User login
Making the World's Skin Crawl: Dermatologic Implications of COVID-19
Coronaviruses (CoVs) are among the most common causes of the common cold but also can lead to severe respiratory disease.1 In recent years, CoVs have been responsible for outbreaks of severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), caused by SARS-CoV and MERS-CoV, respectively. Severe acute respiratory syndrome emerged from China in 2002, and MERS started in Saudi Arabia in 2012. In December 2019, several cases of unexplained pneumonia were reported in Wuhan, China.1 A novel CoV--SARS-CoV-2--was isolated in these patients and is now known to cause coronavirus disease 19 (COVID-19).1 Coronavirus disease 19 can cause acute respiratory distress and multiorgan failure.1,2 It spread quickly throughout the world and was declared a pandemic by the World Health Organization on March 11, 2020. According to the Johns Hopkins University Coronavirus Resource Center (https://coronavirus.jhu.edu/map.html), there were approximately 14,500 COVID-19 cases diagnosed worldwide on February 1, 2020; by May 22, 2020, there were more than 5,159,600 cases. Thus, heightened measures for infection prevention and control were put in place around the globe in an attempt to slow the spread of disease.1
In this article, we describe the dermatologic implications of COVID-19, including the clinical manifestations of the disease, risk reduction techniques for patients and providers, personal protective equipment-associated adverse reactions, and the financial impact on dermatologists.
Clinical Manifestations
At the start of the COVID-19 outbreak, little was known about the skin manifestations of the disease. Providers speculated that COVID-19 could have nonspecific skin findings similar to many other viral illnesses.3,4 Research throughout the pandemic has found many cutaneous manifestations of the disease.3-6 A case report from Thailand described a patient who presented with petechiae in addition to fever and thrombocytopenia, which led to an initial misdiagnosis of Dengue fever; however, when the patient began having respiratory symptoms, the diagnosis of COVID-19 was discovered.5 Furthermore, a study from Italy (N=88) showed dermatologic findings in 20.4% (18/88) of patients, including erythematous rash (77.8% [14/18]), widespread urticaria (16.7% [3/18]), and chickenpoxlike vesicles (5.6% [1/18]). A recent study from Spain (N=375) found 5 cutaneous patterns associated with COVID-19: pseudochilblain--acral areas of erythema with vesicles and/or pustules--lesions (19%), vesicular eruptions (9%), urticarial lesions (19%), maculopapular eruptions (47%), and livedoid/necrotic lesions (6%).6 Pseudochilblain lesions appeared in younger patients, occurred later in the disease course, and were associated with less severe disease. Vesicular lesions often were found in middle-aged patients prior to the onset of other COVID-19 symptoms, and they were associated with intermediate disease severity. Urticarial and maculopapular lesions typically paralleled other COVID-19 symptoms in timing and were associated with more severe disease. Likewise, livedoid and necrotic lesions were associated with more severe disease; they occurred more frequently in older patients.6 Clinicians at Cleveland Clinic found similar cutaneous lesions in COVID-19 patients, including morbilliform rashes, acral purpura resembling perniosis, and livedoid lesions.3 Initial biopsies of these lesions pointed to viral exanthema and thrombotic vasculopathy as potential etiologies of morbilliform and livedoid lesions, respectively. Interestingly, patients may present with multiple cutaneous morphologies of the disease at the same time.3 The acral lesions ("COVID toes") have been popularized throughout the media and thus may be the best-known cutaneous manifestation of the disease at this time. New findings continuously arise, and further research is warranted as lesions that develop in hospitalized COVID-19 patients could be virus related or secondary to hospital-induced skin irritation, stressors, or medications.3 Importantly, clinicians should be aware of these cutaneous signs of COVID-19, especially when triaging patients.
Risk Reduction
The current health crisis could have a drastic impact on dermatology patients and providers. One factor that may increase COVID-19 risk in dermatology patients is immunosuppression. Many patients are on immunomodulators and biologics for skin conditions, which can cause immunosuppression directly and indirectly. Immunosuppression is a risk factor for severe disease in patients with COVID-19, so this population is at higher risk for serious infection.7 Telemedicine for nonemergent cases and follow-ups should be considered to decrease traffic in high-risk hospitals; to limit the number of people in waiting rooms; and to protect staff, providers, and patients alike.1 Recommendations for teledermatology consultation during this time include the following: First, have patients take photographs of their skin lesions and send them remotely to the consulting physician. If the lesion is easily recognizable, treatment recommendations can be made remotely; if the diagnosis is ambiguous, the dermatologist can set up an in-person appointment.1
Personal Protective Equipment
Moreover, the current need to wear personal protective equipment (PPE) and wash hands frequently may lead to skin disease among health care providers. Facial rashes may arise from wearing masks and goggles, and repeated handwashing and wearing gloves may lead to hand dermatitis.8 One study examined adverse skin reactions among health care workers (N=322) during the SARS outbreak in 2003. More than one-third (35.5%) of staff members who wore masks regularly during the outbreak reported adverse skin reactions, including acne (59.6%), facial itching (51.4%), and rash (35.8%).8 The acne etiology likely is multifactorial. Masks increase heat and humidity in the covered facial region, which can cause acne flare-ups due to increased sebum production and Cutibacterium acnes growth.8 Additionally, tight N95 masks may occlude the pilosebaceous glands, causing acne to flare. In the SARS study, facial itchiness and rashes likely were due to irritant contact dermatitis to the N95 masks. All of the respondents with adverse skin reactions from masks developed them after using N95 masks; those who wore surgical masks did not report reactions.8 Because N95 masks are recommended for health care workers caring for patients with highly transmissible respiratory infections such as SARS and COVID-19, it will be difficult to avoid wearing them during the current crisis. For this reason, topical retinoids and topical benzoyl peroxide should be the first-line treatment of mask-induced acne, and moisturization and topical corticosteroids should be used for facial erythema. Additionally, 21.4% of respondents reported adverse skin reactions from latex gloves during the SARS outbreak, including dry skin, itchiness, rash, and wheals.8 These skin reactions may have been type I IgE-mediated hypersensitivity reactions or irritant contact dermatitis due to latex sensitization and frequent handwashing. No respondents reported skin reactions to plastic gloves.8 For this reason, health care providers should consider wearing plastic gloves in lieu of or under latex gloves to prevent hand dermatitis during this time. Moisturization, barrier creams, and topical corticosteroids also can help treat hand dermatitis. Frequently changing PPE may help prevent skin disease among the frontline health care workers,8 which posed a problem at the beginning of the COVID-19 outbreak as there was a PPE shortage. With industry and individuals coming together to make and donate PPE, it is now more widely available for our frontline providers.
Financial Impact
Finally, the pandemic is having an immense financial impact on dermatology.9 At the onset of the outbreak, our role as health care providers was to help slow the spread of COVID-19; for this reason, most elective procedures were cancelled, and many outpatient clinics closed. Both elective procedures and outpatient visits are central to dermatology, so many dermatologists worked less or not at all during this time, leading to a loss of revenue. The goals of these measures were to reduce transmissibility of the disease, to prevent the health care system from being overwhelmed with critical COVID-19 cases, and to allocate resources to the frontline providers.9 Although these measures were beneficial for slowing the spread of disease, they were detrimental to some providers' and practices' financial stability. Many dermatology practices have begun to reopen with COVID-19 precautions in place. For example, practices are limiting the number of patients that can be in the office at one time, mandating temperature readings upon check-in, and requiring masks be worn throughout the entire visit. With continued recommendations for individuals to stay at home as much as possible, the number of patients being seen in dermatology clinics on a daily basis remains less than normal. One potential solution is telemedicine, which would allow patients' concerns to be addressed while keeping providers practicing with a normal patient volume during this time.9 Keeping providers financially afloat is vital for private practices to continue operating after the pandemic. Dermatology appointments are in high demand with long waiting lists during nonpandemic times; without dermatologists practicing at full capacity, there will be an accumulation of patients with dermatologic conditions with even longer waiting times after the pandemic. Telemedicine may help reduce this potential accumulation of patients and allow patients to be treated in a more timely manner while alleviating financial pressures for providers.
Final Thoughts
The COVID-19 pandemic has spread across the world, infecting millions of people. Although the trends have slowed, more than 106,100 cases are still being diagnosed daily according to the Johns Hopkins University Coronavirus Resource Center (https://coronavirus.jhu.edu/map.html). Patients with COVID-19 may present with a variety of cutaneous lesions. Wearing PPE to take care of COVID-19 patients may lead to skin irritation, so care should be taken to address these adverse skin reactions to maintain the safety of providers. Finally, dermatologists should consider telemedicine during this time to protect high-risk patients, prevent a postpandemic surge of patients, and alleviate financial stressors caused by COVID-19.
- Tao J, Song Z, Yang L, et al. Emergency management for preventing and controlling nosocomial infection of 2019 novel coronavirus: implications for the dermatology department [published online March 5, 2020]. Br J Dermatol. doi:10.1111/bjd.19011.
- Lippi G, Plebani M, Michael HB. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis [published online March 13, 2020]. Clin Chim Acta. doi:10.1016/j.cca.2020.03.022.
- Young S, Fernandez AP. Skin manifestations of COVID-19 [published online May 14, 2020]. Cleve Clin J Med. doi:10.3949/ccjm.87a.ccc031.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective [published online March 26, 2020]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.16387.
- Joob B, Wiwanitkit V. COVID-19 can present with a rash and be mistaken for Dengue [published online March 22, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.03.036.
- Casas CG, Catalá A, Hernández GC, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases [published online April 29, 2020]. Br J Dermatol. doi:10.1111/bjd.19163.
- Conforti C, Giuffrida R, Dianzani C, et al. COVID-19 and psoriasis: is it time to limited treatment with immunosuppressants? a call for action [published online March 11, 2020]. Dermatol Ther. doi:10.1111/dth.13298.
- Foo CC, Goon AT, Leow YH, et al. Adverse skin reactions to personal protective equipment against severe respiratory syndrome--a descriptive study in Singapore. Contact Dermatitis. 2006;55:291-294.
- Heymann WR. The profound dermatological manifestations of COVID-19 [published online March 18, 2020]. Dermatology World Insights and Inquiries. https://www.aad.org/dw/dw-insights-and-inquiries/2020-archive/march/dermatological-manifestations-covid-19. Accessed May 21, 2020.
Coronaviruses (CoVs) are among the most common causes of the common cold but also can lead to severe respiratory disease.1 In recent years, CoVs have been responsible for outbreaks of severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), caused by SARS-CoV and MERS-CoV, respectively. Severe acute respiratory syndrome emerged from China in 2002, and MERS started in Saudi Arabia in 2012. In December 2019, several cases of unexplained pneumonia were reported in Wuhan, China.1 A novel CoV--SARS-CoV-2--was isolated in these patients and is now known to cause coronavirus disease 19 (COVID-19).1 Coronavirus disease 19 can cause acute respiratory distress and multiorgan failure.1,2 It spread quickly throughout the world and was declared a pandemic by the World Health Organization on March 11, 2020. According to the Johns Hopkins University Coronavirus Resource Center (https://coronavirus.jhu.edu/map.html), there were approximately 14,500 COVID-19 cases diagnosed worldwide on February 1, 2020; by May 22, 2020, there were more than 5,159,600 cases. Thus, heightened measures for infection prevention and control were put in place around the globe in an attempt to slow the spread of disease.1
In this article, we describe the dermatologic implications of COVID-19, including the clinical manifestations of the disease, risk reduction techniques for patients and providers, personal protective equipment-associated adverse reactions, and the financial impact on dermatologists.
Clinical Manifestations
At the start of the COVID-19 outbreak, little was known about the skin manifestations of the disease. Providers speculated that COVID-19 could have nonspecific skin findings similar to many other viral illnesses.3,4 Research throughout the pandemic has found many cutaneous manifestations of the disease.3-6 A case report from Thailand described a patient who presented with petechiae in addition to fever and thrombocytopenia, which led to an initial misdiagnosis of Dengue fever; however, when the patient began having respiratory symptoms, the diagnosis of COVID-19 was discovered.5 Furthermore, a study from Italy (N=88) showed dermatologic findings in 20.4% (18/88) of patients, including erythematous rash (77.8% [14/18]), widespread urticaria (16.7% [3/18]), and chickenpoxlike vesicles (5.6% [1/18]). A recent study from Spain (N=375) found 5 cutaneous patterns associated with COVID-19: pseudochilblain--acral areas of erythema with vesicles and/or pustules--lesions (19%), vesicular eruptions (9%), urticarial lesions (19%), maculopapular eruptions (47%), and livedoid/necrotic lesions (6%).6 Pseudochilblain lesions appeared in younger patients, occurred later in the disease course, and were associated with less severe disease. Vesicular lesions often were found in middle-aged patients prior to the onset of other COVID-19 symptoms, and they were associated with intermediate disease severity. Urticarial and maculopapular lesions typically paralleled other COVID-19 symptoms in timing and were associated with more severe disease. Likewise, livedoid and necrotic lesions were associated with more severe disease; they occurred more frequently in older patients.6 Clinicians at Cleveland Clinic found similar cutaneous lesions in COVID-19 patients, including morbilliform rashes, acral purpura resembling perniosis, and livedoid lesions.3 Initial biopsies of these lesions pointed to viral exanthema and thrombotic vasculopathy as potential etiologies of morbilliform and livedoid lesions, respectively. Interestingly, patients may present with multiple cutaneous morphologies of the disease at the same time.3 The acral lesions ("COVID toes") have been popularized throughout the media and thus may be the best-known cutaneous manifestation of the disease at this time. New findings continuously arise, and further research is warranted as lesions that develop in hospitalized COVID-19 patients could be virus related or secondary to hospital-induced skin irritation, stressors, or medications.3 Importantly, clinicians should be aware of these cutaneous signs of COVID-19, especially when triaging patients.
Risk Reduction
The current health crisis could have a drastic impact on dermatology patients and providers. One factor that may increase COVID-19 risk in dermatology patients is immunosuppression. Many patients are on immunomodulators and biologics for skin conditions, which can cause immunosuppression directly and indirectly. Immunosuppression is a risk factor for severe disease in patients with COVID-19, so this population is at higher risk for serious infection.7 Telemedicine for nonemergent cases and follow-ups should be considered to decrease traffic in high-risk hospitals; to limit the number of people in waiting rooms; and to protect staff, providers, and patients alike.1 Recommendations for teledermatology consultation during this time include the following: First, have patients take photographs of their skin lesions and send them remotely to the consulting physician. If the lesion is easily recognizable, treatment recommendations can be made remotely; if the diagnosis is ambiguous, the dermatologist can set up an in-person appointment.1
Personal Protective Equipment
Moreover, the current need to wear personal protective equipment (PPE) and wash hands frequently may lead to skin disease among health care providers. Facial rashes may arise from wearing masks and goggles, and repeated handwashing and wearing gloves may lead to hand dermatitis.8 One study examined adverse skin reactions among health care workers (N=322) during the SARS outbreak in 2003. More than one-third (35.5%) of staff members who wore masks regularly during the outbreak reported adverse skin reactions, including acne (59.6%), facial itching (51.4%), and rash (35.8%).8 The acne etiology likely is multifactorial. Masks increase heat and humidity in the covered facial region, which can cause acne flare-ups due to increased sebum production and Cutibacterium acnes growth.8 Additionally, tight N95 masks may occlude the pilosebaceous glands, causing acne to flare. In the SARS study, facial itchiness and rashes likely were due to irritant contact dermatitis to the N95 masks. All of the respondents with adverse skin reactions from masks developed them after using N95 masks; those who wore surgical masks did not report reactions.8 Because N95 masks are recommended for health care workers caring for patients with highly transmissible respiratory infections such as SARS and COVID-19, it will be difficult to avoid wearing them during the current crisis. For this reason, topical retinoids and topical benzoyl peroxide should be the first-line treatment of mask-induced acne, and moisturization and topical corticosteroids should be used for facial erythema. Additionally, 21.4% of respondents reported adverse skin reactions from latex gloves during the SARS outbreak, including dry skin, itchiness, rash, and wheals.8 These skin reactions may have been type I IgE-mediated hypersensitivity reactions or irritant contact dermatitis due to latex sensitization and frequent handwashing. No respondents reported skin reactions to plastic gloves.8 For this reason, health care providers should consider wearing plastic gloves in lieu of or under latex gloves to prevent hand dermatitis during this time. Moisturization, barrier creams, and topical corticosteroids also can help treat hand dermatitis. Frequently changing PPE may help prevent skin disease among the frontline health care workers,8 which posed a problem at the beginning of the COVID-19 outbreak as there was a PPE shortage. With industry and individuals coming together to make and donate PPE, it is now more widely available for our frontline providers.
Financial Impact
Finally, the pandemic is having an immense financial impact on dermatology.9 At the onset of the outbreak, our role as health care providers was to help slow the spread of COVID-19; for this reason, most elective procedures were cancelled, and many outpatient clinics closed. Both elective procedures and outpatient visits are central to dermatology, so many dermatologists worked less or not at all during this time, leading to a loss of revenue. The goals of these measures were to reduce transmissibility of the disease, to prevent the health care system from being overwhelmed with critical COVID-19 cases, and to allocate resources to the frontline providers.9 Although these measures were beneficial for slowing the spread of disease, they were detrimental to some providers' and practices' financial stability. Many dermatology practices have begun to reopen with COVID-19 precautions in place. For example, practices are limiting the number of patients that can be in the office at one time, mandating temperature readings upon check-in, and requiring masks be worn throughout the entire visit. With continued recommendations for individuals to stay at home as much as possible, the number of patients being seen in dermatology clinics on a daily basis remains less than normal. One potential solution is telemedicine, which would allow patients' concerns to be addressed while keeping providers practicing with a normal patient volume during this time.9 Keeping providers financially afloat is vital for private practices to continue operating after the pandemic. Dermatology appointments are in high demand with long waiting lists during nonpandemic times; without dermatologists practicing at full capacity, there will be an accumulation of patients with dermatologic conditions with even longer waiting times after the pandemic. Telemedicine may help reduce this potential accumulation of patients and allow patients to be treated in a more timely manner while alleviating financial pressures for providers.
Final Thoughts
The COVID-19 pandemic has spread across the world, infecting millions of people. Although the trends have slowed, more than 106,100 cases are still being diagnosed daily according to the Johns Hopkins University Coronavirus Resource Center (https://coronavirus.jhu.edu/map.html). Patients with COVID-19 may present with a variety of cutaneous lesions. Wearing PPE to take care of COVID-19 patients may lead to skin irritation, so care should be taken to address these adverse skin reactions to maintain the safety of providers. Finally, dermatologists should consider telemedicine during this time to protect high-risk patients, prevent a postpandemic surge of patients, and alleviate financial stressors caused by COVID-19.
Coronaviruses (CoVs) are among the most common causes of the common cold but also can lead to severe respiratory disease.1 In recent years, CoVs have been responsible for outbreaks of severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), caused by SARS-CoV and MERS-CoV, respectively. Severe acute respiratory syndrome emerged from China in 2002, and MERS started in Saudi Arabia in 2012. In December 2019, several cases of unexplained pneumonia were reported in Wuhan, China.1 A novel CoV--SARS-CoV-2--was isolated in these patients and is now known to cause coronavirus disease 19 (COVID-19).1 Coronavirus disease 19 can cause acute respiratory distress and multiorgan failure.1,2 It spread quickly throughout the world and was declared a pandemic by the World Health Organization on March 11, 2020. According to the Johns Hopkins University Coronavirus Resource Center (https://coronavirus.jhu.edu/map.html), there were approximately 14,500 COVID-19 cases diagnosed worldwide on February 1, 2020; by May 22, 2020, there were more than 5,159,600 cases. Thus, heightened measures for infection prevention and control were put in place around the globe in an attempt to slow the spread of disease.1
In this article, we describe the dermatologic implications of COVID-19, including the clinical manifestations of the disease, risk reduction techniques for patients and providers, personal protective equipment-associated adverse reactions, and the financial impact on dermatologists.
Clinical Manifestations
At the start of the COVID-19 outbreak, little was known about the skin manifestations of the disease. Providers speculated that COVID-19 could have nonspecific skin findings similar to many other viral illnesses.3,4 Research throughout the pandemic has found many cutaneous manifestations of the disease.3-6 A case report from Thailand described a patient who presented with petechiae in addition to fever and thrombocytopenia, which led to an initial misdiagnosis of Dengue fever; however, when the patient began having respiratory symptoms, the diagnosis of COVID-19 was discovered.5 Furthermore, a study from Italy (N=88) showed dermatologic findings in 20.4% (18/88) of patients, including erythematous rash (77.8% [14/18]), widespread urticaria (16.7% [3/18]), and chickenpoxlike vesicles (5.6% [1/18]). A recent study from Spain (N=375) found 5 cutaneous patterns associated with COVID-19: pseudochilblain--acral areas of erythema with vesicles and/or pustules--lesions (19%), vesicular eruptions (9%), urticarial lesions (19%), maculopapular eruptions (47%), and livedoid/necrotic lesions (6%).6 Pseudochilblain lesions appeared in younger patients, occurred later in the disease course, and were associated with less severe disease. Vesicular lesions often were found in middle-aged patients prior to the onset of other COVID-19 symptoms, and they were associated with intermediate disease severity. Urticarial and maculopapular lesions typically paralleled other COVID-19 symptoms in timing and were associated with more severe disease. Likewise, livedoid and necrotic lesions were associated with more severe disease; they occurred more frequently in older patients.6 Clinicians at Cleveland Clinic found similar cutaneous lesions in COVID-19 patients, including morbilliform rashes, acral purpura resembling perniosis, and livedoid lesions.3 Initial biopsies of these lesions pointed to viral exanthema and thrombotic vasculopathy as potential etiologies of morbilliform and livedoid lesions, respectively. Interestingly, patients may present with multiple cutaneous morphologies of the disease at the same time.3 The acral lesions ("COVID toes") have been popularized throughout the media and thus may be the best-known cutaneous manifestation of the disease at this time. New findings continuously arise, and further research is warranted as lesions that develop in hospitalized COVID-19 patients could be virus related or secondary to hospital-induced skin irritation, stressors, or medications.3 Importantly, clinicians should be aware of these cutaneous signs of COVID-19, especially when triaging patients.
Risk Reduction
The current health crisis could have a drastic impact on dermatology patients and providers. One factor that may increase COVID-19 risk in dermatology patients is immunosuppression. Many patients are on immunomodulators and biologics for skin conditions, which can cause immunosuppression directly and indirectly. Immunosuppression is a risk factor for severe disease in patients with COVID-19, so this population is at higher risk for serious infection.7 Telemedicine for nonemergent cases and follow-ups should be considered to decrease traffic in high-risk hospitals; to limit the number of people in waiting rooms; and to protect staff, providers, and patients alike.1 Recommendations for teledermatology consultation during this time include the following: First, have patients take photographs of their skin lesions and send them remotely to the consulting physician. If the lesion is easily recognizable, treatment recommendations can be made remotely; if the diagnosis is ambiguous, the dermatologist can set up an in-person appointment.1
Personal Protective Equipment
Moreover, the current need to wear personal protective equipment (PPE) and wash hands frequently may lead to skin disease among health care providers. Facial rashes may arise from wearing masks and goggles, and repeated handwashing and wearing gloves may lead to hand dermatitis.8 One study examined adverse skin reactions among health care workers (N=322) during the SARS outbreak in 2003. More than one-third (35.5%) of staff members who wore masks regularly during the outbreak reported adverse skin reactions, including acne (59.6%), facial itching (51.4%), and rash (35.8%).8 The acne etiology likely is multifactorial. Masks increase heat and humidity in the covered facial region, which can cause acne flare-ups due to increased sebum production and Cutibacterium acnes growth.8 Additionally, tight N95 masks may occlude the pilosebaceous glands, causing acne to flare. In the SARS study, facial itchiness and rashes likely were due to irritant contact dermatitis to the N95 masks. All of the respondents with adverse skin reactions from masks developed them after using N95 masks; those who wore surgical masks did not report reactions.8 Because N95 masks are recommended for health care workers caring for patients with highly transmissible respiratory infections such as SARS and COVID-19, it will be difficult to avoid wearing them during the current crisis. For this reason, topical retinoids and topical benzoyl peroxide should be the first-line treatment of mask-induced acne, and moisturization and topical corticosteroids should be used for facial erythema. Additionally, 21.4% of respondents reported adverse skin reactions from latex gloves during the SARS outbreak, including dry skin, itchiness, rash, and wheals.8 These skin reactions may have been type I IgE-mediated hypersensitivity reactions or irritant contact dermatitis due to latex sensitization and frequent handwashing. No respondents reported skin reactions to plastic gloves.8 For this reason, health care providers should consider wearing plastic gloves in lieu of or under latex gloves to prevent hand dermatitis during this time. Moisturization, barrier creams, and topical corticosteroids also can help treat hand dermatitis. Frequently changing PPE may help prevent skin disease among the frontline health care workers,8 which posed a problem at the beginning of the COVID-19 outbreak as there was a PPE shortage. With industry and individuals coming together to make and donate PPE, it is now more widely available for our frontline providers.
Financial Impact
Finally, the pandemic is having an immense financial impact on dermatology.9 At the onset of the outbreak, our role as health care providers was to help slow the spread of COVID-19; for this reason, most elective procedures were cancelled, and many outpatient clinics closed. Both elective procedures and outpatient visits are central to dermatology, so many dermatologists worked less or not at all during this time, leading to a loss of revenue. The goals of these measures were to reduce transmissibility of the disease, to prevent the health care system from being overwhelmed with critical COVID-19 cases, and to allocate resources to the frontline providers.9 Although these measures were beneficial for slowing the spread of disease, they were detrimental to some providers' and practices' financial stability. Many dermatology practices have begun to reopen with COVID-19 precautions in place. For example, practices are limiting the number of patients that can be in the office at one time, mandating temperature readings upon check-in, and requiring masks be worn throughout the entire visit. With continued recommendations for individuals to stay at home as much as possible, the number of patients being seen in dermatology clinics on a daily basis remains less than normal. One potential solution is telemedicine, which would allow patients' concerns to be addressed while keeping providers practicing with a normal patient volume during this time.9 Keeping providers financially afloat is vital for private practices to continue operating after the pandemic. Dermatology appointments are in high demand with long waiting lists during nonpandemic times; without dermatologists practicing at full capacity, there will be an accumulation of patients with dermatologic conditions with even longer waiting times after the pandemic. Telemedicine may help reduce this potential accumulation of patients and allow patients to be treated in a more timely manner while alleviating financial pressures for providers.
Final Thoughts
The COVID-19 pandemic has spread across the world, infecting millions of people. Although the trends have slowed, more than 106,100 cases are still being diagnosed daily according to the Johns Hopkins University Coronavirus Resource Center (https://coronavirus.jhu.edu/map.html). Patients with COVID-19 may present with a variety of cutaneous lesions. Wearing PPE to take care of COVID-19 patients may lead to skin irritation, so care should be taken to address these adverse skin reactions to maintain the safety of providers. Finally, dermatologists should consider telemedicine during this time to protect high-risk patients, prevent a postpandemic surge of patients, and alleviate financial stressors caused by COVID-19.
- Tao J, Song Z, Yang L, et al. Emergency management for preventing and controlling nosocomial infection of 2019 novel coronavirus: implications for the dermatology department [published online March 5, 2020]. Br J Dermatol. doi:10.1111/bjd.19011.
- Lippi G, Plebani M, Michael HB. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis [published online March 13, 2020]. Clin Chim Acta. doi:10.1016/j.cca.2020.03.022.
- Young S, Fernandez AP. Skin manifestations of COVID-19 [published online May 14, 2020]. Cleve Clin J Med. doi:10.3949/ccjm.87a.ccc031.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective [published online March 26, 2020]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.16387.
- Joob B, Wiwanitkit V. COVID-19 can present with a rash and be mistaken for Dengue [published online March 22, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.03.036.
- Casas CG, Catalá A, Hernández GC, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases [published online April 29, 2020]. Br J Dermatol. doi:10.1111/bjd.19163.
- Conforti C, Giuffrida R, Dianzani C, et al. COVID-19 and psoriasis: is it time to limited treatment with immunosuppressants? a call for action [published online March 11, 2020]. Dermatol Ther. doi:10.1111/dth.13298.
- Foo CC, Goon AT, Leow YH, et al. Adverse skin reactions to personal protective equipment against severe respiratory syndrome--a descriptive study in Singapore. Contact Dermatitis. 2006;55:291-294.
- Heymann WR. The profound dermatological manifestations of COVID-19 [published online March 18, 2020]. Dermatology World Insights and Inquiries. https://www.aad.org/dw/dw-insights-and-inquiries/2020-archive/march/dermatological-manifestations-covid-19. Accessed May 21, 2020.
- Tao J, Song Z, Yang L, et al. Emergency management for preventing and controlling nosocomial infection of 2019 novel coronavirus: implications for the dermatology department [published online March 5, 2020]. Br J Dermatol. doi:10.1111/bjd.19011.
- Lippi G, Plebani M, Michael HB. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis [published online March 13, 2020]. Clin Chim Acta. doi:10.1016/j.cca.2020.03.022.
- Young S, Fernandez AP. Skin manifestations of COVID-19 [published online May 14, 2020]. Cleve Clin J Med. doi:10.3949/ccjm.87a.ccc031.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective [published online March 26, 2020]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.16387.
- Joob B, Wiwanitkit V. COVID-19 can present with a rash and be mistaken for Dengue [published online March 22, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.03.036.
- Casas CG, Catalá A, Hernández GC, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases [published online April 29, 2020]. Br J Dermatol. doi:10.1111/bjd.19163.
- Conforti C, Giuffrida R, Dianzani C, et al. COVID-19 and psoriasis: is it time to limited treatment with immunosuppressants? a call for action [published online March 11, 2020]. Dermatol Ther. doi:10.1111/dth.13298.
- Foo CC, Goon AT, Leow YH, et al. Adverse skin reactions to personal protective equipment against severe respiratory syndrome--a descriptive study in Singapore. Contact Dermatitis. 2006;55:291-294.
- Heymann WR. The profound dermatological manifestations of COVID-19 [published online March 18, 2020]. Dermatology World Insights and Inquiries. https://www.aad.org/dw/dw-insights-and-inquiries/2020-archive/march/dermatological-manifestations-covid-19. Accessed May 21, 2020.
Practice Points
- Clinicians should be aware of the skin manifesta-tions of coronavirus disease 19, especially when triaging patients.
- Health care providers may develop skin diseases from wearing the extensive personal protective equipment required during the current health crisis.
- Coronavirus disease 19 has had a substantial finan-cial impact on dermatologists, and telemedicine may be a potential solution.
COVID-19 ravaging the Navajo Nation
The Navajo people have dealt with adversity that has tested our strength and resilience since our creation. In Navajo culture, the Holy People or gods challenged us with Naayee (monsters). We endured and learned from each Naayee, hunger, and death to name a few adversities. The COVID-19 pandemic, or “Big Cough” (Dikos Nitsaa’igii -19 in Navajo language) is a monster confronting the Navajo today. It has had significant impact on our nation and people.
The Navajo have the most cases of the COVID-19 virus of any tribe in the United States, and numbers as of May 31, 2020, are 5,348, with 246 confirmed deaths.1 The Navajo Nation, which once lagged behind New York, has reported the largest per-capita infection rate in the United States.
These devastating numbers, which might be leveling off, are associated with Navajo people having higher-than-average numbers of diabetes, heart disease, and cancer. This is compounded with 30%-40% of homes having no electricity or running water, and a poverty rate of about 38%.2
Geographical and cultural factors also contribute to the inability to gain a foothold in mitigating the number of cases. The Navajo Nation is the largest tribe in the United States, covering 27,000 square miles over an arid, red rock expanse with canyons and mountains. The population is over 250,000,3 and Navajo have traditionally lived in matrilineal clan units throughout the reservation, the size of West Virginia. The family traditional dwelling, called a “hogan,” often is clustered together. Multiple generations live together in these units. The COVID-19 virus inflicted many Navajo and rapidly spread to the elderly in these close-proximity living quarters.
Most Navajo live away from services and grocery stores and travel back and forth for food and water, which contributes to the virus rapidly being transmitted among the community members. Education aimed at curbing travel and spread of the virus was issued with curfews, commands to stay at home and keep social distance, and protect elders. The Navajo leadership and traditional medicine people, meanwhile, advised the people to follow their cultural values by caring for family and community members and providing a safe environment.
Resources are spread out
There are only 13 stores in this expansive reservation,4 so tribal members rely on traveling to border towns, such as Farmington and Gallup, N.M., Families usually travel to these towns on weekends to replenish food and supplies. There has been a cluster of cases in Gallup, N.M., so to reduce the numbers, the town shut itself off from outsiders – including the Navajo people coming to buy food, do laundry, and get water and feed for livestock. This has affected and stressed the Navajo further in attempting to access necessities.
Access to health care is already challenging because of lack of transportation and distance. This has made it more difficult to access COVID-19 testing and more challenging to get the results back. The Indian Health Service has been the designated health care system for the Navajo since 1955. The Treaty of Bosque Redondo, signed by the Navajo in 1868, included the provision of health care, as well as education in exchange for tracts of land, that included the Navajo homeland or Dinetah.5
The Indian Health Service provides care with hospitals and clinics throughout the reservation. Some of the IHS facilities have been taken over by the Navajo, so there are four Navajo tribally controlled hospitals, along with one private hospital. Coordination of care for a pandemic is, therefore, more challenging to coordinate. This contributes to problems with coordination of the health care, establishing alternate care sites, accessing personal protective equipment, and providing testing sites. The Navajo Nation Council is working hard to equitably distribute the $600 million from the CARES Act.6
Dealing with the pandemic is compromised by chronic underfunding from the U.S. government. The treaty obligation of the U.S. government is to provide health care to all federally recognized Native Americans. The IHS, which has been designated to provide that care for a tribal person, gets one-third the Medicare dollars for health care provided for a person in the general population.7 Health factors have led to the public health issues of poorly controlled diabetes, obesity, and coronary artery disease, which is related to this underfunding and the high rate of COVID-19 cases. Parts of the reservation are also exposed to high levels of pollution from oil and gas wells from the coal-fueled power plants. Those exposed to these high levels of pollutions have a higher than average number of cases of COVID-19, higher than in areas where the pollution is markedly lower.8
The Navajo are having to rely on the strength and resilience of traditional Navajo culture and philosophy to confront this monster, Dikos Nitsaa’igii’ 19. We have relied on Western medicine and its limited resources but now need to empower the strength from our traditional ways of knowing. We have used this knowledge in times of adversity for hundreds of years. The Navajo elders and medicine people have reminded us we have dealt with monsters and know how to endure hardship and be resilient. This helps to ameliorate mental health conditions, but there are still issues that remain challenging.
Those having the virus go through times of shortness of breath, which produces anxiety and panic. The risk of death adds further stress, and for a family-oriented culture, the need to isolate from family adds further stress. For the elderly and young people with more serious disease having to go to the hospital alone without family, often far from home, is so challenging. Connecting family by phone or social media with those stricken is essential to decrease anxiety and isolation. Those infected with the virus can learn breathing exercises, which can help the damage from the virus and decrease emotional activation and triggers. Specific breathing techniques can be taught by medical providers. An effective breathing technique to reduce anxiety is coherent breathing, which is done by inhaling 6 seconds and exhaling for 6 seconds without holding your breath. Behavioral health practitioners are available in the tribal and IHS mental health clinics to refer patients to therapy support to manage anxiety and are available by telemedicine. Many of these programs are offering social media informational sessions for the Navajo community. Navajo people often access traditional healing for protection prayers and ceremonies. Some of the tribal and IHS programs provide traditional counselors to talk to. The Navajo access healing that focuses on restoring balance to the body, mind, and spirit.
Taking action against the virus by social distancing, hand washing, and wearing masks can go a long way in reducing anxiety and fear about getting the virus. Resources to help the Navajo Nation are coming from all over the world, from as far as Ireland,9 Doctors Without Borders, 10 and University of San Francisco.11
Two resources that provide relief on the reservation are the Navajo Relief Fund and United Natives.
References
1. Navaho Times. 2020 May 27.
2. Ingalls A et al. BMC Obes. 2019 May 6. doi: 10.1186/s40608-019-0233-9.
3. U.S. Census 2010, as reported by discovernavajo.com.
4. Gould C et al. “Addressing food insecurity on the Navajo reservation through sustainable greenhouses.” 2018 Aug.
5. Native Knowledge 360. Smithsonian Institution. “Bosque Redondo.”
6. Personal communication, Carl Roessel Slater, Navajo Nation Council delegate.
7. IHS Profile Fact Sheet.
8. Wu X et al. medRxiv. 2020 Apr 27.
9. Carroll R. ”Irish support for Native American COVID-19 relief highlights historic bond.” The Guardian. 2020 May 9.
10. Capatides C. “Doctors Without Borders dispatches team to the Navajo Nation” CBS News. 2020 May 11.
11. Weiler N. “UCSF sends second wave of health workers to Navajo Nation.” UCSF.edu. 2020 May 21.
Dr. Roessel is a Navajo board-certified psychiatrist practicing in Santa Fe, N.M., working with the local indigenous population. She has special expertise in cultural psychiatry; her childhood was spent growing up in the Navajo Nation with her grandfather, who was a Navajo medicine man. Her psychiatric practice focuses on integrating indigenous knowledge and principles. Dr. Roessel is a distinguished fellow of the American Psychiatric Association. She has no disclosures.
The Navajo people have dealt with adversity that has tested our strength and resilience since our creation. In Navajo culture, the Holy People or gods challenged us with Naayee (monsters). We endured and learned from each Naayee, hunger, and death to name a few adversities. The COVID-19 pandemic, or “Big Cough” (Dikos Nitsaa’igii -19 in Navajo language) is a monster confronting the Navajo today. It has had significant impact on our nation and people.
The Navajo have the most cases of the COVID-19 virus of any tribe in the United States, and numbers as of May 31, 2020, are 5,348, with 246 confirmed deaths.1 The Navajo Nation, which once lagged behind New York, has reported the largest per-capita infection rate in the United States.
These devastating numbers, which might be leveling off, are associated with Navajo people having higher-than-average numbers of diabetes, heart disease, and cancer. This is compounded with 30%-40% of homes having no electricity or running water, and a poverty rate of about 38%.2
Geographical and cultural factors also contribute to the inability to gain a foothold in mitigating the number of cases. The Navajo Nation is the largest tribe in the United States, covering 27,000 square miles over an arid, red rock expanse with canyons and mountains. The population is over 250,000,3 and Navajo have traditionally lived in matrilineal clan units throughout the reservation, the size of West Virginia. The family traditional dwelling, called a “hogan,” often is clustered together. Multiple generations live together in these units. The COVID-19 virus inflicted many Navajo and rapidly spread to the elderly in these close-proximity living quarters.
Most Navajo live away from services and grocery stores and travel back and forth for food and water, which contributes to the virus rapidly being transmitted among the community members. Education aimed at curbing travel and spread of the virus was issued with curfews, commands to stay at home and keep social distance, and protect elders. The Navajo leadership and traditional medicine people, meanwhile, advised the people to follow their cultural values by caring for family and community members and providing a safe environment.
Resources are spread out
There are only 13 stores in this expansive reservation,4 so tribal members rely on traveling to border towns, such as Farmington and Gallup, N.M., Families usually travel to these towns on weekends to replenish food and supplies. There has been a cluster of cases in Gallup, N.M., so to reduce the numbers, the town shut itself off from outsiders – including the Navajo people coming to buy food, do laundry, and get water and feed for livestock. This has affected and stressed the Navajo further in attempting to access necessities.
Access to health care is already challenging because of lack of transportation and distance. This has made it more difficult to access COVID-19 testing and more challenging to get the results back. The Indian Health Service has been the designated health care system for the Navajo since 1955. The Treaty of Bosque Redondo, signed by the Navajo in 1868, included the provision of health care, as well as education in exchange for tracts of land, that included the Navajo homeland or Dinetah.5
The Indian Health Service provides care with hospitals and clinics throughout the reservation. Some of the IHS facilities have been taken over by the Navajo, so there are four Navajo tribally controlled hospitals, along with one private hospital. Coordination of care for a pandemic is, therefore, more challenging to coordinate. This contributes to problems with coordination of the health care, establishing alternate care sites, accessing personal protective equipment, and providing testing sites. The Navajo Nation Council is working hard to equitably distribute the $600 million from the CARES Act.6
Dealing with the pandemic is compromised by chronic underfunding from the U.S. government. The treaty obligation of the U.S. government is to provide health care to all federally recognized Native Americans. The IHS, which has been designated to provide that care for a tribal person, gets one-third the Medicare dollars for health care provided for a person in the general population.7 Health factors have led to the public health issues of poorly controlled diabetes, obesity, and coronary artery disease, which is related to this underfunding and the high rate of COVID-19 cases. Parts of the reservation are also exposed to high levels of pollution from oil and gas wells from the coal-fueled power plants. Those exposed to these high levels of pollutions have a higher than average number of cases of COVID-19, higher than in areas where the pollution is markedly lower.8
The Navajo are having to rely on the strength and resilience of traditional Navajo culture and philosophy to confront this monster, Dikos Nitsaa’igii’ 19. We have relied on Western medicine and its limited resources but now need to empower the strength from our traditional ways of knowing. We have used this knowledge in times of adversity for hundreds of years. The Navajo elders and medicine people have reminded us we have dealt with monsters and know how to endure hardship and be resilient. This helps to ameliorate mental health conditions, but there are still issues that remain challenging.
Those having the virus go through times of shortness of breath, which produces anxiety and panic. The risk of death adds further stress, and for a family-oriented culture, the need to isolate from family adds further stress. For the elderly and young people with more serious disease having to go to the hospital alone without family, often far from home, is so challenging. Connecting family by phone or social media with those stricken is essential to decrease anxiety and isolation. Those infected with the virus can learn breathing exercises, which can help the damage from the virus and decrease emotional activation and triggers. Specific breathing techniques can be taught by medical providers. An effective breathing technique to reduce anxiety is coherent breathing, which is done by inhaling 6 seconds and exhaling for 6 seconds without holding your breath. Behavioral health practitioners are available in the tribal and IHS mental health clinics to refer patients to therapy support to manage anxiety and are available by telemedicine. Many of these programs are offering social media informational sessions for the Navajo community. Navajo people often access traditional healing for protection prayers and ceremonies. Some of the tribal and IHS programs provide traditional counselors to talk to. The Navajo access healing that focuses on restoring balance to the body, mind, and spirit.
Taking action against the virus by social distancing, hand washing, and wearing masks can go a long way in reducing anxiety and fear about getting the virus. Resources to help the Navajo Nation are coming from all over the world, from as far as Ireland,9 Doctors Without Borders, 10 and University of San Francisco.11
Two resources that provide relief on the reservation are the Navajo Relief Fund and United Natives.
References
1. Navaho Times. 2020 May 27.
2. Ingalls A et al. BMC Obes. 2019 May 6. doi: 10.1186/s40608-019-0233-9.
3. U.S. Census 2010, as reported by discovernavajo.com.
4. Gould C et al. “Addressing food insecurity on the Navajo reservation through sustainable greenhouses.” 2018 Aug.
5. Native Knowledge 360. Smithsonian Institution. “Bosque Redondo.”
6. Personal communication, Carl Roessel Slater, Navajo Nation Council delegate.
7. IHS Profile Fact Sheet.
8. Wu X et al. medRxiv. 2020 Apr 27.
9. Carroll R. ”Irish support for Native American COVID-19 relief highlights historic bond.” The Guardian. 2020 May 9.
10. Capatides C. “Doctors Without Borders dispatches team to the Navajo Nation” CBS News. 2020 May 11.
11. Weiler N. “UCSF sends second wave of health workers to Navajo Nation.” UCSF.edu. 2020 May 21.
Dr. Roessel is a Navajo board-certified psychiatrist practicing in Santa Fe, N.M., working with the local indigenous population. She has special expertise in cultural psychiatry; her childhood was spent growing up in the Navajo Nation with her grandfather, who was a Navajo medicine man. Her psychiatric practice focuses on integrating indigenous knowledge and principles. Dr. Roessel is a distinguished fellow of the American Psychiatric Association. She has no disclosures.
The Navajo people have dealt with adversity that has tested our strength and resilience since our creation. In Navajo culture, the Holy People or gods challenged us with Naayee (monsters). We endured and learned from each Naayee, hunger, and death to name a few adversities. The COVID-19 pandemic, or “Big Cough” (Dikos Nitsaa’igii -19 in Navajo language) is a monster confronting the Navajo today. It has had significant impact on our nation and people.
The Navajo have the most cases of the COVID-19 virus of any tribe in the United States, and numbers as of May 31, 2020, are 5,348, with 246 confirmed deaths.1 The Navajo Nation, which once lagged behind New York, has reported the largest per-capita infection rate in the United States.
These devastating numbers, which might be leveling off, are associated with Navajo people having higher-than-average numbers of diabetes, heart disease, and cancer. This is compounded with 30%-40% of homes having no electricity or running water, and a poverty rate of about 38%.2
Geographical and cultural factors also contribute to the inability to gain a foothold in mitigating the number of cases. The Navajo Nation is the largest tribe in the United States, covering 27,000 square miles over an arid, red rock expanse with canyons and mountains. The population is over 250,000,3 and Navajo have traditionally lived in matrilineal clan units throughout the reservation, the size of West Virginia. The family traditional dwelling, called a “hogan,” often is clustered together. Multiple generations live together in these units. The COVID-19 virus inflicted many Navajo and rapidly spread to the elderly in these close-proximity living quarters.
Most Navajo live away from services and grocery stores and travel back and forth for food and water, which contributes to the virus rapidly being transmitted among the community members. Education aimed at curbing travel and spread of the virus was issued with curfews, commands to stay at home and keep social distance, and protect elders. The Navajo leadership and traditional medicine people, meanwhile, advised the people to follow their cultural values by caring for family and community members and providing a safe environment.
Resources are spread out
There are only 13 stores in this expansive reservation,4 so tribal members rely on traveling to border towns, such as Farmington and Gallup, N.M., Families usually travel to these towns on weekends to replenish food and supplies. There has been a cluster of cases in Gallup, N.M., so to reduce the numbers, the town shut itself off from outsiders – including the Navajo people coming to buy food, do laundry, and get water and feed for livestock. This has affected and stressed the Navajo further in attempting to access necessities.
Access to health care is already challenging because of lack of transportation and distance. This has made it more difficult to access COVID-19 testing and more challenging to get the results back. The Indian Health Service has been the designated health care system for the Navajo since 1955. The Treaty of Bosque Redondo, signed by the Navajo in 1868, included the provision of health care, as well as education in exchange for tracts of land, that included the Navajo homeland or Dinetah.5
The Indian Health Service provides care with hospitals and clinics throughout the reservation. Some of the IHS facilities have been taken over by the Navajo, so there are four Navajo tribally controlled hospitals, along with one private hospital. Coordination of care for a pandemic is, therefore, more challenging to coordinate. This contributes to problems with coordination of the health care, establishing alternate care sites, accessing personal protective equipment, and providing testing sites. The Navajo Nation Council is working hard to equitably distribute the $600 million from the CARES Act.6
Dealing with the pandemic is compromised by chronic underfunding from the U.S. government. The treaty obligation of the U.S. government is to provide health care to all federally recognized Native Americans. The IHS, which has been designated to provide that care for a tribal person, gets one-third the Medicare dollars for health care provided for a person in the general population.7 Health factors have led to the public health issues of poorly controlled diabetes, obesity, and coronary artery disease, which is related to this underfunding and the high rate of COVID-19 cases. Parts of the reservation are also exposed to high levels of pollution from oil and gas wells from the coal-fueled power plants. Those exposed to these high levels of pollutions have a higher than average number of cases of COVID-19, higher than in areas where the pollution is markedly lower.8
The Navajo are having to rely on the strength and resilience of traditional Navajo culture and philosophy to confront this monster, Dikos Nitsaa’igii’ 19. We have relied on Western medicine and its limited resources but now need to empower the strength from our traditional ways of knowing. We have used this knowledge in times of adversity for hundreds of years. The Navajo elders and medicine people have reminded us we have dealt with monsters and know how to endure hardship and be resilient. This helps to ameliorate mental health conditions, but there are still issues that remain challenging.
Those having the virus go through times of shortness of breath, which produces anxiety and panic. The risk of death adds further stress, and for a family-oriented culture, the need to isolate from family adds further stress. For the elderly and young people with more serious disease having to go to the hospital alone without family, often far from home, is so challenging. Connecting family by phone or social media with those stricken is essential to decrease anxiety and isolation. Those infected with the virus can learn breathing exercises, which can help the damage from the virus and decrease emotional activation and triggers. Specific breathing techniques can be taught by medical providers. An effective breathing technique to reduce anxiety is coherent breathing, which is done by inhaling 6 seconds and exhaling for 6 seconds without holding your breath. Behavioral health practitioners are available in the tribal and IHS mental health clinics to refer patients to therapy support to manage anxiety and are available by telemedicine. Many of these programs are offering social media informational sessions for the Navajo community. Navajo people often access traditional healing for protection prayers and ceremonies. Some of the tribal and IHS programs provide traditional counselors to talk to. The Navajo access healing that focuses on restoring balance to the body, mind, and spirit.
Taking action against the virus by social distancing, hand washing, and wearing masks can go a long way in reducing anxiety and fear about getting the virus. Resources to help the Navajo Nation are coming from all over the world, from as far as Ireland,9 Doctors Without Borders, 10 and University of San Francisco.11
Two resources that provide relief on the reservation are the Navajo Relief Fund and United Natives.
References
1. Navaho Times. 2020 May 27.
2. Ingalls A et al. BMC Obes. 2019 May 6. doi: 10.1186/s40608-019-0233-9.
3. U.S. Census 2010, as reported by discovernavajo.com.
4. Gould C et al. “Addressing food insecurity on the Navajo reservation through sustainable greenhouses.” 2018 Aug.
5. Native Knowledge 360. Smithsonian Institution. “Bosque Redondo.”
6. Personal communication, Carl Roessel Slater, Navajo Nation Council delegate.
7. IHS Profile Fact Sheet.
8. Wu X et al. medRxiv. 2020 Apr 27.
9. Carroll R. ”Irish support for Native American COVID-19 relief highlights historic bond.” The Guardian. 2020 May 9.
10. Capatides C. “Doctors Without Borders dispatches team to the Navajo Nation” CBS News. 2020 May 11.
11. Weiler N. “UCSF sends second wave of health workers to Navajo Nation.” UCSF.edu. 2020 May 21.
Dr. Roessel is a Navajo board-certified psychiatrist practicing in Santa Fe, N.M., working with the local indigenous population. She has special expertise in cultural psychiatry; her childhood was spent growing up in the Navajo Nation with her grandfather, who was a Navajo medicine man. Her psychiatric practice focuses on integrating indigenous knowledge and principles. Dr. Roessel is a distinguished fellow of the American Psychiatric Association. She has no disclosures.
Short medication regimen noninferior to long regimen for rifampin-resistant TB
Background: Multidrug-resistant TB is more difficult to treat than is drug-susceptible TB. The 2011 World Health Organization (WHO) recommendations for the treatment of multidrug-resistant TB, based on very-low-quality and conditional evidence, consists of an intensive treatment phase of 8 months and total treatment duration of 20 months. Although cohort studies have shown promising cure rates among patients with multidrug-resistant TB who received existing drugs in regimens shorter than that recommended by the WHO, data from phase 3 randomized trials were lacking.
Study design: Randomized phase 3 noninferior trial.
Setting: Multisite, international; countries were selected based on background disease burden of TB, multidrug-resistant TB, and TB-HIV coinfection (Ethiopia, Mongolia, South Africa, Vietnam).
Synopsis: 424 patients were randomized to the short and long medication regimen groups with 369 included in the modified intention-to-treat analysis and 310 included in the final per protocol efficacy analysis. The short regimen included IV moxifloxacin, clofazimine, ethambutol, and pyrazinamide administered over a 40-week period, supplemented by kanamycin, isoniazid, and prothionamide in the first 16 weeks, compared with 8 months of intense treatment and total 20 months of treatment in the long regimen. At 132 weeks after randomization, cultures were negative for Mycobacterium tuberculosis in more than 78 % patients in both long- and short-regimen group. Unfavorable bacteriologic outcome (10.6%), cardiac conduction defects (9.9%), and hepatobiliary problems (8.9%) were more common in the short-regimen group whereas patients in long-regimen group were lost to follow-up more frequently (2.4%) and had more metabolic disorders (7.1%). More deaths were reported in the short-regimen group, especially in those with HIV coinfections (17.5%). Although the results of this trial are encouraging, further studies will be needed to find a short, simple regimen for multidrug-resistant tuberculosis with improved safety outcomes.
Bottom line: Short medication regimen (9-11 months) is noninferior to the traditional WHO-recommended long regimen (20 months) for treating rifampin-resistant tuberculosis.
Citation: Nunn AJ et al. A trial of a shorter regimen for rifampin-resistant tuberculosis. N Engl J Med. 2019 Mar 28; 380:1201-13.
Dr. Kamath is an assistant professor of medicine at Duke University.
Background: Multidrug-resistant TB is more difficult to treat than is drug-susceptible TB. The 2011 World Health Organization (WHO) recommendations for the treatment of multidrug-resistant TB, based on very-low-quality and conditional evidence, consists of an intensive treatment phase of 8 months and total treatment duration of 20 months. Although cohort studies have shown promising cure rates among patients with multidrug-resistant TB who received existing drugs in regimens shorter than that recommended by the WHO, data from phase 3 randomized trials were lacking.
Study design: Randomized phase 3 noninferior trial.
Setting: Multisite, international; countries were selected based on background disease burden of TB, multidrug-resistant TB, and TB-HIV coinfection (Ethiopia, Mongolia, South Africa, Vietnam).
Synopsis: 424 patients were randomized to the short and long medication regimen groups with 369 included in the modified intention-to-treat analysis and 310 included in the final per protocol efficacy analysis. The short regimen included IV moxifloxacin, clofazimine, ethambutol, and pyrazinamide administered over a 40-week period, supplemented by kanamycin, isoniazid, and prothionamide in the first 16 weeks, compared with 8 months of intense treatment and total 20 months of treatment in the long regimen. At 132 weeks after randomization, cultures were negative for Mycobacterium tuberculosis in more than 78 % patients in both long- and short-regimen group. Unfavorable bacteriologic outcome (10.6%), cardiac conduction defects (9.9%), and hepatobiliary problems (8.9%) were more common in the short-regimen group whereas patients in long-regimen group were lost to follow-up more frequently (2.4%) and had more metabolic disorders (7.1%). More deaths were reported in the short-regimen group, especially in those with HIV coinfections (17.5%). Although the results of this trial are encouraging, further studies will be needed to find a short, simple regimen for multidrug-resistant tuberculosis with improved safety outcomes.
Bottom line: Short medication regimen (9-11 months) is noninferior to the traditional WHO-recommended long regimen (20 months) for treating rifampin-resistant tuberculosis.
Citation: Nunn AJ et al. A trial of a shorter regimen for rifampin-resistant tuberculosis. N Engl J Med. 2019 Mar 28; 380:1201-13.
Dr. Kamath is an assistant professor of medicine at Duke University.
Background: Multidrug-resistant TB is more difficult to treat than is drug-susceptible TB. The 2011 World Health Organization (WHO) recommendations for the treatment of multidrug-resistant TB, based on very-low-quality and conditional evidence, consists of an intensive treatment phase of 8 months and total treatment duration of 20 months. Although cohort studies have shown promising cure rates among patients with multidrug-resistant TB who received existing drugs in regimens shorter than that recommended by the WHO, data from phase 3 randomized trials were lacking.
Study design: Randomized phase 3 noninferior trial.
Setting: Multisite, international; countries were selected based on background disease burden of TB, multidrug-resistant TB, and TB-HIV coinfection (Ethiopia, Mongolia, South Africa, Vietnam).
Synopsis: 424 patients were randomized to the short and long medication regimen groups with 369 included in the modified intention-to-treat analysis and 310 included in the final per protocol efficacy analysis. The short regimen included IV moxifloxacin, clofazimine, ethambutol, and pyrazinamide administered over a 40-week period, supplemented by kanamycin, isoniazid, and prothionamide in the first 16 weeks, compared with 8 months of intense treatment and total 20 months of treatment in the long regimen. At 132 weeks after randomization, cultures were negative for Mycobacterium tuberculosis in more than 78 % patients in both long- and short-regimen group. Unfavorable bacteriologic outcome (10.6%), cardiac conduction defects (9.9%), and hepatobiliary problems (8.9%) were more common in the short-regimen group whereas patients in long-regimen group were lost to follow-up more frequently (2.4%) and had more metabolic disorders (7.1%). More deaths were reported in the short-regimen group, especially in those with HIV coinfections (17.5%). Although the results of this trial are encouraging, further studies will be needed to find a short, simple regimen for multidrug-resistant tuberculosis with improved safety outcomes.
Bottom line: Short medication regimen (9-11 months) is noninferior to the traditional WHO-recommended long regimen (20 months) for treating rifampin-resistant tuberculosis.
Citation: Nunn AJ et al. A trial of a shorter regimen for rifampin-resistant tuberculosis. N Engl J Med. 2019 Mar 28; 380:1201-13.
Dr. Kamath is an assistant professor of medicine at Duke University.
SARS-CoV-2 infection rate 16% in asymptomatic pregnant women at delivery
Among women with a planned delivery in a New York City health system during the first half of April, the rate of asymptomatic SARS-CoV-2 infection was 16%, according to a study published in Obstetrics & Gynecology. Among the patients’ designated support persons, the asymptomatic carrier rate was 10%.
“If universal testing of pregnant patients in a high prevalence area is not performed, health care workers will be inadvertently exposed to COVID-19, unless universal precautions with personal protective equipment are taken,” wrote the researchers affiliated with the department of obstetrics, gynecology, and reproductive medicine at Icahn School of Medicine at Mount Sinai, New York.
Angela Bianco, MD, and colleagues conducted an observational study of women who were scheduled for a planned delivery within the Mount Sinai Health System between April 4 and April 15, 2020. Patients and their designated support person completed a telephone screen and underwent COVID-19 testing the day before a scheduled delivery. If support persons screened positive during the telephone interview about COVID-19 symptoms, they could not attend the birth, and patients could contact a different support person to be screened and tested. “All patients and their support persons were informed of their SARS-CoV-2 test results before admission,” the investigators wrote. “Those who tested positive were counseled regarding symptomatology that should prompt medical attention.”
In all, researchers screened 158 patients with a planned delivery, and 155 agreed to undergo COVID-19 testing. Of the 155 women tested, 24 (16%) tested positive for SARS CoV-2 infection. Among 146 support persons who had a negative interview screen and underwent SARS-CoV-2 testing, 14 (10%) tested positive for SARS-CoV-2 infection.
Test results were substantially concordant among patient and support person pairs. “Among patients who tested positive for COVID-19 infection and had a support person present, 11 of 19 (58%) support persons also tested positive for COVID-19 infection,” the authors reported. “Among patients who tested negative for COVID-19 infection and had a support person present, only 3 of 127 (2.4%) support persons tested positive for COVID-19 infection.”
Telephone screening did not identify any of the COVID-19–positive cases. Of the 24 patients with SARS-CoV-2 infection, none of their newborns tested positive at birth.
“Universal testing ... provides a mechanism for more accurate counseling of patients regarding issues such as newborn skin-to-skin contact and breastfeeding,” noted Dr. Bianco and colleagues. At their institution, parents with COVID-19 are instructed to wear a mask and practice proper hand hygiene when caring for their newborns.
Kristina Adams Waldorf, MD, said in an interview that the study by Bianco et al. underscores the high rate of asymptomatic or mildly symptomatic COVID-19 infections detected with universal screening in a hospital at the U.S. epicenter of the pandemic. “Each state and hospital will need to evaluate their own data to determine the value of universal screening for their patient population. In rural parts of America that have yet to see cases, universal screening may not make sense, but these areas are likely to be few and far between. The rest of America will need to quickly get on board with universal screening to protect their labor and delivery staff.”
Testing the partner was a strength of the study. “It is reassuring that when a pregnant woman tested negative for SARS-CoV-2, the rate was very, very low (2.4%) that her partner would test positive. However, it was disconcerting that telephone screening for common symptoms associated with COVID-19 was not very helpful in identifying cases,” said Dr. Waldorf, a professor of obstetrics and gynecology at the University of Washington, Seattle. She was not involved in the study by Bianco et al.
One study author receives payment from the American Board of Obstetrics and Gynecology for serving as a board examiner, receives payment from UpToDate, and serves as an expert witness in malpractice and products liability cases. The other authors did not report any potential conflicts of interest. Dr. Waldorf said she had no relevant financial disclosures.
SOURCE: Bianco A et al. Obstet Gynecol. 2020 May 19. doi: 10.1097/AOG.0000000000003985.
Among women with a planned delivery in a New York City health system during the first half of April, the rate of asymptomatic SARS-CoV-2 infection was 16%, according to a study published in Obstetrics & Gynecology. Among the patients’ designated support persons, the asymptomatic carrier rate was 10%.
“If universal testing of pregnant patients in a high prevalence area is not performed, health care workers will be inadvertently exposed to COVID-19, unless universal precautions with personal protective equipment are taken,” wrote the researchers affiliated with the department of obstetrics, gynecology, and reproductive medicine at Icahn School of Medicine at Mount Sinai, New York.
Angela Bianco, MD, and colleagues conducted an observational study of women who were scheduled for a planned delivery within the Mount Sinai Health System between April 4 and April 15, 2020. Patients and their designated support person completed a telephone screen and underwent COVID-19 testing the day before a scheduled delivery. If support persons screened positive during the telephone interview about COVID-19 symptoms, they could not attend the birth, and patients could contact a different support person to be screened and tested. “All patients and their support persons were informed of their SARS-CoV-2 test results before admission,” the investigators wrote. “Those who tested positive were counseled regarding symptomatology that should prompt medical attention.”
In all, researchers screened 158 patients with a planned delivery, and 155 agreed to undergo COVID-19 testing. Of the 155 women tested, 24 (16%) tested positive for SARS CoV-2 infection. Among 146 support persons who had a negative interview screen and underwent SARS-CoV-2 testing, 14 (10%) tested positive for SARS-CoV-2 infection.
Test results were substantially concordant among patient and support person pairs. “Among patients who tested positive for COVID-19 infection and had a support person present, 11 of 19 (58%) support persons also tested positive for COVID-19 infection,” the authors reported. “Among patients who tested negative for COVID-19 infection and had a support person present, only 3 of 127 (2.4%) support persons tested positive for COVID-19 infection.”
Telephone screening did not identify any of the COVID-19–positive cases. Of the 24 patients with SARS-CoV-2 infection, none of their newborns tested positive at birth.
“Universal testing ... provides a mechanism for more accurate counseling of patients regarding issues such as newborn skin-to-skin contact and breastfeeding,” noted Dr. Bianco and colleagues. At their institution, parents with COVID-19 are instructed to wear a mask and practice proper hand hygiene when caring for their newborns.
Kristina Adams Waldorf, MD, said in an interview that the study by Bianco et al. underscores the high rate of asymptomatic or mildly symptomatic COVID-19 infections detected with universal screening in a hospital at the U.S. epicenter of the pandemic. “Each state and hospital will need to evaluate their own data to determine the value of universal screening for their patient population. In rural parts of America that have yet to see cases, universal screening may not make sense, but these areas are likely to be few and far between. The rest of America will need to quickly get on board with universal screening to protect their labor and delivery staff.”
Testing the partner was a strength of the study. “It is reassuring that when a pregnant woman tested negative for SARS-CoV-2, the rate was very, very low (2.4%) that her partner would test positive. However, it was disconcerting that telephone screening for common symptoms associated with COVID-19 was not very helpful in identifying cases,” said Dr. Waldorf, a professor of obstetrics and gynecology at the University of Washington, Seattle. She was not involved in the study by Bianco et al.
One study author receives payment from the American Board of Obstetrics and Gynecology for serving as a board examiner, receives payment from UpToDate, and serves as an expert witness in malpractice and products liability cases. The other authors did not report any potential conflicts of interest. Dr. Waldorf said she had no relevant financial disclosures.
SOURCE: Bianco A et al. Obstet Gynecol. 2020 May 19. doi: 10.1097/AOG.0000000000003985.
Among women with a planned delivery in a New York City health system during the first half of April, the rate of asymptomatic SARS-CoV-2 infection was 16%, according to a study published in Obstetrics & Gynecology. Among the patients’ designated support persons, the asymptomatic carrier rate was 10%.
“If universal testing of pregnant patients in a high prevalence area is not performed, health care workers will be inadvertently exposed to COVID-19, unless universal precautions with personal protective equipment are taken,” wrote the researchers affiliated with the department of obstetrics, gynecology, and reproductive medicine at Icahn School of Medicine at Mount Sinai, New York.
Angela Bianco, MD, and colleagues conducted an observational study of women who were scheduled for a planned delivery within the Mount Sinai Health System between April 4 and April 15, 2020. Patients and their designated support person completed a telephone screen and underwent COVID-19 testing the day before a scheduled delivery. If support persons screened positive during the telephone interview about COVID-19 symptoms, they could not attend the birth, and patients could contact a different support person to be screened and tested. “All patients and their support persons were informed of their SARS-CoV-2 test results before admission,” the investigators wrote. “Those who tested positive were counseled regarding symptomatology that should prompt medical attention.”
In all, researchers screened 158 patients with a planned delivery, and 155 agreed to undergo COVID-19 testing. Of the 155 women tested, 24 (16%) tested positive for SARS CoV-2 infection. Among 146 support persons who had a negative interview screen and underwent SARS-CoV-2 testing, 14 (10%) tested positive for SARS-CoV-2 infection.
Test results were substantially concordant among patient and support person pairs. “Among patients who tested positive for COVID-19 infection and had a support person present, 11 of 19 (58%) support persons also tested positive for COVID-19 infection,” the authors reported. “Among patients who tested negative for COVID-19 infection and had a support person present, only 3 of 127 (2.4%) support persons tested positive for COVID-19 infection.”
Telephone screening did not identify any of the COVID-19–positive cases. Of the 24 patients with SARS-CoV-2 infection, none of their newborns tested positive at birth.
“Universal testing ... provides a mechanism for more accurate counseling of patients regarding issues such as newborn skin-to-skin contact and breastfeeding,” noted Dr. Bianco and colleagues. At their institution, parents with COVID-19 are instructed to wear a mask and practice proper hand hygiene when caring for their newborns.
Kristina Adams Waldorf, MD, said in an interview that the study by Bianco et al. underscores the high rate of asymptomatic or mildly symptomatic COVID-19 infections detected with universal screening in a hospital at the U.S. epicenter of the pandemic. “Each state and hospital will need to evaluate their own data to determine the value of universal screening for their patient population. In rural parts of America that have yet to see cases, universal screening may not make sense, but these areas are likely to be few and far between. The rest of America will need to quickly get on board with universal screening to protect their labor and delivery staff.”
Testing the partner was a strength of the study. “It is reassuring that when a pregnant woman tested negative for SARS-CoV-2, the rate was very, very low (2.4%) that her partner would test positive. However, it was disconcerting that telephone screening for common symptoms associated with COVID-19 was not very helpful in identifying cases,” said Dr. Waldorf, a professor of obstetrics and gynecology at the University of Washington, Seattle. She was not involved in the study by Bianco et al.
One study author receives payment from the American Board of Obstetrics and Gynecology for serving as a board examiner, receives payment from UpToDate, and serves as an expert witness in malpractice and products liability cases. The other authors did not report any potential conflicts of interest. Dr. Waldorf said she had no relevant financial disclosures.
SOURCE: Bianco A et al. Obstet Gynecol. 2020 May 19. doi: 10.1097/AOG.0000000000003985.
FROM OBSTETRICS & GYNECOLOGY
Whether to test laboring women for SARS-CoV-2 may hinge on regional prevalence
at the time of admission, research published online in Obstetrics & Gynecology suggests.
In Los Angeles, researchers stopped universal testing after none of the first 80 asymptomatic women had positive results. Researchers in Chicago, on the other hand, found a positive rate of approximately 1.6% among 614 asymptomatic patients and continue to test all patients.
“Decisions regarding universal testing need to be made in the context of regional prevalence of COVID-19 infection, with recognition that a ‘one-size-fits-all’ approach is unlikely to be justifiable,” Torri D. Metz, MD,of University of Utah Health in Salt Lake City said in an editorial accompanying research letters that described the experience in Los Angeles and Chicago. “In the setting of low population prevalence of COVID-19 infection or in locations with limited testing availability, deferring universal testing may represent the better part of valor when weighing risks, benefits, economic burden, and unintended consequences of testing for SARS-CoV-2 infection. In high-prevalence regions, universal testing may be a valuable addition to obstetric care that will prevent infections in health care workers and neonates.”
Testing all patients also may provide valuable population-level surveillance, added Dr. Metz, who is an associate professor of obstetrics and gynecology, a maternal-fetal medicine subspecialist, and vice-chair of research in obstetrics and gynecology.
One week of data
After New York hospitals reported an approximately 13% prevalence of SARS-CoV-2 infection among asymptomatic laboring women, Cedars-Sinai Medical Center in Los Angeles changed its policy from testing only women with COVID-19 symptoms to testing all women beginning April 4, 2020. “Data from New York made us very concerned about the possibility of asymptomatic infections among our own pregnant patients,” Mariam Naqvi, MD, a maternal-fetal medicine specialist at Cedars-Sinai Medical Center, said in a news release. “This would have implications for them, their babies, their households, and for the health of our staff caring for them.”
In 1 week, 82 pregnant women admitted to the obstetric unit were tested for SARS-CoV-2 infection. Of two women who reported COVID-19 symptoms, one tested positive for SARS-CoV-2. “Of the remaining 80 asymptomatic women, none tested positive for SARS-CoV-2 infection, and all remained symptom free throughout their hospitalizations,” Dr. Naqvi and colleagues reported. “One asymptomatic patient had an inadequate nasopharyngeal specimen and declined repeat testing.”
Precautions taken during universal testing meant that all members of the treatment team used valuable personal protective equipment. In some cases, mothers and newborns were separated until test results were available.
“We discontinued universal testing after a 7-day period, because we could not justify continued testing of asymptomatic women in the absence of positive test results for SARS-CoV-2 infection,” they noted. “Though universal testing did not yield enough positive results on our obstetric unit to warrant continued testing at this time, our approach may change if local rates of infection increase.”
20 days of testing
In a prospective case series of pregnant women admitted to Northwestern Memorial Hospital in Chicago from April 8 to April 27, 2020, universal testing did detect asymptomatic infections. Women with scheduled admissions were tested 12-36 hours before admission in a drive-through testing center, and women with unscheduled admissions received a test that has a 2- to 3-hour turnaround time. In addition, patients were screened for symptoms such as fever, shortness of breath, cough, sore throat, body aches, chills, new-onset vomiting, diarrhea, loss of taste or smell, and red or painful eyes.
“Asymptomatic women with pending tests were managed on the routine labor floor, but health care workers used personal protective equipment that included a respirator during the second stage of labor and delivery until the test result became available,” wrote Emily S. Miller, MD, MPH, of Northwestern University, Chicago, and colleagues.
During the first 20 days of universal testing, 635 pregnant women were admitted, and 23 (3.6%) tested positive for SARS-CoV-2 infection. Of 21 women with COVID-19 symptoms, 13 (62%) tested positive for SARS-CoV-2 infection. Of 614 women who were asymptomatic, 10 (1.6%) tested positive for SARS-CoV-2. “Our data corroborate the observation that pregnant women with SARS-CoV-2 infection on admission do not seem to be reliably identified using symptom screening alone,” the researchers wrote.
Unintended consequences
Despite a lack of effective treatments for mild to moderate COVID-19, “knowledge of the disease state allows ... health care workers to wear appropriate personal protective equipment to avoid exposure,” Dr. Metz wrote. It also allows “women to be counseled about ways to decrease transmission to neonates” and enables close monitoring of patients with infection.
At the same time, universal testing may have unintended consequences for infected patients, such as stigmatization, separation from the newborn, and delays in care related to health care providers spending more time donning personal protective equipment or changes in medical decision-making regarding cesarean delivery, she emphasized.
“Obstetricians should remain aware of disease prevalence in their communities and consider universal screening of asymptomatic women on an ongoing basis as new ‘hot spots’ for COVID-19 infection are identified,” Dr. Metz concluded.
One of Dr. Naqvi’s coauthors disclosed receiving funds from Contemporary OB/GYN, Keneka, and the American College of Obstetricians and Gynecologists and serving as a board examiner for the American Board of Obstetrics and Gynecology; her coauthors did not report any relevant financial disclosures. Dr. Metz disclosed that money was paid to her institution from Pfizer and GestVision for work related to an RSV vaccination trial and a preeclampsia test, respectively. Dr. Miller and colleagues did not report any potential conflicts of interest.
SOURCES: Naqvi M et al. Obstet Gynecol. 2020 May 19. doi: 10.1097/AOG.0000000000003987; Miller ES et al. Obstet Gynecol. 2020 May 19. doi: 10.1097/AOG.0000000000003983; Metz TD. Obstet Gynecol. 2020 May 19. doi: 10.1097/AOG.0000000000003972.
at the time of admission, research published online in Obstetrics & Gynecology suggests.
In Los Angeles, researchers stopped universal testing after none of the first 80 asymptomatic women had positive results. Researchers in Chicago, on the other hand, found a positive rate of approximately 1.6% among 614 asymptomatic patients and continue to test all patients.
“Decisions regarding universal testing need to be made in the context of regional prevalence of COVID-19 infection, with recognition that a ‘one-size-fits-all’ approach is unlikely to be justifiable,” Torri D. Metz, MD,of University of Utah Health in Salt Lake City said in an editorial accompanying research letters that described the experience in Los Angeles and Chicago. “In the setting of low population prevalence of COVID-19 infection or in locations with limited testing availability, deferring universal testing may represent the better part of valor when weighing risks, benefits, economic burden, and unintended consequences of testing for SARS-CoV-2 infection. In high-prevalence regions, universal testing may be a valuable addition to obstetric care that will prevent infections in health care workers and neonates.”
Testing all patients also may provide valuable population-level surveillance, added Dr. Metz, who is an associate professor of obstetrics and gynecology, a maternal-fetal medicine subspecialist, and vice-chair of research in obstetrics and gynecology.
One week of data
After New York hospitals reported an approximately 13% prevalence of SARS-CoV-2 infection among asymptomatic laboring women, Cedars-Sinai Medical Center in Los Angeles changed its policy from testing only women with COVID-19 symptoms to testing all women beginning April 4, 2020. “Data from New York made us very concerned about the possibility of asymptomatic infections among our own pregnant patients,” Mariam Naqvi, MD, a maternal-fetal medicine specialist at Cedars-Sinai Medical Center, said in a news release. “This would have implications for them, their babies, their households, and for the health of our staff caring for them.”
In 1 week, 82 pregnant women admitted to the obstetric unit were tested for SARS-CoV-2 infection. Of two women who reported COVID-19 symptoms, one tested positive for SARS-CoV-2. “Of the remaining 80 asymptomatic women, none tested positive for SARS-CoV-2 infection, and all remained symptom free throughout their hospitalizations,” Dr. Naqvi and colleagues reported. “One asymptomatic patient had an inadequate nasopharyngeal specimen and declined repeat testing.”
Precautions taken during universal testing meant that all members of the treatment team used valuable personal protective equipment. In some cases, mothers and newborns were separated until test results were available.
“We discontinued universal testing after a 7-day period, because we could not justify continued testing of asymptomatic women in the absence of positive test results for SARS-CoV-2 infection,” they noted. “Though universal testing did not yield enough positive results on our obstetric unit to warrant continued testing at this time, our approach may change if local rates of infection increase.”
20 days of testing
In a prospective case series of pregnant women admitted to Northwestern Memorial Hospital in Chicago from April 8 to April 27, 2020, universal testing did detect asymptomatic infections. Women with scheduled admissions were tested 12-36 hours before admission in a drive-through testing center, and women with unscheduled admissions received a test that has a 2- to 3-hour turnaround time. In addition, patients were screened for symptoms such as fever, shortness of breath, cough, sore throat, body aches, chills, new-onset vomiting, diarrhea, loss of taste or smell, and red or painful eyes.
“Asymptomatic women with pending tests were managed on the routine labor floor, but health care workers used personal protective equipment that included a respirator during the second stage of labor and delivery until the test result became available,” wrote Emily S. Miller, MD, MPH, of Northwestern University, Chicago, and colleagues.
During the first 20 days of universal testing, 635 pregnant women were admitted, and 23 (3.6%) tested positive for SARS-CoV-2 infection. Of 21 women with COVID-19 symptoms, 13 (62%) tested positive for SARS-CoV-2 infection. Of 614 women who were asymptomatic, 10 (1.6%) tested positive for SARS-CoV-2. “Our data corroborate the observation that pregnant women with SARS-CoV-2 infection on admission do not seem to be reliably identified using symptom screening alone,” the researchers wrote.
Unintended consequences
Despite a lack of effective treatments for mild to moderate COVID-19, “knowledge of the disease state allows ... health care workers to wear appropriate personal protective equipment to avoid exposure,” Dr. Metz wrote. It also allows “women to be counseled about ways to decrease transmission to neonates” and enables close monitoring of patients with infection.
At the same time, universal testing may have unintended consequences for infected patients, such as stigmatization, separation from the newborn, and delays in care related to health care providers spending more time donning personal protective equipment or changes in medical decision-making regarding cesarean delivery, she emphasized.
“Obstetricians should remain aware of disease prevalence in their communities and consider universal screening of asymptomatic women on an ongoing basis as new ‘hot spots’ for COVID-19 infection are identified,” Dr. Metz concluded.
One of Dr. Naqvi’s coauthors disclosed receiving funds from Contemporary OB/GYN, Keneka, and the American College of Obstetricians and Gynecologists and serving as a board examiner for the American Board of Obstetrics and Gynecology; her coauthors did not report any relevant financial disclosures. Dr. Metz disclosed that money was paid to her institution from Pfizer and GestVision for work related to an RSV vaccination trial and a preeclampsia test, respectively. Dr. Miller and colleagues did not report any potential conflicts of interest.
SOURCES: Naqvi M et al. Obstet Gynecol. 2020 May 19. doi: 10.1097/AOG.0000000000003987; Miller ES et al. Obstet Gynecol. 2020 May 19. doi: 10.1097/AOG.0000000000003983; Metz TD. Obstet Gynecol. 2020 May 19. doi: 10.1097/AOG.0000000000003972.
at the time of admission, research published online in Obstetrics & Gynecology suggests.
In Los Angeles, researchers stopped universal testing after none of the first 80 asymptomatic women had positive results. Researchers in Chicago, on the other hand, found a positive rate of approximately 1.6% among 614 asymptomatic patients and continue to test all patients.
“Decisions regarding universal testing need to be made in the context of regional prevalence of COVID-19 infection, with recognition that a ‘one-size-fits-all’ approach is unlikely to be justifiable,” Torri D. Metz, MD,of University of Utah Health in Salt Lake City said in an editorial accompanying research letters that described the experience in Los Angeles and Chicago. “In the setting of low population prevalence of COVID-19 infection or in locations with limited testing availability, deferring universal testing may represent the better part of valor when weighing risks, benefits, economic burden, and unintended consequences of testing for SARS-CoV-2 infection. In high-prevalence regions, universal testing may be a valuable addition to obstetric care that will prevent infections in health care workers and neonates.”
Testing all patients also may provide valuable population-level surveillance, added Dr. Metz, who is an associate professor of obstetrics and gynecology, a maternal-fetal medicine subspecialist, and vice-chair of research in obstetrics and gynecology.
One week of data
After New York hospitals reported an approximately 13% prevalence of SARS-CoV-2 infection among asymptomatic laboring women, Cedars-Sinai Medical Center in Los Angeles changed its policy from testing only women with COVID-19 symptoms to testing all women beginning April 4, 2020. “Data from New York made us very concerned about the possibility of asymptomatic infections among our own pregnant patients,” Mariam Naqvi, MD, a maternal-fetal medicine specialist at Cedars-Sinai Medical Center, said in a news release. “This would have implications for them, their babies, their households, and for the health of our staff caring for them.”
In 1 week, 82 pregnant women admitted to the obstetric unit were tested for SARS-CoV-2 infection. Of two women who reported COVID-19 symptoms, one tested positive for SARS-CoV-2. “Of the remaining 80 asymptomatic women, none tested positive for SARS-CoV-2 infection, and all remained symptom free throughout their hospitalizations,” Dr. Naqvi and colleagues reported. “One asymptomatic patient had an inadequate nasopharyngeal specimen and declined repeat testing.”
Precautions taken during universal testing meant that all members of the treatment team used valuable personal protective equipment. In some cases, mothers and newborns were separated until test results were available.
“We discontinued universal testing after a 7-day period, because we could not justify continued testing of asymptomatic women in the absence of positive test results for SARS-CoV-2 infection,” they noted. “Though universal testing did not yield enough positive results on our obstetric unit to warrant continued testing at this time, our approach may change if local rates of infection increase.”
20 days of testing
In a prospective case series of pregnant women admitted to Northwestern Memorial Hospital in Chicago from April 8 to April 27, 2020, universal testing did detect asymptomatic infections. Women with scheduled admissions were tested 12-36 hours before admission in a drive-through testing center, and women with unscheduled admissions received a test that has a 2- to 3-hour turnaround time. In addition, patients were screened for symptoms such as fever, shortness of breath, cough, sore throat, body aches, chills, new-onset vomiting, diarrhea, loss of taste or smell, and red or painful eyes.
“Asymptomatic women with pending tests were managed on the routine labor floor, but health care workers used personal protective equipment that included a respirator during the second stage of labor and delivery until the test result became available,” wrote Emily S. Miller, MD, MPH, of Northwestern University, Chicago, and colleagues.
During the first 20 days of universal testing, 635 pregnant women were admitted, and 23 (3.6%) tested positive for SARS-CoV-2 infection. Of 21 women with COVID-19 symptoms, 13 (62%) tested positive for SARS-CoV-2 infection. Of 614 women who were asymptomatic, 10 (1.6%) tested positive for SARS-CoV-2. “Our data corroborate the observation that pregnant women with SARS-CoV-2 infection on admission do not seem to be reliably identified using symptom screening alone,” the researchers wrote.
Unintended consequences
Despite a lack of effective treatments for mild to moderate COVID-19, “knowledge of the disease state allows ... health care workers to wear appropriate personal protective equipment to avoid exposure,” Dr. Metz wrote. It also allows “women to be counseled about ways to decrease transmission to neonates” and enables close monitoring of patients with infection.
At the same time, universal testing may have unintended consequences for infected patients, such as stigmatization, separation from the newborn, and delays in care related to health care providers spending more time donning personal protective equipment or changes in medical decision-making regarding cesarean delivery, she emphasized.
“Obstetricians should remain aware of disease prevalence in their communities and consider universal screening of asymptomatic women on an ongoing basis as new ‘hot spots’ for COVID-19 infection are identified,” Dr. Metz concluded.
One of Dr. Naqvi’s coauthors disclosed receiving funds from Contemporary OB/GYN, Keneka, and the American College of Obstetricians and Gynecologists and serving as a board examiner for the American Board of Obstetrics and Gynecology; her coauthors did not report any relevant financial disclosures. Dr. Metz disclosed that money was paid to her institution from Pfizer and GestVision for work related to an RSV vaccination trial and a preeclampsia test, respectively. Dr. Miller and colleagues did not report any potential conflicts of interest.
SOURCES: Naqvi M et al. Obstet Gynecol. 2020 May 19. doi: 10.1097/AOG.0000000000003987; Miller ES et al. Obstet Gynecol. 2020 May 19. doi: 10.1097/AOG.0000000000003983; Metz TD. Obstet Gynecol. 2020 May 19. doi: 10.1097/AOG.0000000000003972.
FROM OBSTETRICS & GYNECOLOGY
Half of Americans would get COVID-19 vaccine, poll shows
About half of Americans say they would get a COVID-19 vaccine if one is available, according to the Associated Press.
The poll was conducted May 14-18 and released May 27.
A massive national and international effort is underway to develop a vaccine for the coronavirus. According to the poll, 20% of Americans believe a vaccine will be available before the end of 2020. Another 61% think it will arrive in 2021, and 17% say it will take longer.
“It’s always better to under-promise and over-deliver,” William Schaffner, MD, an infectious disease specialist at Vanderbilt University Medical Center, told the AP.
Americans over age 60 were more likely to say they’ll get a coronavirus vaccine when it’s available. Those who worry that they or someone in their household could become infected with the virus were also more likely to say they’ll get a vaccine. However, Black Americans were more likely than were Hispanic or white responders to say that they don’t plan to get a vaccine.
Among those who plan to get a vaccine, 93% said they want to protect themselves, and 88% said they want to protect their family. About 72% said “life won’t go back to normal until most people are vaccinated,” and 33% said they have a chronic health condition such as asthma or diabetes and believe it’s important to receive a vaccine.
Among those who don’t plan to get a vaccine, 70% said they’re concerned about side effects. Another 42% are worried about getting the coronavirus from the vaccine. Others say they’re not concerned about getting seriously ill from the coronavirus, they don’t think vaccines work well, the COVID-19 outbreak isn’t serious, or they don’t like needles.
The National Institutes of Health says that safety is the top priority and is creating a plan to test the vaccine in thousands of people for safety and efficacy in coming months, according to the AP.
“I would not want people to think that we’re cutting corners because that would be a big mistake,” NIH director Francis Collins, MD, told AP earlier this month. “I think this is an effort to try to achieve efficiencies but not to sacrifice rigor.”
This article first appeared on WebMD.com.
About half of Americans say they would get a COVID-19 vaccine if one is available, according to the Associated Press.
The poll was conducted May 14-18 and released May 27.
A massive national and international effort is underway to develop a vaccine for the coronavirus. According to the poll, 20% of Americans believe a vaccine will be available before the end of 2020. Another 61% think it will arrive in 2021, and 17% say it will take longer.
“It’s always better to under-promise and over-deliver,” William Schaffner, MD, an infectious disease specialist at Vanderbilt University Medical Center, told the AP.
Americans over age 60 were more likely to say they’ll get a coronavirus vaccine when it’s available. Those who worry that they or someone in their household could become infected with the virus were also more likely to say they’ll get a vaccine. However, Black Americans were more likely than were Hispanic or white responders to say that they don’t plan to get a vaccine.
Among those who plan to get a vaccine, 93% said they want to protect themselves, and 88% said they want to protect their family. About 72% said “life won’t go back to normal until most people are vaccinated,” and 33% said they have a chronic health condition such as asthma or diabetes and believe it’s important to receive a vaccine.
Among those who don’t plan to get a vaccine, 70% said they’re concerned about side effects. Another 42% are worried about getting the coronavirus from the vaccine. Others say they’re not concerned about getting seriously ill from the coronavirus, they don’t think vaccines work well, the COVID-19 outbreak isn’t serious, or they don’t like needles.
The National Institutes of Health says that safety is the top priority and is creating a plan to test the vaccine in thousands of people for safety and efficacy in coming months, according to the AP.
“I would not want people to think that we’re cutting corners because that would be a big mistake,” NIH director Francis Collins, MD, told AP earlier this month. “I think this is an effort to try to achieve efficiencies but not to sacrifice rigor.”
This article first appeared on WebMD.com.
About half of Americans say they would get a COVID-19 vaccine if one is available, according to the Associated Press.
The poll was conducted May 14-18 and released May 27.
A massive national and international effort is underway to develop a vaccine for the coronavirus. According to the poll, 20% of Americans believe a vaccine will be available before the end of 2020. Another 61% think it will arrive in 2021, and 17% say it will take longer.
“It’s always better to under-promise and over-deliver,” William Schaffner, MD, an infectious disease specialist at Vanderbilt University Medical Center, told the AP.
Americans over age 60 were more likely to say they’ll get a coronavirus vaccine when it’s available. Those who worry that they or someone in their household could become infected with the virus were also more likely to say they’ll get a vaccine. However, Black Americans were more likely than were Hispanic or white responders to say that they don’t plan to get a vaccine.
Among those who plan to get a vaccine, 93% said they want to protect themselves, and 88% said they want to protect their family. About 72% said “life won’t go back to normal until most people are vaccinated,” and 33% said they have a chronic health condition such as asthma or diabetes and believe it’s important to receive a vaccine.
Among those who don’t plan to get a vaccine, 70% said they’re concerned about side effects. Another 42% are worried about getting the coronavirus from the vaccine. Others say they’re not concerned about getting seriously ill from the coronavirus, they don’t think vaccines work well, the COVID-19 outbreak isn’t serious, or they don’t like needles.
The National Institutes of Health says that safety is the top priority and is creating a plan to test the vaccine in thousands of people for safety and efficacy in coming months, according to the AP.
“I would not want people to think that we’re cutting corners because that would be a big mistake,” NIH director Francis Collins, MD, told AP earlier this month. “I think this is an effort to try to achieve efficiencies but not to sacrifice rigor.”
This article first appeared on WebMD.com.
Can you catch COVID-19 through your eyes?
You can catch COVID-19 if an infected person coughs or sneezes and contagious droplets enter your nose or mouth. But can you become ill if the virus lands in your eyes?
Virologist Joseph Fair, PhD, an NBC News contributor, raised that concern when he became critically ill with COVID-19, the disease caused by the coronavirus. From a hospital bed in his hometown of New Orleans, he told the network that he had flown on a crowded plane where flight attendants weren’t wearing masks. He wore a mask and gloves, but no eye protection.
“My best guess,” he told the interviewer, “was that it came through the eye route.”
Asked if people should start wearing eye protection, Dr. Fair replied, “In my opinion, yes.”
While Dr. Fair is convinced that eye protection helps, other experts aren’t sure. So much remains unknown about the new coronavirus, SARS-CoV-2, that researchers are still trying to establish whether infection can actually happen through the eyes.
“I don’t think we can answer that question with 100% confidence at this time,” said H. Nida Sen, MD, director of the uveitis clinic at the National Eye Institute in Bethesda, Md., and a clinical investigator who is studying the effects of COVID-19 on the eye. But, she says, “I think it is biologically plausible.”
Some research has begun pointing in that direction, according to Elia Duh, MD, a researcher and professor of ophthalmology at Johns Hopkins University in Baltimore.
The clear tissue that covers the white of the eye and lines the inside of the eyelid, known as the conjunctiva, “can be infected by other viruses, such as adenoviruses associated with the common cold and the herpes simplex virus,” he said.
There’s the same chance of infection with SARS-CoV-2, said Dr. Duh. “ just like the nasal passages are exposed. In addition, people rub and touch their eyes a lot. So there’s certainly already the vulnerability.”
To study whether SARS-CoV-2 could infect the eyes, Dr. Duh and fellow researchers at Johns Hopkins looked at whether the eye’s surface cells possess key factors that make the virus more likely to enter and infect them.
In their study (BioRxiv. 2020 May 9. doi: 10.1101/2020.05.09.086165), which is now being peer-reviewed, the team examined 10 postmortem eyes and five surgical samples of conjunctiva from patients who did not have the coronavirus. They wanted to see whether the eyes’ surface cells produced the key receptor for coronavirus, the ACE2 receptor.
For SARS-CoV-2 to enter a cell, “the cell has to have ACE2 on its surface so that the coronavirus can latch onto it and gain entry into the cell,” Dr. Duh said.
Not much research existed on ACE2 and the eye’s surface cells, he said. “We were really struck that ACE2 was clearly present in the surface cells of all of the specimens.” In addition, the researchers found that the eye’s surface cells also produce TMPRSS2, an enzyme that helps the virus enter the cell.
More research is needed for a definitive answer, Dr. Duh said. But “all of this evidence together seems to suggest that there’s a good likelihood that the ocular surface cells are susceptible to infection by coronavirus.”
If that’s the case, the virus then could be transmitted through the tear ducts that connect the eyes to the nasal cavity and subsequently infect the respiratory cells, he said.
Edward E. Manche, MD, professor of ophthalmology at Stanford (Calif.) University, said that while doctors don’t know for sure, many think eye infection can happen. “I think it’s widely believed now that you can acquire it through the eye. The way the virus works, it’s most commonly transmitted through the mouth and nasal passages. We have mucosal tissues where it can get in.”
Dr. Manche said the eyes would be “the least common mode of transmission.”
Besides looking at the eyes as an entryway, researchers are exploring whether people with SARS-CoV-2 in their eyes could infect others through their tears or eye secretions.
“The virus has been detected in tears and conjunctival swab specimens from individuals with COVID-19,” Dr. Duh said. “If someone rubs their eyes and then touches someone else or touches a surface, that kind of transmission mechanism could occur.
“It again highlights how contagious the coronavirus is and how stealthy it can be in its contagiousness,” he said.
If it turns out that the coronavirus can infect the eyes, the virus could persist there as a source of contagion, Dr. Duh said. “The eyes and tears could serve as a source of infection to others for longer.” He noted a case of a COVID-infected woman with conjunctivitis who still had detectable virus in her eyes 3 weeks after her symptoms started.
Conjunctivitis, commonly called pink eye, could be a symptom of COVID-19, said Dr. Sen, who is an ophthalmologist. She recommends that people get tested for COVID-19 if they have this condition, which is marked by redness, itchiness, tearing, discharge, and a gritty sensation in the eye.
Dr. Fair, the virologist, was released from the hospital to recover at home and continued to urge eye protection. “People like to call people like me fearmongers ... but the reality is, we’re just trying to keep them safe,” he told NBC News.
The CDC hasn’t issued such advice. In an email, the agency said it “does not have specific recommendations for the public regarding eye protection. However, in health care settings, the CDC does recommend eye protection for health care workers to prevent transmission via droplets.”
Dr. Sen agrees. “For the general public, I don’t think we have enough data to suggest that they should be covering the eyes in some form,” she said.
When she goes to the grocery store, she doesn’t wear eye protection. “I am only wearing goggles when I’m seeing ophthalmology patients up close, basically because I’m 4 or 5 inches away from them.”
But fuller protection – a mask, gloves, and even eye protection, such as goggles – might help those taking care of a COVID-19 patient at home, Dr. Manche said. “If you’re caring for somebody, that’s a much higher risk because they’re shedding viral load. You lessen the chance of transmission.”
For the public, Dr. Sen stresses the continued importance of hand hygiene. “In an abundance of caution, I would still encourage handwashing and not touching the eye for many reasons, not just COVID. You can transmit simple infections to your eye. We have other viruses and bacteria that are circulating in the environment and in our bodies elsewhere, so we can easily carry those to the eyes.”
Switching from contact lenses to eyeglasses could help cut down on touching the eyes, she says. Eyeglasses can also be a “mechanical barrier” to keep hands away.
Eyeglasses might block some droplets if someone nearby sneezes or coughs, Dr. Manche said, although they “aren’t sealed around the edges. They’re not like true medical goggles that are going to keep out the virus.”
Dr. Duh agrees that health care workers must don eye protection, but he said the public doesn’t need to start wearing goggles, face shields, or other eye protection. “I still think the major mode of transmission is through the nasal passages and the respiratory system,” he said.
It’s unclear whether eye protection is warranted for airplane passengers, Dr. Manche said. “It probably wouldn’t hurt, but I think the more important thing would be to take precautions: wearing a face mask, washing your hands, cleaning the seats and tray tables in front of you, and not touching things and touching your face and eyes.”
A version of this article originally appeared on WebMD.com.
You can catch COVID-19 if an infected person coughs or sneezes and contagious droplets enter your nose or mouth. But can you become ill if the virus lands in your eyes?
Virologist Joseph Fair, PhD, an NBC News contributor, raised that concern when he became critically ill with COVID-19, the disease caused by the coronavirus. From a hospital bed in his hometown of New Orleans, he told the network that he had flown on a crowded plane where flight attendants weren’t wearing masks. He wore a mask and gloves, but no eye protection.
“My best guess,” he told the interviewer, “was that it came through the eye route.”
Asked if people should start wearing eye protection, Dr. Fair replied, “In my opinion, yes.”
While Dr. Fair is convinced that eye protection helps, other experts aren’t sure. So much remains unknown about the new coronavirus, SARS-CoV-2, that researchers are still trying to establish whether infection can actually happen through the eyes.
“I don’t think we can answer that question with 100% confidence at this time,” said H. Nida Sen, MD, director of the uveitis clinic at the National Eye Institute in Bethesda, Md., and a clinical investigator who is studying the effects of COVID-19 on the eye. But, she says, “I think it is biologically plausible.”
Some research has begun pointing in that direction, according to Elia Duh, MD, a researcher and professor of ophthalmology at Johns Hopkins University in Baltimore.
The clear tissue that covers the white of the eye and lines the inside of the eyelid, known as the conjunctiva, “can be infected by other viruses, such as adenoviruses associated with the common cold and the herpes simplex virus,” he said.
There’s the same chance of infection with SARS-CoV-2, said Dr. Duh. “ just like the nasal passages are exposed. In addition, people rub and touch their eyes a lot. So there’s certainly already the vulnerability.”
To study whether SARS-CoV-2 could infect the eyes, Dr. Duh and fellow researchers at Johns Hopkins looked at whether the eye’s surface cells possess key factors that make the virus more likely to enter and infect them.
In their study (BioRxiv. 2020 May 9. doi: 10.1101/2020.05.09.086165), which is now being peer-reviewed, the team examined 10 postmortem eyes and five surgical samples of conjunctiva from patients who did not have the coronavirus. They wanted to see whether the eyes’ surface cells produced the key receptor for coronavirus, the ACE2 receptor.
For SARS-CoV-2 to enter a cell, “the cell has to have ACE2 on its surface so that the coronavirus can latch onto it and gain entry into the cell,” Dr. Duh said.
Not much research existed on ACE2 and the eye’s surface cells, he said. “We were really struck that ACE2 was clearly present in the surface cells of all of the specimens.” In addition, the researchers found that the eye’s surface cells also produce TMPRSS2, an enzyme that helps the virus enter the cell.
More research is needed for a definitive answer, Dr. Duh said. But “all of this evidence together seems to suggest that there’s a good likelihood that the ocular surface cells are susceptible to infection by coronavirus.”
If that’s the case, the virus then could be transmitted through the tear ducts that connect the eyes to the nasal cavity and subsequently infect the respiratory cells, he said.
Edward E. Manche, MD, professor of ophthalmology at Stanford (Calif.) University, said that while doctors don’t know for sure, many think eye infection can happen. “I think it’s widely believed now that you can acquire it through the eye. The way the virus works, it’s most commonly transmitted through the mouth and nasal passages. We have mucosal tissues where it can get in.”
Dr. Manche said the eyes would be “the least common mode of transmission.”
Besides looking at the eyes as an entryway, researchers are exploring whether people with SARS-CoV-2 in their eyes could infect others through their tears or eye secretions.
“The virus has been detected in tears and conjunctival swab specimens from individuals with COVID-19,” Dr. Duh said. “If someone rubs their eyes and then touches someone else or touches a surface, that kind of transmission mechanism could occur.
“It again highlights how contagious the coronavirus is and how stealthy it can be in its contagiousness,” he said.
If it turns out that the coronavirus can infect the eyes, the virus could persist there as a source of contagion, Dr. Duh said. “The eyes and tears could serve as a source of infection to others for longer.” He noted a case of a COVID-infected woman with conjunctivitis who still had detectable virus in her eyes 3 weeks after her symptoms started.
Conjunctivitis, commonly called pink eye, could be a symptom of COVID-19, said Dr. Sen, who is an ophthalmologist. She recommends that people get tested for COVID-19 if they have this condition, which is marked by redness, itchiness, tearing, discharge, and a gritty sensation in the eye.
Dr. Fair, the virologist, was released from the hospital to recover at home and continued to urge eye protection. “People like to call people like me fearmongers ... but the reality is, we’re just trying to keep them safe,” he told NBC News.
The CDC hasn’t issued such advice. In an email, the agency said it “does not have specific recommendations for the public regarding eye protection. However, in health care settings, the CDC does recommend eye protection for health care workers to prevent transmission via droplets.”
Dr. Sen agrees. “For the general public, I don’t think we have enough data to suggest that they should be covering the eyes in some form,” she said.
When she goes to the grocery store, she doesn’t wear eye protection. “I am only wearing goggles when I’m seeing ophthalmology patients up close, basically because I’m 4 or 5 inches away from them.”
But fuller protection – a mask, gloves, and even eye protection, such as goggles – might help those taking care of a COVID-19 patient at home, Dr. Manche said. “If you’re caring for somebody, that’s a much higher risk because they’re shedding viral load. You lessen the chance of transmission.”
For the public, Dr. Sen stresses the continued importance of hand hygiene. “In an abundance of caution, I would still encourage handwashing and not touching the eye for many reasons, not just COVID. You can transmit simple infections to your eye. We have other viruses and bacteria that are circulating in the environment and in our bodies elsewhere, so we can easily carry those to the eyes.”
Switching from contact lenses to eyeglasses could help cut down on touching the eyes, she says. Eyeglasses can also be a “mechanical barrier” to keep hands away.
Eyeglasses might block some droplets if someone nearby sneezes or coughs, Dr. Manche said, although they “aren’t sealed around the edges. They’re not like true medical goggles that are going to keep out the virus.”
Dr. Duh agrees that health care workers must don eye protection, but he said the public doesn’t need to start wearing goggles, face shields, or other eye protection. “I still think the major mode of transmission is through the nasal passages and the respiratory system,” he said.
It’s unclear whether eye protection is warranted for airplane passengers, Dr. Manche said. “It probably wouldn’t hurt, but I think the more important thing would be to take precautions: wearing a face mask, washing your hands, cleaning the seats and tray tables in front of you, and not touching things and touching your face and eyes.”
A version of this article originally appeared on WebMD.com.
You can catch COVID-19 if an infected person coughs or sneezes and contagious droplets enter your nose or mouth. But can you become ill if the virus lands in your eyes?
Virologist Joseph Fair, PhD, an NBC News contributor, raised that concern when he became critically ill with COVID-19, the disease caused by the coronavirus. From a hospital bed in his hometown of New Orleans, he told the network that he had flown on a crowded plane where flight attendants weren’t wearing masks. He wore a mask and gloves, but no eye protection.
“My best guess,” he told the interviewer, “was that it came through the eye route.”
Asked if people should start wearing eye protection, Dr. Fair replied, “In my opinion, yes.”
While Dr. Fair is convinced that eye protection helps, other experts aren’t sure. So much remains unknown about the new coronavirus, SARS-CoV-2, that researchers are still trying to establish whether infection can actually happen through the eyes.
“I don’t think we can answer that question with 100% confidence at this time,” said H. Nida Sen, MD, director of the uveitis clinic at the National Eye Institute in Bethesda, Md., and a clinical investigator who is studying the effects of COVID-19 on the eye. But, she says, “I think it is biologically plausible.”
Some research has begun pointing in that direction, according to Elia Duh, MD, a researcher and professor of ophthalmology at Johns Hopkins University in Baltimore.
The clear tissue that covers the white of the eye and lines the inside of the eyelid, known as the conjunctiva, “can be infected by other viruses, such as adenoviruses associated with the common cold and the herpes simplex virus,” he said.
There’s the same chance of infection with SARS-CoV-2, said Dr. Duh. “ just like the nasal passages are exposed. In addition, people rub and touch their eyes a lot. So there’s certainly already the vulnerability.”
To study whether SARS-CoV-2 could infect the eyes, Dr. Duh and fellow researchers at Johns Hopkins looked at whether the eye’s surface cells possess key factors that make the virus more likely to enter and infect them.
In their study (BioRxiv. 2020 May 9. doi: 10.1101/2020.05.09.086165), which is now being peer-reviewed, the team examined 10 postmortem eyes and five surgical samples of conjunctiva from patients who did not have the coronavirus. They wanted to see whether the eyes’ surface cells produced the key receptor for coronavirus, the ACE2 receptor.
For SARS-CoV-2 to enter a cell, “the cell has to have ACE2 on its surface so that the coronavirus can latch onto it and gain entry into the cell,” Dr. Duh said.
Not much research existed on ACE2 and the eye’s surface cells, he said. “We were really struck that ACE2 was clearly present in the surface cells of all of the specimens.” In addition, the researchers found that the eye’s surface cells also produce TMPRSS2, an enzyme that helps the virus enter the cell.
More research is needed for a definitive answer, Dr. Duh said. But “all of this evidence together seems to suggest that there’s a good likelihood that the ocular surface cells are susceptible to infection by coronavirus.”
If that’s the case, the virus then could be transmitted through the tear ducts that connect the eyes to the nasal cavity and subsequently infect the respiratory cells, he said.
Edward E. Manche, MD, professor of ophthalmology at Stanford (Calif.) University, said that while doctors don’t know for sure, many think eye infection can happen. “I think it’s widely believed now that you can acquire it through the eye. The way the virus works, it’s most commonly transmitted through the mouth and nasal passages. We have mucosal tissues where it can get in.”
Dr. Manche said the eyes would be “the least common mode of transmission.”
Besides looking at the eyes as an entryway, researchers are exploring whether people with SARS-CoV-2 in their eyes could infect others through their tears or eye secretions.
“The virus has been detected in tears and conjunctival swab specimens from individuals with COVID-19,” Dr. Duh said. “If someone rubs their eyes and then touches someone else or touches a surface, that kind of transmission mechanism could occur.
“It again highlights how contagious the coronavirus is and how stealthy it can be in its contagiousness,” he said.
If it turns out that the coronavirus can infect the eyes, the virus could persist there as a source of contagion, Dr. Duh said. “The eyes and tears could serve as a source of infection to others for longer.” He noted a case of a COVID-infected woman with conjunctivitis who still had detectable virus in her eyes 3 weeks after her symptoms started.
Conjunctivitis, commonly called pink eye, could be a symptom of COVID-19, said Dr. Sen, who is an ophthalmologist. She recommends that people get tested for COVID-19 if they have this condition, which is marked by redness, itchiness, tearing, discharge, and a gritty sensation in the eye.
Dr. Fair, the virologist, was released from the hospital to recover at home and continued to urge eye protection. “People like to call people like me fearmongers ... but the reality is, we’re just trying to keep them safe,” he told NBC News.
The CDC hasn’t issued such advice. In an email, the agency said it “does not have specific recommendations for the public regarding eye protection. However, in health care settings, the CDC does recommend eye protection for health care workers to prevent transmission via droplets.”
Dr. Sen agrees. “For the general public, I don’t think we have enough data to suggest that they should be covering the eyes in some form,” she said.
When she goes to the grocery store, she doesn’t wear eye protection. “I am only wearing goggles when I’m seeing ophthalmology patients up close, basically because I’m 4 or 5 inches away from them.”
But fuller protection – a mask, gloves, and even eye protection, such as goggles – might help those taking care of a COVID-19 patient at home, Dr. Manche said. “If you’re caring for somebody, that’s a much higher risk because they’re shedding viral load. You lessen the chance of transmission.”
For the public, Dr. Sen stresses the continued importance of hand hygiene. “In an abundance of caution, I would still encourage handwashing and not touching the eye for many reasons, not just COVID. You can transmit simple infections to your eye. We have other viruses and bacteria that are circulating in the environment and in our bodies elsewhere, so we can easily carry those to the eyes.”
Switching from contact lenses to eyeglasses could help cut down on touching the eyes, she says. Eyeglasses can also be a “mechanical barrier” to keep hands away.
Eyeglasses might block some droplets if someone nearby sneezes or coughs, Dr. Manche said, although they “aren’t sealed around the edges. They’re not like true medical goggles that are going to keep out the virus.”
Dr. Duh agrees that health care workers must don eye protection, but he said the public doesn’t need to start wearing goggles, face shields, or other eye protection. “I still think the major mode of transmission is through the nasal passages and the respiratory system,” he said.
It’s unclear whether eye protection is warranted for airplane passengers, Dr. Manche said. “It probably wouldn’t hurt, but I think the more important thing would be to take precautions: wearing a face mask, washing your hands, cleaning the seats and tray tables in front of you, and not touching things and touching your face and eyes.”
A version of this article originally appeared on WebMD.com.
Severe disease not uncommon in children hospitalized with COVID-19
Children with COVID-19 are more likely to develop severe illness and require intensive care than previously realized, data from a single-center study suggest.
Jerry Y. Chao, MD, of the department of anesthesiology, Albert Einstein College of Medicine, New York, and colleagues reported their findings in an article published online May 11 in the Journal of Pediatrics.
“Thankfully most children with COVID-19 fare well, and some do not have any symptoms at all, but this research is a sobering reminder that children are not immune to this virus and some do require a higher level of care,” senior author Shivanand S. Medar, MD, FAAP, attending physician, Cardiac Intensive Care, Children’s Hospital at Montefiore, and assistant professor of pediatrics, Albert Einstein College of Medicine, said in a Montefiore Medical Center news release.
The study included 67 patients aged 1 month to 21 years (median, 13.1 years) who were treated for COVID-19 at a tertiary care children’s hospital between March 15 and April 13. Of those, 21 (31.3%) were treated as outpatients.
“As the number of patients screened for COVID-19 was restricted during the first weeks of the outbreak because of limited testing availability, the number of mildly symptomatic patients is not known, and therefore these 21 patients are not included in the analysis,” the authors wrote.
Of the 46 hospitalized patients, 33 (72%) were admitted to a general pediatric medical ward, and 13 (28%) were admitted to the pediatric intensive care unit (PICU).
Almost one-third (14 children; 30.4%) of the admitted patients were obese, and almost one-quarter (11 children; 24.4%) had asthma, but neither factor was associated with an increased risk for PICU admission.
“We know that in adults, obesity is a risk factor for more severe disease, however, surprisingly, our study found that children admitted to the intensive care unit did not have a higher prevalence of obesity than those on the general unit,” Dr. Chao said in the news release.
Three of the PICU patients (25%) had preexisting seizure disorders, as did one (3%) patient on the general medical unit. “There was no significant difference in the usage of ibuprofen prior to hospitalization among patients admitted to medical unit compared with those admitted to the PICU,” the authors wrote.
Platelet counts were lower in patients admitted to the PICU compared with those on the general medical unit; however, C-reactive protein, procalcitonin, and pro–brain natriuretic peptide levels were all elevated in patients admitted to the PICU compared with those admitted to the general medical unit.
Patients admitted to the PICU were more likely to need high-flow nasal cannula. Ten (77%) patients in the PICU developed acute respiratory distress syndrome (ARDS), and six (46.2%) of them needed “invasive mechanical ventilation for a median of 9 days.”
The only clinical symptom significantly linked to PICU admission was shortness of breath (92.3% vs 30.3%; P < .001).
Eight (61.5%) of the 13 patients treated in the PICU were discharged to home; four (30.7%) were still hospitalized and receiving ventilatory support on day 14. One patient had metastatic cancer and died as a result of the cancer after life-sustaining therapy was withdrawn.
Those admitted to the PICU were more likely to receive treatment with remdesivir via compassionate use compared with those treated in the general medical unit. Seven (53.8%) patients in the PICU developed severe sepsis and septic shock syndromes.
The average hospital stay was 4 days longer for the children admitted to the PICU than for the children admitted to the general medical unit.
Cough (63%) and fever (60.9%) were the most frequently reported symptoms at admission. The median duration of symptoms before admission was 3 days. None of the children had traveled to an area affected by COVID-19 before becoming ill, and only 20 (43.5%) children were confirmed to have had contact with someone with COVID-19. “The lack of a known sick contact reported in our study may have implications for how healthcare providers identify and screen for potential cases,” the authors explained.
Although children are believed to experience milder SARS-CoV-2 illness, these results and those of an earlier study suggest that some pediatric patients develop illness severe enough to require PICU admission. “This subset had significantly higher markers of inflammation (CRP, pro-BNP, procalcitonin) compared with patients in the medical unit. Inflammation likely contributed to the high rate of ARDS we observed, although serum levels of IL-6 and other cytokines linked to ARDS were not determined,” the authors wrote.
A retrospective cohort study found that of 177 children and young adults treated in a single center, patients younger than 1 year and older than 15 years were more likely to become critically ill with COVID-19 (J Pediatr. 2020 May. doi: 10.1016/j.jpeds.2020.05.007).
Each of the two age groups accounted for 32% of the hospitalized patients.
The authors have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Children with COVID-19 are more likely to develop severe illness and require intensive care than previously realized, data from a single-center study suggest.
Jerry Y. Chao, MD, of the department of anesthesiology, Albert Einstein College of Medicine, New York, and colleagues reported their findings in an article published online May 11 in the Journal of Pediatrics.
“Thankfully most children with COVID-19 fare well, and some do not have any symptoms at all, but this research is a sobering reminder that children are not immune to this virus and some do require a higher level of care,” senior author Shivanand S. Medar, MD, FAAP, attending physician, Cardiac Intensive Care, Children’s Hospital at Montefiore, and assistant professor of pediatrics, Albert Einstein College of Medicine, said in a Montefiore Medical Center news release.
The study included 67 patients aged 1 month to 21 years (median, 13.1 years) who were treated for COVID-19 at a tertiary care children’s hospital between March 15 and April 13. Of those, 21 (31.3%) were treated as outpatients.
“As the number of patients screened for COVID-19 was restricted during the first weeks of the outbreak because of limited testing availability, the number of mildly symptomatic patients is not known, and therefore these 21 patients are not included in the analysis,” the authors wrote.
Of the 46 hospitalized patients, 33 (72%) were admitted to a general pediatric medical ward, and 13 (28%) were admitted to the pediatric intensive care unit (PICU).
Almost one-third (14 children; 30.4%) of the admitted patients were obese, and almost one-quarter (11 children; 24.4%) had asthma, but neither factor was associated with an increased risk for PICU admission.
“We know that in adults, obesity is a risk factor for more severe disease, however, surprisingly, our study found that children admitted to the intensive care unit did not have a higher prevalence of obesity than those on the general unit,” Dr. Chao said in the news release.
Three of the PICU patients (25%) had preexisting seizure disorders, as did one (3%) patient on the general medical unit. “There was no significant difference in the usage of ibuprofen prior to hospitalization among patients admitted to medical unit compared with those admitted to the PICU,” the authors wrote.
Platelet counts were lower in patients admitted to the PICU compared with those on the general medical unit; however, C-reactive protein, procalcitonin, and pro–brain natriuretic peptide levels were all elevated in patients admitted to the PICU compared with those admitted to the general medical unit.
Patients admitted to the PICU were more likely to need high-flow nasal cannula. Ten (77%) patients in the PICU developed acute respiratory distress syndrome (ARDS), and six (46.2%) of them needed “invasive mechanical ventilation for a median of 9 days.”
The only clinical symptom significantly linked to PICU admission was shortness of breath (92.3% vs 30.3%; P < .001).
Eight (61.5%) of the 13 patients treated in the PICU were discharged to home; four (30.7%) were still hospitalized and receiving ventilatory support on day 14. One patient had metastatic cancer and died as a result of the cancer after life-sustaining therapy was withdrawn.
Those admitted to the PICU were more likely to receive treatment with remdesivir via compassionate use compared with those treated in the general medical unit. Seven (53.8%) patients in the PICU developed severe sepsis and septic shock syndromes.
The average hospital stay was 4 days longer for the children admitted to the PICU than for the children admitted to the general medical unit.
Cough (63%) and fever (60.9%) were the most frequently reported symptoms at admission. The median duration of symptoms before admission was 3 days. None of the children had traveled to an area affected by COVID-19 before becoming ill, and only 20 (43.5%) children were confirmed to have had contact with someone with COVID-19. “The lack of a known sick contact reported in our study may have implications for how healthcare providers identify and screen for potential cases,” the authors explained.
Although children are believed to experience milder SARS-CoV-2 illness, these results and those of an earlier study suggest that some pediatric patients develop illness severe enough to require PICU admission. “This subset had significantly higher markers of inflammation (CRP, pro-BNP, procalcitonin) compared with patients in the medical unit. Inflammation likely contributed to the high rate of ARDS we observed, although serum levels of IL-6 and other cytokines linked to ARDS were not determined,” the authors wrote.
A retrospective cohort study found that of 177 children and young adults treated in a single center, patients younger than 1 year and older than 15 years were more likely to become critically ill with COVID-19 (J Pediatr. 2020 May. doi: 10.1016/j.jpeds.2020.05.007).
Each of the two age groups accounted for 32% of the hospitalized patients.
The authors have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Children with COVID-19 are more likely to develop severe illness and require intensive care than previously realized, data from a single-center study suggest.
Jerry Y. Chao, MD, of the department of anesthesiology, Albert Einstein College of Medicine, New York, and colleagues reported their findings in an article published online May 11 in the Journal of Pediatrics.
“Thankfully most children with COVID-19 fare well, and some do not have any symptoms at all, but this research is a sobering reminder that children are not immune to this virus and some do require a higher level of care,” senior author Shivanand S. Medar, MD, FAAP, attending physician, Cardiac Intensive Care, Children’s Hospital at Montefiore, and assistant professor of pediatrics, Albert Einstein College of Medicine, said in a Montefiore Medical Center news release.
The study included 67 patients aged 1 month to 21 years (median, 13.1 years) who were treated for COVID-19 at a tertiary care children’s hospital between March 15 and April 13. Of those, 21 (31.3%) were treated as outpatients.
“As the number of patients screened for COVID-19 was restricted during the first weeks of the outbreak because of limited testing availability, the number of mildly symptomatic patients is not known, and therefore these 21 patients are not included in the analysis,” the authors wrote.
Of the 46 hospitalized patients, 33 (72%) were admitted to a general pediatric medical ward, and 13 (28%) were admitted to the pediatric intensive care unit (PICU).
Almost one-third (14 children; 30.4%) of the admitted patients were obese, and almost one-quarter (11 children; 24.4%) had asthma, but neither factor was associated with an increased risk for PICU admission.
“We know that in adults, obesity is a risk factor for more severe disease, however, surprisingly, our study found that children admitted to the intensive care unit did not have a higher prevalence of obesity than those on the general unit,” Dr. Chao said in the news release.
Three of the PICU patients (25%) had preexisting seizure disorders, as did one (3%) patient on the general medical unit. “There was no significant difference in the usage of ibuprofen prior to hospitalization among patients admitted to medical unit compared with those admitted to the PICU,” the authors wrote.
Platelet counts were lower in patients admitted to the PICU compared with those on the general medical unit; however, C-reactive protein, procalcitonin, and pro–brain natriuretic peptide levels were all elevated in patients admitted to the PICU compared with those admitted to the general medical unit.
Patients admitted to the PICU were more likely to need high-flow nasal cannula. Ten (77%) patients in the PICU developed acute respiratory distress syndrome (ARDS), and six (46.2%) of them needed “invasive mechanical ventilation for a median of 9 days.”
The only clinical symptom significantly linked to PICU admission was shortness of breath (92.3% vs 30.3%; P < .001).
Eight (61.5%) of the 13 patients treated in the PICU were discharged to home; four (30.7%) were still hospitalized and receiving ventilatory support on day 14. One patient had metastatic cancer and died as a result of the cancer after life-sustaining therapy was withdrawn.
Those admitted to the PICU were more likely to receive treatment with remdesivir via compassionate use compared with those treated in the general medical unit. Seven (53.8%) patients in the PICU developed severe sepsis and septic shock syndromes.
The average hospital stay was 4 days longer for the children admitted to the PICU than for the children admitted to the general medical unit.
Cough (63%) and fever (60.9%) were the most frequently reported symptoms at admission. The median duration of symptoms before admission was 3 days. None of the children had traveled to an area affected by COVID-19 before becoming ill, and only 20 (43.5%) children were confirmed to have had contact with someone with COVID-19. “The lack of a known sick contact reported in our study may have implications for how healthcare providers identify and screen for potential cases,” the authors explained.
Although children are believed to experience milder SARS-CoV-2 illness, these results and those of an earlier study suggest that some pediatric patients develop illness severe enough to require PICU admission. “This subset had significantly higher markers of inflammation (CRP, pro-BNP, procalcitonin) compared with patients in the medical unit. Inflammation likely contributed to the high rate of ARDS we observed, although serum levels of IL-6 and other cytokines linked to ARDS were not determined,” the authors wrote.
A retrospective cohort study found that of 177 children and young adults treated in a single center, patients younger than 1 year and older than 15 years were more likely to become critically ill with COVID-19 (J Pediatr. 2020 May. doi: 10.1016/j.jpeds.2020.05.007).
Each of the two age groups accounted for 32% of the hospitalized patients.
The authors have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Remdesivir shortens COVID-19 time to recovery in published study
Much-anticipated results from the National Institute of Allergy and Infectious Diseases’ clinical trial of remdesivir in COVID-19 patients published in the New England Journal of Medicine suggest remdesivir shortens the disease course for hospitalized COVID-19 patients.
The agency reported initial promising results from the study earlier this month, which prompted the Food and Drug Administration to issue an emergency use authorization (EUA) for the drug, but the full data and results have not been widely available until now.
The findings also suggest remdesivir should be started, if possible, before patients have such severe pulmonary disease that they require mechanical ventilation, according to the study authors.
The published results are “completely consistent” with the NIAID’s earlier announcement, H. Clifford Lane, MD, deputy director for clinical research and special projects at the NIAID, said in an interview. “The benefit appeared to be the greatest for the patients who are hospitalized with severe disease who require supplemental oxygen.”
Given the limited supply of remdesivir, physicians have been eager to see the full data to ensure they use the drug most effectively, Daniel Kaul, MD, a professor of infectious diseases at the University of Michigan, Ann Arbor, said in an interview. Hospitals in states across the country, including New York, Michigan, and Washington, have received limited supplies of the drug in the last couple of weeks since the FDA’s authorization.
“I am losing my patience waiting for #remdesivir data. I was willing to give them a week to verify the numbers, triple proof the tables, cautiously frame conclusions. But it’s gone on too long. We are rationing with no rationale. We are floating on whisps [sic] of data, adrift,” Kate Stephenson, MD, an infectious diseases specialist at the Center for Virology and Vaccine Research at Harvard Medical School, Boston, wrote on Twitter May 18. After reading the paper, she tweeted Friday evening that she was “relieved to see convincing benefit – I was starting to worry!”
In the midst of a public health crisis, however, it is not unusual to make an announcement about trial results before the full dataset has been analyzed, said Dr. Lane. The NIAID followed a similar playbook for the PALM trial evaluating possible Ebola treatments in the Democratic Republic of Congo, with the independent monitoring board recommending the trial be terminated early in response to positive results from two of the four candidate drugs.
“When you have a result you think is of public health importance, you don’t wait for it to be published in a peer-reviewed journal,” said Dr. Lane, a coauthor of the study. The lag time from announcement to study publication was a result of the time it took to write up the paper for publication and go through peer review, Dr. Lane added. He also noted that the FDA had access to the data when the agency wrote its guidance for physicians administering the drug to patients under the EUA.
The authors opted not to publish the initial findings on a preprint server because they felt it was important to undergo peer review, said Dr. Lane. “The last thing you want for something this critical is for incomplete data to be out there, or you don’t have everything audited to the level that you want.”
Trial details
In the ACTT-1 randomized, placebo-controlled, double-blinded trial, researchers enrolled 1,063 patients from Feb. 21 to April 19, 2020, at 60 trial sites and 13 subsites worldwide (45 sites in the United States). The remdesivir group had 541 patients, and the placebo group had 522. A small number of patients (49 in the remdesivir group and 53 in the placebo group) discontinued treatment before day 10 because of an adverse event or withdrawn consent. When data collection for this preliminary analysis ended in late April, 301 patients had not recovered and had not completed their final follow-up visit.
Most of the patients had one (27%) or more (52.1%) preexisting conditions, including hypertension (49.6%), obesity (37%), and type 2 diabetes mellitus (29.7%). Mean patient age was 58.9 years, and the majority of patients were men (64.3%). The median number of days from symptom onset to randomization was 9, and 53.6% of the patients were white, 20.6% were black, 12.6% were Asian, 23.4% were Hispanic or Latino, and the ethnicity of 13.6% were not reported or reported as other.
Patients received one 200-mg loading dose on the first day of the trial, and then one 100-mg maintenance dose every day for days 2 through 10, or until discharge or death. Patients in the control group of the study received a matching placebo on the same schedule and volume. The clinical status of each patient was assessed every day, from day 1 through day 29 of his or her hospital stay, according to an eight-category ordinal scale.
Time to recovery was defined as the first day during the 28-day enrollment period that a patient’s clinical status met a 1 (not hospitalization, no activity limitations), 2 (not hospitalized, activity limitation, oxygen requirement or both), or 3 (hospitalized, not requiring supplemental oxygen or medical care if hospitalization was extended for infection-control reasons) on the eight-category scale. A score of 4 indicated a patient was hospitalized and needed ongoing medical care, but did not require supplemental oxygen; a score of 8 signified death.
The analysis found remdesivir patients had a median time to recovery of 11 days, compared with the median 15 days for patients on the placebo (rate ratio for recovery, 1.32; 95% confidence interval, 1.12-1.55; P < .001). Mortality was also lower in the remdesivir group (hazard ratio for death, 0.70; 95% CI, 0.47-1.04), but the result was not statistically significant. By 14 days, the Kaplan-Meier estimate of mortality was 7.1 % in the remdesivir group and 11.9% in the placebo group.
Patients receiving oxygen, but not yet requiring high-flow oxygen, mechanical ventilation, or extracorporeal membrane oxygenation, seemed to fare best from treatment with remdesivir (these patients had a baseline ordinal score of 5). That may be a result of the larger sample size of these patients, the researchers note in the study. The study authors were unable to estimate the recovery time for the most severely ill patients (category 7), possibly because the follow-up time was too short to fully evaluate this subgroup.
“There is clear and consistent evidence of clinically significant benefit for those hospitalized on oxygen but not yet requiring mechanical ventilation,” Dr. Kaul, who was not involved in the study, said after seeing the published results. “Surprisingly, early dosing as measured from time to onset of symptoms did not seem to make a difference.”
Dr. Kaul said there is still the possibility that remdesivir could benefit patients on mechanical ventilation, but “clinicians will have to determine if the evidence suggesting no benefit in those who are intubated is strong enough to justify using this currently scarce resource in that population versus limiting use to those requiring oxygen but not on mechanical ventilation.”
Site investigators estimated that just four serious adverse events (two in each group) in enrolled patients were related to remdesivir or placebo. No deaths were attributed to the treatments, although acute respiratory failure, hypotension, acute kidney injury, and viral pneumonia were slightly more common in patients receiving the placebo than those receiving remdesivir.
The researchers plan to publish a follow-up study in the coming weeks or months, after the full cohort has completed 28 days of follow-up, Dr. Lane said. In future studies, the agency will likely focus on comparing remdesivir with combinations of remdesivir with other treatments, like the anti-inflammatory baricitinib.
A version of this article originally appeared on Medscape.com.
Much-anticipated results from the National Institute of Allergy and Infectious Diseases’ clinical trial of remdesivir in COVID-19 patients published in the New England Journal of Medicine suggest remdesivir shortens the disease course for hospitalized COVID-19 patients.
The agency reported initial promising results from the study earlier this month, which prompted the Food and Drug Administration to issue an emergency use authorization (EUA) for the drug, but the full data and results have not been widely available until now.
The findings also suggest remdesivir should be started, if possible, before patients have such severe pulmonary disease that they require mechanical ventilation, according to the study authors.
The published results are “completely consistent” with the NIAID’s earlier announcement, H. Clifford Lane, MD, deputy director for clinical research and special projects at the NIAID, said in an interview. “The benefit appeared to be the greatest for the patients who are hospitalized with severe disease who require supplemental oxygen.”
Given the limited supply of remdesivir, physicians have been eager to see the full data to ensure they use the drug most effectively, Daniel Kaul, MD, a professor of infectious diseases at the University of Michigan, Ann Arbor, said in an interview. Hospitals in states across the country, including New York, Michigan, and Washington, have received limited supplies of the drug in the last couple of weeks since the FDA’s authorization.
“I am losing my patience waiting for #remdesivir data. I was willing to give them a week to verify the numbers, triple proof the tables, cautiously frame conclusions. But it’s gone on too long. We are rationing with no rationale. We are floating on whisps [sic] of data, adrift,” Kate Stephenson, MD, an infectious diseases specialist at the Center for Virology and Vaccine Research at Harvard Medical School, Boston, wrote on Twitter May 18. After reading the paper, she tweeted Friday evening that she was “relieved to see convincing benefit – I was starting to worry!”
In the midst of a public health crisis, however, it is not unusual to make an announcement about trial results before the full dataset has been analyzed, said Dr. Lane. The NIAID followed a similar playbook for the PALM trial evaluating possible Ebola treatments in the Democratic Republic of Congo, with the independent monitoring board recommending the trial be terminated early in response to positive results from two of the four candidate drugs.
“When you have a result you think is of public health importance, you don’t wait for it to be published in a peer-reviewed journal,” said Dr. Lane, a coauthor of the study. The lag time from announcement to study publication was a result of the time it took to write up the paper for publication and go through peer review, Dr. Lane added. He also noted that the FDA had access to the data when the agency wrote its guidance for physicians administering the drug to patients under the EUA.
The authors opted not to publish the initial findings on a preprint server because they felt it was important to undergo peer review, said Dr. Lane. “The last thing you want for something this critical is for incomplete data to be out there, or you don’t have everything audited to the level that you want.”
Trial details
In the ACTT-1 randomized, placebo-controlled, double-blinded trial, researchers enrolled 1,063 patients from Feb. 21 to April 19, 2020, at 60 trial sites and 13 subsites worldwide (45 sites in the United States). The remdesivir group had 541 patients, and the placebo group had 522. A small number of patients (49 in the remdesivir group and 53 in the placebo group) discontinued treatment before day 10 because of an adverse event or withdrawn consent. When data collection for this preliminary analysis ended in late April, 301 patients had not recovered and had not completed their final follow-up visit.
Most of the patients had one (27%) or more (52.1%) preexisting conditions, including hypertension (49.6%), obesity (37%), and type 2 diabetes mellitus (29.7%). Mean patient age was 58.9 years, and the majority of patients were men (64.3%). The median number of days from symptom onset to randomization was 9, and 53.6% of the patients were white, 20.6% were black, 12.6% were Asian, 23.4% were Hispanic or Latino, and the ethnicity of 13.6% were not reported or reported as other.
Patients received one 200-mg loading dose on the first day of the trial, and then one 100-mg maintenance dose every day for days 2 through 10, or until discharge or death. Patients in the control group of the study received a matching placebo on the same schedule and volume. The clinical status of each patient was assessed every day, from day 1 through day 29 of his or her hospital stay, according to an eight-category ordinal scale.
Time to recovery was defined as the first day during the 28-day enrollment period that a patient’s clinical status met a 1 (not hospitalization, no activity limitations), 2 (not hospitalized, activity limitation, oxygen requirement or both), or 3 (hospitalized, not requiring supplemental oxygen or medical care if hospitalization was extended for infection-control reasons) on the eight-category scale. A score of 4 indicated a patient was hospitalized and needed ongoing medical care, but did not require supplemental oxygen; a score of 8 signified death.
The analysis found remdesivir patients had a median time to recovery of 11 days, compared with the median 15 days for patients on the placebo (rate ratio for recovery, 1.32; 95% confidence interval, 1.12-1.55; P < .001). Mortality was also lower in the remdesivir group (hazard ratio for death, 0.70; 95% CI, 0.47-1.04), but the result was not statistically significant. By 14 days, the Kaplan-Meier estimate of mortality was 7.1 % in the remdesivir group and 11.9% in the placebo group.
Patients receiving oxygen, but not yet requiring high-flow oxygen, mechanical ventilation, or extracorporeal membrane oxygenation, seemed to fare best from treatment with remdesivir (these patients had a baseline ordinal score of 5). That may be a result of the larger sample size of these patients, the researchers note in the study. The study authors were unable to estimate the recovery time for the most severely ill patients (category 7), possibly because the follow-up time was too short to fully evaluate this subgroup.
“There is clear and consistent evidence of clinically significant benefit for those hospitalized on oxygen but not yet requiring mechanical ventilation,” Dr. Kaul, who was not involved in the study, said after seeing the published results. “Surprisingly, early dosing as measured from time to onset of symptoms did not seem to make a difference.”
Dr. Kaul said there is still the possibility that remdesivir could benefit patients on mechanical ventilation, but “clinicians will have to determine if the evidence suggesting no benefit in those who are intubated is strong enough to justify using this currently scarce resource in that population versus limiting use to those requiring oxygen but not on mechanical ventilation.”
Site investigators estimated that just four serious adverse events (two in each group) in enrolled patients were related to remdesivir or placebo. No deaths were attributed to the treatments, although acute respiratory failure, hypotension, acute kidney injury, and viral pneumonia were slightly more common in patients receiving the placebo than those receiving remdesivir.
The researchers plan to publish a follow-up study in the coming weeks or months, after the full cohort has completed 28 days of follow-up, Dr. Lane said. In future studies, the agency will likely focus on comparing remdesivir with combinations of remdesivir with other treatments, like the anti-inflammatory baricitinib.
A version of this article originally appeared on Medscape.com.
Much-anticipated results from the National Institute of Allergy and Infectious Diseases’ clinical trial of remdesivir in COVID-19 patients published in the New England Journal of Medicine suggest remdesivir shortens the disease course for hospitalized COVID-19 patients.
The agency reported initial promising results from the study earlier this month, which prompted the Food and Drug Administration to issue an emergency use authorization (EUA) for the drug, but the full data and results have not been widely available until now.
The findings also suggest remdesivir should be started, if possible, before patients have such severe pulmonary disease that they require mechanical ventilation, according to the study authors.
The published results are “completely consistent” with the NIAID’s earlier announcement, H. Clifford Lane, MD, deputy director for clinical research and special projects at the NIAID, said in an interview. “The benefit appeared to be the greatest for the patients who are hospitalized with severe disease who require supplemental oxygen.”
Given the limited supply of remdesivir, physicians have been eager to see the full data to ensure they use the drug most effectively, Daniel Kaul, MD, a professor of infectious diseases at the University of Michigan, Ann Arbor, said in an interview. Hospitals in states across the country, including New York, Michigan, and Washington, have received limited supplies of the drug in the last couple of weeks since the FDA’s authorization.
“I am losing my patience waiting for #remdesivir data. I was willing to give them a week to verify the numbers, triple proof the tables, cautiously frame conclusions. But it’s gone on too long. We are rationing with no rationale. We are floating on whisps [sic] of data, adrift,” Kate Stephenson, MD, an infectious diseases specialist at the Center for Virology and Vaccine Research at Harvard Medical School, Boston, wrote on Twitter May 18. After reading the paper, she tweeted Friday evening that she was “relieved to see convincing benefit – I was starting to worry!”
In the midst of a public health crisis, however, it is not unusual to make an announcement about trial results before the full dataset has been analyzed, said Dr. Lane. The NIAID followed a similar playbook for the PALM trial evaluating possible Ebola treatments in the Democratic Republic of Congo, with the independent monitoring board recommending the trial be terminated early in response to positive results from two of the four candidate drugs.
“When you have a result you think is of public health importance, you don’t wait for it to be published in a peer-reviewed journal,” said Dr. Lane, a coauthor of the study. The lag time from announcement to study publication was a result of the time it took to write up the paper for publication and go through peer review, Dr. Lane added. He also noted that the FDA had access to the data when the agency wrote its guidance for physicians administering the drug to patients under the EUA.
The authors opted not to publish the initial findings on a preprint server because they felt it was important to undergo peer review, said Dr. Lane. “The last thing you want for something this critical is for incomplete data to be out there, or you don’t have everything audited to the level that you want.”
Trial details
In the ACTT-1 randomized, placebo-controlled, double-blinded trial, researchers enrolled 1,063 patients from Feb. 21 to April 19, 2020, at 60 trial sites and 13 subsites worldwide (45 sites in the United States). The remdesivir group had 541 patients, and the placebo group had 522. A small number of patients (49 in the remdesivir group and 53 in the placebo group) discontinued treatment before day 10 because of an adverse event or withdrawn consent. When data collection for this preliminary analysis ended in late April, 301 patients had not recovered and had not completed their final follow-up visit.
Most of the patients had one (27%) or more (52.1%) preexisting conditions, including hypertension (49.6%), obesity (37%), and type 2 diabetes mellitus (29.7%). Mean patient age was 58.9 years, and the majority of patients were men (64.3%). The median number of days from symptom onset to randomization was 9, and 53.6% of the patients were white, 20.6% were black, 12.6% were Asian, 23.4% were Hispanic or Latino, and the ethnicity of 13.6% were not reported or reported as other.
Patients received one 200-mg loading dose on the first day of the trial, and then one 100-mg maintenance dose every day for days 2 through 10, or until discharge or death. Patients in the control group of the study received a matching placebo on the same schedule and volume. The clinical status of each patient was assessed every day, from day 1 through day 29 of his or her hospital stay, according to an eight-category ordinal scale.
Time to recovery was defined as the first day during the 28-day enrollment period that a patient’s clinical status met a 1 (not hospitalization, no activity limitations), 2 (not hospitalized, activity limitation, oxygen requirement or both), or 3 (hospitalized, not requiring supplemental oxygen or medical care if hospitalization was extended for infection-control reasons) on the eight-category scale. A score of 4 indicated a patient was hospitalized and needed ongoing medical care, but did not require supplemental oxygen; a score of 8 signified death.
The analysis found remdesivir patients had a median time to recovery of 11 days, compared with the median 15 days for patients on the placebo (rate ratio for recovery, 1.32; 95% confidence interval, 1.12-1.55; P < .001). Mortality was also lower in the remdesivir group (hazard ratio for death, 0.70; 95% CI, 0.47-1.04), but the result was not statistically significant. By 14 days, the Kaplan-Meier estimate of mortality was 7.1 % in the remdesivir group and 11.9% in the placebo group.
Patients receiving oxygen, but not yet requiring high-flow oxygen, mechanical ventilation, or extracorporeal membrane oxygenation, seemed to fare best from treatment with remdesivir (these patients had a baseline ordinal score of 5). That may be a result of the larger sample size of these patients, the researchers note in the study. The study authors were unable to estimate the recovery time for the most severely ill patients (category 7), possibly because the follow-up time was too short to fully evaluate this subgroup.
“There is clear and consistent evidence of clinically significant benefit for those hospitalized on oxygen but not yet requiring mechanical ventilation,” Dr. Kaul, who was not involved in the study, said after seeing the published results. “Surprisingly, early dosing as measured from time to onset of symptoms did not seem to make a difference.”
Dr. Kaul said there is still the possibility that remdesivir could benefit patients on mechanical ventilation, but “clinicians will have to determine if the evidence suggesting no benefit in those who are intubated is strong enough to justify using this currently scarce resource in that population versus limiting use to those requiring oxygen but not on mechanical ventilation.”
Site investigators estimated that just four serious adverse events (two in each group) in enrolled patients were related to remdesivir or placebo. No deaths were attributed to the treatments, although acute respiratory failure, hypotension, acute kidney injury, and viral pneumonia were slightly more common in patients receiving the placebo than those receiving remdesivir.
The researchers plan to publish a follow-up study in the coming weeks or months, after the full cohort has completed 28 days of follow-up, Dr. Lane said. In future studies, the agency will likely focus on comparing remdesivir with combinations of remdesivir with other treatments, like the anti-inflammatory baricitinib.
A version of this article originally appeared on Medscape.com.
Procalcitonin-Guided Antibiotic Discontinuation: An Antimicrobial Stewardship Initiative to Assist Providers
From Western Michigan University, Homer Stryker MD School of Medicine, Kalamazoo, MI (Dr. Vaillant and Dr. Kavanaugh), Ferris State University, Grand Rapids, MI (Dr. Mersfelder), and Bronson Methodist Hospital, Kalamazoo, MI (Dr. Maynard).
Abstract
- Background: Procalcitonin has emerged as an important marker of sepsis and lung infections of bacterial origin. The role of procalcitonin in guiding antibiotic stewardship in lower respiratory tract infections and sepsis has been extensively studied, and use of this biomarker has been shown to decrease antibiotic usage in clinical trials. We sought to evaluate the impact of a pharmacist-driven initiative regarding discontinuation of antibiotics utilizing procalcitonin levels at a community teaching hospital.
- Methods: We retrospectively gathered baseline data on adult patients admitted to a community teaching hospital who were 18 years of age and older, under the care of an inpatient service, and had a single procalcitonin level < 0.25 mcg/L obtained during admission. We then prospectively identified an intervention group of similar patients using a web-based, real-time clinical surveillance system. When a low procalcitonin level was identified in the intervention group, the participating clinical pharmacists screened for antibiotic use and the indication(s), determined whether the antibiotic could be discontinued based on the low procalcitonin level and the absence of another indication for antibiotics, and, when appropriate, contacted the patient’s health care provider via telephone to discuss possible antibiotic discontinuation. The total antibiotic treatment duration was compared between the baseline and intervention groups.
- Results: A total of 172 patients were included in this study (86 in each group). The duration of antibiotic use was not significantly different between the baseline (3.14 ± 4.04 days) and the intervention (3.34 ± 2.8 days) groups (P = 0.1083). Other patient demographics did not influence antibiotic duration.
- Conclusion: Our study did not demonstrate a difference in total antibiotic treatment duration with the utilization of procalcitonin and an oral communication intervention made by a clinical pharmacist at a community-based teaching hospital. Outside of clinical trials, and in the absence of an algorithmic approach, procalcitonin has not consistently been shown to aid in the diagnosis and treatment of infectious diseases. It is important to have a comprehensive antimicrobial stewardship program to reduce antibiotic use and effectively use laboratory values.
Keywords: antibiotic use; bacterial infection; biomarkers; procalcitonin.
Procalcitonin is the precursor of the hormone calcitonin, which is normally produced in the parafollicular cells of the thyroid gland under physiological conditions.1 However, procalcitonin is also released in response to a proinflammatory stimulus, especially that of bacterial origin.1 The source of the procalcitonin surge seen during proinflammatory states is not the parafollicular cells of the thyroid, but rather the neuroendocrine cells of the lung and intestine.1 Stimulants of procalcitonin in these scenarios include bacterial endotoxin, tumor necrosis factor, and interleukin-6.1,2 Due to these observations, procalcitonin has emerged as an important marker of sepsis and lung infections of bacterial origin.3
The role of procalcitonin in guiding antibiotic stewardship in lower respiratory tract infections and sepsis has been extensively studied.4,5 Various randomized controlled trials have shown that antibiotic stewardship guided by procalcitonin levels resulted in lower rates of antibiotic initiation and shorter duration of antibiotic use.4-6 Similar results were obtained in prospective studies evaluating its role in patients with chronic obstructive pulmonary disease and sepsis.7,8 Based on these data, protocol-driven procalcitonin-guided antibiotic stewardship appears beneficial.
Many of these studies employed rigorous protocols. Studies of procalcitonin use in a so-called real-world setting, in which the provider can order and use procalcitonin levels without the use of protocols, are limited. The objective of our study was to evaluate the impact of a pharmacist-driven initiative on discontinuing antibiotics, if indicated, utilizing single procalcitonin measurement results of < 0.25 mcg/L at a community teaching hospital.
Methods
Our study utilized a 2-phase approach. The first phase was a retrospective chart review to establish baseline data regarding adult inpatients with a low procalcitonin level; these patients were randomly selected over a 1-year period (2017). Patients were included if they were 18 years of age or older, under the care of an inpatient service, and had a single procalcitonin level < 0.25 mcg/L obtained during their admission. Patients admitted to the intensive care unit were excluded. In the second phase, we prospectively identified similar patients admitted between January and March 2018 using a web-based, real-time clinical surveillance system. When patients with low procalcitonin levels were identified, 2 participating clinical pharmacists screened for antibiotic use and indication. If it was determined that the antibiotic could be discontinued as a result of the low procalcitonin level and no additional indication for antibiotics was present, the pharmacist contacted the patient’s health care provider via telephone to discuss possible antibiotic discontinuation. Data collected before and after the intervention included total antibiotic treatment duration, white blood cell count, maximum temperature, age, and procalcitonin level.
A sample size of 86 was calculated to provide an alpha of 0.05 and a power of 0.8. A nonparametric Wilcoxon 2-sample test was used to test for a difference in duration of antibiotic treatment between the baseline and intervention groups. A nonparametric test was used due to right-skewed data. All patients were included in the group analysis, regardless of antibiotic use, as the procalcitonin level may have been used in the decision to initiate antibiotics, and this is more representative of a real-world application of the test. This allowed for detection of a significant decrease of 2 days in antibiotic duration post intervention, with a 10% margin to compensate for potential missing data. Data from 86 patients obtained prior to the pharmacist intervention acted as a control comparison group. Statistical analysis was performed using SAS 9.4.
Results
A total of 172 patients were included in this study: 86 patients prior to the intervention, and 86 after implementation. Baseline demographics, laboratory values, vitals, and principal diagnoses for both groups are shown in Table 1 and Table 2. The most common indications for procalcitonin measurement were pneumonia (45.9%), chronic obstructive pulmonary disease (15.7%), and sepsis (14.5%). The remaining diagnoses were encephalopathy, fever and leukocytosis, skin and soft tissue infection, urinary tract infection or pyelonephritis, bone and joint infection, meningitis, intra-abdominal infection, and asthma exacerbation.
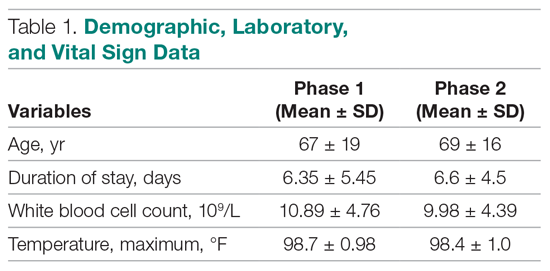
Antibiotic therapy was initiated in 68% of the patients overall, 59% in the baseline group and 76% in the intervention group. The duration of antibiotic use was not significantly different between the baseline (3.14 ± 4.04 days) and intervention (3.34 ± 2.8 days) groups (P = 0.1083). Furthermore, antibiotic treatment duration did not vary significantly with patient age, white blood cell count, maximum temperature, or procalcitonin level in either group. Although there was no difference in total antibiotic treatment duration, a post-hoc analysis revealed a 0.6-day decrease in the interval between the date of procalcitonin measurement and the stop date of antibiotics in the intervention group. The average time from admission to obtaining a procalcitonin level was 3 days in the baseline group and 2 days in the intervention group.
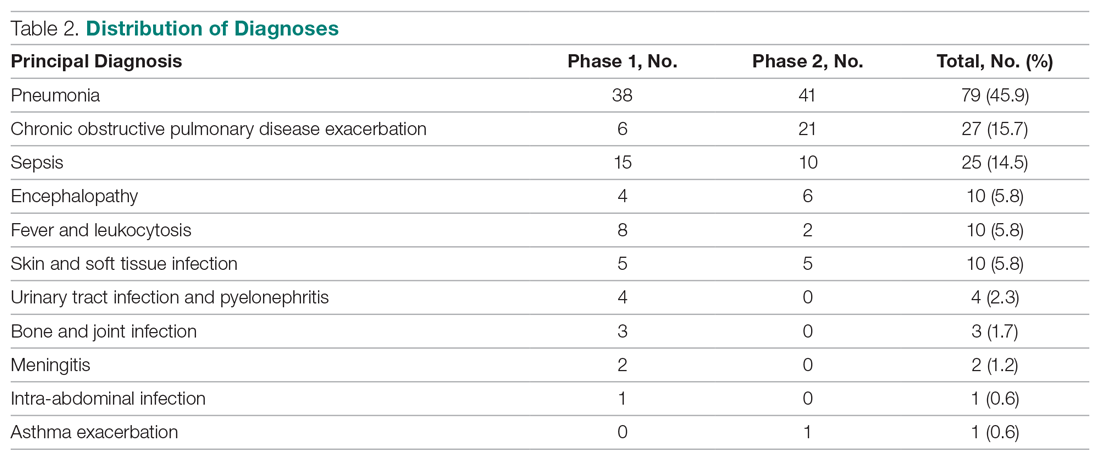
Discussion
Our study did not demonstrate a difference in total antibiotic treatment duration with procalcitonin measurement and an oral communication intervention made by a clinical pharmacist at a community teaching hospital with a well-established antimicrobial stewardship program. This may be due to several factors. First, the providers did not receive ongoing education regarding the appropriate use or interpretation of procalcitonin. The procalcitonin result in the electronic health record references the risk for progression to severe sepsis and/or septic shock, but does not indicate how to use procalcitonin as an aid in antibiotic decision-making. However, a recent study in patients with lower respiratory tract infections treated by providers who had been educated on the use of procalcitonin failed to find a reduction in total antibiotic use.9 Second, our study included hospital-wide use of procalcitonin, and was not limited to infections for which procalcitonin use has the strongest evidence (eg, upper respiratory tract infections, pneumonia, sepsis). Thus, providers may have been less likely to use protocolized guidelines. Last, we did not limit the data on antibiotic duration to patients with a procalcitonin level obtained within a defined time frame from antibiotic initiation or time of admission, and some patients had procalcitonin levels measured several days into their hospital stay. While this is likely to have skewed the data in favor of longer antibiotic treatment courses, it also represents a more realistic way in which this laboratory test is being used. Our post-hoc finding of earlier discontinuation of antibiotics after procalcitonin measurement suggests that our intervention may have influenced the decision to discontinue antibiotics. Such an effect may be augmented if procalcitonin is measured earlier in a hospital admission.
Previous studies have also failed to show that the use of procalcitonin decreased duration of antibiotics.9,10 In the aforementioned study regarding real-world outcomes in patients with lower respiratory tract infections, antibiotic duration was not reduced, despite provider education.9 A large observational study that evaluated real-world outcomes in intensive care unit patients did not find decreased antibiotic use or improved outcomes with procalcitonin use.10 With these large studies evaluating the 2 most common infectious diseases for which procalcitonin has previously been found to have clinical benefit, it is important for institutions to re-evaluate how procalcitonin is being utilized by providers. Furthermore, institutions should explore ways to optimize procalcitonin use and decrease unnecessary health care costs. Notably, the current community-acquired pneumonia guidelines recommend against routine use of procalcitonin.11
Conclusion
Outside of clinical trials, and in the absence of an algorithmic approach, procalcitonin has not consistently been shown to aid in the diagnosis or treatment of infectious diseases. It is important to have a comprehensive antimicrobial stewardship program that includes an algorithmic protocol to promote appropriate laboratory testing and reduce total antibiotic use. In addition to improved communication with providers, other interventions need to be investigated to effectively use this biomarker or limit its use.
Acknowledgment: The authors thank the Western Michigan University Department of Epidemiology and Biostatistics for their assistance in preparing this article.
Corresponding author: James Vaillant, MD, Western Michigan University, Homer Stryker MD School of Medicine, 1000 Oakland Drive, Kalamazoo, MI, 49008; [email protected].
Financial disclosures: None.
1. Maruna P, Nedelníková K, Gürlich R. Physiology and genetics of procalcitonin. Physiol Res. 2000;(49 suppl 1):S57-S61.
2. Becker KL, Snider R, Nylen ES. Procalcitonin in sepsis and systemic inflammation: a harmful biomarker and a therapeutic target. Br J Pharmacol. 2010;159:253-264.
3. Vijayan AL, Vanimaya RS, Saikant R, et al. Procalcitonin: a promising diagnostic marker for sepsis and antibiotic therapy. J Intensive Care. 2017;5:51.
4. Hey J, Thompson-Leduc P, Kirson NY, et al. Procalcitonin guidance in patients with lower respiratory tract infections: A systematic review and meta-analysis. Clin Chem Lab Med. 2018;56:1200-1209.
5. Schuetz P, Wirz Y, Sager R, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2017;10:CD007498.
6. Huang HB, Peng JM, Weng L, et al. Procalcitonin-guided antibiotic therapy in intensive care unit patients: a systematic review and meta-analysis. Ann Intensive Care. 2017;7:114.
7. Stolz D, Christ-Crain M, Bingisser R, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest. 2007;131:9-19.
8. Prkno A, Wacker C, Brunkhorst FM, Schlattmann P. Procalcitonin-guided therapy in intensive care unit patients with severe sepsis and septic shock—a systematic review and meta-analysis. Crit Care. 2013;17:R291.
9. Huang DT, Yealy DM, Filbin MR, et al. Procalcitonin-guided use of antibiotics for lower respiratory tract infections. N Engl J Med. 2018;379:236-249.
10. Chu DC, Mehta AB, Walkey AJ. Practice patterns and outcomes associated with procalcitonin use in critically ill patients with sepsis. Clin Infect Dis. 2017;64:1509-1515.
11. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45-e67.
From Western Michigan University, Homer Stryker MD School of Medicine, Kalamazoo, MI (Dr. Vaillant and Dr. Kavanaugh), Ferris State University, Grand Rapids, MI (Dr. Mersfelder), and Bronson Methodist Hospital, Kalamazoo, MI (Dr. Maynard).
Abstract
- Background: Procalcitonin has emerged as an important marker of sepsis and lung infections of bacterial origin. The role of procalcitonin in guiding antibiotic stewardship in lower respiratory tract infections and sepsis has been extensively studied, and use of this biomarker has been shown to decrease antibiotic usage in clinical trials. We sought to evaluate the impact of a pharmacist-driven initiative regarding discontinuation of antibiotics utilizing procalcitonin levels at a community teaching hospital.
- Methods: We retrospectively gathered baseline data on adult patients admitted to a community teaching hospital who were 18 years of age and older, under the care of an inpatient service, and had a single procalcitonin level < 0.25 mcg/L obtained during admission. We then prospectively identified an intervention group of similar patients using a web-based, real-time clinical surveillance system. When a low procalcitonin level was identified in the intervention group, the participating clinical pharmacists screened for antibiotic use and the indication(s), determined whether the antibiotic could be discontinued based on the low procalcitonin level and the absence of another indication for antibiotics, and, when appropriate, contacted the patient’s health care provider via telephone to discuss possible antibiotic discontinuation. The total antibiotic treatment duration was compared between the baseline and intervention groups.
- Results: A total of 172 patients were included in this study (86 in each group). The duration of antibiotic use was not significantly different between the baseline (3.14 ± 4.04 days) and the intervention (3.34 ± 2.8 days) groups (P = 0.1083). Other patient demographics did not influence antibiotic duration.
- Conclusion: Our study did not demonstrate a difference in total antibiotic treatment duration with the utilization of procalcitonin and an oral communication intervention made by a clinical pharmacist at a community-based teaching hospital. Outside of clinical trials, and in the absence of an algorithmic approach, procalcitonin has not consistently been shown to aid in the diagnosis and treatment of infectious diseases. It is important to have a comprehensive antimicrobial stewardship program to reduce antibiotic use and effectively use laboratory values.
Keywords: antibiotic use; bacterial infection; biomarkers; procalcitonin.
Procalcitonin is the precursor of the hormone calcitonin, which is normally produced in the parafollicular cells of the thyroid gland under physiological conditions.1 However, procalcitonin is also released in response to a proinflammatory stimulus, especially that of bacterial origin.1 The source of the procalcitonin surge seen during proinflammatory states is not the parafollicular cells of the thyroid, but rather the neuroendocrine cells of the lung and intestine.1 Stimulants of procalcitonin in these scenarios include bacterial endotoxin, tumor necrosis factor, and interleukin-6.1,2 Due to these observations, procalcitonin has emerged as an important marker of sepsis and lung infections of bacterial origin.3
The role of procalcitonin in guiding antibiotic stewardship in lower respiratory tract infections and sepsis has been extensively studied.4,5 Various randomized controlled trials have shown that antibiotic stewardship guided by procalcitonin levels resulted in lower rates of antibiotic initiation and shorter duration of antibiotic use.4-6 Similar results were obtained in prospective studies evaluating its role in patients with chronic obstructive pulmonary disease and sepsis.7,8 Based on these data, protocol-driven procalcitonin-guided antibiotic stewardship appears beneficial.
Many of these studies employed rigorous protocols. Studies of procalcitonin use in a so-called real-world setting, in which the provider can order and use procalcitonin levels without the use of protocols, are limited. The objective of our study was to evaluate the impact of a pharmacist-driven initiative on discontinuing antibiotics, if indicated, utilizing single procalcitonin measurement results of < 0.25 mcg/L at a community teaching hospital.
Methods
Our study utilized a 2-phase approach. The first phase was a retrospective chart review to establish baseline data regarding adult inpatients with a low procalcitonin level; these patients were randomly selected over a 1-year period (2017). Patients were included if they were 18 years of age or older, under the care of an inpatient service, and had a single procalcitonin level < 0.25 mcg/L obtained during their admission. Patients admitted to the intensive care unit were excluded. In the second phase, we prospectively identified similar patients admitted between January and March 2018 using a web-based, real-time clinical surveillance system. When patients with low procalcitonin levels were identified, 2 participating clinical pharmacists screened for antibiotic use and indication. If it was determined that the antibiotic could be discontinued as a result of the low procalcitonin level and no additional indication for antibiotics was present, the pharmacist contacted the patient’s health care provider via telephone to discuss possible antibiotic discontinuation. Data collected before and after the intervention included total antibiotic treatment duration, white blood cell count, maximum temperature, age, and procalcitonin level.
A sample size of 86 was calculated to provide an alpha of 0.05 and a power of 0.8. A nonparametric Wilcoxon 2-sample test was used to test for a difference in duration of antibiotic treatment between the baseline and intervention groups. A nonparametric test was used due to right-skewed data. All patients were included in the group analysis, regardless of antibiotic use, as the procalcitonin level may have been used in the decision to initiate antibiotics, and this is more representative of a real-world application of the test. This allowed for detection of a significant decrease of 2 days in antibiotic duration post intervention, with a 10% margin to compensate for potential missing data. Data from 86 patients obtained prior to the pharmacist intervention acted as a control comparison group. Statistical analysis was performed using SAS 9.4.
Results
A total of 172 patients were included in this study: 86 patients prior to the intervention, and 86 after implementation. Baseline demographics, laboratory values, vitals, and principal diagnoses for both groups are shown in Table 1 and Table 2. The most common indications for procalcitonin measurement were pneumonia (45.9%), chronic obstructive pulmonary disease (15.7%), and sepsis (14.5%). The remaining diagnoses were encephalopathy, fever and leukocytosis, skin and soft tissue infection, urinary tract infection or pyelonephritis, bone and joint infection, meningitis, intra-abdominal infection, and asthma exacerbation.

Antibiotic therapy was initiated in 68% of the patients overall, 59% in the baseline group and 76% in the intervention group. The duration of antibiotic use was not significantly different between the baseline (3.14 ± 4.04 days) and intervention (3.34 ± 2.8 days) groups (P = 0.1083). Furthermore, antibiotic treatment duration did not vary significantly with patient age, white blood cell count, maximum temperature, or procalcitonin level in either group. Although there was no difference in total antibiotic treatment duration, a post-hoc analysis revealed a 0.6-day decrease in the interval between the date of procalcitonin measurement and the stop date of antibiotics in the intervention group. The average time from admission to obtaining a procalcitonin level was 3 days in the baseline group and 2 days in the intervention group.

Discussion
Our study did not demonstrate a difference in total antibiotic treatment duration with procalcitonin measurement and an oral communication intervention made by a clinical pharmacist at a community teaching hospital with a well-established antimicrobial stewardship program. This may be due to several factors. First, the providers did not receive ongoing education regarding the appropriate use or interpretation of procalcitonin. The procalcitonin result in the electronic health record references the risk for progression to severe sepsis and/or septic shock, but does not indicate how to use procalcitonin as an aid in antibiotic decision-making. However, a recent study in patients with lower respiratory tract infections treated by providers who had been educated on the use of procalcitonin failed to find a reduction in total antibiotic use.9 Second, our study included hospital-wide use of procalcitonin, and was not limited to infections for which procalcitonin use has the strongest evidence (eg, upper respiratory tract infections, pneumonia, sepsis). Thus, providers may have been less likely to use protocolized guidelines. Last, we did not limit the data on antibiotic duration to patients with a procalcitonin level obtained within a defined time frame from antibiotic initiation or time of admission, and some patients had procalcitonin levels measured several days into their hospital stay. While this is likely to have skewed the data in favor of longer antibiotic treatment courses, it also represents a more realistic way in which this laboratory test is being used. Our post-hoc finding of earlier discontinuation of antibiotics after procalcitonin measurement suggests that our intervention may have influenced the decision to discontinue antibiotics. Such an effect may be augmented if procalcitonin is measured earlier in a hospital admission.
Previous studies have also failed to show that the use of procalcitonin decreased duration of antibiotics.9,10 In the aforementioned study regarding real-world outcomes in patients with lower respiratory tract infections, antibiotic duration was not reduced, despite provider education.9 A large observational study that evaluated real-world outcomes in intensive care unit patients did not find decreased antibiotic use or improved outcomes with procalcitonin use.10 With these large studies evaluating the 2 most common infectious diseases for which procalcitonin has previously been found to have clinical benefit, it is important for institutions to re-evaluate how procalcitonin is being utilized by providers. Furthermore, institutions should explore ways to optimize procalcitonin use and decrease unnecessary health care costs. Notably, the current community-acquired pneumonia guidelines recommend against routine use of procalcitonin.11
Conclusion
Outside of clinical trials, and in the absence of an algorithmic approach, procalcitonin has not consistently been shown to aid in the diagnosis or treatment of infectious diseases. It is important to have a comprehensive antimicrobial stewardship program that includes an algorithmic protocol to promote appropriate laboratory testing and reduce total antibiotic use. In addition to improved communication with providers, other interventions need to be investigated to effectively use this biomarker or limit its use.
Acknowledgment: The authors thank the Western Michigan University Department of Epidemiology and Biostatistics for their assistance in preparing this article.
Corresponding author: James Vaillant, MD, Western Michigan University, Homer Stryker MD School of Medicine, 1000 Oakland Drive, Kalamazoo, MI, 49008; [email protected].
Financial disclosures: None.
From Western Michigan University, Homer Stryker MD School of Medicine, Kalamazoo, MI (Dr. Vaillant and Dr. Kavanaugh), Ferris State University, Grand Rapids, MI (Dr. Mersfelder), and Bronson Methodist Hospital, Kalamazoo, MI (Dr. Maynard).
Abstract
- Background: Procalcitonin has emerged as an important marker of sepsis and lung infections of bacterial origin. The role of procalcitonin in guiding antibiotic stewardship in lower respiratory tract infections and sepsis has been extensively studied, and use of this biomarker has been shown to decrease antibiotic usage in clinical trials. We sought to evaluate the impact of a pharmacist-driven initiative regarding discontinuation of antibiotics utilizing procalcitonin levels at a community teaching hospital.
- Methods: We retrospectively gathered baseline data on adult patients admitted to a community teaching hospital who were 18 years of age and older, under the care of an inpatient service, and had a single procalcitonin level < 0.25 mcg/L obtained during admission. We then prospectively identified an intervention group of similar patients using a web-based, real-time clinical surveillance system. When a low procalcitonin level was identified in the intervention group, the participating clinical pharmacists screened for antibiotic use and the indication(s), determined whether the antibiotic could be discontinued based on the low procalcitonin level and the absence of another indication for antibiotics, and, when appropriate, contacted the patient’s health care provider via telephone to discuss possible antibiotic discontinuation. The total antibiotic treatment duration was compared between the baseline and intervention groups.
- Results: A total of 172 patients were included in this study (86 in each group). The duration of antibiotic use was not significantly different between the baseline (3.14 ± 4.04 days) and the intervention (3.34 ± 2.8 days) groups (P = 0.1083). Other patient demographics did not influence antibiotic duration.
- Conclusion: Our study did not demonstrate a difference in total antibiotic treatment duration with the utilization of procalcitonin and an oral communication intervention made by a clinical pharmacist at a community-based teaching hospital. Outside of clinical trials, and in the absence of an algorithmic approach, procalcitonin has not consistently been shown to aid in the diagnosis and treatment of infectious diseases. It is important to have a comprehensive antimicrobial stewardship program to reduce antibiotic use and effectively use laboratory values.
Keywords: antibiotic use; bacterial infection; biomarkers; procalcitonin.
Procalcitonin is the precursor of the hormone calcitonin, which is normally produced in the parafollicular cells of the thyroid gland under physiological conditions.1 However, procalcitonin is also released in response to a proinflammatory stimulus, especially that of bacterial origin.1 The source of the procalcitonin surge seen during proinflammatory states is not the parafollicular cells of the thyroid, but rather the neuroendocrine cells of the lung and intestine.1 Stimulants of procalcitonin in these scenarios include bacterial endotoxin, tumor necrosis factor, and interleukin-6.1,2 Due to these observations, procalcitonin has emerged as an important marker of sepsis and lung infections of bacterial origin.3
The role of procalcitonin in guiding antibiotic stewardship in lower respiratory tract infections and sepsis has been extensively studied.4,5 Various randomized controlled trials have shown that antibiotic stewardship guided by procalcitonin levels resulted in lower rates of antibiotic initiation and shorter duration of antibiotic use.4-6 Similar results were obtained in prospective studies evaluating its role in patients with chronic obstructive pulmonary disease and sepsis.7,8 Based on these data, protocol-driven procalcitonin-guided antibiotic stewardship appears beneficial.
Many of these studies employed rigorous protocols. Studies of procalcitonin use in a so-called real-world setting, in which the provider can order and use procalcitonin levels without the use of protocols, are limited. The objective of our study was to evaluate the impact of a pharmacist-driven initiative on discontinuing antibiotics, if indicated, utilizing single procalcitonin measurement results of < 0.25 mcg/L at a community teaching hospital.
Methods
Our study utilized a 2-phase approach. The first phase was a retrospective chart review to establish baseline data regarding adult inpatients with a low procalcitonin level; these patients were randomly selected over a 1-year period (2017). Patients were included if they were 18 years of age or older, under the care of an inpatient service, and had a single procalcitonin level < 0.25 mcg/L obtained during their admission. Patients admitted to the intensive care unit were excluded. In the second phase, we prospectively identified similar patients admitted between January and March 2018 using a web-based, real-time clinical surveillance system. When patients with low procalcitonin levels were identified, 2 participating clinical pharmacists screened for antibiotic use and indication. If it was determined that the antibiotic could be discontinued as a result of the low procalcitonin level and no additional indication for antibiotics was present, the pharmacist contacted the patient’s health care provider via telephone to discuss possible antibiotic discontinuation. Data collected before and after the intervention included total antibiotic treatment duration, white blood cell count, maximum temperature, age, and procalcitonin level.
A sample size of 86 was calculated to provide an alpha of 0.05 and a power of 0.8. A nonparametric Wilcoxon 2-sample test was used to test for a difference in duration of antibiotic treatment between the baseline and intervention groups. A nonparametric test was used due to right-skewed data. All patients were included in the group analysis, regardless of antibiotic use, as the procalcitonin level may have been used in the decision to initiate antibiotics, and this is more representative of a real-world application of the test. This allowed for detection of a significant decrease of 2 days in antibiotic duration post intervention, with a 10% margin to compensate for potential missing data. Data from 86 patients obtained prior to the pharmacist intervention acted as a control comparison group. Statistical analysis was performed using SAS 9.4.
Results
A total of 172 patients were included in this study: 86 patients prior to the intervention, and 86 after implementation. Baseline demographics, laboratory values, vitals, and principal diagnoses for both groups are shown in Table 1 and Table 2. The most common indications for procalcitonin measurement were pneumonia (45.9%), chronic obstructive pulmonary disease (15.7%), and sepsis (14.5%). The remaining diagnoses were encephalopathy, fever and leukocytosis, skin and soft tissue infection, urinary tract infection or pyelonephritis, bone and joint infection, meningitis, intra-abdominal infection, and asthma exacerbation.

Antibiotic therapy was initiated in 68% of the patients overall, 59% in the baseline group and 76% in the intervention group. The duration of antibiotic use was not significantly different between the baseline (3.14 ± 4.04 days) and intervention (3.34 ± 2.8 days) groups (P = 0.1083). Furthermore, antibiotic treatment duration did not vary significantly with patient age, white blood cell count, maximum temperature, or procalcitonin level in either group. Although there was no difference in total antibiotic treatment duration, a post-hoc analysis revealed a 0.6-day decrease in the interval between the date of procalcitonin measurement and the stop date of antibiotics in the intervention group. The average time from admission to obtaining a procalcitonin level was 3 days in the baseline group and 2 days in the intervention group.

Discussion
Our study did not demonstrate a difference in total antibiotic treatment duration with procalcitonin measurement and an oral communication intervention made by a clinical pharmacist at a community teaching hospital with a well-established antimicrobial stewardship program. This may be due to several factors. First, the providers did not receive ongoing education regarding the appropriate use or interpretation of procalcitonin. The procalcitonin result in the electronic health record references the risk for progression to severe sepsis and/or septic shock, but does not indicate how to use procalcitonin as an aid in antibiotic decision-making. However, a recent study in patients with lower respiratory tract infections treated by providers who had been educated on the use of procalcitonin failed to find a reduction in total antibiotic use.9 Second, our study included hospital-wide use of procalcitonin, and was not limited to infections for which procalcitonin use has the strongest evidence (eg, upper respiratory tract infections, pneumonia, sepsis). Thus, providers may have been less likely to use protocolized guidelines. Last, we did not limit the data on antibiotic duration to patients with a procalcitonin level obtained within a defined time frame from antibiotic initiation or time of admission, and some patients had procalcitonin levels measured several days into their hospital stay. While this is likely to have skewed the data in favor of longer antibiotic treatment courses, it also represents a more realistic way in which this laboratory test is being used. Our post-hoc finding of earlier discontinuation of antibiotics after procalcitonin measurement suggests that our intervention may have influenced the decision to discontinue antibiotics. Such an effect may be augmented if procalcitonin is measured earlier in a hospital admission.
Previous studies have also failed to show that the use of procalcitonin decreased duration of antibiotics.9,10 In the aforementioned study regarding real-world outcomes in patients with lower respiratory tract infections, antibiotic duration was not reduced, despite provider education.9 A large observational study that evaluated real-world outcomes in intensive care unit patients did not find decreased antibiotic use or improved outcomes with procalcitonin use.10 With these large studies evaluating the 2 most common infectious diseases for which procalcitonin has previously been found to have clinical benefit, it is important for institutions to re-evaluate how procalcitonin is being utilized by providers. Furthermore, institutions should explore ways to optimize procalcitonin use and decrease unnecessary health care costs. Notably, the current community-acquired pneumonia guidelines recommend against routine use of procalcitonin.11
Conclusion
Outside of clinical trials, and in the absence of an algorithmic approach, procalcitonin has not consistently been shown to aid in the diagnosis or treatment of infectious diseases. It is important to have a comprehensive antimicrobial stewardship program that includes an algorithmic protocol to promote appropriate laboratory testing and reduce total antibiotic use. In addition to improved communication with providers, other interventions need to be investigated to effectively use this biomarker or limit its use.
Acknowledgment: The authors thank the Western Michigan University Department of Epidemiology and Biostatistics for their assistance in preparing this article.
Corresponding author: James Vaillant, MD, Western Michigan University, Homer Stryker MD School of Medicine, 1000 Oakland Drive, Kalamazoo, MI, 49008; [email protected].
Financial disclosures: None.
1. Maruna P, Nedelníková K, Gürlich R. Physiology and genetics of procalcitonin. Physiol Res. 2000;(49 suppl 1):S57-S61.
2. Becker KL, Snider R, Nylen ES. Procalcitonin in sepsis and systemic inflammation: a harmful biomarker and a therapeutic target. Br J Pharmacol. 2010;159:253-264.
3. Vijayan AL, Vanimaya RS, Saikant R, et al. Procalcitonin: a promising diagnostic marker for sepsis and antibiotic therapy. J Intensive Care. 2017;5:51.
4. Hey J, Thompson-Leduc P, Kirson NY, et al. Procalcitonin guidance in patients with lower respiratory tract infections: A systematic review and meta-analysis. Clin Chem Lab Med. 2018;56:1200-1209.
5. Schuetz P, Wirz Y, Sager R, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2017;10:CD007498.
6. Huang HB, Peng JM, Weng L, et al. Procalcitonin-guided antibiotic therapy in intensive care unit patients: a systematic review and meta-analysis. Ann Intensive Care. 2017;7:114.
7. Stolz D, Christ-Crain M, Bingisser R, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest. 2007;131:9-19.
8. Prkno A, Wacker C, Brunkhorst FM, Schlattmann P. Procalcitonin-guided therapy in intensive care unit patients with severe sepsis and septic shock—a systematic review and meta-analysis. Crit Care. 2013;17:R291.
9. Huang DT, Yealy DM, Filbin MR, et al. Procalcitonin-guided use of antibiotics for lower respiratory tract infections. N Engl J Med. 2018;379:236-249.
10. Chu DC, Mehta AB, Walkey AJ. Practice patterns and outcomes associated with procalcitonin use in critically ill patients with sepsis. Clin Infect Dis. 2017;64:1509-1515.
11. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45-e67.
1. Maruna P, Nedelníková K, Gürlich R. Physiology and genetics of procalcitonin. Physiol Res. 2000;(49 suppl 1):S57-S61.
2. Becker KL, Snider R, Nylen ES. Procalcitonin in sepsis and systemic inflammation: a harmful biomarker and a therapeutic target. Br J Pharmacol. 2010;159:253-264.
3. Vijayan AL, Vanimaya RS, Saikant R, et al. Procalcitonin: a promising diagnostic marker for sepsis and antibiotic therapy. J Intensive Care. 2017;5:51.
4. Hey J, Thompson-Leduc P, Kirson NY, et al. Procalcitonin guidance in patients with lower respiratory tract infections: A systematic review and meta-analysis. Clin Chem Lab Med. 2018;56:1200-1209.
5. Schuetz P, Wirz Y, Sager R, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2017;10:CD007498.
6. Huang HB, Peng JM, Weng L, et al. Procalcitonin-guided antibiotic therapy in intensive care unit patients: a systematic review and meta-analysis. Ann Intensive Care. 2017;7:114.
7. Stolz D, Christ-Crain M, Bingisser R, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest. 2007;131:9-19.
8. Prkno A, Wacker C, Brunkhorst FM, Schlattmann P. Procalcitonin-guided therapy in intensive care unit patients with severe sepsis and septic shock—a systematic review and meta-analysis. Crit Care. 2013;17:R291.
9. Huang DT, Yealy DM, Filbin MR, et al. Procalcitonin-guided use of antibiotics for lower respiratory tract infections. N Engl J Med. 2018;379:236-249.
10. Chu DC, Mehta AB, Walkey AJ. Practice patterns and outcomes associated with procalcitonin use in critically ill patients with sepsis. Clin Infect Dis. 2017;64:1509-1515.
11. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45-e67.


