User login
Gram Stain Doesn’t Improve UTI Diagnosis in the ED
TOPLINE:
Compared with other urine analysis methods, urine Gram stain has a moderate predictive value for detecting gram-negative bacteria in urine culture but does not significantly improve urinary tract infection (UTI) diagnosis in the emergency department (ED).
METHODOLOGY:
- Researchers conducted an observational cohort study at the University Medical Center Groningen in the Netherlands, encompassing 1358 episodes across 1136 patients suspected of having a UTI.
- The study included the following predefined subgroups: patients using urinary catheters and patients with leukopenia (< 4.0×10⁹ leucocytes/L). Urine dipstick nitrite, automated urinalysis, Gram stain, and urine cultures were performed on urine samples collected from patients presenting at the ED.
- The sensitivity and specificity of Gram stain for “many” bacteria (quantified as > 15/high power field) were compared with those of urine dipstick nitrite and automated bacterial counting in urinalysis.
TAKEAWAY:
- The sensitivity and specificity of Gram stain for “many” bacteria were 51.3% and 91.0%, respectively, with an accuracy of 76.8%.
- Gram stain showed a positive predictive value (PPV) of 84.7% for gram-negative rods in urine culture; however, the PPV was only 38.4% for gram-positive cocci.
- In the catheter subgroup, the presence of monomorphic bacteria quantified as “many” had a higher PPV for diagnosing a UTI than the presence of polymorphic bacteria with the same quantification.
- The overall performance of Gram stain in diagnosing a UTI in the ED was comparable to that of automated bacterial counting in urinalysis but better than that of urine dipstick nitrite.
IN PRACTICE:
“With the exception of a moderate prediction of gram-negative bacteria in the UC [urine culture], urine GS [Gram stain] does not improve UTI diagnosis at the ED compared to other urine parameters,” the authors wrote.
SOURCE:
The study was led by Stephanie J.M. Middelkoop, University of Groningen, University Medical Center Groningen, the Netherlands. It was published online on August 16, 2024, in Infectious Diseases.
LIMITATIONS:
The study’s limitations included a small sample size within the leukopenia subgroup, which may have affected the generalizability of the findings. Additionally, the potential influence of refrigeration of urine samples on bacterial growth could have affected the results. In this study, indwelling catheters were not replaced before urine sample collection, which may have affected the accuracy of UTI diagnosis in patients using catheters.
DISCLOSURES:
No conflicts of interest were disclosed by the authors.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Compared with other urine analysis methods, urine Gram stain has a moderate predictive value for detecting gram-negative bacteria in urine culture but does not significantly improve urinary tract infection (UTI) diagnosis in the emergency department (ED).
METHODOLOGY:
- Researchers conducted an observational cohort study at the University Medical Center Groningen in the Netherlands, encompassing 1358 episodes across 1136 patients suspected of having a UTI.
- The study included the following predefined subgroups: patients using urinary catheters and patients with leukopenia (< 4.0×10⁹ leucocytes/L). Urine dipstick nitrite, automated urinalysis, Gram stain, and urine cultures were performed on urine samples collected from patients presenting at the ED.
- The sensitivity and specificity of Gram stain for “many” bacteria (quantified as > 15/high power field) were compared with those of urine dipstick nitrite and automated bacterial counting in urinalysis.
TAKEAWAY:
- The sensitivity and specificity of Gram stain for “many” bacteria were 51.3% and 91.0%, respectively, with an accuracy of 76.8%.
- Gram stain showed a positive predictive value (PPV) of 84.7% for gram-negative rods in urine culture; however, the PPV was only 38.4% for gram-positive cocci.
- In the catheter subgroup, the presence of monomorphic bacteria quantified as “many” had a higher PPV for diagnosing a UTI than the presence of polymorphic bacteria with the same quantification.
- The overall performance of Gram stain in diagnosing a UTI in the ED was comparable to that of automated bacterial counting in urinalysis but better than that of urine dipstick nitrite.
IN PRACTICE:
“With the exception of a moderate prediction of gram-negative bacteria in the UC [urine culture], urine GS [Gram stain] does not improve UTI diagnosis at the ED compared to other urine parameters,” the authors wrote.
SOURCE:
The study was led by Stephanie J.M. Middelkoop, University of Groningen, University Medical Center Groningen, the Netherlands. It was published online on August 16, 2024, in Infectious Diseases.
LIMITATIONS:
The study’s limitations included a small sample size within the leukopenia subgroup, which may have affected the generalizability of the findings. Additionally, the potential influence of refrigeration of urine samples on bacterial growth could have affected the results. In this study, indwelling catheters were not replaced before urine sample collection, which may have affected the accuracy of UTI diagnosis in patients using catheters.
DISCLOSURES:
No conflicts of interest were disclosed by the authors.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Compared with other urine analysis methods, urine Gram stain has a moderate predictive value for detecting gram-negative bacteria in urine culture but does not significantly improve urinary tract infection (UTI) diagnosis in the emergency department (ED).
METHODOLOGY:
- Researchers conducted an observational cohort study at the University Medical Center Groningen in the Netherlands, encompassing 1358 episodes across 1136 patients suspected of having a UTI.
- The study included the following predefined subgroups: patients using urinary catheters and patients with leukopenia (< 4.0×10⁹ leucocytes/L). Urine dipstick nitrite, automated urinalysis, Gram stain, and urine cultures were performed on urine samples collected from patients presenting at the ED.
- The sensitivity and specificity of Gram stain for “many” bacteria (quantified as > 15/high power field) were compared with those of urine dipstick nitrite and automated bacterial counting in urinalysis.
TAKEAWAY:
- The sensitivity and specificity of Gram stain for “many” bacteria were 51.3% and 91.0%, respectively, with an accuracy of 76.8%.
- Gram stain showed a positive predictive value (PPV) of 84.7% for gram-negative rods in urine culture; however, the PPV was only 38.4% for gram-positive cocci.
- In the catheter subgroup, the presence of monomorphic bacteria quantified as “many” had a higher PPV for diagnosing a UTI than the presence of polymorphic bacteria with the same quantification.
- The overall performance of Gram stain in diagnosing a UTI in the ED was comparable to that of automated bacterial counting in urinalysis but better than that of urine dipstick nitrite.
IN PRACTICE:
“With the exception of a moderate prediction of gram-negative bacteria in the UC [urine culture], urine GS [Gram stain] does not improve UTI diagnosis at the ED compared to other urine parameters,” the authors wrote.
SOURCE:
The study was led by Stephanie J.M. Middelkoop, University of Groningen, University Medical Center Groningen, the Netherlands. It was published online on August 16, 2024, in Infectious Diseases.
LIMITATIONS:
The study’s limitations included a small sample size within the leukopenia subgroup, which may have affected the generalizability of the findings. Additionally, the potential influence of refrigeration of urine samples on bacterial growth could have affected the results. In this study, indwelling catheters were not replaced before urine sample collection, which may have affected the accuracy of UTI diagnosis in patients using catheters.
DISCLOSURES:
No conflicts of interest were disclosed by the authors.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Listeriosis During Pregnancy Can Be Fatal for the Fetus
Listeriosis during pregnancy, when invasive, can be fatal for the fetus, with a rate of fetal loss or neonatal death of 29%, investigators reported in an article alerting clinicians to this condition.
The article was prompted when the Reproductive Infectious Diseases team at The University of British Columbia in Vancouver, British Columbia, Canada, “received many phone calls from concerned doctors and patients after the plant-based milk recall in early July,” Jeffrey Man Hay Wong, MD, told this news organization. “With such concerns, we updated our British Columbia guidelines for our patients but quickly realized that our recommendations would be useful across the country.”
The article was published online in the Canadian Medical Association Journal.
Five Key Points
Dr. Wong and colleagues provided the following five points and recommendations:
First, invasive listeriosis (bacteremia or meningitis) in pregnancy can have major fetal consequences, including fetal loss or neonatal death in 29% of cases. Affected patients can be asymptomatic or experience gastrointestinal symptoms, myalgias, fevers, acute respiratory distress syndrome, or sepsis.
Second, pregnant people should avoid foods at a high risk for Listeria monocytogenes contamination, including unpasteurized dairy products, luncheon meats, refrigerated meat spreads, and prepared salads. They also should stay aware of Health Canada recalls.
Third, it is not necessary to investigate or treat patients who may have ingested contaminated food but are asymptomatic. Listeriosis can present at 2-3 months after exposure because the incubation period can be as long as 70 days.
Fourth, for patients with mild gastroenteritis or flu-like symptoms who may have ingested contaminated food, obtaining blood cultures or starting a 2-week course of oral amoxicillin (500 mg, three times daily) could be considered.
Fifth, for patients with fever and possible exposure to L monocytogenes, blood cultures should be drawn immediately, and high-dose ampicillin should be initiated, along with electronic fetal heart rate monitoring.
“While choosing safer foods in pregnancy is recommended, it is most important to be aware of Health Canada food recalls and pay attention to symptoms if you’ve ingested these foods,” said Dr. Wong. “Working with the BC Centre for Disease Control, our teams are actively monitoring for cases of listeriosis in pregnancy here in British Columbia.
“Thankfully,” he said, “there haven’t been any confirmed cases in British Columbia related to the plant-based milk recall, though the bacteria’s incubation period can be up to 70 days in pregnancy.”
No Increase Suspected
Commenting on the article, Khady Diouf, MD, director of global obstetrics and gynecology at Brigham and Women’s Hospital in Boston, said, “It summarizes the main management, which is based mostly on expert opinion.”
US clinicians also should be reminded about listeriosis in pregnancy, she noted, pointing to “helpful guidance” from the American College of Obstetrics and Gynecology.
Although the United States similarly experienced a recent listeriosis outbreak resulting from contaminated deli meats, both Dr. Wong and Dr. Diouf said that these outbreaks do not seem to signal an increase in listeriosis cases overall.
“Food-borne listeriosis seems to come in waves,” said Dr. Wong. “At a public health level, we certainly have better surveillance programs for Listeria infections. In 2023, Health Canada updated its Policy on L monocytogenes in ready-to-eat foods, which emphasizes the good manufacturing practices recommended for food processing environments to identify outbreaks earlier.”
“I think we get these recalls yearly, and this has been the case for as long as I can remember,” Dr. Diouf agreed.
No funding was declared, and the authors declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Listeriosis during pregnancy, when invasive, can be fatal for the fetus, with a rate of fetal loss or neonatal death of 29%, investigators reported in an article alerting clinicians to this condition.
The article was prompted when the Reproductive Infectious Diseases team at The University of British Columbia in Vancouver, British Columbia, Canada, “received many phone calls from concerned doctors and patients after the plant-based milk recall in early July,” Jeffrey Man Hay Wong, MD, told this news organization. “With such concerns, we updated our British Columbia guidelines for our patients but quickly realized that our recommendations would be useful across the country.”
The article was published online in the Canadian Medical Association Journal.
Five Key Points
Dr. Wong and colleagues provided the following five points and recommendations:
First, invasive listeriosis (bacteremia or meningitis) in pregnancy can have major fetal consequences, including fetal loss or neonatal death in 29% of cases. Affected patients can be asymptomatic or experience gastrointestinal symptoms, myalgias, fevers, acute respiratory distress syndrome, or sepsis.
Second, pregnant people should avoid foods at a high risk for Listeria monocytogenes contamination, including unpasteurized dairy products, luncheon meats, refrigerated meat spreads, and prepared salads. They also should stay aware of Health Canada recalls.
Third, it is not necessary to investigate or treat patients who may have ingested contaminated food but are asymptomatic. Listeriosis can present at 2-3 months after exposure because the incubation period can be as long as 70 days.
Fourth, for patients with mild gastroenteritis or flu-like symptoms who may have ingested contaminated food, obtaining blood cultures or starting a 2-week course of oral amoxicillin (500 mg, three times daily) could be considered.
Fifth, for patients with fever and possible exposure to L monocytogenes, blood cultures should be drawn immediately, and high-dose ampicillin should be initiated, along with electronic fetal heart rate monitoring.
“While choosing safer foods in pregnancy is recommended, it is most important to be aware of Health Canada food recalls and pay attention to symptoms if you’ve ingested these foods,” said Dr. Wong. “Working with the BC Centre for Disease Control, our teams are actively monitoring for cases of listeriosis in pregnancy here in British Columbia.
“Thankfully,” he said, “there haven’t been any confirmed cases in British Columbia related to the plant-based milk recall, though the bacteria’s incubation period can be up to 70 days in pregnancy.”
No Increase Suspected
Commenting on the article, Khady Diouf, MD, director of global obstetrics and gynecology at Brigham and Women’s Hospital in Boston, said, “It summarizes the main management, which is based mostly on expert opinion.”
US clinicians also should be reminded about listeriosis in pregnancy, she noted, pointing to “helpful guidance” from the American College of Obstetrics and Gynecology.
Although the United States similarly experienced a recent listeriosis outbreak resulting from contaminated deli meats, both Dr. Wong and Dr. Diouf said that these outbreaks do not seem to signal an increase in listeriosis cases overall.
“Food-borne listeriosis seems to come in waves,” said Dr. Wong. “At a public health level, we certainly have better surveillance programs for Listeria infections. In 2023, Health Canada updated its Policy on L monocytogenes in ready-to-eat foods, which emphasizes the good manufacturing practices recommended for food processing environments to identify outbreaks earlier.”
“I think we get these recalls yearly, and this has been the case for as long as I can remember,” Dr. Diouf agreed.
No funding was declared, and the authors declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Listeriosis during pregnancy, when invasive, can be fatal for the fetus, with a rate of fetal loss or neonatal death of 29%, investigators reported in an article alerting clinicians to this condition.
The article was prompted when the Reproductive Infectious Diseases team at The University of British Columbia in Vancouver, British Columbia, Canada, “received many phone calls from concerned doctors and patients after the plant-based milk recall in early July,” Jeffrey Man Hay Wong, MD, told this news organization. “With such concerns, we updated our British Columbia guidelines for our patients but quickly realized that our recommendations would be useful across the country.”
The article was published online in the Canadian Medical Association Journal.
Five Key Points
Dr. Wong and colleagues provided the following five points and recommendations:
First, invasive listeriosis (bacteremia or meningitis) in pregnancy can have major fetal consequences, including fetal loss or neonatal death in 29% of cases. Affected patients can be asymptomatic or experience gastrointestinal symptoms, myalgias, fevers, acute respiratory distress syndrome, or sepsis.
Second, pregnant people should avoid foods at a high risk for Listeria monocytogenes contamination, including unpasteurized dairy products, luncheon meats, refrigerated meat spreads, and prepared salads. They also should stay aware of Health Canada recalls.
Third, it is not necessary to investigate or treat patients who may have ingested contaminated food but are asymptomatic. Listeriosis can present at 2-3 months after exposure because the incubation period can be as long as 70 days.
Fourth, for patients with mild gastroenteritis or flu-like symptoms who may have ingested contaminated food, obtaining blood cultures or starting a 2-week course of oral amoxicillin (500 mg, three times daily) could be considered.
Fifth, for patients with fever and possible exposure to L monocytogenes, blood cultures should be drawn immediately, and high-dose ampicillin should be initiated, along with electronic fetal heart rate monitoring.
“While choosing safer foods in pregnancy is recommended, it is most important to be aware of Health Canada food recalls and pay attention to symptoms if you’ve ingested these foods,” said Dr. Wong. “Working with the BC Centre for Disease Control, our teams are actively monitoring for cases of listeriosis in pregnancy here in British Columbia.
“Thankfully,” he said, “there haven’t been any confirmed cases in British Columbia related to the plant-based milk recall, though the bacteria’s incubation period can be up to 70 days in pregnancy.”
No Increase Suspected
Commenting on the article, Khady Diouf, MD, director of global obstetrics and gynecology at Brigham and Women’s Hospital in Boston, said, “It summarizes the main management, which is based mostly on expert opinion.”
US clinicians also should be reminded about listeriosis in pregnancy, she noted, pointing to “helpful guidance” from the American College of Obstetrics and Gynecology.
Although the United States similarly experienced a recent listeriosis outbreak resulting from contaminated deli meats, both Dr. Wong and Dr. Diouf said that these outbreaks do not seem to signal an increase in listeriosis cases overall.
“Food-borne listeriosis seems to come in waves,” said Dr. Wong. “At a public health level, we certainly have better surveillance programs for Listeria infections. In 2023, Health Canada updated its Policy on L monocytogenes in ready-to-eat foods, which emphasizes the good manufacturing practices recommended for food processing environments to identify outbreaks earlier.”
“I think we get these recalls yearly, and this has been the case for as long as I can remember,” Dr. Diouf agreed.
No funding was declared, and the authors declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE CANADIAN MEDICAL ASSOCIATION JOURNAL
Multiple Draining Sinus Tracts on the Thigh
The Diagnosis: Mycobacterial Infection
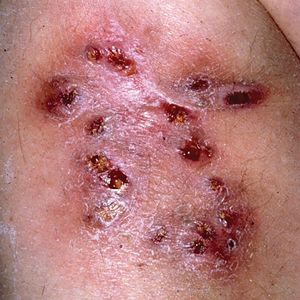
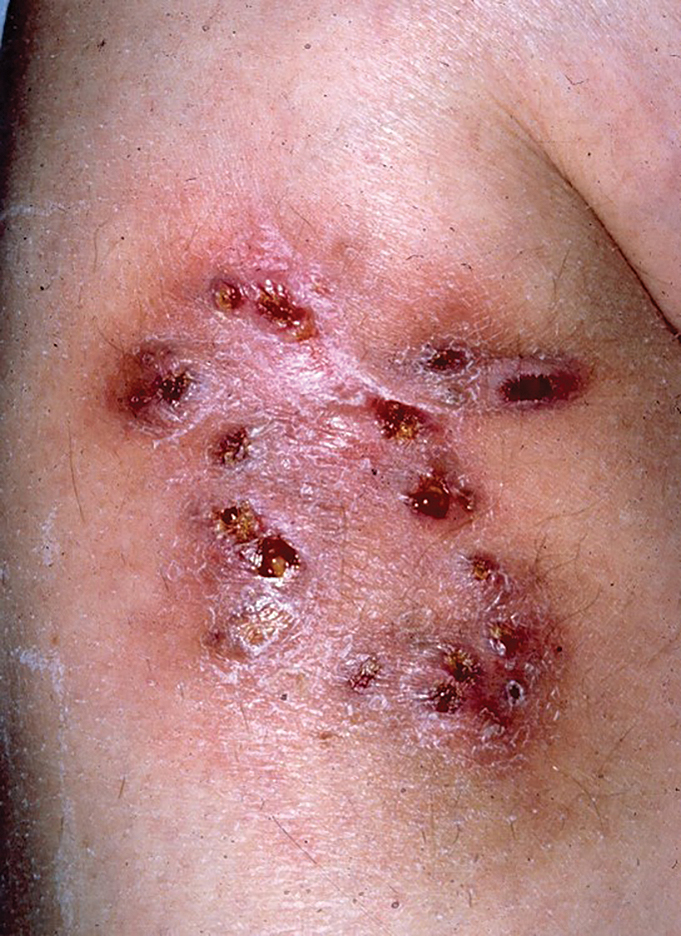
An injury sustained in a wet environment that results in chronic indolent abscesses, nodules, or draining sinus tracts suggests a mycobacterial infection. In our patient, a culture revealed MycobacteriuM fortuitum, which is classified in the rapid grower nontuberculous mycobacteria (NTM) group, along with Mycobacterium chelonae and Mycobacterium abscessus.1 The patient’s history of skin injury while cutting wet grass and the common presence of M fortuitum in the environment suggested that the organism entered the wound. The patient healed completely following surgical excision and a 2-month course of clarithromycin 1 g daily and rifampin 600 mg daily.
MycobacteriuM fortuitum was first isolated from an amphibian source in 1905 and later identified in a human with cutaneous infection in 1938. It commonly is found in soil and water.2 Skin and soft-tissue infections with M fortuitum usually are acquired from direct entry of the organism through a damaged skin barrier from trauma, medical injection, surgery, or tattoo placement.2,3
Skin lesions caused by NTM often are nonspecific and can mimic a variety of other dermatologic conditions, making clinical diagnosis challenging. As such, cutaneous manifestations of M fortuitum infection can include recurrent cutaneous abscesses, nodular lesions, chronic discharging sinuses, cellulitis, and surgical site infections.4 Although cutaneous infection with M fortuitum classically manifests with a single subcutaneous nodule at the site of trauma or surgery,5 it also can manifest as multiple draining sinus tracts, as seen in our patient. Hence, the diagnosis and treatment of cutaneous NTM infection is challenging, especially when M fortuitum skin manifestations can take up to 4 to 6 weeks to develop after inoculation. Diagnosis often requires a detailed patient history, tissue cultures, and histopathology.5
In recent years, rapid detection with polymerase chain reaction (PCR) techniques has been employed more widely. Notably, a molecular system based on multiplex real-time PCR with high-resolution melting was shown to have a sensitivity of up to 54% for distinguishing M fortuitum from other NTM.6 More recently, a 2-step real-time PCR method has demonstrated diagnostic sensitivity and specificity for differentiating NTM from Mycobacterium tuberculosis infections and identifying the causative NTM agent.7
Compared to immunocompetent individuals, those who are immunocompromised are more susceptible to less pathogenic strains of NTM, which can cause dissemination and lead to tenosynovitis, myositis, osteomyelitis, and septic arthritis.8-12 Nonetheless, cases of infections with NTM—including M fortuitum—are becoming harder to treat. Several single nucleotide polymorphisms and point mutations have been demonstrated in the ribosomal RNA methylase gene erm(39) related to clarithromycin resistance and in the rrl gene related to linezolid resistance.13 Due to increasing inducible resistance to common classes of antibiotics, such as macrolides and linezolid, treatment of M fortuitum requires multidrug regimens.13,14 Drug susceptibility testing also may be required, as M fortuitum has shown low resistance to tigecycline, tetracycline, cefmetazole, imipenem, and aminoglycosides (eg, amikacin, tobramycin, neomycin, gentamycin). Surgery is an important adjunctive tool in treating M fortuitum infections; patients with a single lesion are more likely to undergo surgical treatment alone or in combination with antibiotic therapy.15 More recently, antimicrobial photodynamic therapy has been explored as an alternative to eliminate NTM, including M fortuitum.16
The differential diagnosis for skin lesions manifesting with draining fistulae and sinus tracts includes conditions with infectious (cellulitis and chromomycosis) and inflammatory (pyoderma gangrenosum [PG] and hidradenitis suppurativa [HS]) causes.
Cellulitis is a common infection of the skin and subcutaneous tissue that predominantly is caused by gram-positive organisms such as β-hemolytic streptococci.17 Clinical manifestations include acute skin erythema, swelling, tenderness, and warmth. The legs are the most common sites of infection, but any area of the skin can be involved.17 Cellulitis comprises 10% of all infectious disease hospitalizations and up to 11% of all dermatologic admissions.18,19 It frequently is misdiagnosed, perhaps due to the lack of a reliable confirmatory laboratory test or imaging study, in addition to the plethora of diseases that mimic cellulitis, such as stasis dermatitis, lipodermatosclerosis, contact dermatitis, lymphedema, eosinophilic cellulitis, and papular urticaria.20,21 The consequences of misdiagnosis include but are not limited to unnecessary hospitalizations, inappropriate antibiotic use, and delayed management of the disease; thus, there is an urgent need for a reliable standard test to confirm the diagnosis, especially among nonspecialist physicians. 20 Most patients with uncomplicated cellulitis can be treated with empiric oral antibiotics that target β-hemolytic streptococci (ie, penicillin V potassium, amoxicillin).17 Methicillin-resistant Staphylococcus aureus coverage generally is unnecessary for nonpurulent cellulitis, but clinicians can consider adding amoxicillin-clavulanate, dicloxacillin, and cephalexin to the regimen. For purulent cellulitis, incision and drainage should be performed. In severe cases that manifest with sepsis, altered mental status, or hemodynamic instability, inpatient management is required.17
Chromomycosis (also known as chromoblastomycosis) is a chronic, indolent, granulomatous, suppurative mycosis of the skin and subcutaneous tissue22 that is caused by traumatic inoculation of various fungi of the order Chaetothyriales and family Herpotrichiellaceae, which are present in soil, plants, and decomposing wood. Chromomycosis is prevalent in tropical and subtropical regions.23,24 Clinically, it manifests as oligosymptomatic or asymptomatic lesions around an infection site that can manifest as papules with centrifugal growth evolving into nodular, verrucous, plaque, tumoral, or atrophic forms.22 Diagnosis is made with direct microscopy using potassium hydroxide, which reveals muriform bodies. Fungal culture in Sabouraud agar also can be used to isolate the causative pathogen.22 Unfortunately, chromomycosis is difficult to treat, with low cure rates and high relapse rates. Antifungal agents combined with surgery, cryotherapy, or thermotherapy often are used, with cure rates ranging from 15% to 80%.22,25
Pyoderma gangrenosum is a reactive noninfectious inflammatory dermatosis associated with inflammatory bowel disease and rheumatoid arthritis. The exact etiology is not clearly understood, but it generally is considered an autoinflammatory disorder.26 The most common form—classical PG—occurs in approximately 85% of cases and manifests as a painful erythematous lesion that progresses to a blistered or necrotic ulcer. It primarily affects the lower legs but can occur in other body sites.27 The diagnosis is based on clinical symptoms after excluding other similar conditions; histopathology of biopsied wound tissues often are required for confirmation. Treatment of PG starts with fast-acting immunosuppressive drugs (corticosteroids and/or cyclosporine) followed by slowacting immunosuppressive drugs (biologics).26
Hidradenitis suppurativa is a chronic recurrent disease of the hair follicle unit that develops after puberty.28 Clinically, HS manifests with painful nodules, abscesses, chronically draining fistulas, and scarring in areas of the body rich in apocrine glands.29,30 Treatment of HS is challenging due to its diverse clinical manifestations and unclear etiology. Topical therapy, systemic treatments, biologic agents, surgery, and light therapy have shown variable results.28,31
- Franco-Paredes C, Marcos LA, Henao-Martínez AF, et al. Cutaneous mycobacterial infections. Clin Microbiol Rev. 2018;32: E00069-18. doi:10.1128/CMR.00069-18
- Brown TH. The rapidly growing mycobacteria—MycobacteriuM fortuitum and Mycobacterium chelonae. Infect Control. 1985;6:283-238. doi:10.1017/s0195941700061762
- Hooper J; Beltrami EJ; Santoro F; et al. Remember the fite: a case of cutaneous MycobacteriuM fortuitum infection. Am J Dermatopathol. 2023;45:214-215. doi:10.1097/DAD.0000000000002336
- Franco-Paredes C, Chastain DB, Allen L, et al. Overview of cutaneous mycobacterial infections. Curr Trop Med Rep. 2018;5:228-232. doi:10.1007/s40475-018-0161-7
- Gonzalez-Santiago TM, Drage LA. Nontuberculous mycobacteria: skin and soft tissue infections. Dermatol Clin. 2015;33:563-77. doi:10.1016/j.det.2015.03.017
- Peixoto ADS, Montenegro LML, Lima AS, et al. Identification of nontuberculous mycobacteria species by multiplex real-time PCR with high-resolution melting. Rev Soc Bras Med Trop. 2020;53:E20200211. doi:10.1590/0037-8682-0211-2020
- Park J, Kwak N, Chae JC, et al. A two-step real-time PCR method to identify Mycobacterium tuberculosis infections and six dominant nontuberculous mycobacterial infections from clinical specimens. Microbiol Spectr. 2023:E0160623. doi:10.1128/spectrum.01606-23
- Fowler J, Mahlen SD. Localized cutaneous infections in immunocompetent individuals due to rapidly growing mycobacteria. Arch Pathol Lab Med. 2014;138:1106-1109. doi:10.5858/arpa.2012-0203-RS
- Gardini G, Gregori N, Matteelli A, et al. Mycobacterial skin infection. Curr Opin Infect Dis. 2022;35:79-87. doi:10.1097/QCO.0000000000000820
- Wang SH, Pancholi P. Mycobacterial skin and soft tissue infection. Curr Infect Dis Rep. 2014;16:438. doi:10.1007/s11908-014-0438-5
- Griffith DE, Aksamit T, Brown-Elliott BA, et al; ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416. doi:10.1164/rccm.200604-571ST
- Mougari F, Guglielmetti L, Raskine L, et al. Infections caused by Mycobacterium abscessus: epidemiology, diagnostic tools and treatment. Expert Rev Anti Infect Ther. 2016;14:1139-1154. doi:10.1080/14787210.201 6.1238304
- Tu HZ, Lee HS, Chen YS, et al. High rates of antimicrobial resistance in rapidly growing mycobacterial infections in Taiwan. Pathogens. 2022;11:969. doi:10.3390/pathogens11090969
- Hashemzadeh M, Zadegan Dezfuli AA, Khosravi AD, et al. F requency of mutations in erm(39) related to clarithromycin resistance and in rrl related to linezolid resistance in clinical isolates of MycobacteriuM fortuitum in Iran. Acta Microbiol Immunol Hung. 2023;70:167-176. doi:10.1556/030.2023.02020
- Uslan DZ, Kowalski TJ, Wengenack NL, et al. Skin and soft tissue infections due to rapidly growing mycobacteria: comparison of clinical features, treatment, and susceptibility. Arch Dermatol. 2006;142:1287-1292. doi:10.1001/archderm.142.10.1287
- Miretti M, Juri L, Peralta A, et al. Photoinactivation of non-tuberculous mycobacteria using Zn-phthalocyanine loaded into liposomes. Tuberculosis (Edinb). 2022;136:102247. doi:10.1016/j.tube.2022.102247
- Bystritsky RJ. Cellulitis. Infect Dis Clin North Am. 2021;35:49-60. doi:10.1016/j.idc.2020.10.002
- Christensen K, Holman R, Steiner C, et al. Infectious disease hospitalizations in the United States. Clin Infect Dis. 2009;49:1025-1035. doi:10.1086/605562
- Yang JJ, Maloney NJ, Bach DQ, et al. Dermatology in the emergency department: prescriptions, rates of inpatient admission, and predictors of high utilization in the United States from 1996 to 2012. J Am Acad Dermatol. 2021;84:1480-1483. doi:10.1016/J.JAAD.2020.07.055
- Cutler TS, Jannat-Khah DP, Kam B, et al. Prevalence of misdiagnosis of cellulitis: a systematic review and meta-analysis. J Hosp Med. 2023;18:254-261. doi:10.1002/jhm.12977
- Keller EC, Tomecki KJ, Alraies MC. Distinguishing cellulitis from its mimics. Cleve Clin J Med. 2012;79:547-52. doi:10.3949/ccjm.79a.11121
- Brito AC, Bittencourt MJS. Chromoblastomycosis: an etiological, epidemiological, clinical, diagnostic, and treatment update. An Bras Dermatol. 2018;93:495-506. doi:10.1590/abd1806-4841.20187321
- McGinnis MR. Chromoblastomycosis and phaeohyphomycosis: new concepts, diagnosis, and mycology. J Am Acad Dermatol. 1983;8:1-16.
- Rubin HA, Bruce S, Rosen T, et al. Evidence for percutaneous inoculation as the mode of transmission for chromoblastomycosis. J Am Acad Dermatol. 1991;25:951-954.
- Bonifaz A, Paredes-Solís V, Saúl A. Treating chromoblastomycosis with systemic antifungals. Expert Opin Pharmacother. 2004;5:247-254.
- Maverakis E, Marzano AV, Le ST, et al. Pyoderma gangrenosum. Nat Rev Dis Primers. 2020;6:81. doi:10.1038/s41572-020-0213-x
- George C, Deroide F, Rustin M. Pyoderma gangrenosum—a guide to diagnosis and management. Clin Med (Lond). 2019;19:224-228. doi:10.7861/clinmedicine.19-3-224
- Narla S, Lyons AB, Hamzavi IH. The most recent advances in understanding and managing hidradenitis suppurativa. F1000Res. 2020;9:F1000 Faculty Rev-1049. doi:10.12688/f1000research.26083.1
- Garg A, Lavian J, Lin G, et al. Incidence of hidradenitis suppurativa in the United States: a sex- and age-adjusted population analysis. J Am Acad Dermatol. 2017;77:118-122. doi:10.1016/j.jaad.2017.02.005
- Daxhelet M, Suppa M, White J, et al. Proposed definitions of typical lesions in hidradenitis suppurativa. Dermatology. 2020;236:431-438. doi:10.1159/000507348
- Amat-Samaranch V, Agut-Busquet E, Vilarrasa E, et al. New perspectives on the treatment of hidradenitis suppurativa. Ther Adv Chronic Dis. 2021;12:20406223211055920. doi:10.1177/20406223211055920
The Diagnosis: Mycobacterial Infection
An injury sustained in a wet environment that results in chronic indolent abscesses, nodules, or draining sinus tracts suggests a mycobacterial infection. In our patient, a culture revealed MycobacteriuM fortuitum, which is classified in the rapid grower nontuberculous mycobacteria (NTM) group, along with Mycobacterium chelonae and Mycobacterium abscessus.1 The patient’s history of skin injury while cutting wet grass and the common presence of M fortuitum in the environment suggested that the organism entered the wound. The patient healed completely following surgical excision and a 2-month course of clarithromycin 1 g daily and rifampin 600 mg daily.
MycobacteriuM fortuitum was first isolated from an amphibian source in 1905 and later identified in a human with cutaneous infection in 1938. It commonly is found in soil and water.2 Skin and soft-tissue infections with M fortuitum usually are acquired from direct entry of the organism through a damaged skin barrier from trauma, medical injection, surgery, or tattoo placement.2,3
Skin lesions caused by NTM often are nonspecific and can mimic a variety of other dermatologic conditions, making clinical diagnosis challenging. As such, cutaneous manifestations of M fortuitum infection can include recurrent cutaneous abscesses, nodular lesions, chronic discharging sinuses, cellulitis, and surgical site infections.4 Although cutaneous infection with M fortuitum classically manifests with a single subcutaneous nodule at the site of trauma or surgery,5 it also can manifest as multiple draining sinus tracts, as seen in our patient. Hence, the diagnosis and treatment of cutaneous NTM infection is challenging, especially when M fortuitum skin manifestations can take up to 4 to 6 weeks to develop after inoculation. Diagnosis often requires a detailed patient history, tissue cultures, and histopathology.5
In recent years, rapid detection with polymerase chain reaction (PCR) techniques has been employed more widely. Notably, a molecular system based on multiplex real-time PCR with high-resolution melting was shown to have a sensitivity of up to 54% for distinguishing M fortuitum from other NTM.6 More recently, a 2-step real-time PCR method has demonstrated diagnostic sensitivity and specificity for differentiating NTM from Mycobacterium tuberculosis infections and identifying the causative NTM agent.7
Compared to immunocompetent individuals, those who are immunocompromised are more susceptible to less pathogenic strains of NTM, which can cause dissemination and lead to tenosynovitis, myositis, osteomyelitis, and septic arthritis.8-12 Nonetheless, cases of infections with NTM—including M fortuitum—are becoming harder to treat. Several single nucleotide polymorphisms and point mutations have been demonstrated in the ribosomal RNA methylase gene erm(39) related to clarithromycin resistance and in the rrl gene related to linezolid resistance.13 Due to increasing inducible resistance to common classes of antibiotics, such as macrolides and linezolid, treatment of M fortuitum requires multidrug regimens.13,14 Drug susceptibility testing also may be required, as M fortuitum has shown low resistance to tigecycline, tetracycline, cefmetazole, imipenem, and aminoglycosides (eg, amikacin, tobramycin, neomycin, gentamycin). Surgery is an important adjunctive tool in treating M fortuitum infections; patients with a single lesion are more likely to undergo surgical treatment alone or in combination with antibiotic therapy.15 More recently, antimicrobial photodynamic therapy has been explored as an alternative to eliminate NTM, including M fortuitum.16
The differential diagnosis for skin lesions manifesting with draining fistulae and sinus tracts includes conditions with infectious (cellulitis and chromomycosis) and inflammatory (pyoderma gangrenosum [PG] and hidradenitis suppurativa [HS]) causes.
Cellulitis is a common infection of the skin and subcutaneous tissue that predominantly is caused by gram-positive organisms such as β-hemolytic streptococci.17 Clinical manifestations include acute skin erythema, swelling, tenderness, and warmth. The legs are the most common sites of infection, but any area of the skin can be involved.17 Cellulitis comprises 10% of all infectious disease hospitalizations and up to 11% of all dermatologic admissions.18,19 It frequently is misdiagnosed, perhaps due to the lack of a reliable confirmatory laboratory test or imaging study, in addition to the plethora of diseases that mimic cellulitis, such as stasis dermatitis, lipodermatosclerosis, contact dermatitis, lymphedema, eosinophilic cellulitis, and papular urticaria.20,21 The consequences of misdiagnosis include but are not limited to unnecessary hospitalizations, inappropriate antibiotic use, and delayed management of the disease; thus, there is an urgent need for a reliable standard test to confirm the diagnosis, especially among nonspecialist physicians. 20 Most patients with uncomplicated cellulitis can be treated with empiric oral antibiotics that target β-hemolytic streptococci (ie, penicillin V potassium, amoxicillin).17 Methicillin-resistant Staphylococcus aureus coverage generally is unnecessary for nonpurulent cellulitis, but clinicians can consider adding amoxicillin-clavulanate, dicloxacillin, and cephalexin to the regimen. For purulent cellulitis, incision and drainage should be performed. In severe cases that manifest with sepsis, altered mental status, or hemodynamic instability, inpatient management is required.17
Chromomycosis (also known as chromoblastomycosis) is a chronic, indolent, granulomatous, suppurative mycosis of the skin and subcutaneous tissue22 that is caused by traumatic inoculation of various fungi of the order Chaetothyriales and family Herpotrichiellaceae, which are present in soil, plants, and decomposing wood. Chromomycosis is prevalent in tropical and subtropical regions.23,24 Clinically, it manifests as oligosymptomatic or asymptomatic lesions around an infection site that can manifest as papules with centrifugal growth evolving into nodular, verrucous, plaque, tumoral, or atrophic forms.22 Diagnosis is made with direct microscopy using potassium hydroxide, which reveals muriform bodies. Fungal culture in Sabouraud agar also can be used to isolate the causative pathogen.22 Unfortunately, chromomycosis is difficult to treat, with low cure rates and high relapse rates. Antifungal agents combined with surgery, cryotherapy, or thermotherapy often are used, with cure rates ranging from 15% to 80%.22,25
Pyoderma gangrenosum is a reactive noninfectious inflammatory dermatosis associated with inflammatory bowel disease and rheumatoid arthritis. The exact etiology is not clearly understood, but it generally is considered an autoinflammatory disorder.26 The most common form—classical PG—occurs in approximately 85% of cases and manifests as a painful erythematous lesion that progresses to a blistered or necrotic ulcer. It primarily affects the lower legs but can occur in other body sites.27 The diagnosis is based on clinical symptoms after excluding other similar conditions; histopathology of biopsied wound tissues often are required for confirmation. Treatment of PG starts with fast-acting immunosuppressive drugs (corticosteroids and/or cyclosporine) followed by slowacting immunosuppressive drugs (biologics).26
Hidradenitis suppurativa is a chronic recurrent disease of the hair follicle unit that develops after puberty.28 Clinically, HS manifests with painful nodules, abscesses, chronically draining fistulas, and scarring in areas of the body rich in apocrine glands.29,30 Treatment of HS is challenging due to its diverse clinical manifestations and unclear etiology. Topical therapy, systemic treatments, biologic agents, surgery, and light therapy have shown variable results.28,31
The Diagnosis: Mycobacterial Infection
An injury sustained in a wet environment that results in chronic indolent abscesses, nodules, or draining sinus tracts suggests a mycobacterial infection. In our patient, a culture revealed MycobacteriuM fortuitum, which is classified in the rapid grower nontuberculous mycobacteria (NTM) group, along with Mycobacterium chelonae and Mycobacterium abscessus.1 The patient’s history of skin injury while cutting wet grass and the common presence of M fortuitum in the environment suggested that the organism entered the wound. The patient healed completely following surgical excision and a 2-month course of clarithromycin 1 g daily and rifampin 600 mg daily.
MycobacteriuM fortuitum was first isolated from an amphibian source in 1905 and later identified in a human with cutaneous infection in 1938. It commonly is found in soil and water.2 Skin and soft-tissue infections with M fortuitum usually are acquired from direct entry of the organism through a damaged skin barrier from trauma, medical injection, surgery, or tattoo placement.2,3
Skin lesions caused by NTM often are nonspecific and can mimic a variety of other dermatologic conditions, making clinical diagnosis challenging. As such, cutaneous manifestations of M fortuitum infection can include recurrent cutaneous abscesses, nodular lesions, chronic discharging sinuses, cellulitis, and surgical site infections.4 Although cutaneous infection with M fortuitum classically manifests with a single subcutaneous nodule at the site of trauma or surgery,5 it also can manifest as multiple draining sinus tracts, as seen in our patient. Hence, the diagnosis and treatment of cutaneous NTM infection is challenging, especially when M fortuitum skin manifestations can take up to 4 to 6 weeks to develop after inoculation. Diagnosis often requires a detailed patient history, tissue cultures, and histopathology.5
In recent years, rapid detection with polymerase chain reaction (PCR) techniques has been employed more widely. Notably, a molecular system based on multiplex real-time PCR with high-resolution melting was shown to have a sensitivity of up to 54% for distinguishing M fortuitum from other NTM.6 More recently, a 2-step real-time PCR method has demonstrated diagnostic sensitivity and specificity for differentiating NTM from Mycobacterium tuberculosis infections and identifying the causative NTM agent.7
Compared to immunocompetent individuals, those who are immunocompromised are more susceptible to less pathogenic strains of NTM, which can cause dissemination and lead to tenosynovitis, myositis, osteomyelitis, and septic arthritis.8-12 Nonetheless, cases of infections with NTM—including M fortuitum—are becoming harder to treat. Several single nucleotide polymorphisms and point mutations have been demonstrated in the ribosomal RNA methylase gene erm(39) related to clarithromycin resistance and in the rrl gene related to linezolid resistance.13 Due to increasing inducible resistance to common classes of antibiotics, such as macrolides and linezolid, treatment of M fortuitum requires multidrug regimens.13,14 Drug susceptibility testing also may be required, as M fortuitum has shown low resistance to tigecycline, tetracycline, cefmetazole, imipenem, and aminoglycosides (eg, amikacin, tobramycin, neomycin, gentamycin). Surgery is an important adjunctive tool in treating M fortuitum infections; patients with a single lesion are more likely to undergo surgical treatment alone or in combination with antibiotic therapy.15 More recently, antimicrobial photodynamic therapy has been explored as an alternative to eliminate NTM, including M fortuitum.16
The differential diagnosis for skin lesions manifesting with draining fistulae and sinus tracts includes conditions with infectious (cellulitis and chromomycosis) and inflammatory (pyoderma gangrenosum [PG] and hidradenitis suppurativa [HS]) causes.
Cellulitis is a common infection of the skin and subcutaneous tissue that predominantly is caused by gram-positive organisms such as β-hemolytic streptococci.17 Clinical manifestations include acute skin erythema, swelling, tenderness, and warmth. The legs are the most common sites of infection, but any area of the skin can be involved.17 Cellulitis comprises 10% of all infectious disease hospitalizations and up to 11% of all dermatologic admissions.18,19 It frequently is misdiagnosed, perhaps due to the lack of a reliable confirmatory laboratory test or imaging study, in addition to the plethora of diseases that mimic cellulitis, such as stasis dermatitis, lipodermatosclerosis, contact dermatitis, lymphedema, eosinophilic cellulitis, and papular urticaria.20,21 The consequences of misdiagnosis include but are not limited to unnecessary hospitalizations, inappropriate antibiotic use, and delayed management of the disease; thus, there is an urgent need for a reliable standard test to confirm the diagnosis, especially among nonspecialist physicians. 20 Most patients with uncomplicated cellulitis can be treated with empiric oral antibiotics that target β-hemolytic streptococci (ie, penicillin V potassium, amoxicillin).17 Methicillin-resistant Staphylococcus aureus coverage generally is unnecessary for nonpurulent cellulitis, but clinicians can consider adding amoxicillin-clavulanate, dicloxacillin, and cephalexin to the regimen. For purulent cellulitis, incision and drainage should be performed. In severe cases that manifest with sepsis, altered mental status, or hemodynamic instability, inpatient management is required.17
Chromomycosis (also known as chromoblastomycosis) is a chronic, indolent, granulomatous, suppurative mycosis of the skin and subcutaneous tissue22 that is caused by traumatic inoculation of various fungi of the order Chaetothyriales and family Herpotrichiellaceae, which are present in soil, plants, and decomposing wood. Chromomycosis is prevalent in tropical and subtropical regions.23,24 Clinically, it manifests as oligosymptomatic or asymptomatic lesions around an infection site that can manifest as papules with centrifugal growth evolving into nodular, verrucous, plaque, tumoral, or atrophic forms.22 Diagnosis is made with direct microscopy using potassium hydroxide, which reveals muriform bodies. Fungal culture in Sabouraud agar also can be used to isolate the causative pathogen.22 Unfortunately, chromomycosis is difficult to treat, with low cure rates and high relapse rates. Antifungal agents combined with surgery, cryotherapy, or thermotherapy often are used, with cure rates ranging from 15% to 80%.22,25
Pyoderma gangrenosum is a reactive noninfectious inflammatory dermatosis associated with inflammatory bowel disease and rheumatoid arthritis. The exact etiology is not clearly understood, but it generally is considered an autoinflammatory disorder.26 The most common form—classical PG—occurs in approximately 85% of cases and manifests as a painful erythematous lesion that progresses to a blistered or necrotic ulcer. It primarily affects the lower legs but can occur in other body sites.27 The diagnosis is based on clinical symptoms after excluding other similar conditions; histopathology of biopsied wound tissues often are required for confirmation. Treatment of PG starts with fast-acting immunosuppressive drugs (corticosteroids and/or cyclosporine) followed by slowacting immunosuppressive drugs (biologics).26
Hidradenitis suppurativa is a chronic recurrent disease of the hair follicle unit that develops after puberty.28 Clinically, HS manifests with painful nodules, abscesses, chronically draining fistulas, and scarring in areas of the body rich in apocrine glands.29,30 Treatment of HS is challenging due to its diverse clinical manifestations and unclear etiology. Topical therapy, systemic treatments, biologic agents, surgery, and light therapy have shown variable results.28,31
- Franco-Paredes C, Marcos LA, Henao-Martínez AF, et al. Cutaneous mycobacterial infections. Clin Microbiol Rev. 2018;32: E00069-18. doi:10.1128/CMR.00069-18
- Brown TH. The rapidly growing mycobacteria—MycobacteriuM fortuitum and Mycobacterium chelonae. Infect Control. 1985;6:283-238. doi:10.1017/s0195941700061762
- Hooper J; Beltrami EJ; Santoro F; et al. Remember the fite: a case of cutaneous MycobacteriuM fortuitum infection. Am J Dermatopathol. 2023;45:214-215. doi:10.1097/DAD.0000000000002336
- Franco-Paredes C, Chastain DB, Allen L, et al. Overview of cutaneous mycobacterial infections. Curr Trop Med Rep. 2018;5:228-232. doi:10.1007/s40475-018-0161-7
- Gonzalez-Santiago TM, Drage LA. Nontuberculous mycobacteria: skin and soft tissue infections. Dermatol Clin. 2015;33:563-77. doi:10.1016/j.det.2015.03.017
- Peixoto ADS, Montenegro LML, Lima AS, et al. Identification of nontuberculous mycobacteria species by multiplex real-time PCR with high-resolution melting. Rev Soc Bras Med Trop. 2020;53:E20200211. doi:10.1590/0037-8682-0211-2020
- Park J, Kwak N, Chae JC, et al. A two-step real-time PCR method to identify Mycobacterium tuberculosis infections and six dominant nontuberculous mycobacterial infections from clinical specimens. Microbiol Spectr. 2023:E0160623. doi:10.1128/spectrum.01606-23
- Fowler J, Mahlen SD. Localized cutaneous infections in immunocompetent individuals due to rapidly growing mycobacteria. Arch Pathol Lab Med. 2014;138:1106-1109. doi:10.5858/arpa.2012-0203-RS
- Gardini G, Gregori N, Matteelli A, et al. Mycobacterial skin infection. Curr Opin Infect Dis. 2022;35:79-87. doi:10.1097/QCO.0000000000000820
- Wang SH, Pancholi P. Mycobacterial skin and soft tissue infection. Curr Infect Dis Rep. 2014;16:438. doi:10.1007/s11908-014-0438-5
- Griffith DE, Aksamit T, Brown-Elliott BA, et al; ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416. doi:10.1164/rccm.200604-571ST
- Mougari F, Guglielmetti L, Raskine L, et al. Infections caused by Mycobacterium abscessus: epidemiology, diagnostic tools and treatment. Expert Rev Anti Infect Ther. 2016;14:1139-1154. doi:10.1080/14787210.201 6.1238304
- Tu HZ, Lee HS, Chen YS, et al. High rates of antimicrobial resistance in rapidly growing mycobacterial infections in Taiwan. Pathogens. 2022;11:969. doi:10.3390/pathogens11090969
- Hashemzadeh M, Zadegan Dezfuli AA, Khosravi AD, et al. F requency of mutations in erm(39) related to clarithromycin resistance and in rrl related to linezolid resistance in clinical isolates of MycobacteriuM fortuitum in Iran. Acta Microbiol Immunol Hung. 2023;70:167-176. doi:10.1556/030.2023.02020
- Uslan DZ, Kowalski TJ, Wengenack NL, et al. Skin and soft tissue infections due to rapidly growing mycobacteria: comparison of clinical features, treatment, and susceptibility. Arch Dermatol. 2006;142:1287-1292. doi:10.1001/archderm.142.10.1287
- Miretti M, Juri L, Peralta A, et al. Photoinactivation of non-tuberculous mycobacteria using Zn-phthalocyanine loaded into liposomes. Tuberculosis (Edinb). 2022;136:102247. doi:10.1016/j.tube.2022.102247
- Bystritsky RJ. Cellulitis. Infect Dis Clin North Am. 2021;35:49-60. doi:10.1016/j.idc.2020.10.002
- Christensen K, Holman R, Steiner C, et al. Infectious disease hospitalizations in the United States. Clin Infect Dis. 2009;49:1025-1035. doi:10.1086/605562
- Yang JJ, Maloney NJ, Bach DQ, et al. Dermatology in the emergency department: prescriptions, rates of inpatient admission, and predictors of high utilization in the United States from 1996 to 2012. J Am Acad Dermatol. 2021;84:1480-1483. doi:10.1016/J.JAAD.2020.07.055
- Cutler TS, Jannat-Khah DP, Kam B, et al. Prevalence of misdiagnosis of cellulitis: a systematic review and meta-analysis. J Hosp Med. 2023;18:254-261. doi:10.1002/jhm.12977
- Keller EC, Tomecki KJ, Alraies MC. Distinguishing cellulitis from its mimics. Cleve Clin J Med. 2012;79:547-52. doi:10.3949/ccjm.79a.11121
- Brito AC, Bittencourt MJS. Chromoblastomycosis: an etiological, epidemiological, clinical, diagnostic, and treatment update. An Bras Dermatol. 2018;93:495-506. doi:10.1590/abd1806-4841.20187321
- McGinnis MR. Chromoblastomycosis and phaeohyphomycosis: new concepts, diagnosis, and mycology. J Am Acad Dermatol. 1983;8:1-16.
- Rubin HA, Bruce S, Rosen T, et al. Evidence for percutaneous inoculation as the mode of transmission for chromoblastomycosis. J Am Acad Dermatol. 1991;25:951-954.
- Bonifaz A, Paredes-Solís V, Saúl A. Treating chromoblastomycosis with systemic antifungals. Expert Opin Pharmacother. 2004;5:247-254.
- Maverakis E, Marzano AV, Le ST, et al. Pyoderma gangrenosum. Nat Rev Dis Primers. 2020;6:81. doi:10.1038/s41572-020-0213-x
- George C, Deroide F, Rustin M. Pyoderma gangrenosum—a guide to diagnosis and management. Clin Med (Lond). 2019;19:224-228. doi:10.7861/clinmedicine.19-3-224
- Narla S, Lyons AB, Hamzavi IH. The most recent advances in understanding and managing hidradenitis suppurativa. F1000Res. 2020;9:F1000 Faculty Rev-1049. doi:10.12688/f1000research.26083.1
- Garg A, Lavian J, Lin G, et al. Incidence of hidradenitis suppurativa in the United States: a sex- and age-adjusted population analysis. J Am Acad Dermatol. 2017;77:118-122. doi:10.1016/j.jaad.2017.02.005
- Daxhelet M, Suppa M, White J, et al. Proposed definitions of typical lesions in hidradenitis suppurativa. Dermatology. 2020;236:431-438. doi:10.1159/000507348
- Amat-Samaranch V, Agut-Busquet E, Vilarrasa E, et al. New perspectives on the treatment of hidradenitis suppurativa. Ther Adv Chronic Dis. 2021;12:20406223211055920. doi:10.1177/20406223211055920
- Franco-Paredes C, Marcos LA, Henao-Martínez AF, et al. Cutaneous mycobacterial infections. Clin Microbiol Rev. 2018;32: E00069-18. doi:10.1128/CMR.00069-18
- Brown TH. The rapidly growing mycobacteria—MycobacteriuM fortuitum and Mycobacterium chelonae. Infect Control. 1985;6:283-238. doi:10.1017/s0195941700061762
- Hooper J; Beltrami EJ; Santoro F; et al. Remember the fite: a case of cutaneous MycobacteriuM fortuitum infection. Am J Dermatopathol. 2023;45:214-215. doi:10.1097/DAD.0000000000002336
- Franco-Paredes C, Chastain DB, Allen L, et al. Overview of cutaneous mycobacterial infections. Curr Trop Med Rep. 2018;5:228-232. doi:10.1007/s40475-018-0161-7
- Gonzalez-Santiago TM, Drage LA. Nontuberculous mycobacteria: skin and soft tissue infections. Dermatol Clin. 2015;33:563-77. doi:10.1016/j.det.2015.03.017
- Peixoto ADS, Montenegro LML, Lima AS, et al. Identification of nontuberculous mycobacteria species by multiplex real-time PCR with high-resolution melting. Rev Soc Bras Med Trop. 2020;53:E20200211. doi:10.1590/0037-8682-0211-2020
- Park J, Kwak N, Chae JC, et al. A two-step real-time PCR method to identify Mycobacterium tuberculosis infections and six dominant nontuberculous mycobacterial infections from clinical specimens. Microbiol Spectr. 2023:E0160623. doi:10.1128/spectrum.01606-23
- Fowler J, Mahlen SD. Localized cutaneous infections in immunocompetent individuals due to rapidly growing mycobacteria. Arch Pathol Lab Med. 2014;138:1106-1109. doi:10.5858/arpa.2012-0203-RS
- Gardini G, Gregori N, Matteelli A, et al. Mycobacterial skin infection. Curr Opin Infect Dis. 2022;35:79-87. doi:10.1097/QCO.0000000000000820
- Wang SH, Pancholi P. Mycobacterial skin and soft tissue infection. Curr Infect Dis Rep. 2014;16:438. doi:10.1007/s11908-014-0438-5
- Griffith DE, Aksamit T, Brown-Elliott BA, et al; ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416. doi:10.1164/rccm.200604-571ST
- Mougari F, Guglielmetti L, Raskine L, et al. Infections caused by Mycobacterium abscessus: epidemiology, diagnostic tools and treatment. Expert Rev Anti Infect Ther. 2016;14:1139-1154. doi:10.1080/14787210.201 6.1238304
- Tu HZ, Lee HS, Chen YS, et al. High rates of antimicrobial resistance in rapidly growing mycobacterial infections in Taiwan. Pathogens. 2022;11:969. doi:10.3390/pathogens11090969
- Hashemzadeh M, Zadegan Dezfuli AA, Khosravi AD, et al. F requency of mutations in erm(39) related to clarithromycin resistance and in rrl related to linezolid resistance in clinical isolates of MycobacteriuM fortuitum in Iran. Acta Microbiol Immunol Hung. 2023;70:167-176. doi:10.1556/030.2023.02020
- Uslan DZ, Kowalski TJ, Wengenack NL, et al. Skin and soft tissue infections due to rapidly growing mycobacteria: comparison of clinical features, treatment, and susceptibility. Arch Dermatol. 2006;142:1287-1292. doi:10.1001/archderm.142.10.1287
- Miretti M, Juri L, Peralta A, et al. Photoinactivation of non-tuberculous mycobacteria using Zn-phthalocyanine loaded into liposomes. Tuberculosis (Edinb). 2022;136:102247. doi:10.1016/j.tube.2022.102247
- Bystritsky RJ. Cellulitis. Infect Dis Clin North Am. 2021;35:49-60. doi:10.1016/j.idc.2020.10.002
- Christensen K, Holman R, Steiner C, et al. Infectious disease hospitalizations in the United States. Clin Infect Dis. 2009;49:1025-1035. doi:10.1086/605562
- Yang JJ, Maloney NJ, Bach DQ, et al. Dermatology in the emergency department: prescriptions, rates of inpatient admission, and predictors of high utilization in the United States from 1996 to 2012. J Am Acad Dermatol. 2021;84:1480-1483. doi:10.1016/J.JAAD.2020.07.055
- Cutler TS, Jannat-Khah DP, Kam B, et al. Prevalence of misdiagnosis of cellulitis: a systematic review and meta-analysis. J Hosp Med. 2023;18:254-261. doi:10.1002/jhm.12977
- Keller EC, Tomecki KJ, Alraies MC. Distinguishing cellulitis from its mimics. Cleve Clin J Med. 2012;79:547-52. doi:10.3949/ccjm.79a.11121
- Brito AC, Bittencourt MJS. Chromoblastomycosis: an etiological, epidemiological, clinical, diagnostic, and treatment update. An Bras Dermatol. 2018;93:495-506. doi:10.1590/abd1806-4841.20187321
- McGinnis MR. Chromoblastomycosis and phaeohyphomycosis: new concepts, diagnosis, and mycology. J Am Acad Dermatol. 1983;8:1-16.
- Rubin HA, Bruce S, Rosen T, et al. Evidence for percutaneous inoculation as the mode of transmission for chromoblastomycosis. J Am Acad Dermatol. 1991;25:951-954.
- Bonifaz A, Paredes-Solís V, Saúl A. Treating chromoblastomycosis with systemic antifungals. Expert Opin Pharmacother. 2004;5:247-254.
- Maverakis E, Marzano AV, Le ST, et al. Pyoderma gangrenosum. Nat Rev Dis Primers. 2020;6:81. doi:10.1038/s41572-020-0213-x
- George C, Deroide F, Rustin M. Pyoderma gangrenosum—a guide to diagnosis and management. Clin Med (Lond). 2019;19:224-228. doi:10.7861/clinmedicine.19-3-224
- Narla S, Lyons AB, Hamzavi IH. The most recent advances in understanding and managing hidradenitis suppurativa. F1000Res. 2020;9:F1000 Faculty Rev-1049. doi:10.12688/f1000research.26083.1
- Garg A, Lavian J, Lin G, et al. Incidence of hidradenitis suppurativa in the United States: a sex- and age-adjusted population analysis. J Am Acad Dermatol. 2017;77:118-122. doi:10.1016/j.jaad.2017.02.005
- Daxhelet M, Suppa M, White J, et al. Proposed definitions of typical lesions in hidradenitis suppurativa. Dermatology. 2020;236:431-438. doi:10.1159/000507348
- Amat-Samaranch V, Agut-Busquet E, Vilarrasa E, et al. New perspectives on the treatment of hidradenitis suppurativa. Ther Adv Chronic Dis. 2021;12:20406223211055920. doi:10.1177/20406223211055920
A 40-year-old woman presented with multiple draining sinus tracts on the right thigh following an injury sustained weeks earlier while mowing wet grass.
Severe Community-Acquired Pneumonia: Diagnostic Criteria, Treatment, and COVID-19
- Torres A, Cilloniz C, Niederman MS, et al. Pneumonia. Nat Rev Dis Primers. 2021;7(1):25. doi:10.1038/s41572-021-00259-0
- Niederman MS, Torres A. Severe community-acquired pneumonia. Eur Respir Rev. 2022;31(166):220123. doi:10.1183/16000617.0123-2022
- Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45-e67. doi:10.1164/rccm.201908-1581ST
- Memon RA, Rashid MA, Avva S, et al. The use of the SMART-COP score in predicting severity outcomes among patients with community-acquired pneumonia: a meta-analysis. Cureus. 2022;14(7):e27248. doi:10.7759/cureus.27248
- Regunath H, Oba Y. Community-acquired pneumonia. StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2024. Updated January 26, 2024. Accessed May 14, 2024. https://www.ncbi.nlm.nih.gov/books/NBK430749/
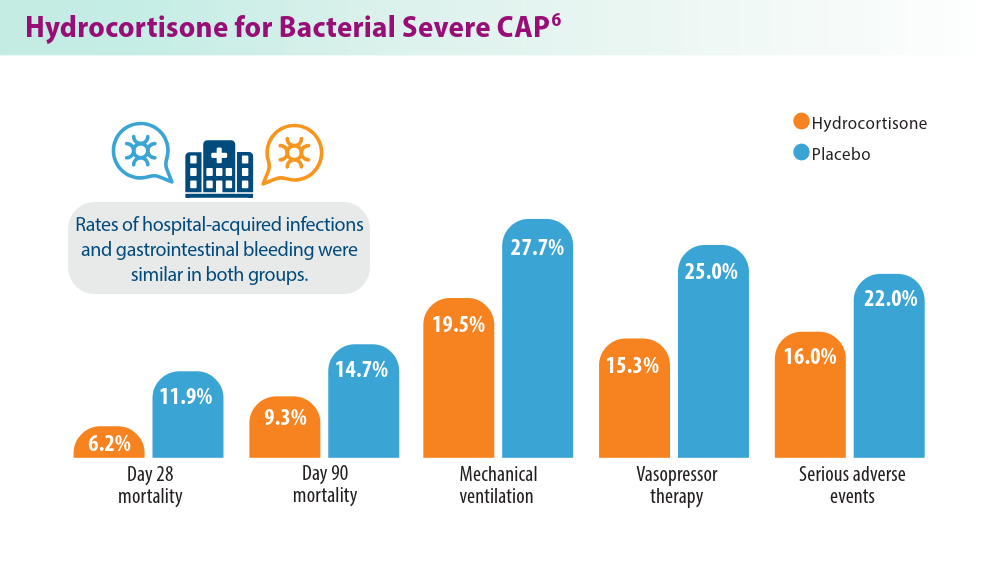
- Dequin PF, Meziani F, Quenot JP, et al; for the CRICS-TriGGERSep Network. Hydrocortisone in severe community-acquired pneumonia. N Engl J Med. 2023;388(21):1931-1941. doi:10.1056/NEJMoa2215145
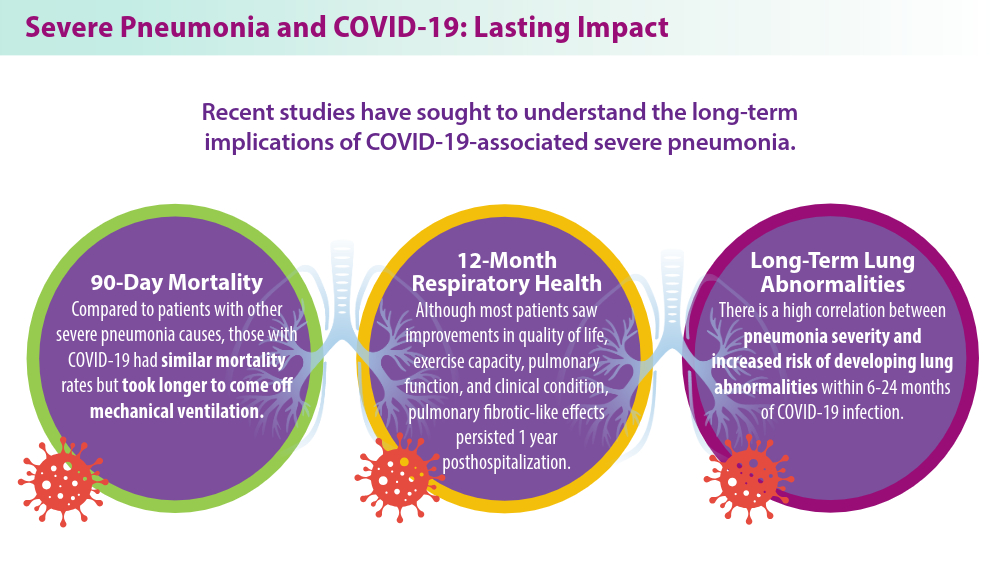
- Eizaguirre S, Sabater G, Belda S, et al. Long-term respiratory consequences of COVID-19 related pneumonia: a cohort study. BMC Pulm Med. 2023;23(1):439. doi:10.1186/s12890-023-02627-w
- Ramirez JA, Wiemken TL, Peyrani P, et al; for the University of Louisville Pneumonia Study Group. Adults hospitalized with pneumonia in the United States: incidence, epidemiology, and mortality. Clin Infect Dis. 2017;65(11):1806-1812. doi:10.1093/cid/cix647
- Morgan AJ, Glossop AJ. Severe community-acquired pneumonia. BJA Educ. 2016;16(5):167-172. doi:10.1093/bjaed/mkv052
- Haessler S, Guo N, Deshpande A, et al. Etiology, treatments, and outcomes of patients with severe community-acquired pneumonia in a large U.S. sample. Crit Care Med. 2022;50(7):1063-1071. doi:10.1097/CCM.0000000000005498
- Nolley EP, Sahetya SK, Hochberg CH, et al. Outcomes among mechanically ventilated patients with severe pneumonia and acute hypoxemic respiratory failure from SARS-CoV-2 and other etiologies. JAMA Netw Open. 2023;6(1):e2250401. doi:10.1001/jamanetworkopen.2022.50401
- Hino T, Nishino M, Valtchinov VI, et al. Severe COVID-19 pneumonia leads to post-COVID-19 lung abnormalities on follow-up CT scans. Eur J Radiol Open. 2023;10:100483. doi:10.1016/j.ejro.2023.100483
- Torres A, Cilloniz C, Niederman MS, et al. Pneumonia. Nat Rev Dis Primers. 2021;7(1):25. doi:10.1038/s41572-021-00259-0
- Niederman MS, Torres A. Severe community-acquired pneumonia. Eur Respir Rev. 2022;31(166):220123. doi:10.1183/16000617.0123-2022
- Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45-e67. doi:10.1164/rccm.201908-1581ST
- Memon RA, Rashid MA, Avva S, et al. The use of the SMART-COP score in predicting severity outcomes among patients with community-acquired pneumonia: a meta-analysis. Cureus. 2022;14(7):e27248. doi:10.7759/cureus.27248
- Regunath H, Oba Y. Community-acquired pneumonia. StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2024. Updated January 26, 2024. Accessed May 14, 2024. https://www.ncbi.nlm.nih.gov/books/NBK430749/
- Dequin PF, Meziani F, Quenot JP, et al; for the CRICS-TriGGERSep Network. Hydrocortisone in severe community-acquired pneumonia. N Engl J Med. 2023;388(21):1931-1941. doi:10.1056/NEJMoa2215145
- Eizaguirre S, Sabater G, Belda S, et al. Long-term respiratory consequences of COVID-19 related pneumonia: a cohort study. BMC Pulm Med. 2023;23(1):439. doi:10.1186/s12890-023-02627-w
- Ramirez JA, Wiemken TL, Peyrani P, et al; for the University of Louisville Pneumonia Study Group. Adults hospitalized with pneumonia in the United States: incidence, epidemiology, and mortality. Clin Infect Dis. 2017;65(11):1806-1812. doi:10.1093/cid/cix647
- Morgan AJ, Glossop AJ. Severe community-acquired pneumonia. BJA Educ. 2016;16(5):167-172. doi:10.1093/bjaed/mkv052
- Haessler S, Guo N, Deshpande A, et al. Etiology, treatments, and outcomes of patients with severe community-acquired pneumonia in a large U.S. sample. Crit Care Med. 2022;50(7):1063-1071. doi:10.1097/CCM.0000000000005498
- Nolley EP, Sahetya SK, Hochberg CH, et al. Outcomes among mechanically ventilated patients with severe pneumonia and acute hypoxemic respiratory failure from SARS-CoV-2 and other etiologies. JAMA Netw Open. 2023;6(1):e2250401. doi:10.1001/jamanetworkopen.2022.50401
- Hino T, Nishino M, Valtchinov VI, et al. Severe COVID-19 pneumonia leads to post-COVID-19 lung abnormalities on follow-up CT scans. Eur J Radiol Open. 2023;10:100483. doi:10.1016/j.ejro.2023.100483
- Torres A, Cilloniz C, Niederman MS, et al. Pneumonia. Nat Rev Dis Primers. 2021;7(1):25. doi:10.1038/s41572-021-00259-0
- Niederman MS, Torres A. Severe community-acquired pneumonia. Eur Respir Rev. 2022;31(166):220123. doi:10.1183/16000617.0123-2022
- Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45-e67. doi:10.1164/rccm.201908-1581ST
- Memon RA, Rashid MA, Avva S, et al. The use of the SMART-COP score in predicting severity outcomes among patients with community-acquired pneumonia: a meta-analysis. Cureus. 2022;14(7):e27248. doi:10.7759/cureus.27248
- Regunath H, Oba Y. Community-acquired pneumonia. StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2024. Updated January 26, 2024. Accessed May 14, 2024. https://www.ncbi.nlm.nih.gov/books/NBK430749/
- Dequin PF, Meziani F, Quenot JP, et al; for the CRICS-TriGGERSep Network. Hydrocortisone in severe community-acquired pneumonia. N Engl J Med. 2023;388(21):1931-1941. doi:10.1056/NEJMoa2215145
- Eizaguirre S, Sabater G, Belda S, et al. Long-term respiratory consequences of COVID-19 related pneumonia: a cohort study. BMC Pulm Med. 2023;23(1):439. doi:10.1186/s12890-023-02627-w
- Ramirez JA, Wiemken TL, Peyrani P, et al; for the University of Louisville Pneumonia Study Group. Adults hospitalized with pneumonia in the United States: incidence, epidemiology, and mortality. Clin Infect Dis. 2017;65(11):1806-1812. doi:10.1093/cid/cix647
- Morgan AJ, Glossop AJ. Severe community-acquired pneumonia. BJA Educ. 2016;16(5):167-172. doi:10.1093/bjaed/mkv052
- Haessler S, Guo N, Deshpande A, et al. Etiology, treatments, and outcomes of patients with severe community-acquired pneumonia in a large U.S. sample. Crit Care Med. 2022;50(7):1063-1071. doi:10.1097/CCM.0000000000005498
- Nolley EP, Sahetya SK, Hochberg CH, et al. Outcomes among mechanically ventilated patients with severe pneumonia and acute hypoxemic respiratory failure from SARS-CoV-2 and other etiologies. JAMA Netw Open. 2023;6(1):e2250401. doi:10.1001/jamanetworkopen.2022.50401
- Hino T, Nishino M, Valtchinov VI, et al. Severe COVID-19 pneumonia leads to post-COVID-19 lung abnormalities on follow-up CT scans. Eur J Radiol Open. 2023;10:100483. doi:10.1016/j.ejro.2023.100483
Acute Tender Papules on the Arms and Legs
The Diagnosis: Erythema Nodosum Leprosum
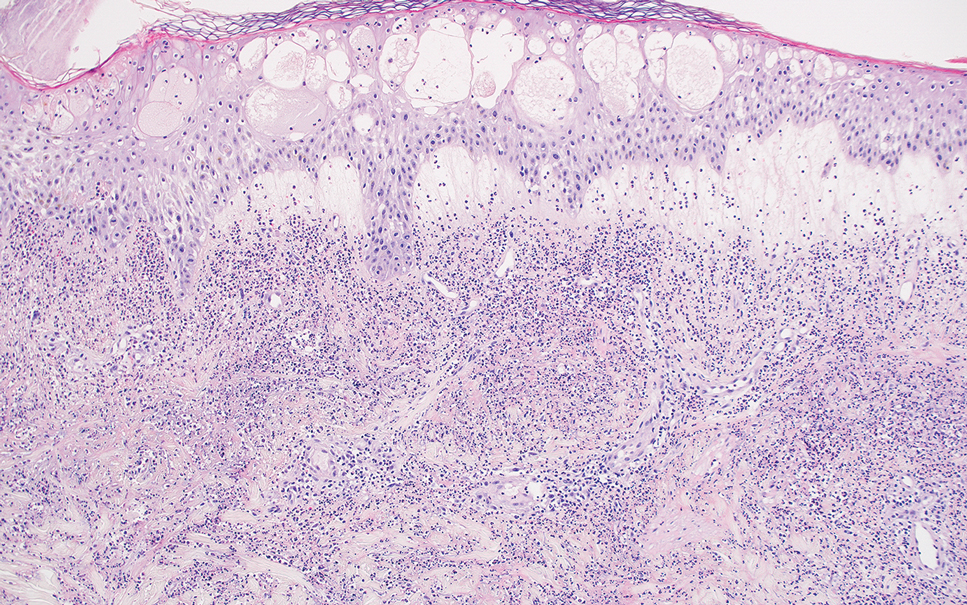
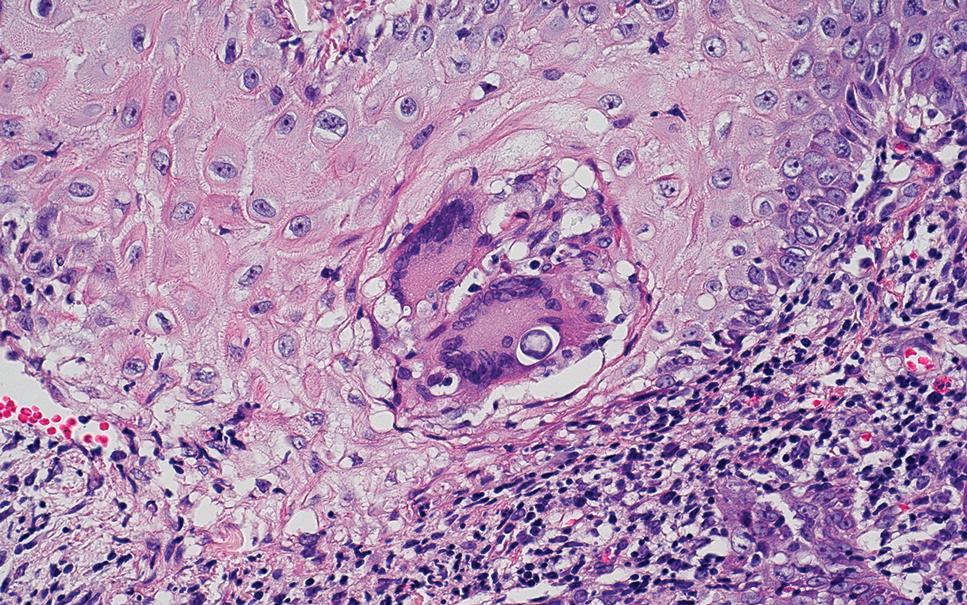
Erythema nodosum leprosum (ENL) is a type 2 reaction sometimes seen in patients infected with Mycobacterium leprae—primarily those with lepromatous or borderline lepromatous subtypes. Clinically, ENL manifests with abrupt onset of tender erythematous papules with associated fevers and general malaise. Studies have demonstrated a complex immune system reaction in ENL, but the detailed pathophysiology is not fully understood.1 Biopsies conducted within 24 hours of lesion formation are most elucidating. Foamy histiocytes admixed with neutrophils are seen in the subcutis, often causing a lobular panniculitis (quiz image).2 Neutrophils rarely are seen in other types of leprosy and thus are a useful diagnostic clue for ENL. Vasculitis of small- to medium-sized vessels can be seen but is not a necessary diagnostic criterion. Fite staining will highlight many acid-fast bacilli within the histiocytes (Figure 1).
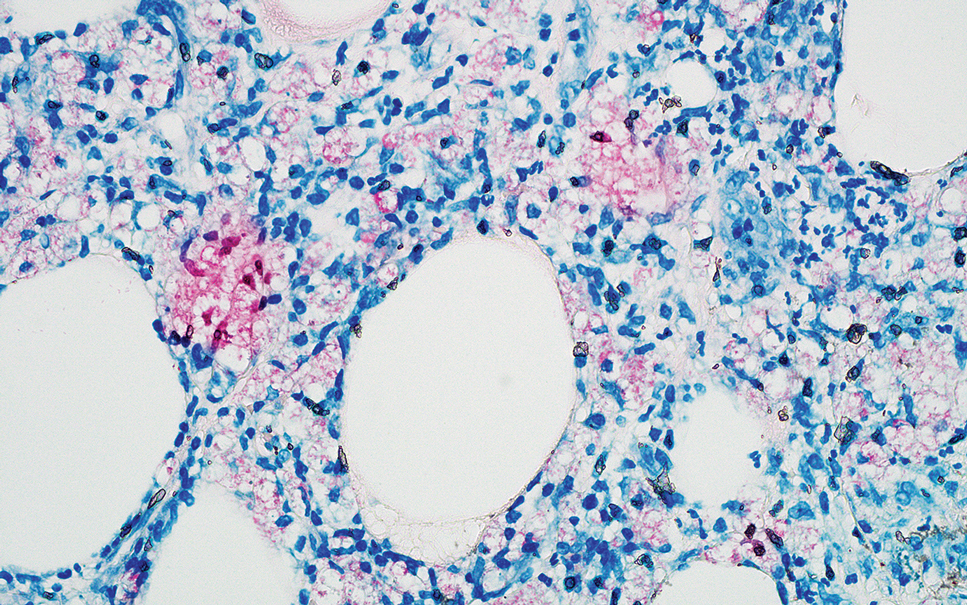
Erythema nodosum leprosum is treated with a combination of immunosuppressants such as prednisone and thalidomide. Our patient was taking triple-antibiotic therapy—dapsone, rifampin, and clofazimine—for lepromatous leprosy when the erythematous papules developed on the arms and legs. After a skin biopsy confirmed the diagnosis of ENL, he was started on prednisone 20 mg daily with plans for close follow-up. Unfortunately, the patient was subsequently lost to follow-up.
Acute febrile neutrophilic dermatosis (also known as Sweet syndrome) is an acute inflammatory disease characterized by abrupt onset of painful erythematous papules, plaques, or nodules on the skin. It often is seen in association with preceding infections (especially those in the upper respiratory or gastrointestinal tracts), hematologic malignancies, inflammatory bowel disease, or exposure to certain classes of medications (eg, granulocyte colony-stimulating factor, tyrosine kinase inhibitors, various antibiotics).3 Histologically, acute febrile neutrophilic dermatosis is characterized by dense neutrophilic infiltrates, often with notable dermal edema (Figure 2).4 Many cases also show leukocytoclastic vasculitis; however, foamy histiocytes are not a notable component of the inflammatory infiltrate, though a histiocytoid form of acute febrile neutrophilic dermatosis has been described.5 Infections must be rigorously ruled out prior to diagnosing a patient with acute febrile neutrophilic dermatosis, making it a diagnosis of exclusion.
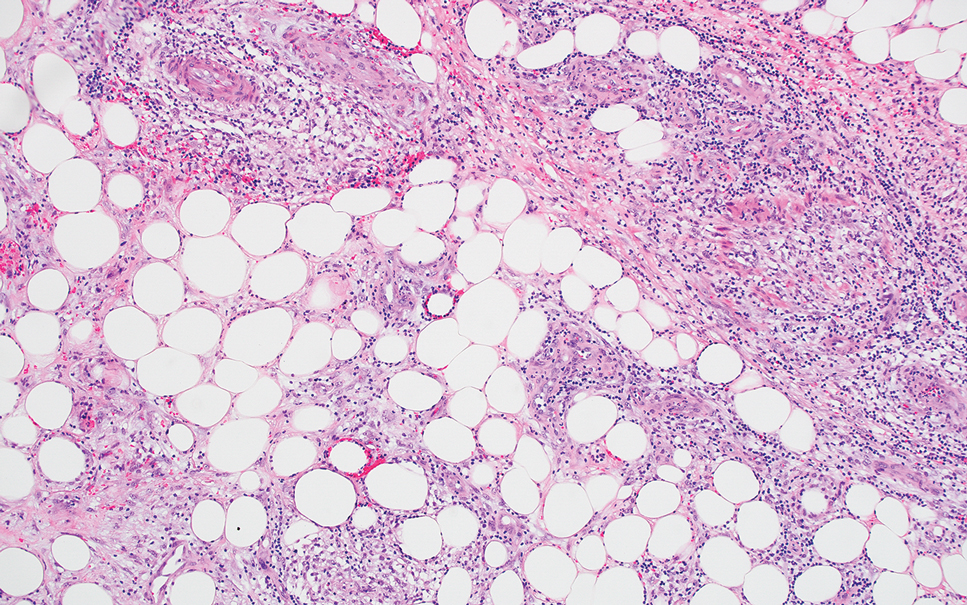
Cutaneous coccidioidomycosis is an infection caused by the dimorphic fungi Coccidioides immitis or Coccidioides posadasii. Cutaneous disease is rare but can occur from direct inoculation or dissemination from pulmonary disease in immunocompetent or immunocompromised patients. Papules, pustules, or plaques are seen clinically. Histologically, cutaneous coccidioidomycosis shows spherules that vary from 10 to 100 μm and are filled with multiple smaller endospores (Figure 3).6 Pseudoepitheliomatous hyperplasia with dense suppurative and granulomatous infiltrates also is seen.
Erythema induratum is characterized by tender nodules on the lower extremities and has a substantial female predominance. Many cases are associated with Mycobacterium tuberculosis infection. The bacteria are not seen directly in the skin but are instead detectable through DNA polymerase chain reaction testing or investigation of other organ systems.7,8 Histologically, lesions show a lobular panniculitis with a mixed infiltrate. Vasculitis is seen in approximately 90% of erythema induratum cases vs approximately 25% of classic ENL cases (Figure 4),2,9 which has led some to use the term nodular vasculitis to describe this disease entity. Nodular vasculitis is considered by others to be a distinct disease entity in which there are clinical and histologic features similar to erythema induratum but no evidence of M tuberculosis infection.9
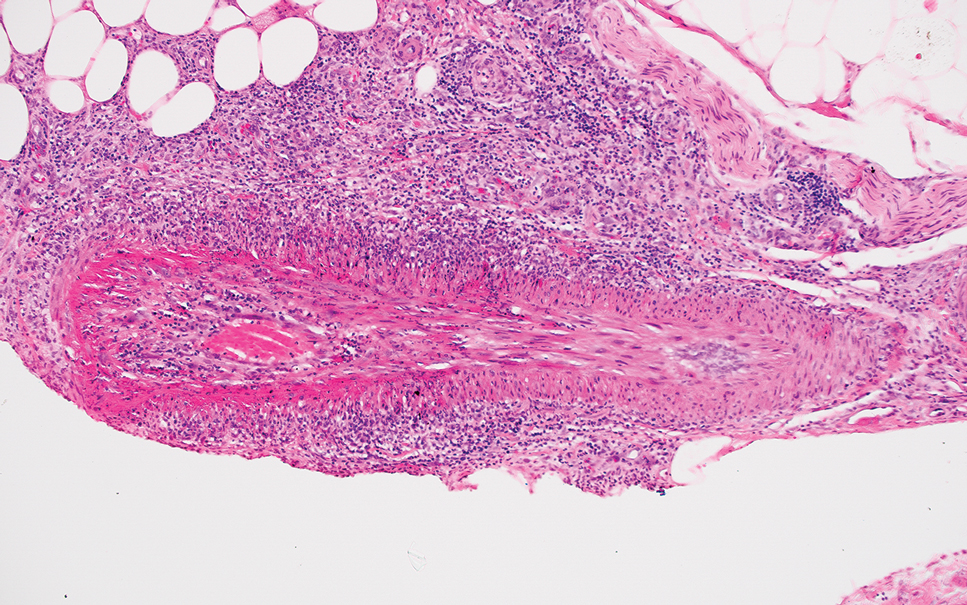
Polyarteritis nodosa is a vasculitis that affects medium- sized vessels of various organ systems. The presenting signs and symptoms vary based on the affected organ systems. Palpable to retiform purpura, livedo racemosa, subcutaneous nodules, or ulcers are seen when the skin is involved. The histologic hallmark is necrotizing vasculitis of medium-sized arterioles (Figure 5), although leukocytoclastic vasculitis of small-caliber vessels also can be seen in biopsies of affected skin.10 The vascular changes are said to be segmental, with uninvolved segments interspersed with involved segments. Antineutrophil cytoplasmic antibody (ANCA)– associated vasculitis also must be considered when one sees leukocytoclastic vasculitis of small-caliber vessels in the skin, as it can be distinguished most readily by detecting circulating antibodies specific for myeloperoxidase (MPO-ANCA) or proteinase 3 (PR3-ANCA).
- Polycarpou A, Walker SL, Lockwood DNJ. A systematic review of immunological studies of erythema nodosum leprosum. Front Immunol. 2017;8:233. doi:10.3389/fimmu.2017.00233
- Massone C, Belachew WA, Schettini A. Histopathology of the lepromatous skin biopsy. Clin Dermatol. 2015;33:38-45. doi:10.1016/j.clindermatol.2014.10.003
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:1-28. doi:10.1186/1750-1172-2-34
- Ratzinger G, Burgdorf W, Zelger BG, et al. Acute febrile neutrophilic dermatosis: a histopathologic study of 31 cases with review of literature. Am J Dermatopathol. 2007;29:125-133. doi:10.1097/01.dad.0000249887.59810.76
- Wilson TC, Stone MS, Swick BL. Histiocytoid Sweet syndrome with haloed myeloid cells masquerading as a cryptococcal infection. Am J Dermatopathology. 2014;36:264-269. doi:10.1097/DAD.0b013e31828b811b
- Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24:247-280. doi:10.1128/CMR.00053-10
- Schneider JW, Jordaan HF, Geiger DH, et al. Erythema induratum of Bazin: a clinicopathological study of 20 cases of Mycobacterium tuberculosis DNA in skin lesions by polymerase chain reaction. Am J Dermatopathol. 1995;17:350-356. doi:10.1097/00000372-199508000-00008
- Boonchai W, Suthipinittharm P, Mahaisavariya P. Panniculitis in tuberculosis: a clinicopathologic study of nodular panniculitis associated with tuberculosis. Int J Dermatol. 1998;37:361-363. doi:10.1046/j.1365-4362.1998.00299.x
- Segura S, Pujol RM, Trindade F, et al. Vasculitis in erythema induratum of Bazin: a histopathologic study of 101 biopsy specimens from 86 patients. J Am Acad Dermatol. 2008;59:839-851. doi:10.1016/j.jaad.2008.07.030
- Ishiguro N, Kawashima M. Cutaneous polyarteritis nodosa: a report of 16 cases with clinical and histopathological analysis and a review of the published work. J Dermatol. 2010;37:85-93. doi:10.1111/j.1346-8138.2009.00752.x
The Diagnosis: Erythema Nodosum Leprosum
Erythema nodosum leprosum (ENL) is a type 2 reaction sometimes seen in patients infected with Mycobacterium leprae—primarily those with lepromatous or borderline lepromatous subtypes. Clinically, ENL manifests with abrupt onset of tender erythematous papules with associated fevers and general malaise. Studies have demonstrated a complex immune system reaction in ENL, but the detailed pathophysiology is not fully understood.1 Biopsies conducted within 24 hours of lesion formation are most elucidating. Foamy histiocytes admixed with neutrophils are seen in the subcutis, often causing a lobular panniculitis (quiz image).2 Neutrophils rarely are seen in other types of leprosy and thus are a useful diagnostic clue for ENL. Vasculitis of small- to medium-sized vessels can be seen but is not a necessary diagnostic criterion. Fite staining will highlight many acid-fast bacilli within the histiocytes (Figure 1).

Erythema nodosum leprosum is treated with a combination of immunosuppressants such as prednisone and thalidomide. Our patient was taking triple-antibiotic therapy—dapsone, rifampin, and clofazimine—for lepromatous leprosy when the erythematous papules developed on the arms and legs. After a skin biopsy confirmed the diagnosis of ENL, he was started on prednisone 20 mg daily with plans for close follow-up. Unfortunately, the patient was subsequently lost to follow-up.
Acute febrile neutrophilic dermatosis (also known as Sweet syndrome) is an acute inflammatory disease characterized by abrupt onset of painful erythematous papules, plaques, or nodules on the skin. It often is seen in association with preceding infections (especially those in the upper respiratory or gastrointestinal tracts), hematologic malignancies, inflammatory bowel disease, or exposure to certain classes of medications (eg, granulocyte colony-stimulating factor, tyrosine kinase inhibitors, various antibiotics).3 Histologically, acute febrile neutrophilic dermatosis is characterized by dense neutrophilic infiltrates, often with notable dermal edema (Figure 2).4 Many cases also show leukocytoclastic vasculitis; however, foamy histiocytes are not a notable component of the inflammatory infiltrate, though a histiocytoid form of acute febrile neutrophilic dermatosis has been described.5 Infections must be rigorously ruled out prior to diagnosing a patient with acute febrile neutrophilic dermatosis, making it a diagnosis of exclusion.

Cutaneous coccidioidomycosis is an infection caused by the dimorphic fungi Coccidioides immitis or Coccidioides posadasii. Cutaneous disease is rare but can occur from direct inoculation or dissemination from pulmonary disease in immunocompetent or immunocompromised patients. Papules, pustules, or plaques are seen clinically. Histologically, cutaneous coccidioidomycosis shows spherules that vary from 10 to 100 μm and are filled with multiple smaller endospores (Figure 3).6 Pseudoepitheliomatous hyperplasia with dense suppurative and granulomatous infiltrates also is seen.

Erythema induratum is characterized by tender nodules on the lower extremities and has a substantial female predominance. Many cases are associated with Mycobacterium tuberculosis infection. The bacteria are not seen directly in the skin but are instead detectable through DNA polymerase chain reaction testing or investigation of other organ systems.7,8 Histologically, lesions show a lobular panniculitis with a mixed infiltrate. Vasculitis is seen in approximately 90% of erythema induratum cases vs approximately 25% of classic ENL cases (Figure 4),2,9 which has led some to use the term nodular vasculitis to describe this disease entity. Nodular vasculitis is considered by others to be a distinct disease entity in which there are clinical and histologic features similar to erythema induratum but no evidence of M tuberculosis infection.9

Polyarteritis nodosa is a vasculitis that affects medium- sized vessels of various organ systems. The presenting signs and symptoms vary based on the affected organ systems. Palpable to retiform purpura, livedo racemosa, subcutaneous nodules, or ulcers are seen when the skin is involved. The histologic hallmark is necrotizing vasculitis of medium-sized arterioles (Figure 5), although leukocytoclastic vasculitis of small-caliber vessels also can be seen in biopsies of affected skin.10 The vascular changes are said to be segmental, with uninvolved segments interspersed with involved segments. Antineutrophil cytoplasmic antibody (ANCA)– associated vasculitis also must be considered when one sees leukocytoclastic vasculitis of small-caliber vessels in the skin, as it can be distinguished most readily by detecting circulating antibodies specific for myeloperoxidase (MPO-ANCA) or proteinase 3 (PR3-ANCA).

The Diagnosis: Erythema Nodosum Leprosum
Erythema nodosum leprosum (ENL) is a type 2 reaction sometimes seen in patients infected with Mycobacterium leprae—primarily those with lepromatous or borderline lepromatous subtypes. Clinically, ENL manifests with abrupt onset of tender erythematous papules with associated fevers and general malaise. Studies have demonstrated a complex immune system reaction in ENL, but the detailed pathophysiology is not fully understood.1 Biopsies conducted within 24 hours of lesion formation are most elucidating. Foamy histiocytes admixed with neutrophils are seen in the subcutis, often causing a lobular panniculitis (quiz image).2 Neutrophils rarely are seen in other types of leprosy and thus are a useful diagnostic clue for ENL. Vasculitis of small- to medium-sized vessels can be seen but is not a necessary diagnostic criterion. Fite staining will highlight many acid-fast bacilli within the histiocytes (Figure 1).

Erythema nodosum leprosum is treated with a combination of immunosuppressants such as prednisone and thalidomide. Our patient was taking triple-antibiotic therapy—dapsone, rifampin, and clofazimine—for lepromatous leprosy when the erythematous papules developed on the arms and legs. After a skin biopsy confirmed the diagnosis of ENL, he was started on prednisone 20 mg daily with plans for close follow-up. Unfortunately, the patient was subsequently lost to follow-up.
Acute febrile neutrophilic dermatosis (also known as Sweet syndrome) is an acute inflammatory disease characterized by abrupt onset of painful erythematous papules, plaques, or nodules on the skin. It often is seen in association with preceding infections (especially those in the upper respiratory or gastrointestinal tracts), hematologic malignancies, inflammatory bowel disease, or exposure to certain classes of medications (eg, granulocyte colony-stimulating factor, tyrosine kinase inhibitors, various antibiotics).3 Histologically, acute febrile neutrophilic dermatosis is characterized by dense neutrophilic infiltrates, often with notable dermal edema (Figure 2).4 Many cases also show leukocytoclastic vasculitis; however, foamy histiocytes are not a notable component of the inflammatory infiltrate, though a histiocytoid form of acute febrile neutrophilic dermatosis has been described.5 Infections must be rigorously ruled out prior to diagnosing a patient with acute febrile neutrophilic dermatosis, making it a diagnosis of exclusion.

Cutaneous coccidioidomycosis is an infection caused by the dimorphic fungi Coccidioides immitis or Coccidioides posadasii. Cutaneous disease is rare but can occur from direct inoculation or dissemination from pulmonary disease in immunocompetent or immunocompromised patients. Papules, pustules, or plaques are seen clinically. Histologically, cutaneous coccidioidomycosis shows spherules that vary from 10 to 100 μm and are filled with multiple smaller endospores (Figure 3).6 Pseudoepitheliomatous hyperplasia with dense suppurative and granulomatous infiltrates also is seen.

Erythema induratum is characterized by tender nodules on the lower extremities and has a substantial female predominance. Many cases are associated with Mycobacterium tuberculosis infection. The bacteria are not seen directly in the skin but are instead detectable through DNA polymerase chain reaction testing or investigation of other organ systems.7,8 Histologically, lesions show a lobular panniculitis with a mixed infiltrate. Vasculitis is seen in approximately 90% of erythema induratum cases vs approximately 25% of classic ENL cases (Figure 4),2,9 which has led some to use the term nodular vasculitis to describe this disease entity. Nodular vasculitis is considered by others to be a distinct disease entity in which there are clinical and histologic features similar to erythema induratum but no evidence of M tuberculosis infection.9

Polyarteritis nodosa is a vasculitis that affects medium- sized vessels of various organ systems. The presenting signs and symptoms vary based on the affected organ systems. Palpable to retiform purpura, livedo racemosa, subcutaneous nodules, or ulcers are seen when the skin is involved. The histologic hallmark is necrotizing vasculitis of medium-sized arterioles (Figure 5), although leukocytoclastic vasculitis of small-caliber vessels also can be seen in biopsies of affected skin.10 The vascular changes are said to be segmental, with uninvolved segments interspersed with involved segments. Antineutrophil cytoplasmic antibody (ANCA)– associated vasculitis also must be considered when one sees leukocytoclastic vasculitis of small-caliber vessels in the skin, as it can be distinguished most readily by detecting circulating antibodies specific for myeloperoxidase (MPO-ANCA) or proteinase 3 (PR3-ANCA).

- Polycarpou A, Walker SL, Lockwood DNJ. A systematic review of immunological studies of erythema nodosum leprosum. Front Immunol. 2017;8:233. doi:10.3389/fimmu.2017.00233
- Massone C, Belachew WA, Schettini A. Histopathology of the lepromatous skin biopsy. Clin Dermatol. 2015;33:38-45. doi:10.1016/j.clindermatol.2014.10.003
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:1-28. doi:10.1186/1750-1172-2-34
- Ratzinger G, Burgdorf W, Zelger BG, et al. Acute febrile neutrophilic dermatosis: a histopathologic study of 31 cases with review of literature. Am J Dermatopathol. 2007;29:125-133. doi:10.1097/01.dad.0000249887.59810.76
- Wilson TC, Stone MS, Swick BL. Histiocytoid Sweet syndrome with haloed myeloid cells masquerading as a cryptococcal infection. Am J Dermatopathology. 2014;36:264-269. doi:10.1097/DAD.0b013e31828b811b
- Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24:247-280. doi:10.1128/CMR.00053-10
- Schneider JW, Jordaan HF, Geiger DH, et al. Erythema induratum of Bazin: a clinicopathological study of 20 cases of Mycobacterium tuberculosis DNA in skin lesions by polymerase chain reaction. Am J Dermatopathol. 1995;17:350-356. doi:10.1097/00000372-199508000-00008
- Boonchai W, Suthipinittharm P, Mahaisavariya P. Panniculitis in tuberculosis: a clinicopathologic study of nodular panniculitis associated with tuberculosis. Int J Dermatol. 1998;37:361-363. doi:10.1046/j.1365-4362.1998.00299.x
- Segura S, Pujol RM, Trindade F, et al. Vasculitis in erythema induratum of Bazin: a histopathologic study of 101 biopsy specimens from 86 patients. J Am Acad Dermatol. 2008;59:839-851. doi:10.1016/j.jaad.2008.07.030
- Ishiguro N, Kawashima M. Cutaneous polyarteritis nodosa: a report of 16 cases with clinical and histopathological analysis and a review of the published work. J Dermatol. 2010;37:85-93. doi:10.1111/j.1346-8138.2009.00752.x
- Polycarpou A, Walker SL, Lockwood DNJ. A systematic review of immunological studies of erythema nodosum leprosum. Front Immunol. 2017;8:233. doi:10.3389/fimmu.2017.00233
- Massone C, Belachew WA, Schettini A. Histopathology of the lepromatous skin biopsy. Clin Dermatol. 2015;33:38-45. doi:10.1016/j.clindermatol.2014.10.003
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:1-28. doi:10.1186/1750-1172-2-34
- Ratzinger G, Burgdorf W, Zelger BG, et al. Acute febrile neutrophilic dermatosis: a histopathologic study of 31 cases with review of literature. Am J Dermatopathol. 2007;29:125-133. doi:10.1097/01.dad.0000249887.59810.76
- Wilson TC, Stone MS, Swick BL. Histiocytoid Sweet syndrome with haloed myeloid cells masquerading as a cryptococcal infection. Am J Dermatopathology. 2014;36:264-269. doi:10.1097/DAD.0b013e31828b811b
- Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24:247-280. doi:10.1128/CMR.00053-10
- Schneider JW, Jordaan HF, Geiger DH, et al. Erythema induratum of Bazin: a clinicopathological study of 20 cases of Mycobacterium tuberculosis DNA in skin lesions by polymerase chain reaction. Am J Dermatopathol. 1995;17:350-356. doi:10.1097/00000372-199508000-00008
- Boonchai W, Suthipinittharm P, Mahaisavariya P. Panniculitis in tuberculosis: a clinicopathologic study of nodular panniculitis associated with tuberculosis. Int J Dermatol. 1998;37:361-363. doi:10.1046/j.1365-4362.1998.00299.x
- Segura S, Pujol RM, Trindade F, et al. Vasculitis in erythema induratum of Bazin: a histopathologic study of 101 biopsy specimens from 86 patients. J Am Acad Dermatol. 2008;59:839-851. doi:10.1016/j.jaad.2008.07.030
- Ishiguro N, Kawashima M. Cutaneous polyarteritis nodosa: a report of 16 cases with clinical and histopathological analysis and a review of the published work. J Dermatol. 2010;37:85-93. doi:10.1111/j.1346-8138.2009.00752.x
A 66-year-old man presented with new tender erythematous papules scattered over the arms and legs. A biopsy of a lesion on the left thigh was performed.
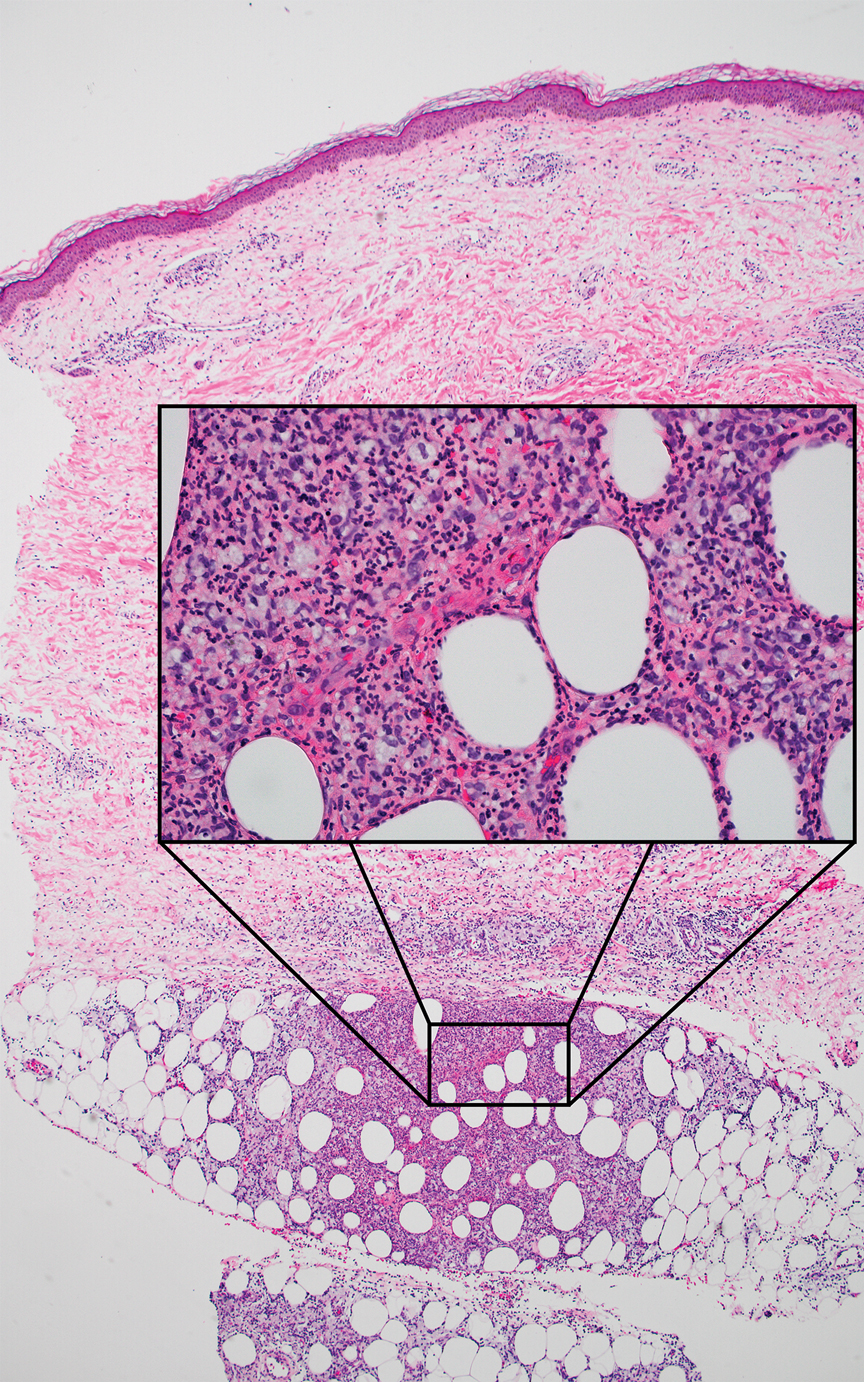
Part of Taking a Good (Human) Patient History Includes Asking About Pet Vaccinations
This transcript has been edited for clarity.
In my job, I spend 99% of my time thinking about ethical issues that arise in the care of human beings. That is the focus of our medical school, and that’s what we do.
However,
Recently, there has been a great increase in the number of pet owners who are saying, “I’m not going to vaccinate my pets.” As horrible as this sounds, what’s happening is vaccine hesitancy about vaccines used in humans is extending through some people to their pets.
The number of people who say they don’t trust things like rabies vaccine to be effective or safe for their pet animals is 40%, at least in surveys, and the American Veterinary Medical Association reports that 15%-18% of pet owners are not, in fact, vaccinating their pets against rabies.
Rabies, as I hope everybody knows, is one horrible disease. Even the treatment of it, should you get bitten by a rabid animal, is no fun, expensive, and hopefully something that can be administered quickly. It’s not always the case. Worldwide, at least 70,000 people die from rabies every year.
Obviously, there are many countries that are so terrified of rabies, they won’t let you bring pets in without quarantining them, say, England, for at least 6 months to a year, I believe, because they don’t want rabies getting into their country. They’re very strict about the movement of pets.
It is inexcusable for people, first, not to give their pets vaccines that prevent them getting distemper, parvovirus, or many other diseases that harm the pet. It’s also inexcusable to shorten your pet’s life or ask your patients to care for pets who get sick from many of these diseases that are vaccine preventable.
Worst of all, it’s inexcusable for any pet owner not to give a rabies vaccine to their pets. Were it up to me, I’d say you have to license your pet, and as part of that, you must mandate rabies vaccines for your dogs, cats, and other pets.
We know what happens when people encounter wild animals like raccoons and rabbits. It is not a good situation. Your pets can easily encounter a rabid animal and then put themselves in a position where they can harm their human owners.
We have an efficacious, safe treatment. If you’re dealing with someone, it might make sense to ask them, “Do you own a pet? Are you vaccinating?” It may not be something you’d ever thought about, but what we don’t need is rabies back in a bigger way in the United States than it’s been in the past.
I think, as a matter of prudence and public health, maybe firing up that question, “Got a pet in the house and are you vaccinating,” could be part of taking a good history.
Dr. Caplan is director of the division of medical ethics at New York University Langone Medical Center, New York City. He disclosed conflicts of interest with Johnson & Johnson and Medscape.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
In my job, I spend 99% of my time thinking about ethical issues that arise in the care of human beings. That is the focus of our medical school, and that’s what we do.
However,
Recently, there has been a great increase in the number of pet owners who are saying, “I’m not going to vaccinate my pets.” As horrible as this sounds, what’s happening is vaccine hesitancy about vaccines used in humans is extending through some people to their pets.
The number of people who say they don’t trust things like rabies vaccine to be effective or safe for their pet animals is 40%, at least in surveys, and the American Veterinary Medical Association reports that 15%-18% of pet owners are not, in fact, vaccinating their pets against rabies.
Rabies, as I hope everybody knows, is one horrible disease. Even the treatment of it, should you get bitten by a rabid animal, is no fun, expensive, and hopefully something that can be administered quickly. It’s not always the case. Worldwide, at least 70,000 people die from rabies every year.
Obviously, there are many countries that are so terrified of rabies, they won’t let you bring pets in without quarantining them, say, England, for at least 6 months to a year, I believe, because they don’t want rabies getting into their country. They’re very strict about the movement of pets.
It is inexcusable for people, first, not to give their pets vaccines that prevent them getting distemper, parvovirus, or many other diseases that harm the pet. It’s also inexcusable to shorten your pet’s life or ask your patients to care for pets who get sick from many of these diseases that are vaccine preventable.
Worst of all, it’s inexcusable for any pet owner not to give a rabies vaccine to their pets. Were it up to me, I’d say you have to license your pet, and as part of that, you must mandate rabies vaccines for your dogs, cats, and other pets.
We know what happens when people encounter wild animals like raccoons and rabbits. It is not a good situation. Your pets can easily encounter a rabid animal and then put themselves in a position where they can harm their human owners.
We have an efficacious, safe treatment. If you’re dealing with someone, it might make sense to ask them, “Do you own a pet? Are you vaccinating?” It may not be something you’d ever thought about, but what we don’t need is rabies back in a bigger way in the United States than it’s been in the past.
I think, as a matter of prudence and public health, maybe firing up that question, “Got a pet in the house and are you vaccinating,” could be part of taking a good history.
Dr. Caplan is director of the division of medical ethics at New York University Langone Medical Center, New York City. He disclosed conflicts of interest with Johnson & Johnson and Medscape.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
In my job, I spend 99% of my time thinking about ethical issues that arise in the care of human beings. That is the focus of our medical school, and that’s what we do.
However,
Recently, there has been a great increase in the number of pet owners who are saying, “I’m not going to vaccinate my pets.” As horrible as this sounds, what’s happening is vaccine hesitancy about vaccines used in humans is extending through some people to their pets.
The number of people who say they don’t trust things like rabies vaccine to be effective or safe for their pet animals is 40%, at least in surveys, and the American Veterinary Medical Association reports that 15%-18% of pet owners are not, in fact, vaccinating their pets against rabies.
Rabies, as I hope everybody knows, is one horrible disease. Even the treatment of it, should you get bitten by a rabid animal, is no fun, expensive, and hopefully something that can be administered quickly. It’s not always the case. Worldwide, at least 70,000 people die from rabies every year.
Obviously, there are many countries that are so terrified of rabies, they won’t let you bring pets in without quarantining them, say, England, for at least 6 months to a year, I believe, because they don’t want rabies getting into their country. They’re very strict about the movement of pets.
It is inexcusable for people, first, not to give their pets vaccines that prevent them getting distemper, parvovirus, or many other diseases that harm the pet. It’s also inexcusable to shorten your pet’s life or ask your patients to care for pets who get sick from many of these diseases that are vaccine preventable.
Worst of all, it’s inexcusable for any pet owner not to give a rabies vaccine to their pets. Were it up to me, I’d say you have to license your pet, and as part of that, you must mandate rabies vaccines for your dogs, cats, and other pets.
We know what happens when people encounter wild animals like raccoons and rabbits. It is not a good situation. Your pets can easily encounter a rabid animal and then put themselves in a position where they can harm their human owners.
We have an efficacious, safe treatment. If you’re dealing with someone, it might make sense to ask them, “Do you own a pet? Are you vaccinating?” It may not be something you’d ever thought about, but what we don’t need is rabies back in a bigger way in the United States than it’s been in the past.
I think, as a matter of prudence and public health, maybe firing up that question, “Got a pet in the house and are you vaccinating,” could be part of taking a good history.
Dr. Caplan is director of the division of medical ethics at New York University Langone Medical Center, New York City. He disclosed conflicts of interest with Johnson & Johnson and Medscape.
A version of this article first appeared on Medscape.com.
The Battle Against Recurrent UTIs in Welsh Women
TOPLINE:
The prevalence of recurrent urinary tract infections (rUTIs) and the use of antibiotics for prevention are substantial among women in Wales, particularly among those over the age of 57 years. A high level of resistance to two recommended antibiotics was observed, suggesting that more frequent urine cultures could better guide antibiotic selection for treatment and prophylaxis.
METHODOLOGY:
- The researchers conducted a retrospective cross-sectional study using a large databank of patients in Wales to describe the characteristics and urine profiles of women with rUTIs between 2010 and 2022.
- They created two cohorts: One with 92,213 women (median age, 60 years) who experienced rUTIs, defined as two or more acute episodes within 6 months or three or more acute episodes within 12 months.
- Another cohort comprised of 26,862 women (median age, 71 years) were prescribed prophylactic antibiotics, which was defined as receiving three or more consecutive prescriptions of the same UTI-specific antibiotic (trimethoprim, nitrofurantoin, or cefalexin), with intervals of 21-56 days between prescriptions.
- Urine culture results in the 12 months before a rUTI diagnosis and 18 months before prophylactic antibiotic initiation and all urine culture results within 7 days of an acute UTI were analyzed to assess antibiotic resistance patterns.
TAKEAWAY:
- Overall, 6% of women had rUTIs, 1.7% of which were prescribed prophylactic antibiotics with proportions increasing sharply after age 57.
- Nearly half of the women (49%) who were prescribed a prophylactic antibiotic qualified as having rUTIs in the 18 months before initiation.
- This study showed that 80.8% of women with rUTIs had a urine culture result documented in the 12 months preceding the diagnosis.
- More than half (64%) of the women taking prophylactic antibiotics had a urine culture result documented before starting treatment, and 18% of those prescribed trimethoprim had resistance to the antibiotic.
IN PRACTICE:
“More frequent urine cultures in the workup of rUTI diagnosis and prophylactic antibiotic initiation could better inform antibiotic choice,” the authors wrote.
SOURCE:
The study was led by Leigh Sanyaolu, BSc (Hons), MRCS, MRCGP, PGDip, a general practitioner from the Division of Population Medicine and PRIME Centre Wales at Cardiff University in Cardiff, and was published online in the British Journal of General Practice.
LIMITATIONS:
The study’s reliance on electronic health records may have led to coding errors and missing data. The diagnosis of UTIs may have been difficult in older women with increased frailty as they can have fewer specific symptoms and asymptomatic bacteriuria, which can be misdiagnosed as a UTI.
DISCLOSURES:
This work was supported by Health and Care Research Wales. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
The prevalence of recurrent urinary tract infections (rUTIs) and the use of antibiotics for prevention are substantial among women in Wales, particularly among those over the age of 57 years. A high level of resistance to two recommended antibiotics was observed, suggesting that more frequent urine cultures could better guide antibiotic selection for treatment and prophylaxis.
METHODOLOGY:
- The researchers conducted a retrospective cross-sectional study using a large databank of patients in Wales to describe the characteristics and urine profiles of women with rUTIs between 2010 and 2022.
- They created two cohorts: One with 92,213 women (median age, 60 years) who experienced rUTIs, defined as two or more acute episodes within 6 months or three or more acute episodes within 12 months.
- Another cohort comprised of 26,862 women (median age, 71 years) were prescribed prophylactic antibiotics, which was defined as receiving three or more consecutive prescriptions of the same UTI-specific antibiotic (trimethoprim, nitrofurantoin, or cefalexin), with intervals of 21-56 days between prescriptions.
- Urine culture results in the 12 months before a rUTI diagnosis and 18 months before prophylactic antibiotic initiation and all urine culture results within 7 days of an acute UTI were analyzed to assess antibiotic resistance patterns.
TAKEAWAY:
- Overall, 6% of women had rUTIs, 1.7% of which were prescribed prophylactic antibiotics with proportions increasing sharply after age 57.
- Nearly half of the women (49%) who were prescribed a prophylactic antibiotic qualified as having rUTIs in the 18 months before initiation.
- This study showed that 80.8% of women with rUTIs had a urine culture result documented in the 12 months preceding the diagnosis.
- More than half (64%) of the women taking prophylactic antibiotics had a urine culture result documented before starting treatment, and 18% of those prescribed trimethoprim had resistance to the antibiotic.
IN PRACTICE:
“More frequent urine cultures in the workup of rUTI diagnosis and prophylactic antibiotic initiation could better inform antibiotic choice,” the authors wrote.
SOURCE:
The study was led by Leigh Sanyaolu, BSc (Hons), MRCS, MRCGP, PGDip, a general practitioner from the Division of Population Medicine and PRIME Centre Wales at Cardiff University in Cardiff, and was published online in the British Journal of General Practice.
LIMITATIONS:
The study’s reliance on electronic health records may have led to coding errors and missing data. The diagnosis of UTIs may have been difficult in older women with increased frailty as they can have fewer specific symptoms and asymptomatic bacteriuria, which can be misdiagnosed as a UTI.
DISCLOSURES:
This work was supported by Health and Care Research Wales. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
The prevalence of recurrent urinary tract infections (rUTIs) and the use of antibiotics for prevention are substantial among women in Wales, particularly among those over the age of 57 years. A high level of resistance to two recommended antibiotics was observed, suggesting that more frequent urine cultures could better guide antibiotic selection for treatment and prophylaxis.
METHODOLOGY:
- The researchers conducted a retrospective cross-sectional study using a large databank of patients in Wales to describe the characteristics and urine profiles of women with rUTIs between 2010 and 2022.
- They created two cohorts: One with 92,213 women (median age, 60 years) who experienced rUTIs, defined as two or more acute episodes within 6 months or three or more acute episodes within 12 months.
- Another cohort comprised of 26,862 women (median age, 71 years) were prescribed prophylactic antibiotics, which was defined as receiving three or more consecutive prescriptions of the same UTI-specific antibiotic (trimethoprim, nitrofurantoin, or cefalexin), with intervals of 21-56 days between prescriptions.
- Urine culture results in the 12 months before a rUTI diagnosis and 18 months before prophylactic antibiotic initiation and all urine culture results within 7 days of an acute UTI were analyzed to assess antibiotic resistance patterns.
TAKEAWAY:
- Overall, 6% of women had rUTIs, 1.7% of which were prescribed prophylactic antibiotics with proportions increasing sharply after age 57.
- Nearly half of the women (49%) who were prescribed a prophylactic antibiotic qualified as having rUTIs in the 18 months before initiation.
- This study showed that 80.8% of women with rUTIs had a urine culture result documented in the 12 months preceding the diagnosis.
- More than half (64%) of the women taking prophylactic antibiotics had a urine culture result documented before starting treatment, and 18% of those prescribed trimethoprim had resistance to the antibiotic.
IN PRACTICE:
“More frequent urine cultures in the workup of rUTI diagnosis and prophylactic antibiotic initiation could better inform antibiotic choice,” the authors wrote.
SOURCE:
The study was led by Leigh Sanyaolu, BSc (Hons), MRCS, MRCGP, PGDip, a general practitioner from the Division of Population Medicine and PRIME Centre Wales at Cardiff University in Cardiff, and was published online in the British Journal of General Practice.
LIMITATIONS:
The study’s reliance on electronic health records may have led to coding errors and missing data. The diagnosis of UTIs may have been difficult in older women with increased frailty as they can have fewer specific symptoms and asymptomatic bacteriuria, which can be misdiagnosed as a UTI.
DISCLOSURES:
This work was supported by Health and Care Research Wales. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
The New Formula for Stronger, Longer-Lasting Vaccines
Vaccines work pretty well. But with a little help, they could work better.
Stanford researchers have developed a new vaccine helper that combines two kinds of adjuvants, ingredients that improve a vaccine’s efficacy, in a novel, customizable system.
In lab tests, the experimental additive improved the effectiveness of COVID-19 and HIV vaccine candidates, though it could be adapted to stimulate immune responses to a variety of pathogens, the researchers said. It could also be used one day to fine-tune vaccines for vulnerable groups like young children, older adults, and those with compromised immune systems.
“Current vaccines are not perfect,” said lead study author Ben Ou, a PhD candidate and researcher in the lab of Eric Appel, PhD, an associate professor of materials science and engineering, at Stanford University in California. “Many fail to generate long-lasting immunity or immunity against closely related strains [such as] flu or COVID vaccines. One way to improve them is to design more potent vaccine adjuvants.”
The study marks an advance in an area of growing scientific interest: Combining different adjuvants to enhance the immune-stimulating effect.
The Stanford scientists developed sphere-shaped nanoparticles, like tiny round cages, made of saponins, immune-stimulating molecules common in adjuvant development. To these nanoparticles, they attached Toll-like receptor (TLR) agonists, molecules that have become a focus in vaccine research because they stimulate a variety of immune responses.
Dr. Ou and the team tested the new adjuvant platform in COVID and HIV vaccines, comparing it to vaccines containing alum, a widely used adjuvant. (Alum is not used in COVID vaccines available in the United States.)
The nanoparticle-adjuvanted vaccines triggered stronger, longer-lasting effects.
Notably, the combination of the new adjuvant system with a SARS-CoV-2 virus vaccine was effective in mice against the original SARS-CoV-2 virus and against Delta, Omicron, and other variants that emerged in the months and years after the initial outbreak.
“Since our nanoparticle adjuvant platform is more potent than traditional/clinical vaccine adjuvants,” Dr. Ou said, “we expected mice to produce broadly neutralizing antibodies and better breadth responses.”
100 Years of Adjuvants
The first vaccine adjuvants were aluminum salts mixed into shots against pertussis, diphtheria, and tetanus in the 1920s. Today, alum is still used in many vaccines, including shots for diphtheria, tetanus, and pertussis; hepatitis A and B; human papillomavirus; and pneumococcal disease.
But since the 1990s, new adjuvants have come on the scene. Saponin-based compounds, harvested from the soapbark tree, are used in the Novavax COVID-19 Vaccine, Adjuvanted; a synthetic DNA adjuvant in the Heplisav-B vaccine against hepatitis B; and oil in water adjuvants using squalene in the Fluad and Fluad Quadrivalent influenza vaccines. Other vaccines, including those for chickenpox, cholera, measles, mumps, rubella, and mRNA-based COVID vaccines from Pfizer-BioNTech and Moderna, don’t contain adjuvants.
TLR agonists have recently become research hotspots in vaccine science.
“TLR agonists activate the innate immune system, putting it on a heightened alert state that can result in a higher antibody production and longer-lasting protection,” said David Burkhart, PhD, a research professor in biomedical and pharmaceutical sciences at the University of Montana in Missoula. He is also the chief operating officer of Inimmune, a biotech company developing vaccines and immunotherapies.
Dr. Burkhart studies TLR agonists in vaccines and other applications. “Different combinations activate different parts of the immune system,” he said. “TLR4 might activate the army, while TLR7 might activate the air force. You might need both in one vaccine.”
TLR agonists have also shown promise against Alzheimer’s disease, allergies, cancer, and even addiction. In immune’s experimental immunotherapy using TLR agonists for advanced solid tumors has just entered human trials, and the company is looking at a TLR agonist therapy for allergic rhinitis.
Combining Forces
In the new study, researchers tested five different combinations of TLR agonists hooked to the saponin nanoparticle framework. Each elicited a slightly different response from the immune cells.
“Our immune systems generate different downstream immune responses based on which TLRs are activated,” Dr. Ou said.
Ultimately, the advance could spur the development of vaccines tuned for stronger immune protection.
“We need different immune responses to fight different types of pathogens,” Dr. Ou said. “Depending on what specific virus/disease the vaccine is formulated for, activation of one specific TLR may confer better protection than another TLR.”
According to Dr. Burkhart, combining a saponin with a TLR agonist has found success before.
Biopharma company GSK (formerly GlaxoSmithKline) used the combination in its AS01 adjuvant, in the vaccine Shingrix against herpes zoster. The live-attenuated yellow fever vaccine, given to more than 600 million people around the world and considered one of the most powerful vaccines ever developed, uses several TLR agonists.
The Stanford paper, Dr. Burkhart said, “is a nice demonstration of the enhanced efficacy [that] adjuvants can provide to vaccines by exploiting the synergy different adjuvants and TLR agonists can provide when used in combination.”
Tailoring Vaccines
The customizable aspect of TLR agonists is important too, Dr. Burkhart said.
“The human immune system changes dramatically from birth to childhood into adulthood into older maturity,” he said. “It’s not a one-size-fits-all. Vaccines need to be tailored to these populations for maximum effectiveness and safety. TLRAs [TLR agonists] are a highly valuable tool in the vaccine toolbox. I think it’s inevitable we’ll have more in the future.”
That’s what the Stanford researchers hope for.
They noted in the study that the nanoparticle platform could easily be used to test different TLR agonist adjuvant combinations in vaccines.
But human studies are still a ways off. Tests in larger animals would likely come next, Dr. Ou said.
“We now have a single nanoparticle adjuvant platform with formulations containing different TLRs,” Dr. Ou said. “Scientists can pick which specific formulation is the most suitable for their needs.”
A version of this article first appeared on Medscape.com.
Vaccines work pretty well. But with a little help, they could work better.
Stanford researchers have developed a new vaccine helper that combines two kinds of adjuvants, ingredients that improve a vaccine’s efficacy, in a novel, customizable system.
In lab tests, the experimental additive improved the effectiveness of COVID-19 and HIV vaccine candidates, though it could be adapted to stimulate immune responses to a variety of pathogens, the researchers said. It could also be used one day to fine-tune vaccines for vulnerable groups like young children, older adults, and those with compromised immune systems.
“Current vaccines are not perfect,” said lead study author Ben Ou, a PhD candidate and researcher in the lab of Eric Appel, PhD, an associate professor of materials science and engineering, at Stanford University in California. “Many fail to generate long-lasting immunity or immunity against closely related strains [such as] flu or COVID vaccines. One way to improve them is to design more potent vaccine adjuvants.”
The study marks an advance in an area of growing scientific interest: Combining different adjuvants to enhance the immune-stimulating effect.
The Stanford scientists developed sphere-shaped nanoparticles, like tiny round cages, made of saponins, immune-stimulating molecules common in adjuvant development. To these nanoparticles, they attached Toll-like receptor (TLR) agonists, molecules that have become a focus in vaccine research because they stimulate a variety of immune responses.
Dr. Ou and the team tested the new adjuvant platform in COVID and HIV vaccines, comparing it to vaccines containing alum, a widely used adjuvant. (Alum is not used in COVID vaccines available in the United States.)
The nanoparticle-adjuvanted vaccines triggered stronger, longer-lasting effects.
Notably, the combination of the new adjuvant system with a SARS-CoV-2 virus vaccine was effective in mice against the original SARS-CoV-2 virus and against Delta, Omicron, and other variants that emerged in the months and years after the initial outbreak.
“Since our nanoparticle adjuvant platform is more potent than traditional/clinical vaccine adjuvants,” Dr. Ou said, “we expected mice to produce broadly neutralizing antibodies and better breadth responses.”
100 Years of Adjuvants
The first vaccine adjuvants were aluminum salts mixed into shots against pertussis, diphtheria, and tetanus in the 1920s. Today, alum is still used in many vaccines, including shots for diphtheria, tetanus, and pertussis; hepatitis A and B; human papillomavirus; and pneumococcal disease.
But since the 1990s, new adjuvants have come on the scene. Saponin-based compounds, harvested from the soapbark tree, are used in the Novavax COVID-19 Vaccine, Adjuvanted; a synthetic DNA adjuvant in the Heplisav-B vaccine against hepatitis B; and oil in water adjuvants using squalene in the Fluad and Fluad Quadrivalent influenza vaccines. Other vaccines, including those for chickenpox, cholera, measles, mumps, rubella, and mRNA-based COVID vaccines from Pfizer-BioNTech and Moderna, don’t contain adjuvants.
TLR agonists have recently become research hotspots in vaccine science.
“TLR agonists activate the innate immune system, putting it on a heightened alert state that can result in a higher antibody production and longer-lasting protection,” said David Burkhart, PhD, a research professor in biomedical and pharmaceutical sciences at the University of Montana in Missoula. He is also the chief operating officer of Inimmune, a biotech company developing vaccines and immunotherapies.
Dr. Burkhart studies TLR agonists in vaccines and other applications. “Different combinations activate different parts of the immune system,” he said. “TLR4 might activate the army, while TLR7 might activate the air force. You might need both in one vaccine.”
TLR agonists have also shown promise against Alzheimer’s disease, allergies, cancer, and even addiction. In immune’s experimental immunotherapy using TLR agonists for advanced solid tumors has just entered human trials, and the company is looking at a TLR agonist therapy for allergic rhinitis.
Combining Forces
In the new study, researchers tested five different combinations of TLR agonists hooked to the saponin nanoparticle framework. Each elicited a slightly different response from the immune cells.
“Our immune systems generate different downstream immune responses based on which TLRs are activated,” Dr. Ou said.
Ultimately, the advance could spur the development of vaccines tuned for stronger immune protection.
“We need different immune responses to fight different types of pathogens,” Dr. Ou said. “Depending on what specific virus/disease the vaccine is formulated for, activation of one specific TLR may confer better protection than another TLR.”
According to Dr. Burkhart, combining a saponin with a TLR agonist has found success before.
Biopharma company GSK (formerly GlaxoSmithKline) used the combination in its AS01 adjuvant, in the vaccine Shingrix against herpes zoster. The live-attenuated yellow fever vaccine, given to more than 600 million people around the world and considered one of the most powerful vaccines ever developed, uses several TLR agonists.
The Stanford paper, Dr. Burkhart said, “is a nice demonstration of the enhanced efficacy [that] adjuvants can provide to vaccines by exploiting the synergy different adjuvants and TLR agonists can provide when used in combination.”
Tailoring Vaccines
The customizable aspect of TLR agonists is important too, Dr. Burkhart said.
“The human immune system changes dramatically from birth to childhood into adulthood into older maturity,” he said. “It’s not a one-size-fits-all. Vaccines need to be tailored to these populations for maximum effectiveness and safety. TLRAs [TLR agonists] are a highly valuable tool in the vaccine toolbox. I think it’s inevitable we’ll have more in the future.”
That’s what the Stanford researchers hope for.
They noted in the study that the nanoparticle platform could easily be used to test different TLR agonist adjuvant combinations in vaccines.
But human studies are still a ways off. Tests in larger animals would likely come next, Dr. Ou said.
“We now have a single nanoparticle adjuvant platform with formulations containing different TLRs,” Dr. Ou said. “Scientists can pick which specific formulation is the most suitable for their needs.”
A version of this article first appeared on Medscape.com.
Vaccines work pretty well. But with a little help, they could work better.
Stanford researchers have developed a new vaccine helper that combines two kinds of adjuvants, ingredients that improve a vaccine’s efficacy, in a novel, customizable system.
In lab tests, the experimental additive improved the effectiveness of COVID-19 and HIV vaccine candidates, though it could be adapted to stimulate immune responses to a variety of pathogens, the researchers said. It could also be used one day to fine-tune vaccines for vulnerable groups like young children, older adults, and those with compromised immune systems.
“Current vaccines are not perfect,” said lead study author Ben Ou, a PhD candidate and researcher in the lab of Eric Appel, PhD, an associate professor of materials science and engineering, at Stanford University in California. “Many fail to generate long-lasting immunity or immunity against closely related strains [such as] flu or COVID vaccines. One way to improve them is to design more potent vaccine adjuvants.”
The study marks an advance in an area of growing scientific interest: Combining different adjuvants to enhance the immune-stimulating effect.
The Stanford scientists developed sphere-shaped nanoparticles, like tiny round cages, made of saponins, immune-stimulating molecules common in adjuvant development. To these nanoparticles, they attached Toll-like receptor (TLR) agonists, molecules that have become a focus in vaccine research because they stimulate a variety of immune responses.
Dr. Ou and the team tested the new adjuvant platform in COVID and HIV vaccines, comparing it to vaccines containing alum, a widely used adjuvant. (Alum is not used in COVID vaccines available in the United States.)
The nanoparticle-adjuvanted vaccines triggered stronger, longer-lasting effects.
Notably, the combination of the new adjuvant system with a SARS-CoV-2 virus vaccine was effective in mice against the original SARS-CoV-2 virus and against Delta, Omicron, and other variants that emerged in the months and years after the initial outbreak.
“Since our nanoparticle adjuvant platform is more potent than traditional/clinical vaccine adjuvants,” Dr. Ou said, “we expected mice to produce broadly neutralizing antibodies and better breadth responses.”
100 Years of Adjuvants
The first vaccine adjuvants were aluminum salts mixed into shots against pertussis, diphtheria, and tetanus in the 1920s. Today, alum is still used in many vaccines, including shots for diphtheria, tetanus, and pertussis; hepatitis A and B; human papillomavirus; and pneumococcal disease.
But since the 1990s, new adjuvants have come on the scene. Saponin-based compounds, harvested from the soapbark tree, are used in the Novavax COVID-19 Vaccine, Adjuvanted; a synthetic DNA adjuvant in the Heplisav-B vaccine against hepatitis B; and oil in water adjuvants using squalene in the Fluad and Fluad Quadrivalent influenza vaccines. Other vaccines, including those for chickenpox, cholera, measles, mumps, rubella, and mRNA-based COVID vaccines from Pfizer-BioNTech and Moderna, don’t contain adjuvants.
TLR agonists have recently become research hotspots in vaccine science.
“TLR agonists activate the innate immune system, putting it on a heightened alert state that can result in a higher antibody production and longer-lasting protection,” said David Burkhart, PhD, a research professor in biomedical and pharmaceutical sciences at the University of Montana in Missoula. He is also the chief operating officer of Inimmune, a biotech company developing vaccines and immunotherapies.
Dr. Burkhart studies TLR agonists in vaccines and other applications. “Different combinations activate different parts of the immune system,” he said. “TLR4 might activate the army, while TLR7 might activate the air force. You might need both in one vaccine.”
TLR agonists have also shown promise against Alzheimer’s disease, allergies, cancer, and even addiction. In immune’s experimental immunotherapy using TLR agonists for advanced solid tumors has just entered human trials, and the company is looking at a TLR agonist therapy for allergic rhinitis.
Combining Forces
In the new study, researchers tested five different combinations of TLR agonists hooked to the saponin nanoparticle framework. Each elicited a slightly different response from the immune cells.
“Our immune systems generate different downstream immune responses based on which TLRs are activated,” Dr. Ou said.
Ultimately, the advance could spur the development of vaccines tuned for stronger immune protection.
“We need different immune responses to fight different types of pathogens,” Dr. Ou said. “Depending on what specific virus/disease the vaccine is formulated for, activation of one specific TLR may confer better protection than another TLR.”
According to Dr. Burkhart, combining a saponin with a TLR agonist has found success before.
Biopharma company GSK (formerly GlaxoSmithKline) used the combination in its AS01 adjuvant, in the vaccine Shingrix against herpes zoster. The live-attenuated yellow fever vaccine, given to more than 600 million people around the world and considered one of the most powerful vaccines ever developed, uses several TLR agonists.
The Stanford paper, Dr. Burkhart said, “is a nice demonstration of the enhanced efficacy [that] adjuvants can provide to vaccines by exploiting the synergy different adjuvants and TLR agonists can provide when used in combination.”
Tailoring Vaccines
The customizable aspect of TLR agonists is important too, Dr. Burkhart said.
“The human immune system changes dramatically from birth to childhood into adulthood into older maturity,” he said. “It’s not a one-size-fits-all. Vaccines need to be tailored to these populations for maximum effectiveness and safety. TLRAs [TLR agonists] are a highly valuable tool in the vaccine toolbox. I think it’s inevitable we’ll have more in the future.”
That’s what the Stanford researchers hope for.
They noted in the study that the nanoparticle platform could easily be used to test different TLR agonist adjuvant combinations in vaccines.
But human studies are still a ways off. Tests in larger animals would likely come next, Dr. Ou said.
“We now have a single nanoparticle adjuvant platform with formulations containing different TLRs,” Dr. Ou said. “Scientists can pick which specific formulation is the most suitable for their needs.”
A version of this article first appeared on Medscape.com.
FROM SCIENCE ADVANCES
The Next Frontier of Antibiotic Discovery: Inside Your Gut
Scientists at Stanford University and the University of Pennsylvania have discovered a new antibiotic candidate in a surprising place: the human gut.
In mice, the antibiotic — a peptide known as prevotellin-2 — showed antimicrobial potency on par with polymyxin B, an antibiotic medication used to treat multidrug-resistant infections. Meanwhile, the peptide mainly left commensal, or beneficial, bacteria alone. The study, published in Cell, also identified several other potent antibiotic peptides with the potential to combat antimicrobial-resistant infections.
The research is part of a larger quest to find new antibiotics that can fight drug-resistant infections, a critical public health threat with more than 2.8 million cases and 35,000 deaths annually in the United States. That quest is urgent, said study author César de la Fuente, PhD, professor of bioengineering at the University of Pennsylvania, Philadelphia.
“The main pillars that have enabled us to almost double our lifespan in the last 100 years or so have been antibiotics, vaccines, and clean water,” said Dr. de la Fuente. “Imagine taking out one of those. I think it would be pretty dramatic.” (Dr. De la Fuente’s lab has become known for finding antibiotic candidates in unusual places, like ancient genetic information of Neanderthals and woolly mammoths.)
The first widely used antibiotic, penicillin, was discovered in 1928, when a physician studying Staphylococcus bacteria returned to his lab after summer break to find mold growing in one of his petri dishes. But many other antibiotics — like streptomycin, tetracycline, and erythromycin — were discovered from soil bacteria, which produce variations of these substances to compete with other microorganisms.
By looking in the gut microbiome, the researchers hoped to identify peptides that the trillions of microbes use against each other in the fight for limited resources — ideally, peptides that wouldn’t broadly kill off the entire microbiome.
Kill the Bad, Spare the Good
Many traditional antibiotics are small molecules. This means they can wipe out the good bacteria in your body, and because each targets a specific bacterial function, bad bacteria can become resistant to them.
Peptide antibiotics, on the other hand, don’t diffuse into the whole body. If taken orally, they stay in the gut; if taken intravenously, they generally stay in the blood. And because of how they kill bacteria, targeting the membrane, they’re also less prone to bacterial resistance.
The microbiome is like a big reservoir of pathogens, said Ami Bhatt, MD, PhD, hematologist at Stanford University in California and one of the study’s authors. Because many antibiotics kill healthy gut bacteria, “what you have left over,” Dr. Bhatt said, “is this big open niche that gets filled up with multidrug-resistant organisms like E coli [Escherichia coli] or vancomycin-resistant Enterococcus.”
Dr. Bhatt has seen cancer patients undergo successful treatment only to die of a multidrug-resistant infection, because current antibiotics fail against those pathogens. “That’s like winning the battle to lose the war.”
By investigating the microbiome, “we wanted to see if we could identify antimicrobial peptides that might spare key members of our regular microbiome, so that we wouldn’t totally disrupt the microbiome the way we do when we use broad-spectrum, small molecule–based antibiotics,” Dr. Bhatt said.
The researchers used artificial intelligence to sift through 400,000 proteins to predict, based on known antibiotics, which peptide sequences might have antimicrobial properties. From the results, they chose 78 peptides to synthesize and test.
“The application of computational approaches combined with experimental validation is very powerful and exciting,” said Jennifer Geddes-McAlister, PhD, professor of cell biology at the University of Guelph in Ontario, Canada, who was not involved in the study. “The study is robust in its approach to microbiome sampling.”
The Long Journey from Lab to Clinic
More than half of the peptides the team tested effectively inhibited the growth of harmful bacteria, and prevotellin-2 (derived from the bacteria Prevotella copri)stood out as the most powerful.
“The study validates experimental data from the lab using animal models, which moves discoveries closer to the clinic,” said Dr. Geddes-McAlister. “Further testing with clinical trials is needed, but the potential for clinical application is promising.”
Unfortunately, that’s not likely to happen anytime soon, said Dr. de la Fuente. “There is not enough economic incentive” for companies to develop new antibiotics. Ten years is his most hopeful guess for when we might see prevotellin-2, or a similar antibiotic, complete clinical trials.
A version of this article first appeared on Medscape.com.
Scientists at Stanford University and the University of Pennsylvania have discovered a new antibiotic candidate in a surprising place: the human gut.
In mice, the antibiotic — a peptide known as prevotellin-2 — showed antimicrobial potency on par with polymyxin B, an antibiotic medication used to treat multidrug-resistant infections. Meanwhile, the peptide mainly left commensal, or beneficial, bacteria alone. The study, published in Cell, also identified several other potent antibiotic peptides with the potential to combat antimicrobial-resistant infections.
The research is part of a larger quest to find new antibiotics that can fight drug-resistant infections, a critical public health threat with more than 2.8 million cases and 35,000 deaths annually in the United States. That quest is urgent, said study author César de la Fuente, PhD, professor of bioengineering at the University of Pennsylvania, Philadelphia.
“The main pillars that have enabled us to almost double our lifespan in the last 100 years or so have been antibiotics, vaccines, and clean water,” said Dr. de la Fuente. “Imagine taking out one of those. I think it would be pretty dramatic.” (Dr. De la Fuente’s lab has become known for finding antibiotic candidates in unusual places, like ancient genetic information of Neanderthals and woolly mammoths.)
The first widely used antibiotic, penicillin, was discovered in 1928, when a physician studying Staphylococcus bacteria returned to his lab after summer break to find mold growing in one of his petri dishes. But many other antibiotics — like streptomycin, tetracycline, and erythromycin — were discovered from soil bacteria, which produce variations of these substances to compete with other microorganisms.
By looking in the gut microbiome, the researchers hoped to identify peptides that the trillions of microbes use against each other in the fight for limited resources — ideally, peptides that wouldn’t broadly kill off the entire microbiome.
Kill the Bad, Spare the Good
Many traditional antibiotics are small molecules. This means they can wipe out the good bacteria in your body, and because each targets a specific bacterial function, bad bacteria can become resistant to them.
Peptide antibiotics, on the other hand, don’t diffuse into the whole body. If taken orally, they stay in the gut; if taken intravenously, they generally stay in the blood. And because of how they kill bacteria, targeting the membrane, they’re also less prone to bacterial resistance.
The microbiome is like a big reservoir of pathogens, said Ami Bhatt, MD, PhD, hematologist at Stanford University in California and one of the study’s authors. Because many antibiotics kill healthy gut bacteria, “what you have left over,” Dr. Bhatt said, “is this big open niche that gets filled up with multidrug-resistant organisms like E coli [Escherichia coli] or vancomycin-resistant Enterococcus.”
Dr. Bhatt has seen cancer patients undergo successful treatment only to die of a multidrug-resistant infection, because current antibiotics fail against those pathogens. “That’s like winning the battle to lose the war.”
By investigating the microbiome, “we wanted to see if we could identify antimicrobial peptides that might spare key members of our regular microbiome, so that we wouldn’t totally disrupt the microbiome the way we do when we use broad-spectrum, small molecule–based antibiotics,” Dr. Bhatt said.
The researchers used artificial intelligence to sift through 400,000 proteins to predict, based on known antibiotics, which peptide sequences might have antimicrobial properties. From the results, they chose 78 peptides to synthesize and test.
“The application of computational approaches combined with experimental validation is very powerful and exciting,” said Jennifer Geddes-McAlister, PhD, professor of cell biology at the University of Guelph in Ontario, Canada, who was not involved in the study. “The study is robust in its approach to microbiome sampling.”
The Long Journey from Lab to Clinic
More than half of the peptides the team tested effectively inhibited the growth of harmful bacteria, and prevotellin-2 (derived from the bacteria Prevotella copri)stood out as the most powerful.
“The study validates experimental data from the lab using animal models, which moves discoveries closer to the clinic,” said Dr. Geddes-McAlister. “Further testing with clinical trials is needed, but the potential for clinical application is promising.”
Unfortunately, that’s not likely to happen anytime soon, said Dr. de la Fuente. “There is not enough economic incentive” for companies to develop new antibiotics. Ten years is his most hopeful guess for when we might see prevotellin-2, or a similar antibiotic, complete clinical trials.
A version of this article first appeared on Medscape.com.
Scientists at Stanford University and the University of Pennsylvania have discovered a new antibiotic candidate in a surprising place: the human gut.
In mice, the antibiotic — a peptide known as prevotellin-2 — showed antimicrobial potency on par with polymyxin B, an antibiotic medication used to treat multidrug-resistant infections. Meanwhile, the peptide mainly left commensal, or beneficial, bacteria alone. The study, published in Cell, also identified several other potent antibiotic peptides with the potential to combat antimicrobial-resistant infections.
The research is part of a larger quest to find new antibiotics that can fight drug-resistant infections, a critical public health threat with more than 2.8 million cases and 35,000 deaths annually in the United States. That quest is urgent, said study author César de la Fuente, PhD, professor of bioengineering at the University of Pennsylvania, Philadelphia.
“The main pillars that have enabled us to almost double our lifespan in the last 100 years or so have been antibiotics, vaccines, and clean water,” said Dr. de la Fuente. “Imagine taking out one of those. I think it would be pretty dramatic.” (Dr. De la Fuente’s lab has become known for finding antibiotic candidates in unusual places, like ancient genetic information of Neanderthals and woolly mammoths.)
The first widely used antibiotic, penicillin, was discovered in 1928, when a physician studying Staphylococcus bacteria returned to his lab after summer break to find mold growing in one of his petri dishes. But many other antibiotics — like streptomycin, tetracycline, and erythromycin — were discovered from soil bacteria, which produce variations of these substances to compete with other microorganisms.
By looking in the gut microbiome, the researchers hoped to identify peptides that the trillions of microbes use against each other in the fight for limited resources — ideally, peptides that wouldn’t broadly kill off the entire microbiome.
Kill the Bad, Spare the Good
Many traditional antibiotics are small molecules. This means they can wipe out the good bacteria in your body, and because each targets a specific bacterial function, bad bacteria can become resistant to them.
Peptide antibiotics, on the other hand, don’t diffuse into the whole body. If taken orally, they stay in the gut; if taken intravenously, they generally stay in the blood. And because of how they kill bacteria, targeting the membrane, they’re also less prone to bacterial resistance.
The microbiome is like a big reservoir of pathogens, said Ami Bhatt, MD, PhD, hematologist at Stanford University in California and one of the study’s authors. Because many antibiotics kill healthy gut bacteria, “what you have left over,” Dr. Bhatt said, “is this big open niche that gets filled up with multidrug-resistant organisms like E coli [Escherichia coli] or vancomycin-resistant Enterococcus.”
Dr. Bhatt has seen cancer patients undergo successful treatment only to die of a multidrug-resistant infection, because current antibiotics fail against those pathogens. “That’s like winning the battle to lose the war.”
By investigating the microbiome, “we wanted to see if we could identify antimicrobial peptides that might spare key members of our regular microbiome, so that we wouldn’t totally disrupt the microbiome the way we do when we use broad-spectrum, small molecule–based antibiotics,” Dr. Bhatt said.
The researchers used artificial intelligence to sift through 400,000 proteins to predict, based on known antibiotics, which peptide sequences might have antimicrobial properties. From the results, they chose 78 peptides to synthesize and test.
“The application of computational approaches combined with experimental validation is very powerful and exciting,” said Jennifer Geddes-McAlister, PhD, professor of cell biology at the University of Guelph in Ontario, Canada, who was not involved in the study. “The study is robust in its approach to microbiome sampling.”
The Long Journey from Lab to Clinic
More than half of the peptides the team tested effectively inhibited the growth of harmful bacteria, and prevotellin-2 (derived from the bacteria Prevotella copri)stood out as the most powerful.
“The study validates experimental data from the lab using animal models, which moves discoveries closer to the clinic,” said Dr. Geddes-McAlister. “Further testing with clinical trials is needed, but the potential for clinical application is promising.”
Unfortunately, that’s not likely to happen anytime soon, said Dr. de la Fuente. “There is not enough economic incentive” for companies to develop new antibiotics. Ten years is his most hopeful guess for when we might see prevotellin-2, or a similar antibiotic, complete clinical trials.
A version of this article first appeared on Medscape.com.
FROM CELL
PrEP Prescription Pickups Vary With Prescriber Specialty
Preexposure prophylaxis prescription reversals and abandonments were lower for patients seen by primary care clinicians than by other non–infectious disease clinicians, based on data from approximately 37,000 individuals.
Although preexposure prophylaxis (PrEP) has been associated with a reduced risk of HIV (human immunodeficiency virus) infection when used as prescribed, the association between PrEP prescription pickup and specialty of the prescribing clinician has not been examined, wrote Lorraine T. Dean, ScD, an epidemiologist at Johns Hopkins University, Baltimore, Maryland, and colleagues.
“HIV PrEP is highly effective at preventing new HIV cases, and while use is on the rise, is still used much less than it should be by people who are at risk of HIV,” Dr. Dean said in an interview. “This study is helpful in pinpointing who is at risk for not picking up PrEP and in helping us think through how to reach them so that they can be better positioned to get PrEP,” she said.
In a study published in JAMA Internal Medicine, the researchers reviewed data for PrEP care. The study population included 37,003 patients aged 18 years and older who received new insurer-approved PrEP prescriptions between 2015 and 2019. Most of the patients (77%) ranged in age from 25 to 64 years; 88% were male.
Pharmacy claims data were matched with clinician data from the US National Plan and Provider Enumeration System.
Clinicians were divided into three groups: primary care providers (PCPs), infectious disease specialists (IDs), and other specialists (defined as any clinician prescribing PrEP but not classified as a PCP or an ID specialist). The main binary outcomes were prescription reversal (defined as when a patient failed to retrieve a prescription) and abandonment (defined as when a patient neglected to pick up a prescription for 1 year).
Overall, of 24,604 patients 67% received prescriptions from PCPs, 3,571 (10%) received prescriptions from ID specialists, and 8828 (24%) received prescriptions from other specialty clinicians.
The prevalence of reversals for patients seen by PCPs, ID specialists, and other specialty clinicians was 18%, 18%, and 25%, respectively. The prevalence of abandonments by clinician group was 12%, 12%, and 20%, respectively.
In a regression analysis, patients prescribed PrEP by ID specialists had 10% lower odds of reversals and 12% lower odds of abandonments compared to those seen by PCPs (odds ratio 0.90 and 0.88, respectively). However, patients seen by other clinicians (not primary care or ID) were 33% and 54% more likely to have reversals and abandonments, respectively, compared with those seen by PCPs.
Many patients at risk for HIV first see a PCP and then are referred to a specialist, such as an ID physician, Dr. Dean said. “The patients who take the time to then follow up with a specialist may be most motivated and able to follow through with the specialist’s request, in this case, accessing their PrEP prescription,” she said. In the current study, the researchers were most surprised by how many other specialty providers are involved in PrEP care, which is very positive given the effectiveness of the medication, she noted.
“Our results suggest that a wide range of prescribers, regardless of specialty, should be equipped to prescribe PrEP as well as offer PrEP counseling,” Dr. Dean said. A key takeaway for clinicians is that PrEP should have no cost for the majority of patients in the United States, she emphasized. The absence of cost expands the population who should be interested and able to access PrEP, she said. Therefore, providers should be prepared to recommend PrEP to eligible patients, and seek training or continuing medical education for themselves so they feel equipped to prescribe and counsel patients on PrEP, she said.
“One limitation of this work is that, while it can point to what is happening, it cannot tell us why the reversals are happening; what is the reason patients prescribed by certain providers are more or less likely to get their PrEP,” Dr. Dean explained. “We have tried to do interviews with patients to understand why this might be happening, but it’s hard to find people who aren’t showing up to do something, compared to finding people who are showing up to do something,” she said. Alternatively, researchers could interview providers to understand their perspective on why differences in prescription pickups occur across specialties, she said.
Looking ahead, “a national PrEP program that includes elements of required clinician training could be beneficial, and research on how a national PrEP program could be implemented and impact HIV rates would be helpful in considering this strategy of prevention,” said Dr. Dean.
Support All Prescribers to Increase PrEP Adherence
Differences in uptake of PrEP prescriptions may be explained by the different populations seen by various specialties, Meredith Green, MD, of Indiana University School of Medicine, Indianapolis, and Lona Mody, MD, of the University of Michigan, Ann Arbor, wrote in an accompanying editorial. However, the key question is how to support all prescribers and promote initiation and adherence to PrEP, they said.
Considerations include whether people at risk for HIV prefer to discuss PrEP with a clinician they already know, vs. a new specialist, but many PCPs are not familiar with the latest PrEP guidelines, they said.
“Interventions that support PrEP provision by PCPs, especially since they prescribed the largest proportion of PrEP prescriptions, can accelerate the uptake of PrEP,” the editorialists wrote.
“Supporting a diverse clinician workforce reflective of communities most impacted by HIV will remain critical, as will acknowledging and addressing HIV stigma,” they said. Educational interventions, including online programs and specialist access for complex cases, would help as well, they said. The approval of additional PrEP agents since the current study was conducted make it even more important to support PrEP prescribers and promote treatment adherence for those at risk for HIV, Dr. Green and Dr. Mody emphasized.
The study was funded by the National Institutes of Health. Dr. Dean had no financial conflicts to disclose. Dr. Green disclosed grants from Gilead and royalties from Wolters Kluwer unrelated to the current study; she also disclosed serving on the Centers for Disease Control and Prevention/Health Resources and Services Administration advisory committee on HIV, viral hepatitis, and sexually transmitted infection prevention and treatment. Dr. Mody disclosed grants from the US National Institute on Aging, Veterans Affairs, Centers for Disease Control and Prevention, NanoVibronix, and UpToDate unrelated to the current study.
Preexposure prophylaxis prescription reversals and abandonments were lower for patients seen by primary care clinicians than by other non–infectious disease clinicians, based on data from approximately 37,000 individuals.
Although preexposure prophylaxis (PrEP) has been associated with a reduced risk of HIV (human immunodeficiency virus) infection when used as prescribed, the association between PrEP prescription pickup and specialty of the prescribing clinician has not been examined, wrote Lorraine T. Dean, ScD, an epidemiologist at Johns Hopkins University, Baltimore, Maryland, and colleagues.
“HIV PrEP is highly effective at preventing new HIV cases, and while use is on the rise, is still used much less than it should be by people who are at risk of HIV,” Dr. Dean said in an interview. “This study is helpful in pinpointing who is at risk for not picking up PrEP and in helping us think through how to reach them so that they can be better positioned to get PrEP,” she said.
In a study published in JAMA Internal Medicine, the researchers reviewed data for PrEP care. The study population included 37,003 patients aged 18 years and older who received new insurer-approved PrEP prescriptions between 2015 and 2019. Most of the patients (77%) ranged in age from 25 to 64 years; 88% were male.
Pharmacy claims data were matched with clinician data from the US National Plan and Provider Enumeration System.
Clinicians were divided into three groups: primary care providers (PCPs), infectious disease specialists (IDs), and other specialists (defined as any clinician prescribing PrEP but not classified as a PCP or an ID specialist). The main binary outcomes were prescription reversal (defined as when a patient failed to retrieve a prescription) and abandonment (defined as when a patient neglected to pick up a prescription for 1 year).
Overall, of 24,604 patients 67% received prescriptions from PCPs, 3,571 (10%) received prescriptions from ID specialists, and 8828 (24%) received prescriptions from other specialty clinicians.
The prevalence of reversals for patients seen by PCPs, ID specialists, and other specialty clinicians was 18%, 18%, and 25%, respectively. The prevalence of abandonments by clinician group was 12%, 12%, and 20%, respectively.
In a regression analysis, patients prescribed PrEP by ID specialists had 10% lower odds of reversals and 12% lower odds of abandonments compared to those seen by PCPs (odds ratio 0.90 and 0.88, respectively). However, patients seen by other clinicians (not primary care or ID) were 33% and 54% more likely to have reversals and abandonments, respectively, compared with those seen by PCPs.
Many patients at risk for HIV first see a PCP and then are referred to a specialist, such as an ID physician, Dr. Dean said. “The patients who take the time to then follow up with a specialist may be most motivated and able to follow through with the specialist’s request, in this case, accessing their PrEP prescription,” she said. In the current study, the researchers were most surprised by how many other specialty providers are involved in PrEP care, which is very positive given the effectiveness of the medication, she noted.
“Our results suggest that a wide range of prescribers, regardless of specialty, should be equipped to prescribe PrEP as well as offer PrEP counseling,” Dr. Dean said. A key takeaway for clinicians is that PrEP should have no cost for the majority of patients in the United States, she emphasized. The absence of cost expands the population who should be interested and able to access PrEP, she said. Therefore, providers should be prepared to recommend PrEP to eligible patients, and seek training or continuing medical education for themselves so they feel equipped to prescribe and counsel patients on PrEP, she said.
“One limitation of this work is that, while it can point to what is happening, it cannot tell us why the reversals are happening; what is the reason patients prescribed by certain providers are more or less likely to get their PrEP,” Dr. Dean explained. “We have tried to do interviews with patients to understand why this might be happening, but it’s hard to find people who aren’t showing up to do something, compared to finding people who are showing up to do something,” she said. Alternatively, researchers could interview providers to understand their perspective on why differences in prescription pickups occur across specialties, she said.
Looking ahead, “a national PrEP program that includes elements of required clinician training could be beneficial, and research on how a national PrEP program could be implemented and impact HIV rates would be helpful in considering this strategy of prevention,” said Dr. Dean.
Support All Prescribers to Increase PrEP Adherence
Differences in uptake of PrEP prescriptions may be explained by the different populations seen by various specialties, Meredith Green, MD, of Indiana University School of Medicine, Indianapolis, and Lona Mody, MD, of the University of Michigan, Ann Arbor, wrote in an accompanying editorial. However, the key question is how to support all prescribers and promote initiation and adherence to PrEP, they said.
Considerations include whether people at risk for HIV prefer to discuss PrEP with a clinician they already know, vs. a new specialist, but many PCPs are not familiar with the latest PrEP guidelines, they said.
“Interventions that support PrEP provision by PCPs, especially since they prescribed the largest proportion of PrEP prescriptions, can accelerate the uptake of PrEP,” the editorialists wrote.
“Supporting a diverse clinician workforce reflective of communities most impacted by HIV will remain critical, as will acknowledging and addressing HIV stigma,” they said. Educational interventions, including online programs and specialist access for complex cases, would help as well, they said. The approval of additional PrEP agents since the current study was conducted make it even more important to support PrEP prescribers and promote treatment adherence for those at risk for HIV, Dr. Green and Dr. Mody emphasized.
The study was funded by the National Institutes of Health. Dr. Dean had no financial conflicts to disclose. Dr. Green disclosed grants from Gilead and royalties from Wolters Kluwer unrelated to the current study; she also disclosed serving on the Centers for Disease Control and Prevention/Health Resources and Services Administration advisory committee on HIV, viral hepatitis, and sexually transmitted infection prevention and treatment. Dr. Mody disclosed grants from the US National Institute on Aging, Veterans Affairs, Centers for Disease Control and Prevention, NanoVibronix, and UpToDate unrelated to the current study.
Preexposure prophylaxis prescription reversals and abandonments were lower for patients seen by primary care clinicians than by other non–infectious disease clinicians, based on data from approximately 37,000 individuals.
Although preexposure prophylaxis (PrEP) has been associated with a reduced risk of HIV (human immunodeficiency virus) infection when used as prescribed, the association between PrEP prescription pickup and specialty of the prescribing clinician has not been examined, wrote Lorraine T. Dean, ScD, an epidemiologist at Johns Hopkins University, Baltimore, Maryland, and colleagues.
“HIV PrEP is highly effective at preventing new HIV cases, and while use is on the rise, is still used much less than it should be by people who are at risk of HIV,” Dr. Dean said in an interview. “This study is helpful in pinpointing who is at risk for not picking up PrEP and in helping us think through how to reach them so that they can be better positioned to get PrEP,” she said.
In a study published in JAMA Internal Medicine, the researchers reviewed data for PrEP care. The study population included 37,003 patients aged 18 years and older who received new insurer-approved PrEP prescriptions between 2015 and 2019. Most of the patients (77%) ranged in age from 25 to 64 years; 88% were male.
Pharmacy claims data were matched with clinician data from the US National Plan and Provider Enumeration System.
Clinicians were divided into three groups: primary care providers (PCPs), infectious disease specialists (IDs), and other specialists (defined as any clinician prescribing PrEP but not classified as a PCP or an ID specialist). The main binary outcomes were prescription reversal (defined as when a patient failed to retrieve a prescription) and abandonment (defined as when a patient neglected to pick up a prescription for 1 year).
Overall, of 24,604 patients 67% received prescriptions from PCPs, 3,571 (10%) received prescriptions from ID specialists, and 8828 (24%) received prescriptions from other specialty clinicians.
The prevalence of reversals for patients seen by PCPs, ID specialists, and other specialty clinicians was 18%, 18%, and 25%, respectively. The prevalence of abandonments by clinician group was 12%, 12%, and 20%, respectively.
In a regression analysis, patients prescribed PrEP by ID specialists had 10% lower odds of reversals and 12% lower odds of abandonments compared to those seen by PCPs (odds ratio 0.90 and 0.88, respectively). However, patients seen by other clinicians (not primary care or ID) were 33% and 54% more likely to have reversals and abandonments, respectively, compared with those seen by PCPs.
Many patients at risk for HIV first see a PCP and then are referred to a specialist, such as an ID physician, Dr. Dean said. “The patients who take the time to then follow up with a specialist may be most motivated and able to follow through with the specialist’s request, in this case, accessing their PrEP prescription,” she said. In the current study, the researchers were most surprised by how many other specialty providers are involved in PrEP care, which is very positive given the effectiveness of the medication, she noted.
“Our results suggest that a wide range of prescribers, regardless of specialty, should be equipped to prescribe PrEP as well as offer PrEP counseling,” Dr. Dean said. A key takeaway for clinicians is that PrEP should have no cost for the majority of patients in the United States, she emphasized. The absence of cost expands the population who should be interested and able to access PrEP, she said. Therefore, providers should be prepared to recommend PrEP to eligible patients, and seek training or continuing medical education for themselves so they feel equipped to prescribe and counsel patients on PrEP, she said.
“One limitation of this work is that, while it can point to what is happening, it cannot tell us why the reversals are happening; what is the reason patients prescribed by certain providers are more or less likely to get their PrEP,” Dr. Dean explained. “We have tried to do interviews with patients to understand why this might be happening, but it’s hard to find people who aren’t showing up to do something, compared to finding people who are showing up to do something,” she said. Alternatively, researchers could interview providers to understand their perspective on why differences in prescription pickups occur across specialties, she said.
Looking ahead, “a national PrEP program that includes elements of required clinician training could be beneficial, and research on how a national PrEP program could be implemented and impact HIV rates would be helpful in considering this strategy of prevention,” said Dr. Dean.
Support All Prescribers to Increase PrEP Adherence
Differences in uptake of PrEP prescriptions may be explained by the different populations seen by various specialties, Meredith Green, MD, of Indiana University School of Medicine, Indianapolis, and Lona Mody, MD, of the University of Michigan, Ann Arbor, wrote in an accompanying editorial. However, the key question is how to support all prescribers and promote initiation and adherence to PrEP, they said.
Considerations include whether people at risk for HIV prefer to discuss PrEP with a clinician they already know, vs. a new specialist, but many PCPs are not familiar with the latest PrEP guidelines, they said.
“Interventions that support PrEP provision by PCPs, especially since they prescribed the largest proportion of PrEP prescriptions, can accelerate the uptake of PrEP,” the editorialists wrote.
“Supporting a diverse clinician workforce reflective of communities most impacted by HIV will remain critical, as will acknowledging and addressing HIV stigma,” they said. Educational interventions, including online programs and specialist access for complex cases, would help as well, they said. The approval of additional PrEP agents since the current study was conducted make it even more important to support PrEP prescribers and promote treatment adherence for those at risk for HIV, Dr. Green and Dr. Mody emphasized.
The study was funded by the National Institutes of Health. Dr. Dean had no financial conflicts to disclose. Dr. Green disclosed grants from Gilead and royalties from Wolters Kluwer unrelated to the current study; she also disclosed serving on the Centers for Disease Control and Prevention/Health Resources and Services Administration advisory committee on HIV, viral hepatitis, and sexually transmitted infection prevention and treatment. Dr. Mody disclosed grants from the US National Institute on Aging, Veterans Affairs, Centers for Disease Control and Prevention, NanoVibronix, and UpToDate unrelated to the current study.
FROM JAMA INTERNAL MEDICINE