User login
COVID infection provides immunity equal to vaccination: Study
People who’ve been infected with COVID reduced their chances of hospitalization and death by 88% over 10 months compared to somebody who hasn’t been infected, according to the study, published in The Lancet.
The natural immunity provided by infection was “at least as high, if not higher” than the immunity provided by two doses of Moderna or Pfizer mRNA vaccines against the ancestral, Alpha, Delta, and Omicron BA.1 variants, the researchers reported.
But protection against the BA.1 subvariant of Omicron was not as high – 36% at 10 months after infection, wrote the research team from the Institute for Health Metrics and Evaluation at the University of Washington.
They examined 65 studies from 19 countries through Sept. 31, 2022. They did not study data about infection from Omicron XBB and its sub-lineages. People who had immunity from both infection and vaccination, known as hybrid immunity, were not studied.
The findings don’t mean people should skip the vaccines and get COVID on purpose, one of the researchers told NBC News.
“The problem of saying ‘I’m gonna get infected to get immunity’ is you might be one of those people that end up in the hospital or die,” said Christopher Murray, MD, DPhil, director of the IHME. “Why would you take the risk when you can get immunity through vaccination quite safely?”
The findings could help people figure out the most effective time to get vaccinated or boosted and guide officials in setting policies on workplace vaccine mandates and rules for high-occupancy indoor settings, the researchers concluded.
This was the largest meta-analysis of immunity following infection to date, NBC News reports.
A version of this article originally appeared on WebMD.com.
People who’ve been infected with COVID reduced their chances of hospitalization and death by 88% over 10 months compared to somebody who hasn’t been infected, according to the study, published in The Lancet.
The natural immunity provided by infection was “at least as high, if not higher” than the immunity provided by two doses of Moderna or Pfizer mRNA vaccines against the ancestral, Alpha, Delta, and Omicron BA.1 variants, the researchers reported.
But protection against the BA.1 subvariant of Omicron was not as high – 36% at 10 months after infection, wrote the research team from the Institute for Health Metrics and Evaluation at the University of Washington.
They examined 65 studies from 19 countries through Sept. 31, 2022. They did not study data about infection from Omicron XBB and its sub-lineages. People who had immunity from both infection and vaccination, known as hybrid immunity, were not studied.
The findings don’t mean people should skip the vaccines and get COVID on purpose, one of the researchers told NBC News.
“The problem of saying ‘I’m gonna get infected to get immunity’ is you might be one of those people that end up in the hospital or die,” said Christopher Murray, MD, DPhil, director of the IHME. “Why would you take the risk when you can get immunity through vaccination quite safely?”
The findings could help people figure out the most effective time to get vaccinated or boosted and guide officials in setting policies on workplace vaccine mandates and rules for high-occupancy indoor settings, the researchers concluded.
This was the largest meta-analysis of immunity following infection to date, NBC News reports.
A version of this article originally appeared on WebMD.com.
People who’ve been infected with COVID reduced their chances of hospitalization and death by 88% over 10 months compared to somebody who hasn’t been infected, according to the study, published in The Lancet.
The natural immunity provided by infection was “at least as high, if not higher” than the immunity provided by two doses of Moderna or Pfizer mRNA vaccines against the ancestral, Alpha, Delta, and Omicron BA.1 variants, the researchers reported.
But protection against the BA.1 subvariant of Omicron was not as high – 36% at 10 months after infection, wrote the research team from the Institute for Health Metrics and Evaluation at the University of Washington.
They examined 65 studies from 19 countries through Sept. 31, 2022. They did not study data about infection from Omicron XBB and its sub-lineages. People who had immunity from both infection and vaccination, known as hybrid immunity, were not studied.
The findings don’t mean people should skip the vaccines and get COVID on purpose, one of the researchers told NBC News.
“The problem of saying ‘I’m gonna get infected to get immunity’ is you might be one of those people that end up in the hospital or die,” said Christopher Murray, MD, DPhil, director of the IHME. “Why would you take the risk when you can get immunity through vaccination quite safely?”
The findings could help people figure out the most effective time to get vaccinated or boosted and guide officials in setting policies on workplace vaccine mandates and rules for high-occupancy indoor settings, the researchers concluded.
This was the largest meta-analysis of immunity following infection to date, NBC News reports.
A version of this article originally appeared on WebMD.com.
FROM THE LANCET
Untreated COVID often involves relapse, clarifying antiviral rebound discussion
These findings offer a natural history of COVID-19 that will inform discussions and research concerning antiviral therapy, lead author Jonathan Z. Li, MD, associate professor of infectious disease at Brigham and Women’s Hospital and Harvard Medical School, both in Boston, and colleagues reported in Annals of Internal Medicine.
“There are increasing reports that high-risk patients are avoiding nirmatrelvir-ritonavir due to concerns about post-Paxlovid rebound, but there remains a gap in our knowledge of the frequency of symptom and viral relapse during untreated natural infection,” Dr. Li said in a written comment.
To address this gap, Dr. Li and colleagues analyzed data from 563 participants from the placebo group of the Adaptive Platform Treatment Trial for Outpatients with COVID-19 (ACTIV-2/A5401).
From days 0-28, patients recorded severity of 13 symptoms, with scores ranging from absent to severe (absent = 0, mild = 1, moderate = 2, severe = 3). RNA testing was performed on samples from nasal swabs on days 0–14, 21, and 28.
“The symptom rebound definition was determined by consensus of the study team, which comprises more than 10 infectious disease, pulmonary, and critical care physicians, as likely representing a clinically meaningful change in symptoms,” Dr. Li said.
Symptom scores needed to increase by at least 4 points to reach the threshold. For instance, a patient would qualify for relapse if they had worsening of four symptoms from mild to moderate, emergence of two new moderate symptoms, or emergence of one new moderate and two new mild symptoms.
The threshold for viral relapse was defined by an increase of at least 0.5 log10 RNA copies/mL from one nasal swab to the next, while high-level viral relapse was defined by an increase of at least 5.0 log10 RNA copies/mL. The former threshold was chosen based on previous analysis of viral rebound after nirmatrelvir treatment in the EPIC-HR phase 3 trial, whereas the high-level relapse point was based on Dr. Li and colleagues’ previous work linking this cutoff with the presence of infectious virus.
Their present analysis revealed that 26% of patients had symptom relapse at a median of 11 days after first symptom onset. Viral relapse occurred in 31% of patients, while high-level viral relapse occurred in 13% of participants. In about 9 out 10 cases, these relapses were detected at only one time point, suggesting they were transient. Of note, symptom relapse and high-level viral relapse occurred simultaneously in only 3% of patients.
This lack of correlation was “surprising” and “highlights that recovery from any infection is not always a linear process,” Dr. Li said.
This finding also suggests that untreated patients with recurring symptoms probably pose a low risk of contagion, according to David Wohl, MD, coauthor of the paper and professor of medicine in the division of infectious diseases at the University of North Carolina at Chapel Hill.
Paxlovid may not be to blame for COVID-19 rebound
“These results provide important context for the reports of Paxlovid rebound and show that baseline rates of symptom and viral relapse should be accounted for when studying the risk of rebound after antiviral therapy,” Dr. Li said.
Dr. Wohl suggested that these data can also play a role in conversations with patients who experience rebound after taking antiviral therapy.
“Many who have a return of their symptoms after taking Paxlovid blame the drug, and that may be justified, but this study suggests it happens in untreated people too,” Dr. Wohl said in a written comment.
Longer antiviral therapy deserves investigation
This is a “very important study” because it offers a baseline for comparing the natural history of COVID-19 with clinical course after antiviral therapy, said Timothy Henrich, MD, associate professor in the division of experimental medicine at University of California, San Francisco.
“Unlike this natural history, where it’s kind of sputtering up and down as it goes down, [after antiviral therapy,] it goes away for several days, and then it comes back up; and when it comes up, people have symptoms again,” Dr. Henrich said in an interview.
This suggests that each type of rebound is a unique phenomenon and, from a clinical perspective, that antiviral therapy may need to be extended.
“We treat for too short a period of time,” Dr. Henrich said. “We’re able to suppress [SARS-CoV-2] to the point where we’re not detecting it in the nasal pharynx, but it’s clearly still there. And it’s clearly still in a place that can replicate without the drug.”
That said, treating for longer may not be a sure-fire solution, especially if antiviral therapy is started early in the clinical course, as this could delay SARS-CoV-2-specific immune responses that are necessary for resolution, Dr. Henrich added,
“We need further study of longer-term therapies,” he said.
An array of research questions need to be addressed, according to Aditya Shah, MBBS, an infectious disease specialist at Mayo Clinic, Rochester, Minn. In a written comment, he probed the significance of rebound in various clinical scenarios.
“What [type of] rebound matters and what doesn’t?” Dr. Shah asked. “Does symptom rebound matter? How many untreated and treated ‘symptom rebounders’ need additional treatment or health care? If rebound does not really matter, but if Paxlovid helps in certain unvaccinated and high-risk patients, then does rebound matter? Future research should also focus on Paxlovid utility in vaccinated but high-risk patients. Is it as beneficial in them as it is in unvaccinated high-risk patients?”
While potentially regimen-altering questions like these remain unanswered, Dr. Henrich advised providers to keep patients focused on what we do know about the benefits of antiviral therapy given the current 5-day course, which is that it reduces the risk of severe disease and hospitalization.
The investigators disclosed relationships with Merck, Gilead, ViiV, and others. Dr. Henrich disclosed grant support from Merck and a consulting role with Roche. Dr. Shah disclosed no conflicts of interest.
These findings offer a natural history of COVID-19 that will inform discussions and research concerning antiviral therapy, lead author Jonathan Z. Li, MD, associate professor of infectious disease at Brigham and Women’s Hospital and Harvard Medical School, both in Boston, and colleagues reported in Annals of Internal Medicine.
“There are increasing reports that high-risk patients are avoiding nirmatrelvir-ritonavir due to concerns about post-Paxlovid rebound, but there remains a gap in our knowledge of the frequency of symptom and viral relapse during untreated natural infection,” Dr. Li said in a written comment.
To address this gap, Dr. Li and colleagues analyzed data from 563 participants from the placebo group of the Adaptive Platform Treatment Trial for Outpatients with COVID-19 (ACTIV-2/A5401).
From days 0-28, patients recorded severity of 13 symptoms, with scores ranging from absent to severe (absent = 0, mild = 1, moderate = 2, severe = 3). RNA testing was performed on samples from nasal swabs on days 0–14, 21, and 28.
“The symptom rebound definition was determined by consensus of the study team, which comprises more than 10 infectious disease, pulmonary, and critical care physicians, as likely representing a clinically meaningful change in symptoms,” Dr. Li said.
Symptom scores needed to increase by at least 4 points to reach the threshold. For instance, a patient would qualify for relapse if they had worsening of four symptoms from mild to moderate, emergence of two new moderate symptoms, or emergence of one new moderate and two new mild symptoms.
The threshold for viral relapse was defined by an increase of at least 0.5 log10 RNA copies/mL from one nasal swab to the next, while high-level viral relapse was defined by an increase of at least 5.0 log10 RNA copies/mL. The former threshold was chosen based on previous analysis of viral rebound after nirmatrelvir treatment in the EPIC-HR phase 3 trial, whereas the high-level relapse point was based on Dr. Li and colleagues’ previous work linking this cutoff with the presence of infectious virus.
Their present analysis revealed that 26% of patients had symptom relapse at a median of 11 days after first symptom onset. Viral relapse occurred in 31% of patients, while high-level viral relapse occurred in 13% of participants. In about 9 out 10 cases, these relapses were detected at only one time point, suggesting they were transient. Of note, symptom relapse and high-level viral relapse occurred simultaneously in only 3% of patients.
This lack of correlation was “surprising” and “highlights that recovery from any infection is not always a linear process,” Dr. Li said.
This finding also suggests that untreated patients with recurring symptoms probably pose a low risk of contagion, according to David Wohl, MD, coauthor of the paper and professor of medicine in the division of infectious diseases at the University of North Carolina at Chapel Hill.
Paxlovid may not be to blame for COVID-19 rebound
“These results provide important context for the reports of Paxlovid rebound and show that baseline rates of symptom and viral relapse should be accounted for when studying the risk of rebound after antiviral therapy,” Dr. Li said.
Dr. Wohl suggested that these data can also play a role in conversations with patients who experience rebound after taking antiviral therapy.
“Many who have a return of their symptoms after taking Paxlovid blame the drug, and that may be justified, but this study suggests it happens in untreated people too,” Dr. Wohl said in a written comment.
Longer antiviral therapy deserves investigation
This is a “very important study” because it offers a baseline for comparing the natural history of COVID-19 with clinical course after antiviral therapy, said Timothy Henrich, MD, associate professor in the division of experimental medicine at University of California, San Francisco.
“Unlike this natural history, where it’s kind of sputtering up and down as it goes down, [after antiviral therapy,] it goes away for several days, and then it comes back up; and when it comes up, people have symptoms again,” Dr. Henrich said in an interview.
This suggests that each type of rebound is a unique phenomenon and, from a clinical perspective, that antiviral therapy may need to be extended.
“We treat for too short a period of time,” Dr. Henrich said. “We’re able to suppress [SARS-CoV-2] to the point where we’re not detecting it in the nasal pharynx, but it’s clearly still there. And it’s clearly still in a place that can replicate without the drug.”
That said, treating for longer may not be a sure-fire solution, especially if antiviral therapy is started early in the clinical course, as this could delay SARS-CoV-2-specific immune responses that are necessary for resolution, Dr. Henrich added,
“We need further study of longer-term therapies,” he said.
An array of research questions need to be addressed, according to Aditya Shah, MBBS, an infectious disease specialist at Mayo Clinic, Rochester, Minn. In a written comment, he probed the significance of rebound in various clinical scenarios.
“What [type of] rebound matters and what doesn’t?” Dr. Shah asked. “Does symptom rebound matter? How many untreated and treated ‘symptom rebounders’ need additional treatment or health care? If rebound does not really matter, but if Paxlovid helps in certain unvaccinated and high-risk patients, then does rebound matter? Future research should also focus on Paxlovid utility in vaccinated but high-risk patients. Is it as beneficial in them as it is in unvaccinated high-risk patients?”
While potentially regimen-altering questions like these remain unanswered, Dr. Henrich advised providers to keep patients focused on what we do know about the benefits of antiviral therapy given the current 5-day course, which is that it reduces the risk of severe disease and hospitalization.
The investigators disclosed relationships with Merck, Gilead, ViiV, and others. Dr. Henrich disclosed grant support from Merck and a consulting role with Roche. Dr. Shah disclosed no conflicts of interest.
These findings offer a natural history of COVID-19 that will inform discussions and research concerning antiviral therapy, lead author Jonathan Z. Li, MD, associate professor of infectious disease at Brigham and Women’s Hospital and Harvard Medical School, both in Boston, and colleagues reported in Annals of Internal Medicine.
“There are increasing reports that high-risk patients are avoiding nirmatrelvir-ritonavir due to concerns about post-Paxlovid rebound, but there remains a gap in our knowledge of the frequency of symptom and viral relapse during untreated natural infection,” Dr. Li said in a written comment.
To address this gap, Dr. Li and colleagues analyzed data from 563 participants from the placebo group of the Adaptive Platform Treatment Trial for Outpatients with COVID-19 (ACTIV-2/A5401).
From days 0-28, patients recorded severity of 13 symptoms, with scores ranging from absent to severe (absent = 0, mild = 1, moderate = 2, severe = 3). RNA testing was performed on samples from nasal swabs on days 0–14, 21, and 28.
“The symptom rebound definition was determined by consensus of the study team, which comprises more than 10 infectious disease, pulmonary, and critical care physicians, as likely representing a clinically meaningful change in symptoms,” Dr. Li said.
Symptom scores needed to increase by at least 4 points to reach the threshold. For instance, a patient would qualify for relapse if they had worsening of four symptoms from mild to moderate, emergence of two new moderate symptoms, or emergence of one new moderate and two new mild symptoms.
The threshold for viral relapse was defined by an increase of at least 0.5 log10 RNA copies/mL from one nasal swab to the next, while high-level viral relapse was defined by an increase of at least 5.0 log10 RNA copies/mL. The former threshold was chosen based on previous analysis of viral rebound after nirmatrelvir treatment in the EPIC-HR phase 3 trial, whereas the high-level relapse point was based on Dr. Li and colleagues’ previous work linking this cutoff with the presence of infectious virus.
Their present analysis revealed that 26% of patients had symptom relapse at a median of 11 days after first symptom onset. Viral relapse occurred in 31% of patients, while high-level viral relapse occurred in 13% of participants. In about 9 out 10 cases, these relapses were detected at only one time point, suggesting they were transient. Of note, symptom relapse and high-level viral relapse occurred simultaneously in only 3% of patients.
This lack of correlation was “surprising” and “highlights that recovery from any infection is not always a linear process,” Dr. Li said.
This finding also suggests that untreated patients with recurring symptoms probably pose a low risk of contagion, according to David Wohl, MD, coauthor of the paper and professor of medicine in the division of infectious diseases at the University of North Carolina at Chapel Hill.
Paxlovid may not be to blame for COVID-19 rebound
“These results provide important context for the reports of Paxlovid rebound and show that baseline rates of symptom and viral relapse should be accounted for when studying the risk of rebound after antiviral therapy,” Dr. Li said.
Dr. Wohl suggested that these data can also play a role in conversations with patients who experience rebound after taking antiviral therapy.
“Many who have a return of their symptoms after taking Paxlovid blame the drug, and that may be justified, but this study suggests it happens in untreated people too,” Dr. Wohl said in a written comment.
Longer antiviral therapy deserves investigation
This is a “very important study” because it offers a baseline for comparing the natural history of COVID-19 with clinical course after antiviral therapy, said Timothy Henrich, MD, associate professor in the division of experimental medicine at University of California, San Francisco.
“Unlike this natural history, where it’s kind of sputtering up and down as it goes down, [after antiviral therapy,] it goes away for several days, and then it comes back up; and when it comes up, people have symptoms again,” Dr. Henrich said in an interview.
This suggests that each type of rebound is a unique phenomenon and, from a clinical perspective, that antiviral therapy may need to be extended.
“We treat for too short a period of time,” Dr. Henrich said. “We’re able to suppress [SARS-CoV-2] to the point where we’re not detecting it in the nasal pharynx, but it’s clearly still there. And it’s clearly still in a place that can replicate without the drug.”
That said, treating for longer may not be a sure-fire solution, especially if antiviral therapy is started early in the clinical course, as this could delay SARS-CoV-2-specific immune responses that are necessary for resolution, Dr. Henrich added,
“We need further study of longer-term therapies,” he said.
An array of research questions need to be addressed, according to Aditya Shah, MBBS, an infectious disease specialist at Mayo Clinic, Rochester, Minn. In a written comment, he probed the significance of rebound in various clinical scenarios.
“What [type of] rebound matters and what doesn’t?” Dr. Shah asked. “Does symptom rebound matter? How many untreated and treated ‘symptom rebounders’ need additional treatment or health care? If rebound does not really matter, but if Paxlovid helps in certain unvaccinated and high-risk patients, then does rebound matter? Future research should also focus on Paxlovid utility in vaccinated but high-risk patients. Is it as beneficial in them as it is in unvaccinated high-risk patients?”
While potentially regimen-altering questions like these remain unanswered, Dr. Henrich advised providers to keep patients focused on what we do know about the benefits of antiviral therapy given the current 5-day course, which is that it reduces the risk of severe disease and hospitalization.
The investigators disclosed relationships with Merck, Gilead, ViiV, and others. Dr. Henrich disclosed grant support from Merck and a consulting role with Roche. Dr. Shah disclosed no conflicts of interest.
FROM ANNALS OF INTERNAL MEDICINE
Immunodeficiencies tied to psychiatric disorders in offspring
new research suggests.
Results from a cohort study of more than 4.2 million individuals showed that offspring of mothers with PIDs had a 17% increased risk for a psychiatric disorder and a 20% increased risk for suicidal behavior, compared with their peers with mothers who did not have PIDs.
The risk was more pronounced in offspring of mothers with both PIDs and autoimmune diseases. These risks remained after strictly controlling for different covariates, such as the parents’ psychiatric history, offspring PIDs, and offspring autoimmune diseases.
The investigators, led by Josef Isung, MD, PhD, Centre for Psychiatry Research, department of clinical neuroscience, Karolinska Institutet, Stockholm, noted that they could not “pinpoint a precise causal mechanism” underlying these findings.
Still, “the results add to the existing literature suggesting that the intrauterine immune environment may have implications for fetal neurodevelopment and that a compromised maternal immune system during pregnancy may be a risk factor for psychiatric disorders and suicidal behavior in their offspring in the long term,” they wrote.
The findings were published online in JAMA Psychiatry.
‘Natural experiment’
Maternal immune activation (MIA) is “an overarching term for aberrant and disrupted immune activity in the mother during gestation [and] has long been of interest in relation to adverse health outcomes in the offspring,” Dr. Isung noted.
“In relation to negative psychiatric outcomes, there is an abundance of preclinical evidence that has shown a negative impact on offspring secondary to MIA. And in humans, there are several observational studies supporting this link,” he said in an interview.
Dr. Isung added that PIDs are “rare conditions” known to be associated with repeated infections and high rates of autoimmune diseases, causing substantial disability.
“PIDs represent an interesting ‘natural experiment’ for researchers to understand more about the association between immune system dysfunctions and mental health,” he said.
Dr. Isung’s group previously showed that individuals with PIDs have increased odds of psychiatric disorders and suicidal behavior. The link was more pronounced in women with PIDs – and was even more pronounced in those with both PIDs and autoimmune diseases.
In the current study, “we wanted to see whether offspring of individuals were differentially at risk of psychiatric disorders and suicidal behavior, depending on being offspring of mothers or fathers with PIDs,” Dr. Isung said.
“Our hypothesis was that mothers with PIDs would have an increased risk of having offspring with neuropsychiatric outcomes, and that this risk could be due to MIA,” he added.
The researchers turned to Swedish nationwide health and administrative registers. They analyzed data on all individuals with diagnoses of PIDs identified between 1973 and 2013. Offspring born prior to 2003 were included, and parent-offspring pairs in which both parents had a history of PIDs were excluded.
The final study sample consisted of 4,294,169 offspring (51.4% boys). Of these participants, 7,270 (0.17%) had a parent with PIDs.
The researchers identified lifetime records of 10 psychiatric disorders: obsessive-compulsive disorder, ADHD, autism spectrum disorders, schizophrenia and other psychotic disorders, bipolar disorders, major depressive disorder and other mood disorders, anxiety and stress-related disorders, eating disorders, substance use disorders, and Tourette syndrome and chronic tic disorders.
The investigators included parental birth year, psychopathology, suicide attempts, suicide deaths, and autoimmune diseases as covariates, as well as offsprings’ birth year and gender.
Elucidation needed
Results showed that, of the 4,676 offspring of mothers with PID, 17.1% had a psychiatric disorder versus 12.7% of offspring of mothers without PIDs. This translated “into a 17% increased risk for offspring of mothers with PIDs in the fully adjusted model,” the investigators reported.
The risk was even higher for offspring of mothers who had not only PIDs but also one of six of the individual psychiatric disorders, with incident rate ratios ranging from 1.15 to 1.71.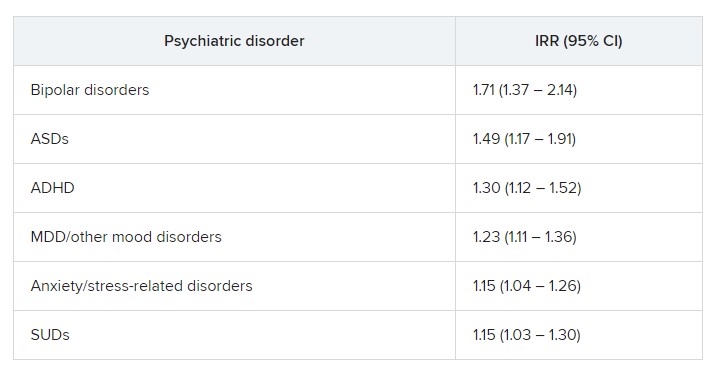
“In fully adjusted models, offspring of mothers with PIDs had an increased risk of any psychiatric disorder, while no such risks were observed in offspring of fathers with PIDs” (IRR, 1.17 vs. 1.03; P < .001), the researchers reported.
A higher risk for suicidal behavior was also observed among offspring of mothers with PIDS, in contrast to those of fathers with PIDs (IRR, 1.2 vs. 1.1; P = .01).
The greatest risk for any psychiatric disorder, as well as suicidal behavior, was found in offspring of mothers who had both PIDs and autoimmune diseases (IRRs, 1.24 and 1.44, respectively).
“The results could be seen as substantiating the hypothesis that immune disruption may be important in the pathophysiology of psychiatric disorders and suicidal behavior,” Dr. Isung said.
“Furthermore, the fact that only offspring of mothers and not offspring of fathers with PIDs had this association would align with our hypothesis that MIA is of importance,” he added.
However, he noted that “the specific mechanisms are most likely multifactorial and remain to be elucidated.”
Important piece of the puzzle?
In a comment, Michael Eriksen Benros, MD, PhD, professor of immunopsychiatry, department of immunology and microbiology, health, and medical sciences, University of Copenhagen, said this was a “high-quality study” that used a “rich data source.”
Dr. Benros, who is also head of research (biological and precision psychiatry) at the Copenhagen Research Centre for Mental Health, Copenhagen University Hospital, was not involved with the current study.
He noted that prior studies, including some conducted by his own group, have shown that maternal infections overall did not seem to be “specifically linked to mental disorders in the offspring.”
However, “specific maternal infections or specific brain-reactive antibodies during the pregnancy period have been shown to be associated with neurodevelopmental outcomes among the children,” such as intellectual disability, he said.
Regarding direct clinical implications of the study, “it is important to note that the increased risk of psychiatric disorders and suicidality in the offspring of mothers with PID were small,” Dr. Benros said.
“However, it adds an important part to the scientific puzzle regarding the role of maternal immune activation during pregnancy and the risk of mental disorders,” he added.
The study was funded by the Söderström König Foundation and the Fredrik and Ingrid Thuring Foundation. Neither Dr. Isung nor Dr. Benros reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests.
Results from a cohort study of more than 4.2 million individuals showed that offspring of mothers with PIDs had a 17% increased risk for a psychiatric disorder and a 20% increased risk for suicidal behavior, compared with their peers with mothers who did not have PIDs.
The risk was more pronounced in offspring of mothers with both PIDs and autoimmune diseases. These risks remained after strictly controlling for different covariates, such as the parents’ psychiatric history, offspring PIDs, and offspring autoimmune diseases.
The investigators, led by Josef Isung, MD, PhD, Centre for Psychiatry Research, department of clinical neuroscience, Karolinska Institutet, Stockholm, noted that they could not “pinpoint a precise causal mechanism” underlying these findings.
Still, “the results add to the existing literature suggesting that the intrauterine immune environment may have implications for fetal neurodevelopment and that a compromised maternal immune system during pregnancy may be a risk factor for psychiatric disorders and suicidal behavior in their offspring in the long term,” they wrote.
The findings were published online in JAMA Psychiatry.
‘Natural experiment’
Maternal immune activation (MIA) is “an overarching term for aberrant and disrupted immune activity in the mother during gestation [and] has long been of interest in relation to adverse health outcomes in the offspring,” Dr. Isung noted.
“In relation to negative psychiatric outcomes, there is an abundance of preclinical evidence that has shown a negative impact on offspring secondary to MIA. And in humans, there are several observational studies supporting this link,” he said in an interview.
Dr. Isung added that PIDs are “rare conditions” known to be associated with repeated infections and high rates of autoimmune diseases, causing substantial disability.
“PIDs represent an interesting ‘natural experiment’ for researchers to understand more about the association between immune system dysfunctions and mental health,” he said.
Dr. Isung’s group previously showed that individuals with PIDs have increased odds of psychiatric disorders and suicidal behavior. The link was more pronounced in women with PIDs – and was even more pronounced in those with both PIDs and autoimmune diseases.
In the current study, “we wanted to see whether offspring of individuals were differentially at risk of psychiatric disorders and suicidal behavior, depending on being offspring of mothers or fathers with PIDs,” Dr. Isung said.
“Our hypothesis was that mothers with PIDs would have an increased risk of having offspring with neuropsychiatric outcomes, and that this risk could be due to MIA,” he added.
The researchers turned to Swedish nationwide health and administrative registers. They analyzed data on all individuals with diagnoses of PIDs identified between 1973 and 2013. Offspring born prior to 2003 were included, and parent-offspring pairs in which both parents had a history of PIDs were excluded.
The final study sample consisted of 4,294,169 offspring (51.4% boys). Of these participants, 7,270 (0.17%) had a parent with PIDs.
The researchers identified lifetime records of 10 psychiatric disorders: obsessive-compulsive disorder, ADHD, autism spectrum disorders, schizophrenia and other psychotic disorders, bipolar disorders, major depressive disorder and other mood disorders, anxiety and stress-related disorders, eating disorders, substance use disorders, and Tourette syndrome and chronic tic disorders.
The investigators included parental birth year, psychopathology, suicide attempts, suicide deaths, and autoimmune diseases as covariates, as well as offsprings’ birth year and gender.
Elucidation needed
Results showed that, of the 4,676 offspring of mothers with PID, 17.1% had a psychiatric disorder versus 12.7% of offspring of mothers without PIDs. This translated “into a 17% increased risk for offspring of mothers with PIDs in the fully adjusted model,” the investigators reported.
The risk was even higher for offspring of mothers who had not only PIDs but also one of six of the individual psychiatric disorders, with incident rate ratios ranging from 1.15 to 1.71.
“In fully adjusted models, offspring of mothers with PIDs had an increased risk of any psychiatric disorder, while no such risks were observed in offspring of fathers with PIDs” (IRR, 1.17 vs. 1.03; P < .001), the researchers reported.
A higher risk for suicidal behavior was also observed among offspring of mothers with PIDS, in contrast to those of fathers with PIDs (IRR, 1.2 vs. 1.1; P = .01).
The greatest risk for any psychiatric disorder, as well as suicidal behavior, was found in offspring of mothers who had both PIDs and autoimmune diseases (IRRs, 1.24 and 1.44, respectively).
“The results could be seen as substantiating the hypothesis that immune disruption may be important in the pathophysiology of psychiatric disorders and suicidal behavior,” Dr. Isung said.
“Furthermore, the fact that only offspring of mothers and not offspring of fathers with PIDs had this association would align with our hypothesis that MIA is of importance,” he added.
However, he noted that “the specific mechanisms are most likely multifactorial and remain to be elucidated.”
Important piece of the puzzle?
In a comment, Michael Eriksen Benros, MD, PhD, professor of immunopsychiatry, department of immunology and microbiology, health, and medical sciences, University of Copenhagen, said this was a “high-quality study” that used a “rich data source.”
Dr. Benros, who is also head of research (biological and precision psychiatry) at the Copenhagen Research Centre for Mental Health, Copenhagen University Hospital, was not involved with the current study.
He noted that prior studies, including some conducted by his own group, have shown that maternal infections overall did not seem to be “specifically linked to mental disorders in the offspring.”
However, “specific maternal infections or specific brain-reactive antibodies during the pregnancy period have been shown to be associated with neurodevelopmental outcomes among the children,” such as intellectual disability, he said.
Regarding direct clinical implications of the study, “it is important to note that the increased risk of psychiatric disorders and suicidality in the offspring of mothers with PID were small,” Dr. Benros said.
“However, it adds an important part to the scientific puzzle regarding the role of maternal immune activation during pregnancy and the risk of mental disorders,” he added.
The study was funded by the Söderström König Foundation and the Fredrik and Ingrid Thuring Foundation. Neither Dr. Isung nor Dr. Benros reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests.
Results from a cohort study of more than 4.2 million individuals showed that offspring of mothers with PIDs had a 17% increased risk for a psychiatric disorder and a 20% increased risk for suicidal behavior, compared with their peers with mothers who did not have PIDs.
The risk was more pronounced in offspring of mothers with both PIDs and autoimmune diseases. These risks remained after strictly controlling for different covariates, such as the parents’ psychiatric history, offspring PIDs, and offspring autoimmune diseases.
The investigators, led by Josef Isung, MD, PhD, Centre for Psychiatry Research, department of clinical neuroscience, Karolinska Institutet, Stockholm, noted that they could not “pinpoint a precise causal mechanism” underlying these findings.
Still, “the results add to the existing literature suggesting that the intrauterine immune environment may have implications for fetal neurodevelopment and that a compromised maternal immune system during pregnancy may be a risk factor for psychiatric disorders and suicidal behavior in their offspring in the long term,” they wrote.
The findings were published online in JAMA Psychiatry.
‘Natural experiment’
Maternal immune activation (MIA) is “an overarching term for aberrant and disrupted immune activity in the mother during gestation [and] has long been of interest in relation to adverse health outcomes in the offspring,” Dr. Isung noted.
“In relation to negative psychiatric outcomes, there is an abundance of preclinical evidence that has shown a negative impact on offspring secondary to MIA. And in humans, there are several observational studies supporting this link,” he said in an interview.
Dr. Isung added that PIDs are “rare conditions” known to be associated with repeated infections and high rates of autoimmune diseases, causing substantial disability.
“PIDs represent an interesting ‘natural experiment’ for researchers to understand more about the association between immune system dysfunctions and mental health,” he said.
Dr. Isung’s group previously showed that individuals with PIDs have increased odds of psychiatric disorders and suicidal behavior. The link was more pronounced in women with PIDs – and was even more pronounced in those with both PIDs and autoimmune diseases.
In the current study, “we wanted to see whether offspring of individuals were differentially at risk of psychiatric disorders and suicidal behavior, depending on being offspring of mothers or fathers with PIDs,” Dr. Isung said.
“Our hypothesis was that mothers with PIDs would have an increased risk of having offspring with neuropsychiatric outcomes, and that this risk could be due to MIA,” he added.
The researchers turned to Swedish nationwide health and administrative registers. They analyzed data on all individuals with diagnoses of PIDs identified between 1973 and 2013. Offspring born prior to 2003 were included, and parent-offspring pairs in which both parents had a history of PIDs were excluded.
The final study sample consisted of 4,294,169 offspring (51.4% boys). Of these participants, 7,270 (0.17%) had a parent with PIDs.
The researchers identified lifetime records of 10 psychiatric disorders: obsessive-compulsive disorder, ADHD, autism spectrum disorders, schizophrenia and other psychotic disorders, bipolar disorders, major depressive disorder and other mood disorders, anxiety and stress-related disorders, eating disorders, substance use disorders, and Tourette syndrome and chronic tic disorders.
The investigators included parental birth year, psychopathology, suicide attempts, suicide deaths, and autoimmune diseases as covariates, as well as offsprings’ birth year and gender.
Elucidation needed
Results showed that, of the 4,676 offspring of mothers with PID, 17.1% had a psychiatric disorder versus 12.7% of offspring of mothers without PIDs. This translated “into a 17% increased risk for offspring of mothers with PIDs in the fully adjusted model,” the investigators reported.
The risk was even higher for offspring of mothers who had not only PIDs but also one of six of the individual psychiatric disorders, with incident rate ratios ranging from 1.15 to 1.71.
“In fully adjusted models, offspring of mothers with PIDs had an increased risk of any psychiatric disorder, while no such risks were observed in offspring of fathers with PIDs” (IRR, 1.17 vs. 1.03; P < .001), the researchers reported.
A higher risk for suicidal behavior was also observed among offspring of mothers with PIDS, in contrast to those of fathers with PIDs (IRR, 1.2 vs. 1.1; P = .01).
The greatest risk for any psychiatric disorder, as well as suicidal behavior, was found in offspring of mothers who had both PIDs and autoimmune diseases (IRRs, 1.24 and 1.44, respectively).
“The results could be seen as substantiating the hypothesis that immune disruption may be important in the pathophysiology of psychiatric disorders and suicidal behavior,” Dr. Isung said.
“Furthermore, the fact that only offspring of mothers and not offspring of fathers with PIDs had this association would align with our hypothesis that MIA is of importance,” he added.
However, he noted that “the specific mechanisms are most likely multifactorial and remain to be elucidated.”
Important piece of the puzzle?
In a comment, Michael Eriksen Benros, MD, PhD, professor of immunopsychiatry, department of immunology and microbiology, health, and medical sciences, University of Copenhagen, said this was a “high-quality study” that used a “rich data source.”
Dr. Benros, who is also head of research (biological and precision psychiatry) at the Copenhagen Research Centre for Mental Health, Copenhagen University Hospital, was not involved with the current study.
He noted that prior studies, including some conducted by his own group, have shown that maternal infections overall did not seem to be “specifically linked to mental disorders in the offspring.”
However, “specific maternal infections or specific brain-reactive antibodies during the pregnancy period have been shown to be associated with neurodevelopmental outcomes among the children,” such as intellectual disability, he said.
Regarding direct clinical implications of the study, “it is important to note that the increased risk of psychiatric disorders and suicidality in the offspring of mothers with PID were small,” Dr. Benros said.
“However, it adds an important part to the scientific puzzle regarding the role of maternal immune activation during pregnancy and the risk of mental disorders,” he added.
The study was funded by the Söderström König Foundation and the Fredrik and Ingrid Thuring Foundation. Neither Dr. Isung nor Dr. Benros reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM JAMA PSYCHIATRY
COVID-19 shot appears to reduce diabetes risk, even after Omicron
new data suggest.
The findings, from more than 20,000 patients in the Cedars-Sinai Health System in Los Angeles, suggest that “continued efforts to prevent COVID-19 infection may be beneficial to patient health until we develop better understanding of the effects of potential long-term effects of COVID-19,” lead author Alan C. Kwan, MD, of the department of cardiology at Cedars Sinai’s Smidt Heart Institute, said in an interview.
Several studies conducted early in the pandemic suggested increased risks for both new-onset diabetes and cardiometabolic diseases following COVID-19 infection, possibly because of persistent inflammation contributing to insulin resistance.
However, it hasn’t been clear if those risks have persisted with the more recent predominance of the less-virulent Omicron variant or whether the COVID-19 vaccine influences the risk. This new study suggests that both are the case.
“Our results verify that the risk of developing type 2 diabetes after a COVID-19 infection was not just an early observation but, in fact, a real risk that has, unfortunately, persisted through the Omicron era,” Dr. Kwan noted.
“While the level of evidence by our study and others may not reach the degree needed to affect formal guidelines at this time, we believe it is reasonable to have increased clinical suspicion for diabetes after COVID-19 infection and a lower threshold for testing,” he added.
Moreover, “we believe that our study and others suggest the potential role of COVID-19 to affect cardiovascular risk, and so both prevention of COVID-19 infection, through reasonable personal practices and vaccination, and an increased attention to cardiovascular health after COVID-19 infection is warranted.”
The findings were published online in JAMA Network Open.
Dr. Kwan and colleagues analyzed data for a total of 23,709 patients treated (inpatient and outpatient) for at least one COVID-19 infection between March 2020 and June 2022.
Rates of new-onset diabetes (using ICD-10 codes, primarily type 2 diabetes), hypertension, and hyperlipidemia were all elevated in the 90 days following COVID-19 infection compared with the 90 days prior. The same was true of two diagnoses unrelated to COVID-19, urinary tract infection and gastroesophageal reflux, used as benchmarks of health care engagement.
The highest odds for post versus preinfection were for diabetes (odds ratio, 2.35; P < .001), followed by hypertension (OR, 1.54; P < .001), the benchmark diagnoses (OR, 1.42; P < .001), and hyperlipidemia (OR, 1.22; P = .03).
Following adjustments, the risk versus the benchmark conditions for new-onset diabetes before versus after COVID-19 was significantly elevated (OR, 1.58; P < .001), while the risks for hypertension and hyperlipidemia versus benchmark diagnoses were not (OR, 1.06; P = .52 and 0.91, P = .43, respectively).
The diabetes risk after versus before COVID-19 infection was higher among those who had not been vaccinated (OR, 1.78; P < .001), compared with those who had received the vaccine (OR, 1.07; P = .80).
However, there was no significant interaction between vaccination and diabetes diagnosis (P = .08). “For this reason, we believe our data are suggestive of a protective effect in the population who received vaccination prior to infection, but [this is] not definitive,” Dr. Kwan said.
There were no apparent interactions by age, sex, or pre-existing cardiovascular risk factors, including hypertension or hyperlipidemia. Age, sex, and timing of index infection regarding the Omicron variant were not associated with an increased risk of a new cardiometabolic diagnosis before or after COVID-19 infection in any of the models.
Dr. Kwan said in an interview: “We have continued to be surprised by the evolving understanding of the SARS-CoV-2 virus and the effects on human health. In the beginning of the pandemic it was framed as a purely respiratory virus, which we now know to be a severely limited description of all of its potential effects on the human body. We believe that our research and others raise a concern for increased cardiometabolic risk after COVID infection.”
He added that, “while knowledge is incomplete on this topic, we believe that clinical providers may wish to have a higher degree of suspicion for both diabetes and risk of future cardiac events in patients after COVID infection, and that continued efforts to prevent COVID infection may be beneficial to patient health until we develop better understanding of the potential long-term effects of COVID.”
This study was funded by the Erika J. Glazer Family Foundation, the Doris Duke Charitable Foundation, and grants from the National Institutes of Health. Dr. Kwan reported receiving grants from the Doris Duke Charitable Foundation during the conduct of the study.
A version of this article originally appeared on Medscape.com.
new data suggest.
The findings, from more than 20,000 patients in the Cedars-Sinai Health System in Los Angeles, suggest that “continued efforts to prevent COVID-19 infection may be beneficial to patient health until we develop better understanding of the effects of potential long-term effects of COVID-19,” lead author Alan C. Kwan, MD, of the department of cardiology at Cedars Sinai’s Smidt Heart Institute, said in an interview.
Several studies conducted early in the pandemic suggested increased risks for both new-onset diabetes and cardiometabolic diseases following COVID-19 infection, possibly because of persistent inflammation contributing to insulin resistance.
However, it hasn’t been clear if those risks have persisted with the more recent predominance of the less-virulent Omicron variant or whether the COVID-19 vaccine influences the risk. This new study suggests that both are the case.
“Our results verify that the risk of developing type 2 diabetes after a COVID-19 infection was not just an early observation but, in fact, a real risk that has, unfortunately, persisted through the Omicron era,” Dr. Kwan noted.
“While the level of evidence by our study and others may not reach the degree needed to affect formal guidelines at this time, we believe it is reasonable to have increased clinical suspicion for diabetes after COVID-19 infection and a lower threshold for testing,” he added.
Moreover, “we believe that our study and others suggest the potential role of COVID-19 to affect cardiovascular risk, and so both prevention of COVID-19 infection, through reasonable personal practices and vaccination, and an increased attention to cardiovascular health after COVID-19 infection is warranted.”
The findings were published online in JAMA Network Open.
Dr. Kwan and colleagues analyzed data for a total of 23,709 patients treated (inpatient and outpatient) for at least one COVID-19 infection between March 2020 and June 2022.
Rates of new-onset diabetes (using ICD-10 codes, primarily type 2 diabetes), hypertension, and hyperlipidemia were all elevated in the 90 days following COVID-19 infection compared with the 90 days prior. The same was true of two diagnoses unrelated to COVID-19, urinary tract infection and gastroesophageal reflux, used as benchmarks of health care engagement.
The highest odds for post versus preinfection were for diabetes (odds ratio, 2.35; P < .001), followed by hypertension (OR, 1.54; P < .001), the benchmark diagnoses (OR, 1.42; P < .001), and hyperlipidemia (OR, 1.22; P = .03).
Following adjustments, the risk versus the benchmark conditions for new-onset diabetes before versus after COVID-19 was significantly elevated (OR, 1.58; P < .001), while the risks for hypertension and hyperlipidemia versus benchmark diagnoses were not (OR, 1.06; P = .52 and 0.91, P = .43, respectively).
The diabetes risk after versus before COVID-19 infection was higher among those who had not been vaccinated (OR, 1.78; P < .001), compared with those who had received the vaccine (OR, 1.07; P = .80).
However, there was no significant interaction between vaccination and diabetes diagnosis (P = .08). “For this reason, we believe our data are suggestive of a protective effect in the population who received vaccination prior to infection, but [this is] not definitive,” Dr. Kwan said.
There were no apparent interactions by age, sex, or pre-existing cardiovascular risk factors, including hypertension or hyperlipidemia. Age, sex, and timing of index infection regarding the Omicron variant were not associated with an increased risk of a new cardiometabolic diagnosis before or after COVID-19 infection in any of the models.
Dr. Kwan said in an interview: “We have continued to be surprised by the evolving understanding of the SARS-CoV-2 virus and the effects on human health. In the beginning of the pandemic it was framed as a purely respiratory virus, which we now know to be a severely limited description of all of its potential effects on the human body. We believe that our research and others raise a concern for increased cardiometabolic risk after COVID infection.”
He added that, “while knowledge is incomplete on this topic, we believe that clinical providers may wish to have a higher degree of suspicion for both diabetes and risk of future cardiac events in patients after COVID infection, and that continued efforts to prevent COVID infection may be beneficial to patient health until we develop better understanding of the potential long-term effects of COVID.”
This study was funded by the Erika J. Glazer Family Foundation, the Doris Duke Charitable Foundation, and grants from the National Institutes of Health. Dr. Kwan reported receiving grants from the Doris Duke Charitable Foundation during the conduct of the study.
A version of this article originally appeared on Medscape.com.
new data suggest.
The findings, from more than 20,000 patients in the Cedars-Sinai Health System in Los Angeles, suggest that “continued efforts to prevent COVID-19 infection may be beneficial to patient health until we develop better understanding of the effects of potential long-term effects of COVID-19,” lead author Alan C. Kwan, MD, of the department of cardiology at Cedars Sinai’s Smidt Heart Institute, said in an interview.
Several studies conducted early in the pandemic suggested increased risks for both new-onset diabetes and cardiometabolic diseases following COVID-19 infection, possibly because of persistent inflammation contributing to insulin resistance.
However, it hasn’t been clear if those risks have persisted with the more recent predominance of the less-virulent Omicron variant or whether the COVID-19 vaccine influences the risk. This new study suggests that both are the case.
“Our results verify that the risk of developing type 2 diabetes after a COVID-19 infection was not just an early observation but, in fact, a real risk that has, unfortunately, persisted through the Omicron era,” Dr. Kwan noted.
“While the level of evidence by our study and others may not reach the degree needed to affect formal guidelines at this time, we believe it is reasonable to have increased clinical suspicion for diabetes after COVID-19 infection and a lower threshold for testing,” he added.
Moreover, “we believe that our study and others suggest the potential role of COVID-19 to affect cardiovascular risk, and so both prevention of COVID-19 infection, through reasonable personal practices and vaccination, and an increased attention to cardiovascular health after COVID-19 infection is warranted.”
The findings were published online in JAMA Network Open.
Dr. Kwan and colleagues analyzed data for a total of 23,709 patients treated (inpatient and outpatient) for at least one COVID-19 infection between March 2020 and June 2022.
Rates of new-onset diabetes (using ICD-10 codes, primarily type 2 diabetes), hypertension, and hyperlipidemia were all elevated in the 90 days following COVID-19 infection compared with the 90 days prior. The same was true of two diagnoses unrelated to COVID-19, urinary tract infection and gastroesophageal reflux, used as benchmarks of health care engagement.
The highest odds for post versus preinfection were for diabetes (odds ratio, 2.35; P < .001), followed by hypertension (OR, 1.54; P < .001), the benchmark diagnoses (OR, 1.42; P < .001), and hyperlipidemia (OR, 1.22; P = .03).
Following adjustments, the risk versus the benchmark conditions for new-onset diabetes before versus after COVID-19 was significantly elevated (OR, 1.58; P < .001), while the risks for hypertension and hyperlipidemia versus benchmark diagnoses were not (OR, 1.06; P = .52 and 0.91, P = .43, respectively).
The diabetes risk after versus before COVID-19 infection was higher among those who had not been vaccinated (OR, 1.78; P < .001), compared with those who had received the vaccine (OR, 1.07; P = .80).
However, there was no significant interaction between vaccination and diabetes diagnosis (P = .08). “For this reason, we believe our data are suggestive of a protective effect in the population who received vaccination prior to infection, but [this is] not definitive,” Dr. Kwan said.
There were no apparent interactions by age, sex, or pre-existing cardiovascular risk factors, including hypertension or hyperlipidemia. Age, sex, and timing of index infection regarding the Omicron variant were not associated with an increased risk of a new cardiometabolic diagnosis before or after COVID-19 infection in any of the models.
Dr. Kwan said in an interview: “We have continued to be surprised by the evolving understanding of the SARS-CoV-2 virus and the effects on human health. In the beginning of the pandemic it was framed as a purely respiratory virus, which we now know to be a severely limited description of all of its potential effects on the human body. We believe that our research and others raise a concern for increased cardiometabolic risk after COVID infection.”
He added that, “while knowledge is incomplete on this topic, we believe that clinical providers may wish to have a higher degree of suspicion for both diabetes and risk of future cardiac events in patients after COVID infection, and that continued efforts to prevent COVID infection may be beneficial to patient health until we develop better understanding of the potential long-term effects of COVID.”
This study was funded by the Erika J. Glazer Family Foundation, the Doris Duke Charitable Foundation, and grants from the National Institutes of Health. Dr. Kwan reported receiving grants from the Doris Duke Charitable Foundation during the conduct of the study.
A version of this article originally appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Must-read acute care medicine articles from 2022
When 2022 began, we started seeing some light at the end of the COVID-19 tunnel. Vaccines were widely available, and even with new variants of the virus still occasionally emerging, the rates of severe morbidity and mortality appeared to be decreasing.
Expectedly, journals appeared to start moving more toward mainstream topics and publications rather than what seemed like a major focus on COVID-19 publications. The resulting literature was fantastic.
Several of those topics were discussed in a prior Emergency Medicine Viewpoint from this news organization, and many more of the research advances of 2022 will be discussed in the near future. However, in this Viewpoint, I would like to present my annual review of my three “must-read” articles of the past year.
As in past years, I am choosing reviews of the literature rather than original research articles (which, all too often, become outdated or debunked within a few years). I choose these articles in the hopes that readers will not simply settle for my brief reviews of the key points but instead will feel compelled to download and read the entire articles. These publications address common conditions and quandaries we face in the daily practice of emergency medicine and are practice-changing.
Myocardial dysfunction after cardiac arrest: Tips and pitfalls
The management of post–cardiac arrest patients remains a hot topic in the resuscitation literature as we continue to understand that the immediate post-arrest period is critical to patient outcome.
Ortuno and colleagues reviewed the current literature on post-arrest care and wrote an outstanding summary of how to optimally care for these patients. More specifically, they focused on post-arrest patients who demonstrate continued shock, or “post–cardiac arrest myocardial dysfunction” (PCAMD).
They propose three mechanisms for the pathogenesis of PCAMD: ischemia reperfusion phenomenon, systemic inflammatory response, and increased catecholamine release
I will skip through the details of the pathophysiology that they describe in the article, but I certainly do recommend that everyone review their descriptions.
Management of these patients begins with a good hemodynamic assessment, which includes clinical markers of perfusion (blood pressure, capillary refill), ECG, and point-of-care ultrasound (POCUS). If the initial assessment reveals an obvious cause of the cardiac arrest (e.g., massive pulmonary embolism, myocardial infarction, pericardial tamponade), then the underlying cause should be treated expeditiously.
In the absence of an obvious treatable cause of the shock, the fluid status and cardiac function should be addressed with POCUS. If the patient is hypovolemic, intravenous fluids should be administered. If the fluid status is adequate, POCUS should be used to estimate the patient’s ventricular function. If the ventricle appears to be hyperdynamic with good contractility, shock should be treated with norepinephrine. On the other hand, if the ventricle is hypodynamic, dobutamine should be substituted for norepinephrine or, more often, added to norepinephrine.
The above represents a simplified summary of the critical points, but the authors do delve into further detail and also discuss some other options for therapies, including steroids, coronary revascularization, extracorporeal membrane oxygenation, and so on. The review is very thoughtful, thorough, and definitely worth a full read.
Top myths of diagnosis and management of infectious diseases in hospital medicine
Most, if not all of us in medicine, have heard the saying that 50% of what we learn in medical school (or residency) will turn out to be wrong. I certainly believe in this concept and consequently, like many of you, I enjoy reading about myths and misconceptions that we have been taught. With that in mind, I have to say that I love this article because it seems to have been written specifically to address what I was taught!
This author group, consisting mostly of clinical PharmDs who are experts in antibiotic use, provide us with an evidence-based discussion of myths and pitfalls in how antibiotics are often used in current clinical practice. The authors review their top 10 myths involving the use of antibiotics in treating infections in the hospital setting. A few of these relate more to the inpatient setting, but here are my favorite emergency department (ED)–related myths that they address:
- “Antibiotics do no harm.” The authors address the risk-benefit of antibiotics based on assumed vs. confirmed infections, including a brief discussion of adverse drug effects.
- “Antibiotic durations of 7, 14, or 21 days are typically necessary.” The authors address appropriate duration of antibiotic use and the fact that unnecessarily long durations of use can lead to resistance. They also provide reassurance that some infections can be treated with quite short durations of antibiotics.
- “If one drug is good, two (or more!) is better.” The use of multiple antibiotics, often with overlapping bacterial coverage, is rampant in medicine and further increases the risk for adverse drug effects and resistance.
- “Oral antibiotics are not as good as intravenous antibiotics for hospitalized patients.” This is definitely a myth that I learned. I recall being taught by many senior physicians that anyone sick enough for admission should be treated with intravenous antibiotics. As it turns out, absorption and effectiveness of most oral antibiotics is just as good as intravenous antibiotics, and the oral formulations are often safer.
- “A history of a penicillin allergy means the patient can never receive a beta-lactam antibiotic.” This is a myth that was debunked quite a few years ago, but it seems that many clinicians still need a reminder.
The authors included five more myths that are worth the read. This is an article that needs to be disseminated among all hospital clinicians.
Guidelines for low-risk, recurrent abdominal pain in the emergency department
The Society for Academic Emergency Medicine (SAEM) recently initiated a program focused on creating evidence-based approaches to challenging chief complaints and presentations in the emergency department (ED). In 2021, they published an approach to managing patients with recurrent, low-risk chest pain in the ED. This past year, they published their second guideline, focused on the management of patients with low-risk, recurrent abdominal pain in the ED.
Recurrent low-risk abdominal pain is a common and vexing presentation to EDs around the world, and there is little prior published guidance. Do all of these patients need repeat imaging? How do we manage their pain? Are there nonabdominal conditions that should be considered?
Broder and colleagues did a fantastic review of the current literature and, on behalf of SAEM, have provided a rational approach to optimal management of these patients. The four major questions they addressed, with brief summaries of their recommendations, are:
- Should adult ED patients with low-risk, recurrent and previously undifferentiated abdominal pain receive a repeat CT abdomen-pelvis (CTAP) after a negative CTAP within the past 12 months? This is a typical question that we all ponder when managing these patients. Unfortunately, the writing group found insufficient evidence to definitively identify populations in whom CTAP was recommended vs could be safely withheld. It is a bit disappointing that there is no definite answer to the question. On the other hand, it is reassuring to know that the world’s best evidence essentially says that it is perfectly appropriate to use your own good clinical judgment.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain with a negative CTAP receive additional imaging with abdominal ultrasound? In this case, the writing group found enough evidence, though low-level, to suggest against routine ultrasound in the absence of concern specifically for pelvic or hepatobiliary pathology. Like most tests, ultrasound is best used when there are specific concerns rather than being used in an undifferentiated fashion.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain receive screening for depression/anxiety? The writing group found enough evidence, though low-level again, to suggest that screening for depression and/or anxiety be performed during the ED evaluation. This could lead to successful therapy for the abdominal pain.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain receive nonopioid and/or nonpharmacologic analgesics? The writing group found little evidence to suggest for or against these analgesics, but they made a consensus recommendation suggesting an opioid-minimizing strategy for pain control.
Although the final recommendations of the writing group were not definitive or based on the strongest level of evidence, I find it helpful to have this guidance, nevertheless, on behalf of a major national organization. I also find it helpful to know that even with the best evidence available, optimal patient care will often boil down to physician experience and gestalt. I should also add that the overall article is chock-full of pearls and helpful information that will further inform the readers’ decisions, and so the full version is definitely worth the read.
In summary
There you have it – my three favorite practice-changing articles of 2022. Although I have tried to provide key points here, the full discussions of those key points in the published articles will provide a great deal more education than I can offer in this brief write-up, and so I strongly encourage everyone to read the full versions. Please be sure to include in the comments section your own pick for favorite or must-read articles from the past year.
Amal Mattu, MD, is a professor, vice chair of education, and codirector of the emergency cardiology fellowship in the department of emergency medicine at the University of Maryland, Baltimore. She reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
When 2022 began, we started seeing some light at the end of the COVID-19 tunnel. Vaccines were widely available, and even with new variants of the virus still occasionally emerging, the rates of severe morbidity and mortality appeared to be decreasing.
Expectedly, journals appeared to start moving more toward mainstream topics and publications rather than what seemed like a major focus on COVID-19 publications. The resulting literature was fantastic.
Several of those topics were discussed in a prior Emergency Medicine Viewpoint from this news organization, and many more of the research advances of 2022 will be discussed in the near future. However, in this Viewpoint, I would like to present my annual review of my three “must-read” articles of the past year.
As in past years, I am choosing reviews of the literature rather than original research articles (which, all too often, become outdated or debunked within a few years). I choose these articles in the hopes that readers will not simply settle for my brief reviews of the key points but instead will feel compelled to download and read the entire articles. These publications address common conditions and quandaries we face in the daily practice of emergency medicine and are practice-changing.
Myocardial dysfunction after cardiac arrest: Tips and pitfalls
The management of post–cardiac arrest patients remains a hot topic in the resuscitation literature as we continue to understand that the immediate post-arrest period is critical to patient outcome.
Ortuno and colleagues reviewed the current literature on post-arrest care and wrote an outstanding summary of how to optimally care for these patients. More specifically, they focused on post-arrest patients who demonstrate continued shock, or “post–cardiac arrest myocardial dysfunction” (PCAMD).
They propose three mechanisms for the pathogenesis of PCAMD: ischemia reperfusion phenomenon, systemic inflammatory response, and increased catecholamine release
I will skip through the details of the pathophysiology that they describe in the article, but I certainly do recommend that everyone review their descriptions.
Management of these patients begins with a good hemodynamic assessment, which includes clinical markers of perfusion (blood pressure, capillary refill), ECG, and point-of-care ultrasound (POCUS). If the initial assessment reveals an obvious cause of the cardiac arrest (e.g., massive pulmonary embolism, myocardial infarction, pericardial tamponade), then the underlying cause should be treated expeditiously.
In the absence of an obvious treatable cause of the shock, the fluid status and cardiac function should be addressed with POCUS. If the patient is hypovolemic, intravenous fluids should be administered. If the fluid status is adequate, POCUS should be used to estimate the patient’s ventricular function. If the ventricle appears to be hyperdynamic with good contractility, shock should be treated with norepinephrine. On the other hand, if the ventricle is hypodynamic, dobutamine should be substituted for norepinephrine or, more often, added to norepinephrine.
The above represents a simplified summary of the critical points, but the authors do delve into further detail and also discuss some other options for therapies, including steroids, coronary revascularization, extracorporeal membrane oxygenation, and so on. The review is very thoughtful, thorough, and definitely worth a full read.
Top myths of diagnosis and management of infectious diseases in hospital medicine
Most, if not all of us in medicine, have heard the saying that 50% of what we learn in medical school (or residency) will turn out to be wrong. I certainly believe in this concept and consequently, like many of you, I enjoy reading about myths and misconceptions that we have been taught. With that in mind, I have to say that I love this article because it seems to have been written specifically to address what I was taught!
This author group, consisting mostly of clinical PharmDs who are experts in antibiotic use, provide us with an evidence-based discussion of myths and pitfalls in how antibiotics are often used in current clinical practice. The authors review their top 10 myths involving the use of antibiotics in treating infections in the hospital setting. A few of these relate more to the inpatient setting, but here are my favorite emergency department (ED)–related myths that they address:
- “Antibiotics do no harm.” The authors address the risk-benefit of antibiotics based on assumed vs. confirmed infections, including a brief discussion of adverse drug effects.
- “Antibiotic durations of 7, 14, or 21 days are typically necessary.” The authors address appropriate duration of antibiotic use and the fact that unnecessarily long durations of use can lead to resistance. They also provide reassurance that some infections can be treated with quite short durations of antibiotics.
- “If one drug is good, two (or more!) is better.” The use of multiple antibiotics, often with overlapping bacterial coverage, is rampant in medicine and further increases the risk for adverse drug effects and resistance.
- “Oral antibiotics are not as good as intravenous antibiotics for hospitalized patients.” This is definitely a myth that I learned. I recall being taught by many senior physicians that anyone sick enough for admission should be treated with intravenous antibiotics. As it turns out, absorption and effectiveness of most oral antibiotics is just as good as intravenous antibiotics, and the oral formulations are often safer.
- “A history of a penicillin allergy means the patient can never receive a beta-lactam antibiotic.” This is a myth that was debunked quite a few years ago, but it seems that many clinicians still need a reminder.
The authors included five more myths that are worth the read. This is an article that needs to be disseminated among all hospital clinicians.
Guidelines for low-risk, recurrent abdominal pain in the emergency department
The Society for Academic Emergency Medicine (SAEM) recently initiated a program focused on creating evidence-based approaches to challenging chief complaints and presentations in the emergency department (ED). In 2021, they published an approach to managing patients with recurrent, low-risk chest pain in the ED. This past year, they published their second guideline, focused on the management of patients with low-risk, recurrent abdominal pain in the ED.
Recurrent low-risk abdominal pain is a common and vexing presentation to EDs around the world, and there is little prior published guidance. Do all of these patients need repeat imaging? How do we manage their pain? Are there nonabdominal conditions that should be considered?
Broder and colleagues did a fantastic review of the current literature and, on behalf of SAEM, have provided a rational approach to optimal management of these patients. The four major questions they addressed, with brief summaries of their recommendations, are:
- Should adult ED patients with low-risk, recurrent and previously undifferentiated abdominal pain receive a repeat CT abdomen-pelvis (CTAP) after a negative CTAP within the past 12 months? This is a typical question that we all ponder when managing these patients. Unfortunately, the writing group found insufficient evidence to definitively identify populations in whom CTAP was recommended vs could be safely withheld. It is a bit disappointing that there is no definite answer to the question. On the other hand, it is reassuring to know that the world’s best evidence essentially says that it is perfectly appropriate to use your own good clinical judgment.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain with a negative CTAP receive additional imaging with abdominal ultrasound? In this case, the writing group found enough evidence, though low-level, to suggest against routine ultrasound in the absence of concern specifically for pelvic or hepatobiliary pathology. Like most tests, ultrasound is best used when there are specific concerns rather than being used in an undifferentiated fashion.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain receive screening for depression/anxiety? The writing group found enough evidence, though low-level again, to suggest that screening for depression and/or anxiety be performed during the ED evaluation. This could lead to successful therapy for the abdominal pain.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain receive nonopioid and/or nonpharmacologic analgesics? The writing group found little evidence to suggest for or against these analgesics, but they made a consensus recommendation suggesting an opioid-minimizing strategy for pain control.
Although the final recommendations of the writing group were not definitive or based on the strongest level of evidence, I find it helpful to have this guidance, nevertheless, on behalf of a major national organization. I also find it helpful to know that even with the best evidence available, optimal patient care will often boil down to physician experience and gestalt. I should also add that the overall article is chock-full of pearls and helpful information that will further inform the readers’ decisions, and so the full version is definitely worth the read.
In summary
There you have it – my three favorite practice-changing articles of 2022. Although I have tried to provide key points here, the full discussions of those key points in the published articles will provide a great deal more education than I can offer in this brief write-up, and so I strongly encourage everyone to read the full versions. Please be sure to include in the comments section your own pick for favorite or must-read articles from the past year.
Amal Mattu, MD, is a professor, vice chair of education, and codirector of the emergency cardiology fellowship in the department of emergency medicine at the University of Maryland, Baltimore. She reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
When 2022 began, we started seeing some light at the end of the COVID-19 tunnel. Vaccines were widely available, and even with new variants of the virus still occasionally emerging, the rates of severe morbidity and mortality appeared to be decreasing.
Expectedly, journals appeared to start moving more toward mainstream topics and publications rather than what seemed like a major focus on COVID-19 publications. The resulting literature was fantastic.
Several of those topics were discussed in a prior Emergency Medicine Viewpoint from this news organization, and many more of the research advances of 2022 will be discussed in the near future. However, in this Viewpoint, I would like to present my annual review of my three “must-read” articles of the past year.
As in past years, I am choosing reviews of the literature rather than original research articles (which, all too often, become outdated or debunked within a few years). I choose these articles in the hopes that readers will not simply settle for my brief reviews of the key points but instead will feel compelled to download and read the entire articles. These publications address common conditions and quandaries we face in the daily practice of emergency medicine and are practice-changing.
Myocardial dysfunction after cardiac arrest: Tips and pitfalls
The management of post–cardiac arrest patients remains a hot topic in the resuscitation literature as we continue to understand that the immediate post-arrest period is critical to patient outcome.
Ortuno and colleagues reviewed the current literature on post-arrest care and wrote an outstanding summary of how to optimally care for these patients. More specifically, they focused on post-arrest patients who demonstrate continued shock, or “post–cardiac arrest myocardial dysfunction” (PCAMD).
They propose three mechanisms for the pathogenesis of PCAMD: ischemia reperfusion phenomenon, systemic inflammatory response, and increased catecholamine release
I will skip through the details of the pathophysiology that they describe in the article, but I certainly do recommend that everyone review their descriptions.
Management of these patients begins with a good hemodynamic assessment, which includes clinical markers of perfusion (blood pressure, capillary refill), ECG, and point-of-care ultrasound (POCUS). If the initial assessment reveals an obvious cause of the cardiac arrest (e.g., massive pulmonary embolism, myocardial infarction, pericardial tamponade), then the underlying cause should be treated expeditiously.
In the absence of an obvious treatable cause of the shock, the fluid status and cardiac function should be addressed with POCUS. If the patient is hypovolemic, intravenous fluids should be administered. If the fluid status is adequate, POCUS should be used to estimate the patient’s ventricular function. If the ventricle appears to be hyperdynamic with good contractility, shock should be treated with norepinephrine. On the other hand, if the ventricle is hypodynamic, dobutamine should be substituted for norepinephrine or, more often, added to norepinephrine.
The above represents a simplified summary of the critical points, but the authors do delve into further detail and also discuss some other options for therapies, including steroids, coronary revascularization, extracorporeal membrane oxygenation, and so on. The review is very thoughtful, thorough, and definitely worth a full read.
Top myths of diagnosis and management of infectious diseases in hospital medicine
Most, if not all of us in medicine, have heard the saying that 50% of what we learn in medical school (or residency) will turn out to be wrong. I certainly believe in this concept and consequently, like many of you, I enjoy reading about myths and misconceptions that we have been taught. With that in mind, I have to say that I love this article because it seems to have been written specifically to address what I was taught!
This author group, consisting mostly of clinical PharmDs who are experts in antibiotic use, provide us with an evidence-based discussion of myths and pitfalls in how antibiotics are often used in current clinical practice. The authors review their top 10 myths involving the use of antibiotics in treating infections in the hospital setting. A few of these relate more to the inpatient setting, but here are my favorite emergency department (ED)–related myths that they address:
- “Antibiotics do no harm.” The authors address the risk-benefit of antibiotics based on assumed vs. confirmed infections, including a brief discussion of adverse drug effects.
- “Antibiotic durations of 7, 14, or 21 days are typically necessary.” The authors address appropriate duration of antibiotic use and the fact that unnecessarily long durations of use can lead to resistance. They also provide reassurance that some infections can be treated with quite short durations of antibiotics.
- “If one drug is good, two (or more!) is better.” The use of multiple antibiotics, often with overlapping bacterial coverage, is rampant in medicine and further increases the risk for adverse drug effects and resistance.
- “Oral antibiotics are not as good as intravenous antibiotics for hospitalized patients.” This is definitely a myth that I learned. I recall being taught by many senior physicians that anyone sick enough for admission should be treated with intravenous antibiotics. As it turns out, absorption and effectiveness of most oral antibiotics is just as good as intravenous antibiotics, and the oral formulations are often safer.
- “A history of a penicillin allergy means the patient can never receive a beta-lactam antibiotic.” This is a myth that was debunked quite a few years ago, but it seems that many clinicians still need a reminder.
The authors included five more myths that are worth the read. This is an article that needs to be disseminated among all hospital clinicians.
Guidelines for low-risk, recurrent abdominal pain in the emergency department
The Society for Academic Emergency Medicine (SAEM) recently initiated a program focused on creating evidence-based approaches to challenging chief complaints and presentations in the emergency department (ED). In 2021, they published an approach to managing patients with recurrent, low-risk chest pain in the ED. This past year, they published their second guideline, focused on the management of patients with low-risk, recurrent abdominal pain in the ED.
Recurrent low-risk abdominal pain is a common and vexing presentation to EDs around the world, and there is little prior published guidance. Do all of these patients need repeat imaging? How do we manage their pain? Are there nonabdominal conditions that should be considered?
Broder and colleagues did a fantastic review of the current literature and, on behalf of SAEM, have provided a rational approach to optimal management of these patients. The four major questions they addressed, with brief summaries of their recommendations, are:
- Should adult ED patients with low-risk, recurrent and previously undifferentiated abdominal pain receive a repeat CT abdomen-pelvis (CTAP) after a negative CTAP within the past 12 months? This is a typical question that we all ponder when managing these patients. Unfortunately, the writing group found insufficient evidence to definitively identify populations in whom CTAP was recommended vs could be safely withheld. It is a bit disappointing that there is no definite answer to the question. On the other hand, it is reassuring to know that the world’s best evidence essentially says that it is perfectly appropriate to use your own good clinical judgment.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain with a negative CTAP receive additional imaging with abdominal ultrasound? In this case, the writing group found enough evidence, though low-level, to suggest against routine ultrasound in the absence of concern specifically for pelvic or hepatobiliary pathology. Like most tests, ultrasound is best used when there are specific concerns rather than being used in an undifferentiated fashion.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain receive screening for depression/anxiety? The writing group found enough evidence, though low-level again, to suggest that screening for depression and/or anxiety be performed during the ED evaluation. This could lead to successful therapy for the abdominal pain.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain receive nonopioid and/or nonpharmacologic analgesics? The writing group found little evidence to suggest for or against these analgesics, but they made a consensus recommendation suggesting an opioid-minimizing strategy for pain control.
Although the final recommendations of the writing group were not definitive or based on the strongest level of evidence, I find it helpful to have this guidance, nevertheless, on behalf of a major national organization. I also find it helpful to know that even with the best evidence available, optimal patient care will often boil down to physician experience and gestalt. I should also add that the overall article is chock-full of pearls and helpful information that will further inform the readers’ decisions, and so the full version is definitely worth the read.
In summary
There you have it – my three favorite practice-changing articles of 2022. Although I have tried to provide key points here, the full discussions of those key points in the published articles will provide a great deal more education than I can offer in this brief write-up, and so I strongly encourage everyone to read the full versions. Please be sure to include in the comments section your own pick for favorite or must-read articles from the past year.
Amal Mattu, MD, is a professor, vice chair of education, and codirector of the emergency cardiology fellowship in the department of emergency medicine at the University of Maryland, Baltimore. She reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Three wild technologies about to change health care
When I was a child, I watched syndicated episodes of the original “Star Trek.” I was dazzled by the space travel, sure, but also the medical technology.
A handheld “tricorder” detected diseases, while an intramuscular injector (“hypospray”) could treat them. Sickbay “biobeds” came with real-time health monitors that looked futuristic at the time but seem primitive today.
Such visions inspired a lot of us kids to pursue science. Little did we know the real-life advances many of us would see in our lifetimes.
Artificial intelligence helping to spot disease, robots performing surgery, even video calls between doctor and patient – all these once sounded fantastical but now happen in clinical care.
Now, in the 23rd year of the 21st century, you might not believe wht we’ll be capable of next. Three especially wild examples are moving closer to clinical reality.
Human hibernation
Captain America, Han Solo, and “Star Trek” villain Khan – all were preserved at low temperatures and then revived, waking up alive and well months, decades, or centuries later. These are fictional examples, to be sure, but the science they’re rooted in is real.
(In one extreme case, a climber survived after almost 9 hours of efforts to revive him.)
Useful for a space traveler? Maybe not. But it’s potentially huge for someone with life-threatening injuries from a car accident or a gunshot wound.
That’s the thinking behind a breakthrough procedure that came after decades of research on pigs and dogs, now in a clinical trial. The idea: A person with massive blood loss whose heart has stopped is injected with an ice-cold fluid, cooling them from the inside, down to about 50° F.
Doctors already induce more modest hypothermia to protect the brain and other organs after cardiac arrest and during surgery on the aortic arch (the main artery carrying blood from the heart).
But this experimental procedure – called emergency preservation and resuscitation (EPR) – goes far beyond that, dramatically “decreasing the body’s need for oxygen and blood flow,” says Samuel Tisherman, MD, a trauma surgeon at the University of Maryland Medical Center and the trial’s lead researcher. This puts the patient in a state of suspended animation that “could buy time for surgeons to stop the bleeding and save more of these patients.”
The technique has been done on at least six patients, though none were reported to survive. The trial is expected to include 20 people by the time it wraps up in December, according to the listing on the U.S. clinical trials database. Though given the strict requirements for candidates (emergency trauma victims who are not likely to survive), one can’t exactly rely on a set schedule.
Still, the technology is promising. Someday we may even use it to keep patients in suspended animation for months or years, experts predict, helping astronauts through decades-long spaceflights, or stalling death in sick patients awaiting a cure.
Artificial womb
Another sci-fi classic: growing human babies outside the womb. Think the fetus fields from “The Matrix,” or the frozen embryos in “Alien: Covenant.”
In 1923, British biologist J.B.S. Haldane coined a term for that – ectogenesis. He predicted that 70% of pregnancies would take place, from fertilization to birth, in artificial wombs by 2074. That many seems unlikely, but the timeline is on track.
Developing an embryo outside the womb is already routine in in vitro fertilization. And technology enables preterm babies to survive through much of the second half of gestation. Normal human pregnancy is 40 weeks, and the youngest preterm baby ever to survive was 21 weeks and 1 day old, just a few days younger than a smattering of others who lived.
The biggest obstacle for babies younger than that is lung viability. Mechanical ventilation can damage the lungs and lead to a chronic (sometimes fatal) lung disease known as bronchopulmonary dysplasia. Avoiding this would mean figuring out a way to maintain fetal circulation – the intricate system that delivers oxygenated blood from the placenta to the fetus via the umbilical cord. Researchers at Children’s Hospital of Philadelphia have done this using a fetal lamb.
The key to their invention is a substitute placenta: an oxygenator connected to the lamb’s umbilical cord. Tubes inserted through the umbilical vein and arteries carry oxygenated blood from the “placenta” to the fetus, and deoxygenated blood back out. The lamb resides in an artificial, fluid-filled amniotic sac until its lungs and other organs are developed.
Fertility treatment could benefit, too. “An artificial womb may substitute in situations in which a gestational carrier – surrogate – is indicated,” says Paula Amato, MD, a professor of obstetrics and gynecology at Oregon Health and Science University, Portland. (Dr. Amato is not involved in the CHOP research.) For example: when the mother is missing a uterus or can’t carry a pregnancy safely.
No date is set for clinical trials yet. But according to the research, the main difference between human and lamb may come down to size. A lamb’s umbilical vessels are larger, so feeding in a tube is easier. With today’s advances in miniaturizing surgical methods, that seems like a challenge scientists can overcome.
Messenger RNA therapeutics
Back to “Star Trek.” The hypospray injector’s contents could cure just about any disease, even one newly discovered on a strange planet. That’s not unlike messenger RNA (mRNA) technology, a breakthrough that enabled scientists to quickly develop some of the first COVID-19 vaccines.
But vaccines are just the beginning of what this technology can do.
A whole field of immunotherapy is emerging that uses mRNA to deliver instructions to produce chimeric antigen receptor–modified immune cells (CAR-modified immune cells). These cells are engineered to target diseased cells and tissues, like cancer cells and harmful fibroblasts (scar tissue) that promote fibrosis in, for example, the heart and lungs.
The field is bursting with rodent research, and clinical trials have started for treating some advanced-stage malignancies.
Actual clinical use may be years away, but if all goes well, these medicines could help treat or even cure the core medical problems facing humanity. We’re talking cancer, heart disease, neurodegenerative disease – transforming one therapy into another by simply changing the mRNA’s “nucleotide sequence,” the blueprint containing instructions telling it what to do, and what disease to attack.
As this technology matures, we may start to feel as if we’re really on “Star Trek,” where Dr. Leonard “Bones” McCoy pulls out the same device to treat just about every disease or injury.
A version of this article first appeared on WebMD.com.
When I was a child, I watched syndicated episodes of the original “Star Trek.” I was dazzled by the space travel, sure, but also the medical technology.
A handheld “tricorder” detected diseases, while an intramuscular injector (“hypospray”) could treat them. Sickbay “biobeds” came with real-time health monitors that looked futuristic at the time but seem primitive today.
Such visions inspired a lot of us kids to pursue science. Little did we know the real-life advances many of us would see in our lifetimes.
Artificial intelligence helping to spot disease, robots performing surgery, even video calls between doctor and patient – all these once sounded fantastical but now happen in clinical care.
Now, in the 23rd year of the 21st century, you might not believe wht we’ll be capable of next. Three especially wild examples are moving closer to clinical reality.
Human hibernation
Captain America, Han Solo, and “Star Trek” villain Khan – all were preserved at low temperatures and then revived, waking up alive and well months, decades, or centuries later. These are fictional examples, to be sure, but the science they’re rooted in is real.
(In one extreme case, a climber survived after almost 9 hours of efforts to revive him.)
Useful for a space traveler? Maybe not. But it’s potentially huge for someone with life-threatening injuries from a car accident or a gunshot wound.
That’s the thinking behind a breakthrough procedure that came after decades of research on pigs and dogs, now in a clinical trial. The idea: A person with massive blood loss whose heart has stopped is injected with an ice-cold fluid, cooling them from the inside, down to about 50° F.
Doctors already induce more modest hypothermia to protect the brain and other organs after cardiac arrest and during surgery on the aortic arch (the main artery carrying blood from the heart).
But this experimental procedure – called emergency preservation and resuscitation (EPR) – goes far beyond that, dramatically “decreasing the body’s need for oxygen and blood flow,” says Samuel Tisherman, MD, a trauma surgeon at the University of Maryland Medical Center and the trial’s lead researcher. This puts the patient in a state of suspended animation that “could buy time for surgeons to stop the bleeding and save more of these patients.”
The technique has been done on at least six patients, though none were reported to survive. The trial is expected to include 20 people by the time it wraps up in December, according to the listing on the U.S. clinical trials database. Though given the strict requirements for candidates (emergency trauma victims who are not likely to survive), one can’t exactly rely on a set schedule.
Still, the technology is promising. Someday we may even use it to keep patients in suspended animation for months or years, experts predict, helping astronauts through decades-long spaceflights, or stalling death in sick patients awaiting a cure.
Artificial womb
Another sci-fi classic: growing human babies outside the womb. Think the fetus fields from “The Matrix,” or the frozen embryos in “Alien: Covenant.”
In 1923, British biologist J.B.S. Haldane coined a term for that – ectogenesis. He predicted that 70% of pregnancies would take place, from fertilization to birth, in artificial wombs by 2074. That many seems unlikely, but the timeline is on track.
Developing an embryo outside the womb is already routine in in vitro fertilization. And technology enables preterm babies to survive through much of the second half of gestation. Normal human pregnancy is 40 weeks, and the youngest preterm baby ever to survive was 21 weeks and 1 day old, just a few days younger than a smattering of others who lived.
The biggest obstacle for babies younger than that is lung viability. Mechanical ventilation can damage the lungs and lead to a chronic (sometimes fatal) lung disease known as bronchopulmonary dysplasia. Avoiding this would mean figuring out a way to maintain fetal circulation – the intricate system that delivers oxygenated blood from the placenta to the fetus via the umbilical cord. Researchers at Children’s Hospital of Philadelphia have done this using a fetal lamb.
The key to their invention is a substitute placenta: an oxygenator connected to the lamb’s umbilical cord. Tubes inserted through the umbilical vein and arteries carry oxygenated blood from the “placenta” to the fetus, and deoxygenated blood back out. The lamb resides in an artificial, fluid-filled amniotic sac until its lungs and other organs are developed.
Fertility treatment could benefit, too. “An artificial womb may substitute in situations in which a gestational carrier – surrogate – is indicated,” says Paula Amato, MD, a professor of obstetrics and gynecology at Oregon Health and Science University, Portland. (Dr. Amato is not involved in the CHOP research.) For example: when the mother is missing a uterus or can’t carry a pregnancy safely.
No date is set for clinical trials yet. But according to the research, the main difference between human and lamb may come down to size. A lamb’s umbilical vessels are larger, so feeding in a tube is easier. With today’s advances in miniaturizing surgical methods, that seems like a challenge scientists can overcome.
Messenger RNA therapeutics
Back to “Star Trek.” The hypospray injector’s contents could cure just about any disease, even one newly discovered on a strange planet. That’s not unlike messenger RNA (mRNA) technology, a breakthrough that enabled scientists to quickly develop some of the first COVID-19 vaccines.
But vaccines are just the beginning of what this technology can do.
A whole field of immunotherapy is emerging that uses mRNA to deliver instructions to produce chimeric antigen receptor–modified immune cells (CAR-modified immune cells). These cells are engineered to target diseased cells and tissues, like cancer cells and harmful fibroblasts (scar tissue) that promote fibrosis in, for example, the heart and lungs.
The field is bursting with rodent research, and clinical trials have started for treating some advanced-stage malignancies.
Actual clinical use may be years away, but if all goes well, these medicines could help treat or even cure the core medical problems facing humanity. We’re talking cancer, heart disease, neurodegenerative disease – transforming one therapy into another by simply changing the mRNA’s “nucleotide sequence,” the blueprint containing instructions telling it what to do, and what disease to attack.
As this technology matures, we may start to feel as if we’re really on “Star Trek,” where Dr. Leonard “Bones” McCoy pulls out the same device to treat just about every disease or injury.
A version of this article first appeared on WebMD.com.
When I was a child, I watched syndicated episodes of the original “Star Trek.” I was dazzled by the space travel, sure, but also the medical technology.
A handheld “tricorder” detected diseases, while an intramuscular injector (“hypospray”) could treat them. Sickbay “biobeds” came with real-time health monitors that looked futuristic at the time but seem primitive today.
Such visions inspired a lot of us kids to pursue science. Little did we know the real-life advances many of us would see in our lifetimes.
Artificial intelligence helping to spot disease, robots performing surgery, even video calls between doctor and patient – all these once sounded fantastical but now happen in clinical care.
Now, in the 23rd year of the 21st century, you might not believe wht we’ll be capable of next. Three especially wild examples are moving closer to clinical reality.
Human hibernation
Captain America, Han Solo, and “Star Trek” villain Khan – all were preserved at low temperatures and then revived, waking up alive and well months, decades, or centuries later. These are fictional examples, to be sure, but the science they’re rooted in is real.
(In one extreme case, a climber survived after almost 9 hours of efforts to revive him.)
Useful for a space traveler? Maybe not. But it’s potentially huge for someone with life-threatening injuries from a car accident or a gunshot wound.
That’s the thinking behind a breakthrough procedure that came after decades of research on pigs and dogs, now in a clinical trial. The idea: A person with massive blood loss whose heart has stopped is injected with an ice-cold fluid, cooling them from the inside, down to about 50° F.
Doctors already induce more modest hypothermia to protect the brain and other organs after cardiac arrest and during surgery on the aortic arch (the main artery carrying blood from the heart).
But this experimental procedure – called emergency preservation and resuscitation (EPR) – goes far beyond that, dramatically “decreasing the body’s need for oxygen and blood flow,” says Samuel Tisherman, MD, a trauma surgeon at the University of Maryland Medical Center and the trial’s lead researcher. This puts the patient in a state of suspended animation that “could buy time for surgeons to stop the bleeding and save more of these patients.”
The technique has been done on at least six patients, though none were reported to survive. The trial is expected to include 20 people by the time it wraps up in December, according to the listing on the U.S. clinical trials database. Though given the strict requirements for candidates (emergency trauma victims who are not likely to survive), one can’t exactly rely on a set schedule.
Still, the technology is promising. Someday we may even use it to keep patients in suspended animation for months or years, experts predict, helping astronauts through decades-long spaceflights, or stalling death in sick patients awaiting a cure.
Artificial womb
Another sci-fi classic: growing human babies outside the womb. Think the fetus fields from “The Matrix,” or the frozen embryos in “Alien: Covenant.”
In 1923, British biologist J.B.S. Haldane coined a term for that – ectogenesis. He predicted that 70% of pregnancies would take place, from fertilization to birth, in artificial wombs by 2074. That many seems unlikely, but the timeline is on track.
Developing an embryo outside the womb is already routine in in vitro fertilization. And technology enables preterm babies to survive through much of the second half of gestation. Normal human pregnancy is 40 weeks, and the youngest preterm baby ever to survive was 21 weeks and 1 day old, just a few days younger than a smattering of others who lived.
The biggest obstacle for babies younger than that is lung viability. Mechanical ventilation can damage the lungs and lead to a chronic (sometimes fatal) lung disease known as bronchopulmonary dysplasia. Avoiding this would mean figuring out a way to maintain fetal circulation – the intricate system that delivers oxygenated blood from the placenta to the fetus via the umbilical cord. Researchers at Children’s Hospital of Philadelphia have done this using a fetal lamb.
The key to their invention is a substitute placenta: an oxygenator connected to the lamb’s umbilical cord. Tubes inserted through the umbilical vein and arteries carry oxygenated blood from the “placenta” to the fetus, and deoxygenated blood back out. The lamb resides in an artificial, fluid-filled amniotic sac until its lungs and other organs are developed.
Fertility treatment could benefit, too. “An artificial womb may substitute in situations in which a gestational carrier – surrogate – is indicated,” says Paula Amato, MD, a professor of obstetrics and gynecology at Oregon Health and Science University, Portland. (Dr. Amato is not involved in the CHOP research.) For example: when the mother is missing a uterus or can’t carry a pregnancy safely.
No date is set for clinical trials yet. But according to the research, the main difference between human and lamb may come down to size. A lamb’s umbilical vessels are larger, so feeding in a tube is easier. With today’s advances in miniaturizing surgical methods, that seems like a challenge scientists can overcome.
Messenger RNA therapeutics
Back to “Star Trek.” The hypospray injector’s contents could cure just about any disease, even one newly discovered on a strange planet. That’s not unlike messenger RNA (mRNA) technology, a breakthrough that enabled scientists to quickly develop some of the first COVID-19 vaccines.
But vaccines are just the beginning of what this technology can do.
A whole field of immunotherapy is emerging that uses mRNA to deliver instructions to produce chimeric antigen receptor–modified immune cells (CAR-modified immune cells). These cells are engineered to target diseased cells and tissues, like cancer cells and harmful fibroblasts (scar tissue) that promote fibrosis in, for example, the heart and lungs.
The field is bursting with rodent research, and clinical trials have started for treating some advanced-stage malignancies.
Actual clinical use may be years away, but if all goes well, these medicines could help treat or even cure the core medical problems facing humanity. We’re talking cancer, heart disease, neurodegenerative disease – transforming one therapy into another by simply changing the mRNA’s “nucleotide sequence,” the blueprint containing instructions telling it what to do, and what disease to attack.
As this technology matures, we may start to feel as if we’re really on “Star Trek,” where Dr. Leonard “Bones” McCoy pulls out the same device to treat just about every disease or injury.
A version of this article first appeared on WebMD.com.
A new (old) drug joins the COVID fray, and guess what? It works
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.

At this point, with the monoclonals found to be essentially useless, we are left with remdesivir with its modest efficacy and Paxlovid, which, for some reason, people don’t seem to be taking.
Part of the reason the monoclonals have failed lately is because of their specificity; they are homogeneous antibodies targeted toward a very specific epitope that may change from variant to variant. We need a broader therapeutic, one that has activity across all variants — maybe even one that has activity against all viruses? We’ve got one. Interferon.
The first mention of interferon as a potential COVID therapy was at the very start of the pandemic, so I’m sort of surprised that the first large, randomized trial is only being reported now in the New England Journal of Medicine.
Before we dig into the results, let’s talk mechanism. This is a trial of interferon-lambda, also known as interleukin-29.
The lambda interferons were only discovered in 2003. They differ from the more familiar interferons only in their cellular receptors; the downstream effects seem quite similar. As opposed to the cellular receptors for interferon alfa, which are widely expressed, the receptors for lambda are restricted to epithelial tissues. This makes it a good choice as a COVID treatment, since the virus also preferentially targets those epithelial cells.
In this study, 1,951 participants from Brazil and Canada, but mostly Brazil, with new COVID infections who were not yet hospitalized were randomized to receive 180 mcg of interferon lambda or placebo.
This was a relatively current COVID trial, as you can see from the participant characteristics. The majority had been vaccinated, and nearly half of the infections were during the Omicron phase of the pandemic.
If you just want to cut to the chase, interferon worked.
The primary outcome – hospitalization or a prolonged emergency room visit for COVID – was 50% lower in the interferon group.
Key secondary outcomes, including death from COVID, were lower in the interferon group as well. These effects persisted across most of the subgroups I was looking out for.
Interferon seemed to help those who were already vaccinated and those who were unvaccinated. There’s a hint that it works better within the first few days of symptoms, which isn’t surprising; we’ve seen this for many of the therapeutics, including Paxlovid. Time is of the essence. Encouragingly, the effect was a bit more pronounced among those infected with Omicron.
Of course, if you have any experience with interferon, you know that the side effects can be pretty rough. In the bad old days when we treated hepatitis C infection with interferon, patients would get their injections on Friday in anticipation of being essentially out of commission with flu-like symptoms through the weekend. But we don’t see much evidence of adverse events in this trial, maybe due to the greater specificity of interferon lambda.
Putting it all together, the state of play for interferons in COVID may be changing. To date, the FDA has not recommended the use of interferon alfa or -beta for COVID-19, citing some data that they are ineffective or even harmful in hospitalized patients with COVID. Interferon lambda is not FDA approved and thus not even available in the United States. But the reason it has not been approved is that there has not been a large, well-conducted interferon lambda trial. Now there is. Will this study be enough to prompt an emergency use authorization? The elephant in the room, of course, is Paxlovid, which at this point has a longer safety track record and, importantly, is oral. I’d love to see a head-to-head trial. Short of that, I tend to be in favor of having more options on the table.
Dr. Perry Wilson is associate professor, department of medicine, and director, Clinical and Translational Research Accelerator, at Yale University, New Haven, Conn. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.

At this point, with the monoclonals found to be essentially useless, we are left with remdesivir with its modest efficacy and Paxlovid, which, for some reason, people don’t seem to be taking.
Part of the reason the monoclonals have failed lately is because of their specificity; they are homogeneous antibodies targeted toward a very specific epitope that may change from variant to variant. We need a broader therapeutic, one that has activity across all variants — maybe even one that has activity against all viruses? We’ve got one. Interferon.
The first mention of interferon as a potential COVID therapy was at the very start of the pandemic, so I’m sort of surprised that the first large, randomized trial is only being reported now in the New England Journal of Medicine.
Before we dig into the results, let’s talk mechanism. This is a trial of interferon-lambda, also known as interleukin-29.
The lambda interferons were only discovered in 2003. They differ from the more familiar interferons only in their cellular receptors; the downstream effects seem quite similar. As opposed to the cellular receptors for interferon alfa, which are widely expressed, the receptors for lambda are restricted to epithelial tissues. This makes it a good choice as a COVID treatment, since the virus also preferentially targets those epithelial cells.
In this study, 1,951 participants from Brazil and Canada, but mostly Brazil, with new COVID infections who were not yet hospitalized were randomized to receive 180 mcg of interferon lambda or placebo.
This was a relatively current COVID trial, as you can see from the participant characteristics. The majority had been vaccinated, and nearly half of the infections were during the Omicron phase of the pandemic.
If you just want to cut to the chase, interferon worked.
The primary outcome – hospitalization or a prolonged emergency room visit for COVID – was 50% lower in the interferon group.
Key secondary outcomes, including death from COVID, were lower in the interferon group as well. These effects persisted across most of the subgroups I was looking out for.
Interferon seemed to help those who were already vaccinated and those who were unvaccinated. There’s a hint that it works better within the first few days of symptoms, which isn’t surprising; we’ve seen this for many of the therapeutics, including Paxlovid. Time is of the essence. Encouragingly, the effect was a bit more pronounced among those infected with Omicron.
Of course, if you have any experience with interferon, you know that the side effects can be pretty rough. In the bad old days when we treated hepatitis C infection with interferon, patients would get their injections on Friday in anticipation of being essentially out of commission with flu-like symptoms through the weekend. But we don’t see much evidence of adverse events in this trial, maybe due to the greater specificity of interferon lambda.
Putting it all together, the state of play for interferons in COVID may be changing. To date, the FDA has not recommended the use of interferon alfa or -beta for COVID-19, citing some data that they are ineffective or even harmful in hospitalized patients with COVID. Interferon lambda is not FDA approved and thus not even available in the United States. But the reason it has not been approved is that there has not been a large, well-conducted interferon lambda trial. Now there is. Will this study be enough to prompt an emergency use authorization? The elephant in the room, of course, is Paxlovid, which at this point has a longer safety track record and, importantly, is oral. I’d love to see a head-to-head trial. Short of that, I tend to be in favor of having more options on the table.
Dr. Perry Wilson is associate professor, department of medicine, and director, Clinical and Translational Research Accelerator, at Yale University, New Haven, Conn. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.

At this point, with the monoclonals found to be essentially useless, we are left with remdesivir with its modest efficacy and Paxlovid, which, for some reason, people don’t seem to be taking.
Part of the reason the monoclonals have failed lately is because of their specificity; they are homogeneous antibodies targeted toward a very specific epitope that may change from variant to variant. We need a broader therapeutic, one that has activity across all variants — maybe even one that has activity against all viruses? We’ve got one. Interferon.
The first mention of interferon as a potential COVID therapy was at the very start of the pandemic, so I’m sort of surprised that the first large, randomized trial is only being reported now in the New England Journal of Medicine.
Before we dig into the results, let’s talk mechanism. This is a trial of interferon-lambda, also known as interleukin-29.
The lambda interferons were only discovered in 2003. They differ from the more familiar interferons only in their cellular receptors; the downstream effects seem quite similar. As opposed to the cellular receptors for interferon alfa, which are widely expressed, the receptors for lambda are restricted to epithelial tissues. This makes it a good choice as a COVID treatment, since the virus also preferentially targets those epithelial cells.
In this study, 1,951 participants from Brazil and Canada, but mostly Brazil, with new COVID infections who were not yet hospitalized were randomized to receive 180 mcg of interferon lambda or placebo.
This was a relatively current COVID trial, as you can see from the participant characteristics. The majority had been vaccinated, and nearly half of the infections were during the Omicron phase of the pandemic.
If you just want to cut to the chase, interferon worked.
The primary outcome – hospitalization or a prolonged emergency room visit for COVID – was 50% lower in the interferon group.
Key secondary outcomes, including death from COVID, were lower in the interferon group as well. These effects persisted across most of the subgroups I was looking out for.
Interferon seemed to help those who were already vaccinated and those who were unvaccinated. There’s a hint that it works better within the first few days of symptoms, which isn’t surprising; we’ve seen this for many of the therapeutics, including Paxlovid. Time is of the essence. Encouragingly, the effect was a bit more pronounced among those infected with Omicron.
Of course, if you have any experience with interferon, you know that the side effects can be pretty rough. In the bad old days when we treated hepatitis C infection with interferon, patients would get their injections on Friday in anticipation of being essentially out of commission with flu-like symptoms through the weekend. But we don’t see much evidence of adverse events in this trial, maybe due to the greater specificity of interferon lambda.
Putting it all together, the state of play for interferons in COVID may be changing. To date, the FDA has not recommended the use of interferon alfa or -beta for COVID-19, citing some data that they are ineffective or even harmful in hospitalized patients with COVID. Interferon lambda is not FDA approved and thus not even available in the United States. But the reason it has not been approved is that there has not been a large, well-conducted interferon lambda trial. Now there is. Will this study be enough to prompt an emergency use authorization? The elephant in the room, of course, is Paxlovid, which at this point has a longer safety track record and, importantly, is oral. I’d love to see a head-to-head trial. Short of that, I tend to be in favor of having more options on the table.
Dr. Perry Wilson is associate professor, department of medicine, and director, Clinical and Translational Research Accelerator, at Yale University, New Haven, Conn. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Maternal COVID-19 vaccine curbs infant infection
a new study shows.
Previous research has confirmed that COVID-19 neutralizing antibodies following maternal vaccination or maternal COVID-19 infection are present in umbilical cord blood, breast milk, and infant serum specimens, wrote Sarah C.J. Jorgensen, PharmD, MPH, of the University of Toronto, and colleagues in their article published in The BMJ.
In the study, the researchers identified maternal and newborn pairs using administrative databases from Canada. The study population included 8,809 infants aged younger than 6 months who were born between May 7, 2021, and March 31, 2022, and who underwent testing for COVID-19 between May 7, 2021, and September 5, 2022.
Maternal vaccination with the primary COVID-19 mRNA monovalent vaccine series was defined as two vaccine doses administered up to 14 days before delivery, with at least one of the doses after the conception date.
Maternal vaccination with the primary series plus one booster was defined as three doses administered up to 14 days before delivery, with at least one of these doses after the conception date.
The primary outcome was the presence of delta or omicron COVID-19 infection or hospital admission of the infants.
The study population included 99 COVID-19 cases with the delta variant (with 4,365 controls) and 1,501 cases with the omicron variant (with 4,847 controls).
Overall, the vaccine effectiveness of maternal doses was 95% against delta infection and 45% against omicron.
The effectiveness against hospital admission in cases of delta and omicron variants were 97% and 53%, respectively.
The effectiveness of three doses was 73% against omicron infant infection and 80% against omicron-related infant hospitalization. Data were not available for the effectiveness of three doses against the delta variant.
The effectiveness of two doses of vaccine against infant omicron infection was highest when mothers received the second dose during the third trimester of pregnancy, compared with during the first trimester or second trimester (53% vs. 47% and 53% vs. 37%, respectively).
Vaccine effectiveness with two doses against infant infection from omicron was highest in the first 8 weeks of life (57%), then decreased to 40% among infants after 16 weeks of age.
Although the study was not designed to assess the mechanism of action of the impact of maternal vaccination on infants, the current study results were consistent with other recent studies showing a reduction in infections and hospitalizations among infants whose mothers received COVID-19 vaccines during pregnancy, the researchers wrote in their discussion.
The findings were limited by several factors including the potential unmeasured confounders not available in databases, such as whether infants were breastfed, the researchers noted. Other limitations included a lack of data on home test results and the inability to assess the waning impact of the vaccine effectiveness against the delta variant because of the small number of delta cases, they said. However, the results suggest that the mRNA COVID-19 vaccine during pregnancy was moderately to highly effective for protection against omicron and delta infection and infection-related hospitalization – especially during the first 8 weeks of life.
Effectiveness is encouraging, but updates are needed
The effectiveness of maternal vaccination to prevent COVID-19 infection and related hospitalizations in infants is promising, especially since those younger than 6 months have no other source of vaccine protection against COVID-19 infection, wrote Dana Danino, MD, of Soroka University Medical Center, Israel, and Ilan Youngster, MD, of Shamir Medical Center, Israel, in an accompanying editorial also published in The BMJ.
They also noted that maternal vaccination during pregnancy is an established method of protecting infants from infections such as influenza and pertussis.
Data from previous studies show that most infants whose mothers were vaccinated against COVID-19 during pregnancy retained maternal antibodies at 6 months, “but evidence for protection against neonatal COVID-19 infection has been deficient,” they said.
The current study findings support the value of vaccination during pregnancy, and the findings were strengthened by the large study population, the editorialists wrote. However, whether the same effectiveness holds for other COVID-19 strains such as BQ.1, BQ.1.1, BF.7, XBB, and XBB.1 remains unknown, they said.
Other areas in need of exploration include the optimal timing of vaccination during pregnancy, the protective effects of a bivalent mRNA vaccine (vs. the primary monovalent vaccine in the current study), and the potential benefits of additional boosters, they added.
“Although Jorgenson and colleagues’ study reinforces the value of maternal vaccination against COVID-19 during pregnancy, more studies are needed to better inform vaccination recommendations in an evolving landscape of new SARS-CoV-2 strains and novel vaccines,” the editorialists concluded.
The study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and the Ministry of Long-term Care; the study also received funding from the Canadian Immunization Research Network and the Public Health Agency of Canada. Dr. Jorgensen and the editorialists had no financial conflicts to disclose.
*This article was updated on 3/2/2023.
a new study shows.
Previous research has confirmed that COVID-19 neutralizing antibodies following maternal vaccination or maternal COVID-19 infection are present in umbilical cord blood, breast milk, and infant serum specimens, wrote Sarah C.J. Jorgensen, PharmD, MPH, of the University of Toronto, and colleagues in their article published in The BMJ.
In the study, the researchers identified maternal and newborn pairs using administrative databases from Canada. The study population included 8,809 infants aged younger than 6 months who were born between May 7, 2021, and March 31, 2022, and who underwent testing for COVID-19 between May 7, 2021, and September 5, 2022.
Maternal vaccination with the primary COVID-19 mRNA monovalent vaccine series was defined as two vaccine doses administered up to 14 days before delivery, with at least one of the doses after the conception date.
Maternal vaccination with the primary series plus one booster was defined as three doses administered up to 14 days before delivery, with at least one of these doses after the conception date.
The primary outcome was the presence of delta or omicron COVID-19 infection or hospital admission of the infants.
The study population included 99 COVID-19 cases with the delta variant (with 4,365 controls) and 1,501 cases with the omicron variant (with 4,847 controls).
Overall, the vaccine effectiveness of maternal doses was 95% against delta infection and 45% against omicron.
The effectiveness against hospital admission in cases of delta and omicron variants were 97% and 53%, respectively.
The effectiveness of three doses was 73% against omicron infant infection and 80% against omicron-related infant hospitalization. Data were not available for the effectiveness of three doses against the delta variant.
The effectiveness of two doses of vaccine against infant omicron infection was highest when mothers received the second dose during the third trimester of pregnancy, compared with during the first trimester or second trimester (53% vs. 47% and 53% vs. 37%, respectively).
Vaccine effectiveness with two doses against infant infection from omicron was highest in the first 8 weeks of life (57%), then decreased to 40% among infants after 16 weeks of age.
Although the study was not designed to assess the mechanism of action of the impact of maternal vaccination on infants, the current study results were consistent with other recent studies showing a reduction in infections and hospitalizations among infants whose mothers received COVID-19 vaccines during pregnancy, the researchers wrote in their discussion.
The findings were limited by several factors including the potential unmeasured confounders not available in databases, such as whether infants were breastfed, the researchers noted. Other limitations included a lack of data on home test results and the inability to assess the waning impact of the vaccine effectiveness against the delta variant because of the small number of delta cases, they said. However, the results suggest that the mRNA COVID-19 vaccine during pregnancy was moderately to highly effective for protection against omicron and delta infection and infection-related hospitalization – especially during the first 8 weeks of life.
Effectiveness is encouraging, but updates are needed
The effectiveness of maternal vaccination to prevent COVID-19 infection and related hospitalizations in infants is promising, especially since those younger than 6 months have no other source of vaccine protection against COVID-19 infection, wrote Dana Danino, MD, of Soroka University Medical Center, Israel, and Ilan Youngster, MD, of Shamir Medical Center, Israel, in an accompanying editorial also published in The BMJ.
They also noted that maternal vaccination during pregnancy is an established method of protecting infants from infections such as influenza and pertussis.
Data from previous studies show that most infants whose mothers were vaccinated against COVID-19 during pregnancy retained maternal antibodies at 6 months, “but evidence for protection against neonatal COVID-19 infection has been deficient,” they said.
The current study findings support the value of vaccination during pregnancy, and the findings were strengthened by the large study population, the editorialists wrote. However, whether the same effectiveness holds for other COVID-19 strains such as BQ.1, BQ.1.1, BF.7, XBB, and XBB.1 remains unknown, they said.
Other areas in need of exploration include the optimal timing of vaccination during pregnancy, the protective effects of a bivalent mRNA vaccine (vs. the primary monovalent vaccine in the current study), and the potential benefits of additional boosters, they added.
“Although Jorgenson and colleagues’ study reinforces the value of maternal vaccination against COVID-19 during pregnancy, more studies are needed to better inform vaccination recommendations in an evolving landscape of new SARS-CoV-2 strains and novel vaccines,” the editorialists concluded.
The study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and the Ministry of Long-term Care; the study also received funding from the Canadian Immunization Research Network and the Public Health Agency of Canada. Dr. Jorgensen and the editorialists had no financial conflicts to disclose.
*This article was updated on 3/2/2023.
a new study shows.
Previous research has confirmed that COVID-19 neutralizing antibodies following maternal vaccination or maternal COVID-19 infection are present in umbilical cord blood, breast milk, and infant serum specimens, wrote Sarah C.J. Jorgensen, PharmD, MPH, of the University of Toronto, and colleagues in their article published in The BMJ.
In the study, the researchers identified maternal and newborn pairs using administrative databases from Canada. The study population included 8,809 infants aged younger than 6 months who were born between May 7, 2021, and March 31, 2022, and who underwent testing for COVID-19 between May 7, 2021, and September 5, 2022.
Maternal vaccination with the primary COVID-19 mRNA monovalent vaccine series was defined as two vaccine doses administered up to 14 days before delivery, with at least one of the doses after the conception date.
Maternal vaccination with the primary series plus one booster was defined as three doses administered up to 14 days before delivery, with at least one of these doses after the conception date.
The primary outcome was the presence of delta or omicron COVID-19 infection or hospital admission of the infants.
The study population included 99 COVID-19 cases with the delta variant (with 4,365 controls) and 1,501 cases with the omicron variant (with 4,847 controls).
Overall, the vaccine effectiveness of maternal doses was 95% against delta infection and 45% against omicron.
The effectiveness against hospital admission in cases of delta and omicron variants were 97% and 53%, respectively.
The effectiveness of three doses was 73% against omicron infant infection and 80% against omicron-related infant hospitalization. Data were not available for the effectiveness of three doses against the delta variant.
The effectiveness of two doses of vaccine against infant omicron infection was highest when mothers received the second dose during the third trimester of pregnancy, compared with during the first trimester or second trimester (53% vs. 47% and 53% vs. 37%, respectively).
Vaccine effectiveness with two doses against infant infection from omicron was highest in the first 8 weeks of life (57%), then decreased to 40% among infants after 16 weeks of age.
Although the study was not designed to assess the mechanism of action of the impact of maternal vaccination on infants, the current study results were consistent with other recent studies showing a reduction in infections and hospitalizations among infants whose mothers received COVID-19 vaccines during pregnancy, the researchers wrote in their discussion.
The findings were limited by several factors including the potential unmeasured confounders not available in databases, such as whether infants were breastfed, the researchers noted. Other limitations included a lack of data on home test results and the inability to assess the waning impact of the vaccine effectiveness against the delta variant because of the small number of delta cases, they said. However, the results suggest that the mRNA COVID-19 vaccine during pregnancy was moderately to highly effective for protection against omicron and delta infection and infection-related hospitalization – especially during the first 8 weeks of life.
Effectiveness is encouraging, but updates are needed
The effectiveness of maternal vaccination to prevent COVID-19 infection and related hospitalizations in infants is promising, especially since those younger than 6 months have no other source of vaccine protection against COVID-19 infection, wrote Dana Danino, MD, of Soroka University Medical Center, Israel, and Ilan Youngster, MD, of Shamir Medical Center, Israel, in an accompanying editorial also published in The BMJ.
They also noted that maternal vaccination during pregnancy is an established method of protecting infants from infections such as influenza and pertussis.
Data from previous studies show that most infants whose mothers were vaccinated against COVID-19 during pregnancy retained maternal antibodies at 6 months, “but evidence for protection against neonatal COVID-19 infection has been deficient,” they said.
The current study findings support the value of vaccination during pregnancy, and the findings were strengthened by the large study population, the editorialists wrote. However, whether the same effectiveness holds for other COVID-19 strains such as BQ.1, BQ.1.1, BF.7, XBB, and XBB.1 remains unknown, they said.
Other areas in need of exploration include the optimal timing of vaccination during pregnancy, the protective effects of a bivalent mRNA vaccine (vs. the primary monovalent vaccine in the current study), and the potential benefits of additional boosters, they added.
“Although Jorgenson and colleagues’ study reinforces the value of maternal vaccination against COVID-19 during pregnancy, more studies are needed to better inform vaccination recommendations in an evolving landscape of new SARS-CoV-2 strains and novel vaccines,” the editorialists concluded.
The study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and the Ministry of Long-term Care; the study also received funding from the Canadian Immunization Research Network and the Public Health Agency of Canada. Dr. Jorgensen and the editorialists had no financial conflicts to disclose.
*This article was updated on 3/2/2023.
FROM THE BMJ
More data back Guillain-Barré risk with Janssen COVID shot
Over 14 months, GBS reporting rates within 21 and 42 days of administration of Janssen’s replication-incompetent adenoviral vector vaccine were approximately 9 to 12 times higher than after administration of the Pfizer-BioNTech (BNT162b2) or the Moderna (mRNA-1273) mRNA COVID vaccines.
Additionally, observed GBS cases after the Janssen shot were 2 to 3 times greater than expected, based on background rates within 21 and 42 days of vaccination.
Conversely, and confirming prior data, there was no increased risk for GBS with the Pfizer or Moderna vaccines and no significant difference between observed and expected numbers of GBS cases after either mRNA COVID-19 vaccine.
The findings were published online in JAMA Network Open.
More precise risk estimates
Winston Abara, MD, with the U.S. Centers for Disease Control and Prevention, and colleagues analyzed GBS reports submitted to the VAERS between December 2020 and January 2022.
Among 487.6 million COVID-19 vaccine doses administered, 3.7% were Janssen’s Ad26.COV2.S vaccine, 54.7% were Pfizer’s BNT162b2 vaccine, and 41.6% were Moderna’s mRNA-1273 vaccine.
There were 295 verified reports of GBS identified after COVID-19 vaccination. Of these, 209 occurred within 21 days of vaccination and 253 within 42 days.
Within 21 days of vaccination, GBS reporting rates per 1 million doses were 3.29 for the Janssen vaccine versus 0.29 and 0.35 for the Pfizer and Moderna vaccines, respectively. Within 42 days of vaccination, reporting rates per 1 million doses were 4.07, 0.34, and 0.44, respectively.
Also within 21 days of vaccination, GBS reporting rates were significantly higher with the Janssen vaccine than the Pfizer vaccine (reporting rate ratio, 11.40) and the Moderna vaccine (RRR, 9.26). Similar findings were observed within 42 days after vaccination.
The observed-to-expected ratios were 3.79 for 21-day and 2.34 for 42-day intervals after receipt of the Janssen vaccine, and less than 1 (not significant) after the Pfizer or Moderna vaccine within both post-vaccination periods.
“Unlike prior studies, our analysis included all U.S. reports of verified GBS cases that met the Brighton Collaboration GBS case definition criteria (Brighton Levels 1, 2, and 3) submitted over a 14-month surveillance period to the to the Vaccine Adverse Event Reporting System,” Dr. Abara said in an interview. “Because we used all U.S. reports, the sample of verified GBS cases in this analysis is larger than other studies. Therefore, it may provide a more precise estimate of the GBS risk within 21 and 42 days after mRNA and Ad26.COV2.S vaccination,” he said.
‘Remarkably low’ use
Nicola Klein, MD, PhD, Kaiser Permanente Vaccine Study Center, Oakland, Calif., noted that this is a “nice confirmatory analysis that supports and further expands what’s been observed before.”
Last year, as reported by this news organization, Dr. Klein and colleagues reported data from the Vaccine Safety Datalink confirming a small but statistically significant increased risk for GBS in the 3 weeks after receipt of the Janssen COVID-19 vaccine but not the Pfizer or Moderna vaccines.
Unlike VAERS, the Vaccine Safety Datalink is not a reporting system. It’s an active surveillance of medical records in the Kaiser Permanente system. The VAERS is a passive system, so it requires individuals to report GBS cases to the VAERS team, Dr. Klein explained.
So although the two studies are slightly different, overall, the VAERS data is “consistent with what we found,” she said.
Also weighing in, C. Buddy Creech, MD, MPH, director of the Vanderbilt Vaccine Research Program and professor of pediatrics at the Vanderbilt University School of Medicine, Nashville, Tenn., said it is “important to realize that GBS had been observed after adenovirus-vectored vaccines earlier in the pandemic, both for the AstraZeneca vaccine and the Janssen vaccine.”
The Advisory Committee on Immunization Practices (ACIP) preferentially recommends that people age 18 years and older receive an mRNA COVID-19 vaccine rather than the Janssen adenoviral vector vaccine when both types of COVID-19 vaccine are available.
“Thus, the use of the Janssen vaccine is remarkably low in the U.S. right now,” Dr. Creech said.
“Nevertheless, we have a firm commitment, both scientifically and ethically, to track potential side effects after vaccination and to make sure that the vaccines in use for COVID, and other important infectious diseases, are safe and effective,” he added.
The study had no commercial funding. Dr. Abara and Dr. Creech have reported no relevant financial relationships. Dr. Klein reported having received grants from Pfizer research support for a COVID vaccine clinical trial, as well as grants from Merck, GlaxoSmithKline, Sanofi Pasteur, and Protein Science (now Sanofi Pasteur).
A version of this article first appeared on Medscape.com.
Over 14 months, GBS reporting rates within 21 and 42 days of administration of Janssen’s replication-incompetent adenoviral vector vaccine were approximately 9 to 12 times higher than after administration of the Pfizer-BioNTech (BNT162b2) or the Moderna (mRNA-1273) mRNA COVID vaccines.
Additionally, observed GBS cases after the Janssen shot were 2 to 3 times greater than expected, based on background rates within 21 and 42 days of vaccination.
Conversely, and confirming prior data, there was no increased risk for GBS with the Pfizer or Moderna vaccines and no significant difference between observed and expected numbers of GBS cases after either mRNA COVID-19 vaccine.
The findings were published online in JAMA Network Open.
More precise risk estimates
Winston Abara, MD, with the U.S. Centers for Disease Control and Prevention, and colleagues analyzed GBS reports submitted to the VAERS between December 2020 and January 2022.
Among 487.6 million COVID-19 vaccine doses administered, 3.7% were Janssen’s Ad26.COV2.S vaccine, 54.7% were Pfizer’s BNT162b2 vaccine, and 41.6% were Moderna’s mRNA-1273 vaccine.
There were 295 verified reports of GBS identified after COVID-19 vaccination. Of these, 209 occurred within 21 days of vaccination and 253 within 42 days.
Within 21 days of vaccination, GBS reporting rates per 1 million doses were 3.29 for the Janssen vaccine versus 0.29 and 0.35 for the Pfizer and Moderna vaccines, respectively. Within 42 days of vaccination, reporting rates per 1 million doses were 4.07, 0.34, and 0.44, respectively.
Also within 21 days of vaccination, GBS reporting rates were significantly higher with the Janssen vaccine than the Pfizer vaccine (reporting rate ratio, 11.40) and the Moderna vaccine (RRR, 9.26). Similar findings were observed within 42 days after vaccination.
The observed-to-expected ratios were 3.79 for 21-day and 2.34 for 42-day intervals after receipt of the Janssen vaccine, and less than 1 (not significant) after the Pfizer or Moderna vaccine within both post-vaccination periods.
“Unlike prior studies, our analysis included all U.S. reports of verified GBS cases that met the Brighton Collaboration GBS case definition criteria (Brighton Levels 1, 2, and 3) submitted over a 14-month surveillance period to the to the Vaccine Adverse Event Reporting System,” Dr. Abara said in an interview. “Because we used all U.S. reports, the sample of verified GBS cases in this analysis is larger than other studies. Therefore, it may provide a more precise estimate of the GBS risk within 21 and 42 days after mRNA and Ad26.COV2.S vaccination,” he said.
‘Remarkably low’ use
Nicola Klein, MD, PhD, Kaiser Permanente Vaccine Study Center, Oakland, Calif., noted that this is a “nice confirmatory analysis that supports and further expands what’s been observed before.”
Last year, as reported by this news organization, Dr. Klein and colleagues reported data from the Vaccine Safety Datalink confirming a small but statistically significant increased risk for GBS in the 3 weeks after receipt of the Janssen COVID-19 vaccine but not the Pfizer or Moderna vaccines.
Unlike VAERS, the Vaccine Safety Datalink is not a reporting system. It’s an active surveillance of medical records in the Kaiser Permanente system. The VAERS is a passive system, so it requires individuals to report GBS cases to the VAERS team, Dr. Klein explained.
So although the two studies are slightly different, overall, the VAERS data is “consistent with what we found,” she said.
Also weighing in, C. Buddy Creech, MD, MPH, director of the Vanderbilt Vaccine Research Program and professor of pediatrics at the Vanderbilt University School of Medicine, Nashville, Tenn., said it is “important to realize that GBS had been observed after adenovirus-vectored vaccines earlier in the pandemic, both for the AstraZeneca vaccine and the Janssen vaccine.”
The Advisory Committee on Immunization Practices (ACIP) preferentially recommends that people age 18 years and older receive an mRNA COVID-19 vaccine rather than the Janssen adenoviral vector vaccine when both types of COVID-19 vaccine are available.
“Thus, the use of the Janssen vaccine is remarkably low in the U.S. right now,” Dr. Creech said.
“Nevertheless, we have a firm commitment, both scientifically and ethically, to track potential side effects after vaccination and to make sure that the vaccines in use for COVID, and other important infectious diseases, are safe and effective,” he added.
The study had no commercial funding. Dr. Abara and Dr. Creech have reported no relevant financial relationships. Dr. Klein reported having received grants from Pfizer research support for a COVID vaccine clinical trial, as well as grants from Merck, GlaxoSmithKline, Sanofi Pasteur, and Protein Science (now Sanofi Pasteur).
A version of this article first appeared on Medscape.com.
Over 14 months, GBS reporting rates within 21 and 42 days of administration of Janssen’s replication-incompetent adenoviral vector vaccine were approximately 9 to 12 times higher than after administration of the Pfizer-BioNTech (BNT162b2) or the Moderna (mRNA-1273) mRNA COVID vaccines.
Additionally, observed GBS cases after the Janssen shot were 2 to 3 times greater than expected, based on background rates within 21 and 42 days of vaccination.
Conversely, and confirming prior data, there was no increased risk for GBS with the Pfizer or Moderna vaccines and no significant difference between observed and expected numbers of GBS cases after either mRNA COVID-19 vaccine.
The findings were published online in JAMA Network Open.
More precise risk estimates
Winston Abara, MD, with the U.S. Centers for Disease Control and Prevention, and colleagues analyzed GBS reports submitted to the VAERS between December 2020 and January 2022.
Among 487.6 million COVID-19 vaccine doses administered, 3.7% were Janssen’s Ad26.COV2.S vaccine, 54.7% were Pfizer’s BNT162b2 vaccine, and 41.6% were Moderna’s mRNA-1273 vaccine.
There were 295 verified reports of GBS identified after COVID-19 vaccination. Of these, 209 occurred within 21 days of vaccination and 253 within 42 days.
Within 21 days of vaccination, GBS reporting rates per 1 million doses were 3.29 for the Janssen vaccine versus 0.29 and 0.35 for the Pfizer and Moderna vaccines, respectively. Within 42 days of vaccination, reporting rates per 1 million doses were 4.07, 0.34, and 0.44, respectively.
Also within 21 days of vaccination, GBS reporting rates were significantly higher with the Janssen vaccine than the Pfizer vaccine (reporting rate ratio, 11.40) and the Moderna vaccine (RRR, 9.26). Similar findings were observed within 42 days after vaccination.
The observed-to-expected ratios were 3.79 for 21-day and 2.34 for 42-day intervals after receipt of the Janssen vaccine, and less than 1 (not significant) after the Pfizer or Moderna vaccine within both post-vaccination periods.
“Unlike prior studies, our analysis included all U.S. reports of verified GBS cases that met the Brighton Collaboration GBS case definition criteria (Brighton Levels 1, 2, and 3) submitted over a 14-month surveillance period to the to the Vaccine Adverse Event Reporting System,” Dr. Abara said in an interview. “Because we used all U.S. reports, the sample of verified GBS cases in this analysis is larger than other studies. Therefore, it may provide a more precise estimate of the GBS risk within 21 and 42 days after mRNA and Ad26.COV2.S vaccination,” he said.
‘Remarkably low’ use
Nicola Klein, MD, PhD, Kaiser Permanente Vaccine Study Center, Oakland, Calif., noted that this is a “nice confirmatory analysis that supports and further expands what’s been observed before.”
Last year, as reported by this news organization, Dr. Klein and colleagues reported data from the Vaccine Safety Datalink confirming a small but statistically significant increased risk for GBS in the 3 weeks after receipt of the Janssen COVID-19 vaccine but not the Pfizer or Moderna vaccines.
Unlike VAERS, the Vaccine Safety Datalink is not a reporting system. It’s an active surveillance of medical records in the Kaiser Permanente system. The VAERS is a passive system, so it requires individuals to report GBS cases to the VAERS team, Dr. Klein explained.
So although the two studies are slightly different, overall, the VAERS data is “consistent with what we found,” she said.
Also weighing in, C. Buddy Creech, MD, MPH, director of the Vanderbilt Vaccine Research Program and professor of pediatrics at the Vanderbilt University School of Medicine, Nashville, Tenn., said it is “important to realize that GBS had been observed after adenovirus-vectored vaccines earlier in the pandemic, both for the AstraZeneca vaccine and the Janssen vaccine.”
The Advisory Committee on Immunization Practices (ACIP) preferentially recommends that people age 18 years and older receive an mRNA COVID-19 vaccine rather than the Janssen adenoviral vector vaccine when both types of COVID-19 vaccine are available.
“Thus, the use of the Janssen vaccine is remarkably low in the U.S. right now,” Dr. Creech said.
“Nevertheless, we have a firm commitment, both scientifically and ethically, to track potential side effects after vaccination and to make sure that the vaccines in use for COVID, and other important infectious diseases, are safe and effective,” he added.
The study had no commercial funding. Dr. Abara and Dr. Creech have reported no relevant financial relationships. Dr. Klein reported having received grants from Pfizer research support for a COVID vaccine clinical trial, as well as grants from Merck, GlaxoSmithKline, Sanofi Pasteur, and Protein Science (now Sanofi Pasteur).
A version of this article first appeared on Medscape.com.
Inflammation and immunity troubles top long-COVID suspect list
“I think that it’s a much more complex picture than just inflammation, or just autoimmunity, or just immune dysregulation. And it’s probably a combination of all three causing a cascade of effects that then manifests itself as brain fog, or shortness of breath, or chronic fatigue,” says Alexander Truong, MD, a pulmonologist and assistant professor at Emory University, Atlanta, who also runs a long-COVID clinic.
Long COVID, post–COVID-19 condition, and postacute sequelae of SARS-CoV-2 (PASC) are among the terms used by the National Institutes of Health to describe the long-term health issues faced by an estimated 10%-30% of people infected with COVID-19. Symptoms – as many as 200 – can range from inconvenient to crippling, damage multiple organ systems, come and go, and relapse. Long COVID increases the risk of worsening existing health problems and triggering new ones, including cardiovascular disease and type 2 diabetes.
So far, research suggests there is no single cause, condition, or disease that explains why some people have an extensive range of symptoms long after the early COVID-19 infection has cleared up. Many experts believe some combination of biological processes – including the virus hanging around in our bodies, inflammation, autoimmunity, tiny blood clots, immune system problems, and even the reactivation of dormant viruses such as the Epstein-Barr virus – could be the culprit, a theory also supported by a comprehensive and in-depth review of long-COVID studies published in the journal Nature Reviews Microbiology.
“It’s become clear over the last couple of years that there are different [symptoms] of long COVID … that cannot all be lumped together,” says Michael Peluso, MD, an assistant professor of medicine and an infectious diseases doctor at the University of California, San Francisco.
Inflammation and a virus that hangs around
Multiple studies have shown that the virus or pieces of it can remain in many parts of the body, including the kidneys, brain, heart, and gastrointestinal system, long after the early infection.
“One major question that I think is the area of most intense investigation now is whether there is viral persistence that is driving immune dysregulation and therefore symptoms,” says Dr. Peluso.
A small Harvard University study, for example, found evidence that reservoirs of the coronavirus could linger in patients up to a year after they’re first diagnosed.
An earlier German study found that patients with post-COVID-19 symptoms had higher levels of three cytokines – small proteins that tell the body’s immune system what to do and are involved in the growth and activity of immune system cells and blood cells. Researchers said the results supported the theory that there is persistent reprogramming of certain immune cells, and that the uncontrolled “self-fueled hyperinflammation” during the early COVID-19 infection can become continued immune cell disruption that drives long-COVID symptoms.
“Long COVID is more likely due to either an inflammatory response by the body or reservoirs of virus that the body is still trying to clear … and the symptoms we’re seeing are a side effect of that,” says Rainu Kaushal, MD, senior associate dean for clinical research at Weill Cornell Medicine in New York.
Australian researchers found that immune system recovery appeared different, compared with those who were infected with other common coronaviruses.
These findings also support concerns that some experts express over the long-term risks of COVID-19 infections in general, but especially repeat infections.
“Anything that kind of revs up inflammation in the body can boil that pot over and make the symptoms worse. That’s very easily an infection or some other insult to the body. So that’s the generalized hypothesis as to why insults to the body may worsen the symptoms,” says Dr. Truong.
An autoimmune condition?
But inflammation alone does not fully explain post–COVID-19 problems.
Dr. Truong and his team, for example, have been documenting inflammatory markers in patients at the post-COVID clinic he cofounded more than 2 years ago at Emory Executive Park in Atlanta. When the clinic was first launched, high-dose nonsteroidal anti-inflammatory drugs – including ibuprofen – and prednisone were prescribed to long-COVID patients.
“It didn’t make a difference at all for any of these folks,” he says, adding that there are signs that autoimmunity is at play. But he cautions that it is still too early to suggest treating long-COVID patients with medications used for other autoimmune conditions.
In autoimmune conditions such as rheumatoid arthritis, lupus, and type 1 diabetes, a person’s immune system can’t tell normal cells from foreign pathogens and attacks healthy cells. There is typically no single diagnostic test, and many share similar symptoms, making detection and diagnosis potentially difficult, according to Johns Hopkins Medicine.
A small study published in the journal Science Translational Medicine found that, among patients who failed to regain their sense of smell long after their initial infection, there was inflammation in the nose tissue where smell nerve cells are found, even though no detectable virus remained. Fewer olfactory sensory neurons were seen, as well – findings that researchers said resembled some kind of “autoimmune-like process.”
Meanwhile, scientists in Canada found signs of autoimmunity in blood samples taken from patients who still had fatigue and shortness of breath after their initial COVID-19 infection. Two specific proteins were present a year after infection in up to 30% of patients, many of whom still had shortness of breath and fatigue, the researchers reported in the Jan. 1 issue of the European Respiratory Journal. These patients had been healthy and had no autoimmune condition or other diseases before they were infected.
Immune system problems
A number of studies have suggested that a problematic immune response could also explain why symptoms persist for some people.
Researchers in France, for example, found that the immune response problems in those with severe COVID-19 infections caused exaggerated or uncontrolled formation of a type of bug-fighting defense mechanism called a neutrophil extracellular trap (NET), which in turn triggers harmful inflammation that can result in multiorgan damage. These traps are netlike structures made from fibers composed mostly of DNA strings that bind, or trap, pathogens.
Long COVID is not like an acute infectious disease, says Alexander Charney, MD, PhD, the lead principal investigator of the RECOVER adult cohort at Mount Sinai in New York, and an associate professor at Icahn School of Medicine at Mount Sinai. It is more similar to other complex chronic diseases that have taken decades to understand, such as heart disease, mental illness, and rheumatologic diseases, he says.
Biomarkers and blood clots
Scientists are homing in on biomarkers, or detectable and measurable traits – in this case, molecular indicators – that can make diagnosing long COVID easier and give better direction for treatment. These biomarkers are also key to helping sort out the complex biology of long COVID.
In one study, data from blood samples taken from hundreds of hospitalized COVID-19 patients suggests changes are happening at the molecular level during initial severe infections. These changes may be tied to the development of longer-term symptoms, according to the study by Dr. Charney and his team at Mount Sinai published in Nature Medicine
Blood clotting issues have also been detected in long COVID patients. At least one study found signs that long-COVID patients had higher levels of a type of auto-antibody linked to the abnormal formation of clots. Researchers suspect that tiny, persistent microclots – undetectable via regular pathology tests – may be cutting off oxygen flow to tissue by blocking capillaries – and could explain many of the post-COVID symptoms described by patients.
While enormous progress has been made toward understanding long COVID, the research is still considered early and faces many challenges, including varying criteria used to define the condition, the types and quality of data used, differences in how patients are defined and recruited, and the small size of many studies. Some research also appears to conflict with other studies. And while there are specialized tools for diagnosing some aspects of the condition, standard tests often don’t detect many of the signs seen in long-COVID patients. But given the urgency and global scale of the problem, experts say more funding and support should be prioritized.
“People are suffering now, and they want answers now. ... It’s not like with COVID, where the path towards a great and meaningful solution to this unbelievable problem was clear – we need a vaccine,” says Dr. Charney.
“It’s going to be a long haul to figure out what is going on.”
A version of this article originally appeared on WebMD.com.
“I think that it’s a much more complex picture than just inflammation, or just autoimmunity, or just immune dysregulation. And it’s probably a combination of all three causing a cascade of effects that then manifests itself as brain fog, or shortness of breath, or chronic fatigue,” says Alexander Truong, MD, a pulmonologist and assistant professor at Emory University, Atlanta, who also runs a long-COVID clinic.
Long COVID, post–COVID-19 condition, and postacute sequelae of SARS-CoV-2 (PASC) are among the terms used by the National Institutes of Health to describe the long-term health issues faced by an estimated 10%-30% of people infected with COVID-19. Symptoms – as many as 200 – can range from inconvenient to crippling, damage multiple organ systems, come and go, and relapse. Long COVID increases the risk of worsening existing health problems and triggering new ones, including cardiovascular disease and type 2 diabetes.
So far, research suggests there is no single cause, condition, or disease that explains why some people have an extensive range of symptoms long after the early COVID-19 infection has cleared up. Many experts believe some combination of biological processes – including the virus hanging around in our bodies, inflammation, autoimmunity, tiny blood clots, immune system problems, and even the reactivation of dormant viruses such as the Epstein-Barr virus – could be the culprit, a theory also supported by a comprehensive and in-depth review of long-COVID studies published in the journal Nature Reviews Microbiology.
“It’s become clear over the last couple of years that there are different [symptoms] of long COVID … that cannot all be lumped together,” says Michael Peluso, MD, an assistant professor of medicine and an infectious diseases doctor at the University of California, San Francisco.
Inflammation and a virus that hangs around
Multiple studies have shown that the virus or pieces of it can remain in many parts of the body, including the kidneys, brain, heart, and gastrointestinal system, long after the early infection.
“One major question that I think is the area of most intense investigation now is whether there is viral persistence that is driving immune dysregulation and therefore symptoms,” says Dr. Peluso.
A small Harvard University study, for example, found evidence that reservoirs of the coronavirus could linger in patients up to a year after they’re first diagnosed.
An earlier German study found that patients with post-COVID-19 symptoms had higher levels of three cytokines – small proteins that tell the body’s immune system what to do and are involved in the growth and activity of immune system cells and blood cells. Researchers said the results supported the theory that there is persistent reprogramming of certain immune cells, and that the uncontrolled “self-fueled hyperinflammation” during the early COVID-19 infection can become continued immune cell disruption that drives long-COVID symptoms.
“Long COVID is more likely due to either an inflammatory response by the body or reservoirs of virus that the body is still trying to clear … and the symptoms we’re seeing are a side effect of that,” says Rainu Kaushal, MD, senior associate dean for clinical research at Weill Cornell Medicine in New York.
Australian researchers found that immune system recovery appeared different, compared with those who were infected with other common coronaviruses.
These findings also support concerns that some experts express over the long-term risks of COVID-19 infections in general, but especially repeat infections.
“Anything that kind of revs up inflammation in the body can boil that pot over and make the symptoms worse. That’s very easily an infection or some other insult to the body. So that’s the generalized hypothesis as to why insults to the body may worsen the symptoms,” says Dr. Truong.
An autoimmune condition?
But inflammation alone does not fully explain post–COVID-19 problems.
Dr. Truong and his team, for example, have been documenting inflammatory markers in patients at the post-COVID clinic he cofounded more than 2 years ago at Emory Executive Park in Atlanta. When the clinic was first launched, high-dose nonsteroidal anti-inflammatory drugs – including ibuprofen – and prednisone were prescribed to long-COVID patients.
“It didn’t make a difference at all for any of these folks,” he says, adding that there are signs that autoimmunity is at play. But he cautions that it is still too early to suggest treating long-COVID patients with medications used for other autoimmune conditions.
In autoimmune conditions such as rheumatoid arthritis, lupus, and type 1 diabetes, a person’s immune system can’t tell normal cells from foreign pathogens and attacks healthy cells. There is typically no single diagnostic test, and many share similar symptoms, making detection and diagnosis potentially difficult, according to Johns Hopkins Medicine.
A small study published in the journal Science Translational Medicine found that, among patients who failed to regain their sense of smell long after their initial infection, there was inflammation in the nose tissue where smell nerve cells are found, even though no detectable virus remained. Fewer olfactory sensory neurons were seen, as well – findings that researchers said resembled some kind of “autoimmune-like process.”
Meanwhile, scientists in Canada found signs of autoimmunity in blood samples taken from patients who still had fatigue and shortness of breath after their initial COVID-19 infection. Two specific proteins were present a year after infection in up to 30% of patients, many of whom still had shortness of breath and fatigue, the researchers reported in the Jan. 1 issue of the European Respiratory Journal. These patients had been healthy and had no autoimmune condition or other diseases before they were infected.
Immune system problems
A number of studies have suggested that a problematic immune response could also explain why symptoms persist for some people.
Researchers in France, for example, found that the immune response problems in those with severe COVID-19 infections caused exaggerated or uncontrolled formation of a type of bug-fighting defense mechanism called a neutrophil extracellular trap (NET), which in turn triggers harmful inflammation that can result in multiorgan damage. These traps are netlike structures made from fibers composed mostly of DNA strings that bind, or trap, pathogens.
Long COVID is not like an acute infectious disease, says Alexander Charney, MD, PhD, the lead principal investigator of the RECOVER adult cohort at Mount Sinai in New York, and an associate professor at Icahn School of Medicine at Mount Sinai. It is more similar to other complex chronic diseases that have taken decades to understand, such as heart disease, mental illness, and rheumatologic diseases, he says.
Biomarkers and blood clots
Scientists are homing in on biomarkers, or detectable and measurable traits – in this case, molecular indicators – that can make diagnosing long COVID easier and give better direction for treatment. These biomarkers are also key to helping sort out the complex biology of long COVID.
In one study, data from blood samples taken from hundreds of hospitalized COVID-19 patients suggests changes are happening at the molecular level during initial severe infections. These changes may be tied to the development of longer-term symptoms, according to the study by Dr. Charney and his team at Mount Sinai published in Nature Medicine
Blood clotting issues have also been detected in long COVID patients. At least one study found signs that long-COVID patients had higher levels of a type of auto-antibody linked to the abnormal formation of clots. Researchers suspect that tiny, persistent microclots – undetectable via regular pathology tests – may be cutting off oxygen flow to tissue by blocking capillaries – and could explain many of the post-COVID symptoms described by patients.
While enormous progress has been made toward understanding long COVID, the research is still considered early and faces many challenges, including varying criteria used to define the condition, the types and quality of data used, differences in how patients are defined and recruited, and the small size of many studies. Some research also appears to conflict with other studies. And while there are specialized tools for diagnosing some aspects of the condition, standard tests often don’t detect many of the signs seen in long-COVID patients. But given the urgency and global scale of the problem, experts say more funding and support should be prioritized.
“People are suffering now, and they want answers now. ... It’s not like with COVID, where the path towards a great and meaningful solution to this unbelievable problem was clear – we need a vaccine,” says Dr. Charney.
“It’s going to be a long haul to figure out what is going on.”
A version of this article originally appeared on WebMD.com.
“I think that it’s a much more complex picture than just inflammation, or just autoimmunity, or just immune dysregulation. And it’s probably a combination of all three causing a cascade of effects that then manifests itself as brain fog, or shortness of breath, or chronic fatigue,” says Alexander Truong, MD, a pulmonologist and assistant professor at Emory University, Atlanta, who also runs a long-COVID clinic.
Long COVID, post–COVID-19 condition, and postacute sequelae of SARS-CoV-2 (PASC) are among the terms used by the National Institutes of Health to describe the long-term health issues faced by an estimated 10%-30% of people infected with COVID-19. Symptoms – as many as 200 – can range from inconvenient to crippling, damage multiple organ systems, come and go, and relapse. Long COVID increases the risk of worsening existing health problems and triggering new ones, including cardiovascular disease and type 2 diabetes.
So far, research suggests there is no single cause, condition, or disease that explains why some people have an extensive range of symptoms long after the early COVID-19 infection has cleared up. Many experts believe some combination of biological processes – including the virus hanging around in our bodies, inflammation, autoimmunity, tiny blood clots, immune system problems, and even the reactivation of dormant viruses such as the Epstein-Barr virus – could be the culprit, a theory also supported by a comprehensive and in-depth review of long-COVID studies published in the journal Nature Reviews Microbiology.
“It’s become clear over the last couple of years that there are different [symptoms] of long COVID … that cannot all be lumped together,” says Michael Peluso, MD, an assistant professor of medicine and an infectious diseases doctor at the University of California, San Francisco.
Inflammation and a virus that hangs around
Multiple studies have shown that the virus or pieces of it can remain in many parts of the body, including the kidneys, brain, heart, and gastrointestinal system, long after the early infection.
“One major question that I think is the area of most intense investigation now is whether there is viral persistence that is driving immune dysregulation and therefore symptoms,” says Dr. Peluso.
A small Harvard University study, for example, found evidence that reservoirs of the coronavirus could linger in patients up to a year after they’re first diagnosed.
An earlier German study found that patients with post-COVID-19 symptoms had higher levels of three cytokines – small proteins that tell the body’s immune system what to do and are involved in the growth and activity of immune system cells and blood cells. Researchers said the results supported the theory that there is persistent reprogramming of certain immune cells, and that the uncontrolled “self-fueled hyperinflammation” during the early COVID-19 infection can become continued immune cell disruption that drives long-COVID symptoms.
“Long COVID is more likely due to either an inflammatory response by the body or reservoirs of virus that the body is still trying to clear … and the symptoms we’re seeing are a side effect of that,” says Rainu Kaushal, MD, senior associate dean for clinical research at Weill Cornell Medicine in New York.
Australian researchers found that immune system recovery appeared different, compared with those who were infected with other common coronaviruses.
These findings also support concerns that some experts express over the long-term risks of COVID-19 infections in general, but especially repeat infections.
“Anything that kind of revs up inflammation in the body can boil that pot over and make the symptoms worse. That’s very easily an infection or some other insult to the body. So that’s the generalized hypothesis as to why insults to the body may worsen the symptoms,” says Dr. Truong.
An autoimmune condition?
But inflammation alone does not fully explain post–COVID-19 problems.
Dr. Truong and his team, for example, have been documenting inflammatory markers in patients at the post-COVID clinic he cofounded more than 2 years ago at Emory Executive Park in Atlanta. When the clinic was first launched, high-dose nonsteroidal anti-inflammatory drugs – including ibuprofen – and prednisone were prescribed to long-COVID patients.
“It didn’t make a difference at all for any of these folks,” he says, adding that there are signs that autoimmunity is at play. But he cautions that it is still too early to suggest treating long-COVID patients with medications used for other autoimmune conditions.
In autoimmune conditions such as rheumatoid arthritis, lupus, and type 1 diabetes, a person’s immune system can’t tell normal cells from foreign pathogens and attacks healthy cells. There is typically no single diagnostic test, and many share similar symptoms, making detection and diagnosis potentially difficult, according to Johns Hopkins Medicine.
A small study published in the journal Science Translational Medicine found that, among patients who failed to regain their sense of smell long after their initial infection, there was inflammation in the nose tissue where smell nerve cells are found, even though no detectable virus remained. Fewer olfactory sensory neurons were seen, as well – findings that researchers said resembled some kind of “autoimmune-like process.”
Meanwhile, scientists in Canada found signs of autoimmunity in blood samples taken from patients who still had fatigue and shortness of breath after their initial COVID-19 infection. Two specific proteins were present a year after infection in up to 30% of patients, many of whom still had shortness of breath and fatigue, the researchers reported in the Jan. 1 issue of the European Respiratory Journal. These patients had been healthy and had no autoimmune condition or other diseases before they were infected.
Immune system problems
A number of studies have suggested that a problematic immune response could also explain why symptoms persist for some people.
Researchers in France, for example, found that the immune response problems in those with severe COVID-19 infections caused exaggerated or uncontrolled formation of a type of bug-fighting defense mechanism called a neutrophil extracellular trap (NET), which in turn triggers harmful inflammation that can result in multiorgan damage. These traps are netlike structures made from fibers composed mostly of DNA strings that bind, or trap, pathogens.
Long COVID is not like an acute infectious disease, says Alexander Charney, MD, PhD, the lead principal investigator of the RECOVER adult cohort at Mount Sinai in New York, and an associate professor at Icahn School of Medicine at Mount Sinai. It is more similar to other complex chronic diseases that have taken decades to understand, such as heart disease, mental illness, and rheumatologic diseases, he says.
Biomarkers and blood clots
Scientists are homing in on biomarkers, or detectable and measurable traits – in this case, molecular indicators – that can make diagnosing long COVID easier and give better direction for treatment. These biomarkers are also key to helping sort out the complex biology of long COVID.
In one study, data from blood samples taken from hundreds of hospitalized COVID-19 patients suggests changes are happening at the molecular level during initial severe infections. These changes may be tied to the development of longer-term symptoms, according to the study by Dr. Charney and his team at Mount Sinai published in Nature Medicine
Blood clotting issues have also been detected in long COVID patients. At least one study found signs that long-COVID patients had higher levels of a type of auto-antibody linked to the abnormal formation of clots. Researchers suspect that tiny, persistent microclots – undetectable via regular pathology tests – may be cutting off oxygen flow to tissue by blocking capillaries – and could explain many of the post-COVID symptoms described by patients.
While enormous progress has been made toward understanding long COVID, the research is still considered early and faces many challenges, including varying criteria used to define the condition, the types and quality of data used, differences in how patients are defined and recruited, and the small size of many studies. Some research also appears to conflict with other studies. And while there are specialized tools for diagnosing some aspects of the condition, standard tests often don’t detect many of the signs seen in long-COVID patients. But given the urgency and global scale of the problem, experts say more funding and support should be prioritized.
“People are suffering now, and they want answers now. ... It’s not like with COVID, where the path towards a great and meaningful solution to this unbelievable problem was clear – we need a vaccine,” says Dr. Charney.
“It’s going to be a long haul to figure out what is going on.”
A version of this article originally appeared on WebMD.com.


