. Veterans enrolled at the VA are more likely to be poorer, older, African American, less well educated, unemployed or underemployed, lack social support, and in poorer physical and mental health compared with the general population or with veterans who do not use VA health care.11-13 Such health disparities have been linked to delays in timely access to health care.11
In a study comparing an age-adjusted ambulatory care population with veterans receiving care at the VA, VA patients were also found to be 3 times more likely to have ever been diagnosed with cancer.12 Exposures to carcinogens during their military service, such as Agent Orange, may contribute to this difference.14 Veterans have higher rates of posttraumatic stress disorder (PTSD) and other mental health disorders from military combat experiences or other traumas; these conditions can be exacerbated by the distress of a cancer diagnosis.15-17
Veterans requiring specialty care, such as cancer-related care, are referred within the VISN and may need to travel long distances in to access these specialty providers. Continuity of care is challenged during cancer diagnosis, staging, treatment, and surveillance when some aspects of care may be completed at geographically distant sites or by community providers if unavailable through the local VA. Appointments for care occur within each specialty service, and staff and clinic availability limit scheduling. There are no formal mechanisms for coordinating visits for efficiency or minimizing travel burden. The electronic medical record (EMR) at the VA can be helpful in accessing information from remote locations but does not easily integrate medical information from different facilities. Clinical data, such as recommendations for follow-up care, may take time and patience to access.
These challenges to the delivery of timely, efficient, patient-centered cancer care were documented in a cancer needs assessment performed in 2012 across VISN 20 (Figure 1). In response, a 3-year pilot program was initiated to implement a network of CCNTs in 8 VA facilities across the region.
Planning and Implementation
The VAPSHCS is a major referral center for cancer care that serves veterans living in VISN 20. On average, about 1,000 new cancers are diagnosed, and VAPSHCS sees 2,000 unique veterans for cancer care annually (Figure 2).One-quarter of these veterans are from out of state. For veterans living in Washington, nearly half traveled 50 miles or more to access cancer services at VAPSHCS. VA Puget Sound implemented its CCNT in the fall of 2014 and consists of an advanced practice registered nurse practitioner (ARNP), registered nurse (RN), social worker (SW), and program support assistant (PSA).
Veterans in identified priority cohorts thought to be at highest risk for barriers to cancer care are enrolled in navigation services. These priority groups include those veterans referred from another regional VA facility, those living more than 100 miles from the VAPSHCS, those referred for multimodality care (eg, surgery with neoadjuvant chemoradiation), and those with significant psychosocial barriers to care. Veterans are identified by the CCNT through a formal consult, notification from the CCNT at another VA facility, a cancer conference, a review of pathology results, and in some cases by veteran self-referral.
As it develops further capacity, CCNT will add other high-risk groups. Ideally, CCNT will eventually be a resource all veterans referred to VAPSHCS for cancer care, so all veterans may be assessed for potential barriers to care and be provided with much needed support and resources.
The CCNT is proactive and systematic in its navigation processes. Where possible, CCNT members are cross-trained to provide role coverage. The team reviews medical records for veterans actively enrolled in CCNT services weekly, to identify new barriers to care and address them in a timely manner. A robust data tracking system (created using a relational database) allows for storage of updated patient information and assigns tasks within the team, tracks upcoming appointments to support coordination, identifies travel and lodging needs, and assures follow-up care is completed. It also generates lists used for routine rounding on patient groups, treatment summary reports, and survivorship care plans.
The CCNT uses standardized assessment tools, including a navigation intake form, the National Comprehensive Cancer Network (NCCN) Distress Thermometer, and a functional assessment. Communication is an essential part of the navigation team, which addresses veteran’s identified needs by conducting weekly rounds within the interdisciplinary team to share information and collaborate.
The team has weekly telephone calls with its CCNTs from referring facilities to discuss veterans at all stages of the cancer continuum and facilitate transfer of information between facilities and providers, including needed diagnostic services and follow-up recommendations. The CCNT also facilitates communication with PSHCS specialty services by actively participating in multidisciplinary rounds and cancer conference.
Finally, although the CCNT follows individual veterans, the team also recognizes its role in identifying and addressing system barriers to cancer care. Collaborating with its partners within the facility and across the network, the team has improved access to services, created teaching tools that can be shared across disciplines, and implemented new procedures and policies to meet the American College of Surgeons Commission on Cancer accreditation standards and improve the cancer care system as a whole.
VAPSHCS Cancer Navigation Model
The VAPSHCS cancer navigation model is divided into 4 main processes based on the cancer care continuum. To illustrate this navigation model, this paper follows the journey of a 57-year-old male veteran referred to PSHCS with newly diagnosed head and neck cancer. He is divorced, with very little social support and lives in a remote area about 60 miles from his primary VA facility and more than 400 miles from PSHCS. His case was presented at the PSHCS facility cancer conference, where concurrent chemotherapy and radiation was recommended. This particular treatment consists of daily radiation and weekly chemotherapy over 6 to 7 weeks. The CCNT staff recognized that this veteran met criteria for navigation, entered him in the tracking database, and notified his referring facility CCNT of the plan of care.
Preconsult
Prior to veterans traveling to VAPSHCS for a new diagnosis or suspicion of cancer, the first goal is to identify any potential barriers to travel. It is a financial burden for many veterans to travel, and in the past, travel has prevented veterans from attending their specialty consult appointments. It is the role of the CCNT PSA to contact the veteran by telephone, introduce their services, provide education about available travel and lodging benefits, and schedule a visit with the CCNT RN to coincide with the veteran’s scheduled other specialty appointments.
In this case, the CCNT PSA contacted the veteran with information about the VAPSHCS, placed a lodging consult to arrange hotel accommodations for the veteran while in Seattle, and provided information regarding transportation from the hotel to the VA. The CCNT also identified that the veteran required a radiation oncology consultation and dental evaluation to proceed with a treatment plan. To decrease travel burden with additional trips to Seattle, the PSA contacted these specialty services to schedule the appointments. The PSA then assembled and mailed a packet of information to the veteran, which included details about how to pack and prepare for the trip, a facility map, and a hotel shuttle schedule.
Consult Visit and Planning
When veterans arrive at VAPSHCS, the CCNT RN meets them and completes an intake form. This standardized questionnaire identifies potential barriers to cancer care and supports the need for referrals to services, such as a dietitian, chaplain, palliative care, social work, physical and occupational therapy, travel, or lodging.
During this visit, the CCNT RN also asks the veteran to complete a NCCN Distress Thermometer. This thermometer assessment tool screens for physical, emotional, and practical needs that are specific to cancer. In this particular veteran’s situation, the distress level was 7 out of 10 (a score of 4 or greater triggersan automatic consult to social work once the results are entered in the EMR). Based on the outcomes information obtained from the intake form and NCCN Distress Thermometer, the CCNT RN made referrals to SW, chaplain services, and the oncology dietitian.
During the CCNT RN visit, nurse identified that the veteran’s financial situation had changed significantly resulting in less income and causing financial distress. The veteran was encouraged to complete an updated benefit renewal form with the SW that would likely eliminate his required copays for medical visits and prescription medications during the 6 weeks of chemotherapy and radiation. This need was communicated to the CCNT SW. The RN provided the veteran with information about VA resources to support him during cancer treatment, including meal options and support groups for both veterans and caregivers. They discussed the likely plan of care, including disease progress, information on prescribed drugs, dental evaluation and extractions as needed, placement of a feeding tube and a central line, and gave the veteran written brochures to review at his convenience. The RN also reviewed the logistics of a prolonged stay for the recommended course of chemotherapy and radiation.
During the initial CCNT intake process, the RN identified that the veteran would be without a caregiver and would be staying alone in lodging throughout his cancer treatment. The RN then completed a functional assessment of safety risks while lodging alone during this extended time. This brief questionnaire identifies any deficits in a veteran’s activity of daily living that may influence safety while lodging alone. The assessment is documented in the EMR, and if any concerns are identified, these are discussed with the veteran and a team of medical providers. If necessary, interventions are put into place before the veteran’s return for treatment. Potential safeguards may include obtaining safety equipment (eg, walker and bath chair), identifying an appropriate caregiver, or referring the veteran to a skilled nursing facility for the duration of treatment.
Following the veteran’s consultation visits, he went home with a return date 2 weeks later to start treatment. The VAPSHCS CCNT discussed the plan of care with his local CCNT, which facilitated placement of his feeding tube and addressed other symptom management concerns. The local CCNT SW completed advanced directives with the veteran and coordinated his travel back to VAPSHCS to begin treatment.
During Treatment
Veterans traveling from other VA facilities are away from their primary care providers (PCPs) for a number of weeks. Other specialty providers see a veteran during cancer treatment; however, the CCNT ARNP supports primary care needs while the veteran is away from their home VA facility. The ARNP is able to address chronic or acute medical issues before the start of treatment to prevent delays in cancer care.
Once the veteran returned to VAPSHCS to initiate therapy, the CCNT ARNP completed a history and physical examination to identify and address any active medical problems and document past medical history and current medication list in the EMR. This provides easy access to a thorough and complete baseline to both the oncology and radiation oncology providers. The ARNP examination revealed a new neck wound on the veteran, likely related to his cancer, and an urgent consult was placed to wound care. The otolaryngology, oncology, and radiation oncology departments were alerted to this development so they could assess the patient and adjust treatment plans as necessary. The veteran also required a refill of his blood pressure medication and had a number of questions regarding his upcoming treatment, which were addressed during the visit.
Within the first 2 days of the veteran’s return, he was scheduled to meet with the CCNT SW who reviewed and documented his advanced directive within the system, assessed his distress, provided therapeutic counseling, and completed the health benefit renewal form. Given the veteran’s financial status, the SW was able to help him apply for financial hardship to cover the costs of the care he had already received and assisted him with securing an appointment with the Social Security Administration (SSA) for disability benefits. The CCNT SW then helped the veteran complete a phone interview with the SSA and complete the application process. The SW also helped him complete the application for VA service-connected compensation and pension disability benefits.
Throughout his treatment course, the CCNT continued to be a resource for the veteran. Because he had PTSD and was uncomfortable attending support groups, the CCNT SW met with him weekly to provide counseling and psychosocial support. He stopped by the CCNT office on several occasions to report how he was doing, and the team provided assistance in obtaining supplies for his feeding tube and managing a complication that arose with his lodging. In preparation for his treatment completion and return home, the VAPSHCS CCNT communicated with his local CCNT to describe follow-up needs and ensure appropriate medical visits were scheduled. His travel home was arranged by the VAPSHCS PSA.
Treatment Completion
Before leaving VAPSHCS, the veteran was scheduled and seen in the clinic by the ARNP, where he received a written comprehensive treatment summary. The summary documented his cancer diagnosis, treatment, complications, and recommendations for follow-up care. He had the opportunity to ask questions about his treatment, and a clinical assessment was made for adverse effects. Appropriate interventions also were identified and addressed. A comprehensive treatment summary note was documented in the EMR and sent to his PCP and other medical specialists at his home facility to assure continuity of care.
The VAPSHCS CCNT continued to communicate weekly with the veteran’s home CCNT following his return, to ensure he received appropriate follow-up care and addressed questions and needs that arose. The veteran’s home CCNT continued to monitor the veteran for 1 year post treatment and communicate with VAPSHCS CCNT.
Conclusion
The VA is in a unique position to meet the needs of veterans by providing comprehensive care with sensitivity to military culture, access to a range of complicated benefits awarded to veterans, particularly those with servicerelated exposures or injuries, and specialists in diagnosis and treatment of physical and mental consequences of their service. Patient navigation helps ensure veterans can access these services, maintain continuity of care despite referrals across large geographic regions, and receive support while receiving cancer treatment at the VA.
Use of an interdisciplinary team, including an ARNP, RN, SW, and PSA is vital to fully address the wide range of physical, psychosocial, and practical barriers to care that a veteran may experience. Since September 2014, PSHCS has enrolled more than 500 veterans with CCNT, and nearly 200 are actively being followed and provided with navigation services at any given time (Figure 3). By proactively identifying and addressing barriers to care, the advocacy provided by CCNT has averted patient safety risks, made better use of limited veteran and VA resources, and provided patient-centered care to veterans.
Evaluation is currently underway to measure the impact of the program and develop metrics for the CCNT. Given the needs of the patient population, the team hopes to see further expansion of CCNT in order to reach more risk groups. Institutional support and funding for patient navigation should be a high priority as the VA strives to provide excellent, patient-centered care.
Acknowledgements
The authors would like first and foremost to give a special thank-you to the veterans for their service to our country. In addition, the authors would like to thank champions for the cancer care navigation team, including Dr. Daniel Wu, chief of oncology; and Dr. Peter Wu, cancer committee chair and surgical oncologist, and Sandra Solomon, nurse manager of the Cancer Care Clinic and inpatient cancer unit at VA Puget Sound Health Care System; Dr. Carol Sprague, staff physician and clinical lead VISN 20 Cancer Care Platform, Judy McConnachie, MPH, administrative director, Clinical Business Intelligence Northwest Innovation Center, VA Portland Health Care System in Portland, Oregon; and Tracy Weistreich, PhD, RN, associate director Patient Care Services at VA Roseburg Healthcare System in Roseburg, Oregon; and the VISN 20 Executive Cancer Care Platform Advisory Board.
The authors would also like to acknowledge all the VISN 20 network cancer care navigation teams at the following sites: Anchorage, Alaska; Boise, Idaho; Portland, Oregon; Roseburg, Oregon; Spokane, Washington; Walla Walla, Washington; and White City, Oregon. Team members at each site have been an integral part of the development and success of the VAPSHCS CCNT.
The authors are also grateful to all of the nurse coordinators and providers within all the specialty services at Puget Sound Health Care Systems, including oncology, radiation oncology, cancer care, otolaryngology, general surgery, palliative care, dental and primary care, for their collaboration with veteran care.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to continue reading.
1. Freeman HP. Patient navigation as a targeted intervention: for patients at high risk
for delays in cancer care. Cancer. 2015;121(22):3930-3932.
2. Moy B, Chabner BA. Patient navigator programs, cancer disparities, and the patient protection and affordable care act. Oncologist. 2011;16(7):926-929.
3. Meade CD, Wells KJ, Arevalo M, et al. Lay navigator model for impacting cancer health disparities. J Cancer Educ. 2014;29(3):449-457.
4. Fillion L, Cook S, Veillette AM, et al. Professional navigation: a comparative study of two Canadian models. Can Oncol Nurs J. 2012;22(4):257-277.
5. Lairson DR, Huo J, Ricks KA, Savas L, Fernández ME. The cost of implementing a 2-1-1 call center-based cancer control navigator program. Eval Program Plann. 2013:39:51-56.
6. Percac-Lima S, Cronin PR, Ryan DP, Chabner BA, Daly E, Kimball AB. Patient navigation based on predictive modeling decreases no-show rates in cancer care. Cancer. 2015;121(10):1662-1670.
7. Percac-Lima S, Ashburner JM, McCarthy AM, Piawah S, Atlas SJ. Patient navigation
to improve follow-up of abnormal mammograms among disadvantaged women. J Womens Health (Larchmt). 2015;24(2):138-143.
8. Ladabaum U, Mannalithara A, Jandorf L, Itzkowitz SH. Cost-effectiveness of patient navigation to increase adherence with screening colonoscopy among minority
individuals. Cancer. 2015;121(7):1088-1097.
9. Baliski C, McGahan CE, Liberto CM, et al. Influence of nurse navigation on wait times for breast cancer care in a Canadian regional cancer center. Am J Surg. 2014;207(5):686-691.
10. Hoffman JH, LaVerda NL, Young HA, et al. Patient navigation significantly reduces delays in breast cancer diagnosis in the District of Columbia. Cancer Epidemiol Biomarkers Prev. 2012;1(10):1655-1663
11. Kazis LE, Miller DR, Clark J, et al. Health-related quality of life in patients served by the Department of Veterans Affairs: results from the Veterans Health Study. Arch Intern Med. 1998;158(6):626-632.
12. Rogers WH, Kazis LE, Miller DR, et al. Comparing the health status of VA and non-VA ambulatory patients: the veterans health and medical outcome studies. J Ambul Care Manage. 2004;27(3):249-262.
13. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257.
14. Institute of Medicine (US) Committee to Review the Health Effects in Vietnam Veterans of Exposure to Herbicides. Veterans and Agent Orange: Health Effects of Herbicides Used in Vietnam. Washington, DC: National Academies Press; 1994.
15. Wachen JS, Patidar SM, Mulligan EA, Naik AD, Moye J. Cancer-related PTSD symptoms in a veteran sample: association with age, combat PTSD, and quality of life. Psychooncology. 2014;23(8):921-927.
16. Mulligan EA, Wachen JS, Naik AD, Gosian J, Moye J. Cancer as a criterion a traumatic stressor for veterans: prevalence and correlates. Psychol Trauma. 2014;6(suppl 1):S73-S81.
17. Dobie DJ, Kivlahan DR, Maynard C, Bush KR, Davis TM, Bradley KA. Posttraumatic stress disorder in female veterans: association with self-reported health problems and functional impairment. Arch Intern Med. 2004;164(4):394-400.
Note: Page numbers differ between the print issue and digital edition.
1. Freeman HP. Patient navigation as a targeted intervention: for patients at high risk
for delays in cancer care. Cancer. 2015;121(22):3930-3932.
2. Moy B, Chabner BA. Patient navigator programs, cancer disparities, and the patient protection and affordable care act. Oncologist. 2011;16(7):926-929.
3. Meade CD, Wells KJ, Arevalo M, et al. Lay navigator model for impacting cancer health disparities. J Cancer Educ. 2014;29(3):449-457.
4. Fillion L, Cook S, Veillette AM, et al. Professional navigation: a comparative study of two Canadian models. Can Oncol Nurs J. 2012;22(4):257-277.
5. Lairson DR, Huo J, Ricks KA, Savas L, Fernández ME. The cost of implementing a 2-1-1 call center-based cancer control navigator program. Eval Program Plann. 2013:39:51-56.
6. Percac-Lima S, Cronin PR, Ryan DP, Chabner BA, Daly E, Kimball AB. Patient navigation based on predictive modeling decreases no-show rates in cancer care. Cancer. 2015;121(10):1662-1670.
7. Percac-Lima S, Ashburner JM, McCarthy AM, Piawah S, Atlas SJ. Patient navigation
to improve follow-up of abnormal mammograms among disadvantaged women. J Womens Health (Larchmt). 2015;24(2):138-143.
8. Ladabaum U, Mannalithara A, Jandorf L, Itzkowitz SH. Cost-effectiveness of patient navigation to increase adherence with screening colonoscopy among minority
individuals. Cancer. 2015;121(7):1088-1097.
9. Baliski C, McGahan CE, Liberto CM, et al. Influence of nurse navigation on wait times for breast cancer care in a Canadian regional cancer center. Am J Surg. 2014;207(5):686-691.
10. Hoffman JH, LaVerda NL, Young HA, et al. Patient navigation significantly reduces delays in breast cancer diagnosis in the District of Columbia. Cancer Epidemiol Biomarkers Prev. 2012;1(10):1655-1663
11. Kazis LE, Miller DR, Clark J, et al. Health-related quality of life in patients served by the Department of Veterans Affairs: results from the Veterans Health Study. Arch Intern Med. 1998;158(6):626-632.
12. Rogers WH, Kazis LE, Miller DR, et al. Comparing the health status of VA and non-VA ambulatory patients: the veterans health and medical outcome studies. J Ambul Care Manage. 2004;27(3):249-262.
13. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257.
14. Institute of Medicine (US) Committee to Review the Health Effects in Vietnam Veterans of Exposure to Herbicides. Veterans and Agent Orange: Health Effects of Herbicides Used in Vietnam. Washington, DC: National Academies Press; 1994.
15. Wachen JS, Patidar SM, Mulligan EA, Naik AD, Moye J. Cancer-related PTSD symptoms in a veteran sample: association with age, combat PTSD, and quality of life. Psychooncology. 2014;23(8):921-927.
16. Mulligan EA, Wachen JS, Naik AD, Gosian J, Moye J. Cancer as a criterion a traumatic stressor for veterans: prevalence and correlates. Psychol Trauma. 2014;6(suppl 1):S73-S81.
17. Dobie DJ, Kivlahan DR, Maynard C, Bush KR, Davis TM, Bradley KA. Posttraumatic stress disorder in female veterans: association with self-reported health problems and functional impairment. Arch Intern Med. 2004;164(4):394-400.
Note: Page numbers differ between the print issue and digital edition.
MAVERIC Precision Oncology Program
Federal Practitioner talks one-on-one with Louis Fiore, MD, MPH, the executive director of the Massachusetts Veterans Epidemiology Research and Information Center (MAVERIC), which the VA created as 1 of 3 epidemiological research centers. At MAVERIC Fiore is spearheading a precision oncology program, which is developing precision oncology best practices; enhancing patient and provider engagement; and fostering collaboration among the VA, National Cancer Institute, academia, and other health care systems to provide cancer patients with access to clinical trial participation.
Federal Practitioner talks one-on-one with Louis Fiore, MD, MPH, the executive director of the Massachusetts Veterans Epidemiology Research and Information Center (MAVERIC), which the VA created as 1 of 3 epidemiological research centers. At MAVERIC Fiore is spearheading a precision oncology program, which is developing precision oncology best practices; enhancing patient and provider engagement; and fostering collaboration among the VA, National Cancer Institute, academia, and other health care systems to provide cancer patients with access to clinical trial participation.
Federal Practitioner talks one-on-one with Louis Fiore, MD, MPH, the executive director of the Massachusetts Veterans Epidemiology Research and Information Center (MAVERIC), which the VA created as 1 of 3 epidemiological research centers. At MAVERIC Fiore is spearheading a precision oncology program, which is developing precision oncology best practices; enhancing patient and provider engagement; and fostering collaboration among the VA, National Cancer Institute, academia, and other health care systems to provide cancer patients with access to clinical trial participation.
Implementation of a Precision Oncology Program as an Exemplar of a Learning Health Care System in the VA
Traditional research methods, well suited for scientific discovery and drug development, fall short of providing health care systems with pragmatic information in 2 important ways: Current funding and institutions cannot support comparative effectiveness studies in sufficient numbers to answer the plethora of important clinical questions that confront health care providers (HCPs). The resultant knowledge gap manifests in treatment variability based on clinician impression rather than on direct evidence. A second equally important deficiency is the inability to make full use of the knowledge acquired in treating past patients to determine the best treatment option for the current patient.
Digitization of medical records, creation of health care system corporate data warehouses, and state-of-the-art analytical tools already allow for this revolutionary approach to patient care. Obstructing progress, however, is a lack of understanding by health care system managers and HCPs of the capability of the approach, and unfamiliarity with the requisite informatics by traditional medical researchers. Furthermore the regulatory approach is tilted against the reuse of medical record data for learning and toward strict adherence to patient confidentiality.
The Case for VA Leadership
A solution to these 2 central dilemmas will result in continued health care improvement and, arguably, meaningful cost reduction through elimination of inferior treatments and optimization of individual patient care strategies. Since the current research culture does not reward such accomplishments, the responsibility for moving forward is left squarely on the health care systems. Said differently, a health care research budget that is a small fraction (5%) of health care expenditures is undersized and too culturally foreign for the task.1
A critical attribute that enables the VA to promote progress to the benefit of both veterans and taxpayers is an accountable care organization incentive to use a long horizon and invest in opportunities that reduce overall cost and improve outcomes for its beneficiariesover their entire lifespan. Although this feature is common to a handful of other large health care providers (Kaiser Permanente, Intermountain Healthcare, Mayo Clinic), those systems lack the assets fundamental to solution design that are broadly represented across VA medical centers: a staff, culture, and apparatus in support of research at most medical centers; an integrated electronic health record (EHR) for data access; and a patient population receptive to participating in activities that will aid fellow veterans.
Ongoing Programs
The VA is in an excellent position to create an efficient and scalable apparatus to perform comparative effectiveness studies.The Point-of-Care clinical trials program, proposed and championed by the Massachusetts Veterans Epidemiology Research and Information Center (MAVERIC) and supported by the VA Cooperative Studies Program, embeds low-risk clinical trials directly into the clinical ecosystem with a resultant decreased cost and increased relevance owing to study designs driven by current patient care processes.
This methodology and program is applauded by the Institute of Medicine and the Society for Clinical Trials, and each has invited MAVERIC to present at national meetings and roundtable discussions.2 Designation as a research “transformative initiative” by the VA Office of Research and Development (ORD) provided sufficient support to culminate in the imminent launch of the first national VA Point-of-Care Clinical Trial—the Diuretic Comparison Study. The VA is proceeding with this trial at 50 VA sites for a significantly lower cost.(VA Cooperative Studies Program study #597, methods manuscript in preparation). Results will inform the optimal initial treatment for hypertension and impact the care of millions of veterans and nonveterans.
Precision Oncology Program
The VA Precision Oncology Program (POP), initiated in VISN 1 and funded through a clinical care budget, goes a step further toward creating learning opportunities. The POP sequences the DNA of tumor tissue from veterans newly diagnosed with cancer to determine the DNA mutations responsible for the tumor development and behavior. Armed with this information, HCPs can optimize therapy based on mutation status by the delivery of drugs that are targeted against particular gene products.
Systematic implementation of the POP across all VAMCs will reduce disparities in cancer care induced by variation in medical center familiarity with treatment options. Features supported by the POP include enhanced enrollment of patients into clinical trials of novel targeted therapeutics and sharing of patient outcomes data to assist in decision support for future patients. In addition, this approach could facilitate the creation of a national VA database of cancer patient characteristics, tumor mutations, and cancer-related treatments and outcomes to accelerate the pace of discovery in VA cancer care.
Million Veteran Program
The Million Veteran Program (MVP) is a VA ORD initiative that asks veterans to share their medical data, lifestyle, and genetic data with researchers to allow for the discovery of correlations between their genetic profile and their health, disease and response totreatments. Currently more than 430,000 veterans have agreed to participate and have donated data and blood samples, and researchers are performing the first projects to use this resource.
Although the knowledge gained from these studies will be indirectly relevant to veterans in general the MVP presents an opportunity to present specific findings to individual participants that will directly affect their care. While reuse of the MVP resource for precision medicine is under consideration, there are important cultural and technical barriers that must be addressed. Like POP, integration of the MVP research program with clinical care should be carried out with consideration of a community of stakeholders and not driven exclusively by a research agenda.
Challenges in Moving Forward
Central to the implementation of a learning mechanism in health care systems is the recognition by administrators of the importance of the activity and appreciation of the business argument favoring the investment. This runs counter to the current notion of separate silos for health care and medical research whereby health care systems are liberated from the cost of investigation but then suffer from a dearth of knowledge relevant to their operation.
Additionally, research enterprises are not structured for such activities. Academic investigators are incentivized to create knowledge and generate publications and they understand best the currency of grant funding. Their world is not geared to reinvent or engineer solutions for health care systems. In light of these considerations, a decentralized approach that creates institutions for local learning needs to be developed and “owned” by individual and groups of medical centers with engagement of administration, patient, scientific, and community stakeholders. The Patient-Centered Outcome Research Institute (PCORI) and the consortia it has funded, PCOR-Net, have adopted this approach.3
Importantly, a new set of ethical and regulatory standards that distinguish it from traditional research must accompany progress in the creation of a learning health care system (LHS). Sharing of patient data to benefit fellow patients must come to be expected and without the formalized sharing agreements that are required in traditional research activities. Although the digitization of medical records makes most of what this article discusses possible, execution requires access to information technology resources and a talented staff.
More than a decade ago, the decision was made to dis-integrate the Office of Information Technology from VHA. This was executed with no provision to support the small army of VA clinician-informaticists who had done much in support of patient care, including the creation of the initial iteration of the VA EHR. Although the VA includes small pockets of this clinical informatics culture throughout its organization, the community has been largely silenced and taken refuge at academic affiliates. Access to VA information systems and funding opportunities for development and implementation of tools essential for learning will draw this intellectual capital back to the VA and allow for the VA to lead in this critical arena.
The VA Precision Oncology Program
Precision medicine is a medical model that incorporates the results of genetic diagnostic testing to customize or tailor medical decision making and treatment for the individual patient. Characteristics of the VA health care system that create a favored environment for introducing precision medicine include the single-payer model, where implementation decision and authority are centralized, a standardized EHR that enables informatics requirements, and a clinician and patient culture that supports innovation. To date, the benefits of precision medicine are most robust in cancer care. Under the leadership of Michael Mayo-Smith, MD, the VA New England Healthcare System has completed a regional pilot project in precision oncology that demonstrated feasibility of incorporating a precision medicine program in the clinical care environment.
For the majority of patients with lung cancer, DNA sequencing of tumor tissue identifies driver mutations—alterations believed responsible for tumor growth and behavior. The abundance of both driver and passenger mutations (those alterations whose significance is unknown) identified within an individual cancer specimen and the diversity of alterations found across the spectrum of all patients with cancer virtually assures the unique genetic profile (hence behavior) of any given patient’s tumor. The new generation of antineoplastic agents are targeted therapies that disrupt the downstream effects of these alterations and result in improved anticancer effects and reduced toxicity compared with conventional chemotherapy. The POP approach to cancer treatment determines the mutation profile of malignancies and identifies targeted therapies with the highest likelihood of treatment success. Although many driver mutation-targeted therapy combinations have been FDA approved, many more are in development and are available only as investigational agents.
Work Accomplished
Developed over the past 2 years in VISN 1, POP is a demonstration project that standardizes the processes necessary to deliver precision oncology care for veterans with lung cancer. With approval of the cancer care specialist, targeted sequencing of cancer genes (multiple biomarker panels) is performed on formalinfixed, paraffin-embedded tissue from newly diagnosed lung cancers as part of routine POP cancer care. Samples are shipped within 48 hours of diagnosis to Personal Genome Diagnostics (CancerSelect-88 targeted genome panel: PGD, Baltimore, MD) or Personalis (ACE Extended Cancer Panel: Menlo Park, CA). Following the sequencing of the targeted gene regions for mutations, a formal report of identified genomic aberrations is collated, annotated, and transmitted for inclusion in patient medical records. Both PGD and Personalis use N-of-One (Lexington, MA) to curate the medical literature and provide mutation annotations. The VA Computerized Patient Record System shares mutation results with the treating clinician, and a consultation service, offered through Specialty Care Access Network-Extension for Community technology, is available to help clinicians incorporate the test results into a treatment plan for the patient.
The POP is highly interdisciplinary: design and implementation required buy-in and coordinated efforts from the clinical medicine, laboratory medicine, pathology, pharmacy, radiology, and research services as well as from contracting, human resources, information technology, and procurement. With more than 150 specimens processed, procedures for tissue selection, processing, shipment, and tracking have been refined, and the informatics challenges met.
A Learning Health Care System Approach
Although the standard of care in oncology is evolving to include sequencing for all solid tumors and hematologic malignancies, the lack of correlated mutation status, patient outcomes data available for analysis, and difficulties in identifying subjects eligible for clinical trials of novel therapeutics combine to slow progress. The former problem arises from the effort required to aggregate EHR data from disparate systems as well as technical and cultural barriers to data sharing. The latter problem stems from the relative rarity of patients (and the difficulty identifying them) with a given mutation that determines eligibility for a clinical trial of a particular targeted therapy.
The POP attempts to overcome these limitations by embracing the principles of a LHS with clinical trials embedded to the extent possible in the clinical care ecosystem. The creation of a precision oncology data repository derived largely from the VA Corporate Data Warehouse makes correlated data available. This repository contains patient demographics and comorbidities, tumor features and mutation status, treatments, and outcomes. Data in the repository are used to both inform individual patient care (ie, what can we learn from past patients that would inform the care of the present patient?) and to allow for generalizable discovery and validation (ie, traditional data-mining research). Given a sufficiently large POP population, clinical trial-matching algorithms will identify patients available for any number of studies open for enrollment, thus reducing the existing bottleneck in clinical trial participation.
Rationale for a National Program
Numerous organizations, including the National Comprehensive Cancer Network, the American Society of Clinical Oncology Institute for Quality, and the Society for Gynecologic Oncology, already propose tumor sequencing as the standard of care for a variety of malignancies, and there is much to suggest that additional recommendations will be forthcoming.4-6 Expanding the VISN 1 POP across the nation provides a mechanism to minimize disparities in the delivery of precision oncology across the VA. The POP will afford opportunities to create VA-centric expertise derived from the POP data repository and filtered through a national tumor board. The POP will also expand opportunities for patients to participate in clinical trials and receive state-of-the-art treatments beyond what can be offered regionally.
Both knowledge generation and the creation of a large-scale clinical trial operation require the numbers of patients that only a national POP can achieve. The economies of scale introduced by wide participation will also reduce the cost of tumor sequencing, therapeutics, and infrastructure development and will eliminate otherwise duplicate efforts that would be required to create a number of smaller regional activities. Importantly, a national POP with sufficient voice would be far more effective at moving forward the LHS agenda.
Research Activities
For the majority of POP participants, the best hope for improved quality and quantity of life lies with targeted therapeutics that are under development and available only through research protocols. The VISN 1 Clinical Trial Network (directed by Mary Brophy, MD) has developed an Oncology Consortium that includes facilities both within and outside of VISN 1. The consortium has partnered with the National Cancer Institute through a storefront mechanism with the Southwest Oncology Group to become the first national VA cancer consortium to participate in intergroup protocols. Novel therapeutics will be available to POP participants through this and other partnerships with a variety of industry sponsors.
Novel, efficient, and nationally scalable mechanisms have been proposed to facilitate clinician participation and patient enrollment in clinical trials. Additionally, MAVERIC is working with the VA Central Institutional Review Board to advance a distributed enrollment innovation, which brings the clinical trial to the patient rather than have patients travel to facilities where studies are open.
Conclusion
Unique features of the VHA enable a national rollout of the POP, which VISN 1 successfully piloted. The first of its kind effort for precision medicine within the VA holds the promise of delivering cutting-edge, life-enhancing therapy to cancer patients.
This interdisciplinary program incorporates LHS principles so that delivery of care is accompanied by analytics that can be applied to decision making for future patients. Participation in clinical trials, facilitated by the consortium model, is a cardinal feature of the POP. Opportunity exists to explore novel trial designs that meet the unique challenges presented in precision medicine, where therapeutics tailored to uncommon mutations limit patient availability.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Research America. Truth and Consequences: Health R&D Spending in the U.S. (FY11-12). Research America Website. http://www.researchamerica.org/sites/default/files/uploads/healthdollar12.pdf. Accessed January 14, 2016.
2. Institute of Medicine. Large Simple Trials and Knowledge Generation in a Learning Healthcare System. Washington, DC: National Academies Press;2013:93-114.
3. Patient-Centered Outcomes Research Institute. About us. Patient-Centered Outcomes Research Institute Website. http://www.pcori.org/about-us. Updated October 14, 2014. Accessed January 21, 2016.
4. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). nonsmall cell lung cancer. National Comprehensive Cancer Network Website. http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Updated January 12, 2016. Accessed January 21, 2016.
5. Leighl NB, Rekhtman N, Biermann WA, et al. Molecular testing for selection of patients with lung cancer for epidermal growth factor receptor and anaplastic lymphoma kinase tyrosine kinase inhibitors: American Society of Clinical Oncology endorsement of the College of American Pathologists/International Association for the study of lung cancer/association for molecular pathology guideline. J Clin Oncol. 2014;32(32):3673-3679.
6. Society of Gynecologic Oncology. SGO clinical practice statement: next generation cancer gene panels versus gene by gene testing. Society of Gynecologic Oncology Website. https://www.sgo.org/clinical-practice/guidelines/next-generation-cancer-gene-panels-versus-gene-by-gene-testing/. Updated March 2014. Accessed January 21, 2016.
Note: Page numbers differ between the print issue and digital edition.
Traditional research methods, well suited for scientific discovery and drug development, fall short of providing health care systems with pragmatic information in 2 important ways: Current funding and institutions cannot support comparative effectiveness studies in sufficient numbers to answer the plethora of important clinical questions that confront health care providers (HCPs). The resultant knowledge gap manifests in treatment variability based on clinician impression rather than on direct evidence. A second equally important deficiency is the inability to make full use of the knowledge acquired in treating past patients to determine the best treatment option for the current patient.
Digitization of medical records, creation of health care system corporate data warehouses, and state-of-the-art analytical tools already allow for this revolutionary approach to patient care. Obstructing progress, however, is a lack of understanding by health care system managers and HCPs of the capability of the approach, and unfamiliarity with the requisite informatics by traditional medical researchers. Furthermore the regulatory approach is tilted against the reuse of medical record data for learning and toward strict adherence to patient confidentiality.
The Case for VA Leadership
A solution to these 2 central dilemmas will result in continued health care improvement and, arguably, meaningful cost reduction through elimination of inferior treatments and optimization of individual patient care strategies. Since the current research culture does not reward such accomplishments, the responsibility for moving forward is left squarely on the health care systems. Said differently, a health care research budget that is a small fraction (5%) of health care expenditures is undersized and too culturally foreign for the task.1
A critical attribute that enables the VA to promote progress to the benefit of both veterans and taxpayers is an accountable care organization incentive to use a long horizon and invest in opportunities that reduce overall cost and improve outcomes for its beneficiariesover their entire lifespan. Although this feature is common to a handful of other large health care providers (Kaiser Permanente, Intermountain Healthcare, Mayo Clinic), those systems lack the assets fundamental to solution design that are broadly represented across VA medical centers: a staff, culture, and apparatus in support of research at most medical centers; an integrated electronic health record (EHR) for data access; and a patient population receptive to participating in activities that will aid fellow veterans.
Ongoing Programs
The VA is in an excellent position to create an efficient and scalable apparatus to perform comparative effectiveness studies.The Point-of-Care clinical trials program, proposed and championed by the Massachusetts Veterans Epidemiology Research and Information Center (MAVERIC) and supported by the VA Cooperative Studies Program, embeds low-risk clinical trials directly into the clinical ecosystem with a resultant decreased cost and increased relevance owing to study designs driven by current patient care processes.
This methodology and program is applauded by the Institute of Medicine and the Society for Clinical Trials, and each has invited MAVERIC to present at national meetings and roundtable discussions.2 Designation as a research “transformative initiative” by the VA Office of Research and Development (ORD) provided sufficient support to culminate in the imminent launch of the first national VA Point-of-Care Clinical Trial—the Diuretic Comparison Study. The VA is proceeding with this trial at 50 VA sites for a significantly lower cost.(VA Cooperative Studies Program study #597, methods manuscript in preparation). Results will inform the optimal initial treatment for hypertension and impact the care of millions of veterans and nonveterans.
Precision Oncology Program
The VA Precision Oncology Program (POP), initiated in VISN 1 and funded through a clinical care budget, goes a step further toward creating learning opportunities. The POP sequences the DNA of tumor tissue from veterans newly diagnosed with cancer to determine the DNA mutations responsible for the tumor development and behavior. Armed with this information, HCPs can optimize therapy based on mutation status by the delivery of drugs that are targeted against particular gene products.
Systematic implementation of the POP across all VAMCs will reduce disparities in cancer care induced by variation in medical center familiarity with treatment options. Features supported by the POP include enhanced enrollment of patients into clinical trials of novel targeted therapeutics and sharing of patient outcomes data to assist in decision support for future patients. In addition, this approach could facilitate the creation of a national VA database of cancer patient characteristics, tumor mutations, and cancer-related treatments and outcomes to accelerate the pace of discovery in VA cancer care.
Million Veteran Program
The Million Veteran Program (MVP) is a VA ORD initiative that asks veterans to share their medical data, lifestyle, and genetic data with researchers to allow for the discovery of correlations between their genetic profile and their health, disease and response totreatments. Currently more than 430,000 veterans have agreed to participate and have donated data and blood samples, and researchers are performing the first projects to use this resource.
Although the knowledge gained from these studies will be indirectly relevant to veterans in general the MVP presents an opportunity to present specific findings to individual participants that will directly affect their care. While reuse of the MVP resource for precision medicine is under consideration, there are important cultural and technical barriers that must be addressed. Like POP, integration of the MVP research program with clinical care should be carried out with consideration of a community of stakeholders and not driven exclusively by a research agenda.
Challenges in Moving Forward
Central to the implementation of a learning mechanism in health care systems is the recognition by administrators of the importance of the activity and appreciation of the business argument favoring the investment. This runs counter to the current notion of separate silos for health care and medical research whereby health care systems are liberated from the cost of investigation but then suffer from a dearth of knowledge relevant to their operation.
Additionally, research enterprises are not structured for such activities. Academic investigators are incentivized to create knowledge and generate publications and they understand best the currency of grant funding. Their world is not geared to reinvent or engineer solutions for health care systems. In light of these considerations, a decentralized approach that creates institutions for local learning needs to be developed and “owned” by individual and groups of medical centers with engagement of administration, patient, scientific, and community stakeholders. The Patient-Centered Outcome Research Institute (PCORI) and the consortia it has funded, PCOR-Net, have adopted this approach.3
Importantly, a new set of ethical and regulatory standards that distinguish it from traditional research must accompany progress in the creation of a learning health care system (LHS). Sharing of patient data to benefit fellow patients must come to be expected and without the formalized sharing agreements that are required in traditional research activities. Although the digitization of medical records makes most of what this article discusses possible, execution requires access to information technology resources and a talented staff.
More than a decade ago, the decision was made to dis-integrate the Office of Information Technology from VHA. This was executed with no provision to support the small army of VA clinician-informaticists who had done much in support of patient care, including the creation of the initial iteration of the VA EHR. Although the VA includes small pockets of this clinical informatics culture throughout its organization, the community has been largely silenced and taken refuge at academic affiliates. Access to VA information systems and funding opportunities for development and implementation of tools essential for learning will draw this intellectual capital back to the VA and allow for the VA to lead in this critical arena.
The VA Precision Oncology Program
Precision medicine is a medical model that incorporates the results of genetic diagnostic testing to customize or tailor medical decision making and treatment for the individual patient. Characteristics of the VA health care system that create a favored environment for introducing precision medicine include the single-payer model, where implementation decision and authority are centralized, a standardized EHR that enables informatics requirements, and a clinician and patient culture that supports innovation. To date, the benefits of precision medicine are most robust in cancer care. Under the leadership of Michael Mayo-Smith, MD, the VA New England Healthcare System has completed a regional pilot project in precision oncology that demonstrated feasibility of incorporating a precision medicine program in the clinical care environment.
For the majority of patients with lung cancer, DNA sequencing of tumor tissue identifies driver mutations—alterations believed responsible for tumor growth and behavior. The abundance of both driver and passenger mutations (those alterations whose significance is unknown) identified within an individual cancer specimen and the diversity of alterations found across the spectrum of all patients with cancer virtually assures the unique genetic profile (hence behavior) of any given patient’s tumor. The new generation of antineoplastic agents are targeted therapies that disrupt the downstream effects of these alterations and result in improved anticancer effects and reduced toxicity compared with conventional chemotherapy. The POP approach to cancer treatment determines the mutation profile of malignancies and identifies targeted therapies with the highest likelihood of treatment success. Although many driver mutation-targeted therapy combinations have been FDA approved, many more are in development and are available only as investigational agents.
Work Accomplished
Developed over the past 2 years in VISN 1, POP is a demonstration project that standardizes the processes necessary to deliver precision oncology care for veterans with lung cancer. With approval of the cancer care specialist, targeted sequencing of cancer genes (multiple biomarker panels) is performed on formalinfixed, paraffin-embedded tissue from newly diagnosed lung cancers as part of routine POP cancer care. Samples are shipped within 48 hours of diagnosis to Personal Genome Diagnostics (CancerSelect-88 targeted genome panel: PGD, Baltimore, MD) or Personalis (ACE Extended Cancer Panel: Menlo Park, CA). Following the sequencing of the targeted gene regions for mutations, a formal report of identified genomic aberrations is collated, annotated, and transmitted for inclusion in patient medical records. Both PGD and Personalis use N-of-One (Lexington, MA) to curate the medical literature and provide mutation annotations. The VA Computerized Patient Record System shares mutation results with the treating clinician, and a consultation service, offered through Specialty Care Access Network-Extension for Community technology, is available to help clinicians incorporate the test results into a treatment plan for the patient.
The POP is highly interdisciplinary: design and implementation required buy-in and coordinated efforts from the clinical medicine, laboratory medicine, pathology, pharmacy, radiology, and research services as well as from contracting, human resources, information technology, and procurement. With more than 150 specimens processed, procedures for tissue selection, processing, shipment, and tracking have been refined, and the informatics challenges met.
A Learning Health Care System Approach
Although the standard of care in oncology is evolving to include sequencing for all solid tumors and hematologic malignancies, the lack of correlated mutation status, patient outcomes data available for analysis, and difficulties in identifying subjects eligible for clinical trials of novel therapeutics combine to slow progress. The former problem arises from the effort required to aggregate EHR data from disparate systems as well as technical and cultural barriers to data sharing. The latter problem stems from the relative rarity of patients (and the difficulty identifying them) with a given mutation that determines eligibility for a clinical trial of a particular targeted therapy.
The POP attempts to overcome these limitations by embracing the principles of a LHS with clinical trials embedded to the extent possible in the clinical care ecosystem. The creation of a precision oncology data repository derived largely from the VA Corporate Data Warehouse makes correlated data available. This repository contains patient demographics and comorbidities, tumor features and mutation status, treatments, and outcomes. Data in the repository are used to both inform individual patient care (ie, what can we learn from past patients that would inform the care of the present patient?) and to allow for generalizable discovery and validation (ie, traditional data-mining research). Given a sufficiently large POP population, clinical trial-matching algorithms will identify patients available for any number of studies open for enrollment, thus reducing the existing bottleneck in clinical trial participation.
Rationale for a National Program
Numerous organizations, including the National Comprehensive Cancer Network, the American Society of Clinical Oncology Institute for Quality, and the Society for Gynecologic Oncology, already propose tumor sequencing as the standard of care for a variety of malignancies, and there is much to suggest that additional recommendations will be forthcoming.4-6 Expanding the VISN 1 POP across the nation provides a mechanism to minimize disparities in the delivery of precision oncology across the VA. The POP will afford opportunities to create VA-centric expertise derived from the POP data repository and filtered through a national tumor board. The POP will also expand opportunities for patients to participate in clinical trials and receive state-of-the-art treatments beyond what can be offered regionally.
Both knowledge generation and the creation of a large-scale clinical trial operation require the numbers of patients that only a national POP can achieve. The economies of scale introduced by wide participation will also reduce the cost of tumor sequencing, therapeutics, and infrastructure development and will eliminate otherwise duplicate efforts that would be required to create a number of smaller regional activities. Importantly, a national POP with sufficient voice would be far more effective at moving forward the LHS agenda.
Research Activities
For the majority of POP participants, the best hope for improved quality and quantity of life lies with targeted therapeutics that are under development and available only through research protocols. The VISN 1 Clinical Trial Network (directed by Mary Brophy, MD) has developed an Oncology Consortium that includes facilities both within and outside of VISN 1. The consortium has partnered with the National Cancer Institute through a storefront mechanism with the Southwest Oncology Group to become the first national VA cancer consortium to participate in intergroup protocols. Novel therapeutics will be available to POP participants through this and other partnerships with a variety of industry sponsors.
Novel, efficient, and nationally scalable mechanisms have been proposed to facilitate clinician participation and patient enrollment in clinical trials. Additionally, MAVERIC is working with the VA Central Institutional Review Board to advance a distributed enrollment innovation, which brings the clinical trial to the patient rather than have patients travel to facilities where studies are open.
Conclusion
Unique features of the VHA enable a national rollout of the POP, which VISN 1 successfully piloted. The first of its kind effort for precision medicine within the VA holds the promise of delivering cutting-edge, life-enhancing therapy to cancer patients.
This interdisciplinary program incorporates LHS principles so that delivery of care is accompanied by analytics that can be applied to decision making for future patients. Participation in clinical trials, facilitated by the consortium model, is a cardinal feature of the POP. Opportunity exists to explore novel trial designs that meet the unique challenges presented in precision medicine, where therapeutics tailored to uncommon mutations limit patient availability.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
Traditional research methods, well suited for scientific discovery and drug development, fall short of providing health care systems with pragmatic information in 2 important ways: Current funding and institutions cannot support comparative effectiveness studies in sufficient numbers to answer the plethora of important clinical questions that confront health care providers (HCPs). The resultant knowledge gap manifests in treatment variability based on clinician impression rather than on direct evidence. A second equally important deficiency is the inability to make full use of the knowledge acquired in treating past patients to determine the best treatment option for the current patient.
Digitization of medical records, creation of health care system corporate data warehouses, and state-of-the-art analytical tools already allow for this revolutionary approach to patient care. Obstructing progress, however, is a lack of understanding by health care system managers and HCPs of the capability of the approach, and unfamiliarity with the requisite informatics by traditional medical researchers. Furthermore the regulatory approach is tilted against the reuse of medical record data for learning and toward strict adherence to patient confidentiality.
The Case for VA Leadership
A solution to these 2 central dilemmas will result in continued health care improvement and, arguably, meaningful cost reduction through elimination of inferior treatments and optimization of individual patient care strategies. Since the current research culture does not reward such accomplishments, the responsibility for moving forward is left squarely on the health care systems. Said differently, a health care research budget that is a small fraction (5%) of health care expenditures is undersized and too culturally foreign for the task.1
A critical attribute that enables the VA to promote progress to the benefit of both veterans and taxpayers is an accountable care organization incentive to use a long horizon and invest in opportunities that reduce overall cost and improve outcomes for its beneficiariesover their entire lifespan. Although this feature is common to a handful of other large health care providers (Kaiser Permanente, Intermountain Healthcare, Mayo Clinic), those systems lack the assets fundamental to solution design that are broadly represented across VA medical centers: a staff, culture, and apparatus in support of research at most medical centers; an integrated electronic health record (EHR) for data access; and a patient population receptive to participating in activities that will aid fellow veterans.
Ongoing Programs
The VA is in an excellent position to create an efficient and scalable apparatus to perform comparative effectiveness studies.The Point-of-Care clinical trials program, proposed and championed by the Massachusetts Veterans Epidemiology Research and Information Center (MAVERIC) and supported by the VA Cooperative Studies Program, embeds low-risk clinical trials directly into the clinical ecosystem with a resultant decreased cost and increased relevance owing to study designs driven by current patient care processes.
This methodology and program is applauded by the Institute of Medicine and the Society for Clinical Trials, and each has invited MAVERIC to present at national meetings and roundtable discussions.2 Designation as a research “transformative initiative” by the VA Office of Research and Development (ORD) provided sufficient support to culminate in the imminent launch of the first national VA Point-of-Care Clinical Trial—the Diuretic Comparison Study. The VA is proceeding with this trial at 50 VA sites for a significantly lower cost.(VA Cooperative Studies Program study #597, methods manuscript in preparation). Results will inform the optimal initial treatment for hypertension and impact the care of millions of veterans and nonveterans.
Precision Oncology Program
The VA Precision Oncology Program (POP), initiated in VISN 1 and funded through a clinical care budget, goes a step further toward creating learning opportunities. The POP sequences the DNA of tumor tissue from veterans newly diagnosed with cancer to determine the DNA mutations responsible for the tumor development and behavior. Armed with this information, HCPs can optimize therapy based on mutation status by the delivery of drugs that are targeted against particular gene products.
Systematic implementation of the POP across all VAMCs will reduce disparities in cancer care induced by variation in medical center familiarity with treatment options. Features supported by the POP include enhanced enrollment of patients into clinical trials of novel targeted therapeutics and sharing of patient outcomes data to assist in decision support for future patients. In addition, this approach could facilitate the creation of a national VA database of cancer patient characteristics, tumor mutations, and cancer-related treatments and outcomes to accelerate the pace of discovery in VA cancer care.
Million Veteran Program
The Million Veteran Program (MVP) is a VA ORD initiative that asks veterans to share their medical data, lifestyle, and genetic data with researchers to allow for the discovery of correlations between their genetic profile and their health, disease and response totreatments. Currently more than 430,000 veterans have agreed to participate and have donated data and blood samples, and researchers are performing the first projects to use this resource.
Although the knowledge gained from these studies will be indirectly relevant to veterans in general the MVP presents an opportunity to present specific findings to individual participants that will directly affect their care. While reuse of the MVP resource for precision medicine is under consideration, there are important cultural and technical barriers that must be addressed. Like POP, integration of the MVP research program with clinical care should be carried out with consideration of a community of stakeholders and not driven exclusively by a research agenda.
Challenges in Moving Forward
Central to the implementation of a learning mechanism in health care systems is the recognition by administrators of the importance of the activity and appreciation of the business argument favoring the investment. This runs counter to the current notion of separate silos for health care and medical research whereby health care systems are liberated from the cost of investigation but then suffer from a dearth of knowledge relevant to their operation.
Additionally, research enterprises are not structured for such activities. Academic investigators are incentivized to create knowledge and generate publications and they understand best the currency of grant funding. Their world is not geared to reinvent or engineer solutions for health care systems. In light of these considerations, a decentralized approach that creates institutions for local learning needs to be developed and “owned” by individual and groups of medical centers with engagement of administration, patient, scientific, and community stakeholders. The Patient-Centered Outcome Research Institute (PCORI) and the consortia it has funded, PCOR-Net, have adopted this approach.3
Importantly, a new set of ethical and regulatory standards that distinguish it from traditional research must accompany progress in the creation of a learning health care system (LHS). Sharing of patient data to benefit fellow patients must come to be expected and without the formalized sharing agreements that are required in traditional research activities. Although the digitization of medical records makes most of what this article discusses possible, execution requires access to information technology resources and a talented staff.
More than a decade ago, the decision was made to dis-integrate the Office of Information Technology from VHA. This was executed with no provision to support the small army of VA clinician-informaticists who had done much in support of patient care, including the creation of the initial iteration of the VA EHR. Although the VA includes small pockets of this clinical informatics culture throughout its organization, the community has been largely silenced and taken refuge at academic affiliates. Access to VA information systems and funding opportunities for development and implementation of tools essential for learning will draw this intellectual capital back to the VA and allow for the VA to lead in this critical arena.
The VA Precision Oncology Program
Precision medicine is a medical model that incorporates the results of genetic diagnostic testing to customize or tailor medical decision making and treatment for the individual patient. Characteristics of the VA health care system that create a favored environment for introducing precision medicine include the single-payer model, where implementation decision and authority are centralized, a standardized EHR that enables informatics requirements, and a clinician and patient culture that supports innovation. To date, the benefits of precision medicine are most robust in cancer care. Under the leadership of Michael Mayo-Smith, MD, the VA New England Healthcare System has completed a regional pilot project in precision oncology that demonstrated feasibility of incorporating a precision medicine program in the clinical care environment.
For the majority of patients with lung cancer, DNA sequencing of tumor tissue identifies driver mutations—alterations believed responsible for tumor growth and behavior. The abundance of both driver and passenger mutations (those alterations whose significance is unknown) identified within an individual cancer specimen and the diversity of alterations found across the spectrum of all patients with cancer virtually assures the unique genetic profile (hence behavior) of any given patient’s tumor. The new generation of antineoplastic agents are targeted therapies that disrupt the downstream effects of these alterations and result in improved anticancer effects and reduced toxicity compared with conventional chemotherapy. The POP approach to cancer treatment determines the mutation profile of malignancies and identifies targeted therapies with the highest likelihood of treatment success. Although many driver mutation-targeted therapy combinations have been FDA approved, many more are in development and are available only as investigational agents.
Work Accomplished
Developed over the past 2 years in VISN 1, POP is a demonstration project that standardizes the processes necessary to deliver precision oncology care for veterans with lung cancer. With approval of the cancer care specialist, targeted sequencing of cancer genes (multiple biomarker panels) is performed on formalinfixed, paraffin-embedded tissue from newly diagnosed lung cancers as part of routine POP cancer care. Samples are shipped within 48 hours of diagnosis to Personal Genome Diagnostics (CancerSelect-88 targeted genome panel: PGD, Baltimore, MD) or Personalis (ACE Extended Cancer Panel: Menlo Park, CA). Following the sequencing of the targeted gene regions for mutations, a formal report of identified genomic aberrations is collated, annotated, and transmitted for inclusion in patient medical records. Both PGD and Personalis use N-of-One (Lexington, MA) to curate the medical literature and provide mutation annotations. The VA Computerized Patient Record System shares mutation results with the treating clinician, and a consultation service, offered through Specialty Care Access Network-Extension for Community technology, is available to help clinicians incorporate the test results into a treatment plan for the patient.
The POP is highly interdisciplinary: design and implementation required buy-in and coordinated efforts from the clinical medicine, laboratory medicine, pathology, pharmacy, radiology, and research services as well as from contracting, human resources, information technology, and procurement. With more than 150 specimens processed, procedures for tissue selection, processing, shipment, and tracking have been refined, and the informatics challenges met.
A Learning Health Care System Approach
Although the standard of care in oncology is evolving to include sequencing for all solid tumors and hematologic malignancies, the lack of correlated mutation status, patient outcomes data available for analysis, and difficulties in identifying subjects eligible for clinical trials of novel therapeutics combine to slow progress. The former problem arises from the effort required to aggregate EHR data from disparate systems as well as technical and cultural barriers to data sharing. The latter problem stems from the relative rarity of patients (and the difficulty identifying them) with a given mutation that determines eligibility for a clinical trial of a particular targeted therapy.
The POP attempts to overcome these limitations by embracing the principles of a LHS with clinical trials embedded to the extent possible in the clinical care ecosystem. The creation of a precision oncology data repository derived largely from the VA Corporate Data Warehouse makes correlated data available. This repository contains patient demographics and comorbidities, tumor features and mutation status, treatments, and outcomes. Data in the repository are used to both inform individual patient care (ie, what can we learn from past patients that would inform the care of the present patient?) and to allow for generalizable discovery and validation (ie, traditional data-mining research). Given a sufficiently large POP population, clinical trial-matching algorithms will identify patients available for any number of studies open for enrollment, thus reducing the existing bottleneck in clinical trial participation.
Rationale for a National Program
Numerous organizations, including the National Comprehensive Cancer Network, the American Society of Clinical Oncology Institute for Quality, and the Society for Gynecologic Oncology, already propose tumor sequencing as the standard of care for a variety of malignancies, and there is much to suggest that additional recommendations will be forthcoming.4-6 Expanding the VISN 1 POP across the nation provides a mechanism to minimize disparities in the delivery of precision oncology across the VA. The POP will afford opportunities to create VA-centric expertise derived from the POP data repository and filtered through a national tumor board. The POP will also expand opportunities for patients to participate in clinical trials and receive state-of-the-art treatments beyond what can be offered regionally.
Both knowledge generation and the creation of a large-scale clinical trial operation require the numbers of patients that only a national POP can achieve. The economies of scale introduced by wide participation will also reduce the cost of tumor sequencing, therapeutics, and infrastructure development and will eliminate otherwise duplicate efforts that would be required to create a number of smaller regional activities. Importantly, a national POP with sufficient voice would be far more effective at moving forward the LHS agenda.
Research Activities
For the majority of POP participants, the best hope for improved quality and quantity of life lies with targeted therapeutics that are under development and available only through research protocols. The VISN 1 Clinical Trial Network (directed by Mary Brophy, MD) has developed an Oncology Consortium that includes facilities both within and outside of VISN 1. The consortium has partnered with the National Cancer Institute through a storefront mechanism with the Southwest Oncology Group to become the first national VA cancer consortium to participate in intergroup protocols. Novel therapeutics will be available to POP participants through this and other partnerships with a variety of industry sponsors.
Novel, efficient, and nationally scalable mechanisms have been proposed to facilitate clinician participation and patient enrollment in clinical trials. Additionally, MAVERIC is working with the VA Central Institutional Review Board to advance a distributed enrollment innovation, which brings the clinical trial to the patient rather than have patients travel to facilities where studies are open.
Conclusion
Unique features of the VHA enable a national rollout of the POP, which VISN 1 successfully piloted. The first of its kind effort for precision medicine within the VA holds the promise of delivering cutting-edge, life-enhancing therapy to cancer patients.
This interdisciplinary program incorporates LHS principles so that delivery of care is accompanied by analytics that can be applied to decision making for future patients. Participation in clinical trials, facilitated by the consortium model, is a cardinal feature of the POP. Opportunity exists to explore novel trial designs that meet the unique challenges presented in precision medicine, where therapeutics tailored to uncommon mutations limit patient availability.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Research America. Truth and Consequences: Health R&D Spending in the U.S. (FY11-12). Research America Website. http://www.researchamerica.org/sites/default/files/uploads/healthdollar12.pdf. Accessed January 14, 2016.
2. Institute of Medicine. Large Simple Trials and Knowledge Generation in a Learning Healthcare System. Washington, DC: National Academies Press;2013:93-114.
3. Patient-Centered Outcomes Research Institute. About us. Patient-Centered Outcomes Research Institute Website. http://www.pcori.org/about-us. Updated October 14, 2014. Accessed January 21, 2016.
4. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). nonsmall cell lung cancer. National Comprehensive Cancer Network Website. http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Updated January 12, 2016. Accessed January 21, 2016.
5. Leighl NB, Rekhtman N, Biermann WA, et al. Molecular testing for selection of patients with lung cancer for epidermal growth factor receptor and anaplastic lymphoma kinase tyrosine kinase inhibitors: American Society of Clinical Oncology endorsement of the College of American Pathologists/International Association for the study of lung cancer/association for molecular pathology guideline. J Clin Oncol. 2014;32(32):3673-3679.
6. Society of Gynecologic Oncology. SGO clinical practice statement: next generation cancer gene panels versus gene by gene testing. Society of Gynecologic Oncology Website. https://www.sgo.org/clinical-practice/guidelines/next-generation-cancer-gene-panels-versus-gene-by-gene-testing/. Updated March 2014. Accessed January 21, 2016.
Note: Page numbers differ between the print issue and digital edition.
1. Research America. Truth and Consequences: Health R&D Spending in the U.S. (FY11-12). Research America Website. http://www.researchamerica.org/sites/default/files/uploads/healthdollar12.pdf. Accessed January 14, 2016.
2. Institute of Medicine. Large Simple Trials and Knowledge Generation in a Learning Healthcare System. Washington, DC: National Academies Press;2013:93-114.
3. Patient-Centered Outcomes Research Institute. About us. Patient-Centered Outcomes Research Institute Website. http://www.pcori.org/about-us. Updated October 14, 2014. Accessed January 21, 2016.
4. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). nonsmall cell lung cancer. National Comprehensive Cancer Network Website. http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Updated January 12, 2016. Accessed January 21, 2016.
5. Leighl NB, Rekhtman N, Biermann WA, et al. Molecular testing for selection of patients with lung cancer for epidermal growth factor receptor and anaplastic lymphoma kinase tyrosine kinase inhibitors: American Society of Clinical Oncology endorsement of the College of American Pathologists/International Association for the study of lung cancer/association for molecular pathology guideline. J Clin Oncol. 2014;32(32):3673-3679.
6. Society of Gynecologic Oncology. SGO clinical practice statement: next generation cancer gene panels versus gene by gene testing. Society of Gynecologic Oncology Website. https://www.sgo.org/clinical-practice/guidelines/next-generation-cancer-gene-panels-versus-gene-by-gene-testing/. Updated March 2014. Accessed January 21, 2016.
Note: Page numbers differ between the print issue and digital edition.