User login
Worried parents scramble to vaccinate kids despite FDA guidance
One week after reporting promising results from the trial of their COVID-19 vaccine in children ages 5-11, Pfizer and BioNTech announced they’d submitted the data to the Food and Drug Administration. But that hasn’t stopped some parents from discreetly getting their children under age 12 vaccinated.
“The FDA, you never want to get ahead of their judgment,” Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, told MSNBC on Sept. 28. “But I would imagine in the next few weeks, they will examine that data and hopefully they’ll give the okay so that we can start vaccinating children, hopefully before the end of October.”
Lying to vaccinate now
More than half of all parents with children under 12 say they plan to get their kids vaccinated, according to a Gallup poll.
And although the FDA and the American Academy of Pediatrics have warned against it, some parents whose children can pass for 12 have lied to get them vaccinated already.
Dawn G. is a mom of two in southwest Missouri, where less than 45% of the population has been fully vaccinated. Her son turns 12 in early October, but in-person school started in mid-August.
“It was scary, thinking of him going to school for even 2 months,” she said. “Some parents thought their kid had a low chance of getting COVID, and their kid died. Nobody expects it to be them.”
In July, she and her husband took their son to a walk-in clinic and lied about his age.
“So many things can happen, from bullying to school shootings, and now this added pandemic risk,” she said. “I’ll do anything I can to protect my child, and a birthdate seems so arbitrary. He’ll be 12 in a matter of weeks. It seems ridiculous that that date would stop me from protecting him.”
In northern California, Carrie S. had a similar thought. When the vaccine was authorized for children ages 12-15 in May, the older of her two children got the shot right away. But her youngest doesn’t turn 12 until November.
“We were tempted to get the younger one vaccinated in May, but it didn’t seem like a rush. We were willing to wait to get the dosage right,” she ssaid. “But as Delta came through, there were no options for online school, the CDC was dropping mask expectations –it seemed like the world was ready to forget the pandemic was happening. It seemed like the least-bad option to get her vaccinated so she could go back to school, and we could find some balance of risk in our lives.”
Adult vs. pediatric doses
For now, experts advise against getting younger children vaccinated, even those who are the size of an adult, because of the way the human immune system develops.
“It’s not really about size,” said Anne Liu, MD, an immunologist and pediatrics professor at Stanford (Calif.) University. “The immune system behaves differently at different ages. Younger kids tend to have a more exuberant innate immune system, which is the part of the immune system that senses danger, even before it has developed a memory response.”
The adult Pfizer-BioNTech vaccine contains 30 mcg of mRNA, while the pediatric dose is just 10 mcg. That smaller dose produces an immune response similar to what’s seen in adults who receive 30 mcg, according to Pfizer.
“We were one of the sites that was involved in the phase 1 trial, a lot of times that’s called a dose-finding trial,” said Michael Smith, MD, a coinvestigator for the COVID vaccine trials done at Duke University. “And basically, if younger kids got a higher dose, they had more of a reaction, so it hurt more. They had fever, they had more redness and swelling at the site of the injection, and they just felt lousy, more than at the lower doses.”
At this point, with Pfizer’s data showing that younger children need a smaller dose, it doesn’t make sense to lie about your child’s age, said Dr. Smith.
“If my two options were having my child get the infection versus getting the vaccine, I’d get the vaccine. But we’re a few weeks away from getting the lower dose approved in kids,” he said. “It’s certainly safer. I don’t expect major, lifelong side effects from the higher dose, but it’s going to hurt, your kid’s going to have a fever, they’re going to feel lousy for a couple days, and they just don’t need that much antigen.”
A version of this article first appeared on WebMD.com.
One week after reporting promising results from the trial of their COVID-19 vaccine in children ages 5-11, Pfizer and BioNTech announced they’d submitted the data to the Food and Drug Administration. But that hasn’t stopped some parents from discreetly getting their children under age 12 vaccinated.
“The FDA, you never want to get ahead of their judgment,” Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, told MSNBC on Sept. 28. “But I would imagine in the next few weeks, they will examine that data and hopefully they’ll give the okay so that we can start vaccinating children, hopefully before the end of October.”
Lying to vaccinate now
More than half of all parents with children under 12 say they plan to get their kids vaccinated, according to a Gallup poll.
And although the FDA and the American Academy of Pediatrics have warned against it, some parents whose children can pass for 12 have lied to get them vaccinated already.
Dawn G. is a mom of two in southwest Missouri, where less than 45% of the population has been fully vaccinated. Her son turns 12 in early October, but in-person school started in mid-August.
“It was scary, thinking of him going to school for even 2 months,” she said. “Some parents thought their kid had a low chance of getting COVID, and their kid died. Nobody expects it to be them.”
In July, she and her husband took their son to a walk-in clinic and lied about his age.
“So many things can happen, from bullying to school shootings, and now this added pandemic risk,” she said. “I’ll do anything I can to protect my child, and a birthdate seems so arbitrary. He’ll be 12 in a matter of weeks. It seems ridiculous that that date would stop me from protecting him.”
In northern California, Carrie S. had a similar thought. When the vaccine was authorized for children ages 12-15 in May, the older of her two children got the shot right away. But her youngest doesn’t turn 12 until November.
“We were tempted to get the younger one vaccinated in May, but it didn’t seem like a rush. We were willing to wait to get the dosage right,” she ssaid. “But as Delta came through, there were no options for online school, the CDC was dropping mask expectations –it seemed like the world was ready to forget the pandemic was happening. It seemed like the least-bad option to get her vaccinated so she could go back to school, and we could find some balance of risk in our lives.”
Adult vs. pediatric doses
For now, experts advise against getting younger children vaccinated, even those who are the size of an adult, because of the way the human immune system develops.
“It’s not really about size,” said Anne Liu, MD, an immunologist and pediatrics professor at Stanford (Calif.) University. “The immune system behaves differently at different ages. Younger kids tend to have a more exuberant innate immune system, which is the part of the immune system that senses danger, even before it has developed a memory response.”
The adult Pfizer-BioNTech vaccine contains 30 mcg of mRNA, while the pediatric dose is just 10 mcg. That smaller dose produces an immune response similar to what’s seen in adults who receive 30 mcg, according to Pfizer.
“We were one of the sites that was involved in the phase 1 trial, a lot of times that’s called a dose-finding trial,” said Michael Smith, MD, a coinvestigator for the COVID vaccine trials done at Duke University. “And basically, if younger kids got a higher dose, they had more of a reaction, so it hurt more. They had fever, they had more redness and swelling at the site of the injection, and they just felt lousy, more than at the lower doses.”
At this point, with Pfizer’s data showing that younger children need a smaller dose, it doesn’t make sense to lie about your child’s age, said Dr. Smith.
“If my two options were having my child get the infection versus getting the vaccine, I’d get the vaccine. But we’re a few weeks away from getting the lower dose approved in kids,” he said. “It’s certainly safer. I don’t expect major, lifelong side effects from the higher dose, but it’s going to hurt, your kid’s going to have a fever, they’re going to feel lousy for a couple days, and they just don’t need that much antigen.”
A version of this article first appeared on WebMD.com.
One week after reporting promising results from the trial of their COVID-19 vaccine in children ages 5-11, Pfizer and BioNTech announced they’d submitted the data to the Food and Drug Administration. But that hasn’t stopped some parents from discreetly getting their children under age 12 vaccinated.
“The FDA, you never want to get ahead of their judgment,” Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, told MSNBC on Sept. 28. “But I would imagine in the next few weeks, they will examine that data and hopefully they’ll give the okay so that we can start vaccinating children, hopefully before the end of October.”
Lying to vaccinate now
More than half of all parents with children under 12 say they plan to get their kids vaccinated, according to a Gallup poll.
And although the FDA and the American Academy of Pediatrics have warned against it, some parents whose children can pass for 12 have lied to get them vaccinated already.
Dawn G. is a mom of two in southwest Missouri, where less than 45% of the population has been fully vaccinated. Her son turns 12 in early October, but in-person school started in mid-August.
“It was scary, thinking of him going to school for even 2 months,” she said. “Some parents thought their kid had a low chance of getting COVID, and their kid died. Nobody expects it to be them.”
In July, she and her husband took their son to a walk-in clinic and lied about his age.
“So many things can happen, from bullying to school shootings, and now this added pandemic risk,” she said. “I’ll do anything I can to protect my child, and a birthdate seems so arbitrary. He’ll be 12 in a matter of weeks. It seems ridiculous that that date would stop me from protecting him.”
In northern California, Carrie S. had a similar thought. When the vaccine was authorized for children ages 12-15 in May, the older of her two children got the shot right away. But her youngest doesn’t turn 12 until November.
“We were tempted to get the younger one vaccinated in May, but it didn’t seem like a rush. We were willing to wait to get the dosage right,” she ssaid. “But as Delta came through, there were no options for online school, the CDC was dropping mask expectations –it seemed like the world was ready to forget the pandemic was happening. It seemed like the least-bad option to get her vaccinated so she could go back to school, and we could find some balance of risk in our lives.”
Adult vs. pediatric doses
For now, experts advise against getting younger children vaccinated, even those who are the size of an adult, because of the way the human immune system develops.
“It’s not really about size,” said Anne Liu, MD, an immunologist and pediatrics professor at Stanford (Calif.) University. “The immune system behaves differently at different ages. Younger kids tend to have a more exuberant innate immune system, which is the part of the immune system that senses danger, even before it has developed a memory response.”
The adult Pfizer-BioNTech vaccine contains 30 mcg of mRNA, while the pediatric dose is just 10 mcg. That smaller dose produces an immune response similar to what’s seen in adults who receive 30 mcg, according to Pfizer.
“We were one of the sites that was involved in the phase 1 trial, a lot of times that’s called a dose-finding trial,” said Michael Smith, MD, a coinvestigator for the COVID vaccine trials done at Duke University. “And basically, if younger kids got a higher dose, they had more of a reaction, so it hurt more. They had fever, they had more redness and swelling at the site of the injection, and they just felt lousy, more than at the lower doses.”
At this point, with Pfizer’s data showing that younger children need a smaller dose, it doesn’t make sense to lie about your child’s age, said Dr. Smith.
“If my two options were having my child get the infection versus getting the vaccine, I’d get the vaccine. But we’re a few weeks away from getting the lower dose approved in kids,” he said. “It’s certainly safer. I don’t expect major, lifelong side effects from the higher dose, but it’s going to hurt, your kid’s going to have a fever, they’re going to feel lousy for a couple days, and they just don’t need that much antigen.”
A version of this article first appeared on WebMD.com.
Urticaria and edema in a 2-year-old boy
A 2-YEAR-OLD BOY presented to the emergency room with a 1-day history of a diffuse, mildly pruritic rash and swelling of his knees, ankles, and feet following treatment of acute otitis media with amoxicillin for the previous 8 days. He was mildly febrile and consolable, but he was refusing to walk. His medical history was unremarkable.
Physical examination revealed erythematous annular wheals on his chest, face, back, and extremities. Lymphadenopathy and mucous membrane involvement were not present. A complete blood count (CBC) with differential, inflammatory marker tests, and a comprehensive metabolic panel were ordered. Given the joint swelling and rash, the patient was admitted for observation.
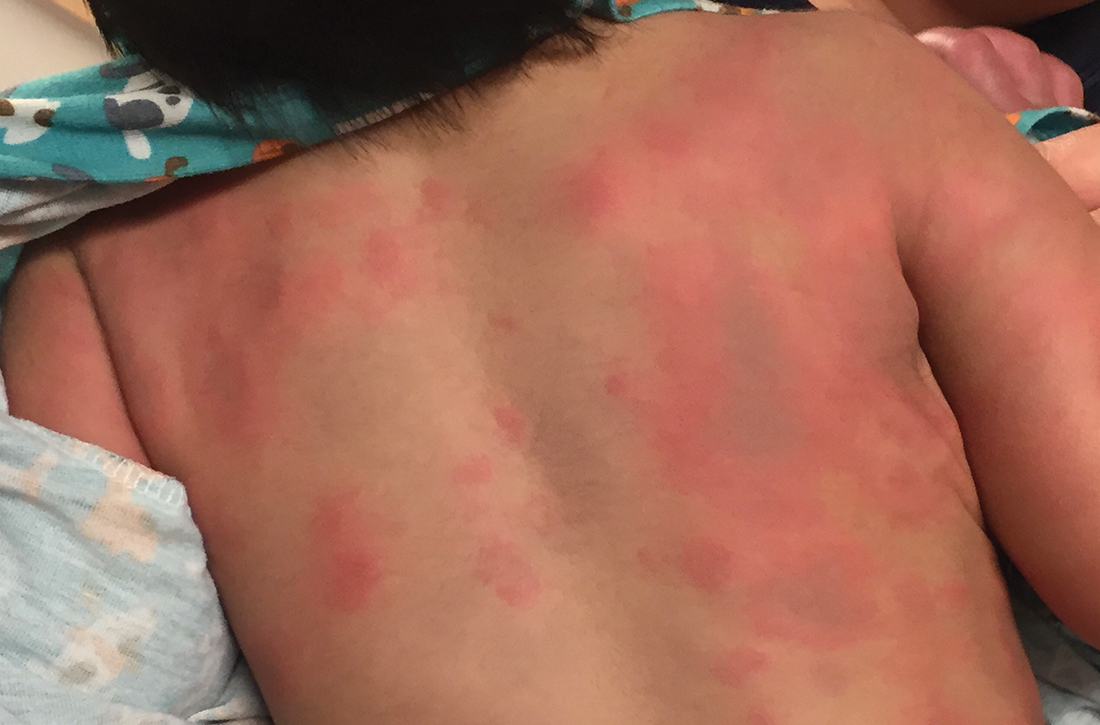
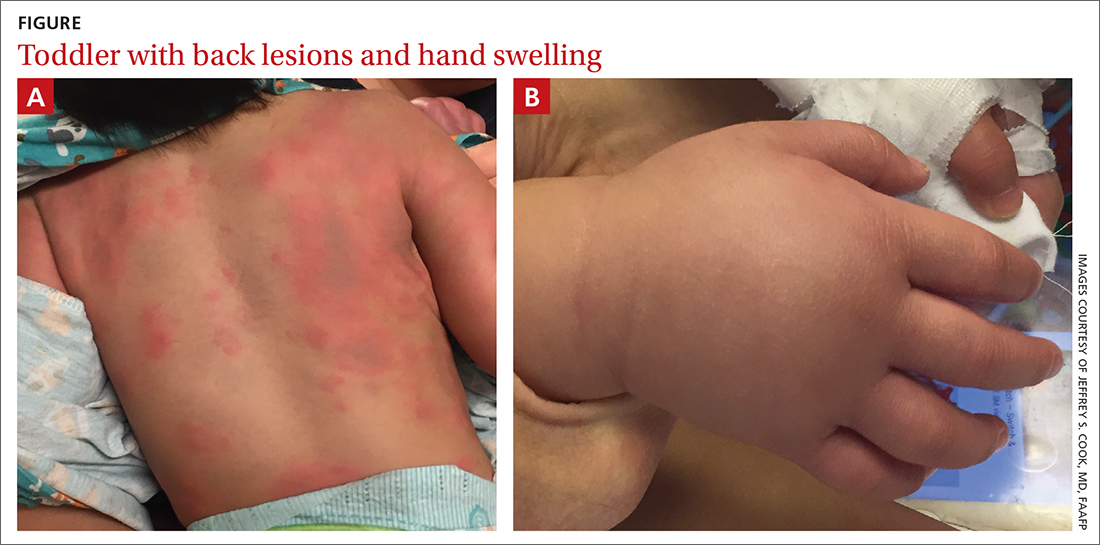
During his second day in the hospital, his skin lesions enlarged and several formed dusky blue centers (FIGURE 1A). He also developed swelling of his hands (FIGURE 1B).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Urticaria multiforme
The patient’s lab work came back within normal range, except for an elevated white blood cell count (19,700/mm3; reference range, 4500-13,500/mm3). His mild systemic symptoms, skin lesions without blistering or necrosis, acral edema, and the absence of lymphadenopathy pointed to a diagnosis of urticaria multiforme.
Urticaria multiforme, also called acute annular urticaria or acute urticarial hypersensitivity syndrome, is a histamine-mediated hypersensitivity reaction characterized by transient annular, polycyclic, urticarial lesions with central ecchymosis. The incidence and prevalence are not known. Urticaria multiforme is considered common, but it is frequently misdiagnosed.1 It typically manifests in children ages 4 months to 4 years and begins with small erythematous macules, papules, and plaques that progress to large blanchable wheals with dusky blue centers.1-3 Lesions are usually located on the face, trunk, and extremities and are often pruritic (60%-94%).1-3 Individual lesions last less than 24 hours, but new ones may appear. The rash generally lasts 2 to 12 days.1,3
Patients often report a preceding viral illness, otitis media, recent use of antibiotics, or recent immunizations. Dermatographism due to mast cell–mediated cutaneous hypersensitivity at sites of minor skin trauma is common (44%).
The diagnosis is made clinically and should not require a skin biopsy or extensive laboratory testing.When performed, laboratory studies, including CBC, erythrocyte sedimentation rate, C-reactive protein, and urinalysis are routinely normal.
Erythema multiforme and urticarial vasculitis are part of the differential
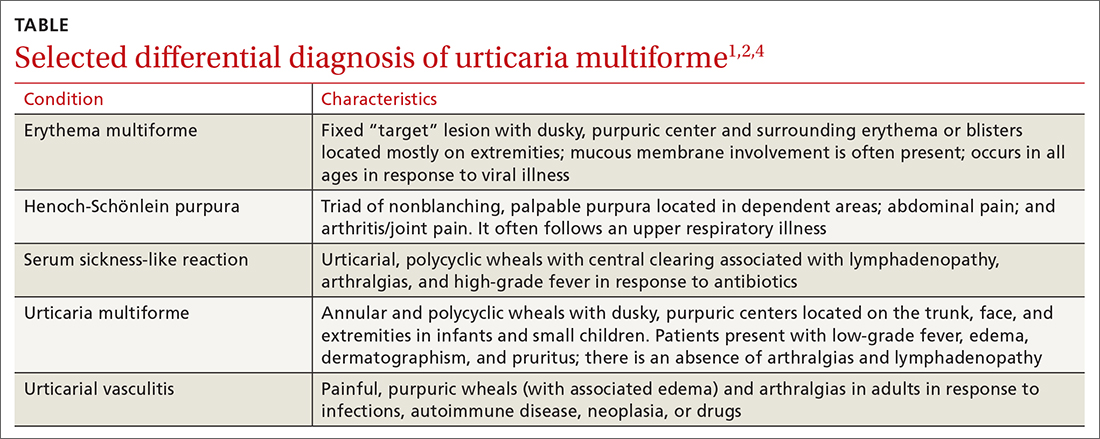
The differential diagnosis in this case includes erythema multiforme, Henoch-Schönlein purpura, serum sickness-like reaction, and urticarial vasculitis (TABLE1,2,4).
Continue to: Erythema multiforme
Erythema multiforme is a common misdiagnosis in patients with urticaria multiforme.1,2 The erythema multiforme rash has a “target” lesion with outer erythema and central ecchymosis, which may develop blisters or necrosis. Lesions are fixed and last 2 to 3 weeks. Unlike urticaria multiforme, patients with erythema multiforme commonly have mucous membrane erosions and occasionally ulcerations. Facial and acral edema is rare. Treatment is largely symptomatic and can include glucocorticoids. Antiviral medications may be used to treat recurrences.1,2
Henoch-Schönlein purpura is an immunoglobulin A–mediated vasculitis that affects the skin, gastrointestinal tract, and joints.4,5 Patients often present with arthralgias, gastrointestinal symptoms such as abdominal pain and bleeding, and a nonpruritic, erythematous rash that progresses to palpable purpura in dependent areas of the body. Treatment is generally symptomatic, but steroids may be used in severe cases.4,5
Serum sickness-like reaction can manifest with angioedema and a similar urticarial rash (with central clearing) that lasts 1 to 6 weeks.1,2,6,7 However, patients tend to have a high-grade fever, arthralgias, myalgias, and lymphadenopathy while dermatographism is absent. Treatment includes discontinuing the offending agent and the use of H1 and H2 antihistamines and steroids, in severe cases.
Urticarial vasculitis manifests as plaques or wheals lasting 1 to 7 days that may cause burning and pain but not pruritis.2,5 Purpura or hypopigmentation may develop as the hives resolve. Angioedema and arthralgias are common, but dermatographism is not present. Triggers include infections, autoimmune disease, malignancy, and the use of certain medications. H1 and H2 blockers and nonsteroidal anti-inflammatory agents are first-line therapy.2
Step 1: Discontinue offending agents; Step 2: Recommend antihistamines
Treatment consists of discontinuing any offending agent (if suspected) and using systemic H1 or H2 antihistamines for symptom relief. Systemic steroids should only be given in refractory cases.
Continue to: Our patient's amoxicillin
Our patient’s amoxicillin was discontinued, and he was started on a 14-day course of cetirizine 5 mg bid and hydroxyzine 10 mg at bedtime. He was also started on triamcinolone 0.1% cream to be applied twice daily for 1 week. During his 3-day hospital stay, his fever resolved and his rash and edema improved.
During an outpatient follow-up visit with a pediatric dermatologist 2 weeks after discharge, the patient’s rash was still present and dermatographism was noted. In light of this, his parents were instructed to continue giving the cetirizine and hydroxyzine once daily for an additional 2 weeks and to return as needed.
1. Shah KN, Honig PJ, Yan AC. “Urticaria multiforme”: a case series and review of acute annular urticarial hypersensitivity syndromes in children. Pediatrics. 2007;119:e1177-e1183. doi: 10.1542/peds.2006-1553
2. Emer JJ, Bernardo SG, Kovalerchik O, et al. Urticaria multiforme. J Clin Aesthet Dermatol. 2013;6:34-39.
3. Starnes L, Patel T, Skinner RB. Urticaria multiforme – a case report. Pediatr Dermatol. 2011; 28:436-438. doi: 10.1111/j.1525-1470.2011.01311.x
4. Reamy BV, Williams PM, Lindsay TJ. Henoch-Schönlein purpura. Am Fam Physician. 2009;80:697-704.
5. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 6th ed. Mosby, Elsevier Inc; 2016.
6. King BA, Geelhoed GC. Adverse skin and joint reactions associated with oral antibiotics in children: the role of cefaclor in serum sickness-like reactions. J Paediatr Child Health. 2003;39:677-681. doi: 10.1046/j.1440-1754.2003.00267.x
7. Misirlioglu ED, Duman H, Ozmen S, et al. Serum sickness-like reaction in children due to cefditoren. Pediatr Dermatol. 2011;29:327-328. doi: 10.1111/j.1525-1470.2011.01539.x
A 2-YEAR-OLD BOY presented to the emergency room with a 1-day history of a diffuse, mildly pruritic rash and swelling of his knees, ankles, and feet following treatment of acute otitis media with amoxicillin for the previous 8 days. He was mildly febrile and consolable, but he was refusing to walk. His medical history was unremarkable.
Physical examination revealed erythematous annular wheals on his chest, face, back, and extremities. Lymphadenopathy and mucous membrane involvement were not present. A complete blood count (CBC) with differential, inflammatory marker tests, and a comprehensive metabolic panel were ordered. Given the joint swelling and rash, the patient was admitted for observation.
During his second day in the hospital, his skin lesions enlarged and several formed dusky blue centers (FIGURE 1A). He also developed swelling of his hands (FIGURE 1B).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Urticaria multiforme
The patient’s lab work came back within normal range, except for an elevated white blood cell count (19,700/mm3; reference range, 4500-13,500/mm3). His mild systemic symptoms, skin lesions without blistering or necrosis, acral edema, and the absence of lymphadenopathy pointed to a diagnosis of urticaria multiforme.
Urticaria multiforme, also called acute annular urticaria or acute urticarial hypersensitivity syndrome, is a histamine-mediated hypersensitivity reaction characterized by transient annular, polycyclic, urticarial lesions with central ecchymosis. The incidence and prevalence are not known. Urticaria multiforme is considered common, but it is frequently misdiagnosed.1 It typically manifests in children ages 4 months to 4 years and begins with small erythematous macules, papules, and plaques that progress to large blanchable wheals with dusky blue centers.1-3 Lesions are usually located on the face, trunk, and extremities and are often pruritic (60%-94%).1-3 Individual lesions last less than 24 hours, but new ones may appear. The rash generally lasts 2 to 12 days.1,3
Patients often report a preceding viral illness, otitis media, recent use of antibiotics, or recent immunizations. Dermatographism due to mast cell–mediated cutaneous hypersensitivity at sites of minor skin trauma is common (44%).
The diagnosis is made clinically and should not require a skin biopsy or extensive laboratory testing.When performed, laboratory studies, including CBC, erythrocyte sedimentation rate, C-reactive protein, and urinalysis are routinely normal.
Erythema multiforme and urticarial vasculitis are part of the differential
The differential diagnosis in this case includes erythema multiforme, Henoch-Schönlein purpura, serum sickness-like reaction, and urticarial vasculitis (TABLE1,2,4).

Continue to: Erythema multiforme
Erythema multiforme is a common misdiagnosis in patients with urticaria multiforme.1,2 The erythema multiforme rash has a “target” lesion with outer erythema and central ecchymosis, which may develop blisters or necrosis. Lesions are fixed and last 2 to 3 weeks. Unlike urticaria multiforme, patients with erythema multiforme commonly have mucous membrane erosions and occasionally ulcerations. Facial and acral edema is rare. Treatment is largely symptomatic and can include glucocorticoids. Antiviral medications may be used to treat recurrences.1,2
Henoch-Schönlein purpura is an immunoglobulin A–mediated vasculitis that affects the skin, gastrointestinal tract, and joints.4,5 Patients often present with arthralgias, gastrointestinal symptoms such as abdominal pain and bleeding, and a nonpruritic, erythematous rash that progresses to palpable purpura in dependent areas of the body. Treatment is generally symptomatic, but steroids may be used in severe cases.4,5
Serum sickness-like reaction can manifest with angioedema and a similar urticarial rash (with central clearing) that lasts 1 to 6 weeks.1,2,6,7 However, patients tend to have a high-grade fever, arthralgias, myalgias, and lymphadenopathy while dermatographism is absent. Treatment includes discontinuing the offending agent and the use of H1 and H2 antihistamines and steroids, in severe cases.
Urticarial vasculitis manifests as plaques or wheals lasting 1 to 7 days that may cause burning and pain but not pruritis.2,5 Purpura or hypopigmentation may develop as the hives resolve. Angioedema and arthralgias are common, but dermatographism is not present. Triggers include infections, autoimmune disease, malignancy, and the use of certain medications. H1 and H2 blockers and nonsteroidal anti-inflammatory agents are first-line therapy.2
Step 1: Discontinue offending agents; Step 2: Recommend antihistamines
Treatment consists of discontinuing any offending agent (if suspected) and using systemic H1 or H2 antihistamines for symptom relief. Systemic steroids should only be given in refractory cases.
Continue to: Our patient's amoxicillin
Our patient’s amoxicillin was discontinued, and he was started on a 14-day course of cetirizine 5 mg bid and hydroxyzine 10 mg at bedtime. He was also started on triamcinolone 0.1% cream to be applied twice daily for 1 week. During his 3-day hospital stay, his fever resolved and his rash and edema improved.
During an outpatient follow-up visit with a pediatric dermatologist 2 weeks after discharge, the patient’s rash was still present and dermatographism was noted. In light of this, his parents were instructed to continue giving the cetirizine and hydroxyzine once daily for an additional 2 weeks and to return as needed.
A 2-YEAR-OLD BOY presented to the emergency room with a 1-day history of a diffuse, mildly pruritic rash and swelling of his knees, ankles, and feet following treatment of acute otitis media with amoxicillin for the previous 8 days. He was mildly febrile and consolable, but he was refusing to walk. His medical history was unremarkable.
Physical examination revealed erythematous annular wheals on his chest, face, back, and extremities. Lymphadenopathy and mucous membrane involvement were not present. A complete blood count (CBC) with differential, inflammatory marker tests, and a comprehensive metabolic panel were ordered. Given the joint swelling and rash, the patient was admitted for observation.
During his second day in the hospital, his skin lesions enlarged and several formed dusky blue centers (FIGURE 1A). He also developed swelling of his hands (FIGURE 1B).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Urticaria multiforme
The patient’s lab work came back within normal range, except for an elevated white blood cell count (19,700/mm3; reference range, 4500-13,500/mm3). His mild systemic symptoms, skin lesions without blistering or necrosis, acral edema, and the absence of lymphadenopathy pointed to a diagnosis of urticaria multiforme.
Urticaria multiforme, also called acute annular urticaria or acute urticarial hypersensitivity syndrome, is a histamine-mediated hypersensitivity reaction characterized by transient annular, polycyclic, urticarial lesions with central ecchymosis. The incidence and prevalence are not known. Urticaria multiforme is considered common, but it is frequently misdiagnosed.1 It typically manifests in children ages 4 months to 4 years and begins with small erythematous macules, papules, and plaques that progress to large blanchable wheals with dusky blue centers.1-3 Lesions are usually located on the face, trunk, and extremities and are often pruritic (60%-94%).1-3 Individual lesions last less than 24 hours, but new ones may appear. The rash generally lasts 2 to 12 days.1,3
Patients often report a preceding viral illness, otitis media, recent use of antibiotics, or recent immunizations. Dermatographism due to mast cell–mediated cutaneous hypersensitivity at sites of minor skin trauma is common (44%).
The diagnosis is made clinically and should not require a skin biopsy or extensive laboratory testing.When performed, laboratory studies, including CBC, erythrocyte sedimentation rate, C-reactive protein, and urinalysis are routinely normal.
Erythema multiforme and urticarial vasculitis are part of the differential
The differential diagnosis in this case includes erythema multiforme, Henoch-Schönlein purpura, serum sickness-like reaction, and urticarial vasculitis (TABLE1,2,4).

Continue to: Erythema multiforme
Erythema multiforme is a common misdiagnosis in patients with urticaria multiforme.1,2 The erythema multiforme rash has a “target” lesion with outer erythema and central ecchymosis, which may develop blisters or necrosis. Lesions are fixed and last 2 to 3 weeks. Unlike urticaria multiforme, patients with erythema multiforme commonly have mucous membrane erosions and occasionally ulcerations. Facial and acral edema is rare. Treatment is largely symptomatic and can include glucocorticoids. Antiviral medications may be used to treat recurrences.1,2
Henoch-Schönlein purpura is an immunoglobulin A–mediated vasculitis that affects the skin, gastrointestinal tract, and joints.4,5 Patients often present with arthralgias, gastrointestinal symptoms such as abdominal pain and bleeding, and a nonpruritic, erythematous rash that progresses to palpable purpura in dependent areas of the body. Treatment is generally symptomatic, but steroids may be used in severe cases.4,5
Serum sickness-like reaction can manifest with angioedema and a similar urticarial rash (with central clearing) that lasts 1 to 6 weeks.1,2,6,7 However, patients tend to have a high-grade fever, arthralgias, myalgias, and lymphadenopathy while dermatographism is absent. Treatment includes discontinuing the offending agent and the use of H1 and H2 antihistamines and steroids, in severe cases.
Urticarial vasculitis manifests as plaques or wheals lasting 1 to 7 days that may cause burning and pain but not pruritis.2,5 Purpura or hypopigmentation may develop as the hives resolve. Angioedema and arthralgias are common, but dermatographism is not present. Triggers include infections, autoimmune disease, malignancy, and the use of certain medications. H1 and H2 blockers and nonsteroidal anti-inflammatory agents are first-line therapy.2
Step 1: Discontinue offending agents; Step 2: Recommend antihistamines
Treatment consists of discontinuing any offending agent (if suspected) and using systemic H1 or H2 antihistamines for symptom relief. Systemic steroids should only be given in refractory cases.
Continue to: Our patient's amoxicillin
Our patient’s amoxicillin was discontinued, and he was started on a 14-day course of cetirizine 5 mg bid and hydroxyzine 10 mg at bedtime. He was also started on triamcinolone 0.1% cream to be applied twice daily for 1 week. During his 3-day hospital stay, his fever resolved and his rash and edema improved.
During an outpatient follow-up visit with a pediatric dermatologist 2 weeks after discharge, the patient’s rash was still present and dermatographism was noted. In light of this, his parents were instructed to continue giving the cetirizine and hydroxyzine once daily for an additional 2 weeks and to return as needed.
1. Shah KN, Honig PJ, Yan AC. “Urticaria multiforme”: a case series and review of acute annular urticarial hypersensitivity syndromes in children. Pediatrics. 2007;119:e1177-e1183. doi: 10.1542/peds.2006-1553
2. Emer JJ, Bernardo SG, Kovalerchik O, et al. Urticaria multiforme. J Clin Aesthet Dermatol. 2013;6:34-39.
3. Starnes L, Patel T, Skinner RB. Urticaria multiforme – a case report. Pediatr Dermatol. 2011; 28:436-438. doi: 10.1111/j.1525-1470.2011.01311.x
4. Reamy BV, Williams PM, Lindsay TJ. Henoch-Schönlein purpura. Am Fam Physician. 2009;80:697-704.
5. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 6th ed. Mosby, Elsevier Inc; 2016.
6. King BA, Geelhoed GC. Adverse skin and joint reactions associated with oral antibiotics in children: the role of cefaclor in serum sickness-like reactions. J Paediatr Child Health. 2003;39:677-681. doi: 10.1046/j.1440-1754.2003.00267.x
7. Misirlioglu ED, Duman H, Ozmen S, et al. Serum sickness-like reaction in children due to cefditoren. Pediatr Dermatol. 2011;29:327-328. doi: 10.1111/j.1525-1470.2011.01539.x
1. Shah KN, Honig PJ, Yan AC. “Urticaria multiforme”: a case series and review of acute annular urticarial hypersensitivity syndromes in children. Pediatrics. 2007;119:e1177-e1183. doi: 10.1542/peds.2006-1553
2. Emer JJ, Bernardo SG, Kovalerchik O, et al. Urticaria multiforme. J Clin Aesthet Dermatol. 2013;6:34-39.
3. Starnes L, Patel T, Skinner RB. Urticaria multiforme – a case report. Pediatr Dermatol. 2011; 28:436-438. doi: 10.1111/j.1525-1470.2011.01311.x
4. Reamy BV, Williams PM, Lindsay TJ. Henoch-Schönlein purpura. Am Fam Physician. 2009;80:697-704.
5. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 6th ed. Mosby, Elsevier Inc; 2016.
6. King BA, Geelhoed GC. Adverse skin and joint reactions associated with oral antibiotics in children: the role of cefaclor in serum sickness-like reactions. J Paediatr Child Health. 2003;39:677-681. doi: 10.1046/j.1440-1754.2003.00267.x
7. Misirlioglu ED, Duman H, Ozmen S, et al. Serum sickness-like reaction in children due to cefditoren. Pediatr Dermatol. 2011;29:327-328. doi: 10.1111/j.1525-1470.2011.01539.x
Strategies to identify and prevent penicillin allergy mislabeling and appropriately de-label patients
In North America and Europe, penicillin allergy is the most common drug-allergy label.1 Carrying a penicillin-allergy label, which has recently gained more attention in health care systems, leads to suboptimal outcomes, increased use of broad-spectrum antibiotics, increased risk of adverse reactions, and increased cost of care.2,3 Despite the high rate of reported reactions, clinically significant immunoglobulin E (IgE)-mediated and T cell–mediated hypersensitivity reactions to penicillins are uncommon.2
Through the Choosing Wisely initiative of the American Board of Internal Medicine Foundation, the American Academy of Allergy, Asthma, and Immunology has issued a recommendation: “Don’t overuse non-beta lactam antibiotics in patients with a history of penicillin allergy without an appropriate evaluation.”4 The primary care physician (PCP) plays a critical role in the appropriate evaluation and accurate initial labeling of penicillin allergy. Furthermore, the PCP plays an integral part, in conjunction with the allergist, in removing the “penicillin allergy” label from a patient’s chart when feasible.
The history of penicillin and prevalence of allergy
History. Penicillin, the first antibiotic, was discovered in 1928 by physician and microbiologist Alexander Fleming when he observed that a mold of the Penicillium genus inhibited growth of gram-positive pathogens.5 Along with pharmacologist Howard Florey and chemist Ernst Chain, both of whom assisted in the large-scale isolation and production of the antibiotic, Fleming won the Nobel Prize in Physiology or Medicine in 1945 for this discovery.5
Antibiotics transformed the practice of medicine across a spectrum, including safer childbirth, surgical procedures, and transplantation.6 Penicillin remains first-line therapy for many infections, such as streptococcal pharyngitis,7 and is the only recommended medication for treating syphilis during pregnancy.8 Continued effectiveness of penicillin in these cases allows broad-spectrum antibiotics to be reserved for more severe infections. Regrettably, incorrect antibiotic allergy labeling poses a significant risk to the patient and health care system.
Epidemiology. As with all medications, the potential for anaphylaxis exists after administration of penicillin. Because its use is widespread, penicillin is the most common cause of drug-induced anaphylaxis. However, the incidence of penicillin-induced anaphylaxis is low9: A 1968 World Health Organization report stated that the rate of penicillin anaphylaxis was between 0.015% and 0.04%.10 A more recent study reported an incidence of 1 in 207,191 patients after an oral dose and 1 in 95,298 after a parenteral dose.11 The most common reactions to penicillins are urticaria and delayed maculopapular rash.8
In the United States, the prevalence of reported penicillin allergy is approximately 10% (estimated range, 8% to 12%)3,12-15; among hospitalized patients, that prevalence is estimated to be as high as 15%.13,15 However, the prevalence of confirmed penicillin allergy is low and has decreased over time—demonstrated in a longitudinal study in which the rate of a positive skin test fell from 15% in 1995 to 0.8% in 2013.16,17
Studies have confirmed that as many as 90% of patients who report penicillin allergy are, in fact, able to tolerate penicillins.14,18-20 This finding might be a consequence of initial mislabeling of penicillin allergy; often, adverse reactions are documented as “allergy” when no risk of anaphylaxis exists. Furthermore, patients can outgrow IgE-mediated penicillin allergy because the presence of penicillin IgE antibodies wanes over time.14,15
Continue to: Consequences of mislabeling
Consequences of mislabeling
Clinical consequences. A multitude of clinical consequences result from carrying a “penicillin allergy” label.
Use of broad-spectrum antibiotics leads to increased risk of Clostridium difficile infection and to development of resistant bacteria, such as methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococcus.2,15
Alternative antibiotics used in the setting of a “penicillin allergy” label might be less efficacious and result in suboptimal outcomes. For example, vancomycin is less effective against methicillin-sensitive S aureus bacteremia than nafcillin or cefazolin.2,21 Beta-lactam antibiotics—in particular, cefazolin—are often first-line for perioperative prophylaxis; patients with reported penicillin allergy often receive a less-optimal alternative, such as clindamycin, vancomycin, or gentamicin.22 These patients are at increased risk of surgical site infection.2,22
In addition, using penicillin alternatives can result in greater risk of drug reactions and adverse effects.2
Increased health care costs. Primarily through observational studies, penicillin allergy has been associated with higher health care costs.23 Patients with reported penicillin allergy had, on average, a longer inpatient stay than patients without penicillin allergy, at a 3-year total estimated additional cost of $64.6 million.24 Inpatients with a listed penicillin allergy had direct drug costs ranging from “no difference” to $609 per patient more than patients without a listed penicillin allergy. Outpatient prescription costs were $14 to $193 higher per patient for patients with a listed penicillin allergy.23
Continue to: Considerations in special populations
Considerations in special populations. Evaluating penicillin allergy during routine care is key to decreasing the necessity for urgent penicillin evaluation and possible desensitization at the time of serious infection. Certain patient populations pose specific challenges:
- Pregnant patients. Unverified penicillin allergy during pregnancy is associated with an increased rate of cesarean section and longer postpartum hospitalization.25 Additionally, group B streptococcus-positive women have increased exposure to alternative antibiotics and an increased incidence of adverse drug reactions.25
- Elderly patients. Drug allergy increases with aging.1 Elderly patients in a long-term care facility are more likely to experience adverse drug effects or drug–drug interactions from the use of penicillin alternatives, such as clindamycin, vancomycin, and fluoroquinolones.2
- Oncology patients often require antibiotic prophylaxis as well as treatment for illnesses, such as neutropenic fever, for which beta-lactam antibiotics are often used as initial treatment.2,26
- Other important populations that present specific challenges include hospitalized patients, pediatric patients, and patients with a sexually transmitted infection.2
Active management of a penicillin-allergy label
Greater recognition of the consequences of penicillin allergy in recent years has led to efforts by hospitals and other health care organizations to develop processes by which patients can be successfully de-labeled as part of antibiotic stewardship programs9 and other initiatives. Ideally, every patient who has a “penicillin allergy” label would be referred to an allergist for evaluation; however, the number of allergy specialists is limited, and access to such specialists might be restricted in some areas, making this approach impracticable. Active management of penicillin allergy requires strategies to both test and de-label patients, as well as proactive approaches to prevent incorrect labeling. These proactive approaches require involvement of all members of the health care team—especially PCPs.
Preventing incorrect labeling. PCPs are the most likely to initially label a patient as allergic to penicillin.27 Most physicians rely on a reported history of allergy alone when selecting medication12; once a patient has been labeled “penicillin allergic,” they often retain that mislabel through adulthood.27,28 A qualitative study of PCPs’ views on prescribing penicillin found that many were aware that documented allergies were incorrect but were uncomfortable using their clinical judgment to prescribe a penicillin or change the record, for fear of a future anaphylactic reaction.29 The first step in the case of any reported reaction should be for you to elicit an accurate drug allergy history (TABLE 1).
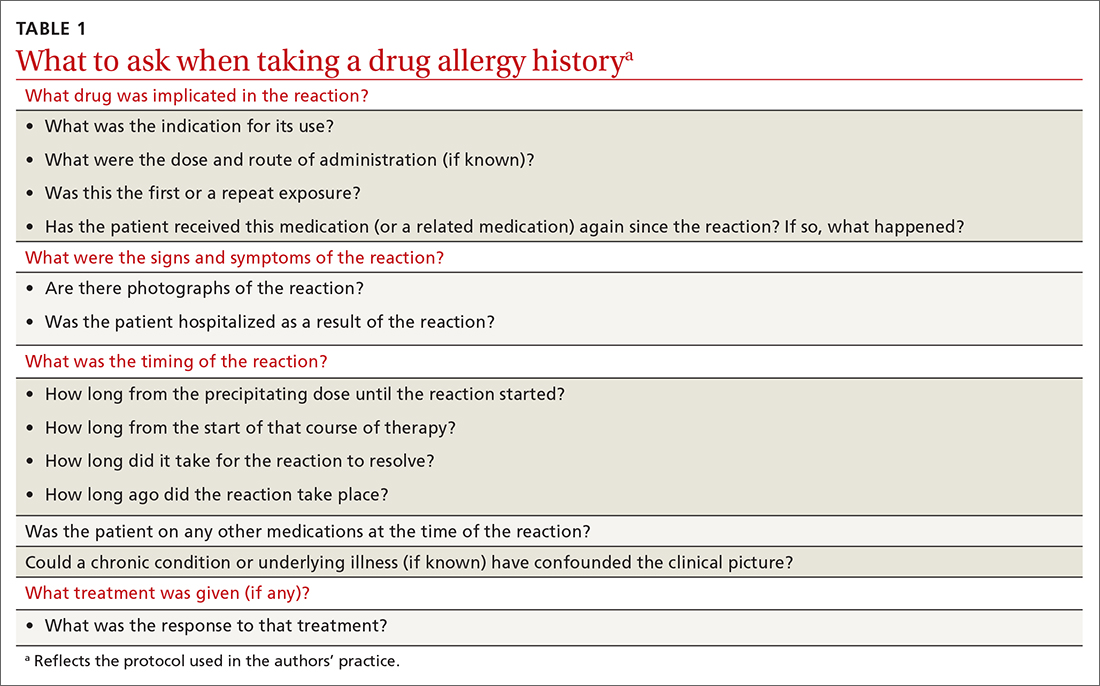
As with other drug reactions, you should consider the context surrounding the reaction to a penicillin. Take care to review signs and symptoms of the reaction to look for clues that make a true allergic reaction more, or less, likely.
Symptoms can generally be divided into low-risk and high-risk categories27 (TABLE 2). An example of a commonly reported low-risk symptom is diarrhea that develops after several doses of a penicillin. In the absence of other symptoms, this finding is most likely due to elimination of normal gut flora,30 not to an allergic reaction to the medication. Symptoms of intolerance to the medication, such as headache and nausea, are also low risk.27,31 In contrast, immediate onset of abdominal pain after a dose of penicillin and lip or throat swelling are considered high risk.
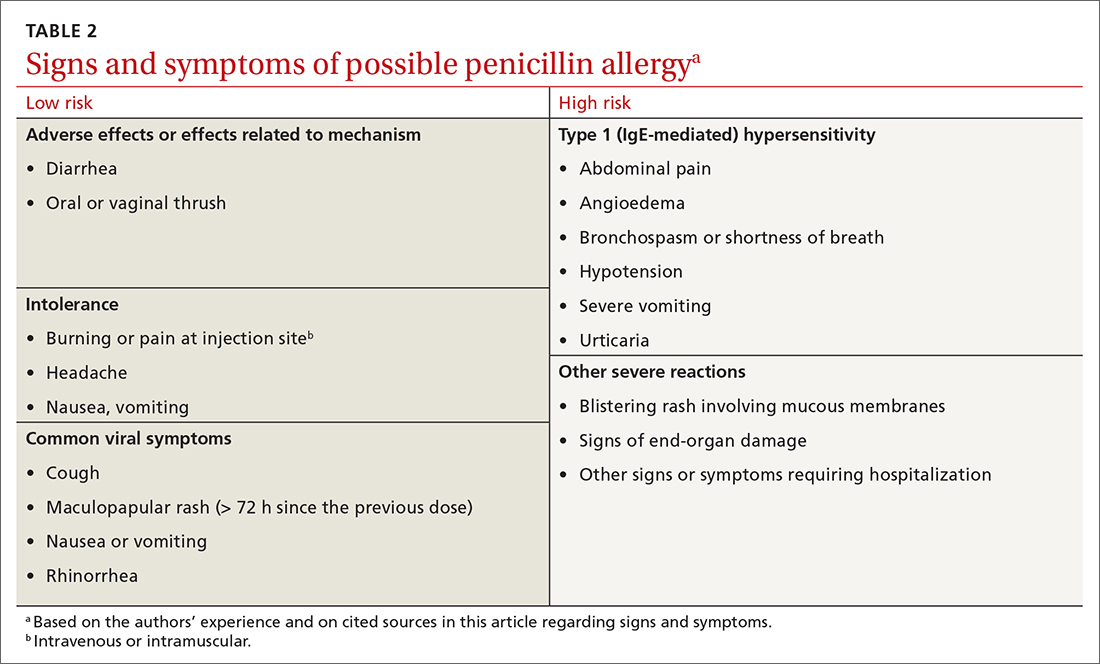
Continue to: Patients presenting with urticaria...
Patients presenting with urticaria or maculopapular rash after taking penicillin are particularly challenging.30 A study of patients in a primary care pediatrics practice found that 7.4% of children receiving a prescription for a penicillin reported a rash.32 Here, timing of onset of symptoms provides some clarity about the likelihood of true allergy. Rashes that manifest during the first hours after exposure are more likely to be IgE mediated, particularly when accompanied by other systemic symptoms; they should be considered high risk. Delayed-onset rashes (> 72 hours after exposure) are usually non-IgE mediated and therefore are generally lower risk,8,30,33 except when associated with certain features, such as mucosal involvement and skin peeling.
Despite acknowledging viral exanthems in the differential, many physicians still label patients presenting with any rash as “allergic.”28 Take care to look for other potential causes of a rash; for example, patients taking amoxicillin who have concurrent Epstein-Barr virus infection frequently develop a maculopapular rash.34 Caubet and colleagues found that 56% of pediatric patients with a history of nonimmediate rash and a negative oral challenge to amoxicillin tested positive for viral infection.28
A family history of penicillin allergy alone should not preclude the use of penicillin.8,27,31 Similarly, if a patient has already received and tolerated a subsequent course of the same penicillin derivative after the initial reaction, the “penicillin allergy” label can be removed. If the reaction history is unknown, refer the patient to an allergist for further evaluation.
Accurate charting is key. With most hospital systems and physician practices now documenting in an electronic health record, there exists the ability to document, in great detail, patients’ reactions to medications. Previous studies have found, however, that such documentation is often done poorly, or not done at all. One such study found that (1) > 20% of patients with a “penicillin allergy” label did not have reaction details listed and (2) when reactions were listed, many were incorrectly labeled as “allergy,” not “intolerance.”35
Many electronic health record systems lump drug allergies, adverse effects, and food and environmental allergies into a single section, leading to a lack of distinction between adverse reactions and true allergy.31 Although many PCPs report that it is easy to change a patient’s allergy label in the record,29 more often, a nurse, resident, or consultant actually documents the reaction.35
Continue to: Documentation at the time of the reaction...
Documentation at the time of the reaction, within the encounter note and the allergy tab, is essential, so that other physicians caring for the patient, in the future, will be knowledgeable about the details of the reaction. Make it your responsibility to accurately document penicillin allergy in patients’ charts, including removing the “penicillin allergy” label from the chart of patients whose history is inconsistent with allergy, who have tolerated subsequent courses of the same penicillin derivative, or who have passed testing in an allergist’s office. In a study of 639 patients who tested negative for penicillin allergy, 51% still had a “penicillin allergy” label in their chart more than 4 years later.36
Penicillin allergy evaluation. When a patient cannot be cleared of a “penicillin allergy” label by history alone, and in the absence of severe features such as mucous membrane involvement, they should be further evaluated through objective testing for potential IgE-mediated allergy. This assessment includes penicillin skin testing or an oral challenge, or both.
Skin testing involves skin-prick testing of major and minor determinants of penicillin; when skin-prick testing is negative, intradermal testing of major and minor determinants should follow. The negative predictive value of penicillin skin testing is high: In a prospective, multicenter investigation, researchers demonstrated that, when both the major penicillin determinant and a minor determinant mixture were used, negative predictive value was 97.9%.37
However, a minor determinant mixture is not commercially available in the United States; therefore, penicillin G is often used alone as the minor determinant. Typically, if a patient passes skin testing, a challenge dose of penicillin or amoxicillin is administered, followed by an observation period. The risk of re-sensitization after oral penicillin is thought to be low and does not preclude future use.38
Although drug testing is most often performed in an allergist’s office, several groups have developed protocols that allow for limited testing of low-risk patients in a primary care setting.8,31 For example, several studies have demonstrated that patients presenting with low-risk skin rash can be safely tested with a supervised oral challenge alone.18,28 The FIGURE8,27,30,31,33,34 outlines our proposed workflow for risk stratification and subsequent management of patients with a “penicillin allergy” label.
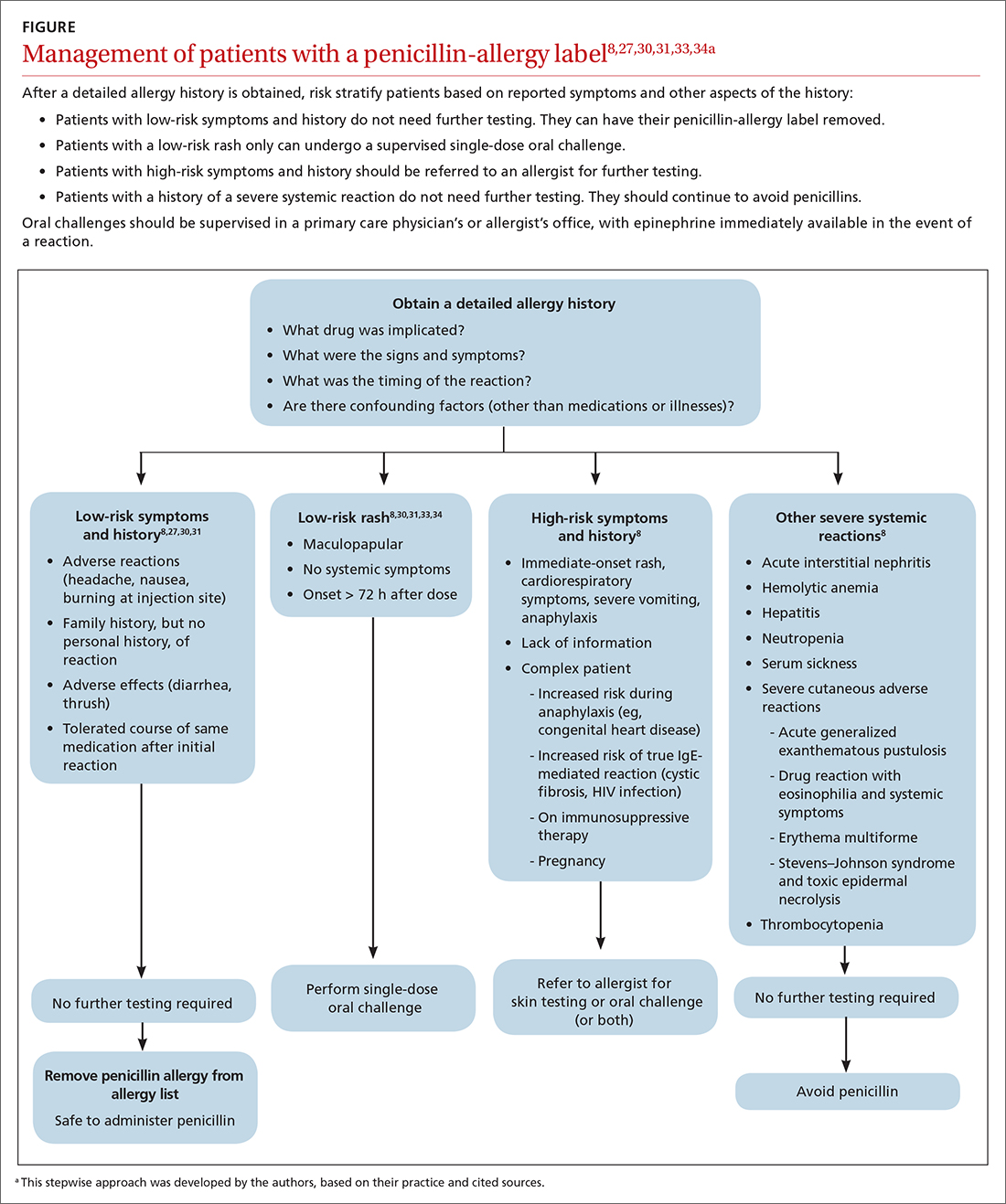
Continue to: De-labeling requires a systems approach
De-labeling requires a systems approach. Given the mismatch between the large number of patients labeled “penicillin allergic” and the few allergy specialists, referral alone is not enough to solve the problem of mislabeling. Targeting specific populations for testing, such as patients presenting to an inner-city sexually transmitted infection clinic19 or preoperative patients, as is done at the Mayo Clinic,9 has been successful. Skin testing in an inpatient setting has also been shown to be safe and effective,13 allowing for protocol-driven testing under the supervision of trained pharmacists (and others), to relieve the burden on allergy specialists.9
CORRESPONDENCE
Andrew Lutzkanin, MD, 500 University Drive, PO Box 850, Hershey, PA 17033; [email protected]
1. Macy E. The clinical evaluation of penicillin allergy: what is necessary, sufficient and safe given the materials currently available? Clin Exp Allergy. 2011;41:1498-1501. doi: 10.1111/j.1365-2222.2011.03837.x
2. Shenoy ES, Macy E, Rowe T, et al. Evaluation and management of penicillin allergy: a review. JAMA. 2019;321:188-199. doi: 10.1001/jama.2018.19283
3. Blumenthal KG, Li Y, Banerji A, et al. The cost of penicillin allergy evaluation. J Allergy Clin Immunol Pract. 2018;6:1019-1027.e2. doi: 10.1016/j.jaip.2017.08.006
4. American Academy of Allergy, Asthma & Immunology: Ten things physicians and patients should question. American Board of Medicine Foundation Choosing Wisely website. 2018. Accessed July 7, 2021. www.choosingwisely.org/doctor-patient-lists/american-academy-of-allergy-asthma-immunology
5. Tan SY, Tatsumura Y. Alexander Fleming (1881-1955): discoverer of penicillin. Singapore Med J. 2015;56:366-367. doi: 10.11622/smedj.2015105
6. Marston HD, Dixon DM, Knisely JM, et al. Antimicrobial resistance. JAMA. 2016;316:1193-1204. doi: 10.1001/jama.2016.11764
7. Spinks A, Glasziou PP, Del Mar CB. Antibiotics for sore throat. Cochrane Database Syst Rev. 2013;2013:CD000023. doi: 10.1002/14651858.CD000023.pub4
8. Castells M, Khan DA, Phillips EJ. Penicillin allergy. N Engl J Med. 2019;381:2338-2351. doi: 10.1056/NEJMra1807761
9. Khan DA. Proactive management of penicillin and other antibiotic allergies. Allergy Asthma Proc. 2020;41:82-89. doi: 10.2500/aap.2020.41.190024
10. Idsoe O, Guthe T, Willcox RR, et al. Nature and extent of penicillin side-reactions, with particular reference to fatalities from anaphylactic shock. Bull World Health Organ. 1968;38:159-188.
11. Chiriac AM, Macy E. Large health system databases and drug hypersensitivity. J Allergy Clin Immunol Pract. 2019;7:2125-2131. doi: 10.1016/j.jaip.2019.04.014
12. Albin S, Agarwal S. Prevalence and characteristics of reported penicillin allergy in an urban outpatient adult population. Allergy Asthma Proc. 2014;35:489-494. doi: 10.2500/aap.2014.35.3791
13. Sacco KA, Bates A, Brigham TJ, et al. Clinical outcomes following inpatient penicillin allergy testing: a systematic review and meta-analysis. Allergy. 2017;72:1288-1296. doi: 10.1111/all.13168
14. Khan DA, Solensky R. Drug allergy. J Allergy Clin Immunol. 2010;125(2 suppl 2):S126-S137. doi: 10.1016/j.jaci.2009.10.028
15. Blumenthal KG, Shenoy ES, Varughese CA, et al. Impact of a clinical guideline for prescribing antibiotics to inpatients reporting penicillin or cephalosporin allergy. Ann Allergy Asthma Immunol. 2015;115:294-300.e2. doi: 10.1016/j.anai.2015.05.011
16. Macy E, Schatz M, Lin C, et al. The falling rate of positive penicillin skin tests from 1995 to 2007. Perm J. 2009;13:12-18. doi: 10.7812/tpp/08-073
17. Macy E, Ngor EW. Safely diagnosing clinically significant penicillin allergy using only penicilloyl-poly-lysine, penicillin, and oral amoxicillin. J Allergy Clin Immunol Pract. 2013;1:258-263. doi: 10.1016/j.jaip.2013.02.002
18. Bourke J, Pavlos R, James I, et al. Improving the effectiveness of penicillin allergy de-labeling. J Allergy Clin Immunol Pract. 2015;3:365-374.e1. doi: 10.1016/j.jaip.2014.11.002
19. Gadde J, Spence M, Wheeler B, et al. Clinical experience with penicillin skin testing in a large inner-city STD clinic. JAMA. 1993;270:2456-2463.
20. Klaustermeyer WB, Gowda VC. Penicillin skin testing: a 20-year study at the West Los Angeles Veterans Affairs Medical Center. Mil Med. 2005;170:701-704. doi: 10.7205/milmed.170.8.701.
21. McDanel JS, Perencevich EN, Diekema DJ, et al. Comparative effectiveness of beta-lactams versus vancomycin for treatment of methicillin-susceptible Staphylococcus aureus bloodstream infections among 122 hospitals. Clin Infect Dis. 2015;61:361-367. doi: 10.1093/cid/civ308
22. Blumenthal KG, Ryan EE, Li Y, et al. The impact of a reported penicillin allergy on surgical site infection risk. Clin Infect Dis. 2018;66:329-336. doi: 10.1093/cid/cix794
23. Mattingly TJ 2nd, Fulton A, Lumish RA, et al. The cost of self-reported penicillin allergy: a systematic review. J Allergy Clin Immunol Pract. 2018;6:1649-1654.e4. doi: 10.1016/j.jaip.2017.12.033
24. Macy E, Contreras R. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: a cohort study. J Allergy Clin Immunol. 2014;133:790-796. doi: 10.1016/j.jaci.2013.09.021
25. Desai SH, Kaplan MS, Chen Q, et al. Morbidity in pregnant women associated with unverified penicillin allergies, antibiotic use, and group B streptococcus infections. Perm J. 2017;21:16-80. doi: 10.7812/TPP/16-080
26. Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52:e56-e93. doi: 10.1093/cid/cir073
27. Vyles D, Mistry RD, Heffner V, et al. Reported knowledge and management of potential penicillin allergy in children. Acad Pediatr. 2019;19:684-690. doi: 10.1016/j.acap.2019.01.002
28. Caubet J-C, Kaiser L, Lemaître B, et al. The role of penicillin in benign skin rashes in childhood: a prospective study based on drug rechallenge. J Allergy Clin Immunol. 2011;127:218-222. doi: 10.1016/j.jaci.2010.08.025
29. Wanat M, Anthierens S, Butler CC, et al. Patient and primary care physician perceptions of penicillin allergy testing and subsequent use of penicillin-containing antibiotics: a qualitative study. J Allergy Clin Immunol Pract. 2019;7:1888-1893.e1. doi: 10.1016/j.jaip.2019.02.036
30. Norton AE, Konvinse K, Phillips EJ, et al. Antibiotic allergy in pediatrics. Pediatrics. 2018;141: e20172497. doi: 10.1542/peds.2017-2497
31. Collins C. The low risks and high rewards of penicillin allergy delabeling: an algorithm to expedite the evaluation. J Pediatr. 2019;212:216-223. doi: 10.1016/j.jpeds.2019.05.060
32. Ibia EO, Schwartz RH, Wiedermann BL. Antibiotic rashes in children: a survey in a private practice setting. Arch Dermatol. 2000;136:849-854. doi: 10.1001/archderm.136.7.849
33. Salkind AR, Cuddy PG, Foxworth JW. The rational clinical examination. Is this patient allergic to penicillin? An evidence-based analysis of the likelihood of penicillin allergy. JAMA. 2001;285:2498-2505. doi: 10.1001/jama.285.19.2498
34. Patel BM. Skin rash with infectious mononucleosis and ampicillin. Pediatrics. 1967;40:910-911.
35. Inglis JM, Caughey GE, Smith W, et al. Documentation of penicillin adverse drug reactions in electronic health records: inconsistent use of allergy and intolerance labels. Intern Med J. 2017;47:1292-1297. doi: 10.1111/imj.13558
36. Lachover-Roth I, Sharon S, Rosman Y, et al. Long-term follow-up after penicillin allergy delabeling in ambulatory patients. J Allergy Clin Immunol Pract. 2019;7:231-235.e1. doi: 10.1016/j.jaip.2018.04.042
37. Solensky R, Jacobs J, Lester M, et al. Penicillin allergy evaluation: a prospective, multicenter, open-label evaluation of a comprehensive penicillin skin test kit. J Allergy Clin Immunol Pract. 2019;7:1876-1885.e3. doi: 10.1016/j.jaip.2019.02.040
38. A; ; . Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105:259-273. doi: 10.1016/j.anai.2010.08.002
In North America and Europe, penicillin allergy is the most common drug-allergy label.1 Carrying a penicillin-allergy label, which has recently gained more attention in health care systems, leads to suboptimal outcomes, increased use of broad-spectrum antibiotics, increased risk of adverse reactions, and increased cost of care.2,3 Despite the high rate of reported reactions, clinically significant immunoglobulin E (IgE)-mediated and T cell–mediated hypersensitivity reactions to penicillins are uncommon.2
Through the Choosing Wisely initiative of the American Board of Internal Medicine Foundation, the American Academy of Allergy, Asthma, and Immunology has issued a recommendation: “Don’t overuse non-beta lactam antibiotics in patients with a history of penicillin allergy without an appropriate evaluation.”4 The primary care physician (PCP) plays a critical role in the appropriate evaluation and accurate initial labeling of penicillin allergy. Furthermore, the PCP plays an integral part, in conjunction with the allergist, in removing the “penicillin allergy” label from a patient’s chart when feasible.

The history of penicillin and prevalence of allergy
History. Penicillin, the first antibiotic, was discovered in 1928 by physician and microbiologist Alexander Fleming when he observed that a mold of the Penicillium genus inhibited growth of gram-positive pathogens.5 Along with pharmacologist Howard Florey and chemist Ernst Chain, both of whom assisted in the large-scale isolation and production of the antibiotic, Fleming won the Nobel Prize in Physiology or Medicine in 1945 for this discovery.5
Antibiotics transformed the practice of medicine across a spectrum, including safer childbirth, surgical procedures, and transplantation.6 Penicillin remains first-line therapy for many infections, such as streptococcal pharyngitis,7 and is the only recommended medication for treating syphilis during pregnancy.8 Continued effectiveness of penicillin in these cases allows broad-spectrum antibiotics to be reserved for more severe infections. Regrettably, incorrect antibiotic allergy labeling poses a significant risk to the patient and health care system.
Epidemiology. As with all medications, the potential for anaphylaxis exists after administration of penicillin. Because its use is widespread, penicillin is the most common cause of drug-induced anaphylaxis. However, the incidence of penicillin-induced anaphylaxis is low9: A 1968 World Health Organization report stated that the rate of penicillin anaphylaxis was between 0.015% and 0.04%.10 A more recent study reported an incidence of 1 in 207,191 patients after an oral dose and 1 in 95,298 after a parenteral dose.11 The most common reactions to penicillins are urticaria and delayed maculopapular rash.8
In the United States, the prevalence of reported penicillin allergy is approximately 10% (estimated range, 8% to 12%)3,12-15; among hospitalized patients, that prevalence is estimated to be as high as 15%.13,15 However, the prevalence of confirmed penicillin allergy is low and has decreased over time—demonstrated in a longitudinal study in which the rate of a positive skin test fell from 15% in 1995 to 0.8% in 2013.16,17
Studies have confirmed that as many as 90% of patients who report penicillin allergy are, in fact, able to tolerate penicillins.14,18-20 This finding might be a consequence of initial mislabeling of penicillin allergy; often, adverse reactions are documented as “allergy” when no risk of anaphylaxis exists. Furthermore, patients can outgrow IgE-mediated penicillin allergy because the presence of penicillin IgE antibodies wanes over time.14,15
Continue to: Consequences of mislabeling
Consequences of mislabeling
Clinical consequences. A multitude of clinical consequences result from carrying a “penicillin allergy” label.
Use of broad-spectrum antibiotics leads to increased risk of Clostridium difficile infection and to development of resistant bacteria, such as methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococcus.2,15
Alternative antibiotics used in the setting of a “penicillin allergy” label might be less efficacious and result in suboptimal outcomes. For example, vancomycin is less effective against methicillin-sensitive S aureus bacteremia than nafcillin or cefazolin.2,21 Beta-lactam antibiotics—in particular, cefazolin—are often first-line for perioperative prophylaxis; patients with reported penicillin allergy often receive a less-optimal alternative, such as clindamycin, vancomycin, or gentamicin.22 These patients are at increased risk of surgical site infection.2,22
In addition, using penicillin alternatives can result in greater risk of drug reactions and adverse effects.2
Increased health care costs. Primarily through observational studies, penicillin allergy has been associated with higher health care costs.23 Patients with reported penicillin allergy had, on average, a longer inpatient stay than patients without penicillin allergy, at a 3-year total estimated additional cost of $64.6 million.24 Inpatients with a listed penicillin allergy had direct drug costs ranging from “no difference” to $609 per patient more than patients without a listed penicillin allergy. Outpatient prescription costs were $14 to $193 higher per patient for patients with a listed penicillin allergy.23
Continue to: Considerations in special populations
Considerations in special populations. Evaluating penicillin allergy during routine care is key to decreasing the necessity for urgent penicillin evaluation and possible desensitization at the time of serious infection. Certain patient populations pose specific challenges:
- Pregnant patients. Unverified penicillin allergy during pregnancy is associated with an increased rate of cesarean section and longer postpartum hospitalization.25 Additionally, group B streptococcus-positive women have increased exposure to alternative antibiotics and an increased incidence of adverse drug reactions.25
- Elderly patients. Drug allergy increases with aging.1 Elderly patients in a long-term care facility are more likely to experience adverse drug effects or drug–drug interactions from the use of penicillin alternatives, such as clindamycin, vancomycin, and fluoroquinolones.2
- Oncology patients often require antibiotic prophylaxis as well as treatment for illnesses, such as neutropenic fever, for which beta-lactam antibiotics are often used as initial treatment.2,26
- Other important populations that present specific challenges include hospitalized patients, pediatric patients, and patients with a sexually transmitted infection.2
Active management of a penicillin-allergy label
Greater recognition of the consequences of penicillin allergy in recent years has led to efforts by hospitals and other health care organizations to develop processes by which patients can be successfully de-labeled as part of antibiotic stewardship programs9 and other initiatives. Ideally, every patient who has a “penicillin allergy” label would be referred to an allergist for evaluation; however, the number of allergy specialists is limited, and access to such specialists might be restricted in some areas, making this approach impracticable. Active management of penicillin allergy requires strategies to both test and de-label patients, as well as proactive approaches to prevent incorrect labeling. These proactive approaches require involvement of all members of the health care team—especially PCPs.
Preventing incorrect labeling. PCPs are the most likely to initially label a patient as allergic to penicillin.27 Most physicians rely on a reported history of allergy alone when selecting medication12; once a patient has been labeled “penicillin allergic,” they often retain that mislabel through adulthood.27,28 A qualitative study of PCPs’ views on prescribing penicillin found that many were aware that documented allergies were incorrect but were uncomfortable using their clinical judgment to prescribe a penicillin or change the record, for fear of a future anaphylactic reaction.29 The first step in the case of any reported reaction should be for you to elicit an accurate drug allergy history (TABLE 1).

As with other drug reactions, you should consider the context surrounding the reaction to a penicillin. Take care to review signs and symptoms of the reaction to look for clues that make a true allergic reaction more, or less, likely.
Symptoms can generally be divided into low-risk and high-risk categories27 (TABLE 2). An example of a commonly reported low-risk symptom is diarrhea that develops after several doses of a penicillin. In the absence of other symptoms, this finding is most likely due to elimination of normal gut flora,30 not to an allergic reaction to the medication. Symptoms of intolerance to the medication, such as headache and nausea, are also low risk.27,31 In contrast, immediate onset of abdominal pain after a dose of penicillin and lip or throat swelling are considered high risk.

Continue to: Patients presenting with urticaria...
Patients presenting with urticaria or maculopapular rash after taking penicillin are particularly challenging.30 A study of patients in a primary care pediatrics practice found that 7.4% of children receiving a prescription for a penicillin reported a rash.32 Here, timing of onset of symptoms provides some clarity about the likelihood of true allergy. Rashes that manifest during the first hours after exposure are more likely to be IgE mediated, particularly when accompanied by other systemic symptoms; they should be considered high risk. Delayed-onset rashes (> 72 hours after exposure) are usually non-IgE mediated and therefore are generally lower risk,8,30,33 except when associated with certain features, such as mucosal involvement and skin peeling.
Despite acknowledging viral exanthems in the differential, many physicians still label patients presenting with any rash as “allergic.”28 Take care to look for other potential causes of a rash; for example, patients taking amoxicillin who have concurrent Epstein-Barr virus infection frequently develop a maculopapular rash.34 Caubet and colleagues found that 56% of pediatric patients with a history of nonimmediate rash and a negative oral challenge to amoxicillin tested positive for viral infection.28
A family history of penicillin allergy alone should not preclude the use of penicillin.8,27,31 Similarly, if a patient has already received and tolerated a subsequent course of the same penicillin derivative after the initial reaction, the “penicillin allergy” label can be removed. If the reaction history is unknown, refer the patient to an allergist for further evaluation.
Accurate charting is key. With most hospital systems and physician practices now documenting in an electronic health record, there exists the ability to document, in great detail, patients’ reactions to medications. Previous studies have found, however, that such documentation is often done poorly, or not done at all. One such study found that (1) > 20% of patients with a “penicillin allergy” label did not have reaction details listed and (2) when reactions were listed, many were incorrectly labeled as “allergy,” not “intolerance.”35
Many electronic health record systems lump drug allergies, adverse effects, and food and environmental allergies into a single section, leading to a lack of distinction between adverse reactions and true allergy.31 Although many PCPs report that it is easy to change a patient’s allergy label in the record,29 more often, a nurse, resident, or consultant actually documents the reaction.35
Continue to: Documentation at the time of the reaction...
Documentation at the time of the reaction, within the encounter note and the allergy tab, is essential, so that other physicians caring for the patient, in the future, will be knowledgeable about the details of the reaction. Make it your responsibility to accurately document penicillin allergy in patients’ charts, including removing the “penicillin allergy” label from the chart of patients whose history is inconsistent with allergy, who have tolerated subsequent courses of the same penicillin derivative, or who have passed testing in an allergist’s office. In a study of 639 patients who tested negative for penicillin allergy, 51% still had a “penicillin allergy” label in their chart more than 4 years later.36
Penicillin allergy evaluation. When a patient cannot be cleared of a “penicillin allergy” label by history alone, and in the absence of severe features such as mucous membrane involvement, they should be further evaluated through objective testing for potential IgE-mediated allergy. This assessment includes penicillin skin testing or an oral challenge, or both.
Skin testing involves skin-prick testing of major and minor determinants of penicillin; when skin-prick testing is negative, intradermal testing of major and minor determinants should follow. The negative predictive value of penicillin skin testing is high: In a prospective, multicenter investigation, researchers demonstrated that, when both the major penicillin determinant and a minor determinant mixture were used, negative predictive value was 97.9%.37
However, a minor determinant mixture is not commercially available in the United States; therefore, penicillin G is often used alone as the minor determinant. Typically, if a patient passes skin testing, a challenge dose of penicillin or amoxicillin is administered, followed by an observation period. The risk of re-sensitization after oral penicillin is thought to be low and does not preclude future use.38
Although drug testing is most often performed in an allergist’s office, several groups have developed protocols that allow for limited testing of low-risk patients in a primary care setting.8,31 For example, several studies have demonstrated that patients presenting with low-risk skin rash can be safely tested with a supervised oral challenge alone.18,28 The FIGURE8,27,30,31,33,34 outlines our proposed workflow for risk stratification and subsequent management of patients with a “penicillin allergy” label.

Continue to: De-labeling requires a systems approach
De-labeling requires a systems approach. Given the mismatch between the large number of patients labeled “penicillin allergic” and the few allergy specialists, referral alone is not enough to solve the problem of mislabeling. Targeting specific populations for testing, such as patients presenting to an inner-city sexually transmitted infection clinic19 or preoperative patients, as is done at the Mayo Clinic,9 has been successful. Skin testing in an inpatient setting has also been shown to be safe and effective,13 allowing for protocol-driven testing under the supervision of trained pharmacists (and others), to relieve the burden on allergy specialists.9
CORRESPONDENCE
Andrew Lutzkanin, MD, 500 University Drive, PO Box 850, Hershey, PA 17033; [email protected]
In North America and Europe, penicillin allergy is the most common drug-allergy label.1 Carrying a penicillin-allergy label, which has recently gained more attention in health care systems, leads to suboptimal outcomes, increased use of broad-spectrum antibiotics, increased risk of adverse reactions, and increased cost of care.2,3 Despite the high rate of reported reactions, clinically significant immunoglobulin E (IgE)-mediated and T cell–mediated hypersensitivity reactions to penicillins are uncommon.2
Through the Choosing Wisely initiative of the American Board of Internal Medicine Foundation, the American Academy of Allergy, Asthma, and Immunology has issued a recommendation: “Don’t overuse non-beta lactam antibiotics in patients with a history of penicillin allergy without an appropriate evaluation.”4 The primary care physician (PCP) plays a critical role in the appropriate evaluation and accurate initial labeling of penicillin allergy. Furthermore, the PCP plays an integral part, in conjunction with the allergist, in removing the “penicillin allergy” label from a patient’s chart when feasible.

The history of penicillin and prevalence of allergy
History. Penicillin, the first antibiotic, was discovered in 1928 by physician and microbiologist Alexander Fleming when he observed that a mold of the Penicillium genus inhibited growth of gram-positive pathogens.5 Along with pharmacologist Howard Florey and chemist Ernst Chain, both of whom assisted in the large-scale isolation and production of the antibiotic, Fleming won the Nobel Prize in Physiology or Medicine in 1945 for this discovery.5
Antibiotics transformed the practice of medicine across a spectrum, including safer childbirth, surgical procedures, and transplantation.6 Penicillin remains first-line therapy for many infections, such as streptococcal pharyngitis,7 and is the only recommended medication for treating syphilis during pregnancy.8 Continued effectiveness of penicillin in these cases allows broad-spectrum antibiotics to be reserved for more severe infections. Regrettably, incorrect antibiotic allergy labeling poses a significant risk to the patient and health care system.
Epidemiology. As with all medications, the potential for anaphylaxis exists after administration of penicillin. Because its use is widespread, penicillin is the most common cause of drug-induced anaphylaxis. However, the incidence of penicillin-induced anaphylaxis is low9: A 1968 World Health Organization report stated that the rate of penicillin anaphylaxis was between 0.015% and 0.04%.10 A more recent study reported an incidence of 1 in 207,191 patients after an oral dose and 1 in 95,298 after a parenteral dose.11 The most common reactions to penicillins are urticaria and delayed maculopapular rash.8
In the United States, the prevalence of reported penicillin allergy is approximately 10% (estimated range, 8% to 12%)3,12-15; among hospitalized patients, that prevalence is estimated to be as high as 15%.13,15 However, the prevalence of confirmed penicillin allergy is low and has decreased over time—demonstrated in a longitudinal study in which the rate of a positive skin test fell from 15% in 1995 to 0.8% in 2013.16,17
Studies have confirmed that as many as 90% of patients who report penicillin allergy are, in fact, able to tolerate penicillins.14,18-20 This finding might be a consequence of initial mislabeling of penicillin allergy; often, adverse reactions are documented as “allergy” when no risk of anaphylaxis exists. Furthermore, patients can outgrow IgE-mediated penicillin allergy because the presence of penicillin IgE antibodies wanes over time.14,15
Continue to: Consequences of mislabeling
Consequences of mislabeling
Clinical consequences. A multitude of clinical consequences result from carrying a “penicillin allergy” label.
Use of broad-spectrum antibiotics leads to increased risk of Clostridium difficile infection and to development of resistant bacteria, such as methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococcus.2,15
Alternative antibiotics used in the setting of a “penicillin allergy” label might be less efficacious and result in suboptimal outcomes. For example, vancomycin is less effective against methicillin-sensitive S aureus bacteremia than nafcillin or cefazolin.2,21 Beta-lactam antibiotics—in particular, cefazolin—are often first-line for perioperative prophylaxis; patients with reported penicillin allergy often receive a less-optimal alternative, such as clindamycin, vancomycin, or gentamicin.22 These patients are at increased risk of surgical site infection.2,22
In addition, using penicillin alternatives can result in greater risk of drug reactions and adverse effects.2
Increased health care costs. Primarily through observational studies, penicillin allergy has been associated with higher health care costs.23 Patients with reported penicillin allergy had, on average, a longer inpatient stay than patients without penicillin allergy, at a 3-year total estimated additional cost of $64.6 million.24 Inpatients with a listed penicillin allergy had direct drug costs ranging from “no difference” to $609 per patient more than patients without a listed penicillin allergy. Outpatient prescription costs were $14 to $193 higher per patient for patients with a listed penicillin allergy.23
Continue to: Considerations in special populations
Considerations in special populations. Evaluating penicillin allergy during routine care is key to decreasing the necessity for urgent penicillin evaluation and possible desensitization at the time of serious infection. Certain patient populations pose specific challenges:
- Pregnant patients. Unverified penicillin allergy during pregnancy is associated with an increased rate of cesarean section and longer postpartum hospitalization.25 Additionally, group B streptococcus-positive women have increased exposure to alternative antibiotics and an increased incidence of adverse drug reactions.25
- Elderly patients. Drug allergy increases with aging.1 Elderly patients in a long-term care facility are more likely to experience adverse drug effects or drug–drug interactions from the use of penicillin alternatives, such as clindamycin, vancomycin, and fluoroquinolones.2
- Oncology patients often require antibiotic prophylaxis as well as treatment for illnesses, such as neutropenic fever, for which beta-lactam antibiotics are often used as initial treatment.2,26
- Other important populations that present specific challenges include hospitalized patients, pediatric patients, and patients with a sexually transmitted infection.2
Active management of a penicillin-allergy label
Greater recognition of the consequences of penicillin allergy in recent years has led to efforts by hospitals and other health care organizations to develop processes by which patients can be successfully de-labeled as part of antibiotic stewardship programs9 and other initiatives. Ideally, every patient who has a “penicillin allergy” label would be referred to an allergist for evaluation; however, the number of allergy specialists is limited, and access to such specialists might be restricted in some areas, making this approach impracticable. Active management of penicillin allergy requires strategies to both test and de-label patients, as well as proactive approaches to prevent incorrect labeling. These proactive approaches require involvement of all members of the health care team—especially PCPs.
Preventing incorrect labeling. PCPs are the most likely to initially label a patient as allergic to penicillin.27 Most physicians rely on a reported history of allergy alone when selecting medication12; once a patient has been labeled “penicillin allergic,” they often retain that mislabel through adulthood.27,28 A qualitative study of PCPs’ views on prescribing penicillin found that many were aware that documented allergies were incorrect but were uncomfortable using their clinical judgment to prescribe a penicillin or change the record, for fear of a future anaphylactic reaction.29 The first step in the case of any reported reaction should be for you to elicit an accurate drug allergy history (TABLE 1).

As with other drug reactions, you should consider the context surrounding the reaction to a penicillin. Take care to review signs and symptoms of the reaction to look for clues that make a true allergic reaction more, or less, likely.
Symptoms can generally be divided into low-risk and high-risk categories27 (TABLE 2). An example of a commonly reported low-risk symptom is diarrhea that develops after several doses of a penicillin. In the absence of other symptoms, this finding is most likely due to elimination of normal gut flora,30 not to an allergic reaction to the medication. Symptoms of intolerance to the medication, such as headache and nausea, are also low risk.27,31 In contrast, immediate onset of abdominal pain after a dose of penicillin and lip or throat swelling are considered high risk.

Continue to: Patients presenting with urticaria...
Patients presenting with urticaria or maculopapular rash after taking penicillin are particularly challenging.30 A study of patients in a primary care pediatrics practice found that 7.4% of children receiving a prescription for a penicillin reported a rash.32 Here, timing of onset of symptoms provides some clarity about the likelihood of true allergy. Rashes that manifest during the first hours after exposure are more likely to be IgE mediated, particularly when accompanied by other systemic symptoms; they should be considered high risk. Delayed-onset rashes (> 72 hours after exposure) are usually non-IgE mediated and therefore are generally lower risk,8,30,33 except when associated with certain features, such as mucosal involvement and skin peeling.
Despite acknowledging viral exanthems in the differential, many physicians still label patients presenting with any rash as “allergic.”28 Take care to look for other potential causes of a rash; for example, patients taking amoxicillin who have concurrent Epstein-Barr virus infection frequently develop a maculopapular rash.34 Caubet and colleagues found that 56% of pediatric patients with a history of nonimmediate rash and a negative oral challenge to amoxicillin tested positive for viral infection.28
A family history of penicillin allergy alone should not preclude the use of penicillin.8,27,31 Similarly, if a patient has already received and tolerated a subsequent course of the same penicillin derivative after the initial reaction, the “penicillin allergy” label can be removed. If the reaction history is unknown, refer the patient to an allergist for further evaluation.
Accurate charting is key. With most hospital systems and physician practices now documenting in an electronic health record, there exists the ability to document, in great detail, patients’ reactions to medications. Previous studies have found, however, that such documentation is often done poorly, or not done at all. One such study found that (1) > 20% of patients with a “penicillin allergy” label did not have reaction details listed and (2) when reactions were listed, many were incorrectly labeled as “allergy,” not “intolerance.”35
Many electronic health record systems lump drug allergies, adverse effects, and food and environmental allergies into a single section, leading to a lack of distinction between adverse reactions and true allergy.31 Although many PCPs report that it is easy to change a patient’s allergy label in the record,29 more often, a nurse, resident, or consultant actually documents the reaction.35
Continue to: Documentation at the time of the reaction...
Documentation at the time of the reaction, within the encounter note and the allergy tab, is essential, so that other physicians caring for the patient, in the future, will be knowledgeable about the details of the reaction. Make it your responsibility to accurately document penicillin allergy in patients’ charts, including removing the “penicillin allergy” label from the chart of patients whose history is inconsistent with allergy, who have tolerated subsequent courses of the same penicillin derivative, or who have passed testing in an allergist’s office. In a study of 639 patients who tested negative for penicillin allergy, 51% still had a “penicillin allergy” label in their chart more than 4 years later.36
Penicillin allergy evaluation. When a patient cannot be cleared of a “penicillin allergy” label by history alone, and in the absence of severe features such as mucous membrane involvement, they should be further evaluated through objective testing for potential IgE-mediated allergy. This assessment includes penicillin skin testing or an oral challenge, or both.
Skin testing involves skin-prick testing of major and minor determinants of penicillin; when skin-prick testing is negative, intradermal testing of major and minor determinants should follow. The negative predictive value of penicillin skin testing is high: In a prospective, multicenter investigation, researchers demonstrated that, when both the major penicillin determinant and a minor determinant mixture were used, negative predictive value was 97.9%.37
However, a minor determinant mixture is not commercially available in the United States; therefore, penicillin G is often used alone as the minor determinant. Typically, if a patient passes skin testing, a challenge dose of penicillin or amoxicillin is administered, followed by an observation period. The risk of re-sensitization after oral penicillin is thought to be low and does not preclude future use.38
Although drug testing is most often performed in an allergist’s office, several groups have developed protocols that allow for limited testing of low-risk patients in a primary care setting.8,31 For example, several studies have demonstrated that patients presenting with low-risk skin rash can be safely tested with a supervised oral challenge alone.18,28 The FIGURE8,27,30,31,33,34 outlines our proposed workflow for risk stratification and subsequent management of patients with a “penicillin allergy” label.

Continue to: De-labeling requires a systems approach
De-labeling requires a systems approach. Given the mismatch between the large number of patients labeled “penicillin allergic” and the few allergy specialists, referral alone is not enough to solve the problem of mislabeling. Targeting specific populations for testing, such as patients presenting to an inner-city sexually transmitted infection clinic19 or preoperative patients, as is done at the Mayo Clinic,9 has been successful. Skin testing in an inpatient setting has also been shown to be safe and effective,13 allowing for protocol-driven testing under the supervision of trained pharmacists (and others), to relieve the burden on allergy specialists.9
CORRESPONDENCE
Andrew Lutzkanin, MD, 500 University Drive, PO Box 850, Hershey, PA 17033; [email protected]
1. Macy E. The clinical evaluation of penicillin allergy: what is necessary, sufficient and safe given the materials currently available? Clin Exp Allergy. 2011;41:1498-1501. doi: 10.1111/j.1365-2222.2011.03837.x
2. Shenoy ES, Macy E, Rowe T, et al. Evaluation and management of penicillin allergy: a review. JAMA. 2019;321:188-199. doi: 10.1001/jama.2018.19283
3. Blumenthal KG, Li Y, Banerji A, et al. The cost of penicillin allergy evaluation. J Allergy Clin Immunol Pract. 2018;6:1019-1027.e2. doi: 10.1016/j.jaip.2017.08.006
4. American Academy of Allergy, Asthma & Immunology: Ten things physicians and patients should question. American Board of Medicine Foundation Choosing Wisely website. 2018. Accessed July 7, 2021. www.choosingwisely.org/doctor-patient-lists/american-academy-of-allergy-asthma-immunology
5. Tan SY, Tatsumura Y. Alexander Fleming (1881-1955): discoverer of penicillin. Singapore Med J. 2015;56:366-367. doi: 10.11622/smedj.2015105
6. Marston HD, Dixon DM, Knisely JM, et al. Antimicrobial resistance. JAMA. 2016;316:1193-1204. doi: 10.1001/jama.2016.11764
7. Spinks A, Glasziou PP, Del Mar CB. Antibiotics for sore throat. Cochrane Database Syst Rev. 2013;2013:CD000023. doi: 10.1002/14651858.CD000023.pub4
8. Castells M, Khan DA, Phillips EJ. Penicillin allergy. N Engl J Med. 2019;381:2338-2351. doi: 10.1056/NEJMra1807761
9. Khan DA. Proactive management of penicillin and other antibiotic allergies. Allergy Asthma Proc. 2020;41:82-89. doi: 10.2500/aap.2020.41.190024
10. Idsoe O, Guthe T, Willcox RR, et al. Nature and extent of penicillin side-reactions, with particular reference to fatalities from anaphylactic shock. Bull World Health Organ. 1968;38:159-188.
11. Chiriac AM, Macy E. Large health system databases and drug hypersensitivity. J Allergy Clin Immunol Pract. 2019;7:2125-2131. doi: 10.1016/j.jaip.2019.04.014
12. Albin S, Agarwal S. Prevalence and characteristics of reported penicillin allergy in an urban outpatient adult population. Allergy Asthma Proc. 2014;35:489-494. doi: 10.2500/aap.2014.35.3791
13. Sacco KA, Bates A, Brigham TJ, et al. Clinical outcomes following inpatient penicillin allergy testing: a systematic review and meta-analysis. Allergy. 2017;72:1288-1296. doi: 10.1111/all.13168
14. Khan DA, Solensky R. Drug allergy. J Allergy Clin Immunol. 2010;125(2 suppl 2):S126-S137. doi: 10.1016/j.jaci.2009.10.028
15. Blumenthal KG, Shenoy ES, Varughese CA, et al. Impact of a clinical guideline for prescribing antibiotics to inpatients reporting penicillin or cephalosporin allergy. Ann Allergy Asthma Immunol. 2015;115:294-300.e2. doi: 10.1016/j.anai.2015.05.011
16. Macy E, Schatz M, Lin C, et al. The falling rate of positive penicillin skin tests from 1995 to 2007. Perm J. 2009;13:12-18. doi: 10.7812/tpp/08-073
17. Macy E, Ngor EW. Safely diagnosing clinically significant penicillin allergy using only penicilloyl-poly-lysine, penicillin, and oral amoxicillin. J Allergy Clin Immunol Pract. 2013;1:258-263. doi: 10.1016/j.jaip.2013.02.002
18. Bourke J, Pavlos R, James I, et al. Improving the effectiveness of penicillin allergy de-labeling. J Allergy Clin Immunol Pract. 2015;3:365-374.e1. doi: 10.1016/j.jaip.2014.11.002
19. Gadde J, Spence M, Wheeler B, et al. Clinical experience with penicillin skin testing in a large inner-city STD clinic. JAMA. 1993;270:2456-2463.
20. Klaustermeyer WB, Gowda VC. Penicillin skin testing: a 20-year study at the West Los Angeles Veterans Affairs Medical Center. Mil Med. 2005;170:701-704. doi: 10.7205/milmed.170.8.701.
21. McDanel JS, Perencevich EN, Diekema DJ, et al. Comparative effectiveness of beta-lactams versus vancomycin for treatment of methicillin-susceptible Staphylococcus aureus bloodstream infections among 122 hospitals. Clin Infect Dis. 2015;61:361-367. doi: 10.1093/cid/civ308
22. Blumenthal KG, Ryan EE, Li Y, et al. The impact of a reported penicillin allergy on surgical site infection risk. Clin Infect Dis. 2018;66:329-336. doi: 10.1093/cid/cix794
23. Mattingly TJ 2nd, Fulton A, Lumish RA, et al. The cost of self-reported penicillin allergy: a systematic review. J Allergy Clin Immunol Pract. 2018;6:1649-1654.e4. doi: 10.1016/j.jaip.2017.12.033
24. Macy E, Contreras R. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: a cohort study. J Allergy Clin Immunol. 2014;133:790-796. doi: 10.1016/j.jaci.2013.09.021
25. Desai SH, Kaplan MS, Chen Q, et al. Morbidity in pregnant women associated with unverified penicillin allergies, antibiotic use, and group B streptococcus infections. Perm J. 2017;21:16-80. doi: 10.7812/TPP/16-080
26. Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52:e56-e93. doi: 10.1093/cid/cir073
27. Vyles D, Mistry RD, Heffner V, et al. Reported knowledge and management of potential penicillin allergy in children. Acad Pediatr. 2019;19:684-690. doi: 10.1016/j.acap.2019.01.002
28. Caubet J-C, Kaiser L, Lemaître B, et al. The role of penicillin in benign skin rashes in childhood: a prospective study based on drug rechallenge. J Allergy Clin Immunol. 2011;127:218-222. doi: 10.1016/j.jaci.2010.08.025
29. Wanat M, Anthierens S, Butler CC, et al. Patient and primary care physician perceptions of penicillin allergy testing and subsequent use of penicillin-containing antibiotics: a qualitative study. J Allergy Clin Immunol Pract. 2019;7:1888-1893.e1. doi: 10.1016/j.jaip.2019.02.036
30. Norton AE, Konvinse K, Phillips EJ, et al. Antibiotic allergy in pediatrics. Pediatrics. 2018;141: e20172497. doi: 10.1542/peds.2017-2497
31. Collins C. The low risks and high rewards of penicillin allergy delabeling: an algorithm to expedite the evaluation. J Pediatr. 2019;212:216-223. doi: 10.1016/j.jpeds.2019.05.060
32. Ibia EO, Schwartz RH, Wiedermann BL. Antibiotic rashes in children: a survey in a private practice setting. Arch Dermatol. 2000;136:849-854. doi: 10.1001/archderm.136.7.849
33. Salkind AR, Cuddy PG, Foxworth JW. The rational clinical examination. Is this patient allergic to penicillin? An evidence-based analysis of the likelihood of penicillin allergy. JAMA. 2001;285:2498-2505. doi: 10.1001/jama.285.19.2498
34. Patel BM. Skin rash with infectious mononucleosis and ampicillin. Pediatrics. 1967;40:910-911.
35. Inglis JM, Caughey GE, Smith W, et al. Documentation of penicillin adverse drug reactions in electronic health records: inconsistent use of allergy and intolerance labels. Intern Med J. 2017;47:1292-1297. doi: 10.1111/imj.13558
36. Lachover-Roth I, Sharon S, Rosman Y, et al. Long-term follow-up after penicillin allergy delabeling in ambulatory patients. J Allergy Clin Immunol Pract. 2019;7:231-235.e1. doi: 10.1016/j.jaip.2018.04.042
37. Solensky R, Jacobs J, Lester M, et al. Penicillin allergy evaluation: a prospective, multicenter, open-label evaluation of a comprehensive penicillin skin test kit. J Allergy Clin Immunol Pract. 2019;7:1876-1885.e3. doi: 10.1016/j.jaip.2019.02.040
38. A; ; . Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105:259-273. doi: 10.1016/j.anai.2010.08.002
1. Macy E. The clinical evaluation of penicillin allergy: what is necessary, sufficient and safe given the materials currently available? Clin Exp Allergy. 2011;41:1498-1501. doi: 10.1111/j.1365-2222.2011.03837.x
2. Shenoy ES, Macy E, Rowe T, et al. Evaluation and management of penicillin allergy: a review. JAMA. 2019;321:188-199. doi: 10.1001/jama.2018.19283
3. Blumenthal KG, Li Y, Banerji A, et al. The cost of penicillin allergy evaluation. J Allergy Clin Immunol Pract. 2018;6:1019-1027.e2. doi: 10.1016/j.jaip.2017.08.006
4. American Academy of Allergy, Asthma & Immunology: Ten things physicians and patients should question. American Board of Medicine Foundation Choosing Wisely website. 2018. Accessed July 7, 2021. www.choosingwisely.org/doctor-patient-lists/american-academy-of-allergy-asthma-immunology
5. Tan SY, Tatsumura Y. Alexander Fleming (1881-1955): discoverer of penicillin. Singapore Med J. 2015;56:366-367. doi: 10.11622/smedj.2015105
6. Marston HD, Dixon DM, Knisely JM, et al. Antimicrobial resistance. JAMA. 2016;316:1193-1204. doi: 10.1001/jama.2016.11764
7. Spinks A, Glasziou PP, Del Mar CB. Antibiotics for sore throat. Cochrane Database Syst Rev. 2013;2013:CD000023. doi: 10.1002/14651858.CD000023.pub4
8. Castells M, Khan DA, Phillips EJ. Penicillin allergy. N Engl J Med. 2019;381:2338-2351. doi: 10.1056/NEJMra1807761
9. Khan DA. Proactive management of penicillin and other antibiotic allergies. Allergy Asthma Proc. 2020;41:82-89. doi: 10.2500/aap.2020.41.190024
10. Idsoe O, Guthe T, Willcox RR, et al. Nature and extent of penicillin side-reactions, with particular reference to fatalities from anaphylactic shock. Bull World Health Organ. 1968;38:159-188.
11. Chiriac AM, Macy E. Large health system databases and drug hypersensitivity. J Allergy Clin Immunol Pract. 2019;7:2125-2131. doi: 10.1016/j.jaip.2019.04.014
12. Albin S, Agarwal S. Prevalence and characteristics of reported penicillin allergy in an urban outpatient adult population. Allergy Asthma Proc. 2014;35:489-494. doi: 10.2500/aap.2014.35.3791
13. Sacco KA, Bates A, Brigham TJ, et al. Clinical outcomes following inpatient penicillin allergy testing: a systematic review and meta-analysis. Allergy. 2017;72:1288-1296. doi: 10.1111/all.13168
14. Khan DA, Solensky R. Drug allergy. J Allergy Clin Immunol. 2010;125(2 suppl 2):S126-S137. doi: 10.1016/j.jaci.2009.10.028
15. Blumenthal KG, Shenoy ES, Varughese CA, et al. Impact of a clinical guideline for prescribing antibiotics to inpatients reporting penicillin or cephalosporin allergy. Ann Allergy Asthma Immunol. 2015;115:294-300.e2. doi: 10.1016/j.anai.2015.05.011
16. Macy E, Schatz M, Lin C, et al. The falling rate of positive penicillin skin tests from 1995 to 2007. Perm J. 2009;13:12-18. doi: 10.7812/tpp/08-073
17. Macy E, Ngor EW. Safely diagnosing clinically significant penicillin allergy using only penicilloyl-poly-lysine, penicillin, and oral amoxicillin. J Allergy Clin Immunol Pract. 2013;1:258-263. doi: 10.1016/j.jaip.2013.02.002
18. Bourke J, Pavlos R, James I, et al. Improving the effectiveness of penicillin allergy de-labeling. J Allergy Clin Immunol Pract. 2015;3:365-374.e1. doi: 10.1016/j.jaip.2014.11.002
19. Gadde J, Spence M, Wheeler B, et al. Clinical experience with penicillin skin testing in a large inner-city STD clinic. JAMA. 1993;270:2456-2463.
20. Klaustermeyer WB, Gowda VC. Penicillin skin testing: a 20-year study at the West Los Angeles Veterans Affairs Medical Center. Mil Med. 2005;170:701-704. doi: 10.7205/milmed.170.8.701.
21. McDanel JS, Perencevich EN, Diekema DJ, et al. Comparative effectiveness of beta-lactams versus vancomycin for treatment of methicillin-susceptible Staphylococcus aureus bloodstream infections among 122 hospitals. Clin Infect Dis. 2015;61:361-367. doi: 10.1093/cid/civ308
22. Blumenthal KG, Ryan EE, Li Y, et al. The impact of a reported penicillin allergy on surgical site infection risk. Clin Infect Dis. 2018;66:329-336. doi: 10.1093/cid/cix794
23. Mattingly TJ 2nd, Fulton A, Lumish RA, et al. The cost of self-reported penicillin allergy: a systematic review. J Allergy Clin Immunol Pract. 2018;6:1649-1654.e4. doi: 10.1016/j.jaip.2017.12.033
24. Macy E, Contreras R. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: a cohort study. J Allergy Clin Immunol. 2014;133:790-796. doi: 10.1016/j.jaci.2013.09.021
25. Desai SH, Kaplan MS, Chen Q, et al. Morbidity in pregnant women associated with unverified penicillin allergies, antibiotic use, and group B streptococcus infections. Perm J. 2017;21:16-80. doi: 10.7812/TPP/16-080
26. Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52:e56-e93. doi: 10.1093/cid/cir073
27. Vyles D, Mistry RD, Heffner V, et al. Reported knowledge and management of potential penicillin allergy in children. Acad Pediatr. 2019;19:684-690. doi: 10.1016/j.acap.2019.01.002
28. Caubet J-C, Kaiser L, Lemaître B, et al. The role of penicillin in benign skin rashes in childhood: a prospective study based on drug rechallenge. J Allergy Clin Immunol. 2011;127:218-222. doi: 10.1016/j.jaci.2010.08.025
29. Wanat M, Anthierens S, Butler CC, et al. Patient and primary care physician perceptions of penicillin allergy testing and subsequent use of penicillin-containing antibiotics: a qualitative study. J Allergy Clin Immunol Pract. 2019;7:1888-1893.e1. doi: 10.1016/j.jaip.2019.02.036
30. Norton AE, Konvinse K, Phillips EJ, et al. Antibiotic allergy in pediatrics. Pediatrics. 2018;141: e20172497. doi: 10.1542/peds.2017-2497
31. Collins C. The low risks and high rewards of penicillin allergy delabeling: an algorithm to expedite the evaluation. J Pediatr. 2019;212:216-223. doi: 10.1016/j.jpeds.2019.05.060
32. Ibia EO, Schwartz RH, Wiedermann BL. Antibiotic rashes in children: a survey in a private practice setting. Arch Dermatol. 2000;136:849-854. doi: 10.1001/archderm.136.7.849
33. Salkind AR, Cuddy PG, Foxworth JW. The rational clinical examination. Is this patient allergic to penicillin? An evidence-based analysis of the likelihood of penicillin allergy. JAMA. 2001;285:2498-2505. doi: 10.1001/jama.285.19.2498
34. Patel BM. Skin rash with infectious mononucleosis and ampicillin. Pediatrics. 1967;40:910-911.
35. Inglis JM, Caughey GE, Smith W, et al. Documentation of penicillin adverse drug reactions in electronic health records: inconsistent use of allergy and intolerance labels. Intern Med J. 2017;47:1292-1297. doi: 10.1111/imj.13558
36. Lachover-Roth I, Sharon S, Rosman Y, et al. Long-term follow-up after penicillin allergy delabeling in ambulatory patients. J Allergy Clin Immunol Pract. 2019;7:231-235.e1. doi: 10.1016/j.jaip.2018.04.042
37. Solensky R, Jacobs J, Lester M, et al. Penicillin allergy evaluation: a prospective, multicenter, open-label evaluation of a comprehensive penicillin skin test kit. J Allergy Clin Immunol Pract. 2019;7:1876-1885.e3. doi: 10.1016/j.jaip.2019.02.040
38. A; ; . Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105:259-273. doi: 10.1016/j.anai.2010.08.002
PRACTICE RECOMMENDATIONS
› Obtain an accurate drug allergy history from all patients who have a listed penicillin allergy. B
› De-label penicillin allergy in patients who report symptoms of an adverse reaction (diarrhea, headache, or nausea) but who (1) do not have other systemic symptoms; (2) do have a family history, but no personal history, of a reaction; or (3) have tolerated the same penicillin derivative since the initial reaction. B
› Refer patients whose reaction history includes hives, shortness of breath, or other allergic-type signs and symptoms for potential skin testing or oral challenge, or both. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Emerging data point to underlying autoimmunity in ME/CFS
Emerging evidence suggests that autoimmunity plays a role in postinfectious myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and that targeting autoantibodies could be a promising treatment approach.
The same may also apply to many cases of “long COVID,” in which many of the symptoms overlap with those of ME/CFS, Carmen Scheibenbogen, MD, professor of clinical immunology and director of the Institute for Medical Immunology, Charité University Medicine, Berlin, said during the annual meeting of the International Association for Chronic Fatigue Syndrome/Myalgic Encephalomyelitis.
Several groups, including Dr. Scheibenbogen’s, have reported finding autoantibodies against neurotransmitter receptor antigens in people with ME/CFS. And, in a paper published in the Journal of Clinical Medicine the day that Dr. Scheibenbogen spoke at the meeting, her team reported significant correlations between autoantibodies to vasoregulative G-protein–coupled receptors and symptom severity, autonomic dysfunction, and disability among 116 patients with infection-triggered ME/CFS who were diagnosed using the symptom-based 2003 Canadian consensus criteria.
People with ME/CFS are also more likely to have genetic risk factors associated with autoimmunity and personal and/or family histories of autoimmune conditions. And, clinical trials have demonstrated early success with various immunomodulatory treatments in subsets of people with ME/CFS, including endoxan, rituximab, and immunoadsorption.
“We have evidence that ME/CFS is an autoantibody-mediated disease, and we have evidence that autoantibody targeting is effective in this disease. So far ... we have few and underfinanced clinical studies, but the good news is we have promising emerging treatment options,” Dr. Scheibenbogen said.
Asked to comment, ME/CFS expert Anthony L. Komaroff, MD, of Brigham and Women’s Hospital and Harvard Medical School, both in Boston, said: “There is already strong evidence that there are autoantibodies in ME/CFS. Dr. Scheibenbogen’s work is the latest and employs the latest technology. ... I would bet that autoantibodies to neural targets are likely to cause some of the symptoms of ME/CFS and some of the symptoms of long COVID.”
However, he cautioned, “that has not been proven, and even if it were proven you would have to demonstrate that treatments based on that theory worked.”
Dr. Komaroff said he views autoimmunity as a likely component of the ME/CFS spectrum, but not the only one. “My current view of this illness is that there’s a final common pathway in the brain that leads to the symptoms of the illness. But that final common pathway can be triggered by a variety of different things, one of which could be autoantibodies while another could be infection or inflammation in the brain.”
Emerging evidence points to autoimmunity
Dr. Scheibenbogen summarized the work published in this area over the past few years by her group and others.
In a comparison of ME/CFS patients with 201 healthy controls, significant associations were seen with two specific autoimmunity-related risk alleles only in the ME/CFS patients who reported acute onset of disease with an infection but not in those with ME/CFS without infection-triggered onset or the controls. Both genes play roles in regulating B- and T-cell activation.
Another recent study found associations with ME/CFS and major histocompatibility complex class II molecules, a typical feature of autoimmune diseases, in a comparison between 426 adult Norwegian ME/CFS patients who were diagnosed with the Canadian consensus criteria and 4,511 healthy, ethnically matched controls.
In a 2020 paper, Dr. Scheibenbogen and pharmacologist Klaus Wirth presented a “unifying hypothesis” of ME/CFS pathophysiology based on the finding of elevations in autoantibodies against beta2-adrenergic receptors and muscarinic acetylcholine receptors in some individuals with the condition. Since both of those receptors are important vasodilators, their functional disturbance would be expected to cause vasoconstriction and hypoxemia, which would explain many of the symptoms of ME/CFS. This mechanism would align with other findings of muscular and cerebral hypoperfusion that correlate with fatigue, particularly post exertion, as well as metabolic changes that are in line with the concepts of hypoxemia and ischemia.
Further evidence for vascular dysfunction in ME/CFS came from her group’s study finding evidence of peripheral endothelial dysfunction that was associated with symptom severity in 35 adult patients. “Vasoconstriction, hypovolemia, and release of vasoactive and algesic mediators is probably a key pathomechanism of the disease,” Dr. Scheibenbogen said.
Treatments: Will targeting autoantibodies work?
In the second part of her talk, Dr. Scheibenbogen summarized clinical trials of the following treatment approaches that involve targeting autoantibodies as a way to alleviate ME/CFS symptoms:
Rituximab: Work on infusions of the B-cell depleting agent has been conducted by Norwegian researchers beginning in 2011 with a small randomized trial and an open-label, phase 2 study in 2015, both showing clinical responses in ME/CFS. However, a subsequent phase 3, randomized clinical trial of 151 patients, again diagnosed using the Canadian criteria, was negative.
There are several possible explanations for this, Dr. Scheibenbogen noted. For one, the maintenance dose had to be reduced because of a lack of financial support. “This was probably critical. The lower dose was insufficient to adequately deplete B cells.” Also, there may have been a strong placebo response in the control group since they were being given better care than they normally would receive during the trial. “I think probably nobody will again do a rituximab trial. This was very disappointing for all of us. But, we still have other opportunities to follow this path,” she said.
Dr. Komaroff agreed. “I don’t think the failure of one drug that hits malignant B cells is proof against the autoimmune hypothesis per se. I think the evidence is that rituximab doesn’t work, but that doesn’t invalidate the autoimmunity hypothesis.”
Cyclophosphamide: The same Norwegian group also showed positive findings in an open-label, phase 2 trial of the immune-modifying drug cyclophosphamide in 22 of 40 patients. Interestingly, HLA risk alleles were much more common in responders than nonresponders, Dr. Scheibenbogen noted.
Immunoadsorption: This technique, similar to dialysis, involves separating out the blood plasma by centrifugation and removing IgG autoantibodies by a binding column, then returning the plasma back to the patient. It is used, primarily in Europe, to treat severe autoimmune diseases including dilative cardiomyopathy and refractory systemic lupus erythematosus (SLE).
Dr. Scheibenbogen’s group has conducted two studies of immunoadsorption in ME/CFS. In one, a 5-day procedure led to rapid symptom improvement in 7 of 10 patients, with sustained improvement in 3 patients after 2 years. Autoantibodies decreased rapidly in 9 of the 10 patients. In a follow-up study of five of the responders 2 years later, retreatment with a modified immunoadsorption protocol led to rapid and sustained improvement in four. Further study has been on hold because of the pandemic.
Next-gen IgG-targeting therapies: Another approach that could offer promise for ME/CFS involves therapies that block the Fc receptors of IgG. Several are in phase 1-3 trials for autoimmune conditions. One candidate drug, the Fc fragment efgartigimod, is currently in phase 3 trials for several conditions, including generalized myasthenia gravis, primary immune thrombocytopenia, and chronic inflammatory demyelinating polyneuropathy. Phase 3 trials are planned for the monoclonal antibody rozanolixizumab in those same conditions.
Newer-generation monoclonal antibodies targeting CD19 or CD20 that show benefit in various autoimmune conditions are another possibility for ME/CFS. These include ocrelizumab (Ocrevus), approved in the United States for treating relapsing and progressive multiple sclerosis and in trials for SLE; obinutuzumab (Gazyva), approved for treating lymphoma and also in development for SLE; and ublituximab, in phase 3 trials for multiple sclerosis.
“Most of them are more effective than rituximab,” Dr. Scheibenbogen noted, adding that “currently the data look quite promising. They are effective in different autoimmune diseases and they are quite well tolerated. There’s great hope now with COVID-19 that we can convince some companies to do such trials in ME/CFS as well.”
Dr. Scheibenbogen’s institution, the Charité Fatigue Center, has a patent for beta2-adrenergic receptor antibodies for diagnosing ME/CFS under her name together with Celltrend. Dr. Komaroff has received personal fees from Serimmune.
Emerging evidence suggests that autoimmunity plays a role in postinfectious myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and that targeting autoantibodies could be a promising treatment approach.
The same may also apply to many cases of “long COVID,” in which many of the symptoms overlap with those of ME/CFS, Carmen Scheibenbogen, MD, professor of clinical immunology and director of the Institute for Medical Immunology, Charité University Medicine, Berlin, said during the annual meeting of the International Association for Chronic Fatigue Syndrome/Myalgic Encephalomyelitis.
Several groups, including Dr. Scheibenbogen’s, have reported finding autoantibodies against neurotransmitter receptor antigens in people with ME/CFS. And, in a paper published in the Journal of Clinical Medicine the day that Dr. Scheibenbogen spoke at the meeting, her team reported significant correlations between autoantibodies to vasoregulative G-protein–coupled receptors and symptom severity, autonomic dysfunction, and disability among 116 patients with infection-triggered ME/CFS who were diagnosed using the symptom-based 2003 Canadian consensus criteria.
People with ME/CFS are also more likely to have genetic risk factors associated with autoimmunity and personal and/or family histories of autoimmune conditions. And, clinical trials have demonstrated early success with various immunomodulatory treatments in subsets of people with ME/CFS, including endoxan, rituximab, and immunoadsorption.
“We have evidence that ME/CFS is an autoantibody-mediated disease, and we have evidence that autoantibody targeting is effective in this disease. So far ... we have few and underfinanced clinical studies, but the good news is we have promising emerging treatment options,” Dr. Scheibenbogen said.
Asked to comment, ME/CFS expert Anthony L. Komaroff, MD, of Brigham and Women’s Hospital and Harvard Medical School, both in Boston, said: “There is already strong evidence that there are autoantibodies in ME/CFS. Dr. Scheibenbogen’s work is the latest and employs the latest technology. ... I would bet that autoantibodies to neural targets are likely to cause some of the symptoms of ME/CFS and some of the symptoms of long COVID.”
However, he cautioned, “that has not been proven, and even if it were proven you would have to demonstrate that treatments based on that theory worked.”
Dr. Komaroff said he views autoimmunity as a likely component of the ME/CFS spectrum, but not the only one. “My current view of this illness is that there’s a final common pathway in the brain that leads to the symptoms of the illness. But that final common pathway can be triggered by a variety of different things, one of which could be autoantibodies while another could be infection or inflammation in the brain.”
Emerging evidence points to autoimmunity
Dr. Scheibenbogen summarized the work published in this area over the past few years by her group and others.
In a comparison of ME/CFS patients with 201 healthy controls, significant associations were seen with two specific autoimmunity-related risk alleles only in the ME/CFS patients who reported acute onset of disease with an infection but not in those with ME/CFS without infection-triggered onset or the controls. Both genes play roles in regulating B- and T-cell activation.
Another recent study found associations with ME/CFS and major histocompatibility complex class II molecules, a typical feature of autoimmune diseases, in a comparison between 426 adult Norwegian ME/CFS patients who were diagnosed with the Canadian consensus criteria and 4,511 healthy, ethnically matched controls.
In a 2020 paper, Dr. Scheibenbogen and pharmacologist Klaus Wirth presented a “unifying hypothesis” of ME/CFS pathophysiology based on the finding of elevations in autoantibodies against beta2-adrenergic receptors and muscarinic acetylcholine receptors in some individuals with the condition. Since both of those receptors are important vasodilators, their functional disturbance would be expected to cause vasoconstriction and hypoxemia, which would explain many of the symptoms of ME/CFS. This mechanism would align with other findings of muscular and cerebral hypoperfusion that correlate with fatigue, particularly post exertion, as well as metabolic changes that are in line with the concepts of hypoxemia and ischemia.
Further evidence for vascular dysfunction in ME/CFS came from her group’s study finding evidence of peripheral endothelial dysfunction that was associated with symptom severity in 35 adult patients. “Vasoconstriction, hypovolemia, and release of vasoactive and algesic mediators is probably a key pathomechanism of the disease,” Dr. Scheibenbogen said.
Treatments: Will targeting autoantibodies work?
In the second part of her talk, Dr. Scheibenbogen summarized clinical trials of the following treatment approaches that involve targeting autoantibodies as a way to alleviate ME/CFS symptoms:
Rituximab: Work on infusions of the B-cell depleting agent has been conducted by Norwegian researchers beginning in 2011 with a small randomized trial and an open-label, phase 2 study in 2015, both showing clinical responses in ME/CFS. However, a subsequent phase 3, randomized clinical trial of 151 patients, again diagnosed using the Canadian criteria, was negative.
There are several possible explanations for this, Dr. Scheibenbogen noted. For one, the maintenance dose had to be reduced because of a lack of financial support. “This was probably critical. The lower dose was insufficient to adequately deplete B cells.” Also, there may have been a strong placebo response in the control group since they were being given better care than they normally would receive during the trial. “I think probably nobody will again do a rituximab trial. This was very disappointing for all of us. But, we still have other opportunities to follow this path,” she said.
Dr. Komaroff agreed. “I don’t think the failure of one drug that hits malignant B cells is proof against the autoimmune hypothesis per se. I think the evidence is that rituximab doesn’t work, but that doesn’t invalidate the autoimmunity hypothesis.”
Cyclophosphamide: The same Norwegian group also showed positive findings in an open-label, phase 2 trial of the immune-modifying drug cyclophosphamide in 22 of 40 patients. Interestingly, HLA risk alleles were much more common in responders than nonresponders, Dr. Scheibenbogen noted.
Immunoadsorption: This technique, similar to dialysis, involves separating out the blood plasma by centrifugation and removing IgG autoantibodies by a binding column, then returning the plasma back to the patient. It is used, primarily in Europe, to treat severe autoimmune diseases including dilative cardiomyopathy and refractory systemic lupus erythematosus (SLE).
Dr. Scheibenbogen’s group has conducted two studies of immunoadsorption in ME/CFS. In one, a 5-day procedure led to rapid symptom improvement in 7 of 10 patients, with sustained improvement in 3 patients after 2 years. Autoantibodies decreased rapidly in 9 of the 10 patients. In a follow-up study of five of the responders 2 years later, retreatment with a modified immunoadsorption protocol led to rapid and sustained improvement in four. Further study has been on hold because of the pandemic.
Next-gen IgG-targeting therapies: Another approach that could offer promise for ME/CFS involves therapies that block the Fc receptors of IgG. Several are in phase 1-3 trials for autoimmune conditions. One candidate drug, the Fc fragment efgartigimod, is currently in phase 3 trials for several conditions, including generalized myasthenia gravis, primary immune thrombocytopenia, and chronic inflammatory demyelinating polyneuropathy. Phase 3 trials are planned for the monoclonal antibody rozanolixizumab in those same conditions.
Newer-generation monoclonal antibodies targeting CD19 or CD20 that show benefit in various autoimmune conditions are another possibility for ME/CFS. These include ocrelizumab (Ocrevus), approved in the United States for treating relapsing and progressive multiple sclerosis and in trials for SLE; obinutuzumab (Gazyva), approved for treating lymphoma and also in development for SLE; and ublituximab, in phase 3 trials for multiple sclerosis.
“Most of them are more effective than rituximab,” Dr. Scheibenbogen noted, adding that “currently the data look quite promising. They are effective in different autoimmune diseases and they are quite well tolerated. There’s great hope now with COVID-19 that we can convince some companies to do such trials in ME/CFS as well.”
Dr. Scheibenbogen’s institution, the Charité Fatigue Center, has a patent for beta2-adrenergic receptor antibodies for diagnosing ME/CFS under her name together with Celltrend. Dr. Komaroff has received personal fees from Serimmune.
Emerging evidence suggests that autoimmunity plays a role in postinfectious myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and that targeting autoantibodies could be a promising treatment approach.
The same may also apply to many cases of “long COVID,” in which many of the symptoms overlap with those of ME/CFS, Carmen Scheibenbogen, MD, professor of clinical immunology and director of the Institute for Medical Immunology, Charité University Medicine, Berlin, said during the annual meeting of the International Association for Chronic Fatigue Syndrome/Myalgic Encephalomyelitis.
Several groups, including Dr. Scheibenbogen’s, have reported finding autoantibodies against neurotransmitter receptor antigens in people with ME/CFS. And, in a paper published in the Journal of Clinical Medicine the day that Dr. Scheibenbogen spoke at the meeting, her team reported significant correlations between autoantibodies to vasoregulative G-protein–coupled receptors and symptom severity, autonomic dysfunction, and disability among 116 patients with infection-triggered ME/CFS who were diagnosed using the symptom-based 2003 Canadian consensus criteria.
People with ME/CFS are also more likely to have genetic risk factors associated with autoimmunity and personal and/or family histories of autoimmune conditions. And, clinical trials have demonstrated early success with various immunomodulatory treatments in subsets of people with ME/CFS, including endoxan, rituximab, and immunoadsorption.
“We have evidence that ME/CFS is an autoantibody-mediated disease, and we have evidence that autoantibody targeting is effective in this disease. So far ... we have few and underfinanced clinical studies, but the good news is we have promising emerging treatment options,” Dr. Scheibenbogen said.
Asked to comment, ME/CFS expert Anthony L. Komaroff, MD, of Brigham and Women’s Hospital and Harvard Medical School, both in Boston, said: “There is already strong evidence that there are autoantibodies in ME/CFS. Dr. Scheibenbogen’s work is the latest and employs the latest technology. ... I would bet that autoantibodies to neural targets are likely to cause some of the symptoms of ME/CFS and some of the symptoms of long COVID.”
However, he cautioned, “that has not been proven, and even if it were proven you would have to demonstrate that treatments based on that theory worked.”
Dr. Komaroff said he views autoimmunity as a likely component of the ME/CFS spectrum, but not the only one. “My current view of this illness is that there’s a final common pathway in the brain that leads to the symptoms of the illness. But that final common pathway can be triggered by a variety of different things, one of which could be autoantibodies while another could be infection or inflammation in the brain.”
Emerging evidence points to autoimmunity
Dr. Scheibenbogen summarized the work published in this area over the past few years by her group and others.
In a comparison of ME/CFS patients with 201 healthy controls, significant associations were seen with two specific autoimmunity-related risk alleles only in the ME/CFS patients who reported acute onset of disease with an infection but not in those with ME/CFS without infection-triggered onset or the controls. Both genes play roles in regulating B- and T-cell activation.
Another recent study found associations with ME/CFS and major histocompatibility complex class II molecules, a typical feature of autoimmune diseases, in a comparison between 426 adult Norwegian ME/CFS patients who were diagnosed with the Canadian consensus criteria and 4,511 healthy, ethnically matched controls.
In a 2020 paper, Dr. Scheibenbogen and pharmacologist Klaus Wirth presented a “unifying hypothesis” of ME/CFS pathophysiology based on the finding of elevations in autoantibodies against beta2-adrenergic receptors and muscarinic acetylcholine receptors in some individuals with the condition. Since both of those receptors are important vasodilators, their functional disturbance would be expected to cause vasoconstriction and hypoxemia, which would explain many of the symptoms of ME/CFS. This mechanism would align with other findings of muscular and cerebral hypoperfusion that correlate with fatigue, particularly post exertion, as well as metabolic changes that are in line with the concepts of hypoxemia and ischemia.
Further evidence for vascular dysfunction in ME/CFS came from her group’s study finding evidence of peripheral endothelial dysfunction that was associated with symptom severity in 35 adult patients. “Vasoconstriction, hypovolemia, and release of vasoactive and algesic mediators is probably a key pathomechanism of the disease,” Dr. Scheibenbogen said.
Treatments: Will targeting autoantibodies work?
In the second part of her talk, Dr. Scheibenbogen summarized clinical trials of the following treatment approaches that involve targeting autoantibodies as a way to alleviate ME/CFS symptoms:
Rituximab: Work on infusions of the B-cell depleting agent has been conducted by Norwegian researchers beginning in 2011 with a small randomized trial and an open-label, phase 2 study in 2015, both showing clinical responses in ME/CFS. However, a subsequent phase 3, randomized clinical trial of 151 patients, again diagnosed using the Canadian criteria, was negative.
There are several possible explanations for this, Dr. Scheibenbogen noted. For one, the maintenance dose had to be reduced because of a lack of financial support. “This was probably critical. The lower dose was insufficient to adequately deplete B cells.” Also, there may have been a strong placebo response in the control group since they were being given better care than they normally would receive during the trial. “I think probably nobody will again do a rituximab trial. This was very disappointing for all of us. But, we still have other opportunities to follow this path,” she said.
Dr. Komaroff agreed. “I don’t think the failure of one drug that hits malignant B cells is proof against the autoimmune hypothesis per se. I think the evidence is that rituximab doesn’t work, but that doesn’t invalidate the autoimmunity hypothesis.”
Cyclophosphamide: The same Norwegian group also showed positive findings in an open-label, phase 2 trial of the immune-modifying drug cyclophosphamide in 22 of 40 patients. Interestingly, HLA risk alleles were much more common in responders than nonresponders, Dr. Scheibenbogen noted.
Immunoadsorption: This technique, similar to dialysis, involves separating out the blood plasma by centrifugation and removing IgG autoantibodies by a binding column, then returning the plasma back to the patient. It is used, primarily in Europe, to treat severe autoimmune diseases including dilative cardiomyopathy and refractory systemic lupus erythematosus (SLE).
Dr. Scheibenbogen’s group has conducted two studies of immunoadsorption in ME/CFS. In one, a 5-day procedure led to rapid symptom improvement in 7 of 10 patients, with sustained improvement in 3 patients after 2 years. Autoantibodies decreased rapidly in 9 of the 10 patients. In a follow-up study of five of the responders 2 years later, retreatment with a modified immunoadsorption protocol led to rapid and sustained improvement in four. Further study has been on hold because of the pandemic.
Next-gen IgG-targeting therapies: Another approach that could offer promise for ME/CFS involves therapies that block the Fc receptors of IgG. Several are in phase 1-3 trials for autoimmune conditions. One candidate drug, the Fc fragment efgartigimod, is currently in phase 3 trials for several conditions, including generalized myasthenia gravis, primary immune thrombocytopenia, and chronic inflammatory demyelinating polyneuropathy. Phase 3 trials are planned for the monoclonal antibody rozanolixizumab in those same conditions.
Newer-generation monoclonal antibodies targeting CD19 or CD20 that show benefit in various autoimmune conditions are another possibility for ME/CFS. These include ocrelizumab (Ocrevus), approved in the United States for treating relapsing and progressive multiple sclerosis and in trials for SLE; obinutuzumab (Gazyva), approved for treating lymphoma and also in development for SLE; and ublituximab, in phase 3 trials for multiple sclerosis.
“Most of them are more effective than rituximab,” Dr. Scheibenbogen noted, adding that “currently the data look quite promising. They are effective in different autoimmune diseases and they are quite well tolerated. There’s great hope now with COVID-19 that we can convince some companies to do such trials in ME/CFS as well.”
Dr. Scheibenbogen’s institution, the Charité Fatigue Center, has a patent for beta2-adrenergic receptor antibodies for diagnosing ME/CFS under her name together with Celltrend. Dr. Komaroff has received personal fees from Serimmune.
FROM IACFS/ME 2021
Peanut allergy patients reap continuing benefits past first year, Palforzia study shows
A recent analysis of 142 peanut-allergic children treated for 1.5 to 2 years with a licensed oral immunotherapy (OIT) product confirms what various smaller studies have shown: Maintaining treatment for longer periods improves protection and reduces adverse effects. The findings offer some reassurance regarding the controversial approach, which has become available at a small number of clinics yet faces an uncertain future.
The new study, published July 28 in Allergy, included a subset of patients who chose to complete an extension of the phase 3 PALISADE trial of Palforzia, a proprietary set of premeasured peanut flour capsules developed by Aimmune Therapeutics.
Palforzia was approved last year for children aged 4 to 17 years with peanut allergy – one of the most common food allergies, affecting around 2% of children in the United States and Europe. The treatment is not a cure – patients must still watch what they eat and carry epinephrine for emergency reactions – but it helps build protection through daily ingestion of gradually increasing amounts of the allergen over a period of months.
In the 1-year PALISADE trial, which enrolled 496 peanut-allergic children at 66 sites in North America and Europe, participants received daily doses of study drug or placebo. The dose of the drug was escalated from 3 mg to 300 mg over 6 months; the 300-mg dose was then maintained for another 6 months. By the end of the study, about two-thirds of the children who underwent treatment could safely consume at least 600 mg of peanut protein, about the equivalent of two peanuts.
Could protection be increased with further treatment, and what would be required to sustain it? To address these questions, PALISADE patients who successfully reached the 600-mg threshold, along with those from the placebo group, were invited to participate in Aimmune’s open-label follow-on study. The extension study also explored whether protection could be maintained with less frequent dosing.
Among the 358 eligible participants who opted into the 1-year extension study, 256 came from the PALISADE treatment arm. These children were assigned to five cohorts to continue for 6 months or 12 months with daily or less frequent doses. Within the 6-month group, all started with the 300-mg daily dose. A subset received two doses a week. Within the 12-month group, some patients maintained daily dosing throughout; others received doses every other day, twice weekly, or once every 2 weeks.
The children who continued daily maintenance dosing the longest gained the most protection. Those in less-frequent dosing groups experienced more adverse events than those who received doses every day, the company reported last December in The Journal of Allergy and Clinical Immunology: In Practice.
More than a quarter (97 of 358, or 27.1%) of participants failed to complete the extension. Families could withdraw any time for any reason. Participating in an OIT trial is demanding – it requires office visits for dosing adjustments and blood tests, rest periods, keeping symptom logs in which daily doses are recorded, and possible allergic reactions from the treatment itself. “A common reason for ‘withdrawal of consent’ in clinical studies is the inconvenience of remaining in a long-term study,” Mohamed Yassine, MD, Aimmune’s senior vice present of medical affairs, said via email.
Attrition was concentrated within certain subgroups. Most participants in (88.7%; 102 of 115) PALISADE who received placebo elected to enter the open-label extension; nearly half did not finish. Dropout rates were also high (29.2%) for non-daily dosing participants who had come from the PALISADE treatment arm.
The authors did not report on those high-dropout groups. Instead, they focused their analysis on the 142 treated PALISADE participants who continued daily dosing through the extension – 110 patients for a total of about 1.5 years and 32 patients for about 2 years. In a subgroup analysis, 48.1% of children in the 1.5-year group upped their tolerance to 2,000 mg peanut protein, and even more (80.8%) in the 2-year group reached that threshold – all while taking a 300-mg maintenance dose.
Those who remained on treatment longer also had fewer adverse events. At the exit food challenge, 24% of the 1.5-year participants had reactions that required epinephrine, but among 2-year participants, only 3.8% needed the rescue medication.
Continuing therapy past the first year seemed to have additional benefits, Sandra Hong, MD, director of the Cleveland Clinic Food Allergy Center of Excellence, said in an interview. Dr. Hong was not involved in the new research and has no financial ties with Aimmune or other food allergy companies. “Not only can you ingest more, but your reaction when you do react is going to be less,” she says.
Palforzia is only available through a risk evaluation and mitigation strategy (REMS) program, which educates patients, health care professionals, and pharmacies about immunotherapy risks and precautionary measures. As of last summer, before Aimmune was acquired by Nestlé Health Science, about 100 allergists in the United States had enrolled patients in the REMS program. Families can find allergists who are certified to prescribe Palforzia using the website’s Certified Participant Locator.
Although the field at large remains apprehensive about OIT and other forms of immunotherapy, an estimated 200 or more U.S. clinics are administering home-grown OIT using commercial food products, says Richard Wasserman, an OIT pioneer whose clinic in Dallas has treated allergies to about 20 foods since the practice started offering the therapy in 2008. OIT practitioners have treated more than 15,000 food allergy patients nationwide, Dr. Wasserman said via email, yet they make up just a tiny fraction of the more than 6,000 board-certified allergists in the United States.
Whether using Palforzia or nonproprietary food products, oral immunotherapy requires a lot of time and effort – not just for patients but also practitioners. “You need more space. You need more staffing. Patients doing oral challenges stay in your office for 4 to 5 hours, and we have one-to-one nursing care for them,” said Dr. Hong. “So it’s a lot of resources.”
Her team has treated about 20 children with Palforzia since the Cleveland Clinic began offering the therapy last summer. Dr. Hong and coworkers have administered OIT using commercial peanut flour and peanut butter to some 80 peanut-allergic toddlers younger than 4 years who are too young to receive for the U.S. Food and Drug Administration–approved treatment. Their early data, which were presented at the annual meeting of the American Academy of Allergy, Asthma, and Immunology in February, suggest that toddlers get complete OIT more quickly with fewer side effects than older children, Dr. Hong says. A recent study of preschoolers in Canada also found that nonproprietary OIT is very safe and effective in this younger set and could be cost-saving in the long run.
By comparison, Palforzia, which has a list price of $890 per month, was judged to be less cost-effective in analyses by academic allergists and by the Institute for Clinical and Economic Review. But through a copay savings program, depending on their insurance coverage, some eligible families can pay as little as $20 per month for the FDA-approved treatment.
Because the therapy is time consuming for families and is resource intensive for practices, questions remain as to how long and how frequently patients need to remain on treatment to sustain protection. Do they need to keep taking Palforzia, or “can we switch them to an equivalent amount of food and not bother with the study drug?” said Edwin Kim, director of the UNC Food Allergy Initiative, Chapel Hill, North Carolina, and study investigator for several Palforzia trials, in an interview.
The Food Allergy Support Team, a nonprofit group started by Dr. Wasserman and colleagues, publishes best practices and meets annually to discuss research and protocols. However, the best maintenance dose, the best dosing frequency, and the duration of daily dosing that yields the best outcomes are not known, Dr. Wasserman says.
“We think the best way to answer that question is with a regulated, pharmaceutical-grade form of peanut protein,” Dr. Yassine said.
The field’s experience with Palforzia raises a dilemma: Does its approval legitimize oral immunotherapy in general, or will rigorous, multi-million dollar trials be needed to approve products for each food or combination of foods? About 32 million people in the United States have food allergies – about 1 in 10 adults and 1 in 13 children.
“I think the field has always grappled with that, honestly,” said Stacie Jones, MD, professor of pediatrics and chief of allergy and immunology at the University of Arkansas for Medical Sciences and Arkansas Children’s Hospital, Little Rock, in an interview. Home-grown OIT is “easier to do when you have high control of your small patient volumes or you’re in a clinical trial,” said Dr. Jones, who has served as an investigator on Palforzia trials and last year received more than $30,000 in consulting fees from Aimmune. “It becomes a very different situation when it becomes a national or an international recommended therapy.”
The Canadian Society of Allergy and Clinical Immunology has published clinical practice guidelines and provides practical information on its website on how to implement OIT – including protocols for dozens of foods and diary sheets for patients to log doses and symptoms.
However, U.S. professional societies still consider OIT investigational and suggest that it will not be approved by the FDA. “As a field, are we willing to wait 4 to 5 more years for an egg product? Should we? Are we willing?” said Dr. Kim. “These are tough questions.”
Stacie M. Jones reports advisory board fees, Aimmune Therapeutics, FARE; personal fees, DBV Technologies; clinical trials grants, Aimmune Therapeutics, DBV Technologies, Astellas, Sanofi, Regeneron, FARE, Genentech, and NIH-NIAID. Edwin Kim reports consultancy with Aimmune Therapeutics, Allako, AllerGenis, Belhaven Pharma, DBV Technologies, Duke Clinical Research Institute, and Nutricia; advisory board membership with ALK, DBV Technologies, Kenota Health, and Ukko; grant support from the NIH’s National Institute of Allergy and Infectious Diseases, National Center for Complementary and Integrative Health and Immune Tolerance Network; Food Allergy Research and Education, and the Wallace Research Foundation. Richard Wasserman receives consulting fees from Aimmune Therapeutics and DBV Technologies. Mohamed Yassine is employed by Aimmune Therapeutics. Sandra Hong has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A recent analysis of 142 peanut-allergic children treated for 1.5 to 2 years with a licensed oral immunotherapy (OIT) product confirms what various smaller studies have shown: Maintaining treatment for longer periods improves protection and reduces adverse effects. The findings offer some reassurance regarding the controversial approach, which has become available at a small number of clinics yet faces an uncertain future.
The new study, published July 28 in Allergy, included a subset of patients who chose to complete an extension of the phase 3 PALISADE trial of Palforzia, a proprietary set of premeasured peanut flour capsules developed by Aimmune Therapeutics.
Palforzia was approved last year for children aged 4 to 17 years with peanut allergy – one of the most common food allergies, affecting around 2% of children in the United States and Europe. The treatment is not a cure – patients must still watch what they eat and carry epinephrine for emergency reactions – but it helps build protection through daily ingestion of gradually increasing amounts of the allergen over a period of months.
In the 1-year PALISADE trial, which enrolled 496 peanut-allergic children at 66 sites in North America and Europe, participants received daily doses of study drug or placebo. The dose of the drug was escalated from 3 mg to 300 mg over 6 months; the 300-mg dose was then maintained for another 6 months. By the end of the study, about two-thirds of the children who underwent treatment could safely consume at least 600 mg of peanut protein, about the equivalent of two peanuts.
Could protection be increased with further treatment, and what would be required to sustain it? To address these questions, PALISADE patients who successfully reached the 600-mg threshold, along with those from the placebo group, were invited to participate in Aimmune’s open-label follow-on study. The extension study also explored whether protection could be maintained with less frequent dosing.
Among the 358 eligible participants who opted into the 1-year extension study, 256 came from the PALISADE treatment arm. These children were assigned to five cohorts to continue for 6 months or 12 months with daily or less frequent doses. Within the 6-month group, all started with the 300-mg daily dose. A subset received two doses a week. Within the 12-month group, some patients maintained daily dosing throughout; others received doses every other day, twice weekly, or once every 2 weeks.
The children who continued daily maintenance dosing the longest gained the most protection. Those in less-frequent dosing groups experienced more adverse events than those who received doses every day, the company reported last December in The Journal of Allergy and Clinical Immunology: In Practice.
More than a quarter (97 of 358, or 27.1%) of participants failed to complete the extension. Families could withdraw any time for any reason. Participating in an OIT trial is demanding – it requires office visits for dosing adjustments and blood tests, rest periods, keeping symptom logs in which daily doses are recorded, and possible allergic reactions from the treatment itself. “A common reason for ‘withdrawal of consent’ in clinical studies is the inconvenience of remaining in a long-term study,” Mohamed Yassine, MD, Aimmune’s senior vice present of medical affairs, said via email.
Attrition was concentrated within certain subgroups. Most participants in (88.7%; 102 of 115) PALISADE who received placebo elected to enter the open-label extension; nearly half did not finish. Dropout rates were also high (29.2%) for non-daily dosing participants who had come from the PALISADE treatment arm.
The authors did not report on those high-dropout groups. Instead, they focused their analysis on the 142 treated PALISADE participants who continued daily dosing through the extension – 110 patients for a total of about 1.5 years and 32 patients for about 2 years. In a subgroup analysis, 48.1% of children in the 1.5-year group upped their tolerance to 2,000 mg peanut protein, and even more (80.8%) in the 2-year group reached that threshold – all while taking a 300-mg maintenance dose.
Those who remained on treatment longer also had fewer adverse events. At the exit food challenge, 24% of the 1.5-year participants had reactions that required epinephrine, but among 2-year participants, only 3.8% needed the rescue medication.
Continuing therapy past the first year seemed to have additional benefits, Sandra Hong, MD, director of the Cleveland Clinic Food Allergy Center of Excellence, said in an interview. Dr. Hong was not involved in the new research and has no financial ties with Aimmune or other food allergy companies. “Not only can you ingest more, but your reaction when you do react is going to be less,” she says.
Palforzia is only available through a risk evaluation and mitigation strategy (REMS) program, which educates patients, health care professionals, and pharmacies about immunotherapy risks and precautionary measures. As of last summer, before Aimmune was acquired by Nestlé Health Science, about 100 allergists in the United States had enrolled patients in the REMS program. Families can find allergists who are certified to prescribe Palforzia using the website’s Certified Participant Locator.
Although the field at large remains apprehensive about OIT and other forms of immunotherapy, an estimated 200 or more U.S. clinics are administering home-grown OIT using commercial food products, says Richard Wasserman, an OIT pioneer whose clinic in Dallas has treated allergies to about 20 foods since the practice started offering the therapy in 2008. OIT practitioners have treated more than 15,000 food allergy patients nationwide, Dr. Wasserman said via email, yet they make up just a tiny fraction of the more than 6,000 board-certified allergists in the United States.
Whether using Palforzia or nonproprietary food products, oral immunotherapy requires a lot of time and effort – not just for patients but also practitioners. “You need more space. You need more staffing. Patients doing oral challenges stay in your office for 4 to 5 hours, and we have one-to-one nursing care for them,” said Dr. Hong. “So it’s a lot of resources.”
Her team has treated about 20 children with Palforzia since the Cleveland Clinic began offering the therapy last summer. Dr. Hong and coworkers have administered OIT using commercial peanut flour and peanut butter to some 80 peanut-allergic toddlers younger than 4 years who are too young to receive for the U.S. Food and Drug Administration–approved treatment. Their early data, which were presented at the annual meeting of the American Academy of Allergy, Asthma, and Immunology in February, suggest that toddlers get complete OIT more quickly with fewer side effects than older children, Dr. Hong says. A recent study of preschoolers in Canada also found that nonproprietary OIT is very safe and effective in this younger set and could be cost-saving in the long run.
By comparison, Palforzia, which has a list price of $890 per month, was judged to be less cost-effective in analyses by academic allergists and by the Institute for Clinical and Economic Review. But through a copay savings program, depending on their insurance coverage, some eligible families can pay as little as $20 per month for the FDA-approved treatment.
Because the therapy is time consuming for families and is resource intensive for practices, questions remain as to how long and how frequently patients need to remain on treatment to sustain protection. Do they need to keep taking Palforzia, or “can we switch them to an equivalent amount of food and not bother with the study drug?” said Edwin Kim, director of the UNC Food Allergy Initiative, Chapel Hill, North Carolina, and study investigator for several Palforzia trials, in an interview.
The Food Allergy Support Team, a nonprofit group started by Dr. Wasserman and colleagues, publishes best practices and meets annually to discuss research and protocols. However, the best maintenance dose, the best dosing frequency, and the duration of daily dosing that yields the best outcomes are not known, Dr. Wasserman says.
“We think the best way to answer that question is with a regulated, pharmaceutical-grade form of peanut protein,” Dr. Yassine said.
The field’s experience with Palforzia raises a dilemma: Does its approval legitimize oral immunotherapy in general, or will rigorous, multi-million dollar trials be needed to approve products for each food or combination of foods? About 32 million people in the United States have food allergies – about 1 in 10 adults and 1 in 13 children.
“I think the field has always grappled with that, honestly,” said Stacie Jones, MD, professor of pediatrics and chief of allergy and immunology at the University of Arkansas for Medical Sciences and Arkansas Children’s Hospital, Little Rock, in an interview. Home-grown OIT is “easier to do when you have high control of your small patient volumes or you’re in a clinical trial,” said Dr. Jones, who has served as an investigator on Palforzia trials and last year received more than $30,000 in consulting fees from Aimmune. “It becomes a very different situation when it becomes a national or an international recommended therapy.”
The Canadian Society of Allergy and Clinical Immunology has published clinical practice guidelines and provides practical information on its website on how to implement OIT – including protocols for dozens of foods and diary sheets for patients to log doses and symptoms.
However, U.S. professional societies still consider OIT investigational and suggest that it will not be approved by the FDA. “As a field, are we willing to wait 4 to 5 more years for an egg product? Should we? Are we willing?” said Dr. Kim. “These are tough questions.”
Stacie M. Jones reports advisory board fees, Aimmune Therapeutics, FARE; personal fees, DBV Technologies; clinical trials grants, Aimmune Therapeutics, DBV Technologies, Astellas, Sanofi, Regeneron, FARE, Genentech, and NIH-NIAID. Edwin Kim reports consultancy with Aimmune Therapeutics, Allako, AllerGenis, Belhaven Pharma, DBV Technologies, Duke Clinical Research Institute, and Nutricia; advisory board membership with ALK, DBV Technologies, Kenota Health, and Ukko; grant support from the NIH’s National Institute of Allergy and Infectious Diseases, National Center for Complementary and Integrative Health and Immune Tolerance Network; Food Allergy Research and Education, and the Wallace Research Foundation. Richard Wasserman receives consulting fees from Aimmune Therapeutics and DBV Technologies. Mohamed Yassine is employed by Aimmune Therapeutics. Sandra Hong has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A recent analysis of 142 peanut-allergic children treated for 1.5 to 2 years with a licensed oral immunotherapy (OIT) product confirms what various smaller studies have shown: Maintaining treatment for longer periods improves protection and reduces adverse effects. The findings offer some reassurance regarding the controversial approach, which has become available at a small number of clinics yet faces an uncertain future.
The new study, published July 28 in Allergy, included a subset of patients who chose to complete an extension of the phase 3 PALISADE trial of Palforzia, a proprietary set of premeasured peanut flour capsules developed by Aimmune Therapeutics.
Palforzia was approved last year for children aged 4 to 17 years with peanut allergy – one of the most common food allergies, affecting around 2% of children in the United States and Europe. The treatment is not a cure – patients must still watch what they eat and carry epinephrine for emergency reactions – but it helps build protection through daily ingestion of gradually increasing amounts of the allergen over a period of months.
In the 1-year PALISADE trial, which enrolled 496 peanut-allergic children at 66 sites in North America and Europe, participants received daily doses of study drug or placebo. The dose of the drug was escalated from 3 mg to 300 mg over 6 months; the 300-mg dose was then maintained for another 6 months. By the end of the study, about two-thirds of the children who underwent treatment could safely consume at least 600 mg of peanut protein, about the equivalent of two peanuts.
Could protection be increased with further treatment, and what would be required to sustain it? To address these questions, PALISADE patients who successfully reached the 600-mg threshold, along with those from the placebo group, were invited to participate in Aimmune’s open-label follow-on study. The extension study also explored whether protection could be maintained with less frequent dosing.
Among the 358 eligible participants who opted into the 1-year extension study, 256 came from the PALISADE treatment arm. These children were assigned to five cohorts to continue for 6 months or 12 months with daily or less frequent doses. Within the 6-month group, all started with the 300-mg daily dose. A subset received two doses a week. Within the 12-month group, some patients maintained daily dosing throughout; others received doses every other day, twice weekly, or once every 2 weeks.
The children who continued daily maintenance dosing the longest gained the most protection. Those in less-frequent dosing groups experienced more adverse events than those who received doses every day, the company reported last December in The Journal of Allergy and Clinical Immunology: In Practice.
More than a quarter (97 of 358, or 27.1%) of participants failed to complete the extension. Families could withdraw any time for any reason. Participating in an OIT trial is demanding – it requires office visits for dosing adjustments and blood tests, rest periods, keeping symptom logs in which daily doses are recorded, and possible allergic reactions from the treatment itself. “A common reason for ‘withdrawal of consent’ in clinical studies is the inconvenience of remaining in a long-term study,” Mohamed Yassine, MD, Aimmune’s senior vice present of medical affairs, said via email.
Attrition was concentrated within certain subgroups. Most participants in (88.7%; 102 of 115) PALISADE who received placebo elected to enter the open-label extension; nearly half did not finish. Dropout rates were also high (29.2%) for non-daily dosing participants who had come from the PALISADE treatment arm.
The authors did not report on those high-dropout groups. Instead, they focused their analysis on the 142 treated PALISADE participants who continued daily dosing through the extension – 110 patients for a total of about 1.5 years and 32 patients for about 2 years. In a subgroup analysis, 48.1% of children in the 1.5-year group upped their tolerance to 2,000 mg peanut protein, and even more (80.8%) in the 2-year group reached that threshold – all while taking a 300-mg maintenance dose.
Those who remained on treatment longer also had fewer adverse events. At the exit food challenge, 24% of the 1.5-year participants had reactions that required epinephrine, but among 2-year participants, only 3.8% needed the rescue medication.
Continuing therapy past the first year seemed to have additional benefits, Sandra Hong, MD, director of the Cleveland Clinic Food Allergy Center of Excellence, said in an interview. Dr. Hong was not involved in the new research and has no financial ties with Aimmune or other food allergy companies. “Not only can you ingest more, but your reaction when you do react is going to be less,” she says.
Palforzia is only available through a risk evaluation and mitigation strategy (REMS) program, which educates patients, health care professionals, and pharmacies about immunotherapy risks and precautionary measures. As of last summer, before Aimmune was acquired by Nestlé Health Science, about 100 allergists in the United States had enrolled patients in the REMS program. Families can find allergists who are certified to prescribe Palforzia using the website’s Certified Participant Locator.
Although the field at large remains apprehensive about OIT and other forms of immunotherapy, an estimated 200 or more U.S. clinics are administering home-grown OIT using commercial food products, says Richard Wasserman, an OIT pioneer whose clinic in Dallas has treated allergies to about 20 foods since the practice started offering the therapy in 2008. OIT practitioners have treated more than 15,000 food allergy patients nationwide, Dr. Wasserman said via email, yet they make up just a tiny fraction of the more than 6,000 board-certified allergists in the United States.
Whether using Palforzia or nonproprietary food products, oral immunotherapy requires a lot of time and effort – not just for patients but also practitioners. “You need more space. You need more staffing. Patients doing oral challenges stay in your office for 4 to 5 hours, and we have one-to-one nursing care for them,” said Dr. Hong. “So it’s a lot of resources.”
Her team has treated about 20 children with Palforzia since the Cleveland Clinic began offering the therapy last summer. Dr. Hong and coworkers have administered OIT using commercial peanut flour and peanut butter to some 80 peanut-allergic toddlers younger than 4 years who are too young to receive for the U.S. Food and Drug Administration–approved treatment. Their early data, which were presented at the annual meeting of the American Academy of Allergy, Asthma, and Immunology in February, suggest that toddlers get complete OIT more quickly with fewer side effects than older children, Dr. Hong says. A recent study of preschoolers in Canada also found that nonproprietary OIT is very safe and effective in this younger set and could be cost-saving in the long run.
By comparison, Palforzia, which has a list price of $890 per month, was judged to be less cost-effective in analyses by academic allergists and by the Institute for Clinical and Economic Review. But through a copay savings program, depending on their insurance coverage, some eligible families can pay as little as $20 per month for the FDA-approved treatment.
Because the therapy is time consuming for families and is resource intensive for practices, questions remain as to how long and how frequently patients need to remain on treatment to sustain protection. Do they need to keep taking Palforzia, or “can we switch them to an equivalent amount of food and not bother with the study drug?” said Edwin Kim, director of the UNC Food Allergy Initiative, Chapel Hill, North Carolina, and study investigator for several Palforzia trials, in an interview.
The Food Allergy Support Team, a nonprofit group started by Dr. Wasserman and colleagues, publishes best practices and meets annually to discuss research and protocols. However, the best maintenance dose, the best dosing frequency, and the duration of daily dosing that yields the best outcomes are not known, Dr. Wasserman says.
“We think the best way to answer that question is with a regulated, pharmaceutical-grade form of peanut protein,” Dr. Yassine said.
The field’s experience with Palforzia raises a dilemma: Does its approval legitimize oral immunotherapy in general, or will rigorous, multi-million dollar trials be needed to approve products for each food or combination of foods? About 32 million people in the United States have food allergies – about 1 in 10 adults and 1 in 13 children.
“I think the field has always grappled with that, honestly,” said Stacie Jones, MD, professor of pediatrics and chief of allergy and immunology at the University of Arkansas for Medical Sciences and Arkansas Children’s Hospital, Little Rock, in an interview. Home-grown OIT is “easier to do when you have high control of your small patient volumes or you’re in a clinical trial,” said Dr. Jones, who has served as an investigator on Palforzia trials and last year received more than $30,000 in consulting fees from Aimmune. “It becomes a very different situation when it becomes a national or an international recommended therapy.”
The Canadian Society of Allergy and Clinical Immunology has published clinical practice guidelines and provides practical information on its website on how to implement OIT – including protocols for dozens of foods and diary sheets for patients to log doses and symptoms.
However, U.S. professional societies still consider OIT investigational and suggest that it will not be approved by the FDA. “As a field, are we willing to wait 4 to 5 more years for an egg product? Should we? Are we willing?” said Dr. Kim. “These are tough questions.”
Stacie M. Jones reports advisory board fees, Aimmune Therapeutics, FARE; personal fees, DBV Technologies; clinical trials grants, Aimmune Therapeutics, DBV Technologies, Astellas, Sanofi, Regeneron, FARE, Genentech, and NIH-NIAID. Edwin Kim reports consultancy with Aimmune Therapeutics, Allako, AllerGenis, Belhaven Pharma, DBV Technologies, Duke Clinical Research Institute, and Nutricia; advisory board membership with ALK, DBV Technologies, Kenota Health, and Ukko; grant support from the NIH’s National Institute of Allergy and Infectious Diseases, National Center for Complementary and Integrative Health and Immune Tolerance Network; Food Allergy Research and Education, and the Wallace Research Foundation. Richard Wasserman receives consulting fees from Aimmune Therapeutics and DBV Technologies. Mohamed Yassine is employed by Aimmune Therapeutics. Sandra Hong has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Exposure to marijuana smoke linked to increased risk of respiratory infections in children
Exposure to secondhand marijuana smoke is more strongly associated with viral respiratory infections in children, compared with children who were exposed to tobacco smoke and those with no smoke exposure, new research shows.
“The findings of this study are interesting and pleasantly raise further questions,” said Kristen Miller, MD, attending physician in the division of pulmonary and sleep medicine at Children’s Hospital of Philadelphia, who was not involved in the study. “Given the robust literature regarding secondhand smoke exposure and the current landscape surrounding marijuana, this is a timely study to evaluate the prevalence of marijuana use and the associated effects of marijuana exposure among children.”
Prior research has linked primary marijuana use with respiratory effects. A 2020 study associated cannabis use with an increased risk of severe bronchitis, lung hyperinflation, and increased central airway resistance. However, according to the Centers for Disease Control and Prevention, there are still a lot of unanswered questions surrounding secondhand marijuana smoke exposure and its effects.
“If kids are exposed to enough secondhand smoke, regardless of what the substance is, they’re going to have some negative health outcomes with it,” study author Adam Johnson, MD, of Wake Forest University, Winston-Salem, N.C., said in an interview.
The study, published in Pediatric Research, looked at rates of reported ED and urgent care visits and specific illnesses – such as otitis media, viral respiratory infections, and asthma exacerbations – among children with marijuana exposure and tobacco exposure.
For the study, Dr. Johnson and colleagues surveyed 1,500 parents and caregivers who went to an academic children’s hospital between Dec. 1, 2015, and July 30, 2017. Researchers found that children exposed to marijuana smoke had higher rates of ED visits at 2.21 within the past 12 months, compared with those exposed to tobacco smoke (2.14 within the past 12 months) and those with no smoke exposure (1.94 within the past 12 months). However, the difference in these visits were not statistically significant.
Researchers saw that children exposed to secondhand marijuana smoke saw a 30% increase in viral respiratory infections, compared with those who were not exposed to tobacco or marijuana smoke, Dr. Johnson said. Caregivers who smoked marijuana reported a rate of 1.31 viral infections in their children within the last year. Meanwhile those who smoked tobacco reported a rate of 1.00 infections within the last 12 months and caregivers who did not smoke reported 1.04 infections within the year.
“It suggests that components in marijuana smoke may depress the body’s immune responses to viral infections in children,” Dr. Miller said in an interview.
When it came to otitis media episodes, children exposed to marijuana had a rate of 0.96 episodes within the past 12 months. Children experiencing secondhand tobacco smoke had a rate of 0.83 episodes and those with no smoke exposure had 0.75 episodes within the past 12 months. Researchers did not note this difference as statistically significant.
When it came to asthma exacerbations, children exposed to marijuana smoke also had statistically insignificantly higher rates of exacerbations, compared with those exposed to tobacco smoke and those not exposed to smoke.
“I think it was surprising that the survey results found that marijuana seemed to be more strongly associated with the viral respiratory infections than tobacco,” Dr. Johnson said. “We know that secondhand tobacco smoke exposure in kids does lead to things like otitis media or ear infections, asthma attacks, and other processes, including colds. It was interesting that we didn’t find that association [in the new study], but we found that with marijuana.”
Dr. Johnson said the findings are especially concerning with increases in the acceptance and accessibility of marijuana as it becomes legalized in many states.
A 2015 study examined the effect of secondhand marijuana smoke exposure. Researchers found that exposure to secondhand marijuana smoke can increase heart rate, have mild to moderate sedative effects and can produce detectable cannabinoid levels in blood and urine. However, another study published in 2012 found that low to moderate primary marijuana use is less harmful to users’ lungs than tobacco exposure.
Dr. Miller added that little is known about how exposure to marijuana smoke can affect the innate responses to pathogens and there is a need to “study this in more detail” to figure out if secondhand marijuana smoke is a risk factor for either an increase in respiratory virus infections or their severity.
“These questions could have considerable implications for the health of our children and public health measures regarding marijuana use,” she explained. “As documented marijuana use increases, health care providers need to be aware of the effects of marijuana use and exposure.”
Neither Dr. Johnson nor Dr. Miller has any relevant financial disclosures.
Exposure to secondhand marijuana smoke is more strongly associated with viral respiratory infections in children, compared with children who were exposed to tobacco smoke and those with no smoke exposure, new research shows.
“The findings of this study are interesting and pleasantly raise further questions,” said Kristen Miller, MD, attending physician in the division of pulmonary and sleep medicine at Children’s Hospital of Philadelphia, who was not involved in the study. “Given the robust literature regarding secondhand smoke exposure and the current landscape surrounding marijuana, this is a timely study to evaluate the prevalence of marijuana use and the associated effects of marijuana exposure among children.”
Prior research has linked primary marijuana use with respiratory effects. A 2020 study associated cannabis use with an increased risk of severe bronchitis, lung hyperinflation, and increased central airway resistance. However, according to the Centers for Disease Control and Prevention, there are still a lot of unanswered questions surrounding secondhand marijuana smoke exposure and its effects.
“If kids are exposed to enough secondhand smoke, regardless of what the substance is, they’re going to have some negative health outcomes with it,” study author Adam Johnson, MD, of Wake Forest University, Winston-Salem, N.C., said in an interview.
The study, published in Pediatric Research, looked at rates of reported ED and urgent care visits and specific illnesses – such as otitis media, viral respiratory infections, and asthma exacerbations – among children with marijuana exposure and tobacco exposure.
For the study, Dr. Johnson and colleagues surveyed 1,500 parents and caregivers who went to an academic children’s hospital between Dec. 1, 2015, and July 30, 2017. Researchers found that children exposed to marijuana smoke had higher rates of ED visits at 2.21 within the past 12 months, compared with those exposed to tobacco smoke (2.14 within the past 12 months) and those with no smoke exposure (1.94 within the past 12 months). However, the difference in these visits were not statistically significant.
Researchers saw that children exposed to secondhand marijuana smoke saw a 30% increase in viral respiratory infections, compared with those who were not exposed to tobacco or marijuana smoke, Dr. Johnson said. Caregivers who smoked marijuana reported a rate of 1.31 viral infections in their children within the last year. Meanwhile those who smoked tobacco reported a rate of 1.00 infections within the last 12 months and caregivers who did not smoke reported 1.04 infections within the year.
“It suggests that components in marijuana smoke may depress the body’s immune responses to viral infections in children,” Dr. Miller said in an interview.
When it came to otitis media episodes, children exposed to marijuana had a rate of 0.96 episodes within the past 12 months. Children experiencing secondhand tobacco smoke had a rate of 0.83 episodes and those with no smoke exposure had 0.75 episodes within the past 12 months. Researchers did not note this difference as statistically significant.
When it came to asthma exacerbations, children exposed to marijuana smoke also had statistically insignificantly higher rates of exacerbations, compared with those exposed to tobacco smoke and those not exposed to smoke.
“I think it was surprising that the survey results found that marijuana seemed to be more strongly associated with the viral respiratory infections than tobacco,” Dr. Johnson said. “We know that secondhand tobacco smoke exposure in kids does lead to things like otitis media or ear infections, asthma attacks, and other processes, including colds. It was interesting that we didn’t find that association [in the new study], but we found that with marijuana.”
Dr. Johnson said the findings are especially concerning with increases in the acceptance and accessibility of marijuana as it becomes legalized in many states.
A 2015 study examined the effect of secondhand marijuana smoke exposure. Researchers found that exposure to secondhand marijuana smoke can increase heart rate, have mild to moderate sedative effects and can produce detectable cannabinoid levels in blood and urine. However, another study published in 2012 found that low to moderate primary marijuana use is less harmful to users’ lungs than tobacco exposure.
Dr. Miller added that little is known about how exposure to marijuana smoke can affect the innate responses to pathogens and there is a need to “study this in more detail” to figure out if secondhand marijuana smoke is a risk factor for either an increase in respiratory virus infections or their severity.
“These questions could have considerable implications for the health of our children and public health measures regarding marijuana use,” she explained. “As documented marijuana use increases, health care providers need to be aware of the effects of marijuana use and exposure.”
Neither Dr. Johnson nor Dr. Miller has any relevant financial disclosures.
Exposure to secondhand marijuana smoke is more strongly associated with viral respiratory infections in children, compared with children who were exposed to tobacco smoke and those with no smoke exposure, new research shows.
“The findings of this study are interesting and pleasantly raise further questions,” said Kristen Miller, MD, attending physician in the division of pulmonary and sleep medicine at Children’s Hospital of Philadelphia, who was not involved in the study. “Given the robust literature regarding secondhand smoke exposure and the current landscape surrounding marijuana, this is a timely study to evaluate the prevalence of marijuana use and the associated effects of marijuana exposure among children.”
Prior research has linked primary marijuana use with respiratory effects. A 2020 study associated cannabis use with an increased risk of severe bronchitis, lung hyperinflation, and increased central airway resistance. However, according to the Centers for Disease Control and Prevention, there are still a lot of unanswered questions surrounding secondhand marijuana smoke exposure and its effects.
“If kids are exposed to enough secondhand smoke, regardless of what the substance is, they’re going to have some negative health outcomes with it,” study author Adam Johnson, MD, of Wake Forest University, Winston-Salem, N.C., said in an interview.
The study, published in Pediatric Research, looked at rates of reported ED and urgent care visits and specific illnesses – such as otitis media, viral respiratory infections, and asthma exacerbations – among children with marijuana exposure and tobacco exposure.
For the study, Dr. Johnson and colleagues surveyed 1,500 parents and caregivers who went to an academic children’s hospital between Dec. 1, 2015, and July 30, 2017. Researchers found that children exposed to marijuana smoke had higher rates of ED visits at 2.21 within the past 12 months, compared with those exposed to tobacco smoke (2.14 within the past 12 months) and those with no smoke exposure (1.94 within the past 12 months). However, the difference in these visits were not statistically significant.
Researchers saw that children exposed to secondhand marijuana smoke saw a 30% increase in viral respiratory infections, compared with those who were not exposed to tobacco or marijuana smoke, Dr. Johnson said. Caregivers who smoked marijuana reported a rate of 1.31 viral infections in their children within the last year. Meanwhile those who smoked tobacco reported a rate of 1.00 infections within the last 12 months and caregivers who did not smoke reported 1.04 infections within the year.
“It suggests that components in marijuana smoke may depress the body’s immune responses to viral infections in children,” Dr. Miller said in an interview.
When it came to otitis media episodes, children exposed to marijuana had a rate of 0.96 episodes within the past 12 months. Children experiencing secondhand tobacco smoke had a rate of 0.83 episodes and those with no smoke exposure had 0.75 episodes within the past 12 months. Researchers did not note this difference as statistically significant.
When it came to asthma exacerbations, children exposed to marijuana smoke also had statistically insignificantly higher rates of exacerbations, compared with those exposed to tobacco smoke and those not exposed to smoke.
“I think it was surprising that the survey results found that marijuana seemed to be more strongly associated with the viral respiratory infections than tobacco,” Dr. Johnson said. “We know that secondhand tobacco smoke exposure in kids does lead to things like otitis media or ear infections, asthma attacks, and other processes, including colds. It was interesting that we didn’t find that association [in the new study], but we found that with marijuana.”
Dr. Johnson said the findings are especially concerning with increases in the acceptance and accessibility of marijuana as it becomes legalized in many states.
A 2015 study examined the effect of secondhand marijuana smoke exposure. Researchers found that exposure to secondhand marijuana smoke can increase heart rate, have mild to moderate sedative effects and can produce detectable cannabinoid levels in blood and urine. However, another study published in 2012 found that low to moderate primary marijuana use is less harmful to users’ lungs than tobacco exposure.
Dr. Miller added that little is known about how exposure to marijuana smoke can affect the innate responses to pathogens and there is a need to “study this in more detail” to figure out if secondhand marijuana smoke is a risk factor for either an increase in respiratory virus infections or their severity.
“These questions could have considerable implications for the health of our children and public health measures regarding marijuana use,” she explained. “As documented marijuana use increases, health care providers need to be aware of the effects of marijuana use and exposure.”
Neither Dr. Johnson nor Dr. Miller has any relevant financial disclosures.
FROM PEDIATRIC RESEARCH
Summer campers spread COVID at home, follow-up finds
In a report published online in The New England Journal of Medicine, researchers found that campers spread COVID to household members after returning home – but transmission was more likely from some than others. Distancing and masking helped reduce the risk.
Victoria T. Chu, MD, MPH, with the Centers for Disease Control and Prevention, Atlanta, and colleagues with the agency and the Georgia Department of Health followed up with 224 camp attendees, aged 7 to 19 years, who had evidence of SARS-CoV-2 infection on laboratory testing.
These index patients – 88% of whom had symptoms – had 526 household contacts, mainly parents and siblings. Of 377 household contacts who underwent testing, 46 (12%) tested positive. Another two cases in household contacts were identified using clinical and epidemiologic criteria.
Family members hospitalized
Of the 41 adult household contacts who were infected, four (about 10%) were hospitalized. Their hospital stays ranged from 5 to 11 days. Of the seven infected household contacts who were younger than 18 years, none were hospitalized.
The four hospitalized adults were parents and grandparents aged 45 to 80 years, Dr. Chu said. Two of the four had underlying conditions. None of the household contacts died.
In an adjusted analysis, campers who had practiced physical distancing were less likely to transmit the virus at home, compared with those who had not practiced physical distancing (adjusted odds ratio, 0.4). Household members who had had close or direct contact with the index patients were more than 5 times more likely to become infected, compared with family members with minimal or no contact, analyses showed.
“This retrospective study showed that the efficient transmission of SARS-CoV-2 from school-age children and adolescents to household members led to the hospitalization of adults with secondary cases of COVID-19,” the researchers write. “In households in which transmission occurred, half the household contacts were infected.”
The secondary attack rates in this report may be an underestimate because testing was voluntary and participants reported the results themselves, the authors note. It is possible that infected household contacts spread the virus further, but this study did not address that question, Dr. Chu said.
For the study, investigators interviewed all camp attendees and their parents or guardians by phone between July 17, 2020 and Aug. 24, 2020, to collect information about demographic and clinical characteristics, SARS-CoV-2 testing, and preventive measures. The researchers’ analysis excluded households in which illness onset in a household contact occurred before or less than 2 days after a camper became sick.
About a third of the index patients began to have symptoms while still at camp. These campers may have been less infectious by the time they got home, compared with those whose symptoms started after they returned.
Two-thirds of the index patients adopted physical distancing at home, which “probably reduced the transmission of SARS-CoV-2 in the household,” Dr. Chu and colleagues wrote.
“Children who have had a known COVID-19 exposure should quarantine and obtain testing if they develop symptoms within the 14 days of returning home,” Dr. Chu advised. “If a child develops COVID-19, the child should be cared for and monitored using the proper combination of physical distancing, isolation when feasible, and mask use to prevent household transmission as much as possible. In addition, any person over the age of 12 is now eligible for vaccination in the United States. If eligible, children attending camp and their family members should get vaccinated to protect themselves and others, as vaccinations are our most effective public health prevention strategy.”
Mitigation can help
Another report regarding four overnight camps in Maine – in which three campers tested positive after they arrived last summer – shows that “aggressive mitigation strategies can be effective” in limiting transmission of the virus, William T. Basco Jr., MD, writes in a commentary for this news organization.
This summer, a range of factors, including vaccination rates at the camp, may influence transmission dynamics, Dr. Chu said in an interview. In July, the Associated Press reported outbreaks tied to summer camps in several states.
“Transmission dynamics will probably vary from summer camp to summer camp depending on many factors, such as vaccination rates of camp attendees, the mitigation measures in place, and the number of individual introductions during camp,” Dr. Chu said. “We would expect that a camp with a low vaccination rate among attendees and no enforcement of mitigation measures” still may experience a large outbreak.
“On the other hand, a large proportion of vaccinated individuals and appropriate implementation of multiple mitigation measures, such as wearing masks, may be quite effective at keeping their transmission rates low,” Dr. Chu added. “For camps with younger children who are not currently eligible for vaccination, implementing layered prevention strategies (e.g., mask use, physical distancing, and encouraging outdoor activities when feasible) is important to prevent transmission.”
Although COVID-19 transmission from children to adults, potentially leading to hospitalization, is not a new phenomenon, “data on the extent of transmission driven by children and adolescents in different settings are still quite sparse,” Dr. Chu said. “A better understanding of their impact on household and community transmission to help guide public health recommendations is particularly important, as most children are still not eligible for vaccination, and in-person schools will be reopening this fall.”
A version of this article first appeared on Medscape.com.
In a report published online in The New England Journal of Medicine, researchers found that campers spread COVID to household members after returning home – but transmission was more likely from some than others. Distancing and masking helped reduce the risk.
Victoria T. Chu, MD, MPH, with the Centers for Disease Control and Prevention, Atlanta, and colleagues with the agency and the Georgia Department of Health followed up with 224 camp attendees, aged 7 to 19 years, who had evidence of SARS-CoV-2 infection on laboratory testing.
These index patients – 88% of whom had symptoms – had 526 household contacts, mainly parents and siblings. Of 377 household contacts who underwent testing, 46 (12%) tested positive. Another two cases in household contacts were identified using clinical and epidemiologic criteria.
Family members hospitalized
Of the 41 adult household contacts who were infected, four (about 10%) were hospitalized. Their hospital stays ranged from 5 to 11 days. Of the seven infected household contacts who were younger than 18 years, none were hospitalized.
The four hospitalized adults were parents and grandparents aged 45 to 80 years, Dr. Chu said. Two of the four had underlying conditions. None of the household contacts died.
In an adjusted analysis, campers who had practiced physical distancing were less likely to transmit the virus at home, compared with those who had not practiced physical distancing (adjusted odds ratio, 0.4). Household members who had had close or direct contact with the index patients were more than 5 times more likely to become infected, compared with family members with minimal or no contact, analyses showed.
“This retrospective study showed that the efficient transmission of SARS-CoV-2 from school-age children and adolescents to household members led to the hospitalization of adults with secondary cases of COVID-19,” the researchers write. “In households in which transmission occurred, half the household contacts were infected.”
The secondary attack rates in this report may be an underestimate because testing was voluntary and participants reported the results themselves, the authors note. It is possible that infected household contacts spread the virus further, but this study did not address that question, Dr. Chu said.
For the study, investigators interviewed all camp attendees and their parents or guardians by phone between July 17, 2020 and Aug. 24, 2020, to collect information about demographic and clinical characteristics, SARS-CoV-2 testing, and preventive measures. The researchers’ analysis excluded households in which illness onset in a household contact occurred before or less than 2 days after a camper became sick.
About a third of the index patients began to have symptoms while still at camp. These campers may have been less infectious by the time they got home, compared with those whose symptoms started after they returned.
Two-thirds of the index patients adopted physical distancing at home, which “probably reduced the transmission of SARS-CoV-2 in the household,” Dr. Chu and colleagues wrote.
“Children who have had a known COVID-19 exposure should quarantine and obtain testing if they develop symptoms within the 14 days of returning home,” Dr. Chu advised. “If a child develops COVID-19, the child should be cared for and monitored using the proper combination of physical distancing, isolation when feasible, and mask use to prevent household transmission as much as possible. In addition, any person over the age of 12 is now eligible for vaccination in the United States. If eligible, children attending camp and their family members should get vaccinated to protect themselves and others, as vaccinations are our most effective public health prevention strategy.”
Mitigation can help
Another report regarding four overnight camps in Maine – in which three campers tested positive after they arrived last summer – shows that “aggressive mitigation strategies can be effective” in limiting transmission of the virus, William T. Basco Jr., MD, writes in a commentary for this news organization.
This summer, a range of factors, including vaccination rates at the camp, may influence transmission dynamics, Dr. Chu said in an interview. In July, the Associated Press reported outbreaks tied to summer camps in several states.
“Transmission dynamics will probably vary from summer camp to summer camp depending on many factors, such as vaccination rates of camp attendees, the mitigation measures in place, and the number of individual introductions during camp,” Dr. Chu said. “We would expect that a camp with a low vaccination rate among attendees and no enforcement of mitigation measures” still may experience a large outbreak.
“On the other hand, a large proportion of vaccinated individuals and appropriate implementation of multiple mitigation measures, such as wearing masks, may be quite effective at keeping their transmission rates low,” Dr. Chu added. “For camps with younger children who are not currently eligible for vaccination, implementing layered prevention strategies (e.g., mask use, physical distancing, and encouraging outdoor activities when feasible) is important to prevent transmission.”
Although COVID-19 transmission from children to adults, potentially leading to hospitalization, is not a new phenomenon, “data on the extent of transmission driven by children and adolescents in different settings are still quite sparse,” Dr. Chu said. “A better understanding of their impact on household and community transmission to help guide public health recommendations is particularly important, as most children are still not eligible for vaccination, and in-person schools will be reopening this fall.”
A version of this article first appeared on Medscape.com.
In a report published online in The New England Journal of Medicine, researchers found that campers spread COVID to household members after returning home – but transmission was more likely from some than others. Distancing and masking helped reduce the risk.
Victoria T. Chu, MD, MPH, with the Centers for Disease Control and Prevention, Atlanta, and colleagues with the agency and the Georgia Department of Health followed up with 224 camp attendees, aged 7 to 19 years, who had evidence of SARS-CoV-2 infection on laboratory testing.
These index patients – 88% of whom had symptoms – had 526 household contacts, mainly parents and siblings. Of 377 household contacts who underwent testing, 46 (12%) tested positive. Another two cases in household contacts were identified using clinical and epidemiologic criteria.
Family members hospitalized
Of the 41 adult household contacts who were infected, four (about 10%) were hospitalized. Their hospital stays ranged from 5 to 11 days. Of the seven infected household contacts who were younger than 18 years, none were hospitalized.
The four hospitalized adults were parents and grandparents aged 45 to 80 years, Dr. Chu said. Two of the four had underlying conditions. None of the household contacts died.
In an adjusted analysis, campers who had practiced physical distancing were less likely to transmit the virus at home, compared with those who had not practiced physical distancing (adjusted odds ratio, 0.4). Household members who had had close or direct contact with the index patients were more than 5 times more likely to become infected, compared with family members with minimal or no contact, analyses showed.
“This retrospective study showed that the efficient transmission of SARS-CoV-2 from school-age children and adolescents to household members led to the hospitalization of adults with secondary cases of COVID-19,” the researchers write. “In households in which transmission occurred, half the household contacts were infected.”
The secondary attack rates in this report may be an underestimate because testing was voluntary and participants reported the results themselves, the authors note. It is possible that infected household contacts spread the virus further, but this study did not address that question, Dr. Chu said.
For the study, investigators interviewed all camp attendees and their parents or guardians by phone between July 17, 2020 and Aug. 24, 2020, to collect information about demographic and clinical characteristics, SARS-CoV-2 testing, and preventive measures. The researchers’ analysis excluded households in which illness onset in a household contact occurred before or less than 2 days after a camper became sick.
About a third of the index patients began to have symptoms while still at camp. These campers may have been less infectious by the time they got home, compared with those whose symptoms started after they returned.
Two-thirds of the index patients adopted physical distancing at home, which “probably reduced the transmission of SARS-CoV-2 in the household,” Dr. Chu and colleagues wrote.
“Children who have had a known COVID-19 exposure should quarantine and obtain testing if they develop symptoms within the 14 days of returning home,” Dr. Chu advised. “If a child develops COVID-19, the child should be cared for and monitored using the proper combination of physical distancing, isolation when feasible, and mask use to prevent household transmission as much as possible. In addition, any person over the age of 12 is now eligible for vaccination in the United States. If eligible, children attending camp and their family members should get vaccinated to protect themselves and others, as vaccinations are our most effective public health prevention strategy.”
Mitigation can help
Another report regarding four overnight camps in Maine – in which three campers tested positive after they arrived last summer – shows that “aggressive mitigation strategies can be effective” in limiting transmission of the virus, William T. Basco Jr., MD, writes in a commentary for this news organization.
This summer, a range of factors, including vaccination rates at the camp, may influence transmission dynamics, Dr. Chu said in an interview. In July, the Associated Press reported outbreaks tied to summer camps in several states.
“Transmission dynamics will probably vary from summer camp to summer camp depending on many factors, such as vaccination rates of camp attendees, the mitigation measures in place, and the number of individual introductions during camp,” Dr. Chu said. “We would expect that a camp with a low vaccination rate among attendees and no enforcement of mitigation measures” still may experience a large outbreak.
“On the other hand, a large proportion of vaccinated individuals and appropriate implementation of multiple mitigation measures, such as wearing masks, may be quite effective at keeping their transmission rates low,” Dr. Chu added. “For camps with younger children who are not currently eligible for vaccination, implementing layered prevention strategies (e.g., mask use, physical distancing, and encouraging outdoor activities when feasible) is important to prevent transmission.”
Although COVID-19 transmission from children to adults, potentially leading to hospitalization, is not a new phenomenon, “data on the extent of transmission driven by children and adolescents in different settings are still quite sparse,” Dr. Chu said. “A better understanding of their impact on household and community transmission to help guide public health recommendations is particularly important, as most children are still not eligible for vaccination, and in-person schools will be reopening this fall.”
A version of this article first appeared on Medscape.com.
No link between childhood vaccinations and allergies or asthma
A meta-analysis by Australian researchers found no link between childhood vaccinations and an increase in allergies and asthma. In fact, children who received the BCG vaccine actually had a lesser incidence of eczema than other children, but there was no difference shown in any of the allergies or asthma.
The researchers, in a report published in the journal Allergy, write, “We found no evidence that childhood vaccination with commonly administered vaccines was associated with increased risk of later allergic disease.”
“Allergies have increased worldwide in the last 50 years, and in developed countries, earlier,” said study author Caroline J. Lodge, PhD, principal research fellow at the University of Melbourne, in an interview. “In developing countries, it is still a crisis.” No one knows why, she said. That was the reason for the recent study.
Allergic diseases such as allergic rhinitis (hay fever) and food allergies have a serious influence on quality of life, and the incidence is growing. According to the Global Asthma Network, there are 334 million people living with asthma. Between 2%-10% of adults have atopic eczema, and more than a 250,000 people have food allergies. This coincides temporally with an increase in mass vaccination of children.
Unlike the controversy surrounding vaccinations and autism, which has long been debunked as baseless, a hygiene hypothesis postulates that when children acquire immunity from many diseases, they become vulnerable to allergic reactions. Thanks to vaccinations, children in the developed world now are routinely immune to dozens of diseases.
That immunity leads to suppression of a major antibody response, increasing sensitivity to allergens and allergic disease. Suspicion of a link with childhood vaccinations has been used by opponents of vaccines in lobbying campaigns jeopardizing the sustainability of vaccine programs. In recent days, for example, the state of Tennessee has halted a program to encourage vaccination for COVID-19 as well as all other vaccinations, the result of pressure on the state by anti-vaccination lobbying.
But the Melbourne researchers reported that the meta-analysis of 42 published research studies doesn’t support the vaccine–allergy hypothesis. Using PubMed and EMBASE records between January 1946 and January 2018, researchers selected studies to be included in the analysis, looking for allergic outcomes in children given BCG or vaccines for measles or pertussis. Thirty-five publications reported cohort studies, and seven were based on randomized controlled trials.
The Australian study is not the only one showing the same lack of linkage between vaccination and allergy. The International Study of Asthma and Allergies in Childhood (ISAAC) found no association between mass vaccination and atopic disease. A 1998 Swedish study of 669 children found no differences in the incidence of allergic diseases between those who received pertussis vaccine and those who did not.
“The bottom line is that vaccines prevent infectious diseases,” said Matthew B. Laurens, associate professor of pediatrics at the University of Maryland, Baltimore, in an interview. Dr. Laurens was not part of the Australian study.
“Large-scale epidemiological studies do not support the theory that vaccines are associated with an increased risk of allergy or asthma,” he stressed. “Parents should not be deterred from vaccinating their children because of fears that this would increase risks of allergy and/or asthma.”
Dr. Lodge and Dr. Laurens have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A meta-analysis by Australian researchers found no link between childhood vaccinations and an increase in allergies and asthma. In fact, children who received the BCG vaccine actually had a lesser incidence of eczema than other children, but there was no difference shown in any of the allergies or asthma.
The researchers, in a report published in the journal Allergy, write, “We found no evidence that childhood vaccination with commonly administered vaccines was associated with increased risk of later allergic disease.”
“Allergies have increased worldwide in the last 50 years, and in developed countries, earlier,” said study author Caroline J. Lodge, PhD, principal research fellow at the University of Melbourne, in an interview. “In developing countries, it is still a crisis.” No one knows why, she said. That was the reason for the recent study.
Allergic diseases such as allergic rhinitis (hay fever) and food allergies have a serious influence on quality of life, and the incidence is growing. According to the Global Asthma Network, there are 334 million people living with asthma. Between 2%-10% of adults have atopic eczema, and more than a 250,000 people have food allergies. This coincides temporally with an increase in mass vaccination of children.
Unlike the controversy surrounding vaccinations and autism, which has long been debunked as baseless, a hygiene hypothesis postulates that when children acquire immunity from many diseases, they become vulnerable to allergic reactions. Thanks to vaccinations, children in the developed world now are routinely immune to dozens of diseases.
That immunity leads to suppression of a major antibody response, increasing sensitivity to allergens and allergic disease. Suspicion of a link with childhood vaccinations has been used by opponents of vaccines in lobbying campaigns jeopardizing the sustainability of vaccine programs. In recent days, for example, the state of Tennessee has halted a program to encourage vaccination for COVID-19 as well as all other vaccinations, the result of pressure on the state by anti-vaccination lobbying.
But the Melbourne researchers reported that the meta-analysis of 42 published research studies doesn’t support the vaccine–allergy hypothesis. Using PubMed and EMBASE records between January 1946 and January 2018, researchers selected studies to be included in the analysis, looking for allergic outcomes in children given BCG or vaccines for measles or pertussis. Thirty-five publications reported cohort studies, and seven were based on randomized controlled trials.
The Australian study is not the only one showing the same lack of linkage between vaccination and allergy. The International Study of Asthma and Allergies in Childhood (ISAAC) found no association between mass vaccination and atopic disease. A 1998 Swedish study of 669 children found no differences in the incidence of allergic diseases between those who received pertussis vaccine and those who did not.
“The bottom line is that vaccines prevent infectious diseases,” said Matthew B. Laurens, associate professor of pediatrics at the University of Maryland, Baltimore, in an interview. Dr. Laurens was not part of the Australian study.
“Large-scale epidemiological studies do not support the theory that vaccines are associated with an increased risk of allergy or asthma,” he stressed. “Parents should not be deterred from vaccinating their children because of fears that this would increase risks of allergy and/or asthma.”
Dr. Lodge and Dr. Laurens have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A meta-analysis by Australian researchers found no link between childhood vaccinations and an increase in allergies and asthma. In fact, children who received the BCG vaccine actually had a lesser incidence of eczema than other children, but there was no difference shown in any of the allergies or asthma.
The researchers, in a report published in the journal Allergy, write, “We found no evidence that childhood vaccination with commonly administered vaccines was associated with increased risk of later allergic disease.”
“Allergies have increased worldwide in the last 50 years, and in developed countries, earlier,” said study author Caroline J. Lodge, PhD, principal research fellow at the University of Melbourne, in an interview. “In developing countries, it is still a crisis.” No one knows why, she said. That was the reason for the recent study.
Allergic diseases such as allergic rhinitis (hay fever) and food allergies have a serious influence on quality of life, and the incidence is growing. According to the Global Asthma Network, there are 334 million people living with asthma. Between 2%-10% of adults have atopic eczema, and more than a 250,000 people have food allergies. This coincides temporally with an increase in mass vaccination of children.
Unlike the controversy surrounding vaccinations and autism, which has long been debunked as baseless, a hygiene hypothesis postulates that when children acquire immunity from many diseases, they become vulnerable to allergic reactions. Thanks to vaccinations, children in the developed world now are routinely immune to dozens of diseases.
That immunity leads to suppression of a major antibody response, increasing sensitivity to allergens and allergic disease. Suspicion of a link with childhood vaccinations has been used by opponents of vaccines in lobbying campaigns jeopardizing the sustainability of vaccine programs. In recent days, for example, the state of Tennessee has halted a program to encourage vaccination for COVID-19 as well as all other vaccinations, the result of pressure on the state by anti-vaccination lobbying.
But the Melbourne researchers reported that the meta-analysis of 42 published research studies doesn’t support the vaccine–allergy hypothesis. Using PubMed and EMBASE records between January 1946 and January 2018, researchers selected studies to be included in the analysis, looking for allergic outcomes in children given BCG or vaccines for measles or pertussis. Thirty-five publications reported cohort studies, and seven were based on randomized controlled trials.
The Australian study is not the only one showing the same lack of linkage between vaccination and allergy. The International Study of Asthma and Allergies in Childhood (ISAAC) found no association between mass vaccination and atopic disease. A 1998 Swedish study of 669 children found no differences in the incidence of allergic diseases between those who received pertussis vaccine and those who did not.
“The bottom line is that vaccines prevent infectious diseases,” said Matthew B. Laurens, associate professor of pediatrics at the University of Maryland, Baltimore, in an interview. Dr. Laurens was not part of the Australian study.
“Large-scale epidemiological studies do not support the theory that vaccines are associated with an increased risk of allergy or asthma,” he stressed. “Parents should not be deterred from vaccinating their children because of fears that this would increase risks of allergy and/or asthma.”
Dr. Lodge and Dr. Laurens have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Sublingual immunotherapy: Where does it stand?
Sublingual immunotherapy (SLIT) emerged over a century ago as a gentler alternative to allergy shots. It uses the same antigens found in allergy shots, delivering them through tablets or drops under the tongue rather than by injecting them into the skin.
Yet injection immunotherapy has been the mainstay of allergy treatment in the United States. Allergy shots are “the bread and butter, keeping the lights on at allergy practices,” said allergist Sakina Bajowala, MD, of Kaneland Allergy and Asthma Center, in the Chicago area. So even “when environmental SLIT showed quite clearly that it had efficacy, people were so slow to adapt.”
SLIT – a daily treatment that builds protection from allergens gradually over years with few side effects – is popular around the globe, particularly for environmental allergies. But only a handful of clinics offer food SLIT. Even though recent trials in peanut-allergic children show that SLIT is far safer than oral immunotherapy and about as effective as the Food and Drug Administration–approved peanut-allergy product and has lasting benefits for toddlers, many allergists lack experience with customized immunotherapies and hesitate to offer an unregulated treatment for which the evidence base is still emerging.
Why hasn’t food allergy SLIT caught on?
One issue is that there is scant evidence from randomized, controlled trials. The treatments that clinics offer often hinge on insurance coverage, and increasingly, insurers only cover FDA-approved products. FDA approval requires thousands of patients being enrolled in long, expensive studies to prove the treatment’s merit. In a similar vein, doctors are trained to question methods that lack a strong publication base, for good reason.
Yet SLIT caught the attention of pioneering physicians who were intrigued by this “low-and-slow” immune-modifying approach, despite limited published evidence, and they sought real-world experience.
The late physician David Morris, MD, came across SLIT in the 1960s while searching for alternative ways to help mold-allergic farmers who were suffering terrible side effects from allergy shots. Dr. Morris attended conferences, learned more about sublingual techniques, got board certified in allergy, and opened Allergy Associates of La Crosse (Wis.), in 1970 to offer SLIT as a treatment for food and environmental allergies.
Dr. Morris and colleagues developed a protocol to create custom SLIT drops tailored to individual patients’ clinical histories and allergy test results. The method has been used to treat more than 200,000 patients. It has been used by allergist Nikhila Schroeder, MD, MEng, who learned SLIT methods while treating nearly 1,000 patients at Allergy Associates. In 2018, she opened her own direct-care SLIT practice, Allergenuity Health, in the Charlotte metropolitan area of North Carolina (see part 2 of this series).
Dr. Bajowala’s clinic offers SLIT in addition to oral immunotherapy (OIT). She was encouraged by the recent toddler SLIT data but wondered whether it would translate to a real-world setting. According to her calculations, the published protocol – according to which participants receive up to 4 mg/d over 6 months and continue receiving a daily maintenance dose of 4 mg for 3 years – would cost $10,000 per patient.
With this dosing regimen, the intervention is unaffordable, Dr. Bajowala said. And “there’s no way to make it cheaper because that’s the raw materials cost. It does not include labor or bottles or profit at all. That’s just $10,000 in peanut extract.”
Owing to cost, Dr. Bajowala’s clinic generally uses SLIT as a bridge to OIT. Her food allergy patients receive up to 1 mg/d and remain at that dose for a month or so before transitioning to OIT, “for which the supplies are orders of magnitude cheaper,” she said.
Dr. Schroeder said there is evidence for efficacy at microgram and even nanogram dosing – much lower than used in the recent food SLIT trials. Maintenance doses range from 50 ng/d to 25 mcg/d for environmental SLIT and 4-37 mcg/d for food SLIT, she said. The La Crosse method uses even lower dose ranges.
However, dosing information is not readily available, Dr. Schroeder noted. She has spent years scrutinizing articles and compiling information from allergen extract suppliers – all the while treating hundreds of SLIT patients. “I have had to expend a lot of time and effort,” said Dr. Schroeder. “It’s really hard to explain quickly.”
In the published literature, SLIT dosing recommendations vary widely. According to a 2007 analysis, environmental allergy symptoms improved with doses over a 1,000-fold range. What’s more, success did not scale with increased dosing and seemed to depend more on frequency and duration of treatment.
There are fewer studies regarding food SLIT. The most promising data come from recent trials of peanut-allergic children led by Edwin Kim, MD, director of the UNC Food Allergy Initiative, University of North Carolina at Chapel Hill. Still, “I am nervous to tell people to go do this based on 150 kids at one site,” Dr. Kim said. “We need to have a gigantic study across multiple sites that actually confirms what we have found in our single center.”
Because there are few published trials of food SLIT, confusion about which doses are optimal, how early to start, and how long the benefits last will be a barrier for many clinicians, said Douglas Mack, MD, FRCPC, assistant clinical professor in pediatrics at McMaster University, Hamilton, Ont.
Much could be learned from Allergy Associates of La Crosse, Allergenuity Health, and other clinics with SLIT experience involving thousands of patients. But that real-world data are messy and difficult to publish. Plus, it is hard for private allergists to find time to review charts, analyze data, and draft papers alongside seeing patients and running a clinic – especially without students and interns, who typically assist with academic research, Dr. Schroeder said.
Ruchi Gupta, MD, MPH, professor of pediatrics and medicine at Northwestern University, Chicago, and colleagues worked with a La Crosse team 6 or 7 years ago to try to analyze and publish SLIT outcomes for 121 peanut-allergic children who were treated for food and environmental allergies at the Wisconsin clinic. The researchers had hoped to publish an article describing caregiver-reported and clinical outcomes.
Among 73 caregivers who responded to a survey, more than half reported improved eczema, asthma, and environmental allergy symptoms, and virtually all families said SLIT calmed anxieties and minimized fear of allergic reactions. However, the clinical outcomes – skinprick test results, immune changes, and oral food challenges – were not as robust. And the data were incomplete. Some patients had traveled to La Crosse for SLIT drops but underwent skin and blood testing with their local allergist. Compiling records is “so much harder when you’re not doing a prospective clinical trial,” Dr. Gupta said.
The caregiver-reported outcomes were presented as a poster at the 2015 annual meeting of the American College of Allergy, Asthma, and Immunology and the 2016 annual meeting of the Pediatric Academic Society, said Jeff Kessler, MBA, FACHE, who is practice executive at La Crosse. However, with only self-reported data and no convincing lab metrics, the findings were never submitted for publication.
Others are eager to see clearer proof that SLIT works at doses lower than those published in the most recent trials. “If we can get efficacy with lower doses, that means we can increase accessibility, because we can lower the cost,” Dr. Bajowala said.
Robert Wood, MD, professor of pediatrics and director of pediatric allergy and immunology at Johns Hopkins University, Baltimore, has a pending grant proposal for a multifood trial of SLIT. “It’s a big missing piece,” he said.
Dr. Mack said that in Canada there was “almost an instant change in group think” when the Canadian Society of Allergy and Clinical Immunology published guidelines in support of OIT. With the new guidelines, “people are less concerned about liability. Once they start getting into OIT, I think you’re going to see SLIT coming right along for the ride.”
The shift will be slower in the United States, which has 20 times as many practicing allergists as Canada. Nevertheless, “I totally think SLIT has a place at the table,” Dr. Mack said. “I hope we start to see more high-quality data and people start to use it and experiment with it a bit and see how it works.”
A version of this article first appeared on Medscape.com. This is part three of a three-part series. Part one is here. Part two is here.
Sublingual immunotherapy (SLIT) emerged over a century ago as a gentler alternative to allergy shots. It uses the same antigens found in allergy shots, delivering them through tablets or drops under the tongue rather than by injecting them into the skin.
Yet injection immunotherapy has been the mainstay of allergy treatment in the United States. Allergy shots are “the bread and butter, keeping the lights on at allergy practices,” said allergist Sakina Bajowala, MD, of Kaneland Allergy and Asthma Center, in the Chicago area. So even “when environmental SLIT showed quite clearly that it had efficacy, people were so slow to adapt.”
SLIT – a daily treatment that builds protection from allergens gradually over years with few side effects – is popular around the globe, particularly for environmental allergies. But only a handful of clinics offer food SLIT. Even though recent trials in peanut-allergic children show that SLIT is far safer than oral immunotherapy and about as effective as the Food and Drug Administration–approved peanut-allergy product and has lasting benefits for toddlers, many allergists lack experience with customized immunotherapies and hesitate to offer an unregulated treatment for which the evidence base is still emerging.
Why hasn’t food allergy SLIT caught on?
One issue is that there is scant evidence from randomized, controlled trials. The treatments that clinics offer often hinge on insurance coverage, and increasingly, insurers only cover FDA-approved products. FDA approval requires thousands of patients being enrolled in long, expensive studies to prove the treatment’s merit. In a similar vein, doctors are trained to question methods that lack a strong publication base, for good reason.
Yet SLIT caught the attention of pioneering physicians who were intrigued by this “low-and-slow” immune-modifying approach, despite limited published evidence, and they sought real-world experience.
The late physician David Morris, MD, came across SLIT in the 1960s while searching for alternative ways to help mold-allergic farmers who were suffering terrible side effects from allergy shots. Dr. Morris attended conferences, learned more about sublingual techniques, got board certified in allergy, and opened Allergy Associates of La Crosse (Wis.), in 1970 to offer SLIT as a treatment for food and environmental allergies.
Dr. Morris and colleagues developed a protocol to create custom SLIT drops tailored to individual patients’ clinical histories and allergy test results. The method has been used to treat more than 200,000 patients. It has been used by allergist Nikhila Schroeder, MD, MEng, who learned SLIT methods while treating nearly 1,000 patients at Allergy Associates. In 2018, she opened her own direct-care SLIT practice, Allergenuity Health, in the Charlotte metropolitan area of North Carolina (see part 2 of this series).
Dr. Bajowala’s clinic offers SLIT in addition to oral immunotherapy (OIT). She was encouraged by the recent toddler SLIT data but wondered whether it would translate to a real-world setting. According to her calculations, the published protocol – according to which participants receive up to 4 mg/d over 6 months and continue receiving a daily maintenance dose of 4 mg for 3 years – would cost $10,000 per patient.
With this dosing regimen, the intervention is unaffordable, Dr. Bajowala said. And “there’s no way to make it cheaper because that’s the raw materials cost. It does not include labor or bottles or profit at all. That’s just $10,000 in peanut extract.”
Owing to cost, Dr. Bajowala’s clinic generally uses SLIT as a bridge to OIT. Her food allergy patients receive up to 1 mg/d and remain at that dose for a month or so before transitioning to OIT, “for which the supplies are orders of magnitude cheaper,” she said.
Dr. Schroeder said there is evidence for efficacy at microgram and even nanogram dosing – much lower than used in the recent food SLIT trials. Maintenance doses range from 50 ng/d to 25 mcg/d for environmental SLIT and 4-37 mcg/d for food SLIT, she said. The La Crosse method uses even lower dose ranges.
However, dosing information is not readily available, Dr. Schroeder noted. She has spent years scrutinizing articles and compiling information from allergen extract suppliers – all the while treating hundreds of SLIT patients. “I have had to expend a lot of time and effort,” said Dr. Schroeder. “It’s really hard to explain quickly.”
In the published literature, SLIT dosing recommendations vary widely. According to a 2007 analysis, environmental allergy symptoms improved with doses over a 1,000-fold range. What’s more, success did not scale with increased dosing and seemed to depend more on frequency and duration of treatment.
There are fewer studies regarding food SLIT. The most promising data come from recent trials of peanut-allergic children led by Edwin Kim, MD, director of the UNC Food Allergy Initiative, University of North Carolina at Chapel Hill. Still, “I am nervous to tell people to go do this based on 150 kids at one site,” Dr. Kim said. “We need to have a gigantic study across multiple sites that actually confirms what we have found in our single center.”
Because there are few published trials of food SLIT, confusion about which doses are optimal, how early to start, and how long the benefits last will be a barrier for many clinicians, said Douglas Mack, MD, FRCPC, assistant clinical professor in pediatrics at McMaster University, Hamilton, Ont.
Much could be learned from Allergy Associates of La Crosse, Allergenuity Health, and other clinics with SLIT experience involving thousands of patients. But that real-world data are messy and difficult to publish. Plus, it is hard for private allergists to find time to review charts, analyze data, and draft papers alongside seeing patients and running a clinic – especially without students and interns, who typically assist with academic research, Dr. Schroeder said.
Ruchi Gupta, MD, MPH, professor of pediatrics and medicine at Northwestern University, Chicago, and colleagues worked with a La Crosse team 6 or 7 years ago to try to analyze and publish SLIT outcomes for 121 peanut-allergic children who were treated for food and environmental allergies at the Wisconsin clinic. The researchers had hoped to publish an article describing caregiver-reported and clinical outcomes.
Among 73 caregivers who responded to a survey, more than half reported improved eczema, asthma, and environmental allergy symptoms, and virtually all families said SLIT calmed anxieties and minimized fear of allergic reactions. However, the clinical outcomes – skinprick test results, immune changes, and oral food challenges – were not as robust. And the data were incomplete. Some patients had traveled to La Crosse for SLIT drops but underwent skin and blood testing with their local allergist. Compiling records is “so much harder when you’re not doing a prospective clinical trial,” Dr. Gupta said.
The caregiver-reported outcomes were presented as a poster at the 2015 annual meeting of the American College of Allergy, Asthma, and Immunology and the 2016 annual meeting of the Pediatric Academic Society, said Jeff Kessler, MBA, FACHE, who is practice executive at La Crosse. However, with only self-reported data and no convincing lab metrics, the findings were never submitted for publication.
Others are eager to see clearer proof that SLIT works at doses lower than those published in the most recent trials. “If we can get efficacy with lower doses, that means we can increase accessibility, because we can lower the cost,” Dr. Bajowala said.
Robert Wood, MD, professor of pediatrics and director of pediatric allergy and immunology at Johns Hopkins University, Baltimore, has a pending grant proposal for a multifood trial of SLIT. “It’s a big missing piece,” he said.
Dr. Mack said that in Canada there was “almost an instant change in group think” when the Canadian Society of Allergy and Clinical Immunology published guidelines in support of OIT. With the new guidelines, “people are less concerned about liability. Once they start getting into OIT, I think you’re going to see SLIT coming right along for the ride.”
The shift will be slower in the United States, which has 20 times as many practicing allergists as Canada. Nevertheless, “I totally think SLIT has a place at the table,” Dr. Mack said. “I hope we start to see more high-quality data and people start to use it and experiment with it a bit and see how it works.”
A version of this article first appeared on Medscape.com. This is part three of a three-part series. Part one is here. Part two is here.
Sublingual immunotherapy (SLIT) emerged over a century ago as a gentler alternative to allergy shots. It uses the same antigens found in allergy shots, delivering them through tablets or drops under the tongue rather than by injecting them into the skin.
Yet injection immunotherapy has been the mainstay of allergy treatment in the United States. Allergy shots are “the bread and butter, keeping the lights on at allergy practices,” said allergist Sakina Bajowala, MD, of Kaneland Allergy and Asthma Center, in the Chicago area. So even “when environmental SLIT showed quite clearly that it had efficacy, people were so slow to adapt.”
SLIT – a daily treatment that builds protection from allergens gradually over years with few side effects – is popular around the globe, particularly for environmental allergies. But only a handful of clinics offer food SLIT. Even though recent trials in peanut-allergic children show that SLIT is far safer than oral immunotherapy and about as effective as the Food and Drug Administration–approved peanut-allergy product and has lasting benefits for toddlers, many allergists lack experience with customized immunotherapies and hesitate to offer an unregulated treatment for which the evidence base is still emerging.
Why hasn’t food allergy SLIT caught on?
One issue is that there is scant evidence from randomized, controlled trials. The treatments that clinics offer often hinge on insurance coverage, and increasingly, insurers only cover FDA-approved products. FDA approval requires thousands of patients being enrolled in long, expensive studies to prove the treatment’s merit. In a similar vein, doctors are trained to question methods that lack a strong publication base, for good reason.
Yet SLIT caught the attention of pioneering physicians who were intrigued by this “low-and-slow” immune-modifying approach, despite limited published evidence, and they sought real-world experience.
The late physician David Morris, MD, came across SLIT in the 1960s while searching for alternative ways to help mold-allergic farmers who were suffering terrible side effects from allergy shots. Dr. Morris attended conferences, learned more about sublingual techniques, got board certified in allergy, and opened Allergy Associates of La Crosse (Wis.), in 1970 to offer SLIT as a treatment for food and environmental allergies.
Dr. Morris and colleagues developed a protocol to create custom SLIT drops tailored to individual patients’ clinical histories and allergy test results. The method has been used to treat more than 200,000 patients. It has been used by allergist Nikhila Schroeder, MD, MEng, who learned SLIT methods while treating nearly 1,000 patients at Allergy Associates. In 2018, she opened her own direct-care SLIT practice, Allergenuity Health, in the Charlotte metropolitan area of North Carolina (see part 2 of this series).
Dr. Bajowala’s clinic offers SLIT in addition to oral immunotherapy (OIT). She was encouraged by the recent toddler SLIT data but wondered whether it would translate to a real-world setting. According to her calculations, the published protocol – according to which participants receive up to 4 mg/d over 6 months and continue receiving a daily maintenance dose of 4 mg for 3 years – would cost $10,000 per patient.
With this dosing regimen, the intervention is unaffordable, Dr. Bajowala said. And “there’s no way to make it cheaper because that’s the raw materials cost. It does not include labor or bottles or profit at all. That’s just $10,000 in peanut extract.”
Owing to cost, Dr. Bajowala’s clinic generally uses SLIT as a bridge to OIT. Her food allergy patients receive up to 1 mg/d and remain at that dose for a month or so before transitioning to OIT, “for which the supplies are orders of magnitude cheaper,” she said.
Dr. Schroeder said there is evidence for efficacy at microgram and even nanogram dosing – much lower than used in the recent food SLIT trials. Maintenance doses range from 50 ng/d to 25 mcg/d for environmental SLIT and 4-37 mcg/d for food SLIT, she said. The La Crosse method uses even lower dose ranges.
However, dosing information is not readily available, Dr. Schroeder noted. She has spent years scrutinizing articles and compiling information from allergen extract suppliers – all the while treating hundreds of SLIT patients. “I have had to expend a lot of time and effort,” said Dr. Schroeder. “It’s really hard to explain quickly.”
In the published literature, SLIT dosing recommendations vary widely. According to a 2007 analysis, environmental allergy symptoms improved with doses over a 1,000-fold range. What’s more, success did not scale with increased dosing and seemed to depend more on frequency and duration of treatment.
There are fewer studies regarding food SLIT. The most promising data come from recent trials of peanut-allergic children led by Edwin Kim, MD, director of the UNC Food Allergy Initiative, University of North Carolina at Chapel Hill. Still, “I am nervous to tell people to go do this based on 150 kids at one site,” Dr. Kim said. “We need to have a gigantic study across multiple sites that actually confirms what we have found in our single center.”
Because there are few published trials of food SLIT, confusion about which doses are optimal, how early to start, and how long the benefits last will be a barrier for many clinicians, said Douglas Mack, MD, FRCPC, assistant clinical professor in pediatrics at McMaster University, Hamilton, Ont.
Much could be learned from Allergy Associates of La Crosse, Allergenuity Health, and other clinics with SLIT experience involving thousands of patients. But that real-world data are messy and difficult to publish. Plus, it is hard for private allergists to find time to review charts, analyze data, and draft papers alongside seeing patients and running a clinic – especially without students and interns, who typically assist with academic research, Dr. Schroeder said.
Ruchi Gupta, MD, MPH, professor of pediatrics and medicine at Northwestern University, Chicago, and colleagues worked with a La Crosse team 6 or 7 years ago to try to analyze and publish SLIT outcomes for 121 peanut-allergic children who were treated for food and environmental allergies at the Wisconsin clinic. The researchers had hoped to publish an article describing caregiver-reported and clinical outcomes.
Among 73 caregivers who responded to a survey, more than half reported improved eczema, asthma, and environmental allergy symptoms, and virtually all families said SLIT calmed anxieties and minimized fear of allergic reactions. However, the clinical outcomes – skinprick test results, immune changes, and oral food challenges – were not as robust. And the data were incomplete. Some patients had traveled to La Crosse for SLIT drops but underwent skin and blood testing with their local allergist. Compiling records is “so much harder when you’re not doing a prospective clinical trial,” Dr. Gupta said.
The caregiver-reported outcomes were presented as a poster at the 2015 annual meeting of the American College of Allergy, Asthma, and Immunology and the 2016 annual meeting of the Pediatric Academic Society, said Jeff Kessler, MBA, FACHE, who is practice executive at La Crosse. However, with only self-reported data and no convincing lab metrics, the findings were never submitted for publication.
Others are eager to see clearer proof that SLIT works at doses lower than those published in the most recent trials. “If we can get efficacy with lower doses, that means we can increase accessibility, because we can lower the cost,” Dr. Bajowala said.
Robert Wood, MD, professor of pediatrics and director of pediatric allergy and immunology at Johns Hopkins University, Baltimore, has a pending grant proposal for a multifood trial of SLIT. “It’s a big missing piece,” he said.
Dr. Mack said that in Canada there was “almost an instant change in group think” when the Canadian Society of Allergy and Clinical Immunology published guidelines in support of OIT. With the new guidelines, “people are less concerned about liability. Once they start getting into OIT, I think you’re going to see SLIT coming right along for the ride.”
The shift will be slower in the United States, which has 20 times as many practicing allergists as Canada. Nevertheless, “I totally think SLIT has a place at the table,” Dr. Mack said. “I hope we start to see more high-quality data and people start to use it and experiment with it a bit and see how it works.”
A version of this article first appeared on Medscape.com. This is part three of a three-part series. Part one is here. Part two is here.
Direct-care allergy clinic specializes in sublingual immunotherapy
With degrees in electrical engineering and computer science from the Massachusetts Institute of Technology, Nikhila Schroeder, MD, MEng, brings a problem-solving mindset to medicine.
Being a doctor means having to “figure out all aspects of [a patient’s] situation and do my best to come up with an answer,” said Dr. Schroeder, who founded Allergenuity Health, a solo allergy practice in Huntersville, N.C., with her husband James, who serves as practice executive. It’s “being a medical detective for your patient.”
Yet, during her training, Dr. Schroeder found that market-driven health care makes it hard to practice medicine with a patient’s best interest foremost. Procedures for diagnosing and treating disease cater to insurance companies’ reimbursement policies. “You wind up having to tailor your care to whatever insurance will cover,” she said.
Insurers, in turn, look for evidence from large, peer-reviewed studies to prove that a treatment works. Many physicians hesitate to offer therapies that aren’t covered by insurance, for both liability and financial reasons. So treatment tends to be limited to those options that were rigorously vetted in long, costly, multisite trials that are difficult to conduct without a corporate sponsor.
This is why there is still only one licensed treatment for people with food allergies – a set of standardized peanut powder capsules (Palforzia) that was approved by the Food and Drug Administration in early 2020 for peanut-allergic children aged 4-17 years. A small but growing number of allergists offer unapproved oral immunotherapy (OIT) using commercial food products to treat allergies to peanuts and other foods.
Even fewer allergists treat food allergy patients with another immune-modifying treatment, sublingual immunotherapy (SLIT), which delivers allergens through liquid drops held for several minutes under the tongue. Since 2018, Allergenuity Health, which offers SLIT to treat food and environmental allergies, has provided care to more than 400 patients. More than a third have come from out of state.
The clinic uses a direct-care approach. Rather than taking insurance, the clinic offers a monthly billing program that includes tests, SLIT bottles, and access to Dr. Schroeder via phone, email, or text. “I’m only contracted with the patient, and my only focus is the patient,” Dr. Schroeder said in an interview.
Unforgettable day
Allergy was not on Dr. Schroeder’s radar in medical school. She wanted to be a surgeon. But she loved working with children, so she did a pediatrics residency at the University of Virginia Children’s Hospital in Charlottesville. There Dr. Schroeder started seeing kids with eczema and allergies. While covering a friend’s clinic shift in 2010, she was thrust into an emergency. A family who didn’t speak English had just brought in their screaming 6-month-old baby, red and puffy with hives. “We didn’t know what was going on with this child,” Dr. Schroeder said. “Somehow I was elected to go in there.”
All of a sudden, things got quiet. Yet the baby was still screaming, mouth wide open. Dr. Schroeder had learned about anaphylaxis but had never witnessed it – until that day. The baby›s airways swelled so much that the crying became hoarse and soft. After working with a nurse to administer epinephrine, Dr. Schroeder saw something equally unforgettable: The baby’s heart rate soared, but within minutes the hives and swelling subsided and smiles returned. “It was incredible how quickly things changed,” Dr. Schroeder said. The baby had a reaction to rice, an uncommon allergen.
Dr. Schroeder stayed at UVA 2 more years to complete an allergy and immunology fellowship. She learned to diagnose food allergies but became frustrated having to tell patients they had little recourse but to avoid the food and to check in every year or 2. “I was, like, aren’t we specialists? Shouldn’t we have a little more expertise and maybe see if there are ways we could change this?” Dr. Schroeder said.
During those years, allergy shots were the only form of immunotherapy being taught to fellows. At clinic, Dr. Schroeder served as backup to the nurses when someone reacted to shots. She was troubled that some patients needed epinephrine to stop asthma attacks caused by injections they had received as treatment. The idea of injecting substances under the skin seemed akin to vaccination – where “you want to aggravate the immune system, you want it to get revved up, you want to build it up to fight,” she said. “But that’s not what you want for allergy. You want to tone it down. It didn’t really, to be honest, make a lot of sense to me.”
Dr. Schroeder started digging and asking questions. How does the immune system decide what is safe? Which cells and molecules communicate these decisions? She thought about babies and how they “learn” by putting stuff into their mouths. “If we don’t tolerate most of what we take in there, we wouldn’t survive,” Dr. Schroeder said. “It makes a lot of sense that a lot of tolerance begins with cells of the mouth.”
Dr. Schroeder discussed these concepts with her attendings. “They were all, like, no, there’s really no good evidence for that,” she said. But at some point, someone mentioned sublingual immunotherapy, and Schroeder came across Allergy Associates of La Crosse (Wis.).
The clinic’s late founder, David Morris, MD, learned about SLIT in the 1960s as an alternative option for farmers who suffered terrible side effects from injection immunotherapy they received to treat their mold allergies. Dr. Morris attended conferences, learned more about sublingual techniques – at times seeking advice from European allergists who offered SLIT – and became board certified in allergy before opening the La Crosse clinic in 1970. According to the clinic, more than 200,000 patients with environmental and food allergies have been treated with its SLIT protocol.
Dr. Schroeder was shocked to discover that this clinic had existed for 40 years, yet “I, as an allergist, had heard nothing about them,” she said.
Toward the end of her fellowship, OIT was becoming more well known. But she felt its risks were often downplayed. After years of talking with food allergy patients, Schroeder realized that most didn’t actually care about eating peanut butter sandwiches or sesame or walnuts. “Often I would hear, through tears: ‘I just want my child to be able to sit with their friends at lunch, to not be put at this other table, to not feel so isolated,’ ” she said. What mattered most to many families was gaining enough protection to not feel anxious about participating in social activities involving food.
Dr. Schroeder had a growing sense that SLIT – given its ease, safety, and sensible route of allergen delivery – seemed more useful. She wanted to learn more.
Her mentors urged her to stay in academia instead. “They were, like, you have a good academic reputation. You’re a solid thinker. You’re great at what you do. Do the traditional stuff,” Dr. Schroeder said.
Despite these admonitions, Dr. Schroeder left academia and took a job at La Crosse after completing her allergy fellowship. Determined to see whether SLIT could be effective, “I decided in the end, you know what, I have to go do this,” she said. “I need to know, and the only way I’m going to know is to do it, because no one was giving me good information.”
Before treating anyone with SLIT, Dr. Schroeder tried it herself – as a La Crosse patient. Growing up with severe eczema, eye swelling, and chronic nasal congestion leading to sinus infections, “I myself was a severely allergic person,” she said. Within several months, Dr. Schroeder saw dramatic improvement in her symptoms – “a night and day difference.” She experienced some mouth tingling, one of SLIT’s most common side effects, but found it “very tolerable, very mild.”
Allergenuity Health doesn’t aim to promote SLIT as the best treatment, said Dr. Schroeder, who has helped some families use avoidance or OIT as a better option. “An initial evaluation is always about proper diagnosis and education about all the treatment options available. Really, the point is education – be a detective for them and figure out what’s going on, be honest about what we know and what we don’t know, and give them the tools to figure out how to proceed.”
A version of this article first appeared on Medscape.com. This is part two of a three-part series. Part one is here. Part three is here.
With degrees in electrical engineering and computer science from the Massachusetts Institute of Technology, Nikhila Schroeder, MD, MEng, brings a problem-solving mindset to medicine.
Being a doctor means having to “figure out all aspects of [a patient’s] situation and do my best to come up with an answer,” said Dr. Schroeder, who founded Allergenuity Health, a solo allergy practice in Huntersville, N.C., with her husband James, who serves as practice executive. It’s “being a medical detective for your patient.”
Yet, during her training, Dr. Schroeder found that market-driven health care makes it hard to practice medicine with a patient’s best interest foremost. Procedures for diagnosing and treating disease cater to insurance companies’ reimbursement policies. “You wind up having to tailor your care to whatever insurance will cover,” she said.
Insurers, in turn, look for evidence from large, peer-reviewed studies to prove that a treatment works. Many physicians hesitate to offer therapies that aren’t covered by insurance, for both liability and financial reasons. So treatment tends to be limited to those options that were rigorously vetted in long, costly, multisite trials that are difficult to conduct without a corporate sponsor.
This is why there is still only one licensed treatment for people with food allergies – a set of standardized peanut powder capsules (Palforzia) that was approved by the Food and Drug Administration in early 2020 for peanut-allergic children aged 4-17 years. A small but growing number of allergists offer unapproved oral immunotherapy (OIT) using commercial food products to treat allergies to peanuts and other foods.
Even fewer allergists treat food allergy patients with another immune-modifying treatment, sublingual immunotherapy (SLIT), which delivers allergens through liquid drops held for several minutes under the tongue. Since 2018, Allergenuity Health, which offers SLIT to treat food and environmental allergies, has provided care to more than 400 patients. More than a third have come from out of state.
The clinic uses a direct-care approach. Rather than taking insurance, the clinic offers a monthly billing program that includes tests, SLIT bottles, and access to Dr. Schroeder via phone, email, or text. “I’m only contracted with the patient, and my only focus is the patient,” Dr. Schroeder said in an interview.
Unforgettable day
Allergy was not on Dr. Schroeder’s radar in medical school. She wanted to be a surgeon. But she loved working with children, so she did a pediatrics residency at the University of Virginia Children’s Hospital in Charlottesville. There Dr. Schroeder started seeing kids with eczema and allergies. While covering a friend’s clinic shift in 2010, she was thrust into an emergency. A family who didn’t speak English had just brought in their screaming 6-month-old baby, red and puffy with hives. “We didn’t know what was going on with this child,” Dr. Schroeder said. “Somehow I was elected to go in there.”
All of a sudden, things got quiet. Yet the baby was still screaming, mouth wide open. Dr. Schroeder had learned about anaphylaxis but had never witnessed it – until that day. The baby›s airways swelled so much that the crying became hoarse and soft. After working with a nurse to administer epinephrine, Dr. Schroeder saw something equally unforgettable: The baby’s heart rate soared, but within minutes the hives and swelling subsided and smiles returned. “It was incredible how quickly things changed,” Dr. Schroeder said. The baby had a reaction to rice, an uncommon allergen.
Dr. Schroeder stayed at UVA 2 more years to complete an allergy and immunology fellowship. She learned to diagnose food allergies but became frustrated having to tell patients they had little recourse but to avoid the food and to check in every year or 2. “I was, like, aren’t we specialists? Shouldn’t we have a little more expertise and maybe see if there are ways we could change this?” Dr. Schroeder said.
During those years, allergy shots were the only form of immunotherapy being taught to fellows. At clinic, Dr. Schroeder served as backup to the nurses when someone reacted to shots. She was troubled that some patients needed epinephrine to stop asthma attacks caused by injections they had received as treatment. The idea of injecting substances under the skin seemed akin to vaccination – where “you want to aggravate the immune system, you want it to get revved up, you want to build it up to fight,” she said. “But that’s not what you want for allergy. You want to tone it down. It didn’t really, to be honest, make a lot of sense to me.”
Dr. Schroeder started digging and asking questions. How does the immune system decide what is safe? Which cells and molecules communicate these decisions? She thought about babies and how they “learn” by putting stuff into their mouths. “If we don’t tolerate most of what we take in there, we wouldn’t survive,” Dr. Schroeder said. “It makes a lot of sense that a lot of tolerance begins with cells of the mouth.”
Dr. Schroeder discussed these concepts with her attendings. “They were all, like, no, there’s really no good evidence for that,” she said. But at some point, someone mentioned sublingual immunotherapy, and Schroeder came across Allergy Associates of La Crosse (Wis.).
The clinic’s late founder, David Morris, MD, learned about SLIT in the 1960s as an alternative option for farmers who suffered terrible side effects from injection immunotherapy they received to treat their mold allergies. Dr. Morris attended conferences, learned more about sublingual techniques – at times seeking advice from European allergists who offered SLIT – and became board certified in allergy before opening the La Crosse clinic in 1970. According to the clinic, more than 200,000 patients with environmental and food allergies have been treated with its SLIT protocol.
Dr. Schroeder was shocked to discover that this clinic had existed for 40 years, yet “I, as an allergist, had heard nothing about them,” she said.
Toward the end of her fellowship, OIT was becoming more well known. But she felt its risks were often downplayed. After years of talking with food allergy patients, Schroeder realized that most didn’t actually care about eating peanut butter sandwiches or sesame or walnuts. “Often I would hear, through tears: ‘I just want my child to be able to sit with their friends at lunch, to not be put at this other table, to not feel so isolated,’ ” she said. What mattered most to many families was gaining enough protection to not feel anxious about participating in social activities involving food.
Dr. Schroeder had a growing sense that SLIT – given its ease, safety, and sensible route of allergen delivery – seemed more useful. She wanted to learn more.
Her mentors urged her to stay in academia instead. “They were, like, you have a good academic reputation. You’re a solid thinker. You’re great at what you do. Do the traditional stuff,” Dr. Schroeder said.
Despite these admonitions, Dr. Schroeder left academia and took a job at La Crosse after completing her allergy fellowship. Determined to see whether SLIT could be effective, “I decided in the end, you know what, I have to go do this,” she said. “I need to know, and the only way I’m going to know is to do it, because no one was giving me good information.”
Before treating anyone with SLIT, Dr. Schroeder tried it herself – as a La Crosse patient. Growing up with severe eczema, eye swelling, and chronic nasal congestion leading to sinus infections, “I myself was a severely allergic person,” she said. Within several months, Dr. Schroeder saw dramatic improvement in her symptoms – “a night and day difference.” She experienced some mouth tingling, one of SLIT’s most common side effects, but found it “very tolerable, very mild.”
Allergenuity Health doesn’t aim to promote SLIT as the best treatment, said Dr. Schroeder, who has helped some families use avoidance or OIT as a better option. “An initial evaluation is always about proper diagnosis and education about all the treatment options available. Really, the point is education – be a detective for them and figure out what’s going on, be honest about what we know and what we don’t know, and give them the tools to figure out how to proceed.”
A version of this article first appeared on Medscape.com. This is part two of a three-part series. Part one is here. Part three is here.
With degrees in electrical engineering and computer science from the Massachusetts Institute of Technology, Nikhila Schroeder, MD, MEng, brings a problem-solving mindset to medicine.
Being a doctor means having to “figure out all aspects of [a patient’s] situation and do my best to come up with an answer,” said Dr. Schroeder, who founded Allergenuity Health, a solo allergy practice in Huntersville, N.C., with her husband James, who serves as practice executive. It’s “being a medical detective for your patient.”
Yet, during her training, Dr. Schroeder found that market-driven health care makes it hard to practice medicine with a patient’s best interest foremost. Procedures for diagnosing and treating disease cater to insurance companies’ reimbursement policies. “You wind up having to tailor your care to whatever insurance will cover,” she said.
Insurers, in turn, look for evidence from large, peer-reviewed studies to prove that a treatment works. Many physicians hesitate to offer therapies that aren’t covered by insurance, for both liability and financial reasons. So treatment tends to be limited to those options that were rigorously vetted in long, costly, multisite trials that are difficult to conduct without a corporate sponsor.
This is why there is still only one licensed treatment for people with food allergies – a set of standardized peanut powder capsules (Palforzia) that was approved by the Food and Drug Administration in early 2020 for peanut-allergic children aged 4-17 years. A small but growing number of allergists offer unapproved oral immunotherapy (OIT) using commercial food products to treat allergies to peanuts and other foods.
Even fewer allergists treat food allergy patients with another immune-modifying treatment, sublingual immunotherapy (SLIT), which delivers allergens through liquid drops held for several minutes under the tongue. Since 2018, Allergenuity Health, which offers SLIT to treat food and environmental allergies, has provided care to more than 400 patients. More than a third have come from out of state.
The clinic uses a direct-care approach. Rather than taking insurance, the clinic offers a monthly billing program that includes tests, SLIT bottles, and access to Dr. Schroeder via phone, email, or text. “I’m only contracted with the patient, and my only focus is the patient,” Dr. Schroeder said in an interview.
Unforgettable day
Allergy was not on Dr. Schroeder’s radar in medical school. She wanted to be a surgeon. But she loved working with children, so she did a pediatrics residency at the University of Virginia Children’s Hospital in Charlottesville. There Dr. Schroeder started seeing kids with eczema and allergies. While covering a friend’s clinic shift in 2010, she was thrust into an emergency. A family who didn’t speak English had just brought in their screaming 6-month-old baby, red and puffy with hives. “We didn’t know what was going on with this child,” Dr. Schroeder said. “Somehow I was elected to go in there.”
All of a sudden, things got quiet. Yet the baby was still screaming, mouth wide open. Dr. Schroeder had learned about anaphylaxis but had never witnessed it – until that day. The baby›s airways swelled so much that the crying became hoarse and soft. After working with a nurse to administer epinephrine, Dr. Schroeder saw something equally unforgettable: The baby’s heart rate soared, but within minutes the hives and swelling subsided and smiles returned. “It was incredible how quickly things changed,” Dr. Schroeder said. The baby had a reaction to rice, an uncommon allergen.
Dr. Schroeder stayed at UVA 2 more years to complete an allergy and immunology fellowship. She learned to diagnose food allergies but became frustrated having to tell patients they had little recourse but to avoid the food and to check in every year or 2. “I was, like, aren’t we specialists? Shouldn’t we have a little more expertise and maybe see if there are ways we could change this?” Dr. Schroeder said.
During those years, allergy shots were the only form of immunotherapy being taught to fellows. At clinic, Dr. Schroeder served as backup to the nurses when someone reacted to shots. She was troubled that some patients needed epinephrine to stop asthma attacks caused by injections they had received as treatment. The idea of injecting substances under the skin seemed akin to vaccination – where “you want to aggravate the immune system, you want it to get revved up, you want to build it up to fight,” she said. “But that’s not what you want for allergy. You want to tone it down. It didn’t really, to be honest, make a lot of sense to me.”
Dr. Schroeder started digging and asking questions. How does the immune system decide what is safe? Which cells and molecules communicate these decisions? She thought about babies and how they “learn” by putting stuff into their mouths. “If we don’t tolerate most of what we take in there, we wouldn’t survive,” Dr. Schroeder said. “It makes a lot of sense that a lot of tolerance begins with cells of the mouth.”
Dr. Schroeder discussed these concepts with her attendings. “They were all, like, no, there’s really no good evidence for that,” she said. But at some point, someone mentioned sublingual immunotherapy, and Schroeder came across Allergy Associates of La Crosse (Wis.).
The clinic’s late founder, David Morris, MD, learned about SLIT in the 1960s as an alternative option for farmers who suffered terrible side effects from injection immunotherapy they received to treat their mold allergies. Dr. Morris attended conferences, learned more about sublingual techniques – at times seeking advice from European allergists who offered SLIT – and became board certified in allergy before opening the La Crosse clinic in 1970. According to the clinic, more than 200,000 patients with environmental and food allergies have been treated with its SLIT protocol.
Dr. Schroeder was shocked to discover that this clinic had existed for 40 years, yet “I, as an allergist, had heard nothing about them,” she said.
Toward the end of her fellowship, OIT was becoming more well known. But she felt its risks were often downplayed. After years of talking with food allergy patients, Schroeder realized that most didn’t actually care about eating peanut butter sandwiches or sesame or walnuts. “Often I would hear, through tears: ‘I just want my child to be able to sit with their friends at lunch, to not be put at this other table, to not feel so isolated,’ ” she said. What mattered most to many families was gaining enough protection to not feel anxious about participating in social activities involving food.
Dr. Schroeder had a growing sense that SLIT – given its ease, safety, and sensible route of allergen delivery – seemed more useful. She wanted to learn more.
Her mentors urged her to stay in academia instead. “They were, like, you have a good academic reputation. You’re a solid thinker. You’re great at what you do. Do the traditional stuff,” Dr. Schroeder said.
Despite these admonitions, Dr. Schroeder left academia and took a job at La Crosse after completing her allergy fellowship. Determined to see whether SLIT could be effective, “I decided in the end, you know what, I have to go do this,” she said. “I need to know, and the only way I’m going to know is to do it, because no one was giving me good information.”
Before treating anyone with SLIT, Dr. Schroeder tried it herself – as a La Crosse patient. Growing up with severe eczema, eye swelling, and chronic nasal congestion leading to sinus infections, “I myself was a severely allergic person,” she said. Within several months, Dr. Schroeder saw dramatic improvement in her symptoms – “a night and day difference.” She experienced some mouth tingling, one of SLIT’s most common side effects, but found it “very tolerable, very mild.”
Allergenuity Health doesn’t aim to promote SLIT as the best treatment, said Dr. Schroeder, who has helped some families use avoidance or OIT as a better option. “An initial evaluation is always about proper diagnosis and education about all the treatment options available. Really, the point is education – be a detective for them and figure out what’s going on, be honest about what we know and what we don’t know, and give them the tools to figure out how to proceed.”
A version of this article first appeared on Medscape.com. This is part two of a three-part series. Part one is here. Part three is here.