User login
Send kids home 2 hours after food challenge testing
HOUSTON – Food-allergic children undergoing a double-blind, placebo-controlled food challenge test can safely be discharged home after 2 hours provided they haven’t experienced a severe immediate reaction in the interim, according to a large retrospective Dutch study.
Late reactions are unpredictable and very seldom severe, Jacquelien Saleh-Langenberg reported at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
She presented a study of 1,142 children who underwent double-blind, placebo-controlled food challenge testing at a tertiary clinic at the University of Groningen in the Netherlands, where she is a combined medical student and Ph.D. candidate. The food-allergic children were challenged with cow’s milk, peanut, cashew, hazelnut, and egg.
A total of 400 children developed late reactions: 20.8% of children reported late reactions only on an active challenge day, 9.6% only on a placebo challenge day, and 4.6% reported reactions on both active and placebo challenge days.
Of particular interest was the finding that 89 subjects developed isolated reactions on an active challenge day and 92 did so on a placebo challenge day.
“Isolated late reactions occurred with comparable frequency after active and placebo challenge and are thus unlikely to be a real phenomenon,” Ms. Saleh-Langenberg concluded.
Late reactions were manifest as gastrointestinal symptoms in 45% of cases and cutaneous symptoms in about one-third, with respiratory symptoms accounting for most of the remainder. Ninety-eight percent of late reactions were rated as mild to moderate, having a score of 1-6 on a 12-point severity scale.
The investigators developed a predictive model for late reactions occurring on an active challenge day. It proved to have little practical value, though. The model, which included age, allergic rhinitis, severity of any immediate reaction, and hazelnut allergy, explained a mere 8% of the variance in the incidence of late reactions.
When late reactions occurred on an active challenge day, they did so a mean of 3.5 hours after testing. When they occurred on a placebo challenge day, they happened a mean of 4 hours after the challenge. The reactions took an average of 2 hours and 1 hour, respectively, to disappear.
Ms. Saleh-Langenberg reported no conflicts of interest with regard to this university-supported study.
HOUSTON – Food-allergic children undergoing a double-blind, placebo-controlled food challenge test can safely be discharged home after 2 hours provided they haven’t experienced a severe immediate reaction in the interim, according to a large retrospective Dutch study.
Late reactions are unpredictable and very seldom severe, Jacquelien Saleh-Langenberg reported at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
She presented a study of 1,142 children who underwent double-blind, placebo-controlled food challenge testing at a tertiary clinic at the University of Groningen in the Netherlands, where she is a combined medical student and Ph.D. candidate. The food-allergic children were challenged with cow’s milk, peanut, cashew, hazelnut, and egg.
A total of 400 children developed late reactions: 20.8% of children reported late reactions only on an active challenge day, 9.6% only on a placebo challenge day, and 4.6% reported reactions on both active and placebo challenge days.
Of particular interest was the finding that 89 subjects developed isolated reactions on an active challenge day and 92 did so on a placebo challenge day.
“Isolated late reactions occurred with comparable frequency after active and placebo challenge and are thus unlikely to be a real phenomenon,” Ms. Saleh-Langenberg concluded.
Late reactions were manifest as gastrointestinal symptoms in 45% of cases and cutaneous symptoms in about one-third, with respiratory symptoms accounting for most of the remainder. Ninety-eight percent of late reactions were rated as mild to moderate, having a score of 1-6 on a 12-point severity scale.
The investigators developed a predictive model for late reactions occurring on an active challenge day. It proved to have little practical value, though. The model, which included age, allergic rhinitis, severity of any immediate reaction, and hazelnut allergy, explained a mere 8% of the variance in the incidence of late reactions.
When late reactions occurred on an active challenge day, they did so a mean of 3.5 hours after testing. When they occurred on a placebo challenge day, they happened a mean of 4 hours after the challenge. The reactions took an average of 2 hours and 1 hour, respectively, to disappear.
Ms. Saleh-Langenberg reported no conflicts of interest with regard to this university-supported study.
HOUSTON – Food-allergic children undergoing a double-blind, placebo-controlled food challenge test can safely be discharged home after 2 hours provided they haven’t experienced a severe immediate reaction in the interim, according to a large retrospective Dutch study.
Late reactions are unpredictable and very seldom severe, Jacquelien Saleh-Langenberg reported at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
She presented a study of 1,142 children who underwent double-blind, placebo-controlled food challenge testing at a tertiary clinic at the University of Groningen in the Netherlands, where she is a combined medical student and Ph.D. candidate. The food-allergic children were challenged with cow’s milk, peanut, cashew, hazelnut, and egg.
A total of 400 children developed late reactions: 20.8% of children reported late reactions only on an active challenge day, 9.6% only on a placebo challenge day, and 4.6% reported reactions on both active and placebo challenge days.
Of particular interest was the finding that 89 subjects developed isolated reactions on an active challenge day and 92 did so on a placebo challenge day.
“Isolated late reactions occurred with comparable frequency after active and placebo challenge and are thus unlikely to be a real phenomenon,” Ms. Saleh-Langenberg concluded.
Late reactions were manifest as gastrointestinal symptoms in 45% of cases and cutaneous symptoms in about one-third, with respiratory symptoms accounting for most of the remainder. Ninety-eight percent of late reactions were rated as mild to moderate, having a score of 1-6 on a 12-point severity scale.
The investigators developed a predictive model for late reactions occurring on an active challenge day. It proved to have little practical value, though. The model, which included age, allergic rhinitis, severity of any immediate reaction, and hazelnut allergy, explained a mere 8% of the variance in the incidence of late reactions.
When late reactions occurred on an active challenge day, they did so a mean of 3.5 hours after testing. When they occurred on a placebo challenge day, they happened a mean of 4 hours after the challenge. The reactions took an average of 2 hours and 1 hour, respectively, to disappear.
Ms. Saleh-Langenberg reported no conflicts of interest with regard to this university-supported study.
AT 2015 AAAAI ANNUAL MEETING
Key clinical point: Food-allergic children who don’t experience a severe immediate reaction following double-blind, placebo-controlled food challenges can safely be discharged home 2 hours post-testing.
Major finding: No severe late reactions occurred in a large cohort of children who underwent double-blind, placebo-controlled food challenges with cow’s milk, cashew, peanut, egg, and hazelnut.
Data source: This retrospective study included 1,142 Dutch children who underwent double-blind, placebo-controlled food challenges at a university clinic.
Disclosures: The presenter reported no conflicts of interest with regard to this university-supported study.
Aspirin desensitization making headway in U.S.
HOUSTON – About 63% of allergists and fellows in training perform aspirin desensitization for aspirin-exacerbated respiratory disease, according to a national survey.
That figure is lower than it should be, given the wealth of published evidence that aspirin desensitization is a safe and effective component of the treatment of aspirin-exacerbated respiratory disease (AERD), Dr. Jeremy D. Waldram asserted in presenting the survey findings at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Moreover, the figure likely overcalls the true rate, since participation in the survey was voluntary and fans of aspirin desensitization were probably more inclined to complete the 16-item questionnaire, added Dr. Waldram, a fellow in allergy and immunology at the Scripps Clinic in San Diego.
Was he surprised to find that aspirin desensitization isn’t more widely utilized?
“I think the number that surprised me more was that among the 37.5% of allergists who don’t do aspirin desensitization, almost 30% of them don’t even refer their patients to others who do the procedure. We don’t know why they don’t refer out; it wasn’t a question included in the survey. Perhaps they see patients who are of a less severe phenotype,” he said in an interview.
The 684 survey responses represented a 15% response rate. While 37.5% of respondents indicated they don’t perform aspirin desensitization, 73% of those who reported doing the procedure said they do an average of 1-5 cases annually.
Among allergists who don’t perform aspirin desensitization, safety concerns were the leading reason cited. Indeed, 70% of those who don’t do aspirin desensitization indicated safety risks were the main reason. More than one reason could be given, however, and 30% of allergists cited poor compensation for the procedure as a deterrent, nearly 60% said the logistics of monitoring care were too onerous, and one-third said they didn’t perform aspirin desensitization because they hadn’t been trained to do it.
Of allergists who reported doing aspirin desensitization, 52% perform the procedure in an outpatient setting unattached to a hospital. Another 21% do so in an outpatient clinic that’s physically attached to a hospital.
Within the past 5 years, 9% of respondents said that they’ve had a patient react severely to aspirin desensitization, requiring an unanticipated transfer to a higher level of care. That’s contrary to the experience among allergists at the Scripps Clinic, which is widely credited with pioneering the outpatient approach.
“We essentially do all our aspirin desensitizations for AERD in the outpatient setting. In 1,500 treated patients we’ve never had one that we had to transfer to a higher level of care. We don’t have any special setup. It’s a typical outpatient clinic. We usually don’t start IVs or do anything above and beyond,” Dr. Waldram said.
While 26% of respondents reported they generally recommend aspirin desensitization immediately upon identifying a patient history that supports the diagnosis of AERD, another 54% said they usually recommend the procedure to patients only after they’ve failed to improve on typical medical therapy.
Twenty percent of physicians rated aspirin desensitization as “extremely helpful for the majority of patients,” another 49% said they find it most beneficial as an adjuvant to ongoing medical therapy.
Forty-four percent of allergists who perform aspirin desensitization reported that they learned to do the procedure during fellowship training. Fourteen percent said they learned to the procedure at an annual meeting, and 36% picked it up by reviewing the relevant literature.
Several allergists commented that had Dr. Waldram’s survey been conducted even a couple of years ago the rate of utilization of aspirin desensitization would have been far lower. They interpreted his reported 62.5% rate as a sign of progress. Dr. Waldram said he believes the key to further boosting utilization of aspirin desensitization lies in increasing exposure to the procedure during fellowship training. He noted that internal medicine-trained fellows who responded to the survey had a significantly higher aspirin desensitization utilization rate than those who came to their allergy fellowship with a background in pediatrics.
The hallmarks of AERD are difficult-to-treat nasal polyps, chronic eosinophilic sinusitis, and asthma in a patient with sensitivity to aspirin and other COX-1 inhibitors.
Dr. Waldram reported having no financial conflicts with regard to his study, which was conducted free of commercial support.
HOUSTON – About 63% of allergists and fellows in training perform aspirin desensitization for aspirin-exacerbated respiratory disease, according to a national survey.
That figure is lower than it should be, given the wealth of published evidence that aspirin desensitization is a safe and effective component of the treatment of aspirin-exacerbated respiratory disease (AERD), Dr. Jeremy D. Waldram asserted in presenting the survey findings at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Moreover, the figure likely overcalls the true rate, since participation in the survey was voluntary and fans of aspirin desensitization were probably more inclined to complete the 16-item questionnaire, added Dr. Waldram, a fellow in allergy and immunology at the Scripps Clinic in San Diego.
Was he surprised to find that aspirin desensitization isn’t more widely utilized?
“I think the number that surprised me more was that among the 37.5% of allergists who don’t do aspirin desensitization, almost 30% of them don’t even refer their patients to others who do the procedure. We don’t know why they don’t refer out; it wasn’t a question included in the survey. Perhaps they see patients who are of a less severe phenotype,” he said in an interview.
The 684 survey responses represented a 15% response rate. While 37.5% of respondents indicated they don’t perform aspirin desensitization, 73% of those who reported doing the procedure said they do an average of 1-5 cases annually.
Among allergists who don’t perform aspirin desensitization, safety concerns were the leading reason cited. Indeed, 70% of those who don’t do aspirin desensitization indicated safety risks were the main reason. More than one reason could be given, however, and 30% of allergists cited poor compensation for the procedure as a deterrent, nearly 60% said the logistics of monitoring care were too onerous, and one-third said they didn’t perform aspirin desensitization because they hadn’t been trained to do it.
Of allergists who reported doing aspirin desensitization, 52% perform the procedure in an outpatient setting unattached to a hospital. Another 21% do so in an outpatient clinic that’s physically attached to a hospital.
Within the past 5 years, 9% of respondents said that they’ve had a patient react severely to aspirin desensitization, requiring an unanticipated transfer to a higher level of care. That’s contrary to the experience among allergists at the Scripps Clinic, which is widely credited with pioneering the outpatient approach.
“We essentially do all our aspirin desensitizations for AERD in the outpatient setting. In 1,500 treated patients we’ve never had one that we had to transfer to a higher level of care. We don’t have any special setup. It’s a typical outpatient clinic. We usually don’t start IVs or do anything above and beyond,” Dr. Waldram said.
While 26% of respondents reported they generally recommend aspirin desensitization immediately upon identifying a patient history that supports the diagnosis of AERD, another 54% said they usually recommend the procedure to patients only after they’ve failed to improve on typical medical therapy.
Twenty percent of physicians rated aspirin desensitization as “extremely helpful for the majority of patients,” another 49% said they find it most beneficial as an adjuvant to ongoing medical therapy.
Forty-four percent of allergists who perform aspirin desensitization reported that they learned to do the procedure during fellowship training. Fourteen percent said they learned to the procedure at an annual meeting, and 36% picked it up by reviewing the relevant literature.
Several allergists commented that had Dr. Waldram’s survey been conducted even a couple of years ago the rate of utilization of aspirin desensitization would have been far lower. They interpreted his reported 62.5% rate as a sign of progress. Dr. Waldram said he believes the key to further boosting utilization of aspirin desensitization lies in increasing exposure to the procedure during fellowship training. He noted that internal medicine-trained fellows who responded to the survey had a significantly higher aspirin desensitization utilization rate than those who came to their allergy fellowship with a background in pediatrics.
The hallmarks of AERD are difficult-to-treat nasal polyps, chronic eosinophilic sinusitis, and asthma in a patient with sensitivity to aspirin and other COX-1 inhibitors.
Dr. Waldram reported having no financial conflicts with regard to his study, which was conducted free of commercial support.
HOUSTON – About 63% of allergists and fellows in training perform aspirin desensitization for aspirin-exacerbated respiratory disease, according to a national survey.
That figure is lower than it should be, given the wealth of published evidence that aspirin desensitization is a safe and effective component of the treatment of aspirin-exacerbated respiratory disease (AERD), Dr. Jeremy D. Waldram asserted in presenting the survey findings at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Moreover, the figure likely overcalls the true rate, since participation in the survey was voluntary and fans of aspirin desensitization were probably more inclined to complete the 16-item questionnaire, added Dr. Waldram, a fellow in allergy and immunology at the Scripps Clinic in San Diego.
Was he surprised to find that aspirin desensitization isn’t more widely utilized?
“I think the number that surprised me more was that among the 37.5% of allergists who don’t do aspirin desensitization, almost 30% of them don’t even refer their patients to others who do the procedure. We don’t know why they don’t refer out; it wasn’t a question included in the survey. Perhaps they see patients who are of a less severe phenotype,” he said in an interview.
The 684 survey responses represented a 15% response rate. While 37.5% of respondents indicated they don’t perform aspirin desensitization, 73% of those who reported doing the procedure said they do an average of 1-5 cases annually.
Among allergists who don’t perform aspirin desensitization, safety concerns were the leading reason cited. Indeed, 70% of those who don’t do aspirin desensitization indicated safety risks were the main reason. More than one reason could be given, however, and 30% of allergists cited poor compensation for the procedure as a deterrent, nearly 60% said the logistics of monitoring care were too onerous, and one-third said they didn’t perform aspirin desensitization because they hadn’t been trained to do it.
Of allergists who reported doing aspirin desensitization, 52% perform the procedure in an outpatient setting unattached to a hospital. Another 21% do so in an outpatient clinic that’s physically attached to a hospital.
Within the past 5 years, 9% of respondents said that they’ve had a patient react severely to aspirin desensitization, requiring an unanticipated transfer to a higher level of care. That’s contrary to the experience among allergists at the Scripps Clinic, which is widely credited with pioneering the outpatient approach.
“We essentially do all our aspirin desensitizations for AERD in the outpatient setting. In 1,500 treated patients we’ve never had one that we had to transfer to a higher level of care. We don’t have any special setup. It’s a typical outpatient clinic. We usually don’t start IVs or do anything above and beyond,” Dr. Waldram said.
While 26% of respondents reported they generally recommend aspirin desensitization immediately upon identifying a patient history that supports the diagnosis of AERD, another 54% said they usually recommend the procedure to patients only after they’ve failed to improve on typical medical therapy.
Twenty percent of physicians rated aspirin desensitization as “extremely helpful for the majority of patients,” another 49% said they find it most beneficial as an adjuvant to ongoing medical therapy.
Forty-four percent of allergists who perform aspirin desensitization reported that they learned to do the procedure during fellowship training. Fourteen percent said they learned to the procedure at an annual meeting, and 36% picked it up by reviewing the relevant literature.
Several allergists commented that had Dr. Waldram’s survey been conducted even a couple of years ago the rate of utilization of aspirin desensitization would have been far lower. They interpreted his reported 62.5% rate as a sign of progress. Dr. Waldram said he believes the key to further boosting utilization of aspirin desensitization lies in increasing exposure to the procedure during fellowship training. He noted that internal medicine-trained fellows who responded to the survey had a significantly higher aspirin desensitization utilization rate than those who came to their allergy fellowship with a background in pediatrics.
The hallmarks of AERD are difficult-to-treat nasal polyps, chronic eosinophilic sinusitis, and asthma in a patient with sensitivity to aspirin and other COX-1 inhibitors.
Dr. Waldram reported having no financial conflicts with regard to his study, which was conducted free of commercial support.
AT 2015 AAAAI ANNUAL MEETING
Key clinical point: Aspirin desensitization for patients with aspirin-exacerbated respiratory disease is catching on among U.S. allergists.
Major finding: Roughly 63% of allergists and allergy fellows who responded to a national survey indicated they perform aspirin desensitization for aspirin-exacerbated respiratory disease.
Data source: This was a 16-question survey of aspirin desensitization practices among U.S. allergists and allergy fellows. The national survey drew 684 responses.
Disclosures: The presenter reported having no financial conflicts with regard to his study, which was funded without commercial support.
Risk factors identified for gestational eczema
HOUSTON– New-onset eczema during pregnancy is a common phenomenon with several newly identified risk factors.
This disease entity deserves a proper name: gestational eczema, Dr. Wilfried J.J. Karmaus asserted at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
In contrast, the likelihood of new-onset asthma arising during pregnancy isn’t significantly more common than in an affected woman’s male partner during the same time frame.
“There was no large difference in wheezing between the women and men. Therefore, we cannot propose the term ‘gestational asthma,’” said Dr. Karmaus, professor of epidemiology at the University of Memphis. “Investigations into how to prevent eczema and asthma in pregnancy are really important, because eczema and asthma in pregnancy can increase the risk of these diseases in the offspring. This is a totally undeveloped field.”
He presented new findings from the Isle of Wight study, a prospective study in which a cohort of women has been followed from birth through pregnancy across three generations.
Eczema and asthma are common atopic diseases, and they are particularly common during pregnancy. Indeed, eczema is the most common skin disease seen in pregnancy, accounting for 35%-50% of all dermatoses in previous studies by other investigators. In those studies, only 20%-40% of women with eczema during pregnancy had a prepregnancy history of the disease.
In the Isle of Wight cohort, women were evaluated for asthma and eczema symptoms at ages 1, 2, 4, 10, and 18 years and again during pregnancy at gestational weeks 20 and 28. A total of 26 of 116 women developed eczema during pregnancy, with eight of them (31%) experiencing the skin disease for the first time in their lives. In contrast, only six of their male partners had eczema during the pregnancy time frame, and just one of them had new-onset eczema.
A history of maternal eczema in the preceding generation was associated with a 52% increased relative risk of having eczema by age 18 and a 3.1-fold increased likelihood of eczema during pregnancy. Also, methylation of the filaggrin gene at the cytosine-phosphate-guanine site cg13447818 when assessed at age 18 was associated with a significantly increased likelihood of eczema in a subject’s mother as well as increased risk of gestational eczema 1-7 years later, Dr. Karmaus continued.
Eighteen percent of women in the Isle of Wight cohort had asthma during pregnancy, as did a similar proportion of their male partners. Twenty-seven percent of women with asthma during pregnancy had no previous history of the respiratory disease, a rate which was again comparable in their male partners with asthma.
DNA methylation of the IL1RL1 gene at cg17738684 was significantly associated with asthma heritability across three generations in the Isle of Wight study. The IL1RL1 gene at cg17738684 is a candidate gene for asthma that encodes for interleukin-33. This finding raises the possibility that addressing this DNA methylation could prove fruitful as a transgenerational asthma prevention strategy.
The Isle of Wight birth cohort study is funded by the National Institutes of Health, Asthma UK, and the Isle of Wight Trust. Dr. Karmaus reported having no financial conflicts of interest.
HOUSTON– New-onset eczema during pregnancy is a common phenomenon with several newly identified risk factors.
This disease entity deserves a proper name: gestational eczema, Dr. Wilfried J.J. Karmaus asserted at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
In contrast, the likelihood of new-onset asthma arising during pregnancy isn’t significantly more common than in an affected woman’s male partner during the same time frame.
“There was no large difference in wheezing between the women and men. Therefore, we cannot propose the term ‘gestational asthma,’” said Dr. Karmaus, professor of epidemiology at the University of Memphis. “Investigations into how to prevent eczema and asthma in pregnancy are really important, because eczema and asthma in pregnancy can increase the risk of these diseases in the offspring. This is a totally undeveloped field.”
He presented new findings from the Isle of Wight study, a prospective study in which a cohort of women has been followed from birth through pregnancy across three generations.
Eczema and asthma are common atopic diseases, and they are particularly common during pregnancy. Indeed, eczema is the most common skin disease seen in pregnancy, accounting for 35%-50% of all dermatoses in previous studies by other investigators. In those studies, only 20%-40% of women with eczema during pregnancy had a prepregnancy history of the disease.
In the Isle of Wight cohort, women were evaluated for asthma and eczema symptoms at ages 1, 2, 4, 10, and 18 years and again during pregnancy at gestational weeks 20 and 28. A total of 26 of 116 women developed eczema during pregnancy, with eight of them (31%) experiencing the skin disease for the first time in their lives. In contrast, only six of their male partners had eczema during the pregnancy time frame, and just one of them had new-onset eczema.
A history of maternal eczema in the preceding generation was associated with a 52% increased relative risk of having eczema by age 18 and a 3.1-fold increased likelihood of eczema during pregnancy. Also, methylation of the filaggrin gene at the cytosine-phosphate-guanine site cg13447818 when assessed at age 18 was associated with a significantly increased likelihood of eczema in a subject’s mother as well as increased risk of gestational eczema 1-7 years later, Dr. Karmaus continued.
Eighteen percent of women in the Isle of Wight cohort had asthma during pregnancy, as did a similar proportion of their male partners. Twenty-seven percent of women with asthma during pregnancy had no previous history of the respiratory disease, a rate which was again comparable in their male partners with asthma.
DNA methylation of the IL1RL1 gene at cg17738684 was significantly associated with asthma heritability across three generations in the Isle of Wight study. The IL1RL1 gene at cg17738684 is a candidate gene for asthma that encodes for interleukin-33. This finding raises the possibility that addressing this DNA methylation could prove fruitful as a transgenerational asthma prevention strategy.
The Isle of Wight birth cohort study is funded by the National Institutes of Health, Asthma UK, and the Isle of Wight Trust. Dr. Karmaus reported having no financial conflicts of interest.
HOUSTON– New-onset eczema during pregnancy is a common phenomenon with several newly identified risk factors.
This disease entity deserves a proper name: gestational eczema, Dr. Wilfried J.J. Karmaus asserted at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
In contrast, the likelihood of new-onset asthma arising during pregnancy isn’t significantly more common than in an affected woman’s male partner during the same time frame.
“There was no large difference in wheezing between the women and men. Therefore, we cannot propose the term ‘gestational asthma,’” said Dr. Karmaus, professor of epidemiology at the University of Memphis. “Investigations into how to prevent eczema and asthma in pregnancy are really important, because eczema and asthma in pregnancy can increase the risk of these diseases in the offspring. This is a totally undeveloped field.”
He presented new findings from the Isle of Wight study, a prospective study in which a cohort of women has been followed from birth through pregnancy across three generations.
Eczema and asthma are common atopic diseases, and they are particularly common during pregnancy. Indeed, eczema is the most common skin disease seen in pregnancy, accounting for 35%-50% of all dermatoses in previous studies by other investigators. In those studies, only 20%-40% of women with eczema during pregnancy had a prepregnancy history of the disease.
In the Isle of Wight cohort, women were evaluated for asthma and eczema symptoms at ages 1, 2, 4, 10, and 18 years and again during pregnancy at gestational weeks 20 and 28. A total of 26 of 116 women developed eczema during pregnancy, with eight of them (31%) experiencing the skin disease for the first time in their lives. In contrast, only six of their male partners had eczema during the pregnancy time frame, and just one of them had new-onset eczema.
A history of maternal eczema in the preceding generation was associated with a 52% increased relative risk of having eczema by age 18 and a 3.1-fold increased likelihood of eczema during pregnancy. Also, methylation of the filaggrin gene at the cytosine-phosphate-guanine site cg13447818 when assessed at age 18 was associated with a significantly increased likelihood of eczema in a subject’s mother as well as increased risk of gestational eczema 1-7 years later, Dr. Karmaus continued.
Eighteen percent of women in the Isle of Wight cohort had asthma during pregnancy, as did a similar proportion of their male partners. Twenty-seven percent of women with asthma during pregnancy had no previous history of the respiratory disease, a rate which was again comparable in their male partners with asthma.
DNA methylation of the IL1RL1 gene at cg17738684 was significantly associated with asthma heritability across three generations in the Isle of Wight study. The IL1RL1 gene at cg17738684 is a candidate gene for asthma that encodes for interleukin-33. This finding raises the possibility that addressing this DNA methylation could prove fruitful as a transgenerational asthma prevention strategy.
The Isle of Wight birth cohort study is funded by the National Institutes of Health, Asthma UK, and the Isle of Wight Trust. Dr. Karmaus reported having no financial conflicts of interest.
AT 2015 AAAAI ANNUAL MEETING
Key clinical point: Thirty-one percent of cases of eczema and 27% of asthma in a group of pregnant women occurred for the first time in the woman’s life.
Major finding: A history of maternal eczema was associated with a 3.1-fold increased likelihood of eczema during the offspring’s pregnancy.
Data source: The Isle of Wight birth cohort study is a prospective study following three generations from birth through pregnancy.
Disclosures: The study is funded by the National Institutes of Health, Asthma UK, and the Isle of Wight Trust. The presenter reported having no financial conflicts of interest.
Aerosolized Measles Vaccine Inferior to Subcutaneous
An aerosolized measles vaccine proved to be immunogenic but inferior to the subcutaneous vaccine in inducing seropositivity among infants in rural India, according to a report published online April 16 in the New England Journal of Medicine.
Aerosolized delivery of a measles vaccine could prove especially helpful in resource-poor countries where major measles outbreaks continue to occur because of poor health service infrastructure, but data concerning the efficacy of this form of delivery have been inconsistent. “Given the established record of injectable measles vaccine, alternative delivery methods should show noninferiority,” wrote Dr. Nicola Low of the Institute of Social and Preventive Medicine, University of Bern (Switzerland), and her associates.
They performed a randomized, open-label noninferiority trial comparing a primary dose of aerosolized measles vaccine (1,001 children) against subcutaneous measles vaccine (1,003 children) among babies aged 9-11.9 months living in villages in rural India. The primary endpoint – seropositivity for serum antibodies against measles at 91 days after vaccination – was 85.4% for aerosolized vaccine and 94.6% for subcutaneous vaccine. This difference of 9.2 percentage points did not reach the threshold for noninferiority, which was 5.0 percentage points, the investigators said (N. Engl. J. Med. 2015;372:1519-29 [doi:10.1056/NEJMoa1407417]).
Among the children who did achieve seropositivity, however, the geometric mean concentrations of measles antibodies were similar between the two study groups.
Adverse-event profiles were similar between aerosolized and subcutaneous vaccine, and adverse events were rare. The most common effects judged likely to be related to the vaccines were rash, coryza, cough, diarrhea, and fever.
After this study was designed, experts recommended providing a second dose of measles vaccine to protect children who did not respond to the first dose. Using this two-dose strategy, the aerosolized formulation induced higher and more sustained levels of seropositivity than the subcutaneous formulation in studies of school-aged children in South Africa. So it is possible that the aerosolized measles vaccine may prove to be noninferior in future studies involving a different dosing schedule and an older pediatric patient population, Dr. Low and her associates noted.
This study was funded by the Bill and Melinda Gates Foundation. The Serum Institute of India provided vaccines free of charge and Aerogen provided the delivery devices free of charge. Dr. Low reported several grants plus monies paid to her institution from the World Health Organization for projects about vaccines and sexually transmitted infections; her associates reported ties to the Serum Institute of India, Aerogen, and Dance Biopharm. One associate has a patent pending on an aerosol device licensed to Novartis and another has a patent pending related to vaccine nebulizers.
An aerosolized measles vaccine proved to be immunogenic but inferior to the subcutaneous vaccine in inducing seropositivity among infants in rural India, according to a report published online April 16 in the New England Journal of Medicine.
Aerosolized delivery of a measles vaccine could prove especially helpful in resource-poor countries where major measles outbreaks continue to occur because of poor health service infrastructure, but data concerning the efficacy of this form of delivery have been inconsistent. “Given the established record of injectable measles vaccine, alternative delivery methods should show noninferiority,” wrote Dr. Nicola Low of the Institute of Social and Preventive Medicine, University of Bern (Switzerland), and her associates.
They performed a randomized, open-label noninferiority trial comparing a primary dose of aerosolized measles vaccine (1,001 children) against subcutaneous measles vaccine (1,003 children) among babies aged 9-11.9 months living in villages in rural India. The primary endpoint – seropositivity for serum antibodies against measles at 91 days after vaccination – was 85.4% for aerosolized vaccine and 94.6% for subcutaneous vaccine. This difference of 9.2 percentage points did not reach the threshold for noninferiority, which was 5.0 percentage points, the investigators said (N. Engl. J. Med. 2015;372:1519-29 [doi:10.1056/NEJMoa1407417]).
Among the children who did achieve seropositivity, however, the geometric mean concentrations of measles antibodies were similar between the two study groups.
Adverse-event profiles were similar between aerosolized and subcutaneous vaccine, and adverse events were rare. The most common effects judged likely to be related to the vaccines were rash, coryza, cough, diarrhea, and fever.
After this study was designed, experts recommended providing a second dose of measles vaccine to protect children who did not respond to the first dose. Using this two-dose strategy, the aerosolized formulation induced higher and more sustained levels of seropositivity than the subcutaneous formulation in studies of school-aged children in South Africa. So it is possible that the aerosolized measles vaccine may prove to be noninferior in future studies involving a different dosing schedule and an older pediatric patient population, Dr. Low and her associates noted.
This study was funded by the Bill and Melinda Gates Foundation. The Serum Institute of India provided vaccines free of charge and Aerogen provided the delivery devices free of charge. Dr. Low reported several grants plus monies paid to her institution from the World Health Organization for projects about vaccines and sexually transmitted infections; her associates reported ties to the Serum Institute of India, Aerogen, and Dance Biopharm. One associate has a patent pending on an aerosol device licensed to Novartis and another has a patent pending related to vaccine nebulizers.
An aerosolized measles vaccine proved to be immunogenic but inferior to the subcutaneous vaccine in inducing seropositivity among infants in rural India, according to a report published online April 16 in the New England Journal of Medicine.
Aerosolized delivery of a measles vaccine could prove especially helpful in resource-poor countries where major measles outbreaks continue to occur because of poor health service infrastructure, but data concerning the efficacy of this form of delivery have been inconsistent. “Given the established record of injectable measles vaccine, alternative delivery methods should show noninferiority,” wrote Dr. Nicola Low of the Institute of Social and Preventive Medicine, University of Bern (Switzerland), and her associates.
They performed a randomized, open-label noninferiority trial comparing a primary dose of aerosolized measles vaccine (1,001 children) against subcutaneous measles vaccine (1,003 children) among babies aged 9-11.9 months living in villages in rural India. The primary endpoint – seropositivity for serum antibodies against measles at 91 days after vaccination – was 85.4% for aerosolized vaccine and 94.6% for subcutaneous vaccine. This difference of 9.2 percentage points did not reach the threshold for noninferiority, which was 5.0 percentage points, the investigators said (N. Engl. J. Med. 2015;372:1519-29 [doi:10.1056/NEJMoa1407417]).
Among the children who did achieve seropositivity, however, the geometric mean concentrations of measles antibodies were similar between the two study groups.
Adverse-event profiles were similar between aerosolized and subcutaneous vaccine, and adverse events were rare. The most common effects judged likely to be related to the vaccines were rash, coryza, cough, diarrhea, and fever.
After this study was designed, experts recommended providing a second dose of measles vaccine to protect children who did not respond to the first dose. Using this two-dose strategy, the aerosolized formulation induced higher and more sustained levels of seropositivity than the subcutaneous formulation in studies of school-aged children in South Africa. So it is possible that the aerosolized measles vaccine may prove to be noninferior in future studies involving a different dosing schedule and an older pediatric patient population, Dr. Low and her associates noted.
This study was funded by the Bill and Melinda Gates Foundation. The Serum Institute of India provided vaccines free of charge and Aerogen provided the delivery devices free of charge. Dr. Low reported several grants plus monies paid to her institution from the World Health Organization for projects about vaccines and sexually transmitted infections; her associates reported ties to the Serum Institute of India, Aerogen, and Dance Biopharm. One associate has a patent pending on an aerosol device licensed to Novartis and another has a patent pending related to vaccine nebulizers.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Respiratory disorders most common cause of childhood hospitalization
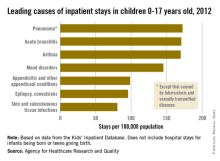
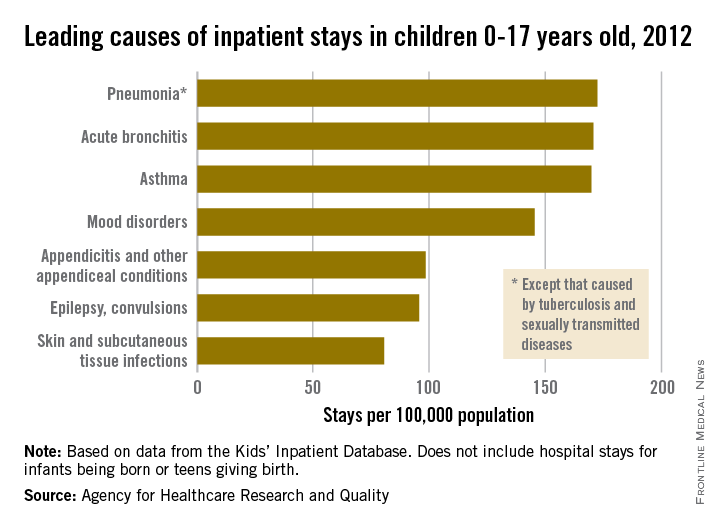
The three most common causes of nonneonatal and nonmaternal hospitalization in 2012 for children under 18 years were all respiratory disorders, according to a report from the Agency for Healthcare Research and Quality.
Pneumonia was the most common cause of hospitalization in children, with stays occurring at a rate of 169/100,000 population. There was very little difference in the rate between pneumonia and the next two most common diagnoses, acute bronchitis and asthma, which occurred at a rate of 168/100,000 and 167/100,000, respectively.
Mood disorders were the most common nonrespiratory and nonmaternal hospitalization diagnosis, with a hospital admission incidence of 144/100,000 population. This was followed by appendicitis (97/100,000), epilepsy and convulsions (95/100,000), and skin and subcutaneous tissue infections (80/100,000). The overall rate of hospitalization in children under age 18 years for nonneonatal and nonmaternal diagnoses was just over 2,500/100,000.
The incidence of six of the seven most common causes of hospitalization, and all but 1 of the 24 reported diagnostic categories, either decreased or remained steady from 2000 to 2012. Only skin and subcutaneous tissue conditions saw a significant increase in that time period. “Understanding the reasons why children are hospitalized and examining trends over time is critical to inform clinical practice and health policy,” the researchers commented.
The AHRQ report used data collected by the Healthcare Cost and Utilization Project Kids’ Inpatient Database.
The three most common causes of nonneonatal and nonmaternal hospitalization in 2012 for children under 18 years were all respiratory disorders, according to a report from the Agency for Healthcare Research and Quality.
Pneumonia was the most common cause of hospitalization in children, with stays occurring at a rate of 169/100,000 population. There was very little difference in the rate between pneumonia and the next two most common diagnoses, acute bronchitis and asthma, which occurred at a rate of 168/100,000 and 167/100,000, respectively.
Mood disorders were the most common nonrespiratory and nonmaternal hospitalization diagnosis, with a hospital admission incidence of 144/100,000 population. This was followed by appendicitis (97/100,000), epilepsy and convulsions (95/100,000), and skin and subcutaneous tissue infections (80/100,000). The overall rate of hospitalization in children under age 18 years for nonneonatal and nonmaternal diagnoses was just over 2,500/100,000.
The incidence of six of the seven most common causes of hospitalization, and all but 1 of the 24 reported diagnostic categories, either decreased or remained steady from 2000 to 2012. Only skin and subcutaneous tissue conditions saw a significant increase in that time period. “Understanding the reasons why children are hospitalized and examining trends over time is critical to inform clinical practice and health policy,” the researchers commented.
The AHRQ report used data collected by the Healthcare Cost and Utilization Project Kids’ Inpatient Database.
The three most common causes of nonneonatal and nonmaternal hospitalization in 2012 for children under 18 years were all respiratory disorders, according to a report from the Agency for Healthcare Research and Quality.
Pneumonia was the most common cause of hospitalization in children, with stays occurring at a rate of 169/100,000 population. There was very little difference in the rate between pneumonia and the next two most common diagnoses, acute bronchitis and asthma, which occurred at a rate of 168/100,000 and 167/100,000, respectively.
Mood disorders were the most common nonrespiratory and nonmaternal hospitalization diagnosis, with a hospital admission incidence of 144/100,000 population. This was followed by appendicitis (97/100,000), epilepsy and convulsions (95/100,000), and skin and subcutaneous tissue infections (80/100,000). The overall rate of hospitalization in children under age 18 years for nonneonatal and nonmaternal diagnoses was just over 2,500/100,000.
The incidence of six of the seven most common causes of hospitalization, and all but 1 of the 24 reported diagnostic categories, either decreased or remained steady from 2000 to 2012. Only skin and subcutaneous tissue conditions saw a significant increase in that time period. “Understanding the reasons why children are hospitalized and examining trends over time is critical to inform clinical practice and health policy,” the researchers commented.
The AHRQ report used data collected by the Healthcare Cost and Utilization Project Kids’ Inpatient Database.
CDC Ebola Vaccine Trial Underway in Sierra Leone
The Centers for Disease Control and Prevention is looking to enroll 6,000 people in an Ebola vaccine trial now underway in Sierra Leone.
Frontline workers are being randomized to the experimental vaccine rVSV-ZEBOV either up front or 6 months after enrollment. Subjects then will be followed for 6 months to see how well the vaccine protects them from infection. The trial will cost about $25 million, funded mostly by the U.S. government.
“The Ebola epidemic has been devastating for West Africa. I really hope that this is the beginning of a new positive chapter for Sierra Leone and the story of battling this virus,” said Dr. Anne Schuchat, director of CDC’s National Center for Immunization and Respiratory Diseases.
So far, about 200 people have enrolled in the study, and more than 90 have gotten the shot. Foreign aid workers are eligible for the trial, but because subjects need to be in country long enough for follow-up, most will be from Sierra Leone. To cover time and transportation costs, participants are paid $10 for enrollment and $30 after they get the vaccine, Dr. Schuchat said in an April 14 teleconference.
Subjects include doctors, nurses, janitors, lab technicians, security guards, pharmacists, administrators, burial workers, and those who wash the bodies of the newly dead. The study is being conducted in hard-hit areas of Sierra Leone, including the capital of Freetown. Similar studies are underway in Liberia and Guinea.
Ebola cases “are way down” in West Africa lately, “but people are continuing to get” the disease, “and some of the Ebola outbreaks in the past have had long tails,” so health care workers remain at risk, according to Dr. Schuchat.
With infection rates down, it’s unclear whether or not the study will have enough cases to demonstrate efficacy for rVSV-ZEBOV. Even so, “we plan to collect information on safety and immune response that could contribute to licensure pathways even if we are not able to estimate efficacy,” she said.
About 800 people have gotten rVSV-ZEBOV in other trials. Common side effects include fatigue, fever, headaches, and muscle aches. There have also been reports of mild, self-limited joint pain and swelling.
CDC is working with the Sierra Leone College of Medicine and Allied Health Sciences and the Sierra Leone Ministry of Health and Sanitation to conduct the trial, dubbed The Sierra Leone Trial to Introduce a Vaccine against Ebola (STRIVE). About 350 local people have been hired and trained to help with the study.
The vaccine uses a vesicular stomatitis virus carrying a noninfectious Ebola virus gene, and has been shown to produce an immune response against the virus. It was developed by the Public Health Agency of Canada’s National Microbiology Laboratory and is licensed to NewLink Genetics. In 2014, NewLink entered into a licensing and collaboration agreement with Merck to develop rVSV-ZEBOV.
Until it is known whether or not the vaccine works, and how well, CDC cautioned that vaccinated people still need to take full protective measures against Ebola.
The Centers for Disease Control and Prevention is looking to enroll 6,000 people in an Ebola vaccine trial now underway in Sierra Leone.
Frontline workers are being randomized to the experimental vaccine rVSV-ZEBOV either up front or 6 months after enrollment. Subjects then will be followed for 6 months to see how well the vaccine protects them from infection. The trial will cost about $25 million, funded mostly by the U.S. government.
“The Ebola epidemic has been devastating for West Africa. I really hope that this is the beginning of a new positive chapter for Sierra Leone and the story of battling this virus,” said Dr. Anne Schuchat, director of CDC’s National Center for Immunization and Respiratory Diseases.
So far, about 200 people have enrolled in the study, and more than 90 have gotten the shot. Foreign aid workers are eligible for the trial, but because subjects need to be in country long enough for follow-up, most will be from Sierra Leone. To cover time and transportation costs, participants are paid $10 for enrollment and $30 after they get the vaccine, Dr. Schuchat said in an April 14 teleconference.
Subjects include doctors, nurses, janitors, lab technicians, security guards, pharmacists, administrators, burial workers, and those who wash the bodies of the newly dead. The study is being conducted in hard-hit areas of Sierra Leone, including the capital of Freetown. Similar studies are underway in Liberia and Guinea.
Ebola cases “are way down” in West Africa lately, “but people are continuing to get” the disease, “and some of the Ebola outbreaks in the past have had long tails,” so health care workers remain at risk, according to Dr. Schuchat.
With infection rates down, it’s unclear whether or not the study will have enough cases to demonstrate efficacy for rVSV-ZEBOV. Even so, “we plan to collect information on safety and immune response that could contribute to licensure pathways even if we are not able to estimate efficacy,” she said.
About 800 people have gotten rVSV-ZEBOV in other trials. Common side effects include fatigue, fever, headaches, and muscle aches. There have also been reports of mild, self-limited joint pain and swelling.
CDC is working with the Sierra Leone College of Medicine and Allied Health Sciences and the Sierra Leone Ministry of Health and Sanitation to conduct the trial, dubbed The Sierra Leone Trial to Introduce a Vaccine against Ebola (STRIVE). About 350 local people have been hired and trained to help with the study.
The vaccine uses a vesicular stomatitis virus carrying a noninfectious Ebola virus gene, and has been shown to produce an immune response against the virus. It was developed by the Public Health Agency of Canada’s National Microbiology Laboratory and is licensed to NewLink Genetics. In 2014, NewLink entered into a licensing and collaboration agreement with Merck to develop rVSV-ZEBOV.
Until it is known whether or not the vaccine works, and how well, CDC cautioned that vaccinated people still need to take full protective measures against Ebola.
The Centers for Disease Control and Prevention is looking to enroll 6,000 people in an Ebola vaccine trial now underway in Sierra Leone.
Frontline workers are being randomized to the experimental vaccine rVSV-ZEBOV either up front or 6 months after enrollment. Subjects then will be followed for 6 months to see how well the vaccine protects them from infection. The trial will cost about $25 million, funded mostly by the U.S. government.
“The Ebola epidemic has been devastating for West Africa. I really hope that this is the beginning of a new positive chapter for Sierra Leone and the story of battling this virus,” said Dr. Anne Schuchat, director of CDC’s National Center for Immunization and Respiratory Diseases.
So far, about 200 people have enrolled in the study, and more than 90 have gotten the shot. Foreign aid workers are eligible for the trial, but because subjects need to be in country long enough for follow-up, most will be from Sierra Leone. To cover time and transportation costs, participants are paid $10 for enrollment and $30 after they get the vaccine, Dr. Schuchat said in an April 14 teleconference.
Subjects include doctors, nurses, janitors, lab technicians, security guards, pharmacists, administrators, burial workers, and those who wash the bodies of the newly dead. The study is being conducted in hard-hit areas of Sierra Leone, including the capital of Freetown. Similar studies are underway in Liberia and Guinea.
Ebola cases “are way down” in West Africa lately, “but people are continuing to get” the disease, “and some of the Ebola outbreaks in the past have had long tails,” so health care workers remain at risk, according to Dr. Schuchat.
With infection rates down, it’s unclear whether or not the study will have enough cases to demonstrate efficacy for rVSV-ZEBOV. Even so, “we plan to collect information on safety and immune response that could contribute to licensure pathways even if we are not able to estimate efficacy,” she said.
About 800 people have gotten rVSV-ZEBOV in other trials. Common side effects include fatigue, fever, headaches, and muscle aches. There have also been reports of mild, self-limited joint pain and swelling.
CDC is working with the Sierra Leone College of Medicine and Allied Health Sciences and the Sierra Leone Ministry of Health and Sanitation to conduct the trial, dubbed The Sierra Leone Trial to Introduce a Vaccine against Ebola (STRIVE). About 350 local people have been hired and trained to help with the study.
The vaccine uses a vesicular stomatitis virus carrying a noninfectious Ebola virus gene, and has been shown to produce an immune response against the virus. It was developed by the Public Health Agency of Canada’s National Microbiology Laboratory and is licensed to NewLink Genetics. In 2014, NewLink entered into a licensing and collaboration agreement with Merck to develop rVSV-ZEBOV.
Until it is known whether or not the vaccine works, and how well, CDC cautioned that vaccinated people still need to take full protective measures against Ebola.
FROM A CDC TELECONFERENCE
Mast cells predict hypersensitive reactions in rituximab desensitization
HOUSTON – For rituximab patients undergoing desensitization, mast cell degranulation is a more effective predictor of hypersensitive reactions (HSR) than are the traditionally employed skin tests, according to a study presented at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
“In this particular study, we were interested in investigating the roles of immunoglobulin E (IgE) in the mast cells in rituximab hypersensitivity,” said Dr. Johnson T. Wong of Massachusetts General Hospital, Boston, who presented the findings during a session dedicated to “Novel Insights in Drug Allergy.”
The study comprised 25 patients with persistent or severe rituximab sensitivity (RITS) who were treated at Massachusetts General between 2008 and 2013. Dr. Wong and his coinvestigators performed skin testing on 18 subjects, of which 7 (39%) reacted positively at least once during the course of testing.
HSR reactions occurred at similar rates in subjects who reacted positively and negatively to skin testing. Tryptase levels were taken in 18 subjects (72%), during 67% of the HSR desensitizations and 21% of the asymptomatic desensitizations.
Overall, elevated levels were noted in 21% of the HSR desensitizations.
The elevated tryptase levels in patients experiencing HSR indicates that mast cell degranulation is likely a major contributing factor in causing HSR, with cytokine release and tumor lysis also playing an important role, Dr. Wong said. Mast cell degranulations also points to an association with IgE mechanisms as a reliable indicator of HSR likelihood.
Dr. Wong and his coauthors concluded via tryptase level testing that asymptomatic mast cell degranulation happened very rarely, as only 1 out of 27 assessments (3%) indicated that result. One patient was excluded from the HSR desensitization cohort’s results because of probable mast cell activation syndrome, which caused abnormally elevated tryptase levels throughout the study period.
“In both positive and negative skin test patients, our desensitization protocol was able to reduce the reaction rate to less than 30%, so around 70% of patients had no reaction at all during desensitization,” said Dr. Wong, adding that nearly all desensitizations were completed successfully.
Dr. Wong had no disclosures.
HOUSTON – For rituximab patients undergoing desensitization, mast cell degranulation is a more effective predictor of hypersensitive reactions (HSR) than are the traditionally employed skin tests, according to a study presented at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
“In this particular study, we were interested in investigating the roles of immunoglobulin E (IgE) in the mast cells in rituximab hypersensitivity,” said Dr. Johnson T. Wong of Massachusetts General Hospital, Boston, who presented the findings during a session dedicated to “Novel Insights in Drug Allergy.”
The study comprised 25 patients with persistent or severe rituximab sensitivity (RITS) who were treated at Massachusetts General between 2008 and 2013. Dr. Wong and his coinvestigators performed skin testing on 18 subjects, of which 7 (39%) reacted positively at least once during the course of testing.
HSR reactions occurred at similar rates in subjects who reacted positively and negatively to skin testing. Tryptase levels were taken in 18 subjects (72%), during 67% of the HSR desensitizations and 21% of the asymptomatic desensitizations.
Overall, elevated levels were noted in 21% of the HSR desensitizations.
The elevated tryptase levels in patients experiencing HSR indicates that mast cell degranulation is likely a major contributing factor in causing HSR, with cytokine release and tumor lysis also playing an important role, Dr. Wong said. Mast cell degranulations also points to an association with IgE mechanisms as a reliable indicator of HSR likelihood.
Dr. Wong and his coauthors concluded via tryptase level testing that asymptomatic mast cell degranulation happened very rarely, as only 1 out of 27 assessments (3%) indicated that result. One patient was excluded from the HSR desensitization cohort’s results because of probable mast cell activation syndrome, which caused abnormally elevated tryptase levels throughout the study period.
“In both positive and negative skin test patients, our desensitization protocol was able to reduce the reaction rate to less than 30%, so around 70% of patients had no reaction at all during desensitization,” said Dr. Wong, adding that nearly all desensitizations were completed successfully.
Dr. Wong had no disclosures.
HOUSTON – For rituximab patients undergoing desensitization, mast cell degranulation is a more effective predictor of hypersensitive reactions (HSR) than are the traditionally employed skin tests, according to a study presented at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
“In this particular study, we were interested in investigating the roles of immunoglobulin E (IgE) in the mast cells in rituximab hypersensitivity,” said Dr. Johnson T. Wong of Massachusetts General Hospital, Boston, who presented the findings during a session dedicated to “Novel Insights in Drug Allergy.”
The study comprised 25 patients with persistent or severe rituximab sensitivity (RITS) who were treated at Massachusetts General between 2008 and 2013. Dr. Wong and his coinvestigators performed skin testing on 18 subjects, of which 7 (39%) reacted positively at least once during the course of testing.
HSR reactions occurred at similar rates in subjects who reacted positively and negatively to skin testing. Tryptase levels were taken in 18 subjects (72%), during 67% of the HSR desensitizations and 21% of the asymptomatic desensitizations.
Overall, elevated levels were noted in 21% of the HSR desensitizations.
The elevated tryptase levels in patients experiencing HSR indicates that mast cell degranulation is likely a major contributing factor in causing HSR, with cytokine release and tumor lysis also playing an important role, Dr. Wong said. Mast cell degranulations also points to an association with IgE mechanisms as a reliable indicator of HSR likelihood.
Dr. Wong and his coauthors concluded via tryptase level testing that asymptomatic mast cell degranulation happened very rarely, as only 1 out of 27 assessments (3%) indicated that result. One patient was excluded from the HSR desensitization cohort’s results because of probable mast cell activation syndrome, which caused abnormally elevated tryptase levels throughout the study period.
“In both positive and negative skin test patients, our desensitization protocol was able to reduce the reaction rate to less than 30%, so around 70% of patients had no reaction at all during desensitization,” said Dr. Wong, adding that nearly all desensitizations were completed successfully.
Dr. Wong had no disclosures.
AT THE 2015 AAAAI ANNUAL MEETING
Key clinical point: Look to mast cell degranulation, not skin tests, for accurate information regarding the likelihood of hypersensitive reactions in rituximab patients undergoing desensitization.
Major finding: Tryptase levels were elevated in 21% of patients who experienced hypersensitive reactions during desensitization.
Data source: Study of 25 patients with rituximab sensitivity at Massachusetts General Hospital.
Disclosures: Dr. Wong reported no relevant financial disclosures.
‘Mossy Oak sign’ suggests delayed anaphylaxis to red meat
HOUSTON– In central Virginia, where IgE-mediated delayed allergic reactions to red meat have become the most common cause of anaphylaxis in adults, physicians have taken to looking for what they call the ‘Mossy Oak sign.’
“If a patient shows up in a blaze-orange cap and hunter’s camouflage fatigues, an allergy fellow will tell me, ‘There’s a positive Mossy Oak sign in room 2,’ and I know that probably means the patient has delayed anaphylaxis to alpha-gal,” Dr. Scott P. Commins said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Alpha-gal is short for galactose-alpha-1,3,-galactose, an oligosaccharide present on thyroglobulin and other tissues in nonprimate mammals. It’s not normally present in humans, but when a lone star tick (Amblyomma americanum) that has fed on a nonprimate mammal bites a human, the alpha-gal is transferred, eliciting serum IgE antibodies.
Mossy Oak is a popular brand of hunter’s camouflage clothing. A positive Mossy Oak sign is useful in clinical practice because a patient who presents to a medical clinic dressed in hunting regalia is someone who spends a lot of time outdoors in the woods and fields where ticks lurk. He’s also typically someone who enjoys eating red meat. And whether it’s venison, beef, pork, lamb, goat, or bison, it contains alpha-gal. The result, in a patient who’s been primed via tick bite, can be a life-threatening anaphylactic or urticarial reaction arising 3-6 hours later, explained Dr. Commins, an allergist at the University of Virginia, Charlottesville.
He and his coinvestigators have played a central role in the still-unfolding story of this novel disease involving late-onset anaphylaxis to mammalian meat. Dr. Commins was the lead author of the paper that first described the syndrome (J. Allergy Clin. Immunol. 2009;123:426-33), as well as a subsequent paper that established the lone star tick as the culprit, making this syndrome the first known example of a response to an ectoparasite giving rise to a serious form of food allergy (J. Allergy Clin. Immunol. 2011;127:1286-93). More recently, the investigators have shown that alpha-gal-specific IgE does not cause or worsen asthma (Am. J. Respir. Crit. Care Med. 2012;185:723-30).
In a wide-ranging talk, Dr. Commins addressed the diagnosis and management of delayed anaphylaxis to red meat. He also touched upon some provocative emerging issues, including the possible risks posed by placing a porcine heart valve or bioprosthetic ligament in a patient with serum IgE antibodies to alpha-gal.
The investigators stumbled upon the phenomenon of tick-transmitted delayed anaphylaxis to red meat while they were trying to unravel the explanation for the markedly regional occurrence of IgE-mediated hypersensitivity reactions to the chimeric monoclonal antibody cetuximab (Erbitux) previously reported in the oncology literature (J. Clin. Oncol. 2007;25:3644-8). Dr. Commins and his colleagues realized that the same southeastern and south-central states where reactions to the initial infusion of cetuximab were concentrated were the states where the lone star tick abounds.
Incidentally, scientists at the Centers for Disease Control and Prevention follow the lone star tick closely because it is the primary vector for ehrlichiosis. CDC researchers say the tick’s range is steadily expanding and now includes 28 states, with New York’s Long Island a hot spot.
There is no public health requirement to report serum IgE-mediated delayed reactions to red meat, so the exact number of affected patients is unknown. But it’s clear that many thousands of individuals are affected, and estimates are being revised upward as the novel syndrome becomes more widely known. The disorder is common in Europe and Australia as well.
Classically, IgE-mediated anaphylactic reactions occur within 5-30 minutes after exposure to the offending agent. Thus, the 3- to 6-hour delay in symptom onset in patients with a reaction to the alpha-gal in red meat is remarkable; the explanation for the time lag remains unclear.
Dr. Commins and others have shown that individuals with serum IgE antibodies to alpha-gal also typically have serum IgE antibodies to cat, dog, beef, and pork, but not to egg, peanut, chicken, fish, or house dust mite.
Diagnosis of IgE-mediated urticarial or anaphylactic reactions to mammalian meat is made on the basis of the presence of serum IgE antibodies to alpha-gal. Dr. Commins recommended considering the diagnosis and ordering the blood test in the setting of new-onset anaphylaxis in a patient who enjoys hunting or other outdoor activities in a state where the lone star tick is found, particularly if the symptoms occur at night, hours after a big meat-heavy meal. A history of recent or persistent tick bites is an obvious clue.
“Also, it’s striking how many patients develop palmar erythema and itching during an episode. Not all report it, but when they do it’s usually a pretty good giveaway that they might have IgE to alpha-gal,” according to the allergist.
He added that it’s entirely reasonable to order a screening test for IgE to alpha-gal in patients in lone star tick–abiding states whose anaphylactic reactions seem to occur randomly without an apparent trigger.
Dr. Commins and his coinvestigators have assembled a database of roughly 500 of their patients with IgE to alpha-gal, about half of whom have a history of atopy. The investigators have found that an individual’s atopic status has no bearing on IgE antibody titer or the severity of the delayed reactions. Moreover, neither the alpha-gal IgE antibody level, the ratio of alpha-gal-specific IgE to total IgE, nor IgG antibodies correlate with reaction severity, he continued.
Based upon their study of 45 affected children, Dr. Commins and coworkers concluded that the clinical presentation and serum IgE pattern are the same as in adults (Pediatrics 2013 May [doi:10.1542/peds.2012-2585]). Since that publication, however, the investigators have realized there is a subgroup of affected teenagers who present with GI symptoms, he added.
Turning to disease management, Dr. Commins said he advocates an avoidance diet that eliminates mammalian meats, rich desserts, and super-premium ice cream.
“I also counsel patients to avoid broths, gravies, and anything that might be a mystery sauce,” he said. “You’d be surprised at how many people with alpha-gal order chicken at a Mexican restaurant thinking that they’re doing the right thing and end up reacting. I don’t know exactly why it happens, so I just say ‘avoid mystery sauces.’ Dairy and cheese are actually fairly well tolerated, although soft cheeses, like brie, can cause a reaction.”
Reactions are inconsistent, and symptoms can vary from episode to episode. Cofactors are a concern, with exercise and alcohol tending to make patients more sensitive to an alpha-gal exposure. The degree of risk posed by vaccines containing gelatin constitutes an emerging and unresolved issue.
“We’ve heard of several reactions to the shingles vaccine because of the gelatin, and the MMR vaccine is also on the radar,” Dr. Commins said.
Implantation of porcine bioprosthetic heart valves in patients with serum IgE antibodies to alpha-gal has been associated with reports of early valve failure; all bioprosthetic valves contain alpha-gal unless they’ve been decellularized. In addition, Dr. Commins is familiar with a case at another university in which three separate attempts to place a bioprosthetic ligament during repeated arthroscopic knee surgeries failed in a patient who had a “screamingly high” level of IgE to alpha-gal.
“I think this bioprosthesis issue is yet to be resolved,” he added.
One audience member, a Texas allergist, said she has a lot of trouble convincing her patients who are avid hunters to give up eating red meats. Dr. Commins said he faces the same issue.
“There is a recalcitrant group that just wants to eat a side of beef every day. I tell them if you’re not going to be on an avoidance diet, at least avoid the fattier cuts and don’t eat tremendous amounts. We believe that the antigen is possibly a glycolipid. Those cuts of meat that are high in fat are the ones patients tell us over and over again give them the worst reactions,” he said.
“The inconsistency of the allergic reactions keeps some patients from taking this disease seriously,” the allergist added. “What eventually happens for some patients is they end up having a really bad reaction. And then that convinces them.”
He monitors affected patients’ alpha-gal IgE levels over time. If and when the IgE becomes negative, he recommends a food challenge test. The patient comes in at 8 a.m., eats three pork sausage patties, and spends the day under observation at the clinic, walking the stairs periodically since exercise is a cofactor. If the challenge goes off without a hitch, the patient is free to go home at 4 or 5 p.m. In the past, that was the patient’s ticket to clearance to safely eat a big meat meal with alcohol, but Dr. Commins has pulled back of late from that recommendation.
“We believe that additional tick bites can make the allergy come back. So if someone passes a challenge in October and then the following spring gets more tick bites, you may have set them up to have a reaction because you’ve told them they can eat meat again. So the utility of a negative food challenge is unclear unless you’re pretty confident a patient is not going to have more tick bites,” he explained.
Dr. Commins reported receiving research grants from the National Institutes of Health to conduct his studies on delayed anaphylactic reactions to red meat. He serves on speakers bureaus for Genentech and Teva.
HOUSTON– In central Virginia, where IgE-mediated delayed allergic reactions to red meat have become the most common cause of anaphylaxis in adults, physicians have taken to looking for what they call the ‘Mossy Oak sign.’
“If a patient shows up in a blaze-orange cap and hunter’s camouflage fatigues, an allergy fellow will tell me, ‘There’s a positive Mossy Oak sign in room 2,’ and I know that probably means the patient has delayed anaphylaxis to alpha-gal,” Dr. Scott P. Commins said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Alpha-gal is short for galactose-alpha-1,3,-galactose, an oligosaccharide present on thyroglobulin and other tissues in nonprimate mammals. It’s not normally present in humans, but when a lone star tick (Amblyomma americanum) that has fed on a nonprimate mammal bites a human, the alpha-gal is transferred, eliciting serum IgE antibodies.
Mossy Oak is a popular brand of hunter’s camouflage clothing. A positive Mossy Oak sign is useful in clinical practice because a patient who presents to a medical clinic dressed in hunting regalia is someone who spends a lot of time outdoors in the woods and fields where ticks lurk. He’s also typically someone who enjoys eating red meat. And whether it’s venison, beef, pork, lamb, goat, or bison, it contains alpha-gal. The result, in a patient who’s been primed via tick bite, can be a life-threatening anaphylactic or urticarial reaction arising 3-6 hours later, explained Dr. Commins, an allergist at the University of Virginia, Charlottesville.
He and his coinvestigators have played a central role in the still-unfolding story of this novel disease involving late-onset anaphylaxis to mammalian meat. Dr. Commins was the lead author of the paper that first described the syndrome (J. Allergy Clin. Immunol. 2009;123:426-33), as well as a subsequent paper that established the lone star tick as the culprit, making this syndrome the first known example of a response to an ectoparasite giving rise to a serious form of food allergy (J. Allergy Clin. Immunol. 2011;127:1286-93). More recently, the investigators have shown that alpha-gal-specific IgE does not cause or worsen asthma (Am. J. Respir. Crit. Care Med. 2012;185:723-30).
In a wide-ranging talk, Dr. Commins addressed the diagnosis and management of delayed anaphylaxis to red meat. He also touched upon some provocative emerging issues, including the possible risks posed by placing a porcine heart valve or bioprosthetic ligament in a patient with serum IgE antibodies to alpha-gal.
The investigators stumbled upon the phenomenon of tick-transmitted delayed anaphylaxis to red meat while they were trying to unravel the explanation for the markedly regional occurrence of IgE-mediated hypersensitivity reactions to the chimeric monoclonal antibody cetuximab (Erbitux) previously reported in the oncology literature (J. Clin. Oncol. 2007;25:3644-8). Dr. Commins and his colleagues realized that the same southeastern and south-central states where reactions to the initial infusion of cetuximab were concentrated were the states where the lone star tick abounds.
Incidentally, scientists at the Centers for Disease Control and Prevention follow the lone star tick closely because it is the primary vector for ehrlichiosis. CDC researchers say the tick’s range is steadily expanding and now includes 28 states, with New York’s Long Island a hot spot.
There is no public health requirement to report serum IgE-mediated delayed reactions to red meat, so the exact number of affected patients is unknown. But it’s clear that many thousands of individuals are affected, and estimates are being revised upward as the novel syndrome becomes more widely known. The disorder is common in Europe and Australia as well.
Classically, IgE-mediated anaphylactic reactions occur within 5-30 minutes after exposure to the offending agent. Thus, the 3- to 6-hour delay in symptom onset in patients with a reaction to the alpha-gal in red meat is remarkable; the explanation for the time lag remains unclear.
Dr. Commins and others have shown that individuals with serum IgE antibodies to alpha-gal also typically have serum IgE antibodies to cat, dog, beef, and pork, but not to egg, peanut, chicken, fish, or house dust mite.
Diagnosis of IgE-mediated urticarial or anaphylactic reactions to mammalian meat is made on the basis of the presence of serum IgE antibodies to alpha-gal. Dr. Commins recommended considering the diagnosis and ordering the blood test in the setting of new-onset anaphylaxis in a patient who enjoys hunting or other outdoor activities in a state where the lone star tick is found, particularly if the symptoms occur at night, hours after a big meat-heavy meal. A history of recent or persistent tick bites is an obvious clue.
“Also, it’s striking how many patients develop palmar erythema and itching during an episode. Not all report it, but when they do it’s usually a pretty good giveaway that they might have IgE to alpha-gal,” according to the allergist.
He added that it’s entirely reasonable to order a screening test for IgE to alpha-gal in patients in lone star tick–abiding states whose anaphylactic reactions seem to occur randomly without an apparent trigger.
Dr. Commins and his coinvestigators have assembled a database of roughly 500 of their patients with IgE to alpha-gal, about half of whom have a history of atopy. The investigators have found that an individual’s atopic status has no bearing on IgE antibody titer or the severity of the delayed reactions. Moreover, neither the alpha-gal IgE antibody level, the ratio of alpha-gal-specific IgE to total IgE, nor IgG antibodies correlate with reaction severity, he continued.
Based upon their study of 45 affected children, Dr. Commins and coworkers concluded that the clinical presentation and serum IgE pattern are the same as in adults (Pediatrics 2013 May [doi:10.1542/peds.2012-2585]). Since that publication, however, the investigators have realized there is a subgroup of affected teenagers who present with GI symptoms, he added.
Turning to disease management, Dr. Commins said he advocates an avoidance diet that eliminates mammalian meats, rich desserts, and super-premium ice cream.
“I also counsel patients to avoid broths, gravies, and anything that might be a mystery sauce,” he said. “You’d be surprised at how many people with alpha-gal order chicken at a Mexican restaurant thinking that they’re doing the right thing and end up reacting. I don’t know exactly why it happens, so I just say ‘avoid mystery sauces.’ Dairy and cheese are actually fairly well tolerated, although soft cheeses, like brie, can cause a reaction.”
Reactions are inconsistent, and symptoms can vary from episode to episode. Cofactors are a concern, with exercise and alcohol tending to make patients more sensitive to an alpha-gal exposure. The degree of risk posed by vaccines containing gelatin constitutes an emerging and unresolved issue.
“We’ve heard of several reactions to the shingles vaccine because of the gelatin, and the MMR vaccine is also on the radar,” Dr. Commins said.
Implantation of porcine bioprosthetic heart valves in patients with serum IgE antibodies to alpha-gal has been associated with reports of early valve failure; all bioprosthetic valves contain alpha-gal unless they’ve been decellularized. In addition, Dr. Commins is familiar with a case at another university in which three separate attempts to place a bioprosthetic ligament during repeated arthroscopic knee surgeries failed in a patient who had a “screamingly high” level of IgE to alpha-gal.
“I think this bioprosthesis issue is yet to be resolved,” he added.
One audience member, a Texas allergist, said she has a lot of trouble convincing her patients who are avid hunters to give up eating red meats. Dr. Commins said he faces the same issue.
“There is a recalcitrant group that just wants to eat a side of beef every day. I tell them if you’re not going to be on an avoidance diet, at least avoid the fattier cuts and don’t eat tremendous amounts. We believe that the antigen is possibly a glycolipid. Those cuts of meat that are high in fat are the ones patients tell us over and over again give them the worst reactions,” he said.
“The inconsistency of the allergic reactions keeps some patients from taking this disease seriously,” the allergist added. “What eventually happens for some patients is they end up having a really bad reaction. And then that convinces them.”
He monitors affected patients’ alpha-gal IgE levels over time. If and when the IgE becomes negative, he recommends a food challenge test. The patient comes in at 8 a.m., eats three pork sausage patties, and spends the day under observation at the clinic, walking the stairs periodically since exercise is a cofactor. If the challenge goes off without a hitch, the patient is free to go home at 4 or 5 p.m. In the past, that was the patient’s ticket to clearance to safely eat a big meat meal with alcohol, but Dr. Commins has pulled back of late from that recommendation.
“We believe that additional tick bites can make the allergy come back. So if someone passes a challenge in October and then the following spring gets more tick bites, you may have set them up to have a reaction because you’ve told them they can eat meat again. So the utility of a negative food challenge is unclear unless you’re pretty confident a patient is not going to have more tick bites,” he explained.
Dr. Commins reported receiving research grants from the National Institutes of Health to conduct his studies on delayed anaphylactic reactions to red meat. He serves on speakers bureaus for Genentech and Teva.
HOUSTON– In central Virginia, where IgE-mediated delayed allergic reactions to red meat have become the most common cause of anaphylaxis in adults, physicians have taken to looking for what they call the ‘Mossy Oak sign.’
“If a patient shows up in a blaze-orange cap and hunter’s camouflage fatigues, an allergy fellow will tell me, ‘There’s a positive Mossy Oak sign in room 2,’ and I know that probably means the patient has delayed anaphylaxis to alpha-gal,” Dr. Scott P. Commins said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Alpha-gal is short for galactose-alpha-1,3,-galactose, an oligosaccharide present on thyroglobulin and other tissues in nonprimate mammals. It’s not normally present in humans, but when a lone star tick (Amblyomma americanum) that has fed on a nonprimate mammal bites a human, the alpha-gal is transferred, eliciting serum IgE antibodies.
Mossy Oak is a popular brand of hunter’s camouflage clothing. A positive Mossy Oak sign is useful in clinical practice because a patient who presents to a medical clinic dressed in hunting regalia is someone who spends a lot of time outdoors in the woods and fields where ticks lurk. He’s also typically someone who enjoys eating red meat. And whether it’s venison, beef, pork, lamb, goat, or bison, it contains alpha-gal. The result, in a patient who’s been primed via tick bite, can be a life-threatening anaphylactic or urticarial reaction arising 3-6 hours later, explained Dr. Commins, an allergist at the University of Virginia, Charlottesville.
He and his coinvestigators have played a central role in the still-unfolding story of this novel disease involving late-onset anaphylaxis to mammalian meat. Dr. Commins was the lead author of the paper that first described the syndrome (J. Allergy Clin. Immunol. 2009;123:426-33), as well as a subsequent paper that established the lone star tick as the culprit, making this syndrome the first known example of a response to an ectoparasite giving rise to a serious form of food allergy (J. Allergy Clin. Immunol. 2011;127:1286-93). More recently, the investigators have shown that alpha-gal-specific IgE does not cause or worsen asthma (Am. J. Respir. Crit. Care Med. 2012;185:723-30).
In a wide-ranging talk, Dr. Commins addressed the diagnosis and management of delayed anaphylaxis to red meat. He also touched upon some provocative emerging issues, including the possible risks posed by placing a porcine heart valve or bioprosthetic ligament in a patient with serum IgE antibodies to alpha-gal.
The investigators stumbled upon the phenomenon of tick-transmitted delayed anaphylaxis to red meat while they were trying to unravel the explanation for the markedly regional occurrence of IgE-mediated hypersensitivity reactions to the chimeric monoclonal antibody cetuximab (Erbitux) previously reported in the oncology literature (J. Clin. Oncol. 2007;25:3644-8). Dr. Commins and his colleagues realized that the same southeastern and south-central states where reactions to the initial infusion of cetuximab were concentrated were the states where the lone star tick abounds.
Incidentally, scientists at the Centers for Disease Control and Prevention follow the lone star tick closely because it is the primary vector for ehrlichiosis. CDC researchers say the tick’s range is steadily expanding and now includes 28 states, with New York’s Long Island a hot spot.
There is no public health requirement to report serum IgE-mediated delayed reactions to red meat, so the exact number of affected patients is unknown. But it’s clear that many thousands of individuals are affected, and estimates are being revised upward as the novel syndrome becomes more widely known. The disorder is common in Europe and Australia as well.
Classically, IgE-mediated anaphylactic reactions occur within 5-30 minutes after exposure to the offending agent. Thus, the 3- to 6-hour delay in symptom onset in patients with a reaction to the alpha-gal in red meat is remarkable; the explanation for the time lag remains unclear.
Dr. Commins and others have shown that individuals with serum IgE antibodies to alpha-gal also typically have serum IgE antibodies to cat, dog, beef, and pork, but not to egg, peanut, chicken, fish, or house dust mite.
Diagnosis of IgE-mediated urticarial or anaphylactic reactions to mammalian meat is made on the basis of the presence of serum IgE antibodies to alpha-gal. Dr. Commins recommended considering the diagnosis and ordering the blood test in the setting of new-onset anaphylaxis in a patient who enjoys hunting or other outdoor activities in a state where the lone star tick is found, particularly if the symptoms occur at night, hours after a big meat-heavy meal. A history of recent or persistent tick bites is an obvious clue.
“Also, it’s striking how many patients develop palmar erythema and itching during an episode. Not all report it, but when they do it’s usually a pretty good giveaway that they might have IgE to alpha-gal,” according to the allergist.
He added that it’s entirely reasonable to order a screening test for IgE to alpha-gal in patients in lone star tick–abiding states whose anaphylactic reactions seem to occur randomly without an apparent trigger.
Dr. Commins and his coinvestigators have assembled a database of roughly 500 of their patients with IgE to alpha-gal, about half of whom have a history of atopy. The investigators have found that an individual’s atopic status has no bearing on IgE antibody titer or the severity of the delayed reactions. Moreover, neither the alpha-gal IgE antibody level, the ratio of alpha-gal-specific IgE to total IgE, nor IgG antibodies correlate with reaction severity, he continued.
Based upon their study of 45 affected children, Dr. Commins and coworkers concluded that the clinical presentation and serum IgE pattern are the same as in adults (Pediatrics 2013 May [doi:10.1542/peds.2012-2585]). Since that publication, however, the investigators have realized there is a subgroup of affected teenagers who present with GI symptoms, he added.
Turning to disease management, Dr. Commins said he advocates an avoidance diet that eliminates mammalian meats, rich desserts, and super-premium ice cream.
“I also counsel patients to avoid broths, gravies, and anything that might be a mystery sauce,” he said. “You’d be surprised at how many people with alpha-gal order chicken at a Mexican restaurant thinking that they’re doing the right thing and end up reacting. I don’t know exactly why it happens, so I just say ‘avoid mystery sauces.’ Dairy and cheese are actually fairly well tolerated, although soft cheeses, like brie, can cause a reaction.”
Reactions are inconsistent, and symptoms can vary from episode to episode. Cofactors are a concern, with exercise and alcohol tending to make patients more sensitive to an alpha-gal exposure. The degree of risk posed by vaccines containing gelatin constitutes an emerging and unresolved issue.
“We’ve heard of several reactions to the shingles vaccine because of the gelatin, and the MMR vaccine is also on the radar,” Dr. Commins said.
Implantation of porcine bioprosthetic heart valves in patients with serum IgE antibodies to alpha-gal has been associated with reports of early valve failure; all bioprosthetic valves contain alpha-gal unless they’ve been decellularized. In addition, Dr. Commins is familiar with a case at another university in which three separate attempts to place a bioprosthetic ligament during repeated arthroscopic knee surgeries failed in a patient who had a “screamingly high” level of IgE to alpha-gal.
“I think this bioprosthesis issue is yet to be resolved,” he added.
One audience member, a Texas allergist, said she has a lot of trouble convincing her patients who are avid hunters to give up eating red meats. Dr. Commins said he faces the same issue.
“There is a recalcitrant group that just wants to eat a side of beef every day. I tell them if you’re not going to be on an avoidance diet, at least avoid the fattier cuts and don’t eat tremendous amounts. We believe that the antigen is possibly a glycolipid. Those cuts of meat that are high in fat are the ones patients tell us over and over again give them the worst reactions,” he said.
“The inconsistency of the allergic reactions keeps some patients from taking this disease seriously,” the allergist added. “What eventually happens for some patients is they end up having a really bad reaction. And then that convinces them.”
He monitors affected patients’ alpha-gal IgE levels over time. If and when the IgE becomes negative, he recommends a food challenge test. The patient comes in at 8 a.m., eats three pork sausage patties, and spends the day under observation at the clinic, walking the stairs periodically since exercise is a cofactor. If the challenge goes off without a hitch, the patient is free to go home at 4 or 5 p.m. In the past, that was the patient’s ticket to clearance to safely eat a big meat meal with alcohol, but Dr. Commins has pulled back of late from that recommendation.
“We believe that additional tick bites can make the allergy come back. So if someone passes a challenge in October and then the following spring gets more tick bites, you may have set them up to have a reaction because you’ve told them they can eat meat again. So the utility of a negative food challenge is unclear unless you’re pretty confident a patient is not going to have more tick bites,” he explained.
Dr. Commins reported receiving research grants from the National Institutes of Health to conduct his studies on delayed anaphylactic reactions to red meat. He serves on speakers bureaus for Genentech and Teva.
EXPERT ANALYSIS FROM THE 2015 AAAAI ANNUAL MEETING
Early pneumonia linked with later asthma and COPD
Lower respiratory illness in childhood is associated with later development of asthma and wheezing that can persist into adulthood, and may be a risk factor for adult chronic obstructive pulmonary disease (COPD), a prospective study has found.
Researchers assessed the lung function of 646 children – 338 of whom had experienced lower respiratory illness before age 3 years, and 308 controls – and found those who had early pneumonia had a nearly twofold increase in the risk of asthma and wheeze up to age 26 years.
They also had the most severe subsequent deficits in lung function, while those with early non-pneumonia lower respiratory illness had smaller but still significant impairments in lung function and increased risk of wheezing, according to a paper published online in Pediatrics (2015;135:607-15 [10.1542/peds.2014-3060]).
“Because there is considerable evidence that asthma associated with airflow limitation is a strong risk factor for subsequent chronic obstructive pulmonary disease, the prevention of early-life pneumonia and of the factors that determine low lung function in infancy may contribute significantly to decrease the public health burden of chronic obstructive pulmonary disease,” wrote Dr. Johnny Y.C. Chan of the Kwong Wah Hospital, Hong Kong, and his associates.
The study was funded by the National Institutes of Health, and no conflicts of interest were declared.
Lower respiratory illness in childhood is associated with later development of asthma and wheezing that can persist into adulthood, and may be a risk factor for adult chronic obstructive pulmonary disease (COPD), a prospective study has found.
Researchers assessed the lung function of 646 children – 338 of whom had experienced lower respiratory illness before age 3 years, and 308 controls – and found those who had early pneumonia had a nearly twofold increase in the risk of asthma and wheeze up to age 26 years.
They also had the most severe subsequent deficits in lung function, while those with early non-pneumonia lower respiratory illness had smaller but still significant impairments in lung function and increased risk of wheezing, according to a paper published online in Pediatrics (2015;135:607-15 [10.1542/peds.2014-3060]).
“Because there is considerable evidence that asthma associated with airflow limitation is a strong risk factor for subsequent chronic obstructive pulmonary disease, the prevention of early-life pneumonia and of the factors that determine low lung function in infancy may contribute significantly to decrease the public health burden of chronic obstructive pulmonary disease,” wrote Dr. Johnny Y.C. Chan of the Kwong Wah Hospital, Hong Kong, and his associates.
The study was funded by the National Institutes of Health, and no conflicts of interest were declared.
Lower respiratory illness in childhood is associated with later development of asthma and wheezing that can persist into adulthood, and may be a risk factor for adult chronic obstructive pulmonary disease (COPD), a prospective study has found.
Researchers assessed the lung function of 646 children – 338 of whom had experienced lower respiratory illness before age 3 years, and 308 controls – and found those who had early pneumonia had a nearly twofold increase in the risk of asthma and wheeze up to age 26 years.
They also had the most severe subsequent deficits in lung function, while those with early non-pneumonia lower respiratory illness had smaller but still significant impairments in lung function and increased risk of wheezing, according to a paper published online in Pediatrics (2015;135:607-15 [10.1542/peds.2014-3060]).
“Because there is considerable evidence that asthma associated with airflow limitation is a strong risk factor for subsequent chronic obstructive pulmonary disease, the prevention of early-life pneumonia and of the factors that determine low lung function in infancy may contribute significantly to decrease the public health burden of chronic obstructive pulmonary disease,” wrote Dr. Johnny Y.C. Chan of the Kwong Wah Hospital, Hong Kong, and his associates.
The study was funded by the National Institutes of Health, and no conflicts of interest were declared.
Key clinical point: Lower respiratory illness in childhood is associated with later development of asthma and wheezing that can persist into adulthood.
Major finding: Children who experienced pneumonia before age 3 years had a nearly twofold increase in the risk of asthma and wheeze up to age 26.
Data source: Prospective cohort study in 338 children with early lower respiratory illness and 308 controls.
Disclosures: The study was funded by the National Institutes of Health, and no conflicts of interest were declared.
Personalized Melanoma Vaccine Evokes Immune Response
A customized vaccine targeting patient-specific, tumor neoantigens evoked an immune response in three adults with advanced cutaneous melanoma in a proof-of-principle study that paves the way for a phase I trial.
The data are “too premature to conclude that the treatment had any therapeutic benefit to the patients,” Dr. Gerald P. Linette of Washington University in St. Louis, Mo., said in a teleconference. However, he noted that a phase I trial would likely begin within the next 9-12 months.
“Our primary goal was to see if this was safe, and if we could elicit an immune response,” Dr. Linette said.
Vaccination not only augmented T-cell immunity directed against naturally occurring, dominant tumor neoantigens, but also expanded the breadth of the immune response by revealing additional subdominant neoantigens, lead author Beatriz M. Carreno, Ph.D., of Washington University in St. Louis and her associates reported online April 2 in Science (doi:10.1126/science.aaa3828).
All three patients had stage IIIC, resected, cutaneous melanoma and had failed prior therapy with ipilimumab (Yervoy).
Some melanoma patients harbor tumor missense mutations, translated into amino acid substitutions (AAS). These mutations are thought to produce antigens that the immune system perceives as foreign, prompting a tumor-specific T-cell response. Heretofore, there has been no systematic evaluation of these neoantigens or whether vaccination can enhance these responses.
The aim of the current study was to determine the safety, tolerability, and immunologic responses to AAS peptides inside a modified dendritic cell vaccine. Seven AAS peptide candidates were selected among validated HLA-A*02:01 binders, along with the melanoma peptides G209-2M and G280-9V as positive controls for vaccination.
In each patient, T cell immunity to one AAS peptide was detected in prevaccine peripheral blood mononuclear cell samples.
The treatment differs from other personalized cancer vaccines in two key ways, Dr. Carreno said in the teleconference.
“We mature the [dendritic] cells before we give them back to the patients; the cells make growth factors that we have previously shown are important to the generation of the immune response,” she said. In addition, the vaccine was given as an infusion, rather than an injection, she noted.
Vaccination augmented the T-cell response to the three neoantigens – TMEM48 F169L, SEC24A P469, EXOC8 Q656P – with observed frequencies of 23%, 64%, and 89%, respectively.
Immune monitoring also detected T-cell immunity to two additional neoantigens per patient, Dr. Carreno and her associates reported.
Robust immune responses were detected as early as week 2 and peaked at weeks 8-9 after the initial dose. Each patient received three vaccine doses, without side effects or toxicity.
“Vaccination against tumor antigens appears safe as all three patients are alive and well, with no autoimmune adverse events,” the researchers wrote. Post vaccine restaging CT revealed stable disease in a 54-year-old man with BRAF V6003 mutation-positive melanoma. A second man, who had achieved a complete response to ipilimumab for BRAF V6003-positive disease, remains in complete remission after receiving the vaccine in the adjuvant setting.
The vaccine induced 30% tumor reduction in a woman with extensive skin metastases and 5-12 mm lung nodules after ipilimumab and vemurafenib (Zelboraf), but tumor size returned back to baseline dimensions 12 weeks later with no new disease sites. Her disease has remained stable for the past 8 months, the researchers said.
However, it is too soon to attribute a clinical benefit to the observed immune response to the vaccine, Dr. Linette emphasized. “I would be speculating if I said the vaccine had any benefit to the patients,” he said.
Vaccination increased the frequency of most existing prevaccine T-cell receptor-beta (TCR-beta) clonotypes and revealed new clonotypes for 6 neoantigens.
For both dominant and subdominant neoantigens, the TCR-beta repertoire was increased significantly after vaccination. For example, 84 clonotypes representing 19 TCR-beta families were detected for TKT R438W, 61 clonotypes representing 12 TCR-beta families were detected for SEC24A P469L, and 12 clonotypes representing 8 TCR-beta families were detected for EXOC8 Q656P.
“The revelation of a highly diverse TCR-beta repertoire specific for dominant and subdominant neoantigens was surprising and points to a potentially rich pool of naive tumor-specific T cells that remain ignorant unless activated by vaccination,” the researchers wrote.
Recent data indicate that CTLA-4 monoclonal antibody use can alter TCR repertoire diversity in patients, suggesting a potential strategy of combination checkpoint inhibitors, including ipilimumab, and neoantigen vaccines. The novel vaccine strategy also could be used to treat other malignancies with high mutational burdens such as lung, bladder, and colorectal cancers, while other genomic alterations may generate potential neoantigens relevant in low–mutational burden cancers such as leukemia, the authors suggest.
Although the cells in this study were grown in a specialized laboratory, “as the technology improves, I think this [strategy] will become reproducible and available at other medical centers,” Dr. Linette said.
This work was supported by Barnes-Jewish Hospital Foundation, Siteman Cancer Frontier Fund, Our Mark on Melanoma (MOM) Foundation, Come Out Swinging (COS) Foundation, Blackout Melanoma Foundation and the National Cancer Institute.
Heidi Splete contributed to this story.
A customized vaccine targeting patient-specific, tumor neoantigens evoked an immune response in three adults with advanced cutaneous melanoma in a proof-of-principle study that paves the way for a phase I trial.
The data are “too premature to conclude that the treatment had any therapeutic benefit to the patients,” Dr. Gerald P. Linette of Washington University in St. Louis, Mo., said in a teleconference. However, he noted that a phase I trial would likely begin within the next 9-12 months.
“Our primary goal was to see if this was safe, and if we could elicit an immune response,” Dr. Linette said.
Vaccination not only augmented T-cell immunity directed against naturally occurring, dominant tumor neoantigens, but also expanded the breadth of the immune response by revealing additional subdominant neoantigens, lead author Beatriz M. Carreno, Ph.D., of Washington University in St. Louis and her associates reported online April 2 in Science (doi:10.1126/science.aaa3828).
All three patients had stage IIIC, resected, cutaneous melanoma and had failed prior therapy with ipilimumab (Yervoy).
Some melanoma patients harbor tumor missense mutations, translated into amino acid substitutions (AAS). These mutations are thought to produce antigens that the immune system perceives as foreign, prompting a tumor-specific T-cell response. Heretofore, there has been no systematic evaluation of these neoantigens or whether vaccination can enhance these responses.
The aim of the current study was to determine the safety, tolerability, and immunologic responses to AAS peptides inside a modified dendritic cell vaccine. Seven AAS peptide candidates were selected among validated HLA-A*02:01 binders, along with the melanoma peptides G209-2M and G280-9V as positive controls for vaccination.
In each patient, T cell immunity to one AAS peptide was detected in prevaccine peripheral blood mononuclear cell samples.
The treatment differs from other personalized cancer vaccines in two key ways, Dr. Carreno said in the teleconference.
“We mature the [dendritic] cells before we give them back to the patients; the cells make growth factors that we have previously shown are important to the generation of the immune response,” she said. In addition, the vaccine was given as an infusion, rather than an injection, she noted.
Vaccination augmented the T-cell response to the three neoantigens – TMEM48 F169L, SEC24A P469, EXOC8 Q656P – with observed frequencies of 23%, 64%, and 89%, respectively.
Immune monitoring also detected T-cell immunity to two additional neoantigens per patient, Dr. Carreno and her associates reported.
Robust immune responses were detected as early as week 2 and peaked at weeks 8-9 after the initial dose. Each patient received three vaccine doses, without side effects or toxicity.
“Vaccination against tumor antigens appears safe as all three patients are alive and well, with no autoimmune adverse events,” the researchers wrote. Post vaccine restaging CT revealed stable disease in a 54-year-old man with BRAF V6003 mutation-positive melanoma. A second man, who had achieved a complete response to ipilimumab for BRAF V6003-positive disease, remains in complete remission after receiving the vaccine in the adjuvant setting.
The vaccine induced 30% tumor reduction in a woman with extensive skin metastases and 5-12 mm lung nodules after ipilimumab and vemurafenib (Zelboraf), but tumor size returned back to baseline dimensions 12 weeks later with no new disease sites. Her disease has remained stable for the past 8 months, the researchers said.
However, it is too soon to attribute a clinical benefit to the observed immune response to the vaccine, Dr. Linette emphasized. “I would be speculating if I said the vaccine had any benefit to the patients,” he said.
Vaccination increased the frequency of most existing prevaccine T-cell receptor-beta (TCR-beta) clonotypes and revealed new clonotypes for 6 neoantigens.
For both dominant and subdominant neoantigens, the TCR-beta repertoire was increased significantly after vaccination. For example, 84 clonotypes representing 19 TCR-beta families were detected for TKT R438W, 61 clonotypes representing 12 TCR-beta families were detected for SEC24A P469L, and 12 clonotypes representing 8 TCR-beta families were detected for EXOC8 Q656P.
“The revelation of a highly diverse TCR-beta repertoire specific for dominant and subdominant neoantigens was surprising and points to a potentially rich pool of naive tumor-specific T cells that remain ignorant unless activated by vaccination,” the researchers wrote.
Recent data indicate that CTLA-4 monoclonal antibody use can alter TCR repertoire diversity in patients, suggesting a potential strategy of combination checkpoint inhibitors, including ipilimumab, and neoantigen vaccines. The novel vaccine strategy also could be used to treat other malignancies with high mutational burdens such as lung, bladder, and colorectal cancers, while other genomic alterations may generate potential neoantigens relevant in low–mutational burden cancers such as leukemia, the authors suggest.
Although the cells in this study were grown in a specialized laboratory, “as the technology improves, I think this [strategy] will become reproducible and available at other medical centers,” Dr. Linette said.
This work was supported by Barnes-Jewish Hospital Foundation, Siteman Cancer Frontier Fund, Our Mark on Melanoma (MOM) Foundation, Come Out Swinging (COS) Foundation, Blackout Melanoma Foundation and the National Cancer Institute.
Heidi Splete contributed to this story.
A customized vaccine targeting patient-specific, tumor neoantigens evoked an immune response in three adults with advanced cutaneous melanoma in a proof-of-principle study that paves the way for a phase I trial.
The data are “too premature to conclude that the treatment had any therapeutic benefit to the patients,” Dr. Gerald P. Linette of Washington University in St. Louis, Mo., said in a teleconference. However, he noted that a phase I trial would likely begin within the next 9-12 months.
“Our primary goal was to see if this was safe, and if we could elicit an immune response,” Dr. Linette said.
Vaccination not only augmented T-cell immunity directed against naturally occurring, dominant tumor neoantigens, but also expanded the breadth of the immune response by revealing additional subdominant neoantigens, lead author Beatriz M. Carreno, Ph.D., of Washington University in St. Louis and her associates reported online April 2 in Science (doi:10.1126/science.aaa3828).
All three patients had stage IIIC, resected, cutaneous melanoma and had failed prior therapy with ipilimumab (Yervoy).
Some melanoma patients harbor tumor missense mutations, translated into amino acid substitutions (AAS). These mutations are thought to produce antigens that the immune system perceives as foreign, prompting a tumor-specific T-cell response. Heretofore, there has been no systematic evaluation of these neoantigens or whether vaccination can enhance these responses.
The aim of the current study was to determine the safety, tolerability, and immunologic responses to AAS peptides inside a modified dendritic cell vaccine. Seven AAS peptide candidates were selected among validated HLA-A*02:01 binders, along with the melanoma peptides G209-2M and G280-9V as positive controls for vaccination.
In each patient, T cell immunity to one AAS peptide was detected in prevaccine peripheral blood mononuclear cell samples.
The treatment differs from other personalized cancer vaccines in two key ways, Dr. Carreno said in the teleconference.
“We mature the [dendritic] cells before we give them back to the patients; the cells make growth factors that we have previously shown are important to the generation of the immune response,” she said. In addition, the vaccine was given as an infusion, rather than an injection, she noted.
Vaccination augmented the T-cell response to the three neoantigens – TMEM48 F169L, SEC24A P469, EXOC8 Q656P – with observed frequencies of 23%, 64%, and 89%, respectively.
Immune monitoring also detected T-cell immunity to two additional neoantigens per patient, Dr. Carreno and her associates reported.
Robust immune responses were detected as early as week 2 and peaked at weeks 8-9 after the initial dose. Each patient received three vaccine doses, without side effects or toxicity.
“Vaccination against tumor antigens appears safe as all three patients are alive and well, with no autoimmune adverse events,” the researchers wrote. Post vaccine restaging CT revealed stable disease in a 54-year-old man with BRAF V6003 mutation-positive melanoma. A second man, who had achieved a complete response to ipilimumab for BRAF V6003-positive disease, remains in complete remission after receiving the vaccine in the adjuvant setting.
The vaccine induced 30% tumor reduction in a woman with extensive skin metastases and 5-12 mm lung nodules after ipilimumab and vemurafenib (Zelboraf), but tumor size returned back to baseline dimensions 12 weeks later with no new disease sites. Her disease has remained stable for the past 8 months, the researchers said.
However, it is too soon to attribute a clinical benefit to the observed immune response to the vaccine, Dr. Linette emphasized. “I would be speculating if I said the vaccine had any benefit to the patients,” he said.
Vaccination increased the frequency of most existing prevaccine T-cell receptor-beta (TCR-beta) clonotypes and revealed new clonotypes for 6 neoantigens.
For both dominant and subdominant neoantigens, the TCR-beta repertoire was increased significantly after vaccination. For example, 84 clonotypes representing 19 TCR-beta families were detected for TKT R438W, 61 clonotypes representing 12 TCR-beta families were detected for SEC24A P469L, and 12 clonotypes representing 8 TCR-beta families were detected for EXOC8 Q656P.
“The revelation of a highly diverse TCR-beta repertoire specific for dominant and subdominant neoantigens was surprising and points to a potentially rich pool of naive tumor-specific T cells that remain ignorant unless activated by vaccination,” the researchers wrote.
Recent data indicate that CTLA-4 monoclonal antibody use can alter TCR repertoire diversity in patients, suggesting a potential strategy of combination checkpoint inhibitors, including ipilimumab, and neoantigen vaccines. The novel vaccine strategy also could be used to treat other malignancies with high mutational burdens such as lung, bladder, and colorectal cancers, while other genomic alterations may generate potential neoantigens relevant in low–mutational burden cancers such as leukemia, the authors suggest.
Although the cells in this study were grown in a specialized laboratory, “as the technology improves, I think this [strategy] will become reproducible and available at other medical centers,” Dr. Linette said.
This work was supported by Barnes-Jewish Hospital Foundation, Siteman Cancer Frontier Fund, Our Mark on Melanoma (MOM) Foundation, Come Out Swinging (COS) Foundation, Blackout Melanoma Foundation and the National Cancer Institute.
Heidi Splete contributed to this story.
FROM SCIENCE