User login
A Systems Engineering and Decision-Support Tool to Enhance Care of Veterans Diagnosed With Prostate Cancer
In the U.S. in 2015, there were more than 220,800 new cases of prostate cancer and about 27,000 deaths due to prostate cancer. Across the VHA, prostate cancer is the most common nonskin cancer malignancy, and more than 25,000 patients are diagnosed yearly.1 Patients who receive treatment for prostate cancer have excellent rates of disease-specific survival: nearly 100% at 5 years, 99% at 10 years, and 94% at 15 years.
Prostate cancer is one of several cancers that can be treated successfully with radiotherapy alone, and its success or failure is defined by a discrete numerical value from the prostate specific antigen (PSA) blood test. Failure occurs when the PSA is 2.0 ng/mL greater than the lowest PSA value posttreatment.2 Multiple clinical trials have used this method to determine whether or not a certain intervention is successful.
Although high rates of survival and clear biochemical indicators exist, patients diagnosed with and treated for prostate cancer are at significant risk of PSA failure. The risk can range from 5% to 70% by 10 years, depending on the the treatment modality, risk group, and series reported.3 These patients require long-term follow-up for disease recurrence and management of adverse effects. The current guidelines recommend annual follow-up care 5 years after treatment.4
The number of veterans requiring follow-up care for prostate cancer constitutes a disproportionately large share of visits compared with those of other cancers, such as cancers of the head and neck region, chest, or gastrointestinal system, and there are many challenges to providing quality long-term care. Veterans in rural locations face barriers to accessing follow-up care for effective management.
Missed appointments can compromise long-term care, escalating the risk of nonadherence over time. Missed appointments occur commonly and may negatively impact outcomes and can restrict care for other patients.5 In a recently published article by Percac-Lima and colleagues, no-show rates among 5 cancer center clinics at the Massachusetts General Hospital were as high as 10%.6
Missed appointments have also been associated with decreased quality of care and increased resource use.7 Patients with prostate cancer who miss follow-up visits are at risk for having their cancer progress to the point it becomes symptomatic and no longer treatable with salvage therapies. These patients also risk lost efficacy of treatments that are still available.
Due to these challenges, automated PSA tracking systems can be an effective way to ensure that quality, longterm care is provided to the patient. The purpose of the PSA tracking system is to identify patients who require intervention before they present with clinical problems. A PSA tracking system helps prevent patients being inappropriately lost to follow-up or missing a needed followup PSA blood test. The tracker would serve to correctly identify, among thousands or millions of patients in the electronic medical record system (EMR), which patients were at risk of failure or active failing biochemically by triggering an alert to the cancer specialist to assess that patient’s chart and determine whether a higher level of intervention is required. It could also serve to avoid unnecessary travel or inconvenience to a patient whose prostate cancer disease status can correctly be confirmed as under control by a simple blood test and related to the patient by phone, letter, or online.
Prostate-specific antigen trackers have been used to monitor patients for postprostatectomy treatment failures on a small scale in Ireland.8 For a PSA tracker to be successful, the system must have access to all posttreatment PSA data. The VHA is uniquely positioned to leverage this information because most patients who receive treatment for prostate cancer at a VHA facility stay within the VHA system for follow-up care. All laboratory data are also collected and stored in the EMR system, which is sent daily to the VA Corporate Data Warehouse (CDW).
Project Proposal
In November 2014, the Office of Rural Health and the National Radiation Oncology Program Office issued a request for proposal for projects that would improve follow-up care for rural patients with prostate cancer following treatment with radiotherapy. A team of health care providers at the Hunter Holmes McGuire VAMC drafted a proposal to address this problem. Veterans Engineering Resource Centers (VERCs) in Pittsburgh and New England were also included in the proposal as key collaborators. Staff from these 2 centers brought expertise in analytics, implementation, and project management to help rapidly innovate and implement a PSA tracking system.
The proposal was submitted on time and required approval at multiple levels, including facility and VISN leadership. It was essential that the perceived value of the proposal be readily apparent to all stakeholders, or the necessary approvals would not have been obtainable.
The proposal was accepted, and funds were transferred in February 2015. Four core team members led rapid cycle design and prototyping of the PSA tracking system. The project lead and sponsor was a radiation oncologist and service line chief at the Hunter Holmes Mc-Guire VAMC who provided overall strategy, direction, and clinical domain knowledge. A VERC engineer provided project management and analytic expertise, and a VERC developer designed code to pull data from the VA CDW and led design of the user interface. Finally, a nurse practitioner dedicated numerous hours to review charts, contact patients, write notes, and provide user feedback on the system.
Development
The purpose of the radiation oncology-centered PSA tracking system within the VA was to identify patients who require intervention following definitive treatment with radiotherapy before they present with clinical problems from disease recurrence. The PSA tracker that the authors developed was based on a relatively simple algorithm that sorts through thousands of patient records and identifies patients who had a diagnosis of prostate cancer but did not have metastatic disease, were treated at the Hunter Homes McGuire VAMC with radiation therapy, were not seen in clinic within the past 400 days, and did not have a PSA drawn within 450 days or had a rising PSA of 0.5 or more above the lowest PSA value posttreatment. In other words, the tracker uses the power of the CDW to successfully identify the exact charts that need to be reviewed and helped ensure that patients were not lost to follow-up or did not receive appropriate care. Without the PSA tracking system, providers would not know whether or not patients were being missed.
Development of the tracker required regular team meetings with well-defined, achievable goals. The team consisted of a physician as team leader, a biostatistician with structured query language experience who had access to the CDW, and a project manager with an industrial engineering background. The team met weekly. The project was broken into several components that were achieved in series and at times in parallel. The first goal was determining whether an algorithm could be written to correctly identify patients with prostate cancer treated with radiotherapy at the Hunter Holmes McGuire VAMC who did not have metastatic disease.
By using various values available within the CDW, such as ICD 9 codes, CPT codes, PSA laboratory values, dates, and other information, the authors were able to create a successful algorithm. The ability to complete the algorithm in a short time frame wasfacilitated by several factors: a very small group, weekly meetings, good communication, easy to understand concepts across all disciplines, ability to quickly determine whether the results of the algorithm were accurate or not, and high perceived value of the end product that served to motivate the team members. Each meeting ended with clear action items and a scheduled time for the next meeting. Throughout the design and implementation process, the team discussed any problems, planned solutions, and reviewed the status of project deliverables.
Results
The tracker has already been useful for reengaging patients in care and ensuring PSA testing is occurring at appropriate intervals. Of the more than 50,000 veterans currently alive who have received care at the Hunter Holmes McGuire VAMC, 1,158 were treated with radiotherapy definitively for prostate cancer. A total of 455 (39%) prostate cancer survivors had not been seen in the clinic in the past 13 months. Of these patients, 294 were being followed appropriately elsewhere within the VA system. Meanwhile, 161 neither had a PSA level nor a prostate cancer follow-up appointment recorded in the past 13 months anywhere within the entire VA system. This yielded a loss-to-follow-up rate of 14% (161/1,158).
The authors found that 21 (13%) of patients had a PSA level > 2.0 ng/mL above the posttreatment nadir.9 The authors were able to review the charts of these 21 patients to assess whether or not they required or were suitable for salvage brachytherapy. Of these, 1 has been set up for salvage high-dose rate brachytherapy treatment. Out of 50,000 patients, the PSA tracker algorithm facilitated a focus on the 21 patients who were most likely to be in need, making it possible for a nurse practitioner and physician to spend just 3 hours looking at charts instead of 3,000 hours.
Sustained use of the tracker is critically important to the Hunter Holmes McGuire VAMC project team and for the care of its veterans. Funds to support sustaining the program have been approved for fiscal year 2016. Efforts are underway to try to scale up the program and test the feasibility of disseminating the program across the enterprise. The authors estimate that an experienced advanced care provider would spend about 8 hours a week reviewing charts, contacting patients in the program, sending letters, and reviewing nuanced cases. The program would still benefit from increased automation as well as identifying a method for obtaining appropriate workload credit for this unique program.
The next phase of development will focus on improving the user interface and allowing easier transfer of information between the tracker and notes within the Computerized Patient Record System. The team will also look into automating additional parts of the process but feels that a clinician (ideally a nurse practitioner or physician assistant working with the radiation oncologist) must be part of the team, because clinical decisions must be made based on multiple variables and patient preferences.
The development of this PSA tracking system has significant future implications for improving biochemical control and extending patient survival. The tracker could be easily adapted to monitor prostatectomy patients and PSA failures requiring early intervention with salvage radiotherapy. It has been shown in several publications that early treatment with radiotherapy while PSA is relatively low results in higher rates of long-term biochemical control.10-22
Conclusions
Access to the VA CDW was essential for the success of the PSA tracking system. Furthermore, veteran patients with prostate cancer tend toward a high rate of adherence and typically stay within the system. Prostate cancer is one of the few cancers where disease recurrence is detected and determined by a quantitative laboratory value, which lends itself well to objective arithmetical tracking and detection.
Patients with prostate cancer are at risk of recurrence years after their treatment and require a long-term follow-up that includes annual PSA checks. Identifying patients who have missed follow-up appointments and not had their PSA checked is essential for combating prostate cancer recurrences. The VA CDW makes it possible to track the majority of the patients with prostate cancer who are treated in the system and identify those most in need of early treatment or early intervention before they become
symptomatic.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. American Cancer Society. What are the key statistics about prostate cancer? American Cancer Society Website. http://www.cancer.org/cancer/prostatecancer/detailedguide/prostate-cancer key-statistics. Last revised March 12, 2015. Accessed January 11, 2016.
2. Roach M III, Hanks G, Thames H Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65(4):965-974.
3. Grimm P, Billiet I, Bostwick D, et al. Comparative analysis of prostate-specific antigen free survival outcomes for patients with low, intermediate and high risk prostate cancer treatment by radical therapy. Results from the Prostate Cancer Results Study Group. BJU Int. 2012;109(suppl 1):22-29.
4. Resnick MJ, Lacchetti C, Bergman J, et al. Prostate cancer survivorship care guideline: American Society of Clinical Oncology Clinical Practice Guidelines endorsement. J Clin Oncol. 2015;33(9):1078-1085.
5. Husain-Gambles M, Neal RD, Dempsey O, Lawlor DA, Hodgson J. Missed appointments in primary care: questionnaire and focus group study of health professionals. Br J Gen Pract. 2004;54(499):108-113.
6. Percac-Lima S, Cronin PR, Ryan DP, Chabner BA, Daly DA, Kimball AB. Patient navigation based on predictive modeling decreases no-show rates in cancer care. Cancer. 2015;121(10):1662-1670.
7. Hwang AS, Atlas SJ, Ashburner JM, et al. Appointment “no-shows” are an independent predictor of subsequent quality of care and resource utilization outcomes. J Gen Intern Med. 2015;30(10):1426-1433.
8. Hennessey DB, Lynn C, Templeton H, Chambers K, Mulholland C. The PSA tracker: a computerised health care system initiative in Northern Ireland. Ulster Med J. 2013;82(3):146-149.
9. Chang M, Troeschel S, DeSotto K, et al. Development of a Post-Radiotherapy Prostate-Specific Antigen Detection and Tracking System. Poster presented at: Genito-Urinary Cancers Symposium Annual Meeting; January 2016; San Francisco, CA.
10. Anscher MS, Clough R, Dodge R. Radiotherapy for a rising prostate-specific
antigen after radical prostatectomy: the first 10 years. Int J Radiat Oncol Biol
Phys. 2000;48(2):369-375.
11. Catton C, Gospodarowicz M, Warde P, et al. Adjuvant and salvage radiation
therapy after radical prostatectomy for adenocarcinoma of the prostate. Radiother
Oncol. 2001;59(1):51-60.
12. Cheung R, Kamat AM, de Crevoisier R, et al. Outcome of salvage radiotherapy for biochemical failure after radical prostatectomy with or without hormonal therapy. Int J Radiat Oncol Biol Phys. 2005;63(1):134-140.
13. Katz MS, Zelefsky MJ, Venkatraman ES, Hummer A, Leibal SA. Predictors of biochemical outcome with salvage conformal radiotherapy after radical prostatectomy for prostate cancer. J Clin Oncol. 2003;21(3):483-489.
14. Leventis AK, Shariat SF, Kattan MW, Butler EB, Wheeler TM, Slawin KM. Prediction of response to salvage radiation therapy in patients with prostate cancer recurrence after radical prostatectomy. J Clin Oncol. 2001;19(4):1030-1039.
15. Liauw SL, Webster WS, Pistenmaa DA, Roehrborn CG. Salvage radiotherapy for
biochemical failure of radical prostatectomy: a single-institution experience. Urology.
2003;61(6):1204-1210.
16. Maier J, Forman J, Tekyi-Mensah S, Bolton S, Patel R, Pontes JE. Salvage radiation
for a rising PSA following radical prostatectomy. Urol Oncol. 2004;22(1):50-56.
17. Perez CA, Michalski JM, Baglan K, Andriole G, Cui Q, Lockett MA. Radiation therapy for increasing prostate-specific antigen levels after radical prostatectomy. Clin Prostate Cancer. 2003;1(4):235-241.
18. Pisansky TM, Kozelsky TF, Myers RP, et al. Radiotherapy for isolated serum prostate specific antigen elevation after prostatectomy for prostate cancer. J Urol. 2000;163(3):845-850.
19. Song DY, Thompson TL, Ramakrishnan V, et al. Salvage radiotherapy for rising or
persistent PSA after radical prostatectomy. Urology. 2002;60(2):281-287.
20. Stephenson AJ, Shariat SF, Zelefsky MJ, et al. Salvage radiotherapy for recurrent prostate cancer after radical prostatectomy. JAMA. 2004;291(11):1325-1332.
21. Valicenti RK, Gomella LG, Ismail M, et al. Durable efficacy of early postoperative radiation therapy for high-risk pT3N0 prostate cancer: the importance of radiation dose. Urology. 1998;52(6):1034-1040.
22. Vicini FA, Ziaja EL, Kestin LL, et al. Treatment outcome with adjuvant and salvage irradiation after radical prostatectomy for prostate cancer. Urology. 1999;54(1):111-117.
Note: Page numbers differ between the print issue and digital edition.
In the U.S. in 2015, there were more than 220,800 new cases of prostate cancer and about 27,000 deaths due to prostate cancer. Across the VHA, prostate cancer is the most common nonskin cancer malignancy, and more than 25,000 patients are diagnosed yearly.1 Patients who receive treatment for prostate cancer have excellent rates of disease-specific survival: nearly 100% at 5 years, 99% at 10 years, and 94% at 15 years.
Prostate cancer is one of several cancers that can be treated successfully with radiotherapy alone, and its success or failure is defined by a discrete numerical value from the prostate specific antigen (PSA) blood test. Failure occurs when the PSA is 2.0 ng/mL greater than the lowest PSA value posttreatment.2 Multiple clinical trials have used this method to determine whether or not a certain intervention is successful.
Although high rates of survival and clear biochemical indicators exist, patients diagnosed with and treated for prostate cancer are at significant risk of PSA failure. The risk can range from 5% to 70% by 10 years, depending on the the treatment modality, risk group, and series reported.3 These patients require long-term follow-up for disease recurrence and management of adverse effects. The current guidelines recommend annual follow-up care 5 years after treatment.4
The number of veterans requiring follow-up care for prostate cancer constitutes a disproportionately large share of visits compared with those of other cancers, such as cancers of the head and neck region, chest, or gastrointestinal system, and there are many challenges to providing quality long-term care. Veterans in rural locations face barriers to accessing follow-up care for effective management.
Missed appointments can compromise long-term care, escalating the risk of nonadherence over time. Missed appointments occur commonly and may negatively impact outcomes and can restrict care for other patients.5 In a recently published article by Percac-Lima and colleagues, no-show rates among 5 cancer center clinics at the Massachusetts General Hospital were as high as 10%.6
Missed appointments have also been associated with decreased quality of care and increased resource use.7 Patients with prostate cancer who miss follow-up visits are at risk for having their cancer progress to the point it becomes symptomatic and no longer treatable with salvage therapies. These patients also risk lost efficacy of treatments that are still available.
Due to these challenges, automated PSA tracking systems can be an effective way to ensure that quality, longterm care is provided to the patient. The purpose of the PSA tracking system is to identify patients who require intervention before they present with clinical problems. A PSA tracking system helps prevent patients being inappropriately lost to follow-up or missing a needed followup PSA blood test. The tracker would serve to correctly identify, among thousands or millions of patients in the electronic medical record system (EMR), which patients were at risk of failure or active failing biochemically by triggering an alert to the cancer specialist to assess that patient’s chart and determine whether a higher level of intervention is required. It could also serve to avoid unnecessary travel or inconvenience to a patient whose prostate cancer disease status can correctly be confirmed as under control by a simple blood test and related to the patient by phone, letter, or online.
Prostate-specific antigen trackers have been used to monitor patients for postprostatectomy treatment failures on a small scale in Ireland.8 For a PSA tracker to be successful, the system must have access to all posttreatment PSA data. The VHA is uniquely positioned to leverage this information because most patients who receive treatment for prostate cancer at a VHA facility stay within the VHA system for follow-up care. All laboratory data are also collected and stored in the EMR system, which is sent daily to the VA Corporate Data Warehouse (CDW).
Project Proposal
In November 2014, the Office of Rural Health and the National Radiation Oncology Program Office issued a request for proposal for projects that would improve follow-up care for rural patients with prostate cancer following treatment with radiotherapy. A team of health care providers at the Hunter Holmes McGuire VAMC drafted a proposal to address this problem. Veterans Engineering Resource Centers (VERCs) in Pittsburgh and New England were also included in the proposal as key collaborators. Staff from these 2 centers brought expertise in analytics, implementation, and project management to help rapidly innovate and implement a PSA tracking system.
The proposal was submitted on time and required approval at multiple levels, including facility and VISN leadership. It was essential that the perceived value of the proposal be readily apparent to all stakeholders, or the necessary approvals would not have been obtainable.
The proposal was accepted, and funds were transferred in February 2015. Four core team members led rapid cycle design and prototyping of the PSA tracking system. The project lead and sponsor was a radiation oncologist and service line chief at the Hunter Holmes Mc-Guire VAMC who provided overall strategy, direction, and clinical domain knowledge. A VERC engineer provided project management and analytic expertise, and a VERC developer designed code to pull data from the VA CDW and led design of the user interface. Finally, a nurse practitioner dedicated numerous hours to review charts, contact patients, write notes, and provide user feedback on the system.
Development
The purpose of the radiation oncology-centered PSA tracking system within the VA was to identify patients who require intervention following definitive treatment with radiotherapy before they present with clinical problems from disease recurrence. The PSA tracker that the authors developed was based on a relatively simple algorithm that sorts through thousands of patient records and identifies patients who had a diagnosis of prostate cancer but did not have metastatic disease, were treated at the Hunter Homes McGuire VAMC with radiation therapy, were not seen in clinic within the past 400 days, and did not have a PSA drawn within 450 days or had a rising PSA of 0.5 or more above the lowest PSA value posttreatment. In other words, the tracker uses the power of the CDW to successfully identify the exact charts that need to be reviewed and helped ensure that patients were not lost to follow-up or did not receive appropriate care. Without the PSA tracking system, providers would not know whether or not patients were being missed.
Development of the tracker required regular team meetings with well-defined, achievable goals. The team consisted of a physician as team leader, a biostatistician with structured query language experience who had access to the CDW, and a project manager with an industrial engineering background. The team met weekly. The project was broken into several components that were achieved in series and at times in parallel. The first goal was determining whether an algorithm could be written to correctly identify patients with prostate cancer treated with radiotherapy at the Hunter Holmes McGuire VAMC who did not have metastatic disease.
By using various values available within the CDW, such as ICD 9 codes, CPT codes, PSA laboratory values, dates, and other information, the authors were able to create a successful algorithm. The ability to complete the algorithm in a short time frame wasfacilitated by several factors: a very small group, weekly meetings, good communication, easy to understand concepts across all disciplines, ability to quickly determine whether the results of the algorithm were accurate or not, and high perceived value of the end product that served to motivate the team members. Each meeting ended with clear action items and a scheduled time for the next meeting. Throughout the design and implementation process, the team discussed any problems, planned solutions, and reviewed the status of project deliverables.
Results
The tracker has already been useful for reengaging patients in care and ensuring PSA testing is occurring at appropriate intervals. Of the more than 50,000 veterans currently alive who have received care at the Hunter Holmes McGuire VAMC, 1,158 were treated with radiotherapy definitively for prostate cancer. A total of 455 (39%) prostate cancer survivors had not been seen in the clinic in the past 13 months. Of these patients, 294 were being followed appropriately elsewhere within the VA system. Meanwhile, 161 neither had a PSA level nor a prostate cancer follow-up appointment recorded in the past 13 months anywhere within the entire VA system. This yielded a loss-to-follow-up rate of 14% (161/1,158).
The authors found that 21 (13%) of patients had a PSA level > 2.0 ng/mL above the posttreatment nadir.9 The authors were able to review the charts of these 21 patients to assess whether or not they required or were suitable for salvage brachytherapy. Of these, 1 has been set up for salvage high-dose rate brachytherapy treatment. Out of 50,000 patients, the PSA tracker algorithm facilitated a focus on the 21 patients who were most likely to be in need, making it possible for a nurse practitioner and physician to spend just 3 hours looking at charts instead of 3,000 hours.
Sustained use of the tracker is critically important to the Hunter Holmes McGuire VAMC project team and for the care of its veterans. Funds to support sustaining the program have been approved for fiscal year 2016. Efforts are underway to try to scale up the program and test the feasibility of disseminating the program across the enterprise. The authors estimate that an experienced advanced care provider would spend about 8 hours a week reviewing charts, contacting patients in the program, sending letters, and reviewing nuanced cases. The program would still benefit from increased automation as well as identifying a method for obtaining appropriate workload credit for this unique program.
The next phase of development will focus on improving the user interface and allowing easier transfer of information between the tracker and notes within the Computerized Patient Record System. The team will also look into automating additional parts of the process but feels that a clinician (ideally a nurse practitioner or physician assistant working with the radiation oncologist) must be part of the team, because clinical decisions must be made based on multiple variables and patient preferences.
The development of this PSA tracking system has significant future implications for improving biochemical control and extending patient survival. The tracker could be easily adapted to monitor prostatectomy patients and PSA failures requiring early intervention with salvage radiotherapy. It has been shown in several publications that early treatment with radiotherapy while PSA is relatively low results in higher rates of long-term biochemical control.10-22
Conclusions
Access to the VA CDW was essential for the success of the PSA tracking system. Furthermore, veteran patients with prostate cancer tend toward a high rate of adherence and typically stay within the system. Prostate cancer is one of the few cancers where disease recurrence is detected and determined by a quantitative laboratory value, which lends itself well to objective arithmetical tracking and detection.
Patients with prostate cancer are at risk of recurrence years after their treatment and require a long-term follow-up that includes annual PSA checks. Identifying patients who have missed follow-up appointments and not had their PSA checked is essential for combating prostate cancer recurrences. The VA CDW makes it possible to track the majority of the patients with prostate cancer who are treated in the system and identify those most in need of early treatment or early intervention before they become
symptomatic.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
In the U.S. in 2015, there were more than 220,800 new cases of prostate cancer and about 27,000 deaths due to prostate cancer. Across the VHA, prostate cancer is the most common nonskin cancer malignancy, and more than 25,000 patients are diagnosed yearly.1 Patients who receive treatment for prostate cancer have excellent rates of disease-specific survival: nearly 100% at 5 years, 99% at 10 years, and 94% at 15 years.
Prostate cancer is one of several cancers that can be treated successfully with radiotherapy alone, and its success or failure is defined by a discrete numerical value from the prostate specific antigen (PSA) blood test. Failure occurs when the PSA is 2.0 ng/mL greater than the lowest PSA value posttreatment.2 Multiple clinical trials have used this method to determine whether or not a certain intervention is successful.
Although high rates of survival and clear biochemical indicators exist, patients diagnosed with and treated for prostate cancer are at significant risk of PSA failure. The risk can range from 5% to 70% by 10 years, depending on the the treatment modality, risk group, and series reported.3 These patients require long-term follow-up for disease recurrence and management of adverse effects. The current guidelines recommend annual follow-up care 5 years after treatment.4
The number of veterans requiring follow-up care for prostate cancer constitutes a disproportionately large share of visits compared with those of other cancers, such as cancers of the head and neck region, chest, or gastrointestinal system, and there are many challenges to providing quality long-term care. Veterans in rural locations face barriers to accessing follow-up care for effective management.
Missed appointments can compromise long-term care, escalating the risk of nonadherence over time. Missed appointments occur commonly and may negatively impact outcomes and can restrict care for other patients.5 In a recently published article by Percac-Lima and colleagues, no-show rates among 5 cancer center clinics at the Massachusetts General Hospital were as high as 10%.6
Missed appointments have also been associated with decreased quality of care and increased resource use.7 Patients with prostate cancer who miss follow-up visits are at risk for having their cancer progress to the point it becomes symptomatic and no longer treatable with salvage therapies. These patients also risk lost efficacy of treatments that are still available.
Due to these challenges, automated PSA tracking systems can be an effective way to ensure that quality, longterm care is provided to the patient. The purpose of the PSA tracking system is to identify patients who require intervention before they present with clinical problems. A PSA tracking system helps prevent patients being inappropriately lost to follow-up or missing a needed followup PSA blood test. The tracker would serve to correctly identify, among thousands or millions of patients in the electronic medical record system (EMR), which patients were at risk of failure or active failing biochemically by triggering an alert to the cancer specialist to assess that patient’s chart and determine whether a higher level of intervention is required. It could also serve to avoid unnecessary travel or inconvenience to a patient whose prostate cancer disease status can correctly be confirmed as under control by a simple blood test and related to the patient by phone, letter, or online.
Prostate-specific antigen trackers have been used to monitor patients for postprostatectomy treatment failures on a small scale in Ireland.8 For a PSA tracker to be successful, the system must have access to all posttreatment PSA data. The VHA is uniquely positioned to leverage this information because most patients who receive treatment for prostate cancer at a VHA facility stay within the VHA system for follow-up care. All laboratory data are also collected and stored in the EMR system, which is sent daily to the VA Corporate Data Warehouse (CDW).
Project Proposal
In November 2014, the Office of Rural Health and the National Radiation Oncology Program Office issued a request for proposal for projects that would improve follow-up care for rural patients with prostate cancer following treatment with radiotherapy. A team of health care providers at the Hunter Holmes McGuire VAMC drafted a proposal to address this problem. Veterans Engineering Resource Centers (VERCs) in Pittsburgh and New England were also included in the proposal as key collaborators. Staff from these 2 centers brought expertise in analytics, implementation, and project management to help rapidly innovate and implement a PSA tracking system.
The proposal was submitted on time and required approval at multiple levels, including facility and VISN leadership. It was essential that the perceived value of the proposal be readily apparent to all stakeholders, or the necessary approvals would not have been obtainable.
The proposal was accepted, and funds were transferred in February 2015. Four core team members led rapid cycle design and prototyping of the PSA tracking system. The project lead and sponsor was a radiation oncologist and service line chief at the Hunter Holmes Mc-Guire VAMC who provided overall strategy, direction, and clinical domain knowledge. A VERC engineer provided project management and analytic expertise, and a VERC developer designed code to pull data from the VA CDW and led design of the user interface. Finally, a nurse practitioner dedicated numerous hours to review charts, contact patients, write notes, and provide user feedback on the system.
Development
The purpose of the radiation oncology-centered PSA tracking system within the VA was to identify patients who require intervention following definitive treatment with radiotherapy before they present with clinical problems from disease recurrence. The PSA tracker that the authors developed was based on a relatively simple algorithm that sorts through thousands of patient records and identifies patients who had a diagnosis of prostate cancer but did not have metastatic disease, were treated at the Hunter Homes McGuire VAMC with radiation therapy, were not seen in clinic within the past 400 days, and did not have a PSA drawn within 450 days or had a rising PSA of 0.5 or more above the lowest PSA value posttreatment. In other words, the tracker uses the power of the CDW to successfully identify the exact charts that need to be reviewed and helped ensure that patients were not lost to follow-up or did not receive appropriate care. Without the PSA tracking system, providers would not know whether or not patients were being missed.
Development of the tracker required regular team meetings with well-defined, achievable goals. The team consisted of a physician as team leader, a biostatistician with structured query language experience who had access to the CDW, and a project manager with an industrial engineering background. The team met weekly. The project was broken into several components that were achieved in series and at times in parallel. The first goal was determining whether an algorithm could be written to correctly identify patients with prostate cancer treated with radiotherapy at the Hunter Holmes McGuire VAMC who did not have metastatic disease.
By using various values available within the CDW, such as ICD 9 codes, CPT codes, PSA laboratory values, dates, and other information, the authors were able to create a successful algorithm. The ability to complete the algorithm in a short time frame wasfacilitated by several factors: a very small group, weekly meetings, good communication, easy to understand concepts across all disciplines, ability to quickly determine whether the results of the algorithm were accurate or not, and high perceived value of the end product that served to motivate the team members. Each meeting ended with clear action items and a scheduled time for the next meeting. Throughout the design and implementation process, the team discussed any problems, planned solutions, and reviewed the status of project deliverables.
Results
The tracker has already been useful for reengaging patients in care and ensuring PSA testing is occurring at appropriate intervals. Of the more than 50,000 veterans currently alive who have received care at the Hunter Holmes McGuire VAMC, 1,158 were treated with radiotherapy definitively for prostate cancer. A total of 455 (39%) prostate cancer survivors had not been seen in the clinic in the past 13 months. Of these patients, 294 were being followed appropriately elsewhere within the VA system. Meanwhile, 161 neither had a PSA level nor a prostate cancer follow-up appointment recorded in the past 13 months anywhere within the entire VA system. This yielded a loss-to-follow-up rate of 14% (161/1,158).
The authors found that 21 (13%) of patients had a PSA level > 2.0 ng/mL above the posttreatment nadir.9 The authors were able to review the charts of these 21 patients to assess whether or not they required or were suitable for salvage brachytherapy. Of these, 1 has been set up for salvage high-dose rate brachytherapy treatment. Out of 50,000 patients, the PSA tracker algorithm facilitated a focus on the 21 patients who were most likely to be in need, making it possible for a nurse practitioner and physician to spend just 3 hours looking at charts instead of 3,000 hours.
Sustained use of the tracker is critically important to the Hunter Holmes McGuire VAMC project team and for the care of its veterans. Funds to support sustaining the program have been approved for fiscal year 2016. Efforts are underway to try to scale up the program and test the feasibility of disseminating the program across the enterprise. The authors estimate that an experienced advanced care provider would spend about 8 hours a week reviewing charts, contacting patients in the program, sending letters, and reviewing nuanced cases. The program would still benefit from increased automation as well as identifying a method for obtaining appropriate workload credit for this unique program.
The next phase of development will focus on improving the user interface and allowing easier transfer of information between the tracker and notes within the Computerized Patient Record System. The team will also look into automating additional parts of the process but feels that a clinician (ideally a nurse practitioner or physician assistant working with the radiation oncologist) must be part of the team, because clinical decisions must be made based on multiple variables and patient preferences.
The development of this PSA tracking system has significant future implications for improving biochemical control and extending patient survival. The tracker could be easily adapted to monitor prostatectomy patients and PSA failures requiring early intervention with salvage radiotherapy. It has been shown in several publications that early treatment with radiotherapy while PSA is relatively low results in higher rates of long-term biochemical control.10-22
Conclusions
Access to the VA CDW was essential for the success of the PSA tracking system. Furthermore, veteran patients with prostate cancer tend toward a high rate of adherence and typically stay within the system. Prostate cancer is one of the few cancers where disease recurrence is detected and determined by a quantitative laboratory value, which lends itself well to objective arithmetical tracking and detection.
Patients with prostate cancer are at risk of recurrence years after their treatment and require a long-term follow-up that includes annual PSA checks. Identifying patients who have missed follow-up appointments and not had their PSA checked is essential for combating prostate cancer recurrences. The VA CDW makes it possible to track the majority of the patients with prostate cancer who are treated in the system and identify those most in need of early treatment or early intervention before they become
symptomatic.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. American Cancer Society. What are the key statistics about prostate cancer? American Cancer Society Website. http://www.cancer.org/cancer/prostatecancer/detailedguide/prostate-cancer key-statistics. Last revised March 12, 2015. Accessed January 11, 2016.
2. Roach M III, Hanks G, Thames H Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65(4):965-974.
3. Grimm P, Billiet I, Bostwick D, et al. Comparative analysis of prostate-specific antigen free survival outcomes for patients with low, intermediate and high risk prostate cancer treatment by radical therapy. Results from the Prostate Cancer Results Study Group. BJU Int. 2012;109(suppl 1):22-29.
4. Resnick MJ, Lacchetti C, Bergman J, et al. Prostate cancer survivorship care guideline: American Society of Clinical Oncology Clinical Practice Guidelines endorsement. J Clin Oncol. 2015;33(9):1078-1085.
5. Husain-Gambles M, Neal RD, Dempsey O, Lawlor DA, Hodgson J. Missed appointments in primary care: questionnaire and focus group study of health professionals. Br J Gen Pract. 2004;54(499):108-113.
6. Percac-Lima S, Cronin PR, Ryan DP, Chabner BA, Daly DA, Kimball AB. Patient navigation based on predictive modeling decreases no-show rates in cancer care. Cancer. 2015;121(10):1662-1670.
7. Hwang AS, Atlas SJ, Ashburner JM, et al. Appointment “no-shows” are an independent predictor of subsequent quality of care and resource utilization outcomes. J Gen Intern Med. 2015;30(10):1426-1433.
8. Hennessey DB, Lynn C, Templeton H, Chambers K, Mulholland C. The PSA tracker: a computerised health care system initiative in Northern Ireland. Ulster Med J. 2013;82(3):146-149.
9. Chang M, Troeschel S, DeSotto K, et al. Development of a Post-Radiotherapy Prostate-Specific Antigen Detection and Tracking System. Poster presented at: Genito-Urinary Cancers Symposium Annual Meeting; January 2016; San Francisco, CA.
10. Anscher MS, Clough R, Dodge R. Radiotherapy for a rising prostate-specific
antigen after radical prostatectomy: the first 10 years. Int J Radiat Oncol Biol
Phys. 2000;48(2):369-375.
11. Catton C, Gospodarowicz M, Warde P, et al. Adjuvant and salvage radiation
therapy after radical prostatectomy for adenocarcinoma of the prostate. Radiother
Oncol. 2001;59(1):51-60.
12. Cheung R, Kamat AM, de Crevoisier R, et al. Outcome of salvage radiotherapy for biochemical failure after radical prostatectomy with or without hormonal therapy. Int J Radiat Oncol Biol Phys. 2005;63(1):134-140.
13. Katz MS, Zelefsky MJ, Venkatraman ES, Hummer A, Leibal SA. Predictors of biochemical outcome with salvage conformal radiotherapy after radical prostatectomy for prostate cancer. J Clin Oncol. 2003;21(3):483-489.
14. Leventis AK, Shariat SF, Kattan MW, Butler EB, Wheeler TM, Slawin KM. Prediction of response to salvage radiation therapy in patients with prostate cancer recurrence after radical prostatectomy. J Clin Oncol. 2001;19(4):1030-1039.
15. Liauw SL, Webster WS, Pistenmaa DA, Roehrborn CG. Salvage radiotherapy for
biochemical failure of radical prostatectomy: a single-institution experience. Urology.
2003;61(6):1204-1210.
16. Maier J, Forman J, Tekyi-Mensah S, Bolton S, Patel R, Pontes JE. Salvage radiation
for a rising PSA following radical prostatectomy. Urol Oncol. 2004;22(1):50-56.
17. Perez CA, Michalski JM, Baglan K, Andriole G, Cui Q, Lockett MA. Radiation therapy for increasing prostate-specific antigen levels after radical prostatectomy. Clin Prostate Cancer. 2003;1(4):235-241.
18. Pisansky TM, Kozelsky TF, Myers RP, et al. Radiotherapy for isolated serum prostate specific antigen elevation after prostatectomy for prostate cancer. J Urol. 2000;163(3):845-850.
19. Song DY, Thompson TL, Ramakrishnan V, et al. Salvage radiotherapy for rising or
persistent PSA after radical prostatectomy. Urology. 2002;60(2):281-287.
20. Stephenson AJ, Shariat SF, Zelefsky MJ, et al. Salvage radiotherapy for recurrent prostate cancer after radical prostatectomy. JAMA. 2004;291(11):1325-1332.
21. Valicenti RK, Gomella LG, Ismail M, et al. Durable efficacy of early postoperative radiation therapy for high-risk pT3N0 prostate cancer: the importance of radiation dose. Urology. 1998;52(6):1034-1040.
22. Vicini FA, Ziaja EL, Kestin LL, et al. Treatment outcome with adjuvant and salvage irradiation after radical prostatectomy for prostate cancer. Urology. 1999;54(1):111-117.
Note: Page numbers differ between the print issue and digital edition.
1. American Cancer Society. What are the key statistics about prostate cancer? American Cancer Society Website. http://www.cancer.org/cancer/prostatecancer/detailedguide/prostate-cancer key-statistics. Last revised March 12, 2015. Accessed January 11, 2016.
2. Roach M III, Hanks G, Thames H Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65(4):965-974.
3. Grimm P, Billiet I, Bostwick D, et al. Comparative analysis of prostate-specific antigen free survival outcomes for patients with low, intermediate and high risk prostate cancer treatment by radical therapy. Results from the Prostate Cancer Results Study Group. BJU Int. 2012;109(suppl 1):22-29.
4. Resnick MJ, Lacchetti C, Bergman J, et al. Prostate cancer survivorship care guideline: American Society of Clinical Oncology Clinical Practice Guidelines endorsement. J Clin Oncol. 2015;33(9):1078-1085.
5. Husain-Gambles M, Neal RD, Dempsey O, Lawlor DA, Hodgson J. Missed appointments in primary care: questionnaire and focus group study of health professionals. Br J Gen Pract. 2004;54(499):108-113.
6. Percac-Lima S, Cronin PR, Ryan DP, Chabner BA, Daly DA, Kimball AB. Patient navigation based on predictive modeling decreases no-show rates in cancer care. Cancer. 2015;121(10):1662-1670.
7. Hwang AS, Atlas SJ, Ashburner JM, et al. Appointment “no-shows” are an independent predictor of subsequent quality of care and resource utilization outcomes. J Gen Intern Med. 2015;30(10):1426-1433.
8. Hennessey DB, Lynn C, Templeton H, Chambers K, Mulholland C. The PSA tracker: a computerised health care system initiative in Northern Ireland. Ulster Med J. 2013;82(3):146-149.
9. Chang M, Troeschel S, DeSotto K, et al. Development of a Post-Radiotherapy Prostate-Specific Antigen Detection and Tracking System. Poster presented at: Genito-Urinary Cancers Symposium Annual Meeting; January 2016; San Francisco, CA.
10. Anscher MS, Clough R, Dodge R. Radiotherapy for a rising prostate-specific
antigen after radical prostatectomy: the first 10 years. Int J Radiat Oncol Biol
Phys. 2000;48(2):369-375.
11. Catton C, Gospodarowicz M, Warde P, et al. Adjuvant and salvage radiation
therapy after radical prostatectomy for adenocarcinoma of the prostate. Radiother
Oncol. 2001;59(1):51-60.
12. Cheung R, Kamat AM, de Crevoisier R, et al. Outcome of salvage radiotherapy for biochemical failure after radical prostatectomy with or without hormonal therapy. Int J Radiat Oncol Biol Phys. 2005;63(1):134-140.
13. Katz MS, Zelefsky MJ, Venkatraman ES, Hummer A, Leibal SA. Predictors of biochemical outcome with salvage conformal radiotherapy after radical prostatectomy for prostate cancer. J Clin Oncol. 2003;21(3):483-489.
14. Leventis AK, Shariat SF, Kattan MW, Butler EB, Wheeler TM, Slawin KM. Prediction of response to salvage radiation therapy in patients with prostate cancer recurrence after radical prostatectomy. J Clin Oncol. 2001;19(4):1030-1039.
15. Liauw SL, Webster WS, Pistenmaa DA, Roehrborn CG. Salvage radiotherapy for
biochemical failure of radical prostatectomy: a single-institution experience. Urology.
2003;61(6):1204-1210.
16. Maier J, Forman J, Tekyi-Mensah S, Bolton S, Patel R, Pontes JE. Salvage radiation
for a rising PSA following radical prostatectomy. Urol Oncol. 2004;22(1):50-56.
17. Perez CA, Michalski JM, Baglan K, Andriole G, Cui Q, Lockett MA. Radiation therapy for increasing prostate-specific antigen levels after radical prostatectomy. Clin Prostate Cancer. 2003;1(4):235-241.
18. Pisansky TM, Kozelsky TF, Myers RP, et al. Radiotherapy for isolated serum prostate specific antigen elevation after prostatectomy for prostate cancer. J Urol. 2000;163(3):845-850.
19. Song DY, Thompson TL, Ramakrishnan V, et al. Salvage radiotherapy for rising or
persistent PSA after radical prostatectomy. Urology. 2002;60(2):281-287.
20. Stephenson AJ, Shariat SF, Zelefsky MJ, et al. Salvage radiotherapy for recurrent prostate cancer after radical prostatectomy. JAMA. 2004;291(11):1325-1332.
21. Valicenti RK, Gomella LG, Ismail M, et al. Durable efficacy of early postoperative radiation therapy for high-risk pT3N0 prostate cancer: the importance of radiation dose. Urology. 1998;52(6):1034-1040.
22. Vicini FA, Ziaja EL, Kestin LL, et al. Treatment outcome with adjuvant and salvage irradiation after radical prostatectomy for prostate cancer. Urology. 1999;54(1):111-117.
Note: Page numbers differ between the print issue and digital edition.
The ethics of ICDs: History and future directions
In 1975, Julia and Joseph Quinlan approached the administrator of St. Clare’s Hospital in Denville, New Jersey, and requested that the mechanical ventilator on which their adopted daughter, Karen, was dependent be turned off. Karen Ann Quinlan, 21 years old, was in a permanent vegetative state after a severe anoxic event, and her parents had been informed by the hospital’s medical staff that she would never regain consciousness.
To the Quinlans’ request to withdraw the ventilator, the hospital administrator replied, “You have to understand our position, Mrs. Quinlan. In this hospital we don’t kill people.”1
The administrator’s response was consistent with prevailing ethical and legal perspectives, analyses, and directives at that time related to discontinuation of life-sustaining treatment. In the mid-1970s, the American Medical Association’s position was that it was permissible to not put a patient on a ventilator (ie, a physician could withhold a life-sustaining treatment), but once a patient was on a ventilator, it was not permissible to take the patient off if the intention was to allow death to occur.1 However, the New Jersey Supreme Court ultimately found this distinction between withholding and withdrawing unconvincing, and ruled unanimously that Karen Quinlan’s ventilator could be turned off.2
THE HASTINGS CENTER REPORT: STOPPING IS THE SAME AS NOT STARTING
During the subsequent decade, further ethical analysis and additional legal cases resulted in new insights and more nuanced thinking about forgoing life-sustaining treatment.
These developments were summarized in a 1987 report by the Hastings Center,3 a leading bioethics research and policy institute. The report provided normative guidance for the termination of life-sustaining treatment and for the care of dying patients. It acknowledged that deciding not to start a life-sustaining treatment can emotionally and psychologically affect healthcare professionals differently than deciding to stop such a treatment. However, the report also asserted that there is no morally important difference between withholding and withdrawing such treatments.
Reflecting a partnership model between patients and professionals for healthcare decision-making, and affirming the ethical significance of both a burden-benefit analysis and patient autonomy, the report stated that when a patient or surrogate in collaboration with a responsible healthcare professional decides that a treatment under way and the life it supports have become more burdensome than beneficial to the patient, that is sufficient reason to stop. There is no ethical requirement that treatment, once initiated, must continue against the patient’s wishes or when the surrogate determines that it is more burdensome than beneficial from the patient’s perspective. In fact, imposing treatment in such circumstances violates the patient’s right to self-determination.3
The report noted further that, because of frequent uncertainty about the efficacy of proposed treatments, it is preferable to initiate time-limited trials of treatments and then later stop them if they prove ineffective or become overly burdensome from a patient’s perspective.
ICDs ARE LIKE OTHER LIFE-SUSTAINING THERAPIES
In this issue of Cleveland Clinic Journal of Medicine, Baibars et al4 address the question of how implantable cardioverter-defibrillators (ICDs) should be managed at the end of life. The historical events and developments recounted above regarding withdrawing life-sustaining technologies are an appropriate context for ethically assessing the management of ICDs for dying patients.
Obviously, ICDs are not ventilators, but like ventilators, they are life-sustaining therapy, as are dialysis machines, blood transfusions, medically supplied nutrition and hydration, ventricular assist devices, and other implantable electronic cardiac devices such as pacemakers. Each of these life-sustaining therapies, depending on a patient’s clinical condition, underlying illness, and comorbidities, can become a death-prolonging technology.
An ethical framework and analysis about whether to continue any life-sustaining therapy, including an ICD, must include an assessment of the benefit-to-burden ratio from the patient’s perspective. Does the therapy enhance or maintain a quality of life acceptable to the patient? Or has it become overly burdensome and does it maintain a quality of life the patient finds (or would find) unacceptable? If the latter is true, and especially in the context of an underlying terminal condition, then shifting the goals of care to focus on comfort is always appropriate and ethically justified. Treatments—including ICDs—that do not contribute to patient comfort should be withdrawn.
TOWARD COMPETENCY IN ETHICAL MANAGEMENT
Baibars et al note that much more needs to be done to enhance competencies, increase proficiencies, and mitigate the moral distress of healthcare professionals caring for dying patients with ICDs and other devices. To help clinicians achieve a personal and professional “comfort zone” for ethically managing patients with ICDs, we recommend that healthcare institutions, medical schools, and nursing schools take the following steps:
Develop comprehensive end-of-life policies, procedures, and protocols that incorporate specific guidance for managing cardiac devices and that have been endorsed by a hospital ethics committee. Such guidance can be informative and educational and can ensure that decisions and resulting actions (including stopping cardiac devices) are ethically supportable.
Provide more palliative care training in medical and nursing schools, residency programs, and continuing education activities so that front-line clinicians can deliver “basic,” “primary” palliative care not requiring specialty palliative medicine. This training, called for in the Institute of Medicine’s 2014 report, Dying in America,5 should include explicit ethics discussions about managing cardiac devices at the end of life.
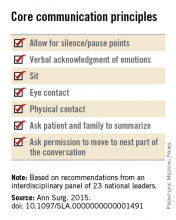
Provide ongoing training in communication skills needed for all patient-professional encounters. Effectively engaging patients in goals-of-care discussions, especially patients with life-limiting illnesses such as heart failure, cannot be achieved without these skills.
- Pence G. Comas: Karen Quinlan and Nancy Cruzan. In: Classic Cases in Medical Ethics: Accounts of Cases That Have Shaped Medical Ethics, With Philosophical, Legal, and Historical Backgrounds, 3rd edition. Boston: McGraw-Hill; 2000:29–55.
- In the matter of Karen Quinlan, an alleged incompetent. In re Quinlan. 70 N.J. 10, 355 A.2d 647 (1976), cert. denied, 429 U.S. 922 (1976).
- Wolf SM. Hastings Center. Guidelines on the Termination of Life-Sustaining Treatment and Care of the Dying: A Report by the Hastings Center. The Hastings Center: Briarcliff Manor, NY; 1987.
- Baibars MM, Alraies MC, Kabach A, Pritzker M. Can patients opt to turn off implantable cardioverter-defibrillators near the end of life? Cleve Clin J Med 2016; 83:97–98.
- National Academy of Sciences. Dying in America: improving quality and honoring individual p near the end of life. www.iom.edu/Reports/2014/Dying-In-America-Improving-Quality-and-Honoring-Individual-P-Near-the-End-of-Life.aspx. Accessed January 4, 2016.
In 1975, Julia and Joseph Quinlan approached the administrator of St. Clare’s Hospital in Denville, New Jersey, and requested that the mechanical ventilator on which their adopted daughter, Karen, was dependent be turned off. Karen Ann Quinlan, 21 years old, was in a permanent vegetative state after a severe anoxic event, and her parents had been informed by the hospital’s medical staff that she would never regain consciousness.
To the Quinlans’ request to withdraw the ventilator, the hospital administrator replied, “You have to understand our position, Mrs. Quinlan. In this hospital we don’t kill people.”1
The administrator’s response was consistent with prevailing ethical and legal perspectives, analyses, and directives at that time related to discontinuation of life-sustaining treatment. In the mid-1970s, the American Medical Association’s position was that it was permissible to not put a patient on a ventilator (ie, a physician could withhold a life-sustaining treatment), but once a patient was on a ventilator, it was not permissible to take the patient off if the intention was to allow death to occur.1 However, the New Jersey Supreme Court ultimately found this distinction between withholding and withdrawing unconvincing, and ruled unanimously that Karen Quinlan’s ventilator could be turned off.2
THE HASTINGS CENTER REPORT: STOPPING IS THE SAME AS NOT STARTING
During the subsequent decade, further ethical analysis and additional legal cases resulted in new insights and more nuanced thinking about forgoing life-sustaining treatment.
These developments were summarized in a 1987 report by the Hastings Center,3 a leading bioethics research and policy institute. The report provided normative guidance for the termination of life-sustaining treatment and for the care of dying patients. It acknowledged that deciding not to start a life-sustaining treatment can emotionally and psychologically affect healthcare professionals differently than deciding to stop such a treatment. However, the report also asserted that there is no morally important difference between withholding and withdrawing such treatments.
Reflecting a partnership model between patients and professionals for healthcare decision-making, and affirming the ethical significance of both a burden-benefit analysis and patient autonomy, the report stated that when a patient or surrogate in collaboration with a responsible healthcare professional decides that a treatment under way and the life it supports have become more burdensome than beneficial to the patient, that is sufficient reason to stop. There is no ethical requirement that treatment, once initiated, must continue against the patient’s wishes or when the surrogate determines that it is more burdensome than beneficial from the patient’s perspective. In fact, imposing treatment in such circumstances violates the patient’s right to self-determination.3
The report noted further that, because of frequent uncertainty about the efficacy of proposed treatments, it is preferable to initiate time-limited trials of treatments and then later stop them if they prove ineffective or become overly burdensome from a patient’s perspective.
ICDs ARE LIKE OTHER LIFE-SUSTAINING THERAPIES
In this issue of Cleveland Clinic Journal of Medicine, Baibars et al4 address the question of how implantable cardioverter-defibrillators (ICDs) should be managed at the end of life. The historical events and developments recounted above regarding withdrawing life-sustaining technologies are an appropriate context for ethically assessing the management of ICDs for dying patients.
Obviously, ICDs are not ventilators, but like ventilators, they are life-sustaining therapy, as are dialysis machines, blood transfusions, medically supplied nutrition and hydration, ventricular assist devices, and other implantable electronic cardiac devices such as pacemakers. Each of these life-sustaining therapies, depending on a patient’s clinical condition, underlying illness, and comorbidities, can become a death-prolonging technology.
An ethical framework and analysis about whether to continue any life-sustaining therapy, including an ICD, must include an assessment of the benefit-to-burden ratio from the patient’s perspective. Does the therapy enhance or maintain a quality of life acceptable to the patient? Or has it become overly burdensome and does it maintain a quality of life the patient finds (or would find) unacceptable? If the latter is true, and especially in the context of an underlying terminal condition, then shifting the goals of care to focus on comfort is always appropriate and ethically justified. Treatments—including ICDs—that do not contribute to patient comfort should be withdrawn.
TOWARD COMPETENCY IN ETHICAL MANAGEMENT
Baibars et al note that much more needs to be done to enhance competencies, increase proficiencies, and mitigate the moral distress of healthcare professionals caring for dying patients with ICDs and other devices. To help clinicians achieve a personal and professional “comfort zone” for ethically managing patients with ICDs, we recommend that healthcare institutions, medical schools, and nursing schools take the following steps:
Develop comprehensive end-of-life policies, procedures, and protocols that incorporate specific guidance for managing cardiac devices and that have been endorsed by a hospital ethics committee. Such guidance can be informative and educational and can ensure that decisions and resulting actions (including stopping cardiac devices) are ethically supportable.
Provide more palliative care training in medical and nursing schools, residency programs, and continuing education activities so that front-line clinicians can deliver “basic,” “primary” palliative care not requiring specialty palliative medicine. This training, called for in the Institute of Medicine’s 2014 report, Dying in America,5 should include explicit ethics discussions about managing cardiac devices at the end of life.
Provide ongoing training in communication skills needed for all patient-professional encounters. Effectively engaging patients in goals-of-care discussions, especially patients with life-limiting illnesses such as heart failure, cannot be achieved without these skills.
In 1975, Julia and Joseph Quinlan approached the administrator of St. Clare’s Hospital in Denville, New Jersey, and requested that the mechanical ventilator on which their adopted daughter, Karen, was dependent be turned off. Karen Ann Quinlan, 21 years old, was in a permanent vegetative state after a severe anoxic event, and her parents had been informed by the hospital’s medical staff that she would never regain consciousness.
To the Quinlans’ request to withdraw the ventilator, the hospital administrator replied, “You have to understand our position, Mrs. Quinlan. In this hospital we don’t kill people.”1
The administrator’s response was consistent with prevailing ethical and legal perspectives, analyses, and directives at that time related to discontinuation of life-sustaining treatment. In the mid-1970s, the American Medical Association’s position was that it was permissible to not put a patient on a ventilator (ie, a physician could withhold a life-sustaining treatment), but once a patient was on a ventilator, it was not permissible to take the patient off if the intention was to allow death to occur.1 However, the New Jersey Supreme Court ultimately found this distinction between withholding and withdrawing unconvincing, and ruled unanimously that Karen Quinlan’s ventilator could be turned off.2
THE HASTINGS CENTER REPORT: STOPPING IS THE SAME AS NOT STARTING
During the subsequent decade, further ethical analysis and additional legal cases resulted in new insights and more nuanced thinking about forgoing life-sustaining treatment.
These developments were summarized in a 1987 report by the Hastings Center,3 a leading bioethics research and policy institute. The report provided normative guidance for the termination of life-sustaining treatment and for the care of dying patients. It acknowledged that deciding not to start a life-sustaining treatment can emotionally and psychologically affect healthcare professionals differently than deciding to stop such a treatment. However, the report also asserted that there is no morally important difference between withholding and withdrawing such treatments.
Reflecting a partnership model between patients and professionals for healthcare decision-making, and affirming the ethical significance of both a burden-benefit analysis and patient autonomy, the report stated that when a patient or surrogate in collaboration with a responsible healthcare professional decides that a treatment under way and the life it supports have become more burdensome than beneficial to the patient, that is sufficient reason to stop. There is no ethical requirement that treatment, once initiated, must continue against the patient’s wishes or when the surrogate determines that it is more burdensome than beneficial from the patient’s perspective. In fact, imposing treatment in such circumstances violates the patient’s right to self-determination.3
The report noted further that, because of frequent uncertainty about the efficacy of proposed treatments, it is preferable to initiate time-limited trials of treatments and then later stop them if they prove ineffective or become overly burdensome from a patient’s perspective.
ICDs ARE LIKE OTHER LIFE-SUSTAINING THERAPIES
In this issue of Cleveland Clinic Journal of Medicine, Baibars et al4 address the question of how implantable cardioverter-defibrillators (ICDs) should be managed at the end of life. The historical events and developments recounted above regarding withdrawing life-sustaining technologies are an appropriate context for ethically assessing the management of ICDs for dying patients.
Obviously, ICDs are not ventilators, but like ventilators, they are life-sustaining therapy, as are dialysis machines, blood transfusions, medically supplied nutrition and hydration, ventricular assist devices, and other implantable electronic cardiac devices such as pacemakers. Each of these life-sustaining therapies, depending on a patient’s clinical condition, underlying illness, and comorbidities, can become a death-prolonging technology.
An ethical framework and analysis about whether to continue any life-sustaining therapy, including an ICD, must include an assessment of the benefit-to-burden ratio from the patient’s perspective. Does the therapy enhance or maintain a quality of life acceptable to the patient? Or has it become overly burdensome and does it maintain a quality of life the patient finds (or would find) unacceptable? If the latter is true, and especially in the context of an underlying terminal condition, then shifting the goals of care to focus on comfort is always appropriate and ethically justified. Treatments—including ICDs—that do not contribute to patient comfort should be withdrawn.
TOWARD COMPETENCY IN ETHICAL MANAGEMENT
Baibars et al note that much more needs to be done to enhance competencies, increase proficiencies, and mitigate the moral distress of healthcare professionals caring for dying patients with ICDs and other devices. To help clinicians achieve a personal and professional “comfort zone” for ethically managing patients with ICDs, we recommend that healthcare institutions, medical schools, and nursing schools take the following steps:
Develop comprehensive end-of-life policies, procedures, and protocols that incorporate specific guidance for managing cardiac devices and that have been endorsed by a hospital ethics committee. Such guidance can be informative and educational and can ensure that decisions and resulting actions (including stopping cardiac devices) are ethically supportable.
Provide more palliative care training in medical and nursing schools, residency programs, and continuing education activities so that front-line clinicians can deliver “basic,” “primary” palliative care not requiring specialty palliative medicine. This training, called for in the Institute of Medicine’s 2014 report, Dying in America,5 should include explicit ethics discussions about managing cardiac devices at the end of life.
Provide ongoing training in communication skills needed for all patient-professional encounters. Effectively engaging patients in goals-of-care discussions, especially patients with life-limiting illnesses such as heart failure, cannot be achieved without these skills.
- Pence G. Comas: Karen Quinlan and Nancy Cruzan. In: Classic Cases in Medical Ethics: Accounts of Cases That Have Shaped Medical Ethics, With Philosophical, Legal, and Historical Backgrounds, 3rd edition. Boston: McGraw-Hill; 2000:29–55.
- In the matter of Karen Quinlan, an alleged incompetent. In re Quinlan. 70 N.J. 10, 355 A.2d 647 (1976), cert. denied, 429 U.S. 922 (1976).
- Wolf SM. Hastings Center. Guidelines on the Termination of Life-Sustaining Treatment and Care of the Dying: A Report by the Hastings Center. The Hastings Center: Briarcliff Manor, NY; 1987.
- Baibars MM, Alraies MC, Kabach A, Pritzker M. Can patients opt to turn off implantable cardioverter-defibrillators near the end of life? Cleve Clin J Med 2016; 83:97–98.
- National Academy of Sciences. Dying in America: improving quality and honoring individual p near the end of life. www.iom.edu/Reports/2014/Dying-In-America-Improving-Quality-and-Honoring-Individual-P-Near-the-End-of-Life.aspx. Accessed January 4, 2016.
- Pence G. Comas: Karen Quinlan and Nancy Cruzan. In: Classic Cases in Medical Ethics: Accounts of Cases That Have Shaped Medical Ethics, With Philosophical, Legal, and Historical Backgrounds, 3rd edition. Boston: McGraw-Hill; 2000:29–55.
- In the matter of Karen Quinlan, an alleged incompetent. In re Quinlan. 70 N.J. 10, 355 A.2d 647 (1976), cert. denied, 429 U.S. 922 (1976).
- Wolf SM. Hastings Center. Guidelines on the Termination of Life-Sustaining Treatment and Care of the Dying: A Report by the Hastings Center. The Hastings Center: Briarcliff Manor, NY; 1987.
- Baibars MM, Alraies MC, Kabach A, Pritzker M. Can patients opt to turn off implantable cardioverter-defibrillators near the end of life? Cleve Clin J Med 2016; 83:97–98.
- National Academy of Sciences. Dying in America: improving quality and honoring individual p near the end of life. www.iom.edu/Reports/2014/Dying-In-America-Improving-Quality-and-Honoring-Individual-P-Near-the-End-of-Life.aspx. Accessed January 4, 2016.
Can patients opt to turn off implantable cardioverter-defibrillators near the end of life?
Yes. Although implantable cardioverter-defibrillators (ICDs) prevent sudden cardiac death in patients with advanced heart failure, their benefit in terminally ill patients is small.1 Furthermore, the shocks they deliver at the end of life can cause distress. Therefore, it is reasonable to consider ICD deactivation if the patient or family wishes.
A DIFFICULT DECISION
End-of-life decisions place significant emotional burdens on patients, their families, and their healthcare providers and can have social and legal consequences.
Turning off an ICD is an especially difficult decision, considering that these devices protect against sudden cardiac death and fatal arrhythmias. Also, patients and their representatives may find it more difficult to withdraw from active care than to forgo further interventions (more on this below), and they may misunderstand discussions about ICD deactivation, perceiving them as the beginning of abandonment.
ICD DEACTIVATION IS OFTEN DONE HAPHAZARDLY OR NOT AT ALL
Many healthcare providers are not trained in or comfortable with discussing end-of-life issues, and many hospitals and hospice programs lack policies and protocols for managing implanted devices at the end of life. Consequently, ICD management at the end of life varies among providers and tends to be suboptimal.2
In a report of a survey in 414 hospice facilities, 97% of facilities reported that they admitted patients with ICDs, but only 10% had a policy on device deactivation.3
In a survey of 47 European medical centers, only 4% said they addressed ICD deactivation with their patients.4
A study of 125 patients with ICDs who had died found that 52% had do-not-resuscitate orders. Nevertheless, in 100 patients the ICD had remained active in the last 24 hours of their life, and 31 of these patients had received shocks during their last 24 hours.5
In a survey of next of kin of patients with ICDs who had died of any cause,6 in only 27 of 100 cases had the clinician discussed ICD deactivation, and about three-fourths of these discussions had occurred during the last few days of life. Twenty-seven patients had received ICD discharges in the last month of life, and 8% had received a discharge during the final minutes.
TRAINING AND PROTOCOLS ARE NEEDED
Healthcare professionals need education about device deactivation at the end of life so that they are comfortable communicating with patients and families about this critical issue. To this end, several cardiac and palliative care societies have jointly released an expert statement on managing ICDs and other implantable devices in end-of-life situations.7
Many providers harbor a misunderstanding of the difference between withholding a device and withdrawing (or turning off) a device that is already implanted.2 Some mistakenly believe they would be committing a crime by deactivating an implanted life-sustaining device. Legally and ethically, there is no difference between withholding a device and withdrawing a device. Legally, carrying out a request to withdraw life-sustaining treatment is neither physician-assisted suicide nor euthanasia.
DISCUSSION SHOULD BEGIN EARLY AND SHOULD BE ONGOING
The discussion of ICD deactivation should begin before the device is implanted and should continue as the patient’s health status changes. In a survey, 40% of patients said they felt that ICD deactivation should be discussed before the device is implanted, and only 5% felt that this discussion should be undertaken in the last days of life.8
At the least, it is important to identify patients with ICDs on admission to hospice and to have policies in place that ensure adequate patient education to make an informed decision about ICD deactivation at the end of life.
The topic should be discussed when goals of care change and when do-not-resuscitate status is addressed, and also when advanced directives are being acknowledged. If the patient or his or her legal representative wishes to keep the ICD turned on, that wish should be respected. The essence of a discussion is not to impose the providers’ choice on the patient, but to help the patient make the right decision for himself or herself. Of note, patients entering hospice do not have to have do-not-resuscitate status.
We believe that device management in end-of-life circumstances should be part of the discussion of the goals of care. Accordingly, healthcare providers need to be familiar with device management and to have a higher comfort level in addressing such sensitive topics with patients facing the end of life, as well as with their families.
It is also advisable to apply protocols within hospice services to address ICD management options for the patient and the legal representative. An early decision regarding end-of-life deactivation will help patients avoid distressing ICD discharges and the related emotional distress in their last moments.
- Barsheshet A, Moss AJ, Huang DT, McNitt S, Zareba W, Goldenberg I. Applicability of a risk score for prediction of the long-term (8-year) benefit of the implantable cardioverter-defibrillator. J Am Coll Cardiol 2012; 59:2075–2079.
- Kapa S, Mueller PS, Hayes DL, Asirvatham SJ. Perspectives on withdrawing pacemaker and implantable cardioverter-defibrillator therapies at end of life: results of a survey of medical and legal professionals and patients. Mayo Clin Proc 2010; 85:981–990.
- Goldstein N, Carlson M, Livote E, Kutner JS. Brief communication: management of implantable cardioverter-defibrillators in hospice: a nationwide survey. Ann Intern Med 2010; 152:296–299.
- Marinskis G, van Erven L; EHRA Scientific Initiatives Committtee. Deactivation of implanted cardioverter-defibrillators at the end of life: results of the EHRA survey. Europace 2010; 12:1176–1177.
- Kinch Westerdahl A, Sjoblom J, Mattiasson AC, Rosenqvist M, Frykman V. Implantable cardioverter-defibrillator therapy before death: high risk for painful shocks at end of life. Circulation 2014; 129:422–429.
- Goldstein NE, Lampert R, Bradley E, Lynn J, Krumholz HM. Management of implantable cardioverter defibrillators in end-of-life care. Ann Intern Med 2004; 141:835–838.
- Lampert R, Hayes DL, Annas GJ, et al; American College of Cardiology; American Geriatrics Society; American Academy of Hospice and Palliative Medicine; American Heart Association; European Heart Rhythm Association; Hospice and Palliative Nurses Association. HRS expert consensus statement on the management of cardiovascular implantable electronic devices (CIEDs) in patients nearing end of life or requesting withdrawal of therapy. Heart Rhythm 2010; 7:1008–1026.
- Raphael CE, Koa-Wing M, Stain N, Wright I, Francis DP, Kanagaratnam P. Implantable cardioverter-defibrillator recipient attitudes towards device activation: how much do patients want to know? Pacing Clin Electrophysiol 2011; 34:1628–1633.
Yes. Although implantable cardioverter-defibrillators (ICDs) prevent sudden cardiac death in patients with advanced heart failure, their benefit in terminally ill patients is small.1 Furthermore, the shocks they deliver at the end of life can cause distress. Therefore, it is reasonable to consider ICD deactivation if the patient or family wishes.
A DIFFICULT DECISION
End-of-life decisions place significant emotional burdens on patients, their families, and their healthcare providers and can have social and legal consequences.
Turning off an ICD is an especially difficult decision, considering that these devices protect against sudden cardiac death and fatal arrhythmias. Also, patients and their representatives may find it more difficult to withdraw from active care than to forgo further interventions (more on this below), and they may misunderstand discussions about ICD deactivation, perceiving them as the beginning of abandonment.
ICD DEACTIVATION IS OFTEN DONE HAPHAZARDLY OR NOT AT ALL
Many healthcare providers are not trained in or comfortable with discussing end-of-life issues, and many hospitals and hospice programs lack policies and protocols for managing implanted devices at the end of life. Consequently, ICD management at the end of life varies among providers and tends to be suboptimal.2
In a report of a survey in 414 hospice facilities, 97% of facilities reported that they admitted patients with ICDs, but only 10% had a policy on device deactivation.3
In a survey of 47 European medical centers, only 4% said they addressed ICD deactivation with their patients.4
A study of 125 patients with ICDs who had died found that 52% had do-not-resuscitate orders. Nevertheless, in 100 patients the ICD had remained active in the last 24 hours of their life, and 31 of these patients had received shocks during their last 24 hours.5
In a survey of next of kin of patients with ICDs who had died of any cause,6 in only 27 of 100 cases had the clinician discussed ICD deactivation, and about three-fourths of these discussions had occurred during the last few days of life. Twenty-seven patients had received ICD discharges in the last month of life, and 8% had received a discharge during the final minutes.
TRAINING AND PROTOCOLS ARE NEEDED
Healthcare professionals need education about device deactivation at the end of life so that they are comfortable communicating with patients and families about this critical issue. To this end, several cardiac and palliative care societies have jointly released an expert statement on managing ICDs and other implantable devices in end-of-life situations.7
Many providers harbor a misunderstanding of the difference between withholding a device and withdrawing (or turning off) a device that is already implanted.2 Some mistakenly believe they would be committing a crime by deactivating an implanted life-sustaining device. Legally and ethically, there is no difference between withholding a device and withdrawing a device. Legally, carrying out a request to withdraw life-sustaining treatment is neither physician-assisted suicide nor euthanasia.
DISCUSSION SHOULD BEGIN EARLY AND SHOULD BE ONGOING
The discussion of ICD deactivation should begin before the device is implanted and should continue as the patient’s health status changes. In a survey, 40% of patients said they felt that ICD deactivation should be discussed before the device is implanted, and only 5% felt that this discussion should be undertaken in the last days of life.8
At the least, it is important to identify patients with ICDs on admission to hospice and to have policies in place that ensure adequate patient education to make an informed decision about ICD deactivation at the end of life.
The topic should be discussed when goals of care change and when do-not-resuscitate status is addressed, and also when advanced directives are being acknowledged. If the patient or his or her legal representative wishes to keep the ICD turned on, that wish should be respected. The essence of a discussion is not to impose the providers’ choice on the patient, but to help the patient make the right decision for himself or herself. Of note, patients entering hospice do not have to have do-not-resuscitate status.
We believe that device management in end-of-life circumstances should be part of the discussion of the goals of care. Accordingly, healthcare providers need to be familiar with device management and to have a higher comfort level in addressing such sensitive topics with patients facing the end of life, as well as with their families.
It is also advisable to apply protocols within hospice services to address ICD management options for the patient and the legal representative. An early decision regarding end-of-life deactivation will help patients avoid distressing ICD discharges and the related emotional distress in their last moments.
Yes. Although implantable cardioverter-defibrillators (ICDs) prevent sudden cardiac death in patients with advanced heart failure, their benefit in terminally ill patients is small.1 Furthermore, the shocks they deliver at the end of life can cause distress. Therefore, it is reasonable to consider ICD deactivation if the patient or family wishes.
A DIFFICULT DECISION
End-of-life decisions place significant emotional burdens on patients, their families, and their healthcare providers and can have social and legal consequences.
Turning off an ICD is an especially difficult decision, considering that these devices protect against sudden cardiac death and fatal arrhythmias. Also, patients and their representatives may find it more difficult to withdraw from active care than to forgo further interventions (more on this below), and they may misunderstand discussions about ICD deactivation, perceiving them as the beginning of abandonment.
ICD DEACTIVATION IS OFTEN DONE HAPHAZARDLY OR NOT AT ALL
Many healthcare providers are not trained in or comfortable with discussing end-of-life issues, and many hospitals and hospice programs lack policies and protocols for managing implanted devices at the end of life. Consequently, ICD management at the end of life varies among providers and tends to be suboptimal.2
In a report of a survey in 414 hospice facilities, 97% of facilities reported that they admitted patients with ICDs, but only 10% had a policy on device deactivation.3
In a survey of 47 European medical centers, only 4% said they addressed ICD deactivation with their patients.4
A study of 125 patients with ICDs who had died found that 52% had do-not-resuscitate orders. Nevertheless, in 100 patients the ICD had remained active in the last 24 hours of their life, and 31 of these patients had received shocks during their last 24 hours.5
In a survey of next of kin of patients with ICDs who had died of any cause,6 in only 27 of 100 cases had the clinician discussed ICD deactivation, and about three-fourths of these discussions had occurred during the last few days of life. Twenty-seven patients had received ICD discharges in the last month of life, and 8% had received a discharge during the final minutes.
TRAINING AND PROTOCOLS ARE NEEDED
Healthcare professionals need education about device deactivation at the end of life so that they are comfortable communicating with patients and families about this critical issue. To this end, several cardiac and palliative care societies have jointly released an expert statement on managing ICDs and other implantable devices in end-of-life situations.7
Many providers harbor a misunderstanding of the difference between withholding a device and withdrawing (or turning off) a device that is already implanted.2 Some mistakenly believe they would be committing a crime by deactivating an implanted life-sustaining device. Legally and ethically, there is no difference between withholding a device and withdrawing a device. Legally, carrying out a request to withdraw life-sustaining treatment is neither physician-assisted suicide nor euthanasia.
DISCUSSION SHOULD BEGIN EARLY AND SHOULD BE ONGOING
The discussion of ICD deactivation should begin before the device is implanted and should continue as the patient’s health status changes. In a survey, 40% of patients said they felt that ICD deactivation should be discussed before the device is implanted, and only 5% felt that this discussion should be undertaken in the last days of life.8
At the least, it is important to identify patients with ICDs on admission to hospice and to have policies in place that ensure adequate patient education to make an informed decision about ICD deactivation at the end of life.
The topic should be discussed when goals of care change and when do-not-resuscitate status is addressed, and also when advanced directives are being acknowledged. If the patient or his or her legal representative wishes to keep the ICD turned on, that wish should be respected. The essence of a discussion is not to impose the providers’ choice on the patient, but to help the patient make the right decision for himself or herself. Of note, patients entering hospice do not have to have do-not-resuscitate status.
We believe that device management in end-of-life circumstances should be part of the discussion of the goals of care. Accordingly, healthcare providers need to be familiar with device management and to have a higher comfort level in addressing such sensitive topics with patients facing the end of life, as well as with their families.
It is also advisable to apply protocols within hospice services to address ICD management options for the patient and the legal representative. An early decision regarding end-of-life deactivation will help patients avoid distressing ICD discharges and the related emotional distress in their last moments.
- Barsheshet A, Moss AJ, Huang DT, McNitt S, Zareba W, Goldenberg I. Applicability of a risk score for prediction of the long-term (8-year) benefit of the implantable cardioverter-defibrillator. J Am Coll Cardiol 2012; 59:2075–2079.
- Kapa S, Mueller PS, Hayes DL, Asirvatham SJ. Perspectives on withdrawing pacemaker and implantable cardioverter-defibrillator therapies at end of life: results of a survey of medical and legal professionals and patients. Mayo Clin Proc 2010; 85:981–990.
- Goldstein N, Carlson M, Livote E, Kutner JS. Brief communication: management of implantable cardioverter-defibrillators in hospice: a nationwide survey. Ann Intern Med 2010; 152:296–299.
- Marinskis G, van Erven L; EHRA Scientific Initiatives Committtee. Deactivation of implanted cardioverter-defibrillators at the end of life: results of the EHRA survey. Europace 2010; 12:1176–1177.
- Kinch Westerdahl A, Sjoblom J, Mattiasson AC, Rosenqvist M, Frykman V. Implantable cardioverter-defibrillator therapy before death: high risk for painful shocks at end of life. Circulation 2014; 129:422–429.
- Goldstein NE, Lampert R, Bradley E, Lynn J, Krumholz HM. Management of implantable cardioverter defibrillators in end-of-life care. Ann Intern Med 2004; 141:835–838.
- Lampert R, Hayes DL, Annas GJ, et al; American College of Cardiology; American Geriatrics Society; American Academy of Hospice and Palliative Medicine; American Heart Association; European Heart Rhythm Association; Hospice and Palliative Nurses Association. HRS expert consensus statement on the management of cardiovascular implantable electronic devices (CIEDs) in patients nearing end of life or requesting withdrawal of therapy. Heart Rhythm 2010; 7:1008–1026.
- Raphael CE, Koa-Wing M, Stain N, Wright I, Francis DP, Kanagaratnam P. Implantable cardioverter-defibrillator recipient attitudes towards device activation: how much do patients want to know? Pacing Clin Electrophysiol 2011; 34:1628–1633.
- Barsheshet A, Moss AJ, Huang DT, McNitt S, Zareba W, Goldenberg I. Applicability of a risk score for prediction of the long-term (8-year) benefit of the implantable cardioverter-defibrillator. J Am Coll Cardiol 2012; 59:2075–2079.
- Kapa S, Mueller PS, Hayes DL, Asirvatham SJ. Perspectives on withdrawing pacemaker and implantable cardioverter-defibrillator therapies at end of life: results of a survey of medical and legal professionals and patients. Mayo Clin Proc 2010; 85:981–990.
- Goldstein N, Carlson M, Livote E, Kutner JS. Brief communication: management of implantable cardioverter-defibrillators in hospice: a nationwide survey. Ann Intern Med 2010; 152:296–299.
- Marinskis G, van Erven L; EHRA Scientific Initiatives Committtee. Deactivation of implanted cardioverter-defibrillators at the end of life: results of the EHRA survey. Europace 2010; 12:1176–1177.
- Kinch Westerdahl A, Sjoblom J, Mattiasson AC, Rosenqvist M, Frykman V. Implantable cardioverter-defibrillator therapy before death: high risk for painful shocks at end of life. Circulation 2014; 129:422–429.
- Goldstein NE, Lampert R, Bradley E, Lynn J, Krumholz HM. Management of implantable cardioverter defibrillators in end-of-life care. Ann Intern Med 2004; 141:835–838.
- Lampert R, Hayes DL, Annas GJ, et al; American College of Cardiology; American Geriatrics Society; American Academy of Hospice and Palliative Medicine; American Heart Association; European Heart Rhythm Association; Hospice and Palliative Nurses Association. HRS expert consensus statement on the management of cardiovascular implantable electronic devices (CIEDs) in patients nearing end of life or requesting withdrawal of therapy. Heart Rhythm 2010; 7:1008–1026.
- Raphael CE, Koa-Wing M, Stain N, Wright I, Francis DP, Kanagaratnam P. Implantable cardioverter-defibrillator recipient attitudes towards device activation: how much do patients want to know? Pacing Clin Electrophysiol 2011; 34:1628–1633.
Families Perceive Few Benefits From Aggressive End-of-Life Care
Bereaved families were substantially more satisfied with end-of-life cancer care when patients did not die in hospital, received more than 3 days of hospice care, and did not enter the ICU within 30 days of dying, according to a multicenter, prospective study published online Jan. 19 in JAMA.
The analysis is one of the first of its type to assess these end-of-life care indicators, said Dr. Alexi Wright of Harvard Medical School, Boston, and her associates. The findings could affect health policy as electronic health records expand under the Health Information Technology for Economic and Clinical Health Act, they said.
End-of-life cancer care has become increasingly aggressive, belying evidence that this approach does not improve patient outcomes, quality of life, or caregiver bereavement. To explore alternatives, the researchers analyzed 1,146 interviews of family members of Medicare patients who died of lung or colorectal cancer by 2011. Their data source was the multiregional, prospective, observational Cancer Care Outcomes Research and Surveillance (CanCORS) study (JAMA 2016;315:284-92).
Family members described end-of-life care as “excellent” 59% of the time when hospice care lasted more 3 days, but 43% of the time otherwise (95% confidence interval for adjusted difference, 11% to 22%). Notably, 73% of patients who received more than 3 days of hospice care died in their preferred location, compared with 40% of patients who received less or no hospice care. Care was rated as excellent 52% of the time when ICU admission was avoided within 30 days of death, and 57% of the time when patients died outside the hospital, compared with 45% and 42% of the time otherwise.
The results support “advance care planning consistent with the preferences of patients,” said the investigators. They recommended more extensive counseling of cancer patients and families, earlier palliative care referrals, and an audit and feedback system to monitor the use of aggressive end-of-life care.
The National Cancer Institute and the Cancer Care Outcomes Research and Surveillance Consortium funded the study. One coinvestigator reported financial relationships with the American Academy of Hospice and Palliative Medicine, National Institute of Nursing Research, National Institute on Aging, Retirement Research Retirement Foundation, California Healthcare Foundation, Commonwealth Fund, West Health Institute, University of Wisconsin, and UpToDate.com. Senior author Dr. Mary Landrum, also of Harvard Medical School, reported grant funding from Pfizer and personal fees from McKinsey and Company and Greylock McKinnon Associates. The other authors had no disclosures.
Bereaved families were substantially more satisfied with end-of-life cancer care when patients did not die in hospital, received more than 3 days of hospice care, and did not enter the ICU within 30 days of dying, according to a multicenter, prospective study published online Jan. 19 in JAMA.
The analysis is one of the first of its type to assess these end-of-life care indicators, said Dr. Alexi Wright of Harvard Medical School, Boston, and her associates. The findings could affect health policy as electronic health records expand under the Health Information Technology for Economic and Clinical Health Act, they said.
End-of-life cancer care has become increasingly aggressive, belying evidence that this approach does not improve patient outcomes, quality of life, or caregiver bereavement. To explore alternatives, the researchers analyzed 1,146 interviews of family members of Medicare patients who died of lung or colorectal cancer by 2011. Their data source was the multiregional, prospective, observational Cancer Care Outcomes Research and Surveillance (CanCORS) study (JAMA 2016;315:284-92).
Family members described end-of-life care as “excellent” 59% of the time when hospice care lasted more 3 days, but 43% of the time otherwise (95% confidence interval for adjusted difference, 11% to 22%). Notably, 73% of patients who received more than 3 days of hospice care died in their preferred location, compared with 40% of patients who received less or no hospice care. Care was rated as excellent 52% of the time when ICU admission was avoided within 30 days of death, and 57% of the time when patients died outside the hospital, compared with 45% and 42% of the time otherwise.
The results support “advance care planning consistent with the preferences of patients,” said the investigators. They recommended more extensive counseling of cancer patients and families, earlier palliative care referrals, and an audit and feedback system to monitor the use of aggressive end-of-life care.
The National Cancer Institute and the Cancer Care Outcomes Research and Surveillance Consortium funded the study. One coinvestigator reported financial relationships with the American Academy of Hospice and Palliative Medicine, National Institute of Nursing Research, National Institute on Aging, Retirement Research Retirement Foundation, California Healthcare Foundation, Commonwealth Fund, West Health Institute, University of Wisconsin, and UpToDate.com. Senior author Dr. Mary Landrum, also of Harvard Medical School, reported grant funding from Pfizer and personal fees from McKinsey and Company and Greylock McKinnon Associates. The other authors had no disclosures.
Bereaved families were substantially more satisfied with end-of-life cancer care when patients did not die in hospital, received more than 3 days of hospice care, and did not enter the ICU within 30 days of dying, according to a multicenter, prospective study published online Jan. 19 in JAMA.
The analysis is one of the first of its type to assess these end-of-life care indicators, said Dr. Alexi Wright of Harvard Medical School, Boston, and her associates. The findings could affect health policy as electronic health records expand under the Health Information Technology for Economic and Clinical Health Act, they said.
End-of-life cancer care has become increasingly aggressive, belying evidence that this approach does not improve patient outcomes, quality of life, or caregiver bereavement. To explore alternatives, the researchers analyzed 1,146 interviews of family members of Medicare patients who died of lung or colorectal cancer by 2011. Their data source was the multiregional, prospective, observational Cancer Care Outcomes Research and Surveillance (CanCORS) study (JAMA 2016;315:284-92).
Family members described end-of-life care as “excellent” 59% of the time when hospice care lasted more 3 days, but 43% of the time otherwise (95% confidence interval for adjusted difference, 11% to 22%). Notably, 73% of patients who received more than 3 days of hospice care died in their preferred location, compared with 40% of patients who received less or no hospice care. Care was rated as excellent 52% of the time when ICU admission was avoided within 30 days of death, and 57% of the time when patients died outside the hospital, compared with 45% and 42% of the time otherwise.
The results support “advance care planning consistent with the preferences of patients,” said the investigators. They recommended more extensive counseling of cancer patients and families, earlier palliative care referrals, and an audit and feedback system to monitor the use of aggressive end-of-life care.
The National Cancer Institute and the Cancer Care Outcomes Research and Surveillance Consortium funded the study. One coinvestigator reported financial relationships with the American Academy of Hospice and Palliative Medicine, National Institute of Nursing Research, National Institute on Aging, Retirement Research Retirement Foundation, California Healthcare Foundation, Commonwealth Fund, West Health Institute, University of Wisconsin, and UpToDate.com. Senior author Dr. Mary Landrum, also of Harvard Medical School, reported grant funding from Pfizer and personal fees from McKinsey and Company and Greylock McKinnon Associates. The other authors had no disclosures.
FROM JAMA
Neurosurgeon memoir illuminates the journey through cancer treatment and acceptance of mortality
Dr. Paul Kalanithi, a neurosurgeon who had just completed his residency at the Stanford (Calif.) University, died of metastatic lung cancer last year, but he left a memoir of his experiences as a physician, a patient, and a dying man that was published on Jan. 12. His book, “When Breath Becomes Air” (New York: Random House, 2016), recounts the many years of working to exhaustion and deferring of life experiences and pleasures that are necessary to complete medical training.
In a review of the book, Janet Maslin wrote, “One of the most poignant things about Dr. Kalanithi’s story is that he had postponed learning how to live while pursuing his career in neurosurgery. By the time he was ready to enjoy a life outside the operating room, what he needed to learn was how to die.”
Dr. Kalanithi reflected on the profound grief and sense of loss that comes with a diagnosis that he knew meant imminent death. The memoir also reveals his search for meaning and joy, and finally, his acceptance of mortality. He opted for palliative care and his memoir, along with the epilogue written by his wife, Dr. Lucy Kalanithi, gives insight into the value of the palliative path to patients and their families in dire medical crises.
Dr. Paul Kalanithi, a neurosurgeon who had just completed his residency at the Stanford (Calif.) University, died of metastatic lung cancer last year, but he left a memoir of his experiences as a physician, a patient, and a dying man that was published on Jan. 12. His book, “When Breath Becomes Air” (New York: Random House, 2016), recounts the many years of working to exhaustion and deferring of life experiences and pleasures that are necessary to complete medical training.
In a review of the book, Janet Maslin wrote, “One of the most poignant things about Dr. Kalanithi’s story is that he had postponed learning how to live while pursuing his career in neurosurgery. By the time he was ready to enjoy a life outside the operating room, what he needed to learn was how to die.”
Dr. Kalanithi reflected on the profound grief and sense of loss that comes with a diagnosis that he knew meant imminent death. The memoir also reveals his search for meaning and joy, and finally, his acceptance of mortality. He opted for palliative care and his memoir, along with the epilogue written by his wife, Dr. Lucy Kalanithi, gives insight into the value of the palliative path to patients and their families in dire medical crises.
Dr. Paul Kalanithi, a neurosurgeon who had just completed his residency at the Stanford (Calif.) University, died of metastatic lung cancer last year, but he left a memoir of his experiences as a physician, a patient, and a dying man that was published on Jan. 12. His book, “When Breath Becomes Air” (New York: Random House, 2016), recounts the many years of working to exhaustion and deferring of life experiences and pleasures that are necessary to complete medical training.
In a review of the book, Janet Maslin wrote, “One of the most poignant things about Dr. Kalanithi’s story is that he had postponed learning how to live while pursuing his career in neurosurgery. By the time he was ready to enjoy a life outside the operating room, what he needed to learn was how to die.”
Dr. Kalanithi reflected on the profound grief and sense of loss that comes with a diagnosis that he knew meant imminent death. The memoir also reveals his search for meaning and joy, and finally, his acceptance of mortality. He opted for palliative care and his memoir, along with the epilogue written by his wife, Dr. Lucy Kalanithi, gives insight into the value of the palliative path to patients and their families in dire medical crises.
David Bowie’s death inspires blog on palliative care
The death of David Bowie, iconic musician and artist, on Jan. 10 inspired palliative care specialist Dr. Mark Taubert to write a blog about end-of-life scenarios and the importance of advance care planning. The blog, which begins by thanking Mr. Bowie for his many artistic contributions, continues by suggesting that his planned death at home will inspire many people in similar health crises to consider palliative care. The palliative care conversation between a doctor and a patient facing death can be challenging but can lead to what Dr. Taubert called “a good death” at home with symptoms managed and loved ones nearby. Mr. Bowie’s son, Duncan Jones, tweeted a link to the blog in the days after his father’s death.
Dr. Taubert found himself speaking with a patient who was facing probable death in the near future, and both doctor and patient found inspiration in Mr. Bowie’s final music project and his death at home with his family. Dr. Taubert and his patient were able to have the conversation about palliative care at end-of-life in part because they were both impressed with what Mr. Bowie was able to achieve in his last months. “Your story became a way for us to communicate very openly about death, something many doctors and nurses struggle to introduce as a topic of conversation,” he wrote.
Dr. Taubert of the Velindre NHS Trust in Cardiff, Wales, noted that, although palliative care is a highly developed skill with many resources to help patients at the end of life, “this essential part of training is not always available for junior healthcare professionals, including doctors and nurses, and is sometimes overlooked or under-prioritized by those who plan their education. I think if you [David Bowie] were ever to return (as Lazarus did), you would be a firm advocate for good palliative care training being available everywhere.”
The death of David Bowie, iconic musician and artist, on Jan. 10 inspired palliative care specialist Dr. Mark Taubert to write a blog about end-of-life scenarios and the importance of advance care planning. The blog, which begins by thanking Mr. Bowie for his many artistic contributions, continues by suggesting that his planned death at home will inspire many people in similar health crises to consider palliative care. The palliative care conversation between a doctor and a patient facing death can be challenging but can lead to what Dr. Taubert called “a good death” at home with symptoms managed and loved ones nearby. Mr. Bowie’s son, Duncan Jones, tweeted a link to the blog in the days after his father’s death.
Dr. Taubert found himself speaking with a patient who was facing probable death in the near future, and both doctor and patient found inspiration in Mr. Bowie’s final music project and his death at home with his family. Dr. Taubert and his patient were able to have the conversation about palliative care at end-of-life in part because they were both impressed with what Mr. Bowie was able to achieve in his last months. “Your story became a way for us to communicate very openly about death, something many doctors and nurses struggle to introduce as a topic of conversation,” he wrote.
Dr. Taubert of the Velindre NHS Trust in Cardiff, Wales, noted that, although palliative care is a highly developed skill with many resources to help patients at the end of life, “this essential part of training is not always available for junior healthcare professionals, including doctors and nurses, and is sometimes overlooked or under-prioritized by those who plan their education. I think if you [David Bowie] were ever to return (as Lazarus did), you would be a firm advocate for good palliative care training being available everywhere.”
The death of David Bowie, iconic musician and artist, on Jan. 10 inspired palliative care specialist Dr. Mark Taubert to write a blog about end-of-life scenarios and the importance of advance care planning. The blog, which begins by thanking Mr. Bowie for his many artistic contributions, continues by suggesting that his planned death at home will inspire many people in similar health crises to consider palliative care. The palliative care conversation between a doctor and a patient facing death can be challenging but can lead to what Dr. Taubert called “a good death” at home with symptoms managed and loved ones nearby. Mr. Bowie’s son, Duncan Jones, tweeted a link to the blog in the days after his father’s death.
Dr. Taubert found himself speaking with a patient who was facing probable death in the near future, and both doctor and patient found inspiration in Mr. Bowie’s final music project and his death at home with his family. Dr. Taubert and his patient were able to have the conversation about palliative care at end-of-life in part because they were both impressed with what Mr. Bowie was able to achieve in his last months. “Your story became a way for us to communicate very openly about death, something many doctors and nurses struggle to introduce as a topic of conversation,” he wrote.
Dr. Taubert of the Velindre NHS Trust in Cardiff, Wales, noted that, although palliative care is a highly developed skill with many resources to help patients at the end of life, “this essential part of training is not always available for junior healthcare professionals, including doctors and nurses, and is sometimes overlooked or under-prioritized by those who plan their education. I think if you [David Bowie] were ever to return (as Lazarus did), you would be a firm advocate for good palliative care training being available everywhere.”
Families perceive few benefits from aggressive end-of-life care
Bereaved families were substantially more satisfied with end-of-life cancer care when patients did not die in hospital, received more than 3 days of hospice care, and did not enter the ICU within 30 days of dying, according to a multicenter, prospective study published online Jan. 19 in JAMA.
The analysis is one of the first of its type to assess these end-of-life care indicators, said Dr. Alexi Wright of Harvard Medical School, Boston, and her associates. The findings could affect health policy as electronic health records expand under the Health Information Technology for Economic and Clinical Health Act, they said.
End-of-life cancer care has become increasingly aggressive, belying evidence that this approach does not improve patient outcomes, quality of life, or caregiver bereavement. To explore alternatives, the researchers analyzed 1,146 interviews of family members of Medicare patients who died of lung or colorectal cancer by 2011. Their data source was the multiregional, prospective, observational Cancer Care Outcomes Research and Surveillance (CanCORS) study (JAMA 2016;315:284-92).
Family members described end-of-life care as “excellent” 59% of the time when hospice care lasted more 3 days, but 43% of the time otherwise (95% confidence interval for adjusted difference, 11% to 22%). Notably, 73% of patients who received more than 3 days of hospice care died in their preferred location, compared with 40% of patients who received less or no hospice care. Care was rated as excellent 52% of the time when ICU admission was avoided within 30 days of death, and 57% of the time when patients died outside the hospital, compared with 45% and 42% of the time otherwise.
The results support “advance care planning consistent with the preferences of patients,” said the investigators. They recommended more extensive counseling of cancer patients and families, earlier palliative care referrals, and an audit and feedback system to monitor the use of aggressive end-of-life care.
The National Cancer Institute and the Cancer Care Outcomes Research and Surveillance Consortium funded the study. One coinvestigator reported financial relationships with the American Academy of Hospice and Palliative Medicine, National Institute of Nursing Research, National Institute on Aging, Retirement Research Retirement Foundation, California Healthcare Foundation, Commonwealth Fund, West Health Institute, University of Wisconsin, and UpToDate.com. Senior author Dr. Mary Landrum, also of Harvard Medical School, reported grant funding from Pfizer and personal fees from McKinsey and Company and Greylock McKinnon Associates. The other authors had no disclosures.
Bereaved families were substantially more satisfied with end-of-life cancer care when patients did not die in hospital, received more than 3 days of hospice care, and did not enter the ICU within 30 days of dying, according to a multicenter, prospective study published online Jan. 19 in JAMA.
The analysis is one of the first of its type to assess these end-of-life care indicators, said Dr. Alexi Wright of Harvard Medical School, Boston, and her associates. The findings could affect health policy as electronic health records expand under the Health Information Technology for Economic and Clinical Health Act, they said.
End-of-life cancer care has become increasingly aggressive, belying evidence that this approach does not improve patient outcomes, quality of life, or caregiver bereavement. To explore alternatives, the researchers analyzed 1,146 interviews of family members of Medicare patients who died of lung or colorectal cancer by 2011. Their data source was the multiregional, prospective, observational Cancer Care Outcomes Research and Surveillance (CanCORS) study (JAMA 2016;315:284-92).
Family members described end-of-life care as “excellent” 59% of the time when hospice care lasted more 3 days, but 43% of the time otherwise (95% confidence interval for adjusted difference, 11% to 22%). Notably, 73% of patients who received more than 3 days of hospice care died in their preferred location, compared with 40% of patients who received less or no hospice care. Care was rated as excellent 52% of the time when ICU admission was avoided within 30 days of death, and 57% of the time when patients died outside the hospital, compared with 45% and 42% of the time otherwise.
The results support “advance care planning consistent with the preferences of patients,” said the investigators. They recommended more extensive counseling of cancer patients and families, earlier palliative care referrals, and an audit and feedback system to monitor the use of aggressive end-of-life care.
The National Cancer Institute and the Cancer Care Outcomes Research and Surveillance Consortium funded the study. One coinvestigator reported financial relationships with the American Academy of Hospice and Palliative Medicine, National Institute of Nursing Research, National Institute on Aging, Retirement Research Retirement Foundation, California Healthcare Foundation, Commonwealth Fund, West Health Institute, University of Wisconsin, and UpToDate.com. Senior author Dr. Mary Landrum, also of Harvard Medical School, reported grant funding from Pfizer and personal fees from McKinsey and Company and Greylock McKinnon Associates. The other authors had no disclosures.
Bereaved families were substantially more satisfied with end-of-life cancer care when patients did not die in hospital, received more than 3 days of hospice care, and did not enter the ICU within 30 days of dying, according to a multicenter, prospective study published online Jan. 19 in JAMA.
The analysis is one of the first of its type to assess these end-of-life care indicators, said Dr. Alexi Wright of Harvard Medical School, Boston, and her associates. The findings could affect health policy as electronic health records expand under the Health Information Technology for Economic and Clinical Health Act, they said.
End-of-life cancer care has become increasingly aggressive, belying evidence that this approach does not improve patient outcomes, quality of life, or caregiver bereavement. To explore alternatives, the researchers analyzed 1,146 interviews of family members of Medicare patients who died of lung or colorectal cancer by 2011. Their data source was the multiregional, prospective, observational Cancer Care Outcomes Research and Surveillance (CanCORS) study (JAMA 2016;315:284-92).
Family members described end-of-life care as “excellent” 59% of the time when hospice care lasted more 3 days, but 43% of the time otherwise (95% confidence interval for adjusted difference, 11% to 22%). Notably, 73% of patients who received more than 3 days of hospice care died in their preferred location, compared with 40% of patients who received less or no hospice care. Care was rated as excellent 52% of the time when ICU admission was avoided within 30 days of death, and 57% of the time when patients died outside the hospital, compared with 45% and 42% of the time otherwise.
The results support “advance care planning consistent with the preferences of patients,” said the investigators. They recommended more extensive counseling of cancer patients and families, earlier palliative care referrals, and an audit and feedback system to monitor the use of aggressive end-of-life care.
The National Cancer Institute and the Cancer Care Outcomes Research and Surveillance Consortium funded the study. One coinvestigator reported financial relationships with the American Academy of Hospice and Palliative Medicine, National Institute of Nursing Research, National Institute on Aging, Retirement Research Retirement Foundation, California Healthcare Foundation, Commonwealth Fund, West Health Institute, University of Wisconsin, and UpToDate.com. Senior author Dr. Mary Landrum, also of Harvard Medical School, reported grant funding from Pfizer and personal fees from McKinsey and Company and Greylock McKinnon Associates. The other authors had no disclosures.
FROM JAMA
Key clinical point: Bereaved family members were more satisfied with end-of-life cancer care when patients spent more than 3 days in hospice, died outside the hospital, and were not admitted to the ICU within 30 days of dying.
Major finding: Care was described as “excellent” about 9%-17% more often when these end-of-life quality indicators were met.
Data source: A multicenter, prospective, observational study of 1,146 family members of patients who died of lung or colorectal cancer.
Disclosures: The National Cancer Institute and the Cancer Care Outcomes Research and Surveillance Consortium funded the analysis. One coinvestigator reported financial relationships with the American Academy of Hospice and Palliative Medicine, National Institute of Nursing Research, National Institute on Aging, Retirement Research Retirement Foundation, California Healthcare Foundation, Commonwealth Fund, West Health Institute, University of Wisconsin, and UpToDate.com. Senior author Dr. Mary Landrum reported grant funding from Pfizer and personal fees from McKinsey and Company and Greylock McKinnon Associates. The other authors had no disclosures.
Neurosurgery at the End of Life
The juxtaposition between my first 2 days of neurosurgery could not have been more profound. On my first day as a third-year medical student, the attending and chief resident let me take the lead on the first case: a straightforward brain biopsy. I got to make the incision, drill the burr hole, and perform the needle biopsy. I still remember the thrill of the technical challenge, the controlled violence of drilling into the skull, and the finesse of accessing the tumor core.
The buzz was so strong that I barely registered the diagnosis that was called back from the pathologist: glioblastoma. It was not until I saw the face of the disease the next morning that I understood the reality of a GBM diagnosis. That face belonged to a 47-year-old man who hadn’t slept all night, wide eyed with apprehension at what news I might bring. He beseeched me with questions, and though his aphasia left him stammering to get the words out, I knew exactly what he was asking: Would he live or die? It was a question I was in no position to answer. Instead, I reassured him that we were waiting on the final pathology, all the while trying to forget the fact that the frozen section suggested an aggressive subtype, surely heralding a poor prognosis.
In his poignant memoir, “Do No Harm: Stories of Life, Death and Brain Surgery” (New York: Thomas Dunne Book, 2015), Dr. Henry Marsh writes beautifully about how difficult it can be to find the balance between optimism and realism. In one memorable passage, Dr. Marsh shows a house officer a scan of a highly malignant brain tumor and asks him what he would say to the patient. The trainee reflexively hides behind jargon, skirting around what he knew to be the truth: This tumor would kill her. Marsh presses him to admit that he’s lying, before lamenting at how hard it is to improve these critical communication skills: “When I have had to break bad news I never know whether I have done it well or not. The patients aren’t going to ring me up afterward and say, ‘Mr. Marsh, I really liked the way you told me that I was going to die,’ or ‘Mr. Marsh, you were crap.’ You can only hope that you haven’t made too much of a mess of it.”
I could certainly relate to Dr. Marsh’s house officer as I walked away from my own patient. I felt almost deceitful withholding diagnostic information from him, even if I did the “right” thing. It made me wonder, why did I want to become a neurosurgeon? Surely to help people through some of the most difficult moments of their lives. But is it possible to be a source of comfort when you are required so often to be a harbinger of death? The answer depends on whether one can envision a role for the neurosurgeon beyond the mandate of “life at all costs.”
While the field has become known for its life-saving procedures, neurosurgeons are called just as often to preside over the end of their patient’s lives – work that requires just as much skill as any technical procedure. Dr. Marsh recognized the tremendous human cost of neglecting that work. For cases that appear “hopeless,” he writes, “We often end up operating because it’s easier than being honest, and it means that we can avoid a painful conversation.”
We are only beginning to understand the many issues that neurosurgical patients face at the end of life, but so far it is clear that neurosurgical trainees require substantive training in prognostication, communication, and palliation (Crit Care Med. 2015 Sep;43[9]:1964-77 1,2; J Neurooncol. 2009 Jan;91[1]:39-43). Is there room in the current training paradigm for more formal education in these domains? As we move further into the 21st century, we must embrace the need for masterful clinicians outside of the operating room if we are to ever challenge the axiom set forth by the renowned French surgeon, René Leriche, some 65 years ago: “Every surgeon carries within himself a small cemetery, where from time to time he goes to pray – a place of bitterness and regret, where he must look for an explanation for his failures.” Let us look forward to the day when this is no longer the case.
Stephen Miranda is a medical student from the University of Rochester, who is now working as a research fellow at Ariadne Labs, a joint center for health systems innovation at Brigham & Women’s Hospital and Harvard T.H. Chan School of Public Health, both in Boston.
The juxtaposition between my first 2 days of neurosurgery could not have been more profound. On my first day as a third-year medical student, the attending and chief resident let me take the lead on the first case: a straightforward brain biopsy. I got to make the incision, drill the burr hole, and perform the needle biopsy. I still remember the thrill of the technical challenge, the controlled violence of drilling into the skull, and the finesse of accessing the tumor core.
The buzz was so strong that I barely registered the diagnosis that was called back from the pathologist: glioblastoma. It was not until I saw the face of the disease the next morning that I understood the reality of a GBM diagnosis. That face belonged to a 47-year-old man who hadn’t slept all night, wide eyed with apprehension at what news I might bring. He beseeched me with questions, and though his aphasia left him stammering to get the words out, I knew exactly what he was asking: Would he live or die? It was a question I was in no position to answer. Instead, I reassured him that we were waiting on the final pathology, all the while trying to forget the fact that the frozen section suggested an aggressive subtype, surely heralding a poor prognosis.
In his poignant memoir, “Do No Harm: Stories of Life, Death and Brain Surgery” (New York: Thomas Dunne Book, 2015), Dr. Henry Marsh writes beautifully about how difficult it can be to find the balance between optimism and realism. In one memorable passage, Dr. Marsh shows a house officer a scan of a highly malignant brain tumor and asks him what he would say to the patient. The trainee reflexively hides behind jargon, skirting around what he knew to be the truth: This tumor would kill her. Marsh presses him to admit that he’s lying, before lamenting at how hard it is to improve these critical communication skills: “When I have had to break bad news I never know whether I have done it well or not. The patients aren’t going to ring me up afterward and say, ‘Mr. Marsh, I really liked the way you told me that I was going to die,’ or ‘Mr. Marsh, you were crap.’ You can only hope that you haven’t made too much of a mess of it.”
I could certainly relate to Dr. Marsh’s house officer as I walked away from my own patient. I felt almost deceitful withholding diagnostic information from him, even if I did the “right” thing. It made me wonder, why did I want to become a neurosurgeon? Surely to help people through some of the most difficult moments of their lives. But is it possible to be a source of comfort when you are required so often to be a harbinger of death? The answer depends on whether one can envision a role for the neurosurgeon beyond the mandate of “life at all costs.”
While the field has become known for its life-saving procedures, neurosurgeons are called just as often to preside over the end of their patient’s lives – work that requires just as much skill as any technical procedure. Dr. Marsh recognized the tremendous human cost of neglecting that work. For cases that appear “hopeless,” he writes, “We often end up operating because it’s easier than being honest, and it means that we can avoid a painful conversation.”
We are only beginning to understand the many issues that neurosurgical patients face at the end of life, but so far it is clear that neurosurgical trainees require substantive training in prognostication, communication, and palliation (Crit Care Med. 2015 Sep;43[9]:1964-77 1,2; J Neurooncol. 2009 Jan;91[1]:39-43). Is there room in the current training paradigm for more formal education in these domains? As we move further into the 21st century, we must embrace the need for masterful clinicians outside of the operating room if we are to ever challenge the axiom set forth by the renowned French surgeon, René Leriche, some 65 years ago: “Every surgeon carries within himself a small cemetery, where from time to time he goes to pray – a place of bitterness and regret, where he must look for an explanation for his failures.” Let us look forward to the day when this is no longer the case.
Stephen Miranda is a medical student from the University of Rochester, who is now working as a research fellow at Ariadne Labs, a joint center for health systems innovation at Brigham & Women’s Hospital and Harvard T.H. Chan School of Public Health, both in Boston.
The juxtaposition between my first 2 days of neurosurgery could not have been more profound. On my first day as a third-year medical student, the attending and chief resident let me take the lead on the first case: a straightforward brain biopsy. I got to make the incision, drill the burr hole, and perform the needle biopsy. I still remember the thrill of the technical challenge, the controlled violence of drilling into the skull, and the finesse of accessing the tumor core.
The buzz was so strong that I barely registered the diagnosis that was called back from the pathologist: glioblastoma. It was not until I saw the face of the disease the next morning that I understood the reality of a GBM diagnosis. That face belonged to a 47-year-old man who hadn’t slept all night, wide eyed with apprehension at what news I might bring. He beseeched me with questions, and though his aphasia left him stammering to get the words out, I knew exactly what he was asking: Would he live or die? It was a question I was in no position to answer. Instead, I reassured him that we were waiting on the final pathology, all the while trying to forget the fact that the frozen section suggested an aggressive subtype, surely heralding a poor prognosis.
In his poignant memoir, “Do No Harm: Stories of Life, Death and Brain Surgery” (New York: Thomas Dunne Book, 2015), Dr. Henry Marsh writes beautifully about how difficult it can be to find the balance between optimism and realism. In one memorable passage, Dr. Marsh shows a house officer a scan of a highly malignant brain tumor and asks him what he would say to the patient. The trainee reflexively hides behind jargon, skirting around what he knew to be the truth: This tumor would kill her. Marsh presses him to admit that he’s lying, before lamenting at how hard it is to improve these critical communication skills: “When I have had to break bad news I never know whether I have done it well or not. The patients aren’t going to ring me up afterward and say, ‘Mr. Marsh, I really liked the way you told me that I was going to die,’ or ‘Mr. Marsh, you were crap.’ You can only hope that you haven’t made too much of a mess of it.”
I could certainly relate to Dr. Marsh’s house officer as I walked away from my own patient. I felt almost deceitful withholding diagnostic information from him, even if I did the “right” thing. It made me wonder, why did I want to become a neurosurgeon? Surely to help people through some of the most difficult moments of their lives. But is it possible to be a source of comfort when you are required so often to be a harbinger of death? The answer depends on whether one can envision a role for the neurosurgeon beyond the mandate of “life at all costs.”
While the field has become known for its life-saving procedures, neurosurgeons are called just as often to preside over the end of their patient’s lives – work that requires just as much skill as any technical procedure. Dr. Marsh recognized the tremendous human cost of neglecting that work. For cases that appear “hopeless,” he writes, “We often end up operating because it’s easier than being honest, and it means that we can avoid a painful conversation.”
We are only beginning to understand the many issues that neurosurgical patients face at the end of life, but so far it is clear that neurosurgical trainees require substantive training in prognostication, communication, and palliation (Crit Care Med. 2015 Sep;43[9]:1964-77 1,2; J Neurooncol. 2009 Jan;91[1]:39-43). Is there room in the current training paradigm for more formal education in these domains? As we move further into the 21st century, we must embrace the need for masterful clinicians outside of the operating room if we are to ever challenge the axiom set forth by the renowned French surgeon, René Leriche, some 65 years ago: “Every surgeon carries within himself a small cemetery, where from time to time he goes to pray – a place of bitterness and regret, where he must look for an explanation for his failures.” Let us look forward to the day when this is no longer the case.
Stephen Miranda is a medical student from the University of Rochester, who is now working as a research fellow at Ariadne Labs, a joint center for health systems innovation at Brigham & Women’s Hospital and Harvard T.H. Chan School of Public Health, both in Boston.
The palliative path: Talking with elderly patients facing emergency surgery
An expert panel has developed a communication framework to improve treatment of older, seriously ill patients who have surgical emergencies, which has been published online in Annals of Surgery.
A substantial portion of older patients who undergo emergency surgeries already have serious life-limiting illnesses such as cardiopulmonary disease, renal failure, liver failure, dementia, severe neurological impairment, or malignancy. The advisory panel based its work on the premise that surgery in these circumstances can lead to significant further morbidity, health care utilization, functional decline, prolonged hospital stay or institutionalization, and death, with attendant physical discomfort and psychological distress at the end of these patients’ lives.
Surgeons consulted in the emergency setting for these patients are hampered by patients unable to communicate well because they are in extremis, by surrogates who are unprepared for their role, and by time constraints, lack of familiarity with the patient, poor understanding of the illness by patients and families, prognostic uncertainty, and inadequate advance care planning. In addition, “many surgeons lack skills to engage in conversations about end-of-life care, or are too unfamiliar with palliative options to discuss them well,” or feel obligated to maintain postoperative life support despite the patient’s wishes, said Dr. Zara Cooper, of Ariadne Labs and the Center for Surgery and Public Health at Brigham and Women’s Hospital, both in Boston, and her associates.
To address these issues and assist surgeons in caring for such patients, an expert panel of 23 national leaders in acute care surgery, general surgery, surgical oncology, palliative medicine, critical care, emergency medicine, anesthesia, and health care innovation was convened at Harvard Medical School, Boston.
The focus of the panel’s recommendations was a structured communications framework prototype to facilitate shared decision-making in these difficult circumstances.
Among the panel’s recommendations for surgeons were the following priorities:
• Review the medical record and consult the treatment team to fully understand the patient’s current condition, comorbidities, expected illness trajectory, and preferences for end-of-life care.
• Assess functional performance as part of the routine history and physical to fully understand the patient’s fitness for surgery.
• Formulate a prognosis regarding the patient’s overall health both with and without surgery.
The panel offered a set of principles and specific elements for the meeting with the patient and family:
• The surgeon should begin by introducing himself or herself; according to reports in the literature, physicians fail to do this approximately half of the time.
• Pay attention to nonverbal communication, such as eye contact and physical contact, as this is critical to building rapport. Immediately address pain, anxiety, and other indicators of distress, to maximize the patients’ and the families’ engagement in subsequent medical discussions. “Although adequate analgesia may render a patient unable to make their own decisions, surrogates are more likely to make appropriate decisions when they feel their loved one is comfortable,” the panel noted.
• Allow pauses and silences to occur. Let the patient and the family process information and their own emotions.
• Elicit the patients’ or the surrogates’ understanding of the illness and their views of the patients’ likely trajectory, correcting any inaccuracies. This substantially influences their decisions regarding the aggressiveness of subsequent treatments.
• Inform the patient and family of the life-threatening nature of the patient’s acute condition and its potential impact on the rest of his or her life, including the possibility of prolonged life support, ICU stay, burdensome treatment, and loss of independence. Use accepted techniques for breaking bad news, and check to be sure the patient understands what was conveyed.
• At this point, the surgeon should synthesize and summarize the information from the patient, the family, and the medical record, then pause to give them time to process the information and to assess their emotional state. It is helpful to label and respond to the patient’s emotions at this juncture, and to build empathy with statements such as “I know this is difficult news, and I wish it were different.”
• Describe the benefits, burdens, and range of likely outcomes if surgery is undertaken and if it is not. The surgeon should use nonmedical language to describe symptoms, and should convey his or her expectations regarding length of hospitalization, need for and duration of life support, burdensome symptoms, discharge to an institution, and functional recovery.
• Surgeons should be able to communicate palliative options possible either in combination with surgery or instead of surgery. Palliative care can aid in managing advanced symptoms, providing psychosocial support for patients and caregivers, facilitating interdisciplinary communication, and facilitating medical decisions and care transitions.
• Avoid describing surgical procedures as “doing everything” and palliative care as “doing nothing.” This can make patients and families “feel abandoned, fearful, isolated, and angry, and fails to encompass palliative care’s practices of proactive communication, aggressive symptom management, and timely emotional support to alleviate suffering and affirm quality of life,” the panel said.
• Surgeons should explicitly support the patients’ medical decisions, whether or not they choose surgery.
The panel also cited a few factors that would assist surgeons in following these recommendations. First, surgeons must recognize the importance of communicating well with seriously ill older patients and acknowledge that this is a crucial clinical skill for them to cultivate. They must also recognize that palliative care is vital to delivering high-quality surgical care. Surgeons should consider discharging patients to hospice, which can improve pain and symptom management, improve patient and family satisfaction with care, and avoid unwanted hospitalization or cardiopulmonary resuscitation.
“There are a number of major barriers to introducing palliative care in these situations. One is an education problem - the perception on the part of patients and clinicians, and surgeons in particular, that palliative care is only limited to end-of-life care, which it is not. It is a misperception of what palliative care means in this equation - that palliative care and hospice are the same thing, which they absolutely are not,”said Dr. Cooper in an interview.
”The definition of palliative care has evolved over the past decade and the focus of palliative care is on quality of life and alleviating symptoms. End-of-life palliative care is part of that, and as patients get closer to the end of life, symptom management and quality of life become more focal than life-prolonging treatment... But for patients with chronic and serious illness, there has to be a role for palliative care because we know that when patients feel better, they tend to live longer. And when patients feel their emotional concerns and physical needs are being addressed, they tend to do better. Patients families have improved satisfaction when their loved one receives palliative care,” she noted.”
However, the number of palliative providers is completely inadequate to meet the needs of the number of seriously ill patients, she said. And a lot of hospital-based palliative care is by necessity limited to end-of-life care because of a lack of palliative resources.
Dr. Atul Gawande, a coauthor of the panel recommendations, wrote a best-selling book, Being Mortal (New York: Metropolitan Books, 2014) addressing the shortcomings and potential remaking of medical care in the context of age-related frailty, grave illness, and death. Dr. Cooper noted that there is a growing sentiment among the general public that they want to have their quality of life addressed in the type of medical care they receive. She said that Dr. Gawande’s book tapped into the perception of a lack of recognition of personhood of seriously ill patients.
“We often focus on diagnosis and we don’t have the ‘bandwidth’ to focus on the person carrying that diagnosis, and our patients and focus on the person carrying that diagnosis, but our patients and their families are demanding different types of care. So, ultimately, the patients will be the ones to push us to do better for them.”
The next steps to further developing a widely used and validated communication framework would be to create educational opportunities for clinicians to develop clinical skills in communication with seriously ill patients and palliative care, and to study the impact of these initiatives on improving outcomes most relevant to older patient. This work was supported by the Ariadne Labs, a Joint Center for Health System Innovation at Brigham and Women’s Hospital. Dr. Cooper and her associates reported having no relevant financial disclosures.
An expert panel has developed a communication framework to improve treatment of older, seriously ill patients who have surgical emergencies, which has been published online in Annals of Surgery.
A substantial portion of older patients who undergo emergency surgeries already have serious life-limiting illnesses such as cardiopulmonary disease, renal failure, liver failure, dementia, severe neurological impairment, or malignancy. The advisory panel based its work on the premise that surgery in these circumstances can lead to significant further morbidity, health care utilization, functional decline, prolonged hospital stay or institutionalization, and death, with attendant physical discomfort and psychological distress at the end of these patients’ lives.
Surgeons consulted in the emergency setting for these patients are hampered by patients unable to communicate well because they are in extremis, by surrogates who are unprepared for their role, and by time constraints, lack of familiarity with the patient, poor understanding of the illness by patients and families, prognostic uncertainty, and inadequate advance care planning. In addition, “many surgeons lack skills to engage in conversations about end-of-life care, or are too unfamiliar with palliative options to discuss them well,” or feel obligated to maintain postoperative life support despite the patient’s wishes, said Dr. Zara Cooper, of Ariadne Labs and the Center for Surgery and Public Health at Brigham and Women’s Hospital, both in Boston, and her associates.
To address these issues and assist surgeons in caring for such patients, an expert panel of 23 national leaders in acute care surgery, general surgery, surgical oncology, palliative medicine, critical care, emergency medicine, anesthesia, and health care innovation was convened at Harvard Medical School, Boston.
The focus of the panel’s recommendations was a structured communications framework prototype to facilitate shared decision-making in these difficult circumstances.
Among the panel’s recommendations for surgeons were the following priorities:
• Review the medical record and consult the treatment team to fully understand the patient’s current condition, comorbidities, expected illness trajectory, and preferences for end-of-life care.
• Assess functional performance as part of the routine history and physical to fully understand the patient’s fitness for surgery.
• Formulate a prognosis regarding the patient’s overall health both with and without surgery.
The panel offered a set of principles and specific elements for the meeting with the patient and family:
• The surgeon should begin by introducing himself or herself; according to reports in the literature, physicians fail to do this approximately half of the time.
• Pay attention to nonverbal communication, such as eye contact and physical contact, as this is critical to building rapport. Immediately address pain, anxiety, and other indicators of distress, to maximize the patients’ and the families’ engagement in subsequent medical discussions. “Although adequate analgesia may render a patient unable to make their own decisions, surrogates are more likely to make appropriate decisions when they feel their loved one is comfortable,” the panel noted.
• Allow pauses and silences to occur. Let the patient and the family process information and their own emotions.
• Elicit the patients’ or the surrogates’ understanding of the illness and their views of the patients’ likely trajectory, correcting any inaccuracies. This substantially influences their decisions regarding the aggressiveness of subsequent treatments.
• Inform the patient and family of the life-threatening nature of the patient’s acute condition and its potential impact on the rest of his or her life, including the possibility of prolonged life support, ICU stay, burdensome treatment, and loss of independence. Use accepted techniques for breaking bad news, and check to be sure the patient understands what was conveyed.
• At this point, the surgeon should synthesize and summarize the information from the patient, the family, and the medical record, then pause to give them time to process the information and to assess their emotional state. It is helpful to label and respond to the patient’s emotions at this juncture, and to build empathy with statements such as “I know this is difficult news, and I wish it were different.”
• Describe the benefits, burdens, and range of likely outcomes if surgery is undertaken and if it is not. The surgeon should use nonmedical language to describe symptoms, and should convey his or her expectations regarding length of hospitalization, need for and duration of life support, burdensome symptoms, discharge to an institution, and functional recovery.
• Surgeons should be able to communicate palliative options possible either in combination with surgery or instead of surgery. Palliative care can aid in managing advanced symptoms, providing psychosocial support for patients and caregivers, facilitating interdisciplinary communication, and facilitating medical decisions and care transitions.
• Avoid describing surgical procedures as “doing everything” and palliative care as “doing nothing.” This can make patients and families “feel abandoned, fearful, isolated, and angry, and fails to encompass palliative care’s practices of proactive communication, aggressive symptom management, and timely emotional support to alleviate suffering and affirm quality of life,” the panel said.
• Surgeons should explicitly support the patients’ medical decisions, whether or not they choose surgery.
The panel also cited a few factors that would assist surgeons in following these recommendations. First, surgeons must recognize the importance of communicating well with seriously ill older patients and acknowledge that this is a crucial clinical skill for them to cultivate. They must also recognize that palliative care is vital to delivering high-quality surgical care. Surgeons should consider discharging patients to hospice, which can improve pain and symptom management, improve patient and family satisfaction with care, and avoid unwanted hospitalization or cardiopulmonary resuscitation.
“There are a number of major barriers to introducing palliative care in these situations. One is an education problem - the perception on the part of patients and clinicians, and surgeons in particular, that palliative care is only limited to end-of-life care, which it is not. It is a misperception of what palliative care means in this equation - that palliative care and hospice are the same thing, which they absolutely are not,”said Dr. Cooper in an interview.
”The definition of palliative care has evolved over the past decade and the focus of palliative care is on quality of life and alleviating symptoms. End-of-life palliative care is part of that, and as patients get closer to the end of life, symptom management and quality of life become more focal than life-prolonging treatment... But for patients with chronic and serious illness, there has to be a role for palliative care because we know that when patients feel better, they tend to live longer. And when patients feel their emotional concerns and physical needs are being addressed, they tend to do better. Patients families have improved satisfaction when their loved one receives palliative care,” she noted.”
However, the number of palliative providers is completely inadequate to meet the needs of the number of seriously ill patients, she said. And a lot of hospital-based palliative care is by necessity limited to end-of-life care because of a lack of palliative resources.
Dr. Atul Gawande, a coauthor of the panel recommendations, wrote a best-selling book, Being Mortal (New York: Metropolitan Books, 2014) addressing the shortcomings and potential remaking of medical care in the context of age-related frailty, grave illness, and death. Dr. Cooper noted that there is a growing sentiment among the general public that they want to have their quality of life addressed in the type of medical care they receive. She said that Dr. Gawande’s book tapped into the perception of a lack of recognition of personhood of seriously ill patients.
“We often focus on diagnosis and we don’t have the ‘bandwidth’ to focus on the person carrying that diagnosis, and our patients and focus on the person carrying that diagnosis, but our patients and their families are demanding different types of care. So, ultimately, the patients will be the ones to push us to do better for them.”
The next steps to further developing a widely used and validated communication framework would be to create educational opportunities for clinicians to develop clinical skills in communication with seriously ill patients and palliative care, and to study the impact of these initiatives on improving outcomes most relevant to older patient. This work was supported by the Ariadne Labs, a Joint Center for Health System Innovation at Brigham and Women’s Hospital. Dr. Cooper and her associates reported having no relevant financial disclosures.
An expert panel has developed a communication framework to improve treatment of older, seriously ill patients who have surgical emergencies, which has been published online in Annals of Surgery.
A substantial portion of older patients who undergo emergency surgeries already have serious life-limiting illnesses such as cardiopulmonary disease, renal failure, liver failure, dementia, severe neurological impairment, or malignancy. The advisory panel based its work on the premise that surgery in these circumstances can lead to significant further morbidity, health care utilization, functional decline, prolonged hospital stay or institutionalization, and death, with attendant physical discomfort and psychological distress at the end of these patients’ lives.
Surgeons consulted in the emergency setting for these patients are hampered by patients unable to communicate well because they are in extremis, by surrogates who are unprepared for their role, and by time constraints, lack of familiarity with the patient, poor understanding of the illness by patients and families, prognostic uncertainty, and inadequate advance care planning. In addition, “many surgeons lack skills to engage in conversations about end-of-life care, or are too unfamiliar with palliative options to discuss them well,” or feel obligated to maintain postoperative life support despite the patient’s wishes, said Dr. Zara Cooper, of Ariadne Labs and the Center for Surgery and Public Health at Brigham and Women’s Hospital, both in Boston, and her associates.
To address these issues and assist surgeons in caring for such patients, an expert panel of 23 national leaders in acute care surgery, general surgery, surgical oncology, palliative medicine, critical care, emergency medicine, anesthesia, and health care innovation was convened at Harvard Medical School, Boston.
The focus of the panel’s recommendations was a structured communications framework prototype to facilitate shared decision-making in these difficult circumstances.
Among the panel’s recommendations for surgeons were the following priorities:
• Review the medical record and consult the treatment team to fully understand the patient’s current condition, comorbidities, expected illness trajectory, and preferences for end-of-life care.
• Assess functional performance as part of the routine history and physical to fully understand the patient’s fitness for surgery.
• Formulate a prognosis regarding the patient’s overall health both with and without surgery.
The panel offered a set of principles and specific elements for the meeting with the patient and family:
• The surgeon should begin by introducing himself or herself; according to reports in the literature, physicians fail to do this approximately half of the time.
• Pay attention to nonverbal communication, such as eye contact and physical contact, as this is critical to building rapport. Immediately address pain, anxiety, and other indicators of distress, to maximize the patients’ and the families’ engagement in subsequent medical discussions. “Although adequate analgesia may render a patient unable to make their own decisions, surrogates are more likely to make appropriate decisions when they feel their loved one is comfortable,” the panel noted.
• Allow pauses and silences to occur. Let the patient and the family process information and their own emotions.
• Elicit the patients’ or the surrogates’ understanding of the illness and their views of the patients’ likely trajectory, correcting any inaccuracies. This substantially influences their decisions regarding the aggressiveness of subsequent treatments.
• Inform the patient and family of the life-threatening nature of the patient’s acute condition and its potential impact on the rest of his or her life, including the possibility of prolonged life support, ICU stay, burdensome treatment, and loss of independence. Use accepted techniques for breaking bad news, and check to be sure the patient understands what was conveyed.
• At this point, the surgeon should synthesize and summarize the information from the patient, the family, and the medical record, then pause to give them time to process the information and to assess their emotional state. It is helpful to label and respond to the patient’s emotions at this juncture, and to build empathy with statements such as “I know this is difficult news, and I wish it were different.”
• Describe the benefits, burdens, and range of likely outcomes if surgery is undertaken and if it is not. The surgeon should use nonmedical language to describe symptoms, and should convey his or her expectations regarding length of hospitalization, need for and duration of life support, burdensome symptoms, discharge to an institution, and functional recovery.
• Surgeons should be able to communicate palliative options possible either in combination with surgery or instead of surgery. Palliative care can aid in managing advanced symptoms, providing psychosocial support for patients and caregivers, facilitating interdisciplinary communication, and facilitating medical decisions and care transitions.
• Avoid describing surgical procedures as “doing everything” and palliative care as “doing nothing.” This can make patients and families “feel abandoned, fearful, isolated, and angry, and fails to encompass palliative care’s practices of proactive communication, aggressive symptom management, and timely emotional support to alleviate suffering and affirm quality of life,” the panel said.
• Surgeons should explicitly support the patients’ medical decisions, whether or not they choose surgery.
The panel also cited a few factors that would assist surgeons in following these recommendations. First, surgeons must recognize the importance of communicating well with seriously ill older patients and acknowledge that this is a crucial clinical skill for them to cultivate. They must also recognize that palliative care is vital to delivering high-quality surgical care. Surgeons should consider discharging patients to hospice, which can improve pain and symptom management, improve patient and family satisfaction with care, and avoid unwanted hospitalization or cardiopulmonary resuscitation.
“There are a number of major barriers to introducing palliative care in these situations. One is an education problem - the perception on the part of patients and clinicians, and surgeons in particular, that palliative care is only limited to end-of-life care, which it is not. It is a misperception of what palliative care means in this equation - that palliative care and hospice are the same thing, which they absolutely are not,”said Dr. Cooper in an interview.
”The definition of palliative care has evolved over the past decade and the focus of palliative care is on quality of life and alleviating symptoms. End-of-life palliative care is part of that, and as patients get closer to the end of life, symptom management and quality of life become more focal than life-prolonging treatment... But for patients with chronic and serious illness, there has to be a role for palliative care because we know that when patients feel better, they tend to live longer. And when patients feel their emotional concerns and physical needs are being addressed, they tend to do better. Patients families have improved satisfaction when their loved one receives palliative care,” she noted.”
However, the number of palliative providers is completely inadequate to meet the needs of the number of seriously ill patients, she said. And a lot of hospital-based palliative care is by necessity limited to end-of-life care because of a lack of palliative resources.
Dr. Atul Gawande, a coauthor of the panel recommendations, wrote a best-selling book, Being Mortal (New York: Metropolitan Books, 2014) addressing the shortcomings and potential remaking of medical care in the context of age-related frailty, grave illness, and death. Dr. Cooper noted that there is a growing sentiment among the general public that they want to have their quality of life addressed in the type of medical care they receive. She said that Dr. Gawande’s book tapped into the perception of a lack of recognition of personhood of seriously ill patients.
“We often focus on diagnosis and we don’t have the ‘bandwidth’ to focus on the person carrying that diagnosis, and our patients and focus on the person carrying that diagnosis, but our patients and their families are demanding different types of care. So, ultimately, the patients will be the ones to push us to do better for them.”
The next steps to further developing a widely used and validated communication framework would be to create educational opportunities for clinicians to develop clinical skills in communication with seriously ill patients and palliative care, and to study the impact of these initiatives on improving outcomes most relevant to older patient. This work was supported by the Ariadne Labs, a Joint Center for Health System Innovation at Brigham and Women’s Hospital. Dr. Cooper and her associates reported having no relevant financial disclosures.
FROM ANNALS OF SURGERY
Early Palliative Care Can Save Money
Clinical question: Does time to consult after admission change the effect palliative care consultation has on cost of care?
Background: Studies have shown that early palliative care involvement improves quality of life and survival among cancer patients while reducing the cost of care. Little is known about the optimal timing of palliative care consultation and its effect on cost.
Study design: Prospective, observational study.
Setting: Multi-site, high-volume, tertiary care hospitals with established palliative care teams.
Synopsis: Clinical and cost data were collected for 969 adult patients with advanced cancer admitted to the five participating hospitals. Among those, 256 patients received palliative care consultation and 713 received usual care. Subsamples were created based on time to consultation after admission.
The study found that earlier consultation yielded larger effects on cost savings. There was a 24% reduction in total cost if consultation occurred within two days (95% CI, -$3,438 to -$1,122; P<0.001), with estimated savings of $2,280. For consultation within six days of admission, there was a $1,312 savings (95% CI, -$2,568 to -$ 1,122; P<0.04), consistent with a 14% reduction in total cost.
There are notable limitations to this study. Half of eligible patients were excluded due to incomplete data collection, resulting in a small sample size. Further, these results can be generalized only to inpatients with advanced cancer.
Bottom line: Reducing the time to consultation with palliative care increases cost savings. In advanced cancer patients, a 24% reduction in total costs was realized for consultation within two days following admission.
Citation: May P, Garrido MM, Cassel JB, et al. Prospective cohort study of hospital palliative care teams for inpatients with advanced cancer: earlier consultation is associated with larger cost-saving effect. J Clin Oncol. 2015;33(25):2745-2752.
Clinical question: Does time to consult after admission change the effect palliative care consultation has on cost of care?
Background: Studies have shown that early palliative care involvement improves quality of life and survival among cancer patients while reducing the cost of care. Little is known about the optimal timing of palliative care consultation and its effect on cost.
Study design: Prospective, observational study.
Setting: Multi-site, high-volume, tertiary care hospitals with established palliative care teams.
Synopsis: Clinical and cost data were collected for 969 adult patients with advanced cancer admitted to the five participating hospitals. Among those, 256 patients received palliative care consultation and 713 received usual care. Subsamples were created based on time to consultation after admission.
The study found that earlier consultation yielded larger effects on cost savings. There was a 24% reduction in total cost if consultation occurred within two days (95% CI, -$3,438 to -$1,122; P<0.001), with estimated savings of $2,280. For consultation within six days of admission, there was a $1,312 savings (95% CI, -$2,568 to -$ 1,122; P<0.04), consistent with a 14% reduction in total cost.
There are notable limitations to this study. Half of eligible patients were excluded due to incomplete data collection, resulting in a small sample size. Further, these results can be generalized only to inpatients with advanced cancer.
Bottom line: Reducing the time to consultation with palliative care increases cost savings. In advanced cancer patients, a 24% reduction in total costs was realized for consultation within two days following admission.
Citation: May P, Garrido MM, Cassel JB, et al. Prospective cohort study of hospital palliative care teams for inpatients with advanced cancer: earlier consultation is associated with larger cost-saving effect. J Clin Oncol. 2015;33(25):2745-2752.
Clinical question: Does time to consult after admission change the effect palliative care consultation has on cost of care?
Background: Studies have shown that early palliative care involvement improves quality of life and survival among cancer patients while reducing the cost of care. Little is known about the optimal timing of palliative care consultation and its effect on cost.
Study design: Prospective, observational study.
Setting: Multi-site, high-volume, tertiary care hospitals with established palliative care teams.
Synopsis: Clinical and cost data were collected for 969 adult patients with advanced cancer admitted to the five participating hospitals. Among those, 256 patients received palliative care consultation and 713 received usual care. Subsamples were created based on time to consultation after admission.
The study found that earlier consultation yielded larger effects on cost savings. There was a 24% reduction in total cost if consultation occurred within two days (95% CI, -$3,438 to -$1,122; P<0.001), with estimated savings of $2,280. For consultation within six days of admission, there was a $1,312 savings (95% CI, -$2,568 to -$ 1,122; P<0.04), consistent with a 14% reduction in total cost.
There are notable limitations to this study. Half of eligible patients were excluded due to incomplete data collection, resulting in a small sample size. Further, these results can be generalized only to inpatients with advanced cancer.
Bottom line: Reducing the time to consultation with palliative care increases cost savings. In advanced cancer patients, a 24% reduction in total costs was realized for consultation within two days following admission.
Citation: May P, Garrido MM, Cassel JB, et al. Prospective cohort study of hospital palliative care teams for inpatients with advanced cancer: earlier consultation is associated with larger cost-saving effect. J Clin Oncol. 2015;33(25):2745-2752.