User login
COVID-19 vaccine won’t be a slam dunk
A successful vaccine for prevention of SARS-CoV-2 infection will probably need to incorporate T-cell epitopes to induce a long-term memory T-cell immune response to the virus, Mehrdad Matloubian, MD, PhD, predicted at the virtual edition of the American College of Rheumatology’s 2020 State-of-the-Art Clinical Symposium.
Vaccine-induced neutralizing antibodies may not be sufficient to reliably provide sustained protection against infection. In mouse studies, T-cell immunity has protected against reinfection with the novel coronaviruses. And in some but not all studies of patients infected with the SARS virus, which shares 80% genetic overlap with the SARS-CoV-2 virus responsible for the COVID-19 pandemic, neutralizing antibodies have waned over time.
“In one study, 20 of 26 patients with SARS had lost their antibody response by 6 years post infection. And they had no B-cell immunity against the SARS antigens. The good news is they did have T-cell memory against SARS virus, and people with more severe disease tended to have more T-cell memory against SARS. All of this has really important implications for vaccine development,” observed Dr. Matloubian, a rheumatologist at the University of California, San Francisco.
Dr. Matloubian is among those who are convinced that the ongoing massive global accelerated effort to develop a safe and effective vaccine affords the best opportunity to gain the upper hand in the COVID-19 pandemic. A large array of vaccines are in development.
A key safety concern to watch for in the coming months is whether a vaccine candidate is able to sidestep the issue of antibody-dependent enhancement, whereby prior infection with a non-SARS coronavirus, such as those that cause the common cold, might result in creation of rogue subneutralizing coronavirus antibodies in response to vaccination. There is concern that these nonneutralizing antibodies could facilitate entry of the virus into monocytes and other cells lacking the ACE2 receptor, its usual portal of entry. This in turn could trigger expanded viral replication, a hyperinflammatory response, and viral spread to sites beyond the lung, such as the heart or kidneys.
Little optimism about antivirals’ impact
Dr. Matloubian predicted that antiviral medications, including the much-ballyhooed remdesivir, are unlikely to be a game changer in the COVID-19 pandemic. That’s because most patients who become symptomatic don’t do so until at least 2 days post infection. By that point, their viral load has already peaked and is waning and the B- and T-cell immune responses are starting to gear up.
“Timing seems to be everything when it comes to treatment with antivirals,” he observed. “The virus titer is usually declining by the time people present with severe COVID-19, suggesting that at this time antiviral therapy might be of little use to change the course of the disease, especially if it’s mainly immune-mediated by then. Even with influenza virus, there’s a really short window where Tamiflu [oseltamivir] is effective. It’s going to be the same case for antivirals used for treatment of COVID-19.”
He noted that in a placebo-controlled, randomized trial of remdesivir in 236 Chinese patients with severe COVID-19, intravenous remdesivir wasn’t associated with a significantly shorter time to clinical improvement, although there was a trend in that direction in the subgroup with symptom duration of 10 days or less at initiation of treatment.
A National Institutes of Health press release announcing that remdesivir had a positive impact on duration of hospitalization in a separate randomized trial drew enormous attention from a public desperate for good news. However, the full study has yet to be published, and it’s unclear when during the disease course the antiviral agent was started.
“We need a blockbuster antiviral that’s oral, highly effective, and doesn’t have any side effects to be used in prophylaxis of health care workers and for people who are exposed by family members being infected. And so far there is no such thing, even on the horizon,” according to the rheumatologist.
Fellow panelist Jinoos Yazdany, MD, concurred.
“As we talk to experts around the country, it seems like there isn’t very much optimism about such a blockbuster drug. Most people are actually putting their hope in a vaccine,” said Dr. Yazdany, professor of medicine at the University of California, San Francisco, and chief of rheumatology at San Francisco General Hospital.
Another research priority is identification of biomarkers in blood or bronchoalveolar lavage fluid to identify early on the subgroup of infected patients who are likely to crash and develop severe disease. That would permit a targeted approach to inhibition of the inflammatory pathways contributing to development of acute respiratory distress syndrome before this full-blown cytokine storm-like syndrome can occur. There is great interest in trying to achieve this by repurposing many biologic agents widely used by rheumatologists, including the interleukin-1 blocker anakinra (Kineret) and the IL-6 blocker tocilizumab (Actemra).
Dr. Matloubian reported having no financial conflicts of interest regarding his presentation.
A successful vaccine for prevention of SARS-CoV-2 infection will probably need to incorporate T-cell epitopes to induce a long-term memory T-cell immune response to the virus, Mehrdad Matloubian, MD, PhD, predicted at the virtual edition of the American College of Rheumatology’s 2020 State-of-the-Art Clinical Symposium.
Vaccine-induced neutralizing antibodies may not be sufficient to reliably provide sustained protection against infection. In mouse studies, T-cell immunity has protected against reinfection with the novel coronaviruses. And in some but not all studies of patients infected with the SARS virus, which shares 80% genetic overlap with the SARS-CoV-2 virus responsible for the COVID-19 pandemic, neutralizing antibodies have waned over time.
“In one study, 20 of 26 patients with SARS had lost their antibody response by 6 years post infection. And they had no B-cell immunity against the SARS antigens. The good news is they did have T-cell memory against SARS virus, and people with more severe disease tended to have more T-cell memory against SARS. All of this has really important implications for vaccine development,” observed Dr. Matloubian, a rheumatologist at the University of California, San Francisco.
Dr. Matloubian is among those who are convinced that the ongoing massive global accelerated effort to develop a safe and effective vaccine affords the best opportunity to gain the upper hand in the COVID-19 pandemic. A large array of vaccines are in development.
A key safety concern to watch for in the coming months is whether a vaccine candidate is able to sidestep the issue of antibody-dependent enhancement, whereby prior infection with a non-SARS coronavirus, such as those that cause the common cold, might result in creation of rogue subneutralizing coronavirus antibodies in response to vaccination. There is concern that these nonneutralizing antibodies could facilitate entry of the virus into monocytes and other cells lacking the ACE2 receptor, its usual portal of entry. This in turn could trigger expanded viral replication, a hyperinflammatory response, and viral spread to sites beyond the lung, such as the heart or kidneys.
Little optimism about antivirals’ impact
Dr. Matloubian predicted that antiviral medications, including the much-ballyhooed remdesivir, are unlikely to be a game changer in the COVID-19 pandemic. That’s because most patients who become symptomatic don’t do so until at least 2 days post infection. By that point, their viral load has already peaked and is waning and the B- and T-cell immune responses are starting to gear up.
“Timing seems to be everything when it comes to treatment with antivirals,” he observed. “The virus titer is usually declining by the time people present with severe COVID-19, suggesting that at this time antiviral therapy might be of little use to change the course of the disease, especially if it’s mainly immune-mediated by then. Even with influenza virus, there’s a really short window where Tamiflu [oseltamivir] is effective. It’s going to be the same case for antivirals used for treatment of COVID-19.”
He noted that in a placebo-controlled, randomized trial of remdesivir in 236 Chinese patients with severe COVID-19, intravenous remdesivir wasn’t associated with a significantly shorter time to clinical improvement, although there was a trend in that direction in the subgroup with symptom duration of 10 days or less at initiation of treatment.
A National Institutes of Health press release announcing that remdesivir had a positive impact on duration of hospitalization in a separate randomized trial drew enormous attention from a public desperate for good news. However, the full study has yet to be published, and it’s unclear when during the disease course the antiviral agent was started.
“We need a blockbuster antiviral that’s oral, highly effective, and doesn’t have any side effects to be used in prophylaxis of health care workers and for people who are exposed by family members being infected. And so far there is no such thing, even on the horizon,” according to the rheumatologist.
Fellow panelist Jinoos Yazdany, MD, concurred.
“As we talk to experts around the country, it seems like there isn’t very much optimism about such a blockbuster drug. Most people are actually putting their hope in a vaccine,” said Dr. Yazdany, professor of medicine at the University of California, San Francisco, and chief of rheumatology at San Francisco General Hospital.
Another research priority is identification of biomarkers in blood or bronchoalveolar lavage fluid to identify early on the subgroup of infected patients who are likely to crash and develop severe disease. That would permit a targeted approach to inhibition of the inflammatory pathways contributing to development of acute respiratory distress syndrome before this full-blown cytokine storm-like syndrome can occur. There is great interest in trying to achieve this by repurposing many biologic agents widely used by rheumatologists, including the interleukin-1 blocker anakinra (Kineret) and the IL-6 blocker tocilizumab (Actemra).
Dr. Matloubian reported having no financial conflicts of interest regarding his presentation.
A successful vaccine for prevention of SARS-CoV-2 infection will probably need to incorporate T-cell epitopes to induce a long-term memory T-cell immune response to the virus, Mehrdad Matloubian, MD, PhD, predicted at the virtual edition of the American College of Rheumatology’s 2020 State-of-the-Art Clinical Symposium.
Vaccine-induced neutralizing antibodies may not be sufficient to reliably provide sustained protection against infection. In mouse studies, T-cell immunity has protected against reinfection with the novel coronaviruses. And in some but not all studies of patients infected with the SARS virus, which shares 80% genetic overlap with the SARS-CoV-2 virus responsible for the COVID-19 pandemic, neutralizing antibodies have waned over time.
“In one study, 20 of 26 patients with SARS had lost their antibody response by 6 years post infection. And they had no B-cell immunity against the SARS antigens. The good news is they did have T-cell memory against SARS virus, and people with more severe disease tended to have more T-cell memory against SARS. All of this has really important implications for vaccine development,” observed Dr. Matloubian, a rheumatologist at the University of California, San Francisco.
Dr. Matloubian is among those who are convinced that the ongoing massive global accelerated effort to develop a safe and effective vaccine affords the best opportunity to gain the upper hand in the COVID-19 pandemic. A large array of vaccines are in development.
A key safety concern to watch for in the coming months is whether a vaccine candidate is able to sidestep the issue of antibody-dependent enhancement, whereby prior infection with a non-SARS coronavirus, such as those that cause the common cold, might result in creation of rogue subneutralizing coronavirus antibodies in response to vaccination. There is concern that these nonneutralizing antibodies could facilitate entry of the virus into monocytes and other cells lacking the ACE2 receptor, its usual portal of entry. This in turn could trigger expanded viral replication, a hyperinflammatory response, and viral spread to sites beyond the lung, such as the heart or kidneys.
Little optimism about antivirals’ impact
Dr. Matloubian predicted that antiviral medications, including the much-ballyhooed remdesivir, are unlikely to be a game changer in the COVID-19 pandemic. That’s because most patients who become symptomatic don’t do so until at least 2 days post infection. By that point, their viral load has already peaked and is waning and the B- and T-cell immune responses are starting to gear up.
“Timing seems to be everything when it comes to treatment with antivirals,” he observed. “The virus titer is usually declining by the time people present with severe COVID-19, suggesting that at this time antiviral therapy might be of little use to change the course of the disease, especially if it’s mainly immune-mediated by then. Even with influenza virus, there’s a really short window where Tamiflu [oseltamivir] is effective. It’s going to be the same case for antivirals used for treatment of COVID-19.”
He noted that in a placebo-controlled, randomized trial of remdesivir in 236 Chinese patients with severe COVID-19, intravenous remdesivir wasn’t associated with a significantly shorter time to clinical improvement, although there was a trend in that direction in the subgroup with symptom duration of 10 days or less at initiation of treatment.
A National Institutes of Health press release announcing that remdesivir had a positive impact on duration of hospitalization in a separate randomized trial drew enormous attention from a public desperate for good news. However, the full study has yet to be published, and it’s unclear when during the disease course the antiviral agent was started.
“We need a blockbuster antiviral that’s oral, highly effective, and doesn’t have any side effects to be used in prophylaxis of health care workers and for people who are exposed by family members being infected. And so far there is no such thing, even on the horizon,” according to the rheumatologist.
Fellow panelist Jinoos Yazdany, MD, concurred.
“As we talk to experts around the country, it seems like there isn’t very much optimism about such a blockbuster drug. Most people are actually putting their hope in a vaccine,” said Dr. Yazdany, professor of medicine at the University of California, San Francisco, and chief of rheumatology at San Francisco General Hospital.
Another research priority is identification of biomarkers in blood or bronchoalveolar lavage fluid to identify early on the subgroup of infected patients who are likely to crash and develop severe disease. That would permit a targeted approach to inhibition of the inflammatory pathways contributing to development of acute respiratory distress syndrome before this full-blown cytokine storm-like syndrome can occur. There is great interest in trying to achieve this by repurposing many biologic agents widely used by rheumatologists, including the interleukin-1 blocker anakinra (Kineret) and the IL-6 blocker tocilizumab (Actemra).
Dr. Matloubian reported having no financial conflicts of interest regarding his presentation.
FROM SOTA 2020
Vaccination regimen effective in preventing pneumonia in MM patients
Patients with hematological malignancies are at high risk of invasive Staphylococcus pneumoniae. Multiple myeloma (MM) patients, in particular, have been found to have one of the highest incidences of invasive pneumococcal disease. However, researchers found that a full three-dose vaccination regimen by 13-valent pneumococcal conjugate (PCV13) vaccine was protective in MM patients when provided between treatment courses, according to a study reported in Vaccine.
The researchers performed a prospective study of 18 adult patients who were vaccinated with PCV13, compared with 18 control-matched patients from 2017 to 2020. The three-dose vaccination regimen was provided between treatment courses with novel target agents (bortezomib, lenalidomide, ixazomib) with a minimum of a 1-month interval. They used the incidence of pneumonias during the one-year observation period as the primary outcome.
Totally there were 12 cases (33.3%) of clinically and radiologically confirmed pneumonias in the entire study group (n = 36), with a distribution between the vaccinated and nonvaccinated groups of 3 (16.7%) and 9 (50%). respectively (P = .037).
The absolute risk reduction seen with vaccination was 33.3%, and the number needed to treat with PCV13 vaccination in MM patients receiving novel agents was 3.0; (95% confidence interval 1.61-22.1). In addition, there were no adverse effects seen from vaccination, according to the authors.
“Despite the expected decrease in immunological response to vaccination during the chemotherapy, we have shown the clinical effectiveness of a PCV13 vaccination schedule based on 3 doses given with a minimum 1 month interval between the courses of novel agents,” the investigators concluded.
The authors reported that they had no relevant disclosures.
SOURCE: Stoma I et al. Vaccine. 2020 May 14; doi.org/10.1016/j.vaccine.2020.05.024.
Patients with hematological malignancies are at high risk of invasive Staphylococcus pneumoniae. Multiple myeloma (MM) patients, in particular, have been found to have one of the highest incidences of invasive pneumococcal disease. However, researchers found that a full three-dose vaccination regimen by 13-valent pneumococcal conjugate (PCV13) vaccine was protective in MM patients when provided between treatment courses, according to a study reported in Vaccine.
The researchers performed a prospective study of 18 adult patients who were vaccinated with PCV13, compared with 18 control-matched patients from 2017 to 2020. The three-dose vaccination regimen was provided between treatment courses with novel target agents (bortezomib, lenalidomide, ixazomib) with a minimum of a 1-month interval. They used the incidence of pneumonias during the one-year observation period as the primary outcome.
Totally there were 12 cases (33.3%) of clinically and radiologically confirmed pneumonias in the entire study group (n = 36), with a distribution between the vaccinated and nonvaccinated groups of 3 (16.7%) and 9 (50%). respectively (P = .037).
The absolute risk reduction seen with vaccination was 33.3%, and the number needed to treat with PCV13 vaccination in MM patients receiving novel agents was 3.0; (95% confidence interval 1.61-22.1). In addition, there were no adverse effects seen from vaccination, according to the authors.
“Despite the expected decrease in immunological response to vaccination during the chemotherapy, we have shown the clinical effectiveness of a PCV13 vaccination schedule based on 3 doses given with a minimum 1 month interval between the courses of novel agents,” the investigators concluded.
The authors reported that they had no relevant disclosures.
SOURCE: Stoma I et al. Vaccine. 2020 May 14; doi.org/10.1016/j.vaccine.2020.05.024.
Patients with hematological malignancies are at high risk of invasive Staphylococcus pneumoniae. Multiple myeloma (MM) patients, in particular, have been found to have one of the highest incidences of invasive pneumococcal disease. However, researchers found that a full three-dose vaccination regimen by 13-valent pneumococcal conjugate (PCV13) vaccine was protective in MM patients when provided between treatment courses, according to a study reported in Vaccine.
The researchers performed a prospective study of 18 adult patients who were vaccinated with PCV13, compared with 18 control-matched patients from 2017 to 2020. The three-dose vaccination regimen was provided between treatment courses with novel target agents (bortezomib, lenalidomide, ixazomib) with a minimum of a 1-month interval. They used the incidence of pneumonias during the one-year observation period as the primary outcome.
Totally there were 12 cases (33.3%) of clinically and radiologically confirmed pneumonias in the entire study group (n = 36), with a distribution between the vaccinated and nonvaccinated groups of 3 (16.7%) and 9 (50%). respectively (P = .037).
The absolute risk reduction seen with vaccination was 33.3%, and the number needed to treat with PCV13 vaccination in MM patients receiving novel agents was 3.0; (95% confidence interval 1.61-22.1). In addition, there were no adverse effects seen from vaccination, according to the authors.
“Despite the expected decrease in immunological response to vaccination during the chemotherapy, we have shown the clinical effectiveness of a PCV13 vaccination schedule based on 3 doses given with a minimum 1 month interval between the courses of novel agents,” the investigators concluded.
The authors reported that they had no relevant disclosures.
SOURCE: Stoma I et al. Vaccine. 2020 May 14; doi.org/10.1016/j.vaccine.2020.05.024.
FROM VACCINE
Atypical Features of COVID-19: A Literature Review
From the University of Florida College of Medicine, Division of Infectious Diseases and Global Medicine, Gainesville, FL.
Abstract
- Objective: To review current reports on atypical manifestations of coronavirus disease 2019 (COVID-19).
- Methods: Review of the literature.
- Results: Evidence regarding atypical features of COVID-19 is accumulating. SARS-CoV-2 can infect human cells that express the angiotensin-converting enzyme 2 receptor, which would allow for a broad spectrum of illnesses affecting the renal, cardiac, and gastrointestinal organ systems. Neurologic, cutaneous, and musculoskeletal manifestations have also been reported. The potential for SARS-CoV-2 to induce a hypercoagulable state provides another avenue for the virus to indirectly damage various organ systems, as evidenced by reports of cerebrovascular disease, myocardial injury, and a chilblain-like rash in patients with COVID-19.
- Conclusion: Because the signs and symptoms of COVID-19 may occur with varying frequency across populations, it is important to keep differentials broad when assessing patients with a clinical illness that may indeed be COVID-19.
Keywords: coronavirus; severe acute respiratory syndrome coronavirus-2; SARS-CoV-2; pandemic.
Coronavirus disease 2019 (COVID-19), the syndrome caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), was first reported in Wuhan, China, in early December 2019.1 Since then, the virus has spread quickly around the world, with the World Health Organization (WHO) declaring the coronavirus outbreak a global pandemic on March 11, 2020. As of May 21, 2020, more than 5,000,000 cases of COVID-19 have been confirmed, and more than 328,000 deaths related to COVID-19 have been reported globally.2 These numbers are expected to increase, due to the reproduction number (R0) of SARS-CoV-2. R0 represents the number of new infections generated by an infectious person in a totally naïve population.3 The WHO estimates that the R0 of SARS-CoV-2 is 1.95, with other estimates ranging from 1.4 to 6.49.3 To control the pathogen, the R0 needs to be brought under a value of 1.
A fundamental tool in lowering the R0 is prompt testing and isolation of those who display signs and symptoms of infection. SARS-CoV-2 is still a novel pathogen about which we know relatively little. The common symptoms of COVID-19 are now well known—including fever, fatigue, anorexia, cough, and shortness of breath—but atypical manifestations of this viral continue to be reported and described. To help clinicians across specialties and settings identify patients with possible infection, we have summarized findings from current reports on COVID-19 manifestations involving the renal, cardiac, gastrointestinal (GI), and other organ systems.
Renal
During the 2003 SARS-CoV-1 outbreak, acute kidney injury (AKI) was an uncommon complication of the infection, but early reports suggest that AKI may occur more commonly with COVID-19.4 In a study of 193 patients with laboratory-confirmed COVID-19 treated in 3 Chinese hospitals, 59% presented with proteinuria, 44% with hematuria, 14% with increased blood urea nitrogen, and 10% with increased levels of serum creatinine.4 These markers, indicative of AKI, may be associated with increased mortality. Among this cohort, those with AKI had a mortality risk 5.3 times higher than those who did not have AKI.4 The pathophysiology of renal disease in COVID-19 may be related to dehydration or inflammatory mediators, causing decreased renal perfusion and cytokine storm, but evidence also suggests that SARS-CoV-2 is able to directly infect kidney cells.5 The virus infects cells by using angiotensin-converting enzyme 2 (ACE2) on the cell membrane as a cell entry receptor; ACE2 is expressed on the kidney, heart, and GI cells, and this may allow SARS-CoV-2 to directly infect and damage these organs. Other potential mechanisms of renal injury include overproduction of proinflammatory cytokines and administration of nephrotoxic drugs. No matter the mechanism, however, increased serum creatinine and blood urea nitrogen correlate with an increased likelihood of requiring intensive care unit (ICU) admission.6 Therefore, clinicians should carefully monitor renal function in patients with COVID-19.
Cardiac
In a report of 138 Chinese patients hospitalized for COVID-19, 36 required ICU admission: 44.4% of these had arrhythmias and 22.2% had developed acute cardiac injury.6 In addition, the cardiac cell injury biomarker troponin I was more likely to be elevated in ICU patients.6 A study of 21 patients admitted to the ICU in Washington State found elevated levels of brain natriuretic peptide.7 These biomarkers reflect the presence of myocardial stress, but do not necessarily indicate direct myocardial infection. Case reports of fulminant myocarditis in those with COVID-19 have begun to surface, however.8,9 An examination of 68 deaths in persons with COVID-19 concluded that 7% were caused by myocarditis with circulatory failure.10
The pathophysiology of myocardial injury in COVID-19 is likely multifactorial. This includes increased inflammatory mediators, hypoxemia, and metabolic changes that can directly damage myocardial tissue. These factors can also exacerbate comorbid conditions, such as coronary artery disease, leading to ischemia and dysfunction of preexisting electrical conduction abnormalities. However, pathologic evidence of myocarditis and the presence of the ACE2 receptor, which may be a mediator of cardiac function, on cardiac muscle cells suggest that SARS-CoV-2 is capable of directly infecting and damaging myocardial cells. Other proposed mechanisms include infection-mediated downregulation of ACE2, causing cardiac dysfunction, or thrombus formation.11 Although respiratory failure is the most common source of advanced illness in COVID-19 patients, myocarditis and arrhythmias can be life-threatening manifestations of the disease.
Gastrointestinal
As noted, ACE2 is expressed in the GI tract. In 73 patients hospitalized for COVID-19, 53.4% tested positive for SARS-CoV-2 RNA in stool, and 23.4% continued to have RNA-positive stool samples even after their respiratory samples tested negative.12 These findings suggest the potential for SARS-CoV-2 to spread through fecal-oral transmission in those who are asymptomatic, pre-symptomatic, or symptomatic. This mode of transmission has yet to be determined conclusively, and more research is needed. However, GI symptoms have been reported in persons with COVID-19. Among 138 hospitalized patients, 10.1% had complaints of diarrhea and nausea and 3.6% reported vomiting.6 Those who reported nausea and diarrhea noted that they developed these symptoms 1 to 2 days before they developed fever.6 Also, among a cohort of 1099 Chinese patients with COVID-19, 3.8% complained of diarrhea.13 Although diarrhea does not occur in a majority of patients, GI complaints, such as nausea, vomiting, or diarrhea, should raise clinical suspicion for COVID-19, and in known areas of active transmission, testing of patients with GI symptoms is likely warranted.
Ocular
Ocular manifestations of COVID-19 are now being described, and should be taken into consideration when examining a patient. In a study of 38 patients with COVID-19 from Hubei province, China, 31.6% had ocular findings consistent with conjunctivitis, including conjunctival hyperemia, chemosis, epiphora, and increased ocular secretions.14 SARS-CoV-2 was detected in conjunctival and nasopharyngeal samples in 2 patients from this cohort. Conjunctival congestion was reported in a cohort of 1099 patients with COVID-19 treated at multiple centers throughout China, but at a much lower incidence, approximately 0.8%.13 Because SARS-CoV-2 can cause conjunctival disease and has been detected in samples from the external surface of the eye, it appears the virus is transmissible from tears or contact with the eye itself.
Neurologic
Common reported neurologic symptoms include dizziness, headache, impaired consciousness, ataxia, and cerebrovascular events. In a cohort of 214 patients from Wuhan, China, 36.4% had some form of neurological insult.15 These symptoms were more common in those with severe illness (P = 0.02).15 Two interesting neurologic symptoms that have been described are anosmia (loss of smell) and ageusia (loss of taste), which are being found primarily in tandem. It is still unclear how many people with COVID-19 are experiencing these symptoms, but a report from Italy estimates 19.4% of 320 patients examined had chemosensory dysfunction.16 The aforementioned report from Wuhan, China, found that 5.1% had anosmia and 5.6% had ageusia.15 The presence of anosmia/ageusia in some patients suggests that SARS-CoV-2 may enter the central nervous system (CNS) through a retrograde neuronal route.15 In addition, a case report from Japan described a 24-year-old man who presented with meningitis/encephalitis and had SARS-CoV-2 RNA present in his cerebrospinal fluid, showing that SARS-CoV-2 can penetrate into the CNS.17
SARS-CoV-2 may also have an association with Guillain–Barré syndrome, as this condition was reported in 5 patients from 3 hospitals in Northern Italy.18 The symptoms of Guillain–Barré syndrome presented 5 to 10 days after the typical COVID-19 symptoms, and evolved over 36 hours to 4 days afterwards. Four of the 5 patients experienced flaccid tetraparesis or tetraplegia, and 3 required mechanical ventilation.18
Another possible cause of neurologic injury in COVID-19 is damage to endothelial cells in cerebral blood vessels, causing thrombus formation and possibly increasing the risk of acute ischemic stroke.15,19 Supporting this mechanism of injury, significantly lower platelet counts were noted in patients with CNS symptoms (P = 0.005).15 Other hematological impacts of COVID-19 have been reported, particularly hypercoagulability, as evidenced by elevated D-dimer levels.13,20 This hypercoagulable state is linked to overproduction of proinflammatory cytokines (cytokine storm), leading to dysregulation of coagulation pathways and reduced concentrations of anticoagulants, such as protein C, antithrombin III, and tissue factor pathway inhibitor.21
Cutaneous
Cutaneous findings emerging in persons with COVID-19 demonstrate features of small-vessel and capillary occlusion, including erythematous skin eruptions and petechial rash. One report from Italy noted that 20.4% of patients with COVID-19 (n = 88) had a cutaneous finding, with a cutaneous manifestation developing in 8 at the onset of illness and in 10 following hospital admission.22 Fourteen patients had an erythematous rash, primarily on the trunk, with 3 patients having a diffuse urticarial appearing rash, and 1 patient developing vesicles.22 The severity of illness did not appear to correlate with the cutaneous manifestation, and the lesions healed within a few days.
One case report described a patient from Bangkok who was thought to be suffering from dengue fever, but was found to have SARS-CoV-2 infection. He initially presented with skin rash and petechiae, and later developed respiratory disease.23
Other dermatologic findings of COVID-19 resemble chilblains disease, colloquially referred to as “COVID toes.” Two women, 27 and 35 years old, presented to a dermatology clinic in Qatar with a chief complaint of skin rash, described as red-purple papules on the dorsal aspects of the fingers bilaterally.22 Both patients had an unremarkable medical and drug history, but recent travel to the United Kingdom dictated SARS-CoV-2 screening, which was positive.24 An Italian case report describes a 23-year-old man who tested positive for SARS-CoV-2 and had violaceous plaques on an erythematous background on his feet, without any lesions on his hands.25 Since chilblains is less common in the warmer months and these events correspond with the COVID-19 pandemic, SARS-CoV-2 infection is the suspected etiology. The pathophysiology of these lesions is unclear, and more research is needed. As more data become available, we may see cutaneous manifestations in patients with COVID-19 similar to those commonly reported with other viral infectious processes.
Musculoskeletal
Of 138 patients hospitalized in Wuhan, China, for COVID-19, 34.8% presented with myalgia; the presence of myalgia does not appear to be correlated with an increased likelihood of ICU admission.6 Myalgia or arthralgia was also reported in 14.9% among the cohort of 1099 COVID-19 patients in China.13 These musculoskeletal symptoms are described among large muscle groups found in the extremities, trunk, and back, and should raise suspicion in patients who present with other signs and symptoms concerning for COVID-19.
Conclusion
Evidence regarding atypical features of COVID-19 is accumulating. SARS-CoV-2 can infect a human cells that express the ACE2 receptor, which would allow for a broad spectrum of illnesses. The potential for SARS-CoV-2 to induce a hypercoagulable state allows it to indirectly damage various organ systems,20 leading to cerebrovascular disease, myocardial injury, and a chilblain-like rash. Clinicians must be aware of these unique features, as early recognition of persons who present with COVID-19 will allow for prompt testing, institution of infection control and isolation practices, and treatment, as needed, among those infected. Also, this is a pandemic involving a novel virus affecting different populations throughout the world, and these signs and symptoms may occur with varying frequency across populations. Therefore, it is important to keep differentials broad when assessing patients with a clinical illness that may indeed be COVID-19.
Corresponding author: Norman L. Beatty, MD, [email protected].
Financial disclosures: None.
1. WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020 [press release]. World Health Organization; March 11, 2020.
2. Coronavirus COVID-19 Global Cases by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. Johns Hopkins CSSE. https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6 Accessed May 15, 2020.
3. Liu Y, Gayle AA, Wilder-Smith A, Rocklöv J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Travel Med. 2020;27(2):taaa021. doi:10.1093/jtm/taaa021
4. Li Z, Wu M, Guo J, et al. Caution on kidney dysfunctions of 2019-nCoV patients. medRxiv preprint. doi: 10.1101/2020.02.08.20021212
5. Li W, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450-454. doi: 10.1038/nature02145.
6. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. doi:10.1001/jama.2020.1585
7. Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323:1612‐1614. doi:10.1001/jama.2020.4326
8. Chen C, Zhou Y, Wang DW. SARS-CoV-2: a potential novel etiology of fulminant myocarditis. Herz. 2020;45:230-232. doi: 10.1007/s00059-020-04909-z
9. Hu H, Ma F, Wei X, Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. 2020 Mar 16;ehaa190. doi: 10.1093/eurheartj/ehaa190
10. Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846-848. doi:10.1007/s00134-020-05991-x
11. Akhmerov A, Marban E. COVID-19 and the heart. Circ Res. 2020;126:1443-1455. doi:10.1161/CIRCRESAHA.120.317055
12. Xiao F, Tang M, Zheng X, et al. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158:1831-1833. doi: 10.1053/j.gastro.2020.02.055
13. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1078-1720. doi: 10.1056/NEJMoa2002032
14. Wu P, Duan F, Luo C, et al. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020 Mar 31;e201291. doi: 10.1001/jamaophthalmol.2020.1291
15. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020 Apr 10. doi: 10.1001/jamaneurol.2020.1127
16. Vaira LA, Salzano G, Deiana G, De Riu G. Anosmia and ageusia: common findings in COVID-19 patients. Laryngoscope. 2020 Apr 1. doi: 10.1002/lary.28692
17. Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS-coronavirus-2. Int J Infect Dis. 2020;94:55-58. doi: 10.1016/j.ijid.2020.03.062
18. Toscano G, Palmerini F, Ravaglia S, et al. Guillain–Barré syndrome associated with SARS-CoV-2. N Engl J Med. 2020 Apr 17;NEJMc2009191. doi:10.1056/nejmc2009191
19. Dafer RM, Osteraas ND, Biller J. Acute stroke care in the coronavirus disease 2019 pandemic. J Stroke Cerebrovascular Dis. 2020 Apr 17:104881. doi: 10.1016/j.jstrokecerebrovasdis.2020.104881
20. Terpos E, Ntanasis-Stathopoulos I, Elalamy I, et al. Hematological findings and complications of COVID-19. Am J Hematol. 2020;10.1002/ajh.25829. doi:10.1002/ajh.25829
21. Jose RJ, Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. 2020;S2213-2600(20)30216-2. doi:10.1016/S2213-2600(20)30216-2
22. Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020 Mar 26. doi: 10.1111/jdv.16387
23. Joob B, Wiwanitkit V. COVID-19 can present with a rash and be mistaken for dengue. J Am Acad Dermatol. 2020;82(5):e177. doi: 10.1016/j.jaad.2020.03.036
24. Alramthan A, Aldaraji W. A Case of COVID‐19 presenting in clinical picture resembling chilblains disease. First report from the Middle East. Clin Exp Dermatol. 2020 Apr 17. doi: 10.1111/ced.14243
25. Kolivras A, Dehavay F, Delplace D, et al. Coronavirus (COVID-19) infection–induced chilblains: a case report with histopathologic findings. JAAD Case Rep. 2020 Apr 18. doi: 10.1016/j.jdcr.2020.04.011
From the University of Florida College of Medicine, Division of Infectious Diseases and Global Medicine, Gainesville, FL.
Abstract
- Objective: To review current reports on atypical manifestations of coronavirus disease 2019 (COVID-19).
- Methods: Review of the literature.
- Results: Evidence regarding atypical features of COVID-19 is accumulating. SARS-CoV-2 can infect human cells that express the angiotensin-converting enzyme 2 receptor, which would allow for a broad spectrum of illnesses affecting the renal, cardiac, and gastrointestinal organ systems. Neurologic, cutaneous, and musculoskeletal manifestations have also been reported. The potential for SARS-CoV-2 to induce a hypercoagulable state provides another avenue for the virus to indirectly damage various organ systems, as evidenced by reports of cerebrovascular disease, myocardial injury, and a chilblain-like rash in patients with COVID-19.
- Conclusion: Because the signs and symptoms of COVID-19 may occur with varying frequency across populations, it is important to keep differentials broad when assessing patients with a clinical illness that may indeed be COVID-19.
Keywords: coronavirus; severe acute respiratory syndrome coronavirus-2; SARS-CoV-2; pandemic.
Coronavirus disease 2019 (COVID-19), the syndrome caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), was first reported in Wuhan, China, in early December 2019.1 Since then, the virus has spread quickly around the world, with the World Health Organization (WHO) declaring the coronavirus outbreak a global pandemic on March 11, 2020. As of May 21, 2020, more than 5,000,000 cases of COVID-19 have been confirmed, and more than 328,000 deaths related to COVID-19 have been reported globally.2 These numbers are expected to increase, due to the reproduction number (R0) of SARS-CoV-2. R0 represents the number of new infections generated by an infectious person in a totally naïve population.3 The WHO estimates that the R0 of SARS-CoV-2 is 1.95, with other estimates ranging from 1.4 to 6.49.3 To control the pathogen, the R0 needs to be brought under a value of 1.
A fundamental tool in lowering the R0 is prompt testing and isolation of those who display signs and symptoms of infection. SARS-CoV-2 is still a novel pathogen about which we know relatively little. The common symptoms of COVID-19 are now well known—including fever, fatigue, anorexia, cough, and shortness of breath—but atypical manifestations of this viral continue to be reported and described. To help clinicians across specialties and settings identify patients with possible infection, we have summarized findings from current reports on COVID-19 manifestations involving the renal, cardiac, gastrointestinal (GI), and other organ systems.
Renal
During the 2003 SARS-CoV-1 outbreak, acute kidney injury (AKI) was an uncommon complication of the infection, but early reports suggest that AKI may occur more commonly with COVID-19.4 In a study of 193 patients with laboratory-confirmed COVID-19 treated in 3 Chinese hospitals, 59% presented with proteinuria, 44% with hematuria, 14% with increased blood urea nitrogen, and 10% with increased levels of serum creatinine.4 These markers, indicative of AKI, may be associated with increased mortality. Among this cohort, those with AKI had a mortality risk 5.3 times higher than those who did not have AKI.4 The pathophysiology of renal disease in COVID-19 may be related to dehydration or inflammatory mediators, causing decreased renal perfusion and cytokine storm, but evidence also suggests that SARS-CoV-2 is able to directly infect kidney cells.5 The virus infects cells by using angiotensin-converting enzyme 2 (ACE2) on the cell membrane as a cell entry receptor; ACE2 is expressed on the kidney, heart, and GI cells, and this may allow SARS-CoV-2 to directly infect and damage these organs. Other potential mechanisms of renal injury include overproduction of proinflammatory cytokines and administration of nephrotoxic drugs. No matter the mechanism, however, increased serum creatinine and blood urea nitrogen correlate with an increased likelihood of requiring intensive care unit (ICU) admission.6 Therefore, clinicians should carefully monitor renal function in patients with COVID-19.
Cardiac
In a report of 138 Chinese patients hospitalized for COVID-19, 36 required ICU admission: 44.4% of these had arrhythmias and 22.2% had developed acute cardiac injury.6 In addition, the cardiac cell injury biomarker troponin I was more likely to be elevated in ICU patients.6 A study of 21 patients admitted to the ICU in Washington State found elevated levels of brain natriuretic peptide.7 These biomarkers reflect the presence of myocardial stress, but do not necessarily indicate direct myocardial infection. Case reports of fulminant myocarditis in those with COVID-19 have begun to surface, however.8,9 An examination of 68 deaths in persons with COVID-19 concluded that 7% were caused by myocarditis with circulatory failure.10
The pathophysiology of myocardial injury in COVID-19 is likely multifactorial. This includes increased inflammatory mediators, hypoxemia, and metabolic changes that can directly damage myocardial tissue. These factors can also exacerbate comorbid conditions, such as coronary artery disease, leading to ischemia and dysfunction of preexisting electrical conduction abnormalities. However, pathologic evidence of myocarditis and the presence of the ACE2 receptor, which may be a mediator of cardiac function, on cardiac muscle cells suggest that SARS-CoV-2 is capable of directly infecting and damaging myocardial cells. Other proposed mechanisms include infection-mediated downregulation of ACE2, causing cardiac dysfunction, or thrombus formation.11 Although respiratory failure is the most common source of advanced illness in COVID-19 patients, myocarditis and arrhythmias can be life-threatening manifestations of the disease.
Gastrointestinal
As noted, ACE2 is expressed in the GI tract. In 73 patients hospitalized for COVID-19, 53.4% tested positive for SARS-CoV-2 RNA in stool, and 23.4% continued to have RNA-positive stool samples even after their respiratory samples tested negative.12 These findings suggest the potential for SARS-CoV-2 to spread through fecal-oral transmission in those who are asymptomatic, pre-symptomatic, or symptomatic. This mode of transmission has yet to be determined conclusively, and more research is needed. However, GI symptoms have been reported in persons with COVID-19. Among 138 hospitalized patients, 10.1% had complaints of diarrhea and nausea and 3.6% reported vomiting.6 Those who reported nausea and diarrhea noted that they developed these symptoms 1 to 2 days before they developed fever.6 Also, among a cohort of 1099 Chinese patients with COVID-19, 3.8% complained of diarrhea.13 Although diarrhea does not occur in a majority of patients, GI complaints, such as nausea, vomiting, or diarrhea, should raise clinical suspicion for COVID-19, and in known areas of active transmission, testing of patients with GI symptoms is likely warranted.
Ocular
Ocular manifestations of COVID-19 are now being described, and should be taken into consideration when examining a patient. In a study of 38 patients with COVID-19 from Hubei province, China, 31.6% had ocular findings consistent with conjunctivitis, including conjunctival hyperemia, chemosis, epiphora, and increased ocular secretions.14 SARS-CoV-2 was detected in conjunctival and nasopharyngeal samples in 2 patients from this cohort. Conjunctival congestion was reported in a cohort of 1099 patients with COVID-19 treated at multiple centers throughout China, but at a much lower incidence, approximately 0.8%.13 Because SARS-CoV-2 can cause conjunctival disease and has been detected in samples from the external surface of the eye, it appears the virus is transmissible from tears or contact with the eye itself.
Neurologic
Common reported neurologic symptoms include dizziness, headache, impaired consciousness, ataxia, and cerebrovascular events. In a cohort of 214 patients from Wuhan, China, 36.4% had some form of neurological insult.15 These symptoms were more common in those with severe illness (P = 0.02).15 Two interesting neurologic symptoms that have been described are anosmia (loss of smell) and ageusia (loss of taste), which are being found primarily in tandem. It is still unclear how many people with COVID-19 are experiencing these symptoms, but a report from Italy estimates 19.4% of 320 patients examined had chemosensory dysfunction.16 The aforementioned report from Wuhan, China, found that 5.1% had anosmia and 5.6% had ageusia.15 The presence of anosmia/ageusia in some patients suggests that SARS-CoV-2 may enter the central nervous system (CNS) through a retrograde neuronal route.15 In addition, a case report from Japan described a 24-year-old man who presented with meningitis/encephalitis and had SARS-CoV-2 RNA present in his cerebrospinal fluid, showing that SARS-CoV-2 can penetrate into the CNS.17
SARS-CoV-2 may also have an association with Guillain–Barré syndrome, as this condition was reported in 5 patients from 3 hospitals in Northern Italy.18 The symptoms of Guillain–Barré syndrome presented 5 to 10 days after the typical COVID-19 symptoms, and evolved over 36 hours to 4 days afterwards. Four of the 5 patients experienced flaccid tetraparesis or tetraplegia, and 3 required mechanical ventilation.18
Another possible cause of neurologic injury in COVID-19 is damage to endothelial cells in cerebral blood vessels, causing thrombus formation and possibly increasing the risk of acute ischemic stroke.15,19 Supporting this mechanism of injury, significantly lower platelet counts were noted in patients with CNS symptoms (P = 0.005).15 Other hematological impacts of COVID-19 have been reported, particularly hypercoagulability, as evidenced by elevated D-dimer levels.13,20 This hypercoagulable state is linked to overproduction of proinflammatory cytokines (cytokine storm), leading to dysregulation of coagulation pathways and reduced concentrations of anticoagulants, such as protein C, antithrombin III, and tissue factor pathway inhibitor.21
Cutaneous
Cutaneous findings emerging in persons with COVID-19 demonstrate features of small-vessel and capillary occlusion, including erythematous skin eruptions and petechial rash. One report from Italy noted that 20.4% of patients with COVID-19 (n = 88) had a cutaneous finding, with a cutaneous manifestation developing in 8 at the onset of illness and in 10 following hospital admission.22 Fourteen patients had an erythematous rash, primarily on the trunk, with 3 patients having a diffuse urticarial appearing rash, and 1 patient developing vesicles.22 The severity of illness did not appear to correlate with the cutaneous manifestation, and the lesions healed within a few days.
One case report described a patient from Bangkok who was thought to be suffering from dengue fever, but was found to have SARS-CoV-2 infection. He initially presented with skin rash and petechiae, and later developed respiratory disease.23
Other dermatologic findings of COVID-19 resemble chilblains disease, colloquially referred to as “COVID toes.” Two women, 27 and 35 years old, presented to a dermatology clinic in Qatar with a chief complaint of skin rash, described as red-purple papules on the dorsal aspects of the fingers bilaterally.22 Both patients had an unremarkable medical and drug history, but recent travel to the United Kingdom dictated SARS-CoV-2 screening, which was positive.24 An Italian case report describes a 23-year-old man who tested positive for SARS-CoV-2 and had violaceous plaques on an erythematous background on his feet, without any lesions on his hands.25 Since chilblains is less common in the warmer months and these events correspond with the COVID-19 pandemic, SARS-CoV-2 infection is the suspected etiology. The pathophysiology of these lesions is unclear, and more research is needed. As more data become available, we may see cutaneous manifestations in patients with COVID-19 similar to those commonly reported with other viral infectious processes.
Musculoskeletal
Of 138 patients hospitalized in Wuhan, China, for COVID-19, 34.8% presented with myalgia; the presence of myalgia does not appear to be correlated with an increased likelihood of ICU admission.6 Myalgia or arthralgia was also reported in 14.9% among the cohort of 1099 COVID-19 patients in China.13 These musculoskeletal symptoms are described among large muscle groups found in the extremities, trunk, and back, and should raise suspicion in patients who present with other signs and symptoms concerning for COVID-19.
Conclusion
Evidence regarding atypical features of COVID-19 is accumulating. SARS-CoV-2 can infect a human cells that express the ACE2 receptor, which would allow for a broad spectrum of illnesses. The potential for SARS-CoV-2 to induce a hypercoagulable state allows it to indirectly damage various organ systems,20 leading to cerebrovascular disease, myocardial injury, and a chilblain-like rash. Clinicians must be aware of these unique features, as early recognition of persons who present with COVID-19 will allow for prompt testing, institution of infection control and isolation practices, and treatment, as needed, among those infected. Also, this is a pandemic involving a novel virus affecting different populations throughout the world, and these signs and symptoms may occur with varying frequency across populations. Therefore, it is important to keep differentials broad when assessing patients with a clinical illness that may indeed be COVID-19.
Corresponding author: Norman L. Beatty, MD, [email protected].
Financial disclosures: None.
From the University of Florida College of Medicine, Division of Infectious Diseases and Global Medicine, Gainesville, FL.
Abstract
- Objective: To review current reports on atypical manifestations of coronavirus disease 2019 (COVID-19).
- Methods: Review of the literature.
- Results: Evidence regarding atypical features of COVID-19 is accumulating. SARS-CoV-2 can infect human cells that express the angiotensin-converting enzyme 2 receptor, which would allow for a broad spectrum of illnesses affecting the renal, cardiac, and gastrointestinal organ systems. Neurologic, cutaneous, and musculoskeletal manifestations have also been reported. The potential for SARS-CoV-2 to induce a hypercoagulable state provides another avenue for the virus to indirectly damage various organ systems, as evidenced by reports of cerebrovascular disease, myocardial injury, and a chilblain-like rash in patients with COVID-19.
- Conclusion: Because the signs and symptoms of COVID-19 may occur with varying frequency across populations, it is important to keep differentials broad when assessing patients with a clinical illness that may indeed be COVID-19.
Keywords: coronavirus; severe acute respiratory syndrome coronavirus-2; SARS-CoV-2; pandemic.
Coronavirus disease 2019 (COVID-19), the syndrome caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), was first reported in Wuhan, China, in early December 2019.1 Since then, the virus has spread quickly around the world, with the World Health Organization (WHO) declaring the coronavirus outbreak a global pandemic on March 11, 2020. As of May 21, 2020, more than 5,000,000 cases of COVID-19 have been confirmed, and more than 328,000 deaths related to COVID-19 have been reported globally.2 These numbers are expected to increase, due to the reproduction number (R0) of SARS-CoV-2. R0 represents the number of new infections generated by an infectious person in a totally naïve population.3 The WHO estimates that the R0 of SARS-CoV-2 is 1.95, with other estimates ranging from 1.4 to 6.49.3 To control the pathogen, the R0 needs to be brought under a value of 1.
A fundamental tool in lowering the R0 is prompt testing and isolation of those who display signs and symptoms of infection. SARS-CoV-2 is still a novel pathogen about which we know relatively little. The common symptoms of COVID-19 are now well known—including fever, fatigue, anorexia, cough, and shortness of breath—but atypical manifestations of this viral continue to be reported and described. To help clinicians across specialties and settings identify patients with possible infection, we have summarized findings from current reports on COVID-19 manifestations involving the renal, cardiac, gastrointestinal (GI), and other organ systems.
Renal
During the 2003 SARS-CoV-1 outbreak, acute kidney injury (AKI) was an uncommon complication of the infection, but early reports suggest that AKI may occur more commonly with COVID-19.4 In a study of 193 patients with laboratory-confirmed COVID-19 treated in 3 Chinese hospitals, 59% presented with proteinuria, 44% with hematuria, 14% with increased blood urea nitrogen, and 10% with increased levels of serum creatinine.4 These markers, indicative of AKI, may be associated with increased mortality. Among this cohort, those with AKI had a mortality risk 5.3 times higher than those who did not have AKI.4 The pathophysiology of renal disease in COVID-19 may be related to dehydration or inflammatory mediators, causing decreased renal perfusion and cytokine storm, but evidence also suggests that SARS-CoV-2 is able to directly infect kidney cells.5 The virus infects cells by using angiotensin-converting enzyme 2 (ACE2) on the cell membrane as a cell entry receptor; ACE2 is expressed on the kidney, heart, and GI cells, and this may allow SARS-CoV-2 to directly infect and damage these organs. Other potential mechanisms of renal injury include overproduction of proinflammatory cytokines and administration of nephrotoxic drugs. No matter the mechanism, however, increased serum creatinine and blood urea nitrogen correlate with an increased likelihood of requiring intensive care unit (ICU) admission.6 Therefore, clinicians should carefully monitor renal function in patients with COVID-19.
Cardiac
In a report of 138 Chinese patients hospitalized for COVID-19, 36 required ICU admission: 44.4% of these had arrhythmias and 22.2% had developed acute cardiac injury.6 In addition, the cardiac cell injury biomarker troponin I was more likely to be elevated in ICU patients.6 A study of 21 patients admitted to the ICU in Washington State found elevated levels of brain natriuretic peptide.7 These biomarkers reflect the presence of myocardial stress, but do not necessarily indicate direct myocardial infection. Case reports of fulminant myocarditis in those with COVID-19 have begun to surface, however.8,9 An examination of 68 deaths in persons with COVID-19 concluded that 7% were caused by myocarditis with circulatory failure.10
The pathophysiology of myocardial injury in COVID-19 is likely multifactorial. This includes increased inflammatory mediators, hypoxemia, and metabolic changes that can directly damage myocardial tissue. These factors can also exacerbate comorbid conditions, such as coronary artery disease, leading to ischemia and dysfunction of preexisting electrical conduction abnormalities. However, pathologic evidence of myocarditis and the presence of the ACE2 receptor, which may be a mediator of cardiac function, on cardiac muscle cells suggest that SARS-CoV-2 is capable of directly infecting and damaging myocardial cells. Other proposed mechanisms include infection-mediated downregulation of ACE2, causing cardiac dysfunction, or thrombus formation.11 Although respiratory failure is the most common source of advanced illness in COVID-19 patients, myocarditis and arrhythmias can be life-threatening manifestations of the disease.
Gastrointestinal
As noted, ACE2 is expressed in the GI tract. In 73 patients hospitalized for COVID-19, 53.4% tested positive for SARS-CoV-2 RNA in stool, and 23.4% continued to have RNA-positive stool samples even after their respiratory samples tested negative.12 These findings suggest the potential for SARS-CoV-2 to spread through fecal-oral transmission in those who are asymptomatic, pre-symptomatic, or symptomatic. This mode of transmission has yet to be determined conclusively, and more research is needed. However, GI symptoms have been reported in persons with COVID-19. Among 138 hospitalized patients, 10.1% had complaints of diarrhea and nausea and 3.6% reported vomiting.6 Those who reported nausea and diarrhea noted that they developed these symptoms 1 to 2 days before they developed fever.6 Also, among a cohort of 1099 Chinese patients with COVID-19, 3.8% complained of diarrhea.13 Although diarrhea does not occur in a majority of patients, GI complaints, such as nausea, vomiting, or diarrhea, should raise clinical suspicion for COVID-19, and in known areas of active transmission, testing of patients with GI symptoms is likely warranted.
Ocular
Ocular manifestations of COVID-19 are now being described, and should be taken into consideration when examining a patient. In a study of 38 patients with COVID-19 from Hubei province, China, 31.6% had ocular findings consistent with conjunctivitis, including conjunctival hyperemia, chemosis, epiphora, and increased ocular secretions.14 SARS-CoV-2 was detected in conjunctival and nasopharyngeal samples in 2 patients from this cohort. Conjunctival congestion was reported in a cohort of 1099 patients with COVID-19 treated at multiple centers throughout China, but at a much lower incidence, approximately 0.8%.13 Because SARS-CoV-2 can cause conjunctival disease and has been detected in samples from the external surface of the eye, it appears the virus is transmissible from tears or contact with the eye itself.
Neurologic
Common reported neurologic symptoms include dizziness, headache, impaired consciousness, ataxia, and cerebrovascular events. In a cohort of 214 patients from Wuhan, China, 36.4% had some form of neurological insult.15 These symptoms were more common in those with severe illness (P = 0.02).15 Two interesting neurologic symptoms that have been described are anosmia (loss of smell) and ageusia (loss of taste), which are being found primarily in tandem. It is still unclear how many people with COVID-19 are experiencing these symptoms, but a report from Italy estimates 19.4% of 320 patients examined had chemosensory dysfunction.16 The aforementioned report from Wuhan, China, found that 5.1% had anosmia and 5.6% had ageusia.15 The presence of anosmia/ageusia in some patients suggests that SARS-CoV-2 may enter the central nervous system (CNS) through a retrograde neuronal route.15 In addition, a case report from Japan described a 24-year-old man who presented with meningitis/encephalitis and had SARS-CoV-2 RNA present in his cerebrospinal fluid, showing that SARS-CoV-2 can penetrate into the CNS.17
SARS-CoV-2 may also have an association with Guillain–Barré syndrome, as this condition was reported in 5 patients from 3 hospitals in Northern Italy.18 The symptoms of Guillain–Barré syndrome presented 5 to 10 days after the typical COVID-19 symptoms, and evolved over 36 hours to 4 days afterwards. Four of the 5 patients experienced flaccid tetraparesis or tetraplegia, and 3 required mechanical ventilation.18
Another possible cause of neurologic injury in COVID-19 is damage to endothelial cells in cerebral blood vessels, causing thrombus formation and possibly increasing the risk of acute ischemic stroke.15,19 Supporting this mechanism of injury, significantly lower platelet counts were noted in patients with CNS symptoms (P = 0.005).15 Other hematological impacts of COVID-19 have been reported, particularly hypercoagulability, as evidenced by elevated D-dimer levels.13,20 This hypercoagulable state is linked to overproduction of proinflammatory cytokines (cytokine storm), leading to dysregulation of coagulation pathways and reduced concentrations of anticoagulants, such as protein C, antithrombin III, and tissue factor pathway inhibitor.21
Cutaneous
Cutaneous findings emerging in persons with COVID-19 demonstrate features of small-vessel and capillary occlusion, including erythematous skin eruptions and petechial rash. One report from Italy noted that 20.4% of patients with COVID-19 (n = 88) had a cutaneous finding, with a cutaneous manifestation developing in 8 at the onset of illness and in 10 following hospital admission.22 Fourteen patients had an erythematous rash, primarily on the trunk, with 3 patients having a diffuse urticarial appearing rash, and 1 patient developing vesicles.22 The severity of illness did not appear to correlate with the cutaneous manifestation, and the lesions healed within a few days.
One case report described a patient from Bangkok who was thought to be suffering from dengue fever, but was found to have SARS-CoV-2 infection. He initially presented with skin rash and petechiae, and later developed respiratory disease.23
Other dermatologic findings of COVID-19 resemble chilblains disease, colloquially referred to as “COVID toes.” Two women, 27 and 35 years old, presented to a dermatology clinic in Qatar with a chief complaint of skin rash, described as red-purple papules on the dorsal aspects of the fingers bilaterally.22 Both patients had an unremarkable medical and drug history, but recent travel to the United Kingdom dictated SARS-CoV-2 screening, which was positive.24 An Italian case report describes a 23-year-old man who tested positive for SARS-CoV-2 and had violaceous plaques on an erythematous background on his feet, without any lesions on his hands.25 Since chilblains is less common in the warmer months and these events correspond with the COVID-19 pandemic, SARS-CoV-2 infection is the suspected etiology. The pathophysiology of these lesions is unclear, and more research is needed. As more data become available, we may see cutaneous manifestations in patients with COVID-19 similar to those commonly reported with other viral infectious processes.
Musculoskeletal
Of 138 patients hospitalized in Wuhan, China, for COVID-19, 34.8% presented with myalgia; the presence of myalgia does not appear to be correlated with an increased likelihood of ICU admission.6 Myalgia or arthralgia was also reported in 14.9% among the cohort of 1099 COVID-19 patients in China.13 These musculoskeletal symptoms are described among large muscle groups found in the extremities, trunk, and back, and should raise suspicion in patients who present with other signs and symptoms concerning for COVID-19.
Conclusion
Evidence regarding atypical features of COVID-19 is accumulating. SARS-CoV-2 can infect a human cells that express the ACE2 receptor, which would allow for a broad spectrum of illnesses. The potential for SARS-CoV-2 to induce a hypercoagulable state allows it to indirectly damage various organ systems,20 leading to cerebrovascular disease, myocardial injury, and a chilblain-like rash. Clinicians must be aware of these unique features, as early recognition of persons who present with COVID-19 will allow for prompt testing, institution of infection control and isolation practices, and treatment, as needed, among those infected. Also, this is a pandemic involving a novel virus affecting different populations throughout the world, and these signs and symptoms may occur with varying frequency across populations. Therefore, it is important to keep differentials broad when assessing patients with a clinical illness that may indeed be COVID-19.
Corresponding author: Norman L. Beatty, MD, [email protected].
Financial disclosures: None.
1. WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020 [press release]. World Health Organization; March 11, 2020.
2. Coronavirus COVID-19 Global Cases by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. Johns Hopkins CSSE. https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6 Accessed May 15, 2020.
3. Liu Y, Gayle AA, Wilder-Smith A, Rocklöv J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Travel Med. 2020;27(2):taaa021. doi:10.1093/jtm/taaa021
4. Li Z, Wu M, Guo J, et al. Caution on kidney dysfunctions of 2019-nCoV patients. medRxiv preprint. doi: 10.1101/2020.02.08.20021212
5. Li W, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450-454. doi: 10.1038/nature02145.
6. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. doi:10.1001/jama.2020.1585
7. Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323:1612‐1614. doi:10.1001/jama.2020.4326
8. Chen C, Zhou Y, Wang DW. SARS-CoV-2: a potential novel etiology of fulminant myocarditis. Herz. 2020;45:230-232. doi: 10.1007/s00059-020-04909-z
9. Hu H, Ma F, Wei X, Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. 2020 Mar 16;ehaa190. doi: 10.1093/eurheartj/ehaa190
10. Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846-848. doi:10.1007/s00134-020-05991-x
11. Akhmerov A, Marban E. COVID-19 and the heart. Circ Res. 2020;126:1443-1455. doi:10.1161/CIRCRESAHA.120.317055
12. Xiao F, Tang M, Zheng X, et al. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158:1831-1833. doi: 10.1053/j.gastro.2020.02.055
13. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1078-1720. doi: 10.1056/NEJMoa2002032
14. Wu P, Duan F, Luo C, et al. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020 Mar 31;e201291. doi: 10.1001/jamaophthalmol.2020.1291
15. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020 Apr 10. doi: 10.1001/jamaneurol.2020.1127
16. Vaira LA, Salzano G, Deiana G, De Riu G. Anosmia and ageusia: common findings in COVID-19 patients. Laryngoscope. 2020 Apr 1. doi: 10.1002/lary.28692
17. Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS-coronavirus-2. Int J Infect Dis. 2020;94:55-58. doi: 10.1016/j.ijid.2020.03.062
18. Toscano G, Palmerini F, Ravaglia S, et al. Guillain–Barré syndrome associated with SARS-CoV-2. N Engl J Med. 2020 Apr 17;NEJMc2009191. doi:10.1056/nejmc2009191
19. Dafer RM, Osteraas ND, Biller J. Acute stroke care in the coronavirus disease 2019 pandemic. J Stroke Cerebrovascular Dis. 2020 Apr 17:104881. doi: 10.1016/j.jstrokecerebrovasdis.2020.104881
20. Terpos E, Ntanasis-Stathopoulos I, Elalamy I, et al. Hematological findings and complications of COVID-19. Am J Hematol. 2020;10.1002/ajh.25829. doi:10.1002/ajh.25829
21. Jose RJ, Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. 2020;S2213-2600(20)30216-2. doi:10.1016/S2213-2600(20)30216-2
22. Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020 Mar 26. doi: 10.1111/jdv.16387
23. Joob B, Wiwanitkit V. COVID-19 can present with a rash and be mistaken for dengue. J Am Acad Dermatol. 2020;82(5):e177. doi: 10.1016/j.jaad.2020.03.036
24. Alramthan A, Aldaraji W. A Case of COVID‐19 presenting in clinical picture resembling chilblains disease. First report from the Middle East. Clin Exp Dermatol. 2020 Apr 17. doi: 10.1111/ced.14243
25. Kolivras A, Dehavay F, Delplace D, et al. Coronavirus (COVID-19) infection–induced chilblains: a case report with histopathologic findings. JAAD Case Rep. 2020 Apr 18. doi: 10.1016/j.jdcr.2020.04.011
1. WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020 [press release]. World Health Organization; March 11, 2020.
2. Coronavirus COVID-19 Global Cases by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. Johns Hopkins CSSE. https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6 Accessed May 15, 2020.
3. Liu Y, Gayle AA, Wilder-Smith A, Rocklöv J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Travel Med. 2020;27(2):taaa021. doi:10.1093/jtm/taaa021
4. Li Z, Wu M, Guo J, et al. Caution on kidney dysfunctions of 2019-nCoV patients. medRxiv preprint. doi: 10.1101/2020.02.08.20021212
5. Li W, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450-454. doi: 10.1038/nature02145.
6. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. doi:10.1001/jama.2020.1585
7. Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323:1612‐1614. doi:10.1001/jama.2020.4326
8. Chen C, Zhou Y, Wang DW. SARS-CoV-2: a potential novel etiology of fulminant myocarditis. Herz. 2020;45:230-232. doi: 10.1007/s00059-020-04909-z
9. Hu H, Ma F, Wei X, Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. 2020 Mar 16;ehaa190. doi: 10.1093/eurheartj/ehaa190
10. Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846-848. doi:10.1007/s00134-020-05991-x
11. Akhmerov A, Marban E. COVID-19 and the heart. Circ Res. 2020;126:1443-1455. doi:10.1161/CIRCRESAHA.120.317055
12. Xiao F, Tang M, Zheng X, et al. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158:1831-1833. doi: 10.1053/j.gastro.2020.02.055
13. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1078-1720. doi: 10.1056/NEJMoa2002032
14. Wu P, Duan F, Luo C, et al. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020 Mar 31;e201291. doi: 10.1001/jamaophthalmol.2020.1291
15. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020 Apr 10. doi: 10.1001/jamaneurol.2020.1127
16. Vaira LA, Salzano G, Deiana G, De Riu G. Anosmia and ageusia: common findings in COVID-19 patients. Laryngoscope. 2020 Apr 1. doi: 10.1002/lary.28692
17. Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS-coronavirus-2. Int J Infect Dis. 2020;94:55-58. doi: 10.1016/j.ijid.2020.03.062
18. Toscano G, Palmerini F, Ravaglia S, et al. Guillain–Barré syndrome associated with SARS-CoV-2. N Engl J Med. 2020 Apr 17;NEJMc2009191. doi:10.1056/nejmc2009191
19. Dafer RM, Osteraas ND, Biller J. Acute stroke care in the coronavirus disease 2019 pandemic. J Stroke Cerebrovascular Dis. 2020 Apr 17:104881. doi: 10.1016/j.jstrokecerebrovasdis.2020.104881
20. Terpos E, Ntanasis-Stathopoulos I, Elalamy I, et al. Hematological findings and complications of COVID-19. Am J Hematol. 2020;10.1002/ajh.25829. doi:10.1002/ajh.25829
21. Jose RJ, Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. 2020;S2213-2600(20)30216-2. doi:10.1016/S2213-2600(20)30216-2
22. Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020 Mar 26. doi: 10.1111/jdv.16387
23. Joob B, Wiwanitkit V. COVID-19 can present with a rash and be mistaken for dengue. J Am Acad Dermatol. 2020;82(5):e177. doi: 10.1016/j.jaad.2020.03.036
24. Alramthan A, Aldaraji W. A Case of COVID‐19 presenting in clinical picture resembling chilblains disease. First report from the Middle East. Clin Exp Dermatol. 2020 Apr 17. doi: 10.1111/ced.14243
25. Kolivras A, Dehavay F, Delplace D, et al. Coronavirus (COVID-19) infection–induced chilblains: a case report with histopathologic findings. JAAD Case Rep. 2020 Apr 18. doi: 10.1016/j.jdcr.2020.04.011
Remdesivir in Hospitalized Adults With Severe COVID-19: Lessons Learned From the First Randomized Trial
Study Overview
Objective. To assess the efficacy, safety, and clinical benefit of remdesivir in hospitalized adults with confirmed pneumonia due to severe SARS-CoV-2 infection.
Design. Randomized, investigator-initiated, placebo-controlled, double-blind, multicenter trial.
Setting and participants. The trial took place between February 6, 2020 and March 12, 2020, at 10 hospitals in Wuhan, China. Study participants included adult patients (aged ≥ 18 years) admitted to hospital who tested positive for SARS-CoV-2 by reverse transcription polymerase chain reaction assay and had the following clinical characteristics: radiographic evidence of pneumonia; hypoxia with oxygen saturation ≤ 94% on room air or a ratio of arterial oxygen partial pressure to fractional inspired oxygen ≤ 300 mm Hg; and symptom onset to enrollment ≤ 12 days. Some of the exclusion criteria for participation in the study were pregnancy or breast feeding, liver cirrhosis, abnormal liver enzymes ≥ 5 times the upper limit of normal, severe renal impairment or receipt of renal replacement therapy, plan for transfer to a non-study hospital, and enrollment in a trial for COVID-19 within the previous month.
Intervention. Participants were randomized in a 2:1 ratio to the remdesivir group or the placebo group and were administered either intravenous infusions of remdesivir (200 mg on day 1 followed by 100 mg daily on days 2-10) or the same volume of placebo for 10 days. Clinical and safety data assessed included laboratory testing, electrocardiogram, and medication adverse effects. Testing of oropharyngeal and nasopharyngeal swab samples, anal swab samples, sputum, and stool was performed for viral RNA detection and quantification on days 1, 3, 5, 7, 10, 14, 21, and 28.
Main outcome measures. The primary endpoint of this study was time to clinical improvement within 28 days after randomization. Clinical improvement was defined as a 2-point reduction in participants’ admission status on a 6-point ordinal scale (1 = discharged or clinical recovery, 6 = death) or live discharge from hospital, whichever came first. Secondary outcomes included all-cause mortality at day 28 and duration of hospital admission, oxygen support, and invasive mechanical ventilation. Virological measures and safety outcomes ascertained included treatment-emergent adverse events, serious adverse events, and premature discontinuation of remdesivir.
The sample size estimate for the original study design was a total of 453 patients (302 in the remdesivir group and 151 in the placebo group). This sample size would provide 80% power, assuming a hazard ratio (HR) of 1.4 comparing remdesivir to placebo, and corresponding to a change in time to clinical improvement of 6 days. The analysis of primary outcome was performed on an intention-to-treat basis. Time to clinical improvement within 28 days was assessed with Kaplan-Meier plots.
Main results. A total of 255 patients were screened, of whom 237 were enrolled and randomized to remdesivir (158) or placebo (79) group. Of the participants in the remdesivir group, 155 started study treatment and 150 completed treatment per protocol. For the participants in the placebo group, 78 started study treatment and 76 completed treatment per-protocol. Study enrollment was terminated after March 12, 2020, before attaining the prespecified sample size, because no additional patients met study eligibility criteria due to various public health measures implemented in Wuhan. The median age of participants was 65 years (IQR, 56-71), the majority were men (56% in remdesivir group vs 65% in placebo group), and the most common comorbidities included hypertension, diabetes, and coronary artery disease. Median time from symptom onset to study enrollment was 10 days (IQR, 9-12). The time to clinical improvement between treatments (21 days for remdesivir group vs 23 days for placebo group) was not significantly different (HR, 1.23; 95% confidence interval [CI], 0.87-1.75). In addition, in participants who received treatment within 10 days of symptom onset, those who were administered remdesivir had a nonsignificant (HR, 1.52; 95% CI, 0.95-2.43) but faster time (18 days) to clinical improvement, compared to those administered placebo (23 days). Moreover, treatment with remdesivir versus placebo did not lead to differences in secondary outcomes (eg, 28-day mortality and duration of hospital stay, oxygen support, and invasive mechanical ventilation), changes in viral load over time, or adverse events between the groups.
Conclusion. This study found that, compared with placebo, intravenous remdesivir did not significantly improve the time to clinical improvement, mortality, or time to clearance of SARS-CoV-2 in hospitalized adults with severe COVID-19. A numeric reduction in time to clinical improvement with early remdesivir treatment (ie, within 10 days of symptom onset) that approached statistical significance was observed in this underpowered study.
Commentary
Within a few short months since its emergence. SARS-CoV-2 infection has caused a global pandemic, posing a dire threat to public health due to its adverse effects on morbidity (eg, respiratory failure, thromboembolic diseases, multiorgan failure) and mortality. To date, no pharmacologic treatment has been shown to effectively improve clinical outcomes in patients with COVID-19. Multiple ongoing clinical trials are being conducted globally to determine potential therapeutic treatments for severe COVID-19. The first clinical trials of hydroxychloroquine and lopinavir-ritonavir, agents traditionally used for other indications, such as malaria and HIV, did not show a clear benefit in COVID-19.1,2 Remdesivir, a nucleoside analogue prodrug, is a broad-spectrum antiviral agent that was previously used for treatment of Ebola and has been shown to have inhibitory effects on pathogenic coronaviruses. The study reported by Wang and colleagues was the first randomized controlled trial (RCT) aimed at evaluating whether remdesivir improves outcomes in patients with severe COVID-19. Thus, the worsening COVID-19 pandemic, coupled with the absence of a curative treatment, underscore the urgency of this trial.
The study was grounded on observational data from several recent case reports and case series centering on the potential efficacy of remdesivir in treating COVID-19.3 The study itself was designed well (ie, randomized, placebo-controlled, double-blind, multicenter) and carefully implemented (ie, high protocol adherence to treatments, no loss to follow-up). The principal limitation of this study was its inability to reach the estimated statistical power of study. Due to successful epidemic control in Wuhan, which led to marked reductions in hospital admission of patients with COVID-19, and implementation of stringent termination criteria per the study protocol, only 237 participants were enrolled, instead of the 453, as specified by the sample estimate. This corresponded to a reduction of statistical power from 80% to 58%. Due to this limitation, the study was underpowered, rendering its findings inconclusive.
Despite this limitation, the study found that those treated with remdesivir within 10 days of symptom onset had a numerically faster time (although not statistically significant) to clinical improvement. This leads to an interesting question: whether remdesivir administration early in COVID-19 course could improve clinical outcomes, a question that warrants further investigation by an adequately powered trial. Also, data from this study provided evidence that intravenous remdesivir administration is likely safe in adults during the treatment period, although the long-term drug effects, as well as the safety profile in pediatric patients, remain unknown at this time.
While the study reported by Wang and colleagues was underpowered and is thus inconclusive, several other ongoing RCTs are evaluating the potential clinical benefit of remdesivir treatment in patients hospitalized with COVID-19. On the date of online publication of this report in The Lancet, the National Institutes of Health (NIH) published a news release summarizing preliminary findings from the Adaptive COVID-19 Treatment Trial (ACTT), which showed positive effects of remdesivir on clinical recovery from advanced COVID-19.4 The ACTT, the first RCT launched in the United States to evaluate experimental treatment for COVID-19, included 1063 hospitalized participants with advanced COVID-19 and lung involvement. Participants who were administered remdesivir had a 31% faster time to recovery compared to those in the placebo group (median time to recovery, 11 days vs 15 days, respectively; P < 0.001), and had near statistically significant improved survival (mortality rate, 8.0% vs 11.6%, respectively; P = 0.059). In response to these findings, the US Food and Drug Administration (FDA) issued an emergency use authorization for remdesivir on May 1, 2020, for the treatment of suspected or laboratory-confirmed COVID-19 in adults and children hospitalized with severe disease.5 While the findings noted from the NIH news release are very encouraging and provide the first evidence of a potentially beneficial antiviral treatment for severe COVID-19 in humans, the scientific community awaits the peer-reviewed publication of the ACTT to better assess the safety and effectiveness of remdesivir therapy and determine the trial’s implications in the management of COVID-19.
Applications for Clinical Practice
The discovery of an effective pharmacologic intervention for COVID-19 is of utmost urgency. While the present study was unable to answer the question of whether remdesivir is effective in improving clinical outcomes in patients with severe COVID-19, other ongoing or completed (ie, ACTT) studies will likely address this knowledge gap in the coming months. The FDA’s emergency use authorization for remdesivir provides a glimpse into this possibility.
–Katerina Oikonomou, MD, Brookdale Department of Geriatrics & Palliative Medicine, Icahn School of Medicine at Mount Sinai, New York, NY
–Fred Ko, MD
1. Tang W, Cao Z, Han M, et al. Hydroxychloroquine in patients with COVID-19: an open-label, randomized, controlled trial [published online April 14, 2020]. medRxiv.org. doi:10.1101/2020.04.10.20060558.
2. Cao B, Wang Y, Wen D, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe COVID-19. N Engl J Med. 2020;382:1787-1799.
3. Grein J, Ohmagari N, Shin D, et al. Compassionate use of remdesivir for patients with severe COVID-19 [published online April 10, 2020]. N Engl J Med. doi:10.1056/NEJMoa2007016.
4. NIH clinical trial shows remdesivir accelerates recovery from advanced COVID-19. www.niaid.nih.gov/news-events/nih-clinical-trial-shows-remdesivir-accelerates-recovery-advanced-covid-19. Accessed May 9, 2020
5. Coronavirus (COVID-19) update: FDA issues Emergency Use Authorization for potential COVID-19 treatment. www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-emergency-use-authorization-potential-covid-19-treatment. Accessed May 9, 2020.
Study Overview
Objective. To assess the efficacy, safety, and clinical benefit of remdesivir in hospitalized adults with confirmed pneumonia due to severe SARS-CoV-2 infection.
Design. Randomized, investigator-initiated, placebo-controlled, double-blind, multicenter trial.
Setting and participants. The trial took place between February 6, 2020 and March 12, 2020, at 10 hospitals in Wuhan, China. Study participants included adult patients (aged ≥ 18 years) admitted to hospital who tested positive for SARS-CoV-2 by reverse transcription polymerase chain reaction assay and had the following clinical characteristics: radiographic evidence of pneumonia; hypoxia with oxygen saturation ≤ 94% on room air or a ratio of arterial oxygen partial pressure to fractional inspired oxygen ≤ 300 mm Hg; and symptom onset to enrollment ≤ 12 days. Some of the exclusion criteria for participation in the study were pregnancy or breast feeding, liver cirrhosis, abnormal liver enzymes ≥ 5 times the upper limit of normal, severe renal impairment or receipt of renal replacement therapy, plan for transfer to a non-study hospital, and enrollment in a trial for COVID-19 within the previous month.
Intervention. Participants were randomized in a 2:1 ratio to the remdesivir group or the placebo group and were administered either intravenous infusions of remdesivir (200 mg on day 1 followed by 100 mg daily on days 2-10) or the same volume of placebo for 10 days. Clinical and safety data assessed included laboratory testing, electrocardiogram, and medication adverse effects. Testing of oropharyngeal and nasopharyngeal swab samples, anal swab samples, sputum, and stool was performed for viral RNA detection and quantification on days 1, 3, 5, 7, 10, 14, 21, and 28.
Main outcome measures. The primary endpoint of this study was time to clinical improvement within 28 days after randomization. Clinical improvement was defined as a 2-point reduction in participants’ admission status on a 6-point ordinal scale (1 = discharged or clinical recovery, 6 = death) or live discharge from hospital, whichever came first. Secondary outcomes included all-cause mortality at day 28 and duration of hospital admission, oxygen support, and invasive mechanical ventilation. Virological measures and safety outcomes ascertained included treatment-emergent adverse events, serious adverse events, and premature discontinuation of remdesivir.
The sample size estimate for the original study design was a total of 453 patients (302 in the remdesivir group and 151 in the placebo group). This sample size would provide 80% power, assuming a hazard ratio (HR) of 1.4 comparing remdesivir to placebo, and corresponding to a change in time to clinical improvement of 6 days. The analysis of primary outcome was performed on an intention-to-treat basis. Time to clinical improvement within 28 days was assessed with Kaplan-Meier plots.
Main results. A total of 255 patients were screened, of whom 237 were enrolled and randomized to remdesivir (158) or placebo (79) group. Of the participants in the remdesivir group, 155 started study treatment and 150 completed treatment per protocol. For the participants in the placebo group, 78 started study treatment and 76 completed treatment per-protocol. Study enrollment was terminated after March 12, 2020, before attaining the prespecified sample size, because no additional patients met study eligibility criteria due to various public health measures implemented in Wuhan. The median age of participants was 65 years (IQR, 56-71), the majority were men (56% in remdesivir group vs 65% in placebo group), and the most common comorbidities included hypertension, diabetes, and coronary artery disease. Median time from symptom onset to study enrollment was 10 days (IQR, 9-12). The time to clinical improvement between treatments (21 days for remdesivir group vs 23 days for placebo group) was not significantly different (HR, 1.23; 95% confidence interval [CI], 0.87-1.75). In addition, in participants who received treatment within 10 days of symptom onset, those who were administered remdesivir had a nonsignificant (HR, 1.52; 95% CI, 0.95-2.43) but faster time (18 days) to clinical improvement, compared to those administered placebo (23 days). Moreover, treatment with remdesivir versus placebo did not lead to differences in secondary outcomes (eg, 28-day mortality and duration of hospital stay, oxygen support, and invasive mechanical ventilation), changes in viral load over time, or adverse events between the groups.
Conclusion. This study found that, compared with placebo, intravenous remdesivir did not significantly improve the time to clinical improvement, mortality, or time to clearance of SARS-CoV-2 in hospitalized adults with severe COVID-19. A numeric reduction in time to clinical improvement with early remdesivir treatment (ie, within 10 days of symptom onset) that approached statistical significance was observed in this underpowered study.
Commentary
Within a few short months since its emergence. SARS-CoV-2 infection has caused a global pandemic, posing a dire threat to public health due to its adverse effects on morbidity (eg, respiratory failure, thromboembolic diseases, multiorgan failure) and mortality. To date, no pharmacologic treatment has been shown to effectively improve clinical outcomes in patients with COVID-19. Multiple ongoing clinical trials are being conducted globally to determine potential therapeutic treatments for severe COVID-19. The first clinical trials of hydroxychloroquine and lopinavir-ritonavir, agents traditionally used for other indications, such as malaria and HIV, did not show a clear benefit in COVID-19.1,2 Remdesivir, a nucleoside analogue prodrug, is a broad-spectrum antiviral agent that was previously used for treatment of Ebola and has been shown to have inhibitory effects on pathogenic coronaviruses. The study reported by Wang and colleagues was the first randomized controlled trial (RCT) aimed at evaluating whether remdesivir improves outcomes in patients with severe COVID-19. Thus, the worsening COVID-19 pandemic, coupled with the absence of a curative treatment, underscore the urgency of this trial.
The study was grounded on observational data from several recent case reports and case series centering on the potential efficacy of remdesivir in treating COVID-19.3 The study itself was designed well (ie, randomized, placebo-controlled, double-blind, multicenter) and carefully implemented (ie, high protocol adherence to treatments, no loss to follow-up). The principal limitation of this study was its inability to reach the estimated statistical power of study. Due to successful epidemic control in Wuhan, which led to marked reductions in hospital admission of patients with COVID-19, and implementation of stringent termination criteria per the study protocol, only 237 participants were enrolled, instead of the 453, as specified by the sample estimate. This corresponded to a reduction of statistical power from 80% to 58%. Due to this limitation, the study was underpowered, rendering its findings inconclusive.
Despite this limitation, the study found that those treated with remdesivir within 10 days of symptom onset had a numerically faster time (although not statistically significant) to clinical improvement. This leads to an interesting question: whether remdesivir administration early in COVID-19 course could improve clinical outcomes, a question that warrants further investigation by an adequately powered trial. Also, data from this study provided evidence that intravenous remdesivir administration is likely safe in adults during the treatment period, although the long-term drug effects, as well as the safety profile in pediatric patients, remain unknown at this time.
While the study reported by Wang and colleagues was underpowered and is thus inconclusive, several other ongoing RCTs are evaluating the potential clinical benefit of remdesivir treatment in patients hospitalized with COVID-19. On the date of online publication of this report in The Lancet, the National Institutes of Health (NIH) published a news release summarizing preliminary findings from the Adaptive COVID-19 Treatment Trial (ACTT), which showed positive effects of remdesivir on clinical recovery from advanced COVID-19.4 The ACTT, the first RCT launched in the United States to evaluate experimental treatment for COVID-19, included 1063 hospitalized participants with advanced COVID-19 and lung involvement. Participants who were administered remdesivir had a 31% faster time to recovery compared to those in the placebo group (median time to recovery, 11 days vs 15 days, respectively; P < 0.001), and had near statistically significant improved survival (mortality rate, 8.0% vs 11.6%, respectively; P = 0.059). In response to these findings, the US Food and Drug Administration (FDA) issued an emergency use authorization for remdesivir on May 1, 2020, for the treatment of suspected or laboratory-confirmed COVID-19 in adults and children hospitalized with severe disease.5 While the findings noted from the NIH news release are very encouraging and provide the first evidence of a potentially beneficial antiviral treatment for severe COVID-19 in humans, the scientific community awaits the peer-reviewed publication of the ACTT to better assess the safety and effectiveness of remdesivir therapy and determine the trial’s implications in the management of COVID-19.
Applications for Clinical Practice
The discovery of an effective pharmacologic intervention for COVID-19 is of utmost urgency. While the present study was unable to answer the question of whether remdesivir is effective in improving clinical outcomes in patients with severe COVID-19, other ongoing or completed (ie, ACTT) studies will likely address this knowledge gap in the coming months. The FDA’s emergency use authorization for remdesivir provides a glimpse into this possibility.
–Katerina Oikonomou, MD, Brookdale Department of Geriatrics & Palliative Medicine, Icahn School of Medicine at Mount Sinai, New York, NY
–Fred Ko, MD
Study Overview
Objective. To assess the efficacy, safety, and clinical benefit of remdesivir in hospitalized adults with confirmed pneumonia due to severe SARS-CoV-2 infection.
Design. Randomized, investigator-initiated, placebo-controlled, double-blind, multicenter trial.
Setting and participants. The trial took place between February 6, 2020 and March 12, 2020, at 10 hospitals in Wuhan, China. Study participants included adult patients (aged ≥ 18 years) admitted to hospital who tested positive for SARS-CoV-2 by reverse transcription polymerase chain reaction assay and had the following clinical characteristics: radiographic evidence of pneumonia; hypoxia with oxygen saturation ≤ 94% on room air or a ratio of arterial oxygen partial pressure to fractional inspired oxygen ≤ 300 mm Hg; and symptom onset to enrollment ≤ 12 days. Some of the exclusion criteria for participation in the study were pregnancy or breast feeding, liver cirrhosis, abnormal liver enzymes ≥ 5 times the upper limit of normal, severe renal impairment or receipt of renal replacement therapy, plan for transfer to a non-study hospital, and enrollment in a trial for COVID-19 within the previous month.
Intervention. Participants were randomized in a 2:1 ratio to the remdesivir group or the placebo group and were administered either intravenous infusions of remdesivir (200 mg on day 1 followed by 100 mg daily on days 2-10) or the same volume of placebo for 10 days. Clinical and safety data assessed included laboratory testing, electrocardiogram, and medication adverse effects. Testing of oropharyngeal and nasopharyngeal swab samples, anal swab samples, sputum, and stool was performed for viral RNA detection and quantification on days 1, 3, 5, 7, 10, 14, 21, and 28.
Main outcome measures. The primary endpoint of this study was time to clinical improvement within 28 days after randomization. Clinical improvement was defined as a 2-point reduction in participants’ admission status on a 6-point ordinal scale (1 = discharged or clinical recovery, 6 = death) or live discharge from hospital, whichever came first. Secondary outcomes included all-cause mortality at day 28 and duration of hospital admission, oxygen support, and invasive mechanical ventilation. Virological measures and safety outcomes ascertained included treatment-emergent adverse events, serious adverse events, and premature discontinuation of remdesivir.
The sample size estimate for the original study design was a total of 453 patients (302 in the remdesivir group and 151 in the placebo group). This sample size would provide 80% power, assuming a hazard ratio (HR) of 1.4 comparing remdesivir to placebo, and corresponding to a change in time to clinical improvement of 6 days. The analysis of primary outcome was performed on an intention-to-treat basis. Time to clinical improvement within 28 days was assessed with Kaplan-Meier plots.
Main results. A total of 255 patients were screened, of whom 237 were enrolled and randomized to remdesivir (158) or placebo (79) group. Of the participants in the remdesivir group, 155 started study treatment and 150 completed treatment per protocol. For the participants in the placebo group, 78 started study treatment and 76 completed treatment per-protocol. Study enrollment was terminated after March 12, 2020, before attaining the prespecified sample size, because no additional patients met study eligibility criteria due to various public health measures implemented in Wuhan. The median age of participants was 65 years (IQR, 56-71), the majority were men (56% in remdesivir group vs 65% in placebo group), and the most common comorbidities included hypertension, diabetes, and coronary artery disease. Median time from symptom onset to study enrollment was 10 days (IQR, 9-12). The time to clinical improvement between treatments (21 days for remdesivir group vs 23 days for placebo group) was not significantly different (HR, 1.23; 95% confidence interval [CI], 0.87-1.75). In addition, in participants who received treatment within 10 days of symptom onset, those who were administered remdesivir had a nonsignificant (HR, 1.52; 95% CI, 0.95-2.43) but faster time (18 days) to clinical improvement, compared to those administered placebo (23 days). Moreover, treatment with remdesivir versus placebo did not lead to differences in secondary outcomes (eg, 28-day mortality and duration of hospital stay, oxygen support, and invasive mechanical ventilation), changes in viral load over time, or adverse events between the groups.
Conclusion. This study found that, compared with placebo, intravenous remdesivir did not significantly improve the time to clinical improvement, mortality, or time to clearance of SARS-CoV-2 in hospitalized adults with severe COVID-19. A numeric reduction in time to clinical improvement with early remdesivir treatment (ie, within 10 days of symptom onset) that approached statistical significance was observed in this underpowered study.
Commentary
Within a few short months since its emergence. SARS-CoV-2 infection has caused a global pandemic, posing a dire threat to public health due to its adverse effects on morbidity (eg, respiratory failure, thromboembolic diseases, multiorgan failure) and mortality. To date, no pharmacologic treatment has been shown to effectively improve clinical outcomes in patients with COVID-19. Multiple ongoing clinical trials are being conducted globally to determine potential therapeutic treatments for severe COVID-19. The first clinical trials of hydroxychloroquine and lopinavir-ritonavir, agents traditionally used for other indications, such as malaria and HIV, did not show a clear benefit in COVID-19.1,2 Remdesivir, a nucleoside analogue prodrug, is a broad-spectrum antiviral agent that was previously used for treatment of Ebola and has been shown to have inhibitory effects on pathogenic coronaviruses. The study reported by Wang and colleagues was the first randomized controlled trial (RCT) aimed at evaluating whether remdesivir improves outcomes in patients with severe COVID-19. Thus, the worsening COVID-19 pandemic, coupled with the absence of a curative treatment, underscore the urgency of this trial.
The study was grounded on observational data from several recent case reports and case series centering on the potential efficacy of remdesivir in treating COVID-19.3 The study itself was designed well (ie, randomized, placebo-controlled, double-blind, multicenter) and carefully implemented (ie, high protocol adherence to treatments, no loss to follow-up). The principal limitation of this study was its inability to reach the estimated statistical power of study. Due to successful epidemic control in Wuhan, which led to marked reductions in hospital admission of patients with COVID-19, and implementation of stringent termination criteria per the study protocol, only 237 participants were enrolled, instead of the 453, as specified by the sample estimate. This corresponded to a reduction of statistical power from 80% to 58%. Due to this limitation, the study was underpowered, rendering its findings inconclusive.
Despite this limitation, the study found that those treated with remdesivir within 10 days of symptom onset had a numerically faster time (although not statistically significant) to clinical improvement. This leads to an interesting question: whether remdesivir administration early in COVID-19 course could improve clinical outcomes, a question that warrants further investigation by an adequately powered trial. Also, data from this study provided evidence that intravenous remdesivir administration is likely safe in adults during the treatment period, although the long-term drug effects, as well as the safety profile in pediatric patients, remain unknown at this time.
While the study reported by Wang and colleagues was underpowered and is thus inconclusive, several other ongoing RCTs are evaluating the potential clinical benefit of remdesivir treatment in patients hospitalized with COVID-19. On the date of online publication of this report in The Lancet, the National Institutes of Health (NIH) published a news release summarizing preliminary findings from the Adaptive COVID-19 Treatment Trial (ACTT), which showed positive effects of remdesivir on clinical recovery from advanced COVID-19.4 The ACTT, the first RCT launched in the United States to evaluate experimental treatment for COVID-19, included 1063 hospitalized participants with advanced COVID-19 and lung involvement. Participants who were administered remdesivir had a 31% faster time to recovery compared to those in the placebo group (median time to recovery, 11 days vs 15 days, respectively; P < 0.001), and had near statistically significant improved survival (mortality rate, 8.0% vs 11.6%, respectively; P = 0.059). In response to these findings, the US Food and Drug Administration (FDA) issued an emergency use authorization for remdesivir on May 1, 2020, for the treatment of suspected or laboratory-confirmed COVID-19 in adults and children hospitalized with severe disease.5 While the findings noted from the NIH news release are very encouraging and provide the first evidence of a potentially beneficial antiviral treatment for severe COVID-19 in humans, the scientific community awaits the peer-reviewed publication of the ACTT to better assess the safety and effectiveness of remdesivir therapy and determine the trial’s implications in the management of COVID-19.
Applications for Clinical Practice
The discovery of an effective pharmacologic intervention for COVID-19 is of utmost urgency. While the present study was unable to answer the question of whether remdesivir is effective in improving clinical outcomes in patients with severe COVID-19, other ongoing or completed (ie, ACTT) studies will likely address this knowledge gap in the coming months. The FDA’s emergency use authorization for remdesivir provides a glimpse into this possibility.
–Katerina Oikonomou, MD, Brookdale Department of Geriatrics & Palliative Medicine, Icahn School of Medicine at Mount Sinai, New York, NY
–Fred Ko, MD
1. Tang W, Cao Z, Han M, et al. Hydroxychloroquine in patients with COVID-19: an open-label, randomized, controlled trial [published online April 14, 2020]. medRxiv.org. doi:10.1101/2020.04.10.20060558.
2. Cao B, Wang Y, Wen D, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe COVID-19. N Engl J Med. 2020;382:1787-1799.
3. Grein J, Ohmagari N, Shin D, et al. Compassionate use of remdesivir for patients with severe COVID-19 [published online April 10, 2020]. N Engl J Med. doi:10.1056/NEJMoa2007016.
4. NIH clinical trial shows remdesivir accelerates recovery from advanced COVID-19. www.niaid.nih.gov/news-events/nih-clinical-trial-shows-remdesivir-accelerates-recovery-advanced-covid-19. Accessed May 9, 2020
5. Coronavirus (COVID-19) update: FDA issues Emergency Use Authorization for potential COVID-19 treatment. www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-emergency-use-authorization-potential-covid-19-treatment. Accessed May 9, 2020.
1. Tang W, Cao Z, Han M, et al. Hydroxychloroquine in patients with COVID-19: an open-label, randomized, controlled trial [published online April 14, 2020]. medRxiv.org. doi:10.1101/2020.04.10.20060558.
2. Cao B, Wang Y, Wen D, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe COVID-19. N Engl J Med. 2020;382:1787-1799.
3. Grein J, Ohmagari N, Shin D, et al. Compassionate use of remdesivir for patients with severe COVID-19 [published online April 10, 2020]. N Engl J Med. doi:10.1056/NEJMoa2007016.
4. NIH clinical trial shows remdesivir accelerates recovery from advanced COVID-19. www.niaid.nih.gov/news-events/nih-clinical-trial-shows-remdesivir-accelerates-recovery-advanced-covid-19. Accessed May 9, 2020
5. Coronavirus (COVID-19) update: FDA issues Emergency Use Authorization for potential COVID-19 treatment. www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-emergency-use-authorization-potential-covid-19-treatment. Accessed May 9, 2020.
Today’s top news highlights: Risks & benefits of universal masking, prostate cancer rising
Here are the stories our MDedge editors across specialties think you need to know about today:
Universal masking: Risks and benefits
The idea of universal masking has been debated extensively. As reported in Science, previous randomized clinical studies performed on other viruses have shown no added protection, though small sample sizes and noncompliance are limiting factors. Leung et al. stated in The Lancet that the lack of proof that masks are effective should not rule them as ineffective. A study in the Journal of Medical Virology demonstrates 99.98%, 97.14%, and 95.15% efficacy for N95, surgical, and homemade masks, respectively, in blocking the avian influenza virus. On the contrary, an Annals of Internal Medicine study of four COVID-19 positive subjects found that “neither surgical masks nor cloth masks effectively filtered SARS-CoV-2 during coughs of infected patients.” READ MORE
Inflammation, thrombosis biomarkers tied to COVID-19 deaths
Biomarkers for inflammation and thrombosis may predict deaths from COVID-19 among critically ill patients, researchers said. Their prospective cohort study of 1,150 patients hospitalized in New York City also revealed a high proportion of racial and ethnic minorities, and confirmed high rates of critical illness and mortality. “Of particular interest is the finding that over three quarters of critically ill patients required a ventilator and almost one third required renal dialysis support,” Max O’Donnell, MD, MPH, assistant professor of medicine and epidemiology at Columbia University in New York, said in a press release. The study was published in The Lancet. READ MORE
Advanced prostate cancers still rising in U.S.
The incidence of advanced prostate cancers in the United States “persistently” increased annually for 5 years after the United States Preventive Services Task Force controversially advised in 2012 against prostate-specific antigen screening in men of all ages. “These data illustrate the trade-off between higher screening rates and more early-stage disease diagnoses (possibly overdiagnosis and overtreatment) and lower screening rates and more late-stage (possibly fatal) disease,” the authors of the study, published in the Journal of the National Cancer Institute, commented. “What is a surprise is that it’s every year,” said Ahmad Shabsigh, MD, a urologic oncologist at the Ohio State University Comprehensive Cancer Center. “To see it so clearly in this study is sad." READ MORE
Testicular sperm may improve IVF outcomes
Use of testicular sperm in nonazoospermic couples who had prior in vitro fertilization failure using ejaculated sperm appears to improve embryo development and rates of clinical pregnancy and live birth, a retrospective observational study has found. The findings offer more evidence “that this might be something we can offer patients who’ve had multiple failures and no other reason as to why,” said M. Blake Evans, DO, a clinical fellow in reproductive endocrinology and infertility. The study, which won the college’s Donald F. Richardson Memorial Prize Research Paper award, was released ahead of a scheduled presentation at the annual American College of Obstetricians and Gynecologists meeting. READ MORE
For more on COVID-19, visit our Resource Center. All of our latest news coverage is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Universal masking: Risks and benefits
The idea of universal masking has been debated extensively. As reported in Science, previous randomized clinical studies performed on other viruses have shown no added protection, though small sample sizes and noncompliance are limiting factors. Leung et al. stated in The Lancet that the lack of proof that masks are effective should not rule them as ineffective. A study in the Journal of Medical Virology demonstrates 99.98%, 97.14%, and 95.15% efficacy for N95, surgical, and homemade masks, respectively, in blocking the avian influenza virus. On the contrary, an Annals of Internal Medicine study of four COVID-19 positive subjects found that “neither surgical masks nor cloth masks effectively filtered SARS-CoV-2 during coughs of infected patients.” READ MORE
Inflammation, thrombosis biomarkers tied to COVID-19 deaths
Biomarkers for inflammation and thrombosis may predict deaths from COVID-19 among critically ill patients, researchers said. Their prospective cohort study of 1,150 patients hospitalized in New York City also revealed a high proportion of racial and ethnic minorities, and confirmed high rates of critical illness and mortality. “Of particular interest is the finding that over three quarters of critically ill patients required a ventilator and almost one third required renal dialysis support,” Max O’Donnell, MD, MPH, assistant professor of medicine and epidemiology at Columbia University in New York, said in a press release. The study was published in The Lancet. READ MORE
Advanced prostate cancers still rising in U.S.
The incidence of advanced prostate cancers in the United States “persistently” increased annually for 5 years after the United States Preventive Services Task Force controversially advised in 2012 against prostate-specific antigen screening in men of all ages. “These data illustrate the trade-off between higher screening rates and more early-stage disease diagnoses (possibly overdiagnosis and overtreatment) and lower screening rates and more late-stage (possibly fatal) disease,” the authors of the study, published in the Journal of the National Cancer Institute, commented. “What is a surprise is that it’s every year,” said Ahmad Shabsigh, MD, a urologic oncologist at the Ohio State University Comprehensive Cancer Center. “To see it so clearly in this study is sad." READ MORE
Testicular sperm may improve IVF outcomes
Use of testicular sperm in nonazoospermic couples who had prior in vitro fertilization failure using ejaculated sperm appears to improve embryo development and rates of clinical pregnancy and live birth, a retrospective observational study has found. The findings offer more evidence “that this might be something we can offer patients who’ve had multiple failures and no other reason as to why,” said M. Blake Evans, DO, a clinical fellow in reproductive endocrinology and infertility. The study, which won the college’s Donald F. Richardson Memorial Prize Research Paper award, was released ahead of a scheduled presentation at the annual American College of Obstetricians and Gynecologists meeting. READ MORE
For more on COVID-19, visit our Resource Center. All of our latest news coverage is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Universal masking: Risks and benefits
The idea of universal masking has been debated extensively. As reported in Science, previous randomized clinical studies performed on other viruses have shown no added protection, though small sample sizes and noncompliance are limiting factors. Leung et al. stated in The Lancet that the lack of proof that masks are effective should not rule them as ineffective. A study in the Journal of Medical Virology demonstrates 99.98%, 97.14%, and 95.15% efficacy for N95, surgical, and homemade masks, respectively, in blocking the avian influenza virus. On the contrary, an Annals of Internal Medicine study of four COVID-19 positive subjects found that “neither surgical masks nor cloth masks effectively filtered SARS-CoV-2 during coughs of infected patients.” READ MORE
Inflammation, thrombosis biomarkers tied to COVID-19 deaths
Biomarkers for inflammation and thrombosis may predict deaths from COVID-19 among critically ill patients, researchers said. Their prospective cohort study of 1,150 patients hospitalized in New York City also revealed a high proportion of racial and ethnic minorities, and confirmed high rates of critical illness and mortality. “Of particular interest is the finding that over three quarters of critically ill patients required a ventilator and almost one third required renal dialysis support,” Max O’Donnell, MD, MPH, assistant professor of medicine and epidemiology at Columbia University in New York, said in a press release. The study was published in The Lancet. READ MORE
Advanced prostate cancers still rising in U.S.
The incidence of advanced prostate cancers in the United States “persistently” increased annually for 5 years after the United States Preventive Services Task Force controversially advised in 2012 against prostate-specific antigen screening in men of all ages. “These data illustrate the trade-off between higher screening rates and more early-stage disease diagnoses (possibly overdiagnosis and overtreatment) and lower screening rates and more late-stage (possibly fatal) disease,” the authors of the study, published in the Journal of the National Cancer Institute, commented. “What is a surprise is that it’s every year,” said Ahmad Shabsigh, MD, a urologic oncologist at the Ohio State University Comprehensive Cancer Center. “To see it so clearly in this study is sad." READ MORE
Testicular sperm may improve IVF outcomes
Use of testicular sperm in nonazoospermic couples who had prior in vitro fertilization failure using ejaculated sperm appears to improve embryo development and rates of clinical pregnancy and live birth, a retrospective observational study has found. The findings offer more evidence “that this might be something we can offer patients who’ve had multiple failures and no other reason as to why,” said M. Blake Evans, DO, a clinical fellow in reproductive endocrinology and infertility. The study, which won the college’s Donald F. Richardson Memorial Prize Research Paper award, was released ahead of a scheduled presentation at the annual American College of Obstetricians and Gynecologists meeting. READ MORE
For more on COVID-19, visit our Resource Center. All of our latest news coverage is available on MDedge.com.
Inflammation, thrombosis biomarkers tied to COVID-19 deaths
Their prospective cohort study of 1150 patients hospitalized with the disease in New York City also revealed a high proportion of racial and ethnic minorities, and confirmed high rates of critical illness and mortality.
“Of particular interest is the finding that over three quarters of critically ill patients required a ventilator and almost one third required renal dialysis support,” Max O’Donnell, MD, MPH, assistant professor of medicine and epidemiology at Columbia University in New York City, said in a press release.
O’Donnell and colleagues published the results of their study online today in The Lancet. It is the largest prospective cohort study published in the United States, they said.
“Although the clinical spectrum of disease has been characterised in reports from China and Italy, until now, detailed understanding of how the virus is affecting critically ill patients in the US has been limited to reports from a small number of cases,” said Natalie Yip, MD, assistant professor of medicine at Columbia University.
In the cohort, drawn from two NewYork-Presbyterian hospitals, the researchers focused on the 257 (22%) patients who required intensive care. When they estimated inflammation through interleukin-6 (IL-6) concentrations and thrombosis through D-dimer concentrations, they found a 10% increased risk for death with every 10% increase of IL-6 (adjusted hazard ratio [aHR], 1.11; 95% confidence interval [CI], 1.02–1.20) or D-dimer concentration (aHR, 1.10; 95% CI, 1.01–1.19).
“The association of mortality with higher concentrations of IL-6 and d-dimer is particularly relevant for two reasons,” write Giacomo Grasselli, from the Fondazione IRCCS Ca’ Granda Ospediale Maggiore Policlinico, and Alberto Zanella, from the University of Milan, Italy, in an accompanying commentary.
“First, it confirms the key pathogenic role played by the activation of systemic inflammation and endothelial-vascular damage in the development of organ dysfunction,” they write. “Second, it provides the rationale for the design of clinical trials for measuring the efficacy of treatment with immunomodulating and anticoagulant drugs.”
Seventeen percent of patients received interleukin receptor antagonists and 26% received corticosteroids, but the authors did not report any data on the effects of these treatments, or any data about anticoagulant therapies administered.
Severe disease common
The study also highlighted a high proportion of ethnic and racial minorities. Sixty-two percent of the critically ill patients were Hispanic or Latinx, 19% Black, 32% White, and 3% Asian.
Their median age was 62 years and 67% were men. Eighty-two percent had at least one chronic illness, most commonly hypertension (63%), followed by diabetes (36%). Forty-six percent were obese.
As of April 28, 2020, 101 (39%) of the critically ill patients had died following a median of 9 days (interquartile range (IQR), 5–15) in the hospital and 94 (37%) remained hospitalized. Of the 203 patients who received invasive mechanical ventilation, 84 (41%) had died.
The poor prognosis of patients requiring ventilation is consistent with data from a report on patients treated in National Health Service intensive care units in England, Wales, and Northern Ireland through May 15. Overall, 11,292 patients with COVID-19 required critical care, and 4855 needed advanced respiratory support. Approximately half of the patients receiving mechanical ventilation had died 30 days after starting critical care.
In the New York study, patients spent an average of 18 days on a ventilator (IQR, 9–28 days). This is a longer period than reported in smaller studies of cases from Washington state, but corresponds with a recent report from Italy, the researchers said.
Remarkably, O’Donnell and colleagues report that almost a third (31%) of critically ill patients developed severe kidney damage and required dialysis.
Mortality was associated with several baseline factors, including older age (aHR, 1.31 [95% CI, 1.09–1.57] per 10-year increase), chronic cardiac disease (aHR, 1.76; 95% CI, 1.08–2·86), and chronic pulmonary disease (aHR, 2.94; 95% CI, 1.48–5.84).
Authors of the New York study reported financial relationships to ICE Neurosystems, ALung Technologies, Baxter, BREETHE, Xenios, Hemovent, Gilead Sciences, Amazon, and Karyopharm Therapeutics. Grasselli reports personal fees from Biotest, Draeger, Fisher & Paykel, Maquet, Merck Sharp & Dohme, and Pfizer, all outside the area of work commented on here. Zanella has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Their prospective cohort study of 1150 patients hospitalized with the disease in New York City also revealed a high proportion of racial and ethnic minorities, and confirmed high rates of critical illness and mortality.
“Of particular interest is the finding that over three quarters of critically ill patients required a ventilator and almost one third required renal dialysis support,” Max O’Donnell, MD, MPH, assistant professor of medicine and epidemiology at Columbia University in New York City, said in a press release.
O’Donnell and colleagues published the results of their study online today in The Lancet. It is the largest prospective cohort study published in the United States, they said.
“Although the clinical spectrum of disease has been characterised in reports from China and Italy, until now, detailed understanding of how the virus is affecting critically ill patients in the US has been limited to reports from a small number of cases,” said Natalie Yip, MD, assistant professor of medicine at Columbia University.
In the cohort, drawn from two NewYork-Presbyterian hospitals, the researchers focused on the 257 (22%) patients who required intensive care. When they estimated inflammation through interleukin-6 (IL-6) concentrations and thrombosis through D-dimer concentrations, they found a 10% increased risk for death with every 10% increase of IL-6 (adjusted hazard ratio [aHR], 1.11; 95% confidence interval [CI], 1.02–1.20) or D-dimer concentration (aHR, 1.10; 95% CI, 1.01–1.19).
“The association of mortality with higher concentrations of IL-6 and d-dimer is particularly relevant for two reasons,” write Giacomo Grasselli, from the Fondazione IRCCS Ca’ Granda Ospediale Maggiore Policlinico, and Alberto Zanella, from the University of Milan, Italy, in an accompanying commentary.
“First, it confirms the key pathogenic role played by the activation of systemic inflammation and endothelial-vascular damage in the development of organ dysfunction,” they write. “Second, it provides the rationale for the design of clinical trials for measuring the efficacy of treatment with immunomodulating and anticoagulant drugs.”
Seventeen percent of patients received interleukin receptor antagonists and 26% received corticosteroids, but the authors did not report any data on the effects of these treatments, or any data about anticoagulant therapies administered.
Severe disease common
The study also highlighted a high proportion of ethnic and racial minorities. Sixty-two percent of the critically ill patients were Hispanic or Latinx, 19% Black, 32% White, and 3% Asian.
Their median age was 62 years and 67% were men. Eighty-two percent had at least one chronic illness, most commonly hypertension (63%), followed by diabetes (36%). Forty-six percent were obese.
As of April 28, 2020, 101 (39%) of the critically ill patients had died following a median of 9 days (interquartile range (IQR), 5–15) in the hospital and 94 (37%) remained hospitalized. Of the 203 patients who received invasive mechanical ventilation, 84 (41%) had died.
The poor prognosis of patients requiring ventilation is consistent with data from a report on patients treated in National Health Service intensive care units in England, Wales, and Northern Ireland through May 15. Overall, 11,292 patients with COVID-19 required critical care, and 4855 needed advanced respiratory support. Approximately half of the patients receiving mechanical ventilation had died 30 days after starting critical care.
In the New York study, patients spent an average of 18 days on a ventilator (IQR, 9–28 days). This is a longer period than reported in smaller studies of cases from Washington state, but corresponds with a recent report from Italy, the researchers said.
Remarkably, O’Donnell and colleagues report that almost a third (31%) of critically ill patients developed severe kidney damage and required dialysis.
Mortality was associated with several baseline factors, including older age (aHR, 1.31 [95% CI, 1.09–1.57] per 10-year increase), chronic cardiac disease (aHR, 1.76; 95% CI, 1.08–2·86), and chronic pulmonary disease (aHR, 2.94; 95% CI, 1.48–5.84).
Authors of the New York study reported financial relationships to ICE Neurosystems, ALung Technologies, Baxter, BREETHE, Xenios, Hemovent, Gilead Sciences, Amazon, and Karyopharm Therapeutics. Grasselli reports personal fees from Biotest, Draeger, Fisher & Paykel, Maquet, Merck Sharp & Dohme, and Pfizer, all outside the area of work commented on here. Zanella has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Their prospective cohort study of 1150 patients hospitalized with the disease in New York City also revealed a high proportion of racial and ethnic minorities, and confirmed high rates of critical illness and mortality.
“Of particular interest is the finding that over three quarters of critically ill patients required a ventilator and almost one third required renal dialysis support,” Max O’Donnell, MD, MPH, assistant professor of medicine and epidemiology at Columbia University in New York City, said in a press release.
O’Donnell and colleagues published the results of their study online today in The Lancet. It is the largest prospective cohort study published in the United States, they said.
“Although the clinical spectrum of disease has been characterised in reports from China and Italy, until now, detailed understanding of how the virus is affecting critically ill patients in the US has been limited to reports from a small number of cases,” said Natalie Yip, MD, assistant professor of medicine at Columbia University.
In the cohort, drawn from two NewYork-Presbyterian hospitals, the researchers focused on the 257 (22%) patients who required intensive care. When they estimated inflammation through interleukin-6 (IL-6) concentrations and thrombosis through D-dimer concentrations, they found a 10% increased risk for death with every 10% increase of IL-6 (adjusted hazard ratio [aHR], 1.11; 95% confidence interval [CI], 1.02–1.20) or D-dimer concentration (aHR, 1.10; 95% CI, 1.01–1.19).
“The association of mortality with higher concentrations of IL-6 and d-dimer is particularly relevant for two reasons,” write Giacomo Grasselli, from the Fondazione IRCCS Ca’ Granda Ospediale Maggiore Policlinico, and Alberto Zanella, from the University of Milan, Italy, in an accompanying commentary.
“First, it confirms the key pathogenic role played by the activation of systemic inflammation and endothelial-vascular damage in the development of organ dysfunction,” they write. “Second, it provides the rationale for the design of clinical trials for measuring the efficacy of treatment with immunomodulating and anticoagulant drugs.”
Seventeen percent of patients received interleukin receptor antagonists and 26% received corticosteroids, but the authors did not report any data on the effects of these treatments, or any data about anticoagulant therapies administered.
Severe disease common
The study also highlighted a high proportion of ethnic and racial minorities. Sixty-two percent of the critically ill patients were Hispanic or Latinx, 19% Black, 32% White, and 3% Asian.
Their median age was 62 years and 67% were men. Eighty-two percent had at least one chronic illness, most commonly hypertension (63%), followed by diabetes (36%). Forty-six percent were obese.
As of April 28, 2020, 101 (39%) of the critically ill patients had died following a median of 9 days (interquartile range (IQR), 5–15) in the hospital and 94 (37%) remained hospitalized. Of the 203 patients who received invasive mechanical ventilation, 84 (41%) had died.
The poor prognosis of patients requiring ventilation is consistent with data from a report on patients treated in National Health Service intensive care units in England, Wales, and Northern Ireland through May 15. Overall, 11,292 patients with COVID-19 required critical care, and 4855 needed advanced respiratory support. Approximately half of the patients receiving mechanical ventilation had died 30 days after starting critical care.
In the New York study, patients spent an average of 18 days on a ventilator (IQR, 9–28 days). This is a longer period than reported in smaller studies of cases from Washington state, but corresponds with a recent report from Italy, the researchers said.
Remarkably, O’Donnell and colleagues report that almost a third (31%) of critically ill patients developed severe kidney damage and required dialysis.
Mortality was associated with several baseline factors, including older age (aHR, 1.31 [95% CI, 1.09–1.57] per 10-year increase), chronic cardiac disease (aHR, 1.76; 95% CI, 1.08–2·86), and chronic pulmonary disease (aHR, 2.94; 95% CI, 1.48–5.84).
Authors of the New York study reported financial relationships to ICE Neurosystems, ALung Technologies, Baxter, BREETHE, Xenios, Hemovent, Gilead Sciences, Amazon, and Karyopharm Therapeutics. Grasselli reports personal fees from Biotest, Draeger, Fisher & Paykel, Maquet, Merck Sharp & Dohme, and Pfizer, all outside the area of work commented on here. Zanella has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Maskomania: Masks and COVID-19
A comprehensive review
On April 3, the Centers for Disease Control and Prevention issued an advisory that the general public wear cloth face masks when outside, particularly those residing in areas with significant severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) community transmission.1 Recent research reveals several factors related to the nature of the virus as well as the epidemiologic spread of the illness that may have led to this decision.
However, controversy still prevails whether this recommendation will alleviate or aggravate disease progression. With many hospitals across America lacking sufficient personal protective equipment (PPE) and scrambling for supplies, universal masking may create more chaos, especially with certain states imposing monetary fines on individuals spotted outdoors without a mask. With new information being discovered each day about COVID-19, it is more imperative than ever to update existing strategies and formulate more effective methods to flatten the curve.
Airborne vs. droplet transmission
According to a scientific brief released by the World Health Organization, there have been studies with mixed evidence and opinions regarding the presence of COVID-19 ribonucleic acid (RNA) in air samples.2 In medRxiv, Santarpia et al., from the University of Nebraska Medical Center, Omaha, detected viral RNA in samples taken from beneath a patient’s bed and from a window ledge, both areas in which neither the patient nor health care personnel had any direct contact. They also found that 66.7% of air samples taken from a hospital hallway carried virus-containing particles.3 It is worth noting that certain aerosol-generating procedures (AGP) may increase the likelihood of airborne dissemination. Whether airborne transmission is a major mode of COVID-19 spread in the community and routine clinical settings (with no aerosol-generating procedures) is still a debatable question without a definitive answer.
We should consider the epidemiology of COVID-19 thus far in the pandemic to determine if transmission patterns are more consistent with that of other common respiratory viral pathogens or more consistent with that of the agents we classically consider to be transmitted by the airborne route (measles, varicella zoster virus, and Mycobacterium tuberculosis). The attack rates in various settings (household, health care, and the public) as well as the expected number of secondary cases from a single infected individual in a susceptible population (R0) are more consistent with those of a droplet spread pathogen.
For measles, the R0 is 12-18, and the secondary household attack rates are ≥ 90%. In case of the varicella zoster virus, the R0 is ~10, and the secondary household attack rate is 85%. The R0 for pulmonary tuberculosis is up to 10 (per year) and the secondary household attack rate has been reported to be >50%. With COVID-19, the R0 appears to be around 2.5-3 and secondary household attack rates are ~ 10% from data available so far, similar to that of influenza viruses. This discrepancy suggests that droplet transmission may be more likely. The dichotomy of airborne versus droplet mode of spread may be better described as a continuum, as pointed out in a recent article in the JAMA. Infectious droplets form turbulent gas clouds allowing the virus particles to travel further and remain in the air longer.4 The necessary precautions for an airborne illness should be chosen over droplet precautions, especially when there is concern for an AGP.
Universal masking: Risks and benefits
The idea of universal masking has been debated extensively since the initial stages of the COVID-19 pandemic. According to public health authorities, significant exposure is defined as “face-to-face contact within 6 feet with a patient with symptomatic COVID-19” in the range of a few minutes up to 30 minutes.5 The researchers wrote in the New England Journal of Medicine that the chance of catching COVID-19 from a passing interaction in a public space is therefore minimal, and it may seem unnecessary to wear a mask at all times in public.
As reported in Science, randomized clinical studies performed on other viruses in the past have shown no added protection conferred by wearing a mask, though small sample sizes and noncompliance are limiting factors to their validity.6 On the contrary, mask wearing has been enforced in many parts of Asia, including Hong Kong and Singapore with promising results.5 Leung et al. stated in The Lancet that the lack of proof that masks are effective should not rule them as ineffective. Also, universal masking would reduce the stigma around symptomatic individuals covering their faces. It has become a cultural phenomenon in many southeast Asian countries and has been cited as one of the reasons for relatively successful containment in Singapore, South Korea, and Taiwan. The most important benefit of universal masking is protection attained by preventing spread from asymptomatic, mildly symptomatic, and presymptomatic carriers.7
In a study in the New England Journal of Medicine that estimated viral loads during various stages of COVID-19, researchers found that asymptomatic patients had similar viral loads to symptomatic patients, thereby suggesting high potential for transmission.8 Furthermore, numerous cases are being reported concerning the spread of illness from asymptomatic carriers.9-12 In an outbreak at a skilled nursing facility in Washington outlined in MMWR, 13 of 23 residents with positive test results were asymptomatic at the time of testing, and of those, 3 never developed any symptoms.12
Many hospitals are now embracing the policy of universal masking. A mask is a critical component of the personal protective equipment (PPE) clinicians need when caring for symptomatic patients with respiratory viral infections, in conjunction with a gown, gloves, and eye protection. Masking in this context is already part of routine operations in most hospitals. There are two scenarios in which there may be possible benefits. One scenario is the lower likelihood of transmission from asymptomatic and minimally symptomatic health care workers with COVID-19 to other providers and patients. The other less plausible benefit of universal masking among health care workers is that it may provide some protection in the possibility of caring for an unrecognized COVID-19 patient. However, universal masking should be coupled with other favorable practices like temperature checks and symptom screening on a daily basis to avail the maximum benefit from masking. Despite varied opinions on the outcomes of universal masking, this measure helps improve health care workers’ safety, psychological well-being, trust in their hospital, and decreases anxiety of acquiring the illness.
Efficacy of various types of masks
With the possibility of airborne transmission of the virus, are cloth masks as recommended by the CDC truly helpful in preventing infection? A study in the Journal of Medical Virology demonstrates 99.98%, 97.14%, and 95.15% efficacy for N95, surgical, and homemade masks, respectively, in blocking the avian influenza virus (comparable to coronavirus in size and physical characteristics). The homemade mask was created using one layer of polyester cloth and a four-layered kitchen filter paper.13
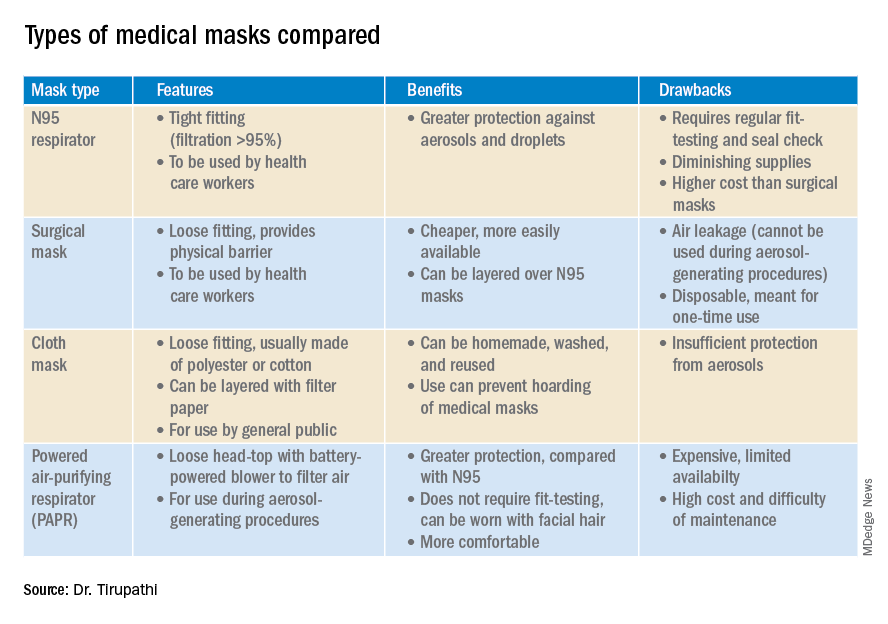
N95 masks (equivalent to FFP/P2 in European countries) are made of electrostatically charged polypropylene microfibers designed to filter particles measuring 100-300nm in diameter with 95% efficacy. A single SARS-CoV-2 molecule measures 125 nm approximately. N99 (FFP3) and N100 (P3) masks are also available, though not as widely used, with 99% and 99.7% efficacy respectively for the same size range. Though cloth masks are the clear-cut last resort for medical professionals, a few studies state no clinically proven difference in protection between surgical masks and N95 respirators.14,15 Even aerosolized droplets (< 5 mcm) were found to be blocked by surgical masks in a Nature Medicine study in which 4/10 subjects tested positive for coronavirus in exhaled breath samples without masks and 0/10 subjects with masks.16
On the contrary, an Annals of Internal Medicine study of four COVID-19 positive subjects that “neither surgical masks nor cloth masks effectively filtered SARS-CoV-2 during coughs of infected patients.” In fact, more contamination was found on the outer surface of the masks when compared to the inner surface, probably owing to the masks’ aerodynamic properties.17 Because of limitations present in the above-mentioned studies, further research is necessary to conclusively determine which types of masks are efficacious in preventing infection by the virus. In a scarcity of surgical masks and respirators for health care personnel, suboptimal masks can be of some use provided there is adherent use, minimal donning and doffing, and it is to be accompanied by adequate hand washing practices.14
In case of severe infections with high viral loads or patients undergoing aerosol-generating procedures, powered air-purifying respirators (PAPRs) also are advisable as they confer greater protection than N95 respirators, according to a study in the Annals of Work Exposures and Health. Despite being more comfortable for long-term use and accommodative of facial hair, their use is limited because of high cost and difficult maintenance.18 3-D printing also is being used to combat the current shortage of masks worldwide. However, a study from the International Journal of Oral & Maxillofacial Surgery reported that virologic testing for leakage between the two reusable components and contamination of the components themselves after one or multiple disinfection cycles is essential before application in real-life situations.19
Ongoing issues
WHO estimates a monthly requirement of nearly 90 million masks exclusively for health care workers to protect themselves against COVID-19.20 In spite of increasing the production rate by 40%, if the general public hoards masks and respirators, the results could be disastrous. Personal protective equipment is currently at 100 times the usual demand and 20 times the usual cost, with stocks backlogged by 4-6 months. The appropriate order of priority in distribution to health care professionals first, followed by those caring for infected patients is critical.20 In a survey conducted by the Association for Professionals in Infection Control and Epidemiology, results revealed that 48% of the U.S. health care facilities that responded were either out or nearly out of respirators as of March 25. 21
The gravest risk behind the universal masking policy is the likely depletion of medical resources.22 A possible solution to this issue could be to modify the policy to stagger the requirement based on the severity of community transmission in that area of residence. In the article appropriately titled “Rational use of face masks in the COVID-19 pandemic” published in The Lancet Respiratory Medicine, researchers described how the Chinese population was classified into moderate, low, and very-low risk of infection categories and advised to wear a surgical or disposable mask, disposable mask, and no mask respectively.23 This curbs widespread panic and eagerness by the general public to stock up on essential medical equipment when it may not even be necessary.
Reuse, extended use, and sterilization
Several studies have been conducted to identify the viability of the COVID-19 on various surfaces.24-25 The CDC and National Institute for Occupational Safety and Health (NIOSH) guidelines state that an N95 respirator can be used up to 8 hours with intermittent or continuous use, though this number is not fixed and heavily depends upon the extent of exposure, risk of contamination, and frequency of donning and doffing26,27. Though traditionally meant for single-time usage, after 8 hours, the mask can be decontaminated and reused. The CDC defines extended use as the “practice of wearing the same N95 respirator for repeated close-contact encounters with several patients, without removing the respirator between patient encounters.” Reuse is defined as “using the same N95 respirator for multiple encounters with patients but removing it (‘doffing’) after each encounter. The respirator is stored in between encounters to be put on again (‘donned’) prior to the next encounter with a patient.”
It has been established that extended use is more advisable than reuse given the lower risk of self-inoculation. Furthermore, health care professionals are urged to wear a cleanable face shield or disposable mask over the respirator to minimize contamination and practice diligent hand hygiene before and after handling the respirator. N95 respirators are to be discarded following aerosol-generating procedures or if they come in contact with blood, respiratory secretions, or bodily fluids. They should also be discarded in case of close contact with an infected patient or if they cause breathing difficulties to the wearer.27 This may not always be possible given the unprecedented shortage of PPE, hence decontamination techniques and repurposing are the need of the hour.
In Anesthesia & Analgesia, Naveen Nathan, MD, of Northwestern University, Chicago, recommends recycling four masks in a series, using one per day, keeping the mask in a dry, clean environment, and then repeating use of the first mask on the 5th day, the second on the 6th day, and so forth. This ensures clearance of the virus particles by the next use. Alternatively, respirators can be sterilized between uses by heating to 70º C (158º F) for 30 minutes. Liquid disinfectants such as alcohol and bleach as well as ultraviolet rays in sunlight tend to damage masks.28 Steam sterilization is the most commonly utilized technique in hospitals. Other methods, described by the N95/PPE Working Group, report include gamma irradiation at 20kGy (2MRad) for large-scale sterilization (though the facilities may not be widely available), vaporized hydrogen peroxide, ozone decontamination, ultraviolet germicidal irradiation, and ethylene oxide.29 Though a discussion on various considerations of decontamination techniques is out of the scope of this article, detailed guidelines have been published by the CDC30 and the COVID-19 Healthcare Coalition.30
Conclusion
A recent startling discovery reported on in Emerging Infectious Diseases suggests that the basic COVID-19 reproductive number (R0) is actually much higher than previously thought. Using expanded data, updated epidemiologic parameters, and the current outbreak dynamics in Wuhan, the team came to the conclusion that the R0 for the novel coronavirus is actually 5.7 (95% CI 3.8-8.9), compared with an initial estimate of 2.2-2.7.31 Concern for transmissibility demands heightened prevention strategies until more data evolves. The latest recommendation by the CDC regarding cloth masking in the public may help slow the progression of the pandemic. However, it is of paramount importance to keep in mind that masks alone are not enough to control the disease and must be coupled with other nonpharmacologic interventions such as social distancing, quarantining/isolation, and diligent hand hygiene.
Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro (Pa.) Hospitals. He also is the lead physician for antibiotic stewardship at these hospitals. Dr. Bharathidasan is a recent medical graduate from India with an interest in public health and community research; she plans to pursue residency training in the United States. Ms. Freshman is currently the regional director of infection prevention for WellSpan Health and has 35 years of experience in nursing. Dr. Palabindala is the medical director, utilization management and physician advisory services, at the University of Mississippi Medical Center, Jackson. He is an associate professor of medicine and academic hospitalist in the UMMC School of Medicine.
References
1. Centers for Disease Control and Prevention. Recommendation regarding the use of cloth face coverings.
2. World Health Organization. Modes of transmission of virus causing COVID-19 : implications for IPC precaution recommendations. Sci Br. 2020 Mar 29:1-3.
3. Santarpia JL et al. Transmission potential of SARS-CoV-2 in viral shedding observed at the University of Nebraska Medical Center. 2020 Mar 26. medRxiv. 2020;2020.03.23.20039446.
4. Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: Potential implications for reducing transmission of COVID-19. JAMA. 2020 Mar 26. doi: 10.1001/jama.2020.4756.
5. Klompas M et al. Universal masking in hospitals in the Covid-19 era. N Engl J Med. 2020 Apr 1. doi: 10.1056/NEJMp2006372.
6. Servick K. Would everyone wearing face masks help us slow the pandemic? Science 2020 Mar 28. doi: 10.1126/science.abb9371.
7. Leung CC et al. Mass masking in the COVID-19 epidemic: People need guidance. Lancet 2020 Mar 21;395(10228):945. doi: 10.1016/S0140-6736(20)30520-1.
8. Zou L et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020 Mar 19;382(12):1177-9.
9. Pan X et al. Asymptomatic cases in a family cluster with SARS-CoV-2 infection. Lancet Infect Dis. 2020 Apr;20(4):410-1.
10. Bai Y et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020 Feb 21;323(14):1406-7.
11. Wei WE et al. Presymptomatic transmission of SARS-CoV-2 – Singapore, Jan. 23–March 16, 2020. MMWR Morb Mortal Wkly Rep 2020;69:411-5.
12. Kimball A et al. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility – King County, Washington, March 2020. 2020 Apr 3. MMWR Morb Mortal Wkly Rep 2020;69:377-81.
13. Ma Q-X et al. Potential utilities of mask wearing and instant hand hygiene for fighting SARS-CoV-2. J Med Virol. 2020 Mar 31;10.1002/jmv.25805. doi: 10.1002/jmv.25805.
14. Abd-Elsayed A et al. Utility of substandard face mask options for health care workers during the COVID-19 pandemic. Anesth Analg. 2020 Mar 31;10.1213/ANE.0000000000004841. doi: 10.1213/ANE.0000000000004841.
15. Long Y et al. Effectiveness of N95 respirators versus surgical masks against influenza: A systematic review and meta-analysis. J Evid Based Med. 2020 Mar 13;10.1111/jebm.12381. doi: 10.1111/jebm.12381.
16. Leung NHL et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med. 2020 May;26(5):676-80.
17. Bae S et al. Effectiveness of surgical and cotton masks in blocking SARS-CoV-2: A controlled comparison in 4 patients. Ann Intern Med. 2020 Apr 6;M20-1342. doi: 10.7326/M20-1342.
18. Brosseau LM. Are powered air purifying respirators a solution for protecting healthcare workers from emerging aerosol-transmissible diseases? Ann Work Expo Health. 2020 Apr 30;64(4):339-41.
19. Swennen GRJ et al. Custom-made 3D-printed face masks in case of pandemic crisis situations with a lack of commercially available FFP2/3 masks. Int J Oral Maxillofac Surg. 2020 May;49(5):673-7.
20. Mahase E. Coronavirus: Global stocks of protective gear are depleted, with demand at “100 times” normal level, WHO warns. BMJ. 2020 Feb 10;368:m543. doi: 10.1136/bmj.m543.
21. National survey shows dire shortages of PPE, hand sanitizer across the U.S. 2020 Mar 27. Association for Professionals in Infection Control and Epidemiology (APIC) press briefing.
22. Wu HL et al. Facemask shortage and the novel coronavirus disease (COVID-19) outbreak: Reflections on public health measures. EClinicalMedicine. 2020 Apr 3:100329. doi: 10.1016/j.eclinm.2020.100329.
23. Feng S et al. Rational use of face masks in the COVID-19 pandemic. Lancet Respir Med. 2020 May;8(5):434-6.
24. Chin AWH et al. Stability of SARS-CoV-2 in different environmental. The Lancet Microbe. 2020 May 1;5247(20):2004973. doi. org/10.1016/S2666-5247(20)30003-3.
25. van Doremalen N et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020 Apr 16;382(16):1564-7.
26. NIOSH – Workplace Safety and Health Topics: Recommended guidance for extended use and limited reuse of n95 filtering facepiece respirators in healthcare settings.
27. Centers for Disease Control and Prevention. COVID-19 decontamination and reuse of filtering facepiece respirators. 2020 Apr 15.
28. Nathan N. Waste not, want not: The re-usability of N95 masks. Anesth Analg. 2020 Mar 31.doi: 10.1213/ane.0000000000004843.
29. European Centre for Disease Prevention and Control technical report. Cloth masks and mask sterilisation as options in case of shortage of surgical masks and respirators. 2020 Mar.
30. N95/PPE Working Group report. Evaluation of decontamination techniques for the reuse of N95 respirators. 2020 Apr 3;2:1-7.
31. Sanche Set al. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020 Jul. doi. org/10.3201/eid2607.200282.
A comprehensive review
A comprehensive review
On April 3, the Centers for Disease Control and Prevention issued an advisory that the general public wear cloth face masks when outside, particularly those residing in areas with significant severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) community transmission.1 Recent research reveals several factors related to the nature of the virus as well as the epidemiologic spread of the illness that may have led to this decision.
However, controversy still prevails whether this recommendation will alleviate or aggravate disease progression. With many hospitals across America lacking sufficient personal protective equipment (PPE) and scrambling for supplies, universal masking may create more chaos, especially with certain states imposing monetary fines on individuals spotted outdoors without a mask. With new information being discovered each day about COVID-19, it is more imperative than ever to update existing strategies and formulate more effective methods to flatten the curve.
Airborne vs. droplet transmission
According to a scientific brief released by the World Health Organization, there have been studies with mixed evidence and opinions regarding the presence of COVID-19 ribonucleic acid (RNA) in air samples.2 In medRxiv, Santarpia et al., from the University of Nebraska Medical Center, Omaha, detected viral RNA in samples taken from beneath a patient’s bed and from a window ledge, both areas in which neither the patient nor health care personnel had any direct contact. They also found that 66.7% of air samples taken from a hospital hallway carried virus-containing particles.3 It is worth noting that certain aerosol-generating procedures (AGP) may increase the likelihood of airborne dissemination. Whether airborne transmission is a major mode of COVID-19 spread in the community and routine clinical settings (with no aerosol-generating procedures) is still a debatable question without a definitive answer.
We should consider the epidemiology of COVID-19 thus far in the pandemic to determine if transmission patterns are more consistent with that of other common respiratory viral pathogens or more consistent with that of the agents we classically consider to be transmitted by the airborne route (measles, varicella zoster virus, and Mycobacterium tuberculosis). The attack rates in various settings (household, health care, and the public) as well as the expected number of secondary cases from a single infected individual in a susceptible population (R0) are more consistent with those of a droplet spread pathogen.
For measles, the R0 is 12-18, and the secondary household attack rates are ≥ 90%. In case of the varicella zoster virus, the R0 is ~10, and the secondary household attack rate is 85%. The R0 for pulmonary tuberculosis is up to 10 (per year) and the secondary household attack rate has been reported to be >50%. With COVID-19, the R0 appears to be around 2.5-3 and secondary household attack rates are ~ 10% from data available so far, similar to that of influenza viruses. This discrepancy suggests that droplet transmission may be more likely. The dichotomy of airborne versus droplet mode of spread may be better described as a continuum, as pointed out in a recent article in the JAMA. Infectious droplets form turbulent gas clouds allowing the virus particles to travel further and remain in the air longer.4 The necessary precautions for an airborne illness should be chosen over droplet precautions, especially when there is concern for an AGP.
Universal masking: Risks and benefits
The idea of universal masking has been debated extensively since the initial stages of the COVID-19 pandemic. According to public health authorities, significant exposure is defined as “face-to-face contact within 6 feet with a patient with symptomatic COVID-19” in the range of a few minutes up to 30 minutes.5 The researchers wrote in the New England Journal of Medicine that the chance of catching COVID-19 from a passing interaction in a public space is therefore minimal, and it may seem unnecessary to wear a mask at all times in public.
As reported in Science, randomized clinical studies performed on other viruses in the past have shown no added protection conferred by wearing a mask, though small sample sizes and noncompliance are limiting factors to their validity.6 On the contrary, mask wearing has been enforced in many parts of Asia, including Hong Kong and Singapore with promising results.5 Leung et al. stated in The Lancet that the lack of proof that masks are effective should not rule them as ineffective. Also, universal masking would reduce the stigma around symptomatic individuals covering their faces. It has become a cultural phenomenon in many southeast Asian countries and has been cited as one of the reasons for relatively successful containment in Singapore, South Korea, and Taiwan. The most important benefit of universal masking is protection attained by preventing spread from asymptomatic, mildly symptomatic, and presymptomatic carriers.7
In a study in the New England Journal of Medicine that estimated viral loads during various stages of COVID-19, researchers found that asymptomatic patients had similar viral loads to symptomatic patients, thereby suggesting high potential for transmission.8 Furthermore, numerous cases are being reported concerning the spread of illness from asymptomatic carriers.9-12 In an outbreak at a skilled nursing facility in Washington outlined in MMWR, 13 of 23 residents with positive test results were asymptomatic at the time of testing, and of those, 3 never developed any symptoms.12
Many hospitals are now embracing the policy of universal masking. A mask is a critical component of the personal protective equipment (PPE) clinicians need when caring for symptomatic patients with respiratory viral infections, in conjunction with a gown, gloves, and eye protection. Masking in this context is already part of routine operations in most hospitals. There are two scenarios in which there may be possible benefits. One scenario is the lower likelihood of transmission from asymptomatic and minimally symptomatic health care workers with COVID-19 to other providers and patients. The other less plausible benefit of universal masking among health care workers is that it may provide some protection in the possibility of caring for an unrecognized COVID-19 patient. However, universal masking should be coupled with other favorable practices like temperature checks and symptom screening on a daily basis to avail the maximum benefit from masking. Despite varied opinions on the outcomes of universal masking, this measure helps improve health care workers’ safety, psychological well-being, trust in their hospital, and decreases anxiety of acquiring the illness.
Efficacy of various types of masks
With the possibility of airborne transmission of the virus, are cloth masks as recommended by the CDC truly helpful in preventing infection? A study in the Journal of Medical Virology demonstrates 99.98%, 97.14%, and 95.15% efficacy for N95, surgical, and homemade masks, respectively, in blocking the avian influenza virus (comparable to coronavirus in size and physical characteristics). The homemade mask was created using one layer of polyester cloth and a four-layered kitchen filter paper.13

N95 masks (equivalent to FFP/P2 in European countries) are made of electrostatically charged polypropylene microfibers designed to filter particles measuring 100-300nm in diameter with 95% efficacy. A single SARS-CoV-2 molecule measures 125 nm approximately. N99 (FFP3) and N100 (P3) masks are also available, though not as widely used, with 99% and 99.7% efficacy respectively for the same size range. Though cloth masks are the clear-cut last resort for medical professionals, a few studies state no clinically proven difference in protection between surgical masks and N95 respirators.14,15 Even aerosolized droplets (< 5 mcm) were found to be blocked by surgical masks in a Nature Medicine study in which 4/10 subjects tested positive for coronavirus in exhaled breath samples without masks and 0/10 subjects with masks.16
On the contrary, an Annals of Internal Medicine study of four COVID-19 positive subjects that “neither surgical masks nor cloth masks effectively filtered SARS-CoV-2 during coughs of infected patients.” In fact, more contamination was found on the outer surface of the masks when compared to the inner surface, probably owing to the masks’ aerodynamic properties.17 Because of limitations present in the above-mentioned studies, further research is necessary to conclusively determine which types of masks are efficacious in preventing infection by the virus. In a scarcity of surgical masks and respirators for health care personnel, suboptimal masks can be of some use provided there is adherent use, minimal donning and doffing, and it is to be accompanied by adequate hand washing practices.14
In case of severe infections with high viral loads or patients undergoing aerosol-generating procedures, powered air-purifying respirators (PAPRs) also are advisable as they confer greater protection than N95 respirators, according to a study in the Annals of Work Exposures and Health. Despite being more comfortable for long-term use and accommodative of facial hair, their use is limited because of high cost and difficult maintenance.18 3-D printing also is being used to combat the current shortage of masks worldwide. However, a study from the International Journal of Oral & Maxillofacial Surgery reported that virologic testing for leakage between the two reusable components and contamination of the components themselves after one or multiple disinfection cycles is essential before application in real-life situations.19
Ongoing issues
WHO estimates a monthly requirement of nearly 90 million masks exclusively for health care workers to protect themselves against COVID-19.20 In spite of increasing the production rate by 40%, if the general public hoards masks and respirators, the results could be disastrous. Personal protective equipment is currently at 100 times the usual demand and 20 times the usual cost, with stocks backlogged by 4-6 months. The appropriate order of priority in distribution to health care professionals first, followed by those caring for infected patients is critical.20 In a survey conducted by the Association for Professionals in Infection Control and Epidemiology, results revealed that 48% of the U.S. health care facilities that responded were either out or nearly out of respirators as of March 25. 21
The gravest risk behind the universal masking policy is the likely depletion of medical resources.22 A possible solution to this issue could be to modify the policy to stagger the requirement based on the severity of community transmission in that area of residence. In the article appropriately titled “Rational use of face masks in the COVID-19 pandemic” published in The Lancet Respiratory Medicine, researchers described how the Chinese population was classified into moderate, low, and very-low risk of infection categories and advised to wear a surgical or disposable mask, disposable mask, and no mask respectively.23 This curbs widespread panic and eagerness by the general public to stock up on essential medical equipment when it may not even be necessary.
Reuse, extended use, and sterilization
Several studies have been conducted to identify the viability of the COVID-19 on various surfaces.24-25 The CDC and National Institute for Occupational Safety and Health (NIOSH) guidelines state that an N95 respirator can be used up to 8 hours with intermittent or continuous use, though this number is not fixed and heavily depends upon the extent of exposure, risk of contamination, and frequency of donning and doffing26,27. Though traditionally meant for single-time usage, after 8 hours, the mask can be decontaminated and reused. The CDC defines extended use as the “practice of wearing the same N95 respirator for repeated close-contact encounters with several patients, without removing the respirator between patient encounters.” Reuse is defined as “using the same N95 respirator for multiple encounters with patients but removing it (‘doffing’) after each encounter. The respirator is stored in between encounters to be put on again (‘donned’) prior to the next encounter with a patient.”
It has been established that extended use is more advisable than reuse given the lower risk of self-inoculation. Furthermore, health care professionals are urged to wear a cleanable face shield or disposable mask over the respirator to minimize contamination and practice diligent hand hygiene before and after handling the respirator. N95 respirators are to be discarded following aerosol-generating procedures or if they come in contact with blood, respiratory secretions, or bodily fluids. They should also be discarded in case of close contact with an infected patient or if they cause breathing difficulties to the wearer.27 This may not always be possible given the unprecedented shortage of PPE, hence decontamination techniques and repurposing are the need of the hour.
In Anesthesia & Analgesia, Naveen Nathan, MD, of Northwestern University, Chicago, recommends recycling four masks in a series, using one per day, keeping the mask in a dry, clean environment, and then repeating use of the first mask on the 5th day, the second on the 6th day, and so forth. This ensures clearance of the virus particles by the next use. Alternatively, respirators can be sterilized between uses by heating to 70º C (158º F) for 30 minutes. Liquid disinfectants such as alcohol and bleach as well as ultraviolet rays in sunlight tend to damage masks.28 Steam sterilization is the most commonly utilized technique in hospitals. Other methods, described by the N95/PPE Working Group, report include gamma irradiation at 20kGy (2MRad) for large-scale sterilization (though the facilities may not be widely available), vaporized hydrogen peroxide, ozone decontamination, ultraviolet germicidal irradiation, and ethylene oxide.29 Though a discussion on various considerations of decontamination techniques is out of the scope of this article, detailed guidelines have been published by the CDC30 and the COVID-19 Healthcare Coalition.30
Conclusion
A recent startling discovery reported on in Emerging Infectious Diseases suggests that the basic COVID-19 reproductive number (R0) is actually much higher than previously thought. Using expanded data, updated epidemiologic parameters, and the current outbreak dynamics in Wuhan, the team came to the conclusion that the R0 for the novel coronavirus is actually 5.7 (95% CI 3.8-8.9), compared with an initial estimate of 2.2-2.7.31 Concern for transmissibility demands heightened prevention strategies until more data evolves. The latest recommendation by the CDC regarding cloth masking in the public may help slow the progression of the pandemic. However, it is of paramount importance to keep in mind that masks alone are not enough to control the disease and must be coupled with other nonpharmacologic interventions such as social distancing, quarantining/isolation, and diligent hand hygiene.
Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro (Pa.) Hospitals. He also is the lead physician for antibiotic stewardship at these hospitals. Dr. Bharathidasan is a recent medical graduate from India with an interest in public health and community research; she plans to pursue residency training in the United States. Ms. Freshman is currently the regional director of infection prevention for WellSpan Health and has 35 years of experience in nursing. Dr. Palabindala is the medical director, utilization management and physician advisory services, at the University of Mississippi Medical Center, Jackson. He is an associate professor of medicine and academic hospitalist in the UMMC School of Medicine.
References
1. Centers for Disease Control and Prevention. Recommendation regarding the use of cloth face coverings.
2. World Health Organization. Modes of transmission of virus causing COVID-19 : implications for IPC precaution recommendations. Sci Br. 2020 Mar 29:1-3.
3. Santarpia JL et al. Transmission potential of SARS-CoV-2 in viral shedding observed at the University of Nebraska Medical Center. 2020 Mar 26. medRxiv. 2020;2020.03.23.20039446.
4. Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: Potential implications for reducing transmission of COVID-19. JAMA. 2020 Mar 26. doi: 10.1001/jama.2020.4756.
5. Klompas M et al. Universal masking in hospitals in the Covid-19 era. N Engl J Med. 2020 Apr 1. doi: 10.1056/NEJMp2006372.
6. Servick K. Would everyone wearing face masks help us slow the pandemic? Science 2020 Mar 28. doi: 10.1126/science.abb9371.
7. Leung CC et al. Mass masking in the COVID-19 epidemic: People need guidance. Lancet 2020 Mar 21;395(10228):945. doi: 10.1016/S0140-6736(20)30520-1.
8. Zou L et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020 Mar 19;382(12):1177-9.
9. Pan X et al. Asymptomatic cases in a family cluster with SARS-CoV-2 infection. Lancet Infect Dis. 2020 Apr;20(4):410-1.
10. Bai Y et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020 Feb 21;323(14):1406-7.
11. Wei WE et al. Presymptomatic transmission of SARS-CoV-2 – Singapore, Jan. 23–March 16, 2020. MMWR Morb Mortal Wkly Rep 2020;69:411-5.
12. Kimball A et al. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility – King County, Washington, March 2020. 2020 Apr 3. MMWR Morb Mortal Wkly Rep 2020;69:377-81.
13. Ma Q-X et al. Potential utilities of mask wearing and instant hand hygiene for fighting SARS-CoV-2. J Med Virol. 2020 Mar 31;10.1002/jmv.25805. doi: 10.1002/jmv.25805.
14. Abd-Elsayed A et al. Utility of substandard face mask options for health care workers during the COVID-19 pandemic. Anesth Analg. 2020 Mar 31;10.1213/ANE.0000000000004841. doi: 10.1213/ANE.0000000000004841.
15. Long Y et al. Effectiveness of N95 respirators versus surgical masks against influenza: A systematic review and meta-analysis. J Evid Based Med. 2020 Mar 13;10.1111/jebm.12381. doi: 10.1111/jebm.12381.
16. Leung NHL et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med. 2020 May;26(5):676-80.
17. Bae S et al. Effectiveness of surgical and cotton masks in blocking SARS-CoV-2: A controlled comparison in 4 patients. Ann Intern Med. 2020 Apr 6;M20-1342. doi: 10.7326/M20-1342.
18. Brosseau LM. Are powered air purifying respirators a solution for protecting healthcare workers from emerging aerosol-transmissible diseases? Ann Work Expo Health. 2020 Apr 30;64(4):339-41.
19. Swennen GRJ et al. Custom-made 3D-printed face masks in case of pandemic crisis situations with a lack of commercially available FFP2/3 masks. Int J Oral Maxillofac Surg. 2020 May;49(5):673-7.
20. Mahase E. Coronavirus: Global stocks of protective gear are depleted, with demand at “100 times” normal level, WHO warns. BMJ. 2020 Feb 10;368:m543. doi: 10.1136/bmj.m543.
21. National survey shows dire shortages of PPE, hand sanitizer across the U.S. 2020 Mar 27. Association for Professionals in Infection Control and Epidemiology (APIC) press briefing.
22. Wu HL et al. Facemask shortage and the novel coronavirus disease (COVID-19) outbreak: Reflections on public health measures. EClinicalMedicine. 2020 Apr 3:100329. doi: 10.1016/j.eclinm.2020.100329.
23. Feng S et al. Rational use of face masks in the COVID-19 pandemic. Lancet Respir Med. 2020 May;8(5):434-6.
24. Chin AWH et al. Stability of SARS-CoV-2 in different environmental. The Lancet Microbe. 2020 May 1;5247(20):2004973. doi. org/10.1016/S2666-5247(20)30003-3.
25. van Doremalen N et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020 Apr 16;382(16):1564-7.
26. NIOSH – Workplace Safety and Health Topics: Recommended guidance for extended use and limited reuse of n95 filtering facepiece respirators in healthcare settings.
27. Centers for Disease Control and Prevention. COVID-19 decontamination and reuse of filtering facepiece respirators. 2020 Apr 15.
28. Nathan N. Waste not, want not: The re-usability of N95 masks. Anesth Analg. 2020 Mar 31.doi: 10.1213/ane.0000000000004843.
29. European Centre for Disease Prevention and Control technical report. Cloth masks and mask sterilisation as options in case of shortage of surgical masks and respirators. 2020 Mar.
30. N95/PPE Working Group report. Evaluation of decontamination techniques for the reuse of N95 respirators. 2020 Apr 3;2:1-7.
31. Sanche Set al. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020 Jul. doi. org/10.3201/eid2607.200282.
On April 3, the Centers for Disease Control and Prevention issued an advisory that the general public wear cloth face masks when outside, particularly those residing in areas with significant severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) community transmission.1 Recent research reveals several factors related to the nature of the virus as well as the epidemiologic spread of the illness that may have led to this decision.
However, controversy still prevails whether this recommendation will alleviate or aggravate disease progression. With many hospitals across America lacking sufficient personal protective equipment (PPE) and scrambling for supplies, universal masking may create more chaos, especially with certain states imposing monetary fines on individuals spotted outdoors without a mask. With new information being discovered each day about COVID-19, it is more imperative than ever to update existing strategies and formulate more effective methods to flatten the curve.
Airborne vs. droplet transmission
According to a scientific brief released by the World Health Organization, there have been studies with mixed evidence and opinions regarding the presence of COVID-19 ribonucleic acid (RNA) in air samples.2 In medRxiv, Santarpia et al., from the University of Nebraska Medical Center, Omaha, detected viral RNA in samples taken from beneath a patient’s bed and from a window ledge, both areas in which neither the patient nor health care personnel had any direct contact. They also found that 66.7% of air samples taken from a hospital hallway carried virus-containing particles.3 It is worth noting that certain aerosol-generating procedures (AGP) may increase the likelihood of airborne dissemination. Whether airborne transmission is a major mode of COVID-19 spread in the community and routine clinical settings (with no aerosol-generating procedures) is still a debatable question without a definitive answer.
We should consider the epidemiology of COVID-19 thus far in the pandemic to determine if transmission patterns are more consistent with that of other common respiratory viral pathogens or more consistent with that of the agents we classically consider to be transmitted by the airborne route (measles, varicella zoster virus, and Mycobacterium tuberculosis). The attack rates in various settings (household, health care, and the public) as well as the expected number of secondary cases from a single infected individual in a susceptible population (R0) are more consistent with those of a droplet spread pathogen.
For measles, the R0 is 12-18, and the secondary household attack rates are ≥ 90%. In case of the varicella zoster virus, the R0 is ~10, and the secondary household attack rate is 85%. The R0 for pulmonary tuberculosis is up to 10 (per year) and the secondary household attack rate has been reported to be >50%. With COVID-19, the R0 appears to be around 2.5-3 and secondary household attack rates are ~ 10% from data available so far, similar to that of influenza viruses. This discrepancy suggests that droplet transmission may be more likely. The dichotomy of airborne versus droplet mode of spread may be better described as a continuum, as pointed out in a recent article in the JAMA. Infectious droplets form turbulent gas clouds allowing the virus particles to travel further and remain in the air longer.4 The necessary precautions for an airborne illness should be chosen over droplet precautions, especially when there is concern for an AGP.
Universal masking: Risks and benefits
The idea of universal masking has been debated extensively since the initial stages of the COVID-19 pandemic. According to public health authorities, significant exposure is defined as “face-to-face contact within 6 feet with a patient with symptomatic COVID-19” in the range of a few minutes up to 30 minutes.5 The researchers wrote in the New England Journal of Medicine that the chance of catching COVID-19 from a passing interaction in a public space is therefore minimal, and it may seem unnecessary to wear a mask at all times in public.
As reported in Science, randomized clinical studies performed on other viruses in the past have shown no added protection conferred by wearing a mask, though small sample sizes and noncompliance are limiting factors to their validity.6 On the contrary, mask wearing has been enforced in many parts of Asia, including Hong Kong and Singapore with promising results.5 Leung et al. stated in The Lancet that the lack of proof that masks are effective should not rule them as ineffective. Also, universal masking would reduce the stigma around symptomatic individuals covering their faces. It has become a cultural phenomenon in many southeast Asian countries and has been cited as one of the reasons for relatively successful containment in Singapore, South Korea, and Taiwan. The most important benefit of universal masking is protection attained by preventing spread from asymptomatic, mildly symptomatic, and presymptomatic carriers.7
In a study in the New England Journal of Medicine that estimated viral loads during various stages of COVID-19, researchers found that asymptomatic patients had similar viral loads to symptomatic patients, thereby suggesting high potential for transmission.8 Furthermore, numerous cases are being reported concerning the spread of illness from asymptomatic carriers.9-12 In an outbreak at a skilled nursing facility in Washington outlined in MMWR, 13 of 23 residents with positive test results were asymptomatic at the time of testing, and of those, 3 never developed any symptoms.12
Many hospitals are now embracing the policy of universal masking. A mask is a critical component of the personal protective equipment (PPE) clinicians need when caring for symptomatic patients with respiratory viral infections, in conjunction with a gown, gloves, and eye protection. Masking in this context is already part of routine operations in most hospitals. There are two scenarios in which there may be possible benefits. One scenario is the lower likelihood of transmission from asymptomatic and minimally symptomatic health care workers with COVID-19 to other providers and patients. The other less plausible benefit of universal masking among health care workers is that it may provide some protection in the possibility of caring for an unrecognized COVID-19 patient. However, universal masking should be coupled with other favorable practices like temperature checks and symptom screening on a daily basis to avail the maximum benefit from masking. Despite varied opinions on the outcomes of universal masking, this measure helps improve health care workers’ safety, psychological well-being, trust in their hospital, and decreases anxiety of acquiring the illness.
Efficacy of various types of masks
With the possibility of airborne transmission of the virus, are cloth masks as recommended by the CDC truly helpful in preventing infection? A study in the Journal of Medical Virology demonstrates 99.98%, 97.14%, and 95.15% efficacy for N95, surgical, and homemade masks, respectively, in blocking the avian influenza virus (comparable to coronavirus in size and physical characteristics). The homemade mask was created using one layer of polyester cloth and a four-layered kitchen filter paper.13

N95 masks (equivalent to FFP/P2 in European countries) are made of electrostatically charged polypropylene microfibers designed to filter particles measuring 100-300nm in diameter with 95% efficacy. A single SARS-CoV-2 molecule measures 125 nm approximately. N99 (FFP3) and N100 (P3) masks are also available, though not as widely used, with 99% and 99.7% efficacy respectively for the same size range. Though cloth masks are the clear-cut last resort for medical professionals, a few studies state no clinically proven difference in protection between surgical masks and N95 respirators.14,15 Even aerosolized droplets (< 5 mcm) were found to be blocked by surgical masks in a Nature Medicine study in which 4/10 subjects tested positive for coronavirus in exhaled breath samples without masks and 0/10 subjects with masks.16
On the contrary, an Annals of Internal Medicine study of four COVID-19 positive subjects that “neither surgical masks nor cloth masks effectively filtered SARS-CoV-2 during coughs of infected patients.” In fact, more contamination was found on the outer surface of the masks when compared to the inner surface, probably owing to the masks’ aerodynamic properties.17 Because of limitations present in the above-mentioned studies, further research is necessary to conclusively determine which types of masks are efficacious in preventing infection by the virus. In a scarcity of surgical masks and respirators for health care personnel, suboptimal masks can be of some use provided there is adherent use, minimal donning and doffing, and it is to be accompanied by adequate hand washing practices.14
In case of severe infections with high viral loads or patients undergoing aerosol-generating procedures, powered air-purifying respirators (PAPRs) also are advisable as they confer greater protection than N95 respirators, according to a study in the Annals of Work Exposures and Health. Despite being more comfortable for long-term use and accommodative of facial hair, their use is limited because of high cost and difficult maintenance.18 3-D printing also is being used to combat the current shortage of masks worldwide. However, a study from the International Journal of Oral & Maxillofacial Surgery reported that virologic testing for leakage between the two reusable components and contamination of the components themselves after one or multiple disinfection cycles is essential before application in real-life situations.19
Ongoing issues
WHO estimates a monthly requirement of nearly 90 million masks exclusively for health care workers to protect themselves against COVID-19.20 In spite of increasing the production rate by 40%, if the general public hoards masks and respirators, the results could be disastrous. Personal protective equipment is currently at 100 times the usual demand and 20 times the usual cost, with stocks backlogged by 4-6 months. The appropriate order of priority in distribution to health care professionals first, followed by those caring for infected patients is critical.20 In a survey conducted by the Association for Professionals in Infection Control and Epidemiology, results revealed that 48% of the U.S. health care facilities that responded were either out or nearly out of respirators as of March 25. 21
The gravest risk behind the universal masking policy is the likely depletion of medical resources.22 A possible solution to this issue could be to modify the policy to stagger the requirement based on the severity of community transmission in that area of residence. In the article appropriately titled “Rational use of face masks in the COVID-19 pandemic” published in The Lancet Respiratory Medicine, researchers described how the Chinese population was classified into moderate, low, and very-low risk of infection categories and advised to wear a surgical or disposable mask, disposable mask, and no mask respectively.23 This curbs widespread panic and eagerness by the general public to stock up on essential medical equipment when it may not even be necessary.
Reuse, extended use, and sterilization
Several studies have been conducted to identify the viability of the COVID-19 on various surfaces.24-25 The CDC and National Institute for Occupational Safety and Health (NIOSH) guidelines state that an N95 respirator can be used up to 8 hours with intermittent or continuous use, though this number is not fixed and heavily depends upon the extent of exposure, risk of contamination, and frequency of donning and doffing26,27. Though traditionally meant for single-time usage, after 8 hours, the mask can be decontaminated and reused. The CDC defines extended use as the “practice of wearing the same N95 respirator for repeated close-contact encounters with several patients, without removing the respirator between patient encounters.” Reuse is defined as “using the same N95 respirator for multiple encounters with patients but removing it (‘doffing’) after each encounter. The respirator is stored in between encounters to be put on again (‘donned’) prior to the next encounter with a patient.”
It has been established that extended use is more advisable than reuse given the lower risk of self-inoculation. Furthermore, health care professionals are urged to wear a cleanable face shield or disposable mask over the respirator to minimize contamination and practice diligent hand hygiene before and after handling the respirator. N95 respirators are to be discarded following aerosol-generating procedures or if they come in contact with blood, respiratory secretions, or bodily fluids. They should also be discarded in case of close contact with an infected patient or if they cause breathing difficulties to the wearer.27 This may not always be possible given the unprecedented shortage of PPE, hence decontamination techniques and repurposing are the need of the hour.
In Anesthesia & Analgesia, Naveen Nathan, MD, of Northwestern University, Chicago, recommends recycling four masks in a series, using one per day, keeping the mask in a dry, clean environment, and then repeating use of the first mask on the 5th day, the second on the 6th day, and so forth. This ensures clearance of the virus particles by the next use. Alternatively, respirators can be sterilized between uses by heating to 70º C (158º F) for 30 minutes. Liquid disinfectants such as alcohol and bleach as well as ultraviolet rays in sunlight tend to damage masks.28 Steam sterilization is the most commonly utilized technique in hospitals. Other methods, described by the N95/PPE Working Group, report include gamma irradiation at 20kGy (2MRad) for large-scale sterilization (though the facilities may not be widely available), vaporized hydrogen peroxide, ozone decontamination, ultraviolet germicidal irradiation, and ethylene oxide.29 Though a discussion on various considerations of decontamination techniques is out of the scope of this article, detailed guidelines have been published by the CDC30 and the COVID-19 Healthcare Coalition.30
Conclusion
A recent startling discovery reported on in Emerging Infectious Diseases suggests that the basic COVID-19 reproductive number (R0) is actually much higher than previously thought. Using expanded data, updated epidemiologic parameters, and the current outbreak dynamics in Wuhan, the team came to the conclusion that the R0 for the novel coronavirus is actually 5.7 (95% CI 3.8-8.9), compared with an initial estimate of 2.2-2.7.31 Concern for transmissibility demands heightened prevention strategies until more data evolves. The latest recommendation by the CDC regarding cloth masking in the public may help slow the progression of the pandemic. However, it is of paramount importance to keep in mind that masks alone are not enough to control the disease and must be coupled with other nonpharmacologic interventions such as social distancing, quarantining/isolation, and diligent hand hygiene.
Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro (Pa.) Hospitals. He also is the lead physician for antibiotic stewardship at these hospitals. Dr. Bharathidasan is a recent medical graduate from India with an interest in public health and community research; she plans to pursue residency training in the United States. Ms. Freshman is currently the regional director of infection prevention for WellSpan Health and has 35 years of experience in nursing. Dr. Palabindala is the medical director, utilization management and physician advisory services, at the University of Mississippi Medical Center, Jackson. He is an associate professor of medicine and academic hospitalist in the UMMC School of Medicine.
References
1. Centers for Disease Control and Prevention. Recommendation regarding the use of cloth face coverings.
2. World Health Organization. Modes of transmission of virus causing COVID-19 : implications for IPC precaution recommendations. Sci Br. 2020 Mar 29:1-3.
3. Santarpia JL et al. Transmission potential of SARS-CoV-2 in viral shedding observed at the University of Nebraska Medical Center. 2020 Mar 26. medRxiv. 2020;2020.03.23.20039446.
4. Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: Potential implications for reducing transmission of COVID-19. JAMA. 2020 Mar 26. doi: 10.1001/jama.2020.4756.
5. Klompas M et al. Universal masking in hospitals in the Covid-19 era. N Engl J Med. 2020 Apr 1. doi: 10.1056/NEJMp2006372.
6. Servick K. Would everyone wearing face masks help us slow the pandemic? Science 2020 Mar 28. doi: 10.1126/science.abb9371.
7. Leung CC et al. Mass masking in the COVID-19 epidemic: People need guidance. Lancet 2020 Mar 21;395(10228):945. doi: 10.1016/S0140-6736(20)30520-1.
8. Zou L et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020 Mar 19;382(12):1177-9.
9. Pan X et al. Asymptomatic cases in a family cluster with SARS-CoV-2 infection. Lancet Infect Dis. 2020 Apr;20(4):410-1.
10. Bai Y et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020 Feb 21;323(14):1406-7.
11. Wei WE et al. Presymptomatic transmission of SARS-CoV-2 – Singapore, Jan. 23–March 16, 2020. MMWR Morb Mortal Wkly Rep 2020;69:411-5.
12. Kimball A et al. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility – King County, Washington, March 2020. 2020 Apr 3. MMWR Morb Mortal Wkly Rep 2020;69:377-81.
13. Ma Q-X et al. Potential utilities of mask wearing and instant hand hygiene for fighting SARS-CoV-2. J Med Virol. 2020 Mar 31;10.1002/jmv.25805. doi: 10.1002/jmv.25805.
14. Abd-Elsayed A et al. Utility of substandard face mask options for health care workers during the COVID-19 pandemic. Anesth Analg. 2020 Mar 31;10.1213/ANE.0000000000004841. doi: 10.1213/ANE.0000000000004841.
15. Long Y et al. Effectiveness of N95 respirators versus surgical masks against influenza: A systematic review and meta-analysis. J Evid Based Med. 2020 Mar 13;10.1111/jebm.12381. doi: 10.1111/jebm.12381.
16. Leung NHL et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med. 2020 May;26(5):676-80.
17. Bae S et al. Effectiveness of surgical and cotton masks in blocking SARS-CoV-2: A controlled comparison in 4 patients. Ann Intern Med. 2020 Apr 6;M20-1342. doi: 10.7326/M20-1342.
18. Brosseau LM. Are powered air purifying respirators a solution for protecting healthcare workers from emerging aerosol-transmissible diseases? Ann Work Expo Health. 2020 Apr 30;64(4):339-41.
19. Swennen GRJ et al. Custom-made 3D-printed face masks in case of pandemic crisis situations with a lack of commercially available FFP2/3 masks. Int J Oral Maxillofac Surg. 2020 May;49(5):673-7.
20. Mahase E. Coronavirus: Global stocks of protective gear are depleted, with demand at “100 times” normal level, WHO warns. BMJ. 2020 Feb 10;368:m543. doi: 10.1136/bmj.m543.
21. National survey shows dire shortages of PPE, hand sanitizer across the U.S. 2020 Mar 27. Association for Professionals in Infection Control and Epidemiology (APIC) press briefing.
22. Wu HL et al. Facemask shortage and the novel coronavirus disease (COVID-19) outbreak: Reflections on public health measures. EClinicalMedicine. 2020 Apr 3:100329. doi: 10.1016/j.eclinm.2020.100329.
23. Feng S et al. Rational use of face masks in the COVID-19 pandemic. Lancet Respir Med. 2020 May;8(5):434-6.
24. Chin AWH et al. Stability of SARS-CoV-2 in different environmental. The Lancet Microbe. 2020 May 1;5247(20):2004973. doi. org/10.1016/S2666-5247(20)30003-3.
25. van Doremalen N et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020 Apr 16;382(16):1564-7.
26. NIOSH – Workplace Safety and Health Topics: Recommended guidance for extended use and limited reuse of n95 filtering facepiece respirators in healthcare settings.
27. Centers for Disease Control and Prevention. COVID-19 decontamination and reuse of filtering facepiece respirators. 2020 Apr 15.
28. Nathan N. Waste not, want not: The re-usability of N95 masks. Anesth Analg. 2020 Mar 31.doi: 10.1213/ane.0000000000004843.
29. European Centre for Disease Prevention and Control technical report. Cloth masks and mask sterilisation as options in case of shortage of surgical masks and respirators. 2020 Mar.
30. N95/PPE Working Group report. Evaluation of decontamination techniques for the reuse of N95 respirators. 2020 Apr 3;2:1-7.
31. Sanche Set al. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020 Jul. doi. org/10.3201/eid2607.200282.
Today’s top news highlights: COVID-19 in kids, addiction-related suicide
Here are the stories our MDedge editors across specialties think you need to know about today:
COVID-19 in kids
Children and young adults in all age groups can develop severe illess after SARS-CoV-2 infection, but infants and teens are most likely to be hospitalized, according to retrospective data from 177 children and young adults at a single center. “One patient had features consistent with the recently emerged Kawasaki disease–like presentation with hyperinflammatory state, hypotension, and profound myocardial depression,” Roberta L. DeBiasi, MD, of Children’s National Hospital, Washington, and colleagues reported in the Journal of Pediatrics. READ MORE
Avoiding ageism in COVID resource allocation
The American Geriatrics Society has issued new policy recommendations for resource allocation during the COVID-19 pandemic that are aimed at protecting seniors for ageism. When allocating scarce resources in an emergency, officials should equally weigh in-hospital survival and severe comorbidities contributing to short-term mortality, the group wrote. “Age per se should never be used as a means for a categorical exclusion from therapeutic interventions that represent the standard of care. ... Likewise, specific age-based cutoffs should not be used in resource allocation strategies,” AGS officials wrote in the statement. READ MORE
Preventing addiction-related suicide
Individuals with substance use disorders are at a significant risk for suicide, but there have been few evidence-based options for their treatment. Now a single intervention is showing promise for this high-risk group. In a large, multicenter randomized effectiveness study, a single 3-hour-long group psychosocial intervention resulted in significantly improved knowledge and attitudes regarding suicide that persisted at 6 months of follow-up. The intervention to prevent future suicide was designed specifically for patients who were in intensive outpatient programs for addiction treatment. “We’ve shown that suicide prevention in intensive outpatient program addiction groups is feasible, easy to train, and highly rated by counselors, and I’d say it’s very adaptable, easy to go national in almost any addiction treatment program, right out of the box,” said Richard K. Ries, MD, director of outpatient psychiatry as well as the psychiatry addiction division at Harborview Medical Center. READ MORE
TNF inhibitors may hamper COVID-19 severity
Early evidence from the COVID-19 Global Rheumatology Alliance Registry has produced an intriguing result: Patients on tumor necrosis factor inhibitors for their rheumatic disease are less likely to require hospitalization when infected with COVID-19. The registry data also show that taking hydroxychloroquine or other antimalarials at the time of COVID-19 infection had no impact on hospitalization. “A strength of the global registry has been that it provides timely data that’s been very helpful for rheumatologists to rapidly dispel misinformation that has been spread about hydroxychloroquine, especially statements about lupus patients not getting COVID-19. We know from these data that’s not true,” said Jinoos Yazdany, MD, professor of medicine at the University of California, San Francisco, and chief of rheumatology at San Francisco General Hospital. READ MORE
Audrey Hepburn’s lessons in pandemic grace
There are a lot of new skills required for praticing medicine during the COVID-19 pandemic. In his latest MDedge column, Jeffrey Benabio, MD, explains that grace is one of them. Dr. Benabio, director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego, looks to Audrey Hepburn for inspiration. “Effort is also required for telephone and video visits,” he writes. “In them, our doctor-patient connection is diminished – no matter how high definition, it’s a virtual affair. Ms. Hepburn would no doubt take the time to ensure she appeared professional, well lit, with a pleasing background. She’d plan for the call to be done in a quiet location and without distraction.” READ MORE
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
COVID-19 in kids
Children and young adults in all age groups can develop severe illess after SARS-CoV-2 infection, but infants and teens are most likely to be hospitalized, according to retrospective data from 177 children and young adults at a single center. “One patient had features consistent with the recently emerged Kawasaki disease–like presentation with hyperinflammatory state, hypotension, and profound myocardial depression,” Roberta L. DeBiasi, MD, of Children’s National Hospital, Washington, and colleagues reported in the Journal of Pediatrics. READ MORE
Avoiding ageism in COVID resource allocation
The American Geriatrics Society has issued new policy recommendations for resource allocation during the COVID-19 pandemic that are aimed at protecting seniors for ageism. When allocating scarce resources in an emergency, officials should equally weigh in-hospital survival and severe comorbidities contributing to short-term mortality, the group wrote. “Age per se should never be used as a means for a categorical exclusion from therapeutic interventions that represent the standard of care. ... Likewise, specific age-based cutoffs should not be used in resource allocation strategies,” AGS officials wrote in the statement. READ MORE
Preventing addiction-related suicide
Individuals with substance use disorders are at a significant risk for suicide, but there have been few evidence-based options for their treatment. Now a single intervention is showing promise for this high-risk group. In a large, multicenter randomized effectiveness study, a single 3-hour-long group psychosocial intervention resulted in significantly improved knowledge and attitudes regarding suicide that persisted at 6 months of follow-up. The intervention to prevent future suicide was designed specifically for patients who were in intensive outpatient programs for addiction treatment. “We’ve shown that suicide prevention in intensive outpatient program addiction groups is feasible, easy to train, and highly rated by counselors, and I’d say it’s very adaptable, easy to go national in almost any addiction treatment program, right out of the box,” said Richard K. Ries, MD, director of outpatient psychiatry as well as the psychiatry addiction division at Harborview Medical Center. READ MORE
TNF inhibitors may hamper COVID-19 severity
Early evidence from the COVID-19 Global Rheumatology Alliance Registry has produced an intriguing result: Patients on tumor necrosis factor inhibitors for their rheumatic disease are less likely to require hospitalization when infected with COVID-19. The registry data also show that taking hydroxychloroquine or other antimalarials at the time of COVID-19 infection had no impact on hospitalization. “A strength of the global registry has been that it provides timely data that’s been very helpful for rheumatologists to rapidly dispel misinformation that has been spread about hydroxychloroquine, especially statements about lupus patients not getting COVID-19. We know from these data that’s not true,” said Jinoos Yazdany, MD, professor of medicine at the University of California, San Francisco, and chief of rheumatology at San Francisco General Hospital. READ MORE
Audrey Hepburn’s lessons in pandemic grace
There are a lot of new skills required for praticing medicine during the COVID-19 pandemic. In his latest MDedge column, Jeffrey Benabio, MD, explains that grace is one of them. Dr. Benabio, director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego, looks to Audrey Hepburn for inspiration. “Effort is also required for telephone and video visits,” he writes. “In them, our doctor-patient connection is diminished – no matter how high definition, it’s a virtual affair. Ms. Hepburn would no doubt take the time to ensure she appeared professional, well lit, with a pleasing background. She’d plan for the call to be done in a quiet location and without distraction.” READ MORE
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
COVID-19 in kids
Children and young adults in all age groups can develop severe illess after SARS-CoV-2 infection, but infants and teens are most likely to be hospitalized, according to retrospective data from 177 children and young adults at a single center. “One patient had features consistent with the recently emerged Kawasaki disease–like presentation with hyperinflammatory state, hypotension, and profound myocardial depression,” Roberta L. DeBiasi, MD, of Children’s National Hospital, Washington, and colleagues reported in the Journal of Pediatrics. READ MORE
Avoiding ageism in COVID resource allocation
The American Geriatrics Society has issued new policy recommendations for resource allocation during the COVID-19 pandemic that are aimed at protecting seniors for ageism. When allocating scarce resources in an emergency, officials should equally weigh in-hospital survival and severe comorbidities contributing to short-term mortality, the group wrote. “Age per se should never be used as a means for a categorical exclusion from therapeutic interventions that represent the standard of care. ... Likewise, specific age-based cutoffs should not be used in resource allocation strategies,” AGS officials wrote in the statement. READ MORE
Preventing addiction-related suicide
Individuals with substance use disorders are at a significant risk for suicide, but there have been few evidence-based options for their treatment. Now a single intervention is showing promise for this high-risk group. In a large, multicenter randomized effectiveness study, a single 3-hour-long group psychosocial intervention resulted in significantly improved knowledge and attitudes regarding suicide that persisted at 6 months of follow-up. The intervention to prevent future suicide was designed specifically for patients who were in intensive outpatient programs for addiction treatment. “We’ve shown that suicide prevention in intensive outpatient program addiction groups is feasible, easy to train, and highly rated by counselors, and I’d say it’s very adaptable, easy to go national in almost any addiction treatment program, right out of the box,” said Richard K. Ries, MD, director of outpatient psychiatry as well as the psychiatry addiction division at Harborview Medical Center. READ MORE
TNF inhibitors may hamper COVID-19 severity
Early evidence from the COVID-19 Global Rheumatology Alliance Registry has produced an intriguing result: Patients on tumor necrosis factor inhibitors for their rheumatic disease are less likely to require hospitalization when infected with COVID-19. The registry data also show that taking hydroxychloroquine or other antimalarials at the time of COVID-19 infection had no impact on hospitalization. “A strength of the global registry has been that it provides timely data that’s been very helpful for rheumatologists to rapidly dispel misinformation that has been spread about hydroxychloroquine, especially statements about lupus patients not getting COVID-19. We know from these data that’s not true,” said Jinoos Yazdany, MD, professor of medicine at the University of California, San Francisco, and chief of rheumatology at San Francisco General Hospital. READ MORE
Audrey Hepburn’s lessons in pandemic grace
There are a lot of new skills required for praticing medicine during the COVID-19 pandemic. In his latest MDedge column, Jeffrey Benabio, MD, explains that grace is one of them. Dr. Benabio, director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego, looks to Audrey Hepburn for inspiration. “Effort is also required for telephone and video visits,” he writes. “In them, our doctor-patient connection is diminished – no matter how high definition, it’s a virtual affair. Ms. Hepburn would no doubt take the time to ensure she appeared professional, well lit, with a pleasing background. She’d plan for the call to be done in a quiet location and without distraction.” READ MORE
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
3 new latent TB preventive regimens dramatically cut Tx time
References
- Sterling TR, Njie G, Zenner D, et al. Guidelines for the treatment of latent tubercular infection: recommendations from the National Tuberculosis Controllers Association and the CDC, 2020. MMWR Recomm Rep. 2020;69:1-11.
- USPSTF. Latent tuberculosis screening [final recommendation statement]. Published September 6, 2016. www.uspreventiveservicestaskforce.org/uspstf/recommendation/latent-tuberculosis-infection-screening. Accessed May 19, 2020.
- CDC. Tuberculosis (TB): data and statistics. Updated September 6, 2019. www.cdc.gov/tb/statistics/default.htm. Accessed May 19, 2020.
References
- Sterling TR, Njie G, Zenner D, et al. Guidelines for the treatment of latent tubercular infection: recommendations from the National Tuberculosis Controllers Association and the CDC, 2020. MMWR Recomm Rep. 2020;69:1-11.
- USPSTF. Latent tuberculosis screening [final recommendation statement]. Published September 6, 2016. www.uspreventiveservicestaskforce.org/uspstf/recommendation/latent-tuberculosis-infection-screening. Accessed May 19, 2020.
- CDC. Tuberculosis (TB): data and statistics. Updated September 6, 2019. www.cdc.gov/tb/statistics/default.htm. Accessed May 19, 2020.
References
- Sterling TR, Njie G, Zenner D, et al. Guidelines for the treatment of latent tubercular infection: recommendations from the National Tuberculosis Controllers Association and the CDC, 2020. MMWR Recomm Rep. 2020;69:1-11.
- USPSTF. Latent tuberculosis screening [final recommendation statement]. Published September 6, 2016. www.uspreventiveservicestaskforce.org/uspstf/recommendation/latent-tuberculosis-infection-screening. Accessed May 19, 2020.
- CDC. Tuberculosis (TB): data and statistics. Updated September 6, 2019. www.cdc.gov/tb/statistics/default.htm. Accessed May 19, 2020.
Vitamin D: A low-hanging fruit in COVID-19?
Mainstream media outlets have been flooded recently with reports speculating on what role, if any, vitamin D may play in reducing the severity of COVID-19 infection.
as well as mortality, with the further suggestion of an effect of vitamin D on the immune response to infection.
But other studies question such a link, including any association between vitamin D concentration and differences in COVID-19 severity by ethnic group.
And while some researchers and clinicians believe people should get tested to see if they have adequate vitamin D levels during this pandemic – in particular frontline health care workers – most doctors say the best way to ensure that people have adequate levels of vitamin D during COVID-19 is to simply take supplements at currently recommended levels.
This is especially important given the fact that, during “lockdown” scenarios, many people are spending more time than usual indoors.
Clifford Rosen, MD, senior scientist at Maine Medical Center’s Research Institute in Scarborough, has been researching vitamin D for 25 years.
“There’s no randomized, controlled trial for sure, and that’s the gold standard,” he said in an interview, and “the observational data are so confounded, it’s difficult to know.”
Whether from diet or supplementation, having adequate vitamin D is important, especially for those at the highest risk of COVID-19, he said. Still, robust data supporting a role of vitamin D in prevention of COVID-19, or as any kind of “therapy” for the infection, are currently lacking.
Rose Anne Kenny, MD, professor of medical gerontology at Trinity College Dublin, recently coauthored an article detailing an inverse association between vitamin D levels and mortality from COVID-19 across countries in Europe.
“At no stage are any of us saying this is a given, but there’s a probability that [vitamin D] – a low-hanging fruit – is a contributory factor and we can do something about it now,” she said in an interview.
Dr. Kenny is calling for the Irish government to formally change their recommendations. “We call on the Irish government to update guidelines as a matter of urgency and encourage all adults to take [vitamin D] supplements during the COVID-19 crisis.” Northern Ireland, part of the United Kingdom, also has not yet made this recommendation, she said.
Meanwhile, Harpreet S. Bajaj, MD, MPH, a practicing endocrinologist from Mount Sinai Hospital, Toronto, said: “Vitamin D could have any of three potential roles in risk for COVID-19 and/or its severity: no role, simply a marker, or a causal factor.”
Dr. Bajaj said – as did Dr. Rosen and Dr. Kenny – that randomized, controlled trials (RCTs) are sorely needed to help ascertain whether there is a specific role of vitamin D.
“Until then, we should continue to follow established public health recommendations for vitamin D supplementation, in addition to following COVID-19 prevention guidance and evolving guidelines for COVID-19 treatment.”
What is the role of vitamin D fortification?
In their study in the Irish Medical Journal, Dr. Kenny and colleagues noted that, in Europe, despite being sunny, Spain and Northern Italy had high rates of vitamin D deficiency and have experienced some of the highest COVID-19 infection and mortality rates in the world.
But these countries do not formally fortify foods or recommend supplementation with vitamin D.
Conversely, the northern countries of Norway, Finland, and Sweden had higher vitamin D levels despite less UVB sunlight exposure, as a result of common supplementation and formal fortification of foods. These Nordic countries also had lower levels of COVID-19 infection and mortality.
Overall, the correlation between low vitamin D levels and mortality from COVID-19 was statistically significant (P = .046), the investigators reported.
“Optimizing vitamin D status to recommendations by national and international public health agencies will certainly have ... potential benefits for COVID-19,” they concluded.
“We’re not saying there aren’t any confounders. This can absolutely be the case, but this [finding] needs to be in the mix of evidence,” Dr. Kenny said.
Dr. Kenny also noted that countries in the Southern Hemisphere have been seeing a relatively low mortality from COVID-19, although she acknowledged the explanation could be that the virus spread later to those countries.
Dr. Rosen has doubts on this issue, too.
“Sure, vitamin D supplementation may have worked for [Nordic countries], their COVID-19 has been better controlled, but there’s no causality here; there’s another step to actually prove this. Other factors might be at play,” he said.
“Look at Brazil, it’s at the equator but the disease is devastating the country. Right now, I just don’t believe it.”
Does vitamin D have a role to play in immune modulation?
One theory currently circulating is that, if vitamin D does have any role to play in modulating response to COVID-19, this may be via a blunting of the immune system reaction to the virus.
In a recent preprint study, Ali Daneshkhah, PhD, and colleagues from Northwestern University, Chicago, interrogated hospital data from China, France, Germany, Italy, Iran, South Korea, Spain, Switzerland, the United Kingdom, and the United States.
Specifically, the risk of severe COVID-19 cases among patients with severe vitamin D deficiency was 17.3%, whereas the equivalent figure for patients with normal vitamin D levels was 14.6% (a reduction of 15.6%).
“This potential effect may be attributed to vitamin D’s ability to suppress the adaptive immune system, regulating cytokine levels and thereby reducing the risk of developing severe COVID-19,” said the researchers.
Likewise, JoAnn E. Manson, MD, chief of the division of preventive medicine at Brigham and Women’s Hospital in Boston, in a recent commentary, noted evidence from an observational study from three South Asian hospitals, in which the prevalence of vitamin D deficiency was much higher among those with severe COVID-19 illness compared with those with mild illness.
“We also know that vitamin D has an immune-modulating effect and can lower inflammation, and this may be relevant to the respiratory response during COVID-19 and the cytokine storm that’s been demonstrated,” she noted.
Dr. Rosen said he is willing to listen on the issue of a potential role of vitamin D in immune modulation.
“I’ve been a huge skeptic from the get-go, and loudly criticized the data for doing nothing. I am surprised at myself for saying there might be some effect,” he said.
“Clearly most people don’t get this [cytokine storm] but of those that do, it’s unclear why they do. Maybe if you are vitamin D sufficient, it might have some impact down the road on your response to an infection,” Dr. Rosen said. “Vitamin D may induce proteins important in modulating the function of macrophages of the immune system.”
Ethnic minorities disproportionately affected
It is also well recognized that COVID-19 disproportionately affects black and Asian minority ethnic individuals.
But on the issue of vitamin D in this context, one recent peer-reviewed study using UK Biobank data found no evidence to support a potential role for vitamin D concentration to explain susceptibility to COVID-19 infection either overall or in explaining differences between ethnic groups.
“Vitamin D is unlikely to be the underlying mechanism for the higher risk observed in black and minority ethnic individuals, and vitamin D supplements are unlikely to provide an effective intervention,” Claire Hastie, PhD, of the University of Glasgow and colleagues concluded.
But this hasn’t stopped two endocrinologists from appealing to members of the British Association of Physicians of Indian Origin (BAPIO) to get their vitamin D levels tested.
The black and Asian minority ethnic population, “especially frontline staff, should get their Vitamin D3 levels checked and get appropriate replacement as required,” said Parag Singhal, MD, of Weston General Hospital, Weston-Super-Mare, England, and David C. Anderson, a retired endocrinologist, said in a letter to BAPIO members.
Indeed, they suggested a booster dose of 100,000 IU as a one-off for black and Asian minority ethnic health care staff that should raise vitamin D levels for 2-3 months. They referred to a systematic review that concludes that “single vitamin D3 doses ≥300,000 IU are most effective at improving vitamin D status ... for up to 3 months”.
Commenting on the idea, Dr. Rosen remarked that, in general, the high-dose 50,000-500,000 IU given as a one-off does not confer any greater benefit than a single dose of 1,000 IU per day, except that the blood levels go up quicker and higher.
“Really there is no evidence that getting to super-high levels of vitamin D confer a greater benefit than normal levels,” he said. “So if health care workers suspect vitamin D deficiency, daily doses of 1,000 IU seem reasonable; even if they miss doses, the blood levels are relatively stable.”
On the specific question of vitamin D needs in ethnic minorities, Dr. Rosen said while such individuals do have lower serum levels of vitamin D, the issue is whether there are meaningful clinical implications related to this.
“The real question is whether [ethnic minority individuals] have physiologically adapted for this in other ways because these low levels have been so for thousands of years. In fact, African Americans have lower vitamin D levels but they absolutely have better bones than [whites],” he pointed out.
Testing and governmental recommendations during COVID-19
The U.S. National Institutes of Health in general advises 400 IU to 800 IU per day intake of vitamin D, depending on age, with those over 70 years requiring the highest daily dose. This will result in blood levels that are sufficient to maintain bone health and normal calcium metabolism in healthy people. There are no additional recommendations specific to vitamin D intake during the COVID-19 pandemic, however.
And Dr. Rosen pointed out that there is no evidence for mass screening of vitamin D levels among the U.S. population.
“U.S. public health guidance was pre-COVID, and I think high-risk individuals might want to think about their levels; for example, someone with inflammatory bowel disease or liver or pancreatic disease. These people are at higher risk anyway, and it could be because their vitamin D is low,” he said.
“Skip the test and ensure you are getting adequate levels of vitamin D whether via diet or supplement [400-800 IU per day],” he suggested. “It won’t harm.”
The U.K.’s Public Health England (PHE) clarified its advice on vitamin D supplementation during COVID-19. Alison Tedstone, PhD, chief nutritionist at PHE, said: “Many people are spending more time indoors and may not get all the vitamin D they need from sunlight. To protect their bone and muscle health, they should consider taking a daily supplement containing 10 micrograms [400 IU] of vitamin D.”
However, “there is no sufficient evidence to support recommending Vitamin D for reducing the risk of COVID-19,” she stressed.
Dr. Bajaj is on the advisory board of Medscape Diabetes & Endocrinology. He has ties with Amgen, AstraZeneca Boehringer Ingelheim, Janssen, Merck, Novo Nordisk, Sanofi, Eli Lilly,Valeant, Canadian Collaborative Research Network, CMS Knowledge Translation, Diabetes Canada Scientific Group, LMC Healthcare,mdBriefCase,Medscape, andMeducom. Dr. Kenny, Dr. Rosen, and Dr. Singhal have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Mainstream media outlets have been flooded recently with reports speculating on what role, if any, vitamin D may play in reducing the severity of COVID-19 infection.
as well as mortality, with the further suggestion of an effect of vitamin D on the immune response to infection.
But other studies question such a link, including any association between vitamin D concentration and differences in COVID-19 severity by ethnic group.
And while some researchers and clinicians believe people should get tested to see if they have adequate vitamin D levels during this pandemic – in particular frontline health care workers – most doctors say the best way to ensure that people have adequate levels of vitamin D during COVID-19 is to simply take supplements at currently recommended levels.
This is especially important given the fact that, during “lockdown” scenarios, many people are spending more time than usual indoors.
Clifford Rosen, MD, senior scientist at Maine Medical Center’s Research Institute in Scarborough, has been researching vitamin D for 25 years.
“There’s no randomized, controlled trial for sure, and that’s the gold standard,” he said in an interview, and “the observational data are so confounded, it’s difficult to know.”
Whether from diet or supplementation, having adequate vitamin D is important, especially for those at the highest risk of COVID-19, he said. Still, robust data supporting a role of vitamin D in prevention of COVID-19, or as any kind of “therapy” for the infection, are currently lacking.
Rose Anne Kenny, MD, professor of medical gerontology at Trinity College Dublin, recently coauthored an article detailing an inverse association between vitamin D levels and mortality from COVID-19 across countries in Europe.
“At no stage are any of us saying this is a given, but there’s a probability that [vitamin D] – a low-hanging fruit – is a contributory factor and we can do something about it now,” she said in an interview.
Dr. Kenny is calling for the Irish government to formally change their recommendations. “We call on the Irish government to update guidelines as a matter of urgency and encourage all adults to take [vitamin D] supplements during the COVID-19 crisis.” Northern Ireland, part of the United Kingdom, also has not yet made this recommendation, she said.
Meanwhile, Harpreet S. Bajaj, MD, MPH, a practicing endocrinologist from Mount Sinai Hospital, Toronto, said: “Vitamin D could have any of three potential roles in risk for COVID-19 and/or its severity: no role, simply a marker, or a causal factor.”
Dr. Bajaj said – as did Dr. Rosen and Dr. Kenny – that randomized, controlled trials (RCTs) are sorely needed to help ascertain whether there is a specific role of vitamin D.
“Until then, we should continue to follow established public health recommendations for vitamin D supplementation, in addition to following COVID-19 prevention guidance and evolving guidelines for COVID-19 treatment.”
What is the role of vitamin D fortification?
In their study in the Irish Medical Journal, Dr. Kenny and colleagues noted that, in Europe, despite being sunny, Spain and Northern Italy had high rates of vitamin D deficiency and have experienced some of the highest COVID-19 infection and mortality rates in the world.
But these countries do not formally fortify foods or recommend supplementation with vitamin D.
Conversely, the northern countries of Norway, Finland, and Sweden had higher vitamin D levels despite less UVB sunlight exposure, as a result of common supplementation and formal fortification of foods. These Nordic countries also had lower levels of COVID-19 infection and mortality.
Overall, the correlation between low vitamin D levels and mortality from COVID-19 was statistically significant (P = .046), the investigators reported.
“Optimizing vitamin D status to recommendations by national and international public health agencies will certainly have ... potential benefits for COVID-19,” they concluded.
“We’re not saying there aren’t any confounders. This can absolutely be the case, but this [finding] needs to be in the mix of evidence,” Dr. Kenny said.
Dr. Kenny also noted that countries in the Southern Hemisphere have been seeing a relatively low mortality from COVID-19, although she acknowledged the explanation could be that the virus spread later to those countries.
Dr. Rosen has doubts on this issue, too.
“Sure, vitamin D supplementation may have worked for [Nordic countries], their COVID-19 has been better controlled, but there’s no causality here; there’s another step to actually prove this. Other factors might be at play,” he said.
“Look at Brazil, it’s at the equator but the disease is devastating the country. Right now, I just don’t believe it.”
Does vitamin D have a role to play in immune modulation?
One theory currently circulating is that, if vitamin D does have any role to play in modulating response to COVID-19, this may be via a blunting of the immune system reaction to the virus.
In a recent preprint study, Ali Daneshkhah, PhD, and colleagues from Northwestern University, Chicago, interrogated hospital data from China, France, Germany, Italy, Iran, South Korea, Spain, Switzerland, the United Kingdom, and the United States.
Specifically, the risk of severe COVID-19 cases among patients with severe vitamin D deficiency was 17.3%, whereas the equivalent figure for patients with normal vitamin D levels was 14.6% (a reduction of 15.6%).
“This potential effect may be attributed to vitamin D’s ability to suppress the adaptive immune system, regulating cytokine levels and thereby reducing the risk of developing severe COVID-19,” said the researchers.
Likewise, JoAnn E. Manson, MD, chief of the division of preventive medicine at Brigham and Women’s Hospital in Boston, in a recent commentary, noted evidence from an observational study from three South Asian hospitals, in which the prevalence of vitamin D deficiency was much higher among those with severe COVID-19 illness compared with those with mild illness.
“We also know that vitamin D has an immune-modulating effect and can lower inflammation, and this may be relevant to the respiratory response during COVID-19 and the cytokine storm that’s been demonstrated,” she noted.
Dr. Rosen said he is willing to listen on the issue of a potential role of vitamin D in immune modulation.
“I’ve been a huge skeptic from the get-go, and loudly criticized the data for doing nothing. I am surprised at myself for saying there might be some effect,” he said.
“Clearly most people don’t get this [cytokine storm] but of those that do, it’s unclear why they do. Maybe if you are vitamin D sufficient, it might have some impact down the road on your response to an infection,” Dr. Rosen said. “Vitamin D may induce proteins important in modulating the function of macrophages of the immune system.”
Ethnic minorities disproportionately affected
It is also well recognized that COVID-19 disproportionately affects black and Asian minority ethnic individuals.
But on the issue of vitamin D in this context, one recent peer-reviewed study using UK Biobank data found no evidence to support a potential role for vitamin D concentration to explain susceptibility to COVID-19 infection either overall or in explaining differences between ethnic groups.
“Vitamin D is unlikely to be the underlying mechanism for the higher risk observed in black and minority ethnic individuals, and vitamin D supplements are unlikely to provide an effective intervention,” Claire Hastie, PhD, of the University of Glasgow and colleagues concluded.
But this hasn’t stopped two endocrinologists from appealing to members of the British Association of Physicians of Indian Origin (BAPIO) to get their vitamin D levels tested.
The black and Asian minority ethnic population, “especially frontline staff, should get their Vitamin D3 levels checked and get appropriate replacement as required,” said Parag Singhal, MD, of Weston General Hospital, Weston-Super-Mare, England, and David C. Anderson, a retired endocrinologist, said in a letter to BAPIO members.
Indeed, they suggested a booster dose of 100,000 IU as a one-off for black and Asian minority ethnic health care staff that should raise vitamin D levels for 2-3 months. They referred to a systematic review that concludes that “single vitamin D3 doses ≥300,000 IU are most effective at improving vitamin D status ... for up to 3 months”.
Commenting on the idea, Dr. Rosen remarked that, in general, the high-dose 50,000-500,000 IU given as a one-off does not confer any greater benefit than a single dose of 1,000 IU per day, except that the blood levels go up quicker and higher.
“Really there is no evidence that getting to super-high levels of vitamin D confer a greater benefit than normal levels,” he said. “So if health care workers suspect vitamin D deficiency, daily doses of 1,000 IU seem reasonable; even if they miss doses, the blood levels are relatively stable.”
On the specific question of vitamin D needs in ethnic minorities, Dr. Rosen said while such individuals do have lower serum levels of vitamin D, the issue is whether there are meaningful clinical implications related to this.
“The real question is whether [ethnic minority individuals] have physiologically adapted for this in other ways because these low levels have been so for thousands of years. In fact, African Americans have lower vitamin D levels but they absolutely have better bones than [whites],” he pointed out.
Testing and governmental recommendations during COVID-19
The U.S. National Institutes of Health in general advises 400 IU to 800 IU per day intake of vitamin D, depending on age, with those over 70 years requiring the highest daily dose. This will result in blood levels that are sufficient to maintain bone health and normal calcium metabolism in healthy people. There are no additional recommendations specific to vitamin D intake during the COVID-19 pandemic, however.
And Dr. Rosen pointed out that there is no evidence for mass screening of vitamin D levels among the U.S. population.
“U.S. public health guidance was pre-COVID, and I think high-risk individuals might want to think about their levels; for example, someone with inflammatory bowel disease or liver or pancreatic disease. These people are at higher risk anyway, and it could be because their vitamin D is low,” he said.
“Skip the test and ensure you are getting adequate levels of vitamin D whether via diet or supplement [400-800 IU per day],” he suggested. “It won’t harm.”
The U.K.’s Public Health England (PHE) clarified its advice on vitamin D supplementation during COVID-19. Alison Tedstone, PhD, chief nutritionist at PHE, said: “Many people are spending more time indoors and may not get all the vitamin D they need from sunlight. To protect their bone and muscle health, they should consider taking a daily supplement containing 10 micrograms [400 IU] of vitamin D.”
However, “there is no sufficient evidence to support recommending Vitamin D for reducing the risk of COVID-19,” she stressed.
Dr. Bajaj is on the advisory board of Medscape Diabetes & Endocrinology. He has ties with Amgen, AstraZeneca Boehringer Ingelheim, Janssen, Merck, Novo Nordisk, Sanofi, Eli Lilly,Valeant, Canadian Collaborative Research Network, CMS Knowledge Translation, Diabetes Canada Scientific Group, LMC Healthcare,mdBriefCase,Medscape, andMeducom. Dr. Kenny, Dr. Rosen, and Dr. Singhal have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Mainstream media outlets have been flooded recently with reports speculating on what role, if any, vitamin D may play in reducing the severity of COVID-19 infection.
as well as mortality, with the further suggestion of an effect of vitamin D on the immune response to infection.
But other studies question such a link, including any association between vitamin D concentration and differences in COVID-19 severity by ethnic group.
And while some researchers and clinicians believe people should get tested to see if they have adequate vitamin D levels during this pandemic – in particular frontline health care workers – most doctors say the best way to ensure that people have adequate levels of vitamin D during COVID-19 is to simply take supplements at currently recommended levels.
This is especially important given the fact that, during “lockdown” scenarios, many people are spending more time than usual indoors.
Clifford Rosen, MD, senior scientist at Maine Medical Center’s Research Institute in Scarborough, has been researching vitamin D for 25 years.
“There’s no randomized, controlled trial for sure, and that’s the gold standard,” he said in an interview, and “the observational data are so confounded, it’s difficult to know.”
Whether from diet or supplementation, having adequate vitamin D is important, especially for those at the highest risk of COVID-19, he said. Still, robust data supporting a role of vitamin D in prevention of COVID-19, or as any kind of “therapy” for the infection, are currently lacking.
Rose Anne Kenny, MD, professor of medical gerontology at Trinity College Dublin, recently coauthored an article detailing an inverse association between vitamin D levels and mortality from COVID-19 across countries in Europe.
“At no stage are any of us saying this is a given, but there’s a probability that [vitamin D] – a low-hanging fruit – is a contributory factor and we can do something about it now,” she said in an interview.
Dr. Kenny is calling for the Irish government to formally change their recommendations. “We call on the Irish government to update guidelines as a matter of urgency and encourage all adults to take [vitamin D] supplements during the COVID-19 crisis.” Northern Ireland, part of the United Kingdom, also has not yet made this recommendation, she said.
Meanwhile, Harpreet S. Bajaj, MD, MPH, a practicing endocrinologist from Mount Sinai Hospital, Toronto, said: “Vitamin D could have any of three potential roles in risk for COVID-19 and/or its severity: no role, simply a marker, or a causal factor.”
Dr. Bajaj said – as did Dr. Rosen and Dr. Kenny – that randomized, controlled trials (RCTs) are sorely needed to help ascertain whether there is a specific role of vitamin D.
“Until then, we should continue to follow established public health recommendations for vitamin D supplementation, in addition to following COVID-19 prevention guidance and evolving guidelines for COVID-19 treatment.”
What is the role of vitamin D fortification?
In their study in the Irish Medical Journal, Dr. Kenny and colleagues noted that, in Europe, despite being sunny, Spain and Northern Italy had high rates of vitamin D deficiency and have experienced some of the highest COVID-19 infection and mortality rates in the world.
But these countries do not formally fortify foods or recommend supplementation with vitamin D.
Conversely, the northern countries of Norway, Finland, and Sweden had higher vitamin D levels despite less UVB sunlight exposure, as a result of common supplementation and formal fortification of foods. These Nordic countries also had lower levels of COVID-19 infection and mortality.
Overall, the correlation between low vitamin D levels and mortality from COVID-19 was statistically significant (P = .046), the investigators reported.
“Optimizing vitamin D status to recommendations by national and international public health agencies will certainly have ... potential benefits for COVID-19,” they concluded.
“We’re not saying there aren’t any confounders. This can absolutely be the case, but this [finding] needs to be in the mix of evidence,” Dr. Kenny said.
Dr. Kenny also noted that countries in the Southern Hemisphere have been seeing a relatively low mortality from COVID-19, although she acknowledged the explanation could be that the virus spread later to those countries.
Dr. Rosen has doubts on this issue, too.
“Sure, vitamin D supplementation may have worked for [Nordic countries], their COVID-19 has been better controlled, but there’s no causality here; there’s another step to actually prove this. Other factors might be at play,” he said.
“Look at Brazil, it’s at the equator but the disease is devastating the country. Right now, I just don’t believe it.”
Does vitamin D have a role to play in immune modulation?
One theory currently circulating is that, if vitamin D does have any role to play in modulating response to COVID-19, this may be via a blunting of the immune system reaction to the virus.
In a recent preprint study, Ali Daneshkhah, PhD, and colleagues from Northwestern University, Chicago, interrogated hospital data from China, France, Germany, Italy, Iran, South Korea, Spain, Switzerland, the United Kingdom, and the United States.
Specifically, the risk of severe COVID-19 cases among patients with severe vitamin D deficiency was 17.3%, whereas the equivalent figure for patients with normal vitamin D levels was 14.6% (a reduction of 15.6%).
“This potential effect may be attributed to vitamin D’s ability to suppress the adaptive immune system, regulating cytokine levels and thereby reducing the risk of developing severe COVID-19,” said the researchers.
Likewise, JoAnn E. Manson, MD, chief of the division of preventive medicine at Brigham and Women’s Hospital in Boston, in a recent commentary, noted evidence from an observational study from three South Asian hospitals, in which the prevalence of vitamin D deficiency was much higher among those with severe COVID-19 illness compared with those with mild illness.
“We also know that vitamin D has an immune-modulating effect and can lower inflammation, and this may be relevant to the respiratory response during COVID-19 and the cytokine storm that’s been demonstrated,” she noted.
Dr. Rosen said he is willing to listen on the issue of a potential role of vitamin D in immune modulation.
“I’ve been a huge skeptic from the get-go, and loudly criticized the data for doing nothing. I am surprised at myself for saying there might be some effect,” he said.
“Clearly most people don’t get this [cytokine storm] but of those that do, it’s unclear why they do. Maybe if you are vitamin D sufficient, it might have some impact down the road on your response to an infection,” Dr. Rosen said. “Vitamin D may induce proteins important in modulating the function of macrophages of the immune system.”
Ethnic minorities disproportionately affected
It is also well recognized that COVID-19 disproportionately affects black and Asian minority ethnic individuals.
But on the issue of vitamin D in this context, one recent peer-reviewed study using UK Biobank data found no evidence to support a potential role for vitamin D concentration to explain susceptibility to COVID-19 infection either overall or in explaining differences between ethnic groups.
“Vitamin D is unlikely to be the underlying mechanism for the higher risk observed in black and minority ethnic individuals, and vitamin D supplements are unlikely to provide an effective intervention,” Claire Hastie, PhD, of the University of Glasgow and colleagues concluded.
But this hasn’t stopped two endocrinologists from appealing to members of the British Association of Physicians of Indian Origin (BAPIO) to get their vitamin D levels tested.
The black and Asian minority ethnic population, “especially frontline staff, should get their Vitamin D3 levels checked and get appropriate replacement as required,” said Parag Singhal, MD, of Weston General Hospital, Weston-Super-Mare, England, and David C. Anderson, a retired endocrinologist, said in a letter to BAPIO members.
Indeed, they suggested a booster dose of 100,000 IU as a one-off for black and Asian minority ethnic health care staff that should raise vitamin D levels for 2-3 months. They referred to a systematic review that concludes that “single vitamin D3 doses ≥300,000 IU are most effective at improving vitamin D status ... for up to 3 months”.
Commenting on the idea, Dr. Rosen remarked that, in general, the high-dose 50,000-500,000 IU given as a one-off does not confer any greater benefit than a single dose of 1,000 IU per day, except that the blood levels go up quicker and higher.
“Really there is no evidence that getting to super-high levels of vitamin D confer a greater benefit than normal levels,” he said. “So if health care workers suspect vitamin D deficiency, daily doses of 1,000 IU seem reasonable; even if they miss doses, the blood levels are relatively stable.”
On the specific question of vitamin D needs in ethnic minorities, Dr. Rosen said while such individuals do have lower serum levels of vitamin D, the issue is whether there are meaningful clinical implications related to this.
“The real question is whether [ethnic minority individuals] have physiologically adapted for this in other ways because these low levels have been so for thousands of years. In fact, African Americans have lower vitamin D levels but they absolutely have better bones than [whites],” he pointed out.
Testing and governmental recommendations during COVID-19
The U.S. National Institutes of Health in general advises 400 IU to 800 IU per day intake of vitamin D, depending on age, with those over 70 years requiring the highest daily dose. This will result in blood levels that are sufficient to maintain bone health and normal calcium metabolism in healthy people. There are no additional recommendations specific to vitamin D intake during the COVID-19 pandemic, however.
And Dr. Rosen pointed out that there is no evidence for mass screening of vitamin D levels among the U.S. population.
“U.S. public health guidance was pre-COVID, and I think high-risk individuals might want to think about their levels; for example, someone with inflammatory bowel disease or liver or pancreatic disease. These people are at higher risk anyway, and it could be because their vitamin D is low,” he said.
“Skip the test and ensure you are getting adequate levels of vitamin D whether via diet or supplement [400-800 IU per day],” he suggested. “It won’t harm.”
The U.K.’s Public Health England (PHE) clarified its advice on vitamin D supplementation during COVID-19. Alison Tedstone, PhD, chief nutritionist at PHE, said: “Many people are spending more time indoors and may not get all the vitamin D they need from sunlight. To protect their bone and muscle health, they should consider taking a daily supplement containing 10 micrograms [400 IU] of vitamin D.”
However, “there is no sufficient evidence to support recommending Vitamin D for reducing the risk of COVID-19,” she stressed.
Dr. Bajaj is on the advisory board of Medscape Diabetes & Endocrinology. He has ties with Amgen, AstraZeneca Boehringer Ingelheim, Janssen, Merck, Novo Nordisk, Sanofi, Eli Lilly,Valeant, Canadian Collaborative Research Network, CMS Knowledge Translation, Diabetes Canada Scientific Group, LMC Healthcare,mdBriefCase,Medscape, andMeducom. Dr. Kenny, Dr. Rosen, and Dr. Singhal have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.