User login
61-year-old woman • nausea • paresthesia • cold allodynia • Dx?
THE CASE
An active 61-year-old woman (140 lbs) in good health became ill during a sailing holiday in the Virgin Islands. During the trip, she ate various fish in local restaurants; after one lunch, she developed nausea, diarrhea, dizziness, headache, and light-headedness. In the following days, she suffered “intense itching” in the ears, dizziness, malaise, a “fluttering feeling” throughout her body, genitourinary sensitivity, and a “rhythmic buzzing sensation near the rectum.”
She said that cold objects and beverages felt uncomfortably hot (cold allodynia). She noted heightened senses of smell and taste, as well as paresthesia down her spine, and described feeling “moody.” She reduced her workload, took many days off from work, and ceased consuming meat and alcohol because these items seemed to aggravate her symptoms.
The paresthesia persisted, and she consulted her family physician one month later. Laboratory tests—including a complete blood count, hematocrit, thyroid-stimulating hormone, antinuclear antibodies, and titers for Ehrlichia chaffeensis, Lyme disease, and Anaplasma phagocytophila—all yielded normal results. Her symptoms continued for 3 more months before referral to Medical Toxicology.
THE DIAGNOSIS
The patient’s symptoms and history were consistent with ciguatera poisoning. Features supporting this diagnosis included an acute gastrointestinal illness after eating fish caught in tropical waters and subsequent persistent paresthesia, including cold allodynia.1 Laboratory testing excluded acute infection, anemia, thyroid dysfunction, vitamin B12 deficiency, lupus, rheumatoid arthritis, Lyme disease, ehrlichiosis, and anaplasmosis.
DISCUSSION
Ciguatera results from ciguatoxin, a class of heat-stable polycyclic toxins produced in warm tropical waters by microscopic dinoflagellates (most often Gambierdiscus toxicus).2,3 Small variations exist in the Caribbean, Pacific, and Indian Ocean forms. Ciguatoxin bio-accumulates in the food chain, and humans most often ingest it by eating larger fish (typically barracuda, snapper, grouper, or amberjack).4 Because ciguatoxin confers no characteristic taste or smell to the fish, people who prepare or eat contaminated seafood have no reliable means to detect and avoid it.
Ciguatoxin opens neuronal voltage-gated sodium channels and blocks delayed-rectifier potassium channels.5 These cause repetitive, spontaneous action potentials that explain the paresthesia. Sodium influx triggers an increase in intracellular calcium concentrations. Increased intracellular sodium and calcium concentrations draw water into the intracellular space and cause neuronal edema.
Death is rarely associated with ciguatera (< 0.1% in the largest observational study).1 Even without treatment (discussed shortly), symptoms of ciguatera will gradually resolve over several weeks to several months in most cases.1,4,5 However, after recovery, patients often briefly experience milder symptoms after consuming fish, alcohol, or nuts.6
Continue to: Treatment of ciguatera
Treatment of ciguatera may include intravenous (IV) mannitol infusion. Other treatments, such as amitriptyline, gabapentin, pregabalin, and tocainide, have been used, but there is limited supporting evidence and they appear variably effective.7
Mannitol reverses the effects of ciguatoxin, with suppression of spontaneous action potentials and reversal of neuronal edema.8,9 It is reasonable to offer mannitol for acute or persistent symptoms of ciguatera fish poisoning even after a delay of several weeks.
A recent systematic review found that mannitol has the largest body of evidence supporting its use, although that evidence is generally of low quality (case reports and large case series).7 While these reports10-13 describe beneficial effects of mannitol, a single randomized trial suggested that mannitol is no more effective than normal saline.14 However, this study was underpowered and had inadequate treatment concealment; twice as many saline control patients as mannitol-treated patients requested a rescue dose of mannitol.14
Mannitol may be most effective when given early in the course of ciguatera but has shown some success when given later.5,12,13 In 1 large case series, the longest interval from symptom onset to successful treatment was 70 days, although most patients with satisfactory results received mannitol in the first few days.5
Our patient was administered an IV infusion of 100 g of 20% mannitol over 1 hour. She received the infusion 140 days after the onset of her symptoms and experienced rapid symptom relief.
Continue to: At a follow-up visit...
At a follow-up visit 2 weeks later, she described increased energy and further improvement in her paresthesia. She returned to a full work schedule and resumed all of her daily activities. However, she continued to avoid alcohol and proteins, as she had experienced a mild recurrence that she temporally related to eating meat and drinking alcohol.
At the 2-month follow-up, the patient reported continued improvement in her paresthesia but continued to experience occasional gastrointestinal symptoms and fatigue associated with meat and alcohol consumption.
The Takeaway
Ciguatera fish poisoning is largely a clinical diagnosis. It is based on early gastrointestinal symptoms followed by persistent paresthesia and cold allodynia after consumption of fish caught in tropical waters. Family physicians may see ciguatera in returning travelers or people who have consumed certain fish imported from endemic areas. Untreated symptoms may last for many weeks or months. IV mannitol may relieve symptoms of ciguatera poisoning even when administered several months after symptom onset.
We are grateful to our patient, who allowed us to share her story in the hope of helping other travelers.
CORRESPONDENCE
Michael E. Mullins, MD, Division of Medical Toxicology, Department of Emergency Medicine, Washington University School of Medicine, Campus Box 8072, 660 South Euclid Avenue, Saint Louis, MO 63110; [email protected]
1. Bagnis R, Kuberski T, Laugier S. Clinical Observations of 3,009 cases of ciguatera (fish poisoning) in the South Pacific. Am J Trop Med Hyg. 1979;28:1067-1073. doi: 10.4269/ajtmh.1979.28.1067
2. Morris JG Jr, Lewin P, Smith CW, et al. Ciguatera fish poisoning: epidemiology of the disease on St. Thomas, US Virgin Islands. Am J Trop Med Hyg. 1982;31:574-578. doi: 10.4269/ajtmh.1982.31.574
3. Radke EG, Grattan LM, Cook RL, et al. Ciguatera incidence in the US Virgin Islands has not increased over a 30-year time period despite rising seawater temperatures. Am J Trop Med Hyg. 2013;88:908-913. doi: 10.4269/ajtmh.12-0676
4. Goodman DM, Rogers J, Livingston EH. Ciguatera fish poisoning.
5. Blythe DG, De Sylva DP, Fleming LE, et al. Clinical experience with IV mannitol in the treatment of ciguatera. Bull Soc Pathol Exot. 1992;85:425-426.
6. Lewis, RJ. The changing face of ciguatera. Toxicon. 2001;39:97-106. doi: 10.1016/s0041-0101(00)00161-6
7. Mullins ME, Hoffman RS. Is mannitol the treatment of choice for ciguatera fish poisoning? Clin Toxicol (Phila). 2017;55:947-955. doi: 10.1080/15563650.2017.1327664
8. Nicholson GM, Lewis, RJ. Ciguatoxins: cyclic polyether modulators of voltage-gated ion channel function. Mar Drugs. 2006;4:82-118.
9. Mattei C, Molgo J, Marquais M, et al. Hyperosmolar D-mannitol reverses the increased membrane excitability and the nodal swelling caused by Caribbean ciguatoxin-1 in single frog myelinated neurons. Brain Res. 1999;847:50-58. doi: 10.1016/s0006-8993(99)02032-6
10. Palafox NA, Jain LG, Pinano AZ, et al. Successful treatment of ciguatera fish poisoning with intravenous mannitol. JAMA. 1988;259:2740-2742.
11. Pearn JH, Lewis RJ, Ruff T, et al. Ciguatera and mannitol: experience with a new treatment regimen. Med J Aust. 1989;151:77-80. doi: 10.5694/j.1326-5377.1989.tb101165.x
12. Eastaugh JA. Delayed use of intravenous mannitol in ciguatera (fish poisoning). Ann Emerg Med. 1996;28:105-106. doi: 10.1016/s0196-0644(96)70151-8
13. Schwarz ES, Mullins ME, Brooks CB. Ciguatera poisoning successfully treated with delayed mannitol. Ann Emerg Med. 2008;52:476-477. doi: 10.1016/j.annemergmed.2008.05.015
14. Schnorf H, Taurarii M, Cundy T. Ciguatera fish poisoning: a double-blind randomized trial of mannitol therapy. Neurology. 2002;58:873-880. doi: 10.1212/wnl.58.6.873
THE CASE
An active 61-year-old woman (140 lbs) in good health became ill during a sailing holiday in the Virgin Islands. During the trip, she ate various fish in local restaurants; after one lunch, she developed nausea, diarrhea, dizziness, headache, and light-headedness. In the following days, she suffered “intense itching” in the ears, dizziness, malaise, a “fluttering feeling” throughout her body, genitourinary sensitivity, and a “rhythmic buzzing sensation near the rectum.”
She said that cold objects and beverages felt uncomfortably hot (cold allodynia). She noted heightened senses of smell and taste, as well as paresthesia down her spine, and described feeling “moody.” She reduced her workload, took many days off from work, and ceased consuming meat and alcohol because these items seemed to aggravate her symptoms.
The paresthesia persisted, and she consulted her family physician one month later. Laboratory tests—including a complete blood count, hematocrit, thyroid-stimulating hormone, antinuclear antibodies, and titers for Ehrlichia chaffeensis, Lyme disease, and Anaplasma phagocytophila—all yielded normal results. Her symptoms continued for 3 more months before referral to Medical Toxicology.
THE DIAGNOSIS
The patient’s symptoms and history were consistent with ciguatera poisoning. Features supporting this diagnosis included an acute gastrointestinal illness after eating fish caught in tropical waters and subsequent persistent paresthesia, including cold allodynia.1 Laboratory testing excluded acute infection, anemia, thyroid dysfunction, vitamin B12 deficiency, lupus, rheumatoid arthritis, Lyme disease, ehrlichiosis, and anaplasmosis.
DISCUSSION
Ciguatera results from ciguatoxin, a class of heat-stable polycyclic toxins produced in warm tropical waters by microscopic dinoflagellates (most often Gambierdiscus toxicus).2,3 Small variations exist in the Caribbean, Pacific, and Indian Ocean forms. Ciguatoxin bio-accumulates in the food chain, and humans most often ingest it by eating larger fish (typically barracuda, snapper, grouper, or amberjack).4 Because ciguatoxin confers no characteristic taste or smell to the fish, people who prepare or eat contaminated seafood have no reliable means to detect and avoid it.
Ciguatoxin opens neuronal voltage-gated sodium channels and blocks delayed-rectifier potassium channels.5 These cause repetitive, spontaneous action potentials that explain the paresthesia. Sodium influx triggers an increase in intracellular calcium concentrations. Increased intracellular sodium and calcium concentrations draw water into the intracellular space and cause neuronal edema.
Death is rarely associated with ciguatera (< 0.1% in the largest observational study).1 Even without treatment (discussed shortly), symptoms of ciguatera will gradually resolve over several weeks to several months in most cases.1,4,5 However, after recovery, patients often briefly experience milder symptoms after consuming fish, alcohol, or nuts.6
Continue to: Treatment of ciguatera
Treatment of ciguatera may include intravenous (IV) mannitol infusion. Other treatments, such as amitriptyline, gabapentin, pregabalin, and tocainide, have been used, but there is limited supporting evidence and they appear variably effective.7
Mannitol reverses the effects of ciguatoxin, with suppression of spontaneous action potentials and reversal of neuronal edema.8,9 It is reasonable to offer mannitol for acute or persistent symptoms of ciguatera fish poisoning even after a delay of several weeks.
A recent systematic review found that mannitol has the largest body of evidence supporting its use, although that evidence is generally of low quality (case reports and large case series).7 While these reports10-13 describe beneficial effects of mannitol, a single randomized trial suggested that mannitol is no more effective than normal saline.14 However, this study was underpowered and had inadequate treatment concealment; twice as many saline control patients as mannitol-treated patients requested a rescue dose of mannitol.14
Mannitol may be most effective when given early in the course of ciguatera but has shown some success when given later.5,12,13 In 1 large case series, the longest interval from symptom onset to successful treatment was 70 days, although most patients with satisfactory results received mannitol in the first few days.5
Our patient was administered an IV infusion of 100 g of 20% mannitol over 1 hour. She received the infusion 140 days after the onset of her symptoms and experienced rapid symptom relief.
Continue to: At a follow-up visit...
At a follow-up visit 2 weeks later, she described increased energy and further improvement in her paresthesia. She returned to a full work schedule and resumed all of her daily activities. However, she continued to avoid alcohol and proteins, as she had experienced a mild recurrence that she temporally related to eating meat and drinking alcohol.
At the 2-month follow-up, the patient reported continued improvement in her paresthesia but continued to experience occasional gastrointestinal symptoms and fatigue associated with meat and alcohol consumption.
The Takeaway
Ciguatera fish poisoning is largely a clinical diagnosis. It is based on early gastrointestinal symptoms followed by persistent paresthesia and cold allodynia after consumption of fish caught in tropical waters. Family physicians may see ciguatera in returning travelers or people who have consumed certain fish imported from endemic areas. Untreated symptoms may last for many weeks or months. IV mannitol may relieve symptoms of ciguatera poisoning even when administered several months after symptom onset.
We are grateful to our patient, who allowed us to share her story in the hope of helping other travelers.
CORRESPONDENCE
Michael E. Mullins, MD, Division of Medical Toxicology, Department of Emergency Medicine, Washington University School of Medicine, Campus Box 8072, 660 South Euclid Avenue, Saint Louis, MO 63110; [email protected]
THE CASE
An active 61-year-old woman (140 lbs) in good health became ill during a sailing holiday in the Virgin Islands. During the trip, she ate various fish in local restaurants; after one lunch, she developed nausea, diarrhea, dizziness, headache, and light-headedness. In the following days, she suffered “intense itching” in the ears, dizziness, malaise, a “fluttering feeling” throughout her body, genitourinary sensitivity, and a “rhythmic buzzing sensation near the rectum.”
She said that cold objects and beverages felt uncomfortably hot (cold allodynia). She noted heightened senses of smell and taste, as well as paresthesia down her spine, and described feeling “moody.” She reduced her workload, took many days off from work, and ceased consuming meat and alcohol because these items seemed to aggravate her symptoms.
The paresthesia persisted, and she consulted her family physician one month later. Laboratory tests—including a complete blood count, hematocrit, thyroid-stimulating hormone, antinuclear antibodies, and titers for Ehrlichia chaffeensis, Lyme disease, and Anaplasma phagocytophila—all yielded normal results. Her symptoms continued for 3 more months before referral to Medical Toxicology.
THE DIAGNOSIS
The patient’s symptoms and history were consistent with ciguatera poisoning. Features supporting this diagnosis included an acute gastrointestinal illness after eating fish caught in tropical waters and subsequent persistent paresthesia, including cold allodynia.1 Laboratory testing excluded acute infection, anemia, thyroid dysfunction, vitamin B12 deficiency, lupus, rheumatoid arthritis, Lyme disease, ehrlichiosis, and anaplasmosis.
DISCUSSION
Ciguatera results from ciguatoxin, a class of heat-stable polycyclic toxins produced in warm tropical waters by microscopic dinoflagellates (most often Gambierdiscus toxicus).2,3 Small variations exist in the Caribbean, Pacific, and Indian Ocean forms. Ciguatoxin bio-accumulates in the food chain, and humans most often ingest it by eating larger fish (typically barracuda, snapper, grouper, or amberjack).4 Because ciguatoxin confers no characteristic taste or smell to the fish, people who prepare or eat contaminated seafood have no reliable means to detect and avoid it.
Ciguatoxin opens neuronal voltage-gated sodium channels and blocks delayed-rectifier potassium channels.5 These cause repetitive, spontaneous action potentials that explain the paresthesia. Sodium influx triggers an increase in intracellular calcium concentrations. Increased intracellular sodium and calcium concentrations draw water into the intracellular space and cause neuronal edema.
Death is rarely associated with ciguatera (< 0.1% in the largest observational study).1 Even without treatment (discussed shortly), symptoms of ciguatera will gradually resolve over several weeks to several months in most cases.1,4,5 However, after recovery, patients often briefly experience milder symptoms after consuming fish, alcohol, or nuts.6
Continue to: Treatment of ciguatera
Treatment of ciguatera may include intravenous (IV) mannitol infusion. Other treatments, such as amitriptyline, gabapentin, pregabalin, and tocainide, have been used, but there is limited supporting evidence and they appear variably effective.7
Mannitol reverses the effects of ciguatoxin, with suppression of spontaneous action potentials and reversal of neuronal edema.8,9 It is reasonable to offer mannitol for acute or persistent symptoms of ciguatera fish poisoning even after a delay of several weeks.
A recent systematic review found that mannitol has the largest body of evidence supporting its use, although that evidence is generally of low quality (case reports and large case series).7 While these reports10-13 describe beneficial effects of mannitol, a single randomized trial suggested that mannitol is no more effective than normal saline.14 However, this study was underpowered and had inadequate treatment concealment; twice as many saline control patients as mannitol-treated patients requested a rescue dose of mannitol.14
Mannitol may be most effective when given early in the course of ciguatera but has shown some success when given later.5,12,13 In 1 large case series, the longest interval from symptom onset to successful treatment was 70 days, although most patients with satisfactory results received mannitol in the first few days.5
Our patient was administered an IV infusion of 100 g of 20% mannitol over 1 hour. She received the infusion 140 days after the onset of her symptoms and experienced rapid symptom relief.
Continue to: At a follow-up visit...
At a follow-up visit 2 weeks later, she described increased energy and further improvement in her paresthesia. She returned to a full work schedule and resumed all of her daily activities. However, she continued to avoid alcohol and proteins, as she had experienced a mild recurrence that she temporally related to eating meat and drinking alcohol.
At the 2-month follow-up, the patient reported continued improvement in her paresthesia but continued to experience occasional gastrointestinal symptoms and fatigue associated with meat and alcohol consumption.
The Takeaway
Ciguatera fish poisoning is largely a clinical diagnosis. It is based on early gastrointestinal symptoms followed by persistent paresthesia and cold allodynia after consumption of fish caught in tropical waters. Family physicians may see ciguatera in returning travelers or people who have consumed certain fish imported from endemic areas. Untreated symptoms may last for many weeks or months. IV mannitol may relieve symptoms of ciguatera poisoning even when administered several months after symptom onset.
We are grateful to our patient, who allowed us to share her story in the hope of helping other travelers.
CORRESPONDENCE
Michael E. Mullins, MD, Division of Medical Toxicology, Department of Emergency Medicine, Washington University School of Medicine, Campus Box 8072, 660 South Euclid Avenue, Saint Louis, MO 63110; [email protected]
1. Bagnis R, Kuberski T, Laugier S. Clinical Observations of 3,009 cases of ciguatera (fish poisoning) in the South Pacific. Am J Trop Med Hyg. 1979;28:1067-1073. doi: 10.4269/ajtmh.1979.28.1067
2. Morris JG Jr, Lewin P, Smith CW, et al. Ciguatera fish poisoning: epidemiology of the disease on St. Thomas, US Virgin Islands. Am J Trop Med Hyg. 1982;31:574-578. doi: 10.4269/ajtmh.1982.31.574
3. Radke EG, Grattan LM, Cook RL, et al. Ciguatera incidence in the US Virgin Islands has not increased over a 30-year time period despite rising seawater temperatures. Am J Trop Med Hyg. 2013;88:908-913. doi: 10.4269/ajtmh.12-0676
4. Goodman DM, Rogers J, Livingston EH. Ciguatera fish poisoning.
5. Blythe DG, De Sylva DP, Fleming LE, et al. Clinical experience with IV mannitol in the treatment of ciguatera. Bull Soc Pathol Exot. 1992;85:425-426.
6. Lewis, RJ. The changing face of ciguatera. Toxicon. 2001;39:97-106. doi: 10.1016/s0041-0101(00)00161-6
7. Mullins ME, Hoffman RS. Is mannitol the treatment of choice for ciguatera fish poisoning? Clin Toxicol (Phila). 2017;55:947-955. doi: 10.1080/15563650.2017.1327664
8. Nicholson GM, Lewis, RJ. Ciguatoxins: cyclic polyether modulators of voltage-gated ion channel function. Mar Drugs. 2006;4:82-118.
9. Mattei C, Molgo J, Marquais M, et al. Hyperosmolar D-mannitol reverses the increased membrane excitability and the nodal swelling caused by Caribbean ciguatoxin-1 in single frog myelinated neurons. Brain Res. 1999;847:50-58. doi: 10.1016/s0006-8993(99)02032-6
10. Palafox NA, Jain LG, Pinano AZ, et al. Successful treatment of ciguatera fish poisoning with intravenous mannitol. JAMA. 1988;259:2740-2742.
11. Pearn JH, Lewis RJ, Ruff T, et al. Ciguatera and mannitol: experience with a new treatment regimen. Med J Aust. 1989;151:77-80. doi: 10.5694/j.1326-5377.1989.tb101165.x
12. Eastaugh JA. Delayed use of intravenous mannitol in ciguatera (fish poisoning). Ann Emerg Med. 1996;28:105-106. doi: 10.1016/s0196-0644(96)70151-8
13. Schwarz ES, Mullins ME, Brooks CB. Ciguatera poisoning successfully treated with delayed mannitol. Ann Emerg Med. 2008;52:476-477. doi: 10.1016/j.annemergmed.2008.05.015
14. Schnorf H, Taurarii M, Cundy T. Ciguatera fish poisoning: a double-blind randomized trial of mannitol therapy. Neurology. 2002;58:873-880. doi: 10.1212/wnl.58.6.873
1. Bagnis R, Kuberski T, Laugier S. Clinical Observations of 3,009 cases of ciguatera (fish poisoning) in the South Pacific. Am J Trop Med Hyg. 1979;28:1067-1073. doi: 10.4269/ajtmh.1979.28.1067
2. Morris JG Jr, Lewin P, Smith CW, et al. Ciguatera fish poisoning: epidemiology of the disease on St. Thomas, US Virgin Islands. Am J Trop Med Hyg. 1982;31:574-578. doi: 10.4269/ajtmh.1982.31.574
3. Radke EG, Grattan LM, Cook RL, et al. Ciguatera incidence in the US Virgin Islands has not increased over a 30-year time period despite rising seawater temperatures. Am J Trop Med Hyg. 2013;88:908-913. doi: 10.4269/ajtmh.12-0676
4. Goodman DM, Rogers J, Livingston EH. Ciguatera fish poisoning.
5. Blythe DG, De Sylva DP, Fleming LE, et al. Clinical experience with IV mannitol in the treatment of ciguatera. Bull Soc Pathol Exot. 1992;85:425-426.
6. Lewis, RJ. The changing face of ciguatera. Toxicon. 2001;39:97-106. doi: 10.1016/s0041-0101(00)00161-6
7. Mullins ME, Hoffman RS. Is mannitol the treatment of choice for ciguatera fish poisoning? Clin Toxicol (Phila). 2017;55:947-955. doi: 10.1080/15563650.2017.1327664
8. Nicholson GM, Lewis, RJ. Ciguatoxins: cyclic polyether modulators of voltage-gated ion channel function. Mar Drugs. 2006;4:82-118.
9. Mattei C, Molgo J, Marquais M, et al. Hyperosmolar D-mannitol reverses the increased membrane excitability and the nodal swelling caused by Caribbean ciguatoxin-1 in single frog myelinated neurons. Brain Res. 1999;847:50-58. doi: 10.1016/s0006-8993(99)02032-6
10. Palafox NA, Jain LG, Pinano AZ, et al. Successful treatment of ciguatera fish poisoning with intravenous mannitol. JAMA. 1988;259:2740-2742.
11. Pearn JH, Lewis RJ, Ruff T, et al. Ciguatera and mannitol: experience with a new treatment regimen. Med J Aust. 1989;151:77-80. doi: 10.5694/j.1326-5377.1989.tb101165.x
12. Eastaugh JA. Delayed use of intravenous mannitol in ciguatera (fish poisoning). Ann Emerg Med. 1996;28:105-106. doi: 10.1016/s0196-0644(96)70151-8
13. Schwarz ES, Mullins ME, Brooks CB. Ciguatera poisoning successfully treated with delayed mannitol. Ann Emerg Med. 2008;52:476-477. doi: 10.1016/j.annemergmed.2008.05.015
14. Schnorf H, Taurarii M, Cundy T. Ciguatera fish poisoning: a double-blind randomized trial of mannitol therapy. Neurology. 2002;58:873-880. doi: 10.1212/wnl.58.6.873
Denosumab boosts bone strength in glucocorticoid users
Bone strength and microarchitecture remained stronger at 24 months after treatment with denosumab compared to risedronate, in a study of 110 adults using glucocorticoids.
Patients using glucocorticoids are at increased risk for vertebral and nonvertebral fractures at both the start of treatment or as treatment continues, wrote Piet Geusens, MD, of Maastricht University, the Netherlands, and colleagues.
Imaging data collected via high-resolution peripheral quantitative computed tomography (HR-pQCT) allow for the assessment of bone microarchitecture and strength, but specific data comparing the impact of bone treatment in patients using glucocorticoids are lacking, they said.
In a study published in the Journal of Bone and Mineral Research, the researchers identified a subset of 56 patients randomized to denosumab and 54 to risedronate patients out of a total of 590 patients who were enrolled in a phase 3 randomized, controlled trial of denosumab vs. risedronate for bone mineral density. The main results of the larger trial – presented at EULAR 2018 – showed greater increases in bone strength with denosumab over risedronate in patients receiving glucocorticoids.
In the current study, the researchers reviewed HR-pQCT scans of the distal radius and tibia at baseline, 12 months, and 24 months. Bone strength and microarchitecture were defined in terms of failure load (FL) as a primary outcome. Patients also were divided into subpopulations of those initiating glucocorticoid treatment (GC-I) and continuing treatment (GC-C).
Baseline characteristics were mainly balanced among the treatment groups within the GC-I and GC-C categories.
Among the GC-I patients, in the denosumab group, FL increased significantly from baseline to 12 months at the radius at tibia (1.8% and 1.7%, respectively) but did not change significantly in the risedronate group, which translated to a significant treatment difference between the drugs of 3.3% for radius and 2.5% for tibia.
At 24 months, the radius measure of FL was unchanged from baseline in denosumab patients but significantly decreased in risedronate patients, with a difference of –4.1%, which translated to a significant between-treatment difference at the radius of 5.6% (P < .001). Changes at the tibia were not significantly different between the groups at 24 months.
Among the GC-C patients, FL was unchanged from baseline to 12 months for both the denosumab and risedronate groups. However, FL significantly increased with denosumab (4.3%) and remained unchanged in the risedronate group.
The researchers also found significant differences between denosumab and risedronate in percentage changes in cortical bone mineral density, and less prominent changes and differences in trabecular bone mineral density.
The study findings were limited by several factors including the use of the HR-pQCT scanner, which limits the measurement of trabecular microarchitecture, and the use of only standard HR-pQCT parameters, which do not allow insight into endosteal changes, and the inability to correct for multiplicity of data, the researchers noted.
However, the results support the superiority of denosumab over risedronate for preventing FL and total bone mineral density loss at the radius and tibia in new glucocorticoid users, and for increasing FL and total bone mineral density at the radius in long-term glucocorticoid users, they said.
Denosumab therefore could be a useful therapeutic option and could inform decision-making in patients initiating GC-therapy or on long-term GC-therapy, they concluded.
The study was supported by Amgen. Dr. Geusens disclosed grants from Amgen, Celgene, Lilly, Merck, Pfizer, Roche, UCB, Fresenius, Mylan, and Sandoz, and grants and other funding from AbbVie, outside the current study.
Bone strength and microarchitecture remained stronger at 24 months after treatment with denosumab compared to risedronate, in a study of 110 adults using glucocorticoids.
Patients using glucocorticoids are at increased risk for vertebral and nonvertebral fractures at both the start of treatment or as treatment continues, wrote Piet Geusens, MD, of Maastricht University, the Netherlands, and colleagues.
Imaging data collected via high-resolution peripheral quantitative computed tomography (HR-pQCT) allow for the assessment of bone microarchitecture and strength, but specific data comparing the impact of bone treatment in patients using glucocorticoids are lacking, they said.
In a study published in the Journal of Bone and Mineral Research, the researchers identified a subset of 56 patients randomized to denosumab and 54 to risedronate patients out of a total of 590 patients who were enrolled in a phase 3 randomized, controlled trial of denosumab vs. risedronate for bone mineral density. The main results of the larger trial – presented at EULAR 2018 – showed greater increases in bone strength with denosumab over risedronate in patients receiving glucocorticoids.
In the current study, the researchers reviewed HR-pQCT scans of the distal radius and tibia at baseline, 12 months, and 24 months. Bone strength and microarchitecture were defined in terms of failure load (FL) as a primary outcome. Patients also were divided into subpopulations of those initiating glucocorticoid treatment (GC-I) and continuing treatment (GC-C).
Baseline characteristics were mainly balanced among the treatment groups within the GC-I and GC-C categories.
Among the GC-I patients, in the denosumab group, FL increased significantly from baseline to 12 months at the radius at tibia (1.8% and 1.7%, respectively) but did not change significantly in the risedronate group, which translated to a significant treatment difference between the drugs of 3.3% for radius and 2.5% for tibia.
At 24 months, the radius measure of FL was unchanged from baseline in denosumab patients but significantly decreased in risedronate patients, with a difference of –4.1%, which translated to a significant between-treatment difference at the radius of 5.6% (P < .001). Changes at the tibia were not significantly different between the groups at 24 months.
Among the GC-C patients, FL was unchanged from baseline to 12 months for both the denosumab and risedronate groups. However, FL significantly increased with denosumab (4.3%) and remained unchanged in the risedronate group.
The researchers also found significant differences between denosumab and risedronate in percentage changes in cortical bone mineral density, and less prominent changes and differences in trabecular bone mineral density.
The study findings were limited by several factors including the use of the HR-pQCT scanner, which limits the measurement of trabecular microarchitecture, and the use of only standard HR-pQCT parameters, which do not allow insight into endosteal changes, and the inability to correct for multiplicity of data, the researchers noted.
However, the results support the superiority of denosumab over risedronate for preventing FL and total bone mineral density loss at the radius and tibia in new glucocorticoid users, and for increasing FL and total bone mineral density at the radius in long-term glucocorticoid users, they said.
Denosumab therefore could be a useful therapeutic option and could inform decision-making in patients initiating GC-therapy or on long-term GC-therapy, they concluded.
The study was supported by Amgen. Dr. Geusens disclosed grants from Amgen, Celgene, Lilly, Merck, Pfizer, Roche, UCB, Fresenius, Mylan, and Sandoz, and grants and other funding from AbbVie, outside the current study.
Bone strength and microarchitecture remained stronger at 24 months after treatment with denosumab compared to risedronate, in a study of 110 adults using glucocorticoids.
Patients using glucocorticoids are at increased risk for vertebral and nonvertebral fractures at both the start of treatment or as treatment continues, wrote Piet Geusens, MD, of Maastricht University, the Netherlands, and colleagues.
Imaging data collected via high-resolution peripheral quantitative computed tomography (HR-pQCT) allow for the assessment of bone microarchitecture and strength, but specific data comparing the impact of bone treatment in patients using glucocorticoids are lacking, they said.
In a study published in the Journal of Bone and Mineral Research, the researchers identified a subset of 56 patients randomized to denosumab and 54 to risedronate patients out of a total of 590 patients who were enrolled in a phase 3 randomized, controlled trial of denosumab vs. risedronate for bone mineral density. The main results of the larger trial – presented at EULAR 2018 – showed greater increases in bone strength with denosumab over risedronate in patients receiving glucocorticoids.
In the current study, the researchers reviewed HR-pQCT scans of the distal radius and tibia at baseline, 12 months, and 24 months. Bone strength and microarchitecture were defined in terms of failure load (FL) as a primary outcome. Patients also were divided into subpopulations of those initiating glucocorticoid treatment (GC-I) and continuing treatment (GC-C).
Baseline characteristics were mainly balanced among the treatment groups within the GC-I and GC-C categories.
Among the GC-I patients, in the denosumab group, FL increased significantly from baseline to 12 months at the radius at tibia (1.8% and 1.7%, respectively) but did not change significantly in the risedronate group, which translated to a significant treatment difference between the drugs of 3.3% for radius and 2.5% for tibia.
At 24 months, the radius measure of FL was unchanged from baseline in denosumab patients but significantly decreased in risedronate patients, with a difference of –4.1%, which translated to a significant between-treatment difference at the radius of 5.6% (P < .001). Changes at the tibia were not significantly different between the groups at 24 months.
Among the GC-C patients, FL was unchanged from baseline to 12 months for both the denosumab and risedronate groups. However, FL significantly increased with denosumab (4.3%) and remained unchanged in the risedronate group.
The researchers also found significant differences between denosumab and risedronate in percentage changes in cortical bone mineral density, and less prominent changes and differences in trabecular bone mineral density.
The study findings were limited by several factors including the use of the HR-pQCT scanner, which limits the measurement of trabecular microarchitecture, and the use of only standard HR-pQCT parameters, which do not allow insight into endosteal changes, and the inability to correct for multiplicity of data, the researchers noted.
However, the results support the superiority of denosumab over risedronate for preventing FL and total bone mineral density loss at the radius and tibia in new glucocorticoid users, and for increasing FL and total bone mineral density at the radius in long-term glucocorticoid users, they said.
Denosumab therefore could be a useful therapeutic option and could inform decision-making in patients initiating GC-therapy or on long-term GC-therapy, they concluded.
The study was supported by Amgen. Dr. Geusens disclosed grants from Amgen, Celgene, Lilly, Merck, Pfizer, Roche, UCB, Fresenius, Mylan, and Sandoz, and grants and other funding from AbbVie, outside the current study.
FROM THE JOURNAL OF BONE AND MINERAL RESEARCH
Children and teens with food allergies face quality-of-life issues
Children and adolescents with food allergies appear to fare worse physically, socially, and emotionally, and have poorer overall health-related quality of life (HRQL) than their food allergy–free peers, a new systematic review suggests.
“Findings from the current review suggest that food allergy has a negative impact on the HRQL of children and teens, particularly older children and those with severe food allergy,” the authors wrote. “By comparison, the link between food allergy and psychosocial functioning is less clear.
“Evidence from the qualitative literature suggests that the burden of childhood food allergy largely stems from worries surrounding exposures outside of the home and the social consequences of the condition,” they added.
Lead study author Michael A. Golding, a research coordinator at Children’s Hospital Research Institute of Manitoba in Winnipeg, Canada, and colleagues searched PubMed, Scopus, PsycInfo, and CINAHL (Cumulative Index to Nursing and Allied Health Literature) databases on several days between November 2019 and March 2021 for peer-reviewed articles published in English in any year.
They reviewed articles focused on HRQL, psychological health, or social well-being in children and teens with food allergy from birth through 19 years of age. Food allergy comprised both immunoglobulin E (IgE)-mediated food allergies and non-IgE-mediated allergies, including food protein–induced enterocolitis, enteropathy, and proctocolitis.
From the 3,789 publications the researchers screened, they included 8,202 patients in 45 studies in their quantitative synthesis and 186 patients in 9 studies in their qualitative synthesis. Using a segregated, mixed research synthesis design, they analyzed and synthesized the quantitative and qualitative articles separately, then integrated those findings.
Navigating through many challenges
The authors found that food allergy lowered the young people’s HRQL. In 11 of the 14 studies (78%) that included a comparison group, young patients with food allergy showed significantly lower HRQL in at least one domain. Most significant differences occurred in domains related to total HRQL (66%), social functioning (58%), emotional functioning (54%), and physical functioning (54%).
Parents were often more likely than their children to perceive that the child’s food allergy was causing problems.
Between 20% and 32% of children reported bullying related to their food allergy. Many children reported that their allergy sometimes isolated them from their classmates.
Many children described feeling comfortable at home but worried in places where they had less control, such as school, restaurants, or when traveling.
Children and teens tended to downplay their limitations and the negative impacts of their condition.
Older children who had been diagnosed early in life tended to accept managing their food allergy as a way of life, whereas those diagnosed when they were older reported the need to adapt, accept, and grieve the loss of foods and experiences.
“This study highlights the importance of addressing the underlying impact that food allergy can have on patients’ mental health and social functioning,” Kelly Marie O’Shea, MD, assistant professor of allergy and immunology at University of Michigan Health in Ann Arbor, said in an interview.
“While it has been shown previously that food-allergic patients have lower HRQL, this systematic review aptly reveals that for children and teens with food allergy, overall quality of life, including psychosocial functioning, can also be negatively affected,” said Dr. O’Shea, who was not involved in the study.
“Symptoms of anxiety and depression are reported at higher rates in the food-allergic population, and social limitations have been shown to play a role,” she explained. “However, as revealed in this study, longitudinal and appropriately controlled studies to investigate the impact of food allergy on psychosocial outcomes in children and teens are scarce.”
Robert Alan Wood, MD, professor of pediatrics at Johns Hopkins University and director of pediatric allergy and immunology at Johns Hopkins Children’s Center, Baltimore, told this news organization that the effects of food allergy on mental health are not fully appreciated by the public or by many clinicians.
“These findings emphasize the need to recognize the emotional consequences of food allergy and to take steps to be proactive in managing these issues among our patients,” said Dr. Wood, who was not associated with the study.
More research is needed
The authors noted that more research is needed to examine links between food allergy, HRQL, and psychosocial outcome; links between food allergy and bullying; and how challenges change over time. They recommend exploring the relative impacts of specific types of food allergy and whether specific traits in young people with food allergy make them more susceptible to its psychological effects. They also call for efforts to identify and help young people with food allergy overcome their many challenges.
The study was funded by the Canadian Institutes for Health Research, the Children’s Hospital Research Institute of Manitoba, and the University of Manitoba.
Study senior author Jennifer L. P. Protudjer, PhD, reported involvement with Canada’s National Food Allergy Action Plan and Allied Health at the Canadian Society of Allergy and Clinical Immunology, and receipt of fees from Novartis. The remaining authors, as well as Dr. O’Shea and Dr. Wood, reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Children and adolescents with food allergies appear to fare worse physically, socially, and emotionally, and have poorer overall health-related quality of life (HRQL) than their food allergy–free peers, a new systematic review suggests.
“Findings from the current review suggest that food allergy has a negative impact on the HRQL of children and teens, particularly older children and those with severe food allergy,” the authors wrote. “By comparison, the link between food allergy and psychosocial functioning is less clear.
“Evidence from the qualitative literature suggests that the burden of childhood food allergy largely stems from worries surrounding exposures outside of the home and the social consequences of the condition,” they added.
Lead study author Michael A. Golding, a research coordinator at Children’s Hospital Research Institute of Manitoba in Winnipeg, Canada, and colleagues searched PubMed, Scopus, PsycInfo, and CINAHL (Cumulative Index to Nursing and Allied Health Literature) databases on several days between November 2019 and March 2021 for peer-reviewed articles published in English in any year.
They reviewed articles focused on HRQL, psychological health, or social well-being in children and teens with food allergy from birth through 19 years of age. Food allergy comprised both immunoglobulin E (IgE)-mediated food allergies and non-IgE-mediated allergies, including food protein–induced enterocolitis, enteropathy, and proctocolitis.
From the 3,789 publications the researchers screened, they included 8,202 patients in 45 studies in their quantitative synthesis and 186 patients in 9 studies in their qualitative synthesis. Using a segregated, mixed research synthesis design, they analyzed and synthesized the quantitative and qualitative articles separately, then integrated those findings.
Navigating through many challenges
The authors found that food allergy lowered the young people’s HRQL. In 11 of the 14 studies (78%) that included a comparison group, young patients with food allergy showed significantly lower HRQL in at least one domain. Most significant differences occurred in domains related to total HRQL (66%), social functioning (58%), emotional functioning (54%), and physical functioning (54%).
Parents were often more likely than their children to perceive that the child’s food allergy was causing problems.
Between 20% and 32% of children reported bullying related to their food allergy. Many children reported that their allergy sometimes isolated them from their classmates.
Many children described feeling comfortable at home but worried in places where they had less control, such as school, restaurants, or when traveling.
Children and teens tended to downplay their limitations and the negative impacts of their condition.
Older children who had been diagnosed early in life tended to accept managing their food allergy as a way of life, whereas those diagnosed when they were older reported the need to adapt, accept, and grieve the loss of foods and experiences.
“This study highlights the importance of addressing the underlying impact that food allergy can have on patients’ mental health and social functioning,” Kelly Marie O’Shea, MD, assistant professor of allergy and immunology at University of Michigan Health in Ann Arbor, said in an interview.
“While it has been shown previously that food-allergic patients have lower HRQL, this systematic review aptly reveals that for children and teens with food allergy, overall quality of life, including psychosocial functioning, can also be negatively affected,” said Dr. O’Shea, who was not involved in the study.
“Symptoms of anxiety and depression are reported at higher rates in the food-allergic population, and social limitations have been shown to play a role,” she explained. “However, as revealed in this study, longitudinal and appropriately controlled studies to investigate the impact of food allergy on psychosocial outcomes in children and teens are scarce.”
Robert Alan Wood, MD, professor of pediatrics at Johns Hopkins University and director of pediatric allergy and immunology at Johns Hopkins Children’s Center, Baltimore, told this news organization that the effects of food allergy on mental health are not fully appreciated by the public or by many clinicians.
“These findings emphasize the need to recognize the emotional consequences of food allergy and to take steps to be proactive in managing these issues among our patients,” said Dr. Wood, who was not associated with the study.
More research is needed
The authors noted that more research is needed to examine links between food allergy, HRQL, and psychosocial outcome; links between food allergy and bullying; and how challenges change over time. They recommend exploring the relative impacts of specific types of food allergy and whether specific traits in young people with food allergy make them more susceptible to its psychological effects. They also call for efforts to identify and help young people with food allergy overcome their many challenges.
The study was funded by the Canadian Institutes for Health Research, the Children’s Hospital Research Institute of Manitoba, and the University of Manitoba.
Study senior author Jennifer L. P. Protudjer, PhD, reported involvement with Canada’s National Food Allergy Action Plan and Allied Health at the Canadian Society of Allergy and Clinical Immunology, and receipt of fees from Novartis. The remaining authors, as well as Dr. O’Shea and Dr. Wood, reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Children and adolescents with food allergies appear to fare worse physically, socially, and emotionally, and have poorer overall health-related quality of life (HRQL) than their food allergy–free peers, a new systematic review suggests.
“Findings from the current review suggest that food allergy has a negative impact on the HRQL of children and teens, particularly older children and those with severe food allergy,” the authors wrote. “By comparison, the link between food allergy and psychosocial functioning is less clear.
“Evidence from the qualitative literature suggests that the burden of childhood food allergy largely stems from worries surrounding exposures outside of the home and the social consequences of the condition,” they added.
Lead study author Michael A. Golding, a research coordinator at Children’s Hospital Research Institute of Manitoba in Winnipeg, Canada, and colleagues searched PubMed, Scopus, PsycInfo, and CINAHL (Cumulative Index to Nursing and Allied Health Literature) databases on several days between November 2019 and March 2021 for peer-reviewed articles published in English in any year.
They reviewed articles focused on HRQL, psychological health, or social well-being in children and teens with food allergy from birth through 19 years of age. Food allergy comprised both immunoglobulin E (IgE)-mediated food allergies and non-IgE-mediated allergies, including food protein–induced enterocolitis, enteropathy, and proctocolitis.
From the 3,789 publications the researchers screened, they included 8,202 patients in 45 studies in their quantitative synthesis and 186 patients in 9 studies in their qualitative synthesis. Using a segregated, mixed research synthesis design, they analyzed and synthesized the quantitative and qualitative articles separately, then integrated those findings.
Navigating through many challenges
The authors found that food allergy lowered the young people’s HRQL. In 11 of the 14 studies (78%) that included a comparison group, young patients with food allergy showed significantly lower HRQL in at least one domain. Most significant differences occurred in domains related to total HRQL (66%), social functioning (58%), emotional functioning (54%), and physical functioning (54%).
Parents were often more likely than their children to perceive that the child’s food allergy was causing problems.
Between 20% and 32% of children reported bullying related to their food allergy. Many children reported that their allergy sometimes isolated them from their classmates.
Many children described feeling comfortable at home but worried in places where they had less control, such as school, restaurants, or when traveling.
Children and teens tended to downplay their limitations and the negative impacts of their condition.
Older children who had been diagnosed early in life tended to accept managing their food allergy as a way of life, whereas those diagnosed when they were older reported the need to adapt, accept, and grieve the loss of foods and experiences.
“This study highlights the importance of addressing the underlying impact that food allergy can have on patients’ mental health and social functioning,” Kelly Marie O’Shea, MD, assistant professor of allergy and immunology at University of Michigan Health in Ann Arbor, said in an interview.
“While it has been shown previously that food-allergic patients have lower HRQL, this systematic review aptly reveals that for children and teens with food allergy, overall quality of life, including psychosocial functioning, can also be negatively affected,” said Dr. O’Shea, who was not involved in the study.
“Symptoms of anxiety and depression are reported at higher rates in the food-allergic population, and social limitations have been shown to play a role,” she explained. “However, as revealed in this study, longitudinal and appropriately controlled studies to investigate the impact of food allergy on psychosocial outcomes in children and teens are scarce.”
Robert Alan Wood, MD, professor of pediatrics at Johns Hopkins University and director of pediatric allergy and immunology at Johns Hopkins Children’s Center, Baltimore, told this news organization that the effects of food allergy on mental health are not fully appreciated by the public or by many clinicians.
“These findings emphasize the need to recognize the emotional consequences of food allergy and to take steps to be proactive in managing these issues among our patients,” said Dr. Wood, who was not associated with the study.
More research is needed
The authors noted that more research is needed to examine links between food allergy, HRQL, and psychosocial outcome; links between food allergy and bullying; and how challenges change over time. They recommend exploring the relative impacts of specific types of food allergy and whether specific traits in young people with food allergy make them more susceptible to its psychological effects. They also call for efforts to identify and help young people with food allergy overcome their many challenges.
The study was funded by the Canadian Institutes for Health Research, the Children’s Hospital Research Institute of Manitoba, and the University of Manitoba.
Study senior author Jennifer L. P. Protudjer, PhD, reported involvement with Canada’s National Food Allergy Action Plan and Allied Health at the Canadian Society of Allergy and Clinical Immunology, and receipt of fees from Novartis. The remaining authors, as well as Dr. O’Shea and Dr. Wood, reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Anaphylaxis risk with IV iron low, but varies with formulation
The results of the new retrospective cohort study were published online March 29 in Annals of Internal Medicine (doi: 10.7326/M21-4009).
“The rates of anaphylaxis were very low with all IV iron products but were three- to eightfold greater for iron dextran and ferumoxytol than for iron sucrose,” wrote Chintan V. Dave, PharmD, PhD, of Rutgers University, New Brunswick, N.J., and colleagues.
Using data from Medicare insurance claims, the researchers evaluated the incidence of anaphylaxis among patients 65 years or older receiving their first dose of one of five different IV iron formulations for the treatment of iron deficiency anemia. Patients were treated between July 2013 and December 2018 and the iron formulations were ferric carboxymaltose, ferumoxytol, ferric gluconate, iron dextran, or iron sucrose.
Overall, 167,925 patients were included and categorized based on the iron supplement they received. Dr. Dave and colleagues found that the adjusted incidence rates (IRs) for anaphylaxis per 10,000 first administrations were 9.8 cases for iron dextran (95% confidence interval [CI], 6.2 to 15.3 cases), 4.0 cases for ferumoxytol (95% CI, 2.5 to 6.6 cases), 1.5 cases for ferric gluconate (95% CI, 0.3 to 6.6 cases), 1.2 cases for iron sucrose (95% CI, 0.6 to 2.5 cases), and 0.8 cases for ferric carboxymaltose (95% CI, 0.3 to 2.6 cases).
Only those patients receiving iron dextran or ferumoxytol had anaphylactic reactions requiring hospitalization.
Using iron sucrose as the referent category, the researchers found that the odds ratios (ORs) for anaphylaxis were 8.3 for iron dextran (95% CI, 3.5-19.8) and 3.4 for ferumoxytol (95% CI, 1.4-8.3).
“Anaphylaxis is just one of many factors one should consider when deciding on the choice of IV iron therapy,” Dr. Dave noted in an interview, when asked whether he feels that these findings will change the use of parenteral iron in practice.
Acknowledging that anaphylaxis is a severe but rare complication, Dr. Dave stated that other factors such as “clinical indication, setting, dose, the number and duration of administrations required to replenish iron reserves, risk of other adverse reactions, and costs,” should also be considered when designing treatment plans using intravenous iron.
In the study, anaphylaxis was defined as reactions that occurred within 24 hours of IV iron administration and was restricted to the following:
- Anaphylaxis resulting in hospitalization.
- An outpatient or emergency department visit due to anaphylactic shock accompanied by codes relating to the administration of cardiopulmonary resuscitation or epinephrine or the occurrence of hypotension.
- Two separate encounters for anaphylactic shock within the same day representing different encounter types, that is, inpatient, outpatient, or emergency department visit.
Dr. Dave and colleagues acknowledged study limitations, such as the fact the anaphylaxis criteria included only the most severe cases and could therefore have missed milder cases of anaphylaxis secondary to IV iron. Further, they noted that these findings may not be applicable to a younger patient population.
Patients were excluded from the study if they had received IV iron between January 2007 and July 2013, had a diagnosis of HIV or end-stage renal disease, had a recent blood transfusion, or had a history of anaphylactic reactions.
The study authors disclosed no relevant financial relationships.
The results of the new retrospective cohort study were published online March 29 in Annals of Internal Medicine (doi: 10.7326/M21-4009).
“The rates of anaphylaxis were very low with all IV iron products but were three- to eightfold greater for iron dextran and ferumoxytol than for iron sucrose,” wrote Chintan V. Dave, PharmD, PhD, of Rutgers University, New Brunswick, N.J., and colleagues.
Using data from Medicare insurance claims, the researchers evaluated the incidence of anaphylaxis among patients 65 years or older receiving their first dose of one of five different IV iron formulations for the treatment of iron deficiency anemia. Patients were treated between July 2013 and December 2018 and the iron formulations were ferric carboxymaltose, ferumoxytol, ferric gluconate, iron dextran, or iron sucrose.
Overall, 167,925 patients were included and categorized based on the iron supplement they received. Dr. Dave and colleagues found that the adjusted incidence rates (IRs) for anaphylaxis per 10,000 first administrations were 9.8 cases for iron dextran (95% confidence interval [CI], 6.2 to 15.3 cases), 4.0 cases for ferumoxytol (95% CI, 2.5 to 6.6 cases), 1.5 cases for ferric gluconate (95% CI, 0.3 to 6.6 cases), 1.2 cases for iron sucrose (95% CI, 0.6 to 2.5 cases), and 0.8 cases for ferric carboxymaltose (95% CI, 0.3 to 2.6 cases).
Only those patients receiving iron dextran or ferumoxytol had anaphylactic reactions requiring hospitalization.
Using iron sucrose as the referent category, the researchers found that the odds ratios (ORs) for anaphylaxis were 8.3 for iron dextran (95% CI, 3.5-19.8) and 3.4 for ferumoxytol (95% CI, 1.4-8.3).
“Anaphylaxis is just one of many factors one should consider when deciding on the choice of IV iron therapy,” Dr. Dave noted in an interview, when asked whether he feels that these findings will change the use of parenteral iron in practice.
Acknowledging that anaphylaxis is a severe but rare complication, Dr. Dave stated that other factors such as “clinical indication, setting, dose, the number and duration of administrations required to replenish iron reserves, risk of other adverse reactions, and costs,” should also be considered when designing treatment plans using intravenous iron.
In the study, anaphylaxis was defined as reactions that occurred within 24 hours of IV iron administration and was restricted to the following:
- Anaphylaxis resulting in hospitalization.
- An outpatient or emergency department visit due to anaphylactic shock accompanied by codes relating to the administration of cardiopulmonary resuscitation or epinephrine or the occurrence of hypotension.
- Two separate encounters for anaphylactic shock within the same day representing different encounter types, that is, inpatient, outpatient, or emergency department visit.
Dr. Dave and colleagues acknowledged study limitations, such as the fact the anaphylaxis criteria included only the most severe cases and could therefore have missed milder cases of anaphylaxis secondary to IV iron. Further, they noted that these findings may not be applicable to a younger patient population.
Patients were excluded from the study if they had received IV iron between January 2007 and July 2013, had a diagnosis of HIV or end-stage renal disease, had a recent blood transfusion, or had a history of anaphylactic reactions.
The study authors disclosed no relevant financial relationships.
The results of the new retrospective cohort study were published online March 29 in Annals of Internal Medicine (doi: 10.7326/M21-4009).
“The rates of anaphylaxis were very low with all IV iron products but were three- to eightfold greater for iron dextran and ferumoxytol than for iron sucrose,” wrote Chintan V. Dave, PharmD, PhD, of Rutgers University, New Brunswick, N.J., and colleagues.
Using data from Medicare insurance claims, the researchers evaluated the incidence of anaphylaxis among patients 65 years or older receiving their first dose of one of five different IV iron formulations for the treatment of iron deficiency anemia. Patients were treated between July 2013 and December 2018 and the iron formulations were ferric carboxymaltose, ferumoxytol, ferric gluconate, iron dextran, or iron sucrose.
Overall, 167,925 patients were included and categorized based on the iron supplement they received. Dr. Dave and colleagues found that the adjusted incidence rates (IRs) for anaphylaxis per 10,000 first administrations were 9.8 cases for iron dextran (95% confidence interval [CI], 6.2 to 15.3 cases), 4.0 cases for ferumoxytol (95% CI, 2.5 to 6.6 cases), 1.5 cases for ferric gluconate (95% CI, 0.3 to 6.6 cases), 1.2 cases for iron sucrose (95% CI, 0.6 to 2.5 cases), and 0.8 cases for ferric carboxymaltose (95% CI, 0.3 to 2.6 cases).
Only those patients receiving iron dextran or ferumoxytol had anaphylactic reactions requiring hospitalization.
Using iron sucrose as the referent category, the researchers found that the odds ratios (ORs) for anaphylaxis were 8.3 for iron dextran (95% CI, 3.5-19.8) and 3.4 for ferumoxytol (95% CI, 1.4-8.3).
“Anaphylaxis is just one of many factors one should consider when deciding on the choice of IV iron therapy,” Dr. Dave noted in an interview, when asked whether he feels that these findings will change the use of parenteral iron in practice.
Acknowledging that anaphylaxis is a severe but rare complication, Dr. Dave stated that other factors such as “clinical indication, setting, dose, the number and duration of administrations required to replenish iron reserves, risk of other adverse reactions, and costs,” should also be considered when designing treatment plans using intravenous iron.
In the study, anaphylaxis was defined as reactions that occurred within 24 hours of IV iron administration and was restricted to the following:
- Anaphylaxis resulting in hospitalization.
- An outpatient or emergency department visit due to anaphylactic shock accompanied by codes relating to the administration of cardiopulmonary resuscitation or epinephrine or the occurrence of hypotension.
- Two separate encounters for anaphylactic shock within the same day representing different encounter types, that is, inpatient, outpatient, or emergency department visit.
Dr. Dave and colleagues acknowledged study limitations, such as the fact the anaphylaxis criteria included only the most severe cases and could therefore have missed milder cases of anaphylaxis secondary to IV iron. Further, they noted that these findings may not be applicable to a younger patient population.
Patients were excluded from the study if they had received IV iron between January 2007 and July 2013, had a diagnosis of HIV or end-stage renal disease, had a recent blood transfusion, or had a history of anaphylactic reactions.
The study authors disclosed no relevant financial relationships.
FROM ANNALS OF INTERNAL MEDICINE
Pollution levels linked to physical and mental health problems
Other analyses of data have found environmental air pollution from sources such as car exhaust and factory output can trigger an inflammatory response in the body. What’s new about a study published in RMD Open is that it explored an association between long-term exposure to pollution and risk of autoimmune diseases, wrote Giovanni Adami, MD, of the University of Verona (Italy) and colleagues.
“Environmental air pollution, according to the World Health Organization, is a major risk to health and 99% of the population worldwide is living in places where recommendations for air quality are not met,” said Dr. Adami in an interview. The limited data on the precise role of air pollution on rheumatic diseases in particular prompted the study, he said.
To explore the potential link between air pollution exposure and autoimmune disease, the researchers reviewed medical information from 81,363 adults via a national medical database in Italy; the data were submitted between June 2016 and November 2020.
The average age of the study population was 65 years, and 92% were women; 22% had at least one coexisting health condition. Each study participant was linked to local environmental monitoring via their residential postcode.
The researchers obtained details about concentrations of particulate matter in the environment from the Italian Institute of Environmental Protection that included 617 monitoring stations in 110 Italian provinces. They focused on concentrations of 10 and 2.5 (PM10 and PM2.5).
Exposure thresholds of 30 mcg/m3 for PM10 and 20 mcg/m3 for PM2.5 are generally considered harmful to health, they noted. On average, the long-term exposure was 16 mcg/m3 for PM2.5 and 25 mcg/m3 for PM10 between 2013 and 2019.
Overall, 9,723 individuals (12%) were diagnosed with an autoimmune disease between 2016 and 2020.
Exposure to PM10 was associated with a 7% higher risk of diagnosis with any autoimmune disease for every 10 mcg/m3 increase in concentration, but no association appeared between PM2.5 exposure and increased risk of autoimmune diseases.
However, in an adjusted model, chronic exposure to PM10 above 30 mcg/m3 and to PM2.5 above 20 mcg/m3 were associated with a 12% and 13% higher risk, respectively, of any autoimmune disease.
Chronic exposure to high levels of PM10 was specifically associated with a higher risk of rheumatoid arthritis, but no other autoimmune diseases. Chronic exposure to high levels of PM2.5 was associated with a higher risk of rheumatoid arthritis, connective tissue diseases, and inflammatory bowel diseases.
In their discussion, the researchers noted that the smaller diameter of PM2.5 molecules fluctuate less in response to rain and other weather, compared with PM10 molecules, which might make them a more accurate predictor of exposure to chronic air pollution.
The study findings were limited by several factors including the observational design, which prohibits the establishment of cause, and a lack of data on the start of symptoms and dates of diagnoses for autoimmune diseases, the researchers noted. Other limitations include the high percentage of older women in the study, which may limit generalizability, and the inability to account for additional personal exposure to pollutants outside of the environmental exposure, they said.
However, the results were strengthened by the large sample size and wide geographic distribution with variable pollution exposure, they said.
“Unfortunately, we were not surprised at all,” by the findings, Dr. Adami said in an interview.
“The biological rationale underpinning our findings is strong. Nevertheless, the magnitude of the effect was overwhelming. In addition, we saw an effect even at threshold of exposure that is widely considered as safe,” Dr. Adami noted.
Clinicians have been taught to consider cigarette smoking or other lifestyle behaviors as major risk factors for the development of several autoimmune diseases, said Dr. Adami. “In the future, we probably should include air pollution exposure as a risk factor as well. Interestingly, there is also accumulating evidence linking acute exposure to environmental air pollution with flares of chronic arthritis,” he said.
“Our study could have direct societal and political consequences,” and might help direct policy makers’ decisions on addressing strategies aimed to reduce fossil emissions, he said. As for additional research, “we certainly need multination studies to confirm our results on a larger scale,” Dr. Adami emphasized. “In addition, it is time to take action and start designing interventions aimed to reduce acute and chronic exposure to air pollution in patients suffering from RMDs.”
Consider the big picture of air quality
The Italian study is especially timely “given our evolving and emerging understanding of environmental risk factors for acute and chronic diseases, which we must first understand before we can address,” said Eileen Barrett, MD, of the University of New Mexico, Albuquerque, in an interview.
“I am largely surprised about the findings, as most physicians aren’t studying ambient air quality and risk for autoimmune disease,” said Dr. Barrett. “More often we think of air quality when we think of risk for respiratory diseases than autoimmune diseases, per se,” she said.
“There are several take-home messages from this study,” said Dr. Barrett. “The first is that we need more research to understand the consequences of air pollutants on health. Second, this study reminds us to think broadly about how air quality and our environment can affect health. And third, all clinicians should be committed to promoting science that can improve public health and reduce death and disability,” she emphasized.
The findings do not specifically reflect associations between pollution and other conditions such as chronic obstructive pulmonary disease and asthma although previous studies have shown an association between asthma and COPD exacerbations and air pollution, Dr. Barrett said.
“Further research will be needed to confirm the associations reported in this study,” Dr. Barrett said.
More research in other countries, including research related to other autoimmune diseases, and with other datasets on population and community level risks from poor air quality, would be helpful, and that information could be used to advise smart public policy, Dr. Barrett added.
Air pollution’s mental health impact
Air pollution’s effects extend beyond physical to the psychological, a new study of depression in teenagers showed. This study was published in Developmental Psychology.
Previous research on the environmental factors associated with depressive symptoms in teens has focused mainly on individual and family level contributors; the impact of the physical environment has not been well studied, the investigators, Erika M. Manczak, PhD, of the University of Denver and colleagues, wrote.
In their paper, the authors found a significant impact of neighborhood ozone exposure on the trajectory of depressive symptoms in teens over a 4-year period.
“Given that inhaling pollution activates biological pathways implicated in the development of depression, including immune, cardiovascular, and neurodevelopmental processes, exposure to ambient air pollution may influence the development and/or trajectory of depressive symptoms in youth,” they said.
The researchers recruited 213 adolescents in the San Francisco Bay area through local advertisements. The participants were aged 9-13 years at baseline, with an average age of 11 years. A total of 121 were female, 47% were white, 8.5% were African American, 12.3% were Asian, 10.4% were nonwhite Latin, and 21.7% were biracial or another ethnicity. The participants self-reported depressive symptoms and other psychopathology symptoms up to three times during the study period. Ozone exposure was calculated based on home addresses.
After controlling for other personal, family, and neighborhood variables, the researchers found that higher levels of ozone exposure were significantly associated with increased depressive symptoms over time, and the slope of trajectory of depressive symptoms became steeper as the ozone levels increased (P less than .001). Ozone did not significantly predict the trajectory of any other psychopathology symptoms.
“The results of this study provide preliminary support for the possibility that ozone is an overlooked contributor to the development or course of youth depressive symptoms,” the researchers wrote in their discussion.
“Interestingly, the association between ozone and symptom trajectories as measured by Anxious/Depressed subscale of the [Youth Self-Report] was not as strong as it was for the [Children’s Depression Inventory-Short Version] or Withdrawn/Depressed scales, suggesting that associations are more robust for behavioral withdrawal symptoms of depression than for other types of symptoms,” they noted.
The study findings were limited by the use of self-reports and by the inability of the study design to show causality, the researchers said. Other limitations include the use of average assessments of ozone that are less precise, lack of assessment of biological pathways for risk, lack of formal psychiatric diagnoses, and the small geographic region included in the study, they said.
However, the results provide preliminary evidence that ozone exposure is a potential contributing factor to depressive symptoms in youth, and serve as a jumping-off point for future research, they noted. Future studies should address changes in systemic inflammation, neurodevelopment, or stress reactivity, as well as concurrent psychosocial or biological factors, and temporal associations between air pollution and mental health symptoms, they concluded.
Environmental factors drive inflammatory responses
Peter L. Loper Jr., MD, considers the findings of the Developmental Psychology study to be unsurprising but important – because air pollution is simply getting worse.
“As the study authors cite, there is sufficient data correlating ozone to negative physical health outcomes in youth, but a paucity of data exploring the impact of poor air quality on mental health outcomes in this demographic,” noted Dr. Loper, of the University of South Carolina, Columbia, in an interview.
“As discussed by the study researchers, any environmental exposure that increases immune-mediated inflammation can result in negative health outcomes. In fact, there is already data to suggest that similar cytokines, or immune cell signalers, that get released by our immune system due to environmental exposures and that contribute to asthma, may also be implicated in depression and other mental health problems,” he noted.
“Just like downstream symptom indicators of physical illnesses such as asthma are secondary to immune-mediated pulmonary inflammation, downstream symptom indicators of mental illness, such as depression, are secondary to immune-mediated neuroinflammation,” Dr. Loper emphasized. “The most well-characterized upstream phenomenon perpetuating the downstream symptom indicators of depression involve neuroinflammatory states due to psychosocial and relational factors such as chronic stress, poor relationships, or substance use. However, any environmental factor that triggers an immune response and inflammation can promote neuroinflammation that manifests as symptoms of mental illness.”
The message for teens with depression and their families is that “we are a product of our environment,” Dr. Loper said. “When our environments are proinflammatory, or cause our immune system to become overactive, then we will develop illness; however, the most potent mediator of inflammation in the brain, and the downstream symptoms of depression, is our relationships with those we love most,” he said.
Dr. Loper suggested research aimed at identifying other sources of immune-mediated inflammation caused by physical environments and better understanding how environmental phenomenon like ozone may compound previously established risk factors for mental illness could be useful.
The RMD Open study received no outside funding, and its authors had no financial conflicts.
The Developmental Psychology study was supported by the National Institute of Mental Health and the Stanford University Precision Health and Integrated Diagnostics Center. The researchers for that report, and Dr. Loper and Dr. Barrett had no conflicts to disclose.
Other analyses of data have found environmental air pollution from sources such as car exhaust and factory output can trigger an inflammatory response in the body. What’s new about a study published in RMD Open is that it explored an association between long-term exposure to pollution and risk of autoimmune diseases, wrote Giovanni Adami, MD, of the University of Verona (Italy) and colleagues.
“Environmental air pollution, according to the World Health Organization, is a major risk to health and 99% of the population worldwide is living in places where recommendations for air quality are not met,” said Dr. Adami in an interview. The limited data on the precise role of air pollution on rheumatic diseases in particular prompted the study, he said.
To explore the potential link between air pollution exposure and autoimmune disease, the researchers reviewed medical information from 81,363 adults via a national medical database in Italy; the data were submitted between June 2016 and November 2020.
The average age of the study population was 65 years, and 92% were women; 22% had at least one coexisting health condition. Each study participant was linked to local environmental monitoring via their residential postcode.
The researchers obtained details about concentrations of particulate matter in the environment from the Italian Institute of Environmental Protection that included 617 monitoring stations in 110 Italian provinces. They focused on concentrations of 10 and 2.5 (PM10 and PM2.5).
Exposure thresholds of 30 mcg/m3 for PM10 and 20 mcg/m3 for PM2.5 are generally considered harmful to health, they noted. On average, the long-term exposure was 16 mcg/m3 for PM2.5 and 25 mcg/m3 for PM10 between 2013 and 2019.
Overall, 9,723 individuals (12%) were diagnosed with an autoimmune disease between 2016 and 2020.
Exposure to PM10 was associated with a 7% higher risk of diagnosis with any autoimmune disease for every 10 mcg/m3 increase in concentration, but no association appeared between PM2.5 exposure and increased risk of autoimmune diseases.
However, in an adjusted model, chronic exposure to PM10 above 30 mcg/m3 and to PM2.5 above 20 mcg/m3 were associated with a 12% and 13% higher risk, respectively, of any autoimmune disease.
Chronic exposure to high levels of PM10 was specifically associated with a higher risk of rheumatoid arthritis, but no other autoimmune diseases. Chronic exposure to high levels of PM2.5 was associated with a higher risk of rheumatoid arthritis, connective tissue diseases, and inflammatory bowel diseases.
In their discussion, the researchers noted that the smaller diameter of PM2.5 molecules fluctuate less in response to rain and other weather, compared with PM10 molecules, which might make them a more accurate predictor of exposure to chronic air pollution.
The study findings were limited by several factors including the observational design, which prohibits the establishment of cause, and a lack of data on the start of symptoms and dates of diagnoses for autoimmune diseases, the researchers noted. Other limitations include the high percentage of older women in the study, which may limit generalizability, and the inability to account for additional personal exposure to pollutants outside of the environmental exposure, they said.
However, the results were strengthened by the large sample size and wide geographic distribution with variable pollution exposure, they said.
“Unfortunately, we were not surprised at all,” by the findings, Dr. Adami said in an interview.
“The biological rationale underpinning our findings is strong. Nevertheless, the magnitude of the effect was overwhelming. In addition, we saw an effect even at threshold of exposure that is widely considered as safe,” Dr. Adami noted.
Clinicians have been taught to consider cigarette smoking or other lifestyle behaviors as major risk factors for the development of several autoimmune diseases, said Dr. Adami. “In the future, we probably should include air pollution exposure as a risk factor as well. Interestingly, there is also accumulating evidence linking acute exposure to environmental air pollution with flares of chronic arthritis,” he said.
“Our study could have direct societal and political consequences,” and might help direct policy makers’ decisions on addressing strategies aimed to reduce fossil emissions, he said. As for additional research, “we certainly need multination studies to confirm our results on a larger scale,” Dr. Adami emphasized. “In addition, it is time to take action and start designing interventions aimed to reduce acute and chronic exposure to air pollution in patients suffering from RMDs.”
Consider the big picture of air quality
The Italian study is especially timely “given our evolving and emerging understanding of environmental risk factors for acute and chronic diseases, which we must first understand before we can address,” said Eileen Barrett, MD, of the University of New Mexico, Albuquerque, in an interview.
“I am largely surprised about the findings, as most physicians aren’t studying ambient air quality and risk for autoimmune disease,” said Dr. Barrett. “More often we think of air quality when we think of risk for respiratory diseases than autoimmune diseases, per se,” she said.
“There are several take-home messages from this study,” said Dr. Barrett. “The first is that we need more research to understand the consequences of air pollutants on health. Second, this study reminds us to think broadly about how air quality and our environment can affect health. And third, all clinicians should be committed to promoting science that can improve public health and reduce death and disability,” she emphasized.
The findings do not specifically reflect associations between pollution and other conditions such as chronic obstructive pulmonary disease and asthma although previous studies have shown an association between asthma and COPD exacerbations and air pollution, Dr. Barrett said.
“Further research will be needed to confirm the associations reported in this study,” Dr. Barrett said.
More research in other countries, including research related to other autoimmune diseases, and with other datasets on population and community level risks from poor air quality, would be helpful, and that information could be used to advise smart public policy, Dr. Barrett added.
Air pollution’s mental health impact
Air pollution’s effects extend beyond physical to the psychological, a new study of depression in teenagers showed. This study was published in Developmental Psychology.
Previous research on the environmental factors associated with depressive symptoms in teens has focused mainly on individual and family level contributors; the impact of the physical environment has not been well studied, the investigators, Erika M. Manczak, PhD, of the University of Denver and colleagues, wrote.
In their paper, the authors found a significant impact of neighborhood ozone exposure on the trajectory of depressive symptoms in teens over a 4-year period.
“Given that inhaling pollution activates biological pathways implicated in the development of depression, including immune, cardiovascular, and neurodevelopmental processes, exposure to ambient air pollution may influence the development and/or trajectory of depressive symptoms in youth,” they said.
The researchers recruited 213 adolescents in the San Francisco Bay area through local advertisements. The participants were aged 9-13 years at baseline, with an average age of 11 years. A total of 121 were female, 47% were white, 8.5% were African American, 12.3% were Asian, 10.4% were nonwhite Latin, and 21.7% were biracial or another ethnicity. The participants self-reported depressive symptoms and other psychopathology symptoms up to three times during the study period. Ozone exposure was calculated based on home addresses.
After controlling for other personal, family, and neighborhood variables, the researchers found that higher levels of ozone exposure were significantly associated with increased depressive symptoms over time, and the slope of trajectory of depressive symptoms became steeper as the ozone levels increased (P less than .001). Ozone did not significantly predict the trajectory of any other psychopathology symptoms.
“The results of this study provide preliminary support for the possibility that ozone is an overlooked contributor to the development or course of youth depressive symptoms,” the researchers wrote in their discussion.
“Interestingly, the association between ozone and symptom trajectories as measured by Anxious/Depressed subscale of the [Youth Self-Report] was not as strong as it was for the [Children’s Depression Inventory-Short Version] or Withdrawn/Depressed scales, suggesting that associations are more robust for behavioral withdrawal symptoms of depression than for other types of symptoms,” they noted.
The study findings were limited by the use of self-reports and by the inability of the study design to show causality, the researchers said. Other limitations include the use of average assessments of ozone that are less precise, lack of assessment of biological pathways for risk, lack of formal psychiatric diagnoses, and the small geographic region included in the study, they said.
However, the results provide preliminary evidence that ozone exposure is a potential contributing factor to depressive symptoms in youth, and serve as a jumping-off point for future research, they noted. Future studies should address changes in systemic inflammation, neurodevelopment, or stress reactivity, as well as concurrent psychosocial or biological factors, and temporal associations between air pollution and mental health symptoms, they concluded.
Environmental factors drive inflammatory responses
Peter L. Loper Jr., MD, considers the findings of the Developmental Psychology study to be unsurprising but important – because air pollution is simply getting worse.
“As the study authors cite, there is sufficient data correlating ozone to negative physical health outcomes in youth, but a paucity of data exploring the impact of poor air quality on mental health outcomes in this demographic,” noted Dr. Loper, of the University of South Carolina, Columbia, in an interview.
“As discussed by the study researchers, any environmental exposure that increases immune-mediated inflammation can result in negative health outcomes. In fact, there is already data to suggest that similar cytokines, or immune cell signalers, that get released by our immune system due to environmental exposures and that contribute to asthma, may also be implicated in depression and other mental health problems,” he noted.
“Just like downstream symptom indicators of physical illnesses such as asthma are secondary to immune-mediated pulmonary inflammation, downstream symptom indicators of mental illness, such as depression, are secondary to immune-mediated neuroinflammation,” Dr. Loper emphasized. “The most well-characterized upstream phenomenon perpetuating the downstream symptom indicators of depression involve neuroinflammatory states due to psychosocial and relational factors such as chronic stress, poor relationships, or substance use. However, any environmental factor that triggers an immune response and inflammation can promote neuroinflammation that manifests as symptoms of mental illness.”
The message for teens with depression and their families is that “we are a product of our environment,” Dr. Loper said. “When our environments are proinflammatory, or cause our immune system to become overactive, then we will develop illness; however, the most potent mediator of inflammation in the brain, and the downstream symptoms of depression, is our relationships with those we love most,” he said.
Dr. Loper suggested research aimed at identifying other sources of immune-mediated inflammation caused by physical environments and better understanding how environmental phenomenon like ozone may compound previously established risk factors for mental illness could be useful.
The RMD Open study received no outside funding, and its authors had no financial conflicts.
The Developmental Psychology study was supported by the National Institute of Mental Health and the Stanford University Precision Health and Integrated Diagnostics Center. The researchers for that report, and Dr. Loper and Dr. Barrett had no conflicts to disclose.
Other analyses of data have found environmental air pollution from sources such as car exhaust and factory output can trigger an inflammatory response in the body. What’s new about a study published in RMD Open is that it explored an association between long-term exposure to pollution and risk of autoimmune diseases, wrote Giovanni Adami, MD, of the University of Verona (Italy) and colleagues.
“Environmental air pollution, according to the World Health Organization, is a major risk to health and 99% of the population worldwide is living in places where recommendations for air quality are not met,” said Dr. Adami in an interview. The limited data on the precise role of air pollution on rheumatic diseases in particular prompted the study, he said.
To explore the potential link between air pollution exposure and autoimmune disease, the researchers reviewed medical information from 81,363 adults via a national medical database in Italy; the data were submitted between June 2016 and November 2020.
The average age of the study population was 65 years, and 92% were women; 22% had at least one coexisting health condition. Each study participant was linked to local environmental monitoring via their residential postcode.
The researchers obtained details about concentrations of particulate matter in the environment from the Italian Institute of Environmental Protection that included 617 monitoring stations in 110 Italian provinces. They focused on concentrations of 10 and 2.5 (PM10 and PM2.5).
Exposure thresholds of 30 mcg/m3 for PM10 and 20 mcg/m3 for PM2.5 are generally considered harmful to health, they noted. On average, the long-term exposure was 16 mcg/m3 for PM2.5 and 25 mcg/m3 for PM10 between 2013 and 2019.
Overall, 9,723 individuals (12%) were diagnosed with an autoimmune disease between 2016 and 2020.
Exposure to PM10 was associated with a 7% higher risk of diagnosis with any autoimmune disease for every 10 mcg/m3 increase in concentration, but no association appeared between PM2.5 exposure and increased risk of autoimmune diseases.
However, in an adjusted model, chronic exposure to PM10 above 30 mcg/m3 and to PM2.5 above 20 mcg/m3 were associated with a 12% and 13% higher risk, respectively, of any autoimmune disease.
Chronic exposure to high levels of PM10 was specifically associated with a higher risk of rheumatoid arthritis, but no other autoimmune diseases. Chronic exposure to high levels of PM2.5 was associated with a higher risk of rheumatoid arthritis, connective tissue diseases, and inflammatory bowel diseases.
In their discussion, the researchers noted that the smaller diameter of PM2.5 molecules fluctuate less in response to rain and other weather, compared with PM10 molecules, which might make them a more accurate predictor of exposure to chronic air pollution.
The study findings were limited by several factors including the observational design, which prohibits the establishment of cause, and a lack of data on the start of symptoms and dates of diagnoses for autoimmune diseases, the researchers noted. Other limitations include the high percentage of older women in the study, which may limit generalizability, and the inability to account for additional personal exposure to pollutants outside of the environmental exposure, they said.
However, the results were strengthened by the large sample size and wide geographic distribution with variable pollution exposure, they said.
“Unfortunately, we were not surprised at all,” by the findings, Dr. Adami said in an interview.
“The biological rationale underpinning our findings is strong. Nevertheless, the magnitude of the effect was overwhelming. In addition, we saw an effect even at threshold of exposure that is widely considered as safe,” Dr. Adami noted.
Clinicians have been taught to consider cigarette smoking or other lifestyle behaviors as major risk factors for the development of several autoimmune diseases, said Dr. Adami. “In the future, we probably should include air pollution exposure as a risk factor as well. Interestingly, there is also accumulating evidence linking acute exposure to environmental air pollution with flares of chronic arthritis,” he said.
“Our study could have direct societal and political consequences,” and might help direct policy makers’ decisions on addressing strategies aimed to reduce fossil emissions, he said. As for additional research, “we certainly need multination studies to confirm our results on a larger scale,” Dr. Adami emphasized. “In addition, it is time to take action and start designing interventions aimed to reduce acute and chronic exposure to air pollution in patients suffering from RMDs.”
Consider the big picture of air quality
The Italian study is especially timely “given our evolving and emerging understanding of environmental risk factors for acute and chronic diseases, which we must first understand before we can address,” said Eileen Barrett, MD, of the University of New Mexico, Albuquerque, in an interview.
“I am largely surprised about the findings, as most physicians aren’t studying ambient air quality and risk for autoimmune disease,” said Dr. Barrett. “More often we think of air quality when we think of risk for respiratory diseases than autoimmune diseases, per se,” she said.
“There are several take-home messages from this study,” said Dr. Barrett. “The first is that we need more research to understand the consequences of air pollutants on health. Second, this study reminds us to think broadly about how air quality and our environment can affect health. And third, all clinicians should be committed to promoting science that can improve public health and reduce death and disability,” she emphasized.
The findings do not specifically reflect associations between pollution and other conditions such as chronic obstructive pulmonary disease and asthma although previous studies have shown an association between asthma and COPD exacerbations and air pollution, Dr. Barrett said.
“Further research will be needed to confirm the associations reported in this study,” Dr. Barrett said.
More research in other countries, including research related to other autoimmune diseases, and with other datasets on population and community level risks from poor air quality, would be helpful, and that information could be used to advise smart public policy, Dr. Barrett added.
Air pollution’s mental health impact
Air pollution’s effects extend beyond physical to the psychological, a new study of depression in teenagers showed. This study was published in Developmental Psychology.
Previous research on the environmental factors associated with depressive symptoms in teens has focused mainly on individual and family level contributors; the impact of the physical environment has not been well studied, the investigators, Erika M. Manczak, PhD, of the University of Denver and colleagues, wrote.
In their paper, the authors found a significant impact of neighborhood ozone exposure on the trajectory of depressive symptoms in teens over a 4-year period.
“Given that inhaling pollution activates biological pathways implicated in the development of depression, including immune, cardiovascular, and neurodevelopmental processes, exposure to ambient air pollution may influence the development and/or trajectory of depressive symptoms in youth,” they said.
The researchers recruited 213 adolescents in the San Francisco Bay area through local advertisements. The participants were aged 9-13 years at baseline, with an average age of 11 years. A total of 121 were female, 47% were white, 8.5% were African American, 12.3% were Asian, 10.4% were nonwhite Latin, and 21.7% were biracial or another ethnicity. The participants self-reported depressive symptoms and other psychopathology symptoms up to three times during the study period. Ozone exposure was calculated based on home addresses.
After controlling for other personal, family, and neighborhood variables, the researchers found that higher levels of ozone exposure were significantly associated with increased depressive symptoms over time, and the slope of trajectory of depressive symptoms became steeper as the ozone levels increased (P less than .001). Ozone did not significantly predict the trajectory of any other psychopathology symptoms.
“The results of this study provide preliminary support for the possibility that ozone is an overlooked contributor to the development or course of youth depressive symptoms,” the researchers wrote in their discussion.
“Interestingly, the association between ozone and symptom trajectories as measured by Anxious/Depressed subscale of the [Youth Self-Report] was not as strong as it was for the [Children’s Depression Inventory-Short Version] or Withdrawn/Depressed scales, suggesting that associations are more robust for behavioral withdrawal symptoms of depression than for other types of symptoms,” they noted.
The study findings were limited by the use of self-reports and by the inability of the study design to show causality, the researchers said. Other limitations include the use of average assessments of ozone that are less precise, lack of assessment of biological pathways for risk, lack of formal psychiatric diagnoses, and the small geographic region included in the study, they said.
However, the results provide preliminary evidence that ozone exposure is a potential contributing factor to depressive symptoms in youth, and serve as a jumping-off point for future research, they noted. Future studies should address changes in systemic inflammation, neurodevelopment, or stress reactivity, as well as concurrent psychosocial or biological factors, and temporal associations between air pollution and mental health symptoms, they concluded.
Environmental factors drive inflammatory responses
Peter L. Loper Jr., MD, considers the findings of the Developmental Psychology study to be unsurprising but important – because air pollution is simply getting worse.
“As the study authors cite, there is sufficient data correlating ozone to negative physical health outcomes in youth, but a paucity of data exploring the impact of poor air quality on mental health outcomes in this demographic,” noted Dr. Loper, of the University of South Carolina, Columbia, in an interview.
“As discussed by the study researchers, any environmental exposure that increases immune-mediated inflammation can result in negative health outcomes. In fact, there is already data to suggest that similar cytokines, or immune cell signalers, that get released by our immune system due to environmental exposures and that contribute to asthma, may also be implicated in depression and other mental health problems,” he noted.
“Just like downstream symptom indicators of physical illnesses such as asthma are secondary to immune-mediated pulmonary inflammation, downstream symptom indicators of mental illness, such as depression, are secondary to immune-mediated neuroinflammation,” Dr. Loper emphasized. “The most well-characterized upstream phenomenon perpetuating the downstream symptom indicators of depression involve neuroinflammatory states due to psychosocial and relational factors such as chronic stress, poor relationships, or substance use. However, any environmental factor that triggers an immune response and inflammation can promote neuroinflammation that manifests as symptoms of mental illness.”
The message for teens with depression and their families is that “we are a product of our environment,” Dr. Loper said. “When our environments are proinflammatory, or cause our immune system to become overactive, then we will develop illness; however, the most potent mediator of inflammation in the brain, and the downstream symptoms of depression, is our relationships with those we love most,” he said.
Dr. Loper suggested research aimed at identifying other sources of immune-mediated inflammation caused by physical environments and better understanding how environmental phenomenon like ozone may compound previously established risk factors for mental illness could be useful.
The RMD Open study received no outside funding, and its authors had no financial conflicts.
The Developmental Psychology study was supported by the National Institute of Mental Health and the Stanford University Precision Health and Integrated Diagnostics Center. The researchers for that report, and Dr. Loper and Dr. Barrett had no conflicts to disclose.
FROM RMD OPEN
New contact lens elutes antihistamine for ocular allergy
“This is the world’s first and only contact lens that’s able to prevent itching associated with allergies, while at the same time providing vision correction,” said Brian Pall, DO, director of clinical science at Johnson & Johnson Vision Care, which is making the lens. “It’s certainly exciting.”
The new lens, Acuvue Theravision With Ketotifenis, is already on the market in Canada and Japan.
The lenses are daily disposable contacts indicated for the prevention of ocular itch caused by allergic conjunctivitis in people who do not have red eyes, are suitable for wearing contact lenses, and do not have more than 1.00 D of astigmatism.
Antihistamine eyedrops are contraindicated for use with contact lenses because eyedrop preservatives could interact with the lenses, and clinical trials generally exclude contact lens wearers.
Johnson & Johnson worked for over a decade to find an antihistamine that paired well with a contact lens material, finally hitting on the combination of ketotifen and etafilcon A, said Pall. The drug is integrated into the polymer during manufacturing.
In contact with the eye, the drug diffuses from the lens into the tear film and is absorbed by the ocular tissues, much like a conventional eyedrop. “The key difference is that this is a slower release,” Dr. Pall said. “Instead of a bolus of this large drop hitting the eye and then being flushed out immediately, we get a much more sustained release.”
Because the lens is kept sterilized until use, no preservatives are added to the medication. This is an advantage because preservatives cause irritation in some patients.
Ketotifen, a well-established treatment for ocular allergies, not only blocks histamine receptors but also stabilizes mast cells so that they don’t release cytokines, and it prevents inflammatory cells from rushing to the site of irritation, Dr. Pall said.
For a pair of identical clinical trials, published in 2019 in Cornea, Dr. Pall and his colleagues recruited 244 people with ocular allergies. For each trial, they divided these subjects into three groups. One group wore the ketotifen lenses in one eye and lenses without the drug in the other eye. The second group wore the ketotifen lenses in both eyes. The third wore the control lenses in both eyes.
The researchers then exposed the subjects to allergens and asked them to rate the itchiness of their eyes on a scale of 0-4 after 3 minutes, 5 minutes, and 7 minutes. Over these periods, the patients rated itchiness of the eyes with the ketotifen contact lenses a mean from 0.42-0.59; they rated the eyes with the control contacts 1.60-1.94. The differences were statistically significant (P < .001).
While about 5% of patients experienced adverse events, most of the events were not judged to be related to the contact lenses. The most common adverse event, reported by about 1% of patients, was installation site irritation. “The good news, what we’re hearing from the field, is it’s very subtle, it’s pretty mild, and it quickly dissipates,” said Dr. Pall.
The new contact lens “is promising for those who have contact lenses and the 20%-40% of the American population who have allergies,” said Leonard Bielory, MD, a professor of medicine, allergy, immunology, and ophthalmology at Hackensack Meridian School of Medicine in Nutley, N.J., who was not involved in the trial or in developing the lens.
“I have patients who wear contacts and have allergies, and they have to work around it,” he said in an interview. “I expected this 15 years ago, because this is a good idea.”
Johnson & Johnson is researching other drugs that might be delivered through contact lenses, Dr. Pall said.
The study was funded by Johnson and Johnson. Dr. Pall is an employee of Johnson & Johnson. Dr. Bielory reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“This is the world’s first and only contact lens that’s able to prevent itching associated with allergies, while at the same time providing vision correction,” said Brian Pall, DO, director of clinical science at Johnson & Johnson Vision Care, which is making the lens. “It’s certainly exciting.”
The new lens, Acuvue Theravision With Ketotifenis, is already on the market in Canada and Japan.
The lenses are daily disposable contacts indicated for the prevention of ocular itch caused by allergic conjunctivitis in people who do not have red eyes, are suitable for wearing contact lenses, and do not have more than 1.00 D of astigmatism.
Antihistamine eyedrops are contraindicated for use with contact lenses because eyedrop preservatives could interact with the lenses, and clinical trials generally exclude contact lens wearers.
Johnson & Johnson worked for over a decade to find an antihistamine that paired well with a contact lens material, finally hitting on the combination of ketotifen and etafilcon A, said Pall. The drug is integrated into the polymer during manufacturing.
In contact with the eye, the drug diffuses from the lens into the tear film and is absorbed by the ocular tissues, much like a conventional eyedrop. “The key difference is that this is a slower release,” Dr. Pall said. “Instead of a bolus of this large drop hitting the eye and then being flushed out immediately, we get a much more sustained release.”
Because the lens is kept sterilized until use, no preservatives are added to the medication. This is an advantage because preservatives cause irritation in some patients.
Ketotifen, a well-established treatment for ocular allergies, not only blocks histamine receptors but also stabilizes mast cells so that they don’t release cytokines, and it prevents inflammatory cells from rushing to the site of irritation, Dr. Pall said.
For a pair of identical clinical trials, published in 2019 in Cornea, Dr. Pall and his colleagues recruited 244 people with ocular allergies. For each trial, they divided these subjects into three groups. One group wore the ketotifen lenses in one eye and lenses without the drug in the other eye. The second group wore the ketotifen lenses in both eyes. The third wore the control lenses in both eyes.
The researchers then exposed the subjects to allergens and asked them to rate the itchiness of their eyes on a scale of 0-4 after 3 minutes, 5 minutes, and 7 minutes. Over these periods, the patients rated itchiness of the eyes with the ketotifen contact lenses a mean from 0.42-0.59; they rated the eyes with the control contacts 1.60-1.94. The differences were statistically significant (P < .001).
While about 5% of patients experienced adverse events, most of the events were not judged to be related to the contact lenses. The most common adverse event, reported by about 1% of patients, was installation site irritation. “The good news, what we’re hearing from the field, is it’s very subtle, it’s pretty mild, and it quickly dissipates,” said Dr. Pall.
The new contact lens “is promising for those who have contact lenses and the 20%-40% of the American population who have allergies,” said Leonard Bielory, MD, a professor of medicine, allergy, immunology, and ophthalmology at Hackensack Meridian School of Medicine in Nutley, N.J., who was not involved in the trial or in developing the lens.
“I have patients who wear contacts and have allergies, and they have to work around it,” he said in an interview. “I expected this 15 years ago, because this is a good idea.”
Johnson & Johnson is researching other drugs that might be delivered through contact lenses, Dr. Pall said.
The study was funded by Johnson and Johnson. Dr. Pall is an employee of Johnson & Johnson. Dr. Bielory reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“This is the world’s first and only contact lens that’s able to prevent itching associated with allergies, while at the same time providing vision correction,” said Brian Pall, DO, director of clinical science at Johnson & Johnson Vision Care, which is making the lens. “It’s certainly exciting.”
The new lens, Acuvue Theravision With Ketotifenis, is already on the market in Canada and Japan.
The lenses are daily disposable contacts indicated for the prevention of ocular itch caused by allergic conjunctivitis in people who do not have red eyes, are suitable for wearing contact lenses, and do not have more than 1.00 D of astigmatism.
Antihistamine eyedrops are contraindicated for use with contact lenses because eyedrop preservatives could interact with the lenses, and clinical trials generally exclude contact lens wearers.
Johnson & Johnson worked for over a decade to find an antihistamine that paired well with a contact lens material, finally hitting on the combination of ketotifen and etafilcon A, said Pall. The drug is integrated into the polymer during manufacturing.
In contact with the eye, the drug diffuses from the lens into the tear film and is absorbed by the ocular tissues, much like a conventional eyedrop. “The key difference is that this is a slower release,” Dr. Pall said. “Instead of a bolus of this large drop hitting the eye and then being flushed out immediately, we get a much more sustained release.”
Because the lens is kept sterilized until use, no preservatives are added to the medication. This is an advantage because preservatives cause irritation in some patients.
Ketotifen, a well-established treatment for ocular allergies, not only blocks histamine receptors but also stabilizes mast cells so that they don’t release cytokines, and it prevents inflammatory cells from rushing to the site of irritation, Dr. Pall said.
For a pair of identical clinical trials, published in 2019 in Cornea, Dr. Pall and his colleagues recruited 244 people with ocular allergies. For each trial, they divided these subjects into three groups. One group wore the ketotifen lenses in one eye and lenses without the drug in the other eye. The second group wore the ketotifen lenses in both eyes. The third wore the control lenses in both eyes.
The researchers then exposed the subjects to allergens and asked them to rate the itchiness of their eyes on a scale of 0-4 after 3 minutes, 5 minutes, and 7 minutes. Over these periods, the patients rated itchiness of the eyes with the ketotifen contact lenses a mean from 0.42-0.59; they rated the eyes with the control contacts 1.60-1.94. The differences were statistically significant (P < .001).
While about 5% of patients experienced adverse events, most of the events were not judged to be related to the contact lenses. The most common adverse event, reported by about 1% of patients, was installation site irritation. “The good news, what we’re hearing from the field, is it’s very subtle, it’s pretty mild, and it quickly dissipates,” said Dr. Pall.
The new contact lens “is promising for those who have contact lenses and the 20%-40% of the American population who have allergies,” said Leonard Bielory, MD, a professor of medicine, allergy, immunology, and ophthalmology at Hackensack Meridian School of Medicine in Nutley, N.J., who was not involved in the trial or in developing the lens.
“I have patients who wear contacts and have allergies, and they have to work around it,” he said in an interview. “I expected this 15 years ago, because this is a good idea.”
Johnson & Johnson is researching other drugs that might be delivered through contact lenses, Dr. Pall said.
The study was funded by Johnson and Johnson. Dr. Pall is an employee of Johnson & Johnson. Dr. Bielory reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 often more severe with congenital heart defects
Adults with a congenital heart defect (CHD) are at increased risk for serious illness and death when hospitalized with COVID-19, making vaccination and other preventive measures even important in this population, say researchers with the Centers for Disease Control and Prevention.
“We found that hospitalized patients with heart defects are up to twice as likely to have critical outcomes of COVID-19 illness (admission to the intensive care unit, use of a ventilator to help with breathing, or death) compared to hospitalized COVID-19 patients without heart defects,” Karrie Downing, MPH, epidemiologist, with the CDC’s National Center on Birth Defects and Developmental Disabilities, said in an interview.
“Additionally, we learned that people with hearts defects who were older or who also had other conditions like heart failure, pulmonary hypertension, Down syndrome, diabetes, or obesity were the most likely to have critical COVID-19 illness, but children and adults with heart defects without these other conditions were still at increased risk,” Ms. Downing said.
The message for health care providers is clear: “Encourage your patients with heart defects to get vaccinated and discuss with your patients the need for other preventive measures to avoid infection that may progress to severe COVID-19 illness,” Ms. Downing added.
The study was published online March 7, 2022, in Circulation.
The researchers analyzed data on 235,638 patients hospitalized with COVID-19 between March 2020 and January 2021, including 421 (0.2%) with CHD. Most CHD patients were older than 30 years (73%) and 61% were men, with 55% non-Hispanic white, 19% Hispanic and 16% non-Hispanic Black.
Overall, 68% of CHD patients had at least one comorbidity, as did 59% of patients without CHD.
Rates of ICU admission were higher in the CHD group (54% vs. 43%), as were rates of invasive mechanical ventilation (24% vs. 15%) and in-hospital death (11% vs. 7%).
After accounting for patient characteristics, ICU admission, invasive mechanical ventilation and death were more prevalent among COVID-19 patients with rather than without CHD, with adjusted prevalence ratios of 1.4, 1.8 and 2.0, respectively.
When stratified by high-risk characteristics, prevalence estimates for ICU admission, invasive mechanical ventilation and death remained higher among patients with COVID-19 and CHD across nearly all strata, including younger age groups and those without heart failure, pulmonary hypertension, Down syndrome, diabetes, or obesity, the researchers reported.
Ms. Downing said more work is needed to identify why the clinical course of COVID-19 disease results in admission to the ICU, the need for a ventilator, or death for some hospitalized patients with CHD and not for others.
“There could be a number of social, environmental, economic, medical, and genetic factors playing a role. But staying up to date with COVID-19 vaccines and following preventive measures for COVID-19 are effective ways to reduce the risk of severe illness from COVID-19,” Ms. Downing said.
The study had no specific funding. The authors reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Adults with a congenital heart defect (CHD) are at increased risk for serious illness and death when hospitalized with COVID-19, making vaccination and other preventive measures even important in this population, say researchers with the Centers for Disease Control and Prevention.
“We found that hospitalized patients with heart defects are up to twice as likely to have critical outcomes of COVID-19 illness (admission to the intensive care unit, use of a ventilator to help with breathing, or death) compared to hospitalized COVID-19 patients without heart defects,” Karrie Downing, MPH, epidemiologist, with the CDC’s National Center on Birth Defects and Developmental Disabilities, said in an interview.
“Additionally, we learned that people with hearts defects who were older or who also had other conditions like heart failure, pulmonary hypertension, Down syndrome, diabetes, or obesity were the most likely to have critical COVID-19 illness, but children and adults with heart defects without these other conditions were still at increased risk,” Ms. Downing said.
The message for health care providers is clear: “Encourage your patients with heart defects to get vaccinated and discuss with your patients the need for other preventive measures to avoid infection that may progress to severe COVID-19 illness,” Ms. Downing added.
The study was published online March 7, 2022, in Circulation.
The researchers analyzed data on 235,638 patients hospitalized with COVID-19 between March 2020 and January 2021, including 421 (0.2%) with CHD. Most CHD patients were older than 30 years (73%) and 61% were men, with 55% non-Hispanic white, 19% Hispanic and 16% non-Hispanic Black.
Overall, 68% of CHD patients had at least one comorbidity, as did 59% of patients without CHD.
Rates of ICU admission were higher in the CHD group (54% vs. 43%), as were rates of invasive mechanical ventilation (24% vs. 15%) and in-hospital death (11% vs. 7%).
After accounting for patient characteristics, ICU admission, invasive mechanical ventilation and death were more prevalent among COVID-19 patients with rather than without CHD, with adjusted prevalence ratios of 1.4, 1.8 and 2.0, respectively.
When stratified by high-risk characteristics, prevalence estimates for ICU admission, invasive mechanical ventilation and death remained higher among patients with COVID-19 and CHD across nearly all strata, including younger age groups and those without heart failure, pulmonary hypertension, Down syndrome, diabetes, or obesity, the researchers reported.
Ms. Downing said more work is needed to identify why the clinical course of COVID-19 disease results in admission to the ICU, the need for a ventilator, or death for some hospitalized patients with CHD and not for others.
“There could be a number of social, environmental, economic, medical, and genetic factors playing a role. But staying up to date with COVID-19 vaccines and following preventive measures for COVID-19 are effective ways to reduce the risk of severe illness from COVID-19,” Ms. Downing said.
The study had no specific funding. The authors reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Adults with a congenital heart defect (CHD) are at increased risk for serious illness and death when hospitalized with COVID-19, making vaccination and other preventive measures even important in this population, say researchers with the Centers for Disease Control and Prevention.
“We found that hospitalized patients with heart defects are up to twice as likely to have critical outcomes of COVID-19 illness (admission to the intensive care unit, use of a ventilator to help with breathing, or death) compared to hospitalized COVID-19 patients without heart defects,” Karrie Downing, MPH, epidemiologist, with the CDC’s National Center on Birth Defects and Developmental Disabilities, said in an interview.
“Additionally, we learned that people with hearts defects who were older or who also had other conditions like heart failure, pulmonary hypertension, Down syndrome, diabetes, or obesity were the most likely to have critical COVID-19 illness, but children and adults with heart defects without these other conditions were still at increased risk,” Ms. Downing said.
The message for health care providers is clear: “Encourage your patients with heart defects to get vaccinated and discuss with your patients the need for other preventive measures to avoid infection that may progress to severe COVID-19 illness,” Ms. Downing added.
The study was published online March 7, 2022, in Circulation.
The researchers analyzed data on 235,638 patients hospitalized with COVID-19 between March 2020 and January 2021, including 421 (0.2%) with CHD. Most CHD patients were older than 30 years (73%) and 61% were men, with 55% non-Hispanic white, 19% Hispanic and 16% non-Hispanic Black.
Overall, 68% of CHD patients had at least one comorbidity, as did 59% of patients without CHD.
Rates of ICU admission were higher in the CHD group (54% vs. 43%), as were rates of invasive mechanical ventilation (24% vs. 15%) and in-hospital death (11% vs. 7%).
After accounting for patient characteristics, ICU admission, invasive mechanical ventilation and death were more prevalent among COVID-19 patients with rather than without CHD, with adjusted prevalence ratios of 1.4, 1.8 and 2.0, respectively.
When stratified by high-risk characteristics, prevalence estimates for ICU admission, invasive mechanical ventilation and death remained higher among patients with COVID-19 and CHD across nearly all strata, including younger age groups and those without heart failure, pulmonary hypertension, Down syndrome, diabetes, or obesity, the researchers reported.
Ms. Downing said more work is needed to identify why the clinical course of COVID-19 disease results in admission to the ICU, the need for a ventilator, or death for some hospitalized patients with CHD and not for others.
“There could be a number of social, environmental, economic, medical, and genetic factors playing a role. But staying up to date with COVID-19 vaccines and following preventive measures for COVID-19 are effective ways to reduce the risk of severe illness from COVID-19,” Ms. Downing said.
The study had no specific funding. The authors reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM CIRCULATION
Vaccine update: The latest recommendations from ACIP
In a typical year, the Advisory Committee on Immunization Practices (ACIP) has three 1.5- to 2-day meetings to make recommendations for the use of new and existing vaccines in the US population. However, 2021 was not a typical year. Last year, ACIP held 17 meetings for a total of 127 hours. Most of these were related to vaccines to prevent COVID-19. There are now 3 COVID-19 vaccines authorized for use in the United States: the 2-dose mRNA-based Pfizer-BioNTech/Comirnaty and Moderna COVID-19 vaccines and the single-dose adenovirus, vector-based Janssen (Johnson & Johnson) COVID-19 vaccine.
TABLE 11 includes the actions taken by the ACIP from late 2020 through 2021 related to COVID-19 vaccines. All of these recommendations except 1 occurred after the US Food and Drug Administration (FDA) approved the product using an emergency use authorization (EUA). The exception is the recommendation for use of the Pfizer-BioNTech COVID-19 vaccine (BNT162b2) for those ages 16 years and older, which was approved under the normal process 8 months after widespread use under an EUA.
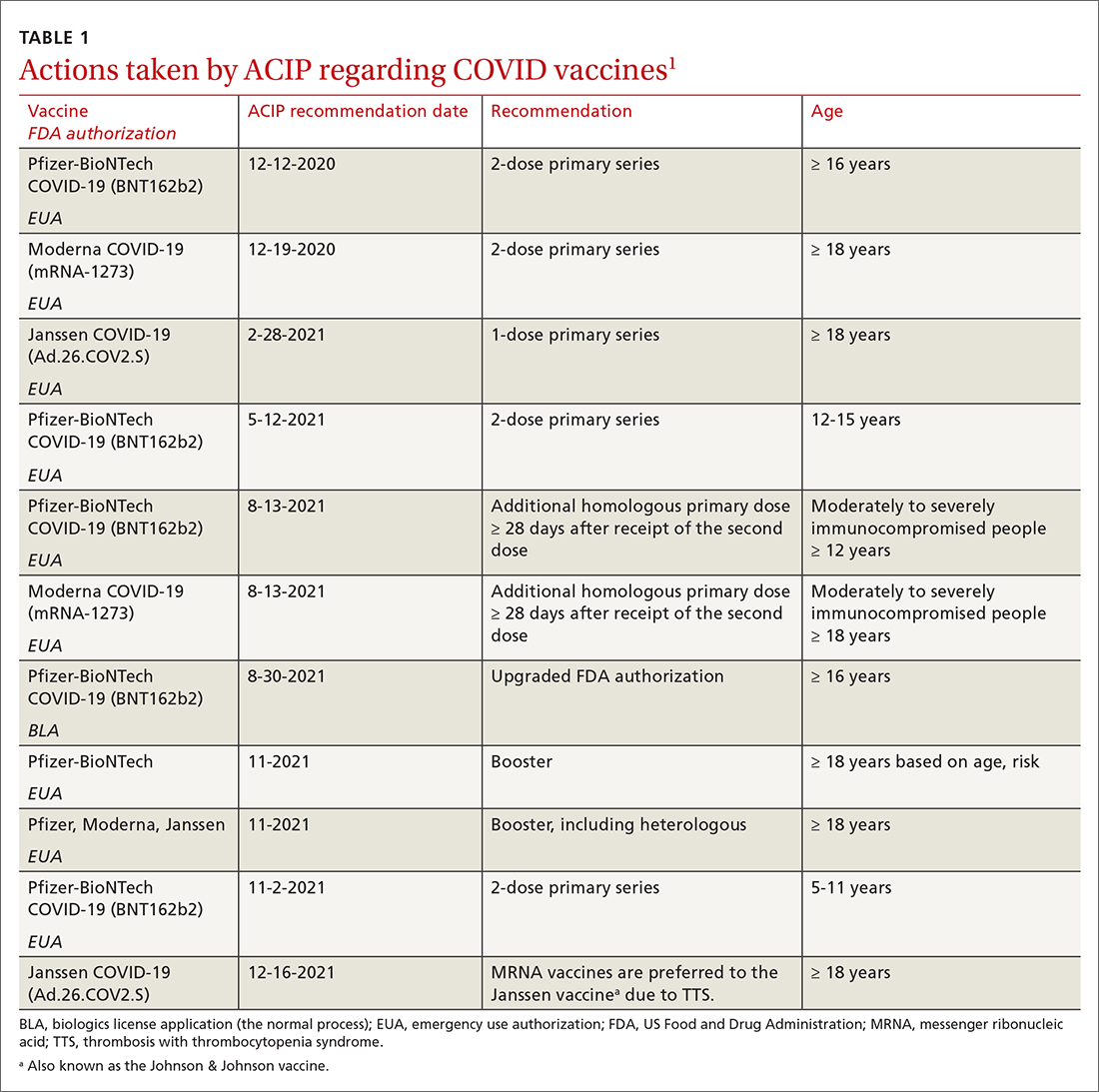
Hepatitis B vaccine now for all nonimmune adults up through 59 years
Since the introduction of hepatitis B (HepB) vaccines in 1980, the incidence of hepatitis B virus (HBV) infections in the United States has been reduced dramatically; there were an estimated 287,000 cases in 19852 and 19,200 in 2014.3 However, the incidence among adults has not declined in recent years and among someage groups has actually increased. Among those ages 40 to 49 years, the rate went from 1.9 per 100,000 in 20114 to 2.7 per 100,000 population in 2019.5 In those ages 50 to 59, there was an increase from 1.1 to 1.6 per 100,000 population over the same period of time.4,5
Recommendations for using HepB vaccine in adults have been based on risk that involves individual behavior, occupation, and medical conditions (TABLE 26). The presence of these risk factors is often unknown to medical professionals, who rarely ask about or document them. And patients can be reluctant to disclose them for fear of being stigmatized. The consequence has been a low rate of vaccination in at-risk adults.

At its November 2021 meeting, ACIP accepted the advice of the Hepatitis Work Group to move to a universal adult recommendation through age 59.7 ACIP believed that the incidence of acute infection in those ages 60 and older was too low to merit a universal recommendation. The new recommendation states that
Multiple HepB vaccine products are available for adults. Two are recombinant-based and require 3 doses: Engerix-B (GlaxoSmithKline) and Recombivax HB (Merck). One is recombinant based and requires only 2 doses: Heplisav-B (Dynavax Technologies). A new product recently approved by the FDA, PREHEVBRIO (VBI Vaccines), is another recombinant 3-dose option that the ACIP will consider early in 2022. HepB and HepA vaccines can also be co-administered with Twinrix (GlaxoSmithKline).
Pneumococcal vaccines: New PCV vaccines alter prescribing choices
The ACIP recommendations for pneumococcal vaccines in adults have been very confusing, involving 2 vaccines: PCV13 (Prevnar13, Pfizer) and PPSV23 (Pneumovax23, Merck). Both PCV13 and PPSV23 given in series were recommended for immunocompromised patients, but only PPSV23 was recommended for those with chronic medical conditions. For those 65 and older, PPSV23 was recommended for all individuals (including those with no chronic or immunocompromising condition), and PCV13 was recommended for those with immunocompromising conditions. Other adults in this older age group could receive PCV13 based on individual risk and shared clinical decision making.8
Continue to: This past year...
This past year, 2 new PCV vaccines were approved by the FDA: PCV15 (Vaxneuvance, Merck) and PCV20 (Prevnar20, Pfizer). While considering these new vaccines, the ACIP re-assessed its entire approval of pneumococcal vaccines. First, they retained the cutoff for universal pneumococcal vaccination at 65 years. For those younger than 65, they combined chronic medical conditions and immunocompromising conditions into a single at-risk group (TABLE 39). They then issued the same recommendation for older adults and those younger than 65 with risks: to receive a PCV vaccine, either PCV15 or PCV20. If they receive PCV15, it should be followed by PPSV23. PPSV23 is not recommended for those who receive PCV20. Therefore,

Recombinant zoster vaccine (RZV) has been licensed and recommended in the United States since 2017 in a 2-dose schedule for adults ages 50 years and older. In the summer of 2021, the FDA expanded the indication for use of RZV to include individuals 18 to 49 years of age who are or will be immunodeficient or immunosuppressed due to known disease or therapy. In October, the ACIP agreed and recommended 2 RZV doses for those 19 years and older in these risk groups (TABLE 410).

This recommendation was based on the elevated risk of herpes zoster documented in those with immune-suppressing conditions and therapies. In the conditions studied, the incidence in these younger adults exceeded that for older adults, for whom the vaccine is recommended.10 There are many immune conditions and immune-suppressing medications. The ACIP Zoster Work Group did not have efficacy and safety information on the use of RZV in each one of them, even though their recommendation includes them all. Many of these patients are under the care of specialists whose specialty societies had been recommending zoster vaccine for their patients, off label, prior to the FDA authorization.
Rabies vaccine is now available in 2-dose schedule
People who should receive rabies pre-exposure prophylaxis (PrEP) with rabies vaccine include laboratory personnel who work with rabies virus, biologists who work with bats, animal care professionals, wildlife biologists, veterinarians, and travelers who may be at risk of encountering rabid dogs. The recommendation has been for 3 doses of rabies vaccine at 0, 7, and 21-28 days. The ACIP voted at its June 2021 meeting to adopt a 2-dose PrEP schedule of 0 and 7 days.11 This will be especially helpful to travelers who want to complete the recommended doses prior to departure. Those who have sustained risk over time can elect to have a third dose after 21 days and before 3 years, or elect to have titers checked. More detailed clinical advice will be published in the CDC’s Morbidity and Mortality Weekly Report in 2022.
Dengue vaccine: New rec for those 9-16 years
In 2019, the FDA approved the first dengue vaccine for use in the United States for children 9 to 16 years old who had laboratory-confirmed previous dengue virus infection and who were living in an area where dengue is endemic. The CYD-TDV dengue vaccine (Dengvaxia) is a live-attenuated tetravalent vaccine built on a yellow fever vaccine backbone. Its effectiveness is 82% for prevention of symptomatic dengue, 79% for prevention of dengue-associated hospitalizations, and 84% against severe dengue.12
Continue to: Dengue viruses...
Dengue viruses (DENV) are transmitted by Aedes mosquitoes. There are 4 serotypes of dengue, and all 4 appear to be circulating in most endemic countries. Clinical disease varies from a mild febrile illness to severe disease. The most common clinical presentation includes sudden onset of fever, headache, retro-orbital pain, myalgia and arthralgia, abdominal pain, and nausea.
Severe disease includes plasma leakage, shock, respiratory distress, severe bleeding, and organ failure. While severe dengue can occur with a primary infection, a second infection with a different DENV increases the risk of severe dengue. A small increased risk of severe dengue occurs when dengue infection occurs after vaccination in those with no evidence of previous dengue infection. It is felt that the vaccine serves as a primary infection that increases the risk of severe dengue with subsequent infections. This is the reason that the vaccine is recommended only for those with a documented previous dengue infection.
At its June 2021 meeting, the ACIP recommended 3-doses of Dengvaxia, administered at 0, 6, and 12 months, for individuals 9 to 16 years of age who have laboratory confirmation of previous dengue infection and live in endemic areas.12 These areas include the territories and affiliated states of Puerto Rico, American Samoa, US Virgin Islands, Federated States of Micronesia, Republic of Marshall Islands, and the Republic of Palau. Puerto Rico accounts for 85% of the population of these areas and 95% of reported dengue cases.12The reason for the delay between FDA approval and the ACIP recommendation was the need to wait for a readily available, accurate laboratory test to confirm previous dengue infection, which is now available. There are other dengue vaccines in development including 2 live-attenuated, tetravalent vaccine candidates in Phase 3 trials.
1. ACIP. COVID-19 vaccine recommendations. Accessed February 8, 2022. www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html
2. CDC. Division of viral hepatitis. Disease burden from viral hepatitis A, B, and C in the United States. Accessed February 8 2022. www.cdc.gov/hepatitis/PDFs/disease_burden.pdf
3. CDC. Surveillance for viral hepatitis – United States, 2014. Hepatitis B. Accessed February 8, 2022. https://www.cdc.gov/hepatitis/statistics/2014surveillance/commentary.htm#:~:text=HEPATITIS%20B-,Acute%20Hepatitis%20B,B%20cases%20occurred%20in%202014
4. CDC. Viral hepatitis surveillance: United States, 2011. Hepatitis B. Accessed February 8, 2022. www.cdc.gov/hepatitis/statistics/2011surveillance/pdfs/2011HepSurveillanceRpt.pdf
5. CDC. Viral hepatitis surveillance report, 2019. Hepatitis B. Accessed February 8, 2022. www.cdc.gov/hepatitis/statistics/2019surveillance/HepB.htm
6. Schillie S, Harris A, Link-Gelles R, et al. Recommendations of the Advisory Committee on Immunization Practices for use of a hepatitis B vaccine with a novel adjuvant. MMWR Morb Mortal Wkly Rep. 2018;67:455-458.
7. CDC. Advisory Committee on Immunization Practices. Meeting recommendations, November 2021. Accessed February 8, 2022. www.cdc.gov/vaccines/acip/index.html
8. Matanock A, Lee G, Gierke R, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:1069-1075.
9. Kobayashi M. Considerations for use of PCV15 and PCV20 in U.S. adults. Accessed February 8, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-02/24-25/05-Pneumococcal-Kobayashi.pdf
10. Anderson TC, Masters NB, Guo A, et al. Use of recombinant zoster vaccine in immunocompromised adults aged ≥19 years: recommendations of the Advisory Committee on Immunization Practices — United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:80-84.
11. CDC. ACIP recommendations. June 2021. Accessed February 8, 2022. www.cdc.gov/vaccines/acip/recommendations.html
12. Paz-Bailey G. Dengue vaccine. Evidence to recommendation framework. Presented to the ACIP June 24, 2021. Accessed February 8, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-06/03-Dengue-Paz-Bailey-508.pdf
In a typical year, the Advisory Committee on Immunization Practices (ACIP) has three 1.5- to 2-day meetings to make recommendations for the use of new and existing vaccines in the US population. However, 2021 was not a typical year. Last year, ACIP held 17 meetings for a total of 127 hours. Most of these were related to vaccines to prevent COVID-19. There are now 3 COVID-19 vaccines authorized for use in the United States: the 2-dose mRNA-based Pfizer-BioNTech/Comirnaty and Moderna COVID-19 vaccines and the single-dose adenovirus, vector-based Janssen (Johnson & Johnson) COVID-19 vaccine.
TABLE 11 includes the actions taken by the ACIP from late 2020 through 2021 related to COVID-19 vaccines. All of these recommendations except 1 occurred after the US Food and Drug Administration (FDA) approved the product using an emergency use authorization (EUA). The exception is the recommendation for use of the Pfizer-BioNTech COVID-19 vaccine (BNT162b2) for those ages 16 years and older, which was approved under the normal process 8 months after widespread use under an EUA.

Hepatitis B vaccine now for all nonimmune adults up through 59 years
Since the introduction of hepatitis B (HepB) vaccines in 1980, the incidence of hepatitis B virus (HBV) infections in the United States has been reduced dramatically; there were an estimated 287,000 cases in 19852 and 19,200 in 2014.3 However, the incidence among adults has not declined in recent years and among someage groups has actually increased. Among those ages 40 to 49 years, the rate went from 1.9 per 100,000 in 20114 to 2.7 per 100,000 population in 2019.5 In those ages 50 to 59, there was an increase from 1.1 to 1.6 per 100,000 population over the same period of time.4,5
Recommendations for using HepB vaccine in adults have been based on risk that involves individual behavior, occupation, and medical conditions (TABLE 26). The presence of these risk factors is often unknown to medical professionals, who rarely ask about or document them. And patients can be reluctant to disclose them for fear of being stigmatized. The consequence has been a low rate of vaccination in at-risk adults.

At its November 2021 meeting, ACIP accepted the advice of the Hepatitis Work Group to move to a universal adult recommendation through age 59.7 ACIP believed that the incidence of acute infection in those ages 60 and older was too low to merit a universal recommendation. The new recommendation states that
Multiple HepB vaccine products are available for adults. Two are recombinant-based and require 3 doses: Engerix-B (GlaxoSmithKline) and Recombivax HB (Merck). One is recombinant based and requires only 2 doses: Heplisav-B (Dynavax Technologies). A new product recently approved by the FDA, PREHEVBRIO (VBI Vaccines), is another recombinant 3-dose option that the ACIP will consider early in 2022. HepB and HepA vaccines can also be co-administered with Twinrix (GlaxoSmithKline).
Pneumococcal vaccines: New PCV vaccines alter prescribing choices
The ACIP recommendations for pneumococcal vaccines in adults have been very confusing, involving 2 vaccines: PCV13 (Prevnar13, Pfizer) and PPSV23 (Pneumovax23, Merck). Both PCV13 and PPSV23 given in series were recommended for immunocompromised patients, but only PPSV23 was recommended for those with chronic medical conditions. For those 65 and older, PPSV23 was recommended for all individuals (including those with no chronic or immunocompromising condition), and PCV13 was recommended for those with immunocompromising conditions. Other adults in this older age group could receive PCV13 based on individual risk and shared clinical decision making.8
Continue to: This past year...
This past year, 2 new PCV vaccines were approved by the FDA: PCV15 (Vaxneuvance, Merck) and PCV20 (Prevnar20, Pfizer). While considering these new vaccines, the ACIP re-assessed its entire approval of pneumococcal vaccines. First, they retained the cutoff for universal pneumococcal vaccination at 65 years. For those younger than 65, they combined chronic medical conditions and immunocompromising conditions into a single at-risk group (TABLE 39). They then issued the same recommendation for older adults and those younger than 65 with risks: to receive a PCV vaccine, either PCV15 or PCV20. If they receive PCV15, it should be followed by PPSV23. PPSV23 is not recommended for those who receive PCV20. Therefore,

Recombinant zoster vaccine (RZV) has been licensed and recommended in the United States since 2017 in a 2-dose schedule for adults ages 50 years and older. In the summer of 2021, the FDA expanded the indication for use of RZV to include individuals 18 to 49 years of age who are or will be immunodeficient or immunosuppressed due to known disease or therapy. In October, the ACIP agreed and recommended 2 RZV doses for those 19 years and older in these risk groups (TABLE 410).

This recommendation was based on the elevated risk of herpes zoster documented in those with immune-suppressing conditions and therapies. In the conditions studied, the incidence in these younger adults exceeded that for older adults, for whom the vaccine is recommended.10 There are many immune conditions and immune-suppressing medications. The ACIP Zoster Work Group did not have efficacy and safety information on the use of RZV in each one of them, even though their recommendation includes them all. Many of these patients are under the care of specialists whose specialty societies had been recommending zoster vaccine for their patients, off label, prior to the FDA authorization.
Rabies vaccine is now available in 2-dose schedule
People who should receive rabies pre-exposure prophylaxis (PrEP) with rabies vaccine include laboratory personnel who work with rabies virus, biologists who work with bats, animal care professionals, wildlife biologists, veterinarians, and travelers who may be at risk of encountering rabid dogs. The recommendation has been for 3 doses of rabies vaccine at 0, 7, and 21-28 days. The ACIP voted at its June 2021 meeting to adopt a 2-dose PrEP schedule of 0 and 7 days.11 This will be especially helpful to travelers who want to complete the recommended doses prior to departure. Those who have sustained risk over time can elect to have a third dose after 21 days and before 3 years, or elect to have titers checked. More detailed clinical advice will be published in the CDC’s Morbidity and Mortality Weekly Report in 2022.
Dengue vaccine: New rec for those 9-16 years
In 2019, the FDA approved the first dengue vaccine for use in the United States for children 9 to 16 years old who had laboratory-confirmed previous dengue virus infection and who were living in an area where dengue is endemic. The CYD-TDV dengue vaccine (Dengvaxia) is a live-attenuated tetravalent vaccine built on a yellow fever vaccine backbone. Its effectiveness is 82% for prevention of symptomatic dengue, 79% for prevention of dengue-associated hospitalizations, and 84% against severe dengue.12
Continue to: Dengue viruses...
Dengue viruses (DENV) are transmitted by Aedes mosquitoes. There are 4 serotypes of dengue, and all 4 appear to be circulating in most endemic countries. Clinical disease varies from a mild febrile illness to severe disease. The most common clinical presentation includes sudden onset of fever, headache, retro-orbital pain, myalgia and arthralgia, abdominal pain, and nausea.
Severe disease includes plasma leakage, shock, respiratory distress, severe bleeding, and organ failure. While severe dengue can occur with a primary infection, a second infection with a different DENV increases the risk of severe dengue. A small increased risk of severe dengue occurs when dengue infection occurs after vaccination in those with no evidence of previous dengue infection. It is felt that the vaccine serves as a primary infection that increases the risk of severe dengue with subsequent infections. This is the reason that the vaccine is recommended only for those with a documented previous dengue infection.
At its June 2021 meeting, the ACIP recommended 3-doses of Dengvaxia, administered at 0, 6, and 12 months, for individuals 9 to 16 years of age who have laboratory confirmation of previous dengue infection and live in endemic areas.12 These areas include the territories and affiliated states of Puerto Rico, American Samoa, US Virgin Islands, Federated States of Micronesia, Republic of Marshall Islands, and the Republic of Palau. Puerto Rico accounts for 85% of the population of these areas and 95% of reported dengue cases.12The reason for the delay between FDA approval and the ACIP recommendation was the need to wait for a readily available, accurate laboratory test to confirm previous dengue infection, which is now available. There are other dengue vaccines in development including 2 live-attenuated, tetravalent vaccine candidates in Phase 3 trials.
In a typical year, the Advisory Committee on Immunization Practices (ACIP) has three 1.5- to 2-day meetings to make recommendations for the use of new and existing vaccines in the US population. However, 2021 was not a typical year. Last year, ACIP held 17 meetings for a total of 127 hours. Most of these were related to vaccines to prevent COVID-19. There are now 3 COVID-19 vaccines authorized for use in the United States: the 2-dose mRNA-based Pfizer-BioNTech/Comirnaty and Moderna COVID-19 vaccines and the single-dose adenovirus, vector-based Janssen (Johnson & Johnson) COVID-19 vaccine.
TABLE 11 includes the actions taken by the ACIP from late 2020 through 2021 related to COVID-19 vaccines. All of these recommendations except 1 occurred after the US Food and Drug Administration (FDA) approved the product using an emergency use authorization (EUA). The exception is the recommendation for use of the Pfizer-BioNTech COVID-19 vaccine (BNT162b2) for those ages 16 years and older, which was approved under the normal process 8 months after widespread use under an EUA.

Hepatitis B vaccine now for all nonimmune adults up through 59 years
Since the introduction of hepatitis B (HepB) vaccines in 1980, the incidence of hepatitis B virus (HBV) infections in the United States has been reduced dramatically; there were an estimated 287,000 cases in 19852 and 19,200 in 2014.3 However, the incidence among adults has not declined in recent years and among someage groups has actually increased. Among those ages 40 to 49 years, the rate went from 1.9 per 100,000 in 20114 to 2.7 per 100,000 population in 2019.5 In those ages 50 to 59, there was an increase from 1.1 to 1.6 per 100,000 population over the same period of time.4,5
Recommendations for using HepB vaccine in adults have been based on risk that involves individual behavior, occupation, and medical conditions (TABLE 26). The presence of these risk factors is often unknown to medical professionals, who rarely ask about or document them. And patients can be reluctant to disclose them for fear of being stigmatized. The consequence has been a low rate of vaccination in at-risk adults.

At its November 2021 meeting, ACIP accepted the advice of the Hepatitis Work Group to move to a universal adult recommendation through age 59.7 ACIP believed that the incidence of acute infection in those ages 60 and older was too low to merit a universal recommendation. The new recommendation states that
Multiple HepB vaccine products are available for adults. Two are recombinant-based and require 3 doses: Engerix-B (GlaxoSmithKline) and Recombivax HB (Merck). One is recombinant based and requires only 2 doses: Heplisav-B (Dynavax Technologies). A new product recently approved by the FDA, PREHEVBRIO (VBI Vaccines), is another recombinant 3-dose option that the ACIP will consider early in 2022. HepB and HepA vaccines can also be co-administered with Twinrix (GlaxoSmithKline).
Pneumococcal vaccines: New PCV vaccines alter prescribing choices
The ACIP recommendations for pneumococcal vaccines in adults have been very confusing, involving 2 vaccines: PCV13 (Prevnar13, Pfizer) and PPSV23 (Pneumovax23, Merck). Both PCV13 and PPSV23 given in series were recommended for immunocompromised patients, but only PPSV23 was recommended for those with chronic medical conditions. For those 65 and older, PPSV23 was recommended for all individuals (including those with no chronic or immunocompromising condition), and PCV13 was recommended for those with immunocompromising conditions. Other adults in this older age group could receive PCV13 based on individual risk and shared clinical decision making.8
Continue to: This past year...
This past year, 2 new PCV vaccines were approved by the FDA: PCV15 (Vaxneuvance, Merck) and PCV20 (Prevnar20, Pfizer). While considering these new vaccines, the ACIP re-assessed its entire approval of pneumococcal vaccines. First, they retained the cutoff for universal pneumococcal vaccination at 65 years. For those younger than 65, they combined chronic medical conditions and immunocompromising conditions into a single at-risk group (TABLE 39). They then issued the same recommendation for older adults and those younger than 65 with risks: to receive a PCV vaccine, either PCV15 or PCV20. If they receive PCV15, it should be followed by PPSV23. PPSV23 is not recommended for those who receive PCV20. Therefore,

Recombinant zoster vaccine (RZV) has been licensed and recommended in the United States since 2017 in a 2-dose schedule for adults ages 50 years and older. In the summer of 2021, the FDA expanded the indication for use of RZV to include individuals 18 to 49 years of age who are or will be immunodeficient or immunosuppressed due to known disease or therapy. In October, the ACIP agreed and recommended 2 RZV doses for those 19 years and older in these risk groups (TABLE 410).

This recommendation was based on the elevated risk of herpes zoster documented in those with immune-suppressing conditions and therapies. In the conditions studied, the incidence in these younger adults exceeded that for older adults, for whom the vaccine is recommended.10 There are many immune conditions and immune-suppressing medications. The ACIP Zoster Work Group did not have efficacy and safety information on the use of RZV in each one of them, even though their recommendation includes them all. Many of these patients are under the care of specialists whose specialty societies had been recommending zoster vaccine for their patients, off label, prior to the FDA authorization.
Rabies vaccine is now available in 2-dose schedule
People who should receive rabies pre-exposure prophylaxis (PrEP) with rabies vaccine include laboratory personnel who work with rabies virus, biologists who work with bats, animal care professionals, wildlife biologists, veterinarians, and travelers who may be at risk of encountering rabid dogs. The recommendation has been for 3 doses of rabies vaccine at 0, 7, and 21-28 days. The ACIP voted at its June 2021 meeting to adopt a 2-dose PrEP schedule of 0 and 7 days.11 This will be especially helpful to travelers who want to complete the recommended doses prior to departure. Those who have sustained risk over time can elect to have a third dose after 21 days and before 3 years, or elect to have titers checked. More detailed clinical advice will be published in the CDC’s Morbidity and Mortality Weekly Report in 2022.
Dengue vaccine: New rec for those 9-16 years
In 2019, the FDA approved the first dengue vaccine for use in the United States for children 9 to 16 years old who had laboratory-confirmed previous dengue virus infection and who were living in an area where dengue is endemic. The CYD-TDV dengue vaccine (Dengvaxia) is a live-attenuated tetravalent vaccine built on a yellow fever vaccine backbone. Its effectiveness is 82% for prevention of symptomatic dengue, 79% for prevention of dengue-associated hospitalizations, and 84% against severe dengue.12
Continue to: Dengue viruses...
Dengue viruses (DENV) are transmitted by Aedes mosquitoes. There are 4 serotypes of dengue, and all 4 appear to be circulating in most endemic countries. Clinical disease varies from a mild febrile illness to severe disease. The most common clinical presentation includes sudden onset of fever, headache, retro-orbital pain, myalgia and arthralgia, abdominal pain, and nausea.
Severe disease includes plasma leakage, shock, respiratory distress, severe bleeding, and organ failure. While severe dengue can occur with a primary infection, a second infection with a different DENV increases the risk of severe dengue. A small increased risk of severe dengue occurs when dengue infection occurs after vaccination in those with no evidence of previous dengue infection. It is felt that the vaccine serves as a primary infection that increases the risk of severe dengue with subsequent infections. This is the reason that the vaccine is recommended only for those with a documented previous dengue infection.
At its June 2021 meeting, the ACIP recommended 3-doses of Dengvaxia, administered at 0, 6, and 12 months, for individuals 9 to 16 years of age who have laboratory confirmation of previous dengue infection and live in endemic areas.12 These areas include the territories and affiliated states of Puerto Rico, American Samoa, US Virgin Islands, Federated States of Micronesia, Republic of Marshall Islands, and the Republic of Palau. Puerto Rico accounts for 85% of the population of these areas and 95% of reported dengue cases.12The reason for the delay between FDA approval and the ACIP recommendation was the need to wait for a readily available, accurate laboratory test to confirm previous dengue infection, which is now available. There are other dengue vaccines in development including 2 live-attenuated, tetravalent vaccine candidates in Phase 3 trials.
1. ACIP. COVID-19 vaccine recommendations. Accessed February 8, 2022. www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html
2. CDC. Division of viral hepatitis. Disease burden from viral hepatitis A, B, and C in the United States. Accessed February 8 2022. www.cdc.gov/hepatitis/PDFs/disease_burden.pdf
3. CDC. Surveillance for viral hepatitis – United States, 2014. Hepatitis B. Accessed February 8, 2022. https://www.cdc.gov/hepatitis/statistics/2014surveillance/commentary.htm#:~:text=HEPATITIS%20B-,Acute%20Hepatitis%20B,B%20cases%20occurred%20in%202014
4. CDC. Viral hepatitis surveillance: United States, 2011. Hepatitis B. Accessed February 8, 2022. www.cdc.gov/hepatitis/statistics/2011surveillance/pdfs/2011HepSurveillanceRpt.pdf
5. CDC. Viral hepatitis surveillance report, 2019. Hepatitis B. Accessed February 8, 2022. www.cdc.gov/hepatitis/statistics/2019surveillance/HepB.htm
6. Schillie S, Harris A, Link-Gelles R, et al. Recommendations of the Advisory Committee on Immunization Practices for use of a hepatitis B vaccine with a novel adjuvant. MMWR Morb Mortal Wkly Rep. 2018;67:455-458.
7. CDC. Advisory Committee on Immunization Practices. Meeting recommendations, November 2021. Accessed February 8, 2022. www.cdc.gov/vaccines/acip/index.html
8. Matanock A, Lee G, Gierke R, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:1069-1075.
9. Kobayashi M. Considerations for use of PCV15 and PCV20 in U.S. adults. Accessed February 8, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-02/24-25/05-Pneumococcal-Kobayashi.pdf
10. Anderson TC, Masters NB, Guo A, et al. Use of recombinant zoster vaccine in immunocompromised adults aged ≥19 years: recommendations of the Advisory Committee on Immunization Practices — United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:80-84.
11. CDC. ACIP recommendations. June 2021. Accessed February 8, 2022. www.cdc.gov/vaccines/acip/recommendations.html
12. Paz-Bailey G. Dengue vaccine. Evidence to recommendation framework. Presented to the ACIP June 24, 2021. Accessed February 8, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-06/03-Dengue-Paz-Bailey-508.pdf
1. ACIP. COVID-19 vaccine recommendations. Accessed February 8, 2022. www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html
2. CDC. Division of viral hepatitis. Disease burden from viral hepatitis A, B, and C in the United States. Accessed February 8 2022. www.cdc.gov/hepatitis/PDFs/disease_burden.pdf
3. CDC. Surveillance for viral hepatitis – United States, 2014. Hepatitis B. Accessed February 8, 2022. https://www.cdc.gov/hepatitis/statistics/2014surveillance/commentary.htm#:~:text=HEPATITIS%20B-,Acute%20Hepatitis%20B,B%20cases%20occurred%20in%202014
4. CDC. Viral hepatitis surveillance: United States, 2011. Hepatitis B. Accessed February 8, 2022. www.cdc.gov/hepatitis/statistics/2011surveillance/pdfs/2011HepSurveillanceRpt.pdf
5. CDC. Viral hepatitis surveillance report, 2019. Hepatitis B. Accessed February 8, 2022. www.cdc.gov/hepatitis/statistics/2019surveillance/HepB.htm
6. Schillie S, Harris A, Link-Gelles R, et al. Recommendations of the Advisory Committee on Immunization Practices for use of a hepatitis B vaccine with a novel adjuvant. MMWR Morb Mortal Wkly Rep. 2018;67:455-458.
7. CDC. Advisory Committee on Immunization Practices. Meeting recommendations, November 2021. Accessed February 8, 2022. www.cdc.gov/vaccines/acip/index.html
8. Matanock A, Lee G, Gierke R, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:1069-1075.
9. Kobayashi M. Considerations for use of PCV15 and PCV20 in U.S. adults. Accessed February 8, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-02/24-25/05-Pneumococcal-Kobayashi.pdf
10. Anderson TC, Masters NB, Guo A, et al. Use of recombinant zoster vaccine in immunocompromised adults aged ≥19 years: recommendations of the Advisory Committee on Immunization Practices — United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:80-84.
11. CDC. ACIP recommendations. June 2021. Accessed February 8, 2022. www.cdc.gov/vaccines/acip/recommendations.html
12. Paz-Bailey G. Dengue vaccine. Evidence to recommendation framework. Presented to the ACIP June 24, 2021. Accessed February 8, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-06/03-Dengue-Paz-Bailey-508.pdf
Managing overuse of food IgE panels: Multiple approaches needed
PHOENIX – For at least a decade, professional allergy and pediatrics societies have urged against using food IgE tests unless the patient has a history consistent with potential IgE-mediated food allergies. Yet virtually every health system offers these blood tests, and their inappropriate use – especially of panels that measure many allergens at once – remains a huge problem.
Beyond wasteful spending, excessive food IgE testing can lead patients to worry needlessly and to avoid foods they aren’t allergic to. For babies and toddlers, avoidance can drive up the risk of developing allergies to those foods later in life – a consequence that was convincingly proven by the LEAP study but has still not translated to a widespread change in practice.
“I think we all know that there’s just a lot of system-wide resistance to making these changes, and we don’t completely understand why,” Nicholas Hartog, MD, an allergist with Spectrum Health in Grand Rapids, Mich., told this news organization.
At the American Academy of Allergy, Asthma & Immunology annual meeting, one of Dr. Hartog’s residents, Courtney Cotter, DO, presented a poster detailing their team’s retrospective review of food panel ordering practices across Spectrum Health, a large, multispecialty physician group in west Michigan.
The team combed Epic health records to evaluate food IgE ordering from January 2016 to December 2021. They tracked monthly figures for the number of patients who underwent food IgE tests, the percentage of tested patients for whom food panels were available, and the number of food panels and total number of food IgE tests ordered. They compared average rates from the final 3 months with rates from the first 3 months, which predated the August 2016 establishment of an academic pediatric allergy/immunology department.
Initially, Dr. Hartog and his colleagues focused on educating doctors on appropriate use of food IgE tests through informal conversations and lectures, but, he said, “It’s really difficult to change physician behavior, so sometimes we have to go about it by making it hard to do the wrong thing.”
To that end, the team tried to eliminate the food panels. However, some lab staff feared the possibility of losing revenue if physicians decided to order these tests elsewhere. After more negotiations, the laboratory agreed in December 2019 to restrict and rework food IgE testing by dropping the number of panels from nine to two and by restricting the number of foods in those panels. For example, in the basic panel, “we limited it to just four allergens, so even if you order a panel, you’re not getting 20 results,” Dr. Hartog told this news organization. “I finally found a friendly pathologist who was very on board with this positive change.”
In December 2020, the team implemented yet another strategy: Epic alerts. Each time doctors request a food panel, they receive a pop-up message stating that panel tests are not recommended and asking if they wish to proceed.
The multipronged effort had a modest impact on the number of food panels ordered per month, which dipped from 112.7 to 84.7 for the first and last 3 months of the study. Monthly totals of individual food IgE tests showed a steeper drop, decreasing from 2,379 to 1,180 in the initial and final 3-month periods – a change Dr. Hartog attributes to the revamped food panels. They estimated the cost savings at around $40 per patient, “and we were getting on average about 200 patients a month, so it adds up,” he said.
But the Epic alerts seemed to have little effect. Over the duration of the study, the monthly number of IgE tests ordered per clinician did not change. Neither did the percentage of patients evaluated with a food panel. “The alerts pop up, but people are still ordering,” Dr. Hartog said.
On the whole, the analysis shows that, “despite major efforts to educate providers and the public about these things, it is rampantly disregarded and is a huge problem for our specialty and is likely causing harm to patients,” said allergist-immunologist Gerald Lee, MD, of Emory University in Atlanta.
Dr. Lee said that a common scenario for inappropriate food IgE testing is severe eczema. Many parents request blood tests because they assume their child’s skin condition is driven by food allergies. When the child turns up positive to various foods on panel tests, which have high false-positive rates, the physician may recommend eliminating those foods to improve the skin rash – which “actually delays introduction of the food and potentially increases the risk for food allergy,” Dr. Lee said. “That was a common practice when I was in fellowship (2011) and is widely prevalent today.”
Edwin Kim, MD, director of the UNC Food Allergy Initiative at the University of North Carolina at Chapel Hill, agrees that food IgE panels are wasteful and harmful. However, he thinks the solution is not to tell primary care physicians and pediatricians to stop using the tests. “We’re insinuating that they’re being used inappropriately, but the problem is that these are people that are patient facing, the patients are asking a question, and the appropriate tests aren’t there,” Dr. Kim said. “A big part of that problem is that the tests we have available to us are not good enough.”
The Spectrum Health analysis did not examine ICD codes associated with the food IgE tests or track which physicians ordered the tests. A 2016 retrospective review published in Pediatrics did evaluate ordering practices by specialty and found that primary care providers ordered “significantly more food allergen panels, tests for uncommon causes of food allergy, and generate higher cost per patient compared with allergists.”
Given the immense challenges with implementing system-wide changes, sometimes it can help to educate parents and families. “When you sit down and take 2 or 3 minutes to explain why this is a bad test and that I care about your kid but just don’t want inappropriate testing, they’re okay with it. They understand,” Dr. Hartog said. “When I teach residents, I make sure to emphasize that we have these conversations all the time.”
Dr. Hartog reports financial relationships with Binding Site (speaker), Regeneron (advisory board), Genentech (advisory board), Horizon Pharmaceuticals (advisory board, consulting, speaker), Takeda (speaker, advisory board) and Pharming Healthcare (advisory board, scientific steering committee, consulting), though none related to food allergy. Dr. Lee has disclosed no relevant financial relationships. Dr. Kim reports consultancy with Aimmune Therapeutics, Allako, AllerGenis, Belhaven Pharma, DBV Technologies, Duke Clinical Research Institute, and Nutricia; advisory board membership with ALK, DBV Technologies, Kenota Health, and Ukko; and grant support from the National Institute of Allergy and Infectious Diseases and the Immune Tolerance Network; the National Center for Complementary and Integrative Health; Food Allergy Research and Education; and the Wallace Research Foundation.
A version of this article first appeared on Medscape.com.
PHOENIX – For at least a decade, professional allergy and pediatrics societies have urged against using food IgE tests unless the patient has a history consistent with potential IgE-mediated food allergies. Yet virtually every health system offers these blood tests, and their inappropriate use – especially of panels that measure many allergens at once – remains a huge problem.
Beyond wasteful spending, excessive food IgE testing can lead patients to worry needlessly and to avoid foods they aren’t allergic to. For babies and toddlers, avoidance can drive up the risk of developing allergies to those foods later in life – a consequence that was convincingly proven by the LEAP study but has still not translated to a widespread change in practice.
“I think we all know that there’s just a lot of system-wide resistance to making these changes, and we don’t completely understand why,” Nicholas Hartog, MD, an allergist with Spectrum Health in Grand Rapids, Mich., told this news organization.
At the American Academy of Allergy, Asthma & Immunology annual meeting, one of Dr. Hartog’s residents, Courtney Cotter, DO, presented a poster detailing their team’s retrospective review of food panel ordering practices across Spectrum Health, a large, multispecialty physician group in west Michigan.
The team combed Epic health records to evaluate food IgE ordering from January 2016 to December 2021. They tracked monthly figures for the number of patients who underwent food IgE tests, the percentage of tested patients for whom food panels were available, and the number of food panels and total number of food IgE tests ordered. They compared average rates from the final 3 months with rates from the first 3 months, which predated the August 2016 establishment of an academic pediatric allergy/immunology department.
Initially, Dr. Hartog and his colleagues focused on educating doctors on appropriate use of food IgE tests through informal conversations and lectures, but, he said, “It’s really difficult to change physician behavior, so sometimes we have to go about it by making it hard to do the wrong thing.”
To that end, the team tried to eliminate the food panels. However, some lab staff feared the possibility of losing revenue if physicians decided to order these tests elsewhere. After more negotiations, the laboratory agreed in December 2019 to restrict and rework food IgE testing by dropping the number of panels from nine to two and by restricting the number of foods in those panels. For example, in the basic panel, “we limited it to just four allergens, so even if you order a panel, you’re not getting 20 results,” Dr. Hartog told this news organization. “I finally found a friendly pathologist who was very on board with this positive change.”
In December 2020, the team implemented yet another strategy: Epic alerts. Each time doctors request a food panel, they receive a pop-up message stating that panel tests are not recommended and asking if they wish to proceed.
The multipronged effort had a modest impact on the number of food panels ordered per month, which dipped from 112.7 to 84.7 for the first and last 3 months of the study. Monthly totals of individual food IgE tests showed a steeper drop, decreasing from 2,379 to 1,180 in the initial and final 3-month periods – a change Dr. Hartog attributes to the revamped food panels. They estimated the cost savings at around $40 per patient, “and we were getting on average about 200 patients a month, so it adds up,” he said.
But the Epic alerts seemed to have little effect. Over the duration of the study, the monthly number of IgE tests ordered per clinician did not change. Neither did the percentage of patients evaluated with a food panel. “The alerts pop up, but people are still ordering,” Dr. Hartog said.
On the whole, the analysis shows that, “despite major efforts to educate providers and the public about these things, it is rampantly disregarded and is a huge problem for our specialty and is likely causing harm to patients,” said allergist-immunologist Gerald Lee, MD, of Emory University in Atlanta.
Dr. Lee said that a common scenario for inappropriate food IgE testing is severe eczema. Many parents request blood tests because they assume their child’s skin condition is driven by food allergies. When the child turns up positive to various foods on panel tests, which have high false-positive rates, the physician may recommend eliminating those foods to improve the skin rash – which “actually delays introduction of the food and potentially increases the risk for food allergy,” Dr. Lee said. “That was a common practice when I was in fellowship (2011) and is widely prevalent today.”
Edwin Kim, MD, director of the UNC Food Allergy Initiative at the University of North Carolina at Chapel Hill, agrees that food IgE panels are wasteful and harmful. However, he thinks the solution is not to tell primary care physicians and pediatricians to stop using the tests. “We’re insinuating that they’re being used inappropriately, but the problem is that these are people that are patient facing, the patients are asking a question, and the appropriate tests aren’t there,” Dr. Kim said. “A big part of that problem is that the tests we have available to us are not good enough.”
The Spectrum Health analysis did not examine ICD codes associated with the food IgE tests or track which physicians ordered the tests. A 2016 retrospective review published in Pediatrics did evaluate ordering practices by specialty and found that primary care providers ordered “significantly more food allergen panels, tests for uncommon causes of food allergy, and generate higher cost per patient compared with allergists.”
Given the immense challenges with implementing system-wide changes, sometimes it can help to educate parents and families. “When you sit down and take 2 or 3 minutes to explain why this is a bad test and that I care about your kid but just don’t want inappropriate testing, they’re okay with it. They understand,” Dr. Hartog said. “When I teach residents, I make sure to emphasize that we have these conversations all the time.”
Dr. Hartog reports financial relationships with Binding Site (speaker), Regeneron (advisory board), Genentech (advisory board), Horizon Pharmaceuticals (advisory board, consulting, speaker), Takeda (speaker, advisory board) and Pharming Healthcare (advisory board, scientific steering committee, consulting), though none related to food allergy. Dr. Lee has disclosed no relevant financial relationships. Dr. Kim reports consultancy with Aimmune Therapeutics, Allako, AllerGenis, Belhaven Pharma, DBV Technologies, Duke Clinical Research Institute, and Nutricia; advisory board membership with ALK, DBV Technologies, Kenota Health, and Ukko; and grant support from the National Institute of Allergy and Infectious Diseases and the Immune Tolerance Network; the National Center for Complementary and Integrative Health; Food Allergy Research and Education; and the Wallace Research Foundation.
A version of this article first appeared on Medscape.com.
PHOENIX – For at least a decade, professional allergy and pediatrics societies have urged against using food IgE tests unless the patient has a history consistent with potential IgE-mediated food allergies. Yet virtually every health system offers these blood tests, and their inappropriate use – especially of panels that measure many allergens at once – remains a huge problem.
Beyond wasteful spending, excessive food IgE testing can lead patients to worry needlessly and to avoid foods they aren’t allergic to. For babies and toddlers, avoidance can drive up the risk of developing allergies to those foods later in life – a consequence that was convincingly proven by the LEAP study but has still not translated to a widespread change in practice.
“I think we all know that there’s just a lot of system-wide resistance to making these changes, and we don’t completely understand why,” Nicholas Hartog, MD, an allergist with Spectrum Health in Grand Rapids, Mich., told this news organization.
At the American Academy of Allergy, Asthma & Immunology annual meeting, one of Dr. Hartog’s residents, Courtney Cotter, DO, presented a poster detailing their team’s retrospective review of food panel ordering practices across Spectrum Health, a large, multispecialty physician group in west Michigan.
The team combed Epic health records to evaluate food IgE ordering from January 2016 to December 2021. They tracked monthly figures for the number of patients who underwent food IgE tests, the percentage of tested patients for whom food panels were available, and the number of food panels and total number of food IgE tests ordered. They compared average rates from the final 3 months with rates from the first 3 months, which predated the August 2016 establishment of an academic pediatric allergy/immunology department.
Initially, Dr. Hartog and his colleagues focused on educating doctors on appropriate use of food IgE tests through informal conversations and lectures, but, he said, “It’s really difficult to change physician behavior, so sometimes we have to go about it by making it hard to do the wrong thing.”
To that end, the team tried to eliminate the food panels. However, some lab staff feared the possibility of losing revenue if physicians decided to order these tests elsewhere. After more negotiations, the laboratory agreed in December 2019 to restrict and rework food IgE testing by dropping the number of panels from nine to two and by restricting the number of foods in those panels. For example, in the basic panel, “we limited it to just four allergens, so even if you order a panel, you’re not getting 20 results,” Dr. Hartog told this news organization. “I finally found a friendly pathologist who was very on board with this positive change.”
In December 2020, the team implemented yet another strategy: Epic alerts. Each time doctors request a food panel, they receive a pop-up message stating that panel tests are not recommended and asking if they wish to proceed.
The multipronged effort had a modest impact on the number of food panels ordered per month, which dipped from 112.7 to 84.7 for the first and last 3 months of the study. Monthly totals of individual food IgE tests showed a steeper drop, decreasing from 2,379 to 1,180 in the initial and final 3-month periods – a change Dr. Hartog attributes to the revamped food panels. They estimated the cost savings at around $40 per patient, “and we were getting on average about 200 patients a month, so it adds up,” he said.
But the Epic alerts seemed to have little effect. Over the duration of the study, the monthly number of IgE tests ordered per clinician did not change. Neither did the percentage of patients evaluated with a food panel. “The alerts pop up, but people are still ordering,” Dr. Hartog said.
On the whole, the analysis shows that, “despite major efforts to educate providers and the public about these things, it is rampantly disregarded and is a huge problem for our specialty and is likely causing harm to patients,” said allergist-immunologist Gerald Lee, MD, of Emory University in Atlanta.
Dr. Lee said that a common scenario for inappropriate food IgE testing is severe eczema. Many parents request blood tests because they assume their child’s skin condition is driven by food allergies. When the child turns up positive to various foods on panel tests, which have high false-positive rates, the physician may recommend eliminating those foods to improve the skin rash – which “actually delays introduction of the food and potentially increases the risk for food allergy,” Dr. Lee said. “That was a common practice when I was in fellowship (2011) and is widely prevalent today.”
Edwin Kim, MD, director of the UNC Food Allergy Initiative at the University of North Carolina at Chapel Hill, agrees that food IgE panels are wasteful and harmful. However, he thinks the solution is not to tell primary care physicians and pediatricians to stop using the tests. “We’re insinuating that they’re being used inappropriately, but the problem is that these are people that are patient facing, the patients are asking a question, and the appropriate tests aren’t there,” Dr. Kim said. “A big part of that problem is that the tests we have available to us are not good enough.”
The Spectrum Health analysis did not examine ICD codes associated with the food IgE tests or track which physicians ordered the tests. A 2016 retrospective review published in Pediatrics did evaluate ordering practices by specialty and found that primary care providers ordered “significantly more food allergen panels, tests for uncommon causes of food allergy, and generate higher cost per patient compared with allergists.”
Given the immense challenges with implementing system-wide changes, sometimes it can help to educate parents and families. “When you sit down and take 2 or 3 minutes to explain why this is a bad test and that I care about your kid but just don’t want inappropriate testing, they’re okay with it. They understand,” Dr. Hartog said. “When I teach residents, I make sure to emphasize that we have these conversations all the time.”
Dr. Hartog reports financial relationships with Binding Site (speaker), Regeneron (advisory board), Genentech (advisory board), Horizon Pharmaceuticals (advisory board, consulting, speaker), Takeda (speaker, advisory board) and Pharming Healthcare (advisory board, scientific steering committee, consulting), though none related to food allergy. Dr. Lee has disclosed no relevant financial relationships. Dr. Kim reports consultancy with Aimmune Therapeutics, Allako, AllerGenis, Belhaven Pharma, DBV Technologies, Duke Clinical Research Institute, and Nutricia; advisory board membership with ALK, DBV Technologies, Kenota Health, and Ukko; and grant support from the National Institute of Allergy and Infectious Diseases and the Immune Tolerance Network; the National Center for Complementary and Integrative Health; Food Allergy Research and Education; and the Wallace Research Foundation.
A version of this article first appeared on Medscape.com.
Dupilumab shows histological and clinical benefit in larger eosinophilic esophagitis cohort
The late-breaking data on Part B of the LIBERTY EoE TREET study drew a standing-room-only crowd at the American Academy of Allergy, Asthma and Immunology (AAAAI) annual meeting.
EoE is a chronic, progressive, type 2 inflammatory disease resulting from esophageal build-up of eosinophils, which injures the tissue and leads to swallowing difficulties. Dupilumab, a monoclonal antibody that blocks type 2 immune responses, is currently approved to treat poorly controlled atopic dermatitis, asthma, and chronic rhinosinusitis with nasal polyposis.
Dupilumab also showed benefits in patients with hard-to-treat EoE in a phase 3 trial (LIBERTY EoE TREET 28-week extension of Part A), reported by Medscape Medical News in October from the American College of Gastroenterology (ACG) annual meeting.
Part B enrolled 159 EoE patients 12 years or older and tested the efficacy and safety of weekly 300 mg dupilumab versus placebo injections for 24 weeks. More than half of the participants had previously tried swallowed topical corticosteroids, and about 30% were on a food elimination diet. (Generally, corticosteroids and elimination diets are about 70% effective in EoE.)
Compared with placebo, 6 months of weekly dupilumab reduced eosinophils in the esophagus and produced statistically significant and clinically meaningful improvements in the ability to swallow.
Treated participants saw a 64% reduction in disease symptoms (23.8-point improvement on the self-reported Dysphagia Symptom Questionnaire [DSQ]), compared with 41% reduction (13.9 point DSQ improvement) in the placebo group.
Histologically, dupilumab reduced peak eosinophil counts to 6 or lower in 59% of patients, whereas only 6% achieved disease remission on placebo.
On safety, dupilumab was generally well tolerated. The most common treatment adverse events were injection site reactions (occurring in about 20% of both groups) or injection site erythema (occurring in 10% of treated patients and 11.5% of placebo patients).
“These results replicate those in Part A in a larger sample size,” Marc Rothenberg, MD, PhD, director of the division of allergy and immunology at Cincinnati Children’s Hospital Medical Center, noted in a prerecorded presentation.
Based on the phase 3 data, dupilumab seems “effective for patients who may have no other options for managing their EoE,” Brian Schroer, MD, director of allergy and immunology at Akron (Ohio) Children’s Hospital, said in an interview. Dr. Schroer expects EoE cases to rise as more food allergy patients begin oral immunotherapy (OIT), where studies have shown EoE as a side effect in about 4% of patients undergoing OIT.
In a live Q&A following the prerecorded talk, Evan Dellon, MD, professor of medicine and epidemiology at the University of North Carolina at Chapel Hill, told attendees that data from Part B’s second arm, which tested dupilumab injections given every other week, have not yet been presented. So far, histological results in this arm look identical to those of patients who received weekly dupilumab, though symptoms “did not meet statistical significance,” he said. “I think we’re going to have much more detail about those results at some conferences to come in the spring.”
LIBERTY EoE TREET was funded by Sanofi and Regeneron. Dr. Dellon and Dr. Rothenberg reported numerous conflicts of interest. Dr. Schroer has received consulting fees from Sanofi and Ready, Set, Food.
A version of this article first appeared on Medscape.com.
The late-breaking data on Part B of the LIBERTY EoE TREET study drew a standing-room-only crowd at the American Academy of Allergy, Asthma and Immunology (AAAAI) annual meeting.
EoE is a chronic, progressive, type 2 inflammatory disease resulting from esophageal build-up of eosinophils, which injures the tissue and leads to swallowing difficulties. Dupilumab, a monoclonal antibody that blocks type 2 immune responses, is currently approved to treat poorly controlled atopic dermatitis, asthma, and chronic rhinosinusitis with nasal polyposis.
Dupilumab also showed benefits in patients with hard-to-treat EoE in a phase 3 trial (LIBERTY EoE TREET 28-week extension of Part A), reported by Medscape Medical News in October from the American College of Gastroenterology (ACG) annual meeting.
Part B enrolled 159 EoE patients 12 years or older and tested the efficacy and safety of weekly 300 mg dupilumab versus placebo injections for 24 weeks. More than half of the participants had previously tried swallowed topical corticosteroids, and about 30% were on a food elimination diet. (Generally, corticosteroids and elimination diets are about 70% effective in EoE.)
Compared with placebo, 6 months of weekly dupilumab reduced eosinophils in the esophagus and produced statistically significant and clinically meaningful improvements in the ability to swallow.
Treated participants saw a 64% reduction in disease symptoms (23.8-point improvement on the self-reported Dysphagia Symptom Questionnaire [DSQ]), compared with 41% reduction (13.9 point DSQ improvement) in the placebo group.
Histologically, dupilumab reduced peak eosinophil counts to 6 or lower in 59% of patients, whereas only 6% achieved disease remission on placebo.
On safety, dupilumab was generally well tolerated. The most common treatment adverse events were injection site reactions (occurring in about 20% of both groups) or injection site erythema (occurring in 10% of treated patients and 11.5% of placebo patients).
“These results replicate those in Part A in a larger sample size,” Marc Rothenberg, MD, PhD, director of the division of allergy and immunology at Cincinnati Children’s Hospital Medical Center, noted in a prerecorded presentation.
Based on the phase 3 data, dupilumab seems “effective for patients who may have no other options for managing their EoE,” Brian Schroer, MD, director of allergy and immunology at Akron (Ohio) Children’s Hospital, said in an interview. Dr. Schroer expects EoE cases to rise as more food allergy patients begin oral immunotherapy (OIT), where studies have shown EoE as a side effect in about 4% of patients undergoing OIT.
In a live Q&A following the prerecorded talk, Evan Dellon, MD, professor of medicine and epidemiology at the University of North Carolina at Chapel Hill, told attendees that data from Part B’s second arm, which tested dupilumab injections given every other week, have not yet been presented. So far, histological results in this arm look identical to those of patients who received weekly dupilumab, though symptoms “did not meet statistical significance,” he said. “I think we’re going to have much more detail about those results at some conferences to come in the spring.”
LIBERTY EoE TREET was funded by Sanofi and Regeneron. Dr. Dellon and Dr. Rothenberg reported numerous conflicts of interest. Dr. Schroer has received consulting fees from Sanofi and Ready, Set, Food.
A version of this article first appeared on Medscape.com.
The late-breaking data on Part B of the LIBERTY EoE TREET study drew a standing-room-only crowd at the American Academy of Allergy, Asthma and Immunology (AAAAI) annual meeting.
EoE is a chronic, progressive, type 2 inflammatory disease resulting from esophageal build-up of eosinophils, which injures the tissue and leads to swallowing difficulties. Dupilumab, a monoclonal antibody that blocks type 2 immune responses, is currently approved to treat poorly controlled atopic dermatitis, asthma, and chronic rhinosinusitis with nasal polyposis.
Dupilumab also showed benefits in patients with hard-to-treat EoE in a phase 3 trial (LIBERTY EoE TREET 28-week extension of Part A), reported by Medscape Medical News in October from the American College of Gastroenterology (ACG) annual meeting.
Part B enrolled 159 EoE patients 12 years or older and tested the efficacy and safety of weekly 300 mg dupilumab versus placebo injections for 24 weeks. More than half of the participants had previously tried swallowed topical corticosteroids, and about 30% were on a food elimination diet. (Generally, corticosteroids and elimination diets are about 70% effective in EoE.)
Compared with placebo, 6 months of weekly dupilumab reduced eosinophils in the esophagus and produced statistically significant and clinically meaningful improvements in the ability to swallow.
Treated participants saw a 64% reduction in disease symptoms (23.8-point improvement on the self-reported Dysphagia Symptom Questionnaire [DSQ]), compared with 41% reduction (13.9 point DSQ improvement) in the placebo group.
Histologically, dupilumab reduced peak eosinophil counts to 6 or lower in 59% of patients, whereas only 6% achieved disease remission on placebo.
On safety, dupilumab was generally well tolerated. The most common treatment adverse events were injection site reactions (occurring in about 20% of both groups) or injection site erythema (occurring in 10% of treated patients and 11.5% of placebo patients).
“These results replicate those in Part A in a larger sample size,” Marc Rothenberg, MD, PhD, director of the division of allergy and immunology at Cincinnati Children’s Hospital Medical Center, noted in a prerecorded presentation.
Based on the phase 3 data, dupilumab seems “effective for patients who may have no other options for managing their EoE,” Brian Schroer, MD, director of allergy and immunology at Akron (Ohio) Children’s Hospital, said in an interview. Dr. Schroer expects EoE cases to rise as more food allergy patients begin oral immunotherapy (OIT), where studies have shown EoE as a side effect in about 4% of patients undergoing OIT.
In a live Q&A following the prerecorded talk, Evan Dellon, MD, professor of medicine and epidemiology at the University of North Carolina at Chapel Hill, told attendees that data from Part B’s second arm, which tested dupilumab injections given every other week, have not yet been presented. So far, histological results in this arm look identical to those of patients who received weekly dupilumab, though symptoms “did not meet statistical significance,” he said. “I think we’re going to have much more detail about those results at some conferences to come in the spring.”
LIBERTY EoE TREET was funded by Sanofi and Regeneron. Dr. Dellon and Dr. Rothenberg reported numerous conflicts of interest. Dr. Schroer has received consulting fees from Sanofi and Ready, Set, Food.
A version of this article first appeared on Medscape.com.
REPORTING FROM AAAAI