User login
Can particles in dairy and beef cause cancer and MS?
In Western diets, dairy and beef are ubiquitous: Milk goes with coffee, melted cheese with pizza, and chili with rice. But what if dairy products and beef contained a new kind of pathogen that could infect you as a child and trigger cancer or multiple sclerosis (MS) 40-70 years later?
However, in two joint statements, the German Federal Institute for Risk Assessment (BfR) and the Max Rubner Institute (MRI) have rejected such theories.
In 2008, Harald zur Hausen, MD, DSc, received the Nobel Prize in Medicine for his discovery that human papillomaviruses cause cervical cancer. His starting point was the observation that sexually abstinent women, such as nuns, rarely develop this cancer. So it was possible to draw the conclusion that pathogens are transmitted during sexual intercourse, explain Dr. zur Hausen and his wife Ethel-Michele de Villiers, PhD, both of DKFZ Heidelberg.
Papillomaviruses, as well as human herpes and Epstein-Barr viruses (EBV), polyomaviruses, and retroviruses, cause cancer in a direct way: by inserting their genes into the DNA of human cells. With a latency of a few years to a few decades, the proteins formed through expression stimulate malignant growth by altering the regulating host gene.
Acid radicals
However, viruses – just like bacteria and parasites – can also indirectly trigger cancer. One mechanism for this triggering is the disruption of immune defenses, as shown by the sometimes drastically increased tumor incidence with AIDS or with immunosuppressants after transplants. Chronic inflammation is a second mechanism that generates acid radicals and thereby causes random mutations in replicating cells. Examples include stomach cancer caused by Helicobacter pylori and liver cancer caused by Schistosoma, liver fluke, and hepatitis B and C viruses.
According to Dr. de Villiers and Dr. zur Hausen, there are good reasons to believe that other pathogens could cause chronic inflammation and thereby lead to cancer. Epidemiologic data suggest that dairy and meat products from European cows (Bos taurus) are a potential source. This is because colon cancer and breast cancer commonly occur in places where these foods are heavily consumed (that is, in North America, Argentina, Europe, and Australia). In contrast, the rate is low in India, where cows are revered as holy animals. Also noteworthy is that women with a lactose intolerance rarely develop breast cancer.
Viral progeny
In fact, the researchers found single-stranded DNA rings that originated in viruses, which they named bovine meat and milk factors (BMMF), in the intestines of patients with colon cancer. They reported, “This new class of pathogen deserves, in our opinion at least, to become the focus of cancer development and further chronic diseases.” They also detected elevated levels of acid radicals in these areas (that is, oxidative stress), which is typical for chronic inflammation.
The researchers assume that infants, whose immune system is not yet fully matured, ingest the BMMF as soon as they have dairy. Therefore, there is no need for adults to avoid dairy or beef because everyone is infected anyway, said Dr. zur Hausen.
‘Breast milk is healthy’
Dr. De Villiers and Dr. zur Hausen outlined more evidence of cancer-triggering pathogens. Mothers who have breastfed are less likely, especially after multiple pregnancies, to develop tumors in various organs or to have MS and type 2 diabetes. The authors attribute the protective effect to oligosaccharides in breast milk, which begin to be formed midway through the pregnancy. They bind to lectin receptors and, in so doing, mask the terminal molecule onto which the viruses need to dock. As a result, their port of entry into the cells is blocked.
The oligosaccharides also protect the baby against life-threatening infections by blocking access by rotaviruses and noroviruses. In this way, especially if breastfeeding lasts a long time – around 1 year – the period of incomplete immunocompetence is bridged.
Colon cancer
To date, it has been assumed that around 20% of all cancerous diseases globally are caused by infections, said the researchers. But if the suspected BMMF cases are included, this figure rises to 50%, even to around 80%, for colon cancer. If the suspicion is confirmed, the consequences for prevention and therapy would be significant.
The voice of a Nobel prize winner undoubtedly carries weight, but at the time, Dr. zur Hausen still had to convince a host of skeptics with his discovery that a viral infection is a major cause of cervical cancer. Nonetheless, some indicators suggest that he and his wife have found a dead end this time.
Institutional skepticism
When his working group made the results public in February 2019, the DKFZ felt the need to give an all-clear signal in response to alarmed press reports. There is no reason to see dairy and meat consumption as something negative. Similarly, in their first joint statement, the BfR and the MRI judged the data to be insufficient and called for further studies. Multiple research teams began to focus on BMMF as a result. In what foods can they be found? Are they more common in patients with cancer than in healthy people? Are they infectious? Do they cause inflammation and cancer?
The findings presented in a second statement by the BfR and MRI at the end of November 2022 contradicted the claims made by the DKFZ scientists across the board. In no way do BMMF represent new pathogens. They are variants of already known DNA sequences. In addition, they are present in numerous animal-based and plant-based foods, including pork, fish, fruit, vegetables, and nuts.
BMMF do not possess the ability to infect human cells, the institutes said. The proof that they are damaging to one’s health was also absent. It is true that the incidence of intestinal tumors correlates positively with the consumption of red and processed meat – which in no way signifies causality – but dairy products are linked to a reduced risk. On the other hand, breast cancer cannot be associated with the consumption of beef or dairy.
Therefore, both institutes recommend continuing to use these products as supplementary diet for infants because of their micronutrients. They further stated that the products are safe for people of all ages.
Association with MS?
Unperturbed, Dr. de Villiers and Dr. zur Hausen went one step further in their current article. They posited that MS is also associated with the consumption of dairy products and beef. Here too geographic distribution prompted the idea to look for BMMF in the brain lesions of patients with MS. The researchers isolated ring-shaped DNA molecules that proved to be closely related to BMMF from dairy and cattle blood. “The result was electrifying for us.”
However, there are several other factors to consider, such as vitamin D3 deficiency. This is because the incidence of MS decreases the further you travel from the poles toward the equator (that is, as solar radiation increases). Also, EBV clearly plays a role because patients with MS display increased titers of EBV antibodies. One study also showed that people in Antarctica excreted reactivated EBV in their saliva during winter and that vitamin D3 stopped the viral secretion.
Under these conditions, the researchers hypothesized that MS is caused by a double infection of brain cells by EBV and BMMF. EBV is reactivated by a lack of vitamin D3, and the BMMF multiply and are eventually converted into proteins. A focal immunoreaction causes the Schwann cells and oligodendrocytes to malfunction, which leads to the destruction of the myelin sheaths around the nerve fibers.
This article was translated from the Medscape German Edition. A version appeared on Medscape.com.
In Western diets, dairy and beef are ubiquitous: Milk goes with coffee, melted cheese with pizza, and chili with rice. But what if dairy products and beef contained a new kind of pathogen that could infect you as a child and trigger cancer or multiple sclerosis (MS) 40-70 years later?
However, in two joint statements, the German Federal Institute for Risk Assessment (BfR) and the Max Rubner Institute (MRI) have rejected such theories.
In 2008, Harald zur Hausen, MD, DSc, received the Nobel Prize in Medicine for his discovery that human papillomaviruses cause cervical cancer. His starting point was the observation that sexually abstinent women, such as nuns, rarely develop this cancer. So it was possible to draw the conclusion that pathogens are transmitted during sexual intercourse, explain Dr. zur Hausen and his wife Ethel-Michele de Villiers, PhD, both of DKFZ Heidelberg.
Papillomaviruses, as well as human herpes and Epstein-Barr viruses (EBV), polyomaviruses, and retroviruses, cause cancer in a direct way: by inserting their genes into the DNA of human cells. With a latency of a few years to a few decades, the proteins formed through expression stimulate malignant growth by altering the regulating host gene.
Acid radicals
However, viruses – just like bacteria and parasites – can also indirectly trigger cancer. One mechanism for this triggering is the disruption of immune defenses, as shown by the sometimes drastically increased tumor incidence with AIDS or with immunosuppressants after transplants. Chronic inflammation is a second mechanism that generates acid radicals and thereby causes random mutations in replicating cells. Examples include stomach cancer caused by Helicobacter pylori and liver cancer caused by Schistosoma, liver fluke, and hepatitis B and C viruses.
According to Dr. de Villiers and Dr. zur Hausen, there are good reasons to believe that other pathogens could cause chronic inflammation and thereby lead to cancer. Epidemiologic data suggest that dairy and meat products from European cows (Bos taurus) are a potential source. This is because colon cancer and breast cancer commonly occur in places where these foods are heavily consumed (that is, in North America, Argentina, Europe, and Australia). In contrast, the rate is low in India, where cows are revered as holy animals. Also noteworthy is that women with a lactose intolerance rarely develop breast cancer.
Viral progeny
In fact, the researchers found single-stranded DNA rings that originated in viruses, which they named bovine meat and milk factors (BMMF), in the intestines of patients with colon cancer. They reported, “This new class of pathogen deserves, in our opinion at least, to become the focus of cancer development and further chronic diseases.” They also detected elevated levels of acid radicals in these areas (that is, oxidative stress), which is typical for chronic inflammation.
The researchers assume that infants, whose immune system is not yet fully matured, ingest the BMMF as soon as they have dairy. Therefore, there is no need for adults to avoid dairy or beef because everyone is infected anyway, said Dr. zur Hausen.
‘Breast milk is healthy’
Dr. De Villiers and Dr. zur Hausen outlined more evidence of cancer-triggering pathogens. Mothers who have breastfed are less likely, especially after multiple pregnancies, to develop tumors in various organs or to have MS and type 2 diabetes. The authors attribute the protective effect to oligosaccharides in breast milk, which begin to be formed midway through the pregnancy. They bind to lectin receptors and, in so doing, mask the terminal molecule onto which the viruses need to dock. As a result, their port of entry into the cells is blocked.
The oligosaccharides also protect the baby against life-threatening infections by blocking access by rotaviruses and noroviruses. In this way, especially if breastfeeding lasts a long time – around 1 year – the period of incomplete immunocompetence is bridged.
Colon cancer
To date, it has been assumed that around 20% of all cancerous diseases globally are caused by infections, said the researchers. But if the suspected BMMF cases are included, this figure rises to 50%, even to around 80%, for colon cancer. If the suspicion is confirmed, the consequences for prevention and therapy would be significant.
The voice of a Nobel prize winner undoubtedly carries weight, but at the time, Dr. zur Hausen still had to convince a host of skeptics with his discovery that a viral infection is a major cause of cervical cancer. Nonetheless, some indicators suggest that he and his wife have found a dead end this time.
Institutional skepticism
When his working group made the results public in February 2019, the DKFZ felt the need to give an all-clear signal in response to alarmed press reports. There is no reason to see dairy and meat consumption as something negative. Similarly, in their first joint statement, the BfR and the MRI judged the data to be insufficient and called for further studies. Multiple research teams began to focus on BMMF as a result. In what foods can they be found? Are they more common in patients with cancer than in healthy people? Are they infectious? Do they cause inflammation and cancer?
The findings presented in a second statement by the BfR and MRI at the end of November 2022 contradicted the claims made by the DKFZ scientists across the board. In no way do BMMF represent new pathogens. They are variants of already known DNA sequences. In addition, they are present in numerous animal-based and plant-based foods, including pork, fish, fruit, vegetables, and nuts.
BMMF do not possess the ability to infect human cells, the institutes said. The proof that they are damaging to one’s health was also absent. It is true that the incidence of intestinal tumors correlates positively with the consumption of red and processed meat – which in no way signifies causality – but dairy products are linked to a reduced risk. On the other hand, breast cancer cannot be associated with the consumption of beef or dairy.
Therefore, both institutes recommend continuing to use these products as supplementary diet for infants because of their micronutrients. They further stated that the products are safe for people of all ages.
Association with MS?
Unperturbed, Dr. de Villiers and Dr. zur Hausen went one step further in their current article. They posited that MS is also associated with the consumption of dairy products and beef. Here too geographic distribution prompted the idea to look for BMMF in the brain lesions of patients with MS. The researchers isolated ring-shaped DNA molecules that proved to be closely related to BMMF from dairy and cattle blood. “The result was electrifying for us.”
However, there are several other factors to consider, such as vitamin D3 deficiency. This is because the incidence of MS decreases the further you travel from the poles toward the equator (that is, as solar radiation increases). Also, EBV clearly plays a role because patients with MS display increased titers of EBV antibodies. One study also showed that people in Antarctica excreted reactivated EBV in their saliva during winter and that vitamin D3 stopped the viral secretion.
Under these conditions, the researchers hypothesized that MS is caused by a double infection of brain cells by EBV and BMMF. EBV is reactivated by a lack of vitamin D3, and the BMMF multiply and are eventually converted into proteins. A focal immunoreaction causes the Schwann cells and oligodendrocytes to malfunction, which leads to the destruction of the myelin sheaths around the nerve fibers.
This article was translated from the Medscape German Edition. A version appeared on Medscape.com.
In Western diets, dairy and beef are ubiquitous: Milk goes with coffee, melted cheese with pizza, and chili with rice. But what if dairy products and beef contained a new kind of pathogen that could infect you as a child and trigger cancer or multiple sclerosis (MS) 40-70 years later?
However, in two joint statements, the German Federal Institute for Risk Assessment (BfR) and the Max Rubner Institute (MRI) have rejected such theories.
In 2008, Harald zur Hausen, MD, DSc, received the Nobel Prize in Medicine for his discovery that human papillomaviruses cause cervical cancer. His starting point was the observation that sexually abstinent women, such as nuns, rarely develop this cancer. So it was possible to draw the conclusion that pathogens are transmitted during sexual intercourse, explain Dr. zur Hausen and his wife Ethel-Michele de Villiers, PhD, both of DKFZ Heidelberg.
Papillomaviruses, as well as human herpes and Epstein-Barr viruses (EBV), polyomaviruses, and retroviruses, cause cancer in a direct way: by inserting their genes into the DNA of human cells. With a latency of a few years to a few decades, the proteins formed through expression stimulate malignant growth by altering the regulating host gene.
Acid radicals
However, viruses – just like bacteria and parasites – can also indirectly trigger cancer. One mechanism for this triggering is the disruption of immune defenses, as shown by the sometimes drastically increased tumor incidence with AIDS or with immunosuppressants after transplants. Chronic inflammation is a second mechanism that generates acid radicals and thereby causes random mutations in replicating cells. Examples include stomach cancer caused by Helicobacter pylori and liver cancer caused by Schistosoma, liver fluke, and hepatitis B and C viruses.
According to Dr. de Villiers and Dr. zur Hausen, there are good reasons to believe that other pathogens could cause chronic inflammation and thereby lead to cancer. Epidemiologic data suggest that dairy and meat products from European cows (Bos taurus) are a potential source. This is because colon cancer and breast cancer commonly occur in places where these foods are heavily consumed (that is, in North America, Argentina, Europe, and Australia). In contrast, the rate is low in India, where cows are revered as holy animals. Also noteworthy is that women with a lactose intolerance rarely develop breast cancer.
Viral progeny
In fact, the researchers found single-stranded DNA rings that originated in viruses, which they named bovine meat and milk factors (BMMF), in the intestines of patients with colon cancer. They reported, “This new class of pathogen deserves, in our opinion at least, to become the focus of cancer development and further chronic diseases.” They also detected elevated levels of acid radicals in these areas (that is, oxidative stress), which is typical for chronic inflammation.
The researchers assume that infants, whose immune system is not yet fully matured, ingest the BMMF as soon as they have dairy. Therefore, there is no need for adults to avoid dairy or beef because everyone is infected anyway, said Dr. zur Hausen.
‘Breast milk is healthy’
Dr. De Villiers and Dr. zur Hausen outlined more evidence of cancer-triggering pathogens. Mothers who have breastfed are less likely, especially after multiple pregnancies, to develop tumors in various organs or to have MS and type 2 diabetes. The authors attribute the protective effect to oligosaccharides in breast milk, which begin to be formed midway through the pregnancy. They bind to lectin receptors and, in so doing, mask the terminal molecule onto which the viruses need to dock. As a result, their port of entry into the cells is blocked.
The oligosaccharides also protect the baby against life-threatening infections by blocking access by rotaviruses and noroviruses. In this way, especially if breastfeeding lasts a long time – around 1 year – the period of incomplete immunocompetence is bridged.
Colon cancer
To date, it has been assumed that around 20% of all cancerous diseases globally are caused by infections, said the researchers. But if the suspected BMMF cases are included, this figure rises to 50%, even to around 80%, for colon cancer. If the suspicion is confirmed, the consequences for prevention and therapy would be significant.
The voice of a Nobel prize winner undoubtedly carries weight, but at the time, Dr. zur Hausen still had to convince a host of skeptics with his discovery that a viral infection is a major cause of cervical cancer. Nonetheless, some indicators suggest that he and his wife have found a dead end this time.
Institutional skepticism
When his working group made the results public in February 2019, the DKFZ felt the need to give an all-clear signal in response to alarmed press reports. There is no reason to see dairy and meat consumption as something negative. Similarly, in their first joint statement, the BfR and the MRI judged the data to be insufficient and called for further studies. Multiple research teams began to focus on BMMF as a result. In what foods can they be found? Are they more common in patients with cancer than in healthy people? Are they infectious? Do they cause inflammation and cancer?
The findings presented in a second statement by the BfR and MRI at the end of November 2022 contradicted the claims made by the DKFZ scientists across the board. In no way do BMMF represent new pathogens. They are variants of already known DNA sequences. In addition, they are present in numerous animal-based and plant-based foods, including pork, fish, fruit, vegetables, and nuts.
BMMF do not possess the ability to infect human cells, the institutes said. The proof that they are damaging to one’s health was also absent. It is true that the incidence of intestinal tumors correlates positively with the consumption of red and processed meat – which in no way signifies causality – but dairy products are linked to a reduced risk. On the other hand, breast cancer cannot be associated with the consumption of beef or dairy.
Therefore, both institutes recommend continuing to use these products as supplementary diet for infants because of their micronutrients. They further stated that the products are safe for people of all ages.
Association with MS?
Unperturbed, Dr. de Villiers and Dr. zur Hausen went one step further in their current article. They posited that MS is also associated with the consumption of dairy products and beef. Here too geographic distribution prompted the idea to look for BMMF in the brain lesions of patients with MS. The researchers isolated ring-shaped DNA molecules that proved to be closely related to BMMF from dairy and cattle blood. “The result was electrifying for us.”
However, there are several other factors to consider, such as vitamin D3 deficiency. This is because the incidence of MS decreases the further you travel from the poles toward the equator (that is, as solar radiation increases). Also, EBV clearly plays a role because patients with MS display increased titers of EBV antibodies. One study also showed that people in Antarctica excreted reactivated EBV in their saliva during winter and that vitamin D3 stopped the viral secretion.
Under these conditions, the researchers hypothesized that MS is caused by a double infection of brain cells by EBV and BMMF. EBV is reactivated by a lack of vitamin D3, and the BMMF multiply and are eventually converted into proteins. A focal immunoreaction causes the Schwann cells and oligodendrocytes to malfunction, which leads to the destruction of the myelin sheaths around the nerve fibers.
This article was translated from the Medscape German Edition. A version appeared on Medscape.com.
FDA warns about anaphylaxis after false-negative allergen tests
The Food and Drug Administration has issued a warning about the potential for patients to experience anaphylactic reactions after a negative skin test with any allergenic extract used to diagnose food allergies.
The FDA is requiring that an anaphylaxis warning after false-negative food allergen skin test results be added to the labels of these products in light of reports to the FDA’s Adverse Event Reporting System (FAERS), according to a March 3 statement.
The action follows the recognition of an increase in adverse event reports of false-negative test results with specific lots of “ALK-Abello’s Allergenic Extract-Peanut (Arachis hypogaea) – For Diagnostic Use Only.” Some of these reports “were associated with life-threatening anaphylaxis from subsequent exposure to peanut,” according to the statement. “FDA determined that the risk of anaphylaxis following false-negative food allergen skin test results is applicable to all allergenic extracts for the diagnosis of food allergies,” the statement notes.
To date, four lots of allergenic extracts have been voluntarily withdrawn from the market by the manufacturer, in November and December 2022, and should not be used.
Although some allergenic extracts are standardized, those used in the diagnosis of food allergy currently licensed by the FDA for use in the United States are nonstandardized, so potency may vary by lot.
The FDA advises health care professionals to consider confirming a negative skin test with serologic testing for peanut-specific IgE or conducting a medically supervised oral food challenge in patients, “based on the patient’s clinical history and the index of suspicion.”
The FDA also urges patients to discuss negative food allergen skin test results with their health care providers to determine the possible need for additional testing and to review the symptoms of a severe allergic reaction.
Any adverse events or side effects associated with allergenic products should be reported to the FDA via the FDA’s MedWatch Safety Information and Adverse Event Reporting Program.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has issued a warning about the potential for patients to experience anaphylactic reactions after a negative skin test with any allergenic extract used to diagnose food allergies.
The FDA is requiring that an anaphylaxis warning after false-negative food allergen skin test results be added to the labels of these products in light of reports to the FDA’s Adverse Event Reporting System (FAERS), according to a March 3 statement.
The action follows the recognition of an increase in adverse event reports of false-negative test results with specific lots of “ALK-Abello’s Allergenic Extract-Peanut (Arachis hypogaea) – For Diagnostic Use Only.” Some of these reports “were associated with life-threatening anaphylaxis from subsequent exposure to peanut,” according to the statement. “FDA determined that the risk of anaphylaxis following false-negative food allergen skin test results is applicable to all allergenic extracts for the diagnosis of food allergies,” the statement notes.
To date, four lots of allergenic extracts have been voluntarily withdrawn from the market by the manufacturer, in November and December 2022, and should not be used.
Although some allergenic extracts are standardized, those used in the diagnosis of food allergy currently licensed by the FDA for use in the United States are nonstandardized, so potency may vary by lot.
The FDA advises health care professionals to consider confirming a negative skin test with serologic testing for peanut-specific IgE or conducting a medically supervised oral food challenge in patients, “based on the patient’s clinical history and the index of suspicion.”
The FDA also urges patients to discuss negative food allergen skin test results with their health care providers to determine the possible need for additional testing and to review the symptoms of a severe allergic reaction.
Any adverse events or side effects associated with allergenic products should be reported to the FDA via the FDA’s MedWatch Safety Information and Adverse Event Reporting Program.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has issued a warning about the potential for patients to experience anaphylactic reactions after a negative skin test with any allergenic extract used to diagnose food allergies.
The FDA is requiring that an anaphylaxis warning after false-negative food allergen skin test results be added to the labels of these products in light of reports to the FDA’s Adverse Event Reporting System (FAERS), according to a March 3 statement.
The action follows the recognition of an increase in adverse event reports of false-negative test results with specific lots of “ALK-Abello’s Allergenic Extract-Peanut (Arachis hypogaea) – For Diagnostic Use Only.” Some of these reports “were associated with life-threatening anaphylaxis from subsequent exposure to peanut,” according to the statement. “FDA determined that the risk of anaphylaxis following false-negative food allergen skin test results is applicable to all allergenic extracts for the diagnosis of food allergies,” the statement notes.
To date, four lots of allergenic extracts have been voluntarily withdrawn from the market by the manufacturer, in November and December 2022, and should not be used.
Although some allergenic extracts are standardized, those used in the diagnosis of food allergy currently licensed by the FDA for use in the United States are nonstandardized, so potency may vary by lot.
The FDA advises health care professionals to consider confirming a negative skin test with serologic testing for peanut-specific IgE or conducting a medically supervised oral food challenge in patients, “based on the patient’s clinical history and the index of suspicion.”
The FDA also urges patients to discuss negative food allergen skin test results with their health care providers to determine the possible need for additional testing and to review the symptoms of a severe allergic reaction.
Any adverse events or side effects associated with allergenic products should be reported to the FDA via the FDA’s MedWatch Safety Information and Adverse Event Reporting Program.
A version of this article first appeared on Medscape.com.
Are you misdiagnosing IBS? Watch out for this mimic
Josh struggled for more than a decade with what his doctors had told him was irritable bowel syndrome (IBS). But curiously, the 39-year-old’s flare-ups were caused by some foods that aren’t typical IBS triggers.
So, Josh (not his real name) sought the care of New York gastroenterologist Yevgenia Pashinsky, MD. She conducted a comprehensive nutritional assessment and sent him for allergy testing. The results: Josh had a little-known condition called systemic nickel allergy syndrome (SNAS), which can mimic some of the symptoms of IBS.
Dr. Pashinsky, of the department of medicine at Icahn School of Medicine at Mount Sinai, New York, and a partner with New York Gastroenterology Associates, presented Josh’s case as part of a seminar on SNAS and IBS “mimickers” at the Food and Nutrition Conference and Expo in Orlando last October, sponsored by the Academy of Nutrition and Dietetics.
She and two registered dietitians in her practice, Suzie Finkel, MS, RD, CDN, and Tamara Duker Freuman, MS, RD, CDN, told seminar attendees that SNAS is rarely diagnosed and can be mistaken for IBS. They noted that it probably strikes more people than doctors suspect.
“Systemic nickel allergy is present in at least 10% of the U.S. population (and much higher in some subgroups),” Dr. Pashinsky told this news organization. “But its connection to GI symptoms and functional GI disorders is still being learned about.
“I think of nickel allergy and other allergic disorders when, in addition to GI symptoms, the patient reports skin and mucous membrane involvement along with their abdominal reactions,” she said.
For patients like Josh with SNAS, the diagnosis and treatment of this condition are surprisingly simple and effective.
“Josh had these really [unusual] symptoms and nontraditional IBS food triggers,” Ms. Finkel said in an interview. “So, that’s a situation where, as dietitians we say, ‘Hmm, that’s weird; if you have IBS, then peanuts and shrimp shouldn’t really cause an issue here.’ But this might be something physicians might not be attuned to because it’s not part of their training.”
Ms. Finkel said that Josh was referred to an allergist. Josh tested positive for skin sensitization to nickel, and he was started on a low-nickel diet, which improved his symptoms.
“So, that was this happy ending,” she added.
The upshot?
“Doctors who treat IBS patients [who are not responding to treatment] need to consider the possibility that they have SNAS and send them for allergy testing,” Ms. Finkel said. “If they come back positive, simple dietary changes can address it.”
An underrecognized condition
There has been very little research regarding SNAS in patients with IBS, and there are no standard guidelines for diagnosing and treating it.
What’s more, many gastroenterologists aren’t familiar with it. More than a dozen gastroenterologists who were contacted for comment declined to be interviewed because they didn’t know about SNAS – or enough about it to provide useful information for the story.
Ms. Finkel said she’s not surprised that many gastroenterologists don’t know much about how SNAS can mimic IBS, which is why she and her colleagues presented the seminar last October in Orlando. “It’s really an allergy and not a GI disease. It manifests with GI symptoms, but the root is not in the digestive tract; the root is in a true allergy – a clinical allergy – to nickel.”
Complicating the issue is that people who have IBS and those with SNAS typically share some common symptoms.
Like IBS, SNAS can cause GI symptoms – such as cramping, abdominal pain, heartburn, constipation, gaseous distension, and mucus in the stool. It can be triggered by certain fresh, cooked, and canned foods.
But the food triggers that cause SNAS are not usually those that cause IBS symptoms. Rather, SNAS flare-ups are nearly always triggered by foods with high levels of nickel. Examples include apricots, artichokes, asparagus, beans, cauliflower, chickpeas, cocoa/chocolate, figs, lentils, licorice, oats, onions, peas, peanuts, potatoes, spinach, tomatoes, and tea.
According to the American Academy of Allergy, Asthma & Immunology, a distinguishing feature of SNAS is that it can cause allergic contact dermatitis when a person touches something made with nickel. Coins, jewelry, eyeglasses, home fixtures, keys, zippers, dental devices, and even stainless-steel cookware can contain allergy-triggering nickel.
What Ms. Finkel sees the most are skin reactions from touching a surface containing nickel or from ingesting it, she said.
The other immediate symptom is abdominal pain or changes in bowel movements, such as diarrhea, she added.
Christopher Randolph, MD, an allergist based in Connecticut, told this news organization that it’s important for doctors to realize that patients who have a skin reaction to nickel may also have inflammatory GI symptoms.
“We definitely need more controlled studies,” said Dr. Randolph, of the department of allergy and immunology at Yale University, New Haven, Conn. “But the takeaway here is for patients and certainly providers to be mindful that you can have systemic reactions to nickel, even though you implicate only the contact dermatitis.”
Diagnosis and treatment recommendations
Skin patch allergy testing – in which a person’s skin is exposed to nickel – can quickly determine whether a patient with IBS is actually experiencing inflammatory reactions to dietary nickel and would benefit from a low-nickel or no-nickel diet, research shows.
For these patients, Dr. Pashinsky recommends the following:
- Avoiding high-nickel foods.
- Limiting canned foods.
- Using nonstainless cookware, especially for acidic foods.
- Boiling foods for potential nickel reduction, especially grains and vegetables.
- Running the tap before using water to drink or cook with first thing in the morning.
Dr. Pashisky and her team also recommend the following guidelines for doctors:
- Ask patients if symptoms occur immediately after eating certain high-nickel foods or worsen with a low-FODMAP (fermentable oligosaccharides, disaccharides, monosaccharides and polyols) diet.
- Determine whether a patient is not responding to typical medical and dietary interventions used to treat IBS.
- Conduct a food/symptom history to identify potential nickel allergy triggers.
- Try a low-nickel dietary intervention to see whether a patient’s symptoms improve in a week or two.
- Refer the patient for additional diagnostic skin-patch testing or treatment.
A multidisciplinary approach
Ms. Finkel said it’s important for doctors, particularly gastroenterologists who treat patients for suspected GI disorders to consider nickel allergy as a cause.
“SNAS is this overlooked condition ... and the research is really in its nascency here,” Ms. Finkel said.
“I would say only give [a low- or no-nickel diet] consideration if the high-nickel foods are a possible trigger,” she said. “It is very specific, looking at their diet history, to have a clear hypothesis based on what their triggers are. It’s not something to try out lightly because it’s a very restrictive diet, so I would never put a patient on a diet that I didn’t think was necessary.”
Ms. Finkel added that treatment of SNAS requires a multidisciplinary approach with a gastroenterologist, an allergist, and a dietitian.
Doctors and dietitians have distinct roles in identifying and treating these patients, Ms. Finkel said.
“If there is a suspicion of IBS symptoms and the patient is not responding to first-line treatments, then it is worth having the input of a dietitian and an allergist,” she said.
A version of this article first appeared on Medscape.com.
Josh struggled for more than a decade with what his doctors had told him was irritable bowel syndrome (IBS). But curiously, the 39-year-old’s flare-ups were caused by some foods that aren’t typical IBS triggers.
So, Josh (not his real name) sought the care of New York gastroenterologist Yevgenia Pashinsky, MD. She conducted a comprehensive nutritional assessment and sent him for allergy testing. The results: Josh had a little-known condition called systemic nickel allergy syndrome (SNAS), which can mimic some of the symptoms of IBS.
Dr. Pashinsky, of the department of medicine at Icahn School of Medicine at Mount Sinai, New York, and a partner with New York Gastroenterology Associates, presented Josh’s case as part of a seminar on SNAS and IBS “mimickers” at the Food and Nutrition Conference and Expo in Orlando last October, sponsored by the Academy of Nutrition and Dietetics.
She and two registered dietitians in her practice, Suzie Finkel, MS, RD, CDN, and Tamara Duker Freuman, MS, RD, CDN, told seminar attendees that SNAS is rarely diagnosed and can be mistaken for IBS. They noted that it probably strikes more people than doctors suspect.
“Systemic nickel allergy is present in at least 10% of the U.S. population (and much higher in some subgroups),” Dr. Pashinsky told this news organization. “But its connection to GI symptoms and functional GI disorders is still being learned about.
“I think of nickel allergy and other allergic disorders when, in addition to GI symptoms, the patient reports skin and mucous membrane involvement along with their abdominal reactions,” she said.
For patients like Josh with SNAS, the diagnosis and treatment of this condition are surprisingly simple and effective.
“Josh had these really [unusual] symptoms and nontraditional IBS food triggers,” Ms. Finkel said in an interview. “So, that’s a situation where, as dietitians we say, ‘Hmm, that’s weird; if you have IBS, then peanuts and shrimp shouldn’t really cause an issue here.’ But this might be something physicians might not be attuned to because it’s not part of their training.”
Ms. Finkel said that Josh was referred to an allergist. Josh tested positive for skin sensitization to nickel, and he was started on a low-nickel diet, which improved his symptoms.
“So, that was this happy ending,” she added.
The upshot?
“Doctors who treat IBS patients [who are not responding to treatment] need to consider the possibility that they have SNAS and send them for allergy testing,” Ms. Finkel said. “If they come back positive, simple dietary changes can address it.”
An underrecognized condition
There has been very little research regarding SNAS in patients with IBS, and there are no standard guidelines for diagnosing and treating it.
What’s more, many gastroenterologists aren’t familiar with it. More than a dozen gastroenterologists who were contacted for comment declined to be interviewed because they didn’t know about SNAS – or enough about it to provide useful information for the story.
Ms. Finkel said she’s not surprised that many gastroenterologists don’t know much about how SNAS can mimic IBS, which is why she and her colleagues presented the seminar last October in Orlando. “It’s really an allergy and not a GI disease. It manifests with GI symptoms, but the root is not in the digestive tract; the root is in a true allergy – a clinical allergy – to nickel.”
Complicating the issue is that people who have IBS and those with SNAS typically share some common symptoms.
Like IBS, SNAS can cause GI symptoms – such as cramping, abdominal pain, heartburn, constipation, gaseous distension, and mucus in the stool. It can be triggered by certain fresh, cooked, and canned foods.
But the food triggers that cause SNAS are not usually those that cause IBS symptoms. Rather, SNAS flare-ups are nearly always triggered by foods with high levels of nickel. Examples include apricots, artichokes, asparagus, beans, cauliflower, chickpeas, cocoa/chocolate, figs, lentils, licorice, oats, onions, peas, peanuts, potatoes, spinach, tomatoes, and tea.
According to the American Academy of Allergy, Asthma & Immunology, a distinguishing feature of SNAS is that it can cause allergic contact dermatitis when a person touches something made with nickel. Coins, jewelry, eyeglasses, home fixtures, keys, zippers, dental devices, and even stainless-steel cookware can contain allergy-triggering nickel.
What Ms. Finkel sees the most are skin reactions from touching a surface containing nickel or from ingesting it, she said.
The other immediate symptom is abdominal pain or changes in bowel movements, such as diarrhea, she added.
Christopher Randolph, MD, an allergist based in Connecticut, told this news organization that it’s important for doctors to realize that patients who have a skin reaction to nickel may also have inflammatory GI symptoms.
“We definitely need more controlled studies,” said Dr. Randolph, of the department of allergy and immunology at Yale University, New Haven, Conn. “But the takeaway here is for patients and certainly providers to be mindful that you can have systemic reactions to nickel, even though you implicate only the contact dermatitis.”
Diagnosis and treatment recommendations
Skin patch allergy testing – in which a person’s skin is exposed to nickel – can quickly determine whether a patient with IBS is actually experiencing inflammatory reactions to dietary nickel and would benefit from a low-nickel or no-nickel diet, research shows.
For these patients, Dr. Pashinsky recommends the following:
- Avoiding high-nickel foods.
- Limiting canned foods.
- Using nonstainless cookware, especially for acidic foods.
- Boiling foods for potential nickel reduction, especially grains and vegetables.
- Running the tap before using water to drink or cook with first thing in the morning.
Dr. Pashisky and her team also recommend the following guidelines for doctors:
- Ask patients if symptoms occur immediately after eating certain high-nickel foods or worsen with a low-FODMAP (fermentable oligosaccharides, disaccharides, monosaccharides and polyols) diet.
- Determine whether a patient is not responding to typical medical and dietary interventions used to treat IBS.
- Conduct a food/symptom history to identify potential nickel allergy triggers.
- Try a low-nickel dietary intervention to see whether a patient’s symptoms improve in a week or two.
- Refer the patient for additional diagnostic skin-patch testing or treatment.
A multidisciplinary approach
Ms. Finkel said it’s important for doctors, particularly gastroenterologists who treat patients for suspected GI disorders to consider nickel allergy as a cause.
“SNAS is this overlooked condition ... and the research is really in its nascency here,” Ms. Finkel said.
“I would say only give [a low- or no-nickel diet] consideration if the high-nickel foods are a possible trigger,” she said. “It is very specific, looking at their diet history, to have a clear hypothesis based on what their triggers are. It’s not something to try out lightly because it’s a very restrictive diet, so I would never put a patient on a diet that I didn’t think was necessary.”
Ms. Finkel added that treatment of SNAS requires a multidisciplinary approach with a gastroenterologist, an allergist, and a dietitian.
Doctors and dietitians have distinct roles in identifying and treating these patients, Ms. Finkel said.
“If there is a suspicion of IBS symptoms and the patient is not responding to first-line treatments, then it is worth having the input of a dietitian and an allergist,” she said.
A version of this article first appeared on Medscape.com.
Josh struggled for more than a decade with what his doctors had told him was irritable bowel syndrome (IBS). But curiously, the 39-year-old’s flare-ups were caused by some foods that aren’t typical IBS triggers.
So, Josh (not his real name) sought the care of New York gastroenterologist Yevgenia Pashinsky, MD. She conducted a comprehensive nutritional assessment and sent him for allergy testing. The results: Josh had a little-known condition called systemic nickel allergy syndrome (SNAS), which can mimic some of the symptoms of IBS.
Dr. Pashinsky, of the department of medicine at Icahn School of Medicine at Mount Sinai, New York, and a partner with New York Gastroenterology Associates, presented Josh’s case as part of a seminar on SNAS and IBS “mimickers” at the Food and Nutrition Conference and Expo in Orlando last October, sponsored by the Academy of Nutrition and Dietetics.
She and two registered dietitians in her practice, Suzie Finkel, MS, RD, CDN, and Tamara Duker Freuman, MS, RD, CDN, told seminar attendees that SNAS is rarely diagnosed and can be mistaken for IBS. They noted that it probably strikes more people than doctors suspect.
“Systemic nickel allergy is present in at least 10% of the U.S. population (and much higher in some subgroups),” Dr. Pashinsky told this news organization. “But its connection to GI symptoms and functional GI disorders is still being learned about.
“I think of nickel allergy and other allergic disorders when, in addition to GI symptoms, the patient reports skin and mucous membrane involvement along with their abdominal reactions,” she said.
For patients like Josh with SNAS, the diagnosis and treatment of this condition are surprisingly simple and effective.
“Josh had these really [unusual] symptoms and nontraditional IBS food triggers,” Ms. Finkel said in an interview. “So, that’s a situation where, as dietitians we say, ‘Hmm, that’s weird; if you have IBS, then peanuts and shrimp shouldn’t really cause an issue here.’ But this might be something physicians might not be attuned to because it’s not part of their training.”
Ms. Finkel said that Josh was referred to an allergist. Josh tested positive for skin sensitization to nickel, and he was started on a low-nickel diet, which improved his symptoms.
“So, that was this happy ending,” she added.
The upshot?
“Doctors who treat IBS patients [who are not responding to treatment] need to consider the possibility that they have SNAS and send them for allergy testing,” Ms. Finkel said. “If they come back positive, simple dietary changes can address it.”
An underrecognized condition
There has been very little research regarding SNAS in patients with IBS, and there are no standard guidelines for diagnosing and treating it.
What’s more, many gastroenterologists aren’t familiar with it. More than a dozen gastroenterologists who were contacted for comment declined to be interviewed because they didn’t know about SNAS – or enough about it to provide useful information for the story.
Ms. Finkel said she’s not surprised that many gastroenterologists don’t know much about how SNAS can mimic IBS, which is why she and her colleagues presented the seminar last October in Orlando. “It’s really an allergy and not a GI disease. It manifests with GI symptoms, but the root is not in the digestive tract; the root is in a true allergy – a clinical allergy – to nickel.”
Complicating the issue is that people who have IBS and those with SNAS typically share some common symptoms.
Like IBS, SNAS can cause GI symptoms – such as cramping, abdominal pain, heartburn, constipation, gaseous distension, and mucus in the stool. It can be triggered by certain fresh, cooked, and canned foods.
But the food triggers that cause SNAS are not usually those that cause IBS symptoms. Rather, SNAS flare-ups are nearly always triggered by foods with high levels of nickel. Examples include apricots, artichokes, asparagus, beans, cauliflower, chickpeas, cocoa/chocolate, figs, lentils, licorice, oats, onions, peas, peanuts, potatoes, spinach, tomatoes, and tea.
According to the American Academy of Allergy, Asthma & Immunology, a distinguishing feature of SNAS is that it can cause allergic contact dermatitis when a person touches something made with nickel. Coins, jewelry, eyeglasses, home fixtures, keys, zippers, dental devices, and even stainless-steel cookware can contain allergy-triggering nickel.
What Ms. Finkel sees the most are skin reactions from touching a surface containing nickel or from ingesting it, she said.
The other immediate symptom is abdominal pain or changes in bowel movements, such as diarrhea, she added.
Christopher Randolph, MD, an allergist based in Connecticut, told this news organization that it’s important for doctors to realize that patients who have a skin reaction to nickel may also have inflammatory GI symptoms.
“We definitely need more controlled studies,” said Dr. Randolph, of the department of allergy and immunology at Yale University, New Haven, Conn. “But the takeaway here is for patients and certainly providers to be mindful that you can have systemic reactions to nickel, even though you implicate only the contact dermatitis.”
Diagnosis and treatment recommendations
Skin patch allergy testing – in which a person’s skin is exposed to nickel – can quickly determine whether a patient with IBS is actually experiencing inflammatory reactions to dietary nickel and would benefit from a low-nickel or no-nickel diet, research shows.
For these patients, Dr. Pashinsky recommends the following:
- Avoiding high-nickel foods.
- Limiting canned foods.
- Using nonstainless cookware, especially for acidic foods.
- Boiling foods for potential nickel reduction, especially grains and vegetables.
- Running the tap before using water to drink or cook with first thing in the morning.
Dr. Pashisky and her team also recommend the following guidelines for doctors:
- Ask patients if symptoms occur immediately after eating certain high-nickel foods or worsen with a low-FODMAP (fermentable oligosaccharides, disaccharides, monosaccharides and polyols) diet.
- Determine whether a patient is not responding to typical medical and dietary interventions used to treat IBS.
- Conduct a food/symptom history to identify potential nickel allergy triggers.
- Try a low-nickel dietary intervention to see whether a patient’s symptoms improve in a week or two.
- Refer the patient for additional diagnostic skin-patch testing or treatment.
A multidisciplinary approach
Ms. Finkel said it’s important for doctors, particularly gastroenterologists who treat patients for suspected GI disorders to consider nickel allergy as a cause.
“SNAS is this overlooked condition ... and the research is really in its nascency here,” Ms. Finkel said.
“I would say only give [a low- or no-nickel diet] consideration if the high-nickel foods are a possible trigger,” she said. “It is very specific, looking at their diet history, to have a clear hypothesis based on what their triggers are. It’s not something to try out lightly because it’s a very restrictive diet, so I would never put a patient on a diet that I didn’t think was necessary.”
Ms. Finkel added that treatment of SNAS requires a multidisciplinary approach with a gastroenterologist, an allergist, and a dietitian.
Doctors and dietitians have distinct roles in identifying and treating these patients, Ms. Finkel said.
“If there is a suspicion of IBS symptoms and the patient is not responding to first-line treatments, then it is worth having the input of a dietitian and an allergist,” she said.
A version of this article first appeared on Medscape.com.
In utero exposure to asthma medication not tied to risks of neurodevelopmental disorders
The drugs included in the study were leukotriene-receptor antagonists (LTRAs), which are often used to treat allergic airway diseases, including asthma and allergic rhinitis.
“Over the years, the U.S. Food and Drug Administration has monitored post-marketing data about the potential harm of neuropsychiatric events (NEs) associated with montelukast, the first type of LTRAs, and issued boxed warnings about serious mental health side effects for montelukast in 2020,” said corresponding author Tsung-Chieh Yao, MD, of Chang Gung Memorial Hospital, Taiwan, in an interview.
However, evidence of a link between NEs and LTRA use has been inconsistent, according to Dr. Yao and colleagues.
“To date, it remains totally unknown whether the exposure to LTRAs during pregnancy is associated with the risk of neuropsychiatric events in offspring,” said Dr. Yao.
To address this question, the researchers used data from National Health Insurance Research Database in Taiwan to identify pregnant women and their offspring from 2009 to 2019. The initial study population included 576,157 mother-offspring pairs, including 1,995 LTRA-exposed and 574,162 nonexposed children.
The women had a diagnosis of asthma or allergic rhinitis; multiple births and children with congenital malformations were excluded. LTRA exposure was defined as any dispensed prescription for LTRAs during pregnancy. Approximately two-thirds of the mothers were aged 30-40 years at the time of delivery.
The findings were published in a research letter in JAMA Network Open.
In the study population at large, the incidence of the three neurodevelopmental disorders ADHD, autism spectrum disorder (ASD), and Tourette syndrome was not significantly different between those children exposed to LTRAs and those not exposed to LTRAs in utero (1.25% vs. 1.32%; 3.31% vs. 4.36%; and 0.45% vs. 0.83%, respectively).
After propensity score matching, the study population included 1,988 LTRA-exposed children and 19,863 nonexposed children. In this group, no significant associations appeared between prenatal LTRA exposure and the risk of attention-deficit/hyperactivity disorder (adjusted hazard ratio, 1.03), autism spectrum disorder (AHR, 1.01), and Tourette syndrome (AHR, 0.63).
Neither duration nor cumulative dose of LTRA use during pregnancy showed an association with ADHD, ASD, or Tourette syndrome in offspring. Duration of LTRA use was categorized as shorter or longer periods of 1-4 weeks vs. more than 4 weeks; cumulative dose was categorized as 1-170 mg vs. 170 mg or higher.
The findings were limited by the lack of randomization, inability to detect long-term risk, and potential lack of generalizability to non-Asian populations, and more research is needed to replicate the results, the researchers noted. However, the current findings were strengthened by the large study population, and suggest that LTRA use in pregnancy does not present a significant risk for NEs in children, which should be reassuring to clinicians and patients, they concluded.
The current study is the first to use the whole of Taiwan population data and extends previous studies by examining the association between LTRA use during pregnancy and risk of neuropsychiatric events in offspring, Dr. Yao said in an interview. “The possibly surprising, but reassuring, finding is that prenatal LTRA exposure did not increase risk of ADHD, ASD, and Tourette syndrome in offspring,” he said.
“Clinicians prescribing LTRAs such as montelukast (Singulair and generics) to pregnant women with asthma or allergic rhinitis may be reassured by our findings,” Dr. Yao added. The results offer real-world evidence to help inform decision-making about the use of LTRAs during pregnancy, although additional research is needed to replicate the study findings in other populations, he said.
The study was supported by the National Health Research Institutes, Taiwan, the Ministry of Science and Technology of Taiwan, the National Science and Technology Council of Taiwan, and the Chang Gung Medical Foundation. The researchers had no financial conflicts to disclose.
The drugs included in the study were leukotriene-receptor antagonists (LTRAs), which are often used to treat allergic airway diseases, including asthma and allergic rhinitis.
“Over the years, the U.S. Food and Drug Administration has monitored post-marketing data about the potential harm of neuropsychiatric events (NEs) associated with montelukast, the first type of LTRAs, and issued boxed warnings about serious mental health side effects for montelukast in 2020,” said corresponding author Tsung-Chieh Yao, MD, of Chang Gung Memorial Hospital, Taiwan, in an interview.
However, evidence of a link between NEs and LTRA use has been inconsistent, according to Dr. Yao and colleagues.
“To date, it remains totally unknown whether the exposure to LTRAs during pregnancy is associated with the risk of neuropsychiatric events in offspring,” said Dr. Yao.
To address this question, the researchers used data from National Health Insurance Research Database in Taiwan to identify pregnant women and their offspring from 2009 to 2019. The initial study population included 576,157 mother-offspring pairs, including 1,995 LTRA-exposed and 574,162 nonexposed children.
The women had a diagnosis of asthma or allergic rhinitis; multiple births and children with congenital malformations were excluded. LTRA exposure was defined as any dispensed prescription for LTRAs during pregnancy. Approximately two-thirds of the mothers were aged 30-40 years at the time of delivery.
The findings were published in a research letter in JAMA Network Open.
In the study population at large, the incidence of the three neurodevelopmental disorders ADHD, autism spectrum disorder (ASD), and Tourette syndrome was not significantly different between those children exposed to LTRAs and those not exposed to LTRAs in utero (1.25% vs. 1.32%; 3.31% vs. 4.36%; and 0.45% vs. 0.83%, respectively).
After propensity score matching, the study population included 1,988 LTRA-exposed children and 19,863 nonexposed children. In this group, no significant associations appeared between prenatal LTRA exposure and the risk of attention-deficit/hyperactivity disorder (adjusted hazard ratio, 1.03), autism spectrum disorder (AHR, 1.01), and Tourette syndrome (AHR, 0.63).
Neither duration nor cumulative dose of LTRA use during pregnancy showed an association with ADHD, ASD, or Tourette syndrome in offspring. Duration of LTRA use was categorized as shorter or longer periods of 1-4 weeks vs. more than 4 weeks; cumulative dose was categorized as 1-170 mg vs. 170 mg or higher.
The findings were limited by the lack of randomization, inability to detect long-term risk, and potential lack of generalizability to non-Asian populations, and more research is needed to replicate the results, the researchers noted. However, the current findings were strengthened by the large study population, and suggest that LTRA use in pregnancy does not present a significant risk for NEs in children, which should be reassuring to clinicians and patients, they concluded.
The current study is the first to use the whole of Taiwan population data and extends previous studies by examining the association between LTRA use during pregnancy and risk of neuropsychiatric events in offspring, Dr. Yao said in an interview. “The possibly surprising, but reassuring, finding is that prenatal LTRA exposure did not increase risk of ADHD, ASD, and Tourette syndrome in offspring,” he said.
“Clinicians prescribing LTRAs such as montelukast (Singulair and generics) to pregnant women with asthma or allergic rhinitis may be reassured by our findings,” Dr. Yao added. The results offer real-world evidence to help inform decision-making about the use of LTRAs during pregnancy, although additional research is needed to replicate the study findings in other populations, he said.
The study was supported by the National Health Research Institutes, Taiwan, the Ministry of Science and Technology of Taiwan, the National Science and Technology Council of Taiwan, and the Chang Gung Medical Foundation. The researchers had no financial conflicts to disclose.
The drugs included in the study were leukotriene-receptor antagonists (LTRAs), which are often used to treat allergic airway diseases, including asthma and allergic rhinitis.
“Over the years, the U.S. Food and Drug Administration has monitored post-marketing data about the potential harm of neuropsychiatric events (NEs) associated with montelukast, the first type of LTRAs, and issued boxed warnings about serious mental health side effects for montelukast in 2020,” said corresponding author Tsung-Chieh Yao, MD, of Chang Gung Memorial Hospital, Taiwan, in an interview.
However, evidence of a link between NEs and LTRA use has been inconsistent, according to Dr. Yao and colleagues.
“To date, it remains totally unknown whether the exposure to LTRAs during pregnancy is associated with the risk of neuropsychiatric events in offspring,” said Dr. Yao.
To address this question, the researchers used data from National Health Insurance Research Database in Taiwan to identify pregnant women and their offspring from 2009 to 2019. The initial study population included 576,157 mother-offspring pairs, including 1,995 LTRA-exposed and 574,162 nonexposed children.
The women had a diagnosis of asthma or allergic rhinitis; multiple births and children with congenital malformations were excluded. LTRA exposure was defined as any dispensed prescription for LTRAs during pregnancy. Approximately two-thirds of the mothers were aged 30-40 years at the time of delivery.
The findings were published in a research letter in JAMA Network Open.
In the study population at large, the incidence of the three neurodevelopmental disorders ADHD, autism spectrum disorder (ASD), and Tourette syndrome was not significantly different between those children exposed to LTRAs and those not exposed to LTRAs in utero (1.25% vs. 1.32%; 3.31% vs. 4.36%; and 0.45% vs. 0.83%, respectively).
After propensity score matching, the study population included 1,988 LTRA-exposed children and 19,863 nonexposed children. In this group, no significant associations appeared between prenatal LTRA exposure and the risk of attention-deficit/hyperactivity disorder (adjusted hazard ratio, 1.03), autism spectrum disorder (AHR, 1.01), and Tourette syndrome (AHR, 0.63).
Neither duration nor cumulative dose of LTRA use during pregnancy showed an association with ADHD, ASD, or Tourette syndrome in offspring. Duration of LTRA use was categorized as shorter or longer periods of 1-4 weeks vs. more than 4 weeks; cumulative dose was categorized as 1-170 mg vs. 170 mg or higher.
The findings were limited by the lack of randomization, inability to detect long-term risk, and potential lack of generalizability to non-Asian populations, and more research is needed to replicate the results, the researchers noted. However, the current findings were strengthened by the large study population, and suggest that LTRA use in pregnancy does not present a significant risk for NEs in children, which should be reassuring to clinicians and patients, they concluded.
The current study is the first to use the whole of Taiwan population data and extends previous studies by examining the association between LTRA use during pregnancy and risk of neuropsychiatric events in offspring, Dr. Yao said in an interview. “The possibly surprising, but reassuring, finding is that prenatal LTRA exposure did not increase risk of ADHD, ASD, and Tourette syndrome in offspring,” he said.
“Clinicians prescribing LTRAs such as montelukast (Singulair and generics) to pregnant women with asthma or allergic rhinitis may be reassured by our findings,” Dr. Yao added. The results offer real-world evidence to help inform decision-making about the use of LTRAs during pregnancy, although additional research is needed to replicate the study findings in other populations, he said.
The study was supported by the National Health Research Institutes, Taiwan, the Ministry of Science and Technology of Taiwan, the National Science and Technology Council of Taiwan, and the Chang Gung Medical Foundation. The researchers had no financial conflicts to disclose.
FROM JAMA NETWORK OPEN
Painful blue fingers
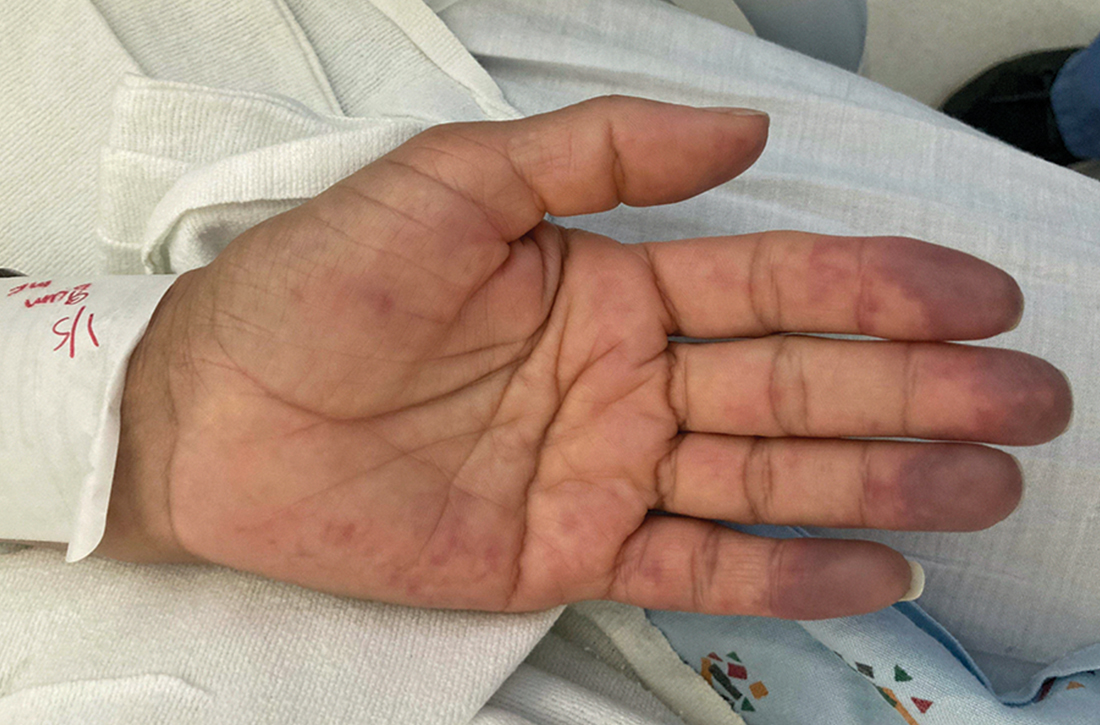
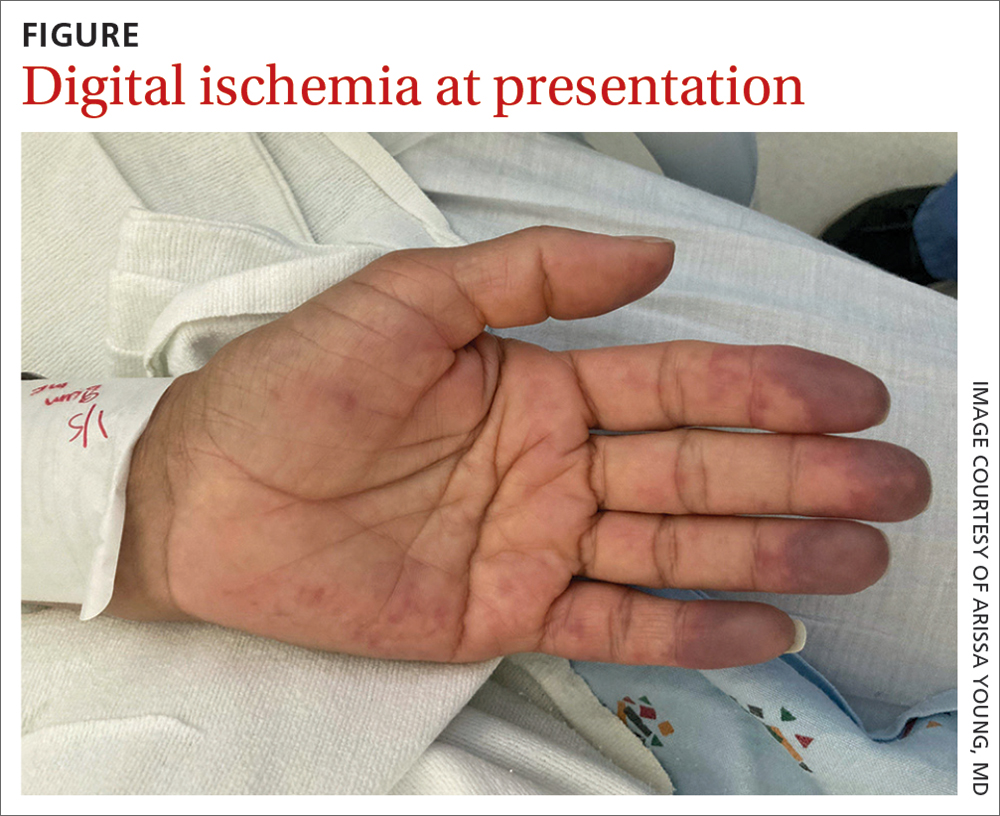
A 48-year-old woman with a history of systemic lupus erythematosus (SLE) presented to the emergency department from the rheumatology clinic for digital ischemia. The clinical manifestations of her SLE consisted predominantly of arthralgias, which had been previously well controlled on hydroxychloroquine 300 mg/d PO. On presentation, she denied oral ulcers, alopecia, shortness of breath, chest pain/pressure, and history of blood clots or miscarriages.
On exam, the patient was afebrile and had a heart rate of 74 bpm; blood pressure, 140/77 mm Hg; and respiratory rate, 18 breaths/min. The fingertips on her left hand were tender and cool to the touch, and the fingertips of her second through fifth digits were blue (FIGURE).
Laboratory workup was notable for the following: hemoglobin, 9.3 g/dL (normal range, 11.6-15.2 g/dL) and erythrocyte sedimentation rate, 44 mm/h (normal range, ≤ 25 mm/h). Double-stranded DNA and complement levels were normal.
Transthoracic echocardiogram did not show any valvular vegetations, and blood cultures from admission were negative. Computed tomography angiography (CTA) with contrast of her left upper extremity showed a filling defect in the origin of the left subclavian artery. Digital plethysmography showed dampened flow signals in the second through fifth digits of the left hand.
Tests for antiphospholipid antibodies were positive for lupus anticoagulant; there were also high titers of anti-β-2-glycoprotein immunoglobulin (Ig) G (58 SGU; normal, ≤ 20 SGU) and anticardiolipin IgG (242.4 CU; normal, ≤ 20 CU).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Antiphospholipid syndrome
Given our patient’s SLE, left subclavian artery thrombosis, digital ischemia, and high-titer antiphospholipid antibodies, we had significant concern for antiphospholipid syndrome (APS). The diagnosis of APS is most often based on the fulfillment of the revised Sapporo classification criteria. These criteria include both clinical criteria (vascular thrombosis or pregnancy morbidity) and laboratory criteria (the presence of antiphospholipid antibodies on at least 2 separate occasions separated by 12 weeks).1 Our patient met clinical criteria given the evidence of subclavian artery thrombosis on CTA as well as digital plethysmography findings consistent with digital emboli. To meet laboratory criteria, she would have needed to have persistent high-titer antiphospholipid antibodies 12 weeks apart.
APS is an autoimmune disease in which the presence of antiphospholipid antibodies is associated with thrombosis; it can be divided into primary and secondary APS. The estimated prevalence of APS is 50 per 100,000 people in the United States.2 Primary APS occurs in the absence of an underlying autoimmune disease, while secondary APS occurs in the presence of an underlying autoimmune disease.
The autoimmune disease most often associated with APS is SLE.3 Among patients with SLE, 15% to 34% have positive lupus anticoagulant and 12% to 30% have anticardiolipin antibodies.4-6 This is compared with young healthy subjects among whom only 1% to 4% have positive lupus anticoagulant and 1% to 5% have anticardiolipin antibodies.7 Previous studies have estimated that 30% to 50% of patients with SLE who test positive for antiphospholipid antibodies will develop thrombosis.5,7
Differential includes Raynaud phenomenon, vasculitis
The differential diagnosis for digital ischemia in a patient with SLE includes APS, Raynaud phenomenon, vasculitis, and septic emboli.
Raynaud phenomenon can manifest in patients with SLE, but the presence of thrombosis on CTA and high-titer positive antiphospholipid antibodies make the diagnosis of APS more likely. Additionally, Raynaud phenomenon is typically temperature dependent with vasospasm in the digital arteries, occurring in cold temperatures and resolving with warming.
Systemic vasculitis may develop in patients with SLE, but in our case was less likely given the patient did not have any evidence of vasculitis on CTA, such as blood vessel wall thickening and/or enhancement.8
Septic emboli from endocarditis can cause digital ischemia but is typically associated with positive blood cultures, fever, and other systemic signs of infection, and/or vegetations on an echocardiogram.
Continue to: Thrombosis determines intensity of lifelong antiocagulation Tx
Thrombosis determines intensity of lifelong anticoagulation Tx
The mainstay of therapy for patients with APS is lifelong anticoagulation with a vitamin K antagonist. The intensity of anticoagulation is determined based on the presence of venous or arterial thrombosis. In patients who present with arterial thrombosis, a higher intensity vitamin K antagonist (ie, international normalized ratio [INR] goal > 3) or the addition of low-dose aspirin should be considered.9,10
Factor Xa inhibitors are generally not recommended at this time due to the lack of evidence to support their use.10 Additionally, a randomized clinical trial comparing rivaroxaban and warfarin in patients with triple antiphospholipid antibody positivity was terminated prematurely due to increased thromboembolic events in the rivaroxaban arm.11
For patients with secondary APS in the setting of SLE, hydroxychloroquine in combination with a vitamin K antagonist has been shown to decrease the risk for recurrent thrombosis compared with treatment with a vitamin K antagonist alone.12
Our patient was started on a heparin drip and transitioned to an oral vitamin K antagonist with an INR goal of 2 to 3. Lifelong anticoagulation was planned. The pain and discoloration in her hands improved on anticoagulation and had nearly resolved by the time of discharge. Given her history of arterial thrombosis, the addition of aspirin was also considered, but this decision was ultimately deferred to her outpatient rheumatologist and hematologist.
1. Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4:295-306. doi: 10.1111/j.1538-7836.2006.01753.x
2. Duarte-García A, Pham MM, Crowson CS, et al. The epidemiology of antiphospholipid syndrome: a population-based study. Arthritis Rheumatol. 2019;71:1545-1552. doi: 10.1002/art.40901
3. Levine JS, Branch DW, Rauch J. The antiphospholipid syndrome. N Engl J Med. 2002;346:752-763. doi: 10.1056/NEJMra002974
4. Cervera R, Khamashta MA, Font J, et al. Systemic lupus erythematosus: clinical and immunologic patterns of disease expression in a cohort of 1,000 patients. Medicine (Baltimore). 1993;72:113-124.
5. Love PE, Santoro SA. Antiphospholipid antibodies: anticardiolipin and the lupus anticoagulant in systemic lupus erythematosus (SLE) and in non-SLE disorders: prevalence and clinical significance. Ann Intern Med. 1990;112:682-698. doi: 10.7326/0003-4819-112-9-682
6. Merkel PA, Chang YC, Pierangeli SS, et al. The prevalence and clinical associations of anticardiolipin antibodies in a large inception cohort of patients with connective tissue diseases. Am J Med. 1996;101:576-583. doi: 10.1016/s0002-9343(96)00335-x
7. Petri M. Epidemiology of the antiphospholipid antibody syndrome. J Autoimmun. 2000;15:145-151. doi: 10.1006/jaut. 2000.0409
8. Bozlar U, Ogur T, Khaja MS, et al. CT angiography of the upper extremity arterial system: Part 2—Clinical applications beyond trauma patients. AJR Am J Roentgenol. 2013;201:753-763. doi: 10.2214/AJR.13.11208
9. Ruiz-Irastorza G, Hunt BJ, Khamashta MA. A systematic review of secondary thromboprophylaxis in patients with antiphospholipid antibodies. Arthritis Rheum. 2007;7:1487-1495. doi: 10.1002/art.23109
10. Tektonidou MG, Andreoli L, Limper M, et al. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann Rheum Dis. 2019;78:1296-1304. doi: 10.1136/annrheumdis-2019-215213
Efficacy and safety of rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome: rationale and design of the Trial on Rivaroxaban in AntiPhospholipid Syndrome (TRAPS) trial. Lupus. 2016;25:301-306. doi: 10.1177/0961203315611495
12. Schmidt-Tanguy A, Voswinkel J, Henrion D, et al. Antithrombotic effects of hydroxychloroquine in primary antiphospholipid syndrome patients. J Thromb Haemost. 2013;11:1927-1929. doi: 10.1111/jth.12363
A 48-year-old woman with a history of systemic lupus erythematosus (SLE) presented to the emergency department from the rheumatology clinic for digital ischemia. The clinical manifestations of her SLE consisted predominantly of arthralgias, which had been previously well controlled on hydroxychloroquine 300 mg/d PO. On presentation, she denied oral ulcers, alopecia, shortness of breath, chest pain/pressure, and history of blood clots or miscarriages.
On exam, the patient was afebrile and had a heart rate of 74 bpm; blood pressure, 140/77 mm Hg; and respiratory rate, 18 breaths/min. The fingertips on her left hand were tender and cool to the touch, and the fingertips of her second through fifth digits were blue (FIGURE).
Laboratory workup was notable for the following: hemoglobin, 9.3 g/dL (normal range, 11.6-15.2 g/dL) and erythrocyte sedimentation rate, 44 mm/h (normal range, ≤ 25 mm/h). Double-stranded DNA and complement levels were normal.
Transthoracic echocardiogram did not show any valvular vegetations, and blood cultures from admission were negative. Computed tomography angiography (CTA) with contrast of her left upper extremity showed a filling defect in the origin of the left subclavian artery. Digital plethysmography showed dampened flow signals in the second through fifth digits of the left hand.
Tests for antiphospholipid antibodies were positive for lupus anticoagulant; there were also high titers of anti-β-2-glycoprotein immunoglobulin (Ig) G (58 SGU; normal, ≤ 20 SGU) and anticardiolipin IgG (242.4 CU; normal, ≤ 20 CU).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Antiphospholipid syndrome
Given our patient’s SLE, left subclavian artery thrombosis, digital ischemia, and high-titer antiphospholipid antibodies, we had significant concern for antiphospholipid syndrome (APS). The diagnosis of APS is most often based on the fulfillment of the revised Sapporo classification criteria. These criteria include both clinical criteria (vascular thrombosis or pregnancy morbidity) and laboratory criteria (the presence of antiphospholipid antibodies on at least 2 separate occasions separated by 12 weeks).1 Our patient met clinical criteria given the evidence of subclavian artery thrombosis on CTA as well as digital plethysmography findings consistent with digital emboli. To meet laboratory criteria, she would have needed to have persistent high-titer antiphospholipid antibodies 12 weeks apart.
APS is an autoimmune disease in which the presence of antiphospholipid antibodies is associated with thrombosis; it can be divided into primary and secondary APS. The estimated prevalence of APS is 50 per 100,000 people in the United States.2 Primary APS occurs in the absence of an underlying autoimmune disease, while secondary APS occurs in the presence of an underlying autoimmune disease.
The autoimmune disease most often associated with APS is SLE.3 Among patients with SLE, 15% to 34% have positive lupus anticoagulant and 12% to 30% have anticardiolipin antibodies.4-6 This is compared with young healthy subjects among whom only 1% to 4% have positive lupus anticoagulant and 1% to 5% have anticardiolipin antibodies.7 Previous studies have estimated that 30% to 50% of patients with SLE who test positive for antiphospholipid antibodies will develop thrombosis.5,7
Differential includes Raynaud phenomenon, vasculitis
The differential diagnosis for digital ischemia in a patient with SLE includes APS, Raynaud phenomenon, vasculitis, and septic emboli.
Raynaud phenomenon can manifest in patients with SLE, but the presence of thrombosis on CTA and high-titer positive antiphospholipid antibodies make the diagnosis of APS more likely. Additionally, Raynaud phenomenon is typically temperature dependent with vasospasm in the digital arteries, occurring in cold temperatures and resolving with warming.
Systemic vasculitis may develop in patients with SLE, but in our case was less likely given the patient did not have any evidence of vasculitis on CTA, such as blood vessel wall thickening and/or enhancement.8
Septic emboli from endocarditis can cause digital ischemia but is typically associated with positive blood cultures, fever, and other systemic signs of infection, and/or vegetations on an echocardiogram.
Continue to: Thrombosis determines intensity of lifelong antiocagulation Tx
Thrombosis determines intensity of lifelong anticoagulation Tx
The mainstay of therapy for patients with APS is lifelong anticoagulation with a vitamin K antagonist. The intensity of anticoagulation is determined based on the presence of venous or arterial thrombosis. In patients who present with arterial thrombosis, a higher intensity vitamin K antagonist (ie, international normalized ratio [INR] goal > 3) or the addition of low-dose aspirin should be considered.9,10
Factor Xa inhibitors are generally not recommended at this time due to the lack of evidence to support their use.10 Additionally, a randomized clinical trial comparing rivaroxaban and warfarin in patients with triple antiphospholipid antibody positivity was terminated prematurely due to increased thromboembolic events in the rivaroxaban arm.11
For patients with secondary APS in the setting of SLE, hydroxychloroquine in combination with a vitamin K antagonist has been shown to decrease the risk for recurrent thrombosis compared with treatment with a vitamin K antagonist alone.12
Our patient was started on a heparin drip and transitioned to an oral vitamin K antagonist with an INR goal of 2 to 3. Lifelong anticoagulation was planned. The pain and discoloration in her hands improved on anticoagulation and had nearly resolved by the time of discharge. Given her history of arterial thrombosis, the addition of aspirin was also considered, but this decision was ultimately deferred to her outpatient rheumatologist and hematologist.
A 48-year-old woman with a history of systemic lupus erythematosus (SLE) presented to the emergency department from the rheumatology clinic for digital ischemia. The clinical manifestations of her SLE consisted predominantly of arthralgias, which had been previously well controlled on hydroxychloroquine 300 mg/d PO. On presentation, she denied oral ulcers, alopecia, shortness of breath, chest pain/pressure, and history of blood clots or miscarriages.
On exam, the patient was afebrile and had a heart rate of 74 bpm; blood pressure, 140/77 mm Hg; and respiratory rate, 18 breaths/min. The fingertips on her left hand were tender and cool to the touch, and the fingertips of her second through fifth digits were blue (FIGURE).
Laboratory workup was notable for the following: hemoglobin, 9.3 g/dL (normal range, 11.6-15.2 g/dL) and erythrocyte sedimentation rate, 44 mm/h (normal range, ≤ 25 mm/h). Double-stranded DNA and complement levels were normal.
Transthoracic echocardiogram did not show any valvular vegetations, and blood cultures from admission were negative. Computed tomography angiography (CTA) with contrast of her left upper extremity showed a filling defect in the origin of the left subclavian artery. Digital plethysmography showed dampened flow signals in the second through fifth digits of the left hand.
Tests for antiphospholipid antibodies were positive for lupus anticoagulant; there were also high titers of anti-β-2-glycoprotein immunoglobulin (Ig) G (58 SGU; normal, ≤ 20 SGU) and anticardiolipin IgG (242.4 CU; normal, ≤ 20 CU).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Antiphospholipid syndrome
Given our patient’s SLE, left subclavian artery thrombosis, digital ischemia, and high-titer antiphospholipid antibodies, we had significant concern for antiphospholipid syndrome (APS). The diagnosis of APS is most often based on the fulfillment of the revised Sapporo classification criteria. These criteria include both clinical criteria (vascular thrombosis or pregnancy morbidity) and laboratory criteria (the presence of antiphospholipid antibodies on at least 2 separate occasions separated by 12 weeks).1 Our patient met clinical criteria given the evidence of subclavian artery thrombosis on CTA as well as digital plethysmography findings consistent with digital emboli. To meet laboratory criteria, she would have needed to have persistent high-titer antiphospholipid antibodies 12 weeks apart.
APS is an autoimmune disease in which the presence of antiphospholipid antibodies is associated with thrombosis; it can be divided into primary and secondary APS. The estimated prevalence of APS is 50 per 100,000 people in the United States.2 Primary APS occurs in the absence of an underlying autoimmune disease, while secondary APS occurs in the presence of an underlying autoimmune disease.
The autoimmune disease most often associated with APS is SLE.3 Among patients with SLE, 15% to 34% have positive lupus anticoagulant and 12% to 30% have anticardiolipin antibodies.4-6 This is compared with young healthy subjects among whom only 1% to 4% have positive lupus anticoagulant and 1% to 5% have anticardiolipin antibodies.7 Previous studies have estimated that 30% to 50% of patients with SLE who test positive for antiphospholipid antibodies will develop thrombosis.5,7
Differential includes Raynaud phenomenon, vasculitis
The differential diagnosis for digital ischemia in a patient with SLE includes APS, Raynaud phenomenon, vasculitis, and septic emboli.
Raynaud phenomenon can manifest in patients with SLE, but the presence of thrombosis on CTA and high-titer positive antiphospholipid antibodies make the diagnosis of APS more likely. Additionally, Raynaud phenomenon is typically temperature dependent with vasospasm in the digital arteries, occurring in cold temperatures and resolving with warming.
Systemic vasculitis may develop in patients with SLE, but in our case was less likely given the patient did not have any evidence of vasculitis on CTA, such as blood vessel wall thickening and/or enhancement.8
Septic emboli from endocarditis can cause digital ischemia but is typically associated with positive blood cultures, fever, and other systemic signs of infection, and/or vegetations on an echocardiogram.
Continue to: Thrombosis determines intensity of lifelong antiocagulation Tx
Thrombosis determines intensity of lifelong anticoagulation Tx
The mainstay of therapy for patients with APS is lifelong anticoagulation with a vitamin K antagonist. The intensity of anticoagulation is determined based on the presence of venous or arterial thrombosis. In patients who present with arterial thrombosis, a higher intensity vitamin K antagonist (ie, international normalized ratio [INR] goal > 3) or the addition of low-dose aspirin should be considered.9,10
Factor Xa inhibitors are generally not recommended at this time due to the lack of evidence to support their use.10 Additionally, a randomized clinical trial comparing rivaroxaban and warfarin in patients with triple antiphospholipid antibody positivity was terminated prematurely due to increased thromboembolic events in the rivaroxaban arm.11
For patients with secondary APS in the setting of SLE, hydroxychloroquine in combination with a vitamin K antagonist has been shown to decrease the risk for recurrent thrombosis compared with treatment with a vitamin K antagonist alone.12
Our patient was started on a heparin drip and transitioned to an oral vitamin K antagonist with an INR goal of 2 to 3. Lifelong anticoagulation was planned. The pain and discoloration in her hands improved on anticoagulation and had nearly resolved by the time of discharge. Given her history of arterial thrombosis, the addition of aspirin was also considered, but this decision was ultimately deferred to her outpatient rheumatologist and hematologist.
1. Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4:295-306. doi: 10.1111/j.1538-7836.2006.01753.x
2. Duarte-García A, Pham MM, Crowson CS, et al. The epidemiology of antiphospholipid syndrome: a population-based study. Arthritis Rheumatol. 2019;71:1545-1552. doi: 10.1002/art.40901
3. Levine JS, Branch DW, Rauch J. The antiphospholipid syndrome. N Engl J Med. 2002;346:752-763. doi: 10.1056/NEJMra002974
4. Cervera R, Khamashta MA, Font J, et al. Systemic lupus erythematosus: clinical and immunologic patterns of disease expression in a cohort of 1,000 patients. Medicine (Baltimore). 1993;72:113-124.
5. Love PE, Santoro SA. Antiphospholipid antibodies: anticardiolipin and the lupus anticoagulant in systemic lupus erythematosus (SLE) and in non-SLE disorders: prevalence and clinical significance. Ann Intern Med. 1990;112:682-698. doi: 10.7326/0003-4819-112-9-682
6. Merkel PA, Chang YC, Pierangeli SS, et al. The prevalence and clinical associations of anticardiolipin antibodies in a large inception cohort of patients with connective tissue diseases. Am J Med. 1996;101:576-583. doi: 10.1016/s0002-9343(96)00335-x
7. Petri M. Epidemiology of the antiphospholipid antibody syndrome. J Autoimmun. 2000;15:145-151. doi: 10.1006/jaut. 2000.0409
8. Bozlar U, Ogur T, Khaja MS, et al. CT angiography of the upper extremity arterial system: Part 2—Clinical applications beyond trauma patients. AJR Am J Roentgenol. 2013;201:753-763. doi: 10.2214/AJR.13.11208
9. Ruiz-Irastorza G, Hunt BJ, Khamashta MA. A systematic review of secondary thromboprophylaxis in patients with antiphospholipid antibodies. Arthritis Rheum. 2007;7:1487-1495. doi: 10.1002/art.23109
10. Tektonidou MG, Andreoli L, Limper M, et al. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann Rheum Dis. 2019;78:1296-1304. doi: 10.1136/annrheumdis-2019-215213
Efficacy and safety of rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome: rationale and design of the Trial on Rivaroxaban in AntiPhospholipid Syndrome (TRAPS) trial. Lupus. 2016;25:301-306. doi: 10.1177/0961203315611495
12. Schmidt-Tanguy A, Voswinkel J, Henrion D, et al. Antithrombotic effects of hydroxychloroquine in primary antiphospholipid syndrome patients. J Thromb Haemost. 2013;11:1927-1929. doi: 10.1111/jth.12363
1. Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4:295-306. doi: 10.1111/j.1538-7836.2006.01753.x
2. Duarte-García A, Pham MM, Crowson CS, et al. The epidemiology of antiphospholipid syndrome: a population-based study. Arthritis Rheumatol. 2019;71:1545-1552. doi: 10.1002/art.40901
3. Levine JS, Branch DW, Rauch J. The antiphospholipid syndrome. N Engl J Med. 2002;346:752-763. doi: 10.1056/NEJMra002974
4. Cervera R, Khamashta MA, Font J, et al. Systemic lupus erythematosus: clinical and immunologic patterns of disease expression in a cohort of 1,000 patients. Medicine (Baltimore). 1993;72:113-124.
5. Love PE, Santoro SA. Antiphospholipid antibodies: anticardiolipin and the lupus anticoagulant in systemic lupus erythematosus (SLE) and in non-SLE disorders: prevalence and clinical significance. Ann Intern Med. 1990;112:682-698. doi: 10.7326/0003-4819-112-9-682
6. Merkel PA, Chang YC, Pierangeli SS, et al. The prevalence and clinical associations of anticardiolipin antibodies in a large inception cohort of patients with connective tissue diseases. Am J Med. 1996;101:576-583. doi: 10.1016/s0002-9343(96)00335-x
7. Petri M. Epidemiology of the antiphospholipid antibody syndrome. J Autoimmun. 2000;15:145-151. doi: 10.1006/jaut. 2000.0409
8. Bozlar U, Ogur T, Khaja MS, et al. CT angiography of the upper extremity arterial system: Part 2—Clinical applications beyond trauma patients. AJR Am J Roentgenol. 2013;201:753-763. doi: 10.2214/AJR.13.11208
9. Ruiz-Irastorza G, Hunt BJ, Khamashta MA. A systematic review of secondary thromboprophylaxis in patients with antiphospholipid antibodies. Arthritis Rheum. 2007;7:1487-1495. doi: 10.1002/art.23109
10. Tektonidou MG, Andreoli L, Limper M, et al. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann Rheum Dis. 2019;78:1296-1304. doi: 10.1136/annrheumdis-2019-215213
Efficacy and safety of rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome: rationale and design of the Trial on Rivaroxaban in AntiPhospholipid Syndrome (TRAPS) trial. Lupus. 2016;25:301-306. doi: 10.1177/0961203315611495
12. Schmidt-Tanguy A, Voswinkel J, Henrion D, et al. Antithrombotic effects of hydroxychloroquine in primary antiphospholipid syndrome patients. J Thromb Haemost. 2013;11:1927-1929. doi: 10.1111/jth.12363
How to help pediatricians apply peanut allergy guidelines
Despite the profound shift in guidelines for preventing peanut allergies in infants after the landmark LEAP study, national surveys in 2021 showed that 70% of parents and caregivers said that they hadn’t heard the new recommendations, and fewer than one-third of pediatricians were following them.
Now, in a 5-year National Institutes of Health–funded study called iREACH, researchers are testing whether a two-part intervention, which includes training videos and a clinical decision support tool, helps pediatricians follow the guidelines and ultimately reduces peanut allergy.
Early results from iREACH, presented at the American Academy of Allergy, Asthma, and Immunology 2023 annual meeting in San Antonio, showed mixed results with a sharp rise in clinician knowledge of the guidelines but only a modest increase in their real-world implementation with high-risk infants.
Raising a food-allergic child while working as a pediatrician herself, Ruchi Gupta, MD, MPH, director of the Center for Food Allergy and Asthma Research at Northwestern University, Chicago, understands the importance and challenge of translating published findings into practice.
During a typical 4- to 6-month well-child visit, pediatricians must check the baby’s growth, perform a physical exam, discuss milestones, field questions about sleep and poop and colic and – if they’re up on the latest guidelines – explain why it’s important to feed peanuts early and often.
“Pediatricians get stuff from every single specialty, and guidelines are always changing,” she told this news organization.
The current feeding guidelines, published in 2017 after the landmark LEAP study, switched from “ ‘don’t introduce peanuts until age 3’ to ‘introduce peanuts now,’ ” said Dr. Gupta.
But the recommendations aren’t entirely straightforward. They require pediatricians to make an assessment when the baby is around 4 months old. If the child is high-risk (has severe eczema or an egg allergy), they need a peanut-specific immunoglobulin E (IgE) test. If the test is negative, the pediatrician should encourage peanut introduction. If positive, they should refer the child to an allergist.
“It’s a little complicated,” Dr. Gupta said.
To boost understanding and adherence, Dr. Gupta’s team created the intervention tested in the iREACH study. It includes a set of training videos, a clinical decision support tool that embeds into the electronic health record (EHR) with pop-ups reminding the physician to discuss early introduction, menus for ordering peanut IgE tests or referring to an allergist if needed, and a caregiver handout that explains how to add peanuts to the baby’s diet. (These resources can be found here.)
The study enrolled 290 pediatric clinicians at 30 local practices, examining 18,460 babies from diverse backgrounds, about one-quarter of whom were from families on public insurance. About half of the clinicians received the intervention, whereas the other half served as the control arm.
The training videos seemed effective. Clinicians’ knowledge of the guidelines rose from 72.6% at baseline to 94.5% after the intervention, and their ability to identify severe eczema went up from 63.4% to 97.6%. This translated to 70.4% success with applying the guidelines when presented various clinical scenarios, up from 29% at baseline. These results are in press at JAMA Network Open.
The next set of analyses, preliminary and unpublished, monitored real-world adherence using natural language processing to pull EHR data from 4- and 6-month well-check visits. It was “AI [artificial intelligence] for notes,” Dr. Gupta said.
For low-risk infants, the training and EHR-embedded support tool greatly improved clinician adherence. Eighty percent of clinicians in the intervention arm followed the guidelines, compared with 26% in the control group.
In high-risk infants, the impact was much weaker. Even after the video-based training, only 17% of pediatric clinicians followed the guidelines – that is, ordered a peanut IgE test or referred to an allergist – compared with 8% in the control group.
Why such a low uptake?
Pediatricians are time-pressed. “How do you add [early introduction] to the other 10 or 15 things you want to talk to a parent about at the 4-month visit?” said Jonathan Necheles, MD, MPH, a pediatrician at Children’s Healthcare Associates in Chicago.
It can also be hard to tell if a baby’s eczema is “severe” or “mild to moderate.” The EHR-integrated support tool included a scorecard for judging eczema severity across a range of skin tones. The condition can be hard to recognize in patients of color. “You don’t get the redness in the same way,” said Dr. Necheles, who worked with Dr. Gupta to develop the iREACH intervention.
Curiously, even though the AI analysis found that less than one-fifth of pediatricians put the guidelines into action for high-risk infants, 69% of them recommended peanut introduction.
One interpretation is that busy pediatricians may be “doing the minimum” – introducing the concept of early introduction and telling parents to try it “but not giving any additional sort of guidance as far as who’s high risk, who’s low risk, who should see the allergist, who should get screened,” said Edwin Kim, MD, allergist-immunologist and director of the Food Allergy Initiative at the University of North Carolina at Chapel Hill.
The ultimate impact of iREACH has yet to be seen. “The end goal is, if pediatricians recommend, will parents follow, and will we reduce peanut allergy?” Dr. Gupta said.
Dr. Gupta consults or serves as an advisor for Genentech, Novartis, Aimmune, Allergenis, and Food Allergy Research & Education; receives research funding from Novartis, Genentech, FARE, Melchiorre Family Foundation, and Sunshine Charitable Foundation; and reports ownership interest from Yobee Care. Dr. Necheles reports no financial disclosures. Dr. Kim reports consultancy with Allergy Therapeutics, Belhaven Biopharma, Duke Clinical Research Institute, Genentech, Nutricia, and Revolo; advisory board membership with ALK, Kenota Health, and Ukko; and grant support from the National Institute of Allergy and Infectious Diseases, Immune Tolerance Network, and Food Allergy Research and Education.
A version of this article first appeared on Medscape.com.
Despite the profound shift in guidelines for preventing peanut allergies in infants after the landmark LEAP study, national surveys in 2021 showed that 70% of parents and caregivers said that they hadn’t heard the new recommendations, and fewer than one-third of pediatricians were following them.
Now, in a 5-year National Institutes of Health–funded study called iREACH, researchers are testing whether a two-part intervention, which includes training videos and a clinical decision support tool, helps pediatricians follow the guidelines and ultimately reduces peanut allergy.
Early results from iREACH, presented at the American Academy of Allergy, Asthma, and Immunology 2023 annual meeting in San Antonio, showed mixed results with a sharp rise in clinician knowledge of the guidelines but only a modest increase in their real-world implementation with high-risk infants.
Raising a food-allergic child while working as a pediatrician herself, Ruchi Gupta, MD, MPH, director of the Center for Food Allergy and Asthma Research at Northwestern University, Chicago, understands the importance and challenge of translating published findings into practice.
During a typical 4- to 6-month well-child visit, pediatricians must check the baby’s growth, perform a physical exam, discuss milestones, field questions about sleep and poop and colic and – if they’re up on the latest guidelines – explain why it’s important to feed peanuts early and often.
“Pediatricians get stuff from every single specialty, and guidelines are always changing,” she told this news organization.
The current feeding guidelines, published in 2017 after the landmark LEAP study, switched from “ ‘don’t introduce peanuts until age 3’ to ‘introduce peanuts now,’ ” said Dr. Gupta.
But the recommendations aren’t entirely straightforward. They require pediatricians to make an assessment when the baby is around 4 months old. If the child is high-risk (has severe eczema or an egg allergy), they need a peanut-specific immunoglobulin E (IgE) test. If the test is negative, the pediatrician should encourage peanut introduction. If positive, they should refer the child to an allergist.
“It’s a little complicated,” Dr. Gupta said.
To boost understanding and adherence, Dr. Gupta’s team created the intervention tested in the iREACH study. It includes a set of training videos, a clinical decision support tool that embeds into the electronic health record (EHR) with pop-ups reminding the physician to discuss early introduction, menus for ordering peanut IgE tests or referring to an allergist if needed, and a caregiver handout that explains how to add peanuts to the baby’s diet. (These resources can be found here.)
The study enrolled 290 pediatric clinicians at 30 local practices, examining 18,460 babies from diverse backgrounds, about one-quarter of whom were from families on public insurance. About half of the clinicians received the intervention, whereas the other half served as the control arm.
The training videos seemed effective. Clinicians’ knowledge of the guidelines rose from 72.6% at baseline to 94.5% after the intervention, and their ability to identify severe eczema went up from 63.4% to 97.6%. This translated to 70.4% success with applying the guidelines when presented various clinical scenarios, up from 29% at baseline. These results are in press at JAMA Network Open.
The next set of analyses, preliminary and unpublished, monitored real-world adherence using natural language processing to pull EHR data from 4- and 6-month well-check visits. It was “AI [artificial intelligence] for notes,” Dr. Gupta said.
For low-risk infants, the training and EHR-embedded support tool greatly improved clinician adherence. Eighty percent of clinicians in the intervention arm followed the guidelines, compared with 26% in the control group.
In high-risk infants, the impact was much weaker. Even after the video-based training, only 17% of pediatric clinicians followed the guidelines – that is, ordered a peanut IgE test or referred to an allergist – compared with 8% in the control group.
Why such a low uptake?
Pediatricians are time-pressed. “How do you add [early introduction] to the other 10 or 15 things you want to talk to a parent about at the 4-month visit?” said Jonathan Necheles, MD, MPH, a pediatrician at Children’s Healthcare Associates in Chicago.
It can also be hard to tell if a baby’s eczema is “severe” or “mild to moderate.” The EHR-integrated support tool included a scorecard for judging eczema severity across a range of skin tones. The condition can be hard to recognize in patients of color. “You don’t get the redness in the same way,” said Dr. Necheles, who worked with Dr. Gupta to develop the iREACH intervention.
Curiously, even though the AI analysis found that less than one-fifth of pediatricians put the guidelines into action for high-risk infants, 69% of them recommended peanut introduction.
One interpretation is that busy pediatricians may be “doing the minimum” – introducing the concept of early introduction and telling parents to try it “but not giving any additional sort of guidance as far as who’s high risk, who’s low risk, who should see the allergist, who should get screened,” said Edwin Kim, MD, allergist-immunologist and director of the Food Allergy Initiative at the University of North Carolina at Chapel Hill.
The ultimate impact of iREACH has yet to be seen. “The end goal is, if pediatricians recommend, will parents follow, and will we reduce peanut allergy?” Dr. Gupta said.
Dr. Gupta consults or serves as an advisor for Genentech, Novartis, Aimmune, Allergenis, and Food Allergy Research & Education; receives research funding from Novartis, Genentech, FARE, Melchiorre Family Foundation, and Sunshine Charitable Foundation; and reports ownership interest from Yobee Care. Dr. Necheles reports no financial disclosures. Dr. Kim reports consultancy with Allergy Therapeutics, Belhaven Biopharma, Duke Clinical Research Institute, Genentech, Nutricia, and Revolo; advisory board membership with ALK, Kenota Health, and Ukko; and grant support from the National Institute of Allergy and Infectious Diseases, Immune Tolerance Network, and Food Allergy Research and Education.
A version of this article first appeared on Medscape.com.
Despite the profound shift in guidelines for preventing peanut allergies in infants after the landmark LEAP study, national surveys in 2021 showed that 70% of parents and caregivers said that they hadn’t heard the new recommendations, and fewer than one-third of pediatricians were following them.
Now, in a 5-year National Institutes of Health–funded study called iREACH, researchers are testing whether a two-part intervention, which includes training videos and a clinical decision support tool, helps pediatricians follow the guidelines and ultimately reduces peanut allergy.
Early results from iREACH, presented at the American Academy of Allergy, Asthma, and Immunology 2023 annual meeting in San Antonio, showed mixed results with a sharp rise in clinician knowledge of the guidelines but only a modest increase in their real-world implementation with high-risk infants.
Raising a food-allergic child while working as a pediatrician herself, Ruchi Gupta, MD, MPH, director of the Center for Food Allergy and Asthma Research at Northwestern University, Chicago, understands the importance and challenge of translating published findings into practice.
During a typical 4- to 6-month well-child visit, pediatricians must check the baby’s growth, perform a physical exam, discuss milestones, field questions about sleep and poop and colic and – if they’re up on the latest guidelines – explain why it’s important to feed peanuts early and often.
“Pediatricians get stuff from every single specialty, and guidelines are always changing,” she told this news organization.
The current feeding guidelines, published in 2017 after the landmark LEAP study, switched from “ ‘don’t introduce peanuts until age 3’ to ‘introduce peanuts now,’ ” said Dr. Gupta.
But the recommendations aren’t entirely straightforward. They require pediatricians to make an assessment when the baby is around 4 months old. If the child is high-risk (has severe eczema or an egg allergy), they need a peanut-specific immunoglobulin E (IgE) test. If the test is negative, the pediatrician should encourage peanut introduction. If positive, they should refer the child to an allergist.
“It’s a little complicated,” Dr. Gupta said.
To boost understanding and adherence, Dr. Gupta’s team created the intervention tested in the iREACH study. It includes a set of training videos, a clinical decision support tool that embeds into the electronic health record (EHR) with pop-ups reminding the physician to discuss early introduction, menus for ordering peanut IgE tests or referring to an allergist if needed, and a caregiver handout that explains how to add peanuts to the baby’s diet. (These resources can be found here.)
The study enrolled 290 pediatric clinicians at 30 local practices, examining 18,460 babies from diverse backgrounds, about one-quarter of whom were from families on public insurance. About half of the clinicians received the intervention, whereas the other half served as the control arm.
The training videos seemed effective. Clinicians’ knowledge of the guidelines rose from 72.6% at baseline to 94.5% after the intervention, and their ability to identify severe eczema went up from 63.4% to 97.6%. This translated to 70.4% success with applying the guidelines when presented various clinical scenarios, up from 29% at baseline. These results are in press at JAMA Network Open.
The next set of analyses, preliminary and unpublished, monitored real-world adherence using natural language processing to pull EHR data from 4- and 6-month well-check visits. It was “AI [artificial intelligence] for notes,” Dr. Gupta said.
For low-risk infants, the training and EHR-embedded support tool greatly improved clinician adherence. Eighty percent of clinicians in the intervention arm followed the guidelines, compared with 26% in the control group.
In high-risk infants, the impact was much weaker. Even after the video-based training, only 17% of pediatric clinicians followed the guidelines – that is, ordered a peanut IgE test or referred to an allergist – compared with 8% in the control group.
Why such a low uptake?
Pediatricians are time-pressed. “How do you add [early introduction] to the other 10 or 15 things you want to talk to a parent about at the 4-month visit?” said Jonathan Necheles, MD, MPH, a pediatrician at Children’s Healthcare Associates in Chicago.
It can also be hard to tell if a baby’s eczema is “severe” or “mild to moderate.” The EHR-integrated support tool included a scorecard for judging eczema severity across a range of skin tones. The condition can be hard to recognize in patients of color. “You don’t get the redness in the same way,” said Dr. Necheles, who worked with Dr. Gupta to develop the iREACH intervention.
Curiously, even though the AI analysis found that less than one-fifth of pediatricians put the guidelines into action for high-risk infants, 69% of them recommended peanut introduction.
One interpretation is that busy pediatricians may be “doing the minimum” – introducing the concept of early introduction and telling parents to try it “but not giving any additional sort of guidance as far as who’s high risk, who’s low risk, who should see the allergist, who should get screened,” said Edwin Kim, MD, allergist-immunologist and director of the Food Allergy Initiative at the University of North Carolina at Chapel Hill.
The ultimate impact of iREACH has yet to be seen. “The end goal is, if pediatricians recommend, will parents follow, and will we reduce peanut allergy?” Dr. Gupta said.
Dr. Gupta consults or serves as an advisor for Genentech, Novartis, Aimmune, Allergenis, and Food Allergy Research & Education; receives research funding from Novartis, Genentech, FARE, Melchiorre Family Foundation, and Sunshine Charitable Foundation; and reports ownership interest from Yobee Care. Dr. Necheles reports no financial disclosures. Dr. Kim reports consultancy with Allergy Therapeutics, Belhaven Biopharma, Duke Clinical Research Institute, Genentech, Nutricia, and Revolo; advisory board membership with ALK, Kenota Health, and Ukko; and grant support from the National Institute of Allergy and Infectious Diseases, Immune Tolerance Network, and Food Allergy Research and Education.
A version of this article first appeared on Medscape.com.
FROM AAAAI 2023
New data strengthen case for oral immunotherapy in tots
The buzz surrounding OIT – which involves ingesting daily doses of the culprit food to raise the threshold that would trigger a reaction – grew with the approval, by the U.S. Food and Drug Administration of Palforzia, of the peanut OIT pill in January 2020. Yet many allergists remained wary about the treatment, a monthslong regimen that can itself trigger allergic reactions.
Now, accumulating research points to “a possible window of opportunity early in life, less than 3 years of age, for more successful disease remission,” Justin Schwartz, MD, PhD, an allergist at Cincinnati Children’s Hospital, told a crowd at the annual meeting of the American Academy of Allergy, Asthma, & Immunology.
His presentation about OIT in toddlers kicked off a 3-hour clinical practice course, one of several dozen conference offerings highlighting this emerging approach.
Several AAAAI posters add to prior studies (for example, DEVIL and IMPACT) suggesting that OIT proceeds more smoothly and faster during a child’s earliest years – a season fraught with accidental exposures and reactions.
One poster described a retrospective study of 73 children younger than 4 years who underwent OIT at the Cleveland Clinic Food Allergy Center. Sixty-four were treated for peanut allergies, and seven patients received OIT for multiple foods, including tree nuts, milk, wheat, and sesame.
Of the 80 total OIT courses, 76 (95%) reached maintenance – meaning the child tolerated a small amount (for example, 1-2 peanuts) without reacting – in a median of 104 days (~3.4 months).
That is “quite impressive,” said allergist Hugh Windom, MD, whose clinic in Sarasota, Fla., has offered OIT since 2012.
Older children typically have 80%-90% success and take longer (6-8 months) to reach maintenance because of busier schedules and reactions that slow them down, he said. In his clinic’s larger retrospective analysis of preschool-aged OIT patients, presented at the 2022 AAAAI meeting, 89% of patients with peanut allergies and 72% of children with multiple food allergies achieved maintenance.
In the Cleveland Clinic study, children with favorable lab test results after receiving the maintenance dose for 6 months were offered an oral food challenge. Of 24 patients who completed the challenge, 75% “passed with a normal serving size of the treated food (for example, two tablespoons of peanut butter),” Sarah Johnson, MD, lead author and Cleveland Clinic allergy/immunology fellow, said in an interview.
Plus, OIT seemed safer for toddlers. Although 41% of the children had reactions during clinic updosing and 48% had reactions at home, only ~3% of toddler OIT courses required epinephrine. By comparison, ~11% of treatments required epinephrine in a large OIT study of older children.
When a child reacts, “you might keep them on the dose or go a little slower,” said Johnson, who worked with allergist Jaclyn Bjelac, MD, on the study. These setbacks occurred less frequently in toddlers, allowing their OIT to “go a lot faster” than in older children. And so far, Dr. Johnson said, none of the toddlers have shown signs of eosinophilic esophagitis, a rare complication that can develop during OIT.
A smaller analysis of real-world outcomes in an academic clinical setting also found that OIT was well tolerated at very young ages. Since 2020, this ongoing study at UVA Children’s Hospital in Charlottesville has enrolled 22 peanut-allergic children (aged 6 months to 3 years) for OIT. Three patients have dropped out, four are in the buildup phrase, and 15 have reached maintenance dosing. None have reported having to use epinephrine.
Three patients have completed 1 year of maintenance therapy, and another patient accidentally consumed ~3,000 mg of peanut protein (equivalent to ~10 peanuts) after 5 months of maintenance. All four “now incorporate peanut into their diets ad lib,” according to lead author and allergist Jonathan Hemler, MD, who directs the UVA pediatric food allergy program.
These findings are “really reassuring – because even if you may not offer OIT, you’re still going to get questions about it,” said Ama Alexis, MD, an allergist/immunologist in private practice in New York and a clinical assistant professor at NYU Grossmann School of Medicine, commenting on the Cleveland Clinic study.
“It’s great that we’re hearing and seeing so much about OIT,” she added. While training as an allergy/immunology fellow 15 years ago, many saw the treatment as dangerous – “an absolute no-no,” she said.
The AAAAI still considers OIT “investigational,” yet this year’s annual meeting featured 22 posters – plus a course, workshop, seminar, and oral abstract session – on the approach.
The “thought process has shifted,” Dr. Alexis said. “It’s good to see all these numbers, these results. I think once you’re comfortable, you should embrace new therapies.”
Dr. Schwartz has consulted for Shire/Takeda and has received research funding from Knopp Biosciences. Dr. Alexis consults for AbbVie, serves on advisory boards for Jansen and Eli Lilli, and is a member of Pfizer’s advisory board and speaker’s bureau. Dr. Johnson, Dr. Windom, and Dr. Hemler report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The buzz surrounding OIT – which involves ingesting daily doses of the culprit food to raise the threshold that would trigger a reaction – grew with the approval, by the U.S. Food and Drug Administration of Palforzia, of the peanut OIT pill in January 2020. Yet many allergists remained wary about the treatment, a monthslong regimen that can itself trigger allergic reactions.
Now, accumulating research points to “a possible window of opportunity early in life, less than 3 years of age, for more successful disease remission,” Justin Schwartz, MD, PhD, an allergist at Cincinnati Children’s Hospital, told a crowd at the annual meeting of the American Academy of Allergy, Asthma, & Immunology.
His presentation about OIT in toddlers kicked off a 3-hour clinical practice course, one of several dozen conference offerings highlighting this emerging approach.
Several AAAAI posters add to prior studies (for example, DEVIL and IMPACT) suggesting that OIT proceeds more smoothly and faster during a child’s earliest years – a season fraught with accidental exposures and reactions.
One poster described a retrospective study of 73 children younger than 4 years who underwent OIT at the Cleveland Clinic Food Allergy Center. Sixty-four were treated for peanut allergies, and seven patients received OIT for multiple foods, including tree nuts, milk, wheat, and sesame.
Of the 80 total OIT courses, 76 (95%) reached maintenance – meaning the child tolerated a small amount (for example, 1-2 peanuts) without reacting – in a median of 104 days (~3.4 months).
That is “quite impressive,” said allergist Hugh Windom, MD, whose clinic in Sarasota, Fla., has offered OIT since 2012.
Older children typically have 80%-90% success and take longer (6-8 months) to reach maintenance because of busier schedules and reactions that slow them down, he said. In his clinic’s larger retrospective analysis of preschool-aged OIT patients, presented at the 2022 AAAAI meeting, 89% of patients with peanut allergies and 72% of children with multiple food allergies achieved maintenance.
In the Cleveland Clinic study, children with favorable lab test results after receiving the maintenance dose for 6 months were offered an oral food challenge. Of 24 patients who completed the challenge, 75% “passed with a normal serving size of the treated food (for example, two tablespoons of peanut butter),” Sarah Johnson, MD, lead author and Cleveland Clinic allergy/immunology fellow, said in an interview.
Plus, OIT seemed safer for toddlers. Although 41% of the children had reactions during clinic updosing and 48% had reactions at home, only ~3% of toddler OIT courses required epinephrine. By comparison, ~11% of treatments required epinephrine in a large OIT study of older children.
When a child reacts, “you might keep them on the dose or go a little slower,” said Johnson, who worked with allergist Jaclyn Bjelac, MD, on the study. These setbacks occurred less frequently in toddlers, allowing their OIT to “go a lot faster” than in older children. And so far, Dr. Johnson said, none of the toddlers have shown signs of eosinophilic esophagitis, a rare complication that can develop during OIT.
A smaller analysis of real-world outcomes in an academic clinical setting also found that OIT was well tolerated at very young ages. Since 2020, this ongoing study at UVA Children’s Hospital in Charlottesville has enrolled 22 peanut-allergic children (aged 6 months to 3 years) for OIT. Three patients have dropped out, four are in the buildup phrase, and 15 have reached maintenance dosing. None have reported having to use epinephrine.
Three patients have completed 1 year of maintenance therapy, and another patient accidentally consumed ~3,000 mg of peanut protein (equivalent to ~10 peanuts) after 5 months of maintenance. All four “now incorporate peanut into their diets ad lib,” according to lead author and allergist Jonathan Hemler, MD, who directs the UVA pediatric food allergy program.
These findings are “really reassuring – because even if you may not offer OIT, you’re still going to get questions about it,” said Ama Alexis, MD, an allergist/immunologist in private practice in New York and a clinical assistant professor at NYU Grossmann School of Medicine, commenting on the Cleveland Clinic study.
“It’s great that we’re hearing and seeing so much about OIT,” she added. While training as an allergy/immunology fellow 15 years ago, many saw the treatment as dangerous – “an absolute no-no,” she said.
The AAAAI still considers OIT “investigational,” yet this year’s annual meeting featured 22 posters – plus a course, workshop, seminar, and oral abstract session – on the approach.
The “thought process has shifted,” Dr. Alexis said. “It’s good to see all these numbers, these results. I think once you’re comfortable, you should embrace new therapies.”
Dr. Schwartz has consulted for Shire/Takeda and has received research funding from Knopp Biosciences. Dr. Alexis consults for AbbVie, serves on advisory boards for Jansen and Eli Lilli, and is a member of Pfizer’s advisory board and speaker’s bureau. Dr. Johnson, Dr. Windom, and Dr. Hemler report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The buzz surrounding OIT – which involves ingesting daily doses of the culprit food to raise the threshold that would trigger a reaction – grew with the approval, by the U.S. Food and Drug Administration of Palforzia, of the peanut OIT pill in January 2020. Yet many allergists remained wary about the treatment, a monthslong regimen that can itself trigger allergic reactions.
Now, accumulating research points to “a possible window of opportunity early in life, less than 3 years of age, for more successful disease remission,” Justin Schwartz, MD, PhD, an allergist at Cincinnati Children’s Hospital, told a crowd at the annual meeting of the American Academy of Allergy, Asthma, & Immunology.
His presentation about OIT in toddlers kicked off a 3-hour clinical practice course, one of several dozen conference offerings highlighting this emerging approach.
Several AAAAI posters add to prior studies (for example, DEVIL and IMPACT) suggesting that OIT proceeds more smoothly and faster during a child’s earliest years – a season fraught with accidental exposures and reactions.
One poster described a retrospective study of 73 children younger than 4 years who underwent OIT at the Cleveland Clinic Food Allergy Center. Sixty-four were treated for peanut allergies, and seven patients received OIT for multiple foods, including tree nuts, milk, wheat, and sesame.
Of the 80 total OIT courses, 76 (95%) reached maintenance – meaning the child tolerated a small amount (for example, 1-2 peanuts) without reacting – in a median of 104 days (~3.4 months).
That is “quite impressive,” said allergist Hugh Windom, MD, whose clinic in Sarasota, Fla., has offered OIT since 2012.
Older children typically have 80%-90% success and take longer (6-8 months) to reach maintenance because of busier schedules and reactions that slow them down, he said. In his clinic’s larger retrospective analysis of preschool-aged OIT patients, presented at the 2022 AAAAI meeting, 89% of patients with peanut allergies and 72% of children with multiple food allergies achieved maintenance.
In the Cleveland Clinic study, children with favorable lab test results after receiving the maintenance dose for 6 months were offered an oral food challenge. Of 24 patients who completed the challenge, 75% “passed with a normal serving size of the treated food (for example, two tablespoons of peanut butter),” Sarah Johnson, MD, lead author and Cleveland Clinic allergy/immunology fellow, said in an interview.
Plus, OIT seemed safer for toddlers. Although 41% of the children had reactions during clinic updosing and 48% had reactions at home, only ~3% of toddler OIT courses required epinephrine. By comparison, ~11% of treatments required epinephrine in a large OIT study of older children.
When a child reacts, “you might keep them on the dose or go a little slower,” said Johnson, who worked with allergist Jaclyn Bjelac, MD, on the study. These setbacks occurred less frequently in toddlers, allowing their OIT to “go a lot faster” than in older children. And so far, Dr. Johnson said, none of the toddlers have shown signs of eosinophilic esophagitis, a rare complication that can develop during OIT.
A smaller analysis of real-world outcomes in an academic clinical setting also found that OIT was well tolerated at very young ages. Since 2020, this ongoing study at UVA Children’s Hospital in Charlottesville has enrolled 22 peanut-allergic children (aged 6 months to 3 years) for OIT. Three patients have dropped out, four are in the buildup phrase, and 15 have reached maintenance dosing. None have reported having to use epinephrine.
Three patients have completed 1 year of maintenance therapy, and another patient accidentally consumed ~3,000 mg of peanut protein (equivalent to ~10 peanuts) after 5 months of maintenance. All four “now incorporate peanut into their diets ad lib,” according to lead author and allergist Jonathan Hemler, MD, who directs the UVA pediatric food allergy program.
These findings are “really reassuring – because even if you may not offer OIT, you’re still going to get questions about it,” said Ama Alexis, MD, an allergist/immunologist in private practice in New York and a clinical assistant professor at NYU Grossmann School of Medicine, commenting on the Cleveland Clinic study.
“It’s great that we’re hearing and seeing so much about OIT,” she added. While training as an allergy/immunology fellow 15 years ago, many saw the treatment as dangerous – “an absolute no-no,” she said.
The AAAAI still considers OIT “investigational,” yet this year’s annual meeting featured 22 posters – plus a course, workshop, seminar, and oral abstract session – on the approach.
The “thought process has shifted,” Dr. Alexis said. “It’s good to see all these numbers, these results. I think once you’re comfortable, you should embrace new therapies.”
Dr. Schwartz has consulted for Shire/Takeda and has received research funding from Knopp Biosciences. Dr. Alexis consults for AbbVie, serves on advisory boards for Jansen and Eli Lilli, and is a member of Pfizer’s advisory board and speaker’s bureau. Dr. Johnson, Dr. Windom, and Dr. Hemler report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AAAAI 2023
Toxic chemicals we consume without knowing it
Life expectancy is falling precipitously. Three-fourths of Americans are overweight or obese, half have diabetes or prediabetes, and a majority are metabolically unhealthy. Furthermore, the rates of allergic, inflammatory, and autoimmune diseases are rising at rates of 3%-9% per year in the West, far faster than the speed of genetic change in this population.
Of course, diet and lifestyle are major factors behind such trends, but a grossly underappreciated driver in what ails us is the role of environmental toxins and endocrine-disrupting chemicals. In years past, these factors have largely evaded the traditional Western medical establishment; however, mounting evidence now supports their significance in fertility, metabolic health, and cancer.
Although several industrial chemicals and toxins have been identified as carcinogens and have subsequently been regulated, many more remain persistent in the environment and continue to be freely used. It is therefore incumbent upon both the general public and clinicians to be knowledgeable about these exposures. Here, we review some of the most common exposures and the substantial health risks associated with them, along with some general guidance around best practices for how to minimize exposure.
Microplastics
“Microplastics” is a term used to describe small fragments or particles of plastic breakdown or microbeads from household or personal care products, measuring less than 5 mm in length.
Plastic waste is accumulating at alarming and devastating proportions – by 2050, it is estimated that by weight, there will be more plastic than fish in the oceans. That translates into hundreds of thousands of tons of microplastics and trillions of these particles in the seas. A recent study demonstrated that microplastics were present in the bloodstream in the majority of 22 otherwise healthy participants.
Since the 1950s, plastic exposure has been shown to promote tumorigenesis in animal studies, and in vitro studies have demonstrated the toxicity of microplastics at the cellular level. However, it is not well known whether the plastic itself is toxic or if it simply serves as a carrier for other environmental toxins to bioaccumulate.
According to Tasha Stoiber, a senior scientist at the Environmental Working Group, “Microplastics have been widely detected in fish and seafood, as well as other products like bottled water, beer, honey, and tap water.” The EWG states there are no formal advisories on fish consumption to avoid exposure to microplastics at the moment.
Pressure also is mounting for a ban on microbeads in personal care products.
Until such bans are put in place, it is advised to avoid single-use plastics, favor reusable tote bags for grocery shopping rather than plastic bags, and opt for loose leaf tea or paper tea bags rather than mesh-based alternatives.
Phthalates
Phthalates are chemicals used to make plastics soft and durable, as well as to bind fragrances. They are commonly found in household items such as vinyl (for example, flooring, shower curtains) and fragrances, air fresheners, and perfumes.
Phthalates are known hormone-disrupting chemicals, exposure to which has been associated with abnormal sexual and brain development in children, as well as lower levels of testosterone in men. Exposures are thought to occur via inhalation, ingestion, and skin contact; however, fasting studies demonstrate that a majority of exposure is probably food related.
To avoid phthalate exposures, recommendations include avoiding polyvinyl chloride plastics (particularly food containers, plastic wrap, and children’s toys), which are identifiable by the recycle code number 3, as well as air fresheners and fragranced products.
The EWG’s Skin Deep database provides an important resource on phthalate-free personal care products.
Despite pressure from consumer advocacy groups, the U.S. Food and Drug Administration has not yet banned phthalates in food packaging.
Bisphenol A (BPA)
BPA is a chemical additive used to make clear and hard polycarbonate plastics, as well as epoxy and thermal papers. BPA is one of the highest-volume chemicals, with roughly 6 billion pounds produced each year. BPA is traditionally found in many clear plastic bottles and sippy cups, as well as in the lining of canned foods.
Structurally, BPA acts as an estrogen mimetic and has been associated with cardiovascular disease, obesity, and male sexual dysfunction. Since 2012, BPA has been banned in sippy cups and baby bottles, but there is some debate as to whether its replacements (bisphenol S and bisphenol F) are any safer; they appear to have similar hormonal effects as BPA.
As with phthalates, the majority of ingestion is thought to be food related. BPA has been found in more than 90% of a representative study population in the United States.
Guidance advises avoiding polycarbonate plastics (identifiable with the recycling code number 7), as well as avoiding handling thermal papers such as tickets and receipts, if possible. Food and beverages should be stored in glass or stainless steel. If plastic must be used, opt for polycarbonate- and polyvinyl chloride–free plastics, and food and beverages should never be reheated in plastic containers or wrapping. Canned foods should ideally be avoided, particularly canned tunas and condensed soups. If canned products are bought, they should ideally be BPA free.
Dioxins and polychlorinated biphenyls (PCBs)
Dioxins are mainly the byproducts of industrial practices; they are released after incineration, trash burning, and fires. PCBs, which are somewhat structurally related to dioxins, were previously found in products such as flame retardants and coolants. Dioxins and PCBs are often grouped in the same category under the umbrella term “persistent organic pollutants” because they break down slowly and remain in the environment even after emissions have been curbed.
Tetrachlorodibenzodioxin, perhaps the best-known dioxin, is a known carcinogen. Dioxins also have been associated with a host of health implications in development, immunity, and reproductive and endocrine systems. Higher levels of PCB exposure have also been associated with an increased risk for mortality from cardiovascular disease.
Notably, dioxin emissions have been reduced by 90% since the 1980s, and the U.S. Environmental Protection Agency has banned the use of PCBs in industrial manufacturing since 1979. However, environmental dioxins and PCBs still enter the food chain and accumulate in fat.
The best ways to avoid exposures are through limiting meat, fish, and dairy consumption and trimming the skin and fat from meats. The level of dioxins and PCBs found in meat, eggs, fish, and dairy are approximately 5-10 times higher than they are in plant-based foods. Research has shown that farmed salmon is likely to be the most PCB-contaminated protein source in the U.S. diet; however, newer forms of land-based and sustainable aquaculture probably avoid this exposure.
Pesticides
The growth of modern monoculture agriculture in the United States over the past century has coincided with a dramatic surge in the use of industrial pesticides. In fact, over 90% of the U.S. population have pesticides in their urine and blood, regardless of where they live. Exposures are thought to be food related.
Approximately 1 billion pounds of pesticides are used annually in the United States, including nearly 300 million pounds of glyphosate, which has been identified as a probable carcinogen by European agencies. The EPA has not yet reached this conclusion, although the matter is currently being litigated.
A large European prospective cohort trial demonstrated a lower risk for cancer in those with a greater frequency of self-reported organic food consumption. In addition to cancer risk, relatively elevated blood levels of a pesticide known as beta-hexachlorocyclohexane (B-HCH) are associated with higher all-cause mortality. Also, exposure to DDE – a metabolite of DDT, a chlorinated pesticide heavily used in the 1940s-1960s that still persists in the environment today – has been shown to increase the risk for Alzheimer’s-type dementia as well as overall cognitive decline.
Because these chlorinated pesticides are often fat soluble, they seem to accumulate in animal products. Therefore, people consuming a vegetarian diet have been found to have lower levels of B-HCH. This has led to the recommendation that consumers of produce should favor organic over conventional, if possible. Here too, the EWG provides an important resource to consumers in the form of shopper guides regarding pesticides in produce.
Per- and polyfluoroalkyl substances (PFAS)
PFAS are a group of fluorinated compounds discovered in the 1930s. Their chemical composition includes a durable carbon-fluoride bond, giving them a persistence within the environment that has led to their being referred to as “forever chemicals.”
PFAS have been detected in the blood of 98% of Americans, and in the rainwater of locations as far afield as Tibet and Antarctica. Even low levels of exposure have been associated with an increased risk for cancer, liver disease, low birth weight, and hormonal disruption.
The properties of PFAS also make them both durable at very high heat and water repellent. Notoriously, the chemical was used by 3M to make Scotchgard for carpets and fabrics and by Dupont to make Teflon for nonstick coating of pots and pans. Although perfluorooctanoic acid (PFOA) was removed from nonstick cookware in 2013, PFAS – a family of thousands of synthetic compounds – remain common in fast-food packaging, water- and stain-repellent clothing, firefighting foam, and personal care products. PFAS are released into the environment during the breakdown of these consumer and industrial products, as well as from dumping from waste facilities.
Alarmingly, the EWG notes that up to 200 million Americans may be exposed to PFAS in their drinking water. In March 2021, the EPA announced that they will be regulating PFAS in drinking water; however, the regulations have not been finalized. Currently, it is up to individual states to test for its presence in the water. The EWG has compiled a map of all known PFAS contamination sites.
To avoid or prevent exposures from PFAS, recommendations include filtering tap water with either reverse osmosis or activated carbon filters, as well as avoiding fast food and carry-out food, if possible, and consumer products labeled as “water resistant,” “stain-resistant,” and “nonstick.”
In a testament to how harmful these chemicals are, the EPA recently revised their lifetime health advisories for PFAS, such as PFOA, to 0.004 parts per trillion, which is more than 10,000 times smaller than the previous limit of 70 parts per trillion. The EPA also has proposed formally designating certain PFAS chemicals as “hazardous substances.”
Dr. Goel, clinical assistant professor of medicine at Weill Cornell Medicine, New York, has disclosed no relevant financial relationships. A version of this article originally appeared on Medscape.com.
Life expectancy is falling precipitously. Three-fourths of Americans are overweight or obese, half have diabetes or prediabetes, and a majority are metabolically unhealthy. Furthermore, the rates of allergic, inflammatory, and autoimmune diseases are rising at rates of 3%-9% per year in the West, far faster than the speed of genetic change in this population.
Of course, diet and lifestyle are major factors behind such trends, but a grossly underappreciated driver in what ails us is the role of environmental toxins and endocrine-disrupting chemicals. In years past, these factors have largely evaded the traditional Western medical establishment; however, mounting evidence now supports their significance in fertility, metabolic health, and cancer.
Although several industrial chemicals and toxins have been identified as carcinogens and have subsequently been regulated, many more remain persistent in the environment and continue to be freely used. It is therefore incumbent upon both the general public and clinicians to be knowledgeable about these exposures. Here, we review some of the most common exposures and the substantial health risks associated with them, along with some general guidance around best practices for how to minimize exposure.
Microplastics
“Microplastics” is a term used to describe small fragments or particles of plastic breakdown or microbeads from household or personal care products, measuring less than 5 mm in length.
Plastic waste is accumulating at alarming and devastating proportions – by 2050, it is estimated that by weight, there will be more plastic than fish in the oceans. That translates into hundreds of thousands of tons of microplastics and trillions of these particles in the seas. A recent study demonstrated that microplastics were present in the bloodstream in the majority of 22 otherwise healthy participants.
Since the 1950s, plastic exposure has been shown to promote tumorigenesis in animal studies, and in vitro studies have demonstrated the toxicity of microplastics at the cellular level. However, it is not well known whether the plastic itself is toxic or if it simply serves as a carrier for other environmental toxins to bioaccumulate.
According to Tasha Stoiber, a senior scientist at the Environmental Working Group, “Microplastics have been widely detected in fish and seafood, as well as other products like bottled water, beer, honey, and tap water.” The EWG states there are no formal advisories on fish consumption to avoid exposure to microplastics at the moment.
Pressure also is mounting for a ban on microbeads in personal care products.
Until such bans are put in place, it is advised to avoid single-use plastics, favor reusable tote bags for grocery shopping rather than plastic bags, and opt for loose leaf tea or paper tea bags rather than mesh-based alternatives.
Phthalates
Phthalates are chemicals used to make plastics soft and durable, as well as to bind fragrances. They are commonly found in household items such as vinyl (for example, flooring, shower curtains) and fragrances, air fresheners, and perfumes.
Phthalates are known hormone-disrupting chemicals, exposure to which has been associated with abnormal sexual and brain development in children, as well as lower levels of testosterone in men. Exposures are thought to occur via inhalation, ingestion, and skin contact; however, fasting studies demonstrate that a majority of exposure is probably food related.
To avoid phthalate exposures, recommendations include avoiding polyvinyl chloride plastics (particularly food containers, plastic wrap, and children’s toys), which are identifiable by the recycle code number 3, as well as air fresheners and fragranced products.
The EWG’s Skin Deep database provides an important resource on phthalate-free personal care products.
Despite pressure from consumer advocacy groups, the U.S. Food and Drug Administration has not yet banned phthalates in food packaging.
Bisphenol A (BPA)
BPA is a chemical additive used to make clear and hard polycarbonate plastics, as well as epoxy and thermal papers. BPA is one of the highest-volume chemicals, with roughly 6 billion pounds produced each year. BPA is traditionally found in many clear plastic bottles and sippy cups, as well as in the lining of canned foods.
Structurally, BPA acts as an estrogen mimetic and has been associated with cardiovascular disease, obesity, and male sexual dysfunction. Since 2012, BPA has been banned in sippy cups and baby bottles, but there is some debate as to whether its replacements (bisphenol S and bisphenol F) are any safer; they appear to have similar hormonal effects as BPA.
As with phthalates, the majority of ingestion is thought to be food related. BPA has been found in more than 90% of a representative study population in the United States.
Guidance advises avoiding polycarbonate plastics (identifiable with the recycling code number 7), as well as avoiding handling thermal papers such as tickets and receipts, if possible. Food and beverages should be stored in glass or stainless steel. If plastic must be used, opt for polycarbonate- and polyvinyl chloride–free plastics, and food and beverages should never be reheated in plastic containers or wrapping. Canned foods should ideally be avoided, particularly canned tunas and condensed soups. If canned products are bought, they should ideally be BPA free.
Dioxins and polychlorinated biphenyls (PCBs)
Dioxins are mainly the byproducts of industrial practices; they are released after incineration, trash burning, and fires. PCBs, which are somewhat structurally related to dioxins, were previously found in products such as flame retardants and coolants. Dioxins and PCBs are often grouped in the same category under the umbrella term “persistent organic pollutants” because they break down slowly and remain in the environment even after emissions have been curbed.
Tetrachlorodibenzodioxin, perhaps the best-known dioxin, is a known carcinogen. Dioxins also have been associated with a host of health implications in development, immunity, and reproductive and endocrine systems. Higher levels of PCB exposure have also been associated with an increased risk for mortality from cardiovascular disease.
Notably, dioxin emissions have been reduced by 90% since the 1980s, and the U.S. Environmental Protection Agency has banned the use of PCBs in industrial manufacturing since 1979. However, environmental dioxins and PCBs still enter the food chain and accumulate in fat.
The best ways to avoid exposures are through limiting meat, fish, and dairy consumption and trimming the skin and fat from meats. The level of dioxins and PCBs found in meat, eggs, fish, and dairy are approximately 5-10 times higher than they are in plant-based foods. Research has shown that farmed salmon is likely to be the most PCB-contaminated protein source in the U.S. diet; however, newer forms of land-based and sustainable aquaculture probably avoid this exposure.
Pesticides
The growth of modern monoculture agriculture in the United States over the past century has coincided with a dramatic surge in the use of industrial pesticides. In fact, over 90% of the U.S. population have pesticides in their urine and blood, regardless of where they live. Exposures are thought to be food related.
Approximately 1 billion pounds of pesticides are used annually in the United States, including nearly 300 million pounds of glyphosate, which has been identified as a probable carcinogen by European agencies. The EPA has not yet reached this conclusion, although the matter is currently being litigated.
A large European prospective cohort trial demonstrated a lower risk for cancer in those with a greater frequency of self-reported organic food consumption. In addition to cancer risk, relatively elevated blood levels of a pesticide known as beta-hexachlorocyclohexane (B-HCH) are associated with higher all-cause mortality. Also, exposure to DDE – a metabolite of DDT, a chlorinated pesticide heavily used in the 1940s-1960s that still persists in the environment today – has been shown to increase the risk for Alzheimer’s-type dementia as well as overall cognitive decline.
Because these chlorinated pesticides are often fat soluble, they seem to accumulate in animal products. Therefore, people consuming a vegetarian diet have been found to have lower levels of B-HCH. This has led to the recommendation that consumers of produce should favor organic over conventional, if possible. Here too, the EWG provides an important resource to consumers in the form of shopper guides regarding pesticides in produce.
Per- and polyfluoroalkyl substances (PFAS)
PFAS are a group of fluorinated compounds discovered in the 1930s. Their chemical composition includes a durable carbon-fluoride bond, giving them a persistence within the environment that has led to their being referred to as “forever chemicals.”
PFAS have been detected in the blood of 98% of Americans, and in the rainwater of locations as far afield as Tibet and Antarctica. Even low levels of exposure have been associated with an increased risk for cancer, liver disease, low birth weight, and hormonal disruption.
The properties of PFAS also make them both durable at very high heat and water repellent. Notoriously, the chemical was used by 3M to make Scotchgard for carpets and fabrics and by Dupont to make Teflon for nonstick coating of pots and pans. Although perfluorooctanoic acid (PFOA) was removed from nonstick cookware in 2013, PFAS – a family of thousands of synthetic compounds – remain common in fast-food packaging, water- and stain-repellent clothing, firefighting foam, and personal care products. PFAS are released into the environment during the breakdown of these consumer and industrial products, as well as from dumping from waste facilities.
Alarmingly, the EWG notes that up to 200 million Americans may be exposed to PFAS in their drinking water. In March 2021, the EPA announced that they will be regulating PFAS in drinking water; however, the regulations have not been finalized. Currently, it is up to individual states to test for its presence in the water. The EWG has compiled a map of all known PFAS contamination sites.
To avoid or prevent exposures from PFAS, recommendations include filtering tap water with either reverse osmosis or activated carbon filters, as well as avoiding fast food and carry-out food, if possible, and consumer products labeled as “water resistant,” “stain-resistant,” and “nonstick.”
In a testament to how harmful these chemicals are, the EPA recently revised their lifetime health advisories for PFAS, such as PFOA, to 0.004 parts per trillion, which is more than 10,000 times smaller than the previous limit of 70 parts per trillion. The EPA also has proposed formally designating certain PFAS chemicals as “hazardous substances.”
Dr. Goel, clinical assistant professor of medicine at Weill Cornell Medicine, New York, has disclosed no relevant financial relationships. A version of this article originally appeared on Medscape.com.
Life expectancy is falling precipitously. Three-fourths of Americans are overweight or obese, half have diabetes or prediabetes, and a majority are metabolically unhealthy. Furthermore, the rates of allergic, inflammatory, and autoimmune diseases are rising at rates of 3%-9% per year in the West, far faster than the speed of genetic change in this population.
Of course, diet and lifestyle are major factors behind such trends, but a grossly underappreciated driver in what ails us is the role of environmental toxins and endocrine-disrupting chemicals. In years past, these factors have largely evaded the traditional Western medical establishment; however, mounting evidence now supports their significance in fertility, metabolic health, and cancer.
Although several industrial chemicals and toxins have been identified as carcinogens and have subsequently been regulated, many more remain persistent in the environment and continue to be freely used. It is therefore incumbent upon both the general public and clinicians to be knowledgeable about these exposures. Here, we review some of the most common exposures and the substantial health risks associated with them, along with some general guidance around best practices for how to minimize exposure.
Microplastics
“Microplastics” is a term used to describe small fragments or particles of plastic breakdown or microbeads from household or personal care products, measuring less than 5 mm in length.
Plastic waste is accumulating at alarming and devastating proportions – by 2050, it is estimated that by weight, there will be more plastic than fish in the oceans. That translates into hundreds of thousands of tons of microplastics and trillions of these particles in the seas. A recent study demonstrated that microplastics were present in the bloodstream in the majority of 22 otherwise healthy participants.
Since the 1950s, plastic exposure has been shown to promote tumorigenesis in animal studies, and in vitro studies have demonstrated the toxicity of microplastics at the cellular level. However, it is not well known whether the plastic itself is toxic or if it simply serves as a carrier for other environmental toxins to bioaccumulate.
According to Tasha Stoiber, a senior scientist at the Environmental Working Group, “Microplastics have been widely detected in fish and seafood, as well as other products like bottled water, beer, honey, and tap water.” The EWG states there are no formal advisories on fish consumption to avoid exposure to microplastics at the moment.
Pressure also is mounting for a ban on microbeads in personal care products.
Until such bans are put in place, it is advised to avoid single-use plastics, favor reusable tote bags for grocery shopping rather than plastic bags, and opt for loose leaf tea or paper tea bags rather than mesh-based alternatives.
Phthalates
Phthalates are chemicals used to make plastics soft and durable, as well as to bind fragrances. They are commonly found in household items such as vinyl (for example, flooring, shower curtains) and fragrances, air fresheners, and perfumes.
Phthalates are known hormone-disrupting chemicals, exposure to which has been associated with abnormal sexual and brain development in children, as well as lower levels of testosterone in men. Exposures are thought to occur via inhalation, ingestion, and skin contact; however, fasting studies demonstrate that a majority of exposure is probably food related.
To avoid phthalate exposures, recommendations include avoiding polyvinyl chloride plastics (particularly food containers, plastic wrap, and children’s toys), which are identifiable by the recycle code number 3, as well as air fresheners and fragranced products.
The EWG’s Skin Deep database provides an important resource on phthalate-free personal care products.
Despite pressure from consumer advocacy groups, the U.S. Food and Drug Administration has not yet banned phthalates in food packaging.
Bisphenol A (BPA)
BPA is a chemical additive used to make clear and hard polycarbonate plastics, as well as epoxy and thermal papers. BPA is one of the highest-volume chemicals, with roughly 6 billion pounds produced each year. BPA is traditionally found in many clear plastic bottles and sippy cups, as well as in the lining of canned foods.
Structurally, BPA acts as an estrogen mimetic and has been associated with cardiovascular disease, obesity, and male sexual dysfunction. Since 2012, BPA has been banned in sippy cups and baby bottles, but there is some debate as to whether its replacements (bisphenol S and bisphenol F) are any safer; they appear to have similar hormonal effects as BPA.
As with phthalates, the majority of ingestion is thought to be food related. BPA has been found in more than 90% of a representative study population in the United States.
Guidance advises avoiding polycarbonate plastics (identifiable with the recycling code number 7), as well as avoiding handling thermal papers such as tickets and receipts, if possible. Food and beverages should be stored in glass or stainless steel. If plastic must be used, opt for polycarbonate- and polyvinyl chloride–free plastics, and food and beverages should never be reheated in plastic containers or wrapping. Canned foods should ideally be avoided, particularly canned tunas and condensed soups. If canned products are bought, they should ideally be BPA free.
Dioxins and polychlorinated biphenyls (PCBs)
Dioxins are mainly the byproducts of industrial practices; they are released after incineration, trash burning, and fires. PCBs, which are somewhat structurally related to dioxins, were previously found in products such as flame retardants and coolants. Dioxins and PCBs are often grouped in the same category under the umbrella term “persistent organic pollutants” because they break down slowly and remain in the environment even after emissions have been curbed.
Tetrachlorodibenzodioxin, perhaps the best-known dioxin, is a known carcinogen. Dioxins also have been associated with a host of health implications in development, immunity, and reproductive and endocrine systems. Higher levels of PCB exposure have also been associated with an increased risk for mortality from cardiovascular disease.
Notably, dioxin emissions have been reduced by 90% since the 1980s, and the U.S. Environmental Protection Agency has banned the use of PCBs in industrial manufacturing since 1979. However, environmental dioxins and PCBs still enter the food chain and accumulate in fat.
The best ways to avoid exposures are through limiting meat, fish, and dairy consumption and trimming the skin and fat from meats. The level of dioxins and PCBs found in meat, eggs, fish, and dairy are approximately 5-10 times higher than they are in plant-based foods. Research has shown that farmed salmon is likely to be the most PCB-contaminated protein source in the U.S. diet; however, newer forms of land-based and sustainable aquaculture probably avoid this exposure.
Pesticides
The growth of modern monoculture agriculture in the United States over the past century has coincided with a dramatic surge in the use of industrial pesticides. In fact, over 90% of the U.S. population have pesticides in their urine and blood, regardless of where they live. Exposures are thought to be food related.
Approximately 1 billion pounds of pesticides are used annually in the United States, including nearly 300 million pounds of glyphosate, which has been identified as a probable carcinogen by European agencies. The EPA has not yet reached this conclusion, although the matter is currently being litigated.
A large European prospective cohort trial demonstrated a lower risk for cancer in those with a greater frequency of self-reported organic food consumption. In addition to cancer risk, relatively elevated blood levels of a pesticide known as beta-hexachlorocyclohexane (B-HCH) are associated with higher all-cause mortality. Also, exposure to DDE – a metabolite of DDT, a chlorinated pesticide heavily used in the 1940s-1960s that still persists in the environment today – has been shown to increase the risk for Alzheimer’s-type dementia as well as overall cognitive decline.
Because these chlorinated pesticides are often fat soluble, they seem to accumulate in animal products. Therefore, people consuming a vegetarian diet have been found to have lower levels of B-HCH. This has led to the recommendation that consumers of produce should favor organic over conventional, if possible. Here too, the EWG provides an important resource to consumers in the form of shopper guides regarding pesticides in produce.
Per- and polyfluoroalkyl substances (PFAS)
PFAS are a group of fluorinated compounds discovered in the 1930s. Their chemical composition includes a durable carbon-fluoride bond, giving them a persistence within the environment that has led to their being referred to as “forever chemicals.”
PFAS have been detected in the blood of 98% of Americans, and in the rainwater of locations as far afield as Tibet and Antarctica. Even low levels of exposure have been associated with an increased risk for cancer, liver disease, low birth weight, and hormonal disruption.
The properties of PFAS also make them both durable at very high heat and water repellent. Notoriously, the chemical was used by 3M to make Scotchgard for carpets and fabrics and by Dupont to make Teflon for nonstick coating of pots and pans. Although perfluorooctanoic acid (PFOA) was removed from nonstick cookware in 2013, PFAS – a family of thousands of synthetic compounds – remain common in fast-food packaging, water- and stain-repellent clothing, firefighting foam, and personal care products. PFAS are released into the environment during the breakdown of these consumer and industrial products, as well as from dumping from waste facilities.
Alarmingly, the EWG notes that up to 200 million Americans may be exposed to PFAS in their drinking water. In March 2021, the EPA announced that they will be regulating PFAS in drinking water; however, the regulations have not been finalized. Currently, it is up to individual states to test for its presence in the water. The EWG has compiled a map of all known PFAS contamination sites.
To avoid or prevent exposures from PFAS, recommendations include filtering tap water with either reverse osmosis or activated carbon filters, as well as avoiding fast food and carry-out food, if possible, and consumer products labeled as “water resistant,” “stain-resistant,” and “nonstick.”
In a testament to how harmful these chemicals are, the EPA recently revised their lifetime health advisories for PFAS, such as PFOA, to 0.004 parts per trillion, which is more than 10,000 times smaller than the previous limit of 70 parts per trillion. The EPA also has proposed formally designating certain PFAS chemicals as “hazardous substances.”
Dr. Goel, clinical assistant professor of medicine at Weill Cornell Medicine, New York, has disclosed no relevant financial relationships. A version of this article originally appeared on Medscape.com.
Physicians and clinicians should be required to get flu shots: Ethicist
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the Division of Medical Ethics at New York University’s Grossman School of Medicine, where I’m the director.
I’ve long believed that every health care institution – nursing homes, hospitals, clinics, home care, hospice – should require flu shots for all doctors and all nurses because it is the easiest, cheapest, and most ethical way to protect the workforce, who you need to be in there when flu outbreaks take place, and to protect patients against getting the flu when they come into hospital settings and get exposed to health care workers who may have the flu already but don’t know it.
In a recent poll, I was happy to see that the majority of physicians surveyed agreed with me: 65% said they supported mandatory flu vaccination in hospitals and only 23% said they did not. I think flu vaccination is something that has already been shown to be useful and important, not only in stopping people from getting the flu but also in making sure that they don’t get as sick when they get the flu.
Just like COVID-19 vaccination, it doesn’t always prevent somebody from getting infected, but if you get it, it keeps you from winding up sick at home, or worse – from dying and winding up in the morgue. Flu kills many, many people every year. We don’t want that to happen. A flu vaccine will really help prevent deaths, help prevent the number of symptoms that somebody gets, and will get people back to work. The benefits are pretty clear.
Does the flu vaccine work equally well every year? It does not. Some years, the strains that are picked for the vaccine don’t match the ones that circulate, and we don’t get as much protection as we hoped for. I think the safety side is so strong that it’s worth making the investment and the effort to promote mandatory flu vaccination.
Can you opt out on religious grounds? Well, some hospitals permit that at New York University. You have to go before a committee and make a case that your exemption on religious grounds is based on an authentic set of beliefs that are deeply held, and not just something you thought up the day before flu vaccine requirements went into effect.
There may be room for some exemptions – obviously, for health reasons. If people think that the flu vaccine is dangerous to them and can get a physician to agree and sign off that they are not appropriate to vaccinate, okay.
On the other hand, if you’re working with an especially vulnerable population – newborns, people who are immunosuppressed – then I think you’ve got to be vaccinated and you shouldn’t be working around people who are at huge risk of getting the flu if you refuse to be vaccinated or, for that matter, can’t be vaccinated.
Would I extend these mandates? Yes, I would. I’d extend them to COVID-19 vaccination and to measles vaccination. I think physicians and nurses should be good role models. They should get vaccinated. We know that the best available evidence says that vaccination for infectious disease is safe. It is really the best thing we can do to combat a variety of diseases such as the flu and COVID-19.
It seems to me that, in addition, the data that are out there in terms of risks from flu and COVID-19 – deaths in places like nursing homes – are overwhelming about the importance of trying to get staff vaccinated so they don’t bring flu into an institutionalized population. This is similar for prison health and many other settings where people are kept close together and staff may move from place to place, rotating from institution to institution, spreading infectious disease.
I’m going to go with the poll. Let’s keep pushing for health care workers to do the right thing and to be good role models. Let’s get everybody a flu vaccination. Let’s extend it to a COVID-19 vaccination and its boosters.
Let’s try to show the nation that health care is going to be guided by good science, a duty to one’s own health, and a duty to one’s patients. It shouldn’t be political. It should be based on what works best for the interests of health care providers and those they care for.
I’m Art Caplan at the New York University Grossman School of Medicine. Thanks for watching.
Dr. Caplan has disclosed the following relevant financial relationships: Served as a director, officer, partner, employee, advisor, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position). Serves as a contributing author and advisor for Medscape. A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the Division of Medical Ethics at New York University’s Grossman School of Medicine, where I’m the director.
I’ve long believed that every health care institution – nursing homes, hospitals, clinics, home care, hospice – should require flu shots for all doctors and all nurses because it is the easiest, cheapest, and most ethical way to protect the workforce, who you need to be in there when flu outbreaks take place, and to protect patients against getting the flu when they come into hospital settings and get exposed to health care workers who may have the flu already but don’t know it.
In a recent poll, I was happy to see that the majority of physicians surveyed agreed with me: 65% said they supported mandatory flu vaccination in hospitals and only 23% said they did not. I think flu vaccination is something that has already been shown to be useful and important, not only in stopping people from getting the flu but also in making sure that they don’t get as sick when they get the flu.
Just like COVID-19 vaccination, it doesn’t always prevent somebody from getting infected, but if you get it, it keeps you from winding up sick at home, or worse – from dying and winding up in the morgue. Flu kills many, many people every year. We don’t want that to happen. A flu vaccine will really help prevent deaths, help prevent the number of symptoms that somebody gets, and will get people back to work. The benefits are pretty clear.
Does the flu vaccine work equally well every year? It does not. Some years, the strains that are picked for the vaccine don’t match the ones that circulate, and we don’t get as much protection as we hoped for. I think the safety side is so strong that it’s worth making the investment and the effort to promote mandatory flu vaccination.
Can you opt out on religious grounds? Well, some hospitals permit that at New York University. You have to go before a committee and make a case that your exemption on religious grounds is based on an authentic set of beliefs that are deeply held, and not just something you thought up the day before flu vaccine requirements went into effect.
There may be room for some exemptions – obviously, for health reasons. If people think that the flu vaccine is dangerous to them and can get a physician to agree and sign off that they are not appropriate to vaccinate, okay.
On the other hand, if you’re working with an especially vulnerable population – newborns, people who are immunosuppressed – then I think you’ve got to be vaccinated and you shouldn’t be working around people who are at huge risk of getting the flu if you refuse to be vaccinated or, for that matter, can’t be vaccinated.
Would I extend these mandates? Yes, I would. I’d extend them to COVID-19 vaccination and to measles vaccination. I think physicians and nurses should be good role models. They should get vaccinated. We know that the best available evidence says that vaccination for infectious disease is safe. It is really the best thing we can do to combat a variety of diseases such as the flu and COVID-19.
It seems to me that, in addition, the data that are out there in terms of risks from flu and COVID-19 – deaths in places like nursing homes – are overwhelming about the importance of trying to get staff vaccinated so they don’t bring flu into an institutionalized population. This is similar for prison health and many other settings where people are kept close together and staff may move from place to place, rotating from institution to institution, spreading infectious disease.
I’m going to go with the poll. Let’s keep pushing for health care workers to do the right thing and to be good role models. Let’s get everybody a flu vaccination. Let’s extend it to a COVID-19 vaccination and its boosters.
Let’s try to show the nation that health care is going to be guided by good science, a duty to one’s own health, and a duty to one’s patients. It shouldn’t be political. It should be based on what works best for the interests of health care providers and those they care for.
I’m Art Caplan at the New York University Grossman School of Medicine. Thanks for watching.
Dr. Caplan has disclosed the following relevant financial relationships: Served as a director, officer, partner, employee, advisor, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position). Serves as a contributing author and advisor for Medscape. A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the Division of Medical Ethics at New York University’s Grossman School of Medicine, where I’m the director.
I’ve long believed that every health care institution – nursing homes, hospitals, clinics, home care, hospice – should require flu shots for all doctors and all nurses because it is the easiest, cheapest, and most ethical way to protect the workforce, who you need to be in there when flu outbreaks take place, and to protect patients against getting the flu when they come into hospital settings and get exposed to health care workers who may have the flu already but don’t know it.
In a recent poll, I was happy to see that the majority of physicians surveyed agreed with me: 65% said they supported mandatory flu vaccination in hospitals and only 23% said they did not. I think flu vaccination is something that has already been shown to be useful and important, not only in stopping people from getting the flu but also in making sure that they don’t get as sick when they get the flu.
Just like COVID-19 vaccination, it doesn’t always prevent somebody from getting infected, but if you get it, it keeps you from winding up sick at home, or worse – from dying and winding up in the morgue. Flu kills many, many people every year. We don’t want that to happen. A flu vaccine will really help prevent deaths, help prevent the number of symptoms that somebody gets, and will get people back to work. The benefits are pretty clear.
Does the flu vaccine work equally well every year? It does not. Some years, the strains that are picked for the vaccine don’t match the ones that circulate, and we don’t get as much protection as we hoped for. I think the safety side is so strong that it’s worth making the investment and the effort to promote mandatory flu vaccination.
Can you opt out on religious grounds? Well, some hospitals permit that at New York University. You have to go before a committee and make a case that your exemption on religious grounds is based on an authentic set of beliefs that are deeply held, and not just something you thought up the day before flu vaccine requirements went into effect.
There may be room for some exemptions – obviously, for health reasons. If people think that the flu vaccine is dangerous to them and can get a physician to agree and sign off that they are not appropriate to vaccinate, okay.
On the other hand, if you’re working with an especially vulnerable population – newborns, people who are immunosuppressed – then I think you’ve got to be vaccinated and you shouldn’t be working around people who are at huge risk of getting the flu if you refuse to be vaccinated or, for that matter, can’t be vaccinated.
Would I extend these mandates? Yes, I would. I’d extend them to COVID-19 vaccination and to measles vaccination. I think physicians and nurses should be good role models. They should get vaccinated. We know that the best available evidence says that vaccination for infectious disease is safe. It is really the best thing we can do to combat a variety of diseases such as the flu and COVID-19.
It seems to me that, in addition, the data that are out there in terms of risks from flu and COVID-19 – deaths in places like nursing homes – are overwhelming about the importance of trying to get staff vaccinated so they don’t bring flu into an institutionalized population. This is similar for prison health and many other settings where people are kept close together and staff may move from place to place, rotating from institution to institution, spreading infectious disease.
I’m going to go with the poll. Let’s keep pushing for health care workers to do the right thing and to be good role models. Let’s get everybody a flu vaccination. Let’s extend it to a COVID-19 vaccination and its boosters.
Let’s try to show the nation that health care is going to be guided by good science, a duty to one’s own health, and a duty to one’s patients. It shouldn’t be political. It should be based on what works best for the interests of health care providers and those they care for.
I’m Art Caplan at the New York University Grossman School of Medicine. Thanks for watching.
Dr. Caplan has disclosed the following relevant financial relationships: Served as a director, officer, partner, employee, advisor, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position). Serves as a contributing author and advisor for Medscape. A version of this article originally appeared on Medscape.com.
How to recognize and treat hidden inflammation
“This then leads to inflammation, the healing of which the body is unable to keep under control,” explained Ulf Müller-Ladner, MD, PhD, chairperson of the German Society of Internal Medicine.
At the DGIM annual press conference, Dr. Müller-Ladner, who is also director of the department of rheumatology and clinical immunology at the Kerckhoff Clinic in Bad Nauheim, Germany, explained how IgG4 inflammation is triggered throughout the body and what therapeutic options are available.
Many manifestations
IgG4-associated inflammation can affect one or more organs or the surrounding connective tissue and cause fibrosis. As a result of fibrosis, the organ gradually loses function and is eventually transformed completely into scarred connective tissue.
“In the case of IgG4-associated inflammation, these fibroses have a histological structure, but extracting a sample is not possible from every affected organ,” said Dr. Müller-Ladner. Liver, bile ducts, blood vessels, skin, eyes, or even the central nervous system – practically every organ system can be affected by these inflammatory reactions.
IgG4-associated diseases have likely been around for some time, but it is only in the past 10 years that awareness has grown that, despite various manifestations, “they are all one and the same disease,” said Dr. Müller-Ladner.
IgG4-associated chronic, inflammatory, fibrosing diseases were only classified together as a single entity in the past few years. In terms of pathophysiology, B lymphocytes, IgG4-positive plasma cells, follicular T-helper cells, cytotoxic CD4-positive T cells, and macrophages work together and trigger an inflammatory reaction, which then encourages fibroblasts to overproduce connective tissue.
Beware inexplicable inflammation
It is estimated that 1 in 100,000 people suffer from the disease, but the number of incorrectly categorized patients may be significantly higher.
The diagnostic challenge lies in the fact that IgG4-associated inflammation occurs in almost every organ. It can cause different symptoms, depending on the organ affected.
Dr. Müller-Ladner provided the following take-home message: “Every inexplicable inflammation event and every organ dysfunction, especially if associated with an increase in connective tissue, could be an IgG4-associated disease. Keeping this in mind is the key to recovery.”
With most people, the inflammation persists for many years before any symptoms of the disease develop. Highly acute courses of progression are also possible.
Classic symptoms, such as fever, are not so characteristic of the latent inflammatory reaction, and according to classification criteria published by specialist rheumatology societies, they are an exclusion criterion. This is true with respect to the differential diagnosis for vasculitis, which also occurs throughout the body.
Histology is key
Blood levels of IgG4 and imaging are not always enough to confirm the diagnosis. In such cases, the histology is often a crucial factor in making a definitive diagnosis. Dominant organs in IgG4-associated diseases are the pancreas, the liver, the gallbladder, the intestines, the retroperitoneum, large blood vessels, the kidneys, the heart, the brain, saliva, tear ducts, as well all of the body’s connective tissue.
The kidneys play host to inflammation in the connective tissue and space-occupying masses in particular. “If the pancreas is affected, the signs can vary from diffuse swelling to the onset of diabetes mellitus. In contrast, if the aorta is affected, then the inflammation is characterized through a thickening of the vessel walls, aneurysms, and the corresponding circulation disorders,” said Dr. Müller-Ladner.
Because of the long period before the diagnosis is made, more than 50% of patients exhibit irreversible organ damage at the time of diagnosis, he added.
Glucocorticoids and immunosuppressants
Despite therapeutic intervention, the disease can have a fatal outcome, even if the patient is young, said Dr. Müller-Ladner. Glucocorticoids are the current therapy of choice. The dose is more than 0.5 mg of prednisolone equivalent per kg of body weight. “This usually leads to a rapid improvement in the inflammation. Subsequently, every organ is thoroughly diagnosed to assess the severity of the disease and to plan further treatment steps.”
In the long term, proven immunosuppressants, such as azathioprine, mycophenolate, leflunomide, and methotrexate, can be used, just as for many other chronic inflammatory diseases. Cyclophosphamide or cyclosporine is used more rarely, owing to their side effect profiles.
Because of the B-cell dominance, B-cell–depleting therapy with rituximab is currently a highly effective therapeutic option but one that must be applied for, because such use is off label. “If the body responds well to the medication, organ function often recovers,” said Dr. Müller-Ladner.
This article was translated from the Medscape German edition. A version appeared on Medscape.com.
“This then leads to inflammation, the healing of which the body is unable to keep under control,” explained Ulf Müller-Ladner, MD, PhD, chairperson of the German Society of Internal Medicine.
At the DGIM annual press conference, Dr. Müller-Ladner, who is also director of the department of rheumatology and clinical immunology at the Kerckhoff Clinic in Bad Nauheim, Germany, explained how IgG4 inflammation is triggered throughout the body and what therapeutic options are available.
Many manifestations
IgG4-associated inflammation can affect one or more organs or the surrounding connective tissue and cause fibrosis. As a result of fibrosis, the organ gradually loses function and is eventually transformed completely into scarred connective tissue.
“In the case of IgG4-associated inflammation, these fibroses have a histological structure, but extracting a sample is not possible from every affected organ,” said Dr. Müller-Ladner. Liver, bile ducts, blood vessels, skin, eyes, or even the central nervous system – practically every organ system can be affected by these inflammatory reactions.
IgG4-associated diseases have likely been around for some time, but it is only in the past 10 years that awareness has grown that, despite various manifestations, “they are all one and the same disease,” said Dr. Müller-Ladner.
IgG4-associated chronic, inflammatory, fibrosing diseases were only classified together as a single entity in the past few years. In terms of pathophysiology, B lymphocytes, IgG4-positive plasma cells, follicular T-helper cells, cytotoxic CD4-positive T cells, and macrophages work together and trigger an inflammatory reaction, which then encourages fibroblasts to overproduce connective tissue.
Beware inexplicable inflammation
It is estimated that 1 in 100,000 people suffer from the disease, but the number of incorrectly categorized patients may be significantly higher.
The diagnostic challenge lies in the fact that IgG4-associated inflammation occurs in almost every organ. It can cause different symptoms, depending on the organ affected.
Dr. Müller-Ladner provided the following take-home message: “Every inexplicable inflammation event and every organ dysfunction, especially if associated with an increase in connective tissue, could be an IgG4-associated disease. Keeping this in mind is the key to recovery.”
With most people, the inflammation persists for many years before any symptoms of the disease develop. Highly acute courses of progression are also possible.
Classic symptoms, such as fever, are not so characteristic of the latent inflammatory reaction, and according to classification criteria published by specialist rheumatology societies, they are an exclusion criterion. This is true with respect to the differential diagnosis for vasculitis, which also occurs throughout the body.
Histology is key
Blood levels of IgG4 and imaging are not always enough to confirm the diagnosis. In such cases, the histology is often a crucial factor in making a definitive diagnosis. Dominant organs in IgG4-associated diseases are the pancreas, the liver, the gallbladder, the intestines, the retroperitoneum, large blood vessels, the kidneys, the heart, the brain, saliva, tear ducts, as well all of the body’s connective tissue.
The kidneys play host to inflammation in the connective tissue and space-occupying masses in particular. “If the pancreas is affected, the signs can vary from diffuse swelling to the onset of diabetes mellitus. In contrast, if the aorta is affected, then the inflammation is characterized through a thickening of the vessel walls, aneurysms, and the corresponding circulation disorders,” said Dr. Müller-Ladner.
Because of the long period before the diagnosis is made, more than 50% of patients exhibit irreversible organ damage at the time of diagnosis, he added.
Glucocorticoids and immunosuppressants
Despite therapeutic intervention, the disease can have a fatal outcome, even if the patient is young, said Dr. Müller-Ladner. Glucocorticoids are the current therapy of choice. The dose is more than 0.5 mg of prednisolone equivalent per kg of body weight. “This usually leads to a rapid improvement in the inflammation. Subsequently, every organ is thoroughly diagnosed to assess the severity of the disease and to plan further treatment steps.”
In the long term, proven immunosuppressants, such as azathioprine, mycophenolate, leflunomide, and methotrexate, can be used, just as for many other chronic inflammatory diseases. Cyclophosphamide or cyclosporine is used more rarely, owing to their side effect profiles.
Because of the B-cell dominance, B-cell–depleting therapy with rituximab is currently a highly effective therapeutic option but one that must be applied for, because such use is off label. “If the body responds well to the medication, organ function often recovers,” said Dr. Müller-Ladner.
This article was translated from the Medscape German edition. A version appeared on Medscape.com.
“This then leads to inflammation, the healing of which the body is unable to keep under control,” explained Ulf Müller-Ladner, MD, PhD, chairperson of the German Society of Internal Medicine.
At the DGIM annual press conference, Dr. Müller-Ladner, who is also director of the department of rheumatology and clinical immunology at the Kerckhoff Clinic in Bad Nauheim, Germany, explained how IgG4 inflammation is triggered throughout the body and what therapeutic options are available.
Many manifestations
IgG4-associated inflammation can affect one or more organs or the surrounding connective tissue and cause fibrosis. As a result of fibrosis, the organ gradually loses function and is eventually transformed completely into scarred connective tissue.
“In the case of IgG4-associated inflammation, these fibroses have a histological structure, but extracting a sample is not possible from every affected organ,” said Dr. Müller-Ladner. Liver, bile ducts, blood vessels, skin, eyes, or even the central nervous system – practically every organ system can be affected by these inflammatory reactions.
IgG4-associated diseases have likely been around for some time, but it is only in the past 10 years that awareness has grown that, despite various manifestations, “they are all one and the same disease,” said Dr. Müller-Ladner.
IgG4-associated chronic, inflammatory, fibrosing diseases were only classified together as a single entity in the past few years. In terms of pathophysiology, B lymphocytes, IgG4-positive plasma cells, follicular T-helper cells, cytotoxic CD4-positive T cells, and macrophages work together and trigger an inflammatory reaction, which then encourages fibroblasts to overproduce connective tissue.
Beware inexplicable inflammation
It is estimated that 1 in 100,000 people suffer from the disease, but the number of incorrectly categorized patients may be significantly higher.
The diagnostic challenge lies in the fact that IgG4-associated inflammation occurs in almost every organ. It can cause different symptoms, depending on the organ affected.
Dr. Müller-Ladner provided the following take-home message: “Every inexplicable inflammation event and every organ dysfunction, especially if associated with an increase in connective tissue, could be an IgG4-associated disease. Keeping this in mind is the key to recovery.”
With most people, the inflammation persists for many years before any symptoms of the disease develop. Highly acute courses of progression are also possible.
Classic symptoms, such as fever, are not so characteristic of the latent inflammatory reaction, and according to classification criteria published by specialist rheumatology societies, they are an exclusion criterion. This is true with respect to the differential diagnosis for vasculitis, which also occurs throughout the body.
Histology is key
Blood levels of IgG4 and imaging are not always enough to confirm the diagnosis. In such cases, the histology is often a crucial factor in making a definitive diagnosis. Dominant organs in IgG4-associated diseases are the pancreas, the liver, the gallbladder, the intestines, the retroperitoneum, large blood vessels, the kidneys, the heart, the brain, saliva, tear ducts, as well all of the body’s connective tissue.
The kidneys play host to inflammation in the connective tissue and space-occupying masses in particular. “If the pancreas is affected, the signs can vary from diffuse swelling to the onset of diabetes mellitus. In contrast, if the aorta is affected, then the inflammation is characterized through a thickening of the vessel walls, aneurysms, and the corresponding circulation disorders,” said Dr. Müller-Ladner.
Because of the long period before the diagnosis is made, more than 50% of patients exhibit irreversible organ damage at the time of diagnosis, he added.
Glucocorticoids and immunosuppressants
Despite therapeutic intervention, the disease can have a fatal outcome, even if the patient is young, said Dr. Müller-Ladner. Glucocorticoids are the current therapy of choice. The dose is more than 0.5 mg of prednisolone equivalent per kg of body weight. “This usually leads to a rapid improvement in the inflammation. Subsequently, every organ is thoroughly diagnosed to assess the severity of the disease and to plan further treatment steps.”
In the long term, proven immunosuppressants, such as azathioprine, mycophenolate, leflunomide, and methotrexate, can be used, just as for many other chronic inflammatory diseases. Cyclophosphamide or cyclosporine is used more rarely, owing to their side effect profiles.
Because of the B-cell dominance, B-cell–depleting therapy with rituximab is currently a highly effective therapeutic option but one that must be applied for, because such use is off label. “If the body responds well to the medication, organ function often recovers,” said Dr. Müller-Ladner.
This article was translated from the Medscape German edition. A version appeared on Medscape.com.